Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0608-10168. The contractual start date was in February 2010. The final report began editorial review in October 2017 and was accepted for publication in August 2018. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Bundred et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
SYNOPSIS
Individualised care aims to improve outcomes, maximising the effectiveness of therapy while minimising its toxicity, taking account of patient variability in terms of recurrent risk (high or low) and patient phenotype (more or less susceptible to complications) and patient choice. In line with the priorities of the NHS Cancer Reform Strategy,1 we addressed current inequalities of care for older women and how to identify and reduce instances of common complications to improve quality of life (QoL). Improved survival has been achieved for most patients aged < 70 years. Undertreatment is common in older patients because practitioners remain concerned about the risk of complications of therapy. Undertreatment is associated with early death within 1 year for patients aged ≥ 70 years and with early recurrence within 5 years.
We investigated perceptions of the surgical decision-making process in order to predict complications and assess the value of surgery for improved survival in the treatment of elderly breast cancer patients in a prospective cohort study in Greater Manchester. In addition, we analysed survival and complication rates in relation to baseline, treatment and process variables to identify the role of age, health status, choice and treatment, etc., in outcome. The elderly patient project was complementary to a Breast Cancer Campaign (BCC) and National Institute for Health Research (NIHR) Fellowship project, focusing on patients’ perceptions of responsibility for surgical discussion in women attending breast units across Greater Manchester and the North West. Two elderly patient cohorts were put together for the subsequent survival data generated at 4 years. This allowed us to understand whether the woman received standard or non-standard treatment, whether this decision was the patient’s or the surgeon’s, and whether it had an impact on her overall survival. It has allowed us to try to identify predictors of surgical risk, which would provide a tool to assess the risk of adverse outcome (fitness for surgery) as part of a complex intervention. Surgery reduced the risk of death [hazard ratio (HR) 0.3] and improved cancer-free survival (regardless of underlying comorbidity).
Multifrequency bioimpedance (BEA) electrical analysis, also referred to as bioimpedance spectroscopy (BIS), is believed to identify lymphoedema development when a 10-fold change (standard deviation from baseline) is detected after axillary node clearance (ANC) surgery. It is claimed to predict lymphoedema by up to 10 months earlier than arm swelling in a small study. We assessed patients’ BEA in 1100 women compared with arm measurement (perometry) for the prediction of lymphoedema and found a positive predictive value (PPV) of 54% but were unable to confirm that BEA monitoring was helpful technology in the prediction of development of lymphoedema. We found a higher risk of lymphoedema in patients developing early arm swelling (4–9% increase on perometry), along with the number of metastatic nodes removed and QoL subscale scores at surgery. A predictive scoring index for lymphoedema has been developed based on these variables.
The PLACE (Prevention of Lymphoedema After Clearance by External compression) trial aimed to prevent lymphoedema after axillary node clearance by applying external compression garments in patients with early arm-volume increase (4–9%). We recruited 143 patients, but recruitment was slow and the IDMC recommended that the trial close to further recruitment while maintaining follow-up of participants, as the rate of lymphoedema in the study was 40% (lower than anticipated).
The rate of lymphoedema in BEA was also lower than expected on follow-up, which we have attributed to the reiterative information and explanation given to patients to protect the arm, combined with the simple lymphatic massage and drainage that the patients were taught after surgery. We intend, however, to continue to follow up patients in the PLACE trial to assess the outcomes on lymphoedema development. Eligible women for our studies were identified preoperatively in nine study sites across the UK initially, but this was increased to 21 sites to improve and expedite recruitment.
During the project, a number of changes occurred, both in staff and to the work planned. The initial Programme Grant co-ordinator was Charlotte Stockton, who left after 36 months and was replaced by Sarah Ashton. Sarah Ashton left after a further 18 months and was replaced by Donna Watterson. Initially, seven NHS sites were planned, but, to improve recruitment to workstream (WS) 3, 14 more sites were added (Figure 1).
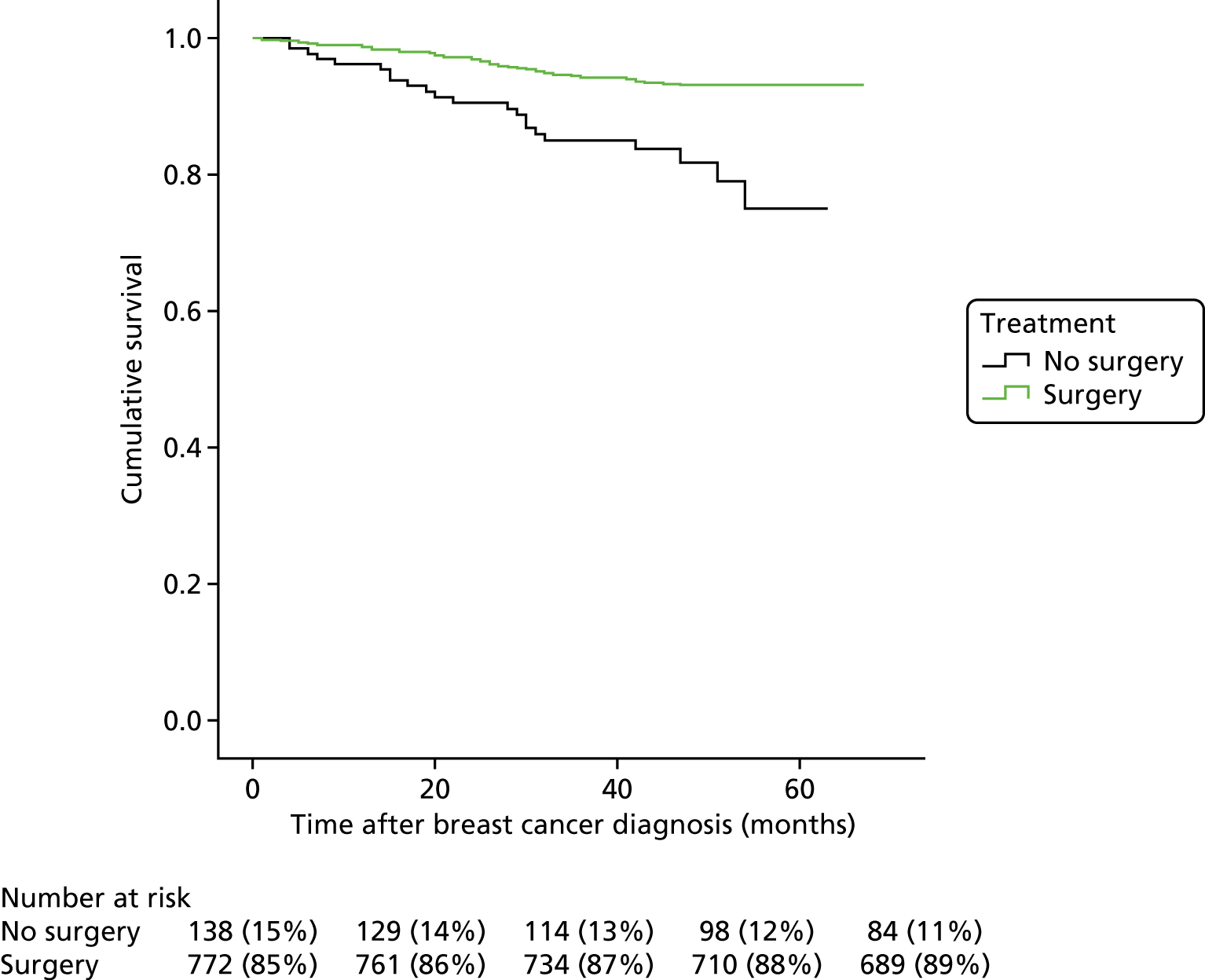
FIGURE 1.
Kaplan–Meier curve for breast cancer-specific survival for patients treated with and without breast surgery. Survival in 910 early breast cancer patients in an elderly population according to surgical treatment.

Workstream 1
The elderly patient study was a prospective cohort study assessing the role of the surgeon and the patient and their agreement as to who made decisions about the use of surgery for their early breast cancer in an elderly population > 70 years of age. It was planned that predictors of surgical risk identified from this study, in terms of either patient fitness or other health parameters, would allow us to develop a screening tool and that we would then conduct feasibility randomised controlled trial (RCT) to determine whether more, appropriate, surgery occurred when assessment of surgical risk took place.
As no predictors of surgical risk were identified, the planned feasibility study could not go ahead and was replaced by further follow-up of the cohort, with several additional analyses of the data as per board approval in January 2016, including outcomes and overall survival in proportion to health risk factors. A comparative analysis with previous work from 1999 and subsequent years was considered, but after a meeting with the NIHR to review, it was agreed that there was greater clinical utility in extending the congruence analysis, to indicate whether desired treatment decision-making had an effect on post-surgical health-related quality of life (HRQoL) (see Appendix 6), and the impact on non-surgical and surgical patients, on the surgery in terms of overall survival Appendix 7. These data have now been published (see Appendix 5).
Workstreams 2 and 3
There was a 6-month delay in the start of recruiting to WS2a and WS3, due to the staggered opening of all initial seven sites and the delay in research and development in certain sites to approve the BEA device, even though it was an external diagnostic device.
Workstream 2a
Comparing bioimpedance with perometer recruited 1100 patients.
Workstream 2b
The diagnostic test accuracy analysis protocol was requested at a NIHR stakeholder meeting in January 2016 to establish the diagnostic test accuracy of bioimpedance, compared with perometry, for the diagnosis of arm lymphoedema and to explore composite measurements to develop a clearer working definition of lymphoedema and implications of alternative definitions. Two hundred and sixty-six patients had a compression garment fitted for lymphoedema in the study (221 by 24 months) and an analysis was performed to understand how this intervention was triggered. Changes in personnel at the Clinical Trials Unit (CTU) also contributed to delay in statistical analysis as a result of data checking, management and cleaning issues within the unit.
Workstream 3
The PLACE trial had a target of 270 patients, but recruitment was slower than anticipated. The PLACE trial was opened at 14 additional centres including King’s Mill Hospital, Macclesfield; Russell Hall Hospital, Dudley; Singleton Hospital, Swansea; Royal Albert Edward Infirmary, Wigan; Homerton University Hospital, London; Macclesfield District General Hospital; Bronglais General Hospital; Peterborough City Hospital; and the George Eliot Hospital, Nuneaton. We explored several other centres, and some of these later centres were open to recruitment only with the addition of tape measurements to assess arm-volume increase. We allowed patients who had had a sentinel node biopsy and who had an arm-volume increase of 4–9% to be recruited to the study (see Appendix 18), as an American study looking at only sentinel node biopsy patients2 reported that these patients had a very high risk of lymphoedema when an arm-volume increase of 4–9% was seen after sentinel node biopsy within 6 months. To provide insight to improve recruitment, a qualitative study was carried out by Karen Spencer, Research Associate, who came into post in August 2015. A number of findings were made that could help improve recruitment procedures, but before we could initiate those findings, the Independent Data Monitoring Committee (IDMC) recommended that as BEA recruitment was complete, and the PLACE trial had recruited only 121 patients, we should close the study to further recruitment as it would not reach its target of 270 patients. In the event, recruitment stayed open until all patients who had been approached to take part in the PLACE trial through BEA decided whether or not to go in the trial. In total, 139 patients were recruited and remain on follow-up. The overall risk of lymphoedema in the study (control and sleeve arm) is 40%, but the IDMC’s decision was taken with data from 65 patients with 2-year follow-up, informed by the CTU statistician (without any chief investigator input). In the light of the extended review of the data (not yet fully quality checked by the CTU) on 139 patients, it is clear that the trial needs to complete follow-up of all patients for 2 years and that the data need to be reviewed. Initial statistical power calculation was that if the difference in lymphoedema was 40% in a control arm and nil or 1% in the sleeve arm, there would be a statistical difference demonstrated with 125 patients.
Additionally, Taghian A et al. (Massachusetts General Hospital, 2016, personal communication) are running a randomised trial of compression sleeves in patients with 4–9% arm increases after sentinel node biopsy or ANC, in Boston, Massachusetts, and have agreed to meta-analyse their data with this study’s data. They currently have around 50 patients recruited to their trial, and it may well be that between the two studies we will have sufficient power to answer the question when follow-up is finished. We have provided the data from WS1, and the publications associated with it, as well as a report, which includes short-term (3.8 years) survival and effects of congruence on QoL, and the finding that having surgery reduced the risk of death from breast cancer by 30%. Data queries from all PLACE trial patients have been updated to allow the PLACE trial data to be finally analysed 2 years after the last patient was randomised in November 2016 (i.e. November 2018).
The number of patients recruited to BEA means that the data permitted us to provide insight into the diagnostic accuracy of BEA, and were presented to the National Institute for Health and Care Excellence (NICE) as part of a Medical Technologies Evaluation Programme in January 2017 for the selection and use of L-Dex® (Carslbad, CA, USA; www.impedimed.com) for detection of lymphoedema (see Report Supplementary Material 1). The NICE review panel appreciated the quality of the evidence and have reported their findings.
Workstream 1: management of elderly breast cancer patients
In line with the priorities of the NHS Cancer Reform Strategy,1 WS1 on the management of older breast cancer patients sought to address inequalities of care for older women. Over recent years, improved survival has been achieved for most patients aged < 70 years. Our earlier work revealed undertreatment to be common in older patients, and practitioners remain concerned about the risk of complications of therapy. Undertreatment is associated with early recurrence and death;3,4 therefore, we proposed to complement our work investigating patient views by investigating surgeons’ perceptions of the surgical decision-making process for the same consultations as those reported by patients. We also planned to develop a risk screening tool based on follow-up of our cohort, which could be administered pre treatment to predict the risk of complications allowing optimisation of treatment for elderly patients with breast cancer.
Study design
In the original application to NIHR, this WS comprised two studies complementing studies funded by BCC and a NIHR fellowship. The original plans had to be modified during the lifetime of the programme (see below).
Study 1
This study complemented the BCC project (protocol submitted to the NIHR with an original application reference of 2008NovPR35) focusing on patients’ perceptions of responsibility for the surgical decision. The BCC study was a prospective cohort study of 550 women aged ≥ 70 years consecutively recruited from newly diagnosed patients with operable (stage I–IIIa) breast cancer attending breast units in Greater Manchester over 21 months. The BCC study collected data on women’s preferences through an interview conducted at home. Study 1 complemented the BCC work by measuring surgeons’ perceptions of who made the treatment decision for the same index cases and related to the same consultations. Thus, we were able to collect a measure of agreed responsibility for treatment decisions. Regardless of whether an older woman received standard or non-standard treatment, we were able to establish whether this decision was a result of the patient’s or the surgeon’s choice. Data on surgeons’ perceptions of responsibility for the surgical decision for individual consultations had to be collected by brief, immediately post-consultation interviews, a resource-intensive method.
Study 2
As part of the research funded by the BCC, NIHR Fellowship and this programme, we planned to identify predictors of surgical risk using multivariate modelling of data from our cohort. For study 2, we planned to develop these predictors into a pre-treatment health assessment/screening tool to assess risk of adverse outcome (i.e. ‘fitness for surgery’). Once we had developed the tool, we planned a feasibility trial following Medical Research Council complex intervention framework and guidelines. However, our modelling revealed no significant clinically novel predictors of surgical risk and therefore we were not able to build a viable screening tool, and hence could not proceed to conduct the planned feasibility trial (see Modelling surgical risk). We thus consulted with programme board and proposed and received board approval for further follow-up of the cohort (IMPACT study) and several additional analyses of the data to investigate outcomes so as to investigate the impact of lack of treatments on older breast cancer patients in the UK. 4 In addition, we undertook an analysis looking at the relationship between congruence (patient getting the treatment decision-making style she preferred) and HRQoL at follow-up.
Workstream 1, aim 1 studies: does patient choice or poor health explain lack of surgery?
The results of study 1 are reported in Lavelle et al. ’s5 paper.
In this first study we investigated whether the lack of surgery for older patients can be explained by patient choice/poor health in a prospective cohort study of 800 women aged ≥ 70 years diagnosed with operable (stage I–IIIa) breast cancer at 22 English breast cancer units in 2010–13 by using interviews and case note review. The outcome measure was surgery for operable breast cancer (stage I–IIIa) < 90 days from diagnosis. Logistic regression adjusting for age, health measures, tumour characteristics, sociodemographics and patients’/surgeons’ perceived responsibility for treatment decisions was undertaken. 6–9
In the univariable analyses, increasing age predicts not undergoing surgery from the age of 75 years, compared with 70- to 74-year-olds. Adjusting for health measures and choice, only women aged ≥ 85 years have reduced odds of surgery (OR 0.18, 95% CI 0.07 to 0.44). Each point increase in activities of daily living (ADL) score (worsening functional status) reduced the odds of surgery by over one-fifth (OR 0.23, 95% CI 0.15 to 0.35). The patient’s role in the treatment decisions made no difference to whether or not they received surgery; those who were active/collaborative were as likely to get surgery as those who were passive, that is, they left the decision up to the surgeon. Lower surgery rates among older women with breast cancer are unlikely to be due to patients actively opting out of having this treatment. However, poorer health explains the difference in surgery between women aged 75–84 years and younger women. The lack of surgery for women aged ≥ 85 years persists even when health and patient choice are adjusted for, revealing that inappropriate undertreatment persists in old age.
To understand these results more fully, we undertook an in-depth qualitative interview study of a group of women who did not receive primary surgery to try to identify how the decision not to have surgery was arrived at. 10 Twenty-eight in-depth interviews were conducted with women aged > 70 years who had operable breast cancer but were receiving primary endocrine therapy (PET) as their primary treatment and had not received, and were not scheduled to receive, surgery. The interviews focused on their perceptions of why they were being treated with PET rather than surgery. Interviews were transcribed verbatim and were analysed using framework analysis. The explanations given varied, but based on reasons for proffered, patients could be divided into three groups: ‘patient declined’, ‘patient considered’ or ‘surgeon decided’. The ‘patient declined’ group ruled out surgery to treat their breast cancer as they were not interested in maximising survival and rejected surgery citing age or concerns about impact of treatment on level of functioning. The ‘patient considered’ group had considered surgery, but chose PET. These patients viewed this as offering them two options; if PET failed, then they could have surgery. The ‘surgeon decided’ group was started on PET by the surgeon and in most cases the surgeon asserted that the patient’s comorbidities were incompatible with surgery.
We conclude that older women are a diverse group and have various reasons for forgoing surgery. Discussions about breast cancer treatment should be patient centred and adapted to differing patient priorities. This issue of patient centeredness is particularly important when we consider the congruence between women’s preferences for involvement in treatment decision-making and their actual involvement, which was addressed in study 2 and is reported below.
As can be seen in Appendix 5, there is little congruence between patients’ preferred and actual roles in the treatment decision-making process, as revealed by their Controlled Preference Score (CPS) scores. Only 163 out of 673 patients (24%) actually received their preferred role in the decision-making, and the vast majority (125; 77%) of these were when they indicated that they wanted decision to be made by the surgeon and indicated this to be the case in actuality. Using Cohen’s kappa, we identify there is only a ‘slight’ level of agreement (κ = 0.039) between preferred and actual role in decision-making. 11 The majority of patients indicated that their actual role was more passive than they would have preferred (442 patients; 66%); only 68 patients (10%) indicated that their actual role was more active than they would have preferred.
These data strongly suggest that it is the surgeon (or at least the surgical team) that is steering the decision in most cases. As revealed by data reported in Lavelle et al. 5 (see Table 3), patients are far more likely than surgeons to indicate that treatment options were not discussed during the consultation. In 112 out of 473 consultations, the patient and surgeon agreed that they did not discuss treatment options. In only 24 out of 136 (17.6%) consultations scored by the surgeons as treatment options not being discussed did patients indicate otherwise; on the other hand, of the 267 consultations scored by patients as not including discussion of treatment options, the surgeons indicated differently in 155 (58%).
Modelling surgical risk
The original plans to identify surgical risk factors create and test a risk assessment tool could not be followed up, because in the final models the novel risk factors proved not to contribute significantly or, if significant, increased the odds only fractionally. These results are reported by Sowerbutts et al. 10 © The Authors. Psycho-Oncology published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
In brief, the ability of pre-treatment health measures to predict complications was investigated in a prospective cohort study of a consecutive series of 664 women aged at least 70 years undergoing surgery for operable (stage I–IIIa) breast cancer at 22 English breast units between 2010 and 2013. 12–14 Data on treatment, surgical complications, health measures and tumour characteristics were collected by case note review and/or patient interview. Outcome measures were all complications and serious complications within 30 days of surgery. One or more complications were experienced by 41% of patients, predominantly seroma or primary or minor infections. Complications were serious in 6.5% of patients. More extensive surgery predicted a higher number of complications, but not serious complications. Older age did not predict complications. Several health measures were associated with complications in univariable analysis, and were included in multivariable analyses, adjusting for type/extent of surgery and tumour characteristics. In the final models, pain predicted a higher count of complications [incidence rate ratio (IRR) 1.01, 95% CI 1.00 to 1.01; p = 0.004]. Fatigue (OR 1.02, 95% CI 1.01 to 1.03; p = 0.004), low platelet count (OR 4.19, 95% CI 1.03 to 17.12; p = 0.046) and pulse rate (OR 0.96, 95% CI 0.93 to 0.99; p = 0.010) predicted serious complications. In conclusion, the risk of serious complications from breast surgery is low for older patients. Surgical decisions should be based on patient fitness rather than on age. Health measures that predict surgical risk were identified in multivariable models, but the effects were weak, with 95% CIs close to unity. They were therefore judged not suitable for building a clinically useful risk screening tool.
Workstream 1, aim 2 studies: impact of lack of treatments on older breast cancer patients in the UK
Introduction
For these studies we used our established cohort of patients aged ≥ 65 years and diagnosed with early-stage invasive breast cancer in 22 trusts in England from 1 July 2010 to 31 March 2013. The extent to which lack of surgery is explained by patient health and choice has been investigated using a range of pre-treatment health measures, tumour characteristics and demographics collected prospectively from patient interview and case note review. 1 However, follow-up of subsequent adjuvant treatment (radiotherapy and/or chemotherapy following surgery) and the impact of lack treatment on survival and long-term HRQoL were not within the remit, resources or timescale of this previous work.
Background
Older women in the UK experience the highest incidence and worst survival for breast cancer, and are less likely to have standard treatment. 1,3,4 The impact of lack of treatment on older patients’ survival needs to be investigated. There is good evidence that poor survival is a particular problem for older breast cancer patients in the UK. Møller et al. 4 found that the 5-year relative survival for women aged ≥ 80 years is 61% in the UK, compared with 74% in Norway and Sweden. They conclude that this ‘leads to important questions about the adequacy of care provided for the oldest patients’. However, Møller et al. did not investigate access to treatment on survival. Moreover, the proportion of patients with comorbidities/frailty and later-stage breast cancer increases with age, and both of these factors also affect survival, and so these variables should also be investigated/adjusted for.
Treatment for breast cancer is based on clinical trials that excluded older women. Moreover, recent trials specific to older patients have closed as a result of failure to recruit. 13 The deficit of evidence on the risks/benefits of treatment for the age group most affected by breast cancer remains. Given the increasing proportion of older people in our population,11,14,15 this presents a growing problem, and studies of older women’s response to therapy are required in order to provide patients, physicians and policy-makers with evidence on which to base decisions about treatment. Surgery is the mainstay of treatment for early breast cancer and yet rates reduce among those aged ≥ 75 years. Omission of surgery leads to lack of local control, particularly at 2 years post diagnosis. 16 Although the only previous trial investigating surgery versus no surgery for older breast cancer patients planned to investigate costs, it closed as a result of failure to recruit. 6
Aims
Workstream 1 set out to address the following research aims in the second set of studies:
-
to investigate the extent to which primary surgery for older women with early breast cancer increases survival and HRQoL and is effective as measured by quality-adjusted life-years (QALYs)
-
to investigate follow-up adjuvant treatment (radiotherapy and/or chemotherapy post surgery) for older breast cancer patients regarding:
-
the extent to which adjuvant treatment increases survival and HRQoL and is effective
-
the extent to which lack of adjuvant treatment can be explained by patient health and choice.
-
Methods
Table 1 and Figure 2 (see Appendix 1) summarise the outcome variables, explanatory variables and main methods for each of the above aims, along with a flow diagram specifying the logic and numbers of patients in the specific analyses performed. Data on our established cohort of 944 women aged ≥ 65 years consecutively diagnosed, from 1 July 2010 to 31 March 2013, already include a wide range of health measures, patient choice, tumour characteristics, demographics and hospital resource variables collected at diagnosis via pre-treatment patient interview and case note review. Follow-up of the cohort involved a further case note review (up to 3 years following diagnosis), postal survey (at 3–4 years post diagnosis – ideally all patients would be surveyed at 3 years, but timings are fixed by diagnosis dates of cohort) and mortality flagging (Figure 19).
| Variable | Category | n | Per cent | Deaths (n) | Log-rank testa p-valueb | |
|---|---|---|---|---|---|---|
| Observed | Expected | |||||
| Primary surgery | Yes | 772 | 84.8 | 49 | 61.99 | |
| No | 138 | 15.2 | 22 | 9.01 | < 0.001 | |
| Age group (years) | 65–69 | 136 | 15.0 | 6 | 11.14 | |
| 70–74 | 265 | 29.1 | 18 | 21.78 | ||
| 75–79 | 225 | 24.7 | 13 | 17.94 | ||
| 80–84 | 148 | 16.3 | 14 | 10.89 | ||
| ≥ 85 | 136 | 15.0 | 20 | 9.26 | 0.001 | |
| Grade | 1 | 168 | 18.5 | 7 | 13.28 | |
| 2 | 489 | 53.7 | 28 | 38.70 | ||
| 3 | 183 | 20.1 | 32 | 13.36 | < 0.001 | |
| Missing | 70 | 7.7 | 4 | 5.67 | < 0.001 | |
| ER or PR positive | Yes | 774 | 85.1 | 50 | 60.77 | |
| No | 81 | 8.9 | 17 | 5.90 | < 0.001 | |
| Missing | 55 | 6.0 | 4 | 4.33 | < 0.001 | |
| Tumour stage | I | 403 | 44.3 | 19 | 32.06 | |
| II and IIIa | 507 | 55.7 | 52 | 38.94 | 0.002 | |
| Charlson Comorbidity Index | 0 | 473 | 52.0 | 38 | 37.98 | |
| 1 | 268 | 29.5 | 21 | 20.53 | ||
| ≥ 2 | 169 | 18.6 | 12 | 12.49 | 0.985 | |
| Functional status | Independent (1–2) | 758 | 83.3 | 55 | 60.38 | |
| Dependent (3–4) | 148 | 16.3 | 16 | 10.38 | 0.061 | |
| Missing | 4 | 0.4 | 0 | 0.24 | 0.153 | |
| Total | 910 | 100 | 71 | 71 | ||
FIGURE 2.
Kaplan–Meier breast cancer-specific survival curve for patients not treated with surgery vs. treated with surgery for breast cancer.
Only patients recruited/diagnosed from 1 July 2010 to 31 December 2012 are included in this study (n = 910) because recruitment was phased out in the final 6 months of the project, with the majority of sites stopping recruitment from 31 December 2012. Only another 34 out of the 944 patients were recruited from 31 December 2012 to 31 March 2013. The inclusion of these final 34 patients in this study is not necessary to support the analyses and would have increased study costs substantially, as it would have required a further wait of 3 months (with concomitant staff salaries, etc.) before the analysis could be conducted with only 34 more patients included (see Appendix 1).
We conducted further case note reviews to follow up each of the included 910 participants up to 3 years post diagnosis, recording adjuvant treatments received. The pro formas for collecting data from case notes were developed and piloted in consultation with clinicians and a health economist (to ensure the correct cost allocation for various procedures). Inter-rater reliability and data quality checks were undertaken on 10% of cases. Pro formas satisfied kappa > 0.6, showing substantial to perfect agreement and data input errors of < 0.3%. 11
The HRQoL survey undertaken at diagnosis was repeated. Ideally, this would have been at 3 years in all women, but timings are fixed to diagnosis date and the first woman was recruited into the study on 1 July 2010. As participants have already consented to further follow-up, ethics approval only required a substantial amendment to specify the follow-up instruments, etc. Patients who did not return the survey were followed up by telephone and reposting 2 weeks later and offered telephone support or a face-to-face interview to complete the survey.
The Office for National Statistics mortality flagging via NHS Digital provided information on both date and cause of death. This enabled analyses of breast cancer survival, the primary outcome of interest.
Outcome/dependent variables
The following outcome measures were specified a priori:
-
aim A (extent to which primary surgery increases survival, HRQoL and is effective)
-
aim Bi (extent to which adjuvant treatment increases survival and HRQoL and is effective)
-
survival to 3 years post diagnosis
-
HRQoL at 3–4 years from diagnosis (see above and Appendices 5–7 for explanation of timings)
-
QALYs at 3–4 years post diagnosis
-
aim Bii (extent to which lack of adjuvant treatment can be explained by patient health and choice)
-
receipt of radiotherapy in addition to primary surgery
-
receipt of chemotherapy in addition to primary surgery.
Explanatory variables
Explanatory variables include measures of health, patient choice, tumour characteristics and demographic variables. Adjusting for these in the analyses enabled us to account for case mix, health status and preferences of older women. Health measures have been selected based on ease of administration, validity, reliability, acceptability to older people, availability of normative data and prediction of non-standard management and/or treatment outcomes. 3,11,15,16 Tumour characteristics have been selected on the basis of management guidelines including TNM stage, grade and steroid receptor status. Choice was determined using the CPS, which has been selected as a validated measure of patient choice. 6–8,14
Analyses
Aim A
The impact of surgery on survival and HRQoL and its effectiveness was investigated in the full sample of 910 women (see Figure 2). For aim Bi, the impact of the adjuvant treatments of radiotherapy and chemotherapy in addition to surgery on these outcomes was tested within a subsample of 759 patients who had surgery (Figure 3). Cox (proportional hazards) regression was used to examine the effect of surgery and adjuvant treatment on survival, adjusting for age, tumour stage, steroid receptor status, comorbidity and functional status. The impact of treatment on difference in HRQoL at diagnosis compared with 3–4 years was adjusted for age, health, choice and tumour characteristics by multiple linear regressions. As recommended by NICE, effectiveness was measured by the difference in QALY gain of treatment adjusted for various age, health, choice and tumour variables (QALY gain of treatment = quality of life lifetime with treatment – quality of life lifetime without treatment). To calculate QALYs, the Short Form Questionnaire-6 Dimensions (SF-6D) utility measure was derived from the SF-12v2, generated using preference weights obtained from a sample of the general population in the UK and following the procedures described at www.sheffield.ac.uk/scharr/sections/heds/mvh/sf-6d (accessed 29 April 2019).
Aim Bii
To assess the extent to which lack of adjuvant treatment can be explained by patient health and choice, surgical patients were included in a logistic regression analysis of receipt of adjuvant chemotherapy and radiotherapy (Figure 20), adjusting for health measures, patient preference, tumour characteristics and demographic variables. Tumour characteristics include those used to determine chemotherapy status in clinical guidelines (steroid receptor status, tumour stage and grade). 13,17 As clinical guidelines indicate that radiotherapy is necessary after lumpectomy but not always necessary following mastectomy,13,17 multivariate logistic regression predicting receipt of radiotherapy was also limited to the number of patients in the cohort receiving lumpectomy.
Results
Aim A
To investigate the extent to which primary surgery for older women with early-stage breast cancer increases survival and HRQoL and is effective.
Sample
All 910 participants could be included in the survival analyses (see Figure 2). Of these, 643 returned the survey at 3–4 years (mean 3.3 years post diagnosis, minimum 3.0 years, maximum 4.4 years), giving an overall response rate of 71%. However, of the 910 participants in the overall sample, 839 completed the Short Form questionnaire-12 items (SF-12) at baseline, of whom 617 returned the survey at 3–4 years, including 501 completed SF-12 surveys. Only those returning completed SF-12 surveys at baseline (diagnosis) and 3–4 years post diagnosis could be included in the analyses of difference in HRQoL from baseline to 3–4 years post diagnosis (n = 501). However, those participants who did not return a survey at 3–4 years because they had died could be included in the QALY calculation and, thus, in the cost-effectiveness analyses (n = 640). Survival results are presented in Appendix 4.
In short, of the 910 women in the study, 178 died before the end point of the study: 71 of breast cancer and 107 of other causes (Table 2 relates to breast cancer deaths only). Patients who had primary surgery (vs. those who did not) had 0.36 times the hazard of dying of breast cancer (95% CI 0.20 to 0.66; p = 0.001) adjusting for other factors. In univariate analysis, women aged ≥ 85 years had an increased hazard of breast cancer death compared with those aged 65–69 years (HR 4.02, 95% CI 1.61 to 10.01; p = 0.003). However, when adjusted for surgery, tumour characteristics and general health, this was at best only borderline significant at the 5% level (p = 0.053). Surgery for older breast cancer patients reduces the hazard of breast cancer death by two-thirds, independent of age, comorbidity and tumour characteristics.
| Coefficient | SE | t | p > t | 95% CI | |
|---|---|---|---|---|---|
| Primary surgery | |||||
| No | (ref) | ||||
| Yes | –0.012 | 0.024 | –0.47 | 0.636 | –0.060 to 0.036 |
| Age group (years) | |||||
| 65–69 | (ref) | ||||
| 70–74 | –0.015 | 0.018 | –0.81 | 0.420 | –0.050 to 0.021 |
| 75–79 | 0.020 | 0.019 | 1.04 | 0.301 | –0.018 to 0.058 |
| 80–84 | –0.015 | 0.022 | –0.67 | 0.504 | –0.059 to 0.029 |
| ≥ 85 | –0.004 | 0.027 | –0.17 | 0.867 | –0.057 to 0.048 |
| Grade | |||||
| 1 | (ref) | ||||
| 2 | 0.004 | 0.017 | 0.26 | 0.794 | –0.029 to 0.038 |
| 3 | –0.019 | 0.021 | –0.88 | 0.382 | –0.061 to 0.023 |
| Missing | 0.019 | 0.028 | 0.67 | 0.501 | –0.036 to 0.073 |
| ER or PR positive | |||||
| Yes | (ref) | ||||
| No | –0.012 | 0.024 | –0.51 | 0.613 | –0.059 to 0.035 |
| Missing | 0.032 | 0.029 | 1.1 | 0.272 | –0.025 to 0.089 |
| Tumour stage | |||||
| I | (ref) | ||||
| II and IIIa | –0.002 | 0.013 | –0.17 | 0.866 | –0.028 to 0.023 |
| Charlson Comorbidity Index | |||||
| 0 | (ref) | ||||
| 1 | 0.009 | 0.015 | 0.61 | 0.545 | –0.020 to 0.038 |
| ≥ 2 | –0.030 | 0.018 | –1.65 | 0.101 | –0.066 to 0.006 |
| Functional status | |||||
| Independent (1–2) | (ref) | ||||
| Dependent (3–4) | 0.108 | 0.023 | 4.77 | < 0.001a | 0.064 to 0.153 |
Health-related quality-of-life results
The average age of the 501 participants in this sample was 75.6 years [standard deviation (SD) 6.4 years]. Their mean SF-6D utility scores (0–1, increase = better health) at diagnosis (0.75, SD 0.15) were higher than 3–4 years later (0.70, SD 0.14), indicating reduced HRQoL for participants over this time (paired t-test p < 0.001). The average decrease in the utility score was –0.05 (SD 0.14). Of the 501 participants, 461 (92.0%) had primary surgery and 40 (8.0%) did not. Although the decrease in utility appears greater for those having surgery (mean –0.06, SD 0.14) than for those not having surgery (mean –0.02, SD 0.16), this difference was not significant (t-test p = 0.175), indicating that having primary surgery does not affect HRQoL in the 3- to 4-year term. This result was confirmed by multiple regression analyses (Table 3) in which only functional status at diagnosis predicted changes in HRQoL. Participants dependent in ADL at diagnosis experienced an increase in HRQoL compared with those who were independent, possibly due to having additional help from supportive services.
| Coefficient | SE | t | p > t | 95% CI | |
|---|---|---|---|---|---|
| Primary surgery | |||||
| No | (ref) | ||||
| Yes | 0.393 | 0.089 | 4.43 | < 0.001 | 0.219 to 0.567 |
| Age group (years) | |||||
| 65–69 | (ref) | ||||
| 70–74 | –0.038 | 0.081 | –0.47 | 0.637 | –0.198 to 0.121 |
| 75–79 | –0.096 | 0.086 | –1.12 | 0.263 | –0.264 to 0.072 |
| 80–84 | –0.350 | 0.095 | –3.69 | < 0.001 | –0.536 to –0.164 |
| ≥ 85 | –0.420 | 0.106 | –3.98 | < 0.001 | –0.627 to –0.212 |
| Grade | |||||
| 1 | (ref) | ||||
| 2 | 0.019 | 0.074 | 0.26 | 0.796 | –0.126 to 0.164 |
| 3 | –0.117 | 0.090 | –1.3 | 0.193 | –0.293 to 0.059 |
| Missing | 0.123 | 0.120 | 1.02 | 0.306 | –0.113 to 0.360 |
| ER or PR positive | |||||
| Yes | (ref) | ||||
| No | –0.260 | 0.095 | –2.73 | 0.007 | –0.446 to –0.073 |
| Missing | –0.209 | 0.117 | –1.79 | 0.074 | –0.439 to 0.021 |
| Tumour stage | |||||
| I | (ref) | ||||
| II and IIIa | –0.026 | 0.055 | –0.47 | 0.637 | –0.134 to 0.082 |
| Charlson Comorbidity Index | |||||
| 0 | (ref) | ||||
| 1 | –0.108 | 0.063 | –1.73 | 0.084 | –0.231 to 0.015 |
| ≥ 2 | –0.158 | 0.075 | –2.11 | 0.035 | –0.305 to –0.011 |
| Functional status | |||||
| Independent (1–2) | (ref) | ||||
| Dependent (3–4) | –0.072 | 0.096 | –0.75 | 0.454 | –0.260 to 0.116 |
| Baseline utility (SF-6D) | 2.149 | 0.207 | 10.36 | < 0.001 | 1.742 to 2.556 |
Quality-adjusted life-years
As recommended by NICE, effectiveness was measured by the difference in QALY gain of treatment adjusted for various age, health, choice and tumour variables (QALY gain of treatment = quality of life × lifetime with treatment – quality of life × lifetime without treatment). QALYs were calculated using the standard procedure described by Manca et al. 18 Lifetime was defined as the time from completion of the SF-12 at baseline (diagnosis) to completion of the follow-up survey 3–4 years later or time to death. Adjustment for baseline HRQoL was made within multiple regression analyses.
The average age of the 640 participants in this sample was 76.5 years (SD 6.8 years). Of these participants, 558 (87.2%) had primary surgery and 82 (12.8%) did not. The average QALY gain was significantly greater for those who had primary surgery (2.08, SD 0.76) than for those who did not (1.32, SD 0.84) (t-test p < 0.001). In the multiple regression analyses, surgery increased QALYs gained by 0.39 (95% CI 0.22 to 0.57), adjusting for baseline utility score as well as age, comorbidity, functional status and tumour characteristics (p < 0.001) (see Table 3).
Aim Bi
To investigate the extent to which adjuvant treatment (radiotherapy and/or chemotherapy) increases survival and HRQoL and is effective for older breast cancer patients undergoing primary surgery. The results reported in this section are more fully explored in Appendix 3.
Sample
Of the 910 participants, 896 had their case notes reviewed and 759 had primary surgery and so could be included in the survival analyses (see Figure 3). Of these, 574 returned the survey at 3–4 years (mean 3.3 years post diagnosis, minimum 3.0 years, maximum 4.4 years), giving an overall response rate of 76%.
However, of the 759 participants in the overall sample, 718 completed the SF-12 at baseline, of whom 555 returned the survey at 3–4 years, including 454 completed SF-12s. Only those returning completed SF-12 surveys at baseline (diagnosis) and 3–4 years post diagnosis could be included in the analyses of difference in HRQoL from baseline to 3–4 years post diagnosis (n = 454). However, those participants who did not return a survey at 3–4 years because they had died could be included in the QALY calculation and thus the effectiveness analyses (n = 640).
Survival results
The primary end point is breast cancer-specific mortality, which was defined as time from diagnosis to death due to breast cancer based on underlying cause of death provided by NHS Digital. 19 Participants who died of other causes were censored at their date of death. Participants were classified as having adjuvant treatment if they received this within 12 months of diagnosis. Therefore, treatment had to be followed up for a minimum of 12 months post diagnosis. Participants who moved away or whose care was transferred to another hospital within 12 months post diagnosis were censored on the date of their last breast clinic visit.
Of the 759 women in the study (mean age 75.99 years, 95% CI 75.53 to 76.44 years), 113 died before the end point of the study (5 February 2016): 48 of breast cancer and 65 of other causes. The mean follow-up time was 3.68 years (95% CI 3.59 to 3.77 years). The baseline characteristics of the sample are detailed in Table 4.
| Variable | Category | n | Per cent | Deaths (n) | Log-rank testa p-valueb | |
|---|---|---|---|---|---|---|
| Observed | Expected | |||||
| Chemotherapy | Yes | 99 | 87.0 | 11 | 6.27 | |
| No | 660 | 13.0 | 37 | 41.73 | 0.043 | |
| Radiotherapy | Yes | 491 | 64.7 | 27 | 31.54 | |
| No | 268 | 35.3 | 21 | 16.46 | 0.167 | |
| Type of surgery | Mastectomy | 353 | 46.5 | 34 | 21.63 | |
| Wide local excision | 406 | 53.5 | 14 | 26.37 | < 0.001 | |
| Age group (years) | 65–69 | 129 | 17.0 | 6 | 8.20 | |
| 70–74 | 244 | 32.2 | 17 | 16.24 | ||
| 75–79 | 188 | 24.8 | 7 | 12.10 | ||
| 80–84 | 121 | 15.9 | 10 | 7.07 | ||
| ≥ 85 | 77 | 10.1 | 8 | 4.39 | 0.137 | |
| Grade | 1 | 142 | 18.7 | 3 | 9.09 | |
| 2 | 397 | 52.3 | 17 | 25.71 | ||
| 3 | 158 | 20.8 | 26 | 9.13 | < 0.001a | |
| Missing | 62 | 8.2 | 2 | 4.07 | < 0.001a | |
| ER or PR positive | Yes | 631 | 83.1 | 28 | 40.37 | |
| No | 77 | 10.1 | 17 | 4.43 | < 0.001a | |
| Missing | 51 | 6.7 | 3 | 3.21 | < 0.001a | |
| Tumour stage | I | 358 | 47.2 | 13 | 23.24 | |
| II and IIIa | 401 | 52.8 | 35 | 24.76 | 0.003a | |
| Charlson Comorbidity Index | 0 | 421 | 55.5 | 33 | 26.87 | |
| 1 | 216 | 28.5 | 10 | 13.54 | ||
| ≥ 2 | 122 | 16.1 | 5 | 7.59 | 0.202 | |
| Functional status | Independent (1–2) | 679 | 89.5 | 42 | 43.1 | |
| Dependent (3–4) | 77 | 10.1 | 6 | 4.76 | 0.555 | |
| Missing | 3 | 0.4 | 0 | 0.14 | 0.780 | |
| Total | 759 | 100% | 48 | 48 | ||
The number of observed breast cancer deaths significantly exceeded those expected for participants whose tumours were of higher grade or stage and steroid receptor negative and warranted chemotherapy and mastectomy [vs. wide local excision (WLE)] (see Table 4). As the number of events (48) per degree of freedom from explanatory variables needs to exceed five in the final model (26), the maximum number of variables could not exceed nine. Therefore, in addition to adjuvant therapy, only variables significant at the 5% level in the univariate analyses were entered into the Cox’s proportional hazards model (Table 5). In this multivariate analysis, breast cancer survival was determined more by tumour characteristics (i.e. grade and receptor status) than by receipt of chemotherapy and radiotherapy.
| Coefficient | SE | t | p > t | 95% CI | |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| No | (ref) | ||||
| Yes | 0.891 | 0.322 | –0.32 | 0.749 | 0.439 to 1.809 |
| Radiotherapy | |||||
| No | (ref) | ||||
| Yes | 0.979 | 0.335 | –0.06 | 0.951 | 0.501 to 1.913 |
| Surgery type | |||||
| Mastectomy | (ref) | ||||
| WLE | 0.475 | 0.185 | –1.91 | 0.056 | 0.221 to 1.019 |
| Grade | |||||
| 1 | (ref) | ||||
| 2 | 1.604 | 1.012 | 0.75 | 0.454 | 0.466 to 5.524 |
| 3 | 4.822 | 3.087 | 2.46 | 0.014a | 1.375 to 16.910 |
| Missing | 0.930 | 0.878 | –0.08 | 0.939 | 0.146 to 5.916 |
| ER or PR positive | |||||
| Yes | (ref) | ||||
| No | 2.720 | 0.949 | 2.87 | 0.004a | 1.373 to 5.390 |
| Missing | 1.785 | 1.133 | 0.91 | 0.361 | 0.515 to 6.194 |
| Tumour stage | |||||
| I | (ref) | ||||
| II and IIIa | 1.502 | 0.527 | 1.16 | 0.246 | 0.756 to 2.987 |
Health-related quality-of-life results
The average age of the 454 participants in this sample was 75.1 years (SD 6.2 years). Their mean SF-6D utility scores (0–1, increase = better health) at diagnosis (0.76, SD 0.15) were higher than at 3–4 years (0.70, SD 0.14), indicating reduced HRQoL for participants over this time (paired t-test p < 0.001). The average decrease in the utility score was –0.06 (SD 0.14). Of the 454 participants, 66 (14.5%) had chemotherapy and 313 (68.9%) had radiotherapy.
The difference in utility from diagnosis (baseline) to 3–4 years later does not differ significantly with receipt of chemotherapy (t-test p = 0.188) or radiotherapy (t-test p = 0.221), indicating that having these adjuvant therapies does not affect HRQoL in the long term. This result was confirmed by multiple regression analyses (Table 6) in which only functional status at diagnosis predicted changes in HRQoL. Participants dependent in ADL at diagnosis experienced an increase in HRQoL compared with those who were independent, possibly because of additional help from supportive services.
| Coefficient | SE | t | p > t | 95% CI | |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| No | (ref) | ||||
| Yes | –0.007 | 0.021 | –0.33 | 0.742 | –0.048 to 0.034 |
| Radiotherapy | |||||
| No | (ref) | ||||
| Yes | –0.016 | 0.019 | –0.86 | 0.392 | –0.054 to 0.021 |
| Surgery type | |||||
| Mastectomy | (ref) | ||||
| WLE | 0.016 | 0.019 | 0.84 | 0.400 | –0.021 to 0.053 |
| Age group (years) | |||||
| 65–69 | (ref) | ||||
| 70–74 | –0.020 | 0.018 | –1.11 | 0.269 | –0.057 to 0.016 |
| 75–79 | 0.019 | 0.020 | 0.92 | 0.356 | –0.021 to 0.059 |
| 80–84 | –0.024 | 0.024 | –1.00 | 0.316 | –0.072 to 0.023 |
| ≥ 85 | –0.015 | 0.030 | –0.49 | 0.623 | –0.074 to 0.044 |
| Grade | |||||
| 1 | (ref) | ||||
| 2 | 0.008 | 0.018 | 0.42 | 0.673 | –0.028 to 0.043 |
| 3 | –0.017 | 0.023 | –0.75 | 0.452 | –0.063 to 0.028 |
| Missing | 0.014 | 0.029 | 0.48 | 0.629 | –0.043 to 0.071 |
| ER or PR positive | |||||
| Yes | (ref) | ||||
| No | –0.011 | 0.025 | –0.44 | 0.658 | –0.060 to 0.038 |
| Missing | 0.025 | 0.029 | 0.84 | 0.400 | –0.033 to 0.082 |
| Tumour stage | |||||
| I | (ref) | ||||
| II and IIIa | 0.002 | 0.014 | 0.16 | 0.869 | –0.026 to 0.031 |
| Charlson Comorbidity Index | |||||
| 0 | (ref) | ||||
| 1 | –0.005 | 0.015 | –0.35 | 0.724 | –0.036 to 0.025 |
| ≥ 2 | –0.031 | 0.019 | –1.60 | 0.111 | –0.069 to 0.007 |
| Functional status | |||||
| Independent (1–2) | (ref) | ||||
| Dependent (3–4) | 0.107 | 0.026 | 4.17 | < 0.001 | 0.057 to 0.158 |
Quality-adjusted life-years
As recommended by NICE, effectiveness was measured by the difference in QALY gain of treatment adjusted for various age, health, choice and tumour variables (QALY gain of treatment = quality of life × lifetime with treatment – quality of life × lifetime without treatment). QALYs were calculated using the standard procedure described by Manca et al. 18 Lifetime was defined as time from completion of the SF-12 at baseline (diagnosis) to completion of the follow-up survey 3–4 years later or time to death. Adjustment for baseline HRQoL was made within multiple regression analyses.
The average age of the 548 participants in this sample was 75.6 years (SD 6.4 years). Of these participants, 15.0% had chemotherapy and 363 (66.2%) had radiotherapy. The average QALY gain was not significantly different for those who had chemotherapy (t-test p = 0.844). Participants having radiotherapy did appear to have significantly greater QALYs (2.17, SD 0.70) than those who did not (1.93, SD 0.85) (t-test p = 0.001). However, this gain did not persist in the multiple regression analyses, adjusting for baseline utility score as well as surgery type, age, comorbidity, functional status and tumour characteristics (Table 7).
| Coefficient | SE | t | p > t | 95% CI | |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| No | (ref) | ||||
| Yes | –0.038 | 0.087 | –0.44 | 0.659 | –0.210 to 0.133 |
| Radiotherapy | |||||
| No | (ref) | ||||
| Yes | 0.033 | 0.076 | 0.43 | 0.669 | –0.117 to 0.183 |
| Surgery type | |||||
| Mastectomy | (ref) | ||||
| WLE | 0.107 | 0.076 | 1.41 | 0.158 | –0.042 to 0.257 |
| Age group (years) | |||||
| 65–69 | (ref) | ||||
| 70–74 | –0.041 | 0.081 | –0.51 | 0.609 | –0.200 to 0.117 |
| 75–79 | –0.063 | 0.088 | –0.71 | 0.478 | –0.237 to 0.111 |
| 80–84 | –0.349 | 0.101 | –3.46 | 0.001 | –0.547 to –0.151 |
| ≥ 85 | –0.419 | 0.120 | –3.49 | 0.001 | –0.654 to –0.183 |
| Grade | |||||
| 1 | (ref) | ||||
| 2 | 0.017 | 0.078 | 0.21 | 0.832 | –0.137 to 0.170 |
| 3 | –0.198 | 0.096 | –2.06 | 0.040 | –0.388 to –0.009 |
| Missing | 0.093 | 0.123 | 0.76 | 0.450 | –0.149 to 0.335 |
| ER or PR positive | |||||
| Yes | (ref) | ||||
| No | –0.213 | 0.094 | –2.25 | 0.025 | –0.398 to –0.027 |
| Missing | –0.201 | 0.117 | –1.72 | 0.087 | –0.431 to 0.029 |
| Tumour stage | |||||
| I | (ref) | ||||
| II and IIIa | –0.011 | 0.061 | –0.17 | 0.862 | –0.130 to 0.109 |
| Charlson Comorbidity Index | |||||
| 0 | (ref) | ||||
| 1 | –0.091 | 0.065 | –1.39 | 0.164 | –0.219 to 0.037 |
| ≥ 2 | –0.074 | 0.081 | –0.92 | 0.358 | –0.233 to 0.085 |
| Functional status | |||||
| Independent (1–2) | (ref) | ||||
| Dependent (3–4) | 0.011 | 0.116 | 0.09 | 0.927 | –0.218 to 0.239 |
| Baseline utility (SF-6D) | 2.327 | 0.214 | 10.89 | < 0.001 | 1.908 to 2.747 |
Workstream 1 summary
In overview:
-
The studies of preference reveal that in about half of consultations the patient and surgeon both chose the same person as making the surgical decision, but the actual agreement between the surgeons and patients is low. In univariate analyses, increasing age predicts not undergoing surgery from the age of 75 years, compared with 70- to 74-year-olds. Adjusting for health measures and choice, only women aged > 85 years have reduced odds of surgery. Patient role in treatment decisions makes no difference to whether or not they receive surgery. Women who were active/collaborative were as likely to get surgery as those who left the decision to the surgeon. The qualitative study of women who did not receive primary surgery revealed three approaches: ‘patient declined’, ‘patient considered’ and ‘surgeon decided’.
-
Older age did not predict complications, and the risk of serious complications from breast surgery is low for older patients. Surgical decisions should be based on patient fitness rather than on age, even though age seems to be a factor taken into account by surgeons, especially for the ‘oldest old’ group as revealed in our study of choice. We were unable to build a pre-treatment risk screening tool as originally planned.
-
In our study of survival, the number of observed cancer deaths exceeded those expected for participants whose tumours were of higher grade or stage and steroid receptor negative, did not undergo surgery and warranted chemotherapy. Adjusting for tumour stage, comorbidity and functional status, women undergoing surgery had one-third the hazard of dying of breast cancer. Given these findings, it is hard to see on what basis surgery should be withheld from older women who are fit for surgery.
-
Following surgery, changes in HRQoL were not associated with getting the treatment decision-making style they preferred. Thus, it seems that the outcomes of consultation with the surgeon in terms of preferences were not detrimental per se to the women’s QoL in the longer term.
-
Many older women do not receive chemotherapy and radiotherapy following surgery, even though they may benefit from these therapies. Can this lack of chemotherapy and radiotherapy be explained by patient choice or health? We demonstrated that women aged ≥ 75 years have lower chemotherapy and radiotherapy rates than women aged 65–69 years. After adjusting for tumour characteristics, health measures and choice, women aged ≥ 75 years still have reduced odds of receiving chemotherapy, whereas age has no impact on the radiotherapy rates of older women. Therefore, lower chemotherapy rates in older women cannot be explained by health or patient choice.
Overall, although over the last decade there have been improvements in the access older women have to breast cancer services, there are still substantial gains to be made by ensuring that treatment decisions are based on ‘fitness’ and ability to benefit, rather than on age.
Workstream 2: comparison of multifrequency bioimpedance with early arm-volume increase in predicting lymphoedema by 18 months
Sentinel lymph node biopsy staging reduces the need for axillary node clearance (ANC), but 30% of breast cancer patients are node positive and require ANC to remove diseased nodes. 18,20
Lymphoedema (gross swelling of the arm) occurs when the lymphatic system is unable to keep up with the normal demands of tissue homeostasis, resulting in fluid accumulating in the interstitial spaces of the subcutaneous tissue. 21–23 If excess protein in the interstitial fluid (that causes the oedema) is allowed to persist, chronic inflammation can lead to fibrotic, thickened skin and tissues and progressive lymphoedema. 21–23 Up to 40% of patients report arm swelling by 18 months post ANC. 20,22,24
The consequences of lymphoedema are multidimensional and can involve physical and psychosocial morbidity. Recurrent infections of the arm (cellulitis) may occur, causing progression of the lymphoedema by further damage to the lymph vessels. 23,25,26 Patients report the limb being heavy and painful, experiencing impaired limb function and reduced shoulder mobility. 21–23 A clinical end point of a > 10% increase in ipsilateral arm volume (vs. contralateral arm) is an accepted criterion for a diagnosis of lymphoedema. 21–24,27
Most women present with established lymphoedema 1–2 years after surgery. 21–23,28 Its management is calculated to cost £350 per patient per year and £10M per annum to the NHS budget, including the cost of treating recurring infection with antibiotics and more intensive treatments when acute exacerbations occur. 23 Intervention before arm swelling becomes chronic may prevent the complications of lymphoedema after ANC. Recent evidence from a prospective cohort study in which preoperative perometer monitoring identified 43 women after ANC with an early RAVI of > 3%, in whom provision of compression garments prevented any further RAVI at 6 months’ follow-up (no lymphoedema developed), has led to claims that the standard of postoperative care should routinely include prospective arm measurement to intervene in the development of so-called ‘preclinical’ lymphoedema. However, this lacks a robust evidence base and the proposed intervention has never been tested in a multicentre randomised trial setting. 29
An alternative definition of lymphoedema is the application of compression sleeve garments, as some women develop hand or lower arm swelling that does not reach the overall 10% arm-volume increase but represents clinical practice by lymphoedema practitioners.
Multifrequency bioimpedance electrical analysis is a non-invasive technique to measure total water content, which involves passing extremely small electrical currents through the body and measuring the impedance (or resistance) to the flow of these currents. In recent years the BEA technique has been refined to measure the impedance over a range of frequencies from 4 to 1000 kHz. By mathematically modelling the measured data, the impedance at zero frequency (i.e. the impedance of the extracellular fluid alone) can be determined. 22,30,31 BEA is used to quantitatively compare the degree of fluid accumulation in the arms using a leg as the reference limb, and a 3SD change in BEA is claimed to accurately diagnose lymphoedema. Small single-centre prospective studies in Australia have claimed that BEA predicts lymphoedema development up to 10 months ahead of arm-volume changes with a sensitivity of 98% and a specificity of 100%. 30,31
Bioimpedance electrical analysis can be measured with a handheld device and is marketed as safe, accurate and diagnostic for lymphoedema (in the absence of confirmed arm swelling of > 10%) to justify early treatment intervention in women after axillary surgery. BEA correlates with arm measurement in lymphoedema patients, but is reported to be more sensitive than and equally as specific as arm circumference measures, particularly in women whose ANC involves the non-dominant arm lymphatics. 30,31
We assessed BEA monitoring compared with perometer arm measurements in women after ANC. BEA monitoring during the study was used to determine its value in predicting response to compression garment therapy. 22,23 Within the study we assessed reproducibility of both methods across all centres and robustly established both intra- and interobserver error rates for both methods in the study population.
Training in the use of L-DEX U400 BIS devices was provided for all centres with the appropriate software and electrodes to carry out a health technology assessment (see BEA protocol). 32,33
All women undergoing ANC in the UK breast units underwent preoperative 1-, 3-, 6-, 12- and 18-monthly bilateral arm measurements with a perometer (Pero-Systems 350S) and circumferential arm tape measurements as well as perometer measurements.
All centres monitored women undergoing ANC from pre-surgery baseline with perometer measurements and BEA to compare the sensitivity and specificity of both techniques for predicting chronic lymphoedema development. Identifying the most sensitive and specific method for detecting chronic lymphoedema would enhance selection of patients for intervention with arm sleeves should the intervention prove cost-effective.
Study design
Women undergoing ANC for breast cancer were approached for baseline (preoperative) and subsequent BEA monitoring, along with perometer arm measurements, in initially seven centres with an increase to 21 across the UK (see flow diagram in protocol34). First, a comparison of the sensitivity and specificity of BEA versus perometer measurement was made in women who developed arm swelling of > 10% by 6 months [based on the ALMANAC (Axillary Lymphatic Mapping Against Nodal Axillary Clearance) trial, we estimated this in advance at 210/1000 (21%) of the initial group]. Second, women with an arm-volume increase of 4–8% at 1, 3 or 6 months where effectively the BEA 6 months readings were to be compared with final 18-month perometer scores to assess the prediction of lymphoedema at 18 months by BEA. Third, women with a < 4% perometer arm-volume increase up to 6 months were to be used to determine the sensitivity and specificity of BEA 6-month measures compared with the perometer 6-month measurement in predicting the 18-month outcome.
Sample size calculation
We were required to screen 1000 patients to enrol enough women into the PLACE trial using perometer measurements, which allowed us to determine if BEA had a > 80% sensitive and a > 80% specific accuracy. Currently the specificity of arm swelling measured by perometer is 87% specific for subsequent lymphoedema at 18 months with a sensitivity of 54% (assessed from ALMANAC data). 34
Older age, increases in body mass index (BMI) and postoperative radiotherapy are claimed to increase lymphoedema development. 21–23,28–31 We built a multivariate model predicting lymphoedema from the following potential predictor variables: BMI, dominant limb, postoperative radiotherapy, previous sentinel node biopsy, cigarette smoking, weight gain and age. This allowed us to identify what factors, as well as early arm-volume changes or BEA, predict subsequent development of lymphoedema. Although we anticipated 1000 patients recruited by 24 months of the programme to allow us to build a multivariate model, delays to sites opening meant that 1100 were recruited by June 2015. Multiple logistic regression modelling techniques were used to identify significant predictors of lymphoedema at early (18 months) and late time points (24 months) in the participants.
Workstream 2 multifrequency bioimpedance study: results of a multicentre prospective study
Among the 1100 women recruited to the trial undergoing ANC surgery for breast cancer from nine centres in England, the median age was 56 years (range 22–90 years). They have undergone preoperative and subsequent regular measurements post surgery (1, 3, 6, 9 and 12 months, then 6-monthly) of arm volume by perometry (Perometer 350 NT; www.pero-system.de) and multifrequency BIS (L-Dex® U400; www.impedimed.com) and currently have a minimum 24 months’ follow-up surveillance. Change in arm volume was calculated using relative arm-volume change (RAVI).
The primary end point of lymphoedema was defined as a ≥ 10% limb volume change, compared with the contralateral arm, by perometry. 24,29 BIS L-Dex change of 10 was considered the diagnostic criterion for lymphoedema. There is considerable variation in the definitions of lymphoedema and methods of measurement, ranging from the more conservative ≥ 10% limb volume change by perometry, through volume increases of 200 ml by perometry, to the more liberal increase of 2 cm in circumference. 20,24 For the purposes of this study, we used a > 10% arm-volume increase (RAVI) since baseline (compared with the contralateral arm) as measured by perometer on at least two occasions to identify women with lymphoedema secondary to ANC. 29
We also used a clinical definition of compression sleeve application (excluding patients who had sleeves applied as part of the intervention arm in the PLACE trial). Lymphoedema determined by BIS was defined as an increase of ≥ 10 units from baseline.
Arms were measured using a 350S perometer with standard perometer software supplied by Pero-System, Wuppertal, Germany. The average of two perometer measurements was used at each visit to exclude intraobserver variability. BIS intracellular fluid was measured using the L-Dex® U400 BIS devices on loan from ImpediMed Ltd (Pinkenba, QLD, Australia). 30,31
At least 50% of breast cancer patients gain weight in the first year after diagnosis, which is associated with increased risk of lymphoedema. Nonetheless, if careful contralateral arm measurements are not performed, weight gain, rather than lymphoedema, can lead to inappropriate fitting of compression sleeves. BIS results are unaltered by weight gain and we tested whether the BIS results were sensitive and/or more specific than perometer measurements in detecting early and later arm swelling.
Self-reported symptoms and quality-of-life measures
Patients were asked to complete a lymphoedema questionnaire, which used three items from the Lymphedema and Breast Cancer Questionnaire about heaviness, numbness and swelling, and the Functional Assessment of Cancer Therapy – Breast Cancer, version 4 (FACT-B+4) Questionnaire (www.facit.org/FACITOrg/Questionnaires) and the EuroQol-5 Dimensions (EQ-5D) (www.euroqol.org/about-eq-5d.html) to assess self-reported upper limb symptoms, physical functioning disease-specific QoL and health utility. All questionnaires were completed preoperatively and then again at 3 and 6 months post surgery, with the exception of the EQ-5D, which was not completed at 3 months post surgery.
Statistical analysis
Statistical analysis included sensitivity and specificity analysis of the BIS L-Dex score against the ‘gold standards’ of perometer assessment at 6, 18 and 24 months (and subsequently clinical sleeve application) using statistical techniques recommended by Bland and Altman. 35 The BIS value cut-off level was checked using receiver operating characteristic (ROC) analysis and confirmed using later results. An assessment of the relationship between the two methods of measurement up to 2 years in predicting lymphoedema was performed. The analysis for the current report involved comparison of the baseline and 6-, 18- and 24-month post-surgery measurements using paired t-tests, and comparison between groups defined by lymphoedema status using independent t-tests, and data were described using means and ranges, sensitivity and specificity, and univariate and multivariate analyses. ROC analysis, Cox proportional hazards regression, log-rank testing and generalised estimating equation (GEE) regression were performed for univariate and multivariate analyses. The GEE regression was chosen as the inference was at the population level so the GEE marginal effects were of interest. Descriptive methods were used for all other data presented.
