Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1067. The contractual start date was in August 2007. The final report began editorial review in January 2016 and was accepted for publication in February 2017. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Moniz-Cook et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction and background
Understanding does not only help find solutions, but it can generate tolerance as the behaviour loses its mystery. With tolerance comes the potential to cope, even with situations that are basically unchanged.
Stokes G. Behavioural, Ecobehavioural and Functional Analysis. In Stokes G, editor. Challenging Behaviour in Dementia: A Person-Centred Approach. pp. 142–541
Chapter overview
In this chapter we describe the background to our programme of assisting mental health practitioners and care home staff to respond effectively to challenging behaviour (CB) from people with dementia living at home and in care homes. First, we provide a conceptual overview and definitional rationale for CB in dementia care. This includes the layers of complexity that need to be considered in the management of CB in dementia within the ‘real world’ of family life and care home settings. Next, we summarise the development of functional analysis, as an approach to systematic assessment and associated management; the findings of our literature review of interventions based on this method; and a second review of the range of psychological and emotional needs of family carers that are associated with caring for people with dementia with CB. The conceptual overview and review findings provide a theoretical and empirically informed grounding for this approach to systematic assessment and the tailoring interventions to individual needs, in the management of CB in dementia care. Finally, we outline and discuss the studies we conducted in the chapters that follow.
Definition of challenging behaviour in dementia
Challenging behaviour associated with dementia includes a wide range of behaviours such as violent resistance to help with personal care and other aggressive responses, repetitive questioning, yelling or screaming, sexual disinhibition and apathy. It causes significant distress to caregivers. Often it is itself a manifestation of distress experienced by the person with dementia, whose cognitive impairment increasingly limits their ability to carry out desired actions, or to express their needs or to inhibit their own behaviour – as would be ‘normal’ for them within their interpersonal and social context. Two influential theories that partially, but far from comprehensively, account for these phenomena are the ‘unmet needs’ hypothesis2 and the ‘progressively lowered stress threshold’ hypothesis. 3
Together with incontinence, CB is the most common reason why family members pass over care responsibilities to residential facilities such as care homes. 4 This may be because the person’s behaviour has passed a threshold of intolerability or is deemed unmanageable at home. In care homes these behaviours then have to be managed by care staff,5–7 many of whom are poorly paid, are accorded low status and are insufficiently resourced and supported. 8,9 Behaviours can become more ‘florid’ (e.g. screaming or violent aggression) and frequent after a move to a care home. Increased frequency of these behaviours in care homes may be attributable to heightened distress, as the person is away from ‘home’ and familiar faces or routines, and does not have the cognitive capacity to adjust to the care home, or possibly because of deficiencies in care.
Phenomena or symptoms associated with CB in dementia are sometimes referred to as neuropsychiatric symptoms (NPSs) or behavioural and psychological symptoms of dementia (BPSD), which are defined as ‘signs and symptoms of disturbed perception, thought content, mood or behaviour that frequently occur in patients with dementia’. 10 This definition has the advantage of acknowledging the strong component of psychological suffering by the person and that there can be comorbid or accompanying mental illness, such as mood disorders, hallucinations and delusions. The definition is less satisfactory, in that it roots the phenomena solidly in the dementia, when this may not necessarily be the case,11 and it mixes up a diverse range of behaviours and mood states. However, its most serious disadvantage is that it takes no account of the context in which the behaviour occurs.
The importance of context in the management of CB in dementia was recognised within the National Institute for Health and Care Excellence (NICE) and Social Care Institute for Excellence (SCIE)’s Dementia Practice Guideline Number 42, in the term ‘behaviour that challenges’ (p. 210). 12 This includes the wide-ranging ways in which people with dementia and others in their environment respond to the phenomena of BPSD. Some of these responses can exacerbate distress for the person and/or their family or staff carers, or others in their environment. In line with these understandings, CB in dementia has been defined as a manifestation of distress or suffering for the person with dementia and/or distress in a carer’ (p. 573),13 and in this programme we extend this definition to describe a person’s behaviour as challenging when it causes distress to the person or the carer or others, thus threatening the quality of life of one or both parties.
There are no good data comparing the level of distress between family members and care home staff, but there is reason to believe that some family members may be more distressed by the unfamiliar and often embarrassing or threatening behaviour of a family member they thought they knew well. In contrast, disturbed behaviour may be seen as part of the job for care home staff, as they can at least look forward to relief at the end of their shift. However, the difficulties14 or ‘occupational disruption’ experienced by care workers faced with CB, regardless of whether or not they actually go on to become distressed later, remains a key factor in them seeking help from specialist services, admissions to hospital, accident and emergency (A&E) use, or transfer to another care home for the person with dementia. This in turn potentially results in increased levels of ‘excess disability’, meaning that functional abilities of people with dementia decline more quickly than can be accounted for by reducing cognition alone over the same period. We use the term CB here because its key component is that, for behaviour to become a clinical problem in need of treatment, it has to challenge the capacity of those exposed to it (usually family or staff carers) to cope.
There are no precise prevalence data because of widely differing perceptions of what is ‘challenging’ among those exposed to it; differences in how symptoms are ascertained and variable thresholds of severity and setting where behaviour problems are said to be ubiquitous. 15,16 Nonetheless, the cost of CB in dementia should not be underestimated, as 35.6 million people and families worldwide live with dementia,17 with an estimated cost of care of US$604B in 2010. 18 Breakdown of care at home becomes an inevitable extra cost if the public purse has to meet the costs of a proportion of the one-third of people with dementia who live in care homes. Although prevalence is hard to estimate, over 80% of people who move to nursing homes can have at least two or more of these behaviours. 19 The Neuropsychiatric Inventory (NPI) is a commonly used measure20 of NPSs and the incremental cost of just a one-point increase in score in a family care setting has been estimated, in the context of the USA, at US$30 per month on average, resulting in urgent calls for targeted intervention to reduce this significant cost. 21
Management of challenging behaviour in dementia
Treatment is problematic. There is extensive evidence of over-reliance on psychotropic medication, in particular antipsychotics,22 despite meta-analyses from 1990 to date showing modest efficacy at best, as well as frequent problematic side effects. 23–25 A rough measure of ineffectiveness is that half to two-thirds of participants referred to intervention studies because of unresolved problem behaviour will already be taking antipsychotic medication. 26,27 Because of the mounting evidence of harm from antipsychotic use in older people with dementia, there are occasional surges of interest in other compounds, in particular anticonvulsants (mood stabilisers), but the evidence is that they are equally ineffective and have equally harmful side effects. 28,29 The dangers of benzodiazepines for older people have long been known30,31 and their use for CB has declined, though a substantial number of medical practitioners are reported as remaining unaware of the literature. 32 The inadequacies of psychotropic drugs mean that there are frequent recommendations to make non-pharmacological interventions the routine first-line treatment. 29 Such calls are honoured more in the breach than the observance but, in any case, the evidence for standard psychosocial approaches is at least as weak as that for psychopharmacology. Systematic reviews of various discrete approaches, such as aromatherapy, light therapy or activity programmes, describe them as showing promise, but most studies lack the methodological rigour required to determine whether or not they are truly effective. 33 In 2005, in one of the most comprehensive meta-analyses of drug trials, Sink et al. 29 concluded that there was ‘no magic pill for neuropsychiatric symptoms of dementia’. This applies equally to standardised psychosocial interventions.
It should be no surprise that where the main clinical target is the suppression of behaviour, standardised pharmacological or psychosocial interventions are only intermittently effective. Interventions often appear to be based on a ‘one syndrome standard treatment’ paradigm. 13 The syndrome is the behaviour or, mostly, a cluster of behaviours and other phenomena usually labelled BPSD or agitation, and treatments applied include interventions for multisensory stimulation such as snoezelen,34 antipsychotics such as risperidone,29 and analgesics. 35 The primary problem is that the behaviour alone is the wrong target for intervention, as the syndrome-standard treatment model takes little or no account of the multifaceted context of the behaviour and its effects. This includes causal or exacerbating factors for the behaviour, why it becomes a clinical or care problem in any one case, and the characteristics and capabilities of those involved. The syndrome is actually very elusive because each of these contextual matters varies widely from case to case and over time. This has profound implications for the nature of clinical interventions, how they are delivered and their utility, as well as for measurement and methodology in intervention research.
Elusiveness of the syndrome: aetiology and other contextual factors
Many current guidelines, including the International Psychogeriatric Association’s Complete Guide to Behavioral and Psychological Symptoms of Dementia36 and Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care (p. 210),12 acknowledge multiple aetiologies for CB, including genetic, neurobiological, psychosocial, medical and physical factors. Given such a complex causal mix, it follows that there will be wide variability between individuals, even if the behaviour is the same, and that a case-specific approach will often be required. Genetic and neurobiological variables are not currently adjustable, but many psychosocial and physical/medical factors are actually or potentially treatable. For example, many treatable or modifiable factors, often in interaction, can contribute to screaming or yelling in dementia, including pain or depression,37 the way care is carried out,38 sensory loss,39 overstimulation40 or loneliness. 41 Similarly, the cause of sleep disturbance or night-wandering can be staff noise or active waking of residents;42 too much sleep/dozing or inactivity during the day;43 inability to find the toilet or the way back to a room at night;44 previous night-time regimes;13 or any combination of these. 45 Addressing potentially treatable case-specific aetiological factors such as these is an obvious first step, and some studies do acknowledge and treat common conditions underlying CB, such as delirium or, in particular, pain, which is grossly undertreated in dementia. 35 However, in the main, trials of standard psychosocial or pharmacological treatments aimed at the behaviour do not typically address causal factors. At best, this is poor practice, but it is also pernicious, leading to non-treatment or wrong treatment of causes of suffering. For example, several studies have shown that, by contrast with cognitively intact older people, people with dementia who are in pain are more likely to be given antipsychotics than analgesics. 46
The elusiveness of the syndrome is not just because of idiosyncratic causes of behaviour. It applies equally to distress among family carers or care staff, and in care homes there may also be distress caused to other residents. Hence, distress in others or the potential for injury to the person and others is what defines the behaviour as ‘challenging’. For example, in family care settings, frequent behaviours are not necessarily the most challenging for carers;47 the carer’s own characteristics (independent of dementia severity) and their sense of a declining relationship with their relative can contribute to the development or maintenance of CB. 48,49 Emotional responses to, and perception of, the behaviour vary widely,26,50 from extreme distress to regarding the behaviour as ‘no problem’. Most people are distressed by behaviours such as screaming, repetitive questions, violence or behaviour of great intensity. Nevertheless, in many cases, individual characteristics of family carers51,52 or care staff are often as important as severity of behaviour in determining whether or not, or to what degree, the behaviour is perceived as challenging. These factors include limited understanding of the changes associated with dementia; lack of support; limited skills; pejorative attitudes to people with dementia or older people; and mood disorders in caregivers. 53,54 In an early example, Hinchliffe et al. 55 found that treating depressed family carers changed their perception of the behaviour, from ‘intolerable’ to ‘no problem’. Thus, in a significant number of cases, though far from all, CB can be described as being in ‘the eye of the beholder’, and a successful intervention can be one that does not change the behaviour but leads to carers no longer perceiving that behaviour as a problem, or at least as not so great a problem.
In residential care, the characteristics of the home contribute to the elusiveness of the syndrome in much the same way. It tends not to depend on the formal classification of the home and nature of funding (subsidised or non-subsidised), but more on individual differences between homes. 56 Even if residents have much the same profiles, in relatively stable facilities where the culture fosters a high level of dementia literacy, empathy and skills, and strong support, disturbed behaviour is less likely to occur, or, where it does occur, it is less likely to be perceived as challenging. 53,57 This means that it is not only the syndrome that is elusive, but also any sense of a standard treatment, because the nature of the intervention and how it is delivered will depend on the existing skill set and culture. However, not even here can stability be assumed. The residential care sector is often in flux; organisational changes, including takeovers and loss of staff, are common, even during intervention studies. 9 Accordingly, attention must be paid to how to engage sufficient staff to make a sustained difference. 58
In summary, because of the multiple interacting contextual factors surrounding BPSD, many of which may have nothing to do with dementia per se, and some of which have little to do with the person with dementia or the objective severity of the behaviour, standardised pharmacological or psychosocial treatments are always going to have strictly limited effectiveness. This is exactly what the literature shows. Treatment should vary in each individual case depending on the aetiology and/or the context in which it occurs, including characteristics of carers, practitioners and the person with dementia – which will determine what is possible in any given case. Thus, the identified focus may be the person with dementia and/or family and/or staff members and/or a whole facility, and treatment may include pharmacological and/or psychosocial methods. Injunctions to use psychosocial methods first are incorrect. If the cause of someone being violent in personal care is painful joints, pharmacological pain relief is likely to be the front-line treatment adjunct with empathic support during personal care. If the cause is poor staff skills, treatment is likely to be psychosocial. If the cause is a combination of both, for example where a care worker with low skills is also insensitive to the fact that the resident is in pain, then the treatment is likely to be both analgesics and training with supportive supervision of the staff member. Where the behaviour is simply dangerous (to self or others), the first line of treatment will often involve psychotropic medication, including antipsychotics, if there is no other alternative (p. 261),12 but usually there are alternatives. 59
Implications for methodology
As there is no standard syndrome based on the behaviour and, therefore, no standard treatment, a range of measures must cover multiple domains in intervention studies. By definition, CB involves both an individual’s behaviour and another’s response to this; therefore, measures of both are needed, and the response must be linked to the referred behaviour. Measures of behaviour must include the actual behaviours that are distressing carers or practitioners, but also generic behaviour measures so that change over time can be aggregated across a diverse range of behaviours of widely varying frequency. Given that severity of behaviour often predicts carer distress, measures of severity as well as frequency are required; for example, the effects of a very low-frequency behaviour, such as physical aggression, can be much more serious than, for example, high-frequency pacing or walking up and down. This often leads to problems in determining what constitutes ‘caseness’, that is, the threshold for offering a clinical intervention, and, equally, what constitutes a successful intervention. In residential care, because of variability between staff and the influence of care culture in determining the quality of care, as well as receptivity to interventions, there must be more general measures (e.g. staff morale, knowledge, skill levels, whether or not organisational change occurs), to enable an analysis of staff and care home factors likely to predict benefit from an intervention. Because of large differences between care homes’ organisation and facilities, there must be a representative sample of them rather than just one or two and, because of the effect of culture, the facility or specific unit rather than individual residents must be the unit of randomisation.
Rationale for current study
A number of trials have delivered education to family carers or care staff as the sole or an important component of interventions, variously covering generic and client-specific skills and knowledge, and/or providing emotional support. That is, they recognise the case- and context-specific nature of CB by attempting to enhance the emotional, attitudinal and practical skills of care providers such that they can flexibly adapt to each new case or change care practices to prevent or minimise CB occurring. Few of these trials have had adequate methodology (for reviews see McCabe et al. 58 and Spector et al. 60), but there have been encouraging, though not conclusive, results in trials of adequate rigour. Changes in behaviour or family carer or staff distress are more likely to occur in programmes that are more case or client specific, that is, education that is person centred or more explicitly links interventions to the specific environment. 60
Examples of outcomes for people with dementia living in residential care have been reductions in frequency and perceived severity of the target behaviour and general practitioner (GP) call-outs,50 reductions in antipsychotic use,27 reductions in agitated behaviour and increases in observed participant pleasure,61 reductions in behaviour frequency and perceived severity, hospitalisations, antipsychotic use, and drug side effects;26,62 and for staff, reductions in stress and short-term improvements in the perception of how challenging staff found the behaviour. 63 Systematic interventions of this type, targeting a variety of outcomes within family care settings, are less common, but they can be found, including within NHS settings in England, where they have demonstrated reductions in CB and improvements in carer mental health. 55,64,65
Many of these studies, especially those involving supervision, required expert clinicians to work with family carers or with residential care facilities. However, Bird et al. 26 showed that the individualised formulaic ‘case-specific’ approach was no more time consuming when compared with ‘usual care’ practice in residential care settings, but it still required a mean of 5.5 clinical visits per case. Applying a similar approach to ‘treatment-resistant cases’, in care homes, Davison et al. 50 required a median of 3 months per case, spread over three visits, to achieve improvements in some, but not necessarily in more complex or treatment-resistant cases. This necessitates significant time/resource investment, namely addressing the shortage of clinicians with the requisite skills to provide the necessary assessment and subsequent intervention to the large and growing number of older people with dementia and CB, and those who care for them. Such ‘expert’ interventions are expensive, so alternative models of service provision are required. In residential care, where the most florid behaviour occurs, there seems to be the potential for staff, if given enough sustained support, to gain and retain these skills for themselves. Furthermore, most of the information required to assess CB, from the resident’s health status to the way intimate personal care is carried out, is already available in the care home, and care staff members are often the primary source of the information that is required by visiting professionals. Support for CB in family care settings in the UK has traditionally been provided by community mental health nurses (CMHNs) working within community mental health teams for older people (CMHTsOP). By providing these practitioners with specialist supervision to target their interventions, reductions in CB and improvements in carer mental health have been demonstrated. 65
Given the elusiveness of the syndrome, our first challenge was to devise a method to assist care home staff and community practitioners themselves to gather and analyse information required for CB interventions in a sufficiently standardised manner to be taught in a generic way, but which also takes as much account as possible of the idiosyncratic biomedical, social, environmental and other contextual factors of each case where behaviour is perceived as challenging. The closest approximation is a well-established range of techniques known as ‘functional analysis’ (see Functional analysis-based interventions), which has been used in a number of single case studies or case series. 66–69 Our second challenge was to devise a means to engage staff in a way that they perceive as relevant to their working experience and with sufficient power to change or expand the way they perceive and respond to behaviour they find challenging. There are preliminary studies of successful interactive online programs using actors to simulate common behaviours in context followed by demonstration of how effective responses can be achieved. 70–73 There is also increasing interest in online education. 72–75 Furthermore, emerging approaches towards personalised electronic decision support systems for targeting dementia care in community settings are being developed. 76 However, these are at an early stage and are yet to be considered for CB.
Functional analysis-based interventions
Functional analysis is a systematic framework for assessment that takes into account potential factors that may cause or contribute to a given behaviour (i.e. the function of the behaviour) for an individual at a given time and within a given social interaction or environmental setting. Following a functional analysis that has fully considered the potential cause(s) or contributory factors underlying the person’s behaviour in their interpersonal situation, the practitioner can then generate ideas of ways of intervening and then test these out in the individual case. If the challenge has not been resolved in an acceptable way, the practitioner can return to other creative ways of addressing the cause and associated challenge. If the challenge has not been resolved in a satisfactory way or if the practitioner wants to consider the function of the same behaviour in a different context or another behaviour, s/he can continue to use the information from the comprehensive assessment, together with observations in the relevant interpersonal setting, to consider other potential ways of intervening. The approach is essentially one of systematic ‘hypothesis generation’, akin to an iterative ‘detective’-like approach to CB in dementia, and overcomes the aforementioned pitfalls of the search for a magic psychosocial or medical ‘pill’ to overcome the challenges that face individual and groups of carers in dementia care. The interventions that arise from a functional analysis are referred to here as functional analysis-based interventions and can include factors, such as pain or infection, that cause discomfort, as well as psychological or social need. Next we outline the development of functional analysis as a means of managing CB in dementia in the UK.
Firmly nested in the tradition of applied behaviour analysis, the functional analytic perspective came to prominence initially in the field of intellectual (learning) disability where, from the early 1980s, conceptualisation and research gradually moved away from the reductionist ‘behaviour modification’ approach to a perspective that encompasses a more person-centred functional analytic perspective. Thus, the term ‘challenging behaviour’ – used in the USA in 1988 by The Association for Persons with Severe Handicaps, replaced that of ‘problem’ or ‘disruptive’ behaviour. 77 This change of terminology signalled an important relocation of responsibility from the individuals displaying the behaviour to the systems around them. A significant body of research emerged over the subsequent two decades attesting to the importance of functional analysis in the field of CB. 78 Although the emerging literature on functional analysis from the USA emphasised the importance of tightly controlled hypotheses-driven behavioural experiments, the literature from the UK also included analysis of the wider context of the person’s life and variables that were closer to the concept of function, where the ‘meaning’ or ‘purpose’ of behaviour was also considered. Thus, the British conceptualisation of functional analysis combined the rigours of experimental analysis with a more anthropological and contextual emphasis on function,79 and some professional societies in the UK provided associated guidance for practitioners. 80,81
In 2000, Stokes1 (see chapter 8) provided an overview of the translation of applied behaviour analysis and the growth of functional analytical approaches to dementia care from around the mid-1980s in the UK. By the mid-1990s a parallel movement associated with the person-centred model of dementia strengthened the potential for application of applied behavioural analysis by allowing researchers to identify the functional significance of many ‘bizarre’ CBs. The challenge was now for services, professionals and carers to find more effective methods of understanding the origins and meaning of a person’s behaviour. This person-centred approach also saw the growth of attempts to find creative ways of responding to the challenges to services. 1 However, although modelling and testing of functional analysis through single case studies exist,1,65–69 these tend to be located in the care home setting. Systematic attempts by professionals and researchers alike to reduce the need of a person with dementia to engage in ‘behaviour that challenges’, where the guideline outlines the same considerations that we have also described previously within a functional analytical assessment framework (see Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care, p. 210),12 have been, on the whole, anecdotal and intuitive. This may be because making systematic choices about the most appropriate and often multicomponent interventions in a given case can be difficult for the practitioner. This is also the case when the practitioner requires to assist a professional or family carer to respond, through understanding of the sometimes ‘idiosyncratic’ meaning of the ‘behaviour that challenges’ in an individual who may be communicating an unmet need and/or distress through their behaviour.
Challenge Demcare
The programme of work reported here sought to use the wider conceptualisation of functional analysis as the basis of its methodology and associated technology, using the internet to expand availability of the method as widely as possible. In order to apply our theoretical stance to a functional analysis-based framework for CB interventions in dementia, we conducted two literature reviews, which will be outlined next.
Literature reviews on the management of challenging behaviour in dementia
First, we conducted a systematic Cochrane review of functional analysis-based interventions for the management of CB in dementia in 2009–10 (updated and published in 2012). 82 This integrated a number of features taken from the conceptual overview described above, including the growth of functional analysis in dementia care and the importance of including the context of CB in the analysis. Thus, although a central tenet of the approach was to teach staff and practitioners to carefully observe the person and their immediate environment before making assumptions about the function of the behaviour, the analysis of function moved beyond the mere completion of antecedent–behaviour–consequence charts and the reductionist assumption that behaviour is a function of its consequences. The review therefore also focused on interventions that included identifying the function of behaviour for an individual, based on knowledge of the person’s life story, to understanding the ‘unmet need’ that was being communicated by the distressed person. Finally, the review included interventions that incorporated training and specialist support of staff in care homes, and trained community practitioners who provided care to families, to apply, monitor, evaluate and adjust individually tailored interventions to reduce CB in dementia. Our primary outcome measure was CB, including the behaviour and responses or reactions to this.
All randomised controlled studies that included functional analysis-based interventions for dementia compared with a control condition were included if they had a valid outcome measure of reported occurrence, in terms of frequency or incidence of CB. Participants included those living at home or in care homes or those cared for in hospital or other dementia facilities, such as assisted living units. The primary outcome was change in reported behaviour or mood on standardised measures, and secondary measures included changes in the caregiver, that is, their reaction, distress, perceived management difficulty and well-being (mood, morale efficacy and burden). The searches located 3335 references, from which 144 abstracts were retrieved. One hundred and twenty-six papers were excluded following our quality ratings and checks for duplication, and 18 were selected for review (see Moniz Cook et al. ,82 table 2), with a baseline total of 2558 care recipients. The majority of these (n = 13) were within family care settings and just three were conducted in care homes. Of these, only two family care studies were conducted in England, in Kent64 and Hull,65 and only one care home study, in Manchester. 83 The review concluded that functional analysis within multicomponent interventions that are geared to the context of either the family or the care home shows promise. A striking finding from the studies reviewed in family settings was the importance of providing support to meet the psychological needs of the family carer. This was consistent with our conceptual understanding of CB that was described earlier, that is, attending to context within the family system is an important target for interventions to reduce CB in dementia. The relatively few randomised controlled trials (RCTs) in care home settings made it hard to properly evaluate the importance of intervening in the system or context of the care home. However, one of these three studies from England,27 in which person-centred care was offered to reduce the use of antipsychotic medication (but did not show change on behaviour outcomes), has been recently upscaled in an implementation study. The authors achieved reductions in antipsychotics equivalent to the original study in some cases, but conclude by outlining contextual barriers to implementation; they recommend revisions to the intervention to address these barriers. 84
Second, given the findings from the Cochrane review on the functional analysis-based interventions,82 that effective interventions usually involve a component of psychotherapy or counselling directed at the family carer, we conducted a second review to gain an in-depth understanding of the potentially ‘hidden’ needs of families living with dementia and CB. This was thought to be important as a focus for the content of a multicomponent intervention for the management of CB in dementia in family settings, as two-thirds of families that receive professional support report an unmet need associated with behaviour management in dementia. 85 A meta-ethnographic approach was chosen to review studies that employed qualitative and quantitative methods of family carer experiences of dementia and CB. Our wide-ranging search strategy identified 10,375 references, of which 70 studies met our initial inclusion criteria and 25 high-quality studies were finally included in the review. Reasons for CB were associated with changes in communication and misunderstandings about the meaning of the relative’s behavior, which was seen by some carers as ‘antisocial’. 86
Our conceptual overview and review findings together provide a theoretical and empirically informed grounding for a functional analysis-based framework for choosing interventions for the management of CB. Thus, we conceived case-specific functional analysis-based interventions for CB to include the health and psychological needs of the person with dementia at a given time, as well as attention to the physical and social environment and the caregiving context. This then included the support needs of staff within a given care home or, for example, the psychological needs of families. In the design of an interactive online intervention for CB in dementia, we created algorithms for intervention within each of these three domains, ensuring that the third domain differed for people living at home or in a care home.
Outline of studies within Challenge Demcare: Chapters 2–6
Our interactive online intervention aimed to provide easy access for care home staff and community practitioners to training and support to meet the needs of people with dementia and CB (see Chapter 2). The ‘e-intervention’ consisted of an e-learning course for staff that was graded across three modules and a decision support system consisting of two e-tools that followed the e-learning course. These e-tools were context specific and tailored for use by staff supporting people with dementia either at home or in a care home. Our aim, in keeping with the applied behaviour analysis roots of functional analysis, was to help care home staff to alter their interactions with the person through an understanding of the basic principles of functional analysis. But we hoped to go beyond behaviour change alone, by changing attitudes as well as behaviour. We anticipated that by paying more attention to the functions of a person’s behaviour, staff would go beyond a ‘rule-governed’ approach that assumed function (‘He’s just doing it to get attention’) and develop a shared sense of humanity and affinity with the person. In this way, we hoped to build a higher tolerance to those CBs that were unlikely to change. For community practitioners, such as CMHNs working in CMHTsOP across England, who provide support to family carers, we expected the training to provide the background and tools for functional analysis-based interventions. Thus, the e-learning course and decision support e-tool were structured in the same way to facilitate the production of a targeted action plan, referred to as functional analysis-based interventions for the management of CB in dementia. Application of the interactive e-learning course for staff from the intervention arm of our care home study, ResCare, and experiences with the decision support e-tool in our family care study, FamCare, are outlined in Chapter 2.
Following adjustments to the procedure of delivery of functional analysis-based interventions in care homes, the clinical effectiveness and cost-effectiveness of the web-assisted intervention were evaluated within a cluster randomised trial (CRT) (see Chapter 3).
We then conducted a comprehensive process evaluation to throw light on the mechanisms responsible for our findings in the ResCare trial (see Chapter 4).
Chapter 5 describes the FamCare observational study, our study of specialist community mental health services for people with dementia and CB living in family care settings in England.
Finally, we conclude by reflecting on our programme across care home and family care settings. We summarise key findings, and, on the basis of observed limitations to our care home intervention, important implications for the design of future research of this type are outlined. We also consider the implications for future research and practice in the delivery of support for CB in dementia care, in the light of changing policies and services for people with dementia and CB (see Chapter 6).
Chapter 2 Development and testing of an online application of functional analysis approaches to intervention for challenging behaviour in dementia
Abstract
Aim
To describe the development and field testing of an interactive online training and decision support intervention, using functional analysis approaches for the management of CB in dementia.
Method
An e-learning course and two decision support e-tools were developed to help staff to use functional analysis-based interventions for up to 25 commonly reported CBs in dementia. The intervention was tested (2011–12) with 92 nominated ‘staff champions’ from 27 care homes and 26 community mental health practitioners from six NHS organisations across England.
Results
The course was well received and strongly recommended by care home staff champions who completed an evaluation sheet (n = 92), but only when this occurred at an external venue, with opportunity for facilitated discussion and practice. Although freely available within homes, e-learning take-up by other staff was limited. Staff selected as champions by their managers were, on average, younger [t(606) = 2.12; p = 0.032], had higher educational attainment (Fisher’s exact test p = 0.0448) and were more likely to have had dementia training (χ2 = 4.38; p = 0.036) than others working in the care homes. E-tool-assisted action plans were developed for 199 residents with CB. Aggression was mostly selected by staff where 58 action plans (29%) were delivered. Immediately after training, staff appeared to have expanded the way in which they viewed some behaviour. They were less likely to perceive behaviour as challenging, with a significant reduction in ratings of CB following training [t(178) = 7.4; p < 0.001]. Community mental health practitioners, who tested the community decision support system for their patients with CB, valued its logical assessment framework and the ‘if–then’ algorithmic method for choosing potentially helpful case-specific interventions.
Conclusions
Worksite-based e-learning opportunities are not at present readily taken up by staff working in care homes in England. Computerised decision support for interventions for CB appears premature in care homes, but shows promise for training community dementia practitioners. However, usability will depend on successful collaboration between clinical experts, information technology (IT) advisors within NHS organisations and software engineers.
Introduction
The concept for the design of the intervention is outlined in Chapter 1. Our aim was to devise a means to engage staff in a way that they perceived as relevant to their working experiences and that had sufficient power to change or expand the way in which they responded to behaviours they found challenging. The intention was to provide an easily available and sustained resource in care homes for staff to learn about behaviours seen as challenging, common contextual reasons why they occur and effective ways to respond, that is, to make them aware that the syndrome is elusive and that, as a consequence, standard responses based only on the nature of the behaviour will be ineffective. By providing a structured framework for capturing the syndrome, with some examples from family and care home settings, we considered this resource to also be relevant for community mental health practitioners working with family carers, in the management of CB in the home setting. Second, when CB does occur, our intervention was designed to enable care home staff and community mental health practitioners to use functional analysis to assess all the parameters of the case sufficiently comprehensively and thus apply systematic support to reduce the impact of CB in dementia care.
Development of the intervention
We conceived a multimedia interactive functional analysis-based intervention for CB in dementia. This comprised a training programme, together with two suites of decision support systems (one for staff in care homes and the other for staff supporting family carers in the community) for the targeting of individualised or person-centred interventions for CB in dementia. A range of options for the platform was considered, including our original plan to develop DVD and CD materials. We explored the potential strengths of an online solution, which were as follows: increased accessibility to information, where content could be standardised, easily updated and revised (see Ruiz et al. 87 for an overview of e-learning in education); options for self-pacing to overcome time pressures;88 immediate feedback with options for improvement, which is an important approach for adult learners;89 and fidelity of presentation with automated documentation, such as tracking and reporting of the learner’s activity,73 to allow monitoring of usage and problems with the program itself and thus facilitate improvements to meet the needs of the learner. In addition, feasibility studies in the USA have noted that direct care workers in nursing homes respond positively to internet-based multimedia training, including the management of aggression. 70,71,73 In family care settings, information and communication technology (ICT)-based solutions have been piloted74 and are also described as important uncharted territory in supporting families to cope with CB at home. 90 Given the huge growth of internet use in recent years, we considered that the online option would offer greater flexibility for those, including care home workers and community practitioners, who may wish to access the e-learning aspect of the resource from other venues, including potentially their own home.
The e-learning course was a multimedia, interactive, skills-based method, to encourage comprehensive assessment, such that knowledge about the person with dementia’s behaviour in their environment could be understood in order to provide solutions. These could include signposting or referral to other professionals when needed. Its aim was to encourage staff to systematically consider variables such as the person’s medical status, life story, communication and a host of other ‘unobservables’ that may give clues about the function of the behaviour. The information management systems (IMSs) for the two decision support e-tools were designed to follow on from a ‘functional analysis’ of the behaviour, in selecting approaches that were likely to ameliorate the behaviour, the emotional response to it or both. Three e-learning modules (outlined below) were developed, using actors to simulate common behaviours seen in people with dementia in context. 70,73,91 The learner is required to observe the potential function of the behaviour from observations and knowledge provided about the person’s past and present circumstances and then consider supportive actions within three domains (i.e. health, psychological and caregiving context) to meet their needs. The e-learning course was underpinned by a learning management system that allowed users to manage their learning and user input to be recorded and potentially assessed and used for targeted feedback. The e-tools that followed led to an assessment summary and algorithms for developing an action plan, in each of the three domains, with the third domain being relevant to the context of either a care home or family care setting. These were structured in line with the third e-learning module, to provide functional analysis-based interventions for up to 25 CBs in dementia. The three e-learning modules are as follows:
-
Module 1: an introductory module, introducing person-centred approaches in which CB is seen as a response to a frightening environment or unmet need. This introduced the notion of a shared sense of humanity and affinity with the person, in line with the philosophy underlying person-centred care. 92
-
Module 2: a skill development module, using interactive video-clips to practise observation and interviewing. This allowed staff to move away from the traditional ‘antecedent–behaviour–consequence’ observational approach to that of observing the relationship between emotion and communication through the behaviour,93 in order to enhance emotion-orientated care94 where relevant. The intention was to enable staff to appreciate the ‘language of behaviour’,95 that is, the feelings and intentions of the person with dementia that were being communicated by the behaviour, using observation of real-life practice in the care setting and thus recognise triggers and early warning signs that can then be acted on to prevent escalation of the behaviour into full-blown CB. 96
-
Module 3: this comprised nine case examples of graded complexity, incorporating video-clips, and an interactive procedure for accessing information about the person, relevant to a functional analysis of the behaviour. This was followed by a case-specific intervention summarised in an ‘action plan’ to address unmet need97 and thus reduce CB. Cases were derived from the clinical situation in which some cases have been documented in the literature. 1,13,68,69,98 Learners were guided through interactive video-clips, resources that are relevant to the person with dementia, such as life story information or case notes, audio-clips on views of others within the care context, and then provided with structured feedback to set their observations of the person’s CB in the context of ‘causation’. 49 The learning focused on guiding staff to use ‘why’ questions63 to consider the potentially multiple causes for what they had observed about the person and the behaviour in the vignette. 13 Through using information about the person’s current health and functional status, their life story and how others respond to the person with dementia during an episode of CB, key concepts for managing CB13,99 were systematically covered to address causation and possible methods of remediation. Three groups of ‘actions’ or interventions were designed and tested in the clinical situation, including those to address unmet somatic and psychological need97,100 in the person with dementia, as well as the caregiving environment, in which consideration of the particular needs of the staff group or family carer were considered. 86,101 These we refer to as functional analysis-based interventions for the management of CB in dementia. These groups were (1) ‘actions to support health needs’, for example signposting for help by the GP, to alleviate pain or discomfort due to constipation, or review of medications, such as antipsychotics or sedatives that may have been overlooked;100 (2) ‘actions to meet the psychological need of the person, such as how to support the person who may be surrounded by a sense of ‘disorder’, perhaps trying to escape from this, or feeling ‘trapped’ or ‘set aside’;102 and (3) ‘actions to support the caregiving context, environment or system’,101 such as accessing support for care home staff or psychological therapy for the family carer. Thus, in addition to the commonly advocated biopsychosocial approach for the management of CB in dementia care,103 our system included interventions to address contextual needs such as those of people providing care, which can be associated with CB,49,86 and are often overlooked (see Chapter 1).
The decision support e-tools were based on clinical practice guidelines76,104 with our assessment questions firmly set within Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care for ‘behaviours that challenge’ in dementia. 12,105 The questions that were used to provide the ‘assessment summary’ are found in Appendix 1. We used case-based reasoning,106 in which information collected about the person with dementia and their setting is stored and can be used as a knowledge source to develop personalised interventions for particular problems. The case-based reasoning method then flows into the action-planning part, in which the individual’s assessment is collated and ‘if–then’ logic is applied to generate choices for the practitioner to consider. The stored information can be used to work on different problems, as a setting-specific (care homes and family care settings) CB checklist is supplied at the beginning. The information source can also be updated; for example, if the person has a change in their health or if they have recently experienced a bereavement or if new knowledge of a person’s life story or personal ways of managing life is discovered. Our e-tool started with the 25-item Challenging Behaviour Scale (CBS) of commonly reported behaviours in care homes107 for staff to select a behaviour to work on. This scale has been recommended as a helpful measure for use in care homes across the UK (see Appendix 2 in Brechin et al. 108). For community settings, we used the Problem Checklist,109 as this was based on the concerns of UK family carers and had been used effectively in our previous study. 65,109 This was seen as important, as many guidelines for the management of aggression, for example, have poor representation of some of the important individualised and contextual characteristics necessary for management. 110 Having chosen the person’s behaviour to work on, the staff member or practitioner is then asked to input selective information about the person with dementia and CB, as they had discovered was necessary in module 3 of the e-learning course. They are guided through the assessment process (see Appendix 1 for a detailed description), concluding with a printable summary of the individual’s assessment. This summary includes documentation of the potential cause(s) or function(s) of the behaviour that was based on the staff member’s or practitioner’s responses (see Appendix 1, Box 7). 65,109 The practitioner is thus provided with a systematic assessment framework where questions they considered covered important aspects of the person’s life story, health status and other factors13 (see also Appendix 1). The next part of the decision support system is the ‘algorithmic’ flow of the information into considering what interventions can be tried. The IMS was conceived to use algorithms (which we tested in the clinical situation using a paper workbook), using the aforementioned ‘if–then’ logic to provide options for treatment – in this case functional analysis-based interventions for given behaviour that is seen as challenging. The interventions in our system are structured within the three action groups that had been used in module 3. These are (1) support for health need, such as considering the effects of commonly encountered medical conditions or the effects and side effects of psychotropic and other medication; (2) support for psychological need, such as needs for reassurance, privacy, comfort and occupation or activity; and (3) support for contextual needs, such as advice on how to optimise the environment or system around the person. For each suggested action, tailored information is provided on what staff can do themselves and when and whom to access for further help. Many of the options for intervention for the first two groups of interventions are outlined in Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care. 12 However, a structured approach to making decisions about treatment based on the particular cause(s) underlying CB (functional analysis) has been absent to date. The algorithms used for the decision support enable the care home staff or community mental health practitioner to apply a structured methodology to choose a set of actions that are possible for them to try within the resources available to them in their routine practice settings. The logic for the case-based reasoning, using tailored assessments, and the algorithms for action plans was developed by the co-chief investigator (BW). It was tested by the chief investigator (EM-C) using a paper workbook with 19 residents who had a score of 4 or more (eight residents) or 10 or more (11 residents) on the CBS. 107 These residents were drawn from eight care homes that were not involved in the CRT (see Chapter 3) and one inpatient dementia unit. All of the 25 items of CB were covered when testing the logic, and actions were refined at this stage. A separate paper workbook for family settings was tested with 15 cases of clinically significant CB determined by a score of 5 and above on a widely used research tool – the Revised Memory and Behaviour Problems Checklist (RMBPC). 111 The practitioner was provided with the Problem Checklist,109 as this was based on the concerns of UK family carers. Action suggestions were further refined to add opportunities that were available in NHS contexts, during our feasibility test with 26 community practitioners from six NHS organisations. The software engineers who were employed to develop the IMS were required to design the system to allow clinical experts (such as physicians, psychiatrists, psychologists and pharmacists) to add content for the functional analysis-based interventions, described within three action plan groupings. Thus, the utility of the action-planning component was that of flexibility for new actions that could be added on an ongoing basis to the system, as experience with the system grew.
Summary of the Challenge Demcare intervention
We used three modules of e-learning to introduce care staff to observational skills and the algorithmic approach to interventions comprising the first two components of the decision support e-tool. This required the practitioner, working with the care staff and the family, to collect important information on key contributory factors associated with CB (see Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care, section 8.6.1.1 on p. 260 and section 8.6.3.1 on pp. 262–312), such as the person’s current health and functional status, their life story, interpersonal and communication style and how others respond to the person during an episode of CB. The decision support system comprised relevant assessment tools and systems to collect this information with algorithms to provide two sets of biopsychosocial groups of ‘action plans’ where practitioners could choose the most relevant way to meet the person’s health or psychosocial need. These are referred to as functional analysis-based interventions, as the approach was to assist practitioners to assess, analyse and then choose the most appropriate set of interventions (‘actions’) for a given episode of CB. Actions for these two components were extracted from the literature, including our overview in Chapter 1, the Cochrane review,82 guidance from the International Psychogeriatric Association (see Complete Guide to Behavioral and Psychological Symptoms of Dementia),36 the range of interventions for CB from Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care12 and policy initiatives to reduce the use of antipsychotics in dementia. 112
Interventions included in the third component of the decision support tool were not related to the person’s unmet need, but arose from a new concept derived from our overview (see Chapter 1), relating to the needs of the caregiving system. These reflected, for example, the needs of the family carer for those living at home (such as counselling or skills training) and those of staff or the environment (such as training, skills enhancement or altering lighting or sound in a room). Algorithms for this component of the interventions were therefore bespoke to the care home or family care setting, where the focus was the need of the caregiving environment or system.
Methods
The e-learning course in care homes
The training, consisting of the three e-learning modules, was planned to occur within the care home, to facilitate ease of access by all staff who wished to engage with this. As noted by researchers in Canada113 and the Netherlands,104 we too found very early on in the process that the majority of the care homes did not have adequate computer equipment to offer online training. For example, most had just one or two computers in the home for sole use by the manager and/or administrator. Therefore, a lengthy process of installing the necessary additional equipment to enable access to the internet ensued. We supplied and installed computers in 19 of the 27 experimental homes (14 laptops and five desktop computers), and provided 16 printers. As noted by researchers in the USA when implementing internet-based training for nursing assistants, buildings were also not always designed to accommodate computers and the internet, in terms of either wiring or space. 73 We arranged for broadband installation in three homes and new telephone lines for broadband in two homes. On one occasion, a wireless internet booster had to be installed in a resident’s bedroom and, to activate the wireless access, the router (located underneath the manager’s desk) had to be switched on each time the staff required to access training. In some homes we provided memory upgrades to the existing computer, wireless access points, power extension cables and headphones to allow staff to access the interactive program without disturbing others. Apart from access to equipment and the internet, organisational obstacles had to be overcome to facilitate staff access to the e-learning. These included support from the research team when home managers were unable to ascertain whether or not they had internet access, obtaining permissions from organisation head offices where their policies would not allow for internet use in the home and negotiating with IT departments where technical support for care homes was outsourced.
Once access to the technology required was in place, as with the US and Canadian research teams,73,113 a specialist dementia care therapist needed to work with individual staff in the first three care homes to assist them with logging in and troubleshooting user errors or computer problems. We additionally offered homes money to backfill staff in order that others could complete the training. Thus, we overcame some of the impeding factors to e-learning in the care home setting, that is, a lack of ready access to computer equipment or internet services and low-speed connections that could detract from the utility of the programs, and staff confidence and motivation to use the training in their work environment. However, despite everyone’s best efforts, our strategy for in-home access to training was not successful because of interruptions, when staff felt that they were needed to help with care tasks or when they felt guilty because they thought that their colleagues might be busy or struggling. An alternative strategy was then used following discussion with care home managers, who nominated staff champions to undertake training, with the intention that they would then support other staff in delivery of support to residents with CB. As noted by others in this field,70,73 we needed to arrange training at an external venue where computer suites were available, to allow staff protected time to complete training within small facilitated group sessions. Funding for travel and time for backfill at the care home was made available. However, training facilities within small groups were still not used to capacity, as it was difficult at times for some homes to release staff to participate. Nonetheless, this worked much better than our attempts at offering in-home access to e-learning, as staff champions had their own uninterrupted learning time, with access to the specialist dementia care therapist and the opportunity to share and discuss with staff from other homes.
The decision support e-tool in care homes
The technological underpinning for, and functionality of, the decision support software proved unreliable, because of what may have been computer coding and software design problems with the IMS. The case-based aspect of the system was mostly, but not always, successful in producing case-specific assessment summaries, although these required some editing for presentation. The rule-based aspect was less successful and the range of actions built into the system remained smaller than desirable. Moreover, the IMS did not allow clinical experts to add management content on an ongoing basis. It had always been envisaged that input from a specialist dementia care therapist, such as a CMHN or a psychologist, would be required to assist with the care home intervention and support the action plans. With the limited range of actions available from the decision support e-tool, this was indeed the case. Therefore, a specialist dementia care therapist checked and, when necessary, edited the assessment summaries produced by the system using the rule-based logic we developed, and worked with staff champions to provide an action plan that the champions considered feasible to deliver in their home.
The decision support e-tool in the community
A second software company was employed to work on the community decision support e-tool. Community practitioners from six NHS organisations were selected by their managers to deliver the intervention. They had access to the e-learning course and were additionally trained in a small group setting by clinical experts from the research team, using relevant cases from module 3 of the e-learning course. This was supplemented by education about the rationale for case-specific functional analysis-based intervention, use of a training manual comprising tools and resources necessary for functional analysis in people with dementia and CB living at home (see Appendix 1, Box 9), and ‘hands-on’ practice with the community e-tool. For this case practice they used the e-tool, with anonymised cases from their own experiences, and engaged in facilitated group discussion with the clinical expert team and each other. These discussions focused on how to use the functional analysis for the anonymised case to develop action plans that were feasible to deliver in their own NHS context and its local resources.
Unlike the care home staff, all community mental health practitioners had access to computers and IT facilities within their NHS organisations. For them, the use of computers was a routine part of their job. Overall, the case-specific aspect leading to the assessment summary was superior to that of the care home e-tool, in terms of functionality, presentation and navigation; that is, the assessment summary was of the quality that was envisaged by the clinical expert research team. However, the software engineers did not deliver a system that was fully populated with actions or one that allowed flexibility for new actions to be added by the clinical experts. Therefore, the community mental health practitioners were provided with our manualised resources, including validated tools to assess common CBs in family settings, and other relevant contributory factors such as pain or discomfort in people with dementia or other family concerns (see Appendix 1, Box 9). This provided them with a workbook, to assist them in adopting a systematic functional analysis-based approach to individualised interventions for clinically significant CB in dementia within family care settings.
Results
The e-learning course in care homes
Ninety-two staff champions across 27 care homes that constituted the experimental arm of the ResCare trial (see Chapter 3) were trained on the e-learning course: 10 managers/deputy managers, 36 senior care assistants, 45 care assistants and one administrator. Of these, seven staff completed the online training within their care home setting and 85 attended sessions over 1.5 days. These occurred at a training centre with a large computer suite. As with e-learning at the care home, this was facilitated by the specialist dementia care therapist, but it additionally offered staff the opportunity for group discussion and demonstration of the e-tool using anonymous cases of residents with dementia and CB from their own care home. In total, 11 training sessions were held outside the home over a 12-month time period (June 2011–June 2012). Attendance ranged from 5 to 10 staff for each cohort (average of eight staff per group).
In all homes the manager agreed to select at least two staff champions for training. Two of the homes were unable to meet this requirement. The number of staff who attended training from the homes varied, ranging from one to nine. Managers were asked to select ‘appropriate’ staff for the training, that is, staff who were involved in care planning for residents with CB and dementia and, to the best of the manager’s knowledge, were likely to be working in that home regularly for the foreseeable future. However, those who ultimately attended the training were not always the most appropriate, as was the case of an administrator who had no involvement in resident care and was not sure why they had been selected by the care home manager.
Characteristics of staff champions
The majority of participating care home staff across both arms of the ResCare trial were female (89.7%), and those designated as champions showed a similar gender balance to the overall pattern, with 89.1% being female. However, staff champions were slightly younger in age [mean 36.3 years, standard deviation (SD) 11.8 years] than other staff (mean 39.6 years, SD 13.3 years), that is, those not trained in experimental homes and all the staff in control homes. The difference in age was statistically significant [t(606) = 2.12; p = 0.032].
Figure 1 shows the age groups of the champions compared with the ‘other’ non-champion staff members in the ResCare trial, with differences particularly evident in the proportion of people aged ≥ 55 years and between 25 years and 34 years.
FIGURE 1.
Age distribution of care home staff selected as champions (n = 92) compared with other staff working in the homes.
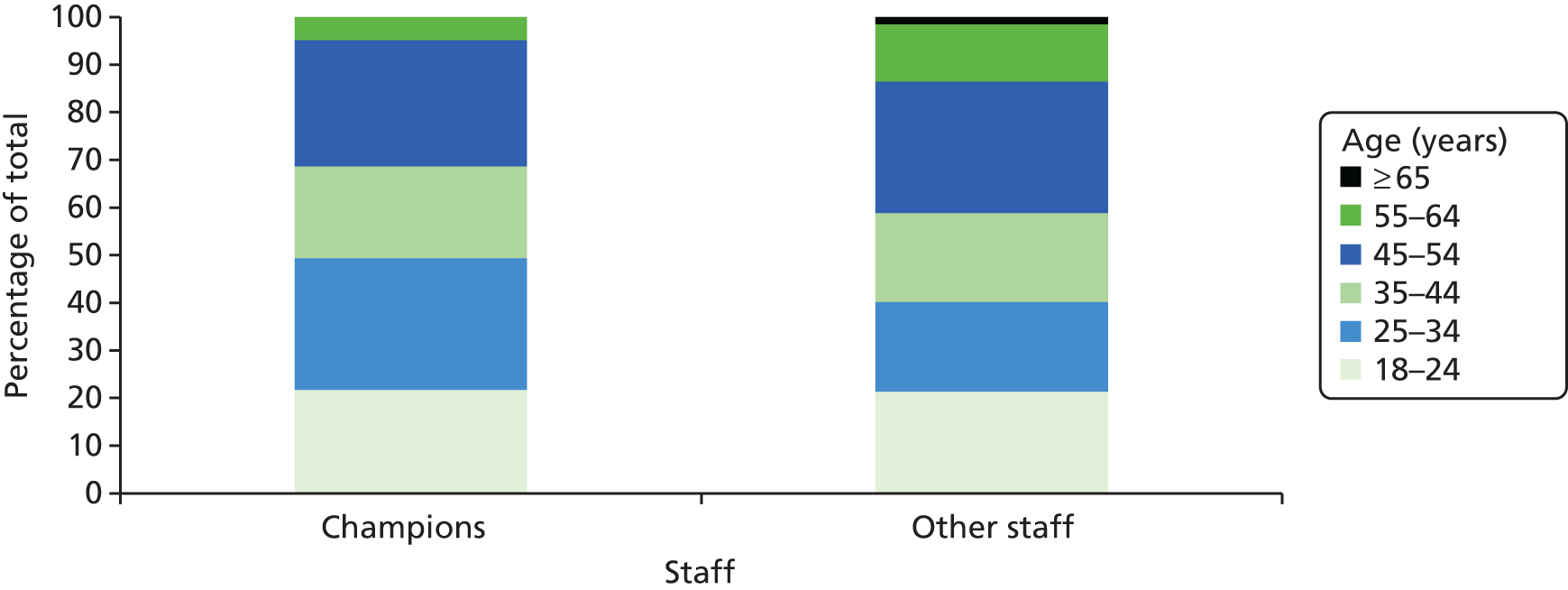
All staff reported whether or not they had received previous training in dementia care or in the use of computers and provided details of their highest qualification. Tables 1 and 2 summarise these data, comparing staff selected as champions with the rest in the ResCare trial. The majority (n = 585) provided most of the details requested.
| Previous training | Staff | Chi-squared test | p-value | All staff (N = 564) | ||||
|---|---|---|---|---|---|---|---|---|
| Champions (N = 77) | Other (N = 487) | |||||||
| n | % | n | % | n | % | |||
| Previous dementia training | ||||||||
| Yes | 62 | 80.5 | 331 | 68.0 | 4.38 | 0.036 | 393 | 69.7 |
| No | 15 | 19.5 | 156 | 32.0 | 171 | 30.3 | ||
| Previous computer training | ||||||||
| Yes | 32 | 41.6 | 171 | 35.1 | 0.94 | 0.333 | 203 | 36.0 |
| No | 45 | 58.4 | 316 | 64.9 | 361 | 64.0 | ||
| Qualification level | Staff | All staff (N = 557) | ||||
|---|---|---|---|---|---|---|
| Champions (N = 76) | Other (N = 481) | |||||
| n | % | n | % | n | % | |
| No qualifications | 3 | 3.9 | 25 | 5.2 | 28 | 5.0 |
| One to four O Levels/CSEs/GCSEs (any grades), entry level, Foundation Diploma, NVQ level 1, Foundation GNVQ, basic skills | 6 | 7.9 | 68 | 14.1 | 74 | 13.3 |
| Five or more O Levels (passes)/CSEs (grade 1)/GCSEs (grades A*–C), School Certificate, one A Level/two or three AS Levels/VCEs, Higher Diploma, NVQ level 2, Intermediate GNVQ, City & Guilds Craft, BTEC First/General Diploma, RSA Diploma | 24 | 31.6 | 175 | 36.4 | 199 | 35.7 |
| Apprenticeship, two or more A Levels/VCEs, four or more AS Levels, Higher School Certificate, Progression/Advanced Diploma, NVQ level 3, Advanced GNVQ, City & Guilds Advanced Craft, ONC, OND, BTEC National, RSA Advanced Diploma | 29 | 38.2 | 139 | 28.9 | 168 | 30.2 |
| Degree (e.g. BA, BSc), higher degree (e.g. MA, PhD, PGCE), NVQ level 4 or 5, HNC, HND, RSA Higher Diploma, BTEC Higher Level | 10 | 13.2 | 36 | 7.5 | 46 | 8.3 |
| Professional qualifications (e.g. teaching, nursing, accountancy) | 3 | 3.9 | 26 | 5.4 | 29 | 5.2 |
| Other | 1 | 1.3 | 12 | 2.5 | 13 | 2.3 |
Champions were significantly more likely to have had previous dementia training (χ2 = 4.38; p = 0.036), but were no more likely to have had computer training. As seen in Table 2, champions were more likely to have reached a higher educational level than other staff. Over half the champions (55.3%) had the equivalent of two Advanced (A) Levels or a National Vocational Qualification (NVQ) of level 3 or above, compared with 41.8% of the non-champions. This difference (higher vs. lower educational attainment) is statistically significant (Fisher’s exact test, p = 0.0448).
It may be that some managers’ decisions in selecting staff to receive training and to oversee the management of residents with CB may have been influenced by their perceptions of strengthening capability for future leaders within the home, by selecting younger staff, with higher educational qualifications and previous dementia care training.
Staff champion feedback
Following the e-learning course, staff champions were asked to complete an anonymous questionnaire using a set of study-specific open and closed questions, to access their views on the training, including how it might be improved in the future. Eighty-five of the 92 care staff who received training completed the evaluation questionnaire.
Only four participants identified aspects for improvement. The majority found the modules interesting, understandable and easy to navigate. Ease of use was reported as follows: module 1, 95%; module 2, 82%; and module 3, 69%. Apart from one participant, the staff rated the modules as manageable to navigate. In a few cases learners had difficulties with the system crashing but, generally, they reported that this was overcome with help from the specialist dementia care therapist, who assisted with restoring connectivity. The majority of participants reported that the additional information provided by the system (such as a glossary and specific help/information boxes, particularly those relating to medication) had been very useful.
Most responses on aspects of the three individual modules were positive (Box 1). Only five people stated that they found parts of the individual modules difficult. The few negative comments were mainly regarding some aspects of the commentary, which was described as repetitive, and when suggestions for improvement ‘to vary the narrators’ were made.
-
People with dementia act the same as other people (36%).
-
I learnt something about myself (25%).
-
Greater awareness about behaviour in general (16%).
-
We are all individuals (13%).
-
A mixture of the first two responses (5%).
-
Strategies for coping with CB (4%).
-
Videos and strategies to manage CB (30%).
-
Similar behaviour may be for different reasons (28%).
-
A basic grasp of functional analysis (i.e. look for reasons behind the behaviour) (17%).
-
Generalised comments about what they had observed and the use of videos of real situations that helped them become more aware of resident ‘communication’ (14%).
-
Greater awareness of self-stimulation for pleasure (8%).
-
Comments about self-development (3%).
-
Each person is unique and this impacts on their behaviour (38%).
-
Comments about usefulness of videos of their situations and strategies to manage CB (29%).
-
Stressed the need for effective care planning (18%).
-
Greater understanding about behaviour in general (10%).
-
Ill health as a causal factor (3%).
All those completing the evaluation form stated they would recommend the e-learning to other colleagues working in similar care roles. Examples of comments taken from the questionnaires from those completing the e-learning course were as follows:
I enjoyed the training because I could listen and watch at the same time – this was important because I have dyslexia.
I liked everything about it. I really enjoyed the course and found it very helpful and have learned a lot . . . would recommend all my work colleagues to take the course.
I would like the lecturer to bring the computer to my residential home so everybody could do the training.
Liked it all – I wish I had this teacher when I was at school.
It’s so lifelike.
The video clips showed just how it can really can be.
I learnt that the same type of behaviour can mean lots of different things.
Some answers were a bit tricky and really made me think.
It was very realistic and well planned out.
Learnt how to manage situations better – and also how to interact with the residents.
To look at the whole person, not just one problem . . . that’s what I learned.
The computer training and the teacher information was helpful and helped me to ask questions and share my ideas. I would like the tutor to come to the home.
That I could do it at my own pace but could ask the teacher questions.
I found this tool very insightful and look forward to putting it into practice.
Staff learning styles
Applying knowledge to practice can depend on how closely training can accommodate the learning preferences of the learner. In the previous section we outlined how preferences associated with self-pacing, training away from the work environment and for facilitated small group learning in the cohort of staff champions were addressed. Some theorists have suggested that the effectiveness of training can also be influenced by how well its methods are matched to an individual’s own learning style. 114 This is thought to be particularly important for online learning, compared with traditional instructor-based classes. 115 Some studies have concluded that learning style makes no difference to learning outcomes,116 but most of these studies were with student or professional populations and employed a variety of instruments, depending on the theoretical underpinnings of the researchers, to measure learning style. 114 One of many approaches used by educators to understand individual preferences for learning among unqualified staff, such as care assistants, is the visual–auditory–kinaesthetic (VAK) learning style model. The VAK questionnaire117 is thought to identify preferences for learning that may influence the effectiveness of practice-based training. As far as we can ascertain there are no normative data, but one study118 indicated that care staff predominantly prefer visual learning (i.e. learning that seeing pictures or visual displays and demonstrations of ‘how to do’). Kinaesthetic learning (i.e. learning through actively doing a task) is less popular, with auditory learning (i.e. listening to a lecture) being the least preferred method among care staff. 118
The Challenge Demcare e-learning course was strongly biased towards visual real-life situations. The course contains an insignificant element of auditory learning (i.e. listening alone), as it does not provide didactic instruction-based training and narration is usually combined with visual and interactive technology to prompt the learner to think creatively.
To examine the hypothesis that the design of the e-learning modules was compatible with the learning needs of staff, the VAK learning styles questionnaire117 was completed by most staff in the ResCare trial. Thus, data were also available for 78 of the 92 staff champions, as well as ‘other staff’ from both intervention (experimental) and control homes across the study. Table 3 shows the learning styles of the 78 staff champions compared with the rest of the care home staff (n = 407).
| Learning style | Staff, n (%) | Total (N = 485), n (%) | |
|---|---|---|---|
| Champions (N = 78) | Other (N = 407) | ||
| V | 34 (43.6) | 200 (49.1) | 234 (48.2) |
| A | 13 (16.7) | 53 (13.0) | 66 (13.6) |
| K | 14 (17.9) | 47 (11.5) | 61 (12.6) |
| VAK | 5 (6.4) | 34 (8.4) | 39 (8.0) |
| VA | 6 (7.7) | 35 (8.6) | 41 (8.5) |
| VK | 3 (3.8) | 32 (7.9) | 35 (7.2) |
| AK | 3 (3.8) | 6 (1.5) | 9 (1.9) |
Nearly two-thirds of the responding staff champions (61.5%, n = 48) had a predominantly visual learning style or a style that included visual preference in combination with others, that is, essentially a preference for seeing or observing things such as films, pictures and demonstrations of how to perform a new task, before trying it out themselves. An even greater proportion (74.0%, n = 301) of the ‘other staff’ had a predominantly visual learning style or a combination that included a visual style preference. Combining the champions who completed the e-learning and the ‘other care home staff’, this strong preference for visual learning represented 71.9% (n = 349) of the total staff group. Only 12.6% (n = 61) of care home staff were classified as having a purely kinaesthetic learning style, meaning that in order to learn they would need someone to show them what to do.
If the widely used VAK learning style model and its questionnaire117 is an appropriate measure of learning preferences in unqualified care workers, our findings suggest that an interactive system using real-life situations through video and related materials is a potentially effective type of training for most care staff, whose training needs/preferences appear to be weighted towards visual demonstrations of ‘how to do’.
Effects of staff training on reports of challenging behaviour in care home residents
Of the 92 staff trained on the e-learning course, 83 provided baseline CBS data for 78 residents with dementia and CB in the ResCare trial. Of these, 55 staff champions subsequently provided follow-up data for 112 residents with dementia and CB. Those staff champions who did not provide follow-up data had not left the care home: in three cases, the care home itself had withdrawn from the study and 25 champions were simply not available at the time of data collection (e.g. were on holiday, off sick or had switched to night shift).
Most CBs were reported to have declined between baseline and after staff training; for example, perseveration was recorded as a problem for 61.1% of residents at baseline, but for only 24.4% of residents following staff training, and levels of this behaviour remained lower at follow-up (reported in 46.1% of residents). Restlessness and lack of self-care also reduced following staff training, but this reduction was not maintained at follow-up. In contrast, small increases in certain behaviours, namely physical and verbal aggression, self-harm and inappropriate sexual behaviour, were reported (see Table 5).
All residents with action plans and for whom questionnaires were completed at all three time points were included in an analysis of change over time. Residents’ CB incidence scores, as measured on the CBS, decreased directly after staff training but returned to the baseline level by follow-up (Figure 2). The difference between baseline and post-staff training was significant [t(178) = 7.4; p < 0.001] and the difference between post-staff training and follow-up was also significant [t(178) = 7.6; p < 0.001].
FIGURE 2.
Average CBS incidence scores over three time points in care homes. Total incidence score = the total number of CBs (score range 0–25).
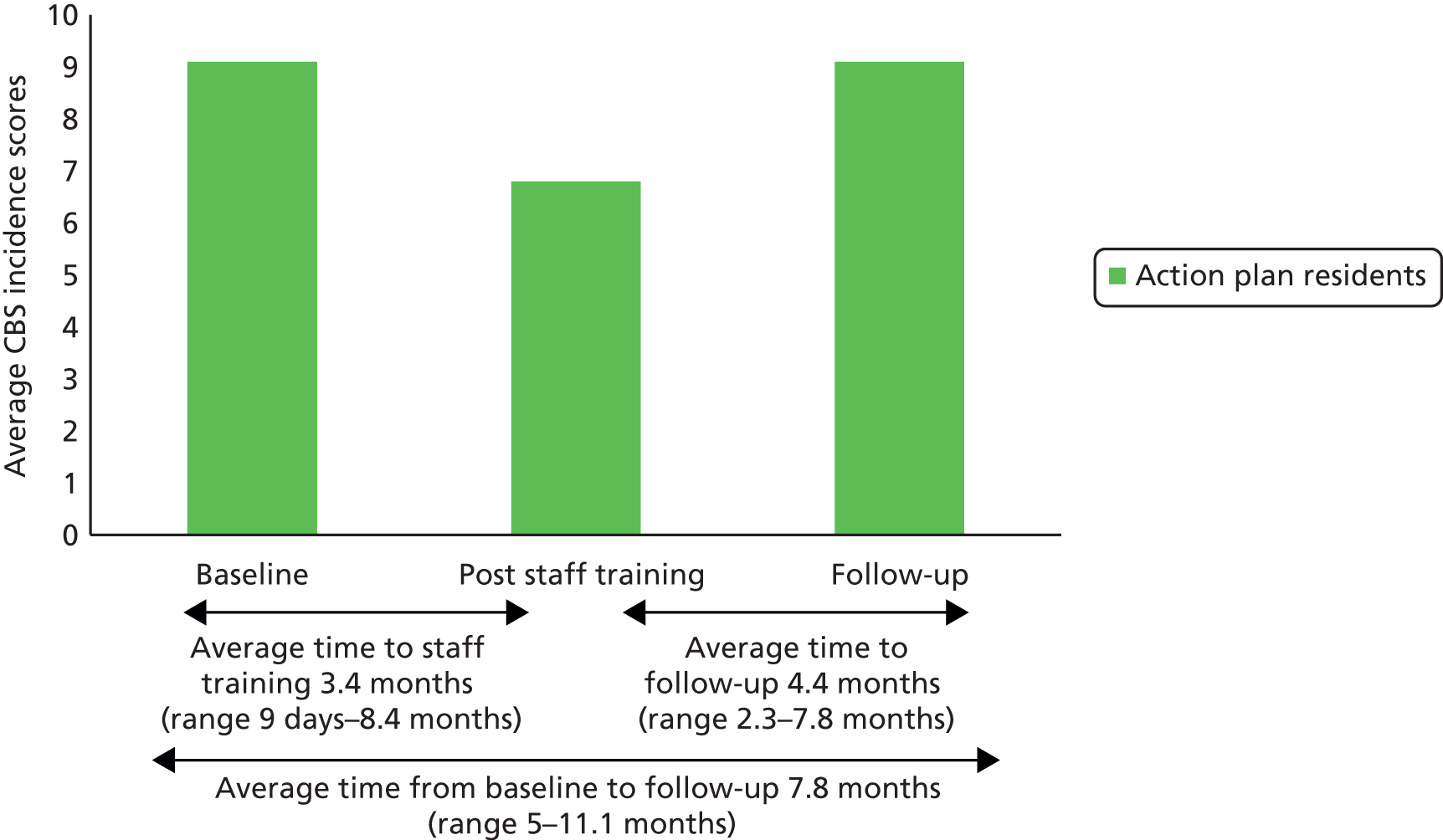
Uptake of the e-learning course after completion of the ResCare trial
Following completion of the ResCare trial, managers at 54 of the study care homes were sent a letter thanking them and their staff for their help. Homes were not sent a letter if they had dropped out of the study or had closed down. In this letter managers were offered free licences for the e-learning course; those in the control homes were offered up to a maximum of five free licences and the experimental homes were offered up to three. Only 30% (n = 16) of homes requested any free licences. In control homes, between three and five licences were requested, and in experimental homes this number ranged from one to three.
In total, we provided 60 free licences (April 2013) to care homes in the study. Sixteen weeks later, data extracted from the learning management system showed that only 12 staff from six homes had activated their licence and started training. Of these, only six accessed training for > 1 hour in total, and a further six accessed training for < 5 minutes. The majority of staff trained during the ResCare trial took around 5 hours in total to complete the three modules of the e-learning course. Therefore, staff who used the e-learning for < 1 hour are unlikely to have completed more than the first module. Once a licence is activated, training can be accessed online for 3 months. Users who successfully complete all three modules are issued with a certificate, which can be downloaded and printed off. Only two staff members completed the full e-learning course in the 16 weeks from the time the licences were provided. They were both from the same care home; one user took 3 hours 35 minutes and the other just under 5 hours to complete the course.
The low take-up rate of free e-learning for functional analysis-based training in dementia and CB is disappointing, particularly as the learning styles analysis showed that the type of training being offered through our e-learning course is compatible with the dominant preferred (i.e. visual) learning style in care home staff. Given that all those who completed an evaluation form following the e-learning said that they would recommend the training package, it was surprising that only 30% of the homes requested the free licences on offer. This perhaps confirms our initial experience that e-learning is hard to implement in care home environments, where staff have other duties to consider and practice development may not be the norm.
The decision support e-tool in care homes
Action plans
Following the training of staff champions using the e-learning course, three research therapists, including the specialist dementia care therapist, then worked with the care home staff on the development of functional analysis-based interventions for 199 residents with dementia and CB (defined as having a score of ≥ 4 on the 25-item CBS107 at baseline data collection). Residents were living in 26 intervention homes, in which an action plan was developed for an average of 7.65 residents (range 2–18 residents). Initially, there were 28 intervention homes; however, one home declined the intervention but remained in the study and another withdrew prior to the intervention. As seen in Figure 3, the main reasons for not providing action plans were that the resident had died or that the care home had either declined the intervention or withdrawn from the study.
FIGURE 3.
Challenging behaviour in residents within the intervention group. a, Two homes withdrew from the study (one before action-planning and one after); b, one intervention home declined the intervention, but contributed to follow-up assessment.
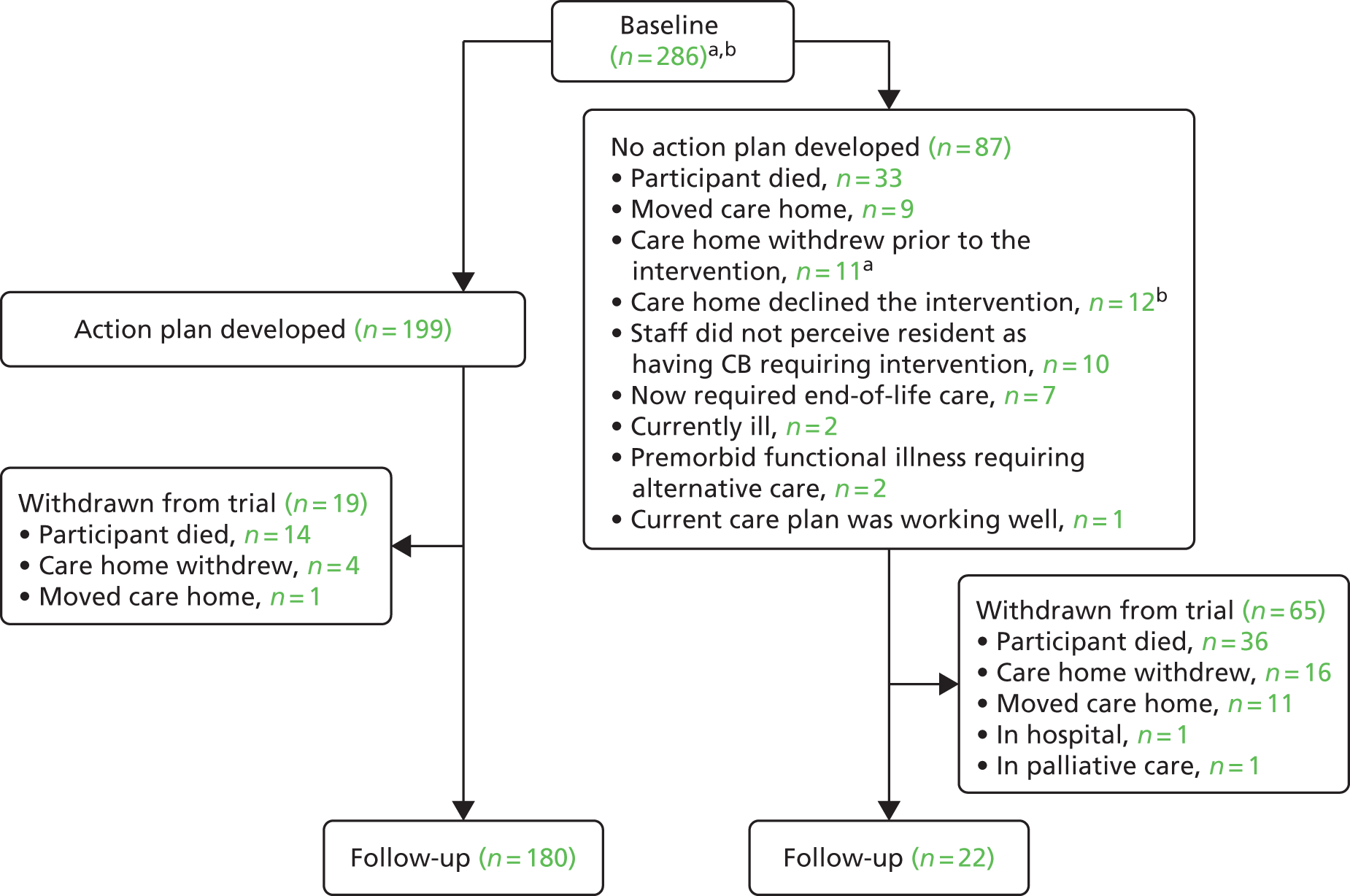
Research therapists gathered the information required about residents using the decision support e-tool. The specialist dementia care therapist was responsible for developing and checking the feasibility of the action plans to address CB for each resident with the relevant staff champion. The other two research therapists assisted with the gathering and inputting of information for populating the action plans in 64% of the 199 residents for whom care plans were developed, but they were not involved in communicating with staff champions in the final development of these. Twenty per cent of the resident action plans were checked and refined, if necessary, by one of the clinical experts from the research team.
Excluding travel time, the research therapists spent 158 hours with care home staff champions (n = 49) gathering information required for the development of action plans. Therapists spent a further 273 hours inputting the information into the e-tool, most of which was done away from the care home. The specialist dementia care therapist then spent an additional 464 hours enhancing the action plans that were produced via the system for 199 residents, and an additional 69 hours with care staff (n = 36) going through them. Additionally, a further 33 hours of resource was provided to homes (n = 17), in the form of ‘booster’ visits or telephone calls, whereby the specialist dementia care therapist provided extra support or information to staff in relation to action plans, for approximately 65% of residents (n = 139). Across all tasks involved in the development of the action plans, 101 hours of research therapist travel time was also incurred, with an additional 8 hours spent on visits to seven of the care homes at various points being unproductive because care home staff were not available for pre-arranged meetings. Excluding travel time and unproductive visits, research therapists spent a total of 997 hours on the generation and delivery of the action plans. This equates to an average of 5 hours per action plan produced. The action-planning aspect of the intervention may be described as ‘low intensity’,119 in terms of specialist input to the care home, particularly as significant time was spent interacting with the technology. Higher-intensity interventions were described in our functional analysis-based interventions review,82 in which just three studies in the care home setting were found.
Selection of the behaviour
The action plans were seen as the potential mechanism for changes in incidence, frequency and severity of CB in the intervention homes, either directly, by making a difference to the targeted behaviour, or indirectly through modelling a problem-solving approach. In practice, staff did not use the option of adding a behaviour that was not included on the 25-item CBS,107 apart from for one resident in whom ‘fear of walking’ was seen as important and for whom a second action plan was developed. This suggests that the CBS107 remains a relevant and comprehensive assessment tool in the care home context. The behaviour most frequently selected by staff champions for an action plan was physical aggression; from 199 action plans, 18.1% focused on this (Table 4). Verbal aggression and shouting were each the focus of more than 10% of action plans, with the remaining 60% spread across 20 other behaviours.
| Behaviour selected for action plan | Number selected for action plans (% of total) |
|---|---|
| Physical aggression | 36 (18.1) |
| Verbal aggression | 22 (11.1) |
| Shouting | 22 (11.1) |
| Wandering | 16 (8.0) |
| Non-compliance | 16 (8.0) |
| Lack of motivation | 10 (5.0) |
| Screaming | 9 (4.5) |
| Restlessness | 8 (4.0) |
| Interfering with other people | 7 (3.5) |
| Lack of self-care | 6 (3.0) |
| Demands attention | 6 (3.0) |
| Lack of occupation | 6 (3.0) |
| Suspiciousness | 5 (2.5) |
| Perseveration | 4 (2.0) |
| Clinging | 4 (2.0) |
| Pilfering/hoarding | 4 (2.0) |
| Inappropriate urination | 4 (2.0) |
| Sleep problems | 4 (2.0) |
| Faecal smearing | 3 (1.5) |
| Inappropriate sexual behaviour | 3 (1.5) |
| Self-harm | 2 (1.0) |
| Spitting | 1 (0.5) |
| Manipulative | 1 (0.5) |
| Dangerous behaviour | 0 |
| Stripping | 0 |
For residents who were the subject of an action plan, incidence scores [i.e. does the behaviour occur? (yes/no)] for each of the 25 CBS items were available from the baseline assessment (time point 1), immediately after the staff training (time point 2) and at the 4-month follow-up assessment (time point 3). Table 5 shows the CBS107 items for 180 residents for whom action plans were developed and follow-up data were also available, at all three time points. The behaviour selected for the action plan is also noted (see Table 5).
| CBS behavioura | Per cent displaying behaviour | Item chosen for action plan from all with this behaviour at time point 1 (%) | ||
|---|---|---|---|---|
| Time point 1: baseline | Time point 2: post staff training | Time point 3: follow-up | ||
| Physical aggression | 49.4 | 53.3 | 61.1 | 33.3 |
| Verbal aggression | 60.6 | 62.2 | 59.4 | 17.0 |
| Self-harm | 6.1 | 6.7 | 6.1 | 8.3 |
| Shouting | 64.4 | 54.4 | 61.7 | 18.4 |
| Screaming | 34.4 | 30.0 | 35.6 | 14.8 |
| Perseveration | 61.1 | 24.4 | 46.1 | 9.1 |
| Wandering | 50.6 | 41.1 | 43.3 | 21.6 |
| Restlessness | 61.7 | 40.0 | 54.4 | 11.1 |
| Lack of motivation | 60.6 | 43.9 | 65.0 | 11.4 |
| Clinging | 27.2 | 17.8 | 27.2 | 12.5 |
| Interfering with other people | 35.6 | 26.7 | 32.8 | 14.6 |
| Pilfering/hoarding | 24.4 | 18.9 | 22.2 | 11.8 |
| Suspiciousness | 36.7 | 21.7 | 32.8 | 10.3 |
| Manipulative | 11.1 | 6.7 | 8.3 | 8.3 |
| Lack of self-care | 77.2 | 50.6 | 88.9 | 5.5 |
| Spitting | 11.1 | 8.9 | 10.0 | 6.3 |
| Faecal smearing | 17.2 | 13.3 | 20.0 | 12.5 |
| Inappropriate urination | 16.7 | 11.7 | 13.9 | 19.0 |
| Stripping | 18.3 | 11.7 | 16.7 | 0.0 |
| Inappropriate sexual behaviour | 3.9 | 5.0 | 5.0 | 33.3 |
| Sleep problems | 35.8 | 29.4 | 30.7 | 7.5 |
| Non-compliance | 54.4 | 41.7 | 56.7 | 21.3 |
| Dangerous behaviour | 9.4 | 3.3 | 10.6 | 0.0 |
| Demands attention | 31.7 | 20.0 | 36.7 | 11.1 |
| Lack of occupation | 51.7 | 33.3 | 60.6 | 8.3 |
The most frequently chosen behaviour for action-planning was physical aggression, selected for one-third of residents who showed this behaviour. On the other hand, over 50% of residents were recorded as having ‘lack of self-care’ at all observational time points, but only 5.5% of residents with self-care problems had an action plan focused on this behaviour. Other behaviours such as wandering (21.6%), non-compliance (21.3%), inappropriate urination (19.0%), shouting (18.4%) and verbal aggression (17.0%) were more likely to be selected if they occurred. Some rarely occurring behaviours, such as inappropriate sexual behaviour, were more likely to be selected (33.3%), but others, such as dangerous behaviour, were not selected for management by any of the staff champions. Perseveration (repeated physical or verbal actions) reduced from 61.1% to 24.4% following training, with just 9.1% selected for a management plan. Restless behaviour was selected in only 11.1% of action plans, though 61.7% of residents were recorded with this CB at baseline (time point 1). These choices may reflect the perceived severity of managing the particular resident’s behaviour or the intention by some staff to consider action plans for other behaviours later. The incidence of some behaviours increased over time; for example, the prevalence of physical aggression increased at each of the three time points.
Booster visits: feedback from the specialist dementia care therapist
The specialist dementia care therapist had access to clinical experts from the research team for development and delivery of the action plans. This specialist therapist also provided ‘booster’ calls to support staff in delivery of the action plans in 17 homes. Key extracts from the clinical expert team’s process notes reflecting themes associated with particular aspects of delivery were those relating to action plans; those relating to residents; and those relating to the therapist’s experience of the e-tool during its delivery. These are summarised as follows:
-
Action plans:
Some action plans had in part, signposted staff to contact the person’s GP to elicit help with interventions such as pain management or review of antipsychotic medication if relevant. In one home the GP had not been forthcoming for a resident and the staff champion appeared to have lost confidence in considering such actions for other residents. The specialist dementia care therapist acting as part of the research team did not feel it appropriate to talk with the GP.
In contrast, within developing multidisciplinary supported ‘in-reach’ for CB to care homes97 a CMHN or other practitioner, acting in the role of a dementia specialist therapist, would have access to expertise of other professionals such as a psychiatrist and may be in a better position to communicate with the GP about the medical care of a particular resident.
-
Residents:
The specialist dementia care therapist and staff have had difficulty in finding a behaviour that requires an action plan. The CBS has just three items identified and none of these are of concern to staff. Although an action plan was developed, the therapist did not feel it a worthwhile task for the particular resident.
-
Specialist dementia care therapist experience:
I have to word process these Assessment Summaries to improve presentation and typos . . .; actions seem quite repetitive for staff . . . they like to see each resident as having ‘different needs’. I made actions read slightly differently to make these palatable for staff. I talked to [staff champion] and needed to adjust this action plan to make it more helpful for other staff to use.
The decision support e-tool in the community
This version of the decision support e-tool was extensively tested for acceptability with 26 community mental health practitioners between November and December 2011. They included CMHNs and others from occupational therapy, social work and clinical psychology disciplines, across six specialist community mental health NHS organisations in England. Prior to use of the e-tool, practitioners practised its logic using the e-learning. They used the system with ‘anonymised cases’, in that they adapted information from their current case to practice, but then ensured that for data protection reasons they did not include any personal identifiable details.
In most cases, but not all, training occurred at the host NHS sponsor site in Yorkshire, allowing practitioners from different parts of the country to discuss their experiences of providing care to families supporting people with CB and dementia at home. Connectivity fluctuated across training sessions, depending on internet speed at a given time or NHS location. For example, at one NHS trust, a thunderstorm delayed use and overall speed was significantly slower than at the sponsor’s NHS site; and, at the sponsor site, practitioners reported varying difficulties with connectivity and speed when using the e-learning course at their own team location. Many practitioners reported that computer and internet use in their routine practice was a predictable pressure on their work, stating connectivity and slow speeds as some common difficulties. Overall, these internet experiences with the e-tool were not perceived as an obstacle to the delivery of computer-assisted functional analysis-based interventions for their patients with dementia and CB, by community mental health practitioners.
One practitioner felt that use of the e-tool did not add anything to their own practice:
Feels like it’s what I do a lot of already without technology to help me – but it’s a different way of formulating. Glad it may help all therapists to work in the same way.
Occupational therapist 1
Overall, practitioners found the e-tool easy to use and understandable, and comments were positive:
Makes you think differently using functional analysis.
CMHN 1
This will help families to feel listened to and give therapists the tools to help decide what needs to be focussed on.
Psychologist
Like the assessment summary as it gives the key relevant information all in one place rather than trawling back through notes.
Occupational therapist 2
Glad it’s not lots of paperwork and glad it’s not just limited to medical interventions. Keen to reduce reliance on meds that are not needed.
CMHTOP practitioner
Think I can use the videos and audio to help families see that they are not alone and we can work together.
CMHN 2
Think the tool will fit in smoothly with work I do already.
CMHN 3
It’s better than I expected, pulls all the information together, action plan printable. I like the format and that the scoring is done for you.
CMHN 4
I like that it’s planning specific and evidenced.
CMHN 5
The behaviour checklist is helpful carer’s can complete whilst waiting and we can use it with the carer to decide how to help.
Psychologist
The behaviour checklist is better than what we use . . . and its evidenced.
CMHN 5
This version of the e-tool was subsequently adapted to take into account the practitioners’ suggestions for improvements, mainly aesthetics and clarifications on wording rather than content. A limitation to our evaluation was that, although participants were trained in a small group setting by clinical experts from the research team, using relevant cases from module 3, we did not take formal data on this aspect of the training. However, our previous participatory workshops with teams (see Chapter 5) to introduce the study suggested that practitioners were enthusiastic about the video material presented of real-life situations in dementia care. Another limitation was that usability of this computerised system could not be fully piloted in routine practice for two main reasons. First, we were impeded by the time required to make arrangements with clinical information governance systems within individual NHS organisations. They were under pressure to develop their own online clinical decision support tool,120 to train practitioners to cluster patients in groups in preparation for the Payment by Results (PbR) initiative (see Chapter 5), which is now mandated for all mental health NHS organisations in England. 121,122 In particular, as was noted by another study of online decision support software for mental health conditions,123 we had yet to resolve complications such as security and privacy concerns with each NHS organisation. Second, the software company was provided with a database of expert interventions, but there were delays in full population of the system. Because of these impeding factors, although we fully investigated acceptability, we were unable to conduct a feasibility study using the e-tool in the NHS within the time scale of our programme. As noted earlier (see Methods), it was not possible for our clinical expert team to update and manage the content of the e-tool, in order to reflect knowledge or resource updates relevant to action-planning.
However, some practitioners tested feasibility of our protocol to deliver functional analysis-based interventions (biopsychosocial) for dementia with CB, within their own work with their patients and families. During our consultations with them and their managers (March–May 2011, see Chapter 5) many had realised that they had been overlooking potential cases for the functional analysis–based intervention as a result of their misunderstandings about the nature of dementia and CB in family settings. This was at a time of significant redesign of specialist dementia services across the NHS in England (see Chapter 5). Consequently, some NHS organisations involved their memory clinic services in order to locate families that might be suitable for the FamCare study. All CMHTsOP and memory clinics involved in the FamCare study had access to clinical expertise with respect to medical (health) and psychosocial (the person with dementia and the family) needs that were identified by the trained practitioner, through use of the functional analysis (see Appendix 1, Box 9). Although practitioners did not have access to the e-tool, paper copies were included in the therapist manual that was supplied during training.
Discussion
This chapter has outlined the development and field testing of a training and decision support intervention. The main findings are threefold, the first located in community NHS settings and the others in the care home context. These will be outlined next.
In the context of specialist community services for the provision of support to people with dementia and families at home, skilled professionals perceived the decision support e-tool for functional analysis-based interventions as useful and relevant to their practice. In the past, this type of service to families was usually provided by the CMHN, with support from a multidisciplinary team for older people. 65 A potential strength of our study, in terms of the NHS, is that the professionals were selected for this study by their managers at a time when they and their organisations were being prepared for a nationally driven new financial regime. 122 The debate across these organisations was about which professional group should deliver this type of support, and the present study recruited a range of professionals, including CMHNs. A potential methodological weakness of this approach was that these professionals were both highly experienced and motivated practitioners. It is out of the scope of our findings to comment on whether or not all qualified practitioners with a remit for providing intervention for CB in dementia care at home would be as enthusiastic about using the computer-assisted intervention in clinical practice. Kortteisto et al. 124 noted that all professional groups have their own perceived duties and practices, and that the perceived usefulness of decision support systems can vary even between professional groups. This will undoubtedly affect the motivation of practitioners to engage directly with the software and indirectly with the intervention.
In the care home context, we overcame most of the commonly reported obstacles to e-learning, such as access to computers, bandwidth and other factors related to the internet, provided funding to release staff to engage in learning,73,104,113 and designed a course that was compatible with the learning styles of most care staff. However, we failed to engage staff at the homes. Several optimal conditions for e-learning for staff working in care homes can be recommended on the basis of this study.
First, management-led selection of staff champions to co-ordinate and support staff in the delivery of interventions was seen as a way forward for most homes. If the goal of the care industry is to develop care leaders for the future, our data suggest that champions will be selected from those who are younger, with higher educational qualifications, and who have had previous dementia training, but are not necessarily any different from other staff in their experience of use of computers (see Characteristics of staff champions). There was some evidence that the method of training, combined with facilitation by the specialist dementia care therapist, was important, perhaps in overcoming stigma attributable to perceived low literacy, which is known to undermine communication with health-care professionals,125 and in this case may also reduce access to training initiatives for some staff. For example, some staff made comments such as ‘I enjoyed the training because I could listen and watch at the same time – this was important because I have dyslexia’ or ‘I wish I had this teacher when I was at school’ (see Staff champion feedback).
Second, training allowed for the preferred option of delivery at an external site with some face-to-face interaction with the specialist dementia care therapist and some group-based discussion. 70,126 Although the training was well received during the project, its take-up beyond the project was disappointing. It is beyond the scope of these findings to offer robust evidence that the care home sector in England is ready to fully embrace an electronic learning culture, on its own. However, the findings outlined in this chapter provide some recommendations on how specialist dementia care therapists can assist care homes to use this e-learning course as a means of sensitising staff to the case-specific elusive nature of CB in dementia, and ways to approach effective responses. This is important as a recent review of e-learning in dementia care, a study of 21 care staff and managers from three care homes in south-east England, observed that staff were ‘unanimously enthusiastic about the potential for e-learning to help them’ deal with CB, using video-clips of different examples. 127 Sensitising staff to an understanding of case-specific causality of CB and functional analysis-based interventions, through online training, did appear to have the power to help staff to change or expand the way in which they perceive behaviour they find challenging (see Figure 2). A previous pilot study of functional analysis-based intervention for CB, which used face-to-face workshops for all care staff in three homes, noted a positive post-training effect at 4 months’ but not 12 months’ follow-up. 63 The effect of the present e-learning course appears to be less sustained. Compared with the 4-month follow-up of the pilot study,63 the planned 4-month follow-up in this present study of 27 care homes averaged 7.8 months, ranging from 5 to 11.5 months (see Figure 2). A study of decision support interventions in care homes104 noted that continued support of staff beyond the initial maintenance phases of a study is of key importance. In the present study this is reflected in the time taken by the need for external support on the assessment, on supporting implementation of action plans and for ‘booster’ visits to the home.
Finally, the decision support e-tool allowed staff, with support from a specialist dementia care therapist, to initially select one of the behaviours from the CBS107 to develop a functional analysis-based action plan for a given resident; for two residents, staff, assisted by the specialist dementia care therapist, developed action plans for two resident behaviours. Residents selected for intervention at baseline were required to have four or more behaviours on the CBS107 at that time point. The effect of training on the perception of staff (see Figure 2) may, to some extent, have influenced their choice of behaviour for developing an action plan (see Table 4). For example, staff sometimes struggled to find challenges in residents that they had previously reported as presenting with at least four behaviours that were challenging (see Figure 3 and Booster visits: feedback from the specialist dementia care therapist). In addition, highly prevalent challenges around self-care, restlessness and perseveration in residents were not often chosen for attention with an action plan (see Table 5). Of note is that restlessness and perseveration include descriptions of pacing or physical agitation, which, together with challenges in assisting residents with self-care, are common antecedents for episodes of aggression. 49,128 Furthermore, poor experiences with other professionals, such as a GP, may have undermined commitment to the action plans for some staff, as an intervention from another study to address unmet need in people with dementia living in care homes suggests that, compared with mental and social health, physical health needs (sensory loss, mobility and medication) were aspects that care staff seemed motivated and able to meet with some success. 129
Limitations
A limitation to our approach is that, apart from the paper workbook-based clinical checks of our e-tool, it was not possible in the project time scale to validate the e-tool itself outside the CRT (see Chapter 3). Reasons for time scale delays are documented in this chapter (see Changes to protocol: the ResCare trial and Changes to protocol: the FamCare trial). In addition, it was also not possible to implement our original plan to build into the action-planning cycle an iterative process that would enable staff to refine and modify a resident’s action plan based on their findings from trying out the first set of actions. According to the principles of a functional analysis approach to understanding behaviour,1 and our modelling of this in clinical practice,13,68,69 an iterative ‘detective’ approach to problem-solving is usually required. Nor did our time scale allow staff to receive assistance from the specialist dementia care therapist to target the range of multiple behaviours, as often there are a number of behaviours that cause distress for the person living with dementia and those supporting them. Moreover, neither the e-tool nor the specialist dementia care therapist monitored detailed implementation of the action plan in the care homes. For example, although clear strategies on contextual issues in the care home, such as assigning responsibility across shifts to ensure that all staff took a consistent approach and time scales for specific aspects of the action plan were documented, these were not actively monitored. A further limitation is that if the success (or not) of the action plan is not reviewed then poor action plans may be implemented in the future. The assumption underlying the decision support e-tool was that encouraging staff to ask ‘why’, to seek fuller understanding of individuals and their behaviour, would lead to change in staff behaviour and perception. Although the training may have contributed to a change in perception, the next stage – of recording actions undertaken and the response – was not part of the e-tool as designed here. The intention was that this aspect should fit with systems already in place in the care home, but this may be a limitation of our approach.
Conclusions
Providing computers, internet facilities, access to a specialist dementia care therapist and backfill funding to release staff to use the training facilities was not enough to engage most care home staff in e-learning. Champions, who were nominated by the care home manager, attended an external centre to use e-learning in facilitated small groups. They were enthusiastic about its potential to help their colleagues support residents with dementia and CB, but take-up by their colleagues was disappointing. The evidence from this study suggests that the care home sector in England is probably not yet ready on its own to engage fully in technology-driven education to assist staff to support people with dementia and CB. This study provides knowledge about important preparatory actions to overcome known obstacles to e-learning as an education method for care home staff who wish to engage in this. Care home organisations and their managers would need to weigh up options such as whether staff would prefer to access e-learning at an external site, in small groups facilitated by a dementia specialist, or if the care home environment would need to arrange computer and internet facilities in the home; or a combination of these options. In contrast, providing specialist community dementia practitioners in the NHS with some training and a computerised rule-based system for making decisions about the most appropriate set of interventions in a given family setting, described here as ‘functional analysis-based interventions’ to support people with dementia and CB at home, shows promise. However, usability will depend on the success of collaboration between clinical experts, IT advisers within individual NHS organisations and the software engineers, as well as financial resources.
Implications for the design of the care home (ResCare) and family care (FamCare) studies: major design changes
Changes to protocol: the ResCare trial
Three main changes to the planned ResCare trial occurred: the delivery of the intervention (reasons for which are described here in Chapter 2); associated implications for follow-up during the CRT (see Chapter 3); and a process evaluation to throw light on the mechanisms for implementation of IT-based intervention in care homes (see Chapter 4). These will be considered next.
Following an initial visit from an unblinded researcher to introduce the care staff to the intervention in the experimental homes (initially these were senior staff who later were designated as champions), we had originally proposed that they would then be left to complete the e-learning course themselves and then have access to the computerised decision support tool within their care home. We envisaged providing some support, including visits and telephone calls from the specialist dementia care therapist, where needed. However, we soon found that staff champions’ other responsibilities within their care homes prevented them from completing the e-learning. Instead, we offered the training course outside the care home environment, where interruptions would be fewer. Furthermore, significant complications in the development and functionality of the decision support e-tool, probably because of coding errors in software design, delayed the start of the trial. The intervention phase consequently took longer than planned as a result of the decision to train care staff at locations outside care homes and the extra input required from the specialist dementia care therapist to apply the most suitable interventions using a poorly developed decision support e-tool. Figure 2 demonstrates the effect of these intervention delivery obstacles on delays in the progress of data collection from baseline to the first follow-up, which had been planned for 4 months post baseline.
Although data collection progressed well following adjustment to the intervention protocol, the planned second follow-up at 12 months was dropped in order to complete the trial within the agreed timetable. Chapter 3 therefore describes our CRT with follow-up at just one time point. An independent research team was commissioned to study the process of implementing functional analysis-based interventions using technology in care homes (see Chapter 4).
Changes to protocol: the FamCare trial
The aim of the FamCare trial was to test a computerised functional analysis-based intervention for use by specialist community mental health practitioners who support people with dementia and their family carers at home across England. The functional analysis-based decision support e-tool was developed by clinical experts and software engineers for three reasons: (1) guidelines suggest that functional analysis is seen as the intervention of choice for CB and dementia;12 (2) although our Cochrane review of the literature suggested that this approach shows promise,82 there do not appear to have been any studies worldwide that have comprehensively tested implementation of the approach in the wide range of settings that provide support for family carers of relatives with dementia and CB; and (3) an IMS would allow improved knowledge on who to approach from the wide range of clinical experts and facilitate adherence to the selection of case-specific interventions for use by practitioners who may not have regular access to the range of clinical experts that can help to formulate key unmet need in a given case. This current chapter has outlined the results of our field testing of the decision support e-tool, which was limited and required further development. The community version of the decision support e-tool was not used in the planned cluster randomised therapeutic study, as we were unable to proceed with the FamCare trial (see Chapter 5 and Appendices 2 and 3).
Chapter 3 Challenge ResCare: a cluster randomised trial of the clinical effectiveness and cost-effectiveness of online training and decision support for care home staff to deliver functional analysis-based interventions for challenging behaviour in dementia
Abstract
Aim
To evaluate the clinical effectiveness and cost-effectiveness relative to usual care of an online application to enable care home staff to understand the function of CB in people with dementia and support them accordingly.
