Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0609-10156. The contractual start date was in March 2011. The final report began editorial review in September 2017 and was accepted for publication in August 2018. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Thomas Craig reports personal fees from Otsuka Pharmaceuticals Europe Ltd, Wexham, UK. Richard Holt reports personal fees from Eli Lilly and Company (IN, USA), Jannssen Pharmaceutica (Beerse, Belgium), Sunovion, Pharmaceuticals Inc. (MA, USA) and Otsuka Pharmaceuticals Europe Ltd and is a member of the Health Technology Assessment (HTA) Women and Children’s Health Panel. Thomas Barnes reports personal fees from Sunovion Pharmaceuticals Inc., Newron Pharmaceuticals SpA (Bresso, Italy), and Otsuka Pharmaceutical Co., Ltd (Tokyo, Japan) and Lundbeck, Copenhagen, Denmark Kate Walters was a member of the Disease Prevention Panel and the Primary Care Commissioning Panel. Susan Michie was a member of the HTA Pandemic Influenza Board. Michael King was a member of the National Institute for Health Research (NIHR)-funded Clinical Trials Units (CTUs), Rapid Trials and Add-on Studies Board. Irwin Nazareth’s membership includes CTUs funded by NIHR, the Disease Prevention Panel, HTA Commissioning Board, HTA Commissioning Sub-board (Expression of Interest) and the HTA Primary Care Themed Call. Rumana Omar was a member of the HTA General Board. Steve Morris sat as a member on the Health Services and Delivery Research (HSDR) board, HSDR Commissioning Board, HSDR Evidence Synthesis Sub-board, HTA Commissioning Board and Public Health Research Research Funding Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Osborn et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Synopsis
Background
Burden of cardiovascular disease in people with severe mental illnesses
People with severe mental illnesses (SMI), including schizophrenia, bipolar disorder and other psychoses, make up around 2% of the UK population. 1
Cardiovascular disease (CVD) is the most important physical problem in people with SMI,2 and those aged < 50 years are three times more likely to die from CVD than those without SMI, whereas those aged 50–75 years have a twofold risk. 3 People with SMI die from CVD up to 20 years earlier than the general population3–6 and recent studies have shown that the mortality gap for people with schizophrenia and bipolar disorder is widening. 7,8
The reasons for increased CVD are multifaceted, including increased levels of smoking, diabetes mellitus, obesity and dyslipidaemia in people with SMI compared with general practice controls. 9,10 Systematic reviews conclude that components of metabolic syndrome (being overweight, abnormal lipids, hypertension and abnormal glucose) are more common in people with SMI. 11 Research has also shown that people with SMI ate a higher-fat diet, ate less fibre and were less likely to participate in physical activity. 12,13 Antipsychotic medications, such as olanzapine and clozapine, have been linked to increased appetite and subsequent weight gain as well as abnormalities of lipid and glucose metabolism. 14 Another theory is that long-term stress of SMI exerts cardiovascular risk via the hypothalamic pituitary axis. 15
Current NHS provision of cardiovascular disease care in severe mental illness
The majority of people with SMI use primary care services and see a general practitioner (GP) more often than people without SMI. 6,16 Routine annual CVD risk screening for people with SMI is recommended in national guidelines17,18 and the responsibility for CVD risk prevention is placed within primary care, while those prescribed antipsychotics should be monitored more regularly. 19
The primary care quality outcomes framework pays GPs for providing an annual physical health review to people with SMI. Indicators for this review have changed over the past few years and glycated haemoglobin (HbA1c) and cholesterol measurements were retired in 2014/15. The 2017/18 indicators consist of a recording of blood pressure, smoking status and alcohol consumption. 20
Studies have shown that if CVD screening is offered to people with SMI, then they are as likely to attend the screening as people without SMI. 21,22 However, in primary care, people with schizophrenia were significantly less likely to receive blood pressure or cholesterol screening than practice controls,23 and BMI and blood pressure recording rates were significantly lower in people with SMI than for those with diabetes mellitus or chronic kidney disease. 24 This study also found that exception reporting rates were higher for people with SMI.
Cardiovascular disease risk prediction tools for people with severe mental illnesses
Cardiovascular risk tools are widely used clinically to predict an individual’s risk of developing CVD, usually over a 5- or 10-year period. The risk scores are algorithms of conventional risk factors, such as smoking, blood pressure and lipids, and the predictive ability of the combined models is greater than that of each single risk factor. The resulting risk scores are also used to determine thresholds at which different risk reduction strategies, such as statins, should be employed.
It is noted in the 2016 National Institute for Health and Care Excellence (NICE) guidelines for CVD disease, risk and reduction that people with SMI constitute a high-risk group for CVD and existing risk prediction tools may underestimate their CVD risk, a picture similar to that observed in South Asian people and people aged < 40 years. 25 Ethnicity-adjusted risk scores have been developed as a result,26 but this has not yet been done for SMI. People with SMI were excluded from the original cohorts, such as the Framingham cohort, from which existing risk scores have been derived. The 10-year risk needs to be accurately determined for CVD scores for people with SMI in order to decide the thresholds at which to intervene.
Evidence for treatments to reduce cardiovascular risk in severe mental illness
Although the evidence shows that there are higher rates of CVD risk factors and higher mortality in people with SMI, we know far less about interventions to decrease this risk. There is a lack of high-quality evidence on CVD risk-reduction strategies in people with SMI.
Statins have been found to reduce severe dyslipidaemia in people with SMI in small studies focused on particularly high-risk populations. 27,28 The best trials of smoking cessation show small changes in smoking and quit rates,29 and studies have shown that medication and behavioural interventions are effective for weight loss. 30,31 A feasibility trial in secondary care to improve screening for CVD risk factors, testing a nurse-led service working across primary and secondary care, improved screening rates, but was too short in duration to reduce CVD risk. 32 Systematic reviews of interventions to increase uptake of lifestyle behaviours found some beneficial impact on CVD risk factors; however, the methodological quality of many of the included studies was low. 33–35
Most studies target single-risk factors and do not seek to address the full CVD risk profile of patients with SMI. Only one trial was found that tested a life goals collaborative care intervention involving management strategies for mental health symptoms and CVD risk factors. The findings were that the intervention improved the primary outcome of quality of life (physical health) compared with usual care. CVD risk factors were measured only as secondary outcomes with significantly lower levels of low-density lipoprotein (LDL) cholesterol in the intervention arm than usual treatment at follow-up, but no significant differences in blood pressure, BMI, waist circumference or other lipid parameters. 36
The Primrose programme overview
The aim of this National Institute for Health Research (NIHR) programme was to develop and test better methods to predict and reduce the risk of excess CVD in people with SMI across three work packages.
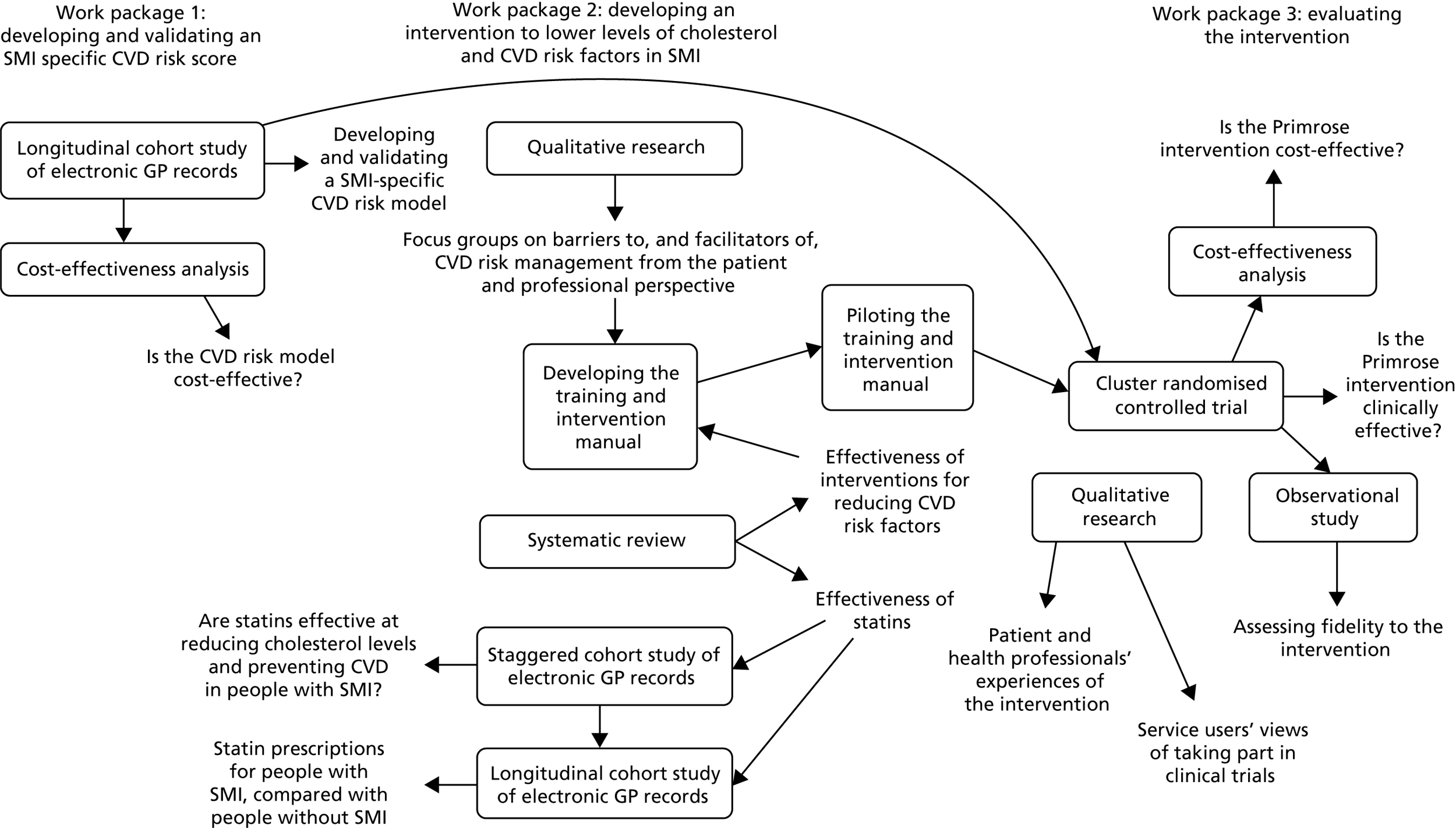
The research was carried out between May 2011 and July 2017. In years 1–2 (May 2011 to May 2013), we developed and validated a CVD risk score tool specifically for people with SMI. We then developed an intervention in primary care to lower levels of cholesterol and reduce CVD risk factors in people with SMI through an update of a systematic review of the literature, focus groups and workshops with clinical and lived experience advisors (years 1–3: June 2011 to December 2013). We also explored statin prescription rates and the effectiveness of statins on lowering levels of cholesterol and preventing CVD in people with SMI using primary care databases (years 2–5: October 2012 to December 2015). Finally, we tested the clinical effectiveness and cost-effectiveness of the new intervention in a cluster randomised controlled trial (RCT) and assessed the extent to which the intervention was delivered as intended (years 3–6: January 2014 to February 2017). The links between the three work packages are summarised in Figure 1.
FIGURE 1.
Overview of the Primrose programme.
Project management
The programme was overseen by a Programme Management Group (PMG) consisting of all authors of this report. The PMG met every 6 months. Subgroups that were drawn from the PMG members met more regularly to deliver each individual work package. A trial management group was formed to oversee the trial delivery and met every 3–6 months. An external trial steering committee was also formed to monitor the conduct of the trial and the trial was supported by the UCL PRIMENT clinical trials unit (www.ucl.ac.uk/priment).
Work package 1: development and validation of a risk model for predicting cardiovascular disease events in people with severe mental illnesses
Work package 1 aimed to address the following research aims during the first 2 years of the Primrose research programme:
-
to develop a CVD risk score tool, specifically for people with SMI and compare its performance with that of existing general population CVD risk tools.
-
to evaluate the cost-effectiveness of the CVD risk score tool.
Effectiveness of a cardiovascular risk prediction algorithm for people with severe mental illnesses
The work package was completed on time and the results have been published in one of the highest-impact international psychiatry journals (JAMA Psychiatry) in 2015 (with an impact factor of 16.6 at the time of writing). 37 To date, this has been cited 30 times on Web of Science and 42 times on Google Scholar. The link to this paper can be found in Appendix 3, Work package 1. 37 The algorithm has been developed and published as a web-based tool [www.ucl.ac.uk/primrose-risk-score (accessed 5 September 2018)].
The published work closely followed the proposed methods in our original funding application. It was a risk score development and validation study.
We used The Health Improvement Network (THIN) UK GP research database to identify a large cohort of 38,824 people with a GP-recorded diagnosis of schizophrenia, other psychoses or bipolar disorder, between 1995 and 2010. We identified 2324 new-onset cardiovascular events within this cohort.
We built a model to predict new-onset CVD using standard regression techniques. We included all the variables usually present in traditional risk scores (e.g. smoking, diabetes mellitus and cholesterol level) and then added SMI-specific variables in addition. These included use of first- and second-generation antipsychotics, use of antidepressants, type of SMI diagnosis and heavy alcohol use.
We developed one model that included blood test results for levels of cholesterol and high-density lipoprotein (HDL) cholesterol: the ‘Primrose lipid model’. We created another ‘Primrose BMI model’, which did not require these results. We compared these new models with the most widely used international models: the Cox Framingham models. We used a variety of methods to assess the performance of our new models in forecasting CVD, using a variety of accepted techniques to create multiple imputed data sets, and by dividing the data up into sections to allow ‘internal cross-validation’.
The validation results demonstrated that the new Primrose models performed better than the existing Cox Framingham models, in both men and women, in predicting future CVD events. In men, the D-statistic for the Primrose lipid model was 1.92 [95% confidence interval (CI) 1.80 to 2.03] and the c-statistic was 0.80 (95% CI 0.76 to 0.83). For published Framingham scores in men, the D-statistic was 1.74 (95% CI 1.54 to 1.86) and the c-statistic was 0.78 (95% CI 0.75 to 0.82). In women, the D-statistic for the Primrose lipid model was 1.87 (95% CI 1.76 to 1.98) and the c-statistic was 0.80 (95% CI 0.76 to 0.83). For published Framingham scores in women, the c-statistic was 1.58 (95% CI 1.48 to 1.68) and the D-statistic was 0.76 (95% CI 0.72 to 0.80).
To assess whether or not this superiority of the new Primrose models reflected a difference in international models (between the US Framingham model and the UK Primrose model), a UK general population risk score model was created from the THIN database. This model would be very similar to UK models, such as QRISK® (University of Nottingham, Nottingham, UK and EMIS Health, Leeds, UK), for which the parameters were not available at the time. The Primrose models still performed better than the UK general population models, which suggested that SMI-specific models are best, although all models performed quite well.
It is concluded that the new Primrose models were the most accurate, but that better evidence is needed regarding their potential impact before they could be recommended for replacing scores, such as the QRISK score or Cox Framingham, as these were models performing at an acceptable level in the SMI cohort and great effort would be required to implement new models across the clinical landscape. The inferior performance of general population algorithms has now been highlighted in clinical guidelines for managing the physical health of people with SMI. Examples are the NHS England Lester tool38 and the British Association for Psychopharmacology (BAP) guidelines on antipsychotic induced weight gain. 39
A limitation of the study was that there may be more missing predictor variables within routine clinical data than in data collected for the purpose of research. In addition, the effectiveness of the CVD risk score models was not evaluated for people from different ethnic backgrounds owing to poor recording.
One risk score study is not sufficient to implement a full change in policy and screening practice for CVD. Therefore, in the Primrose cluster randomised trial, tested in work package 3, our data collection included the variables and calculations for the QRISK score as well as the Primrose new risk scores. Neither risk score was used to determine participant eligibility for the trial, but the risk score work was reassuring that either of the risk scores could be used within the trial for determining 10-year risk and relevant interventions in people with SMI. The risk scores were also used as secondary outcome measures in the trial.
We conducted a further health economics analysis to compare which of Primrose, QRISK or Framingham CVD risk scores would be most cost-effective if combined with statin prescriptions for people with a CVD risk of > 10% over 10 years. The results are outlined in the next section and they show some superiority for the Primrose BMI model in terms of quality-adjusted life-years (QALYs) and net monetary benefit (NMB).
Cost-effectiveness of a cardiovascular risk prediction algorithm for people with severe mental illnesses
The aim of this work package was to evaluate the 10-year cost-effectiveness of the SMI-specific risk algorithm (Primrose) described in the previous section37 compared with a general population CVD risk algorithm. To evaluate this, a 10-year decision model of costs was developed and consequences of CVD in patients with SMI in the UK primary care population were assessed. The full manuscript for this work package has been published in BMJ Open in 2017. 40 The link to this paper can be found in Appendix 3, Work package 1. 40
A patient-level simulation was developed to hypothetically model the progress of people with SMI over 10 years, which was composed of (1) a decision tree to identify those at a risk of CVD over 10 years and eligible for statin therapy and (2) a Markov state transition model of 10 1-year cycles. The patient population in the model was composed of a random sample of 1000 real primary care patients extracted from the THIN UK GP research database.
A CVD risk score was calculated for each of the 1000 patients using four different CVD risk algorithms in four separate analyses. 40 The risk algorithms assessed were:
-
a general population lipid algorithm
-
a general population BMI algorithm
-
a SMI-specific lipid algorithm
-
a SMI-specific BMI algorithm. 40
Algorithms (1) and (2) were based on an adaptation of the widely used Cox Framingham algorithm,41 herein referred to as the general algorithm, which was created and validated using THIN data. 40 Algorithms (3) and (4) were derived from UK SMI patients in THIN, aged 30–90 years. 37,40 The primary analysis was based on a CVD risk threshold of 10%. The primary CVD prevention strategy used was prescription of a statin for patients above the risk threshold. A fifth analysis using no CVD risk algorithm was included to estimate the costs and consequences of not intervening.
The patient-specific probability of having a primary CVD event and the probability of dying in each cycle were based on algorithms developed using the same data set that was used to develop the Primrose risk algorithm37 (38,824 people in THIN with SMI and aged > 18 years). The probability of having a secondary CVD event was calculated from the model in the Reduction of Atherothrombosis for Continued Health Registry. 42
The benefits of statin therapy were modelled by applying the relative risk reduction of CVD from statin use from a Cochrane review (0.73 and 0.78 for coronary heart disease and stroke respectively)43 to the predicted risks of CVD for all patients newly prescribed statins. Costs included in our model were the cost of administering the CVD risk algorithm, CVD risk management and CVD events. All costs were reported in Great British pounds at 2012/13 values, inflated using conversion rates in Curtis (2013). 44
The mortality and morbidity impact was evaluated using QALYs as recommended by NICE,45 in which patients were allocated a utility score assigned to patients with SMI whose symptoms are being managed (0.865). 46 If a patient had a non-fatal CVD event, a utility decrement was applied. All future benefits (QALYs) and costs were discounted at 3.5% per annum. 45
Cost-effectiveness was calculated using the NMB approach47 and probabilistic sensitivity analysis to calculate the probability that each option was cost-effective for a willingness to pay (WTP) for a QALY.
The SMI-specific BMI algorithm classified the highest number of patients as at a ‘high risk’ of CVD (326 patients at 10% and 117 patients at 20%) and resulted in the greatest number of new statin prescriptions (255 patients at 10% and 81 patients at 20%). 40 The general BMI algorithm classified the lowest number of patients as ‘high risk’ (222 patients at 10% and 65 patients at 20%) and generated the lowest number of new statin prescriptions (175 patients at 10% and 44 patients at 20%). 40 The general SMI-specific BMI algorithm also prevented the greatest number of primary CVD events (13 events), equivalent to a 4–6% reduction in primary CVD events, and the highest NMB than the general lipid algorithm, and all other algorithms. 40
The results show that the provision of a relatively low-cost identification tool (the Primrose risk algorithm) and relatively low-cost intervention (statins) compared with the high cost of CVD events means that these combined interventions save up to £53,000 per 1000 patients over 10 years, or £53 per patient administered the Primrose CVD risk algorithm. The Primrose BMI model also gave 15 extra QALYs. The general population-derived lipid model was the next best performing algorithm with 13 extra QALYs and £46,000 saved.
A limitation of the study was that it was not possible to compare the performance of the Primrose models with that of the UK QRISK model, as the algorithm parameters were not available.
Work package 2: development of a practice nurse-/health-care assistant-led intervention for lowering levels of cholesterol and reducing cardiovascular disease risk in people with severe mental illnesses
Work package 2 aimed to address the following research questions using three different methodologies:
-
What are the barriers to, and facilitators of, lowering cardiovascular risk in people with SMI? (Focus groups.)
-
What evidence is there for effective pharmacological and behavioural interventions to manage cardiovascular risk factors in people with SMI? (Update of a systematic review.)
-
What are the patterns of statin prescribing for people with SMI and the general population? (Primary care database study.)
-
What is the effectiveness of statins in people with SMI? (Systematic review and primary care database study.)
The findings from the systematic review and focus group studies were brought together to inform the development of a CVD risk-lowering intervention and training programme for practice nurses and HCAs in primary care (Primrose intervention).
Focus groups with health professionals, patients and carers on the barriers to, and facilitators of, cardiovascular disease prevention in primary care for people with severe mental illnesses
Focus groups were conducted to explore current practices, barriers to, and facilitators of, delivering and accessing CVD risk-lowering interventions for people with SMI in primary care. This work was delivered according to the original programme protocol and was published in the journal PLOS ONE in 2015. 48 The link to this paper can be found in Appendix 3, Work package 2. 48 The findings were used to inform the development of the Primrose intervention and training programme.
A total of 14 focus groups were run with 75 participants, including 32 health professionals working in general practices, 11 staff from community mental health settings, 25 service users with SMI and 7 carers of people with SMI. Topic guides were used to guide the focus group discussions and were developed using domains from an established theoretical domains framework (TDF) for identifying facilitators and barriers to intervention delivery and behaviour change in health-care settings. 49 For this study, the TDF was used to design questions that would help elicit the barriers to, and facilitators of, lowering CVD risk for people with SMI from both the health-care professional and the patient perspective. More specifically, the topic guides were used to explore the resources, systems and training required by health professionals to lower CVD risk in SMI and, to explore with people with SMI and their carers the accessibility of services, motivation and capability to lower their CVD risk. All participants provided written informed consent to participate in the study and focus groups were audio-recorded, transcribed and analysed using a framework analysis approach. 50
A number of factors were identified that may prevent but also encourage people with SMI to access and engage with CVD risk-lowering interventions in general practice. A need for more systematic approaches to delivering CVD risk prevention in this setting was identified; however, the majority of people interviewed agreed that CVD risk monitoring and intervention delivery was the responsibility of health professionals working in general practice.
A number of barriers to CVD risk prevention were identified, including difficulties delivering preventative physical health care because of consultations focusing on mental health rather than physical health problems, scepticism among some health professionals about the effectiveness of stop smoking and weight loss interventions for people with SMI, and limited confidence and training for practice nurses to work with people with SMI. The negative side effects of psychiatric medications, a lack of motivation due to mental health problems and a lack of engagement with CVD risk-lowering interventions and primary care services were all identified as barriers to enacting CVD risk-lowering behaviours in people with SMI.
Potential facilitators were also identified that sought to address some of the barriers. These included practical ideas to increase attendance and engagement (e.g. afternoon appointments, appointment reminders); to involve family members, friends or support workers; to have a named contact at the general practice to ensure continuity; to provide healthy lifestyle advice during appointments; and to agree on and work towards realistic goals.
Stakeholders from different backgrounds and both urban and rural locations attended the focus groups, making the findings applicable to UK general practice. The service users who attended the focus groups may not have been representative of all service users, as they were active participants in their use of health services. It is likely that for people who are not well or not engaged, attending primary care services may be more difficult.
Systematic review of pharmacological and behavioural interventions for reducing cardiovascular disease risk in people with severe mental illnesses
To inform the development of the intervention, we planned to update existing systematic reviews, rather than conduct a review de novo. The updated review was presented and published as a conference abstract at The Lancet Public Health Science Conference51 and the results of the review were incorporated into the Primrose intervention training programme to inform health professionals about effective interventions for losing weight and stopping smoking in people with SMI.
A systematic review was conducted to evaluate and narratively synthesise evidence from published systematic reviews and individual RCTs on the effectiveness of pharmacological and behavioural interventions for reducing modifiable CVD risk factors in people with SMI. A meta-analysis of findings from individual RCTs was not possible because of the heterogeneity of reporting of interventions and outcome measures (see Appendix 1). 31,52–100
The Cochrane Library was searched for existing systematic reviews. The Cochrane Schizophrenia and Cochrane Depression, Anxiety and Neurosis Group Trial Registers were then searched between 1966 and 2014 for additional RCTs not included in the identified reviews. Interventions to manage the following were searched: levels of cholesterol, diabetes mellitus, hypertension, weight, smoking and alcohol consumption.
Fifteen systematic reviews and 28 additional RCTs were included in the review, from 11,028 references. The synthesised data demonstrated that bupropion and nicotine replacement therapy were effective interventions for smoking cessation, or reduction, as was a standardised smoking cessation programme. There was evidence that metformin and topiramate were effective pharmacological interventions for weight loss, and that behavioural interventions aimed at individuals (rather than groups) addressing diet and physical activity were most effective in reducing BMI. Only three trials reported effective interventions to reduce alcohol intake. No trials were found on interventions targeting levels of cholesterol, diabetes mellitus or hypertension as the primary outcome.
Study limitations were that the setting for most of the trials was secondary care, which limits their generalisability to primary care. It was also difficult to synthesise evidence from a wide range of intervention studies that targeted different CVD risk factors and measured outcomes in different ways.
Evidence was found that CVD risk attributable to weight and smoking can be managed effectively in people with SMI using pharmacological and behavioural approaches; however, limited or no evidence on the effectiveness of interventions to manage levels of cholesterol, diabetes mellitus, hypertension, alcohol misuse or multiple CVD risk factors was identified in this population. These findings were taken forward into the development work and used within the training programme.
Investigating patterns of statin prescribing among people with, and without, severe mental illnesses
The aim of this study was to explore the statin prescription rate in people with SMI compared with people without SMI in primary care. This work was published in the journal Schizophrenia Research in 2017 (with an impact factor of 3.958 at the time of writing). 101 The link to this paper can be found in Appendix 3, Work package 2. 101
The uptake of physical health checks in primary care among people with SMI has increased substantially over time, reflecting the introduction of policies and incentives, such as the Quality Outcomes Framework. 20 However, the impact on cardiovascular interventions, such as statin prescribing, was unknown. We used data from THIN to examine the rate of new statin prescriptions in people with, and without, SMI over a 10-year time period. Overall, rates of initiating a statin were at least doubled in people aged 30–59 years with SMI than in those without, even after adjusting for cardiovascular risk factors. Among people 60–74 years, rates were generally similar for people with and without SMI. However, among the oldest (aged ≥ 75 years) with schizophrenia (but not bipolar disorder), the rate of statin prescribing was around 20% lower (IRR 0.81, 95% CI 0.66 to 0.98) relative to those without SMI. These findings suggest that older individuals with schizophrenia are less likely to be initiated on a statin than people of a comparable age without SMI, and that this group may therefore benefit from additional measures to prevent CVD. The higher rate of statin prescribing among 30- to 59-year-olds with SMI (relative to people with SMI) demonstrates that statin prescribing is an important element of CVD prevention for this group and highlights the need for further evaluation.
