Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1045. The contractual start date was in August 2007. The final report began editorial review in August 2013 and was accepted for publication in July 2017. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Declan Murphy has received research funding from Shire (Basingstoke, UK) and leads the European Union (EU) Innovative Medicines Inititative consortium EU Autism Interventions – a Multicentre Study for Developing New Medications that receives funding from both the EU and the European Federation of Pharmaceutical Industries and Associations. Ian Wong received a research grant from the European Commission, Hong Kong Research Grant Council and Janssen-Cilag Ltd (High Wycombe, UK) on research to investigate the safety of antipsychotic drugs and attention deficit hyperactivity disorder treatments. In addition, Ailsa Russell has a patent Authors Copyright – Treatment Manual Cognitive–Behavioural Therapy for Obsessive–Compulsive Disorder in Autism Spectrum Disorder pending. Susan Young has received honoraria for consultancy, travel, educational talks and/or research from the Cognitive Centre of Canada, Janssen Pharmaceutical (Raritan, NJ, USA), Eli Lilly (Indianapolis, IN, USA), Novartis (Frimley, UK), HB Pharma (Sorø, Denmark), Flynn Pharma (Stevenage, UK) and Shire. Philip Asherson has received honoraria for consultancy, travel, educational talks and/or research from Janssen Pharmaceutical, Eli Lilly, Novartis, HB Pharma, Flynn Pharma and Shire.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Murphy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
We need to develop better services and treatments for people with attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) who are ‘in transition’ from childhood to adulthood.
People with ADHD have serious deficits in attention and are hyperactive. In contrast, those with ASD (autism, ‘atypical’ autism and Asperger syndrome) have stereotyped and obsessional behaviours and severe abnormalities in socioemotional behaviour and communication. They are different disorders, but have a lot in common. Thus, we studied them together.
Both ADHD and ASD are neurodevelopmental disorders (NDs) that have lifelong effects. They are of great public concern, much more common than previously thought, very frequently co-occur in the same person and are associated with serious comorbid mental health problems [e.g. drug abuse, anxiety, depression, learning disability (LD) and obsessive–compulsive disorder (OCD)]. This has a large social impact (e.g. the individual lifetime cost for ASD exceeds £2.4M and is significantly increased by comorbid LD).
People with ADHD and ASD have particular problems during the transition from children to adult services. Many ‘drop out’ and go untreated. In addition, few adult services are skilled in managing them, those already in these services are often misdiagnosed and inappropriately treated, and LD people are frequently excluded. This probably has an impact on the clinical outcome and costs – as treatment of the core disorders during childhood greatly reduces comorbidity. However, it is unknown (1) if this is true in young adults or those with LD and (2) if beneficial treatments developed in the general psychiatric population for the comorbid problems frequently found in ASD and ADHD are transferable to, and cost-effective, in these groups [e.g. cognitive–behavioural therapy (CBT) for OCD].
Government policy calls for evidence-based practice, needs-led services and National Institute for Health and Care Excellence (NICE) guidelines that stress psychological treatments. We have previously published instruments, which measure health needs, costs and consumption and gold standard research diagnostic tools. In addition, we carried out pilot studies in the treatment of comorbid disorders using both pharmacological approaches and CBT.
We therefore extended our work in two phases with the themes: (1) more effective services and (2) better treatments.
We used the following research questions to guide our work:
-
What are the needs of affected individuals and their carers and are these met by health services?
-
Are people with ADHD and ASD already in contact with clinical services recognised and treated by the services?
-
Can we improve the diagnosis of ADHD and ASD by clinical services?
-
Can we improve the treatment of ADHD and ASD by clinical services?
We included people with ADHD and/or ASD between the ages of 16 and 25 years during the ‘transition’ from child to adult services and those with a LD. In phase 1 (years 1–3) we worked to improve case identification and management. In phase 2 (years 3–5) we tested if effective treatments developed in the general population reduce comorbidity.
To develop more effective services we (1) developed simple protocols for case identification, (2) ascertained why people ‘drop out’ of services during the ‘transition’ and the consequences of that and (3) determined what interventions help adult services better identify, and meet the needs, of these people.
In this second part, we attempted to reduce disease burden by better use of current pharmacological and psychological treatments, and helping people who are often excluded. The drug studies initially focused on ADHD, as effective medications already existed that can be rapidly tested. In contrast, psychological interventions were aimed at common comorbid symptoms (OCD) in ASD.
We have constructed this report in five sections to match our programme design. In Section 1 (see Chapters 2–6), we present the patient and family perspective, and in Section 2 (see Chapters 7–13) we provide the service perspective. The third section (see Chapters 14–18) focuses on improving outcomes through better diagnosis, whereas the fourth section (see Chapters 19–23) focuses on improving outcomes through intervention. Section 5 (see Chapters 24 and 25) concludes the report by presenting our conclusions, recommendations for future research and examples of the impact of our work.
Within this report, some chapters or parts thereof have been previously published in the following papers:
Patient and public involvement
Our team involved users, carers and their representatives as co-applicants.
They identified the service and treatment priorities we addressed. In addition, they helped design this study and took an active part in carrying out the research. For example, we employed affected individuals as researchers. Furthermore, their representatives monitored our progress by serving on our Study Steering Board.
Dissemination materials and training packages have been prepared in collaboration with users and carers.
Section 1 Patient and family perspective
Chapter 2 Needs at the transition from child to adult services in ADHD and ASD: an overview of rationale and shared methods
Overall aims and objectives
Our aim was to investigate service use and needs among people with ADHD and ASD at the transition from adolescence to young adulthood (from ages 14 to 24 years). We wished to further understand (1) the demographic and medical correlates (e.g. age and comorbid conditions) of service use, (2) if current services are meeting the needs of young people with ADHD and ASD and (3) to what extent, and at what cost, family members of affected young people are able to meet these needs.
Background
Neurodevelopmental disorders such as ASD and ADHD are of increasing concern given their high cost to health services, individuals and families. For example, recent estimates suggest that the cost to the UK economy of supporting children with an ASD is approximately £2.7B (US$4.4B) each year and the cost of supporting adults is much higher (US$40.4B each year). 7 Moreover, although ASD and ADHD are often thought of as childhood disorders, both can persist into adolescence and young adulthood. Approximately 96% of those diagnosed with an ASD as children still warrant diagnosis in young adulthood. 8 The figure is lower for ADHD, with persistence rates estimated between 15% and 65%, depending on the diagnostic criteria used. 9
Not only do young people (adolescents and young adults) with these disorders continue to experience symptoms, they also retain significant impairments. For example, most young people with an ASD show continued impairments in daily living skills, communication, social interaction, employment and education. 10–13 Young adults with ADHD tend to achieve greater independence than their peers with ASDs, but are still more likely than the general population to be unemployed,14 experience more frequent job losses,14,15 underachieve in education,16 experience instability in emotional relationships16,17 and display antisocial behaviours. 18–20
Even though symptoms and impairments continue among young people with ASD and ADHD, few services exist to support those with these disorders through adulthood. 21–23 Additionally, as these conditions have been found to be underdiagnosed among young adults,24,25 many are likely to remain solely reliant on family and friends for assistance. Thus, the situation for most young people with an ASD or ADHD is that they continue to experience symptoms and impairment, but have limited support from services and so rely on families.
Although many studies have investigated service use and needs of children with developmental disorders (e.g. ASD), to date little is known about the service use and needs of these groups as they reach adolescence and transition to adulthood. Nonetheless, concern has been expressed in the UK parliament about the perceived failure to provide effective transitioning because of:
-
inconsistency of referral and treatment criteria
-
poor communication between services
-
lack of continuity
-
conflict between the ‘child/family’ approaches of paediatric mental health services and the individual approach of adult mental health services
-
the disengagement of young adults who drop out of the health-care system. 26
In summary, there is the perception that there are significant problems in the provision of services for ‘youngsters’ as they transition into young adulthood. However, the evidence to help inform the debate is lacking. Hence, research into the needs of young adults with ASD or ADHD and their carers is important in order to design and implement appropriate and effective care programmes and support for carers as well as young adults with this disorder. This first section of our work therefore investigated the following in both ASD and ADHD: (1) service use, met and unmet needs, (2) the role of family members in meeting needs, (3) changes over time in service use and needs and (4) the consequences of these changes for the young person’s and the nominated family member’s well-being. Our approach was to use a core set of measures for both disorders when possible, but to use more appropriate disorder-specific measures when prudent.
Methods used that were common to both ADHD and ASD
We conducted an observational study based on face-to-face interviews and self-completion questionnaires with young people with ADHD and an ASD and their parents (usually mothers) at yearly intervals. The ADHD study was a 3-year prospective study; the ASD group was followed for 2 years [this difference in follow-up time arose owing to delay from Research Ethics Committees (RECs), resource constraints and time limits for the programme set by the National Institute for Health Research (NIHR); for further details, please see Obstacles and solutions]. The study collected information on health service use, needs and social/demographic and health information relating to participants and their family member at the time of the interview. For the ADHD study, self-completion questionnaires were also administered to adolescents/young adults to obtain information regarding drug and alcohol use and problems with the police. Differences in the study design between the ADHD and ASD groups are highlighted in the sections that follow.
Study site
Data collection largely took place in participants’ homes or at the Institute of Psychiatry (IoP). Most participants were from Greater London; the remainder were spread throughout England, extending from Cornwall to Lincolnshire in the north-east.
Sample
Our study included 183 families consisting of young people aged 14–24 years (n = 82 with ADHD and n = 101 with ASD) and their parents. More detailed information on sample sizes is provided in Tables 1 and 4. Families were recruited through their children’s childhood clinical diagnoses of ADHD or an ASD from Child and Adolescent Mental Health Services (CAMHS) and adult clinics, charities and research databases that form part of our clinical research networks. Clinical diagnosis of autism was confirmed in all cases using the Autism Diagnostic Interview – Revised27 (ADI-R) and ADHD (combined type) was defined using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria. As participants in the ADHD group were originally recruited for the International Multi-Centre ADHD Genetics (IMAGE) programme, they were excluded if they had been diagnosed with autism, epilepsy, general learning difficulties, brain disorders and any genetic or medical disorder associated with externalising behaviours that might mimic ADHD based on both history and clinical assessment.
| Variable | % | Mean (SD) | Range | Missing (n) |
|---|---|---|---|---|
| YP age (years) | 17.50 (2.30) | 14–21 | 0 | |
| YP male | 89 | 0 | ||
| YP white or white British | 100 | 0 | ||
| YP education | ||||
| Full time | 57 | 0 | ||
| Part time | 7 | 0 | ||
| Not in education | 34 | 0 | ||
| PR education | ||||
| Low | 63 | 2 | ||
| High | 37 | 2 | ||
| YP accommodation | ||||
| Family home | 87 | 0 | ||
| Other | 13 | 0 | ||
| YP residing in Greater London | 56 | 0 | ||
| YP DIVA above cut-off point (> 6) | 73 | 2 | ||
| YP CIS-R | 7.90 (6.50) | 0–29 | 1 | |
| YP CIS-R above cut-off point (> 12) | 27 | 1 | ||
| YP impairments | 4.46 (2.72) | 0–10 | 3 | |
| YP total needs (parent report) | 5.05 (3.43) | 0–25 | 0 | |
| Met (parent report) | 2.58 (2.30) | 0–25 | 0 | |
| Unmet (parent report) | 2.48 (2.51) | 0–25 | ||
| YP AUDIT-C | 4.99 (3.89) | 0–12 | 6 | |
| YP AUDIT-C above cut-off point (> 4) | 65 | 0–12 | 6 | |
| YP drug use in past month | 24 | 7 | ||
| YP drug use ever | 48 | 7 | ||
| YP problems with police in past 12 months | 25 | 7 | ||
| YP exclusion from school | 50 | 0 | ||
| YP not in contact with services | 41 | 0 | ||
| YP in contact with services | ||||
| Children’s service | 33 | 0 | ||
| Adult service | 10 | 0 | ||
| ADHD service all ages | 16 | 0 | ||
| Childhood | ||||
| CD | 37 | 2 | ||
Recruitment for the ADHD sample began on 1 April 2009 and ended on 23 February 2011, for the ASD sample it began on 7 June 2010 and ended on 20 October 2011. Both studies received REC approval from the London – Camberwell St. Giles REC (previously known as the Joint South London and Maudsley and the IoP REC, South East London REC 4). The ASD REC reference is 04/H0807/71 and the ADHD REC reference is 08/H0807/68.
Instruments common to both ADHD and ASD studies
The instruments common to both studies are described below. Those used only in either the ADHD or the ASD elements of the project are described in the relevant disorder-specific section (see Chapters 2 and 3).
The level of need of the young person was captured using the Camberwell Assessment of Need for Adults with Developmental and Intellectual Disabilities (CANDID). 28 CANDID assesses needs across 25 domains, including social, physical, mental health, self-care and practical needs. For each domain, the young person and the parent were asked whether or not there was a significant need (coded as 1). If so, further questions were asked to ascertain if sufficient formal and/or informal support was being received. If adequate support was received, the need was classified as ‘met’ (regardless of whether or not it was met by formal or informal carers); if insufficient support was received the need was classified as ‘unmet’. Both met and unmet need scores were calculated by summing the number of domains where each was recorded. A total needs score (range 0–25) was calculated by summing the met and unmet need scores. 28
We adapted a brief series of questions on drug use from the Office for National Statistics (ONS) survey Mental Health of Children and Young People in Great Britain, 2004 Report. 29 Young people were asked to self-rate the frequency and nature of drug use from a range of drugs including cannabis, cocaine and heroin. For each individual drug, a question was asked regarding whether or not the participant had ever used this drug, even if just once. If the young person answered yes to this first opening question, two more questions were asked: (1) at what age the young person had first used this drug and (2) whether or not the young person had used this drug in the past month.
Alcohol use was assessed using the Alcohol Use Disorders Identification Test Consumption (AUDIT-C), a brief and validated three-question screen that can help identify hazardous and harmful drinking. 30 The AUDIT-C is an abbreviated version of the 10-question Alcohol Use Disorders Identification Test (AUDIT); the only screening instrument of hazardous alcohol use specifically designed for international use that is consistent with International Classification of Diseases, Tenth Edition (ICD-10), definitions of alcohol dependence and harmful alcohol use. Higher scores indicate greater likelihood of hazardous and harmful drinking and may reflect greater severity of alcohol problems and dependence, as well as a greater need for more intensive treatment.
Problems with police were examined through a series of questions based on those in the background information questionnaire used in the adult ADHD service at the Maudsley Hospital (adapted for this study). All participants were asked whether or not they had been in trouble with the police in the past 12 months. Those who answered yes were asked a brief series of questions regarding the nature and frequency of these problems (e.g. frequency of custodial sentences, times spent in a prison cell, appearances in court).
Caregiver burden was measured using the 12-item Zarit Burden Interview. 31 This captures the psychological and social impact of caring and asks respondents to rate the extent to which they agree or disagree with statements regarding their feelings about the results of caring on a five-point scale, with possible responses ranging from 0 (‘never’) to 4 (‘nearly always’). A total score (0–48) was calculated by summing the scores for each question. 31
We used a modified version of the Client Service Receipt Inventory (CSRI) to capture service use. 32 In particular, participants were asked to state whether or not they were under the formal care of child or adult health services, with those answering yes recorded as currently being in touch with services (coded as 1). The CSRI was completed jointly by the parent and young person (where they took part).
Obstacles and solutions
Our main obstacles related to five main factors:
-
delays with REC approval and the need to submit separate ethics applications for both groups
-
reaching young people
-
attrition, a problem common to all longitudinal studies but of particular concern for the ADHD group
-
the online completion of the Development and Well-Being Assessment (DAWBA) by parents of the ASD group
-
the lack of detail on prescribing patterns in an epidemiological sample that could be accessed in the time scale of this programme.
First, approval of the ASD research project for the purposes of the Mental Capacity Act 200533 led to significant delays due to the higher prevalence of LDs. As there is currently no standardised or accepted protocol for assessing whether or not a person lacks capacity to consent to take part in research it was necessary to develop a protocol for assessing capacity for this research project. Thus, a mental capacity assessment was adapted from one used in an earlier project under the same NIHR programme grant (REC 09/H0807/72). As this took some time to develop and to be approved by the local REC, the ASD study began 1 year after the ADHD study. This meant that ASD participants were followed for only 2 years (instead of 3 years, as for ADHD participants).
Second, our original design for the ADHD sample was based on contacting parents (as participants were contacted as children we only had contact details for parents). However, we soon amended the research design to be more inclusive in approach to young people, rather than approaching only via parents. To this effect, initial letters of invitation were sent both to parents and the adolescents/young adults at their parents’ homes.
Third, in order to reduce attrition (and non-response), especially among those with ADHD, we implemented a variety of measures. As participants were initially recruited through the IMAGE sample, the newsletter was revived and sent on an occasional basis to participants with summaries of the research and key findings (this has been led by Professor Asherson and his colleagues).
Fourth, we encountered difficulties in getting parents to complete the online DAWBA for their children with an ASD. This is because this online assessment is relatively time-consuming. We adopted several solutions to address this issue. Our researchers repeatedly contacted those with incomplete DAWBAs, reminding them of the need to provide this information. Furthermore, our researchers offered to assist parents in completing the forms. Finally, we offered parents a gift voucher of £20 in order to compensate them better for time taken to complete the DAWBAs.
Furthermore, we were concerned by the lack of accurate prescribing data and an epidemiological sample that we could access – as this raises issues about the generalisability of our results. Hence, we developed a collaboration with Professor Ian Wong (who was then at the School of Pharmacy) to examine medication use in a nationally representative data set based on general practitioner (GP) records: The Health Improvement Network (THIN). The data for young people with ADHD have already been published and so we examined psychotropic drug prescribing and neuropsychiatric comorbidities among 0- to 24-year-olds with ASD between 1992 and 2008 in a nationally representative primary care database. Further details outlining the rationale for this component and our results are provided later in this report (see Chapter 20).
Chapter 3 Needs in ADHD at the ‘transition’
Study design
For the ADHD study three separate face-to-face interviews were administered and one self-completion questionnaire. The young person’s questionnaire consisted of:
-
a needs assessment based on the CANDID (i.e. a standardised needs-assessment instrument that assesses need in 25 life domains described above, see Chapter 2)
-
the Clinical Interview Schedule – Revised (CIS-R; a rating scale of comorbid psychological symptoms used in the ONS Psychiatric Morbidity surveys)
-
background information (e.g. current employment situation and educational circumstances).
Self-administered questionnaires gathered information from ADHD adolescents/young adults on:
-
the Barkley Adult ADHD Rating Scale – IV (BAARS-IV; i.e. questions about attention and activity levels over the past 6 months)
-
the Center for Neurologic Study – Liability Scale (CNS-LS), a self-reported measure of the occurrence of moods in the past month and in the past 5 years
-
the AUDIT-C, a widely used self-reported measure of alcohol use described above (see Chapter 2)
-
a brief series of questions on drug use and police contact (e.g. if any problem with police in the past 12 months and number of formal police cautions and times spent in police cell and youth custody).
Parents were asked to:
-
complete the same questions about the their child’s level of symptoms or problems that co-occur with ADHD using the BAARS-IV
-
complete the same questions about their child’s needs using the CANDID
-
assess the impact of their child’s condition on their own employment situation and physical, psychological and social well-being
-
answer questions about the frequency and type of services they used
-
complete a widely used measure of carer burden (an abbreviated version of the Zarit Burden Interview)
-
provide background information (e.g. marital and tenure status, living arrangements).
A joint interview with both the young person and their parent consisted of:
-
the Diagnostic Interview for ADHD in Adults (DIVA) (this was conducted in the first year only)
-
the CSRI adapted for this clinical group asking information about the young person’s frequency and type of service use in the past 3 months, contact with transition teams and their experiences (and satisfaction) of transitioning from child to adult services (described in Chapter 2).
Each of the instruments specific to the ADHD study is described in more detail below.
Instruments specific to ADHD study
We used the DIVA,34 a diagnostic interview measure for adults with ADHD, recommended by the European ADHD Consensus Group,1 to measure current inattentive and hyperactive/impulsive symptoms (that is in the past 6 months). Using the DIVA, three subtypes of ADHD can be identified: predominantly inattentive, predominantly hyperactive–impulsive and combined inattentive and hyperactive–impulsive. 34
Given that there is currently no measure of ADHD symptoms and impairments that is validated for use across the whole age range used in this study (i.e. 14–21 years), the DIVA was chosen for several reasons. First, despite being a relatively new measure, it was used in preference to existing published diagnostic interviews such as the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID),35 because it is briefer, permitted greater freedom in responses and is used increasingly throughout Europe. Second, compared with the CAADID, which has items that are similar to the DIVA, the DIVA is also publicly available. Third, the items in the DIVA were considered to be more realistic for the diagnostic assessment of ADHD in adults by the European ADHD Consensus Group. 1 Finally, it was judged important to keep the outcome data the same across the whole sample, rather than choose one measure for adolescents and one for adults.
We also used the BAARS-IV (informant version),36 a standardised and widely used rating scale for the assessment, diagnosis and monitoring of treatment of ADHD in adults. It assesses 18 symptom items from the diagnostic criteria for ADHD in the DSM-IV and 10 items relating to impairment across different areas of functioning. The informant is asked to rate the frequency and severity of impairments with scores on a four-point scale ranging from 0 to 3 capturing the severity and frequency of the behaviours representing ‘0, not at all or rarely’; ‘1, sometimes’; ‘2, often’; and ‘3, very often’. Although both parent and young person ratings of impairments were obtained only the parent ratings are used here.
Psychiatric-associated conditions were assessed using the CIS-R. 37 It is a standardised, valid and reliable structured diagnostic instrument used for rating psychiatric symptoms across 14 domains (e.g. anxiety, depression), and is widely used in both clinical and the general populations. 37 Scores on each section can range from 0 to 4 (0–5 for the section on depressive ideas), with a total score ranging from 0 to 57. A total score of ≥ 12 is regarded as clinically significant. 37
Results
So far, our study of the ADHD group has focused on addressing the service use (and needs) of this clinical group at the transition to adolescence and young adulthood (that is ages 14–24 years). In addition, we explored the experiences of health-care transition (i.e. the process of moving from child to adult health services) from the young person’s and parents’ perspective.
Our framework for understanding service use among this clinical group was the Andersen–Newman Behavioural Model Of Health Service Use. 38–40 It is an established framework widely used by health economists, psychologists and medical sociologists to explain patterns of service utilisation among diverse populations. 41,42 The model organises service use into predisposing, enabling and need categories, whereby use of services is conceptualised as a function of predisposing (such as the person’s age), enabling (such as parental education) and need factors (such as ADHD symptoms, impairments, psychiatric comorbidities, needs and caregiver burden).
Sample characteristics
We included 82 individuals with ADHD. The average age of ADHD group was 17.5 years (see Table 1), and most were male (89%), still living at home (87%), unmarried (96%) and still in education (66%). Half reported having been excluded from school. Thirty-seven per cent of parents reported having achieved an educational level higher than A level (Advanced level).
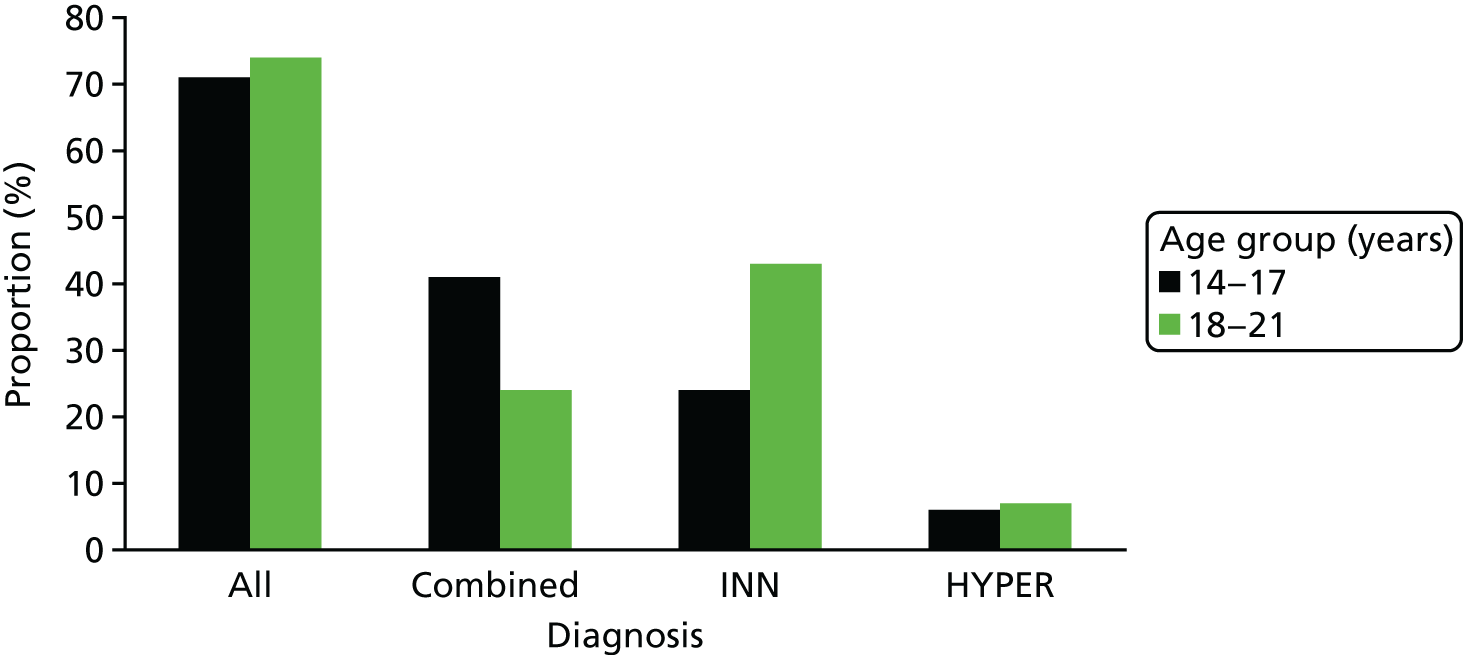
The majority (73%) still met DSM-IV criteria for ADHD. Overall, there was no significant difference between the younger (14–17 years) and the older (18–21 years) age groups in the percentage who met DSM-IV diagnostic criteria for ADHD (χ2 = 0.108; p = 0.742). However, a higher percentage of the younger group met criteria for combined ADHD (41% vs. 24%), whereas the predominantly inattentive subtype was more common in older individuals (44% vs. 24%) (Figure 1).
FIGURE 1.
Distribution of ADHD diagnoses by age according to DSM-IV criteria. HYPER, hyperactive–impulsive; INN, inattentive.
In addition, nearly two-thirds scored above the cut-off score on the AUDIT, suggesting high levels of alcohol consumption. Forty-eight per cent had used illegal drugs at some point and close to one in four had used drugs in the past month. Finally, 25% reported having been in trouble with the police within the past year.
Impairments
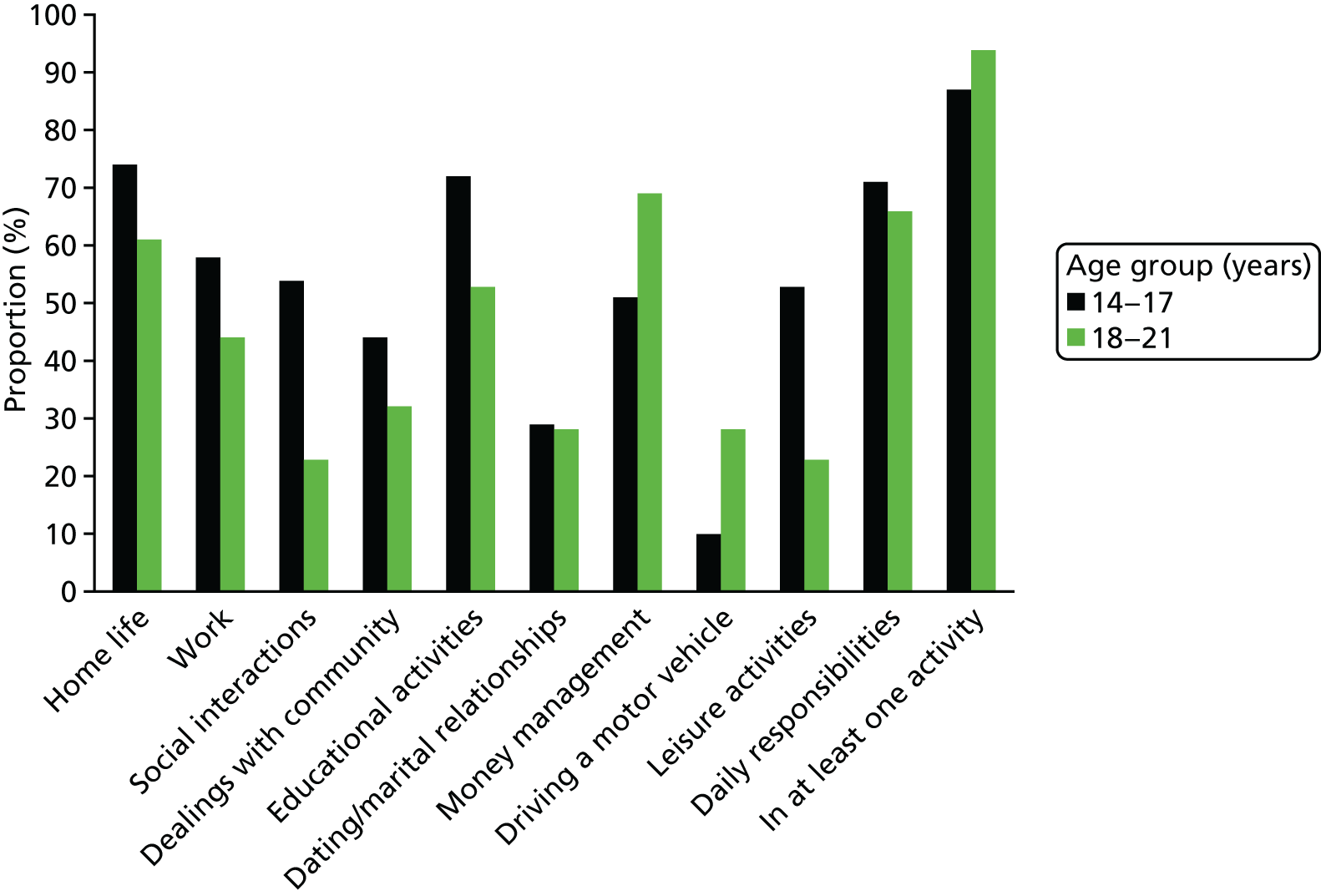
In both age groups nearly all were impaired in at least one life activity (Figure 2). Overall, around two-thirds of parents reported significant impairments in their child’s management of daily responsibilities (68%), home life (67%), educational activities (62%) and money (61%); half of parents reported impairments among their children related to work (49%). There were no significant differences in percentages of impairments reported between the two age groups apart from in social interactions – where parents reported more impairments among those in the 14–17 years age group in comparison with those aged 18–21 years [χ2 = 10.48, degrees of freedom (df) = 3; p = 0.013]. In summary, impairments were not restricted to younger individuals with ADHD – both groups demonstrated marked impairments.
FIGURE 2.
Distribution of impairments in daily activities by age.
Associated psychiatric symptoms
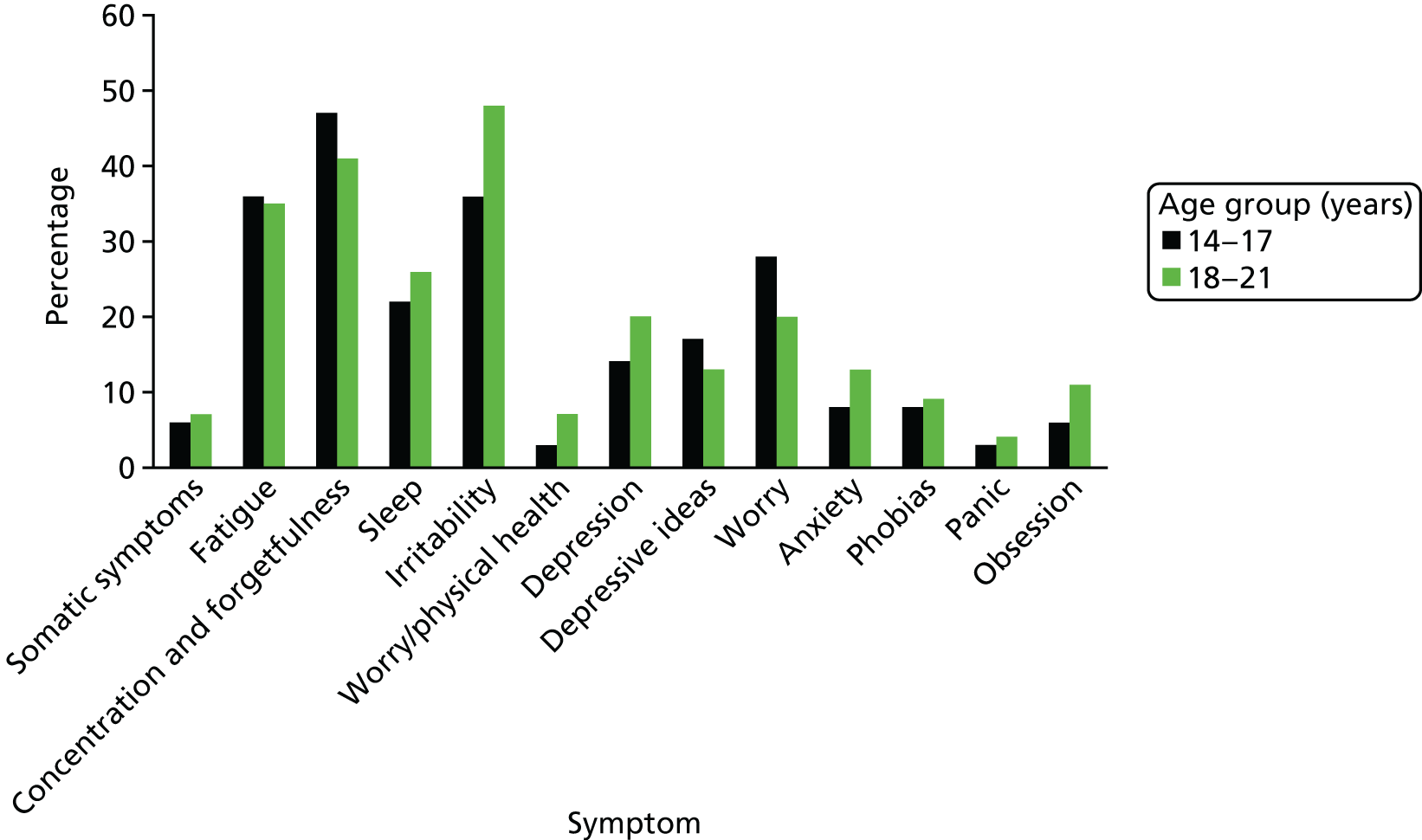
Twenty-seven per cent scored above the cut-off score (> 12) on the CIS-R, indicating the likelihood of comorbid psychopathology. For example, over one-third of participants reported significant levels of fatigue and irritability and around one-quarter reported significant anxieties (Figure 3). There was no significant difference between the two age groups in the prevalence of associated mental health difficulties – including neurotic symptoms (t = –0.452, df = 79; p = 0.652).
FIGURE 3.
Distribution of neurotic symptoms by age according to CIS-R.
Needs
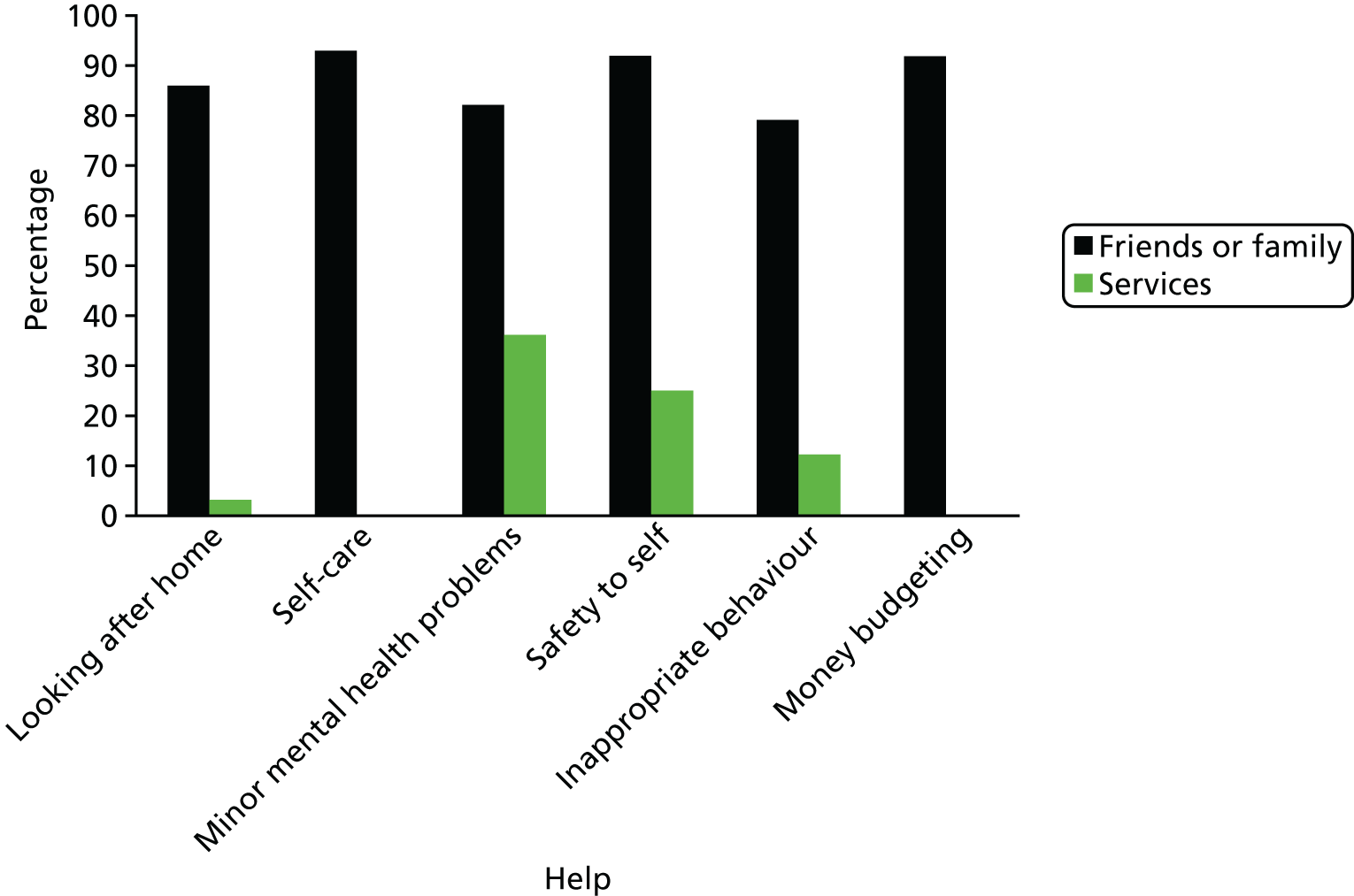
Parents reported that their child had a mean number of five needs (on average 2.58 of which were met and 2.48 of which were unmet; see Table 1). There was no significant difference between the younger and older age groups in the mean number of reported needs (5.25 vs. 4.89, t = 0.47; p = 0.64), met needs (2.78 vs. 2.41, t = 0.71; p = 0.48), or unmet needs (2.47 vs. 2.48, t = –0.01; p = 0.99). There was also no significant difference by age group in the percentages who reported needs within individual domains. The only exception being in the social contact domain, with parents of young people aged 14–17 years reporting more needs than parents of young people aged 18–21 years (χ2 = 6.20, df = 1; p = 0.01) (Figure 4). Most of the help received towards meeting these needs came from family or friends rather than from formal services (Figure 5). Overall, formal services provided little or no help towards needs other than those linked to the participant’s physical health, whereas families or friends or young people provided most of the help towards meeting young people’s needs. For example, whereas 88% of participants received some help from family and friends towards their reported need in the safety to others domain, only 12% reported receiving some help from formal services towards this reducing this need.
FIGURE 4.
Distribution of needs by age.
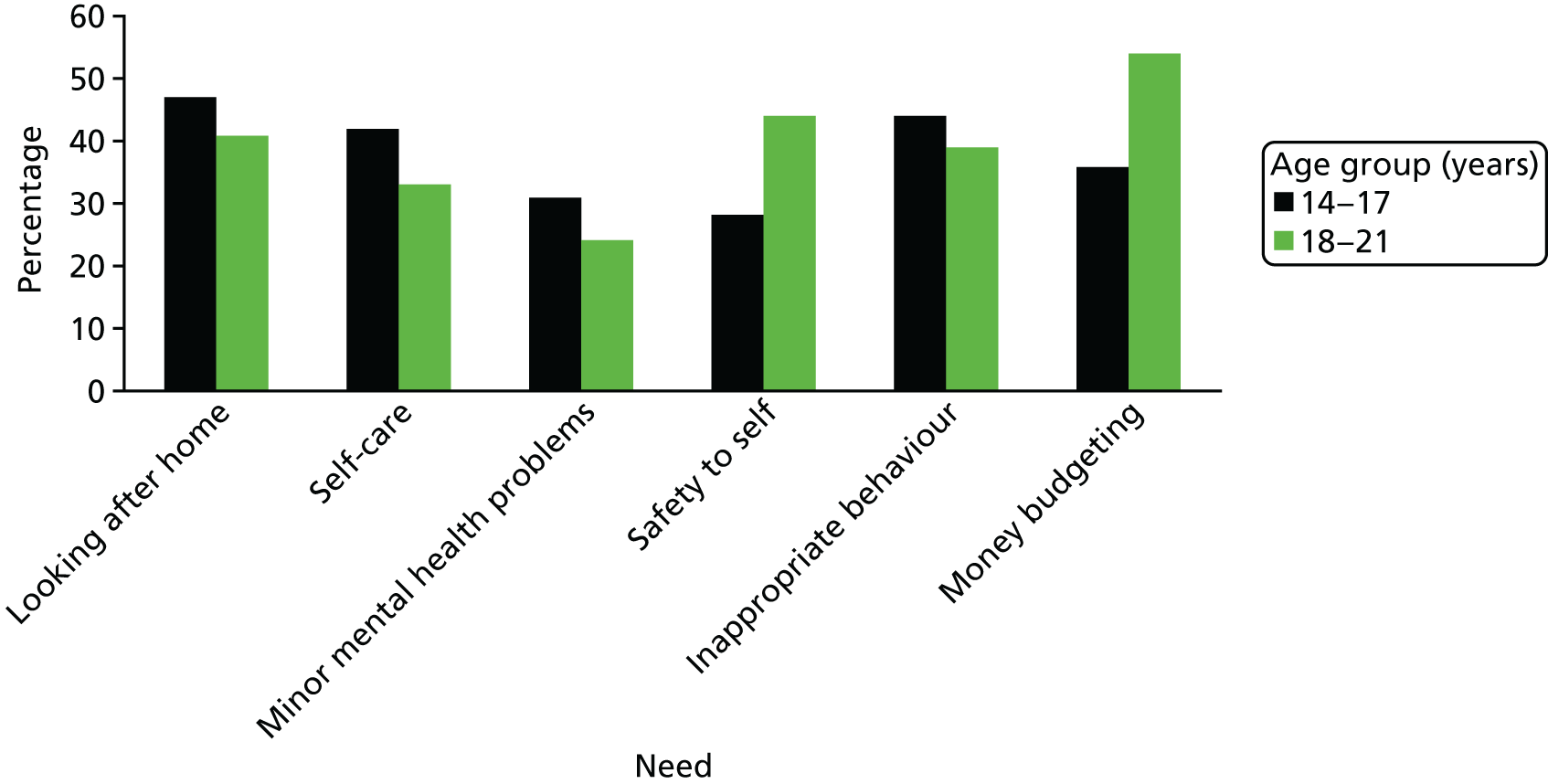
FIGURE 5.
Distribution of help received from friends or family and services towards meeting needs.
Service use
Despite a high prevalence of associated mental health symptoms, 41% of the sample reported that they were no longer in contact with services. Thirty-three per cent of the sample were in contact with children’s services and 16% reported regularly attending an ADHD service without age boundaries (that is one specialising in the management of children, adolescents and adults with neurodevelopmental difficulties). Only eight (10%) of the participants were seen by adult services (Table 2).
| Variable | In contact with servicesa | Significance test | |
|---|---|---|---|
| Yes | No | ||
| Predisposing | |||
| YP age | 16.9 (2.1) | 18.4 (2.3) | df = 79, t = –3.32** |
| Enabling | |||
| PR (parent) education | |||
| Low | 27 (57) | 21 (44) | df = 1, χ2 = 0.041 |
| High | 17 (59) | 12 (41) | |
| Need | |||
| YP DIVA above cut-off point (> 6) | 35 (61) | 22 (39) | df = 1, χ2 = 0.849 |
| YP impairments | 5 (2.8) | 3.7 (2.5) | df = 76, t = 2.12* |
| YP CIS-R | 8.8 (6.8) | 6.8 (5.9) | df = 79, t = 1.39 |
| YP total needs | 5.5 (3.8) | 4.4 (2.9) | df = 79, t = 1.43 |
| YP childhood CD | 17 (57) | 13 (43) | df = 1, χ2 = 0.048 |
| PR carer burden | 19.7 (10.6) | 15.4 (6.8) | df = 79, t = 2.07* |
Those more likely to be in contact with services were younger, reported more impairments and higher parent–carer burden (Table 3 shows the results of the logistic regression model used to analyse correlates of service use). The key outcome measure was whether or not the participant reported being currently seen by child, adult or tertiary services. The predictors captured predisposing (young person’s age), enabling (parental education) and need factors [ADHD symptoms, impairments, psychiatric comorbidities, parental caregiver burden and childhood conduct disorder (CD)]. Needs were not included in the model given their significant association with impairment (r = 0.51, df = 79; p < 0.01). Age was the only significant correlate of service use [odds ratio (OR) 0.62, 95% confidence interval (CI) 0.46 to 0.83; p = 0.001], with a 1-year increase in the young person’s age reducing the odds of being seen by services by 38%. None of the other factors considered was significantly correlated with service use. The proportion of variance explained by the models was 0.24 using Cox and Snell R2 and 0.38 using Nagelkerke R2, indicating that between 24% and 38% of the variance is explained by this model. In summary, we found that in ADHD age (and not level of impairment) determines the chance of being seen by services.
| χ2 | 6.95* | |
| Cox and Snell R2 | 0.24 | |
| Nagelkerke R2 | 0.38 | |
| Predictor | β | OR |
|---|---|---|
| Age | –0.476 | 0.62** |
| Parent education | 0.821 | 2.27 |
| Has ADHD | 0.304 | 1.36 |
| Impairment | 0.221 | 1.28 |
| Psychiatric comorbidity | 0.057 | 1.06 |
| Carer burden | 0.017 | 1.02 |
| Childhood CD | –0.064 | 0.938 |
Health-care transition
As reported above, only 10% (n = 8) of participants reported having experienced a transfer to adult care. However, among this group, seven (≈85%) families reported not having received a written transition plan as recommended in clinical guidelines, and approximately 75% had not been spoken to by a professional about the move from child to adult services (and even the 25% who had said that this occurred reported that it been only a brief conversation). Around half of young people reported that they needed more help during the transition process to help them prepare for the move to adult services. Young people reported a need for more information to help them plan for the future (47%), someone to show them which services are available as they grow up (51%) and someone to explain the transition process to them (49%). In addition, around 70% of parents reported that their child needed more help in relation to information about future options (72%), having someone to talk to about transition (70%) and having someone to explain the transition process to them (69%). In addition, the majority (57%) of the those few young people and parents who had received some help in terms of transition reported that their transition had been poorly managed, with two-thirds reporting that they would have wanted more emotional and practical support.
Discussion
Our data indicate that two-thirds of adolescents and young adults with a childhood diagnosis of ADHD continue to meet DSM-IV diagnostic criteria at follow-up. Many also present with associated psychiatric symptoms and impairments – as well as a wide range of health and social needs. Furthermore, there was no significant age-related difference in the overall prevalence of ADHD symptoms, impairments, psychiatric comorbidity or needs. Despite the continuing burden of symptoms and needs, reported service use decreased significantly with increasing age. For instance, a young person with a childhood diagnosis of ADHD reported 38% lower odds of service use at age 18 years than at age 17 years (irrespective of the severity of ADHD symptoms, impairments, comorbidities, carer burden or diagnosis of CD in childhood). This finding stresses the need for improving access to and/or use of health services among adolescents and young adults with ADHD.
In contrast, we found that most help towards meeting needs for adolescents and young adults came from friends and family rather than services (and families reported being provided with little or no help for the majority of domains). In addition, even where services were reported to have been involved, these were mainly targeted towards meeting the physical – rather than mental health or social – needs of this group.
Finally, we found considerable problems in health-care provision among this group that were specifically related to health-care transition. Only 10% of the sample had been transferred to adult services. Around half of all young people (and two-thirds of all parents) reported a need for more support from services in (1) accessing information regarding which services are available when they grow up, (2) the co-ordination of transition planning and (3) having someone to talk to about their practical as well as emotional needs. 43
Strengths and weaknesses
To our knowledge, this is the first analysis of needs and services use among those diagnosed with ADHD in childhood who are now at transition to adolescence and young adulthood. A key strength of our study includes the use of face-to-face interviews with both young people and their parents using reliable diagnostic and outcome measures. Moreover, our use of separate face-to-face interviews with young people and their parents enabled us to gain a more comprehensive picture of the wide range of needs among this group and to gauge the extent to which these needs are currently being met by services and family or friends. Another key strength of our study lies in our consideration of multiple potential factors that may correlate with service use among this group. This allowed us to better quantify the relative, and independent, contribution of factors that influence service use among this clinical group. Finally, our exploration of young people’s and their parents’ experiences of health-care transition, enabled us to identify key areas where improvements are necessary in health-care delivery. These are required from the perspective of both the affected person and their carers (e.g. a lack of access to sufficient information, problems with co-ordinating transition and lack of sufficient attention to the individual needs of young people and parents).
However, this was not an epidemiologically based sample and so our findings may not generalise. Nonetheless, we suggest that it is likely that the situation may be even worse in the general population, as our sample included individuals who had been in prior research studies of ADHD, and given that our sample had been in contact with CAMHS in childhood. Previous contact with such services may also have resulted in a bias towards presenting with higher levels of psychological problems at follow-up (e.g. in comparison with those in contact only with paediatric services in childhood). Nevertheless, rates of psychiatric comorbidity in our study are consistent with those reported in previous studies of ADHD. 44–48 Furthermore, as only those with a childhood intelligence quotient (IQ) of > 70 were included (with two exceptions), it is possible that our findings represent an underestimate of needs among those with intellectual disabilities. Likewise, as this study involved participants who were mostly males and from a Caucasian background it is not possible to say how representative the current findings are for women and those of ethnic minorities. Finally, due to our sample size we were likely underpowered to show significant effects of some of the predictor variables.
Conclusions
At transition, adolescents and young adults with ADHD represent a vulnerable group who are likely to have continuing needs that are currently poorly met by services. Key to improving transitional care are the health-care professionals and commissioners who design and run services. Without wider organisational support transitional health care is unlikely to become fully integrated into health services. Our initial results confirm Kennedy’s49 suggestion that changes could be made in policy, funding and training to enable the flexibility and continuity needed to put the young person at the centre of care. This comes at a cost. However, set against the considerable costs to the individual, family and society that are associated with untreated ADHD, there are clear clinical, ethical and financial arguments that suggest that investment in transition would realise long-term gains.
Age, rather than symptom severity, level of impairment or comorbidities, is the strongest correlate of service use in ADHD (with younger people being more likely to receive treatments).
Young people with a childhood diagnosis of ADHD at transition to adolescence and young adulthood suffer from persistent ADHD. In addition, they experience ongoing impairments and psychiatric comorbid symptoms. This leads to a wide range of health and social needs that would benefit from evidence-based treatment.
Most needs among adolescents and young adults with a childhood diagnosis of ADHD and their parents are poorly met by services. Most of the help received comes from families or friends.
Young people with a childhood diagnosis of ADHD and their parents report considerable unmet needs in health-care transition.
Chapter 4 Needs in ASD at the ‘transition’
Methods
As with the ADHD group, face-to-face interviews were conducted at yearly intervals with the ASD group. However, this study took place over a 2- rather than a 3-year period. In contrast to the ADHD study (see Chapter 3), interviews were largely undertaken with the parent and (whenever possible) with the young person themselves (we took parental advice into account on how best to manage the interviews in order not to cause any undue distress).
Thus, the first part of the parent interview consisted of a series of questions about their child (including the CANDID, their child’s alcohol and drug use and police contact if any). The second part collected information on the impact of their child’s disorder on their own situation and the final part, conducted when possible together with their child, asked questions about their child’s service use and transitions. When possible, the adolescent/young adult version of the ASD questionnaire consisted of questions on level of need, mood, drug and alcohol use and police contact. With few differences, we collected the same information for both young people and parents in the ASD group as we did for the ADHD group (e.g. the CANDID, the CSRI). The measures unique to the ASD group are briefly discussed below (i.e. those assessing ASD symptoms and associated comorbid psychiatric conditions). We included 101 individuals with ASD.
ASD-specific instruments
Current severity of ASD symptoms were captured using the Autism Quotient (AQ) – informant version. 50 It contains 50 items covering five different areas: social skills, attention switching, attention to detail, communication and imagination. For each question respondents report whether they ‘slightly agree’, ‘definitely agree’, ‘slightly disagree’ or ‘definitely disagree’ that a described behaviour is exhibited. Each question is scored 1 if the respondent reports the autistic symptom (by either ‘definitely’ or ‘slightly’ endorsing the symptom) and 0 if not. A total score (0–50) is calculated by summing the scores for each question. Scores over a cut-off point of 32 are indicative of an ASD. 50
Information on psychological co-occurring mental health symptoms was assessed using the DAWBA. 51 We did not use the CIS-R (as we did in the ADHD study) because there is no informant version of this instrument. The DAWBA assesses psychiatric symptoms and their impact across a number of domains through structured and open-ended questions, which cover the criteria required for a diagnosis according to both DSM-IV and ICD-10 criteria. The domains assessed were specific phobia, social phobia, panic, agoraphobia, OCD, generalised anxiety, depression, deliberate self-harm, impulsivity/hyperactivity and tics/Tourette syndrome. The DAWBA was completed online (www.dawba.net) by parents and young people when possible. Two trained raters (both psychiatrists) reviewed each case and produced consensus diagnoses based on the information provided by both parent and young person (where available). All conditions were rated according to ICD-10 criteria except ADHD, which was rated using DSM-IV criteria (as ADHD is not covered by ICD-10). An additional variable was derived from this measure stating whether or not the young person met criteria for one or more comorbid conditions [e.g. ADHD, Generalised Anxiety Disorder (GAD)].
Mental capacity issues
The ASD (but not ADHD) sample included individuals with intellectual disability (ID). Given the nature of the research, some of those who were eligible to participate lacked capacity to consent. We are aware that the baseline position is that capacity to consent is assumed unless there is reason to suppose otherwise. However, some neurological, psychological and/or behavioural markers may suggest lack of capacity for some things at some times. We also recognise that capacity is fluid in time and with respect to subject.
Indicators of potential lack of capacity include (but are not restricted to) the diagnosis of a LD, an IQ of < 70, significant deficits in adaptive functioning or other signs or symptoms of mental disorder that might compromise capacity to give informed consent. This was of particular concern for the ASD group as we did not exclude participants who had a LD (as was the case for the ADHD group, see above). All individuals with capacity gave informed consent.
Where there were doubts about the capacity of a young adult to give consent the study introduced an easy-read version of the information sheet and a capacity assessment was carried out by a trained researcher. The capacity assessment tool addressed the following questions, which are based on the principles outlined in Section 3 (1) of the Mental Capacity Act 2005:33
-
Can the person understand the information relevant to the decision?
-
Can the person retain that information?
-
Can the person use or weigh that information as part of the process of making the decision?
-
Can the person communicate his/her decision (whether by talking, using sign language or any other means)?
When it was established that the participant did not have capacity, but showed willingness to take part in the study, a relative or close friend (other than the carer) was approached to act as a personal consultee. The role of the consultee is to consider what the wishes and feelings of the participant would be if they had capacity, and if they judge that the person would want to take part, to consent on their behalf. We did our best to ensure that the consultee had no conflict of interest. If there were concerns about a conflict of interest then the parent was asked to contact the research team, at which stage a member of the research team spoke to them about the possibility of finding an alternative personal consultee.
The researchers who interviewed the ASD participants all received training from Dr Dene Robertson (consultant psychiatrist at the Mental Impairment and Evaluation Service, Bethlem Hospital) in assessing mental capacity to consent. In addition, they also undertook additional training; for example, they attended short courses on the Mental Health Act 200533 and Deprivation of Liberty Safeguards awareness training, as well as training on protection of vulnerable adults and the Protection of Children Act 1978. 52 When possible, the researchers conducted assessments in collaboration with a member of the health-care team, known to the potential participant, with experience in assessing mental capacity.
Results
Service use (and needs) among those with an ASD in adolescence and young adulthood
As with the ADHD group, our focus so far has been on addressing the first research question, the correlates of service use. In addition, similar to ADHD, there is a lack of adult services for those with an ASD [and in particular among those with high-functioning autism (HFA) who have ongoing needs and functional impairments]. Appropriate and effective planning of care programmes requires knowledge of the needs of this population (and their carers).
Consequently, our aim was to investigate needs and correlates of service use (e.g. medical and demographic) among those diagnosed with ASD in adolescence and young adulthood (aged 14–24 years), to help identify how they are supported while they move from adolescence to young adulthood.
Similar to the analysis of the ADHD group, we used the Andersen–Newman Behavioural Model of Health Service (see Chapter 3, Results) to investigate the correlates of health service use among young people with an ASD.
ASD sample characteristics
Similar to the ADHD sample the young people were predominantly male (92%) and still living at home (87%). Seventy-three per cent met criteria on the DAWBA for an associated psychiatric condition and 13% had a clinically defined LD. Most of the total sample were still in education (77%) and had received a statement of special educational needs (70%); 40% had been excluded from school at some point in their education. Parental education was assessed and 60% reported a high level of achievement – that is they had received A-level qualifications or above.
Service and medication use
Fifty-six per cent of participants (Table 4) reported currently being in contact with child (33%), adult (22%) or tertiary services (1%). The services most commonly seen in the past 3 months were a GP (used by 43% of participants), psychiatrist (26%), key worker (22%) or psychologist (17%). Those who stated they were in contact with child, adult or tertiary services were significantly more likely than those who were not in contact with formal services to report having seen a psychiatrist (χ2 = 3.66; p = 0.001) or a mental health key worker in the past 3 months (χ2 = 7.92; p = 0.005).
| Variable | % | Mean (SD) | Range | Missing, n |
|---|---|---|---|---|
| YP age (years) | 17.67 (2.9) | 14–24 | 0 | |
| YP male | 92 | 0 | ||
| YP education | ||||
| Full time | 70 | 0 | ||
| Part time | 07 | |||
| Not in education | 23 | |||
| YP employed (of those not in education) | 35 | 0 | ||
| Parental education | ||||
| Low | 41 | 0 | ||
| High | 59 | |||
| YP ethnicity | 1 | |||
| White | 85 | |||
| Black/Asian/other | 14 | |||
| YP accommodation | ||||
| Family home | 87 | 1 | ||
| Other | 12 | |||
| YP symptoms | ||||
| YP AQ symptoms | 35.85 (5.74) | 22–46 | 1 | |
| YP met criteria on DAWBA for one or more psychiatric condition | 73 | 12 | ||
| YP LD | 13 | 3 | ||
| YP needs | ||||
| Total needs (parent rated) | 9.58 (3.64) | 0–18 | 0 | |
| Unmet needs (parent rated) | 3.25 (2.88) | 0–11 | ||
| Met needs (parent rated) | 6.34 (2.72) | 0–12 | ||
| YP service use | ||||
| Children’s services | 33 | 0 | ||
| Adults | 22 | |||
| Tertiary | 1 | |||
| None/do not know | 45 | |||
Thirty-eight per cent of participants were currently taking at least one prescribed medication (Table 5). The most common prescribed medication was for ADHD (taken by 9% of participants), followed by antidepressants (8%) and sleep medication (8%). On average participants were prescribed 0.64 medications.
| Participant information | % | Mean (SD) | Range |
|---|---|---|---|
| Currently using medication | 38 | ||
| Number of medications prescribed | 0.64 (0.48) | 0–4 | |
| Type of medication used | |||
| ADHD | 9 | ||
| Antidepressant | 8 | ||
| Sleep medication | 8 | ||
| Antihistamine | 7 | ||
| Respiratory | 5 | ||
| Epilepsy | 3 | ||
| Beta-blockers | 3 | ||
| Antipsychotic | 2 | ||
Psychiatric associated symptoms
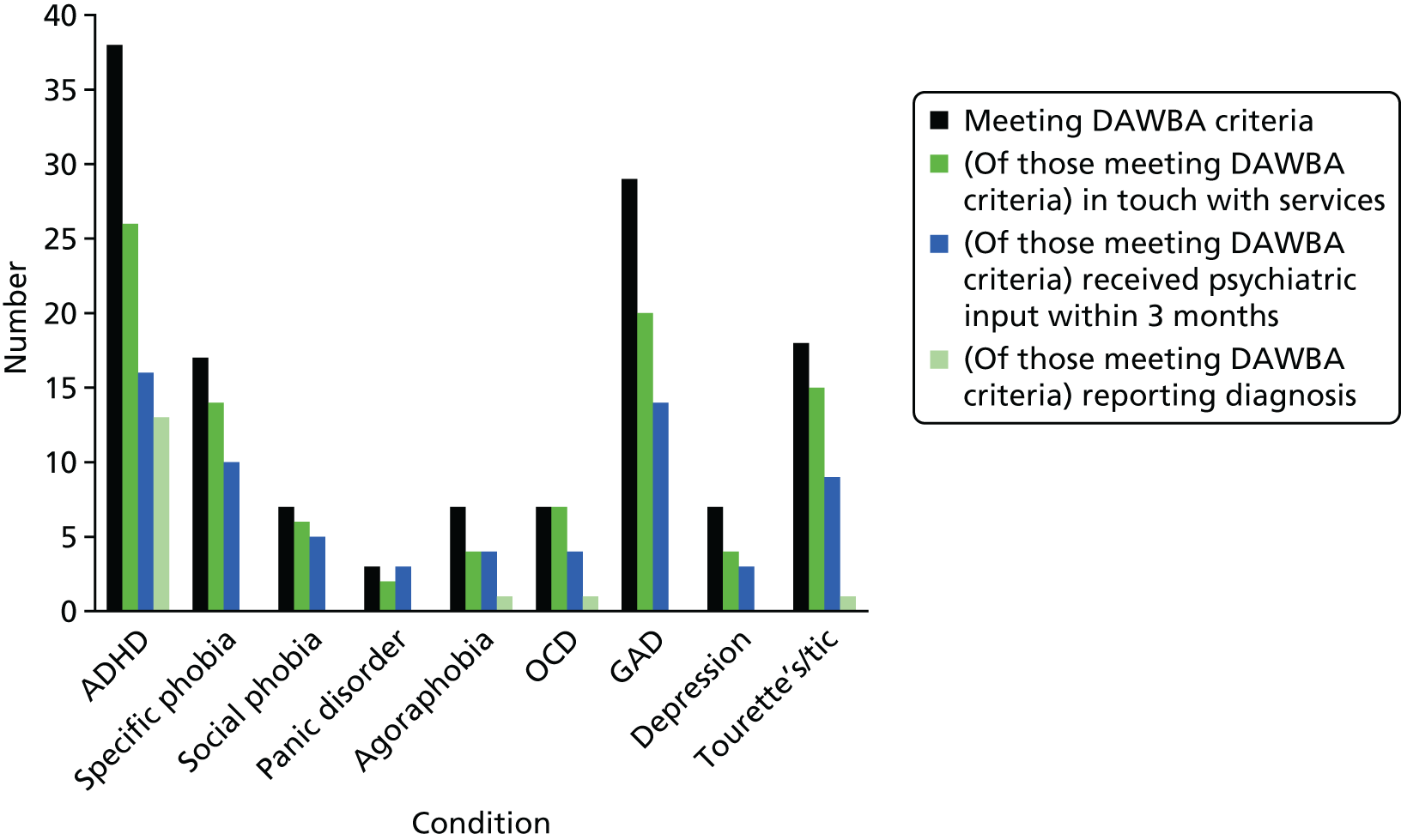
Figure 6 shows the number of ASD participants who also met criteria on the DAWBA for common psychiatric conditions. Figure 6 shows, for those who met the criteria for each condition, the numbers who were in touch with services; had saw a psychiatrist, psychiatric nurse, psychologist or counsellor in the past 3 months; and whose parents reported that their children had previously received a clinical diagnosis for an associated psychiatric disorder. Results show that 43% of the sample met clinical research diagnostic criteria for ADHD (38/89), 33% for GAD (29/89), 19% for a specific phobia (17/89) and 20% for tic/Tourette syndrome (18/89). In addition, a large proportion met criteria for more than one mental health problem. For instance, nearly half (47%) of participants met criteria for two or more co-occurring mental health disorders. However, relatively few of the parents whose children met the criteria for a co-occurring mental health disorder reported having previously been told that their child had a clinical diagnosis of the disorder. The most common additional diagnoses that parents had been made aware of are ADHD [36% (14/39)], agoraphobia [11% (1/9)], OCD [14% (1/7)] and tic/Tourette syndrome [6% (1/18)]. Young people with an ASD who met criteria on the DAWBA for at least one psychiatric disorder were more likely than those who did not to be in touch with services (χ2 = 10.67; p = 0.001). However, of those who currently met criteria for at least one additional psychiatric condition, one-third stated that they were not currently being seen by any services and over half had not seen any psychological or psychiatric services in the past 3 months (see Figure 6).
FIGURE 6.
Number of participants meeting criteria for one or more psychiatric conditions on the DAWBA (n = 89).
Need
Parents reported that their child had a mean number of 10 needs (on average six of which were met and three of which were unmet; see Table 4).
Figure 7 shows the needs most commonly reported by parents and the number of participants who were receiving help from family or services. Eighty-three per cent of parents reported that their child had a need relating to exploitation risk, 73% reported a need related to food, 72% reported a need related to money budgeting and 63% reported social needs. Few of the young people were receiving help for these needs from services; in contrast, almost all were receiving some help for them from friends or families.
FIGURE 7.
Met and unmet needs most frequently reported by participants and help received from family and services (n = 101).

