Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10136. The contractual start date was in July 2008. The final report began editorial review in July 2013 and was accepted for publication in March 2017. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Thompson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction to the programme
The programme of research reported here derives from the National Institute for Health Research (NIHR) Programme Grants for Applied Research project number 11/77/82.
The original aims and objectives were:
-
project 1 – to take an evidence-based self-management support model [Whole system Informing Self-management Engagement (WISE)] for established chronic functional gastrointestinal disorder (FGID) in primary care and to test clinical effectiveness and cost-effectiveness when translated from research settings into routine care
-
project 2 – to determine how well the WISE model prevented patients with new-onset functional gastrointestinal problems from developing chronic ill-health.
Issues related to the delivery of project 2 were identified following NIHR monitoring visits, which acknowledged that recruitment and data quality issues rendered the original plan for project 2 unviable. The focus on project 2 was therefore modified in discussion with the funder to a focus on identification and risk assessment.
The description of the research actually conducted in relation to project 2 is provided in Chapter 7.
Chronic gastrointestinal disorders, including inflammatory bowel disease (IBD) and FGID, account for approximately 8% of the general practice workload in the UK, at an estimated cost of £1B per year. 1,2 Around 50% of these consultations are for FGID. 2,3
Irritable bowel syndrome (IBS), the most prevalent FGID, comprises chronic physical symptoms [abdominal pain (AP) and bloating, erratic bowel habit], which remain unexplained after medical exploration. 4
Improving management of functional gastrointestinal disorder
Over the last decade, members of the research team have developed therapies for these conditions, using patient education and self-management. 5–15 These have been combined with patient-centred psychological approaches, such as cognitive–behavioural therapy (CBT) and hypnotherapy. 16,17
These findings suggested that:
-
information can be improved to incorporate patient experience and expertise alongside medical information about management and treatment
-
clinician training in patient-centred consultation skills and shared decision-making with patients is acceptable and appropriate and leads to positive outcomes
-
health systems that are better aligned to self-management are well received
-
an integrated approach leads to reduced health service utilisation and costs without adverse clinical effect
-
defining the boundaries of care in professional support for self-management is necessary for success.
The results of these studies led us to propose a new integrated WISE model. The WISE model is designed to enhance well-being by improving patient information, drawing on patients’ existing skills in living with long-term conditions, training health professionals to support self-management and improving access to further care. The system is applicable to chronic FGID, such as IBS, and commonalities in the management of conditions suggest that it may be applicable to other long-term conditions.
Although existing evidence shows that the WISE model was effective, we also identified two key issues that required further study:
-
Although results have been demonstrated in research trials, there has been no demonstration that the results could be achieved when translated from research settings into routine care.
-
Psychological ill-health can adversely influence the chronicity and burden of chronic FGID. Psychological therapies could be added to the original WISE model to increase effectiveness, but their uptake, acceptability and cost need to be assessed.
Project 1 research questions
-
What is the clinical effectiveness and cost-effectiveness of an intervention to enhance self-management support for patients with chronic conditions when translated from research settings into routine care? [Phase IV randomised controlled trial (RCT) and economic evaluation.]
-
What are the barriers and facilitators that affect the implementation of the WISE model among patients, clinicians and organisations? (Process evaluation.)
Patient and public involvement
Our patient and public contributors are named in the Acknowledgements. They guided project delivery through attendance at Study Steering Group meetings and provided particular assistance around the challenges of recruiting patients to the project.
Chapter 2 The WISE model of self-management support
Introduction
Long-term conditions are important determinants of quality of life and health-care costs worldwide. 18 Increasing focus has been placed on self-management, defined as:
The care taken by individuals towards their own health and well-being: it comprises the actions they take to lead a healthy lifestyle; to meet their social, emotional and psychological needs; to care for their long-term condition; and to prevent further illness or accidents.
The Wanless report, Securing our Future Health: Taking a Long-term View, suggested that the future costs of health care were very much dependent on:
How well people become fully engaged with their own health. 20
However, realising the potential of self-management requires effective ways of encouraging appropriate behaviour change in patients and professionals. There are a number of factors influencing self-management, including patient factors (e.g. lay epidemiology and health beliefs, self-efficacy, emotional responses to long-term conditions, identity and pre-existing adaptations), and wider influences (such as the organisation of the health-care system and access to material and community resources). 21 A number of models of self-management have been proposed in the literature, including increasing access to health information5 and deployment of assistive technologies. 22 Patient skills training (through the Chronic Disease Self-Management Program and its derivatives) can be used to encourage patients to enhance their individual self-management skills. 23,24 There is evidence for the effectiveness of the programme on some outcomes,25 but there are significant limitations. Intervention ‘reach’ is defined as the:
. . . percentage and risk characteristics of persons who receive or are affected by a policy or program.
Interventions with limited ‘reach’ are unable to translate the effectiveness of an intervention at the individual level to that of the wider population. In the case of the Chronic Disease Self-Management Program, requirements for self-referral or referral from health-care professionals means that levels of uptake can be low, and biased towards certain patient groups, threatening reach and equity.
Models of self-management support
Health policy in the UK has worked with a model that organises care for long-term conditions around three tiers: (1) self-management support for low-risk patients, (2) disease management for patients at some risk and (3) case management for patients with multiple, complex conditions. 27 In the UK, the bulk of disease management is already delivered through primary care. Primary care is generally defined in terms of attributes such as a gatekeeping function and first contact care,28–30 but other attributes also make it an excellent platform for self-management support. Primary care offers open access between the health service and the population, can deliver continuity of care through an extended personal relationship or through informational continuity,28–30 and has a role in helping patients achieve care that balances compliance with clinical guidelines and consistency with patient needs and preferences. Delivering self-management support through primary care also maximises reach.
However, there are major barriers to achieving effective self-management support in primary care. Self-management is only one priority among many facing primary care professionals31 and there is evidence that many primary care professionals do not see self-management as a core part of their remit. 32,33 This is especially true when incentives (financial and otherwise) are focused on specific clinical tasks and biomedical parameters. 34
Achieving the potential of primary care in delivering self-management support
Our research team has engaged in a programme of research over a number of years that has explored the barriers to, and facilitators of, effective self-management support. On the basis of this work, we argue that self-management support requires the following.
-
A whole-systems perspective that involves interventions at the patient, practitioner and service organisation levels in the delivery of self-management support. Many self-management interventions have focused on patient behaviour change or professional training only, but we argue that each level has a different function in encouraging and supporting self-management behaviour, and that effects are maximised when interventions occur at all levels and include attention to patient actions outside the context of contacts with the health service. 13,14,35
-
Widening the evidence base to acknowledge a range of disciplinary perspectives on the way in which patients and professionals respond to, and manage, their long-term conditions. Although psychology has dominated the design of many interventions for self-management support through models such as self-efficacy theory, there are a wide range of applied social science theories that can inform an understanding of the way that patients and professionals understand, respond to and manage long-term conditions. 36–38
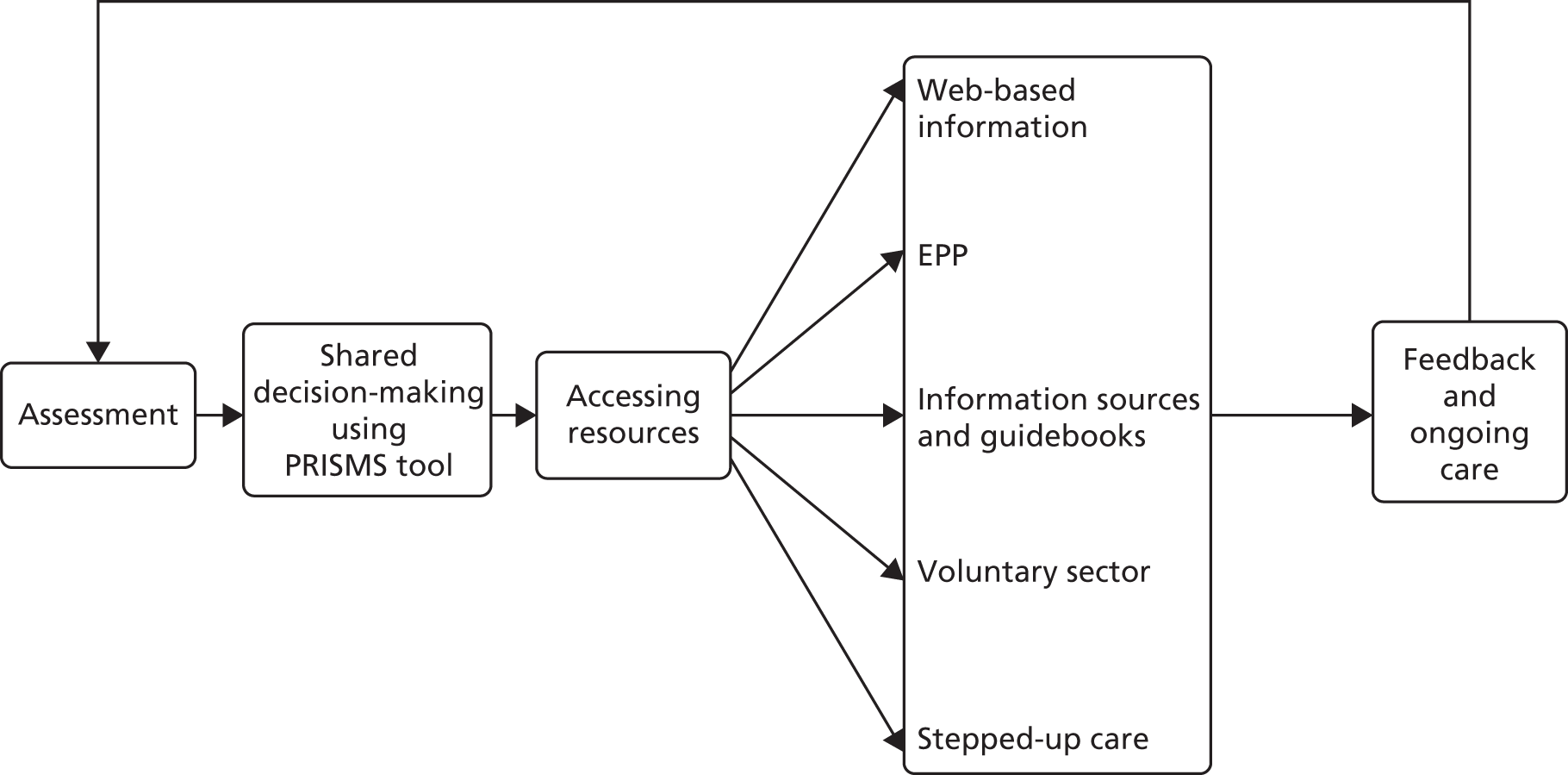
The model has been designed to reflect these findings and provide a feasible and effective model of self-management support. The model (Figure 1) aims to support patients to receive guidance from trained practitioners working within a health-care system geared up to be responsive to patient need.
FIGURE 1.
The WISE model. PRISMS, Patient Report Informing Self-Management Support.

Our approach broadly follows the phased development and evaluation framework outlined for complex interventions by the Medical Research Council (MRC). 39,40 We have developed an evidence base for the elements of the WISE approach using mixed methodology: a combination of RCTs, nested qualitative studies and economic evaluation. In summary, the evidence shows that:
-
information can be effectively improved to incorporate patient experience and expertise alongside medical information about management and treatment5,7,41
-
clinician training in patient-centred consultation skills and shared decision-making with patients to guide and support self-management is acceptable and appropriate, and leads to positive outcomes5
-
health systems that are better aligned to patient practices of self-management are generally well received. 14,35,42
The WISE model as a complex intervention
Complex interventions are defined as those that:
. . . comprise a number of separate elements which seem essential to the proper functioning of the intervention although the ‘active ingredient’ of the intervention that is effective is difficult to specify.
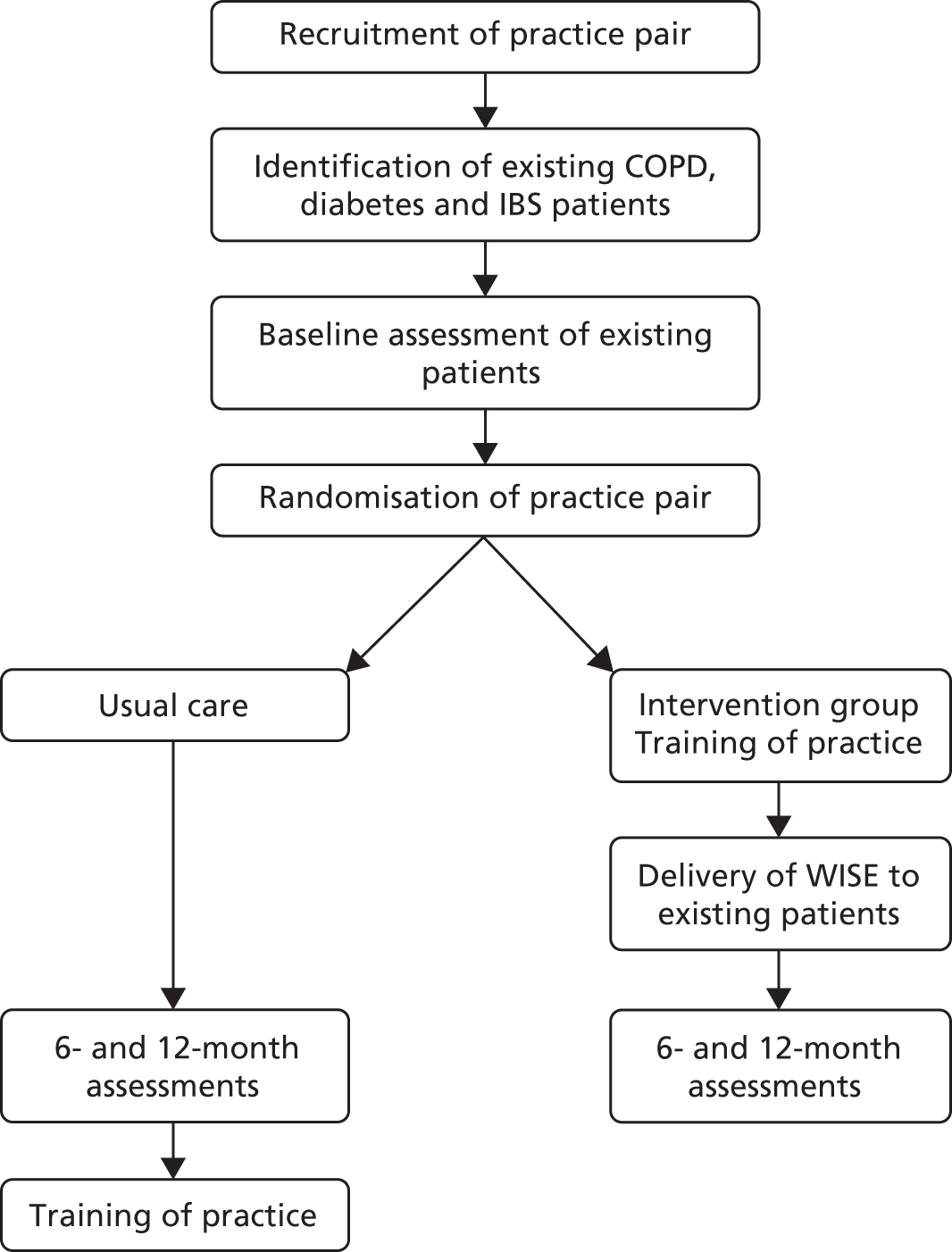
The WISE model, as applied to primary care, met this definition. The intervention was designed to impact on the patient, professional and system levels (see Figure 1). The primary target of the intervention was the practice. The overall aim of the intervention was to encourage practices to adopt a structured and patient-centred approach to the routine management of long-term conditions through providing skills, resources and motivation to make changes to service delivery in line with the principles of the WISE model (Figure 2).
FIGURE 2.
Process of care in the WISE model. PRISMS, Patient Report Informing Self-Management Support. EPP, Expert Patients Programme.
The development, and evaluation, of the training intervention took place prior to this trial, and details have been published elsewhere. 43 The planned approach to training combines evidence-based approaches to changing professional behaviour with approaches to ‘normalise’ those behaviours in current practice. The intervention involved the whole practice and there were also ‘system’ links to the local health organisation, which provided access to additional resources (including a dedicated website of local groups and organisations providing self-management support).
The components of the WISE training intervention include the following:
-
Priority and agenda setting: an intervention, aimed to promote active patient participation in sharing their priorities and management preferences, was developed from the existing published literature and refined in a ‘think aloud’ and qualitative interview study. 44 The Patient Report Informing Self-Management Support (PRISMS) tool was based on a combination of patient-reported outcome measures and a values clarification exercise, intended to encourage patients to clarify and share values and priorities of personal importance. 45,46 The PRISMS tool is intended as a starting point for discussion of patient priorities.
-
Patient-centred information: information can be effective when it incorporates patient experience and expertise alongside medical information about management and treatment, and when it is given in a supportive and timely manner. 10
-
Shared decision-making: shared decision-making about the appropriate type of self-management support, supported by PRISMS and by the use of appropriate ‘explanatory models’. Patients’ explanations and understanding of a condition often differ from the medical model. Explanatory models are ways to make sense of problems and encourage discussion about the causes and consequences of their condition.
-
Referral to community groups: to promote a whole-system approach to self-management, it is essential to engage with relevant community resources. Referral to third-sector providers (i.e. voluntary and community organisations) from primary care has clinically relevant benefits. 47 These groups provide services that are embedded within local community settings to help normalise health-related activities into everyday life. Despite the potential benefits of referral to third-sector providers, there remains an underutilisation of these services by primary care as practitioners report lack of knowledge of the services available. To promote the system change necessary between primary care and relevant third-sector providers, an online database of local self-management support options was developed.
Practice-based training sought to teach the following core skills to primary care staff:
-
Assessment of the individual patient’s self-management support needs, in terms of their current capabilities and current illness trajectory.
-
Shared decision-making about the appropriate type of self-management support based on that assessment (e.g. support from primary care, written information sources, long-term condition support groups or condition-specific education), facilitated by the PRISMS tool and the use of explanatory models.
-
Facilitating patient access to support. This may involve signposting patients to various resources depending on the outcomes of the assessment and shared decision-making processes. These may include access to the Expert Patients Programme, disease-specific courses (such as pulmonary rehabilitation) or generic support (such as befriending). The training encompasses ways that health professionals can negotiate with patients about the more appropriate use of health care.
-
In the case of IBS, this may also involve referral to psychological treatment services (CBT and hypnotherapy) for eligible patients (so-called ‘stepped-up care’). Patients with IBS were informed of the possibility of referral to such services through information leaflets.
As part of the training, primary care professionals received specific assistance in development of the core WISE skills, followed by integration of techniques through role play (with individualised performance feedback based on that role play). 48 The intervention was delivered over two sessions. All relevant staff within the practice were invited to the first session, including general practitioners (GPs), nurses, practice managers and reception staff. Clinical staff were invited to the second session (Box 1). A short intermediate meeting was held between the two main sessions to review progress, and involved the nominated practice lead only.
The first session is delivered to the whole practice by two trainers employed by the PCT who are familiar with primary care. The session involves clinicians, the practice manager and administrative staff and has the following structure:
-
brief introduction to the WISE model
-
team-building exercise
-
exercise on care pathways for patients with long-term conditions
-
WISE tools – PRISMS, explanatory models and menu of local support
-
interactive discussion
-
nomination of practice member to lead on implementation.
This session is a short meeting between the trainers and the nominated practice lead to discuss progress with the WISE approach since session 1.
Session 2This session is delivered by two trainers to all clinicians in the practice team. Through the use of role play and clinical discussion, the training focuses on embedding the three core skills: (1) assessment of self-management needs and capabilities, (2) shared decision-making and (3) facilitating patient access to support into primary care consultations. The session has the following structure:
-
introduction and provision of manual
-
reflection on competencies
-
demonstration of skills to support self-management
-
skills practice
-
discussion on how to ensure sustainability of the WISE model.
PCT, primary care trust.
A training manual was given to all of those who participated in the training for use within the training session and to support practice (see Appendix 1). The training was piloted and modified on the basis of the pilot. The intervention was conducted by trained facilitators working alongside the research team, rather than the research team itself, to test a model of delivery that would be feasible in routine practice and wider implementation.
Enhancement of the WISE model with psychological therapies
Our previous studies had identified a residual group (up to 20%) who fail to benefit and who show high levels of psychological ill-health despite the use of the WISE model.
Evidence demonstrates that physical and psychological factors have an impact on symptom chronicity in patients with chronic gastrointestinal disorders, not only in patients with FGID49 but also in patients with IBD. 50 Such psychological components, particularly anxiety and depression, are important determinants of clinical outcome and health resource use. Although there are limited studies investigating effectiveness of psychological interventions for all FGID, the most common of these disorders (IBS) has been subject to a number of trials. In a systematic review and meta-analysis of the efficacy of psychological treatment for IBS, there was a 50% reduction in symptoms. 51 Our own studies have shown that psychological treatments are of value in chronic gastrointestinal problems. 52 Given the clear evidence of the clinical efficacy of CBT in common mental health problems,53 current best evidence would suggest that either CBT or hypnotherapy would be of utility. We therefore offered both in addition to treatment by the GP.
Cognitive–behavioural therapy
We developed a 12-week CBT intervention comprising an initial assessment of between 60 and 90 minutes, followed by up to 11 weekly, individual, face-to-face sessions of between 45 and 60 minutes. Session 1 consisted of a patient-centred assessment for problem identification, risk assessment and development of a shared problem formulation. The following sessions involved education about the condition and specific CBT techniques (pacing, behavioural activation, diary keeping, identifying and challenging negative and unhelpful thinking patterns, and the development of a longer-term management plan). Participants received a self-management manual with information about IBS, CBT and ‘patient stories’ typical of people’s experience of IBS and how to manage their symptoms.
Hypnotherapy
Gut-focused hypnotherapy consists of giving patients ideas about how the gastrointestinal system works and then using hypnosis to try and control abnormalities of gut function as well as dealing with any other factors that might exacerbate their condition. All sessions last 45–60 minutes on a weekly basis for up to 12 weeks, with the first consisting of an assessment of the patient and their symptom profile, followed by an ‘educational’ tutorial on simple gut physiology and how it might be controlled. Subsequent sessions involve the introduction of relaxation and hypnosis in general, followed by progressively more emphasis being placed on control of gut symptoms by the use of imagery or tactile techniques. All patients are given a compact disc to practise on a regular, preferably daily, basis.
The CBT therapist was an experienced and accredited therapist with the British Association for Behavioural & Cognitive Psychotherapies. A 2-day training workshop, provided by the trial team, consisted of a range of presentations about IBS and applying CBT interventions for people with IBS, with a focus on skills practice. The training was accompanied by a training handbook. The hypnotherapist had been previously fully trained and had been working for 2 years in the local hypnotherapy unit. CBT supervision was provided to the therapist on a fortnightly basis by applicant Karina Lovell (an experienced and accredited CBT therapist). Supervision for the hypnotherapist was provided on a regular basis by applicant Peter Whorwell (an experienced hypnotherapist).
Procedure
Initial and follow-up sessions
Following GP referral, the therapist contacted the participant to arrange the initial session at a convenient time. Each potential patient was given an information sheet prior to the first meeting, and those who agreed to take part signed a consent form to allow access to the self-reported measures (Patient Health Questionnaire-9 and Generalised Anxiety Disorder-7). Treatment sessions were delivered in a range of primary care settings, including practices.
The low-intensity aspects of WISE were rolled out from April 2009 and patients with a history of IBS who did not benefit from the WISE low-intensity intervention after 3 months were then given information about different step-up options by their GP or practice nurse. Following discussion with their GP, patients were directly referred to a therapy.
Recruitment
At the start of the project, both therapists identified the need to introduce themselves to the practices that could potentially refer to step-up, and, when possible, this took place. The aim of these meetings was to educate the practice team about the CBT and hypnotherapy treatments and to reiterate the referral protocol.
However, as a result of the low uptake of step-up via this route, alternative recruitment strategies were developed. This included building a relationship with the local primary care mental health teams in Salford that sought to promote the step-up with the relevant GP practices and facilitated a number of referrals. In addition, an individual letter to every GP in a WISE-trained practice was sent in March 2010, advising GPs about the availability of step-up for their IBS patients. In May 2010, in recognition that the referral rate to step-up remained very low, a leaflet describing the CBT/hypnotherapy options was produced in conjunction with NHS Salford Primary Care Trust (PCT); > 200 leaflets were then directly mailed to patients known to the trial. The step-up treatments were regularly advertised in WISE communications (e-mails and newsletters), and a poster advertising the availability of CBT or hypnotherapy for IBS sufferers was also displayed in patient waiting areas in WISE-trained surgeries.
Chapter 3 Design of the randomised controlled trial
The research question was:
What is the clinical effectiveness and cost-effectiveness of an intervention to enhance self-management support for patients with chronic conditions when translated from research settings into routine care? (Phase IV RCT and economic evaluation.)
The initial iteration of the MRC framework for the development of complex interventions suggested five phases: preclinical and Phases I–IV. Phase III involves comparing ‘a fully defined intervention with an appropriate alternative’ in the context of an appropriately powered RCT. The RCTs completed in the development and initial testing of the WISE model can be considered to be Phase III.
Phase IV has received the least attention of all aspects of the framework. 54 The initial guidance suggested that it concerns replication of Phase III outcomes ‘in uncontrolled settings’. Recent exploration of the differences between Phase III and IV studies suggest other important differences, including:
-
Phase IV RCTs being based on Phase III evidence
-
broad patient inclusion criteria based on those for the clinical service
-
randomisation at the service level
-
outcomes often collected using routine data
-
uptake of the intervention is a crucial variable
-
the implementation of the service is an important part of reporting.
Many of these were present in the study, although not all. For example, measures of self-management and health-related quality of life (HRQoL) are not available in routine data and had to be collected.
Methods
The trial was a pragmatic, two-arm, practice-level cluster Phase IV RCT evaluating outcomes and costs associated with the WISE model (Figure 3). The study was approved by the Salford and Trafford local Research Ethics Committee (reference number 09/H1004/6). We include a summary of the main outcomes of the RCT in this report. A full publication is available elsewhere. 55 The Consolidated Standards of Reporting Trials (CONSORT) checklist is provided in Appendix 2.
FIGURE 3.
Planned trial design. COPD, chronic obstructive pulmonary disease.
Research question
What is the clinical effectiveness and cost-effectiveness of an intervention to enhance self-management support for patients with chronic conditions when translated from research settings into routine care? (Phase IV RCT and economic evaluation.)
Population
The general practice was the cluster and, in line with the Phase IV study design, we aimed to involve all practices in a local ‘health economy’ in the UK. The context was a PCT in Salford, in north-west England. The organisation had a strong commitment to supporting self-management, viewing this as part of a strategic approach to improving the health and well-being of the population. This study took place between 2009 and 2012.
The WISE model is designed to be robust and adaptable enough to use with the vast majority of patients with a long-term condition. We recruited patients with three long-term conditions: diabetes, chronic obstructive pulmonary disease (COPD) and IBS. The particular conditions to be included were chosen on the basis of a number of theoretical, policy and practical criteria, and they have important similarities and differences. Each condition is amenable to self-management interventions, and there is already a significant evidence base that will facilitate comparison of the effects of the WISE model with alternatives. The conditions are also of sufficiently high prevalence within practices to meet the sample size needs of the proposed trial. The conditions also have important differences in symptoms, experience and management. IBS is a more ‘contested’ condition with greater disagreement about diagnosis and appropriate management. The management of both diabetes and COPD is also incentivised in the UK under the Quality and Outcomes Framework (QOF), whereas IBS is not.
As a Phase IV RCT, the inclusion criteria were simple and broad to enhance the external validity of the study.
-
Patients had a clinical diagnosis of COPD, diabetes or IBS, identified from existing primary care systems using appropriate clinical registers (for COPD and diabetes) and Read Codes (for IBS), and verified by primary care professionals.
-
Patients also had to demonstrate sufficient English to be able to complete questionnaires.
-
The practice had to agree that the patient was appropriate for research assessment.
Exclusion criteria included patients in the palliative care stage of condition or the presence of mental health problems that reduced capacity to consent and participate.
Patients who had two or more of our index conditions, or a single index condition and another long-term condition, were still included in the trial. Where a patient was identified as having two or more of the three conditions, clinical staff in the practice were asked to determine the main condition, in order to assist with appropriate outcome measurement (see Outcomes).
Intervention
The intervention was described in detail in Chapter 2.
Intervention practices received training as soon as possible after baseline data collection and subsequent allocation; control practices received training 1 year later. Training was undertaken in two sessions, with the second session approximately 1 month after the first. Practices were asked to select two WISE champions (a health-care professional and a member of the administration team) to help embed the WISE approach in the practice (a short mid-training session for them with the trainers was made available).
Outcomes
All outcomes were at the level of the individual patient. Each practice sent eligible patients a questionnaire at baseline, to be returned directly to the research team. Follow-up questionnaires were sent at 6 and 12 months.
The primary end point was the 12-month follow-up of patient health outcomes and costs.
The trial had three primary outcomes, all at 12 months:
-
shared decision-making (short-form Health Care Climate Questionnaire)56
-
self-efficacy (confidence to undertake chronic disease management)57
These outcomes represent core measures along the ‘causal pathway’ from intervention to health outcomes.
The study also collected a number of secondary outcomes, including disease-specific quality of life, self-management behaviours, service utilisation, empowerment, general health, social/role limitations, well-being and vitality. These are all detailed at URL: www.bmj.com/content/bmj/suppl/2013/05/13/bmj.f2882.DC1/kena009790.ww2_default.pdf (accessed 26 October 2017).
There was no blinding of patients or outcome assessors, although all outcomes were self-report. The analyst remained blind to allocation.
Design
The study was a pragmatic cluster RCT using a waiting list control (see Figure 3). The intervention was designed to impact on all primary care staff in a practice, thus randomisation was at the level of the practice to avoid contamination.
Practice recruitment and randomisation
Practices were recruited via practice visits and asked to identify their preferred time during the year for training. Practices were paired as closely as possible according to their preferred times, and using a minimisation procedure, one practice in each pair was allocated to training in the first year, with the other practice allocated to training at the same time the following year. Research staff recruiting practices were unaware of the next allocation in the sequence at the time of recruitment. Baseline (and subsequent follow-up) data collection then took place at both practices in a pair at the same time. This ensured a balance between the intervention and control groups, to avoid potential bias from changes to care delivery outside the trial context (e.g. new government policy or local system changes on the management of long-term conditions).
The practice (cluster) pairs were allocated – one to the intervention group and the other to the control group – using a minimisation algorithm by the trial statistician. Minimisation variables were practice size, practice deprivation [as measured by the Index of Multiple Deprivation (IMD)] and practice contractual arrangements (i.e. general medical services or personal medical services). This ensured that, barring attrition, the two arms of the trial would have equal numbers of practices and be balanced on the minimisation variables and data collection timeline.
After practice pairing and allocation, potentially eligible patients at each intervention and control group practice were identified from the computer systems and checked for eligibility to be contacted by practice clinical staff.
Sample size
Sample size calculations were made on the basis of data collected from the national evaluation of the Expert Patients Programme. Although all three patient groups were combined in the primary analysis (see Analysis), we powered the trial to detect a fairly small effect of the intervention on diabetes, COPD and IBS separately. Data on outcomes from the national evaluation of the Expert Patients Programme had a range of intraclass correlation coefficients from 0.01 to 0.07. For the power calculations, we assumed an intraclass correlation coefficient of 0.05. Baseline follow-up correlations were taken to be 0.6, that is, towards the lower end of those found in the Expert Patients Programme. On these assumptions, each arm of the trial required 18 practices and 36 patients per condition per practice, to achieve 80% power to detect an effect size of 0.21 per condition. To allow for attrition of practices (estimated to be around 10%), we aimed to recruit 20 practices into each arm of the trial. Questionnaires were to be sent to 80 patients per practice with each condition. For each of the three conditions, this aimed to provide, on average, 48 patients per practice at baseline, reducing to 36 patients at 12 months. We recognised that smaller practices might not have 80 patients with COPD, in which case we compensated by recruiting additional patients from larger practices. On the basis of the above, we aimed to recruit totals of 1728 patients with diabetes, 1728 with COPD and 1728 with IBS.
Analysis
Analysis followed a prespecified analysis plan. Each outcome was subjected to analysis of covariance within a multilevel (patients within practices) regression framework, following intention-to-treat principles and with the analyst (applicant DR) blind to practice allocation. Although we powered the study to detect effects for separate conditions, we maximised power and minimised multiple testing in the analysis by testing for a treatment effect across all three condition groups combined, and for an interaction between trial arm and condition group (controlled for the main effects of condition group). This analysis also controlled for baseline values of each outcome, design factors (practice list size, deprivation and contractual type), and additional covariates (see Process evaluation).
In the case of a statistically non-significant (p > 0.05) interaction between trial arm and condition group, no further condition-specific analyses would be conducted; if the interaction term were significant, this would imply that the effect varied by condition and further analyses would be conducted for each separate condition group.
We applied multiple imputation (five imputed data sets) to baseline variables with missing values (all < 5%), using chained equations and all variables in the model. We did not impute missing follow-up data, but used multivariate logistic regression to identify baseline covariates predictive of missing data and included these (disease condition, age, general health, deprivation index and home ownership) as covariates. Additional prespecified covariates included gender, comorbid conditions count, education and primary care visits 6 months prior to baseline.
Sensitivity analyses assessed the stability of the results to the model specification. All analyses used Stata® v12 (StataCorp LP, College Station, TX, USA) and an alpha value of 5%. For outcome variables with skewness or kurtosis values of ≥ 1.0, confidence intervals (CIs) and p-values were derived using standard errors based on 100 bootstrapped samples.
