Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1043. The contractual start date was in August 2007. The final report began editorial review in February 2016 and was accepted for publication in October 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ulrike Schmidt, Sabine Landau and Janet Treasure received salary support from the South London and Maudsley NHS Foundation Trust’s Mental Health Biomedical Research Centre. Savani Bartholdy reports grants from the National Institute for Health Research (NIHR) Biomedical Research Council outside the submitted work. Charlotte Rhind reports grants from the Psychiatry Research Trust during the conduct of the study. Janet Treasure reports personal fees from Routledge (publishers) outside the submitted work. Rebecca Hibbs reports grants from the NIHR Research for Patient Benefit Programme (PB-PG-0609-19025) during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Schmidt et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and structure of the report
Introduction
Anorexia nervosa (AN) has existed throughout different epochs and cultures. 1 Key symptoms are restricted food intake, weight loss, hyperactivity, and in some cases bingeing and purging. Psychological features include morbid fear of fatness and body image disturbance. AN typically affects young females, although it also affects some men. 2 AN usually starts in adolescence, when brain development is incomplete. 3 Starvation can impair brain function in a lasting way. 4 Early intervention is essential in producing good outcomes. 5 Treatments in the later stages of illness are much less successful. AN is highly heritable but environmental factors are aetiologically important. 6,7 Progress has been made in identifying risk factors for AN (e.g. premorbid feeding problems, obsessive compulsive and anxious traits, high levels of exercising and overinvolved parenting). 6,7 Research on the genetic, epigenetic and neurobiological underpinnings of eating disorder (ED) psychopathology8–13 has identified neurocognitive and social cognitive biomarkers, such as impaired set-shifting, poor central coherence or emotion processing impairments, including poor theory of mind,14–18 which have the potential to inform predictions of treatment outcome and prognosis. A key challenge is to utilise all this knowledge to develop targeted treatments. To this end, we have developed a model of how AN arises and is maintained, informed by these and other clinical neuroscience findings and with the aim of guiding treatment. 19,20
People with AN consult their general practitioner (GP) significantly more than others in the 5 years prior to diagnosis. 21 A single consultation about eating or weight/shape concerns strongly predicts the subsequent emergence of AN. 22 Although GPs exclusively treat 20% of cases with AN,23 they are often not confident at managing AN,24 and there is usually a considerable delay between a diagnosis being made in primary care and the point when more specialist help becomes available. 25
Many parts of the UK lack NHS provision of specialist services for AN. 26,27 Treatment by non-specialists is problematic as many patients are admitted unnecessarily and for lengthy periods,28 with extra costs to the NHS. 29 For example, 35% of people with AN seen in non-specialist Child and Adolescent Mental Health Services (CAMHS) are admitted to hospital, contrasting with only 10% of those seen in specialist ED services. 30 More child and adolescent psychiatric beds (20%) are occupied by young people with AN than any other diagnostic group. 31 Weight gain32 and longer-term outcomes33 are poor in non-specialist units and the mortality is higher. 34,35 Thus, there is a need to disseminate specialist knowledge of this illness. Transitions between services (e.g. from child to adult services, home to university health services, inpatient to follow-up care) are common and can result in fragmented care, thus putting patients at risk. 36,37
Lifetime prevalence rates for AN are 1.6% in women and 0.3% in men. 7 The median duration of illness is 6 years. 38 Physical complications affect all organs39 and the risk of death is the highest of any psychiatric disorder. 40 If they become pregnant the pregnancy is considered high risk and they experience difficulties feeding and playing with their children. 41 Severe psychiatric comorbidity is common. 42 Quality of life is severely impaired,43 more so than in depression. 44 The cost per case of AN is at least that of schizophrenia. 45,46 AN accounts for the highest proportion of admissions of duration > 90 days (26.8%) and the longest median length of stay (36 days). 28 Eating disorders are one of the leading causes of disease burden in terms of years of life lost through death or disability in young women. 47 The family are usually the main carers. They report similar difficulties to carers of people with psychosis, but are more distressed. 25,48 The burden of caregiving and other societal costs have never been examined in economic terms.
This report presents the results of seven independent but integrated work packages (WPs), which form the Applied Research into Anorexia Nervosa and Not Otherwise Specified Eating Disorders (ARIADNE) programme. These WPs focus on optimal disease management for people with AN at all stages of illness, from prevention and detection through to treatment and preventing relapse. The studies focus on a range of populations, including samples from the community, those drawn from inpatient and outpatient settings, as well as specialist groups, such as mothers with an ED and carers of those with an ED. The majority of our WPs focus on building evidence on the efficacy and effectiveness of interventions for these populations, grounded in our clinical neuroscience model of AN. We report on findings from six independent interventions that have been developed and tested by the ARIADNE group during this programme. In addition, we present analyses of the economic and clinical implications of existing care pathways and patterns of service use.
Aims and objectives of the ARIADNE programme
Broad aims
Responding to the need for high-quality research into the management of AN, the overarching aims of the ARIADNE programme were to:
-
produce, validate and disseminate improved evidence-based interventions for AN
-
collaborate with patients and carers throughout the project
-
improve clinical outcomes in AN, by early detection and intervention, by reducing chronicity and relapse, and by improving carer outcomes
-
improve acceptability and cost-effectiveness of AN treatments
-
deliver standardised, trainable and disseminable AN treatments
-
assess service utilisation and NHS costs of AN and implications of changes in clinical practice for patient care and resources.
Objectives
Our objectives were: WP1a, to develop a training programme for school staff to enable them to detect and manage EDs; WP1b, to develop and test a school-based prevention programme for risk factors for EDs; WP2a, to develop an improved treatment for adults with AN that targets disease-maintaining factors, is matched to symptoms, personality and neuropsychological profile and which can be used as a first-line treatment in outpatient settings, and to evaluate the efficacy and cost-effectiveness of this treatment; WP2b, to test and validate components of this treatment, designed as intensive modules for inpatients with AN (i.e. those with severe, chronic or treatment-resistant AN); WP3, to evaluate the efficacy and cost-effectiveness of a carer skills training intervention, and to assess its impact on carer outcomes (e.g. distress, caregiving efficacy) and patient outcomes; WP4, to improve understanding of the nature of a debilitating core symptom of AN (i.e. hyperactivity); WP5, to develop and test a relapse prevention programme for inpatients with AN; WP6, to obtain information on the needs of mothers of children with an ED and the risks of the maternal ED for their offspring and to use this information to inform the development of an intervention for mothers with an ED to minimise the impact of their ED on their children; WP7a, to study existing care pathways for AN, with a focus on the impact of having access to specialist ED services; and WP7b, to study service utilisation and cost of illness in EDs.
Patient and public involvement in the ARIADNE programme
Patient, carer and public involvement has been central to the research in the ARIADNE programme. Mrs Susan Ringwood, the Chief Executive Officer of Beat (the main UK patient carer organisation for EDs) was a co-applicant on the programme. As such, she was involved in the development of the overall programme aims, ensuring that the research questions were aligned with patients’ and carers’ needs. Patients and carers were also part of the Programme Steering Group.
Examples of patient and public involvement (PPI) in specific WPs are as follows.
Example 1: early intervention in schools
Wok package 1a involved the development and pilot testing of a teacher training programme for EDs (see Chapter 2). This research was devised and led by a former ED service user. It involved an extensive period of public consultation, which was used to assess the needs of both school staff and school students in this area. Thorough consultation was achieved through online surveys reaching over 800 school staff and over 500 students. Intervention materials for the training programme were then developed using an iterative process in which two panels of school staff (six members per panel) reviewed draft materials and their feedback was incorporated. PPI ensured that the training materials being developed were responding to a genuine need and were aligned with the needs of the school staff that would be using them.
Example 2: prevention
The intervention development in WP1b for the prevention programme (see Chapter 3) was informed by focus groups with 22 adolescent girls, who provided their experiences of body dissatisfaction, disordered eating and their recommendations for a preventative intervention. The intervention materials were then developed in conjunction with a panel of key stakeholders, which included a young person with a history of an ED, two young people without a history of an ED, and three secondary school teachers. Feedback provided by this panel was incorporated into the materials in an iterative process.
Example 3: carers interventions
Work package 3 evaluated an intervention for carers of people with AN, which was used as an adjunct to inpatient treatment (see Chapter 6). It included extensive PPI, with several members of the research team having personal experiences of an ED. The self-help materials (Experienced Carers Helping Others; ECHO) were developed in collaboration with patients and carers. 49 In addition, the majority of telephone coaching sessions offered during the intervention were provided by trained individuals with personal experience of an ED (either having recovered from the disorder themselves or as a carer). The findings from this trial are being disseminated to the public through a website dedicated to carers of those with an ED [URL: www.thenewmaudsleyapproach.co.uk (accessed 3 July 2017)], a newsletter, the database of ED volunteers and the annual carer’s conference which we run with Beat – the main national organisation for people with EDs and their families.
From the above, it is clear that patients and carers were involved in designing the programme, implementing the WPs and disseminating findings. Such collaboration between researchers and service user representatives has been highlighted as exemplifying good practice in service user involvement by the Mental Health Research Network (MHRN). 50
Report structure
The overarching aims of the ARIADNE programme were realised through seven independent, but integrated, WPs which are presented in detail in this report. An outline of the chapters is as follows:
Work package 1: prevention and early intervention
Chapters 2 and 3 focus on prevention, detection and early intervention of EDs. In Chapter 2, we present the development and evaluation of a learning package for school staff on how to recognise symptoms of EDs, how to communicate about EDs sensitively and how to assess risk. Chapter 3 outlines the development of a teacher-delivered prevention programme for EDs, and the results of a cluster randomised controlled trial (RCT) evaluating its efficacy.
Work package 2: treatment
Chapters 4 and 5 focus on treatment of AN. In Chapter 4 we present the evaluation of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) using a large RCT with individuals in an outpatient setting. Chapter 5 explores the use of components of this treatment in an inpatient setting, designed as intensive modules for severe, chronic or treatment-resistant AN. Here, we evaluate this approach through case report, qualitative evaluation and a pilot trial comparing it to treatment as usual (TAU).
Work package 3: carers interventions
Chapter 6 presents an intervention for those caring for individuals with AN. We present findings from a large RCT assessing the impact of a guided self-help intervention for carers of individuals with AN (ECHO), in addition to standard inpatient care. We report on outcomes for both carers and for patients up to 12 months post discharge from inpatient care.
Work package 4: physical activity in anorexia nervosa
Chapter 7 focuses on the assessment of activity levels and endocrine changes in individuals with AN. We present data from an observational study. Individuals with AN (inpatients and outpatients) are compared with individuals with anxiety and healthy controls (HCs) using a range of methods, including body composition, endocrine measurements, self-report and actimetry.
Work package 5: relapse prevention
Chapter 8 focuses on relapse prevention. Here, we present a feasibility RCT on a novel e-mail-guided manual-based intervention to be used in the post-hospitalisation aftercare of patients with AN.
Work package 6: mothers with an eating disorder
In Chapter 9, we present research on mothers with an ED, a special population that may need tailored services. We report findings regarding fertility difficulties in women with an ED, and associations between maternal EDs and their children’s diet and growth trajectories.
Work package 7: care pathways and economic evaluations
Chapter 10 presents results regarding service use, focusing on how access to specialist services affects rates of referrals, admissions for inpatient treatment, continuity of care and service user experiences. Chapter 11 uses data from ARIADNE WPs (WPs 2, 3, 5 and 7a), plus the British Cohort Study (1970) (BCS-70), to identify the costs of services and treatments used by people with AN and to estimate its annual costs for England.
General discussion
Chapter 12 draws together the findings from the seven WPs and highlights clinical implications and recommendations for future research based on this programme.
Chapter 2 The development and feasibility testing of an eating disorders training programme for UK school staff (work package 1a)
Abstract
Work package 1a composed of four studies.
Study 1: 511 school students aged 11–19 years completed an online questionnaire exploring their experiences of EDs at school. Respondents provided actionable recommendations about improvements that could be made.
Study 2: 826 school staff completed an online questionnaire exploring their ED experiences. Participants highlighted a lack of understanding and knowledge within their schools and a willingness to access training and support.
Study 3: 63 members of staff from 29 UK schools participated in focus groups to further develop the themes explored in study 2. Five salient themes emerged from the focus group discussions.
-
There was little general knowledge about EDs among staff.
-
Mental health issues, including EDs, were not openly talked about among staff.
-
School staff do not feel confident or comfortable teaching students about EDs.
-
When they exist, positive relationships with parents contribute to ED recovery, but sometimes relationships with parents are very negative.
-
More support is needed for school staff involved in the care of students undergoing ED recovery.
Study 4: a 1-day training programme for UK school staff aimed at improving attitudes towards, confidence in supporting and knowledge about EDs was tested for feasibility and acceptability, and was found to have a positive, significant impact with medium and large effect sizes (ESs) that were maintained after 3 months.
Introduction
Eating disorders have a high rate of onset during adolescence,51,52 the period during which young people attend secondary/high school. Research indicates that up to 1.5% of secondary school students suffer from a diagnosable eating disorder53–55 and up to 15% experience subclinical eating disturbance. 56 However, many of these cases go undetected and untreated. 57
As students spend an average of 40 hours a week attending school,58 school staff are in a good position to pick up on the physical and behavioural symptoms that are present during the early stages of eating disorders. 59 Furthermore, school staff are well placed to offer ongoing support as young people have indicated that they are up to nine times more likely to talk to a teacher than a parent about food-related difficulties. 60
Work package 1a is a series of interlinked studies designed to understand the current context of EDs in school and use this understanding alongside school staff and student recommendations in the development of a face-to-face training programme. The WP culminated in the training programme being feasibility tested.
Study 1: student experiences of eating disorders within the school setting – an online survey
Methods
First- and second-hand student experiences of suffering with an ED at school were explored using an online questionnaire (see Appendix 1, Student questionnaire), completed by 511 students aged 11–19 years [mean = 15.4 years, standard deviation (SD) = 2.3 years, 72% female]. Note that n varies by question as not all participants responded to all questions.
The questionnaire included free-text responses, which were coded using content analysis. 61,62 A categorisation system was developed by analysing responses and classifying them into categories ,with care being taken to ensure that the coding system was comprehensive, while avoiding overlapping of categories. A second researcher independently applied the categories, blind to the original researcher’s decisions. An inter-rater reliability of 94% was achieved.
Results
Students’ experiences and recommendations
Thirty-eight per cent (n = 195) reported a current or previous ED, although 49% (n = 96) of these students had not received a diagnosis which confirms this. In total, 53% (n = 115) reported being friends with a student suffering with an ED.
Quantitative data are summarised in Table 1. Qualitative data are summarised in Table 2. Below, both forms of data are considered together under three salient themes which emerged during data analysis:
-
recognition of early symptoms
-
encouraging and supporting sufferer help-seeking
-
providing a supportive school environment for recovery.
| Topic | Response | |||||
|---|---|---|---|---|---|---|
| Could you spot eating disorder warning signs in a friend? (n = 458) | Yes – I have done so before | Yes – I would know the signs | I’m not sure | No | ||
| 257 (56%) | 104 (23%) | 64 (14%) | 33 (7%) | |||
| Have you learnt about eating disorders at school? Was it helpful? (n = 499) | Taught – it was helpful | Taught – it was not helpful | I’ve not been taught | I’m not sure | ||
| 27 (5%) | 124 (25%) | 332 (67%) | 16 (3%) | |||
| What would you do if you spotted eating disorder warning signs in a friend? (n = 505) | I’d help my friend if they came to me | I’d proactively offer my friend my help | I would tell a teacher | I would anonymously tell a teacher | I would talk to a trusted adult outside school | I would watch and wait |
| 137 (27%) | 263 (52%) | 33 (7%) | 15 (3%) | 29 (6%) | 28 (6%) | |
| If you shared your concerns with a teacher, what would you want them to do? (n = 479) | Speak with my friend | Support me in helping my friend | Talk to my friend’s parents | Arrange for support from a counsellor or doctor | Listen | |
| 122 (25%) | 216 (45%) | 11 (2%) | 78 (16%) | 52 (11%) | ||
| If you shared your concerns with a teacher, what would you expect them to actually do? (n = 474) | Speak with my friend | Support me in helping my friend | Talk to my friend’s parents | Arrange for support from a counsellor or doctor | Listen | |
| 106 (22%) | 24 (5%) | 229 (48%) | 50 (11%) | 65 (14%) | ||
| What would be your preferred way of communicating your concerns to a member of school staff? (n = 461) | In person | By telephone | Text/SMS/IM | E-mail/in writing | ||
| 337 (73%) | 5 (1%) | 17 (4%) | 102 (22%) | |||
| Do you consider your school to be a supportive place for someone recovering from an eating disorder? (n = 504) | Strongly agree | Agree | Neutral | Disagree | Strongly disagree | |
| 12 (2%) | 41 (8%) | 81 (16%) | 133 (26%) | 237 (47%) | ||
| Topic | Response | ||||
|---|---|---|---|---|---|
| How could your school help students understand more about eating disorders? (n = 351) | Psychoeducation | Better services available at school | Talk more openly about eating disorders | Improve teacher knowledge | Be less judgemental |
| 185 (59%) | 68 (22%) | 22 (7%) | 19 (14%) | 18 (7%) | |
| Teachers take more caring approach | School cannot help | Improve access to services in school | Better resources | ||
| 16 (6%) | 10 (4%) | 9 (3%) | 4 (1%) | ||
| How could your school be more supportive to students during eating disorder recovery? (n = 342) | Reduced stigma about eating disorders | Bespoke support | Specialist support | Nothing | Support groups |
| 133 (43%) | 72 (23%) | 46 (15%) | 27 (9%) | 25 (8%) | |
| Confidentiality | Peer mentors | Promote school services | |||
| 20 (6%) | 12 (4%) | 7 (2%) | |||
| How has the school helped you or a friend in response to an eating disorder? (n = 169) | They referred the case to a specialist | Staff were supportive | They spoke to parents | They were flexible re schooling | They provided on-going support |
| 67 (22%) | 63 (20%) | 19 (6%) | 10 (3%) | 10 (3%) | |
| Have you had any negative school-based experiences in response to an eating disorder? (n = 321) | No one noticed | I was punished | I was not appropriately consulted | I did not get the specialist help I needed | Staff broke my confidence |
| 89 (27%) | 31 (10%) | 31 (10%) | 27 (8%) | 21 (6%) | |
| Judgemental | Lack of knowledge | Problems with the services | Negative experience not expanded upon | ||
| 12 (4%) | 10 (3%) | 8 (2%) | 92 (28%) | ||
Recognition of early symptoms
Seventy-nine per cent (n = 361) of students surveyed were confident that they would recognise the symptoms of an ED in a friend. Thirty per cent of students had been taught about EDs as part of a planned programme of study at school, but this was not generally well received, with 82% (n = 124) reporting that these lessons could have been better.
Fifty-nine per cent (n = 185) of students surveyed recommendeded that ED education be improved for both students and their teachers. In free-text responses, 16% (n = 46) of students stated that school staff had minimal or no knowledge about EDs. Reducing stigma and enabling friends to recognise and respond to early symptoms were the key motivators outlined in responses.
Encouraging and supporting sufferer help-seeking
Students expressed a reluctance to highlight ED concerns about a friend with a teacher, with only 7% (n = 33) of students stating they would be happy to do so. Several barriers for this type of help seeking emerged including:
-
fear a teacher would dismiss their concerns
-
fear a teacher would over-react to their concerns
-
fear that a teacher would not treat their concerns in confidence.
When school staff help was sought, students shared a clear preference for face-to-face discussions (73%; n = 337) above writing/e-mail (22%; n = 102), or sharing concerns via telephone/text (5%; n = 22).
The positive impact of school staff on student outcomes was shared by 49 respondents. Four students claimed that the support provided by school staff was instrumental in preventing them from dying as a consequence of their ED.
Providing a supportive school environment for recovery
Seventy-three per cent of respondents (n = 370) did not view their schools as a supportive environment for facilitating recovery from an ED. Key reasons cited were:
-
bullying from peers
-
poor reintegration into school
-
staff uncertainty about how to support.
Students whose experiences had been more positive outlined the important role of the school and school staff in their recovery. When asked to describe the ideal approach from school staff, respondents highlighted their desire for honesty (n = 71), openness (n = 19), a non-judgemental approach (n = 23) and someone who felt approachable (n = 29).
Discussion
This was the first UK study of student experiences of EDs. Respondents provided valuable insight into the experiences of UK school students with EDs and, further, provided actionable recommendations about improvements that could be made.
A strength of the study was that both male and female students were surveyed, which has not previously been the norm. 64 Future work could explore moderators (e.g. gender, school type) of student experiences.
The opportunity for students to recognise and respond to early ED symptoms in their friends, and for schools to provide a safe and supportive recovery environment, was clearly highlighted by respondents. However, it is clear that more work needs to be done if this potential is to be realised in UK schools. Psychoeducation and training for students and teachers was suggested by respondents as a key method for addressing this.
Study 2: school staff experiences of eating disorders within the school setting – an online survey
Methods
A total of 1250 UK school staff were invited to respond to an anonymous online survey exploring their experiences of EDs within the school setting (see Appendix 1, Staff questionnaire). A total of 826 (66%) of the convenience sample chose to participate.
Free-text responses were analysed using content analysis. 61,62 A categorisation system was developed by analysing responses and classifying them into categories, with care being taken to ensure that the coding system was comprehensive while avoiding overlapping of categories. A second researcher independently applied the categories, blind to the original researcher’s decisions. An inter-rater reliability of 89% was achieved (κ = 0.471; p < 0.001).
Results
Multiple-choice questions generated quantitative data, which have been summed and recorded in Table 3. Content analysis was used to interrogate the large amount of qualitative data produced in the form of free-text responses to open questions. This is summarised in Table 4.
| Topic | Response | |||
|---|---|---|---|---|
| Is there an eating disorders policy at your school? (n = 774) | No | Yes – within another policy | I’m not sure | Yes – we have a specific policy |
| 320 (41%) | 208 (27%) | 205 (26%) | 41 (5%) | |
| Do you think eating disorders policies are effective? (n = 519) | Very effective | Effective | Ineffective | Very ineffective |
| 42 (8%) | 317 (61%) | 148 (29%) | 12 (2%) | |
| Have you been offered eating disorders training at school? (n = 791) | No | Yes | ||
| 583 (74%) | 208 (26%) | |||
| Who was the training for? (n = 147) | Specialist staff members (three or fewer) | All school staff | All pastoral staff | All senior and middle leaders |
| 82 (56%) | 43 (29%) | 19 (13%) | 3 (2%) | |
| How was the training delivered? (n = 161) | Seminar | Lecture | Written materials | |
| 107 (66%) | 37 (23%) | 17 (11%) | ||
| If you have not received any training, do you think you would find eating disorders training useful? (n = 346) | Very useful | Quite useful | Not very useful | Not at all useful |
| 160 (46%) | 156 (45%) | 30 (9%) | 0 (0%) | |
| Are there any current or past eating disorders cases in your school? (n = 530) | Yes, I am directly involved with at least one case | Yes, but I am not directly involved | No | |
| 266 (50%) | 181 (34%) | 83 (16%) | ||
| At your school, what should a student do if they are worried a friend may have an eating disorder? (n = 781) | They can talk to any member of the school’s staff | This is something that has never been discussed/agreed | There is a specific member of staff they should speak to | There is a system in place for anonymously raising concerns |
| 364 (47%) | 287 (37%) | 112 (14%) | 18 (2%) | |
| How comfortable would you feel teaching students about eating disorders? (n = 785) | I would feel very uncomfortable | I would feel uncomfortable | I would feel comfortable | I would feel very comfortable |
| 419 (54%) | 273 (35%) | 84 (11%) | 9 (1%) | |
| Has your school ever had to reintegrate a student after a period of absence caused by an eating disorder? (n = 487) | Yes we have | No we have not | ||
| 329 (68%) | 158 (32%) | |||
| Did you receive any guidance about how to support students returning following a period of absence? (n = 317) | Yes we did | No we did not | ||
| 240 (76%) | 77 (24%) | |||
| Topic | Response | ||||
|---|---|---|---|---|---|
| Perceived benefits of eating disorders training that had been completed (n = 142) | An increase in confidence supporting eating disorders | I learned how I can support someone with an eating disorder | I now know what warning signs to look out for | It was good to share ideas with other people | Nothing specific just generally useful |
| 39 (27%) | 31 (22%) | 21 (15%) | 15 (11%) | 14 (10%) | |
| Learned about referral processes | Raised awareness of ED | Not useful | |||
| 12 (8%) | 7 (5%) | 3 (2%) | |||
| How could the training have been improved (n = 96) | If it was more in depth | If it had been available to more staff | Booster sessions | The use of real case studies | Nothing the training was comprehensive |
| 24 (25%) | 17 (18%) | 10 (10%) | 10 (10%) | 7 (7%) | |
| More practical suggestions about how to support | If it was more tailored to a student causing concern | A better teacher | Information about developing policies or making referrals | Being taught in a smaller group | |
| 7 (7%) | 6 (6%) | 6 (6%) | 5 (5%) | 4 (4%) | |
| What staff would do next if worried that a student might have an eating disorder (n = 782) | I’m not sure | I’d refer to my school’s policy | I would ask a more experienced colleague | I would speak with the pupil | I would talk to the pupil’s parents |
| 316 (40%) | 168 (21%) | 146 (19%) | 107 (14%) | 25 (3%) | |
| I would make an external referral | I would watch and wait | ||||
| 13 (2%) | 7 (1%) | ||||
| Reasons given for feeling uncomfortable teaching students about eating disorders (n = 546) | Too little knowledge of the topic | Fear of iatrogenic effects | It’s not necessary as students have a good understanding | I would not know how to manage disclosures | |
| 312 (57%) | 109 (20%) | 64 (12%) | 61 (11%) | ||
| How parents have responded to being told their child has an eating disorder (n = 781) | Parent has responded with denial or refusal to speak to us | Both positive and negative reactions | Difficult at first but relations improved | Parents have been angry accusing us of criticising their parenting | |
| 364 (47%) | 287 (37%) | 112 (14%) | 18 (2%) | ||
| Support that staff suggested would be helpful during the reintegration of a student with an eating disorder into school (n = 105) | Tailored information based on the specific student’s needs | More or better training than is currently provided | More people should be involved in training (including e.g. parents, students) | Training should be provided for all relevant staff | Specialist support and advice on an ongoing basis |
| 30 (29%) | 25 (24%) | 17 (16%) | 13 (12%) | 8 (8%) | |
| Students need to be supported in the run up to their friend’s return | Other | ||||
| 8 (8%) | 4 (4%) | ||||
Below, both forms of data are considered together under four salient themes which emerged during data analysis:
-
supporting students with EDs
-
ED training and policies
-
teaching about EDs
-
reintegrating students who were absent as the result of an ED.
Supporting students with eating disorders
Only 40% (n = 316) of respondents stated that they would feel confident following up ED-related concerns in a student.
Eating disorders training and policies
Sixty-one per cent (n = 317) of respondents found policies to be an effective tool within schools. Despite this, only 32% (249) of respondents reported that their school had a policy that made any reference to EDs and, of these, only 5% (n = 41) were specific ED policies.
Thirty-one per cent (n = 160) of respondents found school policies to be ineffective in most cases. This was attributed to a lack of understanding of the role of frontline staff by senior leaders who developed policies.
The majority of respondents (74%; n = 583) reported that their school had never provided training about EDs. Ninety-one per cent (n = 316) of respondents who had not been trained said that they would welcome the opportunity.
Twenty-six per cent (n = 208) of respondents reported that their school had provided training. In most instances (56%; n = 82) this was for specialised groups of three or fewer staff.
Those staff who had received training outlined a range of benefits, including:
-
an increase in confidence (27%; n = 39)
-
practical strategies (22%; n = 31)
-
awareness of early symptoms (11%; n = 15).
They also outlined suggestions for improvement, including:
-
greater depth (25%; n = 24)
-
more widely available (18%; n = 17)
-
booster sessions (10%; n = 10).
Teaching about eating disorders
Eighty-nine per cent (n = 692) of respondents reported that they would not feel comfortable teaching their students about EDs. Reasons given for this included:
-
they lacked the appropriate knowledge (57%; n = 312)
-
fear of iatrogenic effects (20%; n = 109)
-
students’ knowledge exceeded teacher knowledge (n = 64; 12%)
-
uncertainty about how to manage resulting disclosures (n = 61; 11%).
Reintegrating students who were absent as the result of an eating disorder
Sixty-eight per cent (n = 329) of respondents reported that their school had reintegrated one or more students following absence caused by an ED. Twenty-four per cent (n = 77) of these reported receiving no training, support or advice about how best to manage this transition.
Those who had received support outlined suggestions for improvement including:
-
training more heavily tailored to the needs of the specific returning student (24%; n = 25)
-
training for a wider range of people including parents and students as well as the teachers (16%; n = 17).
Discussion
This was the first UK study of school experiences of EDs. Respondents provided valuable insight into the experiences of UK school staff with EDs and, further, provided actionable recommendations about improvements that could be made.
Study 3: recommendations from school staff about spotting and supporting eating disorders
Methods
Participants for study 3 were recruited from those in study 2. Of 826 respondents in study 2, there were 109 expressions of interest in participating in further studies. Sixty-three of these went on to take part in the focus groups conducted in study 3.
A total of eight focus groups were held with 63 members of school staff from 29 schools in the UK. Topic guides were developed to explore school staff experience and opinion with relation to:
-
school culture
-
knowledge of school staff about EDs
-
understanding of school staff about EDs
-
communication with students
-
support strategies
-
working with parents
-
working with external agencies.
The interviews were recorded and transcribed verbatim. Following initial familiarisation with the transcripts, a categorisation system was developed using content analysis. 61,62 Transcripts were analysed and classified into categories, with care being taken to ensure the categorisation system was comprehensive while avoiding overlapping of categories. The transcripts were independently catergorised by two researchers and inter-rater reliability was 87%, with a few minor discrepancies caused by one researcher applying more categories to some items than the other. There were no instances of the two researchers failing to agree on the primary category for a transcript section.
Results
Five salient themes emerged from the focus group discussions:
-
There was little general knowledge about EDs among staff.
-
Mental health issues, including EDs, were not openly talked about among staff.
-
School staff do not feel confident or comfortable teaching students about EDs.
-
When they exist, positive relationships with parents contribute to ED recovery, but sometimes relationships with parents are very negative.
-
More support is needed for school staff involved in the care of students undergoing ED recovery.
These themes are outlined briefly below. An extended discussion, including supporting quotes, is provided by Knightsmith et al. 65
There was little general knowledge about eating disorders among staff
In general, the staff attending the focus groups stated that their own knowledge of EDs was relatively good, but that this was not reflected in colleagues throughout the staff body. Staff reported a lack of knowledge of the major types of EDs and their symptomology.
Staff also reported a lack of misunderstanding of EDs, with many colleagues belieivng that EDs are a teenage phase students will grow out of. Colleagues were reported not to realise that EDs are mental health issues that frequently require specialist medical and psychological intervention.
Mental health issues, including eating disorders, were not openly talked about among staff
Participants frequently reported a lack of open discusion about mental health issues, including EDs, at their schools. Many cited a fear of iatrogenic effects of discussing EDs either among staff or with students, whereas others shared senior management concern that being seen to be focusing on EDs would result in potential students and parents gaining a negative impression of the school.
School staff do not feel confident or comfortable teaching students about eating disorders
Staff expressed a lack of confidence and knowledge about how to talk safely to students about EDs, both in the context of speaking directly to sufferers and in the context of teaching students about EDs as part of a structured curriculum. Staff feared saying or doing the wrong thing and in so doing promoting ED behaviours either in students during their recovery or among the general student population.
When they exist, positive relationships with parents contribute to eating disorder recovery, but sometimes relationships with parents are very negative
Participants highlighted the importance of the role of the parent during recovery and the need for schools and parents to work closely together during the period of recovery. However, many staff also outlined incidents that illustrated very negative school–parent relationships. Negative responses were reported most often as a result of the initial disclosure from school to parents about a child’s ED and negative reactions from parents included:
-
parents seeing the school as interfering unnecssarily
-
parents believing that the school was accusing them of poor parenting
-
parents suggesting that the school was over-reacting.
Focus group participants highlighted the importance of the initial conversation with parents as a key time for setting the tone for the school–parent relationship.
More support is needed for school staff involved in the care of students undergoing eating disorder recovery
Focus group participants highlighted the need for further support for school staff during the recovery period. They expressed a need for practical guidance on a range of topics including:
-
student participation in sports, exercise or physical education lessons
-
supporting mealtimes
-
academic expectations including expectations around homework.
Discussion
Focus group participants provided detailed, actionable insights into the current of confidence, understanding, knowledge and attititudes of UK school staff from a range of geographically and socioeconomically diverse schools.
Although the group reported themselves to be more than usually interested in and informed about EDs and other mental health issues, they drew widely on the knowledge, experience and attitudes of colleagues during the course of the focus groups.
Development of content and outcome measures
A day-long training programme for school staff on the topic of EDs was developed in line with the National Institute for Health and Care Excellence (NICE) Behaviour Change: The Principles for Effective Interventions. 66 Data from studies 1–3 were drawn on significantly during programme development. Furthermore, school staff and clinicians were heavily involved in the authoring and piloting of training materials to ensure that the resulting training programme was relevant and practical for use within a UK school setting, while also drawing on the most recent evidence-based practice.
Content outline
Based on school staff feedback about what is feasible in terms of in-service training days, the intervention was designed to be delivered in four 90-minute sessions, which could be delivered within the space of 1 day.
Each 90-minute session had clearly designed objectives and focus. These were:
-
session 1 – EDs introduction
-
session 2 – when and how to talk to students causing concern
-
session 3 – working with parents, staff and students
-
session 4 – providing a supportive environment during recovery.
Outcome measures
An outcome measure of school staff ED attitude, confidence and knowledge was developed for use during the current study as there was no existing tool. The new tool drew on existing measures of GP attitudes,67 and the feedback of school staff and students shared in studies 1–3.
A self-report style tool was developed which captured attitudes, confidence and knowledge about EDs. A copy of the self-report measure is included in Appendix 1, Eating disorders attitudes and knowledge questionnaire.
Study 4: feasibility study of a 1-day eating disorders training programme for UK secondary school staff
Methods
Forty-five members of UK school staff completed a 1-day face-to-face training programme designed to improve their knowledge about, attitudes towards and confidence in managing EDs. Participants completed self-report measures of knowledge, attitudes and confidence at the beginning of the day, before training commenced (T1, baseline), at the end of the day, once training was completed (T2, post intervention) and again 3 months later (T3, follow-up).
The significance of intragroup changes was determined using generalised estimating equation models.
Results
The full results of the statistical analyses are shown in Tables 5 and 6. There was a statistically significant improvement (all p-values < 0.001) in participants’ self-reported knowledge, attitude and confidence scores post intervention (T2) compared with baseline (T1), with a large ES on all three comparisons. 68 These differences were maintained at the 3-month follow-up (T3) and there was no significant difference between the knowledge, attitude or confidence scores measured post intervention (T2) and at the 3-month follow-up (T3).
| Measure | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline (T1) | Post intervention (T2) | Follow-up (T3) | ||||
| n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | |
| Knowledge | 45 | 17.1 (0.76) | 45 | 29.9 (0.78) | 45 | 29.3 (0.76) |
| Attitude | 45 | 29.9 (0.17) | 45 | 37.9 (0.56) | 45 | 37.2 (0.53) |
| Confidence | 45 | 24.9 (1.44) | 45 | 48.7 (1.40) | 45 | 47.0 (1.38) |
| Measure | β | ES | 95% CI | Hypothesis test | |
|---|---|---|---|---|---|
| Lower | Upper | Sig. | |||
| Baseline (T1) to post intervention (T2) | |||||
| Knowledge | 12.8 | 0.8 | 11.3 | 14.4 | < 0.001 |
| Attitude | 8.1 | 0.8 | 7.0 | 9.1 | < 0.001 |
| Confidence | 23.8 | 0.8 | 21.2 | 26.4 | < 0.001 |
| Baseline (T1) to follow-up (T3) | |||||
| Knowledge | 12.2 | 0.7 | 9.9 | 14.5 | < 0.001 |
| Attitude | 7.4 | 0.8 | 6.3 | 8.4 | < 0.001 |
| Confidence | 22.1 | 0.8 | 17.9 | 26.3 | < 0.001 |
Participants all completed a post-course evaluation form designed to assess the acceptability of the intervention. All delegates (n = 45) considered the course either good (16%) or very good (84%) in terms of course content, course materials and for providing practical strategies they could use at school.
Discussion
A 1-day training programme for UK school staff aimed at improving attitudes towards, confidence in supporting, and knowledge about EDs was tested for feasibility and acceptability and was found to have a positive, significant impact with medium and large ESs, which were maintained after 3 months. However, the study was limited by the use of outcome measures, which were entirely subjective/self-reported and in the lack of a longer-term measure of maintenance of positive outcomes.
General discussion
Strengths
All four studies conducted as part of this WP were the first of their type within the UK setting. The contribution of male viewpoints is a significant strength of the current work as this has not previously been the norm. 64 A further strength of the studies was the large number of participants for studies of this type (511 student survey responses in study 1 and 826 school staff responses in study 2), and the depth of responses provided by these participants. A key strength of study 4 was the immediate and lasting impact of the intervention on participant attitudes, confidence and knowledge about EDs despite its brevity. A programme that took longer to deliver or the effects of which were not lasting would not be feasible or relevant for use in UK schools. Data collection at baseline, post intervention and 12 weeks post intervention is recommended, but not widely practised. 69,70
Limitations of the studies
It was not feasible to include both experimental and control groups within each school participating in study 4 owing to the possibility of trial arm contamination resulting from sharing of information between school staff, especially post intervention. In future studies, a stepped-wedge design could be employed to overcome this, with every participant completing both the control and intervention conditions.
The positive outcomes reported from study 4 in terms of an improvement in staff attitudes, confidence and knowledge were based entirely on self-report measures. Future studies could include some additional objective measures.
Study 4 looked only at school staff outcomes; the impact of the intervention was not extrapolated to explore the impact on student outcomes. Similar studies have similarly failed to provide such evidence,69,70 but appropriate measures should be considered for inclusion in future studies.
Future directions
The implementation of a fully powered stepped-wedge design in order to fully test the training programme developed in study 4 is a key future direction. Another possibility for exploration is the development of online training materials that could be accessed remotely in order to increase the reach and cost-effectiveness of the programme.
Conclusions
The aim of the current WP was to develop and feasibility test an ED training programme for school staff aimed at improving attitudes, confidence and knowledge.
This aim was achieved through a series of studies that drew extensively on the experiences and understanding of UK school staff and students in relation to EDs.
The work of all four studies was unique within the UK setting and the outcomes were promising and provide clear pathways for future research.
Chapter 3 Body image in the classroom: developing and testing a teacher-delivered eating disorder prevention programme using a clustered randomised controlled trial (work package 1b)
An abbreviated version of this chapter has been published in the British Journal of Psychiatry. 71
Abstract
Objectives
To design and evaluate a universal prevention programme for EDs in secondary schools.
Trial design
Clustered RCT comparing intervention lessons with curriculum-as-usual control.
Participants
Students in years 8 and 9.
Intervention
Six-session Me, You & Us programme delivered by teachers.
Outcomes
Questionnaires at baseline, post intervention and at the 3-month follow-up, assessing body esteem (primary outcome), eating pathology, thin-ideal internalisation, appearance conversations, peer support, depressive symptoms and self-esteem.
Randomisation
Unrestricted randomisation of intact classes using random number generator.
Blinding
Teachers, students and researchers were not blinded to group assignment.
Numbers randomised
Sixteen classes were allocated to the intervention (nine classes, 261 students) or control group (seven classes, 187 students). No participants dropped out.
Results
There were significant between-group differences in body esteem favouring the intervention group at post intervention (d = 0.12) and 3-month follow-up (d = 0.19). There were also significant between-group differences in thin-ideal internalisation (d = 0.17, maintained at follow-up, d = 0.16) and self-esteem (d = 0.20, not maintained at follow-up). There were no between-group differences for the other outcomes. Fidelity to intervention material and acceptability of the programme varied across the three schools.
Harms
There was no evidence of reduction in body esteem.
Conclusions
The Me, You & Us programme improved body esteem, thin-ideal internalisation and self-esteem, but no other outcomes. Further work to increase efficacy across the range of outcomes and improve fidelity would be valuable.
Trial registration
Current Controlled Trials International Standard Randomised Controlled Trial Number (ISRCTN) 42594993.
Introduction
Eating disorders are valuable targets for prevention because they are linked with poor outcomes, elevated mortality rates and associated personal and financial costs. 72–77 Universal prevention programmes are a useful element of the prevention portfolio as they allow us to tackle elements of the social environment that are known to be risk factors for EDs, such as perceived pressure from peers towards thinness. 78,79 Universal interventions also do not face some of the difficulties of selective programmes, namely the stigma associated with participating and the low uptake of those identified as being at risk. 80
Secondary school teachers are in a unique position to deliver prevention material widely and with minimal costs, as they have regular contact with almost all of the adolescent population. However, given the difficulties of achieving randomisation in the school setting, very few teacher-delivered interventions have been evaluated by means of a RCT,81–86 and none have done so within the UK. In addition, several of these trials have small sample sizes making them underpowered. 82,85,86 The current state of evidence regarding the efficacy of teacher-delivered interventions for eating disorders is therefore very poor. Given the potential scope for these universal interventions, this lack of high quality evidence is problematic.
In 2012, the UK All Party Parliamentary Group on Body Image recommended that all schools (primary and secondary) include mandatory lessons on body image in response to the high levels of body dissatisfaction and disordered eating in the adolescent population. 87 However, the lack of evidence base in this field means that teachers and school staff are unable to make evidence-based decisions about how best to implement this recommendation. This has resulted in some programmes, such as Media Smart [developed by the Advertising Standards Agency, URL: www.mediasmart.org.uk/resources/bodyimage (accessed 3 July 2017)] being widely disseminated without any evidence for their efficacy. This is problematic as, aside from the potential for wasted resources, some trials of interventions for eating disorders88 and depression89 in schools have found evidence of detrimental effects. There is therefore a need for safe and effective, evidence-based eating disorder prevention programmes that are deliverable by school teachers.
This study describes the evaluation of a universal teacher-delivered eating disorder prevention programme called ‘Me, You & Us’. The intervention material was based on identified risk factors for EDs and body dissatisfaction:6,90,91 thin-ideal internalisation, appearance conversations with friends, negative affect and low self-esteem. Intervention development was supported by a panel of experts and stakeholders, including PPI representatives; clinicians and researchers specialising in EDs; teachers and school nurses; and young people with and without a history of EDs.
In addition, three focus groups with 21 adolescent girls were used to determine young people’s experiences of body dissatisfaction and eating pathology, their understanding of the aetiology of these problems, and their recommendations for preventative strategies. These groups revealed that young people largely endorse a sociocultural approach to body dissatisfaction and EDs, in which media literacy and building networks of support are seen as helpful. These recommendations informed intervention development with the aim of improving the acceptability of the material produced.
Based on this review of empirical literature, including previous universal interventions, and the consultation period, a facilitator’s guide and student workbook were developed (Sharpe H, Treasure J, Schmidt U. King’s College London, London. Me, You & Us: Facilitator’s Guide. Unpublished manual; 2011). There were six interactive 50-minute lessons, with the following topics.
Lessons 1 and 2: media literacy
The aim of the first two lessons was to help participants to critique media presentations of ideal beauty through exploring how conceptions of beauty have varied over time and place, what messages are hidden in media images and how to take action.
Lessons 3 and 4: fat talking
Lessons 3 and 4 focused on perceived peer pressure towards thinness, introducing the concept of fat talking, and examining why we might fat talk as well as possible consequences. The lessons went on to challenge negative appearance-related commentary through exploring the giving and receiving of compliments.
Lessons 5 and 6: personal strengths and well-being
The remaining lessons focused on tackling negative affect and low self-esteem, through learning about personal strengths and how to use them. Simple exercises to promote well-being were explored in the final lesson, including writing a gratitude letter and carrying out small acts of kindness.
Aims and hypotheses
The aims of this study were to assess the efficacy, feasibility and acceptability of Me, You & Us, a teacher-delivered universal prevention programme for EDs. The following hypotheses were generated.
Main hypothesis
-
Students receiving the intervention will show significant improvements in body esteem, internalisation, peer support, appearance conversations, depressive symptoms, self-esteem and eating pathology compared with students in the control group at post intervention and at a 3-month follow-up.
Subsidiary hypotheses
-
Students will find the material in the intervention acceptable, in that they will report enjoying the lessons and perceive them as useful.
-
It will be feasible to train secondary school teachers to deliver an ED prevention programme from a manual and student workbook with high fidelity.
The aims of the trial were to determine the acceptability, feasibility and efficacy of this universal prevention programme for EDs.
Methods
Trial design
The study used a cluster RCT design with intact classes of students allocated in a 1 : 1 ratio to intervention or control arms (trial registration: ISRCTN42594993, including protocol).
Participants
Eligibility criteria
Participants were adolescents in years 8 and 9 in a secondary school in the UK. Secondary schools provided the point of access to participants. Schools were eligible to take part if they:
-
were based in the UK
-
had classes of students in years 8 and/or 9
-
had a sufficiently flexible timetable to manage random allocation of lessons to participating classes.
Participants were eligible to take part if they:
-
attended years 8 or 9 in a participating secondary school
-
were deemed by a member of school staff (head teacher, form teacher, school nurse) to have sufficient English language reading ability to be able to comprehend consent procedures and manage written questionnaires.
Settings and location of data collection
All data were collected within the school setting. School staff administered questionnaires within regular school hours, based on protocols provided by the researcher. Data collection took place between September 2011 and May 2012.
Intervention
The programme involved six 50-minute lessons which teachers delivered to existing classes. The intervention content was described in a Facilitators’ Manual and Student Workbook, both of which are available from the first author (HS). As discussed above, the material targeted risk factors for EDs and body dissatisfaction, namely thin-ideal internalisation, peer factors, depression and low self-esteem. 6,91 Participating teachers received a standardised 2-hour session, involving education about EDs and introduction to the Me, You & Us manual.
The control group received their usual curriculum. The content of these lessons was not determined by the research team.
Outcomes
Age, ethnicity and parental education were provided by participant self-report 1 week before the intervention began (‘pre-intervention’). Participants also completed an ED screening tool, the Eating Disorder Diagnostic Scale,92 which identifies Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) diagnostic criteria for AN, bulimic nervosa and binge ED.
All primary and secondary outcome measures were administered at pre-intervention, 1 week following the intervention period (‘post intervention’) and at approximately 3 months following the intervention period (‘3-month follow-up’).
Primary outcome
Body esteem
Body esteem was assessed using the Body Esteem Scale for Adults and Adolescents,93 a 23-item self-report measure in which participants have to rate the frequency with which they agree with statements about confidence with their appearance on a five-point Likert scale. Note, that higher scores represent greater body esteem (i.e. lower body dissatisfaction).
Secondary outcomes
Presence of binge eating
The presence of binge eating was assessed using the Eating Disorder Diagnostic Scale92 and was defined as self-reported binge eating with loss of control at least once a week for 3 months.
Presence of compensatory behaviours
Compensatory behaviours were also assessed using the Eating Disorder Diagnostic Scale92 and were defined as self-reporting of at least one of the following behaviours at least once per week for 3 months: vomiting, laxative/diuretic use, meal skipping or excessive exercise.
Thin-ideal internalisation
The extent to which participants adhered to the media portrayal of the ideals of thinness was assessed using the General Internalisation subscale of the Sociocultural Attitudes Towards Appearances Scale-3. 94 The General Internalisation subscale consists of nine items about appearances and the media (TV, magazines, films), such as ‘I would like my body to look like the models who appear in magazines’, with which participants have to agree or disagree on a five-point Likert scale. Higher scores represented greater thin-ideal internalisation.
Appearance conversations with peers
The frequency with which participants engaged with friends on the topic of appearances was measured using the Appearance Conversations with Friends Scale. 95 The five items are designed to assess ‘how often students talked with their friends about expectations for their bodies and for appearance enhancements’,95 and take the form of statements such as ‘my friends and I talk about the size and shape of our bodies’, with which participants have to agree or disagree on a five-point Likert scale. Higher scores represent more frequent appearance conversations.
Peer support
Perceived social support was measured using the Friend subscale of the Multidimensional Scale of Perceived Social Support. 96 This four-item scale assesses perceived support from friends through responses on a seven-point Likert scale to items such as ‘My friends really try to help me’. Higher scores represented greater perceived social support.
Depressive symptoms
Depressive symptoms were assessed using the Depression subscale of the short version of the Depression Anxiety and Stress Scales (DASS-21). 97 The DASS-21 Depression subscale is a seven-item scale in which participants are required to state how often particular statements, for example ‘I felt that I had nothing to look forward to’, applied to them over the past week. Higher scores represent greater depressive symptoms.
Self-esteem
A single item – ‘How positive do you feel about yourself?’ – was used to assess self-esteem. Participants were required to respond on a five-point Likert scale from ‘Not at all positive’ to ‘Very positive’. Higher scores represent higher self-esteem.
Acceptability
The programme’s acceptability was assessed using two five-point Likert scales. The first asked ‘How much did you enjoy Me, You & Us?’, and the second asked ‘How useful did you find Me, You & Us?’. The Likert scales ranged from ‘Not at all’ to ‘Very much’. Higher scores represent greater acceptability.
Fidelity to intervention guide
In order to determine fidelity to the intervention manual, two lessons were observed and rated in each school against adherence to planned content. Each activity in the Facilitator’s Guide was scored as ‘completed’ or ‘not completed’. Free text was used to note whether or not any additional material was covered.
Sample size
To account for clustering, the sample size calculation was increased by an inflation factor [1 + (average cluster size – 1) intracluster correlation (ICC)]. The inflation factor for this trial was based on a small ICC (ICC = 0.05). 98 With an average class size of 28 students the estimated inflation factor was 2.35.
G*Power 3 (University of Düsseldorf) was used for sample size calculations. 99 Assuming a 1 : 1 ratio, a small ES (d = 0.20)100 and power set to 0.80, the basic sample size requirement was 394 participants per group, which increased to 926 per group when the estimated inflation factor was taken into account.
Randomisation and blinding
Intact classes were randomly allocated to intervention and control arms. As classes were enrolled into the trial, an unrestricted random allocation was generated by an online random number generator. 101 One researcher (HS) carried out the enrolment of classes, the generation of the random allocation sequence and the allocation of classes to conditions. Participants’ allocation in trial arm was based on their class membership. Informed consent for participants was obtained from all participants’ parents/carers following randomisation. In addition, participants provided written assent post randomisation, when completing the pre-intervention questionnaire measures.
The trial design precluded blinding of school staff or students participants as intervention materials, such as the student workbooks, would have been identifiable to staff and students as being distinct from their usual curriculum. Researchers were also unblinded.
Statistical analyses
Full details of statistical analyses, including managing of missing data, are reported by Sharpe et al. 71 All analyses were based on originally assigned groups. The main hypothesis was tested using linear and logistic mixed-effects models. Two continuous outcomes that were not normally distributed (depression, peer support) were dichotomised for all analyses.
In addition to significance testing, ESs (d) for continuous outcomes were calculated using the differences in adjusted means at each time point. Reliable and clinically significant changes were also computed102 using reliability data and clinical cut-off points from previous work. 103
Results
Participant flow and characteristics
Three schools agreed to participate in the trial. One additional school refused participation because it did not want to trial previously untested resources. Each of the schools was state maintained and had 100% female intake. The three schools varied in their average level of deprivation (free school meal eligibility ranged from 2% to 24%), and in the ethnic background of their students (the percentage of students from black and ethnic minority backgrounds ranged from 28% to 77%). Random allocation of 16 intact classes from year 8 or 9 in these schools resulted in nine classes allocated to the intervention arm and seven classes allocated to the control arm. Of the 479 students from these classes, 31 were excluded because of lack of parental consent. This resulted in 261 students in the intervention arm and 187 students in the control arm. Between 92% and 98% of students provided data at each data collection point. All missing data were due to school absence. No participants withdrew from the trial.
There were no significant differences between the two trial arms at the beginning of the trial. Participants had a mean age of 13.06 (SD = 0.59) years in the intervention group and 12.99 (SD = 0.54) years in the control group. Equal proportions of participants came from ethnic minority backgrounds (intervention = 47%, control = 53%), and had parents with university-level education (intervention = 76%, control = 78%). There was also no difference on reports of body esteem (intervention: mean = 2.30, SD = 0.75; control: mean = 2.27, SD = 0.70), or across any of the other clinical outcomes for the trial. Eight participants scored above the cut-off point in the ED screening tool and so were excluded from analyses on the grounds that the aim of the programme was prevention of future difficulties.
Acceptability and feasibility
Students in the intervention arm were asked to rate how useful and enjoyable they found the lessons in the intervention. The results are shown in Table 7. Looking across both ratings of lessons being enjoyable and useful, the acceptability are notably higher in two schools (A and C) compared with the third school (B). Whereas few students (3–16%) in schools A and C rated the programme negatively, this figure was nearer 50% for school B.
| Acceptability | School, % | ||
|---|---|---|---|
| A (n = 31) | B (n = 79) | C (n = 80) | |
| Enjoyable | |||
| Negative | 3 | 48 | 9 |
| Neutral | 34 | 36 | 48 |
| Positive | 63 | 16 | 43 |
| Useful | |||
| Negative | 10 | 51 | 16 |
| Neutral | 39 | 30 | 41 |
| Positive | 51 | 19 | 43 |
These findings mirror the results of the feasibility assessment, in which the teachers’ fidelity to the intervention manual was assessed. A greater amount of intervention content was delivered in schools A and C than in school B (78% activities rated as ‘completed’ in schools A and C compared with 50% in school B). School B did not deliver material outside the programme, but instead took longer to complete each activity, meaning that fewer activities were completed within the set lesson time.
Body esteem
Results from the mixed-effects models showed an overall marginal effect of the intervention on improvements in body esteem [β = 0.09, standard error (SE) = 0.05; p = 0.08]. Post hoc analyses at each time point showed a marginal difference between the groups post intervention (intervention: mean = 2.31, SE = 0.32; control: mean = 22.2, SE = 0.39; p = 0.07) and a significant difference between the groups at the 3-month follow-up (intervention: mean = 2.37, SE = 0.32; control: mean = 2.22, SE = 0.39; p = 0.006). In each case the results favoured the intervention over the control condition. The ESs for these group differences were small (d = 0.12–0.19).
Reliable and clinically significant change from baseline to post intervention was calculated separately for those above and below the clinical cut-off point at baseline. As this was a community sample, few participants were above the clinical cut-off point at baseline (n = 60). Of those above the cut-off point initially, 50% (n = 17) of participants in the intervention group showed clinically significant improvement, compared with 38% (n = 10) in the control group. However, this difference was not statistically significant [χ2(1) = 0.79; p = 0.37]. When considering reliable change, 32% (n = 11) of participants in the intervention group showed reliable improvement, compared with 8% (n = 2) in the control group. This difference was statistically significant [χ2(1) = 5.97; p = 0.02].
Considering those participants that began the trial in the normal range for body esteem, it was notable that there were no participants that experienced clinically significant worsening of symptoms. Some participants did, however, show reliable improvements in body esteem. In the intervention group, 12% participants (n = 20) experienced reliable improvement compared with 4% in the control group (n = 4). This difference was statistically significant [χ2(1) = 6.58; p = 0.01].
Secondary outcomes
A linear mixed-effects model for continuous secondary outcomes showed that there was a main effect of group for thin-ideal internalisation (β = –1.53, SE = 0.74; p = 0.04) and self-esteem (β = 0.19, SE = 0.09; p = 0.04). There was no significant main effect for appearance conversations (β = –0.04, SE = 0.32; p = 0.90). For thin-ideal internalisation, looking separately at each time point showed significant differences between the groups both post intervention (intervention: mean = 21.94, SE = 0.47; control: mean = 23.47, SE = 0.57; p = 0.04) and at the 3-month follow-up period [χ2(1) = 3.84; p = 0.05]. At each time point the results favoured the intervention. For self-esteem, the comparisons between the groups at each of the time points revealed a significant difference post intervention (intervention: mean = 3.52, SE = 0.06; control: mean = 3.33, SE = 0.07; p = 0.04), but no difference by the 3-month follow-up (intervention: mean = 3.47, SE = 0.06; control: mean = 3.34, SE = 0.07; p = 0.15).
Logistic mixed-effects models were used to explore intervention effects for binary outcomes. There were no main effects of group for binge eating [odds ratio (OR) = 4.44, 95% CI 0.39 to 51.22; p = 0.23], compensatory behaviours [OR = 1.69, 95% CI 0.74 to 3.89; p = 0.22], peer support [OR = 1.40, 95% CI 0.64 to 3.06; p = 0.40] or depressive symptoms [OR = 1.49, 95% CI 0.46 to 4.78; p = 0.50]. Rates of each of these outcomes at each time point are reported in detail elsewhere. 71
Discussion
This study described the design of a teacher-delivered ED-prevention programme and its first rigorous evaluation in UK secondary schools. The results suggest that the approach is feasible and that a programme of this kind can have benefits for adolescents’ mental health.
The programme produced significant improvements in participants’ body esteem compared with their peers who received their usual school curriculum, and this impact was maintained through the 3-month follow-up period. The programme also improved several of the secondary outcomes. First, thin-ideal internalisation was reduced, meaning that the participants were less likely to endorse social ideals associated with thinness. Second, self-esteem was greater in those who received the intervention, although the effects were not maintained at the post-intervention assessment. In contrast to these findings, there were no effects for eating pathology, appearance conversations, peer support or depressive symptoms. There was also clear evidence that intervention delivery and reception varied substantially between the three schools. This suggests that understanding the best way to support delivery of materials such as these in a wide range of schools is a key aspect to take forward from this research.
It is difficult to compare these results with previous work, difficult because most body image interventions in schools have been delivered not by teachers but rather by external expert facilitators. 100,104 The small ESs found in this trial are in line with work outside the UK in which teacher-delivered interventions have been tested. 81,105 Existing work has also reported similar null findings for the broader impact of ED-prevention programmes. For example, other work has found like effect on depressive symptoms despite some content dedicated to this factor. 81,85 Given that interventions focusing specifically on depression are more intensive, it may be that the problem is one of dosage. 106 Similarly, few teacher-delivered prevention programmes have successfully impacted on eating pathology,84,85 although one study with considerably more intensive teacher training has shown promise in this area. 82
Strengths and limitations
A number of factors limit the conclusions of this trial. First, there was a risk of control group contamination, due to random allocation of classes within the same school. Second, we did not use an active control condition. Potentials for sham interventions that have been used in previous similar trials include activities such as expressive writing,107 educational brochures108 and healthy eating programmes. 109 Third, the sample size recruited was below that required for desired statistical power. Post hoc estimates of achieved power using the ES for the primary outcome at the 3-month follow-up (d = 0.19) show that the trial only had 49% power to detect group differences. Fourth, the assessment of intervention fidelity was coarse (two lesson observations per school). Ideally, the trial would have involved recording all sessions and having these rated by independent researchers. Finally, the follow-up period in this trial was limited to 3 months. Valuable information about whether or not effects are maintained in the medium to long term would be gained from longer follow-up.
The generalisability of the results was bolstered by the fact that the schools involved represented a wide range of different participants, including many from ethnic minority backgrounds as well as a range of economic backgrounds. It is also of significance that the trial was conducted in state-funded schools, and was not delivered by specialist school staff. However, it should be pointed out that the reliance on girls’ schools does limit the generalisability of these findings, as typical state-maintained schools in the UK are coeducational. Replication of these findings in coeducational schools is a priority going forwards with this work.
Conclusions
Overall, this study suggests that it is possible to design a teacher-delivered ED-prevention programme that is efficacious, manageable for teachers and liked by students. The between-school differences in acceptability and fidelity suggest that further work is needed to increase the suitability of the materials across a range of school settings and also to examine best practice around training teachers for taking on the delivery of this programme. Efficacy was not found across all outcomes and so there was also scope for improvements to the materials for which intended impacts were not observed. In addition, further trials will be essential to replicate these findings and to ensure that materials provided for schools are a safe and effective use of school resources in tackling EDs.
Chapter 4 A randomised controlled multicentre trial comparing the Maudsley Model of Anorexia Nervosa Treatment for Adults with specialist supportive clinical management in outpatients with broad anorexia nervosa (work package 2a)
An abbreviated version of this chapter has been published in the Journal of Consulting and Clinical Psychology. 110
Abstract
Objectives
To assess the efficacy of a new psychological treatment for AN (MANTRA) versus specialist supportive clinical management (SSCM) in a RCT.
Trial design
Multicentre two-arm superiority trial.
Setting
Four specialist ED services within the south of England.
Participants
Adult outpatients, aged between 18 and 65 years, meeting DSM-IV criteria for AN or an eating disorder not otherwise specified – anorexia nervosa subtype (EDNOS-AN), with a body mass index (BMI) of < 18.5 kg/m2.
Intervention
Twenty once-weekly treatment sessions of MANTRA or SSCM (extended to 30 sessions in those with a BMI of < 15 kg/m2) and optional extras (carers’ sessions, dietetic sessions).
Outcomes
Primary
Body mass index.
Secondary
Eating Disorders Examination (EDE), depression, anxiety and clinical impairment; neurocognitive and social cognitive measures; rates of recovery; and utilisation of other services.
Outcomes were assessed at baseline, 6 months and 12 months.
Randomisation
Completed independently from trial team using a restricted stratified randomisation algorithm with (1) BMI of > or ≤ 15 kg/m2, (2) AN subtype and (3) previous ED admission, as stratifiers.
Blinding
Research assessors were blind, but patients and therapists were not blinded to treatment allocations.
Numbers randomised
Seventy-two patients allocated to MANTRA and 70 allocated to SSCM.
Results
There was no difference in outcome between groups. In both treatments, patients showed significant improvements in BMI, ED symptoms and other clinical outcomes. Patients rated MANTRA as more acceptable and credible than SSCM at 12 months.
Harms
One SSCM patient died during treatment.
Conclusions
Both MANTRA and SSCM can be used as outpatient treatments in adults with AN.
Trial registration
Current Controlled Trials ISRCTN67720902.
Introduction
Anorexia nervosa is a severe mental disorder, and has the highest rate of premature death of any psychiatric disorder. It also is accompanied by much somatic and psychological comorbidity and results in poor quality of life. 111,112 Positive treatment outcomes are difficult to achieve because the disorder is valued by sufferers. In addition, the anxious and obsessional personality traits with which patients commonly present impede recovery. Neurocognitive and socioemotional functioning are often impaired,16,113,114 which makes engagement in treatment an even greater challenge.
Anorexia nervosa puts a great burden on families,115 as relatives are typically closely involved in supporting the person with the illness. Family members carry a burden comparable to that of carers of individuals with psychosis. 48 The financial burden for the health service is also substantial with the cost per case of AN equating to that of schizophrenia. 45,46 Recent reports from the UK and Australia point to considerable costs of EDs to the health-care sector, sufferers and their families. 116–118
Psychotherapy is the recommended first-line treatment for most people with AN, but treatment outcomes greatly depend on the stage of the illness. For adolescents with a shorter duration of illness the response to the usually family-based psychological treatment is excellent. 119 In contrast, for adults with a longer illness duration, treatment outcomes are much less positive and dropout rates are high. 120 Different professional bodies on both sides of the Atlantic have highlighted the need to develop better treatments for adults with AN. 121–124
A Cochrane review of outpatient treatment for AN identified seven small and underpowered treatment trials, two of which included children or adolescents. 125 No treatment approach was consistently better than any other. To date, only one sufficiently powered RCT has been conducted. This compared individual psychodynamic therapy, cognitive–behavioural therapy (CBT) and an optimised TAU. Here, once again, no clear differences in weight outcomes were found between groups at the end of treatment.
A potential reason for the unimpressive treatment outcomes in adults with AN is that treatments that have been tested in RCTs were originally developed in the context of other disorders and have then been modified for use in AN. Available treatments are therefore not suitably tailored to the requirements and/or specific maintenance factors of AN. In an attempt to rectify this problem, we have developed a new treatment for AN, based on our group’s research into neurocognitive, social cognitive and personality characteristics of these patients,19,20,126 the MANTRA. This treatment is unique in including maintenance factors that are intrapersonal or interpersonal. It also includes therapeutic strategies to address these. The treatment is manualised and contains a number of treatment modules. The content of these can be tailored to the needs of each individual. This novel treatment is compared here against a gold-standard comparison treatment, SSCM. SSCM was designed as a comparison treatment in a small trial of outpatient therapies for AN,127 and was found to be superior on a range of outcomes compared with CBT and interpersonal therapy (IPT) in the treatment of adult AN.
Aims
-
The central aim of this work is to test the efficacy and cost-effectiveness of MANTRA in adult outpatients with AN versus SSCM using a randomised controlled design. (Only efficacy data are reported here; cost-effectiveness data are not yet available and will be reported elsewhere when complete.)
-
The subsidiary aim is to assess the impact of the experimental treatment on the requirement for any intensive (i.e. day care or inpatient) treatment and to identify mediators and moderators of treatment outcome. (These results are not currently available and will be published elsewhere when complete.)
Hypotheses
-
MANTRA will be superior to SSCM in producing higher weight increase and greater reduction in ED symptoms in adults with AN at 6 and 12 months.
-
MANTRA will be more cost-effective than SSCM, being less costly at 6 and 12 months. Specifically, MANTRA patients will need fewer and briefer hospitalisations during the study period compared with SSCM. (Only the hospitalisation aspect of this hypothesis is addressed here; the full cost-effectiveness data are not yet available and will be published elsewhere when complete.)
Methods
The methods presented here are taken from the published Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC) trial protocol. 128
Trial design
This is a superiority trial conducted in four NHS specialist ED units. The trial evaluated the efficacy and cost-effectiveness of MANTRA versus SSCM in consecutively referred adult outpatients with AN. Patients were allocated to one of two treatment arms: MANTRA or SSCM. Further details on the randomisation procedure are given below.
Outcomes were measured pre-randomisation, at 6 months post randomisation (i.e. designed to broadly coincide with the end of weekly treatment) and at 12 months post randomisation (follow-up). Outcomes were assessed by researchers who were not involved in the treatment and efforts were made to keep researchers blind to patients’ treatment allocation. As much as possible, patients who had terminated treatment early were followed up to enable intention-to-treat (ITT) analysis.
Ethics approval
We obtained ethics approval for the MOSAIC trial from Central London Research Ethics Committee (REC) 4, National Research Ethics Service, Royal Free Hospital, London (NHS REC Reference: 10/H0714/9). All participants provided informed written consent prior to entering the study. The study was conducted in compliance with the Helsinki Declaration.
Participants
Inclusion criteria
Consecutive patients referred for outpatient treatment to one of the participating centres by their GP were offered participation if they were:
-
aged between 18 and 60 years
-
had a BMI of ≤ 18.5 kg/m2
-
had a DSM-IV diagnosis of AN or eating disorder not otherwise specified (EDNOS). We defined EDNOS as in the meta-analysis by Thomas et al. 129 and included individuals who fulfilled all diagnostic criteria of AN, with the exception of the weight criterion; those who still had menstrual bleeds; those who did not have a fat phobia; and those with a partial AN syndrome (i.e. they had features of AN but failed to meet two or more of the four diagnostic criteria).
The meta-analysis by Thomas et al. 129 shows that EDNOS patients with a more lenient BMI cut-off point and without amenorrhoea are very similar to those with more narrowly defined AN. The BMI cut-off point of 18.5 kg/m2 was selected to be concordant with the World Health Organization’s cut-off point for being underweight. Additionally, this cut-off point was also used in previous studies by our group and others. 128,130,131
Exclusion criteria
We excluded patients if they had severe, medically unstable AN that necessitated inpatient treatment;124 had poor command of English and thus were unable to understand assessment and treatment; had a learning disability; had significant other mental or physical illness that required intervention (e.g. psychotic illness or diabetes mellitus); were alcohol or substance dependent; or were pregnant.
We did not exclude patients on stable doses of antidepressants, defined as having taken the medication for ≥ 4 weeks.
Locations of data collection
Patients were recruited from several centres: South London and Maudsley NHS Foundation Trust; North East London Foundation Trust Eating Disorders Service; Barnet, Enfield & Haringey Mental Health NHS Trust; Oxford Health NHS Foundation Trust.
Interventions
Common features of delivery of Maudsley Model of Anorexia Nervosa Treatment for Adults and specialist supportive clinical management
In both groups patients were offered 20 individual therapy sessions, which took place once a week. In addition, there were 4-monthly follow-up sessions. In severely underweight patients (BMI ≤ 15 kg/m2) sessions were extended to 30 meetings plus four follow-up sessions. In both MANTRA and SSCM, patients were offered two additional sessions with a family member or close other. Finally, in both groups patients could access dietetic sessions if deemed appropriate by their clinician. In both treatments physical risk was monitored on an ongoing basis. Therapy sessions in MANTRA lasted ≈50 minutes throughout treatment, whereas, in SSCM, from the middle of treatment sessions could be briefer (i.e. about 30 minutes) in accordance with the SSCM protocol (available from the authors). 132 This was done to ensure comparability of our study with other trials using SSCM.
Maudsley Model of Anorexia Nervosa Treatment for Adults
The MANTRA model19 suggests that AN typically develops in people with anxious and obsessional personality traits at times of stress or increased developmental demands. Dietary restriction becomes a way of managing negative emotions and coping with stress, and, once established, the illness is maintained by four key maintenance factors in the cognitive, emotional and interpersonal domains. First, this includes an information processing style that is characterised by perfectionism with high standards and fear of making mistakes, excessive detail focus and poor ability to assess ‘gist’ and cognitive rigidity (i.e. an inability to switch between tasks or task demands). Second, there are impairments in emotion generation and regulation (intense emotions, lack of emotional clarity, avoidance and suppression of emotions/emotion expression, reduced ability to adaptively reappraise emotional stimuli). Third, together these cognitive and emotional styles give rise to AN becoming valued by the person (i.e. the person develops positive beliefs about how AN helps them in their life). 133–135 Finally, the inadvertent response of family members or close others, who may be anxious, critical or hostile, may contribute to illness maintenance.
A treatment workbook is given to each patient (see data supplement DS1 in Schmidt et al. 126,136 for details). This has both core and optional chapters, thus the information contained in the manual can be tailored to the requirements of each patient. Throughout the style of therapy is that of motivational interviewing. 137 This means that the therapist draws on the patient’s experience and is responsive and reflective. Based on a thorough biopsychosocial assessment an individual case formulation is developed collaboratively. This also includes a focus on the person’s personality traits, strengths and supports. Normative and ipsative feedback (e.g. about medical risk and thinking style) is given to foster discussions about and interest in change. Health behaviour change principles and techniques are used to guide individuals towards better health and recovery. 138,139 The structure, sequence and hierarchy of treatment procedures are clearly defined. This largely depends on an individual’s clinical severity and also takes into account motivation for change, medical risk and available individual resources and supports. Family members are asked to attend sessions as necessary.
Specialist supportive clinical management
This treatment was designed as a credible comparison against CBT and IPT in a RCT. 127 Full details are given in McIntosh et al. 140 There is a manual for therapists (available from the authors),132 which contains patient handouts giving them relevant information on topics such as the risks of commonly used weight control behaviours and the impact of starvation on body and mind. These handouts are used flexibly throughout treatment. 132 SSCM is designed to be delivered by ED experts and aims ‘to mimic outpatient treatment that could be offered to individuals with AN in usual clinical practice’. SSCM combines principles of good clinical management (emphasising care, safety and expert knowledge) and supportive psychotherapy (emphasising warmth, acceptance, reassurance and information giving as needed). A central feature of SSCM is the focus on the patient’s underweight and abnormal eating behaviour. Patients are given practical advice on how to work on these issues and the key message to them is that if they manage to improve their nutrition their physical and emotional health will also improve. All other therapy content is ad hoc and is dependent on what the patient wishes to discuss in a particular session.
Therapists
Twenty-eight ED therapists delivered the trial therapies across the four centres. Therapists were trained in MANTRA and SSCM (2 days were provided as an introduction to each treatment). Additionally, further training days were offered periodically to ensure uniform delivery of treatments across centres and to protect against ‘therapeutic drift’. Therapists had to deliver both treatments to minimise allegiance effects. Supervision was provided to therapists on a weekly basis by designated trained supervisors in each team. To avoid cross-therapy contamination, this was delivered separately for MANTRA and SSCM. Patients were allocated to therapists based on availability. To ensure that treatments were delivered competently and as planned, audio-recordings of sessions were carried out and three tapes per patient were chosen randomly to assess treatment adherence in both groups.