Design and methods
A CRT that allocated 63 care homes in Yorkshire between intervention and usual care. The primary outcome was the well-validated NPI using frequency and severity scores taken at 4 months to address the primary research question, that is, whether or not the intervention reduces CB in dementia. Secondary outcome measures (n = 21) monitored both residents and staff and included resident quality of life using the EuroQol-5 Dimensions (EQ-5D) self-reported and proxy versions. The basic statistical model for effectiveness analysed follow-up scores by treatment group; corresponding baseline scores; and other covariates for both resident and care home. Resources used by residents with CB were costed by adapting the Client Service Receipt Inventory (CSRI) to focus on health and social care over 4 months; and assuming no marginal change in care home resources, as these are less likely to change and more difficult to cost.
Results
Eight hundred and thirty-two residents (555 with CB) and 609 care staff at baseline were reduced to 658 (79%) residents [428 (77%) with CB] and 436 staff (72%) at follow-up. No participants reported any serious adverse reactions (SARs) to the online intervention or to analogous activities in the control group. Our primary outcome measure, the NPI, showed that the intervention reduced the frequency of NPSs by 0.60 relative to treatment as usual; however, as the 95% confidence interval (CI) from –1.18 to 2.38 includes zero, this finding was not statistically significant. Although the intervention also reduced the severity of those symptoms by 0.45 (95% CI –1.03 to 1.93), this also lacked statistical significance. Although 14 of the 21 secondary outcome measures showed positive effects of the intervention, none reached statistical significance. Furthermore, the intervention generated little change in the prescription of drugs relevant to dementia – notably antipsychotics (χ2 > 0.999), antidepressants (χ2 = 0.635), hypnotics and anxiolytics (χ2 = 0.215), anticonvulsants (χ2 > 0.999) and the anti-dementia (such as the cholinesterase inhibitors) drugs (χ2 > 0.999) or those for pain relief – that is, the opioids (χ2 = 0.399) and non-opioids (χ2 = 0.996). Hence, there is no evidence that the intervention changed the care of CB in dementia. Health- and social-care costs over 4 months did not differ significantly between groups (mean cost was £331 less in the intervention group, with the bootstrapped 95% CI from –£927 to £272) and staff reports of quality-adjusted life-years (QALYs) over 4 months differed little between groups. Hence, there is no evidence that the online intervention was cost-effective.
Conclusions
This computer-assisted intervention was neither clinically effective nor cost-effective. It comprised a comprehensive interactive e-learning program, delivered with group discussion to facilitate case-specific management of CB in dementia, and was followed by assisted decision support to provide case-specific interventions for residents with dementia and clinically significant CB. It was not enough to reduce CB in dementia in care homes.
Trial registration
The International Standard Randomised Controlled Trial Number (ISRCTN) is 02553381.
Introduction
The need for research on case-specific interventions for the management of CB and dementia in care homes was outlined in Chapter 1. Using our Cochrane review, these were conceived as functional analysis-based interventions (see Chapter 1). An online solution was adopted to make the approach available to all care staff working in a home (see Chapter 1) and a mixed-methods study of the process of delivery was described in Chapter 2. We learned that despite enthusiasm and significant support with internet resources from the research team provided to all participating care homes, worksite-based e-learning was not achievable. Specialist dementia care therapist support was appreciated by care staff in the delivery of e-learning (see Chapter 2). Developing interventions with the decision support system also required assistance from a specialist dementia care therapist (see Chapter 2). In this chapter the CRT of effectiveness and cost-effectiveness of computerised functional analysis-based interventions for the management of CB in care homes is described. The study was conducted across Yorkshire.
Research questions
-
Does the experimental intervention described in Chapter 2 reduce CB in residents with dementia, as measured by the frequency and severity scores of the NPI?
-
Does the experimental intervention improve the experience of staff and the quality of life of residents in care homes?
-
Does the experimental intervention reduce the prescription of psychotropic medications and change the usage of medications, such as analgesia for management of pain and laxatives for the management of bowel movements?
-
Is the experimental intervention cost-effective?
Methods
Design
This pragmatic CRT allocated participating care homes to two groups. The experimental (intervention) group received a functional analysis-based intervention and the control group delivered ‘treatment as usual’. Randomisation at care home level ensured that all recruited residents and care staff in each home were allocated to either experimental or control group. Following consent procedures at a care home, a Registered Clinical Trials Unit, the North Wales Organisation for Randomised Trials in Health (NWORTH), undertook computerised randomisation remotely. The researchers who assessed participants were blind to treatment allocation at baseline and follow-up.
Governance and study approvals
The York Research Ethics Committee (REC; reference number 09/H1311/29) approved this study in May 2009 (see Appendix 4). Details of amendments that were approved during the trial by the REC are also found in Appendix 4. In April 2009 the Research and Development (R&D) Department at Humber NHS Foundation Trust confirmed its sponsorship of the trial and gave local research governance approval. Appendix 5 describes the management arrangements for this study, including patient and public involvement.
Changes to the protocol
Chapter 2 outlined reasons for changes to the ResCare trial, namely the decision not to go ahead with a second follow-up assessment at 12 months.
The intervention
Ninety-two nominated champions from homes allocated to the experimental intervention received e-learning (functional analysis training), usually away from their care homes. The purpose was to teach them ‘detective’-like skills to improve their understanding of the ‘language’95 of common behaviours that were seen as challenging. By using simulated case studies of varying complexity, filmed in a care home setting, this course aimed to change or expand the way staff perceived and responded to resident behaviour in their own care home. Staff champions also worked with a specialist dementia care therapist, who used a decision support e-tool to develop action plans for a particular behaviour that was identified by staff as challenging (see Chapter 2, Table 4). The ‘usual’ long-term care services and interventions that were available to residents in control homes were also available to those in the experimental homes, as we were evaluating the additional benefit of our e-learning course and decision support e-tool-assisted intervention. Both control and experimental homes therefore had access to other training materials and courses, as they would normally.
Study population
In July 2010 we invited all care homes in East Yorkshire, Hull and York with 25 or more beds (including nursing care beds if available) listed on the Care Quality Commission (CQC) website (URL: www.cqc.org.uk/) as old-age care homes to take part in the study. Based on data from a previous study,107 homes with 25 beds or over were judged to be the smallest care home sample that could be included to achieve the numbers of people with dementia and CB required per home to meet the sample size calculations of this CRT (see Sample size). To ensure consistency in quality of care across the homes for this trial, we invited only those that the CQC had rated as ‘good’ or excellent’ at the time. All types of homes were invited, including those managed by local authorities, regional and national commercial chains and the not-for-profit sector and small, independently run private homes. Recruitment of care homes began in October 2010, with participants recruited between March 2011 and March 2012. Follow-up was completed in October 2012.
Eligibility criteria for residents
Inclusion criteria
-
Resident lived in the recruited care home.
-
Residents met the diagnostic criteria for dementia in the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV),130 and those in the ‘CB’ stratum were required to exhibit at least four problems on the CBS. 107 Of the residents who did not meet both the DSM-IV and CBS criteria, we selected five residents at random from each home. They constituted the ‘other residents’ stratum and included some residents with dementia and some with CB, but not with both.
Exclusion criteria
-
Residents whose stay in the home was not, in the judgement of the home manager, likely to be long term (e.g. those receiving respite care).
-
Residents who were receiving palliative care.
-
Residents who were unable to speak or understand English.
-
Residents who were out of the home, for example in hospital, at baseline data collection.
Sample size
Originally we planned to recruit 48 homes, expecting to recruit an average of 13 CB participants per home and 624 in total. Mozley et al. ,131 in a study carried out in the same locality as the ResCare trial, reported a 39% loss of care home participants over 12 months because of death, moving elsewhere or illness too severe for follow-up. So we expected to lose two recruited CB residents (15%) per home over 4 months, yielding a completed sample of 48 × 11 = 528 participants. Re-analysis of data from previous studies suggested that intracluster correlation coefficients (ICCs) rarely exceed 0.03. 131 We therefore judged that the ICC would not exceed 0.03, and calculated that 528 CB completers would provide the same power as a simple random sample of 528/[1 + 0.03 × (11 – 1)] = 406. Our original design had > 80% (85%) power using a significance level of 5% to detect a difference in NPI scores equal to 0.3 of their population SD. Though this difference is sometimes regarded as ‘small’, our research team and Programme Steering Committee (PSC) judged that it was both clinically important and feasible. However, as the number of CB residents per home was smaller than expected, we recruited 63 homes to the trial with the aim of achieving 63 × 7 = 441 CB completers, equivalent to a simple random sample of 441/[1 + 0.03 × (7 – 1)] = 374. Using the increased number of care homes with the decreased number of residents in each care home still achieves the > 80% (82%) power of the initial design to detect an effect size of 0.3.
Recruitment procedures
When appropriate, we approached the organisation owning the home for support and then contacted the home manager to discuss the trial, including the implications of participating. We needed to recruit care staff and residents through participating care homes. At this stage, 63 homes provided us with anonymised data on CB, using the 25-item CBS incidence domain,107 for all their residents aged > 65 years (n = 2185). This was in order for us to establish homes where CB was highly prevalent; these cross-sectional data are referred to as the ‘screening data’. Next we began the process of seeking consent for all homes simultaneously in order to seek permissions for residents who lacked capacity to consent to participate in the study. Care home managers introduced research team members to staff, who introduced them to residents. If residents were happy to talk, the researcher explained the study to potential participants, provided information sheets and answered their questions.
Care homes then sent letters advising each resident’s main relative about the research, asking them to inform the home manager or research team if they had any objections to being contacted by us. For residents without capacity to consent, we explained the study to the relative, asked them to act as personal consultees for the resident’s participation and answered their questions. We then asked those willing to act as a consultee whether or not their relative would have wished to take part in this research and to sign a consultee form. For residents with no key relative we approached an alternative consultee (e.g. a solicitor or social worker).
We gave each resident notified to the research team a trial identification number, whether or not they subsequently joined the study. This enabled us to document the flow of participants through the trial, as required by the Consolidated Standards of Reporting Trials (CONSORT). 132
Informed consent
In line with current guidance on the use of the Mental Capacity Act 2005,133 produced by the British Psychological Society,134 we assumed that each person had the capacity to consent, and explained the research in an accessible manner, increasing their likelihood of being able to consent for themselves. We encouraged them to discuss the research with their family when appropriate and gave them as much time as they needed before reaching a decision. Only after residents had been assessed for capacity in this way in accordance with the Mental Capacity Act 2005,133 and they or their consultee had agreed that they would take part, were residents eligible to participate in the trial. We treated consent as a continuing process rather than a single decision, and discontinued interviewing whenever residents did not want to engage or became distressed by the assessments.
We also asked staff to consent to participate in the trial, both by answering questions about themselves and by providing information about consented residents. We emphasised to all participants, both residents and care staff, that choosing not to take part would not disadvantage them in any way. The resident’s GP was also given information about the study and notified that their patient was participating.
Ethical arrangements
We know of no documented harmful effects of using functional analysis-orientated interventions to manage CB. Nevertheless, we devised a trial-specific procedure for reporting serious adverse events (SAEs) to the chief investigator. In addition to agreeing with care home managers that they would update us on any changes to the residents’ circumstances, the researchers contacted each care home every month to check on adverse events that might not have been reported to us. Criteria for judgement of whether or not the event was deemed serious, or could be defined as an ‘untoward occurrence’ for the participant, were as follows:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was considered medically significant by the investigators
-
included alleged or suspected abuse or neglect, according to the protection of vulnerable adults procedure agreed for the trial.
If so, the researcher completed a SAE reporting form for the chief investigator to assess whether that SAE could have been related to the intervention or deemed as ‘unexpected’. We then reported any adjudged intervention related or unexpected (or both a fortiori) to the REC, the trial Data Monitoring and Ethics Committee (DMEC) and the sponsoring NHS trust R&D department, within 15 days.
Randomisation
Given the CRT design, in which the care home was the unit of randomisation, consent was obtained from care home managers prior to computerised randomisation of care homes between the experimental and control groups, undertaken remotely by NWORTH. Our plan was for randomisation to take place gradually, home by home, once the consent process had been completed in each. However, this became problematic at an early stage, as the lengthy process of setting up computers and internet facilities in experimental homes (see Chapter 2) had not been anticipated. Thus, we needed to know which homes would contribute to the experimental arm of the CRT in advance, to ensure that facilities were in place in order to commence intervention as close to the baseline data collection point as possible. Therefore, we gained consent from managers at all homes and began the process of randomisation early on. In order to not affect baseline responses, as far as possible we communicated with managers, but not care staff, about technology required for the intervention, and maintained blinding to the treatment condition for researchers who collected the data.
Homes were randomised using two stratifying criteria:
-
‘high or low CB’, namely whether more or less than 40% of residents had a CBS incidence score of > 10 at screening
-
size of home in terms of registered beds, that is, small (25–39 beds), medium (40–49 beds) or large (≥ 50 beds).
Blinding
As the intervention was pervasive, it was neither possible nor desirable to blind participating staff or residents to homes allocated to the experimental or control group. Instead, the researchers responsible for data collection remained blinded during collection of all baseline and follow-up assessments of care home staff and residents. An unblinded researcher informed the care home manager of their allocation to the experimental or control group, and ensured that experimental homes had the IT equipment and adequate internet (including broadband) facilities that were required for the intervention. An unblinded specialist dementia care therapist then acted as facilitator, supporting staff champions in training and using the decision support e-tool to develop action plans for target behaviours in CB residents identified by care staff.
After follow-up data collection was complete in each home, and in order to take into account the quality of blinding, the blinded researchers completed a perception sheet that asked them to predict the treatment group to which that home had been allocated.
Data analysts applied an agreed analysis plan to the data set, which was blinded for treatment group with anonymous codes.
Data collection
Our aim was to assess all participating residents at baseline and as close to 4 months as possible after that. However, because of problems in delivering the intervention and associated change of plan (see Chapter 2, Changes to protocol: the ResCare trial), follow-up in control homes ranged from 4 to 7 months after baseline [mean 5.15 months (SD 1.31 months)]. In intervention homes, the range was from 5 to 11 months [mean 7.86 months (SD 1.48 months)]. To adjust for this significant difference (p < 0.001) and the potential resulting bias, the agreed analysis plan was updated to use participants’ time to follow-up as a major covariate.
We conducted all interviews with residents and care staff in the care home and this usually took 2 or 3 days per home. In most homes, two researchers interviewed residents and care staff concurrently in separate rooms. In larger homes more than two researchers sometimes worked at the same time; in smaller homes mostly only one researcher conducted all the interviews. Second visits to complete interviews were arranged, for example when participants became tired.
Measures
(See Appendix 6 for details of the instruments and their scoring methods.)
Primary outcome measure
The NPI135 is a validated measure based on interviewing an appropriate informant. It rates ‘frequency’, ‘severity’ and ‘caregiver distress’ for 12 CB categories: delusions, hallucinations, agitation or aggression, depression or dysphoria, anxiety, elation or euphoria, apathy or indifference, disinhibition, irritability or lability, aberrant motor behaviour, sleep and appetite or eating disorders. We addressed our primary research question, that is, whether or not the experimental intervention reduces CB in dementia, by analysing frequency and severity scores.
Secondary outcome measures
-
Frequency and severity of CB in residents with dementia based on staff reports of a resident’s behaviour, assessed using the Cohen–Mansfield Agitation Inventory (CMAI)136 and the CBS. 107
-
Emotional impact of, or reaction to, a resident’s CB for individual staff using the NPI caregiver distress score; the CBS total challenge score; the Maslach Burnout Inventory (MBI);137 and the EQ-5D. 138 The EQ-5D converts staff reports of their current dimensions of quality of life into a single preference-based utility.
-
Attitudes of individual staff towards people with dementia, assessed by the Approaches to Dementia Questionnaire (ADQ). 139
-
Perceived effectiveness of individual staff in caring for people with dementia using the Self-Efficacy Scale (SES),140 based on staff assessments of how effective they believe they are as a care worker.
-
CSRI,141 adapted to estimate the health- and social-care resources, including medication, that each resident with CB uses.
-
Residents’ quality of life using the EQ-5D (self-report and proxy), which is a valid measure in people with mild to moderate dementia;142 and the Quality of Life in Alzheimer’s Disease (QoL-AD) self-report and proxy. 143
-
Residents’ personal utility aggregated over time into QALYs estimated from the EQ-5D by using the trapezium rule at baseline and follow-up.
In addition, we used the Clinical Dementia Rating (CDR)144 not as an outcome, but as a covariate for analysis. During the course of the trial the research team also recorded organisational change, such as change in manager or ownership, in each care home.
Table 6 shows the topics that were addressed, the measures used and whether or not the measure related to the residents or the staff.
| Topic | Measure | Participants |
|---|---|---|
| Behaviour | NPI (frequency) | CB residents |
| NPI (severity) | CB residents | |
| CBS (incidence) | CB residents | |
| CBS (frequency) | CB residents | |
| CBS (difficulty) | CB residents | |
| CMAI (frequency) | CB residents | |
| Emotions | NPI (distress) | CB residents |
| NPI (total score) | CB residents | |
| CBS (total score) | All residents | |
| MBI | Staff | |
| EQ-5D | Staff | |
| Attitude | ADQ | Staff |
| Self-efficacy | SES | Staff |
| Quality of life | EQ-5Da | All residents |
| QoL-ADa | All residents | |
| Resource use | CSRI adapted | CB residents |
Data management
Data were collected in questionnaire packs by researchers, during interviews with participating care home staff and residents, and from residents’ care home records, including the resident’s Medication Administration Record for their prescribed medication. Questionnaires were scanned into Verity Cardiff Teleform (Teleform Desktop V10.4.1; Cardiff Software, Inc., Vista, CA, USA), and were verified and validated. Data were then exported into IBM SPSS Statistics version 19.0 (IBM Corporation, Armonk, NY, USA) and SPSS was used to check data for inconsistencies, values out of range and missing data. All corrections to the SPSS file were logged, along with the reason for each change. A 5% sample of each type of questionnaire was checked against the final SPSS file to ensure consistency and to identify variables needing further in-depth checking. For example, the medication data needed further checking against the questionnaires, because numerous text fields in the recording form had increased the likelihood of human and scanning error.
Data analyses
The main statistical analyses applied SPSS version 20 (similar to version 19 in all relevant respects) to three participant populations: all residents, those with CB and staff. Participants were compared by treatment allocated (previously known as analysis by intention to treat) to draw pragmatic inferences about the real world in which participants vary in their compliance with planned interventions. Health economic analyses used SPSS version 19.
Missing data
The researchers endeavoured to collect as many data as they could. However, two types of missing data were inevitable: missing items within a measure and missing time points. Missing items were attributable to researcher error or participants declining to answer individual questions. When questionnaires had recommended rules for managing such missing items, these were applied.
Several methods to maximise the available data were used. If a resident answered at least 75% of the items for a given subscale, the subscale score was calculated pro rata. For staff-reported measures, 18 residents had missing items for the CBS at baseline and 12 had missing items for the NPI at follow-up. The remaining staff-reported measures had missing items for four or fewer residents at both baseline and follow-up. Imputations to three populations were applied: (1) outcomes for all residents (n = 832); (2) outcomes for the residents with CB (n = 555); and (3) outcomes for all staff (n = 632). Data values were imputed for residents who (1) still had missing values after using the method described above; (2) dropped out; or (3) lived in a care home that withdrew from the study. Values were not imputed for 113 residents who died from the ‘dropout group’ of 174 residents. This procedure was repeated five times to create five sets of data with missing values replaced by imputed values.
The economic cost-effectiveness analyses did not impute missing costs, but used only cases with full cost data. Imputation to replace missing data with statistical estimates is considered the usual procedure to use; however, Briggs et al. 145 outline different approaches to handling missing service use data, noting that simpler methods such as complete-case analysis are acceptable in data sets with a small number of missing data. In the ResCare trial, service use data were available for 428 out of the 474 residents with CB (i.e. 90.3%) who were still alive at the end of the trial. We therefore used complete-case analysis.
For multiple imputations, the method of multivariate imputation by chained equations (MICE)146,147 was used with a two-level modelling structure to capture the clustered trial design (residents in care homes within allocated intervention). The software used for this method was ‘mice 2.9’ in R (The R Foundation for Statistical Computing, Vienna, Austria). 148 Before imputations, a missing value analysis was conducted to seek the level of satisfaction on the assumption of missing at random. If variables that affected the missing mechanism were identified, these were taken into account in imputation and analysis. 149,150 After imputations, the convergence and the marginal distributions of outcomes were investigated to ensure a healthy performance of the MICE algorithm and plausible estimates. The few missing demographics (e.g. age, gender and previous housing) were imputed from all other demographic variables as means from simple regressions, but adjusted when necessary to ensure that imputed values fell within the range of observations. To impute missing outcomes at baseline, we treated home characteristics and demographics as fixed effects, and care homes as random effects. At follow-up we added the same outcome at baseline to the fixed effects, thus keeping care homes as random effects and, again, ensuring that imputed outcomes fell within the range of observations.
Outliers
We checked the data of all outliers identified by statistical analysis, but found no reason to drop any from the main data set. Health economic analysis identified one participant who spent 50 nights on a medical ward; but, after sensitivity analysis to test the effect of removing that resident, we retained the full data set for the main analysis.
Interim analyses
No interim analyses were planned or requested by our DMEC.
Primary effectiveness analyses
The primary research question was: does the experimental intervention reduce CB in residents with dementia, as measured by NPI frequency and severity scores?
Initially we used logistic regression to predict from the covariates measured at baseline whether or not residents were missing at follow-up, and included good predictors of missing status in our main analysis. 151
The NPI frequency and severity scores were analysed by multilevel modelling of follow-up scores; the key factor was allocated group (intervention vs. control) and the main covariate was the corresponding baseline score. Two multilevel models were tested: model I included only allocated group and the baseline score; and model II extended this by adding the other baseline covariates. An adjustment for multiple testing between the primary outcomes was not required because there was no significant difference and, therefore, no change to the results would have been seen.
Two data sets for each resident population were used: the complete-case data set comprised those residents who provided baseline and follow-up scores for the response variable; and the pooled data set of scores imputed for participants’ missing responses at follow-up.
The baseline covariates for care homes were:
-
number of beds in the care home
-
proportion of residents in the care home with CBS incidence scores of > 10
-
type of care home: local authority, not for profit or private
-
the number of days between the baseline and follow-up measures
-
whether or not organisational change occurred between baseline and follow-up.
The baseline covariates for residents were:
-
age
-
gender
-
Clinical Dementia Rating-sum of the boxes (CDR-SB). 144
Secondary effectiveness analyses
The secondary questions were:
-
Does the experimental intervention improve the experience of staff and residents in care homes as follows.
-
Does it reduce the emotional impact of CB on care home staff?
-
Does it give them a more positive and person-centred attitude to people with dementia?
-
Does it improve their self-rated efficacy in caring for people with dementia?
-
Does it improve quality of life of residents, including those without dementia?
-
Does it reduce the number of psychotropic medications used by residents?
-
Does it change the number of relief medications used by residents (e.g. for pain or constipation)?
-
The secondary outcome measures for residents with CB were NPI distress and total scores, CMAI score and the number of psychotropic and relief drugs. For the ‘all residents group’, the secondary outcome measures were CBS, EQ-5D and QoL-AD. For staff, the secondary outcome measures were MBI, EQ-5D, ADQ and SES.
Analysis of quantitative secondary outcomes used the same multilevel model as for the primary outcome measures; the resulting significance levels were adjusted to correct for multiple testing. For staff, the covariates were age, working hours per week and the corresponding baseline score.
Analysis of changes in the proportions of residents taking each medication by home used generalised linear models with logit link function and covariates as follows: treatment group; type and size of home; proportion of residents with a CBS incidence score of > 10 at screening; organisation change between baseline and follow-up; time between baseline and follow-up; and corresponding baseline proportion. Again model I included only treatment groups and baseline proportions; and model II included all the listed covariates.
Economic questions
The main economic question was: what is the incremental cost-effectiveness of the experimental intervention relative to treatment as usual in improving CB in residents?
Other questions were as follows.
-
Secondary question: what is the incremental cost–utility of the experimental intervention relative to treatment as usual in improving the quality of life of residents?
-
Tertiary question: what is the incremental cost-effectiveness of the experimental intervention relative to treatment as usual in reducing emotional impact of CB on care home staff?
Economic analyses
Cost of the intervention
This was derived from the following: costs of developing the e-learning and decision support e-tool for functional analysis in dementia, taken from invoices from the software engineering company to reflect the direct costs of development; costs for equipment including computers, telephone lines and internet use (see Chapter 2); and staff time spent on the e-learning course and in developing action plans. We annuitised all these costs over 5 years, while allowing for staff turnover that would necessitate annual training.
Cost of health and social care
The research team used care home records to extract data for the adapted CSRI. These included residents’ contacts with primary and secondary health care, social care and voluntary services, but not care home fees, as the intervention was neither designed nor likely to change these. Where possible, national unit costs for 2012 were used to estimate the mean total cost of these extra services per resident. 152,153 However, medication costs for 2011154 were the latest available at the time of analysis. We did not discount these service costs as the follow-up period was less than 12 months. Unit costs used in this study are detailed in Appendix 7.
Economic evaluation
A public sector, multiagency perspective for the economic evaluation was adopted and the cost-effectiveness analysis was conducted in accordance with the Medical Research Council (MRC)’s guidelines for the evaluation of complex interventions,155 with a standard operating procedure for economic evaluations carried out alongside RCTs. 156 The main analysis was cost-effectiveness by treatment allocated, with cost per unit of NPI as the criterion. Non-parametric bootstrapping with 1000 replications157 to quantify the uncertainty associated with point estimates of cost-effectiveness ratios was used. Increasing the number of replications to more than 1000 would have been unlikely to change the results, given the lack of significance noted in the effectiveness study.
The cost–utility analysis was conducted using the EQ-5D completed by residents, or staff as proxies for residents, to estimate differences between allocated treatments in residents’ QALYs. If the intervention reduced staff stress, as measured by the MBI, we planned to undertake a secondary cost-effectiveness analysis by treatment allocated, with cost per unit of MBI as the criterion.
To explore whether or not results were robust to our costing assumptions, a sensitivity analysis was carried out to test the effect of changing the staff who created the action plans, from the specialist dementia research therapist to a senior care assistant. An analysis to check whether or not our economic findings were sensitive to size of care home was also undertaken.
Results
Randomised allocation
Figure 4 shows the flow of care homes, staff and resident participants through the trial. In total, 111 care homes were considered for inclusion in the trial, with 63 ultimately being randomised and 58 completing baseline assessment. Table 7 shows the distribution of the 832 residents across the homes, with 555 in the CB group and 277 in the ‘other residents’ group. The main reasons for the lack of baseline data for those residents who were initially assessed for inclusion were withdrawal of care home, death, and non-selection or unwillingness of residents. Baseline assessments were completed by 609 staff.
FIGURE 4.
The ResCare trial: participant flow through the trial by treatment allocated. HN, home numbers; RN, resident numbers; SN, staff numbers. Note that the loss of 43% (SN = 135) from the staff intervention group, but only 21% (SN = 61) from the staff control group and 28% (RN = 117) and 14% (RN = 57), respectively, from the resident group are both largely attributable to the difference in the period of follow-up.
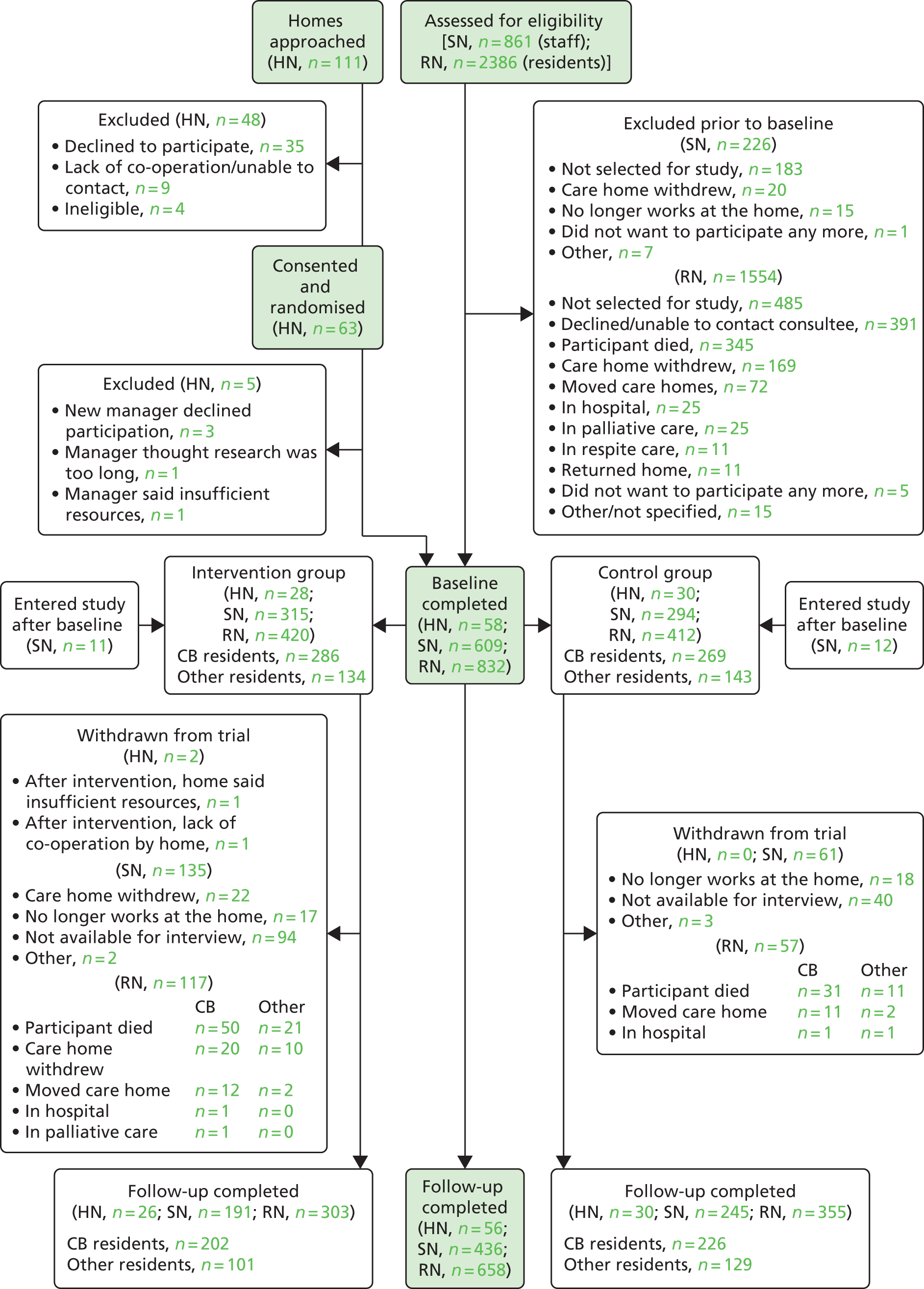
| Residents | Distribution per home (N = 58 homes) | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | First quadrant | Median | Third quadrant | Maximum | |
| Total | 832 | 14.34 | 4.84 | 6 | 11 | 15 | 16.75 | 28 |
| CB group | 555 | 9.57 | 4.81 | 1 | 6 | 10 | 12 | 23 |
| Other residents group | 277 | 4.78 | 0.68 | 2 | 5 | 5 | 5 | 6 |
Of those who completed baseline data collection, in the intervention group there were 28 care homes, 420 residents (286 residents in the CB group and 134 in the other residents group) and 315 staff. In the control group, there were 30 homes, 412 residents (269 residents in the CB group and 143 in the other residents group) and 294 staff. A further 23 staff joined the study after baseline, mainly because some original staff were not available at follow-up and these staff were asked by the manager to answer questions about the residents in their place. Within the intervention homes, 92 staff trained as champions, 88 of whom completed study measures.
Dropouts
Seven homes withdrew from the study, five before baseline data collection and two after. Two homes were lost to follow-up (22 residents at baseline), both from the intervention group: one withdrew after the staff champions had completed the e-learning but before they had developed action plans, and the other withdrew after action plans were completed. Another intervention home failed to identify any staff to act as champions and, therefore, received no intervention, but provided data at follow-up, so these were analysed by treatment allocated (17 residents at baseline and 12 at follow-up). No control homes dropped out of the study between baseline and follow-up. Fifty-six homes were therefore followed up.
The dropout rate for the CB sample was 22.9%, with 428 of the initial 555 in the CB group completing follow-up. This was higher than the original estimate of 15%, which was based on previous local studies. 63,131
The ICCs from the NPI and CMAI data were between 0.03 and 0.06 (except for the NPI distress subscale; see Table 25), and so were higher than the value of 0.03 used in the original sample size estimation. The dropout rate in the other residents group, consisting of residents with dementia or with CB but not both, was 17% and, therefore, closer to our original estimate of 15%.
Table 8 outlines reasons why residents dropped out, broken down by their group allocation. Death was the most common reason, with 10.2% (n = 113) of the overall resident sample being lost to follow-up for that reason.
| Reason | Group, n (%) | Total, n (%) (N = 832) | ||||
|---|---|---|---|---|---|---|
| CB (N = 555) | Other residents (N = 277) | |||||
| Control (N = 269) | Intervention (N = 286) | Control (N = 143) | Intervention (N = 134) | Control (N = 412) | Intervention (N = 420) | |
| Died | 31 (11.5) | 50 (17.5) | 11 (7.7) | 21 (15.7) | 42 (10.2) | 71 (16.9) |
| Moved care home | 11 (4.1) | 12 (4.2) | 2 (1.4) | 2 (1.5) | 13 (3.2) | 14 (3.3) |
| Palliative care/in hospital | 1 (0.4) | 2 (0.7) | 1 (0.7) | 0 | 2 (0.5) | 2 (0.5) |
| Care home withdrew | 0 | 20 (7.0) | 0 | 10 (7.5) | 0 | 30 (7.1) |
| Total | 43 (16.0) | 84 (29.4) | 14 (9.8) | 33 (24.6) | 57 (13.8) | 117 (27.9) |
The mean age of the 113 residents who died before follow-up was 87.85 years (SD 7.01 years) and the mean age of survivors was 84.68 years (SD 7.58 years); 73% of those who died were female, as were 76% of survivors. There appeared to be little or no difference between those who died and those who survived, except for the expected difference in age.
Logistic regression was carried out to identify any factors and covariates affecting the dropout rate, that is, not just those who died. The factors included were gender, smoking status, size of care home, proportion of CB, type of home, treatment group, whether or not there had been organisational change and CB/other resident group. Covariates were age and length of time from baseline to follow-up. Based on the 174 residents who dropped out, the likelihood of dropout from the intervention group was increased by living in a home with a larger number of beds; male gender; older age; and longer time between baseline and follow-up (see Appendix 8, Table 71). The number of residents who dropped out of the intervention group (n = 117) was double that of the control group (n = 57). However, this may be attributed to the longer follow-up times in the intervention homes than those in the control homes because of the delays encountered in training the staff champions following baseline data collection (see Chapter 2).
The dropout analysis, conducted as part of the primary effectiveness analysis, included 31 cases. This figure was arrived at by excluding those residents who had died or whose care home had withdrawn, as these were not considered to be random. Whether or not the resident was in the CB sample group was the only factor found to affect dropout in this case, with these residents having a 2.8 times higher risk of dropout than a resident from the other residents group.
Those who dropped out in the CB group alone (n = 127) were therefore examined further. The proportions dropping out due to death in the intervention (50/64) and control (31/43) groups were compared, excluding those in the care homes that withdrew from the study. No significant difference between the proportions [χ2(1) = 0.51; p = 0.498] was found. Furthermore, no significant differences were found when the CB dropout group was compared with those who remained in the study at follow-up on baseline CBS, NPI and CMAI scores (Table 9).
| Measure | Dropout (n = 127) | Completed follow-up (n = 428) | ||
|---|---|---|---|---|
| Mean score | SD | Mean score | SD | |
| CBS incidence | 9.00 | 3.91 | 8.96 | 3.84 |
| CBS frequency | 28.48 | 13.08 | 27.68 | 13.06 |
| CBS difficulty | 15.15 | 10.92 | 14.61 | 10.79 |
| CMAI | 53.60 | 19.02 | 54.05 | 18.49 |
| NPI incidence | 4.99 | 2.25 | 4.78 | 2.40 |
| NPI total (frequency × severity) | 21.88 | 16.78 | 20.88 | 15.74 |
| NPI distress | 5.22 | 6.46 | 4.67 | 6.60 |
Dropout was the reason why intervention (i.e. an action plan for a resident) was not provided for 87 residents (30% of the original 286 residents with dementia and CB) from the intervention group. This was mainly because the resident had died or the care home had withdrawn or declined the intervention (see Chapter 2, Figure 3).
Analysis of the staff who completed the baseline questionnaire (n = 609) showed that those who dropped out were significantly younger [t(606) = 2.4; p = 0.015], with a mean age of 37.25 years (SD 13.45 years), than those who remained at follow-up [mean age 40.02 years (SD 12.86 years)]. They had also worked with older people for less time, though this difference was not statistically significant.
The most common reason for staff not being followed up was their lack of availability because of either annual leave or sickness (Table 10). As with the figures for resident dropout, more staff dropped out in the intervention homes than in the control homes, but this may again be explained by the longer duration to follow-up in the intervention homes. After excluding the two homes that withdrew from the study, based on the chi-squared/Fisher’s exact test, two factors appear to significantly influence staff dropout: working in a large home and experiencing organisational change (see Appendix 8, Table 72).
| Reason for dropout | Group, n (%) | |
|---|---|---|
| Control (N = 294) | Intervention (N = 315) | |
| Not available for interview | 40 (13.6) | 94 (29.8) |
| Care home withdrew from the study | 0 | 22 (7.0) |
| No longer works at the home | 18 (6.1) | 17 (5.4) |
| Other | 3 (1.0) | 2 (0.6) |
| Total | 61 (20.8) | 135 (42.9) |
Maintenance of ‘blind’ follow-up assessments
One or more researchers completed a blinding perception questionnaire for each of the 26 experimental and 30 control homes at follow-up. Table 11 indicates that, when researchers were able to make a judgement as to which group the care home had been allocated to, they were more likely to be correct than incorrect in their prediction. However, they were certain of their judgement in only 27.4% of instances, most of which were correct. In 41.2% of cases the researchers stated that the allocation could have been ‘either group’ and in another 31.4% researchers only ‘thought they knew’, as opposed to ‘definitely knew’. Some unblinding at the follow-up assessment point may have occurred, but the proportion of correct definite judgements remained low, at just under one-quarter, reflecting the considerable remaining uncertainty.
| Researcher perception | Group, n (%) | Total, n (%) | |
|---|---|---|---|
| Control | Intervention | ||
| I know this home is a . . . home– incorrect | 0 (0.0) | 3 (6.3) | 3 (2.9) |
| I think this home is a . . . home– incorrect | 6 (11.1) | 4 (8.3) | 10 (9.8) |
| This home could be either . . . | 28 (51.9) | 14 (29.2) | 42 (41.2) |
| I think this home is a . . . home– correct | 11 (20.4) | 11 (22.9) | 22 (21.6) |
| I know this home is a . . . home– correct | 9 (16.7) | 16 (33.3) | 25 (24.5) |
| Total | 54 | 48 | 102 |
Descriptive analyses
The baseline descriptive data have been split into a number of tables describing the care homes (see Table 12), the care home staff (see Tables 13–16) and the residents (see Tables 17–20).
Care home descriptives
As shown in Table 12, only 15.5% of care homes were local authority owned, which reflects Lievesley and Crosby’s findings,158 noting that, since the NHS and Community Care Act of 1990,159 private sector care homes have dramatically increased in number and local authority ownership has decreased. The homes in this study were located in a mix of urban, suburban and rural areas, with the majority (63.8%) being categorised as suburban. The suburban category was further split into ‘built up’ (typically more populated areas that include a higher proportion of social housing) and ‘not built up’ (typically more private houses and ‘green’ areas).
| Care home descriptive | Group, n (%) | Total, N (%) (n = 58) | |
|---|---|---|---|
| Control (N = 30) | Intervention (N = 28) | ||
| Type | |||
| Local authority | 7 (23.3) | 2 (7.1) | 9 (15.5) |
| Private without nursing care | 10 (33.3) | 16 (57.1) | 26 (44.8) |
| Private with nursing care | 5 (16.7) | 2 (7.1) | 7 (12.1) |
| Not for profit/voluntary/charity | 8 (26.7) | 8 (28.6) | 16 (27.6) |
| Size of homea | |||
| Small (25–39 beds) | 15 (50.0) | 15 (53.6) | 30 (51.7) |
| Medium (40–49 beds) | 10 (33.3) | 8 (28.6) | 18 (31.0) |
| Large (50+ beds) | 5 (16.7) | 5 (17.9) | 10 (17.2) |
| Location | |||
| Urban | 4 (13.3) | 7 (25.0) | 11 (19.0) |
| Suburban (built up) | 12 (40.0) | 7 (25.0) | 19 (32.8) |
| Suburban (not built up) | 10 (33.3) | 8 (28.6) | 18 (31.0) |
| Rural | 4 (13.3) | 6 (21.4) | 10 (17.2) |
| Proportion with CBS incidence score of > 10 (based on screening data) | |||
| < 0.4 | 25 (83.3) | 24 (85.7) | 49 (84.5) |
| ≥ 0.4 | 5 (16.7) | 4 (14.3) | 9 (15.5) |
| Organisational change | 10 (33.3) | 13 (46.4) | 23 (39.7) |
There appeared to be some differences in the care home characteristics between the two groups; most notably, compared with control homes. The higher percentage of the intervention homes were in the private sector and provided care without nursing (57.1% vs. 33.3%, respectively), a lower percentage were in the private sector and provided care without nursing (7.1% vs. 16.7%) and a higher percentage had undergone major organisational change during the study period (46.4% vs. 33.3%). However, none of these differences or any of the other home demographics was statistically significant.
Staff descriptives
Table 13 outlines demographic information for ResCare trial staff participants. Our data are similar to those from an analysis of the National Minimum Data Set for Social Care (NMDS-SC) in England, which includes a sizeable proportion of all social care staff working in registered services. Nine out of 10 of the ResCare trial participants were female, similar to findings of an 87% female dementia care workforce noted in NMDS-SC dementia care workforce data. 160 The NMDS-SC160 analysis also noted clear incremental mean and median number of years working in the care sector as employee age rises, indicating that the vast majority of older workers in the social care sector are continuing workers, rather than ‘new’ workers hired recently. The ResCare trial participants had worked in their current care home for an average of 4.87 years (SD 5.26 years) and with older people in general for 9.64 years (SD 8.27 years).
| Staff descriptive | Group | Total (N = 609) | ||||
|---|---|---|---|---|---|---|
| Control (N = 294) | Intervention (N = 315) | |||||
| Missing (n) | Mean (SD) | Missing | Mean (SD) | Missing | Mean (SD) | |
| Working with older people (years) | 12 | 10.08 (8.82) | 11 | 9.23 (7.71) | 23 | 9.64 (8.27) |
| Working in current care home (years) | 12 | 4.77 (5.26) | 12 | 4.95 (5.31) | 24 | 4.87 (5.28) |
| Working hours per week | 15 | 34.34 (9.67) | 11 | 33.78 (10.21) | 26 | 34.05 (9.95) |
| Age left full-time education (years) | 16 | 16.90 (2.06) | 13 | 16.61 (1.82) | 29 | 16.75 (1.94) |
| Current age (years) | 13 | 39.13 (13.49) | 11 | 38.23 (12.75) | 24 | 38.66 (13.11) |
| Gender (female) | n = 265 (90.1%) | n = 282 (89.5%) | n = 547 (89.8%) | |||
Overall, our sample is generally representative of this sector in England. Differences are noted in that part-time work is common in social care, yet 84.5% of the ResCare trial participants worked ≥ 24 hours a week, with an overall mean of 34.05 hours per week (SD 9.95 hours) (see Table 13). Perhaps the full-time workforce was more available to participate in the ResCare trial? The NMDS-SC data analysis160 showed that workers in the age range of 50–75 years constitute nearly two-fifths of the whole care workforce and nearly one-eighth of the total are aged between 60 and 75 years. The ResCare trial participants tended to be younger, with only one-quarter aged > 50 years, and just 3.3% aged > 60 years (Table 14).
| Age group (years) | Baseline, n (%) (N = 609)a |
|---|---|
| 16–19 | 25 (4.1) |
| 20–29 | 174 (28.6) |
| 30–39 | 97 (16.0) |
| 40–49 | 159 (26.2) |
| 50–59 | 133 (21.9) |
| 60–69 | 18 (3.0) |
| 70–79 | 2 (0.3) |
Participating staff were asked to provide details on their highest qualification (see Chapter 2, Table 2) and about their computer and dementia training (Table 15). Not all staff chose to complete the questionnaire, and for the 93% (n = 568) returned, not all items were completed. The NMDS-SC data analysis160 found that for ‘highest qualification held’ there was a concentration around NVQ level 2 among dementia care workers (the relevant qualifications at the time of the study), with relatively fewer dementia care workers possessing higher-level qualifications than other social care staff. Staff participating in the ResCare trial held a range of qualifications (see Chapter 2, Table 2): for 54%, the highest qualification level was equivalent to NVQ level 2 or lower, but compared with the national picture the ResCare trial participants were more likely to have higher-level qualifications and only 5%, compared with 9% in the national social care workforce, held no qualifications. Approximately two-thirds of the ResCare trial staff had never received any computer training and a striking 30.3% had never received any dementia training (see Table 15).
| Previous training | Group, n (%) | Total, n (%) (N = 564) | |
|---|---|---|---|
| Control (N = 268) | Intervention (N = 296) | ||
| Previous computer training | |||
| Yes | 101 (37.7) | 102 (34.5) | 203 (36.0) |
| No | 167 (62.3) | 194 (65.5) | 361 (64.0) |
| Previous dementia training | (n = 269) | (n = 295) | (n = 564) |
| Yes | 193 (71.7) | 200 (67.8) | 393 (69.7) |
| No | 76 (28.3) | 95 (32.2) | 171 (30.3) |
There were no noticeable differences in the demographics of the staff across the two treatment groups (see Table 13), apart from the control group having a slightly greater percentage of staff with higher-level qualifications than the staff in intervention homes (see Chapter 2, Table 2).
The mean EQ-5D index value for staff at baseline was similar to the UK population norm of 0.91 (SD 0.16) for the age bracket of 35–44 years. 161 However, the mean staff EQ-5D visual analogue scale (VAS) was slightly lower than the population norm of 86.56 (SD 13.79). The EQ-5D index is scored from –0.59 to 1 (worst health state to full health), and the EQ-5D VAS is scored from 0 to 100 (worst imaginable health state to best imaginable health state), so the staff in this study had reasonable health at baseline (Table 16). Staff attitudes to dementia were consistent with those reported by qualified nurses working in nursing homes in one UK study,139 higher than a pilot study in care homes162 and also more positive than those reported from a large US study,163 where the mean total score of direct care staff on the ADQ was 70.7 (SD 6.4), compared with our mean score of 75.16 (SD 7.04).
| Staff measure | Group | Total (n = 609)a | |||||
|---|---|---|---|---|---|---|---|
| Control (n = 294) | Intervention (n = 315) | ||||||
| Mean score | SD | Mean score | SD | Mean score | SD | Range | |
| EQ-5D index | 0.92 | 0.14 | 0.91 | 0.16 | 0.92 | 0.15 | 0.12–1 |
| EQ-5D VAS | 84.82 | 13.07 | 82.90 | 14.36 | 83.84 | 13.77 | 10–100 |
| ADQ | 75.23 | 7.15 | 75.10 | 6.93 | 75.16 | 7.04 | 54–95 |
| Hope | 27.05 | 4.72 | 26.82 | 4.56 | 26.93 | 4.64 | 12–40 |
| Person centred | 48.18 | 4.12 | 48.28 | 3.98 | 48.23 | 4.05 | 37–55 |
| MBI | 21.82 | 15.51 | 20.27 | 14.58 | 21.03 | 15.05 | 0–84 |
| Emotional exhaustion | 13.31 | 11.43 | 12.31 | 10.40 | 12.80 | 10.92 | 0–49 |
| Personal accomplishment | 42.21 | 5.69 | 42.53 | 5.43 | 42.37 | 5.56 | 18–48 |
| Depersonalisation | 2.72 | 3.41 | 2.48 | 3.41 | 2.60 | 3.35 | 0–21 |
| SES | 54.18 | 7.36 | 53.71 | 7.36 | 53.94 | 7.54 | 25–63 |
On the MBI, average scores showed relatively low levels of emotional exhaustion (Maslach Burnout Inventory-Emotional Exhaustion; MBI-EE) and depersonalisation (Maslach Burnout Inventory-Depersonalisation; MBI-DP) and high levels of personal accomplishment (Maslach Burnout Inventory-Personal Accomplishment; MBI-PA) compared with norms. For the MBI, values greater than the cut-off points, indicating a high degree of burnout137 are MBI-EE = 18, MBI-DP = 10 and MBI-PA = 33. Our sample reported lower MBI-DP scores than with health-care workers in Africa,164 but were in line with a UK pilot study in care homes. 162 Self-efficacy ratings were in line with the original participant sample, where this measure was used. 140
Resident descriptives
Basic demographic details of the residents are detailed in Table 17. Overall, the mean age was 85.11 years (SD 7.58 years), the majority of residents were female (75.7%) and almost half had been living in their own privately owned home prior to moving to the care home. All residents eligible for the CB group had dementia, which was checked using DSM-IV criteria. In the other residents group, 31.3% met the criteria for dementia, but this group did not meet the eligibility criteria of at least four behaviours on the CBS incidence measure. Overall, of the 832 residents in the study at baseline, 473 (56.9%) had a formal diagnosis of dementia according to care home records. The CDR score for those in the CB group is shown in Table 17, with the largest percentage of residents (49.2%) being rated as 3, that is, ‘severe dementia’.
| Resident descriptive | Residents, n (%) | Total by treatment group, n (%) (N = 832) | Total sample, n (%) (N = 832) | ||||
|---|---|---|---|---|---|---|---|
| CB sample (N = 555) | Other (N = 277) | ||||||
| Control (N = 269) | Intervention (N = 286) | Control (N = 143) | Intervention (N = 134) | Control (N = 412) | Intervention (N = 420) | ||
| Gender (female) | 208 (77.3) | 223 (78.0) | 102 (71.3) | 97 (72.4) | 310 (75.2) | 320 (76.2) | 630 (75.7) |
| DSM-IV criteria for dementia met | 269 (100) | 286 (100) | 45 (31.5) | 42 (31.3) | 314 (76.2) | 328 (78.1) | 642 (77.2) |
| CDR | |||||||
| 0 | 2 (0.7) | 2 (0.7) | 4 (0.7) | ||||
| 0.5 | 6 (2.2) | 12 (4.1) | 18 (3.2) | ||||
| 1 | 36 (13.4) | 52 (18.2) | 88 (15.9) | ||||
| 2 | 79 (29.4) | 90 (31.5) | 169 (30.5) | ||||
| 3 | 145 (53.9) | 128 (44.8) | 273 (49.2) | ||||
| Missing | 1 (0.4) | 2 (0.7) | 3 (0.5) | ||||
| Age (years), mean (SD) | 84.84 (7.59) | 85.47 (7.27) | 85.18 (7.62) | 84.81 (8.17) | 84.96 (7.60) | 85.25 (7.56) | 85.11 (7.58) |
There was no noticeable difference in the demographics of the residents across the experimental (intervention) and control groups.
As can be seen from Table 18, the quality-of-life self-report scores (EQ-5D and QoL-AD) were fairly similar for the CB group and the other residents, with the CB group reporting a higher EQ-5D index and VAS score. However, proxy reports on these measures showed lower scores for the CB group, reflecting the contribution made by CB to proxy quality-of-life ratings. 165
| Resident measure | Residents, mean score (SD) | Total sample, mean score (SD) (n = 832) | ||||
|---|---|---|---|---|---|---|
| CB sample (n = 555) | Other (n = 277) | |||||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| CBS incidence | 9.01 (3.84) | 8.94 (3.85) | 3.02 (2.90) | 3.09 (2.41) | 6.93 (4.55) | 7.07 (4.40) |
| CBS frequency | 28.03 (12.92) | 27.55 (13.17) | 8.87 (9.15) | 8.34 (6.75) | 21.38 (14.87) | 21.42 (14.59) |
| CBS difficulty | 14.73 (10.78) | 14.55 (10.71) | 4.08 (5.52) | 4.11 (4.27) | 11.03 (10.59) | 11.22 (10.37) |
| CBS challenge (frequency × difficulty) | 46.67 (36.72) | 46.06 (36.93) | 12.02 (18.52) | 11.36 (12.09) | 34.64 (35.65) | 34.99 (35.16) |
| EQ-5D index (proxy) | 0.31 (0.32) | 0.30 (0.34) | 0.44 (0.32) | 0.44 (0.39) | 0.36 (0.33) | 0.35 (0.36) |
| EQ-5D VAS (proxy) | 57.05 (18.51) | 52.03 (19.07) | 66.36 (17.44) | 62.63 (20.39) | 60.28 (18.66) | 55.42 (20.09) |
| QoL-AD (proxy) | 29.46 (5.82) | 29.38 (5.95) | 34.62 (5.62) | 34.85 (6.92) | 31.25 (6.25) | 31.12 (6.77) |
| EQ-5D index (self-report) | 0.64 (0.34) | 0.69 (0.34) | 0.47 (0.39) | 0.56 (0.38) | 0.57 (0.37) | 0.63 (0.36) |
| EQ-5D VAS (self-report) | 70.67 (22.08) | 73.52 (22.00) | 64.40 (21.54) | 68.05 (18.68) | 67.50 (21.97) | 70.66 (20.44) |
| QoL-AD (self-report) | 33.61 (6.34) | 36.08 (6.33) | 34.37 (5.45) | 35.84 (5.72) | 33.97 (5.94) | 35.96 (6.03) |
| NPI incidence | 4.80 (2.34) | 4.86 (2.40) | – | – | – | – |
| NPI frequency | 12.66 (7.50) | 12.12 (7.12) | – | – | – | – |
| NPI severity | 7.97 (4.87) | 7.55 (4.80) | – | – | – | – |
| NPI total (frequency × severity) | 22.28 (16.22) | 20.06 (15.66) | – | – | – | – |
| NPI distress | 4.82 (6.50) | 4.77 (6.63) | – | – | – | – |
| CMAI total | 53.30 (16.49) | 54.61 (20.43) | – | – | – | – |
| CMAI physical: aggressive | 16.94 (7.79) | 17.20 (9.47) | – | – | – | – |
| CMAI physical: non-aggressive | 19.29 (8.62) | 19.55 (8.93) | – | – | – | – |
| CMAI verbal: aggressive | 5.49 (3.14) | 5.68 (3.21) | – | – | – | – |
| CMAI verbal: non-aggressive | 11.58 (5.68) | 12.13 (6.40) | – | – | – | – |
| CDR-SB | 13.42 (3.49) | 12.70 (3.81) | – | – | – | – |
As shown in Table 19, by far the most common CBs exhibited by residents in the CB sample, as perceived by care home staff, were lack of self-care, lack of motivation, shouting, restlessness, lack of occupation, perseveration, verbal and physical aggression, non-compliance and wandering. This pattern was similar in the other residents group, albeit in smaller proportions, as these were recruited randomly to have dementia or CB but not both. In comparing the CB sample and the other residents, the greater complexity of the CB sample can be seen. For example, for 8 of the 25 CBs (verbal aggression, shouting, perseveration, restlessness, lack of motivation, non-compliance, lack of occupation and lack of self-care), the CB sample percentage incidence scores suggested that those particular CBs were more likely to be present than not present. There were no such occurrences in the other residents group. Another example of this complexity was the higher percentage of CBS incidences of certain types of behaviour, such as faecal smearing, in the CB sample.
| CBS item | Residents | |||||||
|---|---|---|---|---|---|---|---|---|
| CB sample (N = 555) | Other (N = 277) | |||||||
| Control (n = 269) | Intervention (n = 286) | Control (n = 143) | Intervention (n = 134) | |||||
| Incidence, % | Challenge,a mean score (SD) | Incidence, % | Challenge, mean score (SD) | Incidence, % | Challenge, mean score (SD) | Incidence, % | Challenge, mean score (SD) | |
| Physical aggressionb | 49.4 | 2.5 (3.72) | 47.2 | 2.48 (3.81) | 4.9 | 0.31 (1.8) | 7.5 | 0.14 (0.64) |
| Verbal aggressionb | 51.7 | 2.54 (3.52 | 54.9 | 2.58 (3.53) | 18.9 | 0.66 (2.04) | 18.7 | 0.57 (1.8) |
| Self-harm | 8.9 | 0.35 (1.34) | 9.1 | 0.45 (1.89) | 2.1 | 0.13 (0.96) | 2.2 | 0.05 (0.37) |
| Shouting | 61.0 | 3.08 (3.92) | 62.2 | 3.12 (3.86) | 19.6 | 0.63 (1.7) | 21.6 | 0.7 (1.86) |
| Screaming/crying outb | 30.5 | 1.7 (3.33) | 34.6 | 1.98 (3.65) | 6.3 | 0.31 (1.43) | 4.5 | 0.25 (1.61) |
| Perseveration | 53.2 | 2.75 (3.64) | 59.8 | 2.95 (3.48) | 18.2 | 0.64 (1.52) | 14.2 | 0.46 (1.23) |
| Wandering | 48.0 | 2.56 (3.73) | 45.1 | 2.29 (3.33) | 7.7 | 0.29 (1.1) | 8.2 | 0.31 (1.17) |
| Restlessnessb | 60.2 | 3.38 (3.98) | 61.5 | 2.99 (3.52) | 13.3 | 0.45 (1.21) | 23.1 | 0.67 (1.65) |
| Lack of motivationb | 62.3 | 3.09 (3.44) | 62.2 | 3.28 (3.79) | 32.9 | 1.25 (2.19) | 33.6 | 1.51 (2.92) |
| Clinging | 24.9 | 1.33 (2.77) | 25.2 | 1.36 (3.11) | 5.6 | 0.29 (1.39) | 6.0 | 0.22 (0.91) |
| Interfering with others | 32.0 | 2.01 (3.83) | 32.5 | 1.99 (3.69) | 9.1 | 0.29 (1.01) | 8.2 | 0.38 (1.68) |
| Pilfering or hoarding | 22.7 | 1.32 (3.21) | 19.2 | 0.97 (2.46) | 3.5 | 0.09 (0.52) | 3.7 | 0.1 (0.56) |
| Suspiciousness | 33.5 | 1.54 (3.13) | 33.6 | 1.74 (3.27) | 14.7 | 0.51 (1.57) | 11.2 | 0.31 (1.08) |
| Manipulative | 10.8 | 0.67 (2.36) | 10.5 | 0.51 (1.92) | 9.8 | 0.41 (1.59) | 9.0 | 0.4 (1.66) |
| Lack of self-careb | 84.4 | 4.48 (3.39) | 79.7 | 4.51 (3.84) | 52.4 | 2.41 (2.93) | 38.1 | 1.66 (2.74) |
| Spitting | 11.9 | 0.64 (2.3) | 9.8 | 0.63 (2.5) | 1.4 | 0.17 (1.41) | 0.0 | 0 (0) |
| Faecal smearing | 25.3 | 1.09 (2.63) | 19.9 | 1.15 (2.93) | 1.4 | 0.07 (0.76) | 3.0 | 0.07 (0.5) |
| Inappropriate urination | 15.6 | 0.88 (2.66) | 15.7 | 0.69 (2.18) | 4.2 | 0.23 (1.35) | 0.7 | 0.01 (0.09) |
| Stripping | 23.8 | 0.78 (1.83) | 18.9 | 0.63 (1.78) | 3.5 | 0.11 (0.73) | 2.2 | 0.02 (0.15) |
| Inappropriate sexual behaviour | 8.2 | 0.42 (1.75) | 6.6 | 0.29 (1.49) | 1.4 | 0.07 (0.69) | 5.2 | 0.16 (0.87) |
| Sleep problemsb | 31.6 | 1.56 (3.17) | 34.0 | 1.46 (2.88) | 13.3 | 0.4 (1.27) | 17.9 | 0.51 (1.46) |
| Non-compliance | 48.7 | 2.77 (4.02) | 51.4 | 2.82 (3.94) | 10.5 | 0.48 (1.92) | 14.2 | 0.46 (1.78) |
| Dangerous behaviourb | 10.0 | 0.36 (1.55) | 9.4 | 0.36 (1.31) | 1.4 | 0.03 (0.3) | 1.5 | 0.07 (0.58) |
| Demands attention | 32.7 | 1.84 (3.55) | 32.5 | 1.87 (3.57) | 14.7 | 0.51 (1.68) | 16.4 | 0.71 (2.07) |
| Lack of occupationb | 60.2 | 3.06 (3.33) | 57.7 | 3 (3.45) | 31.5 | 1.27 (2.08) | 38.1 | 1.65 (2.49) |
Medication in the challenging behaviour group
In total, 3601 individually prescribed medications were recorded for the CB group, with a mean number of 6.82 medications (range 0–24 medications). This was lower than in a small study in 2008, in which an average of 8.0 medications (range 1–17 medications) was reported for 119 residents in six homes. 166 Males had an average of 7.42 medications (range 1–24 medications) and females averaged 6.64 medications (range 0–19 medications). For residents aged between 90 and 94 years the mean number of medications was 7.02 (range 0–16 medications) and in those aged > 95 years (all female) the mean was 6.71 medications (range 0–17 medications). Six residents (1.08%) did not receive any medication.
Table 20 outlines the drug groups we recorded, showing medications that the residents were prescribed in the 3 months before baseline and whether or not they were taking these for the full 3 months.
| Medication category | Baseline frequency of medication, n (%) (N = 555) | |||
|---|---|---|---|---|
| Not prescribed | < 3 months | Full 3 months (1 drug) | Full 3 months (> 1 drug) | |
| Antipsychotics | 471 (84.9) | 7 (1.3) | 70 (12.6) | 7 (1.3) |
| Atypical | 506 (91.2) | 1 (0.2) | 46 (8.3) | 2 (0.4) |
| Typical | 517 (93.2) | 6 (1.1) | 29 (5.2) | 3 (0.5) |
| Antidepressants | 366 (65.9) | 23 (4.1) | 151 (27.2) | 15 (2.7) |
| SSRI | 438 (78.9) | 16 (2.9) | 97 (17.5) | 4 (0.7) |
| Tricyclic | 514 (92.6) | 4 (0.7) | 36 (6.5) | 1 (0.2) |
| Other | 516 (93.0) | 4 (0.7) | 33 (5.9) | 2 (0.4) |
| Hypnotics and anxiolytics | 443 (79.8) | 13 (2.3) | 91 (16.4) | 8 (1.4) |
| B/Z/A | 444 (80.0) | 13 (2.3) | 90 (16.2) | 8 (1.4) |
| Non-benzodiazepines | 554 (99.8) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Anticonvulsants | 520 (93.7) | 2 (0.4) | 31 (5.6) | 2 (0.4) |
| Dementia drugs | 473 (85.2) | 6 (1.1) | 72 (13.0) | 4 (0.7) |
| Acetylcholinesterase inhibitors | 493 (88.8) | 3 (0.5) | 59 (10.6) | 0 (0.0) |
| Cognitive enhancers | 532 (95.9) | 4 (0.7) | 18 (3.2) | 1 (0.2) |
| Pain relief | 251 (45.2) | 19 (3.4) | 234 (42.2) | 51 (9.2) |
| Opioid | 436 (78.6) | 15 (2.7) | 91 (16.4) | 13 (2.3) |
| Non-opioid | 328 (59.1) | 17 (3.1) | 206 (37.1) | 4 (0.7) |
| Laxatives | 296 (53.3) | 18 (3.2) | 172 (31.0) | 69 (12.4) |
Of interest in the present study were care practices surrounding the prescription of some psychotropic medications, pain relief and relief from discomfort due to constipation. Next we summarise key observations of our baseline data (see Table 20) relating to the antipsychotics, hypnotics and anxiolytics (including benzodiazepines/z-hypnotics, sometimes referred to as the B/Z/A drugs), antidepressants, analgesics and laxatives.
Eighty-four residents (15.14%) were prescribed an antipsychotic, of which the atypical risperidone is the only licensed antipsychotic for the management of serious behavioural symptoms in dementia. Risperidone was prescribed for just 14 residents (2.52%). Contrary to best practice, 38 residents (6.85%) were prescribed a ‘typical antipsychotic’, of which the antipsychotic haloperidol accounted for 28 residents (5.05%) (i.e. double that of the atypical risperidone). Where antipsychotic medication was prescribed to those aged > 90 years, only two residents were treated with risperidone and seven had the atypical antipsychotic quetiapine, a product that is not approved by the US Food and Drug Administration for the treatment of behavioural problems in older people with dementia. Surprisingly, six residents aged > 90 years were prescribed typical antipsychotics, despite the risk factors for adverse drug reactions. Of those receiving at least one antipsychotic prescription, 91.67% (77 residents) were prescribed an antipsychotic for 3 months or more.
The records showed that 111 residents (20%) were prescribed hypnotic and anxiolytic (B/Z/A) drugs. Of these, 88.29% (98 residents) had prescriptions for over 3 months, that is, well over the recommended guidance for prescription. Seventeen residents were prescribed benzodiazepines, despite being aged > 90 years.
Furthermore, 189 residents (34.05%) were being treated with an antidepressant; for 87.83% this was prescribed for over 3 months. Of these, 41 residents (7.39%) were prescribed a tricyclic, including amitriptyline and 117 residents (21.08%) had a selective serotonin reuptake inhibitor (SSRI) or the antidepressant mirtazapine. Fourteen residents were prescribed low-dose amitriptyline (≤ 25 mg) and three had prescriptions for both the antidepressant citalopram and mirtazapine. Forty residents aged > 90 years were prescribed an antidepressant of some description.
In relation to pain relief medication, 304 residents (54.77%), were prescribed some form of analgesia, of whom 59 (10.63%) had co-codamol and 18 (3.24%) an oral non-steroidal anti-inflammatory drug. One hundred and thirteen residents aged > 90 years were prescribed analgesics. Nearly half the resident group (46.6%) was prescribed laxatives, of whom 80 were aged > 90 years.
Analysis of serious adverse events, reactions and unexpected reactions
Our rigorous procedure for identifying, validating and reporting SAEs uncovered no SARs and, therefore, no suspected unexpected SARs.
Our analysis of SAEs focuses on the 58 homes and their 832 residents who completed baseline assessments. The 28 intervention homes reported 80 SAEs, namely 59 in the 286 residents with CB (comprising 50 deaths, seven hospital admissions and two instances of ‘persistent or significant disability’) and 21 deaths among the 134 other residents. The 30 control homes reported 55 SAEs, namely 40 in the 269 residents with CB, (comprising 31 deaths and nine hospital admissions) and 15 among the 143 other residents, comprising 11 deaths and four hospital admissions. As the 420 intervention residents were at risk for an average of 7.86 months, while the 412 control residents were so for an average of 5.15 months, the SAE rates per resident per year were 0.291 in the intervention group and 0.311 in the control group. None of these comparisons is at all close to reaching a statistically significant difference between the intervention and control groups.
Analyses of primary outcome measures
A residual analysis showed that the assumption of normality was acceptable for both NPI frequency and severity. The relationship between the baseline and follow-up measures was similar across all the care homes in both intervention and control groups, so there were no difficulties in using the models, outlined in Methods, for the chosen analysis.
The sample size was 555 residents from the CB sample, and 81 deaths were excluded from the imputed data sets. Fifty-seven care homes were included in the analysis because one home had only one resident in the CB sample at baseline and did not subsequently complete follow-up measures.
Table 21 shows the NPI frequency scores at follow-up for those residents with measures at both baseline and follow-up. There was only a small difference in the scores at follow-up. To be significant at the 5% level, the 95% CI for the mean should not contain zero. Subsequently, because the CIs contain zero for both models for the complete-case analysis, we found no significant difference between the intervention and control groups. This non-significant finding was also found for the imputed data set.
| Group | Follow-up, mean score (SD) | Data set, mean difference in score (95% CI) | |||
|---|---|---|---|---|---|
| Complete case (N = 428)a | Five imputations (N = 474)b | ||||
| Model Ic | Model IId | Model Ic | Model IId | ||
| Control (n = 226) | 11.55 (6.92) | 0.27 (–1.01 to 1.55) | 0.59 (–1.21 to 2.39) | 0.26 (–1.02 to 1.54) | 0.60 (–1.18 to 2.38) |
| Intervention (n = 202) | 11.65 (6.43) | ||||
Table 22 shows the results of the analysis of the NPI severity scores. Again, there was only a small numerical difference between the intervention and control groups at follow-up. The results of fitting models I and II, for both the complete case and multiple imputed data, again failed to find any of the differences to be significant at the 5% level. In model II, we adjusted for the differential duration of follow-up between the two groups, as this was included as a covariate.
| Group | Follow-up, mean score (SD) | Data set, mean difference in score (95% CI) | |||
|---|---|---|---|---|---|
| Complete case (N = 424)a | Five imputations (N = 474)b | ||||
| Model Ic | Model IId | Model Ic | Model IId | ||
| Control (n = 222) | 7.25 (4.45) | 0.27 (–0.67 to 1.21) | 0.37 (–1.03 to 1.77) | 0.30 (–0.76 to 1.36) | 0.45 (–1.03 to 1.93) |
| Intervention (n = 202) | 7.29 (4.44) | ||||
In conclusion, the primary analysis did not demonstrate any evidence of differences between the two treatment groups on the primary outcome measures of NPI frequency and severity. We therefore found no evidence of an effect of the intervention.
Analyses of secondary outcome measures
To determine whether or not the experimental intervention improved staff experience and quality of life for the residents living in the care homes, the following variables for the CB sample were used: NPI distress, NPI total score and CMAI frequency. Variables for the whole resident sample were CBS frequency, CBS total score, EQ-5D and QoL-AD. For the staff the following measures were used: MBI, ADQ, EQ-5D and SES.
The same combination of two models (I and II) and two data sets (complete and pooled results of five imputed data sets) were used for the secondary outcome measures.
A residual analysis showed that the assumption of normality was acceptable for all the variables, and as the relationship between the baseline and follow-up measures was similar across all the care homes in both treatment groups, there were no difficulties in using models I and II for the chosen analyses.
The CBS frequency showed that for model I there was almost a significant effect of the intervention for the complete-case data, whereas the imputed data did find a significant effect. However, model II did not confirm this effect (see Table 23 for the results of the secondary analyses). There was a significant effect of the intervention in relation to the EQ-5D index for model II but not for model I. However, when the correction for multiple comparisons was applied, the possible effect of treatment on EQ-5D index disappeared. No significant effects of the intervention were found in the staff measures for either of the models.
| Measure | Data set, mean difference in score (95% CI) | |||
|---|---|---|---|---|
| Complete case | Five imputations | |||
| Model Ia | Model IIb | Model Ia | Model IIb | |
| CB sample | ||||
| NPI distress | 0.72 (–0.58 to 2.02) | –0.16 (–2.00 to 1.68) | 0.71 (–0.51 to 1.93) | 0.12 (–1.64 to 1.88) |
| NPI total | 1.48 (–1.30 to 4.26) | 0.23 (–5.77 to 6.23) | 1.52 (–1.18 to 4.22) | 0.18 (–3.68 to 4.04) |
| CMAI: physical/aggressive | 0.55 (–1.01 to 2.11) | 0.75 (–1.55 to 3.05) | 0.29 (–1.19 to 1.77) | 0.39 (–1.77 to 2.55) |
| CMAI: physical/non-aggressive | –0.60 (–2.10 to 0.9) | 0.64 (–1.48 to 2.76) | –0.41 (–1.91 to 1.09) | 0.46 (–1.66 to 2.58) |
| CMAI: verbal/aggressive | 0.43 (–0.13 to 0.99) | 0.66 (–0.16 to 1.48) | 0.42 (–0.10 to 0.94) | 0.60 (–0.16 to 1.36) |
| CMAI: verbal/non-aggressive | 0.71 (–0.49 to 1.91) | 0.74 (–1.00 to 2.48) | 0.67 (–0.67 to 2.01) | 0.63 (–1.17 to 2.43) |
| All residents | ||||
| CBS frequency | 1.71 (–0.01 to 3.43) | 0.93 (–1.67 to 3.53) | 1.65 (0.03 to 3.27) | 0.69 (–1.67 to 3.05) |
| CBS (frequency × difficulty) | 4.30 (–0.76 to 9.36) | –0.62 (–7.4 to 6.16) | 4.17 (–0.69 to 9.03) | –0.19 (–6.69 to 6.31) |
| EQ-5D index | –0.001 (–0.06 to 0.059) | 0.09 (0.01 to 0.17) | –0.005 (–0.065 to 0.055) | 0.08 (0.00 to 0.16) |
| EQ-5D VAS | –2.53 (–1.43 to 0.85) | 0.84 (–1.68 to 1.76) | –2.83 (–1.65 to 0.99) | 0.35 (–1.58 to 1.98) |
| QoL-AD | –0.29 (–0.49 to 1.91) | 0.04 (–1.00 to 2.48) | –0.33 (–0.67 to 2.01) | 0.20 (–1.17 to 2.43) |
| Model Ia | Model IIc | Model Ia | Model IIc | |
| All staff | ||||
| MBI total | 0.40 (–2.80 to 3.60) | 1.28 (–3.60 to 6.16) | 0.23 (–2.91 to 3.37) | –0.07 (–4.73 to 4.59) |
| MBI-EE | 0.08 (–2.10 to 2.26) | 0.87 (–2.59 to 4.33) | 0.16 (–2.18 to 2.50) | 1.12 (–3.00 to 5.24) |
| MBI-PA | –0.03 (–1.21 to 1.15) | 0.20 (–1.44 to 1.84) | –0.26 (–1.42 to 0.90) | 0.04 (–1.54 to 1.62) |
| MBI-DP | 0.32 (–0.34 to 0.98) | 0.57 (–0.37 to 1.51) | 0.07 (–0.57 to 0.71) | 0.08 (–0.88 to 1.04) |
| ADQ total | 0.18 (–1.04 to 1.40) | 0.26 (–1.64 to 2.16) | 0.14 (–1.10 to 1.38) | 0.51 (–1.47 to 2.49) |
| ADQ hope | –0.14 (–0.92 to 0.64) | –0.11 (–1.35 to 1.13) | 0.15 (–0.83 to 1.13) | 0.28 (–0.96 to 1.52) |
| ADQ person centred | 0.30 (–0.50 to 1.10) | 0.48 (–0.68 to 1.64) | –0.11 (–1.03 to 0.81) | 0.28 (–1.16 to 1.72) |
| EQ-5D index | –0.02 (–0.04 to 0.00) | –0.01 (–0.05 to 0.03) | –0.02 (–0.04 to 0.00) | –0.01 (–0.07 to 0.05) |
| EQ-5D VAS | –1.24 (–4.92 to 2.44) | –1.38 (–7.20 to 4.44) | –0.28 (–3.94 to 3.38) | –2.29 (–7.61 to 3.03) |
| SES | –0.76 (–1.88 to 0.36) | –0.25 (–2.09 to 1.59) | –0.41 (–1.79 to 0.97) | 0.36 (–1.74 to 2.46) |
Data for the residents’ responses were not imputed, as there were so many missing data (only 376, 174 and 214 out of the total 832 residents answered the EQ-5D index, VAS and QoL-AD, respectively). There were no significant effects.
Other significant covariates were CDR-SB affects CMAI verbal non-aggressive; the time between baseline and follow-up affects EQ-5D index; and the group (CB or other residents group) affects CBS frequency and total score, both EQ-5D measures and QoL-AD.
In conclusion, we did not show that the experimental intervention consistently affected any of the secondary outcomes.
In order to address whether or not the intervention had an impact on the prescriptions of psychotropic and pain/discomfort relief medication, a multilevel analysis as above for the binary outcome of whether or not the resident was prescribed the drug was attempted. However, there were computational difficulties because of the distribution not being normal, and so the preferred analysis could not be performed. We then decided that the clustering effect was less important than the effect of the resident and so the results of comparing those residents who changed medication between baseline and follow-up in the two treatment groups are presented. This potentially results in an analysis that would find more significant effects than would be found if the appropriate multilevel model was fitted. The medication information was collected only for the CB sample and eight medication categories were of particular interest: antipsychotics, antidepressants, hypnotics, anxiolytics, anticonvulsants, dementia drugs, pain relief medication and laxatives.
Table 24 shows details of the residents who were present at both baseline and follow-up (n = 428) and whether or not their medication was changed. The majority of residents were prescribed the medication either at both baseline and follow-up, or at neither time point. Those residents whose medication changed received it either at baseline and not at follow-up, or received it at follow-up but not at baseline. Using only the change figures and comparing them between the two treatment groups, a chi-squared test to compare the proportion found no significant differences between the intervention and control groups.
| Medication category | Time point, number of residents prescribed a medication (% of total) | Chi-squared test | Total number of residents prescribed a medication at baseline | Total number of residents prescribed a medication at follow-up | |||
|---|---|---|---|---|---|---|---|
| At baseline and follow-up | Not prescribed at all | At baseline, but not follow-up | Not prescribed at baseline, but at follow-up | ||||
| Antipsychotics | 60 (14.0) | 349 (81.5) | 6 (1.4) | 13 (3.0) | 66 | 73 | |
| Control (n = 226) | 33 | 184 | 3 | 6 | 36 | 39 | |
| Intervention (n = 202) | 27 | 165 | 3 | 7 | > 0.999 | 30 | 34 |
| Antidepressants SSRI | 126 (29.4) | 268 (62.6) | 15 (3.5) | 19 (4.4) | 141 | 145 | |
| Control (n = 226) | 58 | 148 | 10 | 10 | 68 | 68 | |
| Intervention (n = 202) | 68 | 120 | 5 | 9 | 0.635 | 73 | 77 |
| Hypnotics and anxiolytics | 68 (15.9) | 327 (76.4) | 18 (4.2) | 15 (3.5) | 86 | 83 | |
| Control (n = 226) | 37 | 173 | 11 | 5 | 48 | 42 | |
| Intervention (n = 202) | 31 | 154 | 7 | 10 | 0.215 | 38 | 41 |
| Anticonvulsants | 25 (5.8) | 397 (92.8) | 1 (0.2) | 5 (1.2) | 26 | 30 | |
| Control (n = 226) | 18 | 205 | 1 | 2 | 19 | 20 | |
| Intervention (n = 202) | 7 | 192 | 0 | 3 | > 0.999 | 7 | 10 |
| Dementia drugs | 58 (13.6) | 360 (84.1) | 2 (0.5) | 8 (1.9) | 60 | 66 | |
| Control (n = 226) | 29 | 191 | 1 | 5 | 30 | 34 | |
| Intervention (n = 202) | 29 | 169 | 1 | 3 | > 0.999 | 30 | 32 |
| Pain relief: opioid | 309 (72.2) | 80 (18.7) | 14 (3.3) | 25 (5.8) | 323 | 334 | |
| Control (n = 226) | 167 | 43 | 4 | 12 | 171 | 179 | |
| Intervention (n = 202) | 142 | 37 | 10 | 13 | 0.399 | 152 | 155 |
| Pain relief: non-opioid | 215 (50.2) | 131 (30.6) | 33 (7.7) | 49 (11.4) | 248 | 264 | |
| Control (n = 226) | 113 | 67 | 18 | 28 | 131 | 141 | |
| Intervention (n = 202) | 102 | 64 | 15 | 21 | 0.996 | 117 | 123 |
| Laxatives | 198 (46.3) | 163 (38.1) | 25 (5.8) | 42 (9.8) | 223 | 240 | |
| Control (n = 226) | 115 | 77 | 13 | 21 | 128 | 136 | |
| Intervention (n = 202) | 83 | 86 | 12 | 21 | > 0.999 | 95 | 104 |
In conclusion, we found no evidence of changes in the prescription of medication between the intervention and control groups.
Effect sizes
The effect sizes found were not as large as had been expected when the study was designed, and the largest effect sizes in this study did not occur for the measures that had been expected. Table 25 presents the effect size in two ways: (1) the naive effect size is calculated as the difference between the change from baseline to follow-up (follow-up – baseline) between the two groups (intervention group – control group) divided by the pooled SD of the change; and (2) the effect size with clustering taking account of the clustering in model I.
| Measure | Naive effect size | Effect size with clustering | ICC with clustering |
|---|---|---|---|
| CBS | |||
| Incidence | 0.075 | 0.116 | 0.018 |
| Frequency | 0.140 | 0.173 | 0.010 |
| Difficulty | 0.099 | 0.168 | 0.056 |
| Challenge (frequency × difficulty) | 0.117 | 0.172 | 0.045 |
| EQ-5D (proxy) | |||
| Index | –0.008 | –0.006 | 0.095 |
| VAS | 0.002 | –0.111 | 0.058 |
| QoL-AD (proxy) | –0.033 | –0.058 | 0.081 |
| NPI | |||
| Incidence | –0.102 | –0.066 | 0.028 |
| Frequency | 0.053 | 0.045 | 0.016 |
| Severity | 0.078 | 0.068 | 0.046 |
| Total (frequency × severity) | 0.161 | 0.116 | 0.017 |
| Distress | 0.095 | 0.154 | 0.114 |
| CMAI | |||
| Physical (aggressive) | 0.083 | 0.087 | 0.059 |
| Physical (non-aggressive) | –0.103 | –0.086 | 0.021 |
| Verbal (aggressive) | 0.134 | 0.188 | 0.046 |
| Verbal (non-aggressive) | 0.085 | 0.148 | 0.054 |
| Total | 0.016 | 0.045 | 0.023 |
| CDR-SB | 0.277 | 0.215 | 0.087 |
| EQ-5D (self-report) | |||
| Index | 0.042 | 0.164 | 0.051 |
| VAS | –0.179 | –0.235 | 0.138 |
| QoL-AD (self-report) | 0.114 | 0.236 | 0.000 |
| Staff data | |||
| EQ-5D | |||
| Index | –0.071 | –0.112 | 0.004 |
| VAS | 0.043 | 0.019 | 0.000 |
| ADQ | 0.051 | 0.039 | 0.088 |
| Hope | –0.059 | –0.044 | 0.057 |
| Person-centred | 0.133 | 0.095 | 0.056 |
| MBI | 0.059 | 0.036 | 0.135 |
| MBI-EE | 0.040 | 0.010 | 0.114 |
| MBI-PA | 0.001 | –0.007 | 0.067 |
| MBI-DP | 0.121 | 0.107 | 0.017 |
| SES | –0.098 | –0.151 | 0.014 |
In summary, the effect sizes do not approach the 0.3 value that was used for designing the study. The majority of values are < 0.2, which is usually classified as a small effect size. For the staff-reported residents’ measures the maximum naive effect size is 0.277 for the CDR-SB and with clustering the value for the CDR-SB shows a similar value of 0.215. For the resident-reported residents measures the effect size with clustering value for the QoL-AD is 0.236, but this is not mirrored by the naive effect size value of 0.114. For the staff measures, the effect size with clustering value for the SES is 0.151, but again this is not mirrored by the naive effect size value of 0.098.
Intraclass correlation coefficients
The intraclass correlation coefficients values were calculated from model I output and were found to be of the same magnitude as had been expected when the study was designed assuming a value of 0.03.
Dose effect of the intervention
As we found no evidence of effect of the intervention, we carried out an investigation of the ‘dose effect’. The dose was measured by two home-level outcomes: the number of action plans expressed as a proportion of the number of CB residents per home and the number of champions per home. These two measures were included in models I and II, replacing the treatment groups, to investigate the intervention effect. The number of inpatient days was an additional response besides the primary outcome variables of NPI frequency and severity. For each of the responses, two models were fitted and both considered the clustering. Model 1 included the dose measures and baseline measure of the response. This was to test significant changes of the response caused by the dose measures (the intervention) before adjusting for other factors and covariates. Model 2 added other factors and covariates. At the individual level these were age, gender and baseline measure, and at the home level they were size of home, proportion of residents with a CBS incidence score of > 10, care home type, organisation change, and length of time between baseline and follow-up.
In summary, the proportion of action plans did not affect the NPI frequency significantly according to either model. (See Appendix 9, Tables 73 and 74, for the results of models 1 and 2, respectively, for NPI frequency, Tables 75 and 76 for NPI severity and Tables 77 and 78 for the number of inpatient days.) We found borderline evidence in model 2 that the NPI severity was affected by the number of champions, but this evidence was not strong. The dose measures showed significant effects on the NPI severity using the data after the 25% missing rule, but this was not then confirmed using the pooled results from the imputed data sets. Likewise, the dose measures did not affect the number of inpatient days significantly according to either of the models.
One might have expected that when the number of champions was higher, the NPI severity would have been found to be lower. However, this was not the case: when the number of champions was higher, the NPI severity was also higher. One explanation for this might be that where the NPI severity was higher, the home manager sent more staff to be trained as champions.
Further analysis of residents with inpatient stays
As a point of interest, a further analysis of residents with inpatient stays (medical, surgical, other and mixed) compared with those without any inpatient stays was conducted. The NPI frequency and severity, and the CBS frequency × difficulty scores of the residents were visually compared. Box plots were used to summarise those with no inpatient stays, whereas dot plots summarised those with inpatient stays (see Appendix 10, Figures 17–21). There was no visually apparent difference in the three measures between baseline and follow-up or between those with or without hospital inpatient stays. In relation to the cost of medication in the 4 months up to follow-up, the median costs were similar across all the groups. There was nothing to suggest that those residents with inpatient stays, irrespective of treatment group, were in any way different from those who had no inpatient stays during the study.
Economic analyses
Characteristics of the economic sample
At baseline 555 residents with CB and dementia were randomised (intervention, n = 286; control, n = 269). Follow-up data were available for 428 residents (intervention, n = 202; control, n = 226). Full service use data were available for all 428, so the economic sample size matched the effectiveness sample size. Staff report of the primary outcome measure, the NPI score, was available for all 428 residents at baseline, and for 422 residents at follow-up. The six missing NPI scores were imputed. There were no significant differences between the intervention and control groups with regard to demographic characteristics and the mean cost of service use in the 3 months preceding baseline of the 428 residents for whom both baseline and follow-up data were available (Table 26).
| Characteristic | Group, n (%) | |
|---|---|---|
| Control (N = 226) | Intervention (N = 202) | |
| Resident gender (female) | 176 (77.9) | 161 (79.7) |
| Resident age (years) group | ||
| 60–69 | 9 (4.0) | 8 (4.0) |
| 70–79 | 39 (17.3) | 33 (16.3) |
| 80–89 | 126 (55.8) | 107 (53.0) |
| 90–99 | 48 (21.2) | 52 (25.7) |
| ≥ 100 | 4 (1.8) | 2 (1.0) |
| Group, mean (SD) | ||
| Resident age (years) | 84.60 (7.51) | 84.97 (7.43) |
| NPI total score | 21.89 (15.99) | 19.95 (15.46) |
| EQ-5D (proxy) score | 0.32 (0.31)a | 0.34 (0.34)b |
| EQ-5D (self-report) score | 0.66 (0.33)c | 0.72 (0.32)d |
| QoL-AD (proxy) score | 29.75 (5.87)e | 29.64 (6.07)f |
| QoL-AD (self-report) score | 33.84 (6.31)g | 35.84 (6.63)h |
| CMAI score | 53.65 (17.31) | 54.57 (19.82)i |
| CBS score | 45.99 (37.53) | 45.73 (35.96) |
| Cost (£) of community-based service usei | 171.57 (210.67) | 169.16 (244.57) |
| Cost (£) of hospital-based service usej | 365.87 (2102.23) | 437.96 (2294.18) |
| Cost (£) of medicationj | 257.50 (438.34) | 210.27 (285.29) |
Cost of the e-learning and decision support e-tool package
Table 27 summarises the costs of the ResCare trial intervention. The software development cost was £89,873; however, this did not include costs of staff time incurred by the ResCare trial team developing the e-learning and decision support e-tool package content and subsequent checking. It is anticipated that the package would need to be redeveloped every 5 years. With an assumed discount rate of 3.5%, this is an annuitised cost of £19,905 over 5 years, and a cost of £6635 over 4 months. An unexpected cost was for the installation of computer and telephone equipment in some care homes. The total cost for the additional equipment was £17,455. Annuitising this cost over 5 years at a discount rate of 3.5% leads to an annual cost of £3866 for equipment, which is £1289 over 4 months.
| Cost category | Cost (£) | |||
|---|---|---|---|---|
| Salary | Travela | Total | For 4 months | |
| e-Learning and decision support e-tool package development | 89,873.00 | |||
| Development subtotal | 89,873.00 | 6635.00 | ||
| Equipment for care homes | 17,455.39 | |||
| Equipment subtotal | 17,455.39 | 1288.68 | ||
| Training: therapist and care home staff | 10,065.05 | 2539.10 | 12,604.15 | |
| Backfill to cover staff attending training | 2295.66 | 2295.66 | ||
| Training: venue hire | 757.87 | |||
| Training subtotal | 15,657.68 | 5219.23 | ||
| Information gathering: therapist and care home staff | 4549.38 | 1900.81 | 6450.19 | |
| Information inputting: therapist | 4507.45 | 127.75 | 4635.20 | |
| Action-planning: therapist | 11,078.34 | 0 | 11,078.34 | |
| Disseminating action plans:b therapist and care home staff | 2820.33 | 1052.66 | 3872.99 | |
| Follow-up booster visits: therapist and care home staff | 1313.09 | 564.66 | 1877.75 | |
| Other administration: therapist and care home staff | 43.48 | 45.66 | 89.14 | |
| Action-planning subtotal | 28,003.61 | 28,003.61 | ||
| Total intervention cost | 150,989.68 | 41,146.91 | ||
Training sessions were delivered by one specialist dementia care therapist to groups of up to 10 care home staff. Most of the training sessions took place away from the care homes. Staff costs for training, including venue hire, travel and backfill, were £15,658. It was assumed that because of staff turnover, training would need to be on an annual basis, so spreading the cost over 4 months leads to a total training cost of £5219 for the trial period. Action-planning included information-gathering, and creation and dissemination of action plans. The cost for action-planning over 4 months was £28,004.
The total intervention cost was £150,990. After annuitising costs of software and equipment over 5 years, and training over 1 year, the cost for the 4-month trial period was £41,147. Dividing this cost over the 286 residents randomised to the intervention at baseline results in a mean intervention cost per resident of £144.
Cost of health- and social-care service use
(See Appendix 7 for the national unit costs of health services used in the calculations.)
Tables 28 and 29 provide details of service use in the 3 months before baseline for all residents in the two treatment groups (n = 555) for whom baseline data were collected. The mean overall cost per resident of community-based service use in the intervention group was £160.61 (SD £216.62) and in the control group was £195.52 (SD £227.34), a difference of £34.91. Across the whole sample the overall mean cost for community-based services per resident was £177.53 (SD £222.37). By far the largest cost in both groups was for GP services. In relation to hospital services, the intervention group had higher overall mean costs than the control group [i.e. £552.05 (SD £2587.44) compared with £441.23 (SD £2234.80), which is a difference of £110.82]. Across the whole sample the overall mean cost for hospital services per resident was £498.34 (SD £2423.50), and the largest proportion of the costs across both groups was for medical inpatient stays.
| Service | Group | Difference in mean cost (£) (intervention – control) | |||
|---|---|---|---|---|---|
| Control (n = 269) | Intervention (n = 286) | ||||
| Mean frequency (SD) | Mean cost (£) (SD) | Mean frequency (SD) | Mean cost (£) (SD) | ||
| District nurse | 1.79 (6.07) | 30.62 (82.55) | 2.16 (7.24) | 38.82 (128.63) | 8.20 |
| GP | 1.74 (2.18) | 137.58 (179.53) | 1.27 (1.73) | 88.35 (139.88) | –49.23 |
| Practice nurse | 0.06 (0.47) | 0.87 (6.58) | 0.17 (0.74) | 2.31 (11.31) | 1.44 |
| Health visitor | 0.01 (0.09) | 0.13 (1.53) | 0 | 0 | –0.13 |
| Community psychiatrist | 0.02 (0.16) | 3.04 (29.70) | 0.02 (0.19) | 2.69 (25.69) | –0.35 |
| Psychologist | 0.00 (0.06) | 0.25 (4.15) | 0.00 (0.06) | 0.24 (4.02) | –0.01 |
| CMHN/CMHT | 0.12 (0.67) | 4.15 (31.38) | 0.10 (0.44) | 2.46 (11.30) | –1.69 |
| Physiotherapist | 0.03 (0.25) | 0.48 (3.88) | 0.12 (0.76) | 1.19 (7.48) | 0.71 |
| Occupational therapist | 0 | 0 | 0.05 (0.38) | 0.40 (2.78) | 0.40 |
| Chiropodist | 0.77 (0.87) | 7.74 (10.11) | 0.55 (0.91) | 6.04 (11.11) | –1.70 |
| Dietitian | 0.13 (0.50) | 1.39 (5.57) | 0.13 (0.46) | 1.50 (5.41) | 0.11 |
| Dentist | 0.05 (0.31) | 1.32 (7.77) | 0.06 (0.31) | 1.50 (7.93) | 0.18 |
| Optician | 0.11 (0.36) | 2.31 (7.44) | 0.09 (0.31) | 1.81 (6.35) | –0.50 |
| Social worker | 0.02 (0.15) | 1.33 (9.35) | 0.05 (0.27) | 2.19 (12.70) | 0.86 |
| Speech and language therapist | 0.03 (0.16) | 0.45 (3.52) | 0.03 (0.17) | 0.53 (3.63) | 0.08 |
| Specialist nurse | 0.01 (0.14) | 0.26 (3.01) | 0.01 (0.10) | 0.20 (2.28) | –0.06 |
| Falls team | 0.05 (0.44) | 0.86 (7.92) | 0.03 (0.21) | 0.39 (2.63) | –0.47 |
| Emergency care practitioner | 0.06 (0.35) | 1.63 (11.61) | 0.13 (0.48) | 2.61 (9.85) | 0.98 |
| Other | 0.02 (0.17) | 1.10 (9.00) | 0.03 (0.23) | 7.37 (55.43) | 6.27 |
| Total | 5.02 (7.29) | 195.52 (227.34) | 5.01 (8.08) | 160.61 (216.62) | –34.91 |
| Service | Group | Difference in mean cost (£) (intervention – control) | |||
|---|---|---|---|---|---|
| Control (n = 269) | Intervention (n = 286) | ||||
| Mean frequency (SD) | Mean cost (£) (SD) | Mean frequency (SD) | Mean cost (£) (SD) | ||
| Surgical inpatient ward | 0.07 (1.16) | 63.64 (1043.76) | 0.01 (0.12) | 6.30 (106.55) | –57.34 |
| Medical inpatient ward | 0.35 (1.83) | 318.20 (1646.94) | 0.47 (2.70) | 422.15 (2434.79) | 103.95 |
| Other inpatient ward | 0.05 (0.52) | 46.89 (464.64) | 0.13 (1.00) | 113.41 (901.74) | 66.52 |
| Outpatient service | 0.08 (0.33) | 2.34 (9.93) | 0.08 (0.38) | 2.52 (11.51) | 0.18 |
| A&E | 0.01 (0.09) | 1.03 (11.88) | 0.02 (0.14) | 2.90 (19.81) | 1.87 |
| Day hospital | 0.10 (0.35) | 9.13 (31.59) | 0.05 (0.24) | 4.77 (21.70) | –4.36 |
| Total | 441.23 (2234.80) | 552.05 (2587.44) | 110.82 | ||
Table 30 lists the various groups of medications that the residents were prescribed in the 3 months before baseline. Those residents in the control group had a higher mean cost [£254.12 (SD £412.60)] than those in the intervention group [£222.93 (SD £338.01)], a difference overall of £31.19. There was no significant difference between the intervention and control groups for total medication costs at baseline or follow-up. Across the whole sample, the overall mean cost of medications per resident was £238.05 (SD £375.99).
| Medication category | Group | Difference in mean cost (£) (intervention – control) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 269) | Intervention (n = 286) | ||||||||||
| Number of prescriptions | Number of residents (%) | Mean number (SD) of prescriptions per resident | Total cost (£) | Mean cost (£)(SD) per resident | Number of prescriptions | Number of residents (%) | Mean number (SD) of prescriptions per resident | Total cost (£) | Mean cost (£) (SD) per resident | ||
| Antipsychotics | |||||||||||
| Atypical antipsychotics | 32 | 30 (11.2) | 0.12 (0.35) | 3079.09 | 11.45 (61.40) | 20 | 19 (6.7) | 0.07 (0.27) | 2171.21 | 7.59 (45.22) | –3.86 |
| Typical antipsychotics | 14 | 14 (5.2) | 0.05 (0.22) | 297.20 | 1.10 (7.70) | 28 | 24 (8.4) | 0.10 (0.35) | 386.03 | 1.35 (9.36) | 0.25 |
| Hypnotics and anxiolytics | |||||||||||
| B/Z/A | 59 | 56 (20.8) | 0.22 (0.44) | 1633.59 | 6.07 (70.65) | 60 | 55 (19.2) | 0.21 (0.45) | 1720.01 | 6.01 (49.63) | –0.06 |
| Non-B/Z/A drugs | 1 | 1 (0.4) | 0.00 (0.06) | 78.67 | 0.29 (4.80) | 0 | 0 | 0 | 0 | 0 | –0.29 |
| Antidepressants | |||||||||||
| SSRI | 56 | 53 (19.7) | 0.21 (0.43) | 1070.69 | 3.98 (23.35) | 65 | 64 (22.4) | 0.23 (0.43) | 1241.46 | 4.34 (26.62) | 0.36 |
| Tricyclic | 14 | 14 (5.2) | 0.05 (0.22) | 842.43 | 3.13 (38.19) | 28 | 27 (9.4) | 0.10 (0.31) | 1307.13 | 4.57 (40.04) | 1.44 |
| Other | 22 | 22 (8.2) | 0.08 (0.27) | 331.56 | 1.23 (7.64) | 19 | 17 (5.9) | 0.07 (0.28) | 185.27 | 0.65 (3.92) | –0.58 |
| Anticonvulsants | 22 | 20 (7.4) | 0.08 (0.31) | 641.80 | 2.39 (11.98) | 16 | 15 (5.2) | 0.06 (0.24) | 290.21 | 1.01 (5.66) | –1.38 |
| Dementia drugs | |||||||||||
| Acetylcholinesterase inhibitors | 32 | 32 (11.9) | 0.12 (0.32) | 8536.37 | 31.73 (89.37) | 30 | 30 (10.5) | 0.10 (0.31) | 7566.63 | 26.46 (82.83) | –5.27 |
| Cognitive enhancers | 12 | 8 (3.0) | 0.04 (0.34) | 1338.86 | 4.98 (30.00) | 16 | 15 (5.2) | 0.06 (0.24) | 2947.71 | 10.31 (52.87) | 5.33 |
| Pain relief | |||||||||||
| Opioida | 67 | 57 (21.2) | 0.25 (0.54) | 2106.01 | 7.83 (26.07) | 69 | 62 (21.7) | 0.24 (0.48) | 1855.55 | 6.49 (19.36) | –1.34 |
| Non-opioid | 110 | 109 (40.5) | 0.41 (0.50) | 1489.19 | 5.54 (7.93) | 121 | 118 (41.3) | 0.42 (0.52) | 1722.99 | 6.02 (8.83) | 0.48 |
| Laxatives | 142 | 110 (40.9) | 0.53 (0.74) | 2043.34 | 7.60 (13.10) | 202 | 149 (52.1) | 0.71 (0.81) | 2788.32 | 9.75 (15.24) | 2.15 |
| Other | 1233 | 250 (92.9) | 4.58 (3.05) | 44,868.26 | 166.80 (370.05) | 1278 | 270 (94.4) | 4.47 (2.80) | 39,575.63 | 138.38 (290.63) | –28.40 |
| No medication | – | 9 (3.3) | – | – | – | – | 6 (2.1) | – | – | – | – |
| Total | 1825 | 6.78 (3.78) | 68,357.05 | 254.12 (412.60) | 1958 | 6.85 (3.41) | 63,758.15 | 222.93 (338.01) | –31.19 | ||
Adding together community-based services, hospital services and medications gives a total of £913.92 per resident for the 3 months before baseline. It should be noted that care home fees are not included.