Results
Out of the 1100 patients entered into the study (median follow-up 36 months, minimum 24 months), the mean age was 55.7 years (range 22 to 90 years), 47.0% had a mastectomy and ANC, 90.5% were node positive, 70.9% had a histology of infiltrating ductal carcinoma and the majority (80.6%) were estrogen receptor (ER) positive (Table 8). Eighty-three per cent received postoperative radiotherapy, 67.3% received chemotherapy and 82.4% were given endocrine treatment. Fifty-eight patients (5%) had no post-1-month perometer measurements. Overall, 497 patients have completed 60 months’ follow-up, 105 have died and 204 have been lost to follow-up (or withdrawn from the study).
| Demographic | n (%) |
|---|---|
| Age (N = 1088) | |
| Mean (SD) [range] | 55.7 (12.4) [22 to 90] |
| BMI (kg/m2) (pre-op) (N = 1071) | |
| Median (IQR) [range] | 27.3 (24.0 to 31.2) [16.6 to 60.0] |
| Weight gain at 3 months (as % of baseline) (N = 916) | |
| Mean (SD) [range] | –0.1 (4.0) [–12.8 to 24.2] |
| Side of ANC (N = 1096) | |
| Right : left | 550 (50.2) : 546 (49.8) |
| Dominant hand (N = 1096) | |
| Right : left | 998 (91.0) : 98 (9.0) |
| Smoking history (N = 1094) | |
| Never | 651 (59.5) |
| Ex-smoker | 319 (29.2) |
| Current smoker | 124 (11.3) |
| Previous SN biopsy: yes | 368 (34.3) |
| Type of ANC surgery (N = 1089) | |
| ANC | 257 (23.6) |
| WLE + ANC | 309 (28.4) |
| Mastectomy + ANC | 512 (47.0) |
| Other | 11 (1.0) |
| Histology (N = 1087) | |
| Infiltrating ductal | 771 (70.9) |
| Infiltrating lobular | 125 (11.5) |
| DCIS | 27 (2.5) |
| LCIS | 2 (0.2) |
| Mixed invasive | 91 (8.4) |
| Other | 71 (6.5) |
| Pathological tumour size, (mm) (N = 1078) | |
| Median (IQR) [range] | 26.0 (18.0 to 40.0) [0 to 220] |
| Grade (N = 1080) | |
| 1 | 63 (5.8) |
| 2 | 477 (44.2) |
| 3 | 501 (46.4) |
| Ungraded | 39 (3.6) |
| Number of nodes removed (N = 1088) | |
| Median (IQR) [range] | 17.0 (13.0 to 23.0) [1 to 56] |
| Number of nodes involved (N = 1088) | |
| Median (IQR) [range] | 2.0 (1.0 to 5.8) [0 to 46] |
| Node positive | 985 (90.5) |
| ER negative : ER positive | 208 (19.4) : 864 (80.6) |
| HER-2 (N = 1072) | |
| Negative | 811 (75.7) |
| Amplified | 82 (7.6) |
| 3+ | 179 (16.7) |
| ER, HER–2 combination (N = 1066) | |
| ER negative, HER-2 negative | 152 (14.3) |
| ER negative, HER-2 3+ | 56 (5.3) |
| ER positive, HER-2 negative | 735 (68.9) |
| ER positive, HER-2 3+ | 123 (11.5) |
| Post-operative radiotherapy: yes (N = 1062) | 878 (82.7) |
| Post-operative chemotherapy: yes (N = 1060) | 713 (67.3) |
| Post-operative endocrine therapy: yes (N = 1061) | 874 (82.4) |
| Any disease recurrence: yes | 134 (12.3) |
| Time (years) to first disease recurrence, median (IQR) [range] | 1.44 (0.60 to 2.63) [0.05 to 5.04] |
| Time (years) in study (from definitive surgery) (N = 1072) | |
| Median (IQR) [range] | 3.00 (1.98 to 4.03) [0.06a to 5.51] |
Lymphoedema assessment within the protocol was defined as the development of an arm-volume increase (RAVI) of > 10% but, on reviewing the data, compression sleeves were being applied outside the protocol indications. For some patients, significant swelling of the lower arm or hand or swelling of < 10% RAVI but associated with symptoms led to the application of a compression sleeve by the lymphoedema practitioner. We ascertained this by retrieving the perometer readings for all patients with a sleeve applied. Compression garment application was another surrogate marker of lymphoedema and potentially a better clinical marker. Time to lymphoedema for RAVI of > 10% at follow-up and that for sleeve application are presented.
The median time to developing lymphoedema was 11.3 months (range 2.3–63.1 months). Lymphoedema incidence (sleeve application and RAVI of > 10%) is shown in Tables 9 and 10. The incidence of lymphoedema differed by the definition of either a clinical sleeve application by a lymphoedema nurse or the perometer RAVI of > 10% after 24 months’ follow-up.
| Follow-up date (months) | ||||||
|---|---|---|---|---|---|---|
| ≤ 3 | > 3 and ≤ 6 | > 6 and ≤ 9 | > 9 and ≤ 12 | > 12 and ≤ 18 | > 18 and ≤ 24 | |
| n at risk | 1001 | 925 | 848 | 798 | 722 | 647 |
| Perometer RAVI of ≥ 10% | ||||||
| During interval | 33 | 54 | 27 | 24 | 31 | 25 |
| Total number | 33 | 87 | 114 | 138 | 169 | 194 |
| Kaplan–Meiera probability of event (%) | 3.4 | 9.0 | 11.9 | 14.6 | 18.2 | 21.4 |
| Follow-up date (months) | ||||||
|---|---|---|---|---|---|---|
| ≤ 3 | > 3 and ≤ 6 | > 6 and ≤ 9 | > 9 and ≤ 12 | > 12 and ≤ 18 | > 18 and ≤ 24 | |
| n at risk | 999 | 928 | 856 | 789 | 697 | 622 |
| Lymphoedema | ||||||
| During interval | 29 | 48 | 46 | 43 | 31 | 24 |
| Total number | 29 | 77 | 123 | 166 | 197 | 221 |
| Kaplan–Meiera probability of event (%) | 3.0 | 8.0 | 12.9 | 17.7 | 21.3 | 24.4 |
Using Kaplan–Meier estimates for time to diagnosis of lymphoedema, 14.6% using RAVI and 17.7% by sleeve application were diagnosed by 12 months, and 21.4% and 24.4% were diagnosed by 24 months, respectively (Figure 9).
There was clinical lymphoedema diagnosis/applied sleeve in 24.4% patients by 24 months, compared with 21.4% with RAVI of > 10% during follow-up. The majority of this difference appeared to occur between 6 and 12 months, when more sleeves were applied. This was partly due to the patients who were not eligible for the PLACE trial but had a perometer value RAVI of > 9% and who therefore went on to compression sleeves.
Lymphoedema by 24 months detected in 21.4% of women by perometry whereas using BIS definition in 39.4%. A moderate correlation between perometer and BIS at 6 months (r = 0.61) was found, with a sensitivity of 76% (95% CI 64% to 84%), specificity of 85% (95% CI 83% to 88%) and PPV of BIS of 31% (95% CI 25% to 39%) (Table 11). Sensitivity remained similar at 24 months (75%, 95% CI 64% to 83%), though specificity was higher (91%, 95% CI 89% to 93%), as was PPV of BIS (54%, 95% CI 44% to 63%). The sensitivity and specificity values for BIS fall below the percentage of 95% required according to the study protocol.
| No lymphoedema (perometer definition: RAVI of < 10%) | Lymphoedema (perometer definition: RAVI of > 10%) | Total | |
|---|---|---|---|
| By 6 months | |||
| BIS (< 10) | 698 (82%) true negative | 27 (31%) false negative | 725 |
| BIS (≥ 10) | 153 (18%) false positive | 59 (69%) true positive | 212 |
| Total | 851 | 86 | 937 |
| After 6 months up to 18 months | |||
| BIS (< 10) | 600 (81%) | 25 (32%) | 625 |
| BIS (≥ 10) | 138 (19%) | 53 (68%) | 191 |
| Total | 738 | 78 | 816 |
| After 6 months up to 24 months | |||
| BIS (< 10) | 572 (79%) | 32 (32%) | 604 |
| BIS (≥ 10) | 150 (21%) | 68 (68%) | 218 |
| Total | 722 | 100 | 822 |
Women who developed a RAVI of > 5 to < 10% by 6 months required lymphoedema treatment in 35% of cases by 24 months, whereas a RAVI of < 3% was associated with an 8% lymphoedema rate at 24 months (p < 0.001).
The sensitivity and specificity of BIS and perometer were compared according to sleeve application using sleeve application as the clinical diagnosis of lymphoedema, as well as against the protocol defined perometer, RAVI of > 10%. There were 226 patients with an appropriately applied sleeve, which included patients with a sleeve applied and patients who had > 10% lymphoedema and were offered a sleeve by the lymphoedema nurse but declined it because they already had metastatic disease and were about to die. In addition, 51 patients had their sleeve applied as part of the PLACE trial; nine patients contralateral sleeve application and these patients were excluded from the analysis.
Perometer and bioimpedance spectroscopy comparison
After reviewing the perometer and lymphoedema nursing data from all patients with RAVI of > 10% or a sleeve applied, 226 patients fitted the clinical lymphoedema definition of sleeve appropriately fitted or RAVI of > 10%. There were 25 patients with sleeves applied who were deemed not to have clinical lymphoedema because there was insufficient evidence in the notes; 29 patients did not have a sleeve applied but were deemed to have clinical lymphoedema (predominantly localised lower arm swelling or RAVI of > 10%).
Perometer by 6 months
The perometer by 6 months variable includes those women with lymphoedema at 3 or 6 months.
For those with lymphoedema according to the RAVI of ≥ 10% definition, the BIS value used is the one at the time of the lymphoedema diagnosis. For those without lymphoedema according to the RAVI of ≥ 10% definition, the largest BIS value at either time point was used (Figure 3).
FIGURE 3.
Comparison of perometer and BIS at 6 months. Q1, RAVI only of ≥ 10; Q2, both ≥ 10; Q3, neither ≥ 10; Q4, BIS only of ≥ 10. Sensitivity, 69% (59/86; 95% CI 58% to 77%); specificity, 82% (698/851; 95% CI 79% to 84%); PPV, 28% (59/212; 95% CI 22% to 34%); NPV, 96% (698/725; 95% CI 95% to 97%). NPV, negative predictive value.

At all time points BIS identified high numbers of false-positive patients with a lymphoedema diagnosis (using RAVI of > 10%).
Relative arm-volume increase after 6 months up to 18 months
The RAVI after 6 months up to 18 months variable excludes those with lymphoedema up to and including 6 months.
For those with lymphoedema according to the RAVI of ≥ 10% definition, the BIS value used is the one at the time of the lymphoedema diagnosis. For those without lymphoedema according to the RAVI of ≥ 10% definition, the BIS value is the largest value from 9 to 18 months (Figure 4).
FIGURE 4.
Comparison of RAVI and BIS at 18 months. Q1, RAVI only of ≥ 10; Q2, both ≥ 10; Q3, neither ≥ 10; Q4, BIS only of ≥ 10. Sensitivity, 68% (53/78; 95% CI 57% to 77%); specificity, 81% (600/738; 95% CI 78% to 84%); PPV, 28% (53/191; 95% CI 22% to 34%); NPV, 96% (600/625; 95% CI 94% to 97%). NPV, negative predictive value.
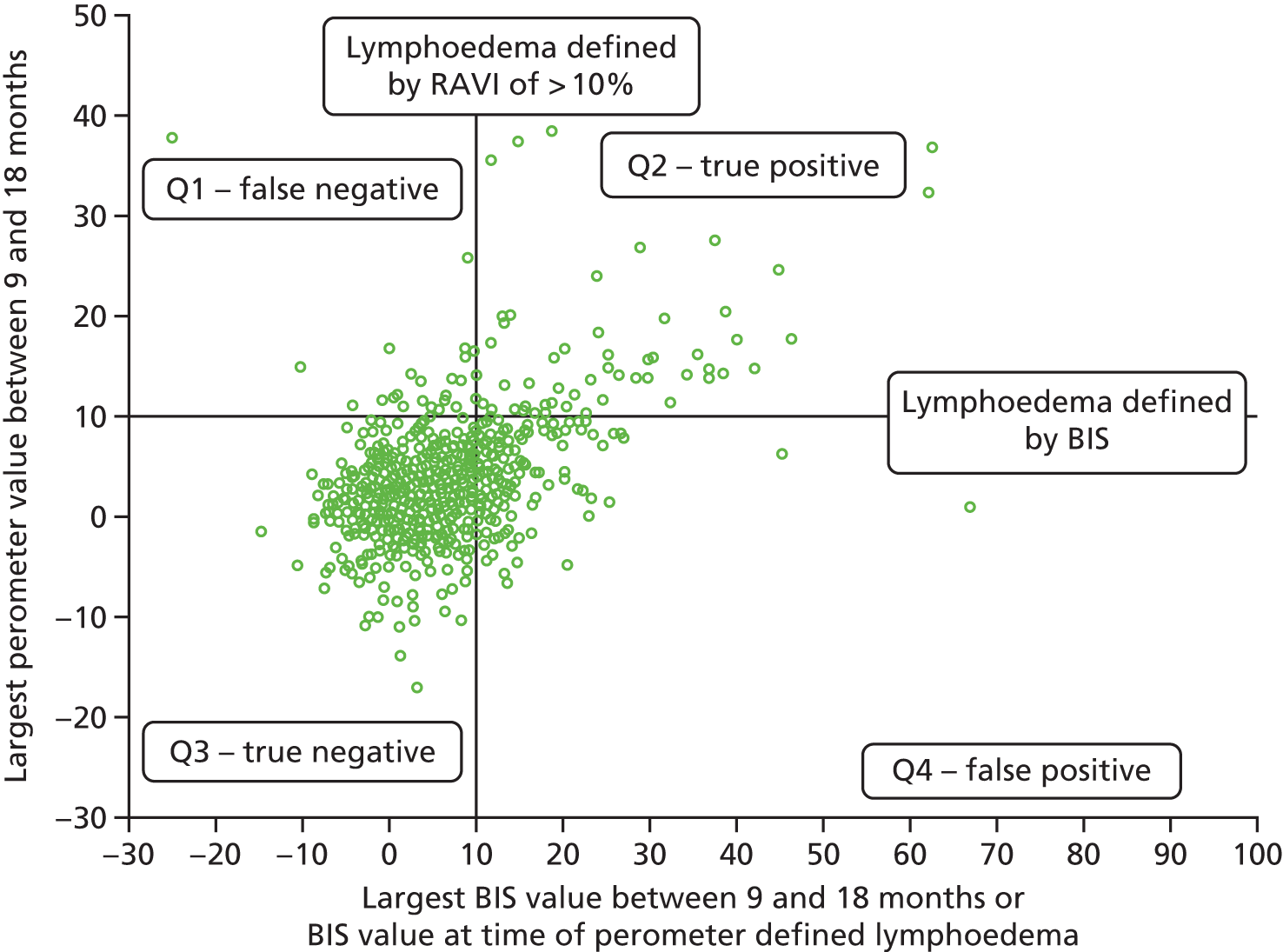
Relative arm-volume increase of > 10% after 6 up to 24 months (excludes those with lymphoedema up to and including 6 months)
For those with lymphoedema according to the RAVI of ≥ 10% definition, the BIS value used is the one at the time of lymphoedema diagnosis. For those without lymphoedema, the BIS value used is the largest value between 9 and 24 months (Figure 5).
FIGURE 5.
Comparison of RAVI and BIS after 6 months up to 24 months. Q1, RAVI only of ≥ 10; Q2, both ≥ 10; Q3, neither ≥ 10; Q4, BIS only of ≥ 10. Sensitivity, 68% (15/22; 95% CI 58% to 76%); specificity, 79% (572/722; 95% CI 76% to 82%); PPV, 31% (68/218; 95% CI 25% to 38%); NPV, 95% (572/604; 95% CI 93% to 96%). NPV, negative predictive value.
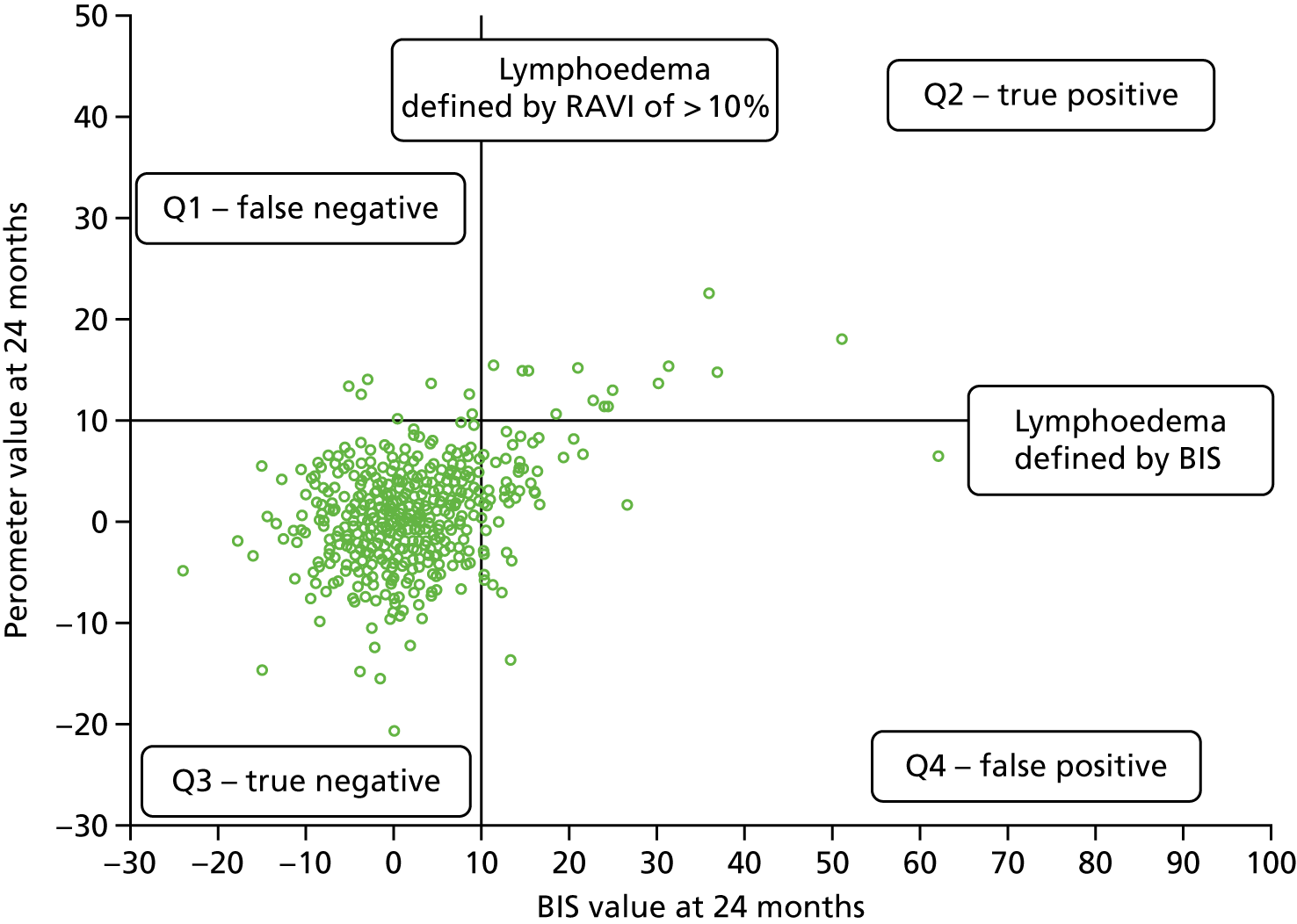
Clinical lymphoedema/appropriately applied sleeve from 6 up to 18 months
The clinical lymphoedema/applied sleeve between 6 and 18 months variable excludes those with lymphoedema up to and including 6 months (see Table 12 and Figure 6).
FIGURE 6.
Comparison of RAVI of > 10% and BIS of > 10% with sleeve application. NB those with no clinical lymphoedema or applied sleeve by 6 months may have had an applied sleeve at a later time point.
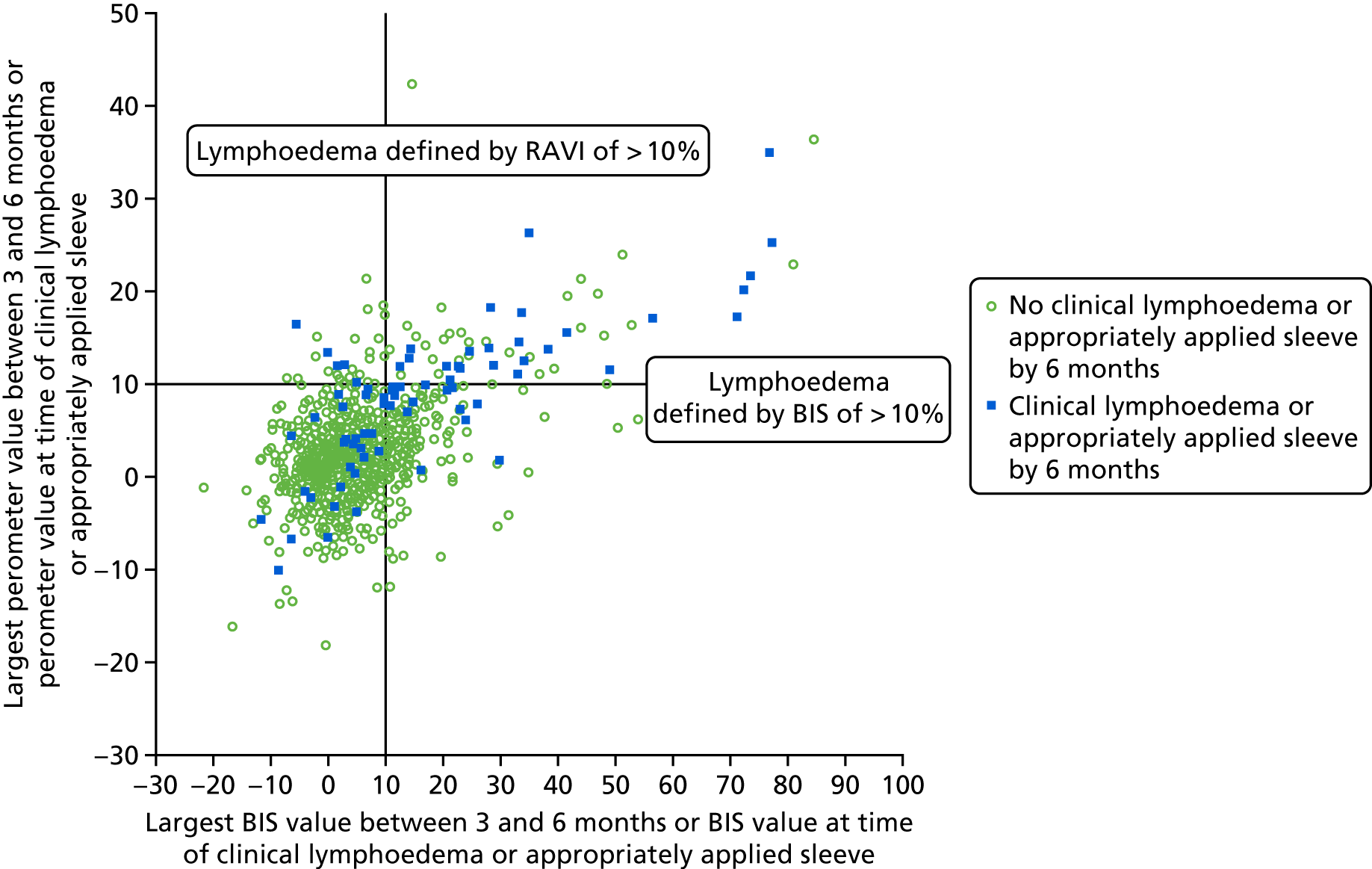
At all time points, BIS would have resulted in more sleeves being applied than RAVI of > 10% incorrectly with lower specificity (Tables 12–15). RAVI of > 10% was better at identifying patients who could be reassured and did not need surveillance proving cost-effective to the NHS. It ruled out patients unlikely to develop lymphoedema and has a higher PPV at 18 months.
| By 6 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| RAVI (< 10%) | 820 (94%) | 45 (60%) | 865 |
| RAVI (≥ 10%) | 55 (6%) | 30 (40%) | 85 |
| Total | 875 | 75 | 950 |
| By 6 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 690 (80%) true negative | 34 (46%) false negative | 724 |
| BIS (≥ 10) | 170 (20%) false positive | 40 (54%) true positive | 210 |
| Total | 860 | 74 | 934 |
| After 6 months up to 18 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| RAVI (< 10%) | 693 (95%) true negative | 72 (62%) false negative | 765 |
| RAVI (≥ 10%) | 33 (5%) false positive | 45 (38%) true positive | 78 |
| Total | 726 | 117 | 843 |
| After 6 months up to 18 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 581 (82%) true negative | 52 (47%) false negative | 633 |
| BIS (≥ 10) | 126 (18%) false positive | 59 (53%) true positive | 185 |
| Total | 707 | 111 | 818 |
For those with lymphoedema, the BIS and perometer values used are those at the time of lymphoedema diagnosis. For those without lymphoedema, the BIS and perometer values used are the largest value between 9 and 18 months.
Up to 6 months: sleeve application in at least one-third of patients appeared to be as a result of a composite of significant arm symptoms (swelling and heaviness) together with arm-volume increases. The PPV for RAVI of > 10% at both time points is superior to BIS, although the negative predictive values (NPVs) are similar (Figure 7).
FIGURE 7.
Comparison of RAVI of > 10% and BIS with sleeve application (6–18 months). Note that those with no clinical lymphoedema or appropriately applied sleeve by 6 months may have had clinical lymphoedema or an applied sleeve at a later time point.
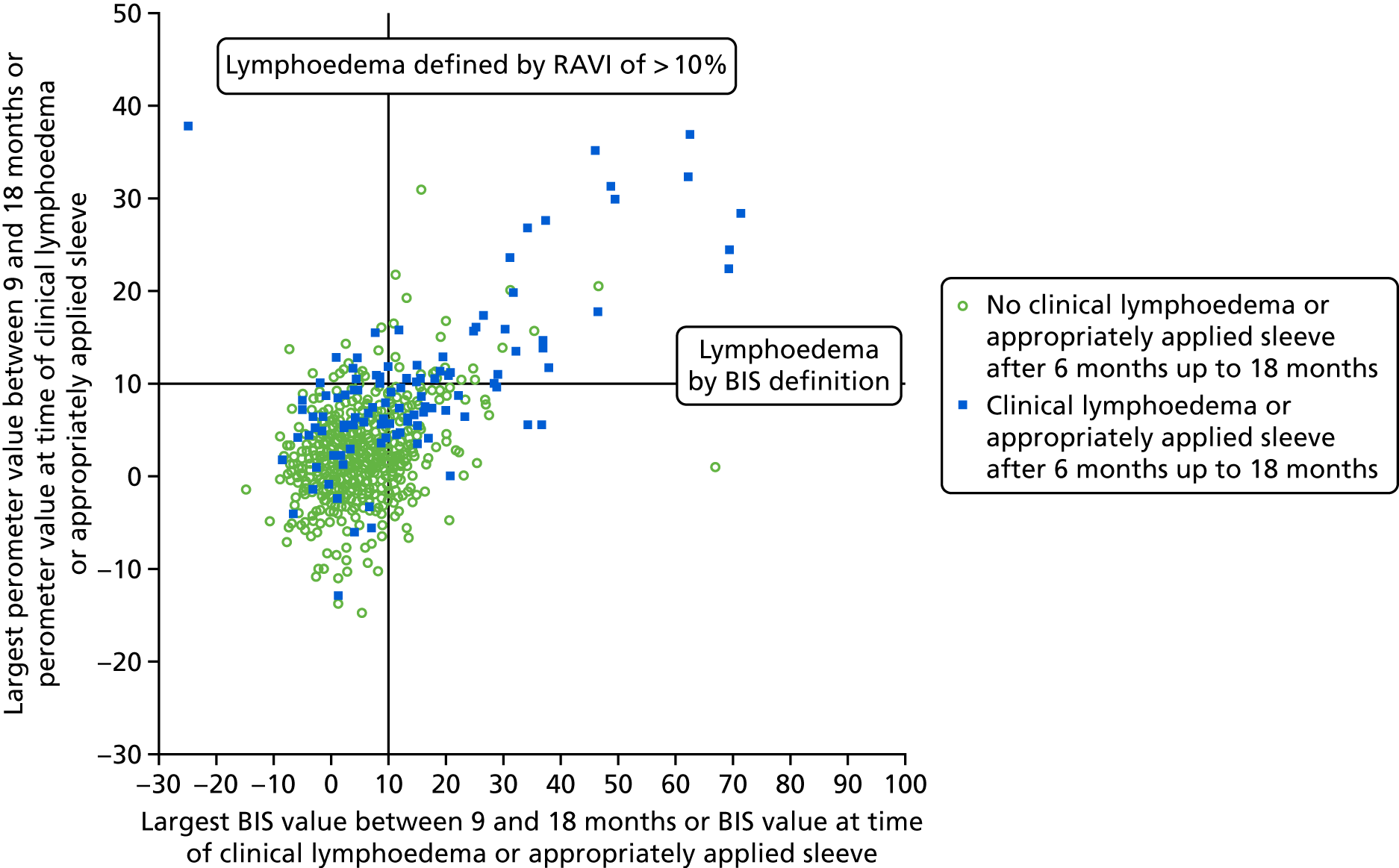
Clinical lymphoedema/applied sleeve after 6 months up to 24 months
The clinical lymphoedema/applied sleeve after 6 up to 24 months variable excludes those with lymphoedema up to and including 6 months.
For those with lymphoedema, the BIS and RAVI values used are those at the time of lymphoedema diagnosis. For those without lymphoedema, the BIS and RAVI values used are the largest value between 9 and 24 months (Table 16).
| After 6 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| RAVI (< 10%) | 667 (94%) | 86 (61%) | 753 |
| RAVI (≥ 10%) | 39 (6%) | 55 (39%) | 94 |
| Total | 706 | 141 | 847 |
It is apparent that perometer is more specific (94–96%) than BIS (80–91%) at all time points. In other words, RAVI measurement gets more diagnoses of sleeve application correct and fewer wrong, particularly at 6 months. Thus, it is noticeable that at 6 months BIS of > 10 had 170 false positives, yet identified only 40 out of the 74 sleeves that were applied, whereas perometer identified 30 out of the 75 sleeves applied but overdiagnosed only 55 rather than 170 patients (Table 17). The differences in sleeve application numbers reflect the fact that some patients did not have a BIS measurement. BIS use would have meant that patients had far more sleeves applied than those using RAVI of > 10%, but neither method was particularly sensitive (see Figure 8).
| After 6 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 556 (80%) | 65 (49%) | 621 |
| BIS (≥ 10) | 136 (20%) | 69 (51%) | 205 |
| Total | 692 | 134 | 826 |
FIGURE 8.
Comparison of RAVI of > 10% and BIS with sleeve application (6–24 months). Note that those women with no clinical lymphoedema or appropriately applied sleeve after 6 months and up to 24 months may have had clinical lymphoedema or a sleeve applied at a later time point.
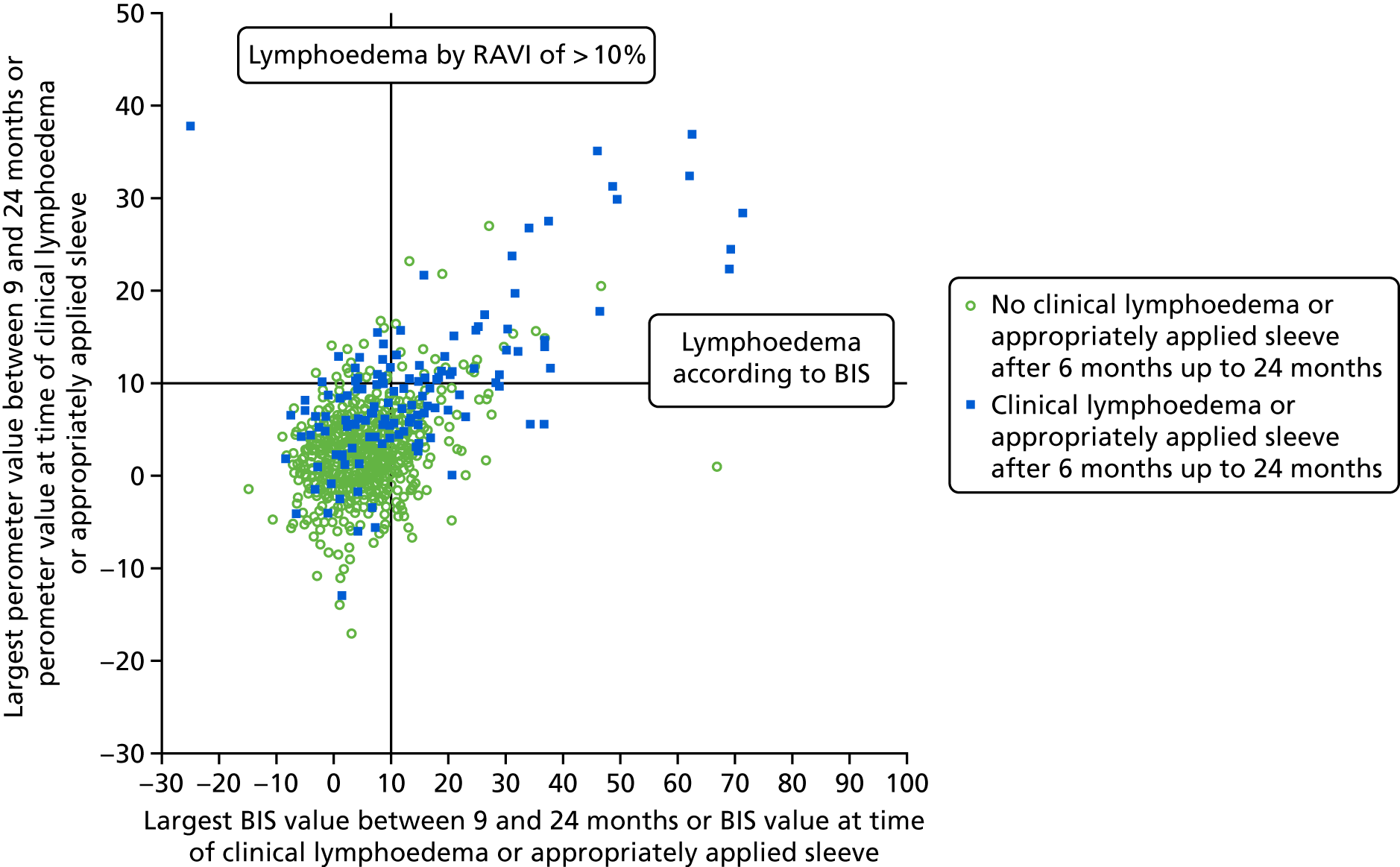
We then looked at the sensitivity and specificity of RAVI and BIS at 6, 18 and 24 months (i.e. comparing both methods with each other). Once again, BIS of > 10 identified those patients with a RAVI of > 10% measurement in 68–76% of cases.
Combined relative arm-volume increase or bioimpedance spectroscopy versus clinical lymphoedema/appropriately applied sleeve
We considered whether combining RAVI of > 10% and BIS of > 10% improved the diagnosis of lymphoedema compared with sleeve application. At all time points it reduced PPV, although sensitivity increased slightly (Table 18).
| By 6 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| RAVI of < 10% and BIS of < 10 | 663 (78%) | 28 (38%) | 691 |
| RAVI of > 10% or BIS of ≥ 10 | 192 (22%) | 45 (62%) | 237 |
| Total | 855 | 73 | 928 |
Clinical lymphoedema/applied sleeve after 6 months up to 24 months (excludes those with lymphoedema up to and including 6 months)
The BIS and RAVI values used are those at the time of the indicated lymphoedema. For those without lymphoedema, the BIS and RAVI values used are the largest value between 9 and 24 months (Table 19).
| After 6 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| RAVI and BIS of < 10 | 537 (78%) | 52 (38%) | 589 |
| RAVI or BIS of ≥ 10 | 154 (22%) | 85 (62%) | 239 |
| Total | 691 | 137 | 828 |
The 85 patients with lymphoedema are made up of 39 with both RAVI and BIS of ≥ 10, 30 with only BIS of ≥ 10 and 16 with only RAVI of ≥ 10%.
Predictive value of bioimpedance spectroscopy value by 6 months against lymphoedema by 18 or 24 months
Lymphoedema defined by RAVI of ≥ 10% and clinical lymphoedema or applied sleeve.
In all of the analyses that follow, any patients diagnosed with a RAVI value of > 10% by 6 months were excluded from the analysis (n = 87) and any patients with a clinical lymphoedema or sleeve applied before 6 months were excluded from the analysis (Table 20).
| BIS value by 6 months | Lymphoedema defined by perometer RAVI > 10% | Clinical lymphoedema or appropriately applied sleeve | ||
|---|---|---|---|---|
| No lymphoedema by 18 months (n = 662) | Lymphoedema by 18 months (n = 77) | No lymphoedema by 18 months (n = 643) | Lymphoedema by 18 months (n = 114) | |
| < 3 | 327 (93%) | 23 (7%) | 324 (93%) | 25 (7%) |
| > 3 to < 5 | 80 (91%) | 8 (9%) | 78 (90%) | 9 (10%) |
| > 5 to < 10 | 156 (92%) | 14 (8%) | 145 (84%) | 27 (16%) |
| > 10 | 99 (76%) | 32 (24%) | 96 (64%) | 53 (36%) |
There is a significant relationship between both BIS category by 6 months and lymphoedema defined by perometer of ≥ 10% by 18 months (p < 0.001) and clinical lymphoedema or applied sleeve by 18 months (p < 0.001).
For lymphoedema defined by RAVI of > 10% the significant relationship appears to be as a result of the higher rate of lymphoedema, 24%, in those with BIS score of ≥ 10 (which is the BIS definition of lymphoedema).
For clinical lymphoedema/applied sleeve there appears to be a small increase in lymphoedema rate across the BIS < 3, ≥ 3 to < 5, and ≥ 5 to < 10 categories from 7% to 16% across the three categories. The significant relationship appears mainly to be as a result of the higher rate of lymphoedema, 36%, in those with BIS of ≥ 10 (Table 21).
| BIS value by 6 months | Lymphoedema defined by RAVI of > 10% | Clinical lymphoedema or appropriately applied sleeve | ||
|---|---|---|---|---|
| No lymphoedema by 24 months (n = 596) | Lymphoedema by 24 months (n = 101) | No lymphoedema by 24 months (n = 577) | Lymphoedema by 24 months (n = 137) | |
| < 3 | 298 (91%) | 30 (9%) | 297 (91%) | 31 (9%) |
| > 3 to < 5 | 68 (85%) | 12 (15%) | 66 (85%) | 12 (15%) |
| > 5 to < 10 | 142 (87%) | 21 (13%) | 128 (78%) | 36 (22%) |
| > 10 | 88 (70%) | 38 (30%) | 86 (60%) | 58 (40%) |
There is a relationship between both BIS category by 6 months and lymphoedema defined by perometer of ≥ 10% (p < 0.001) and clinical lymphoedema or applied sleeve by 24 months (p < 0.001).
For lymphoedema defined by perometer of ≥ 10% there appears to be little difference in the lymphoedema rate in the < 3, ≥ 3 to < 5, and ≥ 5 to < 10 categories; the rate was between 9% and 15% in each of those categories. The relationship appears to be as a result of the higher rate of lymphoedema, 30%, in those with BIS of ≥ 10.
For clinical lymphoedema or applied sleeve, there is an increase in the lymphoedema rate across all four categories, with smaller increases across the first three categories and a larger increase in the rate of lymphoedema in those with BIS of ≥ 10, which is the diagnostic category for lymphoedema according to BIS.
Prediction of lymphoedema
Two analyses were performed: one looked at the situation described above (lymphoedema after 6 months and up to 2 years), and the other looked at the time to first lymphoedema including all follow-up data (1-month visit was excluded as per the protocol, version 5.2, and the NIHR Programme Grants for Applied Research programme response letter). Both RAVI (> 10%) and sleeve application were considered in these analyses.
Factors predicting lymphoedema from baseline
For RAVI of > 10% as the end point, univariate analysis revealed BMI (p = 0.004), age (p = 0.013), previous sentinel node biopsy (p = 0.027), ER status (p = 0.006: ER negativity: HR 1.59, 95% CI 1.14 to 2.21), and number of nodes involved (p < 0.001) all predicted lymphoedema development. If one only considers those with confirmed or absent lymphoedema by 24 months, 25% (47/190) of ER-negative patients and 18% (141/794) of ER positive patients developed lymphoedema.
The multivariable analysis included number of nodes involved (HR 1.04, 95% CI 1.02 to 1.06), age ≥ 70 years compared with age < 70 years (HR 1.67, 95% CI 1.10 to 2.55), BMI of > 30 kg/m2 (HR 1.62, 95% CI 1.14 to 2.32) and ER negativity (HR 1.56, 95% CI 1.11 to 2.19) in the model for predicting lymphoedema development (Table 22).
| Variable | Analysis | |||
|---|---|---|---|---|
| Univariate | Multivariable | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (per year increase) | 1.01 (1.00 to 1.03) | 0.013 | 1.01 (1.00 to 1.02) | 0.074 |
| BMI (kg/m2) at baseline (reference ≤ 25) | 0.004 | 0.005 | ||
| > 25 to ≤ 30 | 1.18 (0.82 to 1.71) | 0.38 | 0.99 (0.68 to 1.44) | 0.95 |
| > 30 | 1.76 (1.24 to 2.51) | 0.002 | 1.62 (1.14 to 2.32) | 0.008 |
| ER negative | 1.59 (1.14 to 2.21) | 0.006 | 1.56 (1.11 to 2.19) | 0.010 |
| Nodes positive (per-node increase) | 1.04 (1.03 to 1.06) | < 0.001 | 1.04 (1.02 to 1.06) | < 0.001 |
| Adjuvant CT (yes) | 1.19 (0.87 to 1.62) | 0.27 | – | – |
| Adjuvant RT (yes) | 1.43 (0.93 to 2.19) | 0.10 | – | – |
| Previous SN biopsy | 0.70 (0.51 to 0.96) | 0.027 | – | – |
Sleeve application from baseline 24 months (excluding 1-month lymphoedema)
Univariate analysis revealed that only two factors predicted the sleeve application, node positivity (per-node increase) (HR 1.04, 95% CI 1.02 to 1.05) and adjuvant radiotherapy (HR 2.08, 95% CI 1.30 to 3.33), and both were independent in the multivariable analysis with a HR of 1.03 (95% CI 1.01 to 1.05; p < 0.001) and a HR of 1.93 (95% CI 1.20 to 3.10; p = 0.007), respectively (Table 23).
| Variable | Analysis | |||
|---|---|---|---|---|
| Univariate | Multivariable | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (per year increase) | 1.00 (0.99 to 1.01) | 0.71 | – | – |
| BMI (kg/m2) at baseline (reference ≤ 25) | 0.53 | – | – | |
| > 25 to ≤ 30 | 0.99 (0.71 to 1.39) | 0.96 | ||
| > 30 | 1.18 (0.84 to 1.66) | 0.35 | ||
| ER negative | 0.80 (0.54 to 1.17) | 0.25 | – | – |
| Nodes positive (per-node increase) | 1.04 (1.02 to 1.05) | < 0.001 | 1.03 (1.01 to 1.05) | 0.001 |
| Adjuvant CT (yes) | 1.34 (0.98 to 1.82) | 0.065 | – | – |
| Adjuvant RT (yes) | 2.08 (1.30 to 3.33) | 0.002 | 1.93 (1.20 to 3.10) | 0.007 |
| Previous SN biopsy | 0.98 (0.73 to 1.31) | 0.89 | – | – |
Lymphoedema development after 6 months’ surveillance (i.e. 6 months up to 2 years and the time to first lymphoedema within that time)
Patients with lymphoedema at 3 or 6 months are excluded because the inclusion of the RAVI variable, which is determined at 6 months, means there would need to be a ≥ 10% category RAVI variable but this is also used as the outcome event. In addition, excluding these patients is part of the study protocol (version 5.2) and the NIHR Programme Grants for Applied Research programme response letter.
The RAVI of ≥ 10% univariate analysis revealed that BMI (p < 0.002), number of nodes involved (median 2, range 0–41; p < 0.001), largest RAVI change by 6 months (p < 0.001; HR 5.58 for ≥ 5% to < 10% vs. < 3%, 95% CI 3.61 to 8.62) and BIS of > 10% (p < 0.001) all predicted lymphoedema development from 6 months up to 2 years.
The multivariable analysis included RAVI change by 6 months (p < 0.001; HR 5.22 for ≥ 5% to < 10%, 95% CI 3.22 to 8.47) along with number of nodes involved (HR 1.05, 95% CI 1.02 to 1.07), adjuvant chemotherapy (HR 1.61, 95% CI 1.01 to 2.55), BMI of > 30 kg/m2 (HR 1.87, 95% CI 1.16 to 3.02) and BIS > 10% (p = 0.069) in the model for predicting lymphoedema development after 6 months up to 2 years (Table 24).
| Variable | Analysis | |||
|---|---|---|---|---|
| Univariate | Multivariable | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (per year increase) | 1.01 (0.99 to 1.02) | 0.31 | – | – |
| BMI (kg/m2) at baseline (reference ≤ 25) | 0.002 | 0.008 | ||
| > 25 to ≤ 30 | 0.81 (0.48 to 1.36) | 0.42 | 0.96 (0.56 to 1.67) | 0.90 |
| > 30 | 1.78 (1.13 to 2.79) | 0.013 | 1.87 (1.16 to 3.02) | 0.010 |
| ER negative | 1.27 (0.79 to 2.05) | 0.33 | – | – |
| Nodes positive (per-node increase) | 1.05 (1.03 to 1.08) | < 0.001 | 1.05 (1.02 to 1.07) | < 0.001 |
| Adjuvant CT (yes) | 1.24 (0.81 to 1.88) | 0.32 | 1.61 (1.01 to 2.55) | 0.044 |
| Adjuvant RT (yes) | 1.43 (0.80 to 2.55) | 0.23 | – | – |
| Previous SN biopsy | 0.68 (0.44 to 1.03) | 0.069 | – | – |
| Arm measurements – 6 months (reference < 3% increase) | < 0.001 | < 0.001 | ||
| RAVI ≥ 3 to < 5% increase | 1.88 (1.06 to 3.33) | 0.030 | 1.87 (1.03 to 3.41) | 0.041 |
| RAVI ≥ 5 to < 10% increase | 5.58 (3.61 to 8.62) | < 0.001 | 5.22 (3.22 to 8.47) | < 0.001 |
| BIS at 6 months (reference < 3% increase) | < 0.001 | 0.069 | ||
| ≥ 3 to < 5% increase | 1.48 (0.74 to 2.95) | 0.26 | 1.54 (0.77 to 3.11) | 0.22 |
| ≥ 5 to < 10% increase | 1.37 (0.79 to 2.39) | 0.26 | 1.25 (0.70 to 2.24) | 0.44 |
| ≥ 10% increase | 3.70 (2.30 to 5.95) | < 0.001 | 1.98 (1.18 to 3.33) | 0.010 |
Smoking, type of surgery, weight gain and histological tumour type were not significant [n = 1100: those with lymphoedema ≤ 6 months have been excluded]. Factors predicting time to lymphoedema (sleeve applied) after 6 months surveillance (excluding lymphoedema at 6 months).
For applied sleeves as the clinical definition of lymphoedema, univariate analysis revealed that adjuvant radiotherapy (p = 0.008), adjuvant chemotherapy (p = 0.005), ER status (p = 0.076), ER negativity (HR 0.63, 95% CI 0.38 to 1.05), BIS of ≥ 10% (p < 0.001) and RAVI of ≥ 10% (p < 0.001) all predicted time to lymphoedema after 6 months to 24 months.
The multivariable analysis included adjuvant radiotherapy (p = 0.021), adjuvant chemotherapy (p = 0.003), ER status (p = 0.012), ER negativity (HR 0.51, 95% CI 0.30 to 0.86), BIS of ≥ 10% (p < 0.001) and perometer of ≥ 10% (p < 0.001) independently predicted time to lymphoedema after 6 months to 24 months (Table 25).
| Variable | Analysis | |||
|---|---|---|---|---|
| Univariate | Multivariable | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (per year increase) | 1.00 (0.99 to 1.01) | > 0.99 | – | – |
| BMI (kg/m2) at baseline (reference ≤ 25) | 0.76 | – | – | |
| > 25 to ≤ 30 | 1.09 (0.72 to 1.65) | 0.67 | ||
| > 30 | 1.18 (0.77 to 1.80) | 0.45 | ||
| ER negative | 0.63 (0.38 to 1.05) | 0.076 | 0.51 (0.30 to 0.86) | 0.012 |
| Nodes positive (per-node increase) | 1.04 (1.02 to 1.06) | < 0.001 | – | – |
| Adjuvant CT (yes) | 1.78 (1.19 to 2.66) | 0.005 | 1.92 (1.24 to 2.96) | 0.003 |
| Adjuvant RT (yes) | 2.23 (1.23 to 4.03) | 0.008 | 2.03 (1.11 to 3.71) | 0.021 |
| Previous SN biopsy | 0.93 (0.65 to 1.33) | 0.68 | – | – |
| Arm measurements – perometer at 6 months (reference < 3% increase) | < 0.001 | < 0.001 | ||
| RAVI of ≥ 3% to < 5% increase | 1.94 (1.15 to 3.26) | 0.013 | 1.57 (0.92 to 2.69) | 0.099 |
| RAVI of ≥ 5% to 10% increase | 3.84 (2.47 to 5.96) | < 0.001 | 3.13 (1.97 to 4.98) | < 0.001 |
| RAVI of ≥ 10% increase | 12.56 (7.84 to 20.14) | < 0.001 | 7.90 (4.78 to 13.06) | < 0.001 |
| Arm measurements BIS at 6 months (reference < 3% increase) | < 0.001 | < 0.001 | ||
| ≥ 3% to < 5% increase | 1.27 (0.60 to 2.67) | 0.53 | 1.48 (0.69 to 3.14) | 0.31 |
| ≥ 5% to 10% increase | 2.39 (1.47 to 3.89) | < 0.001 | 2.51 (1.50 to 4.20) | < 0.001 |
| ≥ 10% increase | 5.65 (3.63 to 8.79) | < 0.001 | 4.06 (2.51 to 6.58) | < 0.001 |
At 1 month, because both chemotherapy and radiotherapy had not commenced, the prediction was not as good as that at 6 months. Early changes in arm volume (RAVI) had the highest HR and were the best single predictor of subsequent lymphoedema.
Quality-of-life analyses: FACT-B scores
There are nine FACT-B+4 summary scores:27 physical well-being (PWB; score range 0–28), social/family well-being (SWB; score range 0–28), emotional well-being (EWB; score range 0–24), functional well-being (FWB; score range 0–28), breast cancer subscale (BCS; score range 0–40), arm subscale (ARM; score range 0–20), FACT-G total score (FACT-G = PWB + SWB + EWB + FWB; score range 0–108), FACT-B total score (FACT-B = PWB + SWB + EWB + FWB + BCS, score range 0–148), and Trial Outcome Index (TOI = PWB + FWB + BCS; score range 0–96). Descriptive summary data are shown in Appendix 16.
A simple comparison of the QoL data at each time point separately revealed that patients with lymphoedema at 6 months (by either definition) had significantly lower FACT-B+4, TOI and ARM subscale scores (Table 26 and 27). Poorer ARM subscale scores were also found at 12, 18 and 24 months (Table 28). At all time points, a significantly higher percentage of patients reporting swelling symptoms was found for those with lymphoedema, and a significantly higher percentage of patients reporting heaviness symptoms was found at 6, 18 and 24 months. Lower FACT-B+4, TOI and ARM subscale scores were found in the two smaller restricted subsets of patients defined by ‘no sleeve’ usage. These lower scores were significant for TOI (Table 27), and for the ARM subscale at 6, 12, 18 and 24 months. Likewise, a higher percentage of patients with reported swelling and patients with reported heaviness symptoms were found for those with lymphoedema in the smaller restricted subsets of patients defined by ‘no sleeve’ usage, and these remained significant (Table 29). The absolute change in FACT-B+4 (p = 0.04), TOI (p = 0.046) and ARM subscale (p = 0.009) scores between 6 and 24 months were significantly related to having lymphoedema at 24 months.
| Time (no : yes) | Lymphoedema, mean (SD) | p-value | |
|---|---|---|---|
| No | Yes | ||
| Perometer of > 10% | |||
| Lymphoedema at 6 months (660 : 58) | 107.4 (21.5) | 101.0 (21.4) | 0.030 |
| Lymphoedema at 12 months (628 : 55) | 112.0 (21.1) | 103.7 (22.8) | 0.005 |
| Lymphoedema at 18 months (566 : 59) | 113.6 (20.2) | 106.2 (21.5) | 0.008 |
| Lymphoedema at 24 months (541 : 68) | 114.1 (20.1) | 108.0 (25.3) | 0.059 |
| Sleeve application | |||
| Lymphoedema by 6 months (683 : 60) | 107.1 (21.5) | 99.6 (23.5) | 0.011 |
| Lymphoedema by 12 months (577 : 121) | 112.9 (20.4) | 104.6 (24.3) | 0.001 |
| Lymphoedema by 18 months (518 : 124) | 114.1 (19.9) | 107.3 (21.6) | 0.001 |
| Lymphoedema by 24 months (466 : 151) | 114.8 (19.8) | 108.5 (23.8) | 0.003 |
| Time (no : yes) | Lymphoedema, mean (SD) | p-value | |
|---|---|---|---|
| No | Yes | ||
| Perometer of > 10% | |||
| Lymphoedema at 6 months (690 : 63) | 64.7 (15.5) | 58.0 (16.1) | 0.001 |
| Lymphoedema at 12 months (585 : 123) | 70.0 (14.2) | 63.6 (17.0) | < 0.001 |
| Lymphoedema at 18 months (523 : 128) | 70.9 (14.0) | 65.6 (14.6) | < 0.001 |
| Lymphoedema at 24 months (472 : 152) | 71.5 (13.7) | 67.0 (16.5) | 0.003 |
| Sleeve application | |||
| Lymphoedema at 6 months (669 : 59) | 65.0 (15.4) | 58.1 (15.3) | 0.001 |
| Lymphoedema at 12 months (637 : 56) | 69.3 (14.6) | 62.9 (16.2) | 0.002 |
| Lymphoedema at 18 months (570 : 63) | 70.4 (14.2) | 64.6 (14.2) | 0.002 |
| Lymphoedema at 24 months (546 : 70) | 71.1 (13.9) | 65.2 (17.8) | 0.009 |
| Time (no : yes) | Lymphoedema, median, IQR (range) | p-value | |
|---|---|---|---|
| No | Yes | ||
| RAVI of > 10% | |||
| Lymphoedema at 6 months (688 : 60) | 16, 13–18 (0–20) | 14, 10–16 (0–20) | < 0.001 |
| Lymphoedema at 12 months (654 : 56) | 16, 14–18 (0–20) | 14, 10–17 (0–20) | < 0.001 |
| Lymphoedema at 18 months (583 : 64) | 16, 14–18 (0–20) | 14, 10–17 (0–20) | < 0.001 |
| Lymphoedema at 24 months (558 : 74) | 17, 14–19 (0–20) | 15, 10–17 (0–20) | < 0.001 |
| Sleeve application | |||
| Lymphoedema by 6 months (712 : 63) | 16, 13–18 (0–20) | 15, 10–16 (0–19) | < 0.001 |
| Lymphoedema by 12 months (598 : 127) | 16, 14–19 (0–20) | 15, 11–17 (0–20) | < 0.001 |
| Lymphoedema by 18 months (531 : 134) | 17, 14–19 (1–20) | 14, 11–16 (0–20) | < 0.001 |
| Lymphoedema by 24 months (483 : 157) | 17, 14–19 (0–20) | 15, 12–17 (0–20) | < 0.001 |
| Time (no : yes) | Lymphoedema, % (n) | p-value | |
|---|---|---|---|
| No | Yes | ||
| RAVI > 10% (swelling) | |||
| Lymphoedema at 6 months (601 : 55) | 31 (186) | 91 (50) | < 0.001 |
| Lymphoedema at 12 months (591 : 53) | 37 (219) | 91 (48) | < 0.001 |
| Lymphoedema at 18 months (524 : 61) | 36 (187) | 89 (54) | < 0.001 |
| Lymphoedema at 24 months (525 : 70) | 35 (185) | 87 (61) | < 0.001 |
| Sleeve application (swelling) | |||
| Lymphoedema by 6 months (620 : 60) | 30 (189) | 90 (54) | < 0.001 |
| Lymphoedema by 12 months (540 : 119) | 31 (167) | 89 (106) | < 0.001 |
| Lymphoedema by 18 months (473 : 127) | 28 (134) | 88 (112) | < 0.001 |
| Lymphoedema by 24 months (449 : 153) | 28 (126) | 80 (123) | < 0.001 |
| RAVI > 10% (heaviness) | |||
| Lymphoedema at 6 months (620 : 57) | 38 (233) | 67 (38) | < 0.001 |
| Lymphoedema at 12 months (590 : 53) | 40 (237) | 66 (35) | < 0.001 |
| Lymphoedema at 18 months (523 : 59) | 39 (202) | 85 (50) | < 0.001 |
| Lymphoedema at 24 months (516 : 67) | 40 (208) | 73 (49) | < 0.001 |
| Sleeve application (heaviness) | |||
| Lymphoedema by 6 months (640 : 60) | 37 (239) | 68 (41) | < 0.001 |
| Lymphoedema by 12 months (544 : 112) | 37 (203) | 67 (75) | < 0.001 |
| Lymphoedema by 18 months (477 : 121) | 35 (169) | 74 (90) | < 0.001 |
| Lymphoedema by 24 months (441 : 149) | 37 (164) | 64 (95) | < 0.001 |
Generalised estimating equation regression analysis to further analyse the changes in quality-of-life scores over time
FACT-B Trial Outcome Index
Owing to the negative skew of the TOI variable, a transformation [log normalised (LN) (120 – TOI)] was used for the GEE analysis to obtain a better approximation to a normal distribution. In a regression model including the time variable, the estimated marginal mean (EMM) of TOI at each time point is presented in Table 30. A total of 997 patients had some data in the model. There was a change in TOI over time (p < 0.001).
| Time point | Estimated marginal mean of TOI | 95% CI |
|---|---|---|
| Pre surgery | 68.0 | 67.2 to 68.9 |
| 3 months | 63.5 | 62.5 to 64.5 |
| 6 months | 65.4 | 64.4 to 66.4 |
| 12 months | 70.2 | 69.2 to 71.1 |
| 18 months | 70.6 | 69.6 to 71.5 |
| 24 months | 71.0 | 70.0 to 71.9 |
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time showed that TOI varied over the time period (p = 0.003), those with lymphoedema by 6 months had significantly lower TOI overall (p = 0.028) and the interaction between time and lymphoedema status was significant (p < 0.001). There was a difference in the pattern of change over time between those with and those without lymphoedema (Table 31 and Figure 10).
The EMMs from the interaction term in the GEE analysis are presented below and in Table 31.
| Time point | Estimated marginal mean of TOI (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 883) | With lymphoedema by 6 months (n = 87) | ||
| Pre surgery | 68.3 (67.4 to 69.2) | 67.0 (63.9 to 70.0) | 0.42 |
| 3 months | 63.8 (62.7 to 64.8) | 61.4 (57.8 to 64.8) | 0.19 |
| 6 months | 66.0 (64.9 to 67.1) | 60.1 (56.4 to 63.5) | 0.001 |
| 12 months | 70.7 (69.8 to 71.7) | 65.3 (61.8 to 68.5) | 0.001 |
| 18 months | 71.1 (70.2 to 72.1) | 65.4 (61.9 to 68.6) | 0.001 |
| 24 months | 71.4 (70.4 to 72.4) | 67.6 (64.0 to 71.0) | 0.033 |
The main effect for the time variable was significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was significant (p = 0.028), showing that there was a difference between the lymphoedema status groups overall. Patients who developed lymphoedema by 6 months did not initially have poorer QoL (TOI) scores than those who did not develop lymphoedema, but by 6 months their scores were poorer and they remained poorer until 24 months, when the difference was no longer significant.
FIGURE 9.
The FACT-B TOI EMMs over time for all patients and those with and those without lymphoedema at 6 months.
It is noteworthy that those without lymphoedema at 6 months begin to regain their QoL (TOI) at 3 months, improving to be above pre-surgery levels by 12 months, whereas those who develop lymphoedema continue to have worsening QoL until 6 months and do not regain QoL to pre-surgery levels until 24 months, and do not surpass their pre-surgery QoL scores. This is an important finding, as it clearly and robustly indicates poorer QoL in the group who develop lymphoedema following surgery.
FACT-B total scores
Owing to the negative skew of the FACT-B total score variable, a transformation [LN(160 – FACT-B)] was used for the GEE analysis to obtain a better approximation to a normal distribution.
There was a change in FACT-B total over time (p < 0.001), and a borderline difference in the overall level of the FACT-B scores between those with and without lymphoedema (p = 0.055) over time (Figure 10). Although the difference between patients with and without lymphoedema indicated deficits in QoL from 3 months onwards, the pattern of change over time between groups was different (p < 0.001). The EMMs from the GEE analysis are in Table 32. By 6 months patients without lymphoedema regained their pre-surgery levels of total FACT-B score, whereas those who developed lymphoedema had persistent FACT-B QoL deficiency until 12 months after surgery (see Table 32 and Figure 10).
FIGURE 10.
The FACT-B total score EMMs over time for all patients and those with and without lymphoedema at 6 months.
| Time point | EMM of FACT-B total score (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 882) | With lymphoedema by 6 months (n = 87) | ||
| Pre surgery | 110.9 (109.7 to 112.2) | 109.4 (105.0 to 113.4) | 0.48 |
| 3 months | 107.8 (106.3 to 109.2) | 105.3 (100.3 to 109.9) | 0.32 |
| 6 months | 110.1 (108.6 to 111.5) | 104.0 (98.7 to 108.8) | 0.018 |
| 12 months | 115.5 (114.1 to 116.8) | 109.5 (104.5 to 114.0) | 0.012 |
| 18 months | 115.9 (114.5 to 117.2) | 109.2 (104.2 to 113.7) | 0.005 |
| 24 months | 116.0 (114.6 to 117.4) | 111.8 (106.5 to 116.7) | 0.11 |
ARM subscale
Owing to the negative skew of the ARM subscale variable, a transformation [LN(22 – ARM)] was used for the GEE analysis to obtain a better approximation to a normal distribution (data for 995 patients).
There was a change in scores over time (p < 0.001) for FACT-B ARM scores (Figure 11 and Table 34), and a difference in the overall level of the ARM scores between those with and without lymphoedema (p = 0.002). The pattern of change over time was different between the two groups (p < 0.001). For ARM subscale values, all patients’ values declined which did not return to baseline by 24 months implying a long-term arm symptom increase with ANC Surgery. ARM scores were persistently worse in those patients who developed lymphoedema, and there was a decrease in ARM subscale EMMs from pre surgery to 3 months in both those with and those without lymphoedema by 6 months. The EMM of those without lymphoedema by 6 months remained similar level to the EMM at 3 months before increasing slightly at 24 months but remained below the pre-surgery EMM. The EMM score at 24 months increased, although it remained below the pre-surgery EMM (Table 33).
FIGURE 11.
Change in FACT-B ARM scores EMMs for patients with and without lymphoedema at 6 months.