For each of their patients, therapists recorded details, including number, duration and content of sessions, and details of any additional treatment.
Outcomes
All outcome measures were collected at baseline, 6 months after randomisation (i.e. approximately end of weekly sessions) and 12 months after randomisation (i.e. follow-up). Treatment credibility and acceptability ratings were taken only at 6 and 12 months. Potential outcome mediator variables were also taken at 3 months (mid-treatment). These results are not yet available and will be published elsewhere when complete.
Primary outcome
-
BMI (kg/m2) at 12 months.
Secondary outcomes
-
BMI (kg/m2) at 6 months.
-
EDE global score and subscales. 141 The EDE is a semistructured interview that has four subscales: dietary restraint, eating concern, weight concern and shape concern. The global score is created from the mean of these four subscales. Patients that were either unwilling or unable to participate in the EDE interview were asked instead to complete the questionnaire version of this measure (Eating Disorders Examination-Questionnaire; EDE-Q). The EDE-Q has comparable psychometric properties to the EDE interview. 142
Potential mediators
The Cognitive Flexibility Scale,144 Beliefs about Emotions Scale,145 the Emotion Regulation Questionnaire146 and a visual analogue scale assessing motivation and social support. These results are not yet available and will be published elsewhere when complete.
Treatment credibility and acceptability
Visual analogue scales of credibility and acceptability of treatment were specifically developed for this trial.
Neurocognitive and social cognitive measures
-
The Wisconsin Card Sorting Test (WCST)147,148 is a widely used measure of set-shifting (i.e. it tests the ability to flexibly switch between different tasks or rules). The participant has to match stimulus cards with one of four category cards. The stimuli are multidimensional according to colour, shape and number. The matching rules change over time and the participant has to adapt to these. We used perseverative errors as the outcome.
-
The Brixton Spatial Anticipation Test149 also assesses set-shifting. Participants have to predict the movement of a blue circle across different positions, adapting their predictions according to the pattern of movement. Error rates are assessed.
-
The Rey–Osterrieth Complex Figure Test (REY)150,151 tests participants’ central coherence (i.e. their ability to plan, organise and assemble complex information). Participants have to copy a complex figure design and are assessed on their approach to this. The more fragmented and detail focused their approach to the task, the lower their score.
-
Baron-Cohen’s Reading the Mind in Film Task (RMF)152 assesses theory of mind based on 22 brief film clips. After viewing a clip the participant is required to select one of four words to describe how the protagonist is feeling in the given situation.
Costs and psychosocial impairment
-
The Client Services Receipt Inventory (CSRI):153 we used a self-report version of this inventory of service use. It was adapted for the present trial to cover a range of mental and physical health services, medications, impact on employment and additional personal expenditure due to the ED. Results relating to the CSRI are not yet available and will be published elsewhwere when complete.
-
The Clinical Impairment Assessment (CIA):154 this is a questionnaire measure assessing psychosocial impairment arising from the individual’s ED behaviours.
Sample size
Full details of our sample size calculation, including the data and assumptions on which this is based, can be found in our protocol paper. 128 In brief, we assumed that we would need a sample size of 69 patients per group to have 90% power to detect a difference in mean weight gain of 2.5 kg, using an independent-samples t-test with a significance level of α = 0.05 and correcting for 20% attrition.
Randomisation and implementation
The randomisation sequence was generated and implemented independently from the study team by the King’s College London Clinical Trials Unit (CTU). After participant recruitment and baseline assessment, the research assessor entered patient identification and stratification details into the online CTU system. Participants were then allocated to one of the two treatments based on a restricted stratified randomisation algorithm. The strata were (1) BMI below or above 15 kg/m2, (2) restricting or binge/purge subtype of AN and (3) previous hospitalisation in an ED unit. These factors are known to affect treatment outcome and rates of potential future inpatient treatment. The stratification was implemented by minimised randomisation with a random component. The first n cases (n would not be disclosed) were allocated entirely at random to further enhance allocation concealment.
Blinding
Although patients and therapists were not blinded, the research assessors were blind to treatment allocation. In order to assess whether or not researcher blinding was successful, they had to make a guess at the end of 12 months’ assessment as to which treatment they believed the person had received.
Statistical methods
The objective of the statistical analyses was the comparison of those offered MANTRA with those offered SSCM on a number of outcomes. The primary clinical outcome was BMI (kg/m2) at 12 months after randomisation. Secondary outcomes were continuous measures at 6 or 12 months’ follow-up (see above). All statistical analyses were based on the ITT principle; thus, patients were analysed in the treatment arm to which they were randomised irrespective of whether or not they received the allocated treatment. All analyses were carried out in Stata® 12 (StataCorp LP, College Station, TX, USA).
All outcomes were analysed using linear mixed models. Full details of the analysis plan can be found elsewhere. 110,128 Outcome variables contained considerable numbers of missing values. Full details of the treatment of missing variables can be found elsewhere. 110
Results
Participant flow and recruitment
Flow of participants through the trial is shown in Figure 1. Of the 319 patients approached, 142 agreed to participate in the trial. Following baseline assessments, random allocation of the 142 participants across all four study sites resulted in 72 patients in the MANTRA arm and 70 in the SSCM arm. Three patients did not receive their allocated therapy (see Figure 1).
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagram (WP2a). Copyright © 2015 by the American Psychological Association (APA). Reproduced with permission. The official citation that should be used in referencing this material is Schmidt U, Magill N, Renwick B, Keyes A, Kenyon M, Dejong H, et al. (2015). The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): Comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with specialist supportive clinical management (SSCM) in outpatients with broadly defined anorexia nervosa: a randomized controlled trial. Journal of Consulting and Clinical Psychology, 83(4), 796–807. 110 The use of APA information does not imply endorsement by APA.
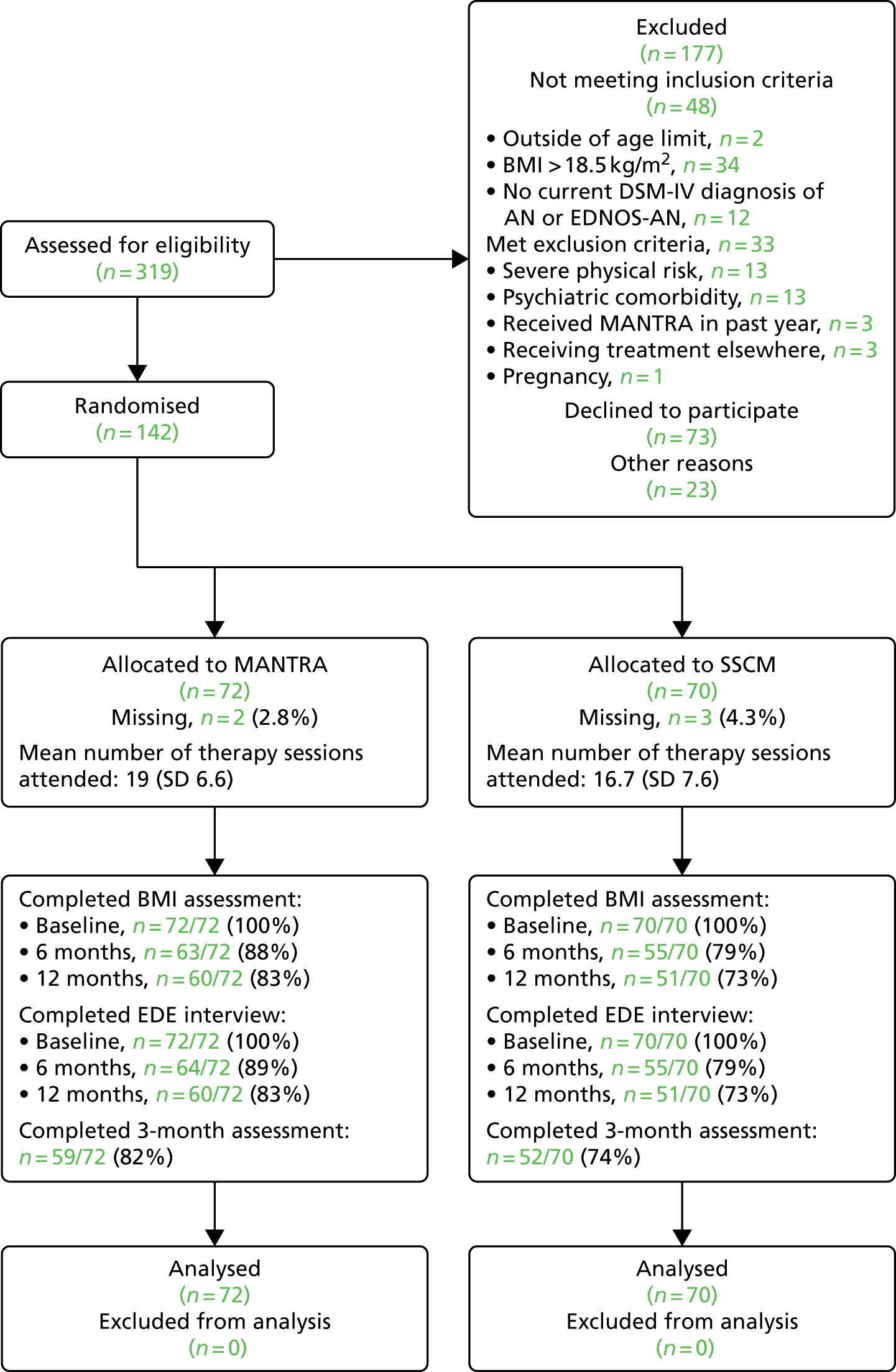
Treatment completion was defined at the start of the study as attending ≥ 15 therapy sessions (i.e. receiving more than three-quarters of the weekly treatment sessions). This definition is in line with other studies’ definitions of treatment adherence. 127,131 Overall, 66.9% of participants were treatment completers, with 75% of MANTRA participants and 59% of SSCM participants completing treatment [χ2(1) = 1.19; p = 0.28].
Non-completion of treatment predicted loss to follow-up at 12 months. At the 12-month follow-up, primary outcome data were missing for 45.2% of non-completing participants, compared with 10.5% of completers. Details of the reasons for missing data can be found in Figure 1.
All 142 participants were included in the primary outcome analysis.
The trial was conducted between June 2010 and November 2013. Between June 2010 and November 2012, participants were recruited into the trial via their initial clinical assessment with the EDs service, which was followed by a pre-intervention assessment. Delivery of the intervention occurred between June 2010 and November 2013. End of weekly treatment session follow-up assessments (month 6) were completed during December 2010 to May 2013 and end of treatment follow-up assessments (month 12) occurred throughout June 2011 to November 2013.
Service utilisation
Use of additional dietitian sessions was not different between groups [MANTRA 33/72 (46%); SSCM 31/70 (44%)]. Sixty-seven of 72 (93%) MANTRA patients and 64 of 70 (91.4%) SSCM patients provided information on additional service utilisation (outside the study protocol) during the study period. Eighteen of 131 patients for whom this information was available (13.7%) had additional treatment during the study period. This was categorised into ED inpatient or day care treatment, general psychiatric inpatient treatment or other treatment. Eight of these patients had been allocated to MANTRA. Two of these had ED inpatient treatment (217 and 66 days, respectively), one of these also had 30 days of ED day care treatment and 1 day of ‘other treatment’. Three further patients only had ED day care (175 days, 144 days and 1 day, respectively). One of these also had a 10-day inpatient alcohol detoxification programme. Two further patients had brief general psychiatric admissions (in one case 7 days and in the other 10 days), because of suicidal or self-harm behaviour; one of these also had 1 day of ‘other treatment’. One further patient had 1 day of ‘other’ treatment only.
Ten patients who needed additional treatment had been allocated to SSCM (15.6%). Five of these had ED inpatient care (of 45, 62, 110, 140 and 198 days), with one of these having a second 8-day ED admission. Three patients had ED day care (for 2, 54, 183 days); one of these also had a 50-day general psychiatric admission following depression and suicidal behaviour. One further patient had a 1-day general psychiatry admission followed by 11 days of home treatment, and one patient had 1 day of ‘other treatment’. Proportions of people needing additional treatment were not different between MANTRA and SSCM. There was also no significant difference between the two treatment arms in the number of days per admission or admission days per patient.
Baseline data
The baseline characteristics of patients in both groups were comparable (Table 8) Overall, the mean baseline BMI was 16.6 (SD 1.2) kg/m2 and the duration of illness was 8.3 (SD 7.3) years. Sixty-three (44.4%) patients presented with restrictive AN.
| Characteristic | Whole group | MANTRA | SSCM | |||
|---|---|---|---|---|---|---|
| n | n | n | ||||
| Demographic details | ||||||
| Age at randomisation (years): mean (SD) | 142 | 26.7 (7.7) | 72 | 27.5 (8.1) | 70 | 25.9 (7.1) |
| Male : female, n | 142 | 3 : 139 | 72 | 0 : 72 | 70 | 3 : 67 |
| Years in education, mean (SD) | 125 | 15.8 (2.3) | 63 | 16.1 (2.1) | 62 | 15.5 (2.5) |
| Has a partner, n (%) | 138 | 50 (35.2) | 72 | 21 (29.2) | 66 | 29 (41.4) |
| Clinical details | ||||||
| Diagnosis, n (%) | 142 | 72 | 70 | |||
| AN-R | 63 (44.4) | 35 (48.6) | 28 (40.0) | |||
| AN-BP | 44 (31.0) | 22 (30.6) | 22 (31.4) | |||
| EDNOS | 35 (24.7) | 15 (20.8) | 20 (28.6) | |||
| BMI (kg/m2), mean (SD) | 142 | 16.64 (1.2) | 72 | 16.61 (1.2) | 70 | 16.62 (1.3) |
| Weight (kg), mean (SD) | 142 | 45.1 (4.9) | 72 | 44.8 (4.5) | 70 | 45.4 (5.4) |
| Age at onset (years), mean (SD) | 132 | 17.7 (6.5) | 67 | 17.3 (6.5) | 65 | 18.1 (6.6) |
| Illness duration (years), mean (SD) | 134 | 8.3 (7.3) | 67 | 9.3 (7.9) | 67 | 7.2 (6.5) |
| Previous eating disorder treatment, n (%) | 140 | 80 (56.3) | 70 | 41 (56.9) | 70 | 39 (55.7) |
| EDE, mean (SD) | 142 | 3.3 (1.3) | 72 | 3.1 (1.3) | 70 | 3.5 (1.3) |
| DASS-21, mean (SD) | 138 | 30.5 (12.7) | 69 | 29.6 (11.5) | 69 | 31.4 (13.8) |
| CIA, mean (SD) | 141 | 32.6 (8.9) | 71 | 32.1 (9.0) | 70 | 33.0 (8.9) |
| Current antidepressant medication, n (%) | 140 | 55 (38.7) | 70 | 29 (40.3) | 70 | 26 (37.1) |
Primary outcome
Tables 9 and 10 show that the two treatments did not differ in their effect on BMI at 6 months (p > 0.05) or at 12 months (p > 0.05) (primary outcome).
| Outcome | Predicted mean (SE), baseline fixed to sample average | Estimated group differencea | Test | 95% CIa | Standardised coefficienta | |
|---|---|---|---|---|---|---|
| MANTRA | SSCM | |||||
| BMI (kg/m2) | 17.52 (0.21) | 17.24 (0.23) | 0.28 | t = 0.91; p = 0.36 | –0.32 to 0.88 | 0.23 |
| EDE global | 2.72 (0.14) | 2.63 (0.15) | 0.09 | t = 0.48; p = 0.63 | –0.29 to 0.48 | 0.07 |
| EDE restraint | 2.48 (0.29) | 2.43 (0.30) | 0.05 | t = 0.13; p = 0.90 | –0.68 to 0.78 | 0.03 |
| EDE eating concern | 2.27 (0.16) | 2.28 (0.18) | –0.01 | t = –0.05; p = 0.96 | –0.47 to 0.45 | –0.01 |
| EDE shape concern | 3.16 (0.18) | 2.01 (0.20) | 0.14 | t = 0.55; p = 0.58 | –0.37 to 0.65 | 0.08 |
| EDE weight concern | 2.74 (0.19) | 2.68 (0.21) | 0.07 | t = 0.24; p = 0.81 | –0.48 to 0.61 | 0.04 |
| DASS-21 | 27.12 (1.54) | 27.25 (1.62) | –0.12 | t = –0.06; p = 0.96 | –4.50 to 4.25 | –0.01 |
| OCI-R | 23.01 (1.86) | 21.58 (1.97) | 1.42 | t = 0.58; p = 0.56 | –3.41 to 6.26 | 0.10 |
| CIA | 26.96 (1.58) | 26.93 (1.69) | 0.03 | t = 0.01; p = 0.99 | –4.25 to 4.31 | < 0.01 |
| WCST | 1.74 (0.13) | 1.58 (0.20) | 0.15 | t = 0.69; p = 0.49 | –0.29 to 0.60 | 0.21 |
| Brixton Spatial Anticipation Test | 10.49 (0.65) | 10.24 (0.79) | 0.25 | t = 0.30; p = 0.77 | –1.43 to 1.94 | 0.04 |
| REY | 1.36 (0.04) | 1.31 (0.05) | 0.05 | t = 0.72; p = 0.48 | –0.08 to 0.17 | 0.18 |
| RMF | 13.91 (0.45) | 13.08 (0.48) | 0.84 | t = 1.38; p = 0.17 | –0.35 to 2.02 | 0.30 |
| Outcome | Predicted mean (SE), baseline fixed to sample average | Estimated group differencea | Test | 95% CIa | Standardised coefficienta | |
|---|---|---|---|---|---|---|
| MANTRA | SSCM | |||||
| BMI (kg/m2) | 18.04 (0.32) | 17.72 (0.35) | 0.31 | t = 0.67; p = 0.50 | –0.60 to 1.22 | 0.25 |
| EDE global | 2.37 (0.16) | 2.58 (0.17) | –0.22 | t = –0.95; p = 0.34 | –0.66 to 0.23 | –0.17 |
| EDE restraint | 2.49 (0.22) | 2.67 (0.24) | –0.18 | t = –0.55; p = 0.58 | –0.82 to 0.46 | –0.12 |
| EDE eating concern | 1.82 (0.18) | 2.17 (0.20) | –0.35 | t = –1.33; p = 0.18 | –0.87 to 0.17 | –0.25 |
| EDE shape concern | 2.83 (0.18) | 2.89 (0.19) | –0.06 | t = –0.24; p = 0.81 | –0.57 to 0.44 | –0.04 |
| EDE weight concern | 2.32 (0.20) | 2.61 (0.22) | –0.29 | t = –1.00; p = 0.32 | –0.86 to 0.28 | –0.18 |
| DASS-21 | 26.00 (1.54) | 25.16 (1.70) | 0.84 | t = 0.37; p = 0.72 | –3.69 to 5.37 | 0.07 |
| OCI-R | 22.01 (1.96) | 20.81 (2.10) | 1.20 | t = 0.46; p = 0.64 | –3.87 to 6.28 | 0.09 |
| CIA | 23.56 (1.57) | 24.62 (1.82) | –1.06 | t = –0.42; p = 0.67 | –6.00 to 3.89 | –0.12 |
| WCST | 1.82 (0.13) | 1.83 (0.20) | –0.01 | t = –0.05; p = 0.96 | –0.44 to 0.42 | –0.01 |
| Brixton Spatial Anticipation Test | 9.34 (1.71) | 5.87 (2.13) | 3.47 | t = 1.41; p = 0.16 | –1.39 to 8.33 | 0.60 |
| REY | 1.43 (0.05) | 1.39 (0.07) | 0.03 | t = 0.42; p = 0.68 | –0.12 to 0.18 | 0.11 |
| RMF | 13.88 (0.68) | 13.39 (0.65) | 0.49 | t = 0.63; p = 0.53 | –1.05 to 2.02 | 0.18 |
Tables 11 and 12 demonstrate, however, that there was a significant overall effect of receiving treatment, with BMI in the total sample increasing from baseline to month 6 by 0.74 [95% confidence interval (CI) 0.4 to 1.08] and from baseline to month 12 by 1.19 (95% CI 0.59 to 1.79).
| Outcome | Estimated change | SE | Test | 95% CI |
|---|---|---|---|---|
| BMI (kg/m2) | 0.74 | 0.17 | t = 4.31; p < 0.001 | 0.40 to 1.08 |
| EDE global | –0.63 | 0.11 | t = –5.80; p < 0.001 | –0.85 to –0.42 |
| EDE restraint | –1.30 | 0.24 | t = –0.13; p < 0.001 | –1.77 to –0.83 |
| EDE eating concern | –0.57 | 0.14 | t = –4.10; p < 0.001 | –0.84 to –0.30 |
| EDE shape concern | –0.41 | 0.15 | t = –2.65; p < 0.01 | –0.71 to –0.11 |
| EDE weight concern | –0.45 | 0.15 | t = –2.94; p < 0.01 | –0.74 to –0.15 |
| DASS-21 | –3.38 | 1.14 | t = –2.97; p < 0.01 | –5.62 to –1.14 |
| OCI-R | –0.90 | 1.62 | t = –0.55; p = 0.58 | –4.12 to 2.32 |
| CIA | –5.41 | 1.43 | t = –3.79; p < 0.001 | –8.23 to –2.59 |
| WCST | –0.36 | 0.13 | t = –2.84; p < 0.01 | –0.61 to –0.11 |
| Brixton Spatial Anticipation Test | –2.02 | 0.50 | t = –4.02; p < 0.001 | –3.02 to –1.03 |
| REY | 0.05 | 0.04 | t = 1.43; p = 0.15 | –0.02 to 0.13 |
| RMF | 0.52 | 0.36 | t = 1.46; p = 0.15 | –0.18 to 1.22 |
| Outcome | Estimated change | SE | Test | 95% CI |
|---|---|---|---|---|
| BMI (kg/m2) | 1.19 | 0.30 | t = 3.91; p < 0.001 | 0.59 to 1.79 |
| EDE global | –0.84 | 0.12 | t = –6.78; p < 0.001 | –1.08 to –0.59 |
| EDE restraint | –1.16 | 0.17 | t = –6.81; p < 0.001 | –1.49 to –0.82 |
| EDE eating concern | –0.85 | 0.17 | t = –5.05; p < 0.001 | –1.18 to –0.52 |
| EDE shape concern | –0.64 | 0.14 | t = –4.55; p < 0.001 | –0.92 to –0.37 |
| EDE weight concern | –0.70 | 0.17 | t = –4.09; p < 0.001 | –1.04 to –0.36 |
| DASS-21 | –5.02 | 1.18 | t = –4.25; p < 0.001 | –7.34 to –2.70 |
| OCI-R | –1.59 | 1.88 | t = –0.85; p = 0.40 | –5.31 to 2.13 |
| CIA | –8.56 | 1.18 | t = –7.28; p < 0.001 | –10.87 to –6.25 |
| WCST | –0.23 | 0.13 | t = –1.74; p = 0.09 | –0.49 to 0.03 |
| Brixton Spatial Anticipation Test | –4.42 | 1.59 | t = –2.78; p < 0.01 | –7.58 to –1.27 |
| REY | 0.12 | 0.05 | t = 2.40; p = 0.02 | 0.02 to 0.23 |
| RMF | 0.64 | 0.55 | t = 1.16; p = 0.25 | –0.46 to 1.74 |
Secondary outcomes
Tables 9 and 10 show that the two treatments did not differ in their effect on the EDE global score at either the 6- or 12-month time point (all p > 0.05).
Tables 11 and 12, however, demonstrate that there was a significant overall effect of receiving treatment, with mean EDE global score decreasing from baseline to month 6 by 0.63 (95% CI –0.85 to –0.42) and from baseline to month 12 by 0.84 (95% CI –1.08 to 10.59).
Tables 9 and 10 show that there was no difference between the two treatments at 6 or 12 months’ follow-up for all other secondary outcomes (all p > 0.05).
Again, Tables 11 and 12 demonstrate that there was a significant overall effect of receiving treatment for all EDE subscales, DASS-21, CIA, WCST and Brixton Spatial Anticipation Test scores after month 6 (all p < 0.01), whereas OCI-R, REY and RMF did not change significantly (all p > 0.05).
At month 12 there was a significant overall effect of treatment for all EDE subscales, DASS-21, CIA, Brixton Spatial Anticipation Test and REY scores (all p < 0.01), whereas OCI-R, WCST and RMF did not change significantly (all p > 0.05).
Recovery rates
At 12 months’ follow-up, participants were split into three groups: recovered, partially recovered and not recovered. These were defined as (1) recovered, BMI of > 18.5 kg/m2 and EDE global score of < 2.77; (b) partially recovered, BMI of ≤ 17.5 kg/m2 and EDE global score of < 2.77, BMI of > 17.5 kg/m2 and ≤ 18.5 kg/m2 or BMI of > 18.5 kg/m2 and EDE global score of > 2.77; and (c) not recovered, BMI of ≤ 17.5 kg/m2 and EDE global score of > 2.77.
The proportions of participants falling into these categories at 12 months were as follows:
-
MANTRA: recovered, 13 of 72 (18.1%); partially recovered, 36 out of 72 (50%); and not recovered, 9 out of 72 (12.5%);
-
SSCM: recovered 8 out of 70 (11.4%); partially recovered, 32 out of 70 (45.7%); and not recovered, 9 out of 70 (12.9%).
There was no association between treatment allocation and recovery [χ2(3) = 2.80; p = 0.42].
Baseline body mass index as a potential moderator of primary and secondary outcomes
We also examined whether or not baseline BMI was a potential moderator of treatment effect on BMI at 6 and 12 months, dividing participants into those with a baseline BMI of < 17.5 kg/m2 (77% of MANTRA and 70% of SSCM participants) and those with a BMI of > 17.5 kg/m2 (18.1% of MANTRA and 25.7% of SSCM participants). (This information was missing for 4.2% and 4.3% of participants in MANTRA and SSCM groups, respectively.)
There was no evidence of a statistically significant interaction between treatment group and whether baseline BMI was above or below 17.5 kg/m2 when BMI was the outcome. However, on visual inspection of the estimated effects, it does appear that the effect of treatment (favouring MANTRA) was larger among those with lower baseline BMI (Table 13).
| Variable | Level | Estimated group differencea | t-score | p-value |
|---|---|---|---|---|
| 6 months after randomisation | ||||
| Baseline BMI | ≤ 17.5 kg/m2 | 0.41 | 1.32 | 0.19 |
| > 17.5 kg/m2 | –0.04 | –0.07 | 0.94 | |
| 12 months after randomisation | ||||
| Baseline BMI | ≤ 17.5 kg/m2 | 0.55 | 1.26 | 0.21 |
| > 17.5 kg/m2 | –0.08 | –0.10 | 0.92 | |
Baseline BMI was also investigated as a potential moderator of secondary outcomes. No evidence of a statistically significant interaction between treatment group and whether baseline BMI was more or less than 17.5 kg/m2 was found for these other outcomes.
Harms
One SSCM patient died. No other harms were noted.
Treatment acceptability and credibility
There were no significant differences in acceptability and credibility ratings between MANTRA and SSCM at 6 months {acceptability: MANTRA = 8.5 [2.0], SSCM = 8.0 [2.2] [t(100) = 1.33; p = 0.18]; credibility: MANTRA = 6.4 [3.1], SSCM = 5.8 [2.7] [t(100) = 1.1; p = 0.29]}. However, at 12 months, MANTRA was given significantly higher ratings on both acceptability and credibility than SSCM {acceptability: MANTRA = 8.6 [1.8], SSCM = 7.8 [2.3] [t(91) = 2.01; p < 0.05]; credibility: MANTRA = 6.8 [3.1], SSCM = 5.5 [2.7] [t(91) = 2.24; p < 0.05]}.
Discussion
Main findings
First, overall, participants showed significant improvements in terms of BMI, ED symptoms, depression, anxiety and stress and psychosocial impairment after 6 months of weekly treatment sessions. These improvements were either maintained or increased at 12 months post randomisation. Second, there were no differential effects between the two treatments in terms of primary and secondary outcomes and further service utilisation. Therefore, our main hypotheses were not confirmed. However, BMI, EDE and proportions of recovered participants all somewhat favour MANTRA at 12 months, and exploratory moderator analysis suggested that the treatment effect in relation to BMI (favouring MANTRA) increased (non-significantly) at 12 months, especially in those patients who were more severely ill at baseline.
There was a mixed picture of results in terms of neurocognitive and social cognitive performance. There was no effect of treatment on social cognition; however, participants were not particularly impaired on this task at baseline,155 so this lack of improvement is unsurprising. Central coherence and cognitive flexibility as measured by the Brixton Spatial Anticipation Test improved significantly by month 12; however, cognitive flexibility as measured by the WCST did not, but again participants were not particularly impaired at baseline. 15
Comparison of main findings with those of other trials
The degree of BMI change observed in the MOSAIC trial is comparable to that found in our earlier single-centre pilot RCT (total n = 72), which used identical study inclusion criteria and which also compared MANTRA and SSCM,136 suggesting that these treatments can be disseminated to other UK centres with relatively brief initial training (2 days) of therapists and booster training (1 day).
Comparison against other trials needs to be approached cautiously because of differences in patient populations. Perhaps the findings from the present study can be compared most easily against the large German psychotherapy trial by Zipfel et al. ,131 which compared focal psychodynamic therapy, CBT and optimised TAU in outpatients with AN. Patients in this trial had comparable baseline BMIs (between 16.6 and 16.8 kg/m2), but received a somewhat higher ‘dose’ of psychotherapy (40 sessions over 10 months). Patients were followed up at 13 months post randomisation and at 22 months post randomisation. Thirteen-month BMI outcomes in the Zipfel et al. 131 study were between 17.6 kg/m2 (focal psychodynamic therapy) and 17.4 kg/m2 (CBT and optimised TAU), whereas our 12-month BMI outcomes were 18.0 kg/m2 in MANTRA and 17.7 kg/m2 in SSCM. Thus, our BMI improvements stand up well in this international comparison.
Two other previous trials have used SSCM. 127,156 Comparison of our data against these trials is difficult. One of these trials was carried out in patients with a significantly milder form of AN (baseline mean BMI of 17.3 kg/m2),127 and the other specifically focused on chronic patients (mean illness duration of 15.5 and 17.7 years in the CBT and SSCM group, respectively). 156 In the McIntosh study, SSCM BMI outcomes were superior to CBT and IPT BMI outcomes at end of treatment, but not at long-term follow-up. 157 In the Touyz et al. 156 study, at 12-month follow-up, BMI gains were modest (0.5 kg/m2) and identical for both groups.
Other findings
Service utilisation
There was no difference in terms of further service utilisation between the two treatments. This is in contrast to findings from our pilot trial, which also compared SSCM with MANTRA,136 and in which all hospital admissions and day care treatment were in the MANTRA group. However, in our earlier smaller trial there was an imbalance between the two groups at baseline, with a significantly higher proportion of SSCM than of MANTRA participants having a partner (and therefore being in a prognostically better group). This may explain the differences between the two treatments in service utilisation in this earlier trial, and suggests that the previously observed difference was a feature of the pilot sample rather than an inadequacy in the MANTRA treatment.
Trial retention, treatment completion and acceptability
Participant retention in the trial was very good, with 83% of MANTRA participants and 73% of SSCM participants, providing BMI and EDE data at 12 months. In comparison, in the trial by Zipfel et al. ,131 at the 13-month assessment between 58.5% (optimised TAU) and 82.5% (CBT) of participants provided data.
Treatment completion rates also compare well against those of other studies that used a similar definition of treatment completion. Seventy-five per cent of MANTRA and 59% of SSCM patients completed treatment. In the McIntosh et al. 127 trial, 62.5% of participants completed treatment, and in the Zipfel et al. 131 trial 66% of focal psychodynamic therapy participants and and 81.3% of CBT participants completed treatment.
Finally, and of note, there was a significant difference between treatment acceptability and credibility ratings at month 12, with MANTRA being rated more favourably than SSCM on both. This may be because MANTRA is a manual-based treatment so that patients continue to have access to key resources during the course of therapy and beyond. The benefits of the manual may become more apparent following the end of weekly sessions, leading to a more positive longer-term experience.
Harms
One SSCM patient died during the study. This patient had very severe and chronic AN. It has been documented in many studies that chronically ill, low-weight AN patients such as the one in our study do have a much increased mortality risk. 72 The causes of death typically are related directly to consequences or complications of the illness (via malnutrition or abnormal weight control methods), and this is thought to be the case here. Previous trials of AN outpatient treatment in adults have reported deaths. For example, in a trial by Dare et al. ,33 one patient (out of 84) died over the course of the trial, and in the study by McIntosh et al. 127 there was one death among 56 participants.
Patient and therapist experience of Maudsley Model of Anorexia Nervosa Treatment for Adults and specialist supportive clinical management
The present trial is so far the only RCT of treatments for AN to include qualitative feedback from patients and therapists, which we published separately prior to the outcome evaluation, as is recommended for such process evaluations. 158–160 In particular, process data from therapists, who of course were able to compare and contrast these treatments, identified marked differences between these two treatments, in terms of the therapy focus, therapeutic strategies and applicability to a wide range of patients. 159 Process data from patients largely agree with these findings, and in addition strongly point to the quality of the therapeutic relationship, as the basis for a successful therapy. 158 Both treatments were perceived as having strengths and weaknesses. A further process evaluation examined written qualitative feedback from all study participants at 12 months. 160 Eighty-two study participants provided such written feedback, significantly more from the MANTRA group than from the SSCM group. MANTRA patients also tended to write in more detail and give more positive feedback than individuals receiving SSCM. Taken together with quantitative acceptability and credibility ratings, these process evaluation findings suggest that patients prefer MANTRA and therapists like it too. It thus provides a good alternative to SSCM. Of note, the greater acceptability of MANTRA may mean that patients are more willing to have further treatment if required or have better outcomes in the longer term. Assessment of these long-term effects of MANTRA and SSCM is currently under way.
Strengths
The strengths of the present study include that it is the largest RCT of first-line psychological treatments for adults outpatients with AN to be carried within the UK and the second largest in the world. 131 The study had good participation rates at follow-up data points, good treatment completion, and high acceptability and good outcomes compared with other studies. As such, the MOSAIC trial improves our limited evidence base regarding outpatient treatments in this hard-to-treat population. This study is also novel in that it is the first AN trial to incorporate both qualitative and quantitative measures of the therapeutic process and therefore offers a greater insight into patient and therapist perceptions of the two treatments.
Limitations
The MOSAIC trial also had limitations. First, as the study compared two active treatments and did not include a no-treatment control group, improvements in clinical outcomes cannot be attributed definitively to treatment effects. Second, although the trial is the largest ever to be carried out within the UK, it is still not large enough to perform conclusive mediation and moderation analyses of the core illness maintenance factors, which MANTRA attempts to target. However, we will be able to amalgamate the data from the present trial with the data from our earlier smaller RCT,136 which used an identical design and overlapping assessments, to allow more definitive moderator and mediator analyses. The combined sample size from both these trials will be 214 patients.
Third, although the ‘lost to follow-up’ rate within our overall sample was acceptable, the rate for participants assigned to SSCM is 10% greater than for those receiving MANTRA. Therefore, information on recovery in individuals in this group is more limited.
Finally, we were restricted by funding to a relatively short-term follow-up of 12 months. Further (2-year) follow-up is necessary to measure the longer-term effects of these two treatments on primary and secondary outcomes.
Generalisability
The MOSAIC trial included four specialist ED services and the interventions were delivered by a large number and broad range of therapeutic staff, with different professional backgrounds and different degrees of experience, so the study can be seen to reflect usual clinical practice. The trial also had very few exclusion criteria, making the sample as generalisable as possible and leading to the inclusion of a sample with a very broad range of clinical severity and BMI. As in usual clinical practice, trial therapists were able to conduct both treatments. Through therapist interviews159 we have been able to confirm that both treatments were experienced very differently and delivered with fidelity. Further confirmation of this will be reported separately through analysis of the therapeutic session recordings.
Conclusions and future outlook
The significant improvement of patients in both groups suggests that both of these two very different treatments have value as first-line outpatient treatments for adults with AN. In the original trial from New Zealand in which SSCM was first used,127 SSCM was found to outperform two active treatments (CBT and IPT) against the investigators’ hypothesis, and therefore provides a very high standard of control treatment. Comparable weight gain and comparable reductions in ED psychopathology between MANTRA and SSCM are therefore encouraging.
The current report includes an ITT analysis only. In future, we will also conduct a more complete analysis and an exploratory analysis of potential treatment mediators. As part of this WP we are also in the process of completing a cost-effectiveness analysis based on the current trial. The results of these analyses will be published as soon as they are available. Finally, we will also conduct analyses of session recordings to assess treatment fidelity and therapeutic process. We have completed a 2-year follow-up of the MOSAIC patients, showing that improvements across clinical outcomes are maintained or increase further. 161
Chapter 5 Cognitive Remediation and Emotional Skills Training for inpatients with anorexia nervosa (work package 2b)
Abstract
Objectives
To evaluate the benefits of Cognitive Remediation and Emotional Skills Training (CREST) for patients with AN receiving inpatient treatment.
Design
This project/chapter has three studies: (1) a case report, (2) a qualitative study of service users’ views about CREST and (3) a quasi-experimental comparison of CREST and TAU in two inpatient settings.
Participants
Adult inpatients with AN.
Interventions
The main novel intervention used in this project is CREST, which consists of simple cognitive exercises and simple emotion regulation, recognition and processing skills training, promoting micro skills in social communication and self-regulation as well as facilitating better self-awareness.
Main outcome measures
Outcomes included qualitative feedback and neuropsychological tests targeting flexibility of thinking, central coherence and theory of mind. Clinical outcomes (BMI, EDE-Q) were also reported.
Results
Qualitative assessments demonstrated CREST to be acceptable and perceived as beneficial by patients. Additionally, self-report questionnaires demonstrated improvements. However, quantitative data were less promising, showing no clear differences between the CREST and TAU groups in terms of cognitive or clinical outcomes.
Conclusions
Future work will focus on revisions to the CREST manual based on the outcomes of this work, as well as greater refinement of the measures used in subsequent quantitative evaluations.
Introduction
Inpatient treatment in the majority of countries is reserved for the most severe patients with AN. NICE guidelines (NICE 2004) encourage treatment innovations for the severely ill eating-disordered patient population. 162
One of the empirically supported models of AN19 suggests inefficiencies in cognitive style and difficulties in emotion generation and regulation as factors maintaining the illness. 14,16,113,163,164 A related division is that into ‘cold’ cognition, which is based on logic and rational thinking, and ‘hot’ cognition, based on feeling, intuition, emotional response and motivation. 165 Targeting cognitive styles (‘cold’ cognition) has been found to be an effective adjunct in treatment of inpatients, using the cognitive remediation treatment, which improves neuropsychological task performance.
The aim of these three studies was to test a novel intervention that targets ‘cold’ and ‘hot’ cognition in a manualised individual format for inpatients. CREST is a brief (1 hour, 10 sessions) psychological intervention. It focuses on inflexible and detail-focused thinking styles (two sessions), and additionally focuses on learning to recognise emotions, the management and expression of emotions, and recognising and acknowledging positive emotions (eight sessions). A range of psychoeducational modules and interactive tasks within CREST have been developed to facilitate individuals to (1) learn about the adaptive function of emotions, (2) learn how to identify own emotions, (3) identify emotions in other people and (4) express emotion.
Study 1: case study
Setting and referral
Anna is a 21-year-old Caucasian British woman informally admitted to the ED unit with AN and self-harming behaviour.
Assessment
On admission, Anna had a BMI of 13.1 kg/m2 (the healthy range is between 19 and 25 kg/m2). She fulfilled diagnostic criteria for the restricting subtype of AN. She was significantly restricting her diet and exercising excessively prior to her admission, which led to significant weight loss. She also described a number of rigid rules and ritualistic behaviours surrounding her eating, and would become distressed if her routines were disrupted. Anna had a distorted body image and body dissatisfaction, and described herself as ‘disgusting’ and ‘fat’, despite being objectively emaciated. She seemed to lack a sense of personal identity, but felt that AN gave her an identity and a sense of specialness. She rated the importance of change as 2/10 and her perceived ability to change as 2/10.
Prior to her admission Anna was frequently engaging in self-harm, including punching, scratching, and burning herself (superficially) on her arms and hands. She described using these behaviours as a means of managing adverse emotional states. She did not have any current suicidal ideation and denied plans to take her own life.
Anna reported growing up in an emotionally invalidating environment, one in which her feelings and thoughts were often marginalised by her parents, and she felt that she was ‘second best’ to her younger brother.
Key triggers for Anna’s eating disorder appeared to be her failing her General Certificate of Secondary Education (GCSE) exams and being bullied by her peers, who told her she was not ‘good enough’ for her boyfriend. These events seem to activate and reinforce negative core beliefs that Anna had about herself. AN may have provided Anna with a sense of achievement and specialness at a time when she was feeling like a failure in other domains. AN and her subsequent self-harm also gave her a way of managing her emotions, which she found confusing and aversive.
Intervention
Anna was offered CREST over 10 individual sessions. This was deemed to be a particularly useful treatment option for Anna, given her difficulties in managing her emotions. Anna’s mother also attended a series of family meetings with Anna, in which they were supported to gain insight into each other’s perspectives. These sessions also helped in supporting Anna’s mother in her own struggle to support Anna.
Outcomes
Anna did not complete the outcome measures given pre and post CREST. However, over the course of her 14-week admission her weight did increase such that her BMI reached 14.7 kg/m2. Her self-reported rating of the importance of change rose from 2/10 to 5/10, and her perceived ability to change also rose from 2/10 to 5/10. Anna reported that she found the intervention (CREST and family work) very useful in helping her to reduce her self-harming and in managing her emotions, as well as allowing for a change in the relationship with her mum to develop. Anna’s mother also said that she found the CREST work very helpful and felt more confident in her ability to support Anna in her recovery.
Study 2: qualitative evaluation
Aims
The aim of this study was to explore patients’ views and experience of individual CREST.
Methods
Participants
Twenty-eight patients in the National Adult Eating Disorder Inpatient Service completed CREST and were included in this study. All participants had a DSM-IV166 diagnosis of AN established by an experienced clinician. Participants’ mean age was 25 (range 13–40) years. The mean BMI before starting CREST was 14.7 (range 11.5–18.1) kg/m2. Eleven patients did not complete all sessions and were excluded from this study. Four patients were discharged from the hospital before they had completed CREST and seven disengaged from the inpatient treatment programme.
Data collection
All patients completed an end of therapy reflection form following CREST. This study was reported in detail by Money et al. 167 and the main idea was to assess patients’ reflections on the helpful aspects of therapy and the points for improvement. Responses were anonymised and then transcribed. All participants were given information about the study and consented to participation.
Analysis
The participants’ qualitative feedback was rated by two independent researchers. Each question was analysed based on the methods described in Elo and Kyngäs168 and Joffe and Yardley. 169 Initially the two researchers searched the data independently for codes or instances; thereafter they met to agree on how these could be grouped together to form different categories. The main categories were, however, predetermined by the questions asked.
Results
Detailed results tables were reported by Money et al. ,167 in which patient responses indicated benefits from the therapy and directions for improvement. In this chapter we will illustrate what the patients had to say about CREST with quotes.
In Table 14 we have provided a few illustrations of what patients had to say about the most helpful aspects of CREST and what we could improve in the future.
| Were there any aspects that were especially helpful? | What do you think could be improved? |
|---|---|
| Finding out that emotions are telling you that you need something – now when I feel upset I look at what needs to be done about it instead of limiting my food intake | Stop making me eat! |
| Learning to identify emotions within myself. Learning a means of communicating these emotions. Realising that I have rights and that emotions are not ‘good’ or ‘bad’, they just tell me something about what I need | More sessions |
| Speaking to psychologists | Longer course |
| Really learning that all feelings are welcome and ok. Learning how to express myself. Putting names to feelings | Proper psychological therapy, support on the unit, being able to talk to someone daily, not being kept waiting and having to wait all day if necessary just to see someone to answer a question |
| I found it useful being in surroundings where you could see the illness in people and you just want to recover so badly | Less basic work at the start. A few more sessions |
| Identifying emotions and needs | Possibly a few more sessions. More about identifying and handling different emotions |
| Set times to talk about emotions. Focusing on positive emotions and not being upset when things go wrong | Generic idea, hard to improve when ultimately cross-section research that is not personal |
| The calm, relaxed environment. The way that the therapies can link into everyday experiences. The visual activities | |
| I found that attempting to identify emotion through body language, facial expression in others, and as a colour/sound/animal helped me to understand my own feelings a little more. Showing me that a negative emotion, i.e. anger, can be a positive thing as it can incite change, helped also | I felt a few more sessions may have been beneficial to me with a bit more focus on different specific emotions. But on the whole both the content and facilitation of the therapy was a good, helpful experience |
| The detail to different types of emotions and also all the challenges and homework that I had to complete. I found these really identified and elicited all the factors I need to work on | I feel another set of CREST should be administered after 1 : 1 psychology is happening with patients. The purpose would be to look at emotions in a different and more in-depth way |
| Everything, talking about different emotions, realising how I deal with my emotions and learning how to express my emotions more instead of bottling them up | No, as I found the sessions helpful |
Discussion
The main aim of the qualitative study was to evaluate the acceptability of the CREST intervention for inpatients with AN. In the national inpatient ward where this study took place, the majority of the patients are nutritionally compromised (according to our annual audit data the mean BMI at admission is 13.0 kg/m2). In a severely ill AN patient, treatment engagement and meaningful psychological intervention delivery is challenging. Research shows that acutely ill AN patients have a major difficulty in correctly identifying and expressing emotions. 16,163,170,171 CREST includes information about basic emotion processing skills and aims to give patients simple tools and strategies to notice their own emotions and express their needs in a safe way. Alongside enhancing these skills, CREST also had a benefit in terms of increasing assertiveness. Many of the patients who received this intervention reported that CREST was helpful and that the strategies they learnt will be useful in the future. This highlights an improvement in patients’ confidence and perceived ability to express thoughts and emotions. From the qualitative outcome analysis we could also conclude that CREST has a positive impact on reducing interpersonal problems known to be present in this population. 19,172
Using qualitative outcomes from the study and learning from patients’ personal experiences were very useful steps in terms of gathering evidence for CREST.
Limitations
This qualitative study had some clear limitations; for example, feedback was not obtained from patients who dropped out of the treatment. There is also the possibility that patients wanted to please clinical and research staff with positive comments; therefore, in the future independent evaluation after discharge from the ward programme might provide more objective data.
A further limitation was that the study was conducted on one inpatient programme only. Future studies may consider evaluating CREST across multiple sites to explore its generalisability.
From the individual work and analysis of the quotes we have obtained very useful ideas about how to help patients to implement some of their new skills in real life. Qualitative feedback from the individual format of CREST led to the idea of developing a group protocol. This allows patients to practise and role play the skills worked on in the individual sessions and express their needs and feelings in the group.
Study 3: controlled trial
Aims
The aims of this study were to use cognitive (neuropsychological) assessments to (1) investigate changes in performance on cognitive flexibility, central coherence and emotion processing tasks in inpatients with AN following CREST and (2) investigate the magnitude of the changes on a cognitive and emotion test battery used in the study with patients who have received CREST plus TAU (referred to from now on as the CREST group), compared with patients who have received TAU only (the TAU group).
Hypotheses
-
In both groups we hypothesised weight gain, since both inpatient programmes focus on nutritional stabilisation.
-
In terms of cognitive and emotion task-related performance, our hypothesis was that improvement after treatment would be greater in the CREST group than in the TAU group.
Method
Ethics approval was obtained from the Oxfordshire REC NHS Ethics Committee (reference 08/H0606/58). Study participants were recruited from specialist inpatient ED units receiving national referrals. Patients receiving CREST were recruited from the South London and Maudsley NHS Foundation Trust inpatient ward and patients receiving TAU were recruited from Manchester (Cheadle Royal Hospital).
Participants
Consecutively referred adult patients were offered participation in the trial if they had a DSM-IV166 diagnosis. Patients were excluded from the study if they had an additional diagnosis of a learning disability, psychosis or major physical illness. Each participant was given a detailed information sheet about the study, and signed consent forms were collected from each study participant.
Interventions
Participants in the London inpatient ward received CREST as detailed above, in addition to TAU. Participants in the TAU group received standard inpatient care with individual and group therapy. At both sites TAU involved typical inpatient care: nutrition management; dietetic input; occupational therapy; family work; individual cognitive behavioural therapy; and group work on self-esteem and body image. In addition, TAU in London included individual work on psychological assessment and formulation. In Manchester, TAU additionally included individual cognitive analytic therapy and schema therapy.
Assessments
Participants in both groups were assessed 10 days after admission to the inpatient wards (within the first 2 weeks, T1). Participants were reassessed 10–11 weeks later [i.e. at the end of treatment (T2)].
Demographic and clinical measures
Pre-morbid intelligence was assessed with the National Adult Reading Test. 173 Clinical characteristics included BMI, illness duration, highest ever BMI and lowest BMI since onset.
Neurocognitive (set-shifting, central coherence) and emotional processing tasks
Set-shifting was assessed using the Brixton Spatial Anticipation Test149 and the WCST. 174 Central coherence was assessed using the Fragmented Pictures Task175 and the Group Embedded Figures Task. 176 Emotion processing was assessed using the Reading the Mind in the Eyes Task (RME)177 and the Pictorial Emotional Stroop Task. 178
Statistical analysis
All statistical analyses were performed in Stata 11. A linear mixed model was used to compare the two treatments. BMI and the six neuropsychological tasks were the outcome measures. Severity of illness (measured by BMI), duration of illness (years since diagnosis was established), intelligence quotient (IQ; measured by National Adult Reading Test total score) and age were included as covariates.
Results
Patient flow
In the CREST group, 46 participants completed the baseline assessments. Nearly one-quarter of these (24%; n = 11) dropped out of the treatment. Seventy-six per cent (n = 35) of the participants completed CREST; however, 8.5% (n = 3) did not participate in the post-treatment assessment and 5.7% (n = 2) were discharged before the post-treatment assessment was due. Thus, 65.2% (n = 30) of CREST and TAU participants completed the follow-up assessment. In the TAU group, 34 participants completed the baseline assessments and 73.5% (n = 25) completed post-treatment assessments.
Patient characteristics
In Table 15, patients’ sociodemographic and clinical characteristics are compared using Wilcoxon rank-sum tests. Both groups were similar in terms of age, IQ and severity of illness.
| Characteristic | CREST (London), mean (SD) | TAU (Manchester), mean (SD) | p-value |
|---|---|---|---|
| Age (years) | 26.1 (7.6) | 26.0 (7.9) | 0.85 |
| IQ | 105.8 (8.1) | 104.5 (7.8) | 0.31 |
| Illness duration (years) | 10.6 (7.0) | 9.9 (7.6) | 0.52 |
| Lowest ever BMI (kg/m2) | 12.0 (1.9) | 12.9 (1.2) | 0.13 |
| Highest ever BMI (kg/m2) | 18.6 (3.5) | 19.8 (3.2) | 0.13 |
Within-group changes
There were significant improvements in BMI in both TAU and CREST groups. In terms of neuropsychological task performance outcomes, there were significant improvements in both groups on the set-shifting performance-based tasks (Brixton Spatial Anticipation Test and WCST) and significant improvements in the CREST group only on the Fragmented Pictures Task, measuring ‘bigger picture’ information processing style. The TAU group showed poorer performance on the second ‘bigger picture’ related task (Group Embedded Figures Task) and no significant differences were observed in either group on the remaining neuropsychological tests (Pictorial Emotional Stroop Task and RME), measuring emotional processing. The test results are reported in detail in Davies et al. 179
Between-group changes
There were no significant differences between the groups concerning the changeover time on any of the outcomes (details of the outcomes are reported in Davies et al. 179).
Additional analysis
An additional analysis was conducted in CREST participants to identify if there was any difference in the baseline neuropsychological assessment scores between intervention completers and those who dropped out. No significant differences were found between these two groups.
Discussion
This study aimed to assess the efficacy of the individual format of CREST for inpatients with AN. Both groups showed significant improvements in BMI, as expected. Our hypothesis, that in within-group analyses neuropsychological measures would not change over time in the TAU group and would improve in the CREST group, was only partially supported by the results. Although the TAU group showed no change over time on central coherence (more ‘bigger picture’ thinking, less attention to detail) or emotion processing outcomes (less bias towards angry faces or recognition of emotions from the RME task), set-shifting task performance did improve in this group (this supports the idea that cognitive flexibility improves with weight restoration). The CREST group also showed set-shifting improvements and additional improvements in global processing (this could be explained by the fact that CREST has introductory sessions on cognitive styles addressing global thinking style strategies). The results of the study showed that the TAU group became more detail focused over time, whereas the CREST group remained stable in their information processing style (central coherence). There were no changes in emotion processing experimental outcomes in the CREST group (e.g. less bias towards angry faces and better recognition of emotions). In the between-group analyses there were no significant differences between the CREST and TAU groups on any of the neuropsychological domains or weight gain.
This study does not support previous findings suggesting that TAU does not improve set-shifting,180 as the TAU group improved in set-shifting in this study. This suggests that more longitudinal studies are required to assess the effects of TAU on set-shifting across different inpatient groups.
It is possible that differences between the groups explain the improvements in cognitive flexibility following TAU and the non-significant differences between the groups on all measures. Although the groups in this study were comparable on sociodemographic and clinical characteristics, there was a trend in the CREST group towards a lower lowest lifetime BMI and a lower highest ever BMI than in the TAU group. Moreover, although the two groups were recruited from specialist inpatient services, the TAU group received intensive individual psychological input from a variety of treatment models as part of their TAU package (e.g. cognitive analytical therapy and schema therapy).
Limitations
This study was designed in a quasi-experimental format, which is less robust than a RCT. As we described above, CREST plus TAU was delivered in London and TAU in Manchester, which means that TAU differed significantly between the study sites. The use of a TAU comparison also means that observed effects may be not due to CREST specifically, but rather to participants receiving additional support of any kind. A small sample size and several neuropsychological outcomes were clear limitations of the study.
Finally, there is evidence from the literature that self-reported measures correspond poorly with neuropsychological assessments. 181 The benefits recognised by patients were not fully reflected in the neurocognitive assessments, which raises the issue of how to measure the outcomes of CREST. We could improve our assessment battery to include tests measuring emotion expression, which is an area into which CREST taps and which has been found to be impaired in AN. 164,179,182 Other important aspects of the CREST intervention are learning how to be with other people and communicating one’s feelings. In future studies a measure of social anhedonia and functional outcomes will be more relevant in the context of measurable benefits of this intervention.
Conclusions
In summary, this study does not provide clear evidence that the addition of CREST to a multifaceted inpatient programme produces changes in neuropsychological task performance. Further studies are needed to corroborate these findings, using a larger sample size and a randomised controlled design with fewer, more targeted, neuropsychological assessments. From the current study it seems that, in the inpatient population with AN, it is harder for psychological treatment to address and change ‘hot’ cognition than ‘cold’ cognition. A systematic review of the existing experimental measures for emotional tasks will inform future studies, enabling us to select fewer and more targeted outcome measures.
General discussion
Cognitive Remediation and Emotional Skills Training was designed to offer support to patients in ward settings who are physically and medically compromised and find it difficult to engage in psychological treatment. Our approach in designing this clinical intervention was based on both extensive input from stakeholders, patients, clinicians and carers,183 and recent findings from experimental studies. 16,184,185
The three studies reported here used mixed methods to provide an initial evaluation of CREST in individual format. The overall results were mixed. The case study (study 1) and qualitative work (study 2) showed that inpatients valued and benefited from the structured approach and taking simple steps to develop awareness and skills to manage emotions. In contrast, we found that the neuropsychological changes were not very impressive or easy to interpret (study 3).
Following on from these findings, our future work will aim to revise the CREST manual in the light of service users’ feedback and our clinical experience from delivering and supervision of the model. Revising the current version of the CREST manual will involve taking into account the qualitative feedback, specifically removing the less relevant exercises (recognising other people’s facial expressions in the social context as well as cold cognitive exercises) and expanding those that patients found to be most helpful (e.g. emotion vocabulary development, thinking, recognising and doing positive things). In addition, we will incorporate recent research findings, such as the lower emotional intelligence relative to the high IQ in groups of AN patients, poor facial expression of positive emotions, limited vocabulary for describing positive emotions, social anhedonia and extreme difficulties in private and social leisure activities. 179,186,187 Finally, outcomes for the next stage (i.e. a randomised treatment trial) will be optimised. We will aim for a balance between experimental and self-report measures, and will use measures that are more tailored to the intervention content in order to detect changes after the treatment.
Chapter 6 A randomised controlled trial to evaluate the efficacy of adding a guided self-help intervention for carers of inpatients with anorexia nervosa (work package 3)
Abstract
Background
Families express a need for information and help to support people with severe and/or enduring AN. We have developed a guided self-help, skills training intervention for carers (ECHO).
Objectives
To examine the impact of the addition of ECHO to standard inpatient care on carer and patient outcomes over time.
Method/design
Patients (aged > 12 years) with a primary diagnosis of AN, and their carers, were ascertained from 15 inpatient services in the UK. Patients were randomised either to receive ECHO [a book, digital versatile discs (DVDs) and 10 telephone coaching sessions per family] or TAU. Patient (n = 178) and carer (n = 268) outcomes were measured at discharge and at 6 and 12 months after discharge.
Results
Carers in the ECHO group spent less time caregiving and had less burden and expressed emotion at discharge and/or 6 months. At 6 months, patients experienced a decrease in ED symptoms and improved quality of life.
Discussion
Skills-based training improves both patient and carer outcomes.
Introduction
Anorexia nervosa develops in early adolescence, and in over 50% of those affected illness persists for over 7 years. 188 Those who fail to respond to treatment and/or in whom there is high medical risk receive inpatient care. The admission and discharge criteria and the form and content of inpatient care vary. For example, the practice in the UK has changed. In the UK, inpatient age at admission has increased since cohorts in 1959–64,189–192 and inpatients present with more severe and long-lasting cases. The parameters of inpatient care are mainly driven by clinical consensus rather than evidence-based decision-making as there has been very little research into this. A recent exception is a RCT that compared standard inpatient care with a short period of inpatient care followed by day patient care for severely ill adolescent patients in the early stage of their illness. The study found similar 1-year weight outcomes between the forms of treatment, but with less expense and better social adjustment with the inpatient/day patient intervention. 193 This suggests that reducing the time separated from the family may be of benefit.
In the earlier stages of AN (less than 3 years’ duration), family-based interventions offer the best patient results. 194,195 However, individuals who develop AN later in life or have an illness duration of more than 3 years respond less well to this intervention. 194 Therefore, there is uncertainty about whether or not and how carers should be involved in those having inpatient care at a later stage of the illness. Families request help and knowledge about the illness196,197 and experience high levels of burden and distress. 198,199 A variety of psychoeducational interventions have been developed to address this need and the data from a recent systematic review and meta-analysis of these interventions showed carers experienced less burden and distress following training. 200
It is of interest to know whether or not these psychoeducational interventions for carers have a secondary beneficial effect on the individuals with an ED. Some of the interventions are based on a theoretical model suggesting that interpersonal factors, including carers’ levels of expressed emotion201 and accommodating and enabling behaviours,202 may contribute to the perpetuation of the illness. 19,20,203 ECHO is a guided self-help, skills training intervention. A recent pre-test post-test study showed an improvement in carers’ well-being after the intervention and, consistent with the theoretical framework,204 found that well-being increased as carers’ expressed emotion, accommodation and enabling decreased. 205 However, as yet there has been no controlled prospective study that has examined the validity of the extended model by examining whether or not the individuals with EDs benefit from giving their carers guided self-help, skills training.
This study aimed to evaluate the impact of a psychoeducational intervention for the carers of patients in the severe and/or enduring stage of AN admitted to day or inpatient care for nutritional rehabilitation. Carers were randomised to receive ECHO plus TAU or TAU alone. Both carer and patient well-being and inpatient treatment was followed in the year following discharge. This RCT was registered with Current Controlled Trials ISRCTN06149665.
Hypotheses
The trial investigated the following experimental hypothesis regarding patient and carer outcomes.
Primary hypotheses
Patient
-
Compared with patients receiving TAU, patients with carers receiving ECHO will be better at maintaining symptom improvement and will be less likely to relapse [measured monthly based on a body mass decrease of 2 points (BMI) or readmission to hospital from ED symptoms, whichever comes first].
Carer
-
At the 12-month follow-up, carers with ECHO training will have less distress than carers with TAU.
Secondary hypotheses
Patient
-
Compared with patients receiving TAU, patients in the ECHO group will have a higher BMI and quality of life, as well as lower ED symptoms and distress.
Carer
-
At all time points, carers of patients in the ECHO group will report fewer negative parenting styles, less burden and time spent caregiving, and an increase in quality of life.
Method
Design
This is a pragmatic, two-arm, multicentre, parallel-group RCT. A detailed account of the trial protocol, including the description of the ECHO carer intervention, has been published. 204 Consenting carers from dyads meeting the inclusion criteria were randomly assigned to either ECHO (plus TAU) or TAU alone. Guidance for the ECHO intervention was delivered by ‘experienced’ coaches [people with lived experience of EDs (n = 15) and postgraduate psychologists without clinical training (n = 5)], who were specifically trained and supervised. Recruitment involved 15 inpatient ED units within the NHS. Baseline and discharge measures were collected, along with follow-up data at 6 and 12 months post discharge. After ceasing intensive treatment, patients were considered ‘discharged’. To meet this criteria, they had to receive inpatient, day patient, or specialised residential treatment on ≥ 4 days per week, and maintain this status for a minimum of 4 weeks. Those who transferred to general supported housing were included too. Data regarding whether patients completed treatment or discharged themselves were not accessible for all sites and are not reported.
Ethics and governance
Main ethics approval was granted by the Royal Free Hospital Ethics Committee (08/H0720/41) with site-specific ethics and governance approval for all participating sites. The study was adopted by the MHRN.
Randomisation
Carers of a patient were randomly allocated to one of the two trial arms (ECHO or TAU). The CTU at King’s College London conducted the randomisation and minimisation was used, stratified by study site (15 NHS England hospital units) and disease severity categories (one or both of BMI < 15 kg/m2, presence of compensatory vomiting). Those in the TAU arm could access the intervention after finishing the study.
Recruitment
Patient outcome
One-year relapse rates are around 70% in comparable patient populations,206 with rates dropping to 30–50% in some relapse prevention studies. 207 A reduction in relapse rates of at least 20% following ECHO was considered clinically significant. To detect this decrease using a survival analysis log-rank test at 2.5% significance (adjusting for patient relapse and carer distress) with 80% power, we would need 110 families per treatment arm to detect a hazard ratio of 0.58. The total sample size required from the power calculation was 316 patients. The limited resources for this trial meant that the final sample size was 178 families. Thus, the study is underpowered, particularly for patient’s relapse rates.
Carer outcome
In our pilot studies, the ES of the decrease in distress for carers who received ECHO with telephone support was 0.55. We therefore predicted that the potency of the intervention should reach an ES of 0.4 at a 12-month follow-up. For continuous outcomes, an effective sample size of 110 per arm would be able to detect an ES of 0.42 or larger with 80% power using a t-test at the 2.5% (adjusting for two primary outcome tests) significance level.
Participating sites
This project involves 15 different centres. Fourteen of the sites are ED specialist inpatient wards in the UK (13 adult, one adolescent), and one is a general psychiatric ward with specialist ED staff (adolescent). One of the specialist sites makes local referrals to specialist ED inpatient wards in the UK. All were NHS units, except one, which is a private collaboration accepting NHS referrals.
Participants
Patients admitted to the treatment facility with a primary diagnosis of AN or an EDNOS with anorexic symptoms were approached. The agreement of at least one carer (proposed by the patient) was required for participation in the study. {Carers are defined as someone who provides unpaid help and support to a child, partner, relative, friend, or neighbour, who could not manage without their help [URL: www.carers.org (accessed 3 July 2017)].}
Inclusion criteria
Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition AN, aged ≥ 12 years, able to speak and comprehend English.
Exclusion criteria
No carer identified (at least one carer had to participate for the patient to be included, although all close carers were encouraged to take part), patient/carers taking part in another treatment study, or discharged from their inpatient stay before baseline assessment completed. In addition, participants with a severe comorbidity at time of admission (e.g. severe learning disability, medical problems, diabetes or psychosis) were not included in the study.
Recruitment
Patients were approached after admission by participating staff on the wards and offered study details. Informed consent was from both patient and carer. Clinical studies officers (CSOs) from the MHRN supported recruitment of patients and administration of the project on eligible sites.
Interventions
Experienced Carers Helping Others
Participants in the ECHO arm received this intervention in addition to TAU. The materials were sent and the coaching begun immediately after randomisation. ECHO uses a skills training approach consisting of a book49 and five DVDs (three theoretical, two practical) that complement the skills offered in the book with role plays and practical examples (presented visually with audio voiceover). A detailed intervention description is described elsewhere204,208 and a professionally produced version is available through the charity SUCCEED. 209
The intervention package additionally included five telephone coaching sessions per individual (up to 10 per family, e.g. mother and father). The coach contacted participants within 2 weeks. Coaches were encouraged to complete the sessions within a 5-month period. Calls were made on a regular basis with time in between (e.g. 2 weeks) for carers to practise the skills. The time taken to complete calls varied between families depending on individual circumstances. The expectation was that telephone calls would last up to 40 minutes and a minimum of five calls (per family) would be required to complete the intervention. Information on coaches, their training and measurement of quality assurance is described in the published protocol. 204
Treatment as usual (inpatient or day patient treatment)
The NICE guidelines specify several grade C recommendations about inpatient care162 and NHS England has set minimal quality criteria for inpatient units. For this study, day patients were defined as patients who required non-residential intensive specialist treatment (≥ 4 days a week).
Intervention delivery
All correspondence with carers on the randomisation outcome was by post. Families in the ECHO group received an intervention pack with the book, five DVDs and supporting documentation (a letter explaining their contact with the telephone coach, action/goal sheets, details on the transtheoretical model of change, frequently asked questions and details for technical support). Those receiving TAU were informed they could access ECHO after completing the study, and were given contact details for Beat, the leading ED charity in the UK.
Data collection
All participants (patients and carers) completed self-report assessments by post at inpatient admission, discharge, and at 6- and 12-month time points post discharge. Finally, for the year following discharge, patients completed a short monthly assessment on core eating symptoms by telephone, e-mail or post. Detailed information about data management is described in the published protocol. 204
Measures
Those completed by the patient are marked with a (P) and those completed by carers are marked with (C).
Primary outcomes
Relapse (P)
Relapse is defined as a readmission to hospital for AN treatment or a drop of 2 points from discharge BMI measured on a monthly basis, whichever came first.
Secondary outcomes
Short Eating Disorders Symptom Scale (P)
A short questionnaire measuring core ED symptoms (e.g. weight, fear of fatness), relating to the degree of ED [AN or bulimia nervosa (BN)] symptoms. Good reliability and validity are reported. 211
Eating Disorder Examination-Questionnaire (P)
A self-report measure assessing ED symptoms over the previous 28 days. 142 The scale yields both behavioural frequency data and information on specific ED psychopathology. The EDE-Q has good reliability and validity in ED samples. 212
Motivation to change (P)
Patients rate the importance of and confidence to change their ED on a Likert scale (0–10).
World Health Organization’s Quality of Life Questionnaire (short version) (P, C)
The World Health Organization’s Quality of Life Questionnaire (WHOQOL-100) measures an individual’s perception of their quality of life in 24 areas. Each facet is represented by one item, a five-point Likert scale, pooled in four domains: physical health, psychological, social relationships and environment. An additional two items evaluate the ‘Overall Quality of Life and General Health’. The quality of life questionnaire has good psychometric properties. 213
Eating Disorder Symptom Impact Scale (C)
A 24-item self-report measure rates carers’ perceptions of ED-specific burden using a five-point Likert scale. A total burden score and subscales can also be calculated. The Eating Disorder Symptom Impact Scale (EDSIS) has good reliability. 208
Accommodation and Enabling Scale for Eating Disorders (C)
This 33-item self-report measure measures the degree of accommodating and enabling behaviours to the ED. A five-point Likert scale is used. A total score and subscale scores can be calculated. This scale has good psychometric properties. 202
Family Questionnaire (C)
This 20-item self-report measures expressed emotion in carers with a four-point Likert scale. Items relate to both criticism and emotional overinvolvement. The Family Questionnaire (FQ) has good psychometric properties. 214
Time spent caregiving (hours/month) (C)
This study utilised a semistructured interview to report the time carers spend with specific demands of their caregiving role across five categories in an average month. Following thematic analysis of 22 female and male carers of someone with an ED describing the demands of their caregiving role, the following categories emerged: ‘medical’, ‘food’, ‘non-food/medical practical support’, ‘emotional’, and ‘communicating with non-ED professionals’. Time spent caregiving is summed across domains to quantify the total amount of care provided to patients in hours per month.
Process evaluation outcomes
Acceptability of intervention (C)
Carers provide quantitative (visual analogue scales) and qualitative feedback on their experience of the study, caring and, for those who received it, ECHO.
Patient feedback (P)
All patients are asked to provide qualitative feedback on their participation in the study (e.g. methodology), their observations of their carers’ responses to the illness since their participation in the project, and their responses to their carers over the course of the study.
Reimbursement
Participants were reimbursed on completion of each set of questionnaires (patients received £25, carers £10).
Statistical analysis
The objective of the statistical analyses was the comparison of those offered ECHO with those allocated to TAU on a number of primary and secondary outcomes. The primary patient clinical outcome is ‘relapse’. A linear change was assumed between monthly measurements of BMI and this was used to extrapolate the day on which two points were estimated to be lost. The variable is right censored since a relapse might not have occurred by the end of the study period (1 year) or follow-up data for BMI could be missing. If five consecutive BMI measurements were missed, time to relapse was censored.
The primary carer clinical outcome is distress (measured by the DASS-21) at 12 months after discharge. To account for two primary outcomes, group differences on these outcomes were tested at a significance level of 2.5%. Secondary patient and carer outcomes are continuous measures at discharge, 6- or 12-month follow-up (see Secondary outcomes). All statistical analyses were based on the ITT principle, that is, participants were analysed in the treatment arm to which they were randomised irrespective of whether or not they received the allocated treatment. Additionally, the statisticians were kept blind to treatment allocation as long as possible.
Time to relapse was analysed using Cox regression. Explanatory variables in this model were the variable of interest (treatment arm) and randomisation stratifiers (site and illness severity categories, based on binary BMI and vomiting). The effect of treatment is estimated by the hazard ratio of relapse comparing ECHO with TAU.
The continuous secondary patient outcomes were analysed using linear mixed models. The dependent variable is the outcome at the relevant time point (e.g. BMI at 12 months post discharge) and (fixed) explanatory variables are given by treatment arm, baseline values of the variable under investigation (e.g. BMI at pre-randomisation) and randomisation stratifiers. The models can contain random intercepts for coaches (in ECHO) to allow for correlation in outcomes due to treatment being facilitated by the same coach. The models were used to estimate differences between treatment arms at each time point. Standardised treatment effect estimates were calculated by dividing group differences by the common pre-randomisation SD of that outcome.
Outcome variables contained considerable numbers of missing values (see Results for details). We empirically identified a number of baseline variables that were predictive of missing values in outcome and also found that the primary carer not adhering to ECHO (coded ‘1’ = completed at least five coaching sessions or read at least half the coaching manual, ‘0’ = did not complete the intervention) was predictive of loss-to-follow up (see Results for details). To allow for these processes driving missingness, in addition to allowing randomised group and values of the outcome under investigation at different time points being predictive of missingness multiple imputation (MI) using chained equations as implemented in the Stata command ice. 215 This allowed us to include predictors of missingness (including the post-randomisation variable adherence) in the imputation step without having to condition on these variables in the analyses models. 216
The continuous carer outcomes were analysed in a similar fashion except that analyses needed to include up to two carers per family (a nominated first and second carer). To deal with this, the analysis and imputation models described for patients were extended for carer outcomes. First, the analysis models contained additional random intercepts that varied at the level of the patient to allow for similar outcomes for carers of the same patient. Second, to ensure that correlations were also reflected, the imputed values imputations in ice were carried out at the level of the patient allowing for two outcome variables – one for the primary carer and another for secondary carer (set to missing when there was no second carer, with resulting imputed values discarded before analysis).