Estimating the effectiveness of statin prescribing for people with severe mental illnesses
This study aimed to examine the effectiveness of statins for people with SMI through a systematic review of existing evidence and a series of staggered cohort studies designed to estimate the effectiveness of statins for the prevention of CVD and for modifying lipids in people with SMI. 102 This study was published in the journal BMJ Open in 2017. 102 The link to this paper can be found in Appendix 3, Work package 2. 102
Although statins form a core part of CVD primary prevention in the general population, this evidence base was not readily transferable to people with SMI. This is because it is not known whether or not patterns of medication adherence differ. Furthermore, some antipsychotic agents interact with sterol regulatory binding elements (which control lipid synthesis) and might therefore counteract the cholesterol-lowering action of statins. In addition, some of the largest statin trials have excluded participants with psychological conditions or excluded individuals perceived as less likely to be compliant with treatment.
Therefore, a systematic review was undertaken to examine the effectiveness of statins for primary prevention of CVD among people with SMI. This review did not identify any information on CVD events, mortality or long-term statin use in people with SMI. However, two studies provided evidence that statin therapy is associated with significant reductions in levels of total cholesterol (decreases of 11%28 and 35%27 for pravastatin and rosuvastatin, respectively) and LDL cholesterol (decreases of 20%28 and 49%27 for pravastatin and rosuvastatin, respectively) over 12 weeks in 60–100 individuals with SMI. These findings suggested that a large-scale study to evaluate the long-term impact of statin prescribing, particularly on CVD outcomes, was needed.
To explore the effectiveness of prescribing statins to people with SMI, we used UK primary care data from THIN to develop a series of cohort studies. These cohorts included 16,854 people aged 40–84 years who had a diagnosis of schizophrenia or bipolar disorder, and did not have pre-existing CVD. Cardiovascular outcomes of statin users and non-users were compared for (1) combined first myocardial infarction (MI) and stroke (primary outcome), (2) all-cause mortality and (3) change in total cholesterol concentration at 1 and 2 years after initiating a statin. We adjusted our results for a wide range of characteristics (such as blood pressure) that are associated with being prescribed a statin and the risk of developing CVD. In the main analysis we used multiple imputation to estimate the value of unobserved data and also conducted a complete-case analysis (for individuals with fully observed data), which produced very similar results to the analysis of the imputed data.
We did not identify statistically significant reductions in the rate of combined MI and stroke [incident rate ratio (IRR) 0.89, 95% CI 0.68 to 1.15] or all-cause mortality (IRR 0.89, 95% CI 0.78 to 1.02) associated with statin prescribing. However, it was found that statin prescribing was associated with statistically significant reductions (equivalent to a 20% decrease) in the level of total cholesterol 2 years after initiating a statin (IRR 1.2 mmol/l, 95% CI 1.1 to 1.3 mmol/l). This finding is similar to the reduction in cholesterol level observed in trial participants without SMI (IRR 1.1 mmol/l, 95% CI 0.8 to 1.4 mmol/l) and suggests that medication adherence in people with SMI is sufficient to support effective lipid modification. This translates to approximately a 25% decrease in mortality and 30% decrease in CVD events. 103
The study investigated a wide range of confounders for which data were captured in THIN; however, we cannot exclude the possibility of residual confounding due to factors (such as diet and exercise) that were unmeasured within our data set. Of note, our estimates of effect for statins were compatible with those from randomised trials examining non-SMI populations, suggesting that the likely impact of unmeasured confounding on our results may be small.
Bringing the evidence together to develop and test the Primrose trial intervention and training programme
The following section describes the development of the Primrose intervention manual and training programme for health professionals working in primary care.
First, the barriers to, and facilitators of, enacting both health professional and patient behaviours for lowering CVD risk in SMI were identified in the focus group work, described in Conclusions and recommendations, Focus groups with health professionals, patients and carers on the barriers and facilitators to cardiovascular disease prevention in primary care for people with severe mental illness, using questions derived from a theory-informed approach for identifying the causes of behaviour (TDF). 49 The identified barriers and facilitators were then mapped to potential intervention components that sought to overcome the identified barriers and harness the facilitators (see Appendix 4, Table 15).
This evidence was supplemented with findings from the systematic review described in Conclusions and recommendations, Systematic review of pharmacological and behavioural interventions for reducing cardiovascular disease risk in people with severe mental illness, and recommendations from workshops with academic, health professionals and lived experience experts. Key intervention components were combined to form an intervention manual and training programme. The key components of the intervention and training programme are described in this section, and supporting tables and figures can be found in Appendix 4. The intervention manual developed as a result of the evidence synthesis can be downloaded on the project web page [(www.ucl.ac.uk/primrose/primrose_manual (accessed 5 September 2018)].
A subgroup consisting of experts in mental health, primary care, behaviour change, expertise from experience and health service research was formed to bring together the evidence into a study manual and 2-day training programme. A logic model was developed by the group to explain the relationship between factors that might influence the effectiveness and implementation of a primary care nurse-HCA-delivered behaviour change intervention. This was also used to guide the clinical and practical aspects of the intervention development and training content (see Appendix 4, Figure 4).
An evidence-based, theory-informed approach (the TDF)49 for identifying factors that might influence behaviour was used to map the barriers and facilitators for lowering CVD risk for people with SMI identified by focus group participants, to potential intervention components. Intervention components were then selected using an established taxonomy of behaviour change techniques104 as well as practical suggestions to address the barriers and incorporate the facilitators identified by the focus group discussions (see Appendix 4, Table 15).
The findings from the systematic review were used in the training programme to educate health professionals on effective interventions for weight reduction and stopping smoking in people with SMI.
Additional workshops were run with academic clinicians and lived experience advisors to elicit expert views on the final design of the intervention and training programme [see Patient and public involvement for a list of recommendations incorporated into the final intervention and training programme from the Lived Experience Advisory Panel (LEAP)]. 105 A review of relevant policy and clinical guidelines was also undertaken to ensure that the intervention followed best clinical practice on CVD prevention in SMI with the aim of addressing the proximal outcomes (behaviours) identified in our logic model (see Appendix 4, Figure 4).
Piloting the Primrose trial intervention and training programme
Seven practice nurses/HCAs attended a pilot session of the training programme before the start of the trial. Some minor changes were suggested that were incorporated into the final training programme, including more opportunities for role play of Primrose appointments and more simplified explanations of the theoretical frameworks used to explain behaviour change.
The first intervention appointment was then piloted with two members of the LEAP. The practice nurse responsible for delivering the training met with each LEAP member and conducted the appointment using the study manual. Feedback from these sessions was incorporated into the final version of the manual and training programme. This included an emphasis on goals being patient led and a need to consider and understand aspects of the person’s life that may make behaviour change difficult.
The Primrose trial intervention and training components
The final intervention consisted of 8–12 appointments with a practice nurse/HCA over 6 months. Nurses/HCAs were trained to support patients to identify and monitor progress with goals on cardiovascular health, including taking medication, improving diet, increasing physical activity, stopping smoking or reducing drinking. They were encouraged to compile a local resource directory and refer patients on to existing support services if available in the local area (e.g. weight management or Stop Smoking Services), or provide support directly if services were unavailable or if the patient requested one-to-one support. They were also encouraged to actively follow-up and monitor attendance at services and progress made towards achieving health goals.
Nurses/HCAs were given a manual to take away with them, which included step-by-step appointment delivery flow charts, help sheets on managing different CVD risk factors and help sheets on strategies to help patients to stay motivated and engaged. A health plan was also given to patients to take away and use in between appointments. The health plan was used to record chosen goals, create an action plan and record progress towards achieving the goal.
The training programme was delivered to between four and eight practice nurses and HCAs at one of six training sessions by a practice nurse with expertise in mental health, a health psychologist, a lived experience trainer and the programme manager. The training comprised lectures and active learning through group discussion and role play of appointments. There were 2 days of learning, which were 2 weeks apart so that the nurses/HCAs could deliver an appointment between sessions and bring any difficulties they might have experienced or areas they felt less confident with to practise at session 2.
Training session 1 consisted of (1) discussing the link between CVD risk and SMI, (2) mental health awareness, (3) effective interventions for lowering CVD risk, (4) behaviour change techniques and (5) practical strategies to encourage motivation and engagement. Appendix 4, Table 16, contains a more detailed description of the content of training session 1. Training session 2 was led by the trainees and involved discussing the appointments that they had delivered and practising strategies and appointment delivery through role play and discussion.
In conclusion, an evidence-based manual and training programme was developed using clinician, patient and clinical academic advice from focus groups and workshops alongside reviews of the best available evidence in both policy and RCTs of CVD risk-lowering interventions. The resulting manual and training programme were piloted with health professionals and patients and found to be acceptable and deliverable. The intervention was then tested in a cluster RCT described in Work package 3.
Work package 3: evaluation of a practice nurse-/health-care assistant-led intervention for lowering levels of cholesterol and reducing cardiovascular disease risk in people with severe mental illnesses in primary care – a cluster randomised controlled trial
This work package evolved from the development work in work package 2 that integrated published evidence, the focus groups and then the work of the PMG and lived experience advisory group (LEAP) to design the nurse-/HCA-led intervention.
Work package 3 took this intervention and subjected it to a full clinical and economic evaluation in a cluster randomised trial, as planned in the original Programme Grants for Applied Research (PGfAR) funding application.
Work package 3 had the following research aims:
-
to establish whether or not the new Primrose intervention reduces levels of total cholesterol in people with SMI over a 12-month period compared with treatment as usual (TAU)
-
to determine whether or not the intervention improves other CVD risk factors over a 12-month period compared with TAU
-
to determine whether or not the intervention is cost-effective when compared with TAU
-
to assess the fidelity of intervention delivery in the Primrose intervention arm.
Clinical effectiveness of an intervention for lowering levels of cholesterol and reducing cardiovascular disease risk for people with severe mental illnesses
The full trial protocol for the cluster randomised trial106 was published in Trials in 2016. This protocol included the final sample size calculations for the trial as well as a description of the interventions and all trial procedures. The link to the protocol paper can be found in Appendix 3, Work package 3. 106
The trial was published in the journal Lancet Psychiatry in 2018. 107 The link to this paper can be found in Appendix 3, Work package 3. 107
The methods and results from the analysis of the cluster randomised trial in terms of clinical effectiveness are summarised below.
Methods
We successfully delivered a cluster randomised trial within which 76 GP practices across England were recruited and then randomised to either the Primrose intervention (n = 38) or the TAU (n = 38) arm. This total number of practices was larger than specified in the original grant application as the numbers of participants (cluster size) recruited in each practice was smaller than expected (mean 4.3 participants).
Participants in the trial were aged 30–75 years with a GP record of SMI, in line with the definitions already outlined in this report. They had a raised lipid profile defined as a cholesterol level of > 5.0 mmol/l or a total-to-HDL cholesterol ratio of > 4 mmol/l; and they needed to have one other cardiovascular risk factor, including hypertension, smoking, obesity, raised HbA1c levels or diabetes mellitus.
For the practices in the intervention arm, allocated nurses or HCAs were trained on two occasions to deliver the Primrose intervention that was developed in the earlier stages of the research programme (work package 2). Briefly, this involved arranging up to 12 appointments with each participant to target the most relevant CVD risk factor and using agreed evidence-based approaches, and established behavioural techniques to maximise the chance of successful risk reduction. In the treatment-as-usual arm, the nurses were not trained in the intervention but they were allowed to offer standard treatment for CVD risk factors in line with routine practice.
Patients and staff could not be masked to the intervention, but researchers were not informed of the allocation. The analysis plan was predetermined and random-effects linear regression (adjusting for baseline characteristics) was performed on the primary outcome of total cholesterol level at 12 months to account for clustering within general practice. Secondary outcomes included CVD risk scores and other cardiometabolic parameters including glucose, smoking, blood pressure and diabetes mellitus as well as BMI. We also included validated measures of well-being, diet and physical activity, patient satisfaction with services and adherence to both psychotropic and physical health medication.
Results
We recruited 327 participants with SMI: 155 participants in the 38 practices within the Primrose intervention arm and 172 participants within the TAU arm. Attrition in the study at 12 months was lower than predicted (12% as opposed to 20%) so that the total number of patients with follow-up data at 12 months (for the primary outcome of total cholesterol level) was greater than the sample size calculation requirements. In general, the patients had a high level of CVD risk factors, as would be expected by the inclusion criteria. For instance, half were current smokers and the mean BMI was above the threshold for obesity in both arms.
For the primary outcome of total cholesterol level there were no differences between arms at 12 months (5.4 mmol/l Primrose vs. 5.5mmol/l TAU; coefficient 0.03, 95% CI –0.22 to 0.29). This remained the case when additional analyses were performed, adjusting for baseline cholesterol levels or for characteristics that differed between arms at baseline. Total cholesterol levels did decrease over 12 months in both arms (mean decrease Primrose 0.22 mmol/l; mean decrease TAU 0.39 mmol/l).
There were also no differences between arms on the secondary outcomes listed in the methods section above, including the cardiometabolic parameters, patient satisfaction with services or adherence to medications. Statin prescriptions were low and did not increase at 12 months in either the Primrose or the TAU arm.
A total of 30 serious adverse events were reported for 25 people. There were fewer serious adverse events in the intervention arm (seven events for seven patients, including one death, three psychiatric admissions and three general admissions) than in the TAU arm (23 events for 18 patients including three deaths, 11 psychiatric admissions for nine people, seven general admissions for six people, one admission to a crisis house and one diagnosis of cancer).
Participant attendance rates at Primrose appointments were moderately good, with only 32 (21%) participants attending no appointments. A total of 72 (46%) participants attended more than six appointments over the 6-month intervention period and 36 (23%) participants attended between two and five appointments, with the remaining 15 participants (10%) attending one appointment.
Care in the TAU arm may have been better than standard general practice care as the GP practices in the study had identified people with raised CVD risk factors, for whom they may have then clinically intervened. Furthermore, participants and the practices were motivated to take part in the trial and to reduce CVD risk factors. This may have minimised the chance to show superiority for the more intensive Primrose intervention.
The choice of primary outcome measure for the trial was a challenge given that the intervention was designed to target multiple CVD risk factors. Nurses/HCAs were trained to discuss cholesterol levels and statin intervention and adherence in the first instance and then move on to other goals relevant to CVD risk reduction; however, this may have been in conflict with goal-setting being patient-led. If other behavioural goals were chosen instead of statins, then they may have had less impact on levels of cholesterol. However, differences were not demonstrated in any other CVD risk factors.
Conclusion
Nurses and HCAs were successfully trained in the Primrose intervention and delivered it to the majority of patients. However, participants in the intervention arm did not do better on the primary or secondary outcomes than participants in the TAU arm in UK primary care. This may reflect good care in both arms, as cholesterol levels did decrease over the study period and satisfaction with services on the validated client satisfaction questionnaire-8 was high in both groups.
This was a pragmatic trial in which participants exhibited a range of clinical characteristics and CVD risk factors, and it may be that this variability made it less likely that the primary outcome of total cholesterol level was targeted by the nurses/participants or that the most effective interventions, especially statins, were chosen. This possibility will be explored in further fidelity and health economics work.
Cost-effectiveness of an intervention for lowering levels of cholesterol and reducing cardiovascular disease risk for people with severe mental illnesses
The trial protocol for the cluster RCT106 was published in Trials in 2016 and included the cost-effectiveness analysis plan. The link to the protocol paper can be found in Appendix 3, Work package 3. 106
The full cost-effectiveness analysis was published as supplementary material to the trial in the journal Lancet Psychiatry in 2018. 107 The link to this paper can be found in Appendix 3, Work package 3. 107 The methods and preliminary results from this paper are summarised below.
Methods
The aim of the economic evaluation was to evaluate if the Primrose intervention was cost-effective compared with TAU, for a range of values of WTP for a QALY gained from a health-care cost perspective over the duration of the trial (12 months).
To calculate QALYs, EuroQol EQ-5D 5 level (EQ-5D-5L) data were collected at baseline, 6 months and 12 months and calculated as the area under the curve adjusting for baseline differences. Data were collected using patient-completed questionnaires asking about health promotion activities over the past 6 months at baseline, 6 months and 12 months. Health promotion activities included services for hazardous and harmful drinking, Stop Smoking Services, nicotine replacement therapy, diabetes mellitus and weight management services. Primary and secondary care resource use was collected from patient medical records for the duration of the trial. The cost of the Primrose intervention was calculated from data collected as part of the trial on the number and duration of Primrose appointments attended, missed appointments and who delivered the intervention (primary care nurse, HCA or GP). Information on the cost of training was also collected.
Results
The Primrose intervention arm showed a mean of 0.769 QALYs (95% CI 0.751 to 0.787) compared with a mean of 0.780 QALYs for TAU (95% CI 0.764 to 0.796). The difference in QALYs was –0.011 (95% CI –0.034 to 0.011).
The total health-care cost for the Primrose intervention group was £1286, with a total cost of £2182 for TAU (mean difference –895, 95% CI –£1631 to –£160; p = 0.012). These lower health costs were mostly a result of fewer mental health inpatient stays and costs (£157 in the Primrose intervention vs. £956 in TAU; –£799, 95% CI –£1480 to –£117; p = 0.018). The total mean 12-month health-care cost per patient for the Primrose intervention (including intervention costs but excluding those who did not attend and training) was £2580 (95% CI £1899 to £3261), with a total mean cost of £3404 (95% CI £2467 to £4340) for TAU. This gave a cost difference of –£824 (95% CI –£568 to £1079) in favour of Primrose. The total incremental cost-effectiveness ratio (–£824/–0.011) is £76,245.
There is some uncertainty as to whether or not using the EQ-5D-5L to calculate QALYs is a suitable methodology for health promotion interventions.
Conclusion
The potential effect of increased contact with a primary care health professional on mental health inpatient admissions warrants further investigation.
Fidelity assessment of the Primrose intervention delivery
We assessed the extent to which the intervention and training programme were delivered as intended through an analysis of a randomly selected sample of transcribed audio-recordings of intervention appointments. The full report of this work can be found in Appendix 2.
Methods
To enhance fidelity in the intervention arm, we developed a study manual in which the detail of each component of the intervention was described. Nurses/HCAs were trained on delivering the intervention through strict adherence to the details provided in the manual. This facilitated the standardised delivery of the intervention across 41 providers in all 38 participating GP practices in the intervention arm.
Nurses/HCAs in the intervention arm were trained in procedures for audio-recording all of their appointments with recruited Primrose patients. After gaining consent from the patients and nurses/HCAs in the intervention arm, nurses/HCAs were asked to record all of their Primrose intervention appointments.
A random 20% sample of audio-recordings was selected for the fidelity assessment as specified in the original grant application. Fidelity was assessed by two independent researchers using first appointment and subsequent appointment checklists adapted from a reliable fidelity assessment method developed for behavioural interventions. 108,109 A score of ‘2’ was assigned if a provider behaviour was achieved, a score of ‘1’ when a provider behaviour was achieved to some extent, a ‘0’ when the provider behaviour was judged appropriate to do but was not delivered, and ‘not applicable’ for provider behaviours judged not appropriate. This scoring system was applied to the appointment transcripts, deriving a percentage fidelity score for each intervention component, appointment and provider. We also derived an overall fidelity percentage score for all sampled appointments and all providers combined. Inter-rater reliability for coding between the two researchers was 86% with a Cohen’s kappa of 0.668 (95% CI 0.63 to 0.70).
Results
One or more appointment audio files were returned by 33 out of 41 (80.5%) providers for 90 out of 123 (73.1%) patients. Out of 831 attended appointments, 431 (53%) audio-recordings were returned. A random selection of 86 out of 431 (20%) audio-recordings covering 23 out of 33 (69.7%) providers and 52 out of 123 (42.3%) patients were transcribed verbatim.
A total of 67.7% of intervention manual-specified components were delivered across all appointments indicating moderate fidelity, with considerable variation among activities. Fidelity was higher in appointment 1 (72.5%) than in subsequent appointments (66.6%). Fidelity also varied between intervention components and was lowest for ‘forming habits’ (47.8%) and highest for ‘reviewing progress’ (90.2%).
Out of 14 first appointments, none of the patients identified a goal that addressed statin adherence or initiation. Eight (57.1%) patients wanted to address diet or physical activity, three (21.4%) patients set a goal around reducing smoking, and two (14.3%) patients chose to reduce their alcohol intake. One patient did not set a goal. Nurses had higher fidelity than HCAs, with 79.5% of intervention components delivered by nurses compared with 64.3% by HCAs. This difference was significant [t(20) = 2.32; p = 0.037].
A potential limitation of the study was whether or not the fidelity sample of intervention practice nurses/HCAs was representative of the trial sample. Nurses were over-represented in the fidelity sample (60.9% vs. 43.9% in the trial) as were providers with previous research experience (52.2% vs. 39% in trial); however, the fidelity sample was randomly generated by an independent statistician.
Conclusion
Observed fidelity to the Primrose intervention was moderate, with some intervention components being delivered more than others. These results are comparable to other fidelity assessments of cardiovascular prevention programmes. 110 Statins were not focused on in initial appointments, which concurs with the main RCT findings in which few statins were initiated. HCAs had a lower fidelity of intervention delivery than practice nurses, which may have implications for future clinical and research work in this field.
Conclusions and recommendations
This section brings together the main conclusions from the Primrose research programme. It reflects on the successes and difficulties we faced over the 6 years, in the context of the original aims and objectives of the funded grant proposal. It then describes the implications of the research findings, the plans for future research building on the programme, and opportunities for other work in the field of cardiovascular comorbidity in people with SMI.
Summary of successes and challenges
We achieved all our main research objectives from our final PGfAR proposal and delivered some additional pieces of work related to cardiovascular health in people with SMI. The risk score work and development work were all delivered and most of that work has been published in peer-reviewed journals and/or presented at scientific meetings. The trial was also completed within a revised timeframe for the larger trial in terms of the increased number of required clusters.
The objectives were achieved despite a number of challenges, especially two major changes in national GP research infrastructure after funding was awarded, namely the closure of both the Medical Research Council General Practice Research Framework (MRC GPRF) and then the Primary Care Research Network (PCRN). We were one of the first research teams to fully run a primary care trial within the new Clinical Research Network (CRN) structure.
The timeframe for the trial in work package 3 required the Primrose programme to run for an additional 15 months to achieve the numbers required in our sample size calculation, mainly because of the challenges of recruiting sufficient participants in each general practice. This meant that we needed to recruit 76 GP practices rather than the original 40. All this was achieved despite a small number of permanent staff in our budget (one programme manager and one research assistant). It is testimony to their skills and hard work that the trial achieved its aims with 76 practices recruited across England, all requiring site initiation, training, liaison with practices and research network staff as well as co-ordination of follow-up data collection and study closure.
Work package 1: development and validation of a risk model for predicting cardiovascular disease events in people with severe mental illnesses
The work package 1 risk score work developed new models for predicting CVD in SMI. These were delivered on time, using all the methods specified in the original proposal. We developed bespoke new Primrose models for predicting CVD in SMI and these performed well in people with SMI. However, existing models from the general population also performed fairly well and we did not generate the objective evidence to suggest that existing CVD risk scores in general practice should be replaced immediately with the bespoke SMI specific models.
A limitation of the study was that the performance of the CVD risk score models among different ethnic groups was not assessed, but the availability of routine ethnicity data is limited and this could be a focus of future work if data quality improves.
Given that a range of guidelines have referenced our work, it is likely that many clinicians are aware that standard risk scores may underestimate risk in people with SMI.
Work package 1: additional economic modelling work over and above the original protocol
The health economics modelling work regarding the new Primrose risk scores has recently been peer reviewed and published. 40 It uses the more recently recommended threshold of 10% CVD 10-year risk to explore which risk models would be better for people with SMI if used to drive statin prescribing. The Primrose BMI models provided the best economic results, which may have been a result of its classification of more individuals at a high risk of CVD and eligible for statin therapy than other algorithms, although the UK QRISK models also performed well.
Given that there was a small difference between the two tools economically, the decision regarding which algorithm to use in routine clinical practice becomes one of implementation, advocacy and ease of use. One could argue in favour of using a general population-derived lipid model as these are already used in UK general practice and hence require no change. On the other hand, the SMI-specific BMI model, although potentially requiring additional training and implementation costs, could confer additional benefit by raising awareness of the need to improve CVD outcomes in people with SMI, and providing a model that requires no blood test to estimate risk, a limitation of other CVD risk algorithms as many people, with and without SMI, decline blood tests. 111 The ease of implementation and delivery of the SMI-specific BMI model means it could be used in any setting, including mental health care and non-clinical settings without blood results. This is particularly important as many people with SMI do not attend primary care and monitoring of CVD risk factors remains low in other settings. 19,111–114 The SMI-specific BMI model provides an opportunity to target more people with SMI, to increase identification of those at a high risk of CVD and decrease the physical, social and financial burden associated with CVD.
We have made the Primrose model available on the internet so that it can be used by interested stakeholders. 40
Although the Primrose risk score results were delivered (and published) on time, the initial validation results were not strikingly different from current CVD screening practice for us to include the new algorithms in the Primrose intervention work or to use them as inclusion criteria for the trial as originally planned. 37 This was partly a timing issue as the risk score work occurred in parallel to the development work packages 2.1–2.3, which involved bringing together this intensive development work to finalise the training and content of the Primrose intervention.
Work package 2: development of a practice nurse-/health-care assistant-led intervention for lowering levels of cholesterol and reducing cardiovascular disease in people with severe mental illnesses
Three major pieces of research were performed to help design the nurse-led intervention for the main trial, as detailed in our original research protocol.
We also worked closely with our patient and public involvement (PPI) LEAP panel throughout this part of the programme and won a national prize for our PPI work, from the NIHR Mental Health Research Network (MHRN).
Focus groups with health professionals, patients and carers on the barriers to, and facilitators of, cardiovascular disease prevention in primary care for people with severe mental illnesses
We had always planned to augment our previous work with stakeholders to derive up-to-date information on the best ways to deliver the Primrose training and the intervention itself in primary care.
We conducted focus groups with nurses, GPs, service users and other stakeholders as planned. This work was peer reviewed and published in PLOS ONE in 2015. 48 We used behavioural science theory49,104 to identify barriers to, and facilitators of, nurses delivering the intervention to reduce CVD risk in people with SMI. A range of important factors emerged and these were incorporated into the intervention for the trial.
Systematic review of pharmacological and behavioural interventions for reducing cardiovascular disease risk in people with severe mental illnesses
As there were existing systematic reviews in this field (for single risk factors, such as weight and smoking), it had only ever been planned to update and summarise this evidence so that the latest research could be incorporated into the training package and intervention. This evidence would include SMI-specific research that targeted the main CVD risk factors in people with SMI, namely levels of cholesterol, blood pressure, dyslipidaemia, raised CVD risk scores, weight/obesity, smoking and diabetes mellitus.
The updated, extensive review was delivered on time and was utilised in the workshops, training and other activities that informed the final content of our intervention for the trial. It did not find many additional pieces of evidence to warrant a new publication.
The findings of our review were specifically useful in demonstrating that smoking and weight reduction interventions have been successful in people with SMI. This was a positive message to incorporate into the intervention training, particularly aimed at tackling negative attitudes towards behaviour change in people with SMI. 27,115–117
The review was presented at a public health conference and the abstract published in The Lancet. 51
Investigating patterns of statin prescribing among people with, and without, severe mental illnesses
We completed primary care database research that showed that statins are generally being used equitably (or at higher levels) in people with SMI, compared with age-matched individuals without SMI, except in older people with schizophrenia for whom there is a disparity and underprescribing of statins. This is an important finding as this age group has the highest absolute rates of CVD.
This work was emerging as the trial and intervention design was under way; therefore, we maintained statins at the top of our hierarchy of interventions for the nurses to focus on in the Primrose intervention arm if the patients met the criteria for statin prescription, which changed to 10% rather than 20% risk at the beginning of the trial in 2014. 25
Additional piece of pharmacological epidemiology: estimating the effectiveness of statin prescribing for people with severe mental illnesses
This work involved a relatively novel methodological approach to assessing the effectiveness of statins in real life for people with SMI, again using the UK THIN database.