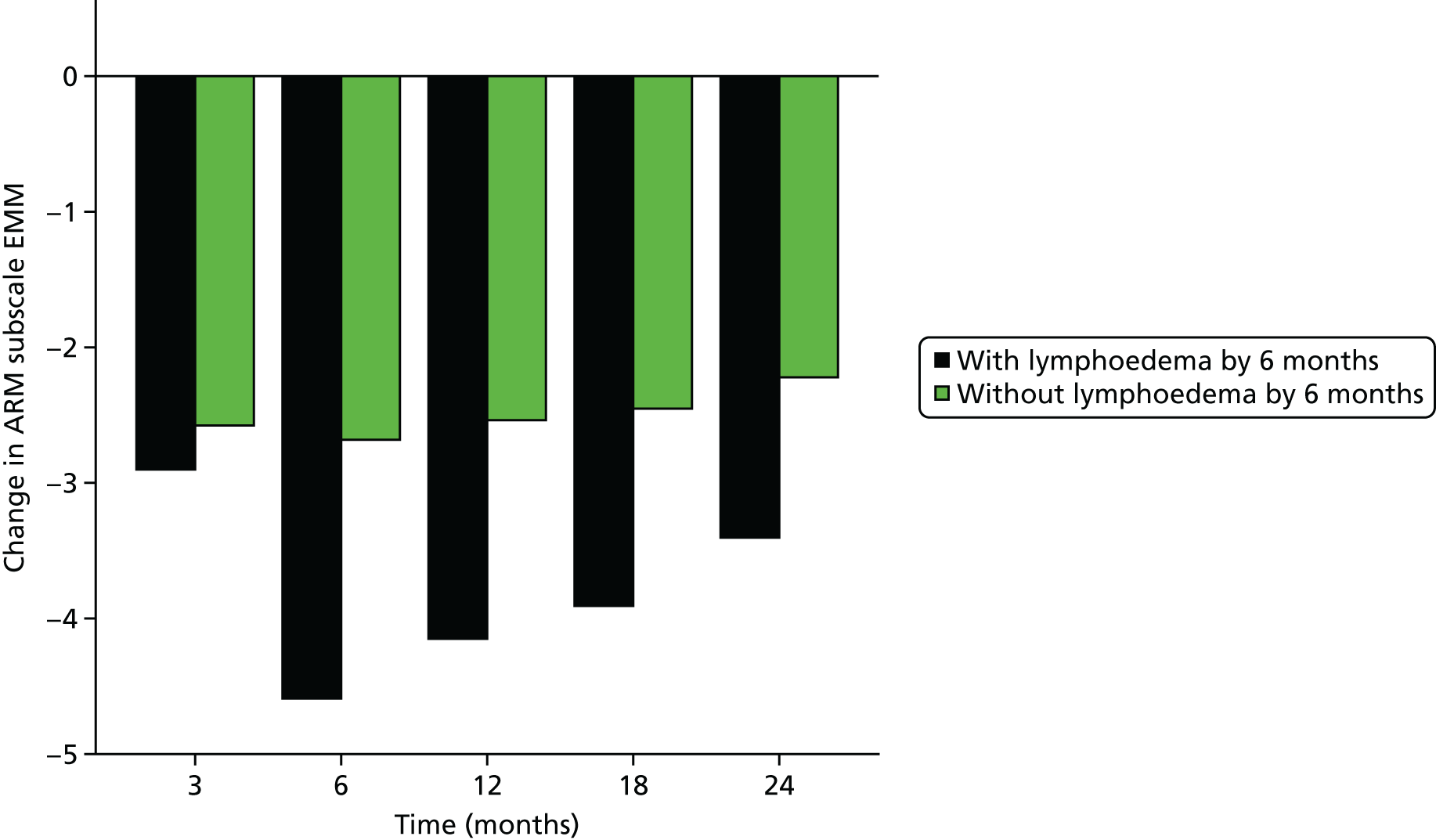
| Time point | EMM of ARM subscale (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 882) | With lymphoedema by 6 months (n = 87) | ||
| Pre surgery | 18.7 (18.6 to 18.9) | 18.6 (17.9 to 19.1) | 0.62 |
| 3 months | 16.2 (15.9 to 16.4) | 15.7 (14.7 to 16.5) | 0.29 |
| 6 months | 16.0 (15.8 to 16.3) | 14.0 (13.0 to 14.8) | < 0.001 |
| 12 months | 16.2 (15.9 to 16.4) | 14.4 (13.4 to 15.3) | < 0.001 |
| 18 months | 16.3 (16.0 to 16.5) | 14.7 (13.5 to 15.6) | 0.001 |
| 24 months | 16.5 (16.3 to 16.8) | 15.2 (14.2 to 16.0) | 0.003 |
| Variable | Analysis | |||
|---|---|---|---|---|
| Univariate | Multivariable (n = 683) | |||
| EMM (95% CI) | p-value | EMM (95% CI) | p-value | |
| Lymphoedema in first 12 months | ||||
| No | 116.9 (115.4 to 118.4) | 114.3 (112.1 to 116.5) | ||
| Yes | 109.9 (105.9 to 113.6) | < 0.001 | 106.4 (101.7 to 110.8) | < 0.001 |
| Adjuvant CT | ||||
| No | 117.9 (115.4 to 120.2) | 0.051 | – | – |
| Yes | 114.9 (113.1 to 116.6) | – | ||
| Adjuvant RT | ||||
| No | 116.6 (113.2 to 119.7) | 0.60 | – | – |
| Yes | 115.6 (114.0 to 117.1) | – | ||
| BMI (kg/m2) at baseline | ||||
| ≤ 25 | 120.3 (118.1 to 122.4) | < 0.001 | 116.5 (113.4 to 119.3) | < 0.001 |
| > 25 to ≤ 30 | 114.8 (112.4 to 117.1) | 109.4 (105.7 to 112.9) | ||
| > 30 | 110.3 (107.1 to 113.2) | 105.0 (100.8 to 108.9) | ||
| Type of surgery | ||||
| ANC/other | 118.4 (115.7 to 120.9) | 0.012 | 112.5 (108.7 to 115.9) | 0.063 |
| WLE + ANC | 116.8 (114.2 to 119.3) | 110.9 (107.1 to 114.5) | ||
| Mastectomy + ANC | 113.4 (111.2 to 115.6) | 108.1 (104.8 to 111.2) | ||
| Smoking | ||||
| Never | 117.1 (115.3 to 118.8) | 0.012 | 113.9 (111.5 to 116.3) | 0.018 |
| Ex-smoker | 114.7 (112.0 to 117.2) | 111.1 (107.9 to 114.2) | ||
| Current | 109.1 (103.0 to 114.5) | 106.2 (99.5 to 112.1) | ||
| Age (years) | ||||
| < 50 | 113.8 (111.1 to 116.3) | 0.058 | 107.8 (103.9 to 111.5) | 0.002 |
| ≥ 50 | 116.7 (115.0 to 118.4) | 113.1 (110.4 to 115.6) | ||
Clinical lymphoedema/appropriately applied sleeve
Whether the clinical lymphoedema or applied sleeve variable is considered or the RAVI of > 10% definition of lymphoedema, the multivariable analysis results are similar. The only difference of note is that type of surgery is no longer associated with FACT-B at 12 months, although the direction of effect is the same as when the RAVI of > 10% definition of lymphoedema was included in the model.
In addition to lymphoedema, the analysis identified BMI, current smoking and older age, which had not previously been reported to influence FACT-B scores.
A similar analysis at 6 months found that chemotherapy significantly influenced scores, but the effect was lost in multivariate analysis by 12 months (see Table 34).
This is the first large prospective analysis of factors affecting QoL using FACT-B+4 in breast cancer patients and has identified several new factors, such as smoking and BMI, which influence QoL. BMI was also found to have important effects on QoL in the health economic analysis, strengthening the case for encouraging weight loss strategies after cancer diagnosis.
Relationship between the lymphoedema checklist variables at 6 months and changes in quality of life from baseline and relative arm-volume increase/bioimpedance spectroscopy from 1 month
The lymphoedema checklist is a patient self-reported symptom checklist; the constituent symptoms were compared with QoL changes in the study.
Greater reductions (from baseline to 6 months) in FACT-B, TOI and ARM subscale scores were found in those with swelling, heaviness (p < 0.001) and numbness (p < 0.035 FACT-B; p = 0.051 TOI; and p < 0.001 ARM).
For patients reporting swelling at 6 months, the reductions (from baseline to 6 months) in FACT-B (p < 0.001), TOI (p < 0.001) and ARM subscale (p < 0.001) were greater than for those not reporting swelling.
For patients reporting numbness at 6 months, the reductions (from baseline to 6 months) in FACT-B (p = 0.035), TOI (p = 0.051) and ARM subscale (p < 0.001) were greater than for those not reporting numbness.
For patients reporting heaviness at 6 months, the reductions (from baseline to 6 months) in FACT-B (p < 0.001), TOI (p < 0.001) and ARM subscale (p < 0.001) were greater than for those not reporting heaviness.
Greater increases (from 1 month to 6 months) in the exact RAVI value and exact BIS values were found in those with swelling (both p < 0.001), and in those with heaviness (RAVI p = 0.038 and exact BIS p = 0.004).
There were greater increases (from 6 to 24 months) in the exact RAVI value in those with swelling (p < 0.001), and in those with heaviness (RAVI p = 0.001) both during the time period and the responses at 24 months. No association was seen between 6 and 24 months with exact BIS values and swelling, numbness or heaviness.
In summary, RAVI of > 10% or changes in RAVI more closely related to patient-reported symptoms of arm swelling and heaviness throughout the BEA study.
Associations between changes in quality of life from baseline to 6 months and perometry/bioimpedance spectroscopy at 6 months
There was a negative association between changes in TOI and RAVI (r = –0.10; p = 0.024) and BIS (r = –0.14; p = 0.001) at 6 months. Larger reductions in QoL scores were found in patients who have had larger RAVI/BIS increases.
Associations between changes in quality of life from baseline to 6 months and changes in perometry/bioimpedance spectroscopy from 1 month to 6 months
There was a negative association between changes in TOI and changes in RAVI (r = –0.10; p = 0.024) and BIS (r = –0.14; p = 0.001), and between changes in FACT-B and changes in BIS (r = –0.11; p = 0.011). Thus, again greater reductions in QoL scores were found in those patients who had bigger increases in RAVI/BIS values.
Quality-of-life overview
These results demonstrate significant and persisting impact of lymphoedema on QoL following diagnosis and treatment for breast cancer. This is true for overall disease-specific HRQoL indicators reflected in the FACT-B TOI and total FACT-B+4 scores and is pronounced in specific symptoms associated with lymphoedema reflected in the ARM subscale scores. While overall QoL (TOI) does return to (or exceed) pre-surgery levels by 12 months for those without lymphoedema and 24 months for those with lymphoedema, the arm-specific measures indicate deficits for all ANC patients continuing until at least 24 months.
Most QoL studies in breast cancer have been cross-sectional and have not used instruments designed specifically for lymphoedema. Most reported reduced QoL with onset of lymphoedema. 23,26,27 The ALMANAC QoL study, reported by Fleissig et al. , found similar TOI and FACT-B+4 reductions in the ANC arm and considered them due to surgery. 26 Whereas 66% of their patients were node negative and the majority did not receive chemotherapy, in contrast in the BEA study, all were node positive and 66% received chemotherapy. There was a significant relationship between chemotherapy and QoL during the 6 months of treatment, but patients developing lymphoedema in the first 6 months had a greater reduction in QoL than those who did not develop lymphoedema. Whereas after the period during which chemotherapy was administered, QoL returned to pre-surgical levels by 12 months in patients who did not develop lymphoedema, the QoL deficit was prolonged after the development of lymphoedema until at least 24 months. Current smoking, BMI and age also affected QoL scores. Self-reported symptoms were associated with lymphoedema development but were not discriminatory predictors on their own. 24 Subjective symptoms such as heaviness and particularly ‘considerable’ swelling correlated with QoL deficits, RAVI and BIS increase and are probably clear and simple markers of adverse effects for many patients. 23,26,27 Such simple to complete self-reported measures could play an important part in clinical practice if widely adapted and combined with objective measures, and as seen below they may also contribute significantly to predicting the development of lymphoedema.
Composite scoring model to define lymphoedema
Reference standard
In a review document published in 2011, the Agency for Health Research and Quality24 concluded that, although rarely identified as gold standards, the frequency of use of different measures of limb volume or circumference would suggest that these measures are the de facto gold standards for diagnosing secondary lymphoedema. 24
The proposed reference standard for this diagnostic test accuracy study is perometry (also known as infrared optoelectric volumetry). Infrared light is used to measure the volume of a limb, at repeated sites along the limb. Numerous studies have reported perometry as a reliable and valid method for determining limb volume with excellent intra- and inter-rater reliability. 6,9,36 Perometry has superseded the use of water displacement as a reference standard. Its adoption into standard clinical practice has been hindered by the relatively high cost of the perometer.
It is acknowledged that in studies of diagnostic test accuracy the reference standard is rarely 100% accurate in practice. An imperfect reference standard can lead to difficulties in the interpretation of test results. If we could assume, based on evidence, that perometry alone provides adequate classification of the target condition, then we had intended to proceed with the diagnostic test accuracy with a reference standard of perometry alone. However, the reference standard did not predict sleeve application and was considered to provide inadequate classification. Given the degree of imperfection of the reference standard, we then considered whether additional information provided adequate classification in the form of a composite reference standard [using, for example, the addition of clinical presentation (application of a sleeve for treatment), presence or absence of arm lymphoedema at 6, 18, 24 months, etc.]. 37–39
There is currently no gold standard for the definition of lymphoedema. Proposed definitions include a 200 ml limb volume difference; a 10% difference in arm volume; and a 2.0 cm circumferential difference at any point on the arm. Widely accepted as diagnostic criteria, the measurements are not equivalent, but constitute explicit, observable clinical definitions. 23,24 The 2011 report for by the Agency for Health Research and Quality concluded that based on the evidence in the extracted studies, there does not appear to be a gold standard to formally grade or measure the severity of lymphoedema. 24
We aimed to identify discriminatory factors for a composite index. 39–41
Diagnostic criteria for lymphoedema
The RAVI of > 10% is the most conservative criterion for diagnosis of lymphoedema, with two-thirds of patients complaining of heaviness or swelling by 24 months. Figure 12 and Table 35 are the same data shown diagrammatically and in tabular form. We considered whether a combination of RAVI and self-reported symptoms might better define lymphoedema, and used both sleeve application and RAVI of > 10% as the basis for redefining lymphoedema diagnosis. Overall, 86% of patients with a RAVI of > 9 and 10% had a sleeve fitted (κ = 0.60).
FIGURE 12.
Temporal changes in self-reported symptoms from ARM subscale and objective measures of arm swelling (≥ 10%, ≥ 9%, ≥ 5%, ≥ 200 ml) compared with sleeve application (clinical lymphoedema).
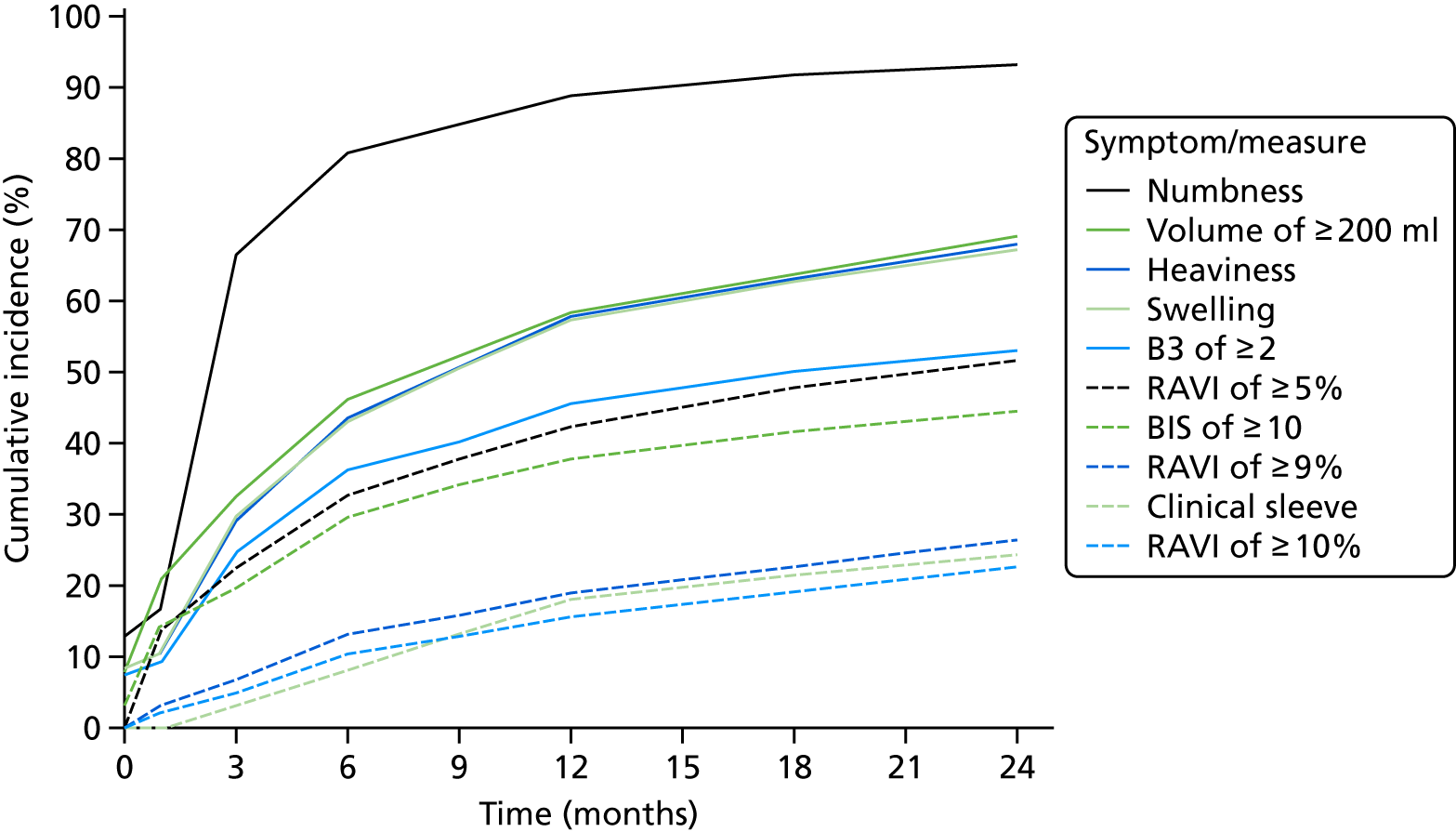
| Symptom/measure | Month (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 9 | 12 | 18 | 24 | |
| Numbness | 12.9 | 17.1 | 66.8 | 80.8 | 84.7 | 88.8 | 91.7 | 93.3 |
| ≥ 200 ml | 8.3 | 21.2 | 32.6 | 46.1 | 52.5 | 58.4 | 64.3 | 69.1 |
| Heaviness | 8.7 | 10.6 | 29.4 | 43.4 | 50.6 | 57.8 | 63.1 | 68.0 |
| Swelling | 8.4 | 10.7 | 29.7 | 43.1 | 50.7 | 57.4 | 62.9 | 67.3 |
| B3 ≥ 2 (from FACT-B+4) | 7.6 | 9.4 | 24.8 | 36.3 | 40.2 | 45.6 | 50.2 | 53.3 |
| ≥ 5% vol | 0.0 | 14.0 | 22.5 | 32.6 | 37.8 | 42.5 | 47.8 | 51.9 |
| BIS of ≥ 10 | 3.8 | 15.0 | 19.7 | 29.8 | 34.2 | 37.8 | 41.8 | 44.6 |
| ≥ 9% vol | 0.0 | 3.3 | 6.9 | 13.2 | 16.0 | 19.1 | 22.8 | 26.5 |
| Clinical sleeve | 0.0 | 0.2 | 3.2 | 8.4 | 13.2 | 18.2 | 21.6 | 24.5 |
| ≥ 10% vol | 0.0 | 2.2 | 5.0 | 10.5 | 13.0 | 15.7 | 19.4 | 22.8 |
Cohort of patients who had clinical lymphoedema or appropriately applied sleeve only (excluding the PLACE trial patients)
A total of 223 patients had clinical lymphoedema (appropriately applied sleeve) by 24 months (79 by 6 months, 168 by 12 months); this excludes those who had a sleeve applied during the PLACE trial.
There were significant increases in the proportion of women with swelling, numbness and heaviness from the time point previous to sleeve fitting to the time point the sleeve was fitted. There was a decrease in the ARM subscale from the FACT-B+4 QoL questionnaire from before the sleeve was fitted to the time the sleeve was fitted (Table 36).
| Mean (SD), range | Paired t-test | ||
|---|---|---|---|
| Time point previous to clinical lymphoedema or appropriately applied sleeve | At time of clinical lymphoedema or appropriately applied sleeve | ||
| Perometer (n = 206) | 6.7 (6.5), –7.0 to 36.4 | 8.7 (8.5), –16.1 to 37.8 | p = 0.001a |
| BIS (n = 199) | 11.3 (14.0), –16.9 to 84.6 | 15.0 (17.9), –25.1 to 77.2 | p = 0.002a |
| FACT-B (n = 169) | 101.7 (23.0), 42.0 to 138.6 | 103.1 (23.0), 28.0 to 141.0 | p = 0.23 |
| TOI (n = 172) | 60.4 (16.4), 20.5 to 88.7 | 61.6 (16.4), 9.0 to 89.0 | p = 0.15 |
| ARM (n = 174) | 14.1 (4.5), 0 to 20 | 13.3 (4.4), 0 to 20 | p = 0.011a |
The rates of symptoms reported in our BEA study are higher than those reported in the ALMANAC trial. 26 The combination of arm swelling symptoms and nurses’ perception of poorer QoL of these women (as reflected by their QoL scores) may have led to the application of compression sleeves in these patients even though the RAVI was < 10% (Table 37). The perometer measurements for these patients are retained at each site and the arm-volume changes over the different segments were reviewed with the case note/source documents to understand the basis for sleeve application in the women for whom RAVI was < 10% in order to be able to produce a composite measure of lymphoedema.
| Symptom/measure | Time point previous to clinical lymphoedema or appropriately applied sleeve (%) | At time of clinical lymphoedema or appropriately applied sleeve (%) | McNemar’s test |
|---|---|---|---|
| ≥ 200 ml (n = 206) | 57 | 73 | p < 0.001a |
| RAVI of ≥ 5% (n = 206) | 55 | 71 | p < 0.001a |
| RAVI of ≥ 10% (n = 206) | 28 | 38 | p = 0.014a |
| BIS of ≥ 10% (n = 199) | 43 | 51 | p = 0.081 |
| Swelling (n = 163) | 56 | 88 | p < 0.001a |
| Numbness (n = 160) | 70 | 84 | p = 0.001a |
| Heaviness (n = 156) | 47 | 65 | p < 0.001a |
We identified localised segmental swelling in the hand or upper, lower arm segments on source perometry measurements such that if the forearm segment had a 10% volume increase (even if the whole arm RAVI was < 10%), a compression sleeve was applied based on these clinical findings, and symptoms. 23
Even after central review of source perometry data, there remained patients for whom decisions regarding sleeve fitting were determined by lymphoedema nurses based on patient reports of worsening symptoms, rather than on objective measurement of arm swelling. This finding is in line with results of the qualitative study reported in WS3 below.
The RAVI and BIS values increased from before lymphoedema to the time of diagnosis but the mean values for RAVI post sleeve application were < 9%, which implies that, in women with subthreshold arm-volume increases whose symptoms worsened, sleeves were used to treat symptoms in the absence of objective volume criteria defining lymphoedema.
Diagnostic accuracy of composite end-points analysis
Using a definition of clinical lymphoedema (applied sleeve), we assessed sensitivity, specificity, PPV and NPV across a range of diagnostic criteria either alone or in combination for increased diagnostic accuracy.
At all time points examined (6, 9, 12, 18 and 24 months), a combination of RAVI of < 5% and B3 score of 3 or 4 (little or no swelling) provided a NPV of 99% and almost guaranteed that the patient would not develop lymphoedema by 24 months.
Positive predictive value was highest overall using RAVI of > 9% and B3 score of < 2 at 9 months (74%). At other time points the optimal criteria varied between RAVI of > 9 or 10% and B3 score of < 2 (PPV 50% at 12 and 18 months), although between 18 and 24 months BIS of > 10% added to PPV, increasing it from 31% with RAVI of > 9%/B3 of < 2 to 41% with all three scores present.
Diagnostic accuracy for these composite end points (number diagnosed added to number excluded with lymphoedema) was 94% (781/834), 94% (668/709) and 95% (526/553) at 6, 12 and 24 months, respectively for a combination of RAVI of > 9%and B3 score of < 2 (self-reported ‘considerable’ swelling) (see Appendix 17 for data analysis).
Using a combination of objective measures (RAVI of > 9%) and self-reported arm swelling (B3 subjective measure) increased diagnostic accuracy for sleeve application. Given that some patients had sleeves applied for arm/shoulder stiffness with little objective swelling, this is a surprisingly good fit for the data.
Changes in quality of life in relation to sleeve application
Overall, for patients who required treatment with a sleeve, an increase in their QoL occurred. Comparing FACT-B+4 at two time points – the time the sleeve was applied and approximately 6 months after – there was a mean increase of 2.96 (p = 0.021: t-test). Using repeated measures [analyses of variance (ANOVAs) shown in Table 43], total FACT-B (p = 0.015), ARM (p < 0.001) and TOI (p < 0.001) all showed that QoL decreased from baseline to the point before their sleeve was applied. FACT-B and TOI showed an improvement at the time at which the sleeve was applied. At 24 months, FACT-B and TOI returned to above pre-surgery levels, but the ARM subscale remained low.
Arm symptoms were not improved as much as overall QoL after sleeve application.
Repeated measures ANOVAs showing the changes in different QoL measurements at various points in relation to the time of sleeve application (Table 38).
| Subscale | n | Estimated marginal mean (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Pre surgery | Before the sleeve was applied | At the time the sleeve was applied | At 24 months | |||
| Total FACT-B+4 | 92 | 107.0 (102.8 to111.1) | 103.5 (99.2 to 107.9) | 106.3 (102.2 to 110.4) | 109.3 (104.6 to 114.0) | 0.015 |
| ARM | 74 | 17.5 (16.5 to18.4) | 13.6 (12.6 to 14.6) | 12.8 (11.8 to 13.8) | 13.1 (12.1 to 14.1) | < 0.001 |
| TOI | 94 | 66.4 (63.5 to 69.3) | 61.8 (58.7 to 64.9) | 63.5 (60.5 to 66.5) | 67.0 (63.7 to 70.3) | < 0.001 |
The patients split into two groups with regard to QoL changes following sleeve application. One group were patients who met the conventional definition of lymphoedema (having a RAVI of ≥ 9% at sleeve application) and another group contained patients who had a RAVI of < 9% at sleeve application. Patients with complete data sets including (both their RAVI and total FACT-B+4) before and after sleeve application were analysed. Sixty patients with sleeves applied were not included because of missing data.
The patients who had a larger increase in arm volume (median RAVI was 11.7%) had a mean FACT-B score of 106.3 when their sleeve was applied. From sleeve application to the first time point afterwards, approximately 6 months after, their QoL showed a large increase to 112.6 (p = 0.004), suggesting that reducing arm swelling by treating lymphoedema is an important factor in improving their QoL.
The group with smaller amounts of arm swelling (i.e. with a median RAVI of 3.6%) had a lower mean FACT-B score of 103.5 when their sleeve was applied, which increased by a small amount, from 105.5 (p = 0.20). However, the arm volume in this group continued to increase, suggesting that the sleeve was not an effective treatment.
Effect of self-reported arm swelling on quality-of-life benefit following sleeve application
Within FACT-B+4 there are five questions, which relate directly to lymphoedema, including B3, which relates to arms being either swollen or tender.
Repeated measures ANOVAs of total FACT-B, TOI and ARM at the time of sleeve application and afterwards, split by the patients’ B3 score at the time of application B3 scores, are reverse coded, so a score of 0 is ‘very much’ arm swelling and 4 is ‘not at all’.
The initial overall QoL scores for patients with little or no swelling are, as expected, significantly higher than those for patients with considerable self-reported arm swelling. Likewise, patients with little arm swelling have higher QoL scores (FACT-B+4, TOI) at 36 months post surgery.
Patients were grouped based on their self-reported B3 scores at the time of sleeve application. The group with large amounts of arm swelling had B3 scores of 0–2 (81% of those with RAVI of ≥ 9% had a B3 score of 0–2 at sleeve application) and the group with little to no arm swelling had B3 scores of 3–4 (54% of those with RAVI of < 9% had a B3 score of 3–4) (p ≤ 0.005).
ARM subscale scores increased (improved QoL) when sleeve was applied for ‘considerable’ arm swelling (B3 score 0–2) but were unaltered when little or no swelling was present (Figure 13).
FIGURE 13.
Change in the ARM subscale score at the time of sleeve application and afterwards, by B3 score.
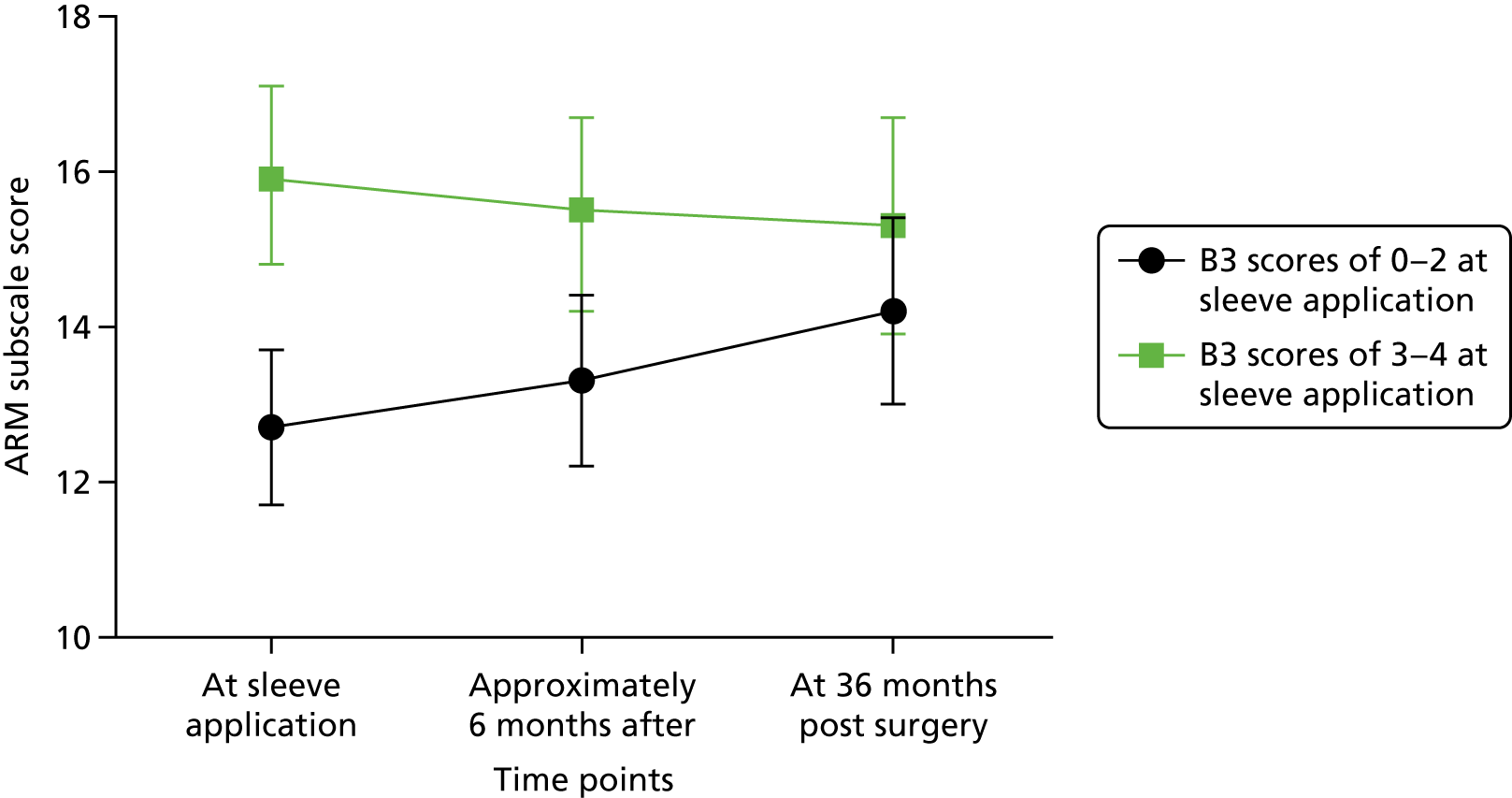
In the ARM subscale (p = 0.044) analysis, there was an interaction between having a B3 score of 0–2 (more arm swelling) or 3–4 (limited arm swelling) and the time points post sleeve application. The interaction term was significant because of the increase in ARM scores after sleeve application in patients with B3 scores 0–2, whereas there was minimal change in the ARM score post sleeve application in patients with a B3 score of 3–4.
In the patients with considerable swelling, mean FACT-B and TOI increased (QoL improved) following sleeve application. Among the group with B3 scores showing limited or no arm swelling, there were small QoL increases. Patients with small amounts of arm swelling had higher QoL scores throughout until 36 months after their surgery (Table 39).
| Subscale | n | Estimated marginal mean (95% CI) | p-value | ||
|---|---|---|---|---|---|
| At sleeve application | Approximately 6 months after sleeve was applied | At 36 months post surgery | |||
| FACT-B+4 total | |||||
| B3 score of 0–2 (considerable swelling) | 36 | 102.9 (97.1 to 108.7) | 105.5 (98.6 to 112.58) | 108.9 (102.6 to 115.1) |
Time: 0.043 B3 score: 0.045 Interaction: 0.72 |
| B3 score of 3–4 (little to no swelling) | 26 | 113.1 (106.3 to 119.9) | 115.2 (107.1 to 123.3) | 116.3 (109.0 to 123.7) | |
| TOI | |||||
| B3 score of 0–2 (considerable swelling) | 37 | 60.0 (56.2 to 63.8) | 64.4 (59.9 to 68.9) | 65.8 (61.6 to 69.9) |
Time: 0.011 B3 score: 0.002 Interaction: 0.33 |
| B3 score of 3–4 (little to no swelling) | 27 | 71.3 (66.9 to 75.7) | 72.6 (67.3 to 77.9) | 73.4 (68.5 to 78.3 | |
| ARM | |||||
| B3 score of 0–2 (considerable swelling) | 38 | 12.7 (11.7 to 13. 7) | 13.3 (12.2 to 14.4) | 14.2 (13.0 to 15.4) |
Time: 0.54 B3 score: 0.002 Interaction: 0.044 |
| B3 score of 3–4 (little to no swelling) | 30 | 15.9 (14.8 to 17.1) | 15.5 (14.2 to 16.7) | 15.3 (13.9 to 16.7) | |
These data indicate that when patients self-report ‘considerable’ arm swelling, application of a sleeve in ‘correctly diagnosed’ lymphoedema successfully improves symptoms and QoL scores. However, if a sleeve is applied without self-reported arm swelling and/or with no RAVI of > 9% (definition of lymphoedema), no benefit in arm symptoms or QoL occurs.
The prescription of compression sleeves in ‘correctly diagnosed’ lymphoedema successfully improves symptoms and QoL. Understanding and developing objective evidence for which patient groups benefit from treatment with a compression sleeve has important implications for compression sleeve prescription and use in the NHS.
Scoring model to predict lymphoedema
The definition of lymphoedema used as the outcome for the logistic regression included both RAVI of > 10% or a sleeve applied after 1 or 6 months up to 24 months.
Of the 1097 patients in the data set, 326 were classified as having either an appropriately applied sleeve or clinical lymphoedema.
Fifty-one patients were identified as being given their sleeve as part of the PLACE trial and nine had a sleeve applied to the contralateral arm (because of deep-vein thrombosis). These 60 patients were excluded from consideration in the following analysis.
There were 266 patients with an appropriately applied sleeve or clinical lymphoedema.
Model at 6 months predicting lymphoedema (relative arm-volume increase of > 10%)
The variables considered for the scoring model were RAVI at 6 months (categorical), BIS at 6 months (categorical), TOI at 6 months, FACT-B total at 6 months, ARM subscale at 6 months, lymphoedema checklist questions at 6 months (swelling, numbness, heaviness), B3 at 6 months (categorical: 0–2, considerable swelling vs. 3–4, little to no swelling), age, BMI at 6 months, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy.
A total of 711 patients were included in this analysis.
A scoring model was produced based on the regression coefficients from the final model (Table 40). The individual scores are the regression coefficients for binary or categorical variables rounded to the nearest 0.5 and the regression coefficients for continuous variables to two decimal places owing to their per-unit increase interpretation. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a 10% RAVI volume increase.
| Variable | OR (95% CI) | p-value | Score |
|---|---|---|---|
| RAVI at 6 months | |||
| < 3% | 1 (–) | < 0.001 | 0 |
| ≥ 3 to < 5% | 1.92 (0.96 to 3.86) | 0.5 | |
| ≥ 5 to < 10% | 7.36 (4.10 to 13.24) | 2 | |
| BIS at 6 months | |||
| < 3 | 1 (–) | 0.030 | 0 |
| ≥ 3 to < 5 | 1.39 (0.57 to 3.38) | 0.5 | |
| ≥ 5 to < 10 | 1.87 (0.96 to 3.64) | 0.5 | |
| ≥ 10 | 2.58 (1.35 to 4.93) | 1 | |
| BMI at 6 months (kg/m2) | |||
| ≤ 25 | 1 (–) | 0.015 | 0 |
| > 25 to ≤ 30 | 1.53 (0.80 to 2.91) | 0.5 | |
| > 30 | 2.53 (1.34 to 4.77) | 1 | |
| Number of positive nodes (per-node increase) | 1.08 (1.04 to 1.12) | < 0.001 | 0.08 × number of positive nodes |
This scoring model gives an area under the receiver operating characteristic (AUROC) of 0.80 (95% CI 0.74 to 0.85). For a cut-off score of 1.58 – where a patient with a score of ≥ 1.58 would be predicted to have a 10% RAVI by perometer – the scoring model would give a sensitivity of 80.0% (68/85), a specificity of 67.7% (424/626), a PPV of 25.2% (68/270) and a NPV of 96.1% (424/441). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to both.
Prediction scoring predicting lymphoedema (relative arm-volume increase of > 10%) at 6 months (excluding bioimpedance spectroscopy)
Bioimpedance spectroscopy is not widely available in the NHS and therefore we concentrated on models that could be used in any lymphoedema clinic in the UK. A total of 740 patients were included in this analysis (Table 41).
| Variable | OR (95% CI) | p-value | Score |
|---|---|---|---|
| RAVI at 6 months | |||
| < 3% | 1 (–) | < 0.001 | 0 |
| ≥ 3 to < 5% | 2.47 (1.27 to 4.79) | 1 | |
| ≥ 5% to < 10% | 9.10 (5.24 to 15.79) | 2 | |
| BMI at 6 months | |||
| ≤ 25 | 1 (–) | 0.025 | 0 |
| > 25 to ≤ 30 | 1.53 (0.82 to 2.86) | 0.5 | |
| > 30 | 2.34 (1.26 to 4.35) | 1 | |
| Number of positive nodes (per-node increase) | 1.08 (1.04 to 1.11) | < 0.001 | 0.07 × number of positive nodes |
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher score is more likely to have a 10% RAVI perometer volume increase.
This scoring model gives an AUROC of 0.77 (95% CI 0.71 to 0.82). For a cut-off score of 1.41, where a patient with a score of ≥ 1.41 is predicted to have a 10% perometer volume increase, the scoring model would give a sensitivity of 72.1% (62/86), a specificity of 72.2% (472/654), a PPV of 25.4% (62/244) and a NPV of 95.2% (472/496). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to both. This model has similar AUROC and would be easy to apply in NHS practice and its components would have few extra costs (i.e. FACT-B+4/lymphoedema checklist) in any NHS setting.
Model of prediction of lymphoedema (relative arm-volume increase of > 10%) from 1 month
Variables considered for the scoring model were RAVI at 1 month (categorical), BIS at 1 month (categorical), TOI at pre-surgery, FACT-B total at pre-surgery, ARM subscale at pre-surgery, lymphoedema checklist questions at pre-surgery (swelling, numbness, heaviness), B3 at pre-surgery (categorical: 0–2, considerable swelling vs. 3–4, little to no swelling), age, BMI at pre-surgery, ER status, number of positive nodes, adjuvant chemotherapy and radiotherapy.
A total of 522 patients were included in this analysis.
A scoring model was produced based on the regression coefficients from the final model as described previously (Table 42). The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher score is more likely to have a 10% RAVI perometer volume increase.
| Variable | OR (95% CI) | p-value | Score |
|---|---|---|---|
| RAVI at 1 month | |||
| < 3% | 1 (–) | < 0.001 | 0 |
| ≥ 3 to < 5% | 2.11 (1.06 to 4.19) | 0.5 | |
| ≥ 5% to < 10% | 4.02 (2.18 to 7.39) | 1.5 | |
| ≥ 10% | 8.89 (2.86 to 27.64) | 2 | |
| Lymphoedema checklist swelling at pre-surgery | |||
| No | 1 (–) | 0.010 | 0 |
| Yes | 2.22 (1.21 to 4.09) | 1 | |
| Number of positive nodes (per-node increase) | 1.08 (1.04 to 1.12) | < 0.001 | 0.07 × number of positive nodes |
This scoring model gives an AUROC of 0.71 (95% CI 0.64 to 0.77). For a cut-off score of 0.82, where a patient with a score of ≥ 0.82 is predicted to have a 10% perometer volume increase, the scoring model would give a sensitivity of 62.9% (56/89), specificity of 70.7% (306/433), PPV of 30.6% (56/183) and NPV of 90.3% (306/339). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to both.
Prediction model: using sleeve as ‘lymphoedema’ definition after 6 months
The variables considered for the scoring model were perometer at 6 months (categorical), BIS at 6 months (categorical), TOI at 6 months, FACT-B total at 6 months, ARM subscale at 6 months, lymphoedema checklist questions at 6 months (swelling, numbness, heaviness), B3 at 6 months (categorical: 0–2, considerable swelling vs. 3–4, little to no swelling), age, BMI at 6 months, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy (Table 43).
| Variable | OR (95% CI) | p-value | Score |
|---|---|---|---|
| RAVI at 6 months | |||
| < 3% | 1 (–) | < 0.001 | 0 |
| ≥ 3 to < 5% | 2.69 (1.36 to 5.31) | 1 | |
| ≥ 5% to < 10% | 5.89 (3.07 to 11.30) | 2 | |
| Lymphoedema checklist swelling at 6 months | |||
| No | 1 (–) | 0.003 | 0 |
| Yes | 2.31 (1.33 to 4.02) | 1 | |
| ER status | |||
| Negative | 1 (–) | 0.045 | 0 |
| Positive | 0.40 (0.16 to 0.98) | 1 | |
| Adjuvant radiotherapy | |||
| No | 1 (–) | 0.005 | 0 |
| Yes | 4.74 (1.61 to 13.92) | 1.5 | |
Patients with a RAVI of ≥ 10% before, or at, 6 months were excluded from the analysis.
A total of 548 patients were included in this analysis.
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a lymphoedema requiring a sleeve.
This scoring model gives an AUROC of 0.76 (95% CI 0.70 to 0.82). For a cut-off score of 4, where a patient with a score of ≥ 4 is predicted to have clinical lymphoedema or a sleeve applied, the scoring model would give a sensitivity of 48.6% (34/70), a specificity of 90.0% (430/478), a PPV of 41.5% (34/82) and a NPV of 92.3% (430/466). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to both.
Again, this model uses simple measures easily available in the NHS and provides good prediction of lymphoedema.
Model predicting lymphoedema (sleeve) development from 1 month post surgery
The variables considered for the scoring model were: perometer at 1 month (categorical), BIS at 1 month (categorical), TOI at pre-surgery, FACT-B total at pre-surgery, ARM subscale at pre-surgery, lymphoedema checklist questions at pre-surgery (swelling, numbness, heaviness), age, BMI at pre-surgery, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy (Table 44).
| Variable | OR (95% CI) | p-value | Score |
|---|---|---|---|
| RAVI at 1 month | |||
| < 3% | 1 (–) | < 0.001 | 0 |
| ≥ 3 to < 5% | 1.45 (0.88 to 2.41) | 0.5 | |
| ≥ 5% to < 10% | 3.61 (2.33 to 5.59) | 1 | |
| ≥ 10% | 5.70 (2.32 to 14.02) | 1.5 | |
| Adjuvant radiotherapy (planned) | |||
| No | 1 (–) | 0.018 | 0 |
| Yes | 1.93 (1.12 to 3.31) | 0.5 | |
| Number of positive nodes (per-node increase) | 1.05 (1.02 to 1.08) | 0.001 | 0.05 × number of positive nodes |
A total of 837 patients were included in this analysis.
A scoring model was produced based on the regression coefficients from the final model. The individual scores are the regression coefficients for binary or categorical variables rounded to the nearest 0.5 and the regression coefficients for continuous variables to two decimal places due to their per-unit increase interpretation. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a clinical lymphoedema or require a sleeve.
This scoring model gives an area under the receiver operator characteristic (AUROC) of 0.67 (95% CI 0.62 to 0.71).
Note that the ARM subscale is significant if included in the above model. However, only 558 patients would be included in the model and the area under the curve (AUC) is not improved by a large amount by its inclusion (AUC 0.67, 95% CI 0.62 to 0.73).
These models provide reasonable prediction of patients at risk of lymphoedema and those for 1 month after surgery have only three variables and are simple to apply in a clinical setting.
Models from 6 months have higher AUROC and are a better fit because patients have completed their treatments at that point.
Summary
The ability to individualise lymphoedema risk is an important step to tailor follow-up and advice to patients. The models predicting at 1 month have lower diagnostic accuracy as patients have not completed adjuvant chemotherapy or radiotherapy. Nonetheless, the main value may be in identifying women at sufficiently low risk of lymphoedema at 6 months post surgery so they can be reassured and released from further arm monitoring.
Health economics: estimation of health-related utility measures – data available for analysis
A data extract was created in July 2017 containing EuroQol-5 Dimensions, three-level version (EQ-5D-3L) data collected from 1100 BEA patients for up to 24 months.
Successful completion of all of the five dimensional questions (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression) required for calculation of the utility measure was disappointing, declining steadily from 82% to 62% during the 2-year period from baseline (Table 45). Most of the incomplete records contained no data for any of the five questions and were therefore unusable for analysis. Completed documents were more often missing for obese patients (BMI of > 30 kg/m2: p < 0.0001) and current smokers (p < 0.004).
| Time from baseline (preoperative) | Complete record | At least one of the five dimension ratings missing | No data provided |
|---|---|---|---|
| Baseline | 904 (82.1%) | 17 (1.5%) | 180 (16.3%) |
| 6 months | 811 (73.7%) | 13 (1.2%) | 277 (25.2%) |
| 12 months | 776 (70.5%) | 15 (1.4%) | 310 (28.2%) |
| 18 months | 711 (64.6%) | 7 (0.6%) | 383 (34.8%) |
| 24 months | 681 (61.9%) | 12 (1.1%) | 408 (37.1%) |
| Overall | 3883 (70.5%) | 64 (1.2%) | 1558 (28.3%) |
The response to the EuroQol visual analogue scale (EQ VAS) question (requiring only a simple cross on a scale between 0 and 100) was similarly disappointing (Table 46).
| Time from baseline (preoperative) | EQ VAS rating, n (%) | |
|---|---|---|
| Provided | Missing | |
| Baseline | 895 (81.3) | 206 (18.7) |
| 6 months | 793 (72.0) | 308 (28.0) |
| 12 months | 773 (70.2) | 328 (29.9) |
| 18 months | 698 (63.4) | 403 (36.6) |
| 24 months | 678 (61.6) | 423 (38.4) |
| Overall | 3837 (69.7) | 1668 (30.3) |
Of particular interest is the number of patients supplying a continuous sequence of complete EQ-5D-3L data from baseline onwards, as this allows temporal changes in estimated health-related utility to be tracked over time, and correlated with clinical events and the development of lymphoedma. As many as possible of the EQ-5D ratings spoiled by missing dimension entries were remedied by tracing similarities in the pattern of response in earlier and later completed forms, and interpolating where the patient showed consistency of response over time. EuroQol forms with missing responses, which could not be remedied by imputation, were excluded from subsequent analyses.
Table 47 shows that a full complete EQ-5D-3L record after imputation of missing values was available for only 37% of the patient sample, and for 17% of patients no data were provided at all.
| Continuous sequence of EQ-5D-3L data from baseline (preoperative) | Patients with complete useable data, n (%) |
|---|---|
| No EQ-5D data provided at any time point | 190 (17.3) |
| Baseline data only | 212 (19.3) |
| Baseline | |
| 6 months complete | 123 (11.2) |
| 12 months complete | 105 (9.5) |
| 18 months complete | 62 (5.6) |
| 24 months complete | 409 (37.2) |
Data imputation
Most of the 40 EQ-5D ratings spoiled by missing dimension entries were remedied by tracing similarities in the pattern of response in earlier and later completed forms and interpolating where the patient showed consistency of response over time. Only four were found to be wholly or partly irredeemably flawed and excluded from subsequent analyses.
Data analysis
The objective of the analysis carried out on this data set was to identify and quantify the mean change in the EQ-5D-3L utility estimate attributable to the presence of confirmed clinical lymphoedema. Ideally, this would be carried out by using complete sequences of utility estimates over 24 months, and comparing those recorded for patients developing lymphoedema with those for patient who remained lymphoedema-free throughout. Unfortunately, the poor completion rates described above result in only 140 patients developing lymphoedema during the trial and also having a full valid sequence of EQ-5D-3L responses from baseline to 24 months after imputation: limiting the reliability of estimates of disutility obtained. However, alternative methods of analysis have been explored in order to obtain an approximation to the magnitude of the effect of lymphoedema on patient experience.
A search for potentially confounding patient characteristics likely to affect the estimation of patient utility values identified a prospective cohort study of lymphoedema patients at the University of Pennsylvania Lymphoedema Clinic, which described 124 patients with upper extremity cancer lymphoedema and reported EQ-5D-3L results. 42 The severity of the lymphoedema had little effect on the mean estimated utility value, but the authors reported strong associations between estimated utility and BMI, higher BMI being associated with lower utility scores.
An initial exploratory regression analysis of the BEA data confirmed a similarly strong association between patient BMI and EQ-5D-3L utility estimates in our study.
Figure 14 demonstrates that obese and very obese patients are more likely to develop lymphoedema (chi-squared test, p = 0.023). As patient recruitment to the BEA study was not randomised, it was necessary in any comparison between subcohorts to apply a corrective adjustment to counter baseline differences in BMI.
FIGURE 14.
Association between the incidence of lymphoedema (assessed by perometry) and patient BMI.
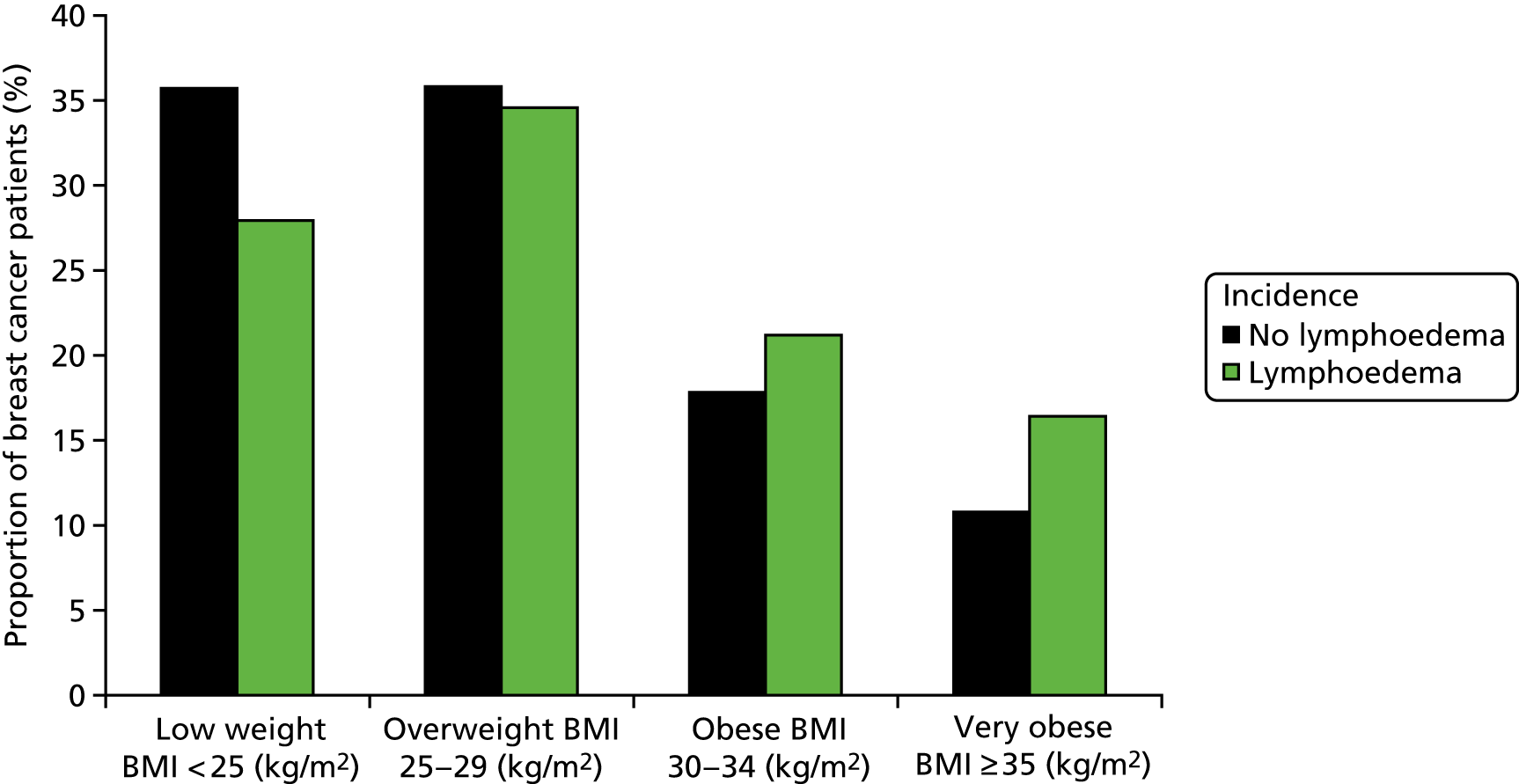
Another patient characteristic known to influence patient-reported utility is the age of patients. An analysis was undertaken of the relationship between the age at which patients entered the BEA study, and their propensity to develop lymphoedema in the 2 years following surgery.
Figure 15 shows that differences in the distribution of patients by age is less pronounced between those who did and did not develop lymphoedema during the study, which is confirmed by a non-significant chi-squared test result (p = 0.39). Correlation analysis between age and utility estimates confirmed that no significant bias is associated with variations by age. Therefore, it was concluded that no adjustment for age was necessary to standardise between the two cohorts.
FIGURE 15.
Association between the incidence of lymphoedema (assessed by perometry) and patient age.
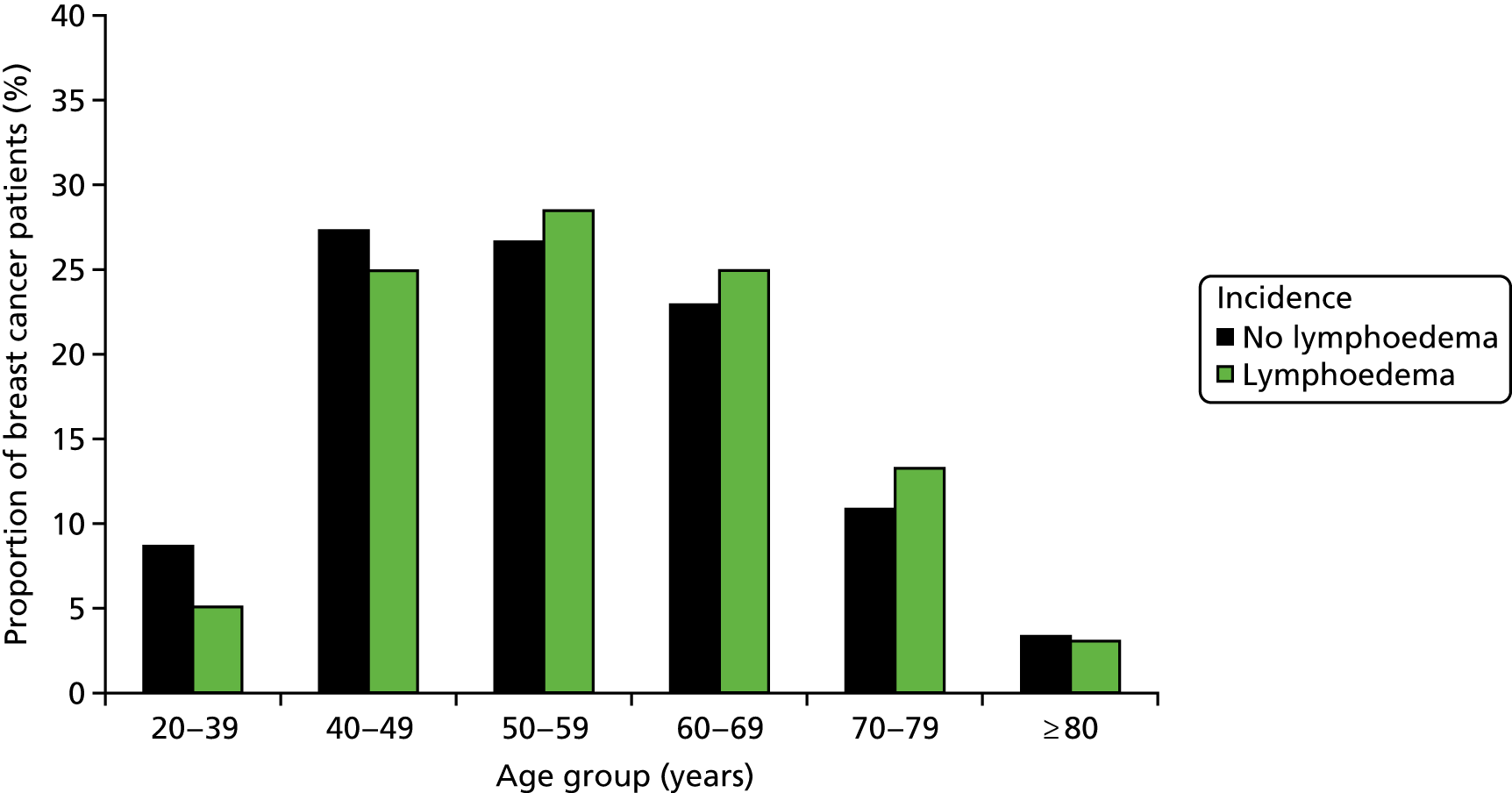
For a total of 403 patients, full EQ-5D responses were submitted or derived by imputation across the 2-year period from baseline (i.e. five data sets at 6-monthly intervals) and a valid BMI could be calculated. Of these, 137 (34%) were identified with primary lymphoedema during the 2-year period, and 266 (66%) were lymphoedema-free throughout. The mean EQ-5D utility estimates are shown in Figure 16, unadjusted for BMI. Patients with lymphoedema are shown in three subgroups according to whether the diagnosis was made by perometry, by clinical assessment with fitting of a compression sleeve, or both in combination.
FIGURE 16.
Mean utility estimates at 6-monthly intervals, by method of assessment leading to diagnosis of lymphoedema, unadjusted for BMI differences.

Figure 17 shows the same comparison following adjustment of utility estimates in the three lymphoedema subgroups to match the mean BMI in the lymphoedema-free group at individual patient level to a common BMI average (26.6 kg/m2). It is important to note that none of the graphical differences in either chart is statistically significant, because of the small number of cases in each of the lymphoedema subgroups.
Nonetheless, it is possible to identify suggestive patterns in these data:
-
There is a consistent loss of estimated patient utility at the 6-month assessment relative to the preoperative (baseline) values, consistent with the impact of recovering from surgery.
-
By the 12-month assessment, there is a general recovery of at least some of the initial utility loss.
-
Patients who do develop lymphoedema in the 24-month period post surgery appear to recover to similar utility levels to those recorded at baseline.
-
The subgroups which featured the need for a compression sleeve to be fitted when lymphoedema was diagnosed (whether or not perometry was used to confirm the diagnosis) generally failed to recover to preoperative utility levels during follow-up.
-
The very small subgroup who were found to have developed lymphoedema by perometry but were not deemed clinically to require a sleeve fitting appear to recover to preoperative utility levels (or possibly better), although this may be related to small number uncertainty.
FIGURE 17.
Mean utility estimates at 6-monthly intervals, adjusted for BMI differences at individual patient level.
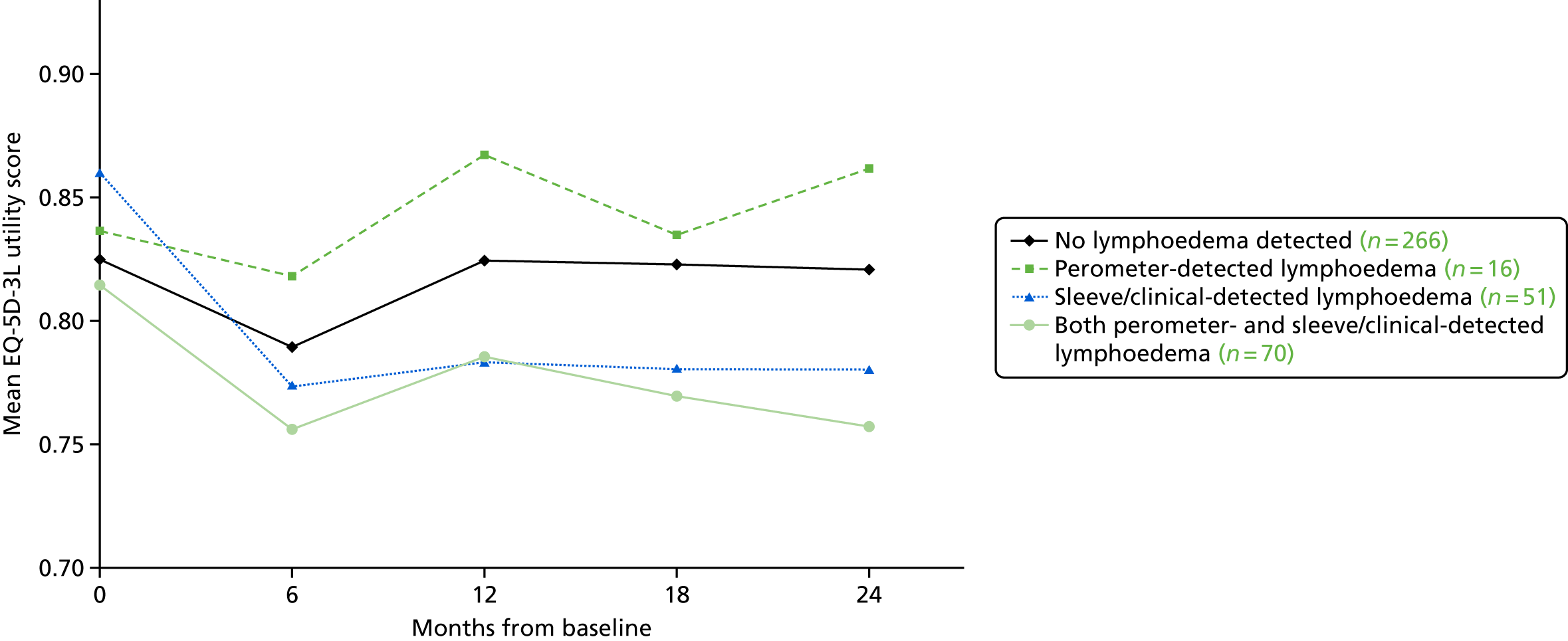
Of particular interest in assessing the cost-effectiveness interventions aimed at reducing the incidence and/or impact of lymphoedema is the estimation of patient-reported disutility attributable to experiencing lymphoedema over an extended period of time. This involves comparing utility estimates for patients with and patients without lymphoedema from the BEA study population.
This has been performed by identifying any patient with a diagnosis of lymphoedema by any method within the first few months after baseline, for whom a full sequence of five utility estimates are available. This limits consideration to patients with at least 6 months experience of the condition. A total of 41 patients fulfilled this criterion, and their utility values at 12, 18 and 24 months were compared with data at the same time points from patients never experiencing lymphoedema.
The results are shown in Table 48 and indicate consistent non-zero disutility estimates at all three time points.
| Patient group | Mean utility | Average estimated utility | ||
|---|---|---|---|---|
| 12 months | 18 months | 24 months | ||
| Patients without lymphoedema (n = 268) | 0.825 | 0.823 | 0.821 | 0.823 |
| Patients with lymphoedema for ≥ 6 months (n = 41) | 0.755 | 0.680 | 0.729 | 0.721 |
| Estimated lymphoedema disutility | –0.070 | –0.143 | –0.092 | –0.102 |
Averaged over this 12-month period of observation, patients with extended experience of lymphoedema recorded a mean utility score of 0.721, compared with 0.823 for patients with no recorded lymphoedema, giving an estimated mean disutility attributable to lymphoedema of –0.102 (95% CI –0.127 to –0.076). If BMI-adjusted utility values are used instead, the size of this effect would reduce to –0.073 (95% CI –0.122 to –0.023).
A search of the literature for comparable research-based estimates of the disutility associated with lymphoedema following breast surgery proved fruitless. Only one published cost-effectiveness study included an assumed value for disutility of –0.03, justified only as the ‘smallest clinically important difference in utility’. 2,43 The estimates obtained using the available BEA data, although not definitive, are statistically significant and evidence-based, and should, therefore, be considered superior.
Further analysis of the BEA data will be possible and will also be performed for PLACE trial participants.
Data analysis: lymphoedema incidence
Another important statistic required to carry out a cost-effectiveness analysis is the incidence rate of the key outcome variable, in this case the proportion of patients confirmed to suffer from clinical lymphoedema, and the timing of such events.
Data on the first recorded time of confirmed lymphoedema have become available for a period exceeding 5 years from baseline. This has made a Kaplan–Meier analysis of the timing of the first recorded lymphoedema event (i.e. the duration of the initial lymphoedema-free period from baseline) possible, as displayed in Figure 18.
FIGURE 18.