As expected, younger people with an ASD with higher levels of need were more likely to be in contact with services than those who were not [mean 10.54, standard deviation (SD) 3.60, in contact vs. mean 8.14, SD 3.57, not in contact, t = –3.13; p = 0.002].
Correlates of service use
Table 6 shows the results of the logistic regression model used to analyse correlates of service use. The dependent variable was whether or not the participant stated that they were currently being seen by child, adult or tertiary health-care services. The predictors captured predisposing (young person’s age), enabling (parental education) and need factors (that is the level of ASD symptomology, total level of need, whether or not they met criteria on the DAWBA for any associated psychiatric conditions and the presence of a LD). The only significant correlate of service use was whether or not the young person met criteria on the DAWBA for one or more psychiatric conditions. For instance, the odds of young people with an ASD and an associated psychiatric condition being in touch with services are four times higher than for those without such a condition. The proportion of variance explained by the model was 0.18 using Cox and Snell R2 and 0.24 using Nagelkerke R2, indicating that between 18% and 24% of the variance in service use is explained by this model.
| Predictor | β | SE | OR | Significance |
|---|---|---|---|---|
| PR education (0 = high) | 0.774 | 0.522 | 2.168 | 0.145 |
| YP age | –0.090 | 0.085 | 0.914 | 0.289 |
| YP autism symptoms (AQ) | 0.002 | 0.044 | 0.998 | 0.973 |
| YP total need (parent rated) | 0.124 | 074 | 1.132 | 0.095 |
| YP met criteria on DAWBA for any condition | 1.353 | 0.587 | 3.870 | 0.021 |
Service use, diagnosis and treatment of comorbid psychiatric conditions
Table 7 examines the relationship between being in touch with services and diagnosis and treatment (in terms of reporting any psychiatric input in the past 3 months and receiving relevant medications), for the two most prevalent psychiatric symptoms (ADHD and GAD). We would expect that those who met criteria for a psychiatric condition and who were in touch with services would be more likely to report having received a clinical diagnosis and treatment. However, we found no such relationship (albeit with one exception). Among those with an ASD meeting DAWBA criteria for an anxiety disorder, 98% (43/44) reported that they had not been informed of (or diagnosed with) this additional problem by any service, 52% (23/44) had received no psychiatric input in the past 3 months and 86% (38/44) reported that they were receiving no relevant medication for anxiety. Only for those with an ASD who met criteria for ADHD was being in touch with services significantly associated with receiving psychiatric input in the past 3 months (see Table 7). However, even among this group, 65% (22/34) reported not having received a clinical diagnosis and 85% (29/34) were not being prescribed relevant medication.
| DAWBA condition | In touch with services | Reporting diagnosis | Fisher’s exact | Any psychiatric input in past 3 months | Fisher’s exact test | Reporting relevant medication | χ2 | Row total for number meeting DAWBA criteria | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||||||
| ADHD | Yes | 10, 38.5% | 16, 61.5% | 14, 53.8% | 12, 41.2% | 4, 18.5% | 22, 81.5% | 26 | |||
| No | 2, 25.0% | 6, 75.0% | 0, 0.0% | 8, 100.0% | 1, 12.5% | 7, 87.5% | 8 | ||||
| Total | 12, 35.3% | 22, 64.7% | p = 0.68 | 14, 41.2% | 20, 58.8% | p = 0.01 | 5, 14.7% | 29, 85.3% | p = 1.00 | 34 | |
| GAD | Yes | 0, 0.0% | 32, 100.0% | 17, 53.1% | 15, 46.9% | 6, 18.8% | 26, 81.2% | 32 | |||
| No | 1, 8.3% | 11, 91.7% | 4, 33.3% | 8, 66.7% | 1, 8.3% | 11, 91.7% | 12 | ||||
| Total | 1, 2.3% | 43, 97.7% | p = 0.27 | 21, 47.7% | 23, 52.3% | p = 0.32 | 7, 15.9% | 38, 84.1% | p = 0.65 | 44 | |
Discussion
We found that young adults with an ASD have a high burden of associated psychiatric symptoms, including ADHD, and this is one of the main drivers of their service use. Even so, 45% were not using any services. In addition, among those who were, most of their parents reported that their children had not been clinically diagnosed or treated for the mental health problem(s) that (we detected) they suffered from. Among our sample, we found that, of those participants reaching DAWBA criteria for GAD and who were in touch with services, no parents had reported that their child had received a formal clinical diagnosis. Eighty-one per cent did not report any relevant medication use for their children. Close to half (49%) reported that their child had no psychiatric input in the past 3 months. Similarly, 85% of those with both ASD and ADHD were untreated.
In addition, we found a very high level of need among this group – and these were more likely to be met by family and friends rather than by services. These needs mainly pertained to core daily living activities and independence and are highlighted in the recent NICE guidelines for adults as important to preparing adults on the spectrum as they transition into adulthood. 53
Our findings support those from a recent study highlighting the lack of treatment and services for adults living with severe and enduring mental health difficulties, including depressive and anxiety disorders and ADHD (URL: http://cep.lse.ac.uk/pubs/download/special/cepsp26.pdf). The report concludes that living with a mental illness such as GAD can be even more debilitating than most chronic physical conditions even though three-quarters get no treatment (URL: http://cep.lse.ac.uk). Thus, our findings highlight the need for better recognition, referral and treatment of psychiatric comorbid conditions among young people with an ASD.
There are few recognised ASD-specific treatments. However, there are a number of effective treatments for ADHD, GAD and depression that have been developed in the general population. In addition, there is recognised success in treating some of the associated psychiatric conditions in developmental disorders. For example, the effective use of optimal dosing of methylphenidate (Concerta XL®; Janssen-Cilag Ltd, High Wycombe, UK) in children with hyperkinetic disorder and ID, ASD54 and for higher-functioning ASD. 55 Yet, despite this, we found very few affected individuals reported that they had been diagnosed or were being treated.
Strengths and weaknesses
This study has a number of limitations. First, the sex balance is not representative of the ASD population as > 90% of our sample is male, compared with a sex ratio of approximately 4 : 1 typically found by others. Second, our sample included relatively high-functioning individuals compared with the ASD samples investigated in other studies. For example, approximately 15% of our sample was diagnosed with a LD compared with a prevalence of 56% in a population cohort of children. 56 Even so, there is increasing recognition that many individuals with an ASD do not have an ID, but still report high prevalence of functional impairments, psychological comorbid disorders and needs. 57,58 Finally, this initial report is from a cross-sectional study and is therefore able to examine associations of need and service use at only one point in time and so causality cannot be inferred.
Nevertheless, this study has significant strengths in comparison with earlier research. Our study is one of the few to focus on adolescents and young adults with an ASD – whereas most studies concentrate on children. 59,60 In addition, our diagnostic assessment, the ADI-R, is a widely recognised and well-validated informant-based (so-called ‘gold standard’) measure of ASD and so the sample contains ‘true’ cases.
Our study has shown a high prevalence of unrecognised psychiatric associated conditions and needs among adolescents and young adults with an ASD. Many of the young people in this clinical group have needs that are met not by services but largely by family and friends. Moreover, our findings show that only severity of psychiatric conditions is associated with service use, and not ASD symptomology. Even those young people with an ASD who met criteria for an additional psychiatric condition and were in touch with services are no more likely to report appropriate diagnosis and treatment. Yet, further research is required into the appropriate use, efficacy and long-term safety of antipsychotics and stimulants in the autistic population before moving towards standardised pharmacological treatments (see Chapter 19). Too few services are ‘autism focused’ and geared towards programmes to help those with an ASD to maintain independence and sustain employment. 61 Our data suggest that needs-led services are required which can meet the complexities of ASD, including the core symptoms (e.g. difficulties with reciprocal social interaction and communication, repetitive behaviours and interests), but which can also diagnose and treat severe associated conditions such as ADHD, anxiety and depression, and thus reduce both the challenges facing the young person and the burden they place on caregivers.
Despite a high prevalence of associated psychiatric symptoms, many adolescents and young adults with an ASD are not being helped by services.
Our findings suggest that needs-led services are required to meet the complexities associated with lifelong disorders.
Chapter 5 Carer burden as people with ASD and ADHD ‘transition’ into adolescence and adulthood in the UK
Background
Here we address the third research question – that is to define the relationship between symptomology, need and service use among people with ADHD or an ASD at transition to adolescence and young adulthood and carer burden. Previous research focused largely on burden to carers of young children with ASD; few studies used adult samples.
The few available studies in ASD reported high levels of caregiver stress and burden; this was greater than in parents caring for adults with other disabilities such as fragile X syndrome and Down syndrome. 62,63 However, to our knowledge, no studies compared levels of burden for ADHD and ASD.
Methods
We used a modified stress appraisal model64 to examine correlates of burden. The model includes the following components as potential correlates of caregiver burden: (1) family background characteristics (parental education), (2) primary stressors (ADHD or ASD symptoms, a LD and measures of psychiatric comorbidities), (3) a primary appraisal (a measure of need) and (4) resources (use of services). This model was based on the stress appraisal model65 incorporating the two main theories regarding caregiver burden: the appraisal model66 and the stress process model. 67 Yates and colleagues’ stress-appraisal model65 consisted of five components: (1) primary stressors (e.g. level and type of disability), (2) primary appraisal (the caregiver’s subjective appraisal of care need), (3) resources (individual and societal resources that may affect the stressor such as the level of formal help), (4) secondary appraisal (often measured as caregiver burden) and (5) outcome (psychological well-being). Casado and Sacco64 altered this model by adding a family background component from Pearlin and colleagues’67 stress process model (family sociodemographic background) and by conceptualising caregiver burden as an outcome. Thus, we propose that family background, the young person’s level of symptoms and psychological comorbidities, and parental perceptions regarding the need for care and resources lead to perceptions of burden.
Results
Sample characteristics
We included 89 individuals with ASD and 81 individuals with ADHD. Young people in the two groups were similar in age, sex (≈90% male), education (≈70% in education) and accommodation (≈90% lived at home) (Table 8). Caregiver sociodemographic characteristics in the two groups were also broadly similar (see Table 8). All of the caregivers were either a biological or an adoptive parent. Caregivers from the two samples showed similar sex and marital status distributions (72% of ASD caregivers were married vs. 78% of ADHD caregivers, χ2 = 0.77; p = 0.38). In addition, around 35% of parents in both groups provided care for someone else aside from their child with ASD/ADHD (χ2 = 0.28; p = 0.60). Caregivers from the two samples were also comparable in terms of age (ASD mean 49 years, SD 6 years vs. ADHD mean 48 years, SD 5 years, t = 1.65; p = 0.10). There were, however, significant differences in occupation of the two caregiver groups: parents of ASD were in higher occupational groups (managerial/professional and associate professional/administrative) (55% ASD vs. 42% ADHD, χ2 = 12.54; p = 0.01).
| Variable | ASD | ADHD | Significance test | ||||
|---|---|---|---|---|---|---|---|
| % | Mean (SD) | Range | % | Mean (SD) | Range | ||
| YP age (years) | 17.57 (2.87) | 14–24 | 17.47 (2.28) | 14–22 | df = 168, t = 0.26 | ||
| YP male | 92 | 89 | df = 1, χ2 = 0.52 | ||||
| PR age (years) | 49.02 (5.89) | 38–64 | 47.60 (5.44) | 36–59 | df = 167, t = 1.65 | ||
| PR female | 93 | 96 | df = 1, χ2 = 0.33 | ||||
| YP education | df = 2, χ2 = 1.67 | ||||||
| Full time | 67 | 57 | |||||
| Part time | 8 | 9 | |||||
| Not in education | 25 | 35 | |||||
| PR education | df = 1, χ2 = 12.58*** | ||||||
| Low | 38 | 65 | |||||
| High | 62 | 35 | |||||
| YP accommodation | df = 1, χ2 = 0.19 | ||||||
| Family home | 90 | 88 | |||||
| Other | 10 | 12 | |||||
| YP symptoms | |||||||
| YP AQ symptoms | 35.56 (5.54) | 22–46 | |||||
| YP ADHD symptoms | 10.63 (4.73) | 0–18 | |||||
| YP LD | 15 | 0 | |||||
| YP SDQ total | 20.51 (7.04) | 4–36 | |||||
| YP CIS-R | 7.85 (6.34) | 0–29 | |||||
| YP needs | |||||||
| YP total needs | 9.90 (3.55) | 0–18 | 5.01 (3.12) | 0–14 | df = 168, t = 9.51*** | ||
| Met | 6.58 (2.72) | 0–12 | 2.60 (2.31) | 0–11 | df = 167.26, t = 10.31*** | ||
| Unmet | 3.31 (2.89) | 0–11 | 2.41 (2.23) | 0–9 | df = 163.70, t = 2.30* | ||
| Service contact and needs | |||||||
| YP In contact with services | 58 | 56 | df = 1, χ2 = 0.14 | ||||
| Zarit Burden Interview | 22.66 (8.84) | 4–41 | 17.80 (9.18) | 0–38 | df = 168, t = 3.52** | ||
Caregiver burden and study variables
Caregiver burden was high in both68,69 groups, but it was significantly higher in ASD than ADHD (ASD mean 22.66, SD 8.84 vs. ADHD mean 17.80, SD 9.18, t = 3.52; p = 0.001). Family background (parental education) was significantly different, with 62% of parents in the ASD group being in the highest educational category, compared with 35% in ADHD (χ2 = 12.58; p < 0.001). Primary stressors (i.e. symptoms, LDs and psychiatric comorbidities) also showed significant differences between the two groups. The mean current severity of ASD as measured by AQ score for the ASD group was 36 (SD 6), with 73% of this group showing AQ scores above the suggested cut-off point of 32. The mean BAARS-IV current symptom score for the ADHD group was 10.63 (SD 4.73). Using a cut-off point of > 5 on the BAARS-IV current symptom score, 58% met threshold for inattentiveness, 42% for hyperactivity and 36% for combined ADHD. Fifteen per cent of the ASD group had been previously diagnosed with a LD, compared with none of the ADHD group. In terms of psychiatric comorbidities, the mean Strengths and Difficulties Questionnaire (SDQ) score for the ASD group was 21 (SD 7), with 70% scoring in the abnormal range (> 16). Fifty-five per cent of the autism group also scored in the abnormal range on the hyperactivity subscale of the SDQ (> 6). The mean CIS-R score for the ADHD group was 8 (SD 6), with 27% per cent of the ADHD group scoring ≥ 12, indicating the presence of a common mental disorder.
Need, our measure of primary appraisal, also showed significant differences between the two groups, with the needs of those with an ASD (as rated by caregivers) being significantly higher (ASD mean 9.90, SD 3.55, vs. ADHD mean 5.01, SD 3.12, t = 9.51; p < 0.001). We used service use to conceptualise resources. There was little difference in this indicator between the two groups; 58% of the ASD group and 56% of the ADHD group were currently being seen by health services.
Tables 9–11 present the sequential multiple regression models used to examine the correlates of caregiver burden. Separate models were produced for each condition, as well as a model combining both conditions. Variables representing family background characteristics (parental education) were first entered into the model, followed by those capturing primary stressors (symptoms, a LD for ASD and psychiatric comorbidities), primary appraisal (need) and resources (use of services). For both samples, caregiver-rated need was a significant correlate of burden even after other factors were controlled for (see Tables 9–11). In addition, once need was controlled for, severity of disorder symptoms was no longer a significant predictor of burden for ASD, but remained significant for ADHD (see Table 10, model 3). Conversely, although psychiatric comorbidities were a significant predictor of burden for ASD, they were not for ADHD. Given that approximately half of the ASD group also met the threshold on the SDQ hyperactivity scale, we also investigated whether or not ADHD symptomology was associated with burden for the ASD group. When the SDQ hyperactivity subscale was included as a separate variable in the model (along with a variable comprising the total score from the other three subscales), the hyperactivity subscale was not significantly associated with burden.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted R2 | 0.01 | 0.28 | 0.35 | 0.34 | ||||
| F change | 0.49 | 9.56*** | 10.37*** | 8.61*** | ||||
| ASD | β | β | β | β | β | β | β | β |
| Correlates (reference groups) | ||||||||
| Family background | ||||||||
| Parent education (high) | –1.36 | –0.08 | –2.64 | –0.15 | –2.31 | –0.13 | –2.44 | –0.14 |
| Primary stressor | ||||||||
| LD | 0.36 | 0.01 | –1.01 | –0.04 | –0.99 | –0.04 | ||
| ASD symptoms | 0.03 | 0.02 | –0.00 | –0.00 | –0.00 | –0.00 | ||
| Psychiatric comorbidities | 0.70*** | 0.56 | 0.52*** | 0.41 | 0.51*** | 0.41 | ||
| Primary appraisal | ||||||||
| Need | 0.77** | 0.31 | 0.74** | 0.30 | ||||
| Resources | ||||||||
| Service use (no service use) | 0.86 | 0.05 | ||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted R2 | –0.01 | 0.16 | 0.23 | 0.23 | ||||
| F change | 0.20 | 5.99** | 6.97*** | 5.75*** | ||||
| ADHD | β | β | β | β | β | β | β | β |
| Correlates (reference group) | ||||||||
| Family background | ||||||||
| Parent education (high) | –0.30 | –0.02 | 0.29 | 0.02 | –0.26 | –0.01 | –0.13 | –0.01 |
| Primary stressor | ||||||||
| ADHD symptoms | 0.69** | 0.36 | 0.48* | 0.25 | 0.44* | 0.23 | ||
| Psychiatric comorbidities | 0.34* | 0.24 | 0.29* | 0.20 | 0.28 | 0.19 | ||
| Primary appraisal | ||||||||
| Need | 0.90** | 0.31 | 0.88** | 0.30 | ||||
| Resources | ||||||||
| Service use (no service use) | 1.78 | 0.10 | ||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted R2 | 0.01 | 0.15 | 0.29 | 0.29 | ||||
| F change | 2.23 | 6.79*** | 12.25*** | 10.87*** | ||||
| ASD and ADHD | β | β | β | β | β | β | β | β |
| Correlates (reference group) | ||||||||
| Family background | ||||||||
| Parent education (high) | –2.12 | –0.12 | –0.14 | –0.01 | –0.64 | 0.03 | –0.72 | 0.04 |
| Primary stressor | ||||||||
| ASD (ADHD) | 2.44 | 0.13 | –1.95 | –0.11 | –1.72 | –0.09 | ||
| LD | 0.05 | 0.00 | –1.77 | –0.05 | –1.73 | –0.05 | ||
| SDQ > 16/CIS-R > 11 (16/11 or less) | 4.87** | 0.26 | 3.16* | 0.17 | 3.15* | 0.17 | ||
| AQ > 32/BAARS-IV inattention/hyperactivity > 5 (32/5 or less) | 3.50* | 0.18 | 2.28 | 0.11 | 2.15 | 0.11 | ||
| Primary appraisal | ||||||||
| Need | 1.10*** | 0.49 | 1.04*** | 0.46 | ||||
| Resources | ||||||||
| Service use (no service use) | 1.81 | 0.10 | ||||||
In the combined model need and comorbid psychopathology were significant predictors of burden, whereas having a diagnosis of either an ASD or ADHD was not (see Table 11). The models show 34% of the variance in caregiver burden among those caring for a young person with an ASD (see Table 9) in comparison with 23% in the ADHD group (see Table 10). Further models were run for both disorders using the young person’s assessment of their needs; however, no relationship was found with parental burden.
In order to further explore the relationship between need and burden the models above were run with unmet and met need instead of total need (not shown). The results showed that only unmet need was significantly associated with caregiver burden. The models using unmet need instead of total need accounted for 42% of the variance in burden for the ASD group and 26% for the ADHD group. To investigate these results further, additional bivariate correlations were calculated between individual CANDID items and caregiver burden. Table 12 shows the individual domains of unmet needs, which were significantly correlated with burden in the two groups. Unmet needs, related to depression/anxiety, inappropriate behaviour, exploitation risk, aggression/violence and daytime activities, were significantly correlated with burden for both samples. Specific to the ASD sample were significant correlations between burden and unmet needs relating to social relationships, mental health problems, safety of self and communication.
| CANDID item of unmet need | Correlation with Zarit Burden Interview (r) | % of sample reporting unmet need |
|---|---|---|
| ASD | ||
| Depression/anxiety | 0.38** | 37 |
| Exploitation risk | 0.36** | 20 |
| Major mental health problems | 0.35** | 11 |
| Social relationships | 0.35** | 43 |
| Inappropriate behaviour | 0.33** | 25 |
| Daytime activities | 0.31** | 25 |
| Safety of self | 0.29** | 10 |
| Communication | 0.28** | 21 |
| Sexual expression | 0.27* | 3 |
| Aggression/violence | 0.24* | 14 |
| Substance misuse | 0.22* | 1 |
| ADHD | ||
| Exploitation risk | 0.32** | 14 |
| Aggression/violence | 0.29** | 14 |
| Inappropriate behaviour | 0.28* | 30 |
| Depression/anxiety | 0.27* | 11 |
| Daytime activities | 0.25* | 20 |
Discussion
We explored (for the first time) the levels, and correlates, of burden among parents caring for a young person with ASD or ADHD at transition from adolescence and young adulthood; and the relationship between caregiver perceptions of their child’s need (met and unmet) and their own burden. Caregivers in both groups had very high levels of burden that equate to those caring for individuals with very serious medical disorders, and there was preliminary evidence that burden was significantly higher in the ASD group. For example, level of burden among caregivers of young people with an ASD was comparable to that among caregivers of those with acquired brain injury, and levels of burden among caregivers of those with ADHD were comparable to those seen in the caregivers of people with dementia. 70
Similar to previous studies, we found that current severity of symptomology of the core disorder is associated with caregiver burden for ADHD but not in ASD32,71 once other potentially relevant factors are controlled for. Contrary to some prior work,72 comorbid psychiatric symptoms were significantly associated with caregiver burden among the ASD group but not among the ADHD group. Previous studies have also found that the presence of physical health problems is associated with higher levels of burden. 69,73 To examine this we explored correlations between the CANDID item assessing physical health needs and caregiver burden; however, no association was found. Similarly, there was no relationship between presence of a LD and caregiver burden. 69,72 Given the fact that few specific autism services (especially for those who are high functioning) are available, our finding that burden is significantly associated with comorbid psychiatric symptoms and needs in the ASD group suggests that autism-specific services targeting multiple conditions may be beneficial in reducing disease burden.
For both disorders, level of unmet need was a significant predictor of burden even after other potential contributing factors (e.g. symptomology) were taken into account. This suggests that it is not just family background factors and primary stressors that contribute to caregiver burden among those caring for a young person with ASD/ADHD, but also the caregiver’s appraisal of the level of unmet need. Unmet needs significantly correlated with burden for both disorders were depression and anxiety, exploitation risk, inappropriate behaviour and aggression/violence. Specific to ASD were significant associations between burden and unmet needs concerning social contact, major mental health problems, appropriate daytime activities, safety of self and communication. Our results suggest that targeted interventions to meet the needs of young people with ASD and ADHD in these domains may also act to reduce caregiver burden. However, it is important to note that burden was associated with parental perceptions of unmet need, not the affected young person’s perceptions of their own unmet needs. This suggests the need for a holistic approach to assessment and treatment of young people; it may be important to consider not only their own assessment of needs, but also their parents’ perceptions of needs, as this is significantly associated with caregiver burden.
Strengths and weaknesses
This study has a number of limitations. First, we did not have a direct measure of maladaptive behaviour, although it is likely that the measures used (e.g. of need and psychiatric comorbidities) also captured behavioural difficulties. Second, our ASD sample appears to be higher functioning than the ASD populations that have been investigated in other studies. For example, approximately 15% of our sample was diagnosed with a LD, in comparison with a prevalence of 56% found by others in a population cohort of children. 7 In addition, there is increasing recognition that many individuals with ASD do not have ID. Even so, caregivers of these higher-functioning individuals still describe a very significant level of burden. Hence, public health policies should not assume that relatively higher-functioning ASD individuals and their caregivers do not also have significant (and unmet) needs. Third, the sex balance of the young people in both the ASD and ADHD samples was not representative of the respective populations. A male to female ratio of 3.3 : 1 was reported in a study of children with ASD62 and a male to female ratio of 1.6 : 1 was reported in a study of adults with ADHD. 63
By contrast, our samples had ratios of approximately 9 : 1 (male : female). In addition, almost all caregivers in the study were mothers. Given that studies in ASD and ADHD have found having a male child to be associated with higher levels of burden,68,69 and that levels of burden have been reported to be higher in mothers than in fathers,74 our results may overestimate the level of burden in this population. Fourth, although a measure of ADHD symptomology was included for the ASD sample (the hyperactivity/inattention subscale of the SDQ), no corresponding measure of ASD symptomology was included for the ADHD sample. Although the ADHD sample was screened for ASD in childhood, mild autism traits could still have been present; however, we were unable to consider the effect of these traits on caregiver burden. Fifth, this initial study was cross-sectional and is therefore able to examine associations of need and caregiver burden at only one point in time. Future longitudinal research is required to determine whether or not input from formal services significantly reduces parental burden. Sixth, there was some reduction in sample size due to missing data. As the CIS-R in the ADHD group was taken from the young person’s interview, analyses focused on those families with both a parent and young person interview (i.e. out of 86 paired interviews, 81 contained complete information). For the ASD group, analyses focused on the parent interviews as these contained the necessary measures. The sample size for this group dropped from 101 to 89, largely as a result of missing data on the SDQ. Seventh, differences in the relationship between comorbidities and caregiver burden in the two groups may be due to differences in the way that this information was collected: through self-reports in the ADHD group using the CIS-R and through informant reports in the ASD group using the SDQ. Finally, our initial analyses were cross-sectional – but we need to also examine change. As all data have now been collected, our future focus will be examining changes over time as outlined in research questions 2 and 3. Thus, we will investigate how needs and associated comorbidities have changed over time in this clinical group and examine the demographic and medical factors associated with this change. Our final analysis, which has not yet begun, will be to examine the main family carer’s role in meeting needs and how this has changed over time and whether or not this is related to changes in service use. We will assess correlates associated with this change and the consequences of changes in the family carer’s role for their own well-being.
Conclusions
This part of our study has shown that caregivers of adolescents and young adults with ASD and ADHD experience very high levels of burden (likened to caregivers of those with acquired brain injury and people with dementia). Many of the young people have needs that are currently unmet by services and the level of unmet need is significantly associated with caregiver burden. These high levels of burden are likely to affect the parents’ health75 and their ability to continue providing care. Interventions to screen for and target depression/anxiety, exploitation risk, inappropriate behaviour and aggression could help reduce burden on caregivers of young people with ASD and ADHD. Interventions to improve the young people’s communication and social relationships, to provide appropriate daytime activities and to treat mental health problems could also act to alleviate burden on caregivers of those with ASD.
Caregivers of young people with an ASD and ADHD have very high (and frequently unrecognised) levels of burden. In ASD this is comparable to that reported in people caring for those with a brain injury.
Psychiatric comorbidities (but not severity of core disorder) are significant sources of this burden in ASD, whereas the opposite is true in ADHD.
In both groups caregiver burden is mainly explained by the affected person’s unmet need, yet there are effective (but unused) treatment interventions available for these needs.
Specific interventions, using currently available treatments, that target unmet needs of young people with ASD and ADHD may reduce caregiver burden.
Chapter 6 Six-year follow-up study of combined-type ADHD from childhood to young adulthood: predictors of functional impairment and comorbid symptoms
Background
In the previous chapters, we reported that individuals with ASD and ADHD who are ‘transitioning’ into young adulthood have high rates of (frequently undiagnosed) co-occurring mental health problems – and that the needs arising from these were not being met by clinical services. In addition, they were associated with significant impact on school/higher education, social performance and carer burden. However, the determinants of these outcomes are unknown. We present, and discuss, our initial findings for ADHD.
There is considerable evidence that the presence of ADHD in childhood is associated with a range of poor outcomes in adulthood, including poorer academic achievement,76 impaired occupational functioning77 and higher risk of criminality,78 substance abuse,79 interpersonal difficulties80 and psychiatric comorbidity,46 particularly antisocial, addiction, mood and anxiety disorders. However, relatively little is known about which childhood and adulthood factors are predictive of poor outcomes. In particular, whether the negative outcomes are linked to earlier ADHD during childhood or current levels of ADHD symptoms reflecting persistence of the disorder, which may therefore be amenable to treatments targeted at ADHD.
It is well established that the hyperactive–impulsive symptoms tend to decline with increasing age, whereas the inattentive symptoms tend to persist and cause the greatest problems to young adults. 81 However, when hyperactive–impulsive symptoms do persist into adulthood they may be particularly impairing (e.g. patients with ADHD and addiction2 or forensic problems20). Meta-analysis of follow-up studies concluded that, although 15% of subjects diagnosed with ADHD during childhood retained the full diagnostic criteria at age 25 years (syndromatic persistence), an additional 50% met criteria for ADHD in partial remission, meaning that they remained impaired by persistent levels of ADHD symptoms, although subthreshold to the diagnostic criteria (symptomatic persistence). 9 We therefore see that ADHD persists to different degrees, with some individuals meeting full criteria as adults, some in partial remission and some showing full recovery.
Previous investigators have investigated predictors of comorbid outcomes. A 5-year follow-up study of girls with ADHD concluded that it was the persistence of ADHD symptoms that predicted the occurrence of later psychiatric comorbidities, behavioural problems and functional impairments in adolescents and young adults. 82 Another study found that it was the persistence of ADHD symptoms in adolescence that was a predictor of antisocial and drug abuse disorders, although in adults these problems appeared to be independent of the persistence of ADHD symptoms. 83 A more recent longer-term follow-up study found that persistence of ADHD was the main predictor of impairments later in life, that could not be accounted for by other comorbid psychopathologies. 84 These earlier studies therefore point towards the persistence of ADHD as a predictor of current impairments and co-occurring behavioural problems such as antisocial behaviour and drug abuse.
To date the reported follow-up studies are predominantly from the USA and most have used broad criteria for ADHD, including all the clinical subtypes. This may have led to relatively low estimates of the persistence rate for the diagnosis and a different pattern of comorbidities. Furthermore, differences in patterns of referral and diagnostic rates between Europe and the USA might reflect overall less impaired groups in the US studies compared with typical ADHD cases seen in child and adolescent clinics in the UK. We therefore set out to follow up a well-defined group of children from UK clinics who all met strict criteria for DSM-IV combined-type ADHD at initial assessment. Here we evaluate the range of outcomes and provide an initial evaluation of the predictors of comorbid outcomes in late adolescence and early adulthood.
We hypothesised that:
-
We would see high rates of persistence due to the selection of combined-type cases, with high levels of both inattentive and hyperactive–impulsive symptoms.
-
The persistent ADHD group would have a significantly higher rate of mental health problems and poorer psychosocial outcomes than population norms.
-
Severity of ADHD at follow-up would be the main predictor of comorbid mental health and behavioural problems.
Method
Sample
Participants were recruited from the IMAGE study,85 a genetic study of ADHD carried out by researchers at the IoP between June 2003 and January 2006. Families were originally recruited to the IMAGE study by referral from child and adolescent clinics in south-east England on the basis that they had received a clinical diagnosis of combined-type ADHD (as defined in the DSM-IV) and had at least one surviving biological sibling aged 5–17 years. Both participants and their siblings were included in the IMAGE study if they were between the ages of 5 and 17 years, had an IQ of ≥ 70, were of European or Caucasian descent and had at least one biological parent willing to provide DNA samples. Participants were excluded from the IMAGE study if they had been diagnosed with autism, epilepsy, an IQ of < 70, brain disorders and/or any genetic or medical disorder associated with externalising behaviours that might mimic ADHD based on both history and clinical assessment.
To compare mental health outcomes with population norms, comparison data were acquired from the Adult Psychiatric Morbidity Study (APMS). 86 Data were included from 411 participants aged 16–23 years who did not meet criteria for ADHD.
Baseline measures
Parent Account of Childhood Symptoms
Parent Account of Childhood Symptoms (PACS) is a semistructured, standardised, investigator-based interview developed as an instrument to provide an objective measure of children’s behaviour. 87 A trained interviewer administered PACS with parents, who were asked for detailed descriptions of the child’s typical behaviour in a range of specified situations. Interviewers then made their own ratings on the basis of a formal training and written definitions of the behaviours to be rated, on a four-point scale of severity and frequency in the previous week and previous year. Inter-rater reliability was high, with product–moment correlations for pairs of interviewers ranging from 0.79 to 0.96.
Parent Account of Childhood Symptoms includes several subscales. The hyperactivity subscale is made up of attention span (time spent on a single activity, rated separately for four different kinds of activity); restlessness (moving about during the same activities); fidgetiness (movements of parts of the body during the same activities); and activity level (rated for structured situations such as mealtimes and car journeys). The defiance subscale is composed of items concerning temper tantrums, lying, stealing, defiance, disobedience, truancy and destructiveness. The emotional disorder subscale is made up of items of misery, worrying, fears and somatic symptoms that describe overt emotional stress rather than inferences concerning the emotional basis of symptoms. The comorbid and other problems section elicits symptoms of ASDs, attachment disorders, manic episode, substance abuse, psychotic symptoms, obsessive–compulsive symptoms and other specific developmental disorder and neurological conditions.
The Long Version of Conners’ Parent Rating Scale
The Long Version of Conners’ Parent Rating Scale consists of 80 items in total, each scored on a Likert scale from 0 to 3. In this scale, 0 represents ‘not true at all (never, seldom)’, 1 represents ‘just a little true (occasionally)’, 2 represents ‘pretty much true (often, quite a bit)’ and 3 represents ‘very much true (very often, very frequent)’. 88
The 80 items yield 14 subscales (from ‘A’ to ‘N’), derived from the published algorithms in the manual. These subscales were constructed based on their factorial validity derived from community samples. The 14 subscales are A, ‘oppositional’ (10 items); B, cognitive problems/inattention (12 items); C, hyperactivity (nine items); D, anxious/shy (eight items); E, perfectionism (seven items); F, social problems (five items); G, psychosomatic (six items); H, ADHD index (12 items); I, Conners’ Global Index: restless-impulsive (7 items); J, Conners’ Global Index: emotional lability (three items); K, Conners’ Global Index: total (10 items); L, DSM-IV symptoms subscales: inattention (nine items); M, DSM-IV symptoms subscales: hyperactive–impulsive (9items); and N, DSM-IV symptoms subscales: total (18 items).
Intelligence quotient
Probands and siblings were screened for global learning difficulties with pro-rated full-scale IQ scores derived from four subtests of the Wechsler Intelligence Scale for Children:89 picture completion, block design, similarities and vocabulary. Individuals with a pro-rated IQ of < 70 were excluded from this analysis.
DSM-IV diagnoses at initial assessment
A standardised algorithm for PACS was applied to all raw PACS data to yield diagnoses based on operational DSM-IV criteria for ADHD. Age adjustment for symptom threshold is built into the PACS algorithm. Situational pervasiveness is captured by the different situations investigated within the PACS interview as well as the presence of at least one symptom in each domain reported by teachers using the Conners’ ADHD subscale. Low teacher ratings for ADHD probands can occur when children are stably maintained on medication at school and, for this reason, situational pervasiveness is also captured in the PACS interview.
Follow-up procedure
Participants were recruited for the present study if they were aged between 14 and 24 years by 1 March 2009. Parents and young people were initially contacted by letter and followed up by telephone. Participants were interviewed either at our research offices or at the participant’s home. Of the original IMAGE sample of 204 families, 118 ADHD probands and their parents were successfully followed up. Mean follow-up duration was 6 (range 3–8) years.
Follow-up measures
ADHD symptoms and diagnostic status
The DIVA was used to assess ADHD symptoms at follow-up. 34 DIVA investigates the DSM-IV criteria of ADHD as well as impairment in five areas of functioning. Although the DIVA is a new measure, it is being widely adopted across Europe for both clinic and research investigations of ADHD in adults. DIVA follows a similar approach with the same enquiries about the DSM-IV symptom items as the more established CAADID. 90 In comparison, it provides more realistic examples of symptoms for the diagnostic assessment of ADHD in adults and has a more detailed impairment section. 1
Functional Impairment
The informant version of the ADHD rating scale for adults36 was also used to measure functional impairment of ADHD symptoms at follow-up. It is a widely used rating scale for the assessment, diagnosis and monitoring of treatment of ADHD in adults. It assesses the same 18 symptom items from the diagnostic criteria for ADHD in the DSM-IV and contains an additional 10 questions assessing impairment across different areas of functioning, including home life, occupation, social interactions, education, driving, money management, leisure time and routine daily responsibilities. Functional impairment scores were calculated by taking the average score for each participant from the 10 impairment questions.
Psychiatric associated symptoms
The CIS-R was used to assess psychiatric comorbidities. 37 It is a standardised, valid and reliable structured diagnostic instrument used for rating psychiatric symptoms across 14 domains (e.g. anxiety, depression), and has been widely used in both clinical and general population surveys. 86,91 The minimum score on each section is zero when the symptoms were either not present in the past week or were present only in mild degree. Symptoms are regarded as severe when the score is ≥ 2 with a maximum score on each section being 4 (5 for the section on depressive ideas). A total score of ≥ 12 is regarded as a clinically significant indicator of general mental health problems. 37
Emotional lability
The auxiliary subscale from the CNS-LS92 was used to measure labile frustration, anger and impatience. It has been validated with patients with amyotrophic lateral sclerosis92 and Pseudobulbar affect. 93 Emotional lability measured with this scale has also been found to be increased in non-comorbid adults with ADHD and to be an independent predictor of impairment. 94 Participants rate the extent to which eight statements apply over the past month. Each statement is rated from 1 to 5, giving a range of 8–40.
Drug and alcohol use and police contact
Drug use was assessed through a series of questions adapted from the ONS survey Mental Health of Children and Young People in Great Britain, 2004 Report. 29 These questions were comparable to questions on drug use used in the APMS 2007. 86 Young people were asked to self-rate the frequency and nature of drug use from a range of drugs such as cannabis, cocaine and heroin. For each drug, participants were asked whether or not they had used the drug within their lifetime and within the past month.
Alcohol use was assessed using the AUDIT-C,30 a brief and validated three-question screen that can help identify hazardous and harmful drinking. A score of ≥ 5 indicates hazardous alcohol use.
Problems with police were examined through a series of questions based on those in the background information questionnaire used in the adult ADHD service at the Maudsley Hospital (adapted for this study). All participants were asked whether or not they had been in trouble with the police in the past 12 months.
Analyses
Descriptive statistics were produced for the sample at baseline and follow-up. To examine the persistence of ADHD, participants were classified into two groups using DIVA scores:
-
persistent ADHD, which included combined type (six or more inattentive and six or more hyperactive/impulsive symptoms), inattentive subtype (six or more inattentive symptoms and fewer than six hyperactive symptoms), and hyperactive–impulsive subtype (six or more hyperactive/impulsive symptoms and fewer than six inattentive symptoms)
-
subthreshold ADHD, which included partial remission (four or five inattentive or hyperactive/impulsive symptoms) and full remission (fewer than four inattentive and hyperactive symptoms).
To investigate the outcomes of persistent ADHD, comparisons were made between participants with persistent ADHD, subthreshold ADHD and the comparison sample from the APMS 2007 study. Comparison data from the APMS study were available for psychiatric comorbidity, drug and alcohol use. To explore correlates of these outcomes, multiple linear regression and logistic regression models were produced for each outcome, including both childhood and adult characteristics as predictors. All analyses were conducted in IBM SPSS Statistics for Windows version 20 (IBM Corporation, Armonk, NY, USA).
Results
Sample characteristics
We included 118 individuals with ADHD. The average age of the ADHD group at follow-up was 17.5 years (Table 13); most were males (89%) and still living at home (88%). Fifty-three per cent had used illegal drugs in their lifetime, 20% had used drugs in the past month and 22% had been in trouble with the police within the past year. Over half of the sample met the cut-off score on the AUDIT, suggesting high levels of alcohol consumption. Twenty-seven per cent exceeded the cut-off score on the CIS-R, which is indicative of comorbid psychopathology (see Table 13). Over half of the sample were in full-time education and 12% were in full-time employment. As expected, the proportion of the sample in education decreases with age and the percentage in employment increases with age. However, 46% of those aged 19–23 years reported being unemployed (Table 14).
| Variable | % | Mean (SD) or percentage of sample | Range | Data available, n (%) |
|---|---|---|---|---|
| Current | ||||
| Age | 17.55 (2.40) | 14–23 | 117 (99) | |
| Male | 89 | 89% | 118 (100) | |
| Parent education | 74 (63) | |||
| Low | 64 | 64% | ||
| High | 37 | 37% | ||
| CIS-R mean | 7.69 (6.63) | 0–29 | 114 (97) | |
| CIS-R above cut-off score (> 11) | 27 | 114 (97) | ||
| Mood | 18.58 (9.51) | 8–40 | 104 (91) | |
| BAARS-IV score | 1.48 (0.61) | 0–2.78 | 107 (91) | |
| AUDIT-C | 4.72 (3.72) | 0–12 | 99 (84) | |
| AUDIT-C above cut-off point (> 4) | 54 | 54% | 99 (84) | |
| Drug use in past month | 20 | 20% | 108 (92) | |
| Drug use ever | 53 | 53% | 98 (84) | |
| Recent police contact? | 22 | 22% | 101 (86) | |
| Childhood | ||||
| IQ | 101.14 (15.78) | 117 (99) | ||
| At least one psychiatric condition on PACS | 84% | 118 (100) | ||
| Taking medication | 87% | 118 (100) | ||
| Participant information | All ages | Age range (years) | ||
|---|---|---|---|---|
| 14–15 (n = 21) | 16–18 (n = 26) | 19–23 (n = 28) | ||
| Education (%) | ||||
| Full time | 55 | 90 | 65 | 21 |
| Part time | 9 | 5 | 12 | 11 |
| Not in education | 34 | 5 | 23 | 68 |
| Employment (%) | ||||
| Full time | 12 | 0 | 4 | 21 |
| Part time | 25 | 19 | 31 | 25 |
| Not in employment | 63 | 81 | 65 | 46 |
| Not in education or full-time employment | 16 | 3 | 15 | 27 |
| Accommodation (%) | ||||
| At home | 88 | 100 | 92 | 75 |
| Not at home | 12 | 0 | 8 | 25 |
Persistence of ADHD at follow-up
At follow-up, 80% of the sample met full diagnostic criteria for DSM-IV ADHD: combined-type ADHD (37%), inattentive subtype (36%) and hyperactive–impulsive subtype (7%). Of the group who did not meet full DSM-IV criteria for ADHD, 15% were in partial remission (subthreshold ADHD), with only 5% in total remission (Tables 15 and 16). Age at follow-up was unrelated to the number of inattentive (r = –0.6; p = 0.54) or number of hyperactive symptoms (r = –0.12; p = 0.21) (Figure 8).
| Participant information | DIVA (%) (n = 106) |
|---|---|
| Persistent ADHD | 80 |
| Combined type | 37 |
| Inattentive subtype | 36 |
| Hyperactive subtype | 7 |
| Remitted ADHD | 20 |
| Partial remission | 15 |
| No ADHD | 5 |
| Rating scale | Childhood, mean (SD) | Adulthood, mean (SD) | t-value/p-value |
|---|---|---|---|
| PACS/DIVA inattentive (n = 108) | 8.31 (0.89) | 6.62 (2.19) | t(107) = –7.36; p < 0.001 |
| PACS/DIVA hyperactive (n = 106) | 8.25 (0.92) | 5.23 (2.42) | t(105) = –13.14; p < 0.001 |
FIGURE 8.
Number of individuals by ADHD outcome in adolescents (aged 14–17 years) and young adults (aged 18–23 years).

Psychiatric comorbidity at follow-up
Significant differences were detected between persistent ADHD, subthreshold ADHD and normative groups in the rate of co-occurring mental health problems as assessed by scores on the CIS-R (F = 8.95; p < 0.001). Total scores on this measure were significantly higher in the persistent ADHD group than in the normative sample, indicating higher rates of comorbidity than in the non-ADHD control sample. No other group differences were present (Figure 9). The CIS-R contains some domains (memory and concentration) that overlap with ADHD diagnostic criteria. These analyses were therefore repeated with the memory and concentration items excluded, with no overall change in the finding for higher CIS-R scores in the ADHD group. We conducted further analyses by defining cases as above or below the recognised cut-off score of 32 on the CIS-R. There was a significant difference between the three groups [χ2(2) = 10.24; p = 0.06], with 17% of the control group above the threshold for other mental health disorders, compared with 40% of the ADHD persistent group. Thus, these results indicate that the individuals with persistent ADHD may be more at risk of psychiatric comorbidity compared with both the subthreshold ADHD group and the general population control sample. The subthreshold and control samples had similar rates of other mental health problems.
FIGURE 9.
Mean CIS-R scores for the normative, subthreshold and persistent ADHD groups. ***p < 0.001. A, persistent vs. normative control groups.

Next, we explored scores on the individual domains of the CIS-R to examine differences in the type of mental health symptoms between the three groups. The persistent ADHD group had significantly higher scores than both the subthreshold ADHD and normative group in the ‘fatigue’ and ‘concentration/memory’ domains and significantly higher scores than the normative group in the ‘anger’ domain. No differences were found in any other clinical domains reflecting a wider range of mental health problems (Figure 10).
FIGURE 10.
Mean CIS-R domain scores by ADHD group (n = 517). *p < 0.05, **p < 0.001, ***p < 0.001. A, persistent vs. normative control groups; B, persistent vs. subthreshold groups; C, subthreshold vs. normative control groups.
Drug use and police contact
The persistent ADHD group showed significantly higher rates of lifetime drug use [χ2(2) = 24.26; p < 0.001] relative to the normative group, but not the subthreshold ADHD group (Figure 11). Both the persistent and subthreshold ADHD groups showed significantly higher rates of police contact than the normative group, but no differences were found between the two ADHD groups [χ2(2) = 0.03; p = 8.72]. No differences were detected between any of the three groups in dangerous alcohol use [χ2(2) = 0.11; p = 0.95].
FIGURE 11.
Percentage of sample exceeding AUDIT cut-off point, reporting drug use and police contact. *p < 0.05, **p < 0.001, ***p < 0.001. A, persistent vs. normative controls groups; B, persistent vs. subthreshold groups; C, subthreshold vs. normative control groups. a, Data were unavailable in the normative control group for police contact over the past year, so data for lifetime police contact were used instead.

