Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0707-10010. The contractual start date was in January 2009. The final report began editorial review in February 2017 and was accepted for publication in December 2018. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sonia Saxena was funded by a National Institute for Health Research (NIHR) Career Development Fellowship (CDF) (NIHR-CDF-2011-04-048). CNAM is funded by the Higher Education Funding Council for England and the NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRC) programme. Kate Costeloe reports that she was a member of the Neonatal Data Analysis Unit Board throughout this research programme. No authors have any financial or business relationships with Clevermed Ltd.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Modi et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Creating the infrastructure: the National Neonatal Research Database
Abstract
Background: Successive UK governments have highlighted the potential of clinical data to advance patient care. Difficulties experienced by high-profile projects exemplify the challenges, including limited population coverage and clinical engagement, unknown data quality and public disquiet.
Aims: To develop the use of electronic patient record (EPR) data to improve neonatal specialised care, a high-cost NHS service.
Methods: We secured approvals from Caldicott Guardians, Lead Clinicians, the National Research Ethics Service and the Health Research Authority Confidentiality Advisory Group. We established a UK Neonatal Collaborative of provider NHS trusts. We collaborated with the Royal College of Paediatrics and Child Health and the national charity Bliss to develop a parent information leaflet. We conducted a systematic review to identify neonatal databases globally. We improved data completeness and quality through close interaction with clinical teams.
Results: We achieved 100% coverage of NHS neonatal units in England, Wales and Scotland (n = 185). We created a new NHS information standard, the Neonatal Data Set (ISB1595) and a National Neonatal Research Database (NNRD) containing a defined extract from real-time, point-of-care, clinician-entered EPRs. The NNRD is now used for a wide range and growing range of purposes including clinical and health services research, quality improvement programmes, national audit, commissioning support and national and regional benchmarking.
Conclusions: We have established proof of principle that EPR data may be employed to support patient care and clinical services through research and evaluation, and reduce the burden placed on busy clinical teams by providing a single national data source to service multiple outputs.
Background
Electronic patient records
Electronic patient records have been used increasingly over the last two to three decades and represent a rich data resource. Successive UK governments have recognised the potential of NHS clinical data to improve patient care and outcomes. 1–3 However, these aspirations have been slow to be adequately realised.
The challenges faced in harnessing the power of clinical data in health care are perhaps exemplified by the lessons of care.data and other high-profile UK projects. These include limited population coverage, weak clinical engagement, unknown data quality, regulatory uncertainty and public disquiet. Confidence in the concept of clinical data as a resource to improve patient care, despite an ambition to use these to improve standards, quality of care, accountability and patient choice, has been further damaged by escalating costs, critical media reports, breaches of data security and loss of public confidence by reports that personal data would be ‘sold’ to commercial organisations.
Neonatal specialised services
In the UK, neonatal specialised services (i.e. services for newborn infants requiring care over and above normal care) are currently provided by neonatal units operating in a series of mature clinical networks, each comprising around six to eight neonatal units. Neonatal networks were introduced as part of the restructuring of neonatal services in response to a report by the Department of Health and Social Care in 2002. Each neonatal network was to be largely self-sufficient in providing care across the complete range of intensities (the traditional intensive, high dependency and special care levels). As a result, large numbers of infants were transferred between neonatal units providing different levels of care in accordance with their care requirements, with ultimate ‘repatriation’ to a neonatal unit closest to home in preparation for discharge. The concurrent need for clinical information to be readily transferable between NHS provider trusts was a cardinal driver for the introduction of EPR technologies into neonatal units.
Prior to the restructuring of neonatal services into networks, the British Association of Perinatal Medicine (BAPM) had commenced developing a ‘minimum’ Neonatal Data Set. 4 Neonatologists had long recognised the benefits of a uniform approach to recording clinical information, including the the ability to evaluate outcomes consistently at a national level. Over the period of the restructuring and subsequently, successive BAPM working parties made refinements to the ‘minimum’ Neonatal Data Set. 4
Neonatal electronic patient records
Over the last decades, a UK-based commercial firm had developed a technical platform for neonatal data in close consultation with neonatal clinicians. This platform has evolved, with successive versions introduced into use over the years. Electronic systems were introduced across all NHS provider trusts from 2005 onwards. The EPR system in most widespread use includes fixed-choice and free-text items, with the NHS number as the principal identifier. Data are recorded daily throughout the neonatal inpatient stay. Clinician-entered diagnoses are converted to the International Classification of Diseases, Tenth Edition (ICD-10) codes. 5
Thus, the reorganisation of neonatal services into managed clinical networks, an established, commercially available technical platform with a user front-end developed in close collaboration with neonatal clinicians, and a ‘minimum’ Neonatal Data Set established by a professional organisation, provided the three prime underpinning requirements on which to develop electronic clinical data for secondary purpose, including research and evaluation. Members of the Medicines for Neonates investigator group had been involved in several of the developments in relation to neonatal data described above, and electronic records more widely (e.g. as members of successive BAPM data working parties) and, hence, brought a wealth of experiential knowledge to the programme.
Aims
Our aim was to develop the use of EPR data for secondary purposes to support neonatal services and facilitate research to improve newborn care and outcomes. We also aimed to secure strong clinician engagement and parental support, implement measures to assess data quality systematically, and establish a new national resource.
Methods
We established a Programme Steering Committee comprising the Medicines for Neonates investigator group, an independent chairperson and independent members, including a patient and public involvement (PPI) representative. We conducted a systematic review to identify and describe existing neonatal databases. We investigated the regulatory processes required, and considered and tested ways in which to establish close clinical engagement, evaluate and improve data completeness and quality, and provide information to parents nationally.
Systematic review methods
We carried out an electronic search on MEDLINE (via Ovid), EMBASE (via Ovid), and CINAHL (Cumulative Index to Nursing and Allied Health Literature; via Athena), of publications covering the period 1 January 2000 to 15 March 2015. We applied language restrictions, including only English, French, German, Italian, Russian and Spanish articles. We employed the following search terms: ‘intensive care units, neonatal/’ OR ‘intensive care, neonatal/’ OR ‘neonatal intensive care units’ OR ‘NNU’ OR ‘NICU’ OR ‘neonatal ICU’ AND ‘infant/’ OR ‘neonat$’ AND ‘database$’ or ‘registry’ OR ‘registries’ OR ‘dataset$’ OR ‘data set$’ OR ‘vital statistics’. The literature search strategy is summarised in Figure 1.
FIGURE 1.
Literature search strategy.

We carried out grey literature searches on the Web of Science and the Ovid Maternity and Infant Care Databases using the free-text terms ‘neonatal intensive care unit’ AND ‘infant’ AND ‘database’.
We exported results, including abstracts, into EndNote X7 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA]. Two researchers reviewed titles and abstracts to identify relevant publications and remove duplicate results. We retained publications that mentioned databases of patient-level information (administrative or clinical) and specified that data covered populations of infants from more than one neonatal unit. We reviewed full-text articles, references and websites. We entered extracted predefined information into Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) (Table 1).
| Name | Original definition from PROSPERO submission | Updated definition for final systematic review |
|---|---|---|
| Study identification | To include main author and year of publication (e.g. Smith, John et al. 2015) | Same as original but also includes websites for databases |
| Database name | The name of the database | No change |
| Primary purpose | Administrative, clinical, research, audit, other | No change |
| Country | Free text for country where database is based | No change |
| Scope | Regional, national or international | No change |
| Scope name | Free text to specify the region of country or countries | No change |
| Population limit | Admissions in hospital, births in hospital | Admission to neonatal units, all infants included in admission to neonatal units, gestational age and/or birthweight cut-off point, admissions or births in enrolled hospitals, health insurance enrolment, no limitations, entire region included |
| Data source | Recorded specifically for database or secondary-use database | Secondary-use database broken down as data extracted from clinical source (electronic health records) or data extracted from administrative source |
| Number of infants reported | Number | No change |
| Time period for number of infants reported | The range of years that the database spans from earliest time period that could be identified to the present (e.g. 2000–15) | Includes if database is still enrolling patients |
| Maternal characteristics | Mother’s ethnicity (yes/no), mother’s age (yes/no), mother’s education (yes/no), mode of delivery (yes/no) | No change |
| Infant characteristics | Gestational age in weeks (yes/no), gestational age in days (yes/no), gestational age definition (free text), birthweight (yes/no), sex (yes/no), multiplicity (yes/no), infant identification (yes/no), infant identification type (free text), intervention (yes/no), intervention type (free text), diagnoses (yes/no), diagnoses coded (yes/no), laboratory samples (yes/no), abdominal X-rays (yes/no), retinopathy of prematurity (yes/no), cranial ultrasound (yes/no), post-discharge information (free text) | Same as before except for the following changes: gestational age definition (yes/no), post-discharge information (yes/no) and blood cultures (yes/no) instead of laboratory samples (yes/no) |
| Funding | Not collected | Hospital subscription, insurance, mixed funding including support from public body. No current funding support identified. Support from public body |
Creating the Neonatal Data Set
We built on and extended the BAPM ‘minimum’ Neonatal Data Set (data items used to derive daily level of care, the currency underpinning the commissioning of neonatal specialised care services) and the mandated National Critical Care Minimum Data Set (NCC-MDS) (used for deriving neonatal Healthcare Resource Groups) to build a national ‘Neonatal Data Set’. This comprised basic demographic details (e.g. date of birth, birthweight), clinical interventions captured daily (e.g. respiratory support, type of feeds, surgical procedures, high-cost drugs), clinical outcomes and diagnoses. Each data item is clearly defined in an accompanying metadata set, and mapped to existing national standards as well as ICD codes. There was a preliminary assessment of the compatibility of Neonatal Data Set items for conversion to Snomed computed tomography (CT) terminology (international medical nomenclature); the conclusion was that the Neonatal Data Set is compatible, but conversion would require clinical and technical resourcing.
With the support of the NHS Information Standards Board (now NHS Digital), we submitted the Neonatal Data Set for approval as a national NHS standard. Following initial submission, the Information Standards Board issued an ‘advance notice’ of the Neonatal Data Set standard. In the process to becoming a national standard, the Neonatal Data Set evolved through changes that came about following public consultation, review by terminology experts at the NHS data dictionary, and alignment to other national data sets. As a result, 25 data items were added to the revised Neonatal Data Set and an existing 28 items were recoded to reflect data dictionary terminology or other national criteria. Full approval of the standard was obtained in December 2013. The stages leading to approval are shown in Table 2. The current approved Neonatal Data Set (SCCI1595) for standard items and age 2 years items are provided as Appendix 3.
| Submission stage | Document reference | Document title | Version | Date |
|---|---|---|---|---|
| Needs | Needs stage | National Neonatal Data Set Needs Stage Submission | 1.7 | 7 September 2012 |
| Requirements gathering | Requirements stage | National Neonatal Data Set ISB 1595 Requirements Stage Submissions | 0.4 | 6 December 2012 |
| Draft and full approval | Review of central returns approval | Review of Central Returns Approval notification OR 2027 FT6 0001PMAND | 1 | 13 August 2013 |
| Draft and full approval | Submission | Neonatal Data Set ISB 1595 Submission | 1.4 | 16 October 2013 |
| Draft and full approval | Specification | Neonatal Data Set ISB 1595 Specification | 0.5 | 16 October 2013 |
| Draft and full approval | Data set | Neonatal Data Set ISB 1595 Release 1 | 2.1 | 16 October 2013 |
| Draft and full approval | Evidence of consultation | Neonatal Data Set ISB 1595 Evidence of consultation | 0.7 | 16 October 2013 |
| Draft and full approval | Data discovery | Neonatal Data Set ISB 1595 Data Discovery | 2.5 | 16 October 2013 |
| Draft and full approval | Implementation and maintenance plan | Neonatal Data Set ISB 1595 Implementation and Maintenance Plan | 0.5 | 16 October 2013 |
| Draft and full approval | Issues and risks | Neonatal Data Set ISB 1595 Issues Log & Risk Register | 10 | 16 October 2013 |
Results
Systematic review
Search results
The search yielded 2037 unique papers. Following a review of the titles and abstracts, 415 papers met our prespecified criteria. From these, we identified 82 databases and, for 52, data were recorded specifically for the database. In 21 papers, data were obtained from a primary administrative source and in nine papers data were obtained from a clinical source (Figure 2). Five countries accounted for the location of more than half (47/82) of all identified databases: the USA (n = 24), Canada (n = 11), the UK (n = 7) and Australia/New Zealand (n = 5). We provide details of the databases identified in Table 3.
FIGURE 2.
Data flows into the Neonatal Data Analysis Unit.
| Number | Name | Primary purpose | Country | Scope | Population limits | Data source | Time period covered by publication (database still enrolling patients) | Maternal variables | Infant variables | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alberta Perinatal Health Program Database6 | A | Canada | Regional (Alberta) | No limitations, entire region included | Extracted from administrative data source | 2002–4 (yes) | Mother’s age, mode of delivery | Birthweight, sex, multiplicity | Support from public body |
| 2 | Alere or Matria Health care/Paradigm7,8 | C | USA | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2003–7 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Hospital subscription |
| 3 | Arizona Newborn Intensive Care Program9 | A | USA | Regional (Arizona) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Extracted from administrative data source | 1994–8 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, identifier, intervention, ROP, post-discharge information | Support from public body |
| 4 | Asian Network on Maternal and Newborn Health10 | R | Asia (Malaysia, Japan, Hong Kong and Singapore) | International | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2003–6 (yes) | Mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, diagnoses, abdominal X-rays, cranial ultrasound | Support from public body |
| 5 | AUDIOPOG Sentinel Network11 | C | France | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 1994–2008 (yes) | Mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | No current funding support identified |
| 6 | Australia and New Zealand Neonatal Network12 | C | Australia/New Zealand | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1994–2012 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, blood cultures, ROP, post-discharge information | Mixed funding, including support from public body |
| 7 | Better Outcomes Registry and Network13 | A | Canada | Regional (Ontario) | No limitations, entire region included | Recorded specifically for database | 2006–10 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, ROP, post-discharge information | Support from public body |
| 8 | California Patient Discharge Linked Birth Cohort Database14,15 | A | USA | Regional (California) | Admissions or births in enrolled hospitals | Extracted from administrative data source | 1999–2004 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity | Support from public body |
| 9 | California Perinatal Quality Care Collaborative16 | C | USA | Regional (California) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2005–11 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 10 | Canadian Institute for Health Information Discharge Abstract Database17 | A | Canada | National | No limitations, entire region included | Extracted from administrative data source | 2002–10 (yes) | Mother’s ethnicity, mother’s age | Gestational age in weeks, birthweight, sex, identifier, intervention, ROP, post-discharge information | Support from public body |
| 11 | Canadian Neonatal Follow-Up Network18 | C | Canada | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2010–11 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, diagnoses, blood cultures, ROP, post-discharge information | Support from public body |
| 12 | Canadian Neonatal Network19 | C | Canada | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2013–14 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, diagnoses, blood cultures | Support from public body |
| 13 | Canadian Paediatric Surgery Network20 | C | Canada | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2013–14 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, identifier, intervention, diagnoses, abdominal X-rays, cranial ultrasound | Support from public body |
| 14 | Children’s Hospital Neonatal Database21 | C | USA | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2010–11 (yes) | Mother’s ethnicity | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Hospital subscription |
| 15 | Colorado Birth Certificate Database22 | A | USA | Regional (Colorado) | No limitations, entire region included | Recorded specifically for database | 2007–12 (yes) | Mother’s ethnicity | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 16 | Consortium of Safe Labor Database23 | R | USA | National | Admissions or births in enrolled hospitals | Extracted from clinical data source (electronic health records) | 2002–8 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 17 | Croatian Intensive Care network24 | C | Croatia | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2004–5 (yes) | No variables identified from those sought | Identifier | Support from public body |
| 18 | Danish Medical Birth Registry25 | A | Denmark | National | No limitations, entire region included | Recorded specifically for database | 1997–2008 (yes) | Mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 19 | Danish Neonatal Clinical Database (NeoBase)26 | C | Denmark | Regional (North And South Jutland) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Extracted from clinical data source (electronic health records) | 2005–6 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight, sex, identifier, intervention | Support from public body |
| 20 | Emilia-Romagna Health Agency27 | A | Italy | Regional (Emilia-Romagna) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2002–9 (yes) | No variables identified from those sought | Gestational age in weeks | Support from public body |
| 21 | EPICure28 | R | UK | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2006–7 (no) | Mother’s ethnicity, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, intervention, ROP, post-discharge information | Support from public body |
| 22 | Erie County Register29 | A | USA | Regional (Erie County, New York) | No limitations, entire region included | Recorded specifically for database | 2006–8 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, identifier, intervention | Support from public body |
| 23 | EuroNeoNet30 | C | European | International (Austria, Belgium, Croatia, Finland, France, Germany, Greece, Italy, Poland, Portugal, Russia, Slovenia, Spain, Switzerland, the Netherlands, Turkey and the UK) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2006–11 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, ROP, post-discharge information | Support from public body |
| 24 | Florida birth registry31 | A | USA | Regional (Florida) | No limitations, entire region included | Recorded specifically for database | 2009–10 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 25 | Intermountain Health care32 | C | USA | Regional (Utah) | Admissions or births in enrolled hospitals | Extracted from clinical data source (electronic health records) | 2003–5 (yes) | No variables identified from those sought | Birthweight | Hospital subscription |
| 26 | Israel National VLBW Infant Database33 | C | Israel | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1995–2003 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 27 | Japanese Vital Statistics34 | A | Japan | National | No limitations, entire region included | Recorded specifically for database | 1999–2008 (yes) | Mother’s age | Gestational age in weeks, birthweight, sex, multiplicity | Support from public body |
| 28 | Kaiser Permanente Medical Care Program35,36 | C | USA | Regional (Northern California and Boston, Massachusetts) | Admissions or births in enrolled hospitals | Extracted from clinical data source (electronic health records) | 1995–6 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Hospital subscription |
| 29 | Kids’ Inpatient Databases37 | R | USA | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2003–12 (yes) | Mother’s ethnicity | Birthweight, sex | Support from public body |
| 30 | Linked Emergency Management and Research Institute – Department of Health and Family Welfare, Government of Gujarat38 | A | India | Regional (Gujarat) | Admissions or births in enrolled hospitals | Extracted from administrative data source | 2008–9 (yes) | Mother’s age, mode of delivery | Gestational age in weeks | Support from public body |
| 31 | London Neonatal Transfer Service39 | C | UK | Regional (London) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2005–11 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight | Support from public body |
| 32 | Massachusetts Community Health Information Profile (MassCHIP) and PELL40,41 | A | USA | Regional (Massachusetts) | No limitations, entire region included | Extracted from administrative data source | 2002–10 (yes) | Mother’s ethnicity, mother’s age | Gestational age in weeks, multiplicity, intervention | Support from public body |
| 33 | Malaysian National Neonatal Registry42 | C | Malaysia | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2006–7 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, blood cultures, abdominal X-rays, cranial ultrasound | Support from public body |
| 34 | Medicaid Analytic eXtract43 | A | USA | National | Health insurance enrolment | Extracted from administrative data source | 2006–8 (yes) | Mode of delivery | Identifier | Support from public body |
| 35 | Memorial Care Medical Centres: Perinatal database, Quality Improvement Database44 | C | USA | Regional (California) | Admissions or births in enrolled hospitals | Recorded specifically for database | 2002–3 (yes) | Mother’s ethnicity, mode of delivery | Gestational age in weeks, identifier, intervention | Hospital subscription |
| 36 | Michigan Linked Records45 | A | USA | Regional (Michigan) | No limitations, entire region included | Extracted from administrative data source | 2003–4 (yes) | Mother’s ethnicity | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 37 | National Centre for Health Statistics linked live birth and infant death cohort file46 | A | USA | National | No limitations, entire region included | Extracted from administrative data source | 1998–9 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity | Support from public body |
| 38 | National Collaborative Perinatal Neonatal Network47 | C | Lebanon | Regional (Greater Beirut) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2009–10 (not identified) | Mother’s age, mother’s education | Gestational age in weeks, birthweight, sex, multiplicity | Support from public body |
| 39 | National Institute for Health and Welfare: Medical Birth Register48 | A | Finland | National | No limitations, entire region included | Recorded specifically for database | 2012–13 (yes) | Mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 40 | National Neonatal Database SEN150049 | C | Spain | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2002–5 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, diagnoses, blood cultures, abdominal X-rays, cranial ultrasound | Support from public body |
| 41 | National Neonatal Perinatal Database50 | C | India | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2002–3 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, intervention | Support from public body |
| 42 | NNRD51 | C | UK | National | Admission to neonatal units, all infants included | Extracted from clinical data source (electronic health records) | 2009–11 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, diagnoses, blood cultures, abdominal X-rays, ROP, cranial ultrasound, post-discharge information | No current funding support identified |
| 43 | National Perinatal Information Centre52 | A | USA | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2004–8 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, sex, identifier | Support from public body |
| 44 | National Perinatal Registry of Slovenia53 | A | Slovenia | National | No limitations, entire region included | Recorded specifically for database | 2012–13 (yes) | No variables identified from those sought | Gestational age in weeks | Support from public body |
| 45 | National Perinatal Registry, the Netherlands54 | C | The Netherlands | National | No limitations, entire region included | Recorded specifically for database | 2003–7 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 46 | National Perinatal Data Collection55 | A | Australia/New Zealand | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Extracted from administrative data source | 2001–5 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 47 | National Registry of Respiratory Distress Syndrome in Romania56 | C | Romania | National | Admissions or births in enrolled hospitals | Recorded specifically for database | 2011–12 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight, sex, identifier, intervention | Support from public body |
| 48 | Neonatal Intensive Care Outcomes and Research Evaluation57 | C | UK | Regional (Northern Ireland) | Admission to neonatal units, all infants included | Extracted from clinical data source (electronic health records) | 1999–2000 (yes) | Mother’s ethnicity, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, blood cultures, abdominal X-rays, cranial ultrasound | Support from public body |
| 49 | Neonatal Research Network of Japan58 | R | Japan | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2003–8 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, intervention, blood cultures | Support from public body |
| 50 | NEOSANO’s Perinatal Network in Mexico59 | A | Mexico | Regional (Mexico City, Tlaxcala City and Oaxaca City) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2006–9 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, multiplicity | Support from public body |
| 51 | New Jersey Perinatal Linked Data-Set60 | A | USA | Regional (New Jersey) | No limitations, entire region included | Extracted from administrative data source | 1997–2005 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, identifier | Support from public body |
| 52 | New South Wales Newborn and Paediatric Emergency Transport Service61 | C | Australia/New Zealand | Regional (New South Wales) | Admissions or births in enrolled hospitals | Recorded specifically for database | 1992–2001 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight, sex, intervention | Support from public body |
| 53 | New York State-wide Perinatal Data System62 | A | USA | Regional (New York) | Admissions or births in enrolled hospitals | Extracted from administrative data source | 1996–2003 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 54 | Newfoundland and Labrador Provincial Perinatal Program Database63 | C | Canada | Regional (Newfoundland and Labrador) | Admissions or births in enrolled hospitals | Extracted from administrative data source | 2001–9 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity | Support from public body |
| 55 | NICHD Neonatal Research Network Generic Database64 | R | USA | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1998–2009 (yes) | Mother’s ethnicity, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, ROP, post-discharge information | Support from public body |
| 56 | Nova Scotia Atlee Perinatal Database65 | A | Canada | Regional (Nova Scotia) | No limitations, entire region included | Extracted from administrative data source | 2002–11 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 57 | NSW Pregnancy and Newborn Services Network66 | C | Australia/New Zealand | Regional (New South Wales And Australian Centralised Territory) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1997–2006 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, intervention | Support from public body |
| 58 | Pediatrix BabySteps Clinical Data Warehouse67 | C | USA | National | Admission to neonatal units, all infants included | Extracted from clinical data source (electronic health records) | 1996–2010 (yes) | Mother’s ethnicity, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, blood cultures | Hospital subscription |
| 59 | Perinatal and Neonatal Surveys in Saxony68 | C | Germany | Regional (Saxony) | No limitations, entire region included | Recorded specifically for database | 2001–5 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 60 | Perinatal database of Middlesex Country, Canada69 | C | Canada | Regional (Middlesex County, Ontario) | Admissions or births in enrolled hospitals | Recorded specifically for database | 2002–11 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex | Support from public body |
| 61 | Perinatal Revision South70 | C | Sweden | Regional (Southern Sweden) | Admissions or births in enrolled hospitals | Recorded specifically for database | 1995–6 (yes) | Mode of delivery | Gestational age in weeks, birthweight, identifier | Support from public body |
| 62 | Perinatal Services British Columbia71 | A | Canada | Regional (British Columbia) | Admissions or births in enrolled hospitals | Extracted from administrative data source | 2004–14 (yes) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 63 | Population Health Research Data Repository72 | A | Canada | Regional (Manitoba) | No limitations, entire region included | Extracted from administrative data source | 2004–9 (yes) | Mother’s age | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 64 | Scottish Administrative Linked Data73 | A | UK | National | No limitations, entire region included | Extracted from administrative data source | 1981–2007 (yes) | Mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 65 | Seguro Medico para una Nueve Generacio74 | A | Mexico | National | Health insurance enrolment | Recorded specifically for database | 2008–9 (yes) | No variables identified from those sought | Gestational age in weeks, sex, identifier | Support from public body |
| 66 | Swedish Neonatal Quality Register75,76 | C | Sweden | National | Admission to neonatal units, all infants included | Extracted from administrative data source | 2001–2 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, blood cultures, ROP, post-discharge information | Support from public body |
| 67 | Swiss Neonatal Network77 | C | Switzerland | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1996–2008 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, intervention, blood cultures, ROP, post-discharge information | Hospital subscription |
| 68 | Taiwan’s National Health Insurance Research Database78 | A | Taiwan | National | Health insurance enrolment | Extracted from administrative data source | 1998–2001 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight, sex, intervention, ROP, post-discharge information | Support from public body |
| 69 | Tennessee Hospital Discharge Data System79 | A | USA | Regional (Tennessee) | Admissions or births in enrolled hospitals | Extracted from administrative data source | 2003–5 (yes) | Mother’s ethnicity | Birthweight, sex, identifier, intervention | Support from public body |
| 70 | The National Neonatology Database80 | C | The Netherlands | National | Admission to neonatal units, all infants included | Recorded specifically for database | 2003–5 (yes) | No variables identified from those sought | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 71 | The Neonatal Survey81 | C | UK | Regional (East Midlands and Yorkshire) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2008–10 (yes) | Mother’s ethnicity, mother’s age, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, infant identification, intervention, diagnoses, laboratory samples, ROP, post-discharge information | Support from public body |
| 72 | The WHO’s Global Survey for Maternal and Perinatal Health82,83 | S | International [Africa (Angola, Democratic Republic of Congo, Algeria, Kenya, Niger, Nigeria and Uganda), Latin America (Argentina, Brazil, Cuba, Ecuador, Mexico, Nicaragua, Paraguay and Peru) and Asia (Cambodia, China, India, Japan, Nepal, Philippines, Sri Lanka, Thailand and Vietnam)] | International | No limitations, entire region included | Recorded specifically for database | 2004–8 (no) | Mother’s age, mother’s education, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier | Support from public body |
| 73 | Vermont Oxford Network84 | C | International (Australia, Brazil, Canada, China, Columbia, Finland, Germany, Hungary, Ireland, Italy, Kuwait, Malaysia, Namibia, Poland, Portugal, Qatar, Romania, Saudi Arabia, Singapore, Slovenia, South Africa, Spain, Switzerland, Taiwan, Turkey, United Arab Emirates, the UK and the USA) | International | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1990–2012 (yes) | Mother’s ethnicity, mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention, blood cultures, abdominal X-rays, cranial ultrasound | Hospital subscription |
| 74 | Victorian Perinatal Data Collection Unit85 | R | Australia/New Zealand | Regional (Victoria) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1979–97 (no) | No variables identified from those sought | Birthweight, sex, identifier, ROP, post-discharge information | Support from public body |
| 75 | West Midlands Perinatal Institute86 | C | UK | Regional (West Midlands) | No limitations, entire region included | Recorded specifically for database | 2008–9 (yes) | No variables identified from those sought | No variables identified from those sought | No current funding support identified |
| 76 | Wisconsin Linked Birth Record File87 | A | USA | Regional (Wisconsin) | Health insurance enrolment | Extracted from administrative data source | 2001–2 (yes) | Mother’s ethnicity, mother’s age, mother’s education, mode of delivery | Birthweight, sex, multiplicity, identifier | Support from public body |
| 77 | AOK National Insurance Entries88 | A | Germany | National | Health insurance enrolment | Recorded specifically for database | 2002–6 (yes) | No variables identified from those sought | No variables identified from those sought | Insurance |
| 78 | Regional Census Data89 | A | Germany | Regional (Westfalen Lippe) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 1990–6 (yes) | No variables identified from those sought | Blood cultures | Support from public body |
| 79 | Neonatal Quality Assurance System90 | A | Germany | Regional (Baden Wuertemberg) | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2003–4 (yes) | No variables identified from those sought | No variables identified from those sought | Support from public body |
| 80 | Bourgogne database91 | A | France | Regional (Bourgogne) | Admissions or births in enrolled hospitals | Extracted from clinical data source (electronic health records) | 2000–1 (yes) | Mode of delivery | Gestational age in weeks, birthweight, sex, multiplicity, identifier, intervention | Support from public body |
| 81 | Multicentre national database92 | R | France | National | Admission to neonatal units, gestational age and/or birthweight cut-off point | Recorded specifically for database | 2005–6 (yes) | No variables identified from those sought | Gestational age in weeks, sex, intervention | Support from public body |
| 82 | Hessen Neonatal Register93 | A | Germany | Regional (Hessen) | No limitations, entire region included | Recorded specifically for database | 1989–2012 (yes) | No variables identified from those sought | No variables identified from those sought | Support from public body |
Primary purpose of databases
Of the 38 national databases, the primary purpose was clinical in 18, administrative in 13 and research in seven. Of the 40 regional databases 15 were clinical, 23 were administrative and two were research orientated. We identified four international databases (two were clinical, one was research and one was surveillance).