Kaplan–Meier survival analysis of primary lymphoedema incidence in BEA patients, compared with the Pennsylvania study. 44
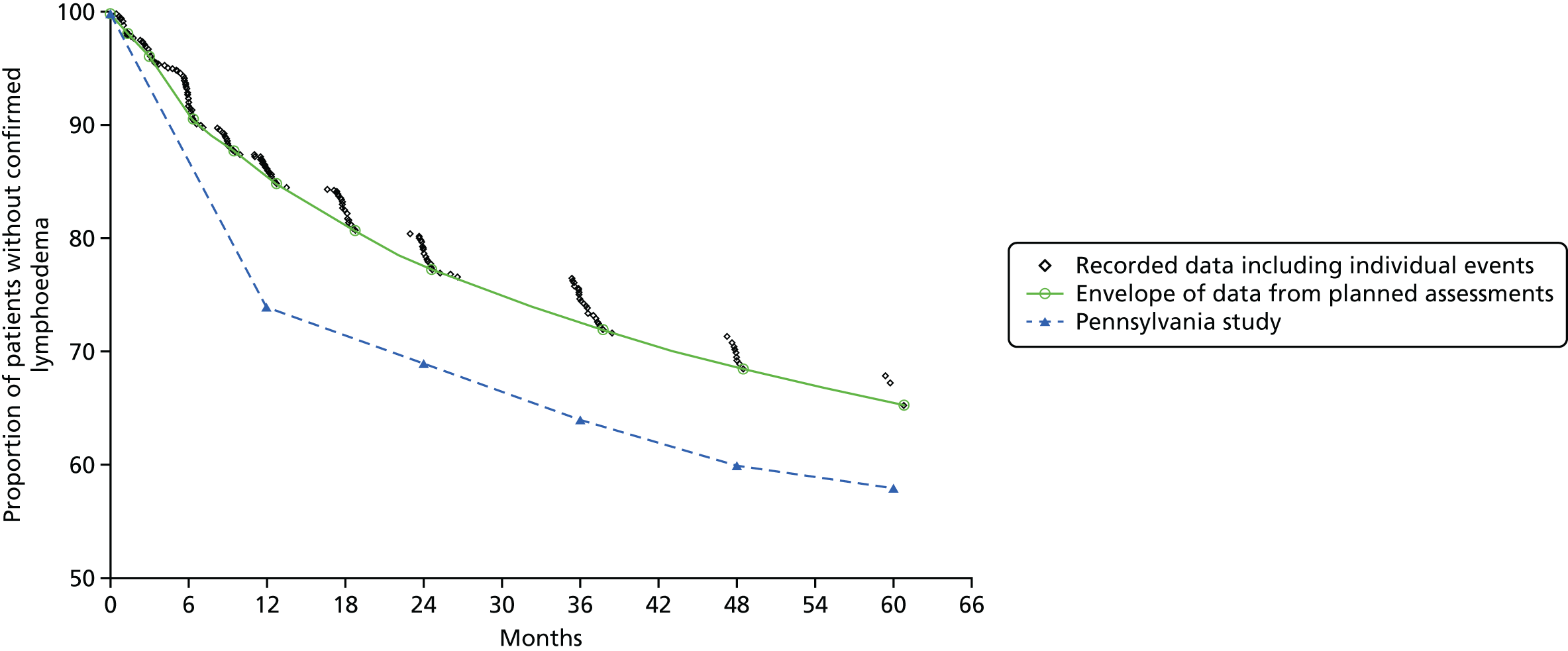
This exhibits a typical profile as seen in studies where assessments are carried out at predetermined intervals, but that the precise timing is spread over several weeks around the target time. This gives rise to periods of time between planned assessments when only a very few ‘opportunistic’ primary lymphoedema events are recorded, followed by multiple events occurring either side of each planned assessment time. To mitigate the bias introduced by the study design, it is necessary to identify an ‘envelope’ of accurate data points corresponding to the time at the end of ‘step-down’ phase of the prespecified assessment times. At these points all planned and opportunistic events up to that time are included. These ‘envelope’ data are represented in the chart by the large circles in Figure 18.
Using sections of the envelope data, it was possible to estimate the annual incidence rate of primary lymphoedema at different periods of time. In the first 6 months from baseline, the incidence rate was 16.4% per annum, contrasting with 9.5% between 9 and 24 months, and 4.9% between 36 and 60 months. Clearly, the risk of lymphoedema-free patients suffering a primary event decreased steadily throughout the 5-year observation period.
Figure 18 also shows comparable 5-year lymphoedema incidence from a study of 631 breast cancer patients in Philadelphia and Delaware Counties, Pennsylvania. 45 Although these patients suffered higher incidence of lymphoedema throughout, the difference between the two trends is wholly attributable to a much higher incidence in the first 12 months (74% lymphoedema-free in the Pennsylvania study, compared with 85% lymphoedema-free in the current study), but the risk of developing lymphoedema thereafter was very similar to that found in the current study.
To estimate the incidence of primary lymphoedema at future times beyond the available data set, a range of standard statistical parametric models was fitted to the envelope data. However, this proved disappointing, with poor correspondence to the trial data when some functions were tested and unrealistic estimates of the mean long-term time spent lymphoedema-free for other functions.
An alternative approach was attempted, which sought to incorporate the existence of an unknown proportion of the study population who were at zero risk of lymphoedema. This method did not generally improve the correspondence of fitted models to the study data and led to a wide variation in the estimates of the zero-risk subset of the population (between 33% and 58%). Therefore, it has been concluded that without additional evidence from other sources it is not possible to obtain reliable estimates of the number of patients suffering lymphoedema beyond the available data and the timing of incident events.
Workstream 3: graduated compression garments to prevent onset of chronic lymphoedema
Sentinel lymph node biopsy staging reduces the need for ANC, but 30% of breast cancer patients are node positive and require ANC to remove diseased nodes. 21–24
A clinical end point of > 10% increase in ipsilateral arm volume (vs. contralateral arm) is an accepted criterion for a diagnosis of lymphoedema. 21–24 Up to 40% of women develop lymphoedema by 18 months post ANC based on this criterion. Intervention before arm swelling becomes chronic may prevent the complications of lymphoedema after ANC.
The management of patients after ANC does not routinely include prospective measurement of the ipsilateral arm. In the absence of prospective arm measurements, early changes preceding lymphoedema are difficult to detect. Consequently, patients present with marked arm swelling before being considered for treatment. 20–22 Initially when a patient presents with concerns about their arm (unless measurement confirms arm swelling equivalent to a 10% arm-volume increase, compared with the contralateral arm), advice is provided regarding arm massage, active movement (series of exercises) and limb elevation, and on avoiding injury and infection of the affected limb(s). 23,28,29
Graduated compression garments, which decrease the amount of interstitial fluid (especially during exercise), are designed to cover the entire area of oedema and are graduated with the greatest compression at the distal end and the least compression at the proximal end. They have been shown to produce reductions in arm swelling by 4–24% in small single-centre randomised trials. 23,28,29 Once arm swelling reduces, to maintain compression on the subcutaneous tissues refitting of a tailored compression sleeve is required. Early intervention before gross arm swelling occurs will reduce the need to refit sleeves because these become looser and no longer fit the arm with the correct pressure gradient.
In the ALMANAC trial, ANC patients had a 40% incidence of lymphoedema by 18 months after surgery overall; however, for those women who developed 4–9% increases in arm volume, there was a 60% lymphoedema incidence at 18 months post surgery. 20,46
Conventional advice is that early arm swelling does not portend chronic swelling and should be treated conservatively. 23,28,29 Arm swelling of 4–9% is usually not clinically apparent unless arm measurements have been made preoperatively, and only 15% of women in the ‘ANC arm’ of the ALMANAC trial complained of significant swelling at 6 and 18 months. 20,27 Early intervention (in a group of patients with 4–9% arm swelling) with a compression garment may prevent the development of chronic lymphoedema. 23 Research has shown that as arm swelling is treated and subsides, QoL significantly improves. 23,26,28 Currently, there is no evidence to support the value of compression garments in preventing lymphoedema after ANC.
There is a need to test early intervention in women after ANC, with a 50–70% risk of lymphoedema at up to 9 months after surgery. We used graduated compression sleeves to test whether prevention in women at high risk of lymphoedema is potentially better than current management and our current inability to cure the condition.
Design
The design was a randomised open controlled trial testing (1) standard care (written advice, arm elevation, exercises and massage) versus (2) the intervention, application of whole arm graduated compression garments (pressure 15–24 mmHg) to the affected arm, together with standard management for 1 year (see Appendix 18).
As women randomised to compression garments were given four compression garments for their wardrobe, we expected that in this group the extra training and reinforcement of the importance of preventing arm swelling will mean that they will reutilise their sleeves if necessary even if they are no longer being prescribed new sleeves at the end of the 1-year intervention to prevent substantial progression of arm swelling between clinic visits.
Primary outcome
-
Time to development of lymphoedema (RAVI of > 10% assessed by perometer scanning) from randomisation.
Secondary outcome
-
Time to development of moderate lymphoedema (RAVI of > 20%) from randomisation.
-
Quality of life in each group (TOI and FACT-B+4 ARM subscale).
-
Costs and utility measurement of individual strategies (EQ-5D-3L utility measures).
-
Incidence of infection/lymphangitis.
-
Incidence of lymphoedema at 5 years post surgery (NB 90% of lymphoedema develops by 3 years post ANC).
Setting
Breast outpatient clinics in teaching and district hospitals affiliated with specialist lymphoedema clinics.
Target population
Women with node-positive, early breast cancer scheduled to undergo ANC who consent to preoperative arm measurements with a perometer and subsequently develop a 4–9% increase in arm volume at 1, 3 or 6 months post surgery.
Inclusion criteria
-
Women aged 18–90 years.
-
Early breast cancer (no metastasis), scheduled to undergo ANC.
-
Consented to prior (pre-surgical) arm measurements who develop arm-volume increases of 4–9% within 6 months after surgery.
-
Written informed consent.
Exclusion criteria
-
Any patients with no pre-surgical baseline measurements.
-
Known distant metastasis.
-
Inoperable breast cancer (T4 category or distant metastasis).
-
Node negative not undergoing axillary clearance.
-
Previous axillary radiotherapy or clearance.
-
Past history of breast/chest wall radiotherapy prior to commencement of monitoring.
-
Bilateral axillary clearance.
Results
One hundred and forty-three patients have been randomised (74 to no sleeve standard care and 69 to compression sleeves plus standard care) between 1 October 2010 and November 2015. On account of slow recruitment, the number of centres was increased from 7 to 21 by November 2013 and a qualitative study commenced to understand the reasons behind the poor recruitment compared with that expected.
Qualitative nested study of recruitment
The qualitative study is more fully reported in Appendix 19.
In-depth interviews were conducted with 38 purposively sampled patients and 16 purposively sampled recruiting staff, from five purposively selected trial sites and two PLACE trial management staff. Recruiting staff were initially invited to participate in focus groups using vignette techniques to explore issues in the recruitment of patients to the trial and were then interviewed one to one. Interviews were audio-recorded, transcribed verbatim and subject to analysis using framework analysis themes. This included patient motivators with patients identifying participating because of altruistic reasons as well as belief that they would receive ‘better care’ due to closer monitoring from the breast cancer research nurses and access to a specialised team. 47 On the other hand there were also patient barriers and a major reason cited was ‘inappropriate timing’ of the trial for their individual circumstances and not wanting to burden themselves with further commitments. One older patient (aged 87 years) considered herself ‘to be too old’ to be bothered with taking part. Some patients declined if their preference for their preferred allocated study arm was not met. Patients also reported withdrawing from the study as they found wearing the study sleeve uncomfortable or stigmatising. Most patients commented on the professional and caring attitude of recruitment staff and this was identified as an organisational facilitator. However, there were also a number of organisational barriers related to procedures/protocol not being followed correctly or misunderstood, lack of training/confidence in explaining the RCT, auditing and trial management issues, as well as staffing issues.
Procedural issues
It became apparent that recruitment staff did not always follow the PLACE trial protocol or study procedures correctly and eligible patients were not always invited to participate. Recruiting staff held variable interpretations of who was eligible for the trial, and there was evidence of a ‘wait and see’ culture, whereby they assumed that they could wait to see if patients were still eligible at later check-up appointments. In some sites this appeared to be the norm, but it had the effect that patients ‘timed out’ at 9 months and thus became de facto ineligible. Most recruiting staff were nurses and they experienced role conflict between their professional roles as clinician and patient advocate and recruiter. These recruiters acted as gatekeepers and often appeared to have assumed that taking part in the RCT would be burdensome, or not beneficial to, for example, patients undergoing chemotherapy. In addition, some patients presenting with reports of distressing symptoms (swelling, heaviness, etc.) or arm swelling towards the upper limit of eligibility for the PLACE trial would be referred directly to the lymphoedema service instead of being entered into the trial.
There were also problems with lack of understanding of the rationale for the trial (misunderstanding of equipoise), explaining the RCT incorrectly to patients, and presenting the randomisation process in ways that may have been off-putting to patients. One recruiter reported that she could not see the benefit of taking part in the PLACE trial, which may have reduced recruitment. A number of patients were interviewed specifically because, according to screening logs, they had been approached and had declined to participate. Some reported no recollection of being approached, and it is not clear if they had been explicitly approached to participate and had forgotten, or if the approach had been rather informal and ‘throw-away’ and not recognised as a request to participate by the patient, or if they had indeed not actually been asked to take part, but that the recruiter had logged this incorrectly, either intentionally or inadvertently.
Although screening logs were completed across recruiting sites, these could not be verified. Staff were not accountable to the research team as they were employed by NIHR Clinical Research Network and had competing trials to recruit to. High turnover of recruitment staff was cited as having a detrimental impact on recruitment. As staff were managed by the network rather than the trial, the trial management team were not made aware of staff changes, and were thus not in a position to ensure training to new staff outside the regular site visits and updates.
In overview, staff struggled with role conflict, problems in understanding and explaining the trial and did not always prioritise this trial. Indeed, nurses reportedly felt that they should use clinical judgement to assess patients’ eligibility to the trial rather than simply base it on arm swelling criteria. They tended to adopt a ‘wait and see’ approach, which resulted in eligible patients timing-out, and/or were overprotective and referred directly to specialist services rather than entering the patient into the trial. As the IDMC closed recruitment before we could feed back results from the qualitative work, we do not know if any of the changes to recruitment procedures that would have followed from our qualitative work could have improved recruitment. These findings concur with other reports in the literature (e.g. Quintet Recruitment Intervention) and suggest that qualitative work of this sort should become an integral part of trials from the outset, to provide insight into recruitment and facilitate improved recruitment rates.
Qualitative study conclusion
Assumptions made by recruitment staff that taking part in the RCT may be burdensome for patients had a significant impact on recruitment behaviour, which in turn led to poor recruitment rates. During recruitment encounters, staff acted as gatekeepers by only suggesting taking part in the PLACE trial with those patients who were deemed suitable for the trial, rather than with all patients who met the inclusion eligibility criteria. Making a clinical judgement not to recruit patients in this way is perceived as paternalistic. For example, PLACE trial recruiters were making decisions on their patients’ behalf with the view that, as clinicians, they knew what was best for patients. Certain recruiters generally described their focus was on protecting and caring for patients’ needs rather than sharing knowledge and information about the RCT.
Current position of the PLACE trial
The IDMC in March 2016 recommended that the trial close to further recruitment as it was unlikely to reach 270 patients in any reasonable time frame and no further centres had been identified. Moreover, BEA had reached 1100 patients and it appeared that around 25% developed an arm-volume increase of 4–9% and were eligible for the PLACE trial. The trial remained open to BEA recruits who had been offered PLACE trial entry if they developed arm-volume increases of 4–9%. Recruitment ceased in late November 2016 and follow-up of participants continued until November 2018.
In general, groups were well matched in BMI, age, dominant arm, side of operation, smoking history, type of surgery and radiotherapy treatments. The median follow-up was 22 months (Table 49).
| Characteristic | Trial arm | |
|---|---|---|
| No sleeve (N = 74) | Sleeve (N = 69) | |
| BMI (kg/m2) (preoperatively) | 27.8 (95% CI 17.2 to 45.3) | 28.7 (95% CI 16.9 to 60.9) |
| BMI (kg/m2) (at PLACE trial entry) | 26.9 (95% CI 18.0 to 47.0) | 28.4 (95% CI 20.7 to 58.4) |
| Difference between arms in % change (at PLACE trial entry) | 5.9 (95% CI 4.1 to 8.9) | 6.4 (95% CI 4.0 to 8.5) |
| Follow-up (months from randomisation) | 23 (95% CI 0 to 59) | 21 (95% CI 0 to 61) |
| Age (years) at randomisation | 55.5 (95% CI 33.5 to 89.9) | 55.8 (95% CI 32.0 to 86.9) |
| Tumour site (n) | ||
| UO | 34 | 37 |
| UI | 9 | 7 |
| LO | 9 | 2 |
| LI | 6 | 2 |
| Central areolar | 5 | 10 |
| Other | 11 | 11 |
| Side (n) | ||
| Right | 38 | 28 |
| Left | 36 | 41 |
| Dominant hand (n) | ||
| Right | 69 | 64 |
| Left | 5 | 5 |
| Smoking history (n) | ||
| Never | 49 | 35 |
| Ex | 20 | 25 |
| Current | 5 | 9 |
| Type of surgery (n) | ||
| ANC | 13 | 15 |
| WLE + ANC | 23 | 17 |
| Mastectomy + ANC | 36 | 34 |
| Other | 2 | 3 |
| Post-surgery radiotherapy: yes (n) | 59 | 58 |
| Dose (cagy) | (n = 59) 4005 (95% CI 3960 to 5605) | (n = 58) 4005 (95% CI 1068 to 6010) |
| Number of fractions | 15 (95% CI 15 to 25) | 15 (95% CI 4 to 30) |
| Site of radiotherapy (n) | ||
| Breast | 28 | 25 |
| Breast + SCF | 18 | 21 |
| Breast + axilla | 3 | 2 |
| Breast + SCF + axilla | 3 | 2 |
| Other | 7 | 8 |
The overall lymphoedema rate is 40% with a 33% Kaplan–Meier lymphoedema rate by 24 months currently. The final results from this trial will not be available until all patients have a minimum 2-year follow-up (November 2018).
After March 2016, when the CTU statistician retired, closer consideration of the PLACE trial data by Julie Morris (trial statistician, appointed May 2016) indicated the overall lymphoedema rate to be 40% and, with longer follow-up, it remains possible that an outcome from the trial will be found, particularly combining the data in a meta-analysis with those from a similar trial being conducted in Boston, MA, USA (principal investigator, A Taghian). The PLACE trial remains the largest multicentre external compression garment trial to prevent lymphoedema, as the previous four trials of compression garments recruited only 85 patients in total and were all single centre. The results from this trial will be crucial to inform the future direction of lymphoedema management and the value of external compression garments to prevent lymphoedema. It appears that baseline and regular arm measurements combined with information leaflets, advice and exercises such as simple lymphatic drainage may reduce rates of lymphoedema development and are valuable in a high-risk population. Although rates of axillary clearance surgery in breast cancer are reducing for low node-positive breast patients (fewer than three nodes involved), clearance surgery remains the treatment for node-positive breast and melanoma patients with involved nodes. Key findings from the PLACE trial are thus likely to be generalisable and applicable in the future.
Patient and public involvement
Patient and public involvement (PPI) occurred during the life of this project from its inception right through to its end. Patient representatives sat on the management committee, giving a patient perspective on how the project was undertaken. Their input of was invaluable in ensuring that the patient point of view was never lost in how the WS were conducted.
Workstream 1
The consumer panels of three cancer research networks were consulted in the development of the research protocol for WS1. The response was positive, supportive and constructive in all cases. For example, one panel wrote, ‘This is a most excellent study that is badly needed’. Recommendations from the panels were integrated into the design of the study.
Similarly, the suggestions of patient representatives on the cancer research networks were followed for training research staff. Patient representatives consented to carry out training interviews with research staff giving them feedback on their interview technique. This proved particularly useful to provide insight into and experience of interacting with supportive ‘patients’ themselves. Recommendations about the design from both WS1 and WS2/3 panels were integrated into the design and helped with the Trial Management Group.
Workstream 2
A group of 10 people with lymphoedema from University Hospital of South Manchester were involved in helping us to develop better treatments for lymphoedema, primarily with regard to different designs of compression sleeves. We involved them in the design of this trial and the quality-of-life measures. They commented that they would have preferred to have had earlier intervention with external compression garments than to have undergone manual lymphatic drainage and compression therapy once they had developed lymphoedema.
Two patients who developed lymphoedema within 2 years of ANC surgery agreed to sit on the patient management group and were both initially involved with this project. Unfortunately, both died during the first 3 years of the project, and two further patient representatives were subsequently involved. A qualitative study consulted patients to understand the poor recruitment in the PLACE trial.
We involved the PPI forum within University Hospital of South Manchester to ensure that patients and the public were formally involved in plans to carry out research, monitor progress, implement findings and monitor the impact on services. We have liaised with charities that have a role in patient support, including BCC and Breakthrough Breast Cancer, to involve the public and patients in this research.
Discussion
Over 2000 NHS patients took part in these studies in nearly 50 NHS breast units and we appreciate the contributions of all the patients, doctors and nursing staff, without whom these studies would not have been possible. Although WS1 commenced on time, delays in opening WS2 and WS3 because of delays in site approval of the BIS device, and the subsequent need to extend the number of centres from 7 to 21 to improve recruitment for the PLACE trial, delayed data collection and follow-up. However, there is now a network of sites for lymphoedema studies developed, which could be built on for further studies.
The studies of preference reveal that in about half of consultations both the patient and the surgeon chose the same person as making the surgical decision, but the actual agreement between surgeons and patients is low. In univariate analyses, increasing age predicts not undergoing surgery from the age of 75 years, compared with those aged 70–74 years. Adjusting for health measures and choice, only women aged > 85 years have reduced odds of surgery. Patient role in treatment decisions makes no difference to whether or not they received surgery. Women who were active/collaborative were as likely to get surgery as those who left the decision to the surgeon. The qualitative study of women who did not receive primary surgery revealed three approaches: ‘patient declined’, ‘patient considered’ and ‘surgeon decided’.
These reductions in surgical rates with increasing age are in broad agreement with previous studies, although previous work reports unadjusted odds. 3,7 Once patient health and choice were adjusted for, both the location and the size of effect changed, and only the oldest women aged > 85 years retained significantly reduced odds of surgery. Moreover, neither patient health nor choice accounts for the lack of surgery for the oldest women aged > 85 years, and this reduction in effect size for 75- to 84-year-olds appears to be largely driven by adjustment for measures of health rather than by patient choice. On the basis of responses to the CPS, there is no evidence that there was any real active choice to not have surgery among those who did not have surgery. These findings suggest that the lack of surgery for the oldest patients is not because they actively opt out of having this treatment. A likely explanation for this is that the option of not having surgery is offered/discussed only if there are concerns about the patient undergoing surgery.
There is some evidence that surgical rates are improving for older women with breast cancer in the UK and our results tend to confirm this. It seems likely that improved surgical rates reflect changes in practice following publication of guidelines and reorganisation of cancer services over the past decade. Nonetheless, although the situation appears to be improving, the lack of surgery for women aged > 85 years persists and, as defined by national policy,1,17 ‘inappropriate undertreatment’ is still occurring for this oldest age group. Older age does not predict complications and the risk of serious complications from breast surgery is low for older patients. Surgical decisions should be based on patient fitness rather than on age. 9,15 The number of observed cancer deaths exceeded those expected for participants whose tumours were of higher grade or stage and steroid receptor negative, but did not undergo surgery and warranted chemotherapy. Adjusting for tumour stage, comorbidity and functional status, women undergoing surgery had one-third the hazard of dying of breast cancer. Given these findings, it is hard to see on what basis surgery should be withheld from older women who are fit for surgery.
Following surgery, many older women do not receive chemotherapy and radiotherapy, even though they may have benefited from these therapies. 15,16,48 Can this lack of chemotherapy and radiotherapy be explained by patient choice or health? We demonstrated that women aged ≥ 75 years have lower chemotherapy and radiotherapy rates than women aged 65–69 years. After adjusting for tumour characteristics, health measures and choice, women aged ≥ 75 years still have reduced odds of receiving chemotherapy, whereas age has no impact on the radiotherapy rates of older women. Thus, lower chemotherapy rates in older women cannot be explained by health or patient choice.
Overall, although over the past decade there have been improvements in the access older women have to breast cancer services, there are still substantial gains to be made by ensuring that treatment decisions are based on ‘fitness’ and ability to benefit rather than on age per se. The endemic ageism of the past may have gone, but there remains room for improvement.
Assessing lymphoedema objectively depends on an agreed international definition. 22–24 We found arm volume measurement (RAVI) to be the optimal choice for assessment of arm swelling, and that BIS, although reasonably specific (85–92%), had a lower sensitivity and only modest correlation with arm volume. It would have led to significantly more patients receiving compression sleeves that were applied inappropriately. If compared with sleeve application as treatment (excluding patients randomised to sleeve in the PLACE trial), BIS missed some patients with lymphoedema and misdiagnosed (false-positive) others. BIS increases of < 10% at 6 months did not aid prediction of lymphoedema, whereas patients with a RAVI of 5–9% had a 35% risk of lymphoedema at 18 months. Moreover, RAVI of > 9% and/or ‘considerable’ arm swelling predicted the clinical benefit of sleeve application.
Some studies have suggested that self-reported symptoms predict lymphoedema,4 whereas others have shown that factors such as being overweight, axillary radiation and chemotherapy are more predictive of lymphoedema after breast cancer surgery. 25 The results indicate that self-report on ARM subscale (particularly B3, ‘considerable swelling’) and the lymphoedema checklist are good predictors of lymphoedema that indicate that patient subjective concerns (probably coupled with anxiety) drive sleeve application, to at least as great an extent as objective measures such as RAVI.
There is, however, some debate over whether gain in weight is a reliable predictor of lymphoedema after surgery for breast cancer, with some conflicting evidence as to whether BMI is significantly related to lymphoedema,26 or not. 27 BMI at surgery was an independent predictor of both QoL and risk of lymphoedema. In our data, substantial change in BMI after surgery was rare, but encouraging interventions to reduce BMI will reduce lymphoedema occurrence and, potentially, improve QoL.
We found that 25% of patients reported symptoms of swelling and/or numbness, and/or heaviness in the limb on their at risk side even before their surgery. 23 These data support the need for a rigorous preoperative baseline assessment and subsequent measurements to determine arm swelling changes. Screening for breast cancer-related lymphoedema would benefit patients by enabling early intervention. 29 Stout Gergich et al. 28 found that in a group of 43 patients with a 3% arm-volume increase, the group wearing compression garments showed a greater decrease in arm-volume than an age-matched control group with a mean follow-up time for the intervention of 4.8 months. 29 The findings support a threshold for intervention of > 4–9% RAVI to prevent progression to lymphoedema, provided that the intervention is demonstrably effective.
The measurement and diagnosis of lymphoedema are inconsistent,22–24 highlighting a need for preoperative baseline measurements against which to monitor early changes in arm volume. The importance of consistent, objective and robust measurement techniques remains and the reliance on symptoms alone to diagnose lymphoedema is insufficient. Perometer has been shown to be the easiest and most objective tool to measure arm swelling given that definitions of lymphoedema are based on arm-volume increases (whether 200 ml, or a RAVI of > 5 or > 10%). Nonetheless, treatment decisions to apply compression sleeves are more subjective and based on patient symptoms such as heaviness and swelling of the arm. Indeed, some staging classifications describe a prodromal or latent phase of lymphoedema characterised by arm heaviness or swelling in the absence of a RAVI of > 10%. We identified a composite definition based on a RAVI of > 5% and B3 of > 2, which identified 99% of patients who would not develop lymphoedema and could be reassured. Composite definition of lymphoedema (utilising a RAVI of > 9% and a B3 score of < 2) produced a diagnostic accuracy of 94–95% for sleeve application.
Patient concerns and anxiety about developing lymphoedema has led to external compression garments described as ‘prophylactic’ with a lower arm compression (10–15 mmHg) being prescribed in the absence of any evidence for either the intervention or the compression pressure (as opposed to therapy garments 15–24 mmHg). The modelling of sleeve application indicates that in a multicentre study, sites used symptoms combined with QoL deficits to justify application of garments despite the absence of objective arm swelling evidence indicating a need for better definitions of lymphoedema which are a composite of RAVI and self-reported symptoms. In particular in the absence of RAVI > 9% and/or self-reported ‘considerable’ arm swelling (B3 scores) little benefit in QoL was seen following sleeve application. Lymphoedema Practitioners need to be clear with their patients who sleeves are not a solution for numbness, painful arm movement or heaviness in the absence of ‘considerable’ arm-volume increases. Such objectivity would reduce NHS costs.
The PLACE trial will help determine the validity of early intervention with external compression garments and their effects on arm-volume increases which affect subjective symptoms and QoL.
The prevalence of lymphoedema at 12 and 24 months varies by the assessment criterion with 25% RAVI of > 10% and 66% having symptoms by 24 months yet only approximately 24% have external compression garments fitted by 24 months. Understanding which factors trigger decisions to apply sleeve therapy is crucial to developing an evidence base for lymphoedema treatment.
Although there are several risk factors commonly associated with the development of lymphoedema, there is still a need to determine some of the underlying pathological and genetic factors associated with the development of secondary lymphoedema after axillary surgery. Specht et al. found that even in patients who underwent sentinel node biopsy, 10–15% still developed lymphoedema, and a RAVI increase of 5–9% also predicted lymphoedema development in sentinel node biopsy patients. 45 The exact threshold for early intervention to prevent progression to lymphoedema postulated at > 4–9% needs confirmation to allow close monitoring or intervention for patients who present with these arm-volume changes. Although there is some correlation between perometer and BIS measurements during the first 6 months after surgery, longer-term data are required to determine their equivalence in predicting and diagnosing lymphoedema.
In addition, identifying genetic markers of lymphoedema would be important, and within the BEA and PLACE study we have 619 patients who have provided paxgene blood samples to investigate this question at a future date.
The study of QoL is the largest in node-positive patients. Fleissig et al. 26 studied QoL in the ALMANAC trial of ANC versus sentinel node biopsy, but the majority (74%) were node negative. In the ANC group they found a TOI reduction of six in the first 6 months corrected to baseline by 12 months and a similar change for FACT-B+4. No attempt to compare effects of Lymphoedema on QoL was made. Likewise, a TOI reduction of five was found by 3 months in the BEA study and in patients who developed Lymphoedema by 6 months the TOI score remained significantly lower even at 18 and 24 months. FACT-B+4 fell by seven points at 6 months but returned to baseline by 12 months. Thus Lymphoedema reduces QoL for sufferers.
A RAVI of > 10% showed greater falls in TOI QoL (fall of –5) than BEA > 10 (fall of –3), suggesting that RAVI of > 10% is a better marker for QoL effects. We will undertake more detailed analysis of FACT-B outcomes from the PLACE trial once full follow-up data on all participants are available. Importantly, understanding the relationship between RAVI increases with symptoms, patient anxiety and QoL reductions may suggest other approaches, such as cognitive–behavioural therapy, to reduce the need for sleeve intervention, as labelling a patient with a lymphoedema diagnosis by applying a sleeve implies the need for interventions for the remainder of a patient’s life.
What was and was not successful in the Programme Grant
The programme of work in elderly breast cancer involving multicentre studies successfully recruited and provided important work on the management of elderly breast cancer. Workstream 2 recruited well after ANC and has produced clear results about the value of arm volume measurements and the use of, and indications for, compression arm sleeves. A health economics analysis was less successful because patients who were acutely affected by their cancer diagnosis and morbidity were reluctant to fill in QoL questionnaires. The trial of compression garments in patients developing early arm swelling failed to recruit sufficient patients because of lack of equipoise among lymphoedema nurses and clinicians. Thus, results of the PLACE trial are still awaited.
Implications for practice
Our findings suggest that older women should be offered surgery, which can be performed under local anaesthetic block if there are concerns over fitness, and surgeons need to make clear the advantages of surgical excision of the cancer on breast cancer survival. The lymphoedema prediction index described will aid communication and individualisation of monitoring of patients after ANC surgery. The use of the Lymphoedema Checklist in women after ANC surgery will aid early recognition of arm problems, particularly if it proves as good a marker of need for intervention as arm measurements. BIS does not reach the expected sensitivity or specificity compared with perometry and the Lymphoedema Checklist to justify its cost and introduction to NHS practice [this was the subject of a recent NICE Medical Technologies Evaluation Programme review www.valueinhealthjournal.com/article/S1098-3015(17)3246-9/fulltext]. Sleeves are not effective in the absence of RAVI of > 9% or ‘considerable’ self-reported arm swelling.
Future work
Understanding the drivers for, and producing more objective measures to understand, sleeve application/prescription in the NHS is required, which we intend to investigate further once source data are further verified. Developing an evidence base for lymphoedema treatment is essential, and ensuring equality of access to a high-quality service throughout the NHS requires a robust understanding of indications for intervention and the benefits of those interventions applied. Trials investigating the value of diet and exercise to prevent or treat lymphoedema in overweight patients are required. Research to understand how self-reported symptoms of lymphoedema (such as heaviness and arm swelling) can be alleviated without the need for sleeve application, by cognitive–behavioural therapy, diet and or various arm or weight-reducing exercise regimes, is required.
Acknowledgements
We are grateful to all of the patients who took part in these studies, the clinicians who enrolled their patients, research nurses and the lymphoedema nurses who helped with the study. We appreciate the work of the interviewers, data managers at the CTU and the trial co-ordinators who worked on this Programme Grant.
Katrina Lavelle aided Chris Todd in leading and writing the WS1 elderly breast cancer patient studies. Katrina Lavelle was also involved in designing, conducting, analysing and writing up the qualitative study of patient choice. Jane Griffiths designed, conducted, analysed and wrote up the qualitative study of recruitment.
Three individuals provided trial co-ordination, Charlotte Stockton, Sarah Ashton and Donna Watterson, and we are grateful for all of their help in arranging the trial management group and liaison with all of the investigators.
The success of this programme is the result of hard work and dedication from a large number of research staff. In WS1 this included research assistants Noshaba Anwar, Lorie Dickinson, Kirsty Ewing, Nisha Patel, Georgina Pennington-Smith, Emma Rhodes and Bernhard Wagner; and administrator Rosie Perry.
Steering Group members included Nigel Bundred, Linda Ashcroft, Katrina Lavelle, Katie Riches, Chris Todd, Vaughan Keeley, Charlotte Stockton, Elizabeth Stein, Maria Bramley, Ash Kothari, Sari, Catherine Fellows, Arnie Purushotham, Janet Walls, Alison Myatt, Amy Cavanagh, Tracy Hodgkiss, Adrian Bagust, Vernie Ramalingam, Norma Hewitt and Sarah Ashton.
We appreciate the work of the IDMC, which was chaired by Professor Mike Bennett with Professor Robert Mansel and Roger Hearn, the IDMC statistician.
The Manchester Academic Health Science Centre – Clinical Trials Unit was responsible for data management, data collection and in the first 3 years the statistical analyses.
Contributions of authors
Nigel Bundred (Professor of Surgical Oncology and Consultant Breast Surgeon) was responsible for writing the report conclusions and recommendations; was responsible for recruitment organising centres and liaising with the CTU for data collection for the BEA in place at trials; and assessed the QoL results, wrote the text and summarised the findings.
Chris Todd (Professor of Primary Care and Community Health) was responsible for writing the report conclusions and recommendations; led and was responsible for writing the WS1 elderly breast cancer patient studies; assessed the QoL results, wrote the text and summarised the findings; designed, conducted, analysed and wrote up the qualitative study of patient choice; and designed, conducted, analysed and wrote up the qualitative study of recruitment.
Julie Morris (Head of Medical Statistics) was responsible for the modelling of the lymphoedema risk and the final statistical analyses presented; and assessed the QoL results, wrote the text and summarised the findings.
Vaughan Keeley was responsible for recruitment organising centres and liaising with the CTU for data collection for the BEA in place at trials.
Arnie Purushotham (Professor of Breast Cancer and Consultant Surgeon) was involved with patient recruitment and reviewed the manuscript.
Adrian Bagust was responsible for the health economics text and summary.
Philip Foden (Medical Statistician) was responsible for the modelling of the lymphoedema risk and the final statistical analyses presented; and assessed the QoL results, wrote the text and summarised the findings.
Maria Bramley (Consultant Breast Surgeon) was involved with patient recruitment and reviewed the manuscript.
Katie Riches was responsible for recruitment organising centres and liaising with the CTU for data collection for the BEA in place at trials.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Please note exclusive use will be retained until the publication of major outputs. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Department of Health and Social Care . Cancer Reform Strategy 2007.
- Nichol MD, Epstein J. Separating gains and losses in health when calculating the minimum important difference for mapped utility measures. Qual Life Res 2008;17:955-61. https://doi.org/10.1007/s11136-008-9369-7.
- Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209-15. https://doi.org/10.1002/bjs.5834.
- Møller H, Sandin F, Bray F, Klint A, Linklater KM, Purushotham A, et al. Breast cancer survival in England, Norway and Sweden: a population-based comparison. Int J Cancer 2010;127:2630-8. https://doi.org/10.1002/ijc.25264.
- Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Degner L, Stockton C, et al. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer 2014;110:573-83. https://doi.org/10.1038/bjc.2013.734.
- Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res 1997;29:21-43.
- Beaver K, Luker KA, Owens RG, Leinster SJ, Degner LF, Sloan JA. Treatment decision making in women newly diagnosed with breast cancer. Cancer Nurs 1996;19:8-19. https://doi.org/10.1097/00002820-199602000-00002.
- Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O’Neil J, et al. Information needs and decisional preferences in women with breast cancer. JAMA 1997;277:1485-92. https://doi.org/10.1001/jama.1997.03540420081039.
- Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient–physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol 2004;22:3091-8. https://doi.org/10.1200/JCO.2004.09.069.
- Sowerbutts AM, Griffiths, J, Todd C, Lavelle K. Why are older women not having surgery for breast cancer?. Psychooncology 2015;24:1036-42. https://doi.org/10.1002/pon.3764.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Audisio RA, Ramesh H, Gennari R, Corsini G, Maffezzini M, Hoekstra HJ, et al. Can preoperative assessment of cancer in the elderly (PACE) predict 30-days postoperative outcomes?. J Clin Oncol 2006;24.
- Ring A, Reed M, Leonard R, Kunkler I, Muss H, Wildiers H, et al. The treatment of early breast cancer in women over the age of 70. Br J Cancer 2011;105:189-93. https://doi.org/10.1038/bjc.2011.234.
- Sturgis P, Thomas R, Purdon S, Bridgwood A, Dodd T. Comparative Review and Assessment of Key Health State Measures of the General Population. London: Department of Health and Social Care; 2001.
- Haywood K, Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Health Status and Quality of Life in Older People: A Structured Review of Patient-assessed Health Instruments Reported from the Patient Assessed Health Instruments Group to the Department of Health. London: Department of Health and Social Care; 2004.
- Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: Cochrane review. Br J Cancer 2007;96:1025-9. https://doi.org/10.1038/sj.bjc.6603600.
- Harnett A, Smallwood J, Titshall V, Champion A. Guideline Development Group. Diagnosis and treatment of early breast cancer, including locally advanced disease – summary of NICE guidance. BMJ 2009;338. https://doi.org/10.1136/bmj.b438.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Office for National Statistics (ONS) . Cancer Survival Rates, Cancer Survival in England: Patients Diagnosed 2007–2011 and Followed up to 2012 n.d. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-320365 (accessed 9 January 2016).
- Brennan MJ. Lymphedema following the surgical treatment of breast cancer: a review of pathophysiology and treatment. J Pain Symptom Manage 1992;7:110-16. https://doi.org/10.1016/0885-3924(92)90122-X.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. https://doi.org/10.1016/S1470-2045(13)70076-7.
- Shaitelman SF, Cromwell KD, Rasmussen JC, Stout NL, Armer JM, Lasinski BB, et al. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin 2015;65:55-81. https://doi.org/10.3322/caac.21253.
- Armer J, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post breast cancer population. Lymphatic Res Biol n.d.;3:208-17. https://doi.org/10.1089/lrb.2005.3.208.
- Agency for Health Research and Quality 2011. www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id66aTA.pdf (accessed 7 May 2019).
- Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat 2003;79:47-5. https://doi.org/10.1023/A:1023330206021.
- Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 2006;95:279-93. https://doi.org/10.1007/s10549-005-9025-7.
- Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol 2007;18:639-46. https://doi.org/10.1093/annonc/mdl182.
- Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008;112:2809-19. https://doi.org/10.1002/cncr.23494.
- Cornish BH, Ward LC, Thomas BJ, Bunce IH. Quantification of lymphoedema using multi-frequency bioimpedance. Appl Radiat Isot 1998;49:651-2. https://doi.org/10.1016/S0969-8043(97)00266-2.
- Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol 2006;4:51-6. https://doi.org/10.1089/lrb.2006.4.51.
- Armer JM, Radine ES, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 2003;52:370-9. https://doi.org/10.1097/00006199-200311000-00004.
- National Institute for Health and Care Excellence (NICE) . L-Dex U400 for Lymphoedema After Breast Cancer Treatment. Medtech Innovation Briefing (MIB111) 2017. www.nice.org.uk/guidance/mib111 (accessed 8 May 2019).
- Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121-9.
- Bundred N, Todd C, Morris J, Keeley V, Purushotham A, Bagust A, et al. Clinical Trial Protocol: Multi-frequency Bioimpedance in the Early Detection of Lymphoedema after Axillary Surgery. National Institute for Health Research and Cancer Research UK; 2015.
- Bland M, Altman M. An Introduction to Medical Statistics. Oxford: Oxford University Press; 2005.
- Deltombe T, Jamart J, Recloux S, Legrand C, Vandenbroeck N, Theys S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007;40:26-34.
- Paskett ED, Naughton MJ, McCoy TP, Case DL, Abbott J. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidem Biomarkers 2007;16:775-82. https://doi.org/10.1158/1055-9965.EPI-06-0168.
- Hui SL, Zhou XH. Evaluation of diagnostic tests without gold standards. Stat Methods Med Res 1998;7:354-70. https://doi.org/10.1177/096228029800700404.
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11500.
- Naaktgeboren CA, Bertens LC, van Smeden M, de Groot JA, Moons KG, Reitsma JB. Value of composite reference standards in diagnostic research. BMJ 2013;347. https://doi.org/10.1136/bmj.f5605.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. London: Routledge; 1994.
- Verry H, Lord SJ, Martin A, Gill G, Lee CK, Howard K, et al. Effectiveness and cost-effectiveness of sentinel lymph node biopsy compared with axillary node dissection in patients with early-stage breast cancer: a decision model analysis. Br J Cancer 2013;106:1045-52. https://doi.org/10.1038/bjc.2012.62.
- Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009;27:390-7. https://doi.org/10.1200/JCO.2008.17.9291.
- Office for National Statistics (ONS) . Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2011, England 2011. https://webarchive.nationalarchives.gov.uk/20151014092800/www.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations--england--series-mb1-/no--42--2011/index.html (accessed 7 May 2019).
- Specht MC, Miller CL, Russell TA, Horick N, Skolny MN, O’Toole JA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression?. Breast Cancer Res Treat 2013;140:485-94. https://doi.org/10.1007/s10549-013-2655-2.
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-60. https://doi.org/10.1093/jnci/djj158.
- Cheville AL, Almoza M, Courmier JN, Basford JR. A prospective cohort study defining utilities using time trade-offs and the Euroqol-5D to assess the impact of cancer-related lymphedema. Cancer 2010;116:3722-31. https://doi.org/10.1002/cncr.25068.
- Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of under treatment. J Clin Oncol 2007;25:1858-69. https://doi.org/10.1200/JCO.2006.10.4208.
- Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Todd C. Pretreatment health measures and complications after surgical management of elderly women with breast cancer. Br J Surg 2015;102:653-67. https://doi.org/10.1002/bjs.9796.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Department of Health and Social Care . Best Research for Best Health – A New National Health Research Strategy 2006.
- UK Clinical Trials Gateway . Public and Patient Survey 2012. www.nihr.ac.uk/documents/about-NIHR/NIHR- Publications/UKCTG-Report-Public-and-Patient-Survey-Report-2012.pdf (accessed 19 April 2018).
- Adams M, Caffrey L, McKevitt C. Barriers and opportunities for enhancing patient recruitment and retention in clinical research: findings from an interview study in an NHS academic health science centre. Health Res Policy Syst 2015;13. https://doi.org/10.1186/1478-4505-13-8.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Mills N, Donovan J, Wade J, Hamdy F, Neal D, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. https://doi.org/10.1016/j.jclinepi.2010.12.017.
- Brown R, Butow PN, Butt DG, Moore AR, Tattersall MH. Developing ethical strategies to assist oncologists in seeking informed consent to cancer clinical trials. Soc Sci Med 2004;58:379-90. https://doi.org/10.1016/S0277-9536(03)00204-1.
- Tomlin Z, deSalis I, Toerien M, Donovan JL. Patient advocacy and patient centredness in participant recruitment to randomized-controlled trials: implications for informed consent. Health Expect 2012;17:670-82. https://doi.org/10.1111/j.1369-7625.2012.00792.x.
- Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-399.
- Townsend D, Mills N, Savović J, Donovan JL. A systematic review of training programmes for recruiters to randomised controlled trials. Trials 2015;16. https://doi.org/10.1186/s13063-015-0908-6.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. J Adm Govern 2009;4:72-9.
- Sugarman J, McCrory DC, Hubal RC. Getting meaningful informed consent from older adults: a structured literature review of empirical research. J Am Geriatr Soc 1998;46:517-24. https://doi.org/10.1111/j.1532-5415.1998.tb02477.x.
Appendix 1 Consolidated Standards of Reporting Trials flow diagrams for workstream 1
FIGURE 19.
Flow diagram of participants in analyses of aim A: impact of primary surgery on survival and HRQoL. Participants included in analyses of breast cancer-specific survival to study end on 5 February 2016 (n = 910). Participants included in analyses of difference in HRQoL by primary surgery (n = 501). SF-6D set at 0 and included in the analysis of QALYs with participants returning the SF-6D (n = 501 + 132 + 7 = 640).
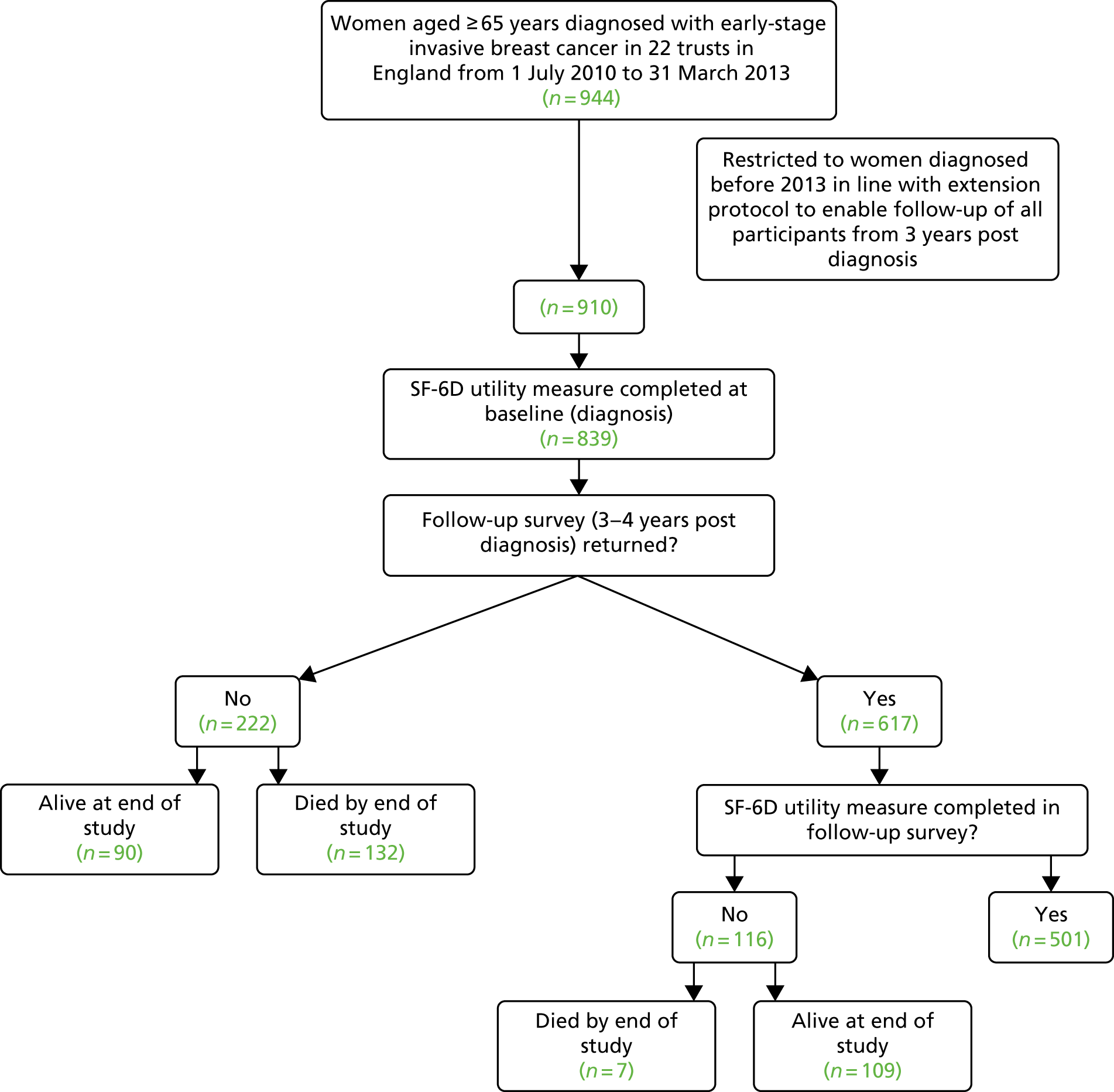
FIGURE 20.
Flow diagram of participants in analyses of aim Bi: impact of adjuvant therapy on survival and HRQoL. Participants included in analyses of impact of adjuvant therapy on survival to study end on 5 February 2016 (n = 910). Participants included in analyses of difference in HRQoL by adjuvant treatment (n = 454). SF-6D set at 0 and included in the analysis of QALYs with participants returning the SF-6D (n = 454 + 88 + 6 = 548).
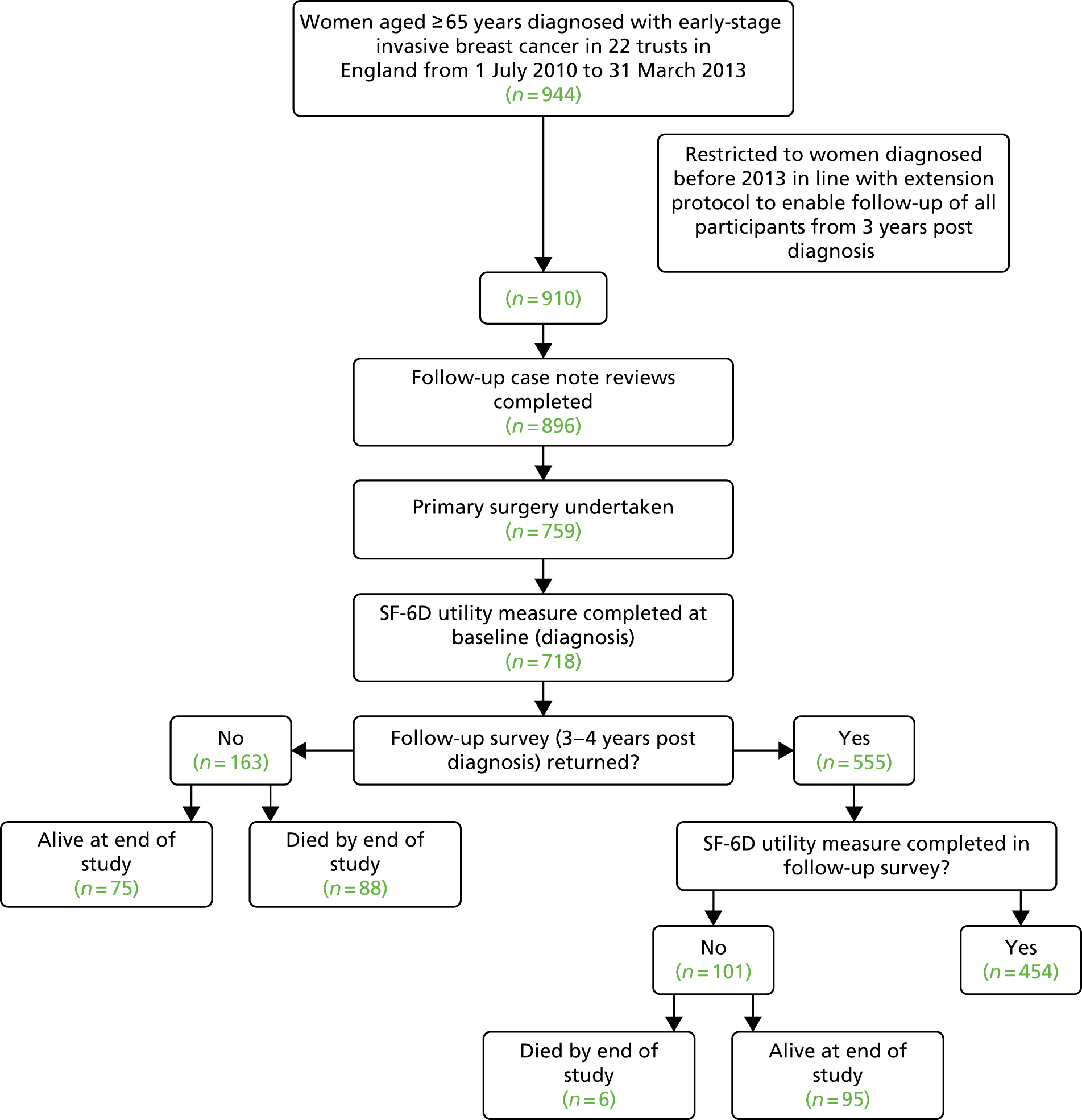
| Aim: to investigate | Outcome(s) | Explanatory variables | Method | Sample |
|---|---|---|---|---|
| A. The extent to which primary surgery for older women with early-stage breast cancer increases survival and HRQoL and is cost-effective |
Survival (to 3 years post diagnosis) Difference in HRQoL (at diagnosis, 3–4 years later) QALYs |
Treatment received (i.e. primary surgery < 3 months of diagnosis) Pre-treatment health Pre-treatment health measures, tumour characteristics and sociodemographics |
We have collected extensive data on health at diagnosis and surgical treatments received < 3 months of diagnosis via interview and case note review, respectively We have set up mortality flagging and investigate effect of surgery on survival at 3 years In addition, we have undertaken a further case note reviews at 3 years and survey at 3–4 years to also measure/investigate outcomes of cost-effectiveness and HRQoL, respectively |
All 910 women in cohort 910 for survival outcome 501 for HRQoL and 640 for QALY outcomes See Figure 2 and submitted paper |
| Bi. The extent to which adjuvant treatment (chemo-/radiotherapy with surgery) increases survival and HRQoL and is cost-effective |
Treatments received (i.e. radiotherapy < 12 months of diagnosis) Chemotherapy commenced < 12 months of diagnosis Pre-treatment health measures, tumour characteristics and sociodemographics |
759 women in cohort who had surgery 759 for survival outcome 454 for HRQoL and 548 for QALY outcomes See Figure 3 |
||
| Bii. The extent to which lack of adjuvant treatment can be explained by patient health and choice |
Radiotherapy < 12 months of diagnosis Chemotherapy commenced < 12 months of diagnosis |
Pre-treatment health measures, patient choice, tumour characteristics and sociodemographics | The effect of pre-treatment health/choice on whether or not patients had surgery has already been investigated.49 We repeat this analyses investigating access to adjuvant treatment by undertaking a further case note review at 3 years and survey at 3–4 years |
688 retained in cohort who had surgery and did not die or move away < 12 months of diagnosis See Appendix 3 |
Appendix 2 Surgical management of older breast cancer patients: which pre-treatment health measures predict 30-day complications?
Figure 21 and Tables 51–56 in this section are based on Lavelle et al. 49
Appendix 3 The impact of health and patient choice on receipt of surgery, radiotherapy or chemotherapy in breast cancer patients on short-term survival of older breast cancer patients in the UK: a prospective cohort study
Appendix 4 Impact of primary surgery on short-term survival of older breast cancer patients in the UK: a prospective cohort study
Appendix 5 Congruence between patients’ preferred and actual role in the surgical treatment decision: impact on post-surgical health-related quality of life
Appendix 6 Relationship between change in perometer/bioimpedance spectroscopy from 6 to 24 months and the lymphoedema checklist questions
| Change from 6 to 24 months | No swelling at 24 months | Swelling at 24 months | p-value |
|---|---|---|---|
| Perometer | n = 323; –0.4 (4.7) | n = 222 2.0 (8.0) | < 0.001 |
| BIS | n = 310; –0.3 (8.0) | n = 206; –1.0 (12.7) | 0.48 |
| No numbness at 24 months | Numbness at 24 months | ||
| Perometer | n = 145; 0.1 (5.7) | n = 408; 0.8 (6.6) | 0.28 |
| BIS | n = 139; –1.8 (9.4) | n = 385; –0.2 (10.2) | 0.12 |
| No heaviness at 24 months | Heaviness at 24 months | ||
| Perometer | n = 303; –0.2 (5.3) | n = 231; 1.7 (7.5) | 0.001 |
| BIS | n = 290; –0.8 (8.4) | n = 216; –0.4 (11.9) | 0.69 |
There were greater increases (from 6 to 24 months) in exact perometer values in those women with swelling at 24 months (p < 0.001) and in those with heaviness at 24 months (p = 0.001).
| Change from 6 month to 24 months | No swelling between 6 and 24 months | Swelling between 6 and 24 months | p-value |
|---|---|---|---|
| Perometer | n = 261; –0.3 (4.5) | n = 372; 1.1 (7.3) | 0.003 |
| BIS | n = 255; 0.4 (8.3) | n = 338; –1.1 (11.6) | 0.076 |
| No numbness between 6 and 24 months | Numbness between 6 and 24 months | ||
| Perometer | n = 64; 0.4 (6.0) | n = 569; 0.5 (6.4) | 0.91 |
| BIS | n = 62; 0.2 (11.9) | n = 531; –0.5 (10.1) | 0.63 |
| No heaviness between 6 and 24 months | Heaviness between 6 and 24 months | ||
| Perometer | n = 245; –0.4 (5.3) | n = 387; 1.0 (6.9) | 0.005 |
| BIS | n = 237; –0.7 (9.5) | n = 356; –0.3 (10.8) | 0.62 |
There were greater increases (from 6 to 24 months) in exact perometer values in those women with swelling between 6 and 24 months (p = 0.003) and in those with heaviness between 6 and 24 months (p = 0.005).
Appendix 7 L-Dex multifrequency bioimpedance lymphoedema
The 85 patients with lymphoedema are made up of 39 with both perometer and BIS ≥ 10, 30 with only BIS ≥ 10 and 16 with only perometer ≥ 10.
Bioimpedance spectroscopy value by 6 months against lymphoedema by 18 or 24 months.
Lymphoedema defined by perometer > 10% and clinical lymphoedema or appropriately applied sleeve.
In all the analyses that follow, any patients diagnosed with a perometer value > 10% by 6 months were excluded from the analysis (n = 87) and any patients with a clinical lymphoedema or sleeve applied before 6 months were excluded from the analysis.
| BIS value by 6 months | Lymphoedema defined by perometer of > 10% | Clinical lymphoedema or appropriately applied sleeve | ||
|---|---|---|---|---|
| No lymphoedema by 18 months (n = 662) | Lymphoedema by 18 months (n = 77) | No lymphoedema by 18 months (n = 643) | Lymphoedema by 18 months (n = 114) | |
| < 3 | 327 (93%) | 23 (7%) | 324 (93%) | 25 (7%) |
| > 3 to < 5 | 80 (91%) | 8 (9%) | 78 (90%) | 9 (10%) |
| > 5 to < 10 | 156 (92%) | 14 (8%) | 145 (84%) | 27 (16%) |
| > 10 | 99 (76%) | 32 (24%) | 96 (64%) | 53 (36%) |
There is a significant relationship between both BIS category by 6 months and lymphoedema defined by perometer of > 10% by 18 months (p < 0.001) and clinical lymphoedema or appropriately applied sleeve by 18 months (p < 0.001).
For lymphoedema defined by perometer of > 10%, the significant relationship appears to be as a result of the higher rate of lymphoedema, 24%, in those with > 10.
For clinical lymphoedema or applied sleeve, there appears to be a small increase in lymphoedema rate across the < 3, > 3 to < 5, and > 5 to < 10 categories; the rate increased from 7% and 16% across the three categories. The significant relationship appears mainly to be as a result of the higher rate of lymphoedema, 36%, in those with BIS of > 10.
24 months
| BIS value by 6 months | Lymphoedema defined by perometer of > 10% | Clinical lymphoedema or appropriately applied sleeve | ||
|---|---|---|---|---|
| No lymphoedema by 24 months (n = 596) | Lymphoedema by 24 months (n = 101) | No lymphoedema by 24 months (n = 577) | Lymphoedema by 24 months (n = 137) | |
| < 3 | 298 (91%) | 30 (9%) | 297 (91%) | 31 (9%) |
| > 3 to < 5 | 68 (85%) | 12 (15%) | 66 (85%) | 12 (15%) |
| > 5 to < 10 | 142 (87%) | 21 (13%) | 128 (78%) | 36 (22%) |
| > 10 | 88 (70%) | 38 (30%) | 86 (60%) | 58 (40%) |
Appendix 8 Lymphoedema scoring models
Lymphoedema scoring model for 10% perometer volume increase definition
Of the 1097 patients in the data set, 326 were classified as having either an appropriately applied sleeve or clinical lymphoedema.
There were 51 patients who were identified as being given their sleeve as part of the PLACE trial; these patients were excluded from consideration in the following analysis.
A further nine patients were excluded because of issues with the sleeve application. These issues included having a sleeve applied to the contralateral arm, having a sleeve when entering the study, and having hand swelling only.
There were 266 patients with an appropriately applied sleeve or clinical lymphoedema in the reduced 1037 patient data set. There were 25 patients with sleeves applied who were deemed not to have clinical lymphoedema due to insufficient evidence in the notes; there were 29 patients who did not have a sleeve applied but were deemed to have clinical lymphoedema.
Model at 6 months
The variables considered for the scoring model were: perometer at 6 months (categorical), BIS at 6 months (categorical), TOI at 6 months, FACT-B total at 6 months, ARM subscale at 6 months, lymphoedema checklist questions at 6 months (swelling, numbness, heaviness), B3 at 6 months (categorical: 0–2, considerable swelling vs. 3–4, little to no swelling), age, BMI at 6 months, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy.
Prediction scoring model 1
A total of 711 patients were included in this analysis.
| Variable at 6 months | OR (95% CI) | p-value |
|---|---|---|
| Perometer | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 1.92 (0.96 to 3.86) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 7.36 (4.10 to 13.24) | |
| BIS | ||
| ≥ 3 to < 5 increase vs. < 3 increase | 1.39 (0.57 to 3.38) | 0.030 |
| ≥ 5 to < 10 increase vs. < 3 increase | 1.87 (0.96 to 3.64) | |
| ≥ 10 increase vs. < 3 increase | ||
| BMI (kg/m2) | ||
| > 25 to ≤ 30 vs. ≤ 25 | 1.53 (0.80 to 2.91) | 0.015 |
| > 30 vs. ≤ 25 | 2.53 (1.34 to 4.77) | |
| Number of positive nodes (per-node increase) | 1.08 (1.04 to 1.12) | < 0.001 |
A scoring model was produced based on the regression coefficients from the final model. The individual scores are the regression coefficients for binary or categorical variables rounded to the nearest 0.5 and the regression coefficients for continuous variables to 2 decimal places due to their per-unit increase interpretation. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a 10% perometer volume increase.
| Variable at 6 months | Score |
|---|---|
| Perometer | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 0.5 |
| ≥ 5% to < 10% increase | 2 |
| BIS | |
| < 3 increase | 0 |
| ≥ 3 to < 5 increase | 0.5 |
| ≥ 5 to < 10 increase | 0.5 |
| ≥ 10 increase | 1 |
| BMI (kg/m2) | |
| ≤ 25 | 0 |
| > 25 to ≤ 30 | 0.5 |
| > 30 | 1 |
| Number of positive nodes | 0.08 × number of positive nodes |
This scoring model gives an AUROC of 0.80 (95% CI 0.74–0.85) (Figure 24).
FIGURE 24.
Prediction scoring model 1: AUROC curve. Diagonal segments are produced by ties.
For a cut-off score of 1.58, where a patient with a score of ≥ 1.58 would be predicted to have a 10% perometer volume increase, the scoring model would give a sensitivity of 80.0% (68/85), specificity of 67.7% (424/626), PPV of 25.2% (68/270) and NPV of 96.1% (424/441). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Prediction scoring model 2: excluding bioimpedance spectroscopy at 6 months
A total of 740 patients were included in this analysis.
| Variable at 6 months | OR (95% CI) | p-value |
|---|---|---|
| Perometer | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 2.47 (1.27 to 4.79) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 9.10 (5.24 to 15.79) | |
| BMI (kg/m2) | ||
| > 25 to ≤ 30 vs. ≤ 25 | 1.53 (0.82 to 2.86) | 0.025 |
| > 30 vs. ≤ 25 | 2.34 (1.26 to 4.35) | |
| Number of positive nodes (per-node increase) | 1.08 (1.04 to 1.11) | < 0.001 |
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a 10% perometer volume increase.
| Variable at 6 months | Score |
|---|---|
| Perometer | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 1 |
| ≥ 5% to < 10% increase | 2 |
| BMI (kg/m2) | |
| ≤ 25 | 0 |
| > 25 to ≤ 30 | 0.5 |
| > 30 | 1 |
| Number of positive nodes | 0.07 × number of positive nodes |
This scoring model gives an AUROC of 0.77 (95% CI 0.71 to 0.82) (Figure 25).