Emotional lability
Normative data were not available for measures of emotional lability or functional impairment, so comparisons were made only between the persistent and subthreshold ADHD groups. Significant differences between the two groups were observed on the emotional lability scale, with the persistent ADHD group scoring higher levels of emotional instability [t(41.10) = –3.93; p < 0.001]. Functional impairment did not differ between the two groups [t(93) = –1.84; p < 0.07].
Predictors of outcome
To explore which child and adulthood characteristics were associated with outcomes, multiple regression and logistic regression models were produced for the main outcome variables. Predictor variables investigated included the number of adult and childhood ADHD symptoms (derived from DIVA and PACS interviews), age, IQ and sex. Emotional lability scores and a variable indicating whether or not they exceeded the cut-off point on the CIS-R were entered for the models for which those variables were not the dependent variable. Childhood medication status and the presence of a comorbid oppositional defiant disorder and CD in childhood were included in preliminary models but were not found to be significantly associated with any outcome measure, so were excluded from the final analysis.
Tables 17–20 list the findings from regression models investigating predictors of comorbid symptoms, behavioural problems and impairment scores. The main findings were as follows:
-
Total functional impairments as rated by informants on the BAARS-IV impairments scale at follow-up was significantly associated with childhood inattentive symptoms and sex (see Table 17). None of the follow-up measures showed any association with impairment.
-
Using a binary variable stating whether or not they met threshold scores for comorbid symptoms on the CIS-R (> 11) as the dependent variable, current level of hyperactive–impulsive symptoms, age, sex and emotional lability were all significant predictors (see Table 18). None of the childhood measures of ADHD showed any association with the adult comorbidity scores.
-
Emotional lability was associated with current comorbidity scores. Childhood characteristics did not predict current emotional lability (see Table 19).
-
Table 20 shows the findings from a linear regression model for alcohol use. Being of male sex was significantly associated with increased alcohol use, but none of the clinical characteristics was significant.
-
The remaining models examined predictors of drug use and police contact. In both models, the only significant predictor was the child’s age; none of the measures of adult or childhood ADHD symptom severity predicted current drug use and police contact.
| Adjusted R2 | 0.20 | |
| F change | 3.41*** | |
| Predictor of impairment | β | β |
|---|---|---|
| Adult inattentive symptoms (DIVA) | 0.06 | 0.03 |
| Adult hyperactive symptoms (DIVA) | 0.04 | 0.03 |
| Childhood inattentive symptoms (PACS) | 0.20 | 0.14*** |
| Childhood hyperactive symptoms (PACS) | –0.11 | –0.07 |
| Age | 0.00 | 0.03 |
| Childhood IQ | –0.00 | –0.00 |
| Above CIS-R cut-off point | –0.02 | –0.16 |
| Sex (0 = male) | 0.52 | 0.16*** |
| Mood lability | 0.00 | 0.01 |
| χ2 | 25.70*** | |
| Cox and Snell R2 | 0.24 | |
| Nagelkerke R2 | 0.35 | |
| Predictor | β | OR |
|---|---|---|
| Adult inattentive symptoms (DIVA) | –0.24 | 0.79 |
| Adult hyperactive symptoms (DIVA) | 0.36 | 1.43* |
| Childhood inattentive symptoms (PACS) | –0.15 | 0.86 |
| Childhood hyperactive symptoms (PACS) | 0.13 | 1.14 |
| Age | 0.25 | 1.28* |
| IQ | 0.01 | 1.00 |
| Sex (0 = male) | 1.71 | 5.55* |
| Mood lability | 0.07 | 1.07* |
| Adjusted R2 | 0.17 | |
| F change | 3.40** | |
| Predictor | β | β |
|---|---|---|
| Adult inattentive symptoms (DIVA) | 0.74 | 0.44 |
| Adult hyperactive symptoms (DIVA) | 0.69 | 0.18 |
| Childhood inattentive symptoms (PACS) | –1.36 | –0.13 |
| Childhood hyperactive symptoms (PACS) | 0.58 | 0.06 |
| Age | 0.08 | 0.02 |
| IQ | –0.10 | –0.17 |
| Above CIS-R cut-off point | 5.18 | 0.24* |
| Sex | 0.45 | 0.02 |
| Adjusted R2 | 0.12 | |
| F change | 2.27* | |
| Predictor | β | β |
|---|---|---|
| Adult inattentive symptoms (DIVA) | 4.77 | 0.10 |
| Adult hyperactive symptoms (DIVA) | –6.49 | –0.15 |
| Childhood inattentive symptoms (PACS) | –12.77 | –0.10 |
| Childhood hyperactive symptoms (PACS) | 12.18 | 0.11 |
| Age | –7.12 | –0.16 |
| IQ | 1.20 | 0.18 |
| Above CIS-R cut-off point | –8.29 | –0.03 |
| Sex | –103.71 | –0.30** |
| Mood lability | –0.07 | –0.01 |
Discussion
We report on the first UK follow-up study of a cohort diagnosed with combined-type ADHD in childhood. The current study had three aims: (1) to describe the rates of persistence of ADHD, (2) to investigate differences in outcome between those with persistent and subthreshold ADHD and compare these to population norms and (3) to explore whether childhood predictors or current symptomology predict follow-up impairment and comorbid outcomes.
Regarding the first aim, we found very high levels of persistence, with 76% of the sample still meeting full criteria for DSM-IV ADHD. This is much higher than the 15% reported from a meta-analysis of follow-up samples in available data sets in 2006. 9 There are likely to be several reasons for this finding. First, in general, the sample was recruited from child and adolescent clinics in south-east England. The diagnostic rates for ADHD are far lower in the UK than in the USA, where the previous outcomes studies were completed. This is likely to be reflected in a greater severity in the ADHD cases in the UK, where recommended criteria include impairments of at least moderate severity. 95 Second, our sample all had combined subtype ADHD when they first came into the study; selecting for a sample with a greater level of ADHD symptoms compared with broader criteria applied in other follow-up studies.
Furthermore, our sample covered a wide age span (from 14 to 23 years) at follow-up and the high rate of ADHD could be explained by the average age of 17 years in this sample. In previous meta-analysis the rate of 15% with the full diagnosis was estimated for age 25 years. To investigate this further we divided the sample into adolescents (aged 14–17 years) and young adults (aged 18–23 years) and found that 76% still met ADHD criteria in the younger group, compared with 83% in the older group. This finding might seem unexpected with a higher rate among the older participants, but is likely to be explained by the selection for combined-type ADHD. This clinical subtype reflects a more severe condition when present in older patients than in younger patients, as the same clinical criteria with the same high symptom threshold are used and some of the younger group will no longer meet combined-type criteria by the time they are adolescents. Combined subtype ADHD has previously been linked to higher rates of persistence in adulthood in an epidemiological study. 96
The other key observation is the change in clinical subtypes, in which around 36% of the combined-type probands progressed to the inattentive subtype at outcome. This is to be expected and is consistent with previous data supporting the change in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), in which the clinical subtypes are viewed as developmentally unstable and the previous subtypes are now referred to as clinical presentations. Overall, we can see that there is a very high rate of persistence of ADHD beyond the age of 17 years in a UK sample of children with combined-type ADHD. This is in stark contrast to service development in the UK, which continues to lag behind the clinical needs of this patient group (see Chapter 3 for discussion of the unmet needs of this clinical group).
For our second aim, we found significantly higher rates of comorbid symptoms among participants with persistent ADHD as compared with both subthreshold ADHD and the general population sample. When this was analysed in detail, the symptoms/syndromes that were increased in the ADHD group included fatigue, concentration/memory and anger, as well as behavioural problems of using drugs and increased police contact. However, increased rates of potential comorbidities such as anxiety and depression were not seen in the patient group at follow-up. The profile of symptoms detected by the CIS-R screen for mental health problems (fatigue, concentration, memory, anger), is most likely to reflect persistence of the core syndrome of ADHD, as both concentration and memory problems are part of diagnostic criteria for ADHD. In this study, we found that emotional lability (related to anger) and current hyperactive–impulsive symptoms were the main predictors of these symptoms. This is consistent with our previous analysis of a sample of adults with ADHD carefully selected for no other mental health problems who reported increased rates of fatigue, anger and irritability. 94 It therefore seems that in this patient group the development of co-occurring mental health disorders, such as anxiety and depression, is not a common outcome compared with population norms, despite the increase in ‘comorbidity’ scores from the CIS-R. Rather, the symptoms detected on this appear to be closely related to the core disorder of ADHD in adolescents and young adults. Similar conclusions were reached in a comparable follow-up study from Mannuzza and colleagues83 in the USA.
The reasons for the low rate of comorbid conditions may be related to this being a clinical follow-up sample, in which most of the participants were actively engaged in treatment programmes as children. In contrast, higher rates of comorbid conditions are reported in cross-sectional studies with mainly untreated ADHD cases (e.g. Fayyad and colleagues97), or in clinic surveys of adult patients, many of whom were not previously treated for ADHD. 94 Further studies are needed to clarify this point, but overall it seems that when children with ADHD are engaged in treatment programmes they report low levels of newly developing adult-onset disorders compared with patients who were untreated during childhood.
In contrast, our study found higher rates of behavioural comorbidities. Both drug use and police contact were found at higher rates than among population norms, reflecting the development of behavioural problems throughout development and into young adult life in young people with ADHD. However, this was equally true for both the persistent and subthreshold groups and, therefore, seems to be related to the childhood diagnosis of ADHD rather than the current clinical status. Similarly, we did not detect differences in ratings of functional impairments between the persistent and non-persistent ADHD groups, which were related to the severity of inattentive symptoms during childhood. Similar observations were made by Mannuzza and colleagues78,83 in some (but not all) of the studies in the USA. Overall, although it is clear that children with ADHD are at risk from the development of problem behaviours, such as drug use and antisocial or criminal behaviour, the predictors remain to some extent uncertain, with discrepancies across different studies. 78 Nevertheless, our findings do emphasise the importance of the severity of ADHD in childhood and the long-term impact on behavioural and functional outcomes in young adults.
This suggestion is supported by a recent epidemiological survey from Sweden that points towards not only a higher risk of criminal behaviour among those with a diagnosis of ADHD, but also the effects of medical treatment [with stimulants or atomoxetine (Strattera®; Eli Lilly and Company, Indianapolis, IN, USA)] on reductions in rates of criminal convictions. 98 Furthermore, in our ongoing study of young ADHD prisoners aged 18–24 years (see Section 5), we can see the ways in which both current and past ADHD affect behaviour. We find high rates of school expulsions, low educational achievement and conduct problems among prisoners with ADHD during their childhood, as well as persistent problems with inattention and impulsivity related more directly to current behavioural problems such as inability to retain themselves in employment and aggressive outbursts.
Further studies are needed to clarify the impact of treating ADHD in older adolescents and young adults on drug use and criminal behaviour, as well as the impact of medical treatment on functional outcomes. The finding of a mixed impact of both present and past ADHD on different aspects of outcomes supports the multimodal approach recommended by NICE, that include symptom control with medication, alongside psychological treatments such as CBT to improve the longer-term negative consequences of childhood ADHD. 99
Strengths and weaknesses
In drawing conclusions from this study, we need to consider several limitations. First, different interview measures were used to assess ADHD in childhood and at follow-up. However, both of these measures are based on the DSM-IV and are recommended for use in the respective age bands as diagnostic instruments. Second, all of our ADHD sample was in touch with services at baseline and had combined-type ADHD. Hence, our ADHD sample is not representative of the broader spectrum of ADHD cases, although in most clinical settings around two-thirds of ADHD cases meet criteria for the combined subtype. The relatively homogeneous sample does, however, bring advantages by reducing clinical heterogeneity and focusing on what are widely considered the most impaired group of children with ADHD. Further work could usefully be completed in the UK to follow groups of children (and girls) with predominantly inattentive symptoms, where outcomes may potentially differ. Finally, due to our relatively small sample size, we were unable to examine separately participants with partially remitted or completely remitted ADHD. Indeed, this problem arose from the very high rate of persistence for the full diagnosis of ADHD that we saw in this sample at follow-up.
Conclusions
Our preliminary analysis demonstrates the poor psychosocial outcomes during the transition years to young adulthood of children who had been previously diagnosed with combined-type ADHD and been in contact with services during childhood. Our work suggests that, as young adults, those patients with persistent ADHD do not have high rates of comorbid psychiatric disorders, but nevertheless suffer from educational and other functional impairments and display symptoms closely linked to ADHD such as fatigue and feelings of irritability or anger. Even when ADHD symptoms decline to subthreshold levels, we still see problems that require continued treatment. Similarly, we also found that high rates of drug use and recent police contact were related to the severity of ADHD in childhood and were seen in both the persistent and non-persistent ADHD groups. Taken together, our results suggest the importance of continuing services for ADHD throughout the transitional years and into adulthood. Future work needs to clarify the impact of different forms of medical and psychological treatments on the various clinical, functional and behavioural impairments seen in children with ADHD when they enter the adult years.
Section 2 Service perspective
Chapter 7 Outline of our work plan
In the previous section (see Section 1), we asked people with ADHD and ASD (and their families) about their needs. We found that many had residual impairments related to the core disorder together with associated mental health symptoms (most of which they were unaware of and/or were untreated). We also found that the main determinant of service provision was age (and not level of need). This led to concern that ASD and ADHD may be over-represented (but unrecognised) in particular parts of the NHS – and perhaps also in prison populations. However, we did not have the perspective of clinical services in our other work streams.
The main aim of this part of the programme, therefore, was to estimate the proportion of ADHD and ASD cases within GP and adult mental health/prison services that are currently unrecognised and untreated. This was achieved by screening adults who attended primary care physicians in the community and adult mental health and forensic/prison services run by one organisation (The South London and Maudsley NHS Trust). As a pragmatic step, we targeted individuals/clinics that were likely to have high rates of ADHD and ASD.
In addition, due to the relatively poor understanding of the rates of ADHD and ASD in forensic settings and the response to treatment in these settings, we:
-
surveyed rates of ADHD and ASD in NHS forensic inpatient units run by one NHS provider (and provide an initial more detailed analysis of ADHD data)
-
carried out a search of electronic databases to identify the literature on ASD in prisons
-
carried out two pilot (proof of concept) studies on current rates of disorder (and associated mental health symptoms) in two different prison settings (one for young prisoners and the other for adults); in this section we report the findings from our analyses of ADHD.
Chapter 8 ASD and ADHD in general practitioner, NHS hospital and prison settings: a general overview
Background
As ADHD and ASD have only recently been recognised in the UK as adult mental health disorders, little is known about the rate of recognition of ADHD and ASD among people already presenting to adult mental health services with co-occurring conditions such as anxiety, depression, personality disorder (PD) and addictions, as well as criminal behaviour. We therefore wished to clarify the extent to which ADHD and ASD goes undiagnosed and untreated within adult mental health and forensic services and whether or not this leads to inappropriate or poorly targeted treatments.
Attention deficit hyperactivity disorder has an estimated prevalence during childhood in the UK of 3.6%. 100 Longitudinal follow-up studies find that around two-thirds of those affected continue to be impaired by ADHD symptoms in adulthood, with an estimated worldwide prevalence for the disorder in adults of around 2.5%. 101 Despite the high rate of ADHD in adults, the disorder remains underdiagnosed and undertreated beyond the adolescent years. 102 This is a particular issue for adult mental health because ADHD symptoms not only lead to impairments in academic, occupational and social functioning, but are also associated with the development of comorbid disorders, including anxiety, depression, PD, antisocial behaviour and substance use disorders (SUDs). 97,103–105
This also suggests that individuals with ADHD may be over-represented in forensic mental health and prison settings. For instance, it has been reported that the prison population106 may have a 3- to 10-fold increase in ADHD (calculated using 4% community prevalence). Despite this, only one study has reported rates of ADHD symptoms in forensic mental health settings and this study, which focused solely on patients with a primary diagnosis of PD, reported that one-third screened positive. 107 This raises the question of whether or not people diagnosed with such conditions may in some cases have undiagnosed and untreated ADHD and whether or not treatment of underlying ADHD may lead to improvements in their comorbid disorder(s).
People with ASD also have high rates of co-existing psychiatric disorders. In young children, our research shows that 90% of 4- to 8-year-olds with ASD have at least one psychiatric disorder that impairs their ability to function in everyday life. 108 Even in older children the rate of impairment is 70%. 58,109 The most common disorders in children with ASD include anxiety, oppositional defiant disorder and ADHD. In adult life, psychiatric disorders are also reported to occur in the majority of people with ASD. For instance, we found that as part of this programme (see Chapter 4), adults with ASD are especially affected by anxiety disorders, OCD and depression. These associated difficulties are long lasting,54 affect quality of life and (as noted in Chapter 5) are associated with significantly increased carer burden. 57 It has been suggested that some individuals with ASD are at increased risk of offending behaviour (but this is based on limited data). In addition, it is unknown how many people within general practice and mental health (and forensic) services have unrecognised ASD.
Hence, it is important to understand how frequently within adult medical and psychiatric (including prison) services ADHD and ASD are currently unrecognised and untreated. Furthermore, within a prison setting, it is important to know if rates of ASD and ADHD are similar in the different prison systems which detain younger and older individuals. Given the known population prevalence of the disorders it is expected that at least 1% of young adult patients within GP clinics will have ADHD or ASD, but that the proportion will be higher (unknown) among ‘attenders’ in adult psychiatric and forensic services.
Methods
We conducted a survey of adult patients attending primary and secondary mental health care services for adults. Patients were screened using rating scales, followed by diagnostic assessments with recognised diagnostic instruments used in both clinical and research practice. We further collected data on impairment and, when possible, on the health service costs related to the care of the identified cases with ADHD and ASD.
Study sites
Patients in the following local primary, forensic and adult mental health services were screened and assessed:
-
primary care patients currently being treated for other common mental health disorders, mainly anxiety and depression
-
patients in the anxiety disorders unit at the Bethlem Hospital, which is an inpatient facility for the treatment of severe anxiety disorders
-
patients in the secure forensic psychiatry inpatient units at Broadmoor Hospital, River House and Bethlem Hospital, which are ‘prison hospitals’ caring for two main groups of patients: around two-thirds with a primary diagnosis of severe mental illness (usually schizophrenia) and one-third with a primary diagnosis of severe and dangerous PD
-
patients in addiction inpatient facilities at the Bethlem Hospital and Maudsley Hospital and a community outpatient drug treatment service
-
sentenced young male prisoners aged 18–25 years, held at Her Majesty’s Prison (HMP) Isis (south London)
-
sentenced adult male prisoners aged 25–65 years, held at HMP Brixton (south London).
Sample
We screened 1604 individuals for ADHD/ASD: 253 individuals from primary care, 153 individuals from hospital anxiety services, 159 individuals from hospital forensic services and 226 individuals from hospital addiction services. Within the prison settings we investigated the rates of ADHD in 592 young people aged 18–25 years and 221 older prisoners aged 25–65 years. As the study of young prisoners was completed as part of a clinical trial of stimulants for ADHD and related behaviours (see Results and discussion), we were unable to complete Autism Diagnostic Observation Schedule (ADOS) interviews in addition to ADHD interviews, because of the already complex protocol and constraints on interview time as advised by the prison.
Diagnosing ADHD
Participants completed the DSM-IV 18-item self-report screening questionnaires for current and childhood ADHD symptoms using either the BAARS-IV44 or, in primary care, the Adult ADHD Self-Report Scale. 110,111 Positive screening for ADHD consisted of six or more ADHD symptoms during childhood, plus four or more symptoms during adulthood. Participants who screened positive in the first stage were invited to a diagnostic interview.
Research diagnosis of ADHD was established using the DIVA,71 conducted by trained research assistants (RAs). Consultant psychiatrists experienced in the diagnosis of ADHD further reviewed the cases. The DIVA interview systematically evaluates each of the DSM-IV symptom items for both current and childhood symptoms and asks additional questions to establish impairment from ADHD symptoms (impairment criteria), in two or more settings (pervasiveness criteria) and the age at onset of symptoms (age at onset criteria). The diagnostic algorithm for ADHD used the following criteria: (1) six or more ADHD symptoms in either domain from retrospective childhood rating scales, (2) four or more ADHD symptoms from either domain from current ADHD symptom rating scales and (3) research diagnosis of DSM-IV ADHD following application of diagnostic interview for DSM-IV ADHD.
Diagnosing ASD
In the screening step, the AQ was used to screen for ASD. 50 Participants with above threshold AQ scores (> 31) were invited to complete the Autism Diagnostic Observation Schedule – Generic (ADOS-G). 27 The ADOS-G algorithm scores participants as below threshold, autism spectrum or autism. The ADOS-G is a structured play and conversational interview that includes a series of social presses and other opportunities to elicit symptoms of ASD. Thirty-one accompanying ratings are divided between the domains of social interaction, communication, imagination/creativity, stereotyped behaviours and restricted interests. According to the coding algorithm, scores in the domains of social interaction, communication and the sum of these two, contribute to the ADOS-G classification of ‘below threshold’ (≤ 6), ‘autism spectrum’ (≥ 7), or ‘autism’ (≥ 10).
Results and discussion
Overview of findings for ADHD
In total, we screened 1468 participants for ADHD from adult mental health and prison mental health services (Table 21). Across all the participants, we estimated an overall prevalence of ADHD of 15.1%, which is around a three- to fivefold increase compared with the population rate of 2.5–4.3%. However, there were marked differences depending on the clinical group being evaluated. The highest rate of ADHD was seen among prison inmates (20.8% for older prisoners; 16.9% for younger prisoners), followed by primary care mental health patients (11.6%), addiction patients (11.7%), forensic psychiatry patients (7.3%) and anxiety disorder patients (6.1%).
| Service | Total screened n = 1604 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary care | Anxiety disorders unit | Prison (aged 18–25 years) | Prison (aged 26–65 years) | Forensic psychiatry unit | Addiction treatment unit | |||||||
| ADHD | ASD | ADHD | ASD | ADHD | ASD | ADHD | ASD | ADHD | ASD | ADHD | ASD | |
| Screeners completed (n) | 250 | 249 | 122 | 101 | 592 | n/a | 221 | 221 | 129 | 158 | 154a | 113 |
| Positive screeners (n) | 50 | 77 | 15 | 30 | 129 | n/a | 60 | 39 | 43 | 43 | 32 | 4 |
| Assessments completed (n) | 31 | 62 | 8 | 18 | 71 | n/a | 60 | 39 | 24 | 24 | 16 | 3 |
| Met research criteria (n) | 18 | 4 | 4 | 3 | 55 | n/a | 46 | 12 | 15 | 15 | 9 | 1 |
| Estimated prevalence (%) | 11.6 | 2.0 | 6.1 | 5.0 | 16.9 | n/a | 20.8 | 5.4 | 7.3 | 17.0 | 11.7 | 1.2 |
The higher rate of ADHD in the older prisoners is not significantly greater than the rate estimated for the younger prisoners.
The rates in the forensic psychiatry unit appear to be only slightly raised above that in the general population; however, further analysis found that this depended on the primary diagnosis. No cases of ADHD were identified among 81 patients diagnosed with a severe mental illness (schizophrenia in most cases), whereas the estimate was 23.5% among the smaller group of 52 forensic patients with a primary diagnosis of severe and dangerous PD. When the criteria were broadened to include those with high levels of ADHD symptoms (four or more symptoms in either domain) persisting from childhood and still causing impairment (ADHD in partial remission), the prevalence within this subgroup was 35.3%.
The increased rate of ADHD in the primary care setting is particularly interesting, as we screened only patients who were currently being treated for common mental health conditions, such as anxiety and depression. This contrasted with the lower rate of ADHD in the specialist anxiety disorder unit, which consisted of patients with a sufficiently severe form of anxiety of OCD to require inpatient treatment.
For the addiction inpatient unit, when patients were entering detoxification programmes, we noted a significant decline in the numbers screening positive for ADHD within 1 week of their admission to the unit. Current ADHD scores dropped significantly between screening assessment session 1 (T1) and screening assessment session 2 (T2) ratings (1 week part) with a mean change of 8.6. We found that 52% of participants with T1 and T2 data had a clinically meaningful reduction of ≥ 8 points on the self-completed ADHD rating scales, which is equivalent to a one-level drop in the Clinical Global Impression (CGI) scale. 112 This finding means that care must be taken when evaluating the level of ADHD symptoms in patients actively addicted to drugs and/or alcohol.
Across the entire set of clinic samples, none of the 90 cases identified as currently meeting diagnostic criteria for ADHD was currently recognised as having ADHD or being treated for the disorder. A small number had been diagnosed during childhood, and this was particularly notable among the young prison study group, who were all aged between 18 and 25 years. None of the cases of ADHD identified in the other services had received a diagnosis during childhood.
Overall, these data find high rates of undiagnosed and untreated ADHD in adult mental health and forensic services, particular among young male prisoners (even though they are routinely screened for other mental health conditions), among the subgroup of male forensic psychiatry patients with a primary diagnosis of severe and dangerous PD, among patients with an addiction disorder and among primary care patients being treated for other common mental health conditions.
A total of 842 participants completed screening for ASD; we did not screen for ASD in young offenders as there were local concerns (expressed by the prison) about the inclusion of the necessary additional screening and diagnostic steps in this population. We were, however, able to carry complete screening in an adult category C prison (Brixton).
An overall rate of ASD was estimated to be 5.5%, which is around eightfold higher than the general population prevalence of 0.6%. However, obtaining accurate diagnoses of ASD was difficult because of the limited access to parent reports of early childhood and developmental onset and course. This was a particular concern within the forensic psychiatry group, in which there was an estimated 17% of patients with ASD according to the ADOS-G assessment, which evaluates current autistic-like behaviours. Many of these patients had a primary diagnosis of schizophrenia and it is possible that ADOS-G scores are explained by the schizophrenic disorders and not autism. Further investigation would require autism diagnostic interviews to be completed with the parents of the forensic psychiatry cases, which was not possible owing to the low rate of agreement to contact families within this patient group. Higher rates were also found in the older adult prisoners (5.4%) and the specialist anxiety unit (5%), but only a relatively small increase in rates within primary care (2.0%) and the addiction service (1.2%). The association of severe anxiety disorders with ASD is interesting because it suggests that in at least some cases ASD may be an important risk factor, leading to sustained difficulties in controlling high levels of anxiety. The rate of ASD was higher in the anxiety unit than within the primary care setting, suggesting that the link is with severe anxiety that has not responded to other forms of treatment, rather than the more common forms of anxiety seen in general practice. ASD, as well as ADHD, appears to be linked to criminal behaviour, leading to incarceration in either forensic psychiatry or prison settings.
In addition to providing an overview of the findings in the section above, we have completed further, more detailed ,analyses of the forensic psychiatry and addiction subpopulations. Here we not only focus on the prevalence of undiagnosed ADHD and ASD, but also start to map out the associated impairments. We have mainly focused on the analysis of impairment data in the ADHD data.
Overall prevalence of ADHD in the adult mental health services investigated was 13.4%, three- to fivefold higher than the population prevalence.
Overall prevalence of ASD in the adult mental health services investigated was 5.3%, approximately fivefold higher than the population prevalence.
ADHD cases are overall more impaired than non-ADHD patients in the clinical settings investigated.
None of the 113 participants with ADHD or ASD identified in this study had been recognised by the services as having ADHD or ASD and none had been referred for, or were in receipt of, appropriate treatment for these disorders.
Chapter 9 Detailed analysis of the different clinical settings: preliminary results on ADHD and ASD in primary care
The primary care study was completed during May 2013. Here we present preliminary findings from this part of the programme.
Background
The population prevalence of adult ADHD is estimated at 2.5–4.4%101,113 and of ASD at 0.6–1%. 114 We know from experience within our specialist services and the literature that both ADHD and ASD are commonly associated with additional mental health problems, including depression, anxiety and substance misuse problems. We therefore expected that many primary care service users offered mental health interventions may have undiagnosed ADHD and/or ASD. Identifying such cases would contribute to service development through the identification of comorbidities that might otherwise be missed and that may have important treatment implications. Hence, we also undertook a preliminary, qualitative, ‘scoping’ pilot study to investigate how treatment is received by affected individuals.
Methods
Recruitment of participants
General practitioner surgeries for recruitment were identified in the local area by the Primary Care Research Network (URL: www.nihr.ac.uk/research-and-impact/nihr-clinical-research-network-portfolio/), and approached by the research team to take part. In accordance with Primary Care Research Network standards, the surgeries/GPs were paid a fee for participating in the study and a ‘per-head’ cost for each participant recruited to the study. Eligible participants included primary care patients aged > 18 years receiving a mental health intervention, including prescription of selective serotonin reuptake inhibitor medication and/or referral to Improving Access to Psychological Therapies services. A list of eligible patients was generated from the surgeries’ computerised databases. These lists were checked by GPs themselves to exclude any patients inappropriate to approach. Researchers prepared invitation letters to post to potential participants. These were signed by the GP before posting on paper or electronically. The letter included information on the study and consent forms to allow participants to opt in. The consent form included permission for researchers to notify GPs of the outcome of their patients’ participation. Inclusion criteria included all patients with non-psychotic disorders attending the clinic. Exclusion criteria for most settings will include major mental disorders such as schizophrenia, bipolar I disorder and dementia. Inclusion/exclusion criteria may differ between units.
Results and discussion
Within the primary care setting, we screened 252 cases and identified 18 cases with ADHD and four cases with ASD. The estimated prevalence rates were 11.6% for ADHD and 2% for ASD, which are around three to four times and twice the population average, respectively. Within the primary care population, we compared the DIVA-positive cases (n = 15) with the ADHD screen-negative cases (n = 39). Individuals with ADHD scored higher in all seven of the impairment domains on the Weiss Functional Impairment Rating Scale (WFIRS); however, only risk and total scores showed significantly more impairment in the ADHD groups than in the ADHD screen-negative patients (Table 22).
| WFIRS item | Screener negative (n = 27–39),a mean (SD) | DIVA positive (n = 12–15),a mean (SD) | t-test |
|---|---|---|---|
| Family | 6.65 (5.43) | 7.38 (3.75) | –0.45 |
| Work | 8.30 (5.58) | 10.83 (5.27) | –1.33 |
| Life skills | 12.92 (7.99) | 17.73 (8.75) | –1.93 |
| Self-concept | 11.18 (4.19) | 12.73 (2.60) | –1.34 |
| Social | 10.49 (5.50) | 10.67 (7.45) | –1.00 |
| Risk | 4.95 (5.24) | 10.07 (6.48) | –2.90** |
| Total score | 51.64 (25.27) | 66.40 (19.52) | –2.04* |
None of the cases was currently identified as suffering from either ADHD or ASD and they were being treated for other conditions. We have yet to analyse all the comorbidity data, but in most of the cases we felt that the individuals were being given treatment for other conditions rather than targeting the NDs that underlined their condition.
ADHD treatment outcome in primary care (qualitative feasibility/pilot study)
From the primary care sample, 15 patients were started on treatment for ADHD. We took baseline measures and after 6 months reviewed their progress. We are currently completing this stage of the project; however, all patients reported improvements in symptoms of ADHD and associated impairments.
We conducted review meetings with the patients on ADHD medication. During the meetings some interesting comments were made about the effect of medication and the treatment process. Patient LL (60-year-old female) reported being able to ‘cope with things better’ and can ‘sit still and watch full programme on TV’, which she had never been able to do before starting medication. Overall, she felt her quality of life had greatly improved. Patient AW (57-year-old male) reported feeling ‘much better’ on medication as it helps get his ‘head together’ and he was getting many more things done. Patient KC (28-year-old female) reported that the medication made her feel ‘more functional’. Medication ‘helped 50% and the rest was just having the diagnosis as it did wonders for my self-esteem . . . used to be down on myself but now I know there is a reason behind how I am’. Others all reported finding the treatment and improved understanding of their condition an enormous help, with some praising the team for finding solutions to long-standing difficulties. For example, one man often had arguments with his daughter who was also diagnosed and treated for ADHD. He now found that his behaviour was much better controlled and he had time to stop and think rather than starting a shouting match with his daughter.
ASD in primary care
One of the most striking findings within the primary care sample was the high rate of false positives. A total of 62 out of 77 patients who were AQ positive had a further ASD assessment (ADOS-G). Only four cases were above threshold (6.45%) and 58 cases were below threshold (93.6%). This suggests that the AQ may not be an effective screening measure in individuals with associated mental health conditions such as generalised anxiety or depression. However, it is important to note the lack of information on developmental history within the primary care sample. The mean age of individuals who were AQ positive was 43 (range 20.6–65.1, SD 11.6) years and many individuals were unable to provide informants to complete the ADI-R assessing key behaviours during early development. Without this essential information it is hard to be say definitively whether the individuals who scored above threshold on the ADOS-G actually had ASD and/or the ADOS-G failed to identify some cases.
Analysis of impairment data revealed that patients who screened positive for ASD had significantly greater social impairments than ASD-negative individuals. There were no other significant group differences on the remaining five scales of the WFIRS (Table 23).
| WFIRS item | ASD screener negative vs. ASD screener positive | ||
|---|---|---|---|
| ASD screener negative (n = 12), mean (SD) | ASD screener positive (n = 56), mean (SD) | t-test | |
| Total | 58.33 (20.09) | 56.15 (24.36) | 0.77 |
| Family | 5.20 (2.57) | 7.31 (5.22) | –1.25 |
| Work | 8.60 (6.02) | 9.49 (6.28) | –0.40 |
| Life skills | 18.17 (6.34) | 14.70 (8.49) | 1.34 |
| Self-concept | 10.58 (4.06) | 11.60 (3.97) | –0.80 |
| Social | 7.50 (6.87) | 11.61 (5.97) | –2.10* |
| Risk | 10.42 (8.31) | 5.87 (5.34) | 2.39** |
When analysis was repeated using a continuous approach, there was a significant positive correlation between AQ total and family (r = 0.28; p = 0.03) and social (r = 0.31; p = 0.01) impairment scores on the WFIRS. The results indicate a significant association between a higher number of autistic traits and greater self-reported impairment in family and social domains (Figure 12). There were no significant differences between ASD screen-positive and ASD screen-negative individuals on the Pittsburgh Sleep Quality Index scale, measuring sleep impairments, or on the Affective Lability Scale, measuring affective liability.
FIGURE 12.
Scatterplot displaying the relationship between AQ total score and self-report family impairment on WFIRS.
It was not possible to compare those above and below the threshold for ASD due to the large difference in numbers in each group (4 vs. 172).
Analysis of impairments in populations
Further analyses on impairments have yet to be completed.
Current screening tools for ASD recommended in NICE guidelines have very high rates of false positives.
ADHD is approximately four times more common in vulnerable people within general practices than in the general population.
Chapter 10 Detailed analysis of the different clinical settings: addiction services
Background
There are relatively few accounts of an increase in drug or alcohol addiction among people with ASDs. In contrast, a bidirectional association of ADHD with addiction disorders is well recognised, with increased rates of SUD within ADHD populations and increased rates of ADHD within SUD populations. 115–117 Previous studies of ADHD in the USA and Europe estimate very high prevalence rates for lifetime SUD of up to 58%. 118 These studies found that alcohol and cannabis are the most frequently abused substances,116 followed by cocaine and amphetamines. 119
There are potentially methodological issues that may inflate prevalence rates, indicated by the wide range in reported prevalence rates for ADHD in SUD populations of around 10–55%. 120,121 Challenges to the correct identification of ADHD in SUD settings include high dropout rates between screening and assessment stages and the potential overlap of symptoms between the two disorders,122 including intoxication and withdrawal states119 and the long-term effects of chronic drug use on brain function123–125 that could mimic ADHD. Retrospective recall of childhood ADHD symptoms is particularly challenging in SUD populations due to impaired memory and the often complex histories with adverse psychosocial risk factors for the development of behavioural problems. Informant reports are often not available in SUD populations because relationships with informants are frequently strained,119 and for this reason most studies depend on self-report. When evaluating ADHD, few papers report the time since last substance use, although delayed screening until 3 weeks of abstinence has been reported. 126
Our study addresses some of these concerns by implementing a systematic screening protocol for ADHD in London SUD clinics, with the aim of estimating the rate of undiagnosed and untreated ADHD within SUD patients; and evaluating whether or not those with comorbid ADHD and SUD experience greater impairment, in terms of comorbid diagnoses, previous convictions or suicide attempts, than those with SUD alone. We also screened for ASD but found low levels in this population (see Results).
Methods
Participants
Sample characteristics are listed in Table 24. Participants (n = 226) were recruited from two inpatient alcohol and drug detoxification and stabilisation units in south-east London. Consecutive admissions to the inpatient units were invited to take part in the study over an 18- (clinic 1) or 11-month (clinic 2) period. Of these, 74% had previously attended a detoxification treatment programme. One participant already had a current adult diagnosis of ADHD and two had a recorded childhood diagnosis of ADHD. We excluded individuals with a history of current or recent psychosis (n = 4), current serious physical illness (n = 3), insufficient understanding of English to give informed consent (n = 6) and lack of capacity to consent (n = 2). Overall, 51% of patients approached agreed to participate. Study participants received no payment for participation.
| Total sample size, n | 226 |
| Male sex, n (%) | 173 (76.5) |
| Mean agea in years (SD) | 39.0 (10.3) |
| Ethnicity,a n (%) | 183 (81.0) white European |
| Alcohol dependence,a n (%) | 112 (49.6) |
| Drug dependence,a n (%) | 29 (12.8) |
| Alcohol and drug dependence,a n (%) | 85 (37.6) |
Study procedures
The study of people undergoing inpatient detoxification included two screening stages using ADHD rating scale data, followed by formal evaluation of the diagnosis of ADHD using a semistructured interview for DSM-IV ADHD. By including two screening steps (T1 and T2) we were able to evaluate the difference between the level of ADHD symptoms on admission (T1) and 1 week later (T2), when they had been detoxified or stabilised on treatments such as methadone.
Screening assessment session 1 (T1) took place soon after admission (mean 5 days, SD 4 days). The second screening assessment session (T2) was completed as close as possible to 7 days after the T1 assessment (mean 8 days, SD 6 days). During the T1 session, patients completed current and childhood symptom checklists for DSM-IV ADHD; however, during the T2 session patients completed current symptoms only, as it was assumed that only the report of current levels of symptoms would be affected by state of detoxification and current drug use and needed to be measured at both time points. In addition, whenever possible, we obtained contact details from informants for childhood and current ADHD symptoms who were asked to complete an informant version of the screening questionnaire. Current informant ratings were obtained for 72 participants and childhood ratings for 48 participants. The self-rated screening data were used to allocate participants into a screen-positive group and a screen-negative group for ADHD.
Participants screening positive for ADHD were invited to complete a research diagnostic interview assessment. Participants who failed to attend the diagnostic interview were offered up to three further appointments. Following three missed appointments, a final letter invited participants to contact the research team to reschedule an appointment before the assessment was recorded as ‘missing’.
Results
All 226 patients completed T1 screening questionnaires, of whom 69% completed T2 ratings. Reasons for missing T2 screeners included early self-discharge or disciplinary discharge (n = 23), refusal to take further part in the study (n = 15) and repeatedly missing T2 appointments (n = 32).
Childhood ADHD screeners
Mean total childhood ADHD symptom scores were highest in the group treated for drug dependency (mean 29, SD 14), followed by the combined drug and alcohol group (mean 24, SD 15) and the alcohol dependency group (mean 17, SD 14). A one-way analysis of variance (ANOVA) showed significant effects of primary diagnosis (drug vs. alcohol vs. combined) on childhood screener total score (p < 0.001). Planned contrasts showed significantly higher ratings of ADHD symptoms for drug than for alcohol participants (p < 0.001), but not between drug and combined drug and alcohol groups (p = 0.12).
T1 compared with T2 ratings
These data are summarised in Table 25. Current ADHD scores dropped significantly between T1 and T2 ratings, with a mean change of 8.6. We found that 52% of participants with T1 and T2 data had a clinically meaningful reduction of ≥ 8 points on the self-completed ADHD rating scales, which is equivalent to a one-level drop in the CGI scale. 112
| ADHD screening score | Childhood | T1 | T2 | Mean change T1 vs. T2 | Paired t-value (T1 vs. T2 change) |
|---|---|---|---|---|---|
| Total score/54 | 21.09 (SD 15.0) | 23.03 (SD 12.5) | 14.65 (SD 12.1) | –8.6 (SD 9.1) | 11.92** |
| Inattentive symptoms/9 | 3.51 (SD 3.2) | 3.59 (SD 2.9) | 1.83 (SD 2.5) | –1.80 (SD 2.2) | 10.21** |
| Hyperactive/impulsive symptoms/9 | 3.29 (SD 3.0) | 3.46 (SD 2.6) | 2.30 (SD 2.6) | –1.23 (SD 2.1) | 7.40** |
There is a significant reduction in ADHD symptoms following 1 week or more of treatment in the addiction units. A 2 × 3 mixed model factorial ANOVA on the time (T1 vs. T2) and primary diagnosis (drug vs. alcohol vs. combined drug and alcohol) found a significant main effect of time (p < 0.001), indicating that self-rated ADHD symptom scores are influenced by the detoxification treatment process and/or withdrawal states following admission. A significant effect of primary diagnosis was also found (p < 0.001), but no significant interaction between time and primary diagnosis (p = 0.34). Between-subjects contrasts showed significant differences for ADHD ratings between the alcohol and combined drug and alcohol groups (p < 0.001), but not between drug and combined (p = 0.28) or alcohol and drug (p = 0.11), indicating higher levels of ADHD symptoms at T1 and T2 among those with drug abuse compared with those with alcohol abuse alone.
Estimated prevalence of ADHD
There was an estimated prevalence rate for ADHD of 12.2%. Of these, 73% met criteria for the combined subtype, 18% for the hyperactive–impulsive subtype and 9% for the inattentive subtype. The prevalence was calculated using the proportion of participants completing each step that did and did not fulfil ADHD screening and assessment criteria. This in turn allowed us to calculate an estimated prevalence rate of positive screens at each stage, which is not affected by attrition rates between screening and assessment stages.
To evaluate whether or not rates of ADHD might differ in the groups that screened positive for ADHD at T1 and T2, but did (or did not) attend for diagnostic interviews, we compared the attendees to the non-attendees. The participants who attended the diagnostic assessments showed no significant differences from those that did not at T1. However, they had significantly higher score for inattentive (but not hyperactive–impulsive) symptoms at T2 (6.1 vs. 4.3; p = 0.025), suggesting that the prevalence estimate based on available data might be slightly inflated.
Broader definitions of ADHD
Broadening the diagnostic threshold, based on the DIVA (diagnostic interview), had only a small impact on the prevalence estimate. For example, reducing the threshold for diagnosing ADHD from six or more symptoms in childhood to four or more increased the overall prevalence estimate only to 14.4%. In addition, no additional cases were identified by decreasing the threshold for current symptoms to four or more.
Informant rating scales
Sixty-four per cent of participants provided contact details for informants of their current behaviour and 43% for childhood behaviour. In addition, 50% of both sets of contacts returned screening questionnaires. Informant ratings of current ADHD symptoms correlated moderately with self-reported symptoms and were similar to the equivalent correlations in the control sample (r = 0.47; p < 0.001). However, the participants with positive screens for ADHD agreed far more strongly with their informants than those who did not screen positive for ADHD (correlations 0.62 to 0.66). Correlations between childhood self and informant ratings were similar to those for current behaviour, but were non-significant. Overall, participants and informants were concordant for the 4+ screening criteria in 64% of cases at T1 and in 50% of cases at T2 and 81% were concordant for the childhood 6+ criteria (Table 26).
| T1 | T2 | |
|---|---|---|
| Self-report: current behaviour informant report | ||
| All participants, n = 72 | 0.372** | 0.375* |
| Positive ADHD screeners [childhood 6+ and T1 and T2 (if applicable) 4+], n = 16 | 0.617* | 0.654* |
| Negative ADHD screeners, n = 56 | 0.258 | 0.169 |
| Childhood screener: childhood behaviour informant report | ||
| All participants, n = 47 | 0.391** | |
| Positive ADHD screeners (childhood 6+), n = 20 | 0.231 | |
| Negative ADHD screeners, n = 27 | 0.293 | |
Impairment and substance use
As expected, the SUD sample rated themselves as significantly more impaired than controls (p < 0.05). Among the individuals with SUD there was a significant positive relationship between self-rated impairment and the severity of ADHD symptoms in childhood and at both T1 and T2 (respectively, r = 0.52, p < 0.001; r = 0.73, p < 0.001; r = 0.51, p < 0.001). Furthermore, impairment was significantly negatively correlated with change scores between T1 and T2 (r = –0.23; p < 0.01), indicating that participants with greater impairment at T1 showed a smaller drop in ADHD symptoms between T1 and T2.
Finally, within the SUD population we compared the diagnosed ADHD cases with the non-ADHD cases. Those with ADHD had significantly higher rates of self-reported impairments and were significantly more likely to have reported using cocaine or amphetamines (Table 27), consumed more units of alcohol per day and had more prior suicide attempts. In addition, they had a trend towards higher rates of depression, previous convictions and previous drug or alcohol dependency treatment (see Table 27).
| Heroin | Amphetamine | Crack cocaine | Cocaine | Marijuana | |
|---|---|---|---|---|---|
| ADHD (n = 11), % | 45.5 | 63.7 | 18.2 | 54.6 | 36.4 |
| Non-ADHD (n = 182), % | 29.7 | 6.6 | 29.1 | 11.0 | 15.4 |
| Likelihood ratio | 1.141 | 21.289 | 0.663 | 11.361 | 2.685 |
| p-value | 0.285 | < 0.001** | 0.416 | 0.001** | 0.101 |
ASD
Only four participants out of 113 who completed the AQ were above the threshold and, of these, only one met criteria for possible ASD. As the rates of ASD in this population, based initially on self-report data, appear to be very low, we did not pursue this line of investigation further. Future studies should consider informant reports of autistic behaviours at the screening stage, to exclude the possibility of low reporting of ASD behaviours using self-report questionnaires.
Discussion
Our main finding was that the estimated prevalence of undiagnosed ADHD within SUD clinic populations’ in south London is around 12%. This is of importance as we also found evidence that those individuals with both SUD and ADHD had significantly higher self-rated impairments across several domains of daily life; and higher rates of substance abuse and alcohol consumption, suicide attempts and depression, recorded in their case notes. Taken together, these two findings highlight the negative impact of ADHD on the individual and the increased burden they most probably place on services. When considering the generalisability of these findings, a limitation of this study is the focus on inpatient detoxification units in London. Further work is therefore required to evaluate rates and impact of ADHD among outpatient SUD populations and other national and international regions.
Impairments associated with comorbid ADHD and substance use disorder
The finding of higher levels of impairment among ADHD cases within the SUD sample appears to be robust, as we found indicators of impairment from both self-report and more objective (case note) measures. Appropriate assessment and management of ADHD in SUD patients would therefore seem to be potentially important to improve the general level of functional impairments, particularly given our finding that ADHD is associated with increased frequency of suicide attempts and depression. Whether or not treating ADHD in the context of SUD improves depression has yet to be adequately studied; however, we know that in people with ADHD symptoms such as mood instability and low self-esteem respond well to treatments for ADHD. 127–130
Another finding was that the ADHD group had significantly higher use of stimulants, such as cocaine and amphetamine (although not crack cocaine). One possible (but untested) explanation for this, given that stimulants are routinely used to treat ADHD in the general population, is that people with ADHD are using stimulants as a form of self-treatment. However, an additional mechanism could be that people with ADHD have a preference for drugs that are more ‘stimulating’, especially when injected or taken at high dose. Further work is needed to address this issue.
Definition of ADHD and methodological issues
Our work attempted to address, as best we could, the inevitable methodological difficulties that might impact on accurate estimation of prevalence rates for ADHD within people with SUD. These include the potential for unreliable information from self-report questionnaires, difficulties with retrospective recall of childhood symptoms and the direct impact of drug and alcohol intoxication and withdrawal states on ADHD-like symptoms. We therefore attempted to measure ADHD symptoms before and after detoxification, included independent informant ratings whenever possible, compared these with population control data and completed research diagnostic interviews. One additional confounder might arise if the study participants considered a positive diagnosis of ADHD as a way to obtain stimulant medication. However, the study was not linked directly to the treatment of ADHD, and expectations for treatment with stimulants are currently low in the UK, because in most cases ADHD in adults goes unrecognised and untreated. 102
Based on the percentage of patients who screened positive for ADHD in childhood and adulthood and the proportion of those interviewed, we estimated an overall rate of ADHD of around 12% in our sample, much higher than the equivalent estimated worldwide prevalence of around 2.5–3.4% in non-SUD populations. 97,101,113 Using slightly broader criteria for ADHD to reflect different approaches to the diagnosis of adult ADHD being taken by different investigators and clinicians, we found the estimated prevalence in our sample increased to a maximum of 15%. Overall, the estimated prevalence of ADHD in our sample is far higher than population rates, yet lower than those cited in some previous studies of ADHD in SUD populations. There are several potential reasons for these differences in the findings from this and previous studies.
One potential reason was our stringent application of the DSM-IV criteria, which might have led to an underestimate of the true rate of ADHD in the SUD population for several reasons. First, the inherent problem in collecting childhood data retrospectively might mean that some participants who met current criteria for ADHD may have been unable to recall sufficient examples of childhood symptoms. We recorded ‘unknown’ ratings in the diagnostic interview assessments if participants could not provide sufficient information to conclude that a symptom was present or absent and found that on average 15% of childhood symptoms could not be scored. Second, DSM-IV criteria only require that ‘some’ symptoms and impairments were present during early childhood and do not specifically require six or more symptoms from childhood so long as the symptom count criteria are currently met as an adult. Nevertheless, we decided to take the more stringent approach because of the lack of prospective data from childhood and to guard against inclusion of ADHD-like syndromes that might arise as a result of chronic drug abuse in the absence of an underlying ADHD diagnosis. Finally, we know that the current DSM-IV criteria are not adjusted to take into account age-related changes in the development of ADHD, and there is evidence that four or more symptoms in adults, rather than the current six or more symptoms, is sufficient. Indeed, this change is being considered for the fifth revision of the DSM that is currently in preparation. 102,131,132 However, taking all of these alternative thresholds into account had only a minor impact on our estimate of the prevalence of ADHD in the SUD population.
The impact of drug detoxification on ADHD symptoms
We investigated the impact of drug intoxication and/or the detoxification process by evaluating self-rated ADHD symptom scores a few days after admission to the detoxification unit and 1 week later, when the participants were detoxified or stabilised on long-term medication and no longer in a withdrawal state. We found significant decreases in ADHD symptoms of around 8 points (15% of the total score), which is a clinically significant reduction in ADHD symptoms and comparable to a one-level drop of the CGI scale. 112 In terms of our screening criteria, this led to 40% of patients no longer meeting screening criteria for ADHD at T2 compared with T1. Hence, prior studies may have reported a higher prevalence than we found due to the confounding effect of drug use and/or withdrawal symptoms. Other researchers have noted mood disturbances during alcohol detoxification133 and it is therefore possible that ADHD-like symptoms are also part of the withdrawal syndrome. Despite this, we found that in 60% of cases self-rated ADHD symptoms remained clinically significant following completion of the detoxification process. One implication is that, although the withdrawal process may impact on the level of ADHD symptoms (and this should be taken into account when evaluating ADHD in SUD patients), there remain a significant number of individuals with clinically significant symptoms of ADHD – and these require treatment.
Clinical implications
Our findings suggest that clinical evaluations for ADHD are probably best completed once detoxification or stabilisation for drug or alcohol dependency has been completed. However, this suggestion may be difficult to implement in community patients. Furthermore, once diagnosis of ADHD has been established it will be important to offer treatment. However, use of some pharmacological treatments (such as stimulants) is complex in those with a current SUD. Nevertheless, therapeutic nihilism is not an option, as treatment of underlying ADHD may be important to the success of drug treatment programmes for some individuals. It might be advisable to use the more stringent screening criteria of six or more symptoms in either domain to identify those that need full clinical evaluation for ADHD, or to recognise the need for detailed ADHD assessment in a higher proportion of cases. Moreover, it may be more appropriate to use non-stimulant medications, such as atomoxetine, as a first-line treatment. Further work is required to address this issue and the potential for using CBT-based therapies for ADHD in this complex population.
Obtaining informant data on ADHD symptoms for childhood and for current symptoms proved difficult in this population. The reasons for this were not investigated here, but probably reflect the often poor relationships that many SUD patients have with their family and friends. Therefore, in clinical practice it will also often be the case that the diagnostic assessment of ADHD will depend on self-report alone. We were able to investigate the validity of these self-reported data by comparison of self-report with informant-reported data in a subset of our sample and a comparison control sample. This investigation revealed moderate correlations between raters which were similar to that seen in non-SUD control populations. For the most impaired subgroup, however, who were screening positive for ADHD based on their self-report, there was a far higher correlation with informant report for current ADHD ratings of around 0.62–0.65. We therefore suggest that, although discrepancies between raters exist for ADHD rating scales, this does not appear to be different for SUD compared with control populations. Furthermore, ratings showed moderately high levels of agreement for the group of patients with ADHD.
Previous research has shown that in general people with ADHD tend to rate their symptoms lower than informants,134 perhaps reflecting difficulties in self-evaluation of ADHD symptoms. Our research does not support this finding within the SUD population investigated here. When we completed diagnostic interviews with 26 people who screened positive for ADHD on the basis of their self-rated symptoms, we found that only 61% met full criteria for ADHD. The clinical implication of this finding is that the diagnosis of ADHD in patients with SUD should depend not solely on rating scale data, but rather on more objective examples of symptoms characteristic of ADHD, as applied here using the DIVA interview. Rating scales are a valuable tool for screening for ADHD, but should not be used as a replacement for a full diagnostic assessment by clinical interview. Furthermore, although informant reports are helpful in supporting the diagnosis, the moderately high correlations with self-report suggest that in most cases self-report alone should be sufficient. For inpatient SUD units it should also be feasible to observe patients for level of restlessness, problems with self-organisation, inattentiveness, impulsive responses and poor emotional regulation, which are characteristic of ADHD in adults.
Conclusions
This study applied stringent ADHD diagnostic criteria, necessary to avoid mistaking withdrawal states and other mental health problems for ADHD. This resulted in identification of a relatively small ADHD group compared with other studies relying only on self-report screening questionnaires. Despite this, our findings confirm high rates of ADHD within SUD populations that are approximately fivefold higher than general population rates. Furthermore, SUD patients with high levels of ADHD were functionally more impaired (including a higher rate of suicide attempts). This study highlights the importance of identifying the subgroup of people with both SUD and ADHD. Further studies are required to evaluate the effectiveness of targeted treatments for ADHD within SUD patient populations.
ADHD in people with SUDs is approximately fivefold more common than in the general population.
People with ADHD and SUDs are more functionally impaired and have a higher rate of suicide attempts.
Screening for ADHD may be better undertaken after, and not before, detoxification.
Chapter 11 Detailed analysis of the different clinical settings: forensic psychiatry – rates of ADHD in NHS medium-secure forensic units
Methods
Participants
Adult patients participated in the study from both high- and medium-security establishments in the Greater London area. Three hundred and forty-one mentally disordered offenders, all of whom were detained under the UK Mental Health Act 2007,135 were resident at these two secure services [241 (71%) at the high-secure service and 100 (29%) at the medium-secure service]. The majority of patients in these settings have a primary diagnosis of either serious mental illness (SMI; e.g. schizophrenia, schizoaffective disorder, bipolar disorder) or PD. Exclusion criteria included age > 65 years and those patients who were too mentally unstable to participate, had severe cognitive deficits due to neurological illness or head injury, posed a risk of violence to the researcher and/or who lacked capacity to consent to participate in the study. Of the 341 patients, 93 (27.3%) were identified as ineligible to participate; 68 (20%) did not meet study criteria and a further 25 (7%) were on trial leave or discharged prior to the assessment. Of the 68 patients who did not meet exclusion criteria, 28 lacked capacity to consent and 40 were mentally unstable. Thus, a total of 248 patients were eligible to take part in the study. From this sample of 248 patients, a further 115 (46%) patients refused to participate in the research. Hence, 133 participated in the study [92 (69.2%) from the high-secure psychiatric service and 41 (30.8%) from the regional medium-secure service].
Assessment procedure
The study took place over a 16-month period. An information sheet describing the study and specifying inclusion and exclusion criteria was sent to clinical teams. Patients meeting these criteria were referred to the researchers, who subsequently approached the patients to explain the study and obtain informed consent. Once consent had been given, patients met with a RA to complete screening measures for ADHD and data were extracted from the clinical records. Four patients in the SMI category consented to participate in the study and met with researchers, but did not consent for their records to be accessed. Hence, there were missing data for these participants.
If a patient screened positive on the BAARS-IV scales (self-rated scales for current and retrospective ADHD symptoms), they were invited to participate in a diagnostic interview. These interviews were administered by a RA who had received training, including observation of these assessments by qualified staff in a clinical setting; specific training by qualified and experienced clinicians who routinely administer the interviews; and practice ratings of recorded clinical interviews to a point of reliability and convergence of ratings. For patients who were positive on the clinical diagnostic interview for ADHD, an informant interview was conducted with the patient’s primary nurse to supplement information from the self-report interview. Once completed, materials for each case were reviewed by a consultant psychiatrist (PA) from the Maudsley Hospital Adult ADHD service.
Behavioural function
Critical incidents were obtained from clinical records recorded by staff over the previous 12-month period. The data were grouped based on harmful impact on others: (1) ‘no harm incidents’ including security issues, verbal aggression and physical injury not requiring treatment or (2) ‘harmful incidents’, classified as any physical injury requiring further assessment or treatment. There were no critical incidents involving grievous bodily harm or homicide during the 12-month period. Seclusion data were obtained from clinical records recorded by staff over the previous 12-month period and categorised by number of episodes and duration of time in seclusion.
Results
Comorbid diagnoses
All participants had a DSM-IV primary diagnosis of SMI (n = 81, 60.90%) or PD (n = 52, 39.10%). Four patients in the SMI category denied us access to records so we were unable to specify their primary diagnosis, offence history, ethnicity, age, critical incidents, episodes and duration of seclusion. The SMI category consisted of patients with a primary diagnosis of psychotic (n = 71, 92.21%) or bipolar disorders (n = 6, 7.79%). The PD category consisted of patients with a primary diagnosis of borderline (n = 24, 46.15%), antisocial (n = 23, 44.23%), schizoid (n = 3, 5.77%), narcissistic (n = 1, 1.92%) or histrionic (n = 1, 1.92%) PD. In common with all forensic mental health settings, there were high rates of comorbidity within the sample; however, only six patients had a history of ADHD recorded in their records (three in the SMI category and three in the PD group), and none had a current diagnosis or was receiving medication for ADHD at the time.
Age, ethnicity and history of offences
Most SMI patients were male (n = 79, 97.53%), with ethnicity classified as white (n = 39, 50.65%), black (n = 32, 41.56%), Asian (n = 2, 2.60%) or mixed race (n = 4; 5.19%). In the PD category, all patients were male (n = 52), with 47 (90.38%) classified as white, two (3.85%) as black, one (1.92%) as Asian and two (3.85%) as mixed race. Participants were aged between 19.3 and 64.3 years with no significant difference in age between the SMI (mean 37.10 years, SD 10.69 years) and PD categories (mean 40.26 years, SD 11.19 years) [t(129) = –1.62; p = 0.11]. Nearly all the participants had a history of violence (n = 96, 74.42%) and/or sexual violence (n = 26, 20.16%). Other offences (n = 7, 5.43%) included arson, burglary and driving offences.
Prevalence estimates
One hundred and thirty-three patients completed the ADHD child and current screeners (SMI, n = 81; PD, n = 52). Compared with patients in the SMI group, in the PD group patients had significantly higher BAARS-IV current total scores [SMI: mean 12.00, SD 9.93; PD: mean 15.92, SD 15.92, t(133) = –2.11; p = 0.04] and childhood scores [SMI: mean 16.59, SD 13.89; PD: mean 27.41, SD 15.07, t(133) = –2.11; p = 0.00].
Twenty-six patients (19.55%) screened positive for ADHD on the BAARS-IV scales and were offered a clinical diagnostic interview. Seven (8.64%) patients had a primary diagnosis of SMI and 19 (36.53%) had a primary diagnosis of PD. Three patients refused further assessment with a clinical diagnostic interview; thus, 23 of the screen-positive participants completed a DIVA (SMI, n = 6; PD, n = 17). Following the DIVA assessment, four participants (23.52%) in the PD category showed persistent ‘syndromatic’ ADHD (one with hyperactive–impulsive type and three with combined type). When applying the more relaxed ‘symptomatic’ criteria, six participants (35.29%) in the PD group had persisting symptoms (three hyperactive–impulsive type and three combined type). Of the six participants in the SMI category who screened positive, none met criteria for either ‘syndromatic’ or ‘symptomatic’ ADHD. Hence, subsequent analysis was conducted on only the PD group.
Self-rated impairments
The forensic patients who met clinical and symptomatic criteria for ADHD (n = 6) and those who screened positive for ADHD (n = 19) rated themselves as significantly more impaired than forensic psychiatry patients who screened negative for ADHD, with a large effect size for all items except for money management (Table 28). Significant positive correlations were also seen between ADHD symptom scores (in adulthood and childhood) and all domains of self-reported impairment (Table 29).
| Item | ADHD-negative screener vs. ADHD-positive screener | ADHD-negative screener vs. DIVA-positive screener | |||
|---|---|---|---|---|---|
| ADHD-negative screener (n = 25–30), mean (SD) | ADHD-positive screener (n = 11–19), mean (SD) | Mann–Whitney U-test, z-value (Cohen’s d) | DIVA-positive screener (n = 6), mean (SD) | Mann–Whitney U-test, z-value (Cohen’s d) | |
| BAARS-IV impairment scores | |||||
| Total impairment score | 7.17 (6.07) | 17.67 (7.30) | –3.62*** (1.6) | 20.33 (6.86) | –3.16*** (2.0) |
| Home life | 0.36 (0.68) | 1.47 (1.23) | 3.17*** (1.1) | 1.67 (1.37) | –2.51* (1.2) |
| Work | 0.60 (0.81) | 1.38 (0.81) | –3.15*** (1.0) | 1.83 (0.41) | –3.29*** (1.9) |
| Social interactions | 0.90 (0.66) | 1.95 (1.03) | –3.55*** (1.2) | 2.67 (0.52) | –3.86*** (2.9) |
| Community activities | 0.89 (0.92) | 2.06 (0.77) | –3.68*** (1.4) | 2.33 (0.82) | –2.91*** (1.7) |
| Education | 1.04 (1.06) | 2.11 (1.10) | –2.97*** (1.0) | 2.50 (0.84) | –2.70*** (1.5) |
| Relationships | 0.73 (1.04) | 1.75 (1.39) | –2.32* (0.8) | 2.40 (1.34) | –2.37* (1.4) |
| Money management | 0.55 (0.87) | 1.26 (1.10) | –2.51*** (0.7) | 1.33 (1.37) | –1.59 (0.7) |
| Driving | 0.24 (0.44) | 1.45 (1.29) | –2.91*** (1.3) | 2.00 (1.23) | –3.14*** (1.9) |
| Leisure activities | 0.53 (0.63) | 1.74 (1.15) | –3.62*** (1.3) | 2.33 (0.82) | –3.65*** (2.5) |
| Daily responsibilities | 0.53 (0.68) | 1.68 (0.89) | –4.08*** (1.5) | 2.00 (0.63) | –3.48*** (2.2) |
| Critical incidents and seclusions | |||||
| Total critical incidents | 2.36 (3.60) | 5.50 (6.56) | –1.46 (0.6) | 4.83 (5.71) | –1.15 (0.5) |
| ‘No harm’ incidents | 2.24 (3.55) | 5.11 (6.08) | –1.55 (0.6) | 4.50 (5.61) | –1.16 (0.5) |
| ‘Harm’ incidents | 0.12 (0.42) | 0.39 (0.85) | –1.34 (0.4) | 0.33 (0.82) | –0.63 (0.7) |
| Episodes of seclusion | 0.09 (0.29) | 0.72 (1.71) | –1.87 (0.5) | 1.50 (2.81) | –1.81 (0.7) |
| Hours in seclusion | 1.0 (5.22) | 106 (348.39) | –1.90* (0.4) | 305.83 (583.50) | –1.81 (0.7) |
| BAARS-IV symptoms scales scores | ||
|---|---|---|
| Child (n = 35–48) | Adult (n = 36–49) | |
| Total impairment score | 0.56*** | 0.66*** |
| Home life | 0.63*** | 0.53*** |
| Work | 0.47*** | 0.52*** |
| Social interactions | 0.55*** | 0.57*** |
| Community activities | 0.56*** | 0.58*** |
| Education | 0.50*** | 0.45*** |
| Relationships | 0.38* | 0.61*** |
| Money management | 0.47*** | 0.33* |
| Driving | 0.65*** | 0.47*** |
| Leisure activities | 0.47*** | 0.62*** |
| Daily responsibilities | 0.63*** | 0.64*** |
| Critical incidents and seclusions | ||
| n = 51 | n = 51 | |
| Total critical incidents | 0.23 | 0.20 |
| ‘No harm’ incidents | 0.25 | 0.19 |
| ‘Harm’ incidents | 0.26 | 0.27 |
| Episodes of seclusion | 0.14 | 0.14 |
| Hours in seclusion | 0.15 | 0.15 |
Critical incidents and seclusion
Critical incident and seclusion data taken from prison records did not show a significant difference (albeit we found trend to higher rates in ADHD cases; p = 0.07). This perhaps reflects the serious nature of the comorbid disorders and behavioural problems, other than those related to ADHD, in this high-risk population and the small sample of identified ADHD cases. Using the broader category of screen-positive cases, there was a significant increase in hours spent in seclusion compared with the screen-negative group (see Table 28).
Discussion
We set out to investigate rates of ADHD in mentally disordered offenders and quantify functional outcomes for these patients in institutional settings. In the SMI group, seven patients scored above threshold on the ADHD screeners, but none fulfilled criteria for ADHD on further assessment. In the PD group, 19 patients screened positive for ADHD. Out of 17 screen-positive PD patients interviewed with the DIVA, six were categorised as having ADHD. This gives an overall estimated prevalence of 8.6% for syndromatic ADHD and 12.9% for symptomatic ADHD, which is two to four times higher than the DSM-IV rate of ADHD (estimated from previous studies to lie in the region of 2.5–4.3% in the adult population97,101,113). Despite the high level of ADHD in the PD group, none of the patients were recognised as having ADHD at the time of the research assessments.
The absence of ADHD within the SMI group suggests that there is no particular association among offenders between ADHD and psychotic disorders, mainly schizophrenia in this sample. This finding suggests that a detailed diagnostic assessment of ADHD should be able to distinguish between ADHD and schizophrenia in most cases and that schizophrenia does not usually generate an ADHD-like syndrome. If this finding generalises to non-forensic psychiatric populations, it increases the likelihood that when ADHD symptoms and impairments are seen in someone with a history of psychosis, they probably reflect two independent co-occurring conditions. Further research is needed to clarify this question.
The screening rate of 36.5% for ADHD in the PD group is consistent with previous reports in a PD population. 107 The screening approach applied in this study had moderate sensitivity within the PD group, whereas it seemed to be less helpful within the SMI population, among which none of the screen-positive participants met clinical criteria for ADHD.
A second aim of the study was to determine the functional impairment of patients with comorbid ADHD compared with their non-ADHD-detained peers in a forensic setting. Overall, significantly greater impairment was reported across all personal, social and occupational domains among the PD and ADHD group compared with those without ADHD, mostly with large effect sizes. ADHD symptoms, both childhood and current, were positively correlated with self-rated impairment.
More specifically, significant differences between groups were found for self-rated impairment across all domains with the exception of money management, which most likely reflects the fact that this environment provides little opportunity for self-management of financial affairs. For the same reasons, patients omitted to complete items on the BAARS-IV impairment scales (e.g. many patients did not attend educational and/or occupational activities). There was wide variation in frequency of incidents, episodes and hours of seclusion. Nevertheless, there was a trend among the comorbid ADHD and PD patients to have a higher number of seclusion interventions and to spend more time in seclusion than their peers. These findings suggest that the staff were more likely to use seclusion to manage PD and ADHD patients than their non-ADHD peers and that the former require more hours in seclusion once this intervention has been applied. This may indicate that, once aroused, individuals with ADHD look agitated and take longer to calm down. This is supported by the finding that level of observed agitation and younger age have been found to be significant predictors of the use of seclusion on psychiatric wards. 136 Further studies are required to establish whether or not treatment of ADHD in these patients will lead to a change in behaviour reflected in a reduction of hours in seclusion.
There was no significant difference between groups in critical incident data, nor was there a significant correlation between ADHD symptoms, incidents and seclusion data. This finding is inconsistent with that reported previously for offenders detained in mental health and prison settings. 20,107,137 The present study included a high proportion of patients detained in high security (69%) where, due to stringent security procedures and sanctions, a relatively low base rate of acting out behaviours are found. Furthermore, it might be the case that for the individuals held within the high-security hospital, other mental health disorders create a comparable degree of behavioural problems regardless of the underlying diagnosis.
Overall, the current study strengthens our understanding of the comorbidity and behavioural consequence of ADHD in adult forensic mental health settings. An advantage of the current research is that it was conducted in a naturalistic setting with patients who have complex presentations and high comorbidity. Furthermore, objective clinical records were used to obtain functional outcomes and diagnostic status was established by comprehensive clinical interviews rather than screening measures.
Strengths and weaknesses
The study is limited by its small sample size, which is partly due to the high rate of refusal (46%), something that is difficult to avoid in the settings investigated. Hence, the sample may be biased as some patients may have refused to participate in a lengthy clinical assessment (including the completion of screening questionnaires) because of problems with attention span and other symptoms associated with ADHD, such as irritability. 94 Alternatively, those with ADHD symptoms might have been more interested in taking part in the study. These considerations might have resulted in a non-representative sample, which was underpowered to detect some of the impairments, such as critical incidents, in within-group analysis. A further limitation is that we did not interview those individuals who screened negative for ADHD, so the specificity of the screening tool in this population remains unknown. Despite these limitations, the use of the DIVA diagnostic interview gives confidence in the finding of high rates of ADHD in the PD group. DIVA is designed to establish the presence or absence of each of the 18 DSM-IV symptoms for ADHD during both childhood and adulthood, in addition to the essential age at onset, situational pervasiveness, symptom chronicity and impairment criteria. As such, it is expected to give lower prevalence rates than the use of symptom rating scales alone. A potential limitation is the ability to accurately identify ADHD symptoms retrospectively when relying on self-report data alone, although in the context of this study this would lead to more conservative estimates of prevalence. Future studies would benefit from diagnostic interviews for ADHD in a wider range of patients and further work to improve on effective screening tools in forensic mental health populations.
Conclusions
Findings from the current study indicate a high prevalence of undetected and untreated ADHD in offenders with a primary diagnosis of PD, but not in patients with a diagnosis of SMI. Patients with both PD and ADHD reported significantly greater functional impairments and spent longer periods in seclusion than their non-ADHD peers. These individuals have complex needs with high rates of comorbid psychiatric conditions such as substance misuse and entrenched antisocial attitudes and thinking styles, and specific interventions are required for their rehabilitation. 20,138–142
There is a high prevalence of undetected and untreated ADHD in offenders in NHS forensic units with a primary diagnosis of PD.
Offenders in NHS forensic units have significantly greater functional impairments and have spent longer periods in seclusion than their non-ADHD peers.
Chapter 12 Detailed analysis of the different clinical setting: forensic psychiatry – ASD in prisoners
Background
The prevalence of ASD among prisoners is unknown although a recent study of screening tools reported ASD to be uncommon in prisons. 143 Hence, as so little is known about ASD in prisoners, we first carried out a search of electronic databases and then carried out a pilot study on rates of ASD and ADHD in ‘vulnerable prisoners’ (VPs) in a local prison (Brixton).
Methods
Step 1: electronic database search
A search of electronic databases (including the Research Autism website, MEDLINE, PsycINFO, EMBASE and PubMed) identified just four relevant articles that focused specifically on ASD in prisons. One was a study in Scotland on the evaluation of a screening tool for ASD in prisons;143 another was on knowledge of ASD among prison staff144 and one reported two case studies of prisoners with Asperger syndrome. 145 These papers are included in the review below. The final article was a Swedish study that could not be obtained in full text. 146
Step 2: rates of ASD and ADHD
Having completed the search of available published data (see Step 1: electronic database search), our next aim was to examine how many adults in a local prison have NDs such as ASD and/or ADHD and to examine rates of associated ID and mental health problems in these individuals in a local prison (HMP Brixton, a category C resettlement prison with capacity for up to 798 category C and D prisoners).
The following screening tools were used: ID, Learning Disability Screening Questionnaire; ASD, Autism Spectrum Quotient; and ADHD, ARSR. Those screening positive were assessed further using the Quick Test (ID) and the so-called ‘gold standard’ research diagnostic tools for ASD and ADHD [Autism Diagnostic Observation Schedule – 2nd Edition/ADI-R (for ASD) and DIVA (ADHD)]. All prisoners screening positive for ID, ASD or ADHD (and all control group participants) were further assessed for mental health diagnoses using the Mini International Neuropsychiatric Interview, which covers 22 DSM-IV/ICD-10 diagnoses.
Results
Step 1
Literature reports on rates of ASD among prisoners
Given that there are an estimated 86,000 prisoners in the UK,147 it might be expected that up to 1000 of these individuals have ASD (applying the 1% rate of ASD among the general population). However, there is little evidence to support or dispute this assumption. 148 A review carried out in 2004 found no studies on the prevalence of ASD among prisoners. 78 Since then, one study has attempted to develop a screening tool for ASD among prisoners but was not able to ascertain prevalence. 143 Despite this lack of evidence, it is commonly hypothesised that people with ASD will be over-represented among offenders in general and among prisoners. 143,149 In fact, although there is evidence that people with ASD are over-represented among high-security psychiatric settings, it is not at all clear whether or not the same is true among prison populations. 150 In many studies, ASD is included among a range of broad ‘learning difficulties’, but it is rare for data on those with ASD to be reported separately. For example, a study of those with an existing diagnosis of intellectual disabilities or ASD found 19 prisoners in 16 prisons; however, the number of these individuals who had ASD was not reported. 78
Rates of ASD appear to vary according to the type of prison, country/geographical location and, as with all estimates of prevalence, the way in which ASD is identified. It is generally agreed that there are likely to be many individuals in prisons who have unrecognised ASD. 78,143,144 Recognising and assessing ASD in prisons78 identified several opportunities for the identification of ASD among prisoners: reception assessment, routine health-care appointments, education assessments and psychological assessments that assess suitability for offender programmes. However, these opportunities continue to be missed and there does not appear to be any policy of routine screening for ASD in UK prisons. A lack of suitable assessment tools is often cited as a reason for this. 78 However, the methods recommended by the NICE guidelines for autism in adults include the 10-item AQ – a self-rating scale that takes around 2 minutes to complete. 53 The AQ has been tested in high-security psychiatric setting but not systematically in prisons. 151
A study in Scotland attempted to screen for ASD using a newly developed screening tool completed by prison officers. 143 Of the 2458 prisoners screened, 4% exceeded the cut-off point for ASD. However, further assessment using other evidence-based tools found that few participants met the criteria for ASD. The specificity and sensitivity of the new tool were low (< 80%) and its routine use was not recommended.
Literature on difficulties experienced by people with ASD in prison
There are a range of the difficulties that may be expected in prison for individuals who have impairment of social communication and issues of sensory over- or understimulation. 143 These characteristics of ASD may affect how an individual interacts with others; their responses may appear inappropriate, discourteous or even confrontational to staff and other prisoners. 144 People with ASD may find the prison environment itself extremely challenging (noisy, brightly lit and enclosed). Potential consequences for people with ASD include being locked in their cell for longer than other prisoners for their own safety;78 difficulty adjusting to the transition from community to prison;152 and vulnerability to bullying or exploitation. 144,150 Paterson’s case studies153 of two young prisoners with Asperger syndrome explored the type of problems experienced by individuals at different levels of the autism spectrum. The participants’ experiences were affected by the family support they received, comorbid mental health problems, their level of social skills and the characteristics of their ASD traits.
Literature on prison staff and prisoners with ASD
Evidence suggests that there is of a lack of awareness and knowledge of ASD and ADHD among prison staff. This does not appear to be restricted to prison officers, but also applies to health and mental health-care staff. A survey of staff in one UK prison found that 34% did not know what autism was and 51% did not know what Asperger syndrome was. 144 Among this sample, which included both officers and health-care staff, 32% did not know that an individual’s senses could be affected by ASD. Many staff believe there are prisoners who have unidentified ASD; they also believe that prisons are not an appropriate environment for these individuals and, furthermore, that prisons are not able to meet their needs. 78
Step 2
As of 16 May 2013 we had approached 351 prisoners, and, of these, 221 were screened. Fifty-nine participants (27%) were housed on the VPs wing. Mean age was 36 years, 41% were white British (around 50% of prisoners in Brixton are from black and minority ethnic backgrounds) and before coming into prison 52% were single, 11% homeless, 47% unemployed and 27% reported past or current mental health problems. Eighty-one prisoners have screened positive for a ND (ASD, n = 39; ADHD, n = 60; ID, n = 32). For the ND group we aim to have a full data set (follow-up diagnostic assessment and the Mini International Neuropsychiatric Interview) on 70 participants. Currently this stands at 60 participants, with eight being followed up.
Overlap of neurodevelopmental disorders and demographic characteristics of prisoners screening positive
Intellectual disabilities
Of those screening positive for ID (n = 32), 75% screened positive for ADHD, 47% positive for ASD and 84% screened positive for either ASD or ADHD. Sixteen (50%) were held on the VP wing, 77% were white, 72% single, 36% homeless before prison and 91% unemployed.
ASD
Of those screening positive for ASD (n = 39), 59% screened positive for ADHD, 38% positive for ID and 67% screened positive for either ASD or ADHD. Eighteen (46%) were held on the VP wing, 75% were white, 62% single, 16% homeless before prison and 75% unemployed.
ADHD
Of those screening positive for ADHD (n = 60), 40% screened positive for ID, 38% positive for ASD and 58% screened positive for either ASD or ADHD. Twenty three (38%) were held on the VP wing, 80% were white, 61% single, 18% homeless before prison and 72% unemployed.
Those screened who met research diagnostic criteria
In total, 57 participants (70% of those screening positive for ND) met diagnostic criteria for ASD, ADHD or ID. Of the 39 participants who screened positive for ASD, 12 (31%) met diagnostic criteria and of the 60 participants who screened positive for ADHD, 46 (77%) met diagnostic criteria.
Comorbidity
Our preliminary analysis suggests that adult prisoners with ASD and/or ADHD have extremely high rates of associated mental health symptoms (Table 30).
| ND | Associated symptom (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Past or current major depressive episode | Current MDE | Social phobia | OCD | PTSD | Alcohol dependence | Drug dependence | Antisocial PD | Psychotic disorder | |
| ADHD | 81 | 46 | 39 | 13 | 19 | 24 | 32 | 68 | 6 |
| ID | 71 | 41 | 41 | 13 | 6 | 24 | 20 | 40 | 0 |
| ASD | 88 | 47 | 44 | 19 | 12 | 19 | 14 | 56 | 0 |
Discussion
The available literature suggests that a considerable proportion of prisoners may have ASD (or ADHD). Our findings support the suggestion that many so-called ‘vulnerable’ prisoners have a ND – including ASD and/or ADHD – and of these nearly all have associated mental health symptoms. However, in most individuals, this had not been diagnosed or treated. Our initial results also suggest that those with a ND are significantly younger, more likely to be white, homeless and unemployed than participants without a ND. Furthermore, our findings challenge predicted current rates of ASD in prisons (of 1%). However, the rates may differ by level of security (category) or diversion system. Nevertheless, our results suggest that we need to improve the recognition and treatment assessment of NDs, such as ASD and ADHD, across the Criminal Justice System. Unfortunately, however, there is currently a lack of access to specialist services and interventions for prisoners diagnosed with ASD and/or ADHD. Furthermore, existing mainstream programmes are not being adapted to meet their needs, with many individuals deemed ineligible because of their developmental and behavioural problems. Indeed, research on and the provision of mental health care in prisons tends to focus on those with a SMI.
Our work also suggests that there should be no room for therapeutic nihilism in treating ASD and/or ADHD in prisons – and prisoners with ASD and/or ADHD should not be denied treatments offered to others. For instance, psychosocial interventions, as described in relevant NICE guidelines,53,95 are suitable for people with ASD and ADHD, but these are not currently being explored to them within prison settings. This is also true for interventions such as Improving Access to Psychological Therapies services and/or guided self-help critical time intervention. Missed opportunities for the assessment of ASD and ADHD (and a failure to acknowledge their extent) hinder the ability of prisons to provide adequate care for affected individuals. 145 The development of provision for this unrecognised group of prisoners with lifelong disabilities requires a coherent approach to planning of health care in prison to improve their long-term health and social outcomes. These issues are further compounded by the current lack of evidence on which to base the development of assessment and intervention services. Our results also suggest that multiagency work across the Ministry of Justice/National Offenders Management Service, Department of Health and Social Care, research organisations and voluntary sectors is required to (1) improve staff knowledge and awareness of ASD and ADHD through training, (2) develop evidence-based screening tools and (3) test the effectiveness of treatments. Unfortunately, however, treatment trials in prisons are difficult to run and to our best knowledge none has been carried out in (either) ASD or ADHD. Hence, as a next step we obtained proof of concept that trials for these disorders can be carried out in prison settings. This issue is addressed in Chapter 21.
ASD and ADHD are common in VPs, but are mostly unrecognised.
Prisoners with ASD or ADHD have very high levels of unrecognised additional mental health problems.
Multiagency work may help:
-
improve forensic mental health and prison staff knowledge and awareness of ASD and ADHD through training
-
develop evidence-based screening tools for use in prisons.
Chapter 13 The economic cost of ASD and ADHD during the ‘transition’
Background
In the previous sections, we reported on the burden of ASD and ADHD as individuals transition from adolescence into adulthood. We have investigated the impact on the affected individual and their carers; and the rates that individuals with these disorders (and their associated mental health symptoms) were identified and treated by clinical services in the community, in hospitals and in forensic/prison settings.
In brief, we found that, as they ‘transition’ into late adolescence and adulthood, most of the needs of people with ASD/ADHD are met by their families (and not services). In addition, the burden on carers is significantly increased by the presence of additional mental health symptoms in their offspring. We also found that most individuals with ASD and/or ADHD in this age group are not in contact with clinical services; an,d even if they are, it is unlikely that they (and their associated mental health symptoms) are recognised or treated.
Given the need to use scarce (health-care) resources wisely, economics adds an important dimension to these findings, that of cost. In particular, and in the knowledge that adolescence and transition to adult services is often accompanied by reductions in support and treatment (as described above), our aim was to identify both the costs of supporting young adults with ADHD or ASD and what might drive those costs.
Our reviews of the cost-related research literature pertaining to the support of young adults with ADHD and ASD found little cost-related evidence either before this research programme started or over its duration. Most of the existing studies come from the USA and simply examined the ‘cost of illness’ – focusing on the costs of supporting children and young people with ADHD or ASD. Commonly, they used billing databases or management information systems. Although there are lessons to be taken from such studies, the very different health-care organisational and financing systems make it difficult to transfer detailed findings between countries. In addition, the commonly used (narrow) focus on health care does not allow estimates of the wider costs associated with the many areas where ADHD or ASD might be expected to have an impact.
For instance, little is known about impacts (in either the USA or the UK) that are likely to be felt across public sector health and social care services, youth and criminal justice services, the welfare benefits system and/or family health and expenditure. Furthermore, as the young adult grows older, wider impacts are likely to be seen, such as reduced employment prospects, lost productivity and problems with family and other relationships, which in turn may generate needs for support. 154
Other types of economic evaluation – such as cost-effectiveness studies – are very rarely found in child and adolescent psychiatry, with only 14 economic evaluations that used primary data published since 2001 across this whole field (Beecham J, University of Kent; 2013). None of the prior studies investigated young people at the point of transition to adult services and adulthood. Nonetheless, reviews of prior research on medication in child psychiatry155,156 found some evidence to support the cost-effectiveness of ADHD medication, albeit with concerns about the use of narrow cost and outcome measures, small sample sizes and short time frames. By contrast there are no evaluations assessing the relative cost-effectiveness of pharmacological interventions for individuals with ASD at any age, although there is some preliminary evidence suggesting that early intervention applied behavioural analysis programmes are effective in very young childhood157 and may reduce public sector costs. 158
Our main aim therefore was to estimate the costs of support for young adults at the point of transition who have ADHD or ASD, and who were identified and treated for these conditions in childhood. The questions that we addressed were the same for both patient groups:
-
What health care and other services do these young adults now use?
-
What are the associated costs to health care and other organisations of supporting these young adults?
-
Do these costs change as young people age?
-
Do support costs change as a consequence of transition to adult services and can reductions in any particular cost domain (particular services or organisations) be identified?
-
Is a young person’s transition to adult services linked to a change in the costs of treatment and support over and above the loss of support they received while within school?
-
To what extent do the young adults’ parents use services because of their child’s difficulties?
-
Over and above age, what are the associations between cost and the young person’s characteristics, needs and family circumstances?
Methods
These analyses are linked to the longer-term follow-up of children discussed previously (see Section 1). To ensure we could add to the small body of evidence on the ‘what is’ scenario provided by the cost of illness studies, we developed a project-specific version of the CSRI159 to be used in the data collection interviews. Used in countless cost-related evaluations, this schedule allows data to be recorded across all domains of support requirements: health and social care services, education and employment status, youth/criminal justice services, etc. The CSRI uses a structured approach that retains sufficient flexibility to ensure that questions about use of services and supports are tailored to the participants’ circumstances. A major focus for these analyses was on (mental) health care, but the CSRI included questions about a range of other supports and services so a comprehensive view could be obtained of service responses in all the areas where ADHD or ASD many have an impact.
To convert the service use data into costs, unit costs for each type of service, treatment or support are required. These were drawn from publicly available sources such as the annual Personal Social Services Research Unit volume of nationally applicable costs160 and the NHS reference costs,161 as well as being estimated specifically for this research using an equivalent methodology (see, for example, Beecham162). Total costs of support were calculated by multiplying the frequency of use of each service by its unit cost. The comprehensive approach to service use has the advantage of allowing costs data to be described and analysed both as a total and in disaggregated form, highlighting the contribution of each agency (e.g. the NHS, or local education authorities) to total support costs. Once costs per person have been calculated over a retrospective period of 3 months, they would be explored using descriptive, bivariate and multivariate analyses to address the research questions set out above.
Results
ADHD and transition
Our analyses were based on the follow-up interviews with young people aged 14–24 years and their parents/partners who were recruited from the IMAGE research database through a childhood clinical diagnoses of ADHD. Participants were included if they provided CSRI data at either wave 1 (n = 86) or wave 2 (n = 79): 66 participants provided data at both waves. Data were also available from the CANDID, the DIVA, the ADHD-specific BAARS-IV rating scale, the DAWBA, the CIS-R, the Zarit Burden Interview and the AUDIT.
Additional questions, developed in the Social Policy Research Unit at the University of York, asked about participants’ experiences of transition processes. 163
Our participants were aged, on average, 17 years; most were male and more than half were in full-time education, although this figure had reduced by the wave 2 interviews (Table 31). Most remained living with their parents and, at wave 1, 43% were taking ADHD medication. The young adults used a wide variety of services, but, in most cases, few people used any one particular service.
| Wave 1 (N = 86), mean (SD) | Wave 2 (N = 79), mean (SD) | |
|---|---|---|
| Age (years) | 17.4 (2.27) | 18.7 (2.45) |
| IQ | 101.9 (15.44) | |
| BAARS-IV checklist (sum) | 4.2 (2.76) | 3.7 (2.79) |
| Mood rating last month | 17.4 (10.07) | 13.2 (8.6) |
| Total number of needs (CANDID) | 2.9 (2.4) | 3.2 (2.92) |
| Total Zarit Burden Interview score | 17.9 (9.28) | 2.4 (2.80) |
| Male (n) | 76 | 60 |
| In full-time education (n) | 48 | 33 |
| Has a job (n) | 30 | 34 |
| Ethnicity white (n) | 82 | 65 |
| Marital status single (n) | 80 | 70 |
| Living with parents (n) | 83 | 72 |
| Drug use (n) | 37 | 25 |
| Medication use (n) | 37 | 28 |
| In trouble with police past year (n) | 19 | 7 |
The most common source of support in both waves was GPs, both for ADHD-specific problems (23% in wave 1 and 15% in wave 2) and for other reasons (33% in both waves). Many participants had repeat prescriptions (52% in wave 1 and 44% in wave 2). Use of specialist mental health services was sparse, with the exception of psychiatrists in wave 1 (26%), but this had dropped to 15% of the sample by the wave 2 interviews. In addition to these formal services, websites and helplines appear to play a fairly important role in supporting young adults, with 19% in wave 1 and 13% in wave 2 reporting their use in the 3 months prior to the interview.
The costs associated with service use and additional education supports (i.e. excluding the costs of mainstream education) are shown in Table 32. In both waves, education was the highest contributor to total costs, accounting for 46% at wave 1 and 34% at wave 2. Mental health services accounted for just 15% and 13% of costs, respectively. Although the average cost of primary care was similar in both waves, the proportion it contributed to total costs was 16% in wave 2 compared with just 8% in wave 1. There was a statistically significant reduction in total costs from wave 1 to wave 2 (£665 vs. £390), with significant differences in the costs (subtotals) for hospital and for mental health-care services. Costs for advice services and self-help supports were similar at each wave.
| Service category | Wave 1 (n = 86) | Wave 2 (n = 79) | ||
|---|---|---|---|---|
| Average cost (£) (SD) | Range (£) | Average cost (£) (SD) | Range (£) | |
| Education | 303 (1199) | 0–10,207 | 133 (426) | 0–2720 |
| Hospitala | 141 (347) | 0–2132 | 56 (153) | 0–755 |
| Primary care | 55 (55) | 0–219 | 63 (67) | 0–263 |
| Mental healtha | 103 (183) | 0–945 | 50 (152) | 0–823 |
| Social work | 10 (45) | 0–344 | 44 (244) | 0–1558 |
| Community therapy | 7 (55) | 0–504 | 2 (11) | 0–84 |
| Advice services | 14 (40) | 0–228 | 16 (73) | 0–587 |
| Other services | 32 (81) | 0–452 | 26 (66) | 0–270 |
| Total costsa | 665 (1283) | 0–10,564 | 390 (594) | 0–2907 |
Our analyses of these data will explore what individual characteristics and needs are associated with these costs at each wave and across both waves. We will also look in more detail at the group of young adults who have transferred from children to adult services (n = 18) over the period; those who do and who do not (n = 13, £0) receive any support; and those who do or do not use specialist mental health services. These analyses will help address the questions identified in Background.
ASD and transition
Young people aged 14–24 years with a diagnosis of ASD were recruited from CAMHS and adult clinics in south London, voluntary organisations and research databases held at the IoP through their child’s childhood clinical diagnosis of ASD. Data for 81 participants were available. As well as the CSRI, data were collected on the CANDID, the AQ – Informant version, the DAWBA, the AUDIT and the Zarit Burden Interview.
The majority of participants were male and white British (85% each). At wave 1, ages ranged from 14 to 25 years, with an average of 17.9 years. Most participants had an International Classification of Diseases (ICD) diagnosis (94%), with > 90% diagnosed as on the autistic spectrum (autism, Asperger syndrome). On average, parents reported needs in nine of the CANDID domains, six of which parents considered to be met needs.
Fifty six (69%) of these young adults were still in full-time education, with a further six in part-time education (Table 33). Two-thirds had a statement of SEN. Additional support in school was common in both waves 1 and 2, with around one in three young adults receiving individual help in class, some lessons taught in smaller classes, support from the special educational needs co-ordinator, or additional meetings with teachers.
| Additional education support | Wave 1 | Wave 2 |
|---|---|---|
| n using service (%) | n using service (%) | |
| Individual tuition | 8 (10) | 5 (6) |
| Individual help in classes | 31 (38) | 25 (31) |
| Lessons in small classes | 24 (30) | 23 (28) |
| School nurse | 6 (7) | 7 (9) |
| Educational psychologist | 7 (9) | 3 (4) |
| Educational welfare officer | 0 (0) | 1 (1) |
| SENCO | 30 (37) | 21 (26) |
| Additional meetings with teacher | 30 (37) | 24 (30) |
| Connexions | 1 (1) | 2 (2) |
| SEN worker | 1 (1) | 0 (0) |
| Learning support assistant | 1 (1) | 1 (1) |
The most commonly used service was the GP, seen by 36% of the sample in wave 1 and 35% in wave 2, although by just 9% in each wave for ASD-related reasons. Repeat prescriptions were made for nearly half the sample at both waves (47% and 46%, respectively). Use of mental health care was similar to the proportion found among the ADHD sample: < 5% of participants used ASD-specific outpatient clinics, therapists or counsellors; 27% had seen a psychiatrist (17% in wave 2); 17% had seen a psychologist (10%); and 6% (2%) had seen a psychiatric nurse specialist. Fewer than 5% of the young adults used community-based therapy services such as occupational or speech and language therapists, but social workers were seen by 16% of participants and 21% had a keyworker.
The costs of support over 3 months are shown in Table 34; nearly half of total costs are absorbed by additional supports provided by the education authorities with higher costs due to attendance at special needs schools. A further one-fifth was accounted for by provision of social care services; higher costs here are for one young adult spending time in a residential care unit and three who used respite care services. Mental health services contributed just 5% to total costs in wave 1 and only slightly less in wave 2 (4%).
| Wave 1 | Wave 2 | |||
|---|---|---|---|---|
| Average cost (£) (SD) | Range (£) | Average cost (£) (SD) | Range (£) | |
| Additional education | 2722 (5920) | 0–45,511 | 1782 (3384) | 0–20,657 |
| Hospital services | 583 (4145) | 0–37,326 | 126 (397) | 0–2946 |
| Primary care | 86 (105) | 0–482 | 74 (90) | 0–407 |
| Mental health services | 239 (531) | 0–3761 | 130 (320) | 0–2101 |
| Social work and social care | 838 (2408) | 0–11,340 | 660 (2027) | 0–11,492 |
| Community therapy | 18 (85) | 0–672 | 58 (247) | 0–1416 |
| Advice/self-help | 46 (177) | 0–1440 | 72 (279) | 0–1440 |
| Other | 26 (69) | 0–339 | 17 (54) | 0–226 |
| Total costs | 4557 (8117) | 0–48,042 | 2919 (4231) | 0–20,657 |
Total average costs were lower in wave 2 than in wave 1 (£4557 vs. £2919), although there was a wide range of costs at both periods; for a number of young adults, total costs were £0 indicating no services or support were used. Mean costs for additional education, hospital services and social work/social care all were lower in wave 2, although again showing wide ranges for each. The only significant difference between wave 1 and wave 2 is mental health service costs (p = 0.02).
As with the ADHD sample, our analyses of these data will explore what individual characteristics and needs are associated with these costs at each wave and across both waves. We will also look in more detail at the group of young adults who have transferred from children to adult services over the period, those who do and who do not receive any support, and those who do or do not use specialist mental health services. These analyses will help address the questions identified in the earlier section (see Background).
Discussion
We carried out the first longitudinal study of the cost of ASD and ADHD as young people with ‘transition’ through adolescence and into young adulthood; and taking into account a wide range of potential service consumption (including health, education and social services). We found that, overall, mean costs for both disorders were mainly borne by education and social care agencies – with much less accounted for by health. In addition, in both groups, mental health supports accounted for a relatively small proportion of the total costs. Finally, as individuals with ADHD and ASD grew older, they continued to consume services, but costs of education, hospital services and social work/social care all reduced for both disorder groups; and for both disorders mental health service costs reduced significantly. The latter finding suggests that although (as noted in prior chapters) individuals with ASD and ADHD continue to experience significant mental health symptoms as they ‘transition’, their contact with treatment and support services reduces significantly.
Few other studies published over the period of our research programme focused on, or separately identified, the age group we investigated (16–25 years). One US analysis showed the distribution of ADHD-related treatment costs by age and found that, for males, > 90% were incurred for children up to age 15 years, 5% between ages 15 and 25 years and < 2% in young adults aged > 25 years. 164 Given that two-thirds of childhood ADHD cases persist into adulthood,101 this means that treatment drop-off among young adults remains high, particularly as children transition out of children’s services (see also Young and colleagues165 and Singh166). However, a recent review of the US literature on the cost of illness related to ADHD suggests that its economic impact is around three times greater among affected adults than children and adolescents. 167
For the UK, one recent study estimated the costs of health care (NHS), social care and education resources associated with ADHD for a cohort of 143 young people aged between 12 and 18 years taking part in the Cardiff Longitudinal ADHD Study, 5 years after their initial diagnosis. 168 Over a 12-month period, average costs were £5493 (median = £2330, 2010 prices), with estimated total costs for the UK of £670M. Snell and colleagues,169 in their analysis of the British Child and Adolescent Mental Health Survey, found observed mean costs for those with hyperkinetic disorders age 5–15 years to be lower, at £3108 per annum. As with the Welsh study, this excluded any costs associated with the criminal justice system and family-borne costs. Just two US studies of childhood ADHD have considered costs to the education sector, and only one included youth justice costs. 170 Notably, children and adolescents with ADHD tend to incur higher medical care costs than their peers without ADHD,171,172 as well as greater other public sector costs and out-of-pocket expenses. 173 Additional problems may include other psychiatric disorders, substance misuse, educational underachievement, difficulties with employment and relationships, and criminality. 134 Each of these can lead to additional costs in (young) adulthood to the (mental) health and social care budgets, welfare payments, as well as in lost productivity.
The economic literature on young adults with ASD is even sparser than that for ADHD. One recent study attempting to estimate the costs of ASD in the UK found no nationally representative samples of children or adults with ASD from which costs could be estimated. 1,174 Costs were estimated for children (aged 0–17 years) and adults (aged ≥ 18 years) using a mixture of prevalence data, analysis of the Children in Need surveys175 and by extracting data on individuals with ASD from ‘in-house’ studies of service use and costs, or from large population psychiatric morbidity surveys. Although the analysis usefully identifies resource use and cost differences between those with ID (higher costs) and those with high-functioning ASD, the data were insufficient to disaggregate the costs in adulthood into different age groups. Only costs attributable to ASD were included and covered all public sector services used by those with ASD and their families, as well as out-of-pocket payments for services, family expenditure and lost employment costs. Informal care time was excluded owing to lack of data. The average annual cost per child with ASD was estimated at £25,400 per person and was £58,900 per adult (2005–6 prices).
Apart from one study based in Sweden,176 we have found no European studies and only one US study177 that estimated the full cost of supporting people with ASD. Ganz177 drew on the published literature on use of services by children with ASD, to create a US prevalence-based cohort from which lifetime costs were calculated. The costs included all hospital and health-care services and equipment, child care, adult care, respite care, home care and modifications, special education and supported employment (2003 prices, discounted to present value at 3%). The average age-specific per capita costs were (1) ages 13–17 years, US$285,082, (2) ages 18–22 years, US$248,446 and (3) ages 23–37 years, US$404,260.
As with ADHD, recent studies (mainly from the USA) have also found higher health-care costs for ASD than for other diagnoses,178 or for children without ASD. 179–181 In the 2003–4 US National Survey of Children’s Health, parents of children with autism reported a significantly higher number of visits to all types of physicians than did parents of children without autism. 182 There is, however, evidence of a fall in the level of support young people with ASD get once they leave school and should be supported by adult services. 163,166,183
Thus, the body of evidence examining the costs of treatment and support for adolescents and young adults with ADHD or ASD is small, often derived from less than ideal data and assumptions, and many have a narrow focus on treatment (medication) or health care. Moreover, much of the literature is USA based, where there are very different organisational and financial incentives at play. None of the existing studies follow participants over the transition period, despite long-standing UK policy concerns such as the National Service Frameworks for Children Young People and Maternity Services and for Mental Health,184,185 which specifically identified the importance of a planned and co-ordinated transition to adult services. Although there is evidence to suggest that the transition to adult services for individuals with a range of disabilities and health conditions is associated with reduced levels of support in England and Wales, the published studies do not allow a full picture to be drawn of the intensity of support that adolescents with ADHD or ASD receive, in which services the reductions in support occur pre or post transition, nor which supports are used more commonly in young adulthood.
The current study can, therefore, inform the evidence base in a number of ways. Our study can clearly show which services are and are not used, by what proportion of this population and by what age groups. Although not all the details are reported here, as well as data on the use of health and social care, we have data on specific education inputs and on young people’s involvement with youth and criminal justice services. The former are more likely to be important for individuals with ASD and the latter for those with ADHD, but both are sadly lacking in existing UK studies. One additional set of data may prove equally revealing: the extent to which parents use services as a result of their child’s condition, for example whether or not perceived carer burden translates into obtaining help for themselves from formal services.
The individual-level support costs can be calculated from these data on each individual’s use of treatment and support services. In turn, these data enable us to identify which service providers bear the brunt of responding to young people’s needs and how these might change as the adolescents grow older. However, we also know very little about what drives treatment and support costs. Is it individuals with greater condition-related symptoms and severity who receive and continue to receive support (as summarised by costs)? Or is it their wider (met or unmet) needs? Or are there wider factors at play, perhaps parental backgrounds or carer burden, leading to request additional support for their child? Or is the level of treatment and support purely a matter of location – or chance? Addressing questions such as these will add substantially to the evidence available to decision-makers and providers alike.
The cost of ASD and ADHD is mainly borne by families, education and special care agencies.
Relatively low costs are borne by the NHS and especially low costs for mental health support.
Treatment and support costs reduce significantly with age – despite persistence of clinical symptoms.
Section 3 Improving outcomes through better diagnosis
Chapter 14 An overview
In the previous two sections we saw that ASD and ADHD (and their associated mental health symptoms) are frequently unrecognised and untreated both in the community by GPs and by the specialist mental health services we investigated. In addition, during the course of this programme, dramatic changes occurred in recommendations for clinical diagnostic practice of ASD in DSM-V. These required urgent clarification due to their potential impact on clinical services – as the effect of this change in young adults was undetermined. Furthermore, breakthroughs were made in brain image analysis, but it was unknown if these could be used to help categorise individuals with and without ASD or ADHD.
In this next section as a first step we:
-
piloted the development of simple/accurate clinical diagnostic interviews for ASD that can be used in community settings
-
developed a tool to aid identification of associated mental health symptoms
-
investigated the effect changes in DSM-V clinical diagnostic criteria would have in young adults with ASD; and carried out a proof of concept study on the potential utility of brain imaging to help categorise individuals with and without ASD/ADHD.
Chapter 15 Improving diagnosis of ASD in the community: tool development
Background
The NICE guidance highlights the increasing need for diagnostic services for children and young people with ASD in the UK. 186 It recommends that every autism diagnostic assessment includes ‘a developmental history, focusing on developmental and behavioural features consistent with ICD-10 or DSM-IV criteria’ using an ‘autism-specific tool’. 186 Currently the best-established diagnostic tools for collecting an autism-specific diagnostic history are the ADI-R; the Diagnostic Interview for Social Communication Disorders; and the Developmental, Dimensional and Diagnostic Interview. These semistructured interviews are carried out by highly trained clinical interviewers and take 2–3 hours to complete. Many community services have struggled to adopt these instruments as part of the diagnostic protocol because of the level of expertise required, the absence of trained staff and the time- and labour-intensive nature of the interview. Instead, they have continued to rely on ‘routine clinical interview’, despite the low inter-rater reliability for clinical subtyping187,188 of this approach.
Hence there is a need for a simpler approach to diagnosis in community settings. Accordingly, we examined the utility of the DAWBA as a diagnostic tool. We chose to pilot the adaption of the DAWBA because it is an assessment tool that can be completed by the parent online, over the telephone or at face-to-face interview with an interviewer. In addition, interviewers need minimal training and this can be done by reviewing information and training materials provided online (URL: www.dawba.info).
The DAWBA has been designed to collect information sufficient to make various psychiatric diagnoses according to ICD and Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. The pervasive developmental disorders (PDDs) module, entitled ‘development of language, routines, play, and social ability’ in the parent interview, gathers information required to diagnose ASDs.
Information from the PDD module is used in a computer algorithm to generate a probability of disorder according to DSM and ICD criteria, ranging from < 0.1% to > 70% probability of meeting diagnostic criteria for ASD. The DAWBA is relatively quick to complete compared with the ADI-R. For instance, the PDD module takes about 20 minutes to complete and data entry is streamlined by online administration. In addition, data entered online, along with the computer-generated probability, diagnosis and comments made by the clinical rater, can be printed out in portable document format (PDF) for each case.
The overall aim of this pilot study, therefore, was to examine the effectiveness of the PDD module of the DAWBA in identifying ASD in a community sample. Specific aims were to (1) compare the DAWBA with the ADI-R as measures of autistic symptoms, (2) establish the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for DAWBA diagnosis assigned by clinical rater and (3) examine the improvement in detection of ASD by combining the DAWBA with an observational tool called the Autism Diagnostic Observation Schedule – Generic (ADOS-G).
Methods
We examined the diagnostic utility of the DAWBA in a population-based sample of 169 twins, who were screened to be at high or low risk for ASD. The sample was drawn from a large community-based twin sample, the Twins Early Development Study (TEDS), which has been described in detail elsewhere. 189 This sample was utilised for a number of reasons. It was important to gain a sample of children and adolescents with ASD who represented the full range of presentations of ASD. Samples ascertained from clinics may be biased in various ways (e.g. children seen in tertiary or quaternary referral clinics are likely to have more complex problems). Using a population-derived sample minimises these types of biases, while also allowing the inclusion of those with subtle difficulties which had not previously been identified as ASD. Children in TEDS were screened and assessed at multiple time points from age 2 years until adolescence, maximising the chances of detecting all cases of ASD in the cohort. There is no evidence that the manifestation of ASD is different in twins and singletons. 190 The study was reviewed and approved by the Joint Schools Research Ethics Sub-Committee for the IoP and the Florence Nightingale School of Nursing and Midwifery, King’s College London. Parents of twins born from 1994 to 1996 in England and Wales were invited to participate in TEDS and the resulting sample is representative of the UK in terms of maternal ethnicity (93.5% white) and education (37.9% with A levels or higher, the equivalent of some college education in the USA).
Selection and diagnostic assessment of ASD sample
Screening for ASD was carried out using the Social Communication Questionnaire (SCQ),191 the Childhood Autism Spectrum Test (CAST)192 and additional questions about whether or not children had previously been given an autism or Asperger syndrome diagnosis. In addition, some families also spontaneously disclosed that one or both of their children was suspected of having ASD during data collection unrelated to ASD. Data were collected via questionnaires mailed to parents when their children were aged 5–8 years (SCQ), 8 years (CAST) and 9 years (questions about previous ASD diagnosis), and via telephone interview when children were aged 7 years (questions about ASD diagnosis). A total of 14,797 families were invited to complete questionnaires or a telephone interview and 8941 (60.4%) returned data at least once. This sample was broadly representative of the UK population and of the TEDS cohort as a whole (maternal ethnicity, 93.2% white; maternal education, 40.1% with A levels or higher).
Children were considered to be at risk of ASD if they met any of the following criteria:
-
They scored ≥ 15 on the SCQ.
-
They scored ≥ 15 on the CAST.
-
Their parents had endorsed questionnaire items at ages 7, 8 or 9 years indicating that their child had been given a diagnosis of ASD.
-
Their parents had spontaneously indicated at other times that they were concerned that their child might have ASD. Families where one or both children were considered to be at risk of ASD were then invited to complete the ASD module of the DAWBA.
Families were then visited at home for further assessment. The ADI-R and the ADOS-G were carried out by trained interviewers. Two interviewers assessed each family. One interviewer carried out the ADI-R for the first twin, while the other interviewer carried out the ADOS-G. The interviewers then reversed roles for the second twin so that the same interviewer did not carry out the ADI-R and the ADOS-G for the same child and did not carry out either for both twins in a pair. A consensus diagnosis was assigned by the study team after reviewing ADI-R and ADOS-G data and other relevant data, such as parent reports of previous clinical diagnosis. Children were classified as ASD (i.e. met criteria for childhood autism, Asperger syndrome, ‘other’ PDD) or unaffected. One hundred and twelve children with a diagnosis of ASD were included in analyses (child sex, 83.0% male; maternal ethnicity, 96.4% white; maternal education, 50.4% with A levels or higher).
Selection of comparison sample
The ‘at-risk’ sample consisted of children likely to have ASD and their co-twins, which included unaffected co-twins. However, as unaffected co-twins were expected to be at higher risk of subthreshold autistic traits,193 parents of twins considered to be low risk (score of < 12 on the CAST) were invited to complete the DAWBA. These families were selected to be matched to the ‘at-risk’ group on sex, zygosity, age and socioeconomic status. Home visits were carried out with the families, during which parents completed the family history interview and children were administered a battery of cognitive measures. Researchers also completed an observation sheet for each child, which included observations about the child’s social interaction with the researcher. Suspicion about ASD was raised for only one child in the control sample and an ADI-R was completed with their mother. No further data were available for this child and so they were excluded from analyses in which a consensus diagnosis was required.
Non-cases included 101 children who were unaffected co-twins of children with ASD and a further 68 children from the low-risk group. For analyses using ADI-R or ADOS-G data, non-cases included unaffected co-twins (n = 101; child sex, 52.5% male; maternal ethnicity, 96.0% white; maternal education, 52.0% with A levels or higher). For analyses using consensus diagnosis data, non-cases included unaffected co-twins and low-risk twins (n = 169; child sex, 60.4% male; maternal ethnicity, 96.4% white; maternal education, 53.0% with A levels or higher).
Measures
Tools used to screen for and identify cases
Social Communication Questionnaire
The SCQ is a 40-item screening questionnaire that is completed by parents of children who are 4 years of age or older. The content parallels that of the ADI-R and the SCQ has shown to be an effective way of screening for PDDs. 194 Parents who indicated that their child had social or communication difficulties during data collection when children were aged 2–4 years were asked to complete the SCQ. A score of ≥ 15 was taken to indicate risk of ASD.
Childhood Autism Spectrum Test
The CAST195 is a 31-item questionnaire that is completed by parents. It was designed to be used in mainstream, non-clinical samples to screen for ASD in primary school-aged children. All parents were asked to complete the CAST when their children were 8 years of age. A score of ≥ 15 was taken to indicate risk of ASD.
Autism Diagnostic Interview – Revised
The ADI-R is an extended semistructured interview designed to elicit sufficient information about developmental history and current day-to-day behaviour to enable diagnosis of ASDs. 196 A trained interviewer carries out the interview with an informant, typically a parent, and the interview takes around 90–150 minutes to complete. A modified diagnostic algorithm based on criteria outlined by the Autism Genetic Resource Exchange was used (URL: http://research.agre.org/program/diag.cfm), which, in addition to the cut-off point for autism, had further cut-off points not quite autism and broad spectrum. Interviews were recorded and consensus coded by the study team: an experienced child psychiatrist (PB) reviewed a subset of cases, including ambiguous cases. A total algorithm score was generated by summing the scores from algorithm items (excluding domain D), giving a quantitative measure of autistic symptoms.
Autism Diagnostic Observation Schedule – Generic
The ADOS-G is a semistructured assessment that provides opportunities to observe social and communication behaviours relevant to diagnosis of ASDs. 197 A trained assessor administers a series of structured and semistructured tasks and interview questions to elicit a range of responses. It takes around 30–60 minutes to administer. Modified diagnostic algorithms were used198 and children were classified as meeting criteria for autism or ASD. A further category, broad spectrum, was defined as being up to 2 points below the cut-off point for ASD. Assessments were videotaped and consensus coded by the study team: an experienced child psychiatrist (PB) reviewed a subset of cases, including ambiguous cases.
Tools used in protocol to be validated
Development and Well-Being Assessment
The module of the DAWBA designed to assess PDDs was administered by telephone interview or completed online. Skip rules, an option available to shorten the interview if the answers to preliminary questions do not indicate ASD, were not used, meaning that parents were asked all questions. Probability bands were generated by computer algorithm, which placed children in one of six bands that ranged from < 0.1% to > 70% probability of having a diagnosis of ASD. An experienced clinician (PB) rated each child, using the probability band, the Social Aptitudes Scale (a measure of current social functioning which is included in the PDD module of the DAWBA)199 score, and the answers to open-ended questions to assign a diagnosis of autism, Asperger syndrome, or other PDD. Rating was carried out blind to diagnostic status and other information gathered during the study. A continuous measure of autistic symptoms was generated by summing scores from the answers to closed questions, giving a total impairment score analogous to the ADI-R total algorithm score.
Statistical analysis
First, the DAWBA ASD module was compared with the ADI-R by examining the correlation between the DAWBA total impairment score and ADI-R total algorithm score using Spearman’s r. The distribution of scores for both measures was also compared in cases and non-cases.
Second, the optimal cut-off point for DAWBA probability bands in this sample was determined using receiver operating characteristic (ROC) curve analysis and by examining the sensitivity, specificity, PPV, NPV and overall percentage of children correctly classified at different cut-off points. The OR for cases versus controls was used to determine if there was a significantly increased risk of ASD associated with probability bands higher than the cut-off point. Robust standard errors, clustered by family, controlled for the relatedness of twins in a pair.
Third, the sensitivity, specificity, PPV, NPV and overall percentage of children correctly classified using diagnosis assigned by clinical rater were compared with the probability bands, to determine to what extent prediction was improved by the clinical rater.
Finally, ADOS-G data were added to the DAWBA clinical rater diagnosis and the sensitivity, specificity, PPV, NPV and overall percentage of children correctly classified were compared for children meeting criteria for ASD on one or both measures.
Results
The sample included in analyses is described in Table 35.
| Participant information | ASD | Non-ASD (co-twins) | Non-ASD (co-twins and low risk) | |||
|---|---|---|---|---|---|---|
| n | 112 | 101 | 169 | |||
| Child sex, % male | 83.0 | a52.5** | a60.4** | |||
| Maternal ethnicity, % white | 96.4 | a96.0 | a96.4 | |||
| Maternal education, % with A level or above | 50.4 | a52.0 | a53.0 | |||
| Child age in years, mean (SD) | 10.04 (1.58) | 9.93 (1.37)a | a11.55 (2.45)** | |||
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| DAWBA ASD total impairment | 30.65 (10.77) | 28.64 to 32.67 | 9.02 (10.30) | 6.99 to 11.05 | 8.67 (8.59) | 7.37 to 9.98 |
| SAS score | 9.23 (6.54) | 8.00 to 10.46 | 22.15 (7.79) | 20.60 to 23.70 | 22.70 (7.17) | 21.61 to 23.79 |
| ADI-R algorithm total | 39.18 (12.77) | 36.79 to 41.57 | 10.59 (10.48) | 8.53 to 12.66 | ||
| ADOS severity score | 6.91 (2.45) | 6.15 to 7.07 | 2.31 (1.85) | 1.94 to 2.67 | ||
One hundred and twelve children with a consensus diagnosis of ASD were included, the majority of whom (83.0%) were male. Two non-ASD comparison groups (unaffected co-twins, n = 101; unaffected co-twins and low-risk twins, n = 169) were included, and both had a significantly lower proportion of males than the ASD group. The two comparison groups did not differ from the ASD group in terms of maternal ethnicity or education. Furthermore, the comparison groups did not differ from each other in terms of DAWBA ASD total impairment scores or Social Aptitudes Scale scores. Children were aged between 8 and 16 years when the DAWBA was completed, with the mean age being slightly higher in low-risk twins.
Performance of Development and Well-Being Assessment in comparison with the Autism Diagnostic Interview – Revised
The distribution of continuous measures of ASD symptoms, the DAWBA ASD total impairment score and the ADI-R algorithm total, were compared with each other in cases and non-cases. Figure 13 shows that the distribution using the DAWBA mirrors that of the ADI-R. Both measures are positively skewed in non-cases, with the majority of children having low scores and roughly normally distributed in cases. There is a strong correlation between the DAWBA ASD total impairment score and ADI-R algorithm score (in the whole sample, r = 0.82, p < 0.001; in cases, r = 0.55, p < 0.001; and in non-cases, r = 0.64, p < 0.001).
FIGURE 13.
Comparison of DAWBA and ADI-R. (a) Distribution of DAWBA ASD total impairment scores (cases n = 112); (b) distribution of DAWBA ASD total impairment scores (non-cases n = 101); (c) ADI-R algorithm total scores (cases n = 112); and (d) ADI-R algorithm total scores (non-cases n = 101). Adapted from McEwen et al. 3 © 2016 McEwen et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