Data sources
Specific data collection is required for 28 out of 38 national databases. Data are extracted from a primary administrative source for seven databases, and from a primary clinical source for three databases (UK: NNRD; USA: Consortium of Safe Labor Database and Pediatrix BabySteps Clinical Data Warehouse) (see Table 3). Twenty-one of the 40 regional databases require specific data collection and, for 14, the source is an administrative database and, for 5, the source is clinical (see Table 3). All four international databases require specific data recording.
Population coverage
Twenty-seven databases hold data on all admissions to neonatal units, and the remaining databases restrict data by gestational and/or birthweight cut-off points, and/or enrolment or insurance cover.
Funding sources
Of the 82 databases identified, 71 receive funding from public sources, eight are funded through hospital subscriptions and one is funded through private insurance. We were unable to identify the funding source for the French AUDIPOG (Association des Utilisateurs de Dossiers Informatisés en Pédiatrie, Obstétrique et Gynécologie) Network;11 the NNRD was developed in part through public research funding, but has no ongoing funding support.
Summary
The NNRD is one of six national neonatal databases with ongoing data acquisition, primarily developed to support research. Uniquely, and in contrast to each of the other five (databases 16, 29, 49, 55 and 81 in Table 3), data in the NNRD are extracted from EPRs rather than being recorded specifically. There is complete national coverage of all admissions to neonatal units and no gestational age, birthweight, insurance cover or other restrictions.
Regulatory approvals
We obtained National Research Ethics Service approval in 2010 to establish a NNRD from extracts from EPRs, undertake projects within the Medicines for Neonates Programme, and employ the NNRD for NHS service evaluations and other research studies (REC reference number 10/H0803/151; provided as Appendices 5 and 6). We obtained approval in 2010 from the Confidentiality Advisory Group of the Health Research Authority [formerly Ethics and Confidentiality Committee of the National Information Governance Board; reference number ECC 8–05(f)/2010; provided as Appendix 4] to receive specific patient identifiers for the purpose of linking to Hospital Episode Statistics (HES) data.
Under standard operating procedures for Research Ethics Committees, site-specific approval is not required for studies conducted using research databases. There is no requirement for specific ethics approval for data collection centres that provide data because, under the Research Governance Framework, data collection centres are not regarded as research sites. NHS trust approval (‘R&D’ approval) is only required from the NNRD host institution (i.e. not from each data collection centre). However, we were informed that ‘local collaborators at Data Collection Centres within the NHS will require internal permission from their NHS care organisation to collect and supply data relating to NHS patients’. 94 We addressed this requirement by seeking Caldicott Guardian and Lead Neonatal Clinician approval from every NHS trust providing neonatal specialised care, to receive a defined extract from their neonatal EPRs, to hold these in the NNRD and to use these in NHS service evaluations and Research Ethics Committee-approved research studies. We obtained approvals incrementally and all NHS trusts in England, Wales and Scotland have now granted approval.
The National Neonatal Research Database
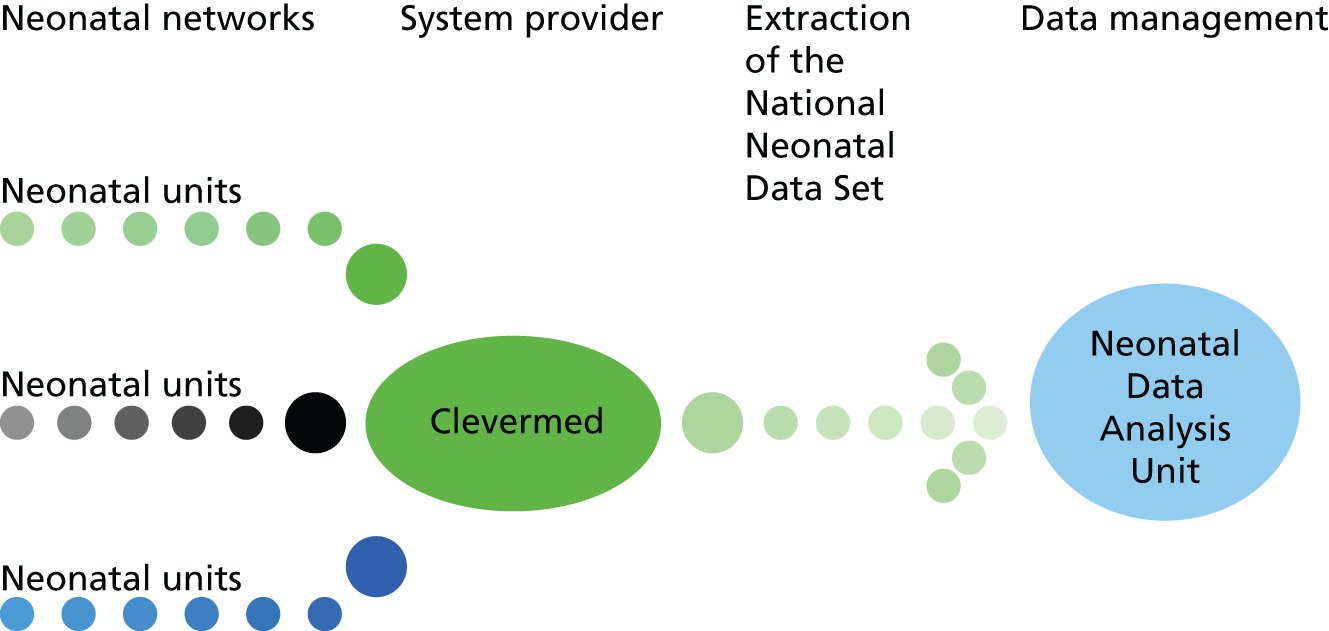
The data items constituting the Neonatal Data Set (NND) are extracted from EPRs created by clinical staff on all admissions to neonatal units in England, Wales and Scotland. Neonatal units in Northern Ireland utilise the same EPR platform but, to date, the regulatory approvals governing data transfer into the NNRD have not been sought. Following receipt of the necessary approvals, retrospective data extraction was undertaken so that the NNRD contains data from 2007 to the present. The NNRD is updated quarterly, and to date it contains data on approximately half a million infants and > 5 million care days. All neonatal units across England, Wales and Scotland have approved the release of Neonatal Data Set data items for inclusion in the NNRD (the total number of neonatal units is approximately 200, which has fluctuated over the course of the Medicines for Neonates programme as neonatal units have merged or reorganised).
An NHS approved supplier, Clevermed Ltd (Edinburgh, UK), provides a web-based data capture platform known as Neonatal.Net or BadgerNet. Data held by Clevermed Ltd are stored on a secure N3 server and transmitted to the Neonatal Data Analysis Unit (NDAU) where they are used to create the NNRD after merging and cleaning of files. The NNRD is held on the NHS servers of Chelsea and Westminster NHS Foundation Trust. Data flows are shown in Figures 2 and 3.
FIGURE 3.
Data flows to create the NNRD. ONS, Office for National Statistics.
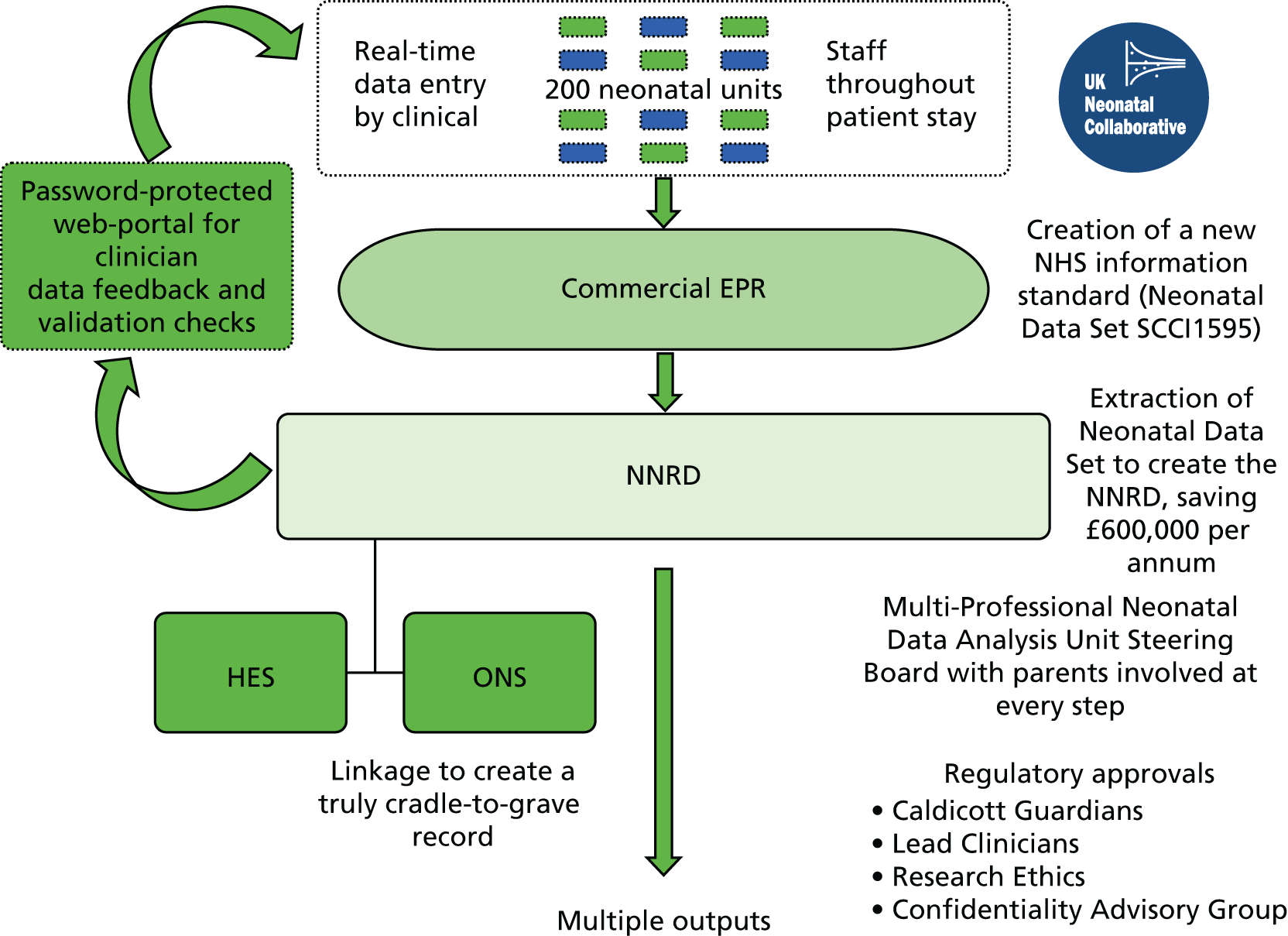
Data management
At the NDAU, all data extracted from the neonatal EPRS are interrogated to identify duplicate, missing and potentially erroneous entries. Items are considered potentially erroneous if they fail a series of out of range, internal logic, and internal inconsistency checks.
A web-based portal was created to notify neonatal unit lead clinicians of missing or potentially erroneous entries. If clinical teams amend errors or complete missing fields, this is done in the baby’s EPR and this is sent to the NDAU at the next download. Initially, this process was confined to the data items (approximately 60 items) used for analyses for the National Neonatal Audit Programme. In addition, as the NNRD has become used for research studies, if key data items are required for specific projects then these are also subjected to the feedback loop process.
At the NDAU, patient episodes across multiple neonatal units are also merged to create a single file for each infant.
Clinician engagement
We termed NHS neonatal units contributing data to the NNRD the ‘UK Neonatal Collaborative’. Of note was that, although only site-specific approval and NHS approvals are required for the NNRD host institution, we would adopt a policy of seeking the approval of each NHS trust’s lead neonatal clinician for their data to be included in research studies. We adopted this practice in order to grow clinician engagement with the concept of the NNRD as a national resource and in recognition of their crucial contribution to acquiring the data.
Parent information leaflet
We collaborated with the Royal College of Paediatrics and Child Health, the national charity Bliss and the parents of newborn babies receiving specialised neonatal care to develop a parent information leaflet (‘A Guide for Parents and Carers’) that explains the multiple uses of the NNRD. This was approved by the National Research Ethics Service and the Ethics and Confidentiality Committee of the National Information Governance Board.
If the parent or carer of the infant does not wish for EPR data on their infant to be extracted for the NNRD, then they can notify the neonatal unit staff who will then notify the data entry system supplier to prevent the flow of the data. To date, no parent or carer has asked that their infant’s data not be extracted.
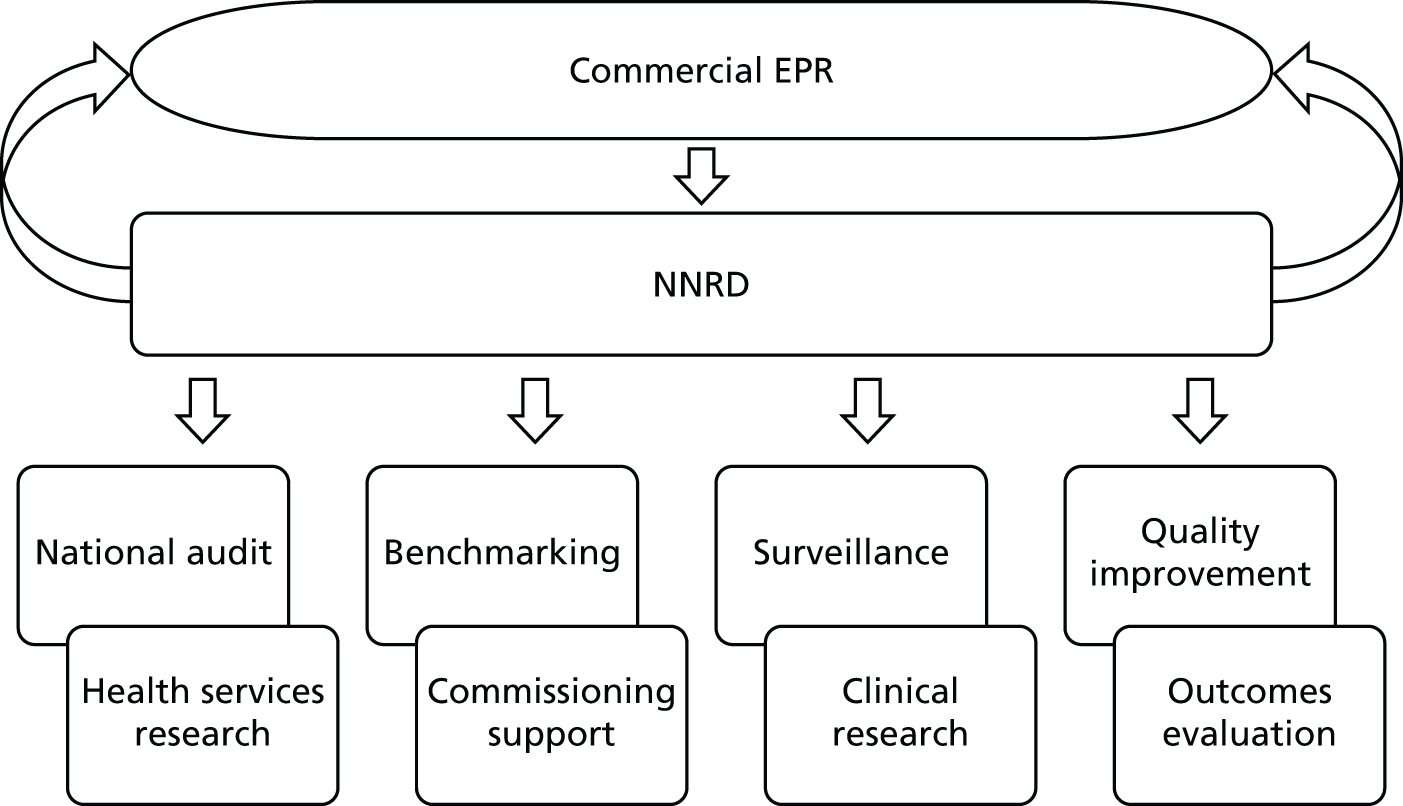
Uses and outputs of the National Neonatal Research Database to date
The use of the NNRD to support a wide range of outputs grew rapidly over the course of the Medicines for Neonates Programme, and continues to expand. Examples of the multiple outputs from the NNRD are shown in Figure 4.
FIGURE 4.
Examples of multiple outputs from the NNRD.
Conclusions
We have shown that it is possible to create a national data resource, the NNRD, from extractions from EPRs, which brings multiple benefits. This eliminates the need for multiple individual collections, with repetitive capture of many commonly required data items. This in turn reduces the burden of data capture all too often imposed on busy clinical teams, and reduces the risk of transcription errors and other errors.
Our literature search identified 82 databases worldwide that hold neonatal information. The NNRD is one of only six national neonatal databases primarily developed to support research, with ongoing data acquisition. Uniquely, data in the NNRD are extracted from EPRs rather than being recorded specifically and there is complete national coverage of all admissions to neonatal units with no gestational age, birthweight, insurance cover or other restrictions.
The Neonatal Data Set incorporates data required to fulfil all currently mandated UK requirements. In addition, the Neonatal Data Set is sufficiently comprehensive to make the need for subsequent addition of new national data items unlikely in the immediate future. However, should this be required, the process for incorporation of new items into the EPR is straightforward (see Chapter 2, Research on an exemplar condition).
We have demonstrated that the approach we have adopted has been successful. The NNRD is now used for a growing number of purposes by a growing number of research groups, professional organisations and government bodies. In effect, our approach has gone a considerable way towards fulfilling the vision set out by Florence Nightingale more than 100 years ago and, more recently, the principles set out in successive national information strategies. These include the Council for Science and Technology report Better Use of Personal Information: Opportunities and Risks,95 the UK Clinical Research Collaboration Research and Development Advisory Group to Connecting for Health,96 the Academy of Medical Sciences report entitled Personal Data for Public Good: Using Health Information in Medical Research,97 the Department of Health and Social Care’s entitled Toolkit for High Quality Neonatal Services,98 and the aspiration articulated by the then Prime Minister, in numerous references to ‘big data’. We believe that the NNRD may reasonably be termed an example of ‘big data’. Although the term has been defined in various ways, Wang and Krishnan99 state that ‘A popular definition of big data is the “3V” model proposed by Gartner, which attributes three fundamental features to big data: high volume of data mass, high velocity of data flow, and high variety of data types’. The data in the NNRD does encompass each of these elements to varying extents, in contrast with many other clinical data sets that are much simpler.
In conclusion, we have established proof of the principle that EPR data may be employed successfully to support patient care and clinical services through research and a range of evaluations. We have shown that it is possible to reduce the burden placed on busy clinical teams by providing a single national data source to service multiple outputs.
Other Medicines for Neonates workstreams deal with issues of data quality, utility and patient (parent) involvement.
Implications for health care
The Medicines or Neonates programme has also established proof of concept for the use of EPR-derived clinical data in a wide range of research and health service evaluations. This opens up the possibility of adapting the road map that we have established for other specialty areas with potential to bring about NHS savings.
The NNRD has been developed and is currently maintained through academic endeavour, but processes to secure the stability of EPR-derived databases as national resources and their ongoing management are uncertain.
Research recommendations
A next step towards seizing the full potential of our approach for the benefit of the NHS and patient care would be to formally test the creation of another specialty database from EPRs using the road map that we have developed.
Chapter 2 Research on an exemplar condition: the use of the National Neonatal Research Database to study neonatal necrotising enterocolitis
Abstract
Background: Necrotising enterocolitis is a feared gastrointestinal inflammatory disease that predominantly affects preterm infants. The aetiology is uncertain and population incidence data are scant. Treatment is supportive and it includes surgery, but research is constrained by the relative rarity of the disease.
Aims and objectives: We utilised EPR-derived data to conduct population surveillance of severe NEC in England. Additional objectives were to inform the development of future clinical trials by identifying factors associated with severe NEC.
Methods: We secured the participation of every NHS neonatal unit in England in a prospective study. We extracted relevant data from the NNRD. We also obtained outcome data for infants who received NEC surgery or died from NEC at stand-alone paediatric surgical centres that do not use the neonatal EPRS.
Results: We identified 531 infants (462 who were born at < 32 weeks’ gestation) with severe NEC (resulting in surgery and/or death) over the complete 2-year period 2013–14. Among the infants born before 32 weeks’ gestation, neonatal network incidence ranged from 19.8 [95% confidence interval (CI) 9.1 to 30.4] to 47.4 (95% CI 32.5 to 62.4) per 1000 babies. We identified no strong evidence of variation between networks following adjustment for gestational age and birthweight standard deviation score (SDS), which were the only factors found to be independently associated with the disease.
Conclusions: The NNRD provides opportunity for rapid population surveillance of neonatal conditions and a source of baseline information to inform clinical trials but it requires strong clinician engagement.
Background
Necrotising enterocolitis is a feared gastrointestinal inflammatory disease that predominantly, but not exclusively, affects preterm infants. NEC is a principal cause of mortality and morbidity in very preterm infants. 100,101 The aetiology of NEC is uncertain and is likely to be multifactorial. Some studies have suggested that the most significant factor in determining NEC incidence is the neonatal unit in which an infant receives care, with the implication that variations in care affect risk. 102,103 In particular, there is a widespread view that enteral feeding regimens, including type of milk, affect the risk of NEC. However, lack of good evidence for specific feed-related interventions that affect the risk of NEC has resulted in variation in neonatal practice, entrenched clinical opinion and bewilderment among parents. Evidence is conflicting regarding whether or not antenatal steroid exposure, a strong predictor of neonatal survival, is associated with NEC. 102,104–110 A further difficulty is that the diagnosis of NEC can be difficult as signs are often non-specific and presentation is variable. No internationally agreed case definition exists, which makes comparisons between studies unreliable. The most frequently applied definitions include modified Bell’s criteria,111,112 which, although developed as criteria for staging after the diagnosis is made, have been widely adopted as a definition worldwide. There are also definitions from the International Classification of Diseases, Ninth Edition (ICD-9) or IC-10 codes, the Vermont Oxford Network (VON), the US Centers for Disease Control and Prevention (CDC) and definitions from individual study authors. None has been developed through evidence-based methodology or has undergone validation.
In the absence of evidence from randomised trials, an approach that is widely believed to be of benefit is to identify variation in NEC incidence between neonatal units or networks, in the hope that this might help highlight potentially beneficial clinical practices that can then be tested in future randomised controlled trials.
Aims
We aimed to build on the establishment of the UK Neonatal Collaborative and the existence of the neonatal EPRS and NNRD to examine aspects of this serious disease. Our objectives were to:
-
conduct population surveillance of severe NEC
-
identify factors associated with severe NEC
-
evaluate variation in incidence across neonatal networks in England.
We also aimed to build engagement with local clinical staff responsible for recording neonatal data, through demonstration of the utility of EPR data in national research in an area considered a priority by parents and clinicians alike.
Methods
Approvals and agreements
We sought and obtained research ethics approval from the National Research Ethics Service (Dulwich Research Ethics Committee; reference number 11/LO/1430) and inclusion into the UK Clinical Research Network Portfolio (ID 11853). We invited the participation of all neonatal units in England. We sought agreement from the local UK Neonatal Collaborative lead clinicians to utilise data from their neonatal unit held in the NNRD on all live-born infants admitted over the complete 2-year period 2012–13.
In order to promote maximal engagement with the neonatal community and optimise data quality and completeness, we asked local clinical staff to ensure that the following data items were recorded in each infant’s EPR: birthweight, gestational age, sex, mother’s race, antenatal steroids, and clinical and abdominal X-ray (AXR) findings for infants in whom abdominal signs were being investigated. We excluded infants of mothers that were unbooked, booked in non-English networks, or for whom network of booking was unknown.
Identifying babies with severe necrotising enterocolitis in the National Neonatal Research Database
We defined ‘severe NEC’ as NEC confirmed at surgery or post-mortem or resulting in death (death certification and/or verified by neonatal team). We initially intended to capture outcomes on a specific section of the neonatal EPRs, the screen used to record details of abdominal X-rays taken to investigate clinical signs consistent with gastrointestinal pathology. However, despite regular quarterly feedback of data completeness to neonatal units, we found that only 25% of infants who proceeded to NEC surgery had completed this screen; hence, sole use of these data would underestimate the incidence of severe NEC. Therefore, we used data from the NNRD to identify infants who may have received surgery for NEC or died from NEC, and verified these outcomes with clinicians at neonatal units. In addition, outcomes were sought for infants who received NEC surgery or who died at the four stand-alone paediatric surgical centres which do not use the BadgerNet neonatal EPRs (Great Ormond Street, Sheffield, Alder Hey and Birmingham Children’s Hospitals). Here, we describe the steps taken to identify and verify infants with severe NEC.
Step 1: data extraction
The variables extracted from the NNRD comprised static data (discharge/died status, cause of death, whether or not the post-mortem-confirmed NEC); daily data (NEC treatment: medical or surgical); episodic data (gastrointestinal diagnoses, discharge diagnoses, procedures during stay); AXR screen (whether or not surgery was required, whether surgery was required but the patient was too sick, whether or not the surgery-confirmed NEC, whether or not histology-confirmed NEC).
Step 2: data verification
We identified infants from the following EPR locations using the predefined field listed:
Discharge diagnoses
-
‘Necrotising enterocolitis – perforated’
-
‘Necrotising enterocolitis – proven (on X-ray or at surgery)’
-
‘Necrotising enterocolitis – confirmed’
-
‘Cause of death includes necrotising enterocolitis’.