FIGURE 25.
Prediction scoring model 2: AUROC curve. Diagonal segments are produced by ties.
For a cut-off score of 1.41, where a patient with a score of 1.41 or above would be predicted to have a 10% perometer volume increase, the scoring model would give a sensitivity of 72.1% (62/86), a specificity of 72.2% (472/654), a PPV of 25.4% (62/244) and a NPV of 95.2% (472/496). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Prediction scoring model at 1 month
The variables considered for the scoring model were perometer at 1 month (categorical), BIS at 1 month (categorical), TOI at pre-surgery, FACT-B total at pre-surgery, ARM subscale at pre-surgery, lymphoedema checklist questions at pre-surgery (swelling, numbness, heaviness), age, BMI at pre-surgery, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy.
Prediction scoring model 3
A total of 506 patients were included in this analysis.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Perometer at 1 month | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 2.21 (1.09 to 4.48) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 3.68 (1.92 to 7.03) | |
| ≥ 10% increase vs. < 3% increase | 7.42 (2.21 to 24.93) | |
| BIS at 1 month | ||
| ≥ 3 to < 5 increase vs. < 3 increase | 2.11 (1.00 to 4.46) | 0.013 |
| ≥ 5 to < 10 increase vs. < 3 increase | 1.00 (0.49 to 2.04) | |
| ≥ 10 increase vs. < 3 increase | 2.54 (1.33 to 4.85) | |
| Lymphoedema checklist swelling at pre surgery (yes vs. no) | 1.89 (1.00 to 3.59) | 0.051 |
| Number of positive nodes (per-node increase) | 1.08 (1.03 to 1.12) | < 0.001 |
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a 10% perometer volume increase.
| Variable | Score |
|---|---|
| Perometer at 1 month | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 1 |
| ≥ 5% to < 10% increase | 1.5 |
| ≥ 10% increase | 2 |
| BIS at 1 month | |
| < 3 increase | 0 |
| ≥ 3 to < 5 increase | 0.5 |
| ≥ 5 to < 10 increase | 0.5 |
| ≥ 10 increase | 1 |
| Lymphoedema checklist swelling at pre surgery | |
| No | 0 |
| Yes | 0.5 |
| Number of positive nodes | 0.07 × number of positive nodes |
This scoring model gives an AUROC of 0.71 (95% CI 0.65 to 0.77) (Figure 26).
FIGURE 26.
Prediction scoring model 3: AUROC curve. Diagonal segments are produced by ties.

For a cut-off score of 1.25, where a patient with a score of ≥ 1.25 would be predicted to have a 10% perometer volume increase, the scoring model would give a sensitivity of 65.5% (55/84), specificity of 69.9% (295/422), PPV of 30.2% (55/182), and NPV of 91.0% (295/324). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Prediction scoring model 4: excluding bioimpedance spectroscopy at 1 month
A total of 522 patients were included in this analysis.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Perometer at 1 month | ||
| ≥ 3 to < 5% increase vs. < 3% increase | 2.11 (1.06 to 4.19) | < 0.001 |
| ≥ 5 to < 10% increase vs. < 3% increase | 4.02 (2.18 to 7.39) | |
| ≥ 10% increase vs. < 3% increase | 8.89 (2.86 to 27.64) | |
| Lymphoedema checklist swelling at pre surgery (yes vs. no) | 2.22 (1.21 to 4.09) | 0.010 |
| Number of positive nodes (per-node increase) | 1.08 (1.04 to 1.12) | < 0.001 |
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a 10% perometer volume increase.
| Variable | Score |
|---|---|
| Perometer at 1 month | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 0.5 |
| ≥ 5% to < 10% increase | 1.5 |
| ≥ 10% increase | 2 |
| Lymphoedema checklist swelling at pre surgery | |
| No | 0 |
| Yes | 1 |
| Number of positive nodes | 0.07 × number of positive nodes |
This scoring model gives an AUROC of 0.71 (95% CI 0.64 to 0.77) (Figure 27).
FIGURE 27.
Clinical lymphoedema prediction scoring model at 6 months. Diagonal segments are produced by ties.
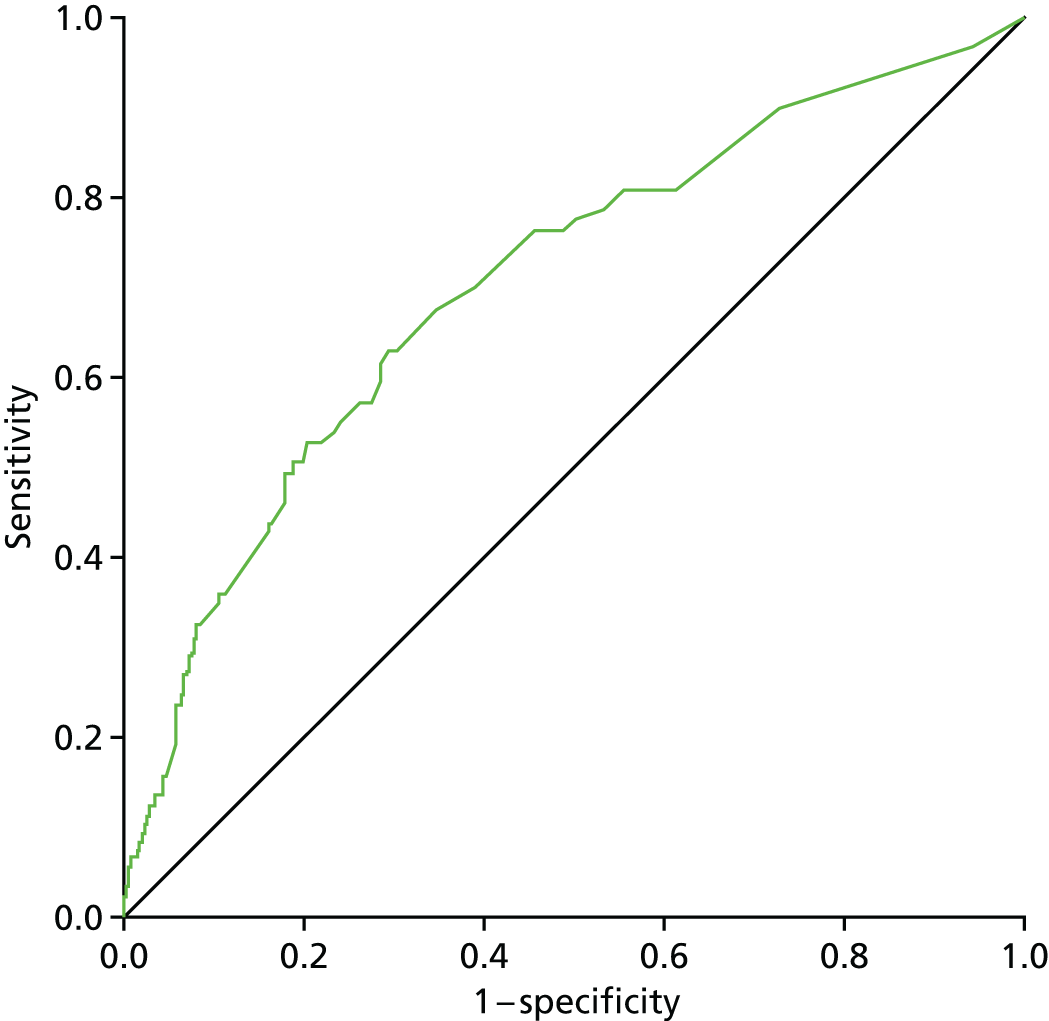
For a cut-off score of 0.82, where a patient with a score of ≥ 0.82 would be predicted to have a 10% perometer volume increase, the scoring model would give a sensitivity of 62.9% (56/89), specificity of 70.7% (306/433), PPV of 30.6% (56/183), and NPV of 90.3% (306/339). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Scoring model with clinical lymphoedema or appropriately applied sleeve
Prediction scoring model at 6 months
The variables considered for the scoring model were perometer at 6 months (categorical), BIS at 6 months (categorical), TOI at 6 months, FACT-B total at 6 months, ARM subscale at 6 months, lymphoedema checklist questions at 6 months (swelling, numbness, heaviness), B3 at 6 months (categorical: 0–2, considerable swelling vs. 3–4, little to no swelling), age, BMI at 6 months, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy.
Patients who had a perometer value ≥ 10 before or at 6 months were excluded from the analysis.
A total of 528 patients were included in this analysis.
Prediction model at 6 months
| Variable at 6 months | OR (95% CI) | p-value |
|---|---|---|
| Perometer | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 1.99 (0.97 to 4.09) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 4.47 (2.25 to 8.85) | |
| BIS | ||
| ≥ 3 to < 5 increase vs. < 3 increase | 1.80 (0.69 to 4.67) | 0.002 |
| ≥ 5 to < 10 increase vs. < 3 increase | 2.85 (1.38 to 5.89) | |
| ≥ 10 increase vs. < 3 increase | 3.68 (1.80 to 7.55) | |
| Lymphoedema checklist swelling (yes vs. no) | 2.15 (1.21 to 3.82) | 0.009 |
| ER status (negative vs. positive) | 0.38 (0.15 to 0.97) | 0.042 |
| Adjuvant radiotherapy (yes vs. no) | 4.52 (1.51 to 13.56) | 0.007 |
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a clinical lymphoedema or require a sleeve.
Prediction scoring model at 6 months
| Variable at 6 months | Score |
|---|---|
| Perometer | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 0.5 |
| ≥ 5% to < 10% increase | 1.5 |
| BIS | |
| < 3 increase | 0 |
| ≥ 3 to < 5 increase | 0.5 |
| ≥ 5 to < 10 increase | 1 |
| ≥ 10 increase | 1.5 |
| Lymphoedema checklist swelling | |
| No | 0 |
| Yes | 1 |
| ER status | |
| Negative | 0 |
| Positive | 1 |
| Adjuvant radiotherapy | |
| No | 0 |
| Yes | 1.5 |
This scoring model gives an AUROC of 0.78 (95% CI 0.72 to 0.84) (Figure 28).
FIGURE 28.
Prediction scoring model 2: AUROC curve. Diagonal segments are produced by ties.

For a cut-off score of 4, where a patient with a score of 4 or above would be predicted to have clinical lymphoedema or a sleeve applied, the scoring model would give a sensitivity of 59.4% (41/69), specificity of 80.4% (369/459), PPV of 31.3% (41/131) and NPV of 92.9% (369/397). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Prediction scoring model: excluding bioimpedance spectroscopy at 6 months
A total of 548 patients were included in this analysis.
Prediction Model –excluding BIS at 6 months.
| Variable at 6 months | OR (95% CI) | p-value |
|---|---|---|
| Perometer | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 2.69 (1.36 to 5.31) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 5.89 (3.07 to 11.30) | |
| Lymphoedema checklist swelling (yes vs. no) | 2.31 (1.33 to 4.02) | 0.003 |
| ER status (negative vs. positive) | 0.40 (0.16 to 0.98) | 0.045 |
| Adjuvant radiotherapy (yes vs. no) | 4.74 (1.61 to 13.92) | 0.005 |
A scoring model was produced based on the regression coefficients from the final model as described previously. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a clinical lymphoedema or require a sleeve.
Prediction scoring model: excluding bioimpedance spectroscopy at 6 months
| Variable at 6 months | Score |
|---|---|
| Perometer | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 1 |
| ≥ 5% to < 10% increase | 2 |
| Lymphoedema checklist swelling | |
| No | 0 |
| Yes | 1 |
| ER status | |
| Negative | 0 |
| Positive | 1 |
| Adjuvant radiotherapy | |
| No | 0 |
| Yes | 1.5 |
This scoring model gives an AUROC of 0.76 (95% CI 0.70 to 0.82) (Figure 29).
FIGURE 29.
Prediction of clinical lymphoedema excluding BIS at 6 months. Diagonal segments are produced by ties.

For a cut-off score of 4, where a patient with a score of ≥ 4 would be predicted to have clinical lymphoedema or a sleeve applied, the scoring model would give a sensitivity of 48.6% (34/70), specificity of 90.0% (430/478), PPV of 41.5% (34/82) and NPV of 92.3% (430/466). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Prediction scoring model at 1 month
The variables considered for the scoring model were: perometer at 1 month (categorical), BIS at 1 month (categorical), TOI at pre-surgery, FACT-B total at pre-surgery, ARM subscale at pre-surgery, lymphoedema checklist questions at pre-surgery (swelling, numbness, heaviness), B3 at pre-surgery (categorical: 0–2, considerable swelling vs. 3–4, little to no swelling), age, BMI at pre-surgery, ER status, number of positive nodes, adjuvant chemotherapy and adjuvant radiotherapy.
A total of 794 patients were included in this analysis.
Prediction model at 1 month
| Variable at 1 month | OR (95% CI) | p-value |
|---|---|---|
| Perometer | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 1.39 (0.82 to 2.36) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 3.40 (2.14 to 5.40) | |
| ≥ 10% increase vs. < 3% increase | 4.07 (1.56 to 10.62) | |
| BIS | ||
| ≥ 3 to < 5 increase vs. < 3 increase | 2.06 (1.19 to 3.58) | 0.005 |
| ≥ 5 to < 10 increase vs. < 3 increase | 1.01 (0.62 to 1.64) | |
| ≥ 10 increase vs. < 3 increase | 1.96 (1.21 to 3.15) | |
| Adjuvant radiotherapy (yes vs. no) | 1.91 (1.09 to 3.34) | 0.023 |
| Number of positive nodes (per-node increase) | 1.05 (1.02 to 1.08) | < 0.001 |
A scoring model was produced based on the regression coefficients from the final model. The individual scores are the regression coefficients for binary or categorical variables rounded to the nearest 0.5 and the regression coefficients for continuous variables to 2 decimal places due to their per-unit increase interpretation. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a clinical lymphoedema or require a sleeve.
Prediction scoring model at 1 month
| Variable at 1 month | Score |
|---|---|
| Perometer | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 0.5 |
| ≥ 5% to < 10% increase | 1 |
| ≥ 10% increase | 1.5 |
| BIS | |
| < 3 increase | 0 |
| ≥ 3 to < 5 increase | 0.5 |
| ≥ 5 to < 10 increase | 0.5 |
| ≥ 10 increase | 0.5 |
| Adjuvant radiotherapy | |
| No | 0 |
| Yes | 0.5 |
| Number of positive nodes | 0.05 × number of positive nodes |
This scoring model gives an AUROC of 0.67 (95% CI 0.63 to 0.72) (Figure 30).
FIGURE 30.
Prediction scoring model of clinical lymphoedema at 1 month. Diagonal segments are produced by ties.
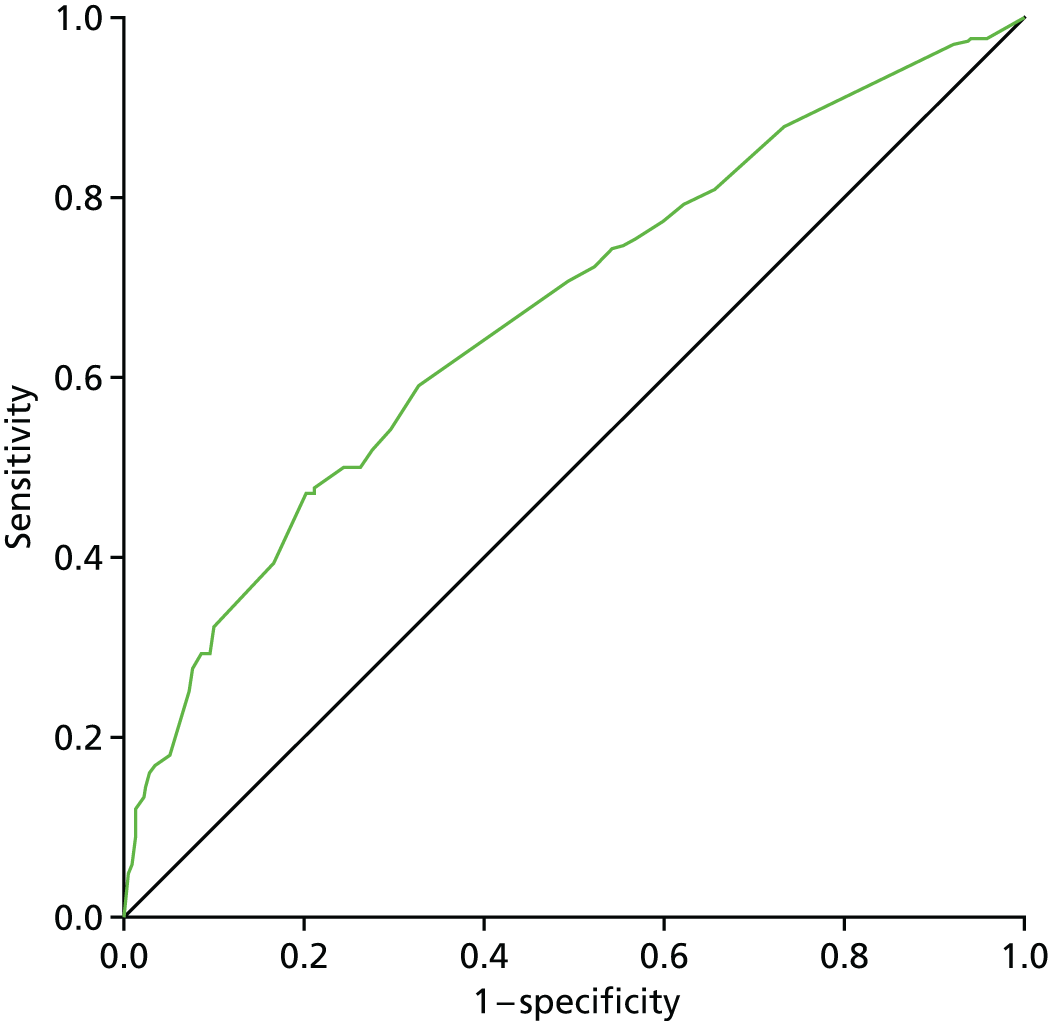
For a cut-off score of 1.55, where a patient with a score of ≥ 1.55 would be predicted to have clinical lymphoedema or a sleeve applied, the scoring model would give a sensitivity of 47.1% (82/174), specificity of 79.8% (495/620), PPV of 39.6% (82/207), and NPV of 84.3% (495/587). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Prediction scoring model: excluding bioimpedance spectroscopy at 1 month
A total of 837 patients were included in this analysis.
| Variable at 1 month | OR (95% CI) | p-value |
|---|---|---|
| Perometer | ||
| ≥ 3% to < 5% increase vs. < 3% increase | 1.45 (0.88 to 2.41) | < 0.001 |
| ≥ 5% to < 10% increase vs. < 3% increase | 3.61 (2.33 to 5.59) | |
| ≥ 10% increase vs. < 3% increase | 5.70 (2.32 to 14.02) | |
| Adjuvant radiotherapy (yes vs. no) | 1.93 (1.12 to 3.31) | 0.018 |
| Number of positive nodes (per-node increase) | 1.05 (1.02 to 1.08) | 0.001 |
A scoring model was produced based on the regression coefficients from the final model. The individual scores are the regression coefficients for binary or categorical variables rounded to the nearest 0.5 and the regression coefficients for continuous variables to 2 decimal places due to their per unit increase interpretation. The total ‘diagnostic’ score is given by summing the individual scores. A patient with a higher total score is more likely to have a clinical lymphoedema or require a sleeve.
| Variable at 1 month | Score |
|---|---|
| Perometer | |
| < 3% increase | 0 |
| ≥ 3% to < 5% increase | 0.5 |
| ≥ 5% to < 10% increase | 1 |
| ≥ 10% increase | 1.5 |
| Adjuvant radiotherapy | |
| No | 0 |
| Yes | 0.5 |
| Number of positive nodes | 0.05 × number of positive nodes |
Note: the ARM subscale is statistically significant if included in the above model. However, only 558 patients would be included in the model and the AUC is not improved by a large amount by its inclusion (AUC 0.67, 95% CI 0.62 to 0.73).
This scoring model gives an AUROC of 0.67 (95% CI 0.62 to 0.71) (Figure 31).
FIGURE 31.
Prediction scoring model of clinical lymphoedema at 1 month excluding BIS. Diagonal segments are produced by ties.

For a cut-off score of 1.55, where a patient with a score of ≥ 1.55 would be predicted to have a clinically identified lymphoedema or sleeve applied, the scoring model would give a sensitivity of 53.6% (98/183), specificity of 70.9% (464/654), PPV of 34.0% (98/288), and NPV of 84.5% (464/549). The cut-off score was chosen to maximise the sum of the sensitivity and specificity, giving equal weight to sensitivity and specificity.
Appendix 9 Composite end-points analysis
| Measure | Time point | |||||
|---|---|---|---|---|---|---|
| At 3 months | At 6 months | At 9 months | At 12 months | At 18 months | At 24 months | |
| 29 | 48 | 46 | 43 | 31 | 24 | |
| Perometer ≥ 10% | 11/27 (41%) | 17 (35%) | 19 (41%) | 14/41 (34%) | 12/30 (40%) | 10 (42%) |
| Perometer ≥ 9% | 11/27 (41%) | 22 (46%) | 24 (52%) | 15/41 (37%) | 12/30 (40%) | 12 (50%) |
| Perometer ≥ 8% | 14/27 (52%) | 23 (48%) | 27 (59%) | 18/41 (44%) | 13/30 (43%) | 13 (54%) |
| Perometer ≥ 5% | 16/27 (59%) | 31 (65%) | 39 (85%) | 28/41 (68%) | 21/30 (70%) | 17 (71%) |
| BIS of ≥ 10 | 10/27 (37%) | 27/47 (57%) | 28/43 (65%) | 17/39 (44%) | 14/29 (48%) | 10/23 (43%) |
| B3 of ≤ 2 | 14/23 (61%) | 28/39 (72%) | 21/42 (50%) | 19/35 (54%) | 13/24 (54%) | 8/21 (38%) |
| Perometer ≥ 10% or B3 of ≤ 2 | 22/26 (85%) | 32/41 (78%) | 28/44 (64%) | 26/38 (68%) | 17/27 (63%) | 15 (63%) |
| Perometer ≥ 9% or B3 of ≤ 2 | 22/26 (85%) | 34/42 (81%) | 31/44 (70%) | 26/38 (68%) | 17/27 (63%) | 16 (67%) |
| Perometer ≥ 8% or B3 of ≤ 2 | 23/26 (88%) | 35/42 (83%) | 33/44 (75%) | 27/38 (71%) | 17/27 (63%) | 17 (71%) |
| Perometer ≥ 5% and B3 of ≤ 2 | 6/23 (26%) | 20/44 (45%) | 19/42 (45%) | 15/37 (41%) | 12/26 (46%) | 5/21 (24%) |
| Perometer ≥ 8% and B3 of ≤ 2 | 5/24 (21%) | 16/45 (36%) | 15/44 (34%) | 10/38 (26%) | 9/27 (33%) | 4/21 (19%) |
In all of the following tables, the best NPV rates with lymphoedema (at that time point) and the best PPV rates [i.e. the best at finding patients with lymphoedema (at that time point)] are shown.
| Measure | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Perometer ≥ 10% | 11/27 (41%) | 828/850 (97%) | 11/33 (33%) | 828/844 (98%) |
| Perometer ≥ 9% | 11/27 (41%) | 818/850 (96%) | 11/43 (26%) | 818/834 (98%) |
| Perometer ≥ 8% | 14/27 (52%) | 801/850 (94%) | 14/63 (22%) | 801/814 (98%) |
| Perometer ≥ 5% | 16/27 (59%) | 728/850 (86%) | 16/138 (12%) | 728/739 (99%) |
| BIS ≥ 10 | 10/27 (37%) | 753/836 (90%) | 10/93 (11%) | 753/770 (98%) |
| B3 of ≤ 2 | 14/23 (61%) | 593/750 (79%) | 14/171 (8%) | 593/602 (99%) |
| Volume ≥ 200 ml | 16/27 (59%) | 640/849 (75%) | 16/225 (7%) | 640/651 (98%) |
| Volume ≥ 250 ml | 10/27 (37%) | 698/849 (82%) | 10/161 (6%) | 698/715 (98%) |
| Volume ≥ 300 ml | 8/27 (30%) | 748/849 (88%) | 8/109 (7%) | 748/767 (98%) |
| Perometer ≥ 10% or B3 of ≤ 2 | 22/26 (85%) | 564/735 (77%) | 22/193 (11%) | 564/568 (99%) |
| Perometer ≥ 9% or B3 of ≤ 2 | 22/26 (85%) | 561/736 (76%) | 22/197 (11%) | 561/565 (99%) |
| Perometer ≥ 8% or B3 of ≤ 2 | 23/26 (88%) | 553/740 (75%) | 23/210 (11%) | 553/556 (99%) |
| Perometer ≥ 5% or B3 of ≤ 2 | 24/27 (89%) | 510/755 (68%) | 24/269 (9%) | 510/513 (99%) |
| BIS ≥ 10 or B3 of ≤ 2 | 19/25 (76%) | 515/735 (70%) | 19/239 (8%) | 515/521 (99%) |
| Perometer ≥ 10% or BIS of ≥ 10 | 13/26 (50%) | 720/817 (88%) | 13/110 (12%) | 720/733 (98%) |
| Perometer ≥ 5% and B3 of ≤ 2 | 6/23 (26%) | 811/845 (96%) | 6/40 (15%) | 811/828 (98%) |
| Perometer ≥ 8% and B3 of ≤ 2 | 5/24 (21%) | 841/860 (98%) | 5/24 (21%) | 841/860 (98%) |
| Perometer ≥ 9% and B3 of ≤ 2 | 3/24 (13%) | 850/864 (98%) | 3/17 (18%) | 850/871 (98%) |
| Perometer ≥ 10% and B3 of ≤ 2 | 3/24 (13%) | 857/865 (99%) | 3/11 (27%) | 857/878 (98%) |
| Volume ≥ 200 ml and B3 of ≤ 2 | 7/23 (30%) | 786/828 (95%) | 7/49 (14%) | 786/802 (98%) |
| Volume ≥ 250 ml and B3 of ≤ 2 | 5/25 (20%) | 808/845 (96%) | 5/42 (12%) | 808/828 (98%) |
| Perometer ≥ 10% and BIS ≥ 10 | 8/28 (29%) | 861/869 (99%) | 8/16 (50%) | 861/881 (98%) |
| Perometer ≥ 9% and BIS ≥ 10 | 8/28 (29%) | 858/869 (99%) | 8/19 (42%) | 858/878 (98%) |
| Perometer ≥ 10% and BIS ≥ 10 and B3 of ≤ 2 | 3/26 (12%) | 871/876 (99%) | 3/8 (38%) | 871/894 (97%) |
| Perometer ≥ 9% and BIS ≥ 10 and B3 of ≤ 2 | 3/26 (12%) | 868/875 (99%) | 3/10 (30%) | 868/891 (97%) |
Those patients with lymphoedema at 3 months are not included in the numbers in Table 74.
| Measure | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Perometer ≥ 10% | 17/48 (35%) | 732/776 (94%) | 17/61 (28%) | 732/763 (96%) |
| Perometer ≥ 9% | 22/48 (46%) | 723/776 (93%) | 22/75 (29%) | 723/749 (97%) |
| Perometer ≥ 8% | 23/48 (48%) | 715/776 (92%) | 23/84 (27%) | 715/740 (97%) |
| Perometer ≥ 5% | 31/48 (65%) | 631/776 (81%) | 31/176 (18%) | 631/648 (97%) |
| BIS ≥ 10 | 24/47 (57%) | 630/759 (83%) | 27/156 (17%) | 630/650 (97%) |
| B3 ≤ 2 | 28/39 (72%) | 556/703 (79%) | 28/175 (16%) | 556/567 (98%) |
| Perometer ≥ 10% or B3 ≤ 2 | 32/41 (78%) | 521/689 (76%) | 32/200 (16%) | 521/530 (98%) |
| Perometer ≥ 9% or B3 ≤ 2 | 34/42 (81%) | 514/690 (74%) | 34/210 (16%) | 514/522 (98%) |
| Perometer ≥ 8% or B3 ≤ 2 | 35/42 (83%) | 508/692 (73%) | 35/219 (16%) | 508/515 (99%) |
| Perometer ≥ 5% or B3 ≤ 2 | 39/43 (91%) | 454/704 (64%) | 39/289 (13%) | 454/458 (99%) |
| BIS ≥ 10 or B3 ≤ 2 | 37/43 (86%) | 455/692 (66%) | 37/274 (14%) | 455/461 (99%) |
| Perometer ≥ 5% and B3 ≤ 2 | 20/44 (45%) | 733/775 (95%) | 20/62 (32%) | 733/757 (97%) |
| Perometer ≥ 8% and B3 ≤ 2 | 16/45 (36%) | 763/787 (97%) | 16/40 (40%) | 763/792 (96%) |
| Perometer ≥ 9% and B3 ≤ 2 | 16/45 (36%) | 765/789 (97%) | 16/40 (40%) | 765/794 (96%) |
| Perometer ≥ 10% and B3 ≤ 2 | 13/46 (28%) | 767/790 (97%) | 13/36 (36%) | 767/800 (96%) |
| Perometer ≥ 10% and BIS ≥ 10 | 15/48 (31%) | 758/789 (96%) | 15/46 (33%) | 758/791 (96%) |
| Perometer ≥ 9% and BIS ≥ 10 | 20/48 (42%) | 753/789 (95%) | 20/56 (36%) | 753/781 (96%) |
| Perometer ≥ 10% and BIS ≥ 10 and B3 ≤ 2 | 13/47 (28%) | 776/792 (98%) | 13/29 (45%) | 776/810 (96%) |
| Perometer ≥ 9% and BIS ≥ 10 and B3 ≤ 2 | 16/46 (35%) | 775/792 (98%) | 16/33 (48%) | 775/805 (96%) |
Those patients with lymphoedema up to 6 months are not included in the numbers in Table 75.
| Measure | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Perometer ≥ 10% | 19/46 (41%) | 580/594 (98%) | 19/33 (58%) | 580/607 (96%) |
| Perometer ≥ 9% | 24/46 (52%) | 577/594 (97%) | 24/41 (59%) | 577/599 (96%) |
| Perometer ≥ 8% | 27/46 (59%) | 568/594 (96%) | 27/53 (51%) | 568/587 (97%) |
| Perometer ≥ 5% | 39/46 (85%) | 522/594 (88%) | 39/111 (35%) | 522/529 (99%) |
| BIS ≥ 10 | 28/43 (65%) | 511/577 (89%) | 28/94 (30%) | 511/526 (97%) |
| B3 ≤ 2 | 21/42 (50%) | 445/526 (85%) | 21/102 (21%) | 445/466 (95%) |
| Perometer ≥ 10% or B3 ≤ 2 | 28/44 (64%) | 428/518 (83%) | 28/118 (24%) | 428/444 (96%) |
| Perometer ≥ 9% or B3 ≤ 2 | 31/44 (70%) | 425/518 (82%) | 31/124 (25%) | 425/438 (97%) |
| Perometer ≥ 8% or B3 ≤ 2 | 33/44 (75%) | 418/519 (81%) | 33/134 (25%) | 418/429 (97%) |
| Perometer ≥ 5% or B3 ≤ 2 | 41/46 (89%) | 385/525 (73%) | 41/181 (23%) | 385/390 (99%) |
| BIS ≥ 10 or B3 ≤ 2 | 35/44 (80%) | 377/513 (73%) | 35/171 (20%) | 377/386 (98%) |
| Perometer ≥ 5% and B3 ≤ 2 | 19/42 (45%) | 582/595 (98%) | 19/32 (59%) | 582/605 (96%) |
| Perometer ≥ 8% and B3 ≤ 2 | 15/44 (34%) | 595/601 (99%) | 15/21 (71%) | 595/624 (95%) |
| Perometer ≥ 9% and B3 ≤ 2 | 14/44 (32%) | 597/602 (99%) | 14/19 (74%) | 597/627 (95%) |
| Perometer ≥ 10% and B3 ≤ 2 | 12/44 (27%) | 597/602 (99%) | 12/17 (71%) | 597/629 (95%) |
| Perometer ≥ 10% and BIS ≥ 10 | 15/46 (33%) | 587/598 (98%) | 15/26 (58%) | 587/618 (95%) |
| Perometer ≥ 9% and BIS ≥ 10 | 18/46 (39%) | 585/598 (98%) | 18/31 (58%) | 585/613 (95%) |
| Perometer ≥ 10% and BIS ≥ 10 and B3 ≤ 2 | 10/44 (23%) | 600/604 (99%) | 10/14 (71%) | 600/634 (95%) |
| Perometer ≥ 9% and BIS ≥ 10 and B3 ≤ 2 | 11/44 (25%) | 600/604 (99%) | 11/15 (73%) | 600/633 (95%) |
Those patients with lymphoedema up to 9 months are not included in the numbers in Table 76.
| Measure | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Perometer ≥ 10% | 14/41 (34%) | 649/666 (97%) | 14/31 (45%) | 649/676 (96%) |
| Perometer ≥ 9% | 15/41 (37%) | 639/666 (96%) | 15/42 (36%) | 639/665 (96%) |
| Perometer ≥ 8% | 18/41 (44%) | 631/666 (95%) | 18/53 (34%) | 631/654 (96%) |
| Perometer ≥ 5% | 28/41 (68%) | 588/666 (88%) | 28/106 (26%) | 588/601 (98%) |
| BIS ≥ 10 | 17/39 (44%) | 573/641 (89%) | 17/85 (20%) | 573/595 (96%) |
| B3 ≤ 2 | 19/35 (54%) | 484/585 (83%) | 19/120 (16%) | 484/500 (97%) |
| Perometer ≥ 10% or B3 ≤ 2 | 26/38 (68%) | 469/580 (81%) | 26/137 (19%) | 469/481 (98%) |
| Perometer ≥ 9% or B3 ≤ 2 | 26/38 (68%) | 463/580 (80%) | 26/143 (18%) | 463/475 (97%) |
| Perometer ≥ 8% or B3 ≤ 2 | 27/38 (71%) | 458/582 (79%) | 27/151 (18%) | 458/469 (98%) |
| Perometer ≥ 5% or B3 ≤ 2 | 32/39 (82%) | 431/588 (73%) | 32/189 (17%) | 431/438 (98%) |
| BIS ≥ 10 or B3 ≤ 2 | 26/37 (70%) | 423/576 (73%) | 26/179 (15%) | 423/434 (97%) |
| Perometer ≥ 5% and B3 ≤ 2 | 15/37 (41%) | 641/663 (97%) | 15/37 (41%) | 641/663 (97%) |
| Perometer ≥ 8% and B3 ≤ 2 | 10/38 (26%) | 657/669 (98%) | 10/22 (45%) | 657/685 (96%) |
| Perometer ≥ 9% and B3 ≤ 2 | 8/38 (21%) | 660/671 (98%) | 8/19 (42%) | 660/690 (96%) |
| Perometer ≥ 10% and B3 ≤ 2 | 7/38 (18%) | 664/671 (99%) | 7/14 (50%) | 664/695 (96%) |
| Perometer ≥ 10% and BIS ≥ 10 | 9/40 (23%) | 658/669 (98%) | 9/20 (45%) | 658/689 (96%) |
| Perometer ≥ 9% and BIS ≥ 10 | 9/40 (23%) | 653/669 (98%) | 9/25 (36%) | 653/684 (95%) |
| Perometer ≥ 10% and BIS ≥ 10 and B3 ≤ 2 | 5/38 (13%) | 668/674 (99%) | 5/11 (45%) | 668/701 (95%) |
| Perometer ≥ 9% and BIS ≥ 10 and B3 ≤ 2 | 5/38 (13%) | 667/674 (99%) | 5/12 (42%) | 667/700 (95%) |
Those patients with lymphoedema up to 12 months are not included in the numbers in Table 77.
| Measure | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Perometer ≥ 10% | 12/30 (40%) | 551/568 (97%) | 12/29 (41%) | 551/569 (97%) |
| Perometer ≥ 9% | 12/30 (40%) | 547/568 (96%) | 12/33 (36%) | 547/565 (97%) |
| Perometer ≥ 8% | 13/30 (43%) | 535/568 (94%) | 13/46 (28%) | 535/552 (97%) |
| Perometer ≥ 5% | 21/30 (70%) | 491/568 (86%) | 21/98 (21%) | 491/500 (98%) |
| BIS ≥ 10 | 14/29 (48%) | 491/542 (91%) | 14/65 (22%) | 491/506 (97%) |
| B3 ≤ 2 | 13/24 (54%) | 434/513 (85%) | 13/92 (14%) | 434/445 (98%) |
| Perometer ≥ 10% or B3 ≤ 2 | 17/27 (63%) | 415/503 (83%) | 17/105 (16%) | 415/425 (98%) |
| Perometer ≥ 9% or B3 ≤ 2 | 17/27 (63%) | 414/504 (82%) | 17/107 (16%) | 414/424 (98%) |
| Perometer ≥ 8% or B3 ≤ 2 | 17/27 (63%) | 404/504 (80%) | 17/117 (15%) | 404/414 (98%) |
| Perometer ≥ 5% or B3 ≤ 2 | 22/28 (79%) | 377/508 (74%) | 22/153 (14%) | 377/383 (98%) |
| BIS ≥ 10 or B3 ≤ 2 | 18/25 (75%) | 370/486 (76%) | 18/134 (13%) | 370/377 (98%) |
| Perometer ≥ 5% and B3 ≤ 2 | 12/26 (46%) | 548/573 (96%) | 12/37 (32%) | 548/562 (98%) |
| Perometer ≥ 8% and B3 ≤ 2 | 9/27 (33%) | 565/577 (98%) | 9/21 (43%) | 565/583 (97%) |
| Perometer ≥ 9% and B3 ≤ 2 | 8/27 (30%) | 567/577 (98%) | 8/18 (44%) | 567/586 (97%) |
| Perometer ≥ 10% and B3 ≤ 2 | 8/27 (30%) | 570/578 (99%) | 8/16 (50%) | 570/589 (97%) |
| Perometer ≥ 10% and BIS ≥ 10 | 9/29 (31%) | 560/569 (98%) | 9/18 (50%) | 560/580 (97%) |
| Perometer ≥ 9% and BIS ≥ 10 | 9/29 (31%) | 560/569 (98%) | 9/18 (50%) | 560/580 (97%) |
| Perometer ≥ 10% and BIS ≥ 10 and B3 ≤ 2 | 6/27 (22%) | 574/580 (99%) | 6/12 (50%) | 574/595 (96%) |
| Perometer ≥ 9% and BIS ≥ 10 and B3 ≤ 2 | 6/27 (22%) | 574/580 (99%) | 6/12 (50%) | 574/595 (96%) |
Those patients with lymphoedema up to 18 months are not included in the numbers in Table 78.
| Measure | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Perometer ≥ 10% | 10/24 (42%) | 511/530 (96%) | 10/29 (34%) | 511/525 (97%) |
| Perometer ≥ 9% | 12/24 (50%) | 507/530 (96%) | 12/35 (34%) | 507/519 (98%) |
| Perometer ≥ 8% | 13/24 (54%) | 503/530 (95%) | 13/40 (33%) | 503/514 (98%) |
| Perometer ≥ 5% | 17/24 (71%) | 454/530 (86%) | 17/93 (18%) | 454/461 (98%) |
| BIS ≥ 10 | 10/23 (43%) | 447/491 (91%) | 10/54 (19%) | 447/460 (97%) |
| B3 ≤ 2 | 8/21 (38%) | 404/467 (87%) | 8/71 (11%) | 404/417 (97%) |
| Perometer ≥ 10% or B3 ≤ 2 | 15/24 (63%) | 391/465 (84%) | 15/89 (17%) | 391/400 (98%) |
| Perometer ≥ 9% or B3 ≤ 2 | 16/24 (67%) | 389/466 (83%) | 16/93 (17%) | 389/397 (98%) |
| Perometer ≥ 8% or B3 ≤ 2 | 17/24 (71%) | 388/467 (83%) | 17/96 (18%) | 388/395 (98%) |
| Perometer ≥ 5% or B3 ≤ 2 | 20/24 (83%) | 343/470 (73%) | 20/147 (14%) | 343/347 (99%) |
| BIS ≥ 10 or B3 ≤ 2 | 13/23 (57%) | 349/444 (79%) | 13/108 (12%) | 349/359 (97%) |
| Perometer ≥ 5% and B3 ≤ 2 | 5/21 (24%) | 515/527 (98%) | 5/17 (29%) | 515/531 (97%) |
| Perometer ≥ 8% and B3 ≤ 2 | 4/21 (19%) | 519/530 (98%) | 4/15 (27%) | 519/536 (97%) |
| Perometer ≥ 9% and B3 ≤ 2 | 4/21 (19%) | 522/531 (98%) | 4/13 (31%) | 522/539 (97%) |
| Perometer ≥ 10% and B3 ≤ 2 | 3/21 (14%) | 524/532 (98%) | 3/11 (27%) | 524/542 (97%) |
| Perometer ≥ 10% and BIS ≥ 10 | 6/23 (26%) | 520/529 (98%) | 6/15 (40%) | 520/537 (97%) |
| Perometer ≥ 9% and BIS ≥ 10 | 7/23 (30%) | 518/528 (98%) | 7/17 (41%) | 518/534 (97%) |
| Perometer ≥ 10% and BIS ≥ 10 and B3 ≤ 2 | 2/21 (10%) | 526/532 (99%) | 2/8 (25%) | 526/545 (97%) |
| Perometer ≥ 9% and BIS ≥ 10 and B3 ≤ 2 | 3/21 (14%) | 525/531 (99%) | 3/9 (33%) | 525/543 (97%) |
Appendix 10 National Institute for Health and Care Excellence medical technology assessment
Appendix 11 Lymphoedema rates
Lymphoedema rates using perometer RAVI of ≥ 10% (primary end point)
| Follow-up date | ||||||
|---|---|---|---|---|---|---|
| ≤ 3 months | > 3 to ≤ 6 months | > 6 to ≤ 9 months | > 9 to ≤ 12 months | > 12 to ≤ 18 months | > 18 to ≤ 24 months | |
| n at risk | 1001 | 925 | 848 | 798 | 722 | 647 |
| Lymphoedema | ||||||
| During interval | 33 | 54 | 27 | 24 | 31 | 25 |
| Total number | 33 | 57 | 114 | 138 | 169 | 194 |
| KMa probability of event | 3.4% | 9.0% | 11.9% | 14.6% | 18.2% | 21.4% |
| Follow-up date | ||||||
|---|---|---|---|---|---|---|
| ≤ 3 months | > 3 to ≤ 6 months | > 6 to ≤ 9 months | > 9 to ≤ 12 months | > 12 to ≤ 18 months | > 18 to ≤ 24 months | |
| n at risk | 999 | 928 | 856 | 789 | 697 | 622 |
| Lymphoedema | ||||||
| During interval | 29 | 48 | 46 | 43 | 31 | 24 |
| Total number | 29 | 77 | 123 | 166 | 197 | 221 |
Lymphoedema by 24 months was detected in 24% of women by perometry and in 45% of women by BIS. There was a moderate correlation between perometer and BIS at 6 months (r = 0.61), with a sensitivity of 75% (95% CI 64% to 84%), specificity of 85% (95% CI 83% to 88%) and PPV of BIS of 31% (95% CI 25% to 39%) (see Table 2). Sensitivity remained similar at 24 months (75%, 95% CI 64% to 83%), although specificity was higher (91%, 95% CI 89% to 93%), as was PPV of BIS (54%, 95% CI 44% to 63%).
The sensitivity and specificity values for BIS fall below the percentage of 95% required according to the study protocol.
Appendix 12 Sensitivity and specificity of perometer and bioimpedance spectroscopy
| Time point | Perometer | Total | |
|---|---|---|---|
| ≥ 10% | < 10% | ||
| 6 months | |||
| BIS (≥ 10) | 29 | 68 | 97 |
| BIS (< 10) | 12 | 382 | 394 |
| Total | 41 | 450 | 491 |
| 24 months | |||
| BIS (≥ 10) | 38 | 29 | 67 |
| BIS (< 10) | 14 | 321 | 335 |
| Total | 52 | 350 | 402 |
Women who developed a relative arm-volume increase of > 5% to < 10% after 6 months required lymphoedema treatment in 44% by 24 months, whereas an arm-volume increase of < 3% was associated with a 9% lymphoedema rate at 24 months (p < 0.0001).
Appendix 13 Comparison of perometer and bioimpedance spectroscopy
FIGURE 32.
Comparison of perometer and BIS at 6 months. Q1, perometer only ≥ 10; Q2, both ≥ 10; Q3, neither ≥ 10; Q4, BIS only ≥ 10. Sensitivity, 69% (59/86; 95% CI 58% to 77%); specificity, 82% (698/851; 95% CI 79% to 84%); PPV, 28% (59/212; 95% CI 22% to 34%); NPV, 96% (698/725; 95% CI 95% to 97%).
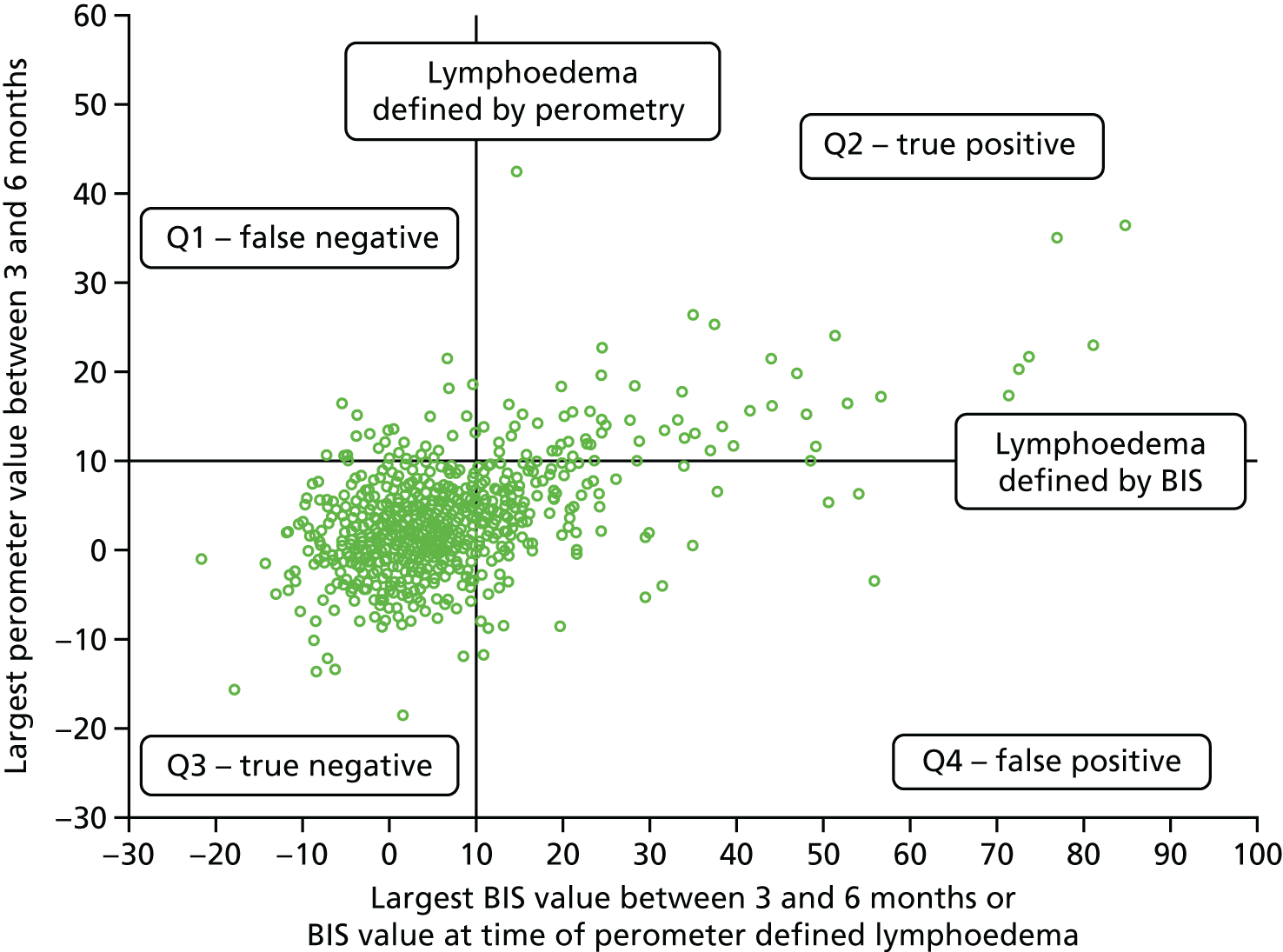
Perometer after 6 months up to 18 months
The perometer after 6 months up to 18 months variable excludes those patients with lymphoedema up to and including 6 months.
For those patients with lymphoedema according to the perometer ≥ 10% definition, the BIS value used is the one at the time of the indicated lymphoedema. For those patients without lymphoedema according to the perometer ≥ 10% definition, the BIS value is the largest value between 9 and 18 months.
FIGURE 33.
Comparison of perometer and BIS at 18 months. Q1, perometer only ≥ 10; Q2, both ≥ 10; Q3, neither ≥ 10; Q4, BIS only ≥ 10. Sensitivity, 68% (53/78; 95% CI 57% to 77%); specificity, 81% (600/738; 95% CI 78% to 84%); PPV, 28% (53/191; 95% CI 22% to 34%); NPV, 96% (600/625; 95% CI 94% to 97%).
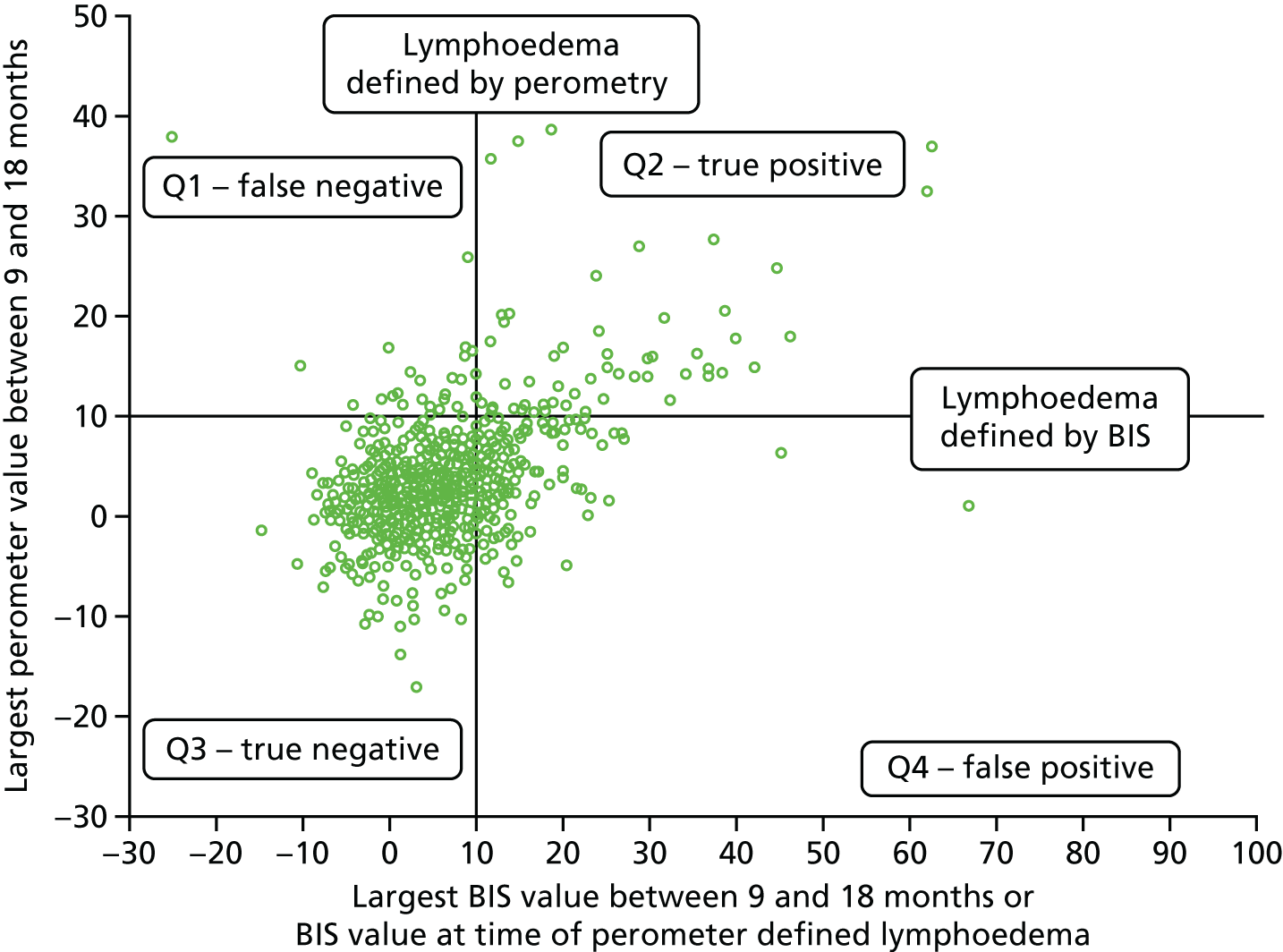
Perometer after 6 months up to 24 months (excludes those patients with lymphoedema up to and including 6 months)
For those patients with lymphoedema according to the perometer ≥ 10% definition, the BIS value used is the one at the time of the indicated lymphoedema. For those patients without lymphoedema, the BIS value used is the largest value between 9 and 24 months.
FIGURE 34.
Comparison of perometer and BIS after 6 months up to 24 months. Q1, perometer only ≥ 10; Q2, both ≥ 10; Q3, neither ≥ 10; Q4, BIS only ≥ 10. Sensitivity, 68% (15/22; 95% CI 58% to 76%); specificity, 79% (572/722; 95% CI 76% to 82%); PPV, 31% (68/218; 95% CI 25% to 38%); NPV, 95% (572/604; 95% CI 93% to 96%).
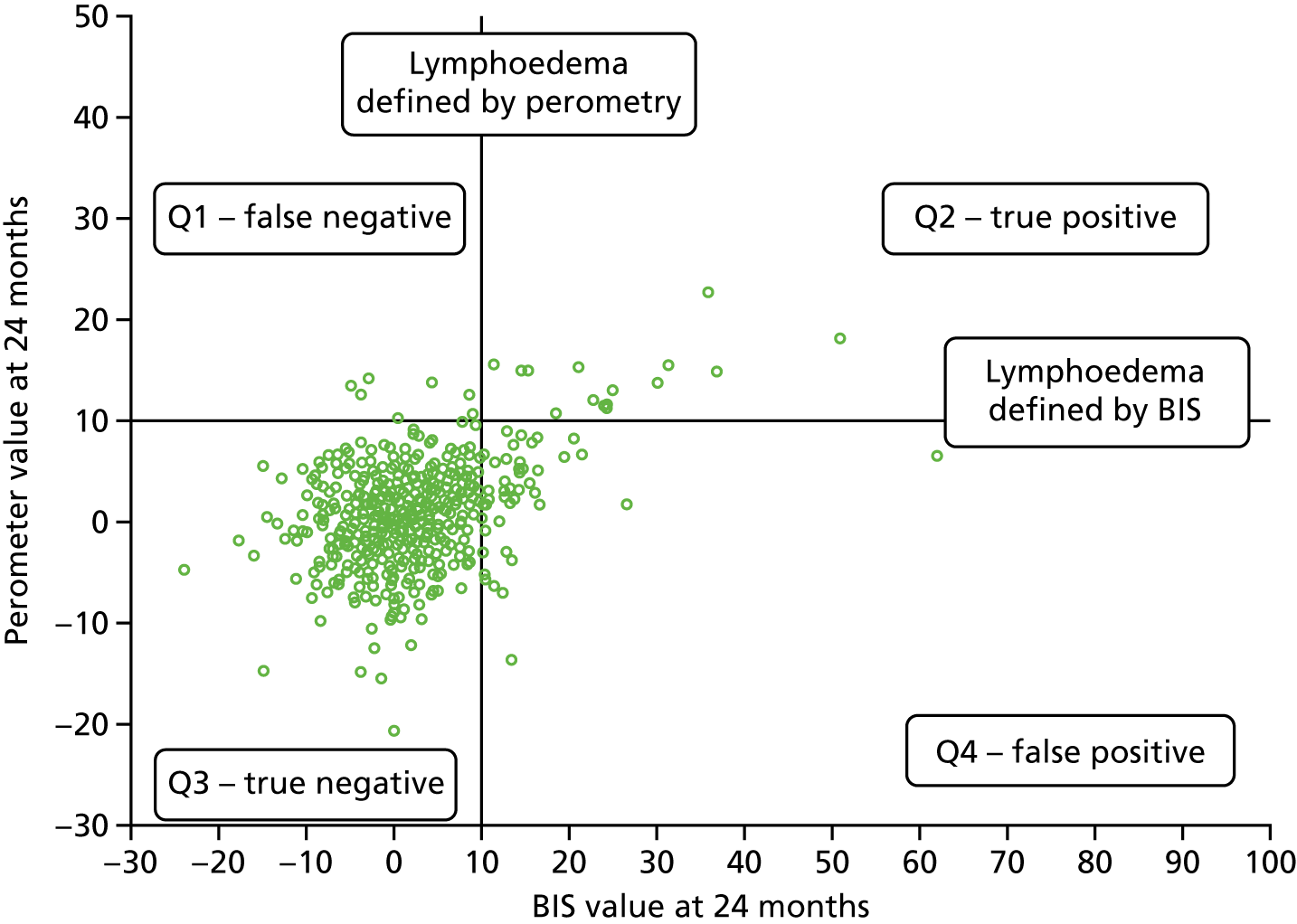
Clinical lymphoedema/appropriately applied sleeve by 6 months
| By 6 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer (< 10%) | 820 (94%) | 45 (60%) | 865 |
| Perometer (≥ 10%) | 55 (6%) | 30 (40%) | 85 |
| Total | 875 | 75 | 950 |
| By 6 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 690 (80%) true negative | 34 (46%) false negative | 724 |
| BIS (≥ 10) | 170 (20%) false positive | 40 (54%) true positive | 210 |
| Total | 860 | 74 | 934 |
FIGURE 35.
Comparison of RAVI > 10% and BIS > 10% with sleeve application. Note that those patients with no clinical lymphoedema or appropriately applied sleeve by 6 months may have had an appropriately applied sleeve at a later time point.
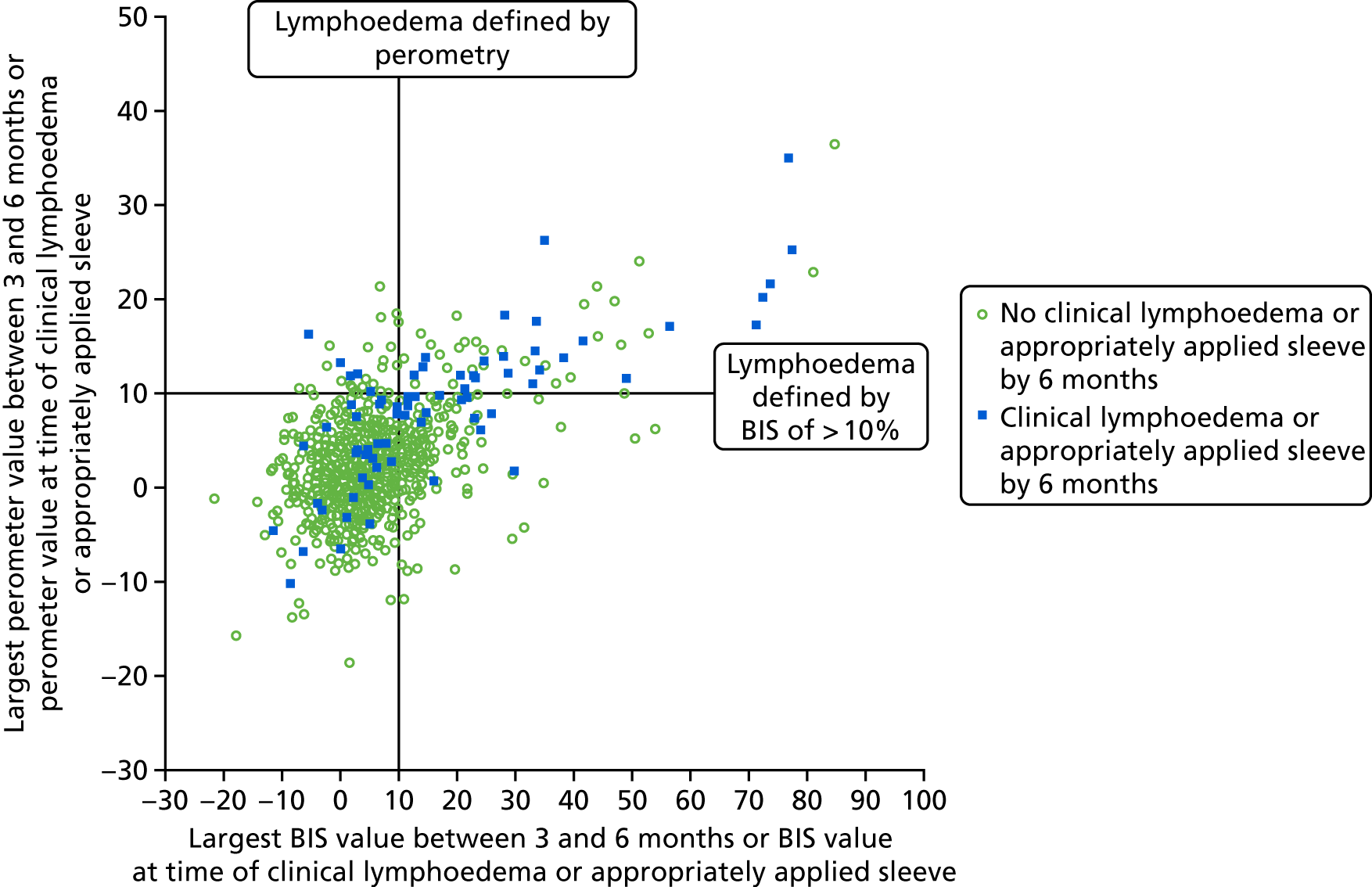
Clinical lymphoedema/appropriately applied sleeve from 6 months up to 18 months
The clinical lymphoedema/appropriately applied sleeve between 6 and 18 months variable excludes those patients with lymphoedema up to and including 6 months.
For those patients with lymphoedema, the BIS and perometer values used are those at the time of the indicated lymphoedema. For those without lymphoedema, the BIS and perometer values used are the largest value between 9 and 18 months.
| After 6 months up to 18 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer (< 10%) | 693 (95%) | 72 (62%) | 765 |
| Perometer (≥ 10%) | 33 (5%) | 45 (38%) | 78 |
| Total | 726 | 117 | 843 |
| After 6 months up to 18 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 581 (82%) true negative | 52 (47%) false negative | 633 |
| BIS (≥ 10) | 126 (18%) false positive | 59 (53%) true positive | 185 |
| Total | 707 | 111 | 818 |
FIGURE 36.
Comparison of RAVI > 10% and BIS with sleeve application (6–18 months). Note that those patients with no clinical lymphoedema or appropriately applied sleeve by 6 months may have had clinical lymphoedema or an appropriately applied sleeve at a later time point.