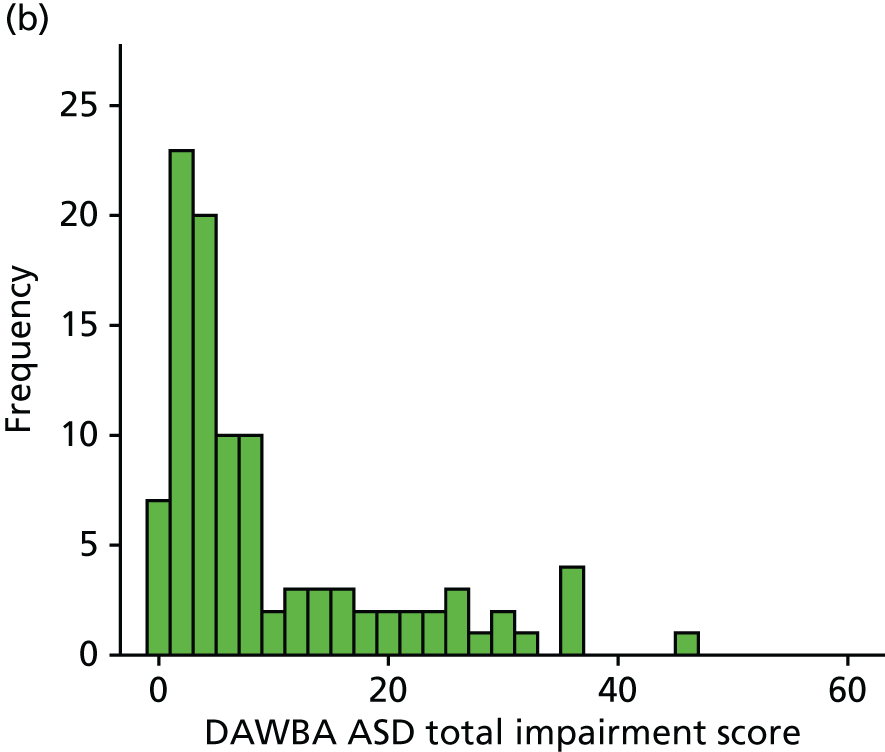

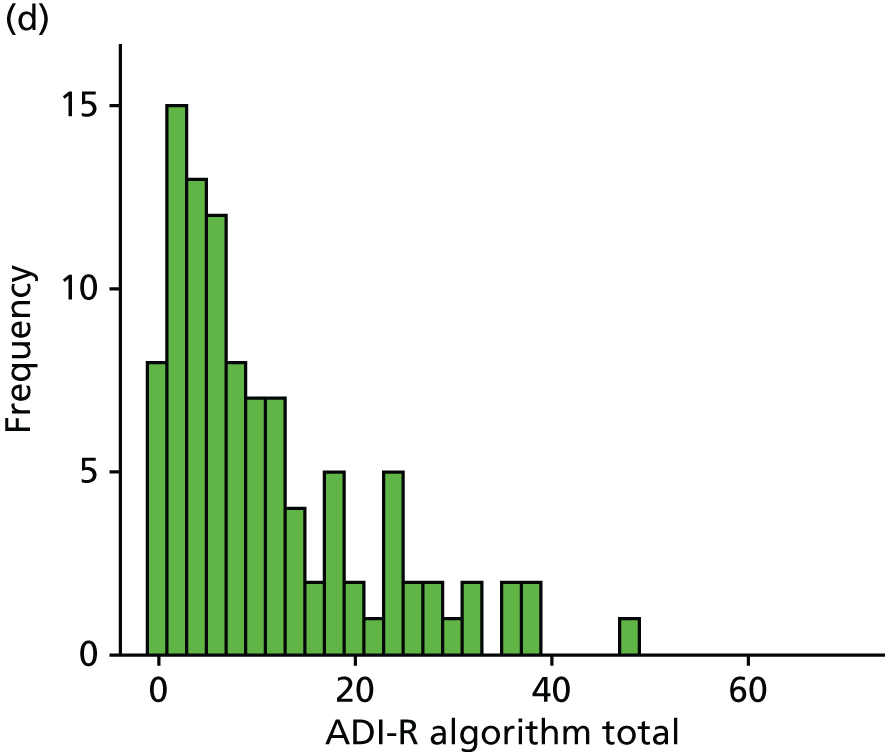
Prediction of ASD by Development and Well-Being Assessment computer-generated probability bands
Receiver operating characteristic curve analysis was used to determine how well DAWBA computer-generated probability bands predicted consensus research diagnosis of ASD (Figure 14). The area under the ROC curve was significant (0.90, 95% CI 0.87 to 0.94; p < 0.001) and the OR, corrected for relatedness of twins in each pair, showed a threefold increase in the odds of an ASD diagnosis for each increasing probability band (OR 3.39, 95% CI 2.60 to 4.42; p < 0.001).
FIGURE 14.
Receiver operating characteristic curve of DAWBA computer-generated probability band predicting consensus research diagnosis of ASD. Area under the curve = 0.90 (95% CI 0.87 to 0.94; p < 0.001). OR 3.39 (95% CI 2.60 to 4.42; p < 0.001) (robust standard error used, clustered by family to control for relatedness of twins in pairs). Adapted from McEwen et al. 3 © 2016 McEwen et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

Receiver operating characteristic curve
Classification statistics for probability bands are shown in Table 36. The optimal cut-off point in this sample was low (3%) – taking those in this band or higher to be cases – with 86.1% of children correctly classified. This achieved a good balance between sensitivity (0.88) and specificity (0.85). The PPV of 0.80 indicated that 8 out of 10 children identified as cases at this cut-off point had a research diagnosis of ASD, whereas 2 out of 10 were false positives. The NPV of 0.91 showed that 9 out of 10 children identified as non-cases were truly non-cases, whereas 1 in 10 identified as non-cases were cases of ASD that had been missed.
| DAWBA diagnosis | Sensitivity | Specificity | PPV | NPV | Correctly classified, % |
|---|---|---|---|---|---|
| Positive if at this computer-generated probability band or higher | |||||
| Very low (0.1) | 1.00 | 0.00 | 0.40 | – | 40.0 |
| Low (0.5) | 0.94 | 0.79 | 0.74 | 0.95 | 84.6 |
| Low (3) | 0.88 | 0.85 | 0.80 | 0.91 | 86.1 |
| Moderate (20) | 0.69 | 0.90 | 0.83 | 0.81 | 81.8 |
| 50/50 | 0.38 | 0.97 | 0.89 | 0.70 | 73.2 |
| High (80) | 0.20 | 0.99 | 0.96 | 0.65 | 67.5 |
| Positive if clinical rating of | |||||
| Any ASD | 0.86 | 0.87 | 0.82 | 0.90 | 86.4 |
| Asperger syndrome or autism | 0.54 | 0.98 | 0.95 | 0.76 | 73.9 |
Ability of DAWBA clinical rater-assigned diagnosis to predict ASD
The numbers of children rated as having autism or Asperger syndrome, other ASD, or not ASD by DAWBA clinical rater are cross-tabulated with consensus research diagnosis of ASD in Table 37. The majority, 86.9%, of children without a research diagnosis of ASD were correctly rated as non-cases by the DAWBA clinical rater.
| Consensus diagnosis | DAWBA diagnosis (clinical rater) | ||
|---|---|---|---|
| Rated as not ASD | Rated as ASD (other) | Rated as Asperger syndrome/autism | |
| Not ASD | 146 | 19 | 3 |
| ASD | 16 | 35 | 61 |
Similarly, 85.7% of children with a research diagnosis of ASD were identified by the DAWBA clinical rater as having an ASD diagnosis. Table 35 shows the classification statistics for cases defined as those with any ASD diagnosis by DAWBA clinical rater, and for cases more narrowly defined as those with a diagnosis of childhood autism or Asperger syndrome. Using the broader category of any ASD diagnosis, there was only minimal improvement over computer probability bands in the overall percentage of children correctly classified, from 86.1% to 86.4%, and minimal change in the sensitivity, specificity, PPV and NPV. Using the narrower category of autism or Asperger syndrome, specificity improved to 0.98 and PPV to 0.95, meaning that virtually all non-cases were correctly classified and there were very few false positives. Conversely, sensitivity decreased to 0.54 and NPV to 0.76, meaning that only around half of the cases of ASD were identified and less confidence was warranted by a negative result.
Does combining the DAWBA with the ADOS improve the classification of cases?
In practice, assessment of children suspected of having ASD should include both a detailed developmental history and direct observation of the child using a tool such as the ADOS-G. Table 38 shows the classification statistics when DAWBA clinical rater-assigned diagnosis was used in conjunction with ADOS-G diagnostic status. Those with DAWBA and ADOS-G data were included (cases, n = 112; non-cases, n = 101; note that this does not include the low-risk non-cases, see Table 37). Children who met criteria according to the DAWBA and/or met criteria for ASD according to the ADOS were assumed to be positive for ASD, and this diagnosis was compared with the research diagnosis of ASD. The DAWBA was used at both the broader category of any ASD and the narrower category of autism and Asperger syndrome.
| DAWBA clinical rater | Sensitivity | Specificity | PPV | NPV | Correctly classified (%) |
|---|---|---|---|---|---|
| Positive if | |||||
| DAWBA clinical rater classified as any ASD | 0.86 | 0.79 | 0.82 | 0.83 | 82.5 |
| AND ADOS-G +ve | 0.74 | 0.95 | 0.94 | 0.77 | 84.0 |
| OR ADOS-G +ve | 0.98 | 0.69 | 0.78 | 0.97 | 84.4 |
| DAWBA clinical rater classified as Asperger syndrome/autism | 0.54 | 0.97 | 0.95 | 0.66 | 74.5 |
| AND ADOS-G +ve | 0.49 | 0.99 | 0.98 | 0.63 | 72.6 |
| OR ADOS-G +ve | 0.92 | 0.82 | 0.85 | 0.90 | 87.3 |
Results of this analysis are shown in Table 38. In summary:
-
Using ADOS-G diagnosis in conjunction with a DAWBA diagnosis of any ASD increased the percentage correctly classified.
-
Using ADOS-G diagnosis in conjunction with the narrower DAWBA diagnosis of autism or Asperger syndrome decreased the percentage correctly classified if cases were taken to be those positive on DAWBA and ADOS-G, but increased it if cases were taken to be those positive on DAWBA or ADOS-G.
-
When a positive result on either the DAWBA or the ADOS-G was used to define cases, sensitivity was very high (nearly all cases were detected), but specificity was lower (some non-cases were incorrectly flagged as having ASD), and the PPV showed that one out of five children identified as cases were false positives.
-
When cases were taken to be those with positive results for both DAWBA and ADOS-G, sensitivity was lower (some cases were missed), but specificity was very high (very few non-cases were flagged as ASD), and PPV was very high (there were very few false positives).
-
Children negative on both the DAWBA and ADOS-G were very unlikely to have ASD.
Sensitivity, specificity, PPV and NPV for diagnosis assigned by DAWBA clinical rater in combination with ADOS-G diagnostic status (if met criteria for ASD or autism according to revised algorithm198)
The level of confidence in positive results is conveyed in Figure 15, which shows the percentage of true and false positives in cases identified through various combinations of DAWBA and ADOS-G results. For example, although a DAWBA rating of any ASD resulted in around 80% true positives, the proportion of true positives increased in those with a narrower DAWBA rating of Asperger syndrome or autism. The proportion also increased in those who met both DAWBA and ADOS-G criteria for ASD, and was 98% in those with a DAWBA rating of Asperger syndrome or autism and who met ADOS-G criteria for ASD.
FIGURE 15.
Percentage of true and false positives for cases identified using DAWBA and ADOS-G. Adapted from McEwen et al. 3 © 2016 McEwen et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The level of confidence in negative results can be seen in Figure 16, which shows the percentage of true and false negatives using the DAWBA and ADOS-G. Nearly 20% of children rated as not having ASD using the DAWBA were false negatives. However, this dropped to only 3% for children who did not meet either DAWBA or ADOS-G criteria for ASD.
FIGURE 16.
Percentage of true and false negatives for cases identified using DAWBA and ADOS-G. Adapted from McEwen et al. 3 © 2016 McEwen et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

Discussion
We examined the utility of the DAWBA in (largely) straightforward cases in a general population sample. Hence, these results are likely to be applicable to community settings. The DAWBA was used in a sample that had been previously screened and that was considered to be at high risk of ASD. This means that the classification statistics and inferences about confidence in positive and negative results will be applicable only to other screened and high-risk samples. In practice, the DAWBA could be used after using an age-appropriate screening tool, such as the CAST or SCQ.
The DAWBA showed good agreement with the ADI-R. Furthermore, the DAWBA predicted consensus diagnosis with good sensitivity and specificity, and this was true using both computer-generated probability bands and clinical rater-assigned diagnosis. Hence, we suggest that the DAWBA could replace the more complicated/expensive ADI-R in assessing straightforward cases in community settings. This is likely to improve the reliability of clinical diagnosis towards the so-called ‘gold standard’ research diagnostic instruments.
Nonetheless, the performance and interpretability of results could be improved by using the DAWBA in conjunction with the ADOS. The greatest confidence in positive results (high PPV) was warranted in cases with a DAWBA rating of the narrower autism or Asperger syndrome diagnosis (regardless of ADOS-G outcome) and in cases with a DAWBA rating of any ASD and who met ADOS-G criteria for ASD. The greatest confidence in negative results (high NPV) was warranted in cases who did not receive a DAWBA rating of ASD and who did not meet ADOS-G criteria for ASD.
The DAWBA may also offer other advantages in community settings, as it can be modified to include modules that assess common co-occurring disorders such as ADHD, anxiety, depression and OCD. Our pilot results suggest, therefore, that the DAWBA could be used as a ‘one-stop shop’ that (in one package) helps information to be gathered about both ‘core symptoms’ and associated disorders, including the perspectives of (more able) affected individuals, parents and teachers. Caution is needed, however, as the majority of the current sample is likely to have consisted of fairly straightforward cases of ASD. The results are therefore not necessarily applicable to more complex clinical samples of children with serious comorbid disorders (e.g. ADHD). Hence, we suggest that the DAWBA should be tested in more complex samples (e.g. tertiary/quaternary referral clinics) before any definitive recommendations can be made about its use in these settings.
Strengths and weaknesses
The strengths of this study include our sample being drawn from a large UK general population sample and so our findings should be reasonably representative of the types of cases seen in community/first opinion settings. The use of staged screening and assessment for ASD at multiple points through childhood and adolescence should also have ensured that cases from across the autism spectrum were detected, including those with more subtle difficulties that may not have been detected until late childhood.
However, our work also has limitations. For instance, the mothers in our sample had a higher level of education than the UK average (≈50% vs. ≈40% with A level or above), and this may have resulted in better-quality data (e.g. more detailed answers given to open questions). However, 50% of the mothers in our sample were of a lower educational level so, despite the slight over-representation of more educated mothers, all educational levels were included. It is therefore unlikely that this would have led to significant bias.
The use of a twin sample has the potential to introduce bias by (1) the relatedness of twins in each pair inflating correlations and (2) contrast effects, whereby parents contrast twins with differing levels of impairment and exaggerate the differences between them. We did control statistically for relatedness of twins in pairs to try to reduce bias introduced by twin similarity. It remains possible that parent rating bias could have exaggerated the difference between discordant pairs, increasing the contrast between affected and unaffected twins. However, in the case of concordant pairs of which one is less severely affected than the other, the less affected twin could be falsely rated by the parent as unaffected. Rater bias could, therefore, result in an increase or decrease in the apparent accuracy of the DAWBA and it is not clear to what extent, or in which direction, this could have acted on the results. However, the benefits of utilising this longitudinal study of a nationally representative cohort, from which results can be generalised to community health settings, are likely to outweigh the potential uncertainties in interpretation of twin data.
Although not necessarily a limitation, it is worth bearing in mind that, in many cases, the DAWBA was administered using telephone interview by RAs. This could have improved the quality of data collection beyond what might be expected if online administration had been used (e.g. interviewers may have asked for clarification or provided guidance if parents had misinterpreted questions). This does not invalidate the results, but it is possible that using an interviewer with some experience of ASD would result in better-quality data than online administration. This needs to be tested as online tools are potentially much more cost-effective than interviewers. Nonetheless, it is likely that some aspects of clinical interview will still be required to be undertaken by trained clinicians to confirm diagnosis.
Conclusions
The DAWBA performed well in this sample and has the potential to be a useful tool in community settings, where it could be used to collect a systematic developmental history and help to identify associated mental health symptoms. The DAWBA should, however, be used in conjunction with a direct observational measure (expert clinical observation or ideally a measure such as the ADOS-G). When both measures agree with each other, there can be confidence in both positive and negative results. In cases where these measures are discrepant, further assessment in more expert centres, using a tool such as the ADI-R, or through observation of behaviour in school or with peers, could help to resolve issues.
The DAWBA will not necessarily perform similarly in other populations, such as more complex cases typical of tertiary or quaternary referral services, and the DAWBA must be tested in these types of populations before any definitive recommendations can be made. Moreover, we piloted this measure in children and adolescents and we need to develop measures that may be more applicable to older populations and examine the effect of recent changes in diagnostic practice. Hence, our next three projects focused on adolescents/young adults and we aimed to (1) pilot a screening tool to help detect associated mental health symptoms, (2) investigate the effect of recent changes in DSM-V and (3) develop proof of concept for a novel brain imaging technique to aid in detecting ASD and ADHD.
Current schedules for diagnosing ASD are time-consuming, require experts and are expensive.
By contrast the DAWBA can be completed online or by untrained staff over the telephone.
In community samples the DAWBA shows promise as an alternative diagnostic tool and has acceptable sensitivity and specificity.
Chapter 16 Improving identification of associated mental health symptoms: tool development
Background
As noted previously (see Sections 1 and 2), we and others found that rates of psychiatric comorbidity are particularly high among individuals with ASD. The most prevalent psychiatric comorbidities associated with ASD are anxiety disorders, depression and ADHD, with prevalence rates of approximately 41%, 37% and 28%, respectively. 58,109,200 The presence of psychiatric comorbidity in ASD can cause significant functional impairment to the affected individual201 and result in significant burden to their caregiver. 57 Therefore, recognising co-occurring conditions is a priority for GPs and specialist ASD services. Unfortunately, comorbidity often goes unrecognised in ASD. 202 To assist in the identification of service users with ASD, clinicians need valid and reliable screening and assessment tools. Owing to the overlapping of symptoms of ASD and certain psychiatric disorders,203 and given the difficulties ASD people have in introspection and communicating their personal state,204 standard psychiatric screening tools may not be suitable for this population. To this end, several instruments have been designed specifically for children with ASD, including the Autism Comorbidity Interview201 and the Psychopathology in Autism Checklist. 205 These instruments have acceptable to good psychometric properties. However, they have not been studied across the autistic spectrum and in the case of the Autism Comorbidity Interview, rely on clinically skilled interviewers and the presence of an informant. 201
The SDQ is one of the most widely used screening tools for psychopathology in children and adolescents in the UK, as the it can be used to screen for psychopathologies that are most commonly found to be comorbid in ASD (i.e. anxiety, ADHD and depression), and because the instrument is relatively brief and can be administered to parents and youths. We considered it a good candidate to investigate its psychometric properties in a group of adolescents and young adults with ASD. Hence, we tested the internal and external validity (and temporal stability) of the SDQ in a sample of adolescents and adults with a diagnosis of ASD.
Methods
Sample
We included 145 families consisting of adolescents and adults with ASD and their parents. Families were recruited through the patient’s clinical diagnosis of ASD from CAMHS, adult clinics, charities and research databases that form part of our clinical research networks. Clinical diagnosis of autism was confirmed using the ADI-R. 27 When a parent was unavailable or unwilling to complete the ADI-R, the ADOS-G197 was used.
Measures
Strengths and Difficulties Questionnaire
The SDQ is a brief, 25-item questionnaire that can be administered as self-report, or to parents and teachers of children and adolescents. Information about the SDQ is available online (URL: www.sdqinfo.com). It covers common areas of emotional and behavioural difficulties and also asks whether or not the informant thinks that the young person has a problem in these areas. It includes five subscales (emotional symptoms, conduct problems, hyperactivity, peer problems and prosocial scales) and an impact score. Scores for each subscale are classified as ‘normal’, ‘borderline’ or ‘abnormal’. Previously validated computerised algorithms were used to predict psychiatric disorder by bringing together information on symptoms and impact from the SDQ completed by multiple informants. The algorithm makes separate predictions for three groups of disorders: (1) conduct-oppositional disorders, (2) hyperactivity–inattention disorders and (3) anxiety–depressive disorder. Each is predicted to be ‘unlikely’, ‘possible’ or ‘probable’. Predictions from these groups are combined to generate a single prediction of the presence of any psychiatric disorder. For a prediction of probable hyperactivity–inattention disorder it is essential to have evidence of pervasiveness through both parent and teacher ratings. As only parent- and self-rated SDQ were available in this study, only unlikely or possible predictions could result from these data.
Psychiatric diagnosis
As noted previously (see Chapter 15), DAWBA51 is a detailed computer-based psychiatric interview which can be completed by parents and patients. Each section of the DAWBA uses skip rules, which start with structured questions that cover the operationalised diagnostic criteria of the DSM-IV. 206 A computer algorithm uses the responses from both informants to generate probability bands for the presence of particular psychiatric morbidity. A clinical rater then reviews all information and assigns a diagnosis for each disorder. Where available, clinical notes were reviewed and the presence or absence of psychiatric disorders was recorded. A patient was considered to be at high risk of particular clinical disorders if they had been assigned a diagnosis by a DAWBA clinical rater, or if they had received a diagnosis in the clinic where they were assessed.
Screening questionnaires
In addition to the DAWBA, we assessed the external validity of the SDQ against standard screening questionnaires and diagnostic measures. Symptoms of ADHD were measured using the BAARS-IV. 207 The BAARS-IV is a commonly used, validated 18-item rating scale of DSM-IV criteria for ADHD.
Anxiety and depression were measured by the relevant subscales of the Hospital Anxiety and Depression Scale (HADS). 208 The HADS is a commonly used, 14-item screening tool for anxiety and depression. It has demonstrated excellent psychometric properties and is used routinely in clinical practice.
The Obsessive–Compulsive Inventory – Revised (OCI-R)209 was used to measure symptoms of OCD. The OCI-R is an 18-item, self-report measure with excellent psychometric properties. 209
Data analysis
Internal reliability was measured by examining the inter-rater reliability, construct validity and temporal stability of the measure. Inter-rater reliability was tested by examining the correlation (Pearson’s r) of total SDQ scores between parent and respondent. Construct validity was tested using multitrait–multimethod analysis (MTMM). MTMMs are based on producing a correlation matrix of multiple traits (e.g. the subscales of the SDQ) measured by multiple methods (e.g. parent and respondent). Construct validity is assessed through comparisons across raters and across subscales. For example, correlations between parent and respondent emotional subscale (a convergent correlation coefficient) would be expected to be higher than between parent emotional and respondent hyperactivity subscales (a discriminant correlation coefficient). Where poor convergent and discriminant correlations are observed, it is inferred that these subscales may not be tapping into the same, distinct constructs across raters. MTMM was performed using Spearman’s correlations calculated in SPSS for Windows version 20. We also present the Cronbach’s alpha for each subscale according to each rater.
Temporal stability was tested by calculating the Pearson’s correlation between baseline and follow-up for each SDQ subscale and the total SDQ score for each rater.
External validity was tested initially by examining the correlation (Spearman’s r) between SDQ subscale scores and other measures of psychiatric disorder, namely DAWBA probability bands, clinical diagnosis (coded as no, possible or definite diagnosis) and standard screening questionnaires. It was hypothesised that SDQ emotional problems would be associated with measures of anxiety disorders, depression and OCD, and that SDQ hyperactivity would be associated with measures of ADHD. Correlations were repeated, controlling for SDQ impact scores (partial r), to ensure that associations were not driven by severity of symptoms (e.g. patients with more severe ASD symptoms being rated highly across SDQ subscales).
Parent- and self-rated SDQ emotional problems and hyperactivity subscales, coded into normal, borderline and abnormal bands, were compared with risk of clinical disorder. Borderline and abnormal bands were collapsed into one category and taken to indicate higher risk. Multi-informant predictive algorithms were also used, with possible and probable categories collapsed for emotional disorders (probable category was not available for hyperactivity, see Strengths and Difficulties Questionnaire). Risk of clinical disorder was measured using DAWBA clinical rater-assigned diagnoses and evidence of diagnoses from clinical notes. Sensitivity, specificity, PPV and NPV were calculated for each rater and for the predictive algorithm. The overall percentage of individuals correctly classified and the OR was also calculated in each case.
Results
Inter-rater correlations
Pearson’s correlations between parent and respondent total SDQ scores were 0.418. This is comparable with the 0.48 inter-rater correlation between parents and youth previously reported in a large-scale epidemiological sample on the SDQ. 210
Construct validity of the Strengths and Difficulties Questionnaire subscales across informants
Table 39 presents a MTMM of the five hypothesised SDQ subscales. The Cronbach’s alpha coefficients ranged between 0.484 and 0.810. The cross-method correlations of the same traits are shaded green; all were significantly different from zero (p < 0.001) and in the moderate magnitude range (0.423–0.573).
| Parent | Respondent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emotional | Peer problems | Behavioural | Hyperactivity | Prosocial | Emotional | Peer problems | Behavioural | Hyperactivity | Prosocial | |
| Parent | ||||||||||
| Emotional | α = 0.784 | |||||||||
| Peer problems | 0.441 | α = 0.550 | ||||||||
| Behavioural | 0.358 | 0.341 | α = 0.763 | |||||||
| Hyperactivity | 0.310 | 0.225 | 0.395 | α = 0.779 | ||||||
| Prosocial | –0.144 | –0.259 | –0.447 | –0.155 | α = 0.810 | |||||
| Youth | ||||||||||
| Emotional | 0.573 | 0.293 | 0.433 | 0.077 | –0.055 | α = 0.762 | ||||
| Peer problems | 0.253 | 0.449 | –0.058 | –0.201 | –0.123 | 0.573 | α = –0.697 | |||
| Behavioural | –0.062 | –0.009 | 0.423 | 0.319 | –0.071 | 0.135 | 0.158 | α = 0.484 | ||
| Hyperactivity | 0.180 | 0.046 | 0.149 | 0.560 | –0.067 | 0.354 | 0.086 | 0.207 | α = –0.775 | |
| Prosocial | –0.018 | –0.066 | –0.098 | –0.019 | 0.469 | –0.154 | –0.182 | –0.226 | –0.134 | α = 0.734 |
In the majority of cases, the convergent correlations were significantly larger than the other correlation coefficients in the same row or column (the discriminant correlations). However, there are some notable exceptions. First, in both informant-rated pairs, the emotional problems subscale demonstrated poor discriminant validity relative to peer problems and parent-rated behavioural problems (relevant cells are in bold). Second, the youth-rated behavioural subscale did not show good discriminant validity relative to the parent-reported hyperactivity subscale and the parent-rated behavioural subscale, which did not show good discriminant validity relative to the youth-reported emotional subscale (relevant cells italicised).
The behavioural, emotional and peer problems subscales therefore demonstrate poor discriminant validity; thus, these findings suggest that the five subscales do not tap into the same, distinct aspects of the youths mental health problems across both informants. However, the three-factor (internalising–externalising–prosocial) contrast provided much better convergent and discriminant validity. Furthermore, Cronbach’s alphas for each subscale were higher on average in the three-factor model (Table 40).
| Parent | Respondent | |||||
|---|---|---|---|---|---|---|
| Internalising | Externalising | Prosocial | Internalising | Externalising | Prosocial | |
| Parent | ||||||
| Internalising | α = 0.748 | |||||
| Externalising | 0.402 | α = 0.820 | ||||
| Prosocial | –0.218 | –0.326 | α = 0.810 | |||
| Youth | ||||||
| Internalising | 0.525 | –0.016 | –0.100 | α = 0.834 | ||
| Externalising | 0.060 | 0.525 | –0.097 | 0.265 | α = 0.797 | |
| Prosocial | –0.024 | –0.097 | 0.469 | –0.189 | –0.228 | α = 0.734 |
Temporal stability
The SDQ was re-administered to parents and youths after an interval of ≈11 months (parents, n = 69; youths, n = 35). The mean retest stability was 0.64. Stability did not differ by informant (parent = 0.62; youth = 0.66) (Table 41).
| SDQ scale | Time 1 × time 2 correlations | |
|---|---|---|
| Parent (n = 69) | Respondent (n = 35) | |
| Emotional problems | 0.547 | 0.644 |
| Peer problems | 0.583 | 0.724 |
| Behavioural problems | 0.695 | 0.642 |
| Hyperactivity | 0.704 | 0.546 |
| Prosocial behaviour | 0.620 | 0.687 |
| Impact | 0.602 | 0.611 |
| Total difficulties | 0.587 | 0.735 |
External validity
Tables 42 and 43 show correlations between SDQ subscales and other measures of comorbid disorders (DAWBA probability bands, clinical diagnosis and standard screening questionnaires). Both parent- and self-report SDQ scores showed the predicted pattern of associations for emotional symptoms (correlating with anxiety disorders, OCD and depression) and hyperactivity (correlating with ADHD). This was true even when the effects of SDQ Impact scores were controlled (using partial correlations), suggesting that these associations are not being driven by severity of problems regardless of domain.
| Emotional | Behavioural | Hyperactivity | Peer problems | Prosocial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | Partial r | r | Partial r | r | Partial r | r | Partial r | r | Partial r | |
| Anxiety | ||||||||||
| DAWBA GAD | 0.73** | 0.60** | 0.30* | 0.00 | 0.09 | –0.20† | 0.38** | 0.08 | –0.15 | 0.13 |
| DAWBA specific phobia | 0.49** | 0.38** | 0.16 | –0.03 | 0.19 | 0.06 | 0.33** | 0.18 | –0.20† | –0.07 |
| DAWBA social phobia | 0.54** | 0.34** | 0.31** | 0.05 | 0.18 | –0.06 | 0.44** | 0.18 | –0.26* | –0.04 |
| DAWBA panic disorder | 0.48** | 0.27* | 0.22† | –0.04 | 0.08 | –0.16 | 0.35** | 0.09 | –0.18 | 0.04 |
| DAWBA agoraphobia | 0.53** | 0.36** | 0.18 | –0.08 | 0.10 | –0.12 | 0.21† | –0.08 | –0.23† | –0.04 |
| Clinical diagnosis | 0.40** | 0.37* | –0.10 | –0.22 | 0.01 | –0.11 | 0.21 | 0.13 | –0.14 | –0.07 |
| HADS anxiety | 0.53* | 0.54* | –0.21 | –0.35 | –0.11 | –0.21 | –0.14 | –0.33 | 0.14 | 0.16 |
| OCD | ||||||||||
| DAWBA OCD | 0.44** | 0.29* | 0.17 | –0.05 | 0.18 | 0.01 | 0.31** | 0.11 | –0.22† | –0.07 |
| Clinical diagnosis | 0.27† | 0.41** | –0.18 | –0.13 | –0.09 | –0.02 | –0.05 | 0.05 | –0.00 | –0.08 |
| OCI-R | 0.42† | 0.31 | 0.23 | 0.07 | 0.24 | 0.10 | 0.07 | –0.22 | 0.25 | 0.31 |
| Depression | ||||||||||
| DAWBA depression | 0.58** | 0.40** | 0.28* | –0.00 | 0.21† | –0.02 | 0.26* | –0.08 | –0.25* | –0.02 |
| Clinical diagnosis | 0.10 | 0.20 | –0.22 | –0.17 | 0.03 | 0.13 | –0.09 | 0.00 | –0.10 | –0.19 |
| HADS depression | 0.47* | 0.36 | –0.16 | –0.47† | –0.23 | –0.51* | 0.21 | –0.06 | –0.05 | –0.00 |
| ADHD | ||||||||||
| DAWBA ADHD | 0.09 | –0.25* | 0.30* | 0.07 | 0.77** | 0.72** | 0.09 | –0.24* | –0.25* | –0.06 |
| Clinical diagnosis | –0.10 | –0.28† | 0.32* | 0.24 | 0.50** | 0.46** | 0.26† | 0.14 | –0.10 | 0.01 |
| BAARS-IV current inattentive (parent) | 0.03 | –0.29 | 0.51* | 0.34 | 0.47* | 0.30 | 0.54* | 0.32 | –0.07 | –0.01 |
| BAARS-IV current hyperactive/impulsive (parent) | –0.13 | –0.28 | 0.36 | 0.30 | 0.55* | 0.53* | 0.47* | 0.46† | 0.26 | 0.29 |
| BAARS-IV current inattentive (self) | –0.08 | –0.33 | 0.39† | 0.24 | 0.32 | 0.17 | 0.34 | 0.13 | –0.21 | –0.18 |
| BAARS-IV current hyperactive/impulsive (self) | –0.34 | –0.56* | 0.32 | 0.22 | 0.31 | 0.23 | 0.41† | 0.34 | 0.28 | 0.33 |
| Emotional | Behavioural | Hyperactivity | Peer problems | Prosocial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | Partial r | r | Partial r | r | Partial r | r | Partial r | r | Partial r | |
| Anxiety | ||||||||||
| DAWBA GAD | 0.66** | 0.52** | 0.09 | –0.08 | 0.34** | 0.20† | 0.33** | 0.14 | 0.01 | –0.06 |
| DAWBA specific phobia | 0.41** | 0.27* | 0.20† | 0.11 | 0.32** | 0.23† | 0.26* | 0.13 | 0.08 | 0.04 |
| DAWBA social phobia | 0.55** | 0.36** | 0.15 | –0.00 | 0.26* | 0.11 | 0.45** | 0.29* | –0.18 | –0.28* |
| DAWBA panic disorder | 0.40** | 0.25* | 0.10 | –0.01 | 0.29* | 0.19 | 0.20† | 0.05 | –0.04 | –0.08 |
| DAWBA agoraphobia | 0.44** | 0.36** | 0.01 | –0.09 | 0.26* | 0.18 | 0.34** | 0.24* | –0.17 | –0.22† |
| Clinical diagnosis | 0.18 | 0.17 | 0.18 | 0.17 | –0.02 | –0.05 | 0.25† | 0.26† | –0.20 | –0.20 |
| HADS anxiety | 0.24 | 0.15 | –0.03 | –0.06 | –0.11 | –0.12 | 0.10 | –0.03 | 0.26 | 0.19 |
| OCD | ||||||||||
| DAWBA OCD | 0.49** | 0.31** | 0.24* | 0.12 | 0.27* | 0.14 | 0.35** | 0.19 | –0.10 | –0.17 |
| Clinical diagnosis | 0.09 | 0.32* | –0.01 | 0.03 | –0.09 | –0.04 | 0.14 | 0.37* | –0.10 | –0.10 |
| OCI-R | 0.52* | 0.38 | 0.37 | 0.33 | 0.27 | 0.27 | 0.07 | –0.28 | 0.31 | 0.14 |
| Depression | ||||||||||
| DAWBA depression | 0.53** | 0.22 † | 0.19 | –0.01 | 0.20† | –0.03 | 0.43** | 0.20† | 0.09 | 0.02 |
| Clinical diagnosis | –0.03 | 0.18 | –0.03 | 0.02 | –0.10 | –0.04 | –0.07 | 0.10 | –0.15 | –0.15 |
| HADS depression | 0.35 | 0.33 | –0.47* | –0.50* | –0.39† | –0.40† | 0.45† | 0.49* | 0.10 | 0.05 |
| ADHD | ||||||||||
| DAWBA ADHD | 0.15 | 0.05 | 0.23† | 0.19 | 0.44** | 0.41** | –0.12 | –0.23† | –0.02 | –0.04 |
| Clinical diagnosis | –0.03 | 0.16 | 0.08 | 0.13 | 0.42** | 0.51** | –0.29† | –0.23 | –0.17 | –0.18 |
| BAARS-IV current inattentive (parent) | 0.12 | 0.08 | 0.17 | 0.15 | 0.30 | 0.30 | 0.19 | 0.17 | 0.29 | 0.29 |
| BAARS-IV current hyperactive/impulsive (parent) | 0.02 | 0.06 | 0.28 | 0.30 | 0.35 | 0.35 | 0.16 | 0.25 | 0.33 | 0.40 |
| BAARS-IV current inattentive (self) | 0.06 | –0.06 | 0.24 | 0.22 | 0.45 † | 0.44 † | 0.11 | –0.01 | –0.03 | –0.14 |
| BAARS-IV current hyperactive/impulsive (self) | 0.17 | 0.01 | 0.23 | 0.19 | 0.45 † | 0.45 † | 0.31 | 0.17 | 0.00 | –0.17 |
Parent- and self-rated SDQ emotional problems and hyperactivity subscales, coded into normal, borderline and abnormal bands, were then compared with risk of clinical disorder.
Emotional disorder
Figure 17 shows ratings for SDQ emotional problems or disorder in those at high and low risk of clinical diagnosis of any emotional disorder. For both parent- and self-rated emotional problems, more than half of those rated as borderline or abnormal were at high risk of emotional disorder and the majority of those rated as normal were at low risk of emotional disorder. Parents were more likely to give abnormal ratings, resulting in more true and false positives, and patients were more likely to rate themselves as normal, resulting in more true and false negatives.
FIGURE 17.
Strengths and Difficulties Questionnaire emotional problems (parent and self-rated) and emotional disorder (multi-informant predictive algorithm) scores in patients at high or low risk of clinical diagnosis of any emotional disorder.

Table 44 shows the sensitivity, specificity, PPV and NPV of each SDQ subscale in predicting risk of relevant clinical disorders. Parent-rated emotional problems had high sensitivity (90% of cases would have been detected), whereas only around half (55%) of cases would have been detected using self-ratings. The PPV showed that 60–70% of cases identified were true positives and 30–40% were false positives. The NPV showed that 60–75% of normal ratings reflected low risk of disorder, whereas 25–40% represented missed cases. The odds of clinical disorder were significantly elevated in those identified using the SDQ, using parent or self-ratings. The multi-informant predictive algorithm performed similarly to parent-rated SDQ, with only slightly improved ability to correctly identify risk of clinical disorder.
| SDQ subscale | Sensitivity | Specificity | PPV | NPV | % correctly classified | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Emotional problems | |||||||
| Parent | 0.90 | 0.32 | 0.59 | 0.75 | 62 | 4.32 | 1.25 to 14.95 |
| Self | 0.55 | 0.70 | 0.67 | 0.59 | 62 | 2.89 | 1.13 to 7.40 |
| Emotional disorder | |||||||
| Multi-informant | 0.90 | 0.41 | 0.62 | 0.79 | 66 | 6.14 | 1.81 to 20.87 |
| Hyperactivity | |||||||
| Parent | 0.86 | 0.62 | 0.65 | 0.84 | 73 | 9.75 | 3.14 to 30.28 |
| Self | 0.60 | 0.67 | 0.60 | 0.67 | 64 | 3.00 | 1.18 to 7.62 |
| Hyperactivity disorder | |||||||
| Multi-informant | 0.86 | 0.64 | 0.67 | 0.84 | 74 | 10.80 | 3.46 to 33.70 |
Hyperactivity
A similar pattern was observed for SDQ hyperactivity. Figure 18 and Table 44 show that the majority (60–65%) of those with borderline or abnormal ratings were at high risk of ADHD and the majority (67–84%) of those with normal ratings were at low risk. ORs were significant for parent ratings, self-ratings and multi-informant predictive algorithm. Parent ratings detected more cases (86%) than self-ratings (60%).
FIGURE 18.
Strengths and Difficulties Questionnaire hyperactivity (parent and self-rated) and hyperactivity disorder (multi-informant predictive algorithm) scores in patients at high or low risk of clinical diagnosis of any type of ADHD/hyperkinesis. NB. Probable band for multi-informant predictive algorithm requires evidence of pervasiveness across settings from parent and teacher SDQ, which was not available in this sample.
Discussion
The psychometric properties of the SDQ were assessed in a sample of adolescents and adults with an ASD. The findings confirm that the SDQ is satisfactorily reliable and valid in this population.
We assessed the reliability of the SDQ by assessing inter-rater agreement, internal consistency and temporal stability. Inter-rater agreement was satisfactory and comparable to the agreement reported in other studies of non-ASD populations using the SDQ. The internal consistencies were reported for each subscale of the five-factor model and the three-factor internalising-externalising model. Internal consistency was generally satisfactory across all subscales. Test–retest reliability is usually measured by re-administering the assessment after a brief interval of approximately 1–4 weeks. In the current study, retest was not carried out until on average 12 months after initial assessment. Therefore, changes in the score may reflect genuine alterations in the respondent’s psychological adjustment as well as from poor measurement reliability. Nevertheless, we report a satisfactory lower bound for true test–retest reliability, which did not seem to differ between rater.
Using a MTMM of the parent and respondent SDQ, we explored the construct validity of the subscales in the two models. The convergent validity coefficients of 0.42–0.57 are higher than those previously reported in a non-ASD sample. Within the five-factor model, we found poor discriminant validity between the emotional subscale and the peer problems and behavioural subscales. The behavioural subscale also showed poor discriminant validity relative to the hyperactivity subscale. Consequently, the labels given to these four subscales (emotional, peer problems, behavioural and hyperactivity) may be misleading, as they cannot be assumed to tap into distinct aspects of psychopathology. Interestingly, despite the association between the behavioural subscale (a component of the externalising subscale in the three-factor model) and the emotional subscale (a component of the internalising subscale), the three-factor model contrasts provided much better discriminant validity.
Good external validity was demonstrated for the emotional problems and hyperactivity subscales. SDQ emotional problems scores correlated with a variety of anxiety disorders, OCD and depression. Similarly, SDQ hyperactivity scores correlated with ADHD. Emotional disorders and ADHD were measured using a variety of methods – DAWBA, standard screening questionnaires and clinical diagnosis – suggesting that the associations with the SDQ are robust and not entirely attributable to common method variance (i.e. SDQ and other questionnaires being completed by the same rater). For example, parent SDQ emotional problems correlated with HADS (anxiety), a self-report questionnaire and with diagnosis assigned by clinician. There was some evidence that common method variance was a factor in hyperactivity: parent SDQ correlated with parent- but not self-rated BAARS-IV scales, and self-rated SDQ correlated with self- but not parent-rated BAARS-IV scales. However, both parent- and self-rated SDQ hyperactivity scores correlated strongly with clinical diagnosis, a more objective measure of ADHD. Using SDQ bands validated in child samples and taking borderline and abnormal scores to indicate risk of disorder, was a reasonably effective way of identifying comorbid emotional disorders and ADHD, at least using parent-rated SDQ (around 90% of cases were identified). Sensitivity was lower using self-rated SDQ (55–60% of cases identified), which suggests that a significant proportion of adults with ASD under-report these difficulties. On this basis, it would be advisable to seek a parent-rated SDQ, when possible, in order to detect the majority of cases of comorbid emotional disorders and ADHD. However, it is important to note that around 4 in 10 cases identified by parents were false positives. The SDQ should be used to identify those in need of further assessment and should not be taken as confirmation of the presence of comorbidity.
The pattern of correlations suggests that the emotional and hyperactivity subscales are fairly specific to these difficulties in adults with ASD: there was no association between SDQ emotional problems and ADHD measures, or between SDQ hyperactivity and emotional disorders measures. Additionally, there were few associations between other SDQ subscales and measures of emotional disorders or ADHD. A notable exception was self-rated peer problems, which correlated with some measures of anxiety, OCD and depression. It may be useful to use self-rated peer problems as an additional indicator of possible emotional disorder, especially if a parent-rated SDQ is not available.
Using the multi-informant predictive algorithm did not markedly improve detection of comorbidities over parent-rated SDQ. In this sample, there is little justification for combining parent- and self-rated SDQ and impact scores using a statistical package. In practice, it may be sufficient, and indeed easier, to use a paper version of the parent SDQ and the published subscale bands.
Older adolescents and adults with ASD have a high prevalence of associated mental health symptoms.
Currently there are no instruments to help identify these comorbid conditions in adults with ASD.
We provide proof of concept that a modified form of the SDQ is an acceptable screening tool for comorbid psychopathology. It can help identify individuals that require more in-depth assessment.
Strengths and weaknesses
The strengths of this study include our comparison of the SDQ with the some of the most widely used screening tools for psychopathology in the UK (BAARS-IV, HADS and the OCI-R). In addition, we compared the SDQ against clinical diagnoses assigned by chartered psychiatrists.
However, our work also has limitations. Our sample primarily consisted of high-functioning individuals, which is not representative of ASD as a whole. For example, approximately 8% of our sample was diagnosed with a LD, in comparison with an estimated prevalence of 56%. 211 Thus, we can generalise our findings only to higher-functioning adults with ASD.
Data used to test external validity were limited in various ways. For example, it was clear from clinical notes that, despite having evidence of comorbid emotional disorder or hyperactivity, some patients had not received further assessment. This means that the absence of clinical diagnosis in these patients did not necessarily reflect the absence of disorder. Furthermore, in some patients the time gap between assessment with the SDQ and with other measures (screening questionnaires, clinical assessment) was relatively long. Consequently, disagreement between the SDQ and other measures may have reflected genuine change in mental state rather than poor performance of the SDQ. Despite these limitations of the clinical data, a consistent pattern of significant associations emerged across measures. Indeed, it is possible that these associations would have been stronger with better-quality data.
Conclusions
The SDQ appears to be a valid way to screen for comorbid anxiety disorders, depression and ADHD in adults with ASD. We suggest that if the screen is used then adults with higher scores on the emotional problems or hyperactivity subscale (using either the parent or self-report version of the SDQ) should be offered further expert assessment. However, the SDQ may not perform similarly in other populations, such as more complex cases or individuals with comorbid ID. Furthermore, it is possible that changes in the diagnostic criteria for ASD (explored in the next study) may have implications for the performance of the SDQ in the new diagnostic categories suggested in DSM-V. In our next work stream, we carried out an initial investigation of the impact that changes in DSM-V have on diagnostic rates of ASD at the transition.
Chapter 17 Improving outcomes through better diagnosis: the effects of changes in DSM-V on clinical diagnosis
Introduction
Autism spectrum disorder is a ND with a prevalence currently estimated at 1 in 80 individuals. 212 In recent years there has been a rise in reported rates of ASD. The reason for this is unclear, but changes in diagnostic practice are likely to have contributed. 213 In addition, a formal diagnosis of an ASD is often used as a ‘gatekeeper’ for services and support. Therefore, changes in diagnostic practice may have important implications – both for clinical prevalence rates and for an individual’s care options.
Autism spectrum disorder is diagnosed on the basis of three domains: (1) impaired social interaction, (2) abnormal communication and (3) restricted and repetitive behaviours and interests. Using the current diagnostic criteria in the ICD-10214 and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),206 ASD comes under the umbrella term of PDD. An individual may be defined as having one of four diagnostic subtypes according to the range of symptoms and the presence/absence of factors such as developmental language delay and ID (i.e. Asperger syndrome, childhood autism/autistic disorder, atypical autism, PDD-unspecified). There are, however, problems with current diagnostic algorithms. These include a lack of evidence for significant differences between ASD subtypes (once IQ matched) in aetiology, neuropsychological profile, treatment or outcome and poor clinical agreement when diagnosing. 215 Hence, the Neurodevelopmental Disorders Workgroup, convened by the American Psychiatric Association, has proposed a number of significant changes to the diagnostic criteria for ASD. 216,217 These include collapsing previously distinct diagnostic subtypes into a single category of ASD, and reducing the current ‘triad of impairments’ to two domains – by combining the social and communication impairment criteria into a single set (as almost any example of communication is social and vice versa). Furthermore, people who do not present with the full range of symptoms will no longer be eligible for an ASD diagnosis, as there is no ‘atypical’ or ‘not otherwise specified’ category (as in ICD-10, DSM-IV). Instead, a new diagnostic category called social communication disorder (SCD) has been proposed. This is defined as being outside the autism spectrum, but will provide ‘diagnostic coverage’ for those individuals with symptoms in the social-communication domain, but who have never displayed repetitive, restricted behaviours or interests.
The effect of the proposed changes on diagnostic outcomes has been investigated in children and adolescents, with several studies reporting that the specificity of the proposed DSM-V criteria is good, but sensitivity is relatively poor, when judged against current ICD-10 or DSM-IV-TR criteria. 218–223 This highlights a key concern of some, that the new criteria will fail to capture individuals currently receiving an ASD diagnosis who are on the ‘broader spectrum’ according to DSM-IV-TR or ICD-10 criteria (e.g. PDD – not otherwise specified). As a consequence, it is feared by some that these individuals will be denied access to services. Reassuringly, however, a large study of children diagnosed within the PDD category according to DSM-IV-TR suggested that sensitivity of DSM-V is very good (0.91), although sensitivity in this study was much lower (0.53). 224
The effect of the proposed changes for adults has received relatively little attention. This is of importance because ASD is a lifelong condition; most people with ASD are adults and an increasing number of individuals are presenting for first diagnosis in adulthood. 225 Furthermore, diagnosis is particularly challenging in this group because a developmental history is often unavailable and/or unreliable and presentation is frequently complicated by additional mental health conditions. 226 The only prior study that explored the agreement between current and proposed ASD criteria in adults included only individuals with (mostly profound) ID living in residential centres;219 they reported that approximately one-third of the individuals who met DSM-IV-TR criteria no longer met them using the draft DSM-V. This study was a valuable first step. However, the majority of the ASD population does not have profound intellectual impairment227 and such people are assessed within mental health or social/educational services. In addition, it is unknown what proportion of individuals would qualify for the new, alternative diagnosis of SCD.
Our primary aim, therefore, was to investigate how diagnostic outcomes of the DSM-V algorithm differed from both ICD-10 and the DSM-IV-TR when applied in a clinical health service; and to compare all three algorithms to so-called ‘gold standard’ research diagnostic assessment tools (the ADI-R27 and ADOS-G197). Our secondary aims were to investigate whether or not diagnostic outcomes were affected by participant characteristics (age, sex, IQ, presence of additional mental health conditions), or alterations to the formulation of the proposed algorithm. Specifically, we examined the impact of reducing the number of criteria required for a formal diagnosis and also the treatment of criteria where the clinician was uncertain or had insufficient information to code the item.
Method
Participants
Participants included 158 individuals consecutively assessed for an ASD diagnosis in a specialist national tertiary ASD diagnostic clinic for adults between January and May 2011. The clinic is situated within the South London and Maudsley NHS Foundation Trust. People are typically referred by their local family physician/GP or consultant psychiatrist for a second opinion. In eight cases diagnosis was inconclusive due to a history of acquired head injury or the presence of severe psychotic symptoms during assessment. Data from these cases were excluded from the study. The remaining 150 participants were aged 18–65 years, with a mean age of 31 years. There were 110 males (mean age 32 years) and 40 females (mean age 31 years). Seventy-three patients already had a diagnosis of a mental health condition, although only seven of these had previously been diagnosed with ASD.
Clinical assessment
Assessment included a detailed psychiatric assessment using ICD-10 research diagnostic criteria and, when possible (i.e. when parents were available, able and willing), an ADI-R. In the event that no parent was available for the ADI-R, we asked the person seeking diagnosis to undergo the ADOS-G. In some cases both assessment tools were required to gather enough relevant information. Seventy-one individuals were assessed using the ADI-R, 62 were assessed with the ADOS-G and 17 were assessed using both the ADI-R and the ADOS-G.
All information obtained was compiled by the multidisciplinary clinical team – a consultant psychiatrist, a junior doctor and an ADI-R/ADOS-G administrator (nurse or psychologist) – who together decided whether or not each criterion on the ICD-10 algorithm was fulfilled. If a patient met the full ICD-10 criteria (a total of at least six symptoms must be present – either currently or by history – with at least two from the first domain and one from each of the second and third domains) and the symptoms were noted before the age of 3 years, they were diagnosed with childhood autism (if they exhibited a language delay) or Asperger syndrome (if there was no evidence of a language delay). In line with ICD-10 guidelines, if a patient exhibited some autistic symptoms but did not meet full ICD-10 diagnostic criteria they were diagnosed with PDD, unspecified (PDD-unspecified), or atypical autism. For the purposes of this study these two subthreshold diagnostic groups were collapsed into a single ‘PDD-unspecified’ group. In some cases, it was not possible to decide confidently whether or not a symptom was present due to a lack of information, or because information obtained from patient and parent was contradictory. In this event the criterion was coded as ‘unclear’ and the team made the clinical diagnostic decision based on the gestalt of the information received. Of the 150 consecutive assessments, 113 were diagnosed with an ASD using ICD-10 criteria. Of these, 28 participants were subtyped as having childhood autism, 48 Asperger syndrome and 37 PDD-unspecified.
Additional mental health conditions were also diagnosed in accordance with the ICD-10 (with the exception of adult ADHD which, in keeping with UK guidelines, was assessed using DSM-IV-TR). Supplementary self-report questionnaires were used to help inform the assessment of OCD, ADHD, depression and anxiety. These were (respectively) the OCI-R,209 the Barkley Current and Childhood Symptom Scales44 and the HADS. 208
Where there was clinical suspicion that a participant might have ID (F70–73 in ICD-10) or a significant lacuna in a neuropsychological function, they were referred for testing of general intellectual and executive functioning. These participants (n = 35) were tested using the Wechsler Adult Intelligence Scale-III,228 which revealed a mean performance IQ of 87 (SD 16, range 55–132) and a mean verbal IQ of 95 (SD 18, range 60–133). Mean full-scale IQ could not be calculated in 16 participants due to large discrepancies between performance IQ and verbal IQ. In the remaining 19 participants mean full-scale IQ was 90 (SD 17, range 53–129) and only two participants had an IQ of < 70. The remaining 115 participants, not referred for such testing, were estimated to be in the normal range of general intellectual function based on education attainment, employment and informant report.
Procedure
For each participant the diagnostic outcome (ASD/not ASD; ICD-10 subtype of ASD; ADI-R and ADOS-G scores; presence of additional mental health problems), was reviewed by the research team. Information from the ICD-10 algorithm was used to determine whether or not each criterion on the proposed DSM-V algorithm would be satisfied and this was supplemented by anonymised reports from the ADI-R, ADOS-G and the psychiatric interview. Each criterion could be coded as ‘yes’, ‘no’ or ‘unclear’. A participant was considered to meet criteria for ASD on the DSM-V only when all three criteria in ‘A’ and at least two out of four criteria in ‘B’ were coded as ‘yes’, as suggested in the criteria last posted by the American Psychiatric Association. If a participant did not meet criteria for ASD on the DSM-V it was determined whether or not they would meet criteria for the alternative diagnosis of SCD (using last posted draft criteria). Recoding was completed by pairs of researchers; and for 40 sets of participant data the recoding was reviewed at consensus meetings with the whole team (10 researchers) to ensure agreement.
Data were also recoded to complete the DSM-IV-TR algorithm, on which participants could be diagnosed as either ‘ASD’ (autistic disorder/Asperger syndrome) if they fulfilled at least six criteria, with at least two in domain A and one from each of domains B and C, or ‘not ASD’.
Factors affecting agreement between ICD-10, DSM-IV-TR and DSM-V were also investigated. Participant characteristics (age, sex, IQ, additional mental health conditions) were compared between ASD-positive and ASD-negative groups. The effect of altering the DSM-V algorithm was examined in two ways: (1) by relaxing the number of criterion required for diagnosis and (2) by considering criteria that were coded as ‘unclear’ as either met or not met. To examine the effect of relaxing the number of criteria required for diagnosis we reduced the thresholds in criteria A (from three to two), or in criteria B (from two to one), or both. To examine the effect of including or excluding the ‘unclear’ items we recoded the DSM-V algorithm by considering ‘unclear’ items to be present. This was relevant because the DSM-V allows criteria to be met by history and allowing or disallowing the ‘unclear’ criteria is likely to be crucial in many adult cases where multiple informants are not available.
We hypothesised that (1) prevalence of childhood autism or Asperger syndrome diagnosed using ICD-10 criteria would be similar to that using DSM-IV-TR and DSM-V, (2) most participants diagnosed with ASD on the ICD-10 but not the DSM-V would be diagnosed with SCD and (3) altering the DSM-V algorithm would have significant effects on the rate of positive DSM-V ASD diagnosis.
Results
Conclusions of initial diagnostic assessments
ICD-10 versus DSM-V
Of the 150 participants, 113 (75%) met criteria for an ASD according to the ICD-10 (childhood autism, Asperger syndrome or PDD-unspecified). In contrast, however, according to the DSM-V, only 63 (42%) met ASD criteria: this was a highly significant decline, χ2 = 35.57; p < 0.001. A further 21 (14%) participants met DSM-V criteria for SCD. Overall, therefore, of those individuals positive for ASD on ICD-10, 74% (84/113) met criteria for ASD or SCD on DSM-V. Nevertheless, the proportion of individuals with no diagnosis at all (ASD or SCD) remained significantly higher when applying the DSM-V criteria instead of ICD-10 (χ2 = 62.5; p < 0.001). None of the participants who was ASD negative using ICD-10 met diagnostic criteria for ASD (or SCD) according to the DSM-V, thus specificity of the DSM-V was 100% (Table 45).
| Diagnostic assessment | Above/below ASD threshold, % (n) | Diagnosis of below ASD threshold participants, % (n) | ||
|---|---|---|---|---|
| ASD full threshold | Below ASD threshold | PDD-unspecified or SCD | No diagnosis | |
| ICD-10 | 51 (76) | 50 (74) | 25 (37) | 25 (37) |
| DSM-IV-TR | 53 (80) | 47 (70) | n/a | n/a |
| DSM-V | 42 (63) | 58 (87) | 14 (21) | 44 (66) |
DSM-IV-TR versus DSM-V
The rate of ASD-positive diagnosis using DSM-V (42%) was also significantly lower than the rate of autistic disorder or Asperger syndrome assessed using DSM-IV-TR (53%), χ2 > 82.5; p < 0.001. In addition, two individuals were diagnosed with ASD on the DSM-V, but not with autistic disorder or Asperger syndrome on the DSM-IV-TR; therefore, the specificity of the DSM-V according to the DSM-IV-TR was 0.97 (see Table 45).
Agreement with Autism Diagnostic Interview – Revised and Autism Diagnostic Observation Schedule – Generic results
Agreement between diagnosis according to the ICD-10, DSM-IV-TR, DSM-V and outcomes of the ADI-R and ADOS-G was calculated when this information was available. When measured against results of the ADI-R, the sensitivity of the ICD-10 and the DSM-IV-TR was higher than the DSM-V (respectively, 0.97, 0.97 and 0.79; both McNemar’s p = 0.07), but their specificity was marginally lower. For the ADOS-G, sensitivity was also higher on the ICD-10 and the DSM-IV-TR than on the DSM-V (respectively, 0.6, 0.7 and 0.5; McNemar’s p = 0.07/0.01) and specificity was very similar (Table 46).
| Diagnostic assessment | ADI-R below cut-off score, % (n)a | ADI-R above cut-off score, % (n)a | ADOS-G below cut-off score, % (n)b | ADOS-G above cut-off score, % (n)b |
|---|---|---|---|---|
| ICD-10: below ASD threshold (not ASD/PDD-unspecified) | 96 (27) | 4 (1) | 75 (35) | 25 (15) |
| ICD-10: ASD (Asperger syndrome/childhood autism) | 36 (17) | 64 (30) | 32 (12) | 68 (25) |
| ICD-10 | Sensitivity 0.97;c specificity 0.61 | Sensitivity 0.63;c specificity 0.74 | ||
| DSM-IV-TR: ASD negative | 96 (27) | 4 (1) | 75 (36) | 25 (12) |
| DSM-IV-TR: ASD positive | 36 (17) | 64 (30) | 28 (11) | 71 (28) |
| DSM-IV-TR | Sensitivity 0.97;c specificity 0.61 | Sensitivity 0.70;d specificity 0.77 | ||
| DSM-V: ASD negative | 83 (29) | 17 (6) | 64 (36) | 36 (20) |
| DSM-V: ASD positive | 38 (15) | 63 (25) | 36 (11) | 65 (20) |
| DSM-V | Sensitivity 0.81; specificity 0.66 | Sensitivity 0.50; specificity 0.77 | ||
Factors affecting agreement between ICD-10 and DSM-V
Participant characteristics: age, sex, intelligence quotient, diagnostic subtype and additional mental health conditions
Among the participants who were ASD positive using the ICD-10, there were no differences with respect to age, sex or IQ (where available) between those individuals who were ASD positive and those who were ASD negative on the DSM-V.
There was, however, a significant difference in rate of DSM-V ASD-positive diagnosis between ICD-10 subtypes (χ2 = 31.58; p < 0.001). Significantly more participants diagnosed with ICD-10 childhood autism or Asperger syndrome met DSM-V criteria for ASD than those in the PDD-unspecified group (Table 47, column 1). The difference between ICD-10 defined childhood autism and Asperger syndrome was not significant (χ2 = 1.64; p = 0.2).
| ICD-10/DSM-IV-TR diagnosis | A | B | C | ||
|---|---|---|---|---|---|
| DSM-V ASD | DSM-V SCD | DSM-V ASD: relax A | DSM-V ASD: relax B | DSM-V ASD: relax A and B | |
| ICD-10, % (n) | |||||
| Not ASD (total n = 37) | 0 | 0 | 0 | 0 | 0 |
| ASD (total n = 113) | 56 (63) | 19 (21) | 69 (78)a | 70 (79)a | 87 (98)a |
| Childhood autism (total n = 28) | 82 (23) | 11 (3) | 86 (24) | 93 (26) | 96 (27) |
| Asperger syndrome (total n = 48) | 69 (33) | 15 (7) | 83 (40)b | 83 (40)b | 98 (47)a |
| PDD-unspecified (total n = 37) | 19 (7) | 30 (11) | 38 (14)b | 35 (13)b | 65 (24)a |
| DSM-IV-TR, % (n) | |||||
| Not ASD (total n = 70) | 3 (2) | 13 (9) | 14 (10)b | 10 (7)b | 27 (19)a |
| ASD (total n = 80) | 77 (61) | 15 (12) | 85 (68)b | 90 (72)a | 99 (79)a |
With respect to additional mental health conditions, a significant difference was found only for OCD: higher rates of OCD were found in the group that were ASD positive on the DSM-V than in the group that were ASD positive only on the ICD-10 (χ2 = 4.58; p = 0.03; Figure 19).
FIGURE 19.
Participants meeting criteria for mental health conditions. *p < 0.05, **p < 0.01. ‘None’, no additional/alternative mental health condition.