To evaluate the effect on primary carer outcome of receiving a sufficient level of intervention (here an adherence score of ‘1’) without bias, the complier-average causal ECHO effect (CACE) on carer distress was estimated using a two-stage least squares (instrumental variables) estimator. 217
Results
Participant flow
This study represents a unique collaboration of major UK ED treatment centres and describes a large group of severely ill patients with AN (178 patients and 268 carers). The Consolidated Standards of Reporting Trials diagram for the study is shown in Figure 2.
FIGURE 2.
Consolidated Standards of Reporting Trials flow diagram (WP3).
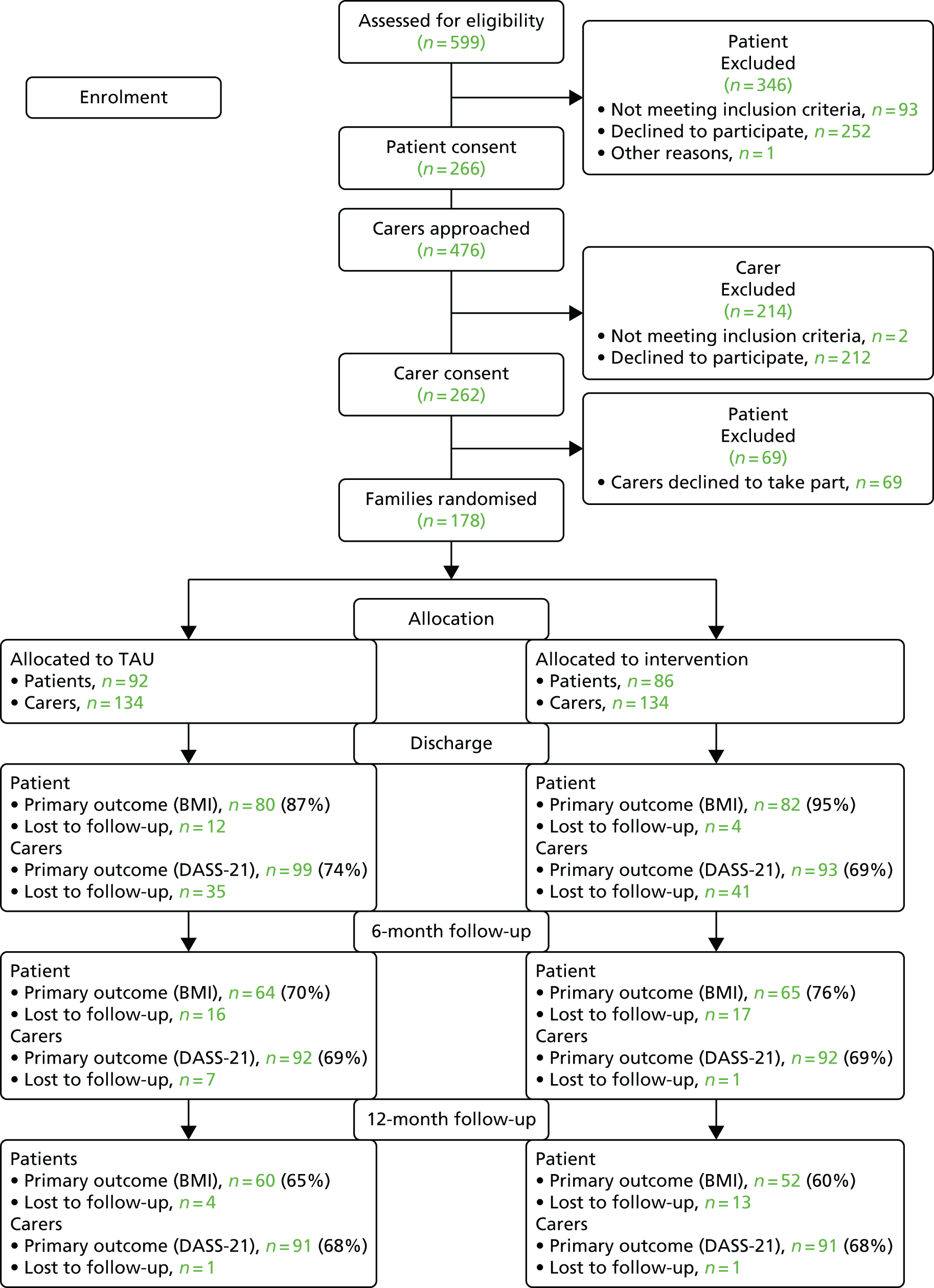
Sample characteristics
Patients
The majority of the patients were white (6% were Asian mixed). Twenty per cent were married, 14% were in work and 41% had higher education. The majority (69%) were living with their carer and prior to admission 48% had > 21 hours of face-to-face contact per week with their carer. The patient group (n = 178) included cases from both adolescent (n = 11) and adult (n = 167) services. The median age at onset was 15 (range 5–45) years. The mean lowest ever BMI was 12.9 (SD = 1.8) kg/m2. Median current age was 24.3 (range 12.5–62.7) years with a median duration of illness of 72 (range 9–480) months. The majority of cases (n = 123, 69%) had been ill for more than 3 years with a subset (n = 83, 55%) having a duration exceeding 6 years (enduring AN). 218 The prevalence of comorbidity with depression was 46%, and 44% of patients were on antidepressants. This was the first admission for approximately one-third of the sample and 8% had five or more admissions.
The short-term effects comparing admission and on discharge symptoms from inpatient care have been published. 219 The median duration of the admission was 153.5 (range 28–991) days and one patient was not discharged throughout the 2 years of the study.
Carers
A total of 268 carers (178 primary carers, 90 secondary carers) were recruited (144 mothers, 81 fathers, 28 partners, seven siblings, five friends and three other relatives). The median age of the carers was 52.7 (range 19.7–78.9) years and 60% were female. Seventy-nine per cent were married or living with a partner, 63% were in work and 46% had higher education. Nineteen per cent of the carers self-reported that they had suffered from an ED themselves, and 24% reported some sort of eating problems in the family other than that of the patient.
Randomisation
Patient and carer outcomes were well balanced across groups. Roughly 25% at discharge, 30% at 6 months after discharge and slightly over one-third of patients at 12 months showed missing questionnaire outcome data. At 12 months, the proportion ranged between 0.33 and 0.37 for the different scales. For the carers, the proportion of missing data was 2–4 percentage points greater at all outcome time points. The proportion of missing outcome data at 12 months ranged between 0.32 and 0.43. Logistic regression was used to explore the relationships between a dependent variable that represented whether outcome data were present or missing at 12 months after discharge, and a number of baseline demographic and clinical variables, such as participant gender and lowest ever BMI. Any variable that showed a statistically significant association with the dependent variable was included in the imputation step of the MI procedure. Non-adherence with ECHO was strongly associated with missingness, with ORs from 1.5 to 2.0 for patients’ data and from 3.3 to 6.2 for carers’ data.
A comparison of inpatient treatment post discharge of the patient groups (carers with Experienced Carers Helping Others and carers with treatment as usual)
The ECHO group had shorter admission length (median = 148 days, range 28–991 days) than the TAU group (median = 163 days, range 33–570 days; Mann–Whitney U-test, z = –0.88, p = 0.38). The readmission rate was 26.7% (n = 23) in the ECHO group and 32% (n = 29) in the TAU group. Relapse in terms of readmission and/or fall in 2 BMI points occurred in 43% of the ECHO group and 52% of the TAU group. The median time to relapse for the ECHO group was 262 days and 240 days for TAU. Survival plots of the time to relapse showed two similar curves (Figure 3). The TAU showed greater relapse-free survival initially. However, after approximately 8 months, the curves crossed over. Thus, the study did not detect a statistically significant difference in survival curves and the main assumption of a Cox regression model, that the hazards are proportional, was broken (a Cox regression which relies on proportional hazards would give a hazard ratio of 0.82 in favour of ECHO and a p-value of 0.42 for the group difference).
FIGURE 3.
Kaplan–Meier curves for time to relapse.
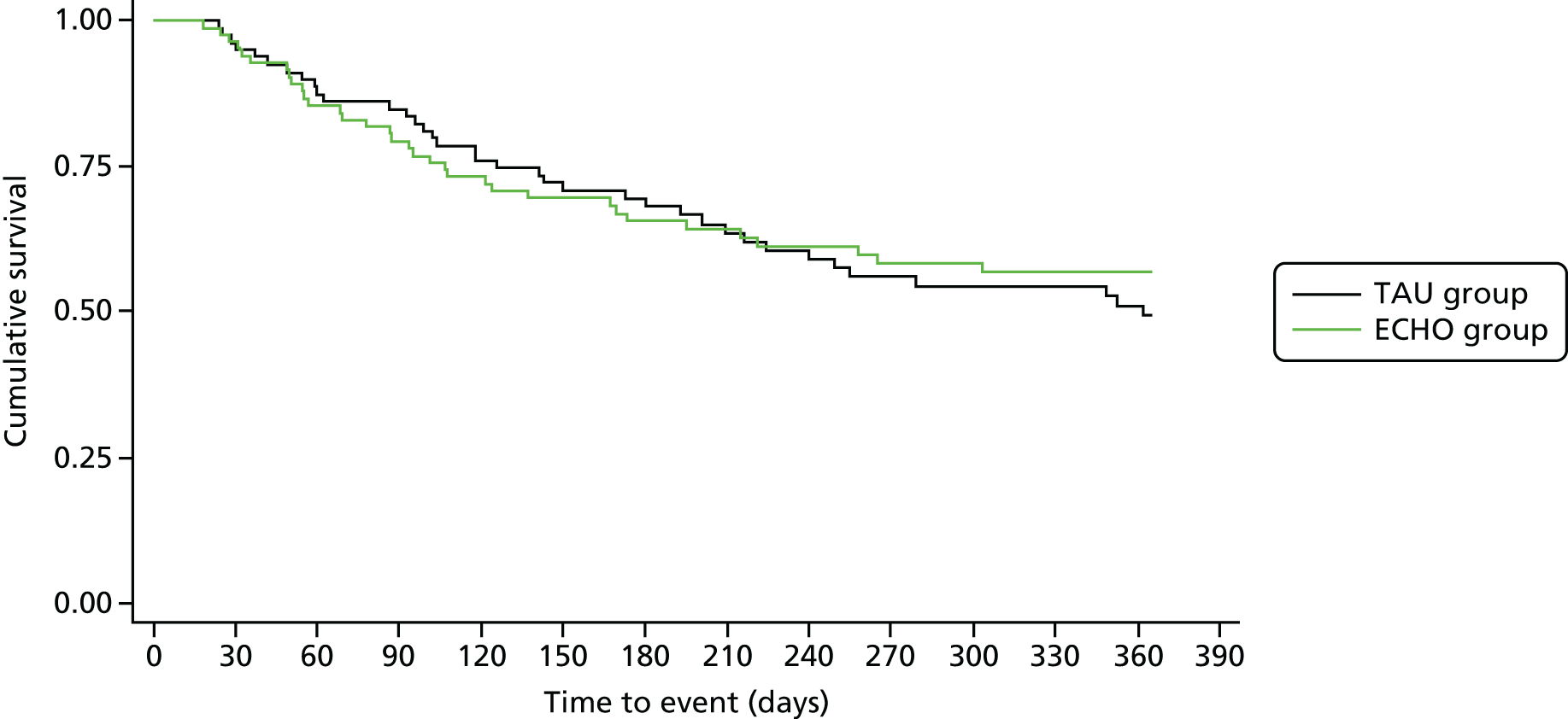
Table 16 summarises the clinical outcomes and Table 17 provides estimated outcome differences between the two treatment arms for both patients and carers at all three time points.
| Outcome | Treatment arm | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECHO | TAU | |||||||||||
| Baseline | Discharge | 6 monthsa | 12 monthsa | Baseline | Discharge | 6 monthsa | 12 monthsa | Baseline | Discharge | 6 monthsa | 12 monthsa | |
| Patient data | ||||||||||||
| BMI (kg/m2) | 14.5 (1.8) | 17.5 (1.9) | 17.1 (2.9) | 17.3 (2.9) | 14.3 (2.3) | 17.4 (2.4) | 17.1 (3.1) | 17.0 (2.5) | 14.4 (2.1) | 17.5 (2.2) | 17.1 (3.0) | 17.2 (2.7) |
| WHOQOL-100 | 10.7 (2.2) | 11.7 (2.6) | 11.9 (2.5) | 11.8 (3.4) | 10.5 (2.6) | 11.6 (2.8) | 11.1 (2.9) | 11.4 (3.2) | 10.6 (2.4) | 11.7 (2.7) | 11.5 (2.7) | 11.6 (3.3) |
| EDE | 4.3 (1.3) | 3.4 (1.5) | 3.4 (1.7) | 3.3 (1.8) | 4.1 (1.2) | 3.3 (1.5) | 3.6 (1.6) | 3.4 (1.6) | 4.2 (1.2) | 3.4 (1.5) | 3.5 (1.6) | 3.3 (1.7) |
| DASS-21 | 77.6 (28.1) | 63.3 (30.1) | 63.2 (31.7) | 61.1 (31.2) | 78.5 (27.8) | 64.3 (33.2) | 65.2 (32.9) | 62.1 (31.5) | 78.1 (27.9) | 63.8 (31.5) | 64.2 (32.2) | 61.6 (31.7) |
| Carer data | ||||||||||||
| DASS-21 | 29.6 (25.6) | 30.5 (25.1) | 26.4 (22.9) | 26.2 (23.6) | 31.5 (26.7) | 34.5 (28.3) | 33.2 (25.5) | 30.9 (29.7) | 30.5 (26.1) | 32.5 (14.3) | 29.8 (24.5) | 28.5 (26.8) |
| FQ | 48.5 (8.2) | 46.0 (9.0) | 44.4 (8.6) | 43.8 (9.5) | 47.9 (9.4) | 48.0 (8.8) | 46.8 (8.4) | 45.0 (9.6) | 48.2 (8.8) | 47.1 (8.9) | 45.6 (8.6) | 44.4 (9.5) |
| AESED | 47.7 (22.1) | 40.7 (21.3) | 35.5 (23.1) | 33.1 (22.6) | 47.3 (24.9) | 43.9 (24.8) | 41.5 (23.8) | 37.7 (24.7) | 47.5 (23.5) | 42.4 (23.3) | 38.5 (23.6) | 35.3 (23.7) |
| EDSIS | 41.4 (12.5) | 32.9 (14.6) | 30.1 (15.1) | 29.0 (16.5) | 41.1 (14.2) | 37.2 (13.8) | 35.6 (14.2) | 33.0 (16.5) | 41.2 (13.4) | 35.2 (14.3) | 33.0 (14.8) | 31.0 (16.6) |
| Time spent caregiving (hours/month)b | 70.5 (0–815.0) | 25.2 (0.6–351.0) | 22.0 (0–478.0) | 17.5 (0–466.0) | 70.6 (0–708.0) | 25.0 (0.3–513.7) | 31.9 (0–378.0) | 20.0 (0–379.0) | 70.6 (0–815.0) | 25.0 (0.3–513.7) | 30.0 (0–478.0) | 20.0 (0–466.0) |
| WHOQOL-100 | 15.1 (2.3) | 14.8 (2.3) | 14.7 (3.1) | 14.3 (4.0) | 14.6 (2.4) | 14.2 (2.4) | 13.7 (2.7) | 13.1 (3.8) | 14.8 (2.3) | 14.5 (2.4) | 14.2 (2.9) | 13.7 (3.9) |
| Outcome | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discharge | 6 months after discharge | 12 months after discharge | ||||||||||
| Estimated group differencea | Test | 95% CIa | Standard coefficienta | Estimated group differencea | Test | 95% CIa | Standard coefficienta | Estimated group differencea | Test | 95% CIa | Standard coefficienta | |
| Patient data | ||||||||||||
| BMI (kg/m2) | –0.05 | z = –0.18, p = 0.86 | –0.57 to 0.48 | –0.02 | –0.07 | z = –0.18, p = 0.86 | –0.82 to 0.69 | –0.03 | –0.30 | z = –0.71, p = 0.48 | –1.11 to 0.52 | –0.14 |
| WHOQOL-100 | 0.13 | z = 0.35, p = 0.73 | –0.62 to 0.89 | 0.05 | 0.91 | z = 2.05, p = 0.04 | 0.04 to 1.78 | 0.38 | 0.23 | z = 0.40, p = 0.69 | –0.90 to 1.37 | 0.10 |
| EDE | –0.12 | z = –0.59, p = 0.56 | –0.54 to 0.29 | –0.10 | –0.47 | z = –2.09, p = 0.04 | –0.92 to –0.03 | –0.38 | –0.40 | z = –1.46, p = 0.15 | –0.93 to 0.14 | –0.32 |
| DASS-21 | –2.74 | z = –0.68, p = 0.50 | –10.60 to 5.13 | –0.10 | –4.45 | z = –1.03, p = 0.31 | –12.97 to 4.06 | –0.16 | –1.58 | z = –0.32, p = 0.75 | –11.30 to 8.13 | –0.06 |
| Carer data | ||||||||||||
| DASS-21 | –0.54 | z = –0.18, p = 0.86 | –7.29 to 6.21b | –0.02 | –4.51 | z = –1.40, p = 0.16 | –11.74 to 2.72b | –0.17 | –3.24 | z = –0.97, p = 0.33 | –10.76 to 4.28b | –0.12 |
| FQ | –1.23 | z = –1.08, p = 0.28 | –3.45 to 1.00 | –0.14 | –2.24 | z = –2.01, p = 0.05 | –4.43 to –0.05 | –0.25 | –0.61 | z = –0.45, p = 0.66 | –3.31 to 2.08 | –0.07 |
| AESED | –1.44 | z = –0.51, p = 0.61 | –7.00 to 4.11 | –0.06 | –3.63 | z = –1.00, p = 0.32 | –10.77 to 3.51 | –0.15 | –0.22 | z = –0.06, p = 0.95 | –7.59 to 7.14 | –0.01 |
| EDSISc | –3.98 | z = –2.05, p = 0.04 | –7.80 to –0.16 | –0.29 | –3.42 | z = –2.11, p = 0.11 | –7.56 to 0.73 | –0.25 | –2.16 | z = –0.83, p = 0.41 | –7.25 to 2.93 | –0.16 |
| Time spent caregiving (hours/month) | 1.02c | z = 0.11, p = 0.91 | 0.68 to 1.52c | 0.01 | 0.63c | z = –1.98, p = 0.05 | 0.40 to 1.00c | –0.34 | 1.04c | z = 0.15, p = 0.88 | 0.58 to 1.90c | 0.03 |
| WHOQOL-100 | 0.32 | z = 1.20, p = 0.23 | –0.20 to 0.84 | 0.14 | 0.12 | z = 0.41, p = 0.77 | –0.69 to 0.94 | 0.05 | 0.40 | z = 0.62, p = 0.54 | –0.88 to 1.69 | 0.17 |
A comparison of carers’ well-being and burden in the Experienced Carers Helping Others and treatment as usual condition
In all of the outcomes measured, the carers in the ECHO group had improved functioning. Carers in the ECHO group reported less distress than those in the TAU group, but this difference was not statistically significant at any time point.
Time spent care giving was reduced 6 months post discharge and was significantly lower in the ECHO group (p = 0.05; ES = –0.34). Expressed emotion was also significantly lower in the ECHO group at 6 months (p = 0.05; ES = –0.25). Burden was significantly lower in the ECHO group at discharge (p = 0.04; ES = –0.29).
At discharge, we estimated that carers’ perception of ED burden in the ECHO group was 3.98 points (95% CI 0.16 to 7.80 points) lower than in the TAU group. At 6 months after discharge, time spent caregiving in the ECHO group was 63% (95% CI 40% to 100%) of that spent in the TAU group. At the same time point, expressed emotion in the ECHO group was estimated as 2.24 points (95% CI 0.05 to 4.43 points) less on the FQ than in the TAU group.
A comparison of the clinical status post discharge of the patient groups (carers with Experienced Carers Helping Others and carers with treatment as usual)
Eating disorder psychopathology and quality of life were significantly better in the ECHO group at 6 months (ES = –0.38 and 0.38, respectively), but these differences were not significant at 12 months post discharge. At 6 months after discharge, we found ED psychopathology among the ECHO group to be 0.47 points (95% CI 0.03 to 0.92 points) less than in the TAU group. At the same time point, we estimated quality of life as measured on the WHOQOL-100 to be 0.91 points (95% CI 0.04 to 1.78 points) lower in the ECHO group compared with the TAU group. Estimated differences in distress and BMI pointed towards a beneficial effect of ECHO, but none of these effects could be shown to be statistically significant.
Important harms or unintended effects
Two patients (one from each trial arm) died during the course of the study.
Semistructured open feedback
Patients
Six months post intervention, 102 of 178 (52 ECHO; 50 TAU) semistructured open feedback forms were returned from patients and analysed using thematic analysis (Table 18). Overall, > 75% of the sample reported positive benefits and changes in their interaction with carers, and within this a greater proportion (66–75%) were from the ECHO group. These aspects included more adaptive, open communication and understanding, continued support, motivation and encouragement, meal support, better goal-setting and a calmer family atmosphere. Over one-third reported empathy and concern for their family [a greater proportion (65%) in the ECHO group].
| Theme | Patients (n) | Utterances (frequency) | ||
|---|---|---|---|---|
| CASIS | TAU | CASIS | TAU | |
| Project involvement | ||||
| Positive aspects of participation | 45 | 44 | 83 | 79 |
| Problematic aspects of participation | 31 | 34 | 48 | 48 |
| Project interest and/or confusion | 9 | 7 | 9 | 7 |
| No impact (carer and/or self) | 19 | 31 | 40 | 57 |
| Suggestions for improvement | 4 | 5 | 4 | 5 |
| Perceived need/appreciation for carer support | 16 | 11 | 25 | 13 |
| Altruism | 13 | 16 | 14 | 18 |
| Perceived changes in carer style | ||||
| Reduced expressed emotion/anxiety | 16 | 6 | 31 | 8 |
| Greater understanding, awareness and coping abilities | 31 | 22 | 75 | 33 |
| Improved relationship and communication | 23 | 13 | 43 | 27 |
| More responsibility, space and trust | 19 | 10 | 32 | 11 |
| Perceived negative changes | 4 | 12 | 4 | 18 |
| Reductions in carer expectations/pressure | 4 | 4 | 6 | 5 |
| Reported general improvements | 14 | 6 | 24 | 11 |
| Helpful strategies | ||||
| Adaptive open communication and understanding | 38 | 33 | 70 | 50 |
| Continued support, motivation and encouragement | 28 | 26 | 50 | 36 |
| Concern for carer(s) and family | 9 | 4 | 10 | 4 |
| Increased responsibility, trust and space | 17 | 15 | 24 | 35 |
| Non-ED communication and behaviours | 8 | 4 | 11 | 4 |
| Calmer family atmosphere | 8 | 7 | 9 | 8 |
| Meal support | 25 | 21 | 36 | 28 |
| Positive role modelling | 1 | 1 | 1 | 1 |
| Practical help | 13 | 17 | 16 | 23 |
| Clear boundary setting and expectations | 18 | 17 | 23 | 28 |
| Reduced pressure and expectations | 4 | 3 | 4 | 4 |
| Collaborative problem-solving and goal-setting | 7 | 5 | 10 | 5 |
| Unhelpful strategies | ||||
| Food, weight and shape talk | 11 | 11 | 14 | 13 |
| High expressed emotion and anxiety | 29 | 21 | 37 | 44 |
| Detachment, avoidance and distance | 15 | 11 | 28 | 18 |
| Pressure of others’ expectations | 7 | 9 | 14 | 13 |
| Intrusive, directive or controlling approach | 11 | 7 | 14 | 8 |
| Loss of the ‘special’ feeling | 1 | 0 | 1 | 0 |
| Lack of understanding, communication and confusion | 4 | 9 | 4 | 14 |
| Lack of clear boundaries | 19 | 15 | 21 | 17 |
| Problematic carer responses and behaviours (general) | 13 | 8 | 22 | 12 |
| Family involvement | 0 | 2 | 0 | 2 |
| Relationship with carers (general) | ||||
| Positive relationship, impact and coping abilities | 36 | 3 | 92 | 97 |
| Problematic aspects of relationship and support | 22 | 31 | 67 | 76 |
| Mixed reports | 11 | 13 | 13 | 14 |
| Dependent attachment | 0 | 6 | 0 | 6 |
| Loss of intimacy | 1 | 1 | 1 | 1 |
| Empathy and concern for carers’ plight | 20 | 11 | 37 | 17 |
| Patient reflections | ||||
| Caring for an adult | 5 | 7 | 5 | 10 |
| Professional services | 6 | 3 | 7 | 7 |
| Recovery process | 4 | 3 | 6 | 3 |
| Own responsibility for recovery | 3 | 5 | 4 | 8 |
| Struggles and reflections on situation | 7 | 9 | 9 | 11 |
Carers
At 6 months, 148 (83%) [n = 73 Carers Assessment, Skills and Information Sharing (CASIS); n = 75 TAU] feedback forms were returned from carers and analysed using thematic analysis (Table 19). Overall, 27% of carers expressed appreciation for the research study, 82.5% of whom were in ECHO group. Interestingly, this aspect of the study revealed partial non-adherence to the trial protocol as seven carers in the TAU group expressed appreciation for the skills training (suggesting that they had possibly purchased the book themselves but received no coaching). Over half of the carers (64%) reported negative perceptions of services’ post-discharge support and the majority (60%) were in the TAU group (TAU, n = 57; CASIS, n = 38). In terms of reports on their observations on the individual with an ED, improved communication was reported by 43% of carers (60% in the ECHO group) and acceptable functioning in 49% of carers (60% in the ECHO group). Overall, this suggests that information and skills training improves expectations and perceptions of both services and the individual with an ED.
| Theme | Carers (n) | Utterances (frequency) | ||
|---|---|---|---|---|
| CASIS | TAU | CASIS | TAU | |
| Service provision and carer support | ||||
| Self-discharge | 4 | 7 | 5 | 8 |
| Negative perceptions of care services’ post-discharge support | 38 | 57 | 79 | 121 |
| Acceptable aspects of post-discharge support/services | 33 | 27 | 50 | 44 |
| Importance/appreciation of carer support and research | 33 | 7 | 51 | 9 |
| Unhelpful support/no need for support | 5 | 4 | 6 | 4 |
| Role perception and relationship with loved one | ||||
| Positive aspects of relationship | 39 | 35 | 55 | 47 |
| Negative or problematic aspects of relationship | 12 | 25 | 14 | 40 |
| Full-time caring and/or dependency | 15 | 20 | 20 | 25 |
| Continued emotional practical support | 54 | 55 | 93 | 117 |
| Reduced caring role | 37 | 31 | 52 | 50 |
| Perceived carer impact | 35 | 36 | 47 | 40 |
| No change or little impact (self or impact on illness) | 12 | 28 | 15 | 38 |
| Familial role differentiation | 7 | 11 | 11 | 14 |
| Apathy and acceptance | 1 | 9 | 1 | 10 |
| Issues unique to partners | 0 | 2 | 0 | 5 |
| Perceived improvements in situation | ||||
| Reports of acceptable functioning in sufferer | 44 | 29 | 76 | 47 |
| Slower progress and determination | 27 | 37 | 43 | 56 |
| Improved communication and relationships | 38 | 25 | 66 | 29 |
| Reduced burden (practicalities) | 3 | 3 | 3 | 3 |
| Perceived carer changes | ||||
| Supporting independence and responsibility | 22 | 24 | 30 | 36 |
| Reduced high expressed emotion | 25 | 20 | 38 | 25 |
| Adaptive communication and techniques | 13 | 11 | 16 | 11 |
| Greater knowledge and understanding | 17 | 15 | 18 | 15 |
| Improved coping skills and self-care | 12 | 8 | 16 | 11 |
| Boundary setting | 5 | 4 | 6 | 4 |
| Separating illness from person | 4 | 3 | 4 | 3 |
| Less ED talk | 1 | 5 | 1 | 5 |
| Continued difficulties and challenges | ||||
| Continued struggles and burden | 46 | 48 | 86 | 135 |
| Problematic behaviours, patient struggles or relapse | 34 | 42 | 52 | 95 |
| Impact on other family members and relationships | 15 | 15 | 25 | 19 |
| Concern for the future | 6 | 15 | 7 | 22 |
| Problematic carer responses | 7 | 10 | 11 | 19 |
| Readmission to hospital | 7 | 11 | 7 | 15 |
| Own health problems | 6 | 10 | 11 | 17 |
| Sense of loss | 5 | 6 | 5 | 8 |
| Poorer communication/relationships | 3 | 6 | 4 | 7 |
| Financial issues | 5 | 6 | 6 | 6 |
| Stigma | 2 | 6 | 2 | 7 |
| Carer coping practices | ||||
| Social support | 41 | 40 | 58 | 60 |
| Hobbies, work | 18 | 16 | 19 | 19 |
| Psychoeducation and self-care | 20 | 15 | 29 | 21 |
| Less contact, detachment or distraction | 10 | 19 | 15 | 25 |
| Spirituality | 1 | 6 | 1 | 10 |
Discussion
The aim was to evaluate whether or not adding a guided self-help, skills training intervention (ECHO) for carers of people with severe and/or enduring AN is of benefit for both carer(s) and for the patient. Patients in the ECHO group had significantly reduced ED symptomatology and improved quality of life at 6 months with no BMI differences. Carers in the ECHO group had a greater reduction in their time caregiving following admission, and this was associated with a small/moderate reduction in carer burden and reduced expressed emotion. The majority of the sample (particularly from the ECHO group) reported positive benefits and changes in their interaction with carers and one-third reported empathy and concern for their family.
We did not find statistically significant effects of ECHO in terms of our distal primary outcomes, patient relapse and carer distress, although differences were in the anticipated direction. It is possible that estimated differences were not found for our primary outcomes because we under-recruited. This will particularly impact binary/time to event outcomes such as our primary patient outcome relapse. In hindsight, due to extraneous variables associated with carer distress, perhaps a more ED-specific outcome such as carer burden should have been used as the primary outcome for carers. It will be important to understand why ECHO effects on variables targeted by this carer intervention (e.g. on expressed emotion) were not translated into stronger effects on distal outcomes, and as a next step we plan to carry out mediation modelling to investigate which hypothesised paths of our theoretical model were active.
The ECHO group made less use of services, as reflected by shorter duration of admission, longer time to relapse and lower number of relapses/readmissions, and carers in this group expressed less dissatisfaction with post-discharge care.
These findings suggest that a degree of sustained benefit can be achieved by patients with severe and/or enduring form of EDs following intensive treatment for nutritional rehabilitation. A previous qualitative study suggests that patients experience benefits from their parents participating in these interventions. 220 The increase in BMI (2.1 kg/m2, 14% increase) at 1 year is smaller than that found in early intervention cases given inpatient/day patient care (3 kg/m2),193 but greater than that seen in outpatient studies with less medically compromised adults [1.3 kg/m2 by Schmidt et al. ,128 1 kg/m2 (4%) by Touyz et al. 156 and 1.4 kg/m2 by Zipfel et al. 218]. On the other hand, the reduction (17%) in ED psychopathology is less than that seen in outpatient care (38%). 136
Approximately two-thirds of the patients lived with their carers, with approximately 50% having > 21 hours of contact time. The levels of parental distress, burden, accommodating and enabling behaviour and expressed emotion at baseline were within the range of that reported in systematic reviews of carer functioning. 198,199 The changes in carer distress, burden and caregiving behaviours are comparable to those found in the meta-analysis from a recent systematic review of psychoeducational interventions for carers. 200 In the meta-analysis, carer burden fell by 0.39 at the end of treatment and by 0.56 at the end of follow-up. On the other hand, the change in distress (DASS-21; ES = 0.12 at 6 months) was less marked than found in previous studies (in the meta-analysis this was 0.31 at the end of treatment and 0.39 at follow-up). Differences in the stage of illness of the patients and in the outcome measures used may explain this variation. It is noteworthy that there was a small increase in carer distress and fall in quality of life during the course of the study in the TAU group. The reduction in accommodating and enabling behaviour was similar to that found in the meta-analysis (0.2–0.5), as was the fall in carers’ expressed emotion (0.3–0.4). In comparison with the high intensity of care that the patients were given, the carers had a very low-intensity intervention. This could explain why the treatment effect occurs at 6 months and then attenuates at the end of 1 year’s follow-up.
We are in the process of conducting cost-effectiveness analyses for ECHO using the outcome data collected in this trial. The results of these analyses will be made available through publication on their completion.
Limitations
This sample is not necessarily representative of the target population as it includes only people who agree for their carers to be involved and who have carers who are willing to help. Moreover, the study includes patients at various stages of illness (19% early and 55% enduring), and it is possible that these subsets have different responses to the interventions. For example, family therapy is effective only as an early intervention. 194 Over half of the patients in this sample (n = 83; 55%) fulfil the criteria for being in a severe and/or enduring stage of illness. Such patients are considered to be resistant to all forms of treatment. 221,222 The sample was powered to detect only moderate overall differences in patient outcome. With 134 carers in each group and an alpha level of 0.025 (adjusting for multiple primary outcomes), we had 80% power to detect an ES of 0.38.
Although there are standard quality criteria for inpatient care, the treatment ethos does vary between services. For example, some services involve carers more than others do. This may have decreased the size of the effect. However, as the sample was stratified by site, it would not have led to any bias in the interpretation of the results. The comparison with TAU (rather than an active control), does mean that we cannot rule out that the apparent benefits of ECHO are non-specific effects of participants receiving a greater intensity of intervention.
Strengths
This study used the strengths of a pragmatic randomised controlled design to examine the effects of adding a low-intensity psychoeducational and skills training intervention for carers to inpatient care for AN. Furthermore, this is a multicentre study in which the majority of NHS England specialised ED centres participated. Considering that the number of patients with AN requiring inpatient care is small, the sample size of this study is unique.
Clinical implications
The possible added benefit for patients and for services attained from giving carers psychoeducational information has been rarely studied, but this study suggests that this is an area that merits more attention. It is possible that increasing the intensity of the intervention and including it within the service model of inpatient care might produce improvements for services, patients and carers. This would mean that carers might become more involved in treatment for adults.
Conclusion
A low-intensity psychoeducational intervention for the carers of patients at a severe and/or enduring stage of AN produced a decrease in burden and time spent caregiving and a small decrease in distress. Unhelpful carer behaviours such as high expressed emotion were also decreased. Carers reported higher levels of functioning in the people with EDs. Moreover, patients had greater improvement in eating psychopathology and quality of life and noted positive changes in carers’ behaviours. Patients felt more empathy for their carer. Moreover, carers’ dissatisfaction with services was lower in the ECHO group. It is possible that increasing the intensity of work with carers may have benefits for clinical services for patients in addition to benefits for carers themselves.
Chapter 7 An investigation of issues associated with physical activity in anorexia nervosa (work package 4)
An abbreviated version of this chapter has been published in the International Journal of Eating Disorders. 223
Abstract
Objectives
This WP examined physical activity (PA) in people with AN and associations between drive to exercise, ED psychopathology, anxiety, endocrine measures and energy expenditure (EE).
Method
Four groups of women were recruited: AN outpatients (n = 37), AN inpatients (n = 18), those with anxiety symptoms (n = 34) and HCs (n = 30). Actigraphy and self-report were used to measure PA, together with the drive and reasons for exercise, ED and general psychopathology, body composition and BMI. Salivary cortisol was also measured and EE was estimated. Measures were made over 24 weeks.
Results
Objective PA levels did not differ significantly between groups cross-sectionally or longitudinally: nonetheless, AN groups reported higher total PA than HCs. AN outpatients reported more walking and moderate PA than HCs. AN inpatients reported more walking but less moderate and vigorous PA than other groups. In the two AN groups, drive to exercise was significantly higher. These groups also rated ‘improving tone’ as being an important motivator to exercise. Estimates of EE due to PA indicated a maximum of 400 kcal/day in patients with AN, out of a total EE of approximately 1600 kcal/day.
Discussion
Elevated PA has been reported in AN, but this may reflect patients’ peception of their activity rather than what is actually done. Drive to exercise in AN seems to be more closely linked to ED psychopathology than to anxiety. Estimates of EE due to PA provide guidance for therapists who want to introduce exercise into therapy.
Introduction
Increased PA in AN can be loosely defined as deliberate exercise and/or indirect behaviours such as pacing and/or fidgeting. These behaviours, however, do not have a well-defined definition,224 yet may contribute to the maintenance of low BMI225,226 and non-recovery at 2 years. 227 High levels of PA prior to admission and following discharge have also been reported to be associated with poorer outcome. 228,229 In relation to treatment outcomes, commitment to exercise is a problem in inpatients and engagement in excessive sport in outpatients may be a barrier to treatment. 230 In addition, high PA may arise prior to illness231,232 and remain following recovery. 225,233 Therefore, excessive exercise behaviours may also contribute to the aetiology of AN rather than simply being a symptom of the illness. Furthermore, as high PA levels are associated with lower BMIs in individuals recovered from AN,225 this may suggest the persistence of illness behaviours, or show that high levels of PA are a trait rather than a state marker.
Research findings on the prevalence of high levels of PA in AN are mixed. This may be due to different methodologies, to perceptual differences in levels of PA undertaken in AN compared with controls, or to high degrees of individual differences in exercise behaviours. Despite these differences, increased PA has often been reported in this patient group. 232,234–237 Recently, a study reported increased PA in a subgroup of AN patients only, suggesting that high PA may not be universal in this patient group. 238
The hypothesised drivers of increased PA in AN are cognitive desire to lose weight,225 to improve mood230 and to reduce anxiety. 239,240 This is supported by the fact that women with AN are reported to value negative affect regulation as a motivator for exercise more than they value exercising to improve health and fitness. 234,241 Individuals with AN are also more likely to rate body tone, attractiveness and weight control as relevant motivators than exercising for enjoyment. 241 Thus, PA may be a deliberate and controlled behaviour that is intended to promote weight loss. 242
The idea that high PA is driven by anxiety in AN is supported by the often reported comorbidity of the two,243–245 and the fact that approximately 50% of AN patients also have a lifetime history of anxiety disorder. 2,246 In some AN patients, anxiety disorders are reported to predate the onset of the ED,245,247 are associated with lower BMI248 and poorer outcome. 249 Therefore, anxiety may be a vulnerability factor that is independent of nutritional state. 245 In terms of PA, an association between anxiety and high levels of PA has been reported in AN patients. Further support for the idea that anxiety may be a driver for PA in AN patients comes from reports that PA helps to reduce depression, stress and anxiety in adolescents250 and adults. 240
This study examined levels of PA, drive to engage in PA, motives for exercise, and a number of associated physiological and psychological measures in female patients with AN, in HCs and in a group with moderate levels of anxiety. The study had the following hypotheses:
-
Subjective and objective measures of PA levels together with the drive to exercise will be increased in AN patients compared with HCs and with the anxious group (these findings will be present both cross-sectionally and longitudinally over 24 weeks).
-
ED psychopathology and anxiety will be associated with PA levels.
-
Reasons for exercising will differ between groups.
-
In patients with AN, estimates of EE due to PA will show that < 500 kcal/day are used.
Methods
Participants
Women were recruited as four groups: inpatients (n = 18) and outpatients (n = 37) with a diagnosis of AN, individuals experiencing moderate anxiety (n = 34) and HCs (n = 30). AN patients (binge-purging or restricting subtype) with a BMI < 17.5 kg/m2 were recruited from in and around London (from specialist ED services). These services included outpatient treatment (n = 26), day care (n = 10) and inpatient treatment (n = 18). Patients attending day care treatment were grouped with the outpatient participants. Anxiety and control participants were recruited via an e-mail circular to students and staff at King’s College London. Anxiety participants were screened using the Generalised Anxiety Disorder questionnaire, and those with a score of ≥ 10 were recruited into the study. Participants had a mean age of 27 years in the anxiety group (range 18–54 years), 29 years in the combined AN groups (range 18–67 years) and 29 years in the HCs (range 20–52 years). All were English speaking. Recruitment commenced following approval by the local REC (09/H0807/4). All participants provided informed consent.
Measures
All participants provided data on the following measures.
Demographic information
Contact details, ethnicity, occupation, marital status, physical or mental health problems and lifestyle habits (such as drinking and smoking).
Weight and height
Weight and height were measured and used to obtain BMI [weight (kg)/height (m2)].
Structured Clinical Interview for DSM
This is used to diagnose DSM-IV axis I disorders. 251 Section H of the research version (Structured Clinical Interview for DSM-I/P, Patient Edition) was used to establish a diagnosis of AN.
Eating Disorder Examination-Questionnaire
This questionnaire (self-report) has been validated in ED studies. 142 It provides a global score and four subscale scores (weight and shape concern, restraint, eating).
Depression Anxiety and Stress Scales 21-version
This measure (a self-report questionnaire) assesses depression, anxiety and stress. 252,253 Scores determine level of severity for each subscale: normal, mild, moderate, severe and extremely severe.
International Physical Activity Questionnaire
This questionnaire uses self-report. It asks participants to estimate days and minutes spent sitting, walking, moderately and vigorously exercising per week. Number of days is multiplied by number of minutes on average spent engaging in each level of exercise to give an estimate (minutes/week) at each level. These are summed to give a total amount of PA (minutes/week). When participants responded ‘I do not know/unsure’, total scores were not calculated. A short version of this measure was used, and has been reported to have good international reliability and validity. 254 This questionnaire has been used to estimate PA in other studies of ED. 255,256
Commitment to Exercise Scale
This eight-item Likert scale-based questionnaire assesses commitment to exercise. It measures the degree to which (1) feelings of well-being are influenced by exercising, (2) adherence to exercise is maintained even in the face of adverse conditions and (3) exercise regimens interfere with social life. 257 It has been used in ED studies. 235,258,259
Obligatory Exercise Questionnaire
This was used to determine the frequency with which participants experience obligatory and compulsory thoughts, feelings and exercise behaviours. 260 The measure has 20 items, where 1 = never, 2 = sometimes, 3 = usually and 4 = always, giving a possible maximum score of 80. Higher scores indicate higher obligation and drive to exercise. The measure has good validity and reliability261 and has been used in ED and general population samples. 262–264
Exercise Addiction Inventory
This measure (self-report) assesses exercise attitudes and its impact on one’s life. 265 It has six items, where 1 = strongly disagree and 5 = strongly agree, giving a possible maximum score of 30. A higher score indicates greater likelihood of ‘exercise addiction’. The Exercise Addiction Inventory (EAI) is a reliable and valid tool for assessing risk of exercise addiction. 265,266
Reasons for Exercise Inventory
This assesses the importance of seven reasons to exercise: improving physical attractiveness, improving body tone, improving mood, enjoyment, weight control, health, and fitness. Each subscale has three or four items scored from 1 = not at all important to 7 = extremely important, and an average for each subscale is obtained. 267 It has been used in studies of AN. 234
Body composition
An InBody 3.0 Biospace Co. Ltd machine (Cerritos, CA, USA) was used to measure skeletal muscle, body fat, total body water and bone mineral content (BMC) in all participants. It is a portable non-invasive device that uses electrical impedance to obtain the measures.
Actimetry
An actometer device (Actiwatch AW4, Cambridge Neurotechnology, Cambridge, UK) was worn continuously by each participant. It was worn on the non-dominant wrist to measure PA over 7 days. This method has been used by other groups assessing PA in ED participants. 268,269 Data from the Actiwatches were analysed using its software (Motionware Software, CamNtech, Cambridge, UK).
Cortisol
Salivary cortisol was measured by obtaining weekly salivary samples in salivettes. Participants were asked to chew for 1 minute on a small cotton swab. As cortisol peaks in the morning, this was done 10 minutes after waking and before smoking or eating breakfast.
Leptin
Serum leptin levels were measured in AN and HC participants only. Blood samples were collected weekly and were sent to the Department of Clinical Biochemistry in the King’s College London Pathology Laboratory for analysis.
Data analysis
Five participants from the HCs with a BMI < 18.5 kg/m2 and six from the anxiety group with EDE-Q global scores > 2.8270 were not used in the analyses. Group means were compared using one-way analyses of variance (ANOVAs) and Tukey post hoc tests. Kruskal–Wallis tests and post hoc Mann–Whitney tests using Bonferroni corrections were used to compare means for variables that violated parametric assumptions [EDE-Q, DASS-21, International Physical Activity Questionnaire (IPAQ) and body fat]. Pearson’s rank correlations were used to infer relationships between variables before hierarchical multiple regressions were performed, with drive to exercise as the dependent variable.
Results
Group differences in outcome measures
Eating Disorders Examination-Questionnaire and Depression Anxiety and Stress Scales
Anorexia nervosa outpatients and inpatients had higher scores than the anxiety group and the HCs on EDE-Q total score (p < 0.001) and across the four subscales (p < 0.001) (Table 20). Restraint and eating concern scores were lower in the AN inpatient group, yet differences between AN groups were not significant. The group with anxiety had higher scores than the HCs on all EDE-Q subscales, and these were significantly higher for eating concern [n = 55, F(3) = 2.76; p < 0.01].
| Measure | HC (n = 27) | Anxiety (n = 28) | AN outpatient (n = 35) | AN inpatient (n = 17) |
|---|---|---|---|---|
| EDE-Q (total) | 0.7 (0.7) | 1.2 (0.9) | 4.2 (1.2) | 4.01 (1.2) |
| DASS-21 (total) | 6.2 (6.3) | 27.1 (11.6) | 34.9 (12.7) | 39.1 (18.4) |
Healthy controls were in the expected range for the DASS-21 subscales (0–7), but the group with anxiety scored in the moderate–severe range for anxiety (7–12) and in the moderate range for stress and depression (6–9). The AN outpatients scored ‘severe’ on all three subscales (8–16) and the inpatient group had scores that assessed them as ‘severe–extremely severe’ for depression (11–14+) and ‘extremely severe’ for stress and anxiety (> 10 and > 17, respectively).
There were significant between-group differences in total DASS-21 scores [F(3) = 56.14; p < 0.001], and for the depression [F(3) = 55.83; p < 0.001], stress [F(3) = 45.1; p < 0.001] and anxiety [F(3) = 49.44; p < 0.001] subscales (see Table 20). The AN groups reported higher anxiety, depression and stress levels than the HCs (p < 0.001). AN outpatients (but not inpatients) had higher depression scores than the anxiety group [F(3) = 3.2; p < 0.01]. The anxious and the AN groups did not significantly differ on other DASS-21 scores. The anxiety group scored higher than HCs on total DASS-21 [F(3) = 5.71; p < 0.001] and on the anxiety [F(3) = 5.55; p < 0.001], depression [F(3) = 5.45; p < 0.001] and stress subscales [F(3) = 5.09; p < 0.001].
Physiology
Among AN outpatients, BMI was consistent with diagnosis (mean = 16.04, SD = 1.44). In 97%, body fat mass was below the normal range and in 67% muscle mass was below the normal range (i.e. muscle was relatively conserved). In 25% of AN outpatients BMC was below the normal range. Among AN inpatients body fat mass was below the normal range in 100%, muscle mass below the normal range in 81% and as BMC below the normal range in 56.3% (presumably reflecting illness severity).
Healthy controls were lean and fit as a group: fat mass (kg) was below the normal range for age and gender in 44%, and muscle mass (kg) was above the normal range in 26%. Values in the anxiety group were similar: fat mass was below the normal range for age and gender in 43% and muscle mass was above the normal range in 21%. However, anxiety participants had a larger range in BMI (a SD of 3.88 vs. 1.5). No anxiety participants had BMC lower than the normal range.
The groups differed significantly in terms of BMI [F(3) = 63.32; p < 0.001], muscle [F(3) = 27.6; p < 0.001], body fat [F(3) = 69.8; p < 0.001], total body water [F(3) = 26.81; p < 0.001] and BMC [F(3) = 37.08; p < 0.001] (Table 21). On all body composition measues, AN participants differed from the HC and anxiety groups (p < 0.001). BMI did not differ significantly between the two AN groups, but the AN outpatients had significantly lower BMC (p < 0.01). The anxiety group and the HCs were similar in terms of body composition.
| Measure | HC (n = 27) | Anxiety (n = 28) | AN outpatient (n = 35) | AN inpatient (n = 17) |
|---|---|---|---|---|
| BMI (kg/m2) | 21.2 (1.5) | 22.24 (3.9) | 16.0 (1.4) | 14.1 (2.1) |
| Body fat (kg) | 12.4 (5.0) | 13.4 (7.7) | 4.1 (2.8) | 1.9 (1.6) |
| Skeletal muscle (kg) | 28.5 (5.5)a | 26.4 (2.9) | 21.5 (3.5)a | 19.1 (3.1)a |
| BMC | 3.1 (0.4) | 3.2 (0.4) | 2.5 (0.4) | 2.1 (0.4) |
| Total body water (litres) | 34.3 (3.9) | 34.5 (3.2) | 28.7 (4.2) | 26.6 (3.5) |
| RMR | 1431.1 (125.9) | 1398.6 (96.6) | 1221.8 (125. 4) | 1151.0 (108.2) |
Physical activity
Actimetry: an objective measure of physical activity
Actimetry showed that all groups had similar levels of activity (average and peak activity) (Table 22) and there were no significant between-group differences [average PA: F(3) = 0.46, p > 0.05; peak PA: F(3) = 0.26, p > 0.05]. The outpatient group had higher peak and average activity levels than the inpatient group (average: AN inpatient, mean = 223.63, SD = 61.67, n = 10; average AN outpatient, mean = 249.73, SD = 73.96, n = 27; peak AN inpatient, mean = 3607.1, SD = 507.83, n = 10; peak AN outpatient, mean = 3961.15, SD = 1329.39, n = 27), but these differences were not significant (both p > 0.013).
| PA | HC | Anxiety | AN outpatient | AN inpatient | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Average PA | 23 | 250.2 (61.5) | 23 | 249.4 (63.1) | 27 | 249.7 (74.1) | 10 | 223.6 (61.7) |
| Peak PA | 22 | 3971.3 (1350) | 22 | 3657.1 (1727.0) | 27 | 3961.2 (1329.4) | 10 | 3607.1 (1507.8) |
International Physical Activity Questionnaire scores: self-reported physical activity
Both of the AN groups recorded higher levels of total PA (minutes/week) than HCs (by 57–92%) (Table 23), and the difference between the outpatient group and HCs [F(3) = 3.05; p < 0.01] was significant. Both outpatient and inpatient AN groups reported doing more walking than HCs (250% and 95% higher, respectively), but the difference was significant only between outpatients and HCs [F(3) = 3.18; p < 0.01]. The outpatient group also reported engaging in more ‘moderate’ exercise than HCs (more than threefold higher), but the difference was not significant. The inpatient group recorded engaging in less ‘moderate’ exercise than the anxious group [F(3) = –2.99; p < 0.01] and less ‘vigorous’ activity than HCs [F(3) = –2.89; p < 0.01] and the anxiety group [F(3) = –2.74; p < 0.01]. The inpatient group reported that they engaged in less moderate and also less vigorous activity than the outpatient group. Finally, the anxiety group, recorded higher total activity (by 43%) and moderate activity (by 80%) and more walking (by 71%) and lower vigorous activity (by 34%) than HCs, but none of these differences was significant.
| IPAQ | HC | Anxiety | AN outpatient | AN inpatient | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | |
| 7 days | ||||||||
| PA (total) | 489.7 (378.7) | 26 | 699.5 (654.1) | 26 | 942.2 (598.6) | 22 | 768.9 (887.3) | 14 |
| Walking | 290.7 (336.1) | 26 | 497.9 (607.5) | 26 | 571.4 (393.4) | 27 | 629.7 (712.4) | 15 |
| Moderate | 70.0 (85.0) | 26 | 125.7 (143.6) | 28 | 219.9 (308.1) | 25 | 67.3 (216.8) | 15 |
| Vigorous | 124.8 (141.4) | 26 | 82.6 (125.5) | 28 | 163.0 (334.0) | 31 | 58.0 (216.5) | 15 |
| Over 5 days | ||||||||
| Sitting | 1872.2 (649.0) | 27 | 2088.0 (748.7) | 25 | 1597.8 (955.8) | 23 | 1406.3 (625.9) | 8 |
Comparison of subjective and objective measures of physical activity
There were no large between-group differences in objective measures of PA (total or peak activity), but, subjectively (as assessed by the IPAQ), the AN and anxiety groups reported undertaking more activity. In addition, in each group, the range is wider at every level of self-reported activity than the actimetry measures. SDs of subjective total PA are 77% (HCs), 94% (anxiety group), 64% (AN outpatients) and 115% (AN inpatients), whereas comparable SDs from objective measures of average PA (actimetry) are 24%, 23%, 29% and 27%, respectively.
Drive to exercise
Both the inpatient and outpatient groups had higher scores than the anxious group and the HCs on the Commitment to Exercise Scale [F(3) = 9.54; p < 0.001], the EAI [F(3) = 8.67; p < 0.001] and the Obligatory Exercise Questionnaire [F(3) = 6.86; p < 0.001] (Table 24). These questionnaires have slightly different elements, but all three show high correlations (r = 0.87; p < 0.01); hence we have created a ‘global drive to exercise’ (GDES) as single score. It was calculated in the following way. Average scores for each questionnaire were converted to a percentage of the highest score by any participant, and averages of these three percentages were then obtained: this has been used previously to create related scales (e.g. for anxiety and depression). 271
| Scale | HC (n = 27) | Anxiety (n = 28) | AN outpatient (n = 35) | AN inpatient (n = 17) | AN groups (n = 52) |
|---|---|---|---|---|---|
| GDTE (%) | 40.9 (12.3) | 45.7 (11.3) | 61.7 (23.0) | 65.9 (30.0) | 63.1 (25.3) |
| CES (%)a | 0.3 (0.2) | 0.3 (0.2) | 0.5 (0.2) | 0.6 (0.3) | 0.5 (0.3) |
| OEQ (%)b | 37.5 (5.9) | 41.1 (7.2) | 48.1 (14.0) | 51.3 (18.3) | 49.1 (15.4) |
| EAI (%)c | 11.2 (4.4) | 13.1 (3.9) | 18.3 (7.4) | 18.5 (9.7) | 18.4 (8.1) |
Significant between-group differences are observed in drive to exercise [F(3) = 9.42; p < 0.001]. Both the outpatients and the inpatients had significantly higher scores than the HCs and the group with anxiety (p < 0.001). No significant differences were seen between anxious participants and HCs or between inpatient and outpatient groups in their drive to exercise.
Reasons for exercise
There were between-group differences for enjoyment [F(3) = 2.91; p < 0.05] and for health [F(3) = 5.42; p < 0.01] (Table 25). Between-group differences for exercising for attractiveness approached significance [F(3) = 2.64; p = 0.054]. Exercising for health reasons was less important for the outpatient group than for HCs and the anxiety group (HCs vs. AN outpatients, 4.83 vs. 3.55, p < 0.013; anxiety vs. AN outpatients, 4.79 vs. 3.55, p < 0.013). Using exercise for enjoyment and to improve attractiveness was rated less important in the outpatient group than in the group with anxiety (attractiveness: mean = 3.11 vs. 4.39; enjoyment: mean = 2.20 vs. 3.17). The results were not, however, significant after applying Bonferroni corrections (p = 0.47 and p = 0.41). Finally, exercising to improve tone was more important in the two patient groups and the anxiety group than in the HCs (e.g. HCs vs. AN outpatients, mean = 3.44 vs. 4.70), but these differences were not significant (p = 0.056).
| Scale | HC (n = 27) | Anxiety (n = 34) | AN outpatients (n = 32) | AN inpatients (n = 15) | AN groups (n = 47) |
|---|---|---|---|---|---|
| Weight control | 4.2a (0.9) | 4.7 (1.1) | 4.6a (2.2) | 5.0 (1.7) | 4.7a (2.1) |
| Fitness | 4.5 (1.2) | 4.4 (1.5) | 4.02 (1.8) | 4.2a (2.0) | 4.1a (1.8) |
| Mood | 4.7 (1.5) | 4.6 (1.8) | 4.7 (1.7) | 4.9a (2.1) | 4.8a (1.8) |
| Health | 4.8 (1.3) | 4.8 (1.4) | 3.6 (1.7) | 3.7 (1.7) | 3.6 (1.7) |
| Attractiveness | 3.3 (1.9) | 4.4 (1.8) | 3.1a (1.8) | 3.4a (2.3) | 3.2a (1.9) |
| Enjoyment | 2.8 (1.5) | 3.2 (1.6) | 2.2 (1.2) | 2.2 (1.2) | 2.2 (1.2) |
| Tone | 3.4 (1.5) | 4.2 (1.7) | 4.7 (1.9) | 4.2 (2.5) | 4.5 (2.1) |
Endocrine data
A significant between-group difference in cortisol was observed (Table 26). Cortisol level was significantly higher in the inpatient group than in HCs [F(3) = 2.73; p < 0.05]. No significant differences in cortisol among the other groups were observed (p > 0.05). Cortisol concentration and the DASS-21 anxiety score were weakly correlated at baseline, and this approached significance (n = 100, r = 0.19, p = 0.07) across the group as a whole. When groups were analysed separately, no significant correlations were found between the DASS-21 anxiety score and baseline cortisol (HC: n = 30, r = –0.05, p = 0.81; anxiety: n = 33, r = 0.21, p = 0.25; AN outpatients: n = 26, r = 0.1, p = 0.65; AN inpatients: n = 11, r = –0.19, p = 0.58).
| Measure | HC | Anxiety | AN outpatients | AN inpatients | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | |
| Cortisol (nmol/l) | 10.0 (3.7) | 30 | 11.4 (10.4) | 31 | 12.3 (5.6) | 26 | 17.9 (11.7) | 11 |
| Leptin (µg/l) | 8.9 (4.7) | 14 | Not measured | 2.8 (2.4) | 30 | 2.6 (3.5) | 16 | |
Serum levels of leptin were measured in the two AN groups and HCs only. There was a significant between-group difference in leptin [F(2) = 18.55; p < 0.001), in that both the inpatient and outpatient groups had significantly lower leptin concentrations than HCs (p < 0.001). AN outpatient and AN inpatient groups did not significantly differ in concentrations of leptin. Across the whole sample, there was a significant correlation between leptin and body fat (%) (n = 57, r = 0.72, p < 0.001). Within-group analysis revealed significant correlations between leptin and percentage body fat in each group (HCs: n = 14, r = 0.71, p < 0.01; AN outpatients: n = 29, r = 0.57, p < 0.001; AN inpatients: n = 14, r = 0.55, p < 0.05).
Associations between outcomes
Subjective and objective measures of activity
When all participants were considered together, average activity (actimetry) was not correlated with total reported activity (IPAQ) and second, peak activity (actimetry) was not correlated with levels of reported vigorous activity. There was a significant but weak correlation between average activity (actimetry) and reported time spent walking (n = 72, r = 0.24; p < 0.05).
Within-group analysis showed that for the HCs, the anxiety group and the AN outpatients, there were no significant correlations between average/peak activity (actimetry) and any levels of self-reported activity. In the AN inpatients group, however, there was a strong correlation between the average activity (actimetry) and self-reported total activity (n = 9, r = 0.76; p < 0.02) and walking (n = 9, r = 0.74; p < 0.02).
Relationship between actimetry data and other measures
Across the study sample as a whole, there were no significant correlations between actimetry scores and other measures [e.g. DASS-21, EDE-Q, drive to exercise and Reasons for Exercise Inventory (REI)]. Within-group analysis showed that, when the two patient groups were combined, peak activity correlated with EDE-Q global scores (n = 36, r = 0.37; p < 0.05). No significant associations were seen between actimetry data and other variables in all other groups.
Self-reported activity (International Physical Activity Questionnaire) and other measures
Self-reported total activity, when examined across the whole group, was weakly correlated with global EDE-Q score (n = 86, r = 0.27; p < 0.01) and with exercising to improve tone (n = 83, r = 0.25; p < 0.02). No significant associations were found between any levels of self-reported activity and other variables when within-group analyses were performed.
Drive to exercise and other variables
Drive to exercise may be an important pathological variable and, as we used three scales to create a single measure (GDES), it was used as one of our main variables.
Across the whole group, drive to exercise (GDES) was most highly correlated with self-reported total activity (n = 86, r = 0.39; p < 0.01). In addition, correlations were present between drive to exercise and self-reported walking (n = 94, r = 0.25; p < 0.02), moderate activity (n = 94, r = 0.29; p < 0.01) and vigorous activity (n = 102, r = 0.37; p < 0.01). All these correlations were, however, < 0.4.
In the AN groups combined, drive to exercise correlated with self-reported total activity (n = 35, r = 0.52; p < 0.01), self-reported walking (n = 42, r = 0.37; p < 0.02) and the following REI subscales: weight control (n = 50, r = 0.55; p < 0.01), fitness (n = 46, r = 0.54; p < 0.01), mood (n = 46, r = 0.74; p < 0.01), health (n = 47, r = 0.43; p < 0.01) and to improve tone (n = 47, r = 0.44; p < 0.01). In addition, correlations were seen between drive to exercise and vigorous activity (n = 47, r = 0.39; p < 0.01) and peak activity (measured by actimetry) (n = 36, r = 0.43; p < 0.01) in these groups. In the anxiety group, drive to exercise correlated with self-reported vigorous activity only (n = 28, r = 0.65; p < 0.01).
Across the groups, drive to exercise correlated (moderately) with EDE-Q global score (n = 107, r = 0.48; p < 0.01), poorly with the DASS-21 anxiety score (n = 107, r = 0.23; p < 0.02) and inversely with (n = 107, r = –0.38; p < 0.01). It was also correlated with exercising to control weight (n = 105, r = 0.5; p < 0.01), to improve tone (n = 102, r = 0.40; p < 0.01), to improve mood (n = 101, r = 0.49; p < 0.01) and for fitness (n = 101, r = 0.3; p < 0.01).
When the two AN groups were combined, drive to exercise was highly correlated with exercising to improve mood (n = 46, r = 0.74; p < 0.01), for fitness (n = 46, r = 0.54; p < 0.01), to control weight (n = 50, r = 0.55; p < 0.01), to improve tone (n = 47, r = 0.44; p < 0.01) and for health (n = 47, r = 0.43; p < 0.0). In the anxiety group, drive to exercise correlated with exercising to improve mood (n = 28, r = 0.44; p < 0.02). Last, in the HCs, drive to exercise was not significantly correlated with other measures.
Multivariate models predicting drive to exercise
Hierarchical multiple regression was conducted to examine the extent to which ED pathology, anxiety, depression, stress and REI subscales predicted drive to exercise (after controlling for percentage muscle and BMI). Across the groups, BMI and muscle (%) were entered at step 1, and explain roughly 15% of the variance in drive to exercise. When REI and DASS-21 subscale scores and global EDE-Q score were entered (step 2), the total variance explained by the model was 57% (p < 0.01). Variables entered at step 2 explained an additional 41.6% of the variance in drive to exercise, after BMI and muscle (%) had been controlled for. In the final model, those variables that contributed most to the overall variance in drive to exercise were exercising to improve mood (6.3%; p < 0.01), exercising for weight control (4.9%; p < 0.01) and global EDE-Q score (3.6%; p < 0.01).
Following the above, the analysis was repeated for each group. In HCs, the DASS-21 and REI subscale scores and the global EDE-Q score explained roughly 35% of the variance in drive to exercise, but overall, the model was not significant (p = 0.74). In the group with anxiety symptoms, these variables explained roughly 70% of the variance [after controlling for BMI and muscle (%)]: the overall model was significant [F(11) = 5.54; p < 0.01]. Specifically, stress (DASS-21 subscale) contributed the most to the variance in drive to exercise (18%, p < 0.01), together with exercising to improve mood (11%; p < 0.01) and global EDE-Q score (8%; p < 0.05). For the outpatient AN group, DASS-21 and REI subscales and the global EDE-Q score explained 86% of the variance in drive to exercise [after controlling for BMI and muscle (%)] [F(11) = 10.48; p < 0.01]. In this group, exercising to improve mood and ED psychopathology contributed the most to the variance in drive to exercise [EDE-Q: 25% (p < 0.01); REI mood: 27% (p < 0.01)]. Other significant contributions were made by exercising to improve tone (10%; p < 0.01), exercising to relieve stress (12%; p < 0.01) and exercising for health (8%; p < 0.01).
The analysis was repeated using self-reported (IPAQ) and objective (actimetry) measures of PA as dependent variables: the resulting models were not significant.
Longitudinal studies related to physical activity
We also investigated PA longitudinally over 24 weeks in patients with AN. The four groups of participants were the same and the methodology was similar to that used in the cross-sectional study. PA was measured continuously for 8 weeks and at follow-up (weeks 12 and 24) by actimetry and self-report (IPAQ). Measures of general and ED-related psychopathology were made in parallel.
Eating disorder psychopathology and physiological measures remained consistent with diagnosis. Drive to exercise, depression, stress and anxiety were, as might have been predicted, consistently higher in the two AN groups than in the HCs and the group with anxiety symptoms. The highest values were seen in the inpatient AN group. In all four groups, there was a wide range in activity, especially on the self-report (IPAC) scores, but mean actimetry values were relatively constant over time. In the AN groups (compared with HCs and the group with anxiety symptoms), self-reported activity was higher on measures of total, walking and moderate PA. In the two AN groups, the drive to exercise was correlated with self-reported exercise. The objective measures (actimetry) showed no statistically significant difference in levels of PA between the four groups.
The data arising from the longitudinal study are in agreement with the results from the cross-sectional investigation. For example, comparison of the objective and the self-report data suggests that, in the AN groups, self-reported overactivity is perceived rather than real and may arise from factors such as drive to exercise. It is also of note that levels of activity remain relatively constant across the duration of the study. Furthermore, the objective data suggest that levels of PA do not differ between the AN groups and the HCs. What is perhaps surprising is that levels of activity are not apparently decreased by being in an inpatient setting, for which limits on activity may have been set. This may be because people in an inpatient setting spend considerable time walking (e.g. pacing). It suggests that levels of PA are difficult to regulate in severely ill patients in whom there is a high drive to exercise. However, given that some of the patients have a very low BMI and high levels of ED pathology, diminished fat reserves and a loss of muscle tissue (i.e. the AN inpatient group), this relatively ‘normal’ level of activity may be of some clinical concern and should be considered. In this respect, it should also be seen in the context of setting a balance that recognises the beneficial effects of exercise on mood and anxiety, and its effects on bone health, EE and PA.
Energy expenditure and physical activity
A final element of the study was an investigation of EE asociated with PA in AN. The data were derived from the measures of self-reported activity and from physiological measures such as BMI. PA-related EE was estimated by converting IPAQ scores to ‘metabolic equivalent of task’ values and then to corrected metabolic equivalent of task values, to adjust for differences in resting metabolic rate (RMR).
Compared with the HC group, RMR was lower in the outpatient group (by 11%) and in the inpatient group (by 17%). When EE is corrected for RMR (corrected metabolic equivalent of task), no group differences in EE emerged. We estimate that EE (i.e. EE due to PA) is roughly 360 kcal/day in the outpatient group and 400 kcal/day in the inpatient group. Based on the use of the self-report (IPAQ) data, combining RMR with EE provides energy neutral points of 1620 kcal/day for the outpatient AN group and 1578 kcal/day for the inpatient AN group. As the two patient groups report that they undertake higher levels of exercise than that suggested by the objective actimetry scores, the actual total daily EE in these two clinical groups is likely to be lower than our estimate (e.g. by roughly 200 kcal/day).
Energy expenditure may be of concern when a patient is at low weight/not eating. However, given that they have a high drive to exercise and that PA may improve affect, reduce stress and possibly improve general health, it is arguable that awareness of the caloric consequences of exercising will enable the desire to exercise to be more easily negotiated in relation to food intake.
Discussion
Summary
The data on psychopathology and on body composition were as expected (i.e. patient groups had significantly higher ED pathology, anxiety, depression and stress and lower BMI, fat and muscle than the HCs and the anxiety groups). This information was fairly consistent both cross-sectionally and longitudinally.
The PA data are less easy to interpret. Self-reported PA (IPAQ scores) showed that the two AN groups reported higher levels of activity across all levels of PA (especially walking) than the anxious and HC groups. These two patient groups reported slightly different exercise profiles: AN outpatients reported undertaking more moderate and vigorous activity than the two control groups and the AN inpatients reported the opposite (i.e. that they engaged in less moderate and vigorous activity than HCs). In contrast, actimetry showed that average and peak PA levels in the AN groups were quite similar to those reported by the HCs and anxious participants.
Both the groups with AN had significantly higher drives to exercise than the HCs and the anxious group, and the groups valued different reasons for exercising as more or less important. For example, the AN groups rated weight control, improving mood and improving tone as more important motivators for engaging in exercise. In contrast, the anxiety and HC groups rated health as the most important exercise motivator.
Physical activity
Physical activity measured by actimetry (both cross-sectionally and longitudinally) shows that the AN groups spent similar amounts of time exercising, and at similar intensities, as HCs, whereas, on the self-report measure, the outpatient AN group have higher PA scores at all intensity levels. The self-report data are therefore consistent with other findings indicating increased PA in AN,232,235,236 and also with our first hypothesis. The objective data do not support our original hypothesis, but do support the findings of Zipfel et al. 238 that individuals with AN and controls showed similar levels of PA when objective measures are used (e.g. the doubly labelled water method). Zipfel et al. also reported that not all individuals with AN have increased levels of PA. However, in the present study, although we saw a range in activity in the AN group, there was no relationship between illness severity and PA and, last, no evidence of activity-based subgroups emerged.
The data from the self-report (IPAQ) measure (both cross-sectional and longitudinal) suggest that there are group differences in the exercise type that is undertaken (e.g. inpatients reported spending significantly more time walking). This suggests that these individuals choose types of exercise that are appropriate for their weight and physical ability in order to satisfy their high drive for exercise. It may also be that it is the most feasible method in an inpatient setting.
Several potential explanations can be provided for the differences between subjective and objective measures of PA. For example, objective measures may not accurately detect different intensities or types of PA. This could occur when arm movements may not be consistent with the intensity of activity (e.g. uphill walking or cycling). There may also be some limitations to self-report measures in terms of recall accuracy. Additionally, it is possible that different groups estimate their levels of PA differently. HC participants may underestimate this whereas, conversely, individuals with AN may perceive themselves to be highly active. Furthermore, AN patients who are physically compromised may perceive PA at any level as a significant physical investment, and this may result in them overestimating their activity. This idea is consistent with the observed large SD and variance in our IPAQ data. Overall, the results suggest that individuals when they are ill (and possibly in an inpatient setting) are undertaking PA at roughly the same level as HCs and this in itself is of some clinical concern.
Drive to exercise and physical activity
Consistent with our second hypothesis, both the AN inpatient and outpatient groups had significantly higher drives to exercise than the HC and the anxiety groups. Second, drive to exercise was not different between the HC and the anxiety groups. It is therefore suggested that this drive to exercise is linked more to ED than to anxiety. The absence of a strong relationship between anxiety and the drive to exercise or objective/subjective measures of PA further supports this. Across the whole sample, there was a moderate correlation between drive to exercise and all measures of self-reported PA (i.e. with total activity, walking, moderate activity and vigorous activity). There were also moderate correlations between drive to exercise and global ED score, exercising to to improve mood, improve tone, for fitness and for weight control. Last, drive to exercise was inversely correlated with BMI and poorly with the DASS-21 anxiety score.
In the AN inpatient and outpatient groups, there was a significant relationship between drive to exercise and exercising to control weight, to improve mood, to improve tone, for fitness and health, and peak activity (based on actimetry). In the group with anxiety symptoms, drive to exercise was highly correlated with self-reported vigorous activity. Finally, drive to exercise was moderately correlated with exercising to improve mood in this group.
Hierarchical multiple regression analysis showed that the global EDE-Q score, exercising to control weight and to improve mood, predicted drive to exercise across the whole sample after controlling for muscle (%) and for BMI. In the anxiety group alone, stress (DASS-21 subscale) contributed most to the variance in drive to exercise. For the AN outpatient group, the biggest contributors to variance in drive to exercise were exercising to improve mood and global EDE-Q score.