The staggered cohort design allowed us to show that after adjusting for multiple factors in the analysis, statins have similar effects on levels of total cholesterol at 12 months, as would be seen in the general population. In other words, statins do work and people with SMI do seem to take them when they are prescribed. This is sometimes questioned given issues around adherence and the multiple risk factor challenges that face people with SMI.
The work has been peer reviewed and published in BMJ Open (with an impact factor of 2.413 at the time of writing). 101,102 The work within work package 2.3 was also the content of a successful PhD awarded to Ruth Blackburn in 2016 as part of Primrose. 118
Bringing the evidence together to develop and test the Primrose trial intervention and training programme
At the end of the development work packages, we entered an intensive period of work, synthesising the evidence that we had identified, running workshops and developing the manual and training programme for the Primrose nurse-led intervention.
We were able to use a rich combination of existing data, expertise, novel research findings and existing clinical guidance to create a contemporary evidence-based intervention.
The intervention appointment structure was piloted with two LEAP members and the training programme was piloted with seven nurses. Feedback was generally positive with suggestions incorporated into the final versions of the manual and training programme. The final intervention included a structure of 8–12 appointments delivered fortnightly over a 6-month period, a 2-day training programme, the manual for the nurses and its component instructions. This specified a hierarchy of risk factors to address during the Primrose appointments, with guidance on how to choose collaborative goals, which were likely to have an impact on CVD risk. The manual and training programme also addressed a range of behavioural techniques to guide the nurses/HCAs through each appointment including goal-setting, creating an action plan and involving supportive others.
Each of the developed products received feedback and input from the full range of Primrose stakeholders including service users, practitioners, researchers and experts in behavioural science, nursing and cardiovascular health. Developing a complex intervention involving different stakeholders from a range of backgrounds was challenging at times, particularly when views on the content of the intervention and training programme were in conflict. One particular area of contention was around statin prescriptions, with clinicians and policy favouring statins as a first-line clinically effective treatment for CVD prevention, but patients expressing concerns about medication and a preference for patient-led behavioural approaches. We decided to maintain our emphasis on statins and statin adherence as the first-line treatment within the intervention, while also emphasising the need to work in partnership with the patient to determine how to tackle raised cholesterol levels and CVD risk, with the option of considering behavioural approaches around diet and physical activity, smoking and alcohol use.
This development process was very comprehensive and it was felt that the resulting intervention had been developed extremely thoroughly and included a high level of scientific and practical specification.
Work package 3: evaluation of a practice nurse-/health-care assistant-led intervention for lowering levels of cholesterol and reducing cardiovascular disease risk in people with severe mental illnesses – a cluster randomised controlled trial
Ethics approval and trial registration were successfully achieved on time to start the trial in 2013. The trial protocol was peer reviewed and published in 2016,106 before any analysis occurred.
The trial commenced in the north London locality and it soon became clear that recruitment procedures for the trial involved large amounts of work to screen for people with SMI in primary care, to check eligibility in terms of cardiovascular risk factors and to invite them to take part. Some potential participants did not have all the required information on CVD risk factors, so they needed to be invited to their GP practice to check eligibility. This was a rate limiting step to recruitment.
We realised that the numbers likely to take part per practice were somewhat smaller than expected, and required a lot of time and effort in the absence of the MRC GPRF and PCRN infrastructure. We therefore decided to increase the number of practices from 40 to a final total of 76, which increased the delivery time of the trial. However, we successfully recruited and retained enough people with SMI and CVD risk factors to achieve the final number of participants with primary outcome data at the 12-month follow-up (total n = 289), meeting the requirements of our published sample size calculation.
This was the first fully powered trial of a primary care-based intervention for lowering CVD risk in people with SMI. The successful delivery demonstrates that there is an appetite for this field in UK primary care and also that the CRNs were able to support this type of work. The large number of GP practices recruited and the geographical spread across both rural and urban areas in England is also a strength in terms of external validity.
The trial finished follow-up on time within our revised timeline (6 months ahead of the end of the overall programme funding as planned), allowing time for data cleaning and analysis of the results.
The training and intervention were successfully delivered across the general practices, and seemed acceptable to providers and participants with almost half attending six or more appointments in the Primrose intervention arm. This contradicts the negative attitudes that are sometimes voiced about uptake of this work in SMI.
The main results showed no differences in the primary outcome of total cholesterol levels at the 12-month follow-up. Furthermore, the trial did not show differences in any of the main secondary outcomes related to cardiometabolic risk factors, such as blood pressure, glucose, weight or smoking. Diet and exercise levels were similar in both arms and levels of satisfaction with services were high in both arms.
The richness and volume of the objective medical records data collected for the economic evaluation of the cluster randomised control trial was a strength of the study, but we did not have the time to conduct all of the analyses we were interested in. Additional work will include an analysis of 12-month data using multiple imputation to account for missing data and an analysis of the relationship between health inputs (health promotion activities) and health outputs (QALYs, reduction in unplanned health-care resource use).
In the assessment of fidelity using the audiotaped appointments, there was evidence that many of the behavioural techniques had been utilised within the appointments. This was true across providers, although fidelity seemed even higher for nurses than for HCAs. There was some evidence that when statin adherence or prescription should have been reviewed and targeted, the participant and provider did not choose statins as their primary focus.
The initial intervention had been envisaged as a nurse-led intervention, but in practice many GPs were unable to provide a nurse with time available to be trained in the Primrose intervention, so a health-care assistant (HCA) was identified instead. These practitioners do deliver CVD screening work to other populations, therefore, it was agreed that they would be trained in Primrose, partly to reflect real life and partly to allow the trial to be delivered practically and on time.
There is evidence in the primary care mental health literature that interventions that include supervision from a physician are more likely to reduce disease risk factors in people with depression,119 but this approach has not been tested in people with SMI. Future intervention studies may wish to test whether or not more intensive supervision and support for staff results in improved outcomes for patients; however, there may be cost implications of additional support.
There were some design issues that are a challenge to the external validity of the trial findings. This included generalisability, as both the GPs and the participants were people interested in physical health in SMI but perhaps not representative of the overall UK population and primary care landscape. A second issue was the cluster design and the fact that practices (and participants) allocated to the TAU arm were aware that there were Primrose participants with identified CVD risk factors who would not receive the Primrose intervention. Therefore, these participants may have received superior, or at least different, care to those undergoing standard care in usual UK general practice, by virtue of the screening process for the trial. In other words, TAU may not have been a ‘fair’ comparison with the Primrose intervention. The finding that levels of total cholesterol were reduced in both the intervention and the TAU arms despite a lack of focus on statins would support this.
It is interesting that mean total cholesterol levels decreased in both arms of the trial over the 12 months. The natural course of cholesterol level is to increase with age, so perhaps this decrease in cholesterol level reflects improvements in CVD risk for people with SMI in both arms and this may be one explanation as to why there were no differences between arms at 12 months.
The choice of an outcome measure for the trial was always going to be a challenge in a study targeting multiple CVD risk factors. The level of cholesterol was eventually chosen because the first aim of the intervention (and the original protocol) was to optimise statin prescribing in the first instance. However, if other behavioural goals were chosen instead of statins, these goals may have been less likely to have an impact on the primary outcome. Nonetheless, we did not show any differences in other CVD risk factors, nor in overall measures of well-being. However, the problem of identifying outcomes in trial settings such as this has been highlighted in other studies. 120 It is also acknowledged that in the longer term, cholesterol level reduction might not necessarily reduce CVD rates.
Recommendations for future research
Future research related to work package 1: the risk score development work, and work package 2.3 – the primary care database work
Our bespoke SMI Primrose risk scores have been validated and can be accessed online (www.ucl.ac.uk/primrose-risk-score/). They have been assessed in terms of economic benefit of using them to guide statin prescribing and would provide a NMB.
This is a particular benefit when people with SMI do not have a blood test result for lipids or decline to have one taken. This may apply to 50% of the UK SMI population. 21
The Primrose risk scores could be validated externally in a separate data set and updated and also compared against newer risk scores, including the new QRISK3 score that was published in 2017. 121 The QRISK3 score should be explicitly evaluated for its benefits for people with SMI, especially as QRISK3 uses a definition of SMI that is inconsistent with the general meaning in UK primary care (i.e. it also includes depression). The inclusion of depression may be valid, but for now we lack evidence as to whether or not this new risk score is superior to the Primrose score for people with schizophrenia, bipolar disorder or other psychoses.
Regarding the statins database research in Primrose,102 it is important that further studies monitor the national prescribing of statins to people with SMI, especially older age groups who are undertreated in current UK practice.
Future study designs may be able to assess the impact of statins on the mortality gap and excess CVD in people with SMI, especially if databases in the UK or internationally achieve larger sample sizes with higher numbers of CVD events, as is currently planned in primary care research.
Future research related to work package 3: the Primrose cluster randomised controlled trial
The Primrose intervention was acceptable and deliverable in real-life practice, with patients attending appointments and behavioural goals being set. However, it was not superior in terms of a difference in levels of total cholesterol between the two arms of the cluster trial.
Future studies might need to move beyond standard trial designs if we are to understand what works for people with SMI in terms of reducing CVD risk. Studies might need to use routinely collected clinical data to assess outcomes for all patients in real-life settings, comparing areas where different interventions are delivered to different people. These naturalistic evaluations would overcome the restrictions imposed by trial inclusion criteria, and the unrepresentativeness of people (and practices) who agree to participate in trials. This could involve more applied methods where routine data are collected within health-care systems to evaluate gaps in CVD risk screening and interventions. These studies should explore the delivery of statins, smoking cessation and weight reduction techniques including pharmacological approaches, such as metformin and behavioural approaches.
Further analysis of the fidelity work could utilise the full data set and explore the content and conversational aspects of the appointments in greater detail to help shed light on the lack of difference in results between intervention and control groups. Future research work should also examine whether or not a conversation regarding statins is happening between professionals and patients, and explore the reasons why statins might not be initiated for those who would benefit.
Further analysis of the Primrose trial data set from work package 3 will be able to assess the content of care within both the intervention arm and the TAU arm and explore what did and did not work, and any predictors of response. Examples will include demographics, provider characteristics, participant characteristics, such as diagnosis, the availability of formal or informal support to the participant and further analysis on service use and prescription data collected for the cost-effectiveness analysis of the trial. The potential effect of increased contact with a primary care health professional on hospital admissions also warrants further investigation.
Additional studies emerging from the Primrose programme
A number of additional studies have emerged from the Primrose programme of work and are currently in application, set-up or analysis phase. This includes:
-
An analysis of data from a qualitative study that used semistructured interviews to explore the experiences of those who delivered and those who took part in the Primrose intervention.
-
As part of the data collection for the cluster RCT, participants consented to provide a saliva sample for deoxyribonucleic acid (DNA) extraction and analysis purposes to determine whether or not any variation in DNA exists, relevant to both SMI diagnosis and physical health outcomes. Participants were given the opportunity to opt out of providing the sample without affecting their entry into the trial. DNA extraction is currently under way with data analysis plans currently being finalised.
-
The programme manager is carrying out secondary analyses on data collected through the trial and fidelity assessments for the purpose of fulfilling a doctorate. The research aims to explore the links between existing social support and adherence to CVD risk reducing treatments and behaviours for people with SMI.
-
A further, independent study funded by the NIHR School for Public Health Research is being commenced to explore inequalities in the provision of smoking cessation interventions for people with SMI, and the effectiveness of interventions in the THIN database 2017–18. This work follows on from the work in work package 2.3, but for smoking rather than statins.
-
Applications are currently being planned for studies to carry out naturalistic evaluations of the different risk scores in real-life settings as well as local interventions, which have been deployed in different regions of the UK to integrate physical and mental health care in people with SMI. An example is the Integrated Practice Unit for psychosis in Camden and Islington Foundation NHS Trust, which brings together health services and other providers to deliver a holistic approach to mental and physical health needs.
-
A further study is being planned, to explore diet and exercise variables and interventions related to these, with a NIHR School for Primary Care Research PhD studentship.
-
Primary care database studies continue to be used to explore CVD outcomes, risk factors and interventions in people with SMI, as new (and better-quality) primary care data become available.
Implications for practice
Work package 1: development and validation of a risk model for predicting cardiovascular disease events in people with severe mental illnesses
We have demonstrated that general population risk scores can underestimate CVD risk in people with SMI. 37 We have also shown that a model that does not require lipid blood tests is most beneficial for deciding when to prescribe statins and prevent CVD in people with SMI. 40
We are now making this tool available for clinicians and will need to update it with contemporary data as it becomes available. We will explore integrating these scores into clinical software packages.
Our findings regarding risk scores in SMI have been highlighted in national guidelines, including the British Association of Psychopharmacology guidelines on weight management in people prescribed antipsychotics. 39
The underperformance of general population risk scores for people with SMI is also highlighted in the adapted ‘Lester’ tool, an algorithm to help clinicians to manage CVD risk in people with SMI. 38 This tool is endorsed by most Royal Colleges and medical/nursing organisations, as well as NICE. 17 The underperformance of general population risk scores in SMI is also recognised in the general 2014 NICE guidelines on lipid modification. 25
In 2017, a new ‘QRISK3’ CVD score was published and it has addressed some of these limitations. 121 This new score included SMI and second-generation antipsychotics. The existing QRISK algorithms do allow calculation of CVD risk without lipid levels, but this is by assigning an average value. The Primrose models use multiple imputation in the BMI model and, therefore, should be more accurate when lipids are missing.
There are no data validating the new QRISK3 tool specifically in SMI, and there are some uncertainties about the new tool as the definition of SMI in the QRISK3 study has needed clarification; it transpires that the authors include depression in their definition of SMI, which is unusual for this field and may limit the utility of QRISK3 for people with schizophrenia, bipolar disorder and other psychoses. The results need further validation and clarification. 122 In contrast, the new Primrose models have been validated and economically evaluated as superior for people with SMI.
Work packages 2.3.1: investigating patterns of statin prescribing, and 2.3.2 – estimating the effectiveness of statin prescribing for people with severe mental illnesses
Some people with SMI who are older may be less likely to receive statins, so this needs to be addressed in clinical practice, especially as this group has the highest absolute number of CVD events. It is important that people with SMI are offered statins, as we have provided robust evidence that they are effective in a large, representative UK sample of people with SMI.
Work package 3: evaluation of a practice nurse-/health-care assistant-led intervention for lowering levels of cholesterol and reducing cardiovascular disease risk in people with severe mental illnesses in primary care – a cluster randomised controlled trial
We demonstrated that a primary care intervention for people with SMI was deliverable across general practices in England. The training was successfully organised and when the nurses and HCAs organised appointments, there was good attendance by people with SMI, with almost a half of patients attending six or more appointments. The providers did deliver the behavioural interventions to a moderate degree of fidelity. This occurred despite some negative attitudes towards the work when the providers initially attended the training for Primrose.
There was some evidence that statins were not chosen as the first goal, even when they should have been initiated or reviewed, but this is preliminary evidence.
The intervention in its current form was not found to be superior for the chosen primary outcome of total cholesterol level, nor the secondary outcomes. However, the success of the trial delivery demonstrates the feasibility of organising care for people with SMI in the primary care setting, at least for a group of people with SMI, with good levels of satisfaction in both arms. It is also of interest that an intervention in primary care with regular appointments was associated with fewer serious adverse events and decreased costs in terms of mental health admissions.
There were some methodological considerations regarding the trial, especially whether or not TAU was superior to usual UK general practice care, and future research designs may need to be more naturalistic, using less constraining methods, which are more inclusive of all people and practices, and which can capture real-life service provision.
In terms of future interventions, our fidelity work demonstrated that behavioural techniques can be delivered during appointments by HCAs/nurses with a manual and with two brief days of training. Patient care may be enhanced through mechanisms to ensure that evidence-based CVD risk reduction strategies are being offered and explained to people with SMI, including reviewing risk scores and explaining the role of statins.
Dissemination plans
It is planned to disseminate the findings from the programme of work through the following mechanisms:
-
Make the CVD risk score available as an online screening tool for use in clinical practice (www.ucl.ac.uk/primrose-risk-score).
-
Communicate key findings through the study website (www.ucl.ac.uk/primrose) and social media (https://twitter.com/UCLprimrose).
-
Publish the trial findings in peer-reviewed academic journals and present the findings at academic conferences.
-
Run workshops with stakeholders who took part in the trial and with leading academics in the field of CVD prevention and SMI to share findings and consider future research and policy implications.
-
Work with the Department of Health and Public Health England to translate the findings into practice.
Patient and public involvement
There was collaboration with both Rethink Mental Illness and the McPin Foundation in an advisory capacity and as active partners in the programme of research to ensure that patient involvement was integrated in the study. An award was received from the MHRN in 2013 for outstanding PPI in the Primrose programme.
A summary of the processes that were designed to ensure that PPI was appropriately delivered is below. Each of these activities is then described in more detail, and a reflection on their perceived impact from the perspective of the study team is provided:
-
Patient consultation prior to funding to assist in the design of the study. This was organised through Rethink Mental Illness. The key service user advisor, Janey Antoniou, died during the study set-up phase and, unfortunately, did not play a role in the delivery of the research.
-
Patient representatives on the management group and as co-applicants in the study (through two charities, first Rethink Mental Illness and second the McPin Foundation).
-
A LEAP during study set up. This group met throughout the life of the programme.
-
A public and patient co-ordinator – Dr Ben Gray. This role was active particularly in the development phases of the programme of research.
-
An intervention development group consisting of members of the LEAP and members of the research team. This group informed the development of the intervention manual and training programme.
-
A service user trainer, Vanessa Robinson, was employed to train practice nurses and HCAs drawing on expertise from experience alongside members of the research team.
-
A small qualitative study designed and delivered by the LEAP supported by the research team and staff at the McPin Foundation.
Design of the study and early development work with the Lived Experience Advisory Panel
We made use of the Camden and Islington Service User Research Forum (SURF) in the early stages of the programme to shape the priorities, research aims and research tools for each work package. Service users consistently endorsed the importance of this work, particularly mentioning weight gain, smoking and ‘diagnostic overshadowing’ whereby physical health is neglected by professionals on account of mental health diagnoses. SURF supported the development work, especially ensuring that statins were not offered too aggressively in the clinical trial as the latest ‘miracle cure’, but collaboratively with careful explanation. The SURF also provided feedback on the development of topic guides for the focus group study.
Shortly after the award of the programme grant, the Independent Service User Consultant co-applicant and researcher at Rethink Mental Illness sadly died. Our partners at Rethink Mental Illness worked closely with us and we were joined by a public and patient co-ordinator from Rethink Mental Illness with expertise in systematic reviews and qualitative work to lead on delivering the PPI arrangements as outlined in the original application. Within Primrose, this role was known as our PPI co-ordinator post. It was responsible for the set up and management of a service user advisory panel, a model pioneered by Rethink Mental Illness and called a LEAP, cofacilitating a service user focus group and assisting in the coding of focus group transcripts to feed into the development of a thematic framework. The PPI co-ordinator also set up a blog for service users, carers and members of the public to share their experiences of mental health and physical health problems and to obtain information on the project. A project website was developed that contained a dedicated service user and carer involvement page with links to Rethink Mental Illness and the blog (www.ucl.ac.uk/primrose).
A ‘virtual’ LEAP of 27 service users and carers with lived experience of mental and physical health problems was formed and met annually to review progress, influence the content of the programme and comment on work as needed. We had so much interest in advising the study that we chose to formulate a large LEAP and smaller face-to-face meetings as needed. The LEAP was co-ordinated by the public and patient co-coordinator employed by Rethink Mental Illness who updated members via e-mail on developments within the programme between meetings. At the first meeting, the purposes of the project were shared and feedback on the development work obtained and incorporated into the protocol. This included recruiting carers through Rethink Mental Illness to take part in an additional focus group, conducting focus groups in rural areas and exploring within the focus groups the potential link between primary care and secondary services in delivering the intervention. Feedback on the development of the project logo was incorporated into the final design. Comments on study documentation including patient information sheets, invitation letters and patient questionnaires and ideas to help improve recruitment and retention in the study were received from panel members in future meetings. This resulted in the redesign of the study recruitment leaflet and the design of a postcard thanking participants for their involvement in the study and to remind them of their follow-up assessment. The LEAP also contributed to the development of the health economics questionnaire for the trial and members were involved in piloting the intervention appointments.
Programme management group
One way to ensure that PPI was integrated into the study was to monitor PPI progress by having a standing item on all management meeting agendas. The PPI standing item tended to be an update report from the PPI co-ordinator, or charity representative. Decisions were taken at these meetings to support PPI, such as a LEAP request, to develop a user-led qualitative study to understand participant motivations for consenting to take part in research.
Intervention development group
A smaller, core LEAP intervention subgroup consisting of eight service users and carers was formed from the larger panel with the remit of translating the findings from the development work packages (focus groups, policy and systematic review) into the design of the intervention. The subgroup met four times over 1 year and developed 11 key recommendations, six of which were incorporated in to the intervention manual and training programme. 105 These recommendations were (1) one step at a time goals for behaviour change, (2) involvement of carers and mental health workers, (3) training of nurses to address attitudes towards mental illness and stigma, (4) appointment reminders to service users, (5) information to take away including the next appointment time and (6) involving service users in the training of nurses. For recommendations that were not incorporated into the intervention, the study team discussed the reasons with the subgroup members and answered any questions arising from these decisions.
Recognition of patient and public involvement in the Primrose study
The Primrose study received an award from the MHRN for outstanding service user involvement in 2013. The study was selected as the winner by three judges that were independent of the MHRN. The award was presented to Professor David Osborn at the annual MHRN Scientific Meeting on 21 March 2013. A service user from the LEAP took up the offer of a fully funded place (awarded to the Primrose study team) to attend the scientific meeting.
The Primrose programme manager, public and patient co-coordinator from Rethink Mental Illness and a member of the LEAP contributed to a case study report written on behalf of the MHRN. 123 The case study documented the impact, experiences and challenges of service user and carer involvement in research.
Training practice nurses and health-care assistants
A lived experience trainer with experience of mental health problems and CVD risk factors shaped the development and piloting of the training programme and co-delivered the training to practice nurses and HCAs. Attendees greatly valued the opportunity to hear the lived experience trainer’s story and ask questions about the experiences of how the trainer managed both their mental and physical health, and this was often cited as the most helpful session of the training. A video of the talk was created for use in the event that the trainer became unwell or was unable to attend, and a written summary document of the experiences was given to all attendees.
Later work of the Lived Experience Advisory Panel
The programme of research across the life of the project required changes in how we approached the LEAP. Once we entered the trial phase there were fewer decisions to make requiring lived experience expertise. We kept in touch with people through a newsletter, and the LEAP met less frequently. One output from the group after the trial began was a change in design for the patient information leaflet. It was updated with new text and images as the group felt that the original, on reflection, was unappealing.
The group also designed a plan for how results might be disseminated. Recommendations for bringing academics together to discuss findings from related studies were made as well as how results would be fed back to participating GP practices and research participants.
Peer-led qualitative research study
A peer-led qualitative project to explore the reasons why people with SMI choose whether or not to engage in research studies was conducted by members of the LEAP supported by staff at the McPin Foundation. This approach is termed Peer Research. 105 The idea for the study came from the LEAP group, and members were asked if they wanted to be involved as service user researchers. We recruited two LEAP members to design the study and conduct the interviews, supported by the Primrose PPI co-ordinator and experienced McPin senior researcher.
User-led interviews to explore decision-making among potential mental health research participants
The study sought to understand how mental health service users living with SMI approach decision-making with regard to being offered the opportunity to join a research trial. The study used the Primrose trial as an example. It also explored individuals’ views on and experiences with research and evaluation activities more generally, considering the impact of these views on specific decisions to take part in trials.
In-depth interviews took place with 12 people who met the criteria for entry into the Primrose trial and were recruited through voluntary sector networks. Interviews were conducted by a peer researcher, and the McPin Foundation team, as well as the Primrose programme manager supported analysis and write-up of findings. The study received research ethics approval from the University College London Ethics Committee (project ID 6357/001).
The findings from this study are currently being analysed.
Reflections from the Lived Experience Advisory Panel members
I felt very involved and included as a member of the LEAP from the beginning. It was good that there was a mix of lived experience of mental distress and carers. We had discussions and I felt my views and everyone’s was valued. We provided feedback on plans, documentation, leaflets and the manual to be used by nurses. The study kept us up to date, especially in the early years of the study. The PPI co-ordinator was excellent at this, along with making sure we all had opportunities to get involved. The group worked well together and were very supportive, along with not being afraid to give their views. I think a lot can be learnt regarding PPI from this study as I felt a very good example of co-production in the true sense. Even with the change from Rethink Mental Illness to McPin Foundation this continued, especially as the PPI co-ordinator moved on to work at McPin Foundation. At all the meetings I could bring my lived experience of taking medication that affected my weight, that left me at greater risk of cardiovascular disease. I could share how I struggled to tackle this, especially the effect that the medication had on me. This experience I feel helped especially when information resources were being developed, as getting the right wording for research participants was so important. I wanted it to be encouraging and supportive, but not telling people, as at the end of the day any person needed to take the decision that they wanted to achieve certain goals themselves. I know only too well how hard is it to make those changes, but with some intervention you never forget what you have learnt and can always return to it further down the line. It would be good if the GP surgeries involved undertook some follow-ups with patients who were part of this programme.
Jackie, LEAP experiences
I had recently joined Rethink Mental Illness as a member, and tentatively answered an online piece regarding research into cardiovascular issues for people with serious mental illness. As I had high cholesterol and was at the time trying to avoid being prescribed statins, I thought maybe I had something to offer the team. I ended up in the main LEAP and intervention development group. We achieved lots in each meeting. I remember the first session we decided on what we considered was an appropriate logo for the project.
We had a co-ordinator who kept us informed of progress in the study. We would meet, were encouraged to express our feelings, doubts and fears; and also put forward helpful ideas to further the research. It may be that the researchers found our input more helpful than we ourselves knew: as we were just mentioning day-to-day experiences that we come across all the time and more or less take for granted (e.g. discrimination, being patronised, or diagnostic overshadowing). We agreed that a gentle approach with a health-care professional at the annual cardiovascular review at the surgery might work well: regular meetings with the same health-care professional, focusing on one issue at a time to address rather than a blanket approach of tackling all the issues, like smoking, dieting, exercise, all at once. And this approach was taken up by the team, into the project and was delivered to patients at GP surgeries across England.
Susie, LEAP experiences
What was learnt about patient and public involvement
Primrose had a well-resourced and comprehensive PPI programme, but not all elements worked as well as we would have liked. There were several challenges during the course of the programme.
-
First, we lost our PPI co-applicant lead early in the programme. This meant that we did not have consistency of input from design through to dissemination.
-
Our PPI co-ordinator became unwell during the study and was off work for periods of time. We did not have immediate contingency plans to replace their input into the development work, particularly the cofacilitation of the service user focus groups. We recruited a service user consultant with experience of running focus groups who was able to cofacilitate two of the service user groups. They continued to co-ordinate LEAP meetings and obtain ad hoc feedback on specific pieces of work including service use input into the development of the health economics questionnaire for the trial, piloting the intervention manual and feedback on patient invitation letters. They continued to update the LEAP members and encourage communication through their blog.
-
There were organisational changes during the study with the PPI co-ordinator at Rethink Mental Illness moving to the McPin Foundation part way through the programme as Rethink Mental Illness closed its research function.
-
During the trial period there was limited work for the LEAP to feedback on, which resulted in less frequent communication. Towards the end of the trial, a smaller LEAP was reformed and met three times to explore the study findings and offer insight and recommendations from the service user and carer perspective on interpreting the results of the trial and qualitative work.
Acknowledgements
Contributions of authors
Professor David Osborn (Professor of Psychiatric Epidemiology) was the chief investigator.
Ms Alexandra Burton (Research Associate/Programme Manager) managed the programme of work.