Effects of relaxing the DSM-V algorithm
For the 113 participants who were ASD positive on the ICD-10, the sensitivity of the DSM-V increased significantly (and specificity remained at 100%) when thresholds for criteria A or B, or both, were relaxed (all χ2 > 20.0; p < 0.001) (see Table 47, column C).
For the 80 participants who were ASD positive on the DSM-IV-TR, the sensitivity of the DSM-V increased to 99% when thresholds A and B were both relaxed; however, specificity was significantly reduced.
Uncertainty on the DSM-V algorithm: ASD diagnostic outcome when criteria coded as ‘unclear’ were considered to be present
Of the participants diagnosed with ASD on the ICD-10, 74% received a diagnosis of ASD on the DSM-V when criteria that were ‘unclear’ were treated as ‘yes’; this was a significant increase from 56% when ‘unclear’ was coded as ‘no’ (χ2 = 51.46; p < 0.001). Specificity remained at 100%.
Discussion
This is the first study to investigate how the DSM-V criteria for ASD might perform in a specialist clinical health service diagnostic clinic for adults without significant ID – who form a large proportion of individuals with ASD.
Our findings suggest that the specificity of the DSM-V criteria, as compared with the currently used ICD-10 and DSM-IV-TR criteria, is good. However, sensitivity is relatively poor. For instance, 44% of the participants who received a diagnosis of an ASD according to ICD-10 did not meet DSM-V criteria. Similarly, 22% of the individuals who met criteria for Asperger syndrome or autistic disorder on DSM-IV-TR would not qualify for a DSM-V diagnosis of ASD. This is of concern, as these individuals, who have genuine difficulties and are most likely on the ‘spectrum’, may not be able to access services available to the ASD population if the DSM-V is used to define eligibility (i.e. if it is used as an ‘entry criterion’).
The decline in sensitivity was highlighted when performance of each diagnostic algorithm was measured against results of so-called ‘gold standard’ assessment tools. Both the ICD-10 and DSM-IV-TR had a 97% chance of reporting a true ASD-positive diagnosis according to the outcome or the ADI-R; and this fell to 81% when using the DSM-V (a marginally significant decline). The chance of reporting a true negative, however, was slightly better when using the DSM-V than when using current diagnostic algorithms. Comparison with the ADOS-G revealed similar results, although there was a lower chance of true positives across all algorithms. This is potentially problematic because poor agreement between different diagnostic algorithms and research assessment tools may lead to confusion in both research and clinical settings; revisions of the assessment tools may therefore be required for use in adult populations.
Given the evidence that a significant proportion of individuals currently considered on the autism spectrum will not be included in the DSM-V ASD category, it is important to clarify what factors are associated with the likelihood that an individual will meet the criteria. Encouragingly, there was no evidence of an effect of age, sex or IQ on diagnostic outcome, suggesting that no particular demographic is more or less likely to receive an ASD diagnosis. This runs counter to some concerns that higher-functioning children (those with Asperger syndrome) are more likely to be missed by the proposed DSM-V criteria than lower-functioning children (those with autistic disorder). 221
In the current study, the difference in rate of DSM-V ASD-positive diagnosis between the ICD-10 subtypes of childhood autism and Asperger syndrome was non-significant. This suggests that adults with an Asperger syndrome diagnosis will be at no greater risk of missing out on an ASD diagnosis when using DSM-V than adults with childhood autism, which perhaps supports the DSM-V proposal to combine these diagnoses into a single category. However, the third ICD-10 subtype – PDD-unspecified – had a significantly lower rate of ASD diagnosis using DSM-V than both of the other two diagnostic subtypes. This is not necessarily cause for concern: people with a PDD-unspecified diagnosis did not actually meet full diagnostic criteria on the ICD-10 either; instead they showed significant ASD traits and were considered to be on the ‘spectrum’. What is perhaps of concern is that one-quarter of people with an ICD-10 ASD diagnosis would not qualify for either ASD or SCD on the DSM-V, and the majority of those affected were of the PDD-unspecified subtype. This is the first study to report the proportion of people who would qualify for the new SCD diagnosis as currently drafted, but our results suggest this alternative category, which was intended to provide diagnostic coverage to many of those who will not qualify for the ASD diagnosis, may not solve the problem of the comparatively poor sensitivity of the DSM-V relative to ICD-10.
The present data suggest that the sensitivity of the draft DSM-V criteria, compared at least with ICD-10 and DSM-IV-TR, can be improved. Several authors have suggested that increased sensitivity without reduced specificity might be achieved by relaxing the proposed criteria. 218,221,229 Our results supported this. We found that relaxing thresholds in DSM-V for social communication and social interaction (criteria A), and/or repetitive patterns of behaviour, interests and activities (criteria B), allowed the inclusion of almost all people currently diagnosed with childhood autism or Asperger syndrome, and the majority of those with PDD-unspecified, while maintaining specificity. Therefore, relaxing one criterion, or both criteria, is likely to allow the inclusion of more people currently considered to have ASD using ICD-10 and DSM-IV-TR, without weakening the boundaries between ASD and non-ASD. In addition, clear guidance on how to deal with uncertainty in the DSM-V classification system will be particularly important; the latest drafts of the DSM-V criteria allow criteria to be met by history or current state, which may help where information is missing or uncertain. Rating criteria that were unclear as present versus absent had significant effects on the rates of DSM-V ASD diagnosis.
Strengths and weaknesses
Although this is the first study to examine the draft DSM-V criteria in able adults with ASD, some limitations should be noted. Like other studies comparing existing criteria with the DSM-V draft, we analysed existing clinical notes and instruments that were used primarily for allocating current (ICD-10) diagnostic categories. It remains to be seen whether or not using the DSM-V criteria in the clinic during assessment in a prospective fashion would result in different findings. For example, sensory sensitivities are not part of current clinical criteria and might be more thoroughly assessed in future in clinics where DSM-V criteria are adopted. Nonetheless, one potentially useful feature of the present study, in contrast to many others, is the relatively large number of participants (25%) who were assessed for ASD but found to be negative using current criteria. This allowed examination of specificity of the different diagnostic measures. Finally, it should be noted, of course, that it remains unclear whether or not existing ICD-10 or DSM-IV-TR criteria should be considered the ‘gold standard’ against which new criteria are compared; in the absence of biomarkers, the exact definition of ASD and its boundaries remains a matter for debate.
Conclusions
The specificity of the DSM-V criteria, as compared with the currently used ICD-10 and DSM-IV-TR criteria, is good but sensitivity is relatively low. This may be improved (without adversely affecting specificity) by relaxing DSM-V criteria and by careful consideration of missing or uncertain symptom information. Nonetheless, it is also important to determine whether or not clinical diagnosis can be aided by the use of objective (non-interview-based) measures – for instance differences in brain anatomy. This was the focus of our next study.
Recent changes in DSM-V criteria have good specificity, but relatively low sensitivity compared with ICD-10.
Nearly half of young adults with an ASD do not meet DSM-V criteria, according to ICD-10.
If DSM-V is used to define eligibility for clinical services in the UK, many young adults will be excluded.
Chapter 18 Improving diagnosis and identifying associated mental health symptoms: can we use recent advances in neuroimaging to help classify ASD and ADHD?
Introduction
As noted in previous chapters, ASD is a highly heterogeneous neurodevelopmental condition with multiple causes and courses, a wide range in symptom severity and many associated comorbid disorders (e.g. see Amaral and colleagues230) – including ADHD. It has thus been suggested that ASD should be thought of as ‘the autisms’ rather than a single autistic phenotype. 231,232 This makes the diagnosis of ASD (and its distinction from ADHD) complex. Moreover, it means that clinical trials (in both disorders) necessarily include heterogeneous samples of individuals who may not share the same biological (or cognitive) deficits. This may account for the failure of many clinical trials in ASD and ADHD. Hence, we need to be able to ‘fractionate’ clinical populations better in order to improve diagnostic accuracy and identify more homogeneous groups for clinical trials. One potential way forward is to use recent advances (gained from neuroimaging) in our understanding of the biology of both disorders.
For instance, there is increasing evidence that both ASD and ADHD are associated with abnormalities in specific brain regions and neural systems. 233–236 However, reports of region-specific differences in ASD (and ADHD) are highly variable (reviewed in Toal and colleagues237 and Amaral and colleagues230). Such variable findings may simply be explained by confounds such as clinical heterogeneity between studies or analytical techniques. Alternatively, variability in findings may indicate that differences in brain anatomy in ASD and ADHD are relatively subtle and spatially distributed, and are difficult to detect using mass-univariate (i.e. voxel-wise) approaches. Finally, given the likely multiaetiology of ASD and ADHD, it is likely that their neuroanatomy is not confined to a single morphological parameter but affects multiple cortical features.
There is already evidence to suggest that several aspects of cerebral morphology are different in people with ASD and ADHD – including both volumetric (i.e. cortical thickness, regional area) and geometric features (i.e. cortical shape)238,239 – and that different morphological features may have different neuropathological and genetic underpinnings. 240 So far, individual aspects of the cortex are generally explored in isolation (i.e. in separate statistical models). Hence, it is not yet known (1) if they equally contribute to differentiating individuals with ASD and ADHD from controls and (2) what the relationship between such multiple cortical abnormalities in ASD is. In the present study, we therefore attempted to establish proof of concept that a multiparameter classification approach employing a support vector machine (SVM) (see Mourão-Miranda and colleagues,241 Davatzikos and colleagues242 and Kloppel and colleagues 243), to investigate brain anatomy in adults with ASD and ADHD. Here, a variety of morphological features, including both volumetric and geometric parameters, were used simultaneously to classify control and ASD/ADHD individuals.
We aimed to demonstrate that the neuroanatomical patterns discriminating individuals with autism and ADHD from controls are truly multidimensional, comprising multiple and most likely independent cortical features. If so, this may guide further exploration of the specific genetic and neuropathological underpinnings of both disorders and provide proof of concept that these may be used to help classify individuals with and without the disorders.
Materials and methods
Participants
Twenty control adults were recruited locally by advertisement and 20 adults with ASD were recruited through a clinical research programme at the Maudsley Hospital/IoP, London. All volunteers (Table 48) gave informed consent (as approved by the IoP and Bethlem and Maudsley Hospital Trust REC), and had a full-scale IQ of > 75 (WASI244).
| Participant information | ASD (n = 20) | Controls (n = 20) |
|---|---|---|
| Age (years) | 33 ± 11 (20–68) | 36 ± 9 (20–49) |
| Full-scale IQ | 103 ± 20 (76–141) | 110 ± 13 (77–129) |
| Verbal IQ | 102 ± 17 (78–133) | 106 ± 14 (71–131) |
| Performance IQ* | 98 ± 19 (77–138) | 110 ± 14 (84–136) |
| ADI-R sociala | 15 ± 4 | – |
| ADI-R communicationa | 10 ± 3 | – |
| ADI-R repetitive behavioura | 4 ± 2 | – |
| ADOS-G totalb | 10 ± 2 | – |
All volunteers were right-handed males and were between 20 and 68 years of age. None had a history of major psychiatric disorder or medical illness affecting brain function (e.g. psychosis or epilepsy). All participants underwent a psychiatric interview and physical examination. Blood tests were used to exclude biochemical, haematological or chromosomal abnormalities (including fragile X syndrome). All participants with ASD were diagnosed with autism according to ICD-10 research criteria245 by a trained and qualified clinician in our adult autism specialist clinic at the South London and Maudsley Hospital. The initial clinical diagnosis was then confirmed using the ADI-R,27 whenever possible (i.e. where parents agreed to take part, n = 17). In 3 out of the 20 included cases, ADI-R scores could not be obtained because informants were unavailable. In these three cases, the clinical diagnosis was confirmed using the ADOS. 196 Both ADI-R and ADOS-G scores were available for two participants. All cases had to reach the ADOS-G or ADI-R algorithm cut-off points in the three domains of impaired reciprocal social interaction, communication and repetitive behaviours and stereotyped patterns, but we did allow failure to reach cut-off point in one of the domains (by one point). Sixteen ASD individuals did not have a delay in development of phrase speech at the age of 36 months (and so may be subtyped by some as having the autistic subtype of Asperger syndrome); the remaining four individuals had a history of delayed phrase speech (and so may be subtyped by some as having the autistic subtype of high-functioning autism). However, due to the small sample size it was not possible to reliably investigate putative differences between people with HFA and Asperger syndrome, and all subjects were analysed in a combined group of individuals with ASD.
Furthermore, we recruited a group of 19 individuals with ADHD from the adult ADHD services at the Maudsley Hospital, London. This group served as a neurodevelopmental control group and was matched to the ASD group in sex, age (mean 31 ± 8.5 years), full-scale IQ (mean 1.7 ± 6) and handedness. Of these, eight individuals fulfilled the criteria for ADHD combined type and 11 for ADHD inattentive type. The diagnosis of ADHD was made by a trained and qualified clinician based on a structured clinical interview according to DSM-IV criteria. ADHD symptoms were also assessed using Barkley Current and Childhood Symptoms Scales207 and the Wender Utah Rating Scale. 246
Magnetic resonance imaging data acquisition
Magnetic resonance imaging (MRI) data were acquired using a 1.5-T GE Signa Neuro-optimized System (General Electric Medical Systems, Milwaukee, WI, USA) fitted with 40 mT/m high-speed gradients at the Maudsley Hospital, London. Whole-brain spoiled gradient recalled acquisition in the steady-state T1-weighted series were collected in the coronal plane with repetition time = 13.8 months, echo time = 2.8 months, yielding 124 contiguous 1.5-mm2 axial slices of 256 × 192 voxels with an in-plane resolution of 1 mm2. Similar T1-weighted MRI scans were acquired for the ADHD group on a 1.5-T GE Signa Neuro-optimized System at the Maudsley Hospital, London, using repetition time = 10.72 months, echo time = 4.86 months, yielding 146 contiguous 1.1-mm2 axial slices. A quadrature birdcage head coil was used for radiofrequency transmission and reception. Foam padding and a forehead strap were used to limit head motion. All scans were visually inspected by a radiologist to assess (1) image contrast, (2) movement artefacts and (3) the existence of clinical abnormalities. Scans displaying low image quality or clinical abnormalities were excluded from this study.
Image processing
Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis suite (Laboratory for Computational Neuroimaging, Charlestown, MA, USA), which is documented and freely available for download online [URL: http://surfer.nmr.mgh.harvard.edu/ (accessed 31 May 2018)]. The technical details of these procedures are described in prior publications. 247–254 Briefly, this processing includes motion correction and averaging of multiple volumetric T1-weighted images (when more than one is available), removal of non-brain tissue using a hybrid watershed/surface deformation procedure,249 automated Talairach transformation, segmentation of the subcortical white matter and deep grey matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles),251,252 intensity normalisation, tessellation of the grey matter–white matter boundary, automated topology correction,255,256 and surface deformation following intensity gradients to optimally place the grey/white and grey/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. 247 Once the cortical models are complete, a number of deformable procedures can be performed for further data processing and analysis, including surface inflation,247 registration to a spherical atlas (which utilised individual cortical folding patterns to match cortical geometry across subjects248), parcellation of the cerebral cortex into units based on gyral and sulcal structure253,257 and creation of a variety of surface-based data, including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three-dimensional magnetic resonance volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the grey/white boundary to the grey/cerebral spinal fluid boundary at each vertex on the tessellated surface. 250 The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data, thus are capable of detecting submillimetre differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis258 and manual measurements. 259,260 FreeSurfer morphometric procedures have been demonstrated to show good test–retest reliability across scanner manufacturers and across field strengths. 261 All reconstructed surfaces were visually inspected for gross anatomical topological defects. Brains that did not reconstruct or showed geometric inaccuracies were excluded from the study.
A set of five morphometric parameters per vertex were used as input to the multimodal classifier. Three parameters [1–3: average convexity or concavity, mean (radial) curvature and metric distortion] accounted for geometric features at each cerebral vertex and two parameters (4–5: cortical thickness and surface area, respectively), measured volumetric features.
The average convexity or concavity was used for quantifying the primary folding pattern of a surface. Convexity/concavity captures large-scale geometric features and is insensitive to noise in the form of small wrinkles in a surface. 248 At each vertex, convexity/concavity indicates the depth/height above the average surface and measures sulcal depth or gyral height, respectively. Differences in sulcal depth have previously been investigated as one important aspect of the cerebral geometry implicated in ASD238 and have been suggested to reflect abnormal pattern of cortical connectivity. Mean (radial) curvature was used to assess folding of the small secondary and tertiary folds in the surface. The selection of this feature was motivated by early findings of polymicrogyria (i.e. excessive number of small convolutions on the surface of the brain) in autism. 262 These would not be captured by the average convexity measure reflecting large-scale geometric features only. Metric distortion (i.e. Jacobian) indicating the degree of cortical folding was calculated as degree of displacement and convolution of the cortical surface relative to the average template. 248,263 Although sulcal depth and radial curvature measure specific aspects of the cortical geometry, metric distortion is a wider measure of the overall degree of cortical folding and thus captures geometric distortions otherwise not specified. There is a large body of evidence to suggest that individuals with ASD display abnormal patterns of cortical gyrification reflecting cerebral development and connectivity (e.g. Levitt and colleagues239 and Hardan and colleagues264). Cortical thickness (see above) and pial area (i.e. the area of a vertex on the grey matter surface, calculated as the average of the area of the triangles touching that vertex) were used to quantify volumetric differences. Although these two features are generally combined to measure regional brain volume, a recent study has shown that cortical thickness and surface are influenced by distinct genetic mechanism240 and we therefore added as separate features into the model. A summary of the set of parameters is displayed in Figure 20.
FIGURE 20.
Summary of the five morphometric parameters measured at each cerebral vertex. (a), Average convexity; (b) cortical thickness; (c) pial area; (d) metric distortion (Jacobian); and (e) mean (radial) curvature. g/w, grey/white. Adapted from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
These included average convexity, cortical thickness, pial area, metric distortion (Jacobian) and mean (radial) curvature.
Group differences in intracranial volume, total brain volume and grey matter volume as estimated by FreeSurfer were assessed using t-tests for independent samples prior to classification. There were no significant differences between groups in any of these parameters at a level of p < 0.05 (Table 49). There were also no significant differences in total brain volume between controls and individuals with ADHD, nor between ASD and ADHD group on p < 0.05. Bartlett’s test for homogeneity of variances was used to examine parameter variability across homologue regions in different hemispheres using a threshold of p < 0.0014 (corrected for multiple comparisons).
| ASD | Control | t(df = 38) | p-value | |
|---|---|---|---|---|
| ICV | 1.67 ± 0.18 × 106 | 1.64 ± 0.21 × 106 | –0.63 | < 0.60 |
| Total brain volume | 9.74 ± 1.14 × 105 | 9.69 ± 0.76 × 105 | –0.14 | < 0.90 |
| Total grey matter volume | 4.89 ± 0.56 × 105 | 4.81 ± 0.41 × 105 | –0.58 | < 0.60 |
| Grey matter volume right | 2.45 ± 0.28 × 105 | 2.41 ± 0.20 × 105 | –0.53 | < 0.60 |
| Grey matter volume left | 2.44 ± 0.28 × 105 | 2.39 ± 0.21 × 105 | –0.62 | < 0.60 |
Classification and support vector machine
A linear SVM was used to classify between patient and control group on the basis of their brain morphology. SVM has previously been applied to MRI data241–243 and a detailed description can be found in Schoelkopf and Smola265 and Burges. 266 Briefly, SVM is a supervised multivariate classification method that treats each image as a point in a high-dimensional space. If SVM is applied to images coming from different modalities, as in the current study, the number of dimensions equals the number of voxels/vertices per image multiplied by the number of modalities. Input data were then classified into two classes (e.g. individuals with ASD and controls), by identifying a separating hyperplane or decision boundary. The algorithm is initially trained on a subset of the data <x,c> to find a hyperplane that best separates the input space according to the class labels c (e.g. –1 for patients, +1 for controls). Here, x represents the input data (i.e. feature vector). The feature vector was generated by concatenating the five image modalities for each subject. Once the decision function is learned from the training data, it can be used to predict the class of a new test example. SVM267 is a maximum margin classifier, which identifies the optimal hyperplane by finding the hyperplane with the maximum margin (i.e. maximal separation between classes). The margin is the distance from the separating hyperplane to the closest training examples. The training examples that lie on the margin are called support vectors. The hyperplane is defined by a weight vector and an offset. The weight vector is a linear combination of the support vectors and is normal to the hyperplane. The linear kernel SVM used in the present study allows direct extraction of the weight vector as an image (i.e. the SVM discrimination map). A parameter C, that controls the trade-off between having zero training errors and allowing misclassifications, was fixed at C = 1 for all cases (default value). The LIBSVM toolbox for MATLAB (ACM Transactions on Intelligent Systems and Technology, Taiwan) was used to perform the classifications [URL: www.csie.ntu.edu.tw/∼cjlin/libsvm/ (accessed 31 May 2018)].
Discrimination maps
The weight vector has the same dimension as the feature vector and is normal to the hyperplane. It can be thought of as a spatial representation of the decision boundary and thus represents a map of the most discriminating regions. Here, the feature vector had n × m dimensions, where n is the number of vertices and m denotes the number of modalities (i.e. five). Given two groups (ASD vs. controls), with the labels +1 and –1, respectively, a positive value in the discrimination map (red/yellow colour scale) indicates relatively higher parameter values in patients than in controls with respect to the hyperplane, and a negative weight (blue/cyan colour scale) means relatively higher parameter values in controls than in patients with respect to the hyperplane. As the classifier is multivariate by nature, the combination of all voxels as a whole is identified as a global spatial pattern by which the groups differ (i.e. the discriminating pattern). To enable visualisation of the discriminating pattern for each image modality, the weight vector was cut into its constituent parts, which were then mapped back onto the average white matter surface. In the present study we coloured all voxels that have values > 30% of the maximum value of the discrimination map. 241,268 This threshold, although ultimately arbitrary, eliminates noise components predominantly (< 30%), thus enabling a better visualisation of the most discriminating regions (31–100%).
Intraregional morphometric profiles
To identify the relative contribution of specific parameters in discriminating between groups at different locations on the cortical surface, we displayed so called intraregional morphometric profiles. These profiles were derived by calculating the average weights of vertices within regions of interest (ROI) for the five different parameters. ROIs were based on contiguous weight clusters from the overall discrimination maps. The choice of the ROIs, although ultimately arbitrary, was motivated by (1) the relevance of a region to the current literature (e.g. sulcal depth differences in intraparietal sulcus238) and (2) generally high parameter weights for a specific morphometric feature (e.g. high weights for cortical thickness in medial temporal sulcus). The ROI analysis aimed to illustrate that different regions can display distinct differences in one or more parameters, rather than displaying the same pattern of feature weights. As all weights were scaled, means and SDs are directly comparable across regions and parameters.
Cross-validation and permutation testing
The performance of the classifier was validated using the commonly used leave-two-out cross-validation approach. This validation approach provides robust parameter estimates particularly for smaller samples. In each trial observations from all but one subject from each group were used to train the classifier. Subsequently, the class assignment of the test subjects was calculated during the test phase. This procedure was repeated S = 20 times (where S is the number of subjects per group), each time leaving observations from a different subject from each group out. The accuracy of the classifier was measured by the proportion of observations that were correctly classified into patient or control group. We also quantified the sensitivity and specificity of the classifier defined as:
where TP is the number of true positives (i.e. the number of patient images correctly classified); TN is the number of true negatives (i.e. number of control images correctly classified); FP is the number of false positives (i.e. number of controls images classified as patients); and FN is the number of false negatives (i.e. number of patients images classified as controls).
Classifications were made, first, by including all five morphological modalities into the feature vector and, second, on the basis of each individual parameter. Different classifiers were trained for each hemisphere. This enabled us to assess the overall classification accuracies for individual parameters and hemispheres. Classifier performance was evaluated using basic ROC graphs as well as permutation testing. Permutation testing can be used to evaluate the probability of getting specificity and sensitivity values higher than the ones obtained during the cross-validation procedure by chance. We permuted the labels 1000 times without replacement, each time randomly assigning patient and control labels to each image and repeated the cross-validation procedure. We then counted the number of times the specificity and sensitivity for the permuted labels were higher than the ones obtained for the real labels. Dividing this number by 1000 we derived a p-value for the classification.
Finally, the established classifier was used to predict group membership of individuals with ADHD. To achieve this, the classifier was first retrained using all ASD–control pairs to provide the best possible prediction model for discriminating the two groups. This trained classifier was then applied to the multiparameter data from the ADHD group and predictions for group membership for these subjects were derived.
To validate the binary classification of the subject groups on a quantitative level and to identify the degree to which the classification is driven by autistic symptoms rather than confounds unrelated to autism, the test margin for each subject coming from the all included classifier was correlated with the level of symptom severity measured by the ADI-R subscales. A similar approach has previously been employed by Ecker et al. 269
Results
Overall classifier performance
Classification accuracies as well as sensitivity and specificity for each classifier are listed in Table 50. On the whole, strong hemispheric asymmetry was observed with regard to the overall classification accuracy. The left hemisphere provided consistently higher and above chance prediction accuracies across all morphometric parameters. Here, individuals with ASD were correctly assigned to the appropriate diagnostic category in 85.0% of all cases when all parameters were considered simultaneously (Figure 21c). The sensitivity of the multiparameter classification in the left hemisphere was 90.0% (i.e. if a volunteer had a clinical diagnosis of ASD, the probability that this participant was correctly assigned to the ASD category was 0.9). The specificity was 80.0%, meaning that 80.0% of the controls subjects were correctly classified as controls. High classification accuracies were also obtained when individual morphological parameters were considered. Figure 21a shows the receiver operator characteristics or ROC graph for the six classifiers in the left hemisphere. In general, one point in ROC space is better than another if it is to the north-west (true-positive rate is high, false-positive rate is low, or both), with the point (0,1) representing perfect classification. Classifiers appearing on the left-hand side of the ROC graph, near the x-axis, may be considered ‘conservative’ (i.e. make positive classifications only with strong evidence but often have low true-positive rates), whereas classifiers on the upper right-hand side of the graph may be thought of as ‘liberal’ (i.e. make positive classifications with weak evidence so they classify nearly all positives correctly but often have high false positives). The diagonal line y = x represents the strategy of randomly guessing a class. Best discrimination was obtained when cortical thickness measures were used to classify between groups with sensitivity and specificity values as high as 90.0%, followed by a classification on the basis of the metric distortion parameter (sensitivity 80.0%, specificity 80.0%). The average convexity, pial area and mean curvature displayed accuracies in the range of 70.0–80.0%. The classification p-value resulting from the permutation test was very low across, as well as within, modalities (< 0.004). The probability of obtaining specificity and sensitivity values higher than the ones obtained during cross-validation procedure by chance is thus extremely low.
| Morphometric feature | Correctly classified (%) | Sensitivity (%) | Specificity (%) | p-value |
|---|---|---|---|---|
| Left hemisphere | ||||
| All parameters | 85 | 90 | 80 | 0a |
| Cortical thickness | 90 | 90 | 90 | 0a |
| Radial curvature | 72.5 | 65 | 80 | < 0.001 |
| Average convexity | 70 | 75 | 65 | < 0.004 |
| Metric distortion | 80 | 80 | 80 | 0a |
| Pial area | 77.5 | 70 | 85 | 0a |
| Right hemisphere | ||||
| All parameters | 65 | 60 | 70 | < 0.03 |
| Cortical thickness | 60 | 65 | 55 | < 0.01 |
| Radial curvature | 52.5 | 50 | 55 | < 0.30 |
| Average convexity | 50 | 40 | 60 | < 0.40 |
| Metric distortion | 57.5 | 45 | 70 | < 0.06 |
| Pial area | 45 | 45 | 45 | < 0.60 |
FIGURE 21.
Receiver operating characteristic graphs for classifiers in the left hemisphere and right hemisphere. (a) Left hemisphere; (b) right hemisphere; (c) classification plots for the left hemisphere; and (d) classification plots for the right hemisphere. A, all parameters; B, cortical thickness; C, metric distortion/Jacobian; D, average convexity; E, pial area; F, mean (radial) curvature. Adapted from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.



A very similar profile of parameter importance was observed in the right hemisphere – despite it having generally lower classification accuracies. Here, individuals with ASD were correctly assigned to the appropriate diagnostic category in 65.0% of all cases (sensitivity 60.0%, specificity 70.0%; p < 0.03), when all parameters were considered simultaneously (see Figure 21a and d). As in the left hemisphere, cortical thickness as well as metric distortion displayed the best classification performance, although only cortical thickness measures reached statistical significance (p < 0.01). The discrepancy in overall classification accuracy between the two hemispheres was not due to differences in parameter variability, as there were no significant differences in parameter variance between homologous regions in different hemispheres (calculated across the whole sample).
Thus, although all parameters provided statistically significant predictions in the left hemisphere, only the full model and cortical thickness displayed significant predictions in the right hemisphere. Highest classification accuracy (90.0%) might thus be obtained by using cortical thickness measures exclusively in the left hemispheres. Correlation coefficients between ADI-R subscales and the test margin coming from the combined model are listed in Table 51. The test margin in the left hemisphere was positively correlated with the ADI-R scores for individuals with ASD in the social (r = 0.414; p < 0.05) and communication domain (r = 0.62; p < 0.01). Therefore, individuals with higher values on these ADI-R subdomains are located on the extreme right relative on the hyperplane, whereas the individuals with a lower level of impairment are mostly located in close proximity to the hyperplane overall.
| Diagnostic test (n = 17) | Left hemisphere | Right hemisphere | ||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| ADI-R social | 0.414* | < 0.04 | –0.152 | < 0.28 |
| ADI-R communication | 0.620** | < 0.01 | –0.074 | < 0.38 |
| ADI-R repetitive behaviour | 0.161 | < 0.26 | –0.198 | < 0.22 |
Classification of individuals with ADHD using the established ASD classifier
In order to establish the degree of clinical specificity of the discrimination algorithm to ASD, rather than to NDs in general, the established classifier was used to predict group membership of individuals with ADHD. To predict group membership, the model including all parameters was chosen as it provided best classification accuracy in the right hemisphere and high accuracy in the left hemisphere. On the basis of the neuroanatomical information available for the left hemisphere, 15 out of the 19 individuals with ADHD were allocated to the control group (78.9%) and four individuals with ADHD were allocated to the ASD group (21%). Using the right hemisphere, the classifier allocated cases with ADHD with approximately equal frequencies (47% allocated to the ASD category; 52% allocated to the control category). The classification plots for individuals with ADHD are shown in Figure 22 for both hemispheres.
FIGURE 22.
Classification plots showing group allocation of individuals with ADHD (blue squares) in the (a) left and (b) right hemisphere using the ASD classifier. Reproduced from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.


Discrimination maps of ASD-specific abnormalities
The spatial maps of brain regions identified as outlined above are shown in Figure 23 and a summary description can be found in Table 52. Each of these maps is a spatial representation of the SVM weight vector, and although they do not directly quantify the information content of each region, each map forms a spatially distributed pattern showing the relative contribution of each voxel to the decision function. Although ‘knockout’ techniques may be used to directly quantify the information content of particular regions, this approach may be hampered by substantial information redundancy across brain regions. Nonetheless, the spatial distribution of the weight vector does provide information about which brain regions contributed to classification and in this case is suggestive of a distributed pattern of relative deficit or excess in ASD with respect to controls. We emphasise that due to the multivariate character of SVM (i.e. it considers inter-regional correlations), each region in the discrimination maps should be interpreted in the context of the entire discriminating pattern and should not be considered in isolation. Furthermore, individual regions may display high classification weights for several reasons [e.g. there is a large difference in volume between groups in that region, or the region is highly intercorrelated with other components of the network (i.e. pattern)]. This is of particular relevance to ASD, as individuals with the disorder most likely have abnormalities in the development of neural systems, in addition to differences in isolated regions. Thus, it is important to highlight that individual network components are not necessarily different between groups, but should be considered as constituent parts of a neuroanatomical network discriminating between groups.
FIGURE 23.
Discrimination maps for the five different morphometric features in the left and right hemispheres. Colour maps represent the weight vector on the basis of the five modality classification for (a) cortical thickness; (b) average convexity; (c) metric distortion; and (d) pial area. Weights for the mean (radial) curvature did not exceed the set threshold. Positive weights (i.e. overall excess patters in ASD relative to controls) are displayed in red, negative weights (i.e. overall deficit patterns in ASD relative to controls) are displayed in blue. Reproduced from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
| Parameter | Region | Hemisphere | x | y | z | Weight of each cluster centroid |
|---|---|---|---|---|---|---|
| Cortical thickness | ||||||
| ASD > controls | Lateral orbitofrontal | R | 27 | 22 | –5 | 5.13 |
| Supramarginal gyrus | L | –49 | –45 | 35 | 4.37 | |
| Superior parietal | R | 20 | –82 | 31 | 3.96 | |
| Inferior temporal | L | –53 | –28 | –22 | 4.53 | |
| Middle temporal | L/R | ± 48 | –28 | –7 | 6.66 | |
| Superior temporal gyrus | L/R | ± 53 | –20 | –1 | 5.85 | |
| Superior temporal sulcus | R | 51 | –34 | 7 | 4.08 | |
| Parahippocampal gyrus | L/R | ± 31 | –27 | –16 | 3.27 | |
| Fusiform gyrus | L/R | –36 | –28 | –18 | 4.64 | |
| Entorhinal cortex | L/R | ± 21 | –17 | –23 | 3.87 | |
| Lateral occipital | L/R | ± 15 | –98 | 6 | 6.92 | |
| Posterior cingulate gyrus | L | –7 | –33 | 28 | 3.97 | |
| Anterior cingulate gyrus | R | –6 | 28 | –7 | –2.71 | |
| Precuneus | L | –11 | –55 | 48 | –3.82 | |
| ASD < controls | Rostral middle frontal | L/R | ± 35 | 36 | 9 | –5.60 |
| Superior frontal | L/R | ± 12 | 61 | 7 | –5.39 | |
| Caudal middle frontal | L | –40 | 20 | 36 | –3.12 | |
| Pars opercularis | L | |||||
| Inferior parietal lobe | R | 41 | –60 | 36 | –3.95 | |
| Superior parietal lobe | R | 17 | –64 | 50 | –5.00 | |
| Precuneus | R | 13 | –37 | 63 | –3.18 | |
| Anterior cingulate gyrus | R | 6 | 30 | –5 | –3.18 | |
| Surface area | ||||||
| ASD > controls | Precentral | R | 56 | 5 | 15 | 3.23 |
| Orbitofrontal | L | –18 | –96 | –7 | 2.60 | |
| Supramarginal gyrus | L | –55 | –40 | 36 | 3.45 | |
| Inferior parietal | R | 49 | –50 | 12 | 4.57 | |
| Inferior temporal lobe | L | –45 | –63 | –1 | 2.69 | |
| Lateral occipital | R | 17 | –95 | 14 | 2.54 | |
| ASD < controls | Superior frontal | R | 15 | 39 | 7 | –2.26 |
| Rostral middle frontal | R | 35 | 36 | 8 | –2.85 | |
| Paracentral | R | 10 | –6 | 46 | –2.20 | |
| Superior temporal | L | –51 | 5 | 13 | –3.98 | |
| Pericalcarine fissure | L | –13 | –76 | 7 | –4.50 | |
As can be seen in Figure 23, the five investigated parameters resulted in different spatially distributed patterns of regions with highest contribution to the discrimination. Although cortical thickness provided best classification accuracy and maximum weight values, differences between groups were observed in both volumetric as well as geometric features.
Colour maps represent the weight vector on the basis of the five modality classification for cortical thickness (see Figure 23a), average convexity (see Figure 23b), metric distortion (see Figure 23c) and pial area (see Figure 23d). Weights for the mean (radial) curvature did not exceed the set threshold. Positive weights (i.e. overall excess patters in ASD relative to controls) are displayed in red, negative weights (i.e. overall deficit patterns in ASD relative to controls) are displayed in blue (see Figure 23).
Cortical thickness
In both hemispheres, the discriminative pattern for cortical thickness in ASD comprised regions in all four lobes of the cortex (see Figure 23a). Regional details are summarised in Table 52. The ‘excess pattern’ (i.e. ASD > controls) comprised several temporal regions, including the right superior temporal sulcus (STS, BA22), medial and superior temporal gyrus (BA21/BA22) as well as the parahippocampal gyrus (BA36), fusiform gyrus (BA20) and enthorinal cortex. In addition, the excess pattern included the inferior/superior parietal lobe (BA39), Brodmann area 18 of the occipital lobe, and the anterior and posterior cingulate gyrus.
Although the excess pattern comprised predominantly occipito-temporal regions, the pattern displaying a relative thinning of the cortex in ASD versus controls (i.e. ‘deficit pattern’) included mainly frontal and parietal regions such as the middle frontal gyrus (BA46), the medial/superior frontal gyrus (BA10) and BA46 in the medial frontal gyrus. In addition, the deficit pattern contained the superior parietal cortex (BA40/7) and in the anterior cingulate gyrus.
Surface area
Only a few surface area (see Figure 23d) differences were observed in relatively small clusters with generally low weights. Details are summarised in Table 52.
Regional geometric characteristics
Apart from volumetric differences, features describing regional geometric characteristics also displayed high discrimination weights, which were summarised in Table 53. In both hemispheres, individuals with ASD displayed a pattern of relative increase in sulcal depth (see Figure 23b) in the intraparietal sulcus as well as in the superior frontal cortex. The discriminative pattern for cortical folding (see Figure 23c), as indicated by the metric distortion (i.e. Jacobian), included mainly bilateral parietal regions such as the inferior parietal lobe (BA39/40), and several regions of the right frontal lobe (e.g. supramarginal gyrus, postcentral gyrus, orbitofrontal regions). In addition, the discriminative pattern in cortical folding comprised the precuneus.
| Parameter and region | Hemisphere | x | y | z | Weight of each cluster centroid |
|---|---|---|---|---|---|
| Metric distortion: ASD > controls | |||||
| Precentral gyrus | L | –50 | –5 | 37 | 3.23 |
| Rostral middle frontal | L | –44 | 24 | 28 | 3.27 |
| Superior frontal gyrus | R | 14 | 40 | 14 | 2.51 |
| Supramarginal gyrus | L | –51 | –50 | 25 | 5.30 |
| Postcentral gyrus | L | –60 | –8 | 20 | 3.67 |
| Posterior cingulate gyrus | L | –7 | –28 | 29 | 3.50 |
| Pericalcarine fissure | L | –17 | –67 | 10 | 3.46 |
| Lateral occipital gyrus | R | 28 | –86 | 16 | 2.87 |
| Inferior parietal lobe | R | 47 | –45 | 37 | 4.90 |
| Middle temporal gyrus | R | 53 | –53 | 2 | 2.69 |
| Paracentral gyrus | R | 8 | –22 | 47 | 3.23 |
| Lingual gyrus | R | 25 | –55 | 2 | 2.50 |
| Metric distortion: ASD < controls | |||||
| Anterior cingulate | L | –6 | 34 | 0 | –2.75 |
| Postcentral gyrus | R | 30 | –28 | 63 | –3.98 |
| Supramarginal gyrus | R | 59 | –35 | 29 | –3.75 |
| Rostral middle frontal | R | 37 | 32 | 7 | –4.27 |
| Precentral gyrus | R | 42 | –6 | 47 | –3.59 |
Morphometric profiles
The discrimination maps show that different morphometric parameters elicited different spatial patterns of weights with some overlap between particular parameter pairs. The weights for individual parameters are thus not uniform across the cortex, but vary from region to region (i.e. vertex to vertex). This can also be seen on the basis of the morphometric profile for individual regions, which are displayed in Figures 24–27 and listed in Table 54. These profiles visualise the mean parameter weights for individual features within ROI.
FIGURE 24.
Visualisation of the morphometric abnormalities in the right intraparietal sulcus. (a) Colour maps represent the weight vector score; (b) outlines of the cortical surface for ASD (red) and control (blue) group (this main discriminating factor in this group was an increase in sulcal depth in ASDs relative to controls); (c) differences in sulcal depth for this ROI are shown for both groups; and (d) morphometric profile for this region. Profiles were derived by averaging the weight vector scores across vertices within this ROI and for the different morphometric parameters. Weights were identified on the basis of the concatenated SVM model, thus showing the relative contribution of parameters in this ROI. Reproduced from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

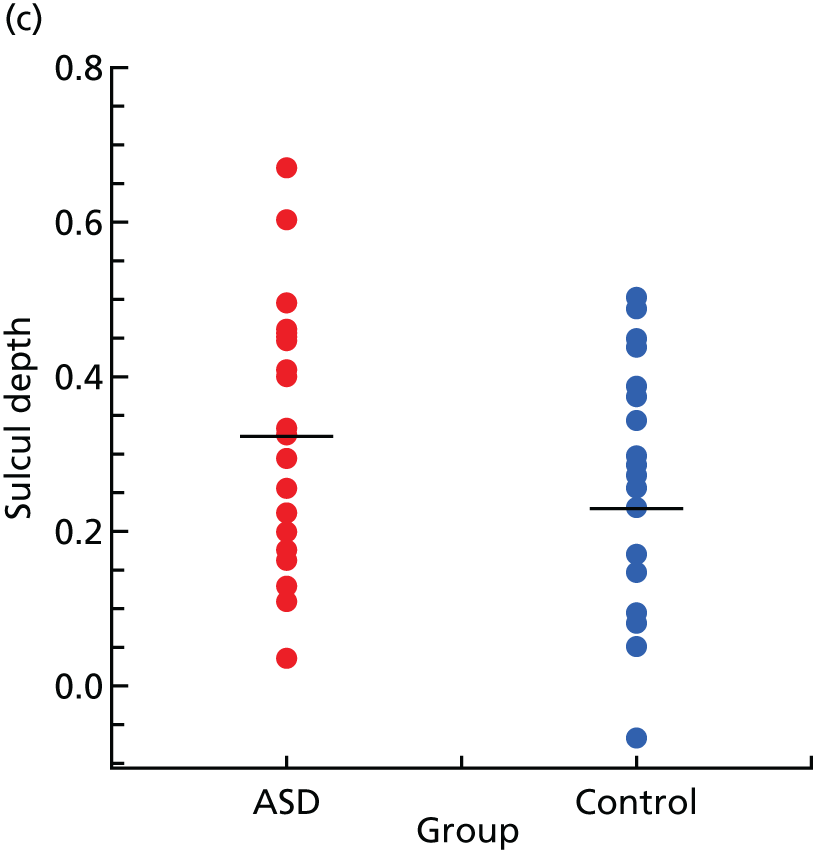
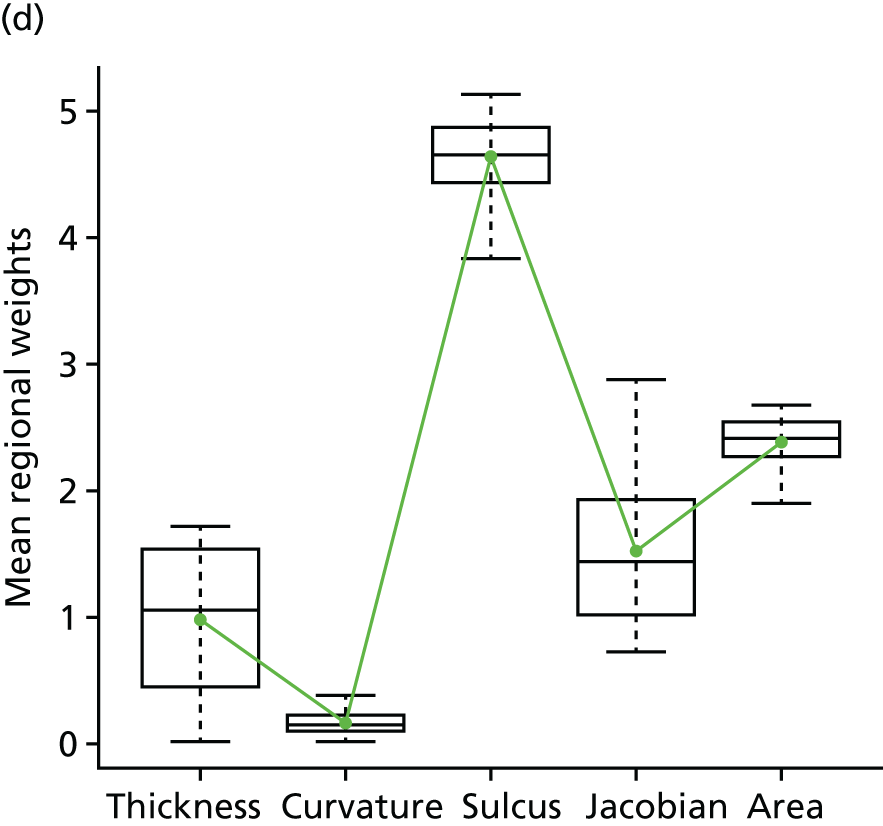
| Region | Mean (weight) | ||||
|---|---|---|---|---|---|
| Cortical thickness | Mean curvature | Sulcal depth | Metric distortion | Surface area | |
| Intraparietal sulcus (R) | 0.97 | 0.16 | 4.63 | 1.51 | 2.38 |
| Inferior parietal lobe (L) | 1.51 | 0.23 | 0.99 | 4.96 | 0.88 |
| Medial temporal sulcus (L) | 5.66 | 0.07 | 0.17 | 0.48 | 0.62 |
| Posterior cingulate gyrus (L) | 3.83 | 0.09 | 0.78 | 3.79 | 0.20 |
FIGURE 25.
(a) Visualisation of the morphometric abnormalities in the left inferior parietal lobe (BA39); (b) outlines of the cortical surface for ASD and control group; (c) differences in metric distortion for this ROI are shown for both groups; and (d) morphometric profile. Reproduced from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.


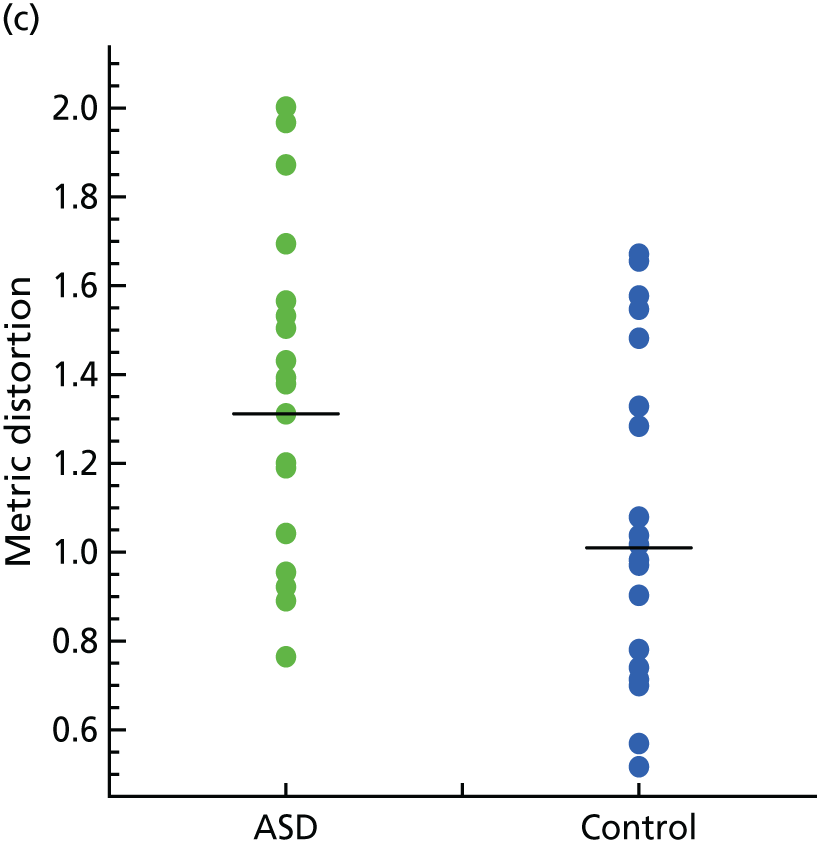
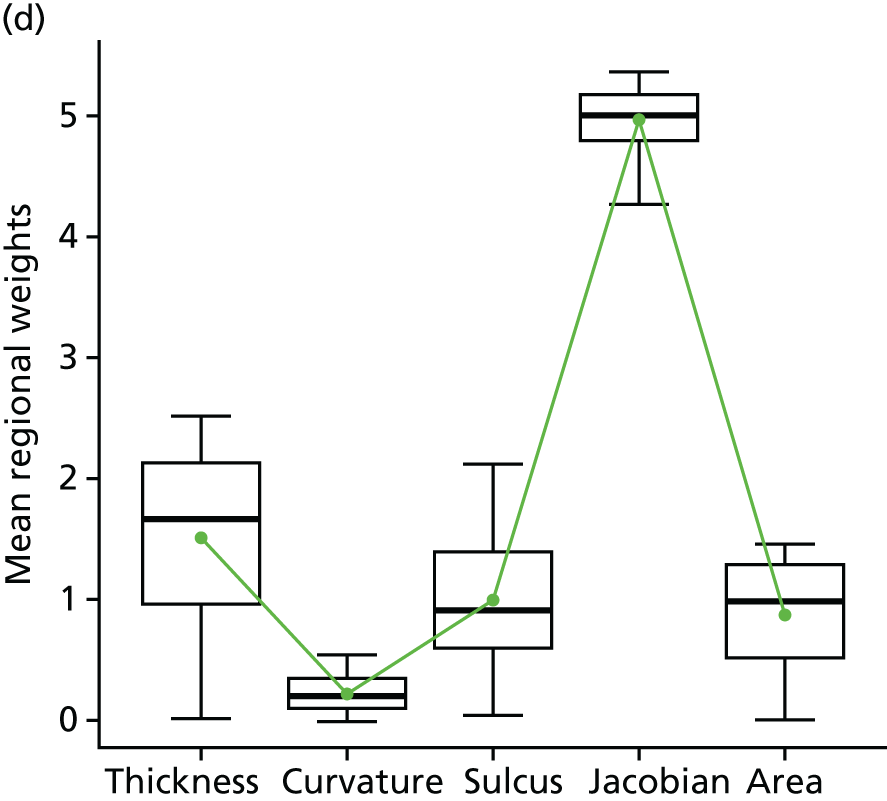
FIGURE 26.
(a) Morphometric abnormalities in the middle temporal sulcus; (b) visualisation of cortical thickness measures within the middle temporal sulcus; (c) and (d) in this region, only cortical thickness discriminated between ASD and control groups, for which individuals with ASD displayed an increase in thickness, relative to controls. Reproduced from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.


FIGURE 27.
(a) Morphometric abnormalities in the posterior cingulate gyrus; (b) and (c) a combination of cortical thickness and folding pattern lead to a high contribution to the classification in that region. Reproduced from Ecker et al. 4 © 2010 Ecker et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.


In the intraparietal sulcus for instance, high discrimination weights were observed for differences in sulcal depth with lower weights for volumetric features such as cortical thickness or surface area (see Figure 24). A similar profile was also seen in the inferior parietal lobe (BA39). Here, the weights for volumetric parameters such as cortical thickness or surface area were very low in comparison with the weights associated with the pattern of cortical folding (i.e. metric distortion) (see Figure 25).
Other regions, however, displayed high weights for volumetric parameters exclusively. For instance, in the temporal sulcus (BA21), high weights were observed for cortical thickness exclusively in the light of low weights in all other parameters (see Figure 27). There were also regions with equally high weights in volumetric as well as geometric features (e.g. the anterior cingulate gyrus). Here, cortical thickness as well as local cortical folding displayed high parameter weights.
In summary, we observed a spatially distributed pattern of regions that can be used to differentiate individuals with ASD from controls. Overall, highest weights were observed for measures of cortical thickness, followed by geometric features such as sulcal depth and metric distortion, which were predominantly observed in parietal regions. This suggests that both volumetric and geometric features play an important role in distinguishing between ASD and control group. Finally, we demonstrated that the parameter weights for individual cortical features were region dependent.
Discussion
Here, we used a multiparameter classification approach to characterise the complex and subtle grey matter differences in adults with ASD and ADHD. SVM achieved good separation between groups and revealed spatially distributed and largely non-overlapping patterns of regions with highest classification weights for each of five morphological features. Our results confirm that the neuroanatomy of ASD and ADHD are truly multidimensional, affecting multiple neural systems. The discriminating patterns detected using SVM may help classify affected individuals.
There is good evidence to suggest that several aspects of cerebral morphology are implemented in ASD and ADHD, including both volumetric and geometric features. 238,239 However, these are normally explored in isolation. Here, we aimed to establish a framework for multiparameter image classification to describe differences in grey matter neuroanatomy in ASD and ADHD in multiple dimensions, and to explore the predictive power of individual parameters for group membership. This was achieved using a multiparameter classifier incorporating volumetric and geometric features at each cerebral vertex. In the left hemisphere, SVM correctly classified 85% of all cases overall at a sensitivity and specificity as high as 90% and 80%, respectively, using all five morphological features. This level of sensitivity compares well with behaviourally guided diagnostic tools, whose accuracies are on average around 80%. Naturally, one would expect lower sensitivity values than the test used for defining the ‘autistic prototype’ itself (i.e. ADI-R). Thus, if a classifier is trained on the basis of true positives identified by diagnostic tools, the maximal classification accuracy that could be reached is only as good as the measurements used to identify true positives.
The significant predictive value of pattern classification approaches may have potential clinical applications. Currently, both ASD and ADHD are diagnosed solely on the basis of behavioural criteria. The behavioural diagnosis is, however, often time-consuming and can be problematic, particularly in adults. In addition, different biological aetiologies might result in the same behavioural phenotype (the ‘autisms’232), which is undetectable using behavioural measures alone. Thus, the existence of an ASD (or ADHD) biomarker such as brain anatomy might be useful to facilitate and guide the behavioural diagnosis. This would, however, require further extensive exploration in the clinical setting, particularly with regard to classifier specificity to ASD or ADHD – rather than neurodevelopmental conditions in general.
To address the issue of clinical specificity, the established ASD classifier was used to classify individuals with ADHD. Bilaterally, the ASD classifier did not allocate the majority of ADHD subjects to the ASD category. This indicates that it does not perform equally well for other neurodevelopmental conditions and is more specific to ASD. To further demonstrate that the classification is driven by autistic symptoms, the test margins of individuals with ASD were correlated with measures of symptom severity. 269 We found that larger margins were associated with more severe impairments in the social and communication domain of the ADI-R. The classifier therefore seems to utilise neuroanatomical information specifically related to ASD rather than simply reflecting non-specific effects introduced by any kind of pathology. However, due to a recent scanner upgrade, ADHD scans were acquired with different acquisition parameters, while manufacturer, field strength and pulse sequence remained the same. FreeSurfer has been demonstrated to show good test–retest reliability particularly within scanner manufacturer and field strength,261 but we cannot exclude the possibility that systematic differences in regional contrast may have affected the ADHD classification. Future research is thus needed to validate the ADHD findings on an independent sample.
The overall classification accuracy varied across hemispheres (79.0% left vs. 65.0% right) in the absence of interhemispheric differences in parameter variability. Hemisphere laterality is an area, which remains relatively unexplored in autism. Although our data suggest that the left hemisphere is better at discriminating between groups (i.e. is more ‘abnormal’), it is unclear whether this discrepancy is due to quantitative differences in parameters or to qualitative aspects of the discriminating patterns (i.e. additional regions). Furthermore, it is also not possible to identify whether or not individuals with ASD or ADHD display a higher (lower) degree of cortical asymmetry relative to controls. There is some evidence to suggest that individuals with ASD show a lower degree of ‘leftward’ (i.e. left > right) cortical symmetry than controls,270 which may explain differences in classification accuracy. There is also evidence to suggest that the left hemisphere is under tighter genetic control than the right hemisphere,271 which may be of relevance to a highly heritable condition such as ASD. However, a direct numerical comparison between hemispheres is needed to address this issue directly.
The classification accuracy varied not only across hemispheres, but also across morphometric parameters. Bilaterally, cortical thickness provided the best classification accuracy and highest regional weights. Differences in cortical thickness have been reported previously in ASD and ADHD for both increases272,273 as well as decreases,272,274 and in similar regions as reported here (i.e. parietal, temporal and frontal areas). The overlap with previous studies indicates that these regions display high classification weights due to a quantitative (i.e. ‘true’) difference rather than high intercorrelations with thickness measures in other brain regions.
Certain geometric features such as average convexity and metric distortion provided above chance classifications as well, particularly in parietal, temporal and frontal regions, and in areas of the cingulum. Average convexity and metric distortion measure different aspects of cortical geometry (see Materials and methods) and have previously been linked to ASD, as has sulcal depth. 238 Such geometric features were suggested to reflect abnormal patterns of cortical connectivity. There have also been reports of abnormal patterns of gyrification262,264 and large-scale displacements of the major sulci. 239 Thus, our study provides further evidence to support the hypothesis that the ‘autistic brain’ is not just bigger or smaller but is also abnormally shaped and that this is ‘separable’ from ADHD.
Strengths and weaknesses
Our findings should be interpreted in the context of a number of methodological limitations.
First, the classification algorithm is highly specific to the particular sample used for ‘training’ the classifier, namely high-functioning adults with ASD. The advantage of this approach is that the classifier offers high specificity with regard to this particular subject group, but is less specific to other cohorts on the spectrum. Owing to the small sample size it was also not possible to reliably investigate differences between HFA and Asperger syndrome. Evidence275 suggests that by adulthood these groups are largely indistinguishable at the phenotypic level. However, the extent to which these groups differ at the level of brain anatomy is unknown and may be worth investigating using SVM in the future. Second, 85% of ASD participants in our sample were diagnosed using the ADI-R and 15% were diagnosed using the ADOS-G. As both diagnostic tools measure autistic symptoms at different developmental stages, the classifier may be biased towards individuals with an early diagnosis of ASD. Although it is not expected that classifier performance on the basis of ADOS-G and ADI-R differ drastically, diagnostic heterogeneity may be a potential limitation. Finally, SVM is a multivariate technique and hence offers a limited degree of interpretability of specific network components. Additional analysis such as ‘searchlight’ or ‘virtual lesions’ approaches276–278 may therefore be combined with SVM in the future to establish the relative contribution of individual regions/parameters to the overall classification performance.
Conclusions
Although classification values and specific patterns we report must be considered as preliminary, our study offers a ‘proof of concept’ that brain imaging may help complement traditional interview-based techniques to more accurately identify young adults with ASD and/or ADHD.
ASD and ADHD are multidimensional and implicate multiple neural systems.
SVM achieves good separation between groups.
The discriminating patterns detected using SVM may help classify affected individuals.
Section 4 Improving outcomes through intervention
Chapter 19 An overview
In the previous chapters, we saw that ASD and ADHD (and their associated mental health symptoms) are frequently unrecognised and untreated – both in the community, by GPs, and by the specialist mental health services we investigated. Therefore, as a next step, we piloted new methods to help identify affected individuals based on clinical interview (and developed proof of concept for the potential utility of biomarkers).
However, simply diagnosing affected individuals is not enough. They need to be offered appropriate treatments based on their individual needs. This is true both in GP community settings and in mental health services.
There are currently no highly effective pharmacological treatments for core symptoms in ASD, but there is initial evidence that some associated symptoms may be modifiable by medication. However, we do not know how medications are currently used in people with ASD. For instance, the current prescribing in the UK by GPs of medication to people with ASD for associated symptoms is unknown. By contrast, in ADHD there are effective treatments for core symptoms and prescribing practice by GPs in the NHS has been extensively investigated. However, relatively little is known about how ADHD medications perform in particular subgroups of individuals who are often excluded from classical clinical trials (e.g. those with ID or prisoners). Finally, people increasingly wish to use cognitive–behavioural (as opposed to medication-based) approaches. The evidence base for their use in these disorders is, however, sparse.
In summary, in order to improve clinical practice we need to understand how current treatments are used across the UK and/or if they can be adapted (or better targeted) to be more effective (especially in people who are often excluded). In addition, we need to improve staff knowledge and clinical practice.
Hence, our overall aims were to:
-
improve understanding of the most commonly used pharmacological treatments in the community through a survey of current prescribing by GPs across the UK to individuals with ASD
-
initiate pilot studies on the effectiveness of ADHD treatments on both core and associated symptoms in people with ID, and in prisoners
-
provide proof of concept for the effectiveness of cognitive behavioural approaches in ASD
-
pilot, and test, the effectiveness of electronic-based staff training from an expert centre to local services.
Chapter 20 Pharmacological treatments prescribed to people with ASD in UK primary health care
Introduction
Individuals with ASD have a very high prevalence of comorbid mental health conditions, including ADHD, LDs, oppositional defiance disorder or CD, emotional disorders, anxiety and other phobic disorders, and chronic tic disorder. 29,58,279 Hence, the development and optimisation of interventions (including behavioural treatment, educational approaches and pharmacotherapy) to alleviate symptoms/impairments of those with ASDs, so as to improve the quality of life of individuals and their families, is imperative.
Medication may be a helpful therapeutic intervention to manage disabling behavioural and/or mental health symptoms in the autistic population; however, there are currently no practice guidelines. 280 A review concluded that although there is no standard medication for treating ASD, people are prescribed a variety of psychotropic medications (and there is scant reliable research evidence supporting this practice in adolescents and adults). 281 In fact, some have suggested that in individuals with ASDs psychiatric medications show decreased efficacy and result in more adverse effects, such as behavioural toxicity with tricyclic antidepressants and social withdrawal and irritability with methylphenidate. 282–284 This creates uncertainty about the appropriateness of using existing pharmacological treatment(s) for comorbid conditions, such as ADHD,280,285 and has led to recent work on drug effectiveness. 54 Despite this uncertainty, drug surveys and studies of health insurance claims databases conducted in the USA have shown that over time more psychotropic drugs (and in particular antipsychotics, antidepressants and stimulants) are being prescribed to children, young people and adults with ASD. 286–290 For example, in North Carolina between 1992–3 and 2001, psychotropic drug use rose significantly from 30.5% of children and adults with autism to 45.2%. Furthermore, antidepressant use increased 3.5-fold (from 6.1% in 1992–3 to 21.4% in 2001). 286 There is also increasing evidence that the likelihood of an ASD person being prescribed medication increases with age. For instance, one study reported that rates of psychotropic drug use rose from 56% for 6- to 11-year-olds to 73% for 18- to 21-year-olds. 288 This is of importance because once an individual has been prescribed a psychotropic medication, they are (1) 11 times more likely to remain on it than they would a non-psychotropic drug289 and (2) much more likely to be exposed to (potentially hazardous) polypharmacy (e.g. some have reported that more than half of children and youths with ASD who were prescribed psychotropic medication experienced this280,291).
Although there is limited evidence to guide psychotropic medication use in the ASD population, two drugs have shown efficacy for the alleviation of behavioural symptoms in children and adolescents with autistic disorder: risperidone (Risperdal®, Janssen-Cilag Ltd) 292,293 and aripiprazole (Abilify®; Otsuka Pharmaceuticals Ltd, Slough, UK). 294,295 In 2006, risperidone was approved by the Food and Drug Administration in the USA for the treatment of irritability associated with autistic disorder in 5- to 16-year-olds; this includes aggression and deliberate self-injuriousness. Aripiprazole was also approved by the Food and Drug Administration in 2009 after demonstrating efficacy in the same indication in 6- to 17-year-olds. 294,295 An application for UK approval was submitted but subsequently withdrawn by the licence holder of risperidone, Janssen-Cilag Ltd, in 2006 after receiving an offer of conditional approval, but limiting its indication to the symptomatic treatment of severe aggression and violence in children with autism. 296 Safety monitoring through a treatment registry formed part of the conditions due to concerns that risperidone could be misused as long-term chemical control in children. In the UK, risperidone remains unlicensed for use in children with autism, but it is approved for the treatment of persistent aggression in CD in children of subaverage intellectual functioning. The reported side effects of antipsychotic drugs include significant weight gain and other metabolic effects such as hyperprolactinaemia and diabetes mellitus,297,298 movement disorders such as tardive dyskinesia, tremor and dystonia,299,300 and life-threatening side effects such as rhabdomyolysis, neuroleptic malignant syndrome and seizures have also been reported. 301–303 However, little is known about the long-term safety of antipsychotics and polypharmacy with other drug classes, especially in the autistic population. This is a valid concern because of the early diagnosis of ASDs and the lifetime persistence of symptoms, which are increasingly managed with psychotropic drugs. 289
Studies have identified substantial support needs for ASD in terms of health, social care and educational services,7,304 but to date we have very few data in the UK about the prescribing of pharmacological treatment to young people with ASDs and associated neuropsychiatric comorbid disorders. Information about prescribing practices and comorbidities is necessary for prioritising further research into the efficacy and short- and long-term safety of psychotropic medications in this particular patient population. Hence, we conducted an observational cohort study based on a primary care database to investigate the pharmacological treatments used among a cohort of children, adolescents and young adults diagnosed with ASD.
Method
Study design
A descriptive cohort study was conducted using THIN database to investigate the incidence and prevalence of ASD diagnoses, psychotropic medication prescribing and neuropsychiatric-related comorbidities of children, adolescents and young adults in UK primary care.
Data source
The Health Improvement Network contains anonymised computerised information systematically recorded by GPs in the UK for patient management. The database provider collates and organises this information in order for it to be used for research. In the UK, almost all patient care is managed by GPs in primary care, who act as gatekeepers of the UK NHS. When required, GPs will refer patients to hospital consultants or specialists in secondary care for diagnosis and initiation of treatment, and GPs usually continue to monitor their patients and issue prescriptions. The diagnosis and management of ASDs and the initiation of drug treatment for symptoms of ASDs (or comorbidities) is usually the responsibility of specialist teams and GPs manage patients in primary care through shared care arrangements with these teams.
The Health Improvement Network covers approximately 5.7% of the UK population with 3.6 million active patients from 464 general practices. 305 The demographic distribution of THIN is broadly representative of that of the UK; therefore, analysis of the clinical and prescribing data will provide information on trends that is representative of national trends. This is particularly useful in evaluating treatment rates, rates of specific diagnoses and changes in these rates.
The Health Improvement Network is a rich source of clinical primary care data and contains information on patient demographics, prescriptions, diagnoses and referrals. Drugs are coded in the database using Multilex® codes (First Databank; THIN). Diagnoses, symptoms and referrals are coded using Read Clinical Terms, a comprehensive hierarchical system. 306 The database has been previously used to study trends in disease incidence and prescribing patterns in paediatric and adult populations. 307–309 A previous study using a similar UK primary care database (the former General Practice Research Database; GPRD), has validated diagnoses of ASDs in GP records by expert review and by a computerised diagnostic algorithm of DSM-IV symptoms ratings, showing they are appropriate for the identification of ASD cases. 310 Practices who contribute to THIN and/or GPRD use the same practice management software (Vision, In Practice Systems Ltd, London, UK), so data from both databases are collected in a similar manner and structure. In fact, a large proportion of practices contributing to THIN also contribute to GPRD (66%, 327/495 practices in 2001–8). 311
Study population
The study population comprised all 5651 individuals in THIN who were children, adolescents and young adults aged < 25 years who had a record of diagnosis of ASD in the study period between 1 January 1992 and 31 December 2009. The start date of each patient was defined as the latest of the following: the date of the patient’s registration at the general practice; the date that the general practice began using the Vision software (a clinical management system); the date that the practice was deemed to meet a key quality indicator known as the acceptable mortality reporting. 312 Patients were included if they had an observation period of least 6 months available from their start date and were registered with the general practice during the study period.
Prevalence of ASD
Patient records dated within 1 year after a patient’s start date (which may or may not be within the study period), were screened to identify diagnoses of ASD. Any individual with an ASD diagnosis within 1 year of the start date were considered affected with ASD (i.e. prevalent) unless they were aged < 2 years at the time of the diagnosis. Autistic disorder can be diagnosed in children as young as 2 years of age, whereas those with Asperger disorder and PDD (not otherwise specified) are frequently not reliably diagnosed until 4 or 5 years of age. 313 Hence, patients with a record of ASD at ages < 2 years are likely to be incident with ASD. Incident patients were defined as (1) those who have a first diagnosis of ASD following the first year screening period; or (2) those with an ASD diagnosis when aged < 2 years during the screening period. The index date for each patient was defined as the date of the first recorded diagnosis of ASD following the patient’s start date. The annual prevalence of ASD was calculated by counting all patient incidents with ASD in a particular year and all prevalent patients in active follow-up of that year divided by the total number of individuals in the THIN mid-year population (MYP; i.e. those who remained registered on the database on 1 July of that calendar year). The annual incidence of ASD was calculated by counting all patients incident with ASD in a particular year divided by the total number of individuals in the THIN MYP. Annual prevalence of ASD was calculated by age group defined as 0–5 years (young children), 6–12 years (children), 13–17 years (adolescents) and 18–24 years (young adults).
Drug prescribing
Nine categories of drugs were defined (by MLM, ES and KG) as stimulants [methylphenidate, dexamfetamine (Dexedrine®; Celltech Pharmaceuticals Ltd, Brussels, Belgium), atomoxetine]; antidepressants; antipsychotics; antiepileptics/mood stabilisers; benzodiazepines; sleep medication [including melatonin (Circadin®; Temmler Pharma GmbH & Co, Marburg, Germany)]; clonidine; beta-blockers; antiparkinsonism drugs which may be co-prescribed with antipsychotics to counteract unwanted motor effects. The prescriptions for the study drugs of each patient recorded on or after their index date were identified and the annual proportions of the ASD cohort prescribed drug treatment were calculated by drug category and by individual drug (with 95% CIs).
Comorbidities
The clinical records of each patient recorded on or after their index date were screened for neuropsychiatric comorbidities. Diagnostic codes (Read Codes) were categorised into 10 groups (by DGM, ES and MLM): psychotic disorders (including schizophrenia and delusions); behavioural, conduct and PDs; mood disorders; anxiety and phobias (including OCD); ADHD; suicidal behaviour and self-harm; anorexia and bulimia; tic disorders; developmental difficulties and LDs; substance and alcohol misuse. The proportions of the cohort with neuropsychiatric comorbidities were calculated.
Data management and analyses were performed using Stata® SE version 11.2 (StataCorp, College Station, TX, USA).
The study protocol was granted approval by the Scientific Review Committee of Cegedim Strategic Data Medical Research UK, the data providers of THIN.
Results
Characteristics of the ASD cohort
There were 5651 patients aged < 25 years in the THIN database with at least one diagnosis record of ASD during the study period: 83% were male (n = 4688). Table 55 provides details on the characteristics of the study cohort. In 4541 patients who became incident with ASD during the study period, the mean age at first recorded diagnosis was 9 years (SD 4.74 years); however, a bimodal distribution of age was observed, with peak frequencies of first diagnoses recorded at 4 years and 8 years of age. First diagnoses of ASD were recorded at a later age in females than in males.
| Cohort characteristic | All | Male | Female |
|---|---|---|---|
| Number of subjects aged 0–24 years (%) with at least one diagnosis of ASD | 5651 (100.0) | 4688 (83.0) | 963 (17.0) |
| Mean age (years) (SD) at first recorded diagnosis of ASD | 9.3 (5.05) | 9.2 (4.92) | 10.1 (5.60) |
| Median age (years) (IQR) at first recorded diagnosis of ASD | 8.4 (7.24) | 8.2 (6.92) | 9.1 (8.64) |
| ASD diagnoses | |||
| Total number of ASD diagnoses | 8602 | 7205 | 1397 |
| Mean number of ASD diagnoses per person (SD) | 1.5 (1.25) | 1.5 (1.27) | 1.5 (1.10) |
| Median number of ASD diagnoses per person (IQR) | 1.0 (1.00) | 1.0 (1.00) | 1.0 (1.00) |
| Prevalent/incident patients | |||
| Number of subjects prevalent with ASD (ASD diagnosis recorded within 1 year of entry into study) | 1110 (19.6) | 919 (19.6) | 191 (19.8) |
| Number of subjects incident with ASD | 4541 (80.4) | 3769 (80.4) | 772 (80.2) |
| Incident patients: mean age (years) (SD) at first recorded diagnosis of ASD | 9.0 (4.74) | 8.9 (4.61) | 9.6 (5.29) |
| Incident patients: median age (years) (IQR) at first recorded diagnosis of ASD | 8.1 (6.72) | 8.0 (6.44) | 8.5 (8.08) |
| Incident patients: mean number of ASD diagnoses per person (SD) | 1.5 (1.33) | 1.5 (1.34) | 1.4 (1.28) |
| Prescribing | |||
| Total number of prescriptions prescribed post index date | 158,285 | 122,587 | 35,698 |
| Number of study drug prescriptions issued at any time post-index date | 47,738 | 36,983 | 10,755 |
| Total number of patients (%) prescribed at least one prescription for a study drug | 1619 (28.6) | 1312 (28.0) | 307 (31.9) |
Prevalence/incidence of ASD diagnoses
The overall prevalence of ASD diagnoses during the study period was 2.23 persons per 1000 MYP; the highest prevalence was seen in children, with 3.87 children per 1000 MYP. In 2008, ASD prevalence for young children was 2.06 per 1000 MYP, 8.89 per 1000 MYP in children, 6.64 per 1000 MYP in adolescents (aged 13–17 years) and 2.44 per 1000 MYP in young adults (aged 18–24 years).
Figure 28 shows the prevalence of ASD in the cohort over the study period. The prevalence of ASD increased 64.6-fold from 0.08 persons per 1000 MYP in 1992 (95% CI 0.05 to 0.12) to 5.04 persons per 1000 MYP in 2008 (95% CI 4.89 to 5.19). The increase was greater in males (77.1-fold) than in females (36.8-fold). The incidence of ASD also rose in the same period by 23.7-fold, from 0.03 persons per 1000 MYP in 1992 (95% CI 0.01 to 0.05) to 0.67 persons per 1000 MYP in 2008 (95% CI 0.61 to 0.72). The rise in incidence was greater in females (32.9-fold) than in males (23.1-fold).
FIGURE 28.
Prevalence of ASD in individuals aged 0–24 years (with 95% CIs). Reproduced from Murray et al. 6 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The figure includes minor formatting changes.
Drug prescribing
There were a total of 1619 patients (28.7%, 95% CI 27.3% to 30.1%) in the cohort, who received 47,738 prescriptions for the study drugs; proportionally more females than males were prescribed these drugs (see Table 55). In 2008, 33.8% of those prescribed psychotropic medications (305/902 patients) were given prescriptions for two or more drugs. The three most commonly prescribed drug groups overall (and in male patients) were sleep medication (9.7% overall, 95% CI 8.9% to 10.6%; 10.0% of males, 95% CI 9.1% to 10.9%), stimulants (7.9% overall, 95% CI 7.2% to 8.7%; 8.7% of males, 95% CI 7.8% to 9.5%) and antipsychotics (7.3% overall, 95% CI 6.7% to 8.1%; 7.0% of males, 95% CI 6.3% to 7.8%). For females they were antiepileptics/mood stabilisers (11.0%, 95% CI 9.0% to 13.3%), antidepressants (9.0%, 95% CI 7.2% to 11.1%) and sleep medication (8.5%, 95% CI 6.8% to 10.6%). Clonidine and beta-blockers were rarely prescribed; only 30 and 36 patients in the total cohort, respectively, were prescribed these drugs. The five most commonly prescribed drugs were methylphenidate (7.1% of the ASD cohort, 95% CI 6.4% to 7.8%), melatonin (5.7%, 95% CI 5.1% to 6.4%), risperidone (5.6%, 95% CI 5.0% to 6.3%), diazepam (3.2%, 95% CI 2.8% to 3.7%) and valproic acid (3.2%, 95% CI 2.8% to 3.7%).
Stimulants accounted for 23% (n = 10,981) of all psychotropic drug prescriptions issued to the cohort and the average number of prescriptions per patient was 24.6. However, the drug group with the most prescriptions was antiepileptics/mood stabilisers, with 33.2% of all prescriptions (n = 15,868; the mean number of prescriptions per patient was 44.1) (Table 56).
| Drug group | Number of patients prescribed (%) | Number of prescriptions (%) | Mean number of prescriptions/patient (SD) | Number of males (%) | Number of prescriptions to males (%) | Mean number of prescriptions/male patient (SD) | Number of females (%) | Number of prescriptions to females (%) | Mean number of prescriptions/female patient (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Stimulants | 446 (27.5) | 10,981 (23.0) | 24.6 (26.8) | 406 (30.9) | 10,159 (27.5) | 25.0 (27.0) | 40 (13.0) | 822 (7.6) | 20.6 (24.2) |
| Antidepressants | 350 (21.6) | 5183 (10.9) | 14.8 (19.1) | 263 (20.0) | 3918 (10.6) | 14.9 (19.8) | 87 (28.3) | 1265 (11.8) | 14.5 (16.8) |
| Antipsychotics | 415 (25.6) | 7934 (16.6) | 19.1 (24.3) | 329 (25.1) | 6105 (16.5) | 18.6 (22.9) | 86 (28.0) | 1829 (17.0) | 21.3 (29.0) |
| Antiepileptics and mood stabilisers | 360 (22.2) | 15,868 (33.2) | 44.1 (61.4) | 254 (19.4) | 10,651 (28.8) | 41.9 (60.9) | 106 (34.5) | 5217 (48.5) | 49.2 (62.5) |
| Benzodiazepines | 275 (17.0) | 1570 (3.3) | 5.7 (12.2) | 195 (14.9) | 934 (2.5) | 4.8 (11.6) | 80 (26.1) | 636 (5.9) | 8.0 (13.3) |
| Clonidine | 30 (1.9) | 395 (0.8) | 13.2 (15.2) | 27 (2.1) | 389 (1.1) | 14.4 (15.6) | 3 (1.0) | 6 (0.1) | 2.0 (1.0) |
| Sleep medication | 550 (34.0) | 4735 (9.9) | 8.6 (13.1) | 468 (35.7) | 4027 (10.9) | 8.6 (13.0) | 82 (26.7) | 708 (6.6) | 8.6 (13.5) |
| Beta-blockers | 36 (2.2) | 312 (0.7) | 8.7 (15.5) | 28 (2.1) | 249 (0.7) | 8.9 (16.8) | 8 (2.6) | 63 (0.6) | 7.9 (10.3) |
| Other | 78 (4.8) | 760 (1.6) | 9.7 (15.5) | 56 (4.3) | 551 (1.5) | 9.8 (16.9) | 22 (7.2) | 209 (1.9) | 9.5 (11.5) |
| Antiparkinsonism drugs | 43 (2.7) | 635 (1.3) | 14.8 (19.1) | 32 (2.4) | 472 (1.3) | 14.8 (20.8) | 11 (3.6) | 163 (1.5) | 14.8 (13.8) |
| Any study drug | 1619 (100.0) | 47,738 (100.0) | 29.5 (48.2) | 1312 (100.0) | 36,983 (100.0) | 28.2 (45.0) | 307 (100.0) | 10,755 (100.0) | 35.0 (59.7) |
The numbers of individuals with ASD diagnoses between 1992 and 1998 were relatively low (see Figure 28), which made prescribing rates unstable. Consequently, Figure 29 shows the prescribing rates of the drug groups from 1999 to 2008 only.
FIGURE 29.
Proportion of cohort with ASD prescribed (a) stimulants, sleep medication or benzodiazepines; (b) antipsychotic or antiparkinsonism drugs; and (c) antidepressants or antiepileptics and mood stabilisers (1999–2008). Reproduced from Murray et al. 6 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The figure includes minor formatting changes.
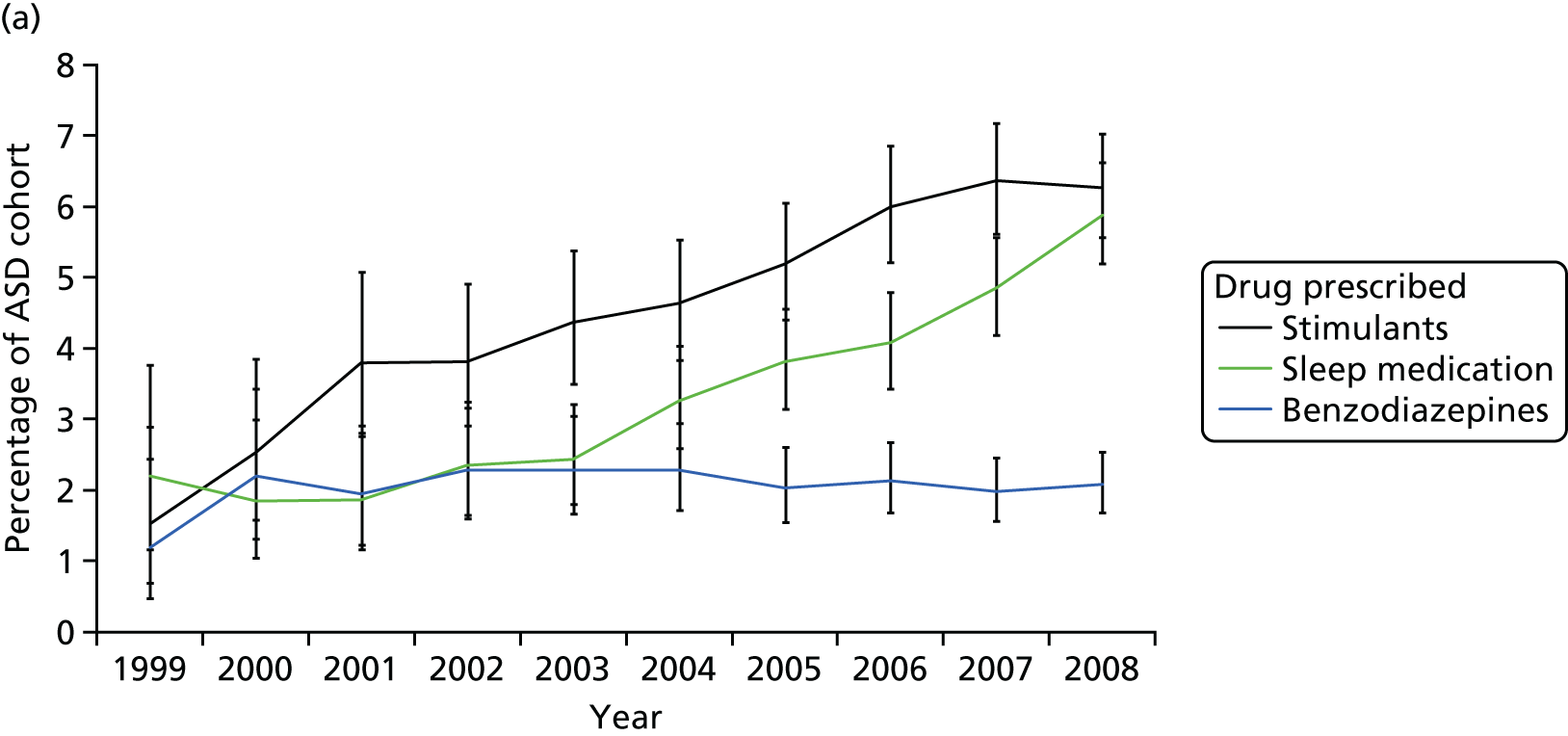