The trial included an economic analysis to compare the costs and outcomes for the trial arms (see Chapter 5 for details of the health economic analysis).
Process evaluation
A process evaluation was designed to complement and provide additional information concerning the trial. 60 Details about the process evaluation and accompanying qualitative study are included in Chapter 6.
Changes to original protocol
The size and complexity of the evaluation meant that it was extremely challenging to implement, and initial delivery of the intervention and research components faced a number of barriers that led to a number of minor changes to the original protocol. We detail these changes and their potential threats to internal and external validity in the following sections.
Single health economy
Our aim was to deliver the proposed intervention across all practices in a single PCT to assess an intervention effect across a complete health economy. Although we were able to recruit 32 practices in Salford (73% response rate), this did not give us the desired level of statistical power. We therefore spread our recruitment to a neighbouring PCT (Bury). This area has a similar socioeconomic profile and the overall spread of IMD scores across practices is quite similar to Salford (4.5–65.5 compared with 6.6–77.2). In addition, the two trusts shared the same chief executive and both are part of a north-west self-management initiative.
However, some aspects of the whole-system intervention detailed previously were not available, such as existence of a dedicated self-management education team and availability of certain community-based support schemes. Differences in the financial arrangements between trusts also meant that we were unable to offer the intervention to control group practices in Bury PCT after 12 months.
Selection of patients before practice allocation
One of the threats to the validity of a cluster randomised trial is recruitment bias, where professionals allocated to different trial arms recruit differently depending on their allocation, leading to selection bias and baseline incomparability. 61 It is preferable in these cases to recruit patients prior to allocation. Although this was our intention, in the event we found that practices required adequate advance notice of their training date; hence it became necessary to inform them of their group allocation prior to patient selection. This does raise the possibility of bias, but we are confident that such bias was small. Initial patient selection was via existing disease registers and Read Codes; therefore, the only way practices could influence recruitment was to request exclusion of a patient after they had been identified through these methods. These exclusions represented a relatively small proportion of patients (11% control and 15% intervention with COPD, 10% and 11%, respectively, for diabetes and 18% and 11%, respectively, for IBS).
Baseline sampling and assessment
Response rates from patients at the practices first to enter the trial were considerably lower than originally expected. In view of this, we made a number of adjustments to improve response. To ensure the patient numbers required for our target level of statistical power, we increased the number of patients surveyed to include all eligible patients at each practice, up to a maximum of 200 per condition (selected at random where this applied). Very few practices had > 200 patients for any condition; hence the study became, in effect, a total population survey. In addition, we introduced a financial incentive to patients for returning a completed questionnaire. We also took the decision to change the baseline questionnaire to focus on a smaller number of core variables. The main effect on the trial of the shortened baseline questionnaire is to reduce our scope for analysis of baseline moderators of treatment effect. However, such analyses are always secondary to the primary intention-to-treat analysis.
Long-term follow-up
Although the original protocol planned for implementation of the model in usual-care practices and a further follow-up at 24 months to assess long-term effects, delays to the project and the lack of effect demonstrated (see Chapter 4) meant that a longer-term follow-up was not possible or appropriate.
Chapter 4 Results
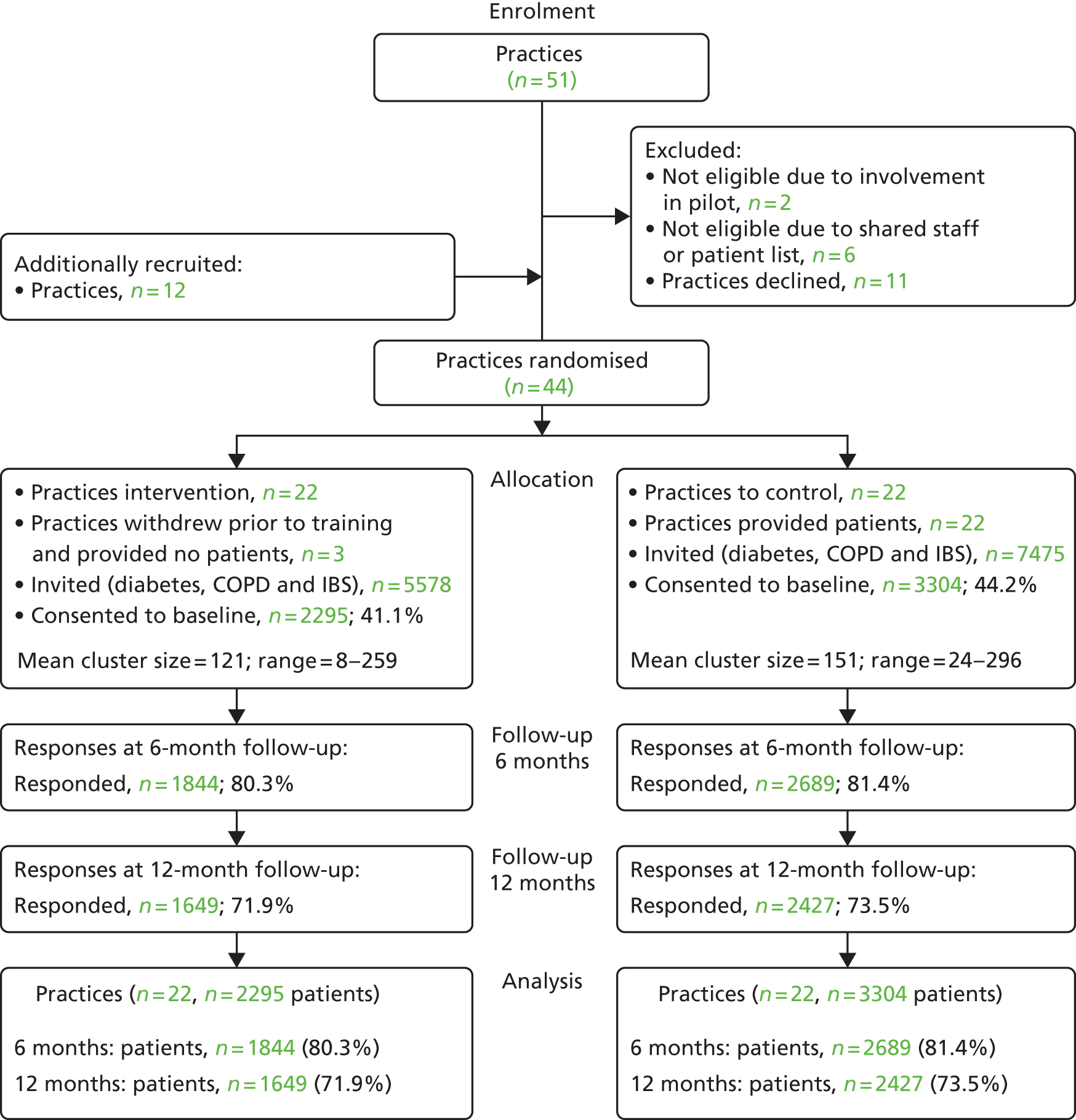
Figure 4 presents the trial CONSORT flow diagram. Practice recruitment from the main PCT (32 practices) fell short of the 40 required to ensure full power. We therefore included additional practices from an adjoining PCT with a very similar demographic profile, resulting in a final total of 44 practices randomised. Three practices randomised to the intervention group withdrew prior to data collection, leaving 19 intervention and 22 control practices.
FIGURE 4.
The CONSORT flow diagram for the trial.
Baseline characteristics of the study participants
A total of 5599 patients (n = 2546 diabetes, n = 1634 COPD and n = 1419 IBS) were recruited, representing 43% of the eligible population. Just over half the sample were female (53.5%) and around half (50.8%) were aged ≥ 65 years (Table 1). Very few (3.4%) were non-white. The great majority (72.5%) had more than one long-term condition, and 23% had visited their GP five times or more in the 6 months prior to the study.
| Characteristic | Trial arm | Total (N = 5599) | |
|---|---|---|---|
| Usual care (N = 3304) | WISE model (N = 2295) | ||
| Main chronic condition, n (%) | |||
| Diabetes | 1486 (45.0) | 1060 (46.2) | 2546 (45.5) |
| COPD | 1009 (30.5) | 625 (27.2) | 1634 (29.2) |
| IBS | 809 (24.5) | 610 (26.6) | 1419 (25.3) |
| Gender, n (%) | |||
| Female | 1728 (52.4) | 1262 (55.1) | 2990 (53.5) |
| Male | 1573 (47.7) | 1030 (44.9) | 2603 (46.5) |
| Age group (years), n (%) | |||
| < 50 | 540 (16.4) | 431 (18.9) | 971 (17.5) |
| 50–64 | 1039 (31.6) | 730 (32.0) | 1769 (31.8) |
| 65–74 | 948 (28.9) | 627 (27.5) | 1575 (28.3) |
| ≥ 75 | 757 (23.1) | 492 (21.6) | 1249 (22.5) |
| Number of chronic conditions, n (%) | |||
| None or one | 909 (27.5) | 628 (27.4) | 1537 (27.5) |
| Two | 999 (30.3) | 709 (30.9) | 1708 (30.5) |
| Three | 780 (23.6) | 532 (23.2) | 1312 (23.4) |
| Four or more | 615 (18.6) | 426 (18.6) | 1041 (18.6) |
| Accommodation, n (%) | |||
| Owner–occupier | 2164 (66.2) | 1498 (66.2) | 3662 (66.2) |
| Renting | 1106 (33.8) | 765 (33.8) | 1871 (33.8) |
| Education, n (%) | |||
| No qualifications | 1044 (31.6) | 699 (30.5) | 1743 (31.1) |
| School-level qualifications | 362 (11.0) | 250 (11.0) | 612 (10.9) |
| Professional or vocational | 949 (28.7) | 649 (28.3) | 1598 (28.5) |
| Bachelor’s degree or higher | 198 (6.0) | 157 (6.84) | 355 (6.3) |
| Missing | 751 (22.7) | 540 (23.5) | 1291 (23.1) |
| IMD, mean ± SD | 30.7 ± 20.0 | 28.9 ± 18.1 | 30.0 ± 19.3 |
| GP visits in prior 6 months, n (%) | |||
| None | 407 (12.9) | 265 (12.1) | 672 (12.6) |
| 1 or 2 | 1215 (38.4) | 881 (40.3) | 2096 (39.2) |
| 3 or 4 | 808 (25.5) | 545 (24.9) | 1353 (25.3) |
| 5 or 6 | 448 (14.2) | 264 (12.1) | 712 (13.3) |
| ≥ 7 | 287 (9.1) | 233 (10.7) | 520 (9.7) |
| Ethnicity, n (%) | |||
| White | 3167 (96.4) | 2207 (97.0) | 5374 (96.7) |
| Not white | 117 (3.6) | 69 (3.0) | 186 (3.4) |
| Shared decision-making, mean ± SD | 76.7 ± 24.0 | 75.7 ± 24.4 | 76.3 ± 24.1 |
| Self-efficacy score, mean ± SD | 71.1 ± 23.0 | 70.5 ± 23.5 | 70.8 ± 23.2 |
| HRQoL, mean ± SD | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| General health, mean ± SD | 41.4 ± 23.7 | 41.2 ± 24.2 | 41.3 ± 23.9 |
| Practice variables | |||
| Number of practices | 22 | 19 | 41 |
| Practice list size, mean ± SD | 4528 ± 2591 | 4003 ± 2211 | 4285 ± 2407 |
| Practice IMD, mean ± SD | 37.9 ± 21.9 | 40.6 ± 19.6 | 39.1 ± 20.6 |
| Contract type, n (%) | |||
| General medical services | 14 (63.6) | 11 (57.9) | 25 (61.0) |
| Personal medical services | 8 (36.4) | 8 (42.1) | 16 (39.0) |
The two trial arms were well balanced on all variables at the patient level, although practices in the intervention group were, on average, slightly smaller (mean list size of n = 4003 patients compared with n = 4528).
Engagement with training
Practice staff attendance rates at the training sessions were generally high: 90% of eligible staff attended session 1 (n = 179) and 82% (n = 85) attended session 2. Training was rated positively (mean score of > 2.5 on a 5-point scale) by 76% of session 1 participants and by 89% of session 2 participants.
Implementation of training
This is detailed in Chapter 6.
Implementation of step-up therapies
Generally, implementation was limited. In total, 94 referrals were received for step-up therapies, 36 referrals for CBT and 58 referrals for hypnotherapy.
Analysis
With one exception, no statistically significant differences were found between patients attending WISE-trained practices and those attending control practices on any primary or secondary outcome (Table 2). The exception was shared decision-making at the 6-month follow-up (p = 0.05), and the difference favoured the control group.
| Outcomea | Trial arm, unadjusted analyses (mean ± SD; n) | Adjusted mean difference (95% CI)b | Effect size (95% CI)c | p-value | p-value for interaction with condition groupd | |
|---|---|---|---|---|---|---|
| Usual care | WISE model | |||||
| Primary outcomes | ||||||
| Shared decision-making | 69.1 ± 26.3; 2379 | 67.7 ± 27.7; 1626 | –0.47 (–2.55 to 1.61) | –0.02 (–0.11 to 0.07) | 0.657 | 0.696 |
| Self-efficacy score | 71.2 ± 22.5; 2394 | 70.4 ± 22.8; 1611 | –0.35 (–1.42 to 0.71) | –0.02 (–0.06 to 0.03) | 0.519 | 0.205 |
| HRQoL | 0.6 ± 0.3; 2382 | 0.6 ± 0.3; 1609 | –0.00 (–0.02 to 0.01) | –0.01 (–0.05 to 0.04) | 0.724 | 0.305 |
| Secondary outcomes | ||||||
| General health | 41.7 ± 24.8; 2413 | 42.2 ± 25.8; 1643 | 0.28 (–1.37 to 0.82) | 0.01 (–0.03 to 0.06) | 0.621 | 0.884 |
| Social/role limitations | 63.3 ± 31.1; 2408 | 62.8 ± 32.3; 1638 | –0.49 (–1.95 to 0.96) | –0.02 (–0.06 to 0.03) | 0.505e | 0.436e |
| Energy/vitality | 46.8 ± 20.9; 2411 | 46.2 ± 21.8; 1638 | –0.42 (–1.53 to 0.69) | –0.02 (–0.07 to 0.03) | 0.456 | 0.332 |
| Self-care activity | 42.4 ± 14.6; 2382 | 42.5 ± 14.9; 1613 | 0.01 (–0.95 to 0.97) | 0.00 (–0.06 to 0.07) | 0.977 | 0.960 |
| Psychological well-being | 64.7 ± 21.9; 2412 | 64.7 ± 22.2; 1640 | 0.49 (–0.75 to 1.73) | 0.02 (–0.03 to 0.08) | 0.436 | 0.303 |
| Enablement | 78.6 ± 28.8; 2365 | 80.7 ± 28.3; 1624 | 0.85 (–1.36 to 3.06) | 0.03 (–0.05 to 0.11) | 0.450e | 0.948e |
| Shared decision-making (6 months) | 70.3 ± 26.1; 2658 | 68.3 ± 27.3; 1818 | –1.77 (–3.53 to 0.0) | –0.07 (–0.15 to 0.0) | 0.050f | 0.065g |
| Self-efficacy (6 months) | 71.1 ± 22.5; 2659 | 70.4 ± 23.1; 1816 | –0.70 (–1.69 to 0.29) | –0.03 (–0.07 to 0.01) | 0.168 | 0.316 |
| HRQoL (6 months) | 0.6 ± 0.3; 2646 | 0.6 ± 0.3; 1803 | 0.00 (–0.01 to 0.01) | 0.00 (–0.04 to 0.05) | 0.862 | 0.824 |
| Self-care activity (6 months) | 42.5 ± 14.6; 2645 | 42.7 ± 15.0; 1813 | 0.03 (–0.88 to 0.93) | 0.00 (–0.06 to 0.06) | 0.955 | 0.776 |
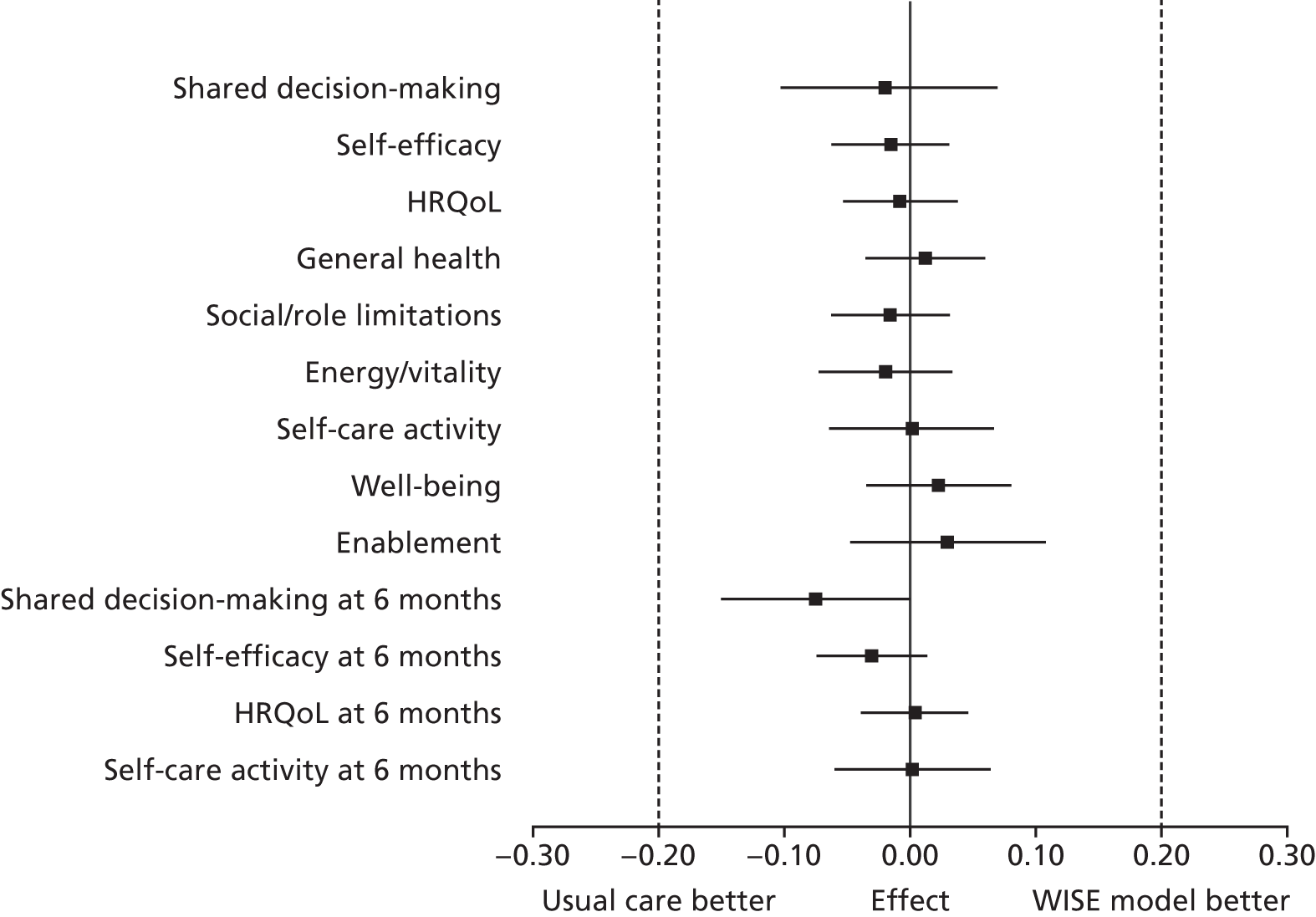
All effect size estimates were very small with narrow 95% CIs and well below the minimally important difference of 0.2 that the trial was powered to detect (Figure 5). The lack of effect applied equally to the intermediate outcomes of shared decision-making, self-efficacy, enablement and self-care activity – which might reasonably be expected to be most directly affected by increased support for self-management – as it did to health-related outcomes. Furthermore, none of the interactions between intervention group and condition group was significant; therefore, we conducted no condition-specific analyses in accordance with the analytic plan. Sensitivity analyses provided no evidence that the results were substantively influenced by model assumptions.
FIGURE 5.
Forest plot of standardised effect sizes (vertical bars indicate minimally important differences).
We repeated the analysis for the IBS sample of 1419 patients, of whom 1119 (79%) completed 6-month follow-up and 1004 (71%) completed 12-month follow-up. Table 3 gives the baseline characteristics for the IBS participants. The analysis, again, found no statistically significant differences between groups on any primary or secondary outcome (Table 4).
| Characteristic | Trial arm | Total (N = 1419) | |
|---|---|---|---|
| Usual care (N = 809) | WISE model (N = 610) | ||
| Gender, n (%) | |||
| Female | 626 (77.4) | 478 (78.5) | 1104 (77.9) |
| Male | 183 (22.6) | 131 (21.5) | 314 (22.1) |
| Age group (years), n (%) | |||
| < 50 | 333 (41.2) | 295 (48.6) | 628 (44.4) |
| 50–64 | 264 (32.7) | 195 (32.1) | 459 (32.4) |
| 65–74 years | 130 (16.1) | 81 (13.3) | 211 (14.9) |
| ≥ 75 | 81 (10.0) | 36 (5.9) | 117 (8.3) |
| Number of chronic conditions, n (%) | |||
| None or one | 323 (39.9) | 251 (41.2) | 574 (40.5) |
| Two | 237 (29.3) | 198 (32.5) | 435 (30.7) |
| Three | 164 (20.3) | 93 (15.3) | 257 (18.1) |
| Four or more | 85 (10.5) | 68 (11.2) | 153 (10.8) |
| Accommodation, n (%) | |||
| Owner–occupier | 561 (69.6) | 456 (75.9) | 1017 (72.3) |
| Renting | 245 (30.4) | 145 (24.1) | 390 (27.7) |
| Education, n (%) | |||
| No qualifications | 142 (17.6) | 99 (16.2) | 241 (17.0) |
| School-level qualifications | 115 (14.2) | 99 (16.2) | 214 (15.1) |
| Professional or vocational | 276 (34.1) | 208 (34.1) | 484 (34.1) |
| Bachelor’s degree or higher | 86 (10.6) | 65 (10.7) | 151 (10.6) |
| Missing | 190 (23.5) | 139 (22.8) | 329 (23.2) |
| IMD, mean ± SD | 27.7 ± 19.2 | 25.3 ± 16.1 | 26.7 ± 17.9 |
| GP visits in prior 6 months, n (%) | |||
| None | 91 (11.7) | 81 (13.8) | 172 (12.6) |
| 1 or 2 | 303 (38.9) | 237 (40.4) | 540 (39.6) |
| 3 or 4 | 217 (27.9) | 134 (22.9) | 351 (25.7) |
| 5 or 6 | 98 (12.6) | 77 (13.1) | 175 (12.8) |
| ≥ 7 | 70 (9.0) | 57 (9.7) | 127 (9.3) |
| Ethnicity, n (%) | |||
| White | 776 (96.4) | 593 (97.9) | 1369 (97.0) |
| Not white | 29 (3.6) | 13 (2.2) | 42 (3.0) |
| Shared decision-making, mean ± SD | 71.9 ± 25.9 | 71.9 ± 24.6 | 71.9 ± 25.3 |
| Self-efficacy score, mean ± SD | 70.2 ± 22.8 | 70.8 ± 22.3 | 70.4 ± 22.6 |
| HRQoL, mean ± SD | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.3 |
| General health, mean ± SD | 3.0 ± 1.0 | 3.0 ± 0.9 | 3.0 ± 1.0 |
| Practice variables | |||
| Number of practices | 21 | 19 | 40 |
| Practice list size, mean ± SD | 4654 ± 2586 | 4003 ± 2211 | 4345 ± 2407 |
| Practice IMD, mean ± SD | 36.1 ± 20.7 | 40.6 ± 19.6 | 38.2 ± 20.1 |
| Contract type, n (%) | |||
| General medical services | 14 (66.7) | 11 (57.9) | 25 (62.5) |
| Personal medical services | 7 (33.3) | 8 (42.1) | 15 (37.5) |
| Outcomea | Trial arm, unadjusted analyses (mean ± SD; n) | Adjusted mean difference (95% CI)b | Effect size (95% CI)c | p-value | |
|---|---|---|---|---|---|
| Usual care | WISE model | ||||
| Primary outcomes | |||||
| Shared decision-making | 66.0 ± 27.7; 560 | 64.5 ± 27.7; 431 | 0.60 (–3.17 to 4.38) | 0.03 (–0.13 to 0.19) | 0.755 |
| Self-efficacy score | 70.6 ± 20.9; 563 | 71.9 ± 21.0; 420 | 0.96 (–0.88 to 2.80) | 0.04 (–0.04 to 0.12) | 0.305 |
| HRQoL | 0.7 ± 0.3; 564 | 0.7 ± 0.3; 423 | 0.01 (–0.02 to 0.04) | 0.03 (–0.06 to 0.12) | 0.521 |
| Secondary outcomes | |||||
| IBS-specific quality of life | 80.3 ± 22.4; 536 | 81.8 ± 21.9; 403 | 2.07 (–0.46 to 4.59) | 0.07 (–0.02 to 0.16) | 0.109d |
| General health | 3.0 ± 1.0; 570 | 3.0 ± 1.0; 432 | 0.02 (–0.06 to 0.11) | 0.02 (–0.06 to 0.11) | 0.588 |
| Social/role limitations | 68.1 ± 29.6; 569 | 69.2 ± 29.7; 428 | 1.14 (–1.76 to 4.04) | 0.04 (–0.06 to 0.13) | 0.441d |
| Energy/vitality | 47.6 ± 20.0; 568 | 47.3 ± 21.5; 431 | 0.40 (–1.73 to 2.53) | 0.02 (–0.08 to 0.12) | 0.714 |
| Self-care activity | 42.4 ± 15.8; 558 | 42.1 ± 16.1; 423 | 0.02 (–1.97 to 2.02) | 0.00 (–0.13 to 0.14) | 0.983 |
| Psychological well-being | 61.1 ± 22.4; 569 | 61.3 ± 22.0; 432 | 1.21 (–1.22 to 3.65) | 0.06 (–0.06 to 0.17) | 0.329 |
| Enablement | 81.0 ± 28.2; 556 | 82.8 ± 26.4; 426 | 1.06 (–2.23 to 4.36) | 0.04 (–0.08 to 0.15) | 0.528d |
| IBS-specific quality of life (6 months) | 80.7 ± 22.4; 598 | 82.3 ± 22.5; 454 | 1.66 (–0.61 to 3.93) | 0.06 (–0.02 to 0.14) | 0.151d |
| HRQoL (6 months) | 0.7 ± 0.3; 626 | 0.7 ± 0.3; 476 | 0.00 (–0.03 to 0.02) | –0.01 (–0.08 to 0.07) | 0.871 |
| Shared decision-making (6 months) | 68.3 ± 26.4; 634 | 64.5 ± 27.4; 479 | –2.87 (–5.81 to 0.06) | –0.12 (–0.25 to 0.00) | 0.055e |
| Self-efficacy (6 months) | 72.4 ± 20.6; 627 | 70.8 ± 22.2; 477 | –1.89 (–3.66 to –0.12) | –0.08 (–0.16 to –0.01) | 0.036f |
| Self-care activity (6 months) | 42.4 ± 15.8; 624 | 41.7 ± 16.1; 478 | –0.37 (–2.25 to 1.50) | –0.03 (–0.15 to 0.10) | 0.697 |
Discussion
This chapter reports one of the largest trials of self-management support in primary care. The WISE model had no significant effects on patient outcomes or on service use. This chapter focuses on trial results, but a separate process evaluation will explore barriers to implementation (see Chapter 6).
Strengths of the study included a very large practice and patient sample size, an intervention based on previous published trials and delivered at an intensity feasible in primary care. A patient recruitment rate of 43% is relatively high for a community-based trial in UK primary care and we achieved excellent levels of follow-up. We also achieved high levels of practice participation. Although it might be argued that effects may have been demonstrated in different long-term conditions or outcomes, our inclusion of a range of conditions and our comprehensive outcome assessment gives us confidence that the lack of effect is robust.
The key threat to trial validity is recruitment bias, which occurs when professionals recruit differently depending on the trial arm to which they are allocated. 61 We intended to recruit patients prior to allocation, but this proved logistically impracticable. Recruitment was via electronic health records rather than professional invitation, but practitioners could exclude patients after identification. 62
A common complaint in health services research is that effective interventions are often not feasible, and feasible interventions are often not effective. Many published self-management trials are conducted in atypical contexts with selected, volunteer samples. Our study took proven components of self-management support and tested whether or not we could implement these as a comprehensive package in routine primary care practice using existing educational structures and applied to an entire local health economy. We sought to sensitise our intervention to the particular nature of primary care, providing a structure and tools to allow practitioners to introduce self-management support into time-limited consultations, to enhance partnerships with patients, and to encourage behaviour change.
The local context included indicators of institutional commitment from the host organisation. This was reflected in the relatively high level of practice engagement. Data from practice staff (see Chapter 6) suggest that training facilitation was successful in parts, with relatively high levels of attendance and acceptability. Limited time was available for training. However, time provided for training was based on our pilot studies and negotiations with practices, and was judged the maximum acceptable to clinical staff, given time demands and the high costs of providing staff cover. Staff self-report data suggest that implementation was variable. We allowed practices flexibility in how they implemented self-management support at the practice level and flexibility can lead to attenuated outcomes. Although a more standardised approach may have enhanced effectiveness, this may have equally jeopardised recruitment and engagement.
In addition, despite our best efforts and the full support of the PCT, no practice was prepared to free up further staff time for reinforcement sessions or fuller engagement of the ‘WISE champions,’ and only one practice allowed access for fidelity checks.
A fuller discussion of the results of the RCT will be presented following the economic analyses and the results of the process evaluation.
Chapter 5 Health economic analysis
In this chapter we assess the cost-effectiveness of the WISE approach to provide useful information for decision-makers. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist is provided in Appendix 3.
Methods
Design
The trial was a two-arm, practice-level cluster RCT evaluating outcomes and costs associated with adoption of the WISE approach in primary care to manage three conditions. The intervention and comparator have been described in Chapter 3, as has the trial design.
Decision problem
To assess the cost-effectiveness of the WISE model in the management of long-term conditions, compared with routine primary care services, and to assess the cost-effectiveness of the model in IBS separately.
Parameter estimates
The following parameter estimates were generated as part of the RCT.
Health-related quality of life
Health-related quality of life was measured in the trial using quality-adjusted life-years (QALYs). QALYs were generated as the product of the health state of each individual and the time spent in that state. The health state of each individual in the study was assessed at entry to the trial (baseline), and at 6- and 12-month follow-up using the EQ-5D descriptive system.
Quality-adjusted life-years were estimated using the area under the curve approach,63 with linear interpolation between EQ-5D scores at each follow-up point. The QALY estimates are presented adjusted for baseline EQ-5D score,64 but also without the adjustment.
Resource use and unit costs
At each follow-up (6 and 12 months post randomisation), patients were asked to recall their use in the last 6 months of hospital services (including inpatient stays and outpatient attendances), visits to GP surgery (GP or practice nurse), home visits (from GP, physiotherapist, or occupational therapist) and other health/social sector resource use.
The unit costs of health services, for example the cost of a visit to a GP, were estimated using the published literature and are presented in Table 5. The unit costs were then applied to the appropriate resource use item.
| Description | Mean (£) | Source |
|---|---|---|
| Cost per elective bed-day | 341.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Outpatient attendance | 105.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| A&E | 119.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Physiotherapist | 47.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Occupational therapist | 74.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| District nurse | 38.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| GP | 25.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Practice nurse | 11.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| GP home visit | 82.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Nurse specialist (community) | 44.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| NHS Direct | 25.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| NHS walk-in centre | 99.00 | Salford Royal NHS Foundation Trust |
| Home help | 9.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Meals on Wheels | 3.50 | Variable |
| Respite care | 500.00 | PSSRU 199867 (inflated to 2010/11) |
| Counsellor | 60.00 | Unit Costs of Health and Social Care 2010/1165,66 |
| Other | 9.00 | Unit Costs of Health and Social Care 2010/1165,66 |
Missing data
All data were available at baseline. There were missing data when follow-up questionnaires were incomplete or patients missed one or more follow-up interviews. In the main analysis missing data were imputed by multiple imputation. Complete-case analysis was conducted as a sensitivity analysis to assess the robustness of results to imputation assumptions.
Where data were missing, the relevant item (e.g. EQ-5D index and/or health-care costs) was imputed by multiple imputation with the Stata v11 program. The ‘mi impute chained (pmm)’ command was employed to generate values for missing data at each follow-up using a predictive mean matching method. Multiple imputation generates several (in this instance, five) data sets rather than a single imputed data set. Each data set contains different imputed values and analysis is then conducted on each of the imputed data sets. The multiple analyses are then combined to yield a single set of results. The major advantage of multiple imputation over single imputation is that it produces standard errors that reflect the degree of uncertainty as a result of the imputation of missing values. In general, multiple imputation techniques require that missing observations are missing at random. This means that, given the observed data, the reason for the observation being missing does not depend on the unobserved data. So, for example, a missing HRQoL observation might be predicted by previous HRQoL, but does not depend on current HRQoL.
EuroQol-5 Dimensions index scores were imputed rather than missing responses to individual EQ-5D domains. Thus, an individual who responded to three of the five EQ-5D domains would have an index score imputed that would reflect their age, gender and other characteristics, but not their responses to the non-missing EQ-5D domains. However, EQ-5D index scores from other time periods were included in the predictive mean matching imputation.
Similarly, because of the complexity of imputing missing resource use by each item, costs were aggregated to levels of primary care, secondary care and community care. Missing data were then imputed at these levels (i.e. primary care costs, secondary care costs or community care costs).
Sensitivity analyses were carried out by excluding patients with missing data. Although complete case can be a useful sensitivity analysis, only a small proportion of individuals completed every question at every follow-up. Therefore, available case data are presented as a sensitivity analysis. The difference between groups was then assessed on this subset of available data.