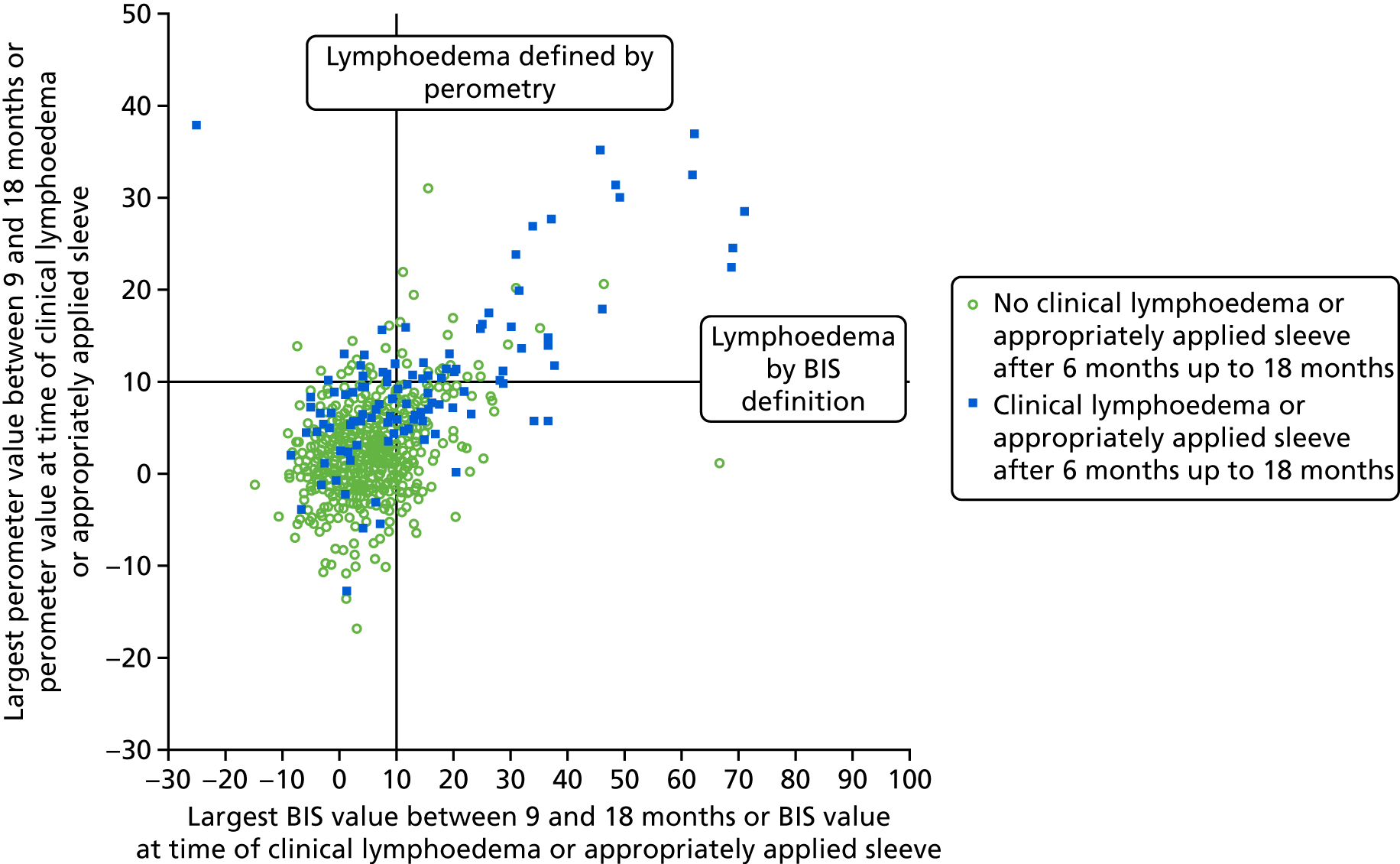
Clinical lymphoedema/appropriately applied sleeve after 6 months up to 24 months
The clinical lymphoedema/appropriately applied sleeve after 6 months up to 24 months variable excludes those patients with lymphoedema up to and including 6 months.
For those patients with lymphoedema, the BIS and perometer values used are those at the time of lymphoedema diagnosis. For those patients without lymphoedema, the BIS and perometer values used are the largest value between 9 and 24 months.
| After 6 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer (< 10%) | 667 (94%) | 86 (61%) | 753 |
| Perometer (≥ 10%) | 39 (6%) | 55 (39%) | 94 |
| Total | 706 | 141 | 847 |
| After 6 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 556 (80%) | 65 (49%) | 621 |
| BIS (≥ 10) | 136 (20%) | 69 (51%) | 205 |
| Total | 692 | 134 | 826 |
FIGURE 37.
Comparison of RAVI of > 10% and BIS with sleeve application (6–24 months). Note that those patients with no clinical lymphoedema or appropriately applied sleeve after 6 months up to 24 months may have had clinical lymphoedema or sleeve applied at a later time point.
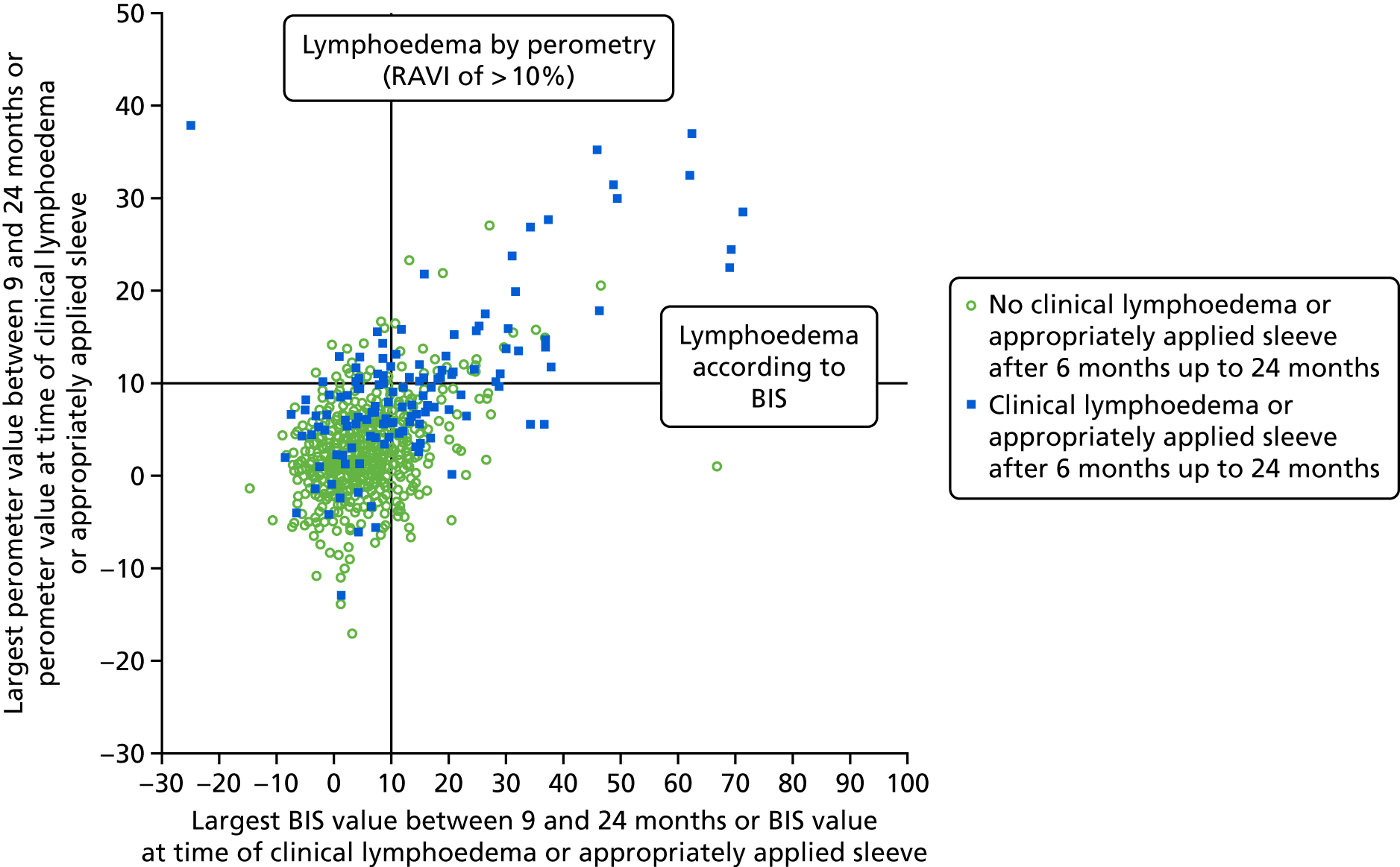
Clinical lymphoedema/appropriately applied sleeve after 18 months up to 24 months
The clinical lymphoedema/appropriately applied sleeve after 18 months up to 24 months variable excludes those patients with lymphoedema up to and including 18 months.
For all patients, the BIS and perometer values used are those at 24 months.
| After 18 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer (< 10%) | 524 (96%) | 14 (58%) | 538 |
| Perometer (≥ 10%) | 21 (4%) | 10 (42%) | 31 |
| Total | 545 | 24 | 569 |
| After 18 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| BIS (< 10) | 460 (91%) true negative | 13 (57%) false negative | 473 |
| BIS (≥ 10) | 45 (9%) false positive | 10 (43%) true positive | 55 |
| Total | 505 | 23 | 528 |
FIGURE 38.
Comparison of BIS and perometer values at 24 months. Note that those with no lymphoedema or appropriately applied sleeve by 24 months may have had an appropriately applied sleeve at a later time point.
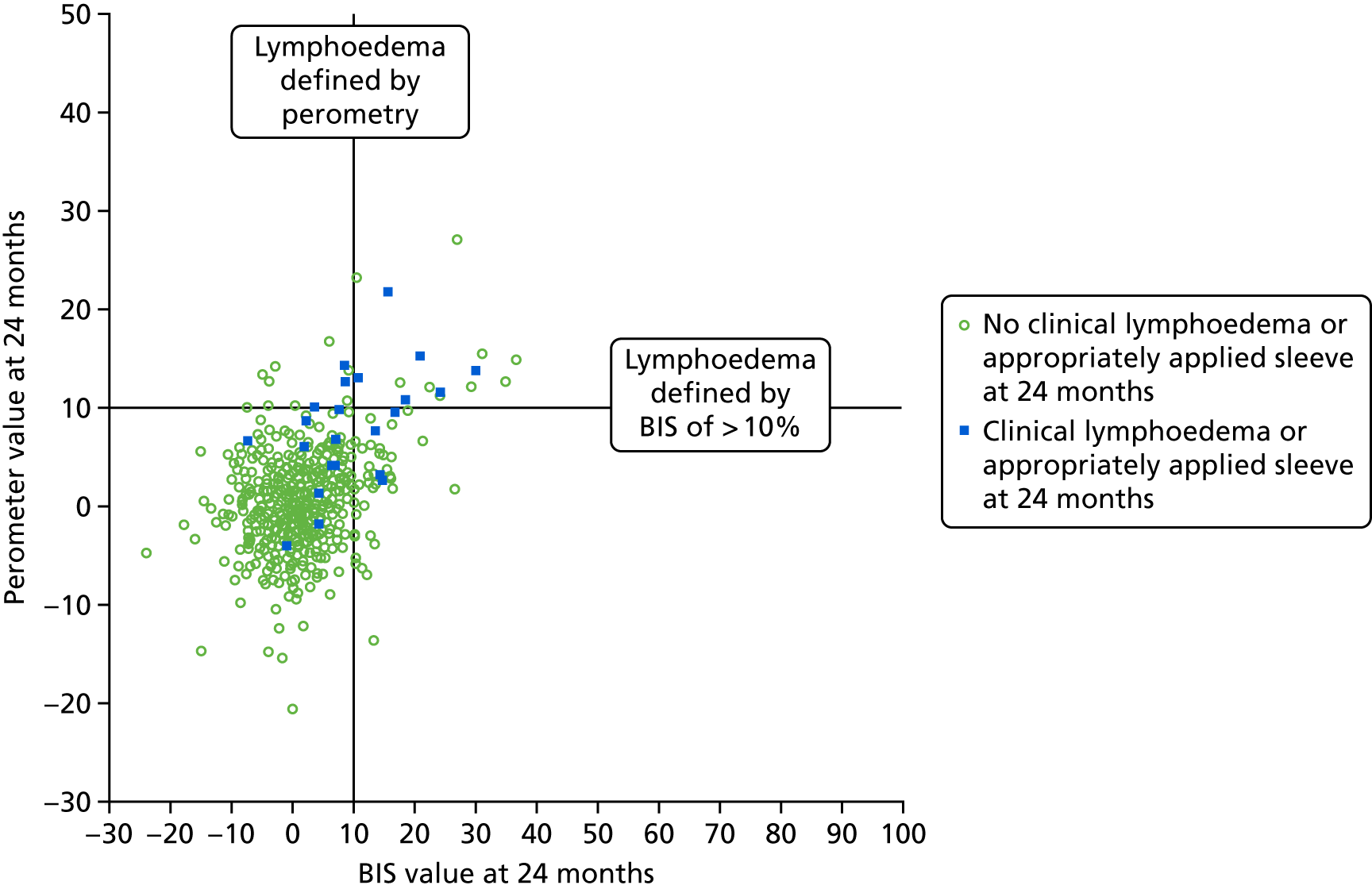
Appendix 14 Combined perometer or bioimpedance spectroscopy versus clinical lymphoedema/appropriately applied sleeve
Combined perometer or bioimpedance spectroscopy versus clinical lymphoedema/appropriately applied sleeve
| By 6 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer and BIS < 10 | 663 (78%) | 28 (38%) | 691 |
| Perometer or BIS ≥ 10 | 192 (22%) | 45 (62%) | 237 |
| Total | 855 | 73 | 928 |
Clinical lymphoedema/appropriately applied sleeve after 6 months up to 18 months
For those patients with lymphoedema, the BIS and perometer values used are those at the time of the indicated lymphoedema. For those patients without lymphoedema, the BIS and perometer values used are the largest value between 9 and 18 months.
| After 6 months up to 18 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer and BIS < 10 | 566 (80%) | 42 (37%) | 608 |
| Perometer or BIS ≥ 10 | 139 (20%) | 71 (63%) | 210 |
| Total | 705 | 113 | 818 |
The 71 patients with lymphoedema are made up of 33 with both perometer and BIS of ≥ 10, 26 with only BIS of ≥ 10 and 12 with only perometer ≥ 10.
Clinical lymphoedema/appropriately applied sleeve after 6 months up to 24 months (excludes those patients with lymphoedema up to and including 6 months).
Bioimpedance spectroscopy and perometer values used are those at the time of the indicated lymphoedema. For those patients without lymphoedema, the BIS and perometer values used are the largest value between 9 and 24 months.
Appendix 15 Factors predicting lymphoedema development after 1 month’s and 6 months’ analysis
Appendix 16 Quality-of-life variables
Change over time in quality-of-life variables analysis notes
Generalised estimating equations (GEEs) were used to assess how the TOI, FACT-B total score and the ARM subscale changed over time.
Trial Outcome Index
Owing to the negative skew of the TOI variable, a transformation [LN(120 – TOI)] was used for the analysis so that a linear GEE model could be used.
In a model only including the time variable, the EMM of TOI at each time point is presented in the table below. A total of 997 patients had some data in the model.
| Time point | Estimated marginal mean of TOI (95% CI) |
|---|---|
| Pre surgery | 68.0 (67.2 to 68.9) |
| 3 months | 63.5 (62.5 to 64.5) |
| 6 months | 65.4 (64.4 to 66.4) |
| 12 months | 70.2 (69.2 to 71.1) |
| 18 months | 70.6 (69.6 to 71.5) |
| 24 months | 71.0 (70.0 to 71.9) |
Time–lymphoedema status interaction
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time found that the interaction was statistically significant (p = 0.003), showing the pattern of change over time was different between the groups.
The main effect for the time variable was statistically significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was statistically significant (p = 0.006), showing that there was a difference between the lymphoedema status groups overall.
The EMMs from the interaction term in the GEE analysis are presented below.
| Time point | Estimated marginal mean of TOI (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 883) | With lymphoedema by 6 months (n = 87) | ||
| Pre surgery | 68.3 (67.4 to 69.2) | 67.0 (63.9 to 70.0) | 0.42 |
| 3 months | 63.8 (62.7 to 64.8) | 61.4 (57.8 to 64.8) | 0.19 |
| 6 months | 66.0 (64.9 to 67.1) | 60.1 (56.4 to 63.5) | 0.001 |
| 12 months | 70.7 (69.8 to 71.7) | 65.3 (61.8 to 68.5) | 0.001 |
| 18 months | 71.1 (70.2 to 72.1) | 65.4 (61.9 to 68.6) | 0.001 |
| 24 months | 71.4 (70.4 to 72.4) | 67.6 (64.0 to 71.0) | 0.033 |
FIGURE 39.
Graph of TOI EMMs.
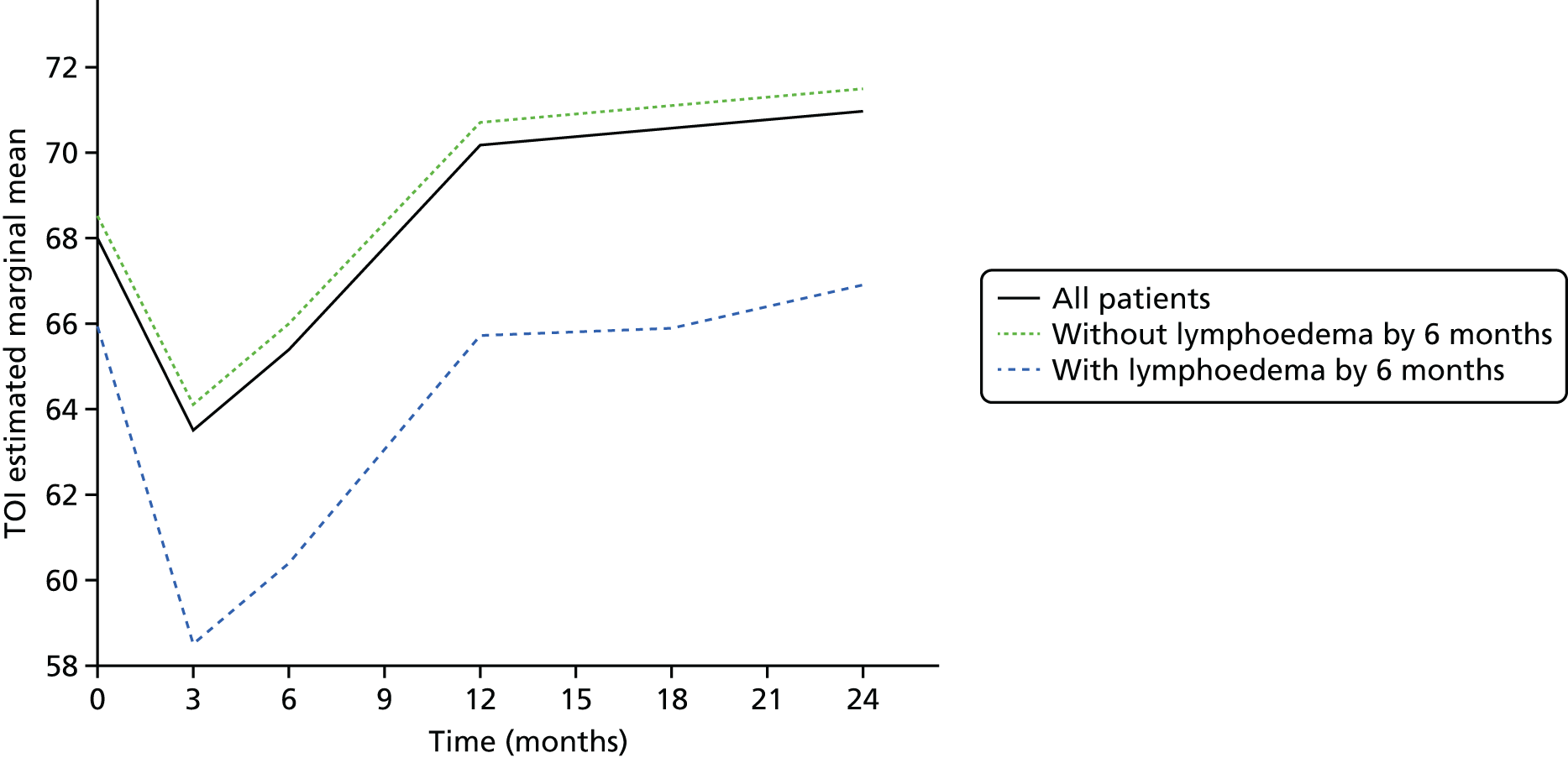
FIGURE 40.
Graph of change in TOI EMMs.
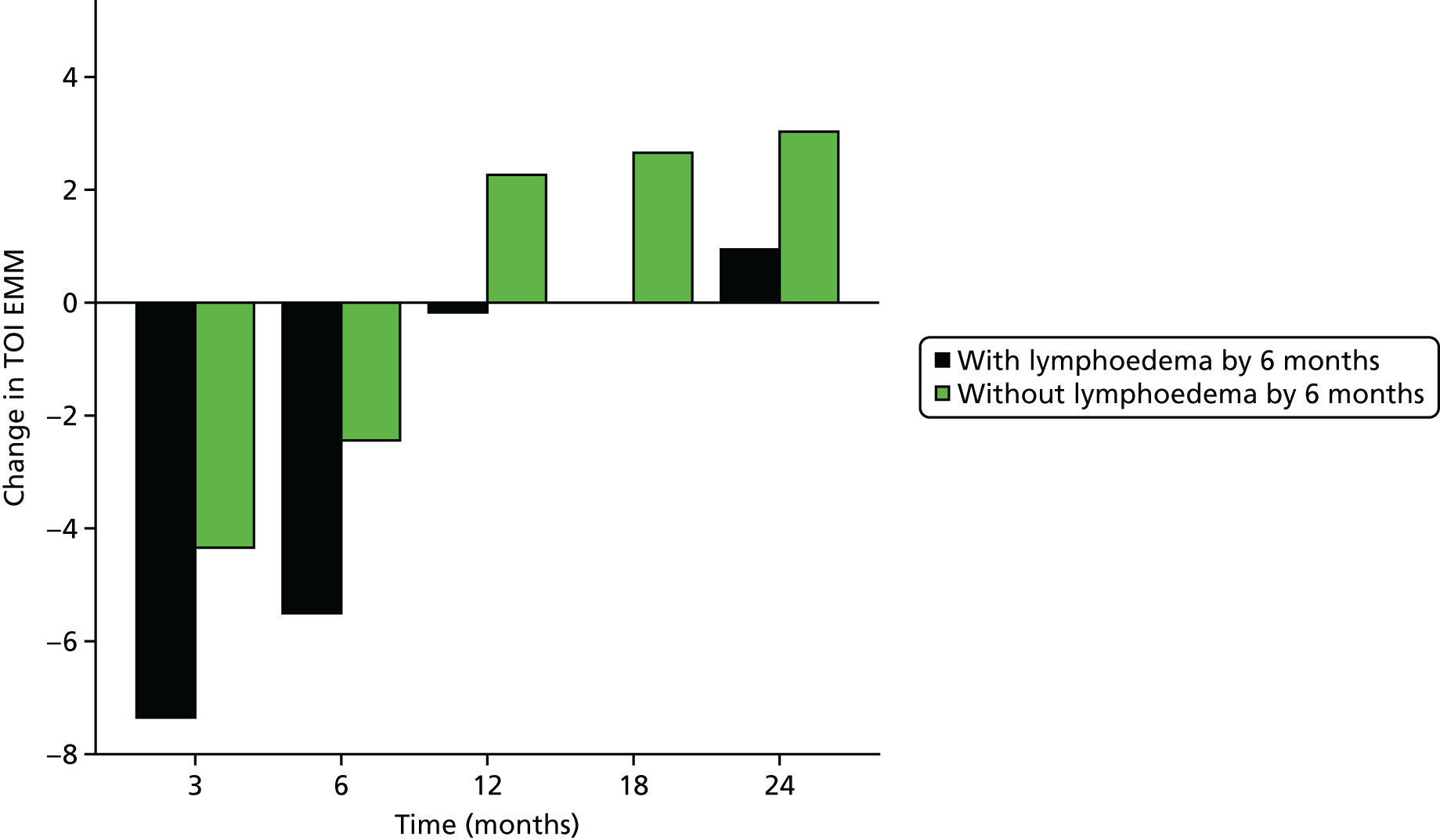
FACT-B total score
Owing to the negative skew of the FACT-B total score variable, a transformation [LN(160 – FACT-B)] was used for the analysis so that a linear GEE model could be used.
In a model only including the time variable, the EMM of FACT-B at each time point is presented in the table below. A total of 996 patients had some data in the model.
| Time point | Estimated marginal mean of FACT-B total score (95% CI) |
|---|---|
| Pre surgery | 110.5 (109.3 to 111.7) |
| 3 months | 107.4 (106.0 to 108.7) |
| 6 months | 109.4 (108.0 to 110.8) |
| 12 months | 114.8 (113.5 to 116.1) |
| 18 months | 115.2 (113.9 to 116.5) |
| 24 months | 115.5 (114.2 to 116.9) |
Time–lymphoedema status interaction
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time found that the interaction was borderline statistically significant (p = 0.055), showing that there was some indication of pattern of change that was different between the groups.
The main effect for the time variable was statistically significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was statistically significant (p = 0.032), showing that there was a difference between the lymphoedema status groups overall.
The EMMs from the interaction term in the GEE analysis are presented below.
| Time point | Estimated marginal mean of FACT-B total score (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 882) | With lymphoedema by 6 months (n = 87) | ||
| Pre surgery | 110.9 (109.7 to 112.2) | 109.4 (105.0 to 113.4) | 0.48 |
| 3 months | 107.8 (106.3 to 109.2) | 105.3 (100.3 to 109.9) | 0.32 |
| 6 months | 110.1 (108.6 to 111.5) | 104.0 (98.7 to 108.8) | 0.018 |
| 12 months | 115.5 (114.1 to 116.8) | 109.5 (104.5 to 114.0) | 0.012 |
| 18 months | 115.9 (114.5 to 117.2) | 109.2 (104.2 to 113.7) | 0.005 |
| 24 months | 116.0 (114.6 to 117.4) | 111.8 (106.5 to 116.7) | 0.11 |
FIGURE 41.
Graph of FACT-B total score EMMs.
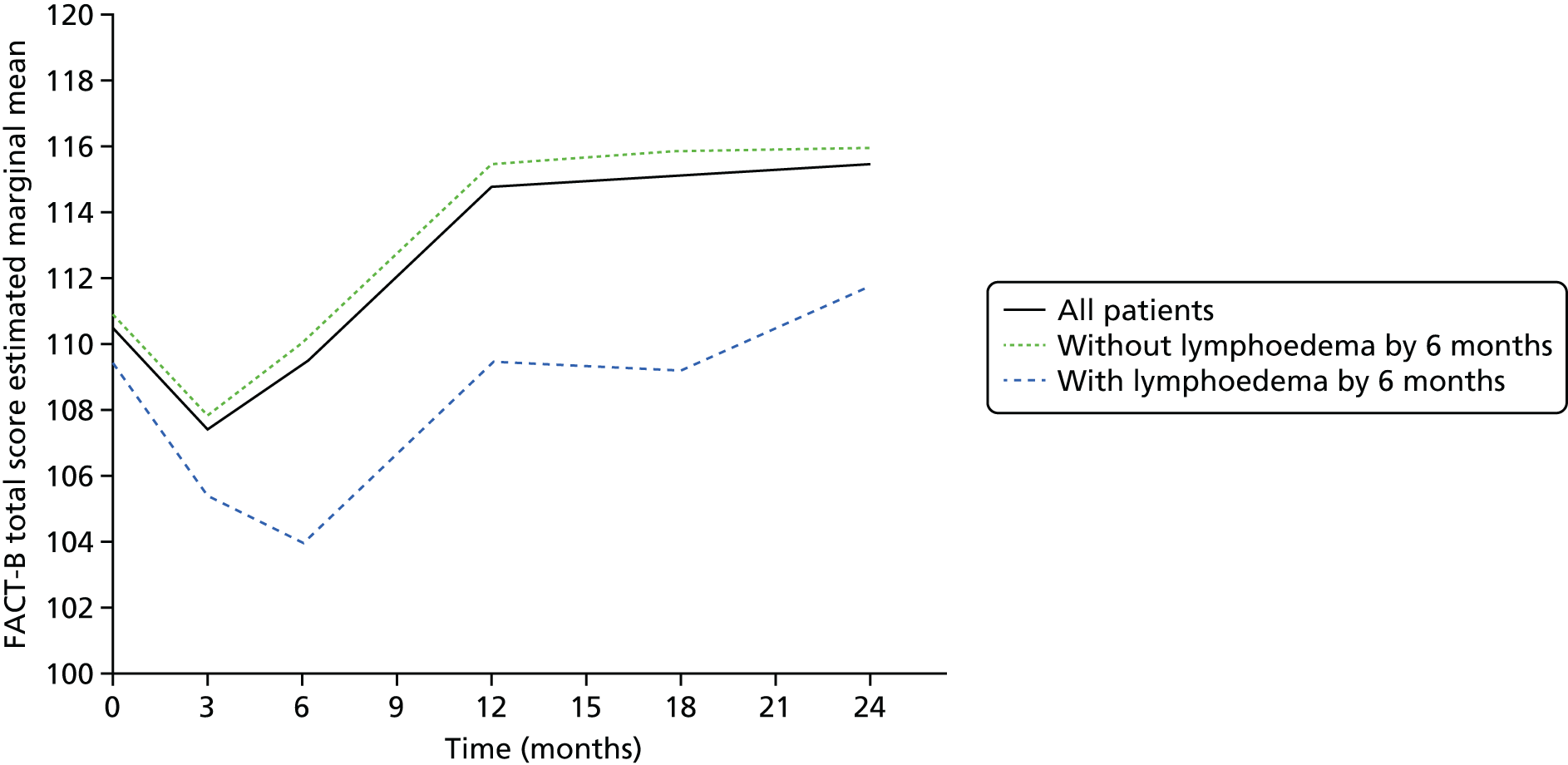
FIGURE 42.
Graph of change in FACT-B total score EMMs.
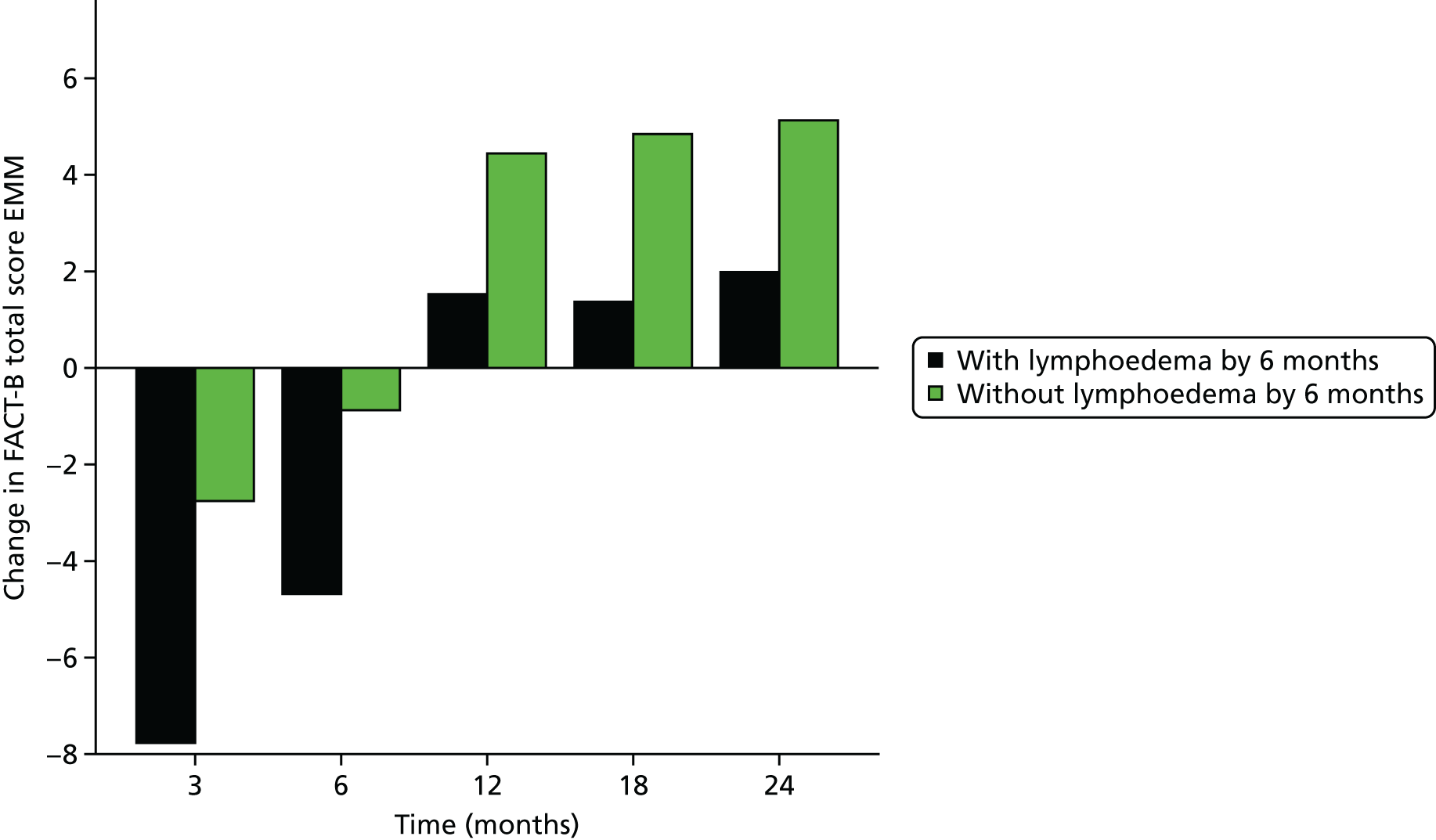
ARM subscale
Owing to the negative skew of the ARM subscale variable, a transformation [LN(22 – ARM)] was used for the analysis so that a linear GEE model could be used.
In a model including only the time variable, the EMM of ARM at each time point is presented in the table below. A total of 995 patients had some data in the model.
| Time point | Estimated marginal mean of ARM subscale (95% CI) |
|---|---|
| Pre surgery | 18.7 (18.5 to 18.9) |
| 3 months | 16.1 (15.9 to 16.3) |
| 6 months | 15.9 (15.6 to 16.1) |
| 12 months | 16.0 (15.8 to 16.3) |
| 18 months | 16.1 (15.9 to 16.4) |
| 24 months | 16.4 (16.1 to 16.6) |
Time–lymphoedema status interaction
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time found that the interaction was not statistically significant (p = 0.25), showing the pattern of change over time was not significantly different between the groups.
The main effect for the time variable was statistically significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was statistically significant (p < 0.001), showing that there was a difference between the lymphoedema status groups overall.
The EMMs from the interaction term in the GEE analysis are presented below.
| Time point | Estimated marginal mean of ARM subscale (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 882) | With lymphoedema by 6 months (n = 87) | ||
| Pre surgery | 18.7 (18.6 to 18.9) | 18.6 (17.9 to 19.1) | 0.62 |
| 3 months | 16.2 (15.9 to 16.4) | 15.7 (14.7 to 16.5) | 0.29 |
| 6 months | 16.2 (15.8 to 16.3) | 14.0 (13.0 to 14.8) | < 0.001 |
| 12 months | 16.2 (15.9 to 16.4) | 14.4 (13.4 to 15.3) | < 0.001 |
| 18 months | 16.3 (16.0 to 16.5) | 14.7 (13.5 to 15.6) | 0.001 |
| 24 months | 16.5 (16.3 to 16.8) | 15.2 (14.2 to 16.0) | 0.003 |
FIGURE 43.
Graph of ARM subscale EMMs.
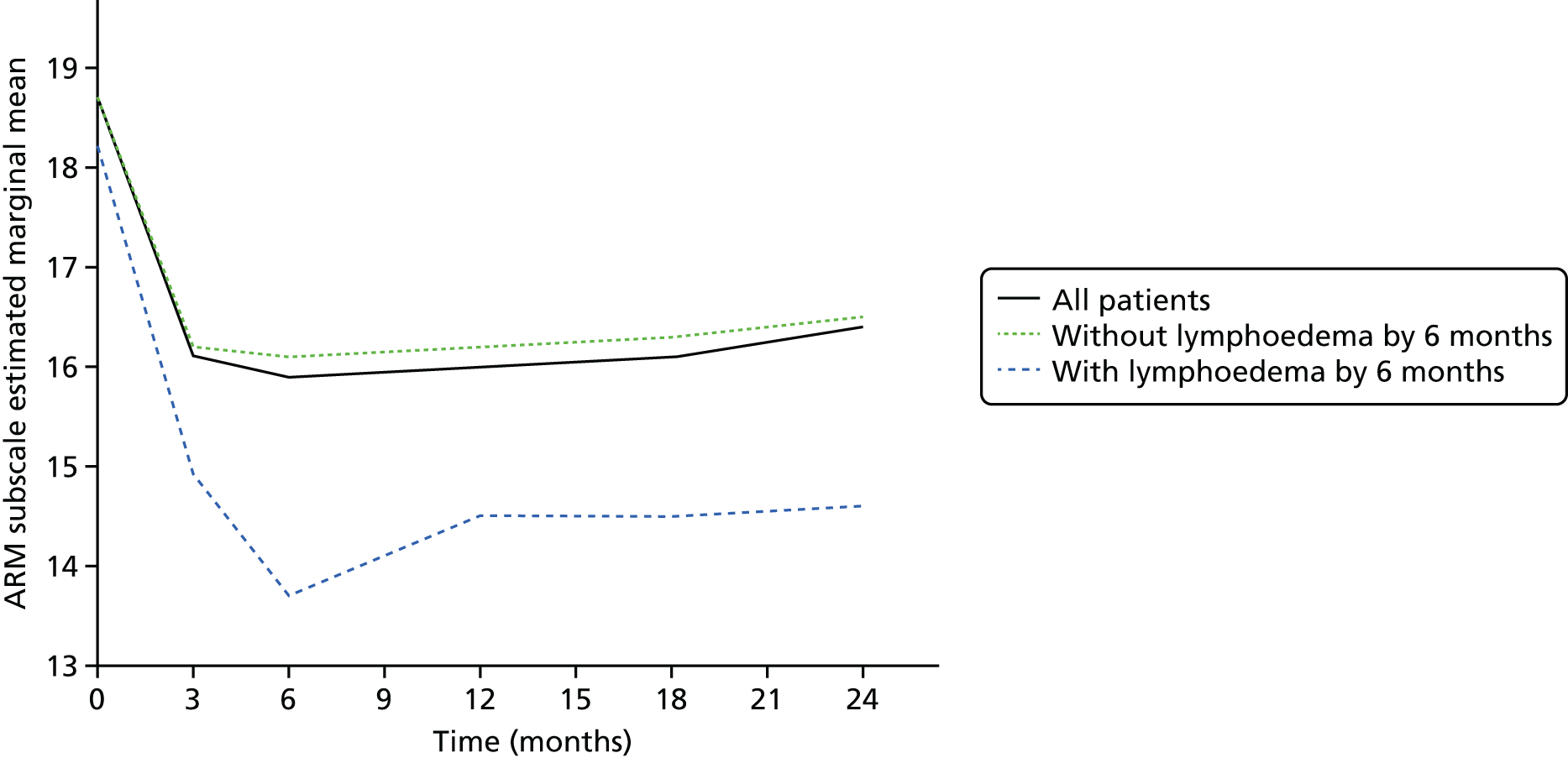
FIGURE 44.
Graph of change in ARM subscale EMMs.
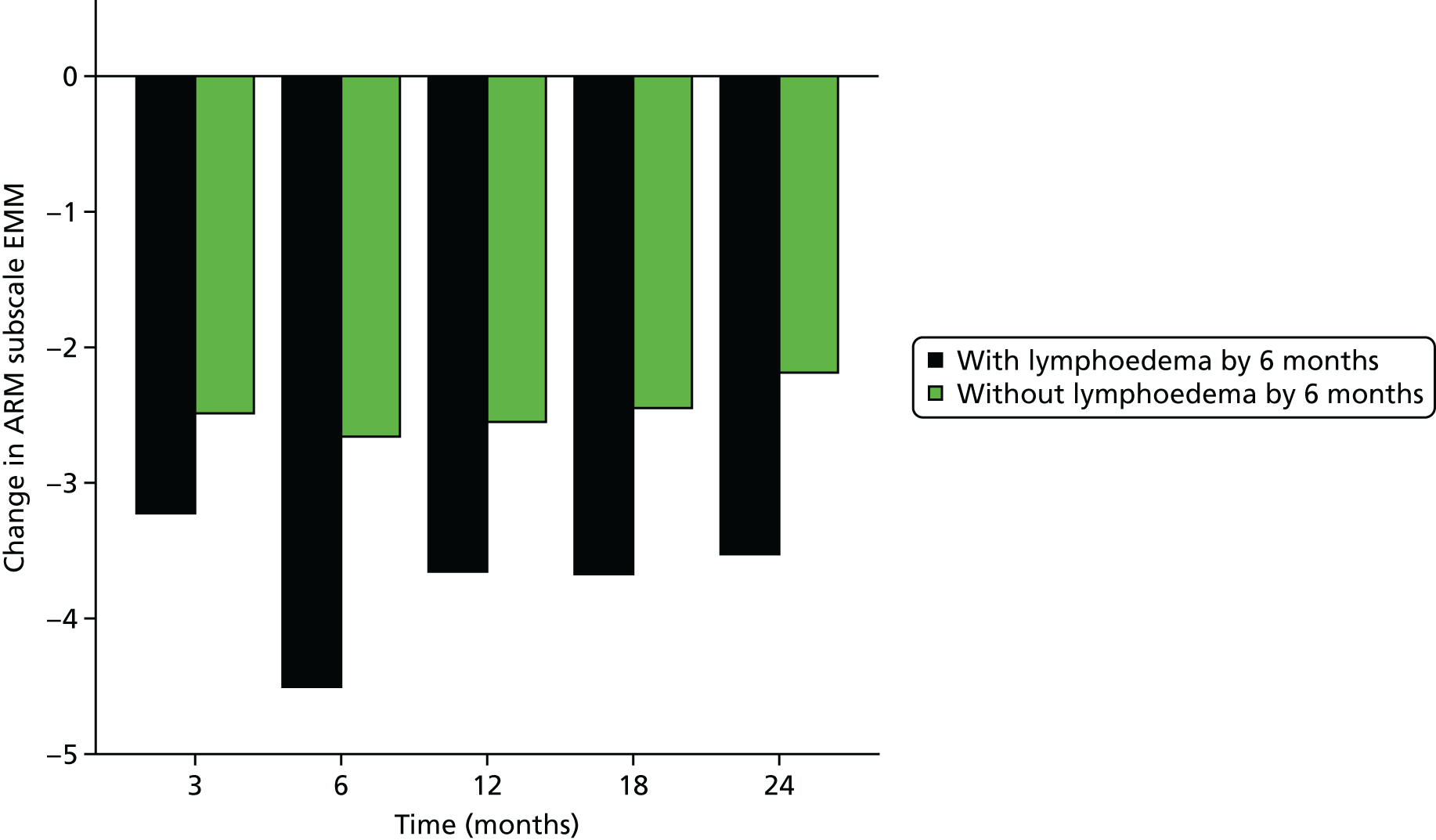
Clinical lymphoedema/appropriately applied sleeve
Trial Outcome Index
Owing to the negative skew of the TOI variable, a transformation [LN(120 – TOI)] was used for the analysis so that a linear GEE model could be used.
Time–lymphoedema status interaction
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time found that the interaction was not statistically significant (p = 0.58), showing the pattern of change over time was not significantly different between the groups.
The main effect for the time variable was statistically significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was statistically significant (p = 0.004), showing that there was a difference between the lymphoedema status groups overall.
The EMMs from the interaction term in the GEE analysis are presented below.
| Time point | Estimated marginal mean of TOI (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 861) | With lymphoedema by 6 months (n = 77) | ||
| Pre surgery | 68.5 (67.6 to 69.4) | 65.9 (62.5 to 69.1) | 0.14 |
| 3 months | 64.1 (63.1 to 65.2) | 58.5 (54.1 to 62.7) | 0.009 |
| 6 months | 66.0 (64.9 to 67.1) | 60.4 (56.3 to 64.2) | 0.005 |
| 12 months | 70.7 (69.8 to 71.7) | 65.7 (61.6 to 69.5) | 0.012 |
| 18 months | 71.1 (70.2 to 72.1) | 65.9 (62.1 to 69.4) | 0.005 |
| 24 months | 71.5 (70.5 to 72.5) | 66.9 (63.1 to 70.4) | 0.013 |
FIGURE 45.
Graph of TOI EMMs.

FIGURE 46.
Graph of change in TOI EMMs.
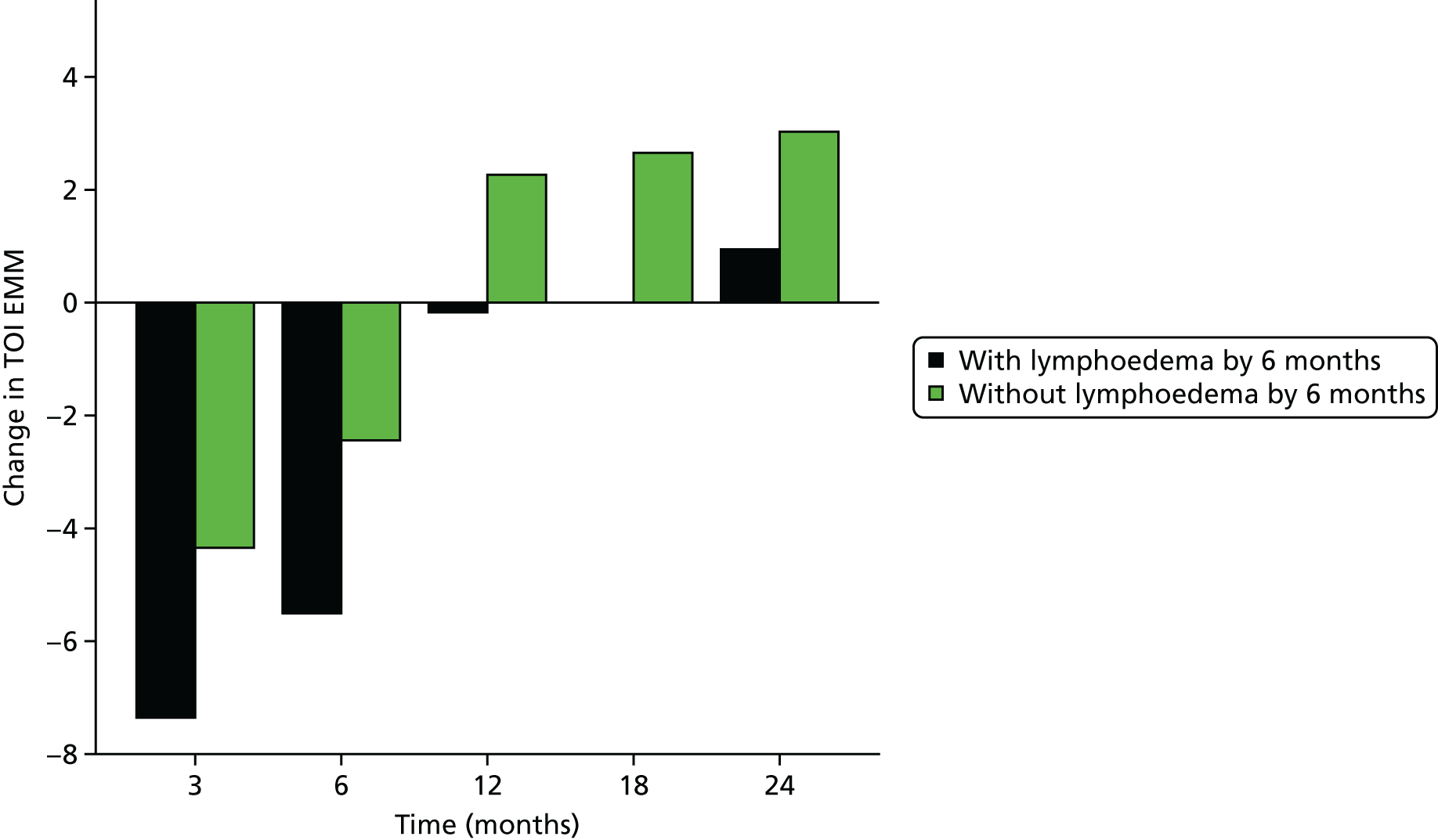
FACT-B total score
Owing to the negative skew of the FACT-B total score variable, a transformation [LN(160 – FACT-B)] was used for the analysis so that a linear GEE model could be used.
Time–lymphoedema status interaction
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time found that the interaction was not statistically significant (p = 0.37), showing the pattern of change over time was not significantly different between the groups.
The main effect for the time variable was statistically significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was statistically significant (p = 0.011), showing that there was a difference between the lymphoedema status groups overall.
The EMMs from the interaction term in the GEE analysis are presented below.
| Time point | Estimated marginal mean of FACT-B total score (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 860) | With lymphoedema by 6 months (n = 77) | ||
| Pre surgery | 111.1 (109.8 to 112.3) | 108.1 (103.1 to 112.7) | 0.23 |
| 3 months | 108.3 (106.9 to 109.7) | 100.4 (94.2 to 106.0) | 0.006 |
| 6 months | 110.2 (108.7 to 111.6) | 103.4 (97.3 to 109.0) | 0.020 |
| 12 months | 115.5 (114.2 to 116.8) | 109.7 (103.5 to 115.2) | 0.042 |
| 18 months | 115.9 (114.6 to 117.2) | 109.5 (104.1 to 114.4) | 0.011 |
| 24 months | 116.2 (114.8 to 117.6) | 110.1 (104.6 to 115.1) | 0.020 |
FIGURE 47.
Graph of FACT-B total score EMMs.
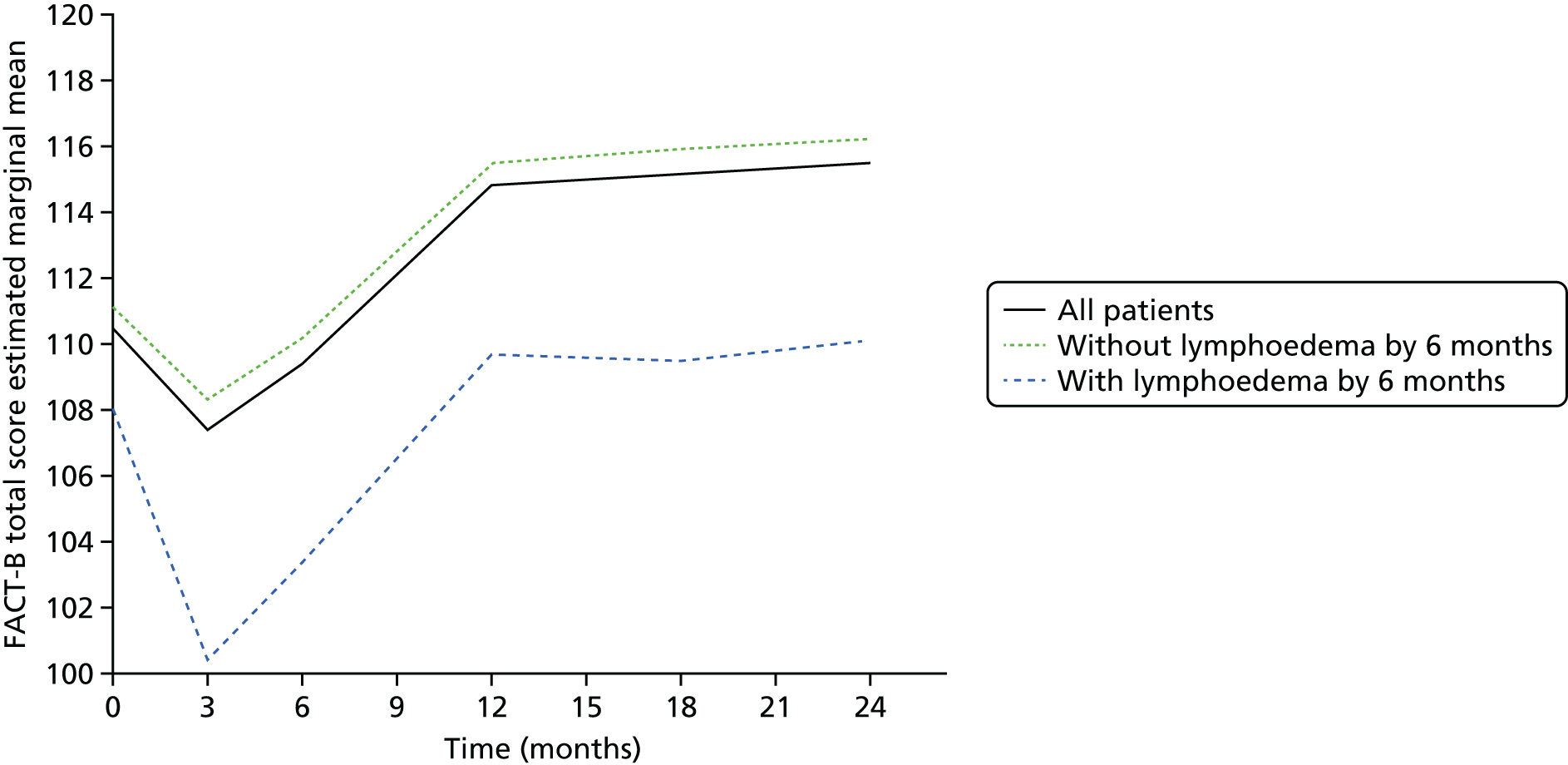
FIGURE 48.
Graph of change in FACT-B total score EMMs.
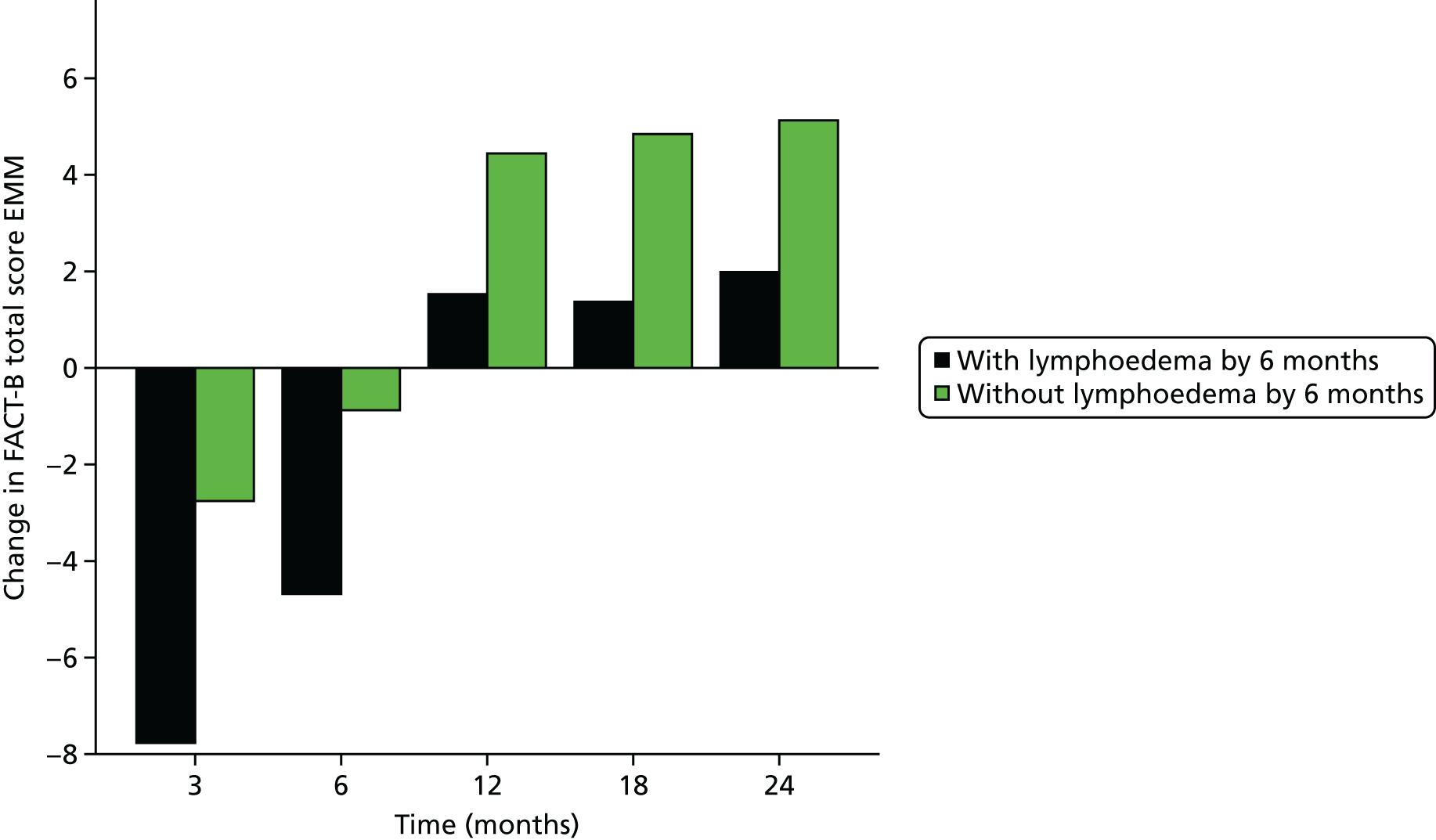
ARM subscale
Owing to the negative skew of the ARM subscale variable, a transformation [LN(22 – ARM)] was used for the analysis so that a linear GEE model could be used.
Time–lymphoedema status interaction
A GEE analysis that included an interaction term between lymphoedema status by 6 months and time found that the interaction was not statistically significant (p = 0.33), showing the pattern of change over time was not significantly different between the groups.
The main effect for the time variable was statistically significant (p < 0.001). The main effect for the lymphoedema status by 6 months variable was statistically significant (p < 0.001), showing that there was a difference between the lymphoedema status groups overall.
The EMMs from the interaction term in the GEE analysis are presented below.
| Time point | Estimated marginal mean of ARM subscale (95% CI) | p-value | |
|---|---|---|---|
| Without lymphoedema by 6 months (n = 859) | With lymphoedema by 6 months (n = 77) | ||
| Pre surgery | 18.7 (18.6 to 18.9) | 18.2 (17.4 to 18.9) | 0.12 |
| 3 months | 16.2 (16.0 to 16.5) | 14.9 (13.7 to 16.0) | 0.014 |
| 6 months | 16.1 (15.8 to 16.3) | 13.7 (12.6 to 14.6) | < 0.001 |
| 12 months | 16.2 (15.9 to 16.4) | 14.5 (13.5 to 15.4) | < 0.001 |
| 18 months | 16.3 (16.0 to 16.5) | 14.5 (13.3 to 15.5) | 0.001 |
| 24 months | 16.5 (16.3 to 16.8) | 14.6 (13.6 to 15.6) | < 0.001 |
FIGURE 49.
Graph of ARM subscale EMMs.
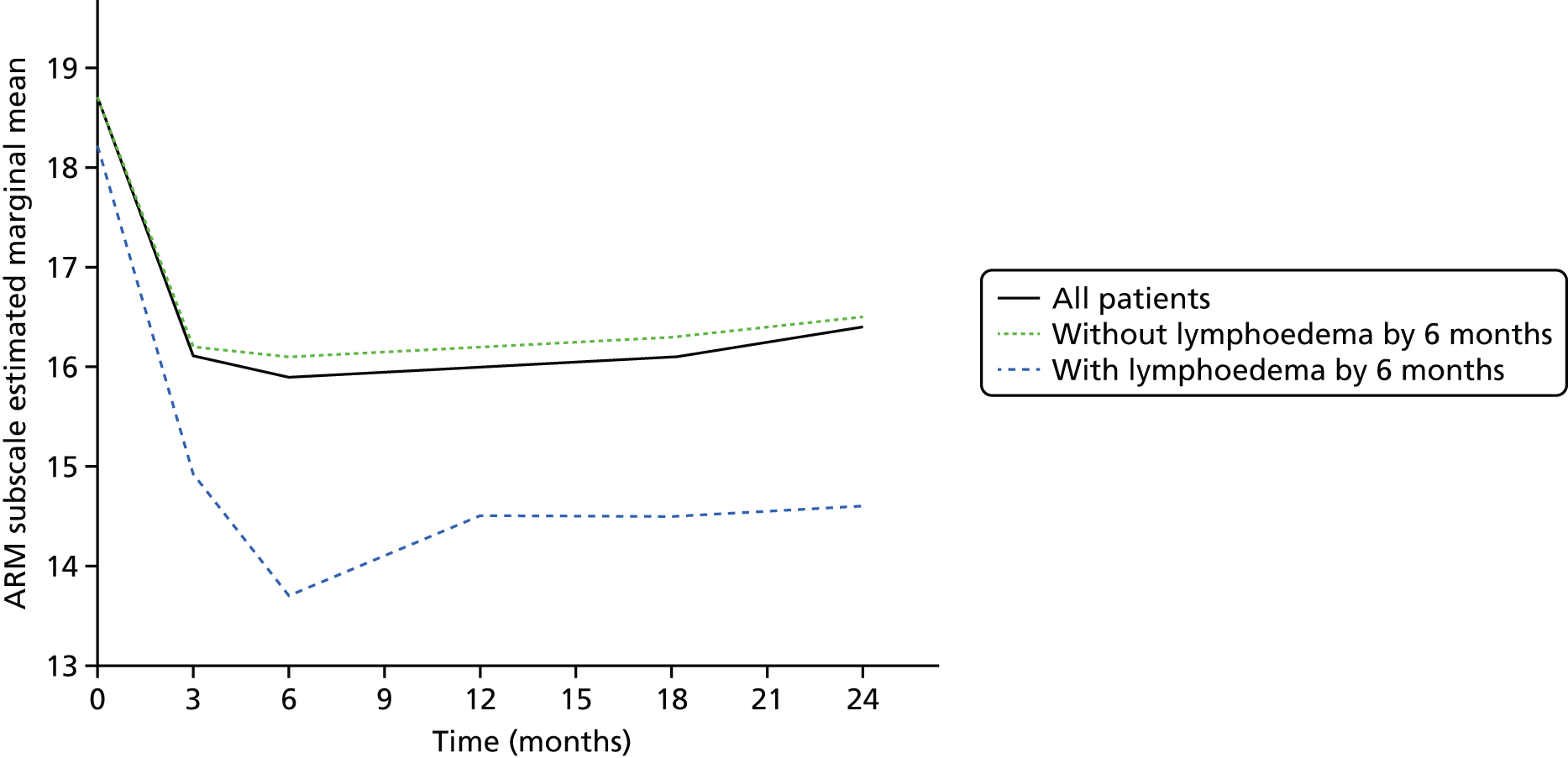
FIGURE 50.
Graph of change in ARM subscale EMMs.
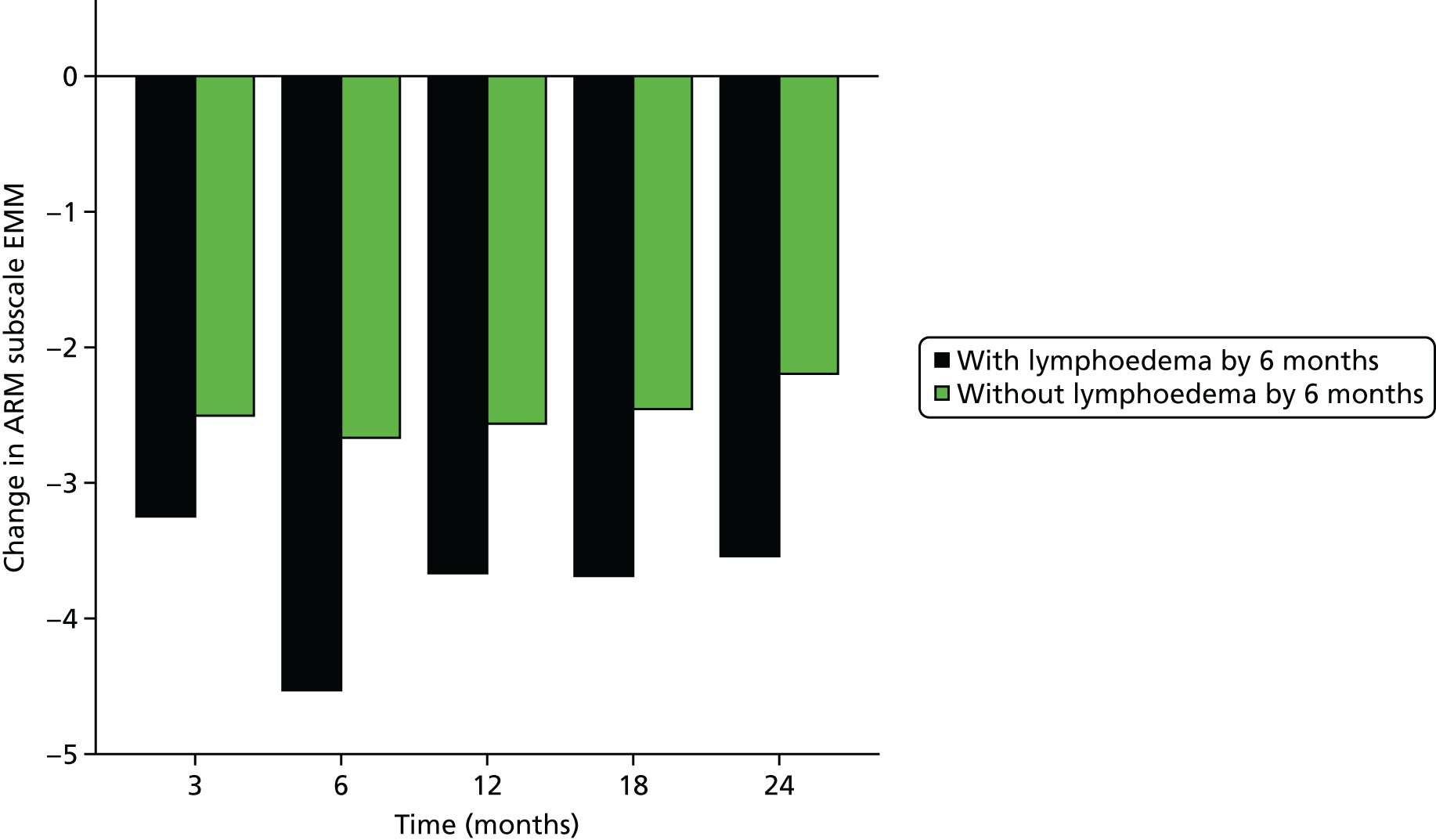
Associations between the 6-month lymphoedema checklist variables and changes in QoL from baseline and perometer/BIS from 1 month.