Overall, results show that PA has several psychological drivers, as well as exercising to improve fitness and tone. Engaging in PA seems to be driven by the desire to cope with stress and to improve mood in the anxiety group. However, in people with AN, the drive may partly be to improve mood, but, in this group, the absence of a relationship between PA and anxiety scores suggests that drive to exercise is not primarily a response to anxiety. It appears that these behaviours are associated with body and weight concerns (i.e. central elements of ED psychopathology). Data thus support our hypotheses and previous findings242 that the drive to exercise in AN is substantially motivated by concerns at the core of ED psychopathology (e.g. the cognitive issues related to losing/maintaining low weight).
Endocrine findings
Anorexia nervosa inpatients have significantly higher cortisol levels than HCs, which is to be expected given the higher level of anxiety in this group. A small correlation was observed between cortisol level and DASS-21 anxiety score. Both AN groups had significantly lower leptin levels than HCs, and there was a strong and highly significant correlation between leptin and body fat percentage (both these findings are consistent with expectations).
Strengths and limitations
A strength of this investigation is that it allowed for PA to be directly compared in people with AN and with moderate anxiety. Second, participants in each group were of similar ages, which is important when comparing PA. Additionally, combining both objective and subjective measures allowed us to assess and compare different methodologies for studying PA. An additional advantage was that measures of ED psychopathology, general psychopathology and biology were collected along with the activity data. Finally, the study was conducted cross-sectionally and longitudinally (over 24 weeks).
The investigation had some limitations. First, the more complex analyses used in this study (e.g. group differences in predictors of reasons for exercise) are probably underpowered. In addition, some data were missing because participants occasionally forgot to wear the Actiwatches and also because self-report data were collected retrospectively over a period of 7 days, making such data slightly less reliable. In addition, there is the possibility of some recruitment bias in the AN groups (i.e. not all of the people with the problem might be willing to join an investigation assessing PA). Finally, participants were predominantly white females and, therefore, it might not be possible to generalise our data to men and/or to different ethnic groups.
Conclusions
Our activity measurements gathered from self-report data indicate that levels of PA are higher in invividuals with AN than in HCs or those with moderate anxiety, whereas the actimetry data show that these groups do not differ in their amount or level of PA. It is possible that self-report overestimates the level of PA in AN patients owing to differences in perception caused by the ED or because these individuals are in poor physical health. Findings also show that patients with AN have a higher drive to exercise than HC and anxiety groups. In AN patients, ED pathology, rather than anxiety, appears to be a more important contributor to their increased drive to exercise. Estimates of RMR and of EE arising from PA will inform decisions related to the clinical management of people with AN and will also inform discussions on the role and use of exercise programmes in treatment.
Chapter 8 Preventing deterioration and relapse in severe anorexia nervosa: randomised controlled feasibility trial of an e-mail-guided manual-based self-care programme based on the Maudsley Model of Anorexia Nervosa Treatment for Adults (work package 5)
Abstract
Background
Relapse rates after inpatient treatment of AN are high. The aims of this study were (1) to assess the feasibility of a relapse prevention programme for people with AN and (2) to acquire information to inform a large RCT of this intervention.
Methods
Participants were inpatients with AN aged ≥ 16 years. Participants were recruited from seven UK specialist ED units and randomly allocated to receive either manual-based e-mail-guided self-care for 12 months combined with TAU or TAU alone. The manual was based on the MANTRA. E-mail support was delivered weekly by therapists. Outcome assessments included BMI, EDE, depression, anxiety, quality of life and service utilisation at 6 and 12 months post randomisation. Treatment ESs (Cohen’s d) were calculated.
Results
Forty-one patients participated. At 6 months post randomisation (and post discharge) there was little difference between groups, with ESs between d = –0.08 and d = 0.3. At 12 months, patients receiving the experimental intervention had a higher BMI (d = 0.41) and lower scores on the DASS-21 (d = 0.64). Readmission rates were 5 out of 22 (22.7%) in the experimental group and 5 out of 16 (31.2%) in the TAU alone group. No harms were detected.
Conclusions
These findings suggest that this low-intensity relapse prevention may be beneficial in the aftercare of inpatients with AN and that a large-scale RCT of this intervention may be justified.
Trial registration
Current Controlled Trials ISRCTN18274621.
Introduction
Anorexia nervosa is a life-threatening illness that is associated with a mortality rate among inpatients that is twice that of those with other psychiatric disorders, and a suicide rate 200 times that of the general population. 72,272,273 There are high levels of physical disability and psychological comorbidity and the median duration of AN is 6 years. 274 Although for most patients with AN the treatment of choice is outpatient psychological therapy, a proportion of cases with severe and potentially life-threatening illness need skilled refeeding in hospital. The threshold for inpatient treatment in AN differs in different countries. In continental Europe, admission to hospital appears to be more common than in the UK, where this treatment is reserved for the most severe cases. Duration of inpatient care also varies in different health-care systems, with longer durations of admissions in European countries than in the USA. 193 Although patients usually manage to gain weight in hospital, deterioration and relapse following inpatient treatment is common and typically occurs within the first year of treatment. Approximately 30–50% of those who remit relapse. 228,275–278 The reasons for these high relapse rates are complex, but may in part be to do with patients’ physical improvements during inpatient refeeding not being mirrored by similar psychological improvements. Thus, effective psychological aftercare following inpatient treatment is important. This is echoed by the NICE guidelines for EDs,279 which recommend that people discharged from inpatient care should be followed up for at least 1 year post discharge. In practice, as patients often live at some distance from a specialist inpatient unit, this follow-up often is delivered by non-specialists, making aftercare practice variable and haphazard.
Only a handful of studies have addressed the question of how best to reduce relapse rates after inpatient weight restoration. Antidepressant treatment alone appears to be unacceptable to the majority of eligible patients,280,281 and ineffective. 206 Two studies in adolescents found family therapy to be superior to individual treatment190,282 or TAU. 283 Conversely, in adults with AN, one study found individual therapy to be somewhat superior to family therapy in preventing relapse,190,282 although a second study using a similar design found no difference between family therapy and two types of individual therapy. 284 In one small study, CBT was superior to nutritional treatment alone in preventing relapse. 207 However, in another study which compared CBT alone or in combination with fluoxetine alone, dropout rates in all three groups were too high to draw any conclusions about efficacy,281 casting doubt on the acceptability of these interventions. Finally, a large-scale RCT (n = 258 participants) from Germany compared an internet-based cognitive–behavioural relapse prevention programme with TAU. In the ITT analysis, patients receiving the internet-based relapse prevention programme gained a small amount of weight, whereas those receiving TAU lost a small amount, although the difference was non-significant. There were significantly more readmissions to hospital in the relapse prevention group. 285
We have designed and piloted a novel manual-based outpatient treatment for adults with AN (MANTRA). 19,126 The content, structure, research underpinning this treatment and iterative development process have been described in detail elsewhere. 20,136,286 In brief, MANTRA is an empirically based cognitive-interpersonal treatment that is trait focused and targets key intra- and interpersonal maintenance factors of AN. It is centred around a patient manual and is modularised with a clear hierarchy of procedures, tailored to the needs of the individual. It has shown promise in pilot studies. 136,286 For the purpose of the present study, this treatment and manual were adapted for use in inpatients following discharge from hospital. We evaluated here the feasibility of using it as an internet-based-guided self-care treatment, supported via e-mail, given that many patients live at a considerable distance from specialist ED units (internet-based Maudsley Model of Anorexia Nervosa Treatment for Adults; iMANTRA).
The aims of the study were (1) to assess the feasibility of using e-mail-guided self-care treatment added to TAU in the post-admission care of hospitalised AN patients and (2) to acquire key information that would inform development of a large-scale RCT that will assess the efficacy and cost-effectiveness of this intervention added to TAU compared with TAU alone. Only efficacy data are reported here; cost-effectiveness data are not yet available and will be published elsewhere when complete.
The specific objectives of the proposed feasibility study were to:
-
assess recruitment and treatment uptake rates
-
determine what is an appropriate frequency of e-mail support for patients
-
determine the best instruments for measuring outcomes in a full trial by examining the quality, completeness, and variability in the data
-
estimate the treatment ESs and SDs for outcome measures to inform the sample size calculations for a large-scale RCT
-
evaluate whether or not the treatment is operating as designed by analysing process measures, such as worry and intolerance of uncertainty
-
determine whether or not patients with AN see this treatment as acceptable and credible.
Method
Design
The trial was a multicentre two-arm feasibility trial that compared iMANTRA added to TAU with TAU alone in consecutive referrals of patients discharged from inpatient treatment for AN. Ethics approval was obtained from the National REC West Midlands – Edgbaston, reference number: 08/H1208/33. The trial was registered with Current Controlled Trials ISRCTN18274621. Recruitment and follow-up procedures took place between March 2009 and December 2012.
Participants and recruitment
Patients were recruited from seven specialist adult or adolescent inpatient units in the UK. These were South London and Maudsley NHS Foundation Trust; Seacroft Eating Disorders Unit, Leeds Teaching Hospitals NHS Trust; St Ann’s Hospital, Barnet, Enfield and Haringey Mental Health Trust; South West London and St George’s Mental Health NHS Trust; Huntercombe Hospitals (Stafford, Edinburgh); The Priory Hospital, Roehampton; and Royal Victoria Hospital, Newcastle upon Tyne. Patients’ suitability for participation in the study was checked by a clinician from the EDs team towards the end of their inpatient treatment (i.e. within 1 month of discharge). If the patient was suitable for participation, their fully informed written consent was sought and after this she/he was introduced to the researcher who completed the research assessment.
Inclusion/exclusion criteria
Inclusion criteria
Patients fulfilling criteria for DSM-IV AN or atypical AN who had undergone a period of inpatient treatment in one of the participating ED services were eligible for inclusion in the study if they were aged ≥ 16 years; had reliable access to broadband internet; were available over the full duration of the study; and had shown clinically significant weight gain during inpatient treatment (a minimum of approximately 3 kg or 1 BMI point).
Exclusion criteria
Unstable AN (i.e. actively losing weight at the end of treatment); insufficient knowledge of English or literacy levels insufficient to allow understanding of the manual and assessment; psychosis; acute suicidality; substance dependence; and diabetes mellitus. We did not exclude patients on psychotropic medication (antidepressants, antipsychotics), which are commonly prescribed in this population.
Comparison groups
Common features
All participants were signed up for regular weighing and physical risk monitoring at their GP surgery. A crisis plan with names and numbers of who to contact in an emergency was also drawn up with patients prior to discharge.
Internet-based Maudsley Model of Anorexia Nervosa Treatment for Adults plus treatment as usual
Those allocated to iMANTRA plus TAU were sent an e-mail informing them of their treatment allocation and giving them some information about their e-mail therapist and the frequency and nature of the e-mail contact. E-mail contact was offered one to three times weekly for the first 6 months following discharge and then more flexibly (once weekly to monthly) during months 7–12. Participants were also told not to use their e-mail therapist for urgent out-of-hours or other crisis support and instead use the crisis contacts on their list. Participants were asked to complete an online questionnaire giving some background information about themselves, to help their e-mail therapist to get to know them better. This questionnaire included questions on their current difficulties, struggles and worries; their hopes, ambitions and goals for the future; their key current relationships, in particular with people who might be able to support them; their personal history; and any important events that have affected or shaped them and any previous treatments that they may have had (a copy of this questionnaire can on request be obtained from the authors).
Patients were also offered an initial telephone call with their online therapist to give both patient and therapist the opportunity to ask questions and clarify information. Finally, patients were sent the revised version of the MANTRA workbook. This included (1) a traffic light system of relapse risk for patients to complete, to increase their awareness of potential indicators of such risk; (2) a nutrition plan that was designed for weight maintenance and also gave information on what to do if more weight gain was needed; (3) a module addressing anxiety-related processes, such as worry and intolerance of uncertainty and strategies to reduce them (this was included as patients with AN generally have high levels of anxiety, worry and intolerance of uncertainty,287–290 and it was thought that during the post-discharge period this would be particularly intensive and therefore an important treatment target); and (4) strategies to prevent and cope with deterioration and lapses.
The role of the therapist was to be motivational and supportive, with the aim to guide patients in their use of the workbook, by suggesting use of relevant modules, thereby tailoring the intervention to the relapse risk and clinical profile of the patient. Patients in this group also received TAU from their local Community Mental Health Team or Child and Adolescent Mental Health Team.
Treatment as usual
Patients randomised to this group did not receive the internet-based intervention. They received TAU from their local Community Mental Health Team or Child and Adolescent Mental Health Team. They were followed up at regular intervals using the same assessment measures as patients who received the iMANTRA intervention.
Measures
Body mass index
Body mass index (weight/height2) was obtained from the medical notes for each patient at admission and at discharge. At 6 and 12 months this was obtained from treating clinicians or from patient self-report.
Interview measures
These were done at baseline and at 6 and 12 months and included (1) the EDE291 and (2) the CSRI. 153 These were completed in person or by telephone.
The EDE is a widely used, semistructured interview that generates four subscale scores: dietary restraint, eating concern, weight concern and shape concern. The mean of these four subscales is used to create a global score.
The CSRI is an inventory of service use that facilitates estimation of support costs. It was adapted for the current study to cover a wide variety of hospital, mental health, and community-based services as well as medications, impact of employment and additional personal expenditure due to the ED.
Questionnaire measures
These were completed at baseline and at 6 and 12 months.
Eating disorders pathology
Other psychopathology
This is a 21-item self-report measure to assess mood state over the past 7 days using a four-point Likert scale. Total score as a measure of general distress or depression, anxiety and stress subscales can be used. High scores indicate higher symptomatology. This measure has good reliability and validity. 210
Quality of life
Items are rated on a five-point Likert scale pooled in four domains: physical health, psychological, social relationships and environment. Good psychometric data have been reported for this scale. High scores indicate better quality of life.
Anxiety-related processes
This is a 16-item questionnaire rated on a five-point scale, with higher scores indicating a greater tendency to worry. The Penn State Worry Questionnaire (PSWQ) has very good psychometric properties293 and is the most established measure of trait worry.
The Intolerance of Uncertainty Scale (IUS) is a 27-item measure designed to assess the cognitive, emotional and behavioural aspects of intolerance of uncertainty. Items are scored on a five-point Likert scale. High scores denote high levels of intolerance of uncertainty. The IUS has very good psychometric properties. 295
Acceptability
At 12 months, patients who had been randomised to the active intervention were asked questions about what they thought had been the most helpful aspects (e-mail, manuals) and also what they thought about content and structure (e.g. frequency of e-mail contact) of the intervention.
Randomisation, blinding and protection against bias
Randomisation was conducted independently from the trial team by the King’s College London CTU using a computerised system. Patients were randomised to the iMANTRA intervention plus TAU versus TAU alone, at a ratio of 1 : 1. Randomisation was carried out using minimisation to balance groups for prognostic factors (previous hospitalisation; illness subtype). Patients were told about the outcome of randomisation by an e-mail sent to them by a study administrator. Throughout the trial every effort was made to ensure that the researcher who conducted outcome assessments remained blind to patients’ treatment allocation.
Therapists
Three experienced ED therapists delivered the e-mail support. All had undergone a 1-day training programme preparing them to deliver the intervention. The focus of the training was to standardise delivery of the e-mail contact. Therapists were instructed to keep e-mails brief, be informal, warm and supportive in tone, point patients in the direction of appropriate materials from their manual that might be relevant in keeping them well or helping them to make further improvement and provide non-intrusive monitoring of symptoms via the traffic light relapse prevention tool.
Statistical analyses
Outcomes were analysed on an ITT basis, that is, participants were analysed in the group to which they were randomised irrespective of their compliance with the assigned treatment. No formal hypothesis testing was undertaken, as this is a feasibility study. ESs (Cohen’s d) were calculated for continuous outcomes at 6 and 12 months from means, SDs and sample sizes using the ES calculator of the Campbell Collaboration [URL: www.campbellcollaboration.org/effect-size-calculato.html (accessed 3 July 2017).
Results
Patient flow
Figure 4 shows the participant flow through the study. Twenty-four participants were randomised to iMANTRA plus TAU and 17 to TAU alone.
FIGURE 4.
Consolidated Standards of Reporting Trials flow diagram (WP5).

Patient characteristics at baseline
Patients in the two treatment groups were similar in terms of baseline sociodemographic and clinical characteristics (Table 27), with the exception of the WHOQOL-100 environmental subscale, according to which the iMANTRA group had significantly poorer quality of life than the TAU group [t(32.6) = –2.34; p = 0.025]. All had severe AN (mean admission BMI of 14.1 kg/m2), with just over half (51%) having had at least one additional previous admission to hospital. Patients had made considerable gains in weight while in hospital (discharge BMI of 18.0 kg/m2) and prior to coming into the study.
| Characteristic | Whole group | iMANTRA plus TAU | TAU only | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) or n (%) | n | Mean (SD) or n (%) | n | Mean (SD) or n (%) | |
| Demographic details | ||||||
| Age | 41 | 24.2 (9.6) | 24 | 24.8 (10.1) | 17 | 23.3 (8.9) |
| Gender ratio (male : female) | 41 | 4 : 37 | 24 | 2 : 22 | 17 | 2 : 15 |
| Proportion with a partner | 41 | 5/41 (12%) | 24 | 4/24 (16.6%) | 17 | 1/17 (6%) |
| Clinical details | ||||||
| Diagnosis | 41 | 24 | 17 | |||
| AN-R | 33/41 (80.5%) | 20/24 (83.3%) | 13/17 (76.5%) | |||
| AN-BP | 8/41 (19.5%) | 4/24 (16.6%) | 4/17 (23.5%) | |||
| Age of onset | 41 | 17.6 (6.0) | 24 | 17.8 (4.8) | 17 | 17.4 (7.6) |
| BMI at hospital admission | 38 | 14.1 (1.4) | 23 | 13.9 (1.7) | 15 | 14.3 (1.0) |
| Proportion with one or more previous admissions | 41 | 21/41 (51.2%) | 24 | 12/24 (50%) | 17 | 9/17 (52.9%) |
| BMI at study entry | 41 | 18.0 (1.9) | 24 | 18.1 (2.2) | 17 | 17.9 (1.4) |
| EDE global | 40 | 2.8 (1.4) | 24 | 2.9 (1.3) | 16 | 2.7 (1.6) |
| DASS-21 total score | 36 | 28.6 (18.0) | 22 | 28.7 (17.6) | 14 | 28.3 (19.2) |
| WHOQOL-100 | 35 | 21 | 14 | |||
| Physical | 47.8 (13.7) | 48.0 (10.7) | 47.5 (17.7) | |||
| Psychological | 40.1 (15.7) | 37.8 (13.4) | 43.4 (18.7) | |||
| Social | 42.1 (22.3) | 37.2 (23.6) | 49.5 (18.5) | |||
| Environmental | 67.2 (19.8) | 61.5 (20.8) | 75.7 (15.10) | |||
| PSWQ | 36 | 62.6 (13.5) | 22 | 63.7 (12.0) | 14 | 60.9 (15.8) |
| IUS | 36 | 87.9 (27.8) | 22 | 88.3 (27.3) | 14 | 87.2 (29.5) |
| Proportion currently taking psychotropic medication | 41 | 24/41 (58.5%) | 24 | 14/24 (58.3%) | 17 | 10/17 (58.8%) |
Acceptability of internet-based Maudsley Model of Anorexia Nervosa Treatment for Adults
Twenty-one of 24 (87.5%) patients allocated to iMANTRA plus TAU took up this treatment. Five iMANTRA patients completed the 12-month acceptability questions. All five said that the e-mail contact was the most helpful element of the intervention. All five participants rated the frequency of e-mails as ‘the right amount’.
Treatment outcomes
Table 28 shows outcomes on interview and questionnaire variables at 6 and 12 months.
| Outcome | iMANTRA plus TAU | TAU alone | ES | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | d | 95% CI | |
| BMI at 6 months | 23 | 17.1 (2.9) | 13 | 17.3 (1.88) | –0.08 | –0.76 to 0.60 |
| BMI at 12 months | 21 | 18.0 (3.3) | 14 | 16.9 (1.4) | 0.41 | –0.28 to 1.09 |
| EDE global at 6 months | 22 | 2.9 (1.7) | 12 | 2.9 (1.8) | 0 | –0.70 to 0.70 |
| EDE global at 12 months | 17 | 2.9 (1.7) | 10 | 2.9 (1.9) | 0 | –0.78 to 0.78 |
| DASS-21 total at 6 months | 18 | 28.6 (16.9) | 9 | 26.6 (20.4) | 0.11 | –0.69 to 0.91 |
| DASS-21 total at 12 months | 15 | 26.5 (16.4) | 6 | 37.8 (20.7) | –0.64 | –1.61 to 0.33 |
| WHOQOL-100 at 6 months | 17 | 8 | ||||
| Physical | 46.5 (14.8) | 43.9 (21.7) | 0.15 | –0.69 to 0.99 | ||
| Psychological | 39.4 (16.9) | 41.6 (20.5) | –0.12 | –0.96 to 0.71 | ||
| Social | 42.4 (25.3) | 39.9 (18.3) | 0.10 | –0.73 to 0.95 | ||
| Environmental | 61.6 (22.1) | 63.5 (15.3) | –0.09 | –0.93 to 0.75 | ||
| WHOQOL-100 at 12 months | 15 | 8 | ||||
| Physical | 47.5 (16.7) | 52.1 (20.6) | –0.25 | –1.12 to 0.61 | ||
| Psychological | 39.3 (19.8) | 32.1 (25.1) | 0.33 | –0.53 to 1.20 | ||
| Social | 41.7 (26.4) | 46.9 (30.2) | –0.19 | –1.05 to 0.67 | ||
| Environmental | 67.2 (20.5) | 66.6 (17.2) | 0.03 | –0.83 to 0.89 | ||
| PSWQ at 6 months | 19 | 63.2 (11.1) | 9 | 59.3 (15.2) | 0.31 | –0.51 to 1.15 |
| PSWQ at 12 months | 15 | 62.5 (11.6) | 8 | 59.9 (14.7) | 0.20 | –0.66 to 1.06 |
| IUS at 6 months | 18 | 91.4 (25.5) | 9 | 87.4 (27.5) | 0.15 | –0.65 to 0.95 |
| IUS at 12 months | 12 | 88.3 (30.2) | 8 | 95.3 (29.8) | –0.23 | –1.13 to 0.66 |
At 6 months there was little difference in outcomes between groups, with ESs between d = –0.08 and d = 0.3 (i.e. negligible to small). At 12 months, patients receiving the experimental intervention had a higher BMI (d = 0.41) and lower total scores on the DASS-21 (d = 0.64). However, CIs were wide and overlapped with zero.
Service utilisation
Ten patients required readmission to hospital during the trial, 22.7% (5/22) in the iMANTRA group and 31.2% (5/16) in the TAU alone group. This difference was not significant [χ2(1) = 0.347; p = 0.556]. Comparable numbers of patients in each group had GP or outpatient care during the study period [iMANTRA: 12/18 (66.6%); and TAU alone: 10/15 (66.6%)]. The proportion of patients with no additional treatment was also similar in both groups [iMANTRA: 1/18 (5.5%); and TAU alone: 1/15 (6.6%)]. No patient died during the study period.
Harms
No harms were detected.
Discussion
This study shows that iMANTRA is a feasible and acceptable intervention when added to TAU in the post-hospitalisation aftercare of patients with AN. Although at 6 months post admission there was little difference in outcomes (with ESs negligible or small), at 12 months between-group ESs favoured iMANTRA in terms of its effects on BMI and depression, anxiety and stress (d = 0.4–0.6). Moreover, readmission rates were somewhat lower in the iMANTRA group. Taken together these findings suggest that this approach has promise and should be studied further. Further evidence supporting this approach comes from a study in Germany,296 which adapted the MANTRA relapse prevention manual for a brief post-admission aftercare intervention with patient and therapist communicating via telemedicine. In an uncontrolled pilot study, this intervention showed promise. 297
In terms of the specific feasibility objectives, the study ascertained that this intervention is not for everyone, as only 19.5% of inpatients screened for the study were found eligible and agreed to participate (feasibility objective a). This raises the question as to how representative of the typical inpatient population in the UK participants was. A recent multicentre cohort study of short-term outcomes of hospital treatment of AN in the UK found a mean admission BMI of 14 kg/m2, a mean discharge BMI of 17.3 kg/m2 and a mean illness duration of 8 years for adults. 219 This suggests that our case mix was very similar to that of a typical adult inpatient with AN.
Feasibility objective b was to determine what is an appropriate frequency of e-mail support. Although only a small number of participating patients (n = 5) answered questions about the acceptability of the intervention offered, all five felt that the offered frequency of e-mail support (i.e. once or twice a week) was ‘about right’.
Feasibility objective c was to determine the best instruments for measuring outcomes in a full trial. The study suggests that both BMI and negative affect (depression, anxiety and stress) are candidate primary outcomes for future large-scale studies and the ESs obtained would inform sample size calculations for such a study (feasibility objective d).
Feasibility objective e was to get an idea of whether or not the treatment was operating as designed by analysing process measures such as worry and intolerance of uncertainty. Between-group ESs at 6 and 12 months were small and mostly not in the expected direction (i.e. did not suggest that reductions in worry or intolerance of uncertainty were specifically associated with receiving iMANTRA).
Finally, feasibility objective f was to determine whether or not patients with AN see iMANTRA as an acceptable and credible intervention. The limited feedback patients gave at 12 months seemed to indicate this. Given the need for regular physical health monitoring in cases of AN, it has previously been said that self-help treatments are contraindicated in this condition. 298 Our findings show that there were no severe untoward events, suggesting that, if delivered with appropriate physical risk monitoring in place, a specialist intervention given at a distance can be delivered safely as an adjunct to TAU.
One important question is how findings from the present study compare with those of the only other (large-scale) study of internet-based aftercare following inpatient treatment. 285 Like the patients in the present study, those in the Fichter et al. 285 study were highly selected [i.e. 1802 patients were screened and 258 of these (14.3%) entered the study]. Age at entry into our study was very similar to that in the study by Fichter et al. , as was patients’ BMI at discharge (i.e. study baseline). However, no information is available about whether or not Fichter et al. ’s patients were as chronically ill or had been as unwell (in terms of their weight at admission) prior to starting treatment.
Finally, one important lesson for a potential future study utilising this approach concerns the fact that patients did not know their e-mail therapist. Informal feedback from trial therapists was that this made it harder for patients and therapists to form a therapeutic relationship. In future studies it would be desirable for therapists to meet the patients to whom they are providing iMANTRA, and perhaps this could even be the therapist the patient had worked with in the inpatient unit.
Strengths
The study recruited patients from a range of specialist units in the UK including those in the NHS and private sector and the patients recruited seemed representative of a typical AN inpatient population.
Limitations
The sample size was small and TAU-only patients were harder to follow up than those allocated to iMANTRA. This may reflect TAU-only patients’ disappointment about not being offered the experimental treatment component. No attempt was made to standardise TAU. Furthermore, the use of a TAU alone comparison also means that observed effects may not be due to iMANTRA specifically, but rather to participants receiving additional support of any kind. Future studies should remedy this.
Conclusion
In conclusion, this study shows that a low-intensity e-mail-guided manual-based self-care intervention has promise in reducing deterioration and relapse in the year following inpatient treatment in patients with severe AN.
Chapter 9 Maternal eating disorders: effects on fertility and child development (work package 6)
Abstract
Objectives
Three studies examining the effects of maternal EDs on (1) fertility, (2) their offspring’s dietary patterns and (3) growth trajectories.
Design
Data from the Avon Longitudinal Study of Parents and Children (ALSPAC).
Participants
A total of 11,088 women from the ALSPAC birth cohort and their children.
Main outcome measures
(1) Maternal self-report on time taken to conceive and whether they had ever received help to conceive, (2) maternal report of child diet at 38, 54, 81 and 103 months, and (3) child weight and height at 1, 2, 5 and 10 years.
Results
(1) Women with AN were more likely than women without an ED to have sought fertility treatment and to have had an unintentional pregnancy; women with AN and BN were more likely than women without an ED to have received help to conceive and to have taken longer than 6 months to conceive. (2) Children of mothers with AN and BN were more likely to be ‘health conscious’ in terms of diet and less likely to have a ‘traditional’ type of diet. (3) There was a complex pattern of differences in trajectories of height, Ponderal Index (PI) and BMI in children of women with an ED compared with children of HCs.
Conclusions
Women with an ED experience difficulties in the pre-conception period and show postnatal differences in their diet patterns. Growth trajectories in their children were found. Continuity of care from pre-conception to the postnatal period is paramount for women with an ED.
Introduction
Psychiatric illness and parenting
Children of parents with a mental illness have an increased risk of developing psychological disturbances and psychiatric disorders themselves. 299,300 Within the study of EDs the topic of pregnancy and motherhood has been investigated only more recently. Nevertheless, there is evidence that an ED can affect parenting ability and have adverse outcomes on children’s development. 301 Furthermore, the offspring of mothers with an ED are at an increased risk of developing disordered eating themselves, and it has been proposed that a cycle of risk may be occurring, perpetuating EDs across generations. 302 Gaining a greater understanding of the associations between EDs, pregnancy and child development may extend our current understanding of the risk of intergenerational transmission of an ED.
Eating disorders and fertility
The presence of an ED can cause significant disruption to a woman’s menstrual cycle, and impaired fertility has been shown to exist across the spectrum of EDs. Women with a history of AN have shown to have greater disturbance in their menses than HCs. 303 Furthermore, these differences have been shown to continue in up to 30% of recovered women. 304 Women with a history of BN or EDNOS have also shown to frequently have greater disturbance in their menses,305,306 even though their body mass is usually within the normal range.
There is not enough research to understand the effect of EDs on fertility. Past research, based on two small studies of women looking for infertility treatment, found similar prevalences: 16–20% of women seeking infertility treatment met clinical criteria for an ED. 256,307 However, there have also been contradictory reports with regards to long-term follow-up studies. For example, Bulik et al. 308 found that the rates and frequencies of pregnancies were similar in women with AN and HCs; furthermore, Crow et al. 309 found the same results in women with BN. 310 On the other hand, when looking at births in women with AN, the number has been reduced to one-third of the expected rate.
A better and more precise measure of fertility may be time taken to conceive. 311,312 A majority of women will become pregnant after trying to conceive for between 3 and 6 months, with 90% of them succeeding after 12 months. 313 Therefore, and based on the data, infertility has been defined as inability to become pregnant after trying to conceive for a period of 12 months or longer,314 with delays in conception being a good measure of a range of underlying fertility problems.
Dietary patterns in children of mothers with eating disorders
There is evidence that mothers with an ED may find catering for the nutritional needs of their children especially challenging. However, only a small number of studies have examined the impact of a maternal ED on their child’s diet. The literature indicates a high risk for feeding difficulties in children of women with an ED. 315 These difficulties may lead to different nutritional intake in their children and have long-term implications for health and development.
Only one previous study has investigated the dietary intake in children whose mothers have an ED;316 although this study found the diet of the children to be generally unaffected, there was some suggestion that children of women with an ED consumed less junk food than children in the control group. However, this particular study was limited by the small sample size and the fact that the sample comprised children at different ages at a single time point. No previous studies have longitudinally investigated dietary patterns and nutritional intake in children of women with an ED.
Growth in the offspring of women with an eating disorder
Taking into consideration the possibility of an association between a maternal ED and their offspring’s feeding and eating habits, it is important to also consider the possibility of an association between a maternal ED and their offspring’s growth.
Maternal EDs, particularly AN, at the time of pregnancy are associated with higher risk of fetal growth restriction and low birthweight. 317,318 Conversely, it has recently been reported that women with binge ED during pregnancy are more likely to deliver babies that are large for gestational age. 319 Although preliminary studies have suggested that these children may reach an adequate postnatal growth in favourable environments,320 others indicate that altered growth patterns may continue throughout infancy and childhood. 321
The large majority of studies in the past have used small samples and included large age ranges, making it difficult to generalise findings. To the best of our knowledge, only one study has investigated growth in the offspring of mothers with an ED in a longitudinal manner, reaching the conclusion that children of women with an ED gained less weight at 1 year compared with HCs;322 however, their BMI was similar to those of controls when the children reached the age of 10 years. 323
Aims
The overall aim of the studies undertaken was to further investigate the effects of EDs on women’s fertility and the dietary patterns and growth trajectories of their offspring. Three separate studies are outlined to achieve the overall aims. The specific aims of each study are outlined below.
Study 1: time taken to conceive among women with eating disorders
The aim of study 1 was to investigate the length of time taken to conceive and the prevalence of fertility problems and unintentional pregnancies in women with EDs, compared with women without an ED.
Study 2: longitudinal dietary patterns in the offspring of mothers with eating disorders
The aim of this study was to explore different dietary patterns among children of women with EDs, compared with children of women without an ED, longitudinally between 3 and 9 years.
Study 3: growth trajectories in the offspring of mothers with eating disorders
The aim of this study was to study the differences in growth trajectories (between birth and 10 years) in children of women with an ED, compared with children of women without an ED and children of women with other psychiatric disorders, in a large prospective population-based cohort.
Methods
Design and participants
Data for this study were collected from the ALSPAC. 324 ALSPAC is a longitudinal birth cohort study, based in Avon, England, which includes all women who were willing to participate and who were due to have a child between 1 April 1991 and 31 December 1992.
A total of 14,663 women were initially enrolled in the study when they were 9 weeks pregnant. Using postal questionnaires, data were obtained from 14,472 women. In the current studies we only included singleton live births (n = 12,254) and excluded all women who were missing information on psychiatric history (n = 2019).
Outcomes and measures
Eating disorder classification
At 12 weeks’ gestation women were asked questions regarding recent or past psychiatric problems, which included depression, schizophrenia, alcohol abuse, AN, BN and other disorders. For all three studies women were grouped according to their response to this question.
A total of 171 (1.5%) women responded yes to the question ‘Have you ever had AN?’, 199 (1.8%) admitted to having ever suffered from BN and another 82 (0.7%) admitted to both AN and BN, with 10,636 (96%) forming the unexposed group (those who answered negatively to the questions on EDs).
In studies 1 and 2, women reporting a psychiatric disorder (n = 1166) other than an ED were excluded from the analysis. To investigate whether or not differences in childhood growth are specific to maternal ED status, this group is included in the analysis as a separate comparison group.
Sociodemographic variables
Highest education, household income, parity and ethnicity were collected via questionnaire from the mothers during pregnancy. Child gender and maternal age were recorded at the time of birth.
Study 1 measures: time taken to conceive
At 12 weeks women were asked ‘if they had ever seen a doctor for infertility problems and if they had received treatment or help to conceive the current pregnancy’. In a later stage, at 18 weeks’ gestation, they were asked how long it took them to conceive. This was a multiple-choice question with four possible answers: < 6 months, 6–11 months, 1–3 years and > 3 years. For the current study, we collapsed the four possible answers into two dichotomous variables: less/more than 12 months (to study rates of infertility) and less/more than 6 months (to study underlying difficulties conceiving). The analyses in the current study were restricted to women who had become pregnant intentionally (n = 7694).
Study 2 measures: dietary patterns
When the children were 38, 54, 81 and 103 months of age, data were collected from Food Frequency Questionnaires and included in the current study. For ease of reporting, these time points will be referred to by the child’s approximate age in years: 3, 4, 7 and 9, respectively. Each Food Frequency Questionnaire contained questions asking about the frequency of consumption of a variety of foods and drinks. The mother, or main carer, indicated how often the child was currently consuming a variety of food items on a scale from one to five. All data were standardised, and a principal component analysis, which is described by North and Emmett,325 and Northstone and Emmett,326,327 was performed on the standardised items.
The principal component analysis yielded three distinct dietary patterns: (1) ‘processed’, (2) ‘health conscious’ and (3) ‘traditional’ at each time point. An additional dietary pattern was identified at the age of 3 years and was labelled ‘snack foods’. For each child, a score was created for each dietary pattern, which is used in the analysis described below to investigate adherence (whereby increasing scores implies greater adherence) to each dietary pattern.
Study 3 measures: height and adiposity
Birthweight and length were obtained via different measures. From medical records, birthweight was obtained and birth length was obtained by ALSPAC staff shortly after birth. Later height and weight data were obtained through health visitor records, parental reports in questionnaires and research clinic attendances. Objective weight and height data were collected from age 7 years onwards. PI was calculated as weight (kg)/height (m3), and BMI was calculated as weight (kg)/height (m3).
Statistical analyses
Study 1: time taken to conceive among women with eating disorders
We used univariate and multivariate logistic regression models to investigate the role of predictors (maternal lifetime ED: AN, BN, AN + BN and no ED) on our main outcome variables. These were frequency of planned pregnancies, fertility-related problems, treatment and time taken to conceive.
Covariates hypothesised as confounders were included at a second stage in analytical models: maternal and paternal age, pre-pregnancy smoking, maternal education level and parity.
Study 2: longitudinal dietary patterns in the offspring of women with eating disorders
Univariate ANOVA and binary logistic regressions were used to assess differences in participant characteristics in groups of women who reported ever having AN, BN, and AN and BN, compared with the unexposed group.
Longitudinal analysis of dietary patterns was investigated using linear mixed-effects models, with random intercepts. Predictors were maternal lifetime ED group and the time point at which childhood dietary patterns were assessed (i.e. mean time of completion at assessment as a continuous variable). A random intercept was also included in the model to take into account the variance due to individual differences at baseline in dietary patterns. All nutrient data were log-transformed to achieve a normal distribution prior to analysis. As the dietary pattern ‘snacks’ was identified only in the 3-year-old children, a linear regression model was used to assess group differences in pattern score. Owing to missing data we used a data set derived from MIs for these analyses.
The models were run unadjusted initially and then adjusted for potential covariates that have been shown to potentially influence outcomes in the same sample: maternal age, education, ethnicity, household income, parity and child gender. 327 We were particularly interested in investigating the role of child gender and age on the main outcome variables, therefore group-by-time point and group-by-child gender interaction terms were tested in all models.
All analyses for studies 1 and 2 were performed using Stata (version 10) and all statistical tests presented are two-tailed.
Study 3: growth trajectories in the offspring of mothers with eating disorders
Statistical modelling
We modelled individual growth trajectories using multilevel models, including two levels: a time and an individual level in the statistical package MLwiN (version 2.02, MLwiN, Centre for Multilevel Modelling, Bristol, UK). We used fractional polynomials to identify the curves that best fit the data. These models allow use of all available data under the assumption of missing at random. They model the change in scale and variance of growth over time, and the individual variance in growth trajectories, allowing each individual to have a unique intercept and slope.
Given the complicated patterns of growth in childhood, we used PI (kg/m3) as the adiposity measure between birth and 2 years. BMI was modelled from 2 to 10 years of age and height from birth to 10 years.
Growth trajectories were modelled by exposure group (maternal lifetime AN, BN and AN + BN). They were estimated by fitting interaction terms between each exposure group and the constant (i.e. PI at birth, length at birth, or BMI at 2 years of age), and each of the polynomial terms in the multilevel models. This method generates specific average growth trajectories for children in each exposure group. z-tests were used to determine statistical differences in outcomes, comparing each group with the unexposed group (no maternal ED) at various time points.
Associations between maternal ED and offspring growth trajectories were investigated in unadjusted and adjusted multilevel models, which included a priori confounders (e.g. maternal education, maternal age, gestational age and parity).
Given known gender differences in childhood growth, and gender-specific effects of maternal lifetime ED, we modelled trajectories separately by gender.
Results
Study 1: time taken to conceive among women with eating disorders
Fertility problems
More women across all four groups had never seen a doctor about treatments for problems with conceiving (n = 9658; 88.1%), or had used any treatment of help to conceive for this pregnancy (n = 10,693; 97.3%). However, women with AN had a higher chance of having been seen for fertility problems during lifetime compared with the general population after we adjusted for pertinent covariates. Women with AN and BN were also two times more likely (6.2%) to receive treatment to help them conceive than unexposed women (2.7%) (Table 29).
| Outcome | Model | ||||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||||
| AN (n = 171) | BN (n = 199) | AN + BN (n = 82) | General population (n = 10,636) | AN (n = 171) | BN (n = 199) | AN + BN (n = 82) | |
| Seen a doctor for fertility problems | |||||||
| n (%) | 35 (20.6) | 22 (11.2) | 16 (19.5) | 1231 (11.7) | |||
| OR (95% CI) | 1.9** (1.3 to 2.8) | 0.9 (0.6 to 1.49) | 1.8* (1.0 to 3.1) | Ref | 1.6* (1.1 to 2.5) | 1.0 (0.6 to 1.6) | 1.9* (1.1 to 3.4), ref |
| Received help to conceive | |||||||
| n (%) | 4 (2.3) | 3 (1.5) | 5 (6.2) | 287 (2.7) | |||
| OR (95% CI) | 0.8 (0.3 to 2.3) | 0.5 (0.2 to 1.8) | 2.3a (0.9 to 5.8) | Ref | 0.8 (0.3 to 2.3) | 0.6 (0.2 to 1.9) | 2.4* (0.9 to 6.3), ref |
| Intentional pregnancy | |||||||
| n (%) | 96 (58.5) | 131 (67.1) | 53 (66.2) | 7414 (71.7) | |||
| OR (95% CI) | 0.5** (0.4 to 0.8) | 0.8 (0.6 to 1.0) | 0.7 (0.5 to 1.2) | Ref | 0.5** (0.4 to 0.7) | 0.8 (0.6 to 1.0) | 0.7 (0.4 to 1.1), ref |
Time taken to conceive
We examined time taken to conceive in our sample. A total of 7694 women had a planned pregnancy and of those, 74.5% reported having conceived in the first 6 months, while 8.3% took longer than 1 year and only 3.6% took over 3 years to conceive. Generally, women in all ED groups were no more likely to take longer than 12 months to conceive compared with women in the general population, and this remained the same for both univariate and adjusted analyses. Women with AN and BN were more likely to report having taken longer than 6 months to become pregnant compared with unexposed women and this difference remained after adjusting for covariates (Table 30).
| Time taken to conceive | OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||||
| AN (n = 89) | BN (n = 116) | AN + BN (n = 48) | General population (n = 6883) | AN | BN | AN + BN | |
| Longer than 12 months | |||||||
| n (%) | 14 (15.7) | 11 (9.4) | 8 (16.6) | 781 (11.3) | |||
| OR (95% CI) | 1.3 (0.8 to 2.4) | 0.9 (0.5 to 1.6) | 1.5 (0.7 to 3.1) | Ref | 1.0 (0.5 to 2.6) | 0.7 (0.4 to 1.4) | 1.5 (0.7 to 3.4) |
| Longer than 6 months | |||||||
| n (%) | 23 (25.8) | 21 (18.1) | 19 (39.5) | 1730 (25) | |||
| OR (95% CI) | 1.0 (0.6 to 1.6) | 0.6 (0.4 to 1.1) | 1.7* (0.9 to 3.1) | Ref | 0.9 (0.5 to 1.5) | 0.6 (0.4 to 1.1) | 1.9* (1.0 to 3.5) |
Intentional pregnancies
The odds of becoming intentionally pregnant were lower in women who had ever suffered from AN than in women who were unexposed (41.5% vs. 28.3%). These differences remained after adjusting for relevant covariates.
Study 2: longitudinal dietary patterns in children of women with lifetime eating disorders
Results from full models are reported in Easter et al. 329
‘Snack’ dietary pattern
Consumption of ‘snacks’ at 3 years among children of women in the three ED groups was similar to that among the unexposed children. After adjusting for confounding variables there were no differences between children in the maternal ED groups and the unexposed group [AN: adjusted coefficient = −0.461 (95% CI −0.227 to 0.135); BN: adjusted coefficient = −0.007 (95% CI−0.145 to 0.159); and AN + BN: adjusted coefficient = 0.903 (95% CI −0.139 to 0.320)].
‘Health-conscious’ dietary pattern
Children of mothers in all three ED groups had higher scores on the ‘health-conscious’ dietary pattern across the four time points than children in the unexposed group. After adjusting for covariates, these differences persisted in the maternal AN and maternal BN groups (AN: adjusted coefficient = 0.355, 95% CI 0.162 to 0.547; BN: adjusted coefficient = 0.209, 95% CI 0.044 to 0.373; and AN + BN: adjusted coefficient = 0.131, 95% CI –0.131 to 0.395).
Boys of women in the AN group were less likely to adhere to this pattern than girls; conversely boys of mothers reporting both AN and BN were more likely than girls to have higher scores on the health-conscious pattern. (Full models can be found in Easter et al. 329)
‘Traditional’ dietary pattern
Children in all exposed groups scored lower on the ‘traditional’ dietary pattern across the four time points than children in the unexposed group. After adjusting for covariates, these differences persisted in the maternal AN and maternal BN groups, and a trend remained in the maternal AN and BN groups but not in the unexposed group (AN: adjusted coefficient = –0.241, 95% CI –0.463 to –0.018; BN: adjusted coefficient = –0.372, 95% CI –0.560 to –0.184; and AN + BN: adjusted coefficient = –0.279, 95% CI –0.592 to 0.032).
‘Processed’ dietary pattern
Scores on the ‘processed’ dietary pattern were similar in the children of maternal ED groups and children in the unexposed group, in both adjusted and unadjusted models. Full models can be found in Easter et al. 329
Study 3: growth trajectories in children of women with lifetime eating disorders
Boys
The sons of women with AN and BN had greater predicted heights than the sons of women with no ED between birth and 5 years of age, and between birth and 10 years of age (Table 31). These differences remained after adjusting for potential confounders. For sons of women with BN, the group difference in predicted heights became larger with age, reaching 1.88 cm by age 10 years (see Table 31). On the other hand, the difference for sons of women with AN was in the opposite direction, as they were, by age 10 years, 0.75 cm shorter than the sons of unexposed women.
| Anthropometry | Mean predicted anthropometry (SD) of offspring of unexposed womena | Mean difference (SD) from unexposed for offspring of women with: | |||
|---|---|---|---|---|---|
| AN | BN | AN + BN | Other psychiatric disorders | ||
| Height (cm) | n = 4588 | n = 74 | n = 85 | n = 38 | n = 501 |
| Birth | 50.3 (5.4) | +0.14 (2.0) | +0.17 (2.08) | –0.10 (2.0) | –0.20 (2.2)* |
| 1 year | 76.1 (5.4) | +0.32 (2.4) | +0.05 (2.4) | –0.09 (2.4) | –0.28 (2.5)** |
| 2 years | 87.3 (5.4) | +0.40 (3.0) | +0.01 (3.1) | –0.10 (3.0) | –0.32 (3.2)* |
| 5 years | 110.2 (6.8) | +0.39 (4.5) | +0.15 (4.5) | –0.17 (4.4) | –0.42 (4.7)* |
| 10 years | 140.7 (8.8) | –0.75 (7.3) | +1.88 (7.5)** | –0.81 (7.1) | –0.64 (7.9)b |
| PI (kg/m3) | n = 4537 | n = 73 | n = 84 | n = 38 | n = 496 |
| Birth | 26.1 (5.4) | –0.43 (2.5) | –0.28 (2.6) | +0.07 (2.5) | +0.13 (2.7) |
| 1 year | 23.3 (4.7) | –0.12 (2.1) | +0.41 (2.2)b | +0.27 (2.1) | +0.22 (2.3)* |
| BMI (kg/m2) | n = 4271 | n = 68 | n = 78 | n = 35 | n = 452 |
| 2 years | 16.8 (3.9) | +0.10 (2.0) | +0.26 (1.9) | +0.32 (1.7) | +0.15 (2.0)b |
| 5 years | 15.9 (3.9) | +0.34 (1.4)* | +0.12 (1.3) | +0.49 (1.4)* | –0.02 (1.5) |
| 10 years | 17.7 (4.6) | +0.09 (3.3) | +0.12 (3.2) | +0.08 (3.2) | +0.09 (3.6) |
The children of women who reported having AN and BN, as well as the children of mothers with other psychiatric disorders, were shorter from birth until aged 10 years. Specifically, we found that the biggest difference in height compared with offspring of unexposed women was in children of mothers with other psychiatric disorders.
Girls
The daughters of women with AN (between birth and 5 years of age) and of women with both AN and BN (between birth and 10 years of age) had lower predicted heights than girls in the unexposed group (Table 32). After adjusting for covariates, daughters of women with AN were expected to be, on average, 0.47 cm shorter than the daughters of women in the unexposed group.
| Anthropometry | Mean predicted anthropometry (SD) of offspring of unexposed womena | Mean difference (SD) from unexposed for offspring of women with | |||
|---|---|---|---|---|---|
| AN | BN | AN + BN | Other psychiatric disorders | ||
| Height (cm) | n = 4416 | n = 65 | n = 82 | n = 30 | n = 432 |
| Birth | 49.7 (5.3) | –0.47 (1.9)b | –0.08 (2.1) | + 0.23 (2.0) | –0.16 (2.2)b |
| 1 year | 74.3 (5.3) | –0.27 (2.4) | +0.05 (2.4) | –0.73 (2.4)b | –0.18 (2.6)b |
| 2 years | 85.6 (5.3) | –0.24 (3.0)* | +0.02 (3.0) | –0.93 (3.0)b | –0.18 (3.2) |
| 5 years | 109.6 (6.0) | –0.31 (4.3) | –0.18 (4.3) | –0.94 (4.2) | –0.17 (4.8) |
| 10 years | 138.9 (8.6) | –0.57 (7.0) | –0.65 (6.9) | –0.32 (6.7) | –0.12 (7.5) |
| PI (kg/m3) | n = 4363 | n = 64 | n = 81 | n = 30 | n = 424 |
| Birth | 26.2 (5.3) | –0.07 (1.9) | +0.48 (2.2)* | –0.16 (2.2) | –0.09 (2.3) |
| 1 year | 23.2 (5.3) | +0.39 (2.4)b | +0.06 (2.5) | +0.39 (2.7) | –0.22 (2.7)b |
| BMI (kg/m2) | n = 4117 | n = 61 | n = 78 | n = 29 | n = 398 |
| 2 years | 16.6 (3.9) | –0.35 (1.9)b | +0.30 (1.9) | +0.25 (1.8) | –0.07 (2.1) |
| 5 years | 16.0 (3.9) | –0.01 (1.5) | +0.26 (1.5)b | +0.32 (1.5) | +0.16 (2.1)* |
| 10 years | 18.1 (5.1) | +0.03 (3.5) | –0.29 (3.4) | –0.52 (3.3) | +0.56 (3.7)** |
On the other hand, early in childhood, the daughters of women with BN were expected to be of similar height to the daugters of mothers in the unexposed group. At the age of 5 years, the daughters of women with BN were slightly shorter, and from 5 to 10 years of age the differences in predicted height were larger, with the daughters of women with BN predicted to be 0.64 cm shorter than the daughters of women in the unexposed group (see Table 32).
Children of women with other psychiatric disorders had a tendency to be shorter during childhood; however, when compared with the daughters of women with AN, or those with AN and BN, these differences were comparably smaller for this group (see Table 32).
Boys
Figures 5 and 6 show group differences in the trajectories of PI and BMI in boys. There was no statistical evidence of growth differences for all periods; in a large number of cases we found large SDs, which could be attributable to the fact that maternal ED groups were small.
FIGURE 5.
Average fractional polynomial curve of PI trajectories for girls and boys by maternal ED, birth to 2 years, adjusted for confounders. Note: values are predicted from the multilevel models, and represent the predicted anthropometry for offspring of mean gestational age (39.4 weeks) and with a mother with the following characteristics: mean age 28.2 years, less than Ordinary level education, parity of zero. (a) Boys’ PI and (b) girls’ PI. This figure is reproduced from Easter et al. 330 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/3.0/.
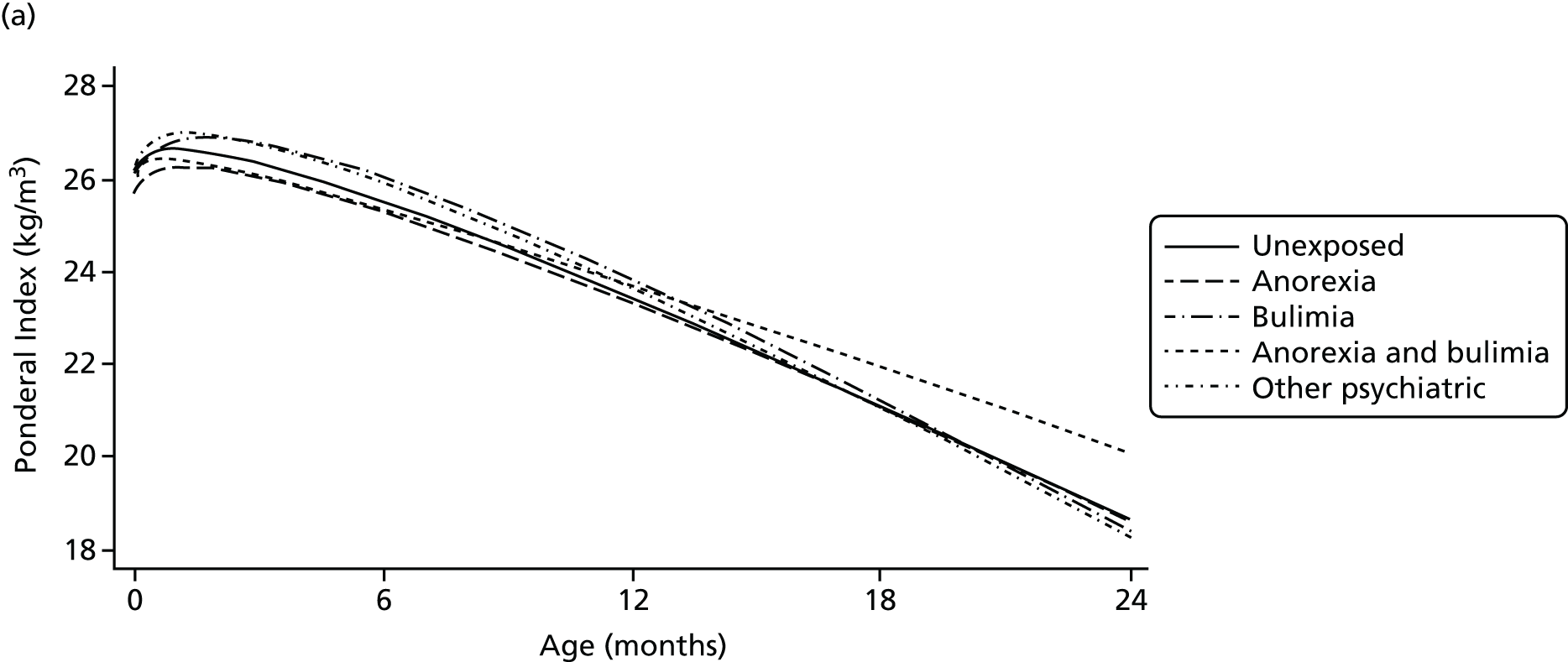
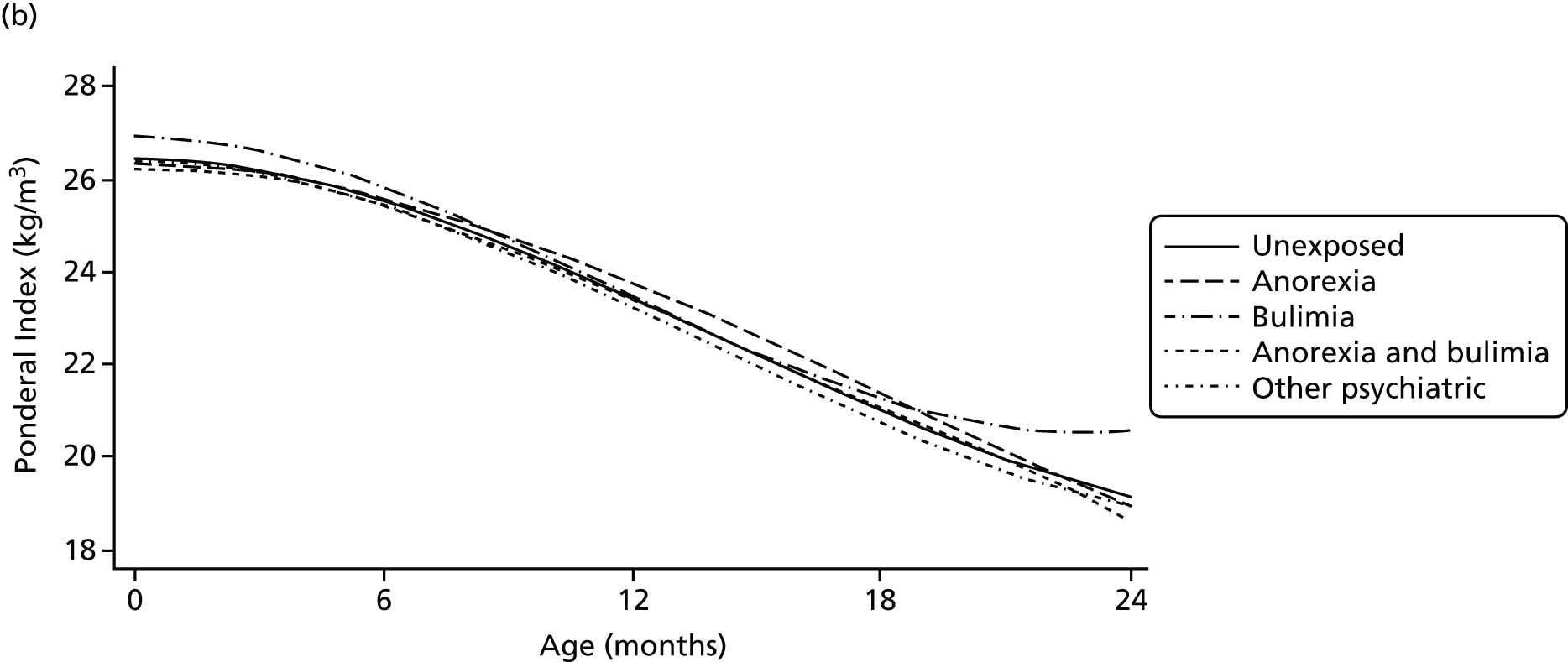
FIGURE 6.
Average fractional polynomial curve of BMI trajectories for girls and boys by maternal ED, aged 2–10 years, adjusted for confounders. Note: values are predicted from the multilevel models, and represent the predicted anthropometry for offspring of mean gestational age (39.4 weeks) and with a mother with the following characteristics: mean age 28.2 years, less than Ordinary level education, parity of zero. (a) Boys’ BMI and (b) girls’ BMI. This figure is reproduced from Easter et al. 330 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/3.0/.
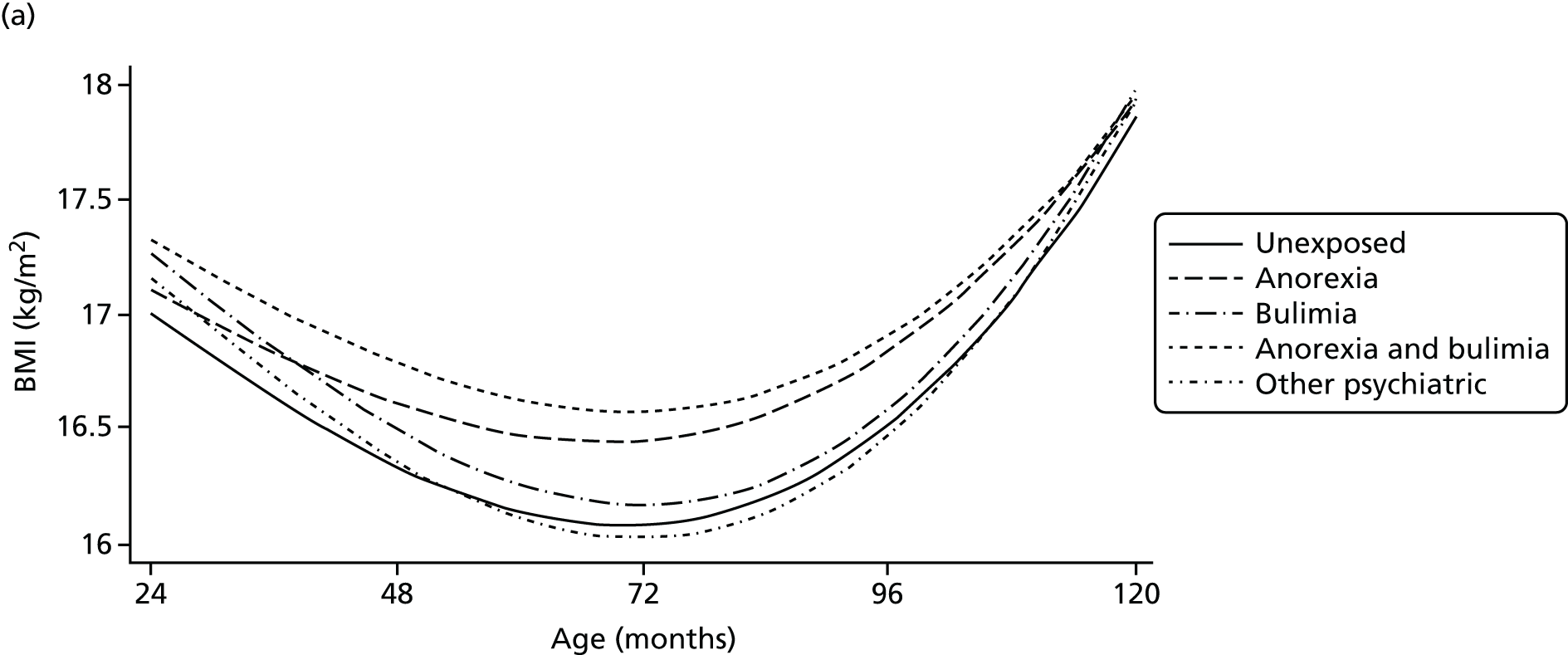
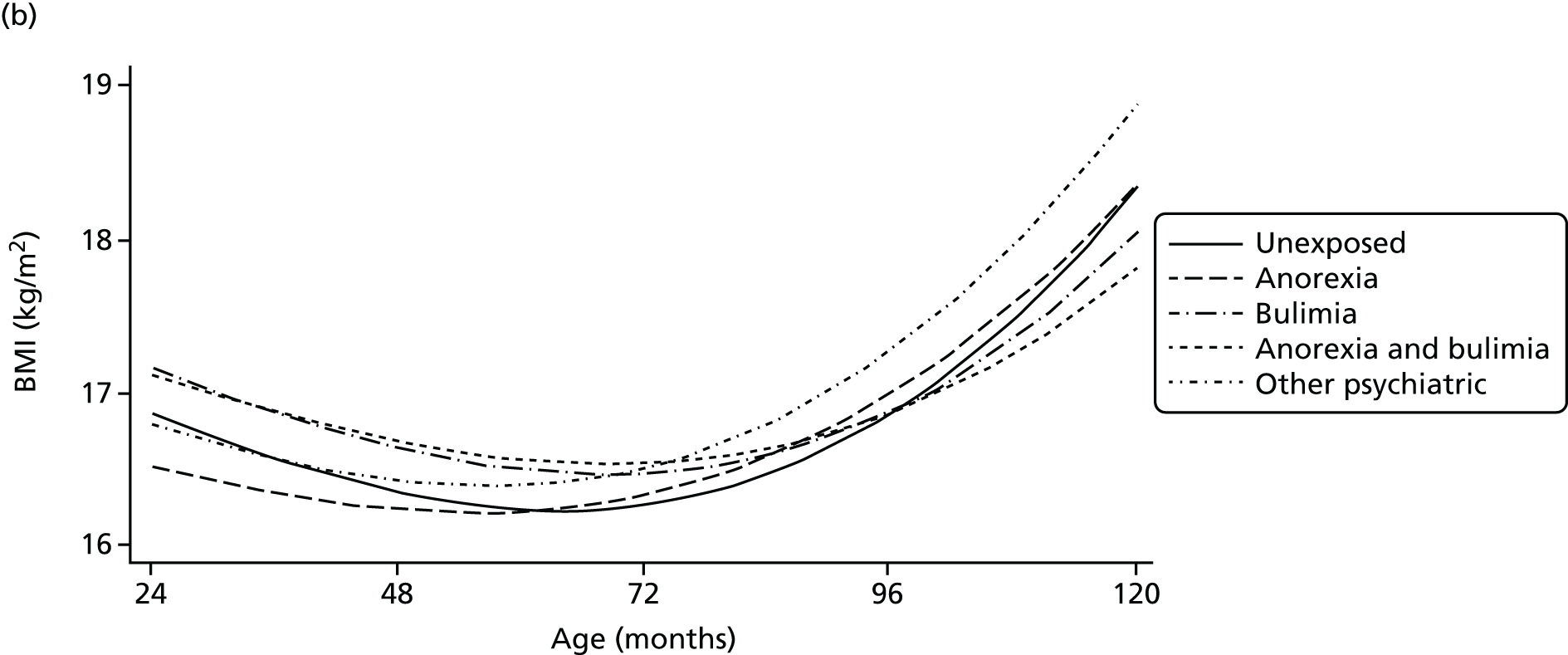
After adjusting for covariates, the sons of women with AN had lower PI than the sons of unexposed women. The children of women across all exposed groups had a higher BMI between the ages of 2 and 10 years than the children of unexposed women. At 5 years of age, the predicted BMI of children of women in the maternal AN group was 0.34 kg/m2 higher than the BMI of children of unexposed mothers. The BMI of sons of women with AN, and women with AN and BN, at 10 years of age was similar to the BMI of the sons of unexposed women (see Table 31). A tendency towards a higher PI at 2 years was found in the sons of mothers with other psychiatric disorders; however, their BMI trajectories a few years later were similar to those of the sons of mothers in the unexposed group.
Girls
The daughters of women with BN had a higher PI at birth than the daughters of unexposed women; however, this difference was reduced by the time they turned 12 months of age.
The daughters of women with BN, and of women with AN and BN, continued to have higher BMI than the daughters of unexposed mothers until the age of 5 (see Figure 6); however, their BMI was comparable to the BMI of daughters of unexposed women by the time they turned 10 years old. On the other hand, the daughters of women with AN had a lower BMI than the daughters of unexposed women during early childhood; however, their BMI was comparable to that of the daughters of unexposed women years later (late childhood) (see Figure 6). The BMI changes through childhood of the daughters of women who reported other psychiatric disorders were comparable to those observed in children of women in the AN group (see Figure 6); conversely, at the age of 10 years, their expected BMI was on average 0.56 points higher than that of the daughters of unexposed mothers.
Discussion
The overall aim of the studies was to investigate the effects of maternal ED on fertility and on their children’s dietary patterns and growth trajectories.
Eating disorders and fertility
Study 1 highlighted that women with an ED experience more difficulties conceiving. The results showed that women with a lifetime diagnosis of AN (either AN or AN + BN) were more likely than unexposed women to visit a doctor because they have problems conceiving. Although only a small percentage of women in this sample actually received treatment during the time trying to conceive, and we found that women with AN and BN were twice as likely to undergo treatment for problems with conception. Based on these results, it could be hypothesised that problems with fertility in this sample may be secondary to low BMI, which is present in both groups.
Our results also showed that the odds of taking longer than 6 months to conceive were higher for women who reported both AN and BN than for unexposed women. Importantly, women with both AN and BN had lower BMI before conception and higher prevalence of lifetime purging than women in the other two groups. 318 The severity in this group may lead to a higher risk for both fertility problems and delayed conception.
Approximately 40% of women in the sample who reported having AN reported that their pregnancy was unintentional. Research has shown that ovulation and pregnancy can still occur in women with an ED who have irregular cycles. 331 Therefore, the large percentage of unintentional pregnancies found in this sample may be due to erroneous beliefs about their ability to become pregnant.
Diet and growth in children of women with eating disorders
Studies 2 and 3 explored potential differences in diet and growth between the ages of 3 and 9 years, and growth trajectories between birth and 10 years, in children of women with an ED.
The children of mothers with an ED were found to be less likely to adhere to a ‘traditional’ dietary pattern than children of women without an ED. This pattern is synonymous with a British ‘meat and two veg diet’, which would typically be eaten at mealtimes. Family mealtimes are particularly difficult for mothers who have experienced an ED,332 and such women have been found to be less likely to regularly cook or eat with their children. 333 Reduced adherence to the ‘traditional’ dietary pattern in this study may reflect less frequent mealtimes in families in which the mother has an ED.
In line with suggestions from previous research that children of women with an ED may consume less junk food,316 the children of mothers reporting either AN or BN were found to consume a more ‘health-conscious’ diet. We have previously found that women with AN in this sample were more likely to follow this dietary pattern in pregnancy themselves;334 these findings might imply a stronger maternal desire for women with an ED to provide a healthy diet for their children.
It is currently not known what effects increased exposure to a health conscious/vegetarian diet during childhood may have on the long-term development and health of the children of women with an ED; previous evidence from this sample suggests associations with positive developmental outcomes. 335,336
Changes across childhood
Over time, children of women with an ED were adhering to a more ‘traditional’ and less ‘health-conscious’ dietary pattern. These findings may reflect the influence of other societal effects on diet, such as school, and consequentially children in the exposed groups having greater influence over their diet. Alternatively, these findings may reflect differences in maternal feeding styles in early childhood. It has previously been reported that mothers with an ED display more restrictive feeding styles;301,337 however, studies suggest that parental restriction of high-fat and palatable foods may in fact increase their desirability. 338–340
Gender differences
Some previous studies have indicated that maternal transmission of weight concerns and dieting behaviours may be stronger for girls than boys. 341,342 In the present investigation, girls of mothers with AN were more likely than males to adhere to the ‘health-conscious’ pattern, suggesting a stronger maternal influence on girls of women with AN.
Past investigations have proposed that the offspring of women with BN may be at risk of becoming obese;315,343 findings from the present study showed that boys of women from all exposed groups had higher adiposity than boys of unexposed women, with the difference being more pronounced at the age of 5 years. Taking into consideration that boys of mothers with other psychiatric problems have similar BMI trajectories to those of unexposed mothers, the differences we found in growth trajectories in the offspring of mothers with EDs may be specific to maternal eating pathology. One possible hypothesis is that a fast increase in growth during earlier years may put them at higher risk for obesity, as well as other health problems, later in life.
Body mass index trajectories in girls of women with an ED were different from those of boys and show a fluctuating pattern throughout childhood. Specifically, girls of women with BN, and with AN and BN, had a tendency for a higher BMI in early years but a lower BMI at 10 years old. On the other hand, girls of women with AN had a higher BMI in earlier years and a more similar BMI to HCs by the age of 10 years. We are not clear on the consequences of the growth trajectories in the long term. However, a recent study found that growth patterns were predictive of later AN. 344 A possibility is that differences in growth trajectories in children of mothers with AN highlighted by this study are in some way related to dietary patterns during childhood; however, we were not able to investigate this association.
Compared with boys of unexposed women, boys of women with BN from the age of 2 years onwards were found to be taller. On the other hand, compared with boys of unexposed women, boys of women with AN were taller only during early childhood, and were found to be shorter by the time they reached 10 years old. Girls of women with ED, predominantly AN, had a tendency to be shorter than unexposed children. These findings may show that this group is at specific risk of having a more restricted growth during childhood. Furthermore, these results are similar to previous case studies and those with younger children that show that children of women with AN do not grow as much as those of unexposed women. 321,343,345
Past research has shown that mothers with an ED are more concerned with their daughters’ weight and shape than with their sons’,346 and that their daughters may have a higher chance of becoming underweight than thier sons. 343 Results from the current studies support gender-specific growth trajectories; however, as our growth models were built separately for each gender, we were unable to examine gender interactions.
Conclusions
In conclusion, the investigations described in this chapter highlight novel findings regarding the effects of ED on women’s fertility and their children’s development. Women with an ED were found to experience difficulties in the pre-conception period and postnatal differences in the dietary patterns and growth trajectories of children born to women with an ED were highlighted.
Our study is the first to investigate longitudinal growth and diet patterns in the children of women with lifetime EDs, and, therefore, replication and additional examination of the pathways are necessary. Nonetheless, health professionals need to be conscious of the associations between EDs in women and their children’s growth so that they can better guide both the mother’s health and the development of the fetus. Continuity of care for this group of women is paramount from before conception into the postnatal period. Furthermore, and at a public health level, it is of extreme importance to further analyse underlying mechanisms of growth differences in children of women with an ED. Following on from this study, we are now developing an intervention for pregnant women with an ED, aimed at preventing adverse child outcomes.