Cost-effectiveness analysis
A NHS and Personal Social Services perspective was considered. All costs and outcomes fell within a 12-month period and, therefore, discounting was not conducted. The analysis presented is a ‘within-trial’ analysis. Thus, only costs and effects observed within the period of the trial were analysed and presented. Where any substantial differences were demonstrated between groups, we intended to investigate the consequences of extending the period of analysis to a longer, more appropriate time horizon.
The mean cost and mean QALYs per patient were calculated for both groups over the period of the trial. The difference in mean costs and mean effects between groups was estimated, and incremental cost-effectiveness ratios (ICERs) were calculated where appropriate. Currently, NHS treatments in England are considered cost-effective by the National Institute for Health and Care Excellence (NICE) if the ICER is < £20,000 per QALY. For interventions that are associated with an ICER between £20,000 and £30,000 per QALY gained, there needs to be evidence that the intervention is innovative and/or that HRQoL is not captured adequately and/or that there is considerable uncertainty around the ICER. As the ICER goes above £30,000 per QALY gained, this evidence needs to be stronger.
Cost-effectiveness analysis is conducted under uncertainty. Uncertainty around the adoption decision is presented graphically using cost-effectiveness acceptability curves. 68,69 Uncertainty in the choice of analysis (e.g. the form of imputation) is addressed using sensitivity analysis.
Results
The results are presented initially for the three conditions combined. The subsequent analysis considers the cost-effectiveness of the WISE model in individuals whose primary condition was IBS only.
The unit costs of the health-related resource use in the trial are presented in Table 5.
The resource use for each item recorded is presented, by trial group, in Table 6. These figures are based on the available cases and will therefore differ from those presented in later tables, which are based on imputed values.
| Resource use | Trial arm, mean resource use | Difference in mean (95% CI) | |
|---|---|---|---|
| WISE model | Usual care | ||
| Length of stay | 1.589 | 1.322 | 0.268 (–0.343 to 0.879) |
| Outpatient attendances | 2.912 | 2.887 | 0.024 (–0.366 to 0.415) |
| A&E attendances | 0.298 | 0.290 | 0.009 (–0.050 to 0.068) |
| GP surgery visits | 4.429 | 4.505 | –0.077 (–0.371 to 0.218) |
| GP home visits | 0.155 | 0.227 | –0.072 (–0.009 to –0.135) |
| GP other | 0.208 | 0.280 | –0.072 (–0.157 to 0.012) |
| Practice nurse visits | 2.783 | 2.893 | –0.110 (–0.307 to 0.086) |
| Community nurse visits | 1.141 | 0.615 | 0.525 (0.129 to 0.922) |
| OT visits | 0.136 | 0.081 | 0.055 (–0.057 to 0.166) |
| Home care worker | 1.156 | 2.290 | –1.133 (–4.405 to 2.139) |
| Meals on Wheels | 0.028 | 0.181 | –0.153 (–0.402 to 0.095) |
| Physiotherapist | 0.104 | 0.090 | 0.014 (–0.041 to 0.070) |
| NHS Direct | 0.172 | 0.136 | 0.036 (–0.030 to 0.101) |
| Walk-in centre | 0.534 | 0.357 | 0.178 (0.034 to 0.321) |
| Respite care | 0.077 | 0.039 | 0.038 (–0.031 to 0.107) |
| Counsellor | 0.252 | 0.281 | –0.029 (–0.156 to 0.098) |
| Other | 0.865 | 0.528 | 0.337 (0.045 to 0.630) |
Table 6 shows that there are few substantial and/or statistically significant differences in resource use between the two trial groups. Although the analysis above describes available cases, these results are completely consistent with the complete-case analysis.
Health-related quality of life
Table 7 shows the percentage of each patient group in each EQ-5D domain by follow-up for those who completed the EQ-5D at the relevant follow-up time point. An examination of the table indicates that there was very little movement between dimensions for either the WISE model or the usual-care group over the 12-month period.
| Intervention and EQ-5D dimension | Time point, % of patients in health state at | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| WISE model | |||||||||
| Mobility | 47.2 | 52.3 | 0.4 | 46.6 | 53.1 | 0.2 | 46.9 | 52.6 | 0.5 |
| Self-care | 76.9 | 22.1 | 1.1 | 76.1 | 22.9 | 1.1 | 76.8 | 22.3 | 1.0 |
| Usual activities | 46.1 | 46.3 | 7.6 | 46.9 | 46.5 | 6.7 | 46.4 | 46.9 | 6.7 |
| Pain/discomfort | 28.8 | 56.3 | 15.0 | 27.2 | 59.0 | 13.9 | 28.4 | 56.9 | 14.7 |
| Anxiety/depression | 55.0 | 39.2 | 5.8 | 54.0 | 39.8 | 6.1 | 54.7 | 38.8 | 6.5 |
| Usual care | |||||||||
| Mobility | 45.0 | 54.8 | 0.2 | 43.8 | 55.9 | 0.3 | 44.7 | 55.0 | 0.3 |
| Self-care | 77.7 | 21.6 | 0.7 | 76.9 | 22.2 | 0.9 | 78.3 | 20.7 | 1.0 |
| Usual activities | 44.8 | 48.8 | 6.4 | 44.9 | 48.8 | 6.3 | 45.6 | 47.6 | 6.8 |
| Pain/discomfort | 27.5 | 59.6 | 12.9 | 26.9 | 59.9 | 13.3 | 28.0 | 59.2 | 12.8 |
| Anxiety/depression | 56.3 | 37.9 | 5.7 | 54.9 | 39.6 | 5.5 | 55.8 | 38.7 | 5.5 |
Imputation of missing data
There were a considerable number of missing resource use data at each follow-up point, although the response rate was > 70% for each resource use variable and > 80% for inpatient stays at 6 months. Thus, the analysis based on multiple imputation is considered as the primary analysis, with the available and complete cases conducted as secondary/sensitivity analyses.
Cost-effectiveness
The mean QALYs for both the WISE model and usual care group are presented in Table 8, together with the CIs around the difference. Unadjusted QALY differences are presented, followed by the adjustment for EQ-5D baseline score, as recommended in the literature. 64 Whether the adjusted or unadjusted analysis is considered, the QALY differences are not substantial. The change in direction of effect that occurs after allowing for baseline differences in EQ-5D score is attributable to the small absolute difference in effectiveness. The mean costs by group are presented in Table 9 and are based on the imputed data.
| Group | Mean QALY | Difference (95% CI) | Difference allowing for baseline characteristics (95% CI) |
|---|---|---|---|
| WISE model | 0.6871 | –0.0029 (–0.0176 to 0.0117) | 0.0044 (–0.0052 to 0.01385) |
| Usual care | 0.6900 |
| Group | Mean total cost (£) | Difference in mean total cost (£) (95% CI) |
|---|---|---|
| WISE model | 1264 | 144 (–99 to 387) |
| Usual care | 1120 |
The difference in cost between the two trial arms is, again, insubstantial. Combining the difference in costs and effects generates the ICER. This statistic is presented in Table 10 with and without adjustment for baseline EQ-5D scores (although the latter is the preferred statistic).
| Cost difference (£) (95% CI) | QALY | ICER (£) | ||
|---|---|---|---|---|
| Difference (95% CI) | Difference allowing for baseline characteristics (95% CI) | Unadjusted QALY | Adjusted QALY | |
| 144 (–98.9 to 386.7) | –0.0029 (–0.0176 to 0.0117) | 0.0044 (–0.0052 to 0.01385) | Dominated | 32,695 per QALY |
There is a considerable amount of uncertainty around the adoption decision, driven by uncertainty in whether the intervention is less or more effective, and less or more costly. This is reflected in the cost-effectiveness acceptability curve (Figure 6), which shows that, at commonly used threshold values of a QALY, we are unsure whether or not the intervention is cost-effective. For example, at a cost-effectiveness threshold of £20,000, there is approximately 30% chance that the WISE model is cost-effective, whereas at £30,000 this rises to almost 50%. The ICER of around £33,000 would not be considered cost-effective at commonly used thresholds. Therefore, the choice of analysis does not affect the adoption decision and the WISE model would not be implemented based on these data.
FIGURE 6.
Cost-effectiveness acceptability curve, controlling for baseline utility.

Sensitivity analysis
To test the robustness of the multiple imputation assumption, costs and effects were estimated for the groups based on the responses received at each follow-up and including the adjustments for zero values described earlier. The results are very similar in magnitude, and direction, for all estimates, thereby adding weight to the conclusion that the WISE model has little impact on either costs or effects on these patient groups (Table 11).
| Cost difference (£) (95% CI) | QALY | ICER (£) | ||
|---|---|---|---|---|
| Difference (95% CI) | Difference allowing for baseline characteristics (95% CI) | Unadjusted QALY | Adjusted QALY | |
| 172 (–27.5 to 372.1) | –0.0025 (–0.0175 to 0.0125) | 0.0050 (–0.0046 to 0.01499) | Dominated | 34,400 per QALY |
Subgroup analysis of irritable bowel syndrome patients
Costs
Based on imputed data for those individuals whose primary condition was IBS, the WISE model was associated with an increased cost of £387 per patient over the duration of the trial. Although this may appear substantial, it would not be considered statistically significant, with a 95% CI of –£133 to £907.
Health-related quality of life
Again, based on imputed data for IBS patients, the WISE model was associated with a small increase in HRQoL. This difference of 0.0015 QALYs is not statistically significant (95% CI –0.0163 to 0.0193 QALYs).
Cost-effectiveness
In the IBS population, the WISE model was associated with small increases in HRQoL observed together with some increases in cost. Thus, it is appropriate to generate an ICER. With an ICER of > £260,000 per QALY for IBS patients, the WISE model would not be considered cost-effective at commonly considered thresholds.
Comparison with other conditions in trial
The three conditions combined yielded a very small improvement in QALYs at an increased cost. The analysis of IBS patients generates very similar results, suggesting that the conclusion that the WISE model has little impact on costs or QALYs over the duration of the trial in any of the conditions considered.
Discussion
The WISE model had little impact on either costs or effects within the time period of the trial. In addition, there is no evidence to suggest that there were any longer-term implications that may not have been captured within the time period of the trial. The results were robust to alternative assumptions about missing data and did not differ between conditions included in the trial. The results of the cost-effectiveness analysis are therefore consistent with the results of the effectiveness analyses.
The strengths of this study are clearly that it is a large RCT so that any demonstration of a treatment effect on costs or outcomes is likely to be reliable. The variety of sensitivity analyses performed also suggests that this result is robust.
A potential weakness of the economic analysis is the time horizon. This assumes that there are no differences between groups after the end of the trial. However, given the lack of movement within EQ-5D dimensions (and single index scores) over the trial period, it is considered a justifiable assumption. Similarly, the trajectory of the EQ-5D scores between follow-up periods is unknown. We have assumed a linear interpolation in the absence of evidence to the contrary. It is feasible that if EQ-5D scores were collected more frequently, differences may have been observed. However, again because of the lack of movement in individuals across dimensions, this is considered unlikely.
Chapter 6 Process evaluation
Sections of this chapter have been reproduced from Kennedy et al. 70 Copyright © 2013 The Authors. Published by Elsevier Ltd.
Primary care potentially provides ready access and continuity of care for patients and, therefore, an appropriate location for guideline-based disease management programmes for patients and, more recently, as a key provider of self-management support. 14 In UK primary care, long-term condition management operates through an increasingly biomedical framework, partly as a result of the QOF, a system of payment to practices for activities done and outcomes achieved.
The organisation of care for people with long-term conditions is in transition and self-management support policies are seen as important to enhance peoples’ self-management capabilities and thus improve health outcomes and reduce the fiscal burden on health-care systems. 71
There are gaps in our knowledge about the implementation of a ‘whole-systems’ approach. A significant degree of complexity was anticipated given the need to incorporate different elements of a relatively ‘open’ system of primary care (compared with secondary care). Thus, there is a need to capture this complexity in understanding how a comprehensive approach to self-management reconfigures existing relationships, communication and practices and how (and if) the principles of a whole-systems patient-centred approach to self-management can become embedded and integrated into routine practice. 72 The latter is particularly salient in a context where the labour of primary care professionals has ostensibly become more biomedical and bureaucratic as a result of the pressures and demands of governance arrangements linked to QOF and pay for performance,34 but at a time when the empowerment and engagement of patients in their own care is also being advocated.
All primary care health professionals are relevant to the implementation of self-management. The role of the practice nurses in long-term condition management grew as a result of the delegation of work associated with QOF. 73 For nurses, two aspects of self-management have been identified as being particularly relevant: using education, techniques and tools to help patients improve their self-management abilities; and a more demanding requirement to transform the patient–caregiver relationship into a collaborative partnership. 74–76
Process evaluation
A process evaluation was designed to complement the RCT (see Chapter 4). Recent MRC guidance defined process evaluation as:
A study which aims to understand the functioning of an intervention, by examining implementation, mechanisms of impact, and contextual factors. Process evaluation is complementary to, but not a substitute for, high quality outcomes evaluation.
The success (or failure) of interventions is predicated on the potential for embedding new interventions within normal ‘everyday’ practices. In this context, normalisation process theory (NPT)77 has utility as a conceptual framework for understanding the incorporation or rejection of the WISE model from a patient and professional perspective.
Process evaluation question
What are the barriers and facilitators that affect the implementation of the WISE model at patient, clinical and organisational levels?
Normalisation process theory provides the conceptual framework for the process evaluation. NPT is designed for the study of implementation processes – to explain how new technologies, ways of acting and working become routinely embedded in everyday practice. Thus, NPT is well orientated to describe and explain the way in which the new practices associated with the WISE model are operationalised in health care. In order to understand the embedding of a practice we must look at what people actually do and how they work.
The theory is concerned with three core problems:
-
implementation – the social organisation of bringing a practice or practices into action
-
embedding – the processes through which practices do or fail to become routinely incorporated in everyday work
-
integration – in which we mean the processes by which a practice or practices are reproduced and sustained among the social matrices of an organisation or institution.
According to NPT, practices become routinely embedded – or normalised – in social contexts as the result of people working, individually and collectively, to enact them. The work of enacting a practice is promoted or inhibited through the operation of generative mechanisms (coherence, cognitive participation, collective action, reflexive monitoring) through which human agency is expressed.
Implementation was explored at three levels: (1) organisational (PCT), (2) practice and professionals and (3) patients.
At the organisational level, the process evaluation investigated how far the knowledge of the intervention had been diffused, taken up, adapted locally and embedded at the level of the PCT and the consequences of any such diffusion.
At the practice and professional level, the key issues were the implementation of the training in the WISE model provided to practices and the implementation of WISE practices and tools (e.g. the use of PRISMS forms).
At the patient level, the process evaluation explored patient perspectives about existing services, their engagement with those services and attitudes to engagement with the new self-management arrangements.
When the results of a RCT are positive, a process evaluation is needed to identify ‘active ingredients’, to aid generalisability (or transferability in qualitative research terms) and to facilitate learning and translation into everyday practice. Similarly, when the results of a RCT are negative or inconclusive, evidence is needed to identify reasons for the lack of effect, which may be found in cultural, organisational or behavioural factors.
Methods
A process evaluation was carried out, in parallel to the RCT, using the following methods.
Organisational level
-
Organisational context: baseline face-to-face interviews with a purposive sample of relevant members of a practice-based consortium and PCT governance bodies.
-
Recruitment of practices: methods to assess this process included contemporaneous researcher notes, e-mails from practices and minutes from meetings.
-
Interviews with relevant individuals who were key to the roll-out of the WISE model.
Practice and professional level
-
Post-training evaluation questionnaire.
-
Collation of documents generated by the training, including patient journey maps created during training; reflections on what the practice does well and on challenges and problems to achieve change; action plans and steps to change identified by the practices; and logos designed by each practice as an ice-breaking task.
-
Training notes (written by the trainers after each training session).
-
The post-training evaluation questionnaires (distributed to all staff who attended each of the training sessions) were collected immediately after each session ended.
-
The survey questionnaires were posted out to the practices.
-
At 3–6 months following the training, all trained practices within Salford PCT were invited to take part in interviews and interviews continued until a broad representation of practice types (based on practice size, population served and number of GPs) was reached. Practice staff interviewed included GPs, nursing staff, the practice manager and a member of the administrative staff. Both trainers were interviewed.
-
Questionnaire to survey the use of tools was conducted 6 months post training.
-
Face-to-face in-depth interviews with practice staff. An interview schedule was used to ask staff about their involvement in supporting patients’ self-management of diabetes, COPD and IBS, their impressions of PRISMS and the guidebooks, and attempts to integrate the tools within their daily routines. The interviews were recorded using digital audio equipment. Field notes summarising the interviews and highlighting key issues were written up soon after each interview.
Patient level
-
Patient experience of the current service arrangements and the WISE model. Face-to-face in-depth interviews with a purposefully selected sample of patients who took part in the trial and a longitudinal study of patients recruited during the exploratory study and early piloting of the WISE model. 78
-
Thirty patients were selected for interview using maximum variation sampling based on the following factors: condition, length of time diagnosed, number of contacts with the GP, self-efficacy scores, help and support from family, choices ever offered by GP, age and gender. For the longitudinal study, participants were recruited for interview when attending two general practices in Salford. Participants were purposefully sampled to have at least one of three conditions in the RCT. We also sampled to include a range of ages and length of time since diagnosis.
-
Analysis: verbatim transcriptions of the audio-recorded interviews were discussed over the course of data collection, enabling an iterative approach to data collection and discussion of emerging codes. The content of the interviews was considered case by case and comparisons drawn across cases to identify similarities and differences in the understanding and values attached to the tools and individuals’ attempts to integrate them in everyday practice. The NPT framework allowed a systematic evaluation of the factors influencing the work required to implement and embed the WISE model. Questions relating to each component within each of the four core constructs were generated.
Results
Organisational level
Seven key individuals were interviewed at the start of the roll-out of the WISE model across Salford. These included the PCT chief executive, two senior PCT managers, the NIHR programme grant principal investigator, the NIHR programme grant project manager and the two WISE model trainers (one of whom had a dual role as a PCT self-care development manager). Interviews took place at baseline. However, as a result of the rapidly changing structure of health management systems and the dismantling of the PCT during the period of the study, follow-up interviews were not conducted.
Findings
Set-up of project
-
Respondents reported that Salford operates as a health economy with close geographical and local networks. It is a PCT with a strong local identity and a reputation for innovation. These factors were seen to have been key in facilitating PCT ‘buy-in’ to the WISE project.
-
The cost of GPs’ time was not included in the budget and so an amount of around £100,000 had to be underwritten by the PCT. This created difficulties because it delayed the start of recruitment of practices and was also a source of anxiety for the project manager.
-
Although there was no risk of the PCT withdrawing support, the processes of getting finances authorised was slow, especially as the project coincided with the introduction of a financial recovery scheme to make savings to prepare for anticipated financial stringencies.
Recruitment of general practices
-
Respondents reported that estimates of buy-in by practices were overstated and did not take account of variation: some practices were keen and motivated to adopt the WISE model but others less so. The WISE model was not viewed as critical to practice business and did not fit with QOF targets, enhanced service or training, development or appraisal targets.
-
This meant that the recruitment process was slower than anticipated, which delayed the start of the training programme.
-
Further practices were recruited from Bury to make up numbers caused by the low recruitment of practices. The chief executive of Salford had oversight of Bury PCT during this period, which facilitated their buy-in to the project.
-
Some interviewees suggested that, in retrospect, the recruitment process could have been improved by getting more key stakeholders engaged earlier on in the project (e.g. GP cluster leads), who could have raised the profile of the WISE model ahead of the implementation process.
The WISE model and the primary care trust
-
During the early stages there were tensions arising from the way in which the PCT involvement in the project had been disseminated from executive level to middle managers. The PCT at an executive level were keen and committed and one of the senior managers interviewed was the executive responsible for the WISE model and a member of the Study Steering Committee. However, their role as Director of Clinical Professional Leadership meant that the WISE model was established in the Clinical Professional Directorate rather than the Commissioning Directorate. This made it more difficult to get engagement from the commissioning managers and also for the WISE model to be integrated with the PCT annual planning cycle. In part, this also seems to relate to the status of the WISE model as a pilot rather than an mature strategy.
-
Respondents reported that the lack of an appropriate ‘home’ for the WISE model or a champion working at managerial level seems to have had considerable consequences on the profile of the WISE intervention across the PCT. There is evidence that the WISE model provided a model to support another initiative around diabetes, but this seem to have happened more by chance than by planning.
-
The status of the WISE model as a research project also meant that PCT managers were uncertain as to its future, which made them less keen to engage fully. One senior manager said she believed it would be useful to have evaluative feedback as soon as possible to give them a sense of whether or not the model was successful enough to be adopted across long-term conditions commissioning.
-
The WISE model was also impacted by turnover of PCT managers. The commissioning manager for long-term conditions was offered an interview, but then left the PCT. The WISE model was then allocated to the commissioning manager for long-term conditions and public health. However, she did not seem to demonstrate ‘ownership’ of the WISE model, although she was keen to receive evaluative feedback as soon as possible.
Training
-
Those attending the first few training sessions did not seem to have a clear idea of the purpose of the session, the project, its funding and its relationship to the PCT. The trainers therefore introduced an overview of the WISE model to the beginning of the training session to address these problems.
-
There were some conflicts around trainers’ responsibilities for the WISE model and other PCT priorities. One trainer in particular stated that she had to ensure that she did not have to take on extra responsibilities for PCT work.
-
The trainers were keen to identify ‘WISE champions’ and introduce follow-up sessions to the training as a way of improving uptake, but had difficulty in engaging practices beyond the initial training.
Communication
-
The appointment of a project manager for the NIHR programme with experience in managing projects in Salford PCT seems to have been key to resolving the initial difficulties. The project manager identified the communication problems between executive and manager level and worked to ensure that all involved were appropriately briefed. The interviews generated positive comments about the project manager’s support.
-
Very positive comments were received about accessibility of the research team and their willingness to support the training scheme.
Summary of organisational-level interviews
Overall, the implementation seems to have progressed well, although more slowly than had been anticipated. The initial problems relating to communication, extra funding for GP time and recruitment of practices appear to have been resolved. However, there was a consistent view that the WISE model had not developed a high enough profile within the PCT. This seems to have arisen because of the tensions created by placing the WISE model outside the Commissioning Directorate. Thus, commissioning managers did not develop much knowledge of the aims of the WISE model. In addition, there is a sense that the fact that it was perceived as a pilot may have contributed to the lack of buy-in at managerial level.
Recruitment of practices
The project started with a list of 56 practices. Practices were not eligible if they shared clinicians or administrative staff with other practices or if they served a very specific population (e.g. drug users, the homeless or young patients). Forty-four practices in the Salford PCT were identified as potentially eligible for the study, and all were contacted. Of these, 32 initially agreed to participate in the trial and were randomised, and three subsequently withdrew from the study before data were collected from 29 practices. To ensure that sufficient power for the study was maintained, we recruited 12 practices from Bury (a neighbouring PCT serving a similar population to Salford).
Salford has a large number of single-handed practices, and 17 were identified as eligible for the trial: 14 of the early sign-ups were from single-handed practices and two of the ineligible practices were single-handers. High sign-up from single-handed practices may reflect the fact that it proved easier to negotiate times of meeting and set dates for training when only one GP was involved.
From the start, practices had different reactions to the introduction of the WISE model training. Reactions ranged from interest and enthusiasm to complete lack of interest and hostility. Few perceived that training in self-management support was needed, so the approach to engaging practices involved the use of incentives (costs of attending training to be met by the PCT), as well as targeting practices known to be well disposed to involvement in research early on and using this to encourage more reluctant GPs to join the programme. The involvement in a research project was deliberately downplayed because of the known difficulties of getting GPs to engage with research. Barriers to engagement included:
-
Practical problems such as staff sickness, arranging time out of the practice to undertake the training and the implications for practice finances (although the PCT provided funding for locum and out-of-hours cover to allow participation in training).
-
Practices faced with incorporating other changes, at the same time, to practice and management.
-
Self-management not being seen as a priority for the practice and a lack of belief that supporting self-management would be effective.
-
A perception of the WISE model as providing nothing new. Staff claimed that they were already providing good care for patients with systems and strategies for long-term conditions in place. Furthermore, they could see no need to change practice and no potential benefit for patients in their attending training.
-
A perception among some practices that patients were unlikely to take-up, or benefit from, a self-management approach.
-
Training being considered by some to be inappropriate for support staff.
-
Signing up to the training and the link to research meaning that clinicians would be expected to invest additional time with patients providing self-management support (i.e. there was concern that using the WISE model approach would generate more work).
-
Negativity towards the inclusion of IBS as one of the chronic conditions being included.
Practice and professional level
Training impact
Evaluation forms were completed by staff at the end of each training session. The scores indicated high satisfaction with the training (Tables 12 and 13).
| Satisfaction question | n | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Did you find the training useful? | 265 | 1 | 4 | 3.05 | 0.820 |
| Did you like the structure? | 264 | 1 | 4 | 3.05 | 0.766 |
| Did you learn from others? | 264 | 0 | 4 | 3.08 | 0.751 |
| Was the patient pathway useful? | 263 | 0 | 4 | 2.99 | 0.803 |
| Was creating opportunities helpful? | 255 | 0 | 4 | 2.91 | 0.791 |
| Were the discussions of benefit? | 263 | 0 | 4 | 3.11 | 0.784 |
| How actively involved were you? | 262 | 1 | 4 | 2.96 | 0.772 |
| Will the practice use PRISMS? | 255 | 0 | 4 | 2.80 | 1.007 |
| How likely is system change? | 252 | 0 | 4 | 2.50 | 0.815 |
| Satisfaction question | n | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Did you find the training useful? | 123 | 1 | 4 | 3.21 | 0.668 |
| Did you like the structure? | 124 | 1 | 4 | 3.18 | 0.663 |
| Did you learn from others? | 124 | 0 | 4 | 3.19 | 0.779 |
| Was the DVD useful? | 120 | 1 | 4 | 2.93 | 0.796 |
| Did you find role play helpful? | 108 | 0 | 4 | 3.06 | 0.818 |
| Were the discussions of benefit? | 124 | 1 | 4 | 3.35 | 0.665 |
| Will you be able to use the skills? | 116 | 1 | 4 | 3.26 | 0.674 |
Questionnaire survey of the use of the WISE model tools
The questionnaire was sent to 302 staff at the 31 practices where training took place (25 in Salford and six in Bury), including 163 administrative staff and 139 clinicians. There was an overall response rate of 48% (88 administration staff and 67 clinicians) (Table 14).
| Use of WISE tools | % reporting use |
|---|---|
| Guidebooks (GPs) | 54% ‘regularly’ |
| Guidebooks (nurses) | 50% ‘regularly’ |
| Guidebooks (administration) | 32% ‘regularly’ |
| PRISMS (GPs) | 11% ‘regularly’ |
| PRISMS (nurses) | 15%, ‘regularly’ |
| PRISMS (administration) | 7% ‘regularly’ |
| Explanatory models (GPs) | 36% ‘regularly’ |
| Explanatory models (nurses) | 19% ‘regularly’ |
| Salford web directory (GPs) | 17% ‘regularly’ |
| Salford web directory (nurses) | 22% ‘regularly’ |
| Salford web directory (administration) | 13% ‘regularly’ |
Face-to-face in-depth interviews with practice staff
The analysis presented here is based on interviews with 11 practice nurses and one assistant practitioner; all were female with between 2 and 21 years of experience of working in general practice. Interviews with other staff, documents and notes collected during the study and the training sessions and the observations carried out in the development stage of the WISE model helped to provide context for the analysis.
Findings
Table 15 provides a summary of how the systematic identification of work undertaken by nurses mapped on to the NPT framework. The analysis focused on the ways nurses spoke about the work of managing patients with long-term conditions and how using the WISE model tools changed their everyday self-management practices. Some elements of the WISE model were reported to work well (such as distribution of the guidebooks), but other elements (such as using the PRISMS tool to help address patient needs and priorities) were rarely taken up. A number of themes emerged. Illustrative data are presented and labelled with the respondent identifier.
| NPT construct | Component | Questions to consider | Findings |
|---|---|---|---|
| Coherence: sense-making work | Differentiation | Does the participant recognise how the WISE model tools and techniques are different from their existing ways of working? | There was difficulty differentiating WISE principles from those underpinning existing SMS practice, which undermined the embedding of the intervention |
| Does the participant understand the purpose of the tools and techniques? | |||
| Communal specification | Does the participant recognise the steps she/he needs to take as part of the practice team to assist in the integration of the tools? | Limited communication within practices post training stifled discussion surrounding the WISE model and its potential benefits | |
| Individual specification | Does the participant identify their personal role and responsibilities with the use of the tools? | Marked variation existed in nurses’ opinion as to the fit of the WISE model tools in their current practice. Nurses reported that the guidebooks fitted well and PRISMS did not | |
| Internalisation | Does the participant identify any benefit in adopting the WISE model tools, and for whom? | Familiarity with information and services provided by long-established, reputable sources undermined efforts to identify the benefits and value of the WISE model guidebooks. One nurse saw the WISE model as improving patient care and relationships | |
| Cognitive participation: relational work | Initiation | To what extent does the participant appear to have been a supporter of the process to integrate the tools? | Failure to engage in a practice-wide strategy discouraged individual commitment to adopt the WISE model |
| Enrolment | Has the participant made any adaptations to their personal routine or assisted in the reorganisation process leading to implementation? | In most cases, no adaptations were made, but nurses who saw themselves as having autonomy were able to take up the WISE model tools in individual practice | |
| Legitimation | Does the participant believe that it is appropriate for them to be involved in integrating the tools? | Health-care assistants and newly qualified nurses did not perceive that their roles required adoption of the WISE model approach | |
| Activation | Has the participant taken steps to sustain the use of the tools? | Assessment and review of the processes involving the tools to sustain their use was afforded little priority. Nurses reported that QOF remained the over-riding practice priority | |
| Collective action: operational work | Interactional workability | What work does the participant describe as having taken place to operationalise the use of the tools? | Difficulty engaging patients in self-management practices limited enthusiasm to invest effort in new ways of working. PRISMS was used (rarely) to widen the content of the consultation, but not in supporting behaviour change |
| Relational integration | To what extent does the integration of the tools and resources help or impede people’s work within the practice? | The convenience of and ready access to information in hard-copy format encouraged use of the guidebooks, but PRISMS got in the way of existing tasks and priorities | |
| Skill-set workability | Who does the participant view as being best placed to make use of the tools? | Nurses were delegated responsibility for SMS by the GPs. Responsibilities as health educators promoted nurses’ role as implementers of the approach of the WISE model | |
| How compatible are the tools with their current tasks? | |||
| Contextual integration | Does the integration of the tools fit with the objectives of the practice? | QOF is the priority of the practice. Nurses were generally happy to do the QOF tasks, but there were tensions with the skills they see themselves as having that are disregarded by the QOF process. QOF priorities means that there is little space for SMS work. The practice systems were not able to integrate PRISMS forms – so ‘not to hand’ | |
| Reflexive monitoring: appraisal work | Systematisation | Has the participant taken practical steps to measure the influence of adopting the new techniques? | Limited, informal gathering of feedback from patients regarding the accessibility and utility of the WISE model guidebooks was recorded, suggesting some use of this resource as a prompt when responding to patient concerns |
| Communal appraisal | Has the practice come together to appraise the impact of implementation? | This was generally limited, as few practitioners recorded engaging colleagues in discussion of their experience of using the tools | |
| Individual appraisal | Does the participant reflect personally on the impact of the WISE model tools on his/her routine? | The limited take-up of the tools and resources was reflected in the prevalent view that the training had produced little change in practice. In contrast, supporters of PRISMS noted the positive impact on patient engagement | |
| Reconfiguration | Has the participant made attempts to modify the way the WISE model tools are used as a result of experience? | For adopters of PRISMS, identifying how the process of using it could be adapted to fit in with existing practice (such as by focusing on the most-pressing concern rather than a range of issues) was important to the sustainability of the tool |
Theme 1: the work associated with self-management support is not a priority for practices
Although all the practices involved had signed up to the RCT and participated in the training, the WISE model approach did not emerge as a priority and failed to ‘disrupt’ the existing work of the nurses.
Practice nurses generally work to a set of tasks and do so in a way that is dictated by practice priorities. One priority is to ensure QOF targets are reached – which involves setting up and carrying out review appointments with patients on the practice disease register. In these appointments, nurses monitor and record vital signs, such blood pressure, blood sugars and lung capacity, as required by QOF. This prioritisation marginalises other non-incentivised work, such as self-management support. GPs, however, viewed nurses as having the specific skills, time and opportunity to do self-management support work with patients and delegated this without necessarily knowing, or being interested in, how this was actually achieved.
So I tend to fiddle with their blood pressure pills and the diabetic pills and their insulin doses and cope with crises when they come along . . . The lifestyle type things, which I know are important, tend to get delegated to the nursing staff to do . . . you know, beyond me being very basic and saying to them well, you need to try and lose weight or you need to try and eat more healthily or you need to do more exercise, that’s almost as much as I can do, really, because of time limitations. Nurses do have more time and they have longer appointments, I would say. So the lifestyle side of things, the nurses are more involved with, that’s my belief anyway.
Dr X, practice 22
This attitude meant that, for nurses, the work of providing self-management support had to be fitted between other, more structured, tasks. This led to cognitive and practical tensions for nurses who were at pains to convey their ability to provide holistic care for their patients, while servicing the QOF agenda for which they had been tasked.
Well now. The thing is I’ve always been taught to focus on the patient and you’ve got to tick . . . you know you’ve got to fulfil your QOF criteria and stuff so . . . It’s COPD, looking if there are any changes in their condition over a period of 12 months. If there is anything they can’t do, if it’s impacting on their lifestyle, are they more breathless, are they getting more exacerbations. You are looking at depression and how it’s impacting on that kind of thing generally.