Appendix 17 Generalised estimating equations analysis
Analysis notes
Generalised estimating equations were used to assess the relationship between arm measurements (perometer and L-Dex) and TOI, the selected QoL measure.
Log-transformation
Owing to the negative skew of the TOI variable, a transformation [LN(120 – TOI)] was used throughout the analysis so that linear GEE models could be used.
Time variable
A time variable was included in each of the models and it was statistically significant in every analysis considered. It appears that, generally, there was an improvement in TOI over time from a minimum at the first time point considered, 3 months, to a maximum at the final time point considered, 24 months.
In a model only including the time variable, the estimated marginal means of TOI at each time point is presented in the table below.
| Time point (months) | Estimated marginal mean of TOI (95% CI) |
|---|---|
| 3 | 64.1 (62.8 to 65.4) |
| 6 | 66.0 (64.7 to 67.3) |
| 9 | 69.7 (68.4 to 70.9) |
| 12 | 70.5 (69.4 to 71.7) |
| 18 | 70.6 (69.4 to 71.8) |
| 24 | 71.0 (69.8 to 72.2) |
| After 6 months up to 24 months | No sleeve or clinical lymphoedema | Sleeve or clinical lymphoedema | Total |
|---|---|---|---|
| Perometer and BIS of < 10 | 537 (78%) | 52 (38%) | 589 |
| Perometer or BIS of ≥ 10 | 154 (22%) | 85 (62%) | 239 |
| Total | 691 | 137 | 828 |
Diagnostic accuracy for composite end points
Those patients with lymphoedema at 3 months are not included in the numbers in Table 98.
| Measure | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy |
|---|---|---|---|---|---|
| Perometer ≥ 10% | 17/48 (35%) | 732/776 (94%) | 17/61 (28%) | 732/763 (96%) | 749/824 (91%) |
| Perometer ≥ 9% | 22/48 (46%) | 723/776 (93%) | 22/75 (29%) | 723/749 (97%) | 745/824 (90%) |
| Perometer ≥ 5% | 31/48 (65%) | 631/776 (81%) | 31/176 (18%) | 631/648 (97%) | 662/824 (80%) |
| BIS ≥ 10 | 24/47 (57%) | 630/759 (83%) | 27/156 (17%) | 630/650 (97%) | 657/806 (82%) |
| B3 ≤ 2 | 28/39 (72%) | 556/703 (79%) | 28/175 (16%) | 556/567 (98%) | 584/742 (79%) |
| Perometer ≥ 5% or B3 ≤ 2 | 39/43 (91%) | 454/704 (64%) | 39/289 (13%) | 454/458 (99%) | 493/747 (66%) |
| Perometer ≥ 9% and B3 ≤ 2 | 16/45 (36%) | 765/789 (97%) | 16/40 (40%) | 765/794 (96%) | 781/834 (94%) |
| Perometer ≥ 10% and B3 ≤ 2 | 13/46 (28%) | 767/790 (97%) | 13/36 (36%) | 767/800 (96%) | 780/836 (93%) |
Those patients with lymphoedema up to 9 months are not included in the numbers in Table 99.
| Measure | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy |
|---|---|---|---|---|---|
| Perometer ≥ 10% | 14/41 (34%) | 649/666 (97%) | 14/31 (45%) | 649/676 (96%) | 663/707 (94%) |
| Perometer ≥ 9% | 15/41 (37%) | 639/666 (96%) | 15/42 (36%) | 639/665 (96%) | 654/707 (93%) |
| Perometer ≥ 5% | 28/41 (68%) | 588/666 (88%) | 28/106 (26%) | 588/601 (98%) | 616/707 (87%) |
| BIS ≥ 10 | 17/39 (44%) | 573/641 (89%) | 17/85 (20%) | 573/595 (96%) | 590/680 (87%) |
| B3 ≤ 2 | 19/35 (54%) | 484/585 (83%) | 19/120 (16%) | 484/500 (97%) | 503/620 (81%) |
| Perometer ≥ 5% or B3 ≤ 2 | 32/39 (82%) | 431/588 (73%) | 32/189 (17%) | 431/438 (98%) | 463/627 (74%) |
| Perometer ≥ 9% and B3 ≤ 2 | 8/38 (21%) | 660/671 (98%) | 8/19 (42%) | 660/690 (96%) | 668/709 (94%) |
| Perometer ≥ 10% and B3 ≤ 2 | 7/38 (18%) | 664/671 (99%) | 7/14 (50%) | 664/695 (96%) | 671/709 (95%) |
Those patients with lymphoedema up to 18 months are not included in the numbers in Table 100.
| Measure | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy |
|---|---|---|---|---|---|
| Perometer ≥ 10% | 10/24 (42%) | 511/530 (96%) | 10/29 (34%) | 511/525 (97%) | 521/554 (94%) |
| Perometer ≥ 9% | 12/24 (50%) | 507/530 (96%) | 12/35 (34%) | 507/519 (98%) | 519/554 (94%) |
| Perometer ≥ 5% | 17/24 (71%) | 454/530 (86%) | 17/93 (18%) | 454/461 (98%) | 471/554 (85%) |
| BIS of ≥ 10 | 10/23 (43%) | 447/491 (91%) | 10/54 (19%) | 447/460 (97%) | 457/514 (89%) |
| B3 ≤ 2 | 8/21 (38%) | 404/467 (87%) | 8/71 (11%) | 404/417 (97%) | 412/488 (84%) |
| Perometer ≥ 5% or B3 ≤ 2 | 20/24 (83%) | 343/470 (73%) | 20/147 (14%) | 343/347 (99%) | 363/494 (73%) |
| Perometer ≥ 9% and B3 ≤ 2 | 4/21 (19%) | 522/531 (98%) | 4/13 (31%) | 522/539 (97%) | 526/552 (95%) |
| Perometer ≥ 10% and B3 ≤ 2 | 3/21 (14%) | 524/532 (98%) | 3/11 (27%) | 524/542 (97%) | 527/553 (95%) |
Appendix 18 Workstream 3
FIGURE 51.
Workstream 3: early intervention after ANC to prevent chronic lymphoedema.
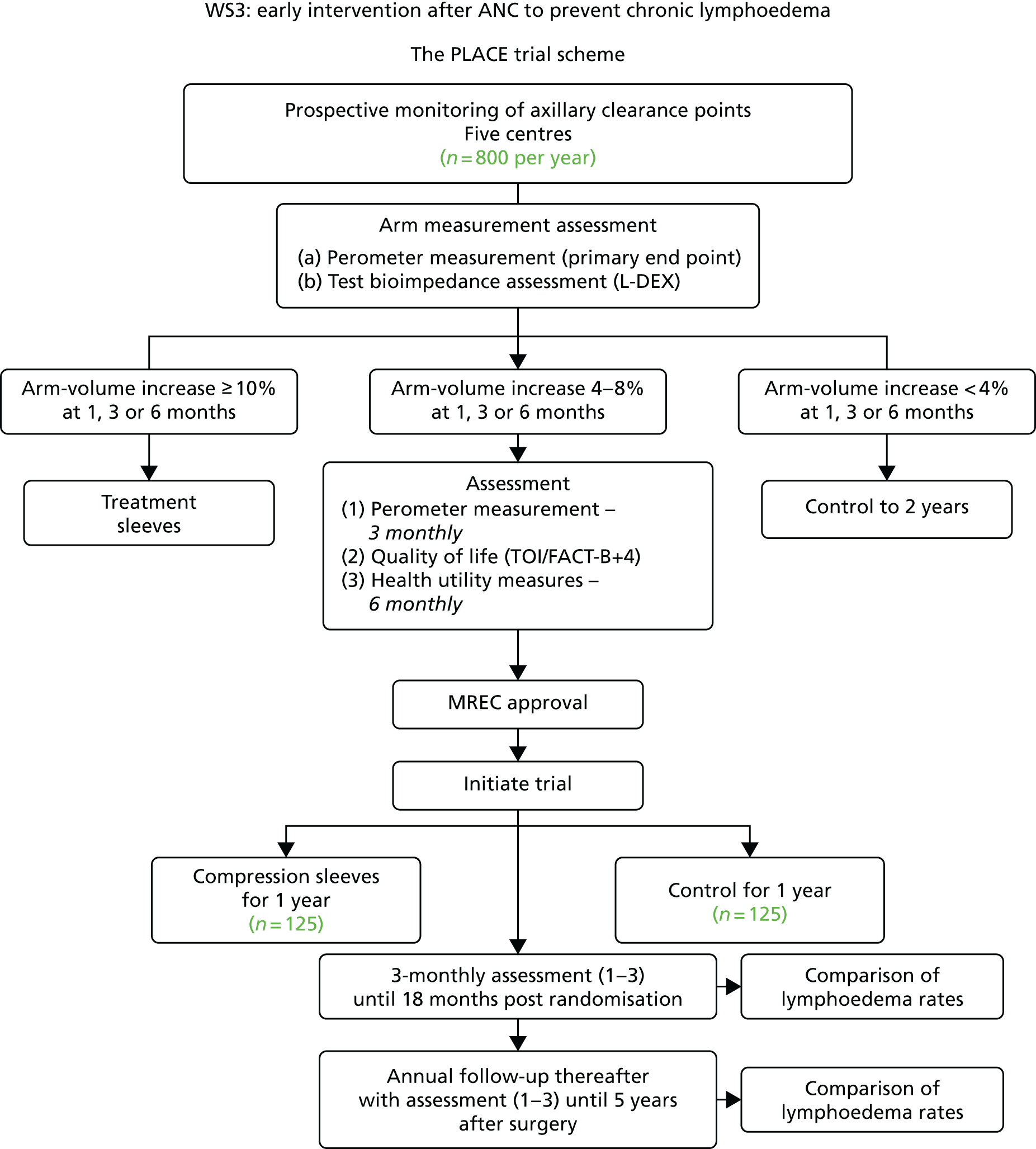
Appendix 19 Findings of the PLACE trial’s qualitative substudy
Introduction
Low recruitment to RCTs can affect internal and external validity, statistical power and successful completion of trials. 50 A recent systematic review of recruitment activity highlighted how common recruitment problems are in health-care RCTs. Currently, 50% of RCTs fail to recruit to target and only 50% of those that successfully recruit do so in a timely manner. 51
Maximising and encouraging more patients to take part in health research is one of the main aims of the National Institute for Health Research. 52 In addition, a key recommendation of the government’s Life Science Strategy is for health-care organisations to provide adequate support to researchers who are recruiting to studies. 53 Although recruitment failure in RCTs has been extensively studied, there remains a lack of substantive research on initiatives that could improve the recruitment activity of research practitioners to improve patient recruitment.
Successful recruitment into trials generally focuses on the patient/recruiter encounter where the impact of communication skills has an important influence on patients’ decision-making processes. 54 Research has highlighted, however, that information conveyed during recruitment varies considerably in quality and content, and that there is little research on actual recruitment encounters. 50 Findings from a qualitative study55 that interviewed recruiting practitioners across six RCTs found that recruiting staff struggled with explaining the rationale for RCTs to patients; willingness to approach all eligible patients; providing accurate information about the trial; and confidence in eliciting patient preferences and exploring underlying reasons for preferences. Another study56 found that patient preferences could change after discussions with the recruiting practitioner. Other research57 has highlighted the importance of shared decision-making between patients and recruiters in obtaining informed consent that is free from coercion.
Predominantly, nurses with a clinical care background are employed as recruiting practitioners within the NHS. However, a number of studies of staff barriers to trial recruitment found that nurses tend to experience conflict between three distinct roles: caring clinical nurse, patient advocate and recruiter/scientist. This conflict leads to discomfort when approaching eligible patients judged as ‘too unwell’ or ‘at the wrong stage of treatment’ to participate in a RCT, such as during chemotherapy. 55,58 These studies also found that problematic recruiter behaviour during encounters, such as interruption, digression, inaccurate information-giving, and inattentiveness, made it difficult for patients’ voices to be heard.
A survey and workshop on interventions to improve recruitment activity into RCTs recommended training recruiter practitioners in generic communication skills and trial-specific skills, such as explaining randomisation and dealing with patient preferences. 59 A recent systematic review60 of training programmes for recruiters suggested that training programmes can improve practitioner recruiters’ self-confidence and communication of some key RCT concepts to patients.
Research aim
Explore staff recruitment practices and shared decision-making during recruitment to the PLACE UK multisite RCT.
Methods
This qualitative study was conducted alongside the UK nationwide PLACE trial. Data were collected between September 2015 and May 2016 from six hospital recruitment sites. Initially, purposive sampling of key recruiting staff was undertaken. All 16 recruiting staff took part in either a focus group (n = 8) or a face-to-face interview (n = 15). The job titles of recruiting staff were senior research nurse (n = 5), research nurse (n = 7) and research practitioner/health-care assistant (n = 4). Open-ended interviews were conducted using a topic guide. The topic guide covered the following areas: staff attitudes to recruitment issues, recruitment procedures, research and study knowledge and information sharing, communication with patients, organisational barriers to recruitment, staff support and research training. Interviews were conducted in a private room at their place of work and lasted between 40 and 70 minutes. All interviews were audio recorded with permission from participants; written informed consent was obtained prior to the start of any discussion. A PPI group (n = 8) was established at the start of the project, which convened quarterly to inform aspects of the study, such as the design of information and interview guides, and to discuss and refine emerging findings from the interviews.
Interviews were transcribed verbatim and NVivo version 10 software (QSR International, Warrington, UK) was used to facilitate analysis. Data were analysed thematically using a framework analysis approach. 61 Analysis of data involved a five-stage process: (1) familiarisation, (2) identifying a thematic framework, (3) indexing, (4) charting and (5) mapping and interpretation. An iterative and inductive approach to analysis was followed so that analysis started alongside data collection and themes and issues identified and informed further questions and probing. Memos and documents were written about emerging categories, to summarise a point, to critique information, and to relate emergent theories to existing literature. All authors met on a regular basis to discuss the development of codes, themes, categories and theories about the phenomenon being studied.
Results
Role of the PLACE trial recruitment practitioners
Health-care professionals with a background in nursing (n = 12), as well as non-clinical staff (n = 4), were employed from established breast cancer research centres (n = 5) to identify patients who fulfilled the inclusion criteria for the PLACE trial. At post-surgery follow-up checks at 1, 3, 6 and 9 months, recruitment practitioners would conduct arm measurements of patients and compare any increase swelling with baseline data.
Four key themes were identified from the focus group and interviews that reflected the main reasons why recruitment rates were low. These were (1) wait and see culture, (2) conflicting roles, (3) misunderstanding the trial arms and (4) paternalism versus shared decision-making.
Wait and see culture
It became apparent that all recruiters held variable interpretations of who was eligible for the trial. Although a detailed protocol had been developed to help identify eligible patients, the majority of sites varied in their consensus of the way patient’s eligibility status was decided. The majority of sites (4 out of 5) never recruited eligible patients at the first 1-month follow-up; usually staff considered that any swelling was mainly due to surgery. Interestingly, recruiters further described that they would provide the standard management arm of the study and defer recruitment into the RCT:
So if a patient has 6% increase at month 1 follow-up, that is not indicative of anything yet, she might have had sermonas or any swellings or infections in the breasts. We’re not worried with a 6% increase. We reiterate about taking care of the arm, we do skin care, we do massage and send them on their merry way.
Clinical nurse
At 1 month we’d tell the patient it’s only been 4 weeks or 6 weeks since your surgery so there might be still post-op swelling, so we leave it at that. But we do say you can do arm exercises that they have been shown by the physio as well. We give people a bit of information from the PLACE [trial] information exercise sheet at the same time as giving good skin care tips, and say see you in 2 months’ time.
Clinical nurse
When you measure patients and there has been quite a jump, you know, from their previous measurements and it is just simply to do with the fact that they have been overusing their arm. So it is put down to that and so patients are more than happy to watch and wait. I don’t know if that, kind of, stops them I suppose from getting on the trial.
Research practitioner
Conflicting roles
The majority of recruiting staff employed within the five PLACE trial sites were nurses (n = 12). All emphasised their dual roles as a clinician/patient advocate and recruiter and reflected on the conflict between the role of health professional, protecting the needs and vulnerability of their patients, and role of recruiter for a clinical trial. This role conflict was experienced by nurses during subsequent recruitment encounters beyond the 1-month follow-up:
If they were over 4% but very fretful I wouldn’t give a PLACE [trial] information sheet and I would let them concentrate on trying to recover from the cancer surgery. But if there’s no good reason that that swelling is there, I would possibly give a PLACE [trial] information sheet just as some background information. Keep it very easy, very light, I’ll just give you this and the next time you come in if you’re over the size again you might want to think about this RCT.
Clinical nurse
I’ve noticed that some patients might start radiotherapy around the 3-month follow-up appointment and, they’ll have the radiotherapy on the same side as well, sometimes that doesn’t help with the measurements, they can’t get their arm in position due to restrictions. If they’ve started their radiotherapy they’re going to be in and out every day, they might not want to come down for follow-up appointments again because they’re quite tired, then the swelling might not go down due to the skin reactions in that area of the body as well. So I think that’s a little bit of a tricky point to recruit also.
Clinical nurse
Sometimes people if they had a high measurement at 3 months, we’d sort of say, look . . . you’re due to see me again in 3 months. If it’s still up then I can recruit you then? I sort of explain that as well, because I think sometimes people don’t want to do anything else. There is enough going on when they’re at 3 months post surgery. Sometimes it’s nice to reassure people that, we are due to see them again at 6 months and 9 months.
Research practitioner
At any stage if a patient seems tearful and a little anxious, that has to be brought into the mix as well, because if you introduce something new to a patient at this point when they’ve actually gotten better then they might think, oh, well, I’ve improved so why are you wanting me to possibly start wearing a sleeve. If I saw a 6% increase at 1 month I might say it will probably settle down, but we’ll have a look at you next time. If I then saw a 4.1% increase from baseline the next time I would then think well, she’s doing well, is there any signs of it [lymphoedema], is she coping well, and I’d kind of leave it, like everyone else, and wait.
Clinical nurse
The ‘wait and see’ nursing culture that was evident across multiple sites became part the way of working for all recruiters regardless of their clinical or non-clinical background. Recruitment teams (generally headed by more senior nurses) followed unwritten procedures embedded in their recruitment teams’ assessment of trial eligibility. The extracts below from research practitioners indicate the powerful way embedded nursing culture affected recruitment rates:
I don’t know about other sites but here it’s not necessarily about recruiting to [the] PLACE [trial]. I’m not saying that, that’s not what people are thinking about, but caring for the patient is more important than recruiting to [the] PLACE [trial]. Here if a lady is looking like they are eligible we tend to give them the information leaflets about standard care and say we will see you next time and see how you are getting on. So sometimes it feels a bit like we’re making them ineligible for [the] PLACE [trial] by making them better, but that’s the paradox of it really. Because of course most of the people I work with, they’re nurses and that’s what they do.
Research practitioner
My view here has been very much that I’m the new person on the team recruiting patients in to the RCT and what will happen is what has always happened. As decided by the people who’ve been here for an awful lot longer than I have and there isn’t a great deal for manoeuvre on that, especially because they are nurses and I am a mere non-nurse.
Research practitioner
Owing to the ‘wait and see’ culture, as well as the role conflict experienced by recruiters who were reluctant to approach vulnerable patients, for example during chemotherapy treatment, some patients were missed during the whole of their eligibility time period. In addition, if patients presented with an arm swelling towards the upper limit of eligibility for the PLACE trial, recruiters would in some cases make a clinical judgement to refer the patient directly to the lymphoedema service, even if the patient was eligible for the study sleeve:
When we had up to 2 years to recruit it was a little bit easier because you had more time to get patients into the study. Now it’s quite a short little burst that we have, so I think it’s probably a little bit too short now because people will only have finished their chemotherapy and radiotherapy in 9 months. I think if it was maybe open to recruitment until 12 months, recruitment rates might improve.
Clinical nurse
At 6 months or even at the 9-month visit if patients swellings have been steadily increasing towards the upper limit of PLACE [trial] eligibility some patients may want something done about it and they don’t really want to go into the study as there is only a 50% chance of them getting a sleeve, they just want an assessment with the lymphoedema service. That’s what I found anyway, we always do have to say that there’s always the referral to the lymphoedema service, because it is part of patient care too. So we can’t just say the only solution for you is the study because it’s not right to do that.
Research practitioner
Misunderstanding the trial arms and equipoise
A common problem among recruiters was that they misunderstood the trial arms of the study. This generally led to recruiters explaining the RCT incorrectly to patients and/or presenting the trial arms in a positive or negative way. Patients’ views and decisions about participating in clinical trials are greatly influenced by how information is presented to them during the recruitment encounter. A number of recruiters inadvertently explained the randomisation process to patients as a ‘fifty–fifty chance of getting the sleeve’, which may have had a detrimental effect on their decision to take part, especially if patients felt that their treatment preferences would not be met. Other recruiters encouraged patients to follow incorrect procedures, for example wearing the compression garment/sleeve day and night:
I think it is very evident, like, it’s that the numbers are lagging and to be honest it is a little bit, kind of, it’s not baffling though to be honest. You know, so if the study was presented to me, if I had an increase in my arm swelling and they give me the option of say fifty, fifty of getting the sleeve or not I personally would want something done about it. So I would take the lymphedema referral. So yeah I get why people haven’t gone on to the study.
Research practitioner
We tell them to wear it all day every day especially day time when doing activity stuff. One patient said she found it uncomfortable wearing it at night so she takes it off. But then she noticed that in the mornings her arm was very swollen and it takes time to put it back on. So I said, if the sleeve isn’t that uncomfortable for you at night time, wear it. I think the only time patients take it off is to wash it.
Research practitioner
So I usually explain that it’s a 50/50 chance of getting either the controller where it’s just arm exercises we give you to do or the computer randomises you to have the sleeve.
Research practitioner
Paternalism versus shared decision-making
Recruiter practitioners involved in the PLACE trial made assumptions that taking part in the RCT may be burdensome, intrusive or not beneficial to individual patients who were deemed by some recruiters as being vulnerable:
I suppose, because they are going through chemo, they are quite . . . you know, they’ve got a lot going on in their lives. You might not want to . . . after discussing about somebody’s life outside surgery, they’re having a tough time then, it might be the last thing you want to do is recruit them into something. It’s our choice it’s about clinical care as well as just the research.
Clinical nurse
There’s two arms to the study, but I think the prospect of patients potentially having to wear a sleeve when potentially there isn’t a benefit for patients, I think that’s one of the things that patients pick up on and realise that and I think especially in some of the younger women, we have approached for [the] PLACE [trial], it’s not necessarily a nice thing to potentially have to wear.
Research practitioner
I think we get a few occasions where patients are quite visibly put off by the fact of having to wear a sleeve, I don’t think that’s a very nice prospect for patients in the first instance especially as, you know, we’re talking to patients, you know, maybe 6 months, 9 months down the line where they’re maybe coming to the end of their treatment, they may have just done the chemotherapy, radiotherapy and then we’re coming back to them and saying actually, we think, potentially there could be some early signs of lymphoedema there and we’re saying that, you know, normally patients wouldn’t necessarily have this treatment, we don’t know if there’s a benefit or not, but we would like to see if there would be a benefit.
Research practitioner
Conclusion
As highlighted earlier, assumptions made by recruitment staff that taking part in the RCT may be burdensome for patients had a significant impact on recruitment behaviour, which, in turn, led to poor recruitment rates. During recruitment encounters, staff acted as gatekeepers by suggesting taking part in the PLACE trial only to those patients who were deemed suitable for the trial, rather than to all patients who met the inclusion eligibility criteria. Making a clinical judgement not to recruit patients in this way is perceived as paternalistic. For example, the PLACE trial recruiters were making decisions on their patients’ behalf with the view that as clinicians they knew what was best for patients. Certain recruiters generally described that their focus was on protecting and caring for patients’ needs rather than sharing knowledge and information about the RCT.
Future research
To develop and test a person-centred communication skills training intervention for clinical research nurses and research practitioners recruiting patients into RCTs. Although extensive research exists that explores barriers to patient participation in clinical trials,62 less work has been untaken that investigates initiatives that could improve the recruiters’ encounters with potential participants. The drive to deliver person-centred care across the NHS has been significantly promoted over the past decade as a means of enhancing the quality of care experienced by patients in everyday clinical settings. However, trial recruitment interactions have been largely neglected and little attention has been given to how the concept of person-centred care is relevant to improving trial recruitment. Within the model of person-centred care, person-centred communication involves providing room for the patient’s story, exploring emotional cues and showing empathy, providing information and advice, explaining things clearly, and involving patients in shared decision-making. Training research practitioners in how to manage these encounters in a more person-centred way has the potential to help recruiters improve their communication skills, increase recruitment rates and collaborate with patients as partners in the development of evidence-based research.
Appendix 20 Consolidated Standards of Reporting Trials flow diagram (from the 1100 multifrequency bioimpedance cohort) based on the data set used in the analyses of workstream 2
FIGURE 52.
Consolidated Standards of Reporting Trials flow diagram. Note that 143 participants were randomised in the PLACE trial (69 to compression sleeves, 74 to control) from the full BEA cohort.
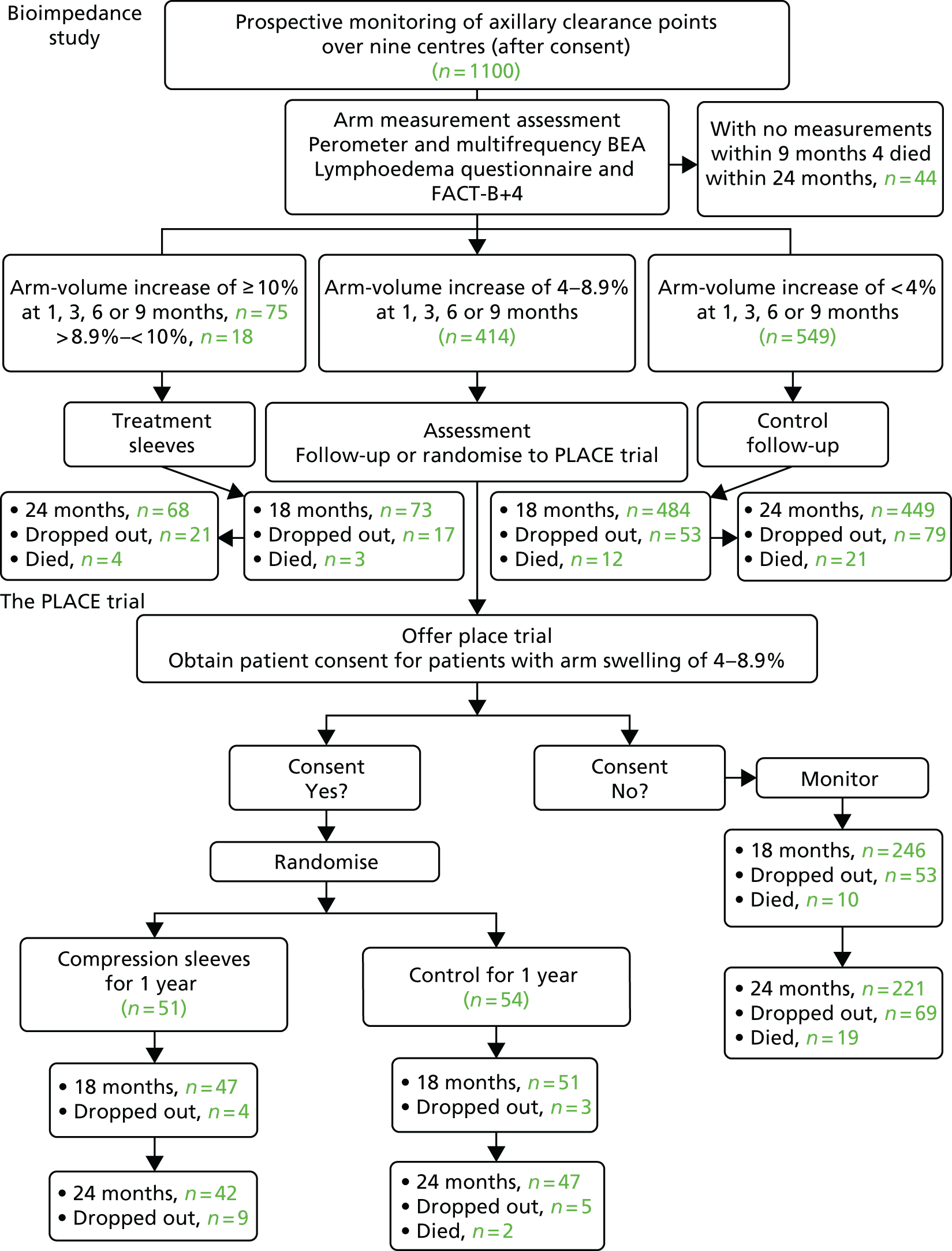
Information from the 600 with final follow-up at 60 months estimated using final perometer measurements:
-
dropout by 24 months – 63, of which 28 had happened by 18 months
-
deaths by 24 months – 50, of which 28 had happened by 18 months (a further 55 patients died after 24 months).
Appendix 21 Site contact list for Programme Grants for Applied Research studies
| Site name | Site type | Site address |
|---|---|---|
| South Manchester |
Sponsor site BEA, PLACE and PLACE qualitative |
Nightingale and Genesis Prevention Centre, Wythenshawe Hospital, Manchester, UK |
| Pennine | BEA, PLACE and PLACE qualitative | The Pennine Acute Hospitals NHS Trust, Oncology Research, North Manchester General Hospital, Trust Headquarters, Manchester, UK |
| North Staffordshire | BEA and PLACE | University Hospital of North Staffordshire NHS Trust, The Cancer Centre, City General Site, Stoke-on-Trent, UK |
| Derby | BEA, PLACE and PLACE qualitative | Derby Hospitals NHS Foundation Trust, Royal Derby Hospital, Nightingale Macmillan Unit, Derby, UK |
| Guy’s | BEA, PLACE and PLACE qualitative | Guy’s & St Thomas’ NHS Foundation Trust, Guy’s Hospital, London, UK |
| Bournemouth | BEA and PLACE | Royal Bournemouth & Christchurch Hospitals NHS Foundation Trust, The Royal Bournemouth Hospital, Bournemouth, UK |
| Poole | BEA and PLACE | Poole Hospital NHS Foundation Trust, Oncology Research, Poole, UK |
| Wolverhampton | BEA and PLACE | The Royal Wolverhampton Hospitals NHS Trust, New Cross Hospital, The McHale Building, New Cross Hospital, Wolverhampton, UK |
| Stockport | BEA screening site | Stepping Hill Hospital, Room Stepping Hill Hospital, Stockport, UK |
| Mansfield | PLACE | King’s Mill Hospital, Sutton in Ashfield, UK |
| Hull | PLACE | Castle Hill Hospital, Breast Care Unit, Cottingham, UK |
| Swansea | PLACE | Singleton Hospital, Swansea, UK |
| Macclesfield | PLACE and PLACE qualitative | Macclesfield District General Hospital, Cancer Resource Centre, Macclesfield, UK |
| Peterborough | PLACE | Peterborough and Stamford Hospitals NHS Foundation Trust, Peterborough City Hospital, Peterborough, UK |
| Dudley | PLACE | Russel Hall Hospital, Dudley, UK |
| Wigan | PLACE | Royal Albert Edward Infirmary, Wigan, UK |
| London | PLACE | Homerton University Hospital, London, UK |
| Nuneaton | PLACE | George Eliot Hospital NHS Trust, Research & Development Office, Nuneaton, UK |
Appendix 22 Authors’ publications that underpin/emanate from the Programme Grant for Applied Research funding
Professor Nigel Bundred
Book chapters
Turner LE, Bundred NJ. Treatment of Menopausal Symptoms in Women with Breast Cancer. In Robert Leonard, Andreas Polychronis, Andrew Miles, editors. The Effective Management of Breast Cancer. 3rd edn. London: Aesculapius Medical Press; 2004. Chapter 21.
Review articles
Holland P, Bundred NJ. Ductal carcinoma in situ. Surgery 1994;12:55–7.
Hadjiloucas I, Bundred NJ. Axillary surgery: is it necessary? The Breast 2000;9:1–3.
Chan KC, Bundred NJ. Chapter 16 Classification and Staging of Breast Cancer: Operable, Locally Advanced and Metastatic. In JM Dixon and V Sacchini, editors. Breast Cancer Diagnosis and Management. London: Elsevier; 2000.
Bundred NJ. Is radiotherapy necessary for all women with DCIS? Oncology Times 2005;1:3.
Original refereed papers in academic journals
(Overall H Index 48, cited over 8405 times.)
To 2000
Hawkins RA, White G, Bundred NJ, Dixon JM, Miller WR, Stewart HJ, Forrest AP. Prognostic significance of oestrogen and progestogen receptor activities in breast cancer. Br J Surg 1987;74:1009–13.
Bundred NJ, Dover MS, Coley S, Morrison JM. Breast abscesses and cigarette smoking. Br J Surg 1992;79:58–9.
Walls J, Boggis CR, Wilson M, Asbury DL, Roberts JV, Bundred NJ, Mansel RE. Treatment of the axilla in patients with screen-detected breast cancer. Br J Surg 1993;80:436–8.
Harding C, Knox WF, Faragher EB, Baildam A, Bundred NJ. Hormone replacement therapy and tumour grade in breast cancer: prospective study in screening unit. BMJ 1996;312:1646–7.
Holland PA, Walls J, Boggis CR, Knox F, Baildam AD, Bundred NJ. A comparison of axillary node status between cancers detected at the prevalence and first incidence breast screening rounds. Br J Cancer 1996;74:1643–6.
Holland PA, Gandhi A, Knox WF, Wilson M, Baildam AD, Bundred NJ. The importance of complete excision in the prevention of local recurrence of ductal carcinoma in situ. Br J Cancer 1998;77:110–14.
Abdullah TI, Iddon J, Barr L, Baildam AD, Bundred NJ. Prospective randomised controlled trial to determine the value of preservation of the intercostobrachial nerve during axillary node clearance for breast cancer. Br J Surg 1998;85:1443–5.
Bundred N, Maguire P, Reynolds J, Grimshaw J, Morris J, Thomson L, et al. Randomised controlled trial of effects of early discharge after surgery for breast cancer. BMJ 1998;317:1275–9.
Hargreaves DF, Knox F, Swindell R, Potten CS, Bundred NJ. Epithelial proliferation and hormone receptor status in the normal post-menopausal breast and the effects of hormone replacement therapy. Br J Cancer 1998;78:945–9.
Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab 1999;84:4017–24. https://doi.org/10.1210/jcem.84.11.6152
2000–10
Chan KC, Knox WF, Sinha G, Gandhi A, Barr L, Baildam AD, Bundred NJ. Extent of excision margin width required in breast conserving surgery for ductal carcinoma in situ. Cancer 2001;91:9–16.
Bundred NJ. Hormone replacement therapy, breast cancer and the future. J Br Menopause Soc 2001;7:13–15.
Bundred NJ. Prognostic and predictive factors in breast cancer. Cancer Treat Rev 2001;27:137–42. https://doi.org/10.1053/ctrv.2000.0207
Dey P, Bundred N, Gibbs A, Hopwood P, Baildam A, Boggis C, et al. Costs and benefits of a one stop clinic compared with a dedicated breast clinic: randomised controlled trial. BMJ 2002;324:507.
Robertson JF, Nicholson RI, Bundred NJ, Anderson E, Rayter Z, Dowsett M, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res 2001;61:6739–46.
Dowsett M, Bundred NJ, Decensi A, Sainsbury RC, Lu Y, Hills M, et al. Effect of Raloxifene on breast cancer cell Ki67 and apoptosis: a double blind, placebo-controlled, randomised clinical trial in postmenopausal patients. Cancer Epidemiol Biomarkers Prevent 2001;10:961–9.
Chan KC, Knox WF, Gee JM, Morris J, Nicholson RI, Potten CS, Bundred NJ. Effect of epidermal growth factor receptor tyrosine kinase inhibition on epithelial proliferation in normal and premalignant breast. Cancer Res 2002;62:122–8.
Wärnberg F, Bundred N. Will early detection of non-axillary sentinel nodes affect treatment decisions? Br J Cancer 2002;87:691–3. https://doi.org/10.1038/sj.bjc.6600557
Bundred NJ, Anderson E, Nicholson RI, Dowsett M, Dixon M, Robertson JF. Fulvestrant, an estrogen receptor downregulator, reduces cell turnover index more effectively than tamoxifen. Anticancer Res 2002;22:2317–19.
Freeman SR, Washington SJ, Pritchard T, Barr L, Baildam AD, Bundred NJ. Long term results of a randomised prospective study of preservation of the intercostobrachial nerve. Eur J Surg Oncol 2003;29:213–15.
Boland GP, McKeown A, Chan KC, Prasad R, Knox WF, Bundred NJ. Biological response to hormonal manipulation in oestrogen receptor positive ductal carcinoma in situ of the breast. Br J Cancer 2003;89:277–83. https://doi.org/10.1038/sj.bjc.6601013
Prasad R, Boland GP, Cramer A, Anderson E, Knox WF, Bundred NJ. Short-term biologic response to withdrawal of hormone replacement therapy in patients with invasive breast carcinoma. Cancer 2003;98:2539–46. https://doi.org/10.1002/cncr.11836
Warnberg F, Bundred NJ. The relationship between oestrogen and breast cancer. J RC Physc Edinburgh 2004;34:25–31.
Bundred NJ, Turner LE. Postmenopausal hormone therapy before and after breast cancer: clinical experiences. Maturitas 2004;49:S22–31. https://doi.org/10.1016/j.maturitas.2004.06.021
Barnes NL, Boland GP, Davenport A, Knox WF, Bundred NJ. Relationship between hormone receptor status and tumour size, grade and comedo necrosis in ductal carcinoma in situ. Br J Surg 2005;92:429–34. https://doi.org/10.1002/bjs.4878
Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, et al. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer 2006;95:1410–14.
Howell A, Clarke RB, Evans G, Bundred N, Cuzick J, Santen R, Allred C. Estrogen deprivation for breast cancer prevention. Recent Results Cancer Res 2007;174:151–67.
Lavelle K, Todd C, Moran A, Howell A, Bundred N, Campbell M. Non-standard management of breast cancer increases with age in the UK: a population based cohort of women > or = 65 years. Br J Cancer 2007;96:1197–203.
Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209–15. https://doi.org/10.1002/bjs.5834
2011
Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12:21–9. https://doi.org/10.1016/S1470-2045(10)70266-7
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84. https://doi.org/10.1016/S0140-6736(11)60993-8
Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al. ; AZURE Investigators. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011;365:1396–405.
2012
Barnes NL, Ooi JL, Yarnold JR, Bundred NJ. Ductal carcinoma in situ of the breast. BMJ 2012;344:e797. https://doi.org/10.1136/bmj.e797
Hadji P, Gnant M, Body JJ, Bundred NJ, Brufsky A, Coleman RE, et al. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer Treat Rev 2012;38:798–806. https://doi.org/10.1016/j.ctrv.2012.02.008
2013
Farnie G, Willan PM, Clarke RB, Bundred NJ. Combined inhibition of ErbB1/2 and Notch receptors effectively targets breast ductal carcinoma in situ (DCIS) stem/progenitor cell activity regardless of ErbB2 status. PLOS ONE 2013;8:e56840. https://doi.org/10.1371/journal.pone.0056840
Singh JK, Farnie G, Bundred NJ, Simões BM, Shergill A, Landberg G, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res 2013;19:643–56. https://doi.org/10.1158/1078-0432.CCR-12-1063
Farnie G, Johnson RL, Williams KE, Clarke RB, Bundred NJ. Lapatinib inhibits stem/progenitor proliferation in preclinical in vitro models of ductal carcinoma in situ (DCIS). Cell Cycle 2014;13:418–25. https://doi.org/10.4161/cc.27201
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 2013;24:398–405. https://doi.org/10.1093/annonc/mds277
Bundred NJ, Prasad R, Morris J, Knox WF, Byrne G, Cheung S, et al. Are symptomatic guidelines for chemotherapy appropriate to ER-positive screen-detected breast cancer (SDBC)? Breast Cancer Res Treat 2013;138:359–68. https://doi.org/10.1007/s10549-011-1652-6
Bundred N, Gardovskis J, Jaskiewicz J, Eglitis J, Paramonov V, McCormack P, et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a phase I multicentre trial in patients scheduled for elective breast cancer surgery. Invest New Drugs 2013;31:949–58. https://doi.org/10.1007/s10637-012-9922-7
Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res 2013;15:R92. https://doi.org/10.1186/bcr3493
Singh JK, Simões BM, Clarke RB, Bundred NJ. Targeting IL-8 signalling to inhibit breast cancer stem cell activity. Expert Opin Ther Targets 2013;17:1235–41. https://doi.org/10.1517/14728222.2013.835398
Bundred N, Gardovskis J, Jaskiewicz J, Eglitis J, Paramonov V, McCormack P, et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a phase I multicentre trial in patients scheduled for elective breast cancer surgery. Invest New Drugs 2013;31:949–58. https://doi.org/10.1007/s10637-012-9922-7
2014
Barnes NL, Dimopoulos N, Williams KE, Howe M, Bundred NJ. The frequency of presentation and clinico-pathological characteristics of symptomatic versus screen detected ductal carcinoma in situ of the breast. Eur J Surg Oncol 2014;40:249–54. https://doi.org/10.1016/j.ejso.2013.12.013
Bundred SM, Zhou J, Whiteside S, Morris J, Wilson M, Hurley E, Bundred N. Impact of full-field digital mammography on pre-operative diagnosis and surgical treatment of mammographic microcalcification. Breast Cancer Res Treat 2014;143:359–66. https://doi.org/10.1007/s10549-013-2803-8
Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Degner L, Stockton C, Todd C. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer 2014;110:573–83. https://doi.org/10.1038/bjc.2013.734
Farnie G, Johnson RL, Williams KE, Clarke RB, Bundred NJ. Lapatinib inhibits stem/progenitor proliferation in preclinical in vitro models of ductal carcinoma in situ (DCIS). Cell Cycle 2014;13:418–25. https://doi.org/10.4161/cc.27201
Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 2014;383:1041–8. https://doi.org/10.1016/S0140-6736(13)62292-8
Topps A, Clay V, Absar M, Howe M, Lim Y, Johnson R, Bundred N. The sensitivity of pre-operative axillary staging in breast cancer: comparison of invasive lobular and ductal carcinoma. Eur J Surg Oncol 2014;40:813–17. https://doi.org/10.1016/j.ejso.2014.03.026
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303–10. https://doi.org/10.1016/S1470-2045(14)70460-7
2015
Bundred NJ, Barnes NL, Rutgers E, Donker M. Is axillary lymph node clearance required in node-positive breast cancer? Nat Rev Clin Oncol 2015;12:55–61. https://doi.org/10.1038/nrclinonc.2014.188
Williams KE, Bundred NJ, Landberg G, Clarke RB, Farnie G. Focal adhesion kinase and Wnt signaling regulate human ductal carcinoma in situ stem cell activity and response to radiotherapy. Stem Cells 2015;33:327–41. https://doi.org/10.1002/stem.1843
Williams KE, Barnes NL, Cramer A, Johnson R, Cheema K, Morris J, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol 2015;26:1019–25. https://doi.org/10.1093/annonc/mdv062
Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121–9. https://doi.org/10.1007/s10549-015-3357-8
Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Todd C. Pretreatment health measures and complications after surgical management of elderly women with breast cancer. Br J Surg 2015;102:653–67. https://doi.org/10.1002/bjs.9796
Leary A, Evans A, Johnston SR, A’Hern R, Bliss JM, Sahoo R, et al. Antiproliferative effect of Lapatinib in HER2-positive and HER2-negative/HER3-high breast cancer: results of the presurgical randomized MAPLE trial (CRUK E/06/039). Clin Cancer Res 2015;21:2932–40. https://doi.org/10.1158/1078-0432.CCR-14-1428
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015;386:1353–61.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C, et al. ; IBIS-II investigators. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet 2016;387:866–73.
2016
Bundred SM, Maxwell AJ, Morris J, Lim YY, Harake MJ, Whiteside S, Bundred NJ. Randomized controlled trial of stereotactic 11-G vacuum-assisted core biopsy for the diagnosis and management of mammographic microcalcification. Br J Radiol 2016;89:20150504. https://doi.org/10.1259/bjr.20150504
Bundred N, Maxwell AJ, Harvey J, Hunt R, Morris J, Lim YY. A randomised pilot study comparing 13 G vacuum-assisted biopsy and conventional 14 G core needle biopsy of axillary lymph nodes in women with breast cancer. Clin Radiol 2016;71:551–7.
Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet 2016;387:866–73.
Al-Himdani S, Timbrell S, Tan KT, Morris J, Bundred NJ. Prediction of margin involvement and local recurrence after skin-sparing and simple mastectomy. Eur J Surg Oncol 2016;42:935–41. https://doi.org/10.1016/j.ejso.2016.04.055
Gellert P, Segal CV, Gao Q, López-Knowles E, Martin L, Dodson A, et al. Impact of mutational profiles on response of primary oestrogen receptor-positive breast cancers to oestrogen deprivation. Nature Comm 2016;7:13294.
2017
Pegington M, Adams JE, Bundred NJ, Campbell AM, Howell A, Howell SJ, et al. Recruitment to the ‘Breast-Activity and Healthy Eating After Diagnosis’ (B-AHEAD) randomized controlled trial. Integr Cancer Ther 2017;17:131–7.
Shaker H, Harrison H, Clarke R, Landberg G, Bundred NJ, Versteeg HH, Kirwan CC. Tissue factor promotes breast cancer stem cell activity in vitro. Oncotarget 2017;8:25915–27. https://doi.org/10.18632/oncotarget.13928
Loncaster J, Armstrong A, Howell S, Wilson G, Welch R, Chittalia A, et al. Impact of Oncotype DX breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: real world experience in Greater Manchester, UK. Eur J Surg Oncol 2017;43:931–7.
Bundred NJ, Thomas J, Dixon JMJ. Whither surgical quality assurance of breast cancer surgery (surgical margins and local recurrence) after Paterson. Breast Cancer Res Treat 2017;165:473–5. https://doi.org/10.1007/s10549-017-4369-3
Published presentations to learned societies
Bundred NJ, Stewart HJ, Sturgeon C, Hawkins RA, Shaw D. In vitro production of CEA and breast cyst protein by breast carcinomas: relationship to receptor status: prognosis and response to hormonal therapy. Eur J Surg Oncol 1987;13:281.
Bundred NJ, Stewart HJ, Sturgeon C, Hawkins RA, Shaw D, Forrest APM, Miller WR. Apocrine differentiation: relationship to receptor status: prognosis and hormonal response in breast cancer. Br J Surg 1987;74:1144–5.
Bundred NJ, Walker RA, White GK, Stewart HJ, Miller WR. The prognosis of apocrine cancer. Breast Cancer Res Treat 1988;12:145.
Bundred NJ, West RR, O’Dowd J, Mansel RE. Is there an increased risk of breast cancer in women who have had a cyst aspirated? Br J Surg 1989;76:626.
Bundred NJ, Walker RA, White GK, Stewart HJ, Miller WR. Apocrine differentiation: a new prognostic factor for breast cancer. Br J Surg 1989;76:625.
Bundred NJ, West RR, Harvey P, Mansel RE. Why do women develop breast cysts? Br J Surgery 1989;76:1230.
Bundred NJ, Walker RA, Miller WR. Comparison of results of assessing apocrine differentiation in breast carcinoma. Breast Cancer Res Treat 1989;14:189.
Bundred NJ, Davis SJ, Scott WN, Miller WR, Mansel RE. Is zinc alpha-2 glycoprotein a marker for apocrine activity. Breast Cancer Res Treat 1989;14:159.
Bundred NJ, Woodhead S, Wheeler MH. Screening for MEN 1. Eur J Surg Oncol 1990;16:262–3. (Winner Alan Edwards Prize.)
Bundred NJ, Woodhead S, Wheeler MH. Screening for multiple endocrine neoplasia type. Br J Surg 1990;77:A1312.
Bundred NJ, Walker RA, Botha H, Everson N. The value of breast cancer screening amongst Asians living in Britain. Br J Cancer 1990;62(Suppl.):26.
Bundred NJ, Ratcliffe WA, Walker RA, Coley S, Morrison JM, Ratcliffe JG. Parathyroid hormone related protein and hypercalcaemia in breast cancer. BMJ 1991;303:1506–9.
Bundred NJ, Jones CE, Wellington M, Walker RA, Morrison JM, Baker PR. The importance of Laminin in the growth of human breast cancer. Br J Cancer 1991;64(S XV):9.
Bundred NJ, Wallace DMA, Chan KK, Fielding F. Salvage surgery for recurrent pelvic malignancy. Br J Cancer 1991;64(S XV):20.
Gateley CA, Bundred NJ, Turkes A, Mansel RE. Electrolyte in gross cystic disease fluid in breast cyst fluid. Br J Cancer 1991;64(S XV):4.
Walls J, Boggis CRM, Wilson M, Asbury DL, Bundred NJ, Mansel RE. Should the axilla be dissected in screen detected breast cancer? Br J Surg 1992;79:A1222.
Walls J, Boggis CRM, Wilson M, Asbury DL, Bundred NJ, Mansel RE. Treatment of the axilla in patients with screen-detected breast cancer. Br J Surg 1993;80:436–8.
Walls J, Ratcliffe WA, Hughes S, McIlwrath A, Howell A, Bundred NJ. A new approach to the diagnosis of hypercalcaemia in a screened population. Eur J Surg Oncol 1993;19:214.
Walls J, Hughes S, McIlwrath A, Ratcliffe WA, Howell A, Bundred NJ. Mechanisms of action of parathyroid hormone related protein (PTHrP) in hypercalcaemia. Br J Surg 1993;80:A648.
Walls J, Ratcliffe WA, Howell A, Bundred NJ. The interaction of parathyroid hormone related protein, hypercalcaemia and bisphosphonate therapy. Br J Cancer 1993; Supplement April.
Edwards RC, Bundred NJ, Ratcliffe WA, Walls J, Morrison M, Ratcliffe JG. Expression of parathyroid hormone related protein (PTHrP) in primary breast cancers. J Endocr 1993;137:30.
Mawer EB, Davies M, Gargan P, Walls J, Howell A, Ratcliffe WA, Bundred NJ. 1,25 Dihydroxyvitamin D (1,25 (OH)2D) and parathyroid hormone related protein (PTHrP) in plasma of patients with breast cancer. J Endocr 1993;137:38.
Walls J, Ratcliffe WA, Hughes S, Howell A, Bundred NJ. The role of parathyroid hormone related protein in the diagnosis of hypercalcaemia in a screened population. Br J Surg 1993;80:1207.
Walls J, Donne A, Knox F, Redford J, Swindell R, Howell A, Bundred NJ. The contribution of full axillary dissection to a prognostic index. The Breast 1993;2:205–6.
Walls J, Mawer EB, Davies M, Gargan P, Ratcliffe WA, Howell A, Bundred NJ. Interaction of Vitamin D and parathyroid hormone related protein in patients with breast cancer. The Breast 1993;2:203.
Bundred NJ, Walls J, Ratcliffe W, Edwards R, O’Donoghue D, Hopkins L, Morrison JM. Cytosol extraction of PTHrP in breast cancer and normal breast tissue. The Breast 1993;2:203.
Walls J, Boggis CRM, Wilson M, Asbury DL, Knox F, Mansel RE, Baildam AD, Bundred NJ. Can mammographic microcalcification predict resection margin involvement in screening breast biopsies. Br J Surg 1993;80:1483.
Baildam AD, Wilson M, Boggis CRM, Walls J, Bundred NJ, Asbury DL. Ultrasound guided biopsy of the breast: an alternative to needle localisation for screen detected impalpable lesions. Breast Cancer Res Treat 1993;26:187.
Walls J, Boggis CRM, Wilson M, Asbury DL, Knox, F, Coyne J, Baildam AD, Bundred NJ, Mansel. Can mammographic microcalcification predict resection margin involvement in screening breast biopsies? Eur J Surg Oncol 1993;19:491.
Walls J, Mawer EB, Davies M, Gargan P, Ratcliffe WA, Howell A, Bundred NJ. The interaction of Vitamin D and parathyroid hormone-related protein in patients with breast cancer. Eur J Surg Oncol 1993;19:495.
Knox F, Walls J, Donne A, Wallis NT, Redford J, Swindell R, Howell A, Bundred NJ. The prognostic value of axillary clearance. J Pathol 1993;170:S384A.
Walls J, Donne A, Knox F, Redford J, Swindell R, Howell A, Bundred NJ. Does axillary node status provide additional information? Breast Cancer Res Treat 1993;27:143.
Walls J, Mawer EB, Davies M, Gargan P, Ratcliffe WA, Howell A, Bundred NJ. The effect of parathyroid hormone-related protein on 1,25(OH)2 vitamin D in patients with breast cancer. Breast Cancer Res Treat 1993;27:168.
Walls J, Ratcliffe W, Edwards R, O’Donoghue D, Hopkins L, Morrison JM, Bundred NJ. Parathyroid hormone-related protein; cytosol extraction in breast cancer, and normal breast tissue. Breast Cancer Res Treat 1993;27:168.
Baildam AD, Higgins RM, Hurley E, Furlong A, Walls J, Venning MC, et al. Cyclosporin A-induced fibroadenomas: a potential model for tumour genesis. Breast Cancer Res Treat 1993;27:185.
Baildam AD, Wilson M, Boggis CRM, Walls J, Bundred NJ, Asbury D, Mansel RE. Ultrasound-guided biopsy of the breast: an alternative to needle-localisation for screen detected impalpable lesions. Breast Cancer Res Treat 1993;27:187.
Walls J, Mawer EB, Davies M, Howell A, Ratcliffe WA, Bundred NJ. The interaction of vitamin D and parathyroid hormone related protein in patients with breast cancer. Br J Surg 1993;80:A1467.
Walls J, Donne A, Knox F, Redford J, Swindell R, Howell A, Bundred NJ. Does axillary node status provide additional prognostic information? Br J Surg 1993;80:1468.
Bowcott M, Ratcliffe WA, Hill-Wilson G, Bundred NJ. Parathyroid hormone-related protein is a growth factor for prostate cancer. Br J Surg 1994;81:767.
Walls J, Assiri AMA, Eastell R, Howell A, Ratcliffe WA, Bundred NJ. Urinary pyridinolines identify bone resorption in humoral hypercalcaemia. Br J Surg 1994;81:767.
Walls J, Assiri AMA, Ratcliffe WA, Howell A, Eastell R, Bundred NJ. Urinary cross links: mechanisms of hypercalcaemia in women with breast cancer. Br J Surg 1994;81:755.
Anderson P, Hoyland J, Knox F, Bowcott M, Freemont AJ, Bundred NJ. A role for Interleukin 6 and IL6 receptor in breast cancer. Eur J Cancer 1994;30A(Suppl. 2):25.
Umaar R, Thomas C, Tetlow L, Harding C, Greenhalgh R, Howell A, Bundred NJ. Does the protein composition of breast secretions alter in malignant breast disease? Eur J Cancer 1994;30A(Suppl. 2):52.
Gateley C, Holland P, El-Teraifi, Baildam AD, Bundred NJ. Prediction of axillary lymph node metastases by cytology in patients with breast cancer. Eur J Cancer 1994;30A(Suppl. 2):73.
Holland PA, Walls J, Wilson M, Boggis C, Asbury DL, Knox F, et al. Incidence screen detected breast cancers require axillary node clearance. Eur J Cancer 1994;30A(Suppl. 2):30.
Holland PA, Walls J, Wilson M, Boggis C, Asbury DL, Knox WF, et al. Incidence screen detected breast cancers require axillary node clearance. Breast Cancer Res Treat 1994;32(Suppl.):40.
Holland PA, Knox F, Howell A, Baildam AD, Bundred NJ. Factors affecting relapse of ductal carcinoma in situ after surgical excision. Breast Cancer Res Treat 1994;32(Suppl.):44.
Bundred NJ, Anderson P, Hoyland J, Knox F, Bowcott M, Freemont AJ. A role for interleukin 6 and its receptor in breast cancer. Breast Cancer Res Treat 1994;32(Suppl.):56.
Bundred NJ, Walls J, Assiri AMA, Ratcliffe WA, Howell A. Eastell R. Urinary cross-links: mechanisms of hypercalcaemia in women with breast cancer. Breast Cancer Res Treat 1994;32(Suppl.):59.
Bundred NJ, Bhatia RK, Bowcott M, Knox F, Walls J. Thrombospondin expression on human breast cancers. Breast Cancer Res Treat 1994;32(Suppl.):60.
Holland PA, Knox WF, Potten CS, Howell A, Baildam AD, Bundred NJ. An in vivo model for human ductal carcinoma in situ of the breast. Br J Surg 1995;82:698.
Downey SE, Bowcott M, Ratcliffe WA, Fraser WD, Bundred NJ. Vitamin D inhibits prostate cancer growth by down-regulating production of PTHrP. Br J Surg 1995;82:704.
Harding C, Knox W, Baildam AD, Bundred NJ. Tumours developing on HRT have a better grade. Eur J Surg Oncol 1995;21:111.
Holland PA, Shah A, Howell A, Baildam AD, Bundred NJ. Lobular carcinoma of the breast can be managed with conserving therapy. Eur J Surg Oncol 1995;21:118.
Howell A, Knox E, Swindell R, Bundred N, Galea M, Blamey RW, et al. Comparability of prognostic indices. The Breast 1995;4:237.
Harding C, Rogers E, Margison J, Faragher B, Howell A, Bundred NJ. Is increased target organ sensitivity of the breast responsible for breast cancer development? The Breast 1995;4:241.
Downey SE, Hoyland JA, Walls J, Ratcliffe WA, Edwards R, Freemont AJ, Bundred NJ. Parathyroid hormone receptor protein and its receptor expression in primary breast carcinomas – prognostic significance. The Breast 1995;4:242.
Holland PA, Knox WF, Baildam AD, Howell A, Bundred NJ. Nuclear grade of DCIS does not predict for early local relapse. The Breast 1995;4:244.
Holland PA, Knox WF, Potten CS, Anderson E, Howell A, Baildam AD, Bundred NJ. Comedo DCIS is hormone independent and will not benefit from antioestrogen therapy. The Breast 1995;4:245.
Harding C, Margison J, Howell A, Bundred NJ. Is increased target organ sensitivity to oestrogen responsible for breast cancer development? Breast Cancer Res Treat 1996;37(Suppl.):109.
Downey SE, Hoyland JA, Walls J, Freemont AJ, Bundred NJ. Primary breast cancers express the receptor for parathyroid hormone related protein. Breast Cancer Research and Treatment. Breast Cancer Res Treat 1996;37(Suppl.):109.
Harding C, Knox WF, Baildam AD, Bundred NJ. Does the use of hormone replacement therapy influence breast cancer prognosis? Breast Cancer Res Treat 1996;37(Suppl.):98.
Harding C, Osudenko S, Tetlow L, Faragher B, Howell A, Bundred NJ. Apolipoprotein D, a new anitoestrogen marker in the breast. Br J Surg 1996;83(Suppl. 1):11.
Harding C, Harvey J, Kirkman R, Bundred NJ. Hormone replacement therapy-induced mastalgia responds to evening primrose oil. Br J Surg 1996;83(Suppl. 1):24.
Gandhi A, Coyne J, Baildam AD, Bundred NJ. The treatment of mammary fistulas. Br J Surg 1996;83(Suppl. 1):25.
Harding C, Osudenko O, Tetlow L, Howell A, Bundred NJ. Non invasive measurement of antioestrogen activity in the breast. Eur J Cancer 1996;32A(Suppl. 2):13.
Dey P, Bundred N, Baildam A, Asbury D, Hopwood P, Readman L, et al. Randomised controlled trial comparing the effectiveness of rapid diagnosis and routine out-patient clinics. Eur J Cancer 1996;32A(Suppl. 2):31.
Bundred NJ, Naylor K, Walls J, Evans G, Eastell R, Howell A. Does tamoxifen (tam) increase bone resorption in premenopausal women? Eur J Cancer 1996;32A(Suppl. 2):35.
Bundred NJ, Naylor K, Walls J, Evans G, Eastell R, Howell A. Does tamoxifen increase bone resorption in premenopausal women? Eur J Surg Oncol 1996;22:553.
Gandhi A, Holland PA, Knox WF, Potten CS, Bundred NJ. Epithelial proliferation and expression of apoptotic genes in human breast ductal carcinoma in situ. Eur J Surg Oncol 1997;23:92.
Michael Phillips D, Harding C, Morton M, Howell A, Potten C, Bundred NJ. The effects of soy supplementation on epithelial proliferation in the normal human breast. Eur J Surg Oncol 1997;23:97.
Downey SE, Hoyland JA, Iddon J, Freemont AJ, Bundred NJ. Expression of Interleukin-6 and its receptor in primary and secondary breast tumours. Eur J Surg Oncol 1997;23:99.
McMichael-Phillips D, Harding C, Morton M, Howell A, Potten C, Bundred NJ. The effects of soy supplementation on epithelial proliferation in the normal human breast. Br Cancer Res Treat 1996;41:263.
Dey P, Hopwood P, Bundred NJ, Baildam A, Asbury D, Woodman CBJ. Randomised Controlled Trial of the Effectiveness of Rapid Diagnosis Clinics – Preliminary Results of Psychological Morbidity. Proceedings of the 7th International Symposium on Benign Breast Disease, London, May 1997.
Gandhi A, Baildam AD, Bundred NJ. Total Duct Excision is the Treatment of Choice for Periareolar Sepsis and Nipple Discharge. Proceedings of the 7th International Symposium on Benign Breast Disease, London, May 1997.
Gandhi A, Holland PA, Knox WF, Baildam AD, Potten CS, Bundred NJ. Antioestrogen therapy does not affect apoptosis or mitosis in comedo ductal carcinoma in situ (DCIS). The Breast 1997;6:229.
Gandhi A, Holland PA, Knox WF, Baildam AD, Bundred NJ. Breast conserving surgery is appropriate for localised ductal carcinoma in situ. The Breast 1997;6:230.
Iddon J, Abdullah K, Knox WF, Baildam AD, Barr L, Bundred NJ. Can mastectomy prevent breast cancer? The Breast 1997;6:230.
Hargreaves DF, Sarwar S, Knox F, Potten CS, Bundred NJ. Epithelial proliferation and hormone receptor status in the normal postmenopausal breast and the effects of HRT use. The Breast 1997;6:238.
Shrestha S, Knox WF, Boggis CRM, Howell A, Bundred NJ. Factors influencing local recurrence following breast conservation therapy. The Breast 1997;6:240.
Abdullah TI, Iddon J, Walls J, Baildam AD, Bundred NJ. A randomised prospective study of preservation of the intercostobrachial nerve in axillary node clearance. The Breast 1997;6:247.
Downey SE, Hoyland JA, Freemont AJ, Bundred NJ. Differential expression of growth factors in metastases to different sites. The Breast 1997;6:247.
Bundred NJ, Reynolds J, Allen D, Rees J, Grimshaw J, Barr L, Baildam AD, Maguire P. A randomised controlled trial of early versus standard discharge after breast cancer surgery. The Breast 1997;6:249.
Iddon J, Downey SE, Freemont A, Hoyland J, Bundred NJ. Expression of parathyroid hormone related protein in prostate cancer. Br J Surg 1997;84:1594.
Iddon J, Downey SE, Freemont A, Hoyland J, Bundred NJ. The expression of parathyroid hormone related protein in prostate cancer. Eur J Surg Oncol 1997;23:376.
Abdullah TI, Iddon J, Walls J, Baildam AD, Bundred NJ. A randomised prospective study of preservation of the intercostobrachial nerve in axillary node clearance. Eur J Surg Oncol 1997;23:379.
Gandhi A, Holland PA, Knox WF, Baildam AD, Bundred NJ. Breast conserving surgery (BCS) is appropriate for localised ductal carcinoma in situ (DCIS). Breast Cancer Res Treat 1997;46:35.
Bronder CS, Byrne GJ, Swindell R, Ashcroft L, Howell A, Bundred NJ. Factors predicting survival after mastectomy and flap recurrence. Breast Cancer Res Treat 1997;46:106.
Pontefract DR, Harding C, Skrimshire K, Tetlow L, Bundred NJ. Why do women develop breast cysts? Breast 1997;6:396.
Gandhi A, Baildam AD, Bundred NJ. Total duct excision is the treatment of choice for peri-areolar sepsis and nipple discharge. Breast 1997;6:397.
Iddon J, Harding C, Baildam AD, Barr L, Bundred NJ. The effect of hormone replacement therapy (HRT) on screen detected breast cancers. Eur Jr Surg Oncol 1998;24-4:350.
Byrne GJ, Hayden KE, Tetlow L, Aggarwal R, Sinha G, Howell AH, Bundred NJ. Plasma thrombospondin (pTSP) in early and advanced breast cancer. Eur Jr Surg Oncol 1998;24-4:355.