Chapter 10 Specialist and non-specialist care pathways for adolescents with anorexia nervosa (work package 7a)
An abbreviated version of this chapter has been published in the International Journal of Eating Disorders. 347
Abstract
Background
There is now a significant body of research demonstrating the efficacy of specific treatments for adolescent AN. Little is known about the way in which different service contexts impact treatment outcome. The present study investigates the role of specialist outpatient services and how the availability of such services influences the rates of referrals, hospital admissions, continuity of care and service user experiences.
Methods
Mental health services in London were asked to identify adolescents who presented for treatment for an ED over a 2-year period. Retrospective data about service use, including the need for hospital admissions, were collected from clinical case notes. A small sample of adolescents and parents were interviewed about their experiences of services.
Results
Direct access to specialist outpatient services was associated with higher referral rates, lower admission rates and greater consistency of care. Service users identified a number of advantages of specialist service provision.
Conclusions
Data from the present study suggest that facilitating direct access to specialist services for adolescents with AN may result in better outcomes, lower costs and higher satisfaction among service users.
Introduction
The rapid physical and psychological deterioration that can occur in young people with AN means that early intervention with the potential for intensification of care is important. Clinical trials suggest that outpatient family-based treatments are effective in the short and longer term. 282,348–350 However, treatment in inpatient settings is relatively common. Data from the UK suggest admission rates for children and adolescents of 35–50%30,351 and, in one survey, young people with EDs occupied more child and adolescent psychiatric beds than any other diagnostic group. 31 Studies of inpatient care have found no evidence of specific benefits arising out of hospital treatment in comparison with outpatient care,352 and there is some evidence that prolonged admissions may result in poorer outcome,353 as well as being more costly. 354
There is considerable variability in the treatment provisions for children and adolescents suffering from AN in the UK, ranging from outpatient treatment provided within general CAMHS to highly specialised ED services. The only RCT to compare general and specialist outpatient services, the Treatment Outcome for Child and adolescent Anorexia Nervosa trial, found no difference in clinical outcomes between services. 355 Prior to the present study, outcomes following TAU in general and specialist outpatient services had not been compared, so to date it has been difficult to separate the effects of specific treatments from the effect of service specialisation.
The present study provides a naturalistic comparison of alternative service provisions for adolescent AN in the UK, using Greater London, with a population of approximately 7.8 million, as an exemplar. We hypothesised that we would find two main care pathways, shaped by the availability of different outpatient services in the form of general CAMHS or specialist child and adolescent ED services. We aimed to explore associations between these different care pathways and (1) the number of cases seen in services beyond primary care and (2) rates of admission for inpatient treatment (excluding admission for acute medical stabilisation only). We also aimed to explore the experiences of adolescent service users and their parents.
Methods
Study 1: quantitative study
The design of the study was a naturalistic, retrospective cohort study, using clinical case notes as the primary data source. Our main hypotheses were (1) accounting for population size, more adolescents with AN and restricting EDNOS from the specialist care pathway than from the general care pathway would present to services beyond primary care and (2) controlling for severity of presentation, a lower proportion of adolescents with AN and EDNOS from the specialist care pathway than from the general care pathway would be admitted for inpatient treatment during the first year of treatment.
Service mapping, recruitment and categorisation
An initial phase was to identify existing services for adolescents with EDs in London. This included searches of the websites of public and private health-care providers, the UK government-funded children’s service mapping exercise (2007–8) and publications by the Royal College of Psychiatrists. 26,356 The identified services were asked to confirm arrangements for managing EDs in their area and return a questionnaire about the treatments their service provided. This information was used to compile a list of eligible services that were then asked to participate in the study.
Outpatient services were categorised either as general CAMHS or as specialist ED services, drawing on existing criteria26,357 to define specialist outpatient ED services: a minimum of 25 new ED referrals per year; a multidisciplinary team, including medical and non-medical staff and more than one person with experience of treating EDs; a team with the expertise to deliver evidence-based treatments; and the resources to offer routine outpatient treatment. Inpatient services were categorised into specialist ED units and general psychiatric units.
Case identification
Each service was asked to identify individuals that met the following criteria:
-
having initial face-to-face contact with the service, or recontact after a treatment break of at least 6 months, between 1 December 2006 and 30 November 2008
-
aged 13–17 years at the time of initial contact (or recontact)
-
a primary diagnosis of an ED
-
being registered with a GP from a London primary care trust (PCT; the commissioning bodies at the time, which determined service accessibility).
In each service, potential participants were identified through searches of electronic patient records, correspondence with service users, clinical diaries, referral lists, funding records or audit data.
Participant recruitment and participant-level data collection
Potential participants were contacted by post, asking for permission for us to access their clinical case notes. A standard item sheet was used to record data for each participant, including their PCT; demographic data, such as age and sex; details of symptoms; and use of treatment and services in the 12 months after entry into the service. Limited, anonymised, data (as agreed with ethics) were collected from services about non-consenters to avoid inclusion of duplicates and enable comparisons between consenters and non-consenters.
Data analysis
To evaluate the number of cases seen beyond primary care (hypothesis 1), pathways were defined reflecting the agreements between PCTs and services defining the type of outpatient service that was typically made available following referral from primary care. The care pathway for each adolescent was therefore defined by the typical funding arrangements of the PCT in the relevant catchment area. For this analysis, data for both consenters and non-consenters were included.
‘Presentation rates’ were calculated for each PCT, using the number of cases identified by services and population statistics from the Office for National Statistics. 358 Separate calculations for females and males were done for each PCT using the following formula:
Mean presentation rates were compared between PCT groups by ANOVA using diagnostic plots to check that required assumptions held. In particular, when PCT groups were compared, we carried out two planned comparisons of CAMHS PCT groups against the specialist service PCT group.
To evaluate differences in admission rates (hypothesis 2), only data from consenting participants could be used. To assess the representativeness of the sample, comparisons of personal, symptomatic and service variables were made between ‘consenters’ and ‘non-consenters’. Independent-samples t-tests were used to compare groups on continuous variables. Chi-squared tests or Fisher’s exact tests were used to compare groups on categorical variables. Care pathway groups were established in two ways: by PCT, as for hypothesis 1, and by the actual care pathway that each participant followed. Binary logistic regression was used to compare rates of admission for inpatient treatment between the groups controlling for the severity of presentation (measured by degree of underweight).
Power calculation
A power calculation was performed, based on the expectation of two main care pathways (starting with either a specialist outpatient ED service or a general CAMHS), and estimated admission rates of 10% (from audit data) and 35%, respectively. A chi-squared test at a significance level of 5% to detect a difference with a power of 80% indicated that 43 participants per group would be needed to show a statistical difference.
Ethics approval
The study was reviewed by the Royal Free Hospital and Medical School REC (reference 07/H0720/119). We were concerned that obtaining consent from participants would affect the extent to which general CAMHS were represented. We anticipated that they would have fewer eligible patients and fewer resources to dedicate to recruitment than specialist services, which could result in both a small total number of participants and a lower proportion of those who were suitable being recruited from general services rather than from specialist services. The Patient Information Advisory Group was approached for permission to access case notes without obtaining consent, but the application was not approved. Subsequently, the National Information Governance Board, which replaced the Patient Information Advisory Group, gave permission to store non-consenters’ initials and dates of birth, enabling removal of duplicates from the non-consenting sample [reference ECC 6-06(I)/209].
Study 2: qualitative study
For the qualitative part of the study, a subsample of adolescents was selected from those who allowed us to access their case notes for the quantitative study. The main aim of sampling was to select people with experience of the different care pathways. Fifty-six adolescents were asked whether or not they would be willing to take part in an interview about their experiences of treatment and services, and were also asked for permission to contact their parents about taking part in a similar interview. Interviews were based on an interview guide, rather than a fixed interview schedule. The focus was on the participant’s journey through services and experience of each stage of treatment. Questions were non-directive and open ended.
Data were analysed by two researchers using thematic analysis. The procedure was based on six phases of analysis, described by Braun and Clarke. 359 In phase 1, interviews were transcribed verbatim, and each researcher read through the transcripts multiple times to become familiar with the content, while making notes about anything they found interesting at a descriptive or interpretative level. Phase 2 involved labelling data with ‘codes’, which highlighted points of interest, and identifying areas of agreement and disagreement between participants. The researchers discussed their coding and, if they had made different interpretations of the data, revisited the transcripts together. Phase 3 involved tentative grouping of codes into themes, which were reviewed and reorganised when necessary in phase 4, and finalised and defined in phase 5. The data were organised to reflect patterns, similarities and differences in participants’ experiences. Themes were then grouped into superordinate themes. Phase 6 was the production of a written report of the findings, including sections of the transcripts (quotes) that led to the development of themes.
Results
Study 1: quantitative study
Service mapping, recruitment and categorisation
Forty-four eligible services were identified, of which 37 provided the required data. Table 33 shows the services by category. Service mapping and recruitment revealed a potentially substantive difference between general CAMHS; some defined themselves as non-specialist in EDs, whereas others described having a specialist ED ‘mini-team’ within the general team. CAMHS were therefore categorised into self-defined ‘specialist ED CAMHS’ and ‘non-specialist CAMHS’. To take this into account the original plan of statistical analysis was modified to include pairwise comparisons between care pathways based on specialist ED services and care pathways based on both types of CAMHS.
| Service | Service | |
|---|---|---|
| Participating | Non-participating | |
| NHS outpatient services | ||
| Specialist child and adolescent ED services | 2 | 0 |
| Other ED services | 3 (1 child, 2 adultsa) | 0 |
| Specialist CAMHS | 7 | 2 |
| Non-specialist CAMHS | 15 | 2 |
| NHS inpatient services | ||
| Specialist ED units | 4 (1 child/adolescent, 3 adultsa) | 0 |
| General psychiatric units | 2 | 1 |
| Private services | ||
| Specialist ED units | 6 (4 children/adolescents, 2 adults) | 2 (1 child/adolescent, 1 adult) |
Presentation rates to services beyond primary care
Primary care trust groups
Three PCT groups were identified, based on the care pathway they typically commissioned to provide outpatient treatment following referral from primary care: (1) a specialist Child and Adolescent Eating Disorders Service (CAEDS), (2) a specialist CAMHS and (3) a non-specialist CAMHS. In the CAEDS group, some PCTs allowed referrals to bypass CAMHS completely, some required CAMHS to authorise referrals, and others required CAMHS to assess adolescents before referring them for treatment in an ED service. In the specialist and non-specialist CAMHS groups, assessment and treatment were provided by the same service. Specialist CAMHS differed from CAEDS in that they each served a single PCT, whereas CAEDS each served a number of PCTs. All PCTs had access to general paediatric medical units. Depending on the area, longer-term inpatient treatment was provided by public or private ED units or general adolescent psychiatric units.
Cases by primary care trust group
Services identified 641 potentially suitable cases. Using initials, dates of birth and PCTs, duplicates were identified and removed, leaving 489 cases. After checking eligibility for the study, 111 cases were excluded. The final number of cases meeting the inclusion criteria was 378. Services from four PCTs did not provide any data and were excluded from the analyses. Therefore, a total of 27 PCTs and 367 cases were included in the subsequent analyses. The grouping of these is shown in Table 34.
| PCT group | AN and EDNOS-AN | BN and EDNOS-BN | EDa | Total | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||
| CAEDS (12 PCTs) | 112 | 8 | 62 | 1 | 25 | 1 | 209 |
| Specialist CAMHS (5 PCTs) | 50 | 3 | 15 | 0 | 5 | 0 | 73 |
| Non-specialist CAMHS (10 PCTs) | 38 | 3 | 10 | 3 | 28 | 3 | 85 |
| Total | 200 | 14 | 87 | 4 | 58 | 4 | 367 |
The total number of males in the overall sample was small (n = 22), making it inappropriate to include them in the analyses. Data from the services could be used to provide a clear diagnosis for 287 females. Of these, 200 had AN or EDNOS-AN and 87 had BN or an eating disorder not otherwise specified – bulimia nervosa subtype (EDNOS-BN). In some of the sample (58 females), however, the services gave a diagnosis only of ‘eating disorder’ and there was insufficient data for a researcher to place these individuals into one of the specific diagnostic groups. Presentation rates were therefore calculated in two ways: first, using cases with a known diagnosis, to give an ‘observed presentation rate’, and, second, based on the assumption that the same proportion of missing and known cases had either AN or EDNOS-AN (69.7%) and BN or EDNOS-BN (30.3%) to give an ‘estimated presentation rate’. As the focus of this study is restricting EDs, results are presented for this subgroup only.
Figure 7 shows the presentation rates of AN and EDNOS-AN by PCT group. Unadjusted pairwise comparisons show that the presentation rates for the non-specialist CAMHS group were significantly lower than those of the specialist ED service group (observed rate: p < 0.01; estimated rate: p = 0.04). The presentation rates of the specialist CAMHS group did not differ significantly from those of the CAEDS group (observed rate: p = 0.41; estimated rate: p = 0.98).
FIGURE 7.
Observed and estimated presentation rates of AN and EDNOS-AN by PCT group.
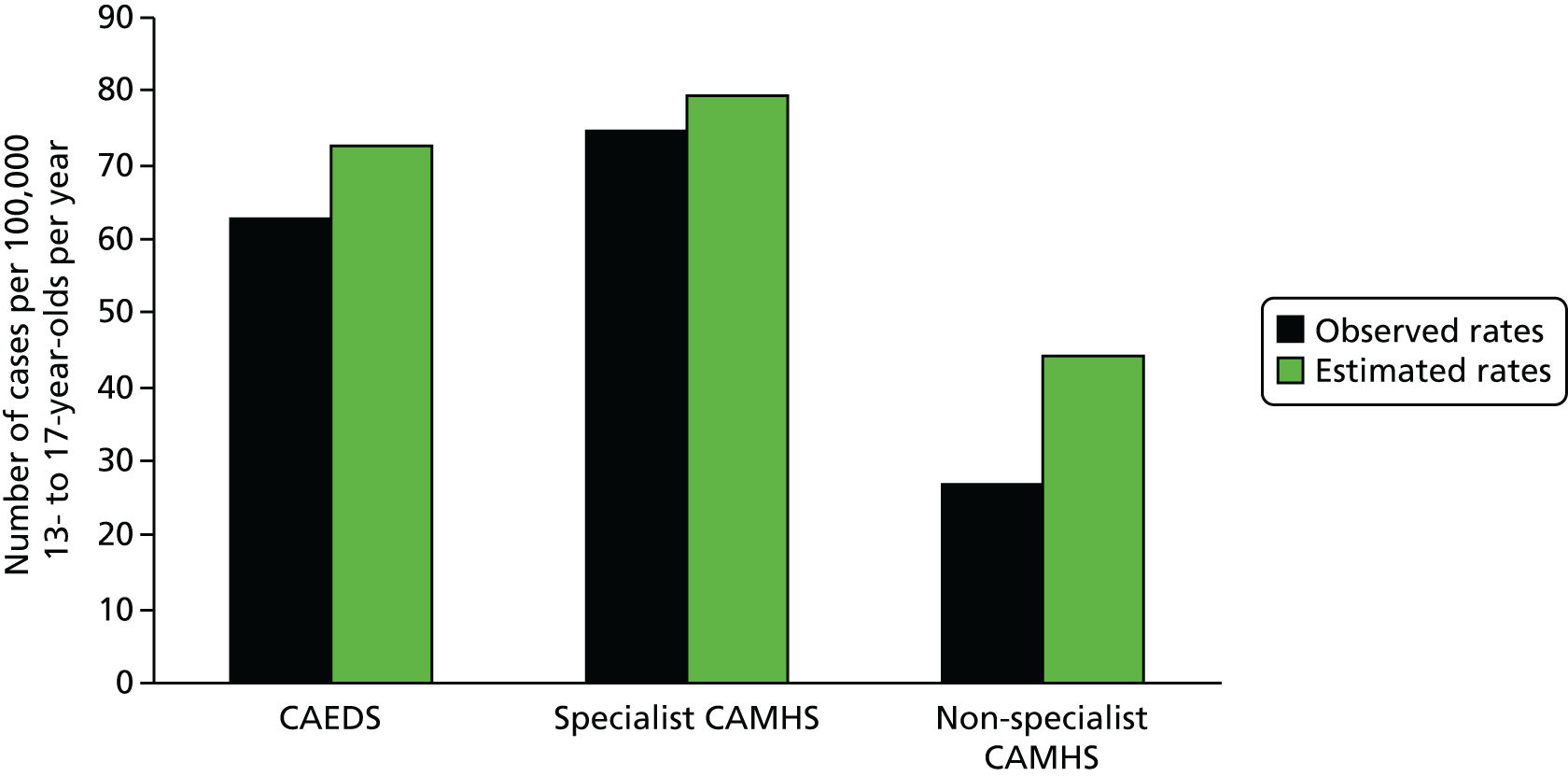
Comparison of participant characteristics of consenters and non-consenters
Of the 378 adolescents meeting entry criteria, 127 (33.6%) gave consent for a researcher to access their clinical case notes, 15 (4.0%) actively refused consent, 235 (62.2%) did not respond and one (0.3%) had died, having withdrawn from treatment in a non-specialist CAMHS. The distribution of diagnoses (AN, EDNOS-AN, BN and EDNOS-BN) did not differ between the consenters and the non-consenters (p = 0.16). Subsequent comparisons include only the 220 adolescents with AN or EDNOS-AN (the 214 cases shown in Table 34, plus six cases from the PCTs that were excluded from the analyses of presentation rates), 93 (42.3%) of whom consented to participate in the study.
No statistically significant differences were found between consenters and non-consenters in mean age at assessment; sex; ethnicity; proportion with a diagnosis of AN (vs. EDNOS-AN); mean BMI at assessment; proportion who were binge eating or self-induced vomiting; first service attended following referral from primary care; or proportion admitted as inpatients during the study period. A higher proportion of consenters (47.3%) than non-consenters (31.8%) accessed more than one participating service during the study period, but this difference did not reach statistical significance (p = 0.06). The only significant difference identified between consenters and non-consenters was the PCT group to which they belonged. The proportion consenting to be part of the study was significantly higher in the CAEDS service group (55.0%) than in the specialist CAMHS group (26.3%; p < 0.01) or the non-specialist CAMHS group (23.3%; p < 0.01).
Characteristics of the consenting sample are shown in Table 35. The average weight is higher than would be expected from a sample of adolescents with AN because of the inclusion of those with EDNOS-AN. Average weight loss prior to assessment was 12.3 kg, which is substantial considering the relatively short mean duration of illness (8 months). There were low rates of binge eating, self-induced vomiting and laxative misuse. No statistically significant differences were found between the groups in mean age at assessment, sex, ethnic background, per cent median BMI at assessment, proportions who were binge eating or vomiting or source of referral.
| Characteristic | Descriptive statistic |
|---|---|
| Mean age | 15.1 years (SD = 1.2 years) |
| Sex | 90 (96.8%) female; 3 (3.2%) male |
| Ethnicity | 64 (68.8%) white British; 23 (24.7%) other; 6 (6.5%) missing |
| Social classa | 62 (66.6%) I/II; 10 (10.8%) IIIN/IIIM; 3 (3.3%) IV/V; 18 (19.4%) missing |
| Parents’ relationship status | 70 (75.3%) intact; 18 (19.4%) separated; 3 (3.3%) other; 2 (2.2%) missing |
| Diagnosis | 57 (62.6%) AN; 34 (36.6%) EDNOS-AN; 2 (2.2%) missing |
| Mean weight for height (%) | 82.8 (SD = 10.4, range = 63.3–131.9) |
| Menstrual status (females) | 55 (61.8%) amenorrhoea; 18 (20.2%) irregular periods; 6 (6.7%) regular periods; 2 (2.2%) on hormonal contraceptives; 8 (9.0%) missing |
| Mean duration of illness (months) | 8.1 (SD = 8.0, range = 1–36) |
| Binge eating | 81 (87.1%) no; 9 (9.7%) yes; 3 (3.2%) missing |
| Self-induced vomiting | 69 (74.2%) no; 21 (22.6%) yes; 3 (3.2%) missing |
| Laxative misuse | 82 (88.2%) no; 27 (7.5%) yes; 4 (4.3%) missing |
| Exercise for weight loss | 44 (47.3%) no; 43 (46.2%) yes; 6 (6.5%) missing |
| Primary care referrer | 65 (69.9%) GP; 9 (9.7%) school/college; 6 (6.5%) paediatric inpatient unit; 3 (3.2%) hospital outpatient department; 5 (5.4%) CAMHS (seen for other issues); 1 (1.1%) counselling service; 1 (1.1%) parent; 3 (3.2%) missing |
Expected and actual care pathways
It became clear during the early part of the study that the PCT agreements did not always predict the actual care pathway that individual patients followed. When the actual care pathways were examined, the data suggested a greater similarity than expected between specialist CAEDS and specialist CAMHS (in terms of the number of cases presenting for treatment and the proportion of admissions for inpatient treatment within 12 months). Therefore, these services were grouped for subsequent analyses, and the following ‘actual care pathway groups’ were formed:
-
specialist assessment to specialist treatment: participants referred directly from primary care to a specialist CAEDS (child and adolescent, or adult) or a specialist CAMHS for assessment and treatment
-
non-specialist assessment to specialist treatment: participants assessed in a non-specialist CAMHS and then immediately referred on to a specialist CAEDS for treatment
-
non-specialist assessment to non-specialist treatment: participants referred from primary care to a non-specialist CAMHS for assessment as well as treatment
-
Private assessment to private treatment: participants who moved from primary care into private ED services (these were excluded from subsequent analyses, as the aim of the present study was to explore care pathways within the NHS). Table 36 compares the actual care pathways with those that would be predicted by PCT agreements. The main difference was that some of the CAMHS services that were in the catchment area of one of the CAED services did not automatically refer on following an assessment and more people than expected were assessed and started treatment in generic CAMHS.
| Care pathway | Pathway, n | |
|---|---|---|
| Expected (based on PCT) | Actual | |
| Specialist assessment to specialist treatment | 71 | 53 |
| Non-specialist assessment to specialist treatment | 10 | 16 |
| Non-specialist assessment to non-specialist treatment | 9 | 15 |
| Private assessment to private treatment | 0 | 6 |
Admissions for inpatient treatment by actual care pathway
In the specialist assessment to specialist treatment group, 8 out of 53 (15.1%) adolescents were admitted to hospital, compared with 3 out of 16 (18.8%) adolescents in the non-specialist assessment to specialist treatment group and 6 out of 15 (40.0%) adolescents in the non-specialist assessment to non-specialist treatment group. The binary logistic regression analysis (controlling for baseline percentage median BMI) indicated that the chance of admission in the non-specialist assessment to specialist treatment group was 32% higher than in the specialist assessment to specialist treatment group (adjusted OR = 1.32, 95% CI 0.30 to 5.81; p = 0.71), and the chance of admission in the non-specialist assessment to non-specialist treatment group was 261% higher than in the specialist assessment to specialist treatment group (adjusted OR = 3.61, 95% CI 1.00 to 13.02; p = 0.049).
Continuity of care
The exploration of the actual care pathways identified an unexpected difference in continuity of care, depending on where outpatient treatment started. In the specialist assessment to specialist treatment group, 83% of participants completed their treatment in the same service, compared with 75% in the non-specialist assessment to specialist treatment group, and 41.7% in the non-specialist assessment to non-specialist treatment group. A post hoc analysis showed that the continuity of care was significantly higher in the specialist assessment to specialist treatment group than in the non-specialist assessment to non-specialist treatment group (χ2 = 6.93; p < 0.01).
Study 2: qualitative study
Participant recruitment and characteristics
Eight adolescents and 11 parents agreed to take part in interviews about their experiences. As with the quantitative study, recruitment was more successful among those who had been to a specialist service. Of the adolescents, five had started treatment in a CAEDS, two in a specialist CAMHS and one in a generic CAMHS. The group of parents included one parent of each of the adolescents who took part in interviews and three parents whose daughters did not want to participate: one of these started treatment in a CAEDS, one in a specialist CAMHS, and one was assessed in a non-specialist CAMHS and then referred for treatment in CAEDS.
The nature of the sample means that the views reported here are not presented as being representative of any group other than that which took part in the interviews; however, they provide an insight into the experiences of these families, and highlight factors that may impact on service user satisfaction. Full details of the studies and results are available elsewhere,360 and only a small number of quotes are presented here to demonstrate particular points.
Experiences of services
In nearly all cases of those we interviewed, help-seeking was prompted by a parent, rather than by the adolescent themselves, with some adolescents describing great resistance to the idea of external intervention:
I don’t really know what I thought was going on, I think I just thought I was losing a bit of weight and everyone was making a massive fuss and I was absolutely fine and please don’t bother me because I’m quite happy just running my own show.
Although some GPs were supportive and facilitated access to treatment, others were viewed as decidedly unhelpful by adolescents and parents. Some GPs appeared to be guided more by physical signs, such as weight, than by parental concerns:
The doctor said [to my parents] ‘Oh no, your daughter’s fine’ . . . that’s when . . . I was like ‘Oh, I’m fine, I can just lose loads of weight’.
The GP basically said that her BMI wasn’t low enough to be anorexic . . . she didn’t mention a referral to CAMHS, she didn’t mention [specialist service] . . . if you got diagnosed with cancer, they would treat it, they wouldn’t . . . wait until you had Stage 3!
Adolescents who received treatment in CAMHS described situations when they felt they were not receiving the help they needed, but were temporarily unable to access more specialist services:
I wasn’t getting any better . . . I maintained a really frighteningly low weight for quite a long time . . . I wanted to get better, I wanted some help . . . I sort of said ‘I’ve never had the opportunity to get better because I’ve never had proper help’.
The parents of these adolescents expressed great relief when they were able to move to more specialist services, and regret that this had not happened immediately:
CAMHS was like a holding bay.
All three of us say ‘If we hadn’t have got to [specialist service], [daughter] would be dead’.
Of course I feel sad when I think ‘Well, what would our story have been if we’d come straight [to specialist service]?’ . . . I don’t think she’d have been allowed to get [as ill as] she got last year.
Adolescents described the difficulty of moving between services and switching therapists, and explained that continuity with helpful relationships was valued:
It’s difficult to build that kind of relationship . . . I felt like I was getting somewhere with [therapist] and then to start all over again with someone that didn’t know me and didn’t know the history . . . it was quite frustrating because it’s kind of like stopping and starting all the time and never really getting anywhere.
Clinicians’ expertise in EDs was an important element of both adolescents’ and parents’ experiences of inpatient and outpatient services:
A lot of it is expertise because I feel like ‘ah, finally someone gets me’ . . . like, everything I say is something that [clinician] has already come across. She knows all of the symptoms so she doesn’t make me feel like I’m a freak . . . she just, she understands.
You cannot compare [paediatric and specialist admission]. It’s chalk and cheese. [The eating disorders unit] know what to look for, they know everything, you know, they know the natures of the beast.
For parents, one of the most unanimously positive experiences (for those who had taken part) was groups with other parents who were in a similar situation – these tended to be organised by specialist services. Those who did not have access to groups were frustrated by this:
It was a feeling that we were all . . . there were all different types of people and different everything else, but we all had this problem.
What I wanted was to talk to other people who were going through the same situation as me . . . to meet up with somebody, to speak . . . to see them face to face.
Discussion
This is the first naturalistic study to examine outcomes following TAU in different types of service for adolescents with an ED. The key findings are (1) once population size was taken into account, the number of adolescents with AN and EDNOS-AN who were referred from primary care in areas with specialist outpatient services for child and adolescent EDs was two to three times that in areas with no specialist services; (2) patients who started treatment in generic CAMHS were 2.5 times more likely to be treated in hospital during the following 12 months than those initially treated in a specialist service; and (3) continuity of care was considerably lower in non-specialist services, in that only 42% of adolescents started and continued treatment for the next 12 months without being referred on, compared with more than 80% of those seen in specialist services.
The number of cases seen in services beyond primary care
This is the most robust finding as it is based on the full sample of 367 cases. The low level of case identification in areas with no specialist services is consistent with previous research. 26 The striking finding from this study is that the rate of referral to specialist services is close to the level that epidemiological studies suggest is the actual incidence of new cases seen in primary care. 51,361 It is possible that GPs with links to specialist services have better knowledge of EDs and their treatment and therefore identify and refer more cases than GPs with no specialist links. Alternatively, the visibility and easy access to specialist services may encourage referrals rather than a ‘wait and see’ approach that is sometimes seen in primary care. The second explanation is supported by Currin’s362 primary care study, which showed that GP’s working in areas where inpatient treatment was the only specialist service provision have higher referral thresholds than those in areas with specialist outpatient services. Our own clinical experience in a specialist context of receiving a growing number of very early referrals also supports this explanation. A third explanation is that availability of services has an effect on patient and/or parent preferences and changes the threshold for seeking help.
Admissions for inpatient treatment
Although there have been previous clinical reports of reductions in admission rates following the development of specialist outpatient services,363 this is the first study that provides clear empirical support for this. Our study showed that admission for inpatient treatment was not predicted by service-level agreements between PCTs and service providers, but was predicted by participants commencing treatment in a non-specialist CAMHS. The high level of admission in the non-specialist care pathway raises a number of concerns. Inpatient treatment of AN is costly,354,364 has high relapse rates,228,365 and there is evidence that hospital treatment is associated with poor outcomes in adolescent AN even when severity of symptoms are controlled for. 353
Continuity of care
In the present study, the differences in the continuity of care between the specialist care and non-specialist care pathway was striking. This is important as continuity potentially reduces duplication of work, and avoids the need for managing transitions between services, which can be problematic for patients and their families. 36
Service user experiences
Adolescent and parents highly valued the level of clinician expertise that they found in specialist services. Those who were dissatisfied with CAMHS were frustrated by barriers to specialist services. Movement between different services could disturb the delicate process of building and maintaining helpful therapeutic relationships, and a lack of continuity of care could be experienced as being ‘sent from pillar to post’ – this was one of the most frequent areas of dissatisfaction for those who were referred to CAMHS. Group interventions were particularly important for parents, and these are likely to be more easily facilitated by specialist services because of the resources available and the greater number of families that can be seen at any one time. Service user reports suggest that work may need to be done with GPs to increase their understanding of EDs beyond the physical symptoms – again, specialist services may be best placed to undertake such work.
Study limitations
Although the differences between the specialist and non-specialist care pathways found in the present study are striking, a degree of caution is needed as we do not know the extent to which this will generalise beyond London, as the study area differs from the rest of the UK in some important ways. The most relevant is that London contains considerably more specialist ED services than rest of the country. We do not know what impact this may have on the attitudes of referrers or the confidence of clinicians working in generic CAMHS. The finding concerning the differences in the continuity of care is probably most susceptible to the specific conditions in London and may not generalise well to areas with no specialist services.
The study did not include every service that could conceivably accept a referral of an adolescent with an ED, but rather focused on services that handle the large majority of such referrals. Although the exclusion of services such as specialist CAMHS targeted at other disorders may have resulted in comorbid cases being excluded, the study was able to obtain data from most of the ED services, CAMHS that specialised in EDs and non-specialist CAMHS in London, with 84% of eligible services providing data. The impact of the different methods of participant identification used by different services is unclear.
Although the sample used to test hypothesis 1 was relatively large, and included cases from the majority of the PCTs in London, the subsample of consenters used to test hypothesis 2 was comparatively small (42.3% of eligible cases), and the analyses of admission rates were underpowered. The logistic regression analyses did not take into account any PCT clusters, so the effect of belonging to an individual PCT on admission was not explored. The differential recruitment rate for specialist and non-specialist services could have biased the sample and influenced the results. However, comparisons between consenters and non-consenters showed no significant differences in the number of services accessed or admissions for inpatient treatment during the study period. Moreover, the findings of high admission rates from general services is consistent with previous reports30,351 as is the lower rate in specialist services. 363 The short follow-up period for this study is a further limitation, as findings relate only to participants’ service use for a year after presentation.
Although the results of the qualitative study cannot be taken as representative of a wider population, they suggest a number of reasons why service users may find specialist services preferable to non-specialist services. These are best described as hypotheses at present, and warrant further study.
Implications for service development and future research
The results of this study are potentially of considerable significance for the way treatment of child and adolescent EDs is provided in the NHS. The data suggest that establishing care pathways in which referrals from primary care go directly to specialist outpatient CAEDS could significantly increase levels of case identification, while at the same time reducing overall costs by reducing admissions for inpatient treatment and the associated readmissions, which are likely to increase cost differentials over time. This study did not examine the nature of the treatments provided within different services or the impact that specific treatments may have had on outcomes. However, it is likely that part of the explanation of the differences found is a greater use of effective, evidence-based treatments by specialist services. One could argue that a further factor in favour of developing specialist services is that this would facilitate dissemination of such treatments.
The fact that CAEDS and specialist CAMHS appeared to be relatively comparable in this study suggests that general CAMHS may be able to achieve similar outcomes to specialist services if they contain some organisation around EDs and clinicians have opportunities to develop necessary expertise. The disadvantage of this arrangement, in comparison with specialist ED services, is that developing a truly multidisciplinary team is often difficult when only a small group of clinicians is involved and such teams are also more vulnerable to the impact of staff turnover (in fact, during the service mapping exercise we heard of several instances when a mini-specialist team was unable to continue when a key member of staff retired or changed jobs).
Conclusion
The study presented here can be considered one of the first stages in the investigation of outcomes following treatment in services with different levels of specialisation in EDs. Findings suggest that starting treatment in a specialist outpatient service may have some advantages. Questions remain, however. The difficulty in recruiting participants from CAMHS in particular raises the question of how to evaluate treatment outside of specialist contexts. Future research will need to address this issue.
Chapter 11 Cost of illness and cost-effective treatments (work package 7b)
Abstract
Background
We aimed to identify services and treatments used by people with AN and associated costs, to estimate the unit costs of ED treatments, to explore cost variations by patient characteristics, to explore the economic consequences of AN and to estimate the annual costs of AN for England.
Methods
We analysed service use data collected alongside three clinical trials and estimated costs by attaching unit costs to service use. Individual variations in costs were explored using regression analyses. Unit costs of ED treatment and service costs for a cohort of young people with AN over 1 year were estimated using data from the Care Pathways Study (see Chapter 10, Study 1: quantitative study). Economic consequences of AN in adulthood were analysed using BCS-70 data,366 comparing women with lifetime AN and women without eating problems. Combining publicly available data and results from the ARIADNE studies, we estimated the annual costs of AN in terms of health service costs, private health-care costs, benefit receipt and years of potential life lost (YPLL).
Results
Service costs were driven by hospital admissions and vary by age, illness severity and treatment history. Those treated in non-specialist outpatient services incurred higher costs than those treated in specialist services only, with no differences in outcome. Women with lifetime AN were more likely to be long-term sick or disabled in adulthood, to receive benefits and to have completed a degree, with no differences in weekly income or employment status compared with cohorts with no eating problems. The annual costs of AN for England were estimated to be between £45M and £230M in 2011.
Introduction
Although it is clear that AN has a severe impact on the health-care system and people’s lives – including patients, their carers, families and partners – to date there have been few attempts at quantifying the economic impact in monetary terms. 115,367 The implementation of cost-effective alternatives to inpatient treatment across the country should be a priority to ensure equitable access and adequate treatment. 26,368,369 Economics evidence can facilitate this by providing a sound ‘cost of illness’ estimate, and associated cost-effectiveness analyses, that allow service providers and planners to estimate the monetary benefit of implementing evidence-based services.
This chapter, therefore, attempts to provide some answers to the following questions:
-
What services and treatments do people with AN use?
-
What are the unit costs of various ED treatments?
-
What are the costs associated with service use by people with AN?
-
Do costs vary by participant characteristics?
-
What are the economic consequences of AN?
-
What are the annual costs of AN for England?
The work was carefully integrated with other studies in the programme and therefore depended on data from other WPs being available. During the first phase of the project, a pragmatic review of the evidence was undertaken with the aim of identifying data on (1) the typical course of illness of AN, including age of onset, duration and outcome; (2) negative outcomes associated with AN, such as negative health and social consequences; (3) service use and costs associated with AN directly and its negative consequences; and (4) cost-effectiveness studies. The review was based on a broad search of the PubMed database, supplemented by snowball searches (clinical studies and policy documents, such as NICE guidance on EDs279), expert submissions, as well as searches of the ‘grey’ literature using the Google search engine (Google Inc., Mountain View, CA, USA). The findings from the evidence review informed the data framework for the cost of illness study. The literature review also revealed that little evidence on the economic consequences of AN was available, and a study using the BCS-70 was designed to fill this gap. 366
In this early phase, we also designed instruments that would facilitate cost estimation within three RCTs (MOSAIC, CASIS and iMANTRA studies; see Chapters 4, 6 and 8). The CSRI,153 originally devised in 1986, is used to record information on participants’ use of services and was carefully adapted in the light of the research questions, service context and participants. A self-report measure was selected as participants were likely to use a wide range of services and there is no one central source of service use data across different agencies. 153 GP records, for example, are an unreliable source for data on hospital and community services,370 but there is high agreement between GP records and self-reported GP contacts. 371
We analysed the baseline service use and costs of people recruited to three RCTs (study 1) and the costs of ED treatment provided in four London PCTs (the Care Pathways Study; study 2). We also present here our analysis of economic outcomes associated with AN based on the BCS-70 (study 3) and our estimate of the annual costs of AN for England (study 4). 366
Study 1: service use and costs associated with anorexia nervosa
Little is known about the service use and costs associated with AN. A recent review of the costs associated with EDs and the cost-effectiveness of treatments up to 2010367 identified just three partial cost-of-illness studies for AN, and none was based in the UK.
There was only one full cost-effectiveness analysis. 354,372 This compared the costs and outcomes of inpatient treatment, specialist outpatient treatment and TAU and found that specialist outpatient treatment had a higher probability of being cost-effective (up to approximately 60%). One limitation of this study was that only 65% of patients adhered to the allocated treatment.
Our literature search identified one study that modelled the value for money generated by different types of ED services in Sheffield, based on cost data provided by the PCT and using a narrow cost perspective. 373 Potential savings to the PCT from preventing admissions were particularly high for residential units out of area or in the private sector at an average of £60,700 per case. The authors of that study recommended that a new commissioning strategy should invest in services that reduce costly inpatient admissions.
Given the paucity of evidence regarding service use, costs and cost-effective treatments, our service use data collected alongside the MOSAIC128 (see Chapter 4), CASIS204 (see Chapter 6) and iMANTRA (see Chapter 8) trials is an important addition to the UK evidence base. Here we present the baseline data collected from patients before receiving any of the trial interventions, exploring patient service use and associated costs (three trials), as well as predictors of total service costs (MOSAIC and CASIS).
Methods
Service use
Participants completed the CSRI153 at baseline and at 6 and 12 months. Note that only baseline data are reported here; full cost data and a cost-effectiveness analysis will be published elsewhere. The schedule covered a retrospective 6-month period and was adapted for each study to include hospital services, specialist mental health services, primary care services and community-based services such as social work and alternative therapy alongside demographic information and details on employment.
Service costs
The costs of service use for each participant were calculated by identifying an appropriate unit cost and duration for each service contact and multiplying these by the number of contacts each person reported. For most hospital, mental health and primary care services as well as social work, unit costs were drawn from publicly available sources. 374,375 Others were taken from previous studies or estimated using an equivalent method376 from data collected as part of the Care Pathways Study (see Study 2: economic analysis of the Care Pathways Study). When service contacts were reported but the number of contacts was missing, the mean for all people in contact with that particular service was entered.
Outcome measures and sample characteristics
Some of our analyses use sociodemographic information and outcome measures collected at baseline in each study. These are described fully in the chapters reporting the outcome findings for each study (see Chapters 4, 6 and 8).
Data analyses
Service use by participants for the 6-month period prior to the baseline assessment is described in terms of the number using a service or providing a type of care and the percentage of the sample. Service costs are presented as means with SDs and ranges by service category.
The relationship between costs, patient characteristics and outcome measures was explored using a cost function approach. 377,378 The aim was to identify if any particular characteristics of people with AN raised or lowered costs as this may influence our cost of illness model. Total service costs are used as the dependent variable, with patient characteristics and measures of clinical severity as explanatory variables in a regression-type framework. To account for the skewed distribution of cost data, regression analysis was performed with 10,000 bootstrap replications and clustering within individuals to obtain robust SEs. Predictors of costs that were statistically significant were selected and a multivariate model was fitted by stepwise removal of non-significant predictors from a full model. A 90% CI was chosen to determine statistical significance because, in economic analyses, there is less risk associated with type II errors than, for example, in clinical studies. Within our set of potential predictors, there are several sets of variables that are closely related: duration of illness and age at onset, for example, are related to age in opposite ways (i.e. age minus duration of illness is age at onset). Similarly, the highest level of education or qualification achieved will be related to age, and the EDE-Q global score is by definition correlated with its subscales. These sets of predictors typically cannot be fitted into the same model, but do give us several options for the final model. In presenting results, we selected the variables that explained the highest proportion of variance while retaining basic principles of parsimony and sense.
Results
Service use
Table 37 shows the number and percentage of participants in each trial using each type of service. Participants reported the highest contact rate with GPs for their EDs in CASIS, followed by outpatient services and dentists, whereas, in MOSAIC, the highest percentage of participants was in contact with outpatient services, followed by GPs for EDs and for other reasons. In iMANTRA, in which participants were recruited from an inpatient population, the high use of inpatient services was followed – by a wide margin – by GPs for EDs and psychiatrists/psychologists.
| Service | Trial | |||||
|---|---|---|---|---|---|---|
| MOSAIC | CASIS | iMANTRA | ||||
| n using | % using | n using | % using | n using | % using | |
| Inpatient: ED | 11 | 8 | 78 | 50 | 41 | 100 |
| Inpatient: other | 15 | 11 | 24 | 15 | 1 | 2 |
| Outpatient: ED | 126 | 89 | 88 | 56 | 0 | 0 |
| Outpatient: other | 34 | 24 | 22 | 14 | 0 | 0 |
| A&E: ED | 27 | 19 | 50 | 32 | 2 | 4 |
| Psychiatrista | 32 | 23 | 51 | 33 | 7 | 15 |
| Psychologista | 28 | 20 | 64 | 41 | ||
| CPN | 17 | 12 | 40 | 26 | 0 | 0 |
| Psychotherapist | 10 | 7 | 25 | 16 | 0 | 0 |
| Family therapist/MFDT | 0 | 0 | 7 | 4 | 4 | 9 |
| Individual therapist/CBT | 2 | 1 | 0 | 0 | 4 | 9 |
| CAMHS/AMHS | 1 | 1 | 3 | 2 | 3 | 7 |
| Crisis team | 0 | 0 | 4 | 3 | 1 | 2 |
| Residential rehabilitation | 1 | 1 | 0 | 0 | 0 | 0 |
| GP: ED | 124 | 88 | 134 | 86 | 6 | 13 |
| GP: other | 96 | 68 | 55 | 35 | 3 | 7 |
| Practice nurse: ED | 32 | 23 | 72 | 46 | 4 | 9 |
| Practice nurse: other | 38 | 27 | 32 | 21 | 0 | 0 |
| Other community nurse | 6 | 4 | 13 | 8 | 0 | 0 |
| Dentist | 67 | 48 | 86 | 55 | 4 | 9 |
| Optician | 36 | 26 | 50 | 32 | 4 | 9 |
| Dietitian/nutritionist | 11 | 8 | 9 | 6 | 5 | 11 |
| Counsellor | 28 | 20 | 44 | 28 | 0 | 0 |
| Alternative therapist | 10 | 7 | 22 | 14 | 0 | 0 |
| Solicitor/lawyer | 7 | 5 | 7 | 4 | 0 | 0 |
| Physiotherapist | 2 | 1 | 1 | 1 | 1 | 2 |
| Occupational therapist | 1 | 1 | 2 | 1 | 0 | 0 |
| Osteopath | 1 | 1 | 0 | 0 | 0 | 0 |
| Police | 0 | 0 | 0 | 0 | 1 | 2 |
| Self-help/support group | 13 | 9 | 22 | 14 | 0 | 0 |
| CAB | 8 | 6 | 12 | 8 | 0 | 0 |
| Helplines | 15 | 11 | 16 | 10 | 0 | 0 |
| Websites | 3 | 2 | 2 | 1 | 0 | 0 |
| Social worker | 9 | 6 | 18 | 12 | 1 | 2 |
| Outreach/family support worker | 4 | 3 | 7 | 4 | 0 | 0 |
| Family centre | 0 | 0 | 0 | 0 | 1 | 2 |
| Carer | 0 | 0 | 1 | 1 | 0 | 0 |
Service costs
Table 38 shows the costs associated with service use, summarising the service use data presented in Table 37 into service categories. Reflecting the pathways of recruitment and the location of the interventions to be evaluated in the RCTs, average costs per person were highest for inpatient treatment in iMANTRA and CASIS, whereas outpatient treatment costs were the largest contributor to total costs in MOSAIC.
| Service category | Trial | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CASIS | MOSAIC | iMANTRA | |||||||
| Mean (£) | SD | Range | Mean (£) | SD | Range | Mean (£) | SD | Range | |
| Hospital | 21,045 | 30,370 | 0–215,172 | 4547 | 14,403 | 0–87,794 | 81,304 | 12,029 | 21,672–87,651 |
| Mental health | 1687 | 2261 | 0–11,232 | 709 | 1564 | 0–11,458 | 1062 | 2894 | 0–13,224 |
| Primary care | 1650 | 1409 | 0–7360 | 1046 | 1304 | 85–12,272 | 229 | 493 | 0–2076 |
| Community services | 286 | 489 | 0–1752 | 115 | 247 | 0–1460 | 3 | 16 | 0–105 |
| Self-help and advice | 32 | 99 | 0–673 | 59 | 394 | 0–4500 | 0 | n/a | n/a |
| Social work | 95 | 397 | 0–3780 | 70 | 383 | 0–3549 | 49 | 293 | 0–1872 |
| Total costs | 24,795 | 31,121 | 318–224,025 | 6546 | 15,316 | 138–96,287 | 82,647 | 11,296 | 25,200–95,124 |
The proportion of total cost absorbed by each cost category for each study can be seen in Figures 8–10. Total costs over the 6 months prior to baseline were highest for the iMANTRA group, in which all participants had used inpatient care over the previous 6 months; hospital costs accounted for 99% of total costs. In the CASIS group, members of which were also recruited from an inpatient population, hospital costs still accounted for 85% of total costs, and only around 6% of this was due to outpatient visits, used by over 60% the participants. In the MOSAIC group – recruited through outpatient services – only 16% reported a hospital admission in the 6 months prior to the intervention. For all three studies, community, self-help and social work services contributed a very small proportion to total costs even though some of these services were used by over 10% of the study samples.
FIGURE 8.
Distribution of costs by service category: MOSAIC.
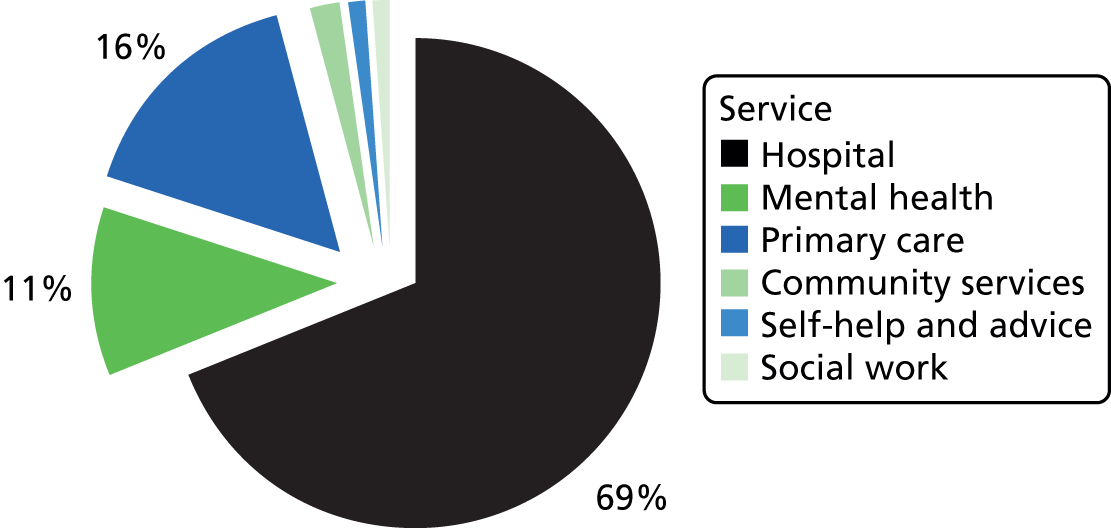
FIGURE 9.
Distribution of costs by service category: CASIS.
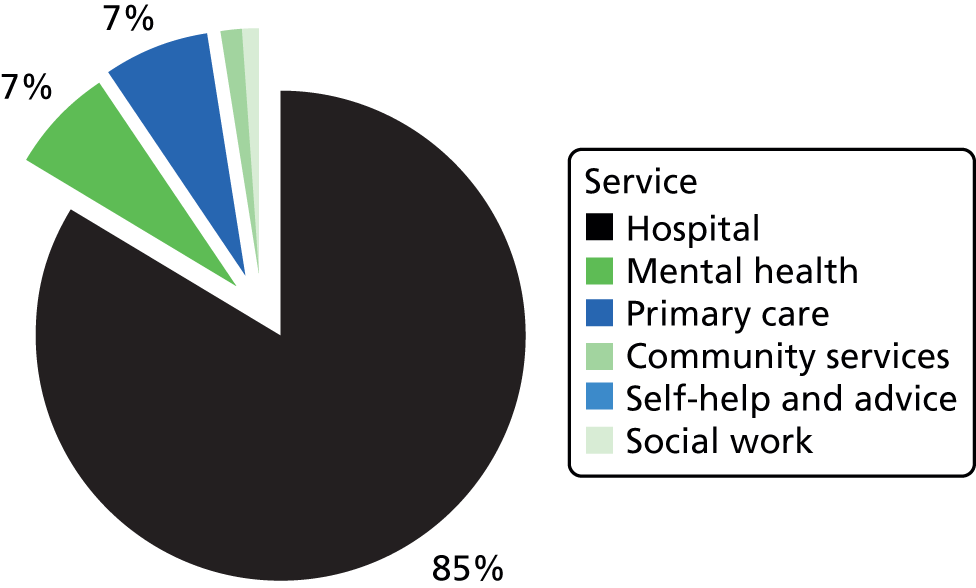
FIGURE 10.
Distribution of costs by service category: iMANTRA.
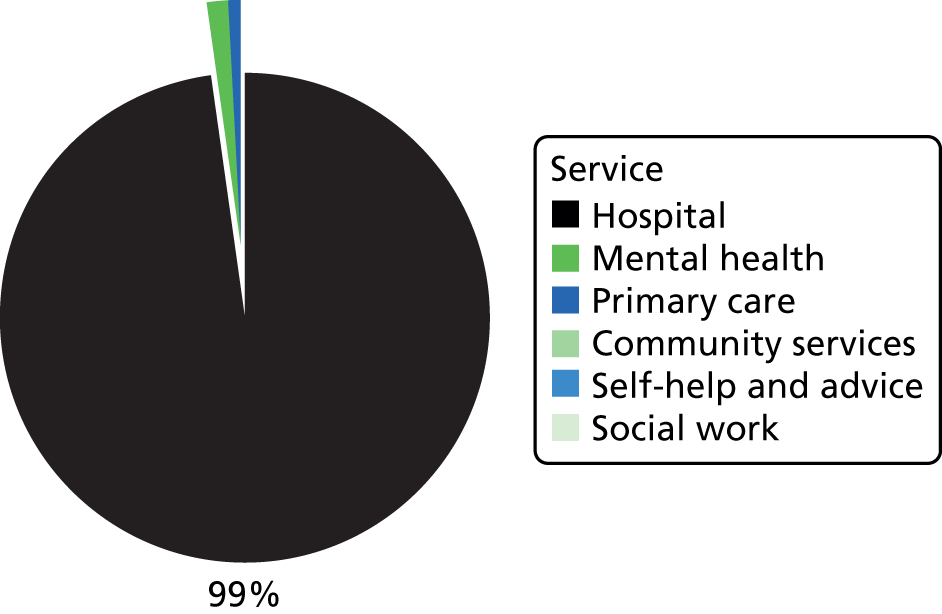
Predictors of service costs
We explored predictors of service costs in the MOSAIC and CASIS groups. Tables 39 and 40 show the results of univariate regression models, relating participant characteristics to total costs, as well as a ‘full’ model which maximises the proportion of variance in total costs explained (R2).
| Predictor | Model | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | n | Full (n = 114) | |||||
| Coefficient | SE | p-value | Coefficient | SE | p-value | ||
| Constant | 39,138 | 22,154 | 0.077 | ||||
| Age | –118 | 235 | 0.615 | 156 | |||
| Gender: male | 7615 | 9121 | 0.404 | 156 | |||
| Ethnicity (other vs. white British) | 3054 | 15,637 | 0.845 | 151 | |||
| English is first language | 21,426 | 2811 | < 0.001 | 153 | 18,685 | 5939 | 0.002 |
| Cohabiting | 1998 | 5033 | 0.691 | 153 | |||
| Has children? | –11,184 | 5060 | 0.027 | 154 | |||
| Number of children | –4783 | 1879 | 0.011 | 154 | |||
| Years of education | 704 | 996 | 0.480 | 145 | |||
| Degree vs. no degree | –4790 | 2696 | 0.076 | 153 | |||
| Full- or part-time employment | 3516 | 5809 | 0.545 | 156 | |||
| Diagnosis AN vs. other | –17,193 | 3836 | < 0.001 | 155 | |||
| BMI (baseline) | –1010 | 951 | 0.288 | 156 | |||
| Lowest BMI ever | –4640 | 1567 | 0.003 | 121 | –4816 | 1747 | 0.006 |
| Age of onset | –635 | 681 | 0.096 | 155 | |||
| Duration of illness | –27 | 18 | 0.138 | 153 | |||
| WHOQOL-100 quality of life rating | 7929 | 5025 | 0.115 | 149 | |||
| WHOQOL-100 health rating | 13,206 | 4812 | 0.006 | 150 | 12,932 | 5603 | 0.021 |
| Depression score | 355 | 264 | 0.178 | 150 | |||
| DASS-21 anxiety score | 287 | 193 | 0.137 | 150 | |||
| DASS-21 stress score | 614 | 171 | < 0.001 | 151 | 596 | 189 | 0.002 |
| Total DASS-21 | 179 | 73 | 0.015 | 150 | |||
| EDE-Q restraint | 17 | 1203 | 0.989 | 149 | |||
| EDE-Q eating concern | 1835 | 2057 | 0.372 | 149 | |||
| EDE-Q shape concern | 714 | 2346 | 0.761 | 149 | |||
| EDE-Q weight concern | 1665 | 1713 | 0.331 | 149 | |||
| EDE-Q global score | 1239 | 1861 | 0.505 | 149 | |||
| Number of hospitalisations | 3723 | 1246 | 0.003 | 149 | 2747 | 1143 | 0.016 |
| Adjusted R2 | 0.24 | ||||||
| Predictor | Model | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | n | Full (n = 140) | p-value | ||||
| Coefficient | SE | p-value | Coefficient | SE | |||
| Constant | 11,917 | 3725 | 0.001 | ||||
| Age | –243 | 137 | 0.075 | 141 | –303 | 125 | 0.015 |
| Ethnicity (other vs. white British) | 3142 | 3586 | 0.381 | 141 | |||
| Living with partner | –3371 | 1693 | 0.047 | 136 | |||
| Degree vs. no degree | –4790 | 2696 | 0.076 | 141 | |||
| Diagnosis AN vs. other | –5563 | 1781 | 0.002 | 141 | –3980 | 1718 | 0.021 |
| BMI (baseline) | –850 | 614 | 0.166 | 133 | |||
| Age of onset | –317 | 147 | 0.031 | 131 | |||
| Duration of illness | –23 | 99 | 0.815 | 133 | |||
| EDE-Q restraint | –1448 | 1306 | 0.271 | 141 | |||
| EDE-Q eating concern | 617 | 847 | 0.466 | 141 | |||
| EDE-Q shape concern | –401 | 1005 | 0.690 | 141 | |||
| EDE-Q weight concern | –965 | 881 | 0.273 | 141 | |||
| EDE-Q global score | –850 | 1305 | 0.521 | 141 | |||
| Previous hospital admission | 16,254 | 5137 | 0.002 | 140 | 16,564 | 6882 | 0.016 |
| Previous treatment for AN | 5120 | 2018 | 0.011 | 139 | |||
| Taking antidepressants | 5433 | 2543 | 0.033 | 139 | |||
| Adjusted R2 | 0.23 | ||||||
Discussion
The data presented were taken from three studies each with a different recruitment pathway. This was reflected in both the service use data and the costs, as, for example, all participants in iMANTRA reported inpatient stays. The MOSAIC group, recruited through outpatient services, was the most diverse in terms of the range of service use, and also reported the lowest average costs.
We found that factors associated with poor outcomes in AN,379 such as low BMI, higher age and longer duration of illness, were also associated with higher treatment costs. These findings suggest that those people with the highest needs in these domains are receiving the most intensive service response when presenting to secondary or tertiary care.
There were some differences between the studies. Higher age was associated with slightly lower treatment costs in MOSAIC. Previous treatment (number of previous hospital admissions in CASIS and a binary indicator of previous hospital admissions in MOSAIC) were associated with higher treatment costs. Interpretation is difficult, as it is unclear whether this simply reflects treatment costs immediately prior to the study, or a prolonged engagement with services due to severity or chronicity of AN.
English as a first language was also associated with higher treatment costs in CASIS, which may point to differences in treatment uptake among minority population groups,360 but this is not reflected in a significant cost impact of the ‘ethnicity’ variable.
Study 2: economic analysis of the Care Pathways Study
Treatment for severe AN often relies on costly inpatient care. 380 In a census of inpatient beds in 1999, 20% of all child and adolescent beds were taken up by ED patients,31 and ED admissions have the longest median length of stay of all adult psychiatric admissions. 28 More recently, in the UK there has been a shift from inpatient to outpatient treatment, driven by a shift in treatment philosophy and to halt the flow of money from the public to the private sector, which provided over 80% of inpatient units in 1998. 31,381,382 However, little is known about the pathways through care in outpatient services, the associated costs, outcomes and cost-effectiveness.
To help address this evidence gap, the Care Pathways Study360 (see Chapter 10) examined the care pathways for adolescents aged 13–18 years with AN across four PCTs in the Greater London area. The economic component of this study aimed to attach costs to the treatment received in each care pathway. To this end, the costs of different types of outpatient treatment sessions were calculated based on the information available from the Care Pathways Study service mapping exercise. These unit costs were then applied to each instance of service use of a cohort of young people with AN to facilitate calculating individual-level costs of care by care pathway. In addition, the study explored the types of ED treatments provided in different services, the professionals providing them and variation in individual-level treatment costs by participant characteristics and care pathway. We also calculated the cost of a ‘good outcome’ based on the Morgan and Russell criterion189 for each pathway.
Methods
Treatment costs (service level)
The information relevant to the economic analysis was compiled from the service-level questionnaires. The cost of providing treatment was calculated for each service individually and an average for each group of services (specialist ED service, including child and adolescent services and adult ED services; CAMHS with ED specialisation; and non-specialist CAMHS). Private sector services were excluded from the economic analysis as the main study focused on the public sector, and costs of private sector provision cannot be estimated reliably. For each type of staff member, a unit cost was calculated based on their likely Agenda for Change pay grade, working hours, ratio of client contact to other tasks, and overheads based on service location (community or hospital), drawing on a compendium of public sector unit costs374 and using a long-run marginal opportunity cost approach. 383
Individual-level service use and costs
The patient-level data were re-entered to better suit the needs of the economic analysis, showing not just the total number of service contacts for each person, but also what specific service provided each treatment session.
Appendix 3, Data availability and assumptions for service-level cost analysis and Individual-level analysis of treatment costs, shows the data available to the economic analysis in more detail.
Data analysis
At the service level, we present the number and per cent of services within each service category providing each type of treatment and the type of staff involved in the most commonly provided treatments. Differences in the odds that a treatment was provided or a professional provided a treatment were tested for statistical significance using univariate logistic regression models. Differences in the number of treatments were tested using simple regression models (equivalent to a t-test), whereas differences in the cost of treatments between services were investigated using regression models with 10,000 bootstrap replications.
At the individual level, the number of participants from each care pathway receiving each type of treatment is shown. The unit costs (calculated from the service-level information) were then applied to the number of contacts reported for each participant to arrive at individual treatment costs over the 1-year period. The unit costs of outpatient treatment, summarised by service type to match the types of outpatient sessions recorded in the individual-level data set, are shown in Appendix 3, Average costs of treatment by ED service type. These averages were used to estimate the cost of treatment for patients who were in contact with services that were not taking part in the study, based on degree of service specialisation. Unit costs for several other treatments were drawn from publicly available sources. 374,375 These unit costs are shown in Appendix 3, Additional unit costs from publicly available sources.
Results
Sample characteristics
Table 41 shows patient demographics, baseline diagnosis, clinical characteristics and distribution between care pathways for the entire cohort (n = 90). Categories with at least five participants in the cell were considered in the analysis of cost variations.
| Patient characteristic | n of N = 90 | % |
|---|---|---|
| Demographics | ||
| Female | 87 | 97 |
| White British | 62 | 69 |
| Parents marital status | ||
| Married or cohabiting | 70 | 78 |
| Other | 20 | 22 |
| Living situation | ||
| Living with parents | 79 | 88 |
| Other | 11 | 12 |
| Baseline diagnosis | ||
| AN | 40 | 44 |
| EDNOS-AN | 50 | 56 |
| Clinical characteristics (baseline) | ||
| Other medical condition | 11 | 12 |
| Taking psychiatric medication | 5 | 6 |
| Any comorbid psychiatric condition | 27 | 30 |
| Comorbid depression | 20 | 22 |
| Comorbid OCD | 1 | 1 |
| Comorbid anxiety | 6 | 7 |
| Self-harm | 6 | 7 |
| Previous outpatient treatment for an ED | 4 | 4 |
| Dietary restriction | 33 | 37 |
| Bingeing | 9 | 10 |
| Vomiting | 21 | 23 |
| Care pathway | ||
| Specialist–specialist | 53 | 59 |
| Non-specialist–specialist | 16 | 18 |
| Non-specialist–non-specialist | 15 | 17 |
| Private services | 6 | 7 |
| Good outcome at follow-up | 32 | 36 |
| Mean (SD) | Range | |
| Age (years), n = 90 | 15.1 (1.21) | 12–17 |
| Duration of illness (months), n = 87 | 8.1 (7.98) | 0–36 |
| Baseline weight/height, n = 88 | 82.8 (10.37) | 63–132 |
Table 42 shows the number of services in each service category that delivered the various treatments for AN (left-hand column) on an outpatient basis. It also identifies the average costs for each treatment.
| Treatment | Service | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Specialist CAEDS including other ED services (n = 5) | General CAMHS with ED specialisation (n = 6) | CAMHS without ED specialisation (n = 15) | |||||||
| Number providing (%) | Mean (£) (SD) | Valid n (cost) | Number providing (%) | Mean (£) (SD) | Valid n (cost) | Number providing (%) | Mean (£) (SD) | Valid n (cost) | |
| Assessment | 5 (100) | 152.79 (49.53) | 5 | 6 (100) | 208.75 (51.86) | 6 | 15 (100) | 152.48 (67.99) | 13 |
| CBT | 5 (100) | 135.52 (20.84) | 5 | 6 (100) | 170.72 (26.46) | 6 | 10 (67) | 163.85 (42.28) | 9 |
| PDT | 3 (60) | 123.91 (10.24) | 3 | 3 (50) | 129.82 (n/a) | 3 | 5 (33) | 137.12 (13.91) | 5 |
| Nurse counselling | 3 (60) | 90.60 (n/a) | 2 | 1 (17) | 71.27 (n/a) | 1 | 3 (20) | 82.09 (n/a) | 2 |
| Other individual therapy | 2 (40) | 129.82 (n/a) | 1 | 2 (33) | 186.17 (n/a) | 1 | 5 (33) | 120.82 (n/a) | 1 |
| Group without parents | 2 (40) | 40.31 (11.69) | 2 | 0 (0) | n/a | 0 | 0 (0) | n/a | 0 |
| Other group therapy | 1 (20) | n/a | 0 | 0 (0) | n/a | 0 | 0 (0) | n/a | 0 |
| IFT | 5 (100) | 204.67 (37.63) | 4 | 6 (100) | 242.26 (86.03) | 6 | 12 (80) | 246.05 (99.51) | 8 |
| MFT | 1 (20) | 525 (n/a) | 1 | 2 (33) | 525 (n/a) | 1 | 2 (13) | 525 (n/a) | 1 |
| Other family therapy | 1 (20) | n/a | 0 | 1 (17) | 21.55 (n/a) | 1 | 2 (13) | 129.54 (n/a) | 2 |
| Refeeding | 2 (40) | n/a | 0 | 2 (33) | n/a | 0 | 3 (20) | n/a | 0 |
| Dietary | 5 (100) | 40.55 (18.55) | 4 | 5 (83) | 38.95 (10.78) | 3 | 8 (53) | 108.68 (n/a) | 2 |
| Medical monitoring | 5 (100) | 121.56 (89.19) | 2 | 5 (83) | 73.85 (n/a) | 1 | 10 (67) | 177.91 (77.60) | 5 |
| Other | 1 (20) | n/a | 0 | 3 (50) | 115.79 (n/a) | 1 | 0 (0) | n/a | 0 |
Across all services, CBT, individual family therapy (IFT) and dietary advice are the most commonly provided treatments. There are differences in average cost per CBT session between service types, which are significant at the 90% level (p = 0.096), but no significant differences in the average cost of IFT sessions (p = 0.667).
The profession and grade of staff delivering the treatment influences the per-session cost of that treatment. The percentages of services (by degree of ED specialisation) in which specific staff members are involved in providing CBT and IFT are shown in Figure 11.
FIGURE 11.
Professionals providing treatment by service type treatments for AN offered in outpatient services. (a) CBT; and (b) IFT.
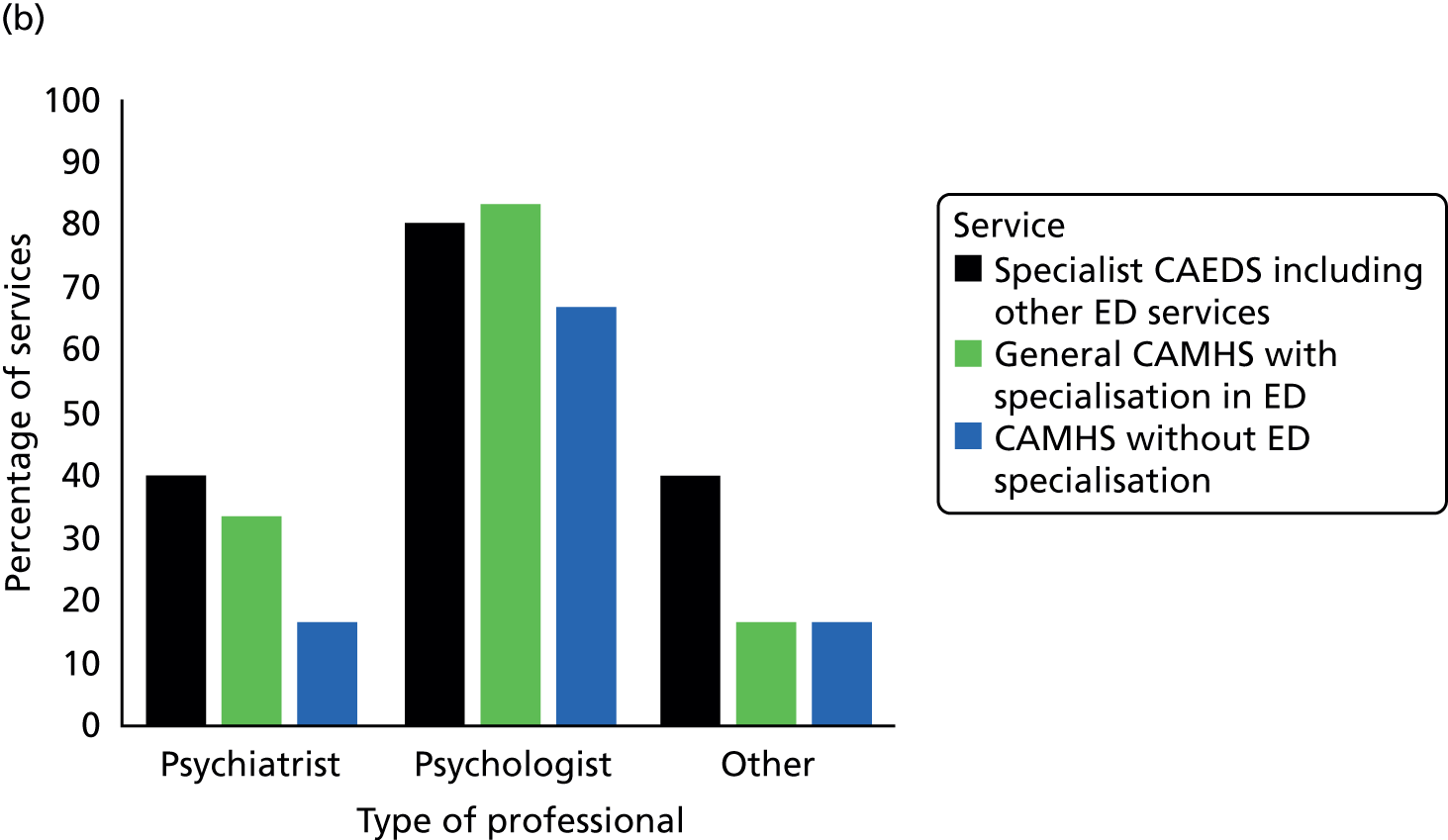
There are no statistically significant differences in terms of delivery of IFT, but psychiatrists are more likely to be involved in CBT in services that are general CAMHS with ED specialisation than in non-specialist CAMHS (p < 0.001). No specialist ED service reported that psychiatrists delivered CBT.
The number of treatments offered differed significantly between service types. Specialist ED services offered on average 2.6 individual treatments, whereas specialist CAMHS offered 2, and non-specialist CAMHS offered 1.6 (p > 0.01). Similarly, specialist ED services offered more than 8 different individual psychological or psychiatric treatments in total, whereas in specialist CAMHS it was 7 and in non-specialist CAMHS it was 5.2 (p > 0.01).
Unit costs of eating disorder treatment
Table 42 also shows that there is some variation in unit costs (per session) between service types. Although a statistical analysis is complicated by the small number of services involved and differences do not reach levels of statistical significance, it is reasonable to conclude that this is due to variations in staff profession (salary) and staff time. In the case of multifamily therapy, specialist services delivered this as whole-day sessions, whereas in the non-specialist CAMHS the sessions lasted only 60–90 minutes. The high cost of dietetic sessions in non-specialist CAMHS arises because psychiatrists provide dietary advice, whereas in other types of services it is more likely to be provided by dieticians or nurses who receive smaller salaries. In the case of parent sessions, the variation in unit costs is mainly due to group provision of sessions for parents in some services (so staff costs per session are shared between several families).
Individual-level service use and costs by care pathway
Patient-level data are available for 84 young people. The main study (see Chapter 10) found that 53 of them were assessed in specialist ED services and remained in specialist ED services for treatment (specialist–specialist care pathway; S–S). Another 16 were assessed in non-specialist CAMHS and referred to specialist services for treatment (non-specialist–specialist care pathway; NS–S), while 15 were assessed in non-specialist CAMHS and remained there for treatment or were directly admitted as inpatients (non-specialist–non-specialist care pathway; NS–NS). The details of service use for participants on each pathway are shown in Table 43.
| Service | Full sample (n = 84) | Care pathway | ||||||
|---|---|---|---|---|---|---|---|---|
| S–S (n = 53) | NS–S (n = 16) | NS–NS (n = 15) | ||||||
| n | % | n | % | n | % | n | % | |
| Assessment | 84 | 100 | 53 | 100 | 16 | 100 | 15 | 100 |
| Individual outpatient | 68 | 81 | 45 | 85 | 9 | 56 | 14 | 93 |
| Family outpatient | 82 | 98 | 51 | 96 | 16 | 100 | 15 | 100 |
| Multifamily outpatient | 13 | 15 | 8 | 15 | 2 | 13 | 3 | 20 |
| Parent only outpatient | 37 | 44 | 23 | 43 | 9 | 56 | 5 | 33 |
| Dietic outpatient | 40 | 48 | 26 | 49 | 6 | 38 | 8 | 53 |
| Medical outpatienta | 52 | 62 | 32 | 60 | 11 | 69 | 9 | 60 |
| Telephone calls | 52 | 62 | 31 | 58 | 11 | 69 | 10 | 67 |
| Psychiatric review | 34 | 40 | 22 | 42 | 6 | 38 | 6 | 40 |
| Day patient | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 13 |
| Inpatient (medical) | 15 | 18 | 5 | 9 | 6 | 38 | 4 | 27 |
| Inpatient (ED) | 17 | 20 | 8 | 15 | 3 | 19 | 6 | 40 |
Across all care pathways, outpatient single family therapy was the most commonly used form of treatment. In the S–S and NS–NS, the next most commonly used services were individual outpatient therapy and medical outpatient appointments. In the NS–S, the order of individual therapy and medical appointments was reversed.