Table 31 shows the frequency and total mean costs of service use by the intervention and control groups, subdivided into primary care, secondary care and medication for the 4 months up to follow-up. For a more detailed breakdown of the services in each category, see Appendix 11. One resident in the control group received 50 nights of medical inpatient care during the trial (aged 100 years and had a fall that required leg pinning), which resulted in the mean costs of hospital service use for the control group being almost double that of the intervention group; however, this was not a statistically significant difference. Mann–Whitney U-tests showed no significant differences in overall service use costs between the intervention and control groups, with a mean cost of £974.85 (SD £1601.40) per resident in the intervention group and £1305.51 (SD £3752.74) per resident in the control group (difference in mean costs of –£331, bootstrapped 95% CI of –£927 to £272).
| Service | Group | Mean difference in cost (£) (intervention – control) (95% CI bootstrapped) | |||
|---|---|---|---|---|---|
| Control (n = 226) | Intervention (n = 202) | ||||
| Mean frequencya (SD) | Mean cost (£) (SD) | Mean frequencya (SD) | Mean cost (£) (SD) | ||
| Primary care total | 116.06 (119.12) | 257.92 (310.16) | 137.84 (151.21) | 277.89 (302.22) | 19.97 (–39.22 to 81.38) |
| Secondary care total | 0.95 (4.05) | 663.37 (3634.81) | 0.59 (1.81) | 362.08 (1544.66) | –301.29 (–924.05 to 160.61) |
| Medication total | 7.47 (3.84) | 384.21 (655.88) | 7.87 (4.06) | 334.88 (386.97) | –49.33 (–153.39 to 51.60) |
| Total service use | 1305.51 (3752.74) | 974.85 (1601.40) | –330.66 (–926.87 to 272.10) | ||
Cost-effectiveness and cost–utility analysis
Follow-up data collection occurred approximately 4 months post intervention; however, due to problems implementing the intervention in some care homes, the length of time between baseline and follow-up was longer than anticipated. To account for the differential timing, it was assumed that the NPI and EQ-5D changed linearly over time and individuals’ outcome changes were adjusted to 4 months. This adjustment meant that our analysis of the data kept closely to our original analysis plan. As shown in Table 32, over 4 months the mean cost per resident in the intervention group was £187 less than the mean cost per resident in the control group (bootstrapped 95% CI of –£744 to £300). For the cost-effectiveness analysis, we reverse scored the total NPI so that an increased score indicated an improvement. NPI total scores range from 0 to 144 points. Residents in the control group had improved their mean NPI score by 2.90 points more than residents in the intervention group (bootstrapped 95% CI of –0.60 to 5.09). No significant difference between groups was found in NPI severity or NPI frequency score in the primary effectiveness analysis; however, the economic analysis used raw change scores and did not include variables accounting for clustering or other characteristics. This was because we adopted a bootstrapping approach to the analysis, and, as there was a very low intraclass correlation between homes, clustering was not seen as necessary. The main base-case economic analysis produced an incremental cost-effectiveness ratio (ICER) of £64 per NPI point improvement (95% bootstrapped CI of £14 to £303). There is no NICE-recommended threshold for a 1-point improvement on the NPI; however, we extrapolated from the £20,000–30,000 per QALY gain threshold that an equivalent threshold for the NPI would be approximately £140–210 per 1-point improvement on the NPI (which has a range of 0–144 points). Figure 5 shows the cost-effectiveness plane with 1000 bootstrapped ICER estimates. The majority of plots (75%) fell in the south-west quadrant of the effectiveness plane, where the intervention is less costly and less effective than the control.
| Measure | Incremental | ICER point estimate (£) (bootstrapped 95% CI) | Probability intervention is cost-effective at | ||
|---|---|---|---|---|---|
| Cost (£) (bootstrapped 95% CI) | Effect (bootstrapped 95% CI) | £20,000 per full unit of effect (%) | £30,000 per full unit of effect (%) | ||
| Main analysis | |||||
| NPI proxy (n = 428) | –187 (–744 to 300) | –2.90 (–5.09 to 0.60) | 64 (14 to 303) | 0 | 0 |
| QALY proxy (n = 428) | –187 (–744 to 303) | 0.01 (–0.01 to 0.02) | N/A: intervention less costly and more effective | 76 | 77 |
| QALY self-report (n = 206) | –72 (–1085 to 714) | 0.02 (0.00 to 0.05) | N/A: intervention less costly and more effective | 85 | 90 |
| Subgroup analysis | |||||
| 25–39 beds (small) (n = 188) | –595 (–1963 to 477) | –2.28 (–4.97 to 0.47) | 261 (44 to 1953) | 6 | 6 |
| 40–49 beds (medium) (n = 148) | 49 (–318 to 419) | –3.24 (–6.77 to 0.60) | N/A: control less costly and more effective | 5 | 5 |
| ≥ 50 beds (large) (n = 92) | –86 (–885 to 617) | –3.81 (–8.80 to 0.94) | 23 (14 to 344) | 7 | 7 |
| Sensitivity analysis | |||||
| Staff cost: action-planning done by senior care assistant (n = 428) | –242 (–776 to 269) | –2.90 (–4.95 to 0.68) | 83 (17 to 327) | 1 | 1 |
| Removing secondary care costs (n = 428) | 115 (–7 to 230) | –2.90 (–5.21 to –0.81) | N/A: control less costly and more effective | 1 | 1 |
| Removing outlier in control group (n = 427) | 14 (–79 to 674) | –2.91 (–5.13 to –0.71) | N/A: control less costly and more effective | 1 | 1 |
FIGURE 5.
The ResCare trial: cost-effectiveness plane for the NPI with 1000 bootstrapped ICER estimates.
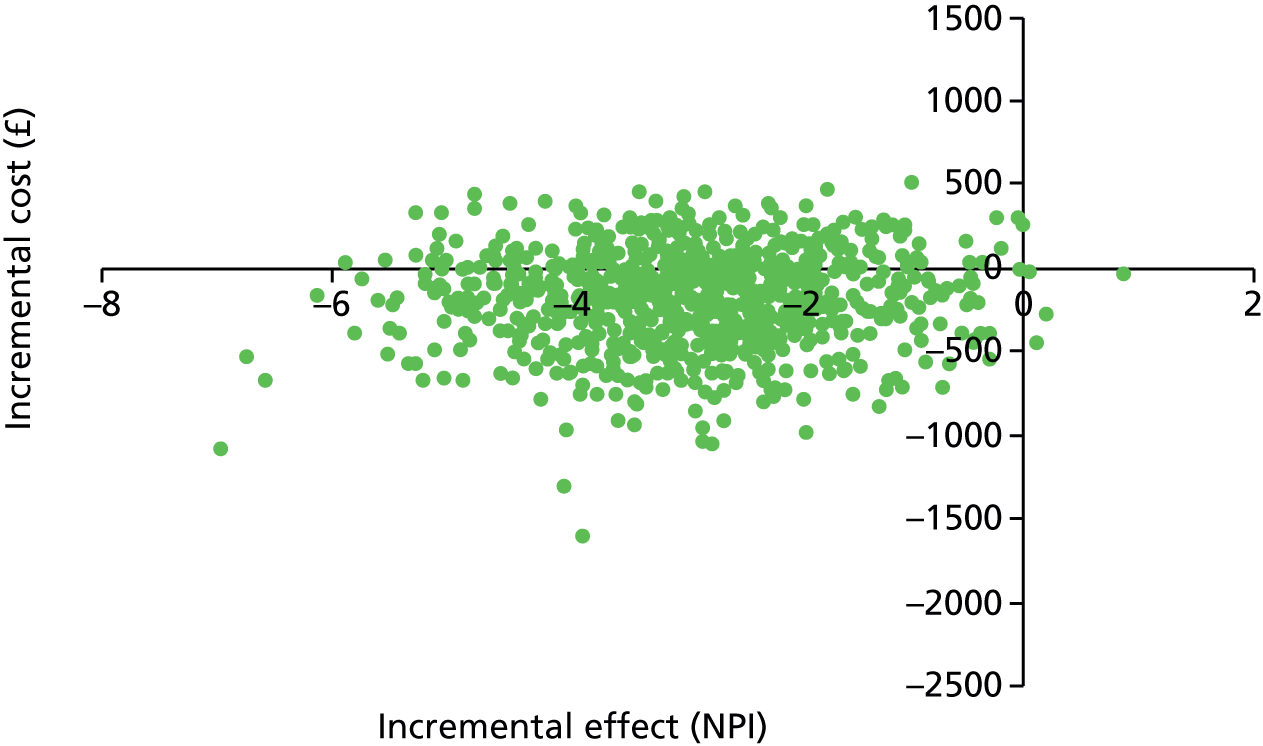
The cost–utility analysis showed a different picture. When repeating the analysis using QALY area under the curve values, calculated using UK tariffs for the EQ-5D completed by staff as proxies for the residents, the intervention group had a QALY gain of 0.01 (equivalent to 3.65 days) larger than the control group (95% bootstrapped CI of –0.01 to 0.02). Figure 6 shows that the majority of the plots in the cost-effectiveness plane fell in the south-east quadrant, where the intervention is less costly and more effective than the control. Using resident self-reported QALY values (Figure 7), the majority of plots in the cost-effectiveness plane fell between the north-east and south-east quadrants, indicating that the intervention was slightly more effective than the control.
FIGURE 6.
The ResCare trial: cost-effectiveness plane for the proxy-rated EQ-5D with 1000 bootstrapped ICER estimates.
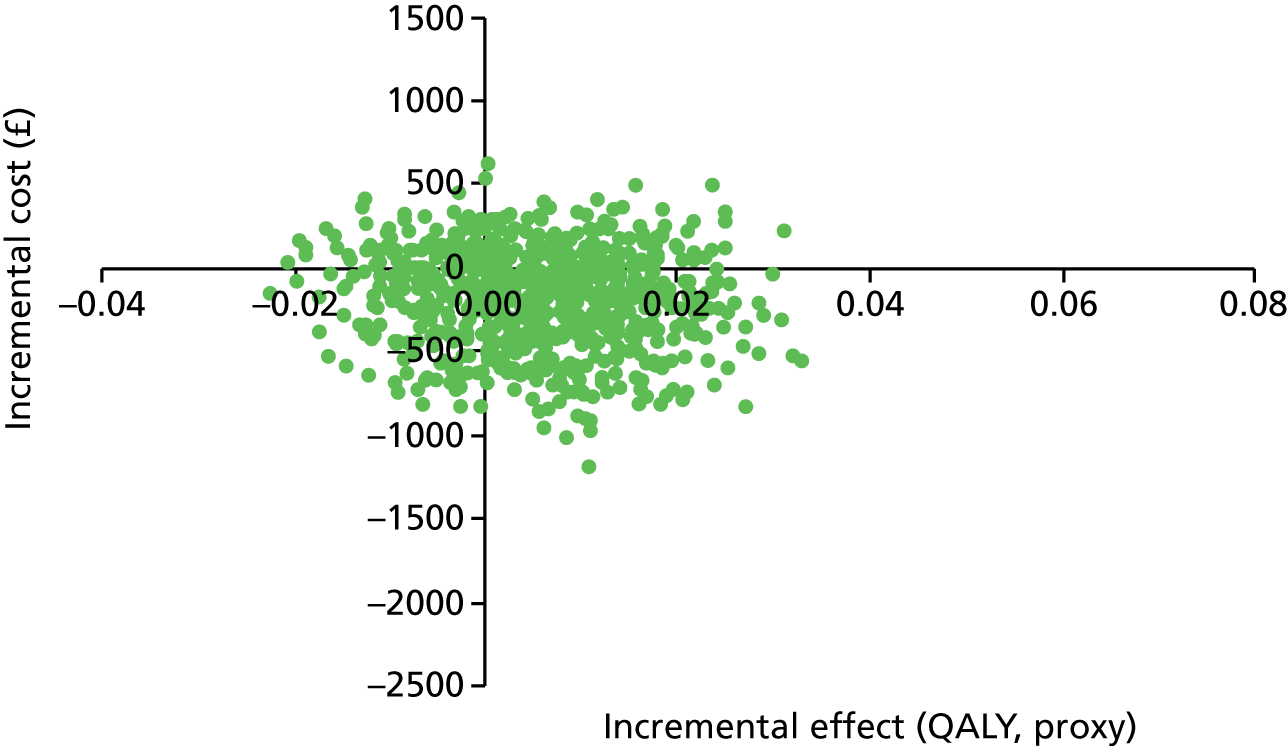
FIGURE 7.
The ResCare trial: cost-effectiveness plane for self-report EQ-5D with 1000 bootstrapped ICER estimates.
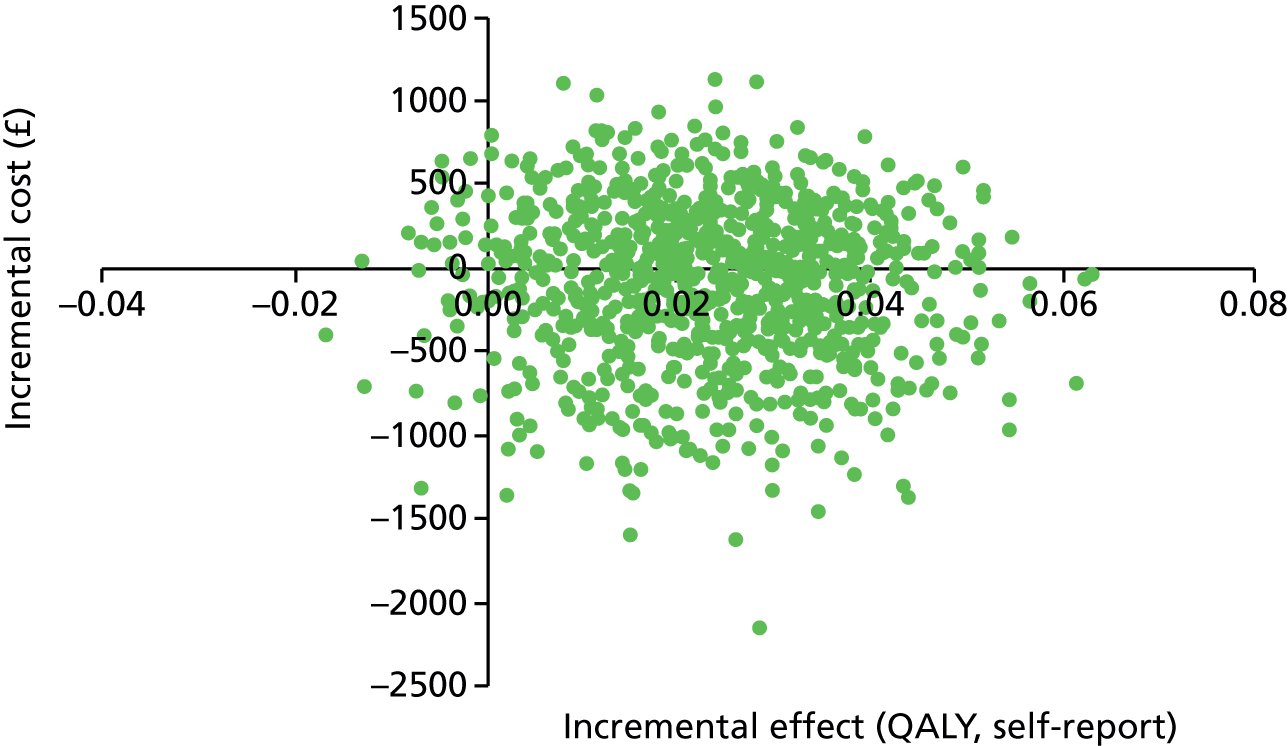
As can be seen in Figure 8, the probability that the intervention is cost-effective at a threshold of £30,000 per full NPI improvement is only 11% when the NPI is used as the measure of effect. The probability that the intervention is cost-effective at a threshold of £30,000 is much higher when the unit of effect is the QALY. In Figure 9 it can be seen that there is a 77% probability of cost-effectiveness at a threshold of £30,000 per QALY using proxy values, and in Figure 10 there is a 90% probability that the intervention is cost-effective at a threshold of £30,000 per QALY when using residents’ self-reported values. The issue is to what extent these two health measures (NPI as a dementia-specific measure and EQ-5D as a generic health-related quality-of-life measure) are in any way comparable. We have concerns for both the NPI and EQ-5D with regard to relating differences in costs to extremely small differences in outcomes, which may not be of clinical significance.
FIGURE 8.
The ResCare trial: cost-effectiveness acceptability curve for NPI.
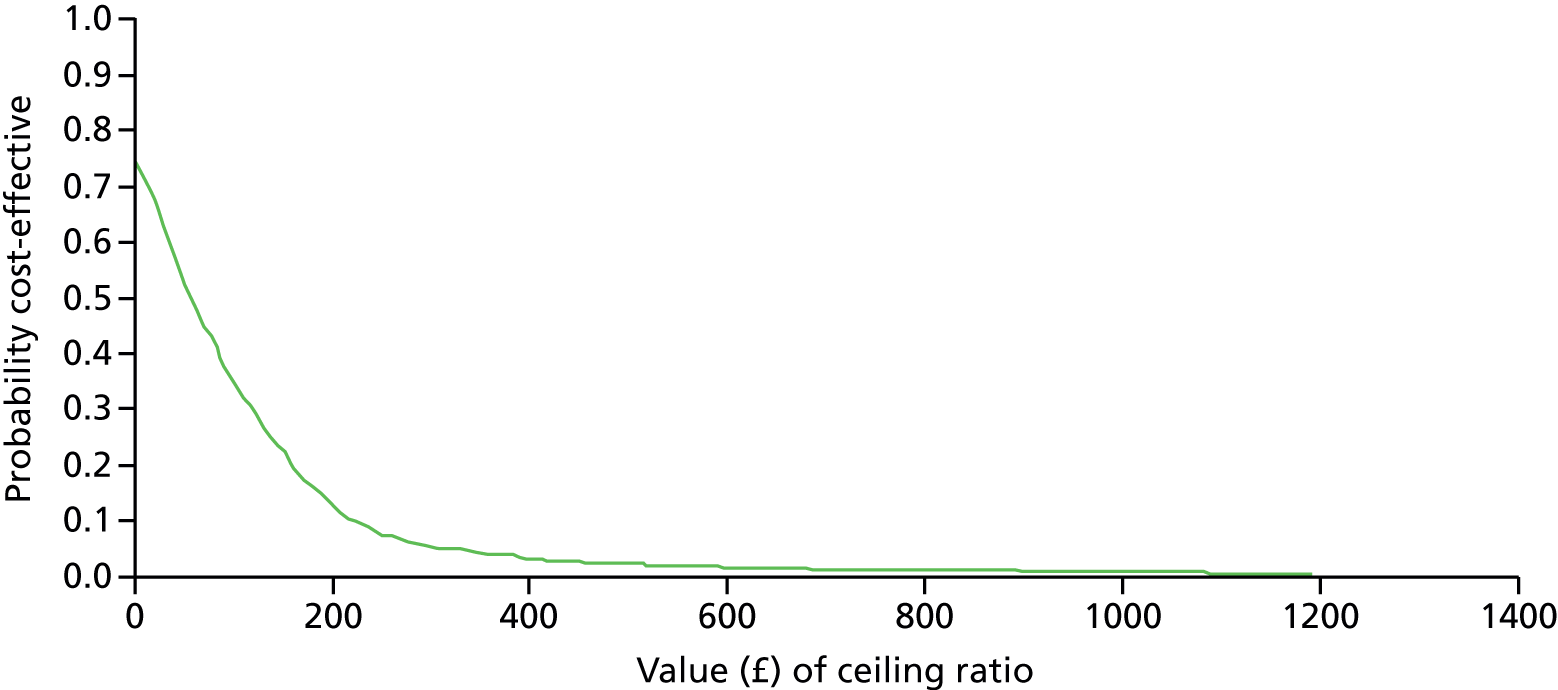
FIGURE 9.
The ResCare trial: cost-effectiveness acceptability curve for proxy-scored EQ-5D.
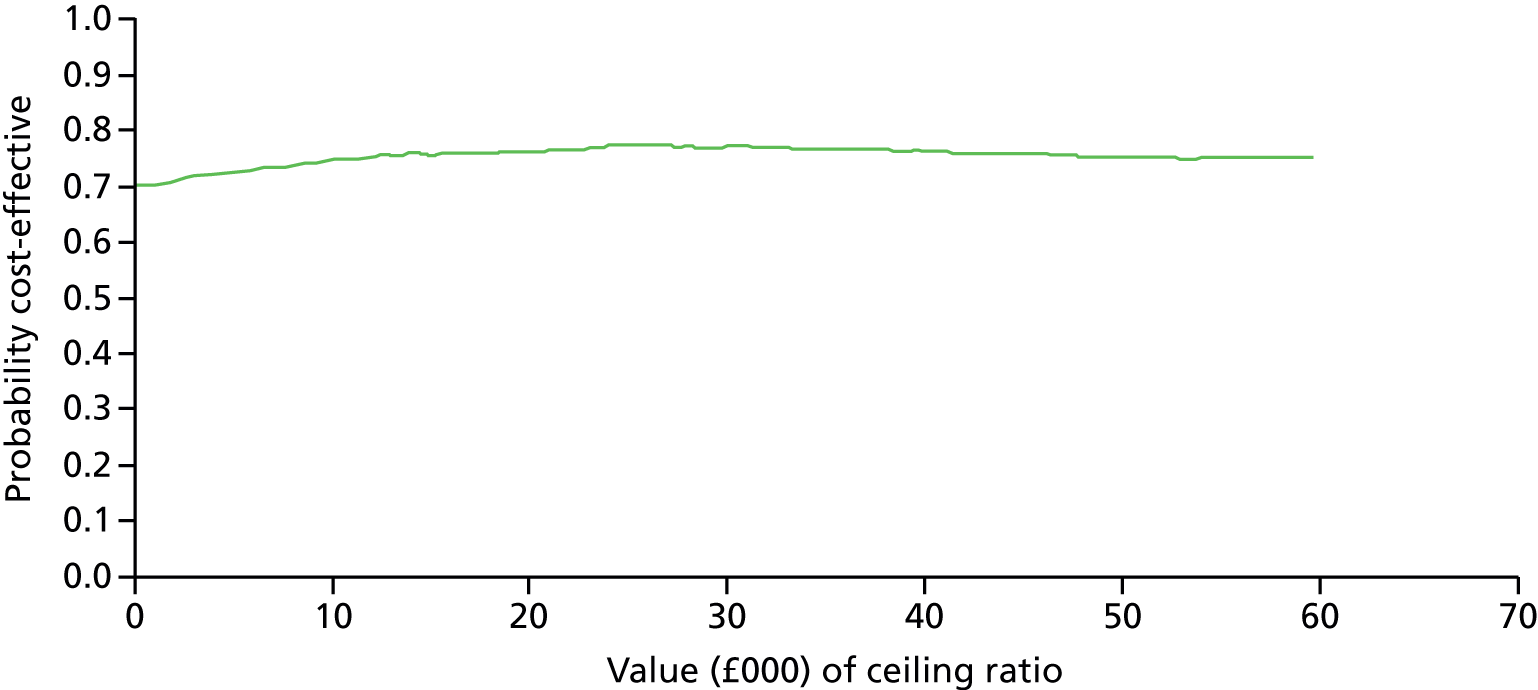
FIGURE 10.
The ResCare trial: cost-effectiveness acceptability curve for self-rated EQ-5D.
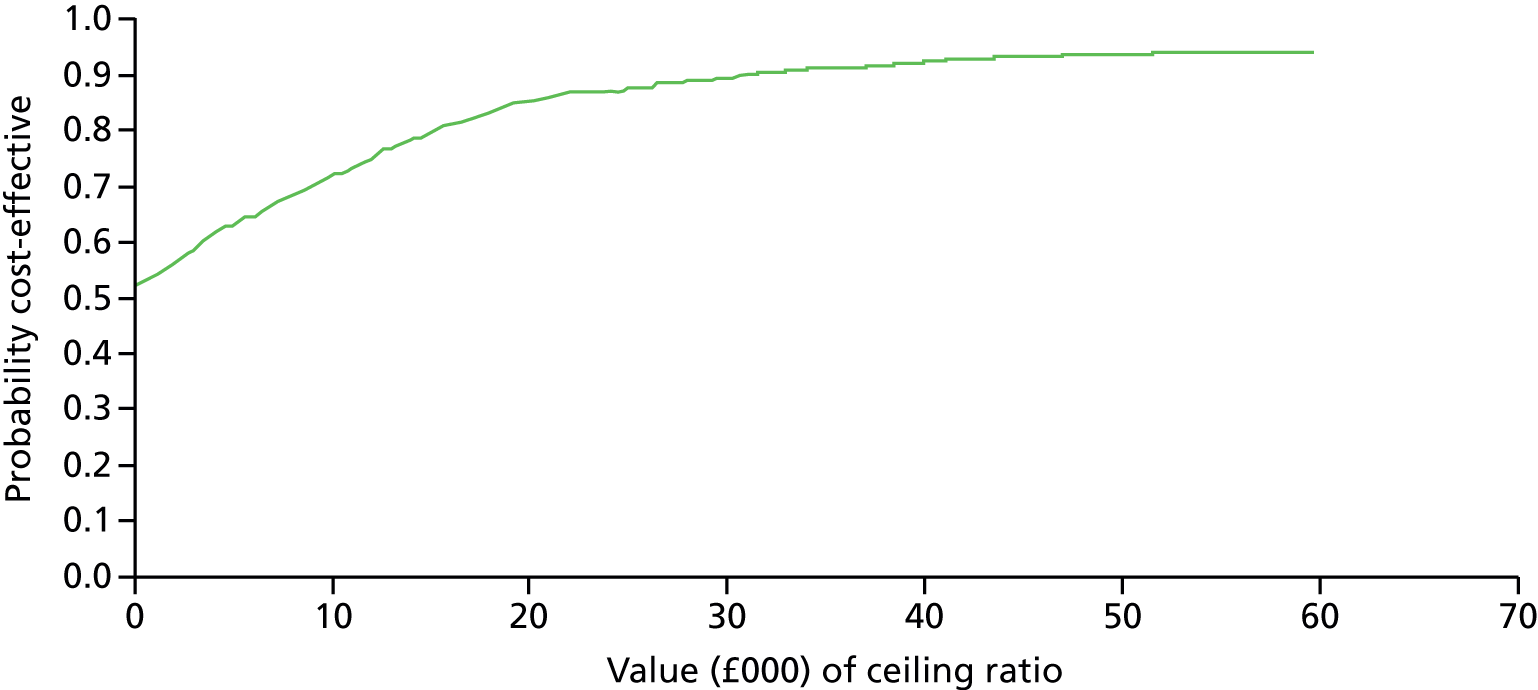
Subgroup analysis
A subgroup analysis (see Table 32) was conducted on the size of care home. The resident in the control group who had spent 50 nights in hospital was in the smallest category of care home size, which led to a larger difference in mean costs between groups in this category than in the main analysis. The difference in mean NPI scores between groups was the largest in the large care homes, with residents in the control group in large homes having greater improvements in their NPI scores.
Sensitivity analysis
The ResCare trial was a pragmatic RCT. During the trial the specialist dementia care therapist created action plans for residents with CBs and worked alongside the care home staff to deliver the plans. The intervention (i.e. the action plans) could, in theory, be developed and delivered by a care home staff champion. Therefore, a sensitivity analysis was conducted using senior care assistant wages instead of the specialist therapist’s wages for the creation of action plans, and the specialist therapist time removed from action plan dissemination costs. The only input from a specialist therapist in this scenario was for training and booster visits. The intervention cost per resident in this scenario fell from £144 to £90. The ICER increased from £64 to £83 per NPI point gained.
Two post hoc sensitivity analyses were conducted to explore the effect that the resident in the control group, who spent 50 nights in inpatient care had on the analyses: one analysis on all participants without secondary care costs and the other with the outlier resident removed completely. In both cases the control group had lower mean costs than the intervention group and a greater improvement in NPI scores, so the control condition dominated (see Table 32).
Cost-effectiveness of the e-learning and decision support e-tool as a means of reducing care home staff stress
Effect size for the range of measures for care home staff was negligible, leading to a conclusion that the e-learning and decision support e-tool package did not reduce care home staff stress. During the development of the ResCare trial, care home staff indicated that they were not willing to give personal information on their own health- and social-care resource use. We were therefore unable to undertake a secondary exploratory cost-effectiveness analysis using one of the carer stress measures as an effectiveness measure and relating this to their service use.
Discussion
Summary of findings
This is the largest study in the UK to date (December 2016) of an intervention for the management of dementia and clinically significant CB in care homes. Two thousand one hundred and eighty-five residents aged > 65 years living in 63 care homes were screened for CB. Homes were then stratified across treatment conditions for high versus low proportions of clinically significant CB. Within the cluster randomised study design, 555 residents with dementia and CB (the CB sample) and 609 staff were recruited to this study. In the intervention homes, 92 staff champions, nominated by their manager, attended training and co-ordinated delivery of action plans for the management of residents with dementia and CB in their home.
There were no significant demographic differences between the residents and staff in the two treatment groups. Comparison of the two groups on the primary measure of NPI (incidence and severity scores) failed to find significant differences that could be attributed to the intervention. This was true for both ‘complete cases’ and those derived by imputing scores for residents who dropped out but did not die, as well as for all of the resident and staff secondary measures, including comparisons of medications that were prescribed for residents. Post hoc analyses found that the proportion of action plans delivered within this intervention in a given home did not affect the NPI scores. There was borderline evidence that the number of champions who received training and contributed to delivery of action plans was associated with an increased NPI severity score. This finding was not examined qualitatively post hoc, but training may have contributed in part. For example, improved observation of communications through behavioural expressions by the resident (see content of e-learning in Chapter 2 and Appendix 1) could have enhanced staff observations of resident behaviour or, conversely, post-intervention completion of the NPI interview may have triggered staff awareness of resident communication of their severe distress through behaviour. Comparisons of health- and social-care costs, over and above care home costs, did not differ between groups over 4 months. However, cost–utility analysis indicated that residents in the intervention group had higher QALYs at the 4-month follow-up than those in the control group.
Limitations and implications for research
There are a number of limitations to design of the study and delivery of the intervention, some of which were unavoidable. We did not conduct extensive feasibility checks, as recommended by the MRC in its framework for complex interventions. 155 For example, we did not survey stakeholder homes prior to inclusion into the study on the availability of computer equipment and opportunities for e-learning (including space for staff to do this) prior to planning the CRT, as we were keen to offer the intervention as widely as possible in the real world of the range of care home settings; nor did we test and validate the e-tool prior to its inclusion in the CRT, as difficulties with the engineers delayed progress of the study. Therefore, the CRT was perhaps premature in relation to the e-tool, which required a more thorough developmental process. Furthermore, we did not evaluate whether or not action plans were being implemented or limit our experimental group data collection to staff (champions) who had attended training and were charged with co-ordinating delivery of the action plans in the home. Finally, although we did not collect data on ethnicity of staff or their migrant status in respect of the national picture, the dementia care workforce includes many migrant workers, and it is likely that the care homes in the ResCare trial were not atypical. Hussein and Manthorpe160 reported that the dementia workforce contains significantly more non-British workers (19.1% vs. 15.3%) than other parts of the care workforce. Non-UK workers among the dementia workforce are most commonly from the Philippines (17%) followed, equally, by Poland and India (10.8%). This may have an influence on training and staff practices, but this study was not designed to investigate these.
Potential contributing factors to the non-significant results of this intervention on CB outcomes in care homes will be considered next.
First, we examine the design of this intervention. Functional analysis-based approaches to intervention for CB and dementia were developed from modelling167 studies of face-to-face staff training workshops for dementia and CB,63 single case studies of functional analysis-based interventions in care homes,13,68,69 and our description of this intervention outlined in our Cochrane review82 (see also Chapter 1). The content of the range of supportive investigations and interventions for the management of CB and dementia was derived from Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care (p. 210). 12 A functional analysis approach offered a systematic means of choosing the most appropriate interventions and testing these out in a given case. In order to widen its impact for future delivery in the present intervention, we additionally conceived an ICT solution for both staff training and intervention planning (see Chapter 2). The ICT solution comprised an e-learning course and a decision support IMS (the e-tool), to develop case-specific functional analysis-based action plans for residents with dementia and clinically significant CB. To this we added support from a specialist dementia care therapist, as this is seen as an important component of delivery in an individualised case-specific intervention such as this. 82 After we had adapted the delivery of staff training to care workers’ need for facilitated learning away from their work environment, this was well received (see Chapter 2). However, the action-planning process required a significant time commitment from the specialist dementia care therapist to interact with the e-tool, rather than supporting care home staff to use the iterative approach that is common in functional analysis-based interventions in dementia (see Chapter 2). This study was not designed to compare therapist-supported functional analysis-based interventions with the present ‘ICT-plus-therapist’ intervention described in Chapter 2. Our aspirations to widen the scope for the delivery of functional analysis-based interventions using ICT may have undermined any potential robust conclusions about use of this promising168 approach to intervention in care homes. A review of computerised clinical decision support systems suggests that these may improve practitioner performance but, to date, the effects on patient outcomes are understudied or inconsistent. 169 The present study found that the decision support software was not enough to impact on resident or staff outcomes. In contrast, an earlier study, FITS (Focused Intervention Training and Support), demonstrated good impact on prescribing practice with the use of bespoke interventions delivered by trained therapists working intensively with a small number of care homes. 27 A trained therapist is usually required for such an intervention,82 as a key skill in working in this area is the ability to be flexible and have the capability to alter care plans as new needs arise, meaning that the process remains iterative, but the functional analytical skills remain at the core of the plan. Future research with decision support software could explore the feasibility of training specialist dementia care therapists in working with care homes to deliver interventions for the management of dementia and clinically significant CB.
Second, this study adds to knowledge about staff training in care home settings. Our e-learning course, with its training on how to deal with common CB using simulated ‘real-world’ scenarios in the care home setting, is what was advocated in a recent focus group study of e-learning with 21 stakeholders in south London. 127 In the present study almost one-third of care home staff had never had any dementia training, let alone specialist training on the management of CB in dementia, which was the focus of our e-learning course. Staff champions were positive about the case-specific individualisation of the approach to dealing with CB and the qualities of the specialist dementia care therapist who facilitated the e-learning course (see Chapter 2). However, our findings indicate that, whatever delivery method is used, training alone is insufficient in its impact on the lives of residents with dementia and CB; future research needs to incorporate a sustained period of joint working and supervision with a trained dementia professional. 60,170 This conclusion stands true even for a course such as ours which, in contrast to many which are not empirically based,170 was informed by the growing evidence on case-specific understandings and intervention for CB13 and also on the NICE–SCIE guidance12 (see Chapters 1 and 2).
Third, organisational factors associated with engagement of care homes impacted on delivery of the intervention. For example, even in the context of providing extra arrangements to deliver the training away from the care home at no financial cost to the home, additional courses had to be arranged, as some homes struggled to send their staff to pre-arranged training (see Chapter 2), whereas homes with high NPI scores nominated more staff champions to support the intervention, suggesting that engagement with our e-intervention would be better focused on homes where there are more residents with CB, as staff may be more aware of their learning needs171 in the management of dementia with CB. Moreover, at the start of the intervention two homes dropped out and a third large facility failed to engage with the intervention at all. In contrast, no control homes were lost to the study. Organisational commitment to an intervention such as this is an important consideration for future research. Barriers to delivery of the intervention to reduce antipsychotics in the FITS implementation study included unclear communication across management levels and values and ethos that contradicted the intervention approach. 84 Other studies have noted that staff perceptions of empowerment are important in the provision of individualised care;172 values and beliefs that guide behaviour in an organisation can have an impact on depressive symptoms in nursing home residents;173 and the impact of training initiatives on staff confidence and standards of care is often dependent on strong leadership and the home culture. 174 We will now consider our findings in the context of fidelity and adherence to the intervention.
Fidelity and adherence to the intervention
Our intervention was designed to empower staff to access clinical expertise for the management of dementia and CB, using a broad view of training that was complemented by an IMS to develop action plans (the decision support e-tool) and access to a specialist dementia care therapist. A study of training in decision-making using the internet in primary care practices also noted disappointing use of the resource. 175 In the present study we can conclude that the effects of training nominated staff champions did not diffuse to non-exposed staff in the homes. This may have been attributable to our unavoidable need to depart from our original plan (see Chapter 2) and deploy research therapists to actively work with staff to gather data for the e-tool, and a specialist dementia care therapist to assist with developing the action plans. It is possible that active intercession of the therapists may have increased feelings of dependency among the staff champions and reduced their sense of ownership of the action plans, which in turn may have reduced the likelihood of them providing leadership in driving forward the steps associated with the intervention. However, a more likely explanation is associated with our proposed mechanism of change, which we will consider next.
The proposed mechanism of change envisaged was that once staff champions had completed the e-learning course, and produced an idiographic action plan for a resident using the decision support tool, they would use the action plan for a given resident. Furthermore, we envisaged that the bespoke training would be used to improve their own approaches and interactive skills and the action plans would further guide them to try solutions to meet the residents’ needs and also to facilitate informed approaches of their colleagues. To achieve the latter, the staff champions needed to be able to influence and motivate their colleagues to work differently and consistently in accordance with the action plan. It was also thought that in those homes in which staff champions operated in this facilitative manner, there would be improvements in residents’ quality of life, staff attitudes and other secondary outcomes. Limiting factors to behaviour change in staff champions include lack of practice in delivering the intervention, as 30% of the residents in the CB intervention group did not receive an action plan (see Chapter 2); no specific component of support or training given to assist them to implement the action plans and/or disseminate their new knowledge to their colleagues; different grades and experiential backgrounds, where some may have been unable within the hierarchy of the home culture to speak up and share insights with their colleagues; the low ‘dosage’ of the intervention in terms of the small percentage of staff champions in each home, the lack of relevant and ongoing supervision, particularly over the implementation phase of the action plan; and the experiences of the specialist dementia care therapist, who provided the perhaps crucial time required to mentor and support staff to implement the action plans, with interaction with the e-tool. This may have also undermined the expert clinical role of the specialist therapist who was cast into a different, more technical role with time taken to troubleshoot computer problems. Therefore, just one action plan was developed for most of the 199 residents, even though residents often displayed more than one CB, which may or may not have been related to each other. In understanding the barriers to integration of internet-based instruction by nurse trainers or other experts working in care homes, Irvine et al. 73 note that the technological role can undermine comfort levels of some clinical experts involved in training, even if they are frequent internet users.
The lack of effect on prescribing practices was particularly disappointing. Our baseline data on prescribing practices indicated that these are contrary to emerging clinical recommendations for antipsychotic use in dementia105,112,176,177 and clinical guidelines advocating benzodiazepines prescription for no longer than 28 days. 178 The action plans for such residents would have signposted staff to seek a review of these from the resident’s GP or a psychiatrist, at a time when high-level NHS targets for the reduction of antipsychotics in dementia care were emerging. 112 Such action plans would have also recommended clinical risk–benefit evaluation for the high use of benzodiazepines, hypnotics and anxiolytics (B/Z/A products) given the cautionary guidelines associated with adverse effects of these in older people. 178
It may be that staff working in care homes were unable to persuade other professionals, for example the GP, to assist with action plans such as review of drugs, as attitudes of professionals, including physicians and psychiatrists, to the management of dementia and CB can vary. 179,180 Indeed, there is some evidence from process notes (see Chapter 2) that the ‘unofficial hierarchy’ that was noted in a study of care home staff and physician interactions104 may have undermined implementation of these action plans. Variability in the culture of some homes, with respect to antipsychotic use,181 could have also contributed. The unwillingness of nurses and GPs to discontinue use of antipsychotics has been noted as a significant barrier to changed practice182 where the authors suggest that these barriers may be overcome with more complex multidisciplinary interventions. The FITS implementation study used the equivalent of specialist dementia care therapists to demonstrate impact on prescribing practices, but only in organisations with commitment to this type of intervention. 84
The fidelity of the intervention could have additionally been compromised by the needs of the specialist dementia care therapist who supported delivery. Although a percentage of action plans were checked by the clinical expert team, this may not have been enough, particularly for the residents who had presentations of severe CB. Routine access by the therapist to the clinical expert team was not incorporated into the planned intervention here. Where care homes are committed to provide care to residents with dementia and clinically significant CB, future cost–benefit evaluation is warranted. However, this will need to include in-reach by a trained dementia care therapist. However, care will be needed to ensure that trained dementia care therapists have access to clinical experts, as one of the lessons learned from integrating IMS into clinical practice is that experts operate at a higher level of complexity,183 and residents with dementia and clinically significant CB often have significant comorbid health conditions184 and live in a complex environment reflecting a system of varying cultures and practices in a care home. This type of cost–benefit study for a training intervention such as this would need to take into account the level of ‘scaffolding’,185,186 such as supervision, mentorship and access to clinical experts needed for the specialist dementia therapist, as well as that provided by the therapist to staff in care homes, as confidence in ongoing management of CB can vary. For example, the relevance of scaffolding new learning was demonstrated empirically in a study of experienced cognitive–behavioural therapy therapists who, following training, demonstrated an early dip in therapy performance, because of an initial undermining of confidence and requirements of the participants to change existing ways of working before further training and support raised their levels of competence. 187 An alternative investigation of ‘dose’ could be to explore substituting many product champions for an in-home specialist dementia care therapist, as our study appears to suggest that this low dose is not effective but that a higher dose, with access to clinical expertise, may be more effective in the management of dementia and clinically significant CB in care homes. For statistical examination of dose effect, our indices were necessarily limited. We were unable to capture the extent to which action plans were implemented and any resulting changes in CB. The likelihood is that many action plans were implemented only partially, if at all, and the good practice of reviewing and adapting plans in the light of the response to the initial set of actions could not be followed through. Future studies need to document each step on the putative pathway to change and allow for longer follow-up, in order to allow the intervention to become embedded in the care home. This was the case in all of the three care home intervention studies in our Cochrane review,82 where the intervention phase was 4, 6 or 10 months, with specialist support following training to support staff in implementing care plans described as occurring weekly or twice weekly.
Health economics findings
The health economic analyses did not identify any significant difference in service use by residents between the intervention and control arms over the follow-up period (health, social care and medicines, excluding residential costs). Although it was possible to calculate an ICER of £64 (95% CI £14 to £303) per 1-point improvement on the NPI, this is difficult to interpret as there is no agreed threshold of societal willingness to pay for such an improvement. In general, the lack of evidence for effectiveness in the trial is not compensated for by any difference in costs. The cost–utility analysis appeared to support the intervention, in that, using EQ-5D as a source of utility weights, the intervention dominated the control group, that is, it was more effective and less costly than usual care. There was a 77% probability that the intervention could be cost-effective at a payer threshold of £30,000. However, the mean QALY gain for the intervention group, as compared with the control group, was only 0.01 and so this finding must be treated with caution.
Conclusions
This study has high ecological validity as, once randomisation had occurred, all care staff and high CBS scoring residents in good-quality care homes were eligible for participation. Thus, the results obtained from our project have direct applicability to clinical services, which is in contrast to the findings obtained from the more selective exploratory types of study. 188 No evidence was identified for the effectiveness of the intervention as implemented, but as the trial progressed it was evident that the tools and resources developed were not sufficient to embed a functional analysis approach in care homes. We cannot then draw conclusions from this trial regarding the effectiveness of using e-learning and decision support software for functional analysis-based interventions, because of the factors associated with implementation error. 189 An implementation error refers to findings that cannot be interpreted as either effective or ineffective in a pragmatic trial, because inadequate attention has been given to intervention fidelity by staff who deliver the new intervention. 189 In this chapter we have outlined some of the factors associated with fidelity that we encountered in our study. These can inform the design of future research on case-specific functional analysis-based interventions for the management of CB in dementia in care homes. In the next chapter, the contextual and process factors that are key to successful implementation of e-learning and decision support software in care homes are explored in depth.
This trial adds further evidence to the literature that suggests that training, in itself, is not enough to change dementia care practice. The next step for future applied research such as this could examine the effectiveness and cost-effectiveness of this training programme for clinically significant CB in dementia supplemented with ‘in-reach’ support from a specialist dementia therapist. 190 However, given the elusiveness of the syndrome and the iterative nature of functional analysis-based intervention for residents with dementia and clinically significant CB, the therapist will need access to clinical experts from the disciplines of psychiatry, geriatric medicine and psychology. This may not necessarily take more contact time with the home than was used in the current study. 26
Chapter 4 Challenge ResCare: a process evaluation of the implementation of e-tools for the management of dementia with challenging behaviour in care homes
Abstract
Aim
To understand key implementation processes for delivery of e-learning and computer-assisted decision support (e-tools) for the management of CB and dementia, including perceived and actual barriers to delivery.
Methods
We used the normalisation process theory (NPT) and framework analysis to re-analyse data from the ResCare trial and examine how innovations may become embedded in everyday work. Barriers to, and facilitators of, change in care homes were studied by considering ‘process problems’ in social care settings; and ‘structural problems’ affecting the integration of new systems into those settings. Following analysis of contextual data collected during the trial for the intervention homes, a typology of ‘organisational cultures’ for computer-assisted intervention was developed. From this, four ‘case study’ homes were extracted and seven (managers, senior care staff and care assistants), from a sample of 14 participants, were interviewed individually; and a specialist dementia care intervention therapist and a research nurse who collected data during the study were interviewed together. Nine additional qualitative interviews with care home staff included those from the control group, and three focus groups using nominal group techniques with a maximum variation sample (n = 22) of wider stakeholders provided opportunities to consider how far the findings of the ResCare trial resonated with their experiences and how they interpreted the trial and its findings.
Results
Three explanatory themes for the findings of the ResCare trial emerged: variation in care home managers’ trust of their staff; variation in the extent to which managers commissioned training; and variation in cultures of training and practices within care homes. The findings also suggest that care homes are not ideal environments for implementing new approaches, but implementation can be feasible, in smaller care homes and in those with less hierarchical structures.
Conclusions
The implementation of interventions for the management of CB and dementia, using specialist NHS support and resources, depends on the readiness of care homes to invest in innovation. Capable leadership and collective willingness are also important. The toolkit developed for implementing online interventions in care homes has scope for informing future practice innovations and research.
Introduction
The need for research on case-specific tailored interventions for the management of CB and dementia in care homes was outlined in Chapter 1. These were described as ‘functional analysis-based interventions’ (see Chapter 1). An online solution was adopted to make the approach available to all care staff working in a home (see Chapter 1) and a mixed-methods study of the process of delivery was described in Chapter 2. The findings of the CRT of computerised functional analysis-based intervention to tailor case-specific support for the management of CB in care homes were described in Chapter 3. In order to make recommendations for how e-learning, computerised and related individually tailored interventions for the management of CB can become embedded in care home settings, key implementation processes are examined in this present chapter.
Studying implementation processes from findings of the ResCare trial
Normalisation process theory is a relatively recently developed method to assist understanding(s) of how new effective ways of working or service improvements may become a ‘normal’ part of daily practice. 191 It has particular strengths in the systematic use of data from complex interventions192 to gain a deeper understanding of the contextual processes involved in delivery of an intervention. By systematically capturing the many human processes at work, when individuals and groups encounter a new set of practices or aspirations to improve service innovations, it allows clear specification of the processes of implementation that can then inform the transfer of the innovation into routine practice.
Normalisation process theory is used in the present investigation as a way of understanding and describing the processes that inhibited or promoted a functional analysis of the causes and consequences of CB in care home residents. Using the theory-based approach provided by NPT and its sociological ‘toolkit’ to understand implementation, we focused on the dynamic, rather than the linear, processes that affected how an innovative approach to training and delivery of functional analysis-based interventions for CB in dementia may become embedded in everyday work in care homes.
There are several ways of using NPT to investigate implementation. Some of these have been developed in care homes193 and have also been used to inform computer-assisted decision support systems in the NHS. 194 In this present study we adopted a retrospective approach, following completion of the ResCare trial (see Chapters 2 and 3), as some implementation variables may have changed during the course of the trial. For example, in a journal dedicated to practitioners, including care home staff and managers in the UK, Flint and Cream127 conclude that there is a pressing need for e-learning programs to manage CB in dementia. Other efforts made in the social care sector to improve care homes’ access to IT have also been recommended for consideration by employers, who have to balance the time staff spend on training against the demands of their work and personal commitments;195 and websites, such as that of the SCIE, have begun investment in dementia management video-assisted demonstration and training resources. 196
In this chapter we report the evaluation that explored the processes at work when people and groups encounter, and seek to embed, a new set of practices or ways of doing things. 191,197 We used process data collected during the trial and also asked a sample of those involved in the study to reflect, with the benefit of hindsight, on the processes of the trial. In addition, during stakeholder consultations, we asked those who had not been engaged in the intervention, or in the trial, to think about the findings of the study and how these might relate to their own current practice or service.
Normalisation process theory was used to address two important problems commonly encountered when attempting to translate an intervention or service improvement into practice:
-
process problems – about the ‘implementation’ of new ways of thinking, acting and organising in health and social care
-
structural problems – about the ‘integration’ of new systems of practice into existing organisational and professional settings.
Normalisation process theory is built around a set of four core constructs and specific components that represent ‘generative’ mechanisms of social action, namely the different kinds of work that people do as they seek to implement; for example, the findings of a trial of a complex intervention. 192 These are the ways that people make sense of the work entailed in implementing and integrating a complex intervention (coherence); how they engage with this work (cognitive participation); how they enact it (collective action); and how they appraise its effects (reflexive monitoring). 198
The present study builds on other recent research conducted in health care, and, to a lesser extent, in social care settings,193,199–202 in which NPT as a conceptual framework has been used to understand the implementation of complex interventions,203 such as the ResCare intervention trial outlined in Chapters 2 and 3. It additionally outlines stakeholder contributions to the knowledge base, in which they discussed and refined the combined findings from the ResCare trial, including those described in Chapters 2 and 3 and from NPT analysis.
Methods
Normalisation process theory can be used at various points in the research cycle including, as here, to re-analyse data from a completed project and to collect new information from those in positions to make observations about findings. We used NPT and framework analysis to link and integrate two sets of data. The first drew from existing qualitative and quantitative process data collected during the ResCare trial; the second was supplementary qualitative data from semistructured interviews and a group discussion collected on completion of the ResCare trial. Thus, NPT was used iteratively to:
-
Examine already collected materials and data from homes (collected by the ResCare trial team) to identify and link the following data sets:
-
Develop a typology of the organisational ‘cultures’ of care homes in which the computer-assisted intervention for reducing CB among people with dementia was delivered. This involved refining and validating the synthesis from point 1 above through additional qualitative data collection, using accounts and updated experiences of the ResCare trial research team (e.g. research nurse, programme manager, intervention therapist and IT engineer).
-
Examine interview data from a sample of four intervention case study homes that were selected on the basis of analysis of the typology for computer-assisted intervention that was developed (see point 2 above), to gain a fuller understanding of implementation processes. This incorporated approaches to identify perceived, as well as actual, barriers to implementation.
-
Engage with a wider group of stakeholders to gain a fuller understanding of the findings and of implementation processes. This used stakeholder interviews and a modified nominal group discussion. Participants were asked to comment on the typology of implementation that emerged from further analysis of the data (see points 1–3, above), and about information derived from contact with study participants. The latter comprised data from interviews with staff sourced from the ResCare trial homes, addressing stages of implementation, homes in which implementation went smoothly and where it did not, and perspectives from the control group of homes, where the intervention did not take place.
-
Cover sheet for each care home (working records for the trial), including numbers of residents, staff, staff turnover, management, etc. In addition, information relating to the attempts to contact homes and researchers’ comments.
-
Computer/broadband information for homes (collected in initial IT scoping exercise).
-
Spreadsheet of data classifying care homes by type of ownership (local authority, private, not-for-profit sector), sole ownership/chains, care homes with and without nursing care, experimental or control, geographical region, numbers of beds (small/medium/large), proportion of CB in care home (high/low).
-
Care staff demographics (e.g. age, gender, age left education, how long working with older people).
-
Researcher-completed questionnaire records of perceptions of helpfulness of staff when visiting, ease of access to staff/data/interview space (some questionnaires completed post study).
-
Study administrative database, researcher (minimal) comments on accessing homes.
-
Researcher (minimal) notes/comments on individual homes.
-
Intervention therapist’s notes.
-
Programme manager’s notes on communication with care home managers.
-
IT engineer’s notes on homes.
-
Focus group transcript: research team discussion of implementing the technology/training in homes and off-site [facilitated by AH (author, Doctor of Philosophy)].
-
Reasons care homes withdrew (recorded by research team).
-
Champions’ evaluation of e-learning (summary of evaluation forms completed).
-
Care staff learning styles questionnaire (summary of data).
-
Research team’s perceived receptivity of homes to e-learning and e-tool.
-
Data detailing organisational change within care homes over the course of the trial.
Normalisation process theory was used retrospectively and deductively in three ways: first, to inform, guide and structure the way in which we collected additional data using interview guides; second, to code and interpret data against the core construct components of NPT; and, third, to inform the way in which we drew conclusions. 199 We also drew on principles of constant comparison204 to inform data collection and analysis, in order to inductively identify any other processes which facilitated or inhibited implementation, and which have been overlooked through a priori application of NPT modelling on its own.
Participants: intervention case study homes
Four candidate intervention case study homes were identified from within the emerging typology of 27 intervention homes by researchers (JK and FP) who were independent of the original ResCare trial. This sample represents approximately 14% of intervention homes and was chosen to best represent the range of contextual factors and implementation processes identified from our analysis of existing data (see Table 33). These included home size, ownership and reported ease of implementation across a range of enabling and disabling implementation processes. All four candidate homes agreed to support the implementation study data collection. We aimed to speak to managers and any staff who attended staff champion training from these case study homes. These additional qualitative data were collected between May and June 2013 by a researcher (JK), unknown to, and independent of, the original research project team. The ResCare trial programme manager (CH) contacted homes to find out who was still employed at the home and available for interview; their contact details were then passed on to the researchers. Interviews were conducted over the telephone, electronically recorded and transcribed verbatim. Topic guides were informed by NPT205 and revised iteratively to explore further matters that emerged as important in the initial analysis. Telephone interviews took between 20 and 50 minutes each.
Reflecting the difficulties experienced by the original research team in contacting care homes, potential participants were often hard to contact, and scheduling interviews proved to be similarly problematic. Care home staff would often schedule dates and times with the researchers, but would later be unavailable. A research nurse, who was involved in data collection during the trial, and the specialist dementia care (intervention) therapist were also interviewed face to face, together, by the researcher (JK), to discuss specific homes. Transcripts were not returned to participants for comment and/or correction, as this was deemed unnecessary for a study of a non-personal nature, and would incur significant delays. Julia Keenan led the analysis to identify salient themes and issues; both Julia Keenan and Fiona Poland independently coded and discussed a subsample of four interviews to develop the initial coding frame, which was largely deductive, as informed by NPT, but tailored to the context of the ResCare trial.
Participants: stakeholders
We aimed to recruit a qualitative maximum variation sample of participants and so, in addition to the case study homes that were sourced from intervention homes (see Participants: intervention case study homes), we expanded our enquiry to participants from the other ResCare trial intervention homes (that were not used as case studies) and control homes; stakeholders from localities where the trial had been carried out; and wider stakeholders from other parts of England. These diverse participant groups are here referred to as ‘stakeholders’. Use of qualitative methodology meant that no sample size was determined in advance for the wider stakeholder group discussion. Instead, individuals with a broad variety of relevant experiences working in different agencies, and others with personal interest in dementia care, were approached and recruited. They included:
-
care home managers and care home workers from different parts of England
-
professionals with experience of supporting care home residents and working with care home staff
-
people with experience of training and professional education
-
people with experience of service improvement (including regulators, inspectors, complaints work), described here as ‘service improvers’
-
lay people who had reflected on their personal experiences of visiting and scrutinising care homes as relatives, or friends of residents; and
-
professionals with experience of purchasing places in care homes on behalf of local authorities (commissioners and senior managers).
Recruitment and data collection ceased when thematic saturation was achieved, that is, when no new themes appeared to be emerging from the data.
Two further researchers who were independent of the original research project (JM and KS) invited 22 stakeholders to a discussion event, which comprised a facilitated meeting involving three discussion groups that used modified nominal group techniques. This was held in London, in April 2013. We (JM and KS) further interviewed nine others in person or over the telephone between June and July 2013. Topic guides were informed by findings from the ResCare trial to include development of the intervention (see Chapter 2), the CRT (see Chapter 3) and the case studies that were used during NPT process investigation. These were tailored to stakeholder participants’ knowledge and role, where stakeholders considered whether or not our findings ‘rang true’ (see section Interview topic guides). Stakeholder interviews and discussions at the facilitated meeting were recorded in note form. These data were anonymised and then analysed thematically. Two researchers (JM and KS) undertook the analysis to identify salient themes and issues. Again, transcripts were not returned to participants for comment and/or correction, as this was deemed unnecessary for a study of a non-personal nature, and would incur significant delays.
Interview topic guides
Semistructured tailored topic guides were developed to explore the research questions posed in the case study interviews with managers and care staff (Boxes 4 and 5), facilitated meeting and in the subsequent interviews. The topic guides were discussed with members of the programme steering group, which included a person with dementia, and the wider research team. The case study topic guides were closely informed by NPT constructs and components, but tailored to the individual characteristics of the care home and interviewee role in relation to the ResCare trial.
What did your home hope to get out of taking part in the ResCare trial? Who decided that your home should take part? . . . Feelings about this?
From your point of view, what did you understand as being the purpose of the research? . . . Was CB among residents with dementia a priority for you at the time, or not?
Was it clear when you joined the research what staff and resources would be involved and what you were all being asked to do? What was your role in all this? Was there someone in your home who was responsible for co-ordinating or leading the research activity?
Were the relevant staff/owners ‘on board’ with the e-learning and the care planning?
Did people want to be involved? Did you have to do any extra work to get people involved? See it as part of their job? How were staff/champions identified to take part?
How did this type of e-learning compare to the usual ways in which you train your staff? Probe perceived learner confidence with IT/education levels. How far had care staff received any previous training to understand CB in residents with dementia? How did the new care planning process (e-tool) compare with your home’s existing care plans?
In your opinion, what were the difficulties encountered in making the intervention run well? What needed to be in place in your home in order to make the intervention run well? What did you have to do (differently) to make this work?
How do you feel staff got on with the e-learning/action-planning? How far did they get the support (time, resources) they needed to complete training and formulate action plans (with confidence)? How were the new action plans used?
How did you judge whether or not the e-learning or action-planning made a difference to residents or staff in dealing with CB? Did you discuss whether or not it was working? Did you get any feedback along the way on how staff managed or performed with the e-learning or with the action-planning? Do you feel this e-learning and action-planning made a difference? Do you feel it made dealing with CB easier or more laborious?
Has anything changed in the day-to-day way you deal with CB in residents with dementia as a result of this intervention? Better/worse? Why?
What did you hope to get out of taking part in the ResCare trial? Who invited you to take part? . . . Feelings about this.
From your point of view, what did you understand as being the purpose of the research? . . . Was CB among residents with dementia a priority for you at the time, or not?
Was it clear when you joined the study what would be involved and what people in the home were being asked to do? What was your role in all this?
Was there someone in your home who was responsible for co-ordinating or leading the research?
Were the relevant staff/owners ‘on board’ with the e-learning and the care planning? Did people want to be involved? See it as part of their job?
How did this type of e-learning compare to the usual ways in which you are trained? Probe perceived learner confidence with IT/education levels. Have you received any other previous training to help you understand and deal with CB in residents with dementia? How did the new action-planning process (e-tool) compare with your home’s existing care plans?
How did you find the e-learning/action-planning? Did you get the right support (time, resources) needed to complete training and formulate action plans (with confidence)? How were the action plans actually used?
In your opinion, were there any problems in your home in implementing the e-learning and action-planning tool? What did you have to do (differently) to make this work?
Did you ever discuss with other colleagues in your home whether or not the e-learning or action-planning made a difference to residents or staff in dealing with CB? Did you get any feedback along the way on how you did with the e-learning or with the action-planning? Do you feel it made a difference? Do you feel it made dealing with CB in residents with dementia easier or harder?
Has anything changed in the day-to-day way you deal with CB in residents with dementia as a result of taking part in this project? What? (Care planning, understanding CB?) Better/worse? Why?
For practitioners and other experts, the interview schedules were tailored to the activities of the specific agency or professional group being interviewed, whereas for family carers, their expressed experiences of managing CB themselves and their observations of care home practice and staffing were both potential sources of information and of their views. The topics covered included:
-
Do the findings of the ResCare trial (summarised), and about implementing an e-learning/e-tool for care home staff (described), and the introduction of functional analysis approached for planning (explained), ‘ring true’ in your experience?
-
Thinking of homes you have worked with or visited, does our typology for computer-assisted interventions (explained) help anticipate and explain these challenges for implementing e-learning/e-tool and functional analysis?
-
What other barriers might be relevant to consider?
-
What potential resources/enablers might be relevant to consider?
Ethical considerations
In line with other parts of the trial, data reported in this chapter were collected with informed consent and participants were assured of anonymity. To help reduce the risk of bias, the interviews were conducted by researchers who had not been part of the trial as the participants may have wished to give socially acceptable answers to members of the trial research team. Each case study home was assigned a pseudonym, chosen to avoid the name of any actual UK care home at the time.
Data analyses
The first phase of analysis involved close reading and re-reading of various overarching and home-specific data sources, related to the process and outcomes of implementing the intervention. These sources were then manually coded (by JK and FP) so as to identify barriers to, and facilitators of, successful implementation, and then to identify the main (sequential) mechanisms of implementation (see the example outlined in Figure 11).
FIGURE 11.
The ResCare trial: mechanisms of implementation taken from the analysis of process and outcome data from the ResCare trial.
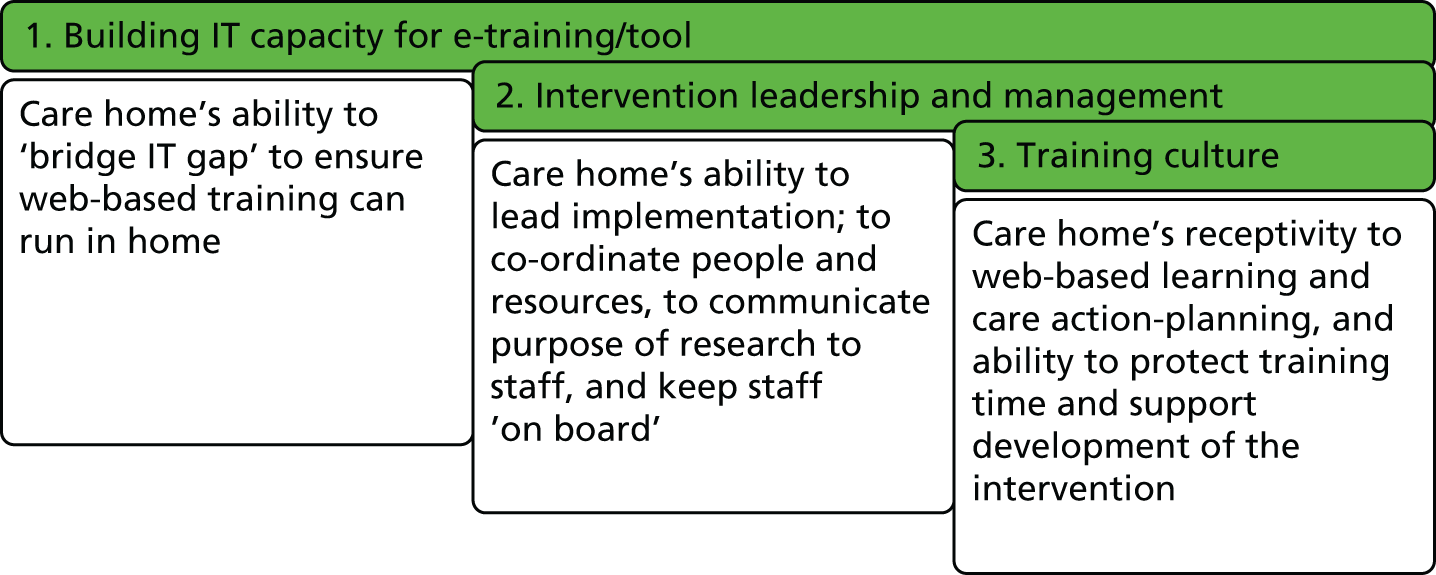
These mechanisms, in conjunction with context data (see Box 2), were used to identify potential factors affecting barriers to, or facilitators of, implementation; and to develop a typology for intervention homes according to whether they provided an enabling or disabling environment for each of these mechanisms. Of the 28 intervention homes in the main study, 27 received the intervention; one home failed to engage with the intervention and one dropped out following intervention (see Chapter 3), so no trial follow-up data were available for this home. Where supporting data were unclear, making initial assignment of a home to an ‘enabling’ or ‘disabling’ environmental category difficult, a note was made, and clarification was achieved in April 2013 during consensus discussions with the research team. NVivo 8 (QSR International, Cambridge, MA, USA) was used to manage the systematic coding of existing and supplementary qualitative data. Participants in any interviews completed for this chapter did not provide feedback on the findings, but the findings from the case study intervention homes’ interviews were shared with the stakeholder group and their reflections recorded as part of the modified nominal group.
Results
Two aspects of our inquiry are outlined in this section: first, those that took part in the intervention study that were included as case study data; and, second, those described as ‘stakeholders’. This group included wider stakeholders from the localities of the trial and also across England, staff at homes that took part in the intervention but were not sourced as case studies and staff in control homes who did not receive the intervention, apart from the offer of free access to the e-learning course if they wished.
First, we report the findings of the study of intervention homes using NPT and framework analysis206,207 by presenting a descriptive typology for all intervention homes, and descriptive data for case study homes. This is followed by a description of the factors that influenced implementation of the intervention, described according to the four NPT constructs: coherence; cognitive participation; collective action; and reflexive monitoring. Interpretation of NPT analysis, relating to requirements for implementation, is outlined in Figure 12.
FIGURE 12.
The ResCare trial: what needs to be in place to support implementation, as identified by NPT analysis. PC, personal computer.
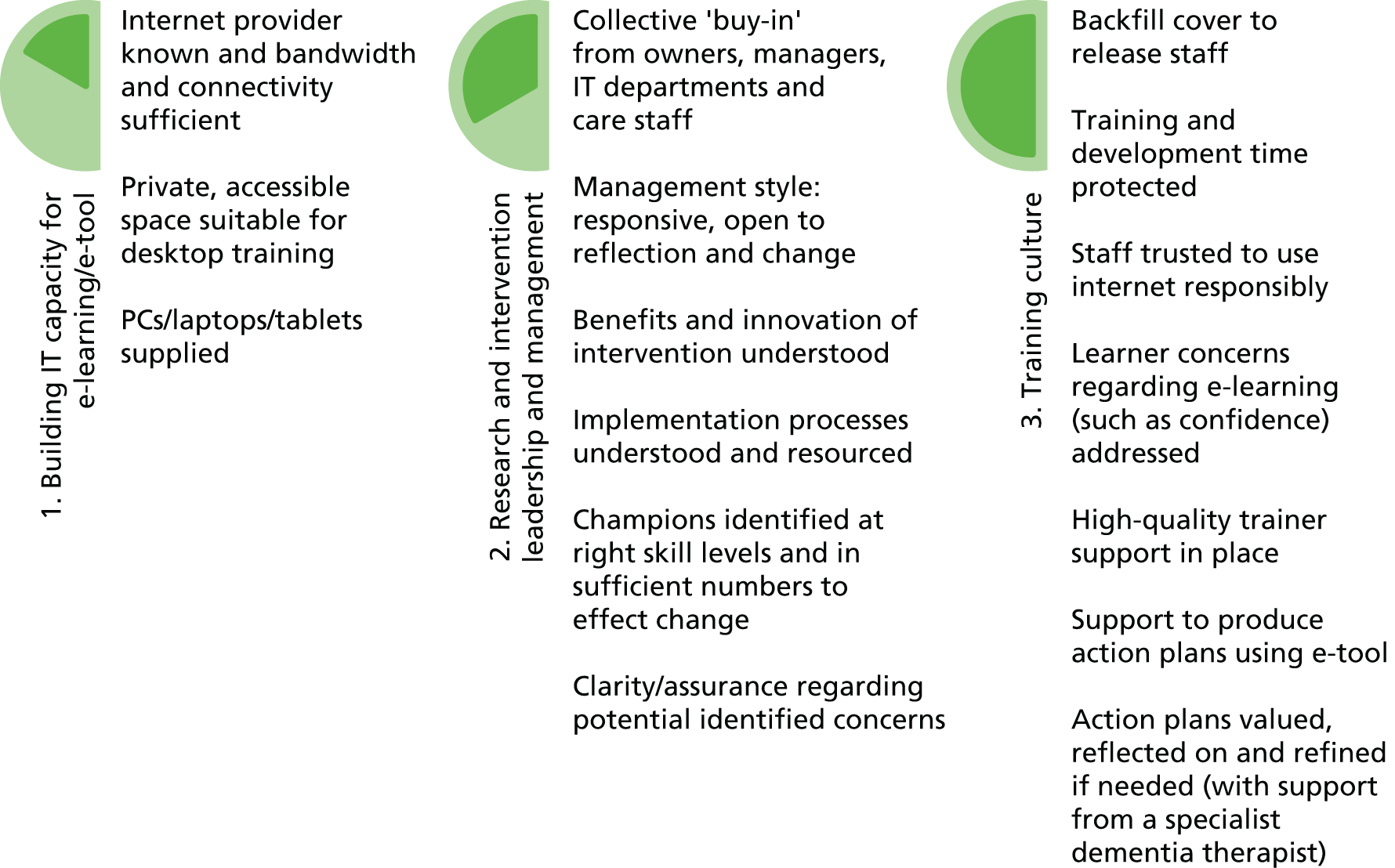
Next, we present the thematic findings of our stakeholder interviews and group discussions, in terms of whether or not our approach to training and functional analysis ‘rang true’ with those not involved in the intervention; whether or not identified enablers and facilitators of implementation are applicable to other parts of the country; and whether or not the problems with and barriers to implementation would be applicable to other homes and areas across England.
Finally, findings on uptake of resources available and how decisions are made about who delivers interventions are outlined.
Implementation of the intervention in homes
Descriptives: typology for all intervention homes
An overview of the implementation process and outcomes across intervention homes is presented within a descriptive typology of care homes (Table 33). The top part of the table shows single homes and the bottom part shows homes that are part of a chain or larger company. The typology of homes is structured according to implementation mechanisms illustrating the three implementation mechanisms (shown as columns in Figure 11): IT (connectivity, equipment and building IT capacity); research and intervention leadership and management; and training culture.
| Ownership | IT | Leadership/management | Training culture | Homes (n = 27) (research team rating – implementing the research trial) |
|---|---|---|---|---|
| Single home (private or charity/voluntary owned) | Enabling | Enabling | Enabling | 1038 (good); 1004a (good); 1028a (good) |
| Disabling | 1016b (average) | |||
| Disabling | Enabling | 1098 (average) | ||
| Disabling | 1073 (challenging); 1093 (average); 1069a (challenging) | |||
| Disabling | Enabling | Enabling | ||
| Disabling | 1111 (average) | |||
| Disabling | Enabling | |||
| Disabling | ||||
| Care group home (private, charity/voluntary or local authority owned) | Enabling | Enabling | Enabling | 1108c (good); 1066c (good); 1015 (good) |
| Disabling | 1043a (challenging); 1072 (average) | |||
| Disabling | Enabling | 1048 (average); 1001c (good); 1079a,c (average) | ||
| Disabling | 1078c (challenging); 1013c,f (challenging); 1103a,d (challenging); 1027 (challenging); 1062 (challenging) | |||
| Disabling | Enabling | Enabling | ||
| Disabling | 1082 (average); 1105a,e (average) | |||
| Disabling | Enabling | 1085e (average) | ||
| Disabling | 1087 (challenging); 1106a (challenging) |
For the first implementation mechanism associated with IT, most single, privately owned independent homes, even when running without modern IT systems before the intervention, could facilitate the required upgrades to run the intervention; only one of the nine single intervention homes (home 1111) struggled here. For the 18 homes that were part of a larger chain or company, just over one-third, struggled at this first hurdle, of having the IT in place in order to deliver the intervention.
The second implementation mechanism associated with enabling or disabling leadership and management environments was equally prevalent across both single homes and homes owned by a chain or larger company. Within homes experiencing changing ownership or management during the trial (shown as codes within the table footnotes), some homes coped better than others in leading the research, with a change of manager sometimes helping the research to progress.
For the final implementation mechanism associated with training culture in homes the picture was also mixed.
As can be seen from Table 33, across the intervention homes, all three implementation mechanisms were ‘enabling’. These were rated as ‘good’ for implementation by the original research team in six homes. Two homes were rated as ‘disabling’, across all three mechanisms, and these were also described as ‘challenging’ by the original research team. Finally, a sizeable cluster of a ‘challenging’ care homes were deemed ‘enabling’ for IT, yet ‘disabling’ in both leadership and training culture.
Descriptives: intervention home case studies
Each of the four case study homes was assigned a pseudonym. Their operational contexts are shown in Table 34.
| Care home pseudonym | |||
|---|---|---|---|
| Happy Haven | Careful Place | Home Court | Lifelong Lodge |
| Single privately owned care home | Private (group) care home with nursing care | Private (group) care home | Not-for-profit (group) care home |
| Mixed client group: organic and functional mental health problems | Registered for those with dementia and for those with physical disability | Registered for those with dementia and for those with physical disability | Registered for those with dementia |
| Small home (29 beds, 27 full-time staffa) | Medium-sized home (45 beds, 12 full-time staff)a | Large home (82 beds) | Small home (30 beds, 37 full-time staff)a |
| Urban area. Home adjoined converted houses, bit ‘run down’ | Suburban – not built up. Converted nurses’ home within own grounds | Suburban – not built up. Staff work on one side of the home or the other | Semi-rural/village location. ‘Not a spacious home’ |
| Home run on ‘relaxed family basis’, staff members related | ‘Cottage hospital’ feel. Care staff described like ‘shop floor girls’ | Designated ‘floor’ for dementia | Specific dementia unit |
To reflect the variety within the intervention homes overall, three case study homes were part of a care group and one was a single privately owned home. We included a small, medium and large care home (with different staffing structures and cultures), and one of the case study homes selected was a care home with nursing (Careful Place). We included homes that had dedicated or specific dementia floors/units for residents. Finally, geographical location and urban or rural setting were likely to influence the pool of available labour and staff turnover, as well as the culture of care; so these were also taken into account.
Fourteen case study home staff (at least one from each home) were identified as potential participants, of whom seven agreed to be interviewed: three managers and four care assistants or senior care assistants (Table 35).
| Staff | Care home pseudonym | |||
|---|---|---|---|---|
| Happy Haven | Careful Place | Home Court | Lifelong Lodge | |
| Number of potential care staff participants (grade) | n = 3 (one manager and two senior care assistants) | n = 3 (one manager and two care assistants) | n = 5 (one manager, two senior care assistants and two care assistants) | n = 3 (one manager and care assistants) |
| Changes in personnel since intervention | No change | One care assistant on maternity leave | Manager stepped down, but in home once a week to train staff; one senior care assistant now acting manager, one left the home and one unable to be released | One care assistant went on long-term leave after training |
| Number of actual participants | n = 3 (one manager and two senior care assistants) | n = 1 (one manager) | n = 1 (one care assistant) | n = 2 (one manager and one care assistant) |
The therapist leading the intervention and a research nurse employed throughout the research process were also interviewed together to discuss implementation in these homes.
Factors influencing implementation of the intervention
Analysis of four NPT-framed factors (i.e. coherence, cognitive participation, collective action and reflexive monitoring) revealed that these varied in their influence during implementation of the intervention. These are described below.
Coherence: making sense of implementing the research and intervention
One emerging finding from the analysis of interviews with care home managers, and for some other staff who attended the training, was that, despite their reported initial enthusiasm for the research and intervention, after allocation to the intervention group and taking part in the research, they struggled to define the unique aim of the intervention. Beyond the novelty of it being specifically about dementia and CB and its delivery by electronic means, none of the three managers interviewed saw the intervention as being about a biopsychological case-specific approach for dementia and CB.
Indeed, the approach was not felt to be entirely new to some homes, but, significantly, no one envisaged it as implementing a somewhat specialised approach to systematically deliver tailored case-specific support or functional analysis-based interventions for the management of CB in the home. Care staff interviewed recollected that the training had addressed ‘thinking about how they [resident] see things’ (Lifelong Lodge, care assistant), the ways in which staff interpreted, understood and could more productively address what might be seen as CB. However, they did not understand themselves as being champions taking forward and modelling the way they understood and supported residents with CB within a cascaded learning approach.
Coherence, that is, how people make sense of the intervention, was vulnerable to fragmentation over the course of the research. This appeared to be the result of the dynamic nature of the research content since, in reality, care homes could be involved in several service improvement initiatives simultaneously:
. . . you know they might have had a patient survey, or they might have had a little university project going on, or they might have been contacted by some audit department, or, . . . changes of management and staff and stuff, so I think they do forget.
PA4 research team focus group
The impact of this within-sector dynamism on maintaining coherence was to some extent foreseen and anticipated by the research team. However, the length of the research process, the gaps created between contact, data collection and intervention activities within individual homes and the necessary implementation changes to the study (Box 6) worked against maintaining coherence. The research team saw this as leading to a considerable increase in workload, as they had to ‘start all over again’ with subsequent contacts.
-
A shift from the e-learning/e-tool in homes running independently without a therapist through a champions/cascade learning model – to delivering e-learning to champions in classes off-site, delivered by a skilled therapist and providing therapist support for action-planning.
-
The e-tool module of the intervention, initially designed to deliver individualised action plans – demanded therapist support and much more input with the therapist from champions in care homes than was originally envisaged.
From the perspectives of care home staff tasked with leading the interventions, they did not see these different components as part of a larger project. Such fragmentation threatened care home managers’ willingness and ability to participate; and was an even greater threat in care homes operating under pressure.
Managers also described fragmentation as a result of several ‘different researchers’ coming into the home at different stages such as those who were there for the purposes of data collection and those who were there to assist with the intervention. The result of this was:
We never got to really form any kind of relationship with the team that was actually doing it.
Careful Place, manager
Finally, the threat of fragmentation to care staff making sense of the intervention arose from changes having to be made in the research process and intervention. The e-tool could not produce the individualised action plans as envisaged without significant additional staff input.
One manager expressed confusion about why the IT equipment from the research was still in the home, having forgotten the original (modified) plan for the intervention to run independently in the homes. Thus, a final significant barrier to coherence was lack of communal specification, within each home, when people who were important to the intervention had lost understanding of its aims and processes. Poor communal specification caused the largest implementation problem in two key areas. The first area was when care staff identified as champions did not understand the intervention’s specific purpose and nature. The second area concerned the need to supply or boost homes’ IT and broadband provision to sufficient levels in order to run the intervention. This was needed to realise the original plan of installing and running the e-learning and e-tool in homes, and to gain the necessary permissions to undertake this work. The research team had sought the agreement of care home owners. These were either heads of groups of privately owned or not-for-profit or local authority-owned care homes or owners or owner/managers of individual care homes. Sometimes permissions from others were required, such as training directors or regional/national company directors or when working with an organisation’s external IT department or an organisation’s outsourced IT provider. This convoluted process of gaining communal understanding and authorisations from different people in the care home system, often located at different sites and often not very contactable, further contributed to a lack of coherence. Staff knowledge of IT systems varied across case study homes, but IT installation was deemed to be relatively straightforward.
Thus, we can conclude that lack of a coherent understanding of the intervention and research process clearly impacted on the willingness of care home staff to fully engage with or ‘buy into’ the intervention, leading to one manager expressing disappointment:
. . . it was very exciting to be able to be picked to take part in it you know, especially as I said at the time it was . . . the timing was right. But I was disappointed with how it progressed.
Careful Place, manager
The lack of coherence was a particular threat in homes operating in conditions of uncertainty, and in those with more hierarchical staffing structures or homes that were part of a larger group. This suggests that smaller homes may be more amenable to the changes needed for implementation of interventions for dementia with CB. The ‘real’ and ‘ideal’ conditions193,199 for making sense of the intervention are summarised in Table 36.
| Conditions | Strategies to promote coherence | |
|---|---|---|
| Real | Ideal | |
| Care home staff struggled to define the unique aim of this research: implementing functional analysis across the home | Recognition of what the research and intervention were trying to deliver | Promote the intervention as a psychosocial framework for CB in dementia, rather than focusing on its novel delivery |
| Coherence vulnerable to fragmentation over the course of the research process | Maintained understanding of the stages of research and intervention implementation | Provide a regularly updated user-friendly timeline to show key research milestones Circulate regular newsletter with research updates and photographs of new research staff |
| Care staff sent for training not understanding themselves as champions within a cascaded learning approach Owners and IT departments did not understand their role in upgrading homes’ IT and broadband provision |
All relevant parties know what the intervention is; and what it requires of them | Create a diagram/poster describing each staff role in implementation Explain/cascade learning model and identify support for champions |
Cognitive participation: investing in implementing the intervention
Cognitive participation, according to NPT, refers to how people engaged with the research process and the intervention. Data were analysed in terms of the barriers to individual and collective investment in delivering interventions for dementia and CB. These were:
-
a manager or other senior member of staff not taking responsibility for initiating and maintaining participation in each home; or not being supported in this role
-
managers being ‘told to take part’, leading to tokenistic engagement
-
managers and care staff resisting the research and intervention.
Embedding innovations requires that key participants drive implementation forward. Not surprisingly, the most important person identified within care homes by the research team to lead implementation was the manager, and it was evident that the manager who ‘invested’ in, or was personally committed to, the research could make a huge difference to implementation. Managers were widely deemed to be more likely to lead participation if they had gained a greater sense of coherence about the research, by themselves engaging in it and showing others through their actions, such as by taking part in data collection (questionnaires), or, more powerfully, as a small number did, choosing to attend the training themselves. For the six homes whose managers attended training, three were rated as being ‘good’ in terms of implementation by the research team, two were rated as ‘average’, and only one as ‘challenging’:
. . . the managers that have been involved in answering questionnaires that have been more hands-on managers have also been much, much easier to work with as well, because they’ve just got it, whereas the managers that have been very distant and we don’t really see, and don’t have any involvement, have been more of a struggle.
PA1 research team focus group
In their focus group discussion, the research team acknowledged ‘in hindsight we should have probably got all managers to come before we then got their staff to come’. This is certainly borne out in the analysis of the case study interviews for this process evaluation; the only manager (Happy Haven) interviewed who attended the training was surprised at its high quality, despite some prior reservations about the quality of learning associated with e-learning.
Having a named manager invested in the research was essential for facilitating implementation, even when aspects were delegated to senior care staff, but that manager also needed to be supported by owners, or their senior organisational managers or directors. Having a supportive key person to promote implementation related to having a collective coherent understanding of the research and intervention. It also now leads us to question how homes initially understood and engaged with the research.
When managers had been ‘told to take part’ by their senior management, this had, on occasion, impacted adversely on their willingness to participate; some were accordingly perceived as resisting by stalling the researchers and/or being ‘a bit stubborn’ (researcher) throughout the process, or engaging only in token form.
In their eagerness to access free training, it was felt that some care homes had signed up without fully thinking through or appreciating what would be required in order to implement the intervention. This is seen in the data collated by the research team describing their perceived receptivity of each home for both the e-learning and the e-tool (separately) on a scale of 1 to 3 (low to high), which strongly indicated that homes in general were more receptive to the e-learning than to the e-tool. Although this made relationships particularly difficult with managers when sorting out IT capacity within each home to enable it to use the computer-assisted intervention, the IT engineer had sympathy for care home managers, who often had poor knowledge of their existing systems and infrastructure.
Thus, managers of homes that were part of a chain or larger company expressed some resistance to taking part in this or any other research, whatever its aims. Other specific reasons managers and care staff had for resisting investing in the research and intervention included:
-
concerns that care staff could not be trusted to do e-learning on computers in the home because they could not be trusted to access the internet responsibly
-
scepticism towards e-learning and the quality of learning delivered through such means
-
concerns that older care staff might lack the necessary experience or confidence to undertake training on an IT platform
-
belief that some staff (in low-paid, transitory jobs) did not want to learn.
Managers’ greatest initial concerns were about broadband internet being put in for the research, and that staff would be accessing the internet in work time for other purposes.
Of the four case study care homes, at least one (Careful Place) had routinely used e-learning for more procedural updates prior to our study, but all managers interviewed expressed doubts about delivering effective training around dementia or CB, or even personalised care, through an e-learning platform. Similarly, managers were more commonly concerned that some older staff would lack confidence with IT platforms. This was the case for one of the older members of care staff at Happy Haven, who was one of a handful who arrived at training not knowing that it was e-learning they had been nominated for. Others felt that having the specialist dementia care therapist available and present to lead training was helpful. The specialist dementia care therapist describes some less confident staff ‘sitting on their hands’ and needing reassurance and support to interact with the computer. Finally, there was evidence of some reluctance on the part of staff selected to attend training and act as champions to do anything other than attend the training. This could have been because they had not been told anything about the important aspect of the intervention involving delivery of case-specific action plans. Some care staff also had concerns over data protection and governance, such as inputting residents’ personal information onto an IT platform ‘in view of others’.
In this theme, several facilitators for staff investment relating to implementation of interventions in the care home were found. A facilitator of implementation was having a named, contactable manager to lead and drive implementation by providing support to staff champions. This was an important mechanism for change, as they had close interaction with the specialist dementia care therapist. Another facilitator was access to, and interaction with, the specialist dementia care therapist,82 who, for the purposes of implementation, can be described here as a ‘service improver’. In the real world of NHS provision for people with dementia with CB living in care homes, this role can be conceived as an equivalent of an ‘in-reach’ specialist NHS dementia therapist. 82 In order to facilitate delivery of the components of the biopsychosocial approaches underpinning functional analysis-based interventions in care homes,97 this role of ‘service improver’, provided by a specialist dementia care therapist, should allow for a therapist who has additional formal arrangements for access to, and clinical support from, a multidisciplinary team. 97,186 Real and ideal conditions for fostering the engagement of care homes and strategies to facilitate investment are summarised in Table 37.
| Conditions | Strategies to promote cognitive participation | |
|---|---|---|
| Real | Ideal | |
| Lack of supported leadership for implementation | Key individuals take a lead role in creating and sustaining momentum for implementation Active support from owners or care group managers to support managers in leadership role |
Payment for managers to co-ordinate implementation Provide guidance on role of ‘service improver’ and avenues for support |
| Scepticism towards e-learning | Care homes understand this e-learning package as based on realistic scenarios, with interactive, problem-solving components and continuous feedback | Managers to undertake training before/with staff Share positive feedback from those who have already completed training |
| Concerns about staff accessing training and using the internet irresponsibly | Care homes trust staff to access internet responsibly for training purposes only | Reassure managers that access to other sites can be blocked, if concerned |
Collective action: enacting the work required for implementing research and the intervention
Home managers were largely responsible for the practical work of co-ordinating resources to ensure that:
-
the research team could collect data from residents and care staff
-
the appropriate staff were identified for training as champions
-
the staff were scheduled to attend training and given time to develop and implement care plans without this undermining the routine running of the home.
Champions also had a central role in enacting the intervention through co-producing and implementing the CB action plans, but there were five barriers identified to enacting the implementation of functional analysis in care homes. These will be outlined next.
First, longitudinal data collected by the research team document various contacts made with individual care homes over the process of the research to initiate and support implementation, and evidence difficulties for researchers in getting in touch with care homes. Telephone calls were the only fruitful way of contacting homes and different homes had different cultures/policies about staff availability to take calls, if they were at work. Absent owners were the most difficult people to reach.
A second barrier, evident in the majority of homes, was that senior staff or managers struggled to co-ordinate staffing to support and enact the research or intervention. The research team tried hard to be flexible and unobtrusive when collecting data in homes; however, this flexibility inevitably incurred difficulties and delays. Staff absences, shift patterns and turnover aside, by far the most significant barrier to managers committing to releasing staff to take part were competing service pressures – when carers would be scheduled to meet with the research team and then called back into service to meet residents’ needs:
. . . It was demands on time; it was like ‘Oh how much longer are you going to be?’ ‘We need you to come and help with bathing,’ or you know, lunch, or somebody’s come to see someone, can you come and deal with it, so there were constant interruptions, nobody was, and they were feeling almost as if ‘I’ve got to rush, I’ve got to rush’.
PA2 focus group
The changes made to (1) deliver e-learning off-site; (2) have a therapist lead the training; (3) deliver the action-planning; and (4) offer to compensate care homes to release staff were appreciated by managers interviewed, although some still complained about sending staff away from the home. Even supportive managers, such as the one at Happy Haven, who managed to schedule all the time and staff required, felt that in hindsight the home should have claimed more money back:
. . . I probably short-changed ourselves to what [staff] we actually did provide to be honest. But if we was to do anything like that again, I’d just have a separate diary for the days the people come in because they’d cancel and then we’d reschedule and all like this but obviously I’d already got them staff members in.
Happy Haven, manager
A more general concern was that managers were seen as unhappy with the amount of staff time implementation would take, irrespective of whether or not it was being paid for by the research team. Many of the managers did not actually claim back the money to cover staff time. From the manager’s point of view, it is burdensome to arrange cover from existing staff to co-ordinate this leave on top of existing training demands:
. . . we don’t have the amount of free staff I suppose, for a better word, to be able to . . . you know, with all the staff training, I mean believe me we have a huge staff training matrix what we have to deliver in a year anyway, [. . .] And if staff aren’t always easy to pin down to say ‘can you do extra shifts to cover the shifts’, that makes it quite difficult really.
Lifelong Lodge, manager
Another barrier was that managers frequently sent inappropriate staff to be trained as champions. Interviews suggested that managers had a strategy for selecting staff for training, but that this was perhaps wrong for the intervention. In Lifelong Lodge, for example, priority was sensibly given to selecting full-time over part-time staff to champion the intervention. However, the therapist’s notes reveal that it had taken several months to get to the stage where the manager at Lifelong Lodge released these staff for training, of whom one was quite junior and another had a booked hospital admission for a knee operation the week after training, and would be off work for a significant period of time. Only one champion actually took part in the action-planning side of the intervention in this home and she was too junior to be able to enact the medications part of the care action plans for residents. More junior staff were usually keen, but they were more likely to complete entries in a resident’s day book than be fully involved in writing individualised care plans within the home, nor did they have a sufficient understanding of residents’ medications to fully engage with care action-planning. Conversely, there were also issues, depending on homes’ staffing structures, when senior staff were distanced from residents and their day-to-day care needs, personalities and life histories:
. . . if you think about it, you’ve got this gap between people writing care plans and people doing the work, so [. . .] it’s not really ideally going to work with just like you know, one person being a bit more senior and they do care planning but don’t do hands-on stuff because you’re not getting that depth of information there. And certainly you know, it’s difficult to then sort of have a prescription of an action plan almost.
Therapist
Thus, in homes with a gap between those doing care planning and those with in-depth knowledge of the person with dementia it can make it difficult to implement this intervention on a one-carer-to-one-resident basis. Across the case study homes there were also differences in how care plans were routinely written and used by those sent to train as champions. In Lifelong Lodge the day seniors and manager write the care plans:
. . . I’m just doing that myself now and updating the care plans and risk assessments and making sure that staff are aware that changes have happened [. . .] she [carer 1] does not take over any care plans or risk assessments . . . they write a daily life record on what’s happened on her shift but . . .
Lifelong Lodge manager
Right and that feeds into the care plan does it, that daily life record or . . .?
Researcher
No, no . . . they’re writing care plans every day but they don’t probably review the changes and . . . they get told back in handover you know, at the beginning of each shift really but it wouldn’t be that they would go look at a care plan and read it thoroughly every shift you know, that doesn’t happen really at all. [. . .] No, I mean major changes or things what are affecting people day by day are changes where they would get given verbally at handover and then like I say it’s left to the senior team to write the care plans.
Lifelong Lodge manager
This is confirmed by the champion from this home, who also noted that neither champion at this home was routinely involved in writing care plans.
Finally, in this section, our NPT analysis in respect of collective action and enacting the research/intervention revealed that a major barrier to implementation was that staff were not given time to develop action plans with the therapist in the homes. The research nurse subsequently felt that this part of the intervention was initially thought to be much more straightforward, that the e-tool would simply produce individualised care action plans; and homes had bought in and signed up to participate on this understanding:
To be fair, right at the very beginning, we told them that this tool . . . there’d be like three trainings [modules] and then the fourth one would write you a care plan. And then it ends up that [Therapist]’s going in with them. So they were hoping to just do that [e-learning] and out comes this piece of paper with like [. . .] and they don’t have to check it because that’s dead right because computer’s done it. [. . .] So they weren’t expecting to spend a lot of time with you [. . .] Press a few buttons and there’s your answer.
Research nurse
However, from the perspective of the research team, if care action plans were to be fine-grained enough to impact on levels of CB, quite detailed information about the person with dementia was required. Once the therapist actually got into the home, champions often were not allowed enough extra time to develop these action plans, and:
. . . a lot of people have had to do a lot of stuff in their own time, and you know, whether they’re prepared to do that or not has made a difference.
Therapist
This was particularly the case at Home Court, where the champion took the main role in providing information about the CB residents for action-planning. This senior care assistant did not appear to see this as a problem and had previously attended training on her non-working days.
To summarise, the five barriers identified above are:
-
difficulties for researchers in getting in touch with care homes
-
managers struggling to co-ordinate staffing to support the research or intervention
-
managers selecting inappropriate staff for training as champions
-
different staffing models within homes sometimes meaning that champions did not have sufficiently detailed knowledge of, or a working relationship with, those residents with dementia to effectively implement care action plans
-
managers not providing adequate time or support for care staff to co-produce action plans.