Abdominal X-ray screen
-
‘Laparotomy-confirmed NEC’
-
‘Histology-confirmed NEC’
-
‘Post-mortem-confirmed NEC’
-
‘Procedures screen’
-
‘Laparotomy approach NEC’
-
‘Colectomy and ileostomy NEC’
-
‘NEC surgery performed’.
Combinations
-
‘Necrotising enterocolitis’ in ‘Discharge diagnoses’ and ‘Laparotomy’ in ‘Procedures’
-
‘Necrotising enterocolitis’ and ‘died’ in discharge status field.
Step 3
The study lead at each neonatal unit or paediatric stand-alone hospital where the surgery was performed or where the infant died was contacted to verify data. The following data were verified:
-
Gestation weeks and days.
-
Birthweight.
-
Did infant die in neonatal unit? (Yes/no.)
-
Age of infant at surgery for NEC (if applicable).
-
Was laparotomy performed? (Yes/no/required but too sick.)
-
Did visualisation confirm NEC? (Yes/no.)
-
Did histology confirm NEC? (Yes/no.)
-
Was a peritoneal drain inserted? (Yes/no.)
-
Was a post-mortem done? (Yes/no.)
-
If yes, did the post-mortem confirm NEC? (Yes/no.)
-
Was NEC a cause of death? (Yes/no).
Other data extraction from the National Neonatal Research Database: data management
We extracted the following data for all babies from the NNRD: booking network, gestational age in completed weeks and days, birthweight, fetus number, antenatal steroids, maternal pyrexia in labour, whether or not mother received antibiotics, mode of delivery, maternal chorioamnionitis and maternal infection. We calculated birthweight SDS, standardised for sex and gestational age from UK World Health Organization (WHO) reference data. 113 We considered a birthweight SDS of < –4 or > 4 to be erroneous, and we treated these as missing values.
Analyses
Incidence and absolute numbers of cases of severe necrotising enterocolitis
We expected all very preterm infants (born before 32 weeks’ gestation) to be admitted to a neonatal unit and, hence, derived the population incidence of severe NEC for this group. To ensure that this is a valid assumption, we compared the numbers of infants for whom data were present in the NNRD against the equivalent number obtained from the most recently available data from the Office for National Statistics (ONS) as this contains complete birth registrations. For preterm infants born before 32 weeks’ gestation, we report the incidence of severe NEC (95% CIs) per 1000 infants admitted to neonatal care. In contrast, not all infants born at a gestational age of ≥ 32 weeks will necessarily be admitted to a neonatal unit and so, for this group, we only present the absolute numbers of cases of severe NEC. For infants who received surgery for NEC, we will report the median and interquartile ranges (IQRs) for postnatal age and postmenstrual age for the day of surgery by gestational bands.
Factors associated with severe necrotising enterocolitis in infants born at a gestational age of < 32 weeks
For preterm infants, we compared baseline characteristics for gestational age in completed weeks, birthweight SDS, fetus number, antenatal steroids, maternal pyrexia in labour, whether or not mother received antibiotics, mode of delivery, maternal chorioamnionitis and maternal infection between those with severe NEC and those who did not develop the condition. We used the chi-squared test, the application of Yate’s correction and the t-test, as appropriate. We performed a stepwise multiple logistic regression; variables found to be significantly associated with severe NEC in the univariate logistic regression analysis (p < 0.15) were considered candidate variables for the multivariable logistic regression model. For the final multivariable model, we retained only variables that were significant independent predictors of NEC. We further investigated the effect of retaining and excluding antenatal steroid exposure in the final multivariable model. The level of statistical significance for all analyses was set at p < 0.05 using two-tailed comparisons.
Variation in the incidence of severe necrotising enterocolitis among infants born before 32 weeks’ gestation
For preterm infants, we report the incidence of severe NEC (95% CIs) per 1000 infants admitted to neonatal care by network of booking. We assessed whether or not there was variation in the incidence of severe NEC at network level in two ways. First, we compared the rate in each neonatal network against the average incidence across England. Second, we compared each individual network against a reference network. We selected the reference as the network contributing the largest number of infants, to minimise the standard errors.
In the first approach, we used methods analogous to those used to calculate standardised mortality ratios (SMRs), assigning infants to the neonatal network of booking. We calculated the standardised severe NEC ratio (SNR) by dividing the observed number of severe NEC cases by the expected number of severe NEC cases. For the unadjusted SNR, the expected number of severe NEC cases was calculated as the total number of infants in the booking network multiplied by the overall severe NEC rate across England. For the adjusted SNR, the expected number of severe NEC cases was calculated by first estimating the probability of severe NEC for each infant using logistic regression, and adding up the probabilities to obtain the expected number of severe NEC cases in each network. The 95% CIs for the SNR were calculated using Byar’s approximation114 with correction for multiple testing, controlling the false discovery rate at 5%. 115 Variables included in the logistic regression to estimate the probability of severe NEC for each infant were gestational age (in completed weeks), birthweight SDS and antenatal steroids.
Funnel plots were used to illustrate the SNR at network level. The prediction limits were drawn corresponding to 95% (SD 2) 99.8% (SD 3) from the target SNR of 1, assuming the observed severe NEC rates follow a Poisson distribution. The limits were adjusted for multiple testing controlling the false discovery rate of 5%.
We expect 5% of networks to lie outside the 95% prediction limits, and 0.2% to lie outside the 99.8% prediction limits. For the second approach, we used multivariable logistic regression adjusted for antenatal steroid exposure and variables independently associated with severe NEC to derive the odds ratio (OR) for severe NEC in each network relative to the reference network. We corrected for multiple testing using the Bonferroni method. All p-values reported are two sided. Statistical analyses were performed in SAS® software, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Data validation
We compared data from the NNRD with data from paper medical notes as part of a project evaluating a quality improvement project conducted by the East of England Neonatal Networks. 116 In brief, between 2011 and 2013, we assessed and fed back completeness and accuracy of NNRD data to participating neonatal teams involving 17 neonatal units. The study lead at each neonatal unit extracted a selection of data items from medical notes for two randomly selected infants discharged in the previous month. These data were sent to the NDAU and compared with NNRD data.
Results
Incidence and numbers of cases of severe necrotising enterocolitis
We extracted data on a total population cohort of 118,073 infants (Figure 5). We identified 531 infants with severe NEC. Table 4 shows the proportion of infants with severe NEC who had surgery and survived to discharge from neonatal care, who had surgery and died, and who died without surgery, by gestational age bands. Of the total number of infants with severe NEC 79.7% (423/531) had surgery; of those who had surgery 32.9% (139/423) died; 20.3% (108/531) of infants with severe NEC had died without surgery (Figure 6).
FIGURE 5.
Flow chart showing derivation of the study population.
| Gestation (completed weeks) | Infants with severe NEC (N = 531), n | Total, n | ||
|---|---|---|---|---|
| Surgery and survived | Surgery and died | Died without surgery | ||
| 22+0 to 25+6 (n = 2035) | 101 | 58 | 41 | 200 |
| 25+0 to 28+6 (n = 4331) | 97 | 56 | 42 | 195 |
| 29+0 to 31+6 (n = 8312) | 42 | 12 | 13 | 67 |
| 32+0 to 36+6 (n = 42,169) | 29 | 11 | 12 | 52 |
| ≥ 37+0 (n = 61,226) | 15 | 2 | 0 | 17 |
| All gestations (n = 118,073) | 284 | 139 | 108 | 531 |
FIGURE 6.
Flow chart showing the population with severe NEC.
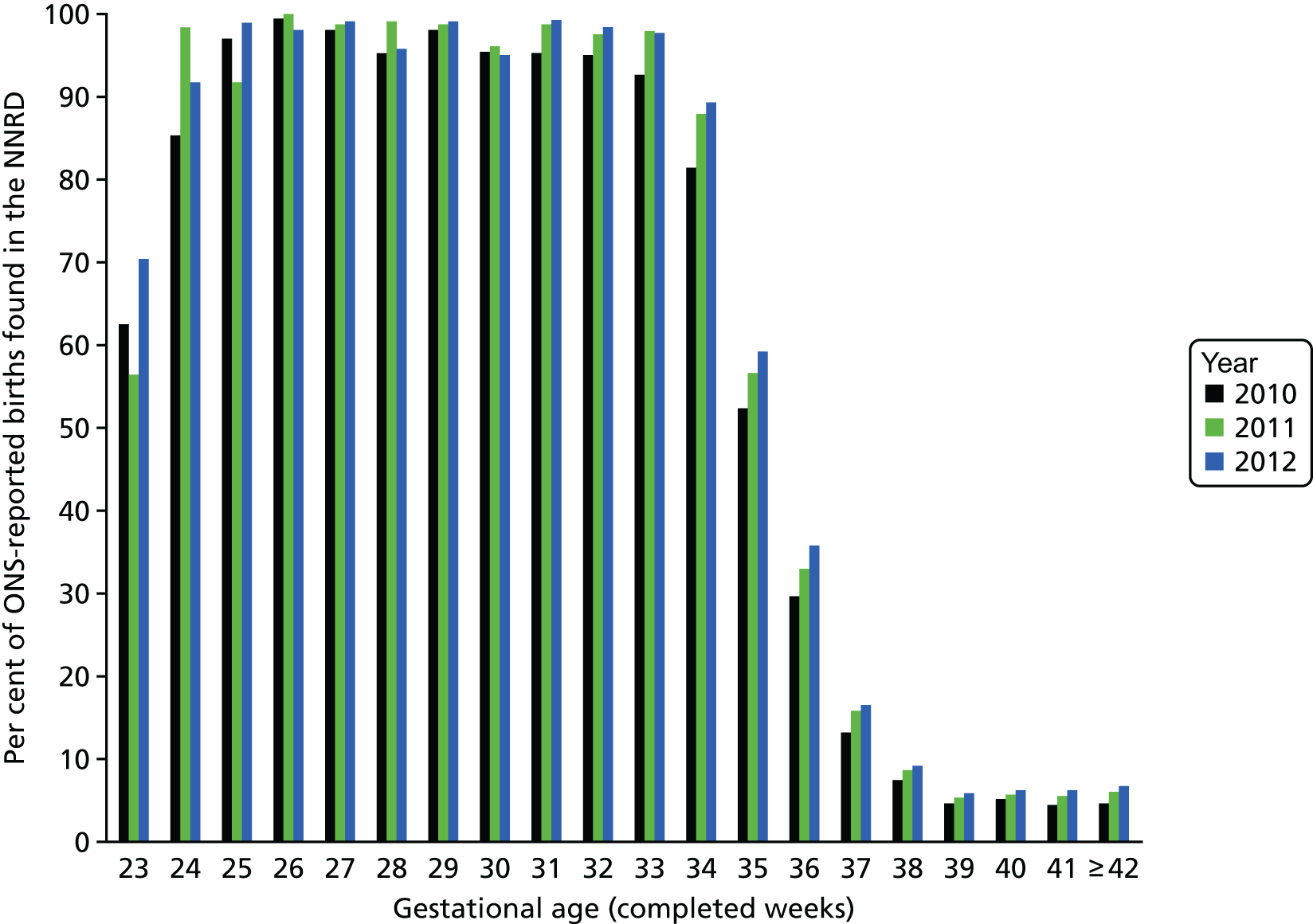
Comparing ONS data with NNRD data from 2012 shows that of the infants who were born alive in England between the gestational ages of 25 and 31+6 weeks, 96–99% were admitted to a neonatal unit (Figure 7); the corresponding figures are 92% and 70% of infants born at 24 and at 23 weeks’ gestation, respectively. The percentage of infants who were admitted to a neonatal unit starts to fall after 32 weeks’ gestation. Therefore, as we have population data for infants born before 32 weeks’ gestation, we present the incidence for these infants; but for infants who were born after 32 weeks’ gestation, we present only the raw numbers.
FIGURE 7.
Percentage of ONS-reported live births in the NNRD.
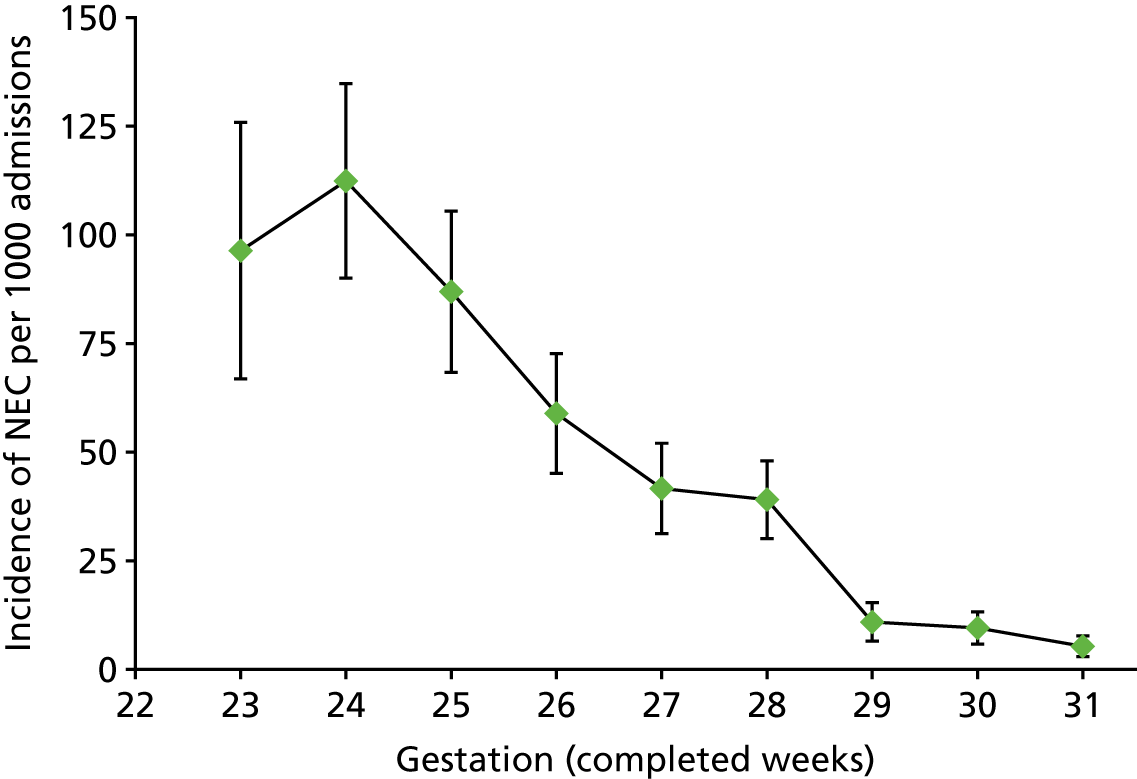
The incidence of severe NEC was inversely related with gestational age (p < 0.001, test for trend). The highest incidence of severe NEC occurred in infants born at 24 weeks’ gestation. The incidence per 1000 infants for all infants born before 32 weeks’ gestation is 31.5 per 1000 (95% CI 28.7 to 34.3 per 1000), and by gestational age is as follows: 23 weeks, 96.4 (95% CI 66.8 to 125.9); 24 weeks, 112.4 (95% CI 90.0 to 134.8); 25 weeks, 86.9 (95% CI 68.4 to 105.5); 26 weeks, 58.9 (95% CI 45.1 to 72.7); 27 weeks, 41.0 (95% CI 30.6 to 51.3); 28 weeks, 39.0 (95% CI 30.1 to 48.0); 29 weeks, 10.9 (95% CI 6.5 to 15.3); 30 weeks, 9.5 (95% CI 5.8 to 13.2); and 31 weeks, 5.3 (95% CI 2.9 to 7.7). Figure 8 shows that there is a sharp decline in the incidence of severe NEC at 29 weeks’ gestation.
FIGURE 8.
Incidence of severe NEC, infants born before 32 weeks’ gestation. Incidence derived from raw data; bars are 95% CI; gestation category labelled 23 weeks represents 22 and 23 weeks (14 infants born at 22 weeks, none with severe NEC).
Of the 531 infants with severe NEC, 462 (87%) were born before 32 weeks’ gestation, of whom 366 received surgery. Table 5 shows the postnatal and postmenstrual age at NEC surgery for infants < 32 weeks’ gestation. There is an inverse relationship between gestational age at birth and postnatal age at surgery (log-rank test < 0.001). The most immature infants born, before 26 weeks’ gestation, receive surgery for NEC around the third to fourth week of life; in contrast infants who are born at 30–31 weeks’ gestation undergo surgery in the second week of life.
| Gestational age (weeks) | Number of infants | Age (days) at NEC surgery (median, IQR) | Postmenstrual age (completed weeks) |
|---|---|---|---|
| 23 | 29 | 25 (12–37) | 27 (25–29) |
| 24 | 66 | 20 (12–38) | 27 (26–30) |
| 25 | 64 | 31 (12–53) | 29 (26–32) |
| 26 | 55 | 29 (15–39) | 30 (28–31) |
| 27 | 41 | 13 (9–31) | 29 (28–31) |
| 28 | 57 | 24 (14–36) | 31 (30–33) |
| 29 | 19 | 18 (9–32) | 32 (30–33) |
| 30 | 19 | 11 (7–25) | 32 (31–33) |
| 31 | 16 | 10 (8–17) | 32 (32–33) |
| Total | 366 | 22 (11–37) | 30 (27–32) |
Factors associated with severe necrotising enterocolitis for infants born before 32 weeks’ gestation
We compared the patient characteristics of the 462 preterm infants who developed severe NEC against the 14,216 preterm infants without severe NEC. Infants who developed severe NEC were more immature, with a mean gestational age of 26.2 weeks compared with 28.5 weeks (p < 0.001). Gestational age, birthweight, birthweight SDS, fetus number, whether or not the mother received antibiotics in labour, and mode of delivery were significantly different between the two groups (see Appendix 1, Table 53).
Using univariate logistic regression for infants born before 32 weeks’ gestation (n = 14,678), we identified fetus number, whether or not the mother had received antibiotics, mode of delivery, gestational age (completed weeks) and birthweight SDS to be significantly associated with severe NEC. After multivariable logistic regression analysis, only gestational age and birthweight SDS were significant independent predictors of severe NEC. We investigated the effect of including antenatal steroid exposure in the multivariable model and found that the conclusions were unchanged. As antenatal steroids are an important determinant of survival, we chose nonetheless to include this is our model. Appendix 1, Table 54, shows parameters for the final multivariable model that include gestational age, birthweight SDS and antenatal steroid exposure.
Incidence of severe necrotising enterocolitis by neonatal network
The incidence of severe NEC per 1000 infants born before 32 weeks’ gestation, by network of booking, is shown in Table 6. The highest incidence was 47.4 cases per 1000 babies (95% CI 32.5 to 62.4); the lowest incidence was 19.8 (95% CI 9.1 to 30.4). The rate of severe NEC in England is 3.15% (462/14,678). We calculated adjusted NEC rates (adjusted for gestational age, birthweight SDS, antenatal steroid exposure) for infants born before 32 weeks’ gestation. The adjusted funnel plot in Figure 9 illustrates that the network-level variation in severe NEC rates is consistent with the pattern we would expect given that the population rate of NEC is 3.15% (i.e. we identified no strong evidence that any neonatal network differed from the mean national incidence of severe NEC).
| Network of booking | Total number of infants (n = 14,678) | Severe NEC cases, n (%) | Incidence of severe NEC per 1000 infants | 95% CI |
|---|---|---|---|---|
| Bedfordshire and Hertfordshire | 391 | 8 (2.05) | 20.5 | 6.4 to 34.5 |
| Cheshire and Merseyside | 635 | 24 (3.8) | 37.8 | 23.0 to 52.6 |
| Eastern | 800 | 37 (4.63) | 46.3 | 31.7 to 60.8 |
| Greater Manchester | 871 | 35 (4.02) | 40.2 | 27.1 to 53.2 |
| Kent | 470 | 15 (3.19) | 31.9 | 16.0 to 47.8 |
| Lancashire and South Cumbria | 409 | 11 (2.69) | 26.9 | 11.2 to 42.6 |
| London North Central | 421 | 9 (2.14) | 21.4 | 7.6 to 35.2 |
| London North East | 1014 | 30 (2.96) | 29.6 | 19.2 to 40.0 |
| London North West | 755 | 31 (4.11) | 41.1 | 26.9 to 55.2 |
| London South East | 600 | 23 (3.83) | 38.3 | 23.0 to 53.7 |
| London South West | 418 | 13 (3.11) | 31.1 | 14.5 to 47.7 |
| Midlands Central | 780 | 37 (4.74) | 47.4 | 32.5 to 62.4 |
| Midlands South West | 797 | 17 (2.13) | 21.3 | 11.3 to 31.4 |
| Midlands North | 649 | 18 (2.77) | 27.7 | 15.1 to 40.4 |
| North Trent | 623 | 20 (3.21) | 32.1 | 18.3 to 45.9 |
| Northern | 773 | 19 (2.46) | 24.6 | 13.7 to 35.5 |
| Peninsula South West | 344 | 7 (2.03) | 20.3 | 5.4 to 35.3 |
| South Central (North) | 589 | 18 (3.06) | 30.6 | 16.7 to 44.5 |
| South Central (South) | 639 | 14 (2.19) | 21.9 | 10.6 to 33.3 |
| Surrey and Sussex | 591 | 26 (4.4) | 44.0 | 27.5 to 60.5 |
| Trent | 520 | 15 (2.88) | 28.8 | 14.5 to 43.2 |
| Western | 658 | 13 (1.98) | 19.8 | 9.1 to 30.4 |
| Yorkshire | 931 | 22 (2.36) | 23.6 | 13.9 to 33.4 |
FIGURE 9.
Funnel plot for infants born before 32 weeks’ gestation, showing percentage of observed to predicted NEC cases estimated from the multivariable logistic regression model (adjusted for gestation, birthweight SDS and antenatal steroids) relative to average percentage of NEC cases in England. 1 = Bedfordshire and Hertfordshire; 2 = Cheshire and Merseyside; 3 = Eastern; 4 = Greater Manchester; 5 = Kent; 6 = Lancashire and South Cumbria; 7 = London North Central; 8 = London North West; 9 = London South East; 10 = London South West; 11 = Midlands Central; 12 = Midlands South West; 13 = Midlands North; 14 = North Trent; 15 = Northern; 16 = Peninsula South West; 17 = South Central (North); 18 = South Central (South); 19 = Surrey and Sussex; 20 = Trent; 21 = Western; 22 = Yorkshire; and 23 = London North East.
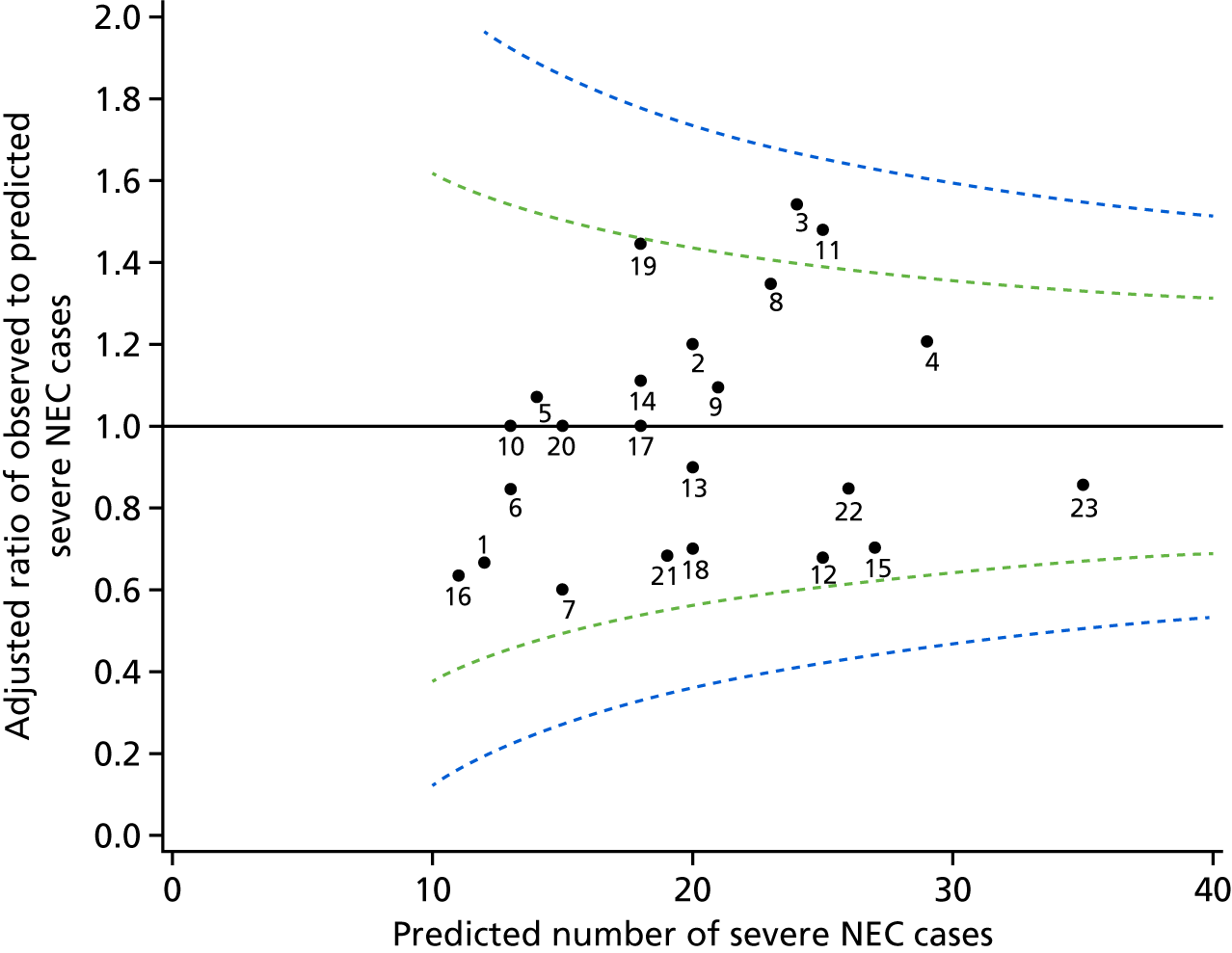
For comparison between networks, we selected a reference network with the largest number of infants born before 32 weeks’ gestation (London North East with 1014 infants) to minimise the standard errors. We identified a statistically significant difference in the incidence of severe NEC between the reference network and all the other 22 networks combined (overall p-value of < 0.001). We therefore proceeded to perform pairwise comparisons between each network and the reference network (see Appendix 1, Table 55). Following correction for multiple testing, we found no statistically significant difference in the incidence of severe NEC between any network and the reference network.
Comparison of National Neonatal Research Database against East of England medical notes
There was high agreement (> 95%) for sex, gestational age, birthweight and discharge destination (see Results). Agreement for the length of parenteral nutrition improved from 80–90% to > 90% over the 26-month study duration (p < 0.029). The agreement for central line days was consistently > 80%, with no significant change over time. Agreement on type of discharge feed improved over time, from 50–60% to 70–80% (p < 0.009). Agreement for numbers of AXR and blood cultures was consistently low, at around 50%. Further results of this work are available in the published manuscript. 116
Conclusions
We identified that over a complete 2-year period, 531 babies admitted to neonatal units in England, required surgery for NEC and/or died as a result of the condition. Of these, one-fifth died before they could receive surgery. Of note, we identify no strong evidence of statistically significant variation between neonatal networks.