Nurse E, practice 12
Nurses identify patient education as key to self-management support, but the need to fulfil QOF criteria leads to a didactic approach to dealing with patients in consultations. Handing out written information while instructing patients what to do is the easiest and quickest way to undertake the task.
. . . have loads of literature, yeah. That’s it. We have a raft of information for diabetes. So I do give out information. And I think COPD . . . diabetes, I’m the same. With the diabetic ones we manage them and we sort of tell them. It sounds awful tell them. We don’t tell anybody but we do sort of tell them, ‘You need to do this. You need to do that. You need to do the other’.
Nurse N, practice 28
Handing out the WISE model guidebooks fitted readily into this way of working, and the guidebooks were seen as filling in the missing ‘patient-centredness’ of their practice. In this respect guidebooks were minimally disruptive so were easily normalised. 40
We give them the WISE book. And we just ask them to read that and if they have any concerns, that there’s a little problem thing at the back they can always talk to us about it when they next come . . . I think it’s made people more aware of how to manage their own condition. The information is in the book. They know they can look at that any time. If there is something there they don’t understand or whatever, I think the information is all in there, you know, and we go through it with them when they come, and we tell them, ‘If you’re ever stuck or worried, please look at the book. Please read and see where you can make these changes if you need it’.
Nurse F, practice 3
Theme 2: the responsibility for self-management is passed ‘down’ from general practitioner to nurse to patient
The demarcation of roles within the practice impacted on how nurses viewed and dealt with self-management support. GPs delegate self-management support to nurses and, in their turn, nurses delegate responsibility to patients. In both cases, this was not necessarily an empowering process based on a partnership and shared decision-making approach: little was shared with patients and work outside the testing for biomedical markers of disease was not considered a central element of consultations. Nurses viewed GPs as having little understanding of the work they did:
They tend to leave us to our own devices, I know it sounds awful, but, to our own devices, because, they don’t really know what we do, in the clinics.
Nurse M, practice 19
The general lack of interest in nurses’ work within the practice was given as justification for why the WISE model approach was not taken up by nurses.
It’s all right being a pilot and stuff, but you’ve got to want to do it and if they’re not . . . Why should one person do it on their own?
Nurse E, practice 12
A few nurses who did make use of the tools to change their practice found that the WISE model provided a structured approach to self-help and brought a more patient-centred focus to consultations previously ‘driven by targets and guidelines’. Patients were felt to need time to understand what they had to do and the work of self-management support could be done in a gradual and shared way.
It sort of has changed my practice quite a lot, but what I mean, I think, is I didn’t necessarily say to the patient, what do you want to talk about today? Whereas maybe I do now, because they’ve gone away with the booklet and they’ve come back with . . . they’ve highlighted what it is that’s really worth talking about.
Nurse B, practice 22
Theme 3: autonomous working practices provide space for optimal self-management support discussions
Some nurses recognised that, although they had certain core tasks to perform, they did have autonomy in planning their work. A few nurses built in elements of the WISE model approach and were enabled to do this by being seen to be efficient managers of QOF work. The recognition and respect they garnered as a result meant that they were left with autonomy to create space for working in other spheres.
I’ve done this job for 21 years now, I’ve been here a long time, so we do have a very good understanding of each other’s roles and I certainly know where my limits are and I don’t overstep that. But within my sphere of expertise I do all the respiratory care, I do all the diabetes care, the CHD [coronary heart disease] stuff, . . . I’m really left to it because my, it’s obvious what I’m doing, it’s in there, it’s all auditable, it’s easy to see. So you know and the QOF has been good for that.
Nurse B, practice 22
This nurse went on to talk about how she had been able to use the PRISMS tool in her consultations and how it had helped to open up the conversation and focus on the priorities of patients rather than the priorities of the practice.
Basically what it does, it enables me to talk about the things that are worrying them, and things like, for example, sexual health. Unless you ask the question they are never, ever, ever going to bring it up in a consultation in a million years. So having something like that does help focus on the whole shebang, really. But I think it just enables the patient to feel that they’re bringing something, it’s not just about me yappy, yacky, yapping on at them, it’s about them sharing more and having, [whispering] (because I do talk a lot at times) . . . you know it’s allowing them to have a little bit of time to . . . for me to shut up.
Nurse B, practice 22
For others, having autonomy allowed nurses to identify with acting in a patient-centred manner through establishing relationships with patients. Relationality is seen as a central part of their work. 25,41 However, in practice, most nurses were not able to use their existing style of relating to engage patients in in-depth conversations concerning self-management support. This was because they gave more priority to building and maintaining comfortable interpersonal relationships over the more negative and hard work of challenging ‘problem’ behaviours.
Yeah. It just depends, because, a lot of the time, you can get them chatting when you’re doing other things, as well.
Nurse M, practice 19
Theme 4: self-management support is not perceived as different enough to warrant investment of time and effort
In terms of making sense of the new innovation, there appeared to be little differentiation made between the WISE model approach and normal self-management support practice. Self-management support was viewed in terms of being patient centred, addressing lifestyle and behaviours and effecting change, and having time to listen – all of which were considered to be ‘normal practice’. This sense of there being nothing new translated into the view that there was no need for change. Indeed, to the following respondent, the time constraints of practice meant that thought of adopting new ways of managing was ridiculous.
I know it sounds awful, it was, like, it was teaching us to suck eggs! . . . Because, we’ve all been clinicians for a long time, I know it gives you another way of looking at things, but, it’s, like, we already know what the patients are going through, we’ve all been experienced clinicians, it’s not, like, we’re new to the post and the fact that, it’s, like, we have a limited amount of time, in a consultation, we’ve not got an hour, per patient, I wish we did, . . . we have 10 minutes and you try and get everything done in them 10 minutes and, then, somebody is coming along and telling you, oh, this is what you should be doing and this is this and this and this and it’s, like, and where are we supposed to fit everything in, in 10 minutes.
Nurse M, practice 19
Theme 5: perceptions of feasibility of changing patient behaviour are low
Changing people’s behaviour is seen as difficult, a view that receives some support from the literature on behavioural change. There was little or no talk about how health professionals could change people’s behaviour in everyday practice. Giving patients information and instructions was seen as easy and routine, but examples of how to motivate and engage people with new practices and behaviours were missing from the narratives of respondents. The PRISMS tool was supposed to assist this process, but the few nurses who reported using the PRISMS tool did not get much further than using it to open the consultation to patient needs and had well-formulated views borne out of previous experience of working with patients shown in the metaphors used to describe non-behaviour change.
We can point . . . take a horse to water but I can’t make him drink. I can give them all these things, but I can’t make them access them. But I can do my best and . . . that’s all.
Nurse E, practice 12
Nurses suggested that their patients were not suitable candidates for a self-management approach, for example because their lives were too chaotic or they had too many other problems. They found it hard to engage patients with lifestyle change and a patient-centred approach was thought to be at odds with providing self-management support in which the shifting of responsibility is a longer-term aim. This has the effect that self-management support work is deferred.
I think COPD, I mean, the main lifestyle is you have to address there is obviously smoking and I will go straight in and say do you smoke, have you thought about stopping . . . If they don’t want to address it, if they’re not interested then I just leave it because it’s pointless trying to force somebody to do something that they’re not prepared to do. And I’ll just leave it open.
Nurse D, practice 12
Theme 6: it is easy to dismiss or under acknowledge the needs of patients
The PRISMS tool was easy to dismiss for several reasons: lack of time, the potential to open up too many complex issues in time-limited consultations, practice systems were not geared up to support it and the cost to the practice.
The happy, smiley face-y thing. We didn’t use it. We primarily, I’ll be honest and say I didn’t use it because I didn’t have the time because there’s only me and I only work part-time. And I think it was another tool, you know what I mean? And I’d love to be able to sit here and have half an hour consultation about patients’ priorities and I’m going to say that I do but in a more roundabout way, and you know. But I didn’t have the time really to be fair.
Nurse N, practice 28
I am doing it, but not quite in the same form as they thought. I’m not doing the PRISMS because nobody has given the forms out. You see we don’t send letters out for appointments because it’s too expensive. If we could send letters we could send the PRISM forms and they could bring it in when they come for an appointment.
Nurse E, practice 12
Theme 7: self-management support resources need to be readily accessible and trustworthy
Nurses work within a structured primary care team. However, their day-to-day work can be distant from other practice staff. Thus, in terms of tools and technologies, they will use what is readily to hand and draw on resources that they trust. The guidebooks were seen as a positive benefit to patients, a nice ‘gift’ for nurses to hand out and superior to computer printouts. So long as the supplies were on the office shelf, they were easy to work with.
Say I have a new diabetic that’s the . . . usually the time that I would introduce the booklet because I find the information is very easy to understand. So and they can take away that is a form it’s not just an A4 piece of paper because we tend to use an awful lot of the patient.co.uk stuff which is excellent, but it’s only a scrap of paper, isn’t it. Whereas the booklet I think [whispering] you know, and they go away with a nice little booklet and it’s nice, but it’s also very pertinent information, it’s easy to read, it has pictures that are coloured in, and I think that helps the eye, and all of that.
Nurse B, practice 22
For some, their use of resources was determined by the trust they had in the organisation that produced the information, rather than any engagement with the content.
I mean the British Lung Foundation is a well-recognised organisation, so they’re the ones that I tend to use. There are also some other booklets which, to be honest with you, I don’t remember where they’re from but they’re . . . they will be from a recognised organisation.
Nurse D, practice 12
Forms such as PRISMS were more troublesome to deal with because more thought was needed into how and when they are utilised in the consultations and integrated into practice systems. The logistics of distributing forms to patients was viewed as problematic. A number of options were considered, including sending them out with patient reminders to attend review appointments. However, practices either lacked the impetus to consider change, or immediately dismissed the possibility of engaging in the work necessary to co-ordinate the adjustments to staff routines.
I’ve got to be 100% and tell you the truth, I don’t know out there because I’m in here [in the consulting room] from 8 o’clock in the morning ‘til half 4. I don’t really go out to be honest. So I’ve got me hand on heart and say I don’t really know.
Nurse G, practice 2
One practice did embrace the WISE model and was able to integrate the forms into its systems, although sustaining the approach is feasible only if there is continual reflection and reappraisal of the benefit of the PRISMS tool at all levels of the practice.
Yes, we have them out in reception for them to complete. Well the reception staff know who’s diabetic or who’s COPD or whatever, the long-standing condition, and they will give them the form at reception when they come in, if they can please fill this form in before you see the nurse.
Nurse F, practice 3
Patient experience and participation with self-management arrangements
Twenty-four participants with an index condition of either COPD (n = 5), diabetes (n = 9) or IBS (n = 10) were interviewed during the process evaluation. Thirty participants from the exploratory trial were also interviewed as part of an embedded longitudinal qualitative study: diabetes (n = 15), IBS (n = 8) and COPD (n = 7). 43
One of the aims of the patient interviews was to explore patient attitudes to engagement with any of the new self-management arrangements. It was apparent from the interviews that patients had not experienced any changes in the nature of their care as a result of the intervention and the interviews explored their views on the tools developed for the WISE model and on their experiences and expectations of self-management support. In order to do this, these instruments were introduced to patients during the interviews.
Meaning and use of the PRISMS tool
The majority of participants in both studies had not used the PRISMS form. When shown the form there was a range of responses about its utility. The participants who did consider it something they could use primarily saw it as a memory prompt and mechanism of introducing relevant issues into the consultation which otherwise would be forgotten or not raised (e.g. if it was a sensitive issue or something that was considered embarrassing, this provided an opportunity that might not occur naturally during the consultation). Of most concern was the receptivity of the GPs to patient-initiated prompts and thus the feasibility of making this work in practice. In this respect patients were rehearsing the needs of professionals and conscious of the problems of disrupting consultation practices established over time.
I haven’t used it. But, it’s um, it is a good idea actually because if you’re like me, you keep forgetting, you can go through it, tick it all off and then just hand it in to your GP when you go. Yeah that’s a good idea that actually . . . Yeah, yeah, that’d be better that, fill it in at your leisure and then when you go down there you can just hand it in. And then they’d know what, rather than trying to remember what you were going to say when you get there . . . it’s a good idea. But, whether the GPs would like it you don’t know, they should do, they can just, alright then, write you a prescription and give it you and you’re away aren’t you?
ID 43309, female, index condition COPD
The perceived usefulness of the PRISMS tool by patients was influenced by the participants’ relationship with their GP and the expected response. One participant who had used the PRISMS form during a primary care consultation reported that this had enabled her to raise ongoing health problems that were not condition specific (i.e. increasing tiredness), and to be referred to appropriate services (Box 2). Use of the PRISMS tool was considered by respondents with reference to previous consultations and encounters with their GP or practice nurse. Participants’ perceived relationship with their GP ranged from good to poor. When participants reported a good relationship with their GP, they described being able to raise concerns easily. However, for the majority of participants, their relationship ranged from ambivalent to negative and this seemingly influenced the perceived acceptability of introducing the PRISMS form. In addition, some participants who considered their conditions to be stable did not feel that the PRISMS tool was applicable to them at that time but might be in the future. This points to the need for tools to be sensitive to patient trajectories and changing needs and circumstances.
The PRISMS form. Do you think that’s useful?
Not to me.
How come? . . .
Most of this is not applicable . . . Maybe because of my age, maybe because it’s diet controlled diabetes . . . It might be more applicable later on.
ID 12188, index condition: diabetes, male
Lyn was a participant in the longitudinal study who had used the PRISMS form. Over the course of the year-long study her health had deteriorated, indicated to her by the number of medications she was taking (seven tablets daily at the outset and 14 tablets daily at the time to the final interview). The way in which she had framed her condition and her well-being had changed for the better. In the final interview, as a result of a number of infections, Lyn’s health had deteriorated. However, she described increasing family support and the valued role of the GP in referring her to a nutritionist. The nutritionist was perceived to have improved her quality of life through dietary changes and the respondent reported being more engaged with managing her conditions, although she did not see any objective improvements in her health. In the final interview the participant recalled using the PRISMS tool, which had reportedly helped her to address ongoing generic health problems, such as increased tiredness, a state which had become normalised over several years. The introduction of this form coincided with the participant being so tired and lacking energy that she believed she was going to die and this critical realisation acted as a tipping point to seeking help.
. . . I filled in what I could . . . like sleep problems, well I always have, I’ve always had big sleep problems . . . support from the family, yeah I get, I get plenty of support . . .
And for the things that it was a problem did this help you sort of start talk, bringing up things with the doctor?
Yeah this is when I said to him I’m going to get myself motivated . . . get myself cracking, I don’t, I, I kept saying I’ll die in the chair if I carry on that cause that’s what I’m saying.
So this sort of helped . . .
Yeah because that’s what he said, that’s what he’s doin’ tests for cause this tiredness but it can be due to the cal-, the calories that I’m burning away . . . that makes you, that can make you tired and all.
Oh OK so this sort of prompted that discussion.
Yeah.
COPD and IBS, Female, age 57
The PRISMS form acted as an initial prompt to the discussion with her GP about how these general symptoms of tiredness were restricting her quality of life. The recommendations made by the GP and nutritionist were then maintained by the ongoing presence of family support, which reinforced personal motivation and engagement with management practices, which in turn continued to improve her sense of well-being and reduction in her symptoms.
The Salford online self-care support guide
None of the participants reported using the self-care guide or being referred to it by their GP or practice nurse.
Condition guidebooks
Participants in the longitudinal study had not received the guidebooks; however, five participants provided feedback on the content in the final interview (Box 3). The majority of participants in the process evaluation of the main trial did not recollect being sent the guidebooks through the post or being given them during a consultation. However, in dialogue with the interviewer, the following participant had received the guidebook and found it useful.
Yeah I sat down and read them. I did sit down, that one [guidebook], that yeah. Yeah you read them and if there’s anything that catches the eye, what I’ll do, yeah I do take notice of what people say and what advice . . . Yeah, they’re good. They are, they are useful.
ID 43309, female, index condition COPD
A very useful book to be given when first diagnosed as it gives other people’s experiences, which may be the same as your own. Also helpful as you can see how other people manage and control the condition and also how some people are not able to do so.
The second chapter is particularly useful as a lot of information in it is not always explained and gone in to in any detail when the condition is being discussed with health-care workers.
Participant ID 110a (index condition: IBS)
I read all of this book and found it really easy to read and understand.
Very informative.
If you are a new patient to IBS you will find this a valuable book so you can refer to it anytime you want to, or maybe you forget sometimes what is inside.
A great book for everybody with problems no matter what you are, male or female.
Participant ID 100a (index condition: diabetes)
I found the guidebook very helpful and I seem to have learned a lot from it. I am sticking more to a healthy diet and trying to do more exercise. I shall keep reading it because it’s useful and interesting.
Participant ID 500s (index condition: COPD)
It is very thorough but rather ‘wordy’ and does rather labour the point about stopping smoking – which does not apply to all patients with these problems.
Stepped-up care
The stepped-up care option was available to IBS participants in the process evaluation only (n = 10) and none had been referred to stepped-up care.
Patient expectations of self-management support
Patients’ expectations of help with self-management were shaped by previous experiences with their GP and expectations of support available. There were generally low expectations of support based on poor previous experiences, and difficult and unhelpful relationships.
. . . but when your doctor says something that really doesn’t help, you know, and I have had her say ‘well you just need to control what you put in your bloody mouth’.
ID 62153, female, diabetes patient
Such events resulted in people avoiding going to their GPs because of their low opinions of the help they would get.
. . . she’s no good as a doctor basically . . . I’d rather suffer than have to go and see her. I only go if I really need to go.
ID 63325, female, IBS patient
The restrictions imposed by the way in which primary care is organised militates against meaningful and supportive discussions and adds to the lack of confidence in obtaining support.
Well, first time I went to her was Thursday morning and apparently you’ve got 10 minutes, but it’s like 2 weeks to get in to see her. So I had a little list, because my memory’s not very good with this fibromyalgia. Sometimes I’m alright, other times I can’t remember things. And I wanted advice on my IBS, seeing if she could come up with something different while I was in the process of suffering as well as this fibromyalgia, as well as menopause, things like that, but she said right, start with the most important, and then when my 10 minutes was up she said would you like to make another appointment please. I thought right, I’ve got another 2 weeks to wait. So I’m not pleased. Not when the person in front of me was in more than 10 minutes.
ID 33304, female, IBS patient
In order to get support that they felt was appropriate, patients often had to work hard to persuade the doctor and the build-up of negative experiences contributed to the low expectations of help.
I had to go to [Town] to a pharmacy that does the programme there . . . you can only purchase it through a pharmacy. And they weigh you every week, they advise you, but they will only let you do the programme with the agreement of the doctor. So they approached my doctor, she was reluctant to do it at first, and it took a month to get her agreement for me to do it.
ID 62153, female, diabetes patient
The examples of positive support occurred when existing relationships were good and when the doctor or nurse was perceived to have genuinely listened; this appeared most likely in nurse consultations.
I usually ask if I can make an appointment with the nurse, and obviously they ask me why. She’s very good. The nurse at my GP centre is very good. They will sit and listen. Listening makes a big difference, while a doctor’s looking at you and looking right through you. You’re just sat there and you’re making me money.
ID 61015, female, COPD patient
Self-management in patients with multiple conditions
One of the objectives of the longitudinal study was to examine self-management priorities for individuals with multiple long-term conditions over time. 78 The study demonstrated the impact of multiple conditions on many aspects of people’s illness management and highlighted the complexities of self-management. Narratives illuminated how individuals’ priorities changed at pivotal points and altered their engagement with self-management practices. This was influenced by contact with health professionals and how people framed illness and lifestyle changes. Medication management was a central point in which individuals took control of their conditions.
Managing additional conditions
Additional conditions were more readily accommodated if people established cognitive links between existing management practices. Thus, multiple conditions were not inevitably experienced as an increasing burden but subject to considerable flux and change. Prioritising one condition over another at a particular time together with a transfer and amalgamation of practices appears to facilitate accommodation of multiple conditions. Clinicians might usefully engage with patients’ understanding to reduce complexity, and enhance engagement of condition management.
However, patients who developed additional long-term conditions could experience that as disruptive.
I don’t know whether it’s I am carrying too much weight or I’m having too much sugar . . . but I’m trying to find out . . . and all I get from [GP], it is your diabetics, they give it a different name all together, I forget, I can’t get my bloody tongue round it and yeah, that’s one main thing and the other is, I have had my eyes done cataract done and that, that were all right but um, it’s just niggly.
ID 0900 (diabetes, knee problems and kidney problems, male, age unknown)
I have, yeah just get on with it. It’s a chronic condition, there’s not a lot I can do about it other than, except I’d like to take more exercise, walking, but at the moment as I say because of my feet problems I can’t, so it’s a double whammy as they say.
ID 110s (COPD, oesophageal problems, feet problems, male, age 69 years)
Conversely, accommodation of further conditions could occur as there was a flow of existing management practices reducing the impact of further conditions on the daily management.
I have to sort of eat regularly for the epilepsy, eat regularly, not get over tired, not drink too much alcohol . . . don’t skip meals, so that is what I do for me epilepsy anyway, so that’s what helps with the diabetes as well.
ID170a (diabetes and epilepsy, female, age 55 years)
Practices that learned and adapted from the management of one condition allowed some respondents to accommodate new conditions if new routines were congruent with existing routines. This contrasts with a view that multimorbidity by definition requires more complex clinical management accompanied by a new set of practices for each ‘new’ condition. Of greatest salience to our respondents was a process of continuation and flow of actions, reactions and self-preservation of strategies that are deemed to work for one condition applied to another.
Well she talked about diet and um, yes, really it was diet really you know, just be careful what I eat.
And has that been a big change?
No not really ‘cos I was already on quite a healthy diet . . . You know, before that I’d um, I’d decided to lose a bit of weight if I could because with having MS it’s better not to carry a lot of weight with it.
ID 100a (diabetes, multiple sclerosis, underactive thyroid and high cholesterol, female, age 66 years)
The dynamic prioritisation of conditions
Shifting prioritisation between conditions was key to the way management was framed and the process of prioritisation over time required renegotiation of available resources. Although for a few participants there was little description of change where one condition remained routinely prioritised, the majority of descriptions were of changing prioritisation of conditions.
But it’s hard (to lose weight) I don’t know. So I put it down me personally to the tablets, whether that’s putting weight on I don’t know . . . just watching what I eat now you know with diabetes really more than anything like . . . But this breathing, this COPD that’s what will affect me in another few weeks when it starts getting cold; it’s the cold that affects me.
ID 600s (diabetes, COPD, high blood pressure, high cholesterol, male, age 65 years)
Interactions with health professionals
Most respondents described a dynamic and variable process of self-management with little separation between conditions. Respondents’ accounts of management practices were viewed across conditions with links in practices and tensions that required active choices between options. This suggests a difference between health professional accounts and patient approaches to multimorbidity, given that participants talked about seeking information and management strategies across conditions and were given little or no information.
The degree of congruence or conflict between views of health professionals and patients regarding management priorities impacted on subsequent management practices and therapeutic relationships. For participants who described similar management goals to their health professionals, contact reinforced and maintained priorities.
What’s kind of, your main priority at the moment with [GP]? . . .
Probably blood pressure, probably and cholesterol . . . so I’m more worried about those because they are more serious things. IBS didn’t kill anybody, you know, but blood pressure is serious and cholesterol is serious so IBS has gone into the background, you know.
ID 110a (IBS and high blood pressure, female, age 57 years)
However, for participants whose illness management priorities conflicted with health professionals, established relationships were challenged. One participant with diabetes and chronic depression described being ‘upset’ and ‘angry’ when her GP told her that she ‘just had to have willpower’ to control her diet for diabetes. However, the respondent’s priority was depression, which she recognised affected her diabetes control, and it was something she had actively tried to change over a number of years but was a complex problem. When she tried to obtain information about managing both conditions ‘they couldn’t offer any help’. The GP’s response conflicted with her priorities and had a negative impact on what she would engage with in managing her health.
I mean, I’ve got to say, when the doctor, when the GP said it to me, I actually came home and felt, er, why should I stick to this diet, because I was depressed I felt, I’ve nothing to live for anyway, the diabetes will kill me anyway.
ID 300s (diabetes, chronic depression, female, age 50 years)
Tipping points
To manage the complexity of multiple conditions, most participants reacted to changes in a process of prioritisation and reprioritisation over time. This involved an interaction of factors such as timing between diagnoses, prior experiences, recommended self-management activities, bereavement, contact with health services and flare-up of conditions.
. . . there’s been a lot of things going on, because I started with epilepsy in 2000 and it’s like I was, I had like a cluster and then they settled down . . . and then out of the blue I started with another cluster, but I think it’s hormonal because that started up, I started with diabetes and blood pressure you know, it seemed just, and I had gone through a period of depression because of [losing] my dad.
ID 170a (diabetes, epilepsy, female, age 55 years)
Prioritisation and reprioritisation occurred around key transition points when the relative importance of different management practices shifted, having either negative or positive effects. These ‘tipping points’ particularly arose around issues of medication management, lifestyle changes and understanding of conditions.
Medication management
Participants’ accounts of medications were framed in one of two ways: as an external event that was ‘being done to them’ and over which they had little control or, conversely, as an internal process whereby they gained control of their illness management by establishing routines for taking tablets and personalising their medication management. For participants whose narratives emphasised taking control, the presence of multiple long-term conditions played a minor role in everyday life.
I am quite vigilant about taking my medication um, and if I’ve missed it I do know that I do start feeling tired um, so I am quite compliant with me medication, but I have to take my epilepsy medication anyway so yeah, I take me medication morning and take my medication at night, like I say I am quite compliant about that.
ID 170a (diabetes and epilepsy, female, age 55 years)
However, other accounts of medicines portray a sense of an ever-increasing burden of tablets and resistance to further tablets. These accounts depicted medicines as objects and a process which they are subject to but have little direction over.
. . . for different ailments and that like and I’ve got bags of tablets there and now I’ve got that many tablets and I don’t know you know, swapping and changing tablets, getting tablets from the hospital and I don’t know where I’m up to with them like, you know.
ID 600s (diabetes, high blood pressure, high cholesterol, male, age 65 years)
Medications were also a point through which change could be negotiated. Some respondents described seeking additional sources of information and verification around medicines to those provided by GPs or specialists, as they did not feel confident that the latter were aware of all medicines being taken or their interactions. The main sources of verification were from the internet, pharmacist and others with similar conditions. These acted as legitimation points through which participants could change their routines as they were able to engage in focused discussions with health professionals to take control and direct illness management. Narratives depicted a change to becoming active self-managers and taking control by seeking out enough information to enable them to make decisions about medications.
I asked the pharmacy yesterday, I rang them up, ‘cos I’m taking so many different drugs, you’ve gotta be careful. And they were saying, I was saying could you take them at the same time, ‘cos you’re supposed to take these with food but you can’t take ‘em all with food . . . there’s one or two more, but . . . you’ve gotta keep a track.
ID 0700 (diabetes, rheumatoid arthritis, high blood pressure, male, age 82 years)
I take a lot of tablets now, every time I go and see him, ‘Don’t give me another tablet.’ . . . He tries me on different things and what you know doesn’t agree with me, he doesn’t give me . . ., he knows that, you know what I mean. Same as painkillers, I won’t take painkillers, I only take paracetamol.
ID 120a (COPD and IBS, female, age 57 years)
Information use in patient self-management
Existing knowledge and direct experience of conditions from other sources played a role in illness prioritisation and interpretation of information. Information was often viewed as contradictory or too general with insufficient information about interactions across conditions. Participants described integrating information from a number of sources. This led respondents either to feel confused or to actively monitor the suitability of information provided by practitioners. Information and advice had to be reinterpreted and verified for it to be useable and allow development of pragmatic routines.
Again, if you start to research it you come across dietary and nutrition, so that one kills all the sugars off and that kills all the fat off and the two of them just clash all the way down . . . You end up with you would be eating absolutely nothing except fruit and the odd slice of bread.
ID 150a (diabetes, high cholesterol, male, age 52 years)
Exercise as part of self-management
Exercise was considered important but also unrealistic because of functional limitations. When conditions placed a physical limitation on an individual, this was often talked about as having the greatest impact on their lives. It caused a tension between which self-management activities they should prioritise and restricted daily activities. Restrictions could accumulate and produce further problems, which influenced what could be attempted and the prioritisation of management practices.
Breathing. Um, only that I can’t do the things I used to, but whether that’s breathing or whether that’s old age I’m not sure. In truth, I suppose I used to walk up to [town centre], now I get a taxi . . . Or somebody takes me, but 70% of that is because of my feet, maybe 30% [because of COPD]. If my feet were fine, I’d still walk up to [town].
ID110s (COPD, oesophageal problems and feet problems, male, age 69 years)
Food and diet as part of self-management support
Dietary information was described as conflicting by many respondents and was not necessarily translatable across conditions, resulting in the accommodation of new practices within pre-existing regimes. Advice was often considered to be too generic and insufficiently individualised. Health-care professionals were not seen as always addressing the impact of other conditions and respondents were left to navigate through conflicting information to find the most appropriate course of action or seek further information.
Well she [practice nurse] gave me, um, information on diet which you could read in any woman’s magazine, there was nothing specific. I did say to her that my problem was binge eating and she had nothing to offer at all. She could not comment you know she knew nothing about it, so she wasn’t able to help. So as I say it was a complete and utter waste of time.
ID 300s (diabetes and chronic depression, female, age 50 years)
Social networks and self-management support
Interviews conducted as part of the longitudinal study with patients also revealed something of the wider relationships influencing self-management support. Analysis reinforced evidence that who is in the network, and the types of relationships that are present, influence how management practices are framed and the extent of engagement. 79 Resources available to an individual through the network can support or undermine engagement, and these change over time. Networks included family, friends, GP, nurses and companion animals. The amalgamation of different relationships that constituted the social networks were characterised by three network typologies. The three types of networks are characterised by differing combinations of features that influence priorities for health, the various roles that network members occupy and the degree to which the network might support and facilitate the normalisation of illness management into everyday life. Networks can be categorised as a family-focused network, a friend-focused network or a health-care professional-focused network.
In the family-focused network, the main source of support and information is provided by multiple family members with various roles in supporting the individual. There are usually multiple ties between network members. For networks that can be defined as family-focused networks, health-care professionals are viewed as important but are not so regularly seen. In a friend-focused network, there is greater variance in the types of relationships present; friends appear to have a more significant role for the individual than members of either the family-focused network or the health-care professional-focused network. For younger participants with a friend-focused network, parents and partners are also important, yet their role is subject to change (e.g. becoming more restricted because of specific events which have altered their relationship). For the participants whose social networks are characterised as friend focused and who are older, family members are less relevant. For these first two types of networks, management is normalised into existing practices and routines through negotiation and discussion with other network members, but, despite multiple conditions, participants in this study identified only a single GP or nurse as significant. The networks described in these first two categories frame health management within existing dimensions of everyday life that are not separate but integrated as routines and practices in daily life. The friend-focused network seems to emerge when there is an absence or loss of adequate family support and alternative lay support is sought instead.
The third type of network, the health-care professional-focused network, represents a network in which health is considered to be something managed more separately from the wider social network. Health and its management are framed as private, which can be a burden to others, and so seeking support is avoided, or limited as much as possible.
Discussion
In the practices, self-management tools failed to be normalised in routine care, apart from handing out the guidebooks. This may explain the lack of change experienced by patients. The WISE model was intended to encourage reassessment of work practices while introducing new elements that fitted with existing work and improved patient care. The long-term condition management work delegated to nurses was a routinised process of monitoring and recording necessary for QOF. Practice nurses viewed themselves as being patient centred and holistic, yet psychosocial and behaviour change support is not generally incorporated, with our respondents reporting use of didactic information giving. Nurses had concerns about the burden of providing enhanced self-management support in terms both of their own workloads and what they felt their patients could accommodate. Provision of the guidebooks was the one element that could be considered minimally disruptive work, fulfilling their need to provide good information while enhancing their ability to be patient centred.
The challenges of changing professional behaviour and attitudes in order to implement self-management support are widely reported. 73 The WISE model approach aimed to pragmatically address existing evidence and recommendations, and provided tools and training in skills to assist self-management support in the context of an organisation geared up to provide appropriate resources. Using NPT allowed us to focus on the everyday work of nurses and to explain why the WISE model did not embed: self-management support was not a practice priority as it was not part of QOF; it was not seen as different enough to existing work to value yet considered too disruptive and time-consuming; and there was lack of communication or support within the practice.
In showing reluctance to engage in behavioural change discussions with patients, nurses demonstrated an awareness that, for patients, self-management involves a complex, embodied, practical knowledge that clashes with the abstract, rationalised models assumed both in biomedical approaches to long-term condition management and in programmes such as the Expert Patients Programme. The claim by nurses to integrate patients’ lived experience and priorities into clinical encounters is not new, but for the most part this is treated as an addition or as something to fit into the tasks of monitoring and testing in a way which represents only marginal movement towards patient needs and knowledge concerning self-management support. In this respect, nurses were not able to deploy the principles of WISE that fitted with patients’ agendas, needs or experience of managing a condition in their daily lives. Rather, they could not adopt and embed a new system at odds with their protocol-based system, which ensures the financial income of the practices which employ them.
The rare occasions when aspects of self-management support were incorporated involved nurses experienced and confident enough to disrupt the prevailing system. Such nurses were prepared to overcome the hidden nature of self-management support and the lack of recognition for providing it. In other words, those individuals were willing to try different approaches and able to reflect on the benefits they saw for their patients. Even so, they were not able to report moving beyond opening up the consultation to address patient priorities – lifestyle change is not readily doable in primary care and falls more within the day-to-day world of the patient.