Dr Kate Walters (Reader in Primary Care and Epidemiology) was the deputy chief investigator.
Dr Lou Atkins (Senior Teaching Fellow in Behaviour Change) led on the systematic review, development of the fidelity protocol and delivered the trial intervention training.
Professor Thomas Barnes (Professor of Clinical Psychiatry) and Professor Thomas Craig (Professor of Community and Social Psychiatry) provided clinical and trial expertise to the intervention development and trial.
Dr Ruth Blackburn (Research Associate in Epidemiology and Statistics) led the statin prescribing and effectiveness database work.
Dr Hazel Gilbert (Honorary Principal Research Associate in Health Psychology) provided health psychology expertise on smoking cessation within the intervention development and trial.
Dr Ben Gray (Senior Research Officer, Service User Expert) and Dr Vanessa Pinfold (Research Director) led on all aspects of PPI within the programme.
Dr Sarah Hardoon (Senior Research Associate in Epidemiology and Statistics) carried out statistical analysis for the risk score work supervised by Professor Irene Petersen (Reader in Epidemiology and Statistics).
Ms Samira Heinkel (Trial Co-ordinator) co-ordinated the trial and led on the fidelity analysis.
Professor Richard Holt (Professor in Diabetes and Endocrinology), Professor Michael King (Professor of Primary Care Psychiatry), Professor Irwin Nazareth (Professor of Primary Care and Population Science) and Professor Robert Peveler (Professor of Liaison Psychiatry) provided clinical and trial expertise to the intervention development, systematic review and trial.
Ms Rachael Hunter (Principal Research Associate in Health Economics) led on the cost-effectiveness analysis for the trial and supervised the economic evaluation of the risk score work.
Ms Claire Johnston (Honorary Clinical Professor of Nursing) and Dr Judy Leibowitz (Consultant Clinical Psychologist) provided clinical expertise on the development of the intervention.
Dr Louise Marston (Senior Research Associate in Statistics) led on the statistical analysis of trial data supervised by Professor Rumana Omar (Professor of Medical Statistics).
Professor Susan Michie (Professor of Health Psychology) provided expertise on behaviour change theory in the intervention development and trial.
Professor Richard Morris (Professor in Medical Statistics) provided expert advice on the risk score work.
Professor Steve Morris (Professor of Health Economics) advised on the economic evaluation of the trial.
Dr Fiona Stevenson (Reader in Medical Sociology) provided qualitative expertise on the focus group work and trial.
Dr Ella Zomer (Research Fellow in Health Economics) led on the economic evaluation of the risk score work.
All authors contributed to the development of the trial intervention and training programme, and provided a critical review and final approval of the report.
Professor David Osborn is supported by the University College London Hospital NIHR Biomedical Research Centre and he was also in part supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Barts Health NHS Trust.
Contributions of others
We would like to thank Dr Sheila Hardy and Ms Vanessa Robinson for their input into the development of the intervention manual and training programme and for delivering the intervention training alongside members of the research team. We would also like to thank Ms Tayla McCloud for her assistance with the fidelity analysis and patient follow-ups, Mr Stuart Brownings for carrying out patient follow-ups in the trial, Dr Maxine Howard and Vinca Tang for their contribution to the systematic review searches and write-up and Ms Nishat Parwin for administrative support to the trial. We would also like to thank Professor Simon Gilbody for chairing our Trial Steering Group, and Ms Jackie Hardy, Dr Fiona Gaughran and Mr Mark Mullee for their advice as Trial Steering Group members. We thank the LEAP for their valuable contributions throughout the programme of work and the CRN network research nurses and researchers for supporting the recruitment and follow-up of trial patients. Finally, we would like to thank all GP practice staff and patients who participated in the focus groups and the trial.
Data-sharing statement
Requests for access to available data should be addressed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, the PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 2007;64:19-28. https://doi.org/10.1001/archpsyc.64.1.19.
- Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334-41. https://doi.org/10.1001/jamapsychiatry.2014.2502.
- Osborn DJ, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s general practice research database. Arch Gen Psychiatry 2007;64:242-9. https://doi.org/10.1001/archpsyc.64.2.242.
- Lawrence DM, Holman CD, Jablensky AV, Hobbs MS. Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980–1998. Br J Psychiatry 2003;182:31-6. https://doi.org/10.1192/bjp.182.1.31.
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005;150:1115-21. https://doi.org/10.1016/j.ahj.2005.02.007.
- Osborn D, Levy G, Nazareth I, King M. Suicide and severe mental illnesses. Cohort study within the UK general practice research database. Schizophr Res 2008;99:134-8. https://doi.org/10.1016/j.schres.2007.11.025.
- Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry 2017;211:175-81. https://doi.org/10.1192/bjp.bp.117.202606.
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172-81. https://doi.org/10.1001/jamapsychiatry.2015.1737.
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 2014;71:1350-63. https://doi.org/10.1001/jamapsychiatry.2014.1314.
- Osborn DP, Nazareth I, King MB. Risk for coronary heart disease in people with severe mental illness: cross-sectional comparative study in primary care. Br J Psychiatry 2006;188:271-7. https://doi.org/10.1192/bjp.bp.104.008060.
- Osborn DP, Wright CA, Levy G, King MB, Deo R, Nazareth I. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry 2008;8. https://doi.org/10.1186/1471-244X-8-84.
- Nenke MA, Hahn LA, Thompson CH, Liu D, Galletly CA. Psychosis and cardiovascular disease: is diet the missing link?. Schizophr Res 2015;161:465-70. https://doi.org/10.1016/j.schres.2014.12.012.
- Osborn DP, Nazareth I, King MB. Physical activity, dietary habits and coronary heart disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol 2007;42:787-93. https://doi.org/10.1007/s00127-007-0247-3.
- Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med 2011;17:97-107. https://doi.org/10.1016/j.molmed.2010.10.010.
- Thakore JH. Metabolic syndrome and schizophrenia. Br J Psychiatr 2005;186:455-6. https://doi.org/10.1192/bjp.186.6.455.
- Kontopantelis E, Olier I, Planner C, Reeves D, Ashcroft DM, Gask L, et al. Primary care consultation rates among people with and without severe mental illness: a UK cohort study using the Clinical Practice Research Datalink. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008650.
- Psychosis and Schizophrenia in Adults: Prevention and Management. NICE Guideline (CG178). London: NICE; 2014.
- Bipolar Disorder: Assessment and Management. NICE Guideline (CG185). London: NICE; 2016.
- Barnes TR, Paton C, Hancock E, Cavanagh MR, Taylor D, Lelliott P. UK Prescribing Observatory for Mental Health . Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: a quality improvement programme. Acta Psychiatr Scand 2008;118:26-33. https://doi.org/10.1111/j.1600-0447.2008.01203.x.
- The NHS Conferderation (employers) . 2016/17/General/Medical/Services/(GMS)/Contract/Quality/and/Outcomes/Framework/(QOF) 2016. www.nhsemployers.org/∼/media/Employers/Documents/Primary%20care%20contracts/QOF/2016-17/2016-17%20QOF%20guidance%20documents.pdf (accessed September 2018).
- Osborn DP, King MB, Nazareth I. Participation in screening for cardiovascular risk by people with schizophrenia or similar mental illnesses: cross sectional study in general practice. BMJ 2003;326:1122-3. https://doi.org/10.1136/bmj.326.7399.1122.
- Hardy S, Gray R. Is the use of an invitation letter effective in prompting patients with severe mental illness to attend a primary care physical health check?. Prim Health Care Res Dev 2012;13:347-52. https://doi.org/10.1017/S1463423612000023.
- Roberts L, Roalfe A, Wilson S, Lester H. Physical health care of patients with schizophrenia in primary care: a comparative study. Fam Pract 2007;24:34-40. https://doi.org/10.1093/fampra/cml054.
- Martin JL, Lowrie R, McConnachie A, McLean G, Mair F, Mercer SW, et al. Physical health indicators in major mental illness: analysis of QOF data across UK general practice. Br J Gen Pract 2014;64:e649-56. https://doi.org/10.3399/bjgp14X681829.
- Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. NICE Guideline (CG181). London: NICE; 2016.
- Brindle P, May M, Gill P, Cappuccio F, D’Agostino R, Fischbacher C, et al. Primary prevention of cardiovascular disease: a web-based risk score for seven British black and minority ethnic groups. Heart 2006;92:1595-602. https://doi.org/10.1136/hrt.2006.092346.
- De Hert M, Kalnicka D, van Winkel R, Wampers M, Hanssens L, Van Eyck D, et al. Treatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2006;67:1889-96. https://doi.org/10.4088/JCP.v67n1208.
- Vincenzi B, Stock S, Borba CP, Cleary SM, Oppenheim CE, Petruzzi LJ, et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res 2014;159:395-403. https://doi.org/10.1016/j.schres.2014.08.021.
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. Am J Psychiatry 2006;163:1934-42. https://doi.org/10.1176/ajp.2006.163.11.1934.
- Naslund JA, Aschbrenner KA, Scherer EA, Pratt SI, Wolfe RS, Bartels SJ. Lifestyle intervention for people with severe obesity and serious mental illness. Am J Prev Med 2016;50:145-53. https://doi.org/10.1016/j.amepre.2015.07.012.
- Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD005148.pub2.
- Osborn DP, Nazareth I, Wright CA, King MB. Impact of a nurse-led intervention to improve screening for cardiovascular risk factors in people with severe mental illnesses. Phase-two cluster randomised feasibility trial of community mental health teams. BMC Health Serv Res 2010;10. https://doi.org/10.1186/1472-6963-10-61.
- Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0112276.
- Baxter AJ, Harris MG, Khatib Y, Brugha TS, Bien H, Bhui K. Reducing excess mortality due to chronic disease in people with severe mental illness: meta-review of health interventions. Br J Psychiatry 2016;208:322-9. https://doi.org/10.1192/bjp.bp.115.163170.
- Bauer IE, Gálvez JF, Hamilton JE, Balanzá-Martínez V, Zunta-Soares GB, Soares JC, et al. Lifestyle interventions targeting dietary habits and exercise in bipolar disorder: a systematic review. J Psychiatr Res 2016;74:1-7. https://doi.org/10.1016/j.jpsychires.2015.12.006.
- Kilbourne AM, Barbaresso MM, Lai Z, Nord KM, Bramlet M, Goodrich DE, et al. Improving physical health in patients with chronic mental disorders: twelve-month results from a randomized controlled collaborative care trial. J Clin Psychiatry 2017;78:129-37. https://doi.org/10.4088/JCP.15m10301.
- Osborn DP, Hardoon S, Omar RZ, Holt RI, King M, Larsen J, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry 2015;72:143-51. https://doi.org/10.1001/jamapsychiatry.2014.2133.
- Shiers DE, Rafi I, Cooper SJ, Holt RIG. 2014 Update (With Acknowledgement to the Late Helen Lester for her Contribution to the Original 2012 Version) Positive Cardiometabolic Health Resource: An Intervention Framework for Patients with Psychosis and Schizophrenia. London: Royal College of Psychiatrists; 2014.
- Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016;30:717-48. https://doi.org/10.1177/0269881116645254.
- Zomer E, Osborn D, Nazareth I, Blackburn R, Burton A, Hardoon S, et al. Effectiveness and cost-effectiveness of a cardiovascular risk prediction algorithm for people with severe mental illness (PRIMROSE). BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-018181.
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
- Wilson PW, D’Agostino R, Bhatt DL, Eagle K, Pencina MJ, Smith SC, et al. An international model to predict recurrent cardiovascular disease. Am J Med 2012;125:695-703.e1. https://doi.org/10.1016/j.amjmed.2012.01.014.
- Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD004816.pub5.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Process and Methods Guides: Guide to the Methods of Technology Appraisal. NICE Guideline. London: NICE; 2013.
- Briggs A, Wild D, Lees M, Reaney M, Dursun S, Parry D, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes 2008;6. https://doi.org/10.1186/1477-7525-6-105.
- Briggs A CK, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Burton A, Osborn D, Atkins L, Michie S, Gray B, Stevenson F, et al. Lowering Cardiovascular Disease Risk for People with Severe Mental Illnesses in Primary Care: A Focus Group Study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0136603.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Burton A, Walters K, Atkins L, Howard M, Michie S, Peveler R, et al. Barriers, facilitators, and effective interventions for lowering cardiovascular disease risk in people with severe mental illnesses: evidence from a systematic review and focus group study. Lancet 2016;388. www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(16)32266-8.pdf (accessed September 2018).
- Banham L, Gilbody S. Smoking cessation in severe mental illness: what works?. Addiction 2010;105:1176-89. https://doi.org/10.1111/j.1360-0443.2010.02946.x.
- Happell B, Davies C, Scott D. Health behaviour interventions to improve physical health in individuals diagnosed with a mental illness: a systematic review. Int J Ment Health Nurs 2012;21:236-47. https://doi.org/10.1111/j.1447-0349.2012.00816.x.
- Tolliver BK, McRae AL, Sonne SC, Brady KT. Safety and tolerability of acamprosate in alcohol-dependent individuals with bipolar disorder: an open-label pilot study. Addict Disord Their Treat 2009;8. https://doi.org/10.1097/ADT.0b013e31816719df.
- Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull 2006;32:644-54. https://doi.org/10.1093/schbul/sbl010.
- McElroy SL, Frye MA, Altshuler LL, Suppes T, Hellemann G, Black D, et al. A 24-week, randomized, controlled trial of adjunctive sibutramine versus topiramate in the treatment of weight gain in overweight or obese patients with bipolar disorders. Bipolar Disord 2007;9:426-34. https://doi.org/10.1111/j.1399-5618.2007.00488.x.
- Roy Chengappa KN, Schwarzman LK, Hulihan JF, Xiang J, Rosenthal NR. Clinical Affairs Product Support Study-168 Investigators . Adjunctive topiramate therapy in patients receiving a mood stabilizer for bipolar I disorder: a randomized, placebo-controlled trial. J Clin Psychiatry 2006;67:1698-706. https://doi.org/10.4088/JCP.v67n1105.
- McElroy SL, Winstanley E, Mori N, Martens B, McCoy J, Moeller D, et al. A randomized, placebo-controlled study of zonisamide to prevent olanzapine-associated weight gain. J Clin Psychopharmacol 2012;32:165-72. https://doi.org/10.1097/JCP.0b013e3182488758.
- Ghanizadeh A, Nikseresht MS, Sahraian A. The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: a randomized, double-blind, placebo-controlled clinical trial. Schizophr Res 2013;147:110-15. https://doi.org/10.1016/j.schres.2013.03.021.
- Ranjbar F, Ghanepour A, Sadeghi-Bazargani H, Asadlo M, Alizadeh A. The effect of ranitidine on olanzapine-induced weight gain. Biomed Res Int 2013;2013. https://doi.org/10.1155/2013/639391.
- Afshar H, Roohafza H, Mousavi G, Golchin S, Toghianifar N, Sadeghi M, et al. Topiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol 2009;23:157-62. https://doi.org/10.1177/0269881108089816.
- Álvarez-Jiménez M, Martínez-García O, Pérez-Iglesias R, Ramírez ML, Vázquez-Barquero JL, Crespo-Facorro B. Prevention of antipsychotic-induced weight gain with early behavioural intervention in first-episode psychosis: 2-year results of a randomized controlled trial. Schizophr Res 2010;116:16-9. https://doi.org/10.1016/j.schres.2009.10.012.
- Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008;299:185-93. https://doi.org/10.1001/jama.2007.56-b.
- Ellinger LK, Ipema HJ, Stachnik JM. Efficacy of metformin and topiramate in prevention and treatment of second-generation antipsychotic-induced weight gain. Ann Pharmacother 2010;44:668-79. https://doi.org/10.1345/aph.1M550.
- Ball MP, Warren KR, Feldman S, McMahon RP, Kelly DL, Buchanan RW. Placebo-controlled trial of atomoxetine for weight reduction in people with schizophrenia treated with clozapine or olanzapine. Clin Schizophr Relat Psychoses 2011;5:17-25. https://doi.org/10.3371/CSRP.5.1.3.
- Borba CP, Fan X, Copeland PM, Paiva A, Freudenreich O, Henderson DC. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol 2011;31:653-8. https://doi.org/10.1097/JCP.0b013e31822bb573.
- Roy Chengappa K, Kupfer DJ, Parepally H, John V, Basu R, Buttenfield J, et al. A placebo-controlled, random-assignment, parallel-group pilot study of adjunctive topiramate for patients with schizoaffective disorder, bipolar type. Bipolar Disord 2007;9:609-17. https://doi.org/10.1111/j.1399-5618.2007.00506.x.
- Elmslie JL, Porter RJ, Joyce PR, Hunt PJ, Mann JI. Carnitine does not improve weight loss outcomes in valproate-treated bipolar patients consuming an energy-restricted, low-fat diet. Bipolar Disord 2006;8:503-7. https://doi.org/10.1111/j.1399-5618.2006.00345.x.
- Henderson DC, Freudenreich O, Borba CP, Wang X, Copeland PM, Macklin E, et al. Effects of modafinil on weight, glucose and lipid metabolism in clozapine-treated patients with schizophrenia. Schizophr Res 2011;130:53-6. https://doi.org/10.1016/j.schres.2011.04.009.
- Narula PK, Rehan HS, Unni KE, Gupta N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophr Res 2010;118:218-23. https://doi.org/10.1016/j.schres.2010.02.001.
- Jarskog LF, Hamer RM, Catellier DJ, Stewart DD, Lavange L, Ray N, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry 2013;170:1032-40. https://doi.org/10.1176/appi.ajp.2013.12010127.
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Weizman R, Weizman A. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology 2013;226:615-22. https://doi.org/10.1007/s00213-012-2935-2.
- Smith RC, Jin H, Li C, Bark N, Shekhar A, Dwivedi S, et al. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophr Res 2013;143:18-24. https://doi.org/10.1016/j.schres.2012.10.023.
- Vancampfort D, Knapen J, De Hert M, van Winkel R, Deckx S, Maurissen K, et al. Cardiometabolic effects of physical activity interventions for people with schizophrenia. Phys Ther Rev 2009;14:388-98. https://doi.org/10.1179/108331909X12540993898053.
- Cabassa LJ, Ezell JM, Lewis-Fernández R. Lifestyle interventions for adults with serious mental illness: a systematic literature review. Psychiatr Serv 2010;61:774-82. https://doi.org/10.1176/ps.2010.61.8.774.
- Verhaeghe N, De Maeseneer J, Maes L, Van Heeringen C, Annemans L. Effectiveness and cost-effectiveness of lifestyle interventions on physical activity and eating habits in persons with severe mental disorders: a systematic review. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-28.
- Gabriele JM, Dubbert PM, Reeves RR. Efficacy of behavioural interventions in managing atypical antipsychotic weight gain. Obes Rev 2009;10:442-55. https://doi.org/10.1111/j.1467-789X.2009.00570.x.
- Álvarez-Jiménez M, Hetrick SE, González-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2008;193:101-7. https://doi.org/10.1192/bjp.bp.107.042853.
- Cordes J, Thunker J, Regenbrecht G, Zielasek J, Correll CU, Schmidt-Kraepelin C, et al. Can an early weight management program (WMP) prevent olanzapine (OLZ)-induced disturbances in body weight, blood glucose and lipid metabolism? Twenty-four- and 48-week results from a 6-month randomized trial. World J Biol Psychiatr 2011;11:1-13.
- Iglesias-García C, Toimil-Iglesias A, Alonso-Villa MJ. Pilot study of the efficacy of an educational programme to reduce weight, on overweight and obese patients with chronic stable schizophrenia. J Psychiatr Ment Health Nurs 2010;17:849-51. https://doi.org/10.1111/j.1365-2850.2010.01590.x.
- Marzolini S, Jensen B, Melville P. Feasibility and effects of a group-based resistance and aerobic exercise program for individuals with severe schizophrenia: a multidisciplinary approach. Ment Health Phys Act 2009;2:29-36. https://doi.org/10.1016/j.mhpa.2008.11.001.
- McCreadie RG, Kelly C, Connolly M, Williams S, Baxter G, Lean M, et al. Dietary improvement in people with schizophrenia: randomised controlled trial. Br J Psychiatry 2005;187:346-51. https://doi.org/10.1192/bjp.187.4.346.
- Methapatara W, Srisurapanont M. Pedometer walking plus motivational interviewing program for Thai schizophrenic patients with obesity or overweight: a 12-week, randomized, controlled trial. Psychiatry Clin Neurosci 2011;65:374-80. https://doi.org/10.1111/j.1440-1819.2011.02225.x.
- Usher K, Park T, Foster K, Buettner P. A randomized controlled trial undertaken to test a nurse-led weight management and exercise intervention designed for people with serious mental illness who take second generation antipsychotics. J Adv Nurs 2013;69:1539-48. https://doi.org/10.1111/jan.12012.
- Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013;368:1594-602. https://doi.org/10.1056/NEJMoa1214530.
- Ratliff JC, Palmese LB, Tonizzo KM, Chwastiak L, Tek C. Contingency management for the treatment of antipsychotic-induced weight gain: a randomized controlled pilot study. Obes Facts 2012;5:919-27. https://doi.org/10.1159/000345975.
- Scocco P, Longo R, Caon F. Weight change in treatment with olanzapine and a psychoeducational approach. Eat Behav 2006;7:115-24. https://doi.org/10.1016/j.eatbeh.2005.08.003.
- Jean-Baptiste M, Tek C, Liskov E, Chakunta UR, Nicholls S, Hassan AQ, et al. A pilot study of a weight management program with food provision in schizophrenia. Schizophr Res 2007;96:198-205. https://doi.org/10.1016/j.schres.2007.05.022.
- Ferron JC, Alterman AI, McHugo GJ, Brunette MF, Drake RE. A review of research on smoking cessation interventions for adults with schizophrenia spectrum disorders. Ment Health Subst Use 2009;2:64-79. https://doi.org/10.1080/17523280802593327.
- Chou KR, Chen R, Lee JF, Ku CH, Lu RB. The effectiveness of nicotine-patch therapy for smoking cessation in patients with schizophrenia. Int J Nurs Stud 2004;41:321-30. https://doi.org/10.1016/j.ijnurstu.2003.07.001.
- Petrakis IL, O’Malley S, Rounsaville B, Poling J, McHugh-Strong C, Krystal JH. VA Naltrexone Study Collaboration Group . Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. Psychopharmacology 2004;172:291-7. https://doi.org/10.1007/s00213-003-1658-9.
- Brown ES, Carmody TJ, Schmitz JM, Caetano R, Adinoff B, Swann AC, et al. A randomized, double-blind, placebo-controlled pilot study of naltrexone in outpatients with bipolar disorder and alcohol dependence. Alcohol Clin Exp Res 2009;33:1863-9. https://doi.org/10.1111/j.1530-0277.2009.01024.x.
- Ralevski E, O’Brien E, Jane JS, Dwan R, Dean E, Edens E, et al. Treatment With Acamprosate in Patients With Schizophrenia Spectrum Disorders and Comorbid Alcohol Dependence. J Dual Diagn 2011;7:64-73. https://doi.org/10.1080/15504263.2011.569440.
- Cleary M, Hunt G, Matheson S, Siegfried N, Walter G. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD001088.pub2.
- Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD007253.pub3.
- Bonfioli E, Berti L, Goss C, Muraro F, Burti L. Health promotion lifestyle interventions for weight management in psychosis: a systematic review and meta-analysis of randomised controlled trials. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-78.
- Gierisch JM, Nieuwsma JA, Bradford DW, Wilder CM, Mann-Wrobel MC, McBroom AJ, et al. Pharmacologic and behavioral interventions to improve cardiovascular risk factors in adults with serious mental illness: a systematic review and meta-analysis. J Clin Psychiatry 2014;75:e424-40. https://doi.org/10.4088/JCP.13r08558.
- Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology 2010;35:1520-30. https://doi.org/10.1038/npp.2010.21.
- Praharaj SK, Jana AK, Goyal N, Sinha VK. Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis. Br J Clin Pharmacol 2011;71:377-82. https://doi.org/10.1111/j.1365-2125.2010.03783.x.
- Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD001088.pub3.
- Blackburn R, Osborn D, Walters K, Nazareth I, Petersen I. Statin prescribing for prevention of cardiovascular disease amongst people with severe mental illness: cohort study in UK primary care. Schizophr Res 2018;192:219-25. https://doi.org/10.1016/j.schres.2017.05.028.
- Blackburn R, Osborn D, Walters K, Falcaro M, Nazareth I, Petersen I. Statin prescribing for people with severe mental illnesses: a staggered cohort study of ‘real-world’ impacts. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013154.
- Action on Cardiovascular Disease: Getting Serious about Prevention. London: Public Health England; 2016.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Gray B, Basset T, Larsen J, Faulkner A. Third sector facilitation of lived experience in research: a case study of service user and carer involvement in the PRIMROSE project. J Ment Health Train Educ Pract 2013;8:141-51. https://doi.org/10.1108/JMHTEP-03-2013-0008.
- Osborn D, Burton A, Walters K, Nazareth I, Heinkel S, Atkins L, et al. Evaluating the clinical and cost effectiveness of a behaviour change intervention for lowering cardiovascular disease risk for people with severe mental illnesses in primary care (PRIMROSE study): study protocol for a cluster randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1176-9.
- Osborn D, Burton A, Hunter R, Marston L, Atkins L, Barnes T, et al. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatr 2018;5:145-54. https://doi.org/10.1016/S2215-0366(18)30007-5.
- Lorencatto F, West R, Christopherson C, Michie S. Assessing fidelity of delivery of smoking cessation behavioural support in practice. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-40.
- Hardeman W, Michie S, Fanshawe T, Prevost AT, Mcloughlin K, Kinmonth AL. Fidelity of delivery of a physical activity intervention: predictors and consequences. Psychol Health 2008;23:11-24. https://doi.org/10.1080/08870440701615948.
- Harting J, van Assema P, van der Molen HT, Ambergen T, de Vries NK. Quality assessment of health counseling: performance of health advisors in cardiovascular prevention. Patient Educ Couns 2004;54:107-18. https://doi.org/10.1016/S0738-3991(03)00194-0.
- Barnes TR, Paton C, Cavanagh MR, Hancock E, Taylor DM. UK Prescribing Observatory for Mental Health . A UK audit of screening for the metabolic side effects of antipsychotics in community patients. Schizophr Bull 2007;33:1397-403. https://doi.org/10.1093/schbul/sbm038.
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52-77. https://doi.org/10.1002/j.2051-5545.2011.tb00014.x.
- Gubbins A, Lally J, McDonald C. Metabolic syndrome in patients attending psychiatric day centres: prevalence and associations. Psychiatrist 2012;36:326-31. https://doi.org/10.1192/pb.bp.111.037374.
- Lack D, Holt RI, Baldwin DS. Poor monitoring of physical health in patients referred to a mood disorders service. Ther Adv Psychopharmacol 2015;5:22-5. https://doi.org/10.1177/2045125314560734.
- Hanssens L, De Hert M, Kalnicka D, van Winkel R, Wampers M, Van Eyck D, et al. Pharmacological treatment of severe dyslipidaemia in patients with schizophrenia. Int Clin Psychopharmacol 2007;22:43-9.
- Landry P, Dimitri E, Tessier S, Legare N. Efficacy of lipid-lowering medications in patients treated with clozapine: a naturalistic study. J Clin Psychopharmacol 2008;28:348-9. https://doi.org/10.1097/JCP.0b013e3181727592.
- Ojala K, Repo-Tiihonen E, Tiihonen J, Niskanen L. Statins are effective in treating dyslipidemia among psychiatric patients using second-generation antipsychotic agents. J Psychopharmacol 2008;22:33-8. https://doi.org/10.1177/0269881107077815.
- Blackburn RM. Exploring the Effectiveness of Statins for Primary Prevention of Cardiovascular Disease in People with Severe Mental Illness. London: University College London; 2016.
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. https://doi.org/10.1056/NEJMoa1003955.