The costs associated with the service use described above are shown in Table 44, again by care pathway. There were differences in the likelihood of admission and in length of stay between the pathways, driving the differences in inpatient costs. Details can be found in House360 and Chapter 10.
| Service | Care pathway | |||||
|---|---|---|---|---|---|---|
| S–S (n = 53) | NS–S (n = 16) | NS–NS (n = 15) | ||||
| Mean (£) (SD) | Range | Mean (£) (SD) | Range | Mean (£) (SD) | Range | |
| Assessment | 170 (36) | 112–293 | 151 (43) | 98–209 | 152 (40) | 98–230 |
| Individual outpatient | 1341 (1195) | 0–4206 | 955 (1229) | 0–3923 | 1933 (1228) | 0–4414 |
| Family outpatient | 2976 (2078) | 0–8005 | 2998 (2944) | 457–11,786 | 2909 (1135) | 965–5099 |
| Multifamily outpatient | 443 (1080) | 0–3829 | 479 (1323) | 0–4376 | 474 (1149) | 0–3282 |
| Parent-only outpatient | 174 (317) | 0–1509 | 15 (172) | 0–591 | 61 (151) | 0–585 |
| Dietic outpatient | 90 (188) | 0–847 | 15 (23) | 0–71 | 78 (105) | 0–382 |
| Medical outpatienta | 2998 (5296) | 0–27,125 | 4892 (6993) | 0–21,700 | 2583 (4023) | 0–13,950 |
| Telephone calls | 43 (68) | 0–314 | 53 (67) | 0–240 | 37 (38) | 0–108 |
| Psychiatric review | 1337 (2644) | 0–12,255 | 784 (1389) | 0–5160 | 562 (1166) | 0–4515 |
| Day patient | – | – | – | – | 1619 (4952) | 0–18,768 |
| Inpatient (medical) | 1529 (6520) | 0–34,112 | 10,260 (22,998) | 0–79,950 | 1421 (2752) | 0–8528 |
| Inpatient (ED) | 6452 (18,073) | 0–73,308 | 14,514 (36,791) | 0–137,760 | 30,242 (44,199) | 0–133,824 |
| Total costs | 17,544 (28,738) | 1323–149,406 | 35,215 (53,575) | 694–165,656 | 42,072 (48,277) | 3649–168,941 |
| Probability of good outcome, % | 39.6 | 31.3 | 33.3 | |||
| Cost per good outcome | 44,306 | 112,508 | 126,342 | |||
The average costs of individual and family outpatient therapy are roughly similar across all pathways, although the average cost of individual outpatient therapy is slightly (but not statistically significantly) lower in the NS–S, and the cost of family outpatient therapy is slightly higher in the S–S. Although dietary advice is a treatment reported to be commonly provided (see Study 3: economic outcomes of anorexia nervosa in a British cohort), the cost of dietetic outpatient sessions for this group of young people is low compared with other cost categories.
Figures 12–14 show the distribution of service costs by care pathway. The largest contributor to total costs for all care pathways is inpatient admissions for an ED. This is followed by individual outpatient treatments and the cost of family-based treatments. Although inpatient stays due to an ED make up a large proportion of costs in the NS–NS, there is a lot of cost variation between individuals (note the large SDs), so the difference between pathways is not statistically significant. Similarly, in the NS–S the cost of medical admissions are high, but this is at least in part due to two participants with unusually long stays (average length of stay 63 days), and the differences in costs are not statistically significant. Together, ED and medical admissions account for over 70% of total costs in both pathways for which the assessment is in a non-specialist service. The lower proportion of costs due to inpatient admissions in the S–S reflects the lower probability of admissions.
FIGURE 12.
Cost distribution: S–S.
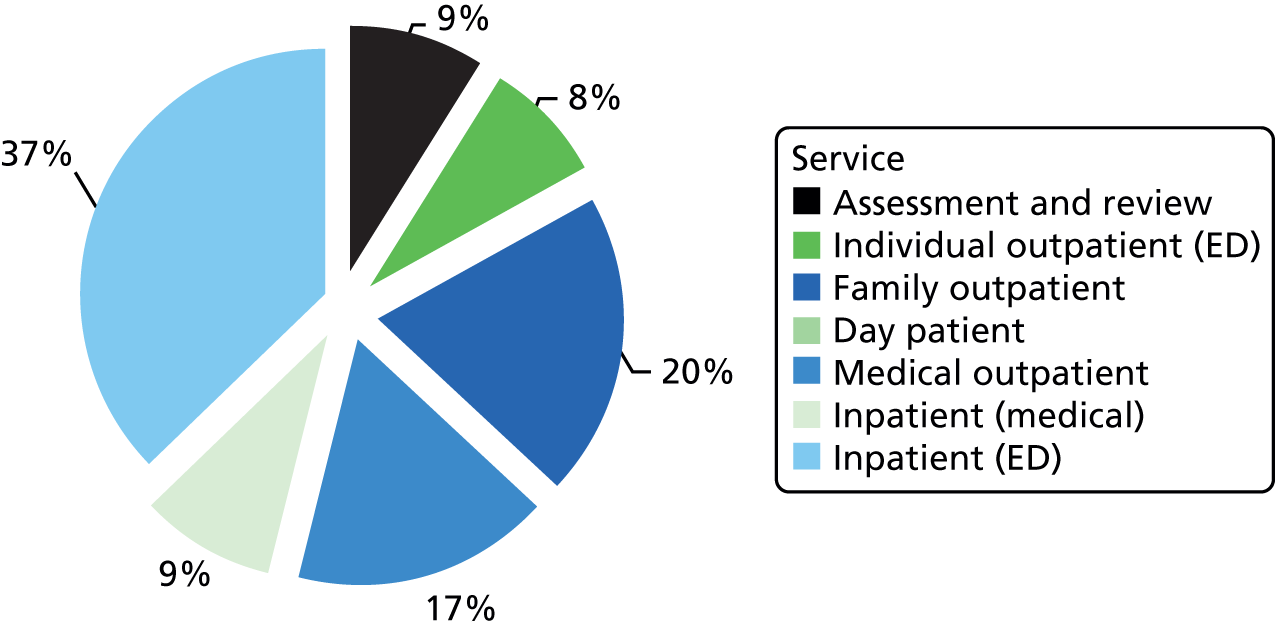
FIGURE 13.
Cost distribution: NS–S.
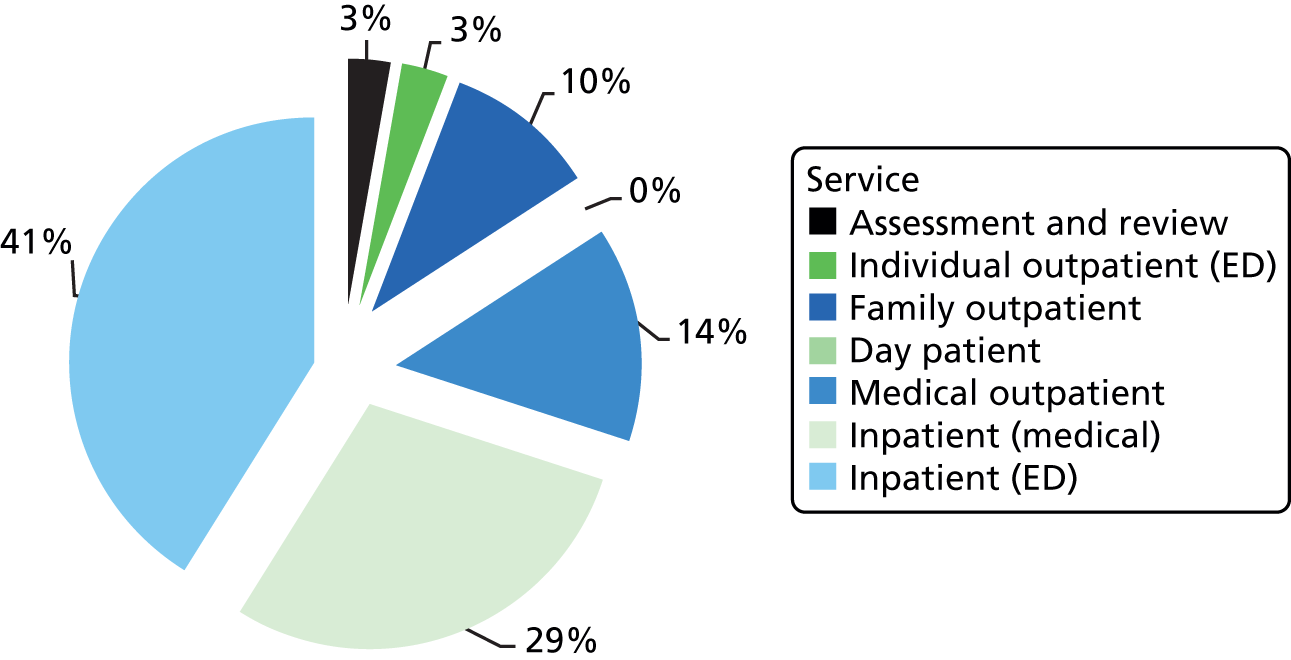
FIGURE 14.
Cost distribution: NS–NS.
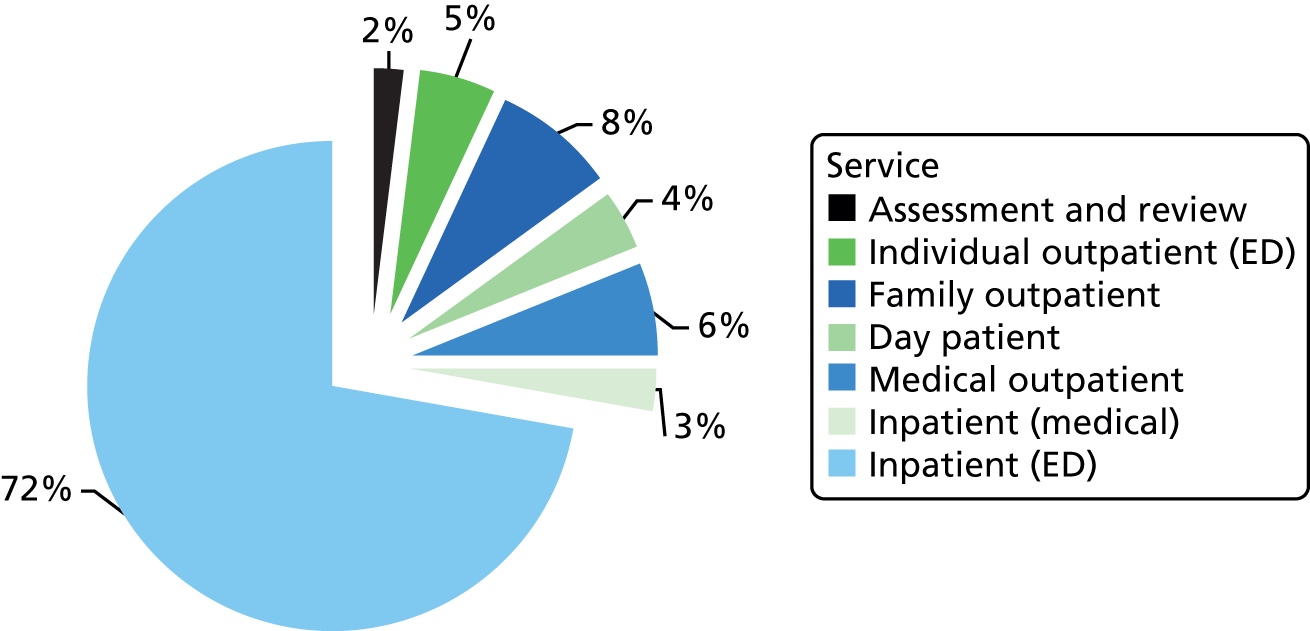
Predictors of treatment cost and cost per ‘good’ outcome
Table 44 shows the total costs over a 1-year period for each pathway (third row from the bottom). Mean costs were lowest for the S–S, and highest for the NS–NS. However, in each case the SD is larger than the mean, suggesting a wide variation in the total cost of treatment for the participants who followed each pathway.
Table 44 also shows the probability of attaining a good outcome (Morgan and Russell criterion189) in each pathway (penultimate row). Although the S–S shows the highest probability (39.6%), this was not a statistically significant difference. The final row shows the cost per good outcome for each pathway.
Table 45 shows the results of the univariate regression analysis identifying whether or not any participants’ characteristics are associated with higher or lower total costs. Costs were positively associated with age and duration of illness (significant at the 90% level), and negatively associated with having another medical condition. There were significant differences by care pathway as a whole, and in pairwise comparisons between the S–S and the NS–S (p = 0.088) and the S–S and the NS–NS (p = 0.016). There was no significant cost difference between the NS–S and NS–NS.
| Predictor | n | Coefficient (contribution to total costs) | SD | p-value |
|---|---|---|---|---|
| White British | 56 | –9442 | 7431 | 0.204 |
| Parents married or cohabiting | 62 | 5759 | 10,317 | 0.577 |
| Living with parents | 70 | 565 | 15,466 | 0.971 |
| Parental social class | ||||
| Class II | 6 | 3631 | 14,965 | 0.808 |
| Class III/IV | 48 | –2121 | 15,878 | 0.834 |
| Class ≥ V | 13 | 26,608 | 13,405 | 0.047 |
| Baseline diagnosis EDNOS vs. AN | 50 | –687 | 12,213 | 0.955 |
| Other medical condition | 11 | –19,991 | 5833 | < 0.001 |
| Any comorbid psychiatric condition | 21 | –5117 | 8544 | 0.549 |
| Dietary restriction | 29 | 1266 | 9529 | 0.894 |
| Vomiting | 18 | –14,237 | 7040 | 0.043 |
| Intense exercise | 39 | –1895 | 9028 | 0.834 |
| Care pathway | ||||
| S–S | 53 | 17,474 | 14,017 | 0.213 |
| NS–S | 16 | 26,156 | 13,962 | 0.061 |
| NS–NS | 15 | 17,741 | 4103 | < 0.001 |
| Age (years), n = 81 | 15.48 (1.2)a | –658 | 379 | 0.083 |
| Duration of illness (months), n = 79 | 7.70 (7.3)a | –858 | 507 | 0.090 |
| Baseline weight/height, n = 80 | 83.11 (10.4)a | –559 | 458 | 0.222 |
Discussion
Across all pathways, inpatient admissions are the main drivers of costs. The composition of total costs is slightly different in the S–S, in which individual and family treatments combined account for almost 45% of costs and the proportion of inpatient admissions is lower.
The service use patterns are reflected in the total costs, with the S–S incurring the lowest total costs and having the highest proportion of cases with good outcome, although only the cost difference is statistically significant. Although there is a difference in costs, caution is needed when interpreting this finding. Given the small sample size and limited data available, in addition to the fact that data collected from London-based services are not generalisable to the rest of England, it would not be appropriate to conclude that this means that the S–S is the more cost-effective option. The data do not allow us to investigate which individual- or service-level factors might have contributed to the observed differences in service use and associated costs. In addition, there were missing data at both the service and individual levels, so that our findings should be regarded as indicative rather than definitive.
Our analysis of treatment provision indicates that a higher degree of specialisation is related to offering a wider variety of treatments, and specialist services appear to be more likely to provide a specific treatment beyond CBT, IFT and dietary advice. Again, caution is advised; given the small number of services, differences are hard to detect. However, our findings regarding the most commonly provided treatments are broadly in line with an analysis of ED services by the Royal College of Psychiatrists. 384
Although there were few differences in the type of professionals providing CBT and IFT, the probability that a psychiatrist is involved in providing CBT is significantly higher in general CAMHS with ED specialisation than in other service types. In part, this may be because generic CAMHS teams tend not to include psychiatrists. 374
Specialist skills may also be important. Specialist ED services are likely to include more staff with expertise specific to EDs, so that staff on lower pay bands (nurses, perhaps) can provide treatments that in specialist CAMHS are more likely to be provided by a psychiatrist. Also, as House360 remarks, ED specialisation in general CAMHS is often due to a consultant taking a special interest.
Although this study makes an important contribution to knowledge about the costs of ED treatment in outpatient services, the small number of services and individuals participating and the focus on one geographical area in south-east England limits the transferability of findings to other parts of the country. The analysis of predictors of treatment costs in particular should be regarded as exploratory.
Study 3: economic outcomes of anorexia nervosa in a British cohort
A number of studies as well as data from this programme have shown the importance of inpatient care in treating AN. Although there is commonly some on-site education, such long hospitalisations can mean that young people spend long stretches of time out of education. 354 It seems that, in the face of severe illness, education often comes second, although this is a concern for parents385 and seen as an important determinant of quality of life. 386
There is some evidence that the illness does not affect educational outcomes in the longer term: there was no statistically significant difference between young women with AN and their healthy co-twins 5 years after recovery from AN,387 and a greater proportion of patients admitted to hospital with AN than of controls had completed post-secondary education. 388 In contrast, Patton et al. 389 found young people with EDNOS-AN more likely than cohort members without ED to be not in education or employment. However, we are not aware of any study looking at educational outcomes controlling for other characteristics, such as parental socioeconomic status.
There is evidence to suggest that the impact of current AN on productivity in adulthood is severe. International studies found that between 21.4%388 and 35%390 of women with AN received state benefits. Long duration of inpatient treatment and psychiatric comorbidity were significant predictors of benefit receipt. It is less clear how a history of adolescent AN affects adult productivity.
Our study describes women who have a self-identified lifetime AN recorded in the BCS-70. 366 We investigate the economic outcomes associated with AN, controlling for risk factors and socioeconomic characteristics, and estimate the size of the effect.
Methods
British Cohort Study (1970)
The BCS-70366 includes over 17,000 babies born in the UK in 1 week in April 1970 and is representative of the UK population. Currently, data are available for seven sweeps up to age 38 years, so people can be tracked well into adulthood. The use of the data for this study has been registered with the Economic and Social Data Service. No formal diagnosis of ED is included in the BCS-70, but, at age 30 years, there is a set of questions about self-reported lifetime ED, age of onset and type of ED.
Inclusion criteria and comparison group
Participants were included if they had answered the question about lifetime ED at age 30 years and reported either AN only (anorexia group) or no eating problems (comparison group). Given that very few males reported AN, analysis by gender was not possible and males were excluded from the analysis.
Data analysis
Given the low population prevalence of AN and the often very detailed categories of outcomes recorded in the BCS-70,366 categorical outcomes were summarised into dummy variables to facilitate analysis and differences in outcomes were tested for statistical significance using Fisher’s exact test, which allows statistical tests for cells with a count < 5.
We created a propensity score predicting the probability that a participant would report AN based on risk factors of lifetime AN identified by Nicholls and Viner. 391 This included frequent feeding problems in infancy, maternal psychological morbidity (Malaise score), separation from the mother for more than 1 month at age 5 years, and child and maternal BMI, undereating, high self-esteem, conduct, hyperactivity and attention problems (teacher-report) at age 10 years.
We then fit logistic regression models – adjusting for the propensity score – to estimate the effects of AN on the following adult outcomes at age 30 years: economic activity (active vs. not), employment-based social class (class I/II vs. lower); benefit receipt (yes/no) and weekly income. We also identified the highest level of educational attainment at age 34 years. The models were estimated using the logit command in Stata 12, with the vce(cluster) option to reflect the clustering within individuals across several time points typical for longitudinal data, and controlling for risk factors of AN (propensity score). The difference in weekly income was estimated using a generalised linear specification with a gamma family and log-link. Missing values were accounted for using MI with chained equations and 20 imputations.
Results
Sample
A total of 116 participants reported lifetime AN. Of these, 101 reported AN only, and 96 were women. In the comparison group, there were 5449 participants. Figures 15 and 16 show the distribution of father’s or mother’s social class at birth of the cohort member (depending on availability) and level of maternal education at age 5 years. There were no statistically significant differences between those with and without AN.
FIGURE 15.
Father’s/mother’s social class at birth.
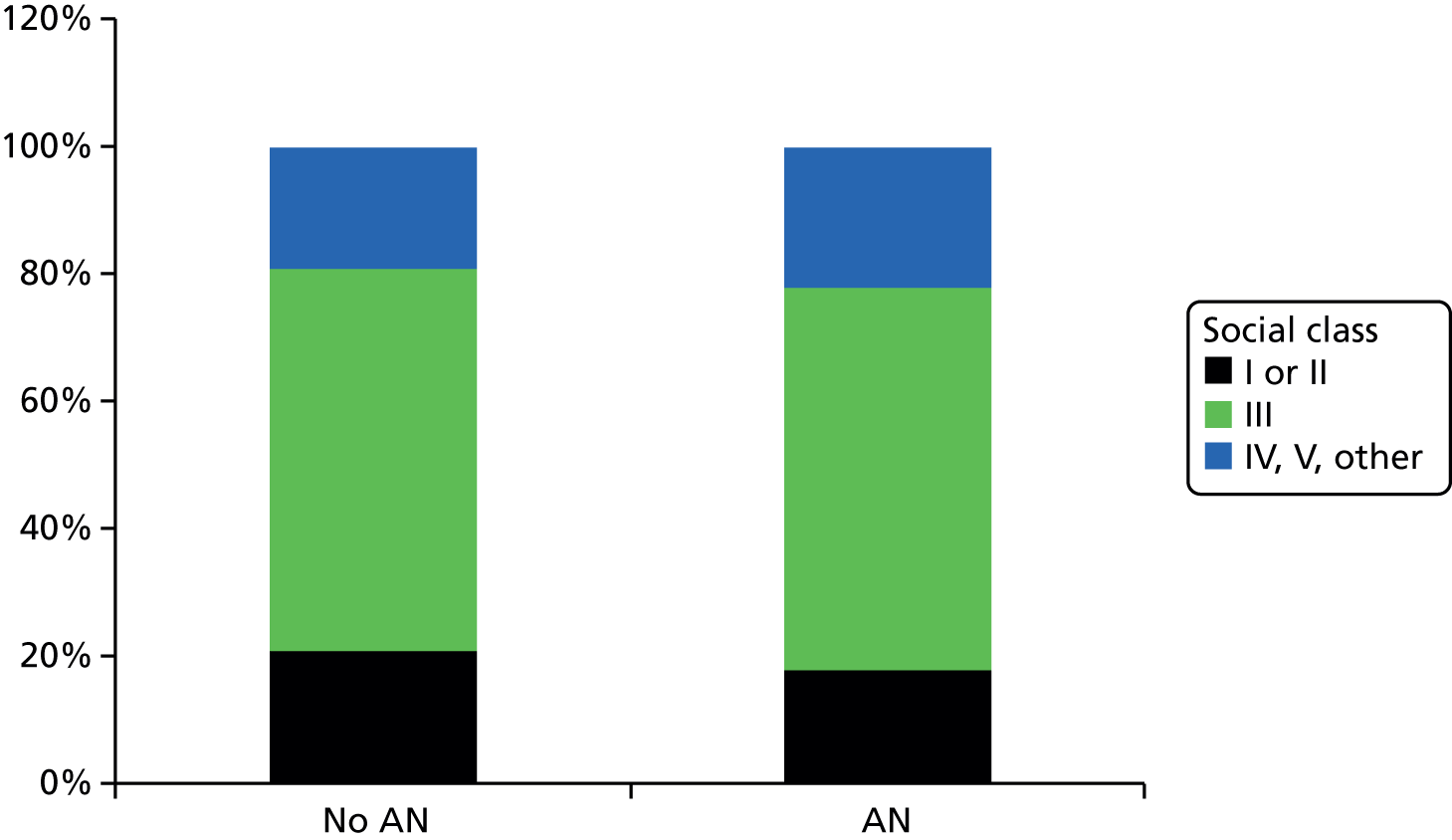
FIGURE 16.
Maternal education at age 5 years.
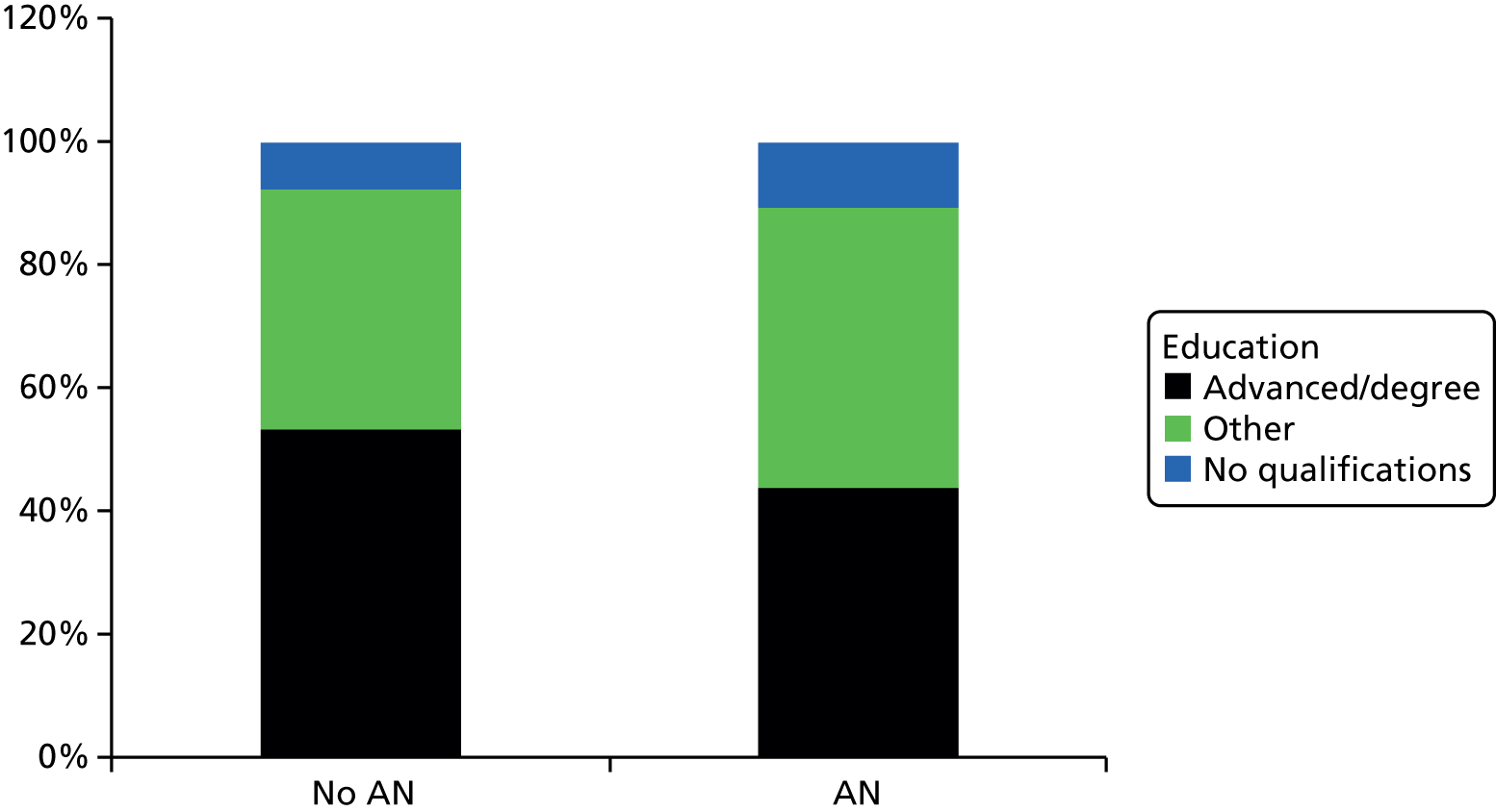
Economic outcomes of anorexia nervosa
Table 46 tabulates economic outcomes in adulthood for those with and without AN. ‘Economically active’ was defined as participation in paid employment or in other activities relating to skills and human capital development. There is a statistically significant difference between those with and without AN for three of the eight outcomes. The statistically significant difference in receipt of income-related benefits reflects the higher probability of those with AN receiving income support (17% vs. 8%) and housing benefit (17% vs. 10%). Mean weekly income at age 30 years was £298 for those with and £279 for those without AN, a non-significant difference.
| Economic outcome | No AN | AN | ||
|---|---|---|---|---|
| % | n | % | n | |
| Economically active vs. not | 76 | 5449 | 72 | 96 |
| Employed | 74 | 5449 | 69 | 96 |
| Sick/disableda | 2 | 5449 | 10 | 96 |
| Full-time work | 51 | 5449 | 48 | 96 |
| Class I or IIa | 40 | 4033 | 54 | 65 |
| Degree vs. nota | 29 | 5449 | 35 | 96 |
| Receiving income-related benefitsa | 14 | 5449 | 22 | 96 |
| Family-related benefits | 53 | 5449 | 50 | 96 |
Table 47 shows the OR for each outcome, adjusting for AN risk factors (the propensity score). Although there were some significant results, the overall models explain very little variation as expressed by the pseudo R2 (final column). Those reporting AN were 6.25 times as likely to be long-term sick or disabled. They were also more likely to be in a high social class (I or II; 1.85 times as likely), and have a degree (twice as likely), although these differences were significant at the 90% level only. For those in employment, there was no difference in weekly income.
| Outcome | Age measured (years) | OR (AN) | p-value | Pseudo R2 |
|---|---|---|---|---|
| Long-term sick/disableda | 30 | 6.25 | < 0.001 | 0.016 |
| Employed | 30 | 0.94 | 0.831 | < 0.001 |
| Social class I or II if employedb | 30 | 1.85 | 0.089 | 0.002 |
| Receives income-related benefits | 30 | 1.01 | 0.970 | < 0.001 |
| Has a degreeb | 34 | 2.06 | 0.085 | 0.003 |
Discussion
We found that, among those surviving into adulthood, a lifetime occurrence of AN does not appear to affect employment prospects, despite the high hospitalisation rate for treatment. The information about the highest level of education provides useful contextual information; Table 46 shows that 35% of the women in the BCS-70 with lifetime AN had a degree, compared with only 29% of those without AN. 366 However, as highest level of education was available for age 34 years, it could not be used when estimating the probability of being employed at age 30 years. Having a degree probably increases the chance of employment, leading to the expectation that employment rates should be higher for people with AN. Unfortunately, this relationship cannot be investigated with the data at hand. Similarly, given the higher level of education, a higher weekly income would be expected for those with AN.
In addition to these structural problems, there are issues with data reliability concerning the wage variable. These have been addressed by Dearden et al.,392 but some unlikely values remain. Although concerns are sometimes raised about the reliability of self-reported diagnoses, simple questions such as the ones used in the BCS-70 have been shown to be as good as more elaborate screening instruments in identifying EDs in community samples. 366,393
The findings from this analysis add to the scant evidence base on the economic circumstances of people with AN and the potential adult consequences of a severe disorder. As more data ‘sweeps’ from the large cohort surveys become available, the relationship between educational attainment, employment and income for those with lifetime AN can be investigated further.
Study 4: societal costs of anorexia nervosa
The final analysis presented here brings together the various strands of WP7b. We draw on our review of existing literature, data collected alongside the RCTs and the Care Pathways Study as well as our analyses of the BCS-70 to present an estimate of the annual costs of AN for England. 366
A recent review of the societal costs associated with EDs and the cost-effectiveness of treatments367 identified several international cost of illness studies, but none included a comprehensive estimate of outpatient service use or wider service use by patients with AN. An early study of the cost of EDs in the UK394 adopted a health service provider perspective and used data from the third National Survey of Morbidity in General Practice and the Hospital Inpatient Enquiry to estimate service use in general practice, inpatient bed-days and prescriptions. Outpatient treatment and intangible costs were not included. The total cost to the NHS was estimated at £4.2M per annum.
The King’s Fund395 estimated the service cost and lost employment due to AN in the UK. Based on the Hospital Episode Statistics (HES), the cost of inpatient care was estimated to be £2.5M for people under age 15 years and £8M for people aged 15–34 years. The cost of outpatient treatment was derived assuming that only 34.4% of all people with AN are in contact with mental health services (following Hoek and van Hoeken396), and that outpatient costs are 41% of inpatient costs (following Striegel-Moore et al. 397), or £4.4M. Lost employment was calculated on the basis that 1830 people received Incapacity Benefits for EDs. Assuming a weighted annual salary of £19,051, the annual cost of unemployment was estimated at £33M. The total cost was £48M per annum, with 69% due to lost productivity.
More recently, the charity Pro Bono Economics estimated the annual cost of EDs to be between £1.26B and £9.6B. 75 This estimate does not distinguish between different types of EDs and estimates are to a large extent based on the previous work by The King’s Fund, which in turn uses cost ratios from international research to estimate cost categories in which English data are lacking. The estimate also includes a burden of disease figure of £950M in the lower-cost scenario, so that only approximately £80M are due to increased health-care costs and £230M due to productivity losses.
Our study endeavoured to reduce the reliance on international figures to reflect the structural idiosyncrasies of the English health-care context more accurately. We present a conservative estimate based on publicly available data such as the HES and benefit data from the Department for Work and Pensions (DWP). In addition, we present a high-cost scenario, incorporating assumptions and results from the literature and the ARIADNE studies. This second estimate includes potential additional admissions and outpatient contacts due to AN recorded under different diagnoses, family (out of pocket or insuranc-based) expenditure on private sector inpatient provision as well as accident and emergency (A&E) visits, primary care costs and an estimate of YPLL. Given the limited availability of reliable data sources, we combine top-down and bottom-up estimates.
Methods
Prevalence of anorexia nervosa
The likely prevalence of AN by age group was estimated based on recent analyses of incidence in the General Practice Research Database,398 and parameters for the average duration399 and mortality72 rates from AN based on a review of the recent literature using the freely available DISMOD II software [Epigear, Sunrise Beach, Australia, URL: www.epigear.com/index_files/dismod_ii.html (accessed 3 July 2017)].
Costs of anorexia nervosa
The assumptions and data sources used to calculate the societal costs of AN are shown in Table 48. Further details can be found in Appendix 3, Assumptions and data sources regarding societal costs of anorexia nervosa.
| Parameter | Assumptions |
|---|---|
| ED inpatient costs children | |
| ED inpatient costs adults | |
| Additional inpatient costs |
|
| Privately funded treatment | |
| Outpatient costs children | |
| Outpatient costs adults | |
| Additional outpatient costs |
|
| A&E |
|
| GP costs |
|
| Wider primary care costs |
|
| Benefit receipt (lower) |
|
| Benefit receipt (higher) |
|
| YPLL |
|
Results
Table 49 shows the results of our DISMOD II analysis, estimating a prevalence of approximately 12,000 cases. Combining these results with an analysis by Harbottle et al. ,402 we estimate that 9000 life-years are lost to AN each year (discounted to present value; see Table 49). Table 50 shows our low and high estimates for 2010/11. Our low estimate shows the annual cost of AN in England to be £45M. At £230M, our high estimate is five times the low estimate, with the value of YPLL absorbing around 60%. In our conservative estimate, nearly 75% of costs are due to inpatient treatment (Figure 17). Outpatient treatment accounts for only 3% or 4% of all health-care costs. In our high estimate, outpatient treatment accounts for 29% of health-care costs (Figure 18).
| Age group (years) | Incidence | Number of new cases | Prevalence | Total cases | % of total cases |
|---|---|---|---|---|---|
| 10–14 | 24.0 | 359 | 46.1 | 690 | 6 |
| 15–19 | 47.6 | 773 | 146.6 | 2379 | 20 |
| 20–24 | 18.9 | 337 | 169.7 | 3021 | 25 |
| 25–29 | 18.9 | 346 | 137.4 | 2516 | 21 |
| 30–34 | 3.0 | 53 | 92.7 | 1636 | 13 |
| 35–39 | 3.0 | 53 | 49.9 | 882 | 7 |
| 40–44 | 1.1 | 22 | 27.7 | 543 | 4 |
| 45–49 | 1.1 | 22 | 15.3 | 301 | 2 |
| 50–54 | 0.0 | 0 | 7.8 | 135 | 1 |
| 55–59 | 0.0 | 0 | 3.1 | 47 | 0 |
| 60–64 | 0.0 | 0 | 1.2 | 19 | 0 |
| 65–69 | 0.0 | 0 | 0.4 | 5 | 0 |
| 70+ | 0.0 | 0 | 0.1 | 1 | 0 |
| Parameter | Conservative estimate (£) | High estimate (£) |
|---|---|---|
| ED inpatient costs: adults | 20.9M | 20.9M |
| ED inpatient costs: children | 12.7M | 12.7M |
| Additional inpatient costs | – | 9.7M |
| Privately funded treatment | – | 1.6M |
| Outpatient costs: adults | 860,000 | 13.7M |
| Outpatient costs: children | 610,000 | 8.1M |
| A&E | – | 400.000 |
| GP costs | 5M | – |
| Wider primary care costs | – | 7M |
| Benefit receipt (lower) | 4.9M | – |
| Benefit receipt (higher) | – | 10.4M |
| YPLL | – | 140M |
| Total costs | 45M | 230M |
FIGURE 17.
Distribution of costs, conservative estimate.
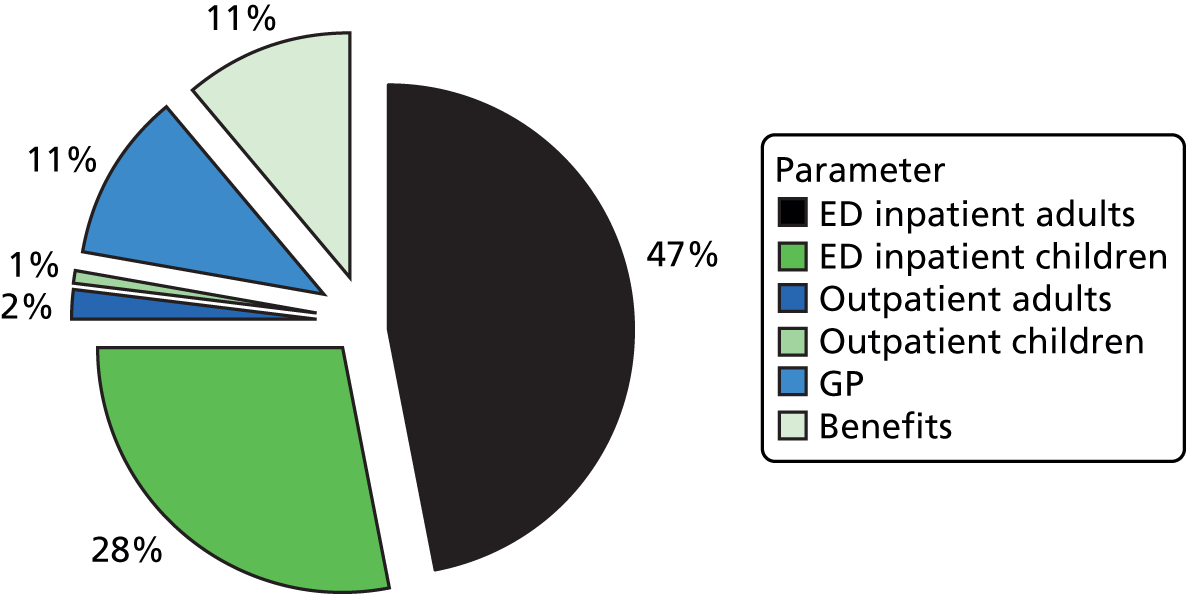
FIGURE 18.
Distribution of costs, high estimate.

Discussion
Any study of the social costs of AN is currently limited by poor data availability, in part due to the small number of cases. Assumptions are therefore needed to come up with reasonable estimates. Although there are many potential points of contention in our assumptions, a few are likely to have a significant impact on results and are worth discussing.
It is unclear how many people with AN receive treatment and in what setting. Our assumption focuses on the main treatment setting, but, in reality, there will be overlaps, with people admitted for inpatient treatment who previously or subsequently receive outpatient treatment, and who may have concurrent input from their GP.
Moreover, it is difficult to account for undetected cases. The ‘true’ prevalence of AN may be two to three times as high as estimated from either self-report or within services,403 and it is unclear what the cost implications of this may be. Similarly, we are unable to estimate costs related to subthreshold AN or EDNOS-AN, as few data are available; there is a tendency to report research findings without distinguishing EDNOS-AN and EDNOS-BN.
Estimating the value of benefits paid as a result of AN is difficult despite the availability of DWP data. Although the majority of claimants received benefits for ≥ 5 years, we may overestimate benefits because the data do not show how many people start or stop claiming benefits within a quarter.
Finally, there are uncertainties surrounding the data from HES. Data are available by diagnosis, but admissions linked to AN may happen for various reasons, such as cardiac problems, self-harm or other medical problems. Furthermore, the average length of stay reported in HES is much lower than that reported by a recent Royal College of Psychiatrists survey of ED services in the UK,384 which reported a length of stay of over 18 weeks, and a recent study on the duration of stay in UK specialist ED units reported an average length of stay of 26 weeks for adults and of 29 weeks for adolescents. 219 One possible explanation is that HES conflates stays in psychiatric or ED units (typically for weight restoration or other mental health concerns such as self-harm) and stays in medical or paediatric units often linked to acute medical issues, which tend to be much shorter. In addition, HES data may not be entirely reliable. 404 To account for this, we assumed that inpatient treatment may be much more frequent that the diagnosis-based data suggest in our high-cost scenario. Given that a high proportion of AN cases are likely to be treated on an outpatient basis, and given that the number of sessions required is generally high, the small contribution of outpatient costs in our conservative estimate is surprising, and it is possible that there are again issues with the underlying data. We therefore estimated potential additional costs in our high-cost scenario.
It is difficult to compare our estimate with the recent work by Pro Bono Economics75 because it did not distinguish costs by type of ED. However, some differences are due to different unit costs applied to incidents of service use and differences in data sources. For example, while HES showed around 8000 outpatient contacts for 2010/11, the Pro Bono Economics estimate cites unpublished data suggesting that the number may be much higher (18,000). Other differences arise from the way in which private sector health-care costs were treated. Although HES reports data on both NHS beds and NHS-commissioned private sector services, the Pro Bono Economics estimate assumed that the HES data referred only to NHS beds, thus arriving at a much larger figure for additional private costs (£45M vs. £1.6M).
Two areas of costs outside the public sector should also be mentioned. Although our study of the BCS-70366 was unable to show an impact on earnings in adulthood owing to a lack of data, there is reason to believe that AN is associated with productivity losses both from absenteeism (time taken off due to illness) and presenteeism (lower productivity when at work due to illness), and that the impact may be large. Although no estimate of the reduction in productivity associated with AN is available, Goetzel et al. 405 reported an average impairment of daily productivity due to depression, sadness or mental illness of 10.7%. Moreover, with an average length of stay of over 50 days, the impact of hospitalisation on the ability to attend work is clearly severe. These reductions in productivity probably affect not only patients, but also carers and partners, who often experience high levels of psychological distress, depression and anxiety. 198
Informal care costs have also been excluded from our model. It is provided to people with AN by parents, carers and partners. The analysis of data collected in the CASIS study (see Chapter 6) shows that up to three-quarters of carers spent nearly a full day per week providing ED-related care,406 with potential impacts not only on their health and well-being but also on their capacity to engage in paid employment.
Although we attempted to integrate findings from the ARIADNE studies into our estimate, the limited data available did not allow us to reliably estimate total costs by ethnicity, gender and ED severity, although we did attempt to distinguish costs by broad age group when possible. As more data from the ARIADNE studies and the large cohort studies become available, it may be possible to address the gaps in our estimate highlighted here.
Conclusions
Although the research agenda on the economics of AN remains long, findings from this programme add considerably to the small evidence base for treatment and support of people with AN in England.
What services and treatments do people with anorexia nervosa use?
To address this question we identified services used at baseline by participants in three ARIADNE trials: CASIS, MOSAIC and iMANTRA. There are two central conclusions from our analyses. First, service use is linked to the recruitment pathway of each study. The iMANTRA group, for example, was recruited from an inpatient population; thus, all of the participants had been in hospital prior to the baseline interview. As hospital admissions tend to be quite extended for people with AN, use of any other services was low. It is, therefore, important to use such data with caution when making inferences about service use for the wider group of people with AN.
Our second conclusion comes from the CASIS and MOSAIC studies, in which participants used a wide range of services and supports. Along with previous studies, we find that inpatient admission is often the mainstay of treatment for AN, but these studies show that outpatient clinics and mental health professionals all provide important components of a treatment package, as does primary care, with more than four-fifths of these two samples seeing the GP about their ED and between one-quarter and a half seeing their practice nurse. Community, self-help and community services all played a part in supporting a small but significant minority of participants.
What are the unit costs of various eating disorder treatments?
Our analysis of the unit costs of ED treatments comes from the Care Pathways Study. The research identified treatments delivered to patients in this cohort from case notes, rather than solely relying on reports from services about what they could provide. CBT and IFT were the most common treatments. Mean per session unit costs for treatment varied considerably. Average unit costs were lowest in the specialist ED services (£136 for CBT and £205 for IFT), with IFT unit costs similar for the CAMHS with ED specialisation and generic services (approximately £245). Unit costs are sensitive to the number and profession of staff delivering the intervention, as well as duration of the session and whether the intervention is provided in a group or individual setting.
What are the costs associated with service use by people with anorexia nervosa?
For the three trials analysed for this chapter mean costs varied, reflecting the recruitment path. However, the range was wide in all studies indicating different intensities of service response. The lowest 6-monthly cost was just £138 (MOSAIC study participant) and the highest was £224,025 (CASIS participant). In all three studies, hospital services absorbed the highest proportion of total costs – between 69% and 99% of total costs.
A similar wide range can be seen for the participants in the Care Pathways Study, which focused solely on health service treatment for AN. The lowest annual cost was £700 and the highest was £169,000.
Do costs vary by participant characteristics?
The large variation in costs warrants further investigation. Our analysis of cost variations in the MOSAIC, CASIS and Care Pathways Study data suggests that previous treatment history (perhaps a marker for severity or chronicity) is associated with costs, alongside age, first language other than English and comorbidity. However, none of these equations explained a large proportion of the cost variation and much, as suggested by the findings of previous studies,24,384 may be related to service availability and pathways to treatment.
What are the economic consequences of anorexia nervosa?
Our study of the BCS-70366 suggests that people with lifetime AN who survive into adulthood have a higher probability of being long-term sick or disabled, of having completed a degree and of being in a high social class if employed. No differences in weekly income could be found, although this analysis was not able to control for highest level of education, severely limiting the conclusions from the study.
What are the annual costs of anorexia nervosa for England?
Our final set of analyses brought together findings from the literature, the three trials, the Care Pathways Study and the BCS-70366 to estimate the societal costs of AN. Our conservative lower-bound estimate suggests an annual cost of AN in England of around £45M. Seventy-five per cent of this is for inpatient treatment, despite the growing use of specialist outpatient clinics. Primary care and receipt of social security benefits account for a further 10% each. Our high estimate is five times higher at £230M per annum, with more than 60% absorbed by the value of ‘years of life lost’.
Chapter 12 Overall discussion and conclusions
This research programme focused on AN, a perplexing and frightening illness affecting mainly young females. In seven integrated WPs it aimed to (1) develop and test tools for ED prevention and early intervention in schools; (2) develop and test targeted, disseminable and cost-effective treatments (first line, adjuncts to inpatient refeeding and relapse prevention) for adults with AN, across the spectrum of illness severity and based on empirical models of AN grounded in clinical neuroscience; (3) develop and test targeted, disseminable and cost-effective interventions for carers of adults with AN; (4) better understand perplexing core symptoms of AN (hyperactivity and its psychological and biological correlates) with the aim of informing future intervention development; (5) better understand the needs of particular populations (i.e. mothers with an ED and their offspring); (6) optimise care pathways for young people with AN; and (7) identify the costs of services used by people with AN and estimate the annual cost of AN for England. Throughout we worked with patients and carers to achieve these aims.
Below, we discuss key findings emanating from the integrated WP in relation to these aims and assess their clinical and research implications. Rather than discussing each WP separately, when appropriate, we have grouped WPs together. A list of the main clinical and research implications emanating from the WPs is also provided in Boxes 1 and 2.
-
The feasibility study in WP1a indicates that, in schools, staff knowledge, attitudes and confidence towards EDs can be improved by brief face-to-face training. Following further effectiveness testing, detection and management of EDs in a school setting may be improved by making this training available to school staff.
-
WP1b shows that it is feasible to train school teachers to deliver efficacious preventative interventions for EDs. How to develop interventions with sufficient flexibility to manage a range of school environments needs further examination.
-
The RCT in WP2a shows that at 6 and 12 months post randomisation both psychological treatments tested (MANTRA and SSCM) significantly improve key clinical outcome measures in outpatients with AN. Patients see MANTRA as more acceptable and credible. Further follow-ups are needed to determine maintenance of treatment effects in the longer term. However, based on available evidence, both treatments can be considered as efficacious first-line outpatient treatments for adults with AN.
-
WP2b demonstrates that CREST is an acceptable therapy, valued by patients and clinicians; however, the addition of CREST to TAU is not superior to TAU alone. Further work is needed to establish the benefits of adding CREST as an adjunct to inpatient care.
-
The RCT in WP3 shows that addition of an intervention for carers of people with AN to standard inpatient treatment reduces carer time caregiving, burden and unhelpful caregiving behaviours. Patients being cared for by those receiving the intervention show reduced ED psychopathology and improved quality of life. Sharing skills and information with family members is of benefit for patients and carers.
-
The observational study in WP4 shows that objectively measured activity levels are not higher in AN; it may, however, be problematic because of their poor health. Patients with AN have an increased drive to exercise that is linked to ED pathology and a desire to improve mood. Reducing this drive to exercise should be a treatment objective.
-
The pilot trial in WP5 indicates that iMANTRA is a feasible and safe intervention, which may have promise in the aftercare of inpatients with AN.
-
WP6 focused on the special population of mothers with EDs. The findings show that EDs are common in pregnancy and are associated with unplanned pregnancies and fertility treatment. In addition, children of mothers with an ED are at risk of growth difficulties and disordered eating patterns. Continuity of care from pre-conception to the postnatal period is paramount for women with an ED and interventions designed for this special population may benefit them and their children.
-
The assessment of care pathways in WP7a shows that there are clear benefits in having specialist community-based outpatient services which are easily accessible directly from primary care as they provide good clinical outcomes with markedly lower rates of hospital admissions (and therefore are at considerably lower costs) and better continuity of care than generic services can deliver. Training in evidence-based treatments should focus on developing skills in specialist multidisciplinary teams.
-
Finally, the economic analyses in W7b demonstrate that AN is associated with a high risk of adult disability. Effective prevention and early intervention to prevent long-term disability are therefore likely to provide significant patient benefit and cost savings. Although inpatient treatment is the largest contributor to treatment costs, participants with AN accessed a wide range of services (primary care, self-help, community services). There may be scope to develop collaborations with community-based services to improve early identification and ensure appropriate treatment.
-
To carry out a fully powered stepped wedge study to test the effectiveness of the school staff training programme developed in WP1a. This research should develop and incorporate objective measures of staff knowledge and confidence and also demonstrate the impact of the intervention on student outcomes.
-
To conduct a large-scale cluster RCT of the teacher-delivered prevention programme developed in WP1b to determine effectiveness of this programme in a range of school environments. This should involve the programme being evaluated for boys as well as girls.
-
To gain longer-term (2-year) follow-up data from the RCT in WP2a, which are essential to determine the relative efficacy of the two psychological treatments evaluated and the maintenance of treatment gains. Given the manual-based nature of MANTRA a future study of recent onset/first episode cases of AN may be useful.
-
To conduct a RCT to explore benefits of CREST (examined in WP2b) in comparison with other manualised treatments of similar length. A larger study of the group format of CREST will be also desirable to consolidate preliminary findings.
-
To examine whether or not adding the carer intervention evaluated in WP3 to standard outpatient care improves outcomes. Another step will be to examine whether or not adding a more intensive family intervention (workshops) improves inpatient care.
-
To conduct a replication of the work in WP4 in a larger study in patients with AN, using new actimetry and physiological measures (e.g. internet based). Studies should test treatment interventions that target the drive to exercise and seek to understand its origins and nature. Finally, studies should investigate how graded exercise might be introduced as a means of reducing anxiety and improving affect.
-
To carry out a large-scale RCT of iMANTRA (piloted in WP5), with economic analyses, longer-term follow-ups and regular assessments to explore detailed relapse/recovery trajectories. A further study exploring the efficacy of iMANTRA using facilitators that have an established therapeutic relationship with the patient would be helpful to determine if this improves outcomes.
-
To develop and test a tailored intervention for pregnant women with EDs. In addition, following the children of women with EDs into adolescence will be essential in exploring the intergenerational risk of an ED.
-
To evaluate the role of specialist ED services beyond the metropolitan London context using a larger-scale study. Further research is also needed to explore the barriers to identifying adolescents suffering from BN, who are poorly identified even in specialist areas, and a high proportion do not receive timely treatment.
-
To conduct a longitudinal study investigating the impact of AN on education, employment and potential earnings differential. It will also be helpful to gather longer-term follow-ups of clinical trials with accompanying economic evaluation to better reflect the longer-term costs of treatment and allowing us to estimate incidence-based costs of AN.
Key findings and their clinical and research implications
Eating disorder prevention and early intervention in schools (work packages 1a and 1b)
A 1-day training programme on early detection and strategies for managing ED in schools resulted in an improvement in the self-reported knowledge, attitudes and confidence of school staff in identifying and managing EDs in an uncontrolled, feasibility study (WP1a). Second, a teacher-delivered intervention was feasible and improved risk factors for EDs (body dissatisfaction, thin-ideal internalisation and self-esteem) in adolescent girls in a cluster randomised trial (WP1b).
Together these studies indicate that teachers and other key school staff can be trained to deliver efficacious preventative interventions for EDs in schools and to feel more knowledgeable, more positive and more confident in relation to identifying and supporting students with an ED. Of note, in both studies, training was brief and fitted in with school staff’s work schedules. More research is needed on these interventions before they are widely disseminated. The teacher training programme outlined in WP1a could best be tested using a fully powered stepped wedge design,407 rather than a RCT. Such a study should develop and incorporate objective measures of staff competence in dealing with common ED scenarios relevant to school settings and also demonstrate the impact of the intervention on student outcomes. The teacher-delivered prevention programme developed in WP1b should be tested in a large-scale, adequately powered cluster RCT to determine the effectiveness of this programme in a range of school environments. This should involve the programme being evaluated for boys as well as girls. Additionally, future research might assess the combination of both interventions in a ‘whole school approach’ to prevention and early detection and management of EDs.
Development and testing of targeted, disseminable and cost-effective treatments (first-line, adjuncts to inpatient refeeding and relapse prevention) for adults with anorexia nervosa (work packages 2a, 2b and 5)
First, a multicentre trial of two first-line outpatient psychological interventions (MANTRA and SSCM) found that both psychological therapies significantly improved ED and other clinical outcomes in outpatients with AN. ED outcomes compare well with those from the only other large-scale RCT in a similar population. 131 Of note, patients significantly preferred MANTRA to SSCM and found it more credible (WP2a). Second, in a quasi-experimental study in inpatients with AN, the addition of CREST to TAU was acceptable with perceived benefits by patients, but it showed no benefits (in relation to EDs or other clinical outcomes) compared with TAU (WP2b). Third, a feasibility RCT in AN patients discharged from inpatient treatment, compared TAU plus an e-mail-guided relapse prevention programme (iMANTRA) with TAU alone, and suggested that there are treatment effects of a medium size in relation to a higher BMI and to lower distress in the experimental group at 12 months after discharge (WP5).
Findings from the large-scale RCT in WP2a suggest that both MANTRA and SSCM can be recommended as first-line outpatient therapies for adults with AN, with the caveat that currently longer-term outcomes for both of these treatments are unknown. Future research should focus on obtaining longer-term (2- and 5-year) follow-up data from the RCT, to determine the relative longer-term efficacy of these two psychological treatments. Moreover, mediators and moderators of outcome should be evaluated. To ensure adequate sample size for mediator and moderator analyses, data from the present study can be combined with those from an earlier pilot RCT. 136 Furthermore, given the manual-based nature of MANTRA (including lots of information, education and skills training), a future study evaluating this approach in recent onset/first episode cases of adults with AN may be useful. Finally, MANTRA might also be a useful adjunct to family-based treatments for adolescents with AN.
Preliminary evidence from WP2b suggests that CREST is an acceptable therapy that is valued by patients and clinicians. The preliminary trial in WP5 indicates that iMANTRA is a feasible and safe intervention that may have promise in the aftercare of inpatients with AN. Thus, both of these interventions should be evaluated further.
For CREST, the next step would be to conduct a RCT to explore benefits of this intervention in comparison with other manualised treatments of similar length. A larger study of the group format of CREST will be also desirable to consolidate preliminary findings.
For iMANTRA (piloted in WP5), the next step would be to carry out a large-scale RCT including health economic analyses, longer-term follow-ups and regular assessments to explore detailed relapse/recovery trajectories. A further study exploring the efficacy of iMANTRA using facilitators that have an established therapeutic relationship with the patient would be helpful to determine if this improves outcomes.
Development and testing of targeted, disseminable and cost-effective interventions for carers of adults with anorexia nervosa (work package 3)
The large-scale, multicentre RCT in WP3 shows that the addition of a psychoeducational and skills sharing intervention (ECHO) for carers of people with severe and/or enduring AN to standard inpatient care reduces carer time spent caregiving, burden and expressed emotion. In addition, patients had reduced ED symptomatology and improved quality of life at 6 months. These findings show that sharing skills and information with family members benefits carers and patients.
Future research should examine whether or not adding the carer intervention evaluated in WP3 to standard outpatient care for adults with AN improves outcomes. Another step will be to examine whether or not adding a more intensive family intervention (workshops) improves inpatient care.
Improved understanding of hyperactivity and its psychological and biological correlates in anorexia nervosa (work package 4)
Key findings from this observational study are that people with AN report that they engage in higher levels of activity than HCs or people with an anxiety disorder, but that an objective measure (actimetry) shows actual activity levels are similar across in- and outpatients with AN, anxiety disorder sufferers and HCs. The study did find that the drive to exercise is significantly higher in AN patients than in HCs, and that it is tied to ED pathology and a desire to improve mood.
The difference between actual and perceived levels of activity in people with AN may reflect the fact that their pathological drive to exercise is resulting in them attempting to exercise even when they are physically compromised by illness (i.e. at a time when they have significantly less skeletal muscle mass than the control participants). Levels of activity did not appear to be related to endocrine measures, such as plasma leptin or salivary cortisol, or to daily average temperature (i.e. activity is not driven by a desire to keep warm). Taken together with other recent evidence, these findings suggest that exercise is driven and rewarding to sufferers with AN. 408 Studies should examine the nature and origins of the increased drive to exercise in AN. Finally, clinicians should develop interventions that address the pathological drive to exercise rather than exercise per se.
Improved understanding of the needs of mothers with an eating disorder and their offspring (work package 6)
Work package 6 focused on a special population: mothers with an ED. Key findings of studies in this WP were that EDs are common in pregnancy and they are associated with unplanned pregnancies and fertility treatment. In addition, children of mothers with an ED are at risk of growth difficulties and disordered eating patterns. A key implication of these findings is that continuity of care from pre-conception to the postnatal period is paramount for women with ED. Findings from this study have provided data for future intervention development. Future research should focus on developing and testing a tailored intervention for pregnant women with EDs, focusing, for example, on giving them information and skills on how to best manage their own nutritional needs during and after pregnancy and the nutritional needs of their offspring in utero and after birth. In addition, following the children of women with EDs into adolescence will be essential for exploring the intergenerational risk of EDs.
Optimising care pathways for young people with anorexia nervosa (work package 7a)
The assessment of the Care Pathways Study in WP7a showed that direct access to specialist ED services for young people with AN was associated with higher referral rates, lower admission rates, greater consistency of care and greater user satisfaction. Thus, there are clear benefits in having specialist community-based outpatient services which are easily accessible directly from primary care. They provide good clinical outcomes with markedly lower rates of hospital admissions (and therefore are at considerably lower costs; see Chapter 11), and better continuity of care than generic services can deliver. Training in evidence-based treatments should focus on developing skills in specialist multidisciplinary teams.
Future research should evaluate the role of specialist ED services beyond the metropolitan London context using a larger-scale study. Further research is also needed to explore the barriers to identifying adolescents suffering from BN as they are poorly identified even in specialist areas, and a high proportion do not receive timely treatment.
Estimating the costs of services used by people with anorexia nervosa and the annual cost of anorexia nervosa for England (work package 7b)
A key finding from this WP is that being treated in specialist services is associated with lower costs. Second, the annual costs of AN in England are estimated at between £45M and £230M. Moreover, the economic analyses in WP7b demonstrate that AN is associated with a high risk of adult disability. Effective prevention and early intervention to prevent long-term disability are therefore likely to provide significant patient benefit and cost savings. Although inpatient treatment is the largest contributor to treatment costs, participants with AN accessed a wide range of services (primary care, self-help, community services). There may be scope to develop collaborations with community-based services to improve early identification and to ensure appropriate treatment. Future research should include a longitudinal study investigating the impact of AN on education, employment and potential earnings differential. It will also be helpful to gather longer-term follow-ups of clinical trials with accompanying economic evaluation to better reflect the longer-term costs of treatment and to allow us to estimate the incidence-based costs of AN. Caution should be used when interpreting results that are based on small sample size, as they may not be generalisable.
Overall strengths of the programme
The strength of the programme is that it took a life- and illness-stage perspective on a disorder that typically starts in adolescence, but which often continues into adulthood. It was also a highly ambitious programme of great depth and breadth. It included:
-
a mixture of well-conducted large-scale adequately powered definitive trials (WP2a: MOSAIC; WP3: CASIS trial)
-
studies (including smaller RCTs, quasi-experimental designs and pre–post designs) assessing feasibility of novel interventions (WP1a: school staff training; WP1b: Me, You & Us; WP2b: CREST; WP5: iMANTRA)
-
longitudinal studies producing evidence that can form the basis for future intervention development (WP4: to reduce the drive for thinness and exercise; WP6: for pregnant women with an ED)
-
a study assessing care pathways for young people with AN (WP7a)
-
studies on the cost of AN in different settings (WP7b).
Although the programme was led by a team from one unit, many of the studies had multiple collaborating sites (WPs 1a, 1b, 2a, 2b, 3, 4, 5) from across the UK or across London (WP7a), thereby ensuring broad generalisability of findings.
Most of the WPs used mixed methods including quantitative and qualitative components. The qualitative components were used to either inform intervention development,63,65 or as process evaluations of the interventions tested. 158–160,167,409 Both approaches have been highly valuable and have added much needed detail to inform the iterative process of intervention development and testing.
The programme has yielded several manual-based interventions for prevention of, training in and treatment of AN and related EDs. Table 51 shows the interventions that have emanated from the programme.
| WP | Name | Purpose, setting and support |
|---|---|---|
| 1a | Teacher training intervention | A 1-day manualised training programme to improve teachers early detection and management of EDs in schools |
| 1b | Me, You & Us | Universal prevention programme for EDs in schools focusing on reducing established risk factors and delivered by school staff |
| 2a | MANTRA | A manualised outpatient treatment focusing on established maintenance factors for AN delivered by therapists |
| 2b | CREST | A manualised treatment focusing on emotion recognition, regulation and social skills in people with severe AN |
| 3 | ECHO | An intervention focusing on carers’ skills training and combining a self-help manual with DVDs and coaching sessions |
| 5 | iMANTRA | An online intervention to prevent relapse in severe AN following inpatient weight restoration |
Most of these interventions are grounded in our model of AN, underpinned by clinical neuroscience (WPs 2a, 2b, 3 and 5). Testing these interventions has involved using neurocognitive and social cognitive measures as outcomes, an approach that is novel in AN treatment studies.
Central to all of the interventions developed and tested here is that they subscribe to the ethos of collaborating with and sharing specialist skills and knowledge with patients, carers and school students and staff. This collaborative approach empowers patients and carers to become experts in understanding and managing their condition.
The interventions developed here can be easily disseminated within schools and the NHS either immediately, or after further research.
Overall limitations
Given the ambitious nature of the programme and the confines of available resources and time, by necessity all of the studies had to make compromises in terms of the size and nature of samples studied, the control samples, number and duration of outcome assessments, and duration of follow-ups. Details of these compromises and limitations are outlined in the relevant chapters of this report.
Conclusions
This programme has focused on development of interventions for prevention, treatment and training in AN and related EDs. The results of the programme’s studies have important implications for the management of AN (and EDs) across the full course of this disorder, from detection through to preventing relapse. Future research is essential to increase our understanding of optimal disease management for AN.
Acknowledgements
This report presents independent research funded by the National Institute for Health Research (NIHR) under a Programme Grant for Applied Research (reference number RP-PG-0606-1043). This work was also supported by the NIHR Biomedical Research Centre for Mental Health, South London and Maudsley NHS Foundation Trust, Institute of Social Psychiatry and Institute of Psychiatry, King’s College London. Ulrike Schmidt, Sabine Landau and Janet Treasure received salary support from the Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The studies in WP2a (CREST), WP2b (MOSAIC), WP3 (CASIS), WP4 (Relationship Between Overactivity, Stress and Anxiety in Anorexia Nervosa; ROSANA) and WP7a (Care Pathways) were adopted by the MHRN.
We would like to thank all the patients, families, young people and school staff who participated in the ARIADNE programme. We would also like to thank all the clinicians in participating services, who recruited and treated patients in the studies, and the researchers, who worked on this programme during its course.
We also thank our colleagues Miriam Grover, Geoffrey Wolff and Mark Allen, who were involved in the original project application.
We also thank our Programme Steering Committee: Simon Gowers, Bob Palmer, Anne Cook and Claire Walker.
Below we acknowledge the contributions of people and teams for individual WPs.
Work package 1: teacher training and prevention
We would like to send our thanks to the students and school staff who gave their time to participate in this research. We would like to acknowledge the researchers and volunteers who contributed to the studies in this WP: Olivia Breen, Katharine Damazer, Peter Musiat, Ulrike Naumann and Ilka Schober.
Work package 2a: outpatient treatment
A big thank you goes to all the patients that participated in the MOSAIC trial. We thank all the researchers and volunteers who worked on the study. These are Hannah Broadbent, Hannah Dejong, Martha Kenyon, Rachel Loomes, Anna Lose, Huma Yasin, Daniella Waterman-Collins, Charlotte Davies, and Kelly Ann Zainal. We would also like to thank the MHRN, which was instrumental in setting up the project and whose CSOs facilitated recruitment and assessments at the local sites: Sandra O’Sullivan, Charlotte Watson, Shreena Ghelani and Lorraine O’Connell.
We extend our thanks to all the lead investigators and other clinical staff in the participating services: Nicky Boughton, Linette Whitehead, Oxford Health NHS Foundation Trust; Dannielle Glennon, Nikola Kern, Eating Disorders Outpatient Service, South London and Maudsley NHS Foundation Trust; Lorna Richards, Eric Johnson-Sabine, St. Ann’s Hospital Eating Disorders Unit, Barnet, Enfield and Haringey Mental Health NHS Trust; Lucy Serpell, North East London Foundation Trust Eating Disorders Service.
We also thank all the study therapists:
North East London Foundation Trust Eating Disorders Service: Rory Harnett, Lucy Serpell, Leonora Spirou, Pjotr Waltos.
Oxford Health NHS Foundation Trust, Eating Disorders Service: Nicky Boughton, Julia Bouska, Joel Hawkins, Jill Roberts, Vanessa Stopp, Linette Whitehead, Rachel Woolrich.
St. Ann’s Hospital Eating Disorders Unit, Barnet, Enfield and Haringey Mental Health NHS Trust: Amy Brown, Kath Delaney-Wetherill, Pramjit Kaur, Mary Meneghetti, Gerry Ruane, Gemma Saunders.
South London and Maudsley NHS Foundation Trust, Eating Disorders Outpatient Service: Yemi Adeniran, Angela Brassett-Harknett, Yael Brown, Philippa East, Nicola Gilbert, Debbie Kanter, Victoria Mountford Tina Pedersen, Penny Rimer, Elaine Smith, Emma Walton.
Work package 2b: inpatient treatment
We would like to thank all the patients who took part in the CREST study and the researchers who contributed to this work: Rebecca Genders, Amy Harrison, Naima Lounes and Ulrike Naumann. We thank the clinicians who delivered CREST (Caroline Fleming, David Hambrook, Claire Money, Anna Oldershaw) and the team leader in the adult inpatient service, Lynn StLouis. We also thank the principal investigators and other clinical staff in the participating services: John Fox, Eating Disorders Service, The Priory Hospital Cheadle Royal, Manchester.
Work package 3: carers’ intervention
We thank all families that participated and the principal investigators and other clinical and research staff on the inpatient units that facilitated the study: Jon Arcelus and Sarvath Abbas, Eating Disorders Service, Brandon Unit, Leicestershire Partnership NHS Trust; Agnes Ayton, The Darwin Centre, North Staffordshire Combined Healthcare NHS Trust; Nicky Boughton, Linette Whitehead and Ian Barkataki, Cotswold House Eating Disorders Service, Oxford Health NHS Foundation Trust; Frances Connan, Vincent Square Eating Disorders Service, Central and North West London NHS Foundation Trust; Rebecca Genders, Gerald Russell Eating Disorders Unit, South London and Maudsley NHS Foundation Trust; Ken Goss, Eating Disorders Service, Coventry and Warwickshire NHS Partnership Trust; Bert Laszlo, Wonford House Hospital, Devon Partnership NHS Trust; John Morgan, Hubert Lacey, Saeideh Saedi and Bryony Bamford, Yorkshire Centre for Eating Disorders, Leeds, and St George’s University of London; Kim Moore, Kinver Centre, Eating Disorders, South Staffordshire and Shropshire NHS Foundation Trust; David Robertson, National Centre for Mental Health, The Barberry, Birmingham and Solihull Mental Health NHS Foundation Trust; Christa Schreiber-Kounine and Dominic Hiles, STEPS Eating Disorders Unit, Avon and Wiltshire Partnership Mental Health NHS Trust; and Sonu Sharma and John Fox, Eating Disorders Service, The Priory Hospital Cheadle Royal, Manchester.
We also thank the MHRN, which was instrumental in setting up the project and whose CSOs facilitated recruitment at the local sites: Emma Anderson, Carly Cooper, Andra Cosma, Shaukat Desai, Hill Finell, John Hiley, Laura Hunt, Honour Josephine, Siobhan Keogh, Sian Lison, Heather Morrison, Sandra O’Sullivan, Ramdip Sandhu, Chantal Sunter, Precina Vara, Michelle Vey, Charlotte Watson, Belinda Williams and Moira Winters.
Our great thanks go to our experienced carer coaches who gave of their time and expertise to be trained in and to deliver the coaching: Snita Ahir, Leigh Best, Yvonne Boughton, Russell Cooper, Suzi Doyle, Jocelyn de Guzman, Amy Harrison, Hannah King, Jenny Langley, Annette Martinez Phillips, Julia Popham, Esther Ritchie, Yvonne Round, Marjeneh Shahabi, Jilly Shipway, Melinda Waldrum and Clare Walker. We would also like to thank Gill Todd for her role in training and supervising the coaching team and Frankie Bishopp for her administrative role throughout the project.
Work package 4: activity and anorexia nervosa
We thank all the patients and HC participants who participated in the ROSANA study. We thank Benita Middleton, University of Surrey, for her help in analysing and interpreting activity data; Tracy Drew, King’s College London, for her help with analysis of biological samples; and Xiaohui Xu, from the Medical Research Council Social, Genetic & Developmental Psychiatry Centre at the Institute of Psychiatry, for her help with processing and storing biological samples.
We also thank the principal investigators and other clinical staff in the participating services: Frances Connan, Vincent Square Eating Disorders Service, Central and North West London NHS Foundation Trust; Peter Webster, The Priory Hospital Chelmsford.