These barriers were addressed by the research team and steps were taken to work around these problems with care homes, with the consequence of significant delays and additional costs. The real and ideal conditions for implementation and strategies to promote the enactment of interventions in care homes are summarised in Table 38.
| Conditions | Strategies to promote collective action | |
|---|---|---|
| Real | Ideal | |
| Managers struggled to co-ordinate staffing to support research or intervention | Managers are supported with appropriate resources and funds to release staff for implementation | Discuss with managers how best to support releasing staff and provide funding |
| Managers selecting inappropriate staff for training as champions | Careful, strategic consideration given to which staff members are sent to train as champions | Discuss with managers which staff would be best placed to effectively champion implementing the intervention across the home |
| Different staffing structures/models meant that champions might lack sufficiently detailed knowledge of, or a working relationship with, residents with CB to effectively implement care action plans | Consideration given to how care homes’ existing care planning processes relate to those of the intervention | Assess each home’s staffing structure with respect to care planning and tailor training/action-planning accordingly Redesign action-planning e-tool to be used collaboratively by more than one member of staff |
| Managers not providing adequate time or support for care staff to produce action plans | Dedicated and protected time to produce care action plans | Discuss with managers how best to support releasing staff and provide funding |
Table 39 provides an overview of findings across the four case study homes with respect to the collective action processes of implementing the intervention.
| Process | Care home pseudonym | |||
|---|---|---|---|---|
| Happy Haven | Careful Place | Home Court | Lifelong Lodge | |
| IT provisioning (engineer’s report) | Installation ‘straight-forward’ | Installation easy and aided by the staff, ‘one of the best’ | Installation ‘straightforward, but Wi-Fi connection is not secure . . . no one was bothered’ | Installation ‘straightforward’, dispute between manager (wanted wireless) and internal IT department who did not |
| Staff knowledge of IT systems | ‘Poor’ | ‘Excellent’ | ‘Non-existent’ | ‘Good’ |
| Service improvement leadership/management | Manager well established Difficult to contact, but ‘very keen’ to implement training and tool |
Manager ‘a very experienced nurse’, enthusiastic and eager to be involved, but extremely busy and difficult to get hold of | Manager keen but has ‘fixed ideas’ about how home should be run and residents cared for Manager busy and difficult to get hold of |
Manager very keen to access hard-to-find CB dementia training Administrator makes it difficult to contact manager or senior staff |
| For research: baseline and follow-up | Manager led strong communal buy-in to help with research Manager available to help researchers, staff available on rota |
Staff informed, but unprepared for research team and intervention – all very busy, no private space in which to conduct research | Manager did not know about needing to see medication records/care plans. Staff informed that researchers were coming, but unaware of the study | Manager rigid about visits not being before 14.00 Care staff on rota for researchers at baseline but not follow-up – unsure as to purpose of research |
| For intervention | Champions understood purpose of the training/tool | Champions did not know about action-planning following e-learning | Champions did not know about action-planning following e-learning | Manager unaware of action plan aspect of research. Champion unaware of their role in this |
| Training culture and management | Manager requested and attended training him/herself. No problems scheduling staff for research and intervention | Manager struggled to release staff to attend training or complete action plans because of staff shortages and/or sickness | Manager had initially only allocated one staff member for the entire study. Took several months to access staff to train as champions Staff shortages prevented release of staff from duties for training |
Took several months to access staff to train as champions. Manager found it difficult to arrange staff to cover shifts Manager noted that some staff in care sector do not want to develop their skills |
| Champions | Two senior care assistants, one ‘initially fearful of using computer’, but both ‘quickly grasped the concept of functional analysis’ Champions not ordinarily involved in writing care plans Champions able to discuss intervention and supported to do action plans in work time Champions question extent of their involvement in action-planning |
Two fairly young, but very keen and IT literate care assistants Champions not suitably qualified to complete action plans for medication (care home with nursing) Champions not ordinarily involved in writing care plans |
Two senior care staff, two care assistants. Senior staff ‘took the main role in providing information about the CB residents for care action-planning’ Champions not ordinarily involved in writing care plans Champions did action plan in their own time, but not likely to be supported implementing action plans |
Two care assistants, one who was about to go on a planned period of leave. Other champion too junior to access medication information Champions not ordinarily involved in writing care plans Care staff not allowed to take telephone calls other than at 14.15 Champions not allowed to contact trainer or get help with action plans in work time – did this in their own time Information provided was patchy and difficult to access from senior staff |
Reflexive monitoring: appraising the effects of implementing the research process and intervention
To successfully embed interventions for CB in residents with dementia, care home staff needed to review their experiences of implementing it and, if necessary, adapt their practice to suit local circumstances and individual residents. Key examples of reflexive monitoring are seen through the changes made by the research team to adapt implementation, such as responding to difficulties about installing computers in homes, providing financial resources to release staff for training and arranging more training events with smaller groups to help some managers who were unable to release staff as planned. However, there were barriers to reflexive monitoring within the homes themselves. These were:
-
lack of any systematic feedback on the impacts of the intervention on residents with dementia from the research team, because the trial was ongoing
-
positive feedback from staff who attended training not being shared with managers
-
lack of momentum and support for staff to share and embed learning and change their practices.
Case study care home managers were aware that the nature of the research design meant that they would not get to know the outcomes of the study (in terms of impacts of the intervention on measured levels of CB among people with dementia) until data had been analysed and the study had ended, but managers at Careful Place and Lifelong Lodge reflected that they felt short-changed in that they had not yet had what they understood as feedback from the research team since data collection had been completed, as described below:
. . . it would have been nice to have had some general feedback you know, to say well the trial’s now finished you know and a general summary at a location.
Careful Place, manager
The manager at Happy Haven, however, recognised that she had received informal feedback along the way and that more formal findings would be coming. Intervention care homes in the study were written to after data collection and thanked and offered three free additional licences (active for 3 months), a certificate and a free place at a dissemination conference.
In terms of appraisals of therapist-led e-learning training, all staff interviewed who had attended training felt that it had exceeded their expectations. This supports the findings of the evaluative questionnaires completed by those who attended training. An aspect of the intervention judged as particularly good was that the e-learning:
. . . wasn’t as complicated on the computers as I’d thought [it would be].
Happy Haven, care staff 1
Most noted was the package providing a much more active and engaging learning experience, which related well to their own experiences of working with people with dementia:
I’m more of a hands-on, ‘let me read it, let me do it’, than having someone just sit there and go on and on and on and on for hours. And then say to you ‘Take a test’ or something, [. . .] it kept your mind going [. . .] some of them scenarios that we did I just thought oh that is so like so and so where I work.
Home Court, care staff 1
Also valued was that the e-learning process gave the learner instant feedback on their understanding:
. . . it made you think ‘Oh, oh yeah, I get it’; do you know what I mean? We put in what we would have done but then the computer told you the correct way.
Happy Haven, care staff 2
Some staff were more effusive in their praise than others, whereas on a basic level the relatively junior champion sent from Lifelong Lodge reflected that:
. . . it does open your eyes a bit.
Others were clear about the specific value the learning added in terms of improving individualised care:
. . . where before you just thought like everybody was the same, dementia was . . . but it was explained better and everybody’s is not the same.
Happy Haven, care staff 2
These individual responses corroborate the therapist’s notes on which champions in each home performed and engaged best with the process – in that these staff could offer a more detailed account of what they learned. The manager from this care home who attended training remarked that:
. . . if it had widely been available, I certainly would have sent more staff on it.
Champions who attended training and completed care action plans appraised its perceived impact on practice in lukewarm ways in interview. Although the impacts could be interpreted as being fairly profound, the intervention prompted some subtle changes, which were perceived to have stayed with staff, as in these comments:
No, I don’t think I’ve changed anything on the way I deal with things. I think it just makes you more aware [. . .] you know, if a situation arises, I think it just gives you a better insight into it, on how to deal with it.
Happy Haven, care staff 1
I don’t know how to explain it really. Something pops up in the back of your mind what you learnt and you do like tend to deal with it differently.
Lifelong Lodge, care staff
. . . it did work sometimes and it still works now sometimes but you just have to find what kind of mood they’re in that day and maybe that one won’t work today but you go and you try it tomorrow and it works fantastically.
Home Court, care staff
When probed, these champions could provide illustrative examples of the intervention in practice as follows:
Just the way you calm them down and things like that, like your tone of voice.
Lifelong Lodge, care staff
Just things like trying to go along with a resident when the resident’s sort of maybe going off on a tangent about their mum or dad or something . . . just to listen and agree [. . .] just things like that instead of constantly battling with them and trying to explain that they actually live here.
Home Court, care staff
Similarly, subtle examples were noted by the therapist when she had returned to homes. One example was the reduction in the number of falls experienced by one resident. Following training, staff had noticed that the resident had difficulty differentiating between a counterpane and the carpet. They changed to counterpane and there were no further falls at this location again.
Given the overwhelmingly positive feedback about the e-learning experience from care staff, it is surprising to find that the two managers interviewed in case study homes who did not attend training saw little value in what staff had learned:
. . . when I spoke to the staff, they said well they didn’t really get a lot out of it and it was kind of dismissed.
Careful Place, manager
And how does that compare to their reaction to other sorts of training or would you expect . . .?
Researcher
No they’d . . . you know, like they’ve just done an end-of-life course and dignity in the home and things like that and their reactions to those were quite positive.
Careful Place, manager
Only the manager at Happy Haven, who had attended training herself and thought that it had been useful for staff, gave any feedback on the perceived impact of the action plans at her home on levels of CB among people with dementia. Furthermore, the therapist’s notes mention that it had been fed back to her that the project action plans had received praise and been identified as being extremely good, in terms of both clarity and person-centred individuality, by the CQC team during their recent audit; the manager in this more recent interview evaluates them differently:
. . . I think it was more like the problem areas, if things was more sort of like . . . we learnt more about the person and sort of like what their conditions was [. . .] [therapist] gave a lot of insight into that which helped staff, rather than more of the individualised . . . we already had it really in the care plans.
Happy Haven, manager
. . . Because we’re not a home that have got a lot of challenging behaviour to other care homes but I saw it more as that we could help like the project develop [. . .] The staff did get something out of it but I think the project got more out of us than we did.
Happy Haven, manager
Again, this reflection that the intervention might have a greater impact on homes or individual residents with higher pre-existing levels of CB should be considered when looking at recommendations for wider implementation.
Interviews with care staff also revealed few existing mechanisms through which champions could share learning with colleagues within the home who had not attended training. In smaller, family-run care homes (Happy Haven), some wider dissemination could be achieved informally and through general face-to-face interactions and a staff comments folder. Staff in larger homes also mentioned being able to discuss and so to reflect with other staff who had attended training and were still employed at the home. However, the lack of formal support either within the home or from the research team to help champions to embed the intervention was seen as a weakness by the therapist:
I think again, what normally happens when something’s like an incremental sort of development thing, if somebody else comes along and shows you what they’re looking at, what type of things that they’re judging it on and then bit by bit you sort of can do that yourself and you learn to judge yourself don’t you and evaluate for yourself.
Therapist
The research team ran out of time to embed the intervention, as it was hard to address and overcome the initial barriers to delivery within the time scale of the CRT.
For care staff, the experience of becoming champions led to fresh insights, and better strategies, to improve the individualised care of residents exhibiting CB. Champions in smaller, less hierarchical homes could share these insights informally with other care staff.
However, overall, there was little feedback to managers who had not attended training about the use of systematically formulated case-specific approaches for managing dementia and CB and ways in which support could be delivered in the context of a given care home. Therefore, there was inadequate support from managers or the research team to further develop and embed a functional analysis approach to the management of residents with dementia and CB. Understanding of real compared with ideal conditions is required to enable staff to evaluate and adapt case-specific interventions for dementia and CB. These should also suit local strategies to facilitate reflexive monitoring and are summarised in Table 40.
| Conditions | Strategies to promote reflexive monitoring | |
|---|---|---|
| Real | Ideal | |
| Lack of systematic feedback on intervention impacts on levels of CB Lack of momentum and support for staff to share and embed learning and change practice |
Research team provides feedback of results to managers and care staff to allow them to reflect on and, if necessary, reconfigure implementation (functional analysis-based interventions), with support | To plan a development phase within the research process Run reflective sessions within homes to evaluate and modify implementation |
| Positive feedback from staff attending training not shared with managers | Managers informed of staff’s assessments of the e-learning | Questionnaire findings from those attending e-learning shared with managers |
Table 41 provides an overview of the outcomes of implementation in the four case study homes. From this and the other data, Figure 12 summarises what needs to be in place to support implementation in individual homes, as identified by NPT analysis.
| Care home pseudonym | |||
|---|---|---|---|
| Happy Haven | Careful Place | Home Court | Lifelong Lodge |
| ‘Good’ | ‘Average’ | ‘Average’ | ‘Challenging’ |
| Action plans ‘well received in the home’ Manager and champions keen to access the tool ‘in house’ and be involved in future research |
Manager feels that care staff did not feed back much about training | Engagement limited by ‘autocratic management style’ – staff wanting to continue with old practices | Manager feels that care staff did not feed back much about training Management style: ‘extremely autocratic’ Home not open to changing care practices provided Manager more focused on successfully meeting audited standards |
| Manager showed CQC team the care action plans during recent audit Both champions feel that they now deal with CB differently |
Staff more task orientated to ensure physical care needs are addressed to high standard | Both champions feel that they now deal with CB differently | Action plans produced were basic, but well received by champion Champion feels that they now deal with CB differently |
| One champion went on to become ‘small change agent’ | ‘More than web-based training required to change practice’ | Champion was subsequently successful in gaining promotion to oversee dementia care floor | ‘It is unlikely that web-based training or action-planning tools would make any impact at this time as basic systems warrant a complete overhaul’ |
Thematic findings from wider stakeholder interviews and groups
The transcripts of interviews with the stakeholder group further informed our findings on implementation, and supplemented the interviews with participants in the experimental and control arms of the trial. In this section, data derived from this second source of enquiry (i.e. the stakeholder group involving interviews with staff from experimental homes who received the intervention; those from control homes who did not receive the intervention, but were offered free access to the e-learning course if they wished; and a discussion group with stakeholders from across the country) are described. These stakeholder interviews and discussions gave rise to potentially generalisable themes, as a wide group of participants were involved (see Methods). The research questions we addressed with stakeholders were as follows.
-
Did the findings of the ResCare trial ‘ring true’ with others not involved in the study?
-
Were the enablers and facilitators found from the case study enquiry applicable to other areas of the country?
-
Were the identified problems and barriers applicable to other areas?
For the purposes of this chapter, overarching themes are presented and between-group differences are highlighted. Initials used for illustrative quotations of key findings are pseudonyms and do not reflect those of the participant.
Training and functional analysis: ‘ringing true’?
Overall, there was general agreement among stakeholders that much could be done to improve the quality of life of care home residents, especially people with dementia and CB. One experienced trainer and professional educator, whose work covers different parts of the UK, observed:
I think many care homes could improve their support for people who are distressed, confused, angry and expressing how they feel in a huge number of ways. I think the way they prepare staff. The way they help staff to even think about what’s happening for that person and what is their emotional experience like and how is that linked to the way that they would behave?
MD
Many of those interviewed had experience of training care home staff or were professionals who visited care home residents for assessment, provision of clinical care or treatment, or to offer staff advice about care practices. However, concern was expressed by some stakeholders that not all training was necessarily helpful. An experienced trainer and professional educator reflected on their experience:
I’ve seen lots of very poor examples, particularly from external professionals coming in who maybe have a general sense of, well, I know what the tasks that care assistant does in a day. But [they] seem to have no insight into where those people are coming from as non-professionals. They [care home workers] haven’t taken three years out to do training [like nurses or occupational therapists].
MJD
Mixed views emerged around the optimum venue for training. Some stakeholders were not surprised to hear of the ResCare trial findings that staff often preferred to undertake training outside their work environment. For example, one stakeholder drew on their broad experience of training (over 25 years) to argue that training needs to relate to the individual’s experience and yet should be challenging:
I would say they definitely prefer to be trained outside their place of work, usually with others doing the same work but in different locations/situations – so they can learn from others’ experiences. Without a shadow of a doubt the training that is most effective falls into two categories, the first is using actual real live case stories of those in need and illustrating how they were supported/helped by the [care home worker]. The second is using more challenging training that in effect places the learner in the same position as those they seek to support. I use exercises that have the effect of making the learner vulnerable and in need of support so they can really ‘feel’ what it is like to be in this position. These can only be used when the Trainer has achieved a strong rapport and very high level of trust within the group of trainees.
PK
This last point reflects the importance expressed by some other participants that a trainer has a key interpersonal role in addressing dynamics and different responses to the emotions that may surface during training. This was something that many commented on, and reflected trial participants’ positive views of the skills of the trainer who facilitated our e-learning course (see Chapter 2).
However, the view of one care home manager from the experimental arm of the ResCare trial was very different. They thought that training at an ‘out-of-home’ venue was often seen as a ‘jolly away from work’ for many of the staff and found that friends tended to go along together, and that their learning was therefore limited. This manager preferred training to be within the home itself. Similarly, an experienced trainer thought that staff might like to leave the care home for training, but that the benefits of this were more to convey to staff that they were valued:
It doesn’t surprise me at all. It’s a completely different change of context and yes, I suppose my experience would be much more about saying, we value our people as a member of staff and people feel that value. In terms of changing people’s skills, confidence, I’m not sure that it addresses that at all.
MJD
A service improver argued that the effects of training were hard to evidence, but that improving staff morale was valuable for care workers and did have a positive effect on practice. They added that in thinking about the wider dementia care services and training the workforce, it could be easier to arrange training for care home staff rather than home care staff ‘where it’s much harder to get people in same place at same time’ (SF).
In contrast, a care home worker (experimental home) who had undertaken the ResCare trial training away from the work location felt that it had been beneficial, for reasons that related to learning with colleagues:
. . . away day . . . helps you get away from everyday work stress . . . [had gone with a couple of friends/colleagues] . . . you have that support when you’re back in the care home to ask questions and like, you know.
CD
In their view, the training had been very good:
I liked the training; I learnt things I hadn’t known before. Maybe I would have learnt it on the job anyway, but it was good to have a day to do it.
CD
Similarly, a care home manager (experimental home) thought that the ‘out-of-home’ venue worked quite well; if staff were able to go away together, they would learn together, and could then ‘bounce ideas off one another’ on their return. Another care home worker said that they had enjoyed the training and the e-learning program as part of the ResCare trial – in their view the trainer was very enthusiastic and ‘taught very well’. They appreciated having a full day for this, but realised that it must have been difficult for the rest of the staff in the care home to cover their shift.
There was an alternative view that a full day was too long and did not reflect what we know about adult learning. One trainer interviewed thought that:
What works best in training is probably a basic outline plus simple case examples – short ongoing sessions comprising revision plus a new aspect each time.
RJ
This trainer suspected that concentration among care staff was often limited and that 15-minute-long at-work sessions were more suitable. They also noted that there seemed to be a much broader range of ability among ‘hands-on’ care staff than among managers, and concluded that opportunities for refresher and repeat sessions should be available for those who needed or wanted them.
Moreover, the value of formal training on its own was not universally accepted. One care worker, working in a control home in the ResCare trial, did not think that there was a need for training because, in their experience:
Just talking about patients with challenging behaviour at case meetings or in the staff room is good, as it helps us get a better understanding of that person . . . it was good to meet the team . . . I was given time to think about the residents when they asked questions and filled in their paperwork . . . just talking about the residents when they [research team] came each time gave me ideas . . .
EF
Although they were not able to comment on the training offered, they thought that discussion with someone with expertise would be more effective:
I’m sure that the training is good, but I can see why that [talking with a specialist dementia CB practitioner] would work.
EF
How this might work was outlined by a manager of a care home that had been part of the experimental arm of the ResCare trial. They described the study as ‘fitting in’ the home and had found that the trained champion was able to answer questions raised by other members of staff. Training without extra support afterwards was not generally seen to be a good investment, and one part of training that appeared to be strongly supported by some interview participants was the desirability of more senior staff (senior care workers or shift leaders) to model good practice ‘on the floor’:
It’s not only about training. For me, the best services are where you have, whoever the shift leader is, a good knowledge of the people and individuals concerned and the way they feel and how their illness or their degeneration of whatever it is affect the way they behave. They are the role model and they will guide that. They will suggest. They will encourage. They will question.
MD
However, a care home manager who expressed their belief in a ‘train the trainers’ model did not think a single champion who could be referred to for all questions was particularly helpful owing to high staff turnover, but that training someone who could regularly cascade this to all other staff in the home was more useful. In their view, sending all staff on training was not always possible, and they expected all staff to be competent in addressing some things like management of CB.
What seemed to emerge in these discussions was a nuanced view of training and learning. Training may make a difference in the short term, if it encourages more material discussion across the team about real incidents and events. It is here that it seemed to be seen as useful in terms of encouraging openness and discussion about the practice that actually happens in a care home. ‘Ringing true’ or a sense of authenticity about service improvement seemed to be a barometer by which the interventions were gauged.
Enablers and facilitators
One service improver commented that training should not have to be argued to be cost-effective, being confident that ‘the big facilitator is that training leads to improved staff morale and job satisfaction’. Similarly, one of the care home managers suggested that training providers would be well received by care homes if they were able to promise refresher training every year, which would incentivise care homes to invest in training. They added:
Most managers have now come to accept that one-off sessions are futile, and without the offer of second phase, when things are likely to have changed, managers see little point in training events.
MJA
Turning to the ResCare trial development of a championing role in each care home, one manager expressed surprise that having a specialist CB practitioner did not work, as they felt that it had been useful in practice. Unfortunately, they reported that most of the staff whom the practitioner had engaged with had subsequently left, being replaced by younger and more junior staff who found training of this sort harder to understand. This manager described how some of these new staff did not understand some of the basics of care home training (such as how to manage restraint and aggression, for example, through breakaway training), and that they themselves had to explain this to the staff.
In contrast, several reflected that some care home staff were not ‘empty vessels’, but with experience they had accumulated substantial skills that were not always acknowledged or respected. For example, a care assistant, working in a control home in the ResCare trial, felt that speaking to others about the topic was helpful as it made them realise how much they actually knew but had not been utilising or had not considered previously. In their view, more intensive training would have been better, as it was about an area that was of particular interest. Similarly, another care home worker had overall enjoyed the ResCare trial training as it had given them the confidence to do what they had known all along, but did not know if it was correct or not – it ‘gave a grounding’.
This contrasted with the view of a former regulator who noted that one enabler of promoting training was when a regulator can insist on staff being trained to do their job. In their experience:
Care homes get away with doing what is mandatory. Issues like dignity, nutrition, and so on, are seen as ‘add ons’ and there is reluctance to release staff for this. I have been commissioned to provide training for care home workers on completing care plans as they had been pulled up by the CQC. [Locally] I have tried engaging with care homes to attend free training and the response was zero. So . . . unless there is evidence of poor practice the regulator can insist only so much. The essential standards only talk about the ‘suitability of staff’ so if the care homes can prove they are meeting this standard by some means CQC will probably have to be satisfied.
ER
Similarly, a local authority manager spoke of the limits of regulation and inspection in prompting training. They pointed to the differences between their role and that of NHS commissioners:
As a purchaser – I use that word because we buy – we don’t actively commission residential care; by and large we are really just a major funder [of people’s fees] we do not currently require a suite of training. We obviously require that providers are registered with CQC and they in turn will check the training records. At the moment, that is as far as we go.
NP
This local authority manager envisaged some changes, as the local authority was working more closely with the local Clinical Commissioning Group to possibly develop a dementia staff competency framework, with validated training attached. In addition, there were aspirations that staff termed dementia care ‘mappers’ (having attended a course on dementia care mapping) might be able to identify specific training needs among a staff group. As a result, if there were severe problems, the contracts monitoring team in the local authority would be able to check if the care home’s staff had undertaken training and thus might be able to block new placements (those paid for by the local authority) until specific training had been undertaken. All this was at the planning stage and would represent considerable management investment, and possibly could be open to questions about infringements of choice.
The stakeholder interview data show that it is important to think about the interactions of content, methods, timing, location and venue of training in order to draw conclusions about its effects. There were several views that the sustainability of current training was limited, partly because of high staff turnover and reorganisation in care homes, but also because of changes in ownership and management. A trainer and professional educator commented:
I’ve worked with many teams who have lots of training – 3 day[s’] training or even more. I’m not sure that that’s reflected in what I see in their practice 6 months later, 12 months later and so, if it is a training that you go away to and you are taught almost in a classroom setting, then I’m not sure a year later that I see the results of that training any more.
MD
Care homes being commercial organisations in the main, even if not for profit, their managers often adopted an economic perspective in discussions of training investment. One care home manager, for example, noted the presence of other preoccupations for managers: ‘There are only so many hours in the day’. They were uncertain as to how care homes could be incentivised to participate in training and e-learning, thinking that this could be over-reliant on one person – ‘sometimes need a champion in the home, but this has to be the manager as they make decisions’. This manager noted that having a champion should help, but that high staff turnover made it hard for managers to decide on investing in a champion who might then decide to leave in a few months. Like many care home managers and other stakeholders, they described high staff turnover as a major problem: hence specific topic training took a lower priority as recruiting new staff, inducting them, and training them for basic care became more important.
In the context of these pressures, a service improver and researcher commented that smaller care homes seemed to benefit from the establishment of a care home or social care-focused organisation that could offer staff opportunities that they could not purchase or arrange by themselves. In the region where they had recently facilitated a learning set for care home managers, such an independent organisation had been financially supported by the local authority, which was able to recommend that some managers might find certain training ‘useful’ when concerns had been raised about their home’s performance.
Problems and barriers
This dissonance between professional expectations and the reality of care staff’s experience and prior skills was expressed as a barrier to receiving professional advice and adopting decision support tools for planning care. In this section we explore stakeholders’ views on care plans. Several commented that detailed care plans were not commonly found in care homes. One manager said:
They are not used ‘on the floor’ and the subtle nuances of how you respond to [a situation of CB]. You end up with things like, ‘please respond sensitively to X’s [resident’s] aggression’. What on earth does that mean? It’s about getting skilled people to role model that to show people; actually this [care planning] works.
MD
Furthermore, although there might be several professionals going in and out of a care home, it seemed to require taking a deliberate decision to respond to a situation where the level of distress was getting beyond the ability of a care home to cope. There seemed no agreement over who should take the lead to challenge, and to say ‘What’s going wrong?’. Part of the explanation offered by one trainer was the lack of a culture of teamworking in some homes:
I still work with teams who don’t meet. They never have a staff meeting. There is no time at all for more skilled perhaps senior staff to be asking those questions. What happened this week? How did it go? I still work with teams that never do that.
MJD
One of the care home managers interviewed ascribed a lack of interest in taking on further activity as a result of some care homes’ economic, and other, uncertainties. This feeling of being under siege arose from the general impression of several changes in the care home sector in recent years, with the recession and the Southern Cross ‘fiasco’ (closure/break-up of a chain of 750 care homes in England 2011, including some that were part of the present study). As a result of general austerity and reductions in local authority expenditure, managers were managing budgets more carefully, and the first expense line to go could be training: ‘Some feel there is no use for it, or not much use, shall I say’. Another care home manager in the ResCare trial control group, although expressing disappointment that they did not have more training, or had not been offered the e-learning provided to the experimental group, considered that ‘we wouldn’t have had time for that anyway [laughs]!’ At an extreme, although voiced by more than one stakeholder, was the view that some care homes were not engaged with policies and strategies about training.
Further barriers raised by a minority of stakeholders were connected with suspicions that e-learning might be adopted as a cost-saving measure. One trainer thought that employers might start to say:
Can you do this in your own time? You all have access to the internet now at home and it will be good for you and your career if you can say you have completed these modules and so some staff will and some staff won’t.
MD
There were other views, that, although it might be theoretically possible to do e-learning at work, shifts were often short staffed and for some staff the ability to switch from care work to computer study would be ‘mentally difficult’, even if they were not interrupted. Others had observed almost a feeling of ‘distrust across some organisations or an anxiety about staff not being on the shop floor’, which would mean that sitting by a computer could be seen as avoiding work or the ‘difficult’ residents.
For one former regulator, its impression was that, in the current state of the public sector economic climate, care homes would do only what was mandatory and had been identified as a requirement by the CQC:
Unfortunately any non-mandatory training is seen as a luxury and in the current financial climate is not something they would do willingly.
ER
Others too were pessimistic about regulation’s potential to improve training uptake:
By chance I’ve studied a few compliance [CQC] reports in the last month. Very, very little mention of training, if the word training in there at all. Even where I would perhaps have identified a training need in relation to an issue that was described. That’s not my experience; I haven’t seen a lot emphasis on that.
DM
However, this did not reflect the stakeholders’ realisation of care homes’ changing customer base, with more residents having high levels of disability. One care home manager felt that ‘selling’ training to junior staff was hard because many initially thought that all people with dementia were ‘nice little grannies just watching TV’. Initially, new staff rarely expected to encounter CB, but they quickly realised it was common. In this manager’s experience this ‘frightens a lot of the younger ones’. Just to get staff ‘to read the care plan’ gave them some confidence that the problem was not their fault. They felt that more discussions and demonstrations, as well as training around management of CB, could do nothing but good. One care home worker from the control group of the ResCare trial said that she had liked having someone:
. . . professional and experienced in challenging behaviour to talk to – it made me appreciate how difficult some of this is.
IJ
The emotional impact of being ‘on the floor’ and being expected to manage CB was communicated by several stakeholders. One added that having someone to talk to made them confident to approach other senior members of staff with any later queries.
Resources and implementation
There were several reasons why the offer of free e-learning licences to the care homes following the ResCare trial was not seized on (see Chapter 2). One care home manager thought that the free licences were not taken up because many younger staff members were being protective of their work–life balance – ‘few are willing to stay late, even if overtime is paid’. Another, in the control group of the ResCare trial, found that taking part in the study had been valuable – ‘everyone is talking about challenging behaviour right now’ – but had mainly found it ‘helpful to have someone [the data collection research team] to talk to about the residents’. This manager thought that free licences, which could enable staff to do the training in their own time, should work, and confessed surprise that they had not been taken up. It was the personal expert approach that seemed to have left a lasting legacy.
One of the care home workers interviewed thought that refresher training would be necessary, ‘as things change so often’. Their expectations for this were not high. By refresher training they meant having someone to remind them about what they had learnt, perhaps to talk to on an annual basis, even over the telephone or during a visit to the care home, rather than having to go along to another day of training ‘like the first aid courses you do’. However, this person seemed little engaged with professional development and could not offer a view about the free licences, other than speculating ‘maybe the manager had other priorities, or didn’t realise it was free or something’. This lack of knowledge was expressed by other care home workers, illustrating the point to be made below that training decisions were largely the business of managers and that front-line care staff felt that they had no role in such decisions and plans. Placing this in context, a trainer thought that many care homes and care home staff were used to abiding by statutory minimum training requirements and added that taking up training in the form of an internet package is ‘not the way they are used to working. It is unusual and it would be new and different’. They further noted that managers would have to free staff from their work and would not necessarily be able to map what staff were doing, a point already discussed above when reflecting on the surveillance of staff in care homes and the limits of trust in the home.
Making decisions
In this section, we report one of the underpinning themes around innovation in care home practice. Who makes decisions about investment and staff time proved to be a helpful diagnostic aid when thinking about system change, which is in effect what the ResCare trial was investigating. Training, for example, was seen by almost all stakeholders as a subject that the manager made decisions about. This was because it necessitated authorisation of expenditure, it affected the resource of staff time, and it was an area where there was some discretion in choosing to go beyond minimum requirements or not. Both managers and their staff agreed that the manager ‘managed’ training. They were described as sometimes shopping around, for example for a ‘knowledgeable good trainer, with appropriate credentials’. However, one care home manager prided themself on being a prudent purchaser, noting, for example, their view that the language used in training needs to be in ‘layman’s’ terms, as specialist knowledge tended to go ‘over the heads’ of younger, less experienced staff. In the experience of a former regulator, managers often ‘shopped locally’ for training as it was cheaper.
There were very few examples of care home staff feeling that they could influence training purchasing. One care home worker in the control group commented that they would have liked more training, but ‘X [name of manager] is in charge, I do what s/he says’. For some of the care homes that were owned by large corporate providers, their managers’ discretion about purchasing training was minimal. One of these, a manager of a care home in the experimental arm of the ResCare trial, commented that in their care home’s company, the national training manager decided whether or not specific training was required across all homes, and then ‘every home has to have it’. This manager expressed surprise that free licences from ResCare were not taken up, but seemed to have passed on responsibility for training to head office: ‘I leave that to the training department’.
Those who were familiar with the world of the corporate care home described how there was little discretion over the training that homes provided for staff. The exception to this was when something had to be commissioned quickly as a response to some failing or perceived problem. Such prompts could emerge from various directions.
The economy of care home systems was often used by stakeholders to explain why care homes operated as they did. For some homes, aspirations to develop training could evaporate if there was no money left in the annual training budget. However, as commented by an experienced trainer interviewed, training could also be part of the marketing budget, or viewed as such:
There is certainly more of (that) – we are marketing ourselves as having this specialism and this specific expertise and therefore everybody will be trained in that . . . That is the way we will sell ourselves.
MD
There was a mix of views expressed about the potential for training during times when care homes were preoccupied with other changes. Generally, it was agreed that during substantial changes (such as change of management or ownership), there would be turmoil and worry about its possible impact. However, changed ownership, management or a new manager was seen to be likely to want to introduce new things to establish their authority. One regulator interviewed commented:
New face – new initiatives are more acceptable than old face – new initiatives – if you know what I mean.
ER
A trainer reported being brought on board at the time of a company takeover of a care home when the new managers were keen to ‘brand’ their new acquisition:
I’ve come across services which will say, we bought up that service and I’ve visited those services with corporate managers and they have said, we are now putting them on board with our dementia methodology whatever it is. Therefore, the staff are now beginning that route of training, so we will start with the managers and then . . . it’s bringing them on board once that company has been subsumed.
MJD
Discussion
The ResCare trial intervention aimed to change ways of thinking and acting in relation to CB and dementia in care homes, rather than to introduce any materially new organisational systems or technologies. The strength of this comprehensive process evaluation, which was embedded within the CRT (see Chapter 3), is its value in enabling us to gain a deeper understanding of the context in which the interventions were used, and to identify ways in which they may be implemented within routine practice in the longer term. 189 Thus, we were able to identify serious limiting factors to implementation and facilitators that can inform practice in care homes. The additional findings from wider stakeholder interviews and discussions allowed us to confirm or explain many of the main ResCare trial findings and the in-depth case study reflections.
First, we note that despite limitations to delivery, some intervention-related changes were found to become embedded in practice, in the ways in which care staff understood, responded to and reflected on such CBs. This finding can be interpreted in two ways. On the one hand, despite the problems noted, some, but not all, aspects of training had translated into everyday thinking and practices. On the other hand, these changes were subtle. They were therefore at risk of being underestimated and undifferentiated in the care home environment, as they could be easily absorbed within common sense ideas of ‘getting to know residents better’ or within pre-existing systems for individualised care planning at some homes. This was not our intention when developing the training and intervention approach. Our aim was to assist staff to focus on a more systematic questioning approach to understand the potential causes of the person’s behaviour, and to thus learn to respond to need in the person with dementia and CB. Based on this functional analysis approach to understanding unmet need in a resident, they could then access timely help from the NHS if necessary. It may be that staff in our sample had similar challenges to those in the USA with respect to finding mental health support for a resident,208 although, at the time of this study, they had access to the specialist dementia care therapist who was working with champions to deliver action plans. More probable is the fact that the dosage or intensity of the intervention as a whole was not enough to change staff behaviour and their responses to resident distress. Additionally, as noted previously (see process notes in Chapter 2, Booster visits: feedback from the specialist dementia care therapist and Chapter 3, Discussion), others, such as the resident’s GP, may not have responded positively to their requests for assistance, thus undermining the efficacy of care staff as change agents, in the timely management of dementia and CB in care homes.
Second, we found that the most important person identified for successful implementation was the home manager. Thus, key to organisational readiness209 for implementation of a new intervention are the qualities and capabilities of the home manager, in leading and supporting others in the home. Managers having an open attitude and a willingness to engage in research and service improvement were found to be key contributors to successful implementation. Managerial support was also highlighted as important for the delivery of interventions in care home settings in a successful intervention in Australia210 and in an implementation study of the FITS care home intervention in the UK. 84 Staff perceptions of empowerment within their work environment can exert considerable influence on the delivery of individualised care,172 which is also a prerequisite for case-specific intervention in dementia and CB. Disappointingly in our case studies, we found examples of managers who were unable to value their ‘staff champions’ by providing encouragement to use the resources, such as the technology and training opportunities we provided. This rang true for the wider stakeholder group, which also pinpointed a tension whereby some managers felt that they needed to observe staff activity to ensure that work is being done, with the feeling among staff that they cannot be trusted, or that their own work and experience are insufficiently valued.
Low commitment to training as a priority for staff development may also be attributable to a sense of a transient workforce. One report found that 31% of care workers leave in their first year. 211 Moreover, high staff turnover was seen as a significant barrier to sustainability of our specialist NHS innovation by our stakeholder group, as, in reality, much energy was directed at recruitment, induction and training in basic care activity. Organisational commitment has been shown to be an important predictor in staff turnover,212 relevant also to the complex systems operating in the care home setting. 213 If, for example, as a consequence of turnover in the workforce, new senior staff employed are unaware that there is a training and intervention methodology available to support residents with dementia and CB, they too will be unable to sustain delivery of interventions to meet the changing needs of residents not because they are disinclined, but because they ‘don’t know what they don’t know’.
Relevant to this is our earlier observation, that our intervention may have become embedded into ‘common sense ideas and practice’, in which staff champions had not fully appreciated the importance of searching for the potential cause or causes of the person’s varying communication of distress due to unmet need. This was our planned mechanism for change in staff behaviour, as our training was designed to engender a more skilled approach involving empathic curiosity214,215 and a ‘detective approach’ to discovering unmet need in the resident who met the diagnostic criteria for both dementia and CB. Indeed, even for existing staff, although intervention champions could identify ways in which functional analysis in part may have become embedded in their own practice, there was little evidence to suggest that this had transferred to other staff in case study homes. This can be explained by different perspectives from stakeholders. They point to the emotional labour of working ‘on the floor’ with people who are distressed and how training and coaching may be useful in providing staff with confidence, resilience and the skill to recognise that they too need support. They highlighted the tendency for members of staff to feel excluded from discussions about training and their lack of involvement in any self-assessment of their own needs, those of the team or shift, or the home generally. This is akin to the phenomenon of working in teams but not as a team,216 in which there may be a disconnect between structure and function of the workers.
Team climate in not-for-profit homes has been shown to moderate staff well-being,217 and recent reports have pointed to the inter-relationship between the well-being of patients and residents and the well-being of care and nursing staff. 218 Moreover, positive staff experiences of their managers’ leadership are critical to workforce retention,219 thus demonstrating that the care industry may be undermined by a vicious circle where managers are overwhelmed with dealing with high turnover and staff cannot be retained because of the limited encouragements made by their managers. Consistent with the international literature, our study also demonstrates that leadership in care homes remains key to the sustainability of service improvements in care homes,219–221 with additional benefits to stabilisation in the workforce. 222 Also consistent with the literature,223 supervision from a specialist was seen as important, but in this case care is needed to avoid the negative effects on staff of reduced supervision and support from their own managers in the home. 224 Organisational investment in improving leadership and management skills is therefore recommended for staff productivity, stability and good resident outcomes. 225
Third, home size is a factor to consider, as, unlike a study carried out over a decade ago in the USA,226 implementation of this intervention was easier to achieve in smaller homes and in those with less hierarchical structures or inflexible managerial styles. To effect change in larger homes in particular, stakeholders noted that this would require a more significant critical mass of senior staff to be trained as champions and then to be made available in sufficient numbers across shifts. This would necessitate a major investment in training for the dementia care home workforce, as levels of training are low overall160 and, as noted previously, high turnover in the industry could dilute such investment, particularly within a context of no clear infrastructure for career development or pay benefits. The potential for large care homes to provide greater autonomy by having small units within them may be one way to encourage more personalised care and support for residents, but this is beyond the scope of this present study. As noted previously, it would also be necessary to address matters of manager support and leadership in the wider organisation.
Fourth, our typology identified homes where the culture of training seemed to be enabling or disabling. The picture here was mixed, in respect of leadership and training culture, as, even across the homes that had a change in ownership and/or management during the study, some coped better than others in leading the research and also in implementing the intervention. This could be in spite or because of, such changes in leadership, and stakeholders agreed that opportunities could arise from adversity. The typology of the organisational ‘training cultures’ for care homes provided a useful overview of, and entry point into, the implementation process and outcomes across intervention homes. This typology highlighted the importance of knowledge of home ownership as particularly important in building IT capacity for the intervention and in commissioning training. Likewise, the typology illustrated which intervention homes had an enabling or disabling environment with respect to research or intervention and management or leadership. These might be related to other pressures or the need to improve quality.
It was no surprise to stakeholders that the implementation of the intervention was inconsistent across care homes, because it proved to be difficult to build a collective understanding of, and thus investment in, the intervention and research process. This was exemplified by the difficulties encountered in upgrading IT/broadband to the standard required to run the intervention within homes. This may be a transitory phenomenon, as there is likely to be further investment in the sector as IT becomes much more common in private and public spheres. Sharing emerging research outcome findings with care homes during the implementation would have biased the study. However, because of the lack of time and resources to fully implement the intervention, there were relatively few observable benefits and very little reflexive monitoring within care homes themselves. The lack of perceived benefit is a predictable barrier to implementation. 193,227
Some care homes proved keen to access CB dementia training for staff, and could be further persuaded of the possibility of improving personalised care through e-learning. This implementation study, however, underlines the importance of achieving better consensus about the potential benefits of functional analysis for addressing different levels of CB, as some staff may not see the use of psychosocial interventions for dementia and CB as part of their job. 228 Not only those working in care homes, but those who are ‘in and out of their doors’ such as medical practitioners, district (community) nurses, mental health practitioners and social workers, would have to display collective willingness and openness to culture change, if they are to persuade those at the frontline of care that residents may benefit from this type of biopsychosocial intervention. This may be because challenges exist in sustaining care innovations because of differing perspectives of what constitutes good care. 229 This can only be further exacerbated as people with dementia and CB represent a more complex group than those who have dementia but do not present with CB, or those without dementia living in care homes. People with dementia with CB often require skilled, case-specific interventions that are more than just ‘good care’, when support to meet their needs may need to draw help from a range of professional disciplines. Senior care home staff therefore need to remain actively engaged in improving their knowledge, skills and confidence230 to allow them to know when to access help from others in the care system, before unmet need in residents escalates to the point of causing harm or reaching a level of perceived crisis.
Overall, it was difficult to maintain individual and collective coherence and investment over the relatively short time frame of the research, especially given the dynamic nature of this sector. Implementation changes made by the research team, such as delivering training off-site with the support of a trained therapist, were largely successful. However, implementation was inconsistent, largely because resources required to ‘enact’ the intervention were underestimated and because managers under pressure struggled to release senior staff, who were seen as best placed to act as champions, to complete training and undertake care action-planning. This was noted even where homes accepted the offer of backfill costs to release staff for training, when some staff continued to worry about the increased overload on their colleagues, suggesting that, even when free training is on offer, with backfill costs covered, the quality of care provided by other staff, sometimes sourced from an agency, appears to remain a concern for some care workers. Within implementation in case study homes, more was achieved where particular staff made personal commitments and sacrifices to undertake the extra communication work required to support collective coherence.
The data from the stakeholder interviews confirmed that the heterogeneity of care homes and ‘one size’ training is not likely to flourish. The data suggested that approaches to training need to consider factors beyond the training’s content, venue, format and timing. Care homes were portrayed as places that change in some respects, but also have great continuity. As the stakeholders and case study homes described, ‘customers’ were changing, staff moved and could be replaced by staff with different experiences and expectations, the economy of care homes was changing and investments were being scrutinised for their profits. However, many care home staff were seen, in this changing landscape, as being committed to their jobs where they lived and worked within their local communities. Additionally, their employers were regarded as perhaps being less often subject to the vagaries of reorganisation than other employers in society at large. Finally, managers were described as exercising some degree of control over a given home, to the extent that some were described as ‘autocratic’.
Limitations to this evaluation
This process evaluation had its limitations. First, the retrospective nature of this exercise meant that there may have been some inaccurate recall by participants over time. Second, although the sample of case study homes was diverse, implementation barriers may have been missed, as staff members who did not attend the training as champions and who would have views about whether learning was shared or cascaded through care homes were not interviewed in large numbers. Third, the sample of stakeholders interviewed may have been tempted to overgeneralise, or to report their own opinions about training and care homes.
Conclusions
Overall, our combined data from the case studies and stakeholder consultations, which were examined in the light of the literature, suggest that the ResCare trial findings appear to be transferable across other settings in England. Although care homes had been initially keen to access training and intervention for the management of dementia with CB, and the potentially unique benefits of a care home-wide functional analysis-based approach to CB were evident to many stakeholders, these benefits were not sufficiently evident to participating care homes. It may be that many homes were not ready for the higher level of systematic work that is required for the management of dementia and clinically significant CB in care homes.
Three key factors are identified for successful implementation. These factors relate particularly to leadership and management, home size and staff turnover, and continued availability and timely use of support and supervision from external professionals. The first two factors appear to reflect potential indicators of organisational readiness209 and commitment. Our study was close to the real world of care homes and pre-dated National Institute for Health Research (NIHR) initiatives, such as Enabling Research In Care Homes (URL: www.enrich.nihr.ac.uk/). Such initiatives may help to develop the capacity of managers over time by fostering positive engagement between them and researchers within the care home setting. However, in applied research such as this, where the aspiration is for implementation in the real world of the variety of contexts of care settings, these ‘research-rich innovations’ may not provide the environment for a full understanding of what is needed for implementation. Moreover, research teams are not always the same, and our findings suggest that staff and managers can lose track with researchers, such as those involved in data collection and those who may be delivering an intervention. Improved communication to enhance a shared understanding between researchers, managers and staff is key to the success of studies such as this. However, as we demonstrate, this is not always easy to achieve given the pressures of lack of time experienced by some managers and care staff.
In the short term, NHS-led training and support initiatives in care homes have a greater likelihood of sustainability in smaller homes, where risks of poor coherence and turnover may be reduced, particularly if leadership is relatively strong or leadership support is also offered. However, systems to monitor the effects of the intervention on the frequency and levels of CB among residents would need to be developed for homes, to ascertain any benefits of taking up the new practice.
Finally, implementing an e-learning program and decision support e-tool by itself, without the support of trainers and the professional support networks from primary and secondary care, was seen to be unlikely to achieve relevant organisational change within homes. This was confirmed by the lack of up-to-date knowledge about the current training requirements within the care home sector among almost all who were interviewed, apart from care home managers and care home trainers with relevant expertise. The publication of Dementia Core Skills Education and Training Framework in 2015,231 subsequent to our research, may have some influence, but this remains to be evaluated. Plans to change the technical regulations that control qualifications in social care (the new Regulated Qualification Framework) may already overtake this development as these will enable care providers to choose a variety of training awards and tailor them to their needs. Again, these changes will need to be evaluated. Our present research suggests that there has been little sustained engagement of NHS professionals within care homes. New recommendations,232,233 that those interested in a nursing career should gain a year’s work experience in a care home may go some way to improve mutual interprofessional and intersectoral learning about what is needed to change current approaches to managing dementia with CB in care homes, but this is not a solution to more widespread problems.
Chapter 5 Challenge FamCare: a naturalistic study of people with dementia and challenging behaviour living at home and their carers
Abstract
Aim
To describe the characteristics and resource use over 6 months of a cohort of people with dementia and CB living at home, and their carers, referred to specialist NHS services across England; and to elicit stakeholder views on CB service provision and the findings from the cohort study.
Design and methods
Cohort study of people with dementia referred for CB to six NHS mental health organisations. Participants were people who met the diagnostic criteria for dementia and CB and their carers (dyads). The primary outcome measure was the RMBPC at baseline, 2 and 6 months; and the extent and cost of formal and informal care – using an adapted CSRI and NHS records of contacts with specialist mental health practitioners. Secondary measures included quality of life for the person with dementia and the family carer; and distress, guilt, mood and coping (sense of competence) in the family carer. Stakeholders debated emerging findings.
Results
Over 15 months we recruited 157 dyads (154 included family carers), in which 61% of people with dementia had mild dementia with clinically significant CB; we followed up 126 and 117 dyads at 2 and 6 months, respectively. Dyads received an average of nine contacts from mental health practitioners over 6 months, but there was little overall change in levels of CB. Increased contact with practitioners significantly reduced levels of guilt (p = 0.016) among carers. There was significant variation in trends for CB among dyads, but no stable clusters of those who improved, remained the same or deteriorated over time were identified. Family carers estimated that they devoted a mean of 112 hours a week to providing care at baseline, rising, though not significantly, to 129 hours at 6 months. They contributed over 80% of the total estimated cost of care. Stakeholder consultations revealed concerns about the equity of access to CB services for these carers.
Conclusions
People living at home with mild dementia can present with clinically significant CB. CB fluctuates for some, even over a short 6-month period. Families require trained practitioners, irrespective of where dementia service pathways are located, to systematically assess their varied needs and provide timely patient-specific interventions. Commissioning practice should reconsider the priority given to specialist assistance for families experiencing CB.
Trial registration
The ISRCTN is 58876649.
Introduction
Two-thirds of people with dementia in the UK live in the community in their own homes, supported by family and friends. One study notes that informal care accounts for £12B, that is, over half of the annual £23B cost of dementia to the UK economy,234 although a more recent study, published in 2014, estimates the current annual spend as £26B, with families shouldering two-thirds (£17.6B) of this themselves, either in unpaid care (£11.6M) or through paying for care. 235 The costs of BPSD in family care settings in other countries such as North America and Israel have been estimated,21,236,237 but these are not known in the UK: the assumption is that, by taking action to address the causes, there will be a reduction in carer stress, thus reducing carers’ reliance on services and delaying breakdown of care at home (p. 7). 238
Addressing the complex causal mix of multiple aetiologies is not straightforward and the elusiveness of the syndrome is outlined in Chapter 1. The important contribution of ‘context’ in the management of CB, such as the way others in the environment respond to BPSD, is emphasised by the term ‘behaviour that challenges’ (p. 210),12 and also calls for a reconceptualisation of BPSD to take account of its impact on the family carer. 239 The Cochrane review on the management of CB in family care settings, which was conducted as part of the present programme (see Chapter 1), also notes the importance of the family context, as 85% of effective interventions incorporated an element of psychological support for the carer. 82
There is a growing empirical rationale for better understanding of the relationship between family context and CB. For example, studies note that frequent BPSD are not necessarily the most challenging for family carers;47 the carer’s own characteristics, such as their coping strategies, independent of dementia severity or other patient factors, can contribute to aggressiveness51 and other BPSD;240 carer responses to BPSD are likely to be influenced by social and cultural setting, and may vary;241 and how the carer accepts their situation and manages dementia-related problems can influence the course of BPSD. 52 This may be why, even when families receive professional support, two-thirds indicate an unmet need associated with how to deal with BPSD. 85 A second systematic review, conducted as part of the present programme, used a meta-ethnographic synthesis to understand why the impact of BPSD in family settings varied from carer to carer. 86 This noted that some reasons for variation in family responses to BPSD were their sense of a ‘declining relationship’; or misunderstandings about their relative’s behaviour, which were perceived as transgressions against social norms, or underlying beliefs that dementia would inevitably undermine the humanity of their relative.
Rationale for and background to the present study
What is not clear from the literature outlined above is the extent of the variation of carer characteristics and their reports of BPSD. 242 This is an important clinical question given our definition of CB in dementia (see Chapter 1) that takes into account distress in both the person with dementia and/or the carer to provide interventions for the management of dementia and CB (see Chapter 2). What is also unknown is whether or not current health- and social-care support, provided early on in the development of CB, that is, while the person with dementia is living at home and the family has been referred to specialist services for mental health support for the management of dementia and CB, makes any difference over time to family carers.
The present longitudinal study was conducted in specialist NHS mental health organisations across England, where the delivery of specialist services for people with dementia and CB was co-ordinated by CMHNs working within specialist multidisciplinary CMHTsOP. During the course of this study, changes within some of the organisations resulted in extending this remit to other professionals, such as occupational therapists, working in these CMHTsOP (see Appendix 2). In this chapter, we describe professionals who deliver specialist NHS mental health support as specialist mental health ‘practitioners’. Additionally, the terms ‘carer’ and ‘family carer’ are used interchangeably in place of ‘informal carer’, as only three participant dyads did not include family members.
This study explores, in detail, the noted variation in carer responses to behaviour in the person with dementia. We examine whether or not it is possible to determine subgroups of carers who might improve, remain stable or worsen in terms of their stress, coping and quality of life. We also examine the impact of the support delivered by specialist mental health practitioners; and the extent of health- and social-care service support over time.
Finally, we examine stakeholder views about CB service provision and the findings from the cohort study.
Changes to protocol
Mental health practitioners were trained to use functional analysis-orientated interventions for CB in people living at home with their family, with a decision support system (see Chapter 2). Appendix 2 outlines detailed changes to the FamCare trial protocol, from a planned CRT to a controlled feasibility trial and then to the present observational naturalistic cohort study.
This was because recruitment to the study across all NHS organisations was slow and we could not therefore proceed with an intervention study. Prior to the study, all organisations had provided estimates of participant recruitment potential based on their recorded figures for performance of the CMHTOP in previous years. Reasons for slow recruitment were as follows.
-
The mental health NHS organisations were in the final preparatory year for the PbR initiative121 and all practitioners were engaged for the first time in using online electronic systems for the assessment and clustering of ‘need’243 for every new patient. Many community mental health services for older people were undergoing redesign,244 in a context of widespread uncertainty,245,246 which could be bewildering and disruptive for some,247,248 whereas others continued to try and make sense of this in their practice. 249
-
Triggered by the National Dementia Strategy of 2009250 was the growing national move towards dementia-specific services, with early diagnosis of dementia carried out in memory clinics or Memory Assessment Services (MASs). During this study period, some NHS organisations delivered all mental health services to older people within the CMHTsOP, others had memory clinics for diagnosis and management of early dementia, with the CMHTsOP providing support to older people with dementia later in the pathway, and yet others were redesigning services to develop either MAS provision within their CMHTOP or new memory clinics. This may have affected thresholds for acceptance as a case by the CMHTOP. At the start of recruitment in August 2010, continuing for an average of 31 weeks (SD 6.00 weeks; range 14–46 weeks), 33 CMHTsOP across seven NHS organisations received 5360 new referrals (see Appendix 3, Table 66), of which only 452 (8.4%) patients were potentially eligible for recruitment to this study of dementia and CB in family settings (see Appendix 3, Table 66). About one-quarter (25.8%) of new cases, that is, 1385 people referred for specialist mental health care, were not accepted by the CMHTOP (see Appendix 3, Table 67). They could therefore not be approached by the research team, as to be eligible for the study the referral had to be accepted by the CMHTsOP. The criterion of recruitment through CMHTsOP was extended to include redesigned CMHTOP services, in which new MASs and memory clinics were emerging in some NHS trusts. Consequently, 16.6% of the FamCare study cohort was recruited from MASs and memory clinics.
-
In 2011/12 the contract for delivering services in one NHS trust was in the process of transfer to another NHS organisation; the reorganisation of roles and responsibilities in this NHS trust resulted in withdrawal of all five of their CMHTsOP from the FamCare study. Although not directly affecting this study, but in order to provide context for this organisation with its five CMHTsOP, we further observed that recommissioning of services for the locality covering this NHS trust occurred for a second time in 2014 with transfer to yet another NHS organisation in 2015.
Research questions
The main aims of this study were to investigate the following questions in respect of people with dementia and CB, living at home, supported by a carer:
-
Do levels of reported CB, and carer reaction to this, change over time as measured by the frequency and reaction domains of the RMBPC?111
-
Does the level of support offered by usual care, determined by the number of specialist mental health care service contacts and time spent with the family, influence CB, family coping and/or quality of life of people with dementia and their family carer?
-
What are the predictors of change in CB, measured by the frequency and reaction domains of the RMBPC and the NPI total and distress domains?
-
What are the patterns of health- and social-care service use and associated costs?
-
What are the estimated costs of informal family care?
-
What are the patterns and costs of prescribing medications?
-
What are the total costs of services, informal care and medication?
An additional aim was to elicit the views of stakeholders in relation to CB service provision in dementia care and the findings from the cohort study.
Methods
Design
A cohort of people with dementia and CB and their carers who were referred to 28 CMHTsOP in six NHS organisations was followed up over a 6-month period. The information collected focused specifically on CB in family care settings, the stress experienced by family carers, the quality of life of people with dementia and their carers and the range, frequency and cost of health- and social-care services (including prescriptions) accessed by participants recruited to the study.
A participatory design development process used consultations with practitioners and managers across the NHS organisations. At the end of data collection, emerging findings were discussed in groups at two further consultation meetings with a wider group of stakeholders. These occurred in July 2013 in two cities in northern England.
Governance and study approvals
Ethics permissions for the protocol and subsequent changes were applied for and granted by the York REC (REC reference number 09/H1311/28), and later Leeds West REC when York ceased to exist, between March 2009 and October 2013 (see Appendix 12).
Confirmation that Humber NHS Foundation Trust would sponsor the trial was provided in April 2009 by the trust’s R&D department. Site-specific approvals for the participating NHS sites were also obtained from all seven of the original participating NHS organisations’ R&D departments, before commencing local recruitment.
For a description of the management arrangements for this study, see Appendix 5.
Study population
Six community mental health NHS organisations in England were involved in the cohort study: Greater Manchester West, Grimsby, Humber, North East London, Oxford and Buckinghamshire, and Sheffield. Of these, Grimsby was a specialist mental health social enterprise and the rest were specialist community mental health NHS trusts. One NHS trust, consisting of five CMHTsOP, withdrew prior to data collection for this study because of the transfer of its contract for services to another NHS trust. However, this NHS trust provided data prior to its withdrawal to inform our review of all new referrals to CMHTsOP (see Appendix 3) and for our iterative consultations of practitioners and managers (see Design). The CMHTsOP were considered to be broadly representative of the national picture; a mixture of rural and city locations, spread geographically across the country and from a mix of affluent and deprived areas. Researchers undertaking data collection were based in NHS mental health services. Within all study sites, the researchers visited each of the CMHTsOP to introduce the study, explain the eligibility criteria, and to collaborate with practitioners to apply these criteria for study recruitment. Recruitment commenced in August 2010 and ended in October 2011, with follow-up data collection continuing until July 2012.
Eligibility criteria
The criteria applied at the point of recruitment are detailed below.
Inclusion criteria
-
Had been reported with dementia.
-
Lived at home.
-
Had a family (or friend) carer.
-
Fulfilled the diagnostic criteria for dementia using the DSM-IV. 130
-
Fulfilled the behavioural criteria for CB, that is, an incidence score of at least five, from a maximum of 24 on the RMBPC. 111 The index for clinically significant CB, set at five on this measure, was based on our longstanding routine use of this measure in clinical practice, where, at the first point of contact, families presented as significantly upset when they reported at least five items on this measure as having occurred one or two times or more in the past week.
Exclusion criteria
-
Living in residential care at the time of recruitment.
-
Being in the palliative stage of disease at the time of recruitment.
-
Not being able to speak or understand English.
Sample size
Each of the six NHS organisations expected to recruit at least 30 dyads and, therefore, this convenience sample had a target of 180 dyads (person with dementia and their carer). Evidence from a previous relevant study65 suggested that approximately 40% of participants with dementia living at home may be lost to follow-up over a period of 6 months because of death, movement to other accommodation or care settings, or illness being too severe to allow for completion of follow-up assessments. If this loss was evenly spread, then 13% of recruited participants, that is, 24 out of the 180 dyads, would be lost by 2 months and 72 by 6 months, leaving a total sample of 108 by the end of the study.
Recruitment procedures
Community mental health teams for older people were asked to approach every new patient referred to them who potentially met the inclusion criteria. Each new patient notified to the research team was allocated a study identification number, whether or not they consented to take part in the FamCare study, or subsequently met the inclusion criteria. This enabled documentation of the flow of participants through each of the stages, as specified in the CONSORT statement. 251
Where agreed with team leaders, a member of the CMHTOP contacted the patient or carer who had been referred to them to explain the study and to ask permission to pass their contact details to the research team. When permission was given, a member of the research team then arranged to visit the person with dementia and their carer to explain the study further, provide information leaflets if not received already, and obtain written consent.
Informed consent
The MRC’s recommended ‘cluster representation mechanism’ (CRM) was adopted for this study. A CRM is an individual, body or mechanism that represents the interests of a cluster, a gatekeeper. In this study, the clusters were the mental health teams and so the team leaders for each were deemed to be the gatekeeper. This accords with the MRC’s guidance that the CRM must be independent of the research team so that they can act in good faith in the interests of the cluster. They had the right to exclude or withdraw the cluster from the trial at any time and to exclude or withdraw individual patients. Patients and family carers who met the inclusion criteria, and were referred to those teams, were approached for consent to be included in the study.
Participants had at least 24 hours to consider taking part in the study. In line with the British Psychological Society’s guidance on the Mental Capacity Act of 2015,134 we assumed that each person had the capacity to consent, and explained the research in an accessible manner, increasing their likelihood of being able to consent for themselves. For each dyad, separate informed consent was sought from the person with dementia and their carer. If the person with dementia was judged to be unable to provide informed consent, then the carer was asked to act as a personal consultee and to indicate whether or not they believed the person would wish to take part. It was made clear to participants that there would be no disadvantage to them if they chose not to participate. Only after people had been assessed for capacity in accordance with the Mental Capacity Act 2005,133 and they or their consultee had agreed that they would take part, were they eligible to participate in the study.
We treated consent as a continuing process rather than a single decision, and discontinued interviewing whenever participants indicated either verbally or in any other way that they did not want to engage, or when they became distressed by the assessments.
Ethical arrangements
Our original design of FamCare, as a trial of functional analysis, included a procedure for reporting SAEs to the chief investigator. On converting the trial to a cohort study, this procedure was no longer necessary, given that interviewing participants with the selected questionnaires carried very little risk of a SAE. Nonetheless, we continued to abide by other ethical obligations, such as being aware of potential safeguarding concerns.
Data collection procedures
After initial baseline data collection, there were two follow-up periods of data collection for this cohort study, at 2 and 6 months. For the first follow-up this ranged from 1.80 to 4.40 months (mean 2.44 months, SD 0.54 months) and for the second follow-up it was 5.77 to 9.50 months (mean 6.55 months, SD 0.72 months).
All interviews were conducted in the home of the person with dementia or their carer, unless they specifically requested an alternative location. The questionnaire measures were arranged into booklets, which facilitated their ease of delivery during the interviews. If a participant became tired, or if it was requested by participants or deemed appropriate by the researcher, an interview was occasionally broken off part-way through and then continued on another day.
As well as the data collected from participants themselves via interview, the number, type and duration of mental health practitioner contacts were also collected from internal patient administration systems, for the full period that participants were in the study. This was from baseline to the first follow-up and then from first follow-up to the second final time point.
Measures
(See Appendix 6 for details of the instruments and their scoring methods.)
Primary outcome measures
The primary outcome measures were the RMBPC frequency and RMBPC reaction, for the sample of persons with dementia and their carers, respectively.
This is a 24-item carer report of observable behavioural problems in the person with dementia living at home. These are known to be of concern to family members and the measure has been widely used in family care studies. 82 It provides one total score and three subscales for problems (memory related, depression and disruptive behaviours) and parallel scores for carer reaction. The frequency dimension, which represents the rate of occurrence of individual behaviours, is seen as a measure of day-to-day problems taken from the perspective of the family carer. Scores vary from zero to four, with a low frequency score indicating that the behaviour arises infrequently and a high score indicating that it arises very frequently. The RMBPC has a second domain relating to the caregiver’s reaction to the reported problem, that is, how ‘bothered or upset’ the caregiver is as a result of the person’s behaviour, rated on a Likert scale from zero to four, with a lower score indicating lower burden. The RMBPC is completed as a structured interview, usually requiring a maximum of 15 minutes of interview time.
Secondary outcome measures
-
Frequency and severity of BPSD assessed, using the NPI, with its caregiver distress domain. 135
-
Emotional impact of CB on carers, using the NPI distress score, where carers report how distressing they find a given BPSD; the 17-item Guilt Scale (GS);252 the Hospital Anxiety and Depression Scale (HADS);253 and the General Health Questionnaire-12 items (GHQ-12). 254
-
Coping and effectiveness in caring for someone with CB, using the Short Sense of Competence Questionnaire (SSCQ),255 and the Relative Stress Scale (RSS),256 which measures stress specific to dementia caregiving.
-
Quality of life of the person with dementia, using the EQ-5D with its index and VAS scorings,138 in which participants are able to indicate their health; the QoL-AD143 and the ICEpop CAPability measure for Older people (ICECAP-O),257 where those people who are able to can report on their perceived quality of life (for EQ-5D and QoL-AD the carers also provide their perception of the person with dementia’s quality of life – proxy report); and the quality of the relationship, assessed by both the person with dementia and their carer using the Quality of Caregiver/Patient Relationship (QCPR) scale. 258
-
Quality of life of the carer, using the EQ-5D (index scoring), ICECAP-O and QCPR.
-
Costs in relation to CB, using the adapted CSRI,141 to establish the level of health- and social-care services and medication being accessed for the couple.
-
Specialist mental health service contacts: data were collected retrospectively from patient administration systems about the number, and duration, of contacts with all mental health practitioners over the 6-month period in which participants were in the study.
In addition, we used the CDR-SB,144 not itself an outcome measure, as a covariate when analysing outcomes. Table 42 shows the topics we addressed, the measures we used and the subjects to whom they relate.
| Topic | Measure | Subjects |
|---|---|---|
| Behaviour | RMBPC (frequency) | Person with dementia |
| RMBPC (incidence) | Person with dementia | |
| Emotions | RMBPC (reaction) | Carer |
| NPI (distress) | Carer | |
| NPI (total score) | Person with dementia | |
| GHQ-12 | Carer | |
| HADS | Carer | |
| GS | Carer | |
| Coping and effectiveness | SSCQ | Carer |
| RSS | Carer | |
| Quality of life | EQ-5Da | Person with dementia and carer |
| QoL-ADa | Person with dementia | |
| QCPR scale | Person with dementia and carer | |
| ICECAP-O | Person with dementia and carer | |
| Resource use | CSRI | Person with dementia and carer |
| Contacts with specialist mental health services staff | Person with dementia and carer |
Data management
Data for the study were collected in questionnaire packs completed by researchers with the person with dementia and their carer. For further details in relation to scanning and data verification see Chapter 3, Data management.
Data analyses
The main statistical analyses for the study applied SPSS version 20 to two participant populations: people with dementia and their carers. Health economic analyses used SPSS version 19.
Missing data
The researchers endeavoured to collect as many data as they could. However, some missing data were inevitable (see Chapter 3, Missing data, for an explanation of the methods used to maximise the available data). We did not impute data for the six people with dementia who died during the study. The first follow-up data sets were imputed simultaneously based on the demographics and corresponding baseline measure. The second follow-up data sets were imputed using demographics and the corresponding measure at baseline and first follow-up.
Outliers
We re-checked the data of all outliers identified during statistical analyses of the cohort, but found no reason to drop any from the data set.
Research question 1
Do levels of reported CB, and carer reaction to this, change over time as measured by the frequency and reaction domains of the RMBPC?
Initially, a linear mixed model (LMM) approach to the analysis of repeated measures was used to assess whether or not there was a significant change in the measures over the three time points. The covariates were baseline measures, NHS organisation, gender, age, carer type and CDR-SB.
As no significant changes were observed, we used a cluster analysis to examine if there were participants who were improving, remaining stable or declining, as measured by the RMBPC frequency and reaction over time. The RMBPC measures were summarised using orthogonal polynomial transformations, and linear and quadratic means. 259 We planned to use different methods of cluster analysis260 on the orthogonal polynomial transformations using those participants with complete data for the RMBPC. If relevant clusters were found, then, for those without complete RMBPC data, we planned to assign them to the cluster they were most similar to, using all available baseline data. The stability of the clustering was assessed by comparing the results from the method described above with those obtained from the fully imputed RMBPC values. We also planned a logistic regression analysis to identify baseline measures that predicted the cluster to which a participant belonged.
Research question 2
Does the level of support offered by usual care, determined by the number of specialist mental health care service contacts and time spent with the family, influence CB, family coping and/or quality of life of people with dementia and their family carer?
The levels of support were measured by the number and length of total contacts with the specialist mental health practitioners during the full period that participants were in the study, that is, between their baseline and first follow-up interviews, and between their first and second follow-up interviews (this measurement of ‘contact’ was taken from the NHS organisation patient administration electronic record and should not be confused with the CSRI data collected during the participant interviews and used in the health economic analyses). The contact data were used to determine their effect on the response measures in a LMM; the response at the two follow-ups were the response measure, the number and duration of contacts were the predictor, and baseline and first follow-up measures acted as the covariate. The response measures here were the primary measures of RMBPC frequency and reaction. The secondary measures were NPI total; for the person with dementia the EQ-5D, using both index and VAS methods, and the QoL-AD; and for the carers they were the GS and the SSCQ.
Research question 3
What are the predictors of change in CB, measured by the frequency and reaction domains of the RMBPC; and the NPI total and distress domains?
The four ‘behaviour’ response measures of RMBPC frequency and reaction, NPI total and distress were further analysed to determine whether or not predictors of change could be identified. We assessed the baseline measures and demographic variables (e.g. NHS organisation, gender, age, carer type, CDR-SB) for inclusion in the LMM used for question 1. The effect of any identified clusters from research question 1 was also included in the analysis. The assumptions of the model were assessed and, where they did not hold, we used transformations of the data to find the most appropriate model. A sensitivity analysis of the imputation results was also conducted.
The missing status of participants was categorised into three groups: complete, monotone missing (dropouts) and non-monotone missing (those who missed first follow-up, but returned for second follow-up). We used logistic regression to assess whether or not the missing status could be predicted from any of the factors and covariates measured at baseline. The variables that were identified to affect the missing status were included in the above models. 261
Economic research questions
Research question 4
What are the patterns of health- and social-care service use and associated costs for people living at home with dementia and CB and their carers?
Research question 5
What are the estimated costs of informal family care?
Research question 6
What are the patterns and costs of prescribing medications?
Research question 7
What are the total costs of services, informal care and medication?
To address the economic questions, questions 4–7, the following methods were used:
First, we interviewed carers (unpaid family or a friend) in order to complete an adapted CSRI141 to record the person’s (with dementia) contacts with community and hospital-based health- and social-care services. They also completed a CSRI for their own contacts and we collected information of prescribed medication extracted from prescriptions held at home for both parties. As described in Chapter 3, we used national unit costs for 2012 and medication costs for 2011.
Second, we categorised the types of services accessed into broad groups: community-based health care, community mental health services, hospital care, social care services and other. This was done to gain a picture of the general pattern of services being accessed by community-dwelling people with dementia and CB, and their family carers. Where possible, we also noted contacts with voluntary sector support services, but these proved very difficult to cost. When looking at prescribed medications, we paid particular attention to drugs prescribed for dementia and CB management, such as antipsychotics, sedatives, antidepressants and anxiolytics, and also drugs for pain management and associated polypharmacy (e.g. drugs to manage bowel movements).
Third, and finally, carers (unpaid family or a friend) were asked to estimate the number of hours spent each week accompanying or caring for the person with dementia. They were also asked how many hours per day they were able to leave the person with dementia unattended. We used two approaches to value this time. First, valuing this time at the replacement cost of hiring a home care worker to replace unpaid carer time; and, second, using a minimum wage value from 2012 for the carers’ time. We did not differentiate between replacement costs and opportunity costs, unlike the recent Alzheimer’s Society report of 2014,235 in which opportunity costs were applied for activities such as hobbies and leisure that a carer might need to give up to support their relative. Instead we treated all hours accompanying or caring for the person with dementia as the same. Many carers were spouses (59.6%) and/or retired (68.4%) and we are aware of the view that assigning a cost to informal care detracts from the perspectives of some carers, who may feel a duty or desire to support their relative with dementia.
Consultations with stakeholders
Two levels of consultation were conducted in order to examine how older people with dementia and CB and their carers received specialist support, and whether or not the process and outcomes of the current FamCare cohort study rang true with stakeholders. In both types of consultation we used a nominal group technique, in which facilitated group discussions were structured around topics relevant to CB in a person with dementia living at home, and their carers. Nominal group techniques have become popular for structuring and focusing understandings in group discussions and in analysing health-care problems to bridge the gap between researchers and practitioners. 262,263
Our practitioner consultations occurred at the participating NHS organisations, early on in the data collection period (March to May 2011). We explored the different perspectives of specialist community mental health practitioners and managers on identification of need, and responses to, referrals of people with dementia and CB living at home, by their specialist mental health services for older people. This occurred at five of the seven participating NHS organisations, in which our initial 33 CMHTsOP were located (see Appendix 3). These discussions were facilitated by the chief investigator, programme manager and principal investigators at each organisation. They involved managers and practitioners, such as CMHNs, support workers and those from occupational therapy, clinical psychology, old age psychiatry, physiotherapy and social work. First, facilitators outlined the emerging data relating to the spread of patient referrals from our examination of every new referral from CMHTsOP (for final data see Appendix 3, Table 66); next, findings from the evidence base and the rationale for our proposed intervention were outlined. We used video-clips and audio-clips to demonstrate real-life presentations of CB in family settings from module 3 (see Appendix 1), such as examples of repeated questioning, accusations, ‘wanting to go home when living in one’s own home’ that could result in miscommunications and conflict between the carer and their relative. Then we asked practitioners of all disciplines to identify three of their recent cases of a ‘typical’ patient with ‘no CB, some CB and severe CB’. For each of these they completed two common measures of CB in dementia, in family settings. 264 These were the RMBPC111 and NPI with its caregiver distress domain135 (see Appendix 6). Finally, in order to examine identification of need, and responses to referrals of people with dementia and CB living at home, practitioners discussed their findings in terms of where people with dementia and CB might be located in their service pathways.
On the basis of data from the initial discussions between March and May 2011, additional facilitated group discussions were held at three organisations between May and October 2011.
A semistructured tailored topic guide was used as follows.
-
How can specialist mental health practitioners identify people with dementia and CB who are living at home, to provide timely support to prevent escalation of CB and distress?
-
What are the contextual obstacles to providing timely support to people with dementia and CB living at home, and their family carer?
-
How might these be overcome in their local setting?
-
What particular supportive strategies or interventions might be used to prevent escalation of upset or distress, for those families who live with dementia and CB and present with low, medium or high levels of CB measured by the RMBPC and NPI measures?
These discussions were recorded in note form; these data were anonymised, then analysed thematically. Two researchers undertook the analysis to identify salient themes, which were then used in the second consultation with a wider stakeholder group, which we outline next.
The second ‘stakeholder’ consultation occurred in July 2013 on the completion of data collection and analysis of the cohort study. We invited stakeholders to a discussion event at each of two study sites. Invitations were sent to service providers, commissioners, lay people and families with current and past experience of caring for a relative with dementia. They were provided with a summary of the combined findings of the referrals to CMHTsOP (see Appendix 3); themes and related quotations from the systematic review, using meta-ethnographic synthesis to understand why the impact of BPSD in family settings varied from carer to carer;86 the findings of the present cohort study, which was designed to examine whether or not there are subgroups of carers who might improve, remain stable or worsen, including findings of the support offered to them by specialist mental health practitioners; and the salient themes that arose from the first set of ‘practitioner’ consultations. A total of six facilitated discussion groups were held at these two events, where stakeholders considered these data from the perspective of the wider context of access to, and delivery of, interventions for people with dementia with CB and their family carers.
Field notes and tape-recordings were taken and transcribed. Two researchers iteratively checked and re-checked the analyses and emerging themes. Checking ceased when thematic saturation was achieved, that is, when no new themes appeared to be emerging from the combined data. The present chapter outlines the findings of this second ‘stakeholder’ consultation.
Results
Recruitment to the study
In total, 289 dyads were considered for inclusion in the study, with 157 completing baseline assessment. Figure 13 shows the flow of participants through the study. Our consultations with practitioners and managers resulted in an awareness of their misunderstandings about the nature of CB in dementia in family care settings; and in-depth discussion to consider where potential participants might be located in their services.
FIGURE 13.
The FamCare study: participant flow through the study.
Consequently, 16.6% were recruited from MASs or memory clinics, as from March 2011 two trusts decided to extend recruitment to within their memory clinics as follows.
-
From May 2011 onwards, one trust, which was redesigning services to develop new memory clinics, recruited 60% of their total contribution to the FamCare study cohort from memory clinics.
-
From October 2011 a second trust, which had two longstanding memory clinics, recruited 28% of its contribution to the FamCare study cohort from one memory clinic.
Of the 132 participants who agreed for their details to be passed to the research team, but then dropped out prior to baseline data collection, 99 (34.3% of the total considered) declined to participate when contacted by researchers. Reasons for this are summarised in Appendix 13. This information suggests that some of the most distressed participant dyads had excluded themselves from this research programme that was specifically designed to help people such as them who may have been distressed because of dementia symptoms.
Follow-up dropout rates
In total, 126 dyads completed the 2-month (first) follow-up, 117 completed the 6-month (second) follow-up and, as three dyads had missed the first follow-up, there were 114 for whom data were collected at all three time points. Therefore, 20% (n = 31) of the initial 157 were lost to follow-up at 2 months; 10% out of 126 (n = 12) were further lost at the second follow-up, and with the three participants who were not observed at all the time points, by the end of the study there were 27% (n = 43) incomplete cases. Three dyads who were lost to follow-up at 2 months returned to the study at second follow-up. Therefore, the overall dropout rate was 25.5%, much less than the 40% predicted at the outset.
Descriptive data
Tables 43 and 44 outline the demographic characteristics of participants. Of the carers recruited to the study, 70.7% were living together with the person with dementia in the same household, 74.5% owned their own homes and most (64.3%) were retired (see Table 43). The majority of the people with dementia were female (59.2%), as were the majority of carers (70.7%). The mean age of people with dementia was 80.34 years (SD 7.66 years) and of carers it was 66.13 years (SD 13.06 years). The most common relationship between dyads (see Table 44) was spousal (52.9%).
| Characteristic (N = 157) | Person with dementia, n (%) | Carer, n (%) |
|---|---|---|
| Gender: female | 93 (59.2) | 111 (70.7) |
| Accommodation | ||
| Owner | 117 (74.5) | 117 (74.5) |
| Privately rented | 12 (7.6) | 9 (5.7) |
| Housing association/local authority | 18 (11.5) | 15 (9.6) |
| Sheltered accommodation | 5 (3.2) | 1 (0.6) |
| Live with friend/relative | 5 (3.2) | 4 (2.5) |
| Not answered | – | 11 (7.0) |
| Employment status | ||
| Paid/self-employed | – | 39 (24.8) |
| Unemployed | – | 13 (8.3) |
| Homemaker | – | 4 (2.5) |
| Retired | – | 101 (64.3) |
| Mean (SD) | Mean (SD) | |
| Age (years) | 80.34 (7.66) | 66.13 (13.06) |
| Age left full-time education (years) | 15.82 (5.00) | 16.59 (2.81) |
| Relationship | Living together (N = 111) | Not living together (N = 46) | Total (N = 157) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Spouse | 81 | 73.0 | 2 | 4.3 | 83 | 52.9 |
| Son/daughter (including in-law) | 28 | 25.2 | 40 | 87.0 | 68 | 43.3 |
| Other relative | 1 | 0.9 | 2 | 4.3 | 3 | 1.9 |
| Other (e.g. friend/neighbour) | 1 | 0.9 | 2 | 4.3 | 3 | 1.9 |
As shown in Table 45, although all participants met the criteria for dementia using the DSM-IV, a very small number (n = 5) at baseline assessment were rated by the researchers as falling within the ‘no cognitive impairment’ range on the CDR. At the opposite end of the spectrum, there were relatively few (n = 14) who were scored as having severe dementia.
| CDR score | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline (N = 157) | First follow-up (N = 126) | Second follow-up (N = 109)a | ||||
| n | % | n | % | n | % | |
| 0: no cognitive impairment | 5 | 3.2 | 3 | 2.4 | 6 | 5.5 |
| 0.5: very mild dementia | 35 | 22.3 | 29 | 23.0 | 18 | 16.5 |
| 1: mild | 59 | 37.6 | 45 | 35.7 | 39 | 35.8 |
| 2: moderate | 44 | 28.0 | 30 | 23.8 | 28 | 25.7 |
| 3: severe | 14 | 8.9 | 19 | 15.1 | 18 | 16.5 |
In the autumn of 2014 the Alzheimer’s Society published an update of its 2007 study on the social and economic impact of dementia in the UK. 235 It did not identify any further UK evidence on the distribution of dementia by severity compared with its estimates in the 2007 Dementia UK: The Full Report265 and, therefore, concluded that estimates for dementia severity remained relevant as follows: 55.4% mild dementia, 32.1% moderate dementia and 12.5% severe dementia. Based on the CDR, the proportions in each group are very similar to those we found in the present FamCare study (see Table 45).
Examination of change over the 6 months of the study (Table 46) indicates that most people either stayed the same or moved up to the next level of severity on the CDR.
| CDR score at second follow-upa | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 3 | ||
| CDR score at baseline | 0 | 1 | 3 | 0 | 0 | 0 |
| 0.5 | 4 | 12 | 14 | 1 | 0 | |
| 1 | 1 | 2 | 21 | 12 | 3 | |
| 2 | 0 | 1 | 4 | 14 | 10 | |
| 3 | 0 | 0 | 0 | 1 | 5 | |
Reported problems at baseline
The RMBPC ratings of the daily concerns of carers show that the most commonly reported problems cited by carers (Table 47) were associated with forgetfulness, including asking the same question and misplacing things. These can be triggers for escalation of upset and other CB in families who have not learned how to respond effectively; the least common ones were destroying property and threatening to hurt themselves or others.
| RMBPC (N = 157) | Incidence | Frequencya | Reactionb | |||
|---|---|---|---|---|---|---|
| n | % | Mean | SD | Mean | SD | |
| Trouble remembering recent events | 154 | 98.1 | 3.65 | 0.71 | 1.55 | 1.26 |
| Forgetting what day it is | 149 | 94.9 | 3.53 | 0.92 | 0.97 | 1.08 |
| Asking the same question | 141 | 89.8 | 3.57 | 0.78 | 1.69 | 1.09 |
| Losing or misplacing things | 131 | 83.4 | 3.28 | 0.97 | 1.47 | 1.16 |
| Difficulty concentrating on a task | 117 | 74.5 | 3.35 | 0.97 | 1.39 | 1.18 |
| Appears anxious or worried | 116 | 73.9 | 2.88 | 1.09 | 2.17 | 1.08 |
| Appears sad or depressed | 109 | 69.4 | 2.65 | 1.16 | 2.22 | 1.13 |
| Starting but not finishing things | 99 | 63.1 | 3.27 | 1.18 | 1.24 | 1.29 |
| Arguing, irritability, complaining | 91 | 58.0 | 2.55 | 1.10 | 1.84 | 1.16 |
| Awaking carer/other family at night | 71 | 45.2 | 2.69 | 1.38 | 2.69 | 1.61 |
| Comments about feeling worthless/burden | 70 | 44.6 | 1.97 | 1.06 | 1.96 | 1.21 |
| Trouble remembering significant past events | 70 | 44.6 | 2.88 | 1.43 | 1.55 | 1.58 |
| Crying and tearfulness | 68 | 43.3 | 2.12 | 1.09 | 2.40 | 1.09 |
| Expressing feelings of hopelessness/sadness about the future | 66 | 42.0 | 2.29 | 1.08 | 1.94 | 1.23 |
| Aggressive to others verbally | 52 | 33.1 | 1.73 | 1.01 | 2.42 | 1.13 |
| Talking about feeling lonely | 49 | 31.2 | 2.19 | 1.14 | 2.23 | 1.05 |
| Doing things that embarrass you | 46 | 29.3 | 2.00 | 1.15 | 2.09 | 1.35 |
| Commenting about death of self or others | 45 | 28.7 | 1.82 | 1.05 | 2.53 | 1.37 |
| Engaging in behaviour dangerous to self or others | 31 | 19.7 | 1.94 | 1.09 | 2.94 | 1.09 |
| Talking loudly and rapidly | 23 | 14.6 | 2.96 | 1.22 | 1.52 | 1.24 |
| Comments about feeling like a failure/not having worthwhile accomplishments | 17 | 10.8 | 2.59 | 2.12 | 2.29 | 2.05 |
| Threats to hurt others | 10 | 6.4 | 1.80 | 1.23 | 3.40 | 1.07 |
| Threats to hurt oneself | 8 | 5.1 | 1.75 | 0.89 | 3.63 | 1.06 |
| Destroying property | 6 | 3.8 | 2.00 | 1.53 | 2.83 | 1.60 |
Main outcomes over time
Table 48 outlines the outcome measures at each time point. From the sample of 157 participants with dementia, 122 completed some of the self-report measures at baseline.
| Measure | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 157) | First follow-up (n = 126) | Second follow-up (n = 117) | |||||||
| Missing | Mean | SD | Missing | Mean | SD | Missing | Mean | SD | |
| RMBPC incidence | 0 | 11.08 | 3.49 | 0 | 10.65 | 3.50 | 1 | 10.76 | 3.73 |
| Memory related | 0 | 5.48 | 1.09 | 0 | 5.45 | 1.18 | 1 | 5.61 | 1.12 |
| Depression | 0 | 3.49 | 2.32 | 0 | 3.08 | 2.21 | 0 | 3.13 | 2.21 |
| Disruptive | 0 | 2.10 | 1.68 | 0 | 2.12 | 1.67 | 0 | 2.09 | 1.81 |
| RMBPC frequency | 1 | 31.66 | 11.97 | 1 | 30.21 | 11.00 | 2 | 31.27 | 12.03 |
| Memory related | 1 | 18.59 | 5.36 | 1 | 18.65 | 5.28 | 2 | 19.32 | 5.38 |
| Depression | 0 | 8.22 | 6.68 | 0 | 7.26 | 6.33 | 0 | 7.28 | 6.18 |
| Disruptive | 0 | 4.83 | 4.58 | 0 | 4.43 | 3.94 | 0 | 4.64 | 4.68 |
| RMBPC reaction | 1 | 19.99 | 12.31 | 8 | 17.31 | 12.47 | 0 | 18.71 | 13.68 |
| Memory related | 1 | 7.64 | 5.32 | 8 | 6.86 | 4.96 | 0 | 7.62 | 5.45 |
| Depression | 0 | 7.58 | 6.83 | 1 | 5.79 | 6.30 | 0 | 6.46 | 6.86 |
| Disruptive | 0 | 4.71 | 4.80 | 1 | 4.52 | 5.08 | 0 | 4.62 | 5.23 |
| NPI incidence | 0 | 5.35 | 2.65 | 0 | 5.03 | 2.67 | 0 | 5.30 | 2.85 |
| NPI total (frequency × severity) | 0 | 25.75 | 19.17 | 0 | 24.21 | 20.14 | 0 | 24.21 | 19.42 |
| NPI distress | 0 | 13.37 | 9.65 | 0 | 11.58 | 9.44 | 0 | 12.37 | 9.31 |
| EQ-5D index (self-report) | 40 | 0.71 | 0.28 | 39 | 0.78 | 0.23 | 43 | 0.81 | 0.22 |
| EQ-5D VAS (self-report) | 40 | 68.16 | 20.23 | 40 | 89.80 | 140.53 | 44 | 84.69 | 109.14 |
| EQ-5D index (proxy) | 0 | 0.47 | 0.32 | 0 | 0.53 | 0.33 | 1 | 0.49 | 0.33 |
| EQ-5D VAS (proxy) | 0 | 52.86 | 20.78 | 0 | 56.25 | 20.58 | 0 | 54.32 | 21.61 |
| EQ-5D index (carer) | 0 | 0.81 | 0.21 | 1 | 0.78 | 0.25 | 0 | 0.80 | 0.23 |
| EQ-5D VAS (carer) | 0 | 74.64 | 18.06 | 0 | 74.81 | 18.43 | 0 | 74.91 | 16.88 |
| QoL-AD (self-report) | 41 | 37.21 | 5.26 | 41 | 37.43 | 5.64 | 44 | 37.01 | 5.94 |
| QoL-AD (proxy) | 1 | 29.73 | 5.91 | 2 | 30.79 | 5.97 | 1 | 30.09 | 6.40 |
| SSCQ | 0 | 24.77 | 5.97 | 0 | 24.74 | 5.53 | 0 | 24.99 | 5.51 |
| ICECAP-O index (person with dementia) | 42 | 0.47 | 0.21 | 40 | 0.45 | 0.21 | 45 | 0.46 | 0.23 |
| ICECAP-O index (carer) | 0 | 0.57 | 0.26 | 0 | 0.52 | 0.28 | 0 | 0.52 | 0.25 |
| GHQ-12 | 0 | 13.86 | 5.01 | 0 | 12.56 | 4.86 | 0 | 12.41 | 5.24 |
| HADS total | 0 | 9.62 | 6.50 | 1 | 9.06 | 6.31 | 0 | 9.07 | 6.07 |
| HADS anxiety | 0 | 5.66 | 4.04 | 1 | 5.26 | 3.91 | 0 | 4.91 | 3.90 |
| HADS depression | 0 | 3.97 | 3.28 | 1 | 3.79 | 3.36 | 0 | 4.15 | 3.20 |
| QCPR total (person with dementia) | 42 | 58.23 | 6.38 | 40 | 57.44 | 7.20 | 45 | 56.58 | 8.13 |
| QCPR warmth | 40 | 34.51 | 3.62 | 39 | 34.01 | 4.26 | 44 | 33.53 | 4.13 |
| QCPR criticism | 42 | 23.70 | 3.47 | 40 | 23.47 | 3.58 | 45 | 23.06 | 4.57 |
| QCPR total (carer) | 0 | 53.03 | 8.71 | 0 | 53.13 | 9.03 | 0 | 53.25 | 9.01 |
| QCPR warmth | 0 | 31.73 | 5.10 | 0 | 31.70 | 5.27 | 0 | 31.66 | 5.09 |
| QCPR criticism | 0 | 21.30 | 4.48 | 0 | 21.43 | 4.52 | 0 | 21.59 | 4.81 |
| GS | 1 | 6.08 | 5.02 | 0 | 4.84 | 4.31 | 0 | 5.25 | 4.94 |
| RSS total | 0 | 20.38 | 10.45 | 1 | 18.04 | 10.60 | 0 | 18.02 | 10.58 |
| CDR-SB | 0 | 7.96 | 4.25 | 0 | 8.20 | 4.76 | 0 | 8.00 | 5.11 |
The average NPI composite score (see Table 48) of 25.75 (SD 19.17) at baseline is strikingly high and is comparable to that found in smaller studies conducted in Spain240 and Australia. 266 There was no discernible change over the 6 months on this measure, or on the RMBPC, which was our primary outcome measure.
The self-reported baseline QoL-AD score averaged at 37.2 (SD 5.3), comparable to values from the REMCARE (REMiniscence groups for people with dementia and their family CAREgivers) study of reminiscence therapy for community-dwelling people with dementia and their carers,267 in which the intervention group average score was 37.5 (SD 5.32) and the control group average score was 37.0 (SD 5.35). As with the REMCARE study,267 carer (proxy) ratings of quality of life of the person with dementia were lower than self-reported ratings, providing further support for the view that patient and proxy ratings of quality of life in people with dementia do not concur with each other;268 perhaps adding weight to findings from another study of BPSD that families may be more aware. FamCare study participants with dementia had a mean EQ-5D index self-reported score of 0.71 (SD 0.28) and carers had a mean self-reported index score of 0.81 (SD 0.21). These values were similar to those found in the REMCARE study. 267 Self-reported scores for participants with dementia were also similar to the age-matched UK general population norms. 161 Although not statistically significant, carers aged ≥ 75 years had a slightly higher average EQ-5D index score (mean 0.79, SD 0.23) than the general population aged ≥ 75 years (mean 0.73, SD 0.27), but younger carers had a slightly lower than normal average EQ-5D index; in the age group of 45–54 years, the average carer EQ-5D index was 0.81 (SD 0.24), compared with the population norm of 0.85 (SD 0.25).