The proportion of the cohort prescribed stimulants rose by 4.7% between 1999 (1.5%, 95% CI 0.7% to 2.9%) and 2008 6.3%, 95% CI 5.6% to 7.0%) (see Figure 29a). Sleep medications also were prescribed to 3.7% more young people during the same period [from 2.2% (95% CI 1.2% to 3.8%) in 1999 to 5.9% (95% CI 5.2% to 6.6%) in 2008]. The prescribing of benzodiazepines, antipsychotics and antiparkinsonism drugs remained stable (see Figure 29a and b), and a slight downwards trend in antidepressant use was seen (see Figure 29c). There was a 1.8% decline in the proportion of patients prescribed antiepileptic drugs between 1999 and 2008, from 6.1% (95% CI 4.3% to 8.4%) to 4.3% (95% CI 3.7% to 4.9%) (see Figure 29c). Finally, prescribing rates (Figure 30) were relatively low in young children/peak in young adolescents and reduce again in young adulthood.
FIGURE 30.
Medications prescribed by age (THIN, 1992–2008).
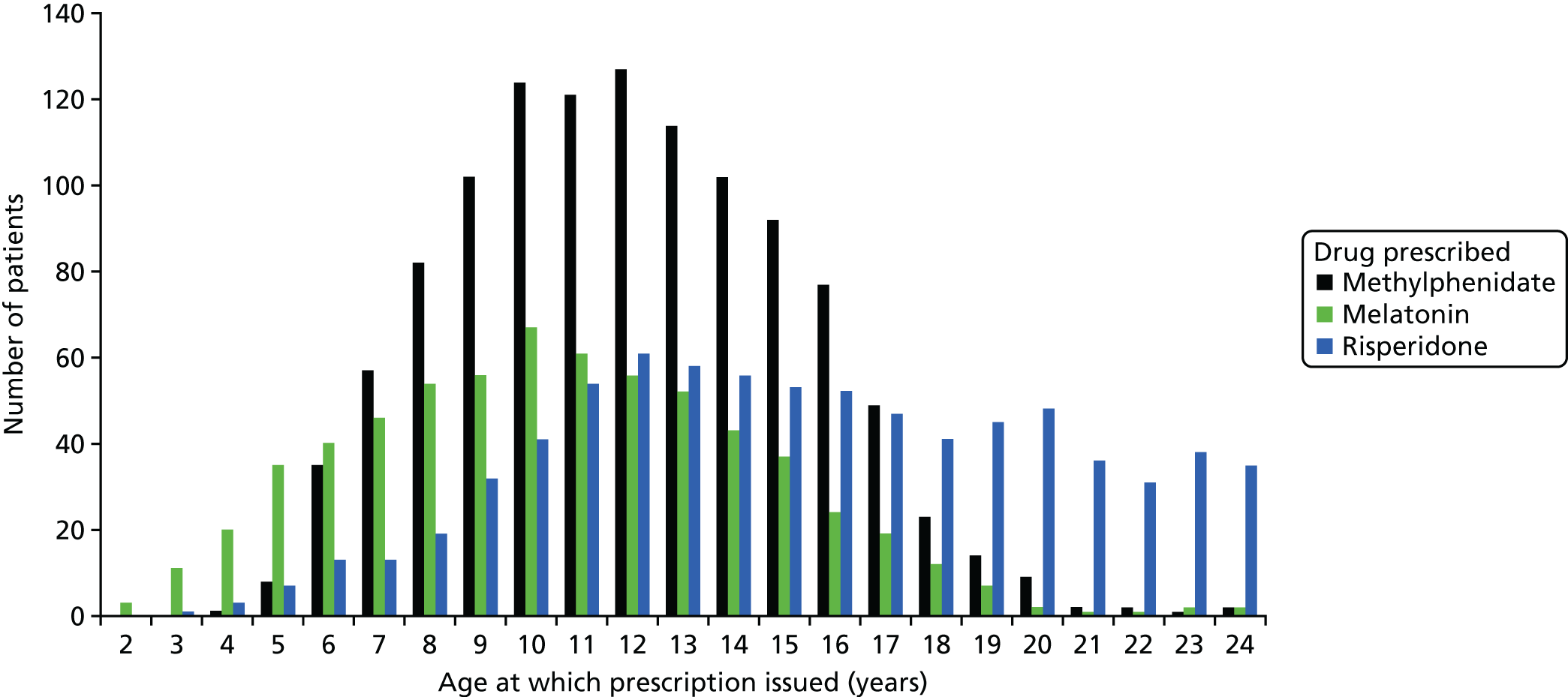
Psychiatric comorbidities
There were 2062 patients (36.5%, 95% CI 34.9% to 38.1%) who had at least one record of a neuropsychiatric comorbidity on or after their first recorded diagnosis of ASD on the database. The most common neuropsychiatric comorbidities were developmental difficulties and LDs (12.6%, 95% CI 11.7% to 13.6%), behavioural disorders, CDs and PDs (11.1%, 95% CI 10.3% to 12.0%), ADHD (7.5%, 95% CI 6.8% to 8.2%) and epilepsy (5.0%, 95% CI 4.4% to 5.6%) (Table 57). Of those with an ADHD diagnosis, 62.0% (n = 261) were prescribed a psychostimulant during the study period and 21.4% (n = 90) received at least one prescription for a sleep medication. Furthermore, 41.5% (185/446) of those given at least one psychostimulant prescription had no comorbidity of ADHD recorded during the period. Almost three-quarters of patients (n = 210) with epilepsy recorded were prescribed an antiepileptic drug.
| Neuropsychiatric comorbidy | All | Male | Female |
|---|---|---|---|
| ADHD | 421 (7.5) | 378 (8.1) | 43 (4.5) |
| Anorexia and bulimia | 47 (0.8) | 30 (0.6) | 17 (1.8) |
| Anxiety and phobias (including OCD) | 251 (4.4) | 192 (4.1) | 59 (6.1) |
| Behavioural, conduct and PDs | 628 (11.1) | 505 (10.8) | 123 (12.8) |
| Mood disorders | 189 (3.3) | 134 (2.9) | 55 (5.7) |
| Epilepsy | 283 (5.0) | 209 (4.5) | 74 (7.7) |
| Developmental difficulties and LDs | 713 (12.6) | 562 (12.0) | 151 (15.7) |
| Psychotic disorders (including schizophrenia and delusions) | 37 (0.7) | 29 (0.6) | 8 (0.8) |
| Substance and alcohol misuse | 18 (0.3) | 13 (0.3) | 5 (0.5) |
| Suicidal behaviour and self-harm | 63 (1.1) | 45 (1.0) | 18 (1.9) |
| Tic disorders | 64 (1.1) | 60 (1.3) | 4 (0.4) |
| Unclassified neuropsychiatric disorders | 287 (5.1) | 227 (4.8) | 60 (6.2) |
| Any neuropsychiatric comorbidity | 2062 (36.5) | 1669 (35.6) | 393 (40.8) |
Discussion
To our knowledge this is the first naturalistic study of psychotropic drug prescribing and neuropsychiatric comorbidities in a large nationally representative cohort of ASD patients in UK primary care. The database has provided rich longitudinal data to enable us to characterise the shared care arrangements with secondary care physicians.
We found that the incidence and prevalence of ASD increased substantially, by 23.7-fold and 64.6-fold, respectively, between 1992 and 2008. These increases may be due to improved recognition and diagnosis of ASD, broadened diagnostic criteria and increased awareness and acceptance of ASD by health-care professionals and parents. 54,314,315 The 2008 prevalence of ASD diagnoses in 6- to 12-year-olds in our cohort (0.89%, 95% CI 0.85% to 0.93%) is comparable to that reported by Baird and colleagues314 (1.16%, 95% CI 0.90% to 1.42%) from their Special Needs and Autism Project sample and the estimate of 1.57% (95% CI 0.9% to 2.46%) in a Cambridgeshire school-based population. 315
Use of psychotropic drugs by British GPs (usually initiated by specialist physicians) is relatively conserative compared with the practices of their American colleagues. Only 29% of our cohort (26% aged 0–21 years) received psychotropic drug prescriptions in UK primary care. In contrast, Mandel and colleagues288 found that 56% of Medicaid-enrolled youths with ASDs aged 0–21 years received at least one psychotropic drug in 2001; likewise, in 2002, 57% of youths aged < 21 years enrolled to another US claims database (MarketScan®, Truven Health Analytics, Michigan, MI, USA) were prescribed psychotropic drugs. 287
Psychotropic drug prescribing practices will be strongly influenced by comorbidities associated with ASDs, which may explain the large proportion of those prescribed psychotropic drugs who were given more than one psychotropic drug (34% of prescribed patients in 2008). One-third of our cohort had at least one neuropsychiatric comorbidity recorded in their primary care records following ASD diagnosis – the most common being developmental difficulties and LDs, behavioural disorders, CDs and PDs, and ADHD. Simonoff and colleagues58 found in their Special Needs and Autism Project cohort that 28.2% were comorbid with ADHD, 30.0% had oppositional disorder or CD and 41.9% had anxiety or phobias; our cohort had much lower proportions of these comorbidities. It is possible that there was under-recording as specialist physicians may find it difficult to diagnose comorbid psychiatric disorders due to communication impairment or cognitive problems in individuals. 201 Alternatively, there may be nosological conventions which exclude the diagnosis of certain symptoms as comorbid psychiatric disorders (e.g. symptoms of hyperactivity and impulsiveness in autistic people may not be reported as ADHD). 55,316 New diagnostic guidelines in DSM-V and almost certainly International Classification of Diseases, Eleventh Edition, will remove the exclusion of making a diagnosis of ADHD in the context of ASD, which has been present. Therefore, a large proportion of stimulant prescribing in our study is likely to be for the treatment of comorbid ADHD or symptoms of hyperactivity,280,317 even though two-fifths of stimulant-prescribed patients did not have an ADHD diagnosis recorded during the study period.
We found that individuals with ASD are much more likely than the UK general population to be prescribed psychostimulants. For example, McCarthy and colleagues307 reported that 0.92% of children aged 6–12 years and and 0.74% of those aged 13–17 years in THIN were prescribed a stimulant drug in 2008. However, 6.60% (of children aged 6–12) and 8.79% (of adolescents aged 13–17 years) of our cohort received at least one prescription for methylphenidate, dexamfetamine or atomoxetine in the same year. So compared with a child in the general population, a child with ASD is seven times more likely to be prescribed a psychostimulant. For autistic adolescents, psychostimulant prescribing is almost 12 times higher than that of the general population.
The increase in prescribing of stimulants over time may reflect the increasing recognition of comorbid ADHD (and its persistence) in young people with ASDs. In addition, secondary care physicians may be more willing to initiate (and GPs more willing to prescribe) methylphenidate following evidence that it is effective in some autistic young people with ADHD. 55 Two-thirds of the autistic population are reported as having moderate sleep disturbances. 318 However, < 10% of our cohort received sleep medications (< 5% were prescribed benzodiazepines, some of which may be used to alleviate sleep problems); it is likely that UK GPs consider prescribing pharmacological treatments only to patients with severe sleeping disorders. It is interesting to note the rising trend of sleep medication prescribing. The trend may be due to increased diagnosis of comorbid ADHD and/or the use of sleeping medications to counteract the effects of stimulants on sleep. Prescribing for antipsychotics and antidepressants in the cohort was relatively stable between 1999 and 2008, at the overall prescribing rates of 4.1% (95% CI 3.85% to 4.37%) and 3.3% (95% CI 3.03% to 3.49%), respectively. This differs from reported increases in antidepressant and antipsychotic use in the US autistic population. Two drug surveys conducted in North Carolina in 1992–3 and 2001 revealed a significant 3.5-fold increase in the proportion of children and adults with autism who were prescribed antidepressants. 286 Williams and colleagues290 found that use of antipsychotics was high (34% in 2008 vs. 7% of our cohort) in their study of Kentucky-based children with autism. This was attributed to the greater use of atypical antipsychotics following the licensing of risperidone in the USA for the treatment of irritability associated with autistic disorder. 290 Although risperidone remains unlicensed in the UK for this indication, we still found that one-fifth of the cohort who were prescribed psychotropic drugs were given risperidone. Although the ratio of the cohort prescribed antipsychotics remained relatively stable between 1999 and 2008, a high proportion of children and teenagers with ASD are prescribed antipsychotics in comparison with the general British population. The prevalence of antipsychotic prescribing in 0- to 18-year-olds was reported to be 0.08% in 2005,319 whereas in the same age group in our cohort the prevalence was 2.98% (a 38.7-fold increased risk).
Antiparkinsonism drugs were prescribed to a very small proportion of patients (0.8% of ASD cohort, n = 43), probably to mitigate extrapyramidal symptoms of antipsychotics, as has been reported elsewhere. 289
Strengths and weaknesses
There may be some underestimation of prescribing as some patients may be treated in specialist centres and THIN does not systematically record prescriptions generated in non-primary care health-care settings (some information on drug treatment may be recorded in discharge summaries and hospital letters). In addition, some GPs may not participate in shared care prescribing arrangements, especially of psychotropic drugs in off-label use and controlled drugs such as the psychostimulants (e.g. methylphenidate and dexamfetamine). The database does not record treatment compliance or dispensing of prescriptions. There may be underestimation of neuropsychiatric comorbidities due to the censoring of patient records, or incomplete transcription of clinical details from correspondence with specialist teams. Finally, prescriptions are not directly linked to diagnoses in THIN, so we cannot definitely identify indications of use.
Further assessment of the efficacy and short- and long-term safety of psychotropic drugs for the treatment of behavioural problems in this population is urgently required, as only two drugs so far (risperidone and aripiprazole) have shown efficacy. 293–295 More recently, optimal dosing of methylphenidate was shown to be effective in reducing ADHD symptoms in about 40% of children with ID (including those with ASDs), and the adverse effects profile was similar to that seen in typically developing children. 54 Safety is an important consideration across all ages due to the lifetime persistence of symptoms and impairment, polypharmacy of psychotropic medications and the difficulties some individuals may have in communicating about adverse effects. More information on psychotropic drug use (and the appropriateness of prescribing for comorbidities) in the ASD population across Europe would be beneficial to focus European drug research activities and to inform clinical practice. In addition, there is a need to prioritise research of the most commonly prescribed drugs in the autistic population: the antipsychotics (in particular risperidone safety), the psychostimulants (especially efficacy and safety in those comorbid with ADHD) and sleep medications.
Conclusions
The increase in prevalence of ASD diagnoses recorded in UK primary care between 1992 and 2008 may be due to improved recognition of ASDs, widening diagnostic criteria and more awareness and acceptance of the conditions. However, there is under-reporting of neuropsychiatric comorbidities in the British autistic population following diagnosis of ASDs, possibly due to diagnostic exclusion or communication difficulties in patients. Fewer than one-third of individuals with ASD receive at least one psychotropic drug prescription, which are mostly for stimulants, antiepileptic drugs and mood stabilisers, and antipsychotics. Of those who are prescribed psychotropic medication, one-fifth are treated with risperidone and at least one-third receive more than one drug. Hence, further research into the appropriate use, efficacy and long-term safety of antipsychotics and stimulants in the autistic population is warranted to support clinical practice for optimal and safe treatment of ASDs and their comorbidities.
Psychotropic drugs were prescribed to 29% of the ASD cohort; the most prescribed drugs were sleep medication, psychostimulants and antipsychotics.
Prescribing rates of psychostimulants and sleep medications are increasing over time.
Polypharmacy was seen in approximately equal to one-third of prescribed patients.
Prescribing levels are highest in young teenagers and lower in young adults.
Chapter 21 Piloting pharmacology trials in frequently excluded groups
Overview
In the previous chapter we established the current prescribing rates for ASD in the UK. We and others had previously published similar data on ADHD outside this programme. Hence, we now had good knowledge of current pharmacological treatments in both ASD and ADHD as used in general practice. However, the effectiveness of current pharmacological treatments in specific subgroups of affected individuals (e.g. those with ID and prisoners), and the effectiveness of CBT-based approaches, remained poorly understood. This is due to lack of clinical trials; one contributing factor to the lack of trials in frequently excluded groups such as these is that investigators typically face significant hurdles (e.g. ethical and practical).
Hence, in order to make the most rapid progress we ran two concurrent work streams to obtain pilot data/proof of concept on the ability to run UK trials on effectiveness of interventions in ADHD and ASD. The first focused on the effectiveness (or otherwise) of current pharmacological treatments for ADHD in people typically excluded from trials (those with ID); and the second piloted a new CBT-based treatment approach for OCD in ASD (discussed in Chapter 22).
Our first step was to pilot an open-label trial of atomoxetine for ADHD in young people with ID (see Pharmacology step 1: atomoxetine trial for ADHD in people with intellectual disability). This was unsuccessful and so resources were diverted to a proof of concept study in a prison setting (see Pharmacology step 2: a pilot study on treating ADHD in prison).
Pharmacology step 1: atomoxetine trial for ADHD in people with intellectual disability
The aim of this project was to obtain initial data about the efficacy and profile of common adverse effects among youngsters with ADHD and ID (global LD) receiving atomoxetine as a second-line treatment. Currently, there is a substantial evidence base for the treatment options among people who are of average cognitive ability. Current guidelines recommend the first-line use of methylphenidate (often in a sustained-release preparation), followed by atomoxetine if methylphenidate is unsuccessful, due either to lack of efficacy or to dose limitation secondary to adverse effects. 320 However, the evidence base does not include young people with global LD, typically defined by an IQ of < 70, along with impairment in adaptive functioning. Considering the latter group separately is important for two main reasons. First, they are disproportionately likely to be affected with ADHD; our research,317 and that of others,321 showed an 8- to 10-fold increase in the rates of ADHD. Second, there are good reasons to consider that the evidence from cognitively average – ‘typically developing’ – young people may not directly extrapolate to them. For instance, studies of methylphenidate indicate that it has a smaller effect size in people with ID – about 0.5 compared with 0.9 in typically developing children. 54 In addition, youngsters with ID have considerably higher rates of adverse effects; much of this appears to be related to the increased rate of ASD in people with ID. 54 In some cases, this is simply greater sensitivity as demonstrated by higher rates of common adverse effects; in others it is idiosyncratic effects. In addition, individuals with ID more commonly have neurological and physical disorders, such as epilepsy or congenital heart disease, that may influence the ability to use ADHD medication in therapeutic doses.
Hence, to address key scientific issues about the efficacy of stimulant treatment for ADHD, we undertook a randomised controlled trial (RCT) of methylphenidate, funded by The Health Foundation. 322 We reported effect sizes of 0.3–0.52. Among parents of children receiving methylphenidate, 40% reported their child to be ‘improved’ or better, compared with 7% on placebo. Nevertheless, despite these promising results, many parents felt that their child had not made substantial improvement and a survey indicated that they would welcome the opportunity to try an alternative treatment. At the time of application, the evidence for atomoxetine came from studies with typically developing children or those with ASD; the latter evidence base was one open-label trial of 16 children. Therefore, to extend the evidence base of atomoxetine as a second-line intervention for youngsters with ADHD and ID, we undertook an open-label trial of carefully titrated medication in which efficacy and common adverse effects were systematically assessed. The trial included young individuals who had not shown an adequate response to methylphenidate, either due to lack of efficacy of an adequate dose or because of dose limitations due to adverse effects. We proposed to recruit affected individuals either from our previous trial, described above, or from routine clinical practice.
What we did
The trial was set up with the involvement of the clinical trials unit at King’s College London. This involved developing a trial protocol, which included genetic testing to determine whether or not genetic polymorphisms were moderating response to atomoxetine. The trial protocol was reviewed by the Joint Clinical Trials Office and the Medicines and Healthcare products Regulatory Agency. However, ethics approval was delayed for several reasons. First, despite using a very similar set of measures that were approved in the previous trial, the REC raised concerns about the inclusion of measures that characterised the sample either for purposes of eligibility assessment or for broader description at the time of publication. We explained the need for these data as part of good clinical practice. Second, we were asked to alter the method of recruitment into the trial from two stages to one stage. In the previous trial we discovered that it was best to consent participants and their families first to an assessment and then, if eligible, to the trial. Although families were aware from the outset that they were being assessed for the trial, we discovered that the two-stage consent process helped to highlight that they might not be eligible, as indeed only a minority were. However, we were asked to change this and did so. We were also asked to give more prominence to certain possible adverse effects, specifically suicidal ideation.
Following ethics approval, we began recruitment from our methylphenidate trial. Although a number of parents had previously indicated an interest, recruitment was much lower than anticipated. There were two main reasons for this. First, a number of the parents who had indicated at the end of the methylphenidate trial that their child had not improved reported, when recontacted, that they thought they were doing well on their present medication and did not want to change. Second, there was a small group of parents who had not waited for the trial to obtain ethics approval and had gone to their local clinician and requested a medication change. Over 1 year, we recruited 12 patients from our methylphenidate trial and received no referrals from clinicians in the NHS, despite monthly e-mails and regular visits to clinics. This reflected several issues. First, there was underidentification – diagnostic overshadowing – of ADHD in people with ID generally (as we had previously noted in our methylphenidate trial). Second, we were trying to identify a subgroup of a subgroup (i.e. those with ADHD and ID who had not responded to medication). Although we were trying to recruit across the South Thames region, this probably was not a large enough region. Hence, we contacted the Medicines for Children Network to determine whether or not they could help with recruitment, but they were unable to take on the study.
The trial was therefore halted and we reallocated resources into a novel opportunity that arose only after the programme started. Specifically, our NHS trust took over the provision of mental health services to prisons for young people in 2012. This allowed us to build on our work in forensic populations (see Chapter 16) and initiate a proof of concept study on the ability to undertake treatment trials for NDs in prison settings (focusing first on ADHD; see Pharmacology step 2: a pilot study on treating ADHD in prison).
What we learned from this failure and how it influenced practice
We learned a number of lessons from the study. First, although we had a robust trial protocol and considerable experience of obtaining ethics approval to undertake clinical trials with the patient population, we noted the considerable variation among RECs and learned that we could not assume that they would have similar concerns. In small part, this reflected changes surrounding expectations in trial conduct, but was largely due to different panel members’ views. However, this led to considerable time delays. The need to approach several different local RECs in addition to a central REC (and despite central ethics approval already been given) leads to very significant delays in clinical trials. For instance, we found that there can be a poor understanding in many RECs about both children and adults with ID, with varying views about the appropriateness of seeking assent (from minors), how to establish capacity in adults and methods for managing consent when capacity is not present. We also learned that the absence of systematic patient databases hinders their recruitment to research, including treatment trials from which they might benefit.
The lessons learned in this research have been incorporated into other work. Specifically, the clinical and research recommendations in the current NICE guidance on the management and support of children with autism, just completing its consultation,323 have been influenced by this research. These guidelines highlight the need for systematic assessment of children to identify co-occurring disorders such as ADHD, which might be treatable in their own right.
Issues raised by this project and other experience of clinical trial among people with LD will be showcased in a proposed editorial for the British Journal of Psychiatry as part of a special edition.
Pharmacology step 2: a pilot study on treating ADHD in prison
Background
Based on the finding of high rates of ADHD, and to a lesser extent ASD, within forensic adult mental health services and adult prisons noted above (see Chapters 11 and 12), there is a need to evaluate rates in prisons and the effectiveness of treatments for both disorders within these settings. We therefore determined, as a first step, to ‘pump prime’ from this programme a pilot study of ADHD treatment in young offenders.
Our rationale was that failure to recognise and treat ADHD most likely has a wide-reaching impact on mental health and behaviour in forensic populations. Our previous work demonstrated that ADHD is associated with high rates of incidents of verbal and physical aggression and other disruptive behaviours in both forensic hospital services, as noted in our work above, and, as we previously published, in prisons. For instance, we previously reported that ADHD accounted for a sixfold increase in such critical incidents among prison inmates, even after controlling for antisocial PD. 137 Furthermore, the symptoms of ADHD are known to interfere with both educational and employment activities due to a combination of restless overactivity and impulsivity, and the problems associated with inattention, forgetfulness and problems with self-organisation. 95,105 This suggests that ADHD itself may lead directly to some forms of disruptive behaviour, potentially linked to difficulties with mood regulation (mood lability/volatile mood states/deficient emotional self-regulation), that are strongly associated with ADHD in adults. 130 Hence, we hypothesised that untreated ADHD may explain a significant portion of aggressive behaviour in offenders.
Within forensic settings, the violent behaviour associated with ADHD significantly impacts on the quality of life of the individual, but also on forensic services and society more widely as these individuals can be caught up in the ‘revolving door’ of the criminal justice system. However, ADHD is a treatable condition with the most recent NICE guidelines95 concluding that stimulant medication (methylphenidate or dexamfphetamine) and atomoxetine provide cost-effective treatments for the control of ADHD symptoms in adults. Nevertheless, as we found earlier (see above), ADHD often remains unrecognised and untreated, which means that known effective treatments for ADHD (and potentially also for the co-occurring challenging behaviours) are not provided. The NICE guidelines for ADHD highlighted the extent of this unmet need and the requirement to establish effective services for the diagnosis and treatment of ADHD in adults, to prevent both immediate and longer-term impairments. Thus, we envisage that medical treatments used for ADHD in the general population will be effective in reducing both the core symptom of ADHD and some of the aggressive/antisocial behaviour and problems with engagement with educational activities associated with ADHD in forensic populations. To date, however, these questions have not been investigated among young offenders in the UK (although rates of ADHD will undoubtedly be high in this group), and there is very limited guidance from international research.
Methods
We initiated an open-label pilot study of an extended release formulation of methylphenidate (Concerta XL®, Janssen-Cilag Ltd) on aggression, in young male offenders with ADHD aged 18–30 years. The primary outcome measure will be a change in disruptive behaviour as recorded in the prison records (number of critical incidents). Secondary outcomes include measures of engagement with educational activities reported by staff within the prison educational programme; measures of aggressive behaviour collected using a behavioural report by prison and education programme staff; and the number of recorded reports of disruptive behaviour reported by the educational programme staff.
Changes will be measured in the level of symptoms ADHD and emotional dysregulation, which previous research has shown responds well to methylphenidate, with an average effect size of around 0.5. 324 This will include ratings of the DSM-IV ADHD items from the Conners’ Adult ADHD Rating Scales – Observer; and the associated symptoms of ‘emotional dysregulation’ from the Wender–Reimherr Adult ADHD Scale. 325
Further analyses will explicitly test the mediation hypothesis that change in symptoms of ADHD and emotional dysregulation mediate changes in aggressive behaviour and engagement with educational activities; and provide estimates of the extent of any mediation effects (partial or complete mediation of the behavioural problems).
Dose escalation from 18 mg to a maximum of 90 mg is intended to maximise the effect of the medication on ADHD symptom levels while limiting potential adverse effects. In addition, to evaluate the longer-term outcomes associated with the use of Concerta XL in this population, the 12-week open-label trial will be followed by a 6-month open-label trial with titration to an optimal dose based on clinician judgement.
The main hypotheses that will be tested are that:
-
ADHD is found in high rates among young male offenders aged 18–30 years (the initial stages of the project allow prevalence estimates for the disorder among the prison population to be estimated).
-
Treating ADHD with Concerta XL will lead to reductions in aggressive behaviour within the prison setting.
-
Treating ADHD with Concerta XL will lead to increased engagement with educational activities within the prison setting.
-
Treatment with Concerta XL will lead to reductions in ADHD symptoms within the prison setting (these changes will be similar in magnitude to those reported in previous clinical and prison populations of adults with ADHD142,324).
-
Treatment with Concerta XL will lead to reductions in symptoms of emotional dysregulation (these changes will be similar in magnitude to those reported in previous clinical trials of the effects of stimulants on symptoms of emotional dysregulation326).
-
The ADHD symptoms and emotional dysregulation symptoms will show strong co-variation during the treatment response.
-
Change in symptoms of ADHD and emotional dysregulation will mediate changes in the measures of aggression and engagement with educational activities.
Study setting
This pilot work is taking place at HMP & YOI Isis, which is in the Belmarsh prison cluster. This is a relatively new prison, which opened in 2010 and holds sentenced young male adults and category C offenders. There are two house blocks with mixed single and double cells and the operational capacity was measured at 480 in August 2010. HMP & YOI Isis has a broad-based curriculum for young sentenced prisoners and available activities include mechanics, construction, bicycle repair, catering, broadcasting, job-related studies and offending behaviour interventions. It therefore has a rehabilitation and resettlement emphasis and all prisoners are offered a full-time occupation.
Progress to date
We have reviewed screening data on 592 prisoners and identified 129 prisoners who screen positive for ADHD. We completed 71 full diagnostic assessments and confirmed a diagnosis of ADHD in 55 of these, giving an overall prevalence rate just below 20%. This means that treatment for ADHD should be considered in around one in five young adult male prisoners.
Recruitment into the treatment trial part of the programme has started, with 16 participants currently entered into the pilot clinical trial. We have identified two main areas of impairment that can impact of risk for repeat offending. First, there is a high rate of dysfunction with education and occupation that is linked to the specific symptoms and impairments of ADHD: poor attention span and being easily distracted, disorganised and forgetful. In addition, many individuals show motor overactivity and impulsive traits that reduce their ability to function in the workplace. The second main area of difficulty is mood instability with temper outbursts that can have a significant role to play in violent offending.
This pilot work is now being pursued as part of a Medical Research Council Efficacy and Mechanism Evaluation-funded grant to Professor Philip Asherson (the principal investigator).
Ethics governing research in vulnerable populations is complicated by considerable variation in local RECs.
The need to approach many local RECs in addition to one central REC slows research considerably.
It is possible to carry out treatment trials for NDs such as ADHD in prisons.
Chapter 22 Piloting psychological approaches: a pilot randomised controlled trial of cognitive–behavioural therapy for comorbid obsessive–compulsive disorder in high functioning ASD
Introduction
In prior chapters we audited – and initiated – pharmacological approaches. However, it is crucial to also offer effective non-pharmacological treatment approaches. As a first step we targeted associated mental health symptoms in ASD as the following:
-
These are very common in this group.
-
We already demonstrated in this programme (see Chapter 5), they very significantly add to the burden experienced by both the affected individual and their carers.
-
There is already substantial evidence for the effectiveness of these approaches in non-autistic populations.
We focused on anxiety disorders, as we found high rates as part of this programme and, likewise, high rates have been reported by others, in both young people316 and adults. 327,328 Rates of disorders in childhood range from 11% to 84%, and a selective pattern of anxiety disorders, namely social anxiety, OCD and specific phobias, has been reported. 329,330
Childhood anxiety reduces social interactions, self-esteem and impoverishes social skills in typically developing children,331 thereby exacerbating problems characteristic of ASD. Furthermore, behavioural problems have been noted to be more likely to berelated to fears in children with ASD than in other groups. 332
Comorbid OCD has been reported to occur in 30% of young people with ASD,328,333 and high rates of OCD have also been reported in adults with ASD both with and without ID. 334,335 OCD has considerable impact on quality of life for both sufferers and carers and is listed in the World Health Organization’s top 20 leading causes of years lived with disability among individuals aged 15–44 years. 336 OCD is a treatable anxiety disorder with good evidence for the effectiveness of empirically based psychological treatments such as CBT. 337
There is emerging evidence that CBT may be effective in ameliorating distressing and debilitating anxiety in people with ASD. Trials of group CBT interventions for anxiety symptoms330 and anxiety disorders338–340 adapted for children with ASD have reported promising results.
To date, most adult treatment studies of CBT in ASD have been confined to single case reports (e.g. its effectiveness for depression341 and social anxiety disorder342). More recently, we reported343 preliminary evidence from an uncontrolled pilot study of CBT for OCD in 24 adults with ASD and comorbid OCD. We found that, of the 12 adults who received CBT for OCD, seven (58%) showed a good treatment response in comparison with two (16%) in the treatment as usual (TAU) group, with a standardised effect size (Cohen’s d) for CBT of 1.01. This is reasonably consistent with published treatment response rates for behaviour therapy (59%) and CBT (67%) in adults with OCD without ASD. 344
In summary, there is evidence of high rates of anxiety disorders, particularly comorbid OCD, in both young people and adults with ASD. The results of both individual and group systematic psychological treatment evaluations for anxiety disorders in children and adolescents with ASD have been promising, but to date none has been OCD specific. There is preliminary evidence that CBT may be more effective for OCD in ASD than TAU, but this requires replication and comparison with other potentially effective approaches for this group. Hence, the aims of the present study were to systematically evaluate CBT for OCD adapted for people with ASD via a RCT comparing the new intervention with a plausible control treatment.
Methods
Participants
Seventy-five participants were recruited from specialist ASD clinics, specialist adult and paediatric OCD clinics, and generic child and adult mental health services. Participants were individuals with a confirmed diagnosis of an ASD, verbal IQ of > 70 and comorbid OCD, aged between 14 and 65 years. Participants were excluded if they had current psychotic symptoms, a current episode of major depression, uncontrolled epilepsy or current substance misuse. Participants were included only if psychiatric medication was stable in the 6 weeks prior to study entry and if they had a baseline Yale–Brown Obsessive Compulsive Scale (YBOCS) severity rating of > 16, typically used for inclusion in clinical trials. 345 Diagnosis of ASD was confirmed using the ADI-R. Diagnostic information was supplemented by the ADOS196 for all participants. The Mini International Neuropsychiatric Interview 5.0 neuropsychiatric interview was used for assessment of other comorbid psychiatric diagnoses and to confirm the presence of OCD. 346
Delineating anxiety-based obsessions and compulsions from the repetitive routines and behaviours and circumscribed interests characteristic of ASD was completed using the YBOCS Symptom Checklist and in accordance with the procedures developed in an earlier phenomenological study,343 which were detailed in the study manual. In brief, at the start of each clinical interview care was taken to ensure that the participant was cognisant of the phenomena to be rated, that the discomfort and anxiety basis for each potential obsessive–compulsive symptom was clearly established using visual tools if necessary. Eliciting of symptoms was achieved if needed by enquiring about daily routines in total before gathering further phenomenological information. Communication style and preferences of each individual were also taken into account when administering the YBOCS. The presence of obsessions/compulsions was not recorded unless the ego-dystonic basis for unwanted internal phenomena and a resistance to/recognition of the excessive nature of compulsions could be established.
All participants read an information sheet and signed consent forms to take part in the study with developmentally appropriate information and assent forms for participants aged 14–16 years.
The study was registered as a controlled trial (ISRCTN87114880) with ethics approval granted by the local REC.
Study design
A manual outlining ASD-specific adaptations to standard CBT for OCD was developed on the basis of a case note review of the pilot study,343 expert recommendations347,348 and the literature on cognitive and neuropsychological function in ASD where deficits in emotion recognition and executive function are reported. Standard CBT for OCD was adapted by:
-
ensuring that the building blocks for treatment (i.e. understanding and differentiating emotions, particularly anxiety, and making links between thoughts, feelings and behaviours) were in place
-
if required, educational sessions about understanding and rating anxiety were provided before moving on to present the rationale for treatment
-
visual tools and concrete/special interest related analogies were used to convey psychological concepts
-
a structured and therapist-directed approach to sessional and homework content.
The CBT treatment was predominantly exposure and response prevention (ERP) based and this was conducted in the usual hierarchal fashion both in sessions and as homework. Post hoc review of the treatment records identified that an average of 10 (SD 5.4) ERP homework tasks were set and the compliance rate for ERP homework tasks was 79%. Cognitive methods were also used to help individuals test out OCD and anxiety-related beliefs if appropriate. Post hoc review identified that a mean of 2.7 (SD 3.2) sessions contained some cognitive techniques in the CBT group.
The control or comparison treatment was specified as anxiety management (AM) to ensure that any treatment effects were solely due to the adapted CBT for OCD, rather than therapist contact, psychoeducation about anxiety or general anxiety reduction techniques. Furthermore, the general lack of access to psychological treatment services for adults with ASD suggested that TAU or a no treatment condition would unfairly advantage the experimental treatment and would not represent an adequate test of effectiveness.
The AM manual was developed for the present study by one of the authors (MF) and was based on previous work. 349,350 It comprised eight modules, which were adapted for ASD by including visual aids or concrete examples. The modules included education about anxiety, diaphragmatic breathing and practice, progressive muscle relaxation education and practice, education about mood, healthy habits and problem-solving. The AM manual did not contain any of the ‘active’ ingredients considered important in effective treatment of OCD (i.e. ERP or any cognitive strategies addressing OCD-related beliefs).
The treatments were matched for duration (up to 20 sessions) and amount of therapist contact (approximately 1 hour per session). Treatment completers were defined as attending at least seven sessions. Treatment duration was specified as up to 20 sessions as prior experience had suggested that a longer assessment and orientation to therapy phase was necessary for some individuals with ASD. See Appendix 1 for further details regarding the treatments.
The treating therapists were all clinical psychologists (n = 4), trained within a behavioural framework, who had extensive experience in treating OCD in both young people and adults. All had received post-qualification training in CBT for OCD, having attended workshops delivered by OCD specialists. All therapists delivered both treatments on a randomly allocated basis. Three pilot cases (two young people and one adult with ASD) were treated with the CBT manual prior to commencing the RCT for feasibility and user perspective purposes. This also allowed the trial therapists to be trained in working more specifically with people with ASD and OCD. As therapists who had worked in specialist OCD clinics, they had previous experience of working with people with ASD. A consultant clinical psychologist with expertise in both adult and paediatric cases (DMC) provided supervision for the CBT cases.
Randomisation procedure
Participants were randomised to the CBT or AM groups using a table of random numbers (1 : 1 ratio) managed by an investigator who was part of the Trial Management Committee but not a treating therapist.
Review of the study protocol by the REC recommended that the ‘other’ treatment should be offered to participants on completion of the first treatment. Thus, participants were informed via the study information sheet that they could try the other treatment 1 month or more following completion of the first treatment, if they wished.
Treatment fidelity and therapist allegiance
A random proportion of cases (20%) were audio-recorded to ensure treatment fidelity. All treatment sessions were recorded and 20% of these recordings were then randomly selected and rated by an independent therapist, blind to treatment condition and outside the clinical trial, as to whether the session contained OCD-targeted interventions (such as ERP) or exploration of OCD-related beliefs. There was no evidence of cross-contamination on the recordings (i.e. none of the AM sessions was recorded as containing any elements of CBT for OCD).
Outcome measurement
Symptom ratings were made by assessors blind to treatment group prior to commencing treatment (i.e. no more than 4 weeks before the first treatment session), end of treatment (1 week after the final treatment session), and at 1, 3, 6 and 12 months’ follow-up. Assessors were all trained clinicians experienced in administering the YBOCS and interviewing people with ASD. In order to address the validity of the blinding procedure, blind assessors were asked to complete a questionnaire at each assessment point noting which they thought was the randomisation group and if this was (a) a random decision, (b) revealed by the participant or (c) due to clinical improvement. 351 Of the treatment completers, this section was not completed in eight (20%) cases. None of the assessors was ‘unblinded’ to treatment group [i.e. cited (b) as the reason for their choice of treatment group]. Blind assessors were accurate in their assignment of treatment group in 24 (60%) of cases. They described their choice as ‘random’ in 30 (75%) of cases. In 18 (45%) cases, clinical improvement was also cited as a reason for group assignment.
Primary outcome
The YBOCS351 total severity rating was the primary outcome measure. In addition to the 10-item severity scale, the insight item from the YBOCS (YBOCS item 11) was also included, with the interviewer being asked to document ‘what is the worst thing that the patient worries will happen if she/he did not respond to obsessive thoughts or urges to perform compulsions?’ and then rating the extent to which the patient is certain that the feared consequence is reasonable and will actually occur ranging from 0, ‘certain that the feared consequence will happen’, to 5, ‘certain that the feared consequence will not happen’.
A reduction of at least 25% on the YBOCS severity rating scale is considered to be a sensitive but not specific measure of treatment response. 352 A YBOCS total score of ≤ 12 for ≥ 1 week was used to define remission,352 with remission lasting for ≥ 1 month being defined as recovery.
Secondary outcomes
A broad range of outcome measures, including the assessment of other anxiety disorders, were employed.
Clinical Global Impression
The CGI and Clinical Global Impression Improvement (CGI-I) rating scales. 353
Dimensional Yale–Brown Obsessive–Compulsive Scale
The Dimensional Yale–Brown Obsessive–Compulsive Scale (D-YBOCS)354 is a semistructured interview to ascertain the presence and severity of six symptom dimensions of OCD. Each symptom dimension is rated for severity (0–15) with a global rating considering severity overall and global impairment (0–30).
Self-report (adult)
Measures comprised the OCI-R,209 Beck Depression Inventory,355 Beck Anxiety Inventory,356 Liebowitz Social Anxiety Scale357 and the Work and Social Adjustment Scale (WSAS). 358
Self-report (youth ages 14–18 years)
Measures comprised the OCI-R, Beck Depression Inventory – Youth,359 WSAS and the Spence Children’s Anxiety Scale. 360
Treatment satisfaction
All participants were asked to rate their satisfaction with the treatment they had received on an eight-point visual analogue scale ranging from 0, ‘not at all satisfied’, to 8, ‘very much satisfied’.
Informant report (all participants)
A person who knew the participant well, such as parent, carer or spouse, was asked to complete the Children’s Obsessive Compulsive Inventory – Parent Version361 at each measurement point. Severity scores can be obtained by summing up the item responses to give ‘compulsions impairment (0–24), obsessions impairment (0–24) and total impairment (0–48)’. Informants were also asked to complete the Family Accommodation Scale – Parent Report,362 where 13 items relating to the provision of reassurance, modification of home routines, etc., are rated on a five-point scale with a possible maximum score of 52. A total score of 13 is generally used as a cut-off point to indicate clinically meaningful accommodation of OCD symptoms and this has been associated with treatment outcome in paediatric OCD. 363
Correspondence
Correspondence between self-, clinician- and informant-administered measures of symptom content and severity.
There were modest but significant correlations between self and informant reports of symptom severity and clinician-administered pre-treatment measures. Furthermore, the subscales of the OCI-R were associated with the relevant symptom dimensions on the D-YBOCS (Table 58).
| Rating | Informant obsessions severity | Informant compulsions severity | Informant total severity | Clinician total severity | Self-report washing | Self-report hoarding | Self-report ordering | Self-report obsessing | Self-report neutralising |
|---|---|---|---|---|---|---|---|---|---|
| Clinician obsessions severity | 0.481;a p = 0.020; n = 23 | ||||||||
| Clinician compulsions severity | 0.276;a p = 0.155; n = 28 | ||||||||
| Clinician total severity | 0.576;a p = 0.001; n = 28 | ||||||||
| Self-report total severity | 0.482;a p = 0.015; n = 25 | 0.265;a p = 0.108; n = 38 | |||||||
| Clinician contamination severity | 0.468;a p = 0.003; n = 39 | ||||||||
| Clinician hoarding severity | 0.394;a p = 0.014; n = 38 | ||||||||
| Clinician symmetry severity | 0.374;a p = 0.019; n = 39 | ||||||||
| Clinician aggression obsessions severity | 0.419;a p = 0.01; n = 37 | 0.462;a p = 0.003; n = 38 |
Power analysis
Based on the data from the pilot study,343 a sample size calculation showed that in order to detect statistically significant differences between the groups on the primary outcome measure (YBOCS total severity rating) at alpha 5% and 80% power, 19 participants would be required for each randomisation group. We recruited 23 participants in each group, allowing for four dropouts in each treatment arm.
Data analysis
Independent t-tests and chi-squared tests were used to consider any pre-treatment differences between the groups on symptom measures and demographics.
An analysis of covariance was carried out on the primary outcome measure controlling for baseline symptom severity to investigate any difference between the two groups at the end of treatment. Repeated measures analyses of variance were used to detect pre–post treatment changes in the AM and CBT groups. Effect sizes were calculated using Cohen’s d. All of the analyses were intention to treat and, where outcome data were not available, pre-treatment scores were not carried forward. 364
Results
Participant flow
Seventy-five people were referred to the study: eight (10.7%) were self-referrals, 24 (32%) were referred from community mental health services, 21 (28%) were referred by specialist ASD services, 11 (14.7%) were referred by specialist anxiety disorder clinics, 4four (5.3%) were referred by voluntary sector services and referral information was missing for seven (9.4%). Seventeen (22.6%) of these 75 individuals did not meet eligibility criteria for the study (Figure 31), two people were eligible but geography prevented participation and 10 people did not consent to take part.
FIGURE 31.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram.

Twenty-three people were randomised to each of the two treatment groups (AM and CBT), with 20 treatment completers in each group.
Participants in the treatment groups were involved in the treatment arm of the study for equivalent periods of time. The mean number of weeks between pre-treatment and end-of-treatment ratings was 23.74 (SD 10.37) in the AM group and 27.06 (SD 10.27) in the CBT group. The mode or most usual length of treatment in weeks was 25.
Demographics and clinical features
Independent-samples t-tests revealed no differences between the groups for the mean domain and total scores on the ADOS-G, verbal IQ, age, or pre-treatment symptom scores (Table 59).
| AM group, mean (SD) | CBT group, mean (SD) | t-test (df) | p-value | |
|---|---|---|---|---|
| Age (years) | 25.2 (13.5); minimum = 14, maximum = 65 | 28.6 (11.3); minimum = 14, maximum = 49 | –0.93 (44) | 1.00 |
| ADOS-G communication and RSI total | 9.9 (4.7) | 10.7 (4.2) | –0.49 (33) | 0.621 |
| WAIS-III/WISC-III verbal IQ | 97.3 (15.2) | 102.5 (16.7) | –0.91 (30) | 0.367 |
| Clinician administered | ||||
| YBOCS obsessions severity | 12.4 (3.1) | 11.6 (2.7) | 0.90 (39) | 0.370 |
| YBOCS compulsions severity | 12.9 (2.8) | 13.2 (1.5) | –0.47 (39) | 0.638 |
| YBOCS total severity | 25.1 (5.2) | 24.8 (3.7) | 0.20 (39) | 0.839 |
| D-YBOCS global severity | 20.4 (5.1) | 20.6 (3.7) | –0.15 (41) | 0.880 |
| CGI | 4.2 (0.8) | 4.1 (0.7) | 0.35 (37) | 0.725 |
| Self-report | ||||
| OCI-R | ||||
| Checking subscale | 5.8 (3.6) | 6.4 (3.7) | –0.37 (33) | 0.708 |
| Hoarding subscale | 5.1 (3.6) | 5.2 (3.6) | –0.19 (33) | 0.849 |
| Neutralising subscale | 2.8 (3.4) | 3.3 (3.0) | –0.58 (33) | 0.566 |
| Obsessing subscale | 6.6 (3.6) | 6.4 (4.0) | 0.26 (32) | 0.790 |
| Ordering subscale | 6.6 (3.2) | 5.3 (4.4) | 0.16 (34) | 0.256 |
| Washing subscale | 4.3 (4.1) | 3.8 (3.8) | 0.16 (34) | 0.869 |
| OCI-R total | 30.9 (13.7) | 30.5 (15.9) | 0.09 (34) | 0.929 |
| BDI | 16.9 (12.0) | 17.3 (13.5) | –0.11 (38) | 0.912 |
| BAI | 15.4 (10.9), n = 15 | 16.2 (11.6), n = 17 | –0.19 (37) | 0.725 |
| SCAS total score | 27.8 (4.7), n = 5 | 28.3 (20.3), n = 3 | –0.05 (6) | 0.955 |
| LSAS total score | 76.8 (26.1) | 67.5 (33.5) | 0.82 (26) | 0.418 |
| WSAS | 18.5 (9.5) | 18.8 (10.9) | –0.069 (30) | 0.946 |
| Parental/carer report | ||||
| CHOCI symptom total | 14.7 (8.1) | 14.4 (6.0) | 0.11 (25) | 0.908 |
| CHOCI impairment total | 28.9 (12.5) | 27.9 (11.6) | 0.21 (25) | 0.832 |
| FAS (n = 27) | 24.1 (13.5) | 27.3 (15.1) | –0.58 (25) | 0.562 |
The treatment groups did not differ with respect to sex distribution (AM group, 69.6% male; CBT group, 82.6% male), or the proportion of those aged < 18 years [AM group, n = 6 (26.1%); CBT group, n = 3 (13%), youth protocol].
The groups did not differ significantly in respect of OCD symptom dimensions, with contamination obsessions and related compulsions reported by 18 (78.2%) in the AM group and 20 (86.9%) in the CBT group; aggressive/harm obsessions by 17 (73.9%) in the AM group and 14 (60.8%) in the CBT group; sexual/religious obsessions by five (21.7%) in the AM group and nine (39.1%) in the CBT group; symmetry obsessions by 16 (69.5%) of the AM and 15 (65.2%) of the CBT group; hoarding obsessions by nine (39.1%) in the AM and 14 (60.8%) in the CBT group; and miscellaneous obsessions/compulsions endorsed by 10 (43.3%) of the AM group and nine (39.1%) of the CBT group. The AM and CBT groups endorsed a mean of 3.1 (SD 1.2) and 3.3 (SD 1.4) OCD symptom dimensions, respectively.
Number of sessions
The mean number of treatment sessions was marginally greater in the CBT (17.43, SD 4.3) than in the AM condition (14.43, SD 5.3) (t = –2.022, df = 42, 95% CI –5.98 to –0.006; p = 0.05).
Treatment response (acute phase)
Table 60 shows, for blind clinical assessor, self and informant ratings, the means and SDs for each measure pre and post treatment and at 1-month follow-up; per cent improvement change between pre and post treatment and between pre treatment and the 1-month follow-up; and the mean difference, 95% CIs and within-group effect sizes. In terms of missing data, YBOCS and D-YBOCS ratings were available for all participants in both groups at the start of treatment, for 20 in the CBT group and 20 in the AM group at the end of treatment and for 18 in the CBT and 17 in the AM group at 1-month follow-up. For the self-report measures, the OCI-R was completed by 20, 17 and 17 in the CBT group and 19, 17 and 17 in the AM group at the start, end and 1 month post treatment, respectively. There was a similar rate of completion with the other self-report measures. The Informant measures were completed by 15, 14 and 11 in the CBT group and by 14, 11 and 9 in the AM group at the start and end of treatment and 1 month post treatment, respectively.
| Rating | Pre treatment, mean (SD) | Post treatment, mean (SD) | 1-month follow-up, mean (SD) | Pre–post difference, mean (95% CI) | Pre 1-month follow-up difference, mean (95% CI) | Pre–post % improvement, mean (SD) | Pre 1-month follow-up % improvement, mean (SD) | Pre–post effect size | Pre 1-month follow-up effect size |
|---|---|---|---|---|---|---|---|---|---|
| CBT: clinician ratings | |||||||||
| YBOCS | |||||||||
| Total severity | 24.8 (3.7) | 17.8 (8.4) | 18.7 (8.2) | 7.0 (3.2 to 10.7) | 5.8.(2 to 9.7) | 27.8 (33.2) | 23.5 (32.1) | 1.078 | 0.958 |
| Obsessions severity | 11.7 (2.8) | 8.7 (4.1) | 8.5 (3.6) | 2.9 (1.1 to 4.7) | 3.1 (1.4 to 4.7) | 24.0 (34.7) | 25.2 (30.9) | 0.854 | 0.922 |
| Compulsions severity | 13.1 (1.5) | 9.0 (4.6) | 9.7 (4.5) | 4.0 (1.7 to 6.3) | 3.2 (0.9 to 5.5) | 29.7 (36.5) | 23.9 (35.7) | 1.198 | 1.013 |
| CGI | 4.2 (0.8) | 3.3 (1.1) | 3.5 (1.3) | 0.9 (0.4 to 1.4) | 0.8 (0.2 to 1.4) | 21.4 (21.8) | 19.6 (25.3) | 0.935 | 0.648 |
| D-YBOCS | |||||||||
| Contamination | 7.3 (4.1) | 3.9 (3.8) | 4.5 (3.8) | 3.4 (1.4 to 5.3) | 2.6 (0.8–4.5) | 41.4 (48.6) | 34.2 (41.3) | 0.860 | 0.708 |
| Hoarding | 3.5 (3.9) | 1.7 (2.8) | 1.7 (2.8) | 1.7 (0.2 to 3.3) | 2.1 (0.3–3.9) | 48.8 (55.7) | 53.4 (49.5) | 0.530 | 0.530 |
| Symmetry | 5.3 (4.5) | 4.2 (4.6) | 4.6 (4.4) | 1.1 (–0.2 to 2.5) | 1.2 (–0.3 to 2.8) | 35.5 (47.1) | 34.9 (48.0) | 0.241 | 0.157 |
| Aggression/harm | 3.8 (4.3) | 2.6 (4.0) | 2.2 (3.5) | 1.2 (–0.1 to 2.5) | 1.8 (0.2 to 3.4) | 29.9 (61.7) | 65.7 (39.3) | 0.288 | 0.408 |
| Sexual/religious | 2.0 (3.1) | 1.2 (2.3) | 1.3 (2.5) | 0.8 (–0.2 to 1.8) | 0.8 (–0.3 to 1.9) | 37.3 (51.4) | 34.7 (52.8) | 0.293 | 0.248 |
| Miscellaneous | 3.2 (4.1) | 1.4 (3.0) | 1.6 (3.5) | 1.8 (0.4 to 3.1) | 1.7.(1 to 3.2) | 60.6 (45.4) | 58.5 (52.6) | 0.501 | 0.419 |
| Global total | 20.7 (3.8) | 15.5 (7.1) | 15.8 (7.0) | 5.2 (2.5 to 7.8) | 5.0 (2.1 to 7.9) | 26.7 (30.1) | 25.4 (30.9) | 0.913 | 0.870 |
| CBT: self-ratings | |||||||||
| OCI-R total | 31.5 (12.7) | 26.8 (15.3) | 29.3 (12.9) | 4.7 (–1.3 to 10.7) | 1.3 (–6.9 to 9.7) | 20.2 (45.8) | –32.1 (97.4) | 0.334 | 0.171 |
| BDI | 16.2 (13.8) | 15.7 (16.5) | 17.5 (15.1) | –0.5 (–3.9 to 4.9) | 2.0 (–2.9 to 6.9) | 17.9 (58.3) | 16.4 (39.1) | 0.032 | –0.089 |
| BAI | 16.4 (10.6) | 14.0 (11.6) | 13.6 (10.1) | 2.3 (–0.8 to 5.5) | 1.5 (–3.8 to 6.9) | 14.1 (52.9) | 26.5 (36.0) | 0.215 | 0.270 |
| LSAS total | 74.7 (27.1) | 67.8 (34.9) | 66.2 (35.7) | 6.9 (–9.8 to 23.7) | –2.2 (–17.6 to 13) | 7.0 (34.7) | –20.3 (46.2.1) | 0.220 | 0.268 |
| SCAS total | 28.3 (20.3) | 49.0 (n = 1) | 20.0 | –28.9 | –1.40 | ||||
| WSAS | 19.0 (10.4) | 22.4 (11.7) | 14.1 (9.1) | 3.4 (–7.8 to 1.0) | 4.6 (–3.9 to 13.1) | –24.4 (43.5) | 8.4 (53.5) | –0.307 | 0.501 |
| CBT: informant | |||||||||
| CHOCI severity | 30.3 (11.1) | 25.8 (10.5) | 28.8 (7.0) | 4.5 (2.1 to 11.1) | 2.7 (–7.5 to 13.0) | 4.9 (41.1) | –3.2 (41.2) | 0.416 | 0.161 |
| FAS | 26.9 (15.2) | 27.9 (15.0) | 21.1 (9.3) | –1 (–11 to 8.9) | 6.3 (–5.8 to 18.4) | –33.1 (90.1) | 1.4 (71.3) | –0.066 | 0.460 |
| AM: clinician ratings | |||||||||
| YBOCS | |||||||||
| Total severity | 25.1 (5.1) | 20.8 (7.8) | 20.7 (5.4) | 4.7 (2.5 to 6.8) | 3.8 (2.1 to 5.6) | 20.3 (23.4) | 16.2 (14.3) | 0.652 | 0.837 |
| Obsessions severity | 12.4 (3.0) | 10.5 (3.8) | 11.9 (2.3) | 2.0 (0.9 to 3.0) | 2.2 (1.2 to 3.3) | 17.6 (21.9) | 19.7 (17.9) | 0.554 | 0.187 |
| Compulsions | 12.9 (2.8) | 10.3 (4.7) | 10.8 (3.0) | 2.7 (1.1 to 4.3) | 1.9 (0.9 to 3.1) | 22.5 (30.6) | 13.1 (20.8) | 0.672 | 0.723 |
| CGI | 4.2 (0.8) | 3.7 (1.1) | 3.7 (1.2) | 0.5 (0.2 to 0.8) | 0.6 (0.1 to 0.9) | 13.9 (18.4) | 14.9 (19.6) | 0.519 | 0.490 |
| D-YBOCS | |||||||||
| Contamination | 5.8 (4.3) | 4.8 (4.8) | 4.9 (4.4) | 1.0 (–0.5 to 2.5) | 0.8 (–0.6 to 2.2) | 22.3 (69.6) | 27.6 (45.0) | 0.219 | 0.206 |
| Hoarding | 2.9 (3.8) | 2.5 (3.6) | 2.0 (3.4) | 0.3 (–1.4 to 2.1) | 0.8 (–1.4 to 3.1) | 54.9 (36.0) | 68.7 (39.2) | 0.108 | 0.249 |
| Symmetry | 5.4 (4.3) | 4.6 (3.9) | 3.7 (3.7) | 0.8 (–0.6 to 2.2) | 2.0 (0.2 to 3.7) | 21.8 (40.8) | 43.1 (43.2) | 0.194 | 0.423 |
| Aggression/harm | 6.5 (4.4) | 5.3 (4.6) | 4.5 (4.1) | 1.2 (–0.1 to 2.6) | 2.1 (0.27 to 3.9) | 29.4 (37.7) | 36.8 (41.7) | 0.266 | 0.470 |
| Sexual/religious | 1.6 (3.4) | 2.2 (4.2) | 2.2 (4.2) | –0.6 (–2.4 to 1.2) | –1.0 (–2.8 to 0.8) | 52.5 (55.0) | 19.5 (14.7) | –0.157 | –0.157 |
| Miscellaneous | 3.3 (4.2) | 1.6 (4.7) | 1.1 (2.2) | 1.6 (0.2 to 3.0) | 1.9 (–0.2 to 3.9) | 58.9 (46.8) | 51.5 (50.8) | 0.381 | 0.656 |
| Global total | 20.3 (4.7) | 17.1 (7.5) | 17 (5.9) | 3.2 (1.1 to 5.2) | 2.9 (1.3 to 4.5) | 18.5 (27.1) | 15.7 (14.9) | 0.511 | 0.524 |
Analysis of covariance, controlling for pre-treatment YBOCS severity ratings, detected no significant differences between the treatment groups on the primary outcome measure (YBOCS total severity scores) at end of treatment (F1,37 = 1.127; p = 0.295).
In the CBT group, univariate repeated measures ANOVAs established significant changes in YBOCS total severity scores from pre treatment to end of treatment (F1,19 = 15.089; p = 0.001). In the AM group, there were also significant changes in YBOCS total severity ratings from pre treatment to end of treatment (F1,19 = 20.169; p < 0.0001).
Within-group treatment effect sizes on the YBOCS were large and could be considered clinically meaningful in the CBT group (1.15) and medium in the AM group (0.6).
There were more treatment responders (i.e. had a > 25% reduction in YBOCS total severity ratings) in the CBT group than in the AM group [9/20 (45%) vs. 5/20 (20%), respectively]. However, this difference in response rate was not statistically significant (χ2 = 1.72, df = 1; p = 0.160). When a more stringent rating of treatment response (i.e. a CGI ‘much or very much improved’ combined with a > 35% reduction in YBOCS total severity ratings) was considered, 6 out of 20 (30%) in the CBT group achieved treatment response, compared with 2 out of 20 (10%) in the AM group. Again, the groups did not differ significantly in the proportion of treatment responders. The number participants classified as remitted cases (i.e. with a YBOCS total severity rating of ≤ 12 1 week after treatment ended) was slightly higher in the CBT group than in the AM group [5/20 (20%) vs. 3/20 (15%)], but the difference was not statistically significant.
Standardised effect sizes to further compare the two treatments were calculated for end of treatment primary outcome ratings using Cohen’s d (mean CBT – mean AM/σpooled). Effect sizes were 0.4 for the YBOCS total severity rating, 0.4 for YBOCS obsessions severity, 0.2 for YBOCS compulsions and 0.3 for CGI, all indicating a small advantage for CBT over AM after.
Maintenance of treatment gains at long-term follow-up
Treatment satisfaction
There were no differences between the two treatment groups as to their reports of satisfaction with the treatments they had received: AM group mean satisfaction score = 5.60 (SD 2.131); CBT group mean satisfaction score = 4.9 (SD 2.3), t = 0.809 (df = 27); p = 0.425.
In the CBT group, there were significant changes in YBOCS total severity scores from pre treatment to 1-month follow-up (F1,17 = 10.530; p = 0.005), 3-month follow-up (n = 10, F1,9 = 11.602; p = 0.008), 6-month follow-up (n = 12, F1,11 = 10.823; p = 0.007) and 12-month follow-up (n = 11, F1,10 = 9.831; p = 0.011). The stability of the change over time can be seen in Figure 32.
FIGURE 32.
Yale–Brown Obsessive Compulsive Scale total severity (pre and end of treatment, 1-month follow-up) by treatment group and 3 (n = 10), 6 (n = 12) and 12 months (n = 11) follow-up for CBT group cross-over cases.

