Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10036 . The contractual start date was in July 2008. The final report began editorial review in August 2014 and was accepted for publication in June 2017. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Karim Brohi reports that TEM Innovations GmbH (ROTEM®) provided unrestricted support to the programme in the form of equipment and reagents for the observational study [National Institute for Health Research (NIHR) portfolio ID 5637].
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Brohi et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
SYNOPSIS
Introduction
Trauma haemorrhage: background
Worldwide, 6 million people die from injuries every year and about 40% of these deaths are due to bleeding. 1 Theoretically, all haemorrhage can be stopped and therefore upwards of 2 million deaths are potentially preventable each year. People still bleed to death, however, because of a number of factors. They may not have their bleeding stopped in time because they do not reach surgery quickly enough; they reach surgery but their bleeding cannot be controlled; or their bleeding is controlled but the severe prolonged shock was too much for the body to recover from. Combined, these factors make trauma haemorrhage exceedingly difficult to manage and around the world mortality from severe bleeding (requiring a massive transfusion) is around 50%. 2
Trauma haemorrhage resuscitation in 2008
Trauma resuscitation in 2008 was very different from today. The focus at this time was fully on restoring the volume of circulating blood to maintain a normal blood pressure and try to maintain oxygen delivery to cells. This was initially performed with high doses of crystalloids, or colloids in some parts of the world. After two or more litres of saline had been administered, blood transfusion was started using packed red blood cells (PRBCs). 3 The goal was to normalise blood pressure and, therefore, perfusion.
It was known that after transfusion of large volumes of red cells (a massive transfusion), complications could occur because of the lack of clotting factors. After the replacement of an entire circulating blood volume (10 units of PRBCs) it was recommended that laboratory parameters of clotting were checked and, if abnormal, this coagulopathy should be managed with the administration of some volume of fresh-frozen plasma (FFP) (usually 4 units initially). Thus, massive transfusion protocols were principally focused on managing a late dilutional coagulopathy caused by large-volume fluid and PRBC transfusion. 4
We now know, from our work and the work of others, that our understanding of the disease processes was wrong, that our resuscitation goals were wrong and that our treatment was too little, too late.
What we knew about trauma-induced coagulopathy in 2008
In 2003, the discovery of a clotting disorder present immediately on arrival in the emergency department prompted a change in our understanding of the disease and the way it was treated. This acute traumatic coagulopathy (ATC) was first described in our study with London’s Air Ambulance. We looked at > 1000 helicopter patients and found that one in four patients arrived with ATC and that, if present, one in four patients would die. 5 By the start of this study several other groups around the world had confirmed the existence of ATC and we had begun to characterise it and explore its pathophysiology. 6,7
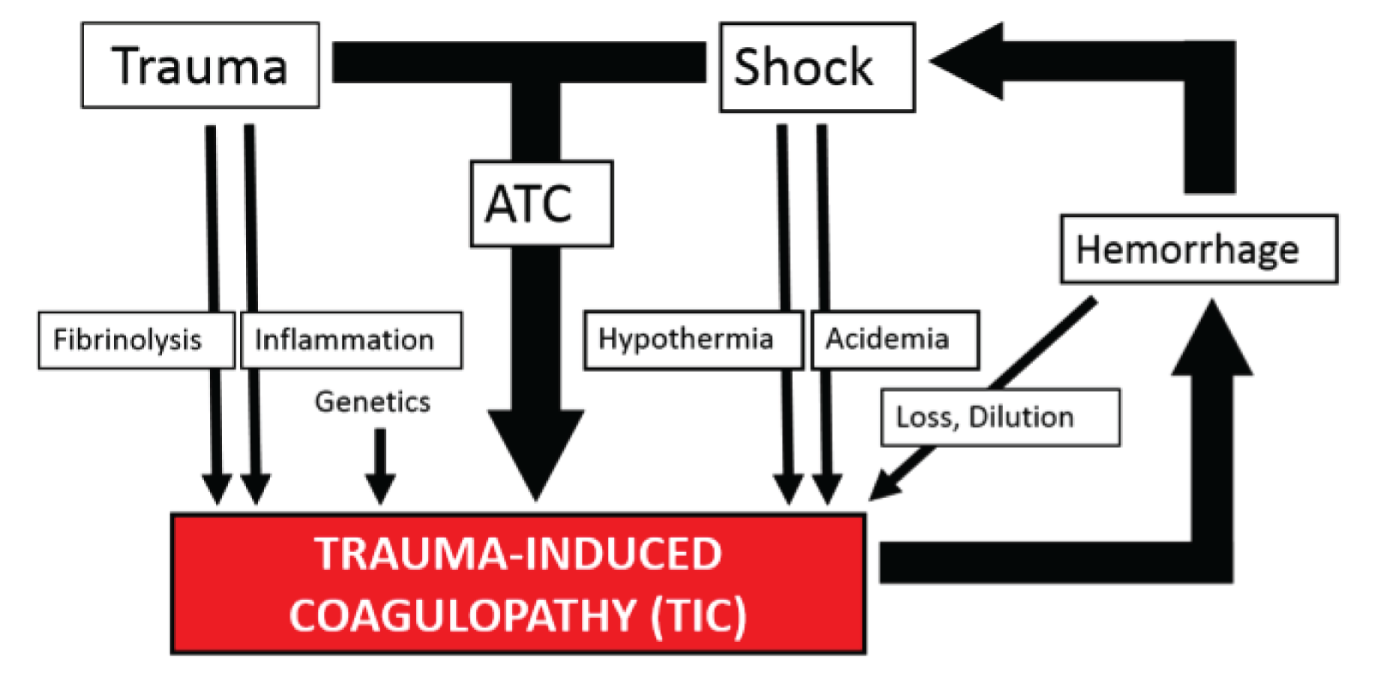
Trauma-induced coagulopathy (TIC; all different possible clotting disorders that a trauma patient could develop) could therefore take multiple forms at different times in their clinical course and several different coagulopathies might exist at the same time (Figure 1). It was becoming clear that TIC was not a single disease entity and therefore probably could not be managed in the same way at all times. 8
FIGURE 1.
Proposed model for the mechanisms and drivers of TIC in 2008. Adapted from Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma, vol. 65, iss. 4, pp. 748–54. 2008. 8 URL: http://journals.lww.com/jtrauma/pages/default.aspx. Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact healthpermissions@wolterskluwer.com for further information.
The identification of ATC provided a potential therapeutic focus for new management strategies and therapeutics. This was first approached in retrospective studies, with patients who had received higher doses of plasma during their haemorrhage resuscitation appearing to have significantly better outcomes than those given standard resuscitation regimens. 9 Patients given less crystalloid also seemed to do better. 10 In 2007 a new paradigm of resuscitation was postulated, damage control resuscitation (DCR), a strategy specifically targeting ATC. 11 The evidence for this position paper was weak, but the collective approach had four central tenets: stop bleeding, permissive hypotension until control, avoid fluids and target coagulopathy. The DCR approach was almost the complete opposite of the then current approach to haemorrhage resuscitation. Much more work needed to be carried out to show that this strategy was effective and did not expose patients to unnecessary risks and waste precious blood resources.
What we did not know
At the beginning of 2008, therefore, we understood that the underlying coagulopathies of trauma were more prevalent, more complex and occurred earlier than previously thought. We understood that the existing resuscitation strategies probably did not address these clotting disorders and in many cases may have exacerbated them. However, much of the underlying data were based on relatively weak evidence and there were areas where there were significant gaps in our knowledge. Our programme of work was designed to address the most pressing and central of these to patients, and to the NHS.
-
How common is major trauma haemorrhage nationally and what are its immediate and long-term outcomes?
-
How are bleeding trauma patients managed, especially in terms of the delivery of blood transfusion therapy?
-
What is the cost to the NHS of treating major haemorrhage? What are the most significant areas of cost burden?
-
What is the existing evidence for the use of blood products for the treatment of TIC?
-
Can we predict who is bleeding or has coagulopathy on arrival to enable activation of the appropriate treatment pathways?
-
What is the fastest and most accurate way to diagnose ATC? How can we diagnose various forms of TIC?
-
What is the best treatment for the different forms of TIC?
-
What are the implications of any changes in the context of a disaster or mass casualty event?
We proposed a multimodal collaborative programme of work to try to address these knowledge gaps, develop a more personalised approach to the management of the bleeding trauma patient and assure the timely, appropriate use of blood products.
What has changed in the management of bleeding trauma patients?
The Royal London Hospital is one of the busiest major trauma centres in the world, with nearly 3000 trauma team activations each year, and treats some of the most severely injured patients in the country. In 2008, patients activating our traditional massive transfusion protocol had a 50% mortality rate. 12
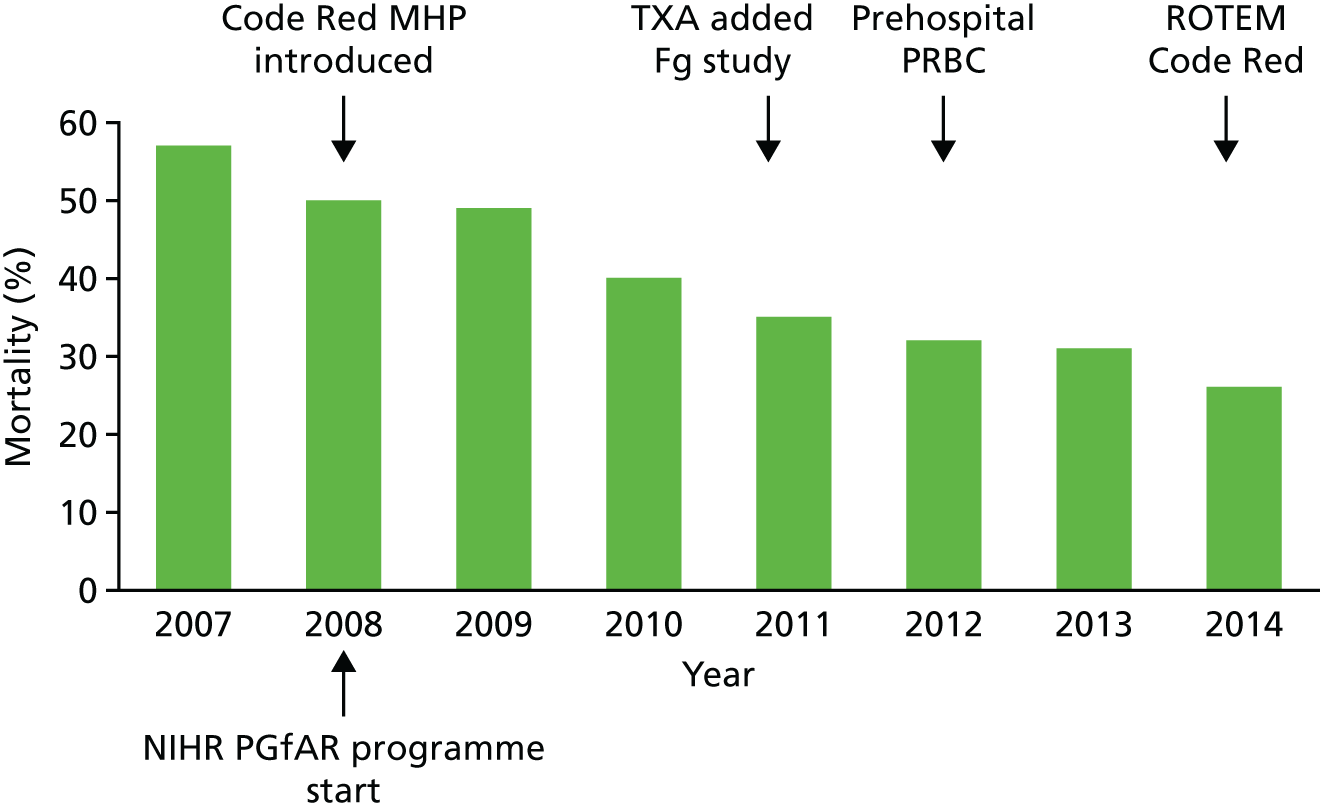
In partnership with the Barts Centre for Trauma Sciences at Queen Mary University of London we have iteratively implemented our research findings into practice with a multispecialty team of clinicians and transfusion specialists. By the end of our programme grant in 2014 we had more than halved the mortality of this group of patients to 26% (Figure 2). We had also halved the number of blood products that they required and almost halved their critical care and hospital stays.
FIGURE 2.
Outcomes of ‘Code Red’ patients at the Royal London Hospital 2007–14. Mortality fell from 57% to 26% and 24-hour PRBC requirements halved from 12 to 6 units.

We have a much deeper understanding of TIC in all its forms and a better understanding of the role of clotting therapy in the management of these conditions. We have new therapeutic agents and others in clinical trial pipelines. These changes have been incorporated in new major haemorrhage ‘Code Red’ protocols, which have been disseminated nationally and internationally and incorporated into clinical guidelines around the world.
In the following sections we describe the programme of work and the central findings that have led to these changes in practice.
The programme of work
The original stated objective of our programme of work was to improve outcomes for severely injured bleeding trauma patients. It was designed around the principle that early identification of patients who present with a TIC and effective, directed therapy will lead to improved outcomes, reduced complications, rationalised transfusions and reduced costs to the NHS.
We had six stated aims to achieve this objective.
-
Aim 1: evidence. To systematically review the current evidence and develop systematic reviews on the identification of coagulopathy and blood component use in trauma.
-
Aim 2: epidemiology. To investigate the national incidence of massive transfusion for trauma, national practice patterns and outcomes.
-
Aim 3: identification. To investigate the utility of clinical, point-of-care laboratory analyses for the identification of the need for massive transfusion.
-
Aim 4: treatment. To identify the optimum methods to guide therapy and rationalise blood product administration during haemorrhage.
-
Aim 5: disaster. To model the hospital use of transfusion services in mass casualty events and develop best practice guidelines.
-
Aim 6: economics. To describe the health economic implications of massive transfusion and the impact of changes in practice on the NHS.
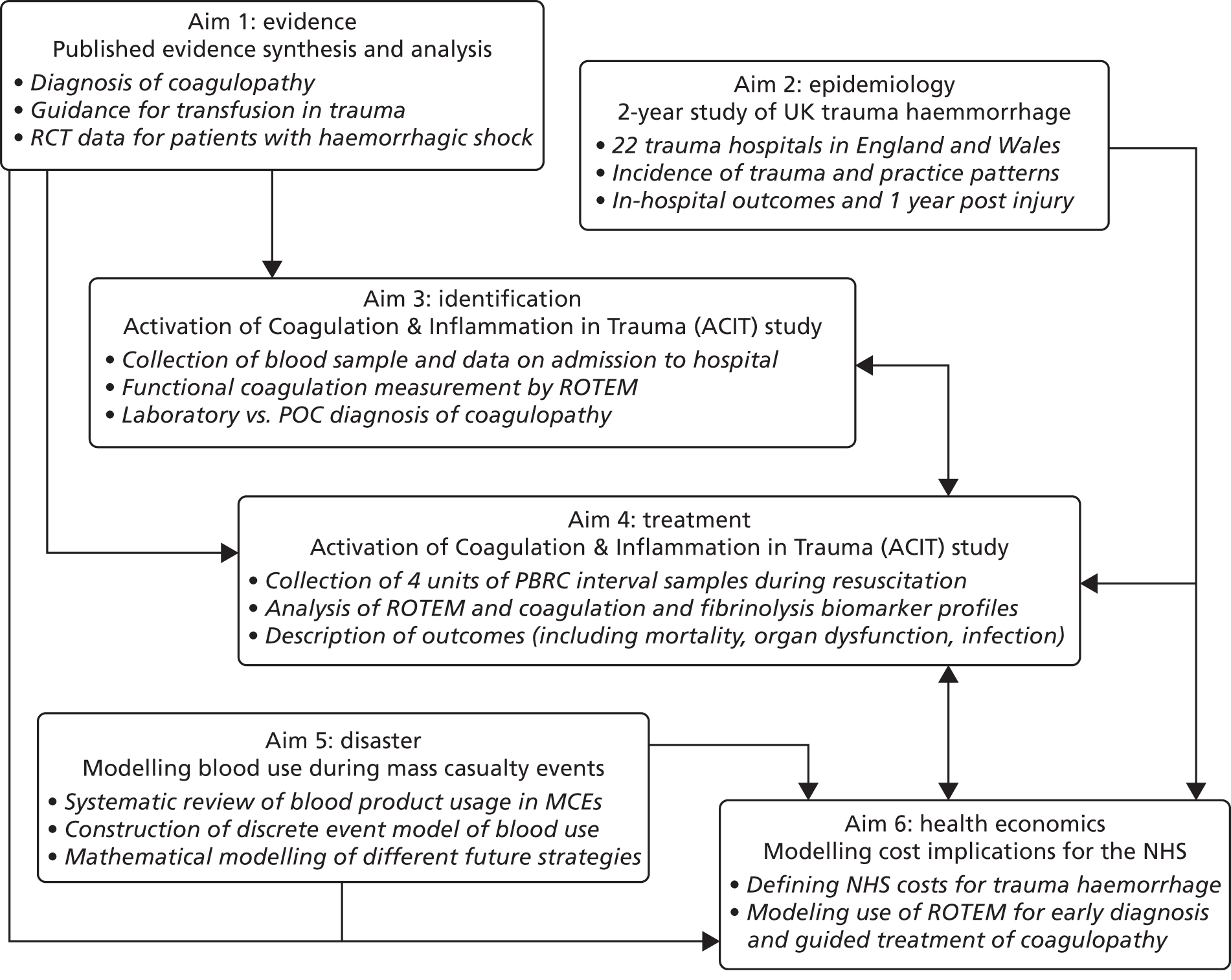
What we did
We performed multimodal parallel studies to achieve the overall objectives (Figure 3). Although these studies were discrete, the conduct and results of each informed aspects of the others.