Prescribed medication 3 months prior to baseline
Table 49 outlines the various groups of medications that people with dementia and their carers were prescribed in the 3 months before baseline, whether or not they were taking them for the full 3 months and whether they were on one or more medication in a category. From this, we can see that 8.9% (n = 14) of people with dementia were taking at least one antipsychotic at some point in the 3 months before baseline, 21.6% (n = 34) antidepressants and 6.3% (n = 10) hypnotics and anxiolytics (B/Z/A drugs). Furthermore, 29.9% (n = 47) were prescribed dementia drugs. Baseline data in Table 49 cover an important period between August 2010 and October 2011. This period follows the publication in October 2009 of the landmark report commissioned by the Department of Health, The Use of Antipsychotic Medication for People with Dementia: Time for Action,112 which recommended that reduction of the use of antipsychotics in dementia should be a clinical governance priority for primary and secondary care. Its author estimated that this could be done safely over a 36-month period. In March 2011, the Prescribing Observatory for Mental Health (POMH-UK) programme conducted an audit-based quality improvement programme of prescribing in dementia care within 54 specialist NHS organisations. 269 This noted that 64% of people with dementia lived at home and, of this group, 9.6% were prescribed an antipsychotic. Our data, which were taken from patient prescriptions rather than from audits carried out by staff within their organisation – and where some but not all NHS organisations had participated in the POMH-UK audit – show that 91% of our sample were not prescribed an antipsychotic and thus concur with the POMH-UK data. Disappointingly, the quality of prescribing was suboptimal, as very few people had been taking an antipsychotic for less than 3 months. This may be because participants had been managed in primary care prior to referral to specialist services for CB, and primary care physicians may be less confident about withdrawing patients from antipsychotics than their colleagues in secondary care. 269 Similar to other studies, a large number of participants with dementia (i.e. around 20%) were prescribed at least one antidepressant, but our data note a low likelihood of discontinuing in 3 months, despite evidence indicating limited or no benefit in the management of depression in dementia. 270,271 This pattern of suboptimal prescribing for the hypnotics and anxiolytic (B/Z/A) drugs is also seen, as 6.4% of participants continued with prescription for 3 months or more, contrary to guidelines advocating benzodiazepine prescription for no longer than 28 days. 178
| Category (N = 157) | Person with dementia, n (%) | Carer, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Not prescribed | Less than 3 months | Full 3 months (1 drug) | Full 3 months (> 1 drug) | Not prescribed | Less than 3 months | Full 3 months (1 drug) | Full 3 months (> 1 drug) | |
| Antipsychotics | 143 (91.1) | 2 (1.3) | 11 (7.0) | 1 (0.6) | 156 (99.4) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Atypical | 145 (92.4) | 2 (1.3) | 9 (5.7) | 1 (0.6) | 157 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Typical | 155 (98.7) | 0 (0.0) | 2 (1.3) | 0 (0.0) | 156 (99.4) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Antidepressants | 123 (78.3) | 0 (0.0) | 31 (19.7) | 3 (1.9) | 139 (88.5) | 2 (1.3) | 13 (8.3) | 3 (1.9) |
| SSRI | 139 (88.5) | 0 (0.0) | 18 (11.5) | 0 (0.0) | 142 (90.4) | 2 (1.3) | 13 (8.3) | 0 (0.0) |
| Tricyclic | 147 (93.6) | 0 (0.0) | 10 (6.4) | 0 (0.0) | 155 (98.7) | 0 (0.0) | 2 (1.3) | 0 (0.0) |
| Other | 149 (94.9) | 0 (0.0) | 7 (4.5) | 1 (0.6) | 154 (98.1) | 0 (0.0) | 2 (1.3) | 1 (0.6) |
| Hypnotics and anxiolytics | 147 (93.6) | 0 (0.0) | 9 (5.7) | 1 (0.6) | 152 (96.8) | 0 (0.0) | 5 (3.2) | 0 (0.0) |
| B/Z/A drugs | 148 (94.3) | 0 (0.0) | 8 (5.1) | 1 (0.6) | 152 (96.8) | 0 (0.0) | 5 (3.2) | 0 (0.0) |
| Non-benzodiazepines | 156 (99.4) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 157 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anticonvulsants | 154 (98.1) | 0 (0.0) | 3 (1.9) | 0 (0.0) | 155 (98.7) | 0 (0.0) | 2 (1.3) | 0 (0.0) |
| Dementia drugs | 110 (70.1) | 6 (3.8) | 39 (24.8) | 2 (1.3) | 157 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acetylcholinesterase inhibitors | 113 (72.0) | 5 (3.2) | 38 (24.2) | 1 (0.6) | 157 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cognitive enhancers | 153 (97.5) | 2 (1.3) | 2 (1.3) | 0 (0.0) | 157 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pain relief | 125 (79.6) | 0 (0.0) | 24 (15.3) | 8 (5.1) | 133 (84.7) | 1 (0.6) | 18 (11.5) | 5 (3.2) |
| Opioida | 141 (89.8) | 1 (0.6) | 12 (7.6) | 3 (1.9) | 138 (87.9) | 1 (0.6) | 15 (9.6) | 3 (1.9) |
| Non-opioid | 134 (85.4) | 0 (0.0) | 23 (14.6) | 0 (0.0) | 149 (94.9) | 0 (0.0) | 8 (5.1) | 0 (0.0) |
| Laxatives | 145 (92.4) | 1 (0.6) | 8 (5.1) | 3 (1.9) | 153 (97.5) | 0 (0.0) | 3 (1.9) | 1 (0.6) |
Contacts with practitioners from the community mental health teams for older people for treatment
During the study, dyads continued with their treatment as usual and as such were contacted by specialist mental health services such as psychiatrists, psychologists and other mental health practitioners (such as CMHNs, occupational therapists, physiotherapists and support workers). Data were collected for 157 participants between baseline and first follow-up (2 months) and 129 between first and second follow-ups (4 months) in the six NHS organisations from which baseline data were collected. There were 20 (of the 157) dyads that had no contacts at all with specialist mental health care services during the period in which they were in the study. Table 50 summarises the total number, length (minutes) and type of contacts during the two time periods. The most common were face-to-face contacts, with 116 of the 157 dyads having this type in the first period and 94 out of 129 in the second period.
| Contact type | Time period | |||||
|---|---|---|---|---|---|---|
| Baseline to first follow-up (2-month period; n = 157) | First to second follow-up (4-month period; n = 129) | |||||
| n | Mean | SD | n | Mean | SD | |
| Face to face | ||||||
| Number | 116 | 3.60 | 3.86 | 94 | 5.50 | 6.72 |
| Length (minutes)a | 235.83 | 320.81 | 375.68 | 659.15 | ||
| Telephone | ||||||
| Number | 53 | 2.36 | 2.02 | 42 | 2.57 | 2.12 |
| Length (minutes)a | 34.49 | 32.80 | 36.02 | 31.26 | ||
| Group | ||||||
| Number | 7 | 3.57 | 2.94 | 7 | 5.29 | 5.56 |
| Length (minutes)a | 461.43 | 646.93 | 950.29 | 1344.77 | ||
| Not specified | ||||||
| Number | 4 | 1.50 | 0.58 | 5 | 1.00 | 0.00 |
| Length (minutes)a | 65.00 | 41.23 | 41.00 | 20.12 | ||
| Other | ||||||
| Number | 2 | 1.00 | 0.00 | 3 | 1.33 | 0.58 |
| Length (minutes)a | 52.50 | 10.61 | 41.67 | 32.53 | ||
| Zero contacts | 35 | 30 | ||||
As shown in Table 51, mental health practitioners, such as CMHNs or occupational therapists, accounted for the majority of contacts with dyads (i.e. almost two-thirds of our sample). Dyads had the fewest contacts with psychologists, with just 7.6% receiving a contact with them during the first time period and 6.2% during the second. The percentage of dyads receiving each type of contact was similar between the two time periods.
| Profession | Time period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to first follow-up (2-month period; n = 157) | First follow-up to second follow-up (4-month period; n = 129) | |||||||||
| Total number of contactsa | Number of dyads (%) | Mean (SD) [range] number of contacts | Total length of contacts (minutes)b | Mean (SD) [range] total length of contacts | Total number of contactsa | Number of dyads (%) | Mean (SD) [range] number of contacts | Total length of contacts (minutes)b | Mean (SD) [range] total length of contacts | |
| Mental health practitionerc | 383 | 98 (62.4) | 2.44 (3.19) [0–19] | 20,284 | 129.20 (247.87) [0–2110] | 368 | 81 (62.8) | 2.85 (3.67) [0–18] | 19,138 | 148.36 (255.08) [0–1651] |
| Psychiatrist | 57 | 48 (30.6) | 0.36 (0.60) [0–3] | 3185 | 20.29 (46.92) [0–480] | 74 | 47 (36.4) | 0.57 (1.00) [0–5] | 3126 | 24.23 (43.22) [0–240] |
| Psychologist | 38 | 12 (7.6) | 0.24 (1.05) [0–9] | 2780 | 17.71 (132.21) [0–1620] | 17 | 8 (6.2) | 0.13 (0.90) [0–10] | 2715 | 21.05 (191.33) [0–2160] |
| Support worker | 98 | 25 (15.9) | 0.62 (2.29) [0–22] | 6530 | 41.59 (163.74) [0–1501] | 211 | 23 (17.8) | 1.64 (5.14) [0–32] | 18,820 | 144.50 (544.98) [0–3810] |
| Zero contacts | 35 (22.3) | 30 (23.3) | ||||||||
| Total | 576 | 3.67 (4.53) [0–29] | 32,779 | 208.78 (349.99) [0–2190] | 701 | 5.19 (7.36) [0–43] | 43,799 | 339.53 (696.46) [0–3820] | ||
As seen in Table 51, the average number of contacts per month with a mental health practitioner (rather than across each of the two periods) was 1.22 contacts (SD 1.60 contacts) per dyad in the first time period, dropping to 0.71 contacts (SD 0.92 contacts) per month in the second time period. The mean number and length of contacts per month with mental health practitioners, psychiatrists and psychologists decreased between the two time periods. At the same time, the mean number and length of support worker contacts per month increased, in accordance with reports of an increased reliance on practitioners without professional registration working in CMHTsOP in England. 272 Overall (Table 52), the average contact time with mental health services across the 6 months was just over 9 hours (mean 559.71 minutes, range 0–5490 minutes), and the total number of contacts was nine (mean 8.78 contacts, range 0–57 contacts).
| Profession | Baseline to second follow-up (6 month period; n = 129) | ||||
|---|---|---|---|---|---|
| Total number of contactsa | Per cent of dyads (%) | Mean (SD) [range] number of contacts | Total length of contacts (minutes)b | Mean (SD) [range] total length of contacts | |
| Mental health practitionerc | 677 | 99 | 5.25 (5.51) [0–25] | 36,648 | 284.09 (413.93) [0–2281] |
| Psychiatrist | 119 | 62 | 0.922 (1.31) [0–6] | 5736 | 44.47 (72.57) [0–536] |
| Psychologist | 47 | 14 | 0.36 (1.45) [0–10] | 5195 | 40.27 (251.42) [0–2160] |
| Support worker | 289 | 27 | 2.24 (6.96) [0–45] | 24,623 | 190.88 (638.12) [0–4005] |
| Zero contacts | 16 | ||||
| Total | 1132 | 8.78 (10.49) [0–57] | 72,202 | 559.71 (970.94) [0–5490] | |
Dropout analysis
Baseline data were used to identify the factors that could be used as the predictors for dropouts from our study. The demographic variables considered were participant’s age, participant’s gender, relationship between participant and carer, whether or not participant and carer are living together and NHS organisation. Other demographic variables could have been included, but, because of a large number of missing values, they were not considered; for example, the time that the person with dementia is left alone per day was excluded because 29% of values were missing. Only the baseline information was used in the model to predict the dropouts. Other key predictors involved RMBPC incidence, frequency and reaction total scores, NPI incidence, frequency, severity and distress scores, EQ-5D index and VAS of participants with dementia, QoL-AD score, SSCQ, ICECAP-O, GHQ-12, HADS, QCPR, GS, RSS and CDR-SB.
A chi-squared test was used to explore the relationships between dropouts and demographic variables. If the expected value of one cell was smaller than five, then Fisher’s exact test was used instead of the chi-squared test. It was found that age, NHS organisation, relationship and CDR had a significant impact on the number of dropouts.
Logistic regression was used to identify the predictors for the dropouts at second follow-up. The factors and covariates were selected by the forward (Wald) selection method and this was implemented by the SPSS logistic program. The sensitivity analysis used the data after applying the 25% missing rule on the subscales/scales, as well as five imputed data sets from the multiple imputations. The predictors contained two sets of variables: demographic factors and questionnaire measures. All the selected models agreed that participant’s age and NHS organisation were two significant factors affecting the number of dropouts. As a result, the final model included two variables: age and NHS organisation. Applying this model on the original data showed that increasing age of the participant increases the chance of dropout by 10% (95% CI 4% to 16%). NHS organisation 1 [exp (β) = 0.25, standard error (SE) (β) = 0.83] and organisation 2 [exp (β) = 0.77, SE (β) = 0.63] had lower dropout rates than NHS organisation 3. NHS organisation 4 [exp (β) = 1.48, SE (β) = 0.58] had a 48% higher dropout rate. NHS organisation 5 [exp (β) = 2.11, SE (β) = 0.78] had over twice as high a chance to dropout than NHS organisation 3, although organisation 6 [exp (β) = 3.27, SE (β) = 0.61] had over three times more dropouts than organisation 3.
Research question 1
Do levels of reported CB, and carer reaction to this, change over time as measured by the frequency and reaction domains of the RMBPC?
As noted previously, the primary outcome measures were the RMBPC frequency and RMBPC reaction for the sample of people with dementia and carers, respectively.
Results for the linear mixed-model analysis
Analysis was performed on three versions of the data set: the observed data with no imputations of missing items or missing time points; after using the 25% missing item rule; and missing values replaced by five imputed values. The basic analysis used a LMM to analyse the repeated measurements made at baseline, first follow-up and second follow-up. An extended analysis included covariates in the LMM.
The assumptions of the LMM were assessed. They held for RMBPC frequency but a 0.4 power transformation was needed for the RMBPC reaction.
Time was included in the model as both a fixed and a random effect. The fixed effect of time showed whether or not there was a trend among the whole group, whereas the random effect of time showed whether or not there was variation among the participants in their trend. In the basic LMM, the fixed effects of time combining five imputations were not significant for RMBPC frequency or for RMBPC reaction. The same general findings were found for the other versions of the data set, as shown in Table 53.
| Imputation | RMBPC frequency | RMBPC reaction | ||||
|---|---|---|---|---|---|---|
| β | SE | t (p-value) | β | SE | t (p-value) | |
| After 25% missing rule | 0.009 | 0.027 | 0.333 (0.740) | –0.040 | 0.033 | –1.242 (0.215) |
| 1 | –0.004 | 0.026 | –0.169 (0.866) | –0.039 | 0.028 | –1.38 (0.168) |
| 2 | 0.009 | 0.023 | 0.383 (0.702) | –0.032 | 0.028 | –1.136 (0.257) |
| 3 | 0.019 | 0.024 | 0.808 (0.420) | 0.010 | 0.030 | 0.329 (0.743) |
| 4 | 0.028 | 0.023 | 1.207 (0.228) | –0.031 | 0.030 | –1.056 (0.292) |
| 5 | 0.009 | 0.024 | 0.375 (0.708) | 0.007 | 0.030 | –0.236 (0.813) |
| Pooled | 0.012 | 0.027 | 0.442 (0.660) | –0.017 | 0.002 | –0.440 (0.665) |
As for the basic model, the extended model included time as both a fixed and random effect. However, we now also included the effects of the participant’s age and gender; the carer’s age, gender and relationship; NHS organisation; whether or not the dyad lives together; carer’s job type; and CDR-SB. Carer’s gender and CDR-SB were consistently found across the three versions of the data as predictors of RMBPC frequency, whereas, for RMBPC reaction, the carer’s age was also a predictor. There was no significant change in RMBPC frequency or reaction over time. The significant results are shown in Tables 54 and 55.
| Imputation | Carer’s gender | CDR-SB | ||
|---|---|---|---|---|
| β (SE) | t (p-value) | β (SE) | t (p-value) | |
| After 25% missing rule | –0.75 (0.31) | –2.40 (0.018) | 0.17 (0.03) | 4.99 (< 0.001) |
| 1 | –0.81 (0.29) | –2.77 (0.006) | 0.15 (0.03) | 4.85 (< 0.001) |
| 2 | –0.80 (0.30) | –2.62 (0.010) | 0.16 (0.03) | 4.82 (< 0.001) |
| 3 | –0.88 (0.29) | –3.00 (0.003) | 0.17 (0.03) | 5.37 (< 0.001) |
| 4 | –0.88 (0.30) | –2.96 (0.004) | 0.17 (0.03) | 5.19 (< 0.001) |
| 5 | –0.69 (0.31) | –2.25 (0.026) | 0.16 (0.03) | 4.79 (< 0.001) |
| Pooled | –0.81 (0.31) | –2.61 (0.010) | 0.16 (0.03) | 4.86 (< 0.001) |
| Imputation (n = 157) | Carer’s gender | Carer’s age | CDR-SB | |||
|---|---|---|---|---|---|---|
| β (SE) | t (p-value) | β (SE) | t (p-value) | β (SE) | t (p-value) | |
| After 25% missing rule | –0.94 (0.35) | –2.72 (0.007) | –0.03 (0.01) | –2.23 (0.027) | 0.08 (0.04) | 2.10 (0.038) |
| 1 | –0.84 (0.34) | –2.46 (0.015) | –0.03 (0.01) | –2.33 (0.021) | 0.08 (0.04) | 2.06 (0.041) |
| 2 | –0.90 (0.34) | –2.62 (0.010) | –0.03 (0.01) | –2.23 (0.027) | 0.07 (0.04) | 2.01 (0.046) |
| 3 | –1.05 (0.34) | –3.04 (0.003) | –0.03 (0.01) | –2.52 (0.013) | 0.07 (0.04) | 2.02 (0.046) |
| 4 | –1.07 (0.34) | –3.18 (0.002) | –0.03 (0.01) | –2.28 (0.024) | 0.08 (0.04) | 2.34 (0.020) |
| 5 | –1.00 (0.35) | –2.90 (0.004) | –0.03 (0.01) | –2.45 (0.015) | 0.08 (0.04) | 2.21 (0.029) |
| Pooled | –0.97 (0.36) | –2.71 (0.008) | –0.03 (0.01) | –2.34 (0.021) | 0.08 (0.04) | 2.11 (0.037) |
In conclusion, there was no significant trend for either RMBPC frequency or reaction. However, there was evidence of significant variation among participants in their trends for both RMBPC frequency and reaction. Carer’s gender (female) and CDR-SB affected the trend for RMBPC frequency and carer’s age, gender (female) and CDR-SB affected the trend for RMBPC reaction, meaning that female carers had lower predicted scores than males in their reported frequency of, and coping with, CB. As there was no significant change over time, a cluster analysis was performed.
Results for the cluster analysis
Four commonly used methods of cluster analysis were used: hierarchical clustering with complete, single or Ward’s linkage, and k-means clustering. The data used were the mean, linear and quadratic orthogonal polynomial transformation of the RMBPC frequency and reaction scores. These transformations were chosen so that participants following different response patterns over time could be identified. The expectation was that the three clusters would represent those participants whose condition improved over time, those who stayed the same and those who got worse. To assess stability of the findings, the analyses were run on those with complete data (n = 82), after application of the 25% missing rule (n = 104) and for the five full imputations (n = 157). The clustering with Ward’s linkage tended to find three clusters for all versions of the data, but only 25 (16%), three (2%) and five (3%) participants were consistently in the same improving, stable and worse clusters.
In conclusion, no stable clusters were able to identify participants who could be described as improvers, those who were stable or those who got worse. As no stable clusters were identified, no logistic regression was subsequently used to identify the clusters from the baseline measures.
Research question 2
Does the level of support offered by usual care, determined by the number of specialist mental health-care service contacts and time spent with the family, influence CB, family coping and/or quality of life of people with dementia and their family carer?
In order to determine whether or not the level of support provided by specialist mental health-care services influenced CB, family coping and/or the quality of life of people with dementia and their carer, the number and length of total contacts with these services were measured.
We assessed whether or not the number and total length (duration) of specialist mental health service contacts between baseline and the first follow-up and between the first follow-up and second follow-up (see Table 51) predicted the value of each response variable at the later time point. To do this, we co-varied for the effect of the response variable, that is, the number and total length of contacts at the earlier time point. The stability of the results was assessed using the complete data set, after using the 25% missing rule and the pooled results from the five imputations. The assumptions of the analyses were found to hold for measures as follows: RMBPC frequency and reaction; proxy EQ-5D index and VAS; QoL-AD; and, for carers, the GS and SSCQ.
Table 56 shows the coefficients, SE and associated p-value for the relationship between the response variable and each of the number and length of mental health service contacts (‘N_contact’ and ‘L_contact’), the value of the response variable at the time point prior to the period (‘previous’), the time period (‘period’) and the three interactions between the covariates and the period factor. The results from the five pooled imputations are summarised giving the minimum and maximum coefficient (β), the minimum and maximum observed SE and the number of times out of five that the coefficient was significant. It was found that the number of contacts was a significant (p < 0.05) predictor of RMBPC frequency and proxy EQ-5D VAS, whereas the total length of contacts predicted the carer GS score (p < 0.05). These findings were found in at least four of the five imputed data sets. For all three responses there were significant effects of the response at the previous time point period, and the interaction between the period and the response at the previous time point, indicating that the way dyads performed initially on a given measure affected how they continued to perform on that measure.
| Measure | Data after 25% missing rule | Summary of five imputations | Number of times p < 0.05 | |||||
|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | Minimum β | Maximum β | Minimum SE | Maximum SE | ||
| RMBPC frequency | ||||||||
| N_contact | 0.62 | 0.27 | 0.023 | 0.53 | 0.59 | 0.23 | 0.25 | 5 |
| L_contact | –0.006 | 0.003 | 0.074 | –0.006 | –0.005 | 0.003 | 0.003 | 1 |
| Previous | 0.68 | 0.06 | < 0.001 | 0.65 | 0.69 | 0.05 | 0.05 | 5 |
| Period | –6.76 | 3.38 | 0.048 | –8.88 | –6.31 | 3.07 | 3.17 | 5 |
| N_contact × period | 0.13 | 0.47 | 0.779 | –0.07 | 0.17 | 0.43 | 0.45 | 0 |
| L_contact × period | 0.001 | 0.01 | 0.827 | 0.00 | 0.02 | 0.006 | 0.01 | 0 |
| Previous × period | 0.23 | 0.11 | 0.032 | 0.2 | 0.32 | 0.09 | 0.1 | 5 |
| RMBPC reaction | ||||||||
| N_contact | –0.03 | 0.08 | 0.68 | –0.109 | –0.033 | 0.071 | 0.074 | 0 |
| L_contact | 0.002 | 0.001 | 0.1 | 0.001 | 0.002 | 0.001 | 0.001 | 0 |
| Previous | 0.10 | 0.02 | < 0.001 | 0.109 | 0.118 | 0.014 | 0.015 | 5 |
| Period | 0.34 | 0.45 | 0.45 | 0.148 | 0.368 | 0.443 | 0.477 | 0 |
| N_contact × period | 0.12 | 0.11 | 0.29 | 0.106 | 0.187 | 0.108 | 0.112 | 0 |
| L_contact × period | –0.003 | 0.001 | 0.07 | –0.003 | –0.003 | 0.001 | 0.002 | 2 |
| Previous × period | 0.01 | 0.02 | 0.62 | 0.007 | 0.019 | 0.02 | 0.021 | 0 |
| EQ-5D index (proxy) | ||||||||
| N_contact | –0.02 | 0.01 | 0.08 | –0.025 | –0.013 | 0.008 | 0.009 | 3 |
| L_contact | 0.00 | 0 | 0.15 | 0 | 0 | 0 | 0 | 1 |
| Previous | 0.69 | 0.07 | < 0.001 | 0.659 | 0.711 | 0.062 | 0.067 | 5 |
| Period | –0.2 | 0.08 | 0.01 | –0.237 | –0.08 | 0.062 | 0.067 | 4 |
| N_contact × period | 0.01 | 0.01 | 0.62 | 0.002 | 0.014 | 0.013 | 0.014 | 0 |
| L_contact × period | 0.00 | 0 | 0.57 | 0 | 0 | 0 | 0 | 0 |
| Previous × period | 0.25 | 0.11 | 0.022 | 0.118 | 0.302 | 0.098 | 0.103 | 3 |
| EQ-5D VAS (proxy) | ||||||||
| N_contact | –1.47 | 0.65 | 0.027 | –1.63 | –1.012 | 0.59 | 0.634 | 4 |
| L_contact | 0.01 | 0.65 | 0.13 | 0.009 | 0.012 | 0.007 | 0.008 | 0 |
| Previous | 0.44 | 0.07 | < 0.001 | 0.362 | 0.464 | 0.067 | 0.072 | 5 |
| Period | –31.5 | 8.45 | < 0.001 | –36.79 | –28.89 | 6.95 | 7.512 | 5 |
| N_contact × period | 2.49 | 1.13 | 0.03 | 2.08 | 2.718 | 1.024 | 1.114 | 4 |
| L_contact × period | –0.02 | 0.01 | 0.11 | –0.024 | –0.022 | 0.014 | 0.016 | 0 |
| Previous × period | 0.46 | 0.13 | < 0.001 | 0.388 | 0.583 | 0.109 | 0.119 | 5 |
| QoL-AD | ||||||||
| N_contact | –0.17 | 0.13 | 0.21 | –0.18 | –0.099 | 0.114 | 0.129 | 0 |
| L_contact | 0.00 | 0 | 0.8 | 0 | 0.001 | 0.001 | 0.002 | 0 |
| Previous | 0.74 | 0.06 | < 0.001 | 0.699 | 0.801 | 0.051 | 0.055 | 5 |
| Period | –12.68 | 3.34 | < 0.001 | –19.53 | –8.131 | 2.88 | 3.138 | 5 |
| N_contact × period | –0.03 | 0.23 | 0.92 | –0.008 | 0.064 | 0.211 | 0.23 | 0 |
| L_contact × period | 0.00 | 0 | 0.99 | –0.001 | 0 | 0.003 | 0.003 | 0 |
| Previous × period | 0.38 | 0.11 | < 0.001 | 0.232 | 0.585 | 0.093 | 0.1 | 5 |
| GS | ||||||||
| N_contact | 0.20 | 0.12 | 0.092 | 0.115 | 0.23 | 0.097 | 0.117 | 2 |
| L_contact | –0.003 | 0.001 | 0.016 | –0.004 | –0.003 | 0.001 | 0.001 | 4 |
| Previous | 0.55 | 0.06 | < 0.001 | 0.529 | 0.597 | 0.052 | 0.061 | 5 |
| Period | –2.15 | 1 | 0.03 | –2.821 | –1.295 | 0.861 | 0.948 | 3 |
| N_contact × period | –0.04 | 0.22 | 0.86 | –0.166 | 0.057 | 0.19 | 0.221 | 0 |
| L_contact × period | 0.001 | 0.003 | 0.77 | 0 | 0.001 | 0.003 | 0.003 | 0 |
| Previous × period | 0.63 | 0.13 | < 0.001 | 0.463 | 0.725 | 0.105 | 0.113 | 5 |
| SSCQ | ||||||||
| N_contact | 0.13 | 0.13 | 0.32 | 0.016 | 0.195 | 0.109 | 0.117 | 0 |
| L_contact | 0.00 | 0 | 0.46 | –0.002 | 0 | 0.001 | 0.001 | 0 |
| Previous | 0.68 | 0.05 | < 0.001 | 0.632 | 0.72 | 0.047 | 0.05 | 5 |
| Period | –7.23 | 2.62 | 0.01 | –9.883 | –3.868 | 2.293 | 2.431 | 4 |
| N_contact × period | –0.22 | 0.22 | 0.32 | –0.421 | –0.031 | 0.194 | 0.211 | 1 |
| L_contact × period | 0.00 | 0 | 0.44 | 0.001 | 0.003 | 0.003 | 0.003 | 0 |
| Previous × period | 0.32 | 0.1 | < 0.001 | 0.207 | 0.429 | 0.088 | 0.092 | 4 |
In conclusion, the number of contacts was a significant predictor of RMBPC frequency (p = 0.023) and for proxy EQ-5D VAS (p = 0.027), that is, the quality of life of the person with dementia reported by the carer. The coefficient was positive for RMBPC frequency and negative for the proxy EQ-5D VAS, suggesting that, where more mental health contacts were provided to dyads, those carers reported more CB and also reported lower ratings of quality of life for their relative with dementia. This may be because mental health service practitioners have more contact with families when they notice them as having more CB. The total length of contacts was a significant (p = 0.016) predictor of guilt among carers. As the coefficient was negative, this showed that the greater the total duration of contact by the practitioner, the lower the levels of GS in the family carer. When comparing the first time period with the second time period to examine whether or not the timing of contacts (earlier vs. later) made any difference to the outcomes measured, significant effects were noted for RMBPC frequency (coefficient = 0.23; p = 0.0382), GS (coefficient = 0.63; p < 0.001), SSCQ (coefficient = 0.32; p ≤ 0.0021), proxy EQ-5D VAS (coefficient = 0.46; p < 0.001), proxy EQ-5D index (coefficient = 0.25; p = 0.0224) and the QoL-AD (coefficient = 0.38; p < 0.001). The direction of these coefficients is difficult to interpret, so no definitive conclusions can be reached on the timing of contacts over this 6-month period.
Research question 3
What are the predictors of change in CB, measured by the frequency and reaction domains of the RMBPC; and the NPI total and distress domains?
This analysis looked at whether or not predictors of change in CB could be determined. Here, CB was measured by four responses: the frequency and reaction domains of the RMBPC and the NPI total and distress domains.
As stable clusters could not be identified in the primary analyses, they could not be included in this analysis. Table 57 shows the changes across the three time periods after the 25% missing rule had been applied to the data. The change between baseline and second follow-up was analysed using backward regression analysis (see Appendix 14), with the potential predictors being demographic variables (person with dementia’s age and gender, carer’s age and gender, relationship, whether or not the dyad is living together, carer’s job type and baseline CDR-SB), and the mean of the baseline and second follow-up measures (RMBPC incidence, frequency and reaction, NPI total and distress, EQ-5D index and VAS of participants and carers, QoL-AD, SSCQ, ICECAP-O, GHQ-12 HADS, QCPR, GS and RSS). The mean of the baseline and second follow-up measure is included as a predictor to account for the expected relationship between the change over time and the mean over time; the predictors of change after the level of the response has been accounted for are being looked for here.
| Measure | First follow-up – baseline (N = 126) | Second follow-up – first follow-up (N = 114) | Second follow-up – baseline (N = 117) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| RMBPC frequency | 124 | –0.42 | 8.46 | 111 | 0.58 | 8.83 | 114 | 0.70 | 9.83 |
| RMBPC reaction | 118 | –2.12 | 11.02 | 106 | 1.37 | 11.04 | 116 | –0.02 | 11.52 |
| NPI total | 126 | –0.43 | 15.69 | 114 | 0.16 | 15.10 | 117 | –0.51 | 18.81 |
| NPI distress | 126 | –1.29 | 8.06 | 114 | 0.78 | 7.36 | 117 | –0.26 | 9.02 |
For all four responses, the mean of the baseline and second follow-up measures was found to predict the change between baseline and follow-up. Carer gender predicted RMBPC frequency, and both NPI total score and distress (see Appendix 14). No other predictor variables were consistently found across the data set using the five imputed data sets, showing that there are no other stable predictors of change. Male carers showed the larger decrease in the measures.
Economic analyses
Characteristics of the sample
Table 58 shows the baseline characteristics of dyads for whom data at all the time points were collected (n = 114). For the purposes of the main health and care economic analyses we used only the data from this sample, rather than the full available sample at each of the time points.
| Descriptive | Person with dementia (N = 114) | Carer (N = 114) |
|---|---|---|
| Gender (female), n (%) | 63 (55.3) | 77 (67.5) |
| Age group (years), n (%) | ||
| 30–39 | – | 1 (0.9) |
| 40–49 | – | 10 (8.8) |
| 50–59 | – | 20 (17.5) |
| 60–69 | 12 (10.5) | 34 (29.8) |
| 70–79 | 48 (42.1) | 28 (24.6) |
| 80–89 | 44 (38.6) | 19 (16.7) |
| 90–99 | 10 (8.8) | 2 (1.8) |
| Mean (SD) | 79.33 (7.48) | 67.46 (12.63) |
| Relationship to person with dementia, n (%) | ||
| Spouse | – | 68 (59.6) |
| Son/daughter (including in-law) | – | 40 (35.1) |
| Other relative | – | 4 (3.5) |
| Other (e.g. friend/neighbour) | – | 2 (1.8) |
| Carer employment status, n (%): | ||
| Paid employment | – | 28 (24.6) |
| Unemployed | – | 6 (5.3) |
| Homemaker | – | 2 (1.8) |
| Retired | – | 78 (68.4) |
| NPI total score: mean (SD) | 24.8 (19.1) | – |
| EQ-5D index (proxy), mean (SD) | 0.51 (0.31) | – |
| EQ-5D index (self-report), mean (SD) | 0.75 (0.24)a | 0.80 (0.23) |
| EQ-5D VAS (proxy), mean (SD) | 52.9 (20.78) | – |
| EQ-5D VAS (self-report), mean (SD) | 69.91 (17.94)a | 73.72 (18.21) |
| QoL-AD (proxy), mean (SD) | 30.36 (5.73) | – |
| QoL-AD (self-report), mean (SD) | 37.38 (4.99)b | – |
| SSCQ, mean (SD) | – | 24.9 (6.10) |
| GHQ-12, mean (SD) | – | 13.8 (4.80) |
| HADS, mean (SD) | – | 9.1 (6.08) |
| QCPR, mean (SD) | 57.81 (6.56)a | 52.9 (9.01) |
| GS, mean (SD) | – | 5.7 (4.39) |
| RSS, mean (SD) | – | 19.2 (10.12) |
Service use at baseline
Full service use frequencies and costs for the 114 participants available at all time points are presented in Appendix 15 and for the whole cohort that were available at each of the individual time points in Appendix 16. For the 114 at baseline, when carers were asked to recall the person with dementia’s contacts with health- and social-care services using the CSRI, 71.9% of people with dementia in the FamCare study had seen a GP, 71.1% had seen a CMHN and 19.3% had seen a social worker in the previous 3 months. Twenty per cent of participants with dementia had seen a community-based old age psychiatrist over the preceding 3 months. Contacts with hospital services, including A&E admission and overnight stays, were infrequent. These patterns were similar for the full baseline cohort of 157 and the 114 dyads for which data were available for all three time points.
When asked to recall type and frequency of services accessed, 52.6% of family carers had themselves accessed the GP in the preceding 3 months and 24.6% had seen a practice nurse. A social worker had separately been seen by 3.2% of family carers. Use of hospital services was low; 18.4% of family carers had themselves visited outpatient clinics.
Research question 4
What are the patterns of health- and social-care service use and associated costs?
Figures 14 and 15 provide a snapshot of the proportions of people with dementia and their carers accessing selected services over the study period. High proportions of both participants with dementia and their carers saw a GP over the study period. The proportions of contacts with a GP that took place at home, rather than at the GP surgery, increased from 8.3% at baseline to 27.7% at the second follow-up.
FIGURE 14.
The FamCare study: proportion of people with dementia accessing selected services at each time point (n = 114).
FIGURE 15.
The FamCare study: proportion of carers accessing selected services at each time point (n = 114).
In order to be able to explore patterns of service use and costs over commensurate study periods, we converted these into monthly frequencies and monthly mean costs. Table 59 shows mean monthly frequencies of health- and social-care contacts at each time point for dyads interviewed at all time points (n = 114), and Table 60 shows mean monthly costs. For a more detailed breakdown of the health- and social-care categories for people with dementia and carers see Appendix 17.
| Service | Time point, mean monthly frequencya (SD) | |||||
|---|---|---|---|---|---|---|
| Person with dementia (n = 114) | Carer (n = 114) | |||||
| Baseline | First follow-up | Second follow-up | Baseline | First follow-up | Second follow-up | |
| Community health care, excluding mental health | 1.40 (1.62) | 1.14 (1.45) | 1.58 (4.27) | 0.55 (0.64) | 0.54 (0.70) | 0.53 (0.87) |
| Community mental health services | 0.57 (0.73) | 0.58 (0.75) | 0.37 (0.98) | 0.04 (0.24) | 0.03 (0.16) | 0.07 (0.39) |
| Hospital services | 0.59 (1.39) | 0.70 (2.87) | 0.83 (2.19) | 0.18 (0.38) | 0.23 (0.89) | 0.28 (0.91) |
| Social care services, including day care | 4.99 (13.59) | 6.30 (14.83) | 8.19 (18.83) | 0.10 (0.79) | 0.09 (0.55) | 0.04 (0.33) |
| Otherb | 0.63 (2.95) | 0.39 (2.62) | 0.32 (1.97) | 0.14 (0.26) | 0.22 (0.44) | 0.11 (0.21) |
| Mean monthly service use | 8.19 (15.69) | 9.13 (15.20) | 10.87 (19.65) | 1.03 (1.32) | 1.10 (1.69) | 1.04 (1.52) |
| Service | Time point, mean monthly cost (£) (SD) | |||||
|---|---|---|---|---|---|---|
| Person with dementia (n = 114) | Carer (n = 114) | |||||
| Baseline | First follow-up | Second follow-up | Baseline | First follow-up | Second follow-up | |
| Community health care, excluding mental health | 58.05 (57.54) | 43.45 (63.41) | 57.19 (136.88) | 34.40 (69.37) | 28.01 (45.75) | 27.57 (51.36) |
| Community mental health services | 60.10 (220.48) | 53.63 (90.82) | 17.44 (57.55) | 4.30 (26.61) | 1.82 (10.46) | 4.60 (24.41) |
| Hospital services | 214.07 (753.75) | 210.26 (1117.22) | 147.17 (627.12) | 48.30 (235.72) | 31.91 (185.06) | 102.04 (466.30) |
| Social care services, including day care | 106.40 (247.65) | 137.59 (299.45) | 163.11 (334.67) | 6.10 (41.27) | 6.01 (47.55) | 1.29 (6.33) |
| Othera | 12.17 (41.10) | 6.13 (19.13) | 4.03 (12.42) | 3.43 (6.26) | 10.47 (57.86) | 3.20 (7.87) |
| Mean monthly service use costs | 453.33 (877.99) | 451.06 (1150.31) | 388.95 (748.37) | 96.96 (255.04) | 78.21 (231.76) | 138.70 (478.13) |
Service use across community- and hospital-based health- and social-care services was low for both participants with dementia and carers across the study period.
During the 3 months before baseline, one (0.9%) person with dementia and 28 (24.6%) carers recorded no contacts with community-based health- and social-care services. This increased to five (4.4%) people with dementia and 36 (31.6%) carers for the 2 months between baseline and the first follow-up. During the 4 months between the first and second follow-ups, six (5.3%) people with dementia and 29 (25.4%) carers recorded no contacts with community-based health- and social-care services.
When looking at community-based health service use for dyads, the average number of contacts with a GP during the 3 months before baseline was three. There were 37 dyads with higher than average GP contacts (i.e. four or more contacts). These high GP-using dyads had an average of 11 contacts with community-based health- and social-care services, excluding GPs; dyads with three or fewer GP contacts had an average of 22 contacts with other services.
The cost of social care was higher than the cost of community health care (excluding mental health services) for participants with dementia (see Table 60), because participants used these services more often. The main driver of costs in this category was the costs of the home care worker (see Appendix 17, Table 108), with participants having an average of 2.72 (SD 12.69) contacts per month at baseline, increasing to 5.52 (SD 17.80) contacts per month at second follow-up. Care attendants other than social care staff were also seen regularly, with an average monthly frequency of 0.75 (SD 4.20) contacts per month at baseline, increasing to 0.99 (SD 5.22) contacts per month at second follow-up.
Conversely, carers had higher community health-care costs than social care costs (see Table 60). Mean social worker care costs per carer were minimal and decreased over the study period. Only 3.5% of carers had seen a social worker in the 3 months preceding baseline, which is a considerably lower proportion than the 19.3% of participants with dementia who saw a social worker. It may be, of course, that the carers and their relatives were seen together by social workers at their own request.
Research question 5
What are the estimated costs of informal family care?
Over two-thirds of the carers were living with the person with dementia. Resident carers were asked how many hours they felt that they could leave the person with dementia alone for in a typical day. The majority of carers responded ‘not at all’ or ‘an hour or two’. The numbers of hours per week spent in caring for the person with dementia are shown in Table 61. At baseline, resident carers spent an average of 112.15 (SD 58.48) hours per week caring (approximately 16 hours per day), compared with 21.71 (SD 33.54) hours per week (approximately 3 hours per day) spent by carers who did not live with the person with dementia. At the first follow-up, the average number of hours spent caring had increased slightly for resident carers and dropped for non-resident carers. At the second follow-up, the average number of hours spent caring had increased for both resident and non-resident carers.
| Resident/non-resident | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 114) | First follow-up (n = 114) | Second follow-up (n = 114) | |||||||
| Valid/n | Mean (SD) | Total (minimum–maximum) | Valid/n | Mean (SD) | Total (minimum–maximum) | Valid/n | Mean (SD) | Total (minimum–maximum) | |
| Resident carer | 74/83 | 112.15 (58.48) | 8299 (0–168) | 72/82 | 121.81 (56.56) | 8770 (0–168) | 72/75 | 129.18 (51.07) | 9301 (0–168) |
| Non-resident carer | 24/31 | 21.71 (33.54) | 521 (3–168) | 24/32 | 16.46 (10.10) | 395 (2–35) | 31/39 | 22.68 (34.82) | 703 (2–158) |
| Total | 98/114 | 90.00 (66.07) | 8820 (0–168) | 96/114 | 95.47 (67.22) | 9165 (0–168) | 103/114 | 97.13 (67.69) | 10,004 (0–168) |
Over the period, the estimated average weekly time devoted to care by families was 112 hours at baseline, rising to 129 hours at 6 months.
Table 62 shows the costs of care if the hours spent by carers in our cohort were undertaken by a paid home care worker at the rate of £23 per hour of face-to-face contact,153 this reflecting the cost of the total service and not the amount paid to an individual home care worker. Here we refer to these costs as ‘replacement costs’. Across resident and non-resident carers, the average weekly cost of care per person was £2070 at baseline, rising to £2234 at the 6-month follow-up. If the minimum wage (£6.19 per hour in 2012) is used, instead, to estimate unpaid carer time, weekly costs per person at baseline are lower, at £557, rising to £601 at 6 months.
| Resident/non-resident | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | First follow-up | Second follow-up | |||||||
| Valid/n | Total cost (£) | Mean (SD) (£) | Valid/n | Total cost (£) | Mean (SD) (£) | Valid/n | Total cost (£) | Mean (SD) (£) | |
| Resident carer: home care worker wage | 74/83 | 190,877 | 2579.42 (1344.29) | 72/82 | 201,710 | 2801.53 (1300.86) | 72/75 | 213,923 | 2971.15 (1174.52) |
| Non-resident carer: home care worker wage | 24/31 | 11,983 | 499.29 (771.44) | 24/32 | 9085 | 403.32 (232.39) | 31/39 | 16,169 | 521.58 (800.92) |
| Care worker wage total | 98/114 | 202,860 | 2070.00 (1519.71) | 96/114 | 210,795 | 2195.78 (1546.02) | 103/114 | 230,092 | 2233.90 (1556.82) |
| Resident carer: minimum wage | 74/83 | 51,371 | 694.20 (361.71) | 72/82 | 54,286 | 753.98 (350.10) | 72/75 | 57,573 | 799.63 (316.10) |
| Non-resident carer: minimum wage | 24/31 | 3225 | 134.37 (207.62) | 24/32 | 2445 | 108.55 (62.54) | 31/39 | 4352 | 521.58 (140.37) |
| Minimum wage total | 98/114 | 54,596 | 557.10 (409.00) | 96/114 | 56,731 | 590.95 (416.08) | 103/114 | 61,925 | 601.21 (418.99) |
Overall, based on figures in Table 62, the estimated annual costs at baseline, costed at paid home care worker rates of £23 an hour, were £107,640 (£2070 per week); and, costed at the minimum wage rate of £6.19 per hour, these were £28,969 (£557.10 per week). At the 6-month follow-up these rose to £116,168 (£2234 per week) at paid home carer rates and £31,263 (£601.21 per week) at minimum wage rates.
The costs of family care accounted for 80.6% of the total costs of health and social care for people with dementia living in the community.
Comparable to our findings are those of the Alzheimer’s Research Trust in 2010, which reported estimated costs of unpaid carer time at 86% of the total costs of health and social care. 234 The Alzheimer’s Society, in its recent update (2014) of its report of 2007,235 also estimated costs of family care for people with dementia living at home and estimated unpaid carer time at 74.9% of total costs. Unlike our method of costing, the Alzheimer’s Society assigned ‘replacement costs’ for unpaid care equal to the total organisational cost of employing a professional carer (at £19 per hour) for help with personal care activities, and ‘opportunity costs’ to represent the value to individual carers of any activities they can no longer take part in (such as paid work, housework, leisure activities and caring for children), while ‘supervising’ or ensuring that the person with dementia is safe and comfortable. For the FamCare study, we used replacement costs for all care, not just the personal care activities. The Alzheimer’s Society study reported the average annual cost of informal care to be £19,714, £32,237 and £33,482 for people with mild, moderate and severe dementia, respectively. Compared with this, our FamCare study annual estimated costs at the paid home care worker rate of £23,153 for people living at home with additional CB, was, at baseline, £107,640 (£2070 per week; see Table 62); costed at the minimum wage rate of £6.19 per hour, this fell to £28,969 (£557.10 per week; see Table 62). The method we used in the FamCare study would be expected to produce cost estimates that are higher than would be the case if none or only a proportion of the opportunity costs were applied, although, for our purposes of understanding costings for informal care, we have discussed these at the minimum wage rate rather than at the higher rate for paid home care. The authors of the Alzheimer’s Society study note that, should all unpaid hours of care become paid care, at the cost of a paid home care worker, uptake by carers would be unlikely. 235 However, the costings of informal care are not straightforward, as personal circumstances and motivations within families may vary and are therefore hard to operationalise and measure. For example, an adult family member of the person with dementia may take annual leave, or retire or give up paid employment to support well-being in their relative. For these families, this not unsubstantial contribution is perhaps a justifiable cost consideration, in addition to the more stringent calculation of replacement costs for ‘help with personal care activities’.
Later we will return to the issue of whether or not an understanding of the true costs of the experience of some families in supporting a person with dementia and CB can be measured, using estimates of either or both replacement and opportunity costs. Irrespective of the method of costing, our findings note that, for this cohort of people with dementia and significant CB, cost estimates associated with informal care provided by family members and friends, at 80.6% of the total costs of care, significantly dwarf those provided by health- and social-care services.
The number of nights spent by participants with dementia in alternative accommodation was also examined (see Appendix 18). The majority of participants did not live elsewhere during the study period. At baseline, only 2.6% (n = 3) had spent time in a care home during the previous 3 months; this increased to 11.4% (n = 13) at 6 months. The mean number of nights per month in a care home increased from 0.12 (SD 0.80) per participant at baseline to 1.47 (SD 5.47) per participant at 6 months.
Type of care home (i.e. private sector or local authority funded) was not specified. The average cost of residential care in 2012 was £545 per resident per week for private sector residential homes and £1030 per resident per week for local authority homes. 153 In comparison, private sector care homes with nursing cost an average of £758 per resident per week. Assuming that all residential care in the FamCare study occurred in private sector care homes, the mean cost of combined residential and nursing care home use was £9 per participant per month at baseline. This increased to £31 per participant per month at first follow-up and £131 per participant per month at second follow-up. However, this increase is largely caused by a few users, rather than a general trend towards everyone using a bit more respite or short break care. In the 3 months before baseline, three people (2.6%) with dementia had used respite services in a care home. At the second follow-up, this had increased to 13 (11.4%). Of the 13, eight had spent at least 1 month in respite care, with two of these spending 90 nights in respite care over the previous 4 months. Additionally, it is worth noting that 7 out of the 43 who dropped out of the study moved into a care home over the study period. The reasons for this are unclear, but could include deterioration in the patient’s condition or a reduction in ability of the family carer to provide support.
Research question 6
What are the patterns and costs of prescribing medications?
Table 63 shows medication usage for people with dementia and carers, over the 6-month period. To examine patterns of prescribing and change over time, a McNemar’s test was performed for those participants with dementia and their carers who had a change in their prescription between baseline and the second follow-up. The only changes noted related to the person with dementia, when prescriptions for dementia drugs increased and use of non-opioid pain relief decreased. No other drug categories changed significantly.
| Categorya | Prescription frequency | McNemar’s test p-value | |||
|---|---|---|---|---|---|
| At baseline and second follow-up, n (%) | Not at all, n (%) | At baseline but not second follow-up, n (%) | Not at baseline, but at second follow-up, n (%) | ||
| Person with dementia | |||||
| Antipsychotics | 8 (6.8) | 105 (89.7) | 2 (1.7) | 2 (1.7) | > 0.999 |
| Antidepressants | 22 (18.8) | 87 (74.4) | 2 (1.7) | 6 (5.1) | 0.289 |
| Hypnotics and anxiolytics | 5 (4.3) | 108 (92.3) | 1 (0.9) | 3 (2.6) | 0.617 |
| Anticonvulsants | 2 (1.7) | 112 (95.7) | 1 (0.9) | 2 (1.7) | > 0.999 |
| Dementia drugs | 35 (29.9) | 53 (45.3) | 4 (3.4) | 25 (21.4) | < 0.001 |
| Pain relief: opioid | 8 (6.8) | 102 (87.2) | 4 (3.4) | 3 (2.6) | > 0.999 |
| Pain relief: non-opioid | 7 (6.0) | 97 (82.9) | 11 (9.4) | 2 (1.7) | 0.027 |
| Laxatives | 7 (6.0) | 105 (89.7) | 2 (1.7) | 3 (2.6) | > 0.999 |
| Carer | |||||
| Antipsychotics | 0 (0) | 117 (100) | 0 (0) | 0 (0) | |
| Antidepressants | 10 (8.5) | 102 (87.2) | 1 (0.9) | 4 (3.4) | 0.371 |
| Hypnotics and anxiolytics | 2 (1.7) | 113 (96.6) | 1 (0.9) | 1 (0.9) | > 0.999 |
| Anticonvulsants | 1 (0.9) | 116 (99.1) | 0 (0) | 0 (0) | |
| Pain relief: opioid | 10 (8.5) | 102 (87.2) | 3 (2.6) | 2 (1.7) | > 0.999 |
| Pain relief: non-opioid | 3 (2.6) | 108 (92.3) | 3 (2.6) | 3 (2.6) | > 0.999 |
| Laxatives | 2 (1.7) | 114 (97.4) | 0 (0) | 1 (0.9) | > 0.999 |
The total cost of prescribing for people with dementia who were interviewed at all time points in this cohort (n = 114) was £70,029 over the 6-month period. The pro rata prescribing costs were estimated to be over £93,000, with an estimated cost per year per participant with dementia of £819 (SD £693); these costs were more than double that of the carer. Acetylcholinesterase inhibitors represented the highest prescribing cost for any individual medication category. For a breakdown of the prescribing costs for the 3 months before baseline for the whole sample (n = 157) of people with dementia for whom data were available at baseline, see Appendix 19.
Table 64 shows the mean cost of prescriptions per dyad (person with dementia and carer) per time point for each drug category of interest. Figure 16 shows the average number of participants with dementia prescribed each drug category per time point, and the mean cost per participant per month. In this cohort, prescribing expenditure over 9 months for people with dementia was highest for dementia drugs, that is, acetylcholinesterase inhibitors (£30,466), and, for some, cognitive enhancers (£2448). The drug group accounting for the next highest prescribing expenditure was ‘Other’ medications (£29,692). Unsurprisingly, given the age of the cohort, costs related to opioid analgesia were relatively high (£3588).
| Category | Time point, mean (SD) monthly cost (£) per participant | |||||
|---|---|---|---|---|---|---|
| People with dementia (n = 114) | Carers (n = 114) | |||||
| At baseline | At first follow-up | At second follow-up | At baseline | At first follow-up | At second follow-up | |
| Antipsychotics | ||||||
| Atypical antipsychotics | 1.51 (6.73) | 2.05 (8.31) | 1.48 (7.13) | – | – | – |
| Typical antipsychotics | 0.15 (1.14) | 0.57 (6.07) | 0.35 (3.78) | – | – | – |
| Hypnotics and anxiolytics | ||||||
| B/Z/A drugs | 0.22 (1.02) | 0.22 (0.96) | 0.18 (0.76) | 0.03 (0.22) | 0.01 (0.07) | 0.04 (0.23) |
| Non-B/Z/A drugs | – | – | – | – | – | – |
| Antidepressants | ||||||
| SSRI | 0.36 (1.41) | 0.35 (1.46) | 0.37 (1.40) | 1.10 (5.25) | 0.42 (2.23) | 0.48 (2.49) |
| Tricyclic | 0.52 (4.72) | 0.30 (2.39) | 0.29 (2.39) | 0.01 (0.08) | 0.07 (0.53) | 0.03 (0.16) |
| Other | 0.16 (0.67) | 0.13 (0.59) | 0.12 (0.55) | 0.47 (3.56) | 0.21 (2.25) | 0.21 (2.25) |
| Anticonvulsants | 0.14 (0.88) | 0.15 (0.90) | 0.09 (0.68) | 0.13 (1.37) | 0.29 (3.08) | 0.03 (0.34) |
| Dementia drugs | ||||||
| Acetylcholinesterase inhibitors | 23.75 (39.98) | 29.70 (41.63) | 34.08 (44.05) | – | – | – |
| Cognitive enhancers | 2.03 (15.44) | 1.31 (8.42) | 3.20 (12.74) | – | – | – |
| Pain relief | ||||||
| Opioida | 4.53 (31.62) | 7.94 (50.84) | 0.51 (2.22) | 3.32 (17.07) | 5.37 (28.53) | 3.22 (22.98) |
| Non-opioid | 0.39 (1.24) | 0.42 (1.53) | 0.21 (0.92) | 0.13 (0.77) | 0.47 (1.68) | 0.23 (1.20) |
| Laxatives | 0.31 (1.45) | 0.27 (1.30) | 0.29 (1.19) | 0.09 (0.81) | 0.15 (1.02) | 0.12 (0.99) |
| Otherb | 27.46 (38.85) | 27.83 (40.81) | 30.61 (45.68) | 20.62 (40.44) | 23.61 (46.91) | 24.60 (46.14) |
| No medication | – | – | – | – | – | – |
| Total | 61.61 (64.60) | 71.23 (80.09) | 71.76 (67.14) | 25.89 (48.26) | 30.59 (60.30) | 28.96 (52.10) |
FIGURE 16.
The FamCare study: mean number of participants with dementia prescribed each medication category and mean cost per participant per month (n = 114). Note that the number is averaged over all three time points and ‘other’ medications have been excluded.
The average cost of prescribing per month per person with dementia varied slightly between time points [baseline: £61.61 (SD £64.60); first follow-up: £71.23 (SD £80.09); and second follow-up: £71.76 (SD £67.14)], but was more than double that of carers at each time point [baseline: £25.89 (SD £48.26); first follow-up: £30.59 (SD £60.30); and second follow-up: £28.96 (SD £52.10)], with the main difference in prescribing costs, not surprisingly, relating to carers not being prescribed an acetylcholinesterase inhibitor, a cognitive enhancer or an antipsychotic. The total cost of prescribing for carers in this cohort (n = 114) was £29,036, with ‘other’ medications (£23,654) and opioid analgesia (£3825) accounting for the highest prescribing expenditure.
Research question 7
What are the total costs of services, informal care and medication?
Table 65 summarises the total costs for the 114 people with dementia available at all time points. The mean annual cost of community-based service use (including day care) and hospital-based service use for each participant with dementia prorated is £5076. Participants had low use of respite services, but we calculated this additional service at £676 per person per year. Our estimate of the annual cost of unpaid carer time, using minimum wage (£6.19), is £27,369 per participant when using weekly means prorated up across the sample of 114 (see Table 65); this is as opposed to the actual data available at each time point used in Table 62. Our estimate of the mean annual cost of care per person with dementia with CB is £33,941 (SD £19,268), of which the majority of the costs are borne by family carers.
| Cost categorya | Cost (£) | ||
|---|---|---|---|
| Total over 9-month period | Pro rata total | Pro rata per participant | |
| Service use (community care, social care, day care and hospital use) | 434,016 | 578,688 | 5076 (6525) |
| Informal (family) careb | 2,340,080 | 3,120,107 | 27,369 (18,288) |
| Residential and nursing respite care | 57,848 | 77,130 | 676 (2147) |
| Prescribing | 70,029 | 93,371 | 819 (693) |
| Total costs | 2,901,972 | 3,869,296 | 33,941 (19,268) |
We also compared our findings with those of the Alzheimer’s Research Trust 2010 report234 and the Alzheimer’s Society 2007 report (which was updated in 2014). 265 The Alzheimer’s Research Trust 2010 report234 estimated the total annual cost of dementia to the economy as £23B. This report did not estimate the cost per person, but provided a breakdown of the allocation of costs between health care attached to GPs, nurse contacts, hospital use and prescribing costs; residential care; and ‘non-health or social care’ which referred to carer time, mortality and morbidity. Our findings, that family care accounts for a considerable proportion of the total costs for people with dementia living in the community, concur with those noted in the Alzheimer’s Research Trust 2010 report. 234 Its estimated cost of unpaid carer time accounted for 86% of the total costs and is comparable to our findings of 80.6% for this cohort of people with dementia and clinically significant CB. The Alzheimer’s Society’s Dementia UK report of 2007,265 gave estimates of costs of care for people with dementia living in the community, stratified by disease severity. Annual costs reported by the authors in 2007 for mild, moderate and severe dementia were estimated at an average of £16,689, £25,877 and £37,473, respectively. The increase in cost as the disease progresses probably reflects the additional services required to manage the condition, including additional time from family carers. Even accounting for inflation to 2012 prices, our estimate of £33,941 per person per year is comparable to the estimates of 2007.
However, the Alzheimer’s Society’s 2014 update,235 which reported 2012/13 costs, noted that the direct costs of health and social care, that is, excluding informal care and prescribing costs, in 2012/13 were very different from those in 2005/6; the annual average for people with mild dementia living at home in 2012/13 was lower at £5872 than 2005/6 when these were £8634; the corresponding figures for people with moderate dementia were similar at £10,467 in 2012/13 compared with £10,039 in 2005/6; and those for severe dementia were much higher at £21,579 in 2012/13 than £12,037 in 2005/6. The Alzheimer’s Society’s data were collected at one time point and we therefore compare these data with our baseline FamCare study data: our estimated annual cost of caring for people with dementia and significant CB at home is £5440 per participant, with a monthly cost of £453.33 (see Table 60). In our cohort 63.1% of patients had mild dementia, 28% had moderate dementia and 8.9% had severe dementia.
From these data, we can see that the direct health- and social-care costs (excluding informal care and prescribing costs) of supporting people with dementia and significant levels of CB at home are lower than any of those for the range of people with dementia, of any severity, that were noted by the Alzheimer’s Society in its 2014 update of the health- and social-care costs of dementia. 235
Consultation with stakeholders
In the first set of consultations, in which different perspectives of CB among people with dementia were explored with 83 specialist community mental health practitioners and their managers from participating NHS organisations, it emerged that there was limited knowledge about the prevalence and impact of CB among people with dementia living in community settings; or where and how the families caring for such individuals might be supported by local NHS community mental health services. For example, many practitioners felt that their service may have missed potential cases of clinically significant CB (measured by the RMBPC111 and the NPI135) for inclusion in our trial during early evaluations of referral to them or their initial interviews. Reasons put forward for this were that some carers could have under-reported CB and more detailed interviewing may have helped; or some carers may have appeared to be over-reacting to minor changes in their relative; and many felt that their service protocols lacked a structured assessment tool for CB. One NHS trust had tried the NPI,135 but non-medical practitioners had found this unhelpful in their work with family carers, so this was not used routinely. Some practitioners also indicated surprise that families with a score of five on the RMBPC111 or one on the NPI135,273 qualified as a case for clinically significant CB in dementia.
Salient themes from the first five sets of consultations with practitioners were followed by thematic analysis of data from two further discussion events held with the wider stakeholders in July 2013. Stakeholders attending included HealthWatch and other advocacy representatives (n = 3), carers and former carers (n = 6), commissioners of social care services (n = 10), voluntary sector organisations (n = 6) (such as the Alzheimer’s Society, Mind, British Red Cross and Age UK), other community-based health-care practitioners and managers (n = 11) and NHS commissioners (n = 2). A person with dementia, who was a member of the Programme Steering Group, also attended.
These diverse participant groups are here referred to as ‘stakeholders’. Use of qualitative methodology meant that no sample size was determined in advance for the wider stakeholder group discussion. Instead, individuals with a broad variety of relevant experiences working in different agencies, and others with a personal interest in dementia care, were approached and recruited by the research team. Their travel costs and any carer costs were met by the study.
Topic guides were informed by the emerging findings from the FamCare study data, but were tailored to stakeholder participants’ knowledge and role. Questions and discussion topics were devised to explore whether or not our findings ‘rang true’ with the wider stakeholder group.
A realist evaluation approach274 was used to synthesise the data from the combined groups in order to obtain a comprehensive picture of the contexts within which practitioners worked, the mechanisms which promoted or impeded changes, and the consequent outcomes for family carers supporting people with dementia with CB. Realist evaluation was chosen as it addresses the immediate priorities of empirical qualitative research, can be adapted to the research aims, addresses substantive issues and contributes to policy and practice development, rather than aiming for methodological purity. 275 Both consultation events were facilitated by members of the research team, who provided emerging results from the FamCare study fieldwork and asked those attending to debate the findings for their face validity and to offer some interpretations of the findings in the context of practice or service use. Both events were organised in ‘café style’, with tables around which mixed groups could debate the emerging findings. Members of the research team and their colleagues kept notes of the points made and these records were produced as non-verbatim transcripts and then analysed to consider within- and between-group themes. In order to protect confidentiality, all quotations are referred to anonymously and personal details have been redacted.
Consultation with stakeholders: findings
Changes in the NHS and local authorities in England, as they became dominated by reactions to the commissioning imperatives, were a common theme among the overarching reasons given by stakeholders as to why systematic, case-specific interventions for people with dementia and CB living at home and their families could not be accessed or delivered. Commissioning activity entailed prioritisation of new service models to respond to national imperatives, such as the National Dementia Strategy’s250 emphasis on early diagnosis and reductions in the use of antipsychotic medications in dementia. Other broader contextual factors concerned planning around implementation of the controversial Health and Social Care Act 2012,276 which in particular redefined the roles of GPs in England, and the financial pressures affecting local authorities and their contracts with the voluntary sector. Managers and practitioners also noted how new imperatives affected their services’ priorities and, as a consequence, some reorganisation was taking place or was mooted. Issues relating to practice and the way services are organised were found.
First, two areas of specific importance in delivering timely support for dementia with CB at home were identified as relevant to the present study. These were timely recognition of the problems and effective responses to them. Second, two particular imperatives were identified as profoundly affecting what dementia services were able to offer to family carers. These were pressures to avoid hospital admission or for rapid hospital discharge, and early diagnosis of dementia. These are outlined next.
Timely recognition and responses to challenging behaviour
There were some reflections that primary prevention measures to address the risk of CB could be effective if they were properly resourced, and some of these ‘low-level’ interventions were seen as applicable and acceptable to family carers:
Well we are creating peer support groups . . . people are lonely . . .
Voluntary sector worker
We help carers to cope with minor problems like forgetting, putting the kettle in the fridge . . .
Specialist CMHN J
However, many practitioners perceived that the problem remained that family carers did not always see CB as sufficiently difficult to justify seeking help, and that they often had little confidence that help would be forthcoming if they did raise such difficulties. Some carers were thought to see behavioural symptoms as something that were only ‘deserving’ of help if they included physical aggression or similar, whereas other practitioners themselves classed some symptoms as not ‘real’ CB:
[Carers are] . . . surprised that CB can include clinging, not letting carer out of sight, non-stop questioning.
CMHN G
CB . . . well I thought it was all about behavioural problems . . . aggression, wandering, hallucinations . . .
Specialist CMHN J
It’s not real CB . . . [s/he] can’t help forgetting . . . it’s [XXX the carer] who is demanding . . . [s/he] doesn’t seem able to accept dementia . . . I tried to explain to [him/her] that it’s the dementia [s/he the person with dementia] just can’t help it . . . not sure [s/he the carer] took this on board . . .
CMHN E
Several practitioners identified deficiencies in current services that needed to be addressed so that carers could cope better with CB symptoms. Such ideas were concentrated around the need for early identification of CB and work with carers to recognise that CB symptoms were becoming difficult to manage:
We need to know how to identify early . . . before there are huge problems . . . like who are the carers who may not cope.
Dementia nurse specialist
The short checklist . . . of problems that we were given during training helps . . . I can go though it with the carer and well . . . they admit to things a bit . . .
CMHN G
Carers say they are coping with low-level CB . . . repeated questioning . . . but I don’t think they are . . .
Voluntary sector carer advice worker
Carers don’t share information with family . . . wife doesn’t want to tell kids that husband is incontinent/hitting her as it’s their Dad. Doesn’t want to burden them.
Former carer
Such ideas about timely identification were confirmed by some carers and former carers reflecting on their own situations:
You don’t like to admit . . . too early that it’s difficult . . . even to yourself . . . s/he was still independent . . . dressing . . . going for the newspaper . . . to the bank . . . but then s/he would think I was his/her mother.
Former carer
I went to the clinic . . . s/he was upset that I was there . . . but I had to take him/her . . . They asked me if s/he had any problems . . . couldn’t dress him/herself, or shop and cook or if s/he had ever been aggressive . . . I didn’t like to say the small things . . . s/he does nothing all day . . . stopped going to church things since his/her fall . . . always ringing me up to ask about the pension . . . losing money and thinks I have taken it . . . well I was relieved when we left . . . the clinic . . . I didn’t want to complain about my dad/mum.
Daughter
It’s the small things in life . . . incessant checking . . . putting things in the wrong place and we spend hours looking . . . or always harping on and on about the same things . . . looking for [his/her] dead brother . . . not aggressive or anything and she still knows me . . .
Carer
Many practitioners were optimistic that skilled support, accessed in a timely way, could be effective in supporting carers to manage CB if it is addressed early on:
Carers need skilling up and support to practice strategies that may work . . . not just tips and pointers . . .
NHS psychologist
The CPNs [CMHNs] should show them [the carers] how to deal with the person . . . they may be accusing them [carers] of stealing or taking their property . . .
Social worker
The effectiveness of this was confirmed by a small group of carers who felt that they had been listened to; that evaluation of their needs had been comprehensive and helped them to understand and thus cope with their relative with dementia’s behaviour:
It was quite good for me . . . at the clinic . . . they asked me questions and filled in a form . . . when I next saw the nurse I was able to tell them more . . . I noticed things more by the next week . . . not a problem or anything . . . but silly things like clothes not being changed . . . quite smelly . . . s/he got upset and pushed me away when I tried to help him/her with a change of clothes.
Carer
S/he was trying to leave the house by the window at night . . . everyone was worried but we . . . the psychologist at the clinic and I discovered it was only because it looked like a toilet door . . . s/he needs to go a lot . . . to the toilet I mean . . . at night.
Carer
In contrast, recognition of CB too late meant that breakdown of care could occur or crises develop where there were very few options for acceptable resolution:
. . . a lot of pressure to ‘put her’ into a care home.
Former carer
I went to the memory clinic . . . [XXX the person with dementia] blamed me . . . refused to return . . . my [son/daughter] told me to wait and not go to the clinic . . . I didn’t say . . . [XXX the person with dementia] was just not the person I knew . . . irritable, quieter, getting aggressive . . . I mean s/he shouted at me . . . we never shout or argue . . . finally my GP arranged it all [move to care home] with social services and he/she [XXX the person with dementia] then didn’t know me when I visited . . . called me by my daughter’s name once . . . I still feel guilty now after all this time . . . but [XXX the person with dementia] never really hit me . . . or anything like that . . . I couldn’t manage anymore. It was making me ill . . .
Former carer
Hospital pressures and early dementia diagnosis
From different sectors, there were accounts of responding to the demands to avoid hospital admissions or to ensure that discharges were not delayed, as the following comments illustrate.
First, was the ‘hospital admission’ imperative:
We have to keep people out of hospitals so we need to work in care homes . . . that’s where people with dementia and CB are making demands on us.
NHS manager D
We have to concentrate on re-enablement now . . . this may be an opportunity but we have to work this out locally . . . rehabilitation means different things to different people it seems.
Local authority commissioner
We have to reconfigure our rapid response dementia team which worked in one part of the trust . . .
NHS manager N
Second, comments were made about the new priorities given in dementia services to early diagnosis, to the extent that these commanded professional and managerial attention:
We have to get people through the service and diagnose dementia . . . the anti-dementia drugs . . . perhaps some brief other intervention is all we can manage at targets of 600–800 a year . . .
NHS psychiatrist
In contrast, many of the carers participating, and some practitioners working outside the NHS, interpreted such re-engineering of services as cuts to carers’ support and a withdrawal from preventative work:
My nurse [CMHN] who came occasionally had to discharge me . . . she said to ask the doctor to re-refer me if I needed . . . maybe it’s the cuts in the health service.
Former carer
I rang the carers centre . . . they were very nice and we talked a lot . . . they told me I should ask my doctor to refer my [husband/wife] and me to the CPN [CMHN] . . . but the nurse had told me to go to the carers centre if I needed to . . .
Carer
I was lucky . . . they still have me on the books at the clinic . . . I know that I can phone but I don’t often . . . shame they stop doing this now.
Carer
I would like to help carers look after people with dementia at home . . . usually it’s too late and we get called in to help arrange for a care home.
Social worker
Stakeholder consultation: summary
The stakeholder discussions provide a context to the data reported earlier in this chapter and help to explain some of the apparent dissonance between high levels of need among family carers and their uncertain focus in services. When we compared the views of managers, practitioners and carers, some different perspectives emerged between those whose role was to deliver broad strategic aims (such as hospital discharge or admission avoidance, or early dementia diagnosis) and those who were more concerned about the implications of commissioners’ and government decisions for their own organisation and the people they were supporting. High thresholds for service access and high levels of CB were a prime example of a self-excluding explanation of why carers felt that they were not deserving of interventions to prevent escalation of CB at home. Across participants there was some cynicism that the purpose of early recognition of dementia and service reorganisation was seen as more about driving down costs than creating improvements. There was a strong theme among those who were optimistic about functional analysis or similar interventions to minimise the impact of CB. This was that early or timely recognition should be applied to CB symptoms and not just to the diagnostic recognition of dementia. Both practitioners and carers voiced concerns that high thresholds for accessing specialist support led to carers not seeing their situation as ‘deserving’ of help, with the risk of care breakdown and distress. In their view effective responses to CB need to start early on with recognition of the risks; practitioners and advocates should develop strategies to encourage carers to accept such interventions; and services should publicise their availability before escalation of family distress precipitates a crisis or breakdown of care at home.
Discussion
This is, as far as we know, the first naturalistic observational cohort study in family settings across England since publication of the National Dementia Strategy of 2009. 250 A previous longitudinal study of Alzheimer’s Disease in London and the Southeast Region (LASER-AD)277 of England reported in 2005. In these studies participants with other dementia subtypes were excluded; they lived in both care homes and at home; and those with and without clinically significant BPSD were included. In our FamCare study cohort, index participants met the diagnostic criteria for both dementia and clinically significant CB, and family carers also participated. The FamCare study was conducted between August 2010 and July 2012 across England, at a time of major contextual changes in the delivery of NHS and social care services. Some of these were widespread considerations about the Health and Social Care Act 2012;276 introduction of PbR in NHS mental health organisations;121 and financial pressures affecting the amount of social care commissioned by local authorities. Also, stimulated by work associated with the National Dementia Strategy,177 there were several reports or guidance about dementia care from the Department of Health and campaigning groups such as the Alzheimer’s Society. These included, for example, those about the prescribing of antipsychotics in dementia care; mental health care in acute general hospitals; end-of-life care; workforce needs; and calls for more and better support to maintain independence in people with dementia living at home. 112,176,278–281 The FamCare study is also one of the first recent in-depth studies in England investigating the range and cost of health- and social-care services accessed by this group of people with dementia and significant CB, and their family carer. Our participatory design development process,282 using stakeholder consultations, provides a realistic understanding of, and potentially trustworthy conclusions about, the access to, and delivery of, support to families and community-dwelling people with dementia and CB.
Next we will examine our findings in terms of the recognition and management of CB in dementia in family settings, by specialist mental health services for older people, and discuss our health economics findings.
Recognition of dementia and challenging behaviour in family settings
Delivery of support by NHS mental health organisations, to people with dementia and CB living at home, and their family carers, was difficult to examine. They were hard to locate within the records of the organisations (see Appendix 3). From 5360 referrals to 33 CMHTsOP over a 7-month period, in seven NHS organisations, only 8.4% of people who were living at home with potential dementia were accepted by the teams, for evaluation or assessment. The present study provides new detailed data on the type of referrals that are received by CMHTsOP in England, and how these are managed by teams. Practitioners and managers in the teams and organisations were surprised by these findings, as their perception was that their work was predominantly with people with dementia. Just under one-quarter (22%) of new referrals to CMHTsOP were in respect of people living in a care home (see Appendix 3, Table 66). Research suggests that only 38% of people with dementia live in a care home. 235 The present study did not set out to understand in-reach services by CMHTsOP to care homes, much of which is reported to operate informally. 283 Our interest was in the management of dementia and CB in family care settings. Our data confirm the findings of another study of 15 CMHTsOP carried out in different locations from those of the present study, during a similar period, between October 2010 and June 2011. The authors, like us, note a weighting within CMHTsOP towards cases where the individual has a functional mental health problem, rather than dementia conditions. 284 Wilberforce et al. 284 also note a huge variation of practices in CMHTsOP in England, similar to the findings of their literature review of 2008, which concluded that there was patchy evidence for CMHTsOP practices, compared with policy and practice guidance. 285 Our examination of every new case referred to 33 CMHTsOP suggests that only a very small part of work accepted by CMHTsOP focuses on supporting new cases of people with dementia and family carers at home. This, combined with the stakeholder consultations, confirms that the majority of people with dementia living in their own homes, and potentially requiring specialist NHS mental health care, may not be found using services provided by CMHTsOP. Thus, people with dementia with developing CB, and their family carers, may be deprived of timely support to prevent escalation of their concerns and distress, because they are not recognised early enough by services and offered support.
Management of dementia and challenging behaviour in family settings
Our examination of every new referral to CMHTsOP noted that, in the 7-month period, 61.5% (852 cases) were referred, prior to evaluation by the team, on to memory clinics (see Appendix 3, Table 67). Indeed, our observational 6-month cohort study of 157 dyads living at home with dementia and CB recruited 16.6% of participants with dementia and CB from memory clinics, with the rest from CMHTsOP. Consultation with 83 practitioners and managers in participating NHS organisations noted views that, at the points of referral services, some may have missed potential cases of people with dementia and CB, because of the lack of a structured assessment with thresholds for clinically significant CB combined with under-reporting of CB by the family carer, early on. Additional stakeholder findings point to the carers themselves not knowing when they should ask for help.
Although dyads in this cohort varied considerably in their CB, statistical analysis could not identify subgroups that improved, were stable or deteriorated over time. This finding provides further confirmation of our rationale of the elusiveness of the syndrome (see Chapter 1) and the complexity of symptoms,277 and it also lends further support for our rationale (see Chapter 1), that the treatment approach of choice, for this particular group involves, case-specific interventions to address the cause(s) of behaviour, unmet need in the person and needs of the family carer.
Specialist practitioners (not all with professional registration) had, on average, nine contacts with participants with dementia and CB. These took the form of face-to-face support for the majority (116 dyads), but 12.7% of dyads had no contact with a practitioner at all over the 6 months following their referral to specialist mental health services for CB. As 16.6% of this cohort were being seen in memory clinics, it is fair to assume that some of these contacts would have focused on diagnostics and review for the dementia symptom-modifying medications. Over one-quarter (26.1%) of people with dementia and clinically significant CB were already in receipt of a dementia-related medication such as an acetylcholinesterase inhibitor or cognitive enhancer, prior to referral for CB to specialist CMHTsOP (see Table 49). Over the 6 months, prescriptions of these medications, and the associated costs, increased (see Tables 63 and 64): the acetylcholinesterase inhibitors represented the highest prescribing cost (£30,466) for any individual medication category. Disappointingly, the quality of prescribing practice for the antipsychotics was suboptimal, despite policy and best practice initiatives for judicious use of these, which include, as a minimum, regular review and a maximum treatment period of 12 weeks. 59,112 Very few participants had been on an antipsychotic for less than 3 months prior to referral (see Table 49). There was no change over 6 months in antipsychotic prescription (see Table 63). Additionally, the noted high use of antidepressants prior to referral (see Table 49) did not change over the 6 months, despite studies showing that they are of little or no benefit;270,271 nor was there a change in hypnotic (B/Z/A) prescription (see Table 63). Moreover, in the past, people with dementia were more likely to be given antipsychotics than analgesics,46 but there is emerging evidence for the effectiveness of analgesics in reducing agitation in dementia. 35 In our study, we may not have captured the use of self-administered medications, such as paracetamol. However, the overall use of analgesics is surprisingly low for a cohort of this age, among whom comorbid conditions are likely to be common. We did not examine participant case notes to determine the content of interventions offered by specialist mental health practitioners, but these findings have the potential to undermine the view that specialist psychiatric teams are better than primary care at withdrawing antipsychotics. 286
Overall, support from practitioners over the 6 months did not have an impact on the strikingly high levels of BPSD, measured by the NPI or the RMBPC (see Table 48), at baseline. This is consistent with the LASER-AD study of over a decade ago,277 whose authors noted no association between psychiatric treatment and outcome of BPSD, and no change in NPI scores. Our findings of mental health support conclude that, although supportive contact with a practitioner may help families feel less guilty, this was not enough for a sustained management approach to reduce measurable CB in this group. Families need to be helped to learn to identify triggers266 of ‘behaviours that challenge’, including their own interactions with their relative; and practitioners need to support them on an ongoing basis, to modify these triggers and thus reduce their own distress. Specialist practitioners working from multidisciplinary CMHTsOP or memory services also need, when relevant, to collaborate with the GP to address unmet health needs. For example, our Cochrane review82 included a study by Gitlin et al. ,287 who used this type of approach in a randomised controlled study. Their treatment involved up to 11 home or telephone contacts over 16 weeks, including investigating health needs (such as taking blood and urine for tests when relevant); they reported significant reductions in CB, including carer upset, as well as improvements in carer confidence.
In our cohort of people with dementia and clinically significant CB, 87.3% were recorded as being in receipt of specialist multidisciplinary mental health care. In an earlier trial,65 in which CMHNsOP working in multidisciplinary dementia-specific community mental health teams (CMHTs) were trained to apply a functional analysis approach in family settings, a positive impact on CB was noted. There are a number of possible explanations for these disappointing findings of both suboptimal prescribing and no change in levels of CB when families received an average of nine practitioner contacts over a 6-month period. First, practitioners may not have the skill set needed to recognise and treat people with dementia and CB, as their work appeared to be weighted towards people without dementia; they accepted only a very small group of potential cases of dementia for evaluation, often referring people onwards without evaluation (see Appendix 3). However, even in specialist mental health nursing services that do not focus specifically on the management of CB at home, family needs may be missed. 288 Second, the multidisciplinary teams may not have had access to the range of professionals; for example, old age psychiatrists or geriatricians can provide expertise for medical concerns, psychologists can provide expertise in maintaining the micro-skills needed to formulate psychological needs, including those of the care,187 and social workers may also have perspectives about family need and resources. An obstacle to delivery of interventions for dementia with CB may have therefore been the absence of particular professional expertise. It is hard to see why some prescribing practices were suboptimal, as old age psychiatrists were always available in these services; it may be that some practitioners may have been working ‘in silos’272 and that the timing of access of available professional expertise by the practitioner may have been an obstacle to the evaluation for, and delivery of, some interventions for CB. The sometimes hidden ‘scaffolding’185,186 that practitioners require for the delivery of these types of interventions is implicit in most successful applications of case-specific dementia care interventions,289 including those for CB. 26,50 Routine supervision and support from skilled professionals with relevant expertise are therefore particularly important for the management of dementia with CB at home.
Our programme of work has brought together manualised tools for the assessment and recognition and management of people with dementia and clinically significant CB in family settings. These tools can assist practitioners to detect unmet health, psychological and carer need. The tools were viewed by specialist community mental health practitioners as helpful as well as both feasible and acceptable for use. They valued the systematic structure for assessment and decision-making to meet the needs of families who live with or closely support a person with dementia and CB (see Chapter 2). This is important, as practitioners have different requirements for decision-making about treatment in dementia care, compared with other long-term conditions. 290
Health economics
This study set out to identify the frequency of health- and social-care service use and associated costs for people with dementia and CB living at home. It included community-based health- and social-care service use, prescribed medication, hospital use, day care, respite services and unpaid carer time.
The highest mean monthly use of mental health services for people with dementia occurred in the 3 months before baseline. This is unsurprising, as a recent referral to a mental health team was an eligibility criterion for the FamCare study. The costs of mean monthly mental health services were two-thirds lower by the second follow-up. Community-based health-care costs, excluding mental health services, did not vary considerably over time. However, the increase over the 6 months in the proportions of GP home visits, and corresponding decrease in visits to the GP surgery by patients or carers, is interesting. We did not collect detailed information that might explain this change in practice by the GP; it could reflect reduced independence or increased distress in dyads over time or perhaps increased attention for those prescribed an acetylcholinesterase inhibitor,291 as prescriptions for these drugs also increased over the study period. Annual prescribing costs for the individuals with dementia were more than double those of family carers, with acetylcholinesterase inhibitors, cognitive enhancers and antipsychotic prescriptions accounting for most of this difference. Social care use from formal sources, including day care, increased for people with dementia over the study period. For carers, local authority social care use remained low throughout the study. Social care, such as home care, is a major support for family carers, although it is not always seen as a service to them, as the ‘user’, such as the person with dementia who is provided with home or day care, is seen as the focus of intervention. 292
Two striking findings arise from our data of the costs of care for people with dementia and significant CB living at home. First is the level of contribution borne by family carers, which accounted for 80.6% of the total costs for this group. Second is the economic assumption that increases in cost are determined by dementia severity, defined as mild, moderate or severe. What this economic assumption can overlook are the costly challenges of BPSD, which are common at the earliest stages of dementia and can be persistent in the first year of a dementia diagnosis; they are also associated with depression in carers and high costs later in the caregiving career. 235,239,293 In the present cohort, we found high levels of BPSD, even though 63.1% were categorised as having mild dementia. High levels of BPSD among people with mild dementia have also been noted by others. 294 In our cohort, the overall monthly health- and social-care costs were £2828 (mean annual cost £33,941; see Table 65, with all informal care costed at minimum wage); this is comparable to the costs quoted in the Alzheimer’s Society 2014 update235 for all disease severity levels of £29,298. The authors235 also noted costs of £25,723 for mild, £42,841 for moderate and £55,197 for severe dementia. In addition, they reported that since 2007 there has been a reduction in the level of formal care support for people with mild dementia and a sizeable increase in support for people with severe dementia, consistent with efforts to support people at home rather than in a care home. This change in focus may, according to the authors, reflect the tightening of health- and social-care budgets over this period. However, an alternative hypothesis based on our observation of low response by CMHTsOP to referrals of people with dementia, and lack of impact on BPSD over time, may also be attributable to the perception that mild dementia reflects less complexity and associated low need in families.
Health economics: consideration of financial benefits for family carers
Two-thirds of people with dementia live at home, with one-third of these living on their own. 295 The FamCare study cohort was representative of this finding, with 70.7% of participants living with someone who described themselves as a carer. As such a high proportion of the cost of dementia is borne by family carers, it is relevant to consider the adequacy of financial benefits available. In the UK, attendance allowance is paid directly to individuals aged ≥ 65 years with physical or mental disabilities to help with personal care costs. The allowance rate for 2014/15 was capped at £82.30 per week (URL: www.gov.uk/attendance-allowance/overview, accessed 17 July 2015), which would pay for less than 4 hours of a home care worker’s time (costed at £23 per hour to include employment costs). Additionally, a carer’s allowance of £62.10 per week is available for people who spend a minimum of 35 hours per week caring (URL: www.gov.uk/carers-allowance/overview, accessed 17 July 2015). However, the carer’s allowance is taxable and is affected by the level of other benefits received, for example the state pension. The majority of carers in the FamCare study were retired, and unlikely to be eligible for the full carer’s allowance after their pension was taken into account. Carers in receipt of the full carer’s allowance would be able to purchase less than 3 hours of a home care worker’s time, although for some it may be commensurate with employment and its associated costs. However, these observations should be considered in the context of the views outlined previously, that families may provide care to their relative out of duty or desire, and that, should all unpaid hours of care become paid care, uptake by many carers would be unlikely. 235
Limitations
This study was conducted across England, including the south-east, southern Midlands, the north-west, Lincolnshire and Yorkshire. However, one important limitation to the generalisability of our findings is the lack of data on ethnicity and migration status from within our cohort of participants or from the workforce within the 33 CMHTsOP across the country. All but one of the 26 practitioners who were trained to use functional analysis-based interventions were from white British backgrounds and none was from Asian ethnicities. This observation is consistent with the observations of a study by Hussein and Manthorpe. 292 They note that there are wide local variations in diversity among the dementia workforce. In our consultations we recruited more diverse stakeholders to help redress this limitation. Similarly, we did not collect information on socioeconomic status. Such data are rarely collected and analysed in dementia research, but would be useful to the understanding of family and individual resource decisions and priorities.
A second, unavoidable, limitation is consideration of those who did not wish to join our study and the consequent loss of potentially the most distressed families to this cohort investigation (see Appendix 13). A third limitation is that it was not possible to conduct the planned CRT of the intervention (see Chapter 5, Changes to protocol, and Appendix 2).
A fourth limitation is that recruitment from the MAS and memory clinic (16.6% of the cohort) began later in the recruitment period, after workshops had been conducted with stakeholders in NHS organisations. We do not have data on how many of the 61.5% (852 cases) that were referred on by the CMHTsOP to memory services (see Appendix 3, Table 67) might have met our criteria of dementia with clinically significant CB. However, the present study has developed tools for specialist services to use in CMHTsOP, MASs, memory clinics and other commissioned services for the recognition and management of dementia with CB in family settings.
Conclusions
Implications for services
Fundamental questions arise from these data. These are about the limited access regarding equity of support from specialist services, for family carers living with a person with dementia who also has clinically significant CB, compared with other groups of carers providing support. We did not collect comparative data for other groups, but this study provides baseline cost data, for the purposes of such comparisons. This 6-month cohort study notes that over 80% of the total costs of care were borne by family carers, and over two-thirds of the carers were living with the person with dementia. Co-resident carers reduce the risk of breakdown of care at home,296 thus justifying their need for higher priority to timely evaluation and support with CB, by specialist and other services.
A better understanding of views about equity of specialist mental health support for older people without dementia297 may explain our observations of the flow of new referrals to CMHTsOP, in which 1385 of these were not accepted for any assessment by the teams. Of these, we would argue that 61.5% were probably referred for dementia symptoms, as they were signposted on, prior to any evaluation, to memory clinics (see Appendix 3). A new feature of our findings is the context of delivery of diagnosis, and the redirection of investment towards memory clinics and MASs. This appears to have resulted in a perverse consequence of creating a new care gap298 between diagnosis and support, when symptoms of CB are high. The image of a pathway299 appears difficult to justify when there is a stark break between initial contacts and later interventions.
Our practitioner workshops of 83 multiprofessional practitioners combined with our stakeholder interviews, which included carers, service managers, practitioners, advocates and commissioners of health- and social-care services, concluded that effective responses to CB need to start early on with recognition of the risks; practitioners and advocates should develop strategies to encourage carers to accept such interventions; and services should publicise their accessibility and availability in order to avoid escalation of family distress, which then precipitates breakdown of care at home. Although diagnosing dementia has been the ‘dementia challenge’ for services until recently, these findings suggest that providing families with evidence-informed specialist support to manage CB at home could be the challenge for the next 5 years. Specialist mental health NHS organisations in England now have a formal framework of grouping needs of people with dementia within the PbR programme,121 and the initiative has been rolled out. Dementia cases are clustered into four groups, namely ‘clusters 18–21’, where it is anticipated resources will follow complexity of need. These stakeholder contributions therefore have scope to guide commissioning practice for services for dementia with clinically significant CB, to provide assistance for family carers.
Implications for practice
Another important finding is confirmation of the variability over time in a cohort, in which index participants met diagnostic criteria for dementia and CB. This highlights the need for a skilled community-based workforce to co-ordinate and deliver case-specific support to people with dementia and CB living at home, taking into account their health, psychological status, family context and social capital, at any given time. The circumstance of the family carers, in an increasingly diverse society, is a key to understanding what can be achieved in terms of preventing escalation of CB at home, to the point of crisis. Our study provides an evidence-informed framework with appropriate tools for such an approach, particularly about the family context. For example, we have field tested two common outcome measures used in research with potential for use in routine practice,264 namely the RMBPC and the NPI. The RMBPC is a short self-report measure completed by family carers where score of five and above was capable of detecting high levels of BPSD on the NPI. Some non-medical practitioner stakeholders had discontinued their use of the NPI, but the RMBPC may be a useful tool in assisting practitioners early on in their assessments to target their support towards dementia with clinically significant CB; it is a self-report carer measure that can be then evaluated by specialist practitioners of all professional disciplines, irrespective of whether they work in memory clinics or CMHTsOP, or in other integrated services. By harnessing a more focused approach to the practice, and delivery, of case-specific interventions for people with dementia and CB living at home, trained dementia practitioners or ‘therapists’, perhaps described as ‘dementia behaviour therapists’, can use this framework to make rational decisions about care and treatment plans, about which they can then have greater confidence of their efficacy. They will also need to draw on expertise from their multidisciplinary team and wider care providers where relevant, to co-ordinate and deliver sustained ongoing interventions for the management of dementia with CB at home.
Implications for research
In the present cohort, high levels of BPSD were observed even though 63.1% were categorised as having mild dementia. This is consistent with findings from Finland, noting BPSD in 76.5% of people with mild Alzheimer’s disease. 294 BPSD in community-dwelling people with dementia can be severe throughout the course of the condition. 300,301 Longitudinal studies are needed to improve our understanding of the characteristics of people with mild dementia with CB, changes in BPSD over time and what interventions might moderate CB to reduce distress for them, their families and others. Our findings add support to recommendations from studies carried out in other counties for a focused structured assessment of BPSD before deciding on treatment, even in mild dementia,294 and timely recognition with adequate professional support. 300 Future applied research studies such as ours will need careful longitudinal evaluation of specialised individualised interventions for home-dwelling people with clinically significant CB, early on following diagnosis of dementia. Given the gaps in knowledge about the management of CB in family settings which have been noted in an independent review of progress of the National Dementia Strategy of 2009 (pp. 3, 68 and 69),302 the changing landscape of commissioned dementia care in England and the findings from our stakeholder consultation, there is scope, using outputs and tools from the FamCare study, for a future study of the delivery of case-specific interventions for CB in family settings. This could have an impact on NHS, and other services for this group, in the next 2–4 years.
Chapter 6 Discussion: overview of key findings from Challenge Demcare and implications for future research and practice
Overview of Challenge Demcare
Background to the programme
The aim of this programme was to study the management of CB in people with dementia living at home and in care homes. The phenomena associated with CB in dementia are referred to as NPSs or BPSD. These concepts acknowledge the psychological suffering in people with dementia, but they are limited in their reach. First, they root the phenomena solely in the dementia when other factors such as undetected pain and discomfort35 may contribute to an episode of CB; and, second, they take no account of the environmental context in which the behaviour occurs, such as when an episode of aggression is precipitated by the way care is carried out38 or by how a carer perceives a behaviour and responds to it. 48–50
Therefore, we defined CB as ‘a manifestation of distress or suffering for the person with dementia or of distress in a carer or others, thus threatening the quality of life of one or both parties’.
Overall, two related but distinct programmes of work were planned, with development work leading to two CRTs. These were set within the real world of care homes in England’s largest county of Yorkshire; and in seven large NHS mental health organisations across England, with 33 mental health teams that were commissioned to provide care to people with dementia and CB living at home. The first CRT (The ResCare trial) examined an individualised intervention consisting of e-learning and e-tool decision support for ‘action plans’ to assist staff working in care homes in the management of people with dementia and clinically significant CB, with an embedded process evaluation. The second (the FamCare trial) aimed to assist specialist community mental health practitioners working with families to deliver such interventions for people with dementia and clinically significant CB living in their own homes; and to evaluate this within a CRT.
Background to the intervention
The aim of the intervention was to assist practitioners who support families and care home staff to respond effectively to CB. It was refined from the behaviour management literature and outlined in Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care as ‘behavioural and functional analysis conducted by professionals with specific skills, in conjunction with carers and care workers’ (p. 260). 12 This biopsychosocial approach to assessment, analysis and the testing of interventions for CB in dementia, which we describe as functional analysis-based interventions,82 is also known as individualised formulation-led interventions for CB in dementia care. 97,186,303,304 They are usually algorithmic to enhance case specificity62 and are delivered by trained practitioners who have access to knowledge and expertise from other professionals such as psychiatrists, geriatricians and experienced psychologists. 15,26,27,50,97 A trained therapist is usually required for such an intervention,82 as a key skill in working in this area is the ability to be flexible and have the capability to alter care plans as new needs arise, meaning that the process remains iterative but the functional analytical skills remain at the core of the plan. A review of computerised clinical decision support systems suggests that these may improve practitioner performance, but to date the effects on patient outcomes are understudied or inconsistent. 169 The present study found that the decision support software was not enough to impact on resident or staff outcomes. In contrast, an earlier study, FITS, demonstrated good impact on prescribing practice with the use of bespoke interventions delivered by trained therapists working intensively with a small number of care homes. The practitioner works with staff, families or other carers to test out the most appropriate strategies and adjust these where required, to reduce CB in dementia. 1,65–69 In Germany they are delivered through ‘case conferences’,305–309 in England this approach is best known as the Newcastle model for the management of CB,97,186 and others simply use practitioners supported by specialists working in multidisciplinary teams to deliver formulated biopsychosocial case-specific support to staff in care homes. 26 The Newcastle clinical protocols grew out of the FITS RCT. 310 The FITS study was one of the three care home interventions that contributed to our Cochrane review of functional analysis-based interventions for the management of CB in dementia. 82 Many of these formulaic clinical innovations have been conducted in care home settings,97,186,303,304 often growing out of experience by dedicated clinical teams. 60
Rationale for the intervention
Previous work,63 and more recent studies,97,311 demonstrated the successful use of algorithm-based protocols in the management of CB in care homes. One of the most recent studies311 used a pencil-and-paper workbook format in care homes. The workbook, using functional analysis, was evaluated favourably in an Australian RCT311 with four conditions: clinical protocol alone; clinical protocol, plus extra clinical support; extra clinical support alone, plus a workshop on CB; and treatment as usual. All conditions showed an improvement on at least one measure (behavioural frequency; perceived disruptiveness; carer stress; carer attitude). However, the clinical protocol plus clinical support was most effective, and the only condition to maintain the majority of its effects at follow-up.
We were interested in determining whether or not the training provided within the e-learning modules would permit staff to utilise clinical protocols effectively with minimal supervision and support. In essence we were building on preliminary work in the dementia literature that had separately demonstrated the value of clinical algorithms,63,97,311 and current interest in and the use of e-tool technology,70–76 as care staff appeared enthusiastic about access to e-learning dementia care opportunities. 127 If successful we would have produced a cost-effective programme that enabled staff to assess problematic presentations; identify causes and underlying needs; develop appropriate care plans; and, based on the learning from the earlier training modules, execute the plans effectively, with minimal external supervision. Hence, in order to widen the scope for delivery of interventions for the management of CB in dementia, it seemed appropriate to develop an online application of intervention algorithms based on functional analysis.
Chapter outline
This final chapter summarises key findings from our ResCare trial and the FamCare observational study, including our data on prescribing, utilisation of our innovative tools in care homes and our study of the management of dementia and CB in people living at home. The tools were based on a functional analytic approach to individualising interventions for people with dementia and CB, with added attention to strategies to meet the needs of the environment and the caregiving system. Functional analysis is recommended by Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care12 and is also seen as a promising method for the management of dementia with CB in our updated Cochrane review. 82 We will examine why it was not as effective as previous care home studies that did not include state-of-the-art, user-friendly technology.
We will also consider the limitations of our studies; discuss improvements for research methodology; and compare key data from care home (the ResCare trial) and family (the FamCare observational study) settings, to gain a deeper understanding about CB in dementia, and implications for its management in these two settings.
Finally, we will review the findings and conclusions of the ResCare trial (see Chapters 2–4) and the FamCare study (see Chapters 2 and 5) and consider their implications for clinical/organisational practice and future applied research about the management of dementia and CB. The focus of this discussion is on how the NHS in England might take a step forward to improve the delivery of interventions for the management of CB in dementia care.
Key findings
Key findings: the ResCare trial
The e-learning and decision support tool in its current form, without review of whether or not action plans were being implemented or needed adjustment, was not more effective than in usual care, at reducing CB. Neither did it demonstrate significant effects against usual care on secondary outcomes such as quality of life in residents with dementia and CB, or staff coping and burden (see Chapter 3). This is despite huge technological effort and resources, such as arranging broadband, and providing of computers, agreeing costs of backfill for staff to attend training and access to an external dementia care facilitator to support learning.
Individualised action plans using the decision support system of a resident’s profile, with recommendations to request medical review of those in receipt of an antipsychotic or other psychotropic drugs, did not impact on prescribing practices. This was despite the policy-driven environment to review use of antipsychotics112 and an observed weighting by practitioners in studies of interventions, such as ours, towards medical aspects of biopsychosocial interventions. 303
The care home industry in England does not at present appear ready to embrace, on its own, in-house e-learning opportunities or technology-based innovation of this type (see Chapters 2–4). This observation applies to care homes that were rated as ‘good and above’ by the CQC (see Chapter 3).
Staff gave positive feedback when we arranged off-worksite e-learning combined with facilitated group discussion on the application of case-specific interventions for residents with dementia and CB (see Chapter 2). They were particularly appreciative of the skills of the dementia care therapist who facilitated training (see Chapters 2 and 4). They were also enthusiastic about the simulated real-life video material of resident communications during episodes of CB in their settings (see Chapter 2). This has implications for augmenting staff training interventions for the management of CB in dementia.
The embedded process evaluation (see Chapter 4) provided explanations for these findings in terms of variation in care home managers’ trust of their staff; and variation in cultures of training and practices within care homes. Smaller homes that were less hierarchically managed appeared to be more ready to utilise innovation. Capable leadership and collective willingness were also important considerations in understanding the readiness of a care home to invest time in innovation.
Using our analysis of the factors influencing implementation of the intervention in care homes, a toolkit for future research and practice in the delivery of innovation, including digital technology in these settings has been developed (see Chapter 4, Tables 36–38 and 40).
Key findings: the FamCare observational study
It was not possible to carry out the CRT of community mental health practitioners working with families to deliver such interventions with people with dementia and clinically significant CB living in their own homes. This low referral rate was hypothesised to be a new phenomenon, relating to the emergence of MASs; this ‘re-routing’ of the referral pathway in community settings is important in relation to the commissioning of new services.
The cohort study noted that 63.1% of home-dwelling people with dementia and clinically significant CB, referred to 28 specialist mental health services by their GP, were categorised as having mild dementia. The observed low response by CMHTsOP to referrals of people with dementia (see Appendix 3, Tables 66 and 67) and lack of impact on BPSD over time (see Chapter 5) may have been in part attributable to the perception that mild dementia reflects less complexity and associated low need in families. This striking finding has implications for redesign of service pathways and the delivery of post-diagnostic interventions for people with dementia and clinically significant CB living at home, when these are limited to those with severe dementia or care home settings.
This study and its stakeholder consultations noted an emerging care gap, across England, in the treatment offered to families supporting a relative with dementia and clinically significant CB at home. This may be attributable in part to the aforementioned potential for rerouting of services or a perception that mild dementia reflects less complexity associated with BPSD. Other possible reasons are as follows:
-
Evidence from this community study of people with dementia and clinically significant CB shows that patient outcomes are variable; perhaps, as with those living in care homes, symptoms fluctuated312 even over the short period over which we observed these.
-
The study showed that ‘usual’ treatment in family care settings did not have an overall positive effect on CB. Indeed, over a 6-month period of input from specialist mental health services, averaging nine contacts per family dyad, there was no change in the high levels of BPSD. This is not to say that increased contact with mental health services was not at all helpful, as families may have reported more of their concerns as they began to trust the practitioner and more contact with practitioners appeared to have a positive impact on levels of guilt in family carers (see Chapter 5).
-
Practitioners of all disciplines who attended stakeholder consultations were aware of the difficulties in determining what constitutes ‘caseness’ or the threshold for offering a clinical intervention (see Chapter 5). The majority of community mental health practitioners were positive about the bespoke version of the e-tool and appreciated the workbook resources provided (see Chapter 2). This included a self-report checklist of problem behaviours that are common concerns of family carers as a means of structuring the initial assessment. Implications for practice using the manualised tools from this programme in the screening for clinically significant CB in people with dementia living at home will be considered later in this chapter (see Implications for service improvements and practice).