Nine (39%) participants in the AM group, compared with three (13%) participants in the CBT group, asked to ‘cross over’ or try the other treatment either at or after the 1-month follow-up point (χ2 = 4.05, df = 1; p = 0.044).
Eight out of the nine participants originally randomised to AM who ‘crossed over’ to CBT completed the second treatment and attended for symptom ratings (AM + CBT). One participant was not available for end of treatment ratings despite completing the treatment. There was a significant effect of this second treatment (F1,7 = 7.703; p = 0.027) on the primary outcome measure when the end of second treatment scores were compared with those pre treatment. There was no change in YBOCS severity ratings for the three participants who completed AM following CBT, although the individuals attended readily and qualitatively commented that they found the treatment helpful in general stress management.
Secondary outcome measures
Although clinician ratings of CGI changed significantly between pre and post treatment (F = 29.1, df = 1,34; p < 0.001), this did not vary by treatment group (F = 2.28, df = 1,34; p = 0.140). Figure 33 depicts the percentage of participants in each group rated as ‘much or very much improved’ and ‘minimally improved, unchanged or worse’ on the CGI-I scale. On the basis of this dichotomous rating, the treatment groups differ significantly in terms of the proportion of participants in each group rated as ‘much or very much improved’ [CBT group, n = 11; AM group, n = 5 (χ2 = 3.886, df = 1); Fisher’s exact test p = 0.050]. Regarding self-report measures, neither of the groups showed significant differences between pre, post and 1-month follow-up mean scores on any of the self-ratings (see Table 60). Informant ratings differed significantly between pre and post treatment only for the AM group.
FIGURE 33.
Percentage of participants rated as ‘minimally improved, unchanged or worse’ and ‘much or very much improved’ on the CGI-I scale by treatment group.