This study has several strengths. To our best knowledge, it is the largest complete population-based study of NEC. We obtained data from the NNRD in turn derived from the neonatal EPRs and, thus, the data recorded as part of this study remain a permanent part of the infant’s clinical record. Through comparison with ONS data, we show that the NNRD contains data from the majority of infants born alive below 32 weeks’ gestation. Uniquely, unlike previous studies in England and elsewhere,117,118 we engaged the participation of every neonatal unit in the country. The use of the NNRD and the complete population coverage despite frequent transfers between neonatal units has meant that we were able to ascertain the final outcome for every baby. Incomplete ascertainment have been a common limitation of other studies. This has also allowed us to report the incidence of NEC by neonatal network. This is an important consideration; in a networked-based model of care, the delivery of care is a collaborative responsibility and clinical outcomes are in large part attributable to the network rather than to individual neonatal units. Previous studies that have only included tertiary centres are severely limited by selection bias, first, because these neonatal units are likely to care for only the sickest and most complex infants and, second, because of the omission of infants that die before transfer to a tertiary centre. Our study also provides complete ascertainment of all cases of severe NEC, regardless of birthweight or gestational age. We applied a stringent, consistent definition for NEC and confirmed each case individually with clinical teams to minimise contamination from diagnoses such as spontaneous intestinal perforation, dysmotility, feed intolerance, septic ileus or other ambiguous abdominal pathologies.
A limitation of our study is that we did not attempt to determine the incidence of less severe NEC. This is because the value of attempting to do so is open to question given the absence of an agreed case definition, the high degree of diagnostic subjectivity, and the poor sensitivity and specificity of many indicative clinical signs. NEC remains a clinical diagnosis using radiographic and clinical findings and there is increasing recognition of the difficulties caused by inconsistencies in the application of these criteria. This problem requires the identification of reliable biomarkers of NEC or the consistent application of an agreed case-definition purely for surveillance purposes. 118,119
Consistent with other studies, we found the incidence of NEC to be inversely related to gestational age120,121 other than for a lower incidence at 22 and 23 weeks’ gestation. As the median time to onset of NEC in babies born before 32 weeks’ gestation is 22 days, this is likely to be a consequence of the higher proportion of the most immature infants dying in the early neonatal period. We confirm a strong independent association between severe NEC and both gestational age and birthweight SDS. Previous studies are inconsistent regarding other risk factors for NEC including infant sex,117,122,123 race,120,124 mode of delivery,102,104,125 antenatal steroids102,104–110 and prolonged rupture of membranes. 102,126
The lack of strong evidence of variation in the incidence of NEC at neonatal network level in England contrasts with widely held beliefs. Although we identified two neonatal networks with an incidence of severe NEC falling outside the upper 95% control limits of the funnel plot, this is not incompatible with what would be expected by chance alone. Adjusting for gestational age, birthweight and antenatal steroids did not alter our conclusions. Other population-based incidence data are limited. The EPICure 2 study, a population-based study, reported that 8% (95% CI 6% to 9%) of infants born at 22–26 weeks’ gestation in England in 2006 that survived to discharge received a laparotomy for NEC,127 a figure comparable to the equivalent figure of 6% (138/2275) that we found. Our data are also broadly similar to other published studies from Canada,128 Australia,129 and the USA,130 but these largely employed birthweight- or neonatal unit-based selection criteria.
Implications for health care
A clear implication for health care is that the lack of significant variation in NEC between networks despite differences in many clinical practices, such as the use of probiotics and pasteurised human donor milk, the time to commence enteral feeds and the rate of enteral feed advancement, that are widely believed to affect risk, justifies caution in the imposition of inadequately evidence-based guidelines or quality-improvement approaches. The imposition of poorly evidence-based guidance is often justified on the basis that this provides consistency in care; an alternative conclusion is that this places patient safety at risk by exposing all patients, rather than just some, to a potentially less beneficial treatment approach. 131 A corollary is the paramount importance of assisting health-care staff to support the delivery of randomised controlled trials that seek to reduce uncertainties in everyday care practices.
Gestational age-specific population denominators are required to derive population incidences. In the UK, the ONS is the gold-standard source of population denominators based on birth registrations. The data available in the NNRD are timely, as these are downloaded from EPRs at quarterly intervals. In contrast, we were able to obtain ONS data only up to 2012. Improving the timeliness of ONS data would go some way towards improving the scope and utility of health-care evaluations.
Research recommendations
Necrotising enterocolitis is a cardinal cause of morbidity and mortality in the most immature infants. The low incidence rates require national and international collaboration to test preventative strategies in adequately powered randomised controlled trials. An important national advance would be the development of efficient approaches based on routinely recorded data. Implementation of EPR or database trials internationally requires agreement of core data sets that include predefined outcome measures and ancillary variables. Clinical engagement and contribution to reliable data entry into the neonatal EPRs was paramount to this study.
Chapter 3 Clinical outcomes assessed using the National Neonatal Research Database: mortality of very preterm babies admitted to NHS neonatal units
Abstract
Background: Although preterm survival analyses are widely used a health indicator, they have generally been based on historical data, which limits their relevance.
Aims: To evaluate (1) survival trends in relation to geographical region and socioeconomic status for infants born 22+0–31+6 weeks’ gestation and admitted to neonatal units in England over 2008–14, and (2) variations between neonatal networks in mortality to discharge over 2013–14.
Methods: We used logistic regression to model survival probability, joinpoint regression for trend analyses and multiple imputation for missing outcome and covariate data. We calculated unadjusted, risk-adjusted and gestation-specific survival.
Findings: (1) The cohort comprised 50,112 infants. There was an increase in survival to discharge from 88% in 2008 to 91.3% in 2014 [adjusted annual percentage change (APC) 0.46% (95% CI 0.3% to 0.62%); p < 0.001] and 28 days [2008: 91.4%; 2014: 93.5%; APC 0.27% (95% CI 0.11% to 0.44%); p = 0.002] with the greatest improvement for infants born at 22+0–23+6 weeks’ gestation (6.03%, 95% CI 2.47 to 3.53%; p = 0.002). Crude survival was lower for infants from the most deprived quintile than from the least deprived [89.5% (95% CI 88.9% to 90.1%) vs. 91.1% (95% CI 90.2% to 92.1%)] and it was lower in the Midlands and the East of England than in London [89.3% (95% CI 88.6% to 89.9%) vs. 91.1% (95% CI 90.3% to 91.8%)]. Regional variation remained after adjustment for socioeconomic status. (2) We analysed data on 15,255 infants. We identified no strong evidence that any neonatal network differed significantly from the national mean.
Interpretation: Analysis of population data over time is required to identify variation unlikely to be due to chance. Continued national improvement in very preterm survival masks significant north–south variation that is not explained by population characteristics.
Background
Preterm birth is now the primary cause of neonatal death and is associated with risks to health and well-being into childhood and beyond. The preterm birth rate is rising worldwide, and this growing population presents a substantial public health issue. Advances in obstetric and neonatal care have resulted in improved survival of preterm infants over the last few decades. In England, the EPICure study found that survival to discharge from hospital among admitted babies born between 22+0 and 25+6 weeks’ gestation increased from 40% in 1995 to 53% in 2006. 124 Data such as these have been invaluable, but are now several years out of date. Evaluation of survival is a widely used health indicator. Keeping pace with changes in preterm survival is important for counselling parents and planning clinical services, but undertaking population-based studies is challenging.
The NNRD holds extracts from point-of-care EPRs for infants admitted to neonatal units in the UK, providing an opportunity to obtain precise, up-to-date estimates of neonatal survival.
Aims
We aimed to use data from the NNRD to conduct two analyses relating to infants born at 22+0 to 31+6 weeks’ gestation and admitted to neonatal units in England.
First, we describe trends in survival between 2008 and 2014, evaluate regional variation and relationship to socioeconomic deprivation, and compare survival rates with those from the EPICure studies. Secondary aims were to examine changes in the time of death, develop a statistical model to predict the probability of survival to discharge for a given set of infant characteristics, and cross-validate NNRD data with data from the ONS.
In England, neonatal specialised care is delivered through Operation Delivery Networks (ODNs). Our second aim was to present unadjusted and adjusted SMRs for each ODN for admissions to neonatal specialised care over 2013–14.
Methods
We obtained data for infants born between 22+0 and 31+6 weeks’ gestation between January 2008 and December 2014, admitted to a neonatal unit in England. We excluded infants with a birthweight SDS that was > 4 SDs from the gestation and sex-specific mean (UK-WHO preterm standards), as we considered these likely to be erroneous. 132
We described the following population characteristics: gestational age (based on ultrasound or best obstetric estimate), birthweight, birthweight SDS (UK-WHO preterm growth charts), small for gestational age (SGA), singleton/multiple pregnancy, administration of any antenatal steroids (complete or incomplete course), vaginal/caesarean delivery, maternal age, maternal ethnicity, any smoking during pregnancy, the Index of Multiple Deprivation (IMD) 2010133 quintile [based on rank of lower-layer super output area (LSOA)] by year of birth and for the cohort overall.
The primary outcome was survival to discharge from neonatal care. Secondary outcomes were survival to 28 days to facilitate comparison with other neonatal survival data, and time of death in days. The outcomes were determined by the discharge record for the last episode of care. If the last discharge was a transfer to another location for further clinical care and no subsequent data were available, the outcome was coded as missing.
To reduce the number of missing data, we attempted to link infants with missing outcomes to the ONS–HES mortality data set. 134 The NDAU has requested permission from all neonatal units to receive infants’ NHS numbers for the purposes of data linkage following approval by the Confidentiality Advisory Group of the Health Research Authority; at the time of this study, permission had been received from 159 neonatal units (93%). Deterministic linkage was carried out on the basis of NHS number and HES ID, when available. If the NNRD record could be linked to a death in the ONS–HES mortality data set, this information was used for the 28-day survival outcome but not for the survival to discharge outcome as we were unable to determine whether or not the infant was still in neonatal care at the time of death.
All data extraction and linkage was carried out using SAS.
Statistical analysis
Direct standardisation was used to control for population differences, as this permits comparison of rates over time, unlike indirect standardisation. Survival rates were directly standardised for risk. Infants were grouped into 10 categories based on the probability of death predicted by the regression model. The thresholds for the 10 categories were calculated such that each group had an equal number of predicted deaths. The directly standardised rate for each period is the weighted sum of the survival rates in each risk group, with the weights determined by the proportion of infants in each risk group in the whole study cohort. Sensitivity analyses were performed using 5 and 15 risk categories.
Prediction model
We used multivariable logistic regression to model the probability of death before discharge from neonatal care. Variables included in the regression model were gestational age, birthweight, sex, multiplicity of pregnancy (singleton/multiple) and administration of any antenatal steroids (no/yes). Previous research has shown these variables to be significant predictors of mortality. We used spline terms to model gestational age and birthweight and their interaction, using simpler functions if the fit was comparable. We included interactions between multiple birth, gestational age and birthweight, as the influence of these variables on survival is different for singleton and multiple pregnancies. As outcomes for babies from the same pregnancy are likely to be correlated, we used generalised estimating equations (GEEs) to account for the lack of independence.
We excluded observations if > 1% were missing. Otherwise, missing outcome and covariate data were imputed 25 times using multiple imputation with chained equations based on all other variables in the prediction model. Sensitivity analysis using complete cases was performed.
We carried out modelling using Stata® 12 (StataCorp LP, College Station, TX, USA). We present results as regression coefficients with standard errors (SEs). We produced isosurv graphs135 using the ggplot2 package in R 2.13.2 (The R Foundation for Statistical Computing, Vienna, Austria) to show contours of survival probability by gestation and birthweight. We added birth year to the model used to generate the graphs so that predictions would be calibrated to the most recent year.
Model performance
We checked discrimination (ability to differentiate between babies that survived and those who died) by calculating the area under the receiver operating characteristic curve (AUC). We calculated the Brier score as a measure of overall model fit (range 0–1; 0 means better fit). We checked calibration (comparability of actual and predicted survival) using Cox’s calibration in gestational age subgroups (< 28+0 weeks and ≥ 28+0 weeks). If the model predicts perfectly across all survival probabilities, the intercept α will equal 0 and the slope β will equal 1. As model performance was assessed on the same data set used to build the model, these measures were corrected for optimism using 200 bootstrap samples.
Comparison with existing models
We compared model performance with three previously published models to predict death before discharge in preterm babies admitted to neonatal units: (1) the Clinical Risk Index for Babies II (CRIB II), a frequently used model based on data from infants born in 1998–9 and admitted to 35 UK neonatal units; (2) a more recent UK model using data from neonatal units in the East Midlands and Yorkshire region (The Neonatal Survey); and (3) the National Institute of Child Health and Development Neonatal Research Network model based on infants born at 22+0–25+6 weeks’ gestation in 1998–2003 who were admitted to 19 hospitals in the USA. The relevant subset of the NNRD cohort was used to match the population characteristics of the comparator model. The predicted survival rate, AUC, Brier score and the intercept and slope from Cox’s calibration were compared between the NNRD and comparator models.
Time trend analysis
For survival to discharge and to 28 days, trends over time were analysed using joinpoint regression applied to quarterly periods using Joinpoint 4.2.0 software (National Cancer Institute, Bethesda, MD, USA). This method allows detection of changes in trend when the number and location of the changes are unknown. Rates were log-transformed so trends are presented as annual percentage change, which is the annual rate of change of the survival rate. Heteroskedastic errors were allowed using weighted least squares, with weights inversely proportional to the variance. As the number of contributing neonatal units increased over time, we repeated all time trend analyses using data from complete neonatal networks only as a sensitivity analysis. Differences in the time of death across years were tested using quantile regression.
Variation by region and Index of Multiple Deprivation quintile
We restricted this analysis to data from 2011 onwards as lower population coverage in earlier years may bias regional estimates. Infants were assigned to one of the four NHS commissioning regions (London, Midlands and East of England, North of England and South of England) based on LSOA of mother’s residence. Crude and directly standardised rates of survival to discharge and associated 95% CIs were calculated for each region. Trends in crude survival were estimated and compared for each region using joinpoint regression; standardised trends by region were not calculated because of the low quarterly numbers in each risk group.
To examine whether or not survival experiences differ by socioeconomic deprivation, crude and directly standardised rates of survival to discharge were calculated for the highest and lowest IMD quintile and compared using RR. NHS commissioning region (categorical) and IMD decile (continuous) were added in the risk adjustment model to test whether or not there was evidence of residual variation across regions.
Validation with Office for National Statistics data
For validation purposes, the number of deaths before 28 days of infants born in England and Wales at 22 to 31 weeks’ gestation in 2012 was compared in the NNRD data (denominator is neonatal unit admissions) and published ONS data (denominator is live births). 136 Data were compared for 2012 only, owing to comparability of sources.
Comparison with previous national data
The EPICure studies examined survival and morbidity outcomes for all infants born 22+0 to 25+6 weeks’ gestation during 10 months of 1995 in the UK (EPICure) and all infants born at 22+0 to 26+6 weeks’ gestation in 2006 (EPICure 2) in England. 124,137 Survival outcomes were reported separately for infants admitted to neonatal units, giving a population comparable to the NNRD cohort. We compared data on survival to discharge of admitted infants born at 22+0 to 25+6 weeks’ gestation in England in EPICure, EPICure 2 and this study cohort using joinpoint regression to see whether or not the rate of improvement has changed.
Mortality by Operational Delivery Network
We obtained data from the NNRD on infants born in 2013 and 2014 at ≤ 31+6 weeks’ gestation and admitted to neonatal care for whom the neonatal network of booking was known. Death was defined as death before discharge from neonatal care. We used multiple imputation (applying the mi routine in Stata, version 12) to impute missing outcome and covariate data; analysis of complete-case data was also performed. We present standardised mortality ratios (SMRs) for each ODN, assigning infants to the network of booking. Crude and adjusted SMRs are presented for 2013 and 2014 combined.
The SMR was calculated as the observed number of deaths divided by the expected number of deaths. The observed number of deaths was averaged over the imputed data sets so that infants with missing outcomes were included. For the unadjusted SMR, the expected number of deaths was calculated as the total number of infants multiplied by the overall mortality rate across all networks. For the adjusted SMR, the expected number of deaths was calculated by estimating the probability of death for each infant using logistic regression, and adding up the probabilities to obtain the expected number of deaths. The 95% CIs for the SMRs were calculated using Byar’s approximation138 with correction for multiple testing, controlling the false discovery rate at 5%. 115
The logistic model used to estimate the probability of death was derived using data from babies born at ≤ 31+6 weeks’ gestation in England in 2008–14. Multivariable logistic regression was used with survival to discharge from neonatal care as the outcome. Predictor variables were gestational age (typically the best obstetric estimate from antenatal ultrasound), birthweight, sex, multiplicity of pregnancy (singleton/multiple), administration of any antenatal steroids (no/yes). Spline terms were used to model gestational age and birthweight and their interaction. The association between gestation and mortality is known to be different among singletons and multiples;139 a similar interaction effect has been shown for birthweight. 140 Interactions between multiple birth and gestational age/birthweight terms were therefore included.
As outcomes for infants from the same pregnancy are likely to be correlated, we used GEEs to account for the lack of independence. We used funnel plots to illustrate the variation in SMR. Funnel plot limits were drawn corresponding to 2 and 3 standard deviations (SDs) from the target SMR of 1, assuming the observed deaths follow a Poisson distribution. The limits were adjusted for multiple testing141 controlling the false discovery rate at 5%.
Results
Population
Data were available for 71% of neonatal units from the beginning of 2008, 80% in 2009, 86% in 2010, 97% in 2011, 99% in 2012, and 100% in 2013 and 2014. There were 50,467 infants born between January 2008 and December 2014 at 22+0 to 31+6 weeks’ gestation whose mothers were resident in England and who were admitted to a contributing neonatal unit. Thirty-eight infants were excluded owing to implausible birthweight for gestation. A further 317 observations (0.6%) were excluded because birthweight, sex or multiple birth status was missing, leaving 50,112 infants included in the study. Population characteristics were fairly similar across all 5 years (Table 7), although some differences were statistically significant. There was a slight increase in the proportion of infants born 30+0 to 31+6 weeks’ gestation, from 39.6% in 2008 to 42.9% in 2014. The proportion of babies delivered by caesarean section increased every year, from 55% in 2008 to 59% in 2014, but this outcome was missing for around 9% of infants. Infants admitted to neonatal units tend to be from more deprived areas than the general population, and this became more marked over the study period: the 20% most deprived LSOAs contribute > 30% of the study population (increasing from 29% to 33% over the period), whereas the 20% least deprived LSOAs contribute only 13% (decreasing from 14% to 12%).
| Characteristics | Year, n (%) | Total (N = 50,112), n (%) | p-value for trend | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2008 (N = 6103) | 2009 (N = 6487) | 2010 (N = 7386) | 2011 (N = 7733) | 2012 (N = 7667) | 2013 (N = 7367) | 2014 (N = 7369) | |||
| Gestational age (complete weeks) | |||||||||
| 22+0 to 23+6 | 195 (3.2) | 160 (2.5) | 198 (2.7) | 165 (2.1) | 205 (2.7) | 198 (2.7) | 228 (3.1) | 1349 (2.7) | p < 0.01 |
| 24+0 to 25+6 | 760 (12.5) | 694 (10.7) | 759 (10.3) | 890 (11.5) | 872 (11.4) | 842 (11.4) | 820 (11.1) | 5637 (11.2) | |
| 26+0 to 27+6 | 1121 (18.4) | 1219 (18.8) | 1306 (17.7) | 1401 (18.1) | 1373 (17.9) | 1238 (16.8) | 1232 (16.7) | 8890 (17.7) | |
| 28+0 to 29+6 | 1610 (26.4) | 1734 (26.7) | 2029 (27.5) | 2064 (26.7) | 1992 (26) | 1997 (27.1) | 1925 (26.1) | 13,351 (26.6) | |
| 30+0 to 31+6 | 2417 (39.6) | 2680 (41.3) | 3094 (41.9) | 3213 (41.5) | 3225 (42.1) | 3092 (42) | 3164 (42.9) | 20,885 (41.7) | |
| Birthweight (g) | |||||||||
| < 500 | 53 (0.9) | 47 (0.7) | 45 (0.6) | 40 (0.5) | 52 (0.7) | 74 (1) | 71 (1) | 382 (0.8) | p = 0.74 |
| 500 to 999 | 2053 (33.6) | 2061 (31.8) | 2286 (31) | 2523 (32.6) | 2446 (31.9) | 2332 (31.7) | 2360 (32) | 16,061 (32.1) | |
| 1000 to 1499 | 2519 (41.3) | 2811 (43.3) | 3209 (43.4) | 3310 (42.8) | 3297 (43) | 3148 (42.7) | 3160 (42.9) | 21,454 (42.8) | |
| 1500 to 1999 | 1358 (22.3) | 1472 (22.7) | 1716 (23.2) | 1737 (22.5) | 1757 (22.9) | 1700 (23.1) | 1667 (22.6) | 11,407 (22.8) | |
| ≥ 2000 | 120 (2) | 96 (1.5) | 130 (1.8) | 123 (1.6) | 115 (1.5) | 113 (1.5) | 111 (1.5) | 808 (1.6) | |
| SGA | |||||||||
| No | 5211 (85.4) | 5530 (85.2) | 6305 (85.4) | 6540 (84.6) | 6569 (85.7) | 6271 (85.1) | 6261 (85) | 42,687 (85.2) | p = 0.62 |
| Yes | 892 (14.6) | 957 (14.8) | 1081 (14.6) | 1193 (15.4) | 1098 (14.3) | 1096 (14.9) | 1108 (15) | 7425 (14.8) | |
| Sex | |||||||||
| Female | 2831 (46.4) | 3099 (47.8) | 3367 (45.6) | 3547 (45.9) | 3513 (45.8) | 3278 (44.5) | 3376 (45.8) | 23,011 (45.9) | p = 0.01 |
| Male | 3272 (53.6) | 3388 (52.2) | 4019 (54.4) | 4186 (54.1) | 4154 (54.2) | 4089 (55.5) | 3993 (54.2) | 27,101 (54.1) | |
| Multiplicity of pregnancy | |||||||||
| Singleton | 4456 (73) | 4714 (72.7) | 5364 (72.6) | 5628 (72.8) | 5609 (73.2) | 5522 (75) | 5416 (73.5) | 36,709 (73.3) | p = 0.02 |
| Twins | 1514 (24.8) | 1626 (25.1) | 1828 (24.7) | 1889 (24.4) | 1852 (24.2) | 1675 (22.7) | 1777 (24.1) | 12,161 (24.3) | |
| Triplets or more | 133 (2.2) | 147 (2.3) | 194 (2.6) | 216 (2.8) | 206 (2.7) | 170 (2.3) | 176 (2.4) | 1242 (2.5) | |
| Any antenatal steroids given | |||||||||
| No | 738 (12.6) | 728 (11.5) | 868 (12.1) | 864 (11.4) | 879 (11.6) | 773 (10.6) | 766 (10.4) | 5616 (11.4) | p < 0.01 |
| Yes | 5137 (87.4) | 5585 (88.5) | 6312 (87.9) | 6724 (88.6) | 6704 (88.4) | 6552 (89.4) | 6579 (89.6) | 43,593 (88.6) | |
| Missing | 228 | 174 | 206 | 145 | 84 | 42 | 24 | 903 | |
| Mode of delivery | |||||||||
| Vaginal | 2344 (45.2) | 2557 (44.1) | 2949 (43.6) | 3080 (43.1) | 3001 (42.6) | 2848 (42.2) | 2793 (41.1) | 19,572 (43) | p < 0.01 |
| Caesarean | 2843 (54.8) | 3246 (55.9) | 3817 (56.4) | 4070 (56.9) | 4044 (57.4) | 3896 (57.8) | 3996 (58.9) | 25,912 (57) | |
| Missing | 916 | 684 | 620 | 583 | 622 | 623 | 580 | 4626 | |
| Maternal age (years) | |||||||||
| < 20 | 531 (8.9) | 520 (8.1) | 630 (8.6) | 581 (7.5) | 527 (6.9) | 469 (6.4) | 450 (6.2) | 3708 (7.5) | p < 0.01 |
| 20 to 24 | 1088 (18.3) | 1201 (18.6) | 1342 (18.2) | 1498 (19.4) | 1390 (18.2) | 1248 (17) | 1175 (16.1) | 8942 (18) | |
| 25 to 29 | 1526 (25.7) | 1658 (25.7) | 1900 (25.8) | 1984 (25.7) | 1986 (26) | 1892 (25.8) | 1934 (26.5) | 12,880 (25.9) | |
| 30 to 34 | 1499 (25.2) | 1721 (26.7) | 1962 (26.7) | 2072 (26.9) | 2123 (27.8) | 2085 (28.5) | 2165 (29.6) | 13,627 (27.4) | |
| 35 to 40 | 1023 (17.2) | 1063 (16.5) | 1206 (16.4) | 1235 (16) | 1216 (15.9) | 1245 (17) | 1192 (16.3) | 8180 (16.5) | |
| > 40 | 270 (4.5) | 290 (4.5) | 321 (4.4) | 335 (4.3) | 396 (5.2) | 389 (5.3) | 386 (5.3) | 2387 (4.8) | |
| Missing | 166 | 34 | 25 | 28 | 29 | 39 | 67 | 388 | |
Predictive model
Parameter estimates from the logistic regression model are shown in Appendix 1, Table 56. Gestational age was modelled with a five-knot spline and birthweight was modelled as birthweight and birthweight2 with interactions between linear birthweight with all gestational age terms, and birthweight2 with linear gestational age included. Appendix 2, Figures 28–35, show isosurv plots for survival prediction. After correcting for optimism, the AUC was 0.84 and the Brier score was 0.07. The model was well calibrated for both gestational subgroups, with optimism-corrected slopes of 1.01 and 0.97, and intercepts of 0.01 and –0.1 for infants born at < 28+0 weeks’ and ≥ 28+0 weeks’ gestation, respectively. Table 8 shows the results comparing performance with comparator models. The NNRD model was better calibrated than the other models, with calibration intercepts and slopes closer to the target values of 0 and 1, respectively. The AUC was higher than the National Institute of Child Health and Human Development (NICHD) model, but still fairly low at 0.70. There were no other differences in the AUC or the Brier score.
| Statistic | Model | |||||
|---|---|---|---|---|---|---|
| CRIB II | NNRD | Draper | NNRD | NICHD | NNRD | |
| Number | 16,652 | 16,445 | 6986 | |||
| Observed survival (%) | 91.7 | 90.5 | 60.5 | |||
| Predicted survival (%) | 92.6 | 91.5 | 89.3 | 90.5 | 52.2 | 60.2 |
| AUC (95% CI) | 0.82 (0.80 to 0.83) | 0.81 (0.80 to 0.83) | 0.81 (0.80 to 0.82) | 0.82 (0.81 to 0.83) | 0.59 (0.57 to 0.60) | 0.71 (0.69 to 0.72) |
| Brier score | 0.064 | 0.064 | 0.071 | 0.070 | 0.250 | 0.208 |
| Cox | ||||||
| α (95% CI) | –0.27 (–0.37 to –0.17) | –0.08 (–0.19 to 0.02) | –0.36 (–0.45 to –0.27) | 0.03 (–0.07 to 0.13) | –0.40 (–0.45 to –0.35) | 0.02 (–0.04 to 0.08) |
| β (95% CI) | 0.77 (0.73 to 0.81) | 0.97 (0.92 to 1.02) | 0.88 (0.84 to 0.92) | 1.01 (0.97 to 1.06) | 0.42 (0.36 to 0.49) | 1.09 (1.01 to 1.17) |
Survival to discharge from 2008 to 2014
Of the 48,422 admitted infants for whom outcomes were known, 43,444 (89.7%) survived to discharge. There was no evidence of autocorrelation in any analyses (no change when altering the autocorrelation parameter), so results are presented without autocorrelation. There was an increase in the percentage of admitted infants who survived to discharge from 88% in 2008 to 91.3% in 2014. Survival increased with gestational age, from 35% for 22+0 to 23+6 weeks’ gestation to 98% for 30+0 to 31+6 weeks’ gestation. Appendix 1, Table 57, shows the associations between survival and infant characteristics for the whole cohort, based on complete data only. Crude survival rates were lower for boys, infants whose mothers did not receive antenatal steroids and infants born by vaginal delivery. Infants born to younger mothers, mothers who smoked and mothers from more deprived areas had lower crude survival rates.