- Chwastiak L, Fortney J. Learning to integrate cardiometabolic care in serious mental illness. Am J Psychiatry 2017;174:199-201. https://doi.org/10.1176/appi.ajp.2016.16121399.
- Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017;357. https://doi.org/10.1136/bmj.j2099.
- Osborn D, Walters K. Re: Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017;357.
- Staley K. A Series of Case Studies Illustrating the Impact of Service User and Carer Involvement on Research. London: NIHR Clinical Research Network: Mental Health; 2013.
- Parks J, Svendsen D, Singer P, Foti ME. Morbidity and Mortality in People with Serious Mental Illness. Alexandria, VA: National Association of State Mental Health Program Directors Medical Directors Council; 2006.
- Methods for the Development of NICE Public Health Guidance. London: NICE; 2009.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Management of Schizophrenia. Edinburgh: SIGN; 2013.
- Michie S, Abraham C. Advancing the science of behaviour change: a plea for scientific reporting. Addiction 2008;103:1409-10. https://doi.org/10.1111/j.1360-0443.2008.02291.x.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol 2005;73:852-60. https://doi.org/10.1037/0022-006X.73.5.852.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Perepletchikova F, Treat TA, Kazdin AE. Treatment integrity in psychotherapy research: analysis of the studies and examination of the associated factors. J Consult Clin Psychol 2007;75:829-41. https://doi.org/10.1037/0022-006X.75.6.829.
- Michie S. What works and how? Designing more effective interventions needs answers to both questions. Addiction 2008;103:886-7. https://doi.org/10.1111/j.1360-0443.2007.02112.x.
- Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour change techniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol Assess 2015;19. https://doi.org/10.3310/hta19990.
- Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011;36:315-19. https://doi.org/10.1016/j.addbeh.2010.11.016.
- Soltani H, Arden MA, Duxbury AM, Fair FJ. An analysis of behaviour change techniques used in a sample of gestational weight management trials. J Pregnancy 2016;2016. https://doi.org/10.1155/2016/1085916.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. https://doi.org/10.1080/08870446.2010.540664.
- Noell G, Gresham F, Gansle K. Does treatment integrity matter? A preliminary investigation of instructional implementation and mathematical performance. J Behav Educ 2002;11:51-67. https://doi.org/10.1023/A:1014385321849.
- Holcombe A, Wolery M, Snyder E. Effects of two levels of procedural fidelity with constant time delay on children’s learning. J Behav Educ 1994;4:49-73. https://doi.org/10.1007/BF01560509.
- Sweeney-Magee M, Kale D, Galton S, Hamill A, Gilbert H. Assessing the fidelity of delivery of an intervention to increase attendance at the English Stop Smoking Services. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0498-z.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276-82. https://doi.org/10.11613/BM.2012.031.
- Schlosser R. On the importance of being earnest about treatment integrity. Augment Altern Commun 2002;18:36-44. https://doi.org/10.1080/aac.18.1.36.44.
- Gresham FM, Franklin RD, Allison DB, Gorman BS. Design and Analysis of Single-case Research. Mahwah, NJ: Lawrence Erlbaum Associates; 1996.
- Burke LE, Fair J. Promoting prevention. Skills sets and attributes of health care providers who deliver behavioural interventions. J Cardiovasc Nurs 2003;18:256-66. https://doi.org/10.1097/00005082-200309000-00004.
Appendix 1 Systematic review of pharmacological and behavioural interventions for reducing cardiovascular disease risk in people with severe mental illnesses
This section presents the full report of the methods and results of a systematic review that was conducted to inform the development of Primrose intervention and training programme.
Summary
Background
People with SMI are at increased risk of developing CVD and die at a younger age than the general population. This study evaluated and synthesised evidence from published systematic reviews and individual RCTs on the effectiveness of pharmacological and behavioural interventions for reducing modifiable CVD risk factors in people with SMI.
Methods
We searched The Cochrane Library for existing systematic reviews. We then searched the Cochrane Schizophrenia and Cochrane Depression, Anxiety and Neurosis Group Trial Registers between 1966 and 2014 for additional RCTs not included in the identified reviews. We searched for interventions to manage levels of cholesterol, diabetes mellitus, hypertension, weight, smoking and alcohol consumption.
Findings
Fifteen systematic reviews and 28 additional RCTs were included in the review, from 11,028 references. The synthesised data demonstrated good evidence of effective pharmacological and behavioural interventions for weight management and some evidence that combined pharmacological and behavioural interventions might be more effective than either alone. There was good evidence that pharmacological interventions were effective for smoking cessation, or reducing smoking, and some evidence for behavioural or combined interventions. Three studies reported effective interventions to reduce alcohol misuse. No studies were found on effective interventions targeting levels of cholesterol, diabetes mellitus or hypertension in SMI.
Conclusion
There is evidence that CVD risk attributable to weight and smoking can be managed effectively in SMI, using pharmacological and behavioural approaches. Future research should determine the effectiveness of interventions to manage cholesterol levels, diabetes mellitus, hypertension and alcohol misuse in this population.
Introduction
People with SMI die at a significantly younger age than the general population. 124 The majority of these premature deaths (66%) are due to potentially preventable or treatable CVD. 3,5
Increased mortality risk for CVD is seen in people with SMI compared with the general population. Those aged 50–75 years have a twofold increased risk of death from CVD, rising to threefold for those < 50 years old. 3 There is evidence that CVD risk screening for people with SMI does not meet international or national guidelines. 23,111
Increased risk for CVD is attributable to metabolic and anthropomorphic effects of antipsychotic medication as well as lifestyle factors including poor diet, low levels of physical activity, smoking and, in some cases, higher than recommended levels of alcohol consumption.
The challenge is to design and deliver effective interventions to prevent CVD in this group and this requires synthesis of evidence about modifying the different risk factors that contribute to CVD in SMI. Our overarching aim was to identify the best international evidence regarding effective CVD risk management for people with SMI to allow clinicians, policy-makers and researchers to meet the challenge of implementing and appraising CVD risk reduction strategies.
In primary care, multiple risk factors for CVD are often addressed in one consultation but existing reviews of pharmacological or behavioural interventions to reduce CVD risk in people with SMI tend to be limited to one or two contributing risk factors/behaviours (e.g. smoking52,95 diet and physical activity96–99 or alcohol use). 100 Furthermore much existing research has largely been generated in secondary care rather than primary care where, in the UK, evidence-based guidelines suggest CVD risk in SMI should be managed. 17 One review has synthesised evidence across CVD risk factors up to 2010. 53 We aimed to update and improve on this review by including (1) more recently published studies, (2) existing systematic reviews and meta-analyses in addition to individual studies and (3) including only the highest-quality studies using a randomised experimental design. This review informed the development of a primary care-based intervention to reduce CVD risk. 106
Methods
We searched for published systematic reviews and additional RCTs that were not included in existing systematic reviews.
Inclusion/exclusion criteria
Studies were included if they met the following criteria: (1) they used a randomised controlled design, (2) ≥ 50% of participants were adults with a diagnosis of schizophrenia, bipolar disorder or other non-organic psychotic disorders including schizoaffective disorder, (3) they evaluated interventions aimed at managing levels of cholesterol, diabetes mellitus, hypertension, weight, smoking and/or alcohol consumption, (4) title and abstract were written in English (papers were only excluded on the basis of being written in a non-English language where translation of the full text was not possible) and (5) were published between 1966 and 2014.
Search strategy
We conducted the search strategy in five stages in the following order: (1) we searched the Cochrane group publications lists and Cochrane Library database for systematic reviews, (2) an information specialist from the Cochrane Schizophrenia Group searched the Cochrane Schizophrenia Group Trials Register for RCTs with schizophrenia and psychosis populations, (3) we searched The Cochrane Library database for additional RCTs using an adapted search strategy to include populations with bipolar disorder provided by the Cochrane Depression, Anxiety and Neurosis Group, (4) an expert reference group searched for non-peer-reviewed literature, (5) experts in the field were asked to identify any relevant publications not captured by the electronic search strategies and (6) principal investigators identified on trials registers were contacted for relevant publications.
The following search terms were used:
Schizophrenia* OR severe mental illness* OR bipolar or mania* OR manic* OR hypomani* OR psychos* OR psychotic OR postpsychotic OR “post psychotic” OR “rapid cycling” OR schizoaffective OR bipolar OR mania* OR manic* OR hypomani*
AND
*physical* OR *cardio* OR *metabolic* OR *weight* OR *Tobacc* OR *Smok* OR *medical* OR *alcohol* OR *nutrition* OR *diet* OR *health* OR *diabete* OR *blood pressure* OR *hypertension* OR *cholesterol*OR *statin*
Screening
Titles and abstracts of reports of RCTs identified in the electronic searches were screened by four researchers (LA, AB, MH and VT; see Acknowledgements). References not meeting the inclusion criteria and duplicates (including individual RCTs in included systematic reviews) were removed. To assess reliability of screening, reviewers independently screened 10% of each other’s allotted references resulting in < 5% level of disagreement. Disagreements were resolved by discussion.
Full-text reports were obtained and screened by the same researchers using the criteria described above. Data were extracted from reports meeting the inclusion criteria.
Data extraction
Data within the RCTs were extracted using a modified template from Methods for the Development of NICE Public Health Guidance125 to record methodological (publication, design, etc.) and substantive characteristics (participant, setting, etc.) of included studies.
Quality assessment
The quality of evidence in included reports was assessed using The Cochrane Collaboration’s tool for assessing risk of bias. 126
Data synthesis
Reference lists of published systematic reviews were cross-checked and reviews including the greatest number of relevant references were summarised. Papers from less comprehensive reviews were also included if they were not covered by the more comprehensive reviews. A meta-analysis of findings from individual RCTs was not possible owing to the heterogeneity of reporting, so a narrative synthesis of findings was created. Findings from RCTs with fewer than 10 participants, which may not be sufficiently powered to detect change, are summarised separately.
We performed a narrative synthesis of the evidence, first by selecting the most comprehensive systematic review and summarising this and all the additional trial results.
Findings
Fifteen systematic reviews and 28 additional individual RCTs of interventions met the inclusion criteria [Table 1 and Figure 2 for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of individual RCTs].
| Target risk factor and type of intervention | Systematic reviews (number of RCTs included in each review) | Total number of additional RCTs identified from electronic searches |
|---|---|---|
| Weight | ||
| Pharmacological | 3 (32, 11, 4) | 14 |
| Behavioural | 6 (13, 11, 10, 10, 9, 6) | 9 |
| Pharmacological or behavioural | 1a (23) | 0 |
| Combined pharmacological and behavioural | 0 | 0 |
| Smoking | ||
| Pharmacological | 0 | 0 |
| Behavioural | 0 | 0 |
| Combined pharmacological and behavioural | 3 (34, 9, 8) | 0 |
| Alcohol | ||
| Pharmacological | 0 | 5 |
| Behavioural | 1 (32) | 0 |
| Combined pharmacological and behavioural | 0 | 0 |
| Weight, or alcohol or smoking | 1 (16) | 0 |
| Total | 15 (228) | 28 |
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of additional individual RCTs. CCDAN, Cochrane Depression, Anxiety and Neurosis Group; CSzG, Cochrane Schizophrenia Group.

No trials or reviews of interventions directly targeting levels of cholesterol, diabetes mellitus or hypertension in SMI were identified. Systematic reviews and individual RCTs meeting the inclusion criteria were therefore grouped into three categories of interventions: (1) weight, (2) smoking and (3) alcohol consumption.
In this section, we summarise the main findings of the systematic reviews, grouping the interventions according to their main target CVD risk factor and whether they were behavioural, pharmacological or both.
Risk of bias
Risk of bias was assessed in individual RCTs identified through the electronic searches (n = 28) and in an additional five RCTs that were taken from systematic reviews that were discarded from this review because the remaining studies had all been included in one of the 15 more comprehensive reviews, making a total of 33 assessments.
Three RCTs were rated as being at a high risk of bias. Of these, two were open-label studies of pharmacological interventions54,55 and one had high attrition, which differed across treatment groups. 56
Seven RCTs were rated as being at a low risk of bias. 57–63
The remaining 23 RCTs were rated as being at an unclear risk of bias. Although all studies reported using randomisation, 20 of the 23 RCTs did not adequately describe methods of allocation concealment and 17 out of 23 did not describe how participants were randomised.
Systematic reviews and randomised controlled trials targeting weight
Pharmacological interventions targeting weight
Systematic reviews of pharmacological interventions targeting weight
Four systematic reviews were identified which assessed pharmacological interventions to prevent antipsychotic-related weight gain in people with SMI. 31,64,98,99 The most comprehensive review98 is summarised.
Maayan et al. 98 included 32 studies (n = 1482 participants) of the effectiveness of 15 different medications on weight gain for people prescribed olanzapine, clozapine, risperidone, quetiapine or mixed first- or second-generation antipsychotics. Studies were conducted in the USA, Venezuela, Brazil, the UK, Finland, Turkey, Israel, Iran, China and South Korea. These studies explored a range of outcomes including weight, lipids and glucose.
Anthropometric outcomes
A pooled weight change of –1.99 kg (95% CI –2.77 to –1.20 kg) compared with placebo was reported after a mean of 13 weeks. Although modest and heterogeneous, greatest weight loss was reported for metformin (7 RCTs, n = 334, –2.94 kg, 95% CI –4.89 to –0.99 kg), followed by d-fenfluramine (1 RCT, n = 16, –2.60 kg, 95% CI –5.14 to –0.06 kg), sibutramine (2 RCTs, n = 55, –2.56 kg, 95% CI –3.91 to –1.22 kg) and topiramate (2 RCTs, n = 133, –2.52 kg, 95% CI –4.87 to –0.16 kg).
Metabolic outcomes
Compared with placebo, triglyceride levels decreased significantly more with metformin augmented with sibutramine (1 RCT, n = 28; weighted mean difference (WMD) –36.8 mg per 100 ml, 95% CI –63.94 to –9.66 mg per 100 ml; p = 0.008); metformin alone (2 RCTs, n = 109; WMD –28.07 mg per 100 ml, 95% CI –53.22 to –2.92 mg per 100 ml; p = 0.04) and fluvoxamine (1 RCT, n = 68, WMD –22.70 mg per 100 ml, 95% CI –44.59 to –0.81 mg per 100 ml; p = 0.04). Compared with placebo, LDL cholesterol end-point values were significantly lower only with sibutramine (1 RCT, n = 37, WMD –33.80 mg per 100 ml, 95% CI –60.41 to –7.19 ml; p = 0.01).
No significant effects on glucose levels were identified in placebo-controlled RCTs of metformin, sibutramine, metformin augmented with sibutramine, or rosiglitazone (Avandia®; GlaxoSmithKline plc, GSK House, Middlesex, UK).
Additional individual randomised controlled trials of pharmacological interventions targeting weight
A further 14 RCTs of pharmacological interventions to promote weight loss or prevent antipsychotic-related weight gain were identified by searching electronic databases. 56–60,65–73 Five of these studies were trials of topiramate, two were of zonisamide and the others evaluated seven other pharmacological interventions. Findings from these studies, plus an additional study by Afshar et al. ,61 included in a different systematic review64 are summarised below and in more detail (along with effect sizes where available) in Table 2.
| Author and year of publication | Country | Number of participants (diagnosis), follow-up | Intervention and comparator (duration) | Setting | Risk of bias | Weight/BMI | Lipids | Glucose | Blood pressure |
|---|---|---|---|---|---|---|---|---|---|
| a,bAfshar et al. (2009)61 | Iran | 32 (schizophrenia), 8 weeks | Topiramate vs. placebo (8 weeks) | Secondary care outpatients | Low | No significant between-group differences for BMIc | N/A | No significant between-group differences (specific type of glucose measure not reported)c | No significant between-group differences (specific type of blood pressure measure not reported)c |
| Roy Chengappa et al. (2006)57 | USA | 287 (bipolar disorder), 12 weeks | Topiramate vs. placebo (12 weeks) | Secondary care outpatients | Low | BMI change:c intervention: –0.8 kg/m2 (SD 1.2 kg/m2); control: 0.1 kg/m2 (SD 1.0 kg/m2) (p < 0.001) | N/A | N/A | N/A |
| Weight change:c intervention: –2.5 kg (SD 3.4 kg); control: 0.2 kg (SD 3.0 kg) (p < 0.001) | |||||||||
| Roy Chengappa et al. (2007)67 | USA | 48 (schizoaffective disorder), 8 weeks | Topiramate vs. placebo (8 weeks) | Secondary care outpatients | Unclear | BMI change:c intervention: –0.5 kg/m2 (SD 1.3 kg/m2); control: 1.0 kg/m2 (SD 2.2 kg/m2) [F(143) = 6.5, p < 0.02] | N/A | N/A | N/A |
| Weight change:c intervention: –3.3 lb (SD 8.5 lb); control: 6.6 lb (SD 14.2 lb) [F(1,43) = 6.5, p < 0.02] | |||||||||
| Narula et al. (2010)70 | India | 67 (schizophrenia), 12 weeks | Topiramate vs. placebo (12 weeks) | Secondary care outpatients | Unclear | BMI (baseline; 12 weeks):b intervention: 20.6 kg/m2 (SD 3.9 kg/m2) – 20.1 kg/m2 (SD 4.0 kg/m2); control: 20.2 kg/m2 (SD 3.9 kg/m2) – 22.6 kg/m2 (SD 4.1 kg/m2) (t = 2.4; p = 0.017) | Total cholesterol (baseline; 12 weeks):c intervention: 135 mg% (SD 30 mg%) –133 mg% (SD 31 mg%); control: 133 mg% (SD 31 mg%) –156 (SD 6 mg%) (t = 2.7; p = 0.008) | Fasting blood glucose (baseline; 12 weeks):c intervention: 79.8 mg% (SD 9.4 mg%) –78.2 mg% (SD 6.7 mg%) control: 77.8 mg% (SD 7.4 mg%) –88.5 mg% (SD 12.0 mg%) (t = 4.3; p < 0.001) | Systolic blood pressure (baseline; 12 weeks):c intervention: 118.8 mmHg (SD 7.8 mmHg) –117.9 mmHg (SD 7 mmHg); control: 119.9 mmHg (SD 7.1 mmHg) –122.5 mmHg (SD 7.7 mmHg) (t = 2.6; p = 0.012) |
| Weight (baseline; 12 weeks):b intervention: 54 kg (SD 12.9 kg) 52.73 kg (SD 12.9 kg); control: 52.8 kg (SD 12.6 kg) 58.9 kg (SD 13.1 kg) (t = 1.9; p = 0.05) | Diastolic blood pressure (baseline; 12 weeks):c intervention: 78.6 mmHg (SD 5.7) –77.9 mmHg (SD 4.8 mmHg); control: 80.2 mmHg (SD 6.3 mmHg) –81.4 mmHg (SD 6.2 mmHg) (t = 2.5; p = 0.014) | ||||||||
| McElroy et al. (2007)56 | USA | 46 (bipolar disorder or schizoaffective disorder with a BMI of ≥ 30 kg/m2 or ≥ 27 kg/m2 with concomitant obesity-related risk factors), 24 weeks | Topiramate vs. sibutramine (24 weeks) | Secondary care outpatients | High (high attrition differing across treatment groups) | No significant between-group differences for BMIc or weight lossb | N/A | N/A | N/A |
| Ball et al. (2011)65 | USA | 37 (schizophrenia or schizoaffective disorder with weight gain ≥ 7% since starting antipsychotic medication), 24 weeks | Atomoxetine + weight management, group counselling and physical activity vs. weight management, group counselling and physical activity (24 weeks) | Tertiary care outpatients | Unclear | No significant between-group differences for weight or BMIb | No significant between-group differences for HDL, LDL, triglycerides, very-low-density lipoproteinb | No significant between-group differences for fasting glucosec | No significant between-group differences for systolic or diastolic blood pressurec |
| Borba et al. (2011)66 | USA | 20 (schizophrenia or schizoaffective disorder with a BMI of ≥ 30 kg/m2 or ≥ 27 kg/m2 with evidence of insulin resistance or any component of metabolic syndrome), 8 weeks | Ramelteon vs. placebo (8 weeks) | Secondary care outpatients | Unclear | No significant between-group differences for waist circumferenceb | Total cholesterol (baseline; 8 weeks):c intervention: 205 mg/dl (SD 39 mg/dl) –195 mg/dl (SD 42 mg/dl); control: 171 mg/dl (SD 37 mg/dl) –175 mg/dl (SD 36 mg/dl) [F(1,18) = 5.26; p = 0.03] | No significant between-group differences for fasting glucosec | N/A |
| Cholesterol-to-HDL ratio (baseline; 8 weeks): intervention: 5.1 mg/dl (SD 1.4 mg/dl) –4.9 mg/dl (SD 1.3 mg/dl); control: 4 mg/dl (SD 1 mg/dl) –4.6 mg/dl (SD 1.4 mg/dl) [F(1,17) = 7.57; p = 0.01] | |||||||||
| Henderson et al. (2011)69 | USA | 35 (schizophrenia or schizoaffective disorder), 8 weeks | Modafinil vs. placebo (8 weeks) | Secondary care outpatients | Unclear | No significant between-group differences for BMI or weightb | No significant between-group differences for levels of total cholesterol, HDL, non-HDL and triglyceridesc | No significant between-group differences for fasting glucosec | No significant between-group differences for systolic or diastolic blood pressurec |
| McElroy et al. (2012)58 | USA | 42 (psychotic or bipolar disorder with a BMI of ≥ 22 kg/m2), 16 weeks | Zonisamide vs. placebo (16 weeks) | Secondary care outpatients | Low | Weight change:c intervention: 0.9 kg (SD 3.3 kg0; control: 5.0 kg (SD 5.5 kg) (mean difference –4.4 kg (95% CI –1.5 to –7.4; p = 0.01) | N/A | No significant between-group differences for fasting glucosea | No significant between-group differences for systolic or diastolic blood pressurea |
| Ghanizadeh et al. (2013)59 | Iran | 41 (schizophrenia), 10 weeks | Zonisamide vs. placebo (10 weeks) | Outpatient and inpatient | Low | BMI change:c intervention: –0.3 kg/m2 (SD 0.4 kg/m2); control: 2.2 kg/m2 (SD 6.9 kg/m2) (p = 0.0001) | N/A | N/A | N/A |
| Weight change:c intervention: –1.1 kg (SD 1.4 kg); control: 1.4 kg (SD 2.2 kg) (mean difference –4.4 kg (p = 0.0001) | |||||||||
| Elmslie et al. (2006)68 | New Zealand | n = 60 (bipolar disorder with a BMI of > 25 kg/m2), 26 weeks | L-carnitine supplement + dietary counselling sessions vs. placebo + dietary counselling sessions (26 weeks) | Outpatients | Unclear | No significant between-group differences for weight change,c BMIa or waist circumference | N/A | N/A | N/A |
| Ranjbar et al. (2013)60 | Iran | n = 52 (schizophrenia, schizoaffective and schizophreniform disorders), 16 weeks | Ranitidine vs. placebo (16 weeks) | Inpatients | Low | No significant between-group differences for BMI changea | N/A | N/A | N/A |
| Poyurovsky et al. (2013)72 | Israel | n = 43 (schizophrenia), 6 weeks | Reboxetine + betahistine vs. placebo (16 weeks) | Inpatients | Unclear | BMI change:c intervention: 0.7 kg/m2 (SD 0.8 kg/m2); control: 1.5 kg/m2 (SD 1 kg/m2) (t = 2.92, df = 41, p = 0.008) | N/A | N/A | N/A |
| Weight change:c intervention: 2 kg (SD 2.4 kg); control: 4.8 kg (SD 3.2 kg) (t = 2.89, df = 41, p = 0.006) | |||||||||
| Jarskog et al. (2013)71 | USA | n = 148 (schizophrenia and schizoaffective disorder), 16 weeks | Metformin + weekly diet and physical activity intervention vs. weekly diet and physical activity intervention (16 weeks) | Outpatients | Unclear | BMI change:a intervention: –1 kg/m2 (95% CI –1.3 to –0.7); control: –0.3 (95% CI –0.7 to 0) (t = –2.27; p = 0.006) | Triglycerides change:a intervention: –7.0 mg/dl (95% CI –20.4 to 6.3 mg/dl); control: 13.2mg/dl (95% CI –0.3 to 26.7 mg/dl) (t = –2.11; p = 0.037) | No significant between-group differences for fasting glucosea | N/A |
| Weight change:c intervention: –3 kg (95% CI –2 to –4 kg); control: –1 kg (95% CI –2 to 0 kg) (t = –2.61; p = 0.007) | No significant between-group differences for levels of total cholesterol, HDL, non-HDL and LDLa | ||||||||
| Smith et al. (2013)73 | USA | n = 44 (schizophrenia and schizoaffective disorder), 12 weeks | Pioglitazone + manualised diet–exercise intervention vs. placebo + diet–exercise intervention (12 weeks) | Not reported | Unclear | No significant between-group differences for weight and BMI changea | Total cholesterol change:c intervention: 2.3 mg/dl (SD 7.6 mg/dl); control: 2 mg/dl (SD 8 mg/dl) (F = 2.9; p = 0.048) | Fasting blood glucose change:c intervention: 0.02 mg/dl (SD 8.1 mg/dl); control: 24.2 mg/dl (SD 8.6 mg/dl) (FL = 3.88; p = 0.016) | N/A |
| HDL change:c intervention: 4.6 SD 1.8 mg/dl; control: –2.6 SD 2.1mg/dl (F = 6.50, p = 0.001) |
Anthropometric outcomes in additional randomised controlled trials (two randomised controlled trials, n = 55, –2.56 kg, 95% CI –3.91 to –1.22 kg)
Three out of the five RCTs of topiramate reported a significant beneficial effect on BMI and/or weight loss. 57,67,70 Two studies reported no significant effects of topiramate on weight loss or BMI,56,61 one of which was in comparison to sibutramine. 56 Significant effects on BMI and/or weight and/or waist circumference were reported in two RCTs of zonisamide,58,59 one RCT of reboxetine plus betashistine72 and a RCT comparing metformin plus weekly diet and physical activity intervention with a weekly diet and physical activity intervention alone. 71 No significant effects on BMI or weight were reported in RCTs of ramelteon,66 modafinil,69 atomoxetine,65 the supplement L-carnitine,68 ranitidine60 or pioglitazone. 73
Metabolic outcomes
Only one of the five RCTs of topiramate examined the effect on lipids and reported a significant effect on preventing an increase in the level of total cholesterol compared with placebo. 70 A single, small (n = 20) RCT of ramelteon reported a significant effect on reducing levels of cholesterol and reducing the cholesterol-to-HDL ratio. 66 A RCT comparing metformin plus weekly diet and physical activity intervention with a weekly diet and physical activity intervention alone reported a significant reduction in the level of triglycerides in the metformin arm, but no effect on the levels of total, HDL, non-HDL and LDL cholesterol. 71 A RCT comparing pioglitazone plus a manualised diet–exercise intervention with placebo plus a manualised diet–exercise intervention reported a significant effect on the levels of total and HDL cholesterol. 73 Placebo-controlled RCTs of modafinil and atomoxetine reported no significant effect on lipids. 65,69
Out of the eight RCTs examining the effect of pharmacological interventions on glucose58,61,65,66,69–71,73 one RCT of topiramate and one RCT of pioglitazone reported significant effects in reducing glucose. 70,73
Out of the five RCTs examining the effect of pharmacological interventions on blood pressure,58,61,65,69,70 only one RCT (of topiramate) reported a significant reduction in systolic and diastolic blood pressure. 70
Synthesising systematic review and individual randomised controlled trial findings
The most comprehensive systematic review98 reported the greatest effects for metformin on weight loss and blood lipids. Findings from more recently identified individual RCTs report varied effects for topiramate on weight management and limited evidence regarding topiramate and ramelteon on blood lipids.