Moderating factors
Symptom severity
The five participants classified as treatment responders in the AM group had significantly lower YBOCS severity ratings pre treatment (mean 21.20, SD 3.2) than with the non-responders in the AM group [mean 26.8, SD 4.8, t = 2.37 (df = 17), 95% CI 0.6 to 10.6; p = 0.029]. This was not the case in the CBT group, in which responders and non-responders were equivalent with respect to symptom severity before starting treatment [CBT responder: mean 24.8, SD 3.2; CBT non-responder: mean 24.1, SD 4.4, t = –0.41 (df = 16); p = 0.687]. Similarly, treatment responders in the AM group had a significantly lower CGI rating pre treatment than non-responders [AM responders: mean 3.2, SD 0.5; AM non-responders: mean 4.5, SD 0.6, t = 3.56 (df = 15), 95% CI 0.5 to 2.0; p = 0.003]. The AM responders did not differ in terms of number of obsessive–compulsive symptoms from non-responders.
Age
Age was not significantly associated with treatment outcome. The main outcome analysis was also repeated excluding those participants who entered the youth protocol (age 14–16 years), and this did not affect the pattern of results.
Other variables
Other variables purported to be of interest as potential moderators of treatment, including verbal IQ, ADOS-G scores and performance on executive function measures, were investigated in terms of their association with treatment response (i.e. the percentage change in total YBOCS severity scores). None of these factors showed any association. However, the group categorised as treatment responders (i.e. > 25% reduction in YBOCS ratings) differed significantly from non-responders on the Family Accommodation Scale (FAS) at baseline [mean treatment responder: FAS score 18.22, SD 15.91; mean treatment non-responder: FAS score 29.53, SD 12.30; t = 2.015 (df = 24), 95% CI of the difference –0.275 to 22.89; p = 0.055]. The treatment responder and non-responder groups did not differ on the FAS at the end of treatment. There was a wide range of scores on the FAS in the treatment response group at baseline and this reduced by the end of treatment, suggesting that family factors may have changed over the course of treatment.
In terms of insight as ascertained by the YBOCS, 33 participants (71.7% of the total) could identify a specific feared consequence if they did not respond to the obsessive thoughts or compulsive urges. Certainty about the feared consequences differed post treatment according to treatment response at the trend level [responder: mean rating 1.00 (SD 1.15); non-responder: mean rating 1.95 (SD 1.07); t = 2.00 (df = 26); p = 0.056], indicating that non-responders tended to have worse insight scores.
Discussion
This is the first clinical trial to provide evidence for the effectiveness of CBT for comorbid OCD in young people and adults with ASD. The effect of CBT treatment in the present study was comparable with clinical trials of OCD in people without ASD, where aggregated effect sizes of 1.12 and 1.45 have been reported from meta-analyses of CBT trials in adult364 and paediatric365 OCD studies, respectively. Importantly, the treatment gains were sustained over a 12-month follow-up period.
Unexpectedly, AM, a plausible control treatment, was also effective in bringing about a reduction in OCD symptoms in people with ASD, particularly those with milder symptoms. It was not possible to separate the two treatment groups at the end of treatment in terms of symptom severity, although there were twice as many responders in the CBTgroup than in the AM group. Comparison between the effect sizes of the two treatments afforded some small advantage for ERP-based CBT over AM.
This advantage for CBT was greater for ratings of obsessions. In an earlier uncontrolled pilot study,343 in which CBT was compared with TAU, we noted an overall advantage for CBT, with a significant change in obsessions severity ratings but not compulsions. It is possible that measurement issues and in particular difficulties in disentangling ASD-preferred routines and stereotyped behaviours from anxiety-based compulsions has a role to play here. Alternatively, unwanted intrusive thoughts/images/impulses may be associated with greater distress and thus motivation for treatment than compulsive rituals where a preference for routine and sameness in ASD may infer greater tolerance of these symptoms. Furthermore, outcome for adults with ASD is known to be poor with low levels of employment and independent living. In the absence of full occupation, compulsive rituals may not present with a high level of daily interference.
Significantly more patients in the AM group requested crossover to the CBT group than did CBT patients to the AM group after the 1-month follow-up, indicating that the AM treatment, although receiving similar satisfaction ratings, was not perceived as being as potentially useful as the ERP-based treatment. The eight patients who crossed over from AM to CBT and provided data at the 1-month follow-up point achieved statistically significant reductions in OCD severity, whereas those who crossed over from CBT to AM (n = 3) did not improve.
Setting aside the not inconsequential issues of sample size and statistical power (discussed below), it is worth considering why AM might have performed relatively well in people with ASD and comorbid OCD, particularly those with mild symptoms.
First, this is not an unprecedented finding. For example, Whittal and colleagues,366 in a study investigating cognitive therapy for obsessions, found that stress management (a credible control treatment) was also helpful in reducing obsessions with equivalent pre–post effect sizes for both treatments on the primary outcome measure. Unexpectedly, changes in OCD-related cognitions and threat appraisals occurred in the control group. In an internet-based trial of self-help for panic and phobias,350 a similar AM control group did as well as the exposure therapy group on some outcome measures. In an ASD-specific study of group treatment for anxiety in children, a social recreational programme was found to have a similar effect on anxiety symptoms as CBT. 367 Thus, under certain circumstances, non-specific interventions can lead to clinically significant improvements in disorder-specific measures. In the current trial, it is important to note that patients who responded to the control treatment had significantly less severe OCD.
Second, it is important to consider that OCD is not the only commonly occurring anxiety disorder in ASD. There is evidence that processing and identification of emotions may be impaired in this group368 and the AM intervention in the present study (with its focus on education about anxiety and teaching of relaxation techniques) may have made anxiety a more predictable and manageable emotion for some individuals. Risk factors for anxiety disorders, such as increased intolerance of uncertainty and anxiety sensitivity, may be elevated in people with ASD, who have a preference for routine and sameness, and this may represent a pathway to OCD. Increasing an individual’s capacity to manage the emotional consequences brought about by uncertainty and anxiety may thus bring about a reduction in OCD symptoms. Furthermore, the non-specifics of therapy may be more potent in a group who can be socially isolated and lack support. The majority of clinical trials in people with ASD have to date employed a wait list or TAU comparison condition. However, a RCT evaluating CBT for anxiety compared with a social recreational programme in young people with ASD had similar difficulties to the present study in significantly separating the treatments in terms of their effects on anxiety. 367
There were no therapist effects in terms of numbers of treatment responders. Prior to their work on the present study, the majority of the therapists had gained some experience of working with people with ASD as part of generic anxiety/OCD clinic work. Only one of the therapists had previously worked in an ASD-specific service. Thus, the results should be generalisable to clinicians and psychological therapists trained in treating OCD.
Self-report of OCD and other anxiety symptoms were correlated with clinician and informant ratings pre treatment but not at follow-up, suggesting that they were not sensitive to change. Nonetheless, it is of benefit to know that self-report measures can be used as an assessment tool to accurately assess the content of anxiety-related problems in young people and adults with ASD and this is consistent with findings from other studies. 369 It is also important to note that many of the participants in the study had good insight into their OCD and that obsessive–compulsive-related beliefs were prevalent.
There was some indication that family factors (family accommodation) were associated with treatment outcome in the present study, and this is consistent with findings of other anxiety intervention studies in ASD330 and outcome in general paediatric OCD treatment studies. 363 Group and individual psychological interventions for anxiety disorders in this group should then seek the engagement of relevant caregiving/supportive individuals in treatment.
Strengths and weaknesses
Although the study was well powered to detect within-group changes in symptom severity, it may have lacked statistical power to detect a difference between the two treatment groups. The power calculation was based on an earlier pilot study where treatment was compared with no-treatment. 343 As discussed above, the AM intervention clearly had some modest effect, particularly in individuals with mild OCD symptoms. This indicates that larger sample sizes will be needed to detect a statistically significant difference between the groups. The results of the current trial will be useful to help more accurate power calculations for future trials. However, the main message of the current study is that standard CBT for OCD can be successfully adapted for ASD participants who are traditionally perceived as difficult to treat.
The disproportionately high number of participants who endorsed hoarding symptoms in the CBT (60%) compared with the AM (40%) groups may also have contributed to the lack of clearly significant differences between the groups because hoarding symptoms are usually considered difficult to treat with conventional CBT for OCD. 370 Given the current proposals to separate hoarding from OCD in DSM-V,371 future treatment studies would probably benefit from excluding any severe hoarding individuals from their samples.
The wide ranges of age and symptom severity may have had an impact on the findings. Rounsaville and colleagues372 recommend reducing therapist and participant heterogeneity and choosing narrow parameters with which to define the treatment setting, participants and therapists in order to optimise the power available in a small pilot trial. However, we felt that, as a proof of concept trial, it would be important to recruit a wide range of individuals.
The lack of follow-up information on the AM group is a weakness imposed by our design, as it was unethical to withhold the evidence-based treatment (CBT) for longer than the 1-month follow-up and these patients had to be offered the option of a crossover. A further limitation was the absence of detailed information about homework compliance and its association with treatment response.
Not everyone did well as a result of treatment. A proportion of participants in both groups (15% AM and 10% CBT) were rated as minimally or much worse at 1-month follow-up on the Beck Depression Inventory, one of the secondary outcome measures. In addition to the lack of longer-term follow-up previously mentioned in the AM group, just over 50% of the treatment completers in the CBT group remained in follow-up for 12 months. It is possible that those lost to follow-up may also have deteriorated during that period and thus the positive effects of the intervention may have been limited to just those who stayed in the study.
This study may be best considered as a feasibility pilot study. Ideally, randomisation should be carried out by somebody entirely independent of the trials team. In this study participants were randomised to the CBT or AM groups using a table of random numbers (1 : 1 ratio) managed by an investigator who was part of the Trial Management Committee, but they were not part of the team delivering therapies, were blind to individuals and not involved in data analysis.
Conclusions
Psychological treatments for OCD can be successfully adapted for comorbid ASD and OCD. Our results suggest that further development of this intervention is required, together with testing in a larger sample that better represents the whole spectrum of people with ASD.
Both AM and CBT are effective in treating comorbid OCD in young people and adults with ASD.
More people responded to CBT than AM and it may have some advantage for treating obsessions.
The treatments are well accepted by affected individuals.
Chapter 23 Piloting trials to improve knowledge: a pilot study of web-based learning and clinical supervision from expert centres to local clinical teams working with ADHD and ASD
Introduction
An essential component in delivering effective treatments (and needs-led services) is that clinical teams need to both accurately identify individuals with ASD/ADHD and diagnose their associated mental health symptoms. They also need to communicate their findings to the affected individuals and their carers (where relevant). Put simply, there is little point developing new treatments in research settings if these are not applied by clinical services. However, as part of this programme (see Sections 1 and 2), we found that clinical services frequently failed to recognise and treat affected individuals (and especially young adults). Given a number of recent inquiries,373 the need to improve patient care and increase standards particularly for more vulnerable groups is a high priority for health providers. There has been an increase in education programmes to train health professionals over the last 10 years. 374 However, these have not always translated to highly specialist areas including the care of people with ADHD and ASD.
In current supervising practice in medicine there is very little empirical data to inform the structure and approaches to be used in supporting the implementation of national guidelines. 375 In addition, there is little understanding of the factors that are key to the successful implementation of clinical supervision in the development of new competencies for senior practitioners responsible for implementing national guidelines. For instance the National Strategy for Adults with Autism titled Fulfilling and Rewarding Lives: The Strategy for Adults with Autism in England376 is committed to increasing understanding and awareness across all public sector services. Teaching resources such as e-learning modules were developed with the Royal College of Psychiatrists and British Psychological Society to increase knowledge and diagnostic skills in ASD and to improve care for people with ASD with complex presentations such as those requiring mental health care. However, to support professionals across different types of health-care settings in their development of local services, there was a need for professionals developing ASD services to access established experts in regional or tertiary services. Similarly, NICE produced guidelines in 2008 on the diagnosis and management of ADHD in children, young people and adults, which again required opportunities for professionals to develop skills and expertise over time in the development of services for adults with ADHD. 95 Hence, as part of this programme we helped launch the UK Adult ADHD Network in 2009 following the publication of national guidelines to support training among mental health professionals working with ADHD (URL: www.ukaan.org). Therefore, the importance of supervision in developing skills following on from these national strategies and guidelines was essential for the ongoing development of services for adults with ASD or ADHD. Prior to those national initiatives there were a small number of experts working in national services and so the task of offering supervision across the UK can only be achieved on a scale through the use of technology near to the professional’s place of work and practice.
Hence, the aims of this study were to carry out a scoping exercise and:
-
examine if clinical supervision using web-based learning technology can bring about an improvement in participant’s knowledge and clinical assessment, management and diagnosis of ASD and ADHD.
-
evaluate participants’ experience of web-based learning to deliver clinical supervision for the ADHD and ASD.
Methods
Participants were recruited through a series of 1-day training workshops organised across England. These covered either the assessment or management of ASD and or ADHD in adults.
This study uses a simple randomised comparison of online supervision with no online supervision, to evaluate the effectiveness of web-based learning to deliver clinical supervision relating to the recognition, diagnosis and management of ADHD and ASD. The study is divided into two, with both the ADHD and ASD studies incorporating a separate series of three web-based supervision seminars facilitated by clinical and academic experts.
The ADHD study consisted of 13 participants (seven were randomised to the web-based online supervision and six to the control group), whereas 15 participants took part in the ASD study (with six randomised to the supervision group and nine to the control group). Prior to the start of the supervision process (time 1), each participant underwent an assessment of knowledge, skills and attitudes, which was repeated at the end of the study (time 2).
Participants for the ADHD web-based seminars were all psychiatrists, with the majority working in general adult psychiatry and seeing between 11 and 50 ADHD cases per year. Participants for ASD web-based seminars were multidisciplinary and included psychiatrists (53%), psychologists (33%) and others such as speech and language therapists. Forty per cent worked in adult mental health services, 33% worked in ID services and 20% worked in designated ASD services with 20% working in forensic services. Over two-thirds of the group had undertaken at least 1–10 diagnostic assessments with a remaining one-third completing ≥ 11 assessments.
For both studies prior to the start of the supervision process (time 1), each participant underwent an assessment of knowledge, skills and attitudes, which was repeated at the end of the study (time 2). The pre-study assessment at time 1 consisted of multiple-choice questions (MCQs) to test knowledge, an attitude questionnaire (see Appendix 2) and a clinical assessment through a serious of case studies of increasing complexity using videos. All participants (both intervention and control groups) completed the MCQs, clinical skills assessment and attitude questionnaire at time 1 and completed the MCQs clinical skills assessment and attitudes questionnaire at time 2 following the three web-based supervision workshops. The clinical skills assessment for both the ADHD and ASD studies followed three people who had traits of the disorders, but where it was also unclear whether or not they met diagnostic criteria. Background information was given to accompany the series of video clips for each case. Participants were asked a series of questions to elicit their clinical opinion based on their observation of the video and background information. The vignettes portrayed situations of increasing complexity relating to the assessment, diagnosis and management of ADHD and ASD.
Web-based clinical supervision
The three web-based supervision workshops on ADHD and ASD were hosted on the WebEx system (Cisco Systems, San Jose, CA, USA). To access these, each participant in the intervention arm logged into a secure online site through the King’s College London server.
Description of ADHD web-based supervision
The three ADHD supervision sessions were held over a 4-week period in July 2012. Seven participants were invited to attend and all were able to complete at least two out of the three sessions. Each session was attended by two experts (senior clinicians with experience of running a specialist adult ADHD service), in addition to a chairperson. The participants were invited to submit anonymised clinical cases and vignettes for discussion prior to supervision and over the course of the three sessions a total of 13 cases and clinical scenarios were discussed, with six out of the seven participants presenting cases for discussion. The sessions lasted from 1 hour to 1 hour 20 minutes. The WebEx system allowed experts and participants to attend via a video link or telephone connection. For the three sessions, the two experts and chairperson attended by video link so participants were able to see and hear them. The participants (who also had this option) largely attended via a telephone connection.
A wide variety of clinical cases were brought for discussion, reflecting the range of clinical environments that participants worked in. The experience of the participants ranged from clinicians just starting to see adults with ADHD presenting to their service and had not yet undertaken assessments for ADHD, to clinicians experienced in the management of ADHD in adults. In addition, there were clinicians with expertise in subspecialties such as addictions and ID. Cases for discussion were divided between diagnostic and management questions. There were a number of cases relating to the difficulty in diagnosing ADHD in the presence of comorbidity, including mood disorder, PD and ID, as well as discussion involving the role of rating scales in assessment and monitoring of progress and the use of neuropsychological assessment. Questions about the management of ADHD included how to treatment refractory initial insomnia, how to manage comorbid anxiety and the use of venlafaxine (Efexor®; Actavis, Parsippany-Troy Hills, NJ, USA) and antipsychotics in ADHD.
The experts tended to lead discussion with factual information related to their practice, as well as current guidance such as NICE guidelines (e.g. the use of antipsychotics is not recommended as an effective treatment of ADHD). However, the group interaction was made richer by discussion between the participants who had specific expertise (e.g. addictions), and were able to comment on variation in clinical experience in different parts of the UK (e.g. the availability of CBT for ADHD) and the ability to make use of second- and third-line treatment options for ADHD. Abridged versions of the ADHD case studies are provided in Appendix 2.
Description of ASD web-based supervision
The three ASD supervision sessions were held over a 3-week period in September 2012. Six participants were invited to attend and all were able to complete at least two out of the three sessions. Each session was attended by two experts (senior clinicians with experience of running specialist adult ASD services). In addition, a chairperson with similar experience was present. It was agreed with participants that each of them would submit anonymised clinical cases. These cases were distributed prior to the supervision sessions so participants could familiarise themselves. This left an hour discussion for each case and the opportunity for peer review. The sessions lasted from 1 hour 40 minutes to 2 hours 10 minutes. The mode of attendance was the same as the ADHD study.
The six cases presented over the course of the three sessions were a mixture of people with ASD with complex cases histories of comorbid mental health problems and offending and/or challenging behaviours from a variety of community and inpatient services. Unlike the ADHD study, all participants had significant experience in the management of adults with ASD. In addition, many had expertise in subspecialties such as ID, general psychiatry and offender health care. There were a number of cases relating to the assessment strategies, for example in forensic settings, corroborating evidence and what to consider when making recommendations to courts. Abridged versions of the ASD case studies are also provided in Appendix 2. Discussion during the supervision sessions was framed from the presented case studies with the peer and expert opinion offered greatly enhanced by the wide range and extensive experience of the supervision group. The format of supervision (for both the ADHD and ASD) allowed participants to present cases in a safe environment and receive peer support, through suggestion and interrogation of the information provided.
Preliminary results and discussion
Can clinical supervision using web-based learning technology bring about an improvement in participant’s knowledge and clinical assessment, management and diagnosis of ASD and ADHD?
Table 61 provides scoring for knowledge and clinical skills at time 1 and time 2 for both the ADHD and ASD groups. The knowledge scores apply to the MCQs, whereas the clinical skills scores refer to answers given by participants following scrutiny of three video vignettes. Neither study reached significance although modest increases were demonstrated for three of the four groups, with no change in knowledge scores for the participants of the ASD supervision group.
| Participant group | n pre and post | Pre knowledge scores, mean (SD) | Post knowledge scores, mean (SD) | Change in mean scores | Pre clinical skills scores, mean (SD) | Post clinical skills scores, mean (SD) | Change in mean scores |
|---|---|---|---|---|---|---|---|
| ADHD control | 6–4 | 10.7 (1.4) | 10.8 (1.5) | 1 | 12.3 (6.8) | 13.5 (5.3) | 2.3 |
| ADHD supervision | 7–6 | 10.4 (1.9) | 10.7 (2.0) | 1.7 | 11.9 (4.5) | 13.3 (3.2) | 1.5 |
| ASD control | 9–9 | 21.6 (2.6) | 21.9 (1.7) | 0.25 | 67.8 (12.8) | 71.9 (9.3) | 4.1 |
| ASD supervision | 6–6 | 22.8 (2.1) | 22.8 (1.9) | 0.33 | 70.8 (7.1) | 75.8 (11.6) | 5 |
Attitude questionnaire scores
In terms of attitudes, the ADHD clinicians showed slight improvement in scores between times 1 and 2 for both the control (41.8–42.5) and intervention groups (38.9–41.8). In the ASD study there was no improvement in the attitude score of the control group (39.1) between times 1 and 2. The intervention group showed a slight improvement in attitude score between times 1 and 2 (42.2–44.5).
Following the study, participants in the control group were more likely to say they did not have enough support from colleagues to manage adults with ADHD and ASD than those in the supervision group.
Evaluation of participants’ experience of web-based learning to deliver clinical supervision for the ADHD and ASD
ADHD
The feedback from participants during the supervision was generally positive regarding the content of discussion and the opportunity to discuss cases with experts but also with colleagues working in different areas. There was some frustration with the technology because at times it was difficult to hear other participants due to background noise on the telephone connection and more than one participant talking at once.
Five out the seven participants completed written feedback at the end of the three sessions. None of them reported that they had used e-supervision before. All the respondents reported that online supervision was more convenient than face-to-face supervision. The majority (four out of five) rated the experience of e-supervision the same or better than face-to-face supervision with respect to skills learning and the ability to improve clinical practice. However, participants were divided whether or not face-to-face supervision or e-supervision allowed better connection with peers and supervisors (one reported that online offered better connection to supervisors, two felt that face to face was better for connection to peers and supervisors, and three felt that there was no difference between the mediums). With respect to the practicality of receiving online supervision, all respondents were satisfied with their ability to access and participate in the sessions.
With regard to the quality of supervision offered, the respondents agreed that the supervision met their expectations, the approach was relevant, the time allocated was appropriate and their clinical practice was supported. Only one respondent felt that the supervision was not suited to their level of knowledge and expertise. All the respondents were satisfied with the expertise of the supervisors.
The particular strengths of the online supervision were described as ‘easy access . . . [and] able to relate to others’, ‘panel of approachable clinicians maintained focus and application’, ‘not having to travel to London [for supervision]’ and ‘it was very useful to be able to communication with experts and fellow participants from a distance’. One respondent commented on the mix of participants, suggesting that separating supervision for doctors and psychologists would help clinical applicability of advice and for this reason they felt they were not sure they had learnt much from the sessions. Other participants reported that the sessions ‘improved my confidence in managing more complex clinical presentation’ and ‘clarified lots of assessment issues [so I] feel more confident’. All respondents reported that they would recommend this supervision to a colleague.
ASD
Again, there were concerns about the quality of the technology used by participants to utilise the chosen web-based system to its full potential. There were issues because of this, including difficulty in consistently hearing each other. The majority of participants relied on a telephone connection as they could either not access the internet adequately or did not have access to web cams. In terms of general feedback the following comments were received: there was an issue with the information technology (IT) infrastructure in some NHS and university facilities not being up to date (e.g. web cameras, software, sound cards). The ASD supervision group benefited from a high level of expertise within the supervision group who found it very useful to be able to communicate with experts and fellow participants from a distance. The online assessments were also well received and a number of participants commented on the need for similar future initiatives such as e-groups where clinicians could post questions and answers in their own time and focus on material that is relevant to them. Only one person suggested the need for a discipline-specific forum.
Future directions
The original intention was to offer training to more junior staff; however, the recruitment of senior experienced staff may be an indication of the dearth of forums and peer support networks available for experienced clinicians working on complex atypical presentations. The presence of senior people enhanced the process and gave an opportunity to engage with other professionals not usually accessible because of organisational and team boundaries. The expected increase in skills and knowledge that was anticipated did not materialise, probably as a result of the entry level in terms of experience of the participants, but each found it valuable and thought a similar type forum would be beneficial.
In terms of future development, there have been recent calls to scale up the use of telemedicine in psychiatry and specifically in the area of ADHD in order to disseminate good practice and allow training and consultation. Our findings suggest, however, that issues such as accessibility to technology required for web-based learning may be a barrier to widespread implementation. This may also be a contributory to the lack of published evidence for the effectiveness of telehealth for ADHD, despite calls for its use and implementation of some services. 377
This study has highlighted the need when implementing policy (and particularly for those conditions poorly understood at national level) to focus not just on those starting to develop services but also on those who are working to develop services in relative professional isolation. The use of web-based learning to support those with people requiring secondary services with a high level of specialism require access to supervision either through experts at tertiary centres or through peer-to-peer supervision. The main limiting factor at the present time is accessibility to adequate and user-friendly forms of technology being widely available across health-care and academic institutions. Finally, our study suggests the need for the development of a peer-to-peer support structure and networks among experts outside the current organisational boundaries and individual collaborations.
e-learning and training is difficult to achieve in NHS settings due to lack of IT support.
e-support was reported by participants to be useful.
However, e-support did not lead to marked improvement in knowledge (or shift attitudes) in clinical teams about ASD or ADHD.
This may partially reflect quality issues secondary to IT difficulties.
Section 5 Conclusions and research
Chapter 24 Conclusions
Background
Both ASD and ADHD frequently persist into adolescence and young adulthood and there are more teenagers and adults in the UK affected by ASD and/or ADHD than children. However, there are few clinical services that support those with these disorders through adulthood. Research into the needs of young adults with an ASD or ADHD (and their carers) is important in order to design effective care programmes, but little is known about the service use and needs of these groups as they reach adolescence and transition to adulthood.
Objectives
The objectives of our research were to determine:
-
What are the needs of affected individuals and their carers, and are these met by health services?
-
Are people with ADHD and ASD already in contact with clinical services recognised and treated by the services?
-
Can we improve the diagnosis of ADHD and ASD by clinical services?
-
Can we improve the treatment of ADHD and ASD by clinical services?
Methods
We (1) interviewed > 180 affected individuals (and their families) with a confirmed diagnosis of ASD and/or ADHD, (2) screened for ASD and ADHD in approximately 1600 patients currently in receipt of clinical services in community medical (general practice) and mental health services (including general adult, forensic and prison settings) and (3) surveyed GP prescribing to 5651 ASD individuals across the UK (as prescribing in ADHD has already been reported). Finally, we tested the effectivness of (1) new ASD diagnostic interview measures in 169 twins, 145 familes and 150 non-twins, (2) a MRI-based diagnostic aid in 40 ASD individuals, (3) psycholgical treatements in 46 ASD individuals and (4) the feasability of e-learning in 28 clinicians.
Results
What are the needs of affected individuals and their carers, and from their perspective are these met by health services?
Young people with ASD and ADHD have very significant needs as they transition through to adolescence and young adulthood. A major contributor is the presence of associated mental health symptoms and/or ongoing educational and other functional impairments related to the ‘core disorder’. For instance, most young people with ASD suffered from additional mental health problems (e.g. depression and anxiety), and most of those who had suffered from ADHD as children continued to display residual symptoms closely linked to the disorder such as fatigue and feelings of irritability or anger. Effective treatments for these additional mental health problems already exist and if used would be expected to reduce disease burden. However, the additional/residual mental health problems in ASD and ADHD are mostly undiagnosed (and untreated) by clinical services and so the needs of affected individuals are mostly met by their families. Furthermore, we found that the largest determinant of service provision was age and not severity of symptoms (e.g. in ADHD each 1-year increase in a young person’s age, reduced the odds of being seen by services by 38%). This leads to a carer burden that is similar to looking after somebody with a traumatic brain injury or dementia.
This part of our programme suggests that needs-led services are required which can identify individuals with the ‘core symptoms’ of ASD and ADHD at the ‘transition’ and treat their residual symptoms and associated conditions such anxiety and depression.
Are people with ADHD and ASD already in contact with clinical services recognised and treated?
We found that many clinical services have a relatively high prevalence of people with ASD and ADHD, but that these are mainly unrecognised. For instance, rates of ADHD within clinical services for SUD populations are approximately fivefold higher than general population rates, and some (but not all) forensic populations have significantly increased rates of both ADHD and ASD. Furthermore, these (unrecognised) individuals were functionally more impaired, including a higher rate of suicide attempts and spending longer periods in seclusion. These findings begged the question – if people with ASD and/or ADHD are not being recognised by clinical services who is bearing the cost?
We found that, overall, costs for both disorders were mainly borne by education and social care agencies, with much less accounted for by physical (and especially mental) health. Finally, as individuals with ADHD and ASD grew older they continued to consume services, but costs of education, hospital services and social work/social care all reduced (and for both disorders mental health service costs reduced very significantly). This suggests that, although (as noted in other parts of our programme) individuals with ASD and ADHD continue to experience significant symptoms as they ‘transition’, their contact with treatment and support services reduces significantly.
This part of our programme revealed diagnostic unawareness of both ASD and ADHD in a variety of clinical service settings; with the burden of care mostly falling on families and service costs for both disorders mainly borne by education and social care agencies. This situation most likely reflects both a paucity of instruments to aid case identification and lack of clinical staff knowledge.
Can we aid the diagnosis of ADHD and ASD by clinical services?
We took the pragmatic decision to adapt existing instruments (rather than developing completely new ones). In addition, relatively easy-to-use instruments for screening/diagnosing ADHD already exist. Hence, as a first step we focused on ASD by testing the utility of the DAWBA and SDQ for (respectively) diagnosing the core disorder and for identifying associated mental health symptoms.
The DAWBA performed well in our study of children, and our initial results suggest that it has the potential to be a useful tool in community settings. Our results also suggest, however, that the DAWBA should be used in conjunction with a direct observational measure (expert clinical observation or ideally a measure such as the ADOS-G). When both measures agree with each other there can be confidence in both positive and negative results. In cases where these measures are discrepant then further assessment in more expert centres, using a tool such as the ADI-R, or through observation of behaviour in school or with peers could help to resolve issues. In addition, our initial results suggested that the SDQ is also a valid way to screen for comorbid anxiety disorders, depression and ADHD in adults with ASD. However, it is possible that changes in the diagnostic criteria for ASD may have implications for the performance of the SDQ in the new diagnostic categories suggested in DSM-V.
In the next study, therefore, we explored the effect that changes to clinical diagnostic practice as recommended in DSM-V would have on affected individuals. We found that DSM-V is a relatively insensitive measure of ASD in young adults compared with alternatives that are currently used in the UK (such as ICD-10). This is noteworthy, as ICD-10 is about to be revised, and if approaches adopted in International Classification of Diseases, Eleventh Edition are similar to those now used in DSM-V, or if DSM-V is adopted in the UK as a service ‘gatekeeper’, then many affected individuals with ASD will be excluded from services. A natural corollary is that changes in clinical diagnostic practice (that may not reflect the underlying pathology) may worsen the already high level of unmet meets in affected individuals and carer burden.
We therefore next determined if, in young adults, we could establish proof of concept that new advances in brain imaging provide objective measures that may help categorise young adults with ASD and/or ADHD. We used a multiparameter classification approach to characterise the complex and subtle grey matter differences as measured using MRI in ASD and ADHD. SVM achieved good separation between groups and revealed spatially distributed and largely non-overlapping patterns of regions with highest classification weights for each of five morphological features. For instance, in the left hemisphere, SVM correctly classified 85% of all cases overall at a sensitivity and specificity as high as 90% and 80%, respectively, using all five morphological features. Our results provide proof of concept that that the neuroanatomy of ASD and ADHD are truly multidimensional and that the discriminating patterns detected using SVM may help classify affected individuals. The significant predictive value of pattern classification approaches may have potential clinical applications. Currently, both ASD and ADHD are diagnosed solely based on behavioural criteria. The behavioural diagnosis is, however, often time-consuming and can be problematic, particularly in adults. In addition, different biological aetiologies might result in the same behavioural phenotype, which is undetectable using behavioural measures alone. Thus, the existence of an ASD (or ADHD) biomarker such as brain anatomy might be useful to facilitate the behavioural diagnosis. This would, however, require further extensive exploration in the clinical setting.
In summary, this part of our programme provided interview-based tools to help diagnose the core disorder and associated symptoms in ASD, and proof of concept for the potential utility of brain biomarkers to help aid clinical classification of ASD and ADHD in young adults.
Can we improve the treatment of ADHD and ASD?
An essential step in improving treatments is to understand how current treatments are used. We focused on pharmacological prescriptions to affected individuals by GPs across the UK, as these data (unlike the use of psychological approaches) are available from an electronic source called THIN. We had previously reported on prescriptions to young adults with ADHD, but there was no published information on ASD. Hence, we carried out the first naturalistic study of psychotropic drug prescribing and neuropsychiatric comorbidities in a large nationally representative cohort of ASD patients in UK primary care. We found that there were 5651 patients aged < 25 years in the THIN database with at least one diagnostic record of ASD during the study period. In other parts of this programme, we found very high rates of associated mental health symptoms in ASD (e.g. depression and anxiety). However, relatively few individuals had been diagnosed with these symptoms by their GPs and, although approximately one-third of individuals with ASD received at least one psychotropic drug prescription, these were mostly for stimulants, antiepileptic drugs and mood stabilisers, and antipsychotics. Of those who were prescribed psychotropic medication, one-fifth were treated with the antipsychotic risperidone and at least one-third received more than one drug. Furthermore, as people aged, prescribing rates for risperidone remained high (whereas others decreased), suggesting that those adolescents and young adults who are prescribed antipsychotics are (relatively) unlikely to be taken off them. Hence, further research into the appropriate use, efficacy and long-term safety of antipsychotics and stimulants is warranted to support for optimal and safe treatment of ASDs and their comorbidities. One likely contributing factor to the lack of trials in frequently excluded groups such as these is that investigators typically face significant hurdles (e.g. ethical and practical).
Hence, in order to make the most rapid progress we ran two concurrent work streams to obtain pilot data/proof of concept on the ability to run UK trials on effectiveness of interventions in ADHD and ASD. The first focused on the effectiveness (or otherwise) of current pharmacological treatments for ADHD in people typically excluded from trials (those with ID). The second piloted a new CBT-based treatment approach for OCD in ASD. We were unable to complete the first trial in the time frame of this grant (mainly due to delays inherent in obtaining approval from multiple ethical and regulatory authorities) and so, given our findings on high rates of unrecognised disorder in forensic populations, we switched resources into a prison study of ADHD treatment that is ongoing. However, it is crucial to also offer effective non-pharmacological treatment approaches. The effect of CBT treatment in the present study was comparable to clinical trials of OCD in people without ASD. Unexpectedly, however, AM training was also effective in bringing about a reduction in OCD symptoms and particularly in those with milder symptoms. Hence, further work is required to determine if OCD symptoms in ASD are underpinned by anxiety to a greater extent than in non ASD individuals. If this is the case then it will allow more people to be treated (and at lower cost), as AM training can be provided more simply, and with a lower degree of staff expertise, than CBT.
Finally, in order to improve outcomes we need to improve staff knowledge. However, e-learning and training is difficult to achieve in NHS settings due to lack of IT support and it did not lead to marked improvement in knowledge (or shift attitudes) in clinical teams.
In summary, we found that individuals with ASD and ADHD at the ‘transition’ have significant unmet needs and a high carer burden. In addition, the needs of affected individuals are mostly not being met by clinical services and a main determinant of service delivery was age (and not need). Furthermore, many services (including GP, mental health and prisons) contain relatively large numbers of people with ASD and ADHD that have not been recognised; and these individuals suffer from high levels of impairment. To help this situation we provide proof of concept for the potential utility of new interview-based diagnostic/screening tools, biomarkers and behavioural treatments.
Recommendations for future research
Based on our findings, and the limitations of our studies, we suggest that future work is required to address the following:
-
If we can reduce the burden of ASD and ADHD by targeting associated mental health symptoms. We need to develop tools (and training) so that these are recognised and treated. In addition, we need to test the effectiveness of treatments for mental health disorders that are currently used in the general population – it is largely unknown if they are equally effective (or have similar rates of adverse events) in people with ASD or ADHD.
-
The need for quicker, easier and more reliable screening, diagnostic and predictive tools (including perhaps biomarkers) that work in ‘real-world’ clinical settings.
-
The barriers to providing lifelong services for what are lifelong disorders. We need to understand what prevents provision of services that are based on need – and not age.
-
The acceptability to affected individuals and their carers of putative new screening, diagnostic and treatment approaches.
-
The validity, reliability and acceptability of objective outcome markers for treatment interventions.
Chapter 25 Impact
Capacity building
During this programme we endeavoured to develop capacity, increase and disseminate knowledge and help initiate progress. Our outputs are summarised below.
Undergraduate progression
During the course of the grant, two placement students from the University of Surrey and the University of Westminster and a summer work experience student worked on the project. All three individuals reported thoroughly enjoying their experience and two have gone on to further education within related fields; one is applying for a Masters in Child Mental Health to commence in 2014 at the IoP and the other has just been accepted at University of East London to study for a Doctorate in Counselling Psychology. The remaining student will return to university for his final year in September 2013. He wishes to pursue a career in Clinical Psychology.
Career progress of individuals employed by the programme
We are happy to report significant career progress of our RAs. This includes:
-
Tim Cadman (RA) has obtained a placement on a Clinical Psychology training programme (this is extremely competitive in the UK).
-
Hanna Eklund (RA) was awarded a Doctor of Philosophy (PhD) for her work.
-
Dee Howley (RA) has trained as a paediatric research nurse and is now leading a European study on infants at risk of ASD.
-
Hannah Hayward (RA) is leading a study on ADHD and ASD in prison settings.
-
James Findon (RA) has been awarded an extremely prestigious Medical Research Council PhD training fellowship.
-
Jenna Vyas (RA) has obtained a placement on a Clinical Psychology training programme (this is extremely competitive in the UK).
-
Helen Todman (affiliated Master of Science student) obtained a place on the accelerated promotion scheme for NHS management.
-
Eddie Chaplin (research nurse) has completed his PhD on ASD and ADHD in forensic settings
-
two of our other RAs continued into further education: with Zoe Huntley in her final year of Clinical Psychology training at University College London and Amy Hammon completing her Doctorate in Counselling at the University of East London.
-
Emma Woodhouse (RA) is currently working as a RA on a project examining Tuberous Sclerosis and ASD led by Professor Patrick Bolton at the IoP; she is now a registered Member of the British Association for Counselling & Psychotherapy.
-
Karen Ashwood (RA) is currently a post-doctoral research worker at the IoP, working with Professor Tony Charman to develop a clinical European Clinical Trials network for ASD; the project is part of European Autism Interventions – a Multicentre Study for Developing New Medications, one of the largest ever collaborations between academia and industry to develop new medications for ASD.
-
Dr Fiona McEwen (RA) has secured Post-doctoral researcher/study co-ordinator position on a further study at the IoP and is continuing in the field of NDs.
-
Dr Catherine Ames (RA) subsequently completed her doctorate in clinical psychology and secured a position as a clinical psychologist at the Maudsley Hospital, South London & Maudsley NHS Foundation Trust.
Student dissertations
A number of student dissertations (undergraduate, postgraduate taught and PhD dissertations) are listed in Acknowledgements, Publications.
Conference presentations/keynote talks
A number of papers have been presented at conferences and these are listed in Acknowledgements, Publications.
Peer-reviewed publications
A list of peer-reviewed publications is presented in Acknowledgements, Publications.
Books and training materials
Bonell S, et al. 378: this book offers a unique inside line on the subject as each chapter is considered through the eyes of a different person. This compilation of personal insights has been written by consultant psychiatrists, nurses, a GP and service users. Although it is designed to inspire medical professionals to pay more consideration to the needs of this vulnerable and often excluded group; it assumes no prior knowledge and is distributed free on request or by download from URL: www.rcpsych.ac.uk/pdf/Neurodevelopmental%20psychiatry%20-%20an%20introduction%20for%20medical%20students.pdf.
The following books and training materials have been published by Pavilion in Brighton. A number of these resources are available on a per chapter or section basis on individual compact discs (CDs). For further details of the content of these publications go to URL: www.pavpub.com/Common/SearchResult.aspx?type=2&tax-1=224906&tax-2=226290.
Chaplin E, et al. 379: this book provides a comprehensive introduction to working with people who have autism spectrum conditions. The book addresses the needs of people with autism spectrum conditions across the lifespan and across the range of intellectual functioning. Although the content is grounded in evidence-based practice and recent research, the text is intended to be as practical as possible, offering insight into the everyday lives of people with autism spectrum conditions and how staff can best support them.
Chaplin E, et al. 380: written by a range of contributors from multidisciplinary backgrounds, this accessible resource provides background information and learning opportunities for all those working in forensic services for people with LDs. The training material is organised into 12 modules, each supported by a chapter in the accompanying handbook. The training can be provided as standalone modules or as a comprehensive course and is designed to be delivered by trainers and/or clinicians who have experience in providing forensic services to people with LDs.
Relationship with media
Newspapers and magazines
Articles about our research have been featured in Nature (London), Science, New Scientist, MRC News, The Times, The Sunday Times, The Guardian,381 The Independent, Daily Telegraph, Sunday Telegraph, Daily Express, Daily Mail,382 The Washington Post, The New York Times and in numerous different baby, parenting and women’s magazines.
Television and radio
The British Broadcasting Corporation (BBC) National TV News, Channel 4 National TV News, ITV National News, CBS National News, Good Morning America, Sky TV News, The Discovery Channel, Al Jazeera and numerous other local and international TV news programs.
The Breakthrough Appeal and the BBC Radio 4 Appeal. 383
URL: www.bbc.co.uk/news/health-10929032. 384
The work from this NIHR-funded programme that underpins the new grant was launched at a reception hosted by Mrs Samantha Cameron at the Prime Minister’s residence (10 Downing Street) on 2 July 2013. It also was selected for a BBC Radio 4 appeal. 383
Acknowledgements
We wish to thank all the individuals with ADHD and ASD (and their carers), together with the clinical services, for their invaluable help and support while carrying out this programme of work.
Contributions of authors
Professor Declan Murphy (Professor of Psychiatry and Brain Maturation) was the principal investigator on the proposal.
Professor Karen Glaser (Professor in Gerontology) led the analysis of user and carer burdens.
Ms Hannah Hayward (RA) conducted the data gathering of user and carer involvement.
Dr Hanna Eklund (Post-Doctoral Researcher) conducted the data gathering and data analysis of user and carer involvement.
Dr Tim Cadman (RA) conducted the data gathering of user and carer involvement.
Mr James Findon (RA) conducted the data gathering of user and carer involvement.
Ms Emma Woodhouse (RA) conducted the analysis of service use and provision.
Dr Karen Ashwood (Post-Doctoral Researcher) conducted the analysis of service use and provision.
Professor Jennifer Beecham (Professor of Health and Social Care Economics) conducted the health economics analysis.
Professor Patrick Bolton (Professor of Child and Adolescent Psychiatry) led work on designing new interview-based screening and diagnostic measures.
Dr Fiona McEwen (Post-Doctoral Researcher) worked on, and analysed, new interview-based screening and diagnostic measures.
Dr Ellie Wilson (Post-Doctoral Researcher) led the investigation on the effects of changes in clinical diagnostic criteria in DSM-V.
Dr Christine Ecker (Lecturer in Neuroimaging Sciences) led the work investigating the potential utility of biomarkers.
Professor Ian Wong (Professor of Pharmacology) led the study on GP prescribing.
Professor Emily Simonoff (Professor of Child and Adolescent Psychiatry) led the work investigating treatment response of ADHD in LDs.
Dr Ailsa Russell (Clinical Psychologist) led the work examining the effectiveness of CBT.
Dr Jane McCarthy (Consultant Psychiatrist) co-led the work examining the potential utility of e-learning and rates of ADHD and ASD in Brixton prison.
Dr Eddie Chaplin (Research Nurse) co-led the work examining the potential utility of e-learning and rates of ADHD and ASD in Brixton prison.
Dr Susan Young (Senior Lecturer in Forensic and Clinical Psychology) aided the work examining rates of ASD and ADHD in forensic settings.
Professor Philip Asherson (Professor of Molecular Psychiatry) led the work examining diagnostic rates of ADHD in various clinical settings.
Publications
Student dissertations
Undergraduate
Clarke H. To What Extent do Special and Mainstream Schools Accommodate the Needs of Young People with Autism Spectrum Disorders. BSc (Hons) Psychology. Surrey: University of Surrey; 2013.
Findon J. To What Extent Does Stimulant Medication Use for the Treatment of ADHD in Adolescents and Young Adults Meet Their Needs? BSc (Hons) Psychology. Kent: University of Kent; 2011.
Postgraduate taught
Cadman T. Psychological Well-Being of Carers of Adolescents and Young Adults with Autism: The Relationship Between Needs, Service Use and Caregiver Burden. PGDip Psychology. Middlesex: University of Middlesex; 2011.
Todman H. Predictors of Discrepancies Between Self and Informant Reports of Needs in Young Adults with Autistic Spectrum Disorder and Attentional-Deficit Hyperactivity Disorder. MSc Neuroscience. London: IoP, King’s College London; 2012.
PhD dissertations
D’Astous V. How and by Whom are the Current and Anticipated Future Support Needs of Adults with Autism Spectrum Disorder Met? PhD thesis. London: King’s College London; 2017.
Conference presentations/keynote talks
Ecker C, Murphy D. Brain Anatomy – A Biomarker for Autism Spectrum Disorder(s). Conference of the British Radiological Society, London, UK, March 2011.
Murphy D, Ecker C. Debate ‘Advances in Brain Imaging Technology Will Significantly Enhance the Lives of People with Autism’. National Autistic Society Professional Conference, Manchester, UK, March 2011.
Gillan N, Murphy CM, Robertson D, Spain D, Doyle MJ, Wilson E, et al. A Preliminary Investigation of Service Transition as People with Autism Spectrum Disorder Move from Childhood into Young Adulthood. International Meeting for Autism Research (IMFAR), San Diego, CA, USA, May 2011.
Glaser K, Eklund H, Cadman T, Howley D, Beecham J, Findon J, et al. Are Current or Childhood ADHD Symptoms Good Predictors of Psychopathology in Young Adults with ADHD. UK Adult ADHD Network, Expert Workshop, London, UK, September 2011.
Murphy D, Ecker C. Can We Use the ‘New Biology’ to Diagnose ASD and ADHD in Adults? International Congress of the Royal College of Psychiatrists, Brighton, UK, June/July 2011.
Asherson P, Maltezos S, Murphy D, Williams C, Morinan A, Brinsford A. Undiagnosed ADHD in Substance Use Disorder (SUD) Populations. Poster presented at the International Congress for the European Network of Adult ADHD, London, UK, September 2011.
Ashwood K, Woodhouse, E. ASD and ADHD in Adults: Symptoms, Diagnosis and Treatment. Educational Talk given to General Practitioners in Southwark, London, UK, March 2012.
Ward H, Gillan N, Cadman T, Eklund H, Howley D, Findon J, et al. Service Use and Needs Among Those with an ASD at Transition from Child to Adult Services. International Meeting for Autism Research (IMFAR), Toronto, ON, Canada, May 2012.
Woodhouse E, Ashwood K, Hammon A, Young S, Perkins D, Murphy D, et al. Autistic Traits in Patients Within Secure Forensic Mental Health Settings. Poster presented at the International Meeting for Autism Research (IMFAR), Toronto, ON, Canada, May 2012.
Hayward H, Gillan N, Cadman T, Eklund H, Howley D, Findon J, et al. Service Use and Needs Among Those with an ASD at Transition from Child to Adult Services. International Meeting for Autism Research (IMFAR), Toronto, ON, Canada, May 2012.
Cadman T, Eklund H, Howley D, Hayward H, Findon J, Clarke H, et al. Carer Burden in ASD. International Meeting for Autism Research (IMFAR), Toronto, ON, Canada, May 2012.
Wilson E, Robertson DM, Gillan N, Roberts G, Coghlan S, Mendez MA, et al. Prevalence of Co-Morbid Psychiatric Conditions in Men and Women with Autism Spectrum Disorder. International Meeting for Autism Research (IMFAR), Toronto, ON, Canada, May 2012.
Gillan N, Roberts G, Johnston K, Maltezos S, Murphy CM, Murphy DG, et al. The Implications of DSM V: Changes in Diagnostic Outcomes in an Adult Clinical Sample Re-Diagnosed According to the Proposed DSM V. International Meeting for Autism Research (IMFAR), Toronto, ON, Canada, May 2012.
Woodhouse E, Ashwood, K. Understanding Autism Spectrum Disorder. Educational talk given to members of the Environment Agency, London, UK, June 2012.
Murphy CM, Gillan N, Dube T, Pitts M, Dove H, Asherson P. Transition of Young Adults with ADHD: Reporting Symptoms by Young Adults and their Parents/Caregivers. UK Adult ADHD Network 3rd Annual Congress, London, UK, June 2012.
Murphy CM, Gillan N. 22q11.2DS: Transition of Care From Child to Adult Mental Health Services. The 8th Biennial 22q11.2 Deletion Syndrome Meeting, Lake Buena Vista, FL, USA, July 2012.
Underwood L, Forrester A, Chaplin E, McCarthy J. Prisoners with Intellectual Disability: Comorbid Neurodevelopmental Disorders and Mental Health Problems. 47th Annual Conference for Australasian Society for Intellectual Disability: Research to Practice, Wellington, New Zealand, November 2012.
Murphy D, Ecker C, Loth E. Autism – Insights From Neuroimaging and Imaging-Based Autism Subject-Classification and Perspectives on Therapeutics. Neuroscience School of Advanced Studies, Siena, Italy, November 2012.
Ashwood K, Woodhouse E. Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD). Educational talk given to Southwark North Assessment and Liaison Team, London, UK, November 2012.
Ecker C. Translational Neuroimaging in Autism Spectrum Disorder – From Biomarkers to Therapeutics. Wellcome Trust ‘Biomarkers for Brain Disorders’ Conference, Cambridge, UK, February 2013.
McCarthy J, Underwood L, Chaplin E, Sabet J, Young S, Asherson P, et al. Intellectual Disability Among a Prison Population: Recognition, Pathways and Comorbid Mental Health Problems. 12th International Conference on the Care and Treatment of Offenders with a Learning Disability, Northumbria University, Newcastle-upon-Tyne, UK, April 2013.
Gillan N, Wilson CE, O’Rourke L, Murphy CM, Hayward H, D’Almeida V, et al. Using the Autism Quotient and Empathy Quotient to Aid Diagnosis in Adults: Performance in a ‘Real World’ Clinical Setting. International Meeting for Autism Research (IMFAR), San Sebastian, Spain, May 2013.
Hayward H, Gillan N, Cadman T, Eklund H, Howley D, Findon J, et al. Service Use and Needs Among Those with an ASD at Transition from Child to Adult Services. International Meeting for Autism Research (IMFAR), San Sebastien, Spain, 3–5 May 2013.
McEwen FS, Ames C, Woodhouse E, Colvert E, Curran S, Ronald A, et al. Using the Development and Well-Being Assessment (DAWBA) to Identify Autism Spectrum Disorder in a Community Sample of Adolescents. International Meeting for Autism Research (IMFAR), San Sebastian, Spain, May 2013.
McCarthy J, Underwood L, Forrester A, Chaplin E. Intellectual Disability in a Local Prison. 33rd International Congress of Law and Mental Health, Amsterdam, July 2013.
Underwood L, Chaplin E, Hayward H, Sabet J, Young S, Asherson P, et al. Intellectual Disability and Comorbidity in Prisons. IASSIDD Asia Pacific Regional Conference, Tokyo, Japan, August 2013.
Chaplin E, Underwood L, Forrester A, McCarthy J. Prison Pathways for Adults with Neurodevelopmental Disorders. 9th European Congress, ‘New horizons for Mental Health in Intellectual and Developmental Disabilities (IDD)’, Estoril Congress Centre, Estoril, Lisbon, Portugal, September 2013.
Forrester A, Chaplin E, McCarthy J. Prisoners with Neurodevelopmental Disorders. 9th European Congress, ‘New horizons for Mental Health in Intellectual and Developmental Disabilities (IDD)’, Estoril Congress Centre, Estoril, Lisbon, Portugal, September 2013.
Peer-reviewed publications
Russell AJ, Mataix-Cols D, Anson, MA, Murphy DG. Psychological treatment for obsessive–compulsive disorder in people with autism spectrum disorders – a pilot study. Psychother Psychosom 2009;78:59–61.
Bramham J, Ambery F, Young S, Morris R, Russell AJ, Xenitidis K, et al. Executive functioning differences between adults with attention deficit hyperactivity disorder and autistic spectrum disorder in initiation, planning and strategy formation. Autism 2009;13:245–64.
Ecker C, Rocha-Rego V, Johnston P, Mourao-Miranda J, Marquad A, Daly EM, et al. Investigating the predictive value of whole brain structural MR scans in Autism: a pattern classification approach. Neuroimage 2010;49:44–56.
Ecker C, Marquand A, Mourão-Miranda J, Johnston P, Daly EM, Brammer MJ, et al. Describing the brain in autism in five dimensions – magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci 2010;30:10612–23.
Mack H, Fullana MA, Russell AJ, Mataix-Cols D, Nakatani E, Heyman I. Obsessions and compulsions in children with Asperger’s syndrome or high-functioning autism: a case–control study. Aust N Z J Psychiatry 2010;44:1082–88.
Asherson P, Adamou M, Bolea B, Muller U, Morua SD, Pitts M, et al. Is ADHD a valid diagnosis in adults? Yes. BMJ 2010;340:c549.
Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugue M, Carpentier PJ, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry 2010;10:67.
Murphy D, Beecham J, Craig M, Ecker C. Autism in adults: new biological findings and their translational implications to the cost of clinical services. Brain Res 2011;1380:22–33.
Lai MC, Lombardo MV, Pasco G, Ruigrok AN, Wheelwright SJ, Sadek SA, et al. A behavioural comparison of male and female adults with high functioning autism spectrum conditions. PLOS ONE 2011;6:e20835.
Piven J, Rabins P. Autism spectrum disorders in older adults: toward defining a research agenda. Autism-in-Older Adults Working Group. J Am Geriatr Soc 2011;59:2151–5.
Young S, Murphy CM, Coghill D. Avoiding the ‘twilight zone’: recommendations for the transition of services from adolescence to adulthood for young people with ADHD. BMC Psychiatry 2011;11:174.
Young SJ, Adamou M, Bolea B, Gudjonsson G, Muller U, Pitts M, et al. The identification and management of ADHD offenders within the criminal justice system: a consensus statement from the UK Adult ADHD Network and criminal justice agencies. BMC Psychiatry 2011;11:32.
Johnston K, Madden AK, Bramham J, Russell AJ. Response inhibition in adults with autism spectrum disorder compared to attention deficit/hyperactivity disorder. J Autism Dev Disord 2011;41:903–12.
Asherson P, Akehurst R, Kooij JJ, Huss MM, Beusterien K, Sasane R, et al. Under diagnosis of adult ADHD: cultural influences and societal burden. J Atten Disord 2012;16(Suppl. 5):20S–38S.
Bolea B, Adamou M, Arif M, Asherson P, Gudjonsson G, Muller U, et al. ADHD matures: time for practitioners to do the same? J Psychopharmacol 2012;26:766–70.
Cadman T, Eklund H, Howley D, Hayward H, Clarke H, Findon J, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Academy Child Adolesc Psychiatry 2012;51:879–88.
Murphy DG, Spooren W. Translational approaches to drug discovery in autism. Nat Rev Drug Discov 2012;11:815–16.
Christakou A, Murphy CM, Chantiluke K, Cubillo AL, Smith AB, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with Autism. Mol Psychiatry 2012;18:236–44.
Catani M, Dell’Acqua F, Bizzi A, Forkel S, Williams S, Simmons A, et al. Beyond cortical localisation in clinico-anatomical correlation. Cortex 2012;48:1262–87.
Millan MM, Agid Y, Brune M, Bullmore ET, Carter SC, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 2012;11:141–68.
Pertusa A, Bejerot S, Eriksson J, de la Cruz LF, Bonde S, Russell A, et al. Do patients with hoarding disorder have autistic traits? Depress Anxiety 2012;29:210–18.
Bramham J, Murphy D, Xenitidis K, Asherson P, Hopkin G, Young S. Adults with ADHD; An investigation of age-related differences in behavioural symptoms, neuropsychological function and comorbidity. Psychol Med 2012;42:2225–34.
Ecker C, Spooren W, Murphy DGM. Translational approaches to the biology of Autism: false dawn or a new era? Mol Psychiatry 2013;18:435–42.
Russell AJ, Jassi A, Fullana MA, Mack H, Johnston K, Heyman I, et al. Cognitive behavior therapy for co-morbid obsessive–compulsive disorder in high functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013;30:697–708.
Underwood L, Forrester A, Chaplin E, McCarthy J. Prisoners with neurodevelopmental disorders. J Intellect Disabil Offending Behav 2013;4:17–23.
Ecker C, Spooren W, Murphy D. Developing new pharmacotherapies for autism. J Internal Med 2013;274:308–20.
Wilson CE, Gillan N, Spain D, Robertson D, Roberts G, Murphy C, et al. Comparison of ICD-10R, DSM-IV-TR and DSM-5 in an adult autism spectrum disorder diagnostic clinic. J Autism Dev Disord 2013;43:2515–25.
Chantiluke KC, Barrett N, Giampietro VP, Brammer MJ, Simmons A, Murphy DG, et al. Inverse effect of fluoxentine on medical prefrontal cortex activation during reward in ADHD and Autism. Cerebral Cortex 2014;25:1757–70.
Ecker C, Murphy D. Neuroimaging in autism – from basic science to translational research. Nat Rev Neurol 2014;10:82–91.
Steckler T, Spooren W, Murphy D. Autism spectrum disorders – an emerging area in psychopharmacology. Psychopharmacology 2014;231:977–8.
Hsia Y, Wong AYS, Murphy DGM, Simonoff E, Buitelaar JK, Wong ICK. Psychopharmacological prescriptions for people with autism spectrum disorder (ASD): a multinational study. Psychopharmacology 2014;231:999–1009.
Horder J, Wilson CE, Mendez Hernandez A, Murphy DG. Autistic traits and abnormal sensory experiences in adults. J Autism Dev Disord 2014;44:1461–9.
Chantiluke KC, Christakou A, Murphy C, Giampietro VP, Daly EM, Ecker C, et al. Disorder-specific functional abnormalities during temporal discounting in youth with attention deficit hyperactivity disorder (ADHD), Autism and comorbid ADHD and Autism. Psychiatry Res 2014;223:113–20.
Young S, Gudjonsson G, O’Rourke L, Woodhouse E, Ashwood K, Murphy D, et al. Attention-deficit-hyperactivity disorder and associated functional impairments in mentally disordered offenders. Psychiatry Res 2014;230:387–93.
Spain D, Sin PHJ, Chalder T, Murphy DG, Happe FGE. Cognitive-behavioural interventions for adults with ASD and psychiatric co-morbidity: a systematic review. Res Autism Spectr Disord 2015;9:151–62.
Cadman T, Spain D, Johnston P, Russell A, Mataix-Cols D, Craig M, et al. Obsessive–compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R Tell Us? Autism Res 2015;8:477–85.
Spain DC, Sin PHJ, Paliokostas E, Furuta M, Chalder T, Murphy DG, et al. Family therapy for autism spectrum disorders. Cochrane Database Syst Rev 2015;10:CD011894.
McCarthy J, Chaplin E, Underwood L, Forrester A, Hayward H, Sabet J, et al. Characteristics of prisoners with neurodevelopmental disorders and difficulties. J Intellect Disabil Res 2016;60:201–6.
McEwen FS, Stewart C, Colvert EJ, Woodhouse EL, Curran S, Gillan NC, et al. Diagnosing autism spectrum disorder in community settings using the Development and Well-Being Assessment: validation in a UK population-based twin sample. J Child Psychol Psychiatry 2016;57:161–70.
Findon JL, Cadman TJ, Stewart C, Woodhouse EL, Eklund H, Hayward H, et al. Screening for co-occurring conditions in adults with autism spectrum disorder using the Strengths and Difficulties Questionnaire: a pilot study. Autism Res 2016;9:1353–63.
Ashwood K, Gillan N, Horder J, Hayward H, Woodhouse EL, McEwen FS, et al. Predicting the diagnosis of autism in adults using the Autism-Spectrum Quotient (AQ) questionnaire. Psychol Med 2016;46:2595–604.
Russell AJ, Murphy C, Wilson E, Gillan N, Brown C, Robertson DM, et al. The mental health of individuals referred for assessment of autism spectrum disorder in adulthood: a clinic report. Autism 2016;20:623–7.
Murphy C, Wilson CE, Robertson DM, Ecker C, Daly EM, Hammond N, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development Neuropsychiatr Disease Treat 2016;12:1669–86.
Eklund H, Cadman T, Findon J, Hayward H, Howley D, Beecham J, et al. Clinical service use as people with attention deficit hyperactivity disorder transition into adolescence and adulthood: a prospective longitudinal study. BMC Health Serv Res 2016;16:248.
Spain D, Happé F, Johnston P, Campbell M, Sin J, Daly E, et al. Social anxiety in adult males with autism spectrum disorders. Res Autism Spectr Disord 2016;32:13–23.
Wilson CE, Murphy CM, McAlonan G, Robertson DM, Spain D, Hayward H, et al. Does sex influence the diagnostic evaluation of autism spectrum disorder in adults? Autism 2016;20:808–19.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 2010;10. https://doi.org/10.1186/1471-244X-10-67.
- Huntley Z, Maltezos S, Williams C, Morinan A, Hammon A, Ball D, et al. Rates of undiagnosed attention deficit detoxification units. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-223.
- McEwen FS, Stewart CS, Colvert E, Woodhouse E, Curran S, Gillan N, et al. Diagnosing autism spectrum disorder in community settings using the Development and Well-being Assessment: validation in a UK population-based twin sample. J Child Psychol Psychiatr 2016;57:161-70. https://doi.org/10.1111/jcpp.12447.
- Ecker C, Marquand A, Mourão-Miranda J, Johnston P, Daly EM, Brammer MJ, et al. Describing the brain in autism in five dimensions – magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci 2010;30:10612-23. https://doi.org/10.1523/JNEUROSCI.5413-09.2010.
- Lai MC, Lombardo MV, Pasco G, Ruigrok AN, Wheelwright SJ, Sadek SA, et al. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0020835.
- Murray ML, Hsia Y, Glaser K, Simonoff E, Murphy DGM, Asherson PJ, et al. Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology 2014;231:1011-21. https://doi.org/10.1007/s00213-013-3140-7.
- Knapp M, Romeo R, Beecham J. Economic cost of autism in the UK. Autism 2009;13:317-36. https://doi.org/10.1177/1362361309104246.
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. J Child Psychol Psychiatry 2005;46:401-8. https://doi.org/10.1111/j.1469-7610.2004.00361.x.
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med 2006;36:159-65. https://doi.org/10.1017/S003329170500471X.
- Levy A, Perry A. outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011;5:1271-82. https://doi.org/10.1016/j.rasd.2011.01.023.
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29. https://doi.org/10.1111/j.1469-7610.2004.00215.x.
- Barnard J, Harvey V, Potter D, Prior A. Ignored or Ineligible? The Reality for Adults with Autism Spectrum Disorders. London: NAS Publications; 2001.
- Ballaban-Gil K, Rapin I, Tuchman R, Shinnar S. Longitudinal examination of the behavioral, language, and social changes in a population of adolescents and young adults with autistic disorder. Pediatr Neurol 1996;15:217-23. https://doi.org/10.1016/S0887-8994(96)00219-6.
- Biederman J, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, Aleardi M. Functional impairments in adults with self-reports of diagnosed ADHD: a controlled study of 1001 adults in the community. J Clin Psychiatry 2006;67:524-40. https://doi.org/10.4088/JCP.v67n0403.
- Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: comorbidities and adaptive impairments. Compr Psychiatry 1996;37:393-401. https://doi.org/10.1016/S0010-440X(96)90022-X.
- Biederman J, Petty CR, Fried R, Kaiser R, Dolan CR, Schoenfeld S, et al. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: a controlled study. J Clin Psychiatry 2008;69:1217-22. https://doi.org/10.4088/JCP.v69n0803.
- Eakin L, Minde K, Hechtman L, Ochs E, Krane E, Bouffard R, et al. The marital and family functioning of adults with ADHD and their spouses. J Atten Disord 2004;8:1-10. https://doi.org/10.1177/108705470400800101.
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry 2004;45:195-211. https://doi.org/10.1111/j.1469-7610.2004.00214.x.
- Barkley R. ADHD in Adults: Diagnosis and Management. St. Louis, MO: Compact Clinicals – Dean Press; 2009.
- Young SJ, Adamou M, Bolea B, Gudjonsson G, Müller U, Pitts M, et al. The identification and management of ADHD offenders within the criminal justice system: a consensus statement from the UK Adult ADHD Network and criminal justice agencies. BMC Psychiatry 2011;11. https://doi.org/10.1186/1471-244X-11-32.
- Supporting People with Autism Throughout Adulthood. London: The Stationery Office; 2009.
- Edwin F, McDonald J. Services for adults with attention-deficit hyperactivity disorder: national survey. Psychiatr Bull 2007;31:286-8. https://doi.org/10.1192/pb.bp.106.012237.
- Marcer H, Finlay F, Baverstock A. ADHD and transition to adult services – the experience of community paediatricians. Child Care Health Dev 2008;34:564-6. https://doi.org/10.1111/j.1365-2214.2008.00857.x.
- Able SL, Johnston JA, Adler LA, Swindle RW. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med 2007;37:97-107. https://doi.org/10.1017/S0033291706008713.
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry 2011;68:459-65. https://doi.org/10.1001/archgenpsychiatry.2011.38.
- Provision of NHS Mental Health Services (Fourth Report). London: HMSO; 2000.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994;24:659-85. https://doi.org/10.1007/BF02172145.
- Xenitidis K, Thornicroft G, Leese M, Slade M, Fotiadou M, Philp H, et al. Reliability and validity of the CANDID – a needs assessment instrument for adults with learning disabilities and mental health problems. Br J Psychiatry 2000;176:473-8. https://doi.org/10.1192/bjp.176.5.473.
- Green H, McGinnity A, Meltzer H, Ford T, Goodman R. Mental Health of Children and Young People in Great Britain, 2004 Report. London: National Statistics Office, Controller of Her Majesty’s Stationery Office; 2005.
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789-95. https://doi.org/10.1001/archinte.158.16.1789.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992.
- Mental Capacity Act 2005. London: HMSO; 2005.
- Kooij S, Francken M. Diagnostic Interview for ADHD (DIVA) in Adults 2007 n.d. www.divacenter.eu (accessed August 2007).
- Frazier TW, Youngstrom EA, Naugle RI. The latent structure of attention-deficit/hyperactivity disorder in a clinic-referred sample. Neuropsychology 2007;21:45-64. https://doi.org/10.1037/0894-4105.21.1.45.
- Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder. A Clinical Workbook. New York, NY: Guildford Press; 1998.
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 1992;22:465-86. https://doi.org/10.1017/S0033291700030415.
- Andersen R. A Behavioral Model of Families’ Use of Health Services. Chicago, IL: Center for Health Administration Studies, University of Chicago; 1968.
- Andersen RM. Revisiting the behavioral model and access to medical care: does it matter?. J Health Soc Behav 1995;36:1-10. https://doi.org/10.2307/2137284.
- Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Mem Fund Q Health Soc 1973;51:95-124. https://doi.org/10.2307/3349613.
- Fosu GB. Childhood morbidity and health services utilization: cross-national comparisons of user-related factors from DHS data. Soc Sci Med 1994;38:1209-20. https://doi.org/10.1016/0277-9536(94)90186-4.
- Smith SR, Kirking DM. Access and use of medications in HIV disease. Health Serv Res 1999;34:123-44.
- Singh SP, Paul M, Ford T, Kramer T, Weaver T, McLaren S, et al. Process, outcome and experience of transition from child to adult mental healthcare: multiperspective study. Br J Psychiatry 2010;197:305-12. https://doi.org/10.1192/bjp.bp.109.075135.
- Barkley RA, Murphy KR. Attention Deficit Hyperactivity Disorder: A Clinical Workbook (Vol. 2). New York, NY: Guildford Press; 2006.
- Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry 1996;35:343-51. https://doi.org/10.1097/00004583-199603000-00016.
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psycholog Med 2006;36:167-79. https://doi.org/10.1017/S0033291705006410.
- Lee SI, Schachar RJ, Chen SX, Ornstein TJ, Charach A, Barr C, et al. Predictive validity of DSM-IV and ICD-10 criteria for ADHD and hyperkinetic disorder. J Child Psychol Psychiatry 2008;49:70-8. https://doi.org/10.1111/j.1469-7610.2007.01784.x.
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007;164:942-8. https://doi.org/10.1176/ajp.2007.164.6.942.
- Kennedy I. Getting it Right for Children and Young People: Overcoming Cultural Barriers in the NHS so as to Meet Their Needs. London: Department of Health and Social Care; 2010.
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 2001;31:5-17. https://doi.org/10.1023/A:1005653411471.
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000;41:645-55. https://doi.org/10.1111/j.1469-7610.2000.tb02345.x.
- Protection of Children Act 1978. London: HMSO; 1978.
- Autism: Recognition, Referral, Diagnosis and Management of Adults on the Autism Spectrum. Clinical Guideline 142. Leicester: NICE; 2012.
- Simonoff E, Taylor E, Baird G, Bernard S, Chadwick O, Liang H, et al. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry 2013;54:527-35. https://doi.org/10.1111/j.1469-7610.2012.02569.x.
- Research Units on Pediatric Psychopharmacology Autism Network . Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psych 2005;62:1266-74. https://doi.org/10.1001/archpsyc.62.11.1266.
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol Med 2011;41:619-27. https://doi.org/10.1017/S0033291710000991.
- Cadman T, Eklund H, Howley D, Hayward H, Clarke H, Findon J, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012;51:879-88. https://doi.org/10.1016/j.jaac.2012.06.017.
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity and associated factors. J Am Acad Child Adolesc Psychiatry 2008;47:921-9. https://doi.org/10.1097/CHI.0b013e318179964f.
- Ruble LA, Heflinger CA, Renfrew JW, Saunders RC. Access and service use by children with autism spectrum disorders in Medicaid Managed Care. J Autism Dev Disord 2005;35:3-13. https://doi.org/10.1007/s10803-004-1026-6.
- Järbrink K, Fombonne E, Knapp M. Measuring the parental, service and cost impacts of children with autistic spectrum disorder: a pilot study. J Autism Dev Disord 2003;33:395-402. https://doi.org/10.1023/A:1025058711465.
- Taylor JL, Seltzer MM. Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. J Autism Dev Disord 2011;41:566-74. https://doi.org/10.1007/s10803-010-1070-3.
- Abbeduto L, Seltzer MM, Shattuck P, Krauss MW, Orsmond G, Murphy MM. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. Am J Ment Retard 2004;109:237-54. https://doi.org/10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2.
- Blacher J, McIntyre LL. Syndrome specificity and behavioural disorders in young adults with intellectual disability: cultural differences in family impact. J Intellect Disabil Res 2006;50:184-98. https://doi.org/10.1111/j.1365-2788.2005.00768.x.
- Casado B, Sacco P. Correlates of caregiver burden among family caregivers of older Korean Americans. J Gerontol B Psychol Sci Soc Sci 2012;67:331-6. https://doi.org/10.1093/geronb/gbr115.
- Yates ME, Tennstedt S, Chang BH. Contributors to and mediators of psychological well-being for informal caregivers. J Gerontol B Psychol Sci Soc Sci 1999;54:P12-22. https://doi.org/10.1093/geronb/54B.1.P12.
- Lawton MP, Kleban MH, Moss M, Rovine M, Glicksman A. Measuring caregiving appraisal. J Gerontol 1989;44:P61-71. https://doi.org/10.1093/geronj/44.3.P61.
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist 1990;30:583-94. https://doi.org/10.1093/geront/30.5.583.
- Theule J, Wiener J, Tannock R, Jenkins JM. Parenting stress in families of children with ADHD a meta-analysis. J Emot Behav Disord 2013;21:3-17. https://doi.org/10.1177/1063426610387433.
- Lounds J, Seltzer MM, Greenberg JS, Shattuck PT. Transition and change in adolescents and young adults with autism: longitudinal effects on maternal well-being. Am J Ment Retard 2007;112:401-17. https://doi.org/10.1352/0895-8017(2007)112[401:TACIAA]2.0.CO;2.
- Higginson IJ, Gao W, Jackson D, Murray J, Harding R. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol 2010;63:535-42. https://doi.org/10.1016/j.jclinepi.2009.06.014.
- Kooij JJS. Diagnostic Interview for ADHD in Adults (DIVA 2.0). Adult ADHD Diagnostic Assessment and Treatment. Amsterdam: Pearson Assessment and Information BV; 2010.
- Kring SR, Greenberg JS, Seltzer MM. Adolescents and adults with autism with and without co-morbid psychiatric disorders: differences in maternal well-being. J Ment Health Res Intellect Disabil 2008;1:53-74. https://doi.org/10.1080/19315860801988228.
- Kring SR, Greenberg JS, Seltzer MM. The impact of health problems on behavior problems in adolescents and adults with autism spectrum disorders: implications for maternal burden. Soc Work Ment Health 2010;8:54-71. https://doi.org/10.1080/15332980902932441.
- Tehee E, Honan R, Hevey D. Factors contributing to stress in parents of individuals with autistic spectrum disorders. J Appl Res Intellect Disabil 2009;22:34-42. https://doi.org/10.1111/j.1468-3148.2008.00437.x.
- Anderson CS, Linto J, Stewart-Wynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke 1995;26:843-9. https://doi.org/10.1161/01.STR.26.5.843.
- Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement: meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. J Learn Disabil 2007;40:49-65. https://doi.org/10.1177/00222194070400010401.
- Küpper T, Haavik J, Drexler H, Ramos-Quiroga JA, Wermelskirchen D, Prutz C, et al. The negative impact of attention-deficit/hyperactivity disorder on occupational health in adults and adolescents. Int Arch Occup Environ Health 2012;85:837-47. https://doi.org/10.1007/s00420-012-0794-0.
- Mannuzza S, Klein RG, Moulton JL. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res 2008;160:237-46. https://doi.org/10.1016/j.psychres.2007.11.003.
- Molina BS, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol 2003;112:497-50. https://doi.org/10.1037/0021-843X.112.3.497.
- Barkley RA, Murphy K, Kwasnik D. Psychological adjustment and adaptive impairments in young adults with ADHD. J Atten Disord 1996;1:41-54. https://doi.org/10.1177/108705479600100104.
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry 2000;157:816-18. https://doi.org/10.1176/appi.ajp.157.5.816.
- Mick E, Byrne D, Fried R, Monuteaux M, Faraone SV, Biederman J. Predictors of ADHD persistence in girls at 5-year follow-up. J Atten Disord 2011;15:183-92. https://doi.org/10.1177/1087054710362217.
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76. https://doi.org/10.1001/archpsyc.1993.01820190067007.
- Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry 2012;73:941-50. https://doi.org/10.4088/JCP.11m07529.
- Kuntsi J, Neale BM, Chen W, Faraone SV, Asherson P. The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behav Brain Funct 2006;2. https://doi.org/10.1186/1744-9081-2-27.
- Bebbington P, Brugha T, Coid J, Crawford M, Deverill C, D’Souza J, et al. Adult Psychiatric Morbidity in England, 2007: Results of a Household Survey. London: The Health & Social Care Information Centre, Social Care Statistics; 2009.
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, et al. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet 2008;147B:1450-60. https://doi.org/10.1002/ajmg.b.30672.
- Conners CK. Conners Rating Scales-Revised Technical Manual. New York, NY: Multi-Health Systems; 1997.
- Wechsler D. Wechsler Intelligence Scale for Children. New York, NY: Psychological Corporation; 1991.
- Epstein JN, Johnson DE, Varia IM, Conners CK. Neuropsychological assessment of response inhibition in adults with ADHD. J Clin Exp Neuropsychol 2001;23:362-71. https://doi.org/10.1076/jcen.23.3.362.1186.
- Deb S, Burns J. Neuropsychiatric consequences of traumatic brain injury: a comparison between two age groups. Brain Inj 2007;21:301-7. https://doi.org/10.1080/02699050701253137.
- Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA. A self report measure of affective lability. J Neurol Neurosurg Psychiatr 1997;63:89-93. https://doi.org/10.1136/jnnp.63.1.89.
- Smith RA, Berg JE, Pope LE, Callahan JD, Wynn D, Thisted RA. Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult Scler 2004;10:679-85. https://doi.org/10.1191/1352458504ms1106oa.
- Skirrow C, Asherson P. Emotional lability, comorbidity and impairment in adults with attention-deficit hyperactivity disorder. J Affect Disord 2013;147:80-6. https://doi.org/10.1016/j.jad.2012.10.011.
- Attention Deficit Hyperactivity Disorder: The NICE Guideline on Diagnosis and Management of ADHD in Children, Young People and Adults. London: The British Psychological Society and The Royal College of Psychiatrists; 2008.
- Lara C, Fayyad J, de Graaf R, Kessler R, Aguilar-Gaxiola S, Angermeyer M, et al. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiat 2009;65:46-54. https://doi.org/10.1016/j.biopsych.2008.10.005.
- Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry 2007;190:402-9. https://doi.org/10.1192/bjp.bp.106.034389.
- Lichtenstein P, Halldner L, Zetterqvist J, Sjölander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med 2012;367:2006-14. https://doi.org/10.1056/NEJMoa1203241.
- Young S, Amarasinghe JM. Practitioner review: Non-pharmacological treatments for ADHD: a lifespan approach. J Child Psychol Psychiatry 2010;51:116-33. https://doi.org/10.1111/j.1469-7610.2009.02191.x.
- Ford T, Goodman R, Meltzer H. The British Child and Adolescent Mental Health Survey 1999: the prevalence of DSM-IV disorders. J Am Acad Child Adolesc Psychiatry 2003;42:1203-11. https://doi.org/10.1097/00004583-200310000-00011.
- Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 2009;194:204-11. https://doi.org/10.1192/bjp.bp.107.048827.
- McCarthy S, Asherson P, Coghill D, Hollis C, Murray M, Potts L, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry 2009;194:273-7. https://doi.org/10.1192/bjp.bp.107.045245.
- Kooij JJS, Aeckerlin LP, Buitelaar JK. Functioning, comorbidity and treatment of 141 adults with attention deficit hyperactivity disorder (ADHD) at a Psychiatric Outpatients’ Department. Nederlands Tijdschrift Voor Geneeskunde 2001;145:1498-501.
- Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry 2005;57:1442-51. https://doi.org/10.1016/j.biopsych.2005.04.001.
- Kessler RC, Adler LE, Ames M, Barkley RA, Birnbaum H, Greenberg P, et al. The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med 2005;47:565-72. https://doi.org/10.1097/01.jom.0000166863.33541.39.
- Berman G. Prison Population Statistics. London: House of Commons Library; 2012.
- Young S, Gudjonsson G, Ball S, Lam J. Attention Deficit Hyperactivity Disorder (ADHD) in personality disordered offenders and the association with disruptive behavioural problems. J Forens Psychiatry Psychol 2003;14:491-505. https://doi.org/10.1080/14789940310001615461.
- Salazar F, Baird G, Chandler S, O’Sullican T, Tseng E, Simonoff E. Co-occurring psychiatric disorders in preschool and elementary aged children with autism spectrum disorder. J Autism Dev Disord 2015;45:2283-94. https://doi.org/10.1007/s10803-015-2361-5.
- Gjevik E, Eldevik S, Fjæran-Granum T, Sponheim E. Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. J Autism Dev Disord 2011;41:761-9. https://doi.org/10.1007/s10803-010-1095-7.
- Adler L, Kessler RC, Spencer T. Adult ADHD Self-Report Scale (ASRS-v1.1) n.d. https://add.org/wp-content/uploads/2015/03/adhd-questionnaire-ASRS111.pdf.
- Adler LA, Spencer T, Faraone SV, Kessler RC, Howes MJ, Biederman J, et al. Validity of pilot Adult ADHD Self- Report Scale (ASRS) to rate adult ADHD symptoms. Ann Clin Psychiatry 2006;18:145-8. https://doi.org/10.1080/10401230600801077.
- Goodman D, Faraone SV, Adler LA, Dirks B, Hamdani M, Weisler R. Interpreting ADHD rating scale scores: Linking ADHD rating scale scores and CGI levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Prim Psychiatry 2010;17:44-52.
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163:716-23. https://doi.org/10.1176/ajp.2006.163.4.716.
- Green H, McGinnity A, Meltzer H, Ford T, Goodman R. Mental Health of Children and Young People in Great Britain, 2004. UK: Palgrave Macmillan; 2005.
- Sobanski E. Psychiatric comorbidity in adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2006;256:i26-31. https://doi.org/10.1007/s00406-006-1004-4.
- Faraone SV, Wilens TE, Petty C, Antshel K, Spencer T, Biederman J. Substance use among ADHD adults: Implications of late onset and subthreshold diagnoses. Am J Addict 2007;16:24-32. https://doi.org/10.1080/10550490601082767.
- Upadhyaya HP. Substance use disorders in children and adolescents with attention-deficit/hyperactivity disorder: implications for treatment and the role of the primary care physician. Primary Care Companion J Clin Psychiatry 2008;10:211-21. https://doi.org/10.4088/PCC.v10n0306.
- Sizoo B, van den Brink W, Koeter M, Gorissen van Eenige M, van Wijngaarden-Cremers P, van der Gaag RJ. Treatment seeking adults with autism or ADHD and co-morbid substance use disorder: prevalence, risk factors and functional disability. Drug Alcohol Depend 2010;107:44-50. https://doi.org/10.1016/j.drugalcdep.2009.09.003.
- Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann N Y Acad Sci 2001;931:251-70. https://doi.org/10.1111/j.1749-6632.2001.tb05783.x.
- Modestin J, Matutat B, Würmle O. Antecedents of opioid dependence and personality disorder: attention-deficit/hyperactivity disorder and conduct disorder. Eur Arch Psychiatry Clin Neurosci 2001;251:42-7. https://doi.org/10.1007/s004060170067.
- Ohlmeier MD, Peters K, Te Wildt BT, Zedler M, Ziegenbein M, Wiese B, et al. Comorbidity of alcohol and substance dependence with attention-deficit/hyperactivity disorder (ADHD). Alcohol Alcohol 2008;43:300-4. https://doi.org/10.1093/alcalc/agn014.
- Wilson JJ, Levin FR. Attention-deficit/hyperactivity disorder and early-onset substance use disorders. J Child Adolesc Psychopharmacol 2005;15:751-63. https://doi.org/10.1089/cap.2005.15.751.
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry 2004;12:305-20. https://doi.org/10.1080/10673220490910844.
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, et al. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci 2005;25:577-83. https://doi.org/10.1523/JNEUROSCI.4196-04.2005.
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 2009;42:32-41. https://doi.org/10.1055/s-0029-1216356.
- Carpentier PJ, de Jong CA, Dijkstra BA, Verbrugge CA, Krabbe PF. A controlled trial of methylphenidate in adults with attention deficit/hyperactivity disorder and substance use disorders. Addiction 2005;100:1868-74. https://doi.org/10.1111/j.1360-0443.2005.01272.x.
- Reimherr FW, Marchant BK, Strong RE, Hedges DW, Adler L, Spencer TJ, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31. https://doi.org/10.1016/j.biopsych.2005.04.040.
- Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007;68:93-101. https://doi.org/10.4088/JCP.v68n0113.
- Rösler M, Fischer R, Ammer R, Ose C, Retz W. A randomised, placebo-controlled, 24-week, study of low-dose extended-release methylphenidate in adults with attention-deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci 2009;259:120-9. https://doi.org/10.1007/s00406-008-0845-4.
- Skirrow C, McLoughlin G, Kuntsi J, Asherson P. Behavioral, neurocognitive and treatment overlap between attention-deficit/hyperactivity disorder and mood instability. Expert Rev Neurother 2009;9:489-503. https://doi.org/10.1586/ern.09.2.
- Kooij JJ, Buitelaar JK, van den Oord EJ, Furer JW, Rijnders CA, Hodiamont PP. Internal and external validity of attention-deficit hyperactivity disorder in a population-based sample of adults. Psychol Med 2005;35:817-27. https://doi.org/10.1017/S003329170400337X.
- Bell AS. A critical review of ADHD diagnostic criteria: what to address in the DSM-V. J Atten Disord 2010;20:1-8.
- Keedwell PA, Poon L, Papadopoulos AS, Marshall EJ, Checkley SA. Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol 2001;6:247-56. https://doi.org/10.1080/13556210120056580.
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 2002;111:279-89. https://doi.org/10.1037/0021-843X.111.2.279.
- Mental Health Act 2007. London: HMSO; 2007.
- Gudjonsson GH, Rabe-Hesketh S, Szmukler G. Management of psychiatric in-patient violence: patient ethnicity and use of medication, restraint and seclusion. Br J Psychiatry 2004;184:258-62. https://doi.org/10.1192/bjp.184.3.258.
- Young S, Gudjonsson G, Wells J, Asherson P, Theobald D, Oliver B, et al. Attention deficit hyperactivity disorder and critical incidents in a Scottish prison population. Pers Indiv Differ 2009;46:265-9. https://doi.org/10.1016/j.paid.2008.10.003.
- Gudjonsson G, Young S, Yates M. Motivating mentally disordered offenders to change. Instruments for measuring patients’ perception and motivation. J Forens Psych Psychol 2007;18:74-89. https://doi.org/10.1080/14789940601063261.
- Young S, Chesney S, Sperlinger D, Misch P, Collins P. A qualitative study exploring the life-course experiences of young offenders with symptoms and signs of ADHD who were detained in a residential care setting. Crim Behav Ment Health 2009;19:54-63. https://doi.org/10.1002/cbm.721.
- Young S, Goodwin E. Attention-deficit/hyperactivity disorder in persistent criminal offenders: the need for specialist treatment programs. Expert Rev Neurother 2010;10:1497-500. https://doi.org/10.1586/ern.10.142.
- Ginsberg Y, Hirvikoski T, Grann M, Lindefors N. Long-term functional outcome in adult prison inmates with ADHD receiving OROS-methylphenidate. Eur Arch Psychiatry Clin Neurosci 2012;262:705-24. https://doi.org/10.1007/s00406-012-0317-8.
- Ginsberg Y, Lindefors N. Methylphenidate treatment of adult male prison inmates with attention-deficit hyperactivity disorder: randomised double-blind placebo-controlled trial with open-label extension. Br J Psychiatry 2012;200:68-73. https://doi.org/10.1192/bjp.bp.111.092940.
- Robinson L, Spencer MD, Thomson LD, Stanfield AC, Owens DG, Hall J, et al. Evaluation of a screening instrument for autism spectrum disorders in prisoners. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0036078.
- McAdam P. Knowledge and understanding of the autism spectrum amongst prison staff. Prison Service J 2012;202:26-30.
- Wilens TE, Biederman J, Faraone SV, Martelon M, Westerberg D, Spencer TJ. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. J Clin Psychiatry 2009;70:1557-62. https://doi.org/10.4088/JCP.08m04785pur.
- Faraone SV, Biederman J, Spencer T, Mick E, Murray K, Petty C, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid?. Am J Psychiatry 2006;163:1720-9. https://doi.org/10.1176/ajp.2006.163.10.1720.
- Jordan M. Transforming the quality of life for people with dementia through contact with the natural world: fresh air on my face. Ageing Soc 2012;32:1263-4. https://doi.org/10.1017/S0144686X12000475.
- Browning A, Caulfield L. The prevalence and treatment of people with Asperger’s Syndrome in the criminal justice system. Criminol Crim Justic 2011;11:165-80. https://doi.org/10.1177/1748895811398455.
- Howlin P. Outcome in adult life for more able individuals with autism or Asperger syndrome. Autism 2000;4:63-8. https://doi.org/10.1177/1362361300004001005.
- Cashin A, Newman C. Autism in the criminal justice detention system: a review of the literature. J Forensic Nurs 2009;5:70-5. https://doi.org/10.1111/j.1939-3938.2009.01037.x.
- Murphy D. Autism Spectrum Quotient (AQ) profiles among male patients within high security psychiatric care: comparison with personality and cognitive functioning. J Forens Psych Psychol 2011;22:518-34. https://doi.org/10.1080/14789949.2011.596942.
- Loucks N, Talbot J. No One Knows: Identifying and Supporting Prisoners with Learning Difficulties and Learning Disabilities: the Views of Prison Staff. London: Prison Reform Trust; 2007.
- Paterson P. How well do young offenders with Asperger syndrome cope in custody? Two prison case studies. Br J Learn Disabil 2008;36:54-8. https://doi.org/10.1111/j.1468-3156.2007.00466.x.
- Bernfort L, Nordfeldt S, Persson J. ADHD from a socio-economic perspective. Acta Paediatr 2008;97:239-45. https://doi.org/10.1111/j.1651-2227.2007.00611.x.
- Scheffler RM, Hinshaw SP, Modrek S, Levine P. The global market for ADHD medications. Health Aff 2007;26:450-7. https://doi.org/10.1377/hlthaff.26.2.450.
- Wu EQ, Hodgkins P, Ben-Hamadi R, Setyawan J, Xie J, Sikirica V, et al. Cost effectiveness of pharmacotherapies for attention-deficit hyperactivity disorder: a systematic literature review. CNS Drugs 2012;26:581-600. https://doi.org/10.2165/11633900-000000000-00000.
- Peters-Scheffer N, Didden R, Korzilius H, Sturmey P. A meta-analytic study on the effectiveness of comprehensive ABA-based early intervention programs for children with Autism Spectrum Disorders. Res Autism Spectr Disord 2011;5:60-9. https://doi.org/10.1016/j.rasd.2010.03.011.
- Motiwala S, Gupta S, Lilly M, Ungar W, Coyte P. The cost-effectiveness of expanding intensive behavioural intervention to all autistic children in Ontario. Healthc Policy 2006;1:135-51. https://doi.org/10.12927/hcpol.2006.17884.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Department of Health and Social Care . Published Reference Costs n.d. www.gov.uk/government/collections/nhs-reference-costs#published-reference-costs (accessed 11 May 2018).
- Beecham J. Unit Costs: Not Exactly Child’s Play – A Guide to Estimating Unit Costs for Children’s Social Care 2000. www.pssru.ac.uk/pdf/B062.pdf.
- Clarke S, Sloper P, Moran N, Cusworth L, Franklin A, Beecham J. Multi-agency transition services: greater collaboration needed to meet the priorities of young disabled people with complex needs as they move into adulthood. J Integrat Care 2011;19:74-85. https://doi.org/10.1108/14769011111176734.
- Schöffski O, Sohn S, Happich M. Overall burden to society caused by hyperkinetic syndrome (HKS) and attention deficit hyperactivity disorder (ADHD). Gesundheitswesen 2008;70:398-403. https://doi.org/10.1055/s-0028-1082049.
- Young S, Murphy CM, Coghill D. Avoiding the ‘twilight zone’: recommendations for the transition of services from adolescence to adulthood for young people with ADHD. BMC Psychiatry 2011;11. https://doi.org/10.1186/1471-244X-11-174.
- Singh SP. Transition of care from child to adult mental health services: the great divide. Curr Opin Psychiatry 2009;22:386-90. https://doi.org/10.1097/YCO.0b013e32832c9221.
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 2012;51:990-1002. https://doi.org/10.1016/j.jaac.2012.07.008.
- Telford C, Green C, Logan S, Langley K, Thapar A, Ford T. Estimating the costs of ongoing care for adolescents with attention-deficit hyperactivity disorder. Soc Psychiatry Psychiatr Epidemiol 2012;48:337-44. https://doi.org/10.1007/s00127-012-0530-9.
- Snell T, Knapp M, Healey A, Guglani S, Evans-Lacko S, Fernández J-L, et al. Economic impact of childhood psychiatric disorder on public sector services in Britain: estimates from national survey data. J Child Psychol Psychiatry 2013;54:977-85. https://doi.org/10.1111/jcpp.12055.
- Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J Pediatr Psychol 2007;32:711-27. https://doi.org/10.1093/jpepsy/jsm022.
- Hakkart-van Roijen L, Zwirs BW, Bouwmans C, Tan SS, Schulpen TW, Vlasveld L, et al. Societal costs and quality of life of children suffering from attention deficient hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry 2007;16:316-26. https://doi.org/10.1007/s00787-007-0603-6.
- Swensen AR, Birnbaum HG, Secnik K, Marynchenko M, Greenberg P, Claxton A. Attention-deficit/hyperactivity disorder: increased costs for patients and their families. J Am Acad Child Adolesc Psychiatry 2003;42:1415-23. https://doi.org/10.1097/00004583-200312000-00008.
- De Ridder A, De Graeve D. Healthcare use, social burden and costs of children with and without ADHD in Flanders, Belgium. Clin Drug Investig 2006;26:75-90. https://doi.org/10.2165/00044011-200626020-00003.
- Knapp M, Romeo R, Beecham J. The Economic Consequences of Autism in the UK. London: Foundation for People with Learning Disabilities; 2007.
- Bebbington A, Beecham J. Social services support and expenditure for children with autism. Autism 2007;11:43-61. https://doi.org/10.1177/1362361307070911.
- Järbrink K. The economic consequences of autistic spectrum disorder among children in a Swedish municipality. Autism 2007;11:453-63. https://doi.org/10.1177/1362361307079602.
- Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med 2007;161:343-9. https://doi.org/10.1001/archpedi.161.4.343.
- Flanders SC, Engelhart L, Pandina GJ, McCracken JT. Direct health care costs for children with pervasive developmental disorders: 1996-2002. Adm Policy Ment Health 2007;34:213-20. https://doi.org/10.1007/s10488-006-0098-3.
- Croen LA, Najjar DV, Ray GT, Lotspeich L, Bernal P. A comparison of health care utilization and costs of children with and without autism spectrum disorders in a large group-model health plan. Pediatrics 2006;118:e1203-11. https://doi.org/10.1542/peds.2006-0127.
- Liptak GS, Stuart T, Auinger P. Health care utilization and expenditures for children with autism: data from U.S. national samples. J Autism Dev Disord 2006;36:871-9. https://doi.org/10.1007/s10803-006-0119-9.
- Shimabukuro TT, Grosse SD, Rice C. Medical expenditures for children with an autism spectrum disorder in a privately insured population. J Autism Dev Disord 2008;38:546-52. https://doi.org/10.1007/s10803-007-0424-y.
- Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: National Survey of Children’s Health. Arch Pediatr Adolesc Med 2006;160:825-30. https://doi.org/10.1001/archpedi.160.8.825.
- Shattuck PT, Wagner M, Narendorf S, Sterzing P, Hensley M. Post-high school service use among young adults with an autism spectrum disorder. Arch Pediatr Adolesc Med 2011;165:141-6. https://doi.org/10.1001/archpediatrics.2010.279.
- Department of Health and Social Care . National Service Framework for Children, Young People and Maternity Services. Core Standards n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/199952/National_Service_Framework_for_Children_Young_People_and_Maternity_Services_-_Core_Standards.pdf.
- Department of Health and Social Care . National Service Framework for Children, Young People and Maternity Services – The Mental Health and Well-Being of Children and Young People n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/199959/National_Service_Framework_for_Children_Young_People_and_Maternity_Services_-_The_Mental_Health__and_Psychological_Well-being_of_Children_and_Young_People.pdf.
- Autism Diagnosis in Children and Young People: Recognition, Referral and Diagnosis of Children and Young People on the Autism Spectrum. London: NICE; 2011.
- Mahoney WJ, Szatmari P, MacLean JE, Bryson SE, Bartolucci G, Walter SD, et al. Reliability and accuracy of differentiating pervasive developmental disorder subtypes. J Am Acad Child Adolesc Psychiatry 1998;37:278-85. https://doi.org/10.1097/00004583-199803000-00012.
- Klin A, Lang J, Cicchetti DV, Volkmar FR. Brief report: interrater reliability of clinical diagnosis and DSM-IV criteria for autistic disorder: results of the DSM-IV autism field trial. J Autism Dev Disord 2000;30:163-7. https://doi.org/10.1023/A:1005415823867.
- Oliver BR, Plomin R. Twins’ Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Res Hum Genet 2007;10:96-105. https://doi.org/10.1375/twin.10.1.96.
- Curran S, Dworzynski K, Happé F, Ronald A, Allison C, Baron-Cohen S, et al. No major effect of twinning on autistic traits. Autism Res 2011;4:377-82. https://doi.org/10.1002/aur.207.
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ). Los Angeles, CA: Western Psychological Services; 2003.
- Williams J, Scott F, Stott C, Allison C, Bolton P, Baron-Cohen S, et al. The CAST (Childhood Asperger Syndrome Test): test accuracy. Autism 2005;9:45-68. https://doi.org/10.1177/1362361305049029.
- Gerdts J, Bernier R. The broader autism phenotype and its implications on the etiology and treatment of autism spectrum disorders. Autism Res Treat 2011;2011. https://doi.org/10.1155/2011/545901.
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry 1999;175:444-51. https://doi.org/10.1192/bjp.175.5.444.
- Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): preliminary development of a UK screen for mainstream primary-school-age children. Autism 2002;6:9-31. https://doi.org/10.1177/1362361302006001003.
- Lord C. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 1989;19:185-212. https://doi.org/10.1007/BF02211841.
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23. https://doi.org/10.1023/A:1005592401947.
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 2007;37:613-27. https://doi.org/10.1007/s10803-006-0280-1.
- Liddle EB, Batty MJ, Goodman R. The Social Aptitudes Scale: an initial validation. Soc Psychiatry Psychiatr Epidemiol 2009;44:508-13. https://doi.org/10.1007/s00127-008-0456-4.
- Ghaziuddin M, Weidmer-Mikhail E, Ghaziuddin N. Comorbidity of Asperger syndrome: a preliminary report. J Intellect Disabil Res 1998;42:279-83. https://doi.org/10.1111/j.1365-2788.1998.tb01647.x.
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord 2006;36:849-61. https://doi.org/10.1007/s10803-006-0123-0.
- Hayward H, Cadman T, Eklund H, Findon J, Howley D, Gillan N, et al. Service Use and Needs Among Those With an ASD in Adolescence and Young Adulthood n.d.
- Cath DC, Ran N, Smit JH, van Balkom AJ, Comijs HC. Symptom overlap between autism spectrum disorder, generalized social anxiety disorder and obsessive–compulsive disorder in adults: a preliminary case-controlled study. Psychopathology 2008;41:101-10. https://doi.org/10.1159/000111555.
- Ghaziuddin M. Mental Health Aspects of Autism and Asperger Syndrome. London: Jessica Kingsley Publishers; 2005.
- Helverschou SB, Bakken TL, Martinsen H. The Psychopathology in Autism Checklist (PAC): a pilot study. Res Autism Spectr Disord 2009;3:179-95. https://doi.org/10.1016/j.rasd.2008.05.004.
- Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Publishing Inc.; 2000.
- Barkley RA. Attention-Deficit Hyperactivity Disorder – A Handbook for Diagnosis and Treatment. New York, NY: The Guilford Press; 2006.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, et al. The Obsessive–Compulsive Inventory: development and validation of a short version. Psychol Assess 2002;14:485-96. https://doi.org/10.1037/1040-3590.14.4.485.
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry 2001;40:1337-45. https://doi.org/10.1097/00004583-200111000-00015.
- Mash EJ, Barkley RA. Child Psychopathology. New York, NY: The Guilford Press; 2003.
- Pinborough-Zimmerman J, Bakian AV, Fombonne E, Bilder D, Taylor J, McMahon WM. Changes in the administrative prevalence of autism spectrum disorders: contribution of special education and health from 2002-2008. J Autism Dev Disord 2012;42:521-30. https://doi.org/10.1007/s10803-011-1265-2.
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry 2005;66:3-8.
- The International Classification of Diseases, Tenth Revision. Geneva: World Health Organization; 1993.
- Ozonoff S. DSM-5 and autism spectrum disorders – two decades of perspectives from the JCPP. J Child Psychol Psychiatry 2012;53:e4-6. https://doi.org/10.1111/j.1469-7610.2012.02587.x.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011;50:540-2. https://doi.org/10.1016/j.jaac.2011.03.015.
- Swedo SE, Baird G, Cook EHJ, Happé FG, Harris JC, Kaufmann WE, et al. Commentary from the DSM-5 workgroup on neurodevelopmental disorders. J Am Acad Child Adolesc Psychiatry 2012;51:347-9. https://doi.org/10.1016/j.jaac.2012.02.013.
- Frazier TW, Youngstrom EA, Speer L, Embacher R, Law P, Constantino J, et al. Validation of proposed DSM-5 criteria for autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2012;51:28-40.e3. https://doi.org/10.1016/j.jaac.2011.09.021.
- Matson JL, Belva CB, Horovitz M, Kozlowski AM, Bamburg JW. Comparing symptoms of autism spectrum disorders in a developmentally disabled adult population using the current DSM-IV-TR diagnostic criteria and the proposed DSM-5 diagnostic criteria. J Dev Phys Disabil 2012;24:403-14. https://doi.org/10.1007/s10882-012-9278-0.
- Mattila ML, Kielinen M, Linna SL, Jussila K, Ebeling H, Bloigu R, et al. Autism spectrum disorders according to DSM-IV-TR and comparison with DSM-5 draft criteria: an epidemiological study. J Am Acad Child Adolesc Psychiatry 2011;50:583-92. https://doi.org/10.1016/j.jaac.2011.04.001.
- McPartland JC, Reichow B, Volkmar FR. Sensitivity and specificity of proposed DSM-5 diagnostic criteria for autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2012;51:368-83. https://doi.org/10.1016/j.jaac.2012.01.007.
- Taheri A, Perry A. Exploring the proposed DSM-5 criteria in a clinical sample. J Autism Dev Disord 2012;42:1810-17. https://doi.org/10.1007/s10803-012-1599-4.
- Worley JA, Matson JL. Comparing symptoms of autism spectrum disorders using the current ‘DSM-IV-TR’ diagnostic criteria and the proposed ‘DSM-V’ diagnostic criteria. Res Autism Spectr Disord 2012;6:965-70. https://doi.org/10.1016/j.rasd.2011.12.012.
- Huerta M, Bishop SL, Duncan A, Hus V, Lord C. Application of DSM-5 criteria for autism spectrum disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry 2012;169:1056-64. https://doi.org/10.1176/appi.ajp.2012.12020276.
- Murphy D, Beecham J, Craig M, Ecker C. Autism in adults: new biological findings and their translational implications to the cost of clinical services. Brain Res 2011;1380:22-33. https://doi.org/10.1016/j.brainres.2010.10.042.
- Carpenter P. Diagnosis and assessment in autism spectrum disorders. Adv Ment Health Intellect Disabil 2012;6:121-9. https://doi.org/10.1108/20441281211227184.
- Baird G, Charman T, Baron-Cohen S, Cox A, Swettenham J, Wheelwright S, et al. A screening instrument for autism at 18 months of age: a 6-year follow-up study. J Am Acad Child Adolesc Psychiatry 2000;39:694-702. https://doi.org/10.1097/00004583-200006000-00007.
- Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III). San Antonio, TX: Psychological Corporation; 1997.
- Kapp S, Ne’eman A. ASD in DSM-5: What the Research Shows and Recommendations for Change. Autistic Self Advocacy Network Policy Brief (June). Washington, DC: Autistic Self Advocacy Network; 2012.
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci 2008;31:137-45. https://doi.org/10.1016/j.tins.2007.12.005.
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007;17:103-11. https://doi.org/10.1016/j.conb.2007.01.009.
- Geschwind D. Autism: searching for coherence. Biol Psychiatry 2007;62:949-50. https://doi.org/10.1016/j.biopsych.2007.09.001.
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain 2005;128:268-76. https://doi.org/10.1093/brain/awh332.
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain 2002;125:1594-606. https://doi.org/10.1093/brain/awf150.
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 2004;22:619-25. https://doi.org/10.1016/j.neuroimage.2004.02.029.
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 2006;6. https://doi.org/10.1186/1471-244X-6-56.
- Toal F, Murphy DG, Murphy KC. Autistic-spectrum disorders: lessons from neuroimaging. Br J Psychiatry 2005;187:395-7. https://doi.org/10.1192/bjp.187.5.395.
- Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci 2007;27:11725-35. https://doi.org/10.1523/JNEUROSCI.0777-07.2007.
- Levitt JG, Blanton RE, Smalley S, Thompson PM, Guthrie D, McCracken JT, et al. Cortical sulcal maps in autism. Cereb Cortex 2003;13:728-35. https://doi.org/10.1093/cercor/13.7.728.
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 2009;19:2728-35. https://doi.org/10.1093/cercor/bhp026.
- Mourão-Miranda J, Bokde AL, Born C, Hampel H, Stetter M. Classifying brain states and determining the discriminating activation patterns: Support Vector Machine on functional MRI data. Neuroimage 2005;28:980-95. https://doi.org/10.1016/j.neuroimage.2005.06.070.
- Davatzikos C, Fan Y, Wu X, Shen D, Resnick SM. Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiol Aging 2008;29:514-23. https://doi.org/10.1016/j.neurobiolaging.2006.11.010.
- Kloppel S, Stonnington CM, Chu C, Draganski B, Scahill RI, Rohrer JD, et al. Automatic classification of MR scans in Alzheimer’s disease. Brain 2008;131:681-9. https://doi.org/10.1093/brain/awm319.
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Assessment; 1999.
- The ICD-10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:885-90. https://doi.org/10.1176/ajp.150.6.885.
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179-94. https://doi.org/10.1006/nimg.1998.0395.
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195-207. https://doi.org/10.1006/nimg.1998.0396.
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004;22:1060-75. https://doi.org/10.1016/j.neuroimage.2004.03.032.
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050-5. https://doi.org/10.1073/pnas.200033797.
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341-55. https://doi.org/10.1016/S0896-6273(02)00569-X.
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11-22. https://doi.org/10.1093/cercor/bhg087.
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23:S69-84. https://doi.org/10.1016/j.neuroimage.2004.07.016.
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006;30:436-43. https://doi.org/10.1016/j.neuroimage.2005.09.046.
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001;20:70-8. https://doi.org/10.1109/42.906426.
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 2007;26:518-29. https://doi.org/10.1109/TMI.2006.887364.
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968-80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 2002;58:695-701. https://doi.org/10.1212/WNL.58.5.695.
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 2003;60:878-88. https://doi.org/10.1001/archpsyc.60.9.878.
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004;14:721-30. https://doi.org/10.1093/cercor/bhh032.
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006;32:180-94. https://doi.org/10.1016/j.neuroimage.2006.02.051.
- Piven J, Berthier ML, Starkstein SE, Nehme E, Pearlson G, Folstein S. Magnetic resonance imaging evidence for a defect of cerebral cortical development in autism. Am J Psychiatry 1990;147:734-9. https://doi.org/10.1176/ajp.147.6.734.
- Wisco JJ, Kuperberg G, Manoach D, Quinn BT, Busa E, Fischl B, et al. Abnormal cortical folding patterns within Broca’s area in schizophrenia: evidence from structural MRI. Schizophr Res 2007;94:317-27. https://doi.org/10.1016/j.schres.2007.03.031.
- Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res 2004;131:263-8. https://doi.org/10.1016/j.pscychresns.2004.06.001.
- Schoelkopf M, Smola AJ. Learning with Kernes. Cambridge, MA: MIT Press; 2002.
- Burges CJC. A tutorial on support vector machines for pattern recognition. Data Min Knowl Discov 1998;2:121-67. https://doi.org/10.1023/A:1009715923555.
- Vapnik VN. The Nature of Statistical Learning Theory. New York, NY: Springer; 1995.
- Mourão-Miranda J, Reynaud E, McGlone F, Calvert G, Brammer M. The impact of temporal compression and space selection on SVM analysis of single-subject and multi-subject fMRI data. NeuroImage 2006;33:1055-65. https://doi.org/10.1016/j.neuroimage.2006.08.016.
- Ecker C, Rocha-Rego V, Johnston P, Mourao-Miranda J, Marquand A, Daly EM, et al. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage 2010;49:44-56. https://doi.org/10.1016/j.neuroimage.2009.08.024.
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 2005;128:213-26. https://doi.org/10.1093/brain/awh330.
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nat Neurosci 2001;4:1253-8. https://doi.org/10.1038/nn758.
- Chung MK, Robbins S, Evans AC. Unified statistical approach to cortical thickness analysis. Inf Process Med Imaging 2005;19:627-38. https://doi.org/10.1007/11505730_52.
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. Am J Psychiatry 2006;163:1290-2. https://doi.org/10.1176/ajp.2006.163.7.1290.
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 2006;16:1276-82. https://doi.org/10.1093/cercor/bhj069.
- Howlin P. Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. J Autism Dev Disord 2003;33:3-13. https://doi.org/10.1023/A:1022270118899.
- Pessoa L, Padmala S. Decoding near-threshold perception of fear from distributed single-trial brain activation. Cereb Cortex 2007;17:691-70. https://doi.org/10.1093/cercor/bhk020.
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci 2006;7:358-66. https://doi.org/10.1038/nrn1888.
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci USA 2006;103:3863-8. https://doi.org/10.1073/pnas.0600244103.
- Bradley E, Bolton P. Episodic psychiatric disorders in teenagers with learning disabilities with and without autism. Br J Psychiatry 2006;189:361-6. https://doi.org/10.1192/bjp.bp.105.018127.
- Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2011;21:571-9. https://doi.org/10.1089/cap.2011.0057.
- Broadstock M, Doughty C, Eggleston M. Systematic review of the effectiveness of pharmacological treatments for adolescents and adults with autism spectrum disorder. Autism 2007;11:335-48. https://doi.org/10.1177/1362361307078132.
- Sanchez LE, Campbell M, Small AM, Cueva JE, Armenteros JL, Adams PB. A pilot study of clomipramine in young autistic children. J Am Acad Child Adolesc Psychiatry 1996;35:537-44. https://doi.org/10.1097/00004583-199604000-00021.
- Aman MG, Van Bourgondien ME, Osborne PW, Sarphare G. Side effects associated with psychoactive medication in individuals with autism. J Autism Dev Disord 1997;27:342-4.
- Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30:245-55. https://doi.org/10.1023/A:1005548619694.
- Aman MG, Buican B, Arnold LE. Methylphenidate treatment in children with borderline IQ and mental retardation: analysis of three aggregated studies. J Child Adolesc Psychopharmacol 2003;13:29-40. https://doi.org/10.1089/104454603321666171.
- Aman MG, Lam KS, Van Bourgondien ME. Medication patterns in patients with autism: temporal, regional, and demographic influences. J Child Adolesc Psychopharmacol 2005;15:116-26. https://doi.org/10.1089/cap.2005.15.116.
- Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol 2007;17:348-55. https://doi.org/10.1089/cap.2006.17303.
- Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics 2008;121:e441-8. https://doi.org/10.1542/peds.2007-0984.
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009;39:1339-49. https://doi.org/10.1007/s10803-009-0750-3.
- Williams PG, Woods C, Stevenson M, Davis DW, Radmacher P, Smith M. Psychotropic medication use in children with autism in the Kentucky Medicaid population. Clin Pediatr 2012;51:923-7. https://doi.org/10.1177/0009922812440837.
- Logan SL, Nicholas JS, Carpenter LA, King LB, Garrett-Mayer E, Charles JM. High prescription drug use and associated costs among Medicaid-eligible children with autism spectrum disorders identified by a population-based surveillance network. Ann Epidemiol 2012;22:1-8. https://doi.org/10.1016/j.annepidem.2011.10.007.
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21. https://doi.org/10.1056/NEJMoa013171.
- Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-41. https://doi.org/10.1542/peds.2003-0264-F.
- Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009;124:1533-40. https://doi.org/10.1542/peds.2008-3782.
- Marcus RN, Owen R, Kame L, Manos G, McQuade RD, Carson WH, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry 2009;48:1110-19. https://doi.org/10.1097/CHI.0b013e3181b76658.
- Morgan S, Taylor E. Antipsychotic drugs in children with autism. BMJ 2007;334:1069-70. https://doi.org/10.1136/bmj.39216.583333.80.
- Almandil NB, Wong IC. Review on the current use of antipsychotic drugs in children and adolescents. Arch Dis Child Educ Pract Ed 2011;96:192-6. https://doi.org/10.1136/archdischild-2011-300054.
- Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs 2013;15:139-50. https://doi.org/10.1007/s40272-013-0016-6.
- Fleischhaker C, Heiser P, Hennighausen K, Herpertz-Dahlmann B, Holtkamp K, Mehler-Wex C, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol 2006;16:308-16. https://doi.org/10.1089/cap.2006.16.308.
- Robb AS, Andersson C, Bellocchio EE, Manos G, Rojas-Fernandez C, Mathew S, et al. Safety and tolerability of aripiprazole in the treatment of irritability associated with autistic disorder in pediatric subjects (6–17 years old): results from a pooled analysis of 2 studies. Prim Care Companion CNS Disord 2011;13. https://doi.org/10.4088/PCC.10m01008gry.
- Star K, Iessa N, Almandil NB, Wilton L, Curran S, Edwards IR, et al. Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis. J Child Adolesc Psychopharmacol 2012;22:440-51. https://doi.org/10.1089/cap.2011.0134.
- Rani FA, Byrne PJ, Murray ML, Carter P, Wong IC. Paediatric atypical antipsychotic monitoring safety (PAMS) study: pilot study in children and adolescents in secondary- and tertiary-care settings. Drug Saf 2009;32:325-33. https://doi.org/10.2165/00002018-200932040-00006.
- Rani FA, Byrne P, Cranswick N, Murray ML, Wong IC. Mortality in children and adolescents prescribed antipsychotic medication: a retrospective cohort study using the UK general practice research database. Drug Saf 2011;34:773-81. https://doi.org/10.2165/11591120-000000000-00000.
- Barrett B, Byford S, Sharac J, Hudry K, Leadbitter K, Temple K, et al. Service and wider societal costs of very young children with autism in the UK. J Autism Dev Disord 2012;42:797-804. https://doi.org/10.1007/s10803-011-1306-x.
- THIN Data from EPIC: A Guide for Researchers. Chertsey: Cegedim Strategic Data Medical Research; 2009.
- Chisholm J. The Read clinical classification. BMJ 1990;300. https://doi.org/10.1136/bmj.300.6732.1092.
- McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC Pediatr 2012;12:78-89. https://doi.org/10.1186/1471-2431-12-78.
- McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. Persistence of pharmacological treatment into adulthood, in UK primary care, for ADHD patients who started treatment in childhood or adolescence. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-219.
- Wijlaars LP, Nazareth I, Petersen I. Trends in depression and antidepressant prescribing in children and adolescents: a cohort study in The Health Improvement Network (THIN). PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0033181.
- Fombonne E, Heavey L, Smeeth L, Rodrigues LC, Cook C, Smith PG, et al. Validation of the diagnosis of autism in general practitioner records. BMC Public Health 2004;4. https://doi.org/10.1186/1471-2458-4-5.
- Cai B, Xu W, Bortnichak E, Watson D. An algorithm to identify medical practices common to both the General Practice Research Database and The Health Improvement Network database. Pharmacoepidemiol Drug Saf 2012;21:770-4. https://doi.org/10.1002/pds.3277.
- Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 2009;18:76-83. https://doi.org/10.1002/pds.1688.
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry 2009;48:474-83. https://doi.org/10.1097/CHI.0b013e31819b3848.
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 2006;368:210-15. https://doi.org/10.1016/S0140-6736(06)69041-7.
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry 2009;194:500-9. https://doi.org/10.1192/bjp.bp.108.059345.
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev 2009;29:216-29. https://doi.org/10.1016/j.cpr.2009.01.003.
- Simonoff E, Pickles A, Wood N, Gringras P, Chadwick O. ADHD symptoms in children with mild intellectual disability. J Am Acad Child Adolesc Psychiatry 2007;46:591-600. https://doi.org/10.1097/chi.0b013e3180323330.
- Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep 2009;32:1566-78. https://doi.org/10.1093/sleep/32.12.1566.
- Rani F, Murray ML, Byrne PJ, Wong IC. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics 2008;121:1002-9. https://doi.org/10.1542/peds.2007-2008 (accessed 31 May 2018).
- Methylphenidate for the Treatment of Attention Deficit Hyperactivity Disorder in Children and Adolescents 2000. London: NICE; 2000.
- Emerson E. Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. J Intell Disabil Res 2003;47:51-8. https://doi.org/10.1046/j.1365-2788.2003.00464.x.
- Simonoff E, Taylor E, Baird G, Bernard S. Commentary: RCT of optimal dose methylphenidate in children and adolescents with severe ADHD and ID – a reply to Arnold (2013). J Child Psychol Psychiatry 2013;54:703-4. https://doi.org/10.1111/jcpp.12098.
- Royal college of Psychiatrists . National Collaborating Centre for Mental Health n.d. www.rcpsych.ac.uk/workinpsychiatry/nccmh.aspx (accessed 31 May 2018).
- Koesters M, Becker T, Kilian R, Fegert JM, Weinmann S. Limits of meta-analysis: methylphenidate in the treatment of adult attention-deficit hyperactivity disorder. J Psychopharmacol 2009;23:733-44. https://doi.org/10.1177/0269881108092338.
- Rösler M, Retz W, Retz-Junginger P, Stieglitz RD, Kessler H, Reimherr F, et al. Attention deficit hyperactivity disorder in adults. Benchmarking diagnosis using the Wender-Reimherr adult rating scale. Nervenarzt 2008;79:320-7. https://doi.org/10.1007/s00115-007-2375-0.
- Oliver MN, Simons J. The affective lability scales: development of a short-form measure. Pers Individ Dif 2004;37:1279-88. https://doi.org/10.1016/j.paid.2003.12.013.
- Bakken TL, Helverschou SB, Eilertsen DE, Heggelund T, Myrbakk E, Martinsen H. Psychiatric disorders in adolescents and adults with autism and intellectual disability: a representative study in one county in Norway. Res Dev Disabil 2010;31:1669-77. https://doi.org/10.1016/j.ridd.2010.04.009.
- Szatmari P, Bartolucci G, Bremner R. Asperger’s syndrome and autism: comparison of early history and outcome. Dev Med Child Neurol 1989;31:709-20. https://doi.org/10.1111/j.1469-8749.1989.tb04066.x.
- Ollendick TH, King NJ, Muris P. Fears and phobias in children: phenomenology, epidemiology and aetiology. Child Adolesc Ment Health 2002;7:98-106. https://doi.org/10.1111/1475-3588.00019.
- Sofronoff K, Attwood T, Hinton S. A randomised controlled trial of a CBT intervention for anxiety in children with Asperger syndrome. J Child Psychol Psychiatry 2005;46:1152-60. https://doi.org/10.1111/j.1469-7610.2005.00411.x.
- McLoone J, Hudson JL, Rapee R. Treating anxiety disorders in a school setting. Educ Treat Children 2006;29:219-42.
- Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, Taga K. The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: comparisons with developmentally and chronologically age matched children. Child Psychol Human Devel 2005;36:3-26. https://doi.org/10.1007/s10578-004-3619-x.
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J Autism Dev Disord 2005;35:145-58. https://doi.org/10.1007/s10803-004-1992-8.
- McDougle CJ, Kresch LE, Goodman WK, Naylor ST, Volkmar FR, Cohen DJ, et al. A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive–compulsive disorder. Am J Psychiatry 1995;152:772-7. https://doi.org/10.1176/ajp.152.5.772.
- Russell AJ, Mataix-Cols D, Anson M, Murphy DG. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry 2005;186:525-8. https://doi.org/10.1192/bjp.186.6.525.
- Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ 2004;82:858-66.
- van Oppen P, de Haan E, van Balkom AJ, Spinhoven P, Hoogduin K, van Dyck R. Cognitive therapy and exposure in vivo in the treatment of obsessive compulsive disorder. Behav Res Ther 1995;33:379-90. https://doi.org/10.1016/0005-7967(94)00052-L.
- Chalfant AM, Rapee R, Carroll L. Treating anxiety disorders in children with high functioning autism spectrum disorders: a controlled trial. J Autism Dev Disord 2007;37:1842-57. https://doi.org/10.1007/s10803-006-0318-4.
- Wood JJ, Drahota A, Sze K, Har K, Chiu A, Langer DA. Cognitive behavioral therapy for anxiety in children with autism spectrum disorders: a randomized, controlled trial. J Child Psychol Psychiatry 2009;50:224-34. https://doi.org/10.1111/j.1469-7610.2008.01948.x.
- Reaven J, Blakeley-Smith, A, Culhane-Shelburne, K, Hepburn S. Group cognitive behavior therapy for children with high-functioning autism spectrum disorders and anxiety: a randomized trial. J Child Psychol Psychiatry 2012;53:410-19. https://doi.org/10.1111/j.1469-7610.2011.02486.x.
- Hare DJ. The use of cognitive-behaviour therapy with people with Asperger syndrome: a case study. Autism 1997;1:215-25. https://doi.org/10.1177/1362361397012007.
- Cardaciotto LA, Herbert JD. Cognitive behaviour therapy for social anxiety disorder in the context of Asperger’s syndrome: a single subject report. Cog Behav Pract 2004;11:75-81. https://doi.org/10.1016/S1077-7229(04)80009-9.
- Russell AJ, Mataix-Cols D, Anson MA, Murphy DG. Psychological treatment for obsessive compulsive disorder in people with autism spectrum disorders – a pilot study. Psychother Psychosom 2009;78:59-61. https://doi.org/10.1159/000172622.
- Whittal ML, Thordarson DS, McLean PD. Treatment of obsessive–compulsive disorder: cognitive behavior therapy vs. exposure and response prevention. Behav Res Ther 2005;43:1559-76. https://doi.org/10.1016/j.brat.2004.11.012.
- The Clomipramine Collaborative Study Group . Clomipramine in the treatment of patients with Obsessive Compulsive Disorder. Arch Gen Psychiatry 1991;48:730-8. https://doi.org/10.1001/archpsyc.1991.01810320054008.
- Sheehan DV, LeCrubier Y. MINI International Neuropsychiatric Interview English Version 5.0. USA: Harm Research Institute; 2000.
- Attwood T. Modifications to Cognitive Behaviour Therapy to Accommodate the Cognitive Profile of People With Asperger’s Syndrome 1999. www.tonyattwood.com/paper2.htm.
- Anderson S, Morris J. Cognitive behaviour therapy for people with Asperger syndrome. Behav Cogn Psychother 2006;34:293-30. https://doi.org/10.1017/S1352465805002651.
- Cautela JR, Groden J. Relaxation: A Comprehensive Manual for Adults, Children, and Children With Special Needs. Champaign, IL: Research Press; 1978.
- Schneider AJ, Mataix-Cols D, Marks IM, Bachofen M. Internet-guided self-help with or without exposure therapy for phobic and panic disorders. Psychother Psychosom 2005;74:154-64. https://doi.org/10.1159/000084000.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46:1006-11. https://doi.org/10.1001/archpsyc.1989.01810110048007.
- Simpson HB, Huppert JD, Petkova E, Foa EB, Liebowitz MR. Response versus remission in obsessive–compulsive disorder. J Clin Psychiatry 2006;67:269-76. https://doi.org/10.4088/JCP.v67n0214.
- Guy W. Clinical Global Impression ECDEU Assessment Manual for Psychopharmacology-Revised. Rockville, MD: National Institute of Mental Health; 1976.
- Rosario-Camposm MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, et al. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry 2006;11:495-504. https://doi.org/10.1038/sj.mp.4001798.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. San Antonio, TX: The Psychological Corporation; 1996.
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893-7. https://doi.org/10.1037/0022-006X.56.6.893.
- Liebowitz MR, Gorman JM, Fyer AJ, Klein DF. Social phobia. Review of a neglected anxiety disorder. Arch Gen Psychiatry 1985;42:729-36. https://doi.org/10.1001/archpsyc.1985.01790300097013.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. https://doi.org/10.1192/bjp.180.5.461.
- Beck JS, Beck AT, Jolly JB. Beck Youth Inventories. San Antonio, TX: Psychological Corporation; 2001.
- Spence SH. A measure of anxiety symptoms among children. Behav Res Ther 1998;36:545-66. https://doi.org/10.1016/S0005-7967(98)00034-5.
- Shafran R, Frampton I, Heyman I, Reynolds M, Teachman B, Rachman S. The preliminary development of a new self-report measure for OCD in young people. J Adolesc 2003;26:137-42. https://doi.org/10.1016/S0140-1971(02)00083-0.
- Storch EA, Merlo LJ, Geffken GR. The Family Accommodation Scale – Parent Report. Gainesville, FL: University of Florida; 2005.
- Merlo LJ, Lehmkuhl HD, Geffken GR, Storch EA. Decreased family accommodation associated with improved therapy outcome in pediatric obsessive–compulsive disorder. J Consult Clin Psychol 2009;77:355-60. https://doi.org/10.1037/a0012652.
- Rosa-Alcázar AI, Sánchez-Meca J, Gómez-Conesa A, Marín-Martínez F. Psychological treatment of obsessive–compulsive disorder: a meta-analysis. Clin Psychol Rev 2008;28:1310-25. https://doi.org/10.1016/j.cpr.2008.07.001.
- Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive–compulsive disorder. J Child Psychol Psychiatry 2008;49:489-98. https://doi.org/10.1111/j.1469-7610.2007.01875.x.
- Whittal ML, Woody SR, McLean PD, Rachman SJ, Robichaud M. Treatment of obsessions: a randomized controlled trial. Behav Res Ther 2010;48:295-303. https://doi.org/10.1016/j.brat.2009.11.010.
- Sung M, Ooi YP, Goh TJ, Pathy P, Fung DS, Ang RP, et al. Effects of cognitive-behavioral therapy on anxiety in children with autism spectrum disorders: a randomized controlled trial. Child Psychiatry Hum Dev 2011;42:634-49. https://doi.org/10.1007/s10578-011-0238-1.
- Hill E, Berthoz S, Frith U. Brief report: cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J Autism Dev Disord 2004;34:229-35. https://doi.org/10.1023/B:JADD.0000022613.41399.14.
- White SW, Schry AR, Maddox BB. Brief report: the assessment of anxiety in high-functioning adolescents with autism spectrum disorder. J Autism Dev Disord 2012;42:1138-45. https://doi.org/10.1007/s10803-011-1353-3.
- Pertusa A, Frost RO, Fullana MA, Samuels J, Steketee G, Tolin D, et al. Refining the diagnostic boundaries of compulsive hoarding: a critical review. Clin Psychol Rev 2010;30:371-86. https://doi.org/10.1016/j.cpr.2010.01.007.
- Mataix-Cols D, Frost RO, Pertusa A, Clark LA, Saxena S, Leckman JF, et al. Hoarding disorder: a new diagnosis for DSM-V?. Depress Anxiety 2010;27:556-72. https://doi.org/10.1002/da.20693.
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioural therapies research: getting started and moving on from stage I. Clin Psychol: Science Pract 2001;8:133-42. https://doi.org/10.1093/clipsy.8.2.133.
- Francis R. Independent Inquiry into Care Provided by Mid Staffordshire NHS Foundation Trust: January 2005–March 2009: Hearing before the Chaired by Robert Francis QC. London: HMSO; 2010.
- Wong BM, Levinson W, Shojania KG. Quality improvement in medical education: current state and future directions. Med Educ 2012;46:107-19. https://doi.org/10.1111/j.1365-2923.2011.04154.x.
- Kilminster SM, Jolly BC. Effective supervision in clinical practice settings: a literature review. Med Educ 2000;34:827-40. https://doi.org/10.1046/j.1365-2923.2000.00758.x.
- Fulfilling and Rewarding Lives: The Strategy for Adults with Autism in England. London: HM Government; 2010.
- Pukyurek M, Schweitzer J, Yellowless P. Telepsychiatry and ADHD. New York, NY: The Guilford Press; 2013.
- Bonell S, McInerny T, O’Hara J. Neurodevelopmental Psychiatry: An Introduction for Medical Students. London: South London and Maudsley NHS Foundation Trust/King’s Health Partners; 2011.
- Chaplin E, Hardy S, Underwood L. Autism Spectrum Conditions: A Guide. Brighton: Pavilion; 2013.
- Chaplin E, Henry J, Hardy S. Working with People with Learning Disabilities and Offending Behaviour. Brighton: Pavilion; 2009.
- Jha A. Autism Can Be Diagnosed With Brain Scan – Study n.d. www.guardian.co.uk/science/2010/aug/10/autism-brain-scan (accessed 31 May 2018).
- Hope J. 15-Minute Brain Scan Developed by British Scientists Could Spot Child Autism Earlier n.d. www.dailymail.co.uk/health/article-1302044/15-minute-brain-scan-spot-child-autism-earlier.html (accessed 31 May 2018).
- BBC Radio 4 Appeal . Autistica n.d. www.bbc.co.uk/programmes/b036tnb2 (accessed 31 May 2018).
- Hughes J. New Brain Scan to Diagnose Autism n.d. www.bbc.co.uk/news/health-10929032 (accessed 31 May 2018).
- Autism Speaks . Top Ten Autism Research Findings of 2010 n.d. www.autismspeaks.org/about-us/press-releases/top-ten-autism-research-findings-2010 (accessed 31 May 2018).
Appendix 1 Description of the treatments
Cognitive–behavioural therapy treatment
The duration of each session ranged from 41 to 74 minutes (mean session length = 60 minutes, SD 7.5 minutes). Homework was set at every session, which included reading materials from the session, completing OCD diaries and exposure tasks. The compliance rate for homework was 90%, although this included even partial completion of the homework tasks. On average 10 (SD 5.4) ERP tasks were set as homework throughout treatment and the compliance rate for these was 79%. The mean compliance rate for other homework, such as reading materials and keeping records, was 89%. In terms of session content and how the treatment was generally structured, a mean of 2.7 (range 1–6, SD 1.6) sessions comprised anxiety education; OCD education was included in 3–13 sessions (mean 5.8, SD 2.9) sessions; ERP was covered in up to 16 (mean 8.6, SD 5.3) sessions and a mean of 2.7 (SD 3.2) sessions included cognitive intervention techniques. Relapse prevention took between 0 and 2 (mean 1, SD 0.7) sessions.
In terms of the frequency of using ASD-specific modifications, work with one participant incorporated the use of their special interest to help convey concepts; therapists were noted on the record forms as needing to be ‘more directive’ in sessions for 13 (68%) participants; six (32%) participants needed ‘rules’ to engage with the structure of the sessions (e.g. how much time to spend talking on non-OCD topics); nine (47%) participants’ sessions record forms had reference to concrete examples being needed; and use of visual material to convey concepts was incorporated in 11 (58%) participants’ sessions. Nine (47%) participants in the CBT group had direct involvement of parents/carers in sessions.
Anxiety management treatment
The duration of the sessions ranged from 28 to 71 (mean 57, SD 8.3) minutes. Homework was set at every session, which included reading materials, practising techniques learnt in the sessions and keeping records. The compliance rate for homework was 95%, which included even partial completion of the homework tasks.
With regard to content of the treatment, between one and eight sessions were focused on learning and practising breathing exercises (mean 3.9, SD 1.8) and between 1 and 13 sessions included relaxation practice (mean 5.5, SD 3.1). Sessions on mood ranged from 0 to 3 (mean 1.4, SD 0.82), 0–10 sessions were on healthy habits (mean 3.9, SD 2.7) and 0–4 sessions were spent on problem-solving (mean 1.9, SD 1.2).
In terms of modifying the AM treatment to make it suitable for people with ASD, there were no records suggesting any participants had their special interests incorporated into the session. Therapists were noted as being ‘more directive’ in sessions for five (25%) participants, one (5%) participant needed rules for the sessions, six (30%) participants needed reference to concrete examples, nine (45%) participants needed visual aids and six (30%) participants’ parents/carers were directly involved in sessions.
Appendix 2 Cases presented during web-based clinical supervision
Summary of case discussed during web-based clinical supervision on ADHD
Session 1 cases presented
Case 1
Mr A is a 23-year-old man with a history of contact with mental health services, including a history of depression. His score on the Adult ADHD Self-Report Scale (version 1.1) suggested ADHD. This was the first assessment the participant planned to undertake regarding ADHD and advice was requested about how to identify ADHD as being distinct from symptoms, which may be associated with other mental illness.
Case 2
Mr B is a 28-year-old man with inattention, obsessionality, anger and regular cannabis use. He has a history of low self-esteem and depression since his early teens. He was diagnosed with ADHD as a child and treated with methylphenidate until age 15 years. He has a family history of ADHD. His score on the Wender–Reimherr Adult ADHD Scale self-report questionnaire was suggestive of adult ADHD. He was unable to tolerate Concerta XL® and treatment was changed to atomoxetine, which he was tolerating well. The question was whether or not the group agreed with the current management of this case.
Case 3
Ms A is a 18-year-old woman with borderline ID and a background of childhood adversity, including neglect presenting with impulsivity, sexually inappropriate behaviour, social difficulties and inattention. Screening on Brown Attention-Deficit Disorder Scales were highly suggestive of ADHD and eight of nine symptoms of inattention according to DSM-IV criteria. The question presented for supervision was the overlap between global LD and specific inattention secondary to ADHD, and whether or not it is appropriate to consider treatment for this patient.
Session 2 cases presented
Case 1
Ms B, a 34-year-old woman, presented with a diagnosis of bipolar affective disorder with a suggestion of borderline personality traits presenting with recurrent self-harm and with a background of childhood abuse. Use of antidepressant medication precipitated elevated mood enhancing impulsivity and substance misuse. Previous treatment to manage mood symptoms was of limited value. Assessment for adult ADHD met the criteria for inattentive subtype. The challenge was to separate whether this was just ADHD, or comorbid bipolar affective disorder and personality aspects, and how to manage case.
Case 2
Mr C is a 22-year-old man with predominantly attentive problems from childhood, which appeared to be masked by high intelligence. The case was complicated by an extensive psychiatric history with a diagnosis of PD, the possibility of Asperger syndrome and possible paranoid PD. The question was whether or not antipsychotics had a place in ADHD treatment.
Case 3
Ms C is a 32-year-old woman, living with her son, diagnosed with ADHD on the basis of her clinic presentation and her high score on the Wender Utah Rating Scale. She rejected the diagnosis and asked to be treated for anxiety and depression. She was struggling to cope day to day, particularly with marked emotional dysregulation. There was a personal history and strong family history of alcohol dependence and substance misuse, marked anxiety at presentation. The participants wanted to discuss the treatment of ADHD in the presence of marked anxiety.
Case 4
Case 4 presented the scenario of an assessment for an adult with suspected ADHD and asked what is the role of neuropsychological testing.
Case 5
Case 5 presented the scenario of a young man with marked sleep problems in the context of ADHD and asked what sleep management treatment options are available.
Session 3 cases presented
Case 1
Case 1 presented the scenario of an assessment and follow-up for an adult with suspected ADHD and asked what is the role of rating scales.
Case 2
Mr D is a 31-year-old man seeking assessment for potential adult ADHD. On DIVA diagnostic assessment he met criteria for ADHD combined subtype. Presentation complicated by comorbid Huntington’s disease. He commenced a trial of treatment with non-stimulant ADHD medication, atomoxetine, due to concerns about the effect of stimulants in comorbid Huntington’s disease and he appears to be improving. The question was whether or not the group agreed with the current clinical management of this case.
Case 3
Case 3 presented the scenario of treating an adult with ADHD and asked what are the types of social interventions (including skills training) for which there is an evidence base for to use in adult ADHD.
Case 4
Case 4 presented the scenario of treating an adult with ADHD and asked can patients when diagnosed with ADHD take medication on a pro re nata (as needed) basis.
Case 5
Ms D is a 40-year-old woman, referred for assessment by her employer’s occupational health team after presenting with severe anxiety, obsessionality and depression over the past 6 months. Following continuing psychological therapy these symptoms improved. As she got better, other aspects of her presentation emerged suggesting a diagnosis of ADHD. She was prescribed stimulant treatment, which she was unable to tolerate due to side effects, and refused further treatment for ADHD. In the light of ongoing anxiety and depression, venlafaxine was prescribed and a marked improvement in ADHD symptoms noted. The participant was interested to know if others have had similar experience using the serotonin–noradrenaline reuptake inhibitors.
Summary of case discussed during web-based clinical supervision on ASD
Session 1
Case 1
Mr A is a 35-year-old white British man referred by his probation officer for a psychological risk assessment. He has pleaded guilty to one count of causing or inciting a minor to engage in sexual activity and 10 counts of possessing and distributing indecent images. To date, Mr A had been interviewed on one occasion with the plan to offer him one further appointment.
Questions
Can Mr A’s difficulties be understood within the context of Asperger syndrome? Attachment disorder? If Asperger syndrome is a possibility, how is it best to assess/measure this when time is limited?
If Asperger syndrome is contributing to Mr A’s difficulties, how might this guide sentencing (e.g. custody, response to treatment)?
In this case can excessive viewing of online indecent images be an example of ‘preoccupation with one or more stereotyped and restricted patterns of interest that is abnormal either in intensity or focus’?
Case 2
Mr P is a 41-year-old white British man who has had no involvement with services in childhood and adolescence. He initially came to the attention of services when he was referred to a Community Mental Health Team in 2003 with depression. His history is characterised by a number of brief contacts with services and, although there were concerns from a number of agencies over his lifestyle and maladaptive coping, he often failed to meet the eligibility criteria to mental health services. This was in spite of major concerns over his vulnerability, which included living in his grandmother’s shed. Other concerns noted included his lack of willingness to engagement while not thriving in his current environment has led to concerns over his physical health. Mr P also has a number of rituals interfering with activities of daily living.
Questions
Where next? We have been trying to support Mr P in the community and allow him to develop. However, he does not appear to be doing well. His physical health remains poor and he only really engages with services as they bring his money.
What other input might be useful to this man?
What other approaches may work to engage Mr P? We have found no way of engaging him which works consistently.
Session 2
Case 3
Ms X is a 25-year-old woman with a long-standing diagnosis of emotionally unstable PD who was transferred from prison to a medium-secure unit on Sections 47 and 49 of the Mental Health Act 2007. 135 This followed a conviction for simple arson. The unit to which she was admitted was populated by women with PD diagnoses and the staff had considerable experience in working with this group. Ms X was noted to be significantly different from her peers and did not respond to the unit’s treatment programme. Her responsible clinician was of the opinion that she could have a comorbid diagnosis of ASD and asked for a second opinion.
Questions
My question is about the inter-relationship between trauma and ASD – I would like people to share their experience in formulating and managing these individuals as my service is receiving an increasing number of women with features of ASD, emotionally unstable PD and a history of trauma.
Case 4
A 41-year-old white British man referred by his local community team for LDs to an inpatient service for assessment and treatment under Section 37 of the Mental Health Act 2007. 135 He pleaded guilty to the indecent assault of the 2-year-old daughter of a neighbour, at a barbecue.
Questions
How can Mr P’s difficulties best be understood? In particular, his ‘healing’: circumscribed interest or means to gain access to vulnerable women?
What assessments should we be carrying out, given Mr P’s limited intellectual ability?
Session 3
Case 5
Mr G is a 20-year-old man with a diagnosis of ASD (diagnosis in childhood prior to 2006), mild/moderate ID (various views on diagnosis from different assessors).
Questions
Given his family history – genetic testing (fragile X testing was carried out and was negative) – is there a strong argument for array comparative genomic hybridisation? What is the current understanding regarding different genetic associations with ASD?
What are the panel’s thoughts on most effective psychological and pharmacological approaches to agitation and anxiety?
Given the suspicion of father having ASD, how would they approach the difficulties that this presents? Should he be made aware of it?
Given Mr G’s dislike and suspicion of authority, how best to motivate him to engage with the service and occupational/leisure recommendations?
Given the overlapping nature of psychology and speech and language therapy interventions, how can the delivery of these be best organised?
Case study 6
Mr Z is a 24-year-old current inpatient who has moderate ID, ASD and bipolar affective disorder. He was born by caesarean section for fetal distress. He has limited vocabulary and is echolalic, using the Treatment and Education of Autistic and related Communication Handicapped Children (TEACCH) system to communicate through single words. He was raised in London and went to a special school and respite care in a residential placement. At both his school and respite centre, he displayed sexualised behaviour and used the toilet inappropriately, often refusing to go or relieving himself in inappropriately places.
Questions
Are there any other diagnoses that can better explain Mr Z’s history and presentation?
If admitted to your unit, what investigations (if any) and management strategies would you employ?
List of abbreviations
- ADHD
- attention deficit hyperactivity disorder
- ADI-R
- Autism Diagnostic Interview – Revised
- ADOS
- Autism Diagnostic Observation Schedule
- ADOS-G
- Autism Diagnostic Observation Schedule – Generic
- A level
- Advanced level
- AM
- anxiety management
- ANOVA
- analysis of variance
- APMS
- Adult Psychiatric Morbidity Study
- AQ
- Autism Quotient
- ASD
- autism spectrum disorder
- AUDIT
- Alcohol Use Disorders Identification Test
- AUDIT-C
- Alcohol Use Disorders Identification Test Consumption
- BAARS-IV
- Barkley Adult ADHD Rating Scale – IV
- BBC
- British Broadcasting Corporation
- CAADID
- Conners’ Adult ADHD Diagnostic Interview for DSM-IV
- CAMHS
- Child and Adolescent Mental Health Services
- CANDID
- Camberwell Assessment of Need for Adults with Developmental and Intellectual Disabilities
- CAST
- Childhood Autism Spectrum Test
- CBT
- cognitive–behavioural therapy
- CD
- conduct disorder
- CGI
- Clinical Global Impression
- CGI-I
- Clinical Global Impression Improvement
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised
- CNS-LS
- Centre for Neurologic Study – Liability Scale
- CSRI
- Client Service Receipt Inventory
- DAWBA
- Development and Well-Being Assessment
- df
- degrees of freedom
- DIVA
- Diagnostic Interview for ADHD in Adults
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- DSM-IV-TR
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision
- DSM-V
- Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- D-YBOCS
- Dimensional Yale–Brown Obsessive–Compulsive Scale
- ERP
- exposure and response prevention
- FAS
- Family Accommodation Scale
- GAD
- Generalised Anxiety Disorder
- GP
- general practitioner
- GPRD
- General Practice Research Database
- HADS
- Hospital Anxiety and Depression Scale
- HFA
- high-functioning autism
- HMP
- Her Majesty’s Prison
- ICD
- International Classification of Diseases
- ICD-10
- International Classification of Diseases, Tenth Edition
- ID
- intellectual disability
- IMAGE
- International Multi-Centre ADHD Genetics
- IoP
- Institute of Psychiatry
- IQ
- intelligence quotient
- IT
- information technology
- LD
- learning disability
- MCQ
- multiple-choice question
- MRI
- magnetic resonance imaging
- MTMM
- multitrait–multimethod analysis
- MYP
- mid-year population
- ND
- neurodevelopmental disorder
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- OCD
- obsessive–compulsive disorder
- OCI-R
- Obsessive–Compulsive Inventory – Revised
- ONS
- Office for National Statistics
- OR
- odds ratio
- PACS
- Parent Account of Childhood Symptoms
- PD
- personality disorder
- PDD
- pervasive developmental disorder
- PhD
- Doctor of Philosophy
- PPV
- positive predictive value
- RA
- research assistant
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- ROC
- receiver operating characteristic
- ROI
- regions of interest
- SCD
- social communication disorder
- SCQ
- Social Communication Questionnaire
- SD
- standard deviation
- SDQ
- Strengths and Difficulties Questionnaire
- SMI
- serious mental illness
- SUD
- substance use disorder
- SVM
- support vector machine
- T1
- screening assessment session 1
- T2
- screening assessment session 2
- TAU
- treatment as usual
- TEACCH
- Treatment and Education of Autistic and related Communication handicapped Children
- TEDS
- Twins Early Development Study
- THIN
- The Health Improvement Network
- VP
- vulnerable prisoner
- WFIRS
- Weiss Functional Impairment Rating Scale
- WSAS
- Work and Social Adjustment Scale
- YBOCS
- Yale–Brown Obsessive Compulsive Scale