-
Family carers supporting people with dementia and clinically significant CB contributed over 80% of the total estimated cost of care, with an average of 112 hours a week at baseline, rising to 129 hours a week at 6 months. The overall monthly health- and social-care costs were £2828 with an annual cost average of £33,941 (see Table 65), where all informal care is costed at the minimum wage.
Key findings: prescribing practices, antipsychotics and costs across the Challenge Demcare programme
Prescribing practices in care homes (see Chapter 3, Tables 20 and 24) indicated that these were contrary to emerging clinical recommendations for antipsychotic use in dementia105,112,176,177 and clinical guidelines advocating benzodiazepines prescription for no longer than 28 days. 178
Changes in prescribed medication
There was very little change over time in prescribed medication for people living in care homes. However, an interesting feature, as little is known about prescribing practice of the dementia drugs in care homes, is that just under 15% living in care homes were prescribed a dementia drug (see Chapter 3, Table 20). There appeared to be some review of these drugs for a small number of these residents (see Chapter 3, Table 24). In contrast, there were large increases in use of the dementia drugs in the FamCare study sample, and review of non-opioid pain relief treatment was also common (see Chapter 5, Table 63). Raised expectations for early diagnosis to enhance treatment following launch of the National Dementia Strategy in 2009250 may have contributed to these findings. Perhaps prescribing practices for some medications, such as review of the non-opioids, was superior for participants living at home than for those in care homes; and people on a dementia drug in care homes may have had a better opportunity to receive medical attention than those residents not on a dementia drug, through organised review for this particular type of medication. As we shall see next, review of the antipsychotics and other psychotropic medication for BPSD was suboptimal in both the ResCare trial and FamCare study cohorts.
Antipsychotics
Overall, we found that 15.4% of participants in care homes and 9% of those living at home were prescribed an antipsychotic. This is lower than the 25% estimates by Banerjee,112 and similar to the value for care homes (but not the overall data which included family care settings), which was noted by Barnes et al. 269 However, disappointingly, in this subsample of participants in receipt of one or more antipsychotic, prescribing practice, which has been the focus of initiatives to improve quality of prescribing,112 did not alter in either setting. We did not examine records for data on whether participant prescriptions were reviewed by the GP or an old age psychiatrist during the 3-month period before baseline, or for the 6 months (this being the average duration for both groups) between baseline and final follow-up. However, consistent with the findings of Ballard et al. ,313 both the ResCare trial and FamCare study participants remained on one or more antipsychotic (see Tables 24 and 63) for much longer than the 12 weeks recommended by good practice guidance. 314 Suboptimal quality of prescribing practices was also noted in the POMH-UK audit of quality improvement. 269
Other psychotropic drugs
Prescribing practices for other psychotropic medication, particularly in the care home setting, were also suboptimal (see Chapter 3). Disappointingly, for the FamCare study cohort, who received support from multidisciplinary specialist old age psychiatry services, around 20% with dementia were prescribed at least one antidepressant with a low likelihood of discontinuing in 3 months, despite knowledge of little clinical benefit from some of these agents;270,271 and this pattern of suboptimal prescribing was also seen for the hypnotics and anxiolytic (B/Z/A) drugs (see Chapter 5).
Pain relief
Prescriptions for pain relief were surprisingly low in people with dementia and CB living at home, when this may be a legitimate pharmacological intervention for some of this group. 35
Dementia drugs
The main medication intervention for our cohort with dementia and CB appeared to be driven by practitioner understandings of mild dementia and prescription of the dementia drugs, such as the acetylcholinesterase inhibitors and other cognitive enhancers. These findings need to be considered in the light of reports of the practical, emotional and ethical problems that some family carers may be confronted with, in terms of all medication interventions for people with dementia living at home. 315
Quality of medical care
For both cohorts, the findings of suboptimal prescribing practice concur with those of a study in one locality in the UK from over a decade ago, which noted inadequate medical care for older people, particularly those in nursing homes. 316 This is further supported by a more recent examination of the quality of the annual dementia review in general practice in five primary care trusts in the north-west of England. 317 In a separate analysis of the ResCare trial baseline data, we found that prescribing of individual psychotropic medications was not related to symptomology of BPSD (Hilton et al. unpublished); the data were consistent with a study carried out in primary care in Germany, which found associations between antipsychotic prescribing and non-psychotic domains of behavioural symptoms. 318
Costs of medication
Over a 7-month period (3 months before baseline and the 4 months up to final follow-up), the total mean medication cost per person was £598.98 in the ResCare trial and was £479.43 in the FamCare study. In the 3 months before baseline, prescribing costs for the ResCare trial participants were higher than for the FamCare study participants across antipsychotics, laxatives, hypnotics and anxiolytics, antidepressants, anticonvulsants and pain relief. Only prescribing costs for acetylcholinesterase inhibitors were higher for FamCare study participants in the 3 months before baseline, when for the FamCare study the average cost was £67.15 (SD £115.67) and for the ResCare trial this was £29.01 (SD £86.02).
Summary of key findings
Owing to the low referral rate, the design of the programme was changed and the e-tool was tested only empirically in the care home setting. The results showed that the computer-assisted intervention was neither effective nor cost-effective, demonstrating that our comprehensive technology for CB was not enough to change practice in care homes. This finding is in contrast with our now old pilot study of functional analysis-based intervention for CB, which used face-to-face workshops for all care staff in three homes,63 and a recent systematic review of staff training interventions suggesting some evidence of their impact on BPSD. 60 A further, but related, finding was that the attempted use of e-learning in care homes was highly problematic, with many of the care homes having neither the appropriate resources nor the technology to engage in such an approach. The e-learning element and bespoke paper-and-pencil workbook versions of the e-tool were tested separately by psychologists working alongside care staff and by community mental health practitioners working with families. Both versions received positive feedback from these practitioners. However, we were unable to test the e-tool in community settings. Take-up of e-learning by staff in care homes and utilisation of the interventions (action plans) were poor. This was disappointing, but is perhaps an indication that the intervention was never truly embedded within the working practices.
An emerging care gap in the delivery of timely post-diagnostic interventions for home-dwelling people with dementia and clinically significant CB, and their families is noted. The observational study of community mental practitioners’ impact on CB presentations failed to demonstrate significant value for the management of CB in dementia, with respect to their input. The inability to interpret the variable nature of the clinicians’ input is in part attributable to the limitations of the methodology, resulting from the difficulties recruiting to the original FamCare study design.
The quality of prescribing for the antipsychotics and some psychotropic medications was suboptimal, in both care home and family care settings.
Limitations
Our intervention was conceived as an evidence-informed development to improve practice associated with clinical guidelines. It additionally drew on studies from the Cochrane review on the management of CB. 82 A recent literature review on the effectiveness of staff training for BPSD60 also indicated some promise for the content of the training and intervention approach we adopted, as did a recent study of clinical algorithm-based protocols for the management of CB in dementia. 311 However, we additionally chose to develop an online intervention, as we were keen to widen the scope of use in the real world of the full range of ‘good-quality’ (as rated at the time by the CQC) care home settings. We anticipated that ready access to knowledge and resources available for all staff and practitioners who delivered formal care to people with dementia living at home and in care homes would be a way of sustaining ongoing intervention for dementia with CB. Two key difficulties arose from our plan to widen the scope of delivery of the intervention in care homes. First, the study was not designed to compare therapist-supported functional analysis-based interventions with the present ‘ICT-plus-therapist’ intervention described in Chapter 2. Second, in the event, only 92 staff champions attended training and became responsible for co-ordinating delivery of the action plans, but data collection in intervention homes, including that of staff experience, proceeded as we had planned and was not limited to those trained as staff champions.
Although we were keen to offer the intervention as widely as possible, in retrospect, the programme may have benefited from prior scoping of the care homes’ ‘readiness’ to engage in an e-learning and technology-assisted method of delivering the intervention. Earlier stakeholder involvement with respect to this issue would have been beneficial and may have resulted in a non-technology-based intervention for our CRT. Indeed, because of the difficulties concerning initial engagement, and ongoing training, the content and timeliness of delivery of the intervention was in many ways undermined by the technological difficulties.
Some of the difficulties with the study were attributable to the slow development of the e-tool and associated implementation problems with the decision support software. Difficulties encountered by the engineers to provide us with a stable e-tool delayed progress of the study. Thus, we were unable to fully complete our developmental process evaluation and the e-tool was not fully tested and validated prior to its inclusion in the CRT. Although an understandable consequence of such innovative programs, it is evident that the e-tool was not of the standard we had envisaged, and had intended to use. These difficulties impacted on the training and learning experience of the trainees, as well as on the scope of the specialist dementia therapist for monitoring and supporting staff in delivery of the intervention. Therefore, our aspirations to widen the scope for the delivery of functional analysis-based interventions using ICT may have undermined any potential robust conclusions about the use of this promising82,168 approach to intervention in care homes.
The change of plan involving moving the training venues out of the care homes is worth highlighting. First, staff inability to undertake training in the care home suggests a lack of prioritisation, difficulties in ring-fencing training and commitment to the study, combined with general perceptions about the added value of the ResCare programme. Second, staff who were selected as champions by their managers were significantly younger than others in the home. This may have undermined diffusion and delivery of the intervention, such as, the perceived need to use e-learning opportunities or co-operation with ‘younger’ staff to deliver action plans. Third, moving the training outside the homes, potentially provided a notion of ‘separateness’ between academic training and the day-to-day support by clinicians that underlies successful models of specialist ‘in-reach’ support services to care homes. 190
As noted in Chapter 3, although clear care plans were developed, their appropriate and successful implementation was not sufficiently monitored. In the Newcastle clinical protocol, which has grown out of the FITS study,310 a trained therapist continues to monitor the delivery of care plans for a further 6 weeks after initial implementation. 97 Nor were actions plans adjusted when necessary, in line with the iterative ‘detective-like’ approach to problem-solving the external dementia care therapist to target the range of multiple behaviours in this group of people with clinically significant CB, in which a resident could present with several behaviours that challenged staff at different times. Thus, we highlight limitations in terms of care planning, adherence and fidelity, issues that need to be addressed in future programmes.
Staff feedback about the e-learning course was positive, but it did not appear to strongly reflect the aims and content of the course itself. For example, staff may not have understood that, additional to person-centred dementia care, this was an evidence-informed course for the management of clinically significant cases of dementia with CB (see Chapters 2 and 4). The assumption underlying the training was that by encouraging by staff to ask ‘why’, to seek fuller understanding of individuals and their behaviour, they would adopt a case-specific ‘detective-like’ enquiry about the potential multiple cause(s) of the particular resident’s behaviour, during or shortly following an episode of CB, and thus take action, with their colleagues, to address an unmet need. There were not enough qualitative data from our studies to demonstrate that staff had fully understood the importance of this approach to management. The methods of data collection that we used for feedback (see Chapter 2) and for our retrospective process evaluation (see Chapter 4) are too limited in scope to draw conclusions about the effect of our interactive training course on staff behaviour. Nor did we take outcome measures of staff knowledge, skills and efficacy about the management of dementia with CB, when our mechanism for change was staff behaviour. Perhaps more detailed secondary measurement of these aspects of staff behaviour would have been beneficial.
The retrospective nature of the process evaluation (see Chapter 4) may have been compromised by the accuracy of participant recall. Other difficulties with the process evaluation were our inability to access and interview large numbers of staff who had not attended the training as champions. Thus, it was not possible to explore, from differing perspectives, why the effects of training nominated staff champions did not diffuse to non-exposed staff in the homes or how use of strategies documented in action plans might be used in practice. For example, the third group of actions within the ResCare trial included strategies to address system needs, such as assigning responsibility across shifts to encourage all staff to use strategies within the action plan and adopt an ongoing consistent approach to management. However, we did not examine in detail what might have helped staff champions to adhere to this group of actions.
The absence of detailed interviews with staff who were not champions also weakens our conclusions about the generalisability of the positive reports from staff about our e-learning course (see Chapter 2), as staff champions who were selected by their managers had some significantly different characteristics when compared with other staff in the homes: they were younger, had higher levels of educational attainment and were more likely to have had dementia training. A wider range of views about e-learning and technology-based support in care homes would need to be explored with staff of all ages, educational status and motivations for dementia training.
Finally, our sample of the ‘ResCare’ trial stakeholders who were interviewed (see Chapter 2) may have been tempted to overgeneralise, or to report their own opinions about training and care homes.
The alterations to the design of the FamCare trial mid-project meant that we were unable to control for variables that we would have controlled for if the programme had been planned as an observational longitudinal study at the outset. Hence, some of our explanations were limited to information taken from interviews with practitioners and ongoing stakeholder consultations. Another important consequence of the change in design was that we were no longer able to measure the impact of the training on the community staff who had received the e-learning, thus preventing more detailed comparisons with the ResCare trial participants. Furthermore, a serious, but unavoidable, limitation in the FamCare study was that those families who chose not to participate in the study may have been the most distressed, and thus the most likely to benefit from an intervention.
Community mental health practitioners who delivered care to people with dementia and CB were already engaged in using online electronic systems for the assessment and clustering of ‘need’243 for every new patient. This was in preparation for the PbR initiative. 121 They were therefore co-operative in engaging with our technology. However, due to the change in plan from a CRT to an observational study, we did not address the perceived obstacle associated with security and use of the decision support tool for the purposes of research within each NHS organisation. Practitioners were in receipt of our manual of resources, which was used during training to augment decision-making about the three groups of interventions associated with health, psychological and family carer need (see Chapter 2). However, as noted previously, our observational cohort study did not allow us to examine their use of this compared with those practitioners who did not have access to the manualised intervention.
Many of the studies included in our Cochrane review involved highly controlled research environments, including close monitoring of the staff providing the intervention. 82 In contrast, we planned to deliver the intervention in wide-ranging ‘real-world’ settings where care is provided for people with dementia and CB. We did not, for example, source research-rich environments, but adopted a broad approach to engagement of care homes for the ResCare trial and NHS mental health teams across England for the FamCare study. Although we cannot be sure that engaging research-rich facilities would have facilitated the progress of our studies, the plan for wide engagement may have contributed to some of the difficulties we then encountered with delivery of e-learning within care homes, and with recruiting people with dementia and CB from community mental health services across England to a multicentre CRT.
A limitation to the generalisability of our findings for both the ResCare trial and FamCare observational study is the lack of data on ethnicity and migration status from participants and the workforce. Future study of dementia and CB in subgroups of the diverse population across England is warranted.
Implications for research methodology
The pragmatic RCT has become popular in comparative effectiveness research,188 as well as in social care research. 319 However, many challenges exist, including around delivery, which requires close ongoing collaboration between researchers and the organisations for which the evidence is generated. 188 The ResCare study was a CRT entailing a much larger participant group than would have been required for a RCT; with much effort we recruited the necessary numbers for a CRT. However, although we overcame the obstacles of resources, including providing financial contributions for staff backfill, it remained hard for some managers to release staff for training and to attend case discussions with the dementia care therapist at the care home. These ‘real-world’ delivery problems undermined our study – perhaps most significantly there was a mismatch of follow-up time points, with shorter periods occurring for the control condition (see Chapter 3). The FamCare study was also conceived as a CRT, but did not proceed because of changes in NHS locations and audiences for which the evidence was being generated. Additionally, we noted that some potential participants who would have been eligible for treatment excluded themselves from the research (see Appendix 13), thus highlighting the difficulties in developing and testing new interventions for those most in need, even in an applied research programme such as ours. More recently, there have been recruitment initiatives such as the Enabling Research In Care Homes programme (URL: www.enrich.nihr.ac.uk/) and JDR (Join Dementia Research, URL: www.joindementiaresearch.nihr.ac.uk/) initiative. However, use of selected participants from research-rich registers such as these has its own methodological constraints. For example, recruitment from registers alone may not capture the range of patient, carer or care home motivations and needs for services, and thus undermine conclusions about generalisability of findings.
In an overview of large-scale RCTs of psychosocial interventions in dementia and their translation to other settings, we concluded that effectiveness may be undermined because of the weak consideration of implementation strategies at an early stage of the research process. 189 Although we did not draw strongly on implementation science in our programme, as we discovered gaps in our methodology (which was informed by the MRC framework for complex interventions155), we embedded a mixed-methods process enquiry within the ResCare trial. 203 This provided new knowledge about how the intervention might be implemented in routine practice (see Chapter 4). We also utilised high-level stakeholder consultations within a participatory process design, to iteratively understand the progress and findings of the FamCare study. Proactive study of the process of delivery of our intervention, may have addressed some of the limitations to our studies outlined in this chapter and in previous chapters (see Chapters 2–5, Limitations). We have therefore outlined new approaches to methodology,189 which refine the current MRC framework for complex interventions155 that is commonly used in applied health-related research programmes. These go some way to addressing the type III implementation error,320 which can threaten internal validity and undermine the credibility of the findings of an intervention study. A subsequent NIHR applied programme of dementia care in care homes has already benefited from this learning by early adoption of our suggestions in this way. 227
The range of approaches to widen methodological options within applied health programmes has been outlined321 and a rationale, with guidance for a more detailed consideration of the complexities involved in dementia care research, also exists. 322 Although large-scale group designs that underpin RCTs remain the gold standard, other designs may also be considered when difficulties arise in the progress of applied research, as was the case in this programme of work. For example, Steingrimsdottir and Arntzen323 note that researchers may be faced with a problem of too few participants available for randomisation, as was the case in the FamCare study; or they may be limited to addressing the experimental question, thus hindering the possibility of providing reliable information about which treatment can be used in the clinic setting, as was the case in the ResCare trial, in which application of the technology hindered the enquiry of the evidence-informed functional analysis-based interventions. Steingrimsdottir and Arntzen323 outline a range of within-participant research designs which may provide valid information about whether the observed changes are caused by manipulations of the independent variable or if they are attributable to other variables. 323 Although we used a within-participant design to model functional analysis-based interventions in care homes,13,68,69 adopting this approach during the feasibility phase of the ResCare trial may have assisted us in determining the effect of independent variables, such as technology versus face-to face alternatives to staff support, on particular dependent variables, such as uptake of worksite opportunities. Similarly, the effect of independent variables on the dependent variables, such as staff behaviour or levels of external supervision required, of clinical protocols using technology versus a pencil-and-paper workbook format could have been investigated at the feasibility stage of the ResCare trial. This may also have had more meaning for the audiences in which research is delivered and allowed a more informed design for the large-scale enquiry. There is, we suggest, also scope for a wider range of designs, when implementation and co-production components are embedded at an early stage of the research process. Thus, even at the modelling phase, knowledge of practitioner qualities, training and skills, or varied organisational contexts within which an intervention is delivered, can be studied using the wide range of implementation strategies. 324,325 Enquiry using implementation strategies can also be gained from feasibility, pilot or exploratory controlled studies, which can then be embedded in a large-scale RCT. 189
Another method to improve translation of research in practice is to conduct a realist evaluation alongside a RCT. 326 This may help the researcher to elucidate what might work for whom and in what clinical context. Realist evaluation is an emerging area of enquiry that has strong potential for informing research questions that take into account the different contexts of delivery on health-care innovation and interventions, and show scope for refined approaches to process evaluation in applied health-care research327,328 and training. 327,329,330
Challenging behaviour in dementia care home and family care settings: a comparison of clinical presentations
At the time of our study development – and potentially to the present time as well – multidisciplinary CMHTsOP across many NHS organisations were commissioned to deliver support to people with dementia and CB who live at home or in care homes. Some services, such as those replicating the Newcastle model, had also developed ‘in-reach’ care home services for dementia and CB, based on knowledge of the extra support that is required to engage and maintain the system that provides care for the resident, in successful implementation of care home interventions.
In bringing descriptive knowledge from this programme to the clinical setting of specialist mental health support for people with dementia and CB in the NHS, we were interested in comparing CB presentations between care home and home care settings. This could inform a better understanding of symptoms of BPSD and the associated caregiving distress within these differing contextual environments. We therefore used the NPI,135 which is identical to its care home counterpart, the Neuropsychiatric Inventory-Nursing Home (NPI-NH) version,14 in measurement of symptoms and behaviours that constitute BPSD. The NPI135 ‘carer distress’ domain is replaced by the interviewer with the term ‘occupational disruptiveness’ when using the NPI-NH14 in a care home setting.
There were a number of differences between the ResCare and FamCare arms of the programme with respect to the interventions or support that people with dementia and CB had received. For example, for the ResCare CRT some participants had ‘action plans’, which were mostly for just one reported CB, as time scales did not allow us to work on multiple behaviours, while others were in receipt of ‘usual care’, and all FamCare study participants were in receipt of ‘usual care’ with an average of nine intervention contacts from a mental health practitioner. However, participants in both arms of the programme had clinically significant CB; the average duration of our observations, from baseline to final follow-up, was roughly equivalent at 6.42 months for the ResCare trial and 6.55 months for the FamCare observational study; and data collection occurred during the same time frame at a time when NHS policies were growing to improve care for people with dementia. Therefore, some comparison about participants between the two studies is perhaps justifiable, to inform services and practice improvements in the future. We did not examine changes over time across the two studies, as the comparative time period was relatively short, conclusions based on the findings from this comparison would be compromised by differing study designs and fluctuations of individual symptoms are in any case known to occur in care home residents. 312
In comparing the two cohorts, we first note that, unsurprisingly, participants recruited to the FamCare study were, at baseline, less severely impaired, as measured by the CDR, than those from the ResCare trial (see Appendix 20, Table 112). However, at baseline and follow-up, BPSD, as measured by NPI incidence, frequency, severity and total scores (see Appendix 20, Table 113), were all higher in the FamCare study group.
Second, at baseline the types of BPSD differed in these two settings. NPI scores for apathy, depression, anxiety and eating disorders were higher in family settings than for participants in care homes, whereas in care homes agitation and aggression was more common (see Appendix 20, Figure 22). Despite lower scores for levels of agitation and aggression in family settings, BPSD-related distress in families was higher than among care staff (see Appendix 20, Table 113).
What is clear from these findings is that family carers, who made up 98% of the FamCare study group, are, as we have suggested earlier, personally invested in their relative: they spend an average of 16 hours per day (see Chapter 5, Table 61) with their relative, and are highly distressed (see Appendix 20, Table 113). Their distress may be due to the perceived changes in their relative,86 including when the person appears unable to initiate action and is therefore ‘doing nothing’ (‘apathy’) and/or appears depressed or anxious or has changes in appetite (see Appendix 20, Figure 22).
Setting-specific differences in the presentation of CB need to be understood by practitioners who provide care to people with dementia and CB in both care home and home care settings. Otherwise there is the risk that some CB can go unrecognised or untreated with the best available evidence for its management, particularly in family care settings, as demonstrated from our data and stakeholder consultations (see Chapter 5).
Discussion: the management of challenging behaviour in dementia
This programme of work explored the same questions associated with the management of CB in dementia in two different settings: the care home and the family home. These environmental settings and systems of care are broadly different from each other: care home provision reflects systems of care that involve many care providers, each with their own perspectives, experiences, skills and work responsibilities. In contrast, home care systems have one or just a few ‘care providers,’ particularly at the start of developing dementia, or perhaps when people with dementia are categorised as having ‘mild dementia’. These ‘informal care providers’ are mostly family or friends, with personal relationship investment in their relative or friend who has dementia. A single, perhaps co-resident, family member can, in this case, become key to the delivery of interventions for dementia and CB at home, whereas within the care home numerous care staff are necessarily involved in the delivery of these interventions. The initial concepts underlying this programme, the high-level synthesis of the Cochrane review and the subsequent methodological improvements that we have suggested for the design and delivery of a programme such as this, both highlight in several ways the importance of understanding and attending in detail to the context within which a person lives and is supported, when delivering interventions for the management of dementia and CB.
First, by studying dementia and CB in two different care contexts, that is, within care homes and family homes during the same time period, we have compared cohorts from the ResCare trial and FamCare study in terms of dementia severity; BPSD; aspects of the psychosocial system associated with BPSD, such as the distress of staff or families; and aspects of management, such as prescribing practices. This will help to inform future practice and service improvements and research on the management of CB in dementia care, as summarised later in this chapter.
Second, our programme has highlighted how the same intervention, in this case functional analysis-based interventions, can be tailored to the setting and system in which it is to be delivered; the care home and family care settings had the same biopsychosocial functional analysis-based intervention, but we also conceived a third group of setting-specific actions comprising differing strategies to address the system supporting the person. Thus, one set of interventions was geared to the needs of the care home and its staff, whereas the other was geared to the range of needs of the family carer (see Chapters 1 and 2). The interventions conceived in the third component of action plans in the ResCare trial may not have been of the strength necessary to impact on delivery of the other two sets of biopsychosocial action. However, this paradigm of intervening in dementia care, when delivery involves incorporating the environment and needs of the caregiving system, remains important and has been reconceptualised from ‘non-pharmacological’ interventions to ‘ecopsychosocial’ interventions. 331
The ResCare studies concluded that the care home industry in England was perhaps not yet ready to fully embrace an infrastructure comprising computerised learning or an IMS for decision-making about the management of CB in dementia (see Chapters 3 and 4). However, the potential for better uptake of e-learning in smaller care homes with less hierarchical leadership and support from the equivalent of an external supervisor, such as trained dementia therapist, may have potential (see Chapter 4). The toolkit for the delivery of innovation, including digital technology in care homes, has implications for future service improvements (see Chapter 4, Tables 36–38 and 40). In contrast, the FamCare study concluded that specialist mental health practitioners working in NHS organisations across England are ready to engage with e-learning and decision support tools to deliver interventions, if the obstacles associated with security and shared patient information systems can be overcome (see Chapter 2).
Third, the findings from our wide-ranging stakeholder consultations, supported by the expert consensus information,332 highlight the importance of other contextual considerations that underpin care for people with dementia and CB in care homes. Relevant features include having better links with vocational qualifications; training and commissioning practices (e.g. the NVQ, now the Quality and Credit Framework initiative) to improve skills of the workforce; clarity about commissioning purpose, as, for example, in determining training needs and relevant support associated with general person-centred dementia training versus the more specialised higher order problem-solving333 approach required for interventions for dementia with CB; and, finally, within models of specialist ‘in-reach’ support services,190 an appreciation of the often locally produced shifting and delicate cultures, which often require careful consideration. 334 Supportive cultures are important because even skilled managers need to employ a lot of effort to support their staff to deal with the daily challenges in the care homes. 334 The wider ‘context’ of specialist NHS services to people with dementia was also important in the delivery of the FamCare study, where the changing landscape of services across England undermined the CRT of an intervention that was planned for delivery by mental health nurses working in their CMHTsOP (see Chapter 5 and Appendix 3). Of concern were the findings from our review of every new case referred to CMHTsOP that a number of patients referred by their GP to these specialist services were returned to the care of their GP or signposted elsewhere without evaluation by the specialist mental health services (see Appendix 3, Table 67).
Thus, we note a significant emerging care gap within specialist NHS mental health organisations in the delivery of timely support to families. The potential to miss families supporting a relative with dementia and CB through rerouting of referrals to memory clinics, combined with the families’ own uncertainties in knowing when to seek help for CB (see Chapter 5), highlight some of the potential hazards of early recognition of dementia and the complex dilemmas associated with re-engineering dementia care services, that were outlined over a decade ago. 298
Fourth, our findings suggest that care homes need a much higher ‘dosage’ of external support from specialist-trained practitioners than was provided in the present intervention. Aspects of adequate dosage would include co-ordinated treatment support from a trained external dementia practitioner who has formal access to experts, including medical and psychology professionals; attention to multiple behaviours in the resident; and ongoing monitoring of potentially fluctuating CB over time in those residents with clinically significant CB. Many of the UK’s specialist CB teams, which were conceived using the Newcastle clinical protocols, now use a 12-week protocol when treating BPSD,97 with the final 6 weeks being a monitoring/support phase for the implementation of the care plan. In the present study, process observations suggested that some action plans, which can be seen as equivalent to care plans,335 may have been undermined by the unwillingness of GPs to engage with care home staff to consider identified health needs or medication review (see Chapter 2), although they may have engaged with external practitioners or medical colleagues from the multidisciplinary team. Moreover, qualitative data from control group care staff suggested that their positive appreciation of in-depth discussion with research staff external to their setting about residents with CB (see Chapter 4) may have contributed to a Hawthorne effect for the control condition. The role of the specialist mental health support to further ensure the appropriate implementation of care plans must not be underestimated, because poor adherence and lack of fidelity are common problems encountered in formulation-led approaches. 97
Staff training interventions for dementia with challenging behaviour in care homes
A key feature of the change mechanism in our intervention was staff behaviour, whereas care plans were conceived as a tool to facilitate change. The process evaluation (see Chapter 4) suggested that the intervention may have become embedded into ‘common sense ideas and practice’, in which staff champions had not fully appreciated the importance of searching for the potential cause or causes of the person’s varying communication of distress as a result of unmet need. This search for the potential causes of CB was our planned mechanism for change in staff behaviour. Our training was designed to engender a more skilled approach involving empathic curiosity,214,215 allowing a ‘detective-like’ approach to discovering unmet need in the resident with dementia and CB. The training programme had indeed used simulated video material to enhance observation skills of emotion and the meaning of non-verbal communication in residents with dementia and CB, in a given situation (see Chapter 2), but others have noted that even staff who have received training may not act on their observations to prevent escalation of CB. 96 Therefore, it is unlikely that all staff can, at any one point in time, understand enough of the perspective of the resident who has dementia and CB, and respond to their need in an effective manner at all times. The data from the stakeholder interviews confirmed the heterogeneity of care homes and that ‘one size’ training is not likely to flourish, as ‘customers’ were changing, staff moved and could be replaced by staff with different experiences or expectations, and the economy of care homes was changing, with investments often scrutinised for their profits. Thus, we conclude that a staff training e-learning intervention is unlikely to hold traction for the management of dementia with clinically significant CB. However, beneficial effects may be seen if training interventions are combined with additional on-site visits, when care staff are given assistance to work with individuals under supervision. 60,170
In community mental health settings, the impact of staff training seen was not reflected in a CRT. Nor did we replicate findings from an earlier exploratory RCT. 65 This was because the FamCare study participants constituted a ‘treatment as usual’ condition in which the revised design allowed us to observe usual practice within specialist mental health services for home-dwelling people with dementia and CB. This cohort study found that when experienced mental health practitioners provided care to participants with dementia and clinically significant CB, with an average of nine contacts, there was no overall change in CB. This suggests that they, and perhaps other specialist mental health nursing services,288 or indeed other professionals commissioned to provide care for people with dementia and CB, would benefit from specialised training, multidisciplinary support and supervision protocols for the effective delivery of interventions for CB in dementia care. As with our observations for care home systems, the key change mechanism in the application and delivery of case-specific interventions for people with dementia and CB living at home is the trained dementia practitioner82 who works with the family carer. Empirically informed care plans are a helpful tool in the co-ordination and iterative monitoring of an intervention and can improve the process of delivery. 336 Nevertheless, these do not in themselves affect outcomes for people with dementia and families. 336,337 Indeed, without support from a trained dementia practitioner, the care plans (no matter how comprehensive) are frequently ineffective. 338 Thus, one of the key roles of such a clinical practitioner – whether they are CMHTsOP or Admiral nurses or dementia advisors, or any other type of practitioner with knowledge about dementia – is to support implementation of the care plans, as poor adherence and lack of fidelity are common problems encountered in formulation-led approaches. 97
Implications for service improvements and practice
In Chapter 1 we outline a rationale for the elusiveness of the syndrome and the need to avoid limiting the content of interventions to biomedical constructs,339 as this runs the risk of overlooking the complexity of causation and maintenance of CBs in dementia care. Moreover, in the care home context, staff may not see it as their role to manage BPSD,228 whereas in community settings, practitioners find it hard to make rational decisions about treatment options in dementia care than in other chronic conditions. 290 Additionally, although educational interventions can meet the needs of GPs and staff caring for people with dementia, participation in these programmes can be low,340 as was the case in the ResCare trial (see Chapter 2). We have argued that case-specific interventions to address the causes of CB are superior to other, simpler solutions. Although interventions such as aromatherapy, activity programming, carer education or carer counselling are worthy approaches, they can be effective only if they address the potential cause(s) of CB and unmet need in a given situation at a given time. It is therefore not surprising that practitioners and services that support people with dementia and CB, have difficulty in focusing their efforts on those with significant CB. For this reason, algorithms for case-specific biopsychosocial interventions, conceived in the current programme as functional analysis-based approaches (see Chapters 1 and 2), remain a promising intervention for the management of CB in dementia. 82 We will consider next what our programme adds to delivery of NHS-led interventions for the management of CB in dementia care.
Five key aspects are discussed, including the notion of ‘caseness’, which affects our awareness and responsiveness to treat in a timely manner. We also discuss the role of scaffolding185,186 via the use of experienced therapists, and how this could better embed the approach and support the development and delivery of functionally derived care plans. Next we highlight a clearer rationale for a setting-specific content for future training innovation in the management of dementia with CB and then we consider the notion of responsibility, helping to clarify roles and goals. Finally, we discuss the lack of internet facilities in care homes within a modern economy in which greater efficiency increasingly depends on availability of IT and use of the internet.
First, the possibility arises that the intervention may not in itself have been ineffective, but that there were significant obstacles to its delivery in the real world of routine NHS practice. One obstacle that was considered in Chapter 1 was the difficulty for practitioners and specialist services, of determining what constitutes ‘caseness’, that is, the threshold for offering a clinical intervention. There are many measures to ascertain levels of BPSD,341 and the NPI135 and NPI-NH14 are commonly used in research. However, the NPI and NPI-NH are less easy to use in routine practice and do not describe the everyday concerns or challenges experienced by staff and family carers. In the present programme we therefore used the CBS107 and the CMAI136 for care home settings and the RMBPC111 for family care settings, as these reflect the language and the reported challenges of staff and family carers. Additionally, we incorporated the CBS for care homes and its equivalent for family settings (see Chapter 2 and Appendix 1)65,109 within our intervention approach. These offered practitioners the means of structured assessment that is needed before treatment options are considered.
First, in both studies we used cut-off points to determine clinically significant CB in people with dementia. A score of four and above on the 25-item CBS is a validated cut-off point for CB incidence, with a score of > 10 reflecting severe CB in a resident. 107 In the present study, in which residents were included if they had dementia with a score of four and above, comparisons of average scores at baseline with the 12-item NPI were as follows: control group: CBS incidence = 9.01 (SD 3.84) and NPI incidence = 4.80 (SD 2.34); and, experimental group: CBS incidence = 8.94 (SD 3.85) and NPI incidence = 4.86 (SD 2.40) (see Table 18). The 25-item CBS is now widely used by practitioners who provide in-reach services to care homes in the UK (see Brechin et al. 108) and clinicians using a cut-off point of four (or 10 for severe CB) can be more confident of its value in detecting clinically significant BPSD against the NPI. This observation is relevant, as stakeholder practitioners who were not medically trained reported some difficulties about using the NPI as a measure to detect CB in people with dementia. The same observation applies to use of the 24-item RMPBC in the FamCare study, in which comparisons of baseline average scores using a cut-off point of five and above against the 12-item NPI were as follows: RMBPC incidence = 11.08 (SD 3.84); NPI incidence = 5.35 (SD 2.65); NPI total = 25.75 (SD 19.17); and, NPI distress = 13.37 (SD 9.65) (see Table 48). Thus, we suggest that these measures may be useful in service pathways where practitioners need to assess behaviour and its management difficulty to detect CB in people with dementia in care homes and family settings.
Second, service-improvement models will also require the dedicated expertise of key professionals that we have described as ‘scaffolding’,185,186 if practitioners are to maintain their effectiveness of delivery in each setting. There are emerging multidisciplinary post-diagnostic dementia services for CB within which a dementia practitioner has access to ‘diagnostics’, described as ‘formulations’, and where contextual factors within the family or the care home setting can be systematically addressed, alongside unmet need in the person with dementia. 97,186,304 Newer practice models include professional pharmacy review,342 which may go some way to address some of the suboptimal practices we found. This type of ‘scaffolding’185,187 includes the important role of a supervisor,223 which adds to the quality of intervention, as professional dementia therapists are not commonly found across Europe. 343 Indeed, trained dementia practitioners who can provide therapeutic interventions have yet to be conceived as a workforce priority in England. For care homes, where commissioning priorities are targeted towards dementia and significant CB, models could incorporate links to NHS-led and co-ordinated centres of excellence described in the USA as ‘the teaching nursing home’. 344 Here responsibilities for training, supervision and monitoring of care can be operationalised within models of collaborative care. For family care settings models of intermediate care that are currently in place345 could be improved in terms of clarity of purpose, to focus on people and families with dementia and significant CB, with goals that address behavioural symptoms and associated distress in families. This will require practitioner-led relationships with families and co-ordination with ongoing support, as symptoms fluctuate or needs change within family systems.
Third, an important implication for practice for this subgroup of people with dementia who also have clinically significant CB is that, once this has been detected, care staff and family carers should not be left to make decisions and manage on their own, through the trajectory of the condition. Thus, we suggest that there is an important role for co-ordinated care by a trained dementia practitioner,346 as there appears to be a skill gap related to the management of dementia and CB in the current workforce. Given our observations on the differing contextual resources required for delivery within care homes and family care settings, a priority for service improvements for effective management of dementia and CB is the development of separate service models for care home and family care settings within which specialist dementia practitioners may be trained and resourced to have clarity of purpose in their goals for patient care and related contextual outcomes. To determine the nature and content of the training required for each group, a closer examination of the change mechanisms used by the carers in dealing with BPSD is required. Our attempts to provide a third (additional) component to the biopsychosocial functional analysis-based intervention using written action plans to attend to the caregiving system needs in the ResCare trial was not strong enough to impact on CB. Thus, we need to consider improvements to our intervention to overcome the weak adherence to actions for the caregiving system in care homes. One of our research team has therefore developed a training course as an extension of Newcastle approach to CB in care homes. The following principles have been used to determine the course content for this third component of our intervention.
-
Owing to the prevalence of CBs, the carers already must be dealing with problematic situations on a daily basis. Therefore, carers have existing skills that may be used and developed further.
-
The key mechanisms of change used by carers to deal with CBs are communication and interactive skills. Some carers appear to be able to negotiate well and de-escalate situations that could have become problematic.
-
Carers are poor at articulating their CB skills. This is problematic, because they are then unable to appreciate ‘what works and what doesn’t’. One of the goals of the training is to teach the carers aspects of what they do already, but giving them a language (and rationale) to explain their actions.
-
The vehicle through which the communication skills are delivered is a care plan. The care plan is developed bespoke via a functional analytical approach.
Although the training requires adaptation to the different needs of care homes and families, the same general principles apply. The initial manifestation of our communication training is CAIT (communication and interaction training). 338 As outlined above, this training acknowledges carers’ existing communication and negotiating skills in dealing with CB, and supports the development of new approaches, both general and patient specific. CAIT is currently being used in the training of all 62 care homes over a 3-year period in one local authority area in the north-east of England. CAIT teaches a functional analytical approach, but supports the workshop training with 18 weeks of supervision delivered to two key individuals per care home. The comprehensive supervision, a feature missing in the e-tool programme, is being used to (1) embed the CAIT approach, and (2) support the implementation of care plans to ensure that they are adhered to with fidelity.
Fourth, as we now have tools for screening of clinically significant CB in dementia, consideration must be given to service responsibilities for the delivery of interventions. There is the potential for minimisation of the level of need, and ‘everybody’s business’347 becoming ‘nobody’s business’. For example, professionals appeared to expect other professionals to deliver interventions, even before they had evaluated the patient need (see Appendix 3, Table 67), and families appeared unclear about whether they were worthy or deserving of specialist support (see Chapter 5). Furthermore, many people with potential CB were simply signposted onto memory clinics for which some organisations had waiting lists had emerged in some organisations, while some teams had developed long and unwieldy procedures for diagnosis that had the potential to exacerbate distress for some family carers. In care homes, suboptimal medical care and prescribing was also noted. Furthermore, effective management of CB is considered difficult to deal with, even in a NHS continuing care unit, as a result of a lack of treatment options348 when, as we have noted in Chapter 1, usually there are avenues to explore even in the most difficult of cases.
Given the noted elusive nature of the syndrome and the complexity of the condition, particularly for those with clinically significant CB, services and practitioners need to be cautious about adopting one solution as an alternative to a case-specific approach to intervention. For example, even a recent well-designed family caregiving intervention, which incorporated all of the elements of functional analysis-based interventions and demonstrated impressive effectiveness on carer mood, did not have an impact on CB349 or on caregiver abusive behaviour. 350 Similarly, caution at this stage is needed regarding internet solutions for the management of dementia with CB by family carers, in the absence of support by a trained practitioner. Although this may be a seductive option to consider, this type of dosage might be helpful for some clinical presentations, but would probably not be enough for sustained changes in behavioural symptoms, and associated distress in those with clinically significant CB. For example, a recent internet-based dementia support solution has been applied in family settings with good outcomes on some aspects of carer well-being,351 but the authors do not report any impact on their measures of behavioural symptoms.
Finally, the lack of broadband internet availability in some care homes and the low use of electronic records are of some concern for the future of care delivery in an environment where most of the economy is IT based. Use of e-mails or electronic records in care homes has scope for better communication between care home staff and external care providers, such as the GP, nurse practitioners or social workers. Furthermore, internet access has the potential of allowing better communication between families and their relatives who reside in care homes, for example through skype™ (Microsoft Corporation, Redmond, WA, USA) or use of personal tablets and iPads (Apple Inc., Cupertino, CA, USA). This observation has implications for regulators such as the CQC, as internet access at care homes could be a major focus for their inspections.
Recommendations for future research
Staff training and support for dementia with challenging behaviour
There have been at least two recent reviews of training programmes currently on offer. Spector et al. 60 conducted a systematic review of staff training interventions for BPSD and found 13 RCTs and seven non-randomised study designs. They note that effectiveness was enhanced in 10 studies in which individual supervision sessions were offered to help staff incorporate strategies into everyday practice. 60 In a second study, Fossey et al. 170 reviewed available dementia training materials and found that, of the 170 located, only four had a robust evidence base. They too noted that, in addition to education, efficacious programmes included a sustained period supervision with a trained mental health practitioner. 170 The present programme and an initial pilot study about the current day-to-day practices of carer approaches to CB352 suggest that a next step in studying the impact of interventions for dementia with CB is to examine the nature of the support required from external specialists in dementia care.
Future research on interventions for dementia with challenging behaviour
Future interventions based on the approaches we have developed may use conventional (face to face) or other (e.g. online) innovative technology, or a combination of these, to enhance practice. However, the design of studies requires systematic work programmes to examine in detail what might work for whom in a given stakeholder context. A more collaborative approach than has been used to date, such as building on existing skills of practitioners and paid staff carers, may, we suggest, facilitate an improved service for the support of patients and carers. It is also important that potential improvements are measured in ways that appear relevant to the range of stakeholders. Attention needs to be paid to current organisational structures to determine whether or not we have the most appropriate service in terms of structures and functions to effectively treat CB. This work is required because, as we have noted in the FamCare study, the changing organisational structures, including those relating to the then changes associated with the PbR initiative121 and those resulting from the implementation of the National Dementia Strategy,250 may have proved to be a disservice to the timely and potentially effective management of home-dwelling people with dementia and CB. A related issue concerns the treatment of people with dementia receiving treatment in acute settings. Research is required to examine both the patients’ treatment and staff training. The above ideas are discussed in more detail next.
First, we note that within our programme we perhaps paid inadequate attention to the care system, where providing written strategies within action plans for staff champions to support others to deliver the interventions was not enough to impact on CB. Development and evaluation of treatment programmes built around existing carer skills seems sensible. An example of this, and an extension to Challenge Demcare, has been the aforementioned CAIT programme. 338 CAIT is a strength-based relationship approach that involves developing good rapport with carers, and building on their current strengths and knowledge about BPSD. Strength-based relationship principles are embedded in the style employed in the teaching of CAIT, which works via the key concept that talking to people about using their strengths (what the trainee brings to the training, including their hopes and expectations) accounts for the highest level of success in teaching programmes.
Second, NHS-led service models for the formalised support of CB in dementia are important for the co-ordination and delivery of interventions for dementia with CB. This will also require care homes that are ready and perhaps commissioned, to support people with significant CB. Research can then be designed to evaluate relevant outcomes for people with dementia and CB, and coping by care staff.
Third, the significant ‘care gap’ across England that appears to have emerged concerning the support of home-dwelling people with dementia and CB and their families is highlighted. This may have resulted from, for example, difficulties that practitioners may have in determining the threshold for what constitutes clinically significant CB in dementia, from their misperceptions of the complexity of CB in people with mild dementia or from the changing commissioning arrangements of health- and social-care services that has left the variety of service providers, including care staff and practitioners, with uncertainties about their roles and responsibilities. These factors may in turn undermine timely responses to prevent escalation of distress in families. To address this gap, service improvements within a dementia care pathway could involve models in which redesigned roles of the range of practitioners within the current landscape of provision of dementia care may be supported by skills training to improve access to someone akin to a ‘dementia behavioural therapist’. Health service research may then investigate what additional support is required to support these practitioners by way of scaffolding185,187 from professionals with expertise in medical, psychological and social care. Clarity of purpose would require local agreements between stakeholders, including commissioners, GPs, specialist NHS organisations or providers of social care. Research can then be designed to evaluate relevant outcomes for people with dementia and CB, and sustained coping by family carers.
A gap in the care industry around the use of IT in the care of people with dementia living in care homes may also exist. Future health service research on the availability of IT resources or motivations by care staff to use this could also be undertaken, in readiness for future internet solutions to improving the efficiency of care within care homes.
An analysis of the referral pathways for people with dementia and CB is important, and will have implications for where commissioners choose to direct resources across the NHS and social care. For example, following reorganisation of services in line with implementation of the National Dementia Strategy250 and a subsequent growth of memory clinics, research suggests that cognitive stimulation therapy, already provided in many memory clinics, may be an effective alternative to tranquillising medication. 353 Such findings require replication and reflect positive practice shifts in dementia care. However, what is important for the future management of CBs in dementia is to avoid simplistic global practice interventions to reduce antipsychotics or other tranquillising medications with ‘therapies’, such as cognitive stimulation therapy. There are, we suggest, few alternatives to the case-specific formula-led types of interventions based on both biopsychosocial intervention for unmet needs and attention to the caregiver needs in supporting such interventions, in the management of clinically significant CB.
Involving stakeholders early in the research process in the development and delivery of complex interventions for CB is an important strategy for the conduct of future research in dementia. It was particularly relevant for our team to hear the views of frontline practitioners, care providers and family carers about the findings. Furthermore, it is appropriate to examine the views of staff working in acute settings, who are frequently dealing with CBs from their physically ill older patients. A recent paper on the use of communication issues in a general hospital setting354 suggests that many staff feel untrained and unsupported. Also, it is evident that the lower grades of staff (support workers) are left to respond to the problematic behaviours. In contrast, the qualified staff (medical staff, nurses) do not necessarily see it as their responsibility to respond to people who are disorientated or ‘time-shifted’. 354
Conclusions
The NICE guidance, updated by our Cochrane review of functional analysis-based interventions for the management of dementia with CB at home and in care homes, concludes that the approach shows promise. 82 An e-learning program to assist staff working in care homes to develop and deliver such interventions gained little traction in care homes. Access to the internet was patchy in some care homes, and worksite e-learning opportunities were not readily taken up by care home staff. Furthermore, training with group discussion and decision support for individualised interventions did not change practice enough to have an impact on CB in dementia. Smaller homes with a less hierarchical management appear more ready than others to engage in innovation. Capable leadership and collective willingness are also important considerations for the delivery of innovation in care homes.
People with dementia and CB were referred by their GP to specialist NHS services, but treatment over 6 months, averaging nine contacts per family, had no impact on high levels of CB. Over 60% of home-dwelling people with clinically significant CB had mild dementia. Some mental health practitioners appear unaware of thresholds for clinically significant CB in families. In this study, a cut-off point of five on the 24-item RMBPC111 was adequate for the detection of clinically significant CB when compared with NPI,135 which is a measure that non-medical practitioners find difficult to use. Families bear the majority of the care costs of dementia with CB and a care gap in the delivery of evidence-informed support to families and people with dementia with clinically significant CB has emerged.
Higher levels of CB were recorded in family settings than in care homes; and prescribing practices were suboptimal in both care home and family settings.
The contexts of the care home and the family care setting are not equivalent, and delivery of interventions for CB in dementia in these settings therefore requires similar approaches, but different resources and service pathways. For example, care home support, staff training and ongoing resource provision will differ from that of family carers and, when relevant, paid home care staff. Moreover, the differing skills and time resource of trained dementia practitioners who co-ordinate and provide case-specific interventions for CB will also need to be conceived as separate service-improvement developments for each of these two settings. In both cases, these will additionally require clarity of purpose, as one size of service resource will not fit both settings. This is particularly important when service pathways with care homes that are commissioned specifically for people with dementia with CB are absent. Therefore, process and outcome measures for each of the family care and care home settings will need to be set within formal agreements between stakeholders, including commissioners and providers.
This programme has developed manualised instruments and decision support resources for the recognition and targeting of interventions towards unmet need of the person with dementia and the care home or family system that they live in. The resources have been met with enthusiasm by specialist mental health practitioners. These manualised tools may be of value to practitioners within ‘in-reach’ models of specialist mental health support to care homes. They have the potential to assist mental health practitioners in recognition and provision of empirically informed interventions for CB in family settings. This may then have the effect of reducing the impact of behavioural symptoms on the family carer. A toolkit for future research and practice to facilitate the delivery digital technology and other innovative interventions within care homes has also been developed.
No one size of intervention can address the complexity of CB in dementia. However, case specificity62 to include examination of relevant biopsychosocial interventions for the person as well as good attention to the needs of the caregiving system still appears to be the way forward for the management of CB in dementia. A functional analysis approach to interventions for CB in dementia care provides a framework for assessment and targeting of case-specific formulaic interventions. This programme has highlighted a gap in attention to the needs of the caregiving system and has led to the development of the CAIT programme,338 by one of our research team. Application within ‘in-reach’ service models for the management of CB in care homes is an area for future NHS applied research. As noted previously, functional analysis-based intervention and its extension with CAIT338 is now being piloted in local authority care homes in the north-east of England, thus bridging the gap between NHS and local authority care provision for people with dementia. Given the changing landscape of the organisation of dementia care, there is scope for research on the delivery of this specialist intervention across both NHS and social care provision in the future. The intervention we have developed also has strong potential to fill the emerging care gap associated with timely support for CB in family care settings. This is important as families can have deep underlying beliefs that contribute to the variation in their responses to the relative’s unmet need and associated behaviour that they find difficult to deal with. 86 These need to be captured at an early stage,355 by trained dementia practitioners using a structured assessment to decide on appropriate treatment,294 with a focus on minimising the occurrence of CB and distress within families.
Approaches to the management of CB can be efficiently delivered only if there is a shift in current emphases, from the early diagnosis of dementia to early recognition and interventions for CB, and a sustainable pathway of dementia care within specialist mental health organisations in England. To deliver case-specific interventions for CB, trained dementia practitioners (therapists), working within ‘in-reach’ services in care homes or in family settings, will need to work within commissioned systems, where they have formalised access to professional experts who have responsibility for advising on the range of medical and psychological interventions that underpin functional analysis approaches in care home and family care systems. They may then be in a position to train and support care home and home care workers and allay legitimate current concerns by the Alzheimer’s Society on the lack of this, particularly among the formal home care workforce. 356
An independent assessment of improvements in dementia care since 2009 commissioned by the Department of Health, outlines priorities for action in the final year of the ‘Prime Minister’s Challenge on Dementia’, including the need for psychosocial and other alternatives to pharmacological management of CB, to guide commissioners or providers (see Knapp et al. pp. 3, 62). 302 Our programme of empirically informed knowledge about practices and recommendations for post-diagnostic service improvements that are urgently required for the management of CB in dementia care will go some way to address this priority. To realise its full impact the next step is to disseminate the learning and resources from this comprehensive programme of work. The mechanism for achieving this is planned within road shows and workshops involving wide-ranging stakeholders, including commissioners and providers of care.
Acknowledgements
The Challenge Demcare programme was funded by the NIHR Programme Grants for Applied Research programme.
We are very grateful to all of our patients, families, care home residents, NHS and care home staff and stakeholders who generously gave us their time for this programme of research across the country.
The Challenge Demcare team would also like to thank the management of Humber NHS Foundation Trust for supporting the research team; Humber NHS Foundation Trust IT Department for helping with computing requirements in the care homes; other NHS study site personnel at North East London NHS Foundation Trust, Greater Manchester West NHS Foundation Trust, Sheffield Health and Social Care NHS Foundation Trust, Oxford NHS Foundation Trust, NAViGO Health and Social Care Community Interest Company, North Yorkshire and York Primary Care Trust (then Leeds and York Partnership NHS Foundation Trust, now Tees Esk and Wear Valleys NHS Foundation Trust); the research assistants who engaged the many participants during data collection; staff at the NWORTH; Professor Martin Bland (chairperson of the DMEC); and Dr David Jolley (chairperson of the External Steering Group).
Challenge Demcare study team
Professor Esme Moniz-Cook (Chief Investigator and supervision as part of the clinical expert research team for delivery of the care home intervention); Mrs Cathryn Hart (Programme Manager); Professor Bob Woods (Co-Chief Investigator; intervention innovation design, e-learning and e-tool algorithms development for CB intervention; care home and family care studies, ResCare, FamCare); Professor Peter Campion (care home study, ResCare); Dr Andrea Hilton (care home study, ResCare); Professor (Hon) Ian James (care home and family care studies, ResCare, FamCare); Professor Martin Orrell (family care study, FamCare); Professor Ian Russell (trial methodology and interpretation of study results); Professor (Hon) Graham Stokes (intervention innovation design support; care home study, ResCare); Mr Chris Whitaker (methodology and statistics); Professor Rhiannon Tudor Edwards (methodology and health economics); Professor Robert Jones (intervention innovation and design); Dr Ivana Markova (support with intervention design and supervision as part of the clinical expert research team for delivery of the care home intervention); Mrs Caroline Mozley (study design support); Professor Murna Downs (methodology support); Dr Daphne Russell (methodology support); Mr Marcus Tredinnick (Trial Co-ordinator, care home study, ResCare); Dr Andrew Walker (support with intervention design); Mrs Irene Carr (intervention delivery, care home study, ResCare); Mrs Katie Swift (Cochrane review); Mr Gavin Dawson (administrative support for the final report); Dr Barry Hounsome (health economics support); Dr Carys Jones (health economics support); Ms Lu Zou (statistical support); Ms Rachel Waddington (statistical support); Ms Zoe Hoare (statistical support); Professor Fiona Poland (qualitative study, Chapter 4); Dr Julia Keenan (qualitative study support, Chapter 4); Professor Jill Manthorpe (qualitative studies, Chapters 4 and 5); Dr Kritika Samsi (qualitative studies support, Chapters 4 and 5); Professor Carl May (qualitative study advice); Professor Clive Ballard (data interpretation advice); Dr Mike Bird (data interpretation advice and drafting Chapter 1); and the NHS principal investigators for the FamCare study Dr Fiona Goudie, Dr Jane Fossey, Ms Sue Watts, Dr Georgina Charlesworth, Dr Chris Clarke and Ms Janine Smith.
Programme Steering Group
Dr David Jolley (chairperson, University of Manchester/retired Consultant Old Age Psychiatrist); Dr Daphne Wallace (person with dementia/retired Consultant Old Age Psychiatrist); Mr David Stutt (Alzheimer’s Society, Hull and East Riding of Yorkshire); Ms Louise Eastwood (care home/home care provider, local authority, Hull); Professor Roger Watson (University of Sheffield and later University of Hull, Gerontological Nursing); Ms June Cooke (carer and dementia champion, Hull); and Mr Mark Lawton (Old Age Mental Health Nurse Consultant, Doncaster, now retired).
Study team in attendance: Professor Esme Moniz-Cook; Mrs Cathryn Hart; Professor Bob Woods; Mr Chris Whitaker; Professor Peter Campion; Professor Ian James; Professor Graham Stokes; Professor Ian Russell; Professor Martin Orrell; and Dr Andrea Hilton.
Data Monitoring and Ethics Committee
Professor Martin Bland (chairperson, University of York); Dr Chris Fox (Old Age Psychiatry University of East Anglia); and Dr Louisa Shirley (Clinical Psychologist, Northumberland Tyne and Wear NHS Foundation Trust).
Contributions of authors
Professor Esme Moniz-Cook (former lead of the Center of Dementia Research and Practice at Humber NHS Foundation Trust) is the Chief Investigator and the guarantor of this report. She is Professor of Psychology, Ageing and Dementia Care at the University of Hull and an Honorary Consultant Clinical Psychologist at Humber NHS Foundation Trust. She oversaw and was involved in all stages of the studies, from inception to final submission and completion of this report.
Mrs Cathryn Hart is Programme Manager for Challenge Demcare and Head of R&D at Humber NHS Foundation Trust. She managed all stages of the studies, including design and writing this final report.
Professor Bob Woods is Professor of Clinical Psychology of the Elderly at the Dementia Services Development Centre, at Bangor University. He acted as Co-Chief Investigator, co-developed the intervention and prepared Chapter 2. He also contributed to all stages of the studies, from inception to reviewing all chapters of this final report.
Mr Chris Whitaker is a Principal Statistician at NWORTH at Bangor University (now Consultant Statistician, Whitaker Research Ltd). He contributed substantially to the design of the studies, the statistical analyses of the data and the writing of Chapters 3 and 5 of this final report.
Professor Ian James is a Consultant Clinical Psychologist at the Northumberland Tyne and Wear NHS Foundation Trust. He provided substantial advice during development of the intervention, contributed to the design, methodology and interpretation of the studies, and writing of Chapters 3 and 6, and reviewed all chapters of this final report.
Professor Ian Russell is Emeritus Professor of Clinical Trials at Swansea University. He contributed substantially to the design of the studies and the review of Chapters 3 and 5.
Professor Rhiannon Tudor Edwards is Professor of Health Economics and Co-Director of Centre for Health Economics and Medicines Evaluation at Bangor University. She designed and oversaw the economic evaluation and contributed to Chapters 3 and 5.
Dr Andrea Hilton is a Pharmacist and Senior Lecturer in Pharmacy at the University of Hull. She contributed to design of the studies, interpretation of data from the care home study, and the review of Chapter 3. She also contributed the design of the care home intervention and supervised its delivery as part of the clinical expert team.
Professor Martin Orrell is Professor of Ageing and Mental Health at University College London (now Director for Institute of Mental Health, University of Nottingham) and Consultant Old Age Psychiatrist. He contributed to the family care study and review of Chapter 5.
Professor Peter Campion is Professor (now Emeritus) of Primary Care Medicine at the University of Hull. He contributed to the design and interpretation of both studies, and medical aspects of the intervention, and supervised the delivery of the care home intervention as part of the clinical expert team.
Professor Graham Stokes is Global Director of Dementia Care, Bupa and Honorary Visiting Professor of Person-Centred Dementia Care at University of Bradford. He advised on the intervention for the care home study, and on methodology and interpretation of the data for the studies.
Professor Robert SP Jones is Programme Director of North Wales Clinical Psychology Programme at Bangor University and Consultant Clinical Psychologist. He co-designed the staff training intervention, and contributed to preparation and review of Chapter 1.
Dr Mike Bird is a Senior Research Fellow in Dementia Care at the Dementia Services Development Centre, Bangor University. He prepared and reviewed Chapter 1 and contributed to the interpretation of data for Chapter 3.
Professor Fiona Poland is Professor of Social Research Methodology at the University of East Anglia. She contributed to the design of, and oversaw, the qualitative study of care homes, and wrote and reviewed Chapter 4.
Professor Jill Manthorpe is Director of the Social Care Workforce Research Unit at King’s College London. She contributed to the design of the qualitative study of care homes and, wrote and reviewed Chapter 4, designed, analysed and wrote the process evaluation section of Chapter 5, and engaged stakeholders, including representatives of patients and the public.
Data sharing statement
Data from Challenge Demcare can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Stokes G, Stokes G,. Challenging Behaviour in Dementia: A Person Centred Approach. Bicester: Winslow Press; 2000.
- Cohen-Mansfield J. Non-pharmacological interventions for persons with dementia. Alzheimers Care Q 2005;6:129-45.
- Smith M, Gerdner LA, Hall GR, Buckwalter KC. History, development, and future of the progressively lowered stress threshold: a conceptual model for dementia care. J Am Geriatr Soc 2004;52:1755-60. http://dx.doi.org/10.1111/j.1532-5415.2004.52473.x.
- Gilley DW, Bienias JL, Wilson RS, Bennett DA, Beck TL, Evans DA. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer’s disease. Psychol Med 2004;34:1129-35. https://doi.org/10.1017/S0033291703001831.
- Balestreri L, Grossberg A, Grossberg G. Behavioral and psychological symptoms of dementia as a risk factor for nursing home placement. Int Psychogeriatr 2000;12:59-62. https://doi.org/10.1017/S1041610200006773.
- Evers W, Tomic W, Brouwers A. Aggressive behaviour and burnout among staff of homes for the elderly. Int J Ment Health Nurs 2002;11:2-9. https://doi.org/10.1046/j.1440-0979.2002.00219.x.
- Opie J, Doyle C, O’Connor DW. Challenging behaviours in nursing home residents with dementia: a randomized controlled trial of multidisciplinary interventions. Int J Geriatr Psychiatry 2002;17:6-13. https://doi.org/10.1002/gps.493.
- Edberg AK, Bird M, Richards DA, Woods R, Keeley P, Davis-Quarrell V. Strain in nursing care of people with dementia: nurses’ experience in Australia, Sweden and United Kingdom. Aging Ment Health 2008;12:236-43. http://dx.doi.org/10.1080/13607860701616374.
- Luff R, Ferreira Z, Meyer J. Care Homes. Improving the Evidence Base for Adult Social Care Practice. Methods Review 8 no. 978-0-85328-451-2. London: NIHR School for Social Care Research; 2011.
- Finkel S, Costa E, Silva J, Cohen G, Miller S, Sartorius N. Behavioural and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int J Geriatr Psychiatry 1997;12:1060-1. http://dx.doi.org/10.1002/(SICI)1099-1166(199711)12:11<1060::AID-GPS658>3.0.CO;2-M.
- Savva GM, Zaccai J, Matthews FE, Davidson JE, McKeith I, Brayne C, et al. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry 2009;194:212-19. http://dx.doi.org/10.1192/bjp.bp.108.049619.
- Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care. Leicester: The British Psychological Society and the Royal College of Psychiatrists; 2007.
- Bird M, Moniz-Cook E, Woods B, Clare L. Handbook of the Clinical Psychology of Ageing. Chichester: John Wiley & Sons, Ltd; 2008.
- Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry 2000;8:75-83. http://dx.doi.org/10.1097/00019442-200002000-00010.
- Bird M, Ames D, Burns A, O’Brien J. Dementia. London: Arnold; 2010.
- Brodaty H, Draper BM, Low LF. Behavioural and psychological symptoms of dementia: a seven-tiered model of service delivery. Med J Aust 2003;178:231-4.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63-75.e2. http://dx.doi.org/10.1016/j.jalz.2012.11.007.
- Wimo A, Jönsson L, Bond J, Prince M, Winblad B. Alzheimer Disease International . The worldwide economic impact of dementia 2010. Alzheimers Dement 2013;9:1-11.e3. http://dx.doi.org/10.1016/j.jalz.2012.11.006.
- Bakker TJ, Duivenvoorden HJ, van der Lee J, Olde Rikkert MG, Beekman AT, Ribbe MW. Integrative psychotherapeutic nursing home program to reduce multiple psychiatric symptoms of cognitively impaired patients and caregiver burden: randomized controlled trial. Am J Geriatr Psychiatry 2011;19:507-20. http://dx.doi.org/10.1097/JGP.0b013e3181eafdc6.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. http://dx.doi.org/10.1212/WNL.44.12.2308.
- Herrmann N, Lanctôt KL, Sambrook R, Lesnikova N, Hébert R, McCracken P, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry 2006;21:972-6. http://dx.doi.org/10.1002/gps.1594.
- Ballard C, Waite J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.cd003476.pub2.
- Deberdt WG, Dysken MW, Rappaport SA, Feldman PD, Young CA, Hay DP, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry 2005;13:722-30. https://doi.org/10.1097/00019442-200508000-00012.
- Herrmann N. Recommendations for the management of behavioral and psychological symptoms of dementia. Can J Neurol Sci 2001;28:96-107. https://doi.org/10.1017/S0317167100001268.
- Margallo-Lana M, Swann A, O’Brien J, Fairbairn A, Reichelt K, Potkins D, et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry 2001;16:39-44. https://doi.org/10.1002/1099-1166(200101)16:1<39::aid-gps269>3.0.co;2-f.
- Bird M, Llewellyn-Jones R, Korten A, Smithers H. A controlled trial of a predominantly psychosocial approach to BPSD: treating causality. Int Psychogeriatr 2007;19:874-91. http://dx.doi.org/10.1017/S1041610206004790.
- Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332:756-61. https://doi.org/10.1136/bmj.38782.575868.7C.
- Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr 2008;20:293-308. http://dx.doi.org/10.1017/S1041610207006540.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005;293:596-608. https://doi.org/10.1001/jama.293.5.596.
- Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des 2002;8:45-58. https://doi.org/10.2174/1381612023396654.
- Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc 2000;48:682-5. http://dx.doi.org/10.1111/j.1532-5415.2000.tb04729.x.
- Tucker M, Hosford I. Use of psychotropic medicines in residential care facilities for older people in Hawke’s Bay, New Zealand. N Z Med J 2008;121:18-25.
- O’Connor DW, Ames D, Gardner B, King M. Psychosocial treatments of behavior symptoms in dementia: a systematic review of reports meeting quality standards. Int Psychogeriatr 2009;21:225-40. http://dx.doi.org/10.1017/S1041610208007588.
- Baillon S, Van Diepen E, Prettyman R, Redman J, Rooke N, Campbell R. A comparison of the effects of Snoezelen and reminiscence therapy on the agitated behaviour of patients with dementia. Int J Geriatr Psychiatry 2004;19:1047-52. http://dx.doi.org/10.1002/gps.1208.
- Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d4065.
- Complete Guide to Behavioral and Psychological Symptoms of Dementia. IPA; 2010.
- Cohen-Mansfield J, Werner P. Longitudinal predictors of non-aggressive agitated behaviors in the elderly. Int J Geriatr Psychiatry 1999;14:831-44.
- Bird M, Blair A. Clinical psychology and anxiety and depression in dementia. Nord Psychol 2010;62:44-5. https://doi.org/10.1027/1901-2276/a000010.
- Hallberg I, Norberg A, Erikson S. Functional impairment and behavioral disturbances in vocally disruptive patients in psychogeriatric wards compared with controls. Int J Geriatr Psychiatry 1990;5:53-61. https://doi.org/10.1002/gps.930050109.
- Meares S, Draper B. Treatment of vocally disruptive behaviour of multifactorial aetiology. Int J Geriatr Psychiatry 1999;14:285-90. http://dx.doi.org/10.1002/(SICI)1099-1166(199904)14:4<285::AID-GPS900>3.0.CO;2-2.
- Hallberg IR, Edberg A-K, Nordmark A, Johnsson K. Daytime vocal activity in institutionalized severely demented patients identified as vocally disruptive by nurses. Int J Geriatr Psychiatry 1993;8:155-64. https://doi.org/10.1002/gps.930080208.
- Schnelle JF, Alessi CA, Al-Samarrai NR, Fricker RD, Ouslander JG. The nursing home at night: effects of an intervention on noise, light, and sleep. J Am Geriatr Soc 1999;47:430-8. https://doi.org/10.1111/j.1532-5415.1999.tb07235.x.
- Ancoli-Israel S, Parker L, Sinaee R, Fell RL, Kripke DF. Sleep fragmentation in patients from a nursing home. J Gerontol 1989;44:M18-21. https://doi.org/10.1093/geronj/44.1.M18.
- Ersser S, Wiles A, Taylor H, Wade S, Walsh R, Bentley T. The sleep of older people in hospital and nursing homes. J Clin Nurs 1999;8:360-8. https://doi.org/10.1046/j.1365-2702.1999.00267.x.
- Moniz-Cook E, Bird M. Sensory stimulation in dementia. Cause of behavioural and psychological symptoms of dementia needs to be established first. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7390.661.
- Elmståhl S, Stenberg I, Annerstedt L, Ingvad B. Behavioral disturbances and pharmacological treatment of patients with dementia in family caregiving: a 2-year follow-up. Int Psychogeriatr 1998;10:239-52. https://doi.org/10.1017/S1041610298005353.
- Fauth E, Gibbons A. Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int J Geriatr Psychiat 2014;29:263-71. http://dx.doi.org/10.1002/gps.4002.
- Sink KM, Covinsky KE, Barnes DE, Newcomer RJ, Yaffe K. Caregiver characteristics are associated with neuropsychiatric symptoms of dementia. J Am Geriatr Soc 2006;54:796-803. https://doi.org/10.1111/j.1532-5415.2006.00697.x.
- Morgan RO, Sail KR, Snow AL, Davila JA, Fouladi NN, Kunik ME. Modeling causes of aggressive behavior in patients with dementia. Gerontologist 2013;53:738-47. http://dx.doi.org/10.1093/geront/gns129.
- Davison TE, Hudgson C, McCabe MP, George K, Buchanan G. An individualized psychosocial approach for ‘treatment resistant’ behavioral symptoms of dementia among aged care residents. Int Psychogeriatr 2007;19:859-73. http://dx.doi.org/10.1017/S1041610206004224.
- Hamel M, Gold DP, Andres D, Reis M, Dastoor D, Grauer H, et al. Predictors and consequences of aggressive behavior by community-based dementia patients. Gerontologist 1990;30:206-11. https://doi.org/10.1093/geront/30.2.206.
- de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Winkens I, et al. Do caregiver management strategies influence patient behaviour in dementia?. Int J Geriatr Psychiatry 2004;19:85-92. http://dx.doi.org/10.1002/gps.1044.
- Moniz-Cook E, Woods R, Gardiner E. Staff factors associated with perception of behaviour as ‘challenging’ in residential and nursing homes. Aging Ment Health 2000;4:48-55. http://dx.doi.org/10.1080/13607860055973.
- Baillon S, Scothern G, Neville PG, Boyle A. Factors that contribute to stress in care staff in residential homes for the elderly. Int J Geriatr Psychiatry 1996;11:219-26. http://dx.doi.org/10.1002/(sici)1099-1166(199603)11:3<219::aid-gps342>3.0.co;2-l.
- Hinchliffe AC, Hyman IL, Blizard B, Livingston G. Behavioural complications of dementia – can they be treated?. Int J Geriatr Psychiatry 1995;10:839-47. http://dx.doi.org/10.1002/gps.930101005.
- Moniz-Cook ED. Behavioural Disturbance in Care Homes 2001.
- Edvardsson D, Sandman PO, Nay R, Karlsson S. Associations between the working characteristics of nursing staff and the prevalence of behavioral symptoms in people with dementia in residential care. Int Psychogeriatr 2008;20:764-76. http://dx.doi.org/10.1017/S1041610208006716.
- McCabe MP, Davison TE, George K. Effectiveness of staff training programs for behavioral problems among older people with dementia. Aging Ment Health 2007;11:505-19. https://doi.org/10.1080/13607860601086405.
- Ballard C. Barriers to evidence-based prescribing in Alzheimer’s disease. Br J Ment Health Nurs 2013;2:16-21. http://dx.doi.org/10.12968/bjmh.2013.2.1.97534.
- Spector A, Orrell M, Goyder J. A systematic review of staff training interventions to reduce the behavioural and psychological symptoms of dementia. Ageing Res Rev 2013;12:354-64. http://dx.doi.org/10.1016/j.arr.2012.06.005.
- Cohen-Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. J Gerontol A Biol Sci Med Sci 2007;62A:908-16. http://dx.doi.org/10.1093/gerona/62.8.908.
- Bird M, Llewellyn-Jones RH, Korten A. An evaluation of the effectiveness of a case-specific approach to challenging behaviour associated with dementia. Aging Ment Health 2009;13:73-8. http://dx.doi.org/10.1080/13607860802154499.
- Moniz-Cook E, Agar S, Silver M, Woods R, Wang M, Elston C, et al. Can staff training reduce behavioural problems in residential care for the elderly mentally ill?. Int J Geriatr Psychiatry 1998;13:149-58. http://dx.doi.org/10.1002/(SICI)1099-1166(199803)13:3<149::AID-GPS746>3.0.CO;2-Q.
- Gormley N, Lyons D, Howard R. Behavioural management of aggression in dementia: a randomized controlled trial. Age Ageing 2001;30:141-5. https://doi.org/10.1093/ageing/30.2.141.
- Moniz-Cook E, Elston C, Gardiner E, Agar S, Silver M, Win T, et al. Can training community mental health nurses to support family carers reduce behavioural problems in dementia? An exploratory pragmatic randomised controlled trial. Int J Geriatr Psychiatry 2008;23:185-91. http://dx.doi.org/10.1002/gps.1860.
- Baker JC, Hanley GP, Mathews RM. Staff-administered functional analysis and treatment of aggression by an elder with dementia. J Appl Behav Anal 2006;39:469-74. https://doi.org/10.1901/jaba.2006.80-05.
- Dwyer-Moore KJ, Dixon MR. Functional analysis and treatment of problem behavior of elderly adults in long-term care. J Appl Behav Anal 2007;40:679-83. https://doi.org/10.1901/jaba.2007.679-683.
- Moniz-Cook E, Woods RT, Richards K. Functional analysis of challenging behaviour in dementia: the role of superstition. Int J Geriatr Psychiatry 2001;16:45-56. http://dx.doi.org/10.1002/1099-1166(200101)16:1<45::AID-GPS270>3.0.CO;2-F.
- Moniz-Cook E, Stokes G, Agar S. Difficult behaviour and dementia in nursing homes: five cases of psychosocial intervention. Clin Psychol Psychother 2003;10:197-208. http://dx.doi.org/10.1002/cpp.370.
- Hobday JV, Savik K, Gaugler JE. An internet-based multimedia education prototype to enhance late-stage dementia care: formative research results. Geriatr Nurs 2010;31:402-11. http://dx.doi.org/10.1016/j.gerinurse.2010.06.001.
- Hobday JV, Savik K, Smith S, Gaugler JE. Feasibility of internet training for care staff of residents with dementia: the CARES program. J Gerontol Nurs 2010;36:13-21. http://dx.doi.org/10.3928/00989134-20100302-01.
- Coleman CK, Medvene LJ, Van Haitsma K. A person-centered care intervention for geriatric certified nursing assistants. Gerontologist 2013;53:687-98. http://dx.doi.org/10.1093/geront/gns135.
- Irvine AB, Billow MB, Gates DM, Fitzwater EL, Seeley JR, Bourgeois M. Internet training to respond to aggressive resident behaviors. Gerontologist 2012;52:13-2. http://dx.doi.org/10.1093/geront/gnr069.
- Beauchamp N, Irvine AB, Seeley J, Johnson B. Worksite-based internet multimedia program for family caregivers of persons with dementia. Gerontologist 2005;45:793-801. https://doi.org/10.1093/geront/45.6.793.
- Innes A, Mackay K, McCabe L. Dementia studies online: reflections on the opportunities and drawbacks of eLearning. J Vocat Educ Train 2006;58:303-17. https://doi.org/10.1080/13636820600955567.
- Lindgren H. Towards personalized decision support in the dementia domain based on clinical practice guidelines. User Model User-Adapt Interact 2011;21:377-406. http://dx.doi.org/10.1007/s11257-010-9090-4.
- Jones RSP, Eayrs CB. Challenging Behaviour and Intellectual Disabilities: A Psychological Perspective. Avon: B.I.L.D. Publications; 1993.
- Jones RSP, Dowey A, Taylor JL, Lindsay WR, Hastings RP, Hatton C. Psychological Therapy for Adults with Intellectual Disabilities. Oxford: Wiley-Blackwell; 2013.
- Owens RG, Ashcroft JB. Functional analysis in applied psychology. Br J Clin Psychol 1982;21:181-9. http://dx.doi.org/10.1111/j.2044-8260.1982.tb00550.x.
- Psychological Interventions for Severely Challenging Behaviours Shown by People with Learning Disabilities. Clinical Practice Guidelines. Leicester: The British Psychological Society; 2004.
- Challenging Behaviour: A Unified Approach. London: British Psychological Society/Royal College of Psychiatrists; 2007.
- Moniz Cook ED, Swift K, James I, Malouf R, De Vugt M, Verhey F. Functional analysis-based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev 2012;2. http://dx.doi.org/10.1002/14651858.CD006929.pub2.
- Proctor R, Burns A, Powell HS, Tarrier N, Faragher B, Richardson G, et al. Behavioural management in nursing and residential homes: a randomised controlled trial. Lancet 1999;354:26-9. https://doi.org/10.1016/S0140-6736(98)08237-3.
- Brooker DJ, Latham I, Evans SC, Jacobson N, Perry W, Bray J, et al. FITS into practice: translating research into practice in reducing the use of anti-psychotic medication for people with dementia living in care homes. Aging Ment Health 2016;20:709-18. http://dx.doi.org/10.1080/13607863.2015.1063102.
- Peeters JM, Van Beek AP, Meerveld JH, Spreeuwenberg PM, Francke AL. Informal caregivers of persons with dementia, their use of and needs for specific professional support: a survey of the National Dementia Programme. BMC Nurs 2010;9. http://dx.doi.org/10.1186/1472-6955-9-9.
- Feast A, Orrell M, Charlesworth G, Melunsky N, Poland F, Moniz-Cook E. Behavioural and psychological symptoms in dementia and the challenges for family carers: systematic review. Br J Psychiatry 2016;208:429-34. http://dx.doi.org/10.1192/bjp.bp.114.153684.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of E-learning in medical education. Acad Med 2006;81:207-12. http://dx.doi.org/10.1097/00001888-200603000-00002.
- Beer C, Horner B, Almeida OP, Scherer S, Lautenschlager NT, Bretland N, et al. Current experiences and educational preferences of general practitioners and staff caring for people with dementia living in residential facilities. BMC Geriatr 2009;9. http://dx.doi.org/10.1186/1471-2318-9-36.
- Summers S, Carty S, Hoffman J. Educational implications of nurses’ brain dominance, learning styles, and attitudes toward computerization. J Nurs Staff Dev 1993;9:35-9.
- Lauriks S, Reinersmann A, Van der Roest HG, Meiland FJ, Davies RJ, Moelaert F, et al. Review of ICT-based services for identified unmet needs in people with dementia. Ageing Res Rev 2007;6:223-46. https://doi.org/10.1016/j.arr.2007.07.002.
- Rosen J, Mittal V, Mulsant BH, Degenholtz H, Castle N, Fox D. Educating the families of nursing home residents: a pilot study using a computer-based system. J Am Med Dir Assoc 2003;4:128-34. http://dx.doi.org/10.1097/01.JAM.0000062035.91165.A0.
- Røsvik J, Brooker D, Mjorud M, Kirkevold Ø. What is person-centred care in dementia? Clinical reviews into practice: the development of the VIPS practice model. Rev Clin Gerontol 2013;23:155-63. https://doi.org/10.1017/S0959259813000014.
- Lee KH, Algase DL, McConnell ES. Relationship between observable emotional expression and wandering behavior of people with dementia. Int J Geriatr Psychiatry 2014;29:85-92. http://dx.doi.org/10.1002/gps.3977.
- Finnema E, Droes RM, Ettema T, Ooms M, Ader H, Ribbe M, et al. The effect of integrated emotion-oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomized clinical trial. Int J Geriatr Psychiatry 2005;20:330-43. http://dx.doi.org/10.1002/gps.1286.
- Cohen-Mansfield J, Downs M, Bowers B. Excellence in Dementia Care: Research into Practice. Maidenhead: Open University Press; 2008.
- Chrzescijanski D, Moyle W, Creedy D. Reducing dementia-related aggression through a staff education intervention. Dementia 2007;6:271-86. https://doi.org/10.1177/1471301207080369.
- James IA. Understanding Behaviour in Dementia that Challenges: A guide to Assessment and Treatment. London: Jessica Kingsley; 2011.
- Stokes G. And Still the Music Plays. London: Hawker Publications; 2008.
- Vickland V, Chilko N, Draper B, Low LF, O’Connor D, Brodaty H. Individualized guidelines for the management of aggression in dementia – part 1: key concepts. Int Psychogeriatr 2012;24:1112-24. http://dx.doi.org/10.1017/S1041610212000014.
- Collet J, de Vugt ME, Verhey FR, Schols JM. Efficacy of integrated interventions combining psychiatric care and nursing home care for nursing home residents: a review of the literature. Int J Geriatr Psychiatry 2010;25:3-13. http://dx.doi.org/10.1002/gps.2307.
- Hurt C, Bhattacharyya S, Burns A, Camus V, Liperoti R, Marriott A, et al. Patient and caregiver perspectives of quality of life in dementia. An investigation of the relationship to behavioural and psychological symptoms in dementia. Dement Geriatr Cogn Disord 2008;26:138-46. http://dx.doi.org/10.1159/000149584.
- Graneheim UH, Jansson L. The meaning of living with dementia and disturbing behaviour as narrated by three persons admitted to a residential home. J Clin Nurs 2006;15:1397-403. https://doi.org/10.1111/j.1365-2702.2006.01476.x.
- Spector A, Orrell M. Using a biopsychosocial model of dementia as a tool to guide clinical practice. Int Psychogeriatr 2010;22:957-65. http://dx.doi.org/10.1017/S1041610210000840.
- Boorsma M, Langedijk E, Frijters DH, Nijpels G, Elfring T, van Hout HP. Implementation of geriatric assessment and decision support in residential care homes: facilitating and impeding factors during initial and maintenance phase. BMC Health Serv Res 2013;13. http://dx.doi.org/10.1186/1472-6963-13-8.
- Supporting People with Dementia and Their Carers in Health and Social Care: NICE Clinical Guideline 42. London: NICE; 2006.
- Bellazzi R, Montani S, Portinale L, Riva A, Althoff KD, Bergmann R, et al. Case-Based Reasoning Research and Development. Berlin: Springer; 1990.
- Moniz-Cook E, Woods R, Gardiner E, Silver M, Agar S. The Challenging Behaviour Scale (CBS): development of a scale for staff caring for older people in residential and nursing homes. Br J Clin Psychol 2001;40:309-22. https://doi.org/10.1348/014466501163715.
- Brechin D, Murphy G, James IA, Codner J. Briefing Paper – Alternatives to Antipsychotic Medication: Psychological Approaches in Managing Psychological and Behavioural Distress in People with Dementia. Leicester: British Psychological Society; 2013.
- Agar S, Moniz-Cook E, Orbell S, Elston C, Wang M. Measuring the outcome of psychosocial intervention for family caregivers of dementia sufferers: a factor analytic study. Aging Ment Health 1997;1:166-75. http://dx.doi.org/10.1080/13607869757263.
- Vickland V, Chilko N, Draper B, Low LF, O’Connor D, Brodaty H. Individualized guidelines for the management of aggression in dementia – part 2: appraisal of current guidelines. Int Psychogeriatr 2012;24:1125-32. http://dx.doi.org/10.1017/S104161021200004X.
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging 1992;7:622-31. https://doi.org/10.1037/0882-7974.7.4.622.
- Banerjee S. The Use of Antipsychotic Medication for People with Dementia: Time for Action. London: Department of Health; 2009.
- Macdonald C, Stodel E, Coulson I. Planning an eLearning dementia care program for healthcare teams in long-term care facilities: the learners perspectives. Educ Gerontol 2004;30:845-64. http://dx.doi.org/10.1080/03601270490507295.
- Romanelli F, Bird E, Ryan M. Learning styles: a review of theory, application, and best practices. Am J Pharm Educ 2009;73. http://dx.doi.org/10.5688/aj730109.
- Manochehr N. The influence of learning styles on learners in e-Learning environments: an empirical study. Comput High Educ Econ Rev 2006;18:10-4.
- Cook DA, Gelula MH, Dupras DM, Schwartz A. Instructional methods and cognitive and learning styles in web-based learning: report of two randomised trials. Med Educ 2007;41:897-905. http://dx.doi.org/10.1111/j.1365-2923.2007.02822.x.
- Chislett V, Chapman A. Visual Auditory Kinesthetic (VAK) Learning Styles Test 2005. www.businessballs.com/vaklearningstylestest.htm (accessed 6 July 2015).
- Frankel A. Nurses’ learning styles: promoting better integration of theory into practice. Nurs Times 2009;105:24-7.
- James IA, Hope A. Relevance of emotions and beliefs in the treatment of behaviors that challenge in dementia patients. Neurodegen Dis Manage 2013;3:575-88. https://doi.org/10.2217/nmt.13.60.
- Self R, Rigby A, Leggett C, Paxton R. Clinical decision support tool: a rational needs-based approach to making clinical decisions. J Ment Health 2008;17:33-48. http://dx.doi.org/10.1080/09638230701505806.
- Rigby A. Cluster Development Story. Care Pathways &Amp; Packages Project CPPP: Developing Currencies for Mental Health Payment by Results 2013. www.cppconsortium.nhs.uk (accessed 6 July 2015).
- Jacobs R. Payment by results for mental health services: economic considerations of case-mix funding. Adv Psychiatr Treat 2014;20:155-64. http://dx.doi.org/10.1192/apt.bp.113.011312.
- Andrews SB, Drake T, Haslett W, Munusamy R. Developing web-based online support tools: the Dartmouth decision support software. Psychiatr Rehabil J 2010;34:37-41. http://dx.doi.org/10.2975/34.1.2010.37.41.
- Kortteisto T, Komulainen J, Mäkelä M, Kunnamo I, Kaila M. Clinical decision support must be useful, functional is not enough: a qualitative study of computer-based clinical decision support in primary care. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-349.
- Easton P, Entwhistle VA, Williams B. How the stigma of low literacy can impair patient-professional spoken interactions and affect health: insights from a qualitative investigation. BMC Health Serv Res 2013;13. http://dx.doi.org/10.1186/1472-6963-13-319.
- Howatson-Jones IL. Translating an academic module into an online resource. Nurs Stand 2012;26:44-9. http://dx.doi.org/10.7748/ns2012.04.26.31.44.c9029.
- Flint V, Cream J. E-learning: does it work in dementia care?. J Dement Care 2014;22:22-5.
- Whall AL, Colling KB, Kolanowski A, Kim H, Son Hong GR, DeCicco B, et al. Factors associated with aggressive behavior among nursing home residents with dementia. Gerontologist 2008;48:721-31. https://doi.org/10.1093/geront/48.6.721.
- Orrell M, Hancock G, Hoe J, Woods B, Livingston G, Challis D. A cluster randomised controlled trial to reduce the unmet needs of people with dementia living in residential care. Int J Geriatr Psychiatry 2007;22:1127-34. http://dx.doi.org/10.1002/gps.1801.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- Mozley CG, Schneider J, Cordingley L, Molineux M, Duggan S, Hart C, et al. The care home activity project: does introducing an occupational therapy programme reduce depression in care homes?. Aging Ment Health 2007;11:99-107. https://doi.org/10.1080/13607860600637810.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Dobson C. Conducting Research with People Not Having the Capacity to Consent to Their Participation: A Practical Guide for Researchers. Leicester: British Psychological Society; 2008.
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998;46:210-15. http://dx.doi.org/10.1111/j.1532-5415.1998.tb02542.x.
- Cohen-Mansfield J. Agitated behaviors in the elderly. II. Preliminary results in the cognitively deteriorated. J Am Geriatr Soc 1986;34:722-7. http://dx.doi.org/10.1111/j.1532-5415.1986.tb04303.x.
- Maslach C, Jackson SE, Leiter MP. Maslach Burnout Inventory Manual 3rd Edition. Palo Alto, CA: Consulting Psychologists Press; 1996.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Macdonald AJ, Woods RT. Attitudes to dementia and dementia care held by nursing staff in U.K. ‘non-EMI’ care homes: what difference do they make?. Int Psychogeriatr 2005;17:383-91. https://doi.org/10.1017/s104161020500150x.
- Mackenzie CS, Peragine G. Measuring and enhancing self-efficacy among professional caregivers of individuals with dementia. Am J Alzheimers Dis Other Demen 2003;18:291-9. http://dx.doi.org/10.1177/153331750301800507.
- Beecham J, Knapp M, Thornicroft G, Brewin CR, Wing J. Measuring Mental Health Needs. London: Gaskell//Royal College of Psychiatrists; 1992.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9. http://dx.doi.org/10.1016/j.jval.2010.08.002.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19.
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9:173-6. https://doi.org/10.1017/S1041610297004870.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67. http://dx.doi.org/10.18637/jss.v045.i03.
- van Buuren S, Oudshoorn CGM. Multivariate Imputation by Chained Equations: MICE V1.0 User’s Manual. Leiden: TNO Prevention and Health; 2000.
- van Buuren S. Flexible Imputation of Missing Data. Boca Raton, FL: Chapman & Hall/CRC Press; 2012.
- He Y. Missing data analysis using multiple imputation: getting to the heart of the matter. Circ Cardiovasc Qual Outcomes 2010;3:98-105. http://dx.doi.org/10.1161/circoutcomes.109.875658.
- Gelman A, Hill J, Alvarez RM, Beck NL, Wu L. Data Analysis Using Regression and Multilevel/Hierarchical Models. New York, NY: Cambridge University Press; 2007.
- Carpenter J, Kenward M. Guidelines for Handling Missing Data in Social Science Research. London: School of Hygiene and Tropical Medicine; 2004.
- Department of Health . NHS Reference Costs 2011–12 2012. http://data.gov.uk/publisher/nhs-reference-costs (accessed August 2015).
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- NHS . Prescription Cost Analysis – England 2011. http://data.gov.uk/dataset/prescription-cost-analysis-england (accessed August 2015).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Edwards RT, Hounsome B, Linck P, Russell IT. Economic evaluation alongside pragmatic randomised trials: developing a standard operating procedure for clinical trials units. Trials 2008;9. http://dx.doi.org/10.1186/1745-6215-9-64.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. http://dx.doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Lievesley N, Crosby G. The Changing Role of Care Homes. London: Bupa Care Services and Centre for Policy on Ageing; 2011.
- The National Health Service and Community Care Act 1990. London: HMSO; 1990.
- Hussein S, Manthorpe J. The dementia social care workforce in England: secondary analysis of a national workforce dataset. Aging Ment Health 2012;16:110-18. http://dx.doi.org/10.1080/13607863.2011.596808.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Goyder J, Orrell M, Wenborn J, Spector A. Staff training using STAR: a pilot study in UK care homes. Int Psychogeriatr 2012;24:911-20. http://dx.doi.org/10.1017/S1041610211002559.
- Zimmerman S, Williams CS, Reed PS, Boustani M, Preisser JS, Heck E, et al. Attitudes, stress, and satisfaction of staff who care for residents with dementia. Gerontologist 2005;45:96-105.
- Thorsen VC, Tharp AL, Meguid T. High rates of burnout among maternal health staff at a referral hospital in Malawi: a cross-sectional study. BMC Nurs 2011;10. http://dx.doi.org/10.1186/1472-6955-10-9.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Parsons C, Johnston S, Mathie E, Baron N, Machen I, Amador S, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging 2012;29:143-55. https://doi.org/10.2165/11598560-000000000-00000.
- Medical Research Council . Complex Interventions Guidance 2008. www.mrc.ac.uk/complexinterventionsguidance (accessed August 2015).
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological Psychiatry. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. http://dx.doi.org/10.1176/appi.ajp.162.11.1996.
- Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223-38. http://dx.doi.org/10.1001/jama.293.10.1223.
- Fossey J, Masson S, Stafford J, Lawrence V, Corbett A, Ballard C. The disconnect between evidence and practice: a systematic review of person-centred interventions and training manuals for care home staff working with people with dementia. Int J Geriatr Psychiatry 2014;29:797-80. http://dx.doi.org/10.1002/gps.4072.
- Kaasalainen S. Staff development and long-term care of patients with dementia. J Gerontol Nurs 2002;28:39-46. https://doi.org/10.3928/0098-9134-20020701-08.
- Caspar S, Cooke HA, O’Rourke N, MacDonald SW. Influence of individual and contextual characteristics on the provision of individualized care in long-term care facilities. Gerontologist 2013;53:790-80. http://dx.doi.org/10.1093/geront/gns165.
- Cassie KM, Cassie WE. Organizational and individual conditions associated with depressive symptoms among nursing home residents over time. Gerontologist 2012;52:812-21. http://dx.doi.org/10.1093/geront/gns059.
- Hughes J, Bagley H, Reilly S, Burns A, Challis D. Care staff working with people with dementia: training, knowledge and confidence. Dementia 2008;7:227-38. https://doi.org/10.1177/1471301208091159.
- Homa K, Schifferdecker KE, Reed VA. Do lessons learned in a training intervention on web-based health care resources diffuse to nonexposed members in the primary care setting? A comparative study. Qual Manag Health Care 2008;17:330-40. http://dx.doi.org/10.1097/01.QMH.0000338554.90264.2d.
- Reducing the Use of Antipsychotic Drugs: A Guide to the Treatment and Care of Behavioural and Psychological Symptoms of Dementia. London: Alzheimer’s Society; 2011.
- Department of Health . Living Well With Dementia: A National Dementia Strategy Good Practice Compendium 2011. www.gov.uk/government/publications/living-well-with-dementia-a-national-dementia-strategy-good-practice-compendium (accessed 30 January 2017).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Cohen-Mansfield J, Jensen B, Resnick B, Norris M. Knowledge of and attitudes toward nonpharmacological interventions for treatment of behavior symptoms associated with dementia: a comparison of physicians, psychologists, and nurse practitioners. Gerontologist 2012;52:34-45. http://dx.doi.org/10.1093/geront/gnr081.
- Wood-Mitchell A, James IA, Waterworth A, Swann A, Ballard C. Factors influencing the prescribing of medications by old age psychiatrists for behavioural and psychological symptoms of dementia: a qualitative study. Age Ageing 2008;37:547-52. http://dx.doi.org/10.1093/ageing/afn135.
- Kleijer BC, van Marum RJ, Frijters DH, Jansen PA, Ribbe MW, Egberts AC, et al. Variability between nursing homes in prevalence of antipsychotic use in patients with dementia. Int Psychogeriatr 2014;26:363-71. http://dx.doi.org/10.1017/S1041610213002019.
- Azermai M, Vander Stichele RR, Van Bortel LM, Elseviers MM. Barriers to antipsychotic discontinuation in nursing homes: an exploratory study. Aging Ment Health 2014;18:346-53. http://dx.doi.org/10.1080/13607863.2013.832732.
- Lindgren H. Integrating Clinical Decision Support System Development into a Development Process of Clinical Practice – Experiences from Dementia Care n.d.:129-38.
- Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JR. Health status of UK care home residents: a cohort study. Age Ageing 2014;43:97-103. http://dx.doi.org/10.1093/ageing/aft077.
- James IA, Milne D, Morse R. Micro-skills of clinical supervision: scaffolding skills. J Cogn Psychother 2008;22:29-36. https://doi.org/10.1891/0889.8391.22.1.29.
- Jackman LJ, Wood-Mitchell A, James IA. Micro-skills of group formulations in care settings. Dementia 2014;13:23-32. http://dx.doi.org/10.1177/1471301212442463.
- James IA, Milne D, Morse R, Blackburn IM. Conducting successful supervision: novel elements towards an integrative approach. Behav Cogn Psychother 2007;35:191-200. https://doi.org/10.1017/S1352465806003407.
- Chalkidou K, Tunis S, Whicher D, Fowler R, Zwarenstein M. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials 2012;9:436-46. http://dx.doi.org/10.1177/1740774512450097.
- Vernooij-Dassen M, Moniz-Cook E. Raising the standard of applied dementia care research: addressing the implementation error. Aging Ment Health 2014;18:809-14. http://dx.doi.org/10.1080/13607863.2014.899977.
- Szczepura A, Nelson S, Wild D. In-reach specialist nursing teams for residential care homes: uptake of services, impact on care provision and cost-effectiveness. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-269.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-63.
- Bamford C, Heaven B, May C, Moynihan P. Implementing nutrition guidelines for older people in residential care homes: a qualitative study using Normalization Process Theory. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-106.
- Pope C, Halford S, Turnbull J, Prichard J, Calestani M, May C. Using computer decision support systems in NHS emergency and urgent care: ethnographic study using normalisation process theory. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-111.
- Social Care Institute for Excellence (SCIE) . Dementia E-Learning Course 2009 n.d. www.scie.org.uk/dementia/e-learning/ (accessed 11 April 2017).
- Social Care Institute For Excellence (SCIE) . Social Care TV n.d. www.scie.org.uk/socialcaretv/ (accessed 30 July 2015).
- Gask L, Bower P, Lovell K, Escott D, Archer J, Gilbody S, et al. What work has to be done to implement collaborative care for depression? Process evaluation of a trial utilizing the normalization process model. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-15.