FIGURE 3.
Inter-relationships of the different aims of the research programme. MCE, mass casualty event; POC, point of care; RCT, randomised controlled trial; ROTEM, rotational thromboelastometry.
The core studies are described in the following sections.
Aim 1: evidence
We conducted several systematic reviews of the existing literature, specifically focusing on the known utility of prediction tools and diagnostic devices to identify patients with TIC and the efficacy of existing blood product transfusions for the treatment of TIC and major haemorrhage. 13–15 All studies were carried out with full adherence to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines16 and systematic review best practice. 17
Aim 2: epidemiology
We conducted a prospective cohort study of patients admitted to 22 hospitals across the country (see Appendix 1), with 2 years of data collection (2009–11) and 1 year of follow up (see Appendix 2). The study involved a close collaboration between clinicians and transfusion services at each hospital and was co-ordinated nationally through the Trauma Audit and Research Network (TARN) and NHS Blood and Transplant. 18 Hospitals were selected to give a range of large and medium-sized trauma-receiving units, both academic and non-academic. We specifically collected data on the incidence and outcomes of major haemorrhage and on transfusion practice at a national level. In particular, we looked at the hourly delivery and utilisation of blood products by haemorrhaging trauma patients in the first 24 hours. In parallel, we conducted a health economic assessment of the epidemiology study data to determine the baseline resource utilisation and costs associated with major trauma haemorrhage (Aim 6: economics). 19 In total, 442 trauma patients with major haemorrhage (requiring at least 4 units of PRBCs) were enrolled over a 2-year period at the 22 representative hospitals across England and Wales.
Aim 3: identification and aim 4: treatment
We conducted a prospective observational cohort study of trauma patients at two major trauma centres in England (Royal London Hospital and the John Radcliffe Hospital, Oxford). In this study, the Activation of Coagulation & Inflammation in Trauma (ACIT) study, blood samples were collected from trauma patients immediately on arrival in the emergency department and while they were bleeding and requiring blood product transfusions (see Appendices 3–5). 20 The blood was analysed for functional and molecular markers of coagulation. As we had detailed data on what blood, fluid and other therapy patients had received between sampling intervals, we could closely examine the response of the body to our therapeutic attempts to normalise the clotting system. In particular, we were interested in whether point-of-care diagnostics such as thromboelastometry could be used in the emergency setting to identify TIC and guide transfusion management. Patients were closely followed for outcomes including mortality, organ dysfunction, infection, thromboembolism and quality of life. We also collected data on this cohort to evaluate the baseline costs and potential savings associated with new diagnostic and therapeutic approaches (Aim 6: economics).
Over the duration of the programme grant 1095 patients were enrolled into the ACIT study at the two UK sites. Additionally, we opened new ACIT sites in the largest trauma centres in Oslo, Copenhagen, Amsterdam and Cologne, under the umbrella of a new research collaborative, the International Trauma Research Network (INTRN). Over the duration of the programme grant 2201 patients were recruited into the ACIT study across the INTRN partners. Recruitment continues on this important platform and to date we have recruited > 3500 patients into the ACIT study. As the study progressed we also retrospectively reviewed the effect of changes made to practice on outcomes and blood product usage to track the performance of our work against our stated overall objective. 12 From 2008 to 2014, 800 trauma patients activating the major haemorrhage protocol were treated at the Royal London Hospital major trauma centre and we tracked the progress and outcomes of these patients.
Aim 5: disaster
We performed a systematic review of blood product utilisation in mass casualty events over the past century, in part to evaluate the potential to predict the PRBC and component requirements during potential events. 21 We then constructed a mathematical discrete-event model of PRBC utilisation and flow during mass casualty events, based on data from the London bombings of 7 July 2005. We used this model to test different strategies to preserve blood stocks during such events.
Key findings and outputs
The epidemiology of trauma haemorrhage in England and Wales
Incidence
Our national epidemiology study (see Appendix 2) identified 442 patients with major haemorrhage (requiring at least 4 units of PRBCs for resuscitation) and 146 cases of massive haemorrhage (requiring at least 10 units of PRBCs) at 22 hospitals (see Appendix 1). 18
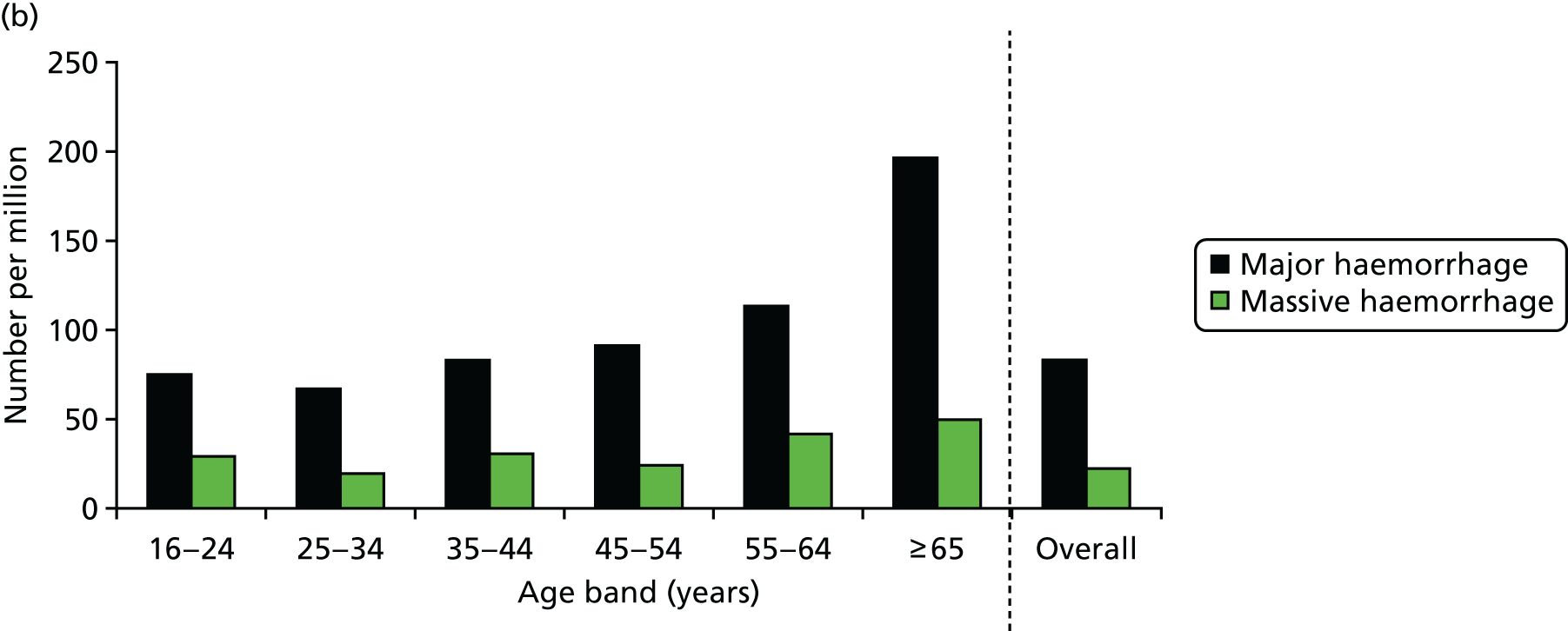
Nationally (for England and Wales), this gives an estimated incidence of 4700 trauma patients a year with major haemorrhage, 1300 of whom will have massive haemorrhage. We unexpectedly found that the incidence was much higher in elderly patients (Figure 4), with more than double the incidence of trauma haemorrhage than in the general population (196 per million vs. 83 per million).
FIGURE 4.
National incidence of major and massive haemorrhage due to trauma: (a) distribution of the 22 participating UK hospitals; and (b) population-based incidence by age group. Source: data from Stanworth et al. 18 Reproduced with permission from Stanworth SJ, Davenport R, Curry N, Seeney F, Eaglestone S, Edwards A, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg 2016;103:357–6518 © 2016 BJS Society Ltd Published by John Wiley & Sons Ltd.
Mortality
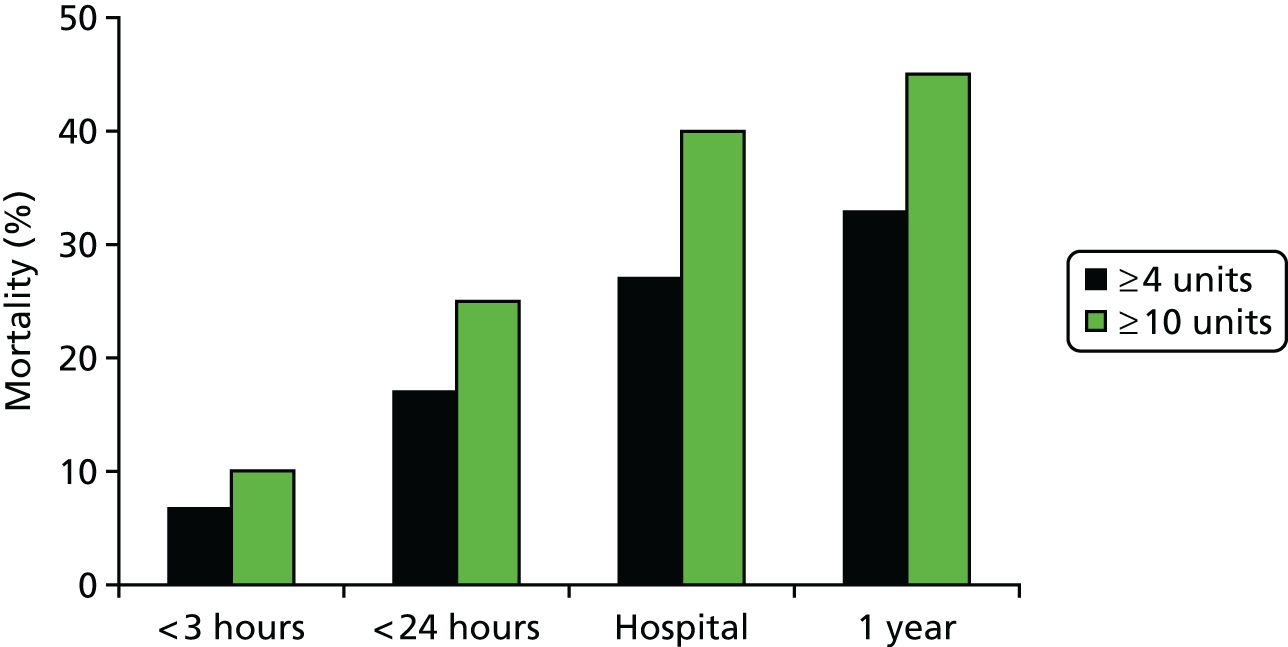
Mortality in our study was high. Nearly half of trauma patients with massive haemorrhage died (and one-third of major haemorrhage patients). This extrapolates to > 1550 people dying of major haemorrhage each year in England and Wales (585 dying of massive haemorrhage). 18
Deaths occurred early, with over half occurring within the first 24 hours of injury and half of these occurring within the first 4 hours of injury (Figure 5). It is clear that improvements in practice have to occur in this critical post-injury window if outcomes from trauma haemorrhage are to improve.
FIGURE 5.
National mortality associated with trauma haemorrhage. One-year mortality approached 50% for patients with massive haemorrhage. Half of all deaths occurred within the first 24 hours and half of these deaths occurred within the first 4 hours of arrival. Source: data from Stanworth et al. 18 Reproduced with permission from Stanworth SJ, Davenport R, Curry N, Seeney F, Eaglestone S, Edwards A, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg 2016;103:357–6518 © 2016 BJS Society Ltd Published by John Wiley & Sons Ltd.
Resource utilisation and health-care costs
On average, major haemorrhage patients received 11 units of blood products (PRBCS, plasma, platelets or cryoprecipitate) and massive haemorrhage patients received 26 blood product transfusions in the first 24 hours. Patients spent just under 3 hours in the emergency department and over half required urgent surgery. 19
Over 80% of trauma patients with major haemorrhage required critical care admission and they spent on average 7 days in critical care and 3 days on a ventilator. They spent on average an additional 20 days in hospital.
Intensive care unit (ICU) costs accounted for the largest proportion of health-care costs, at £4600–9500 per patient. The next largest cost utilisation areas were operating theatres and other ward areas. Blood product transfusions cost approximately £2300 per major haemorrhage patient (£4200 for massive haemorrhage). 19
In total, the estimated cost of treating a major haemorrhage patient was £20,600 (£24,000 for massive haemorrhage). Extrapolating nationally this gives an estimated cost of major haemorrhage after trauma of approximately £85M annually. One-third of all costs are accounted for by elderly trauma patients. 19
Evolution of Code Red major haemorrhage protocols
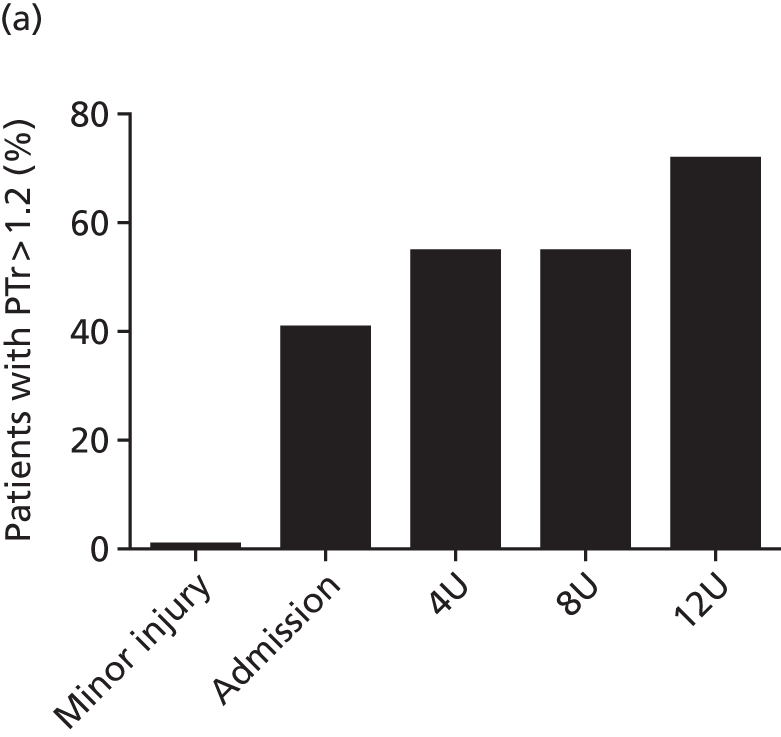
Before 2008, the Royal London Hospital major trauma centre had a massive transfusion protocol in place, based on traditional guidelines such as the British Commitee for Standards in Haematology4 and College of American Pathologists22 recommendations. These massive transfusion protocols were activated for patients likely to require large volumes of red cells, although without clear criteria for activation. Furthermore, they were primarily targeted at the treatment of late complications of large-volume blood and fluid administration, most specifically dilutional coagulopathy. The protocol was associated with a wide variation in the amount of blood products (e.g. plasma) given to patients. There was also a significant amount of waste, especially of platelets. The mortality rate in patients who activated the protocol was 57%. 12
Activation was tightly controlled so that wastage would be reduced. We reviewed the performance of massive transfusion prediction tools and used data from nearly 5700 trauma patients to develop our own prediction algorithm. 23 We determined that there was no specific prediction tool that performs better than another and no algorithm suitable for early (even pre-hospital) activation of a protocol. Additionally, we recognised that activating haemorrhage protocols only on the basis of a specific number of PRBCs transfused precludes improvements in care that reduce PRBC requirements in the future. 23
In late 2008, coinciding with the start of the programme grant, the trauma centre instituted a new major haemorrhage protocol for trauma patients. This Code Red protocol aimed to identify early patients who were bleeding and prevent (rather than treat) dilutional coagulopathy. Treatment followed the principles of DCR based on the limited evidence available at the time. As our research (and that of others) progressed we iteratively updated the protocol to include new research findings and practices.