Chapter 7 Project 2: identification and risk assessment
Some patients with IBS have symptoms that are difficult to control and suffer considerable ill health, whereas other patients have symptoms that are well controlled and they cope well. Approximately 30% of patients who consult their GP with FGIDs go on to develop chronic symptoms. 80 Previous studies have shown that an intervention with self-management tools reduces distressing symptoms for hospital outpatients, consequently reducing health-care utilisation. Provision of a self-help guidebook reduced primary care consultations for IBS by 60%. 15
The medical management of functional lower gastrointestinal symptoms usually involves antispasmodics, antidiarrhoeals or laxatives, as appropriate, and antidepressants, particularly low-dose tricyclic antidepressants. Unfortunately, for some patients medical management yields far from satisfactory results. For patients with intractable symptoms, which are resistant to conventional medical therapy, current guidelines recommend referral for psychological intervention to CBT or hypnotherapy. 81
It has been shown that within primary care it might be possible to predict those patients at risk of having persistent symptoms: 87% of patients exposed to a small number of factors at the time of initial consultation were found to have persistent gastrointestinal symptoms at 6 months. 82 It may be possible to use a screening tool to identify patients at risk of persistent symptoms. 82
This study aimed to test this finding prospectively on a larger scale. If this finding was validated it might be possible to identify those patients at risk of poor outcomes and fast-track them to therapies that are known to be effective, such as CBT or hypnotherapy. This should lead to less distress and lower health-care utilisation for these patients in the long run.
One of the difficulties encountered in previous work on IBS within primary care is the identification of patients. GPs use a variety of Read Codes to describe patients presenting with IBS and, to our knowledge, the range of Read Codes used has not previously been investigated. This would enable future studies to evaluate the ‘true’ burden of IBS in primary care. The work on the use of Read Codes used by GPs to describe functional lower gastrointestinal symptoms is supplemented further by conducting qualitative face-to-face semistructured interviews with GPs to explore their attitudes and approaches to diagnosis and management of functional gastrointestinal symptoms.
Project 2 therefore comprised the following four separate studies.
-
The first study consisted of a GP database study (GP database study 1) to describe how clinicians in primary care record consultations with patients who experience functional lower gastrointestinal symptoms.
To meet this aim we used a computational mapping approach using data from NorthWest EHealth, a health data repository.
-
The second study was a risk assessment study. The aim of the study was to validate a risk assessment tool for predicting symptom distress for IBS.
We used a 12-month prospective case–cohort study in which patients consulting their GP with IBS were recruited prospectively in order to validate a risk assessment questionnaire.
-
The third study was a qualitative study to explore GPs’ views and experiences of defining, diagnosing and managing of functional lower gastrointestinal symptoms in primary care.
-
The fourth study was a further GP database study (GP database study 2) to investigate patient profiles in IBS, IBD and AP, and to explore whether or not GPs follow NICE guidelines81 for patients with IBS.
General practitioner database study (study 1)
Consultations in general practice are recorded and coded using a clinical diagnostic coding system, known as Read Codes. Consultations tend to be coded either by symptom presentation or by problem title, and the current computerised systems for clinical coding seem to promote diversity rather than consistency. 83 GPs tend to make a positive diagnosis of IBS when the risk profile of the patient for that condition is high (and their characteristics fit the profile for functional disease) and the risk of serious bowel disease is low. 84 This approach is recommended in current NICE guidelines81 to help practitioners identify patients with IBS more easily and make a ‘positive diagnosis’ for the condition. This method is distinct from applying diagnostic criteria to the patient’s symptoms, such as Rome III criteria,85 to define IBS. Thus, there might be considerable variation in the way that consultations for patients with IBS are coded. This might be compounded by the fact that IBS is not one of the chronic conditions contained in the QOF – a system of paying GPs for achieving clinical targets. Variations in coding practice, and exclusion of IBS from the QOF, may militate against quickly and reliably identifying IBS patients who might benefit from therapeutic interventions.
Aim
The aim of GP database study 1 was to determine the extent to which there was a recognisable and reliable phenotype for IBS patients presenting to general practice. This was deemed important so that we could identify a range of Read Codes used by GPs serving the study population to denote patients with IBS.
Methods
Setting
The setting for this study was the city of Salford, in north-west England, with an estimated population of 228,992 in 2010.
Sampling frame
The sampling frame comprised all patients registered with the 52 general practices in Salford PCT.
Data collection and coding
The Salford Integrated Record (SIR) is a local patient-sharing record system that integrates primary care, community care and secondary care information into one continuous electronic health record per patient. An anonymised data set is made available for research through NorthWest EHealth. Patients may opt out of the SIR; however, the proportion who do so is relatively small.
We obtained electronic clinical records from the SIR for January 2003 to December 2009. The data set comprises anonymised patient identifiers, anonymised general practice identifiers and the set of Read Codes used to code clinical consultations. In total, the data set contained > 136 million Read Codes derived from 34,200 distinct codes. Two gastroenterologists independently identified all Read Codes potentially relating to gastrointestinal disorders or IBS.
Ethics permission for this study was granted by the NorthWest EHealth Board.
Analysis
The analysis was conducted in two parts. First, we conducted analyses of patients with gastrointestinal symptoms. Second, we refined this analysis to consider only those patients with IBS.
Gastrointestinal symptom space
First, we selected patients with evidence of at least one Read Code for gastrointestinal disorders in 2009, which generated 19,869 patient records. The records for these patients were then searched for those with at least one diagnosis of IBS (Read Code J521) at any time since 2003. For each of these patients we collected together all the Read Codes from their records in general practice in 2009. Each patient was therefore represented by their set of Read Codes from 2009. To investigate the importance of gastrointestinal symptoms we then generated a second data set, which included only the Read Codes for gastrointestinal symptoms for these patients.
These data sets were then mapped onto a two-dimensional plane using a methodology that calculates the Resnik similarity between the concepts. It then maps these data from similarity space to the appropriate vector space and uses principal components analysis (PCA) to represent the data on a plane.
Each patient was therefore represented as a point on the plane and patients with similar symptoms occupied similar positions on the plot. These plots were then presented according to particular sets of Read Codes contained within the records.
The total data set available for analysis in diagnosis space was too large to make an analysis computationally tractable. We therefore examined 16,584 patient records from the three GP surgeries with the highest number of patient records with a diagnosis of IBS. All diagnosis codes for all these patients were collected and projected into diagnosis space using the same computational strategies discussed above.
Results
Representation of patient records in gastrointestinal symptom space
Patient records were represented in the space of gastrointestinal symptoms. Figure 7 shows the results obtained by taking just the gastrointestinal symptoms from the 2009 Salford data set, and performing the semantic similarity analysis and PCA for dimensionality reduction.
FIGURE 7.
Principal component analysis plot of gastrointestinal symptom data. Each dot represents a particular set of patient data from 2009. These were then coloured according to the types of code found within each set of records, as indicated in the figure legend. GIT, gastrointestinal tract.

The gastrointestinal symptoms recorded by GPs were separated on the basis of two predominant symptoms, diarrhoea and gastrointestinal tract (GIT) pain. Patient records marked in light blue do not have any record of diarrhoea or GIT pain. The region highlighted in light green represents the patient records that are positive in recording of diarrhoea symptoms and negative GIT pain. Therefore, the largest source of variation within the data set concerns diarrhoea and GIT pain. Furthermore, patients with particular sets of symptoms occupy particular regions of gastrointestinal diagnosis space.
We considered next how other symptoms are distributed in symptom space. In Figure 8 we present several common gastrointestinal problems in this space: heartburn, flatulence, abdominal distension and indigestion. These symptoms are scattered across most of the diagnosis space without being clustered in any particular area.
FIGURE 8.
Common gastrointestinal problems mapped into symptom space. Shown in green are patients who have been given a diagnosis of IBS. (a) Heartburn; (b) flatulence/wind; (c) abdominal distension symptom; and (d) indigestion.

Representation of irritable bowel syndrome patient records in gastrointestinal symptom space
Figures 7 and 8 provided some insights into symptom space and the ways in which the patients are distributed. Next, we examined whether or not there was any clustering of patients diagnosed with IBS in this space by looking for the IBS diagnosis code in all patient records from 2003 to 2009 and highlighted whether or not the IBS code was present. These records were then mapped onto gastrointestinal symptom space from 2009, as previously calculated (Figure 9).
FIGURE 9.
A representation of patients in gastrointestinal symptom space.
The IBS patients were scattered across all of symptom space. There was no evidence that IBS patients cluster in any part of this space.
Irritable bowel syndrome patients in diagnosis space
Figure 10 shows the results of the PCA applied to all the diagnosis data from all the patients from the three general practices in Salford that had the most patients with an IBS diagnosis. Patient records were classified in different regions of vector space in terms of three different groups of diseases: ‘nervous system and sense organ diseases’; ‘genitourinary system diseases’; and ‘infectious and parasitic diseases’. The light-green area belongs to the patients who were recorded to have ‘infectious and parasitic diseases’ but not ‘nervous system and sense organ diseases’, or ‘genitourinary system diseases’. The light-blue area encompasses those patients who have none of the diseases. The area in black represents the patients who were recorded to have ‘nervous system and sense organ diseases’ but not ‘genitourinary system diseases’. The dark-green region contains the patients who were recorded as having ‘genitourinary system diseases’ but not ‘nervous system and sense organ diseases’. Finally, the dark-blue area belongs to patients who were recorded as having both ‘nervous system and sense organ diseases’ and ‘genitourinary system diseases’. The various diagnoses do cluster very effectively in the plot.
FIGURE 10.
Classification of patient records in terms of three different criteria (F%, K%, A%). F% refers to the patients who have any Read Code describing ‘nervous system and sense organ diseases’ in their records; K% presents the patients who have any Read Code describing ‘genitourinary system diseases’ in their records; and A% indicates patients who has any Read Code encoding the ‘infectious and parasitic diseases’ in their records.

Figure 11 Shows this same data, but this time with patients labelled either by the presence of a gastrointestinal symptom code in their data (see Figure 11a), or by a diagnosis of IBS (see Figure 11b).
FIGURE 11.
A representation of all diagnosis information collected in 2009 for patients from three GP surgeries in Salford. (a) any patients showing gastrointestinal symptoms are shown in green and (b) patients with a diagnosis of IBS are shown in green.

Comparing Figures 10 and 11 provides an insight into the areas of diagnosis space in which patients with gastrointestinal symptoms and a diagnosis of IBS fall. It is clear that gastrointestinal symptoms are found in patients across all of this space. The picture for patients with IBS is more informative. There is a cluster of IBS patients in the area associated with infectious disease, another in the area associated with nervous system and sense organ disease and a third area clustering in genitourinary system disease.
Discussion
In this study we attempted a novel analysis of data regarding the diagnosis and description of patients with IBS from data coded in GP electronic patient records from Salford. Using this approach we were unable to reliably identify a phenotype for IBS patients in primary care.
A major advantage of the SIR is the complete linkage between primary care, community care and secondary care data sets, providing a continuous electronic record for each patient in contact with health services in Salford. However, there were certain limitations in using these data to try to identify patients with IBS in our study.
First, a variety of computerised systems are available for capturing clinical consultation data in primary care. Furthermore, there are several different versions of the various operating systems in use so that there might be slight variations at computer operating system level that would have impeded our research.
Second, the Read Codes used to code a consultation are at the discretion of the individual clinician, which means that there can be considerable variation in their use to describe the same set of symptoms in practice. However, research requires a disciplined approach to data entry and retrieval,86 so that inconsistency in coding potentially presents an important source of information bias.
A further challenge was validating the data set. Although two clinicians generated the list of Read Codes used in the study, independently of each other, we were unable to validate the GP diagnoses. Ideally we would have created a cohort of IBS patients from SIR and validated the diagnosis by asking their GPs,87 but this was not possible as the SIR for research is anonymised at both the patient and GP level. Our analysis of IBS patients in diagnosis space was confined to data from three practices in Salford. This was because of limitations in computational power. However, it is possible that the diagnostic coding behaviour of GPs in those practices with more IBS patients was not typical of the diagnostic coding behaviour of other GPs in Salford. Finally, there are idiosyncrasies in the Read Code taxonomy, in particular the fact that codes for a lack of existence of a symptom can occur as leaf nodes describing that symptom. This type of structure in the codes breaks the semantic relationship between ‘parent’ and ‘child’ nodes in the taxonomy; a ‘child’ does not inherit properties from the parent. However, it seems that GPs might be reluctant to document patients using such negated child node Read Codes as none was found in the data set we used.
Despite these limitations, our analyses generated some useful insights. We first looked at symptoms associated with IBS to investigate whether or not there were any particular gastrointestinal symptom data that could be used to identify IBS patients. However, there was no such clustering of IBS symptoms – the symptoms associated with patient records containing a diagnosis of IBS were distributed throughout this space. This accords with evidence that approximately two-thirds of the symptoms presented by patients in primary care remain unexplained. 88 It is clear from the scattering pattern of IBS in the gastrointestinal symptom space that these symptoms are not unique to, or characteristic of, IBS and probably reflect the relatively subjective criteria used for establishing an IBS diagnosis. Therefore, it would be difficult to find any linear combination of gastrointestinal symptoms as recorded in the GP data in Salford that could be used to reliably identify IBS patients. Cases with diagnosis of any kind of digestive system diseases showed a similarly fragmented pattern.
We looked next in diagnosis space to determine if IBS patients clustered in any way in this space. Here the findings were less clear cut. There was no unique cluster of IBS patients. However, this is unsurprising, as patients with IBS are known to display a range of other symptomatologies. There were concentrations of IBS patients associated with infectious disease, genitourinary problems and nervous system disease. The links with infectious disease and nervous system disease have been highlighted previously,89 and it is reassuring that we found these associations using this novel methodology. There is less literature about a link between IBS and genitourinary disease. However, this work suggests that this is an area that might be worth exploring further.
In epidemiological studies, case definitions are paramount for describing patients for inclusion in a study systematically. Our analyses of data from the SIR, in particular our inability to define an IBS phenotype, suggest that it is not yet possible to develop case definitions for primary care-based research studies of IBS patients using Read Codes. To do so would require a more rigorous, standardised approach to implementing Read diagnostic coding in primary care.
Risk assessment study (study 2)
It has been reported that over half of patients with IBS have consulted their GP within the past 6 months. 90 A significant proportion (approximately 30%) of patients consulting their GP with FGID go on to develop chronic symptoms. 80 However, it might be possible to predict those at risk of having persistent symptoms in patients with gastrointestinal symptoms in primary care. 82
A previous study found that 87% of patients exposed to a small number of factors at the time of initial consultation had persistent gastrointestinal symptoms at 6 months. These factors included higher levels of psychological distress, no change in diet, symptom duration, no recent gastroenteritis and interrupted activities as a result of bowel problems. Consequently, it may be possible to use a simple screening tool to identify patients at risk of persistent symptoms in the future. 82
The current study aimed to test this finding prospectively in a larger-scale study. If the finding was replicated it might be possible to stratify patients into those at low and high risk of persistent symptoms and to fast-track those at risk of doing worse to therapies that are known to be effective, such as CBT or hypnotherapy. In turn, this should lead to less distress and lower health-care utilisation for these patients in the longer term.
Aim
The aim of this study was to test the validity of the risk assessment questionnaire for predicting symptom distress in patients with IBS.
Methods
Design
The risk assessment study was a 12-month prospective case–cohort study in which patients consulting their GP with newly identified (incident) or existing (prevalent) IBS were recruited prospectively in order to validate the risk assessment questionnaire.
Setting
Initially we approached those practices that had a service-level agreement with the Greater Manchester Comprehensive Local Research Network. We then took a staged approach to roll the study out to general practices within Greater Manchester.
Study population
Patients consulting their GP for existing (prevalent) or new episodes (incident) of IBS.
Sample size calculation
The sample size calculation was based on separate samples of newly diagnosed and prevalent (i.e. existing) patients. Assuming an intracluster correlation coefficient of 0.05, 320 patients would be required to provide a 95% CI of ± 10 points or less for true sensitivity of ≥ 70%. The CI for an equivalent level of specificity is considerably smaller (± 6%). Assuming a dropout rate of approximately 25%, 420 newly diagnosed and 420 prevalent (i.e. existing) participants would be required.
Recruitment method: via consultation
General practitioners were asked to invite potential participants to take part in the study during their consultation for IBS and forwarded a permission to contact slip for those participants who expressed an interest in taking part to the study team.
During the consultation, GPs were asked to recruit patients with new or prevalent cases using the following definitions.
-
New diagnoses of functional lower gastrointestinal symptoms were defined clinically as a patient reporting symptoms for at least 3 months but less than 1 year by using Read Codes for:
-
IBS
-
bloating
-
AP
-
diarrhoea
-
constipation
-
change in bowel habit.
-
-
Prevalent episodes were defined clinically as patients with an onset of functional lower gastrointestinal symptoms/IBS more than 1 year ago for:
-
IBS
-
bloating
-
AP
-
diarrhoea
-
constipation
-
change in bowel habit.
-
In addition, patients had to meet the following inclusion criteria:
-
adults aged > 18 years
-
must have consulted the health-care team in the past 3 months for functional lower gastrointestinal symptoms/IBS as defined at (1) and (2) above.
Exclusion criteria
The following are exclusion criteria for both new and prevalent episodes:
-
patients with a terminal illness
-
patients who do not speak English and for whom a suitable interpreter is not available
-
patients with severe mental incapacity
-
patients with known chronic gastrointestinal disorders:
-
IBD (Crohn’s disease and ulcerative colitis)
-
coeliac disease
-
cystic fibrosis
-
surgical obstruction
-
-
patients with NICE ‘red flag’ indicators:
-
unintentional and unexplained weight loss
-
rectal bleeding
-
a family history of bowel or ovarian cancer
-
a change in bowel habit to looser and/or more frequent stools persisting for > 6 weeks in a person aged > 60 years
-
anaemia
-
abdominal masses
-
rectal masses
-
raised inflammatory markers.
-
Recruitment method: Read Code searches
Owing to the difficulties recruiting patients via consultation, GPs were asked to conduct a Read Code search to identify participants. Initially we focused on those practices that had not referred any patients and assistance from the Primary Care Research Network (PCRN) was offered. GPs were asked to screen participants to ensure their suitability for the study.
For the Read Code searches in practices, the inclusion criteria differed slightly from those for GPs who recruited patients via the consultation. Practices were asked to recruit patients on the basis of the IBS Read Code, by identifying those who had received this code within the last 10 years, but patients must have consulted their GP within the past 3 months for functional lower gastrointestinal symptoms (e.g. bloating, AP, diarrhoea, constipation or change in bowel habit). By restricting the search to those who had received the IBS label within the last 10 years and who had consulted recently for lower functional gastrointestinal symptoms, it was felt that this would produce a more manageable number of patients and, therefore, reduce the burden on practices.
Potential participants were sent a study pack containing an invitation letter (either from the GP or the research team), study information sheet, questionnaire and consent form. Those participants who wished to take part in the study were asked to complete the consent form and return it with the questionnaire to the research team. Those who did not wish to participate were asked to return a blank copy of the questionnaire. One reminder was sent to non-responders after approximately 2 weeks. Participants were followed up at 3, 6 and 12 months. One reminder was sent to non-responders after approximately 2 weeks.
Questionnaires
Practice questionnaire
Practices that agreed to take part in study 2 were asked to complete a short questionnaire about the characteristics of their practice. This included details on the number of male and female partners, assistants, retainers, salaried GPs and practice nurses working full or part time; numbers of patients on the practice list; and the number of sites that the practice operated from. Practices were also asked to describe the type of location of their practice (rural, semi-rural, suburban, town/city or inner city), as well as the type of practice population (deprived, mixed – poor, average, mixed – well off or affluent). Finally, they were asked about their contract: General Medical Services, Personal Medical Services, Alternative Provider Medical Services or other; and whether or not the practice was a teaching/training practice.
Patient questionnaire
This consisted of the risk assessment questionnaire, the General Health Questionnaire (GHQ), IBS Symptom Severity Scale, Irritable Bowel Syndrome Quality of Life Questionnaire (IBS-QOL) and the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), as well as patient demographics (see Appendix 4).
The risk assessment questionnaire consisted of the following questions:
-
When your symptoms occurred how long did they last? (≤ 2 hours or less, > 2 hour)
-
When your symptoms occurred had you changed your diet? (yes, no)
-
Did you (i) have gastroenteritis or food poisoning (yes, no) or (ii) have contact with anyone with diarrhoea and vomiting? (yes, no)
-
Have your activities (e.g. work or social activities) been interrupted in the last year because of problems with your bowels? (yes, no)
Participants were asked to answer the questions thinking about the symptoms they consulted their GP with most recently.
Participants were also asked to complete the 12-item GHQ. 91 The GHQ is a self-administered questionnaire aimed at detecting psychological distress. It consists of 12 questions in relation to symptoms or behaviours, which participants are asked to score from four categories ranging from ‘not at all’ to ‘much more than usual’. Scores are then summed for each of the 12 items to produce a total score out of 12, with higher scores representing increasing levels of distress.
The scoring for the risk assessment tool was based on participants’ responses to the above questions (see Appendix 5). 82 In addition, those who scored ≥ 3 on the GHQ were scored an additional point on the risk assessment tool. Thus, participants were given a total score (0–5).
The IBS Symptom Severity Scale92 is a four-item questionnaire. Participants are asked to score on individual visual analogue scales the severity of their AP and distension, satisfaction with bowel habit and interference with life in general. They are also asked how many days out of the last 10 days they had AP. They are given a total score up to a maximum of 500. Those with a score of < 75 are considered healthy patients, 75–174 indicates mild IBS, 175–299 moderate IBS and ≥ 300 severe IBS.
The IBS-QOL93 is a 34-item questionnaire that asks participants to provide responses to statements about their bowel problems over the last 30 days. Responses range from ‘not at all’ to ‘extremely’. The IBS-QOL produces an overall score and eight subscales, including dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual and relationships. Scores are transformed to a 0–100 scale ranging from poor quality of life (0) to maximum quality of life (100).
The EQ-5D-3L58,59 is a self-administered standardised measure of health status. It consists of five dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety/depression. Participants are asked to rate each of these dimensions as having no problems, some problems, extreme problems. Participants are also asked to indicate their own health state today by completing a visual analogue scale from worst (0) to best imaginable health (100). 59
The questionnaire also contained questions in relation to age, gender, postcode (as a measure for socioeconomic status), ethnicity, occupation and employment status. Follow-up questionnaires at 3, 6 and 12 months consisted of the IBS Symptom Severity Scale, GHQ, IBS-QOL and EQ-5D-3L.
Data cleaning and validation
Questionnaires were entered into the study database; data were double entered and checked. This process did not reveal any systematic data entry discrepancies. Frequencies and cross-tabulations were run to check for out-of-range values and data inconsistencies.
Analysis
Responders versus non-responders
Where possible, anonymised data were collected on non-responders, to provide comparison between participants and non-respondents. These data included age, gender and postcode sector. IMD scores were derived using GeoConvert (UK Data Service Census Support, Colchester, UK),94 which provides an IMD score for individual postcodes. For those non-responders identified via Read Code searches, we had information only on postcode sector rather than full postcode. To ensure comparability between responders and non-responders, we used postcode sector for both groups. For each postcode sector GeoConvert was used to obtain a list of postcodes within that postcode sector and assigned an IMD score based on the weighted mean of the postcodes within that postcode sector.
Referrals and response rates
Referrals and response rates were reported for those recruited via the consultation and Read Code searches.
Sensitivity and specificity
Receiver operating characteristic curve analysis was used to examine the ability of the risk assessment questionnaire to predict patients classified as having severe IBS according to the irritable bowel severity scoring system 3, 6 and 12 months later. Sensitivity and specificity were calculated for a range of cut-off points on the questionnaire, from which it was determined whether or not a cut-off point existed that yielded adequately high levels of both sensitivity and specificity. Positive predictive values were used to assess the extent to which those patients rated as high risk by the risk assessment tool went on to have high symptom distress.
Comparison of participants at baseline, 3 and 6 months
Symptom distress, IBS quality of life and EQ-5D scores were presented for all participants at baseline (unmatched), as well as for those who completed the questionnaire at baseline and at 3 and 6 months (matched). We do not report changes at 12 months because responses were not collected from all participants.
Results
Referrals
A total of 58 individual practices were recruited. Table 16 gives further details of practices recruited to the study.
| Practice demographic variable | Number of practices | % of practices |
|---|---|---|
| Location | ||
| Rural/semi-rural | 4 | 7 |
| Suburban | 28 | 48 |
| Town/city | 20 | 35 |
| Inner city | 6 | 10 |
| Population type | ||
| Deprived | 8 | 14 |
| Mixed: poor | 11 | 19 |
| Average | 25 | 43 |
| Mixed: well off | 12 | 21 |
| Affluent | 2 | 3 |
| Type of contract | ||
| General Medical Services | 36 | 62 |
| Personal Medical Services | 20 | 35 |
| Alternative Provider Medical Services | 2 | 3 |
| Teaching practice | ||
| Yes | 40 | 69 |
| No | 18 | 31 |
| Practice list size (mean) | 7166 | |
The majority of practices recruited described their location as suburban (48%) or town/city (35%). The population type was described as average by 43% of practices, deprived or mixed (poor) by 33% and mixed (well off) or affluent by 24% of the practices. The majority of practices worked under a General Medical Services contract (62%) and were training practices (69%). The mean list size was 7166 patients, ranging from 1344 to 16,583 patients. The median number of full- and part-time GP partners was two and the median number of full- and part-time nurses was zero and one, respectively.
Of the 58 practices recruited to the study, 34 referred patients to the study either via permission to contact slips or through the use of Read Code searches. In total, there were 606 referrals to the study, of which just over half (n = 325, 54%) were referred via practices using permission to contact slips (Table 17). The remaining 281 (46%) referrals were made through the use of Read Code searches at the practice. The mean number of referrals from all practices was 10 and ranged from zero referrals to 207 referrals from one practice.
| Referral method | Referrals, n | Responders, n (%) |
|---|---|---|
| Via consultation | 325 | 153 (47) |
| Via record search | 281 | 104 (37) |
| Total | 606 | 257 (42) |
Response rates
Of those referred to the study, 257 patients from 30 different practices agreed to take part (the overall response rate was 42%). The response rate was higher among those patients who were identified via the consultation (47%) than among those who were identified via Read Code searches (37%). For those practices that recruited patients to the study, the mean number of patients recruited was nine, the mode was one and the number of patients recruited ranged from 1 to 94 from one practice.
Completed responses at 3, 6 and 12 months were received from 188 (73%), 161 (63%) and 73 (28%) participants, respectively. Owing to time constraints, complete 12-month data were not available for all participants; of those who posted a 12-month questionnaire, the response rate was 60% (73/121). Of those who returned the initial questionnaire, 54 (21%) completed the initial questionnaire only, 38 (15%) completed the initial and 3-month questionnaires, 82 (32%) completed the initial, 3- and 6-month questionnaires and 65 (25%) completed the initial, 3-, 6- and 12-month questionnaires. A further 18 (7%) participants completed the initial questionnaire but missed one or more follow-up questionnaires.
Table 18 shows the patient characteristics for those recruited to the study. The majority of participants were female (80%) and white (88%). The mean age was 41 years and the mean IMD score was 23.65.
| Patient demographic variable | Participants, n (%) |
|---|---|
| Gender | |
| Female | 206 (80) |
| Male | 51 (20) |
| Age group (years) | |
| 16–24 | 56 (22) |
| 25–34 | 63 (25) |
| 35–44 | 34 (13) |
| 45–54 | 37 (14) |
| 55–64 | 29 (11) |
| ≥ 65 | 37 (14) |
| Age (years), mean (SD) | 41.3 (17.9) |
| Ethnic origin | |
| White | 225 (88) |
| Mixed | 5 (2) |
| Asian or Asian British | 14 (5) |
| Black or black British | 7 (3) |
| Other | 4 (2) |
| Missing | 2 (1) |
| IMD score, mean (SD) | 23.65 (13.95) |
Responders versus non-responders
Demographic data were available for 81% of non-responders (281/345). Males were less likely to respond (56%) than females (52%), and non-responders tended to be slightly younger (39.8 years) than responders (41.3 years). These differences were not statistically significant. There was also no significant difference in the mean IMD scores for responders and non-responders based on the postcode sector analysis (24.9 vs. 26.5, respectively).
Sensitivity, specificity and positive predictive values
Table 19 shows the number and percentage of participants by symptom severity and risk assessment score at baseline, and at 3, 6 and 12 months. At baseline, one-quarter of participants were classified as having severe symptom distress; this proportion fell to about 15% at each of the follow-up periods. The majority of participants scored 4 or 5 on the risk assessment tool at each time point.
| Risk assessment score | Symptom severity scale | |||
|---|---|---|---|---|
| Severe (300–500) | Not severe (0–299) | |||
| n | % | n | % | |
| Baseline | ||||
| 4 or 5 | 50 | 32.1 | 106 | 67.9 |
| 1–3 | 11 | 12.6 | 76 | 87.4 |
| Total | 61 | 25.1 | 182 | 74.9 |
| 3 months | ||||
| 4 or 5 | 23 | 19.3 | 96 | 80.7 |
| 1–3 | 4 | 7.0 | 53 | 93.0 |
| Total | 27 | 15.3 | 149 | 84.7 |
| 6 months | ||||
| 4 or 5 | 20 | 21.5 | 73 | 78.5 |
| 1–3 | 4 | 7.1 | 52 | 92.9 |
| Total | 24 | 16.1 | 125 | 83.9 |
| 12 months | ||||
| 4 or 5 | 8 | 19.0 | 34 | 81.0 |
| 1–3 | 3 | 10.0 | 27 | 90.0 |
| Total | 11 | 15.3 | 61 | 84.7 |
Table 20 shows the results from the receiver operating characteristic curve analysis. A score of ≥ 4 on the risk assessment tool yielded the best cut-off point to predict severe symptom severity at follow-up; however, the number of participants included in the analysis was much lower than that proposed in the original sample size calculation and included both those with prevalent and incident IBS. At 3 and 6 months, sensitivity (the probability that the score on the risk assessment tool was high when symptom severity was severe) was > 80%, but specificity (the probability that the score on the risk assessment tool was low when symptom severity was not severe) was low (36% and 42% at 3 and 6 months, respectively). Positive predictive values (the probability that symptom severity was severe when the score on the risk assessment tool was high) was poor (approximately 20%) at all three time points.
| ROC curve analysis value | Time point, n | ||
|---|---|---|---|
| 3 months | 6 months | 12 months | |
| n | 176 | 149 | 72 |
| Area under the curve | 68 | 62 | 70 |
| Sensitivity | 85 | 83 | 73 |
| Specificity | 36 | 42 | 44 |
| Positive predictive value | 19 | 22 | 19 |
Comparison of participants at baseline and at 3 and 6 months
Of those who completed the baseline questionnaire, 147 (57%) participants also went on to complete questionnaires at 3 and 6 months.
Figure 12 shows the IBS-QOL subscales and overall score for those participants who completed the baseline questionnaire and did not have matched data for 3 and 6 months’ follow-up (baseline unmatched) and those with matched data for baseline, 3 and 6 months. Those with unmatched data tended to have lower scores on all subscales and on the overall IBS-QOL scale. At all time points the lowest scores were reported on the food avoidance subscale and were highest on the relationship subscale. The data also suggest that there were small improvements in most HRQoL subscales over time.
FIGURE 12.
Irritable Bowel Syndrome Quality of Life Questionnaire subscales. Higher values indicate better HRQoL.
Likewise, Figure 13 shows the data for participants according to their symptom severity classification. The proportion of patients within each category was similar for both matched and unmatched participants at baseline, with the majority being classified as having moderate disease. The proportion of those with moderate and severe disease fell at 3 and 6 months, as more participants reported disease remission or mild disease.
FIGURE 13.
Symptom severity classification.

Figure 14 shows the proportion of patients reporting problems (some or extreme problems) on the individual EQ-5D scales. The majority of patients reported no problems on the mobility and self-care scales, about one-third reported problems with their usual activities and a much larger proportion reported problems with pain and/or discomfort or with anxiety and/or depression. The chart suggests that there was a small improvement in the usual activities, pain/discomfort and anxiety/depression scales at 3 months’ follow-up, but this was less obvious at 6 months’ follow-up.
FIGURE 14.
Per cent reporting problems on EQ-5D scales.

Discussion
Summary of findings
The number of patients recruited to the study was much lower than anticipated. However, we were able to calculate sensitivity and specificity based on the sample recruited. The risk assessment tool appeared to be sensitive in predicting those participants with severe disease; however, it was not specific in predicting those without severe disease. Patients reported reduced quality of life; however, the data suggested that there were modest improvements over time.
Strengths and limitations of the study
The main strength of this study was the large number of practices that agreed to take part. Practices were recruited from across Greater Manchester and, therefore, covered a wide geographical area. However, despite the large number of participating practices, the number of referrals to the study was disappointing. Initially, GPs were asked to recruit patients during the consultation and we therefore relied on GPs to remember about the study during the consultation. For a small number of practices this method worked well, but the majority of practices did not refer any patients via this method. This recruitment strategy was later supplemented with Read Code searches at a number of practices. As a result, recruitment to the study was boosted to some extent, but not all practices agreed to carry out a Read Code search despite the offer of assistance from the local PCRN. An inadvertent and unexplained limitation of the current study was selection bias, as the majority of referrals came from a single practice, which is likely to affect the validity of the results. This practice was a particularly large one, with a large student population.
Why is the prevalence of irritable bowel syndrome so low?
Even with the use of Read Code searches, the prevalence of IBS was much lower than expected when compared with the literature. Prevalence estimates of IBS in Western countries range from 2% to 22%. 95 More specifically in the UK, a questionnaire survey estimated IBS prevalence to be 10.5%. 90 However, estimates of the prevalence of IBS are likely to be influenced by a number of factors, including the criteria used, population studied, mode of study delivery and type of prevalence estimate.