The annual percentage change (APC) for crude survival was 0.51% (95% CI 0.35% to 0.67%; p < 0.001), and 0.46% (95% CI 0.30% to 0.62%; p < 0.001) after direct standardisation for risk of death. Results were similar when the only neonatal networks where all hospitals contributed data for the whole period were examined [crude APC 0.56% (95% CI 0.35% to 0.77%); adjusted APC 0.53% (95% CI 0.33% to 0.73%)]. Sensitivity analysis of complete-case data and standardising for 5 and 15 categories gave very similar results.
Survival to 28 days
Fifty deaths were established by linkage with ONS, of which 20 were within 28 days of birth. There was an increase in the percentage of infants who survived to 28 days, from 91.4% in 2008 to 93.5% in 2014. Survival increased with gestational age from 48.4% for 22+0 to 23+6 weeks’ gestation to 98.2% for 30+0 to 31+6 weeks’ gestation. The APC for crude 28-day survival was 0.3% (95% CI 0.15% to 0.45%; p < 0.001), and 0.27% (95% CI 0.11% to 0.44%; p = 0.002) after direct standardisation for risk of death. Results were similar when only the neonatal networks in which all hospitals contributed data for the whole period were examined (crude APC 0.35%, 95% CI 0.19% to 0.52%; adjusted APC 0.3%; 95% CI 0.14% to 0.47%).
Time of death
A total of 24% of deaths occurred within 24 hours, 28% between 25 hours and 7 days, 26% between 8 days and 28 days and 23% beyond 28 days. There was no evidence of a change in the median and 25th percentile time of death, whereas the 75th percentile reduced from 2008 (27.2 days) to 2013 (20.8 days), but rose to 24.3 days in 2014 (estimated annual decrease 2008–14: 0.92 days, 95% CI 0.2 to 1.7 days; p = 0.02).
Trends in survival to discharge by gestational age
Figure 10 shows the joinpoint regression analysis for survival to discharge by gestational age group. Improvements were less marked with increasing gestation, ranging from an APC of 6.03% (95% CI 2.47% to 3.53%; p = 0.002) in infants born 22+0 to 23+6 weeks’ gestation to no change in infants born 30+0 to 31+6 weeks’ gestation (APC 0.01%, 95% CI –0.08% to 0.09%; p = 0.9).
FIGURE 10.
Joinpoint regression analysis for crude rates of survival to discharge for admitted infants born at (upper plot) 22+0 to 25+6 weeks’ gestation and (lower plot) 26+0 to 31+6 weeks’ gestation by birth year. APC, average percentage change. NNU, neonatal unit.
Variation by region and Index of Multiple Deprivation quintile using data from 2011 onwards
Crude survival varied from 89.3% (95% CI 88.6% to 89.9%) in the Midlands and the East of England to 91.1% (95% CI 90.3% to 91.8%) in London; after direct standardisation, the range was 89.2% (95% CI 87.3% to 91.1%) to 91.6% (95% CI 89.1% to 94.2%). Only London and the South of England showed improvements in crude survival (Figure 11).
FIGURE 11.
Joinpoint regression analysis for crude rates of survival to discharge for admitted infants born in 2011–14 at 22–31 weeks’ gestation by NHS commissioning region. A, adjusted survival (%) over years 2011–14; C, crude survival (%) over years 2011–14. NNU, neonatal unit.
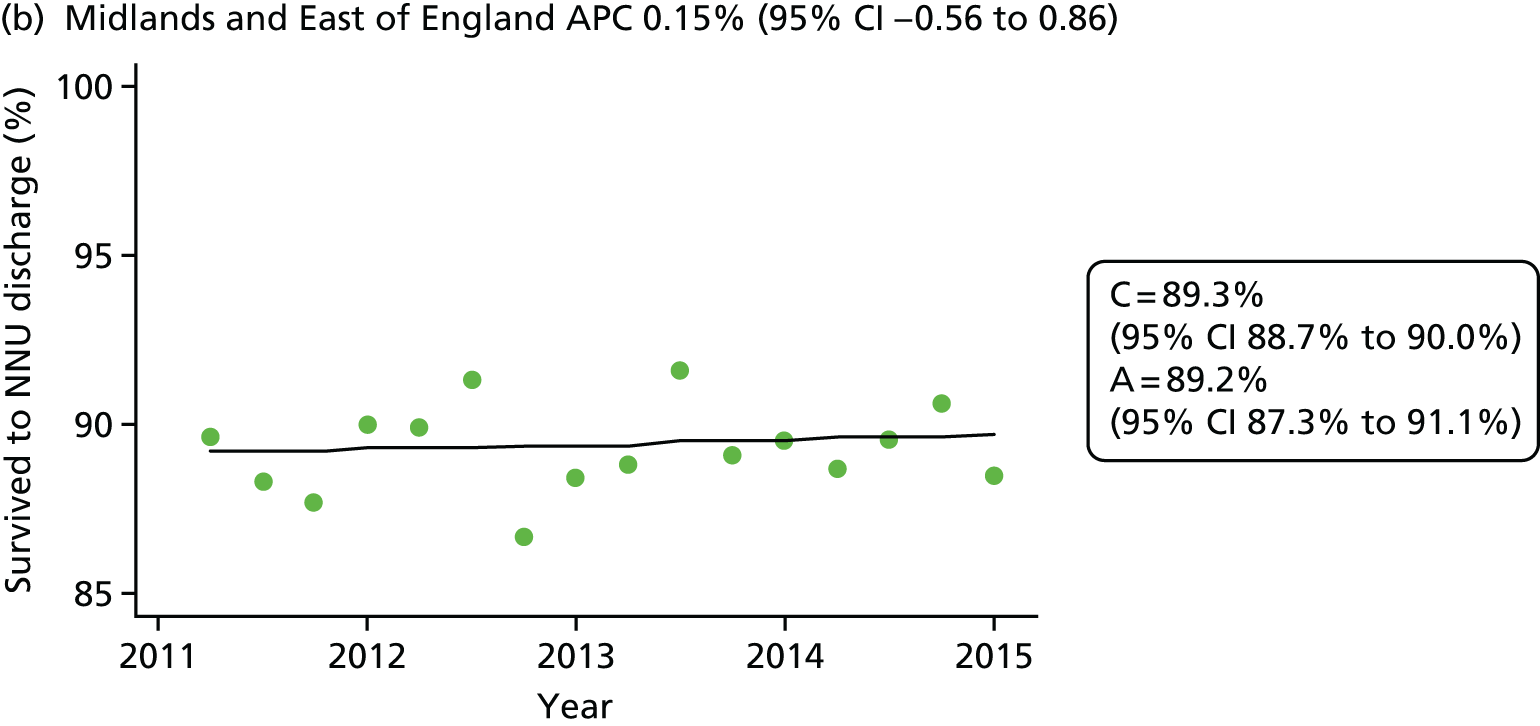
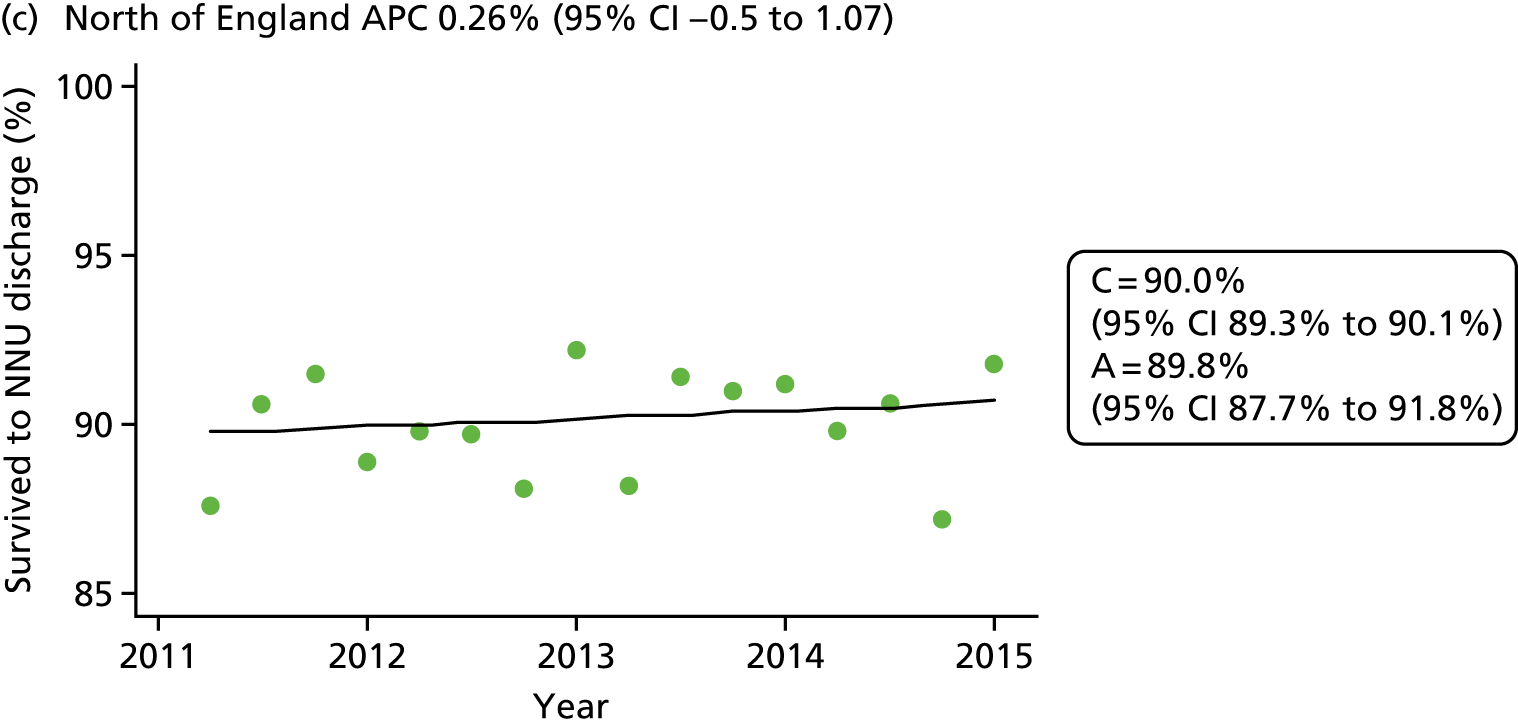

Infants from the most deprived quintile had lower survival rates than those from the least deprived quintile [89.5% (95% CI 88.9% to 90.1%) versus 91.1% (95% CI 90.2% to 92.1%)]; little difference remained after standardisation [89.8% (95% CI 87.9% to 91.5%) versus 90.1% (95% CI 87.1% to 93.2%)]. Inclusion of IMD decile in the risk adjustment model did not change results for each region, with evidence of residual variation across regions (p < 0.001).
Comparison with Office for National Statistics and EPICure data
The number of deaths before 28 days among admitted infants born 22+0 to 31+6 weeks’ gestation recorded in the NNRD for England and Wales in 2012 was 801. This represents 81% (801/989) of the deaths recorded among live births for the same gestation range in England and Wales by the ONS. Most of the discrepancy occurred at earlier gestations: there were seven deaths among infants born 22 weeks’ gestation in the NNRD, compared with 154 in the ONS.
There was no evidence of a change in the rate of improvement since the first EPICure study. Improvements in survival to discharge of infants born at 22+0 to 25+6 weeks’ gestation and admitted to neonatal care in 1995 (EPICure),124 2006 (EPICure 2)135 and 2008–14 (NNRD)137 have continued at a similar rate (Figure 12).
FIGURE 12.
Survival to NNU discharge from 1995 to 2015 based on data from EPICure (triangle), EPICure 2 (cross) and NNRD (circles), for infants born at 23 (blue), 24 (green) and 25 (black) weeks’ gestation. NNU, neonatal unit.
Mortality by Operational Delivery Network
The number of infants were born in 2013–14 at ≤ 31+6 weeks’ gestation and admitted to neonatal care for whom the neonatal network of booking was known was 15,255; 8.9% of those for whom the outcome was known (1327/14,837) died before discharge. The outcome was missing for 2.7% of infants. Infants with a missing outcome tended to be more vulnerable based on other neonatal characteristics. Antenatal steroid entries were missing for 0.5% of infants, and sex and multiple birth status SDSs for < 0.01%. The prediction model fit the data well, giving an area under the receiver operating characteristic (ROC) curve of 0.83 (95% CI 0.82 to 0.84).
The SMRs are shown on funnel plots, both unadjusted (Figure 13) and adjusted (Figure 14), with neonatal networks numbered (Table 9 contains the key). Analysis of complete cases gave very similar results (largest absolute difference in SMR of 0.04).
FIGURE 13.
Funnel plot for unadjusted SMR for babies live-born ≤ 31+6 weeks’ gestation in 2013–14 and admitted to neonatal care, by neonatal network of booking; control limits show 2 and 3 SDs from the mean after correction for multiple testing, assuming observed deaths follow a Poisson distribution; numbers correspond to neonatal networks in Table 9.

FIGURE 14.
Funnel plot for adjusted SMR for babies live-born ≤ 31+6 weeks’ gestation in 2013–14 and admitted to neonatal care, by neonatal network of booking; control limits show 2 and 3 SDs from the mean after correction for multiple testing, assuming observed deaths follow a Poisson distribution. Adjusted for gestation, birthweight SDS, sex, antenatal steroids accounting for correlation within multiple birth sets and missing data; numbers correspond to neonatal networks in Table 9.
| Code | Booked neonatal network | Total infants | SMR (95% CI) | |
|---|---|---|---|---|
| Raw | Adjusted | |||
| 1 | Bedfordshire and Hertfordshire | 422 | 0.64 (0.33 to 1.1) | 0.62 (0.32 to 1.07) |
| 2 | Cheshire and Merseyside | 659 | 1.11 (0.76 to 1.56) | 1.14 (0.78 to 1.6) |
| 3 | Eastern | 773 | 1 (0.69 to 1.4) | 1.02 (0.71 to 1.42) |
| 4 | Greater Manchester | 885 | 1.17 (0.86 to 1.56) | 0.94 (0.69 to 1.26) |
| 5 | Kent | 447 | 0.79 (0.45 to 1.28) | 0.81 (0.46 to 1.31) |
| 6 | Lancashire and South Cumbria | 448 | 1.13 (0.71 to 1.7) | 1.15 (0.72 to 1.72) |
| 7 | London (North Central) | 399 | 0.64 (0.32 to 1.12) | 0.57 (0.29 to 1) |
| 8 | London (North East) | 1029 | 0.92 (0.66 to 1.24) | 0.83 (0.6 to 1.12) |
| 9 | London (North West) | 691 | 0.95 (0.63 to 1.36) | 0.98 (0.65 to 1.4) |
| 10 | London (South East) | 614 | 0.87 (0.56 to 1.3) | 0.8 (0.51 to 1.19) |
| 11 | London (South West) | 402 | 0.77 (0.42 to 1.28) | 0.83 (0.45 to 1.4) |
| 12 | Midlands (Central) | 744 | 1.2 (0.85 to 1.63) | 1.16 (0.83 to 1.58) |
| 13 | Midlands (South West) | 781 | 1.18 (0.85 to 1.6) | 1.04 (0.75 to 1.41) |
| 14 | Staffordshire, Shropshire and Black Country | 596 | 1.25 (0.86 to 1.75) | 1.26 (0.86 to 1.76) |
| 15 | North Trent | 599 | 1.02 (0.67 to 1.48) | 1.1 (0.72 to 1.59) |
| 16 | Northern | 757 | 1.08 (0.76 to 1.49) | 1 (0.7 to 1.38) |
| 17 | Peninsula (South West) | 318 | 0.93 (0.5 to 1.57) | 1.01 (0.54 to 1.71) |
| 18 | South Central (North) | 602 | 1.14 (0.77 to 1.62) | 1.05 (0.71 to 1.48) |
| 19 | South Central (South) | 615 | 0.65 (0.38 to 1.02) | 0.62 (0.37 to 0.98) |
| 20 | Surrey and Sussex | 643 | 0.89 (0.58 to 1.31) | 0.87 (0.57 to 1.28) |
| 21 | Trent | 562 | 1.19 (0.8 to 1.7) | 1.15 (0.77 to 1.64) |
| 22 | Wales | 705 | 1.08 (0.74 to 1.5) | 1.07 (0.74 to 1.5) |
| 23 | Western | 646 | 0.97 (0.65 to 1.4) | 0.98 (0.65 to 1.41) |
| 24 | Yorkshire | 918 | 0.95 (0.67 to 1.3) | 0.97 (0.69 to 1.33) |
Conclusions
Survival between 2008 and 2014
We demonstrate that survival of very preterm infants admitted to neonatal units in England improved between 2008 and 2014, with the greatest improvement seen among infants born at the lowest number of weeks of gestation. However, survival did not improve consistently across the four NHS commissioning regions, with London and the South of England performing better than the Midlands, the East of England, and the North. Survival was lower for infants from more deprived areas, but regional differences in survival persisted after adjustment for socioeconomic differences.
A key strength of the study is the data. Over 50,000 very preterm infants were included, representing the vast majority of neonatal unit admissions in the country during the study period. Assurance on the quality and completeness of the data was provided through comparison with ONS data. Furthermore, neonatal units had the opportunity to validate survival outcomes and clinical characteristics for infants in the study population born after 2012 as part of work by the NDAU. The risk adjustment variables used in the study were limited to key, unambiguous clinical characteristics to minimise the risk of incomplete or inaccurate data; < 3% of records had any missing data, and only 38 records had implausible birthweight for gestation. Several steps were taken to limit or investigate potential bias in the analysis. The improvements in survival remained when we examined only neonatal networks contributing data throughout the period, changed the number of categories used in risk adjustment and looked at infants with complete data only.
A limitation is that the population comprises infants admitted to a neonatal unit, thus excluding live-born infants who died before admission; this is because data capture in the EPRs is triggered by neonatal unit admission. However, this is the relevant population for neonatal services, and comparison with ONS data showed that > 80% of known deaths in this gestational age range were captured in the NNRD, with the shortfall at the earliest gestations likely to represent deaths before admission. Furthermore, analysis of live births does not guarantee a consistent population as there is variation across England in whether or not infants born at < 24 weeks’ gestation are registered as live births. We have imputed missing outcomes on the assumption that the missingness presents no additional information beyond the other neonatal characteristics. This may not be so, as some missing outcomes might reflect infants who were transferred to specialist surgical providers that are not standard neonatal units and do not contribute data to the NNRD. However, no patterns were seen (e.g. with gestational age) for infants according to the reason for transfer, and the proportion of infants with missing outcomes was small.
Improvements in survival of very preterm infants have been demonstrated in other countries, but most examine survival of live-born infants rather than neonatal unit admissions. A population study from New South Wales and the Australian Capital Territory showed improved survival of admitted babies at 24 (50–60%), 25 (60–75%) and 26 (80–85%) weeks’ gestation between 2000–1 and 2007–11, which is similar to results seen here. 142
Mortality by Operational Delivery Network
We combined 2 years’ data to provide improved power to detect significant deviation from average national performance. The average number of expected deaths in a network is 60. If a neonatal network with a patient case-mix leading to 60 expected deaths had a true underlying SMR of 1.3, the probability of the network’s observed data falling above the 2 SD upper control limit is around 60% (i.e. there would be 60% power to alert the network as having potentially unusual performance). This is before widening the limits to allow for multiple testing, which reduces power further.
Note that if the SMR for a neonatal network lies outside the funnel, it will not necessarily have a CI that excludes 1. This is because they have different interpretations: the CI reflects uncertainty about the true SMR for that particular network, whereas the funnel plot limits reflect the variability we would expect to see in the SMR for similar neonatal networks. More specifically, the CI is the range in which we are 95% confident that the true SMR for the neonatal network lies, whereas the funnel plot limits represent the range in which we expect 95% of neonatal networks to lie.
Implications for health care
We have established the feasibility of monitoring neonatal outcomes at the national level using near-contemporaneous, routinely recorded EPR data. This has been achieved with wide professional support and our methods provide a template for future evaluations. We hope the opportunity to monitor survival will be adopted by health-care managers, clinicians and commissioners.
Many clinical outcomes, including mortality, are relatively rare. Hence, achieving adequate power to detect changes over time and in relation to factors, such as geographical region and patient or neonatal unit characteristics, requires a large sample. The use of EPR data facilitates the capture of large samples and, hence, offers opportunity for rigorous evaluation of clinical outcomes. The use of EPR data has also enabled up-to-date, rapid assessment ensuring that the information available to clinicians and health service mangers is near-contemporaneous, with minimal burden to clinical staff.
As data capture in the EPRs is triggered by neonatal unit admission, a limitation is that the total population denominator for all live births is not available in the NNRD. Through comparison with ONS data, we showed that > 80% of known deaths in the preterm gestational age range studied were captured in the NNRD, with the shortfall occurring among the earliest gestations and thus likely to represent deaths before admission rather than incomplete NNRD data. We and others have suggested that the neonatal EPR is modified to enable capture of live-born infants who die before admission, but this will not guarantee a consistent or complete population until the variation across England regarding whether or not infants born live at less than 24 weeks’ gestation are registered as live births is addressed.
Research recommendations
Improved short-term survival over time has been previously recognised but the ensuing trends in later life outcomes are not well defined. A potential extension to our work is to evaluate national trends in developmental outcomes at 2 years, as these data are captured in the NNRD as part of the Royal College of Paediatrics and Child Health National Neonatal Audit Programme.
Our finding that preterm survival has not improved consistently across the four NHS commissioning regions and that this is not accounted for by socioeconomic differences is important. Factors, such as staffing levels and care practices, that might explain geographical variation in survival require consideration in order to reduce potential inequities in health-care delivery.
Chapter 4 Testing the quality of Electronic Patient Record data held in the National Neonatal Research Database to support clinical trials
Abstract
Background: Because data recorded in a trial case report form (CRF), widely considered ‘gold standard’, may already exist within an EPR, repeated collection is wasteful.
Aim: We tested the null hypothesis that EPR data from the NNRD are not of comparable quality to research data.
Methods: We compared NNRD data with data recorded independently in a NIHR trial CRF. We selected a broad range of patient characteristics, processes and outcomes. For each variable, we calculated major and minor discordance rates using predefined criteria, and the sensitivity, specificity and positive predictive values (PPV) of NNRD outcome variables in comparison with the gold standard CRF source.
Results: We assessed 2257 episodes of care in 1258 infants. Major discordance rates were low for 14 out of 15 patient characteristics, 9 out of 12 process measures and 10 out of 11 outcomes. The prevalence of adverse outcomes was < 6% with the exception of bronchopulmonary dysplasia (49.0%) and medical treatment for patent ductus arteriosus (PDA) (20.3%). Specificity was high (> 85%) for all outcomes, sensitivity ranged from 50% to 100%, and PPV ranged from 58.8% (95% CI 40.7% to 75.4%) for a report of a porencephalic cyst to 99.7% (95% CI 99.2% to 99.9%) for survival to discharge.
Conclusions: Patient characteristics and the majority of NNRD items tested compare well against CRF data. A small number of important outcomes are not currently reliably recorded in the EPRs. We recommend minor changes to EPR entry screens to improve outcome data, and testing of NNRD data use in a clinical trial.
Background
Randomised clinical trials are the gold standard method for evaluating therapeutic interventions. The data set recorded for trials involving hospital inpatients usually overlaps with, and may exist completely within, an EPR. Despite this, data collection for clinical trials is usually conducted independently of routine care. This results in duplication of effort for clinical staff and may increase the risk of transcription errors and missing data. The additional workload contributes to the high cost of trials and may act as a disincentive to busy clinicians to participate.
One of the most important principles of the Medicines for Neonates programme is that recording data only once and using them for a multitude of purposes will lead to higher-quality NHS data. This workstream addresses the question of whether or not the data recorded in neonatal EPRs as part of clinical care are of sufficient quality to support a clinical trial. Our objective was to compare routinely recorded EPR data with data recorded specifically for a NIHR-funded multicentre trial of an investigational medicinal product conducted in accordance with the principles of good clinical practice. The trial selected for comparison was the ‘Probiotic in Preterm infants Study (PiPS)’, funded by the Health Technology Assessment (HTA) programme. 143
The PiPS trial143 was a multicentre, double-blind, placebo-controlled, randomised trial of probiotic administration in preterm infants, designed to study the possible benefits of early administration of the probiotic Bifidobacterium breve BBG-001 (hereafter referred to in brief as ‘the probiotic’) to infants born before 31 weeks’ gestation and recruited within 48 hours of birth. There were three primary outcomes: (1) any episode of NEC to Bell stage II or III, (2) any positive blood culture of an organism that is not recognised as a skin commensal on a sample drawn > 72 hours after birth and before 46 weeks’ postmenstrual age or discharge if sooner (hereafter, sepsis for brevity) and (3) death before discharge from hospital.
Infants (n = 1315) from 24 neonatal units in the south-east of England were recruited to PiPS between July 2010 and July 2013. All of the recruiting hospitals and those to which the babies were likely to be transferred before their initial discharge home, with the exception of the Great Ormond Street and the Royal Brompton Hospitals (to which infants are occasionally referred for specialist care), use the neonatal EPRs and submit data to the NDAU.
Professor Kate Costeloe, who is a co-applicant for the Medicines for Neonates Programme, is also the chief investigator for the PiPS trial and, thus, was able to provide a facilitated opportunity to compare data held in the trial CRFs and those derived from the neonatal EPRs.
Aims
-
To assess the agreement between EPR-derived demographic, process and outcome variables held in the NNRD and equivalent CRF-derived variables held in the PiPS trial database.
-
To evaluate whether or not there was any decrease in discordance rates of compared items over the course of recruitment to the PiPS trial.
Methods
Data
Neonatal EPR data obtained from the NNRD were compared with those recorded independently on trial CRFs and held in the PiPS database. Items for comparison were selected either because the definitions were identical or because data in the NNRD could be used to derive the item as defined for PiPS trial requirements. Variables were selected to represent a broad range of patient characteristics, processes and outcome measures.