Immediately after institution we found that we reduced the wastage of products but there was no immediate impact on survival. 12 We progressed, limiting crystalloids, improving the delivery of products and introducing new therapeutics and evidence-based point-of-care coagulation assessments, and we saw a stepwise reduction in mortality. By the end of the programme grant mortality had reduced to 26%, with an associated reduction in all blood product administration and waste, as well as reductions in critical care and hospital stays.
The Royal London Hospital’s Code Red protocol (Figure 6) has been adopted by almost all major trauma centres in the UK (modified for local practice) and has been disseminated internationally. Further refinements continue to occur as more research results become available from this programme of work and the work of others.
FIGURE 6.
Evolution of the Royal London Hospital major trauma centre Code Red protocols: (a) 2008; and (b) 2011. ABG, arterial blood gas; APTT, activated partial thromboplastin time; BP, blood pressure; Ca++, calcium; CA5, clot amplitude at 5 minutes; CT, clotting time; DCS, damage control surgery; ED, emergency department; FBC, full blood count; G&S, Group & Save; HEMS, Helicopter Emergency Medical Services; IV, intravenous; ML, Maximum Lysis; O NEG, O negative; O POS, O positive; POC, point of care; PT, prothrombin time; RBC, red blood cell; ROTEM, rotational thromboelastometry; SpR, specialist registrar.

We have subsequently revised our Code Red protocol again based on our subsequent work using point-of-care functional coagulation assessment. In the following sections we detail our work on using these point-of-care devices to diagnose coagulopathy, predict major haemorrhage and individualise therapy to optimise blood product utilisation and patient outcomes. The protocol is currently being implemented and will be evaluated in a randomised controlled trial as part of a subsequent European Union FP7 programme of work. 24
Rapid diagnosis and characterisation of acute traumatic coagulopathy
Aim 3 of the programme grant was specifically focused on the early identification of coagulopathy and, specifically, ATC. By 2008 ATC had been identified and defined as a prolongation of the laboratory prothrombin time (PT) and was thought to be caused by the systemic activation of anticoagulant factors. 25 Data from our ACIT study (see Appendices 3–5) showed that the laboratory PT was not available in a clinically useful time frame, on average becoming available to the treating trauma team only after 90 minutes. 20 We also investigated a hand-held PT device, used for home warfarin monitoring. These devices are not, however, calibrated for low haematocrit levels found in bleeding patients and we found they significantly under-read compared with the laboratory value. 20
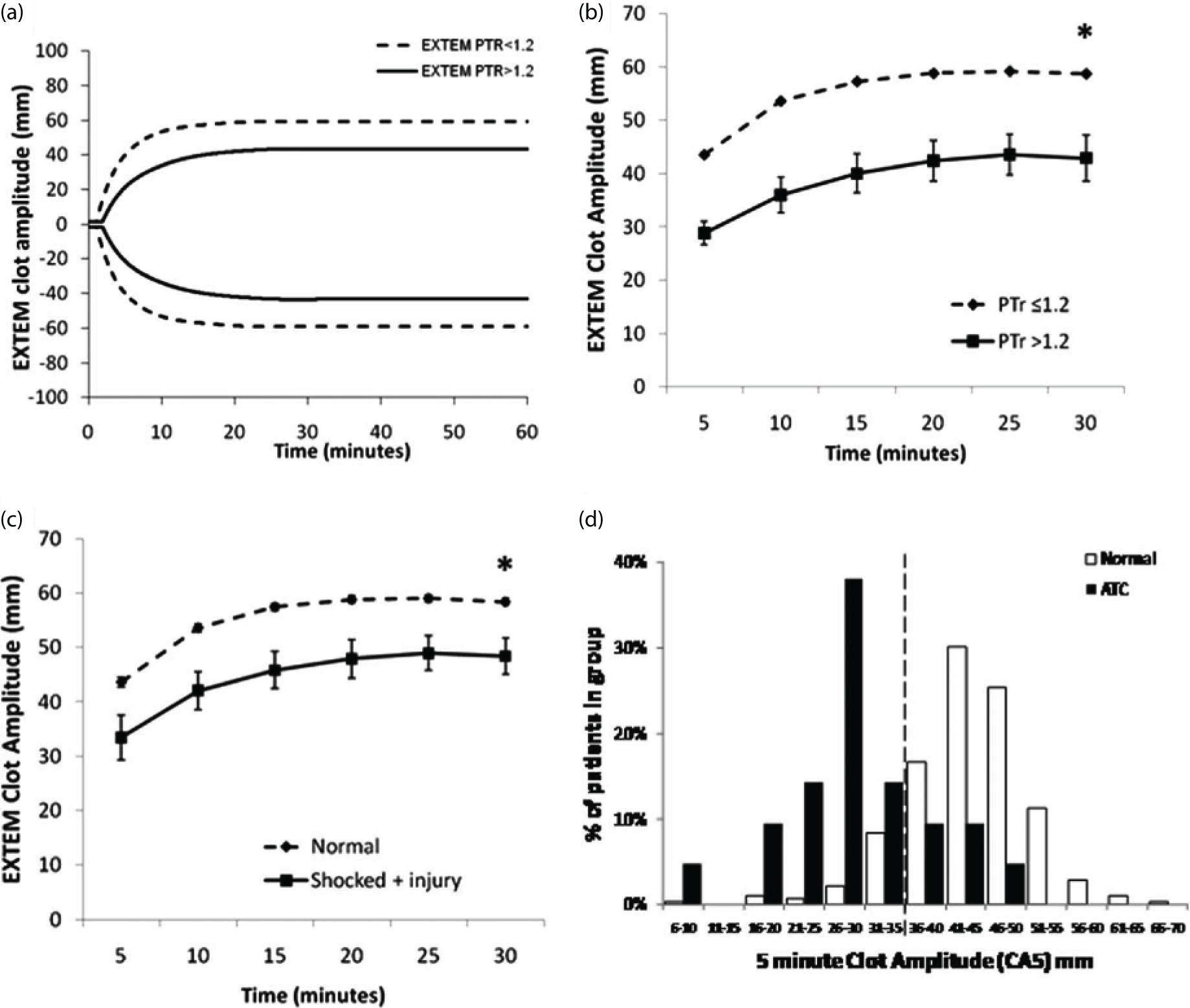
We wished to evaluate the potential for a point-of-care functional coagulation analyser – rotational thromboelastometry [ROTEM® (Tem International GmbH, Munich, Germany)] – to identify patients with ATC. ROTEM measures the strength of a blood clot as it forms over time in a small well of the device. Different activators and inhibitors can be added to assess different parts of the coagulation system.
In our first study we analysed the ROTEM traces of 300 trauma patients. 20 For the first time we were able to fully characterise the functional characteristics of ATC. We found that the primary functional disorder of ATC is a loss of clot strength [reduction in maximum clot firmness (MCF)]. In contrast, prolongation of clot activation (the PT) is a mild and rather late component of the coagulopathy.
Although measurement of the MCF takes half an hour or more, we were able to identify a ROTEM parameter available within 5 minutes that correlated closely with the MCF (Figure 7). The clot amplitude at 5 minutes (CA5) was able to accurately identify ATC and to predict the need for massive transfusion. It also showed an excellent negative predictive value, suggesting that it could also be used to curtail a major haemorrhage protocol to reduce waste or overtransfusion. We have since validated our finding in a later cohort and across the six INTRN partner sites in a study of 808 ACIT patients. 26 The CA5 remains the fastest and most effective point-of-care diagnostic marker of ATC.
FIGURE 7.
Functional characterisation of ATC: (a) averaged ROTEM curves for normal and ATC patients; (b) identification of CA5 of ≤ 35 mm to identify patients with ATC; (c) combined effects of trauma (injury severity score > 15) and shock (base deficit > 6) on the ROTEM trace; and (d) histogram demonstrating the distribution of CA5 among normal and ATC patients. The dotted line represents the new threshold for ATC (CA5 ≤ 35 mm). Source: reproduced with permission from Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, Stanworth S, Brohi K. Functional definition and characterisation of acute traumatic coagulopathy. Critical Care Medicine, vol. 39, issue 12, pp. 2652–8, URL: http://journals.lww.com/ccmjournal/pages/default.aspx. 20 Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact healthpermissions@wolterskluwer.com for further information.
Resuscitation of trauma haemorrhage: effectiveness of high-dose plasma therapy
Early approaches to the treatment of TIC focused on treatment with high-dose FFP. This was based on early assumptions about the mechanism of TIC: at this time it was thought to be caused by a loss or consumption of procoagulant factors. It also appeared to be supported by retrospective military and civilian studies27 that showed improved outcomes when patients were given higher doses of FFP than in standard therapy. 9 The best outcomes appeared to be obtained when 1 unit of FFP was transfused for each 1 or 2 units of PRBCs.
There were significant concerns about this approach, however, which involved transfusion of four to five times the amount of emergency FFP than patients usually received. This would have a major resource impact on blood services worldwide and expose patients to a lot more blood products, with the associated potential risks of immunosuppression and infection. No group had ever been able to study the effect of FFP transfusions on coagulation during the resuscitation of a bleeding trauma patient.
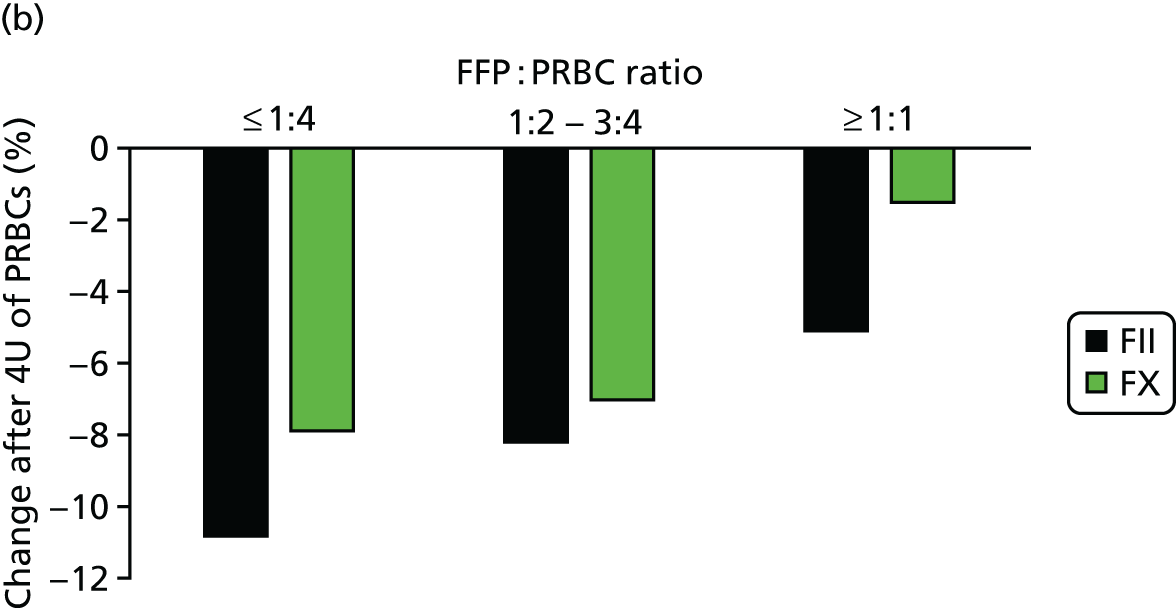
We examined the coagulation system response to high-dose FFP transfusion (Figure 8). 28 We were able to show that giving FFP in high doses certainly appeared to reduce the dilutional coagulopathy that occurred as patients continued to bleed and receive PRBC resuscitation. With low-dose FFP, plasma levels of procoagulant factors such as factors II and X fell to low levels after transfusion of 12 PRBC units, whereas, with high-dose FFP, levels were somewhat protected.
FIGURE 8.
Progression of coagulopathy during bleeding and resuscitation: (a) overall incidence of coagulopathy during haemorrhage and resuscitation; and (b) reduced loss of activity of some procoagulant factors with high-dose plasma administration. FII, factor II; FX, factor X; PTr, prothrombin ratio; U, units. Source: reproduced with permission from Intensive Care Medicine, Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma haemorrhage, vol. 41, 2015, pp. 239–47, Khan S, Davenport R, Raza I, Glasgow S, De’Ath HD, Johansson PI, Curry N, Stanworth S, Gaarder C, Brohi K, © Springer-Verlag Berlin Heidelberg and ESICM 2014 with permission of Springer. 26
However, these plasma regimens did not treat an existing coagulopathy. They were protective against further deterioration, but trauma patients presenting with ATC never corrected their coagulation parameters to normal until bleeding had stopped and compensatory mechanisms had taken over. Although high-dose plasma was effective in avoiding dilutional coagulopathy, there was still an opportunity to improve outcomes with focused, targeted anti-ATC therapy early in the clinical course.
We also reviewed transfusion practice nationally (Aim 2) as part of our prospective cohort study. 18 We found that there were a number of opportunities for improvement in practice that could directly translate to improved outcomes. We particularly looked at delays in the delivery of FFP and other blood products (Figure 9) and times when the unavailability of products would leave patients exposed to a risk of dilution with PRBCs or intravenous fluid replacement (e.g. crystalloids).
FIGURE 9.
National patterns of transfusion therapy in trauma haemorrhage: (a) late FFP availability results in < 50% of patients achieving a FFP : PRBC ratio of ≥ 1 : 2 by 4 hours after admission; (b) the FFP : PRBC ratio varies and balanced resuscitation is not delivered consistently during resuscitation; and (c) mortality by hour showing the concentration of deaths when FFP is not available or administered. Source: data from Stanworth et al. 18 Reproduced with permission from Stanworth SJ, Davenport R, Curry N, Seeney F, Eaglestone S, Edwards A, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg 2016;103:357–6518 © 2016 BJS Society Ltd Published by John Wiley & Sons Ltd.