- Beck AM, Damkjaer K, Tetens I. Lack of compliance of staff in an intervention study with focus on nutrition, exercise and oral care among old (65+ yrs) Danish nursing home residents. Aging Clin Exp Res 2009;21:143-9. https://doi.org/10.1007/BF03325222.
- Macfarlane A, O’Reilly-de Brun M. Using a theory-driven conceptual framework in qualitative health research. Qual Health Res 2012;22:607-18. http://dx.doi.org/10.1177/1049732311431898.
- Mair FS, Hiscock J, Beaton SC. Understanding factors that inhibit or promote the utilization of telecare in chronic lung disease. Chronic Illn 2008;4:110-17. http://dx.doi.org/10.1177/1742395308092482.
- Estabrooks CA, Squires JE, Cummings GG, Teare GF, Norton PG. Study protocol for the translating research in elder care (TREC): building context – an organizational monitoring program in long-term care project (project one). Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-52.
- Masso M, McCarthy G. Literature review to identify factors that support implementation of evidence-based practice in residential aged care. Int J Evid Based Healthc 2009;7:145-56. http://dx.doi.org/10.1111/j.1744-1609.2009.00132.x.
- Evans CJ, Stone KA, Manthorpe J, Higginson IJ. MRC guidance on developing and evaluating complex interventions: application to research on palliative and end of life care. NIHR School for Social Care Research Methods Reviews 2013;15.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. London: Transaction Books; 2009.
- May C, Murray E, Finch T, Mair F, Treweek S, Ballini L, et al. Normalization Process Theory On-Line Users’ Manual and Toolkit 2010. www.normalizationprocess.org (accessed 21 August 2013).
- Ritchie J, Spencer L, O’Connor W, Ritchie JE, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage Publications; 2003.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. JOAAG 2009;4:72-9.
- Ellis ML, Molinari V, Dobbs D, Smith K, Hyer K. Assessing approaches and barriers to reduce antipsychotic drug use in Florida nursing homes. Aging Ment Health 2015;19:507-16. http://dx.doi.org/10.1080/13607863.2014.952710.
- Weiner BJ. A theory of organizational readiness for change. Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-67.
- Jeon YH, Luscombe G, Chenoweth L, Stein-Parbury J, Brodaty H, King M, et al. Staff outcomes from the caring for aged dementia care resident study (CADRES): a cluster randomised trial. Int J Nurs Stud 2012;49:508-18. http://dx.doi.org/10.1016/j.ijnurstu.2011.10.020.
- The National Care Forum . Caring for the Future: NCF Survey Finds Staff Recruitment a Priority 2015. www.nationalcareforum.org.uk/viewNews.asp?news_id=2829 (accessed August 2015).
- Wagner CM. Organizational commitment as a predictor variable in nursing turnover research: literature review. J Adv Nurs 2007;60:235-47. https://doi.org/10.1111/j.1365-2648.2007.04421.x.
- Chalfont G, Hafford-Letchfield T. Leadership from the bottom up: reinventing dementia care in residential and nursing home settings. Soc Work Soc Sci Rev 2010;14:27-46. https://doi.org/10.1921/095352210x557600.
- McEvoy P, Plant R. Dementia care: using empathic curiosity to establish the common ground that is necessary for meaningful communication. J Psychiatr Ment Health Nurs 2014;21:477-82. http://dx.doi.org/10.1111/jpm.12148.
- McEvoy P, Baker D, Plant R, Hylton K, Mansell W. Empathic curiosity: resolving goal conflicts that generate emotional distress. J Psychiatr Ment Health Nurs 2013;20:273-8. http://dx.doi.org/10.1111/j.1365-2850.2012.01926.x.
- Havig AK, Skogstad A, Veenstra M, Romøren TI. Real teams and their effect on the quality of care in nursing homes. BMC Health Serv Res 2013;13. http://dx.doi.org/10.1186/1472-6963-13-499.
- Heponiemi T, Elovainio M, Kouvonen A, Noro A, Finne-Soveri H, Sinervo T. Ownership type and team climate in elderly care facilities: the moderating effect of stress factors. J Adv Nurs 2012;68:647-57. http://dx.doi.org/10.1111/j.1365-2648.2011.05777.x.
- Francis R. Final Report of the Independent Inquiry into Care Provided By Mid Staffordshire NHS Foundation Trust. The Francis Report. London: The Stationery Office; 2013.
- Jeon YH, Glasgow NJ, Merlyn T, Sansoni E. Policy options to improve leadership of middle managers in the Australian residential aged care setting: a narrative synthesis. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-190.
- Rantz MJ, Zwygart-Stauffacher M, Flesner M, Hicks L, Mehr D, Russell T, et al. The influence of teams to sustain quality improvement in nursing homes that ‘need improvement’. J Am Med Dir Assoc 2013;14:48-52. http://dx.doi.org/10.1016/j.jamda.2012.09.008.
- Kjøs BØ, Botten G, Gjevjon ER, Romøren TI. Quality work in long-term care: the role of first-line leaders. Int J Qual Health Care 2010;22:351-7. http://dx.doi.org/10.1093/intqhc/mzq035.
- Harvath TA, Swafford K, Smith K, Miller LL, Volpin M, Sexson K, et al. Enhancing nursing leadership in long-term care. A review of the literature. Res Gerontol Nurs 2008;1:187-96. http://dx.doi.org/10.3928/00220124-20091301-06.
- Rodwell J, Martin A. The importance of the supervisor for the mental health and work attitudes of Australian aged care nurses. Int Psychogeriatr 2012;25:382-9. http://dx.doi.org/10.1017/S1041610212001883.
- Zimmerman S, Mitchell CM, Reed D, Preisser JS, Fletcher S, Beeber AS, et al. Outcomes of a dementia care training program for staff in nursing homes and residential care/assisted living settings. Alzheimers Care Today 2010;11:83-99.
- Jeon YH, Merlyn T, Chenoweth L. Leadership and management in the aged care sector: a narrative synthesis. Australas J Ageing 2010;29:54-60. http://dx.doi.org/10.1111/j.1741-6612.2010.00426.x.
- Lucas JA, Avi-Itzhak T, Robinson JP, Morris CG, Koren MJ, Reinhard SC. Continuous quality improvement as an innovation: which nursing facilities adopt it?. Gerontologist 2005;45:68-77.
- Lawrence V, Fossey J, Ballard C, Ferreira N, Murray J. Helping staff to implement psychosocial interventions in care homes: augmenting existing practices and meeting needs for support. Int J Geriatr Psychiatry 2016;31:284-93. http://dx.doi.org/10.1002/gps.4322.
- Ervin K, Cross M, Koschel A. Barriers to managing behavioural and psychological symptoms of dementia: staff perceptions. Collegian 2014;21:201-7. https://doi.org/10.1016/j.colegn.2013.04.002.
- Moyle W, Venturato L, Cooke M, Hughes J, van Wyk S, Marshall J. Promoting value in dementia care: staff, resident and family experience of the capabilities model of dementia care. Aging Ment Health 2013;17:587-94. http://dx.doi.org/10.1080/13607863.2012.758233.
- Braun KL, Cheang M, Shigeta D. Increasing knowledge, skills, and empathy among direct care workers in elder care: a preliminary study of an active-Learning model. Gerontologist 2005;45:118-24. http://dx.doi.org/10.1093/geront/45.1.118.
- Sallah D, Pond C, Koslowska O, Wright C. Dementia Core Skills Education and Training Framework. Skills for Health, Health Education England and Skills for Care 2015. www.skillsforhealth.org.uk/services/item/176-dementia-core-skills-education-and-training-framework (accessed 30 January 2017).
- Cavendish C. The Cavendish Review: An Independent Review into Healthcare Assistants and Support Workers in the NHS and Social Care Settings. London: HM Government; 2013.
- Lupton C, Croft-White C. Respect and Protect: The Experience of Older People and Staff in Care Homes and Hospitals. 2013.
- Luengo-Fernandez R, Leal J, Gray A. Dementia 2010: The Economic Burden of Dementia and Associated Research Funding in the United Kingdom. Oxford: Health Economics Research Centre, University of Oxford, Alzheimer’s Research Trust; 2010.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Update Second Edition. London: Alzheimer’s Society; 2014.
- Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology 2002;59:1721-9. https://doi.org/10.1212/01.WNL.0000036904.73393.E4.
- Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002;17:403-8. http://dx.doi.org/10.1002/gps.490.
- NICE . Dementia Quality Standard Programme: NICE Cost Impact and Commissioning Assessment: Quality Standard for Dementia 2010. http://guidance.nice.org.uk/QS1 (accessed 30 January 2017).
- Ornstein KA, Gaugler JE, Devanand DP, Scarmeas N, Zhu CW, Stern Y. Are there sensitive time periods for dementia caregivers? The occurrence of behavioral and psychological symptoms in the early stages of dementia. Int Psychogeriatr 2013;25:1453-62. http://dx.doi.org/10.1017/S1041610213000768.
- García-Alberca JM, Cruz B, Lara JP, Garrido V, Lara A, Gris E, et al. The experience of caregiving: the influence of coping strategies on behavioral and psychological symptoms in patients with Alzheimer’s disease. Aging Ment Health 2013;17:615-22. http://dx.doi.org/10.1080/13607863.2013.765833.
- Ward S, Opie J, O’Connor DW. Family carers’ responses to behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 2003;18:1007-12. http://dx.doi.org/10.1002/gps.1005.
- Kwok YT, Chen CY, Chiu MJ, Tang LY, Leung KK. Assessment of behavioral and psychological symptoms of dementia by family caregivers. Arch Gerontol Geriatr 2011;52:60-5. http://dx.doi.org/10.1016/j.archger.2010.01.021.
- Department of Health . Mental Health Clustering Booklet 2011. www.cppconsortium.nhs.uk/admin/files/1296142999MHCT%20Cluster%20Booklet%20v%202%2011%202011%2012.pdf (accessed 7 July 2015).
- Whelan P, Zoha M, Ramcharitar K, Andrews T. Care pathway clustering: issues for older adult mental health services. Prog Neurol Psychiatry 2011;15:6-9. http://dx.doi.org/10.1002/pnp.204.
- Hawkes N. Payment by results works against integrated care and needs to be replaced. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d2135.
- Macdonald AJ, Elphick M. Combining routine outcomes measurement and ‘Payment by Results’: will it work and is it worth it?. Br J Psychiatry 2011;199:178-9. http://dx.doi.org/10.1192/bjp.bp.110.090993.
- Turner A. A Quick Guide to Mental Health Payment By Results and Its Impact on Social Work 2013. www.communitycare.co.uk/2013/04/16/a-quick-guide-to-mental-health-payment-by-results-and-its-impact-on-social-work/ (accessed 13 July 2015).
- Yeomans D. Clustering in mental health payment by results: a critical summary for the clinician. Adv Psychiatr Treat 2014;20:227-34. http://dx.doi.org/10.1192/apt.bp.113.011320.
- Bekas S, Michev O. Payment by results: validating care cluster allocation in the real world. Psychiatrist 2013;37:349-55. http://dx.doi.org/10.1192/pb.bp.112.041780.
- National Dementia Strategy. London: The Stationery Office; 2009.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94. https://doi.org/10.7326/0003-4819-134-8-200104170-00012.
- Woods B. Guilt in Relatives of Older People in Residential Care. Manchester: British Congress of Gerontology; 1996.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Goldberg DP, Williams PDPM. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson Publishing Company Ltd; 1988.
- Vernooij-Dassen MJ, Felling AJ, Brummelkamp E, Dauzenberg MG, van den Bos GA, Grol R. Assessment of caregiver’s competence in dealing with the burden of caregiving for a dementia patient: a Short Sense of Competence Questionnaire (SSCQ) suitable for clinical practice. J Am Geriatr Soc 1999;47:256-7. http://dx.doi.org/10.1111/j.1532-5415.1999.tb04588.x.
- Greene JG, Smith R, Gardiner M, Timbury GC. Measuring behavioural disturbance of elderly demented patients in the community and its effects on relatives: a factor analytic study. Age Ageing 1982;11:121-6. http://dx.doi.org/10.1093/ageing/11.2.121.
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med 2008;67:874-82. http://dx.doi.org/10.1016/j.socscimed.2008.05.015.
- Spruytte N, Van Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychol Psychother 2002;75:295-311. http://dx.doi.org/10.1348/147608302320365208.
- Hubert LJ. The use of orthogonal polynomials for trend analysis. Am Educ Res J 1973;10:241-4. http://dx.doi.org/10.3102/00028312010003241.
- Everitt BS, Landau S, Leese M, Stahl D. Cluster Analysis. Chichester: John Wiley & Sons; 2011.
- Carpenter RJ, Kenward GM. Monograph – Missing Data in Clinical Trials. London: London School of Hygiene and Tropical Medicine; 2007.
- Delbecq AL, Van der Ven AH. A group process model for problem identification and programme planning. J Appl Behav Sci 1971;7:466-92. https://doi.org/10.1177/002188637100700404.
- Dockery G, De Koning K, Martin M. Participatory Research in Health: Issues and Experiences. London: Zed books; 1996.
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14-29. http://dx.doi.org/10.1080/13607860801919850.
- Knapp M, Prince M, Albanese E, Banerjee S, Dhanasiri S, Fernández JL, et al. Dementia UK: The Full Report. London: Alzheimer’s Society; 2007.
- Moore K, Ozanne E, Ames D, Dow B. How do family carers respond to behavioral and psychological symptoms of dementia?. Int Psychogeriatr 2013;25:743-53. http://dx.doi.org/10.1017/S1041610213000070.
- Woods RT, Bruce E, Edwards RT, Elvish R, Hoare Z, Hounsome B, et al. REMCARE: reminiscence groups for people with dementia and their family caregivers – effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16480.
- Schulz R, Cook TB, Beach SR, Lingler JH, Martire LM, Monin JK, et al. Magnitude and causes of bias among family caregivers rating Alzheimer disease patients. Am J Geriatr Psychiatry 2013;21:14-25. http://dx.doi.org/10.1016/j.jagp.2012.10.002.
- Barnes TR, Banerjee S, Collins N, Treloar A, McIntyre SM, Paton C. Antipsychotics in dementia: prevalence and quality of antipsychotic drug prescribing in UK mental health services. Br J Psychiatry 2012;201:221-6. http://dx.doi.org/10.1192/bjp.bp.111.107631.
- Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database Syst Rev 2002;4. http://dx.doi.org/10.1002/14651858.CD003944.
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet 2011;378:403-11. http://dx.doi.org/10.1016/S0140-6736(11)60830-1.
- Wilberforce M, Tucker S, Abendstern M, Brand C, Giebel CM, Challis D. Membership and management: structures of inter-professional working in community mental health teams for older people in England. Int Psychogeriatr 2013;25:1485-92. http://dx.doi.org/10.1017/S104161021300077X.
- Robert P, Verhey FR, Aalten P, Cortes F, Byrne EJ. Neuropsychiatric outcome for clinical trials. J Nutr Health Aging 2007;11:345-7.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3496.
- Pawson R, Manzano-Santaella A. A realist diagnostic workshop. Evaluation 2012;18:176-91. http://dx.doi.org/10.1177/1356389012440912.
- Great Britain . Health and Social Care Act 2012 2012. www.legislation.gov.uk/ukpga/2012/7/contents/enacted (accessed 8 January 2014).
- Ryu SH, Katona C, Rive B, Livingston G. Persistence of and changes in neuropsychiatric symptoms in Alzheimer disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry 2005;13:976-83. https://doi.org/10.1176/appi.ajgp.13.11.976.
- Prepared to Care: Challenging the Dementia Skills Gap. London: All-Party Parliamentary Group on Dementia; 2009.
- Department of Health . Case for Change – Mental Health Liaison Service for Dementia Care in Hospitals 2011. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_128585.pdf (accessed 20 July 2017).
- Department of Health . End of Life Care Strategy: Third Annual Report 2011. www.gov.uk/government/publications/end-of-life-care-strategy-third-annual-report (accessed 20 July 2017).
- Support. Stay. Save. Care and Support of People with Dementia in their Own Homes. London: Alzheimer’s Society; 2011.
- Van Velsen L, Illario M, Jansen-Kosterink S, Crola C, Di Somma C, Colao A, et al. A community-based, technology-supported health service for detecting and preventing frailty among older adults: a participatory design development process. J Aging Res 2015;2015:1-9. http://dx.doi.org/10.1155/2015/216084.
- Tucker S, Wilberforce M, Brand C, Abendstern M, Challis D. All things to all people? The provision of outreach by community mental health teams for older people in England: findings from a national survey. Int J Geriatr Psychiatry 2014;29:489-96. http://dx.doi.org/10.1002/gps.4031.
- Wilberforce M, Tucker S, Brand C, Abendstern M, Jasper R, Stewart K, et al. Community mental health teams for older people: variations in case mix and service receipt (II). Int J Geriatr Psychiatry 2015;30:605-13. http://dx.doi.org/10.1002/gps.4190.
- Abendstern M, Harrington V, Brand C, Tucker S, Wilberforce M, Challis D. Variations in structures, processes and outcomes of community mental health teams for older people: a systematic review of the literature. Aging Ment Health 2012;16:861-73. http://dx.doi.org/10.1080/13607863.2011.651431.
- Malone D, Bradley P, Lindesay J. Olanzapine and risperidone prescriptions for people with dementia in care. Psychiatr Bull 2007;31:459-62. http://dx.doi.org/10.1192/pb.bp.106.012484.
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc 2010;58:1465-74. http://dx.doi.org/10.1111/j.1532-5415.2010.02971.x.
- Maio L, Botsford J, Iliffe S. Family carers’ experiences of the Admiral Nursing Service: a quantitative analysis of carer feedback. Aging Ment Health 2016;20:669-75. https://doi.org/10.1080/13607863.2015.1052776.
- Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA 2006;295:2148-57. https://doi.org/10.1001/jama.295.18.2148.
- Wolfs CA, de Vugt ME, Verkaaik M, Haufe M, Verkade PJ, Verhey FR, et al. Rational decision-making about treatment and care in dementia: a contradiction in terms?. Patient Educ Couns 2012;87:43-8. http://dx.doi.org/10.1016/j.pec.2011.07.023.
- Thune-Boyle I, Wilcock J, Iliffe S. Communicating with carers about dementia. Int J Geriatr Psychiatry 2013;28:433-40. http://dx.doi.org/10.1002/gps.3882.
- Hussein S, Manthorpe J. The diversity of staff supporting family carers in England: findings from an analysis of a national data set. Divers Equal Health Care 2012;9:101-11.
- Gaugler JE, Kane RL, Kane RA, Newcomer R. The longitudinal effects of early behavior problems in the dementia caregiving career. Psychol Aging 2005;20:100-16. https://doi.org/10.1037/0882-7974.20.1.100.
- Karttunen K, Karppi P, Hiltunen A, Vanhanen M, Välimäki T, Martikainen J, et al. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int J Geriatr Psychiatry 2011;26:473-82. http://dx.doi.org/10.1002/gps.2550.
- The Hidden Voice of Loneliness. London: Alzheimer’s Society; 2013.
- McCann M, Donnelly M, O’Reilly D. Living arrangements, relationship to people in the household and admission to care homes for older people. Age Ageing 2011;40:358-63. http://dx.doi.org/10.1093/ageing/afr031.
- Anderson D, Connelly P, Meier R, McCracken C. Mental health service discrimination against older people. Psychiatrist 2013;37:98-103. http://dx.doi.org/10.1192/pb.bp.112.040097.
- Lliffe S, Manthorpe J. The hazards of early recognition of dementia: a risk assessment. Aging Ment Health 2004;8:99-105. http://dx.doi.org/10.1080/13607860410001649653.
- Samsi K, Manthorpe J. Care pathways for dementia: current perspectives. Clin Interv Aging 2014;9:2055-63. http://dx.doi.org/10.2147/CIA.S70628.
- Borsje P, Wetzels RB, Lucassen PL, Pot AM, Koopmans RT. The course of neuropsychiatric symptoms in community-dwelling patients with dementia: a systematic review. Int Psychogeriatr 2015;27:385-40. http://dx.doi.org/10.1017/S1041610214002282.
- van der Linde RM, Dening T, Stephan BC, Prina AM, Evans E, Brayne C. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry 2016;209:366-77. https://doi.org/10.1192/bjp.bp.114.148403.
- Knapp M, Black N, Dixon J, Damant J, Rehill A, Tan S. Independent Assessment of Improvements in Dementia Care and Support Since 2009. London: Policy Innovation Research Unit and NIHR School for Social Care Research; 2014.
- Holle D, Halek M, Holle B, Pinkert C. Individualized formulation-led interventions for analyzing and managing challenging behavior of people with dementia – an integrative review. Aging Ment Health 2016;10:1-19. http://dx.doi.org/10.1080/13607863.2016.1247429.
- Tew V. Formulating with staff teams around behaviour that challenges in people with dementia. FPOP Newsletter 2014;127:18-24.
- Holle D, Roes M, Buscher I, Reuther S, Muller R, Halek M. Process evaluation of the implementation of dementia-specific case conferences in nursing homes (FallDem): study protocol for a randomized controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-485.
- Reuther S, Holle D, Buscher I, Dortmann O, Muller R, Bartholomeyczik S, et al. Effect evaluation of two types of dementia-specific case conferences in German nursing homes (FallDem) using a stepped-wedge design: study protocol for a randomized controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-319.
- Palm R, Trutschel D, Simon M, Bartholomeyczik S, Holle B. Differences in case conferences in dementia specific vs traditional care units in German nursing homes: results from a cross-sectional study. J Am Med Dir Assoc 2016;17:91.e9-13. http://dx.doi.org/10.1016/j.jamda.2015.08.018.
- Albert SM. Role of case conferences in dementia-specific vs traditional care units in German nursing homes. J Am Med Dir Assoc 2016;17:12-3. http://dx.doi.org/10.1016/j.jamda.2015.10.005.
- Reuther S, Dichter MN, Buscher I, Vollmar HC, Holle D, Bartholomeyczik S, et al. Case conferences as interventions dealing with the challenging behavior of people with dementia in nursing homes: a systematic review. Int Psychogeriatr 2012;24:1891-903. http://dx.doi.org/10.1017/S1041610212001342.
- Fossey J, James IA. Evidence-Based Approaches for Improving Dementia Care in Care Homes. London: Alzheimer’s Society; 2007.
- McCabe MP, Bird M, Davison TE, Mellor D, MacPherson S, Hallford D, et al. An RCT to evaluate the utility of a clinical protocol for staff in the management of behavioral and psychological symptoms of dementia in residential aged-care settings. Aging Ment Health 2015;19:799-807. http://dx.doi.org/10.1080/13607863.2014.967659.
- Selbaek G, Engedal K, Benth JS, Bergh S. The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int Psychogeriatr 2014;26:81-9. http://dx.doi.org/10.1017/S1041610213001609.
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 2009;8:151-7. http://dx.doi.org/10.1016/s1474-4422(08)70295-3.
- Corbett A, Burns A, Ballard C. Don’t use antipsychotics routinely to treat agitation and aggression in people with dementia. BMJ 2014;349. http://dx.doi.org/10.1136/bmj.g6420.
- Poland F, Mapes S, Pinnock H, Katona C, Sorensen S, Fox C, et al. Perspectives of carers on medication management in dementia: lessons from collaboratively developing a research proposal. BMC Res Notes 2014;7. http://dx.doi.org/10.1186/1756-0500-7-463.
- Fahey T, Montgomery AA, Barnes J, Protheroe J. Quality of care for elderly residents in nursing homes and elderly people living at home: controlled observational study. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7389.580.
- Connolly A, Iliffe S, Gaehl E, Campbell S, Drake R, Morris J, et al. Quality of care provided to people with dementia: utilisation and quality of the annual dementia review in general practice. Br J Gen Pract 2012;62:e91-8. http://dx.doi.org/10.3399/bjgp12X625148.
- Teipel SJ, Thyrian JR, Hertel J, Eichler T, Wucherer D, Michalowsky B, et al. Neuropsychiatric symptoms in people screened positive for dementia in primary care. Int Psychogeriatr 2015;27:39-48. http://dx.doi.org/10.1017/S1041610214001987.
- Woods R, Russell I. Methods Review: Randomisation and Chance-Based Designs in Social Care Research. London: NIHR SSCR; 2014.
- Hulscher M, Laurant M, Grol R, Grol R, Wensing M, Eccles M. Improving Patient Care: The Implementation of Change in Clinical Practice. London: Elsevier; 2005.
- Woods B. Evidence-based practice in psychosocial intervention in early dementia: how can it be achieved?. Aging Ment Health 2003;7:5-6.
- Zarit S, Femia E. Behavioral and psychosocial interventions for family caregivers. Am J Nurs 2008;108:47-53. http://dx.doi.org/10.1097/01.NAJ.0000336415.60495.34.
- Steingrimsdottir HS, Arntzen E. On the utility of within-participant research design when working with patients with neurocognitive disorders. Clin Interv Aging 2015;10:1189-99. http://dx.doi.org/10.2147/CIA.S81868.
- Tolson D. Promoting Evidence Informed Improvements in Care Homes: Nursing Perspectives 2010.
- Tolson D, Schofield I. Football reminiscence for men with dementia: lessons from a realistic evaluation. Nurs Inq 2012;19:63-70. http://dx.doi.org/10.1111/j.1440-1800.2011.00581.x.
- Hawkins AJ. Realist evaluation and randomised controlled trials for testing program theory in complex social systems. Evaluation 2016;22:270-85. http://dx.doi.org/10.1177/1356389016652744.
- Wong G, Greenhalgh T, Westhorp G, Pawson R. Realist methods in medical education research: what are they and what can they contribute?. Med Educ 2012;46:89-96. http://dx.doi.org/10.1111/j.1365-2923.2011.04045.x.
- Marchal B, Dedzo M, Kegels G. A realist evaluation of the management of a well-performing regional hospital in Ghana. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-24.
- Wong G, Greenhalgh T, Pawson R. Internet-based medical education: a realist review of what works, for whom and in what circumstances. BMC Med Educ 2010;10. http://dx.doi.org/10.1186/1472-6920-10-12.
- Yardley L, Spring BJ, Riper H, Morrison LG, Crane DH, Curtis K, et al. Understanding and promoting effective engagement with digital behavior change interventions. Am J Prev Med 2016;51:833-42. https://doi.org/10.1016/j.amepre.2016.06.015.
- Zeisel J, Reisberg B, Whitehouse P, Woods R, Verheul A. Ecopsychosocial interventions in cognitive decline and dementia: a new terminology and a new paradigm. Am J Alzheimers Dis Other Demen 2016;31:502-7. http://dx.doi.org/10.1177/1533317516650806.
- Tolson D, Rolland Y, Andrieu S, Aquino JP, Beard J, Benetos A, et al. International Association of Gerontology and Geriatrics: a global agenda for clinical research and quality of care in nursing homes. J Am Med Dir Assoc 2011;12:184-9. http://dx.doi.org/10.1016/j.jamda.2010.12.013.
- Bidewell JW, Chang E. Managing dementia agitation in residential aged care. Dementia 2011;10:299-315. http://dx.doi.org/10.1177/1471301211407789.
- Killett A, Burns D, Kelly F, Brooker D, Bowes A, La Fontaine J, et al. Digging deep: how organisational culture affects care home residents’ experiences. Ageing Soc 2014;36:160-88. http://dx.doi.org/10.1017/s0144686x14001111.
- Jeon YH, Govett J, Low LF, Chenoweth L, McNeill G, Hoolahan A, et al. Care planning practices for behavioural and psychological symptoms of dementia in residential aged care: a pilot of an education toolkit informed by the Aged Care Funding Instrument. Contemp Nurse 2013;44:156-69. http://dx.doi.org/10.5172/conu.2013.44.2.156.
- Van Houdt S, De Lepeleire J. Does the use of care plans improve the quality of home care?. Qual Prim Care 2010;18:161-72.
- Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Giraudeau B, Cantet C, Coley N, et al. Effectiveness of a specific care plan in patients with Alzheimer’s disease: cluster randomised trial (PLASA study). BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c2466.
- James IA. The use of CBT in dementia care: a rationale for Communication and Interaction Therapy (CAIT) and therapeutic lies. Cogn Behav Ther 2015;8. http://dx.doi.org/10.1017/s1754470x15000185.
- Caspi E. Time for change: persons with dementia and ‘behavioral expressions,’ not ‘behavior symptoms’. J Am Med Dir Assoc 2013;14:768-9. http://dx.doi.org/10.1016/j.jamda.2013.05.019.
- Beer C, Lowry R, Horner B, Almeida OP, Scherer S, Lautenschlager NT, et al. Development and evaluation of an educational intervention for general practitioners and staff caring for people with dementia living in residential facilities. Int Psychogeriatr 2011;23:221-9. http://dx.doi.org/10.1017/S104161021000195X.
- Gitlin LN, Marx KA, Stanley IH, Hansen BR, Van Haitsma KS. Assessing neuropsychiatric symptoms in people with dementia: a systematic review of measures. Int Psychogeriatr 2014;26:1805-48. http://dx.doi.org/10.1017/S1041610214001537.
- Rojas-Fernandez CH, Patel T, Lee L. An interdisciplinary memory clinic: a novel practice setting for pharmacists in primary care. Ann Pharmacother 2014;48:785-95. http://dx.doi.org/10.1177/1060028014526857.
- Hallberg IR, Cabrera E, Jolley D, Raamat K, Renom-Guiteras A, Verbeek H, et al. Professional care providers in dementia care in eight European countries; their training and involvement in early dementia stage and in home care. Dementia 2014;15:931-57. http://dx.doi.org/10.1177/1471301214548520.
- Lipsitz LA. The teaching nursing home: an educational and economic resource for geriatric training. Am J Med 1994;97:24S-26S. http://dx.doi.org/10.1016/0002-9343(94)90204-6.
- Culverwell A, Milne A. Intermediate care: evaluating a specialist home treatment service. J Dement Care 2010;18:32-5.
- Ament BH, Wolfs CA, Kempen GI, Ambergen T, Verhey FR, De Vugt ME. The benefit of a geriatric nurse practitioner in a multidisciplinary diagnostic service for people with cognitive disorders. BMC Res Notes 2015;8. http://dx.doi.org/10.1186/s13104-015-1189-6.
- Everybody’s Business: Integral Mental Health Services for Older Adults. Leeds: Care Services Improvement Partnership; 2005.
- Balalle C, Jayalath D, Shankar K, Ashaye K. Prevalence and management of behavioural and psychiatric symptoms on a continuing care unit for patients with dementia. Int J Psychiatry Med 2010;40:425-38. http://dx.doi.org/10.2190/PM.40.4.f.
- Livingston G, Barber J, Rapaport P, Knapp M, Griffin M, King D, et al. Clinical effectiveness of a manual based coping strategy programme (START STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f6276.
- Cooper C, Barber J, Griffin M, Rapaport P, Livingston G. Effectiveness of START psychological intervention in reducing abuse by dementia family carers: randomized controlled trial. Int Psychogeriatr 2016;28:881-7. http://dx.doi.org/10.1017/S1041610215002033.
- Blom MM, Zarit SH, Groot Zwaaftink RB, Cuijpers P, Pot AM. Effectiveness of an Internet intervention for family caregivers of people with dementia: results of a randomized controlled trial. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0116622.
- Mallon CM. Managing Behaviours That Challenge Within English Care Homes: An Exploration of Current Practices. Kent: University of Kent; 2015.
- An Economic Evaluation of Alternatives to Antipsychotic Drugs for Individuals Living With Dementia. Coventry: NHS Institute for Innovation and Improvement; 2011.
- Turner A, Eccles F, Keady J, Simpson J, Elvish R. The use of the truth and deception in dementia care amongst general hospital staff. Aging Ment Health 2017;21:862-19. http://dx.doi.org/10.1080/13607863.2016.1179261.
- Wergeland JN, Selbaek G, Hogset LD, Soderhamn U, Kirkevold O. Dementia, neuropsychiatric symptoms, and the use of psychotropic drugs among older people who receive domiciliary care: a cross-sectional study. Int Psychogeriatr 2014;26:383-91. http://dx.doi.org/10.1017/S1041610213002032.
- Carter D. Fix Dementia Care: Homecare. London: Alzheimer’s Society; 2016.
- NHS Business Services Authority . England Statement of Dental Remuneration – Amendment 93 2005. www.nhsbsa.nhs.uk/DentalServices/2878.aspx (accessed 30 January 2017).
- Department of Health . General Ophthalmic Services Letter 2010. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/dr_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_114691.pdf (accessed August 2015).
- Charlesworth GM, Shepstone L, Wilson E, Reynolds S, Mugford M, Price D, et al. Befriending carers of people with dementia: randomised controlled trial. BMJ 2008;336. https://doi.org/10.1136/bmj.39549.548831.AE.
- Blackpool Council . Shared Lives Scheme, Appendix 10a n.d. http://webcache.googleusercontent.com/search?q=cache:qJ-gkIy0QUMJ:www2.blackpool.gov.uk/democracy//members/admin/files/9097ec71-745e-4956-bcd8-2949cd5fd785/Appendix%252010(a).doc+&cd=1&hl=en&ct=clnk&gl=uk (accessed 20 July 2017).
- National Schedule of Unit Costs 2011–12. London: Department of Health; 2012.
Appendix 1 Description of the e-learning and decision support tool
In preparing the content and focus of the modules, consultations were held in the first half of 2008 with international experts in the field (including Professor Linda Teri, Seattle, USA, and Dr Mike Bird, then in Canberra, Australia), as well as with leading authorities on dementia care training in the UK (co-applicants Professor Ian James, Professor Graham Stokes, Professor Bob Woods and Professor Esme Moniz-Cook). The approach set out by Bird,13 encouraging careful holistic assessment and development of a highly individualised action plan, was seen as a good basis from which to develop the modules. Co-applicant Professor Ian James worked with Professors Esme Moniz-Cook and Bob Woods on the detailed development of the modules, bringing many years of experience of work on functional analysis in the context of working with people with intellectual disability.
Storyboards were prepared for the first three modules and an intensive period of filming the required clips took place in September 2008. These modules formed an integrated e-learning course, suitable for staff and care workers in care homes, but also with relevance for people with dementia living in the community.
Module 1: ‘it could happen to you’
The learner is invited to consider how they might themselves behave in difficult circumstances, and through a series of video-clips reflect on the way in which actions that in dementia care are described as CB may in certain situations appear an understandable human response. This module reinforces our shared humanity with people with dementia, reducing the tendency to ‘them and us’ thinking, and situates CB in the interaction with the environment and the perceived reactions and intentions of others.
Module 2: ‘developing observational skills’
This module introduces functional analysis and requires careful observation of video-clips showing CB in a care home context. In each case the staff member is required to decide on the immediate function of the behaviour, focusing on three primary functions: eliciting interaction with others, avoiding contact with others and experiencing enjoyment or pleasure from sensation. Following several teaching examples, where the learner is guided step by step through the process, there are a number of clips for the person to work through. If a given criterion level is not reached, further guidance and additional training clips are provided before the learner can move onto the next module.
Module 3: ‘developing skills in investigating and responding to behaviour that challenges: the search for meaning’
This module takes the learner systematically through assessment and action-planning, with nine case examples, increasing in levels of complexity, to work through. The cases cover a range of scenarios, including people with dementia living in the community in their own homes, as well as in care homes. A range of CB is depicted, from repeated questioning to shouting or aggression. Each is introduced with a video-clip of the CB shown by the person with dementia. The learner answers a number of questions regarding the clip, building on the skills developed in module 2. Interviews with relatives and care staff offer supplementary information providing context and background. If an incorrect answer is given, additional support and guidance are automatically provided to assist the learner in extracting the relevant information. Following a detailed analysis of the specific CB, the assessment process moves on to consider the physical health of the person with dementia, including medication, their biography, important relationships, preferences and disposition. The learner is encouraged to undertake ‘detective work’ to find the information needed from a number of resources that are provided for each person; for example, life story album, medical notes, care home notes and audio-recordings of family members or care staff. In relation to medication and physical health problems, the learner can click on a particular item to obtain more detailed information about the medication or health condition. Subject experts, a physician and pharmacist who was also a trainer in prescribing (Professor Peter Campion and Dr Andrea Hilton), contributed to and verified these sections. The learner’s responses then feed into an assessment summary, detailing the CB, when and in what circumstances it occurs, who are the people affected by it, what its function appears to be and what appears to influence its occurrence. This is placed in the context of the health of the person with dementia, their biography and social environment. For each case example, this then leads onto consideration of potential actions that could be taken to reduce the impact of the CB. In each instance, actions can be selected in relation to three domains: actions to support physical health needs, to alleviate, for example, pain or discomfort due to constipation, or review of drugs, such as antipsychotics or sedatives that may not be helping, or to manage nutrition in those who declined food; the care approach aimed at meeting psychological need in the person with dementia, for example aspects of carer communication or interaction with the person with dementia that might be relevant; and actions to support the caregiving environment. This last set of actions included the social as well as the physical environment, to encompass needs such as distress experienced by relatives and/or care staff; actions by other residents; and particular aspects of the context or system surrounding the person. In addition, this last group of actions included actions to address the regime and needs of staff in a care home, or the family carer’s personal needs, such as considering their physical health and medication issues as well as their psychological needs. The actions selected in each domain were then collated to form an action plan to address factors identified as potentially contributing to, or relevant to, the identified CB for the particular case.
From November 2012 these three training modules have been made available as a self-contained, online, interactive e-learning program on functional analysis-based interventions for CB in dementia: www.livingwithdementia.tv.
The decision support tool
The e-tool was designed to follow the approach used in module 3, but allowed the learner to respond to an actual person with dementia under their care. Two versions were designed, one for care home settings and one for the community, starting with the care home version.
The initial section of the assessment, following completion of basic personal information, focused on immediate risk, seeking to identify if the person had either an acute confusional state (using a standard assessment for confusion), which would require immediate medical assessment, or had severe mental health difficulties, posing an immediate risk to self and/or others, requiring assessment by a specialist mental health service. If risks of these kinds were identified, the assessment process would not proceed until the staff member could confirm that there was no longer an immediate risk.
The system was designed so that for each person with dementia, the staff member would choose one CB, selected from a 25-item CBS,107 which covers the common CBs reported by care home staff. The system allowed staff the option to indicate a behaviour if it was not included on the CBS. The staff member was encouraged to consider the function(s) of the specific behaviour, using their skills developed in modules 2 and 3, from a generic list provided (Box 7). As with module 3, an assessment summary and an action plan (which could be saved and printed out) were the outputs from the process, enabling a functional analysis-based approach to be taken arising from detailed consideration of the specific CB. The assessment summary covered a number of domains, including those shown in Box 8.
-
Frustration.
-
Being upset.
-
Behaving impulsively.
-
Enjoyment or pleasure.
-
Acting in self-defence.
-
Appearing to be trying to escape from the situation.
-
Showing signs of being alarmed.
-
Appearing to be trying to avoid something.
-
Misunderstanding the actions of others.
-
Actively seeking the attention of others.
-
Appearing to blame others.
-
Experiencing failure at something he/she is trying to do.
-
Appearing to be in pain.
-
Showing signs of fear.
-
Showing signs of curiosity.
-
Showing signs of embarrassment.
-
Showing signs of insecurity.
-
Trying to do something that is being prevented by others.
-
Trying to change something in the surroundings.
-
Showing signs of affection.
-
Trying to maintain privacy or personal space.
-
Seeking reassurance.
-
Seeking comfort.
-
Appearing to be helpful (e.g. cleaning or dusting, folding laundry, etc.).
-
Looking for someone.
-
Putting self in a dangerous situation.
-
Personal information (age, marital status, family).
-
Personality and attachment style.
-
The behaviour.
-
Duration.
-
Frequency.
-
Location.
-
Function.
-
Impact on others.
-
-
Health.
-
Dementia diagnosis: type, duration, location.
-
Physical abilities (activities of daily living).
-
Health problems.
-
Medication.
-
Pain (assessed with a validated scale).
-
-
Mood (assessed with validated scales).
-
Depression.
-
Anxiety.
-
-
Relationships.
-
Family.
-
Friends.
-
Visitors.
-
-
Life story.
-
Schooling.
-
Occupation.
-
Losses and difficult experiences.
-
Hobbies and interests (current and past).
-
-
Environmental preferences.
-
Number of people.
-
Quiet/busy.
-
Therefore, as far as possible, information entered by the staff member could be incorporated readily into the ensuing algorithm-driven action-planning process, a wide range of applicable options for each response were provided, with free text used exceptionally. For example, rather than the staff member typing in a list of medication taken by the person with dementia, the e-tool included the great majority of applicable medications, from which the staff member selected those being taken. This then enabled the system to later suggest actions relevant to the specific class of medication (e.g. antipsychotics, antidepressants, benzodiazepines, laxatives, analgesics) being taken.
The action-planning component decision support e-tool used a number of ‘if–then’ algorithms to suggest potential actions based on the information provided during the assessment process and documented in the assessment summary. For example, if the score on the pain scale included in the assessment was above a certain threshold, actions relating to initiating further assessment of pain, or considering pain relief, would be suggested; or if the person was in long-standing receipt of an antipsychotic or benzodiazepine then approaching the GP to arrange review of this was suggested. Similarly, if the person had been reported to be showing signs of curiosity, provision of activities, based on the person’s interests, would be among the actions suggested. If the person had a raised score on the anxiety scale, potential actions relating to relaxation and calming would be offered. The apparent function of the behaviour was targeted with a suggested action wherever possible; for example, if the function was seen as experiencing failure, the suggested action would be to seek to break the activity/task into manageable components. As with module 3, actions were grouped in three domains: health, the care approach and the care environment. Each plan would aim to include actions from each of these areas. Suggested actions were only included in the final action plan if the staff member judged them to be relevant and feasible. This process was intended to be akin to the creative brainstorming that is associated with functional analysis-based interventions (see chapter 8 in Stokes1), in which a wide range of suggestions are made, from which those with the best fit could be selected.
A second version of the decision support e-tool was developed for use with people with dementia living in the community. In this instance, the system was designed for a community mental health practitioner, such as a CMHN, to input information gathered directly from the person with dementia and his/her family carer. Again, a number of brief validated measures were used. For example, an adapted Problem Checklist,109 based on common concerns of family carers in the UK, replaced the CBS107 that was used in the care home setting, as this has been used in a previous family care study. 65,109 All measures were made available to the practitioner in an electronic form to either have them filled in directly by the carer or person with dementia, or to be completed using the paper format in a face-to-face interview. In this version, there was particular emphasis on the caregiving context to include assessments of the family carer’s mood, health, personality and coping style, as well as assessments of the quality of interaction and relationship between the carer and the person with dementia.
The sections of the assessment are shown in Box 9.
-
1a. Core information regarding person with dementia and a specified carer (age, gender, diagnosis, relationship of carer to person with dementia).
-
1b. Risk and delirium/confusion assessment: screen to ensure that there is not an immediate risk of harm, or of health problem requiring urgent assessment.
-
2. Physical abilities, sight and hearing.
-
3. Problem Checklist (completed with carer).
-
4. Health conditions: (a) person with dementia; and (b) carer (only if spouse or sibling).
-
5. Medication (person with dementia).
-
6. Pain scale.
-
7. Mood scale for person with dementia (completed by carer).
-
8. Function of the behaviour of concern and attributions of carer regarding the behaviour.
-
9. Personality of person with dementia.
-
10. Roles and goals: what is important to the person?
-
11. RSS (completed by carer).
-
12. Quality of relationship scale (completed by carer).
-
13. Personality of carer (including attachment style, self-efficacy, neuroticism).
-
14. Mood of carer (PHQ-9 scale to be completed by carer).
-
15. Support available for carer: family, friends, services.
-
16. Demands on carer: other stressors, roles, etc.
-
17. Carer approach.
PHQ-9, Patient Health Questionnaire (nine questions).
Appendix 2 The FamCare study: changes to protocol
Challenge FamCare was conceived as a CRT of a computerised functional analysis-based intervention for use by specialist CMHNs who support people with dementia living at home and their family carers. We developed an online decision support system to improve adherence by practitioners to a selection of case-specific interventions, as many may not have had regular access to supervision or support from clinicians in psychiatry, geriatric medicine and clinical psychology. Additionally, in a previous exploratory RCT, where access to clinical experts was available, this was not always taken up by CMHNs, and variation in CMHN behaviour reported some ICCs as over 0.1. 65
Following consultation with NHS organisations, the delivery of the intervention was widened to include other specialist professional staff as well as CMHNs, which we described as specialist CMHTOP ‘practitioners’ who would act as therapists. This was necessary as roles and responsibilities for the delivery of specialist support by CMHTsOP to people with dementia and CB at home were no longer limited to CMHNs. For the development and field-testing of the intervention in all participating FamCare study NHS organisations see Chapter 2.
Here we outline the progress of the CRT and reasons why it did not proceed.
Summary of design and methods
The pragmatic CRT had been designed to test the experimental intervention in a random sample of CMHTsOP, stratified by specialist community mental health NHS organisation. Not all organisations were NHS trusts (one was a social enterprise) and CMHTsOP were used for recruitment, as this was the commissioned NHS service for support of people with dementia and CB living at home, and their family carers. Thus, all practitioners in each participating team for older people (CMHTOP) would be randomised to either the experimental or control group. Randomisation by CMHTOP accounted for the fact that practitioners work together within teams (see Chapter 1). We anticipated conducting the trial in five NHS organisations (with back-up of a further two, should some organisations have difficulty in assisting with delivery). These five organisations reported a total of 30 CMHTsOP, with estimates of an average of 100 new dementia referrals per CMHTOP per year. As noted previously, only one study65 took account of interpractitioner variation and found a significant difference between them; no study has investigated the effect on outcomes for participants because of differences in practices across CMHTsOP, either within or between organisations. Therefore, our planned study design and proposed three-level analysis had anticipated lower ICCs and more precise estimates. With an (intraCMHTOP) correlation of 0.03, the variance inflation factor was approximately (1 + 20 × 0.03), that is, 1.6. Therefore, the likely usable sample of 600 participants from 30 CMHTsOP would provide an effective sample size of about 360, with 80% power using a significance level of 5% to detect an effect size of about 0.3 SDs. We judged this as a plausible, and clinically important, effect size.
Recruitment of participants was therefore planned to stop at a target of 600 and we had agreed with each participating organisation that each CMHTOP would attempt to recruit every new eligible patient referred to them over a 12-month period or until a total of 600 dyads of patients and carers were reached. Thus, we anticipated an average of 20 dyads (up to a maximum of 40 dyads) per CMHTOP, or eight dyads per practitioner, whichever was the larger in number.
Next, given the aforementioned variation we found in practitioner behaviour,65 we had included a pilot phase of 3 months in all CMHTsOP, which directly preceded the introduction of the intervention to the experimental CMHTsOP. This would allow us to monitor variation within practitioners and CMHTsOP for both experimental and control groups. For this pilot phase, we had planned to recruit seven participant dyads per CMHTOP and 200 in total (i.e. up to 15 for each CMHTOP or three per practitioner), whichever was the larger.
For the CRT, we estimated a likely loss to follow-up at 2 months of, on average, 16 of the 20 participant dyads. This would have given a usable sample of 480 (80%) from the original 600 dyads. With an estimate of 60% (120 dyads) of follow-up data from the pilot study added to the control arm of the CRT, a usable sample of 600 (240 intervention and 360 control) participants at an average of 20 per CMHTOP would reduce power by < 2%.
In conclusion, we had planned a pilot study and follow-up at 2 and 6 months to include 200 dyads from 30 CMHTsOP in five organisations; and a CRT of 600 dyads from these teams.
Progress
Following approvals from the REC (see Appendix 12) and consultation with NHS organisations, we extended the study to seven NHS organisations involving 33 CMHTsOP, of which one organisation was a social enterprise.
Recruitment across all seven organisations was slow from August 2010 to September 2011, requiring us to request a number of amendments which were approved, including a change of design from a CRT to a ‘controlled feasibility trial of a functional analysis-based intervention for community mental health therapists to support people living at home with dementia and CB; and their family carers’ (see Appendix 12).
However, the reasons for slow recruitment in the NHS organisations (see Chapter 5, Changes to protocol) did not change and recruitment remained slow. We failed to recruit our planned pilot recruitment of 200 dyads across 33 CMHTsOP from seven NHS organisations and, therefore, did not complete our planned controlled feasibility study of the intervention.
At the request of the NIHR funders, and in order to complete the programme within time scales, we curtailed our plans to commence the intervention study and redesigned the FamCare trial to be an observational cohort study, which was submitted to, and approved by, the REC (see Appendix 12).
Chapter 5 describes this cohort study, which used data from six NHS organisations and 28 CMHTsOP. One NHS trust with five CMHTsOP withdrew from the study when, during the course of the study, its services were recommissioned to another NHS organisation. During the process of an anticipated change of contract, CMHTsOP at this organisation were unable to support recruitment and we therefore failed to complete any baseline measures for dyads.
Appendix 3 The FamCare study: summary of the recruitment context
Background
Seven community mental health NHS organisations across England initially participated in the FamCare study. They were in Greater Manchester West, Grimsby, Humber, North East London, Oxford and Buckinghamshire, Sheffield and York. Nationally, CMHTsOP served older people with and without dementia, but many organisations were redesigning the roles and responsibilities of teams at the time of this study. Of the 33 CMHTsOP included in the audit of referrals, 14 had access to a discrete early MAS or a memory clinic, whereas another 19 had their memory services subsumed within their CMHTOP. Six CMHTsOP received referrals through a single point of access in their organisation, whereas 27 received referrals into their team direct from the referrer. Twelve had access to an intensive home treatment or crisis resolution team and 21 did not. In these seven NHS organisations, an audit of all new referrals to 33 participating CMHTsOP was conducted, from August 2010 to October 2011, in order to understand what proportion of people with dementia living at home were served by CMHTsOP.
Procedure
The characteristics of every ‘new referral’, over an average of 30.97 weeks (SD 6.00 weeks; range 14–46 weeks), across 33 CMHTsOP were documented. These included people with a non-dementia diagnosis (e.g. depression, psychosis, personality disorders, alcohol problems, delirium) and people with dementia (including those residing in care homes and those living at home). Each CMHTOP recorded the types of new referrals coming into their team each week, but no patient names or identification numbers were entered. These data were taken from the information available to them from the initial referral record that came into the team for each patient. The anonymised records were then collected on a weekly basis and collated by research assistants at each site. Discharged patients, who had previously been on the CMHTsOP case loads, but were referred back during this period, were classed as a new referral, as this was seen as a new episode of care of potential eligibility for the planned FamCare intervention study.
Results
Table 66 shows the groupings of new referrals using the data collected by the 33 participating CMHTsOP.
| New referral categories | Number of new referrals | % of total number of referrals |
|---|---|---|
| Non-dementia diagnosisa | 1985 | 37.0 |
| Dementia: referred straight to other services (see Table 67) | 1385 | 25.8 |
| Dementia: residential careb | 1190 | 22.0 |
| Dementia: at home with carerc | 452 | 8.4 |
| Dementia: no carer | 307 | 5.7 |
| Dementia: died or admitted to hospital before seen | 41 | 0.8 |
| Total | 5360 |
The majority of new referrals to the CMHTsOP during this period were ineligible for the FamCare study as they did not appear to have dementia, but were referred for other conditions; were primarily associated with functional mental health problems (37.0%); they were not living in their own home and were in a residential or nursing care home (22.0%); or they were accepted by the CMHTOP and referred straight out to other services (25.8%). Of particular interest was the striking movement of patients referred to CMHTsOP who were passed onto memory clinic waiting lists (852 cases) prior to evaluation, and the smaller number of people (235 cases) who were returned to primary care without evaluation by the specialist service that they were referred to (Table 67).
| Type of service | Number of referrals made straight to other services | % of total number of referrals made straight to other services |
|---|---|---|
| Early MASs/clinicsa | 852 | 61.5 |
| GPs and other health professionalsb | 235 | 17.0 |
| Other mental health specific servicesc | 138 | 9.96 |
| Hospital services (acute or mental health) | 53 | 3.83 |
| Type of service not specified | 107 | 7.73 |
| Total | 1385 |
People with dementia and challenging behaviour living at home and their family carers: recruitment challenges
The FamCare study was conceived as an intervention trial for dementia and CB. It was to be delivered by community mental health practitioners working in CMHTsOP, as this was where patients with dementia and CB were usually referred for support. To be eligible for the study, the referral had to therefore be accepted by the CMHTsOP and be placed on a practitioner’s case load.
Prior to data collection, practitioners and managers from CMHTsOP estimated significant numbers of people referred to the CMHTsOP within the eligibility criteria, as it was thought that this constituted the majority of their case work. However, this audit noted that the bulk of referrals to CMHTsOP appeared to be focused on patients without potential dementia or those living in residential and nursing care homes (see Table 66). Patients living at home with potential dementia were often redirected by CMHTsOP to other services such as MAS (see Table 67).
Conclusion
Over a period of approximately 7 months of data collection, between August 2010 and October 2011, at each of the seven NHS organisations, only 452 people with dementia living at home (around 8%), from a total of 5360 new cases to CMHTsOP, were accepted for support by a practitioner in the team.
Chapter 5 describes a cohort study where we extended recruitment to MAS, to include the period of this audit. Some participants (16.6%) in this cohort were located in newly emerging MASs or in memory clinics. Follow-up data collection comprising data at 2 and 6 months continued until July 2012 (see Chapter 5).
Appendix 4 The ResCare trial: ethical permissions
Issues that were addressed prior to approval
The issues that the REC requested be addressed were as follows:
-
time given between the provision of information and the taking of consent to be amended to 1 week rather than 1 day
-
alter the wording ‘medical team’ to ‘team of experts’ in the participant information sheet, as reference to ‘medical team’ was felt to be confusing and could be misinterpreted
-
amend the title of the ‘Relative/Friend Assent Form’ to read ‘Consultee Form – Record of Consultation’ instead.
Amendments
During the trial approval was sought and granted for a number of additional amendments as detailed below.
-
In June 2010, a request was made to the following:
-
Change the 7 days required for residents to consent to the trial after receiving information about the trial to ‘as long as they may wish to consent to consider taking part’; this provided the option of consent being taken as soon as the information sheet and explanation of the study had been provided, but would allow for longer periods too.
-
Remove the Short Form questionnaire-12 items (SF-12) from the trial as it was no longer considered necessary.
-
Replace the GHQ-12 with the MBI Human Service Survey,137 which is more specific to care workers.
-
Remove the Structured Medication Inventory from the care staff measures, as feedback from some feasibility work indicated that this measure would make staff feel uncomfortable and suspicious about providing information about personal medication use, and is not vital to the outcome of the economic analyses.
-
Include an abbreviated version of the CBS107 as an initial screen for all residents in the home prior to taking consent from residents. Knowing the incidence of CB enabled the quick identification of numbers of residents with CB in each home. As homes containing fewer than six residents with CB were to be dropped from the trial, this enabled a quick evaluation of whether or not a home was suitable to include without putting residents through the study process only to find the home had insufficient numbers of residents with CB.
Each of these requests was granted in June 2010.
-
-
Later in June 2010 an additional request was submitted to extend the start date of the trial to 12 May 2011 because of the computerised intervention taking longer than anticipated to develop.
This extension to the start date was approved in July 2010.
In January 2011 a request was made for the following:
-
Add the CMAI135 to the current measures based on the positive outcomes of the use of this measure found in the Cochrane review. 82
-
That care homes should no longer be stratified by ‘type of home’ during randomisation, due to the blurring in the distinction of types of homes away from distinct ‘nursing home’ and ‘residential home’ to homes that essentially operate as both.
-
Change the point at which homes were randomised. The original protocol had said we planned to randomise homes as we went along. However, a larger than originally anticipated amount of work was going to be required following consent for homes randomised to the intervention (e.g. installing computers and broadband) and, therefore, it was decided that all homes would be randomised in advance prior to baseline measures.
Approval was granted in January 2011 to all of these requested changes.
-
-
In August 2011 a final request was made to add the VAK Learning Styles Self-Assessment Questionnaire117 for care home staff to complete.
This was granted in August 2011.
Appendix 5 Management of the research
The research studies were managed as part of the overall NIHR Collaborative Research Programme, Challenge Demcare (Dementia Care for Behaviours that Challenge). Day-to-day operation of the programme was managed by the research programme manager, Cathryn Hart, with overall responsibility being that of the chief investigator, Professor Esme Moniz-Cook. In addition, the following management structures were in place.
Programme Management Committee
The Programme Management Committee comprised a programme grant holder, the programme manager, representatives from NWORTH Clinical Trials Unit, host organisation representatives from finance, business, service operations and R&D, and the chief investigator (who was also the chairperson of the committee).
The purpose of the Programme Management Committee was to oversee the running of the programme and ensure its smooth operation. It met on a regular basis, mostly every other month, and was split into two parts: (1) core business relating specifically to the Humber NHS Foundation Trust, for example, monitoring contracts and expenditure; and (2) other programme issues, for example, monitoring overall timetables, study recruitment, promoting the programme and liaising with services.
Programme Steering Committee
The PSC comprised programme grant holders, other independent professionals, care providers, service user representatives and programme staff, and had an independent chairperson, namely, David Jolley, Professor of Old Age Psychiatry, University of Manchester. For the main part it met every 6 months, with the frequency only reducing towards the end of the programme when the studies were closing.
The overall responsibility of the PSC was to ensure the scientific integrity and quality of the ResCare trial and the FamCare study, with its specific aims being to (1) approve the study protocols; (2) monitor and supervise the progress of the studies; (3) approve substantial amendments to the research; (4) consider the recommendations of the DMEC; (5) review relevant information from other sources and recommend appropriate action, if required; and (6) advise on dissemination and implementation.
Data Monitoring and Ethics Committee
The DMEC included independent clinical experts and was chaired by Martin Bland, Professor of Health Statistics in the Department of Health Sciences, University of York. This committee was responsible for looking at the data from an ethical standpoint, with the safety, rights and well-being of the trial participants being paramount.
The DMEC usually conducted its business electronically (via telephone and e-mail) a few weeks prior to the PSC and then submitted a report to the chairperson of the PSC. Its main functions were to (1) determine if additional interim analyses of study data should be undertaken; (2) consider any safety issues for the studies and relevant information from other sources; and (3) provide independent advice on analysis plans.
Patient and public involvement
The PSC included a number of service user representatives, including a person with dementia and family carers, some of whom also attended methodology meetings held during the course of the programme. Once the studies were complete, three consultation events were held to discuss the emerging findings from the studies and to explore implementation issues (see Chapters 4 and 5 for further details). Most, but not all, stakeholders attending were volunteers, carers, representatives from charitable organisations, and others who support those with dementia and their families.
Appendix 6 Measures
The Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
The DSM-IV130 criteria are used by a researcher to assess the likelihood that a person has dementia.
To meet the criteria for dementia the participant must have:
(1) A memory impairment.
AND
(2) One or more of the following: aphasia, apraxia, agnosia or disturbance in executive functioning.
AND
(3) The above must cause significant impairment in social or occupational functioning, represented by a significant decline from previous level of functioning.
AND
(4) Cannot be better explained by delirium or other mental health problems (e.g. depression, schizophrenia, etc.).
Challenging Behaviour Scale
The CBS107 is a 25-item interview whereby the researcher works down the list of 25 behaviours and for each one asks the care staff member if they consider the resident to display that behaviour. For each that is identified, the frequency of that behaviour is then selected from ‘occasionally’ (1) to ‘daily or more’ (4), and the difficulty for that care staff member is from ‘no problem’ (1) to ‘lots of problems’ (4). A challenge score for each behaviour is obtained by multiplying the frequency score by the difficulty score.
Item challenge score = frequency × difficulty (score range 0–16).
Total incidence score = the total number of CBs (score range 0–25).
Total frequency score = sum of frequency scores (score range 0–100).
Total difficulty score = sum of difficulty scores (score range 0–100).
Total challenge score = sum of 25-item challenge scores (score range 0–400).
EuroQol-5 Dimensions
The EQ-5D138 is a two-part questionnaire that measures perceived levels of health.
The first part (index) consists of a five-item questionnaire based on a three-point Likert scale. Responders are asked to rate on the following areas of health: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The rating scale goes from ‘no problems’ (1) to, depending on the question, ‘confined to/unable to/ extreme’ (3).
EuroQol-5 Dimensions index (score range –0.59 to 1).
The second part consists of a thermometer-type VAS where people are asked to indicate current levels of health from ‘worst imaginable health state’ (0) to ‘best imaginable health state’ (100).
EuroQol-5 Dimensions VAS (score range 0–100).
Quality of Life in Alzheimer’s Disease
The QoL-AD143 is a 13-item quality-of-life questionnaire based on a four-point Likert scale. It asks questions on areas such as physical health, energy levels and mood, and responders answer from a range of options between ‘poor’ (1) and ‘excellent’ (4).
Total score = sum of all the items (score range 13–52).
Neuropsychiatric Inventory
The NPI135 is an informant interview and is designed to rate ‘frequency’ and ‘severity’, and the version used for this study also included ratings for ‘caregiver distress’. The NPI has 12 CB categories: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviour, sleep, appetite/eating disorders (plus subquestions for each where more specific areas of disorder can be recorded). Each of the 12 categories has three Likert scales for frequency, severity and caregiver distress. Frequency is rated from ‘occasionally’ (1) to ‘very frequently’ (4). Severity is rated from ‘mild’ (1) to ‘marked’ (3). Distress is rated from ‘not at all’ (0) to ‘extremely’ (5).
Total CB category score = frequency × severity (score range 0–4).
Incidence score = the total number of CBs (score range 0–12).
Frequency score = the total frequency of the 12 behaviours (score range 0–48).
Severity score = sum of severity scores of the 12 behaviours (score range 0–36).
Total score (frequency × severity) = sum of total category scores of the 12 behaviours (score range 0–144).
Distress score = sum of distress scores of the 12 behaviours (score range 0–60).
Cohen–Mansfield Agitation Inventory
The CMAI136 is a 29-item questionnaire based on a seven-point Likert scale. Care staff are asked to rate how often a number of agitated behaviours occur. Answers range from ‘never’ (1) to ‘a few times an hour’ (7).
The measure has four subscales:
-
physical (aggressive) = sum of question 1 to question 11 (score range 11–77)
-
physical (non-aggressive) = sum of question 12 to question 21 (score range 10–70)
-
verbal (aggressive) = sum of question 22 to question 24 (score range 3–21)
-
verbal (non-aggressive) = sum of question 25 to question 29 (score range 5–35).
Total score = sum of all items (score range 29–203).
Clinical Dementia Rating
The CDR144 is a rating scale to be completed by the researcher and is based on how they rate the person with dementia’s performance during an interview (which should last around 1 hour). The researcher rates on six domains: memory, orientation, judgement and problem-solving, community affairs, home and hobbies, and personal care.
The CDR scores are healthy = 0; questionable dementia = 0.5; mild dementia = 1; moderate dementia = 2; severe dementia = 3.
Sum of boxes score (CDR-SB) = sum of domain scores (score range 0–18).
Maslach Burnout Inventory
The MBI137 is a 22-item questionnaire and uses a seven-point Likert scale. Staff report on their levels of burnout working with residents with CB. The measure consists of questions such as ‘I feel emotionally drained from my work’ and staff can answer from a range of options from ‘never’ to ‘everyday’.
The following questions require reverse scoring: questions 4, 7, 9, 12, 17–19 and 21.
There are three subscales:
-
emotional exhaustion: questions 1–3, 6, 8, 13, 14, 16 and 20 (score range 0–54)
-
personal accomplishment: questions 4, 7, 9, 12, 17–19 and 21 (score range 0–48)
-
depersonalisation: questions 5, 10, 11, 15 and 22 (score range 0–30).
Self-Efficacy Scale
The SES140 is a nine-item questionnaire that uses a seven-point Likert scale and asks care staff how effective they believe they are in their role as a care worker. The measure consists of challenging scenarios in care homes and the person has to rate how confident they feel in dealing with that scenario: ‘not at all confident’ (1) to ‘very confident’ (7).
Total score = sum of all the items.
Approaches to Dementia Questionnaire
The ADQ139 is a 19-item questionnaire that uses a five-point Likert scale and asks care staff to indicate their attitudes towards people with dementia. The measures consist of questions such as ‘there is no hope for people with dementia’ and staff can answer a range of options from ‘strongly agree’ to ‘strongly disagree’.
Questions 1–4, 6, 8, 10, 13 and 14 are scored with ‘strongly disagree’ as (5).
Questions 5, 7, 9, 11, 12 and 15–19 are scored with ‘strongly agree’ as (5).
This measure has two subscales:
-
hope = sum of questions 1–4, 6, 8, 10 and 13 (score range 8–40)
-
person-centred = sum of questions 5, 7, 9, 11, 12 and 14–19 (score range 11–55).
Total score = sum of all items (score range 19–95).
Visual–auditory–kinaesthetic
The VAK117 is a 30-item self-completion learning styles questionnaire. For each question people have to indicate if they would choose option (a), (b) or (c).
Mostly (a) = visual learning style.
Mostly (b) = auditory learning style.
Mostly (a) = kinaesthetic learning style.
Some people have a blend of two or three different styles.
Revised Memory and Behaviour Problems Checklist
The RMBPC111 is a 24-item questionnaire on behavioural problems in the person with dementia. It records incidence, and frequency, of the behaviour and the caregiver’s reaction to the behaviour. The higher the score on this scale, the greater the level of problems.
The incidence is scored as ‘yes’ (1) or ‘no’ (0). Frequency ranges from ‘never’ (0) to ‘daily or more often’ (4) and ‘don’t know/not applicable’ (9). The caregiver’s reaction to the reported problem (how much the behaviour bothers them) is rated from ‘not at all’ (0) to ‘extremely’ (4) and ‘don’t know/not applicable’ (9).
The measure has three subscales:
-
memory-related = sum of questions 1–7 (score range 0–7)
-
depression = sum of questions 12, 14, 17–22 and 23 (score range 0–9)
-
disruptive behaviours = sum of questions 8–11, 13, 15, 16 and 24 (score range 0–8).
Quality of the Caregiver/Patient Relationship
The QCPR258 is a 14-item questionnaire that measures the quality of the relationship between the person with dementia and the caregiver via 14 five-point Likert items. A higher score overall on this scale indicates a higher quality of relationship.
The measure consists of statements such as ‘my carer/relative and I often spend time together in an enjoyable way’. The responder can answer from ‘totally disagree’ to ‘totally agree’ with the statement and the answers are scored from 1 to 5, with five being the most positive response to each statement.
The measure has two subscales:
-
warmth = sum of questions 1, 4–7, 9, 12 and 14; scored with ‘totally disagree’ as (1) (score range 8–40)
-
criticism = sum of questions 2, 3, 8, 10, 11 and 13; scored with totally disagree as (5) (score range 6–30).
Total score = sum of two subscales (score range 14–70).
ICEpop CAPability measure for older people
The ICECAP-O257 is a five-item questionnaire which measures the quality of life of the responder via five four-point Likert items. A higher score overall on this scale indicates a higher quality of life.
The measures cover the categories of (1) love and friendship; (2) thinking about the future; (3) doing things that make you feel valued; (4) enjoyment and pleasure; and (5) independence.
The responder can answer from a range of options, which vary from category to category. For the love and friendship category the responder can answer from ‘I can have all of the love and friendship that I want’ (4) to ‘I cannot have any of the love and friendship that I want’ (1). The answers are scored from 1 to 4, with 4 being the most positive response in each category.
ICECAP-O index (score range 0–1).
Short Sense of Competence Questionnaire
The SSCQ255 is a seven-item questionnaire that measures the quality of the relationship between the carer and the person with dementia via seven five-point Likert items. A higher score overall on this scale indicates a higher quality of relationship.
The measures consist of statements such as ‘I feel that my relative behaves the way he/she does to have his/her own way’ and the carer can answer from ‘agree very strongly’ (1) to ‘strongly disagree’ (5). The answers are scored from 1 to 5, with 5 being the most positive response to each of the statements.
Total score = sum of the items (score range 7–35).
General Health Questionnaire
The GHQ-12254 is a 12-item questionnaire that measures the condition of the responder’s recent and present mental health via 12 four-point Likert items. A higher score overall on this scale indicates a lower mental health condition.
The measure consists of questions (Qs) such as ‘Have you recently been able to concentrate on what you are doing?’ The responder can answer from a range of options, which vary from question to question. For the question above about ability to concentrate, the responder can answer from ‘Better than usual’ (0) to ‘Much less than usual’ (3). The answers are scored from 0 to 3, with 0 being the most positive response to each question.
The measure has two subscales:
-
positive = sum of questions 1, 3, 4, 7, 8 and 12 (score range 0–18)
-
negative = sum of questions 2, 5, 6 and 9–11 (score range 0–18).
Total score = sum of the positive and negative subscales (score range 0–36).
Hospital Anxiety and Depression Scale
The HADS253 is a 14-item questionnaire that measures the responder’s anxiety and depression state via 14 four-point Likert items. A higher score overall on this scale indicates a higher level of anxiety and depression.
The measure consists of statements such as ‘I feel tense or ‘wound up’. The responder can answer from a range of options, which vary from statement to statement. For the statement above about feeling tense, the responder can answer from ‘Most of the time’ (3) to ‘Not at all’ (0). The answers are scored from 0 to 3, with 0 being the most positive response to each statement.
The measure has two subscales:
-
anxiety = sum of questions 1, 3, 5, 7, 9, 11 and 13 (score range 0–21)
-
depression = sum of questions 2, 4, 6, 8, 10, 12 and 14 (score range 0–21).
Total score = sum of anxiety and depression subscales (score range 0–42).
Guilt Scale
The GS252 is a 10-item questionnaire that measures the carer’s feelings of guilt via 10 five-point Likert items. A higher score overall on this scale indicates more feelings of guilt.
The measures consist of statements such as ‘Do you worry that you might unintentionally hurt your relative’s feelings?’ The carer can answer from ‘always’ (4) to ‘never’ (0). The answers are scored from 0 to 4, with 0 being the most positive response to each question.
Total score = sum of the items (score range 0–40).
Relative Stress Scale
The RSS256 is a 15-item questionnaire that measures stress specific to caregiving via 15 five-point Likert items. A higher score overall on this scale indicates more stress.
The measures consist of questions such as ‘Do you ever feel that you can no longer cope with the situation?’ The carer can answer from ‘never’ (0) to ‘always/considerably’ (4). The answers are scored from 0 to 4, with 0 being the most positive response to each question.
The measure has three subscales:
-
emotional distress = sum of questions 1–6 (score range 0–24)
-
social distress = sum of questions 7–11 (score range 0–20)
-
negative feelings = sum of questions 12–15 (score range 0–16).
Total score = sum of three subscales (score range 0–60).
Appendix 7 Unit costs and sources
Figures have been taken from Curtis,153 the NHS’s England Statement of Dental Remuneration – Amendment 93357 and the Department of Health’s General Ophthalmic Services Letter. 358
| Item | Unit | Cost (£) | Source/notes |
|---|---|---|---|
| NHS district nurse, home visit | Per hour | 70 | District nurse home visit, including qualification and travel costs (PSSRU 2012, p. 175) |
| NHS district nurse, clinic visit | Per hour | 58 | District nurse per hour of patient-related work, including qualification costs (PSSRU 2012, p. 175) |
| GP surgery visit | Per minute | 4 | GP per surgery/clinic minute including qualification costs and direct care staff costs (PSSRU 2012, p. 183) |
| GP home visit | Per minute | 5 | Per minute of home visit, including travel time, qualification costs and direct care staff costs (PSSRU 2012, p. 183). Assuming out-of-hours costs are same as for home visit |
| Community specialist doctor | Per minute | 4 | Assumed same as GP per surgery/clinic visit. This category includes audiologists, dermatologists, ophthalmologists, oncologists, gynaecologists and general medics |
| Practice nurse | Per hour | 53 | Assumed home visit is the same as clinic visit. Per hour of face-to-face contact, including qualification costs (PSSRU 2012, p. 180) |
| NHS health visitor, home visit | Per hour | 71 | Health visitor home visit, including qualification costs (PSSRU 2012, p. 177) |
| NHS health visitor, clinic visit | Per hour | 59 | Health visitor per hour of clinic contact, including qualification costs (PSSRU 2012, p. 177) |
| NHS psychiatrist | Per hour | 289 | Assumed home visit is the same as clinic visit. Consultant: psychiatric cost per hour of patient contact, including qualification costs (PSSRU 2012, p. 237) |
| NHS psychologist | Per hour | 136 | Assumed home visit is the same as clinic visit. Per hour of client contact, clinical psychologist. £1.50 per visit for travel (PSSRU 2012, p. 171) |
| NHS counsellor | Per hour | 65 | Assumed to be the same as clinic visit. Per hour of client contact for counselling service in primary care (PSSRU 2012, p. 53) |
| NHS CMHN | Per hour | 53 | Assumed home visit is the same as clinic visit. Per hour of face-to-face contact, including qualification costs. £1.50 per visit for travel (PSSRU 2012, p. 176) |
| Rapid response team | Per hour | 37 | Per delivered hour (excludes cost for enhanced payments, cost of assessments, discharge and travel costs) (PSSRU 2012, p. 113) |
| NHS physiotherapist | Per hour | 33 | Assuming home visit is the same as clinic visit. Per physiotherapist hour, including qualification costs (PSSRU 2013, p. 167) |
| NHS occupational therapist | Per hour | 33 | Assumed home visit is the same as clinic visit. NHS community occupational therapist, including qualification costs (PSSRU 2012, p. 168) |
| Home care worker | Per hour | 23 | Per hour of weekday face-to-face contact, Based on the price multipliers for the independent sector provided for social services (PSSRU 2012, p. 193) |
| Care attendant | Per hour | 23 | Assumed same as home care worker |
| Sitting service | Per session | 14.50 | Based on five agencies in Torbay 2005 (Charlesworth et al. 2008, p. 52359); adjusted to 2012 prices |
| Carer’s support worker | Per hour | 49 | Assumed same as clinic visit, per hour of client-related work for family support worker, including training costs (PSSRU 2012, p. 196) |
| NHS chiropodist/podiatrist | Per hour | 30 | Assumed home visit and clinic visit the same. Per hour, community chiropodist (PSSRU 2012, p. 170) |
| NHS dietitian | Per hour | 34 | Per hour, hospital-based dietitian including qualification costs (PSSRU 2012, p. 216) |
| Alternative therapist | Per hour | 17 | Taken as mid-point of NHS pay band 5 including national insurance and pension |
| Meals on Wheels | Per visit | 6 | Cost per meal (PSSRU 2012, p. 125) |
| NHS optician | Per visit | 21 | NHS sight test fee for optometrists and ophthalmic medical practitioners: £20.70 (Department of Health, General Ophthalmic Services letter 2010; URL: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/dr_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_114691.pdf) |
| NHS dentist | Per visit | 25 | Fee code 0121 provision of care and treatment for a patient (NHS Statement of Dental Remuneration 2012, p. 6) |
| Social worker/care co-ordinator | Per hour | 74 | Per hour of client-related work (adult services), including qualification costs (PSSRU 2012, p. 190) |
| Speech and language therapist | Per hour | 33 | Per hour, including qualification costs (PSSRU 2012, p. 169) |
| Specialist nurse | Per hour | 70 | Assumed same as district nurse home visit, including qualification and travel costs (PSSRU 2012, p. 175). Includes asthma nurses, stroke nurses, Parkinson’s nurses, diabetes nurses, ‘stop smoking’ nurses |
| Falls team | Per hour | 42 | Falls teams consist of occupational therapists, physiotherapists, specialist nurses and chiropodists. The unit cost has been assumed as the mean hourly rate of the above |
| Emergency care practitioner | Per hour | 70 | Assumed same as district nurse home visit, including qualification costs (PSSRU 2012, p. 175) |
| Crisis team | Per hour | 38 | Per hour, per team member (PSSRU 2012, p. 201) |
| Paramedic | Per visit | 230 | Per visit (PSSRU 2012, p. 109) |
| Independent mental capacity advocate | Per visit | 110 | Per visit (PSSRU 2012, p. 56) |
| Home care manager | Per hour | 57 | Per hour of client-related work (PSSRU 2012, p. 195) |
| Safeguarding practitioner | Per visit | 358 | Per assessment by mental health assessor, including travel costs (PSSRU 2012, p. 56) |
| Adult family placement/shared lives arrangement | Per hour | 9.05 | Per hour of day time support, including on costs. Taken from appendix 10a of Blackpool Council’s Shared Lives scheme360 |
| Chiropractor | Per visit | 32 | Cost for chiropractic adjustment, taken from www.the-chiropractors.co.uk/our-fees/ |
| Item | Unit | Cost (£) | Source/notes |
|---|---|---|---|
| Inpatient ward | Per night | 919 | PA32B, elective inpatient: minor Injury without intracranial injury without CC |
| Assessment/rehabilitation inpatient | Per night | 308 | VC28Z, ’non-specialist’ rehabilitation services (NSRS) – bed-days: admitted patient care |
| Respite inpatient | Per night | 327 | MHIPE1, mental health inpatients, elderly |
| Outpatient services | Per visit | 30 | WA20Y, outpatient procedures: examination, follow-up, special screening, without CC |
| Day surgery | Per visit | 138 | WA21Y, outpatient procedures: other procedures or health-care problems, without CC |
| A&E | Per visit | 91 | VB09Z, A&E services: not leading to admitted; emergency medicine, category 1 investigation with category 1–2 treatment |
| Ambulance | Per transfer | 263 | Paramedic services, emergency transfer (PSSRU 2012, p. 109) |
| Item | Unit | Cost (£) | Source/notes |
|---|---|---|---|
| Day care: local authority social services | Per session | 40 | Local authority day care for older people (PSSRU 2012, p. 40) |
| Day care: voluntary organisation | Per session | 18 | Taken from a Sheffield charitable day care centre (URL: www.sheffieldhelpyourself.org.uk/keyword_search.asp) |
| Day care: NHS | Per session | 40 | Assumed same as local authority day care |
| Lunch club | Per meal | 3.17 | Taken from an average of 57 lunch clubs in Sheffield (URL: www.sheffieldhelpyourself.org.uk/keyword_search.asp) |
| Social club | Per session | 3.17 | Assumed same as lunch club |
Appendix 8 ResCare: factors affecting resident and staff dropout
| Variable | Dropouts (n = 174), odds ratio (95% CI) |
|---|---|
| Treatment group | 1.69 (1.06 2.70) |
| Number of beds | |
| 40–49 | 2.10 (1.37 3.20) |
| ≥ 50 | 3.07 (1.96 4.83) |
| Gender | 0.57 (0.38 0.86) |
| Age | 1.04 (1.02 1.07) |
| Length between baseline and follow-up at home level | 1.01 (1.00 1.01) |
| Demographic factor | Staff | |
|---|---|---|
| Stayed (n = 413) | Dropouts at follow-up (n = 174) | |
| Age group (years) | ||
| < 25 | 87 | 48 |
| 25–49 | 227 | 93 |
| ≥ 50 | 98 | 33 |
| Missing data | 1 | |
| Sig. | 0.169 | |
| Gender | ||
| Male | 45 | 15 |
| Female | 368 | 159 |
| Sig. | 0.495 | |
| Another paid employment | ||
| Yes | 18 | 12 |
| No | 395 | 162 |
| Sig. | 0.285 | |
| Smoking | ||
| Yes | 177 | 85 |
| No | 236 | 89 |
| Sig. | 0.214 | |
| Number of beds | ||
| 25–39 | 223 | 85 |
| 40–49 | 136 | 45 |
| ≥ 50 | 54 | 44 |
| Sig. | 0.001 | |
| Proportion of residents with a CBS incidence score of > 10 | ||
| < 0.4 | 342 | 153 |
| ≥ 0.4 | 71 | 21 |
| Sig. | 0.151 | |
| Organisation change | ||
| Yes | 262 | 93 |
| No | 151 | 81 |
| Sig. | 0.030 | |
| Home type | ||
| Local | 69 | 17 |
| Private | 180 | 93 |
| Private nursing | 57 | 19 |
| Voluntary/charity | 107 | 45 |
| Sig. | 0.061 | |
| Accommodation | ||
| Missing data | 6 | 2 |
| Owned | 239 | 91 |
| Rented | 116 | 57 |
| Housing Association | 52 | 24 |
| Sig. | 0.424 | |
| Treatment groups | ||
| Control | 233 | 61 |
| Intervention | 180 | 113 |
| Sig. | < 0.001 | |
Appendix 9 The ResCare trial: tables relating to the analysis of the dose effect of the intervention
| Descriptive | Results | |||||
|---|---|---|---|---|---|---|
| Before imputation | Pooled | |||||
| β | SE | t (p-value) | β | SE | t (p-value) | |
| Number of champions | 0.67 | 0.62 | 1.08 (0.284) | 0.72 | 0.55 | 1.30 (0.194) |
| Proportion of action plans/CB residents | 2.62 | 2.33 | 1.12 (0.266) | 2.89 | 2.05 | 1.41 (0.161) |
| Baseline frequency | 0.36 | 0.04 | 8.69 (< 0.001) | 0.32 | 0.04 | 7.38 (< 0.001) |
| Descriptive | Results | |||||
|---|---|---|---|---|---|---|
| Before imputation | Pooled | |||||
| β | SE | t (p-value) | β | SE | t (p-value) | |
| Number of champions | 1.31 | 0.64 | 2.04 (0.042) | 1.13 | 0.58 | 1.97 (0.050) |
| Proportion of action plans/CB residents | 3.09 | 2.31 | 1.34 (0.181) | 2.90 | 2.06 | 1.40 (0.161) |
| Baseline frequency | 0.36 | 0.04 | 8.55 (< 0.001) | 0.32 | 0.04 | 7.38 (< 0.001) |
| Organisational change | 1.22 | 0.79 | 1.55 (0.122) | 1.17 | 0.80 | 1.46 (0.146) |
| Gender | 0.36 | 0.76 | 0.48 (0.634) | 0.30 | 0.81 | 0.37 (0.715) |
| Number of beds | ||||||
| 40–49 | 0.74 | 0.70 | 1.05 (0.296) | 0.82 | 0.72 | 1.14 (0.253) |
| ≥ 50 | 1.83 | 0.96 | 1.91 (0.057) | 1.55 | 1.07 | 1.44 (0.155) |
| Home type | ||||||
| Private | 2.35 | 1.19 | 1.97 (0.049) | 2.00 | 1.19 | 1.67 (0.094) |
| Private (with nursing) | 1.04 | 1.48 | 0.70 (0.485) | 0.92 | 1.51 | 0.61 (0.542) |
| Voluntary/charity | 1.29 | 1.22 | 1.05 (0.293) | 0.83 | 1.24 | 0.67 (0.542) |
| Proportion of residents with a CBS incidence score of > 10 | ||||||
| ≥ 0.4 | 0.94 | 0.91 | 1.04 (0.299) | 0.82 | 0.97 | 0.85 (0.398) |
| Age | –0.02 | 0.04 | –0.44 (0.660) | –0.01 | 0.04 | –0.33 (0.743) |
| Length between baseline and follow-up (days) | 0.01 | 0.01 | 1.51 (0.133) | 0.01 | 0.01 | 1.06 (0.290) |
| Descriptive | Results | |||||
|---|---|---|---|---|---|---|
| Before imputation | Pooled | |||||
| β | SE | t (p-value) | β | SE | t (p-value) | |
| Number of champions | 1.03 | 0.43 | 2.40 (0.020) | 0.50 | 0.52 | 0.95 (0.351) |
| Proportion of action plans/CB residents | 4.07 | 1.60 | 2.54 (0.014) | 2.14 | 1.94 | 1.10 (0.285) |
| Baseline severity | 0.37 | 0.04 | 9.13 (< 0.001) | 0.33 | 0.04 | 7.78 (< 0.001) |
| Descriptive | Results | |||||
|---|---|---|---|---|---|---|
| Before imputation | Pooled | |||||
| β | SE | t (p-value) | β | SE | t (p-value) | |
| Number of champions | 1.24 | 0.48 | 2.62 (0.013) | 0.63 | 0.53 | 1.19 (0.243) |
| Proportion of action plans/CB residents | 4.75 | 1.71 | 2.78 (0.008) | 2.62 | 2.03 | 1.29 (0.209) |
| Baseline severity | 0.38 | 0.04 | 9.01 (< 0.001) | 0.33 | 0.04 | 7.63 (< 0.001) |
| Organisational change | 0.70 | 0.58 | –1.20 (0.240) | 0.56 | 0.64 | –0.87 (0.385) |
| Gender | –0.15 | 0.50 | –0.31 (0.759) | –0.01 | 0.52 | –0.02 (0.982) |
| Number of beds | ||||||
| 40–49 | 1.02 | 0.53 | 1.94 (0.060) | 0.94 | 0.60 | 1.57 (0.117) |
| ≥ 50 | 1.09 | 0.73 | 1.50 (0.144) | 0.39 | 0.77 | 0.51 (0.612) |
| Home type | ||||||
| Private | 0.54 | 0.90 | 0.60 (0.553) | 0.83 | 0.96 | 0.87 (0.386) |
| Private (with nursing) | 0.64 | 1.12 | 0.57 (0.572) | 1.17 | 1.22 | 0.96 (0.337) |
| Voluntary/charity | 0.34 | 0.91 | 0.37 (0.712) | 0.45 | 0.98 | 0.46 (0.645) |
| Proportion of residents with a CBS incidence score of > 10 | ||||||
| ≥ 0.4 | 0.01 | 0.69 | 0.01 (0.992) | 0.16 | 0.77 | 0.20 (0.839) |
| Age | –0.01 | 0.03 | –0.54 (0.591) | –0.02 | 0.03 | –0.60 (0.547) |
| Length between baseline and follow-up (days) | 0.001 | 0.006 | 0.17 (0.863) | –0.001 | 0.006 | –0.23 (0.821) |
| Descriptive | Results | ||
|---|---|---|---|
| β | SE | t (p-value) | |
| Number of champions | –0.13 | 0.38 | –0.34 (0.736) |
| Proportion of action plans/CB residents | –0.84 | 1.43 | –0.59 (0.564) |
| Baseline inpatient days | 0.03 | 0.06 | 0.42 (0.678) |
| Descriptive | Results | ||
|---|---|---|---|
| β | SE | t (p-value) | |
| Champions | –0.25 | 0.48 | –0.53 (0.601) |
| Proportion of action plans/CB residents | –1.29 | 1.71 | –0.76 (0.460) |
| Baseline inpatient days | 0.03 | 0.06 | 0.42 (0.677) |
| Organisational change | 0.28 | 0.59 | 0.47 (0.644) |
| Gender | –0.17 | 0.37 | –0.46 (0.648) |
| Number of beds | |||
| Medium (40–49) | –0.64 | 0.53 | –1.21 (0.240) |
| Large (≥ 50) | –0.56 | 0.74 | –0.76 (0.457) |
| Home type | |||
| Private | –1.25 | 0.89 | –1.41 (0.176) |
| Private (with nursing) | –1.59 | 1.12 | –1.41 (0.174) |
| Voluntary/charity | –1.41 | 0.91 | –1.54 (0.140) |
| Proportion of residents with a CBS incidence score of > 10 | |||
| ≥ 0.4 | –0.88 | 0.69 | –1.27 (0.223) |
| Age | 0.02 | 0.02 | 0.80 (0.427) |
| Length between baseline and follow-up (days) | –0.002 | 0.006 | –0.32 (0.754) |
Appendix 10 The ResCare trial: analysis of residents with inpatient stays
FIGURE 17.
The ResCare trial: box dot plot of NPI frequency by categories of inpatient stays.
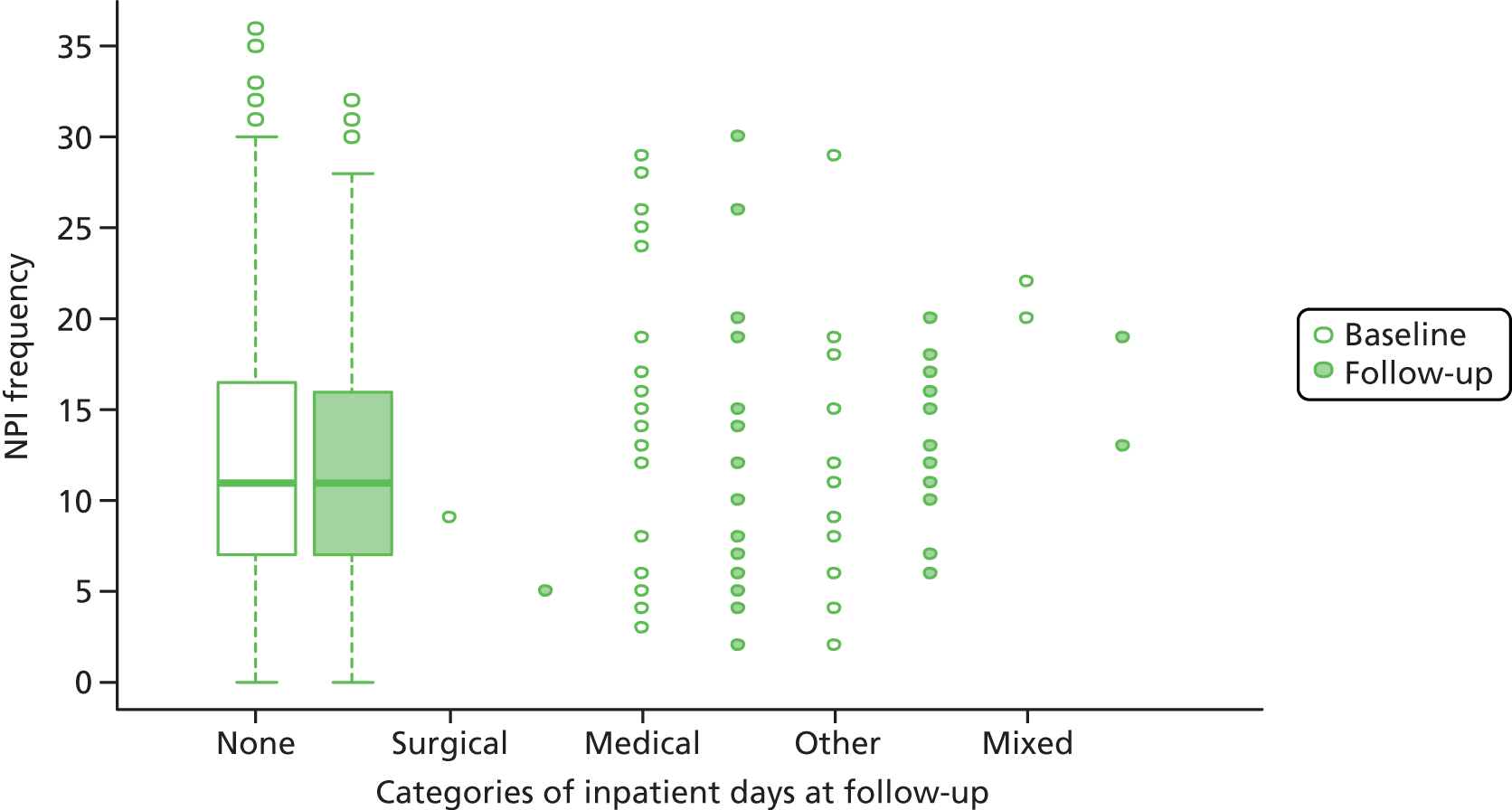
FIGURE 18.
The ResCare trial: box dot plot of NPI severity by categories of inpatient stays.
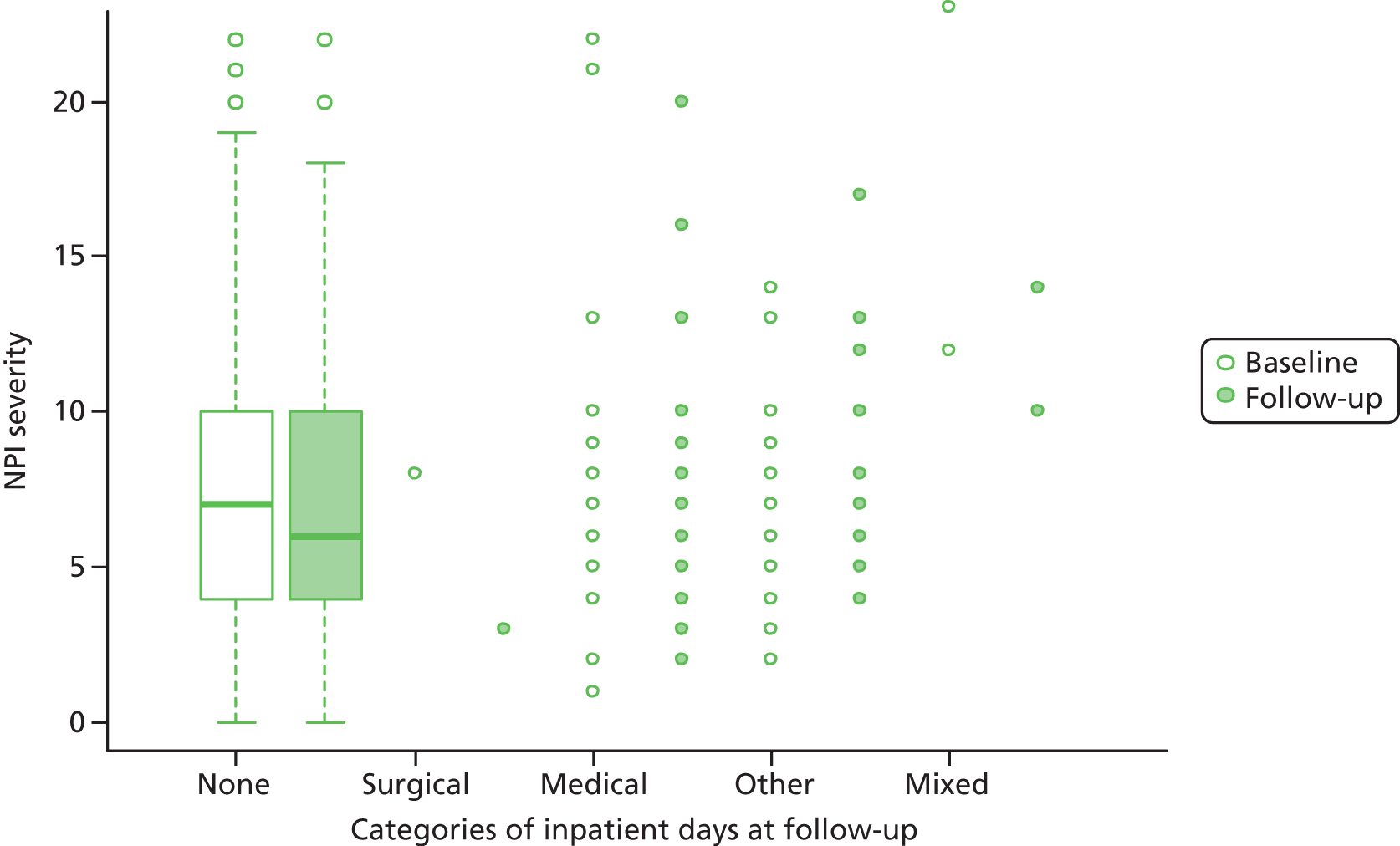
FIGURE 19.
The ResCare trial: box dot plot of CBS frequency × difficulty by categories of inpatient stays.

FIGURE 20.
The ResCare trial: box dot plot of total inpatient cost by categories of inpatient stays. Note that 1028005 is the ID of an outlier.
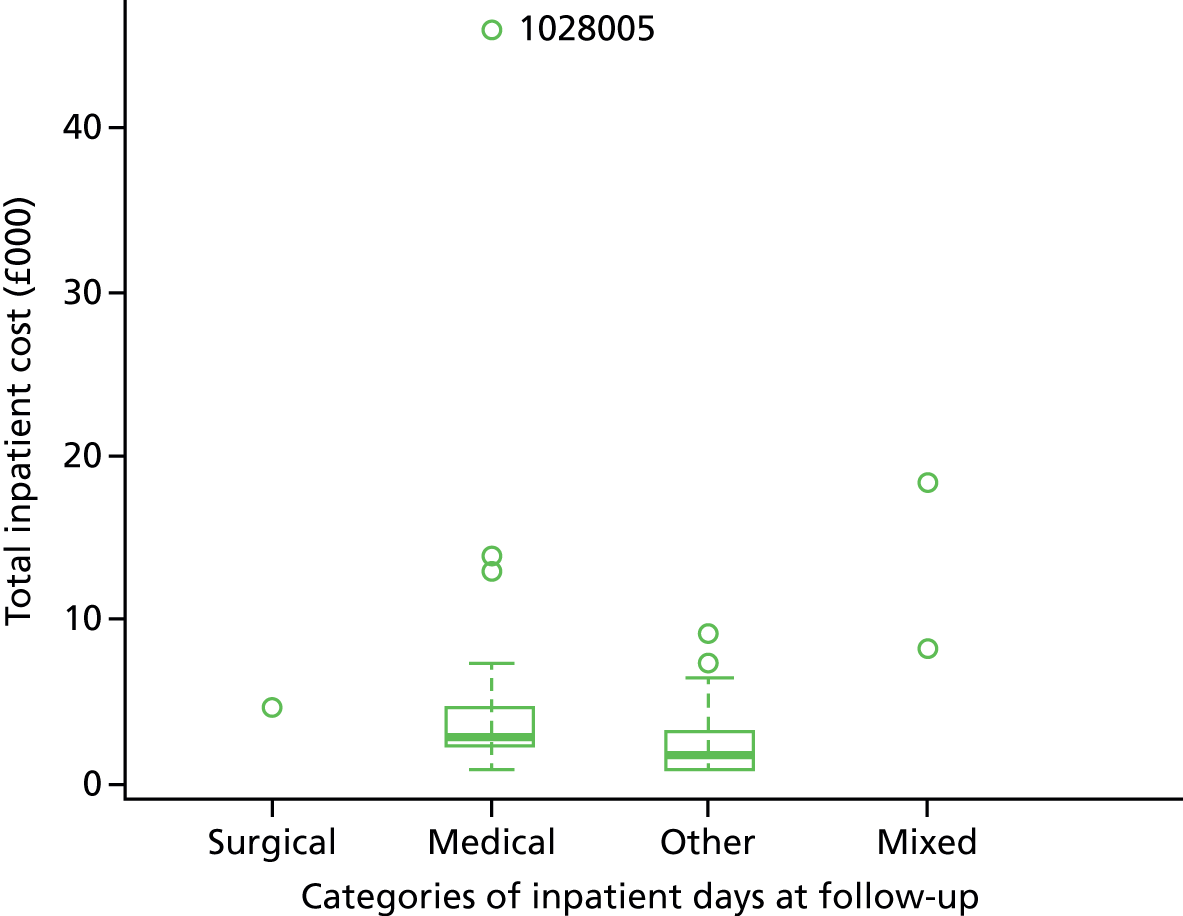
FIGURE 21.