The 15 baseline patient data items comprised (1) expected date of delivery, (2) gestational age (weeks and days), (3) month and year of birth, (4) birthweight (g), (5) sex, (6) Apgar score at 5 minutes, (7) whether inborn or transferred, (8) singleton or multiple, (9) birth order, (10) maternal year of birth, (11) maternal ethnicity by NHS category, (12) LSOAS, as derived from postcode, (13) any antenatal corticosteroid given, (14) mode of delivery (vaginal vs. caesarean) and (15) instrumental delivery (Table 10).
| Item to be compared | Data held | Definition | |||
|---|---|---|---|---|---|
| PiPS | NNRD | Limits of agreement | Minor disagreement | Major disagreement | |
| EDD | EDD | EDD | Up to ± 2 days | 3–6 days | ± 1 week |
| Gestational age (weeks and days) | Gestational age is computed from EDD | Gestational age (recorded independently of EDD) | Up to ± 2 days | 3–6 days | ± 1 week |
| Date of birth (month and year) | Date and time of birth | Month and year of birth | No difference | N/A | N/A |
| Birthweight (g) | Infant’s birthweight (g) | Infant’s birthweight (g) | 30 g | 30–100 g | > 100 g |
| Sex | Infant’s sex (male/female/indeterminate) | Infant’s sex (male/female/indeterminate) | No difference | N/A | N/A |
| Apgar score at 5 minutes | Apgar score at 5 minutes | Apgar score at 5 minutes | ± 1 | ± 2 | ± 3 or more |
| Born in this hospital | Whether or not infant was born in this hospital | Place of birth | No difference | N/A | N/A |
| Singleton or multiple birth | Whether infant is a singleton or multiple birth | Whether infant is a singleton or multiple birth | No difference | N/A | N/A |
| Birth order | Birth order of infant | Birth order of infant | No difference | N/A | N/A |
| Maternal year of birth | Maternal date of birth | Maternal birth year | No difference | N/A | N/A |
| Maternal ethnicity | Maternal ethnicity (NHS categories) | Maternal ethnicity (NHS categories) | No difference | N/A | N/A |
| Maternal LSOA at time of infant’s birth | Maternal LSOA derived from postcode | Maternal LSOA derived from postcode | No difference | N/A | N/A |
| Whether or not any antenatal steroids were given | Antenatal steroids and exact timing | Any antenatal steroids given, no detail of timing | No difference | N/A | N/A |
| Mode of delivery: caesarean or vaginal | Mode of delivery | Mode of delivery | No difference | N/A | N/A |
| Whether or not instrumental delivery | Whether forceps or ventouse were used for delivery | Mode of delivery | No difference | N/A | N/A |
The 13 processes or interventions during admission were (1) surgery for PDA, (2) medical treatment of PDA, (3) retinopathy of prematurity (ROP) treatment by laser or cryotherapy, (4) central venous line days, (5) intensive care days, (6) high-dependency care days, (7) whether or not transferred to another hospital, (8) discharge month and year (Table 11) and, in the first 14 days, (9) day of first milk feed, (10) type or types of milk (maternal milk, donor milk or formula) given on the first day of feeding, (11) a summary of all types of milk received over all of the days on which feeds were reported on both databases, (12) duration of exposure to any antibiotic or (13) duration of exposure to any antacid (Table 12).
| Item to be compared | Data held | Definition | |||
|---|---|---|---|---|---|
| PiPS | NNRD | Limits of agreement | Minor disagreement | Major disagreement | |
| Surgery for PDA | While in this hospital, did the infant receive surgical ligation for PDA? |
Daily data: surgery for PDA today Discharge diagnoses Procedures during stay |
No difference | N/A | Any difference |
| Medical treatment for PDA with indometacin or ibuprofen | While in this hospital, has the infant received medical treatment with indometacin and/or ibuprofen for PDA? |
Daily data: treatment for PDA Daily drugs |
No difference | N/A | Any difference |
| Treatment for ROP with laser or cryotherapy | While in this hospital, has infant had ROP treated with laser/cryotherapy? |
Daily data: treatment Discharge diagnoses Procedures during stay? |
No difference | N/A | Any difference |
| Central venous line days | While in this hospital, what was the total number of days for which the infant had a central venous line [UVC, peripheral long line, BROVIAC® (Bard Access Systems Inc., Salt Lake City, UT, USA), etc.] | Daily data: lines in situ | ± 2 days | 3–4 days | ± 5 or more days |
| Intensive care days | While in this hospital, what was the total number of days of intensive care days? | Daily data | ± 2 days | 3–4 days | ± 5 or more days |
| High-dependency care days | While in this hospital, what was the total number of high-dependency care days? | Daily data | ± 2 days | 3–4 days | ± 5 or more days |
| Transfer to another hospital | Whether or not was transferred to another hospital | Discharge details | No difference | N/A | Any difference |
| Discharge month and year | Date of discharge or death | Discharge details | No difference | N/A | Any difference |
| Item to be compared | Data held | Definition | |||
|---|---|---|---|---|---|
| PiPS | NNRD | Limits of agreement | Minor disagreement | Major disagreement | |
| Day of first milk feed | What day of life was milk commenced? | Daily feeding data | ± 1 day | ± 2 days | > 2 days |
| Type(s) of first milk feed | Milk on first day of receiving milk feed | Daily feeding data | No difference | N/A | Any difference |
| Summary of all types of milk in first 14 days | Daily feeding data for first 14 postnatal days | Daily feeding data | No difference | N/A | Any difference |
| Total number of days of antibiotics received during first 14 postnatal days | Names and total days of antibiotics received during the first 14 postnatal days | Daily drugs | ± 1 day | ± 2 days | > 2 days |
| Total number of days of antacid received during first 14 postnatal days | Total days of antacid use during the first 14 postnatal days | Daily drugs | ± 1 day | ± 2 days | > 2 days |
The nine outcome data items were (1) worst stage of ROP in either eye, (2) bronchopulmonary dysplasia (BPD), defined by whether or not the infant required supplementary oxygen at 36 weeks’ postmenstrual age, (3) mechanical respiratory support at 36 weeks’ postmenstrual age, (4) cranial ultrasound findings, (5) survival to discharge, (6) any diagnosis of perforated NEC, (7) any abdominal surgery for NEC, (8) any gastrointestinal perforation and (9) length of stay (Table 13).
| Variable to be compared, definitions | Data held | Definition | |||
|---|---|---|---|---|---|
| PiPS | NNRD | Limits of agreement | Minor disagreement | Major disagreement | |
| Worst stage of ROP in any eye | Worst stage of ROP in ANY eye? (Stage 1–5) |
Discharge diagnoses Ad hoc forms for each ROP examination |
No difference | N/A | Any difference |
| BPD requiring oxygen at 36 weeks’ postmenstrual age | If still in hospital at 36 weeks’ postmenstrual age: date reached 36 weeks’ postmenstrual age and whether receiving supplementary oxygen? | Daily data for oxygen use | No difference | N/A | Any difference |
| Requirement of mechanical respiratory support at 36 weeks’ postmenstrual age | If still in hospital at 36 weeks’ postmenstrual age was the infant receiving mechanical respiratory support | Daily data for respiratory support received | No difference | N/A | Any difference |
| Cranial ultrasound findings | While in this hospital, did the infant have any of the following abnormalities in their cranial ultrasound scan?
|
Discharge diagnoses Ad hoc forms for each cranial ultrasound examination |
No difference | N/A | Any difference |
| Survival to discharge from neonatal care | Survival to discharge | Discharge details | No difference | N/A | Any difference |
| Gastrointestinal diagnoses |
|
Discharge diagnoses Ad hoc form reporting abdominal radiography Daily surgery/NEC data |
No difference | N/A | Any difference |
| Length of stay | What was the total length of stay in neonatal care? |
Daily data Discharge details |
± 1 day | ± 2 days | ± 3 or more days |
Changes to the original protocol
When this study was designed it was anticipated that recruitment to the PiPS trial would begin in 2009. The analysis could be conducted only once PiPS data for the individual infant were complete and any queries had been addressed. Although it was always appreciated that the order in which the data for trial recruits were signed off would not be sequential, it was nonetheless expected that it would be possible to receive data in batches every few months. The original protocol involved comparison of PiPS and NNRD data from the first 200 infants recruited, as well as feedback of rates of minor and major discrepancies to neonatal units, with the final analysis undertaken on the next 200 infants. There were delays to the start of PiPS recruitment, which did not begin until July 2010 and which did not achieve its target rates for over 1 year. There were further delays to PiPS data being signed off as complete because of problems with introducing a new automated data query system. It became clear that we could not provide feedback to neonatal unit staff after 200 cases and that we were likely to receive any PiPS data at the NDAU only towards the end of PiPS trial recruitment. It was agreed within the MfN Steering Committee that we would request an initial download of all available complete data for piloting the database merger and would then perform the comparative analysis on the final PiPS data set, including all recruits, and this was received in October 2013. There is a constant process of data scrutiny and feedback aimed at improving data completeness and accuracy in the NNRD. It was therefore agreed that we should examine the whole data set for changes in discrepancy rates over time, in order to address the second aim listed above.
Preparation of data for comparison
Data acquisition differs between the PiPS trial and the NNRD, the former being recorded specifically for trial purposes and the latter containing data extracted from point-of-care EPRs, designed to provide a complete clinical record. In this section, we will describe how the data sets were prepared to ensure that the comparisons related to the same infant and the same episode of care.
Neonatal units in NHS hospitals in England function as clinical networks. Neonatal units vary in the care that they provide, in that some provide intensive care only in an emergency, whereas others that do provide ongoing intensive care might not do so for extremely preterm infants. A small number of neonatal units provide specialist services, such as surgery and cardiology. In so far as is possible, infants are looked after in the neonatal unit closest to the family home. Those infants needing specialist care may require transfer to a neonatal unit offering the required expertise; hence the entire course between birth and eventual discharge home may comprise a series of ‘episodes of care’ of varying durations in different neonatal units. The variables that were compared for each infant between the PiPS database and the NNRD comprise ‘once only’ data (e.g. baseline demographic items such as birthweight), ‘episodic’ data (e.g. processes and interventions during a defined episode of care) and ‘infant-level’ data (e.g. outcomes summarised from multiple episodes of care).
Infants born between 23+0 and 30+6 weeks’ gestational age and who were < 48 hours old were eligible for recruitment to the PiPS trial. Infants with a lethal congenital anomaly or any known gastrointestinal malformation known at birth, or with no realistic chance of survival, were excluded.
The CRF data collection was paper based, with four main collection forms: form 1 (entry), form 2 (daily data), form 3 (transfer/discharge) and form 4 (abdominal pathology). Form 1 (entry) is completed within 7 days of recruitment and returned to the National Perinatal Epidemiology Unit (NPEU), and it contains ‘once only’ information regarding the infant and maternal history [e.g. infant sex, expected date of delivery (EDD), maternal and obstetric details and infant’s condition at birth). Form 2 (daily data) requires daily recording for the first 14 postnatal days from the day of birth of the type of milk received, the total daily volume of milk (ml/kg/day), the antibiotics by type, the antifungals and the antacids. Form 3 (transfer/discharge) is completed for each episode of care, terminating at discharge (whether to another hospital or home or at death). It contains only events occurring during that episode (e.g. diagnoses, procedures and treatments received, including, if the admission covers 36 weeks’ postmenstrual age, whether or not the baby is still receiving supplementary oxygen or mechanical ventilatory support). Form 4 (abdominal pathology) is completed for any episode of proven or suspected abdominal pathology including NEC, for which the severity is staged using modified Bell criteria.
On receipt at the PiPS trial office at the NPEU, validation included a series of range, logic and missing data checks to identify inconsistencies within and across forms for the same baby. Some queries were resolved in-house according to predefined protocols; those that could not be resolved were reconciled between the staff in the trial office, the PiPS trial research nurses, the chief investigator and principal investigators with reference to the clinical notes, and documented accordingly. Data were double-entered onto a dedicated trial database.
The NNRD is organised into different files, including static ‘once only’ data, ‘episodic’ data for each admission to a different neonatal unit, ‘daily data’ recorded on a daily basis, and ‘if and only’ data recorded only if applicable (e.g. ad hoc abdominal X-ray forms, blood cultures).
Episode numbering and matching
Episode number 1 on the PiPS database is the admission to the neonatal unit where the infant was recruited to the trial [Form 1 (entry)] and may not be where the infant was born. In contrast, the first episode on the NNRD is always at the hospital of birth (i.e. episode 1 for PiPS may be episode 2 for the NNRD if a baby was transferred from the hospital of birth to a second hospital where PiPS recruitment took place).
It emerged that during processes of addressing missing data and queries, some PiPS episodes had been renumbered and did not appear on the database sequentially. An additional problem arose because data for PiPS were recorded for all episodes, including those spent on paediatric wards and in specialist surgical or cardiac centres that do not use the neonatal EPRs and, thus, these episodes were not present in the NNRD. Consequently, it was necessary to check the dates, anonymised patient ID and hospital name of each episode on each database and renumber, when necessary, to ensure that comparisons were indeed for the same episode. This was further complicated because of inconsistencies of the names of hospitals and NHS trusts entered as free text on the PiPS database. By contrast, the names of hospitals and NHS trusts are standardised in the EPRs through the use of drop-down menus.
Sources of items within the databases
Although many of the data points recorded for the PiPS trial are also present in the NNRD, there are differences in how some data were obtained. In general, data for PiPS were obtained by asking a direct question (e.g. ‘during this episode of care did the infant have surgical ligation for a PDA?’); by contrast, information on whether or not an infant has surgery for a PDA can be entered into the NNRD by a range of routes including variables in the ‘daily data’, ‘discharge diagnoses’ and ‘procedures’ EPR fields.
Preparation of data sets for linkage
We compared data items only for episodes present in both the PiPS and the NNRD databases. We excluded episodes that could not be linked.
Step 1: linking infants to National Neonatal Research Database by matching electronic patient record ID (‘Badger ID’)
We carried out linkage using the unique Badger ID as identifier. This is held as an identifier within the NNRD and is generated at EPR level. The NPEU provided to Clevermed Ltd the NHS numbers and date of birth of all PiPS recruits, requesting the Badger ID. The PiPS data were then anonymised and provided to the NDAU, identified by the Badger ID only.
Step 2: linking episodic data
We linked individual PiPS episodes of care, after any necessary renumbering as described above, to episodes on the NNRD, using the Badger ID and episode number.
Step 3: linking daily data
The NNRD does not hold any dates. The time of events is indicated by a variable ‘minutes from birth’. We used this variable to link data describing feeding, antibiotic and antacid use in the first 14 days.
Methods of comparison
Infant and maternal baseline characteristics: infant-level comparison
These data from the PiPS database were recorded from episode 1 when the infant was recruited into the PiPS study. Data from the corresponding episode at the same neonatal unit on the NNRD were extracted for comparison of all baseline characteristics.
Processes of care and interventions: episodic and infant level comparisons
The majority of processes of care and interventions were compared on an episodic level. Exceptions were as follows: first, the interventions specifically recorded for PDA and ROP that may be entered into multiple EPR fields and, hence, are available in multiple locations in the NNRD and are often carried over across episodes of care to provide the full medical history, and, second, the details of enteral feeds, antibiotics and antacids in the first 14 postnatal days, which are available across episodes on both databases regardless of transfer status, were all compared at an infant level.
To be confident of the accuracy of data for those infants included in the analysis of ‘first day of milk feed’ and ‘type(s) of milk received on first day of feeding’, we included only those that had complete daily data on the NNRD for all days prior to the first feed (i.e. this analysis differs from others in that the PiPS data are linked to selected eligible infants on the NNRD rather than linking NNRD to PiPS data). For the summary variable ‘all types of milk received during first 14 postnatal days’ we included all infants with linkable days on both databases on which detailed daily feeding data (maternal, donor breast or formula milk) were available.
Statistical methods for assessing agreement
We assessed items that were identical, and items with minor and major discordance, using predefined criteria based on clinical judgement and set a priori by KC and CB to mitigate bias.
We calculated for each variable of interest the proportion of babies for whom the NNRD and PiPS trial data differed and the 95% CIs for the proportion. For variables with discordance rates of < 5%, we used the Poisson approximation to the binomial to calculate CIs; otherwise we used the Agresti and Coull method145 for binomial CIs, as this method has better coverage properties. 146 As observations from the same hospital are not independent, we calculated the CIs for discordance using generalised linear models with variances estimated to allow for within-hospital correlation. 147 To test whether or not the discordance rate had changed over the course of recruitment, we calculated discordance rates for sequential quarters for five key variables (i.e. antenatal steroids, mode of delivery, day of first milk feed, type of first milk and central line days) and tested a time trend using linear regression with weights, to allow for a varying number of observations at each time point and adjusting for clustering by hospital. We assessed autocorrelation using residual plots and the Breusch–Godfrey test and accounted for this using the Prais–Winsten procedure, if necessary. To investigate whether or not discordance varied by recruitment site, we calculated discordance rates separately for each hospital for five key variables (i.e. antenatal steroids, mode of delivery, birthweight, EDD and central line days).
To check case ascertainment for binary clinical outcomes, we calculated sensitivity and specificity, treating PiPS data as the gold standard. For continuous variables, we calculated mean and median differences and 95% limits of agreement for the differences.
Sensitivity, specificity and positive predictive values
For binary outcome variables, we calculated the sensitivity, specificity and PPVs of NNRD data in comparison with PiPS data.
In the context of this data comparison, we have taken the prevalence of an outcome as the proportion of infants on the PiPS database with that outcome reported. The following definitions have been used:
-
Sensitivity is the ability of the NNRD database to correctly classify an individual as ‘diseased’ as indicated by the gold standard PiPS database.
-
Specificity is the ability of the NNRD database to correctly classify an individual as disease free as indicated on the PiPS database.
-
Positive predictive value is the percentage of individuals who are identified on the NNRD as having the disease who actually do have the disease as indicated on PiPS database.
Sensitivity and specificity are characteristics of the test and are, in contrast to the PPV, unaffected by the prevalence of the outcome.
Regulatory issues
The establishment of the NNRD and the PiPS trial had each been approved by a Research Ethics Committee (REC) (10/H0803/151 and 09/H0604/30 respectively; patient information leaflet is provided in Appendix 5). Advice was sought from the REC chairperson regarding whether or not an additional application either as a stand-alone project or as an amendment to the existing approval for the PiPS trial was required before undertaking this analysis. We were advised that no such application was necessary.
A data-sharing agreement was then put in place between the NPEU, University of Oxford, where the PiPS trial data are held, and Imperial College London for the transfer of PiPS trial data. These data were stripped of identifiers, other than the Badger ID, and were sent to the NDAU. Database merging and all subsequent analyses took place at the NDAU.
Results
Linkage
A total of 1315 babies were recruited into the PiPS trial; the parents of five babies withdrew consent including consent for the use of any data and, therefore, data for 1310 babies were available for analysis. Clevermed Ltd was able to provide Badger ID for 1280 (98%) infants [no EPR data could be identified for 30 (2%) recruits into the PiPS trial]. This resulted in data for 2360 episodes of care being available on the PiPS database (Figure 15).
FIGURE 15.
Records from PiPS CRF and the NNRD.

Of the second episodes on the NNRD, 81 were the first episode on PiPS because the baby was recruited at a different hospital from that of birth. There were 103 episodes on PiPS from 22 infants who could not be reliably matched on the NNRD, because they were in a ward or a hospital that was not entering data onto BadgerNet, because of duplicate reports of the same episode on the NNRD or, in the case of two infants, because they could not be found in the NNRD. All data for these 22 infants were excluded from the analyses, leaving 2257 episodes of care from 1258 infants eligible for comparison (see Figure 15).
Infant and maternal characteristics
We compared baseline infant and maternal baseline characteristics for 1258 infants. The numbers of infants with missing data for each variable in both databases are reported along with the minor and major discordances calculated using the predefined criteria (Table 14). Gestational age on the PiPS database is calculated from the EDD, whereas gestational age in weeks and days is recorded directly on the EPR and, hence, the NNRD.
| Baseline variable | Number of comparable infants | Missing data, n (%) | Discordancea | ||||
|---|---|---|---|---|---|---|---|
| Any | Major | ||||||
| PiPS | NNRD | Rate (%) | 95% CI (%) | Rate (%) | 95% CI (%) | ||
| EDD | 1142 | 0 | 116 (9.2) | 9.9 | 8.3 to 11.8 | 4.1 | 3.0 to 5.5 |
| Gestational age | 1142 | 0 | 1 | 20.4 | 18.2 to 22.7 | 3.0 | 2.1 to 4.1 |
| Month of birth | 1257 | 0 | 1 (0.08) | 0 | 0 | ||
| Year of birth | 1257 | 0 | 1 (0.08) | 0 | 0 | ||
| Birthweight | 1257 | 0 | 1 (0.08) | 1.7 | 1.0 to 2.6 | 0.9 | 0.4 to 1.6 |
| Sex | 1256 | 0 | 2 (0.16) | 0.2 | 0.02 to 0.6 | ||
| Apgar score at 5 minutes | 1192 | 33 (2.62) | 63 (5.01) | 2.6 | 1.8 to 3.7 | 0.8 | 0.4 to 1.5 |
| Born in this hospital | 1257 | 1 (0.08) | 0 | 1.5 | 0.9 to 2.4 | ||
| Singleton or multiple | 1257 | 0 | 1 (0.08) | 1.1 | 0.6 to 1.9 | ||
| Birth order | 1257 | 0 | 1 (0.08) | 0.6 | 0.3 to 1.3 | ||
| Maternal year of birth | 1255 | 0 | 3 (0.24) | 1.4 | 0.9 to 2.3 | ||
| Maternal ethnicity (NHS categories) | 1185 | 10 (0.79) | 64 (5.09) | 10.2 | 8.6 to 12.1 | ||
| Maternal LSOA | 1090 | 24 (1.91) | 151 (12.08) | 16.5 | 14.4 to 18.8 | ||
| Any antenatal steroids given | 1243 | 9 (0.7) | 6 (0.58) | 2.4 | 1.76 to 3.4 | ||
| Caesarean or vaginal delivery | 1201 | 1 (0.08) | 56 (4.5) | 8.7 | 7.2 to 10.4 | ||
| Instrumental delivery | 1248 | 9 (0.72) | 1 (0.08) | 1.1 | 0.6 to 1.9 | ||
Missing data on the PiPS database were few (< 3%): EDD was missing for 9.2% of infants in the NNRD, LSOA for 12.1% and maternal ethnicity for 5.1%. Rates of ‘any discordance’, defined as ±3 days, were 9.9% and 20.4% for EDD and gestational age, respectively. Major discordance rates were < 10% for all variables except for maternal ethnicity (10.2%) and maternal LSOA (16.5%).
Processes
We were able to compare 2257 linked episodes for 1258 infants (Table 15) using the predefined criteria. Major discordances, defined as ± 5 days, were 10.2% and 11.2% for the duration of high-dependency care and central venous lines, respectively. Discordance for medical treatment of a PDA was 6.0%. For all other variables, discordancies were < 5%.
| Variable | Number of comparable records | Discordance | |||
|---|---|---|---|---|---|
| Any | Major | ||||
| Rate (%) | 95% CI (%) | Rate (%) | 95% CI (%) | ||
| Comparison by episode | |||||
| Intensive care days | 2257 | 8.5 | 7.4 to 9.7 | 3.9 | 3.1 to 4.8 |
| High-dependency care days | 2257 | 14.2 | 12.8 to 15.7 | 10.2 | 9.0 to 11.5 |
| Central venous line | 2257 | 20.5 | 18.9 to 22.2 | 11.2 | 10.0 to 12.6 |
| Length of stay | 2257 | 4.0 | 3.2 to 4.9 | 3.3 | 2.6 to 4.2 |
| Transfer to another hospital | 2257 | 2.2 | 1.6 to 2.9 | ||
| Discharge month | 2257 | 2.3 | 1.8 to 3.1 | ||
| Discharge year | 2257 | 0.5 | 0.3 to 0.9 | ||
| Comparison at infant level | |||||
| Surgery for PDA | 1258 | 1.7 | 1.1,2.6 | ||
| Medical treatment of PDA with ibuprofen or indometacin | 1258 | 6.0 | 4.8 to 7.5 | ||
| ROP treatment by laser or cryotherapy | 1258 | 1.6 | 1.0 to 2.5 | ||
Feeding data
Of the 1258 infants whose records could be matched, 29 on the PiPS database and 35 on the NNRD were reported as having no enteral feeding in the first 14 days. Of the 1223 for whom both databases contained any days with completed details of feeds given, 343 on the NNRD had missing days before the first reported feed and so they could not reliably be included in the analysis of first feeding.
The analysis of the summary of all milk feeds received over the first 14 days includes days when a report was completed confirming that no milk had been given. There were five infants for whom the NNRD contained no reports on any day on whether or not any feed was given; thus, this analysis includes data for 16,203 days from 1253 infants for whom feeding data were complete on both databases.
There was high agreement for day of first milk feed, with 2.8% major discordance (≥ 2 days difference) (Table 16). However, there was high disagreement for the type or types of milk given on first day of milk feed (22.3%) and for the summary of different milks given over the first 14 days (13.8%).
| Variable | Number of comparable records | Discordance | |||
|---|---|---|---|---|---|
| Any | Major | ||||
| Rate (%) | 95% CI (%) | Rate (%) | 95% CI (%) | ||
| First 14 postnatal days | |||||
| Day of first milk feed | 880 | 6.7 | 5.2 to 8.6 | 2.8 | 1.8 to 4.2 |
| Type(s) of first milk feed | 880 | 22.3 | 19.6 to 25.1 | ||
| Summary of all types of milk in first 14 days | 1253 | 13.8 | 12.0 to 15.8 | ||
| Whether or not any antibiotic given in first 14 days | 1258 | 0.6 | 0.2 to 1.1 | ||
| Number of days that antibiotics were given | 1258 | 21.4 | 19.2 to 23.7 | 9.0 | 7.6 to 10.8 |
| Whether or not any antacid was given | 1258 | 5.1 | 4.0 to 6.4 | ||
| Number of days antacid given | 1258 | 6.8 | 5.6 to 8.4 | 4.8 | 3.7 to 6.2 |
Antacids and antibiotics
Antacid and antibiotic administration details are recorded on the EPRs in the ‘daily medications’ field by selecting from a drop-down menu, the completion of which is not essential. Absent data were included in the analysis as indicating that antibiotic and/or antacid was not given. All 1258 infants were considered eligible to be included in this comparison (see Table 16). Although whether or not any antibiotics were given in the first 14 days had high agreement and only 0.6% discordance, the number of days of antibiotic use had a major discordance (> 2 days) of 9.0% and a high ‘any discordance’ rate (±2 days) of 21.4%. Reporting of antacid indicated 5.1% discordance for any use and 9.0% for the number of days given.