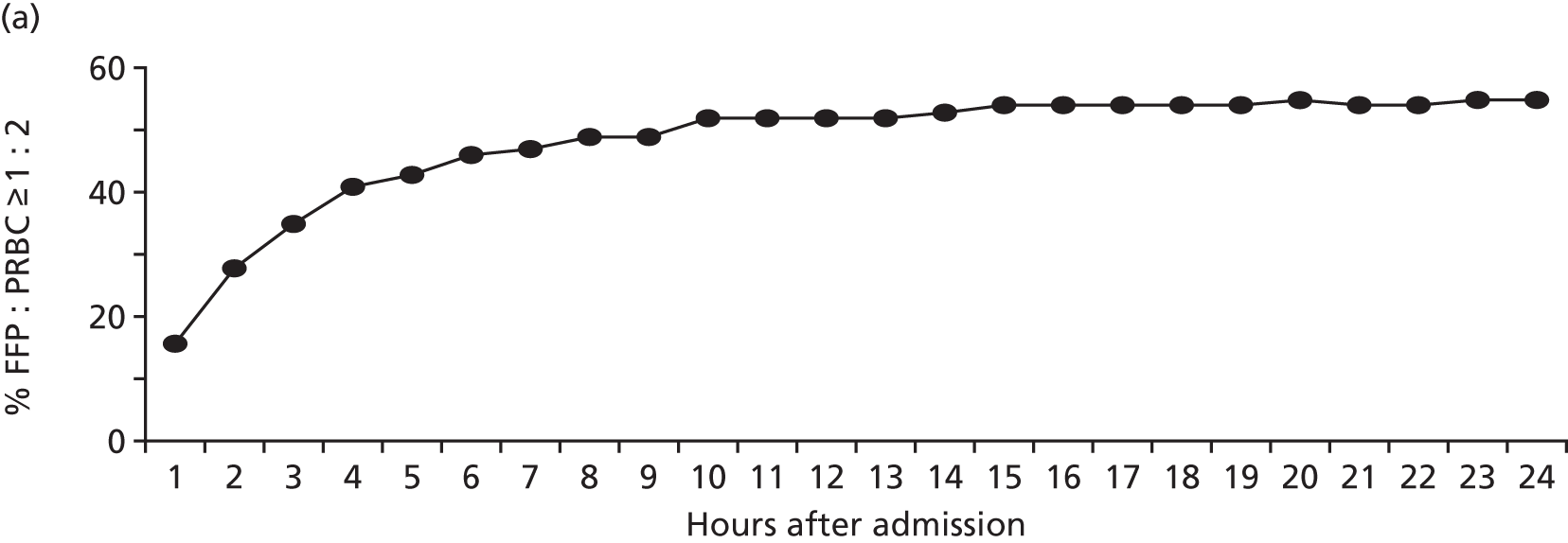
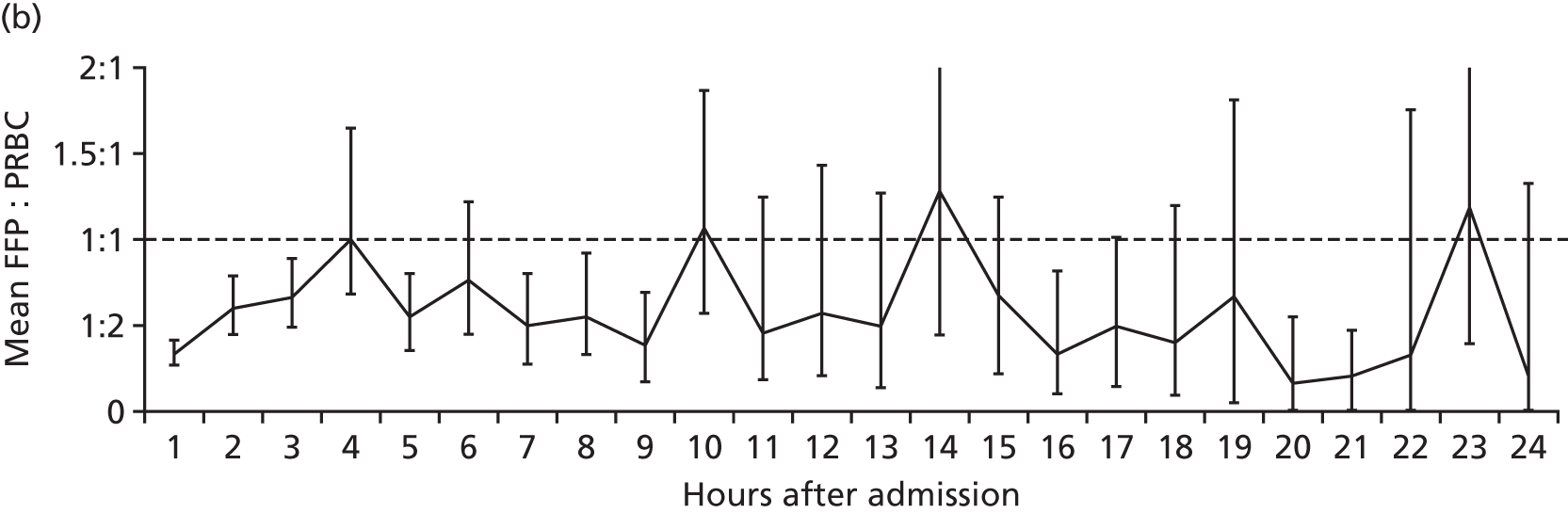
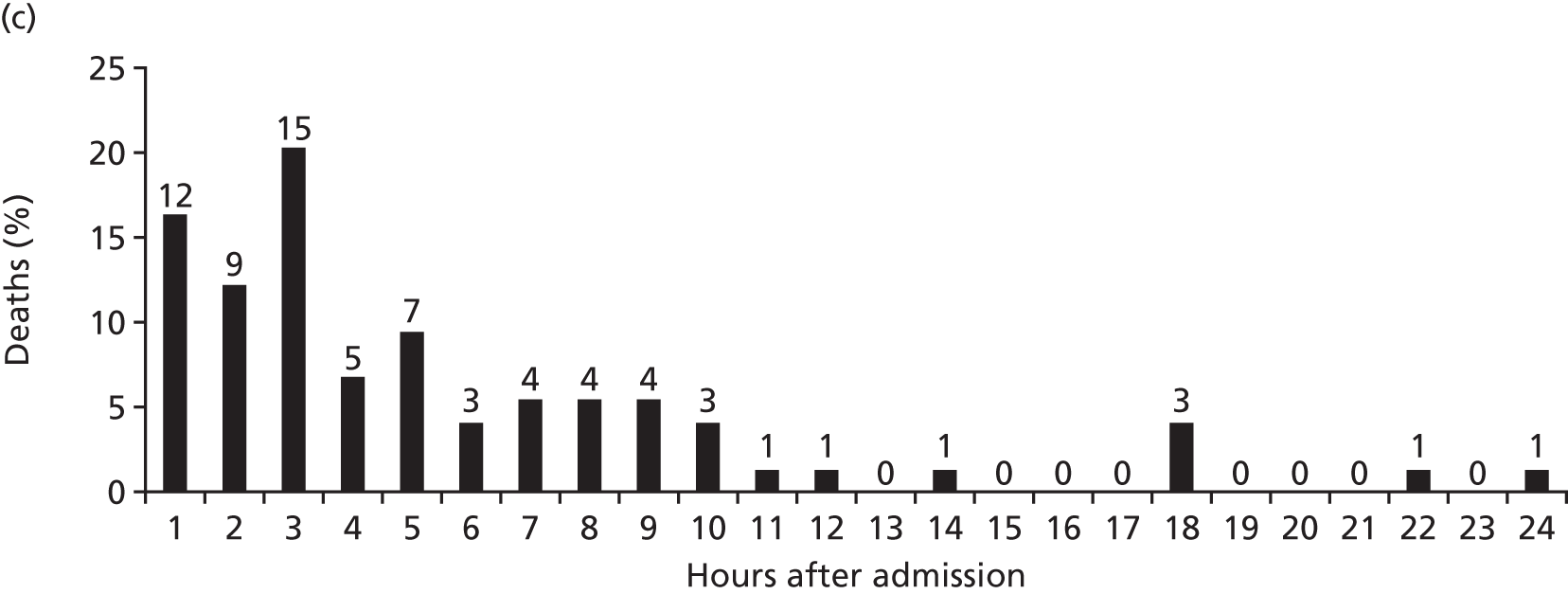
Bleeding trauma patients tended to receive PRBCs first and there was a delay of 1–3 hours before receiving the first doses of FFP. During resuscitation, there was variation in the delivery of FFP leading to variation in the ratio delivered. There were times when no blood products were available. A large proportion of deaths occurred in the early phase of care when plasma and other blood product delivery was absent or inefficient. There are therefore important opportunities for practice improvement on a national level based on our current understanding of the optimal management of trauma haemorrhage.
Acute trauma coagulopathy: fibrinogen loss and replacement
We identified reduced clot strength as a central functional component of ATC early in the course of this programme. 20 We investigated the cause of this and rapidly turned our attention to fibrinogen, the substrate for all blood clot formation. 29 We found that trauma patients with ATC arrive with a dramatically reduced level of fibrinogen (Figure 10) – around half of the normal level – and that this level decreases rapidly as bleeding continues. When no fibrinogen-containing blood products were administered, fibrinogen levels were near zero after transfusion of 8 units of PRBCs. Even with high-dose FFP (which contains some fibrinogen), fibrinogen levels continued to decline during bleeding. Only when fibrinogen was replaced with cryoprecipitate were fibrinogen levels maintained. Much closer attention is paid now to fibrinogen levels during haemorrhage and cryoprecipitate usage has increased worldwide over the last few years.
FIGURE 10.
Fibrinogen levels during trauma haemorrhage: (a) fibrinogen levels are low on admission and decrease dramatically in the absence of any fibrinogen replacement (FFP or platelets or cryoprecipitate); and (b) cryoprecipitate as administered in the Code Red protocol maintains but does not correct fibrinogen levels. Fg, fibrinogen. Source: data from Khan et al. 26
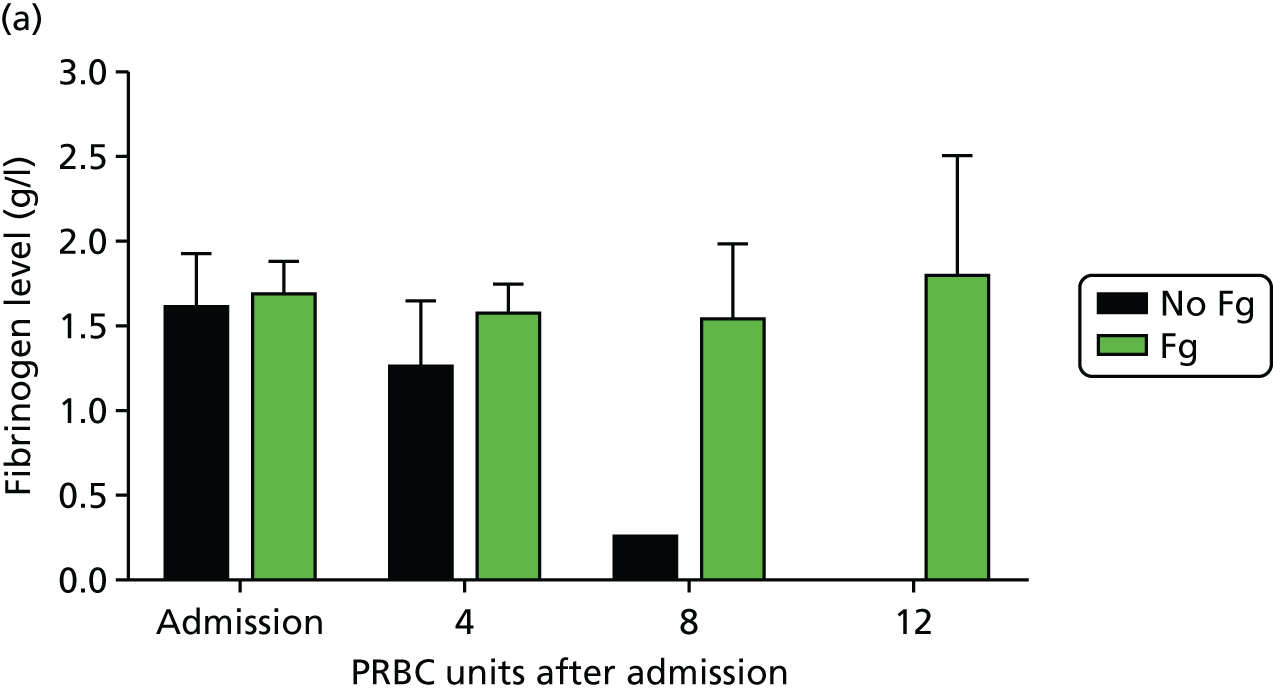
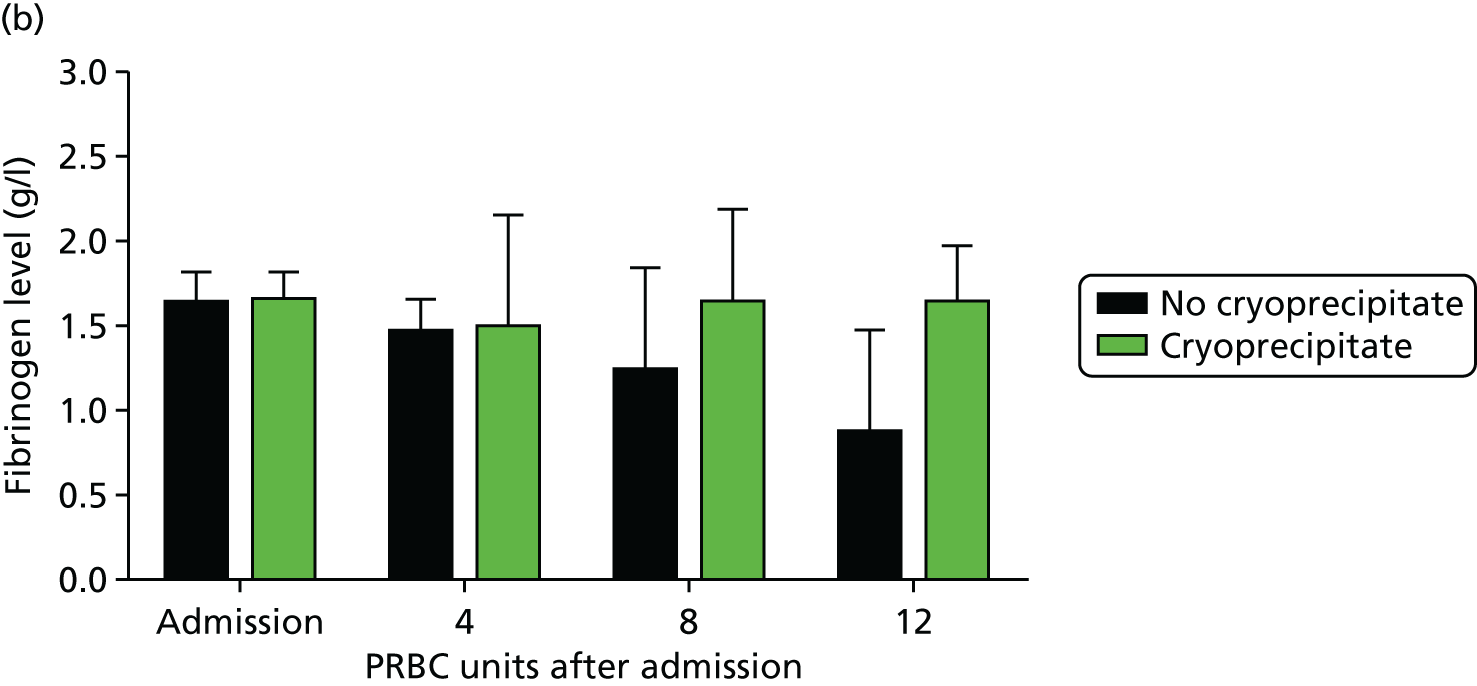
Although cryoprecipitate administered in standard doses can maintain fibrinogen levels during bleeding, it does not correct fibrinogen to normal levels. 29 Trauma patients who arrive with ATC with insufficient fibrinogen substrate to form clots never achieve normal levels of fibrinogen. We therefore conducted ex vivo spiking experiments to determine how much fibrinogen would be necessary to normalise levels. In parallel with the programme grant we then conducted a pilot randomised controlled trial (CRYOSTAT) of early, high-dose cryoprecipitate in bleeding trauma patients. 30 We wanted to see whether or not we could deliver high-dose cryoprecipitate quickly to trauma patients and, in doing so, normalise fibrinogen levels and hence potentially improve outcomes.