Prevalence estimates reported in the literature vary widely depending on the diagnostic criteria (e.g. Kruis,96 Manning97 or Rome I,98 II99 or III4). A study across eight European countries with > 40,000 participants reported that the overall prevalence of IBS was 11.5%; however, only 2.9%, 4.2% and 6.5% of participants met the Rome II, Rome I or Manning criteria, respectively. 100 The usefulness of diagnostic criteria is frequently debated, and they tend to be of little relevance within primary care, as few GPs use them to make a diagnosis. We adopted a symptom-based approach to identify patients consulting with IBS within primary care and, therefore, we would have expected the prevalence of IBS to be higher than in those studies using formal diagnostic criteria such as Rome II. 99 However, there are a number of further possible explanations.
First, many studies used to estimate the prevalence of IBS have been self-reported questionnaire surveys and are therefore likely to overestimate the proportion of patients who have been formally diagnosed with IBS. Nevertheless, Wilson et al. 90 reported that over half of patients with IBS consulted their GP within the past 6 months. In addition, Rey and Talley95 suggest that consultation rates are higher in more recent studies owing to increasing public awareness. They suggest that 30–70% of patients have consulted their GP owing to their symptoms in the previous year. Furthermore, a survey of UK GPs also suggests that IBS is not an insignificant problem. 3 Based on the Read Code searches, we were able to calculate an estimate of IBS prevalence in a 3-month period. This was estimated at 0.2%, and it ranged from 0.03% to 0.4% across practices. However, patients recruited via the Read Code searches had to have had the IBS Read Code recorded in the last 10 years and to have consulted within the past 3 months for symptoms of abdominal pain, bloating, constipation, diarrhoea or change in bowel habit. Despite this, the rate was much lower than expected.
Second, IBS or gastrointestinal symptoms are not a part of the QOF, a scheme that incentivises practices and rewards them according to how well they care for patients. Consequently, they are not likely to be a priority for GPs.
Third, GPs may be reluctant to code patients for IBS or lower gastrointestinal symptoms. One reason for this may be the uncertainty of the diagnosis. Although IBS is no longer regarded as a diagnosis of exclusion, and NICE has produced guidelines,81 GPs may still take a ‘diagnosis of exclusion’ approach. As a result, GPs may not use Read Codes when they first consult and use other Read Codes or free-text fields. Consequently, if patients disengage with primary care they would not receive an IBS label.
The predictive value of the risk assessment tool
We were able to calculate sensitivity, specificity and positive predictive values for the risk assessment tool based on the data collected. Based on a cut-off point of 4 on the risk assessment tool, it was possible to obtain sensitivity values of > 80%. However, specificity was around 40% and the positive predictive value was only about 20%. Therefore, the tool appeared to be quite good at predicting those who did actually have severe IBS, but was not good at predicting those that did not actually have severe IBS. However, the small number of patients recruited to the study meant that the study was underpowered. The sample size calculation estimated that a sample size of 420 prevalent and 420 incident cases would be required in order to detect an effect. Early on it became apparent that we would not be able to recruit incident cases to the study because of the difficulties in establishing a diagnosis and the fact that many patients present with longstanding symptoms. Indeed, of the 257 patients recruited to the study, 95% reported having symptoms of > 3 months’ duration. As a result, the study was expanded to include prevalent cases; however, difficulties with recruitment persisted. Despite these difficulties, it is questionable whether or not such a tool would be useful in predicting which patients have severe disease at a later stage.
Natural history of irritable bowel syndrome
The data suggest that patients with IBS have poor HRQoL, as measured by the IBS-QOL and the EQ-5D. These findings are comparable with those reported in the literature. 101 Those patients who did not complete the questionnaire at all three time points tended to score lower on these measures at baseline. Data from participants who completed the baseline, and 3- and 6-month questionnaires tended to suggest that there were small improvements in the IBS quality of life, symptom severity scores and EQ-5D over time. Although we did not formally test these differences, this appears to contrast with previous findings of no significant difference in the IBS-QOL subscales and overall score from baseline to follow-up 3 months later. 101 No specific intervention was administered in that study. Similarly, in our study, no specific intervention was administered between baseline and 3 and 6 months. However, GPs were asked to recruit patients during the consultation and it may be that treatment prescribed during the course of that consultation resulted in improved outcomes for patients. Alternatively, these improvements may simply reflect the fluctuating nature of the condition.
Owing to the problems with recruitment, we felt that it was important to gather the views of GPs about the management of IBS in primary care. We therefore proceeded to the qualitative study to explore how GPs diagnose and manage patients with IBS (study 3). We also decided to investigate further the patient profiles of patients with gastrointestinal symptoms (IBS, AP and IBD) using data from the SIR (study 4).
Qualitative study (study 3)
The NICE guideline for IBS81 emphasises establishing a positive diagnosis, identifying symptoms that require prompt referral, but avoiding unnecessary investigations and referrals, and working in partnership with the person with IBS.
There is evidence of dissatisfaction and frustration experienced and voiced by patients and doctors alike, arising from uncertainties in aetiology and diagnosis, ineffective treatments and a mismatch between GP and patient explanatory models. 102,103 This may lead to negative stereotyping of patients with IBS by doctors,102 and for patients it may lead to a breakdown in trust and disengagement from services. 104
Patients and GPs may share similar views on aetiology and symptomology, but differ in the treatment approaches found acceptable. 105 GPs can hold hostile views about patients with IBS who are frequent attenders and do not improve. 103 There is little previous work on how a diagnosis of IBS is made in primary care and how this label is applied. Only a small proportion of IBS cases, as recorded in medical records, meet case definition criteria, suggesting that diagnosis in primary care may be problematic. 106
Aim
The qualitative study aimed to explore how GPs currently diagnose and manage IBS and their attitudes towards the use of a predictive, risk assessment tool within general practice.
Methods
Design
Face-to-face, in-depth, semistructured interviews were conducted with GPs in north-west England who were participating in the study of the risk assessment tool. Data were collected between March and December 2011.
Sampling
General practitioners from practices invited to take part in the validation of the risk assessment tool were invited to participate in a semistructured interview. Purposive sampling was used to maximise the variation of the sample in terms of age, length of experience, ethnicity, practice size and location. Recruitment was continued until theme saturation was reached.
Data collection
Interviews were conducted, with written consent, at GP premises by two of the researchers. Interviews lasted between 20 and 70 minutes (mean duration 42 minutes). The topic guide was developed from review of the existing literature and discussion within the research team. The topic guide, designed to be used flexibly, was modified in the light of emerging themes. The main areas explored were GPs’ views on the aetiology of IBS, how the diagnosis of IBS is made, how GPs explain IBS to patients, treatments offered and the potential usefulness of a tool to predict chronicity.
Interviews were fully transcribed. Data coding was by constant comparison across interviews by individual researchers and emerging themes were agreed through discussion among all authors from different professional backgrounds (academic primary care, health services research, epidemiology). An iterative approach to data collection and analysis was taken: coding and conceptual categories were constantly reviewed and refined in the light of new interview data and ongoing discussion in the research team. The topic guide was modified to allow for further exploration of emerging themes. NVivo 9 (QSR International, Warrington, UK) was used to store and manage the data.
Results
Nineteen interviews with GPs were conducted (Table 21). Seventeen GPs had agreed to recruit patients for the larger programme. Two GPs agreed to be interviewed but the practice declined to participate in the main study.
| GP demographic variable | Number of GPs |
|---|---|
| Gender | |
| Male | 11 |
| Female | 8 |
| Age group (years) | |
| 30–39 | 7 |
| 40–49 | 5 |
| ≥ 50 | 7 |
| Ethnic origin | |
| White | 13 |
| Asian/Asian British | 5 |
| Black/black British | 1 |
| Status | |
| Principal | 17 |
| Salaried | 2 |
| Type of practice | |
| Inner city | 2 |
| Urban | 9 |
| Suburban | 8 |
| Mean (range) years in practice | 15.4 (4–28) |
The following themes are presented: understanding IBS, making the diagnosis, explaining the label, management strategies, frequent attenders or disengagers and the utility of a risk assessment tool. Data are presented verbatim from transcripts and identified by a code attributed to the respondent.
Understanding irritable bowel syndrome
All GPs recognised the existence of IBS as a syndrome, a collection of symptoms, affecting different people in different ways.
I do think it exists, I really do. I think people’s lives can be made – it’s a label isn’t? People who have got constipation predominant IBS might – you could just call them constipated. It’s a real condition, people get a lot of pain from it. There are people that seem to get more anxious about it. But we all get a dodgy tummy if you’ve got an exam or something like that. So I definitely think it exists.
GP 11
General practitioners described IBS as a complex condition with a biopsychosocial aetiology, alluding to the notion of susceptibility, triggers and precipitants, with patients often presenting symptoms related to ‘stress’ and lifestyle.
I think there’s a lot of psychology with irritable bowel, not that necessarily the psychology causes the irritable bowel but I think for a fairly benign condition it can cause a lot more upset than you’d expect.
GP 9
General practitioners defined IBS by the lack of demonstrable organic pathology and focused on abnormal behaviour of the bowel.
I think it is an exclusion of other pathology, but it’s often a change in bowel habit, people become constipated, they get increased frequency of bowel motions, bloatedness, colicky abdominal pains, sometimes I think there’s a bit of reflux associated with that, with IBS.
GP 11
So they’d be likely to have, sort of, crampy abdominal pain with bloating, sensitive to bowel disturbance whether it be diarrhoea, constipation, erm, nausea, erm, those types of symptoms, but none of the serious red flag-type symptoms, not usually any sign of any bleeding from the back passage, no sign of weight loss, erm, and that type of pain, sort of, colicky pain.
GP 1
General practitioners were aware of the chronicity of IBS, with the potential for ‘flare-ups’.
. . . well irritable bowel syndrome often it just flares up, they come and they get their month of Mebeverine, they take it and then they’re not troubled for another year.
GP 14
Some GPs alluded to their own experiences, which made symptoms in patients more real and more understandable.
I think I’ve dealt with it so much, I’ve experienced it as well and so I think when you’ve experienced it you know how severe some of the symptoms can be.
GP 11
Making the diagnosis
General practitioners suggested that the first step in making the diagnosis and managing people with IBS is ensuring that the patient’s concerns are listened to.
. . . it helps just to reassure the patient that you’re taking the symptoms seriously. I think that’s probably the biggest worry they have, because when you have some functional symptom and you’ve nothing to show the doctor, it’s almost inevitable that you’re going to wonder whether he’ll take it seriously . . .
GP 3
Some GPs described a traditional doctor-led encounter driven almost entirely by clinical factors, but most portrayed a two-way process in which their actions are shaped by the patient’s expectations. Most GPs were aware of the NICE guideline81 for IBS and the suggestion that the diagnosis should be made in a positive manner, considering the symptoms which pointed towards the diagnosis. Many GPs felt uncomfortable with this approach and, although few described referring to secondary care unless there were clear ‘red flag’ symptoms, they described IBS as a diagnosis of exclusion, driven by investigations and their normality, and the diagnostic process as tentative and iterative.
So to a certain extent there’s always a degree of diagnostic uncertainty because . . . although I know the term isn’t really favoured any more, but pretty much it’s a diagnosis of exclusion.
GP 18
The iterative process of diagnosis, by exclusion of sinister symptoms, was described as the approach taught to them in medical school.
So now it’s very difficult, yeah, I read the NICE guidelines and it is about positive, it’s not a diagnosis of exclusion any more, it’s about positive symptoms. I’ve read all that but it’s still very hard to go away from something that was drummed in.
GP 11
General practitioners suggested that they made a diagnosis based on the patient response to treatments.
. . . and, you know, their full blood count would be alright, their coeliac screen would be negative, erm . . . and then we’d . . . you know, I’d often give them a trial of Mebeverine or something and that quite often does help, and then they just stay on that.
GP 14
The majority of GPs reported that they did not initially add a Read Code for IBS to the patient record, but delayed until they were more confident in the diagnosis. Thus, codes such as ‘abdominal pain’, ‘diarrhoea’ or ‘constipation’ would remain on the patient record, rather than IBS, and patients who do not return with their gastrointestinal symptoms would not be coded as having IBS.
I’d probably put a symptom as the coding on the computer at that stage; the most predominant symptom, but if the patient has come in with the same sorts of things over and over again and it’s looking very much like IBS, and it responds to IBS type treatment, then I’d code it.
GP 1
Explaining the label
General practitioners described various models of IBS, including the metaphor of ‘a string of lights that are lighting up randomly rather than in a regular pattern’ and ‘the bowel as a muscle that can be irritated like any other muscle’. Language often emphasised lack of co-ordination or rhythm (‘higgledy-piggledy’), and the use of analogies was common. The GPs described tailoring their explanations for the individual patient.
I try and explain that this is a, sort of, not particularly well understood problem, which can have a variety of different causes and can cause quite dramatic symptoms, but, isn’t, sort of, generally, sort of, serious in terms of long term, sort of, prognosis.
GP 19
General practitioners suggested that most patients are ready to accept the link between psychosocial issues and bowel symptoms, and may even have considered this before consulting.
Whereas if I involve them throughout the process right from the beginning, er, they adhere to it. I haven’t had a single problem with bringing up psychological issues with any of my patients, yeah.
GP 13
Management strategies
All GPs described giving lifestyle advice, particularly around diet, exercise and activity, although most suggested that patients had already tried to modify their diet.
Some patients have already identified it – ‘My problems seem far worse when I eat such and such . . .’ so then we say hang on, why don’t you just not eat such and such and see how you do and if they feel that their symptoms have been looked into adequately enough, and they’re reassured that it’s IBS then they’re happy to do that.
GP 18
Some GPs suggested that a focus on the related psychological symptoms initially might lead to improvement in gastrointestinal symptoms.
Then I tend to sort of not label them as IBS straightaway, yeah. I will manage the stress, I will manage their anxiety, I’ll manage the depression and see what happens with the symptoms.
GP 13
Despite acknowledging the link between psychological symptoms and IBS, GPs described a reluctance to refer patients with IBS to mental health services, as such resources are scarce and waiting times are long, reserving these interventions for those with more overt mental health symptoms:
We can refer to Mental Health Team for the depression, with the hope that it might help the IBS symptoms at the same time. We couldn’t refer primarily for the IBS.
GP 1
Although CBT and hypnotherapy are recommended interventions for IBS in the NICE guideline,81 some GPs expressed doubt about the evidence base for either intervention.
. . . the NHS does need to be careful about where it puts its money because . . . perhaps we ought to be putting the money into more evidenced-based things.
GP 12
I need to be sort of . . . I’m not quite sure of the link between why CBT might work, the connection between the psychological component and the patient’s IBS, and CBT. I’m assuming there are studies to show that it does work?
GP 2
General practitioners recognised that patients may not always be satisfied with treatments offered by them or the NHS and may seek complementary therapies, which respondents said they would not challenge if not felt to be harmful.
Um, but yeah, like again, they’re placebos, but it’s an area where placebos do work. I certainly wouldn’t get in the way of anyone who suggested trying something, um, provided it sounded safe. Like if they wanted to try a homeopathic remedy or massage remedy, I’d say ‘Yeah, go for it. Why not?’
GP 3
No, I’ll say try what works for you. Some of them will be very specific things. Or they buy some seaweed product from a health shop and that works fine. I don’t care what works, so long as it’s not going to harm them. If they find that going for private acupuncture helps, great. Whatever, I don’t care what it is. I just don’t want them to be suffering without anything working for them. If they find a solution that’s great.
GP 12
Frequent attenders or disengagers?
General practitioners stated that many patients do not return after an initial consultation and attributed this to patients either being satisfied with management or ‘putting up with’ their symptoms.
But sometimes they just never come back and then you just assume they’ve got better [laughter].
GP 14
Whether it’s that they just manage it or whether it’s just that we don’t ask them about it anymore and they just put up with it I don’t know.
GP 8
However, most GPs described the chronicity of IBS and some suggested that there were a minority of patients who did not improve, attended frequently and were difficult to manage.
I suspect to patients it’s a complete nightmare ’cos it’s the daily thing of living with it but in general I have to say my experience is that we can help patients but, you know, to improve their quality of life enormously is not necessarily that easy with irritable bowel.
GP 9
A minority of GPs felt there was little that they could offer to patients with IBS.
It’s only really if it’s . . . if there’s no obvious trigger and if it’s causing significant impact on their life. That’s when it becomes very challenging because I think it’s relatively limited what you can do.
GP 6
General practitioners used negative language to describe such patients, suggesting that frequent attenders were ‘complainers’ or ‘mithered’, and suggested that, in such circumstances, they might be forced to agree to ‘un-necessary’ investigations or referrals.
And if you did refer somebody, what are you expecting from the specialist?
That the patient might not keep hassling me. Usually I would refer, it sounds awful but to get them off my back if they’re persistently coming in. I’m really worried, it’s not getting better, what can you do? It’s not that I think the specialist will do particularly anymore. They might give some advice, but it’s probably not going to be different to what I’ve been able to do myself.
GP 12
I think the other groups of people who find it hard to accept that their body is giving them symptoms, and who are constantly looking for a cause which they can see on an X-ray or on an ultrasound or something, and who are, who are . . . who don’t sort of accept the functional nature, and who are always kind of mithering about wanting more investigations and tests, and then disappointed when they are all normal, and I think that’s very difficult, and I do a lot of warning of people that yes, we can do X or Y, my expectation could be normal, so how will you feel if it’s normal? Because they sort of pin their hopes on the idea that I’ll have a coeliac screen and that will show they’ve got coeliac disease, and then when they haven’t they kind of go, what is it then, you know, that kind of question; so that needs quite careful handling, I think.
GP 4
It was thought that only a minority of patients, however, would become frequent attenders.
And, those with straightforward IBS, with the pattern of our consultation, it makes it easier for us to attend to . . . and, I would say very good, indeed, only a very small percentage fall in the category that are frequent attenders.
GP 15
I mean there are, I guess a subgroup of patients with IBS who are the real sort of somatisers I guess, the real patients that, erm, could be termed doctors heart sink patients, erm, so patients that, that are generally very anxious but are anxious about their health, they’re anxious about every little symptom. There is a small group of patients with IBS who fit into that group and so that group of patients have certain patterns, but I wouldn’t say that all patients with IBS are like that.
GP 18
Utility of a risk assessment tool in primary care
Despite the recognition that there might be a group of patients who disengaged or who became frequent attenders, GPs did not feel that a risk assessment tool to predict which patients might become high users of care would have any utility, particularly given the perceived lack of availability of any proven or cost-effective treatments.
But don’t we intervene anyway? It’s not as if we sit there doing nothing unless we think someone’s going to be a high consulter, so what actual outcome is that going to have to me and my practice?
GP 6
Some GPs expressed concern about the practicalities of administering such a tool in a time-limited primary care consultation.
. . . I can’t be doing with having piles of bits of paper, every specialty has got dozens of bits of paper like this and we work across every single specialty, so having bits of paper in the room is a complete loss.
GP 4
. . . they’re a pain though, using questionnaires in consultations because you have to find it, you have to print it off if you’ve got it on your computer, if you give it to your patient they then . . . you can send them away to fill it in but if they fill it in there and that’s in a 10-minute consultation.
GP 11
Other GPs suggested that such tools do not fit with the realities of the consultation process, attempting to impose an artificial structure on what ought to be a patient-centred encounter.
. . . first of all patients don’t present in this kind of neat tidy way, they come and they say oh God I feel terrible, oh there is this and there is that and I want to tell you about my toe nails and blah blah, so it doesn’t lend itself to that . . . what you have to do is listen to what they are saying not force, you know, it’s not about asking a series of yes–no questions usually.
GP 4
So the value of a tool to predict which patients might benefit from early intervention for their abdominal symptoms and direct management decisions was perceived to be limited.
Discussion
Summary of main findings
This study illustrates how the perspectives of GPs about the diagnosis and management of IBS influence their views on the value of a risk assessment tool to predict chronicity. GPs reported that IBS is not a difficult condition to diagnose or manage, yet most described reluctance to add the Read Code for IBS to the record. Respondents acknowledged the link between IBS and psychological distress, but were reluctant to refer for therapies such as CBT or hypnotherapy and did not see the value of a risk assessment tool to predict chronicity. Most GPs suggested that patients did not return because their symptoms had settled, rather than because they may be dissatisfied with care.
Strength and limitations of the study
Data are presented from interviews with GPs over a wide geographical area; this purposive sampling enabled us to access a range of views. However, because we employed a theoretical, rather than a statistical, sampling approach, the proportion of GPs holding different views cannot be inferred. Seventeen out of 19 GPs were already taking part in an evaluation of the risk assessment tool for IBS and were possibly more likely to feel comfortable managing IBS. The two GPs who agreed to be interviewed but not to participate in the main study might have been expected to have more negative views about IBS; however, this was not apparent. Data were analysed by researchers from different professional backgrounds, increasing trustworthiness. 107
Comparison with existing literature
The diagnosis of medically unexplained symptoms is often contentious;108 however, GPs in the current study did acknowledge the existence of IBS and reported that they had no difficulty in diagnosis or management. These findings are similar to published data reporting that compared with pelvic and back pain, IBS was not considered difficult in terms of distinguishing functional from organic disease. 109 When GPs experienced IBS themselves, or knew someone with IBS, it helped them to understand the condition, resonating with studies describing a similar finding in GPs who have first-hand experience of chronic fatigue syndrome. 110
Respondents recognised the link between IBS and psychological distress;109 however, they were uncertain of the benefit of psychological therapies in patients with IBS. It has been reported that GPs in the UK do not refer patients with IBS for psychological treatment,109 whereas doctors in the Netherlands are more likely to do so. 102
Although the diagnosis of IBS is often made by excluding red flag indicators, in one study 72% of GPs considered that they were usually, or often, able to diagnose IBS at the initial visit. 109 This contrasts with a previous study reporting that 19% of patients formally diagnosed with IBS had been given the diagnosis on their first visit and 56% after a further 1–5 visits. 100 These studies were conducted prior to the introduction of the NICE guideline81 for the diagnosis and management of IBS. However, although most GPs in our study were aware of this guideline, many were reluctant to apply a label of IBS to patients. Read Codes were reported as being applied only if the patient reconsults and probably reflecting the fluctuating nature of the condition. 111
General practitioners emphasised the importance of self-management in patients with IBS and felt that offering advice and reassurance was important in the first instance. Dietary advice was emphasised by respondents, but it is not always appreciated by patients, who are likely to have already tried such measures before consulting. 105
A strong physician–patient relationship and empowering explanations given by GPs are reported to be important to the successful management of IBS. 112,113 General practitioners often give the problem back to the patient102 and health-care professionals tend to distinguish between ‘good’ and ‘bad’ patients, with so-called ‘bad’ patients being unaccepting of the diagnosis of IBS, recurrent attenders, demanding further investigations, failing to cope or respond to treatment and resentful of the IBS label and psychological explanations given. 103 ‘Good patients’, on the other hand, were those who GPs suggested had a sense of relief with the label given and were accepting of the diagnosis. 103 In the current study, GPs suggested that most patients did not return, attributing this to patients either getting better or learning to live with their symptoms. However, there is evidence to suggest that patients with IBS disengage with services because of dissatisfaction with GP interactions,114 a belief that there is little the NHS can offer, or attributing the onset or worsening of symptoms to previous poor medical care. 104
Of those patients who consult their GP with a FGID, approximately 30% go on to develop chronic symptoms. 82 Providing a self-help guidebook, designed with the aid of patients, reduces primary care consultations by 60%. 15 In addition, for patients with intractable symptoms resistant to conventional medical therapy, there may be therapeutic benefit from CBT or hypnotherapy. 16,17 A risk assessment tool may aid GP decisions about the management of patients at risk of poorer outcomes and fast-track them to therapies that are known to be effective, such as CBT or hypnotherapy, leading to lower levels of distress and health-care utilisation in the long term. However, the majority of GPs interviewed did not feel that a risk assessment tool would be useful to them, whereas others felt that it would be impracticable to administer such a tool in a 10-minute consultation.
Implications for future research and clinical practice
Patients might be appreciative of such a tool within the consultation – it may demonstrate that the doctor is taking their symptoms seriously and demonstrate patient involvement in decision-making about treatment. There is a parallel with the use of the Patient Health Questionnaire-9, a tool to which GPs are less receptive than patients,115 and the value of a tool based on prognostic information to determine the appropriate intervention for patients with back pain has been shown to be effective116 but not well used by GPs. 117
This study highlights tensions between research evidence identifying the potential role of a risk assessment tool to support primary care management of IBS and GPs’ perspectives that it is not needed. The study reinforces the need to take account of current clinical practice and practitioners’ perspectives and researchers and clinicians should work together when developing research programmes to tackle conditions that create challenges for clinicians.
General practitioner database study (study 4)
As a result of the difficulties in recruiting to the risk assessment study, and in the light of earlier findings, we decided to investigate further the profiles of patients with IBS using data from the SIR.
Aim
The aim of study 4 was to investigate profiles in patients with IBS, IBD and AP; and to explore whether or not GPs follow NICE guidelines for patients with IBS.
Methods
Setting
The setting for this study was Salford, north-west England, with an estimated population of 228,992 in 2010.
Sampling frame
The sampling frame comprised all patients registered with the 52 practices in Salford. Patients may opt out of the SIR; however, the proportion that do so is relatively small.
Data collection and coding
An application was made to NorthWest EHealth requesting details on a cohort of patients based on various codes for gastrointestinal disorders.
The SIR data were obtained for the period January 2002 to December 2011. Information was supplied in two separate files. The first file contained anonymised patient identifiers, gender and year of birth, whereas the second file contained journal entry identifier, anonymised patient identifier, date of journal entry, Read Code description and Read Code. These two files were matched on patient identifier. The file was checked for duplicates and frequencies were run on all variables to check for any anomalies. Duplicate records and patients under the age of 17 years were removed. Read Codes were then recoded into a new variable to distinguish between symptom and/or diagnostic codes, medication codes and referral codes. Ethics permission for this study was granted by the NorthWest EHealth Board (reference number 177) and the individual health-care organisations.
Analysis
For this study we carried out analysis on three patient groups. These were patients with:
-
IBS (Read Codes 14CF. and J521., including any subheadings)
-
AP (Read Codes 196.., 197.. and R090., including any subheadings)
-
IBD [Read Codes J4... (without subheadings), J40.. and J41.., including any subheadings].
Patients with IBS were our primary group of interest, but we were also interested in how GPs coded patients with similar conditions, to determine whether or not there were any differences in coding practices across conditions. Patients with AP were chosen because it is the main feature of IBS and is required to be present in addition to two other symptoms for a diagnosis of IBS to be given. 81 In addition, we selected patients with IBD because IBD is an organic disorder and patients present with similar symptoms to IBS.
For each group of patients we identified an index episode of IBS, AP or IBD. For each index episode, an index date was created based on the date for that particular journal entry. For each condition we then looked 1 year pre and post the index date to determine:
-
symptoms/diagnoses recorded pre and post IBS, AP and IBD
-
medications prescribed pre and post IBS, AP and IBD
-
referrals to gastrointestinal specialists pre and post IBS, AP and IBD.
The index episode was taken as the first occurrence of IBS, AP or IBD. The date of this episode was then used to calculate the number of days between the index episode and all corresponding journal entries for the same patient. Where the number of days was less than or equal to ± 365, then these were recorded as 1 year pre or post for that particular journal entry. Where the number of days was equal to 0, this was included in the year prior to the index episode for symptoms/diagnoses and included in the year after the index episode for medications and referrals. For the SIR, pre data were based on those with an index episode between 2003 and 2011 (so that only those with a complete year of data available before the index year were included), whereas post data were based on those with an index episode between 2002 and 2010 (so that only those with a complete year of data available after the index year were included).
Symptoms/diagnoses were defined as in Table 22.
| Symptom/diagnoses | Read Code | Rubric |
|---|---|---|
| IBS | 14CF. | History of IBS |
| J521. | IBS | |
| AP | 196.. | Type of GIT pain |
| 197.. | Site of GIT pain | |
| R090. | [D] APa | |
| Bloating | 19A.. | Abdominal distension symptom |
| 19B.. | Flatulence/wind | |
| R0734 | [D] Bloatinga | |
| Constipation symptom | 19C.. | Constipation |
| Functional constipation | J520. | Constipation – functional |
| Change in bowel habit | 19EA. | Change in bowel habit |
| R078. | [D] Change in bowel habita | |
| Diarrhoea | 19F.. | Diarrhoea symptoms |
| 19G.. | Diarrhoea and vomiting | |
| Functional diarrhoea | J525. | Functional diarrhoea |
| J43z. | Chronic diarrhoea | |
| J4z.. | Presumed non-infectious diarrhoea | |
| Nausea | 198.. | Nausea |
| Vomiting | 199.. | Vomiting |
| Diarrhoea and vomiting | 19G.. | Diarrhoea and vomiting |
| Tenesmus | 19D.. | Tenesmus symptom |
| Faeces/motions symptoms | 19E.. | Faeces/motions symptoms |
| Gastrointestinal infection | A0... | Intestinal infectious diseases |
| IBD | J40.. | Crohn’s disease |
| J41.. | Ulcerative colitis or proctitis |
Medications were defined on the basis of the NICE guidelines81 and coded as shown in Table 23.
| NICE medications | Read Code | Rubric |
|---|---|---|
| Bulk-forming laxatives | ab2.. | Ispaghula husk |
| ab3.. | Methylcellulose | |
| ab4.. | Sterculia | |
| Stimulant laxatives | ac5.. | Docusate sodium |
| ac7.. | Senna | |
| ac8.. | Sodium picosulfate | |
| af1.. | Rectal laxatives (glycerol, bisacodyl) | |
| ac1.. | Bisacodyl | |
| Faecal softeners | ad1.. | Liquid paraffin |
| Osmotic laxatives | ae4.. | Polyethylene glycols |
| a12.. | Magnesium salts – antacid | |
| ae2.. | Magnesium hydroxide | |
| ae3.. | Magnesium sulphate | |
| ae7.. | Sodium phosphate | |
| Antimotility agents | a81.. | Codeine phosphate |
| a82.. | Diphenoxylate hydrochloride | |
| a83.. | Loperamide: single drug | |
| a85.. | Loperamide: compound preparation | |
| a842. | Kaolin and morphine mixture | |
| Antispasmodics | a41.. | Atropine sulphate |
| a45.. | Dicycloverine hydrochloride | |
| a47.. | Hyoscine butylbromide | |
| a4c.. | Propantheline bromide | |
| a4d.. | Alverine citrate | |
| a4e.. | Mebeverine hydrochloride | |
| a4f.. | Peppermint oil | |
| Antidepressants | ||
| Tricyclics and related antidepressants | d71.. | Amitriptyline |
| d91.. | Triptafen | |
| d73.. | Clomipramine hydrochloride | |
| d75.. | Dosulepin hydrochloride | |
| d76.. | Doxepin | |
| d77.. | Imipramine hydrochloride | |
| d79.. | Lofepramine | |
| d7c.. | Nortriptyline | |
| d7f.. | Trimipramine | |
| d7b.. | Mianserin hydrochloride | |
| d7e.. | Trazodone hydrochloride | |
| SSRIs | da9.. | Citalopram |
| daC.. | Escitalopram | |
| da4.. | Fluoxetine hydrochloride | |
| da3.. | Fluvoxamine maleate | |
| da6.. | Paroxetine hydrochloride | |
| da5.. | Sertraline hydrochloride | |
| MAOIs | d81.. | Phenelzine |
| d83.. | Isocarboxazid | |
| d84.. | Tranylcypromine | |
| Reversible MAOIs | d85.. | Moclobemide |
| Other antidepressants | gde.. | Duloxetine |
| da1.. | Flupentixol | |
| daB.. | Mirtazapine | |
| daA.. | Reboxetine | |
| da2.. | Tryptophan | |
Referrals to specialists or for further for investigation of gastrointestinal symptoms were defined as shown in Table 24.
| Referral | Read Code | Rubric |
|---|---|---|
| Specialist referral | 8h48. | Gastroenterological referral |
| 8h5J. | Referral to colorectal surgeon | |
| 8H5K. | Referral to upper gastrointestinal surgeon | |
| 8HL8. | Gastroenterology DV done | |
| 8HM8. | Listed for gastroenterology admission | |
| 8Hn4. | Fast-track referral for suspected colorectal cancer | |
| 8Hn9. | Fast-track referral for suspected upper gastrointestinal cancer | |
| 8HS.. | Refer for gastroscopy | |
| 8HS0. | Refer for sigmoidoscopy | |
| 8HU1. | Referral for colonoscopy | |
| 8HU2. | Referral for sigmoidoscopy | |
| 8HVc. | Private referral to colorectal surgeon | |
| 8HVN. | Private referral to gastroenterologist |
We also calculated prevalence estimates based on the Salford population data. Population data were obtained from the Office for National Statistics website for Salford primary care organisation. Rates were standardised to the Greater Manchester population for 2006. This includes the population of the 10 primary care organisations within the Greater Manchester area, which is roughly 2.5 million.