Outcomes compared at infant level
Only 877 infants who were still inpatients at 36 weeks’ postmenstrual age were eligible for comparison of oxygen supplementation and ventilatory support at that time; all other outcomes were summarised and compared for all 1258 infants from all linkable episodes of care. Any disagreement was prespecified as ‘major’. Discordance is < 10% for all outcomes except the continued use of oxygen at 36 weeks’ postmenstrual age, which had a discordance rate of 13.3% (Table 17).
| Variable | Number of comparable records | Major discordance | |
|---|---|---|---|
| Rate (%) | 95% CI (%) | ||
| Outcomes | |||
| Worse stage of ROP in any eye | 1258 | 2.0 | 1.3 to 2.9 |
| Whether or not infant was receiving supplementary oxygen at 36 weeks postmenstrual age | 877 | 13.3 | 11.2 to 15.8 |
| Whether or not infant was receiving mechanical respiratory support at 36 weeks postmenstrual age | 877 | 9.2 | 7.4 to 11.3 |
| Any diagnosis of perforated NEC | 1258 | 2.1 | 1.4 to 3.1 |
| Any gastrointestinal perforation | 1258 | 1.7 | 1.1 to 2.6 |
| Any abdominal surgery for NEC | 1258 | 2.8 | 1.9 to 3.9 |
| Haemorrhagic parenchymal infarct | 1258 | 2.7 | 1.9 to 3.8 |
| Hydrocephalus | 1258 | 1.4 | 0.8 to 2.2 |
| Periventricular leucomalacia | 1258 | 1.7 | 1.1 to 2.6 |
| Porencephalic cyst | 1258 | 2.8 | 1.9 to 3.9 |
| Survival to discharge from neonatal care | 1258 | 0.2 | 0.02 to 0.6 |
Sensitivity and specificity
We report sensitivity, specificity and PPVs for NNRD data in Table 18. In the conventional context, the sensitivity would be the probability of a test that correctly identifies an individual with a disease as ‘diseased’; in this context, it is the probability of being ‘NNRD disease positive’ when disease is present. Sensitivity for outcomes other than survival to discharge, which is 100%, ranges between 50% and 87%. There is a 50–87% chance that infants with the disease are ‘NNRD disease positive’. Therefore, there is under-reporting of disease in the NNRD. Specificity was > 85% for all outcomes, with the majority being > 90%. Infants without the disease have a high probability of being ‘NNRD disease negative’, NNRD correctly identifying infants without disease. With the exception of BPD and medical treatment for PDA, which have a prevalence of 49.0% and 20.3% respectively, the prevalence of these adverse outcomes is low, at < 6%.
| Variable | PiPS, n | Prevalence, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | |
|---|---|---|---|---|---|---|
| Positives | Negatives | |||||
| Processes | ||||||
| Surgery for PDA | 60 | 1198 | 4.8 (3.7 to 6.1) | 70.0 (56.8 to 81.2) | 99.4 (98.8 to 99.8) | 85.7 (72.8 to 94.1) |
| Medical treatment of PDA with ibuprofen or indometacin | 256 | 1002 | 20.3 (18.2 to 22.7) | 71.9 (65.9 to 77.3) | 99.5 (98.8 to 99.8) | 97.4 (93.9 to 99.1) |
| ROP treatment by laser or cryotherapy | 41 | 1217 | 3.3 (2.3 to –4.4) | 85.4 (70.8 to 94.4) | 98.8 (98.1 to 99.4) | 71.4 (56.7 to 83.4) |
| Outcomes | ||||||
| Whether or not infant required supplementary oxygen at 36 weeks’ postmenstrual age | 430 | 447 | 49.0 (45.7 to 52.4) | 86.7 (83.2 to 89.8) | 86.6 (83.1 to 89.6) | 86.1 (82.5 to 89.3) |
| Whether or not infant required mechanical respiratory support at 36 weeks’ postmenstrual age | 214 | 663 | 24.4 (21.6 to 27.4) | 90.7 (85.9 to 942.2) | 90.8 (88.3 to 92.9) | 76.1 (70.4 to 81.2) |
| Any diagnosis of perforated NEC | 43 | 1215 | 3.4 (2.5 to 4.6) | 76.7 (61.4 to 88.2) | 98.6 (97.8 to 99.2) | 66.0 (51.2 to 78.8) |
| Any gastrointestinal perforation | 55 | 1203 | 4.4 (3.3 to 5.7) | 83.6 (71.2 to 92.2) | 98.9 (98.2 to 99.4) | 78.0 (65.3 to 87.7) |
| Any abdominal surgery for NEC | 73 | 1185 | 5.8 (4.6 to 7.2) | 67.1 (55.1 to 77.7) | 99.1 (98.3 to 99.5) | 81.7 (69.6 to 90.5) |
| Haemorrhagic parenchymal infarct | 53 | 1205 | 4.2 (3.2 to 5.5) | 69.8 (55.7 to 81.7) | 98.8 (98.0 to 99.3) | 71.2 (56.9 to 82.9) |
| Hydrocephalus | 24 | 1234 | 1.9 (1.2 to 2.8) | 50.0 (29.1 to 70.9) | 99.5 (98.9 to 99.8) | 66.7 (41.0 to 86.7) |
| Porencephalic cyst | 39 | 1219 | 3.1 (2.2 to 4.2) | 51.3 (34.8 to 67.6) | 98.9 (98.1 to 99.4) | 58.8 (40.7 to 75.4) |
| Periventricular leucomalacia | 40 | 1218 | 3.2 (2.3 to 4.3) | 62.5 (45.8 to 77.3) | 99.5 (98.9 to 99.8) | 80.6 (62.5 to 92.5) |
| Survival to discharge from neonatal care | 1159 | 99 | 92.1 (90.5 to 93.6) | 100.0 (99.7 to 100.0) | 97.0 (91.4 to 99.4) | 99.7 (99.2 to 99.9) |
The PPV of all outcomes with the exception of treated ROP (71.4, 95% CI 56.7 to 83.4), perforated NEC (66.0, 95% CI 51.2 to 78.8) and a range of details of cerebral ultrasound scans was > 75.
By hospital analysis
We compared major discordance rates for five variables (i.e. antenatal steroids, mode of delivery, birthweight, EDD and days with a central line) across the 24 PiPS recruiting hospitals (Table 19). The discordance rates of mode of delivery and central line days, which in general have higher major discordance, show striking variation at different hospitals, with mode of delivery varying from 0.0% to 18.5% and central line days from 2.7% to 28.6%.
| Hospital | Key variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antenatal steroids | Mode of delivery | Birthweight | EDD | Central line days | ||||||
| Total comparable records (episodes) | Major discordance, % (95% CI) | Total comparable records (episodes) | Major discordance, % (95% CI) | Total comparable records (episodes) | Major discordance, % (95% CI) | Total comparable records (episodes) | Major discordance, % (95% CI) | Total comparable records (episodes) | Major discordance, % (95% CI) | |
| Barnet | 29 | 3.45 (0.09 to 19.20) | 27 | 7.4 (0.9 to 2.4) | 29 | 0 | 27 | 0 | 65 | 9.2 (4.0 to 19.0) |
| Basildon | 10 | 0 | 10 | 0 | 10 | 10.0 (0.0 to 42.6) | 8 | 12.5 (0.1 to 49.2) | 25 | 28 (14.1 to 47.8) |
| Croydon | 10 | 0 | 11 | 0 | 11 | 0 | 8 | 0 | 17 | 5.9 (0.0 to 28.9) |
| Guy’s & St Thomas’ | 93 | 0 | 90 | 10.0 (5.1 to 18.1) | 94 | 0 | 91 | 1.1 (0.03 to 6.10) | 146 | 13.7 (9.0 to 20.3) |
| Homerton | 248 | 2.4 (0.9 to 5.3) | 237 | 6.3 (3.8 to 10.3) | 249 | 0.4 (0.01 to 2.20) | 236 | 4.7 (2.3 to 8.3) | 309 | 12.3 (9.1 to 16.5) |
| King’s College Hospital | 22 | 4.5 (0.1 to 25.3) | 23 | 8.7 (1.2 to 28.0) | 23 | 4.3 (0.1 to 24.2) | 23 | 4.3 (0.1 to 24.2) | 44 | 18.2 (9.2 to 32.2) |
| Luton and Dunstable | 31 | 0 | 29 | 10.3 (2.8 to 27.2) | 31 | 3.2 (0.08 to 18.00) | 27 | 7.4 (1.0 to 24.5) | 42 | 7.1 (1.8 to 19.7) |
| Medway Maritime | 73 | 2.7 (0.3 to 9.9) | 72 | 13.9 (7.5 to 23.9) | 73 | 0 | 69 | 1.4 (0.04 to 8.10) | 93 | 14.0 (8.2 to 22.6) |
| Newham General Hospital | 59 | 6.8 (2.2 to 16.6) | 57 | 17.5 (9.6 to 29.6) | 59 | 0 | 53 | 9.4 (3.7 to 20.7) | 110 | 7.3 (3.5 to 13.9) |
| North Middlesex | 22 | 4.55 (0.1 to 25.3) | 21 | 4.8 (0.1 to 26.5) | 22 | 0 | 22 | 0 | 69 | 15.9 (9.0 to 26.5) |
| Oxford John Radcliffe | 69 | 1.4 (0.04 to 8.10) | 67 | 4.4 (0.9 to 13.1) | 71 | 0 | 61 | 0 | 74 | 2.7 (0.3 to 9.8) |
| Queen’s Hospital Romford | 56 | 8.9 (3.5 to 19.7) | 53 | 7.5 (2.5 to 18.4) | 56 | 1.8 (0.05 to 10.00) | 52 | 1.9 (0.05 to 10.70) | 80 | 7.5 (3.2 to 15.7) |
| Royal Sussex County | 26 | 0 | 21 | 9.5 (1.4 to 30.1) | 27 | 7.4 (1.0 to 24.5) | 23 | 4.3 (0.1 to 24.2) | 42 | 28.6 (17.1 to 43.7) |
| Southend Hospital | 19 | 0 | 18 | 5.6 (0.0 to 27.6) | 20 | 0 | 18 | 0 | 31 | 3.2 (0.08 to 18.0) |
| St George’s Hospital | 54 | 0 | 52 | 13.5 (6.4 to 25.6) | 56 | 1.8 (0.05 to 1.0) | 52 | 1.9 (0.05 to 10.7) | 67 | 9.0 (3.8 to 18.5) |
| St Peter’s Hospital | 91 | 0 | 90 | 8.9 (4.4 to 16.8) | 91 | 0 | 83 | 4.8 (1.3 to 12.3) | 102 | 10.8 (6.0 to 18.4) |
| The Royal London Hospital | 68 | 2.9 (0.4 to 10.6) | 65 | 4.6 (1.0 to 13.5) | 69 | 1.4 (0.04 to 8.1) | 60 | 1.7 (0.04 to 9.3) | 156 | 10.9 (6.8 to 16.8) |
| Tunbridge Wells Hospital | 32 | 3.1 (0.08 to 17.40) | 32 | 12.5 (4.4 to 28.7) | 32 | 0 | 31 | 6.5 (0.8 to 21.7) | 54 | 3.7 (0.4 to 13.4) |
| University College London | 90 | 4.4 (1.2 to 11.4) | 86 | 11.6 (6.3 to 20.3) | 90 | 0 | 79 | 5.1 (1.6 to 12.7) | 125 | 7.2 (3.7 to 13.3) |
| University Hospital Lewisham | 21 | 0 | 21 | 4.7 (0.1 to 26.5) | 21 | 0 | 20 | 0 | 33 | 6.1 (0.7 to 20.6) |
| Watford General Hospital | 26 | 0 | 27 | 18.5 (7.7 to 37.2) | 27 | 0 | 25 | 16 (5.8 to 35.3) | 43 | 7.0 (1.7 to 19.3) |
| Whipps Cross University Hospital | 28 | 0 | 27 | 0 | 28 | 0 | 25 | 0 | 83 | 4.8 (1.3 to 12.3) |
| Whittington Hospital | 7 | 0 | 5 | 0 | 7 | 0 | 7 | 14.3 (0.5 to 53.3) | 26 | 7.7 (1.0 to 25.3) |
| William Harvey Hospital | 59 | 3.3 (0.4 to 12.2) | 60 | 6.7 (2.2 to 16.4) | 61 | 3.3 (0.4 to 11.8) | 42 | 14.3 (6.3 to 28.2) | 68 | 14.7 (8.0 to 25.2) |
| Total | 1243 | 1201 | 1257 | 1142 | 1904 | |||||
Trends over time
There were no significant changes in discordance rates over time for any of the five selected variables: any discordance in antenatal steroids (–0.5%, 95% CI –1.4% to 0.4%; p = 0.27) or mode of delivery (–0.5%, 95% CI –2.9% to 1.8%; p = 0.64), major discordance in day of first milk (–0.2%, 95% CI –2.0% to 1.5%; p = 0.78), major discordance in type of milk on first day (0.8%, 95% CI –5.2% to 6.8%; p = 0.78) and central line days (–1.2%, 95% CI –6.2% to 3.8%; p = 0.64).
Conclusions
Our study was designed to test whether or not data entered onto the neonatal EPRs, after the processes they undergo before being entered into the NNRD, are of sufficient completeness and accuracy to be used for a ‘gold standard’ assessment of a therapeutic intervention, that is, for a randomised controlled trial. For the majority of data items, discordance was low and was comparable to research data. For some of the rarer outcomes, sensitivity was low but PPV was relatively high, suggesting that infants diagnosed as having disease on the NNRD are correctly identified, but that a high proportion with disease are missed. It is not possible, with the configuration of the electronic data system, to distinguish between missing and discordant data. By comparing like items within NNRD and the database of the PiPS trial involving an investigational medical product performed to standards compliant with ICH-GCP, our study not only addresses the primary research question but also stands as a valuable audit of NNRD quality. For this analysis, and to preserve the integrity of the PiPS trial, the trial data had to be considered to be accurate and, indeed, the extent to which missing data were chased and inconsistencies were queried between trial and local clinical staff far exceeded what would be possible for population-based routine data. However, items such as types of different milk feeds are often poorly recorded and inaccuracies were probably present in both data sources. For some items, such as days of intensive care and days with central lines in place, we had assumed that those completing trial CRF would be likely to refer to the EPRs and that there might be bias in favour of low discordance. In the event, the discordance of these items was relatively high, which possibly suggests that, rather than trust the routine data, researchers extracted the data from the clinical notes.
Much of the complexity of our analysis is not pertinent to the primary question but arose because of the preliminary work involved in linking episodes of care, which was essential in order to be confident that the process and outcome data being compared were for the same baby at the same time. It is important in considering the results of this study not to be side-tracked by these issues and lose sight of the main objective. In general, we found that simple objective baseline items compare well with those recorded for trial purposes.
Our study also identifies areas for improvement. We considered data completeness in the NNRD at three levels: first, whether or not an infant recruited into PiPS appeared on the NNRD; second, whether or not all of the episodes of care reported to PiPS were identified; and, third, whether or not individual clinical items, recorded once, daily or across episodes of care, were identified. For 2% of recruits into the PiPS trial, no EPR data could be identified. Whether this was because of errors in the date of birth and NHS number on either the PiPS database or the NNRD or, which however seems unlikely, because the infants were never entered onto the EPR is unclear, but this certainly needs to be better understood.
Data for a further 103 episodes, including all of the data for 22 babies, were lost because they could not be linked. Episodes were linked by dates of admission and discharge and by hospital name. Possible reasons for failure to link are inaccuracies in these dates, inconsistency in whether or not short stay episodes (e.g. transfer out of a neonatal unit for specialist ophthalmological assessment for a few hours) was considered an inpatient or an outpatient episode, and inconsistencies in the names of hospitals and NHS trusts. The first and second reasons above apply equally to EPR and PiPS entries and overcoming them requires clear rules to be applied, and the third reason could easily be addressed by ensuring that hospital names are standardised for research data.
We found the completeness of baseline data on the NNRD to generally be good, with the exception of maternal ethnicity and LSOA (derived from maternal postcode), 5-minute Apgar score and vaginal/caesarean birth. The last two items are particularly surprising as the variables are important clinically. In time, it is probable that real-time linkage between maternal and infant records will exist so that key items, such as these, feed directly into the infant record. In contrast to the CRF, where process and outcome data are recorded in answer to specific questions, a number of important NNRD data items are acquired by entries into EPR tick-box lists, or opportunistic entries in response to episodic events. As a consequence, if an item is ticked it is likely to be true, but in the absence of a tick it is impossible to know definitely if an intervention was not performed, if a condition was not present or if the item was simply overlooked and the data are genuinely missing. This difficulty could be readily addressed through reformatting of EPR entries.
We found low discordance for most baseline data. An exception, the apparent high discordance for the type of feed given on the first day, probably arises from an unrealistic expectation that we could capture the full extent of variability in patient care practices. With this exception, the other principal reason for the high discordance of some items describing process and outcomes appears to reside in the organisation of data entries within the EPR and the consequent impossibility of distinguishing negative from missing items.
We identified high specificity but with low sensitivity for some important outcomes. A probable explanation for this is that the computer screens completed for the EPR in general lack direct questions about presence and absence of outcomes. Instead, reporting is dependent on the outcome being recorded in one of a number of places, which may include an ‘ad hoc’ form that a busy junior doctor may overlook. The probable result of this is under-reporting of outcomes and uncertainty as to whether outcomes that are not recorded are true negatives or simply missing.
The PPV is influenced by the prevalence of a condition. If prevalence is low, a positive report on the NNRD is less likely to true. The fact that the PPV is generally high, despite low overall prevalence for key outcomes, highlights the potential utility of the NNRD as a large and growing population database. Smaller local or regional databases would be unlikely to have adequate statistical power to detect clinically important signals.
The accurate reporting of BPD in this comparison is problematic in that it is dependent on the correct identification of the date on which the infant reaches 36 weeks’ postmenstrual age. In the PiPS trial, it was agreed a priori that gestational age and all subsequent assessments of age should be based on the EDD entered at birth with no later changes, whereas on the NNRD a baseline gestation is entered independently of EDD and there is the possibility that clinicians might subsequently revise their view around gestation. It was notable that one of the most frequent reasons why the PiPS staff had to query trial data was because the date the clinical staff had taken as 36 weeks’ postmenstrual age was inconsistent with the EDD. As with other areas where differences exist, this could be improved with agreed adoption of standard rules for the determination of these data.
While we were unable to conduct the study as originally planned, providing specific feedback on preliminary data concordance before the final analysis, it was nonetheless disappointing that we were unable to identify any decrease in discordance over time. However, this is at variance with the experience of data required for the National Neonatal Audit Programme, which also utilises data from the NNRD, where a year-on-year improvement has been identified. 148 This may be a consequence of the introduction of regular feedback of missing and potentially erroneous entries, with opportunity for clinical teams to address these and make corrections to the EPR as part of a logged, auditable process.
It is beyond the scope of this study to explore the variation in discordance at different hospitals, and indeed the variation in recruitment rates and the generally low prevalence of adverse outcomes reduce the statistical power of these analyses. However, variation in outcome between hospitals and neonatal clinical networks is an important area of health services research and these data demonstrate the potential utility of NNRD data for this purpose. One area that would be helpful to explore is the possibility that the presence of dedicated staff for data entry and an identified lead for data collection are associated with increased completeness of data and lower discordance between data collected for routine and research purposes.
With the increased adoption of EPRs into clinical practice and the recognition of the importance of extracting the maximum value from the resultant databases, there is increasing interest in their use to support clinical trials. This has included the use of routine data to facilitate the identification of eligible participants149 and the integration into routine systems of specific items needed for the trial data set. 150,151 In neonatal medicine, data repositories established primarily for observational research and/or benchmarking and audit purposes are increasingly used to support both the identification of recruits and trial conduct152,153 and to obtain trial outcomes directly from the database. 154 We are unaware of any previous exploration of the possibility of extracting neonatal trial data from repositories of EPR data.
Implications for health care
Our study indicates that the use of NNRD data derived from the neonatal EPRs offers a good opportunity to facilitate clinical research and to reduce the burdens imposed on clinical teams and investigators by data recording requirements. However, our study also identifies areas that require attention before this potential can be exploited. A further important implication of our study is in revealing deficiencies in neonatal medical records. Not only are these used in day-to-day neonatal patient care, but these data are also used to inform the clinical summary and are the basis of hospital performance reports including quality indicators, benchmarking and national audit. Formal examination of the quality and completeness of NHS data is rarely if ever undertaken. This has potentially grave implications for the reliability of the inferences that can be drawn from interrogation of much NHS data. An important strength of our study is in bringing this issue to attention.
In order to provide complete national coverage, EPR coverage needs to be extended to those few inpatient sites not currently providing data to the BadgerNet platform, principally some neonatal surgical centres and independent (private) hospitals.
Research recommendations
The problems relating to data entry that we describe could be readily addressed, in theory, through redesign and reorganisation of EPR entry screens. The intention would be to ensure that, in so far as possible, entries are made in response to simple objective questions with options to provide unambiguous answers. Therefore, we intend to engage with the commercial supplier of the neonatal EPR data to request incorporation of certain relatively minor and straightforward adaptations that are necessary.
Our study highlights the necessity of implementing systematic examination of NHS data quality and completeness and testing methods to improve these measures. These include the involvement of parents (or patients) in quality assuring their data, formal ‘sign off’ by a senior manager, and incentives [e.g. Commissioning for Quality and Innovation (CQUIN) payments] for achieving predefined data quality standards.
Finally, our study highlights the importance of close clinical involvement in EPR data entry. This issue is considered further in subsequent chapters.
Chapter 5 Two-year neurodevelopmental outcomes of children who were born preterm, assessed using the National Neonatal Research Database
Abstract
Background: Information on the neurodevelopmental outcomes of children who were born very preterm is an important health metric that is required for multiple purposes.
Aims: To assess (1) the agreement between neurodevelopmental outcome information obtained from EPR data held in the NNRD and a gold-standard assessment, (2) the social communication skills of children using a parent-completed questionnaire and (3) the predictive value of early assessments for later cognitive deficits.
Methods: We assessed children at the age of 2 years to a research standard and obtained equivalent information from the NNRD. We invited parents to complete a questionnaire: the Quantitative Checklist of Autism in Toddlers (Q-CHAT). We conducted a systematic review and meta-analysis of early developmental assessment for identifying school-age cognitive deficits.
Results: We completed a formal neurodevelopmental assessment of 190 children; the parents of 141 children completed the Q-CHAT. The neurodevelopmental assessment conducted during NHS follow-up and recorded in the EPRs has low sensitivity but high specificity for identifying children with neurodevelopmental impairment. Very preterm children display greater early childhood social communication difficulties and autistic behaviour than the general population. Early neurodevelopmental assessment has low sensitivity but high specificity for identifying later school-age cognitive deficits.
Conclusions: Neurodevelopmental data in the EPRs underestimate population prevalence of impairment following preterm birth. Very preterm children may benefit from systematic approaches beyond the age of 2 years to identify autistic spectrum disorder (ASD) characteristics and cognitive deficits.
Background
Overview
Around 6000–7000 children who were born very preterm (< 32 weeks’ gestation) are admitted to NHS neonatal units each year. They are at substantial risk of adverse neurodevelopmental outcomes. Severe disability rates of between 5% and 56% are reported155 and long-term studies show that the adverse consequences of preterm birth are still apparent in adolescence and adulthood. 156,157
Information on the later neurodevelopmental outcomes of preterm infants is necessary for several reasons. For an individual child, outcome assessment is needed to ensure that disability, when present, is identified and timely intervention is provided. Professionals require up-to-date outcome information to counsel, advise and support parents. Neonatal unit and population-based outcome data are essential for service planning, benchmarking and evaluation of the impact of neonatal specialised services and their cost. Neurodevelopment is also a common outcome measure in epidemiological, observational and clinical research.
Most neonatal services attempt to provide follow-up assessments up to around 2 years of age. Ceasing systematic assessment at an earlier age would risk confounding by transient neurological dystonia, which mimics cerebral palsy but which improves or resolves completely during the first year. 158 The literature suggests that a reliable early diagnosis of moderate to severe cerebral palsy can be made by 18 months corrected age, and a reliable early diagnosis of mild cerebral palsy can be made by 24 months corrected age. 159 Although assessment tools, such as the Bayley Scales of Infant Development (BSID), provide standardised mental (cognitive) scores from as early as 12 months of age, the correlation of the early mental scores with subsequent IQ at school age is unclear. Two recent cohort studies reported moderate to substantial agreement between BSID, second edition (BSID-II), Mental Development Index (MDI) at the age of 2 years and full-scale IQ at the age of 5 years among infants born before 30 weeks’ gestation or with very low birthweight (VLBW) (i.e. birthweight of < 1500 g). 160,161 Conversely, Hack et al. 162 described a considerable reduction in the proportions of extremely low-birthweight infants (i.e. birthweight of < 1000 g) who were diagnosed with cognitive impairment (defined as standardised cognitive scores < 70), from 39% at 20 months to 16% at 8 years of age, when the children were tested sequentially. Applying the same diagnostic criteria, Roberts et al. 163 also found a reduction in the proportions of very preterm (< 27 weeks’ gestation) and extremely low-birthweight infants with cognitive impairment, from 27.3% at the age of 2 years to 19.3% at the age of 8 years.
All follow-up programmes, whether for clinical or research purposes, incur significant costs related to the employment of trained staff, interim assessments, long-term tracking, data management and analysis, and the need for financial and logistic support to be sustained long term. This is often the main constraint on maintaining follow-up assessments. In the UK, there are currently no nationally agreed, implemented and funded policies for very preterm follow-up.
Types of neurodevelopmental outcome measures
Cerebral palsy is the most commonly quoted outcome in neonatal follow-up studies. It is an umbrella term used to describe a group of non-progressive permanent disorders of movement and posture that occur following damage to the developing fetal or infant brain. It is most commonly described based on the nature of the neurological abnormality (e.g. spastic, dyskinetic or dystonic) and the topography of limb involvement.
Even in the absence of cerebral palsy, preterm infants experience abnormal patterns of motor development and neuromotor dysfunction. 164 Several authors have described the presence of transient dystonia, which may mimic cerebral palsy, in the first year of life in almost one-third of VLBW cohorts. 158,165 A meta-analysis of studies of children who were born very preterm (≤ 32 weeks’ gestation) reported motor scores of between 0.57 and 0.88 SDs behind their term-born peers. 166
The most common disability among preterm children is developmental or cognitive delay. 167,168 Cognitive ability can be described using developmental quotients (DQs) or intelligence quotients (IQs) derived through standardised developmental or intelligence tests. Conventionally, a standardised DQ or IQ > 2 SDs below the population mean is used to define impairment or disability, as it represents the lowest functioning 2.3% of the population. The prevalence of developmental or cognitive impairment exists as a gradient that is inversely related to gestational age. 169 A population-based comparison of school-age children who were born before 28 weeks’ gestation or with a birthweight of < 1000 g with term-born controls revealed a 0.7 SD reduction in IQ points in the preterm children, after adjusting for sociodemographic factors and exclusion of children with neurosensory impairment. 170 In the EPICure 2 study, which followed infants born before 27 weeks’ gestation in 2006 in England, 35% of survivors assessed at the age of 3 years had cognitive scores (predicted MDI) that were > 1 SD below the normative mean. 28
Preterm infants have delays in receptive language processing,171 expressive language acquisition,172,173 articulation and phonological short-term memory. 172,174,175 A meta-analysis of 12 studies published by Barre et al. 176 in 2011 reported that very preterm (< 32 weeks’ gestation) infants perform between 0.38 and 0.77 SDs below their term-born counterparts in areas of expressive and receptive language. A metaregression of six studies for the difference in language scores between very preterm infants and term-born controls against the age at assessment between 3 and 12 years suggested that the deficit in language function deteriorated with increasing age. 177
Published data in the past 20 years estimate that hearing impairment affects between 1.5% and 9% of infants born very preterm, although < 1% had severe bilateral sensorineural hearing loss uncorrectable with hearing aids. 28,167,178–181
Retinopathy of prematurity resulting from disordered retinal vascular development is a major threat for vision loss in preterm infants and high-risk groups receive regular screening ophthalmic screening examinations. 182 In the UK, ROP affects approximately 17% of infants born very preterm and/or VLBW183 and it accounts for around 3% of all childhood vision loss. 184
There is an increased risk of attention deficit hyperactivity disorder (ADHD) and emotional and social disorders, including ASD, among very preterm/VLBW children, compared with the general population. 185–188 Case–control studies have indicated a twofold to threefold increase in the risk of ADHD in very preterm/VLBW infants, compared with term-born controls. 188 The estimated prevalence of ASD has been reported to be 5% in children with a birthweight of < 2000 g189 and 8% in children who were born at < 26 weeks’ gestation. 190 This represents an approximate tenfold increase over the 2–9 per 1000 prevalence estimate in the general population. 191,192
Nine out of 12 case–control studies published in 1980–2001 and included in a meta-analysis reported an increase in internalising behaviour among the very preterm/VLBW cases at ages 5–12 years; 9 out of 11 studies also reported an increase in externalising behaviour. 169 However, in a more recent meta-analysis based on parents’ and teachers’ ratings, the difference in internalising behaviour scores reported between preterm/VLBW cases and full-term controls was small (preterm cases’ scores were < 0.28 SDs below the scores of the term-born controls), and for externalising behaviour the difference was negligible. 193 The EPICure 1 study reported that extremely preterm children were 3.5 times more likely to have anxiety disorders than their term-born classmate controls. 194
Standardised developmental and neuropsychological tests
Overview
Standardised developmental tests are considered the ‘gold-standard’ method for assessing a child’s development. The tests provide an inventory of key developmental milestones and are ‘standardised’ through administration to a large group of children (the normative sample). 195 Standardised scores are age adjusted with a normalised distribution and typically have a mean of 100 and SD of 15. Standardised tests are designed to be administered by qualified examiners who adhere to stringent administration and scoring protocols. From around 1930 to the present day, there has been a continuous and approximately linear increase in the standardised test score. 196,197 Therefore, tests need to be updated and standardised with a contemporary normative sample to remain valid.