The ResCare trial: box dot plot of follow-up medication cost by categories of inpatient stays.
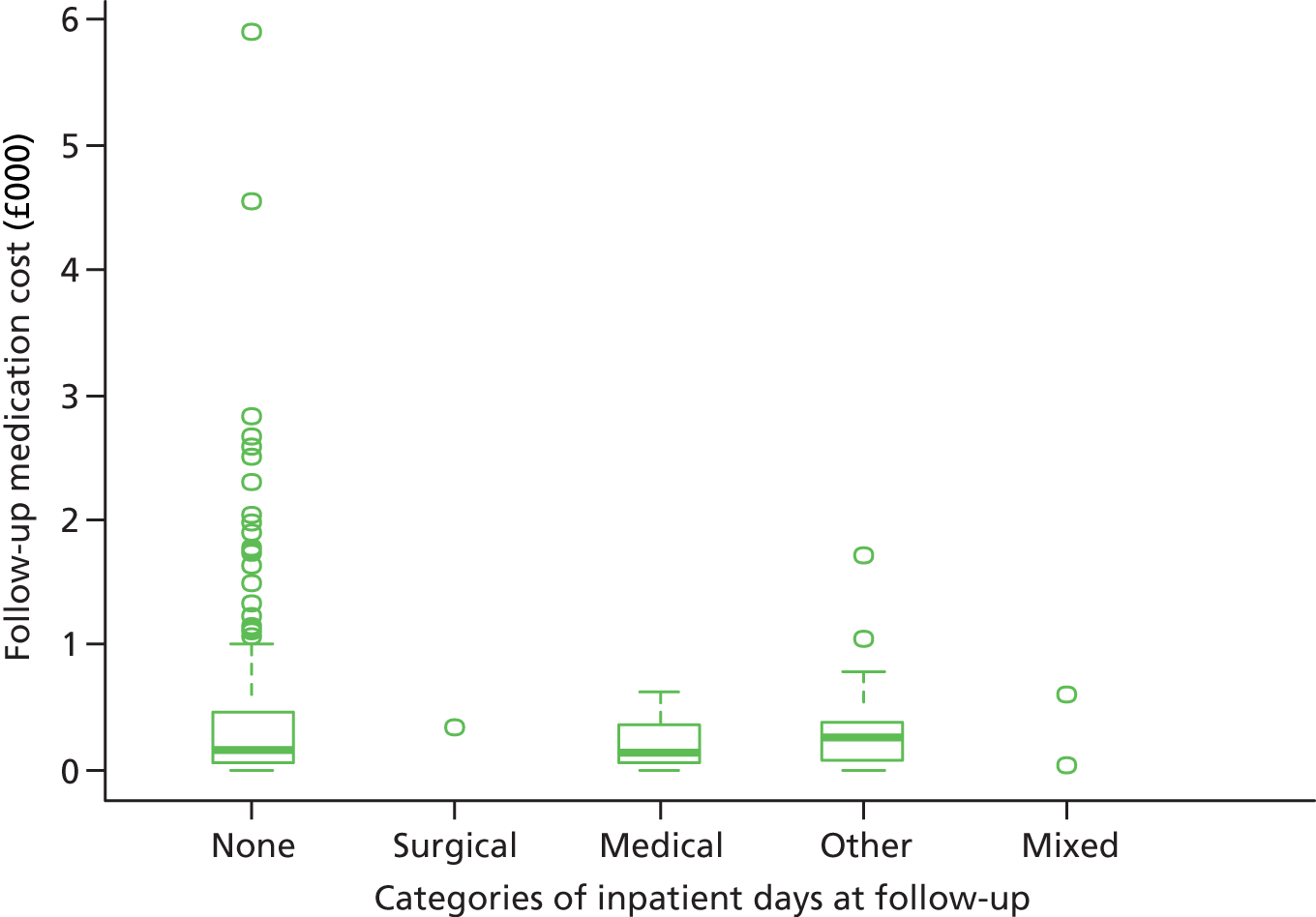
Appendix 11 The ResCare trial: breakdown of resource use and costs in the 4 months up to follow-up
| Service | Group, mean (SD) | Mean difference (£) (intervention – control) (95% CI bootstrapped) | |||
|---|---|---|---|---|---|
| Control (n = 226) | Intervention (n = 202) | ||||
| Frequencya | Cost (£) | Frequencya | Cost (£) | ||
| District nurse | 32.64 (68.35) | 38.06 (79.74) | 41.44 (105.47) | 48.34 (123.05) | 10.28 |
| GP | 39.80 (57.38) | 186.47 (269.40) | 37.97 (46.50) | 177.79 (218.36) | –8.68 |
| Practice nurse | 1.86 (10.29) | 1.64 (9.09) | 1.83 (12.33) | 1.62 (10.89) | –0.02 |
| Community psychiatrist | 0.46 (6.07) | 2.24 (29.21) | 0.15 (2.11) | 0.72 (10.17) | –1.52 |
| Psychologist | 0.93 (12.13) | 2.11 (27.49) | 0.47 (5.72) | 1.07 (12.97) | –1.04 |
| CMHN/CMHT | 4.87 (18.94) | 4.30 (16.73) | 1.56 (7.05) | 1.38 (6.22) | –2.92 |
| Physiotherapist | 1.37 (8.62) | 0.75 (4.74) | 6.61 (33.84) | 3.64 (18.61) | 2.89 |
| Occupational health therapist | 0.62 (4.77) | 0.34 (2.62) | 1.19 (8.56) | 0.65 (4.71) | 0.31 |
| Chiropodist | 17.94 (22.53) | 8.97 (11.27) | 25.23 (28.96) | 12.61 (14.48) | 3.64 |
| Dietitian | 4.69 (14.03) | 2.66 (7.95) | 6.13 (17.44) | 3.47 (9.88) | 0.81 |
| Optician | 3.69 (10.02) | 3.11 (7.91) | 3.66 (10.37) | 3.17 (8.54) | 0.06 |
| Dentist | 1.06 (10.56) | 0.67 (5.80) | 2.28 (13.67) | 2.00 (11.94) | 1.33 |
| Social worker | 1.70 (8.67) | 2.10 (10.69) | 1.81 (9.53) | 2.24 (11.76) | 0.14 |
| Speech and language therapist | 0.62 (4.28) | 0.34 (2.35) | 0.99 (6.77) | 0.55 (3.72) | 0.21 |
| Specialist nurse | 0.27 (2.82) | 0.31 (3.29) | 0.45 (3.00) | 0.52 (3.50) | 0.21 |
| Falls team | 0.22 (2.39) | 0.15 (1.66) | 0.50 (2.91) | 0.34 (2.01) | 0.19 |
| Emergency care practitioner | 2.65 (13.03) | 3.10 (15.20) | 3.00 (13.57) | 3.49 (15.83) | 0.39 |
| Otherb | 0.66 (5.96) | 0.60 (5.52) | 2.60 (12.54) | 14.11 (63.10) | 13.51 |
| Primary care total | 116.06 (119.12) | 257.92 (310.16) | 137.84 (151.21) | 277.89 (302.22) | 19.97 (–39.22 to 81.38) |
| Surgical inpatient | 0 | 0 | 0.02 (0.35) | 22.75 (323.30) | 22.75 |
| Medical inpatient | 0.55 (3.74) | 508.30 (3436.32) | 0.25 (1.33) | 227.48 (1218.96) | –280.82 |
| Other inpatient | 0.15 (0.92) | 138.26 (846.47) | 0.10 (0.80) | 95.54 (735.71) | –42.72 |
| Outpatient | 0.10 (0.33) | 2.92 (9.77) | 0.09 (0.37) | 2.82 (11.04) | –0.10 |
| Day case | 0.00 (0.07) | 0.61 (9.18) | 0.02 (0.14) | 2.73 (19.27) | 2.12 |
| A&E | 0.15 (0.49) | 13.29 (44.66) | 0.10 (0.34) | 10.76 (41.64) | –2.53 |
| Secondary care total | 0.95 (4.05) | 663.37 (3634.81) | 0.59 (1.81) | 362.08 (1544.66) | –301.29 (–924.05 to 160.61) |
| Medication total | 7.47 (3.84) | 384.21 (655.88) | 7.87 (4.06) | 334.88 (386.97) | –49.33 (–153.39 to 51.60) |
| Total service use | 1305.51 (3752.74) | 974.85 (1601.40) | –330.66 (–926.87 to 272.10) | ||
Appendix 12 The FamCare study: ethical permissions
Prior to approval (April 2009)
The REC requested that we address the following:
-
a change in the title of the consultee form
-
the addition of a separate information leaflet and consent form for carers as participants, rather than it being combined with the consultee information
-
a change to some of the language in the information leaflets to be more neutral
-
an extension of the time period given between the provision of information and the taking of consent to 1 week rather than 1 day (this was returned to the original 24 hours, following application – and approval – in a subsequent amendment).
Final approval (May 2009)
-
This was following alterations to the protocol as requested above by the REC.
Amendments (February 2010–October 2011)
During the study, approval was sought and gained for a number of additional amendments as listed below.
-
In February 2010, a request was made and approved to return to the original proposal of participants having 24 hours to consider taking part.
-
In June 2010, a request was made to:
-
obtain the consent of cluster gatekeepers (CMHTOP manager) rather than individual CMHTOP staff, with the consent of people with dementia and their carers remaining the same; this was because the pragmatic CRT was designed to test the experimental intervention in a random sample of CMHTsOP stratified by NHS organisation, thus this was an improved process reflecting the CMHTOP rather than individual practitioners, and also facilitated engagement with governance requirements of some participating NHS organisations
-
add the ICECAP-O Index of Capability;257 the purpose of the ICECAP-O questionnaire was to test alternative hypotheses concerning the quality of life of both people with dementia and their carers
-
to remove the SF-12, as the EQ-5D was already included in the study and so the SF-12 was no longer felt to be necessary.
-
These requests were approved in July 2010.
-
In March 2011, a request was made and approved to change the named principal investigator at one site.
-
In October 2011, as a result of slow recruitment between August 2010 and September 2011, and our consequent inability to conduct the planned pilot study, a request was made and approved (protocol version 7.0) to change a large number of aspects of the study.
-
In July 2012, because of ongoing delays to recruitment and the view that completion was unlikely within our time scale, a site visit by the funders recommended that we discontinued the FamCare study as an intervention trial and altered the design to that of an observational cohort study. To achieve this, we designed a cohort study and submitted this with an analysis plan to the NIHR funders. We used some, but not all, changes that had been approved in October 2011 (protocol version 7.0). These were:
-
alteration of the study design so it was no longer a pragmatic cluster RCT
-
collection of data retrospectively from NHS organisation patient administration systems about the number of contacts with mental health professionals over the 6-month period in which participants were in the study
-
revision of the data analysis to take account of the change in study design; for example, multilevel modelling was no longer appropriate
-
use of the term ‘therapist’ in study documentation as a result of the move in some organisations towards generic mental health therapists or practitioners rather than staff specifically known by their professional registration
-
withdrawal of one NHS trust, to now include six NHS organisations and 28 CMHTsOP.
-
Final amendment: following National Institute for Health Research funder review of this study (October 2013)
The REC approved this final change to the protocol. It was now described as a naturalistic cohort study entitled ‘Challenge FamCare: a study of people with dementia and CBs living at home; and their carers’.
Appendix 13 The FamCare study: reasons for declining participation
The researchers recorded reasons for why 83 of the 99 (83.8%) potential participants declined (Box 10): 32% stated ‘not enough time to participate/too busy’, 19% stated ‘just don’t want to take part’ and 11% stated ‘too stressed/anxious’.
Not enough time to participate/too busy, n = 32.
Just do not want to take part, n = 19.
Too stressed/anxious, n = 11.
Do not like research, n = 5.
Unable to contact, n = 5.
Do not like answering questions, n = 4.
Family querying diagnosis, n = 4.
Illness, n = 3.
Declined, reason not specified, n = 16.
Additional researcher notes were recorded for 56 potential participants who declined, which allowed an in-depth qualitative perusal of why some people felt unable to participate in the study. Anonymised examples from the notes taken for this group are noted below. These are people who would have potentially been eligible for the CRT of the intervention for CB (see Appendix 2), had they not declined to participate. Not all 56 responses documented by researchers are included below, as some addressed similar issues and others did not provide enough detail to be described. Two types of circumstances appeared to affect carer engagement in this research study which was designed to help them manage distress and CB in dementia: first, their own psychological state, including their distress anxiety and perceived burden; and, second, the complexities of their understandings and current circumstances. These are outlined next.
Distress, anxiety and perceived time burden: examples from researcher notes
Patient diagnosed with vascular dementia by neurologist, currently experiencing an episode of high anxiety and depression, gets anxious about her forgetting – carer called CMHN to say they just don’t want anything else at the moment.
Very challenging – been very verbally and physically aggressive towards wife, has not been aggressive to nurses, presentation fluctuates, major hallucinations, dizziness, query mixed dementia/Lewy, wife very down – carer felt that they had too much going on at the moment s/he did not feel s/he wanted to take part. (Researcher suggested that s/he calls the CMHN for help after this phone call.)
Couple have had a lot of medical matters to sort recently (physiotherapy, hospital visits etc.) and feel they would not have time to participate.
CMHN offered social services; not wanting social services; carer feels that s/he has enough going on at the minute and therefore does not have the time to take part.
Daughter is too stressed due to mother’s cognitive impairment and father going through chemotherapy for cancer.
Person with dementia worried s/he will be taken into a home – refused to let us visit. Daughter very restricted on time.
Wife quite stressed, Vascular Dementia – diagnosed 3 years ago. Appeared stressed due to current family circumstances; including own illness, husband’s memory problems and her brother’s deteriorating physical health. Wife stated that she ‘wished she didn’t have to do it [the research]’ due to current stresses within the family. Researcher reassured carer that she did not have to take part.
Carer felt it was too much as she is unwell too.
Carer said she did not want to talk about her feelings she wanted ‘some action’.
Complexities of understandings and circumstances: examples from researcher notes
Carer thinks no diagnosis as yet but wanting a diagnosis and medication. Patient has expressive and receptive dysphasia and dyspraxia; gets very frustrated. CMHN says diagnosis has been given and CMHN due next visit in 3 weeks. Carer reports too busy with family matters so feels they don’t have the time to participate.
Carer tells me that problems relate to alcohol and not dementia.
Person does not believe he has dementia. Carer does not want to take part in research as may upset them.
Carer felt that she was struggling coming to terms with diagnosis, too many people wanting to visit at current time.
Living with daughter at the moment – person not accepted memory problems – do not mention dementia. Carer has a lot going on at the moment.
Carer requested contact be made through daughter to arrange interview. Spoke to her and appointments with CMHTOP cancelled, as patient has been put on meds for heart and is now 90% improved, so psychiatry not considered necessary.
Carer phoned researcher to cancel appointment – said waiting to see psychologist first and agreed to discuss again in a month; CMHN said that after they have seen psychiatrist s/he would call and let us know if they want to take part in research. Status update after 1 month: currently they have a lot going on, son is unwell with heart condition, and still not seen the psychiatrist.
Psychiatrist exploring medication options with patient and carer; CMHN due to go back in a month’s time. Patient is currently trying new medication and things up in the air at the moment.
Telephone call from carer – person with dementia to go into a care home when s/he leaves hospital.
One daughter has no transport. Other daughter away; awaiting contact from her sister on arrival back from holiday. They called – a message was left informing us that they did not wish to take part.
Carer works full time and has various other commitments and would not have the time to commit to the study.
Wife very distressed, may appear quite forgetful herself. Participant has very poor literacy skills. This case will be discussed in multi-disciplinary team meeting in 2 weeks. Called to see if interested in taking part – carer reported that she was still not sure and wouldn’t like to say no or yes. Carer very unwell – chronic obstructive pulmonary disease.
Appendix 14 The FamCare study: results of the backward regression analysis (using the data set after the 25% missing rule for predictors of change)
| Descriptive | After 25% missing rule | ||
|---|---|---|---|
| Regression coefficient | SE | 95% CI | |
| Carer’s gender | –3.59 | 1.96 | –7.43 to 0.25 |
| RMBPC reaction | 0.28 | 0.11 | 0.06 to 0.50 |
| NPI total | –0.21 | 0.08 | –0.37 to –0.05 |
| EQ-5D VAS (participant) | –0.10 | 0.06 | –0.22 to 0.02 |
| Descriptive | After 25% missing rule | ||
|---|---|---|---|
| Regression coefficient | SE | 95% CI | |
| Carer’s gender | –4.67 | 2.26 | –9.10 to –0.24 |
| Living together | –5.79 | 2.53 | –10.75 to –0.83 |
| RMBPC frequency | 0.46 | 0.14 | 0.19 to 0.73 |
| NPI total | –0.21 | 0.09 | –0.39 to –0.03 |
| Descriptive | After 25% missing rule | ||
|---|---|---|---|
| Regression coefficient | SE | 95% CI | |
| Carer’s gender | –9.73 | 3.59 | –16.77 to –2.69 |
| RMBPC incidence | 2.17 | 1.07 | 0.07 to 4.27 |
| RMBPC frequency | –0.86 | 0.33 | –1.51 to –0.21 |
| GS | 1.22 | 0.42 | 0.40 to 2.04 |
| Descriptive | After 25% missing rule | ||
|---|---|---|---|
| Regression coefficient | SE | 95% CI | |
| Carer’s gender | –5.30 | 1.81 | –8.85 to –1.75 |
| NPI incidence | 0.68 | 0.34 | 0.01 to 1.35 |
| NPID distress | –0.33 | 0.14 | –0.60 to –0.06 |
| GS | 0.60 | 0.21 | 0.19 to 1.01 |
Appendix 15 The FamCare study service use frequencies and costs for participants with data recorded at all time points
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total, minimum–maximum | Mean (SD) | ||
| District nurse | 20 (17.5) | 69 (0–23) | 0.61 (2.46) | 1628 (0–322) | 14.28 (47.46) | 1880 (0–376) | 16.49 (55.14) |
| GP | 82 (71.9) | 228 (0–12) | 2.00 (2.27) | 3084 (0–120) | 27.05 (31.24) | 15,100 (0–600) | 132.46 (153.64) |
| Practice nurse | 49 (43.0) | 92 (0–12) | 0.81 (1.50) | 1057 (0–120) | 9.27 (17.12) | 934 (0–106) | 8.19 (15.13) |
| Health visitor | 4 (3.5) | 6 (0–3) | 0.05 (0.32) | 215 (0–180) | 1.89 (16.94) | 250 (0–213) | 2.20 (20.03) |
| Community psychiatrist | 23 (20.2) | 36 (0–12) | 0.32 (1.19) | 2526 (0–1440) | 22.16 (136.44) | 12,167 (0–6936) | 106.73 (657.20) |
| Psychologist | 5 (4.4) | 6 (0–2) | 0.05 (0.26) | 330 (0–120) | 2.89 (14.92) | 748 (0–272) | 6.56 (33.81) |
| CMHN/CMHTa | 81 (71.1) | 154 (0–15) | 1.35 (1.85) | 8648 (0–600) | 75.86 (100.64) | 7639 (0–530) | 67.01 (88.90) |
| Physiotherapist | 3 (2.6) | 19 (0–15) | 0.17 (1.43) | 690 (0–450) | 6.05 (45.54) | 380 (0–248) | 3.33 (25.05) |
| Occupational therapist | 14 (12.3) | 24 (0–3) | 0.21 (0.63) | 1165 (0–180) | 10.22 (32.93) | 641 (0–99) | 5.62 (18.11) |
| Care manager | 3 (2.6) | 5 (0–3) | 0.04 (0.31) | 255 (0–180) | 2.24 (17.54) | 242 (0–171) | 2.13 (16.66) |
| Social worker | 22 (19.3) | 30 (0–3) | 0.26 (0.62) | 1635 (0–120) | 14.34 (32.89) | 2017 (0–148) | 17.69 (40.56) |
| Home care worker | 15 (13.2) | 930 (0–299) | 8.16 (38.07) | 37,573 (0–8970) | 329.59 (1 to 298.01) | 14,403 (0–3439) | 126.34 (497.57) |
| Care attendant | 6 (5.3) | 258 (0–99) | 2.26 (12.59) | 17,985 (0–10,395) | 157.76 (1036.35) | 6894 (0–3985) | 60.48 (397.27) |
| Sitting service | 4 (3.5) | 42 (0–24) | 0.37 (2.48) | 5160 (0–4320) | 45.26 (407.06) | 609 (0–348) | 5.34 (35.90) |
| Carer support worker | 2 (1.8) | 3 (0–2) | 0.03 (0.21) | 240 (0–120) | 2.11 (15.82) | 196 (0–98) | 1.72 (12.92) |
| Chiropodist | 30 (26.3) | 40 (0–3) | 0.35 (0.65) | 1270 (0–135) | 11.14 (23.71) | 635 (0–68) | 5.57 (11.85) |
| Dietitian | 2 (1.8) | 2 (0–1) | 0.02 (0.13) | 60 (0–30) | 0.53 (3.96) | 34 (0–17) | 0.30 (2.24) |
| Meals on Wheels | 4 (3.5) | 124 (0–90) | 1.09 (8.60) | 320 (0–200) | 2.81 (20.24) | 744 (0–540) | 6.53 (51.59) |
| Dentist | 19 (16.7) | 30 (0–6) | 0.26 (0.82) | 845 (0–210) | 7.41 (26.13) | 750 (0–150) | 6.58 (20.52) |
| Optician | 20 (17.5) | 25 (0–2) | 0.22 (0.51) | 965 (0–120) | 8.46 (23.25) | 525 (0–42) | 4.61 (10.73) |
| Other | 7 (6.1) | 38 (0–12) | 0.33 (1.67) | 8160 (0–3840) | 71.58 (453.07) | 2106 (0–750) | 18.47 (97.83) |
| Total | 113 (99.1) | 2161 (0–335) | 18.96 (45.44) | 93,811 (0–11,115) | 822.90 (1 to 900.26) | 68,893 (0–8217) | 604.33 (1037.61) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 3 (2.6) | 7 (0–5) | 0.06 (0.48) | 170 (0–150) | 1.49 (14.09) | 194 (0–175) | 1.70 (16.42) |
| GP | 60 (52.6) | 116 (0–6) | 1.02 (1.31) | 2136 (0–360) | 18.74 (40.22) | 10,600 (0–1800) | 92.98 (200.93) |
| Practice nurse | 28 (24.6) | 37 (0–4) | 0.32 (0.67) | 543 (0–80) | 4.76 (11.42) | 480 (0–71) | 4.21 (10.09) |
| Psychologist | 2 (1.8) | 9 (0–6) | 0.08 (0.63) | 480 (0–300) | 4.21 (32.64) | 1088 (0–680) | 9.54 (73.98) |
| CMHN/CMHTa | 3 (2.6) | 6 (0–3) | 0.05 (0.40) | 480 (0–360) | 4.21 (34.54) | 424 (0–318) | 3.72 (30.51) |
| Physiotherapist | 4 (3.5) | 5 (0–2) | 0.04 (0.24) | 160 (0–60) | 1.40 (7.74) | 88 (0–33) | 0.77 (4.26) |
| Occupational therapist | 2 (1.3) | 4 (0–3) | 0.04 (0.30) | 120 (0–90) | 1.05 (8.86) | 66 (0–50) | 0.58 (4.87) |
| Social worker | 4 (3.5) | 8 (0–3) | 0.07 (0.41) | 1020 (0–900) | 8.95 (84.47) | 1258 (0–1110) | 11.04 (104.18) |
| Home care worker | 1 (0.9) | 24 (0–24) | 0.21 (2.25) | 1728 (0–1728) | 15.16 (161.84) | 662 (0–662) | 5.81 (62.04) |
| Carer support worker | 2 (1.3) | 2 (0–1) | 0.02 (0.13) | 180 (0–120) | 1.58 (12.52) | 147 (0–98) | 1.29 (10.23) |
| Chiropodist | 7 (6.1) | 13 (0–5) | 0.11 (0.56) | 415 (0–120 | 3.64 (17.68) | 208 (0–60) | 1.82 (8.84) |
| Dietitian | 1 (0.9) | 6 (0–6) | 0.05 (0.56) | 180 (0–180) | 1.58 (16.86) | 102 (0–102) | 0.89 (9.55) |
| Dentist | 24 (21.1) | 33 (0–4) | 0.29 (0.66) | 710 (0–80) | 6.23 (13.90) | 825 (0–100) | 7.24 (16.54) |
| Optician | 13 (11.4) | 13 (0–1) | 0.11 (0.32) | 350 (0–60) | 3.07 (9.72) | 325 (0–25) | 2.85 (7.98) |
| Other | 1 (0.9) | 3 (0–3) | 0.03 (0.28) | 60 (0–60) | 0.53 (5.62) | 12 (0–12) | 0.11 (1.12) |
| Total | 86 (75.4) | 288 (0–30) | 2.53 (3.60) | 8852 (0–1833) | 77.65 (208.46) | 16,479 (0–1895) | 144.55 (261.85) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 20 (17.5) | 57 (0–11) | 0.50 (1.56) | 1030 (0–270) | 9.04 (32.01) | 1172 (0–315) | 10.28 (37.20) |
| GP | 49 (43.0) | 91 (0–11) | 0.80 (1.56) | 1435 (0–165) | 12.59 (22.74) | 6840 (0–825) | 60.00 (110.27) |
| Practice nurse | 35 (30.7) | 49 (0–4) | 0.43 (0.82) | 615 (0–60) | 5.39 (10.81) | 543 (0–53) | 4.77 (9.55) |
| Community psychiatrist | 21 (18.4) | 25 (0–2) | 0.22 (0.49) | 1075 (0–120) | 9.43 (22.31) | 5178 (0–578) | 45.42 (107.45) |
| Psychologist | 6 (5.3) | 10 (0–3) | 0.09 (0.41) | 1065 (0–360) | 9.34 (46.73) | 2414 (0–816) | 21.18 (105.93) |
| CMHN/CMHTa | 41 (36.0) | 97 (0–8) | 0.85 (1.40) | 5255 (0–960) | 46.10 (107.79) | 4637 (0–848) | 40.67 (95.23) |
| Physiotherapist | 7 (6.1) | 23 (0–8) | 0.20 (1.07) | 1120 (0–480) | 9.82 (57.18) | 616 (0–264) | 5.40 (31.45) |
| Occupational therapist | 10 (8.8) | 11 (0–2) | 0.10 (0.33) | 545 (0–240) | 4.78 (24.26) | 300 (0–132) | 2.63 (13.34) |
| Care manager | 3 (2.6) | 8 (0–6) | 0.07 (0.58) | 690 (0–360) | 6.05 (42.27) | 656 (0–342) | 5.75 (40.16) |
| Social worker | 18 (15.8) | 30 (0–4) | 0.26 (0.72) | 1885 (0–540) | 16.54 (61.45) | 2325 (0–666) | 20.39 (75.79) |
| Home care worker | 13 (11.4) | 604 (0–120) | 5.30 (20.90) | 21,630 (0–3600) | 189.74 (685.51) | 8292 (0–1380) | 72.73 (262.78) |
| Care attendant | 9 (7.9) | 182 (0–56) | 1.60 (7.73) | 11,905 (0–2880) | 104.43 (458.55) | 4564 (0–1104) | 40.03 (175.78) |
| Sitting service | 4 (3.5) | 64 (0–24) | 0.56 (3.14) | 11,280 (0–4800) | 98.95 (616.50) | 928 (0–348) | 8.14 (45.57) |
| Carer support worker | 2 (1.8) | 172 (0–168) | 1.51 (15.74) | 5160 (0–5040) | 45.26 (472.07) | 4214 (0–4116) | 36.96 (385.53) |
| Chiropodist | 23 (20.2) | 27 (0–2) | 0.24 (0.50) | 810 (0–120) | 7.11 (19.22) | 405 (0–60) | 3.55 (9.61) |
| Dietitian | 3 (2.6) | 3 (0–1) | 0.03 (0.16) | 55 (0–30) | 0.48 (3.26) | 31 (0–17) | 0.27 (1.85) |
| Meals on wheels | 1 (0.9) | 56 (0–56) | 0.49 (5.24) | 280 (0–280) | 16.51 (162.09) | 336 (0–336) | 2.95 (31.47) |
| Dentist | 16 (14.0) | 17 (0–2) | 0.15 (0.38) | 390 (0–60) | 3.42 (10.57) | 425 (0–50) | 3.73 (9.54) |
| Optician | 11 (9.6) | 11 (0–1) | 0.10 (0.30) | 350 (0–60) | 3.07 (10.59) | 231 (0–21) | 2.03 (6.23) |
| Other | 5 (4.4) | 6 (0–2) | 0.05 (0.26) | 260 (0–120) | 2.28 (12.95) | 406 (0–180) | 3.56 (20.34) |
| Total | 109 (95.6) | 1543 (0–187) | 13.54 (28.63) | 66,835 (0–6460) | 586.27 (1–235.77) | 44,511 (0–4469) | 390.45 (580.76) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 7 (6.1) | 10 (0–3) | 0.09 (0.39) | 160 (0–45) | 1.40 (6.26) | 161 (0–44) | 1.41 (6.31) |
| GP | 44 (38.6) | 66 (0–4) | 0.58 (0.85) | 1131 (0–120) | 9.92 (18.60) | 5505 (0–480) | 48.29 (86.97) |
| Practice nurse | 23 (20.2) | 27 (0–3) | 0.24 (0.52) | 390 (0–40) | 3.42 (8.05) | 345 (0–35) | 3.02 (7.11) |
| Psychologist | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 60 (0–60) | 0.53 (5.62) | 136 (0–136) | 1.19 (12.74) |
| CMHN/CMHTa | 3 (2.6) | 5 (0–3) | 0.04 (0.31) | 315 (0–150) | 2.76 (18.99) | 278 (0–133) | 2.44 (16.78) |
| Physiotherapist | 4 (3.5) | 8 (0–3) | 0.07 (0.41) | 260 (0–90) | 2.28 (13.17) | 143 (0–50) | 1.25 (7.24) |
| Care manager | 3 (2.6) | 4 (0–2) | 0.04 (0.23) | 150 (0–60) | 1.32 (8.36) | 143 (0–57) | 1.25 (7.94) |
| Social worker | 3 (2.6) | 7 (0–3) | 0.06 (0.41) | 330 (0–180) | 3.10 (19.29) | 407 (0–222) | 3.57 (24.10) |
| Carer support worker | 2 (1.8) | 9 (0–8) | 0.08 (0.75) | 1005 (0–960) | 8.82 (89.97) | 821 (0–784) | 7.20 (73.48) |
| Chiropodist | 10 (8.8) | 12 (0–2) | 0.11 (0.36) | 465 (0–120) | 4.08 (16.66) | 233 (0–60) | 2.04 (8.33) |
| Dentist | 22 (19.2) | 25 (0–2) | 0.71 (0.62) | 575 (0–90) | 5.04 (13.38) | 625 (0–50) | 5.48 (11.88) |
| Optician | 10 (8.8) | 15 (0–4) | 0.13 (0.51) | 550 (0–150) | 4.82 (20.22) | 315 (0–84) | 2.76 (10.64) |
| Other | 5 (4.4) | 10 (0–3) | 0.09 (0.45) | 735 (0–300) | 6.45 (36.90) | 1447 (0–1200) | 12.69 (112.83) |
| Total | 78 (68.4) | 199 (0–16) | 1.75 (2.38) | 6126 (0–1335) | 53.74 (137.69) | 10,557 (0–1450) | 92.61 (205.64) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 16 (14.0) | 216 (0–116) | 1.89 (12.19) | 3200 (0–1740) | 28.07 (173.29) | 3715 (0–2030) | 32.59 (202.16) |
| GP | 72 (63.2) | 198 (0–20) | 1.74 (2.70) | 3075 (0–240) | 26.97 (44.10) | 14,305 (0–1140) | 125.48 (201.64) |
| Practice nurse | 40 (35.1) | 67 (0–8) | 0.59 (1.08) | 1000 (0–80) | 8.77 (16.12) | 883 (0–71) | 7.75 (14.24) |
| Health visitor | 20 (17.5) | 25 (0–3) | 0.22 (0.53) | 1175 (0–240) | 10.31 (29.82) | 1204 (0–236) | 10.57 (30.20) |
| Community psychiatrist | 3 (2.6) | 3 (0–1) | 0.03 (0.16) | 150 (0–60) | 1.32 (8.36) | 723 (0–289) | 6.34 (40.28) |
| Psychologist | 1 (0.9) | 4 (0–4) | 0.04 (0.37) | 80 (0–80) | 0.70 (7.49) | 181 (0–181) | 1.59 (16.98) |
| CMHN/CMHTa | 40 (35.1) | 160 (0–38) | 1.40 (3.91) | 9905 (0–4560) | 86.89 (431.58) | 7049 (0–2328) | 61.84 (227.60) |
| Physiotherapist | 7 (6.1) | 141 (0–120) | 1.24 (11.26) | 7875 (0–7200) | 69.08 (674.48) | 4331 (0–3960) | 37.99 (370.97) |
| Occupational therapist | 8 (7.0) | 17 (0–5) | 0.15 (0.68) | 740 (0–300) | 6.49 (34.13) | 407 (0–165) | 3.57 (18.77) |
| Care manager | 1 (0.9) | 4 (0–4) | 0.04 (0.37) | 180 (0–180) | 1.58 (16.86) | 171 (0–171) | 1.50 (16.02) |
| Social worker | 15 (13.2) | 32 (0–8) | 0.28 (0.98) | 1560 (0–480) | 13.68 (54.31) | 1924 (0–592) | 16.88 (66.98) |
| Home care worker | 22 (19.3) | 2515 (0–448) | 22.06 (71.20) | 90,955 (0–21,600) | 797.85 (2 to 659.59) | 34,866 (0–8280) | 305.84 (1019.51) |
| Care attendant | 6 (5.3) | 449 (0–160) | 3.94 (20.89) | 41,400 (0–14,400) | 363.16 (1 to 872.04) | 15,870 (0–5520) | 139.21 (717.62) |
| Sitting service | 2 (1.8) | 46 (0–30) | 0.40 (3.17) | 4560 (0–3600) | 40.00 (348.18) | 667 (0–435) | 5.85 (46.00) |
| Carer support worker | 2 (1.8) | 25 (0–24) | 0.22 (2.25) | 1470 (0–1440) | 12.89 (134.87) | 1201 (0–1176) | 10.53 (110.15) |
| Chiropodist | 33 (28.9) | 53 (0–4) | 0.46 (0.92) | 2400 (0–960) | 21.05 (93.14) | 1200 (0–480) | 10.53 (46.57) |
| Dietitian | 3 (2.6) | 3 (0–3) | 0.03 (0.16) | 60 (0–60) | 0.53 (3.22) | 34 (0–34) | 0.30 (1.82) |
| Meals on wheels | 2 (1.8) | 92 (0–84) | 0.81 (7.90) | 500 (0–420) | 4.39 (39.98) | 552 (0–504) | 4.84 (47.38) |
| Dentist | 25 (21.9) | 33 (0–3) | 0.29 (0.61) | 950 (0–160) | 8.33 (21.89) | 825 (0–75) | 7.24 (15.14) |
| Optician | 18 (15.8) | 22 (0–3) | 0.19 (0.50) | 760 (0–90) | 6.67 (17.99) | 462 (0–63) | 4.05 (10.41) |
| Total | 108 (4.7) | 4105 (0–53) | 36.01 (77.03) | 171,995 (0–21,600) | 1508.73 (3–442.68) | 90,571 (0–8280) | 794.48 (1433.86) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 5 (4.4) | 11 (0–6) | 0.10 (0.61) | 170 (0–90) | 1.49 (9.33) | 176 (0–87) | 1.55 (9.38) |
| GP | 60 (52.6) | 136 (0–19) | 1.19 (2.17) | 2280 (0–380) | 20.00 (42.10) | 11,075 (0–1660) | 97.15 (192.35) |
| Practice nurse | 32 (28.1) | 48 (0–5) | 0.42 (0.86) | 707 (0–75) | 6.20 (13.42) | 625 (0–66) | 5.48 (11.85) |
| Psychologist | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 60 (0–60) | 0.53 (5.62) | 136 (0–136) | 1.19 (12.74) |
| Counsellor | 1 (0.9) | 14 (0–14) | 0.12 (1.31) | 840 (0–840) | 7.37 (78.67) | 910 (0–910) | 7.98 (85.23) |
| CMHN/CMHTa | 7 (6.1) | 18 (0–8) | 0.16 (0.87) | 1190 (0–480) | 10.44 (54.24) | 1051 (0–424) | 9.22 (47.91) |
| Physiotherapist | 7 (6.1) | 25 (0–16) | 0.22 (1.54) | 1020 (0–720) | 8.95 (68.75) | 561 (0–396) | 4.92 (37.81) |
| Occupational therapist | 1 (0.9) | 2 (0–2) | 0.02 (0.19) | 120 (0–120) | 1.05 (11.24) | 66 (0–66) | 0.58 (6.18) |
| Social worker | 5 (4.4) | 5 (0–1) | 0.04 (0.21) | 345 (0–150) | 3.03 (16.72) | 426 (0–185) | 3.73 (20.62) |
| Home care worker | 1 (0.9) | 14 (0–14) | 0.12 (1.31) | 420 (0–420) | 3.68 (39.34) | 161 (0–161) | 1.41 (15.08) |
| Chiropodist | 9 (7.9) | 17 (0–4) | 0.15 (0.58) | 455 (0–180) | 3.99 (20.94) | 228 (0–90) | 2.00 (10.47) |
| Dietitian | 2 (1.8) | 2 (0–1) | 0.02 (0.13) | 35 (0–20) | 0.31 (2.33) | 20 (0–11) | 0.17 (1.32) |
| Dentist | 27 (23.7) | 35 (0–3) | 0.31 (0.64) | 1085 (0–240) | 9.52 (32.51) | 875 (0–75) | 7.68 (15.99) |
| Optician | 10 (8.8) | 12 (0–2) | 0.11 (0.36) | 315 (0–60) | 2.76 (9.89) | 252 (0–42) | 2.21 (7.58) |
| Other | 2 (1.8) | 4 (0–3) | 0.04 (0.30) | 585 (0–540) | 5.13 (50.71) | 333 (0–180) | 2.92 (22.03) |
| Total | 85 (74.6) | 344 (0–26) | 3.02 (4.54) | 9627 (0–1045) | 84.45 (162.32) | 16,894 (0–1673) | 148.19 (238.44) |
| Service | n (%) | Day care use | |||
|---|---|---|---|---|---|
| Frequency | Cost (£) | ||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| Day care: local authority | 13 (11.4) | 196 (0–40) | 1.72 (6.38) | 7840 (0–1600) | 68.77 (225.35) |
| Day care: voluntary | 5 (4.4) | 70 (0–32) | 0.61 (3.81) | 1260 (0–576) | 11.05 (68.59) |
| Day care: NHS (not hospital) | 5 (4.4) | 56 (0–24) | 0.49 (2.78) | 2240 (0–960) | 19.65 (111.02) |
| Lunch club | 5 (4.4) | 39 (0–20) | 0.34 (2.20) | 124 (0–63) | 1.08 (6.98) |
| Social club | 10 (8.8) | 77 (0–12) | 0.68 (2.61) | 244 (0–38) | 2.14 (8.27) |
| Total | 34 (29.8) | 438 (0–40) | 3.84 (8.4) | 11,708 (0–1600) | 102.70 (278.20) |
| Service | n (%) | Day care use | |||
|---|---|---|---|---|---|
| Frequency | Cost (£) | ||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| Day care: local authority | 13 (11.4) | 204 (0–24) | 1.79 (5.58) | 8160 (0–960) | 71.58 (223.34) |
| Day care: voluntary | 8 (7.0) | 59 (0–16) | 0.52 (2.42) | 1062 (0–288) | 9.32 (43.52) |
| Day care: NHS (not hospital) | 2 (1.8) | 22 (0–16) | 0.19 (1.60) | 880 (0–640) | 7.72 (63.83) |
| Lunch club | 4 (3.5) | 18 (0–12) | 0.16 (1.16) | 57 (0–38) | 0.50 (3.69) |
| Social club | 7 (6.1) | 74 (0–24) | 0.65 (3.04) | 235 (0–76) | 2.06 (9.63) |
| Total | 33 (28.9) | 377 (0–36) | 3.31 (6.94) | 10,394 (0–960) | 91.17 (230.09) |
| Service | n (%) | Day care use | |||
|---|---|---|---|---|---|
| Frequency | Cost (£) | ||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| Day care: local authority | 19 (16.7) | 428 (0–64) | 3.79 (11.78) | 17,680 (0–2560) | 155.09 (470.68) |
| Day care: voluntary | 3 (2.6) | 58 (0–32) | 0.51 (3.73) | 1044 (0–576) | 9.16 (67.19) |
| Day care: NHS (not hospital) | 3 (2.6) | 12 (0–6) | 0.10 (0.70) | 480 (0–240) | 4.21 (27.84) |
| Lunch club | 5 (4.4) | 24 (0–8) | 0.21 (1.14) | 76 (0–25) | 0.67 (3.61) |
| Social club | 6 (5.3) | 126 (0–36) | 1.11 (5.46) | 399 (0–114) | 3.50 (17.31) |
| Total | 35 (30.7) | 662 (0–64) | 5.81 (13.01) | 19,680 (0–2560) | 172.63 (470.96) |
| Service | Hospital use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person with dementia | Carer | |||||||||
| n (%) | Frequency | Cost (£) | n (%) | Frequency | Cost (£) | |||||
| Total, minimum–maximuma | Mean (SD) | Total, minimum–maximum | Mean (SD) | Total, minimum–maximum | Mean (SD) | Total, minimum–maximum | Mean (SD) | |||
| Assessment/rehabilitation inpatient ward | 5 (4.4) | 30 (0–21) | 0.26 (2.03) | 9240 (0–6468) | 81.05 (624.19) | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 308 (0–308) | 2.70 (28.85) |
| Continuing care/respite inpatient ward | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 327 (0–327) | 2.87 (30.63) | 0 | 0 | 0 | 0 | 0 |
| Medical inpatient ward | 6 (5.7) | 41 (0–14) | 0.36 (1.89) | 37,679 (0–12,866) | 330.52 (1738.22) | 4 (3.5) | 12 (0–7) | 0.11 (0.72) | 11,028 (0–6433) | 96.74 (662.57) |
| Other inpatient ward | 2 (1.8) | 21 (0–14) | 0.18 (1.46) | 19,299 (0–12,866) | 169.29 (1342.47) | 1 (0.9) | 3 (0–3) | 0.03 (0.28) | 2757 (0–2757) | 24.18 (258.22) |
| Outpatient service | 25 (21.9) | 71 (0–15) | 0.62 (2.17) | 2130 (0–450) | 18.68 (65.02) | 21 (18.4) | 34 (0–4) | 0.30 (0.77) | 1050 (0–120) | 9.21 (22.97) |
| A&E | 11 (9.6) | 18 (0–7) | 0.16 (0.72) | 1638 (0–637) | 14.37 (65.84) | 5 (4.4) | 6 (0–2) | 0.05 (0.26) | 546 (0–182) | 4.79 (23.73) |
| Day hospital | 5 (4.4) | 21 (0–15) | 0.18 (1.43) | 2898 (0–2070) | 25.42 (197.36) | 4 (3.5) | 6 (0–2) | 0.05 (0.29) | 828 (0–276) | 7.26 (40.40) |
| Total | 41 (36.0) | – | – | 73,211 (0–12,866) | 642.20 (2261.26) | 34 (29.8) | – | – | 16,517 (0–6433) | 144.89 (707.15) |
| Service | Hospital use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person with dementia | Carer | |||||||||
| n (%) | Frequency | Cost (£) | n (%) | Frequency | Cost (£) | |||||
| Total (minimum–maximum)a | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | |||
| Assessment/rehabilitation inpatient ward | 8 (7.0) | 9 (0–2) | 0.08 (0.30) | 2772 (0–616) | 24.32 (92.94) | 3 (2.6) | 4 (0–2) | 0.04 (0.23) | 1232 (0–616) | 10.81 (70.14) |
| Continuing care/respite inpatient ward | 4 (3.5) | 78 (0–56) | 0.68 (5.43) | 25,506 (0–18,312) | 223.74 (1774.77) | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 327 (0–327) | 2.87 (30.63) |
| Medical inpatient ward | 2 (1.8) | 17 (0–16) | 0.15 (1.50) | 15,623 (0–14,704) | 137.04 (1379.08) | 1 (0.9) | 4 (0–4) | 0.04 (0.37) | 3676 (0–3676) | 32.25 (344.29) |
| Other inpatient ward | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Outpatient service | 23 (20.2) | 32 (0–4) | 0.28 (0.65) | 960 (0–120) | 8.42 (19.35) | 14 (12.3) | 30 (0–15) | 0.26 (1.45) | 900 (0–450) | 7.89 (43.55) |
| A&E | 4 (3.5) | 5 (0–2) | 0.04 (0.24) | 455 (0–182) | 3.99 (22.29) | 2 (1.8) | 11 (0–10) | 0.10 (0.94) | 1001 (0–910) | 8.78 (85.58) |
| Day hospital | 5 (4.4) | 19 (0–14) | 0.17 (1.33) | 2622 (0–1932) | 23.00 (183.52) | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 138 (0–138) | 1.21 (12.92) |
| Total | 41 (36.0) | – | – | 47,938 (0–18,312) | 420.51 (2234.44) | 20 (17.5) | – | – | 7274 (0–3767) | 63.81 (370.11) |
| Service | Hospital use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person with dementia | Carer | |||||||||
| n (%) | Frequency | Cost (£) | n (%) | Frequency | Cost (£) | |||||
| Total (minimum–maximum)a | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | |||
| Assessment/rehabilitation inpatient ward | 4 (3.5) | 7 (0–4) | 0.06 (0.41) | 2156 (0–1232) | 18.91 (124.86) | 7 (6.1) | 8 (0–2) | 0.07 (0.29) | 2464 (0–616) | 21.61 (89.02) |
| Continuing care/respite inpatient ward | 2 (1.8) | 8 (0–7) | 0.07 (0.66) | 2616 (0–2289) | 22.95 (216.29) | 0 | 0 | 0 | 0 | 0 |
| Medical inpatient ward | 3 (2.6) | 30 (0–21) | 0.26 (2.07) | 27,570 (0–19,299) | 241.84 (1906.08) | 6 (5.3) | 38 (0–14) | 0.33 (1.90) | 34,922 (0–12,866) | 306.33 (1749.10) |
| Other inpatient ward | 4 (3.5) | 31 (0–14) | 0.27 (1.76) | 28,489 (0–12,866) | 249.90 (1614.07) | 1 (0.9) | 7 (0–7) | 0.06 (0.66) | 6433 (0–6433) | 56.43 (602.51) |
| Outpatient service | 30 (26.3) | 78 (0–20) | 0.68 (2.68) | 2340 (0–600) | 20.53 (80.53) | 25 (21.9) | 69 (0–30) | 0.61 (2.88) | 2070 (0–900) | 18.16 (86.31) |
| A&E | 11 (9.6) | 16 (0–3) | 0.14 (0.48) | 1456 (0–273) | 12.77 (43.44) | 4 (3.5) | 4 (0–4) | 0.04 (0.18) | 364 (0–91) | 3.19 (16.82) |
| Day hospital | 4 (3.5) | 18 (0–14) | 0.15 (1.33) | 2484 (0–1932) | 21.79 (183.21) | 2 (1.8) | 2 (0–2) | 0.02 (0.13) | 276 (0–138) | 2.42 (18.20) |
| Total | 40 (35.1) | – | – | 67,111 (0–19,390) | 588.69 (2508.48) | 36 (31.6) | – | – | 46,529 (0–12,926) | 408.15 (1865.19) |
Appendix 16 The FamCare study service use frequencies and costs for all participants available at each of the individual time points
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 37 (23.6) | 136 (0–23) | 0.87 (2.91) | 2438 (0–322) | 15.53 (44.81) | 2803 (0–376) | 17.86 (52.04) |
| GP | 118 (75.2) | 333 (0–13) | 2.12 (2.47) | 4924 (0–390) | 31.36 (46.37) | 23,435 (0–1650) | 149.27 (209.97) |
| Practice nurse | 64 (40.8) | 161 (0–50) | 1.03 (4.16) | 1797 (0–500) | 11.45 (42.29) | 1587 (0–442) | 10.11 (37.36) |
| Health visitor | 5 (3.2) | 7 (0–3) | 0.04 (0.29) | 220 (0–180) | 1.40 (14.44) | 256 (0–213) | 1.63 (17.08) |
| Community psychiatrist | 31 (19.7) | 47 (0–12) | 0.30 (1.06) | 3036 (0–1440) | 19.34 (117.46) | 14,623 (0–6936) | 93.14 (565.76) |
| Psychologist | 7 (4.5) | 9 (0–2) | 0.06 (0.28) | 510 (0–120) | 3.25 (16.49) | 1156 (0–272) | 7.36 (37.39) |
| CMHN/CMHTa | 112 (71.3) | 217 (0–15) | 1.38 (1.89) | 11,738 (0–600) | 74.76 (93.73) | 10,369 (0–530) | 66.04 (82.79) |
| Physiotherapist | 4 (2.5) | 22 (0–15) | 0.14 (1.24) | 870 (0–450) | 5.54 (41.30) | 479 (0–248) | 3.05 (22.71) |
| Occupational therapist | 18 (11.5) | 30 (0–3) | 0.19 (0.59) | 1505 (0–180) | 9.59 (31.42) | 828 (0–99) | 5.27 (17.28) |
| Care manager | 6 (3.8) | 11 (0–3) | 0.07 (0.39) | 495 (0–180) | 3.15 (20.87) | 470 (0–171) | 3.00 (19.83) |
| Social worker | 34 (21.7) | 141 (0–84) | 0.90 (6.75) | 6585 (0–2520) | 41.94 (20.77) | 8122 (0–3108) | 51.73 (272.28) |
| Home care worker | 24 (15.3) | 2013 (0–299) | 12.82 (46.12) | 75,488 (0–12,600) | 480.82 (1–714.19) | 28,937 (0–4830) | 184.31 (657.11) |
| Care attendant | 7 (4.5) | 282 (0–99) | 1.80 (10.91) | 20,145 (0–10,395) | 128.31 (899.73) | 7722 (0–3985) | 49.19 (344.90) |
| Sitting service | 6 (3.8) | 87 (0–39) | 0.55 (3.77) | 25,029 (0–19,149) | 159.42 (1–565.19) | 1262 (0–566) | 8.04 (54.66) |
| Carer support worker | 6 (3.8) | 255 (0–168) | 1.62 (14.06) | 6480 (0–3360) | 41.27 (301.29) | 5292 (0–2744) | 33.71 (246.06) |
| Chiropodist | 46 (29.3) | 69 (0–10) | 0.44 (1.01) | 2490 (0–600) | 15.86 (53.75) | 1245 (0–300) | 7.93 (26.88) |
| Dietitian | 3 (1.9) | 3 (0–3) | 0.02 (0.14) | 80 (0–30) | 0.51 (3.72) | 45 (0–17) | 0.29 (2.11) |
| Meals on wheels | 9 (5.7) | 472 (0–90) | 3.01 (15.12) | 2570 (0–900) | 16.37 (92.61) | 2832 (0–540) | 18.04 (90.71) |
| Dentist | 21 (13.4) | 32 (0–6) | 0.20 (0.71) | 885 (0–210) | 5.64 (22.56) | 800 (0–150) | 5.10 (17.84) |
| Optician | 24 (15.3) | 30 (0–2) | 0.19 (0.48) | 1139 (0–120) | 7.25 (21.10) | 630 (0–42) | 4.01 (10.13) |
| Other | 9 (5.7) | 42 (0–12) | 0.27 (1.45) | 8390 (0–3840) | 53.44 (387.00) | 2420 (0–750) | 15.41 (86.41) |
| Total | 156 (99.4) | 4399 (0–343) | 28.02 (59.91) | 176,814 (0–27,429) | 1,126.20 (2–967.73) | 115,313 (0–8217) | 734.48 (1150.64) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 7 (4.5) | 18 (0–8) | 0.11 (0.78) | 565 (0–360) | 3.60 (31.09) | 652 (0–420) | 4.15 (36.26) |
| GP | 78 (49.7) | 167 (0–21) | 1.06 (2.03) | 2871 (0–360) | 18.29 (39.28) | 14,215 (0–1800) | 90.54 (195.91) |
| Practice nurse | 37 (23.6) | 47 (0–4) | 0.30 (0.62) | 633 (0–80) | 4.03 (10.09) | 559 (0–71) | 3.56 (8.91) |
| Psychologist | 3 (1.9) | 10 (0–6) | 0.06 (0.54) | 510 (0–300) | 3.25 (27.92) | 1156 (0–680) | 7.36 (63.30) |
| CMHN/CMHTa | 3 (1.9) | 6 (0–4) | 0.04 (0.34) | 480 (0–360) | 3.06 (29.45) | 424 (0–318) | 2.70 (26.02) |
| Physiotherapist | 6 (3.8) | 14 (0–8) | 0.09 (0.67) | 670 (0–480) | 4.27 (38.85) | 369 (0–264) | 2.35 (21.37) |
| Occupational therapist | 2 (1.3) | 4 (0–3) | 0.03 (0.25) | 120 (0–90) | 0.76 (7.56) | 66 (0–50) | 0.42 (4.16) |
| Social worker | 5 (3.2) | 92 (0–84) | 0.59 (6.71) | 3540 (0–2520) | 22.55 (213.13) | 4366 (0–3108) | 27.81 (262.86) |
| Home care worker | 1 (0.6) | 24 (0–24) | 0.15 (1.92) | 1728 (0–1728) | 11.01 (137.91) | 662 (0–662) | 4.22 (52.87) |
| Carer support worker | 3 (1.9) | 3 (0–1) | 0.02 (0.14) | 240 (0–120) | 1.53 (11.67) | 196 (0–98) | 1.25 (9.53) |
| Chiropodist | 8 (5.1) | 14 (0–5) | 0.09 (0.49) | 445 (0–120) | 2.83 (15.29) | 223 (0–60) | 1.42 (7.64) |
| Dietitian | 1 (0.6) | 6 (0–6) | 0.04 (0.48) | 180 (0–180) | 1.15 (14.37) | 102 (0–102) | 0.65 (8.14) |
| Meals on wheels | 1 (0.6) | 12 (0–12) | 0.08 (0.96) | 120 (0–120) | 0.76 (9.58) | 72 (0–72) | 0.46 (5.75) |
| Dentist | 30 (19.1) | 51 (0–4) | 0.32 (0.83) | 1139 (0–120) | 7.25 (19.35) | 1275 (0–100) | 8.12 (20.84) |
| Optician | 17 (10.8) | 18 (0–2) | 0.11 (0.34) | 580 (0–120) | 3.69 (13.61) | 450 (0–50) | 2.87 (8.48) |
| Other | 2 (1.3) | 11 (0–8) | 0.07 (0.68) | 300 (0–240) | 1.91 (19.71) | 308 (0–240) | 1.96 (19.87) |
| Total | 114 (72.6) | 499 (0–100) | 3.18 (8.61) | 14,241 (0–2775) | 90.71 (285.37) | 25,095 (0–3487) | 159.84 (362.90) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 23 (18.3) | 74 (0–14) | 0.59 (1.92) | 1525 (0–420) | 12.10 (47.91) | 1737 (0–490) | 13.79 (55.73) |
| GP | 54 (42.9) | 104 (0–11) | 0.83 (1.61) | 1635 (0–165) | 12.98 (23.50) | 7700 (0–825) | 61.11 (111.36) |
| Practice nurse | 38 (30.2) | 82 (0–30) | 0.65 (2.75) | 1130 (0–450) | 8.97 (41.23) | 998 (0–398) | 7.92 (36.42) |
| Health visitor | 1 (0.8) | 1 (0–1) | 0.01 (0.09) | 30 (0–30) | 0.24 (2.67) | 36 (0–36) | 0.28 (3.16) |
| Community psychiatrist | 21 (16.7) | 25 (0–2) | 0.20 (0.47) | 1075 (0–120) | 8.53 (21.39) | 5178 (0–578) | 41.09 (103.04) |
| Psychologist | 6 (4.8) | 10 (0–3) | 0.08 (0.39) | 1065 (0–360) | 8.45 (44.52) | 2414 (0–816) | 19.16 (100.91) |
| CMHN/CMHTa | 46 (36.5) | 112 (0–8) | 0.89 (1.52) | 5875 (0–960) | 46.63 (106.95) | 5184 (0–848) | 41.14 (94.48) |
| Physiotherapist | 7 (5.6) | 23 (0–8) | 0.18 (1.02) | 1120 (0–480) | 8.89 (54.44) | 616 (0–264) | 4.89 (29.94) |
| Occupational therapist | 11 (8.7) | 12 (0–2) | 0.10 (0.32) | 605 (0–240) | 4.80 (23.63) | 333 (0–132) | 2.64 (13.00) |
| Care manager | 4 (3.2) | 9 (0–6) | 0.07 (0.55) | 750 (0–360) | 5.95 (40.52) | 713 (0–342) | 5.65 (38.49) |
| Social worker | 22 (17.5) | 34 (0–4) | 0.27 (0.70) | 2135 (0–540) | 16.94 (59.44) | 2633 (0–666) | 20.90 (73.31) |
| Home care worker | 16 (12.7) | 956 (0–152) | 7.59 (27.07) | 36,270 (0–6840) | 287.86 (996.59) | 13,904 (0–2622) | 110.35 (382.03) |
| Care attendant | 10 (7.9) | 186 (0–56) | 1.48 (7.37) | 12,385 (0–2880) | 98.29 (438.33) | 4748 (0–1104) | 37.68 (168.03) |
| Sitting service | 5 (4.0) | 72 (0–24) | 0.57 (3.06) | 11,520 (0–4800) | 91.43 (586.98) | 1044 (0–348) | 8.29 (44.45) |
| Carer support worker | 4 (3.2) | 183 (0–168) | 1.45 (14.98) | 6300 (0–5040) | 50.00 (456.55) | 5145 (0–4116) | 40.83 (372.85) |
| Chiropodist | 26 (20.6) | 30 (0–2) | 0.24 (0.50) | 950 (0–120) | 7.54 (19.58) | 475 (0–60) | 3.77 (9.79) |
| Dietitian | 3 (2.4) | 3 (0–1) | 0.02 (0.15) | 55 (0–30) | 0.44 (3.10) | 31 (0–17) | 0.25 (1.76) |
| Meals on wheels | 2 (1.6) | 116 (0–60) | 0.92 (7.28) | 2080 (0–1800) | 16.51 (162.09) | 696 (0–360) | 5.52 (43.69) |
| Dentist | 16 (12.7) | 17 (0–2) | 0.13 (0.37) | 390 (0–60) | 3.10 (10.10) | 425 (0–50) | 3.37 (9.14) |
| Optician | 14 (11.1) | 14 (0–1) | 0.11 (0.32) | 560 (0–90) | 4.44 (14.58) | 294 (0–21) | 2.33 (6.63) |
| Other | 5 (4.0) | 6 (0–2) | 0.05 (0.25) | 260 (0–120) | 2.06 (12.33) | 406 (0–180) | 3.22 (19.36) |
| Total | 120 (95.2) | 2069 (0–188) | 16.42 (35.08) | 87,715 (0–9090) | 696.15 (1–511.27) | 54,709 (0–4469) | 434.20 (685.14) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 7 (5.6) | 10 (0–3) | 0.08 (0.37) | 160 (0–45) | 1.27 (5.96) | 161 (0–44) | 1.28 (6.02) |
| GP | 49 (38.9) | 75 (0–4) | 0.60 (0.86) | 1250 (0–120) | 9.92 (18.11) | 6100 (0–480) | 48.41 (84.96) |
| Practice nurse | 24 (19.0) | 28 (0–3) | 0.22 (0.50) | 400 (0–40) | 3.17 (7.74) | 353 (0–35) | 2.80 (6.83) |
| Psychologist | 1 (0.8) | 1 (0–1) | 0.01 (0.09) | 60 (0–60) | 0.48 (5.35) | 136 (0–136) | 1.08 (12.12) |
| CMHN/CMHTa | 5 (4.0) | 17 (0–8) | 0.13 (0.84) | 915 (0–360) | 7.26 (42.16) | 808 (0–318) | 6.41 (37.24) |
| Physiotherapist | 4 (3.2) | 8 (0–3) | 0.06 (0.39) | 260 (0–90) | 2.06 (12.54) | 143 (0–50) | 1.13 (6.90) |
| Care manager | 3 (2.4) | 4 (0–2) | 0.03 (0.22) | 150 (0–60) | 1.19 (7.96) | 143 (0–57) | 1.13 (7.56) |
| Social worker | 4 (3.2) | 8 (0–3) | 0.06 (0.39) | 390 (0–180) | 3.10 (19.29) | 481 (0–222) | 3.82 (23.79) |
| Sitting service | 1 (0.8) | 18 (0–18) | 0.14 (1.60) | 2160 (0–2160) | 17.14 (192.43) | 261 (0–261) | 2.07 (23.25) |
| Carer support worker | 2 (1.6) | 9 (0–8) | 0.07 (0.72) | 1005 (0–960) | 7.98 (85.59) | 821 (0–784) | 6.51 (69.89) |
| Chiropodist | 10 (7.9) | 12 (0–2) | 0.10 (0.34) | 465 (0–120) | 3.69 (15.89) | 233 (0–60) | 1.85 (7.94) |
| Dentist | 25 (19.8) | 30 (0–3) | 0.75 (0.71) | 725 (0–120) | 5.75 (16.44) | 750 (0–75) | 5.95 (13.20) |
| Optician | 12 (9.5) | 23 (0–7) | 0.18 (0.78) | 790 (0–210) | 6.27 (26.67) | 483 (0–147) | 3.83 (16.46) |
| Other | 5 (4.0) | 10 (0–3) | 0.08 (0.43) | 735 (0–300) | 5.83 (35.14) | 1447 (0–1200) | 11.48 (107.35) |
| Total | 86 (68.3) | 253 (0–26) | 2.01 (3.29) | 9465 (0–2520) | 75.12 (258.62) | 12,319 (0–1450) | 97.77 (203.32) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 17 (14.5) | 226 (0–116) | 1.93 (12.06) | 3400 (0–1740) | 29.06 (171.81) | 3949 (0–2030) | 33.75 (200.44) |
| GP | 75 (64.1) | 209 (0–20) | 1.79 (2.73) | 3235 (0–240) | 27.65 (44.40) | 15,080 (0–1140) | 128.89 (203.96) |
| Practice nurse | 41 (35.0) | 68 (0–8) | 0.58 (1.07) | 1015 (0–80) | 8.68 (15.97) | 897 (0–71) | 7.66 (14.10) |
| Health visitor | 21 (17.9) | 26 (0–3) | 0.22 (0.53) | 1205 (0–240) | 10.30 (29.52) | 1234 (0–236) | 10.55 (29.89) |
| Community psychiatrist | 3 (2.6) | 3 (0–1) | 0.03 (0.16) | 150 (0–60) | 1.28 (8.26) | 723 (0–289) | 6.18 (39.77) |
| Psychologist | 1 (0.9) | 4 (0–4) | 0.03 (0.37) | 80 (0–80) | 0.68 (7.40) | 181 (0–181) | 1.55 (16.76) |
| CMHN/CMHTa | 41 (35.0) | 162 (0–38) | 1.38 (3.86) | 9935 (0–4560) | 84.91 (426.15) | 7076 (0–2328) | 60.48 (224.80) |
| Physiotherapist | 7 (6.0) | 141 (0–120) | 1.21 (11.11) | 7875 (0–7200) | 67.31 (665.80) | 4331 (0–3960) | 37.02 (366.19) |
| Occupational therapist | 8 (6.8) | 17 (0–5) | 0.15 (0.67) | 740 (0–300) | 6.32 (33.70) | 407 (0–165) | 3.48 (18.54) |
| Care manager | 1 (0.9) | 4 (0–4) | 0.03 (0.37) | 180 (0–180) | 1.54 (16.64) | 171 (0–171) | 1.46 (15.81) |
| Social worker | 15 (12.8) | 32 (0–8) | 0.27 (0.97) | 1560 (0–480) | 13.33 (53.64) | 1924 (0–592) | 16.44 (66.16) |
| Home care worker | 22 (18.8) | 2515 (0–448) | 21.50 (70.37) | 90,955 (0–21,600) | 777.39 (2–628.03) | 34,866 (0–8280) | 298.00 (1077.41) |
| Care attendant | 7 (6.0) | 473 (0–160) | 4.04 (20.71) | 41,760 (0–14,400) | 356.92 (1–848.28) | 16,008 (0–5520) | 136.82 (708.51) |
| Sitting service | 2 (1.7) | 46 (0–30) | 0.39 (3.13) | 4560 (0–3600) | 38.97 (343.71) | 667 (0–435) | 5.70 (45.41) |
| Carer support worker | 2 (1.7) | 25 (0–24) | 0.21 (2.22) | 1470 (0–1440) | 12.56 (133.13) | 1201 (0–1176) | 10.26 (108.73) |
| Chiropodist | 33 (28.2) | 53 (0–4) | 0.45 (0.91) | 2400 (0–960) | 20.51 (91.99) | 1200 (0–480) | 10.36 (45.99) |
| Dietitian | 3 (2.6) | 3 (0–3) | 0.03 (0.16) | 60 (0–60) | 0.51 (3.17) | 34 (0–34) | 0.29 (1.80) |
| Meals on wheels | 2 (1.7) | 92 (0–84) | 0.79 (7.79) | 500 (0–420) | 4.27 (39.46) | 552 (0–504) | 4.72 (46.77) |
| Dentist | 25 (21.4) | 33 (0–3) | 0.28 (0.60) | 950 (0–160) | 8.12 (21.64) | 825 (0–75) | 7.05 (14.99) |
| Optician | 19 (16.2) | 23 (0–3) | 0.20 (0.50) | 780 (0–90) | 6.67 (17.82) | 483 (0–63) | 4.13 (10.41) |
| Total | 111 (94.9) | 4155 (0–453) | 35.51 (76.13) | 172,810 (0–21,600) | 1477.01 (3–403.73) | 91,808 (0–8280) | 784.68 (1417.32) |
| Service | n (%) | Community-based service use | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Minutes | Cost (£) | |||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| District nurse | 5 (4.3) | 11 (0–6) | 0.09 (0.60) | 170 (0–90) | 1.45 (9.22) | 176 (0–87) | 1.51 (9.26) |
| GP | 62 (53.0) | 138 (0–19) | 1.18 (2.14) | 2305 (0–380) | 19.70 (41.61) | 11,200 (0–1660) | 95.73 (190.12) |
| Practice nurse | 34 (29.1) | 50 (0–5) | 0.43 (0.85) | 732 (0–75) | 6.26 (13.32) | 647 (0–66) | 5.53 (11.76) |
| Psychologist | 1 (0.9) | 1 (0–1) | 0.01 (0.09) | 60 (0–60) | 0.51 (5.55) | 136 (0–136) | 1.16 (12.57) |
| Counsellor | 1 (0.9) | 14 (0–14) | 0.12 (1.29) | 840 (0–840) | 7.18 (77.66) | 910 (0–910) | 7.78 (84.13) |
| CMHN/CMHTa | 7 (6.0) | 18 (0–8) | 0.15 (0.86) | 1190 (0–480) | 10.17 (53.56) | 1051 (0–424) | 8.98 (47.31) |
| Physiotherapist | 7 (6.0) | 25 (0–16) | 0.21 (1.52) | 1020 (0–720) | 8.72 (67.87) | 561 (0–396) | 4.79 (37.33) |
| Occupational therapist | 1 (0.9) | 2 (0–2) | 0.02 (0.18) | 120 (0–120) | 1.03 (11.09) | 66 (0–66) | 0.56 (6.10) |
| Social worker | 5 (4.3) | 5 (0–1) | 0.04 (0.20) | 345 (0–150) | 2.95 (16.51) | 426 (0–185) | 3.64 (20.36) |
| Home care worker | 1 (0.9) | 14 (0–14) | 0.12 (1.29) | 420 (0–420) | 3.59 (38.83) | 161 (0–161) | 1.38 (14.88) |
| Chiropodist | 9 (7.7) | 17 (0–4) | 0.15 (0.58) | 455 (0–180) | 3.89 (20.68) | 228 (0–90) | 1.94 (10.34) |
| Dietitian | 2 (1.7) | 2 (0–1) | 0.02 (0.13) | 35 (0–20) | 0.30 (2.30) | 20 (0–11) | 0.17 (1.30) |
| Dentist | 27 (23.1) | 35 (0–3) | 0.30 (0.63) | 1085 (0–240) | 9.27 (32.12) | 875 (0–75) | 7.48 (15.83) |
| Optician | 10 (8.5) | 12 (0–2) | 0.10 (0.36) | 315 (0–60) | 2.69 (9.77) | 252 (0–42) | 2.15 (7.49) |
| Other | 3 (2.6) | 8 (0–4) | 0.07 (0.47) | 705 (0–540) | 6.03 (51.17) | 453 (0–180) | 3.87 (24.29) |
| Total | 87 (74.4) | 352 (0–26) | 3.01 (4.49) | 9797 (0–1045) | 83.74 (160.63) | 17,161 (0–1673) | 146.67 (236.22) |
| Service | n (%) | Day care use | |||
|---|---|---|---|---|---|
| Frequency | Cost (£) | ||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| Day care: local authority | 19 (12.1) | 235 (0–40) | 1.50 (5.63) | 9400 (0–1600) | 59.87 (225.17) |
| Day care: voluntary | 7 (4.5) | 100 (0–32) | 0.64 (3.66) | 1800 (0–576) | 11.46 (65.86) |
| Day care: NHS (not hospital) | 5 (3.2) | 56 (0–24) | 0.36 (2.37) | 2240 (0–960) | 14.27 (94.90) |
| Lunch club | 7 (4.5) | 69 (0–20) | 0.44 (2.53) | 219 (0–63) | 1.39 (8.02) |
| Social club | 12 (7.6) | 104 (0–26) | 0.66 (3.03) | 330 (0–82) | 2.10 (9.59) |
| Total | 44 (28.0) | 564 (0–44) | 3.59 (8.36) | 13,988 (0–1600) | 89.10 (246.20) |
| Service | n (%) | Day care use | |||
|---|---|---|---|---|---|
| Frequency | Cost (£) | ||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| Day care: local authority | 15 (11.9) | 212 (0–24) | 1.68 (5.35) | 8480 (0–960) | 67.30 (213.81) |
| Day care: voluntary | 9 (7.1) | 67 (0–16) | 0.53 (2.40) | 1206 (0–288) | 9.57 (43.18) |
| Day care: NHS (not hospital) | 2 (1.6) | 22 (0–16) | 0.17 (1.52) | 880 (0–640) | 6.98 (60.73) |
| Lunch club | 5 (4.0) | 34 (0–16) | 0.27 (1.80) | 108 (0–51) | 0.86 (5.69) |
| Social club | 8 (6.3) | 75 (0–24) | 0.60 (2.90) | 295 (0–76) | 2.34 (10.40) |
| Total | 39 (31.0) | 428 (0–36) | 3.40 (6.88) | 10,969 (0–960) | 87.05 (220.29) |
| Service | n (%) | Day care use | |||
|---|---|---|---|---|---|
| Frequency | Cost (£) | ||||
| Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | ||
| Day care: local authority | 20 (17.1) | 460 (0–64) | 3.97 (11.93) | 18,960 (0–2560) | 162.05 (476.53) |
| Day care: voluntary | 3 (2.6) | 58 (0–32) | 0.50 (3.69) | 1044 (0–576) | 8.92 (66.33) |
| Day care: NHS (not hospital) | 3 (2.6) | 12 (0–6) | 0.10 (0.69) | 480 (0–240) | 4.10 (27.49) |
| Lunch club | 5 (4.3) | 24 (0–8) | 0.21 (1.13) | 76 (0–25) | 0.65 (3.57) |
| Social club | 6 (5.1) | 126 (0–36) | 1.08 (5.39) | 399 (0–114) | 3.41 (17.09) |
| Total | 36 (30.8) | 694 (0–64) | 5.93 (13.09) | 20,960 (0–2560) | 179.14 (476.56) |
| Service | Hospital use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person with dementia | Carer | |||||||||
| n (%) | Frequency | Cost (£) | n (%) | Frequency | Cost (£) | |||||
| Total (minimum–maximum)a | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | |||
| Assessment/rehabilitation inpatient ward | 7 (4.5) | 45 (0–21) | 0.29 (2.05) | 13,860 (0–6468) | 88.28 (631.65) | 1 (0.6) | 1 (0–1) | 0.01 (0.08) | 308 (0–308) | 1.96 (24.58) |
| Continuing care/respite inpatient ward | 3 (1.9) | 17 (0–9) | 0.11 (0.91) | 5559 (0–2943) | 35.41 (297.54) | 0 | 0 | 0 | 0 | 0 |
| Medical inpatient ward | 8 (5.1) | 56 (0–14) | 0.36 (1.95) | 51,464 (0–12,866) | 327.80 (1796.33) | 7 (4.5) | 23 (0–7) | 0.15 (0.86) | 21,137 (0–6433) | 134.63 (791.21) |
| Other inpatient ward | 4 (2.5) | 23 (0–14) | 0.15 (1.25) | 21,137 (0–12,866) | 134.63 (1148.47) | 1 (0.6) | 3 (0–3) | 0.02 (0.24) | 2757 (0–2757) | 17.56 (220.03) |
| Outpatient service | 32 (20.4) | 90 (0–15) | 0.57 (1.94) | 2700 (0–450) | 17.20 (58.26) | 25 (15.9) | 41 (0–4) | 0.26 (0.71) | 1260 (0–120) | 8.03 (21.32) |
| A&E | 17 (10.8) | 24 (0–7) | 0.15 (0.64) | 2184 (0–637) | 13.91 (58.44) | 7 (4.5) | 8 (0–2) | 0.05 (0.25) | 728 (0–182) | 4.64 (22.57) |
| Day hospital | 5 (3.2) | 21 (0–15) | 0.13 (1.22) | 2898 (0–2070) | 18.46 (168.36) | 5 (3.2) | 7 (0–2) | 0.04 (0.26) | 996 (0–276) | 6.15 (36.12) |
| Total | 56 (35.7) | – | – | 99,802 (0–12,866) | 635.68 (2247.49) | 42 (26.8) | – | – | 27,156 (0–6433) | 172.97 (819.43) |
| Service | Hospital use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person with dementia | Carer | |||||||||
| n (%) | Frequency | Cost (£) | n (%) | Frequency | Cost (£) | |||||
| Total (minimum–maximum)a | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | |||
| Assessment/rehabilitation inpatient ward | 8 (6.3) | 9 (0–2) | 0.07 (0.29) | 2772 (0–616) | 22.00 (88.66) | 3 (2.4) | 4 (0–2) | 0.03 (0.22) | 1232 (0–616) | 9.78 (66.76) |
| Continuing care/respite inpatient ward | 5 (4.0) | 113 (0–56) | 0.90 (6.00) | 36,951 (0–18,312) | 293.26 (1963.23) | 1 (0.8) | 1 (0–1) | 0.01 (0.09) | 327 (0–327) | 2.60 (29.13) |
| Medical inpatient ward | 2 (1.6) | 17 (0–16) | 0.13 (1.43) | 15,623 (0–14,704) | 123.99 (1311.84) | 1 (0.8) | 4 (0–4) | 0.03 (0.36) | 3676 (0–3676) | 29.17 (327.48) |
| Outpatient service | 26 (20.6) | 45 (0–10) | 0.36 (1.08) | 1350 (0–300) | 10.71 (32.30) | 17 (13.5) | 33 (0–15) | 0.26 (1.39) | 990 (0–450) | 7.86 (41.60) |
| A&E | 5 (4.0) | 6 (0–2) | 0.05 (0.25) | 546 (0–182) | 4.33 (22.61) | 3 (2.4) | 12 (0–10) | 0.10 (0.90) | 1092 (0–910) | 8.67 (81.74) |
| Day hospital | 6 (3.8) | 21 (0–14) | 0.17 (1.28) | 2898 (0–1932) | 23.00 (176.08) | 1 (0.8) | 1 (0–1 | 0.01 (0.09) | 138 (0–138) | 1.10 (12.29) |
| Total | 46 (36.5) | – | – | 60,140 (0–18,312) | 477.30 (2344.10) | 23 (18.3) | – | – | 7455 (0–3767) | 59.17 (352.35) |
| Service | Hospital use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person with dementia | Carer | |||||||||
| n (%) | Frequency | Cost (£) | n (%) | Frequency | Cost (£) | |||||
| Total (minimum–maximum)a | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | Total (minimum–maximum) | Mean (SD) | |||
| Assessment/rehabilitation inpatient ward | 4 (3.4) | 7 (0–4) | 0.06 (0.40) | 2156 (0–1232) | 18.43 (123.27) | 7 (6.0) | 8 (0–2) | 0.07 (0.29) | 2464 (0–616) | 21.06 (87.92) |
| Continuing care/respite inpatient ward | 2 (1.7) | 8 (0–7) | 0.07 (0.65) | 2616 (0–2289) | 22.36 (213.51) | 0 | 0 | 0 | 0 | 0 |
| Medical inpatient ward | 3 (2.6) | 30 (0–21) | 0.26 (2.05) | 27,570 (0–19,299) | 235.64 (1881.66) | 6 (5.1) | 38 (0–14) | 0.32 (1.88) | 34,922 (0–12,866) | 298.48 (1727.02) |
| Other inpatient ward | 4 (3.4) | 31 (0–14) | 0.26 (1.73) | 28,489 (0–12,866) | 243.50 (1593.55) | 1 (0.9) | 7 (0–7) | 0.06 (0.65) | 6433 (0–6433) | 54.98 (594.73) |
| Outpatient service | 31 (26.5) | 80 (0–20) | 0.68 (2.65) | 2400 (0–600) | 20.51 (79.44) | 25 (21.4) | 69 (0–30) | 0.59 (2.84) | 2070 (0–900) | 17.69 (85.23) |
| A&E | 12 (10.3) | 17 (0–3) | 0.15 (0.48) | 1547 (0–273) | 13.22 (43.52) | 4 (3.4) | 4 (0–4) | 0.03 (0.18) | 364 (0–91) | 3.11 (16.61) |
| Day hospital | 4 (3.4) | 18 (0–14) | 0.15 (1.31) | 2484 (0–1932) | 21.23 (180.85) | 2 (1.7) | 2 (0–2) | 0.02 (0.13) | 276 (0–138) | 2.36 (17.96) |
| Total | 41 (35.0) | – | – | 67,262 (0–19,390) | 574.89 (2477.33) | 36 (30.8) | – | – | 46,529 (0–12,926) | 397.68 (1842.06) |
Appendix 17 The FamCare study: mean monthly frequencies of health- and social-care contacts for participants with data recorded at all time points
| Service | Time point, mean frequencya (SD) | ||
|---|---|---|---|
| Baseline | First follow-up | Second follow-up | |
| District nurse | 0.20 (0.82) | 0.25 (0.78) | 0.47 (3.05) |
| GP | 0.67 (0.76) | 0.40 (0.78) | 0.44 (0.68) |
| Practice nurse | 0.27 (0.50) | 0.22 (0.41) | 0.15 (0.27) |
| Health visitor | 0.02 (0.11) | 0 | 0.06 (0.13) |
| Chiropodist | 0.12 (0.22) | 0.12 (0.25) | 0.12 (0.23) |
| Dietitian | 0.01 (0.04) | 0.02 (0.08) | 0.01 (0.04) |
| Physiotherapist | 0.06 (0.48) | 0.10 (0.54) | 0.31 (2.82) |
| OT | 0.07 (0.21) | 0.05 (0.17) | 0.04 (0.17) |
| Community care excluding mental health | 1.40 (1.62) | 1.14 (1.45) | 1.58 (4.27) |
| Community psychiatrist | 0.11 (0.40) | 0.11 (0.25) | 0.01 (0.04) |
| Psychologist | 0.02 (0.09) | 0.05 (0.21) | 0.01 (0.09) |
| Counsellor | 0 | 0 | 0 |
| CMHT/rapid response/admiral nursing | 0.45 (0.62) | 0.43 (0.70) | 0.35 (0.98) |
| Community mental health services | 0.57 (0.73) | 0.58 (0.75) | 0.37 (0.98) |
| Care manager | 0.01 (0.10) | 0.04 (0.29) | 0.01 (0.09) |
| Social worker | 0.09 (0.21) | 0.13 (0.36) | 0.07 (0.25) |
| Home care worker | 2.72 (12.69) | 2.65 (10.50) | 5.52 (17.80) |
| Care attendant | 0.75 (4.20) | 0.80 (3.87) | 0.99 (5.22) |
| Sitting service | 0.12 (0.83) | 0.28 (1.57) | 0.10 (0.79) |
| Carer support worker | 0.01 (0.07) | 0.78 (7.87) | 0.06 (0.56) |
| Day care: local authority | 0.57 (2.13) | 0.90 (2.79) | 0.95 (2.95) |
| Day care: voluntary | 0.20 (1.27) | 0.26 (1.21) | 0.13 (0.93) |
| Day care: NHS (not hospital) | 0.16 (0.93) | 0.10 (0.80) | 0.03 (0.18) |
| Lunch club | 0.11 (0.73) | 0.08 (0.58) | 0.05 (0.29) |
| Social club | 0.23 (0.87) | 0.33 (1.52) | 0.28 (1.37) |
| Social services, including day care | 4.99 (13.59) | 6.30 (14.83) | 8.19 (18.83) |
| Dentist | 0.09 (0.27) | 0.08 (0.19) | 0.07 (0.15) |
| Optician | 0.07 (0.17) | 0.05 (0.15) | 0.05 (0.13) |
| Other | 0.11 (0.56) | 0.03 (0.13) | 0 |
| Meals on Wheels | 0.36 (2.87) | 0.25 (2.62) | 0.20 (1.98) |
| Other | 0.63 (2.95) | 0.39 (2.62) | 0.32 (1.97) |
| Assessment/rehabilitation inpatient | 0.09 (0.68) | 0.04 (0.15) | 0.02 (0.10) |
| Continuing care/respite inpatient | 0.00 (0.03) | 0.32 (2.72) | 0.02 (0.17) |
| Medical inpatient | 0.12 (0.63) | 0.08 (0.75) | 0.07 (0.52) |
| Other inpatient | 0.06 (0.49) | 0 | 0.07 (0.44) |
| Outpatient | 0.21 (0.72) | 0.14 (0.33) | 0.17 (0.67) |
| Day case | 0.06 (0.48) | 0.09 (0.44) | 0.04 (0.33) |
| A&E | 0.05 (0.48) | 0.02 (0.12) | 0.04 (0.12) |
| Hospital services | 0.59 (1.39) | 0.70 (2.87) | 0.83 (2.19) |
| Mean monthly service use | 8.19 (15.69) | 9.13 (15.20) | 10.87 (19.65) |
| Service | Time point, mean frequencya (SD) | ||
|---|---|---|---|
| Baseline | First follow-up | Second follow-up | |
| District nurse | 0.02 (0.16) | 0.05 (0.20) | 0.03 (0.15) |
| GP | 0.34 (0.44) | 0.29 (0.43) | 0.30 (0.54) |
| Practice nurse | 0.11 (0.22) | 0.12 (0.26) | 0.11 (0.22) |
| Health visitor | 0 | 0 | 0 |
| Chiropodist | 0.04 (0.19) | 0.06 (0.18) | 0.04 (0.15) |
| Dietitian | 0.02 (0.19) | 0 | 0.01 (0.03) |
| Physiotherapist | 0.01 (0.08) | 0.04 (0.16) | 0.06 (0.39) |
| OT | 0.01 (0.10) | 0 | 0.01 (0.05) |
| Community care excluding mental health | 0.55 (0.64) | 0.54 (0.70) | 0.53 (0.87) |
| Community psychiatrist | 0 | 0 | 0 |
| Psychologist | 0.03 (0.21) | 0.01 (0.05) | 0.00 (0.02) |
| Counsellor | 0 | 0 | 0.03 (0.33) |
| CMHT/rapid response/admiral nursing | 0.02 (0.13) | 0.02 (0.16) | 0.04 (0.22) |
| Community mental health services | 0.04 (0.24) | 0.03 (0.16) | 0.07 (0.39) |
| Care manager | 0 | 0.02 (0.12) | 0 |
| Social worker | 0.02 (0.14) | 0.03 (0.21) | 0.01 (0.05) |
| Home care worker | 0.07 (0.75) | 0 | 0.03 (0.33) |
| Care attendant | 0 | 0 | 0 |
| Sitting service | 0 | 0 | 0 |
| Carer support worker | 0.01 (0.04) | 0.04 (0.38) | 0 |
| Day care: local authority | – | – | – |
| Day care: voluntary | – | – | – |
| Day care: NHS (not hospital) | – | – | – |
| Lunch club | – | – | – |
| Social club | – | – | – |
| Social services, including day care | 0.10 (0.79) | 0.09 (0.55) | 0.04 (0.33) |
| Dentist | 0.10 (0.22) | 0.36 (0.31) | 0.08 (0.16) |
| Optician | 0.04 (0.11) | 0.07 (0.26) | 0.03 (0.09) |
| Other | 0.01 (0.09) | 0.05 (0.23) | 0.01 (0.08) |
| Meals on Wheels | 0 | 0 | 0 |
| Other | 0.14 (0.26) | 0.22 (0.44) | 0.11 (0.21) |
| Assessment/rehabilitation inpatient | 0.00 (0.03) | 0.02 (0.12) | 0.02 (0.07) |
| Continuing care/respite inpatient | 0 | 0.01 (0.05) | 0 |
| Medical inpatient | 0.04 (0.24) | 0.02 (0.19) | 0.08 (0.48) |
| Other inpatient | 0.01 (0.09) | 0 | 0.02 (0.17) |
| Outpatient | 0.10 (0.26) | 0.13 (0.73) | 0.15 (0.72) |
| Day case | 0.02 (0.09) | 0.01 (0.05) | 0.01 (0.03) |
| A&E | 0.02 (0.10) | 0.05 (0.47) | 0.01 (0.05) |
| Hospital services | 0.18 (0.38) | 0.23 (0.89) | 0.28 (0.91) |
| Mean monthly service use | 1.03 (1.32) | 1.10 (1.69) | 1.04 (1.52) |
Appendix 18 The FamCare study: alternative accommodation
| Type of accommodation | Time point, nights | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (N = 114) | First follow-up (N = 114) | Second follow-up (N = 114) | |||||||
| n (%) | Total (minimum–maximum) | Mean per month (SD) | n (%) | Total (minimum–maximum) | Mean per month (SD) | n (%) | Total nights (minimum–maximum) | Mean per month (SD) | |
| Not lived anywhere else | 102 (89.5) | – | – | 96 (84.2) | – | – | 93 (81.6) | – | – |
| Owner-occupied house/flat | 1 (0.9) | 16 (0–16) | 0.14 (1.51) | 3 (2.6) | 46 (0–30) | 0.41 (3.11) | 5 (4.4) | 71 (0–29) | 0.63 (3.55) |
| Privately rented house/flat | – | – | – | – | – | – | 1 (0.9) | 15 (0–15) | 0.13 (1.38) |
| House/flat rented from housing association/local authority | – | – | – | 1 (0.9) | 10 (0–10) | 0.09 (0.94) | 1 (0.9) | 5 (0–5) | 0.05 (0.50) |
| Residential home | 3 (2.6) | 13 (0–7) | 0.12 (0.80) | 6 (5.3) | 45 (0–20) | 0.40 (2.37) | 10 (8.8) | 166 (0–30) | 1.47 (5.47) |
| Nursing home | – | – | – | – | – | – | 3 (2.6) | 24 (0–10) | 0.21 (1.35) |
| Other family | 1 (0.9) | Missing | Missing | 2 (1.8) | 2a (0–2) | 0.02 (0.22) | 2 (1.7) | 29 (0–15) | 0.25 (1.90) |
| Missing | 2 (1.8) | – | – | 1 (0.9) | – | – | 1 (0.9) | – | – |
| Total | – | 29 (0–16) | 0.26 (1.70) | – | 112 (0–30) | 0.93 (4.42) | – | 315 (0–30) | 2.78 (7.40) |
Appendix 19 The FamCare study: medication prescribing for people with dementia in the 3 months before baseline (whole sample)
| Category | Number of | Mean (SD) number of prescriptions per participant | Cost (£) | ||
|---|---|---|---|---|---|
| Prescriptions | Participants (%) | Total | Mean (SD) per participant | ||
| Antipsychotics | |||||
| Atypical antipsychotic | 13 | 12 (7.6) | 0.08 (0.30) | 544.30 | 3.47 (17.32) |
| Typical antipsychotic | 2 | 2 (1.3) | 0.01 (0.11) | 51.11 | 0.33 (2.91) |
| Hypnotics and anxiolytics | |||||
| B/Z/A drug | 10 | 9 (5.7) | 0.06 (0.27) | 89.75 | 0.57 (2.70) |
| Non-B/Z/A drug | 1 | 1 (0.6) | 0.01 (0.08) | 57.86 | 0.37 (4.62) |
| Antidepressants | |||||
| SSRI | 18 | 18 (11.5) | 0.11 (0.32) | 300.19 | 1.91 (6.69) |
| Tricyclic | 10 | 10 (6.4) | 0.06 (0.24) | 211.84 | 1.35 (12.12) |
| Other | 9 | 8 (5.1) | 0.06 (0.26) | 65.66 | 0.42 (1.88) |
| Anticonvulsants | 3 | 3 (1.9) | 0.02 (0.14) | 46.76 | 0.30 (2.27) |
| Dementia drugs | |||||
| Acetylcholinesterase inhibitors | 45 | 44 (28) | 0.29 (0.47) | 10,542.48 | 67.15 (115.67) |
| Cognitive enhancers | 4 | 4 (2.5) | 0.03 (0.16) | 847.82 | 5.40 (41.31) |
| Pain relief | |||||
| Opioida | 20 | 16 (10.2) | 0.13 (0.42) | 1782 | 11.35 (81.50) |
| Non-opioid | 23 | 23 (14.6) | 0.15 (0.35) | 192.54 | 1.23 (3.76) |
| Laxatives | 15 | 12 (7.6) | 0.10 (0.35) | 172.02 | 1.10 (4.99) |
| Otherb | 647 | 142 (90.5) | 4.12 (3.02) | 14,344.45 | 91.37 (145.99) |
| No medication | 7 (4.5) | ||||
| Total | 820 | – | 5.22 (3.30) | 29,248.77 | 186.30 (196.05) |
Appendix 20 Comparisons of the ResCare trial and the FamCare study
| CDR score | Time point, % | |||
|---|---|---|---|---|
| Baseline | Follow-up | |||
| ResCare CB group (n = 555) | FamCare (n = 157) | ResCare CB group (n = 428) | FamCare (n = 109)a | |
| 0: no cognitive impairment | 0.7 | 3.2 | 0.2 | 5.5 |
| 0.5: very mild dementia | 3.3 | 22.3 | 4.48 | 16.5 |
| 1: mild | 15.9 | 37.6 | 14.9 | 35.8 |
| 2: moderate | 30.6 | 28.0 | 23.8 | 25.7 |
| 3: severe | 49.5 | 8.9 | 56.6 | 16.5 |
| NPI | Time point, mean score (SD; range) | |||
|---|---|---|---|---|
| Baseline | Follow-up | |||
| ResCare CB group (n = 555) | FamCare (n = 157) | ResCare CB group (n = 428) | FamCare (n = 117) | |
| Incidence | 4.83 (2.37; 0–12) | 5.35 (2.65; 0–11) | 4.64 (2.3; 0–12) | 5.30 (2.85; 0–11) |
| Frequency | 12.38 (7.3; 0–36) | 14.05 (8.32; 0–34) | 11.6 (6.69; 0–32) | 13.11 (8.41; 0–38) |
| Severity | 7.76 (4.83; 0–23) | 9.12 (5.73; 0–27) | 7.19 (4.48; 0–26) | 8.96 (6.04; 0–26) |
| Total (frequency × severity) | 21.14 (15.96; 0–84) | 25.75 (19.17; 0–94) | 19.08 (14; 0–87) | 24.21 (19.42; 0–89) |
| Distress | 4.80 (6.56; 0–38) | 13.37 (9.65; 0–48) | 3.34 (5.23; 0–36) | 12.37(9.31; 0–39) |
FIGURE 22.
The ResCare trial and FamCare study: incidence of NPI behaviours at baseline.

List of abbreviations
- A&E
- accident and emergency
- ADQ
- Approaches to Dementia Questionnaire
- B/Z/A
- benzodiazepines/z-hypnotics
- BPSD
- behavioural and psychological symptoms of dementia
- CAIT
- communication and interaction training
- CB
- challenging behaviour
- CBS
- Challenging Behaviour Scale
- CDR
- Clinical Dementia Rating
- CDR-SB
- Clinical Dementia Rating-sum of the boxes
- CI
- confidence interval
- CMAI
- Cohen–Mansfield Agitation Inventory
- CMHN
- community mental health nurse
- CMHT
- community mental health team
- CMHTOP
- community mental health team for older people
- CONSORT
- Consolidated Standards of Reporting Trials
- CQC
- Care Quality Commission
- CRM
- cluster representation mechanism
- CRT
- cluster randomised trial
- CSRI
- Client Service Receipt Inventory
- DMEC
- Data Monitoring and Ethics Committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D
- EuroQol-5 Dimensions
- FITS
- Focused Intervention Training and Support
- GHQ-12
- General Health Questionnaire-12 items
- GP
- general practitioner
- GS
- Guilt Scale
- HADS
- Hospital Anxiety and Depression Scale
- ICC
- intracluster correlation coefficient
- ICECAP-O
- ICEpop CAPability measure for Older people
- ICER
- incremental cost-effectiveness ratio
- ICT
- information and communication technology
- IMS
- information management system
- ISRCTN
- International Standard Randomised Controlled Trial Number
- IT
- information technology
- LMM
- linear mixed model
- MAS
- Memory Assessment Service
- MBI
- Maslach Burnout Inventory
- MBI-DP
- Maslach Burnout Inventory-Depersonalisation
- MBI-EE
- Maslach Burnout Inventory-Emotional Exhaustion
- MBI-PA
- Maslach Burnout Inventory-Personal Accomplishment
- MICE
- multivariate imputation by chained equations
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMDS-SC
- National Minimum Data Set for Social Care
- NPI
- Neuropsychiatric Inventory
- NPI-NH
- Neuropsychiatric Inventory-Nursing Home
- NPS
- neuropsychiatric symptom
- NPT
- normalisation process theory
- NVQ
- National Vocational Qualification
- NWORTH
- North Wales Organisation for Randomised Trials in Health
- PbR
- Payment by Results
- POMH-UK
- Prescribing Observatory for Mental Health
- PSC
- Programme Steering Committee
- QALY
- quality-adjusted life-year
- QCPR
- Quality of Caregiver/Patient Relationship scale
- QoL-AD
- Quality of Life in Alzheimer’s Disease
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- REMCARE
- REMiniscence groups for people with dementia and their family CAREgivers
- RMBPC
- Revised Memory and Behaviour Problems Checklist
- RSS
- Relative Stress Scale
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SCIE
- Social Care Institute for Excellence
- SD
- standard deviation
- SE
- standard error
- SES
- Self-Efficacy Scale
- SF-12
- Short Form questionnaire-12 items
- SSCQ
- Short Sense of Competence Questionnaire
- SSRI
- selective serotonin reuptake inhibitor
- VAK
- visual–auditory–kinaesthetic
- VAS
- visual analogue scale