Results
Table 25 shows the number of patients with an IBS, AP or IBD diagnosis in the time period 2002–11. AP was the most commonly reported symptom, followed by IBS and IBD.
| Condition | Number of patients |
|---|---|
| IBS | 8444 |
| AP | 42,490 |
| IBD | 1510 |
Table 26 shows patients’ demographic details. IBS and AP were much more common in females, whereas IBD occurred with similar frequency in males and females. IBS, AP and IBD were most likely to be found in those aged 18–39 years and the frequency declined with age. The proportion of patients aged ≥ 60 years was higher in the IBD group (26%) than in the IBS (16%) or AP group (22%).
| Patient demographic characteristic | Condition | |||||
|---|---|---|---|---|---|---|
| IBS | AP | IBD | ||||
| n | % | n | % | n | % | |
| Female | 6137 | 72.7 | 26,071 | 61.4 | 755 | 50.0 |
| Age (years) | ||||||
| 18–39 | 4373 | 51.8 | 19,662 | 46.3 | 629 | 41.7 |
| 40–59 | 2703 | 32.0 | 13,379 | 31.5 | 487 | 32.3 |
| ≥ 60 | 1368 | 16.2 | 9449 | 22.2 | 394 | 26.1 |
Symptoms pre and post irritable bowel syndrome, abdominal pain or inflammatory bowel disease
Table 27 shows the number of symptoms/diagnoses 1 year prior to and 1 year after the index episode of IBS, AP and IBD. The most commonly reported symptoms in the year prior to IBS were AP (19.5%), diarrhoea (6.1%) and bloating (3.8%). However, a large majority of patients (69.4%) did not have any gastrointestinal symptoms recorded in the year prior to their IBS. Likewise, in the year after the index episode of IBS, the majority of patients did not have any gastrointestinal symptoms recorded (79.1%). AP was the most commonly recorded symptom (13.4%), and 14% of patients had a further episode of IBS recorded in the year after their index episode. IBD showed a similar pattern to IBS with the majority of patients (69.6%) not having any symptoms recorded in the year prior to their IBD diagnosis. The most commonly reported symptoms were AP (15.9%) and diarrhoea (11.8%). In the year after the index episode of IBD, AP and diarrhoea were still the most commonly reported symptoms, but the proportions tended to be lower (10.4% and 5.4%, respectively). Almost one-third of patients had a further episode of IBD recorded in the year after their index episode.
| Symptom/diagnosisa | Condition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS | AP | IBD | ||||||||||
| Pre (N = 7728) | Post (N = 7665) | Pre (N = 39,974) | Post (N = 37,851) | Pre (N = 1336) | Post (N = 1382) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| AP | 1509 | 19.5 | 1028 | 13.4 | – | – | 8683 | 22.9 | 212 | 15.9 | 144 | 10.4 |
| Bloating | 291 | 3.8 | 137 | 1.8 | 411 | 1.0 | 374 | 1.0 | 8 | 0.6 | 7 | 0.5 |
| Constipation symptom | 166 | 2.1 | 138 | 1.8 | 639 | 1.6 | 700 | 1.8 | 16 | 1.2 | 14 | 1.0 |
| Change in bowel habit | 130 | 1.7 | 45 | 0.6 | 175 | 0.4 | 174 | 0.5 | 46 | 3.4 | 5 | 0.4 |
| Diarrhoea symptoms | 475 | 6.1 | 256 | 3.3 | 1046 | 2.6 | 972 | 2.6 | 158 | 11.8 | 74 | 5.4 |
| Nausea | 104 | 1.3 | 100 | 1.3 | 483 | 1.2 | 513 | 1.4 | 6 | 0.4 | 12 | 0.9 |
| Vomiting | 72 | 0.9 | 67 | 0.9 | 583 | 1.5 | 583 | 1.5 | 11 | 0.8 | 19 | 1.4 |
| Diarrhoea and vomiting | 47 | 0.6 | 43 | 0.6 | 202 | 0.5 | 192 | 0.5 | 12 | 0.9 | 6 | 0.4 |
| Tenesmus | 2 | 0.0 | 2 | 0.0 | 6 | 0.0 | 12 | 0.0 | 2 | 0.1 | 0 | 0.0 |
| Faeces/motion symptoms | 31 | 0.4 | 34 | 0.4 | 72 | 0.2 | 80 | 0.2 | 8 | 0.6 | 4 | 0.3 |
| None of the above symptoms | 5367 | 69.4 | 6062 | 79.1 | 36,994 | 92.5 | 27,010 | 71.4 | 930 | 69.6 | 1143 | 82.7 |
| One of the above symptoms | 1950 | 25.2 | 1380 | 18.0 | 2699 | 6.8 | 9555 | 25.2 | 339 | 25.4 | 200 | 14.5 |
| Two or more of the above symptoms | 411 | 5.3 | 223 | 2.9 | 281 | 0.7 | 1286 | 3.4 | 67 | 5.0 | 39 | 2.8 |
| Functional constipation | 151 | 2.0 | 121 | 1.6 | 511 | 1.3 | 573 | 1.5 | 20 | 1.5 | 21 | 1.5 |
| Functional diarrhoea | 31 | 0.4 | 31 | 0.4 | 75 | 0.2 | 118 | 0.3 | 22 | 1.6 | 15 | 1.1 |
| Gastrointestinal infection | 84 | 1.1 | 63 | 0.8 | 295 | 0.7 | 295 | 0.8 | 18 | 1.3 | 8 | 0.6 |
| IBS | – | – | 1090 | 14.2 | 747 | 1.9 | 1061 | 2.8 | 42 | 3.1 | 25 | 1.8 |
| IBD | 33 | 0.4 | 40 | 0.5 | 128 | 0.3 | 204 | 0.5 | – | – | 391 | 28.3 |
Most patients with AP (92.5%) had no symptoms recorded in the year prior to their index episode of AP. In the year after their index episode, 23% of patients had a further AP episode and 2.8% of patients had an IBS diagnosis.
The proportion of patients who reported two or more symptoms in the previous year or the year after IBS, AP or IBD was < 5% in most cases.
Medications pre and post irritable bowel syndrome, abdominal pain or inflammatory bowel disease
Table 28 shows the number of NICE medications prescribed in the year prior to, and after, the index episode of IBS, AP or IBD. NICE medications for IBS were prescribed for 31% of patients in the year prior to their index episode of IBS and for 54% in the year after the index episode. The change was most marked for antispasmodics, prescriptions for which increased from 17% in the year prior to the index episode of IBS to 44% in the year after the index episode. IBS NICE medications were also commonly prescribed in patients with AP and IBD, with about one-third of patients being prescribed NICE medications in the year after the index episode of AP or IBD. Prescribing of antispasmodics increased markedly in those with AP, from 5% before the index episode to 19% after. Approximately 10% of patients with any of the three conditions were prescribed selective serotonin reuptake inhibitors (SSRIs) and about 5% tricyclic antidepressants.
| Medicationa | Condition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS | AP | IBD | ||||||||||
| Pre (N = 7728) | Post (N = 7665) | Pre (N = 39,974) | Post (N = 37,851) | Pre (N = 1336) | Post (N = 1382) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| NICE medications | 2395 | 31.0 | 4099 | 53.5 | 8560 | 21.4 | 13,871 | 36.6 | 389 | 29.1 | 442 | 32.0 |
| Bulking laxatives | 288 | 3.7 | 629 | 8.2 | 754 | 1.9 | 1561 | 4.1 | 40 | 3.0 | 50 | 3.6 |
| Stimulant laxatives | 204 | 2.6 | 247 | 3.2 | 1106 | 2.8 | 1688 | 4.5 | 36 | 2.7 | 59 | 4.3 |
| Faecal softeners | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Osmotic laxatives | 216 | 2.8 | 289 | 3.8 | 845 | 2.1 | 1550 | 4.1 | 27 | 2.0 | 50 | 3.6 |
| Antimotility drugs | 246 | 3.2 | 429 | 5.6 | 727 | 1.8 | 979 | 2.6 | 132 | 9.9 | 169 | 12.2 |
| Antispasmodic drugs | 1328 | 17.2 | 3397 | 44.3 | 1890 | 4.7 | 7035 | 18.6 | 157 | 11.8 | 149 | 10.8 |
| Tricyclic antidepressants | 397 | 5.1 | 553 | 7.2 | 1924 | 4.8 | 2221 | 5.9 | 66 | 4.9 | 78 | 5.6 |
| SSRI | 808 | 10.5 | 957 | 12.5 | 3530 | 8.8 | 3955 | 10.4 | 101 | 7.6 | 129 | 9.3 |
| Other antidepressants | 124 | 1.6 | 147 | 1.9 | 554 | 1.4 | 660 | 1.7 | 12 | 0.9 | 15 | 1.1 |
Tables 29 and 30 show the number of NICE medications prescribed in the year prior to, and the year after, the index episodes of IBS, AP and IBD for those with and without symptoms, respectively. Where gastrointestinal symptoms were recorded in the year prior to, or the year after, the index episode, the proportion of patients prescribed NICE medications was much higher than in those without symptoms. However, there were still a number of patients with no symptoms who received NICE medications in the year prior to their index episode of IBS (22%), AP (18%) or IBD (20%). Among those with IBS, approximately 50% of those with no symptoms recorded in the year after their index episode were prescribed NICE medications, compared with approximately 30% of those with AP or IBD.
| Medicationa | Condition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS | AP | IBD | ||||||||||
| Pre (N = 5367) | Post (N = 6062) | Pre (N = 36,670) | Post (N = 27,010) | Pre (N = 930) | Post (N = 1143) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| NICE medications | 1176 | 21.9 | 2974 | 49.1 | 6720 | 18.3 | 8018 | 29.7 | 191 | 19.9 | 300 | 26.2 |
| Bulking laxatives | 119 | 2.2 | 437 | 7.2 | 569 | 1.6 | 840 | 3.1 | 21 | 2.2 | 32 | 2.8 |
| Stimulant laxatives | 77 | 1.4 | 129 | 2.1 | 795 | 2.2 | 896 | 3.3 | 19 | 2.0 | 33 | 2.9 |
| Faecal softeners | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Osmotic laxativesb | 93 | 1.7 | 152 | 2.5 | 571 | 1.6 | 745 | 2.8 | 13 | 1.4 | 25 | 2.2 |
| Antimotility drugs | 109 | 2.0 | 284 | 4.7 | 423 | 1.2 | 450 | 1.7 | 66 | 6.9 | 119 | 10.4 |
| Antispasmodic drugs | 533 | 9.9 | 2430 | 40.1 | 1414 | 3.9 | 3870 | 14.3 | 55 | 5.7 | 85 | 7.4 |
| Tricyclic antidepressants | 228 | 4.2 | 372 | 6.1 | 1647 | 4.5 | 1364 | 5.0 | 46 | 4.8 | 52 | 4.5 |
| SSRI | 471 | 8.8 | 680 | 11.2 | 3061 | 8.3 | 2488 | 9.2 | 59 | 6.1 | 92 | 8.0 |
| Other antidepressants | 55 | 1.0 | 84 | 1.4 | 472 | 1.3 | 383 | 1.4 | 8 | 0.8 | 10 | 0.9 |
| Medicationa | Condition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS | AP | IBD | ||||||||||
| Pre (N = 2361) | Post (N = 1603) | Pre (N = 3304) | Post (N = 10,841) | Pre (N = 406) | Post (N = 239) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| NICE medications | 1181 | 50.0 | 1103 | 68.8 | 1466 | 44.4 | 5314 | 49.0 | 189 | 46.6 | 135 | 56.5 |
| Bulking laxatives | 169 | 7.2 | 192 | 12.0 | 185 | 5.6 | 721 | 6.7 | 19 | 4.7 | 18 | 7.5 |
| Stimulant laxatives | 127 | 5.4 | 118 | 7.4 | 311 | 9.4 | 792 | 7.3 | 17 | 4.2 | 26 | 10.9 |
| Faecal softeners | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Osmotic laxativesb | 123 | 5.2 | 137 | 8.5 | 274 | 8.3 | 805 | 7.4 | 14 | 3.4 | 25 | 10.5 |
| Antimotility drugs | 137 | 5.8 | 145 | 9.0 | 304 | 9.2 | 529 | 4.9 | 66 | 16.3 | 50 | 20.9 |
| Antispasmodic drugs | 795 | 33.7 | 967 | 60.3 | 476 | 14.4 | 3165 | 29.2 | 102 | 25.1 | 64 | 26.8 |
| Tricyclic antidepressants | 169 | 7.2 | 181 | 11.3 | 277 | 8.4 | 857 | 7.9 | 20 | 4.9 | 26 | 10.9 |
| SSRI | 337 | 14.3 | 277 | 17.3 | 469 | 14.2 | 1467 | 13.5 | 42 | 10.3 | 37 | 15.5 |
| Other antidepressants | 69 | 2.9 | 63 | 3.9 | 82 | 2.5 | 277 | 2.6 | 4 | 1.0 | 5 | 2.1 |
Referrals pre and post irritable bowel syndrome, abdominal pain or inflammatory bowel disease
About 4% of patients had a gastrointestinal referral in either the year prior to or the year after their index episode of IBS (Table 31). This was in contrast to patients with IBD, of whom 9% had a gastrointestinal referral in the year prior to their index episode of IBD and 22% had a gastrointestinal referral in the year after their index episode of IBD. Few patients with AP had a gastrointestinal referral in the year prior to their AP. The number of mental health and lifestyle referrals were low for all three conditions.
| Referralsa | Condition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS | AP | IBD | ||||||||||
| Pre (N = 7728) | Post (N = 7665) | Pre (N = 39,974) | Post (N = 37,851) | Pre (N = 1336) | Post (N = 1382) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Gastrointestinal | 295 | 3.8 | 288 | 3.8 | 358 | 0.9 | 1230 | 3.2 | 123 | 9.2 | 303 | 21.9 |
| Mental health | 3 | 0.0 | 3 | 0.0 | 19 | 0.0 | 21 | 0.1 | 0 | 0.0 | 0 | 0.0 |
| Lifestyle | 24 | 0.3 | 24 | 0.3 | 79 | 0.2 | 99 | 0.3 | 6 | 0.4 | 9 | 0.7 |
Prevalence estimates
Figures 15–17 show the age-specific rates per 100,000 population for IBS, AP and IBD, respectively, for those coded in the SIR data. IBS was much more prevalent in females than in males and peaked in those aged 25–29 years. In females, after this age there is a gradual decline in prevalence by age. In males, the prevalence remains stable until age 50–54 years, after which there is a staged decline in the prevalence rates.
FIGURE 15.
Irritable bowel syndrome age-specific rates: 2002–11.

FIGURE 16.
Abdominal pain age-specific rates: 2002–11.

FIGURE 17.
Inflammatory bowel disease age-specific rates: 2002–11.

The prevalence of AP is much higher than that of IBS or IBD, and higher in females than in males. In females, rates are highest in the youngest age groups up to the age of 30–34 years, after which rates decline until the age of 55–59 years, where the rates gradually increase again to the age of 65–69 years before declining in the oldest age groups. In males, there is a gradual increase in the age-specific rates up to about 60 years, when the rates level out, until they decline in the eldest age group. The rates of IBD are much more similar in males and females and tend to fluctuate across the age groups, with a decline in the older age groups.
Figure 18 shows the age-standardised rates per 100,000 population for IBS, AP and IBD in Salford for the years 2002–11. Rates have been standardised to the Greater Manchester population. The rates for IBD remained stable across the 10-year period for both males and females. IBS shows a similar pattern in males and females, with a slight increase in prevalence rates up to 2006 before levelling out over the second half of the 10-year period. The rates of AP, on the other hand, increased considerably in both males and females from 2002 to about 2009, after which they levelled out for both males and females.
FIGURE 18.
Age-standardised rates: 2002–11. F, female; M, male.
Discussion
The findings from this study demonstrate that the prevalence of IBS in patients who consult, and are recorded by, their GP is low. As highlighted earlier, this may be attributable to a number of factors. The majority of patients who had an IBS Read Code recorded did not have any symptoms prior to, or after, their index date. Antispasmodics were the most prescribed medications for patients with IBS. A small number of patients with IBS were referred to gastrointestinal specialists.
Strengths and limitations
The strengths and limitations of this study are similar to those reported for study 1. This study confirms that the recorded prevalence of IBS in patients who consult, and are recorded by, their GP is low. Several possible explanations for this are given in the Discussion section.
This study also found that the majority of patients who were coded as having IBS did not have gastrointestinal symptoms recorded in the year prior to, or the year after, their index episode. NICE recommends assessment for IBS in patients having any of the following for at least 6 months: AP or discomfort, bloating or change in bowel habit. Therefore, one might expect evidence of this in the recording of symptom codes in the year prior to, or after, the index episode of IBS. GPs in the qualitative study also alluded to the fact that patients may not receive a Read Code for IBS initially, but that they would use Read Codes for AP, diarrhoea or constipation. However, this was not reflected in the data from the SIR. Interestingly, however, IBS medications as recommended by NICE were often prescribed in those without symptoms in the year prior to their IBS. This suggests that the use of medication codes may be an alternative approach to identifying patients via symptom or diagnostic Read Codes. For patients to receive a prescription, GPs must enter a description of the medication, which is then issued electronically. However, some IBS medications (e.g. laxatives) may also be prescribed for other conditions.
The proportion of patients with IBS who were referred to a gastrointestinal specialist was relatively small compared with the proportion of patients with a diagnosis of IBD. Few patients in all three groups had been referred to mental health specialists or for lifestyle advice. This concurs with the findings from the qualitative study where GPs reported being fairly comfortable in managing patients with IBS.
Project 2 overall discussion of findings from the four studies
The use of Read Codes
Read Codes were first introduced in the 1980s and their continual evolution appears to cause problems between users and may be detrimental to the consistency of coding. 118 In addition, the number of Read Codes used to describe a concept can vary widely. For instance, a recent study on the consultation prevalence of musculoskeletal problems in primary care identified over 100 Read Codes to classify knee pain from several different diagnostic chapters (N – musculoskeletal; R – symptoms, signs and ill-defined conditions; S – injury and poisoning; 1 – history/symptoms). 119 About 25% of musculoskeletal consultations were given codes outside the musculoskeletal chapter, including symptoms codes, which some GPs may use prior to making a definite diagnosis. In addition, widespread pain was often recorded using multiple regional pain sites rather than using codes for widespread or generalised pain. Thus, using only codes from the musculoskeletal chapter would underestimate the consultation prevalence for musculoskeletal problems. 119 There is a lack of consistency in the use of Read Codes for diabetes mellitus: only one Read Code was used in all practices, yet just 63% of patients were given this code and other patients were given a diabetes-related code or a prescription code. 120 This study highlights the problems associated with using Read Codes in research for a functional condition. Using Read Codes to identify patients with IBS brings with it further problems, as IBS is typically difficult to diagnose.
Primary care records compared with patient recall
It is apparent that the prevalence of IBS reported in surveys is an overestimate of the consultation prevalence within primary care. Although we did not expect the consultation prevalence for IBS to be as high as that reported in the literature through the use of self-report surveys, we were surprised by how low it was. 95 Previous studies had reported that around 30–70% of patients consulted primary care for their IBS. 97 Furthermore, the literature suggests that GPs do not see IBS as an insignificant problem. 3
One study has found large discrepancies in estimates of consultation prevalence in patients with knee pain when comparing primary care records with patient recall. These discrepancies were a result of ‘telescoping’ on the part of the patients. Telescoping represents a form of recall bias whereby patients underestimate the time since the consultation took place; for example patients may recall a consultation having taken place 12 months ago when it actually occurred 18 months ago. This would lead to an overestimation of the consultation frequency. 121 Another possible reason for the discrepancy was that GPs may not record subsequent consultations if patients have previously consulted for the same condition, in particular if treatment is not provided or changed. Under-recording by GPs may also occur when patients present with multiple problems at consultation.
Interestingly, studies have found that those patients who recalled consultations for knee pain and had widespread pain or greater levels of depression were less likely to have a recorded consultation for their knee pain. 121 Like knee pain, IBS does not have a definite diagnosis and symptoms fluctuate over time; thus, primary care records may reflect the consultation prevalence of IBS only when IBS is a major part of the consultation or when treatment is provided or changed. In addition, Jordan et al. 119 found that the consultation rates increased when the text of the consultations was used in addition to Read Codes alone. Of those patients who recalled having consulted their GP, only 27% had a knee-related Read Code in their medical records, which increased to 40% when the search was extended to include knee problems mentioned in the text. 119 Others have discussed the benefit of narratives during the consultation rather than reducing the clinical encounter to a limited number of codes. 122 Findings from our qualitative study also suggest that this may have been the approach taken by some GPs. However, although using the text from consultations may have increased the number of patients referred to the risk assessment study, it is still unlikely that we would have met our recruitment target.
Health care seeking for irritable bowel syndrome
The findings from the current study show that, within primary care, the consultation rate for coded IBS is not as high as expected from the literature. However, that is not to say that patients do not suffer from symptoms of IBS or that it is a condition that they feel comfortable in managing. There may be a number of explanations as to why patients do not appear to consult for their IBS.
First, patients may consult their GP only when their symptoms are severe and, as a consequence, they perhaps feel less able to cope. Indeed, data from the risk assessment study suggest that the majority of patients had moderate or severe symptoms. However, evidence suggests that symptom severity may have an influence on seeking health care, but that it does explain the majority of the consultation behaviour. 123 Psychological and psychosocial factors have also been implicated in health-care-seeking behaviours among patients with IBS. 124 The present study suggests that a proportion of patients with IBS were prescribed antidepressants in the year before and the year after their index episode. However, we cannot infer whether these medications were prescribed for their gastrointestinal symptoms, for related anxiety or depression, or for unrelated anxiety and depression. GPs in study 3 also suggested that they may focus on related psychological symptoms initially, which may in turn lead to an improvement in gastrointestinal symptoms.
Second, patients may not consult their GP for their IBS because it is in remission, or their symptoms are under control through the use of medication or self-management. A large proportion of patients in the study did not have any symptoms in the year prior to their index episode of IBS but had been prescribed IBS medications, as recommended in the NICE guidelines. However, we are not able to say whether or not these patients had ever been recorded as having IBS prior to the year before their index episode and whether or not they had received a repeat prescription from their GP for their symptoms. Data from our risk assessment study suggested modest improvements in symptom severity and HRQoL over time. However, GPs recruited patients during the consultation or through the use of Read Code searches (with a consultation having occurred in the last 3 months), and it may be that treatment prescribed during the course of that consultation resulted in improved outcomes for patients. Alternatively, these improvements may simply reflect the fluctuating nature of the condition.
Finally, patients may feel that there is little that primary care can offer and, therefore, learn to live with their symptoms. A study reported that patients felt that doctors were unsympathetic and ignorant about IBS and often considered IBS as ‘all being in the mind’ of the patient. Some also felt that GPs were responsible for the worsening of their condition as a result of their ignorance of IBS or ‘through the iatrogenic effects of treatment’. 125 Another study also found that IBS patients report alienation from the health services for similar reasons such as the poor doctor–patient relationship, belief that there is little the NHS can offer and worsening of symptoms as a result of previous medical care. 104 Others have found that moderate to high levels of perceived stigma are significantly more common in IBS patients (27%) than in IBD patients (8%), with the largest difference being for health-care providers. 126 It is therefore perhaps unsurprising that a proportion of those suffering from the symptoms of IBS do not consult their GP and decide to self-medicate or seek alternative therapies. Although alternative therapies are not recommended within the NICE guidance,81 our qualitative study showed that GPs did not discourage their use.
Would a risk assessment tool be helpful?
Despite the problems encountered during the recruitment phase of the study, we were still able to perform some sensitivity and specificity analysis on the risk assessment tool. Even though the study was underpowered, the analysis suggests that the risk assessment tool was sensitive in identifying those who did have severe IBS, but it was poor in identifying those who did not have severe IBS. Whether or not these findings would change as a result of increased study power is unclear. The fact that the reported prevalence of patients consulting for IBS is low also limits the feasibility of such a tool within primary care. The results should also be considered in relation to the qualitative interviews. GPs felt that the use of such an instrument during the consultation was limited, primarily because of the lack of available resources to provide interventions. Many also felt that it would be impracticable to administer such instruments during a 10-minute consultation.
Similarities with other medically unexplained symptoms
Similarities can be seen with other medically unexplained symptoms such as fibromyalgia and chronic fatigue syndrome. For example, a study found the recorded annual prevalence of fibromyalgia in primary care to be 8 per 10,000, which is much lower than the estimated general population prevalence of 2%. This implies that the label of fibromyalgia is rarely used within general practice. 127 This study also found that these patients are similar to those with overlapping functional syndromes or medically unexplained symptoms. Similarly, in a review of medically unexplained symptoms in primary care, many patients with IBS met the criteria for fibromyalgia and chronic pelvic pain. 128 This overlap with other medically unexplained symptoms, which often appear to share similar psychosocial characteristics, creates further complexities.
Implications for future research and clinical practice
The use of symptom or diagnostic Read Codes to identify patients with IBS in primary care is questionable. It is evident that the label IBS, through the use of the IBS diagnostic Read Code, is rarely applied in practice. Similarities can be seen with many other medically unexplained symptoms that are difficult to diagnose in clinical practice, but which may have a common psychological component.
The discrepancies between the self-reported prevalence rates in the literature and those for consultations within the primary care record suggest that there may be conflicting priorities between patients and health-care professionals and that database studies are useful in only identifying the ‘tip of the iceberg’.
Chapter 8 General discussion of programme findings
This general discussion section identifies messages relevant for current clinical practice and for future research into self-management.
Programme organisation
The trial represents one of the largest pragmatic assessments of patient self-management ever conducted, and the level of recruitment and follow-up represents a major logistical achievement.
Despite the achievement of goals in terms of research logistics, the impact of the intervention on organisation of care and patient outcomes was limited. This reflected limits to the engagement with the WISE model and the embedding of the WISE model’s principles and practices. This became evident during the process evaluation and suggests strongly that the process of ‘internalisation’ of the fundamental concepts underpinning the WISE model did not really take place in most practices. As a result, the WISE model was, at best, only minimally adopted. The small number of practices that did show greater adoption of the model could have been usefully targeted for more detailed study, but this was difficult to predict. With regard to the step-up therapies, we again identified a major discrepancy between uptake of therapies that might have been predicted by previous studies and actual uptake in practice.
Implementation of the WISE model: why was this not successful?
We set out to implement a practice-based training programme to enhance outcomes through enhanced self-management, which involved a number of steps:
-
engaging a high proportion of practices with the programme
-
delivering training to a high number of clinicians and other staff
-
ensuring that training was relevant and acceptable
-
encouraging implementation of the training in routine practice
-
enhancing shared decision-making and self-management
-
improving outcomes.
Our data show that, although the study had reasonable success at some steps, we probably lost significant ‘potency’ (in terms of translating outcomes from research into routine practice) at step 4, and what was achieved at stage 4 completely failed to generate changes to steps 5 and 6. Achieving the first three steps may have required so many compromises in terms of time and ongoing support that it failed to translate further. It is also possible that our judgements about impact at step 3 (in terms of the acceptability of training) were overstated, given the limited nature of the evaluations (i.e. self-report staff evaluations).
We consider some of the reasons for the failure below.
Despite some evidence of PCT engagement (organisational ‘buy-in’ at board level, reconfiguration of its self-management programme around the WISE model and reallocation of a sizable proportion of its training budget to support the roll out), it is evident that the PCT was able to exert only minimal pressure on GP behaviour.
Although a research project, implementation of the WISE model within primary care may have been more successful if treated like the implementation of any other service redesign, with full engagement of the PCT commissioning staff. This may have involved consultation with practices prior to implementation and recognition that different approaches may have been needed for different practices. As individual providers, GP practices work in a variety of ways (including the role of practice nurses in the management of patients). Implementation may have been improved if, as part of the study, dedicated Salford commissioning staff had been funded. These staff would be experienced in introducing new services in Salford and working with practices.
As suggested by the process evaluation, implementation in the context of a QOF ‘pay for performance’ climate may mean that general behaviours (such as self-management support) will continue to take second place to those that are ‘mandatory’. GPs, as small independent providers, need to recover the cost of providing services through income. The nature of the GP contract means that practices receive income through a contract to provide a core service and to deliver key measures defined in the QOF. Additional activities can be contracted via the use of direct and local enhanced services contracts. The development of local enhanced services contracts within primary care has proved successful in embedding new ways of working within primary care. These generate additional practice income, helping practices to invest time and other resources to deliver the desired outcomes of the local enhanced services. Some of the difficulties experienced with practice engagement in the training and implementation of the WISE model could have been mitigated by the use of such a contract.
Engagement with step-up therapies was very low. A major issue to be considered further is the extent to which patients with IBS symptoms in primary care are accepting of a psychological component of their condition as an explanation or a route to management. Our results suggest that many patients coded as IBS in general practice are generally happy with the label and do not request to take matters further, either by seeking secondary care investigations or more complex therapy. It also seems evident that at least a proportion of GPs felt that referral for psychological interventions was not in the interest of the patient, as it took the condition beyond a simple description and into a psychiatric dimension.
It is suggested that future endeavours to both explain to and console patients with medically unexplained abdominal symptoms in a health-care system need to recognise the spectrum of the disorder and recognise the dimension of severity in addition to that of the nature and duration of symptoms, which is not adequately expressed by the currently employed symptom-based criteria.
Although a proportion of patients who are genuinely distressed by their symptoms fall into the symptom-based categorisation of IBS, it is apparent that many others who also meet the IBS ‘case definition’ have much less debilitating symptoms.
Several strategies were used to increase referrals to the therapies. It may also have been beneficial for communications to practices to have been incorporated into regular PCT commissioning communications. This includes monthly key messages about new services or pathways. However, practices receive so much information that it is difficult for it all to be effectively received.
The implementation of the WISE model and the changing primary care context
What are the implications of the study for the WISE model? We have identified a number of potential barriers to implementation that led to a failure to deliver the core features of the intervention in routine practice and it is possible that the WISE model, backed by a more effective implementation programme, could still be clinically effective and cost-effective. However, there is potential to modify the model itself. This might include adoption of digital technologies to better support patient and practitioner behaviour change, targeting other conditions or patient groups and alignment with other aspects of the long-term condition management infrastructure, such as care planning. 129,130
As part of the Health and Social Care Act, PCTs were abolished. 131 From 1 April 2013, the duties of PCTs transferred to various organisations, primarily Clinical Commissioning Groups (CCGs) for the commissioning of most hospital, community and mental health services, NHS England for the commissioning of primary care and specialist services and local authorities in respect of public health. CCG budgets are identified at individual practice level and practices are held to account for budget management. This creates an incentive for GP practices to modify their working practices to make the most efficient use of their commissioning budget. This could include embedding within general practice any activities or services that reduce secondary care utilisation and costs, such as self-care. CCGs also have a duty to improve the quality of primary medical care, which will involve an emphasis on GP and practice staff training and a reduction in the variation within general practice. Should a CCG consider self-care as a strategic priority and choose to implement the WISE model approach, this could have been identified from its strategic business planning process and be included as a priority area of work within the strategic commissioning plan. In this way, self-care and the WISE model would become a priority.
It is possible that, should the WISE model be identified as a strategic priority, implementation by CCGs could be more successful than experienced in this study, as greater levers exist in CCGs than in PCTs to implement commissioning priorities. Within CCGs, practices are able to access management and financial resources to help them implement changes. The experience of the NIHR-funded 3D study (which uses a similar practice-based model involving elements of self-management support) will be a potential test case for this hypothesis. 132,133
This study provides insight into implementing new ways of working in primary care, which is valuable to CCGs in their duty of improving the quality of primary care. Lessons learnt include the importance of incentivising practices and reimbursing them for the cost of implementation, using appropriate service improvement and commissioning methodologies and aligning pilots and roll-outs with mainstream commissioning processes and resources.
With regard to the issues of symptom chronicity, and the use of the assessment tool, several messages arise. One particular issue is the ability of GPs to cope with an increasing number of such tools across all specialties and assimilate them into daily activity. The study also showed that, with regard to IBS, the case definition used in primary care is highly inconsistent. This remains so despite attempts for over a decade to identify useable case definitions (in the form of ROME diagnostic criteria4,98,99).
We found that few patients who were given the term IBS had been, or were currently, attending secondary care for the condition, leading to the suspicion that in a population such as Salford the overwhelming proportion of patients meeting the diagnostic symptom criteria for IBS have a relatively mild condition. The qualitative interviews with GPs about IBS suggest that a strongly pragmatic, empirical approach is utilised by most. This almost certainly explains why the prevalence of IBS in our population was substantially lower than that which was predicted from either previous data or case definitions obtained by direct patient contact. In addition, our results show how difficult it is to use symptom-based clinical criteria for health services research in the NHS. A case for tighter case definition in primary care (exemplified by the ROME diagnostic criteria4,98,99) could be made, but it is difficult to see how and why this would provide beneficial.
The lack of uptake of aspects of the programme among patients and professionals suggests that our assumptions about the need for improvements in management of FGID are not necessarily shared.
Implications of the study for research
The Programme Grants for Applied Research programme provided significant financial support for a long-term programme of implementation and evaluation, but this did not result in a clinically effective or cost-effective intervention.
The results highlight the need for further research into large-scale change in management of long-term conditions, given the modest results of recent interventions, including telehealth, the Expert Patients Programme and integration of care.
There is an argument that the intervention was insufficiently piloted, given the focus placed on the use of pilot and feasibility studies within the NIHR research programmes. A counterargument is that piloting of some form had been done of aspects of the WISE model in other trials (see Chapter 1), and there was some preliminary work within practices to assess likely problems, although this work lacked the specificity and detail that would be expected of a formal pilot. Piloting would have potentially led to an earlier indication of the likely problems, leading either to a change in the model of implementation or a change in the research itself (including ending the RCT). However, the potential of pilots should not be overstated. Testing models in a small number of volunteer pilots can raise important issues, but is unlikely to ever provide a complete ‘diagnosis’ of the likely barriers to be faced in scaling up across multiple practices and contexts. The addition of a pilot also potentially adds significantly to the cost and duration of a trial, with that investment only potentially being recouped in a more effective intervention.
The results of the RCT might have more profound implications for large-scale implementation efforts. A more radical alternative suggested by a reviewer of this report would be to undertake a ‘small-scale, formative and more collaborative action–research-style pilot with a handful of practices which treated them as active participants in shaping the intervention’. This would involve much closer working between the research team and the local commissioners and providers, and involve practice teams much more in the development and implementation of the intervention. ‘Working with’ primary care to implement changes around long-term conditions and self-management support might be a preferable model to the more standardised implementation adopted here. However, such an approach would again extend study timelines beyond even the generous confines of the Programme Grants for Applied Research funding, increasing cost, making studies even more vulnerable to contextual change outside the trial and putting potentially serious limits on the ability to undertake rigorous evaluation of the type that is currently prioritised in the UK. This reflects the ongoing debate between those who advocate a conventional (although modified) model of evaluation, even in the context of large-scale service change, and those who call for a different model. 134–136
More research needs to be conducted exploring professional and patient attitudes and experiences of the management of functional disorders, to ensure that assumptions about the need for improvements in management of FGID are shared, and that interventions are seen as having utility by stakeholders.