Finally, our thanks go to the MHRN, which was instrumental in setting up the project and whose CSOs facilitated recruitment at the local sites: Shreena Ghelani, Tara Harvey and Charlotte Watson.
Work package 5: relapse prevention
We thank all the patients that participated in the iMANTRA trial.
We would also like to thank the MHRN, which helped to set up the project and whose CSOs facilitated recruitment and assessments at the local sites: Andra Cosma, Zoe Given-Wilson, Sarah Dickens and Maria Sampson.
Finally, we extend our thanks to the lead investigators and other clinical staff in the participating services: John Morgan, Saeideh Saeidi, Seacroft Eating Disorders Unit, Leeds Teaching Hospitals NHS Trust; Eric Johnson-Sabine, Matthew Cahill, St. Ann’s Hospital Eating Disorders Unit, Barnet, Enfield and Haringey Mental Health Trust; Pippa Hugo, Bryony Bamford, South West London and St. George’s Mental Health NHS Trust; Mark Tattersall, Huntercombe Hospital, Maidenhead; Hind Al Khairulla, Huntercombe Hospital Stafford; Sarah Cassar, Hannah Austin-Payne, Huntercombe Hospital, Edinburgh; and Adrienne Key, Eric Shur, Barbara Rooney, The Priory Hospital Roehampton.
Work package 6: maternal eating disorders
We thank Emma Taborelli and Amanda Bye for contributing to the research in WP6. We would like to thank Kate Northstone and the ALSPAC team. We are extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council (grant reference 74882) the Wellcome Trust (grant reference 076467) and the University of Bristol provide core support for ALSPAC.
Work package 7: service use and economic analyses
We thank Meghan Craig and Emma Porter for contributing to the research in WP7a.
We would also like to thank the lead investigators and other clinical staff in the participating services: Dasha Nicholls, Feeding and Eating Disorders Service, Great Ormond Street Hospital; Pippa Hugo, CAEDS; South West London and St. George’s Mental Health NHS Trust; and Mark Berelowitz, CAEDS, Royal Free London NHS Foundation Trust.
Finally, we would like to thank the MHRN and its CSOs, Kofi Kramo and Gaby Illingworth, who supported this study.
Contributions of authors
Professor Ulrike Schmidt (Professor, EDs) was the principal investigator for the programme and contributed to the study design, intervention development, study management, analysis and report writing across all WPs, and contributed to writing Chapters 1 and 12.
Ms Helen Sharpe (PhD Student, EDs) co-ordinated the final report and contributed to the study design, intervention development, data collection, analysis and report writing for WP1b (see Chapter 3), and contributed to writing Chapters 1 and 12.
Ms Savani Bartholdy (Research Worker, EDs) contributed to the study design, data collection and report writing for WP4 (see Chapter 7).
Ms Eva-Maria Bonin (Research Officer, Health Economics) contributed to the study design, data collection, analysis and report writing across WP2a (see Chapter 4), WP3 (see Chapter 6), WP5 (see Chapter 8), WP7a (see Chapter 10) and WP7b (see Chapter 11).
Ms Helen Davies (PhD Student, EDs) contributed to the study design, data collection, analysis and report writing for WP2a (see Chapter 5).
Ms Abigail Easter (PhD Student, EDs) contributed to the study design, data collection, analysis and report writing for WP6 (see Chapter 9).
Ms Elizabeth Goddard (PhD Student and Research Worker, EDs) contributed to the study design, data collection, analysis and report writing for WP3 (see Chapter 6).
Ms Rebecca Hibbs (PhD Student, EDs) contributed to the study design, data collection, analysis and report writing for WP3 (see Chapter 6).
Ms Jennifer House (PhD Student, EDs) contributed to the study design, data collection, analysis and report writing for WP7a (see Chapter 10) and WP7b (see Chapter 11).
Ms Alexandra Keyes (Research Worker, EDs) contributed to the study design, data collection, analysis and report writing across WP2a (see Chapter 4) and WP4 (see Chapter 7).
Ms Pooky Knightsmith (PhD Student) contributed to the intervention development, study design, data collection, analysis and report writing for WP1a (see Chapter 2).
Ms Antonia Koskina (Research Worker, EDs) contributed to the study design, data collection, analysis and report writing for WP4 (see Chapter 7).
Mr Nicholas Magill (Statistician, Biostatistics) contributed to analysis and report writing across WP2a (see Chapter 4) and WP3 (see Chapter 6).
Ms Jessica McClelland (PhD Student, EDs) contributed to data collection, analysis and report writing for WP5 (see Chapter 8).
Dr Nadia Micali (Senior Lecturer, EDs) contributed to the study design, study management, analysis and report writing for WP6 (see Chapter 3).
Ms Simone Raenker (PhD Student and Research Worker, EDs) contributed to the study design, data collection, analysis and report writing for WP3 (see Chapter 6).
Ms Bethany Renwick (Research Worker, EDs) contributed to the data collection, analysis and report writing for WP2a (see Chapter 4).
Ms Charlotte Rhind (PhD Student, EDs) contributed to the study design, data collection, analysis and report writing for WP3 (see Chapter 6).
Dr Mima Simic (Consultant Child & Adolescent Psychiatrist, EDs) contributed to the study design, study management and report writing for WP7a (see Chapter 10).
Ms Lot Sternheim (PhD Student and Research Worker, EDs) contributed to the study design, data collection, analysis and report writing for WP5 (see Chapter 8).
Dr Sabine Woerwag-Mehta (PhD Student, EDs) contributed to the study design, data collection, analysis and report writing for WP4 (see Chapter 7).
Professor Jennifer Beecham (Professor, Health Economics) contributed to the study design, study management, analysis and report writing across WP2a (see Chapter 4), WP3 (see Chapter 6), WP5 (see Chapter 8), WP7a (see Chapter 10) and WP7b (see Chapter 11).
Professor Iain C Campbell (Professor, EDs) contributed to the study design, study management, analysis and report writing for WP4 (see Chapter 7).
Professor Ivan Eisler (Professor, Family Psychology and Family Therapy) contributed to the study design, study management, analysis and report writing across WP7a (see Chapter 10) and WP7b (see Chapter 11).
Professor Sabine Landau (Professor, Biostatistics) contributed to the study design, analysis and report writing across WP2a (see Chapter 4), WP3 (see Chapter 6) and WP7a (see Chapter 10).
Mrs Susan Ringwood (Chief Executive Officer, Beat) was the PPI representative for the programme and contributed to the study design and report writing for Chapter 1.
Dr Helen Startup (Clinical Psychologist, EDs) contributed to study design, recruitment, analysis and report writing for WP5 (see Chapter 8).
Dr Kate Tchanturia (Reader, EDs) contributed to the study design, study management, analysis and report writing for WP2b (see Chapter 5).
Professor Janet Treasure (Professor, EDs), contributed to study design, intervention development, study management, analysis and report writing across WP1a (see Chapter 2), WP1b (see Chapter 3), WP2a (see Chapter 4), WP3 (see Chapter 6), WP6 (see Chapter 9), WP7a (see Chapter 10) and WP7b (see Chapter 11).
Publications
Work package 1a: training school staff
Peer-reviewed publications
Knightsmith P, Treasure J, Schmidt U. Spotting and supporting eating disorders in school: recommendations from school staff. Health Educ Res 2013;28:1004–13.
Knightsmith P, Sharpe H, Breen O, Treasure J, Schmidt U. ‘My teacher saved my life’ versus ‘Teachers don’t have a clue’: an online survey of pupils’ experiences of eating disorders. Child Adolesc Ment Health 2014;19:131–7.
Knightsmith P, Treasure J, Schmidt U. We don’t know how to help: an online survey of school staff. Child Adolesc Ment Health 2014;19:208–14.
Other publications
Knightsmith P. Eating Disorders Pocketbook. Ayresford, Hampshire: Teachers Pocketbooks; 2012.
Knightsmith P. Guidelines for School Staff. In Treasure J, Alexander J, editors. Anorexia Nervosa: A Recovery Guide for Sufferers, Families and Friends. Oxford: Routledge; 2013. pp. 154–60.
Work package 1b: prevention
Peer-reviewed publications
Sharpe H, Musiat P, Knapton O, Schmidt U. Pro-eating disorder websites: facts, fictions and fixes. J Public Ment Health 2011;10:34–44.
Schober I, Sharpe H, Schmidt U. The reporting of fidelity measures in primary prevention programs for eating disorders in schools. Eur Eat Disord Rev 2013;21:374–81.
Sharpe H, Damazer K, Treasure J, Schmidt U. What are adolescents’ experiences of body dissatisfaction and dieting, and what do they recommend for prevention? A qualitative study. Eat Weight Disord 2013;18:133–41.
Sharpe H, Naumann U, Treasure J, Schmidt U. Is fat talking a causal risk factor for body dissatisfaction? A systematic review and meta-analysis. Int J Eat Disord 2013;46:643–52.
Sharpe H, Schober I, Treasure J, Schmidt U. A cluster randomised controlled trial assessing the feasibility, acceptability and efficacy of a school-based prevention programme for eating disorders. Br J Psychiatry 2013;203:428–35.
Sharpe H, Schober I, Treasure J, Schmidt U. The role of high quality friendships in female adolescents’ eating pathology and body dissatisfaction. Eat Weight Disord 2014;19:159–68.
Other publications
Schmidt U. Cognitive behavioral approaches in adolescent anorexia and bulimia nervosa. Child Adolesc Psychiatr Clin N Am 2009;18:147–58.
Campbell M, Schmidt U. Cognitive–Behavioural Therapy for Adolescent Bulimia Nervosa and Binge-Eating Disorder. In Lock J, Le Grange D, editors. Eating Disorders in Children and Adolescents: A Clinical Handbook. New York, NY: Guilford Press; 2011. pp. 305–18.
Musiat P, Schmidt U. Self-Help and Stepped Care in Eating Disorders. In Agras S, editor. Oxford Handbook of Eating Disorders. Oxford: Oxford University Press; 2011. pp. 386–401.
Sánchez-Ortiz V, Schmidt U. Self-Help Approaches for Bulimia and Binge Eating Disorder. In Grilos C, Mitchell J, editors. The Treatment of Eating Disorders. New York, NY: Guilford Press; 2011. pp. 359–71.
Work package 2a: outpatient treatment
Peer-reviewed publications
Oldershaw A, Hambrook D, Tchanturia K, Treasure J, Schmidt U. Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom Med 2010;72:73–9.
Broadbent HJ, van den Eynde F, Guillaume S, Hanif EL, Stahl D, David AS, et al. Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham-controlled trials: a systematic review. World J Biol Psychiatry 2011;12:240–8.
Claudino A, Van den Eynde F, Stahl D, Dew T, Andiappan M, Kalthoff J, et al. Repetitive transcranial magnetic stimulation reduces cortisol concentrations in bulimic disorders. Psychol Med 2011;41:1329–36.
DeJong H, Perkins S, Grover M, Schmidt U. The prevalence of irritable bowel syndrome in outpatients with bulimia nervosa. Int J Eat Disord 2011;44:661–4.
Guillaume S, Jaussent I, Olié E, Genty C, Bringer J, Courtet P, et al. Characteristics of suicide attempts in anorexia and bulimia nervosa: a case–control study. PLOS ONE 2011;6:e23578.
Hambrook D, Oldershaw A, Rimes K, Schmidt U, Tchanturia K, Treasure J, et al. Emotional expression, self-silencing, and distress tolerance in anorexia nervosa and chronic fatigue syndrome. Br J Clin Psychol 2011;50:310–25.
Oldershaw A, Hambrook D, Rimes KA, Tchanturia K, Treasure J, Richards S, et al. Emotion recognition and emotional theory of mind in chronic fatigue syndrome. Psychol Health 2011;26:989–1005.
Oldershaw A, Hambrook D, Stahl D, Tchanturia K, Treasure J, Schmidt U. The socio-emotional processing stream in Anorexia Nervosa. Neurosci Biobehav Rev 2011;35:970–88.
Oldershaw A, Treasure J, Hambrook D, Tchanturia K, Schmidt U. Is anorexia nervosa a version of autism spectrum disorders? Eur Eat Disord Rev 2011;19:462–74.
Sánchez-Ortiz V, House J, Munro C, Treasure J, Startup H, Williams C, et al. ‘A computer isn’t gonna judge you’: a qualitative study of users’ views of an internet-based cognitive behavioural guided self-care treatment package for bulimia nervosa and related disorders. Eat Weight Disord 2011;16:93–101.
Sánchez-Ortiz VC, Munro C, Startup H, Treasure J, Schmidt U. The role of email guidance in internet-based cognitive-behavioural self-care treatment for bulimia nervosa. Eur Eat Disord Rev 2011;19:342–8.
Schmidt U, Oldershaw A, van Elburg A. Translating experimental neuroscience into treatment of eating disorders: two examples. Curr Top Behav Neurosci 2011;6:253–68.
Van den Eynde F, Claudino AM, Campbell I, Horrell L, Andiappan M, Stahl D, et al. Cardiac safety of repetitive transcranial magnetic stimulation in bulimic eating disorders. Brain Stimulat 2011;4:112–14.
Van den Eynde F, Claudino A, Campbell I, Schmidt U. Immediate cognitive effects of repetitive Transcranial Magnetic Stimulation in eating disorders: a pilot study. Eat Weight Disord 2011;16:e45.
Van den Eynde F, Guillaume S, Broadbent H, Campbell IC, Schmidt U. Repetitive transcranial magnetic stimulation in anorexia nervosa: a pilot study. Eur Psychiatry 2011;28:98–101.
Van den Eynde F, Guillaume S, Broadbent H, Stahl D, Campbell I, Schmidt U, et al. Neurocognition in bulimic eating disorders: a systematic review. Acta Psychiatr Scand 2011;124:120–40.
DeJong H, Broadbent H, Schmidt U. A systematic review of dropout from treatment in outpatients with anorexia nervosa. Int J Eat Disord 2012;45:635–47.
DeJong H, Hillcoat J, Perkins S, Grover M, Schmidt U. Illness perception in bulimia nervosa. J Health Psychol 2012;17:399–408.
Kenyon M, Samarawickrema N, DeJong H, Van den Eynde F, Startup H, Lavender A, et al. Theory of mind in bulimia nervosa. Int J Eat Disord 2012;45:377–84.
Oldershaw A, DeJong H, Hambrook D, Broadbent H, Tchanturia K, Treasure J, et al. Emotional processing following recovery from anorexia nervosa. Eur Eat Disord Rev 2012;20:502–9.
Schmidt U, Oldershaw A, Jichi F, Sternheim L, Startup H, McIntosh V, et al. Out-patient psychological therapies for adults with anorexia nervosa: randomised controlled trial. Br J Psychiatry 2012;201:392–9.
Van den Eynde F, Samarawickrema N, Kenyon M, DeJong H, Lavender A, Startup H, et al. A study of neurocognition in bulimia nervosa and eating disorder not otherwise specified–bulimia type. J Clin Exp Neuropsychol 2012;34:67–77.
Van den Eynde F, Broadbent H, Guillaume S, Claudino A, Campbell I, Schmidt U. Handedness, repetitive transcranial magnetic stimulation and bulimic disorders. Eur Psychiatry 2012;27:290–3.
Van den Eynde F, Suda M, Broadbent H, Guillaume S, Eynde M, Steiger H, et al. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur Eat Disord Rev 2012;20:94–105.
DeJong H, Van den Eynde F, Broadbent H, Kenyon MD, Lavender A, Startup H, et al. Social cognition in bulimia nervosa: a systematic review. Eur Psychiatry 2013;28:1–6.
DeJong H, Oldershaw A, Sternheim L, Samarawickrema N, Kenyon MD, Broadbent H, et al. Quality of life in anorexia nervosa, bulimia nervosa and eating disorder not-otherwise-specified. J Eat Disord 2013;1:43.
Renwick B, Campbell IC, Schmidt U. Review of attentional bias modification: a brain-directed treatment for eating disorders. Eur Eat Disord Rev 2013;21:464–74.
Renwick B, Campbell IC, Schmidt U. Attention bias modification: a new approach to the treatment of eating disorders? Int J Eat Disord 2013;46:496–500.
Renwick B, DeJong H, Kenyon M, Samarawickrema N, Loomes R, Watson C, et al. Social perception in people with eating disorders. Eur Psychiatry 2013;28:436–41.
Schmidt U, Renwick B, Lose A, Kenyon M, DeJong H, Broadbent H, et al. The MOSAIC study – comparison of the Maudsley Model of Treatment for Adults with Anorexia Nervosa (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with anorexia nervosa or eating disorder not otherwise specified, anorexia nervosa type: study protocol for a randomized controlled trial. Trials 2013;30:160.
Startup H, Lavender A, Oldershaw A, Stott R, Tchanturia K, Treasure J, et al. Worry and rumination in anorexia nervosa. Behav Cogn Psychother 2013;41:301–16.
Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord 2013;1:1–13.
Lose A, Davies C, Renwick B, Kenyon M, Treasure J, Schmidt U. Process Evaluation of the MOSAIC trial. Part II: patient experiences of two psychological therapies for treatment of anorexia nervosa. Eur Eat Disord Rev 2014;22:131–9.
Lose A, Davies C, Renwick B, Kenyon M, Treasure J, Schmidt U. Process evaluation of the Maudsley Model for Treatment of Adults with Anorexia Nervosa trial. Part II: patient experiences of two psychological therapies for treatment of anorexia nervosa. Eur Eat Disord Rev 2014;22:131–9.
Schmidt U, Wade TD, Treasure J. The Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA): development, key features and preliminary evidence. J Cogn Psychother 2014;28:48–71.
Schober I, Renwick B, Kenyon M, DeJong H, Sharpe H, Jacobi C, et al. Threat-related attentional bias in anorexia nervosa. Int J Eat Disord 2014;47:168–73.
Waterman-Collins D, Renwick B, Lose A, Kenyon M, Serpell L, Richards L, et al. Process Evaluation of the MOSAIC trial, part I: therapist experiences of delivering two psychological therapies for treatment of anorexia nervosa. Eur Eat Disord Rev 2014;22:122–30.
Renwick B, Musiat P, Lose A, DeJong H, Broadbent H, Kenyon M, et al. Neuro- and social-cognitive clustering highlights distinct profiles in adults with anorexia nervosa. Int J Eat Disord 2015:48:26–34.
Schmidt U, Magill N, Renwick B, Keyes A, Kenyon M, Dejong H, et al. The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with broadly defined anorexia nervosa: a randomized controlled trial. J Consult Clin Psychol 2015;83:796–807.
Work package 2b: inpatient treatment
Peer-reviewed publications
Tchanturia K, Davies H, Lopez C, Schmidt U, Treasure J, Wykes T. Neuropsychological task performance before and after cognitive remediation in anorexia nervosa: a pilot case-series. Psychol Med 2008;38:1371–3.
Davies H, Liao P-C, Campbell IC, Tchanturia K. Multidimensional self reports as a measure of characteristics in people with eating disorders. Eat Weight Disord 2009;14(2–3):84–91.
Kyriacou O, Easter A, Tchanturia K. Comparing views of patients, parents, and clinicians on emotions in anorexia: a qualitative study. J Health Psychol 2009;14:843–54.
Russell TA, Schmidt U, Doherty L, Young V, Tchanturia K. Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind. Psychiatry Res 2009;168:181–5.
Castro L, Davies H, Hale L, Surguladze S, Tchanturia K. Facial affect recognition in anorexia nervosa: is obsessionality a missing piece of the puzzle? Aus N Z J Psychiatry 2010;44:1118–25.
Genders R, Tchanturia K. Cognitive Remediation Therapy (CRT) for anorexia in group format: a pilot study. Eat Weight Disord 2010;15:234–9.
Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychol Med 2010;40:1887–97.
Lopez C, Stahl D, Tchanturia K. Estimated intelligence quotient in anorexia nervosa: a systematic review and meta-analysis of the literature. Ann Gen Psychiatry 2010;9:1–10.
Davies H, Schmidt U, Stahl D, Tchanturia K. Evoked facial emotional expression and emotional experience in people with anorexia nervosa. Int J Eat Disord 2011;44:531–9.
Easter A, Tchanturia K. Therapists’ experiences of cognitive remediation therapy for anorexia nervosa: implications for working with adolescents. Clin Child Psychol Psychiatry 2011;16:233–46.
Harrison A, Genders R, Davies H, Treasure J, Tchanturia K. Experimental measurement of the regulation of anger and aggression in women with anorexia nervosa. Clin Psychol Psychother 2011;18:445–52.
Harrison A, Tchanturia K, Treasure J. Measuring state trait properties of detail processing and global integration ability in eating disorders. World J Biol Psychiatry 2011;12:462–72.
Lounes N, Curtis H, Mountfoud V, Tchanturia K. From systematic review to quality standards and clinical reality: inpatient treatment of anorexia nervosa. Clin Psychol Forum 2011;227:42–6.
Money C, Davies H, Tchanturia K. A case study introducing cognitive remediation and emotion skills training for anorexia nervosa inpatient care. Clin Case Stud 2011;10:110–21.
Money C, Genders R, Treasure J, Schmidt U, Tchanturia K. A brief emotion focused intervention for inpatients with anorexia nervosa: A qualitative study. J Health Psychol 2011;16:947–58.
Tchanturia K, Harrison A, Davies H, Roberts M, Oldershaw A, Nakazato M, et al. Cognitive flexibility and clinical severity in eating disorders. PLOS ONE 2011;6:e20462.
Davies H, Fox J, Naumann U, Treasure J, Schmidt U, Tchanturia K. Cognitive Remediation and Emotion Skills Training (CREST) for anorexia nervosa: an observational study using neuropsychological outcomes. Eur Eat Disord Rev 2012;20:211–17.
Davies H, Swan N, Schmidt U, Tchanturia K. An experimental investigation of verbal expression of emotion in anorexia and bulimia nervosa. Eur Eat Disord Rev 2012;20:476–83.
Lopez C, Davies H, Tchanturia K. Neuropsychological inefficiences in anorexia nervosa targeted in clinical practice: the development of a module of cognitive remediation therapy. Eat Disord 2012;185–97.
Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmid, U, et al. Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Card Sorting Task. PLOS ONE 2012;7:e28331.
Tchanturia K, Liao PC, Forcano L, Fernández-Aranda F, Uher R, Treasure J, et al. Poor decision making in male patients with anorexia nervosa. Eur Eat Disord Rev 2012;20:169–173.
Davies H, Schmidt U, Tchanturia K. Emotional facial expression in women recovered from anorexia nervosa. BMC Psychiatry 2013;13:291.
Tchanturia K, Curtis H, Liao T, Uher R, Schmidt U, Campbell IC. Decision-making and ‘gut feeling’ in males with anorexia nervosa. Adv Eat Disord 2013;1:51–60.
Work package 3: carer intervention
Peer-reviewed publications
Goddard E, Macdonald P, Sepulveda AR, Naumann U, Landau S, Schmidt U, et al. Cognitive interpersonal maintenance model of eating disorders: intervention for carers. Br J Psychiatry 2011;199:225–31.
Goddard E, Macdonald P, Treasure J. An examination of the impact of the Maudsley Collaborative Care skills training workshops on patients with anorexia nerovsa: a qualitative study. Eur Eat Disord Rev 2011;19:150–61.
Grover M, Naumann U, Mohammad-Dar L, Glennon D, Ringwood S, Eisler I, et al. A randomized controlled trial of an internet-based cognitive-behavioural skills package for carers of people with anorexia nervosa. Psychol Med 2011;41:2581–91.
Macdonald P, Murray J, Goddard E, Treasure J. Carer’s experience and perceived effects of a skills based training programme for families of people with eating disorders: a qualitative study. Eur Eat Disord Rev 2011;19:475–86.
Goddard E, Hibbs R, Raenker S, Salerno L, Arcelus J, Boughton N, et al. A multi-centre cohort study of short term outcomes of hospital treatment for anorexia nervosa in the UK. BMC Psychiatry 2013;13:287.
Goddard E, Raenker S, Macdonald P, Todd G, Beecham J, Naumann U, et al. Carers’ assessment, skills and information sharing: theoretical framework and trial protocol for a randomised controlled trial evaluating the efficacy of a complex intervention for carers of inpatients with anorexia nervosa. Eur Eat Disord Rev 2013;21:60–71.
Goddard E, Salerno L, Hibbs R, Raenker S, Naumann U, Arcelus J, et al. Empirical examination of the interpersonal maintenance model of anorexia nervosa. Int J Eat Disord 2013;46:867–74.
Raenker S, Hibbs R, Goddard E, Naumann U, Arcelus J, Ayton A, et al. Caregiving and coping in carers of people with anorexia nervosa admitted for intensive hospital care. Int J Eat Disord 2013;46:346–54.
Hibbs R, Rhind C, Sallis H, Goddard E, Raenker S, Ayton A, et al. Confirmatory factor analysis for two questionnaires of caregiving in eating disorders. Health Psychol Behav Med 2014;2:322–34.
Other publications
Goddard E, Macdonald P, Treasure J. Caring for someone with an eating Disorder. In Grilo C, Mitchell J, editors. The Treatment of Eating Disorders: A Clinical Handbook. New York, NY: Guilford Press; 2010. pp. 487–99.
Kyriacou O, Treasure J, Raenker S. The influence and importance of parents in care and treatment of an eating disorder. In Treasure J, Schmidt U, MacDonald P, editors. The Clinician’s Guide To Collaborative Caring In Eating Disorders The New Maudsley Method. New York, NY: Routledge; 2010. pp. 241–9.
Treasure J, Macdonald P, Goddard E. What the patients say: an examination of what patients think about family interventions. In Treasure J, Schmidt U, Macdonald P, editors. The Clinician’s Guide to Collaborative Caring in Eating Disorders: The New Maudsley Method. Hove: Routledge; 2010. pp. 253–63.
Treasure J, Schmidt U, Macdonald P. The Clinician’s Guide to Collaborative Caring in Eating Disorders: The New Maudsley Method. Hove: Routledge; 2010.
Goddard E, Raenker S, Treasure J. Involving carers: a skills based learning approach. In Alexander J, Treasure J, editors. A Collaborative Approach to Eating Disorders. Hove: Routledge; 2011. pp. 149–62.
Work package 4: activity and anorexia nervosa
Peer-reviewed publications
Campbell IC, Mill J, Uher R, Schmidt U. Eating disorders, gene–environment interactions and epigenetics. Neurosci Biobehav Rev 2011;35:784–93.
Koskina A, Van den Eynde F, Meisel S, Campbell I, Schmidt U. Social appearance anxiety and bulimia nervosa. Eat Weight Disord 2011;16:142–5.
Koskina A, Campbell IC, Schmidt U. Exposure therapy in eating disorders revisited. Neurosci Biobehav Rev 2012;37:193–208.
Uher R, Brooks SJ, Bartholdy S, Tchanturia K, Campbell IC. Increasing cognitive load reduces interference from masked appetitive and aversive but not neutral stimuli. PLOS ONE 2014;9:e94417.
Keyes A, Woerwag-Mehta S, Bartholdy S, Koskina A, Middleton B, Connan F, et al. Physical activity and the drive to exercise in anorexia nervosa. Int J Eat Disord 2015;48:46–54.
Work package 5: relapse prevention
Peer-reviewed publications
Sternheim L, Konstantellou A, Startup H, Schmidt U. What does uncertainty mean to women with anorexia nervosa? An interpretative phenomenological analysis. Eur Eat Disord Rev 2011;19:12–24.
Sternheim L, Startup H, Schmidt U. An experimental exploration of behavioral and cognitive–emotional aspects of intolerance of uncertainty in eating disorder patients. J Anxiety Disord 2011;25:806–12.
Sternheim L, Startup H, Pretorius N, Johnson-Sabine E, Schmidt U, Channon S. An experimental exploration of social problem solving and its associated processes in anorexia nervosa. Psychiatry Res 2012;200:524–9.
Sternheim L, Startup H, Saeidi S, Morgan J, Hugo P, Russell A, et al. Understanding catastrophic worry in eating disorders: process and content characteristics. J Behav Ther Exp Psychiatry 2012;43:1095–103.
Papers submitted or in advanced stages of preparation
Sternheim L, Startup H, Schmidt U. Anxiety-related processes in anorexia nervosa and their relation to eating disorder pathology, depression and anxiety. Adv Eat Disord 2015;3:13–19.
Work package 6: maternal eating disorders
Micali N, Treasure J. Biological effects of a maternal ED on pregnancy and foetal development: a review. Eur Eat Disord Rev 2009;17:448–54.
Easter A, Taborelli E, Micali N. Obstetric outcomes amongst women with a history of anorexia nervosa. Minerva Psichiatr 2010;51:161–75.
Micali N. Management of eating disorders during pregnancy. Prog Neurol Psychiatry 2010;14:24–6.
Easter A, Treasure J, Micali N. Fertility and prenatal attitudes towards pregnancy in women with eating disorders: results from the Avon Longitudinal Study of Parents and Children. BJOG 2011;118:1491–8.
Micali N, Northstone K, Emett P, Naumann U, Treasure J. Nutritional intake and dietary patterns in pregnancy: a longitudinal study of women with lifetime eating disorders. Br J Nutr 2012;108:2093–9.
Easter A, Bye A, Taborelli E, Corfield F, Schmidt U, Treasure J, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev 2013;21:340–4.
Easter A, Naumann U, Northstone K, Schmidt U, Treasure J, Micali N. A longitudinal investigation of nutrition and dietary patterns in children of mothers with eating disorders. J Pediatr 2013;163:173–8.
Easter A, Howe LD, Tilling K, Schmidt U, Treasure J, Micali N. Growth trajectories in the children of mothers with eating disorders: a longitudinal study. BMJ Open 2014;4:e004453.
Work package 7a: care pathways
Peer-reviewed publications
Nishizono-Maher A, Escobar-Koch T, Ringwood S, Banker J, van Furth E, Schmidt U. What are the top five essential features of a high quality eating disorder service? A comparison of the views of US and UK eating disorder sufferers, carers and health professionals. Eur Eat Disord Rev 2011;19:411–16.
House J, Schmidt U, Craig M, Landau S, Simic M, Nicholls D, et al. Comparison of specialist and nonspecialist care pathways for adolescents with anorexia nervosa and related eating disorders. Int J Eat Disord 2012;45:949–56.
Koskina A, Arkell J, Butcher G, Currie A, Gowers S, Key A, et al. Service providers’ perceptions of the strengths and prospective improvements in UK eating disorder services: Findings from a royal college survey. Eur Eat Disord Rev 2012;20:225–31.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Keel PK, Klump KL. Are eating disorders culture-bound syndromes? Implications for conceptualizing their etiology. Psychol Bull 2003;129:747-69. http://dx.doi.org/10.1037/0033-2909.129.5.747.
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 2007;61:348-58. https://doi.org/10.1016/j.biopsych.2006.03.040.
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med 2005;35:163-74. https://doi.org/10.1017/S0033291704003915.
- Southgate L, Tchanturia K, Treasure J. Building a model of the aetiology of eating disorders by translating experimental neuroscience into clinical practice. J Ment Health 2005;14:553-66. https://doi.org/10.1080/09638230500347541.
- Currin L, Schmidt U. A critical analysis of the utility of an early intervention approach in the eating disorders. J Ment Health 2005;14:611-24. https://doi.org/10.1080/09638230500347939.
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull 2004;130:19-65. http://dx.doi.org/10.1037/0033-2909.130.1.19.
- Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry 2006;63:305-12. https://doi.org/10.1001/archpsyc.63.3.305.
- Zhu Y, Hu X, Wang J, Chen J, Guo Q, Li C, et al. Processing of food, body and emotional stimuli in anorexia nervosa: a systematic review and meta-analysis of functional magnetic resonance imaging studies. Eur Eat Disord Rev 2012;20:439-50. http://dx.doi.org/10.1002/erv.2197.
- Frank GK, Kaye WH. Current status of functional imaging in eating disorders. Int J Eat Disord 2012;45:723-36. http://dx.doi.org/10.1002/eat.22016.
- Van den Eynde F, Suda M, Broadbent H, Guillaume S, Van den Eynde M, Steiger H, et al. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur Eat Disord Rev 2012;20:94-105. http://dx.doi.org/10.1002/erv.1163.
- Campbell IC, Mill J, Uher R, Schmidt U. Eating disorders, gene-environment interactions and epigenetics. Neurosci Biobehav Rev 2011;35:784-93. http://dx.doi.org/10.1016/j.neubiorev.2010.09.012.
- Boraska V, Davis OS, Cherkas LF, Helder SG, Harris J, Krug I, et al. Genome-wide association analysis of eating disorder-related symptoms, behaviors, and personality traits. Am J Med Genet B Neuropsychiatr Genet 2012;159B:803-11. http://dx.doi.org/10.1002/ajmg.b.32087.
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 2009;10:573-84. http://dx.doi.org/10.1038/nrn2682.
- Tchanturia K, Harrison A, Davies H, Roberts M, Oldershaw A, Nakazato M, et al. Cognitive flexibility and clinical severity in eating disorders. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0020462.
- Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Card Sorting Task. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0028331.
- Oldershaw A, Hambrook D, Stahl D, Tchanturia K, Treasure J, Schmidt U. The socio-emotional processing stream in Anorexia Nervosa. Neurosci Biobehav Rev 2011;35:970-88. http://dx.doi.org/10.1016/j.neubiorev.2010.11.001.
- Oldershaw A, Hambrook D, Tchanturia K, Treasure J, Schmidt U. Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom Med 2010;72:73-9. http://dx.doi.org/10.1097/PSY.0b013e3181c6c7ca.
- Lopez C, Tchanturia K, Stahl D, Treasure J. Central coherence in eating disorders: a systematic review. Psychol Med 2008;38:1393-404. http://dx.doi.org/10.1017/S0033291708003486.
- Schmidt U, Treasure J. Anorexia nervosa: valued and visible. A cognitive-interpersonal maintenance model and its implications for research and practice. Br J Clin Psychol 2006;45:343-66. https://doi.org/10.1348/014466505X53902.
- Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord 2013;1. http://dx.doi.org/10.1186/2050-2974-1-13.
- Ogg EC, Millar HR, Pusztai EE, Thom AS. General practice consultation patterns preceding diagnosis of eating disorders. Int J Eat Disord 1997;22:89-93. https://doi.org/10.1002/(SICI)1098-108X(199707)22:1<89::AID-EAT12>3.0.CO;2-D.
- Lask B, Bryant-Waugh R, Wright F, Campbell M, Willoughby K, Waller G. Family physician consultation patterns indicate high risk for early-onset anorexia nervosa. Int J Eat Disord 2005;38:269-72. http://dx.doi.org/10.1002/eat.20163.
- Turnbull S, Ward A, Treasure J, Jick H, Derby L. The demand for eating disorder care. An epidemiological study using the general practice research database. Br J Psychiatry 1996;169:705-12. https://doi.org/10.1192/bjp.169.6.705.
- Currin L, Schmidt U, Yeomans M, Ellis G, Nodder J, Stone C, et al. Entry Into Specialist Services For The Eating Disorders: Audit of Clinical Pathways Through Primary and Secondary Care. Report from the Specialist Clinical Audit Programme for South London, Kent, Surry and Sussex. Bexley: NHS Specialist Clinical Audit Office; 2006.
- Whitney J, Murray J, Gavan K, Todd G, Whitaker W, Treasure J. Experience of caring for someone with anorexia nervosa: qualitative study. Br J Psychiatry 2005;187:444-9. https://doi.org/10.1192/bjp.187.5.444.
- Eating Disorders in the UK: Policies for Service Development and Training. London: Royal College of Psychiatrists; 2000.
- Getting Better? Is the Quality of Treatment for Eating Disorders in the UK Getter Better?. Norwich: Eating Disorders Association; 2005.
- Thompson A, Shaw M, Harrison G, Ho D, Gunnell D, Verne J. Patterns of hospital admission for adult psychiatric illness in England: analysis of Hospital Episode Statistics data. Br J Psychiatry 2004;185:334-41. http://dx.doi.org/10.1192/bjp.185.4.334.
- Howlett M, McClelland L, Crisp AH. The cost of the illness that defies. Postgrad Med J 1995;71:705-6. https://doi.org/10.1136/pgmj.71.842.705.
- National CAMHS Audit 2005. www.childrensmappingorg.uk/topics/camhs (accessed 1 April 2014).
- O’Herlihy A, Worrall A, Lelliott P, Jaffa T, Hill P, Banerjee S. Distribution and characteristics of in-patient child and adolescent mental health services in England and Wales. Br J Psychiatry 2003;183:547-51. https://doi.org/10.1192/bjp.183.6.547.
- Eating Disorders. Council Report 14. London: Royal College of Psychiatrists; 1992.
- Dare C, Eisler I, Russell G, Treasure J, Dodge L. Psychological therapies for adults with anorexia nervosa: randomised controlled trial of out-patient treatments. Br J Psychiatry 2001;178:216-21. https://doi.org/10.1192/bjp.178.3.216.
- Crisp AH, Callender JS, Halek C, Hsu LK. Long-term mortality in anorexia nervosa. A 20-year follow-up of the St George’s and Aberdeen cohorts. Br J Psychiatry 1992;161:104-7. https://doi.org/10.1192/bjp.161.1.104.
- Lindblad F, Lindberg L, Hjern A. Improved survival in adolescent patients with anorexia nervosa: a comparison of two Swedish national cohorts of female inpatients. Am J Psychiatry 2006;163:1433-5. https://doi.org/10.1176/ajp.2006.163.8.1433.
- Treasure J, Schmidt U, Hugo P. Mind the gap: service transition and interface problems for patients with eating disorders. Br J Psychiatry 2005;187:398-400. https://doi.org/10.1192/bjp.187.5.398.
- The Mental Health of Students in Higher Education. CR 112. London: Royal College of Psychiatrists; 2003.
- Herzog W, Deter HC, Fiehn W, Petzold E. Medical findings and predictors of long-term physical outcome in anorexia nervosa: a prospective, 12-year follow-up study. Psychol Med 1997;27:269-79. https://doi.org/10.1017/S0033291796004394.
- Zipfel S, Lowe B, Herzog W, Treasure J, Schmidt U, van Furth E. Handbook of Eating Disorders: Theory, Treatment and Research. Chichester: John Wiley and Sons; 2003.
- Nielsen S, Bara-Carril N, Treasure J, Schmidt U, van Furth E. Handbook of Eating Disorders: Theory, Treatment and Research. Chichester: John Wiley and Sons; 2003.
- Micali N, Schmidt U, O’Keane V, Seneviratne T, Marsh M. Psychiatric Disorders and Pregnancy: Obstetric and Psychiatric Care. London: Taylor and Francis; 2006.
- Pomeroy C, Mitchell JE, Fairburn CG, Brownell KD. Eating Disorders and Obesity: A Comprehensive Handbook. New York, NY: Guilford Press; 2002.
- Keilen M, Treasure T, Schmidt U, Treasure J. Quality of life measurements in eating disorders, angina, and transplant candidates: are they comparable?. J R Soc Med 1994;87:441-4.
- de la Rie SM, Noordenbos G, van Furth EF. Quality of life and eating disorders. Qual Life Res 2005;14:1511-22. https://doi.org/10.1007/s11136-005-0585-0.
- Crow SJ, Nyman JA. The cost-effectiveness of anorexia nervosa treatment. Int J Eat Disord 2004;35:155-60. http://dx.doi.org/10.1002/eat.10258.
- Striegel-Moore RH, Leslie D, Petrill SA, Garvin V, Rosenheck RA. One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: evidence from a national database of health insurance claims. Int J Eat Disord 2000;27:381-9. https://doi.org/10.1002/(SICI)1098-108X(200005)27:4<381::AID-EAT2>3.0.CO;2-U.
- Mathers C, Vos T, Stevenson C. The Burden of Disease and Injury in Australia. Canberra: Australian Institute of Health and Welfare; 1999.
- Treasure J, Murphy T, Szmukler G, Todd G, Gavan K, Joyce J. The experience of caregiving for severe mental illness: a comparison between anorexia nervosa and psychosis. Soc Psychiatry Psychiatr Epidemiol 2001;36:343-7. https://doi.org/10.1007/s001270170039.
- Treasure J, Smith G, Crane A. Skills-Based Learning for Caring for a Loved One With an Eating Disorder: The New Maudsley Method. East Sussex: Routledge; 2007.
- Staley K. An Evaluation of Service User Involvement in Studies Adopted by the Mental Health Research Network. London: Mental Health Research Network; 2012.
- Currin L, Schmidt U, Treasure J, Jick H. Time trends in eating disorder incidence. Br J Psychiatry 2005;186:132-5. https://doi.org/10.1192/bjp.186.2.132.
- NHS Digital . Provisional Monthly Hospital Episode Statistics for Admitted Patient Care, Outpatient and Accident &Amp; Emergency Data: April–June 2012 2012. http://content.digital.nhs.uk/catalogue/PUB08574 (accessed 4 April 2017).
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep 2012;14:406-14. http://dx.doi.org/10.1007/s11920-012-0282-y.
- Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 2006;19:389-94. http://dx.doi.org/10.1097/01.yco.0000228759.95237.78.
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry 2011;68:714-23. http://dx.doi.org/10.1001/archgenpsychiatry.2011.22.
- Neumark-Sztainer D, Hannan PJ. Weight-related behaviors among adolescent girls and boys: results from a national survey. Arch Pediatr Adolesc Med 2000;154:569-77. https://doi.org/10.1001/archpedi.154.6.569.
- Jaite C, Hoffmann F, Glaeske G, Bachmann CJ. Prevalence, comorbidities and outpatient treatment of anorexia and bulimia nervosa in German children and adolescents. Eat Weight Disord 2013;18:157-65. http://dx.doi.org/10.1007/s40519-013-0020-4.
- Smolak L, Harris B, Levine MP, Shisslak CM. Teachers: the forgotten influence on the success of prevention programs. Eat Disord 2001;9:261-5. https://doi.org/10.1080/10640260127553.
- Shaw H, Stice E, Becker CB. Preventing eating disorders. Child Adolesc Psychiatr Clin N Am 2009;18:199-207. http://dx.doi.org/10.1016/j.chc.2008.07.012.
- ‘Something’s Got to Change’ – Eating Disorder Sufferer Survey. Norwich: Beat; 2007.
- Weber RP. Basic Content Analysis. Thousand Oaks, CA: Sage; 1990.
- Flick U. An Introduction to Qualitative Research. London: Sage; 2009.
- Knightsmith P, Sharpe H, Breen O, Treasure J, Schmidt U. ‘My teacher saved my life’ versus ‘Teachers don’t have a clue’: an online survey of pupils’ experiences of eating disorders. Child Adolesc Ment Health 2014;19:131-7. http://dx.doi.org/10.1111/camh.12027.
- Mond JM, Arrighi A. Gender differences in perceptions of the severity and prevalence of eating disorders. Early Interv Psychiatry 2011;5:41-9. http://dx.doi.org/10.1111/j.1751-7893.2010.00257.x.
- Knightsmith P, Treasure J, Schmidt U. We don’t know how to help: an online survey of school staff. Child Adolesc Ment Health 2014;19:208-14. http://dx.doi.org/10.1111/camh.12039.
- Behaviour Change: The Principles for Effective Interventions. UK: NICE Public Health Guidance; 2007.
- Currin L, Waller G, Schmidt U. Primary care physicians' knowledge of and attitudes toward the eating disorders: do they affect clinical actions?. Int J Eat Disord 2009;42:453-8. https://doi.org/10.1002/eat.20636.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. London: Lawrence Erlbaum; 1988.
- Deutschlander S. An analysis of training effects on school personnel’s knowledge, attitudes, comfort, and confidence levels toward educating students about HIV/AIDS in Pennsylvania. Int J Mental Health Addict 2010;8:444-52. https://doi.org/10.1007/s11469-009-9221-5.
- Hussein SA, Vostanis P. Teacher training intervention for early identification of common child mental health problems in Pakistan. Emotional Behav Difficulties 2013;18:284-96. https://doi.org/10.1080/13632752.2013.819254.
- Sharpe H, Schober I, Treasure J, Schmidt U. Feasibility, acceptability and efficacy of a school-based prevention programme for eating disorders: cluster randomised controlled trial. Br J Psychiatry 2013;203:428-35. http://dx.doi.org/10.1192/bjp.bp.113.128199.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011;68:724-31. http://dx.doi.org/10.1001/archgenpsychiatry.2011.74.
- Klump KL, Bulik CM, Kaye WH, Treasure J, Tyson E. Academy for eating disorders position paper: eating disorders are serious mental illnesses. Int J Eat Disord 2009;42:97-103. http://dx.doi.org/10.1002/eat.20589.
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry 2006;19:438-43. http://dx.doi.org/10.1097/01.yco.0000228768.79097.3e.
- Henderson J. Pro Bono Economics . Costs of Eating Disorders in England: Economic Impacts of Anorexia Nervosa, Bulimia Nervosa and Other Disorders, Focussing on Young People 2012. www.b-eat.co.uk/assets/000/000/146/CostsofEatingDisordersinEnglandReport_original.pdf?1403538000 (accessed 4 April 2107).
- Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 2002;159:1284-93. http://dx.doi.org/10.1176/appi.ajp.159.8.1284.
- Steinhausen HC, Weber S. The outcome of bulimia nervosa: findings from one-quarter century of research. Am J Psychiatry 2009;166:1331-41. http://dx.doi.org/10.1176/appi.ajp.2009.09040582.
- Keery H, van den Berg P, Thompson JK. An evaluation of the Tripartite Influence Model of body dissatisfaction and eating disturbance with adolescent girls. Body Image 2004;1:237-51. https://doi.org/10.1016/j.bodyim.2004.03.001.
- Stice E. A prospective test of the dual-pathway model of bulimic pathology: mediating effects of dieting and negative affect. J Abnorm Psychol 2001;110:124-35. https://doi.org/10.1037/0021-843X.110.1.124.
- Varnado-Sullivan PJ, Zucker N, Williamson DA, Reas D, Thaw J, Netemeyer SB. Development and implementation of the body logic program for adolescents: a two-stage prevention program for eating disorders. Cogni Behav Pract 2001;8:248-59. https://doi.org/10.1016/S1077-7229(01)80061-4.
- O’Dea JA, Abraham S. Improving the body image, eating attitudes, and behaviors of young male and female adolescents: a new educational approach that focuses on self-esteem. Int J Eat Disord 2000;28:43-57. https://doi.org/10.1002/(SICI)1098-108X(200007)28:1<43::AID-EAT6>3.0.CO;2-D.
- Favaro A, Zanetti T, Huon G, Santonastaso P. Engaging teachers in an eating disorder preventive intervention. Int J Eat Disord 2005;38:73-7. http://dx.doi.org/10.1002/eat.20148.
- Kater KJ, Rohwer J, Londre K. Evaluation of an upper elementary school program to prevent body image, eating, and weight concerns. J Sch Health 2002;72:199-204. https://doi.org/10.1111/j.1746-1561.2002.tb06546.x.
- Phelps L, Sapia J, Nathanson D, Nelson L. An empirically supported eating disorder prevention program. Psychol Schools 2000;37:443-52. https://doi.org/10.1002/1520-6807(200009)37:5<443::AID-PITS4>3.0.CO;2-8.
- Ghaderi A, Mårtensson M, Schwan H. ‘Everybody’s Different’: a primary prevention program among fifth grade school children. Eat Disord 2005;13:245-59. https://doi.org/10.1080/10640260590932869.
- Wade TD, Davidson S, O’Dea JA. A preliminary controlled evaluation of a school-based media literacy program and self-esteem program for reducing eating disorder risk factors. Int J Eat Disord 2003;33:371-83. http://dx.doi.org/10.1002/eat.10136.
- Reflections on Body Image. London: The Stationery Office; 2012.
- Carter JC, Stewart DA, Dunn VJ, Fairburn CG. Primary prevention of eating disorders: might it do more harm than good?. Int J Eat Disord 1997;22:167-72. https://doi.org/10.1002/(SICI)1098-108X(199709)22:2<167::AID-EAT8>3.0.CO;2-D.
- Stallard P, Sayal K, Phillips R, Taylor JA, Spears M, Anderson R, et al. Classroom based cognitive behavioural therapy in reducing symptoms of depression in high risk adolescents: pragmatic cluster randomised controlled trial. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6058.
- Sharpe H, Naumann U, Treasure J, Schmidt U. Is fat talking a causal risk factor for body dissatisfaction? A systematic review and meta-analysis. Int J Eat Disord 2013;46:643-52. http://dx.doi.org/10.1002/eat.22151.
- Stice E. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol Bull 2002;128:825-48. https://doi.org/10.1037/0033-2909.128.5.825.
- Stice E, Telch CF, Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess 2000;12. https://doi.org/10.1037/1040-3590.12.2.123.
- Mendelson BK, Mendelson MJ, White DR. Body-esteem scale for adolescents and adults. J Pers Assess 2001;76:90-106. http://dx.doi.org/10.1207/S15327752JPA7601_6.
- Thompson JK, van den Berg P, Roehrig M, Guarda AS, Heinberg LJ. The sociocultural attitudes towards appearance scale-3 (SATAQ-3): development and validation. Int J Eat Disord 2004;35:293-304. http://dx.doi.org/10.1002/eat.10257.
- Jones DC, Vigfusdottir TH, Lee Y. Body image and the appearance culture among adolescent girls and boys. J Adolesc Res 2004;19:323-39. http://dx.doi.org/10.1177/0743558403258847.
- Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess 1990;55:610-17. http://dx.doi.org/10.1080/00223891.1990.9674095.
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Sydney, Australia: Sydney Psychology Foundation; 1995.
- Hox J. Multilevel Analysis: Techniques and Applications. London: Routledge; 2002.
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-91. https://doi.org/10.3758/BF03193146.
- Wilksch SM, Wade TD. Reduction of shape and weight concern in young adolescents: a 30-month controlled evaluation of a media literacy program. J Am Acad Child Adolesc Psychiatry 2009;48:652-61. http://dx.doi.org/10.1097/CHI.0b013e3181a1f559.
- Haahr M. Random.Org 2012. www.random.org/ (accessed 1 August 2010).
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12-9. https://doi.org/10.1037/0022-006X.59.1.12.
- Mendelson BK, McLaren L, Gauvin L, Steiger H. The relationship of self-esteem and body esteem in women with and without eating disorders. Int J Eat Disord 2002;31:318-23. https://doi.org/10.1002/eat.10011.
- Richardson SM, Paxton SJ. An evaluation of a body image intervention based on risk factors for body dissatisfaction: a controlled study with adolescent girls. Int J Eat Disord 2010;43:112-22. http://dx.doi.org/10.1002/eat.20682.
- Steiner-Adair C, Sjostrom L, Franko DL, Pai S, Tucker R, Becker AE, et al. Primary prevention of risk factors for eating disorders in adolescent girls: learning from practice. Int J Eat Disord 2002;32:401-11. http://dx.doi.org/10.1002/eat.10089.
- Calear AL, Christensen H. Systematic review of school-based prevention and early intervention programs for depression. J Adolesc 2010;33:429-38. http://dx.doi.org/10.1016/j.adolescence.2009.07.004.
- Stice E, Shaw H, Burton E, Wade E. Dissonance and healthy weight eating disorder prevention programs: a randomized efficacy trial. J Consult Clin Psychol 2006;74:263-75. https://doi.org/10.1037/0022-006X.74.2.263.
- Stice E, Rohde P, Shaw H, Gau J. An effectiveness trial of a selected dissonance-based eating disorder prevention program for female high school students: long-term effects. J Consult Clin Psychol 2011;79:500-8. http://dx.doi.org/10.1037/a0024351.
- Baranowski MJ, Hetherington MM. Testing the efficacy of an eating disorder prevention program. Int J Eat Disord 2001;29:119-24. https://doi.org/10.1002/1098-108X(200103)29:2<119::AID-EAT1001>3.0.CO;2-U.
- Schmidt U, Magill N, Renwick B, Keyes A, Kenyon M, Dejong H, et al. The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with broadly defined anorexia nervosa: a randomized controlled trial. J Consult Clin Psychol 2015;83:796-807. http://dx.doi.org/10.1037/ccp0000019.
- DeJong H, Oldershaw A, Sternheim L, Samarawickrema N, Kenyon MD, Broadbent H, et al. Quality of life in anorexia nervosa, bulimia nervosa and eating disorder not-otherwise-specified. J Eat Disord 2013;1. https://doi.org/10.1186/2050-2974-1-43.
- Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet 2010;375:583-93. http://dx.doi.org/10.1016/S0140-6736(09)61748-7.
- Lopez C, Tchanturia K, Stahl D, Treasure J. Weak central coherence in eating disorders: a step towards looking for an endophenotype of eating disorders. J Clin Exp Neuropsychol 2009;31:117-25. http://dx.doi.org/10.1080/13803390802036092.
- Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychol Med 2007;37:1075-84. https://doi.org/10.1017/S0033291707009877.
- Simon J, Schmidt U, Pilling S. The health service use and cost of eating disorders. Psychol Med 2005;35:1543-51. https://doi.org/10.1017/S0033291705004708.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. https://doi.org/10.1001/jama.283.15.2008.
- The Cost of Eating Disorders. London: PricewaterhouseCoopers; 2015.
- The Butterfly Foundation . Paying the Price: The Economic and Social Impact of Eating Disorders in Australia 2012. https://thebutterflyfoundation.org.au/assets/Uploads/Butterfly-report-Paying-the-Price-Executive-Summary.pdf (accessed 4 April 2017).
- le Grange D, Eisler I. Family interventions in adolescent anorexia nervosa. Child Adolesc PsychiatrClin N Am 2009;18:159-73. http://dx.doi.org/10.1016/j.chc.2008.07.004.
- Dejong H, Broadbent H, Schmidt U. A systematic review of dropout from treatment in outpatients with anorexia nervosa. Int J Eat Disord 2012;45:635-47. http://dx.doi.org/10.1002/eat.20956.
- Berkman ND, Bulik CM, Brownley KA, Lohr KN, Sedway JA, Rooks A, et al. Management of eating disorders. Evid Rep Technol Assess 2006;135:1-166.
- Watson HJ, Bulik CM. Update on the treatment of anorexia nervosa: review of clinical trials, practice guidelines and emerging interventions. Psychol Med 2013;43:2477-500. http://dx.doi.org/10.1017/S0033291712002620.
- Fairburn CG. Evidence-based treatment of anorexia nervosa. Int J Eat Disord 2005;37:S26-30. http://dx.doi.org/10.1002/eat.20112.
- Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa and Related Eating Disorders. Leicester & London: British Psychological Society; 2004.
- Hay P, Bacaltchuk J, Claudino A, Ben-Tovim D, Yong PY. Individual psychotherapy in the outpatient treatment of adults with anorexia nervosa. Cochrane Database Syst Rev 2003;4. http://dx.doi.org/10.1002/14651858.CD003909.
- Schmidt U, Wade TD, Treasure J. The Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA): development, key features and preliminary evidence. Jo Cogn Psychother 2014;28:48-71. https://doi.org/10.1891/0889-8391.28.1.48.
- McIntosh VV, Jordan J, Carter FA, Luty SE, McKenzie JM, Bulik CM, et al. Three psychotherapies for anorexia nervosa: a randomized, controlled trial. Am J Psychiatry 2005;162:741-7. https://doi.org/10.1176/appi.ajp.162.4.741.
- Schmidt U, Renwick B, Lose A, Kenyon M, DeJong H, Broadbent H, et al. The MOSAIC study – comparison of the Maudsley Model of Treatment for Adults with Anorexia Nervosa (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with anorexia nervosa or eating disorder not otherwise specified, anorexia nervosa type: study protocol for a randomized controlled trial. Trials 2013;30. https://doi.org/10.1186/1745-6215-14-160.
- Thomas JJ, Vartanian LR, Brownell KD. The relationship between eating disorder not otherwise specified (EDNOS) and officially recognized eating disorders: meta-analysis and implications for DSM. Psychol Bull 2009;135:407-33. http://dx.doi.org/10.1037/a0015326.
- Wild B, Friederich HC, Gross G, Teufel M, Herzog W, Giel KE, et al. The ANTOP study: focal psychodynamic psychotherapy, cognitive-behavioural therapy, and treatment-as-usual in outpatients with anorexia nervosa – a randomized controlled trial. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-23.
- Zipfel S, Wild B, Gross G, Friederich HC, Teufel M, Schellberg D, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet 2014;383:127-37. http://dx.doi.org/10.1016/S0140-6736(13)61746-8.
- McIntosh V, Jordan JM, Joyce PR, McKenzie JM, Luty SE, Carter FA, et al. Specialist Supportive Clinical Care: Therapist Manual 1997.
- Serpell L, Neiderman M, Haworth E, Emmanueli F, Lask B. The use of the Pros and Cons of Anorexia Nervosa (P-CAN) scale with children and adolescents. J Psychosom Res 2003;54:567-71. https://doi.org/10.1016/S0022-3999(02)00464-6.
- Serpell L, Teasdale JD, Troop NA, Treasure J. The development of the P-CAN, a measure to operationalize the pros and cons of anorexia nervosa. Int J Eat Disord 2004;36:416-33. http://dx.doi.org/10.1002/eat.20040.
- Serpell L, Treasure J, Teasdale J, Sullivan V. Anorexia nervosa: friend or foe?. Int J Eat Disord 1999;25:177-86. https://doi.org/10.1002/(SICI)1098-108X(199903)25:2<177::AID-EAT7>3.0.CO;2-D.
- Schmidt U, Oldershaw A, Jichi F, Sternheim L, Startup H, McIntosh V, et al. Out-patient psychological therapies for adults with anorexia nervosa: randomised controlled trial. Br J Psychiatry 2012;201:392-9. http://dx.doi.org/10.1192/bjp.bp.112.112078.
- Miller WR, Rollnick S. Motivational Interviewing. Preparing People for Change. New York, NY: Guilford Press; 2002.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Behavior Change at Population, Community and Individual Levels. London: NICE; 2007.
- McIntosh VV, Jordan J, Luty SE, Carter FA, McKenzie JM, Bulik CM, et al. Specialist supportive clinical management for anorexia nervosa. Int J Eat Disord 2006;39:625-32. http://dx.doi.org/10.1002/eat.20297.
- Fairburn CG, Beglin S, Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. New York, NY: Guildford; 2008.
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire?. Int J Eat Disord 1994;16:363-70.
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, et al. The obsessive-compulsive inventory: development and validation of a short version. Psychol Assess 2002;14:485-96. https://doi.org/10.1037/1040-3590.14.4.485.
- Martin MM, Rubin RB. A new measure of cognitive flexibility. Psychol Rep 1995;76:623-6. https://doi.org/10.2466/pr0.1995.76.2.623.
- Rimes KA, Chalder T. The Beliefs about Emotions Scale: validity, reliability and sensitivity to change. J Psychosom Res 2010;68:285-92. http://dx.doi.org/10.1016/j.jpsychores.2009.09.014.
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 2003;85:348-62. https://doi.org/10.1037/0022-3514.85.2.348.
- Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol 1948;38:404-11. https://doi.org/10.1037/h0059831.
- Computerised Wisconsin Card Sort Task Version 4 (WCST). Lutz, FL: Psychological Assessment Resources; 2003.
- Burgess PW, Shallice T. The Hayling and Brixton Tests. Bury St Edmunds: Thames Valley Test Company; 1997.
- Osterrieth PA. Le test de copie d’une figure complexe. Arch Psychol 1944;30:206-35.
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol 1941;28:215-85.
- Golan O, Baron-Cohen S, Hill JJ, Golan Y. The ‘reading the mind in films’ task: complex emotion recognition in adults with and without autism spectrum conditions. Soc Neurosci 2006;1:111-23. http://dx.doi.org/10.1080/17470910600980986.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Royal College of Psychiatrists; 2001.
- Bohn K, Fairburn CG, Fairburn CG. Cognitive Behaviour Therapy and Eating Disorders. New York, NY: Guilford Press; 2008.
- Kenyon M, Samarawickrema N, Dejong H, Van den Eynde F, Startup H, Lavender A, et al. Theory of mind in bulimia nervosa. Int J Eat Disord 2012;45:377-84. http://dx.doi.org/10.1002/eat.20967.
- Touyz S, Le Grange D, Lacey H, Hay P, Smith R, Maguire S, et al. Treating severe and enduring anorexia nervosa: a randomized controlled trial. Psychol Med 2013;43:2501-11. http://dx.doi.org/10.1017/S0033291713000949.
- Carter FA, Jordan J, McIntosh VV, Luty SE, McKenzie JM, Frampton CM, et al. The long-term efficacy of three psychotherapies for anorexia nervosa: a randomized, controlled trial. Int J Eat Disord 2011;44:647-54. http://dx.doi.org/10.1002/eat.20879.
- Lose A, Davies C, Renwick B, Kenyon M, Treasure J, Schmidt U. Process Evaluation of the MOSAIC trial. Part II: patient experiences of two psychological therapies for treatment of anorexia nervosa. Eur Eat Disord Rev 2014;22:131-9.
- Waterman-Collins D, Renwick B, Lose A, Kenyon M, Serpell L, Richards L, et al. Process evaluation of the MOSAIC Trial. Part I: therapist experiences of delivering two psychological therapies for treatment of anorexia nervosa. Eur Eat Disord Rev 2014;22:122-30. http://dx.doi.org/10.1002/erv.2278.
- Zainal KA, Renwick B, Keyes A, Lose A, Kenyon M, DeJong H, et al. Process evaluation of the MOSAIC trial: the acceptability, credibility and overall treatment experience of two psychological therapies for the treatment of anorexia nervosa. J Eat Disord 2016;4. https://doi.org/10.1186/s40337-016-0091-5.
- Schmidt U, Ryan EG, Bartholdy S, Renwick B, Keyes A, O’Hara C, et al. Two-year follow-up of the MOSAIC trial: A multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord 2016;49:793-800. https://doi.org/10.1002/eat.22523.
- Guidelines for Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa, and Related Eating Disorders. London: NICE; 2004.
- Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychol Med 2010;40:1887-97. http://dx.doi.org/10.1017/S0033291710000036.
- Davies H, Schmidt U, Stahl D, Tchanturia K. Evoked facial emotional expression and emotional experience in people with anorexia nervosa. Int J Eat Disord 2011;44:531-9. http://dx.doi.org/10.1002/eat.20852.
- Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol 2008;23:201-16. https://doi.org/10.1016/j.acn.2007.08.010.
- Diagnostics and Statistical Manual. Washington, DC: American Psychiatric Association; 2000.
- Money C, Genders R, Treasure J, Schmidt U, Tchanturia K. A brief emotion focused intervention for inpatients with anorexia nervosa: a qualitative study. J Health Psychol 2011;16:947-58. http://dx.doi.org/10.1177/1359105310396395.
- Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs 2008;62:107-15. http://dx.doi.org/10.1111/j.1365-2648.2007.04569.x.
- Joffe H, Yardley L, Marks DF, Yardley L. Research Methods for Clinical and Health Psychology. London: Sage; 2004.
- Castro L, Davies H, Hale L, Surguladze S, Tchanturia K. Facial affect recognition in anorexia nervosa: is obsessionality a missing piece of the puzzle?. Aust N Z J Psychiatry 2010;44:1118-25. http://dx.doi.org/10.3109/00048674.2010.524625.
- Jänsch C, Harmer C, Cooper MJ. Emotional processing in women with anorexia nervosa and in healthy volunteers. Eat Behav 2009;10:184-91. http://dx.doi.org/10.1016/j.eatbeh.2009.06.001.
- Hartmann A, Zeeck A, Barrett MS. Interpersonal problems in eating disorders. Int J Eat Disord 2010;43:619-27. http://dx.doi.org/10.1002/eat.20747.
- Nelson HE, Willison J. National Adult Reading Test (NART). Windsor: Nfer-Nelson; 1991.
- Heaton RK. Wisconsin Card Sorting Test: Computer Version 2. Odessa, FL: Psychological Assessment Resources; 1993.
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn 1980;6:174-215. https://doi.org/10.1037/0278-7393.6.2.174.
- Witkin H, Oltman P, Raskin E, Karp S. Group Embedded Figures Test Manual. Redwood City, CA: Mind Garden Inc.; 2002.
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘Reading the Mind in the Eyes’ Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 2001;42:241-51. https://doi.org/10.1111/1469-7610.00715.
- Ashwin C, Wheelwright S, Baron-Cohen S. Attention bias to faces in Asperger syndrome: a pictorial emotion Stroop study. Psychol Med 2006;36:835-43. https://doi.org/10.1017/S0033291706007203.
- Davies H, Fox J, Naumann U, Treasure J, Schmidt U, Tchanturia K. Cognitive remediation and emotion skills training for anorexia nervosa: an observational study using neuropsychological outcomes. Eur Eat Disord Rev 2012;20:211-17. http://dx.doi.org/10.1002/erv.2170.
- Tchanturia K, Morris RG, Anderluh MB, Collier DA, Nikolaou V, Treasure J. Set shifting in anorexia nervosa: an examination before and after weight gain, in full recovery and relationship to childhood and adult OCPD traits. J Psychiatr Res 2004;38:545-52. http://dx.doi.org/10.1016/j.jpsychires.2004.03.001.
- Lounes N, Curtis H, Mountfoud V, Tchanturia K. From systematic review to quality standards and clinical reality: inpatient treatment of anorexia nervosa. Clin Psychol Forum 2011;227:42-6.
- Davies H, Swan N, Schmidt U, Tchanturia K. An experimental investigation of verbal expression of emotion in anorexia and bulimia nervosa. Eur Eat Disord Rev 2012;20:476-83. http://dx.doi.org/10.1002/erv.1157.
- Kyriacou O, Easter A, Tchanturia K. Comparing views of patients, parents, and clinicians on emotions in anorexia: a qualitative study. J Health Psychol 2009;14:843-54. http://dx.doi.org/10.1177/1359105309340977.
- Tchanturia K, Anderluh MB, Morris RG, Rabe-Hesketh S, Collier DA, Sanchez P, et al. Cognitive flexibility in anorexia nervosa and bulimia nervosa. J Int Neuropsychol Soc 2004;10:513-20. http://dx.doi.org/10.1017/S1355617704104086.
- Russell TA, Schmidt U, Doherty L, Young V, Tchanturia K. Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind. Psychiatry Res 2009;168:181-5. http://dx.doi.org/10.1016/j.psychres.2008.10.028.
- Hambrook D, Brown G, Tchanturia K. Emotional intelligence in anorexia nervosa: is anxiety a missing piece of the puzzle?. Psychiatry Res 2012;200:12-9. http://dx.doi.org/10.1016/j.psychres.2012.05.017.
- Tchanturia K, Hambrook D, Curtis H, Jones T, Lounes N, Fenn K, et al. Work and social adjustment in patients with anorexia nervosa. Compr Psychiatry 2013;54:41-5. http://dx.doi.org/10.1016/j.comppsych.2012.03.014.
- Støving RK, Andries A, Brixen K, Bilenberg N, Hørder K. Gender differences in outcome of eating disorders: a retrospective cohort study. Psychiatry Res 2011;186:362-6. http://dx.doi.org/10.1016/j.psychres.2010.08.005.
- Morgan HG, Russell GF. Value of family background and clinical features as predictors of long-term outcome in anorexia nervosa: four-year follow-up study of 41 patients. Psychol Med 1975;5:355-71. https://doi.org/10.1017/S0033291700056981.
- Russell GF, Szmukler GI, Dare C, Eisler I. An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry 1987;44:1047-56. https://doi.org/10.1001/archpsyc.1987.01800240021004.
- Collins RL, Marlatt AG, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 2005.
- Long CG, Fitzgerald K, Hollin CR. Treatment of chronic anorexia nervosa: a 4-year follow up of adult patients treated in an acute inpatient setting. Clin Psychol Psychother 2011;19:1-13. http://dx.doi.org/10.1002/cpp.738.
- Herpertz-Dahlman B, Schwarte R, Krei M, Egberts K, Warnke A, Wewetzer C, et al. Day patient treatment after short inpatient care vs. inpatient treatment in adoelscent anorexia nervosa (ANDI): a multicentre, randomised, open-label, non-inferiority trial. Lancet 2014;383:1222-9. https://doi.org/10.1016/S0140-6736(13)62411-3.
- Fisher CA, Hetrick SE, Rushford N. Family therapy for anorexia nervosa. Cochrane Database Syst Rev 2010;4. http://dx.doi.org/10.1002/14651858.CD004780.pub2.
- Couturier J, Kimber M, Jack S, Niccols A, Van Blyderveen S, McVey G. Understanding the uptake of family-based treatment for adolescents with anorexia nervosa: therapist perspectives. Int J Eat Disord 2013;46:177-88. http://dx.doi.org/10.1002/eat.22049.
- Haigh R, Treasure J. Investigating the needs of carers in the area of eating disorders: development of the Carers’ Needs Assessment Measure (CaNAM). Eur Eat Disord Rev 2003;11:125-41. https://doi.org/10.1002/erv.487.
- De Zwaan M, Zipfel S, Herzog W, Herpertz-Dahlmann B, Konrad K, Hebebrand J, et al. EDNET – eating disorders diagnostic and treatment network. Psychother Psychosom Med Psychol 2009;59:110-16.
- Zabala MJ, Macdonald P, Treasure J. Appraisal of caregiving burden, expressed emotion and psychological distress in families of people with eating disorders: a systematic review. Eur Eat Disord Rev 2009;17:338-49. http://dx.doi.org/10.1002/erv.925.
- Anastasiadou D, Medina-Pradas C, Sepulveda A, Treasure J. A systematic review of family caregiving in eating disorders. Eat Behav 2014;15:464-77. https://doi.org/10.1016/j.eatbeh.2014.06.001.
- Hibbs R, Rhind C, Leppanen J, Treasure J. Interventions for caregivers of someone with an eating disorder: a systematic review and meta-analysis. Int J Eat Disord 2015;48:349-61. https://doi.org/10.1002/eat.22298.
- Kyriacou O, Treasure J, Schmidt U. Expressed emotion in eating disorders assessed via self-report: an examination of factors associated with expressed emotion in carers of people with anorexia nervosa in comparison to control families. Int J Eat Disord 2008;41:37-46. http://dx.doi.org/10.1002/eat.20469.
- Sepulveda AR, Kyriacou O, Treasure J. Development and validation of the accommodation and enabling scale for eating disorders (AESED) for caregivers in eating disorders. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-171.
- Goddard E, Salerno L, Hibbs R, Raenker S, Naumann U, Arcelus J, et al. Empirical examination of the interpersonal maintenance model of anorexia nervosa. Int J Eat Disord 2013;46:867-74. http://dx.doi.org/10.1002/eat.22172.
- Goddard E, Raenker S, Macdonald P, Todd G, Beecham J, Naumann U, et al. Carers’ assessment, skills and information sharing: theoretical framework and trial protocol for a randomised controlled trial evaluating the efficacy of a complex intervention for carers of inpatients with anorexia nervosa. Eur Eat Disord Rev 2013;21:60-71. http://dx.doi.org/10.1002/erv.2193.
- Goddard E, Macdonald P, Sepulveda AR, Naumann U, Landau S, Schmidt U, et al. Cognitive interpersonal maintenance model of eating disorders: intervention for carers. Br J Psychiatry 2011;199:225-31. http://dx.doi.org/10.1192/bjp.bp.110.088401.
- Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, et al. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA 2006;295:2605-12. https://doi.org/10.1001/jama.295.22.2605.
- Pike KM, Walsh BT, Vitousek K, Wilson GT, Bauer J. Cognitive behavior therapy in the posthospitalization treatment of anorexia nervosa. Am J Psychiatry 2003;160:2046-9. http://dx.doi.org/10.1176/appi.ajp.160.11.2046.
- Sepulveda AR, Whitney J, Hankins M, Treasure J. Development and validation of an Eating Disorders Symptom Impact Scale (EDSIS) for carers of people with eating disorders. Health Qual Life Outcomes 2008;6. http://dx.doi.org/10.1186/1477-7525-6-28.
- Succeed . How to Care for Someone With an Eating Disorder – The New Maudsley Approach – SUCCEED 2013.
- Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther 1997;35:79-8. https://doi.org/10.1016/S0005-7967(96)00068-X.
- Bauer S, Winn S, Schmidt U, Kordy H. Construction, scoring and validation of the Short Evaluation of Eating Disorders (SEED). Eur Eat Disord Rev 2005;13:191-200. https://doi.org/10.1002/erv.637.