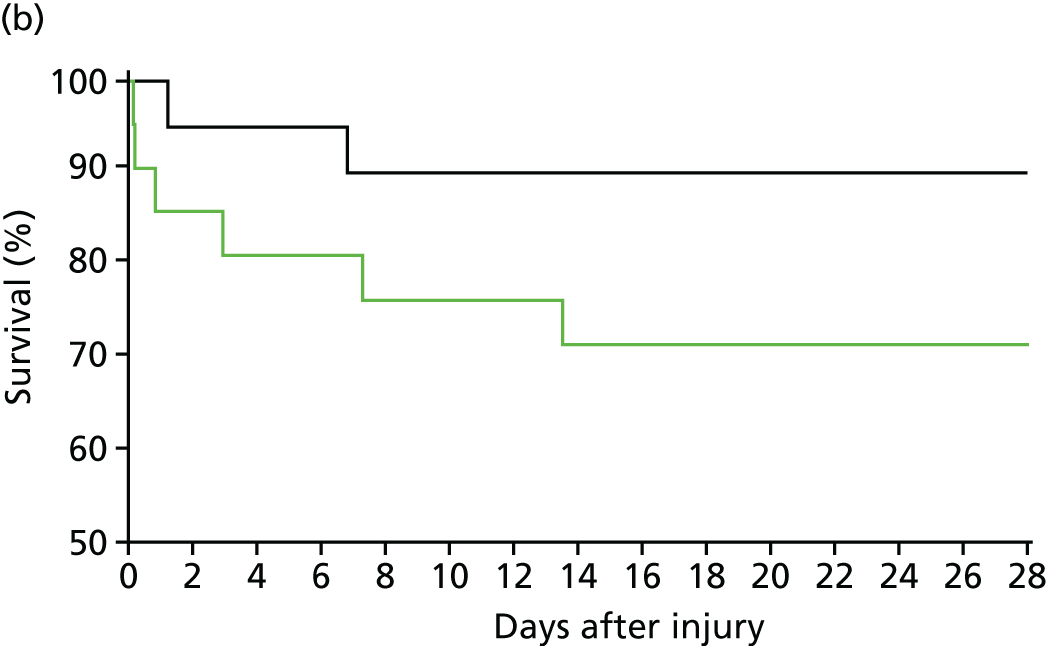
The trial was conducted between the Royal London Hospital and the John Radcliffe Hospital, Oxford. 30 We also recruited patients at the Camp Bastion Military Hospital in Afghanistan. We recruited 40 patients and showed that we could deliver high-dose cryoprecipitate (equivalent to 4 g of fibrinogen supplementation) within 90 minutes to bleeding trauma patients who activated the major haemorrhage protocol. Patients in the early high-dose cryoprecipitate arm maintained their fibrinogen levels throughout the bleeding phase (Figure 11). Furthermore, these patients had a much lower mortality rate than patients in the control arm (less than half of our current Code Red mortality rate), although this was not statistically significant in this small pilot trial.
FIGURE 11.
CRYOSTAT study results: (a) early high-dose cryoprecipitate is able to correct fibrinogen levels during trauma haemorrhage; and (b) survival curves for the high-dose cryoprecipitate (green) and standard therapy (black) groups (not significant). Source: data from Curry et al. 29
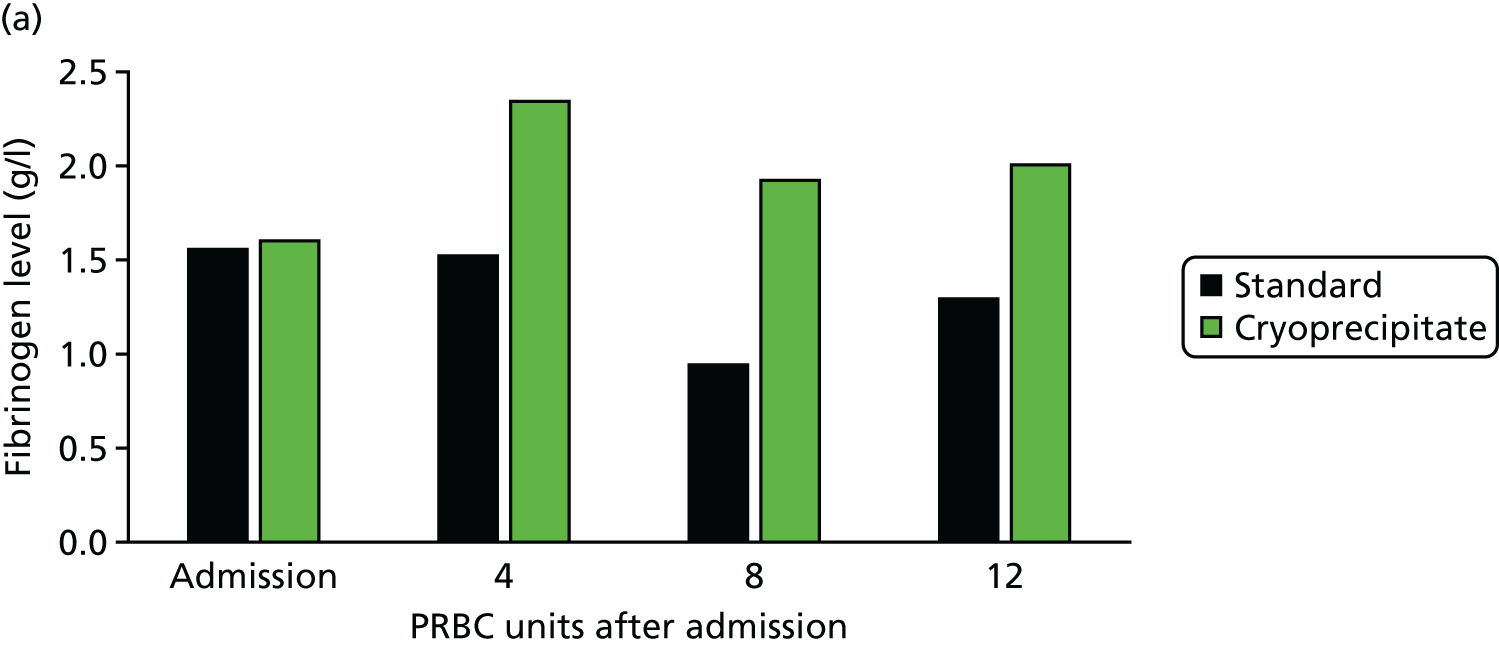
We are currently recruiting to the Phase III CRYOSTAT2 trial to determine whether or not early high-dose fibrinogen replacement significantly improves outcomes in trauma haemorrhage.
Acute trauma coagulopathy: fibrinolysis and tranexamic acid
In earlier work we had identified excessive clot breakdown (hyperfibrinolysis) as a key characteristic of ATC. 25 However, there was conflicting evidence on hyperfibrinolysis. In studies using functional coagulation tests such as ROTEM, hyperfibrinolysis was very rare and almost uniformly fatal. In a large randomised controlled trial [Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage (CRASH-2)] by our close collaborators at the London School of Hygiene & Tropical Medicine, a relatively low dose of an antifibrinolytic agent, tranexamic acid, led to significant improvements in survival. 30 This seeming paradox, together with a potential risk of thromboembolism, had restricted the implementation and uptake of tranexamic acid by the NHS and internationally. Some bodies mandated the use of functional clotting assays to guide therapy, whereas others refused to adopt this treatment.
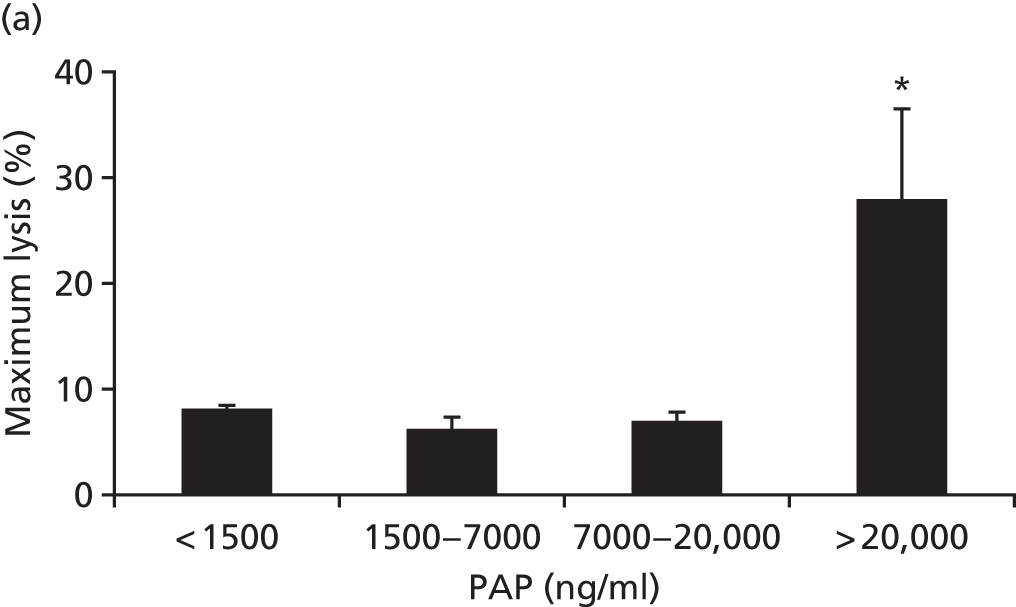
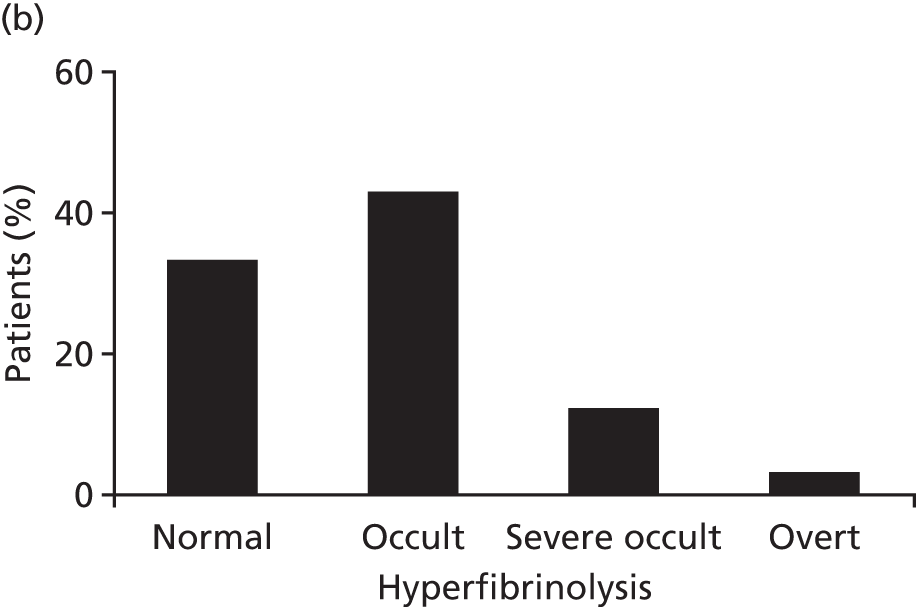
We explored fibrinolysis in trauma patients by examining the fibrinolytic pathway in our ACIT cohort. 31 We found that > 60% of trauma patients have extreme fibrinolysis even in the first minutes after injury (Figure 12). More importantly, this lytic activity is not detectable in viscoelastic assays until endogenous antifibrinolytic proteins are exhausted. This fibrinolysis in trauma patients is common and occult and, therefore, viscoelastic assays should not be used as criteria for antifibrinolytic therapy.
FIGURE 12.
Thromboelastometry is insensitive to fibrinolysis in TIC: (a) maximum lysis occurs only at extremely high levels of plasmin production; and (b) 60% of patients have occult lysis evidenced by PAP levels of > 10× normal and which is not visible on thromboelastometry. PAP, plasmin–antiplasmin. Source: data from Raza et al. 31
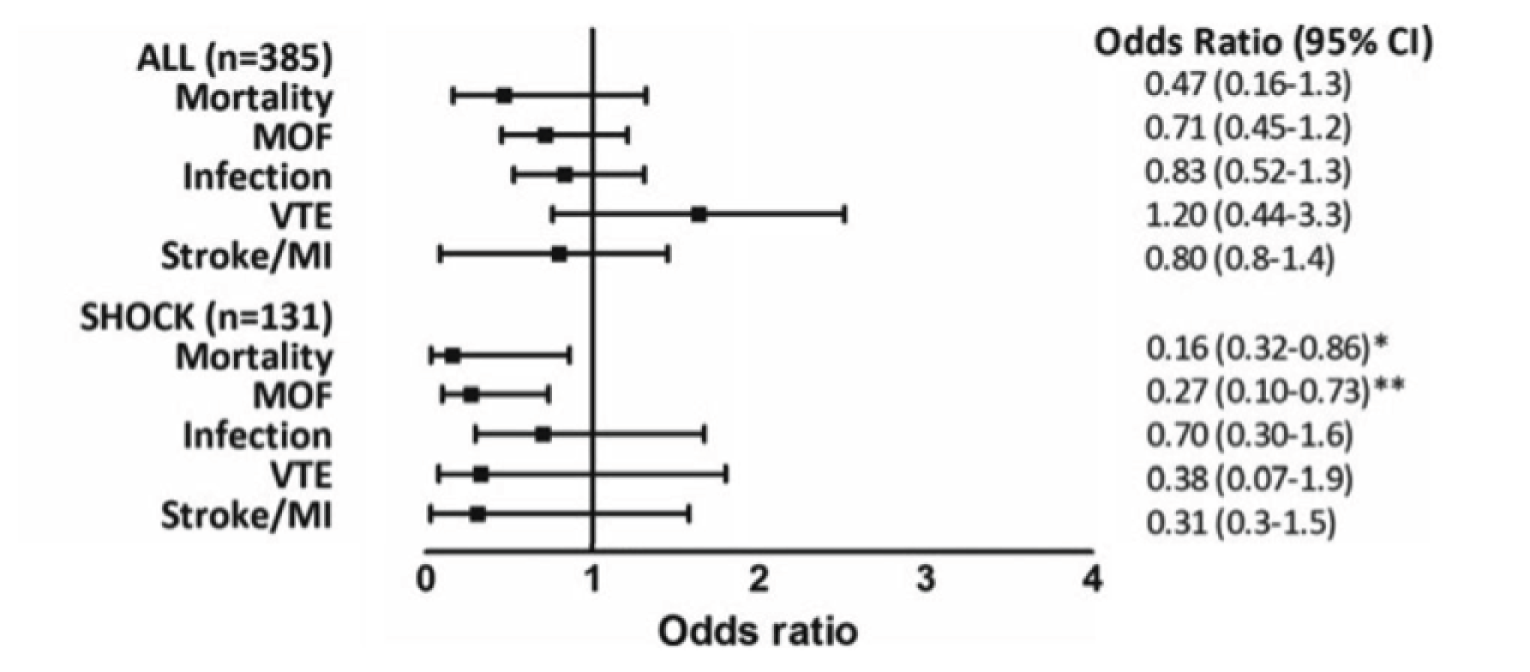
We incorporated tranexamic acid into our Code Red protocol in 2011. In the first civilian study of tranexamic acid in a developed civilian trauma system setting, we showed improved outcomes including survival (Figure 13). 32 Patients presenting in shock had the greatest benefit, with a fourfold reduction in the relative risk of death. We also showed that early tranexamic acid administration not only treats hyperfibrinolysis but also protects clot strength throughout trauma haemorrhage.
FIGURE 13.
Tranexamic acid reduces mortality and multiple organ failure (MOF) in trauma patients presenting with shock (which is defined by base deficit of > 6 mmol/l). MI, myocardial infarction; VTE, venous thromboembolism. Source: reproduced with permission from Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg vol. 261, iss. 2, pp. 390–4. 32 2015. URL: http://journals.lww.com/annalsofsurgery/pages/default.aspx. Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact healthpermissions@wolterskluwer.com for further information.
This work has supported the uptake of tranexamic acid into clinical guidelines and major haemorrhage protocols. It is now widely used across the country and is given by pre-hospital teams to patients suspected to have haemorrhage before arrival at trauma centres.
The evolved concepts of damage control resuscitation and trauma-induced coagulopathy
In overview, we have used the principles of DCR as a starting point and refined its principles, practice and treatment, iteratively generating evidence and implementing it into practice (Figure 14).
FIGURE 14.
Interventions associated with Code Red protocol evolution at the Royal London Hospital major trauma centre. Fg, fibrinogen; MHP, major haemorrhage protocol; NIHR, National Institute for Health Research; PGfAR, Programme Grant for Applied Research; TXA, tranexamic acid.
Our new concept of TIC is simpler, with a more mechanistic basis. Essentially, there are two main coagulopathies: the endogenous ATC and a subsequent dilution/consumption phenotype that progresses during haemorrhage and resuscitation.