Behavioural interventions targeting weight
Systematic reviews of behavioural interventions targeting weight management
We identified six systematic reviews of physical activity and/or diet interventions. 75–78,96,97 The systematic review and meta-analysis by Bonfioli et al. 96 provided the most comprehensive review of the literature and this is summarised here. Two RCTs identified in other reviews but not included in Bonfioli et al. 96 are summarised together with additional individual trials identified by our search.
Bonfioli et al. 96 reviewed 13 RCTs of behavioural interventions to prevent weight gain or promote weight loss. Studies were conducted in the USA (n = 4), Italy (n = 2), the UK (n = 1), Spain (n = 1), Sweden (n = 1), Switzerland (n = 1), Korea (n = 1), China (n = 1) and Australia (n = 1).
Interventions were delivered to both individuals (n = 4) and groups (n = 9) and included different combinations of dietary advice and diet programmes, physical activity, self-monitoring techniques (e.g. keeping diaries), goals and planning techniques (e.g. setting and reviewing goals) as well as general behavioural support approaches, such as motivational interviewing.
The meta-analysis reported an effect on BMI reduction favouring intervention groups compared with control (–0.98 kg/m2, 95% CI –1.31 to –0.65 kg/m2).
Subgroup analyses revealed that interventions delivered to individuals (–1.20 kg/m2, 95% CI –1.57 to –0.83 kg/m2), targeting weight gain prevention (–1.09 kg/m2, 95% CI –1.51 to –0.68 kg/m2) that include dietary (–1.31 kg/m2, 95% CI –1.78 to –0.83 kg/m2) or physical activity (–1.22 kg/m2, 95% CI –1.59 to –0.85 kg/m2) components had the greatest effect in reducing BMI.
Additional individual randomised controlled trials of behavioural interventions targeting weight
We identified a further nine RCTs of behavioural interventions to promote weight loss or prevent antipsychotic-related weight gain through searching electronic databases62,79–86 and an additional two RCTs in other reviews (Scocco et al. 87 in Álvarez-Jiménez et al. 78 and Jean-Baptiste et al. 88 in Cabassa et al. 75). Findings are summarised in more detail in Table 3, apart from four RCTs, which had < 10 participants in each trial arm and are summarised separately. 78–80,88
| Author and year of publication | Country | Number of participants (diagnosis), follow-up | Intervention and comparator | Setting | Risk of bias | Weight/BMI | Lipids | Glucose | Blood pressure |
|---|---|---|---|---|---|---|---|---|---|
| Behavioural interventions | |||||||||
| Álvarez-Jiménez et al. (2010)62 | Spain | n = 61 (schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, brief reactive psychosis, or psychosis not otherwise specified), 52 weeks | 10–14 individual, face-to-face sessions delivered over 12 weeks by a clinical psychologist. Sessions included psychoeducation, dietary counselling, physical activity, and behaviour therapy vs. usual care + advice on weight monitoring strategies and general dietary recommendations | Outpatients (assumed secondary care) | Low risk | No significant group differences in weighta or BMIa at 52-week follow-up | N/A | N/A | N/A |
| Cordes et al. (2011)79 | Germany | n = 100, (schizophrenia or schizoaffective disorder and had gained 1.5 kg since starting antipsychotic medication), 48 weeks | 12, 90-minute group sessions delivered over 6 weeks by a dietitian. Sessions covered nutrition, physical activity and behavioural support vs. usual care | Secondary care inpatients | Unclear | Waist circumference change:b intervention: 4.6 cm (SD 8.3 cm); control: 10.1 cm (SD 7.3 cm) F(8,19.073) = 3.144; p = 0.019) | No significant between-group differences for the levels of total cholesterol or triglyceridesb | Fasting glucose change: intervention: 86.4 mg/dl (SD 21.4 mg/dl); control = 110.9 mg/dl (SD 32.4 mg/dl) (t(23) = 2.159; p = 0.042) | No significant group differences for blood pressureb |
| No significant group differences in body weight at 24 weeksa | Significant group × time effects for fasting glucose: (F(2,26.3) = 3.97; p = 0.03) | ||||||||
| Methapatara and Srisurapanont (2011)83 | Thailand | n = 64 (schizophrenia, BMI of ≥ 23 kg/m2), 12 weeks | Five, 60-minute group sessions delivered over 1 week by study researcher. Sessions included motivational interviewing, education on nutrition and physical activity, information and practice using a pedometer + booster session at 4 weeks healthy lifestyle leaflet vs. healthy lifestyle leaflet | Tertiary care (intervention delivered to inpatients prior to discharge) | Unclear | Weight change:a intervention: –0.8 kg (SD 3.6 kg); control: 1.4 kg (SD 4.1 kg) (95% CI 4.12 to 0.29, t = –2.30; p = 0.03) | N/A | N/A | N/A |
| BMI change: intervention: –0.3 kg/m2 (SD 1.3 kg/m2); control: 0.5 kg/m2 (SD 1.6 kg/m2) (95% CI 1.5 to 0.6, t = –2.2; p = 0.03) | |||||||||
| Waist circumference change: intervention: –3.4 cm (SD 4.4 cm); control: 0.9 cm (SD 5.2 cm) (t = –3.53; p < 0.01) | |||||||||
| McCreadie et al. (2005)82 | UK | n = 100 (schizophrenia), 78 weeks | Free fruit and vegetables for 6 months with diet instructions vs. without instructions vs. usual care | Community | Unclear | No significant group differences for BMIb | N/A | No significant group differences for glucoseb | N/A |
| Usher et al. (2013)84 | Australia | n = 101 (schizophrenia, bipolar disorder), 12 weeks | 12 group sessions, once per week delivered by a nurse; sessions included education and discussion on the healthy lifestyle topic of the week (e.g. five food groups), participants’ progress implementing components of the programme into their everyday life. This session was followed by a 30-minute exercise activity vs. healthy lifestyle booklet | Primary care | Unclear | No significant group differences for weighta or BMIa | N/A | N/A | N/A |
| Daumit et al. (2013)85 | USA | n = 291 (schizophrenia, bipolar disorder), 18 months | 12 group sessions, once per week delivered by a nurse; sessions included education and discussion on the healthy lifestyle topic of the week (e.g. five food groups), participants’ progress implementing components of the programme into their everyday life. This session was followed by a 30-minute exercise activity vs. healthy lifestyle booklet | Primary care | Unclear | BMI change:a intervention: –1.2 kg/m2 (95% CI –1.7 to –0.8 kg/m2); control: –0.1 kg/m2 (95% CI –0.6 to 0.4 kg/m2) [mean between-group change –1.1 kg/m2 (95% CI –1.8 to –0.5 kg/m2; p = 0.001)] | No significant group differences for lipidsb | No significant group differences for glucoseb | No significant group differences for diastolic or systolic blood pressureb |
| Weight change:a intervention: –3.4 kg (95% CI –4.7 to –2.1 kg); control: –0.2 kg (95% CI –1.7 to 1.3 kg) [mean between-group change –3.2 kg (95% CI –5.1 to –1.2 kg; p = 0.002)] | |||||||||
| Ratliff et al. (2012)86 | USA | n = 30 (schizophrenia, schizoaffective disorder), 8 weeks | 8 individual sessions, once per week of a standardised lifestyle programme (SIMPLE programme) supporting participants to choose healthy food and identify opportunities for physical activity (e.g. taking stairs instead of the lift) plus payment for attendance vs. SIMPLE programme plus payment for weight loss vs. control | Community mental health centre | Unclear | No significant group differences for weighta or BMIa | No significant group differences for lipidsb | No significant group differences for glucoseb | N/A |
| Combined pharmacological and behavioural interventions | |||||||||
| Wu et al., (2008)63 | China | n = 128 (schizophrenia), 12 weeks | 12-week diet and physical activity intervention (daily in week 1, weekly in weeks 2–12) delivered by dietitian and exercise psychologist + metformin alone or in combination vs. placebo | Secondary care inpatients | Low risk | Significant differences in BMI change between groups (diet and physical activity plus metformin significantly superior to either alone or placebo in reducing BMI): diet and physical activity plus metformin = –1.8 kg/m2 (95% CI –2.3 to –1.3 kg/m2); metformin = –1.2k g/m2 (95% CI –0.9 to –1.5 kg/m2); diet and physical activity = –0.5 kg/m2 (95% CI –0.8 to –0.3 kg/m2); placebo = 1.2 kg/m2 (95% CI 0.9 to 1.5 kg/m2) | N/A | Significant differences in fasting glucose change between groups (diet and physical activity plus metformin significantly superior to either alone or placebo in reducing fasting glucose): diet and physical activity plus metformin = –7.2 mg/dl (95% CI –10.8 to –5.4 mg/dl); metformin = –10.8 mg/dl (95% CI –16.2 to –7.2 mg/dl); diet and physical activity = –7.2 mg/dl (95% CI –9.0 to –3.6 mg/dl); placebo = 1.8 mg/dl (95% CI –1.8 to 3.6 mg/dl) | N/A |
| Significant differences in weight change between groups (diet and physical activity plus metformin significantly superior to either alone or placebo in reducing weight): diet and physical activity plus metformin = –4.7 kg (95% CI –5.7 to –3.4 kg); metformin = –3.2 kg (95% CI –3.9 to –2.5 kg); diet and physical activity = –1.4 kg (95% CI –2.0 to –0.7 kg); placebo = 3.1 kg (95% CI 2.4 to 3.8 kg) | |||||||||
Anthropometric outcomes
One RCT of a diet and physical activity intervention with behavioural support that also encouraged patients to use a pedometer reported small but significant effects on reducing baseline measures in the intervention group of BMI (–0.32 kg/m2), weight (0.8 kg) and waist circumference (3.38 cm). 83 A RCT of a diet, physical activity and behavioural support intervention also reported a significant reduction on waist circumference. 79 One trial reporting significant effects on weight and BMI immediately after the intervention period reported no effect at 12 months. 62
Three RCTs, one of a group diet and physical activity intervention, one providing free fruit and vegetables to participants for 6 months, and one of a nutrition, physical activity and contingency management intervention [two arms: (1) payment for weight loss and (2) payment for attendance] reported no effect on weight or BMI. 82,84,86
Metabolic outcomes
Two RCTs, one of a nutrition, physical activity and behavioural support intervention, and one of nutrition, physical activity and contingency management, both reported no effect on the levels of triglycerides or total cholesterol. 79,86
Three RCTs examined the effect of behavioural interventions on glucose: two reported no effect,82,86 and one reported a significant reduction in the intervention arm. 62
One RCT examining the effects of interventions on blood pressure reported no effect. 79
Randomised controlled trial with < 10 participants in trial arms
One RCT reported significant effects on weight. This involved an intensive, 16-week intervention of diet (including cooking demonstration, visits to supermarkets) physical activity (participants were given a pedometer) and behavioural support resulting in a –6.4 lb (–2.9 kg) weight change in the intervention group;88 however, only eight participants were included in the intervention arm.
Two RCTs reported no effect on BMI, weight or waist circumference80,81 and one of these RCTs did not have an effect on systolic blood pressure. 81 One was an educational programme providing information and counselling on exercise and nutrition80 and the other was a 12-week exercise programme. 81 A third RCT focusing on diet alone did not find an effect on weight gain. 87
Combined pharmacological and behavioural interventions targeting weight
No systematic reviews of combined pharmacological and behavioural interventions targeting weight were identified.
Additional individual randomised controlled trials of combined pharmacological and behavioural interventions targeting weight
We did not identify any RCTs of combined pharmacological and behavioural interventions to promote weight loss or prevent antipsychotic-related weight gain through searching electronic databases, but we did identify one RCT,63 in a review focusing on behavioural interventions. 77 This study compared metformin and a diet and physical activity intervention alone, in combination and with placebo and reported that diet and physical activity plus metformin were significantly superior to metformin or diet and physical activity alone in reducing fasting BMI, weight and fasting glucose.
Systematic reviews and randomised controlled trials targeting smoking
We identified three systematic reviews of pharmacological, behavioural or combined interventions to promote smoking cessation or reduction in smoking. 52,89,95
Tsoi et al. 95 was the most comprehensive review. There was one additional trial included in a different review which is described later alongside other additional trials we identified. 91 Tsoi et al. 95 reviewed 34 RCTs of pharmacological and/or behavioural interventions to promote smoking cessation, smoking reduction or relapse prevention (16 smoking cessation RCTs; nine smoking reduction RCTs; one relapse prevention RCT and eight RCTs reporting smoking outcomes in interventions aimed at other purposes). The majority of RCTs (28/34) were conducted in the USA.
Pharmacological interventions targeting smoking
Systematic reviews of pharmacological interventions targeting smoking
Seventeen trials of pharmacological interventions were included in the Tsoi et al. 95 review, a meta-analysis of seven placebo-controlled RCTs of bupropion showed that smoking cessation rates were significantly higher in treatment groups at the end of treatment [N = 7, n = 340; risk ratio (RR) 3.03, 95% CI 1.69 to 5.42] and a further meta-analysis of five RCTs confirmed this at 6 months (N = 5, n = 214, RR 2.78, 95% CI 1.02 to 7.58).
A meta-analysis of two trials comparing varenicline with placebo reported significantly higher smoking cessation rates in treatment groups at the end of treatment (N = 2, n = 137, RR 4.74, 95% CI 1.34 to 16.71).
Additional individual randomised controlled trials of pharmacological interventions targeting smoking
We identified one RCT in a systematic review of pharmacological and/or behavioural interventions. 89 The RCT compared nicotine replacement therapy (NRT) with usual care and reported significant effects on point prevalence abstinence, carbon monoxide (CO) levels and cigarettes smoked per day. 90 Findings are summarised in more detail in Table 4.
| Reference | Country | Number of participants (diagnosis), duration (weeks) | Intervention and comparator (duration) | Setting | Risk of bias | Smoking abstinence | Number of cigarettes per day/cotinine/CO levels |
|---|---|---|---|---|---|---|---|
| Pharmacological interventions | |||||||
| aChou et al. (2004)90 | Taiwan | n = 68 (schizophrenia), 12 weeks | NRT vs. TAU (8 weeks) | Secondary care outpatients | Unclear | Point prevalenceb (3 months): NRT = 23%, TAU = 0% | Cigarettes per day:b significant difference between groups (z = –5:8, p < 0.0001) |
| CO levels:b significant difference between groups (z = –8.8, p < 0.0001) | |||||||
Behavioural interventions targeting smoking
Systematic reviews of behavioural interventions targeting smoking
Five trials of behavioural interventions were included in Tsoi et al. ’s95 review. One trial compared a standard smoking cessation programme (American Lung Association programme) with a specialised group therapy intervention for people with schizophrenia and reported significantly higher abstinence rates 6 months post treatment in the standard programme (17.6% vs. 10.7%, p < 0.03). One trial compared a hospital staff-delivered group intervention with one lecture on the dangers of smoking and reported a significant reduction in cigarettes per day at 3 months [F(1,51) = 9.2; p < 0.05]. Other trials compared a single session of motivational interviewing vs. didactic psychoeducation vs. minimal intervention, active repetitive transcranial magnetic stimulation vs. sham repetitive transcranial magnetic stimulation, and high-intensity behaviour support plus NRT vs. low intensity monitoring and information plus NRT. All reported no significant increase in abstinence or reduction in cigarette use or expired CO levels.
Additional individual randomised controlled trials of behavioural interventions targeting smoking
No further RCTs were identified.
Combined pharmacological and behavioural interventions targeting smoking
Systematic reviews of combined pharmacological and behavioural interventions targeting smoking
Three trials of combined pharmacological and behavioural interventions were included in Tsoi et al. 95 A contingent reinforcement intervention plus a nicotine patch was significantly better than both contingent reinforcement alone and minimal intervention in promoting smoking cessation at 9 months (50% vs. 28% vs. 10%).
Individual motivational interviewing plus cognitive-behavioural therapy (CBT) plus a nicotine patch was not effective in promoting smoking cessation compared with usual care but did have a significant effect on reducing cigarette consumption by 50% at 3 months (intervention vs. control, 42.5% vs. 15.7%; odds ratio 3.96, 99% CI 1.53 to 10.23; p < 0.001).
A four-arm RCT comparing financial incentives with no incentive and bupropion with placebo reported significantly reduced biomarkers for cigarette smoking [cotinine levels, F(3,144) = 6.40; p < 0.001)] and carbon monoxide levels [F(3,144) = 5.02; p < 0.01] in the financial incentive groups, but no added value of bupropion.
Additional individual randomised controlled trials of combined pharmacological and behavioural interventions targeting smoking
No further RCTs were identified.
Systematic reviews and randomised controlled trials targeting alcohol
Pharmacological interventions targeting alcohol
Systematic reviews of pharmacological interventions targeting alcohol
No systematic reviews of pharmacological interventions targeting alcohol were identified.
Additional individual randomised controlled trials of pharmacological interventions targeting alcohol
We identified five RCTs of pharmacological interventions to reduce alcohol misuse in people with SMI54,55,81,92,93 (Table 5). Two compared naltrexone plus behavioural support with behavioural support alone. They reported contrasting findings: one reported a significant effect on drinking days (number of days on which participants drank) and number of drinks,91 and one reported no effect on these outcomes. 92 A placebo-controlled RCT of naltrexone plus disulfiram alone or in combination reported a significant increase on days abstinent and reduction in heavy drinking days (days on which five or more drinks consumed) when compared with no drug. 55 Two RCTs of acamprosate54,93 reported no effect on frequency of alcohol, and one reported a significant reduction on number of drinks per week54 but included only nine participants.
| Author and date of publication | Country | Number of participants (diagnosis), follow-up (weeks) | Intervention and comparator (duration) | Setting | Risk of bias | Frequency of alcohol use | Quantity of alcohol use |
|---|---|---|---|---|---|---|---|
| Pharmacological interventions | |||||||
| Petrakis et al. (2004)91 | USA | n = 31 (schizophrenia or schizoaffective disorder and alcohol dependence), 12 weeks | Naltrexone + behavioural support (12 × weekly cognitive behavioural relapse prevention support encouraging abstinence) vs. placebo + behavioural support | Secondary care outpatients | Unclear |
Drinking days:a[F(1,248) = 13.4; p < 0.0001] Heavy drinking days:[F(1,248) = 9.32; p = 0.003] |
Number of drinks during treatment:a
|
| Petrakis et al. (2006)55 | USA | n = 66 (schizophrenia or bipolar disorder and alcohol dependence), 12 weeks | Naltrexone + disulfiram vs. naltrexone vs. disulfiram vs. placebo (12 weeks) | Secondary care outpatient | High risk (all conditions double blind apart from one open label due to the side effects of disulfiram) |
Total days abstinent:a(Medication vs. placebo: t = 2.8; p = 0.01) Heavy drinking daysa:(Medication vs. placebo: t = 2.31; p = 0.02) No significant differences between medications for either outcome |
N/A |
| Brown et al. (2009)92 | USA | n = 50 (bipolar disorder and alcohol dependence), 12 weeks | Naltrexone (12 weeks) + CBT for bipolar disorder and substance use (16 sessions delivered by CBT therapist) vs. placebo + CBT | Outpatients (assumed mixture of primary and secondary care) | Unclear | No significant differences between groups for drinking daysa | No significant differences between groups for drinks per daya |
| Ralevski et al. (2011)93 | USA | n = 34 [schizophrenia (n = 9), schizoaffective disorder (n = 10), non-specified psychosis (n = 4), cocaine and/or cannabis dependence (n = 11)], 12 weeks | Acamprosate vs. placebo (12 weeks) | Secondary care outpatients | Unclear | No significant differences between groups for drinking daysa or heavy drinking daysa | No significant differences between groups for number of drinks per drinking daya |
| Tolliver et al. (2009)54 | USA | n = 9 (bipolar disorder and alcohol dependence), 8 weeks | Acamprosate vs. usual care (8 weeks) | Secondary care outpatients | High (open label RCT) | No significant group × time effect on drinking days per weeka |
Drinks per week:aSignificant group × time effect [F(1,7) = 11.55; p < 0.02] No significant time × group effect on drinks per day |
Behavioural interventions targeting alcohol
Systematic reviews of behavioural interventions targeting alcohol
We identified one systematic review of RCTs of 32 behavioural interventions to reduce alcohol or other substance misuse (n = 3165). 94 Interventions included motivational interviewing and CBT alone and in combination, integrated and non-integrated models of care and skills training. Studies were conducted mostly in the USA (n = 19), six in Australia, three in the UK and one each from Denmark, Germany, Ireland and Switzerland. There were no significant effects of any intervention on outcome measures of alcohol or substance use apart from one motivational interviewing intervention on alcohol abstinence at 6 months (n = 28, RR 0.36, 95% CI 0.2 to 0.8; number needed to treat = 2, 95% CI 2 to 5) though this study was rated as ‘very low quality of evidence’.
Additional individual randomised controlled trials of behavioural interventions targeting alcohol
No further RCTs were identified.
Combined pharmacological and behavioural interventions targeting alcohol
No systematic reviews or RCTs were identified.
Interpretation
Summary of main findings
We performed a comprehensive review of international evidence regarding interventions to reduce cardiovascular risk in people with SMI. We included existing systematic reviews as well as more recent RCTs. We found evidence for effective pharmacological and behavioural interventions to decrease weight and smoking, but limited evidence on effective interventions to manage alcohol misuse. We did not find RCT evidence regarding interventions directly targeting cholesterol levels, hypertension, diabetes mellitus or CVD multiple risk factors.
Regarding pharmacological interventions for managing weight, metformin appeared to be most effective over an average 13-week period, with a modest weight loss of nearly 3 kg on average. 98 Evidence from the 10 more recent individual trials focused mainly on topiramate, which was also shown to be similarly effective in managing weight,57,67,70 although adverse effects may limit its tolerability. 17 Incidentally, it also prevented an increase in the level of cholesterol. 70
For behavioural interventions for managing weight, those aimed at individuals (rather than groups), with both dietary and physical activity components were most effective in reducing BMI. 96 No trials were identified that assessed the effectiveness of physical activity alone. The most effective interventions to reduce BMI were those combining dietary, physical activity and behavioural support. 63,83,85 although adverse effects may limit its tolerability. 17 There was some evidence of effectiveness of behavioural interventions on metabolic outcomes with one of three RCTs reporting a significant effect on plasma glucose levels. 62
The one trial of a combined pharmacological and behavioural (diet and physical activity) intervention was significantly more effective in reducing BMI, weight and fasting glucose than the pharmacological or behavioural components of the intervention alone. 63
Smoking
A meta-analysis demonstrated significant effects of bupropion on smoking abstinence and reduction. 95 One trial of NRT reported significant effects on abstinence, number of cigarettes smoked and CO levels. 90
One trial of a standardised smoking cessation programme included in a published review was significantly more effective than a SMI-tailored intervention in promoting abstinence. 95 Limited conclusions can be drawn from the two RCTs of behavioural interventions identified in this review as their findings conflict: one reported significant effects on reducing smoking; the other reported no significant effect on abstinence. 95
One RCT comparing contingency management and bupropion alone or in combination reported a significant effect of contingency management on CO levels but no additional effects of bupropion. 95
Most of the trials of smoking cessation or reduction interventions included in the review and RCTs identified by this review were conducted in secondary care. It may be that delivering these interventions to patients in primary care who are likely to be more stable may result in a better response.
Alcohol
There was less evidence regarding alcohol in SMI. Two trials of acamprosate reported no significant effects on frequency of alcohol use. 54,93 Two RCTs compared naltrexone plus behavioural support with behavioural support alone; one reported a significant effect in reducing quantity and frequency of use,91 while the other reported no effect. One RCT reported no differentiation in the effectiveness of naltrexone over disulfiram. 55
A published review of 32 interventions reported that only one intervention based on motivational interviewing was effective in promoting abstinence but was rated as very low quality of evidence. 94
Clinical implications
CVD risk could be reduced in people with SMI by delivering combined pharmacological and behavioural interventions for both smoking and weight reduction. The exact content of interventions could be tailored to the preference of the individual as a variety of elements have been shown to be effective, mostly for individuals but also in groups.
There is little specific evidence regarding the effectiveness of interventions to reduce other CVD risk factors commonly found in SMI, including dyslipidaemia, diabetes mellitus and hypertension; however, there is evidence from the general population on what interventions might work to reduce and manage CVD risk. Algorithms are also available to guide treatment recommendations for reducing CVD risk in the general population in both Australia and the UK. 38
Our research findings are in line with recent international guidelines in both England17 and Scotland127 regarding the management of physical health in people with psychosis or bipolar disorder. The lack of evidence regarding managing lipids, diabetes mellitus and hypertension in SMI requires attention. We need to know whether this disadvantaged group of people are accessing and receiving the best interventions to prevent CVD, and then whether interventions, such as statins and antihypertensive drugs, are decreasing excessive rates of CVD. This will require international research to determine whether or not these effective interventions are actually being offered and accepted by people with SMI and ultimately to assess whether or not the stubborn mortality gaps for people with SMI can be diminished in years to come.
Review limitations
It is not straightforward to synthesise all the evidence regarding CVD risk management in SMI. The individual studies frequently have their own design problems and limitations, and evidence is extremely heterogeneous in terms of interventions, settings, target groups and outcome measures. Factors limiting interpretation of findings included small sample sizes, varying or undefined inclusion criteria, setting, short length of follow-up and completeness of intervention description.
Nine of the 23 RCTs not included in the systematic reviews of pharmacological or behavioural interventions to manage weight were tested on SMI patients with a minimum BMI or minimum weight gain since commencing antipsychotic medication (e.g. BMI of > 25 kg/m2 was one of the inclusion criteria). 56–58,65–68,79,83 This limits the generalisability of findings to all SMI patients and highlights a reactive rather than proactive approach to weight management in SMI.
Most of the trials identified by this review were conducted in secondary care limiting their generalisability to other settings, such as primary care.
A further limitation is the length of time between intervention end and collection of follow-up data. As is the nature of pharmacological interventions, follow-up data were collected at the end of the intervention which was, on average, 13 weeks after baseline assessment (aside from one that collected data 4 weeks after intervention end). Of the behavioural and combined pharmacological and behavioural interventions the average length of time between intervention end and collection of follow-up data was 19 weeks. This is a relatively short amount of time in the lifespan of people with SMI, so conclusions regarding longer term effectiveness cannot be made.
There was a lack of detail used to describe behavioural interventions. This problem has been acknowledged in the wider literature on interventions to change behaviour. 128 Although guidance exists to promote detailed reporting to allow replication;129 for some interventions, replication would not be possible based on the descriptions in published reports we have reviewed.
Conclusion
Although we can be optimistic about the efficacy of interventions to address smoking and weight gain in SMI, we also reveal a lack of evidence for managing a range of CVD risk factors including lipids, diabetes mellitus and hypertension as well as a lack of evidence on managing multiple CVD risk factors. These are the challenges for clinical researchers and service planners alike.
Appendix 2 Fidelity assessment of the Primrose intervention delivery
Introduction
In the Primrose cluster RCT, nurses/HCAs were trained to deliver an intervention consisting of simple behaviour change strategies to help patients identify and make progress with goals to lower their level of cholesterol and other CVD risk factors using clinically appropriate treatments and interventions. The results of this trial have been outlined above. This report describes the degree to which the intervention manual and training programme were adhered to, involving a fidelity assessment of audiotapes of the intervention appointments returned by the nurses/HCAs who delivered the intervention.