- Luce KH, Crowther JH. The reliability of the Eating Disorder Examination-Self-Report Questionnaire Version (EDE-Q). Int J Eat Disord 1999;25:349-51. https://doi.org/10.1002/(SICI)1098-108X(199904)25:3<349::AID-EAT15>3.0.CO;2-M.
- WHOQoL-Group . Development of the World Health Organization WHOQUOL-Brief quality of life assessment. Psychol Med 1998;28:551-8. https://doi.org/10.1017/S0033291798006667.
- Wiedemann G, Rayki O, Feinstein E, Hahlweg K. The Family Questionnaire: development and validation of a new self-report scale for assessing expressed emotion. Psychiatry Res 2002;109:265-79. https://doi.org/10.1016/S0165-1781(02)00023-9.
- Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2393.
- Dunn G, Maracy M, Dowrick C, Ayuso-Mateos JL, Dalgard OS, Page H, et al. Estimating psychological treatment effects from a randomised controlled trial with both non-compliance and loss to follow-up. Br J Psychiatry 2003;183:323-31. https://doi.org/10.1192/bjp.183.4.323.
- Zipfel S, Wild B, Gross G, Friederich HC, Teufel M, Schellberg D, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet 2014;383:127-37. https://doi.org/10.1016/S0140-6736(13)61746-8.
- Goddard E, Hibbs R, Raenker S, Salerno L, Arcelus J, Boughton N, et al. A multi-centre cohort study of short term outcomes of hospital treatment for anorexia nervosa in the UK. BMC Psychiatry 2013;13. http://dx.doi.org/10.1186/1471-244X-13-287.
- Goddard E, Macdonald P, Treasure J. An examination of the impact of the Maudsley Collaborative Care skills training workshops on patients with anorexia nervosa: a qualitative study. Eur Eat Disord Rev 2011;19:150-61. https://doi.org/10.1002/erv.1042.
- Hay PJ, Claudino AM. Clinical psychopharmacology of eating disorders: a research update. Int J Neuropsychopharmacol 2012;15:209-22. http://dx.doi.org/10.1017/S1461145711000460.
- Wonderlich S, Mitchell JE, Crosby RD, Myers TC, Kadlec K, Lahaise K, et al. Minimizing and treating chronicity in the eating disorders: a clinical overview. Int J Eat Disord 2012;45:467-75. http://dx.doi.org/10.1002/eat.20978.
- Keyes A, Woerwag-Mehta S, Bartholdy S, Koskina A, Middleton B, Connan F, et al. Physical activity and the drive to exercise in anorexia nervosa. Int J Eat Disord 2015;48:46-54. http://dx.doi.org/10.1002/eat.22354.
- Klein DA, Mayer LE, Schebendach JE, Walsh BT. Physical activity and cortisol in anorexia nervosa. Psychoneuroendocrinology 2007;32:539-47. https://doi.org/10.1016/j.psyneuen.2007.03.007.
- Dellava JE, Hamer RM, Kanodia A, Reyes-Rodríguez ML, Bulik CM. Diet and physical activity in women recovered from anorexia nervosa: a pilot study. Int J Eat Disord 2011;44:376-82. http://dx.doi.org/10.1002/eat.20865.
- Holtkamp K, Hebebrand J, Herpertz-Dahlmann B. The contribution of anxiety and food restriction on physical activity levels in acute anorexia nervosa. Int J Eat Disord 2004;36:163-71. http://dx.doi.org/10.1002/eat.20035.
- Rigaud D, Pennacchio H, Bizeul C, Reveillard V, Vergès B. Outcome in AN adult patients: a 13-year follow-up in 484 patients. Diabetes Metab 2011;37:305-11. http://dx.doi.org/10.1016/j.diabet.2010.11.020.
- Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10-15 years in a prospective study. Int J Eat Disord 1997;22:339-60. https://doi.org/10.1002/(SICI)1098-108X(199712)22:4<339::AID-EAT1>3.0.CO;2-N.
- Casper RC, Jabine LN. An eight-year follow-up: outcome from adolescent compared to adult onset anorexia nervosa. J Youth Adolesc 1996;25:499-517. https://doi.org/10.1007/BF01537545.
- Hechler T, Beaumont P, Touyz S, Marks P, Vocks S. The importance of physical activity in anorexia nervosa – dimensions, assessment and treatment strategies from clinical experts’ point of view. Verhaltenstherapie 2005;15:140-7. https://doi.org/10.1159/000087374.
- Davis C, Blackmore E, Katzman DK, Fox J. Female adolescents with anorexia nervosa and their parents: a case-control study of exercise attitudes and behaviours. Psychol Med 2005;35:377-86. https://doi.org/10.1017/S0033291704003447.
- Kron L, Katz JL, Gorzynski G, Weiner H. Hyperactivity in anorexia nervosa: a fundamental clinical feature. Compr Psychiatry 1978;19:433-40. https://doi.org/10.1016/0010-440X(78)90072-X.
- Falk JR, Halmi KA, Tryon WW. Activity measures in anorexia nervosa. Arch Gen Psychiatry 1985;42:811-14. https://doi.org/10.1001/archpsyc.1985.01790310073010.
- Bratland-Sanda S, Sundgot-Borgen J, Rø O, Rosenvinge JH, Hoffart A, Martinsen EW. ‘I’m not physically active – I only go for walks’: physical activity in patients with longstanding eating disorders. Int J Eat Disord 2010;43:88-92. http://dx.doi.org/10.1002/eat.20753.
- Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K, et al. The prevalence of high-level exercise in the eating disorders: etiological implications. Compr Psychiatry 1997;38:321-6. https://doi.org/10.1016/S0010-440X(97)90927-5.
- Favaro A, Caregaro L, Burlina AB, Santonastaso P. Tryptophan levels, excessive exercise, and nutritional status in anorexia nervosa. Psychosom Med 2000;62:535-8. https://doi.org/10.1097/00006842-200007000-00012.
- El Ghoch M, Calugi S, Pellegrini M, Milanese C, Busacchi M, Battistini NC, et al. Measured physical activity in anorexia nervosa: features and treatment outcome. Int J Eat Disord 2013;46:709-12. http://dx.doi.org/10.1002/eat.22140.
- Zipfel S, Mack I, Baur LA, Hebebrand J, Touyz S, Herzog W, et al. Impact of exercise on energy metabolism in anorexia nervosa. J Eat Disord 2013;1. http://dx.doi.org/10.1186/2050-2974-1-37.
- Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J Psychosom Res 1992;36:55-6. https://doi.org/10.1016/0022-3999(92)90114-H.
- Sexton H, Maere A, Dahl NH. Exercise intensity and reduction in neurotic symptoms. A controlled follow-up study. Acta Psychiatr Scand 1989;80:231-5. https://doi.org/10.1111/j.1600-0447.1989.tb01332.x.
- Mond JM, Calogero RM. Excessive exercise in eating disorder patients and in healthy women. Aust N Z J Psychiatry 2009;43:227-34. http://dx.doi.org/10.1080/00048670802653323.
- Carrera O, Adan RA, Gutierrez E, Danner UN, Hoek HW, van Elburg AA, et al. Hyperactivity in anorexia nervosa: warming up not just burning-off calories. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0041851.
- Lavender JM, De Young KP, Wonderlich SA, Crosby RD, Engel SG, Mitchell JE, et al. Daily patterns of anxiety in anorexia nervosa: associations with eating disorder behaviors in the natural environment. J Abnorm Psychol 2013;122:672-83. http://dx.doi.org/10.1037/a0031823.
- Jordan J, Joyce PR, Carter FA, Horn J, McIntosh VV, Luty SE, et al. Specific and nonspecific comorbidity in anorexia nervosa. Int J Eat Disord 2008;41:47-56. http://dx.doi.org/10.1002/eat.20463.
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry 2004;161:2215-21. https://doi.org/10.1176/appi.ajp.161.12.2215.
- Raney TJ, Thornton LM, Berrettini W, Brandt H, Crawford S, Fichter MM, et al. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. Int J Eat Disord 2008;41:326-32. http://dx.doi.org/10.1002/eat.20508.
- Bulik CM, Sullivan PF, Fear JL, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatr Scand 1997;96:101-7. https://doi.org/10.1111/j.1600-0447.1997.tb09913.x.
- Dellava JE, Thornton LM, Hamer RM, Strober M, Plotnicov K, Klump KL, et al. Childhood anxiety associated with low BMI in women with anorexia nervosa. Behav Res Ther 2010;48:60-7. http://dx.doi.org/10.1016/j.brat.2009.09.009.
- Bloss CS, Berrettini W, Bergen AW, Magistretti P, Duvvuri V, Strober M, et al. Genetic association of recovery from eating disorders: the role of GABA receptor SNPs. Neuropsychopharmacology 2011;36:2222-32. http://dx.doi.org/10.1038/npp.2011.108.
- Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M, et al. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur J Public Health 2006;16:179-84. https://doi.org/10.1093/eurpub/cki159.
- First M, Gibbon M, Spitzer RL, Williams J. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorder – Research Version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 1995;33:335-43. https://doi.org/10.1016/0005-7967(94)00075-U.
- Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol 2005;44:227-39. http://dx.doi.org/10.1348/014466505X29657.
- Craig C, Marshall A, Sjöström M, Bauman A, Booth M, Ainsworth B, et al. IPAQ Reliability and Validity Study Group . International Physical Activity Questionnaire (IPAQ): 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Alberti M, Galvani C, El Ghoch M, Capelli C, Lanza M, Calugi S, et al. Assessment of physical activity in anorexia nervosa and treatment outcome. Med Sci Sports Exerc 2013;45:1643-8. http://dx.doi.org/10.1249/MSS.0b013e31828e8f07.
- Freizinger M, Franko DL, Dacey M, Okun B, Domar AD. The prevalence of eating disorders in infertile women. Fertil Steril 2010;93:72-8. http://dx.doi.org/10.1016/j.fertnstert.2008.09.055.
- Davis C, Brewer H, Ratusny D. Behavioral frequency and psychological commitment: necessary concepts in the study of excessive exercising. J Behav Med 1993;16:611-28. https://doi.org/10.1007/BF00844722.
- Davis C, Kennedy SH, Ralevski E, Dionne M, Brewer H, Neitzert C, et al. Obsessive compulsiveness and physical activity in anorexia nervosa and high-level exercising. J Psychosom Res 1995;39:967-76. https://doi.org/10.1016/0022-3999(95)00064-X.
- Mond JM, Hay PJ, Rodgers B, Owen C. An update on the definition of ‘excessive exercise’ in eating disorders research. Int J Eat Disord 2006;39:147-53. http://dx.doi.org/10.1002/eat.20214.
- Thompson J, Pasman L. The obligatory exercise questionnaire. Behavior Therapist 1991;14:33620-8200.
- Pasman L, Thompson JK. Body image and eating disturbance in obligatory runners, obligatory weightlifters, and sedentary individuals. Int J Eat Disord 1988;7:759-69. https://doi.org/10.1002/1098-108X(198811)7:6<759::AID-EAT2260070605>3.0.CO;2-G.
- Ackard DM, Croll JK, Kearney-Cooke A. Dieting frequency among college females: association with disordered eating, body image, and related psychological problems. J Psychosom Res 2002;52:129-36. https://doi.org/10.1016/S0022-3999(01)00269-0.
- Brehm BJ, Steffen JJ. Relation between obligatory exercise and eating disorders. Am J Health Behav 1998;22:108-19.
- Krejci RC, Sargent R, Forand KJ, Ureda JR, Saunders RP, Larry Durstine J. Psychological and behavioral differences among females classified as bulimic, obligatory exerciser and normal control. Psychiatry 1992;55:185-93. http://dx.doi.org/10.1080/00332747.1992.11024592.
- Terry A, Szabo A, Griffiths M. The exercise addiction inventory: a new brief screening tool. Addict Res Theory 2004;12:489-99. https://doi.org/10.1080/16066350310001637363.
- Griffiths MD, Szabo A, Terry A. The exercise addiction inventory: a quick and easy screening tool for health practitioners. Br J Sports Med 2005;39. https://doi.org/10.1136/bjsm.2004.017020.
- Silberstein L, Striegel-Moore R, Timko C, Rodin J. Behavioral and psychological implications of body dissatisfaction: do men and women differ?. Sex Roles 1988;19:219-32. http://dx.doi.org/10.1007/BF00290156.
- Holtkamp K, Herpertz-Dahlmann B, Hebebrand K, Mika C, Kratzsch J, Hebebrand J. Physical activity and restlessness correlate with leptin levels in patients with adolescent anorexia nervosa. Biol Psychiatry 2006;60:311-13. https://doi.org/10.1016/j.biopsych.2005.11.001.
- van Elburg AA, Hoek HW, Kas MJ, van Engeland H. Nurse evaluation of hyperactivity in anorexia nervosa: a comparative study. Eur Eat Disord Rev 2007;15:425-9. http://dx.doi.org/10.1002/erv.803.
- Mond JM, Myers TC, Crosby RD, Hay PJ, Rodgers B, Morgan JF, et al. Screening for eating disorders in primary care: EDE-Q versus SCOFF. Behav Res Ther 2008;46:612-22. http://dx.doi.org/10.1016/j.brat.2008.02.003.
- Connan F, Troop N, Landau S, Campbell IC, Treasure J. Poor social comparison and the tendency to submissive behavior in anorexia nervosa. Int J Eat Disord 2007;40:733-9. http://dx.doi.org/10.1002/eat.20427.
- Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry 1995;152:1073-4. http://dx.doi.org/10.1176/ajp.152.7.1073.
- Guillaume S, Jaussent I, Olié E, Genty C, Bringer J, Courtet P, et al. Characteristics of suicide attempts in anorexia and bulimia nervosa: a case-control study. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0023578.
- Herzog DB, Dorer DJ, Keel PK, Selwyn SE, Ekeblad ER, Flores AT, et al. Recovery and relapse in anorexia and bulimia nervosa: a 7.5-year follow-up study. J Am Acad Child Adolesc Psychiatry 1999;38:829-37. https://doi.org/10.1097/00004583-199907000-00012.
- Carter JC, Blackmore E, Sutandar-Pinnock K, Woodside DB. Relapse in anorexia nervosa: a survival analysis. Psychol Med 2004;34:671-9. http://dx.doi.org/10.1017/S0033291703001168.
- Carter JC, Mercer-Lynn KB, Norwood SJ, Bewell-Weiss CV, Crosby RD, Woodside DB, et al. A prospective study of predictors of relapse in anorexia nervosa: implications for relapse prevention. Psychiatry Res 2012;200:518-23. http://dx.doi.org/10.1016/j.psychres.2012.04.037.
- Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R. Ten-year follow-up of anorexia nervosa: clinical course and outcome. Psychol Med 1995;25:143-56. https://doi.org/10.1017/S0033291700028166.
- Norring CE, Sohlberg SS. Outcome, recovery, relapse and mortality across six years in patients with clinical eating disorders. Acta Psychiatr Scand 1993;87:437-44. https://doi.org/10.1111/j.1600-0447.1993.tb03401.x.
- Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa and Related Eating Disorders (CG9). London: NICE; 2004.
- Kaye WH, Nagata T, Weltzin TE, Hsu LK, Sokol MS, McConaha C, et al. Double-blind placebo-controlled administration of fluoxetine in restricting- and restricting-purging-type anorexia nervosa. Biol Psychiatry 2001;49:644-52. https://doi.org/10.1016/S0006-3223(00)01013-1.
- Halmi KA, Agras WS, Mitchell J, Wilson GT, Crow S, Bryson SW, et al. Relapse predictors of patients with bulimia nervosa who achieved abstinence through cognitive behavioral therapy. Arch Gen Psychiatry 2002;59:1105-9. https://doi.org/10.1001/archpsyc.59.12.1105.
- Eisler I, Dare C, Russell GF, Szmukler G, le Grange D, Dodge E. Family and individual therapy in anorexia nervosa. A 5-year follow-up. Arch Gen Psychiatry 1997;54:1025-30. https://doi.org/10.1001/archpsyc.1997.01830230063008.
- Godart N, Berthoz S, Curt F, Perdereau F, Rein Z, Wallier J, et al. A randomized controlled trial of adjunctive family therapy and treatment as usual following inpatient treatment for anorexia nervosa adolescents. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0028249.
- Eisler I, Le Grange D, Asen E, Treasure J, Schmidt U, van Furth E. Handbook of Eating Disorders. Chichester: John Wiley & sons; 2003.
- Fichter MM, Quadflieg N, Nisslmüller K, Lindner S, Osen B, Huber T, et al. Does internet-based prevention reduce the risk of relapse for anorexia nervosa?. Behav Res Ther 2012;50:180-90. http://dx.doi.org/10.1016/j.brat.2011.12.003.
- Wade TD, Treasure J, Schmidt U. A case series evaluation of the Maudsley Model for treatment of adults with anorexia nervosa. Eur Eat Disord Rev 2011;19:382-9. http://dx.doi.org/10.1002/erv.1078.
- Startup H, Lavender A, Oldershaw A, Stott R, Tchanturia K, Treasure J, et al. Worry and rumination in anorexia nervosa. Behav Cogn Psychother 2013;41:301-16. http://dx.doi.org/10.1017/S1352465812000847.
- Sternheim L, Konstantellou A, Startup H, Schmidt U. What does uncertainty mean to women with anorexia nervosa? An interpretative phenomenological analysis. Eur Eat Disord Rev 2011;19:12-24. http://dx.doi.org/10.1002/erv.1029.
- Sternheim L, Startup H, Schmidt U. An experimental exploration of behavioral and cognitive-emotional aspects of intolerance of uncertainty in eating disorder patients. J Anxiety Disord 2011;25:806-12. http://dx.doi.org/10.1016/j.janxdis.2011.03.020.
- Sternheim L, Startup H, Saeidi S, Morgan J, Hugo P, Russell A, et al. Understanding catastrophic worry in eating disorders: process and content characteristics. J Behav Ther Exp Psychiatry 2012;43:1095-103. http://dx.doi.org/10.1016/j.jbtep.2012.05.006.
- Fairburn CG, Cooper Z, Fairburn CG, Wilson G. Binge Eating: Nature, Assessment, and Treatment. New York, NY: Guilford Press; 1993.
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 1990;28:487-95. https://doi.org/10.1016/0005-7967(90)90135-6.
- Startup H, Erickson T, Davey G, Wells A. Worry and its Psychological Disorders. Chichester: Wiley; 2006.
- Freeston MH, Rhéaume J, Letarte H, Dugas MJ, Ladouceur R. Why do people worry?. Pers Indiv Dif 1994;17:791-802. https://doi.org/10.1016/0191-8869(94)90048-5.
- Buhr K, Dugas MJ. The Intolerance of Uncertainty Scale: psychometric properties of the English version. Behav Res Ther 2002;40:931-45. https://doi.org/10.1016/S0005-7967(01)00092-4.
- Giel K, Leehr E, Becker S, Startup H, Zipfel S, Schmidt U. Relapse prevention in anorexia nervosa. Psychother Psychosom Med Psychol 2013;63:290-5. http://dx.doi.org/10.1055/s-0033-1334957.
- Giel KE, Leehr EJ, Becker S, Herzog W, Junne F, Schmidt U, et al. Relapse prevention via videoconference for anorexia nervosa – findings from the RESTART pilot study. Psychother Psychosom 2015;84:381-3. http://dx.doi.org/10.1159/000431044.
- Wilson GT, Zandberg LJ. Cognitive-behavioral guided self-help for eating disorders: effectiveness and scalability. Clin Psychol Rev 2012;32:343-57. http://dx.doi.org/10.1016/j.cpr.2012.03.001.
- Rutter M, Brown GW. The reliability and validity of measures of family life and relationships in families containing a psychiatric patient. Soc Psychiatry Psychiatr Epidemiol 1966;1:38-53. https://doi.org/10.1007/bf00583828.
- Rutter M, Quinton D. Parental psychiatric disorder: effects on children. Psychol Med 1984;14:853-80. https://doi.org/10.1017/S0033291700019838.
- Patel P, Wheatcroft R, Park RJ, Stein A. The children of mothers with eating disorders. Clin Child Fam Psychol Rev 2002;5:1-19. https://doi.org/10.1023/A:1014524207660.
- Bulik CM, Reba L, Siega-Riz AM, Reichborn-Kjennerud T. Anorexia nervosa: definition, epidemiology, and cycle of risk. Int J Eat Disord 2005;37:S2-9. http://dx.doi.org/10.1002/eat.20107.
- Kohmura H, Miyake A, Aono T, Tanizawa O. Recovery of reproductive function in patients with anorexia nervosa: a 10-year follow-up study. Eur J Obstet Gynecol Reprod Biol 1986;22:293-6. https://doi.org/10.1016/0028-2243(86)90117-6.
- Falk JR, Halmi KA. Amenorrhea in anorexia nervosa: examination of the critical body weight hypothesis. Biol Psychiatry 1982;17:799-806.
- Pirke KM, Dogs M, Fichter MM, Tuschl RJ. Gonadotrophins, oestradiol and progesterone during the menstrual cycle in bulimia nervosa. Clin Endocrinol 1988;29:265-70. https://doi.org/10.1111/j.1365-2265.1988.tb01224.x.
- Pirke KM, Schweiger U, Lemmel W, Krieg JC, Berger M. The influence of dieting on the menstrual cycle of healthy young women. J Clin Endocrinol Metab 1985;60:1174-9. http://dx.doi.org/10.1210/jcem-60-6-1174.
- Stewart DE, Robinson E, Goldbloom DS, Wright C. Infertility and eating disorders. Am J Obstet Gynecol 1990;163:1196-9. https://doi.org/10.1016/0002-9378(90)90688-4.
- Bulik CM, Sullivan PF, Fear JL, Pickering A, Dawn A, McCullin M. Fertility and reproduction in women with anorexia nervosa: a controlled study. J Clin Psychiatry 1999;60:130-5. https://doi.org/10.4088/JCP.v60n0212.
- Brinch M, Isager T, Tolstrup K. Anorexia nervosa and motherhood: reproduction pattern and mothering behavior of 50 women. Acta Psychiatr Scand 1988;77:611-17. https://doi.org/10.1111/j.1600-0447.1988.tb05175.x.
- Crow SJ, Thuras P, Keel PK, Mitchell JE. Long-term menstrual and reproductive function in patients with bulimia nervosa. Am J Psychiatry 2002;159:1048-50. http://dx.doi.org/10.1176/appi.ajp.159.6.1048.
- Joffe M. Time to pregnancy: a measure of reproductive function in either sex. Asclepios Project. Occup Environ Med 1997;54:289-95. https://doi.org/10.1136/oem.54.5.289.
- Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol 1986;124:470-80. https://doi.org/10.1093/oxfordjournals.aje.a114417.
- Tietze C. Statistical contributions to the study of human fertility. Fertil Steril 1956;7:88-95. https://doi.org/10.1016/S0015-0282(16)32231-2.
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J 1985;291:1693-7. https://doi.org/10.1136/bmj.291.6510.1693.
- Micali N, Simonoff E, Treasure J. Infant feeding and weight in the first year of life in babies of women with eating disorders. J Pediatr 2009;154:55-60.e1. http://dx.doi.org/10.1016/j.jpeds.2008.07.003.
- Waugh E, Bulik CM. Offspring of women with eating disorders. Int J Eat Disord 1999;25:123-33. https://doi.org/10.1002/(SICI)1098-108X(199903)25:2<123::AID-EAT1>3.0.CO;2-B.
- Treasure JL, Russell GF. Intrauterine growth and neonatal weight gain in babies of women with anorexia nervosa. Br Med J 1988;296. https://doi.org/10.1136/bmj.296.6628.1038.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry 2007;190:255-9. https://doi.org/10.1192/bjp.bp.106.020768.
- Siega-Riz AM, Von Holle A, Haugen M, Meltzer HM, Hamer R, Torgersen L, et al. Gestational weight gain of women with eating disorders in the Norwegian pregnancy cohort. Int J Eat Disord 2011;44:428-34. http://dx.doi.org/10.1002/eat.20835.
- Stein A, Murray L, Cooper P, Fairburn CG. Infant growth in the context of maternal eating disorders and maternal depression: a comparative study. Psychol Med 1996;26:569-74. https://doi.org/10.1017/S0033291700035649.
- Timimi S, Robinson P. Disturbances in children of patients with eating disorders. Eur Eat Disord Rev 1996;4:183-8. https://doi.org/10.1002/(SICI)1099-0968(199609)4:3<183::AID-ERV155>3.0.CO;2-C.
- Stein A, Woolley H, Cooper SD, Fairburn CG. An observational study of mothers with eating disorders and their infants. J Child Psychol Psychiatry 1994;35:733-48. https://doi.org/10.1111/j.1469-7610.1994.tb01218.x.
- Stein A, Woolley H, Cooper S, Winterbottom J, Fairburn CG, Cortina-Borja M. Eating habits and attitudes among 10-year-old children of mothers with eating disorders: longitudinal study. Br J Psychiatry 2006;189:324-9. https://doi.org/10.1192/bjp.bp.105.014316.
- Golding J, Pembrey M, Jones R. ALSPAC Study Team . ALSPAC – the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 2001;15:74-87. https://doi.org/10.1046/j.1365-3016.2001.00325.x.
- North K, Emmett P. Multivariate analysis of diet among three-year-old children and associations with socio-demographic characteristics. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Study Team. Eur J Clin Nutr 2000;54:73-80. https://doi.org/10.1038/sj.ejcn.1600896.
- Northstone K, Emmett P. Multivariate analysis of diet in children at four and seven years of age and associations with socio-demographic characteristics. Eur J Clin Nutr 2005;59:751-60. https://doi.org/10.1038/sj.ejcn.1602136.
- Northstone K, Emmett PM. Are dietary patterns stable throughout early and mid-childhood? A birth cohort study. Br J Nutr 2008;100:1069-76. http://dx.doi.org/10.1017/S0007114508968264.
- Easter A, Treasure J, Micali N. Fertility and prenatal attitudes towards pregnancy in women with eating disorders: results from the Avon Longitudinal Study of Parents and Children. BJOG 2011;118:1491-8. http://dx.doi.org/10.1111/j.1471-0528.2011.03077.x.
- Easter A, Naumann U, Northstone K, Schmidt U, Treasure J, Micali N. A longitudinal investigation of nutrition and dietary patterns in children of mothers with eating disorders. J Pediatr 2013;163:173-8. http://dx.doi.org/10.1016/j.jpeds.2012.11.092.
- Easter A, Howe LD, Tilling K, Schmidt U, Treasure J, Micali N. Growth trajectories in the children of mothers with eating disorders: a longitudinal study. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004453.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol 2009;114:1353-67. http://dx.doi.org/10.1097/AOG.0b013e3181c070f9.
- Stein A, Woolley H, McPherson K. Conflict between mothers with eating disorders and their infants during mealtimes. Br J Psychiatry 1999;175:455-61. https://doi.org/10.1192/bjp.175.5.455.
- Woodside DB, Shekter-Wolfson LF. Parenting by patients with anorexia nervosa and bulimia nervosa. Int J Eat Disord 1990;9:303-9. https://doi.org/10.1002/1098-108X(199005)9:3<303::AID-EAT2260090308>3.0.CO;2-3.
- Micali N, Northstone K, Emmett P, Naumann U, Treasure JL. Nutritional intake and dietary patterns in pregnancy: a longitudinal study of women with lifetime eating disorders. Br J Nutr 2012;108:2093-9. http://dx.doi.org/10.1017/S0007114512000256.
- Feinstein L, Sabates R, Sorhaindo A, Rogers I, Herrick D, Northstone K, et al. Dietary patterns related to attainment in school: the importance of early eating patterns. J Epidemiol Community Health 2008;62:734-9. http://dx.doi.org/10.1136/jech.2007.068213.
- Northstone K, Joinson C, Emmett P, Ness A, Paus T. Are dietary patterns in childhood associated with IQ at 8 years of age? A population-based cohort study. J Epidemiol Community Health 2012;66:624-8. https://doi.org/10.1136/jech.2010.111955.
- Park RJ, Senior R, Stein A. The offspring of mothers with eating disorders. Eur Child Adolesc Psychiatry 2003;12:I110-19. http://dx.doi.org/10.1007/s00787-003-1114-8.
- Johnson SL, Birch LL. Parents’ and children’s adiposity and eating style. Pediatrics 1994;94:653-61.
- Clark HR, Goyder E, Bissell P, Blank L, Peters J. How do parents’ child-feeding behaviours influence child weight? Implications for childhood obesity policy. J Public Health 2007;29:132-41. https://doi.org/10.1093/pubmed/fdm012.
- Fisher JO, Birch LL. Restricting access to foods and children’s eating. Appetite 1999;32:405-19. http://dx.doi.org/10.1006/appe.1999.0231.
- Smolak L, Levine MP, Schermer F. Parental input and weight concerns among elementary school children. Int J Eat Disord 1999;25:263-71. https://doi.org/10.1002/(SICI)1098-108X(199904)25:3<263::AID-EAT3>3.0.CO;2-V.
- Edmunds H, Hill AJ. Dieting and the family context of eating in young adolescent children. Int J Eat Disord 1999;25:435-40. https://doi.org/10.1002/(SICI)1098-108X(199905)25:4<435::AID-EAT8>3.0.CO;2-3.
- Hodes M, Timimi S, Robinson P. Children of mothers with eating disorders: a preliminary study. Eur Eat Disord Rev 1997;5:11-24. https://doi.org/10.1002/(SICI)1099-0968(199703)5:1<11::AID-ERV179>3.0.CO;2-7.
- Neveu R, Neveu D, Carrier E, Ourrad N, Perroud A, Nicolas A. Body mass index kinetics around adiposity rebound in anorexia nervosa: a case-control study. Clin Nutr ESPEN 2016;15:32-7. http://doi.org/10.1016/j.clnesp.2016.05.005.
- van Wezel-Meijler G, Wit JM. The offspring of mothers with anorexia nervosa: a high-risk group for undernutrition and stunting?. Eur J Pediatr 1989;149:130-5. https://doi.org/10.1007/BF01995864.
- Agras S, Hammer L, McNicholas F. A prospective study of the influence of eating-disordered mothers on their children. Int J Eat Disord 1999;25:253-62. https://doi.org/10.1002/(SICI)1098-108X(199904)25:3<253::AID-EAT2>3.0.CO;2-Z.
- House J, Schmidt U, Craig M, Landau S, Simic M, Nicholls D, et al. Comparison of specialist and nonspecialist care pathways for adolescents with anorexia nervosa and related eating disorders. Int J Eat Disord 2012;45:949-56. http://dx.doi.org/10.1002/eat.22065.
- Eisler I, Simic M, Russell GF, Dare C. A randomised controlled treatment trial of two forms of family therapy in adolescent anorexia nervosa: a five-year follow-up. J Child Psychol Psychiatry 2007;48:552-60. https://doi.org/10.1111/j.1469-7610.2007.01726.x.
- Lock J, Couturier J, Agras WS. Comparison of long-term outcomes in adolescents with anorexia nervosa treated with family therapy. J Am Acad Child Adolesc Psychiatry 2006;45:666-72. http://dx.doi.org/10.1097/01.chi.0000215152.61400.ca.
- Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, Jo B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry 2010;67:1025-32. http://dx.doi.org/10.1001/archgenpsychiatry.2010.128.
- Nicholls DE, Lynn R, Viner RM. Childhood eating disorders: British national surveillance study. Br J Psychiatry 2011;198:295-301. http://dx.doi.org/10.1192/bjp.bp.110.081356.
- Meads C, Gold L, Burls A. How effective is outpatient care compared to inpatient care for the treatment of anorexia nervosa? A systematic review. Eur Eat Disord Rev 2001;9:229-41. https://doi.org/10.1002/erv.406.
- Gowers SG, Weetman J, Shore A, Hossain F, Elvins R. Impact of hospitalisation on the outcome of adolescent anorexia nervosa. Br J Psychiatry 2000;176:138-41. https://doi.org/10.1192/bjp.176.2.138.
- Byford S, Barrett B, Roberts C, Clark A, Edwards V, Smethurst N, et al. Economic evaluation of a randomised controlled trial for anorexia nervosa in adolescents. Br J Psychiatry 2007;191:436-40. https://doi.org/10.1192/bjp.bp.107.036806.
- Gowers SG, Clark A, Roberts C, Griffiths A, Edwards V, Bryan C, et al. Clinical effectiveness of treatments for anorexia nervosa in adolescents: randomised controlled trial. Br J Psychiatry 2007;191:427-35. https://doi.org/10.1192/bjp.bp.107.036764.
- Farr H, O’Herlihy A. Child and Adolescent Mental Health In-Patient Units in England, Scotland, Wales & Belfast: Unit Directory. London: Royal College of Psychiatrists Research Unit; 2004.
- A Guide to Purchasing and Providing Services. Norwich: Eating Disorders Association; 1995.
- Office for National Statistics . Population Statistics n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates#datasets (accessed 4 April 2017).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- House J. Service Utilisation and Alternative Care Pathways for Adolescents with Anorexia Nervosa and Related Eating Disorders. London: King’s College London; 2011.
- van Son GE, van Hoeken D, Bartelds AI, van Furth EF, Hoek HW. Time trends in the incidence of eating disorders: a primary care study in the Netherlands. Int J Eat Disord 2006;39:565-9. http://dx.doi.org/10.1002/eat.20316.
- Currin L. Primary Management of Eating Disorders: From Diagnosis to Referral. London: King’s College London; 2006.
- Berelowitz M. Management of anorexia nervosa revisited: emphasis needs to continue to shift to outpatient care. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.328.7447.1075-b.
- Lock J, Couturier J, Agras WS. Costs of remission and recovery using family therapy for adolescent anorexia nervosa: a descriptive report. Eat Disord 2008;16:322-30. http://dx.doi.org/10.1080/10640260802115969.
- Lay B, Jennen-Steinmetz C, Reinhard I, Schmidt MH. Characteristics of inpatient weight gain in adolescent anorexia nervosa: relation to speed of relapse and re-admission. Eur Eat Disord Rev 2002;10:22-40. https://doi.org/10.1002/erv.432.
- Centre for Longitudinal Studies . Welcome to the 1970 British Cohort Study n.d. www.cls.ioe.ac.uk/page.aspx?&sitesectionid=795&sitesectiontitle=Welcome+to+the+1970+British+Cohort+Study (accessed 3 July 2017).
- Stuhldreher N, Konnopka A, Wild B, Herzog W, Zipfel S, Löwe B, et al. Cost-of-illness studies and cost-effectiveness analyses in eating disorders: a systematic review. Int J Eat Disord 2012;45:476-91. http://dx.doi.org/10.1002/eat.20977.
- National Service Framework for Mental Health: Modern Standards and Service Models. London: The Stationery Office; 1999.
- Mental Health of Children and Young People in Great Britain, 2004. London: HMSO; 2005.
- Byford S, Leese M, Knapp M, Seivewright H, Cameron S, Jones V, et al. Comparison of alternative methods of collection of service use data for the economic evaluation of health care interventions. Health Econ 2007;16:531-6. http://dx.doi.org/10.1002/hec.1175.
- Patel A, Rendu A, Moran P, Leese M, Mann A, Knapp M. A comparison of two methods of collecting economic data in primary care. Fam Pract 2005;22:323-7. https://doi.org/10.1093/fampra/cmi027.
- Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al. A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14150.
- Airoldi M. Value-For-Money of Sheffield PCT Spend in Eating Disorders. A Socio-Technical Approach to Support the Development of the Commissioning Strategy. London: London School of Economics and Political Science; 2009.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- NHS Reference Costs 2009–10. London: HMSO; 2011.
- Berridge D, Beecham J, Brodie I, Cole T, Daniels H, Knapp M, et al. Costs and Consequences of Services for Troubles Adolescents: An Exploratory, Analytic Study. Luton: University of Luton; 2002.
- Beecham J, Knapp M, Fenyo A. Costs, needs, and outcomes. Schizophr Bull 1991;17:427-39. https://doi.org/10.1093/schbul/17.3.427.
- Knapp M. Making music out of noise – the cost function approach to evaluation. Br J Psychiatry Suppl 1998;36:7-11.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011;199:5-7. http://dx.doi.org/10.1192/bjp.bp.110.087585.
- Gowers S, Bryant-Waugh R. Management of child and adolescent eating disorders: the current evidence base and future directions. J Child Psychol Psychiatry 2004;45:63-8. https://doi.org/10.1046/j.0021-9630.2003.00309.x.
- Palmer RL, Treasure J. Providing specialised services for anorexia nervosa. Br J Psychiatry 1999;175:306-9. https://doi.org/10.1192/bjp.175.4.306.
- Brown A. Eating disorders – the developing mixed economy. Ment Health Res Rev 1997;4:32-5.
- Beecham J. Unit Costs – Not Exactly Child’s Play. A Guide to Estimating Unit Costs for Children’s Social Care. Dartington: Department of Health, Dartington Social Research Unit & PSSRU; 2000.
- Eating Disorders in the UK. Service Distribution, Service Development and Training. London: Royal College of Psychiatrists; 2011.
- Tierney S. The treatment of adolescent anorexia nervosa: a qualitative study of the views of parents. Eat Disord 2005;13:369-79. https://doi.org/10.1080/10640260591005254.
- de la Rie S, Noordenbos G, Donker M, van Furth E. The patient’s view on quality of life and eating disorders. Int J Eat Disord 2007;40:13-20. http://dx.doi.org/10.1002/eat.20338.
- Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 2007;164:1259-65. https://doi.org/10.1176/appi.ajp.2007.06081388.
- Hjern A, Lindberg L, Lindblad F. Outcome and prognostic factors for adolescent female in-patients with anorexia nervosa: 9- to 14-year follow-up. Br J Psychiatry 2006;189:428-32. https://doi.org/10.1192/bjp.bp.105.018820.
- Patton GC, Coffey C, Carlin JB, Sanci L, Sawyer S. Prognosis of adolescent partial syndromes of eating disorder. Br J Psychiatry 2008;192:294-9. http://dx.doi.org/10.1192/bjp.bp.106.031112.
- Su JC, Birmingham CL. Anorexia nervosa: the cost of long-term disability. Eat Weight Disord 2003;8:76-9. https://doi.org/10.1007/BF03324993.
- Nicholls DE, Viner RM. Childhood risk factors for lifetime anorexia nervosa by age 30 years in a national birth cohort. J Am Acad Child Adolesc Psychiatry 2009;48:791-9. http://dx.doi.org/10.1097/CHI.0b013e3181ab8b75.
- Dearden L, McGranahan L, Sianesi B. Returns to Education for the ‘Marginal Learner’: Evidence From the BCS70 2014. http://cee.lse.ac.uk/cee%20dps/ceedp45.pdf (accessed 7 April 2017).
- Keski-Rahkonen A, Sihvola E, Raevuori A, Kaukoranta J, Bulik CM, Hoek HW, et al. Reliability of self-reported eating disorders: optimizing population screening. Int J Eat Disord 2006;39:754-62. http://dx.doi.org/10.1002/eat.20277.
- Eating Disorders: Anorexia Nervosa and Bulimia Nervosa. No. 111. London: Office of Health Economics; 1994.
- Paying the Price: The Cost of Mental Health Care in England to 2026. London: The King’s Fund; 2008.
- Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34:383-96. http://dx.doi.org/10.1002/eat.10222.
- Striegel-Moore RH, DeBar L, Wilson GT, Dickerson J, Rosselli F, Perrin N, et al. Health services use in eating disorders. Psychol Med 2008;38:1465-74. https://doi.org/10.1017/S0033291707001833.
- Micali N, Hagberg KW, Petersen I, Treasure JL. The incidence of eating disorders in the UK in 2000-2009: findings from the General Practice Research Database. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002646.
- Steinhausen HC. Outcome of eating disorders. Child Adolesc Psychiatr Clin N Am 2009;18:225-42. https://doi.org/10.1016/j.chc.2008.07.013.
- HESonline . Hospital Episode Statistics: 2010 11 2011. www.hscic.gov.uk/catalogue/PUB02570/hosp-epis-stat-admi-prim-diag-4cha-10-11-tab.xls (accessed 4 April 2017).
- HESonline . Hospital Episode Statistic Topic of Interest: Eating Disorders 2010–2011 2011. www.hscic.gov.uk/catalogue/PUB05022 (accessed 4 April 2017).
- Harbottle EJ, Birmingham CL, Sayani F. Anorexia nervosa: a survival analysis. Eat Weight Disord 2008;13:e32-4.
- Hoek HW. The incidence and prevalence of anorexia nervosa and bulimia nervosa in primary care. Psychol Med 1991;21:455-60. https://doi.org/10.1017/S0033291700020560.
- Brennan L, Watson M, Klaber R, Charles T. The importance of knowing context of hospital episode statistics when reconfiguring the NHS. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2432.
- Goetzel RZ, Long SR, Ozminkowski RJ, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med 2004;46:398-412. https://doi.org/10.1097/01.jom.0000121151.40413.bd.
- Raenker S, Hibbs R, Goddard E, Naumann U, Arcelus J, Ayton A, et al. Caregiving and coping in carers of people with anorexia nervosa admitted for intensive hospital care. Int J Eat Disord 2013;46:346-54. http://dx.doi.org/10.1002/eat.22068.
- Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 2007;28:182-91. https://doi.org/10.1016/j.cct.2006.05.007.
- Giel KE, Kullmann S, Preiβl H, Bischoff SC, Thiel A, Schmidt U, et al. Understanding the reward system functioning in anorexia nervosa: crucial role of physical activity. Biol Psychol 2013;94:575-81. http://dx.doi.org/10.1016/j.biopsycho.2013.10.004.
- Macdonald P, Murray J, Goddard E, Treasure J. Carer’s experience and perceived effects of a skills based training programme for families of people with eating disorders: a qualitative study. Eur Eat Disord Rev 2011;19:475-86. http://dx.doi.org/10.1002/erv.1065.
- Curtis L. The Unit Costs of Health and Social Care 2009 2009. www.pssru.ac.uk/project-pages/unit-costs/2009/ (accessed 7 April 2017).
- Ball J, Philippou J, Pike G, Sethi J. Survey of District and Community Nurses in 2013, Report to the Royal College of Nursing, King’s College 2014. www.kcl.ac.uk/nursing/research/nnru/publications/Reports/DN-community-RCN-survey-report---UPDATED-27-05-14.pdf (accessed 7 April 2017).
- HESonline . Hospital Episode Statistics. 2010 11 n.d. www.hesonline.nhs.uk (accessed 4 April 2017).
- Office for National Statistics . Life Expectancy for Females at Birth, for England 2011. http://webarchive.nationalarchives.gov.uk/20140721132900/http://www.statistics.gov.uk/hub/population/deaths/life-expectancies (accessed 13 January 2014).
- Kruijshaar ME, Hoeymans N, Spijker J, Stouthard ME, Essink-Bot ML. Has the burden of depression been overestimated?. Bull World Health Organ 2005;83:443-8.
Appendix 1 Training school teachers (work package 1a)
Student questionnaire
-
People with eating disorders can be very good at hiding their problems – do you think you would know enough to tell whether a friend was at risk?
-
– Yes – I have spotted the signs in the past.
-
– Yes – I am confident I would know what I’m looking for.
-
– I’m unsure.
-
– No – I think it’s unlikely I would see the early signs.
-
– Other (free text).
-
-
Has your school ever taught you about eating disorders, and what to do if you’re worried about yourself or a friend?
-
– Yes, I have been taught and it was helpful.
-
– Yes, I have been taught but it was NOT very helpful.
-
– No, I have never been taught at school.
-
– I can’t remember.
-
– Other (free text).
-
-
Can you think of anything your school could do to help you understand as much as you’d like to about eating disorders and how to help your friends if they’re in difficulty? (Free text.)
-
If you were worried that a friend might have an eating disorder what would you do?
-
– My friend would probably talk to me, and I would listen and try to help.
-
– I would approach my friend and raise the issue and we would work out together what to do.
-
– I would talk to a teacher I trusted and ask for advice.
-
– I would anonymously let a teacher know so they could help my friend, but my friend wouldn’t know I had told on them.
-
– I would talk to an adult outside of school (e.g. my parents or youth group worker).
-
– I wouldn’t do anything at first, I would wait and see if things got worse or better.
-
– Other (free text).
-
-
If you told a teacher that you were concerned about a friend, what would you want them to do?
-
– Talk to my friend and find out what was wrong.
-
– Help me to help my friend.
-
– Tell my friend’s parents and get them to help.
-
– Make sure my friend got support from a counsellor or doctor.
-
– Nothing, just listen.
-
– Other (free text).
-
-
If you told a teacher that you were concerned about a friend, what do you think they would actually do?
-
– Talk to my friend and find out what was wrong.
-
– Help me to help my friend.
-
– Tell my friend’s parents and get them to help.
-
– Make sure my friend got support from a counsellor or doctor.
-
– Nothing, just listen.
-
– Other (free text).
-
-
If you felt you should tell a teacher about a friend you were worried about – how would you most like to do it (even if it’s not possible at the moment)?
-
– Face to face.
-
– On the phone.
-
– Text/short message service.
-
– E-mail.
-
– Instant messaging.
-
– In writing.
-
– Other (free text).
-
-
If you were suffering from an eating disorder – do you think your school would feel like a safe and supportive place to recover?
-
– Strongly agree.
-
– Agree.
-
– Neutral.
-
– Disagree.
-
– Strongly disagree.
-
– Other (free text).
-
-
Can you think of anything that would make your school an even better place for people recovering from an eating disorder? (Free text.)
-
Has your school ever helped you, or a friend, when you’ve needed help with regards to an eating disorder? (Please explain.) (Free text.)
-
Has your school ever failed to help/not noticed/made the situation worse when you or a friend needed help with regards to an eating disorder? (Please explain.) (Free text.)
Staff questionnaire
-
Does your school/college have an eating disorders policy in place?
-
– Yes we have specific policies relating to eating disorders.
-
– Yes – eating disorders are covered in another policy (e.g. child protection).
-
– Unsure.
-
– No we definitely do not refer to eating disorders in any of our policies.
-
– Any further comments (free text).
-
-
Do you think that policies that refer to eating disorders are effective? (Only asked if answered yes to question 1.)
-
– Very effective.
-
– Effective.
-
– Ineffective.
-
– Very ineffective.
-
-
Has your school offered any form of training/briefing about eating disorders?
-
– Yes.
-
– No.
-
-
Who’s attended the training? (Only asked if answered yes to question 3.)
-
– Just me.
-
– All pastoral staff.
-
– All middle and senior managers.
-
– Whole staff.
-
– Not applicable.
-
– Other (free text).
-
-
How was the training delivered? (Only asked if answered yes to question 3.)
-
– Written materials.
-
– Face to face – workshop or seminar.
-
– Face to face – lecture.
-
– Over the internet.
-
– Over the phone.
-
– Not applicable.
-
– Other (free text).
-
-
What did you find useful about the training? (Free text.) (Only asked if answered yes to question 3.)
-
What would have made the training more useful? (Free text.) (Only asked if answered yes to question 3.)
-
If you have not received any training on eating disorders. Do you think you would find some training useful? (Only asked if answered no to question 3.)
-
– Yes – very useful.
-
– Yes – quite useful.
-
– No – not very useful.
-
– No – not at all useful.
-
– Any further comments (free text).
-
-
Are you aware of any current or past cases of eating disorders in your school/college?
-
– Yes, I have been directly involved with cases.
-
– Yes, I have been aware but not involved with any cases.
-
– No, I have not been aware of any cases.
-
– No, there have been no cases.
-
– Any further comments (free text).
-
-
What would you do if you were concerned that a student may be suffering from an eating disorder? (Free text.)
-
In your school/college; if a student is concerned that one of their peers may have an eating disorder – what are they encouraged to do?
-
– All concerns are passed on to a specific member of staff.
-
– The student could talk to any member of staff.
-
– We have a texting/e-mailing/post box service that students can anonymously use.
-
– This is not something we have discussed with students.
-
– Other (free text).
-
-
Have you had any particularly positive or negative experiences when communicating with parents regarding eating concerns? If so please briefly outline (free text).
-
Have you worked with any outside agencies to support students with eating disorders? Please outline which agencies you have used and any particularly positive or negative experiences you have had (free text).
-
Has your school/college ever had to re-integrate a student following a period away from school caused by an eating disorder?
-
– Yes.
-
– No.
-
-
Did staff or students receive any advice on how to best support the returning student? (Only asked if answered yes to question 14.)
-
– Yes.
-
– No.
-
-
Can you think of any further support that could have been given to staff or students that would have helped the returning student to reintegrate more successfully? (Please outline.) (Free text.)
-
When we develop our training materials for schools, we want to make sure they are as useful as possible. In order to do this, instead of inventing examples, we are collecting anonymous case studies from school staff which will be used for training purposes. If you have can think of any instances surrounding students, staff and/or parents when dealing with eating disorders it would be helpful if you could provide a brief outline below (free text).
Eating disorders attitudes and knowledge questionnaire
| No knowledge | Full knowledge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No knowledge | Full knowledge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No knowledge | Full knowledge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No knowledge | Full knowledge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| With reference to students with eating disorders | Strongly disagree | Disagree | Agree | Strongly agree |
|---|---|---|---|---|
| Symptoms of eating disorders are fairly common and will resolve over time without treatment | ||||
| Eating disorders are severe mental illnesses | ||||
| Students with eating disorders are to blame for their own condition | ||||
| Eating disorders have major consequences on the sufferer’s quality of life | ||||
| Students with eating disorders cause difficulties for school staff | ||||
| Students with eating disorders cause difficulties for their peers | ||||
| Teaching students about eating disorders will make them more likely to develop one | ||||
| School staff should be involved in the treatment and recovery process for students with eating disorders | ||||
| Sufferers should not continue to attend school where other students may copy their behaviour | ||||
| School staff should review their academic expectations of students with eating disorders | ||||
| The school has a responsibility to support students suffering from eating disorders | ||||
| There is a lot the school can do to help during the recovery process |
Each of the following case studies is a real situation that has been outlined by a member of school staff in the past. As such, there are no right or wrong answers – but please answer the questions following each case study as honestly as possible.
12-year-old Zeena is a model student. She works very hard at everything she does and excels academically. She is usually top of the class and is disappointed even to come second. She always submits her homework on time and it is clear that she dedicates a lot of time to her studies at home as well as at school.
Zeena has recently been fasting for Ramadan and decided to show her dedication to her faith by fasting beyond Ramadan. She has had her parents full support on this – they are very proud of Zeena’s dedication to her faith. In fact, her parents play a huge role in her life; they are very encouraging of her academic achievements too and always encourage her to achieve to her very best and believe that she can achieve anything she wants to if she works hard enough.
Zeena is well liked by her classmates though she seems a little more withdrawn in class than usual and has taken to spending many of her lunchtimes in the library alone. Although she is only in year 8, she has ambitions to study veterinary science and she understands from her parents and older brother that in order to realise her goal she must work very hard.
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
15-year-old Simon is not a natural sportsman, but this year he has been putting his all into the football team. He has been attending every practice and keeping his fitness up by frequently visiting the gym between practices.
The slightly chubby looks that earned him the nickname ‘Podge’ in Year 9 are a thing of the past and instead Simon is building up quite a six-pack. He’s even beginning to get some rather giggly attention from the girls – though he doesn’t seem interested at all, preferring to shy away from the attention and work out in the gym during his lunch breaks and after school.
Simon lives with his younger brother and his mum, who has been a single parent to both boys for as long as you have known them. She is very supportive of Simon and his brother, but isn’t always able to attend parents evenings as she works long hours to support the family.
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
16-year-old Karly is in your tutor group. She has recently been absent from school for half a term receiving treatment for bulimia. She is due to start back at school soon. While her condition is much improved and her physical health no longer in immediate danger, it has been made clear to you that Karly will require quite significant support to ensure that she does not relapse. She is still in the process of recovery and will be for quite some time, but her doctors and parents have agreed that, with an appropriately supportive environment, it would be beneficial for her to continue her recovery in school. She’s a few months away from sitting her GCSEs and has several good friends at school though they have not seen much of her during her absence.
| Not at all confident | Completely confident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Appendix 2 Inpatient treatment (work package 2b)
Detailed results from qualitative evaluation
| Main category | Generic category | Subcategory | Frequency | Example |
|---|---|---|---|---|
| What have you learnt about yourself from this therapy? | Things I have learnt about myself | Bottle up emotions | 8 | How I deal with emotions – that I bottle them up until they explode . . . |
| Block/avoid my emotions | 6 | How I used to block my emotions and not be able to sit with them . . . | ||
| It’s OK to express your feelings | 6 | It’s ok to talk about your problems, it makes you feel better afterwards | ||
| Not getting needs met | 5 | . . . run into difficulties when I don’t look after own needs | ||
| Good at caring for others | 5 | I have also learnt that I am a great friend to others but not a great friend to myself | ||
| Skills I have begun using | Expressing/communicating more | 7 | . . . better at being able to express feelings – more open in communicating positive and negatives | |
| Positive intentions of negative emotions | 6 | Negative feelings aren’t always bad – they teach/protect . . . | ||
| Identifying emotions | 5 | How to name emotions and how to find the right words for how I’m feeling | ||
| Look at the bigger picture | 4 | . . . it’s alright not to worry about the little details being perfect and to look at the bigger picture | ||
| Emotions are fluid | 4 | . . . that emotions are fluid and change throughout the day | ||
| Other people’s perspectives | 3 | Being more aware of other people’s perspectives | ||
| Acknowledging/accepting emotions | 3 | . . . it is ok and acceptable to feel sad at times | ||
| Skills I would like to use | Be more assertive | 3 | Need to be assertive | |
| Be more open | 3 | That I need to focus on communicating my emotions to those around me . . . | ||
| Be more positive | 3 | I would like to be more positive. Being more pro-active can make positive things happen | ||
| Were there any aspects which were particularly helpful? | Emotional strategies learnt | Labelling | 5 | Looking more specifically at different emotions (e.g. labelling) |
| Acting before emotions spiral | 4 | Also for me looking at ways to reduce emotions getting to that extreme point by acting on feelings before they start to spiral out of control | ||
| Communicating feelings | 3 | . . . talking about your problems can actually help you deal with them | ||
| Non-specific strategies | 3 | Strategies to manage difficult situations that occurred during the week | ||
| Recognising the positive intentions of emotions | 3 | Looking at emotions in a different way e.g. positive meanings of negative emotions | ||
| Learning about emotions | Relation to needs | 7 | The idea that each and every emotion is simply an indicator that I need something, or a guide to determine how I behave | |
| Awareness of own difficulties | 5 | . . . being more aware of the issues and difficulties that need attending to | ||
| Fluidity of emotions | 4 | Knowing there are different emotions that we feel, it is ok to have other emotions rather than just feeling the same all the time | ||
| Aspects of the approach | Reflection time | 6 | Having time/space to reflect on emotions and what they actually mean to me in everyday life | |
| Doing homework | 5 | Homework allows time to come up with good examples/reflect more | ||
| Empathy of therapist | 4 | I was met with a very caring and compassionate approach and someone listening to how it was for me | ||
| Structure/themes | 3 | Structure/having themes to each session | ||
| All sessions were helpful | n/a | 6 | To be honest every session has been beneficial . . . | |
| Were there any aspects that were particularly unhelpful? | Aspects of the approach | Initially too basic | 3 | First few sessions seemed a little basic |
| Not personalised | 2 | Tasks [were] statistic based rather than personal based | ||
| Too structured | 1 | Sometimes too structured | ||
| Ended too quickly | 1 | . . . it came to an end too quickly | ||
| Certain tasks/sessions | Facial expressions | 4 | Didn’t gain a lot from the facial expressions exercises | |
| Cognitive remediation therapy sessions | 2 | Not sure at first – thinking about thinking | ||
| Physical manifestations | 2 | Not helpful looking at how emotions feel in the body | ||
| Emotion switching | 1 | Didn’t take that much from emotion switching session – already aware of switching emotions | ||
| No aspects unhelpful | n/a | 10 | None – learned something from everything | |
| What could be improved? | More sessions | Longer therapy | 8 | The therapy here is the best I’ve encountered, the only criticism is there isn’t more of it |
| Follow-up sessions | 1 | . . . a follow-up course of CREST would be highly beneficial | ||
| Tailored more to individual | More in depth | 4 | Taking the therapy to a more in depth level, relating emotions to an underlying thinking and beliefs and working from that level | |
| More personalised | 2 | If it was personalised, tailored to me/individual rather than generic to all | ||
| More on certain topics | Managing emotions | 2 | More strategies – what to do in emotional situations | |
| Emotions and needs | 2 | More about emotions and their relationship to needs | ||
| Less on certain topics | Facial expression | 2 | A few less introductory sessions e.g. looking at faces | |
| Nothing could be improved | n/a | 4 | Nothing. I found the number of sessions to be just right and the length of sessions appropriate | |
| What strategies have you learnt to use in the future? | Having emotional awareness | Benefits of emotions | 6 | Negative emotions can have a positive effect as they can alert me to the fact that something needs to change and inspire me to take positive action |
| Bottling up is not helpful | 4 | Remembering bottling up doesn’t get me anywhere apart from frustrated and angry | ||
| Practical skills | Talking/communicating | 17 | . . . expressing emotions in relation to difficult situations – voice what I am thinking or feeling | |
| Being more assertive | 9 | Being more assertive by speaking up more when needed | ||
| Having a positive attitude | 7 | Focusing on positive things – reflecting on positives and being pro-active at making positive things happen | ||
| Labelling | 6 | Able to identify feelings more accurately | ||
| Getting needs met | 6 | Asking for help when I need it | ||
| Acknowledging emotions | 6 | Acknowledging how I feel | ||
| Looking at the bigger picture | 5 | I have learnt to look at the bigger picture when I am feeling anxious or worried about a certain situation |
Appendix 3 Economic analyses (work package 7b)
Data availability and assumptions for service-level cost analysis
The service-level data available to the economic evaluation included:
-
level of service specialisation with regard to ED
-
service location (hospital or community)
-
details on ED assessment
-
typical length of assessment
-
staff typically involved in assessment
-
outpatient treatment provided for AN
-
type of treatments available
-
typical length of session
-
typical number of sessions
-
staff typically providing the session
-
details of other treatments provided (e.g. inpatient, day patient).
-
-
The approximate cost of each type of treatment session was calculated by applying a set of assumptions:
-
the unit costs for all staff members involved in the session were summed
-
where the questionnaire stated that one or another type of staff member provided the treatment (e.g. ‘psychiatrist or psychologist’), the average unit cost was used
-
where the typical length of treatment was missing, the length from a similar service (e.g. other non-specialist CAMHS) was applied
-
for group treatments, a group size of six patients or families was assumed.
Individual-level analysis of treatment costs
The patient-level data provided the following information relevant to the economic analysis:
-
patient characteristics
-
patient clinical data (weight and height, Morgan–Russell criterion)
-
outpatient treatment
-
number of assessments
-
number of individual or family sessions
-
number of group, dietetic and medical outpatient sessions
-
number and type of outpatient appointments for physical tests
-
number of telephone consultations
-
number of psychiatric reviews
-
-
number of inpatient days for an ED or other reasons
-
services that had provided treatments or assessments.
Average costs of treatment by eating disorder service type
| Treatment | Specialist CAEDS | Other ED services | Specialist CAMHS | Non-specialist CAMHS | Independent CAEDS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Valid, n | Mean (£) | Valid, n | Mean (£) | Valid, n | Mean (£) | Valid, n | Mean (£) | Valid, n | Mean (£) | |
| Individual | 3 | 135 | 2 | 124 | 6 | 163 | 11 | 163 | 3 | 111 |
| Group session | 1 | 32 | 2 | 46 | 0 | – | 0 | – | 2 | 78 |
| IFT | 3 | 187 | 1 | 258 | 5 | 235 | 8 | 246 | 2 | 237 |
| MFT day/session | 1 | 547 | 0 | – | 1 | 547 | 1 | 114 | 0 | – |
| Parent session | 1 | 101 | 0 | – | 1 | 22 | 1 | 98 | 1 | 120 |
| Dietic session | 3 | 33 | 1 | 62 | 3 | 39 | 2 | 109 | 2 | 45 |
| Occupational therapy | 0 | – | 0 | – | 0 | – | 0 | – | 1 | 36 |
| Physiotherapy | 0 | – | 0 | – | 0 | – | 0 | – | 1 | 18 |
Additional unit costs from publicly available sources
| Role | £ per hour | Source374 | Notes |
|---|---|---|---|
| Doctors | |||
| Associate specialist | 166 | PSSRU 2011, p. 202 | Time ratio as consultant (medical) |
| Consultant (assume medical) | 202 | PSSRU 2011, p. 203 | Time ratio from PSSRU 2009, p. 170410 |
| GP (hospital) | 229 | PSSRU 2011, p. 148 | |
| GP (community) | 138 | PSSRU 2011, p. 148 | |
| Paediatrician (hospital) | 202 | Medical consultant | |
| Paediatrician (community) | 138 | As GP | |
| Senior house officer | 61 | PSSRU 2011, p. 199 (foundation house officer year 2) | Time ratio as consultant (medical) |
| Specialist registrar | 89 | Based on PSSRU 2011, p. 201 | Time ratio as consultant (medical) |
| Staff doctor/ward doctor | 117 | Based on medical consultant, PSSRU 2011, p. 203 | Time ratio as consultant (medical) |
| Nurses | |||
| CAMHS nurse | 75 | Based on CAMHS member schema with band 5 median salary | |
| Clinical specialist nurse | 73 | PSSRU 2011, p. 142 | Community mental health nurse |
| Key nurse | 120 | PSSRU 2011, p. 192 | |
| Nurse (hospital) | 104 | PSSRU 2011, p. 193 | |
| Nurse (community) | 71 | PSSRU 2011, p. 141 | |
| Psychology nurse | 104 | As specialist nurse | |
| Senior staff nurse | 120 | PSSRU 2011, p. 192 | |
| Specialist nurse | 104 | PSSRU 2011, p. 193 | |
| Specialist nurse (community) | 89 | Based on PSSRU 2011, p. 144 nurse specialist | Time ratio from RCN report411 |
| Psychologists and psychiatrists | |||
| Child and adolescent psychiatrist | 295 | As generic psychiatrist | |
| Child psychiatrist | 295 | As generic psychiatrist | |
| Child psychiatrist (community) | 293 | As psychiatrists, overheads from CAMHS team | |
| Child psychologist (hospital) | 156 | As clinical psychologist, overheads as psychiatrist | |
| Child psychologist (community) | 152 | As clinical psychologist | |
| Clinical psychologist (hospital) | 156 | As clinical psychologist, overheads as psychiatrist | |
| Clinical psychologist (community) | 152 | PSSRU 2011, p. 137 | Face-to-face contact |
| Consultant psychiatrist (hospital) | 295 | PSSRU 2011, p. 205 | Per hour patient related |
| Psychiatrist (community) | 293 | As psychiatrists, overheads from CAMHS team | |
| Psychologist (hospital) | 156 | As clinical psychologist, overheads as psychiatrist | |
| Psychologist (community) | 152 | As clinical psychologist | |
| Psychology assistant | 123 | As clinical psychologist, median salary grade 6 | |
| Therapists | |||
| Art therapist (hospital) | 139 | Clinical psychologist, AfC band 7 | |
| Art therapist (community) | 136 | Clinical psychologist, AfC band 7 | |
| Child psychotherapist | 156 | As clinical psychologist | |
| Cognitive analytical therapist | 139 | Clinical psychologist, AfC band 7 | |
| Counselling psychotherapist | 156 | As clinical psychologist | |
| Drama therapist | 139 | As art therapist | |
| Family therapist (community) | 183 | Clinical psychologist, AfC band 8b | |
| Family therapist (hospital) | 186 | ||
| Psychotherapist (hospital) | 156 | As clinical psychologist | |
| Psychotherapist (community) | 152 | As clinical psychologist | |
| Systemic psychotherapist | 156 | As clinical psychologist | |
| Therapist | 156 | As clinical psychologist | |
| Dieticians | |||
| Dietitian (hospital) | 36 | PSSRU 2011, p. 184 | |
| Dietitian (community) | 34 | Based on UC volume (hospital), capital overheads from CAMHS teams | |
| Paediatric dietitian | 47 | As specialist dietitian | |
| Specialist dietitian | 47 | Based on UC volume (hospital), salary band 6 (median) | |
| Other | |||
| Behaviourist | 156 | As clinical psychologist | |
| CAMHS professional (hospital) | 101 | See CAMHS professional (community) | |
| CAMHS professional (community) | 98 | PSSRU 2011, p. 175 (targeted) | |
| Occupational therapist (community) | 35 | PSSRU 2011, p. 134 | No time ratio applied |
| Occupational therapist (hospital) | 36 | PSSRU 2011, p. 182 | No time ratio applied |
| Occupational therapist assistant | 31 | Based on occupational therapist; AfC band 4 (assistant practitioner) | No time ratio applied |
| Physiotherapist (community) | 35 | PSSRU 2011, p. 133 | No time ratio applied |
| Physiotherapist (hospital) | 37 | PSSRU 2011, p. 181 | No time ratio applied |
| Social worker (child) | 146 | PSSRU 2011, p. 157 | |
| Therapeutic carer | 104 | As hospital nurse | |
Assumptions and data sources regarding societal costs of anorexia nervosa
Distribution of treatment
Around a third of AN cases are treated exclusively in primary care. 362 Based on HES, we estimate that around 11% of cases are treated as inpatients each year. This suggests that 56% are treated primarily on an outpatient basis.
Inpatient and outpatient treatment
The number of inpatient admissions, number of bed-days and number of outpatient contacts recorded under a primary diagnosis of AN were obtained from the HES for 2010/11412 and the Special Interest Topic on ED for the same year. 401 As a detailed breakdown by age was not available, the ratio of adult (63%) to child (37%) of finished consultant episodes from the Special Interest Topic report was applied when costing inpatient stays. This served as a lower-bound estimate for inpatient costs because admissions that are causally related to AN may not be recorded under a primary diagnosis of AN, such as cardiac problems. To obtain a higher-bound estimate, we applied the ratio of costs for medical inpatient admissions to ED admissions from the Care Pathways Study (see Chapter 11, Study 2: economic analysis of the Care Pathways Study). For our higher estimate, we also included potential additional outpatient care costs, based on the ratio of the costs of ED-related and medical outpatient appointments to ED inpatient costs from the Care Pathways Study, across all three pathways (38% and 27%, respectively). This includes treatment provided in community settings. Our provisional analysis of data relating to patients treated in the private sector indicates that outpatient treatment makes up a much smaller proportion of costs than in the public sector. We therefore apply this additional cost only to NHS beds (51% of ED inpatient costs).
Cost of independent sector provision
We identify the likely cost to the NHS of inpatient treatment provided by the independent sector, and additional costs of privately funded treatment based on the assumption that 49% of ED beds are provided by the private sector384 and 90% of independent beds are NHS funded. 31
Primary care costs
Our lower estimate of primary care costs draws on estimates from the Pro Bono Economics report, in which it was assumed that each person with AN saw their GP three times per year. 75 Little is known about the service use of people with AN prior to entering treatment, but there is some evidence on elevated service use up to 5 years prior to diagnosis. 21,22 For our higher cost estimate, we draw on baseline information from the three trials analysed in Chapter 11 to estimate a plausible range of primary care costs (GP, nurse, dietitian) incurred prior to an inpatient admission or outpatient treatment. When treatment is provided exclusively in primary care, we assume that each person is in contact with their GP three times per year (NICE 2004 ED guidance162).
Benefit payments
The DWP provided data on social security benefit payments made to people because of an ED. There were on average 810 females claiming Employment Support Allowance for an ED per quarter, receiving an average weekly amount of £90.25. Incapacity Benefit or Severe Disability Allowance was paid to 1308 females each quarter, with a weighted average weekly amount of £56.04. As the statistic does not distinguish between different EDs, we assumed that the proportion of benefits paid to people with AN corresponded to the proportion of ED admissions for AN in HES (71%).
Years of potential life lost
Years of potential life lost were calculated based on a survival analysis by Harbottle et al. 402 The study assumed a standardised mortality rate of around 10. We assumed a standardised mortality rate of around 5 and halved the number of life-years lost to ensure a conservative estimate. 72 We present the total annual number of YPLL due to AN, discounted using a 3.5% rate and based on a life expectancy for females at birth of 82.40. 413 This is a simplified approach allowing a rough estimate only. YPLL were valued at £30,000, the threshold commonly recommended by NICE for a healthy life-year gained. As it is unlikely that these additional years will be lived at full health, we applied the disability weight for depression (46% reduction414) to the final figure. The results of our analysis are shown in Table 52.
| Age at onset (years) | Reduction in life expectancy402 | Number of new cases per year | Life-years lost per case (discounted at 3.5%) | Total life-years lost |
|---|---|---|---|---|
| 10–14 | 24.8 | 359 | 2.8 | 1005 |
| 15–19 | 24.6 | 773 | 3.55 | 2744 |
| 20–24 | 23.9 | 337 | 4.65 | 1567 |
| 25–29 | 23.3 | 346 | 5.96 | 2062 |
| 30–34 | 22.7 | 53 | 7.52 | 399 |
| 35–39 | 22.1 | 53 | 8.97 | 475 |
| 40–44 | 21.6 | 22 | 11.09 | 244 |
| 45–49 | Assume 21.6 | 22 | 13.6 | 299 |
| Total | 8796 |
List of abbreviations
- A&E
- accident and emergency
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- AN
- anorexia nervosa
- ANOVA
- analysis of variance
- ARIADNE
- Applied Research into Anorexia Nervosa and Not Otherwise Specified Eating Disorders
- BCS-70
- British Cohort Study (1970)
- BMC
- bone mineral content
- BMI
- body mass index
- BN
- bulimia nervosa
- CAEDS
- Child and Adolescent Eating Disorders Service
- CAMHS
- Child and Adolescent Mental Health Services
- CASIS
- Carers Assessment, Skills and Information Sharing
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CIA
- Clinical Impairment Assessment
- CREST
- Cognitive Remediation and Emotional Skills Training
- CSO
- clinical studies officer
- CSRI
- Client Services Receipt Inventory
- CTU
- clinical trials unit
- DASS-21
- Depression Anxiety and Stress Scales
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- DVD
- digital versatile disc
- DWP
- Department for Work and Pensions
- EAI
- Exercise Addiction Inventory
- ECHO
- Experienced Carers Helping Others
- ED
- eating disorder
- EDE
- Eating Disorders Examination
- EDE-Q
- Eating Disorders Examination-Questionnaire
- EDNOS
- eating disorder not otherwise specified
- EDNOS-AN
- eating disorder not otherwise specified – anorexia nervosa subtype
- EDNOS-BN
- eating disorder not otherwise specified – bulimia nervosa subtype
- EDSIS
- Eating Disorder Symptom Impact Scale
- EE
- energy expenditure
- ES
- effect size
- FQ
- Family Questionnaire
- GCSE
- General Certificate of Secondary Education
- GDES
- global drive to exercise
- GP
- general practitioner
- HC
- healthy control
- HES
- Hospital Episode Statistics
- ICC
- intracluster correlation
- IFT
- individual family therapy
- iMANTRA
- internet-based Maudsley Model of Anorexia Nervosa Treatment for Adults
- IPAQ
- International Physical Activity Questionnaire
- IPT
- interpersonal therapy
- IQ
- intelligence quotient
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- IUS
- Intolerance of Uncertainty Scale
- MANTRA
- Maudsley Model of Anorexia Nervosa Treatment for Adults
- MHRN
- Mental Health Research Network
- MI
- multiple imputation
- MOSAIC
- Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NS–NS
- non-specialist–non-specialist care pathway
- NS–S
- non-specialist–specialist care pathway
- OCI-R
- Obsessive Compulsive Inventory-Revised
- OR
- odds ratio
- PA
- physical activity
- PCT
- primary care trust
- PI
- Ponderal Index
- PPI
- patient and public involvement
- PSWQ
- Penn State Worry Questionnaire
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- REI
- Reasons for Exercise Inventory
- REY
- Rey–Osterrieth Complex Figure Test
- RME
- Reading the Mind in the Eyes Task
- RMF
- Reading the Mind in Film Task
- RMR
- resting metabolic rate
- ROSANA
- Relationship Between Overactivity, Stress and Anxiety in Anorexia Nervosa
- SD
- standard deviation
- SE
- standard error
- S–S
- specialist–specialist care pathway
- SSCM
- specialist supportive clinical management
- TAU
- treatment as usual
- WCST
- Wisconsin Card Sorting Test
- WHOQOL-100
- World Health Organization’s Quality of Life Questionnaire
- WP
- work package
- YPLL
- years of potential life lost