We now believe that the central features of ATC are fibrinogen loss and hyperfibrinolysis. Tranexamic acid has been introduced into practice for hyperfibrinolysis and early fibrinogen replacement is awaiting a next-phase clinical trial.
Dilutional/consumption coagulopathy is now managed by avoiding crystalloids, even in the pre-hospital phase of care; streamlining the delivery of transfusion products; and delivering plasma in a 1 : 1 ratio with PRBCs.
We believe that we have demonstrated that robust implementation of these principles and practices can have a dramatic impact on outcomes for trauma patients. Nationally, trauma haemorrhage mortality remains very high, but we now have a roadmap to begin to reduce these deaths.
Mass casualty and event response plannings
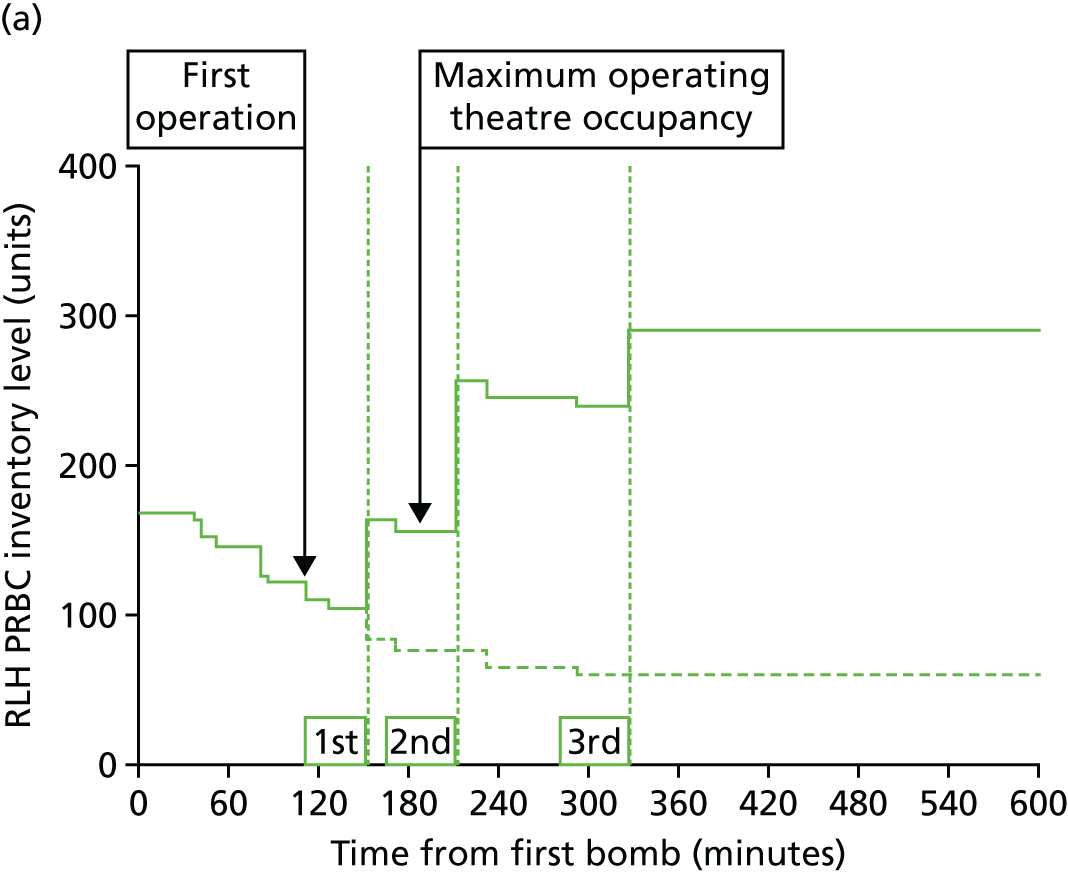
Blood is one of the most constrained resources in the response to mass casualty events such as the London bombings of 2005. 21,33 Restocking of the hospitals involved in a response is difficult or impossible during the early phases of the event. At least initially, hospitals must rely on the stocks already present before the event occurs (Figure 15). New DCR paradigms requiring transfusion of more blood products per casualty will also increase the strain on these limited resources. 34 There are important implications, therefore, for major incident preparedness, as well as for planning for expected events such as the London Olympics.
FIGURE 15.
Provision of red cell transfusions during mass casualty events: (a) red cell stocks at the Royal London Hospital during the bombings of 7 July 2005; and (b) the best correlation of red cell requirements is with the number of critically injured casualties in a mass casualty event. ISS, Injury Severity Score; RBC, red blood cell; RLH, Royal London Hospital. Source: reproduced with permission from Glasgow S, Davenport R, Perkins Z, Tai N, Brohi K. A comprehensive review of blood product use in civilian mass casualty events. J Trauma Acute Care Surg vol. 75, iss. 3, pp. 468–74. 2013. 21 URL: http://journals.lww.com/jtrauma/pages/default.aspx. Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact healthpermissions@wolterskluwer.com for further information.
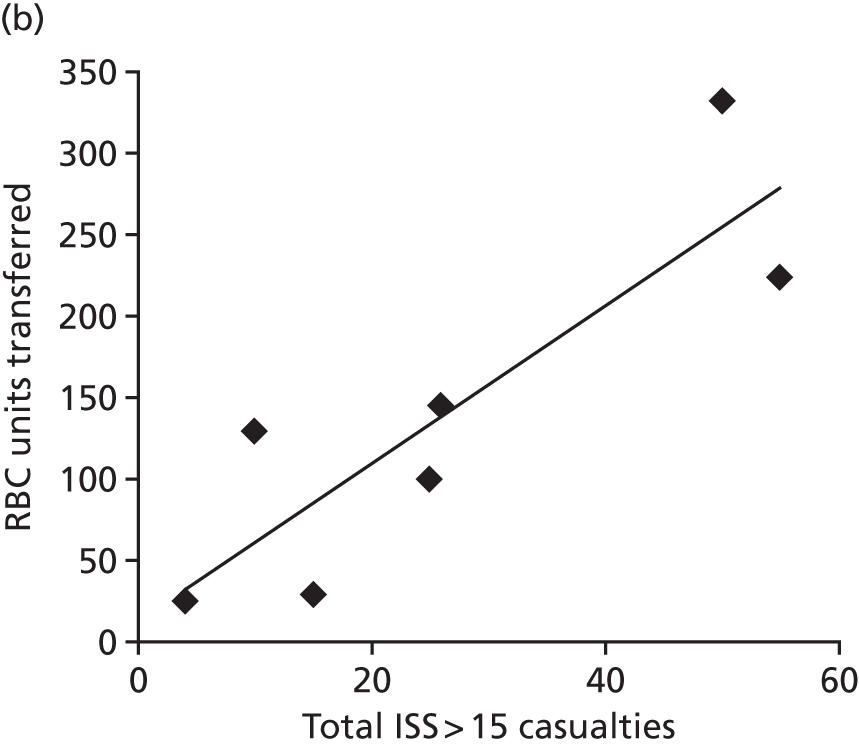
We studied the utilisation of blood stocks during mass casualty events through a systematic review of the literature and a detailed examination of blood utilisation during the London bombings and Norway attacks. 21
We were able to correlate PRBC transfusions with the number of critically injured casualties (as opposed to other measures of casualty load). As these numbers are relatively constant for specific types of events, this can be used to plan the numbers of PRBC and blood product stocks that need to be held for specific events. We were able to feed this information into the planning process for the 2012 London Olympics. 34
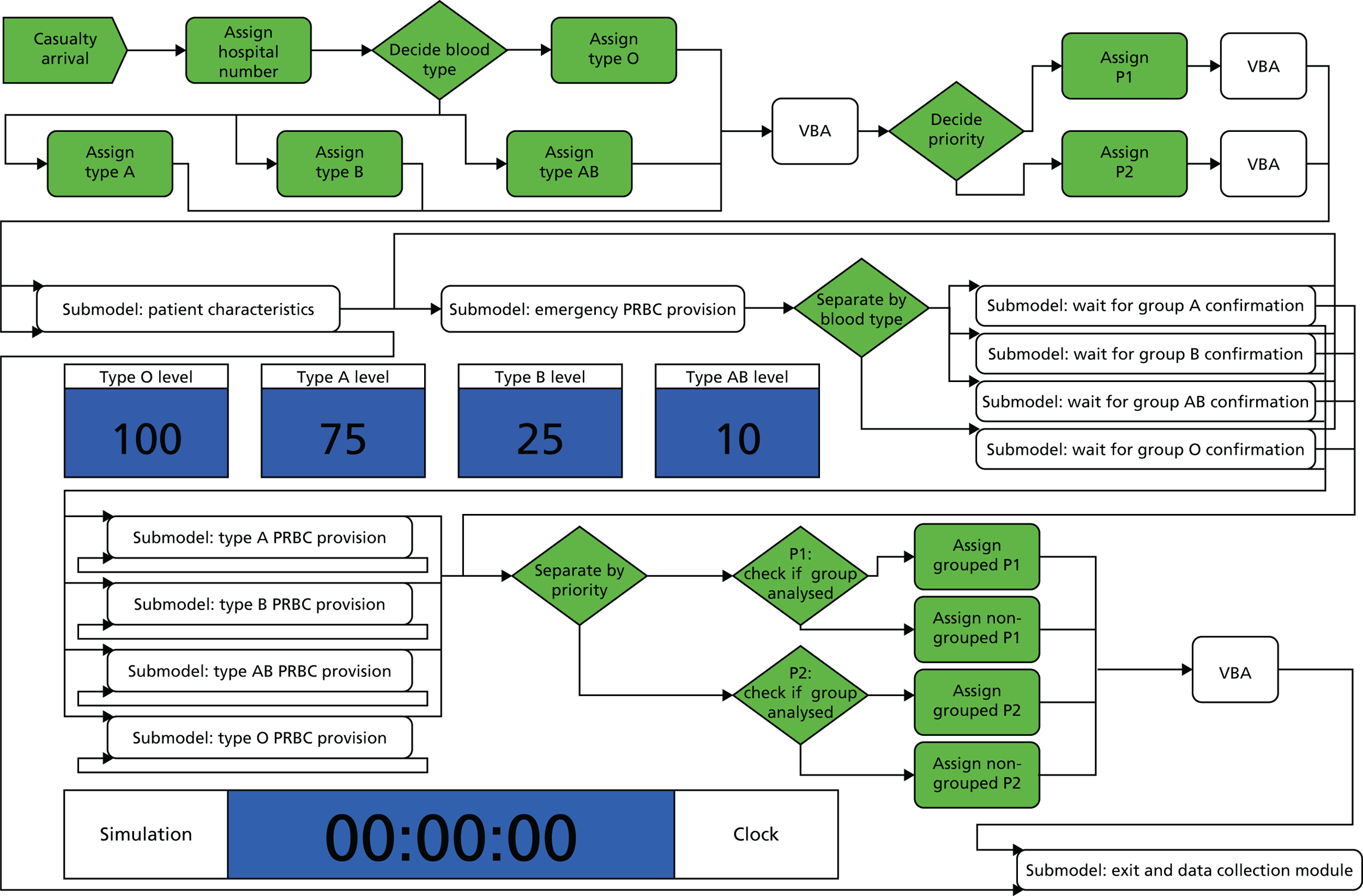
We then created a model of casualty flow and blood utilisation by hospitals during a mass casualty event. 35 We populated this with the data collected from the literature, our specific investigations and the results of interviews with hospital staff and specialists. Once the model was constructed (Figure 16), we could modify parameters to investigate the benefits of alternative methods of utilising and preserving blood resources during such events.
FIGURE 16.
Overview of the queuing model for examining red cell management during mass casualty events. VBA, Visual Basic for Applications.
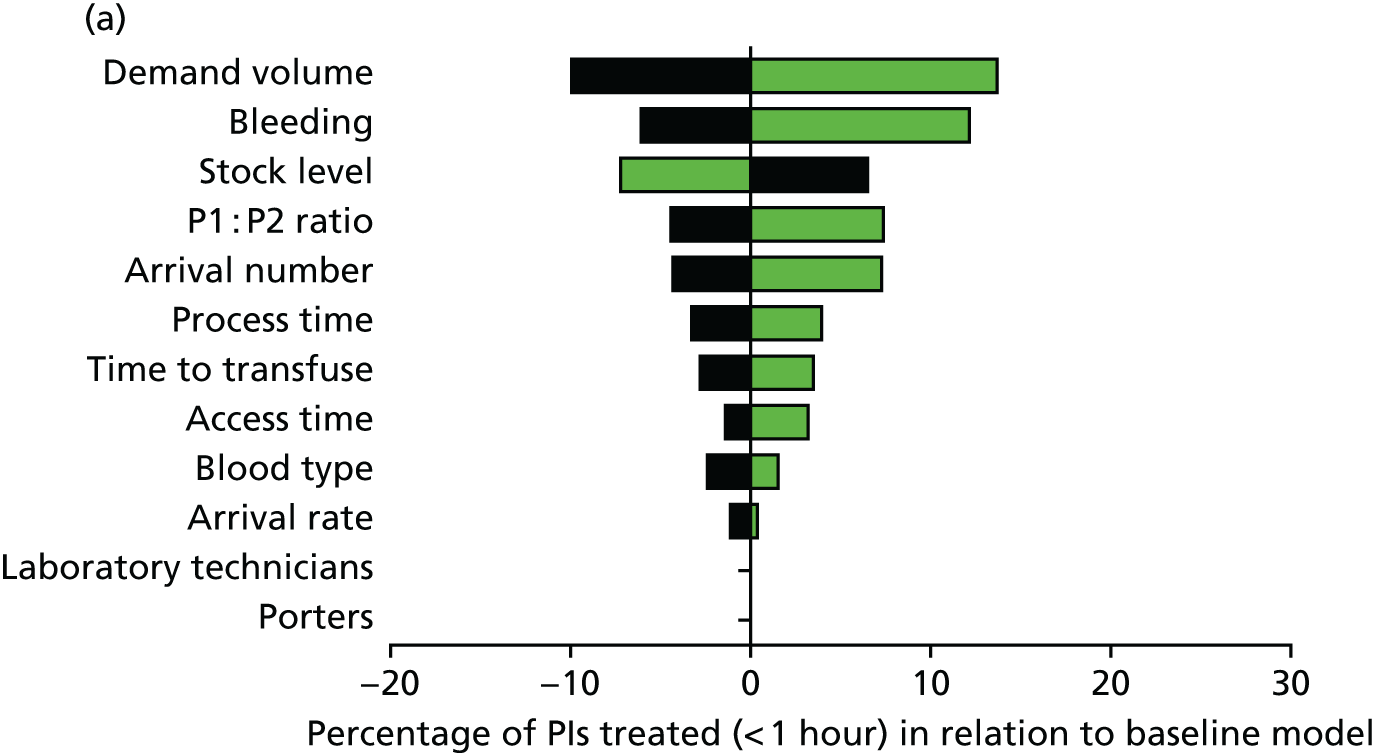
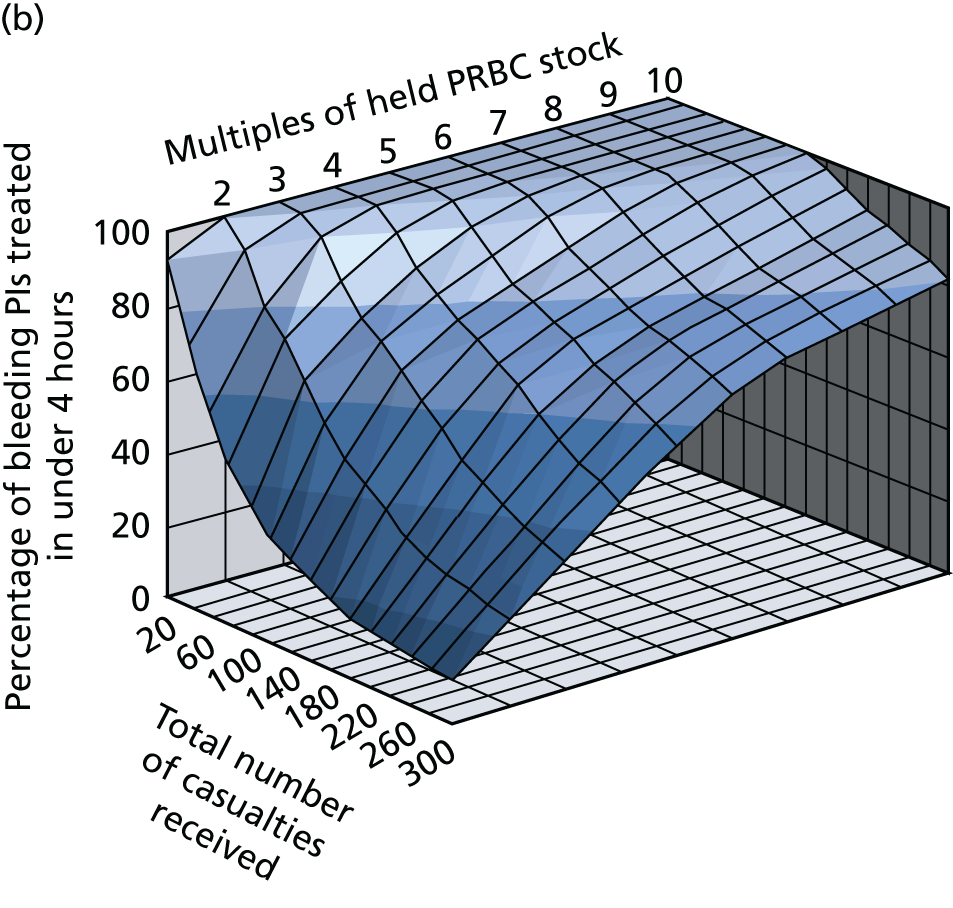
Exploring the model we can see how increased casualty loads and stock levels have the most profound effect on how quickly casualties receive the blood products they need and how blood stocks are preserved (Figure 17). These cannot easily be mitigated by increasing the number of technicians, reducing processing time or otherwise trying to increase efficiencies within the system.