Treatment fidelity refers to the extent to which a treatment or intervention is delivered as planned and specified to protocol. 130 Bellg et al. 131 argue that without a thorough fidelity assessment, it is not possible to determine how much and why any given intervention may be the cause of any observed changes in outcomes. In order to assess fidelity, ongoing monitoring of the intervention and reliable and valid measuring of treatment components is essential. 132 Audio or videotaping intervention sessions is considered to be the gold standard when monitoring fidelity of treatment delivery. 130 Guidance from the Medical Research Council also emphasises the importance of a systematic approach to process evaluation. 133
Fidelity should therefore be assessed and reported, as recommended in both the MRC framework for developing and evaluating complex interventions134 and the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting RCTs. 129
The concept of fidelity sits within a broader framework for health behaviour change trials, such as that developed by the National Institutes of Health Behaviour Change Consortium,131 and involves evaluating whether or not intervention components were delivered as intended over the duration of the intervention. However, a systematic review of psychosocial treatments found that only 3.5% of studies conducted any evaluation of fidelity of treatment delivery. 135
In addition to evaluating the extent of delivery of a behavioural intervention, it is also important to recognise and understand the active ingredients of an intervention. 136 Interventions encompassing different behaviour change elements are often complex and comprise many potentially interrelating components which address different aspects of the behaviour being targeted for change. 134 The precise components of such interventions are difficult to establish as there is a lack of standardised language and confusions may arise when different labels are used to identify the same behaviour change techniques, or different techniques are identified by the same label. 137
In response to this lack of standardised language, a taxonomy of behaviour change techniques (BCTs) has been developed to systematically describe specific elements of behaviour change interventions. BCTs are defined as the components of an intervention aimed at changing behaviour which are observable and replicable. Goal-setting, positive feedback and action planning are all examples of BCTs.
This BCT taxonomy has been modified for use in various health behaviour contexts, such as behavioural support for smoking cessation,138 weight management,139 and physical activity and healthy eating behaviours. 140 We used this taxonomy to inform the development of intervention components for lowering levels of cholesterol and other CVD risk factors in people with SMI (Primrose). 106
The Primrose cluster randomised controlled trial
A total of 326 patients and 76 GP practices were recruited to the trial between January 2014 and January 2016. Patients with a diagnosis of schizophrenia, bipolar disorder or other psychosis, aged 18–75 years, with raised levels of cholesterol and one or more other CVD risk factors were recruited to the study through the UK CRN. GP practices were randomised to receive training and deliver the Primrose intervention or standard care. The primary outcome of interest was the level of total cholesterol at the 12-month follow-up.
Nurses, HCAs and one GP (providers) from 38 GP practices were randomly allocated to deliver the Primrose intervention and attend 2 days of training. The training days were delivered 2 weeks apart to enable the providers to deliver an appointment in between training sessions and rehearse any aspects that they felt less confident with at training session 2. Providers were given a study manual that included help sheets for each intervention component and clinical outcome, appointment flow charts and practical advice on preparing for and organising appointments.
Providers were trained to identify the patient’s primary CVD risk factors at the first intervention appointment and to work collaboratively with the patient to set a behavioural goal targeting the CVD risk factor. Providers were given a flow chart that set out the procedure for deciding on an appropriate behavioural goal. The flow chart detailed potential interventions and behaviours that would have the largest impact on lowering levels of cholesterol, through to blood pressure, pre-diabetes, diabetes mellitus, smoking, weight and alcohol misuse. To lower levels of cholesterol, the provider was guided to begin the consultation by checking whether or not the patient was adhering to their statin prescription (if prescribed). If the patient was on a statin but was not taking it regularly, the suggested goal was to help the patient improve their statin adherence. If the patient had been prescribed a statin and was adherent, the next appropriate intervention was to set a goal around improving diet and increasing physical activity.
The training was delivered by a health psychologist, a practice nurse with mental health expertise, a lived experience trainer and the programme manager and covered the rationale for the study and intervention design, SMI and role play of appointments using the following eight BCTs: (1) setting a behavioural goal, (2) involving supportive others, (3) action planning, (4) recording progress towards the goal, (5) reviewing progress, (6) giving positive feedback, (7) coping with setbacks and (8) forming habits.
Study aim
The aim of this study was to assess the fidelity of delivery (adherence to the manual and training programme) of the Primrose intervention. The study also aimed to identify whether or not there were differences in the extent to which key intervention components were delivered.
Objectives
-
To assess whether or not pre-specified intervention components of the Primrose intervention are delivered by nurses and HCAs.
-
To assess whether or not appropriate behavioural goals that targeted cholesterol level were set in the first appointment.
-
To determine whether or not particular intervention components are delivered more than others.
-
To assess whether or not there is a difference in fidelity of delivery between nurses and HCAs.
Method
A fidelity assessment of audio-recorded Primrose intervention appointments. The intervention was designed to lower levels of cholesterol and other CVD risk factors in people with SMI in primary care and its effectiveness tested in a cluster RCT (Primrose programme).
Sample
A random 20% sample of received audio files were selected for the fidelity assessment as specified in the original funding application.
Data collection
Patients and providers were asked to provide written consent to audio-record all Primrose intervention appointments. Providers were requested to spend 60 minutes delivering the first appointment and 15–20 minutes on subsequent appointments and were supplied with a digital audio-recorder and written instructions on operating and returning the audio files to the study team. Providers were asked to audio-record all appointments with participants who had given consent and to upload each file via a secure data protection system (IDHS Safe Haven, University College London, London, UK).
Measures
Fidelity of delivery
Appointment fidelity checklists were developed by the study authors to assess fidelity of delivery of the intervention, along with coding guidelines and examples, using a procedure that has been found to be effective in other fidelity studies. 108 The checklists consisted of intervention components, provider behaviours necessary to perform each activity and scoring criteria for each provider behaviour. First appointment checklists (Table 6) consisted of three main sections: (1) introduction (explaining the purpose of Primrose), (2) delivery of six behaviour change strategies (setting a relevant behavioural goal, involving supportive others, developing an action plan, recording behaviour, forming habits and providing positive feedback) and (3) finishing the appointment (arranging the next appointment and allowing the opportunity for the patient to ask questions).
| First appointment checklist | |||
|---|---|---|---|
| Patient ID number: | |||
| Intervention component | Provider behaviour | Scoring criteria (2 = done, 1 = done to some extent, 0 = judged appropriate to do but not done, N/A = judged not appropriate to do) | Score |
| Introduction | The purpose of the Primrose service is explained | 2 = The purpose of the Primrose service was explained to the patient | |
| 1 = The purpose of the Primrose service was partially explained to the patient e.g. | |||
|
|||
| 0 = The purpose of the Primrose service was not explained to the patient | |||
| 1. Set a relevant behavioural goal | What is the patient’s primary risk factor(s)? | Cholesterol | |
| Blood pressure | |||
| Pre-diabetes | |||
| Diabetes mellitus | |||
| Smoking | |||
| Weight | |||
| Alcohol | |||
| What risk factor was targeted? | Cholesterol | ||
| Blood pressure | |||
| Pre-diabetes | |||
| Diabetes mellitus | |||
| Smoking | |||
| Weight | |||
| Alcohol | |||
| Is the patient’s CVD risk profile discussed? | 2 = The patient’s full CVD risk profile was discussed | ||
| 1 = Some but not all of the patient’s CVD risk profile was discussed (e.g. only one risk factor discussed when more are present) | |||
| 0 = The patient’s CVD risk profile was not discussed | |||
| Is the patient asked which area of physical health they would like to work on? | 2 = The patient was asked about which aspect of their physical health they would like to work on | ||
| 0 = The patient was not asked about which aspect of their physical health they would like to work on | |||
| Is a behavioural or outcome goal set? | 2 = A goal was set | ||
| 0 = A goal was not set | |||
| What was the goal that was set? | Adherence | ||
| Diet | |||
| Exercise | |||
| Smoking | |||
| Alcohol | |||
| Other | |||
| Is the goal relevant to the patient’s primary or a relevant risk factor? | 2 = If the risk score is > 10% and/or the patient is on a statin the goal made was on taking or starting medication. If the risk score is < 10% and/or the patient is not on a statin the goal made was on diet and/or physical activity | ||
| 1 = If the risk score is > 10% and/or the patient is on a statin the goal made was on diet and/or physical activity. If the risk score is < 10% and/or the patient is not on a statin the goal made was targeting a different but relevant risk factor (e.g. the patient was a smoker and the goal was stop smoking) | |||
| 0 = The goal was not relevant to any of the patient’s risk factors | |||
| Is the goal SMART? | 2 = The goal was SMART (e.g. walk to the park for 30 minutes a day) | ||
| 1 = The goal was S, M, A, R or T but did not cover all 5 components | |||
| 0 = The goal was not SMART (e.g. lose weight, lower levels of cholesterol) | |||
| 2. Involve supportive others | Is the patient asked whether they would like to involve anyone in their care? | 2 = The patient was asked if they would like to involve someone in their care | |
| 0 = The patient was not asked if they would like to involve someone in their care | |||
| Is there an exploration of how they would they like them to be involved? | 2 = There was an exploration of how the supportive other might be involved or this was not applicable as the patient did not want to involve anyone | ||
| 0 = There was no exploration of how the supportive other might be involved | |||
| 3. Develop an action plan | Is an action plan made? | 2 = An action plan was made | |
| 0 = An action plan was not made | |||
| Does the action plan include: | 2 = The action plan included when, where and with whom (if applicable) the goal would be performed. If whom is not applicable still award a score of two if the other two components were addressed | ||
| 1. When? | |||
| 2. Where? | 1 = The action plan included when, where or with whom (if applicable) the goal would be performed but did not cover all three components. If whom is not applicable award a score of one if one of the other two components was not addressed | ||
| 3. With whom the behaviour would be performed? | |||
| 0 = The action plan did not include when, where or with whom (if applicable) the goal would be performed (None of the elements) | |||
| 4. Record behaviour | Is there a discussion on how progress would be recorded? | 2 = There was a discussion on how progress would be recorded (e.g. recording number of cigarettes smoked, food diary, daily physical activity, daily medication taking) | |
| 0 = There was no discussion on how progress would be recorded | |||
| Is the patient encouraged to record progress in ‘My Health Plan’? | 2 = Yes | ||
| 0 = No | |||
| 5. Form habits | Is the patient encouraged to form a habit by linking the action plan to activities that they do regularly? | 2 = The patient was encouraged to form a habit by linking the action plan to activities that they do regularly (e.g. walking to the shops rather than getting the bus, taking medication when they brush their teeth) | |
| 1 = The patient was encouraged to form a habit but there was no discussion with them on how to do it | |||
| 0 = The patient was not encouraged to form a habit | |||
| 6. Positive feedback | Is positive feedback given for attending the appointment? | 2 = Positive feedback was given for attending the appointment (e.g. thank you for coming, it is good to see you, well done for coming) | |
| 0 = No positive feedback was given for attending the appointment | |||
| Is the patient asked if they are happy with the decisions made? | 2 = The patient was asked whether they are happy with the decisions made | ||
| 0 = The patient was not asked whether they are happy with the decisions made | |||
| 7. Finishing the appointment | Is the patient given the opportunity to ask questions? | 2 = The patient was asked whether they had any questions and the questions were addressed | |
| 1 = The patient was asked whether they had any questions but the questions were not addressed | |||
| 0 = The patient was not asked whether they had any questions | |||
| Is the next appointment arranged? | 2 = The date and time of the next appointment was arranged | ||
| 0 = The date and time of the next appointment was not arranged | |||
Subsequent appointment checklists (Table 7) included two sections: (1) delivery of seven behaviour change strategies (reviewing progress with behavioural goals, coping with setbacks, developing an action plan, recording behaviour, forming habits, providing positive feedback, following up supportive others) and (2) finishing the appointment.
| Subsequent appointments checklist | |||
|---|---|---|---|
| Patient ID number: | Appointment number: | ||
| Intervention component | Provider behaviour | Scoring Criteria (2 = done, 1 = done to some extent, 0 = judged appropriate to do but not done, N/A = judged not appropriate to do) | Score |
| 1. Review progress with behavioural goal | Is progress with goals discussed? | 2 = Progress with goals was discussed | |
| 1 = Progress was discussed however closed questions were asked, ‘Did you do X?’ rather than open questions ‘How did you get on?’ | |||
| 0 = Progress with goals was not discussed | |||
| Is the goal reviewed? | 2 = If the goal was achieved either another goal was set or the goal was maintained. If the goal was partly achieved or not achieved the goal was reduced or a new goal was set | ||
| 0 = The goal was reviewed but there was no discussion about setting a new goal, maintaining or reducing the goal | |||
| 2. Cope with setbacks | Are setbacks identified? | 2 = Set backs were identified if the goal was not achieved or only partly achieved. If the goal was achieved potential setbacks were explored | |
| 0 = Set backs were not identified. If the goal was achieved potential setbacks were not explored | |||
| [N/A = Goal was completely accomplished and so it was not applicable to discuss setbacks] | |||
| Is the patient told that setbacks are part of the process? | 2 = The patient was told that setbacks are part of the process | ||
| 0 = The patient was not told that setbacks are part of the process | |||
| Is the patient encouraged to see setbacks as a learning opportunity? | 2 = The patient was encouraged to see setbacks as a learning opportunity | ||
| 0 = The patient was not encouraged to see setbacks as a learning opportunity | |||
| Are strategies agreed with the patient for coping with situations that could lead to a setback? | 2 = Strategies were discussed and agreed for coping with situations that could lead to a setback | ||
| 1 = Strategies for coping with situations that could lead to a setback were discussed but not agreed | |||
| 0 = Strategies were neither discussed or agreed for coping with situations that could lead to a setback | |||
| 3. Develop an action plan | Is an action plan made? | 2 = An action plan was made | |
| 0 = An action plan was not made | |||
| Does the action plan include: | 2 = The action plan included when, where and with whom (if applicable) the goal would be performed. If whom is not applicable still award a score of two if the other two components were addressed | ||
| When? | 1 = The action plan included when, where or with whom (if applicable) the goal would be performed but did not cover all three components. If whom is not applicable award a score of one if one of the other two components was not addressed | ||
| Where? | 0 = The action plan did not include when, where or with whom (if applicable) the goal would be performed (None of the elements) | ||
| With whom the behaviour would be performed? | |||
| 4. Record behaviour | Is there a discussion on how progress would be recorded? | 2 = There was a discussion on how progress would be recorded (e.g. recording number of cigarettes smoked, food diary, daily physical activity, daily medication taking) | |
| 0 = There was no discussion on how progress would be recorded | |||
| Is the patient encouraged to record progress in ‘My Health Plan’? | 2 = Yes | ||
| 0 = No | |||
| 5. Form habits | Is the patient encouraged to form a habit by linking the action plan to activities that they do regularly? | 2 = The patient was encouraged to form a habit by linking the action plan to activities that they do regularly (e.g. walking to the shops rather than getting the bus, taking medication when they brush their teeth) | |
| 1 = The patient was encouraged to form a habit but there was no discussion with them on how to do it | |||
| 0 = The patient was not encouraged to form a habit | |||
| 6. Positive feedback | Is positive feedback given for attending the appointment? | 2 = Positive feedback was given for attending the appointment (e.g. thank you for coming, it is good to see you, well done for coming) | |
| 0 = No positive feedback was given for attending the appointment | |||
| Is positive feedback given for any attempts or progress towards achieving their goal? | 2 = Positive feedback was given on progress towards achieving the goal | ||
| 1 = Positive feedback was given for some but not all achievements | |||
| 0 = Positive feedback was not given on progress towards achieving the goal | |||
| 7. Follow-up supportive others | Was there follow-up on whether the patient would like to involve anyone in their care and how they would like them to be involved? | 2 = There was discussion on how the supportive other has been involved | |
| 0 = There was no discussion on involving supportive others | |||
| 8. Finishing the appointment | Is the next appointment arranged? | 2 = The date and time of the next appointment was arranged | |
| 0 = The date and time of the next appointment was not arranged | |||
| Is the patient given the opportunity to ask questions? | 2 = The patient was asked whether they had any questions and the questions were addressed | ||
| 1 = The patient was asked whether they had any questions but the questions were not addressed | |||
| 0 = The patient was not asked whether they had any questions | |||
Manual-specified intervention components, such as the ‘introduction’ and ‘forming habits’, in appointment 1 constituted a single-provider behaviour, whereas ‘setting a relevant behavioural goal’ constituted up to five different provider behaviours, such as ‘was a goal set?’ and ’was the goal relevant to the patient’s CVD risk factors?’. All other intervention components contained two required provider behaviours. In the subsequent appointments, all possible intervention components consisted of two required provider behaviours apart from ‘forming habits’ and ‘involving supportive others’, which consisted of one provider behaviour. ‘Coping with setbacks’ consisted of four provider behaviours.
Scoring
The checklists were applied to the transcripts and a score was generated for each intervention component. The scoring methods of Hardeman et al. 109 were used. The total number of behaviours delivered within an appointment, were divided by the maximum score possible on the checklist.
A score of ‘2’ was given for provider behaviours that were performed; a ‘1’ for provider behaviours that were ‘performed to some extent’; a ‘0’ for provider behaviours that were judged appropriate to do but were not performed; and ‘not applicable’ for provider behaviours judged not appropriate to perform. The scoring criteria for strategies that were ‘done to some extent’ were developed iteratively by two researchers reviewing the transcripts and comparing their individually assigned codes.
Scoring for ‘setting a behavioural goal’
For each of the 14 initial appointments selected for the fidelity assessment, a descriptive analysis was conducted to (1) determine each patient’s CVD risk factor profile, (2) identify which risk factor was then targeted by the provider at the first appointment and (3) identify whether or not the subsequent behavioural goal was appropriate to the risk factor. A score of ‘2’ was given if the goal was relevant to the patient’s primary risk factor and an appropriate goal was agreed. A score of ‘1’ was given if a goal was set that was relevant to the patient’s risk factor, but not the primary risk factor of cholesterol level. For example, a score of 1 was given if a goal was set around reducing alcohol consumption (a patient’s secondary risk factor) but their primary risk factor of cholesterol level was not targeted by either statin prescription or adherence (as per clinical guidelines, statin prescription is recommended if a patient has a QRISK2 score of > 10%), improving diet or by increasing physical activity. Finally a score of ‘0’ was given to providers who did not set a goal or where no relevant (primary or otherwise) risk factor was targeted.
Analysis
Audio files were transcribed verbatim and anonymised by a professional third-party transcription service. All transcripts were checked by members of the research team to ensure content accuracy and anonymity. Each transcript was then coded independently by two researchers and scores were compared to determine the reliability of the scoring system. Any disagreements or variations in coding were resolved through discussion between researchers until a final score was agreed on.
Researchers scored intervention components as ‘missing’ when there was insufficient information available in the transcript to be able to allocate a score of ‘0’, ‘1’, ‘2’ or ‘N/A’. All fields scored as ‘missing’ or ‘N/A’ were excluded from the denominator in the final analysis.
The fidelity score for each appointment was expressed as the total mean percentage of overall manual-specified intervention components that were delivered in the appointment divided by the total number of activities deemed applicable. In addition, to allow for comparisons between intervention components and to determine whether or not some activities were delivered more than others, the mean total percentage score for each individual intervention component was calculated across all appointments included in the fidelity assessment. A higher percentage score indicates greater fidelity of delivery. We adopted thresholds used in other intervention fidelity work,141,142 where 81–100% constituted high fidelity, 51–80% was moderate fidelity and ≤ 50% constituted low fidelity.
Results
Out of a total of 813 appointments attended, 431 audio files (53%) were received by the study team. One or more appointment audio files were returned by 33 providers (80.5%) for 90 patients (73.1%). A number of appointments were not audio-recorded although patients’ consent had been given; providers gave the following reasons: telephone appointment, audio-recorder batteries ran out, faulty audio-recorder, provider forgot to record and patient retracted consent to record the appointment.
For the purpose of this study, we randomly sampled 20% of first appointment audio files (n = 14) and 20% of all subsequent appointment audio files (n = 72) to assess whether or not the intervention was delivered to protocol. Table 8 presents the total sample and the fidelity sample for patients, providers and audio files.
| Sample | Patients | Providers | Audio files |
|---|---|---|---|
| Total, n | 123 | 41 | 431 |
| Fidelity, n (%) | 52 (42) | 23 (56) | 86 (20) |
Provider characteristics
A total of 41 providers (18 nurses, 22 HCAs and one GP) from 38 GP practices were randomised to receive training to deliver the Primrose intervention to all recruited patients at their GP practice. In three GP practices, only two members of staff were trained because the original staff member left the practice part way through the study. All providers gave their consent to be audio-recorded.
Twenty-three providers were included in the fidelity analysis, of whom 13 (57%) were nurses, 9 (39%) were HCAs and one was a GP (4%). The majority of providers were female (95.7%) and of white ethnic background (95.7%). The mean age of providers was 45.9 years (range 30–60 years) and the average length of experience working as a nurse, HCA or GP was 10 years (range 10 months to 27 years). Eleven providers (48%) had no previous experience of being involved in research. Table 9 presents the characteristics of the fidelity and trial samples.
| Characteristic | Sample, n (%) | |
|---|---|---|
| Fidelity (N = 23) | Trial (N = 41) | |
| Provider type | ||
| HCA | 9 (39.1) | 22 (53.7) |
| Nurse | 13 (56.5) | 18 (43.9) |
| GP | 1 (4.3) | 1 (2.4) |
| Ethnicity | ||
| White | 22 (95.7) | 39 (95.12) |
| Asian | 1 (4.3) | 2 (4.88) |
| Age (years) | ||
| > 25 | 0 (0) | 1 (2.4) |
| 25–35 | 5 (21.7) | 8 (19.5) |
| 36–45 | 5 (21.7) | 9 (22) |
| 46–55 | 7 (30.4) | 15 (36.6) |
| 56–65 | 6 (26.1) | 8 (19.5) |
| > 65 | 0 (0) | 0 (0) |
| Gender | ||
| Female | 22 (95.7) | 39 (95.1) |
| Male | 1 (4.3) | 2 (4.9) |
| Length of experience (as nurse or HCA) (years) | ||
| > 1 | 1 (4.35) | 1 (2.44) |
| 1 to 2 | 3 (13.04) | 4 (9.76) |
| 3 to 5 | 4 (17.39) | 9 (21.95) |
| 6 to 10 | 6 (26.09) | 10 (24.39) |
| 11 to 15 | 2 (8.70) | 5 (12.20) |
| 16 to 20 | 2 (8.70) | 6 (14.63) |
| 21 to 30 | 4 (17.39) | 5 (12.20) |
| Missing | 1 (4.35) | 1 (2.44) |
| Previous experience of research | ||
| Yes | 12 (52.2) | 16 (39) |
| No | 11 (47.8) | 25 (61) |
Patient characteristics
A total of 155 patients were randomised to receive the intervention and 139 patients (89.7%) gave their consent to be audio-recorded. A total of 123 (79.4%) patients attended one or more intervention appointments and one or more appointments were included in the fidelity sample for 52 patients (42.3%). Table 10 presents the characteristics of the fidelity and overall intervention samples. The samples appear to be evenly matched with the exception of a lower number of patients in the ≥ 70-year age category in the fidelity sample compared with the overall intervention sample. The majority of patients in the fidelity sample were female (54%) and of white ethnic background (90%). The mean age of patients was 51 years in both groups with most patients falling into the 40- to 49-year age category (29%) and 46% of patients had a diagnosis of bipolar disorder.
| Characteristic | Sample, n (%) | |
|---|---|---|
| Fidelity subset (N = 52) | All intervention (N = 155) | |
| Ethnicity (n = 154)a | ||
| White | 47 (90) | 134 (87) |
| Black | 3 (6) | 11 (7) |
| Asian | 1 (2) | 5 (3) |
| Other | 1 (2) | 4 (3) |
| Age (years) | ||
| 30–39 | 9 (17) | 25 (16) |
| 40–49 | 15 (29) | 48 (31) |
| 50–59 | 14 (27) | 38 (25) |
| 60–69 | 13 (25) | 32 (21) |
| ≥ 70 | 1 (2) | 12 (8) |
| Gender | ||
| Female | 28 (54) | 88 (57) |
| Male | 24 (46) | 67 (43) |
| Diagnosis | ||
| Schizophrenia/schizoaffective disorder | 20 (38) | 54 (35) |
| Bipolar disorder | 24 (46) | 71 (46) |
| Other psychosis | 8 (15) | 30 (19) |
Intervention appointment characteristics
The mean length of the first appointments in the fidelity sample was 28 minutes (range 19–42 minutes) and in subsequent appointments 14 minutes (range 4–32 minutes). More than half of the fidelity sample appointments (n = 49, 57%) were delivered by a practice nurse, 36 (41.9%) by HCAs and one (1.2%) appointment was delivered by a GP.
Fidelity
A total of 67.7% (median of 65.2%) of intervention manual-specified activities were delivered across all appointments, although there was considerable variation among activities. Mean fidelity to specific intervention components ranged from 47.8% for ‘forming habits’ to 90.2% for ‘reviewing progress’ across all appointments. Average inter-rater reliability for coding was 86% with a Cohen’s kappa of 0.668 (95% CI 0.63 to 0.70). Table 11 presents the percentage adherence to each specified intervention component in appointment 1, subsequent and all appointments.
| Intervention component | Adherence n/N (%) | ||
|---|---|---|---|
| Appointment 1 | Subsequent appointments | All appointments | |
| Introduction | 17/28 (60.7) | N/Aa | 17/28 (60.7) |
| Finishing the appointment | 24/56 (42.9) | 190/276 (68.8) | 214/332 (64.5) |
| Goal-setting | 112/140 (80) | N/Ab | 112/140 (80) |
| Involve supportive others | 34/48 (70.8) | 60/64 (93.8) | 94/112 (83.9) |
| Develop an action plan | 43/54 (79.6) | 161/260 (61.9) | 204/314 (65) |
| Record behaviour | 50/56 (89.3) | 158/260 (61) | 208/316 (65.8) |
| Form habits | 18/28 (64.3) | 68/152 (44.7) | 86/180 (47.8) |
| Positive feedback | 40/56 (71.4) | 229/286 (80.1) | 269/342 (78.7) |
| Review progress | N/A | 220/244 (90.2) | 220/244(90.2) |
| Cope with setbacks | N/A | 203/394 (51.5) | 203/394 (51.5) |
| Total | 338/466 (72.5) | 1289/1936 (66.6) | 1627/2402 (67.7) |
Missing and not applicable intervention components
In total, 8.2% of all intervention components were not applicable and 4.8% were ‘missing’. Table 12 provides a breakdown of not applicable and missing scores by intervention component. Follow-up of supportive others had the highest amount of missing information in subsequent appointments, whereas ‘coping with setbacks’ was the intervention component that was the most likely to be not applicable.
| Intervention component | N/A, n | ‘Missing’, n |
|---|---|---|
| Introduction | 0 | 0 |
| Finishing the appointment | 1 | 0 |
| Goal-setting | 0 | 0 |
| Follow-up supportive others | 4 | 40 |
| Develop an action plan | 2 | 11 |
| Record behaviour | 2 | 15 |
| Form habits | 3 | 0 |
| Positive feedback | 3 | 0 |
| Review progress | 3 | 0 |
| Cope with setbacks | 95 | 0 |
| Total, n/N (%) | 113/1376 (8.2) | 66/1376 (4.8) |
Fidelity to setting a behavioural goal
‘Setting a behaviour goal’ was specified as an intervention component in appointment 1 and, therefore, only the CVD risk factors and agreed goals for the appointment 1 sample (n = 14) were explored. Table 13 presents each patient’s primary CVD risk factor, the risk factor that was targeted in appointment 1, the goal that was actually set, the goal that we would have expected to have been set and the awarded score.
| Patient | Primary CVD risk factor(s) | QRISK (%) | CVD risk factor targeted | Goal set | Goal that should have been set | Score given |
|---|---|---|---|---|---|---|
| 1 | Cholesterol | 6.6 | Cholesterol/weight | Improve diet and increase physical activity | Improve diet | 2 |
| 2 | Cholesterol | 1.1 | Smoking | Stop smoking | Improve diet | 1 |
| 3 | Cholesterol/on statin | 19.8 | Alcohol | Reduce alcohol consumption | Statin adherence | 1 |
| 4 | Cholesterol/on statin | 31.8 | Cholesterol/weight | Increase physical activity | Statin adherence | 1 |
| 5 | Cholesterol | 2.0 | Alcohol/weight | Reduce alcohol consumption | Improve diet | 1 |
| 6 | Cholesterol | 20.5 | None | None | Statin prescription | 0 |
| 7 | Cholesterol/on statin | 17.6 | Cholesterol/weight | Improve diet | Statin adherence | 1 |
| 8 | Cholesterol/pre diabetes | 4.5 | Weight | Improve diet | Improve diet | 2 |
| 9 | Cholesterol/on statin | 8.0 | Smoking | Stop smoking | Statin adherence | 1 |
| 10 | Cholesterol/pre diabetes | 3.9 | Cholesterol | Improve diet | Improve diet | 2 |
| 11 | Cholesterol | 1.8 | Cholesterol | Improve diet and increase physical activity | Improve diet | 2 |
| 12 | Cholesterol | 3.4 | Smoking | Stop smoking | Improve diet | 1 |
| 13 | Cholesterol/on statin | 13.5 | Weight | Improve diet | Improve dieta | 2 |
| 14 | Cholesterol | 13.4 | Weight | Increase physical activity | Statin prescription | 1 |
Eight (57.1%) patients set a goal around behaviours that had an impact on lowering levels of cholesterol, while three (21.4%) patients set a goal around smoking cessation, two (14.3%) patients set a goal around reducing alcohol intake and one (7.1%) patient did not set a goal. No patients addressed statin adherence or initiation (Table 14).
| Goal category | Lower level of cholesterol | ||||||
|---|---|---|---|---|---|---|---|
| Statin prescription/adherence | Improve diet | Increase physical activity | Stop smoking | Reduce alcohol intake | No goal set | Total | |
| Number of patients (%) | 0 (0.0) | 6 (42.9) | 2 (14.3) | 3 (21.4) | 2 (14.3) | 1 (7.1) | 14 (100) |
Fidelity by appointment number
Higher adherence to intervention components was seen in appointment 1 (72.5%) than in subsequent appointments (66.6%) and adherence within individual appointments varied from 40% (appointment 12, n = 1) to 81.4% (appointment 8, n = 5) (Figure 3).