Acknowledgements
We would like to thank the following non-academic contributors: Karen Proctor (Head of Performance & Commissioning Support, Salford CCG); Mike Burrows (Chief Executive, Salford PCT); Jim Nuttall (lay representative); David Backhouse (lay representative); Karen Armstrong (lay representative); and Martin White (Reporting Accountant, Salford Royal NHS Foundation Trust).
We would like to thank all the GPs who gave up their time to be interviewed and all the patients who agreed to take part in the risk assessment study. We thank Julie Want, Victoria Lee and Kris Mackay for their assistance with practice recruitment and training, and the wider members of the research team for their work (Tom Blakeman, Robert Bowen, Martin Eden, Catherine Fullwood, Hannah Gaffney, Caroline Gardner, Rebecca Morris, Joanne Protheroe, Caroline Sanders and Angela Swallow). Linda Gask helped develop the training programme and the National PCRN and Development Centre (funded by the Department of Health) also provided funding for the WISE trial.
We thank Val Harrington for her assistance with the GP interviews and for her contribution to the data analysis and report writing for the qualitative study; Sue Hinder for her contribution to the data analysis and report writing for the qualitative study; Hannah Jackson, Andy Brass and Leila Kalankesh for their contribution to GP database study 1; Laura Grant for her contribution to the data analysis and report writing for the risk assessment tool and GP database study 2; and Ann Durie and Kinjiro Amano for all their help and assistance with administrative aspects of the study.
We also thank Dr Joanna Roberts (CBT therapist) and Lisa Childs (hypnotherapist) who were responsible for delivering the psychological therapies. We acknowledge Yasmin Moore for her support in the completion of the final report.
The research team acknowledges the support of the NIHR, through the PCRN.
Contributions of authors
David G Thompson was an applicant on the grant and was responsible for project 2.
Sarah O’Brien was an applicant on the grant and was responsible for project 2.
Anne Kennedy was an applicant on the grant and was responsible for project 1.
Anne Rogers was an applicant on the grant and was responsible for project 1.
Peter Whorwell was an applicant on the grant and was responsible for project 1.
Karina Lovell was an applicant on the grant and was responsible for project 1.
Gerry Richardson was an applicant on the grant and was responsible for project 1.
David Reeves was an applicant on the grant and was responsible for project 1.
Peter Bower was an applicant on the grant, was responsible for project 1 and co-ordinated the report.
Carolyn Chew-Graham was an applicant on the grant and was responsible for project 1.
Elaine Harkness was responsible for project 2.
Paula Beech was responsible for project management of the grant.
Publications
Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trail of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long-term conditions in primary care: trial protocol. Implement Sci 2012;7:7.
Fullwood C, Kennedy A, Rogers A, Eden M, Gardner C, Protheroe J, et al. Patients’ experiences of shared decision making in primary care practices in the United Kingdom. Med Decis Making 2013;33:26–36.
Harkness E, Grant L, O’Brien SJ, Chew-Graham CA, Thompson DG. Using read codes to identify patients with irritable bowel syndrome in general practice: a database study. BMC Fam Pract 2013;14:183.
Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self-management support for long-term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;13:346.
Harkness EF, Harrington V, Hinder S, O’Brien SJ, Thompson DG, Beech P, et al. GP perspectives of irritable bowel syndrome – an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract 2013;14:92.
Kennedy A, Rogers A, Bowen R, Lee V, Blakeman T, Gardner C, et al. Implementing, embedding and integrating self-management support tools for people with long-term conditions in primary care nursing: a qualitative study. Int J Nurs Stud 2014;51:1103–13.
Presentations and abstracts
Kennedy A. Whole System Primary Care Based Models For Support For Self-Management. Oral presentation, University of Stirling, Stirling, UK, 2009.
Kennedy A. Providing Self-Care Support in the NHS: a Trial of the WISE Approach in Primary Care. Oral presentation, Barts and London School of Medicine, London, UK, 2009.
Kennedy A. Using the WISE Approach to Support Patient Self-Management in Primary Care. Oral presentation, National Primary Care Research & Development Centre, Manchester, UK, 2009.
Protheroe J. Whole System Primary Care Based Models for Support for Self-Management. Oral presentation, the Centre for Clinical Epidemiology and Evaluation, Vancouver, BC, Canada, 2009.
Protheroe J. PRISMS. Oral presentation, Stirling International Conference on Support for Self-Management of Health, Stirling, UK, 2010.
Kennedy A. Supporting Patient Self-Management: The WISE (Whole System Informing Self-management Engagement) Approach. Oral presentation, Canadian Institutes of Health Research Primary Healthcare Summit, Toronto, ON, Canada. 2010.
WISE Team. A Day-Long Workshop/Seminar on the WISE Approach and Related Research. Oral presentation, visiting academics from Glasgow and Stirling Universities. National Primary Care Research & Development Centre, Manchester, UK, 2010.
Kennedy A and Protheroe J. WISE Approach. Webinar and teleconference, Hamilton, BC, Canada, 2010.
Kennedy A. Using the WISE Approach to Support Self-Care in People with Long-Term Conditions. Oral presentation, Keele University, Newcastle, UK, 2010.
Kennedy A. Making Self-Care Support Work in the Health Service: The WISE Approach. Oral-plenary, International Conference on Support for Self-Management of Health, University of Stirling, Stirling, UK, 2010
Kennedy A. Development and Roll Out of an Innovative Approach to Supporting Self-Care in Primary Care. Oral presentation, Society for Academic Primary Care Conference, Norwich, UK, 2010.
Protheroe J. PRISMS. Oral presentation, Annual Society for Academic Primary Care Conference, Norwich, UK, 2010.
Kennedy A. NPT Questionnaire and WISE Process Evaluation. Oral presentation, NPT workshop, London, UK, 2010.
Kennedy A. The WISE Approach to Self-Care Support in Primary Care: Development and Evaluation. Oral presentation, Manchester Academic Health Science Centre, Manchester, UK, 2011.
Kennedy A. Developing and Evaluating an Innovative Approach to Supporting Self-Care in the English Health Service. Oral presentation, Australian Disease Management Association Conference, Canberra, ACT, Australia, 2011.
Kennedy A. Promoting and Supporting Self-Management – Exploring a Health Services Approach. Oral workshop, Queensland Self-Management Alliance Forum, Brisbane, QLD, Australia, 2011.
Kennedy A. How Can Primary Health Care Services Support People to Self-Manage Long-Term Conditions? Oral presentation, University of Queensland, Brisbane, QLD, Australia, 2011.
Protheroe J. PRISMS. Oral presentation, International Conference of Communication in Healthcare, Chicago, IL, USA, 2011.
Protheroe J. WISE Baseline Data. Poster presentation, International Conference of Communication in Healthcare, Chicago, IL, USA, 2011.
Blakeman T. WISE. Oral presentation, Mayo Clinic, Rochester, NY, USA, 2012.
Harrington V and Chew-Graham C. Results from Qualitative Interviews with GPs in the BITMAP Project. Poster presentation, Royal College of General Practitioners Conference, London, UK, 2012.
Kennedy A. Building Healthcare Systems to Support Self-Care. Oral plenary, 6th World Conference of the International Primary Care Respiratory Group, Edinburgh, UK, 2012.
Kennedy A. Implementing Self-Care and Self-Management. Oral presentation, 8th Annual World Health Care Congress Europe, Amsterdam, the Netherlands, 2012.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Requests must include a short proposal and proposed research and analysis plan. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Jones RH. Clinical economics review: gastrointestinal disease in primary care. Aliment Pharmacol Ther 1996;10:233-9. https://doi.org/10.1111/j.0953-0673.1996.00233.x.
- Switz DM. What the gastroenterologist does all day. A survey of a state society’s practice. Gastroenterology 1976;70:1048-50.
- Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46:78-82. https://doi.org/10.1136/gut.46.1.78.
- Drossman D, Corazziari E, Delvaux M, Spiller R, Talley N, Thompson W, et al. ROME III: The Functional Gastrointestinal Disorders. New Haven, CT: Yale University Section of Digestive Disease, Degnon Associates; 2006.
- Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al. A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self-help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7280.
- Kennedy A, Nelson E, Reeves D, Richardson G, Robinson A, Rogers A, et al. A randomised controlled trial to assess effectiveness and cost of a patient orientated self-management approach to chronic inflammatory bowel disease. Gut 2004;53:1639-45. https://doi.org/10.1136/gut.2003.034256.
- Kennedy A, Robinson A. A Handy Guide to Managing Irritable Bowel Syndrome. Southampton: RTFB Publishing Limited; 2000.
- Kennedy A, Robinson A. A Handy Guide to Managing Ulcerative Colitis. Southampton: RTFB Publishing Limited; 2002.
- Kennedy A, Robinson A. A Handy Guide to Managing Crohn’s Disease. Southampton: RTFB Publishing Limited; 2000.
- Kennedy A, Robinson A, Rogers A. Incorporating patients’ views and experiences of life with IBS in the development of an evidence based self-help guidebook. Patient Educ Couns 2003;50:303-10. https://doi.org/10.1016/S0738-3991(03)00054-5.
- Kennedy AP, Robinson AJ, Thompson DG, Wilkin D. Development of a guidebook to promote patient participation in the management of ulcerative colitis. Health Soc Care Community 1999;7:177-86. https://doi.org/10.1046/j.1365-2524.1999.00174.x.
- Kennedy A, Robinson J, Hann M, Thompson D, Wilkin D. A cluster randomized controlled trial of a patient-centred guidebook for patients with ulcerative colitis: effect on knowledge, anxiety and quality of life. Health Soc Care Community 2003;11:64-72. https://doi.org/10.1046/j.1365-2524.2003.00399.x.
- Kennedy AP, Rogers AE. Improving patient involvement in chronic disease management: the views of patients, GPs and specialists on a guidebook for ulcerative colitis. Patient Educ Couns 2002;47:257-63. https://doi.org/10.1016/S0738-3991(01)00228-2.
- Kennedy A, Rogers A, Bower P. Support for self care for patients with chronic disease. BMJ 2007;335:968-70. https://doi.org/10.1136/bmj.39372.540903.94.
- Robinson A, Lee V, Kennedy A, Middleton L, Rogers A, Thompson DG, et al. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of irritable bowel syndrome. Gut 2006;55:643-8. https://doi.org/10.1136/gut.2004.062901.
- Miller V, Whorwell PJ. Hypnotherapy for functional gastrointestinal disorders: a review. Int J Clin Exp Hypn 2009;57:279-92. https://doi.org/10.1080/00207140902881098.
- Whorwell PJ. Behavioral therapy for IBS. Nat Clin Pract Gastroenterol Hepatol 2009;6:148-9. https://doi.org/10.1038/ncpgasthep1361.
- Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard School of Public Health, Harvard University Press; 1996.
- Clark N, Becker M, Janz N, Lorig K, Rawkowski W, Anderson L. Self management of chronic disease by older adults: a review and questions for research. J Aging Health 1991;3:3-27. https://doi.org/10.1177/089826439100300101.
- Wanless D. Securing Our Future Health: Taking a Long-term View. London: HM Treasury; 2002.
- Bower P, Blakeman T, Kennedy A, Protheroe J, Richardson G, Rogers A, et al. What Influences People to Self Care?. Manchester: University of Manchester; 2009.
- Cartwright M, Hirani S, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (whole systems demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f653.
- Lorig KR, Ritter PL, Laurent DD, Fries JF. Long-term randomized controlled trials of tailored-print and small-group arthritis self-management interventions. Med Care 2004;42:346-54. https://doi.org/10.1097/01.mlr.0000118709.74348.65.
- Lorig KR, Sobel DS, Stewart AL, Brown BW, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care 1999;37:5-14. https://doi.org/10.1097/00005650-199901000-00003.
- Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, et al. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254-61. https://doi.org/10.1136/jech.2006.053538.
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322-7. https://doi.org/10.2105/AJPH.89.9.1322.
- Ham C. Chronic care in the English National Health Service: progress and challenges. Health Aff 2009;28:190-201. https://doi.org/10.1377/hlthaff.28.1.190.
- Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ 2003;327:1219-21. https://doi.org/10.1136/bmj.327.7425.1219.
- Starfield B. Is primary care essential?. Lancet 1994;344:1129-33. https://doi.org/10.1016/S0140-6736(94)90634-3.
- Starfield B. Primary Care: Concept, Evaluation and Policy. New York, NY: Oxford University Press; 1992.
- Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med 2007;5:196-201. https://doi.org/10.1370/afm.679.
- Blakeman T, Bower P, Reeves D, Chew-Graham C. Bringing self-management into clinical view: a qualitative study of long-term condition management in primary care consultations. Chronic Illn 2010;6:136-50. https://doi.org/10.1177/1742395309358333.
- Blakeman T, Macdonald W, Bower P, Gately C, Chew-Graham C. A qualitative study of GPs’ attitudes to self-management of chronic disease. Br J Gen Pract 2006;56:407-14.
- Checkland K, Harrison S, McDonald R, Grant S, Campbell S, Guthrie B. Biomedicine, holism and general medical practice: responses to the 2004 General Practitioner contract. Sociol Health Illness 2008;30:788-803. https://doi.org/10.1111/j.1467-9566.2008.01081.x.
- Kennedy A, Rogers A. Improving self-management skills: a whole systems approach. Br J Nurs 2001;10:734-7. https://doi.org/10.12968/bjon.2001.10.11.10435.
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167-82. https://doi.org/10.1111/1467-9566.ep11339939.
- Bury M. The sociology of chronic illness: a review of research and prospects. Sociol Health Illn 1991;13:451-68. https://doi.org/10.1111/j.1467-9566.1991.tb00522.x.
- Bury M, Newbould J, Taylor D. A Rapid Review of the Current State of Knowledge Regarding Lay Led Self-management of Chronic Illness: Evidence Review. London: National Institute for Health and Care Excellence; 2005.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London; 2008.
- Kennedy A, Lovell K. A Handy Guide to Managing Depression and Anxiety. Southampton: RTFB Publishing Limited; 2002.
- Kennedy A, Gask L, Rogers A. Training professionals to engage with and promote self-management. Health Educ Res 2005;20:567-78. https://doi.org/10.1093/her/cyh018.
- Kennedy A, Chew-Graham C, Blakeman T, Bowen A, Gardner C, Protheroe J, et al. Delivering the WISE (Whole Systems Informing Self-Management Engagement) training package in primary care: learning from formative evaluation. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-7.
- Protheroe J, Blakeman T, Bower P, Chew-Graham C, Kennedy A. An intervention to promote patient participation and self-management in long term conditions: development and feasibility testing. BMC Health Serv Res 2010;10. https://doi.org/10.1186/1472-6963-10-206.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999;49:651-61. https://doi.org/10.1016/S0277-9536(99)00145-8.
- Charles C, Gafni A, Whelan T, O’Brien MA. Treatment decision aids: conceptual issues and future directions. Health Expect 2005;8:114-25. https://doi.org/10.1111/j.1369-7625.2005.00325.x.
- Grant C, Goodenough T, Harvey I, Hine C. A randomised controlled trial and economic evaluation of a referrals facilitator between primary care and the voluntary sector. BMJ 2000;320:419-23. https://doi.org/10.1136/bmj.320.7232.419.
- Gask L. Small group interactive techniques utilizing video feedback. Int J Psychiatry Med 1998;28:97-113. https://doi.org/10.2190/U8MM-JX7Y-LT0T-RKPX.
- Houghton L, Hetyman D, Whorwell P. Symptomatology, quality of life and economic features of irritable bowel syndrome – the effect of hypnotherapy. Aliment Pharmacol Ther 1996;10:91-5. https://doi.org/10.1111/j.1365-2036.1996.tb00181.x.
- Mikocka-Walus A, Turnbull D, Moulding N, Wilson I, Andrews JM, Holtmann G. Psychological comorbidity and complexity of gastrointestinal symptoms in clinically diagnosed irritable bowel syndrome patients. J Gastroenterol Hepatol 2008;23:1137-43. https://doi.org/10.1111/j.1440-1746.2007.05245.x.
- Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol 2004;72:1100-13. https://doi.org/10.1037/0022-006X.72.6.1100.
- Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet 1984;2:1232-4. https://doi.org/10.1016/S0140-6736(84)92793-4.
- Depression: Management of Depression in Primary and Secondary Care. London: National Institute for Health and Care Excellence; 2007.
- Pinnock H, Epiphaniou E, Taylor S. Phase IV implementation studies. The forgotten finale to the complex intervention methodology framework. Ann Am Thorac Soc 2014;11:S118-22. https://doi.org/10.1513/AnnalsATS.201308-259RM.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f2882.
- Williams G, McGregor H, Kind D, Nelson C, Glasgow R. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Educ Couns 2005;57:39-45. https://doi.org/10.1016/j.pec.2004.04.001.
- Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage; 1996.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1258.
- Torgerson DJ. Contamination in trials: is cluster randomisation the answer?. BMJ 2001;322:355-7. https://doi.org/10.1136/bmj.322.7282.355.
- Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long- term conditions in primary care: trial protocol. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-7.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. https://doi.org/10.1136/bmj.300.6719.230.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- Curtis L. Unit Costs of Health and Social Care 1998. Canterbury: Personal Social Services Research Unit, University of Kent; 1998.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Kennedy A, Rogers A, Bowen R, Lee V, Blakeman T, Gardner C, et al. Implementing, embedding and integrating self-management support tools for people with long-term conditions in primary care nursing: a qualitative study. Int J Nurs Stud 2014;51:1103-13. https://doi.org/10.1016/j.ijnurstu.2013.11.008.
- Wanless D, Forder J. Securing Good Care for Older People: Taking a Long-Term View 2006. www.kingsfund.org.uk/sites/default/files/field/field_publication_file/securing-good-care-for-older-people-wanless-2006.pdf (accessed 27 October 2017).
- May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-86.
- Macdonald W, Rogers A, Blakeman T, Bower P. Practice nurses and the facilitation of self-management in primary care. J Adv Nurs 2008;62:191-9. https://doi.org/10.1111/j.1365-2648.2007.04585.x.
- Bodenheimer T, Berry-Millet R. Care Management of Patients with Complex Health Care Needs. Research Synthesis report No. 19. San Francisco, CA: University of California Center for Excellence in Primary Care; 2009.
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469-75. https://doi.org/10.1001/jama.288.19.2469.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA 2002;288:1909-14. https://doi.org/10.1001/jama.288.15.1909.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: Normalization Process Theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Morris R, Sanders C, Kennedy A, Rogers A. Shifting priorities in multimorbidity: a longitudinal qualitative study of patient’s prioritization of multiple conditions. Chronic Illn 2011;7:147-61. https://doi.org/10.1177/1742395310393365.
- Rogers A, Vassilev I, Sanders C, Kirk S, Chew-Graham C, Kennedy A, et al. Social networks, work and network-based resources for the management of long-term conditions: a framework and study protocol for developing self-care support. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-56.
- Halder SL, Locke GR, Schleck CD, Zinsmeister AR, Melton LJ, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007;133:799-807. https://doi.org/10.1053/j.gastro.2007.06.010.
- Irritable Bowel Syndrome in Adults: Diagnosis and Management Clinical Guideline. London: National Institute for Health and Care Excellence; 2008.
- Halder S, Macfarlane GJ, Thompson D, O’Brien SJ, Musleh M, McBeth J. Predictors of persistent gastrointestinal symptoms among new presenters to primary care. Eur J Gastroenterol Hepatol 2010;22:296-305. https://doi.org/10.1097/MEG.0b013e32832bab61.
- Tai TW, Anandarajah S, Dhoul N, de Lusignan S. Variation in clinical coding lists in UK general practice: a barrier to consistent data entry?. Inform Prim Care 2007;15:143-50.
- Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ 2002;324:477-80. https://doi.org/10.1136/bmj.324.7335.477.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480-91. https://doi.org/10.1053/j.gastro.2005.11.061.
- Soler JK, Pringle M. Research using electronic patient records in general practice. The EGPRN meeting in Bertinoro, Italy, May 2009. Eur J Gen Pract 2010;16:186-9. https://doi.org/10.3109/13814788.2010.501374.
- Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011;164:602-9. https://doi.org/10.1111/j.1365-2133.2010.10134.x.
- Seaburn DB, Morse D, McDaniel SH, Beckman H, Silberman J, Epstein R. Physician responses to ambiguous patient symptoms. J Gen Intern Med 2005;20:525-30. https://doi.org/10.1111/j.1525-1497.2005.0093.x.
- Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 1997;314:779-82. https://doi.org/10.1136/bmj.314.7083.779.
- Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract 2004;54:495-502.
- Goldberg D. Manual of the General Health Questionnaire. Windsor: Nelson National Foundation for Educational Research; 1978.
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395-402. https://doi.org/10.1046/j.1365-2036.1997.142318000.x.
- Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998;43:400-11. https://doi.org/10.1023/A:1018831127942.
- Office for National Statistics, Postcode Directories . GeoConvert 2015. http://geoconvert.mimas.ac.uk/ (accessed 27 October 2017).
- Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis 2009;41:772-80. https://doi.org/10.1016/j.dld.2009.07.005.
- Kruis W, Thieme C, Weinzierl M, Schussler P, Holl J, Paulus W. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterol 1984;87:1-7.
- Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J 1978;2:653-4. https://doi.org/10.1136/bmj.2.6138.653.
- Thompson WG, Creed FH, Drossman DA, Heaton KW, Mazzacca G. Functional bowel disorders and functional abdominal pain. Gastroenterol Int 1992;5:75-91.
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut 1999;45:1143-7. https://doi.org/10.1136/gut.45.2008.ii43.
- Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 2003;17:643-50. https://doi.org/10.1046/j.1365-2036.2003.01456.x.
- Akehurst RL, Brazier JE, Mathers N, O’Keefe C, Kaltenthaler E, Morgan A, et al. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. PharmacoEconomics 2002;20:455-62. https://doi.org/10.2165/00019053-200220070-00003.
- Casiday RE, Hungin AP, Cornford CS, de Wit NJ, Blell MT. Patients’ explanatory models for irritable bowel syndrome: symptoms and treatment more important than explaining aetiology. Fam Pract 2009;26:40-7. https://doi.org/10.1093/fampra/cmn087.
- Dixon-Woods M, Critchley S. Medical and lay views of irritable bowel syndrome. Fam Pract 2000;17:108-13. https://doi.org/10.1093/fampra/17.2.108.
- Farndale R, Roberts L. Long-term impact of irritable bowel syndrome: a qualitative study. Prim Health Care Res Dev 2011;12:52-67. https://doi.org/10.1017/S1463423610000095.
- Bijkerk CJ, de Wit NJ, Stalman WA, Knottnerus JA, Hoes AW, Muris JW. Irritable bowel syndrome in primary care: the patients’ and doctors’ views on symptoms, etiology and management. Can J Gastroenterol 2003;17:363-8. https://doi.org/10.1155/2003/532138.
- Yale SH, Musana AK, Kieke A, Hayes J, Glurich I, Chyou PH. Applying case definition criteria to irritable bowel syndrome. Clin Med Res 2008;6:9-16. https://doi.org/10.3121/cmr.2008.788.
- Henwood KL, Pidgeon NF. Qualitative research and psychological theorizing. Br J Psychol 1992;83:97-111. https://doi.org/10.1111/j.2044-8295.1992.tb02426.x.
- McGowan L, Escott D, Luker K, Creed F, Chew-Graham C. Is chronic pelvic pain a comfortable diagnosis for primary care practitioners: a qualitative study. BMC Fam Pract 2010;11. https://doi.org/10.1186/1471-2296-11-7.
- Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome: the view from general practice. Eur J Gastroenterol Hepatol 1997;9:689-92. https://doi.org/10.1097/00042737-199707000-00008.
- Chew-Graham CA, Cahill G, Dowrick C, Wearden A, Peters S. Using multiple sources of knowledge to reach clinical understanding of chronic fatigue syndrome. Ann Fam Med 2008;6:340-8. https://doi.org/10.1370/afm.867.
- Williams RE, Black CL, Kim HY, Andrews EB, Mangel AW, Buda JJ, et al. Stability of irritable bowel syndrome using a Rome II-based classification. Aliment Pharmacol Ther 2006;23:197-205. https://doi.org/10.1111/j.1365-2036.2006.02723.x.
- Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther 2004;20:31-9. https://doi.org/10.1111/j.1365-2036.2004.02183.x.
- Guthrie E. Medically unexplained symptoms in primary care. Advances in Psychiatric Treatment 2008;14:432-40. https://doi.org/10.1192/apt.bp.106.003335.
- Dhaliwal SK, Hunt RH. Doctor-patient interaction for irritable bowel syndrome in primary care: a systematic perspective. Eur J Gastroenterol Hepatol 2004;16:1161-6. https://doi.org/10.1097/00042737-200411000-00013.
- Malpass A, Shaw A, Kessler D, Sharp D. Concordance between PHQ-9 scores and patients’ experiences of depression: a mixed methods study. Br J Gen Pract 2010;60:e231-e8. https://doi.org/10.3399/bjgp10X502119.
- Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet 2011;378:1560-71. https://doi.org/10.1016/S0140-6736(11)60937-9.
- Sanders T, Foster NE, Ong BN. Perceptions of general practitioners towards the use of a new system for treating back pain: a qualitative interview study. BMC Med 2011;9. https://doi.org/10.1186/1741-7015-9-49.
- Robinson D, Schulz E, Brown P, Price C. Updating the Read Codes: user-interactive maintenance of a dynamic clinical vocabulary. J Am Med Inform Assoc 1997;4:465-72. https://doi.org/10.1136/jamia.1997.0040465.
- Jordan K, Porcheret M, Croft P. Quality of morbidity coding in general practice computerized medical records: a systematic review. Fam Pract 2004;21:396-412. https://doi.org/10.1093/fampra/cmh409.
- Gray J, Orr D, Majeed A. Use of Read codes in diabetes management in a south London primary care group: implications for establishing disease registers. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7399.1130.
- Jordan K, Jinks C, Croft P. Health care utilization: measurement using primary care records and patient recall both showed bias. J Clin Epidemiol 2006;59:791-7. https://doi.org/10.1016/j.jclinepi.2005.12.008.
- Walsh SH. The clinician’s perspective on electronic health records and how they can affect patient care. BMJ 2004;328:1184-7. https://doi.org/10.1136/bmj.328.7449.1184.
- Koloski NA, Talley NJ, Boyce PM. Predictors of health care seeking for irritable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychosocial factors. Am J Gastroenterol 2001;96:1340-9. https://doi.org/10.1111/j.1572-0241.2001.03789.x.
- Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. Am J Gastroenterol 2002;97:2290-9. https://doi.org/10.1111/j.1572-0241.2002.05783.x.
- Stenner PH, Dancey CP, Watts S. The understanding of their illness amongst people with irritable bowel syndrome: a Q methodological study. Soc Sci Med 2000;51:439-52. https://doi.org/10.1016/S0277-9536(99)00475-X.
- Taft TH, Keefer L, Artz C, Bratten J, Jones MP. Perceptions of illness stigma in patients with inflammatory bowel disease and irritable bowel syndrome. Qual Life Res 2011;20:1391-9. https://doi.org/10.1007/s11136-011-9883-x.
- Rohrbeck J, Jordan K, Croft P. The frequency and characteristics of chronic widespread pain in general practice: a case-control study. Br J Gen Pract 2007;57:109-15.
- Burton C. Beyond somatisation: a review of the understanding and treatment of medically unexplained physical symptoms (MUPS). Br J Gen Pract 2003;53:231-9.
- Burt J, Rick J, Blakeman T, Protheroe J, Roland M, Bower P. Care plans and care planning in long-term conditions: a conceptual model. Prim Health Care Res Dev 2014;15:342-54. https://doi.org/10.1017/S1463423613000327.
- Reeves D, Hann M, Rick J, Rowe K, Small N, Burt J, et al. Care plans and care planning in the management of long-term conditions in the UK: a controlled prospective cohort study. Br J Gen Pract 2014;64:e568-75. https://doi.org/10.3399/bjgp14X681385.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Man M-S, Chaplin K, Mann C, Bower P, Brookes S, Fitzpatrick B, et al. Improving the management of multimorbidity in general practice: protocol of a cluster randomised controlled trial (The 3D Study). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011261.
- Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012;380:7-9. https://doi.org/10.1016/S0140-6736(12)60482-6.
- Lamont T, Barber N, de Pury J, Fulop N, Garfield-Birkbeck S, Lilford R, et al. New approaches to evaluating complex health and care systems. BMJ 2016;352. https://doi.org/10.1136/bmj.i154.
- Raine R, Fitzpatrick R, Barratt H, Bevan G, Black N, Boaden R, et al. Challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Health Serv Deliv Res 2016;4.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:S21-34. https://doi.org/10.1258/1355819054308530.
Appendix 1 Training manual
Appendix 2 Consolidated Standards of Reporting Trials checklist
| Section/topic | Item number | Checklist item | Reported on page number |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | i | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | Not applicable, as trial is subset of main report | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 4–9 |
| 2b | Specific objectives or hypotheses | 11 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 11–13 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 15–16 | |
| Participants | 4a | Eligibility criteria for participants | 11–12 |
| 4b | Settings and locations where the data were collected | 11 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | See Chapter 2 |
| Outcomes | 6a | Completely defined prespecified primary and secondary outcome measures, including how and when they were assessed | 13 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | Not applicable | |
| Sample size | 7a | How sample size was determined | 14 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | Not applicable | |
| Randomisation | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 14 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 14 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 14 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants and who assigned participants to interventions | 14 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (e.g. participants, care providers, those assessing outcomes) and how | 13 |
| 11b | If relevant, description of the similarity of interventions | Not applicable | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 14–15 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 14–15 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment and were analysed for the primary outcome | 17 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 17 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 11 |
| 14b | Why the trial ended or was stopped | Not applicable | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | See Tables 1 and 3 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether or not the analysis was by original assigned groups | 14, see Figure 4 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% CI) | See Table 2 and Figure 5 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | Not applicable | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing prespecified from exploratory | See Table 4 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | Not applicable |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 24 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 24 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 24 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | viii |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | URL: https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-7-7 (accessed 27 October 2017) |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | viii |
Appendix 3 The CHEERS checklist
| Section/item | Item number | Recommendation | Reported on page number |
|---|---|---|---|
| Title and abstract | |||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms such as ‘cost-effectiveness analysis’, and describe the interventions compared | i |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base case and uncertainty analyses), and conclusions | Not applicable as trial is subset of main report |
| Introduction | |||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study | 4–9 |
| Present the study question and its relevance for health policy or practice decisions | 11 | ||
| Methods | |||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analysed, including why they were chosen | 11–13 |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made | 15–16 |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated | 27 |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen | See Chapter 2 |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate | 27 |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate | 27 |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed | 25–26 |
| Measurement of effectiveness | 11a | Single study-based estimates: describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data | See Chapter 4 and 5 |
| 11b | Synthesis-based estimates: describe fully the methods used for identification of included studies and synthesis of clinical effectiveness data | Not applicable | |
| Measurement and valuation of preference based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes | Not applicable |
| Estimating resources and costs | 13a | Single study-based economic evaluation: describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | 25–27 |
| 13b | Model-based economic evaluation: describe approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | Not applicable | |
| Currency, price date and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate | See Table 5 |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used. Providing a figure to show model structure is strongly recommended | Not applicable |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model | Not applicable |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty | 25–27 |
| Results | |||
| Study parameters | 18 | Report the values, ranges, references and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate. Providing a table to show the input values is strongly recommended | See Tables 6 and 7 |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report ICERs | 29 and Table 9 |
| Characterising uncertainty | 20a | Single study-based economic evaluation: describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective) | See Figure 6 |
| 20b | Model-based economic evaluation: describe the effects on the results of uncertainty for all input parameters and uncertainty related to the structure of the model and assumptions | Not applicable | |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes, or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information | 30 |
| Discussion | |||
| Study findings, limitations, generalisability and current knowledge | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge | 31 |
| Other | |||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support | viii |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with the recommendations of the International Committee of Medical Journal Editors | i |
Appendix 4 Project 2 patient questionnaire
Appendix 5 Scoring for risk assessment tool
| Measure | Score |
|---|---|
| GHQ score | |
| 0–2 | 0 |
| 3–12 | 1 |
| Change in diet | |
| No | 1 |
| Yes | 0 |
| Duration of symptom episode (hours) | |
| ≥ 2 | 0 |
| > 2 | 1 |
| Recent gastroenteritis/food poisoning? | |
| No | 1 |
| Yes | 0 |
| Activities interrupted as a result of bowel problems? | |
| No | 0 |
| Yes | 1 |
List of abbreviations
- AP
- abdominal pain
- CBT
- cognitive–behavioural therapy
- CCG
- Clinical Commissioning Group
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FGID
- functional gastrointestinal disorder
- GHQ
- General Health Questionnaire
- GIT
- gastrointestinal tract
- GP
- general practitioner
- HRQoL
- health-related quality of life
- IBD
- inflammatory bowel disease
- IBS
- irritable bowel syndrome
- IBS-QOL
- Irritable Bowel Syndrome Quality of Life Questionnaire
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- PCA
- principal components analysis
- PCRN
- Primary Care Research Network
- PCT
- primary care trust
- PRISMS
- Patient Report Informing Self-Management Support
- QALY
- quality-adjusted Life-year
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- SIR
- Salford Integrated Record
- SSRI
- selective serotonin reuptake inhibitor
- WISE
- Whole system Informing Self-management Engagement