FIGURE 17.
Example modelling outputs for red cell provision during mass casualty events: (a) tornado plot showing sensitivity analysis for how changing event variables affects the number of P1s receiving timely red cell transfusions; and (b) waterfall plot showing the effect of casualty numbers and the size of red cell stocks on the timely transfusion of P1 casualties.
Mass casualty response planning therefore has to focus on methods for restocking supplies. In addition, blood utilisation may need to be restricted during these events. We examined several strategies and found that both overall limitation of total PRBC transfusions and the restriction of emergency type O blood transfusions were effective in providing the best care for the most casualties. However, the clinical acceptability of these strategies still needs to be explored.
Limitations
There are several limitations to our studies and the findings. These are discussed in detail in our papers and are summarised in the following sections.
Epidemiology
In our national study we used a representative sample of large and small hospitals with an element of selection as we relied on the submission of TARN data to assist with data collection. As this work mostly predated the introduction of regional trauma systems, it is possible that we have underestimated the incidence of trauma haemorrhage because of incomplete data reporting. It is also possible that our national estimate is inaccurate because of the hospitals not being representative of the country as a whole. However, the picture of national mortality and other outcomes and of overall transfusion practice and blood product delivery is unlikely to be affected by these potential variations.
Our health economic analysis was as detailed and accurate as possible based on the cost and resource utilisation information available to us. However, these are cost estimates and there was wide variation across the country and between patients. Nevertheless, the total figures represent the most accurate assessment to date of the costs of treating patients with trauma haemorrhage and help to understand where the main cost burden – and potential savings – may lie.
Diagnosis and treatments
The studies on diagnosis and treatment were based principally on the examination of a large cohort from two major trauma centres. As such, although trends and associations can be investigated and hypotheses generated, it is not possible to determine causality from these studies. However, we have looked deeply into mechanisms to support the clinical research findings and, when possible, we have taken these findings into a randomised controlled trial (CRYOSTAT) or collaborated with other trial groups (e.g. CRASH-2). Our findings on diagnostic test accuracy and thresholds for treatment need external validation and utilisation and these projects are ongoing through the INTRN. 36 Our cohorts were not large enough to look at responses to other blood products such as platelet transfusions and further work is required in this area.
Mass casualty management
As with all models the quality of the output is dependent on the base information available for model development. We gathered as much evidence as possible from the literature and specific event information available to us. However, this may not be representative of all possible events or even the average event. However, the model does allow us to vary the input parameters and assess the effects of such variations within the model response. We also have not been able to develop a model that includes plasma and other blood components and this is an area for future work.
What we know now the programme grant is complete
This integrated programme of work has generated a lot of new knowledge and, combined with parallel research by other groups, has led to a major shift in our understanding of trauma haemorrhage and coagulopathy. The work has of course also led to new questions and, therefore, new directions for research and innovation.
We have a much stronger understanding of the epidemiology of trauma haemorrhage nationally. We know not only that nearly 5000 adults will suffer major haemorrhage after trauma each year but also that older patients seem to have a much higher rate of bleeding after trauma, with the reasons for this not being clear. Our study did not include children and paediatric trauma haemorrhage remains an area for further study.
Massive haemorrhage carries an unacceptably high mortality rate nationally, approaching nearly 50%. We found that one-quarter of these deaths occurred in the first hours after injury. These deaths are probably due to exsanguination and reducing this mortality will require innovations in the control of internal haemorrhage and faster, more effective ways to augment blood clotting ability in this critical time window.
One-quarter of deaths in people who survived the initial exsanguination phase occurred in the first 24 hours. These deaths are likely to result from a fulminant physiological failure and the inability of resuscitation to rapidly and effectively restore homeostasis. Improved management during haemorrhage including precision haemorrhage control is required to have an impact on these deaths – and we believe that this is the cohort on whom we have probably made the most impact with work done in this programme. Better diagnostics, therapeutics and focus on consistent delivery of clotting products has allowed us to provide a much more effective, balanced resuscitation.
We demonstrated that, currently, patients tend to receive coagulation therapy late in their clinical course and are often subject to gaps in the provision of blood-derived clotting products while they are bleeding. Our investigations into the underlying pathophysiology of coagulopathy have shown that the central disorders are loss of fibrinogen and increased clot breakdown. Previously, loss of procoagulant factors was thought to be the central problem and efforts were focused on this area. Our evidence has supported the uptake of the antifibrinolytic tranexamic acid into national guidance and it is now given by ambulance crews in the field. We continue to explore the potential benefit of early high-dose fibrinogen replacement therapy, which may also allow early augmentation of clot strength in the immediate post-injury phase. There are undoubtedly further gains to be made in this area in the future with novel haemostatic agents and blood products.
We have also shown that point-of-care devices can identify changes in coagulopathy during haemorrhage and that thresholds can be derived to initiate (or stop) clotting therapy. Use of these devices in trauma care might allow us to move away from our current blind ‘blunderbuss’ approach to a more individualised, precise approach that specifically treats the coagulopathy identified at that time, in that patient. Timely, effective treatment of coagulopathy may reduce bleeding, support resuscitation and improve survival. These devices may also reduce the total number of blood products required and thus reduce wastage of these precious resources. Logistic hurdles still exist for the uptake of these devices but our work is informing the design and implementation of the next generation of point-of-care coagulation analysers for emergency environments.
Half of all deaths occurred after the first 24 hours. Many of these deaths will be the result of multiple organ dysfunction syndrome or its consequences. Better resuscitation should reduce the incidence and severity of later multiple organ dysfunction and it is likely that improvements have also been seen here based on our observed reductions in organ support requirements and critical care stays. Organ dysfunction is responsible for a large proportion of the morbidity experienced by patients and nearly half of all resource use costs were incurred in critical care. Understanding post-traumatic organ dysfunction and developing new therapeutic and management strategies should also be a focus of future research efforts.
Our parallel work on modelling blood use in mass casualty events has shown that initial blood stocks and planned restocking must be central to plans for these events. We have shown that aspects traditionally thought to make a difference, such as the number of available technicians or cross-matching time, have a minimal impact on the provision of blood to critically injured casualties. This work informed London Olympics 2012 planning and is now being used nationally to plan stocks and restocking in the response to larger-scale terrorist attacks such as that seen in Paris in 2015.
Future work and directions
There are multiple avenues for further research and development based on our current findings. Key areas include methods to manage bleeding and coagulopathy within the first hours after injury; understanding how coagulopathy and bleeding lead to organ dysfunction and late deaths; and understanding TIC in specific populations including children, the elderly and patients with traumatic brain injury. The immediate imperatives centre on clinical studies of fibrinogen concentrates; personalised ROTEM-/thromboelastography (TEG)-guided TIC therapy; and the role of platelet dysfunction and transfusion.
Further validation of our findings is required in relation to diagnostic test accuracy and the role of ROTEM/TEG in guiding individualised coagulation therapy. We have formed a collaboration with other trauma research centres around the world (INTRN) to continue to recruit patients to the ACIT study in order to validate our findings in new patient cohorts.
As there was such large variation between patients we were not able to look in detail at the response of coagulation to other coagulation therapies, such as platelet transfusion. This is an expensive and difficult resource to manage and more work is required to understand the role of platelet transfusions in the management of trauma haemorrhage.
Work needs to be continued on how functional coagulation analysers can be incorporated into major haemorrhage guidelines and how they can be efficiently and effectively implemented in major trauma centres. We have been working with the device manufacturers on refinement of the devices for the emergency environment, as well as on improvements to the user interface to aid diagnosis and subsequent management. Implementation research in this area will be important in parallel with standard clinical trials of effectiveness.
Our work also highlights the opportunities for very early intervention to correct ATC, something only partially addressed by current management paradigms. Tranexamic acid has been introduced into practice. Early high-dose fibrinogen supplementation is the clear next opportunity, either with cryoprecipitate or fibrinogen concentrates. There is also a potential role for devices for immediate haemorrhage control and cardiac support in the pre-hospital and hyperacute phase, or when blood products are not available.
In parallel with this, further work is required to specify the different phenotypes of and mechanisms responsible for ATC and other forms of TIC. This work requires further analysis of samples already collected and further studies of the cellular components of blood as well as the response of the endothelium and other components of the inflammatory system.
Further mechanistic work will be particularly important in targeting the 50% of deaths that occur after the first 24 hours. These deaths are not purely due to exsanguination and coagulopathy, but have an intrinsic component of inflammation, multiple organ failure and immunosuppression. These processes are likely to be initiated in the immediate post-injury phase and the quality and nature of immediate resuscitation will set patients on a specific clinical trajectory. A reduction in these late deaths will require further investigation into these aspects of the coagulation and inflammatory response.
Outcomes other than mortality also need to be investigated in these patients. The burden of organ dysfunction, infection and thromboembolism and the effect on reconstruction and rehabilitation all need further investigation. In particular, long-term quality of life and capacity are important in the assessment of both the clinical effectiveness and the cost-effectiveness of new therapeutics and management strategies in the NHS.
Conclusion
In conclusion, this multimodal programme of work has led to new understandings of the epidemiology of trauma haemorrhage and its underlying mechanisms and clinical course. We have identified diagnostic tools and trigger thresholds for identification and management and increased our understanding of how blood components and other therapeutics affect TIC and when they are likely to be most effective. Through a parallel programme of research implementation and engagement we have disseminated findings into clinical practice throughout the NHS and internationally. We have shown major improvements in clinical outcomes over the course of the programme and beyond. The programme has also allowed us to grow in capacity and capability as a trauma sciences research centre, to widen national and international collaborations and to train the next generation of trauma clinician scientists.
Acknowledgements
Contributions of authors
Karim Brohi (Consultant, Trauma) was the chief investigator for the programme.
Simon Eaglestone (Research Manager, Trauma) co-ordinated the programme, conducted the analysis of the national incidence and current practice of transfusion in trauma haemorrhage and prepared the results for publication and final reporting.
Contributions of others
Shubha Allard (Consultant, Haematology) co-managed the collaborative observational study to describe the national incidence and current practice of transfusion in trauma haemorrhage.
Helen Campbell (Senior Researcher, Health Economics) conducted the review of health-care costs in trauma and led the analyses employing economic models.
Nicola Curry (Clinical Research Fellow, Haematology) conducted the reviews of coagulopathy, transfusion and randomised controlled trials, led the prospective recruitment to the observational studies at John Radcliffe Hospital and co-led the study of fibrinogen depletion in clinical studies.
Ross Davenport (Clinical Research Fellow, Trauma) led the prospective recruitment to the observational studies at the Royal London Hospital, conducted the identification of ATC by ROTEM and led the analyses of transfusion treatment and outcomes.
Antoinette Edwards (Project Co-ordinator, TARN) conducted the analysis of the national incidence and current practice of transfusion in trauma haemorrhage.
Simon Glasgow (Clinical Research Fellow, Trauma) conducted the review of and model development for blood resource usage in mass casualty events.
Harriet Hunt (Research Fellow, Public Health) conducted the systematic review of diagnostic test accuracy.
Chris Hyde (Professor, Public Health and Clinical Epidemiology) was the study lead for the review of diagnostic test accuracy.
Sirat Khan (Clinical Research Fellow, Trauma) conducted the analysis of major haemorrage protocol practice and transfusion ratio effectiveness in the treatment of trauma haemorrhage.
Charlotte Llewelyn (Manager, NHS Blood and Transplant Clinical Studies Unit) co-managed the collaborative observational study to describe the national incidence and current practice of transfusion in trauma haemorrhage.
Imran Raza (Clinical Research Fellow, Trauma) conducted the biomarker analysis and characterisation of elevated fibrinolysis in trauma patients.
Claire Rourke (Research Assistant, Trauma) co-led the study of fibrinogen depletion in clinical studies and conducted the ex vivo analysis of coagulopathic patient blood ROTEM.
Frances Seeney (Principal Statistician, Transfusion Medicine) led the analysis of data from the observational study of the national incidence and current practice of transfusion in trauma haemorrhage.
Simon Stanworth (Consultant, Haematology) was co-investigator for the programme.
Elizabeth Stokes (Researcher, Health Economics) conducted the analysis of health-care costs in trauma and the development of the economic models.
Maralyn Woodford (Executive Director, TARN) co-managed the collaborative observational study to describe the national incidence and current practice of transfusion in trauma haemorrhage.
Publications
Aim 1: evidence
Curry N, Hopewell S, Dorée C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomised controlled trials. Crit Care 2011;15(Suppl. 2):R92. https://doi.org/10.1186/cc10096
Curry N, Stanworth S, Hopewell S, Dorée C, Brohi K, Hyde C. Trauma-induced coagulopathy – a review of the systematic reviews: is there sufficient evidence to guide clinical transfusion practice? Transfus Med Rev 2011;25(Suppl. 3):217–31. https://doi.org/10.1016/j.tmrv.2011.01.001 (Restricted link: the corresponding author should be contacted for access to the article.)
Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, et al. Thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev 2015;2:CD010438. https://doi.org/10.1002/14651858.CD010438.pub2
Aim 2: epidemiology
Fuller G, Bouamra O, Woodford M, Jenks T, Stanworth S, Allard S, et al. Recent massive blood transfusion practice in England and Wales: view from a trauma registry. Emerg Med J 2012;29(Suppl. 2):118–23. https://doi.org/10.1136/emj.2010.104349 (Restricted link: the corresponding author should be contacted for access to the article.)
Stanworth SJ, Davenport R, Curry N, Seeney F, Eaglestone S, Edwards A, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice: a national observational study. Br J Surg 2016;103(Suppl. 4): 357–65. https://doi.org/10.1002/bjs.10052 (Restricted link: the corresponding author should be contacted for access to the article.)
Aim 3: identification
Brohi K. Prediction of acute traumatic coagulopathy and massive transfusion – is this the best we can do? Resuscitation 2011;82(Suppl. 9):1128–9. https://doi.org/10.1016/j.resuscitation.2011.06.022 (Restricted link: the corresponding author should be contacted for access to the article.)
Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, et al. Functional definition and characterisation of acute traumatic coagulopathy. Crit Care Med 2011;39(Suppl. 12):2652–8. https://doi.org/10.1097/CCM.0b013e3182281af5
Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012;10(Suppl. 7):1342–51. https://doi.org/10.1111/j.1538-7836.2012.04752.x
Davenport R, Brohi K. Fibrinogen depletion in trauma: early, easy to estimate and central to trauma-induced coagulopathy. Crit Care 2013;17(Suppl. 5):190. https://doi.org/10.1186/cc13021
Hagemo JS, Næss PA, Johansson P, Windeløv NA, Cohen MJ, Røislien J, et al. Evaluation of TEG® and RoTEM® inter-changeability in trauma patients. Injury 2013;44(Suppl. 5):600–5. https://doi.org/10.1016/j.injury.2012.11.016 (Restricted link: the corresponding author should be contacted for access to the article.)