FIGURE 3.
Average fidelity score per appointment.
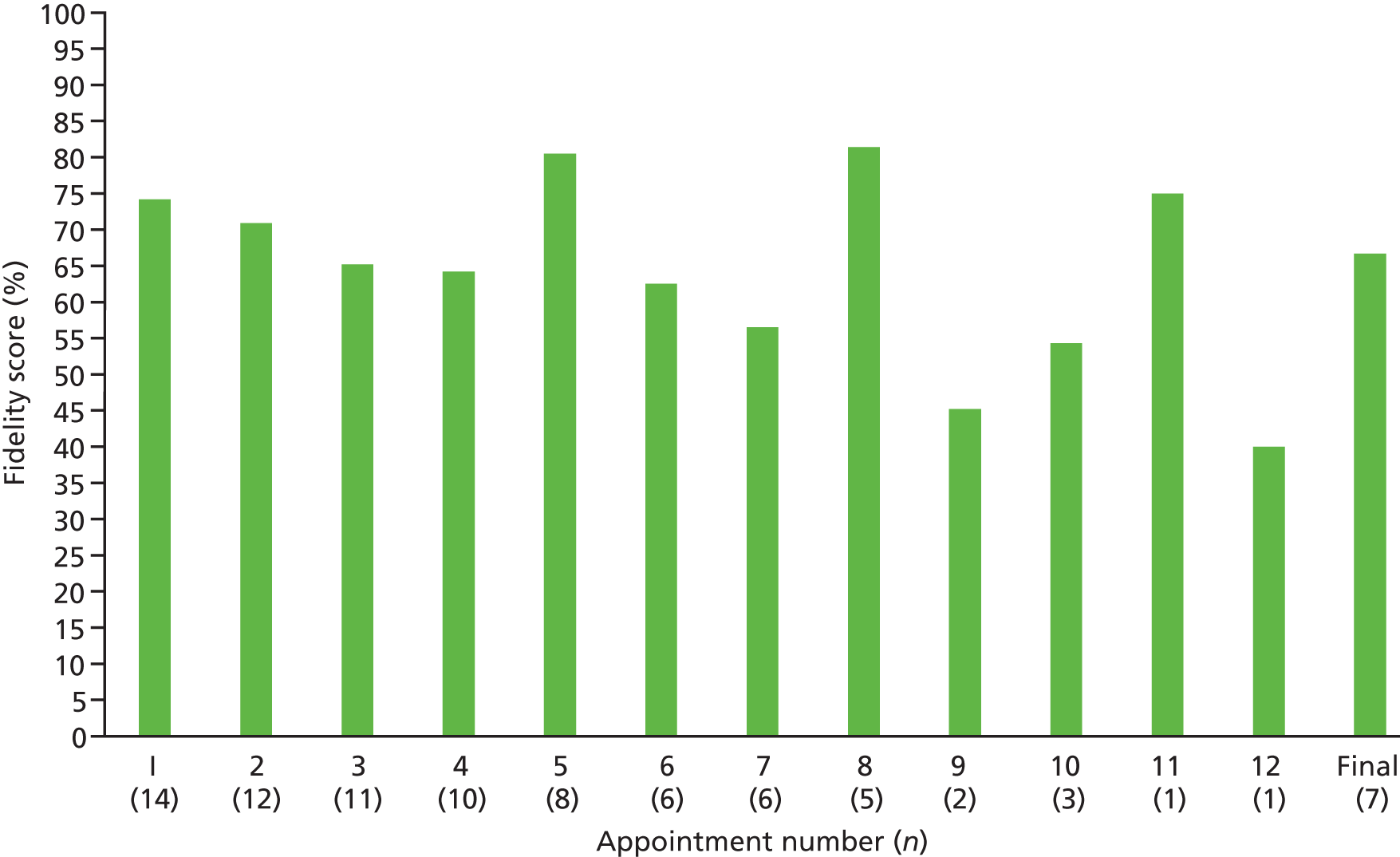
Fidelity by provider type
A mean of 79.5% of intervention manual specified activities were delivered during appointments by nurses (n = 13) in comparison to 64.3% in appointments delivered by HCAs (n = 9). The GP was excluded from this analysis. The difference in fidelity scores between nurses and HCAs was significant [t(20) = 2.32; p = 0.037].
Discussion
Summary of findings
This study presents a thorough assessment of fidelity to a behaviour change intervention for lowering levels of cholesterol and other CVD risk factors delivered in a primary care trial (Primrose).
In response to the first objective of whether or not the intervention components of the Primrose intervention were delivered as specified, our results showed that fidelity was 67.7%. This score indicates moderate fidelity to the intervention. 141,142 This result is comparable to similar fidelity assessments of behavioural interventions including an assessment of a cardiovascular prevention programme110 but higher than the fidelity of delivery of a physical activity intervention (44%). 109 The fidelity observed in this study was slightly lower than for an intervention to increase attendance at a stop smoking service (71.3%);143 however, it was recognised by the authors that the sessions were delivered by two advisors who created a sense of shared responsibility, mitigated feelings of nervousness and encouraged higher adherence to the protocol in an environment where one assessor was being observed by the other.
The second objective sought to determine whether or not a relevant behavioural goal targeting cholesterol level was set at the first appointment. All 14 patients sampled for the first appointment assessment had a primary CVD risk factor of raised cholesterol levels and should have been supported by the provider to set a behavioural goal which would have had a direct impact on lowering levels of cholesterol. Although six of the patients had a risk score that exceeded the threshold for statin prescription (> 10%) and five of those patients were already prescribed a statin, none of these patients set a goal around increasing adherence to statins. Only eight (57.1%) patients set a goal related to behavioural modification (e.g. diet or physical activity) to lower levels of cholesterol, while three (21.4%) patients set a goal on smoking cessation, two (14.3%) patients on reducing alcohol intake and one (7.1%) patient did not set a goal.
As behavioural goals were intended to be patient-led with the support of the provider, the patient may have declined to work on reducing their levels of cholesterol and instead chose to set a goal that they felt was more relevant to their health profile and could produce more tangible results on their health, such as stopping smoking or reducing alcohol.
For the third objective, investigating whether or not particular intervention components were delivered more than others, the highest adherence was seen for ‘reviewing progress with goals’ (90.2%) and the lowest for ‘forming habits’ (47.8%). Providers may have been more comfortable reviewing patient progress as they may already be familiar with providing short-term support in usual practice, such as monitoring blood test results, weight change and blood pressure. Longer-term monitoring and support for behavioural change in primary care is perhaps less common because of short consultation times, limited resources and less continuity of care, which could explain why providers may have found it difficult to address habit formation. This finding may also suggest that if forming habits is considered a crucial element for behaviour change then low adherence to this activity may lead to decreased effectiveness of the intervention on health outcomes over time.
‘Goal-setting’ (80%) was discussed in depth at the start of the intervention training, whereas those BCTs with the lowest adherence scores (‘forming habits’ and ‘coping with setbacks’) were covered towards the end of the training in less detail. It is possible that providers remembered the earlier, more detailed aspects of the training over later topics when fatigue may have set in. A way of improving adherence to these techniques could be to revisit these BCTs at the start of the second training day or include specific role-play activities which explore their applicability in more detail.
The fourth research question assessed whether or not there was a difference in fidelity between provider types. Nurses were more adherent than HCAs, suggesting that the previous experience and skills of providers may need to be taken into consideration for future intervention training programmes. The Primrose trial originally aimed to train practice nurses as intervention providers but, owing to a shortage of nurses in some GP practices and the increasing involvement of HCAs in CVD prevention work, HCAs were trained to deliver the intervention to recruited patients if a nurse was unavailable. If the intervention was delivered solely by nurses, as originally intended, then adherence to the intervention may have been higher, clinical goals may have been more relevant and the intervention may have had a stronger impact on study outcomes.
First appointments were on average 28 minutes (range 19–42 minutes) in duration. This was less than half the time that providers were asked to spend with patients at the first appointment (60 minutes). This observation could be used to explain the moderate fidelity as there may not have been enough time to cover all of the specified components of the intervention.
Strengths
A strength of this study was the robust methodology. Transcripts were double coded and anonymised to remove potential bias. Inter-rater reliability of scoring between two researchers was 86% with a Cohen’s kappa of 0.668 (95% CI 0.63 to 0.70), indicating a moderate level of agreement between raters. 144 Our assessment of a random 20% of the sample is also seen as the recommended amount for fidelity studies. 145
An audio file return rate of 53% was achieved but the number of data provided was large. Health professionals with limited research experience and who had limited time for administrative tasks and a lack of technological support were responsible for operating the recorders and uploading the files rather than trained researchers. The complexity of co-ordinating the return of audio files across 41 providers in 38 GP practices must also be taken into consideration.
A further strength of the study was its external validity. Assessing the fidelity of delivery of an intervention in a real-world primary care setting with primary care nurses and HCAs, rather than in an artificial research setting with trained intervention specialists may increase the ability to apply the results of this study to training and intervention recommendations for health professionals working in primary care.
Limitations
A potential limitation of the study was the representativeness of the fidelity sample compared with the trial sample of intervention providers. There was an over-representation of nurses in the fidelity sample (60.9% compared with 43.9% in the trial) as well as an over-representation of providers who had previous experience of research (52.2% compared with 39% in trial). Although the sample was randomly generated by a statistician not involved in the fidelity analysis, the over-representation of nurses could be explained by a higher return of audio files and a greater number of overall appointments being delivered by nurses than being delivered by HCAs, meaning that nurses comprised a larger proportion of the sample in the fidelity study than in the trial. Similarly, those involved in previous research may also have been more likely to return audio files meaning they were more likely to appear in our fidelity sample. Future intervention training may need to be adapted to troubleshoot potential barriers for HCAs and those with limited research experience for providing the suggested number of intervention appointments to patients and returning the audio files.
Another limitation of the study was that, owing to a decreasing number of subsequent appointments from appointment nine onwards, the potential pool of audio files that the sample could be chosen from was small and for some appointments only one audio file was randomly selected for the fidelity assessment. Interpretation of the results of these subsequent appointments should therefore be treated with caution.
The Primrose intervention included up to 17 provider behaviours across seven different intervention components in the first appointment and 16 provider behaviours across eight different intervention components in subsequent appointments. Furthermore, patients presented with a range of CVD risk factors meaning that providers had to tailor the intervention to each individual. This was a complex intervention and previous research has shown that intervention complexity has a negative impact on fidelity. 146 This might contribute to the moderate score that was observed.
The structure and content of the intervention training could have been another contributing factor to explain the moderate fidelity score as the training may not have been detailed enough, or even too complex for providers who had little previous experience of delivering behaviour change interventions. 147 Providers were instructed to complete their first intervention appointments soon after having attended the intervention training sessions which could explain why overall adherence was higher in the first appointment (72.5%) than in subsequent appointments (66.6%).
Future work could take into consideration the wider content and conversational aspects of the intervention appointments, which were beyond the scope of this study.
Conclusion
This study presents the preliminary findings from an analysis of data collected from a cluster randomised trial to assess fidelity of delivery of an intervention for lowering CVD risk in people with SMI in primary care. The findings from this fidelity assessment suggest that moderate fidelity was achieved, particular intervention components were delivered more than others, and nurses were more adherent than HCAs to intervention delivery. None of the goals that were set targeted the most effective interventions and behaviours for lowering levels of cholesterol (statin prescription and/or statin adherence). Future research on similar interventions should ensure that there is enhanced support and supervision throughout the intervention period and greater consideration of the existing clinical skills of those delivering the intervention. These findings now need to be integrated with the overall trial results and used to help to explain and disseminate the results of the Primrose cluster RCT.
Appendix 3 Links to full text of published studies
Work package 1
Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016;30:717–48. https://doi.org/10.1177/0269881116645254
Osborn DP, Hardoon S, Omar RZ, Holt RI, King M, Larsen J, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry 2015;72:143–51. https://doi.org/10.1001/jamapsychiatry.2014.2133
Zomer E, Osborn D, Nazareth I, Blackburn R, Burton A, Hardoon S, et al. Effectiveness and cost-effectiveness of a cardiovascular risk prediction algorithm for people with severe mental illness (PRIMROSE). BMJ Open 2017;7:e018181. https://doi.org/10.1136/bmjopen-2017-018181
Work package 2
Blackburn R, Osborn D, Walters K, Nazareth I, Petersen I. Statin prescribing for prevention of cardiovascular disease among people with severe mental illness: cohort study in UK primary care. Schizophr Res 2018;192:219–25. https://doi.org/10.1016/j.schres.2017.05.028
Blackburn R, Osborn D, Walters K, Falcaro M, Nazareth I, Petersen I. Statin prescribing for people with severe mental illnesses: a staggered cohort study of ‘real-world’ impacts. BMJ Open 2017;7:e013154. https://doi.org/10.1136/bmjopen-2016-013154
Blackburn R. Exploring the Effectiveness of Statins for Primary Prevention of Cardiovascular Disease in People with Severe Mental Illness. PhD dissertation. London. University College London; 2016. URL: http://discovery.ucl.ac.uk/1476263 (accessed 24 April 2018).
Burton A, Osborn D, Atkins L, Michie S, Gray B, Stevenson F, et al. Lowering cardiovascular disease risk for people with severe mental illnesses in primary care: a focus group study. PLOS ONE 2015;10:e0136603. https://doi.org/10.1371/journal.pone.0136603
Burton A, Walters K, Atkins L, Howard M, Michie S, Peveler R, et al. Barriers, facilitators, and effective interventions for lowering cardiovascular disease risk in people with severe mental illnesses: evidence from a systematic review and focus group study. 2016, National Conference on Public Health Science, Cardiff, UK. Lancet 388(Suppl. 2):30.
Work package 3
Osborn D, Burton A, Walters K, Nazareth I, Heinkel S, Atkins L, et al. Evaluating the clinical and cost effectiveness of a behaviour change intervention for lowering cardiovascular disease risk for people with severe mental illnesses in primary care (PRIMROSE study): study protocol for a cluster randomised controlled trial. Trials 2016;17:80. https://doi.org/10.1186/s13063-016-1176-9
Osborn D, Burton A, Hunter R, Marston L, Atkins L, Barnes T, et al. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatr 2018;5:145–54.
Other outputs arising from the programme
Atkins L, Michie S. Changing eating behaviour: what can we learn from behavioural science? Nutr Bull 2013;8:30–5. https://doi.org/10.1111/nbu.12004
Gray B, Larsen J, Faulkner A. Third sector facilitation of lived experience in research: a case study of service user and carer involvement in the Primrose project. JMHTEP 2013;8:141–51. https://doi.org/10.1108/JMHTEP-03-2013-0008
Hardoon S, Hayes JF, Blackburn R, Petersen I, Walters K, Nazareth I, Osborn DP. Recording of severe mental illness in United Kingdom primary care, 2000–2010. PLOS ONE 2013;8:e82365. https://doi.org/10.1371/journal.pone.0082365
Staley K. A Series of Case Studies Illustrating the Impact of Service User and Carer Involvement on Research. London: NIHR Network: Mental Health; 2013. URL: www.nihr.ac.uk/nihr-in-your-area/mental-health/documents/Impact-of-service-user-and-carer-involvement%20PDF.pdf (accessed 5 May 2018).
Conference proceedings
Atkins L, Michie S. Using Theoretical Domains Framework to Inform an Intervention to Manage CVD Risk in People with Severe Mental Illness: Primrose Programme. Paper presented at Workshop Exploring the Theoretical Domains Framework in Behaviour Change Research, Ottawa, ON, December 2012. URL: https://ktcanada.ohri.ca/workshop_tdf/TDF_Atkins.pdf (accessed 19 November 2018).
Atkins L, Burton A, Walters K, Michie S, Osborn D. Developing a Theory-based Intervention to Reduce Cardiovascular Disease Risk in People with Severe Mental Illness: Findings from a Systematic Review and Focus Group Study. University of Oxford, Oxford: UK Society of Behavioural Medicine; 2013.
Burton A, Osborn D. Developing an Intervention to Manage and Reduce Cardiovascular Disease (CVD) Risk in People with Severe Mental Illness: A Focus Group Study. University College London, London: Qualitative Health Conference; 2013.
Burton A, Osborn D, Atkins L, Michie S, Gray B, Stevenson F, et al. Developing an Intervention To Manage Cardiovascular Disease Risk in People with Severe Mental Illness: A Focus Group Study. University of Manchester, Manchester: Primary Care Mental Health Conference; 2013.
Blackburn R, Osborn D, Walters K, Petersen I, Nazareth I. Inequalities in Statin Prescribing for Primary Prevention of Cardiovascular Disease in People with Severe Mental Illness. University of Exeter, Exeter: Primary Care Mental Health Conference; 2014.
Blackburn R, Osborn D, Petersen I, Walters K, Nazareth I. Statin Prescribing for the Primary Prevention of Cardiovascular Disease in People with Severe Mental Illness. Edinburgh: Society for Academic Primary Care (SAPC); 2014.
Blackburn R, Osborn D, Petersen I, Walters K, Nazareth I. Systematic Review of the Evidence for the Effectiveness of Statins for the Primary Prevention of Cardiovascular Disease in People with Severe Mental Illness. Edinburgh: Society for Academic Primary Care (SAPC); 2014.
Blackburn R, Osborn D, Petersen I, Walters K, Nazareth I. Patterns of Statin Prescribing for the Primary Prevention of Cardiovascular Disease in People with Severe Mental Illness. Taipai, Taiwan: International Society for Pharmacoepidemiology; 2014.
Blackburn R, Osborn D, Petersen I, Walters K, Nazareth I. Statin Prescribing Cardiovascular Disease Prevention in People with Severe Mental Illness. New York, NY: North American Primary Care Research Group (NAPCRG); 2014.
Burton A, Osborn D, Atkins L, Michie S, Gray B, Stevenson F, et al. Development of a Theory and Evidence Based Intervention to Manage Cardiovascular Disease Risk in People with Severe Mental Illnesses in Primary Care (Primrose). University of Exeter, Exeter: Primary Care Mental Health Conference; 2014.
Osborn D. CVD Risk Assessment – Evidence from Primrose Regarding Who and What to Assess/Manage. Malaga: European Network for Mental Health Service Evaluation (ENMESH). 2015. URL: http://enmesh.eu/BookOfAbstracts.pdf (accessed 29 January 2017).
Blackburn R, Osborn D, Petersen I, Walters K, Nazareth I. Reducing Inequalities in Cardiovascular Health in People with Severe Mental Illness: Evaluating the Real-Life Impact of Statin Prescribing. Warwick University, Coventry: Public Health England Applied Epidemiology Scientific Conference; 2016.
Burton A, Heinkel S, Osborn D. Lowering Cardiovascular Risk for People with Severe Mental Illnesses (Primrose). A Cluster Randomised Controlled Trial in Primary Care. Middlesbrough: Clinical Research Network North East and North Cumbria (CRN NENC) Primary Care Research Forum; 2016.
Zomer E, Osborn D, Nazareth I, Blackburn R, Burton A, Hardoon S, et al. Effectiveness and Cost-effectiveness of a Cardiovascular Risk Prediction Algorithm for People with Severe Mental Illness. Madrid: European Congress of Psychiatry (EPA); 2016. URL: www.sciencedirect.com/science/article/pii/S0924933816004375?via%3Dihub (accessed 18 March 2017).
Burton A, Zomer E, Walters K, Atkins L, Howard M, Michie S, et al. A Systematic Review of Interventions to Manage Cardiovascular Disease Risk Factors in People with Severe Mental Illnesses. Melbourne, VIC: 15th World Congress on Public Health; 2017.
Zomer E, Osborn D, Nazareth I, Hunter R. Effectiveness and Cost-effectiveness of a Cardiovascular Risk Prediction Algorithm for People with Severe Mental Illness. Melbourne, VIC: 15th World Congress on Public Health; 2017.
Books/chapters
Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. London: Silverback Publishing; 2014. URL: www.behaviourchangewheel.com (accessed 3 November 2017).
Appendix 4 Development of the Primrose intervention and training programme: figures and tables
The following section presents supplementary information on the development of the Primrose intervention and training programme. Evidence from focus groups, a systematic review and workshops with academic, clinical and lived experience experts was brought together and considered by a subgroup of the programme management team. A logic model (Figure 4) was developed using this evidence, to describe the relationship between factors that might influence the effectiveness and implementation of a primary care nurse-HCA-delivered behaviour change intervention. The logic model was then used as a guide to identifying key intervention and training components for delivering CVD prevention for people with SMI in primary care. Table 15 presents a mapping of the barriers and facilitators identified in the focus groups to the TDF. Potential intervention components were then identified that could be used to address or facilitate the enactment of the domains. Table 16 provides a detailed description of the content in each module of day 1 of the intervention training programme.
FIGURE 4.
Logic model of delivery of a CVD risk management intervention.

| Which barriers need to be addressed and facilitators harnessed? | Who needs to be targeted? | Theoretical domains in which the barriers and facilitators lie | Suggested intervention components to address the barriers and harness the facilitators |
|---|---|---|---|
| Negative attitudes of some health professionals towards CVD intervention in people with SMI (e.g. losing weight and stopping smoking) | Health professionals | Optimism (pessimism) | Nurse training on:
|
| Difficulties for service users to access GP services | Patients and health professionals | Opportunity – environmental context and resources | Direct telephone number of health professional |
| Difficulties in managing a healthy lifestyle due to the side effects of antipsychotic medication | Patients and health professionals | Knowledge and skills | Training health professionals on the effects of antipsychotics |
| Difficulties in managing a healthy lifestyle due to the impact of mental health symptoms on the patient’s ability to engage in healthy behaviours | Patients and health professionals | Capability | Training – only initiate CVD risk prevention when the service user is well |
| Action planning | |||
| Recording and reviewing progress | |||
| Identifying small achievable goals to work towards | |||
| Coping with setbacks | |||
| Difficulties in managing a healthy lifestyle due to a lack of incentivised services | Patients and health professionals | Opportunity – environmental context and resources | Local directory of services to refer people with SMI to |
| Lack of awareness of increased risk and effective interventions for managing CVD risk in people with SMI | Patients and health professionals | Knowledge | Training on:
|
| Patients not turning up to scheduled appointments at their GP practice | Patients and health professionals | Behavioural regulation | Have one named practice nurse/GP overseeing care to ensure continuity and to build a relationship |
| Contact details of supportive others to follow-up non-attendees | |||
| Involving supportive others in the patient’s care | Patients and health professionals | Social opportunity/social influences | Involve supportive others in monitoring adherence to treatments and progress with healthy lifestyle goals |
| Continuity of care | Health professionals | Physical opportunity | Have one named practice nurse/GP overseeing care to ensure continuity and to build a relationship |
| Environmental context and resources | |||
| Providing positive feedback to patients during the appointments | Health professionals | Reflective motivation | Positive feedback |
| Reinforcement | |||
| Setting small, achievable patient-led goals | Patients and health professionals | Capabilty | Setting a behavioural goal |
| Goals |
| Summary of training module | Content of training module | Mode of delivery |
|---|---|---|
| Increasing health professional awareness on the link between SMI and increased risk of CVD | The rationale and reasons for the study and delivering the intervention in primary care were presented | Lectures by a practice nurse with expertise in SMI and the programme manager |
| Discussions were held on the different factors that might lead to an increased risk of CVD in people with SMI and the need to take these factors into consideration when working with patients | Group discussion | |
| Increasing mental health awareness | To tackle any potential stigma and increase confidence in working with people with SMI, we incorporated a session on mental health awareness and education including: | Lecture by a practice nurse with expertise in SMI |
|
Lecture by a lived experience trainer | |
|
Group discussion | |
| Lowering CVD risk factors | Nurses/HCAs were prompted to review each patient’s medical record to establish their CVD risk profile | Lecture by a practice nurse with expertise in SMI |
| They were trained to work through appointment flow charts contained within the study manual to arrive at an appropriate goal relevant to both the patient’s risk profile and in collaboration with the patient and then follow-up and monitor the goal at subsequent appointments | Lecture by health psychologist | |
| They were trained to follow a decision aid beginning with a review of cholesterol level and to consider with the patient, potential interventions that would have the greatest impact on lowering levels of cholesterol | Group discussion | |
| Behaviour change techniques | Nurses/HCAs were trained to use eight behaviour techniques with the aim of addressing the barriers and harnessing the facilitators identified by the focus groups. These were: | Lecture by health psychologist |
|
Role-play observation | |
| Role-play delivery | ||
| Group discussions | ||
| Maintaining engagement | Nurses/HCAs were trained in ways of keeping patients motivated and engaged. These included the importance of seeing the same person throughout the intervention to ensure continuity of care and to establish a relationship, booking the next appointment with the patient in person and writing this in their diary or health plan, to offer flexibility of appointment days and times, to book longer appointments and to give the option of a telephone consultation if the patient was unable to attend | Lectures by health psychologist and programme manager |
List of abbreviations
- BCT
- behaviour change technique
- BMI
- body mass index
- CI
- confidence interval
- CRN
- Clinical Research Network
- CVD
- cardiovascular disease
- DNA
- deoxyribonucleic acid
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HCA
- health-care assistant
- HDL
- high-density lipoprotein
- IRR
- incident rate ratio
- LDL
- low-density lipoprotein
- LEAP
- Lived Experience Advisory Panel
- MHRN
- Mental Health Research Network
- MI
- myocardial infarction
- MRC GPRF
- Medical Research Council General Practice Research Framework
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NRT
- nicotine replacement therapy
- PCRN
- Primary Care Research Network
- PGfAR
- Programme Grants for Applied Research
- PMG
- Programme Management Group
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SMI
- severe mental illness
- SURF
- Service User Research Forum
- TAU
- treatment as usual
- TDF
- theoretical domains framework
- THIN
- The Health Improvement Network
- WTP
- willingness to pay