Byrne GJ, Agarwal R, Sinha G, Bundred NJ. Circulating VEGF in pre- and post-menopausal women and women with early breast cancer. Eur Jr Surg Oncol 1998;24-4:356.
Bundred NJ, Reynolds J, Allen D, Rees J, Grimshaw J, Barr L, Baildam AD, Maguire P. A randomised controlled trial of early versus standard discharge after breast cancer surgery. Eur J Surg Oncol 1998;24-4.
Byrne GJ, Hayden KE, Tetlow L, Aggarwal R, Sinha G, Howell A, Bundred NJ. Plasma thrombospondin in early and advanced breast cancer. BJS 1998;85:1570.
Iddon J, Harding C, Boggis C, Baildam AD, Barr L, Bundred NJ. Effect of hormone replacement therapy on screen-detected breast cancers. BJS 1998;85:1570.
Bramley MD, Harake J, Boggis CRM, Howell A, Bundred NJ. Neoadjuvant chemotherapy for primary breast cancer – how many breasts are saved? Eur J Cancer 1998;34:S53.
Anderson E, Cramer A, Taylor P, Clarke RB, Baildam AD, Bundred NJ, Howell A. Prognostic value of proliferation steroid receptor and epidermal growth factor receptor content determined immunohistochemically in a large series of primary breast tumours. Breast Cancer Res Treat 1998;50:276.
Iddon J, Harding C, Boggis C, Baildam AC, Barr L, Bundred NJ. Breast cancers developing in women taking HRT are of a lower grade. Breast Cancer Res Treat 1998;50:300.
Shaw LE, Hargreaves DF, Knox F, Bundred NJ, Potten CS. The effects of androgen therapy on normal premenopausal female breast. Breast Cancer Res Treat 1998;50:312.
Chan KC, Knox WF, Woodburn JR, Potten CS, Bundred NJ. An Epidermal growth factor receptor (EGFR) Tyrosine kinase inhibitor (ZD1839) inhibits proliferation in normal and preinvasive epithelia. Breast Cancer Res Treat 1999;57:27.
Dowsett M, Lu Y, Hills M, Bundred NJ, Costa A, Decensi A, Sainsbury R, O’Brien M, Scott T, Muchmore DB. Effect of Raloxifene on Ki67 and apoptosis. Breast Cancer Res Treat 1999;57:31.
Robertson JFR, Dixon M, Bundred N, Anderson E, Dowsett M, Nicholson R, Ellis I. A partially-blind randomised multicentre study comparing the anti-tumour effects of single doses (50, 125 and 250 mg) of long-acting (LA) Faslodex (ICI 182,780) with tamoxifen in postmenopausal women with primary breast cancer prior to surgery. Breast Cancer Res Treat 1999;57:31.
Seward J, Byrne GJ, Howell A, Bundred NJ, McCollum CN. Does Cytotoxic chemotherapy precipitate venous thromboembolism in patients with cancer? Breast Cancer Res Treat 1999;57:57.
Byrne GJ, Stringfellow J, Hawnaur JM, Chan KC. Boggis CRM, Bundred NJ. Breast magnetic resonance imaging (MRI): a useful investigation in patients with primary breast cancer? Breast Cancer Res Treat 1999;57:58.
Chan KC, Knox WF, Sinha G, Barr L, Baildam AD, Bundred NJ. Extent of excision margin required in breast conserving surgery (BCS) for ductal carcinoma in situ (DCIS). Breast Cancer Res Treat 1999;57:70.
Byrne GJ, Hayden KE, Tetlow L, Aggarwal R, Howell AH, Bundred NJ. Plasma thrombospondin (PTSP) in early and advanced breast cancer: a marker for metastasis. Breast Cancer Res Treat 1999;57:121.
Hadjiloucas I, Anderson NG, Salem RJ, Garrod DR, Bundred NJ. The expression of desmosomal proteins in invasive breast cancer. Eur J Surg Oncol 1999;25:658.
Chan KC, Knox WF, Sinha G, Gandhi A and Bundred NJ. Extent of excision margin required for ductal carcinoma in situ (DCIS) breast conservation surgery. Br J Surg 1999;86:86.
Iddon J, Byrne G, Baird P, Hoyland J, Freemont AJ, Howell A, Bundred NJ. Macrophage colony stimulating factor and its role in metastatic breast cancer. Br J Surg 1999;86:35.
Chan KC, Knox WF, Sinha G, Gandhi A, Bundred NJ. What is the optimal excision margin distance for ductal carcinoma in situ (DCIS) breast conservation surgery. Breast 1999;8:217.
Iddon J, Byrne G, Baird P, Hoyland J, Freemont AJ, Howell A, Bundred NJ. Macrophage colony-stimulating factor (MCSF) and its role in metastatic breast cancer. Breast 1999;8:222.
Bundred NJ, Reynolds J, Allen D, Rees J, Grimshaw J, Barr L, Baildam AD, Maguire P. A randomised controlled trial of early versus standard discharge after breast cancer surgery. Breast 1999;8:232.
Hargreaves DF, Roberts SA, Howell A, Morton M, Potten CS, Bundred NJ. Effect of phyto-oestrogens on epithelial proliferation in the breast. Breast 1999;8:236.
Chan KC, Knox WF, Woodburn JR, Slamon D, Potten CS, Bundred NJ. Epidermal growth factor receptor inhibition decreases epithelial proliferation in ductal carcinoma in situ of the breast, whereas c-erbB2 blocking does not. Br J Surg 2000;87:628.
Chan KC, Knox WF, Woodburn JR, Potten CS, Bundred NJ. Proliferation in normal breast epithelium is decreased by epidermal growth factor receptor inhibition. Br J Surg 2000;87:679.
Anderson NG, Hoey RP, Linforth RA, Bundred NJ. Characterization of the mitogenic actions of parathyroid hormone-related protein in MCF-7 breast carcinoma cells. Br J Surg 2000;87:26.
Linforth RA, Morris J, Bundred NJ. Cigarette smoking is an aetiological factor in hidradenitis suppurativa. Br J Surg 2000;87:34.
Dobson RRH, Chan CK, Knox F, Potten CS, Bundred NJ. The effects of prolonged HRT treatment in normal postmenopausal breast epithelium. Breast Cancer Res Treat 2000;64:106.
Hurley E, Reaney S, Bundred N. Randomized controlled trial comparing 14g core biopsy with minimally invasive breast biopsy (MIBB) in pre-operative diagnosis of mammographically detected breast lesions. The Breast 2001;10:361.
Down S, Barr L, Baildam A, Bundred N. Axillary accessory breast tissue: incidence, surgical excision and operative complications. The Breast 2001;10:362.
Dey P, Arnold D, Ellen E, Hindmarsh P, Bundred N. Survey of the management of thyroid cancer in the north west. Br J Surg 2001;88:66.
Boland GP, Chan KC, Knox WF, Bundred NJ. Comparison of margin status with the Van Nuys Prognostic Index to predict recurrence of Ductal carcinoma in situ (DCIS) after breast conserving surgery. Br J Surg 2001;88(Suppl. 1):41.
Boland GP, McKeown A, Chan KC, Knox WK, Potten CS, Bundred NJ. Oestrogen withdrawal reduces cell proliferation in Oestrogen receptor positive but not in Oestrogen receptor negative DCIS. Eur J Cancer 2001;37(Suppl. 5):29.
Boland GP, Chan KC, Knox WF, Bundred NJ. Comparison of margin status with the Van Nuys Prognostic Index to predict recurrence of Ductal carcinoma in situ (DCIS) after breast conserving surgery. Eur J Cancer 2001;37(Suppl.):15.
Boland GP, Brown I, Can KC, Baildam AD, Barr L, Bundred NJ. Reliability of stereotactic core biopsy diagnosed ductal carcinoma in situ (DCIS) for screen detected disease. Eur J Cancer 2001;37(Suppl.):13–14.
Bundred NJ. Hormone replacement therapy, breast cancer, and the future. J Br Menopause Soc 2001;7(Suppl. 2):13–15.
Bundred NJ, Chan K, Anderson NG. Studies of epidermal growth factor receptor inhibition in breast cancer. Endocr Relat Cancer 2001;8:183–9.
Boland GP, IS Butt, WF Knox & NJ Bundred. Cox-2 Expression in Breast Neoplasia. Eur J Surg Oncol 2001;27:775.
Baildam A, Keeling F, Noblet M, Thomson L, Bundred N, Hopwood P. Nurse led follow-up for women treated for breast cancer: a randomised controlled trial. EJSO 2001;27:792.
Prasad R, Boland GP, Cramer A, Anderson E, Bundred NJ. Stopping of hormone replacement therapy (HRT) leads to decreased epithelial proliferation in estrogen receptor positive breast cancer. EJSO 2001;27:797.
Boland GP, McKeown A, Chan KC, Knox WF, Potten CS, Bundred NJ. Oestrogen Withdrawal Reduces Cell Proliferation in Oestrogen Receptor (ER) Positive Ductal Carcinoma In Situ (DCIS). 24th Annual San Antonio Breast Cancer Symposium poster presentation, 2001.
Elangovan AE, Wilson M, Knox FW, Barr L, Bundred NJ. Predicting Sentinel Node Involvement: Manchester Experience. 24th Annual San Antonio Breast Cancer Symposium poster presentation, 2001.
Boland GP, Holland PA, Knox WF, Epstein M, Slamon DJ. Potten CS, Bundred NJ. Receptor Expression in Ductal Carcinoma In Situ (DCIS). 24th San Antonio Breast Cancer Symposium poster presentation, 2001.
Boland GP, Brown I, Prasad R, Knox WF, Wilson M, Bundred NJ. Preoperative factors do not predict which patients require reoperation for ductal carcinoma in situ (DCIS). Br J Surg 2002;89:9.
Boland GP, Brown I, Prasad R, Knox WF, Wilson M, Bundred NJ. Impact of preoperative core biopsy on reoperation rates for screen-detected ductal carcinoma in situ (DCIS). Br J Surg 2002;89:9.
Moran T, Gibbs A, Woodman C, Bundred NJ. Do older women get poorer treatment after breast conserving surgery? BJS 2002;89:75.
Prasad R, Boland GP, Cramer A, Anderson E, Bundred NJ. Expression of cerbB-2 does not prevent the withdrawal response to hormone replacement therapy (HRT) in breast cancers. BJS 2002;89:74.
Boland G, Davenport A, Barnes N, Knox WF, Bundred NJ. Prospective study of hormone receptor status is ductal carcinoma in situ (DCIS). Implications for therapy. Br J Surg 2003;S1.
Prasad R, Byrne G, Wilson M, Barr L, Baildam A, Morris J, Bundred NJ. Which women with screen detected breast cancers (SDBC) require chemotherapy? (new index predicting recurrence and mortality). Br J Surg 2003;(90):S1.
Prasad R, Byrne G, Wilson M, Barr L, Baildam AD, Morris J, Bundred NJ. Comparison of 5 year survival of screen detected breast cancers (SDBC) with symptomatic ones of same age (50–60 years) in one unit. Br J Surg 2003;(90):S1.
Warnberg F, White D, Anderson E, Peristerakis I, Knox F, Clarke RB, Bundred NJ. Effect of a RAS farnesyl transferase inhibitor on DCIS and HER-2 positive tumours in vivo. Br J Surg 2003;(90):S1.
Absar M, Prasad R, Wicks P, Burke M, Byrne G, Bundred NJ. Does radiotherapy (DXT) affect survival after breast conserving surgery in elderly patients? Br J Surg 2003;90(Suppl. 1):27.
Warnberg F, White D, Anderson E, Peristerakis I, Kox F, Clarke RB, Bundred NJ. Effect of a ras farnesyl transferase inhibitor on DCIS and HER-2 positive tumours in vivo. Br J Surg 2003;90(Suppl. 1):29.
Prasad R, Byrne G, Wilson M, Barr L, Baildam A, Morris J, Bundred NJ. Which women with screen detected breast cancers (SDBC) require chemotherapy? (New index predicting recurrence and mortality.) Br J Surg 2003;90(Suppl. 1):99.
Prasad R, Byrne G, Wilson M, Barr L, Baildam AD, Morris J, Bundred NJ. Comparison of 5 year survival of screen detected breast cancers (SDBC) with symptomatic ones of same age (50-66 years) in one unit. Br J Surg 2003;90(Suppl. 1):101
Byrne GJ, Kirwan C, Kumar S, Bundred NJ. High levels of serum VEGF and VCAM-1 predict recurrence in women with early breast cancer. Br J Surg 2003;90(Suppl. 1):107.
Barnes NLP, Boland G, Davenport A, Knox WF, Bundred NJ. A prospective study of oestrogen and progesterone receptor status in ductal carcinoma in situ. Breast Cancer Res Treat 2003;82:Abstract No. 150.
Burke MM, Pravica V, Hutchinson IV, Bundred NJ. Association between EGFR ligand gene polymorphisms and the risk and severity of breast cancer. Breast Cancer Res Treat 2003;82:Abstract No. 269.
Warnberg F, White D, Anderson E, Knox F, Clarke RB, Bundred NJ. Effect of a farnesyl transferase inhibitor on HER2 positive tumours and ductal carcinoma in situ of the breast. Breast Cancer Res Treat 2003;82:Abstract No. 347.
Prasad R, Wilson M, Morris J, Byrne G, Bundred NJ. Current HRT (hormone replacement therapy) use at diagnosis of breast cancer does not adversely affect survival from symptomatic or screen detected breast cancers in postmenopausal women. Breast Cancer Res Treat 2003;82:Abstract No. 542.
Barnes NLP, Warnberg F, White D, Anderson E, Bundred NJ. Cyclooxygenase-2 inhibition and tumour growth:celecoxib increases apoptosis in HER2 positive cell lines. Breast Cancer Res Treat 2003;82:Abstract No. 667.
Moore HA, Anderson E, Clarke RB, Warnberg F, Barnes NL, Wakeling AE, Bundred NJ. The effects of fulvestrant and gefitinib on proliferation, progesterone-receptor and pS2 expression in normal human breast epithelium in vivo. Breast Cancer Res Treat 2003;82:Abstract No. 672.
Barnes N, Boland G, Davenport A, Knox WF, Bundred NJ. Hormone Receptor status in ductal carcinoma in situ (DCIS): implications for therapy. EJC 2003;1:14 abstract No. O-43.
Barnes N, Warnberg F, White D, Anderson E, Bundred NJ. The effect of cyclooxygenase-2 (COX-2) inhibition on tumour growth in a xenograft model. EJC 2003;1:14 abstract No. O-141.
Barnes NLP, Warnberg F, White D, Anderson E, Bundred NJ. Cyclooxygenase-2 inhibition: effects on tumour growth and apoptosis in HER2 positive cell lines. EJSO 2003;29:791 abstract No. 29.
Barnes NLP, Boland GP, Khavari S, Cramer A, Knox WF, Bundred NJ. Type 1 Tyrosine kinase receptor co-expression is a predictor of recurrence in ductal carcinoma in situ of the breast. BJS 2004;91:1214.
Barnes NLP, Boland GP, Cramer A, Knox WF, Bundred NJ. Recurrence of ductal carcinoma in situ: the role of COX-2 expression. BJS 2004;91(S1):37 abstract No. 02.
Boland GP, Prasad R, Barnes N, Knox WF, Bundred NJ. HRT use leads to development of oestrogen receptor positive DCIS. BJS 2004;91(S1):135 abstract No. 27.
Barnes NLP, Warnberg F, White D, Anderson E, Bundred NJ. Celecoxib treatment leads to decreased cyclooxygenase-2 (COX-2) expression in a xenograft model of breast cancer. BJC 2004;91(S1):S9 abstract No. 1.8.
Prasad R, Morris J, Wilson M, Knox F, Byrne G, Bundred NJ. The lower proliferation rate of screen detected breast cancers underlies their better survival. BJS 2005;92:1032–327.
Barnes NLP, Haywood PB, Flint PJ, Knox WF, Bundred NJ. Survivin is related to COX-2 expression and recurrence in DCIS. BJS 2005;92:31.
Farnie G, Clarke RB, Bundred NJ. The influence of tumour grade on DCIS stem cell growth. Nottingham Meeting September 2005. Eur J Cancer 2005;3:37.
Prasad R, Knox WF, Morris J, Wilson M, Bundred NJ. Comparison of local recurrence between screen detected and symptomatic breast cancer. Nottingham Meeting. Eur J Cancer 2005;3(S1):14.
Barnes NLP, Flint P, Clarke R, Bundred NJ. Cox-2 inhibition increases apoptosis in human ductal carcinoma in situ of the breast (DCIS) in a xenograft model. Nottingham Meeting. Eur J Cancer 2005;3(S1):16.
Wilson G, Barnes NLP, Knox F, Swindell R, Kawakatsu H, Dive C, Bundred NJ. Activated c-src in dcis correlates with high grade and HER2 expression. Nottingham Meeting. Eur J Cancer 2005;3(S1):17.
Wilson G, Remoush F, Barnes NLP, Knox F, Swindell R, Bundred NJ. Factors predicting recurrence in DCIS after breast conserving surgery with clear margins. Nottingham Meeting. Eur J Cancer 2005;3(S1):17.
Farnie G, Brennan K, Clarke RB, Bundred NJ. Ductal Carcinoma in Situ (DCIS) mammosphere formation; Effect of EGF and NOTCH signalling pathways on self renewal capacity. San Antonio Breast Cancer Conference, 2005.
Pegington M, Adams JE, Bundred NJ, Campbell AM, Howell A, Howell SJ, et al. Recruitment to the ‘Breast-Activity and Healthy Eating After Diagnosis’ (B-AHEAD) randomized controlled trial. Integr Cancer Ther 2018;17:131–7. https://doi.org/10.1177/1534735416687850
Professor Chris Todd
Author/co-author of more than 240 peer-reviewed papers (45% first/last author). Lifetime citation Google 17,795, h-index = 71; WoS = 9477, h-index = 52. Scopus 10,900, h-index = 55.
To 2000
Hollingworth W, Todd CJ, Bell MI, Arafat Q, Girling S, Karia KR, Dixon AK. The diagnostic and therapeutic impact of MRI: an observational multi-centre study. Clin Radiol 2000;55:825–31. https://doi.org/10.1053/crad.2000.0546
Hobby JL, Tom BD, Todd C, Bearcroft PW, Dixon AK. Communication of doubt and certainty in radiological reports. Br J Radiol 2000;73:999–1001. https://doi.org/10.1259/bjr.73.873.11064655
Grande GE, Todd CJ, Barclay SI, Farquhar MC. A randomized controlled trial of a hospital at home service for the terminally ill. Palliat Med 2000;14:375–85. https://doi.org/10.1191/026921600701536200
Sharples LD, Todd CJ, Caine N, Tait S. Measurement properties of the Nottingham Health Profile and the Short Form 36 health status measures in a population sample of elderly people living at home: results from ELPHS. Br J Health Psychol 2000;5:217–34.
Rogers MS, Todd CJ. The ‘right kind’ of pain: talking about symptoms in outpatient oncology consultations. Palliat Med 2000;14:299–307. https://doi.org/10.1191/026921600669288537
Farquhar M, Camilleri-Ferrante C, Todd C. Continuity of care in maternity services: women’s views of one team midwifery scheme. Midwifery 2000;16:35–47.
Farquhar M, Camilleri-Ferrante C, Todd C. General practitioners’ views of working with team midwifery. Br J Gen Pract 2000;50:211–13.
Grande GE, Todd CJ. Why are trials in palliative care so difficult? Palliat Med 2000;14:69–74. https://doi.org/10.1191/026921600677940614
Ismail AA, O’Neill TW, Cooper C, Silman AJ. Risk factors for vertebral deformities in men: relationship to number of vertebral deformities. European Vertebral Osteoporosis Study Group. J Bone Miner Res 2000;15:278–83. https://doi.org/10.1359/jbmr.2000.15.2.278
Ismail AA, O’Neill TW, Cockerill W, Finn JD, Cannata JB, Hoszowski K, et al. Validity of self-report of fractures: results from a prospective study in men and women across Europe. EPOS Study Group. European Prospective Osteoporosis Study Group. Osteoporos Int 2000;11:248–54.
Cockerill W et al (including Todd) on behalf of the EPOS group. Does location of vertebral deformity within the spine influence back pain and disability? Ann Rheumatic Dis 2000;59:368–71.
Grande GE, Todd CJ, Barclay SI, Farquhar MC. Does hospital at home for palliative care facilitate death at home? Randomised controlled trial. BMJ 1999;319:1472–5.
Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, et al. Quality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Working Party for Quality of Life of the European Foundation for Osteoporosis. Osteoporos Int 1999;10:150–60.
Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res 1999;8:79–91.
O’Neill TW, McCloskey EV, Kanis JA, Bhalla AK, Reeve J, Reid DM, et al. The distribution, determinants, and clinical correlates of vertebral osteophytosis: a population based survey. J Rheumatol 1999;26:842–8.
Barclay S, Todd C, McCabe J, Hunt T. Primary care group commissioning of services: the differing priorities of general practitioners and district nurses for palliative care services. Br J Gen Pract 1999;49:181–6.
Ismail AA, O’Neill TW, Cooper C, Finn JD, Bhalla AK, Cannata JB, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998;8:291–7.
Hollingworth W, Dixon AK, Todd CJ, Bell MI, Antoun NM, Arafat Q, et al. Self reported health status and magnetic resonance imaging findings in patients with low back pain. Eur Spine J 1998;7:369–75.
Todd CJ, Farquhar MC, Camilleri-Ferrante C. Team midwifery: the views and job satisfaction of midwives. Midwifery 1998;14:214–24.
Hollingworth W, Bell MI, Dixon AK, Antoun NM, Moffat DA, Todd CJ. Measuring the effects of medical imaging in patients with possible cerebellopontine angle lesions: a four centre study. Acad Radiol 1998;5:S306–9.
Grande GE, Addington-Hall JM, Todd CJ. Place of death and access to home care services: are certain patient groups at a disadvantage? Soc Sci Med 1998;47:565–79.
Parker M, Todd C, Palmer C, Camilleri-Ferrante C, Freeman C, Laxton C, Payne B, Rushton N. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing 1998;27:333–7.
Rogers MS, Barclay SI, Todd CJ. Developing the Cambridge palliative audit schedule (CAMPAS): a palliative care audit for primary health care teams. Br J Gen Pract 1998;48:1224–7.
Farquhar M, Camilleri-Ferrante C, Todd C. Working with team midwifery: health visitors’ views of one team midwifery scheme. J Adv Nurs 1998;27:546–52.
Still A, Todd C. When technical rationality fails: thinking about terminally ill patients. J Health Psychol 1998;3:137–48. https://doi.org/10.1177/135910539800300111
Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, O’Neill TW. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. European Vertebral Osteoporosis Study Group. Osteoporos Int 1999;9:206–13.
Matthis C and the EVOS group. (Including Todd) Health impact of vertebral deformities: Results from the European Vertebral Osteoporosis Study. Osteoporosis Int 1998;8:364–72.
Barclay S, Todd C, Grande G, Lipscombe J. How common is medical training in palliative care? A postal survey of general practitioners. Br J Gen Pract 1997;47:800–4.
Laxton C, Freeman C, Todd C, Payne B, Camilleri-Ferrante C, Palmer C, Parker M, Rushton N. Morbidity at 3 months after hip fracture: data from the East Anglian audit. Health Trends 1997;29:55–60.
Grande GE, Barclay SI, Todd CJ. Difficulty of symptom control and general practitioners’ knowledge of patients’ symptoms. Palliat Med 1997;11:399–406. https://doi.org/10.1177/026921639701100511
Grande GE, Todd CJ, Barclay SI. Support needs in the last year of life: patient and carer dilemmas. Palliat Med 1997;11:202–8. https://doi.org/10.1177/026921639701100304
Silman A and the EVOS group (including Todd). Influence of physical activity on vertebral deformity in men and women: results from the European Vertebral Osteoporosis Study. J Bone Mineral Res 1997;12:813–19.
Reeve J, Silman A, EPOS Study Group. Epidemiology of osteoporotic fractures in Europe: towards biologic mechanisms, The European Prospective Osteoporosis Study. Osteoporosis Int 1997;7(S3):78–83.
O’Neill TW, Silman AJ, Naves Diaz M, Cooper C, Kanis J, Felsenberg D. Influence of hormonal and reproductive factors on the risk of vertebral deformity in European women. European Vertebral Osteoporosis Study Group. Osteoporos Int 1997;7:72–8.
Naves Diaz M, O’Neill TW, Silman AJ. The influence of alcohol consumption on the risk of vertebral deformity. European Vertebral Osteoporosis Study Group. Osteoporos Int 1997;7:65–71.
Diaz MN, O’Neill TW, Silman AJ. The influence of family history of hip fracture on the risk of vertebral deformity in men and women: the European Vertebral Osteoporosis Study. Bone 1997;20:145–9.
Johnell O, the EVOS study group. Anthropometric measurements and vertebral deformities. Am J Epidemiol 1997;146:287–93.
Bedford S, Melzer D, Dening T, Lawton C, Todd C, Badger G, Brayne C. Carers and the monitoring of psychogeriatric community teams. Int J Geriatr Psychiatry 1996;11:1057–61.
Bedford S, Melzer D, Dening T, Lawton C, Todd C, Badger G, Brayne C. What becomes of people with dementia referred to community psychogeriatric teams? Int J Geriatr Psychiatry 1996;11:1051–56.
Hollingworth W, Todd CJ, Parker MJ. The cost of treating hip fractures in the twenty-first century: short report. Osteoporos Int 1996;6(Suppl. 2):13–15.
Grande GE, Todd CJ, Barclay SI, Doyle JH. What terminally ill patients value in the support provided by GPs, district and Macmillan nurses. Int J Palliat Nurs 1996;2:138–43. https://doi.org/10.12968/ijpn.1996.2.3.138
O’Neill TW, the EVOS group. The prevalence of vertebral deformity in European men and women – The European Vertebral Osteoporosis Study. J Bone Mineral Res 1996;11:1010–18.
Hollingworth W, Mackenzie R, Todd CJ, Dixon AK. Measuring changes in quality of life following magnetic resonance imaging of the knee: SF-36, EuroQol or Rosser index? Qual Life Res 1995;4:325–34.
Chi LY, Brayne C, Todd CJ, O’Connor DW, Pollitt PA. Predictors of hospital contact by very elderly people: a pilot study from a cohort of people aged 75 years and over. Age Ageing 1995;24:382–8.
Hollingworth W, Todd CJ, Parker MJ. The cost of treating hip fractures in the twenty-first century. J Public Health Med 1995;17:269–76.
Todd CJ, Freeman CJ, Camilleri-Ferrante C, Palmer CR, Hyder A, Laxton CE, et al. Differences in mortality after fracture of hip: the east Anglian audit. BMJ 1995;310:904–8.
O’Neill TW, Marsden D, Silman AJ. Differences in the characteristics of responders and non-responders in a prevalence survey of vertebral osteoporosis. European Vertebral Osteoporosis Study Group. Osteoporos Int 1995;5:327–34.
O’Neill TW, the EVOS group. Survey response rates – national and regional differences in a European multicenter study of vertebral osteoporosis. J Epidemiol Community Health 1995;49:87–93.
Meyer HE, Falch JA, O’Neill T, Tverdal A, Varlow J. Height and body mass index in Oslo, Norway, compared to other regions of Europe: do they explain differences in the incidence of hip fracture? European Vertebral Osteoporosis Study Group. Bone 1995;17:347–50.
O’Neill TW, the EVOS group. Design and development of a questionnaire for use in a multicentre survey of vertebral osteoporosis in Europe: The European Vertebral Osteoporosis Study. Rheumatology in Europe 1995;24:75–81.
Vaughan NJA, Bradshaw C, Bradley C, Brown F, Cleary R, Clements D, et al. Measuring the outcomes of diabetes care. Diabetic Med 1994;11:418–23.
Reid N, Robinson G, Todd C. The 12-hour shift: the views of nurse educators and students. J Adv Nurs 1994;19:938–46.
O’Neill TW, Varlow J, Felsenberg D, Johnell O, Weber K. Marchant F, et al. Variation in vertebral height ratios in population studies. European Vertebral Osteoporosis Study Group. J Bone Mineral Res 1994;9:1895–907.
O’Neill TW, Cooper C, Cannata JB, Diaz Lopez JB, Hoszowski K, Johnell O, et al. Reproducibility of a questionnaire on risk factors for osteoporosis in a multicentre prevalence survey: the European Vertebral Osteoporosis Study. Int J Epidemiol 1994;23:559–65.
Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ 1993;307:903–6.
Todd C, Robinson G, Reid N. 12-hour shifts: job satisfaction of nurses. J Nurs Manag 1993;1:215–20.
Reid N, Robinson G, Todd C. The quantity of nursing care on wards working eight hour and twelve hour shifts. Int J Nurs Stud 1993;30:403–13.
Todd C, Still A. General practitioners’ strategies and tactics of communication with the terminally ill. Fam Pract 1993;10:268–76.
Wilson AE, Home PD. A dataset to allow exchange of information for monitoring continuing diabetes care. The Diabetes Audit Working Group. Diabetic Med 1993;10:378–90.
Working Group of the Research Unit of the Royal College of Physicians and the British Diabetic Association. A proposal for continuing audit of diabetes services. Diabetic Med 1992;9:759–64.
Todd CJ. Reduction in the incidence of suicide: a health gain objective for the NHS. J Psychopharmacol 1992;6(Suppl. 2):318–24. https://doi.org/10.1177/0269881192006002061
Reid N, Todd C, Robinson G. Educational activities on wards under 12 hour shifts. Int J Nurs Stud 1991;28:47–54.
Todd C, Reid N, Robinson G. The quality of nursing care on wards working eight and twelve hour shifts: a repeated measures study using the MONITOR index of quality of care. Int J Nurs Stud 1989;26:359–68.
Still AW, Todd CJ. Role ambiguity in general practice: the care of patients dying at home. Soc Sci Med 1986;23:519–25.
Still A, Todd C. Differences between terminally ill patients who know, and those who do not know, that they are dying. J Clin Psychol 1986;42:287–96.
Todd CJ, Still AW. Communication between general practitioners and patients dying at home. Soc Sci Med 1984;18:667–72.
2001
O’Leary D, Paykel E, Todd C, Vardulaki K. Suicide in primary affective disorders revisited: a systematic review by treatment era. J Clin Psychiatry 2001;62:804–11.
Walter FM, Kinmonth AL, Hyland F, Murrell P, Marteau TM, Todd C. Experiences and expectations of the new genetics in relation to familial risk of breast cancer: a comparison of the views of GPs and practice nurses. Fam Pract 2001;18:491–4.
Murrell P, Todd CJ, Martin A, Walton J, Lips P, Reeve J, Working Party for Quality of Life of the International Osteoporosis Foundation. Postal administration compared with nurse-supported administration of the QUALEFFO-41 in a population sample: comparison of results and assessment of psychometric properties. Osteoporos Int 2001;12:672–9.
The Women’s Concerns Study Group. Raising concerns about family history of breast cancer in primary care consultations: prospective, population based study. BMJ 2001;322:27–8.
Ismail AA, Cockerill W, Cooper C, Finn JD, Abendroth K, Parisi G, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European Prospective Osteoporosis Study. Osteoporosis Int 2001;12:85–90.
2002
Coomber S, Todd C, Park G, Baxter P, Firth-Cozens J, Shore S. Stress in UK intensive care unit doctors. Br J Anaesth 2002;89:873–81.
Freeman C, Todd C, Camilleri-Ferrante C, Laxton C, Murrell P, Palmer CR, et al. Quality improvement for patients with hip fracture: experience from a multi-site audit. Qual Saf Health Care 2002;11:239–45.
Rogers M, Todd C. Information exchange in oncology outpatient clinics: source, valence and uncertainty. Psycho-Oncology 2002;11:336–45. https://doi.org/10.1002/pon.575
Barrett J, Goh S, Todd C, Barclay S, Daza-Ramirez P, Vardulaki K. A description of an intermediate care service using routinely collected data. J Nurs Manag 2002;10:221–7.
Canoy DS, Hart AR, Todd CJ. Epidemiology of duodenal ulcer perforation: a study on hospital admissions in Norfolk, United Kingdom. Dig Liver Dis 2002;34:322–7.
Barclay S, Todd C, Grande G, Lipscombe J. Controlling cancer pain in primary care: the prescribing habits and knowledge base of general practitioners. J Pain Symptom Manage 2002;23:383–92.
Hollingworth W, Todd CJ, King H, Males T, Dixon AK, Karia KR, Kinmonth AL. Primary care referrals for lumbar spine radiography: diagnostic yield and clinical guidelines. Br J Gen Pract 2002;52:475–80.
Burbeck R, Coomber S, Robinson SM, Todd C. Occupational stress in consultants in accident and emergency medicine: a national survey of levels of stress at work. Emerg Med J 2002;19:234–8.
Barclay S, Todd C, Finlay I, Grande G, Wyatt P. Not another questionnaire! Maximizing the response rate, predicting non-response and assessing non-response bias in postal questionnaire studies of GPs. Fam Pract 2002;19:105–11.
Todd CJ, Grande GE, Barclay SI, Farquhar MC. General practitioners’ and district nurses’ views of hospital at home for palliative care. Palliat Med 2002;16:251–4. https://doi.org/10.1191/0269216302pm513oa
Farquhar M, Grande G, Todd C, Barclay S. Defining patients as palliative: hospital doctors’ versus general practitioners’ perceptions. Palliat Med 2002;16:247–50. https://doi.org/10.1191/0269216302pm520oa
Grande GE, McKerral A, Todd CJ. Which cancer patients are referred to Hospital at Home for palliative care? Palliat Med 2002;16:115–23. https://doi.org/10.1191/0269216302pm519oa
European Prospective Osteoporosis Study (EPOS) Group, Felsenberg D, Silman AJ, Lunt M, Armbrecht G, Ismail AA, et al. Incidence of vertebral fractures in Europe: results from the European Prospective Osteoporosis Study (EPOS). J Bone Mineral Res 2002;17:716–24.
Lunt M, Ismail AA, Felsenberg D, Cooper C, Kanis JA, Reeve J, et al. ; the EPOS Study Group (including Todd). Defining incident vertebral deformities in population studies: a comparison of morphometric criteria. Osteoporosis Int 2002;13:809–15.
The European Prospective Osteoporosis Study (EPOS) Group, O’Neill TW. The relationship between bone density and incident vertebral fracture in men and women. J Bone Mineral Res 2002;17:2214–21.
Ismail AA, Pye SR, Cockerill WC, Lunt M, Silman AJ, Reeve J, et al. Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 2002;13:565–71. https://doi.org/10.1007/s001980200074
Canoy DS, Hart AR, Todd CJ. Epidemiology of duodenal ulcer perforation: a study on hospital admissions in Norfolk, United Kingdom. Dig Liver Dis 2002;34:322–7.
2003
Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care 2003;1:337–44.
Grande GE, McKerral A, Addington-Hall JM, Todd CJ. Place of death and use of health services in the last year of life. J Palliat Care 2003;19:263–70.
Boyle A, Todd C. Incidence and prevalence of domestic violence in a UK emergency department. Emerg Med J 2003;20:438–42.
Barclay S, Wyatt P, Shore S, Finlay I, Grande G, Todd C. Caring for the dying: how well prepared are general practitioners? A questionnaire study in Wales. Palliat Med 2003;17:27–39. https://doi.org/10.1191/0269216303pm665oa
Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut off should we use? Int J Geriatr Psychiatry 2003;18:358–9.
Roy DK, O’Neill TW, Finn JD, Lunt M, Silman AJ, Felsenberg D, et al. Determinants of incident vertebral fracture in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 2003;14:19–26. https://doi.org/10.1007/s00198-002-1317-8
Reeve J, Lunt M, Felsenberg D, Silman AJ, Scheidt-Nave A, Poor G, et al. Determinants of the size of incident vertebral deformities in European men and women in the 6th–9th decades of age: the European Prospective Osteoporosis Study (EPOS). J Bone Mineral Res 2003;18:1664–73.
2004
Walshe CE, Caress AL, Chew-Graham C, Todd CJ. Case studies: a research strategy appropriate for palliative care? Palliat Med 2004;18:677–84. https://doi.org/10.1191/0269216304pm962ra
Grande GE, Farquhar MC, Barclay SI, Todd CJ. Valued aspects of primary palliative care: content analysis of bereaved carers’ descriptions. Br J Gen Pract 2004;54:772–8.
Ewing G, Rogers M, Barclay S, McCabe J, Martin A, Todd C. Recruiting patients into a primary care based study of palliative care: why is it so difficult? Palliat Med 2004;18:452–9. https://doi.org/10.1191/0269216304pm905oa
Grande GE, Farquhar MC, Barclay SI, Todd CJ. Caregiver bereavement outcome: relationship with hospice at home, satisfaction with care, and home death. J Palliat Care 2004;20:69–77.
Ewing G, Todd C, Rogers M, Barclay S, McCabe J, Martin A. Validation of a symptom measure suitable for use among palliative care patients in the community: CAMPAS-R. J Pain Symptom Manage 2004;27:287–99. https://doi.org/10.1016/j.jpainsymman.2003.12.012
Todd C, Griffiths J. Commentary on Gallagher LP, Truglio-Londrigan M. Community support: older adults’ perceptions. Clin Nurs Res 2004;13:24–32.
Raspe H, Matthis C, Croft P, O’Neill T, European Vertebral Osteoporosis Study Group. Variation in back pain between countries: the example of Britain and Germany. Spine 2004;29:1017–21.
O’Neill T, Cockerill W, Matthis C, Raspe HH, Lunt M, Cooper C, et al. Back pain, disability and prevalent vertebral fracture: a prospective study. Osteoporosis Int 2004;15:760–5.
Cockerill W, Lunt M, Silman A, Cooper C, Lips P, Bhalla AK, et al. Health related quality of life and radiographic vertebral fracture. Osteoporosis Int 2004;15:113–19.
European Prospective Osteoporosis Study Group. Risk factors for Colles’ fracture in men and women: results from the European Prospective Osteoporosis Study. Osteoporosis Int 2003;14:213–18. [Erratum published in Osteoporosis Int 2004;15:927.]
2005
Burden ST, Stoppard E, Shaffer J, Makin A, Todd C. Can we use mid upper arm anthropometry to detect malnutrition in medical inpatients? A validation study. J Hum Nutr Diet 2005;18:287–94.
Coventry PA, Grande GE, Richards DA, Todd CJ. Prediction of appropriate timing of palliative care for older adults with non-malignant life-threatening disease: a systematic review. Age Ageing 2005;34:218–27.
2006
Uitterlinden AG, Ralston SH, Brandi ML, Carey AH, Grinberg D, Langdahl BL, et al. ; APOSS Investigators; EPOS Investigators; EPOLOS Investigators; FAMOS Investigators; LASA Investigators; Rotterdam Study Investigators; GENOMOS Study. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med 2006;145:255–64.
Grande GE, Farquhar MC, Barclay SI, Todd CJ. The influence of patient and carer age in access to palliative care services. Age Ageing 2006;35:267–73.
Ewing G, Rogers M, Barclay S, McCabe J, Martin A, Campbell M, Todd C. Palliative care in primary care: a study to determine whether patients and professionals agree on symptoms. Br J Gen Pract 2006;56:27–34.
Kaptoge S, Armbrecht G, Felsenberg D, Lunt M, Weber K, Boonen S, et al. Whom to treat? The contribution of vertebral X-rays to risk-based algorithms for fracture prediction. Results from the European Prospective Osteoporosis Study. Osteoporos Int 2006;17:1369–81. https://doi.org/10.1007/s00198-005-0067-9
2007
Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209–15. https://doi.org/10.1002/bjs.5834
Cullum S, Tucker S, Todd C, Brayne C. Effectiveness of liaison psychiatric nursing in older medical inpatients with depression: a randomised controlled trial. Age Ageing 2007;36:436–42.
Yardley L, Donovan-Hall M, Francis K, Todd C. Attitudes and beliefs that predict older people’s intention to undertake strength and balance training. J Gerontol B Psychol Sci Soc Sci 2007;62:P119–25.
Lavelle K, Todd C, Moran A, Howell A, Bundred N, Campbell M. Non-standard management of breast cancer increases with age in the UK: a population based cohort of women > or = 65 years. Br J Cancer 2007;96:1197–203.
Berman R, Campbell M, Makin W, Todd C. Occupational stress in palliative medicine, medical oncology and clinical oncology specialist registrars. Clin Med 2007;7:235–42.
Walshe C, Caress A, Chew-Graham C, Todd C. Evaluating partnership working: lessons for palliative care. Eur J Cancer Care 2007;16:48–54.
2008
Walshe C, Caress A, Chew-Graham C, Todd C. Implementation and impact of the Gold Standards Framework in community palliative care: a qualitative study of three primary care trusts. Palliat Med 2008;22:736–43. https://doi.org/10.1177/0269216308094103
2009
Preston N, Payne S, Todd C. Conducting research in palliative care patients: a burden or an opportunity? Int J Palliat Nurs 2009;15:524–5.
Grande G, Stajduhar K, Aoun S, Toye C, Funk L, Addington-Hall J, et al. Supporting lay carers in end of life care: current gaps and future priorities. Palliat Med 2009;23:339–44. https://doi.org/10.1177/0269216309104875
Mackereth P, Carter A, Parkin S, Stringer J, Caress A, Todd C, et al. Complementary therapists’ training and cancer care: a multi-site study. Eur J Oncol Nurs 2009;13:330–5. https://doi.org/10.1016/j.ejon.2009.04.009
Mackereth P, Carter A, Parkin S, Stringer J, Roberts D, Long A, et al. Complementary therapists’ motivation to work in cancer/supportive and palliative care: a multi-centre case study. Complement Ther Clin Pract 2009;15:161–5.
Pye SR, Tobias J, Silman AJ, Reeve J, O’Neill; EPOS Study Group. Childhood fractures do not predict future fractures: results from the European Prospective Osteoporosis Study. J Bone Mineral Res 2009;24:1314–18.
2011
Walshe C, Caress A, Chew-Graham C, Todd C. Equity, choice or chance? Community palliative care. J Community Nurs 2011;25:4–7.
McAllister M, Wood AM, Dunn G, Shiloh S, Todd C. The Genetic Counseling Outcome Scale: a new patient-reported outcome measure for clinical genetics services. Clin Genet 2011;79:413–24. https://doi.org/10.1111/j.1399-0004.2011.01636.x
2012
Burden S, Todd C, Hill J, Lal S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012;11:CD008879. https://doi.org/10.1002/14651858.CD008879.pub2
Stone P, Gwilliam B, Keeley V, Todd C, Gittins M, Kelly L, et al. Patients’ reports or clinicians’ assessments: which are better for prognosticating? BMJ Support Palliat Care 2012;2:219–23. https://doi.org/10.1136/bmjspcare-2012-000216
Hawley, H. Skelton, D.A. Campbell, M. Todd. C. Are the attitudes of exercise instructors who work with older adults influenced by training and personal characteristics? J Ageing Physical Activity 2012;20:47–63.
McAllister M, Wood AM, Dunn G, Shiloh S, Todd C. The Perceived Personal Control (PPC) Questionnaire: reliability and validity in a UK sample. Am J Med Genet A 2012;158A:367–72.
2013
Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Degner L, Stockton C, Todd C. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer 2014;110:573–83.
Evans CJ, Harding R, Higginson IJ, MORECare. ‘Best practice’ in developing and evaluating palliative and end-of-life care services: a meta-synthesis of research methods for the MORECare project. Palliat Med 2013;27:885–98. https://doi.org/10.1177/0269216312467489
Preston NJ, Fayers P, Walters SJ, Pilling M, Grande GE, Short V, et al. Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliat Med 2013;27:899–907. https://doi.org/10.1177/0269216313486952
Roberts D, Wilson C, Todd C, Long AF, Mackereth P, Stringer J, et al. Complementary therapies in cancer: patients’ views on their purposes and value pre and post receipt of complementary therapy – a multi-centre case study. Eur J Integr Med 2013;5:443–9.
2015
Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Todd, C. Pre-treatment health measures and complications after surgical management of elderly women with breast cancer. Br J Surg 2015;102:653–67.
Sowerbutts AM, Griffiths, J, Todd C, Lavelle K. Why are older women not having surgery for breast cancer? Psychooncology 2015;24:1036–42.
2016
Hawley-Hague H, Horne M, Skelton DA, Todd C. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open 2016;6:e011560. https://doi.org/10.1136/bmjopen-2016-011560
Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. The clinical and cost effectiveness of a Breathlessness Intervention Service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials 2016;17:185. https://doi.org/10.1186/s13063-016-1304-6
Preston NJ, Farquhar MC, Walshe CE, Stevinson C, Ewing G, Calman LA, et al. Strategies designed to help healthcare professionals to recruit participants to research studies. Cochrane Database Syst Rev 2016;2:MR000036.
Hawley-Hague H, Horne M, Skelton DA, Todd C. Older adults’ uptake and adherence to exercise classes: instructors’ perspectives. J Aging Phys Act 2016;24:119–28. https://doi.org/10.1123/japa.2014-0108
2017
Helbostad JL, Vereijken B, Becker C, Todd C, Taraldsen K, Pijnappels M, et al. Mobile Health Applications to Promote Active and Healthy Ageing. Sensors 2017;17:E622.
Bundred N, Lavelle K, Sowerbutts AM, Pilling M, Todd C. Impact of primary surgery on short-term survival of older breast cancer patients in the UK. Feb 2017. Cancer Res (4 suppl.) ed. American Association for Cancer Research, Vol. 77, p. P3-13-021.
Boulton E, Horne M, Todd C. Multiple influences on participating in physical activity in older age: developing a social ecological approach. Health Expect 2018;21:239–48.
Julie Morris
2015
Williams KE, Barnes NL, Cramer A, Johnson R, Cheema K, Morris J, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol 2015;26:1019–25. https://doi.org/10.1093/annonc/mdv062
Jayanti A, Foden P, Wearden A, Morris J, Brenchley P, Mitra S, BASIC-HHD Study Group. Self-cannulation for haemodialysis: patient attributes, clinical correlates and self-cannulation predilection models. PLOS ONE 2015;10:e0125606. https://doi.org/10.1371/journal.pone.0125606
2016
Al-Himdani S, Timbrell S, Tan KT, Morris J, Bundred NJ. Prediction of margin involvement and local recurrence after skin-sparing and simple mastectomy. Eur J Surg Oncol 2016;42:935–41. https://doi.org/10.1016/j.ejso.2016.04.055
Dr Vaughan Keeley
2003
Hunt J, Cobb M, Keeley VL, Ahmedzai SH. The quality of spiritual care – developing a standard. Int J Palliat Nurs 2003;9:208–15. https://doi.org/10.12968/ijpn.2003.9.5.11493
2004
Hunt J, Keeley VL, Cobb M, Ahmedzai SH. A new quality assurance package for hospital palliative care teams: the Trent Hospice Audit Group model. Br J Cancer 2004;91:248–53. https://doi.org/10.1038/sj.bjc.6601945
2008
Keeley V. Pharmacological treatment for chronic oedema. Br J Community Nurs 2008;13:S4, S6, S8–10. https://doi.org/10.12968/bjcn.2008.13.Sup2.29394
Keeley V. Quality of life assessment tools in chronic oedema. Br J Community Nurs 2008;13:S22–7. https://doi.org/10.12968/bjcn.2008.13.Sup5.31193
Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008;158:1175–6. https://doi.org/10.1111/j.1365-2133.2008.08590.x
2010
Connell F, Brice G, Jeffery S, Keeley V, Mortimer P, Mansour S. A new classification system for primary lymphatic dysplasias based on phenotype. Clin Genet 2010;77:438–52. https://doi.org/10.1111/j.1399-0004.2010.01394.x
2012
Gwilliam B, Keeley V, Todd C, Gittins M, Roberts C, Kelly L, et al. Development of Prognosis in Palliative care Study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ Support Palliat Care 2012;2:63–71. https://doi.org/10.1136/bmjspcare.2012.d4920rep
Stone P, Gwilliam B, Keeley V, Todd C, Gittins M, Kelly L, et al. Patients’ reports or clinicians’ assessments: which are better for prognosticating? BMJ Support Palliat Care 2012;2:219–23. https://doi.org/10.1136/bmjspcare-2012-000216
2013
Connell FC, Gordon K, Brice G, Keeley V, Jeffery S, Mortimer PS, et al. The classification and diagnostic algorithm for primary lymphatic dysplasia: an update from 2010 to include molecular findings. Clin Genet 2013;84:303–14. https://doi.org/10.1111/cge.12173
Connell F, Brice G, Jeffery S, Keeley V, Mortimer P, Mansour S. A new classification system for primary lymphatic dysplasias based on phenotype. Clin Genet 2010;77:438–52. https://doi.org/10.1111/j.1399-0004.2010.01394.x
Stone PC, Gwilliam B, Keeley V, Todd C, Kelly LC, Barclay S. Factors affecting recruitment to an observational multicentre palliative care study. BMJ Support Palliat Care 2013;3:318–23. https://doi.org/10.1136/bmjspcare-2012-000396
Gwilliam B, Keeley V, Todd C, Roberts C, Gittins M, Kelly L, et al. Prognosticating in patients with advanced cancer – observational study comparing the accuracy of clinicians’ and patients’ estimates of survival. Ann Oncol 2013;24:482–8. https://doi.org/10.1093/annonc/mds341
2015
Atton G, Gordon K, Brice G, Keeley V, Riches K, Ostergaard P, et al. The lymphatic phenotype in Turner syndrome: an evaluation of nineteen patients and literature review. Eur J Hum Genet 2015;23:1634–9. https://doi.org/10.1038/ejhg.2015.41
Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121–9. https://doi.org/10.1007/s10549-015-3357-8
2017
Narahari SR, Aggithaya MG, Moffatt C, Ryan TJ, Keeley V, Vijaya B, et al. Future research priorities for morbidity control of lymphedema. Indian J Dermatol 2017;62:33–40. https://doi.org/10.4103/0019-5154.198039
Moffatt CJ, Keeley V, Franks PJ, Rich A, Pinnington LL. Chronic oedema: a prevalent health care problem for UK health services. Int Wound J 2017;14:772–81. https://doi.org/10.1111/iwj.12694
Keeley V. Advances in understanding and management of lymphoedema (cancer, primary). Curr Opin Support Palliat Care 2017;11:355–60. https://doi.org/10.1097/SPC.0000000000000311
Professor Arnie Purushotham
2004
Evans JR, Helmy AE, Cluroe A, Purushotham AD. Lymphoma of the breast - case report and review of the literature. J BUON 2004;9:307–11.
Lynch MD, Jones AE, Marker A, Grant JW, Purushotham AD. Malignant eccrine poroma in breast cancer-related lymphoedema. Ann R Coll Surg Engl 2004;86:W32–5. https://doi.org/10.1308/147870804155
2005
Tait R, Pinder SE, Ellis IO, Purushotham AD. Adenomyoepithelioma of the breast; a case report and literature review. J BUON 2005;10:393–5.
De Silva NK, Pinder S, Purushotham AD. Invasive micropapillary carcinoma of breast: case report with literature review. J BUON 2005;10:271–5.
Lynch MD, Cariati M, Purushotham AD. Breast cancer, stem cells and prospects for therapy. Breast Cancer Res 2006;8:211.
Pain SJ, Barber RW, Solanki CK, Ballinger JR, Britton TB, Mortimer PS, et al. Short-term effects of axillary lymph node clearance surgery on lymphatic physiology of the arm in breast cancer. J Appl Physiol 2005;99:2345–51.
2006
Patkar V, Hurt C, Steele R, Love S, Purushotham A, Williams M, et al. Evidence-based guidelines and decision support services: a discussion and evaluation in triple assessment of suspected breast cancer. Br J Cancer 2006;95:1490–6.
O’Mahony S, Solanki CK, Barber RW, Mortimer PS, Purushotham AD, Peters AM. Imaging of lymphatic vessels in breast cancer-related lymphedema: intradermal versus subcutaneous injection of 99mTc-immunoglobulin. AJR Am J Roentgenol 2006;186:1349–55.
2007
Cash CJ, Coles CE, Treece GM, Purushotham AD, Britton P, Sinnatamby R, et al. Breast cancers: noninvasive method of preoperative localization with three-dimensional US and surface contour mapping. Radiology 2007;245:556–66.
Purushotham AD, Bennett Britton TM, Klevesath MB, Chou P, Agbaje OF, Duffy SW. Lymph node status and breast cancer-related lymphedema. Ann Surg 2007;246:42–5. https://doi.org/10.1097/01.sla.0000259390.51203.7b
O’Mahony S, Britton TM, Solanki CK, Ballinger JR, Pain SJ, Mortimer PS, et al. Lymphatic transfer studies with immunoglobulin scintigraphy after axillary surgery. Eur J Surg Oncol 2007;33:1052–60.
Bennett Britton TM, Buczacki SJ, Turner CL, Vowler SL, Pain SJ, Purushotham AD. Venous changes and lymphoedema 4 years after axillary surgery for breast cancer. Br J Surg 2007;94:833–4. https://doi.org/10.1002/bjs.5711
2008
Wishart GC, Greenberg DC, Britton PD, Chou P, Brown CH, Purushotham AD, Duffy SW. Screen-detected vs symptomatic breast cancer: is improved survival due to stage migration alone? Br J Cancer 2008;98:1741-4.
Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg 2008;95:302–9. https://doi.org/10.1002/bjs.5943
2009
Peters AM, Fowler JC, Britton TB, Solanki CK, Ballinger JR, Ravichandran D, et al. Functional variation in lymph node arrangements within the axilla. Lymphat Res Biol 2009;7:139–44. https://doi.org/10.1089/lrb.2008.1021
Bennett Britton TM, Wallace SM, Wilkinson IB, Mortimer PS, Peters AM, Purushotham AD. Sympathetic nerve damage as a potential cause of lymphoedema after axillary dissection for breast cancer. Br J Surg 2009;96:865–9. https://doi.org/10.1002/bjs.6660
Britton TB, Solanki CK, Pinder SE, Mortimer PS, Peters AM, Purushotham AD. Lymphatic drainage pathways of the breast and the upper limb. Nucl Med Commun 2009;30:427–30. https://doi.org/10.1097/MNM.0b013e328315a6c6
Bennett Britton TM, Purushotham AD. Understanding breast cancer-related lymphoedema. Surgeon 2009;7:120–4.
Fowler JC, Solanki CK, Guenther I, Barber R, Miller F, Bobrow L, et al. A pilot study of dual-isotope lymphoscintigraphy for breast sentinel node biopsy comparing intradermal and intraparenchymal injection. Eur J Surg Oncol 2009;35:1041–7. https://doi.org/10.1016/j.ejso.2009.02.018
2010
Purushotham A, Pinder S, Cariati M, Harries M, Goldhirsch A. Neoadjuvant chemotherapy: not the best option in estrogen receptor-positive, HER2-negative, invasive classical lobular carcinoma of the breast? J Clin Oncol 2010;28:3552-4.
Fowler JC, Britton TB, Provenzano E, Ravichandran D, Lawrence D, Solanki CK, et al. Measurement of lymph node function from the extraction of immunoglobulin in lymph. Scand J Clin Lab Invest 2010;70:112–15. https://doi.org/10.3109/00365510903572040
Lewison G, Purushotham A, Mason M, McVie G, Sullivan R. Understanding the impact of public policy on cancer research: a bibliometric approach. Eur J Cancer 2010;46:912–19. https://doi.org/10.1016/j.ejca.2009.12.020
O’Mahony S, Britton TB, Ballinger JR, Solanki CK, Barber RW, Mortimer PS, et al. Delivery of radiolabelled blood cells to lymphatic vessels by intradermal injection: a means of investigating lymphovenous communications in the upper limb. Nucl Med Commun 2010;31:121–7. https://doi.org/10.1097/MNM.0b013e328330dd14
2011
Sullivan R, Purushotham AD. Avoiding the zero sum game in global cancer policy: beyond 2011 UN high level summit. Eur J Cancer 2011;47:2375–80. https://doi.org/10.1016/j.ejca.2011.08.017
Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011;12:933–80. https://doi.org/10.1016/S1470-2045(11)70141-3
2012
Sullivan R, Homberg L, Purushotham AD. Cancer risk and prevention in a globalised world: solving the public policy mismatch. Eur J Cancer 2012;48:2043–5. https://doi.org/10.1016/j.ejca.2012.01.019
Purushotham AD, Lewison G, Sullivan R. The state of research and development in global cancer surgery. Ann Surg 2012;255:427–32. https://doi.org/10.1097/SLA.0b013e318246591f
Haire K, Burton C, Park R, Reynolds J, Stewart D, Purushotham AD. Integrated Cancer System: a perspective on developing an integrated system for cancer services in London. London J Prim Care 2012;5:29–34.
2013
Purushotham A, Bains S, Lewison G, Szmukler G, Sullivan R. Cancer and mental health – a clinical and research unmet need. Ann Oncol 2013;24:2274–8. https://doi.org/10.1093/annonc/mdt214
Klevesath MB, Pantel K, Agbaje O, Provenzano E, Wishart GC, Gough P, et al. Patterns of metastatic spread in early breast cancer. Breast 2013;22:449–54. https://doi.org/10.1016/j.breast.2013.04.017
Enfield L, Cantanhede G, Douek M, Ramalingam V, Purushotham A, Hebden J, Gibson A. Monitoring the response to neoadjuvant hormone therapy for locally advanced breast cancer using three-dimensional time-resolved optical mammography. J Biomed Opt 2013;18:56012. https://doi.org/10.1117/1.JBO.18.5.056012
Purushotham A, Cornwell J, Burton C, Stewart D, Sullivan R. What really matters in cancer?: Putting people back into the heart of cancer policy. Eur J Cancer 2013;49:1669–72. https://doi.org/10.1016/j.ejca.2013.01.004
2014
Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast. Ann Surg Oncol 2014;21:1237–45. https://doi.org/10.1245/s10434-013-3379-6
Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:e351–62. https://doi.org/10.1016/S1470-2045(13)70590-4
Honeth G, Lombardi S, Ginestier C, Hur M, Marlow R, Buchupalli B, et al. Aldehyde dehydrogenase and estrogen receptor define a hierarchy of cellular differentiation in the normal human mammary epithelium. Breast Cancer Res 2014;16:R52. https://doi.org/10.1186/bcr3663
Purushotham A, Shamil E, Cariati M, Agbaje O, Muhidin A, Gillett C, et al. Age at diagnosis and distant metastasis in breast cancer – a surprising inverse relationship. Eur J Cancer 2014;50:1697–705. https://doi.org/10.1016/j.ejca.2014.04.002
Suyoi A, Bains SK, Kothari A, Douek M, Agbaje O, Hamed H, et al. When is a completion axillary lymph node dissection necessary in the presence of a positive sentinel lymph node? Eur J Cancer 2014;50:690–7. https://doi.org/10.1016/j.ejca.2013.11.024
Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol 2014;21:1237–45.
2015
Bains SK, Peters AM, Zammit C, Ryan N, Ballinger J, Glass DM, et al. Global abnormalities in lymphatic function following systemic therapy in patients with breast cancer. Br J Surg 2015;102:534–40. https://doi.org/10.1002/bjs.9766
Bains SK, Ballinger J, Allen S, Stanton AW, Levick JR, Mortimer PS, et al. An investigation of lymphovenous communications in the upper limbs of breast cancer patients. Eur J Surg Oncol 2015;41:433–8. https://doi.org/10.1016/j.ejso.2014.11.036
Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015;16:1193–224. https://doi.org/10.1016/S1470-2045(15)00223-5
Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121–9. https://doi.org/10.1007/s10549-015-3357-8
Cariati M, Bains SK, Grootendorst MR, Suyoi A, Peters AM, Mortimer P, et al. Adjuvant taxanes and the development of breast cancer-related arm lymphoedema. Br J Surg 2015;102:1071–8. https://doi.org/10.1002/bjs.9846
Harris J, Tsianakas V, Ream E, Van Hemelrijck M, Purushotham A, Mucci L, et al. CanWalk: study protocol for a randomized feasibility trial of a walking intervention for people with recurrent or metastatic cancer. Pilot Feasibility Stud 2015;1:7. https://doi.org/10.1186/s40814-015-0003-5
Robert G, Cornwell J, Locock L, Purushotham A, Sturmey G, Gager M. Patients and staff as codesigners of healthcare services. BMJ 2015;350:g7714. https://doi.org/10.1136/bmj.g7714
Ahmed M, Purushotham AD, Horgan K, Klaase JM, Douek M. Meta-analysis of superficial versus deep injection of radioactive tracer and blue dye for lymphatic mapping and detection of sentinel lymph nodes in breast cancer. Br J Surg 2015;102:169–81. https://doi.org/10.1002/bjs.9673
Vollan HK, Rueda OM, Chin SF, Curtis C, Turashvili G, Shah S, et al. A tumor DNA complex aberration index is an independent predictor of survival in breast and ovarian cancer. Mol Oncol 2015;9:115–27. https://doi.org/10.1016/j.molonc.2014.07.019
2016
Møller H, Henson K, Lüchtenborg M, Broggio J, Charman J, Coupland VH, et al. Short-term breast cancer survival in relation to ethnicity, stage, grade and receptor status: national cohort study in England. Br J Cancer 2016;115:1408–15. https://doi.org/10.1038/bjc.2016.335
Weitsman G, Barber PR, Nguyen LK, Lawler K, Patel G, Woodman N, et al. HER2-HER3 dimer quantification by FLIM-FRET predicts breast cancer metastatic relapse independently of HER2 IHC status. Oncotarget 2016;7:51012–51026. https://doi.org/10.18632/oncotarget.9963
Cintolesi V, Stanton AW, Bains SK, Cousins E, Peters AM, Purushotham AD, et al. Constitutively Enhanced Lymphatic Pumping in the Upper Limbs of Women Who Later Develop Breast Cancer-Related Lymphedema. Lymphat Res Biol 2016;14:50–61. https://doi.org/10.1089/lrb.2016.0005
Melvin JC, Wulaningsih W, Hana Z, Purushotham AD, Pinder SE, Fentiman I, et al. Family history of breast cancer and its association with disease severity and mortality. Cancer Med 2016;5:942–9. https://doi.org/10.1002/cam4.648
Jeffs E, Purushotham A. The prevalence of lymphoedema in women who attended an information and exercise class to reduce the risk of breast cancer-related upper limb lymphoedema. Springerplus 2016;5:21. https://doi.org/10.1186/s40064-015-1629-8
2017
Lawler K, Papouli E, Naceur-Lombardelli C, Mera A, Ougham K, Tutt A, et al. Gene expression modules in primary breast cancers as risk factors for organotropic patterns of first metastatic spread: a case control study. Breast Cancer Res 2017;19:113. https://doi.org/10.1186/s13058-017-0881-y
Aggarwal A, Lewis D, Mason M, Purushotham A, Sullivan R, van der Meulen J. Effect of patient choice and hospital competition on service configuration and technology adoption within cancer surgery: a national, population-based study. Lancet Oncol 2017;18:1445–53.
Tsianakas V, Harris J, Ream E, Van Hemelrijck M, Purushotham A, Mucci L, et al. CanWalk: a feasibility study with embedded randomised controlled trial pilot of a walking intervention for people with recurrent or metastatic cancer. BMJ Open 2017;7:e013719. https://doi.org/10.1136/bmjopen-2016-013719
Professor Adrian Bagust
Up to 2000
Bagust A, Burrows J, Oakley J. Quality or quantity. Health Serv J 1992;102:23–5.
Haycox A, Bagust A, Walley T. Importance of health economics must be recognised when trials are designed. BMJ 1999;318:1696–7.
2010
Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLOS ONE 2010;5:e8933. https://doi.org/10.1371/journal.pone.0008933
Maria Bramley
2015
Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121–9. https://doi.org/10.1007/s10549-015-3357-8
Katie Riches
2015
Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121–9. https://doi.org/10.1007/s10549-015-3357-8
List of abbreviations
- ADL
- activities of daily living
- ALMANAC
- Axillary Lymphatic Mapping Against Nodal Axillary Clearance
- ANC
- axillary node clearance
- ANOVA
- analysis of variance
- AUC
- area under the curve
- AUROC
- area under the receiver operating characteristic
- BCC
- Breast Cancer Campaign
- BEA
- multifrequency bioimpedance
- BIS
- bioimpedance spectroscopy
- BMI
- body mass index
- CI
- confidence interval
- CPS
- Controlled Preference Score
- CTU
- Clinical Trials Unit
- EMM
- estimated marginal mean
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ VAS
- EuroQol visual analogue scale
- ER
- estrogen receptor
- FACT-B
- Functional Assessment of Cancer Therapy – Breast Cancer
- FACT-B+4
- Functional Assessment of Cancer Therapy – Breast Cancer, version 4
- GEE
- general estimating equation
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- IDMC
- Independent Data Monitoring Committee
- IRR
- incidence rate ratio
- LN
- log normalised
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- NSQIP
- National Surgical Quality Improvement Program
- OR
- odds ratio
- PET
- primary endocrine therapy
- PLACE
- Prevention of Lymphoedema After Clearance by External compression
- PPI
- patient and public involvement
- PPV
- positive predictive value
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RAVI
- relative arm-volume increase
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SF-6D
- Short Form Questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- TOI
- Trial Outcome Index
- WLE
- wide local excision
- WS
- workstream