Hunt BJ, Raza I, Brohi K. The incidence and magnitude of fibrinolytic activation in trauma patients: a reply to a rebuttal. J Thromb Haemost 2013;11(Suppl. 7):1437–8. https://doi.org/10.1111/jth.12296
Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost 2013;11(Suppl. 2):307–14. https://doi.org/10.1111/jth.12078
Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care 2015;19:97. https://doi.org/10.1186/s13054-015-0823-y
Hagemo JS, Stanworth S, Juffermans NP, Brohi K, Cohen M, Johansson PI, et al. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: a multicentre observational study. Crit Care 2014;18(Suppl. 2):R52. https://doi.org/10.1186/cc13798
Aim 4: treatment
Stanworth SJ, Morris TP, Gaarder C, Goslings JC, Maegele M, Cohen MJ, et al. Reappraising the concept of massive transfusion in trauma. Crit Care 2010;14(Suppl. 6):R239. https://doi.org/10.1186/cc9394
Davenport R, Curry N, Manson J, De’Ath H, Coates A, Rourke C, et al. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1 : 2. J Trauma 2011;70(Suppl. 1):90–5. https://doi.org/10.1097/TA.0b013e318202e486 (Restricted link: the corresponding author should be contacted for access to the article.)
Khan S, Allard S, Weaver A, Barber C, Davenport R, Brohi K. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury 2013;44(Suppl. 5):587–92. https://doi.org/10.1016/j.injury.2012.09.029 (Restricted link: the corresponding author should be contacted for access to the article.)
Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, et al. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg 2014;76(Suppl. 3):561–7; discussion 567–8. https://doi.org/10.1097/TA.0000000000000146 (Restricted link: the corresponding author should be contacted for access to the article.)
Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg 2015;261(Suppl. 2):390–4. https://doi.org/10.1097/SLA.0000000000000717 (Restricted link: the corresponding author should be contacted for access to the article.)
Curry N, Rourke C, Davenport R, Beer S, Pankhurst L, Deary A, et al. Early cryoprecipitate for major haemorrhage in trauma: a randomised controlled feasibility trial. Br J Anaesth 2015;115:76–83. https://doi.org/10.1093/bja/aev134 (Restricted link: the corresponding author should be contacted for access to the article.)
Khan S, Davenport R, Raza I, Glasgow S, De’Ath HD, Johansson PI, et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med 2015;41(Suppl. 2):239–47. https://doi.org/10.1007/s00134-014-3584-1 (Restricted link: the corresponding author should be contacted for access to the article.)
Aim 5: disaster
Glasgow SM, Allard S, Doughty H, Spreadborough P, Watkins E. Blood and bombs: the demand and use of blood following the London bombings of 7 July 2005 – a retrospective review. Transfus Med 2012;22:244–50. https://doi.org/10.1111/j.1365-3148.2012.01173.x (Restricted link: the corresponding author should be contacted for access to the article.)
Glasgow S, Davenport R, Perkins Z, Tai N, Brohi K. A comprehensive review of blood product use in civilian mass casualty events. J Trauma Acute Care Surg 2013;75(Suppl. 3):468–74. https://doi.org/10.1097/TA.0b013e318298efb9 (Restricted link: the corresponding author should be contacted for access to the article.)
Glasgow SM, Allard S, Rackham R, Doughty H. Going for gold: blood planning for the London 2012 Olympic Games. Transfus Med 2014;24(Suppl. 3):145–53. https://doi.org/10.1111/tme.12116 (Restricted link: the corresponding author should be contacted for access to the article.)
Aim 6: economics
Campbell HE, Stokes EA, Bargo DN, Curry N, Lecky FE, Edwards A, et al. Quantifying the healthcare costs of treating severely bleeding major trauma patients: a national study for England. Crit Care 2015;19:276. https://doi.org/10.1186/s13054-015-0987-5
Input into clinical guidelines and systematic reviews
Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013;17:R76. https://doi.org/10.1186/cc12685 [European bleeding guidelines (2013)]
Mitra B, O’Reilly G, Cameron PA, Zatta A, Gruen RL. Effectiveness of massive transfusion protocols on mortality in trauma: a systematic review and meta-analysis. ANZ J Surg 2013;83:918–23. https://doi.org/10.1111/ans.12417 (Restricted link: the corresponding author should be contacted for access to the article.)
National Institute for Health and Care Excellence. Detecting, Managing and Monitoring Haemostasis: Viscoelastometric Point-of-Care Testing (ROTEM, TEG and Sonoclot systems). Diagnostics guidance DG13. London: NICE; 2014. URL: www.nice.org.uk/guidance/dg13 (accessed 14 August 2017).
Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K; British Committee for Standards in Haematology. A practical guideline for the haematological management of major haemorrhage [published online ahead of print 6 July 2015]. Br J Haematol 2015. [British Committee for Standards in Haematology Guidelines (2015)]. (Restricted link: the corresponding author should be contacted for access to the article.)
Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia 2016;71(Suppl. 7):829–42. https://doi.org/10.1111/anae.13489 (Restricted link: the corresponding author should be contacted for access to the article.)
Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, et al. Thromboelastography (TEG) and rotational thromboelastometry for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev 2015;2:CD010438. https://doi.org/10.1002/14651858.CD010438.pub2 (Restricted link: the corresponding author should be contacted for access to the article.)
Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19(58). https://doi.org/10.3310/hta19580
National Institute for Health and Care Excellence. Major Trauma: Assessment and Initial Management. NICE guideline NG39. London: NICE; 2016. URL: www.nice.org.uk/guidance/ng39 (accessed 14 August 2017).
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care 2005;9:1-9. https://doi.org/10.1186/cc3779.
- Mock CN, Jurkovich GJ, nii-Amon-Kotei D, Arreola-Risa C, Maier RV. Trauma mortality patterns in three nations at different economic levels: implications for global trauma system development. J Trauma 1998;44:804-12. https://doi.org/10.1097/00005373-199805000-00011.
- Kortbeek JB, Al Turki SA, Ali J, Antoine JA, Bouillon B, Brasel K, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma 2008;64:1638-50. https://doi.org/10.1097/TA.0b013e3181744b03.
- Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. British Committee for Standards in Haematology . Guidelines on the management of massive blood loss. Br J Haematol 2006;135:634-41. https://doi.org/10.1111/j.1365-2141.2006.06355.x.
- Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma 2003;54:1127-30. https://doi.org/10.1097/01.TA.0000069184.82147.06.
- Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 2007;38:298-304. https://doi.org/10.1016/j.injury.2006.10.003.
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma 2003;55:39-44. https://doi.org/10.1097/01.TA.0000075338.21177.EF.
- Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 2008;65:748-54. https://doi.org/10.1097/TA.0b013e3181877a9c.
- Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg 2008;248:447-58. https://doi.org/10.1097/SLA.0b013e318185a9ad.
- Neal MD, Hoffman MK, Cuschieri J, Minei JP, Maier RV, Harbrecht BG, et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: when a little goes a long way. J Trauma Acute Care Surg 2012;72:892-8. https://doi.org/10.1097/TA.0b013e31823d84a7.
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 2007;62:307-10. https://doi.org/10.1097/TA.0b013e3180324124.
- Khan S, Allard S, Weaver A, Barber C, Davenport R, Brohi K. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury 2013;44:587-92. https://doi.org/10.1016/j.injury.2012.09.029.
- Curry N, Stanworth S, Hopewell S, Dorée C, Brohi K, Hyde C. Trauma-induced coagulopathy – a review of the systematic reviews: is there sufficient evidence to guide clinical transfusion practice?. Transfus Med Rev 2011;25:217-31. https://doi.org/10.1016/j.tmrv.2011.01.001.
- Curry N, Hopewell S, Dorée C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care 2011;15. https://doi.org/10.1186/cc10096.
- Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD010438.pub2.
- PRISMA Guidelines n.d. www.prisma-statement.org/ (accessed 1 March 2017).
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
- Stanworth SJ, Davenport R, Curry N, Seeney F, Eaglestone S, Edwards A, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg 2016;103:357-65. https://doi.org/10.1002/bjs.10052.
- Campbell HE, Stokes EA, Bargo DN, Curry N, Lecky FE, Edwards A, et al. Quantifying the healthcare costs of treating severely bleeding major trauma patients: a national study for England. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0987-5.
- Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 2011;39:2652-8. https://doi.org/10.1097/CCM.0b013e3182281af5.
- Glasgow S, Davenport R, Perkins Z, Tai N, Brohi K. A comprehensive review of blood product use in civilian mass casualty events. J Trauma Acute Care Surg 2013;75:468-74. https://doi.org/10.1097/TA.0b013e318298efb9.
- Cooper ES, Bracey AW, Horvath AE, Shanberge JN, Simon TL, Yawn DH. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets . Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA 1994;271:777-81. https://doi.org/10.1001/jama.271.10.777.
- Stanworth SJ, Morris TP, Gaarder C, Goslings JC, Maegele M, Cohen MJ, et al. Reappraising the concept of massive transfusion in trauma. Crit Care 2010;14. https://doi.org/10.1186/cc9394.
- Section of Transfusion Medicine Capital Region Blood Bank, Department of Clinical Immunology . TACTIC: Targeted Action for Curing Trauma Induced Coagulopathy n.d. www.tacticgroup.dk (accessed 6 February 2016).
- Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma 2008;64:1211-17. https://doi.org/10.1097/TA.0b013e318169cd3c.
- Khan S, Davenport R, Raza I, Glasgow S, De’Ath HD, Johansson PI, et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med 2015;41:239-47. https://doi.org/10.1007/s00134-014-3584-1.
- Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007;63:805-13. https://doi.org/10.1097/TA.0b013e3181271ba3.
- Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012;10:1342-51. https://doi.org/10.1111/j.1538-7836.2012.04752.x.
- Curry N, Rourke C, Davenport R, Beer S, Pankhurst L, Deary A, et al. Early cryoprecipitate for major haemorrhage in trauma: a randomised controlled feasibility trial. Br J Anaesth 2015;115:76-83. https://doi.org/10.1093/bja/aev134.
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Lancet 2010;376:23-32. https://doi.org/10.1016/S0140-6736(10)60835-5.
- Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost 2013;11:307-14. https://doi.org/10.1111/jth.12078.
- Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg 2015;261:390-4. https://doi.org/10.1097/SLA.0000000000000717.
- Glasgow SM, Allard S, Doughty H, Spreadborough P, Watkins E. Blood and bombs: the demand and use of blood following the London bombings of 7 July 2005 – a retrospective review. Transfus Med 2012;22:244-50. https://doi.org/10.1111/j.1365-3148.2012.01173.x.
- Glasgow SM, Allard S, Rackham R, Doughty H. Going for gold: blood planning for the London 2012 Olympic Games. Transfus Med 2014;24:145-53. https://doi.org/10.1111/tme.12116.
- Glasgow S, Vasilakis C, Perkins Z, Brundage S, Tai N, Brohi K. Managing the surge in demand for blood following mass casualty event: early automatic restocking may preserve red cell supply. J Trauma Acute Care Surg 2016;81:50-7. https://doi.org/10.1097/TA.0000000000001101.
- Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0823-y.
Appendix 1 Aim 2 (epidemiology): participating trauma-receiving hospitals
| Major trauma centres | Medium-sized hospitals with trauma units |
|---|---|
|
|
Consecutive enrolment of eligible patients across all centres commenced on 1 April 2009 and continued until 30 March 2011.
Appendix 2 Aim 2 (epidemiology): Trauma Audit and Research Network standard operating procedure
Appendix 3 Aim 3 (identification) and aim 4 (treatment): study protocol
Appendix 4 Aim 3 (identification) and aim 4 (treatment): exemplar information sheet
Appendix 5 Aim 3 (identification) and aim 4 (treatment): exemplar consent form
Appendix 6 Patient and public involvement
Patients and the public were not involved in the initial development of the programme as this was early in the development of the trauma centre as well as our research programme and PPI in general. Over the course of the programme, however, we formed a group consisting of ex-patients who were active in reviewing all aspects of the studies, including ethics and consent issues, study recruitment, preliminary results and final outcomes. This group has continued and grown and also includes relatives and members of the public. Out of this has grown a patient experience and support website, AfterTrauma.org [see www.aftertrauma.org (accessed 14 August 2017)], which we continue to develop and have expanded to national scope. From a research perspective, members of the group, and others, have been important in reflecting on the outputs of our study and highlighting priorities for future studies.
A number of outputs and engagement events have been directed at patients and the public during the course of the programme grant and are ongoing.
Media
Cohen D. Code Red – Repairing Blood in the Emergency Room. New Scientist, 19 October 2011. URL: www.newscientist.com/article/mg21228352–900-code-red-repairing-blood-in-the-emergency-room/ (accessed 9 September 2015).
BBC World Service and BBC Radio 4. Frontline Medicine: Survival. 2012. URL: www.bbc.co.uk/programmes/b017Ld83 (accessed 28 August 2017).
BBC Television. Trauma Medicine: the Fight for Life. 2014. URL: www.bbc.co.uk/programmes/b04svfs5 (accessed 28 August 2017).
Science and medicine education
Royal Society Summer Science Exhibition, London, 2011.
Moscow Science Fair, Moscow, 2011.
Big Bang Fair, Birmingham, 2012 (Wellcome Trust People Award WT098159A1A).
Centre of the Cell: Trauma Surgery: Science of the Bleeding Obvious, 2011–2014. Shortlisted for a Times Higher Education Widening Participation Award, 2012.
Appendix 7 Research training
PhDs completed
Ross Davenport. Acute Traumatic Coagulopathy: Functional Characterisation of the Protein C Pathway and Haemostatic Response to Transfusion. PhD Thesis. Glasgow: University of Glasgow; 2011.
Nicola Curry. The Coagulopathy of Trauma Related Major Haemorrhage. PhD Thesis. London: Queen Mary University of London; 2014.
Sirat Khan. Blood Component Therapy in Trauma Haemorrhage. PhD Thesis. Glasgow: University of Glasgow; 2015.
Simon Glasgow. Modelling Red Blood Cell Provision in Mass Casualty Events. PhD Thesis. Glasgow: University of Glasgow; 2015.
Appendix 8 Affiliated and follow-on grant income
Academic Department of Military Surgery and Trauma (2010) – Bayesian modelling of ATC (£63,209).
Royal College of Surgeons of England (2010) – RCS Fellowship for PhD student (£51,183).
Unrestricted Grant, Octapharma Ltd (2010) – Optimising procoagulant therapy and response (£72,280).
Central and East London Comprehensive Local Research Network (2011) – Research capacity (£95,017).
Barts and The London Charity (2012) – Saving lives in bleeding trauma patients (£104,075).
Central and East London Comprehensive Local Research Network (2012) – Research capacity (£41,809).
Engineering and Physical Sciences Research Council (2012) – Doctoral Training Award (£71,000).
Fulbright Commission (2012) – UK–US Scholarship for early career clinical scientist (£50,000).
Royal College of Surgeons of England Fellowship (2012) – RCS Fellowship for PhD student (£49,984).
European Union FP7 Award (2013) – TACTIC – Targeted Action for Trauma-Induced Coagulopathy (€5,880,192).
Haemonetics, Inc. (2013) – TEG in trauma haemorrhage ($1,000,000).
NHS Blood and Transplant (2013) – CRYOSTAT (£34,846).
List of abbreviations
- ACIT
- Activation of Coagulation & Inflammation in Trauma
- ATC
- acute traumatic coagulopathy
- CA5
- clot amplitude at 5 minutes
- CRASH-2
- Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage
- DCR
- damage control resuscitation
- FFP
- fresh-frozen plasma
- INTRN
- International Trauma Research Network
- MCF
- maximum clot firmness
- PRBC
- packed red blood cell
- PT
- prothrombin time
- ROTEM
- rotational thromboelastometry
- TARN
- Trauma Audit and Research Network
- TEG
- thromboelastography
- TIC
- trauma-induced coagulopathy