There is a range of standardised assessment tools available. A review commissioned by the Department of Health Policy Research Programme to consider tools that can be used as part of the 2- to 2.5-year Healthy Child Programme198 to monitor child development at population level was completed by the Policy Research Unit in the Health of Children, Young People and Families at the University College London Institute of Child Health. The report included a comprehensive analysis of the advantages and disadvantages of 13 different measures identified through a systematic literature search. In neonatal outcome studies, the BSID is the most commonly used.
Bayley Scales of Infant Development
The Bayley Scales of Infant Development, second edition (BSID-II), recognised to be highly reliable and valid, was the developmental test of choice among most major neonatal research studies, including the EPICure studies,167,168 the Victorian Infant Collaborative Study199 and the National Institute of Child Health and Human Development Neonatal Research Network. 200 Despite its popularity, the BSID-II has been criticised for the lack of separate assessments for language and non-verbal skills and for gross and fine motor performance.
The Bayley Scales of Infant and Toddler Development, third edition (Bayley-III),201 standardised on a cohort of 1700 children in the USA in 2004, ameliorated these shortcomings by providing a more comprehensive assessment in separate cognitive, communication and motor domains, with subscale scores in receptive and expressive languages and fine and gross motor skills. However, several studies have raised concerns that, when compared with the BSID-II, the Bayley-III underestimates neurodevelopmental impairment. 202–205 Crucially, one of the key differences in the standardisation procedure between the two editions was the inclusion of ‘clinical cases’ (children with cognitive, physical and behavioural issues) to constitute approximately 10% of the Bayley-III standardisation sample. This was made on the basis that excluding these conditions with higher risk for developmental impairment that are normally present in the general population would falsely inflate the average test scores. However, the effect of these clinical cases in the normative sample appeared to be an increase in discrepancy between BSID-II and Bayley-III scores particularly in the lower functioning range,203,204 leading to an overestimation of ability when the Bayley-III is used in children with suboptimal development. Some studies have developed conversion algorithms or suggested different cut-off scores to determine developmental delay, in order to allow comparison between cohorts. 203,204,206
The Bayley-III Social-Emotional questionnaire was derived from the Greenspan Social-Emotional Growth Chart, which was reported to have a sensitivity of 67.2% and a specificity of 97.8% in identifying children with ASD. 207 The questionnaire is designed to be completed by parents and is structured according to the anticipated acquisition of functional emotional milestones between birth and 42 months of age. It was standardised on the same normative cohort as the Bayley-III and, therefore, produces a composite score with a mean of 100 and SD of 15.
Standard neurological examination
The use of a standard neurological examination in conjunction with a gross motor functional assessment increases the diagnostic accuracy for cerebral palsy. 159,208 The Hammersmith Infant Neurological Examination (HINE) is a simple, quantitative method for assessing children between the ages of 2 and 24 months to assess their cranial function, posture, movement, tone and reflexes, and it yields an optimality score. 209 The optimality score is valid for use in children who were born preterm. 210
Assessment of autistic features
Several authors have studied the use of the Modified Checklist for Autism in Toddlers (M-CHAT) among preterm populations. The M-CHAT has promising test characteristics (sensitivity 87%, specificity 99%, PPV 80%, NPV 99%) when validated in a mixed population of unselected and high-risk children. 211 When applied to the preterm population, high positive screening rates of 25% in VLBW infants212 and 21–41% in infants born before 28 weeks’ gestation213,214 were found. The M-CHAT is poor at differentiating autistic symptoms from neurosensory, cognitive and motor impairments and the specificity of screening for ASD in the preterm population is confounded by the high prevalence of these coexisting morbidities. 213–215 High positive screening rates were also found with other screening tools, such as the Communication and Symbolic Behaviour Scales Developmental Profile Infant-Toddler Checklist,216 the Infant/Toddler Sensory Profile,217 and the Pervasive Developmental Disorders Screening Test, 2nd edition. 218,219 A major revision of the M-CHAT, the Quantitative Checklist for Autism in Toddlers (Q-CHAT), has been published. 220
The Q-CHAT is a parent-completed questionnaire that aims to identify children at risk for autism with a 5-point rating scale (0–4) instead of a binary scoring system for each item. In a preliminary report, Q-CHAT scores from an unselected group of 754 toddlers aged between 17 and 26 months (mean age 21.2 months), living in Cambridgeshire in the UK, followed a near-normal distribution and were significantly lower (more normal) than the scores of children with ASD. 220 The Q-CHAT has not yet been validated as an ASD screening tool.
Classification of neurodevelopmental outcomes
National Perinatal Epidemiology Unit/Oxford classification of functional status at 2 years
In 1993, a working group of experts formed by NPEU and the former Oxford Regional Health Authority developed a standard minimum data set relevant to the measurement of health status in early childhood. 221 This consisted of patient identifiers (NHS numbers of mother and child, and child’s date of birth), sociodemographic measures (postcode, mother’s age at delivery, age last in full-time education and support status at birth), perinatal variables (birthweight, gestation, gender, plurality, hospital of birth, and presence of congenital anomaly) and information on the child’s health and functional status in eight clinical domains at the age of 2 years, based on responses to 11 key questions. This set of 11 key questions became known as the ‘Health Status Questionnaire’ or the ‘NPEU/Oxford criteria for disability’.
In 2007, a working group of the British Association of Perinatal Medicine (BAPM) and the National Neonatal Audit Project based in the Royal College of Paediatrics and Child Health222 specified a data set based on the model of the NPEU/Oxford criteria to allow standardised classification of preterm children at 2 years corrected age into one of three outcome groups: (1) normal, (2) impairment without severe disability (or mild–moderate disability) or (3) severe disability. Moderate agreement between the NPEU/Oxford criteria and other methods of assessing disability had been reported. 223 The NPEU/Oxford classification had been used by several studies in the UK to report 2-year outcomes of preterm children,179,224 most notably the EPICure 1 study. 167,168 Comparing the disability profile of the EPICure 1 cohort at 30 months and at 6 years, the use of the NPEU/Oxford classification at 30 months corrected age had 50% sensitivity and 93% specificity for moderate or severe disability at 6 years of age. 225
Functional classification of cerebral palsy
The Gross Motor Function Classification System (GMFCS) is a method for categorising the gross motor functional abilities of children with cerebral palsy. 226 It is widely used internationally and it has proven to be reliable for classifying children with cerebral palsy to allow comparisons between different studies. 227–229 Classification using GMFCS at the age of 2 years has been found to be stable over time. 229 The Manual Ability Classification System is designed for children between the ages of 4 and 18 years and it provides a description of how children with cerebral palsy use their hands to handle objects in daily activities. 230
Neonatal follow-up programmes in the UK
Neonatal follow-up programmes are not universal in the UK. Some neonatal networks have set up regional projects,231 but the cost of setting up and running a follow-up programme, which includes training staff, is considerable. 232 Most neonatal units offer routine clinical follow-up for infants born very preterm, but the proportion that actually receive the assessment is unknown. In addition, the approach to the assessment of neurodevelopment during routine clinical follow-up varies widely. Children may be assessed by a neonatal or community paediatrics consultant, staff grade doctor, associate specialist, trainee doctor, advanced neonatal nurse practitioner or an occupational or developmental therapist.
In the UK, there is an established surveillance programme to monitor the health and development of all children. 198,199 In the 1990s, several studies investigated the extent to which data recorded during routine service delivery can be used to report the outcomes of survivors of neonatal intensive care. 224,233,234 The Trent Neonatal Follow-up Project reported that most of the data required to meet the NPEU/Oxford minimum data set could be extracted from routinely available information systems. 233 However, the quality of the data was variable and there was no standardisation in the interpretation or documentation of clinical assessments. An exercise on data linkage between the neonatal register and the community child health surveillance database produced ‘error-free’ linkage (using the identifiers date of birth, birthweight and gestation) in only 53.9% of children who had received neonatal intensive care. Modi and Carpenter235 reported similar problems when they reviewed the use of district and regional child health database in the North Thames Region to ascertain the 2-year health status of children who were born at < 29 weeks’ gestation. They were able to retrieve child health surveillance records for only 2 out of 80 children surviving to 2 years. When Johnson and King234 used the routine child health information system to compile a list of children with motor or sensory disability, they failed to identify 162 out of 446 (36.3%) children listed on the coexisting population register of cerebral palsy, sensorineural deafness and severe vision loss. Since 1992, several reports have highlighted the need for data collection on the later morbidity of survivors of neonatal intensive care. 236–239 The Audit Commission237 proposed that all neonatal units collect data in a nationally agreed format. The Department of Health and Social Care (DHSC)’s report Changing Childbirth recommended the development of a system of data collection to enable meaningful comparison of perinatal statistics. 239
The British Association of Perinatal Medicine (BAPM) first published standards for hospitals providing neonatal services in 2001, recommending that the later health status of survivors at particular risk of disability should be ascertained up to at least a corrected age of 2 years and standardised guidelines for the definition of disability should be used. 240
Despite these recommendations, routine outcome reporting of health outcomes following preterm births remains largely unavailable. In 2007, the National Audit Office reported that evidence of neonatal outcomes, other than the traditional indicator of mortality rates, was still sparse. 241
The National Institute for Health and Care Excellence (NICE), in 2010, published a list of statements that define high-quality specialist neonatal care. 242 This included evidence of processes to enable collection of health outcome data on babies who receive specialist neonatal care. The NICE guideline for developmental follow-up of preterm infants is also currently being developed and is anticipated to be published in full in 2017. Some neonatal networks have included a target for 2-year assessment of very preterm infants in the CQUIN payment framework to encourage follow-up and data collection.
Parent-completed questionnaires
Parent-completed questionnaires have been developed as a low-cost alternative to developmental tests to identify children with disabilities. The level of agreement between parental perceptions and paediatrician assessments is inconsistent243–245 and may be influenced by parent sociodemographic factors. The validity of the revised Parent Report of Children’s Abilities-Revised (PARCA-R),246,247 the Parent’s Evaluation of Developmental Status (PEDS),248 the Functional Status II (FS-II) questionnaire,249 the Ages and Stages Questionnaire (ASQ)250 and a questionnaire adapted from the Griffiths Developmental Scales251 had been evaluated in the preterm population. In particular, the PARCA-R was found to have good diagnostic utility for moderate to severe cognitive and language impairment when validated against the BSID-II (reported sensitivity 85%; specificity 87%)247 and the Bayley-III (sensitivity 75–94%; specificity 79–89%),252 and had been used for outcome reporting in neonatal studies. 253,254 Although the typical response rates to postal questionnaires were reported to be between 52% and 61%,255 Field et al.,256 when testing parent-completed questionnaires as a source of outcome data at 2 years following neonatal discharge, recorded a 90% response rate by maintaining contact with the families in the form of Christmas and birthday cards.
Electronic patient records
In the UK, most community child health services hold clinical information from child health surveillance programmes on electronic information systems, although these systems vary from one NHS trust to another and the data are not routinely passed back to neonatal units. In the past decade, all neonatal units in the UK have moved towards routinely recording clinical information in an EPR to facilitate shared care within neonatal networks. The BadgerNet platform is most widely used (www.clevermed.com/). In 2007, a standardised format for the recording of 2-year neurodevelopmental and health status, adapted from the NPEU/Oxford classification of disability, was developed by the Thames Regional Perinatal Group Outcomes Group. This was incorporated into the EPR in 2008. Since 2009, the National Neonatal Audit Programme, delivered by the Royal College of Paediatrics and Child Health, has been using data held in the NNRD for audit purposes, including 2-year health status of children who were born at < 30 weeks’ gestation. The programme has promoted outcome data recording, with an increase in the number of participating neonatal units documenting any 2-year outcome data on the eligible infants from 51 out of 170 units (30%) in 2009 to 158 out of 179 units (88%) in 2013.
Aims and objectives
The aim of this workstream was to evaluate the reliability and utility of neurodevelopmental outcome information on children who were born very preterm obtained in the course of NHS follow-up care. Specific objectives were to:
-
compare the agreement between outcome data obtained during NHS follow-up assessments and recorded in the EPR, and outcomes obtained through a formal neurodevelopmental assessment conducted to a research standard
-
characterise the early social communication skills and autistic-like traits in children at the age of 2 years who were born very preterm
-
perform a systematic review of published literature and meta-analysis of the sensitivity and specificity of early developmental assessment in predicting school-age cognitive deficit in children who were born very preterm.
Methods
Approvals and registration
Approval was received from the National Research Ethics Committee (REC 10/H0720/35) and the NHS Research and Development Department of each study site. The study was adopted onto the UK Clinical Research Network Portfolio (ID 8626). The systematic review meta-analysis was registered on PROSPERO, an international prospective register of systematic reviews (CRD42012002168).
Study sites
Study sites were selected to provide representation of infants from a wide range of ethnic and socioeconomic backgrounds as well as include neonatal units where clinicians of different grades and specialties provide follow-up assessments of preterm infants. Study sites were restricted to within Greater London and Cambridge for practical reasons. The lead consultant responsible for post-discharge follow-up at each hospital was invited to be the local collaborator for the study. No hospital declined participation.
Participants
Eligible participants were children who were born at < 30 weeks’ gestation and attended the routine NHS follow-up assessments at participating hospital sites between the corrected ages of 20 and 28 months (age adjusted for prematurity) during the recruitment period (June 2010 to July 2012).
To prevent ‘practice effect’ bias from repeated testing, children who had received Bayley-III assessment, either as part of their routine NHS assessment or owing to enrolment in other research studies, were excluded. Children from non-English-speaking families were also excluded because the Bayley-III neurodevelopmental assessment was developed to be administered in English and parents would not be able to complete the Q-CHAT and Bayley-III Social-Emotional questionnaires independently.
Recruitment
Local collaborators (i.e. clinical consultants) identified eligible participants and approached parents, who were sent the study information sheet and a letter of invitation to participate (see Appendix 5). If they were interested in participating or wished to discuss the study, they were asked to provide their contact details on a pre-printed response form and send it to the researcher in a pre-paid envelope. Alternatively, parents were given study information at the time of their child’s NHS follow-up appointment.
Researcher training
The researcher received training on Bayley-III assessment techniques through attendance at a 2-day training workshop, followed by practice sessions that were supervised by Bayley-III expert trainers. Techniques were accredited through a pilot assessment that was independently scored. A score of 100% agreement on all items on the assessment scales was achieved. To ensure reliability and consistency during the study, validation sessions were attended by an observer, who scored assessments administered by the researcher on non-study participants who were born at < 30 weeks’ gestation and were 20–28 months old (corrected age). The interobserver agreement between scores was evaluated and the researcher received feedback. The researcher also received training in the standardised neurological examination based on the HINE209 from an expert trainer.
The research assessment
At the time of assessing the participant, the researcher was blinded to the results from the NHS assessment. The assessments took place in an outpatient clinic room at the same site as the routine NHS assessments. Each participant was accompanied by one or both parent(s). For the Bayley-III assessment, the participant was seated either at a children’s table or on his/her parent’s lap at the office desk. The test items were administered sitting across the table facing the participant. In the case of twins or triplets, one child was assessed at a time.
Timing of research assessment
The intention was to complete the research assessment within 1 month before or after the participant’s NHS follow-up assessment. To minimise potential information bias caused by changes in development during the interval between the NHS and the research assessments, the intention was to administer the research assessment before the routine assessment in approximately half the cohort and after in the other half.
Assessment of cognition, language and neuromotor development
Participants’ cognitive, language and motor development were assessed using the Bayley-III. 201 Each test item was scored as 1 (pass) or 0 (fail). If the participant refused to respond to any test item, it was scored as ‘failed’. For each scale, the sum of the scores for all items tested between the basal and ceiling levels constitute the participant’s raw score. Two types of norm-referenced scores were obtained: scaled scores, which are standardised to a mean of 10 and SD of 3; and composite scores, which have a mean of 100 and SD of 15. For the cognitive scale, both the scaled score and the composite score were derived from the raw score. The language composite score was derived from the sum of the receptive communication and expressive communication scaled scores. Similarly, the motor composite score was obtained from the sum of the fine motor and gross motor scaled scores. The algorithm developed by Moore et al. 204 was used to convert the Bayley-III cognitive and language scores into a predicted BSID-II MDI, for the purpose of comparing the classification of neurodevelopmental outcomes into categories of severity based on the two scores. The algorithm is:
On the Bayley-III Social-Emotional questionnaire, parents were asked to rate how often their child demonstrated certain behaviours. Scores for each item were allocated according to behaviour frequency as follows: all of the time (5 points), most of the time (4 points), half of the time (3 points), some of the time (2 points), none of the time (1 point) and can’t tell (0 point). A score of 0 (equivalent to ‘can’t tell’) was given to questions with incomplete responses; if more than one response was given, the response with the highest score was used.
Assessment for neurological deficits and cerebral palsy
The tools utilised were the standardised neurological examination, based on the HINE,209 and the ‘extremely low gestational age newborn’ algorithm, as a structured guide to diagnose and classify cerebral palsy into topography-based categories of quadriparesis (at least three-limb involvement), diparesis (involvement of one or both lower limbs) and hemiparesis (involvement of one side of the body). Functional severity of cerebral palsy was classified into five levels based on the GMFCS;226,228,257 social communication abilities were judged using the parent-completed Q-CHAT220 and Bayley-III Social-Emotional201 questionnaires; parents were sent the questionnaires prior to the appointment; the Q-CHAT consisting of 25 items used a 5-point Likert scale (0–4 points) and scores were allocated according to the methods described by the research team that developed it. 220 Questionnaires with more than six incomplete responses were excluded. The scores from all items were summed to obtain a total Q-CHAT score within a possible range of 0–100.
Record of observed behaviour during the research assessment
The Behavioural Observation Inventory included in the standard Bayley-III was used to record behaviour observed during the assessment. Thirteen types of behaviour were noted: positive affect, enthusiasm, exploration, ease of engagement, co-operativeness, appropriate activity level, adaptability to change, alertness, distractibility, appropriate motor tone, tactile defensiveness, fear or anxiety, and negative affect. Numerical scores were assigned for each behaviour: a score of 2 was given if the behaviour was ‘observed most of the time’, a score of 1 was given if it was ‘observed some of the time’ and a score of 0 was given if it was ‘never or rarely observed’. The presence of ‘distractibility’, ‘tactile defensiveness’, ‘fear/anxiety’ and ‘negative affect’ were reverse-scored. Using the same form, the parent(s) or caregiver accompanying the participant was asked to rate how much the child’s behaviour during the assessment was representative of his/her usual conduct. A score of 2 points was given for ‘very typical (child is like this most of the time)’, a score of 1 was given for ‘somewhat typical’ and a score of 0 was given for ‘not at all typical’. Hence, two behavioural rating scores (each with maximum score of 26) were obtained; an examiner rated the behavioural score for the frequency of positive behaviour and a parent rated the score for the typicality of behaviour.
Classification of impairment from the research assessment
Participants were classified into categories of neurodevelopmental status using two methods:
-
SD score groups ‘higher than –1 SD’, ‘–1 to –2 SDs’ and ‘lower than –2 SDs’ based on their Bayley-III scores.
-
‘No’, ‘mild–moderate’ and ‘severe’ impairment groups according to the modified NPEU/Oxford criteria.
Classification of impairment
The Bayley-III composite score was used to assign the SD score group in the cognitive domain. In the language and motor domains, composite scores were derived from combining scaled scores from the receptive and expressive communication subtests and the fine motor and gross motor subtests, respectively. Therefore, if a child had a specific impairment in only one subtest, it is possible for compensation from the other subtest to occur, resulting in a composite score within the normal range. Hence, the scaled score was used to identify specific impairment in the subdomains of receptive communication, expressive communication, fine motor skills and gross motor skills. In the combined language and motor domains, impairment was taken as the worst category of outcome assigned in the respective subdomains and based on the Bayley-III composite scores. The overall outcome of each participant was based on the worst category of impairment from the cognitive, language and motor domains. Participants who received Bayley-III scores of lower than –1 SD were considered to have at least a mild form of impairment and scores of lower than –2 SDs were considered to represent at least moderate to severe impairment.
Impairments were also classified according to the modified NPEU/Oxford criteria.
Outcome data from NHS follow-up assessments
Participants were assessed by their local clinicians as part of their routine NHS post-discharge follow-up. Assessors were blinded to the results of the research assessment. Results were entered into the electronic ‘2-year outcome’ form on the BadgerNet EPR as required for the National Neonatal Audit Programme. The specific questions for the development (cognitive), communication and motor domains were as shown in Box 1.
D1: Is the child’s development between 3 and 6 months behind corrected age?
D2: Is the child’s development between 6 and 12 months behind corrected age?
D3: Is the child’s development > 12 months behind corrected age?
Receptive communicationRC1: Does this child have difficulty with understanding outside of familiar context?
RC2: Is this child unable to understand words or signs?
Expressive communicationEC1: Does this child have any difficulty with communication?
EC2: Does this child have difficulty with speech (< 10 words/signs)?
EC3: Does the child have fewer than five meaningful words, vocalisation or signs?
Fine motorFM1: Does this child have any difficulty with the use of one hand?
FM2: Does this child have difficulty with the use of both hands?
FM3: Is this child unable to use hands (i.e. to feed)?
Gross motorGM1: Does this child have any difficulty walking?
GM2: Is this child’s gait non-fluent or abnormal reducing mobility?
GM3: Is this child unable to walk without assistance?
GM4: Is this child unstable or needs to be supported when sitting?
GM5: Is this child unable to sit?
A positive response to any of the questions implied the presence of impairment. Questions D3, RC2, EC3, FM3, GM3 and GM5 denote the criteria for severe impairment. Additional information on whether or not the child was diagnosed with cerebral palsy, whether or not a standardised neurodevelopment test was used during the NHS assessment and whether or not the child was difficult to assess were also entered. The electronic form could be completed by the examining health professional or by administrators, such as secretaries or data entry clerks, based on the information given to them by the examiner.
With parental consent, the participants’ unique identifier on the NNRD (the Badger ID) was obtained from the local collaborator at each study site. Neonatal and 2-year outcome data were then obtained from the NNRD with assistance from the NDAU data managers.
Classification of disability based on National Neonatal Research Database data
Participants were classified into categories of ‘no’, ‘mild–moderate’ and ‘severe’ impairment within each outcome domain (i.e. cognitive, receptive communication, expressive communication, fine motor and gross motor) using the electronic 2-year outcome data and according to the algorithm outlined in Figure 16. A missing response did not count as a ‘no’; therefore, complete data entry is required to assign participants as having no impairment. An overall level of impairment was defined based on the worst outcome from the five domains.
FIGURE 16.
Algorithm for the classification of impairment using data from NHS assessments.
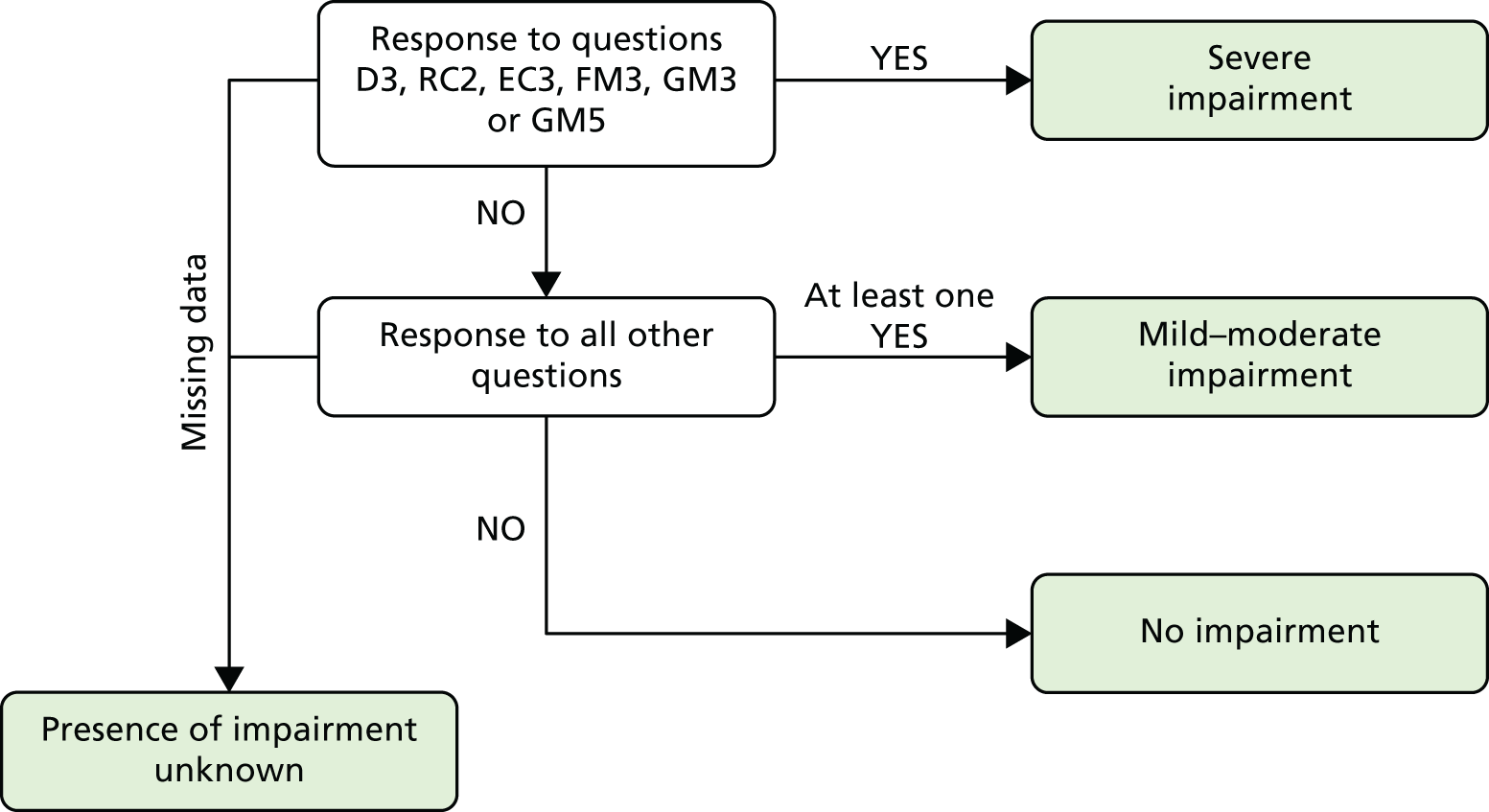
In addition, for the purpose of assessing selection bias, the following data were extracted from the NNRD for all infants born between 1 January 2008 and 31 December 2010 at gestational ages of < 30 weeks and discharged from the participating study sites (the ‘baseline population’): gestation at birth, birthweight, sex, ethnicity, singleton or multiple pregnancy, mode of delivery, days of mechanical ventilation, oxygen therapy at 36 weeks’ corrected gestational age, maternal age and the IMD based on maternal residence at the time of birth. The IMD is a summary measure of relative area deprivation, calculated through a weighted combination of scores from 38 different indicators covering factors, such as income, employment, education, health, living environment and crime, for each area in England, using national census data. The IMD was obtained based on the postcode of the mother at the time of birth of her child and according to the English Indices of Deprivation 2010. 258
Statistical tests
Data were coded for analysis using Microsoft Excel® 2007 (Microsoft Corporation, Redmond, WA, USA). Data were double-entered, examined and outliers were verified. All analyses were performed using Stata statistical package, version 11.0.
Quantitative variables are presented as means and SDs for normally distributed data, or medians and IQRs when the distribution was skewed. Qualitative variables are presented as numbers of subjects and percentages. Differences between categorical variables were analysed using Pearson’s chi-squared test. For continuous variables, Student’s t-test was used for parametric comparison and the Mann–Whitney U-test was used for non-parametric comparison. The p-values derived from statistical tests are presented and the conventional 5% level is used to define statistical significance. Several key statistical measures used in the analyses are described below.
The validity of an assessment, in the context of this research, refers to the ability of the assessment to differentiate accurately between children with and without neurodevelopmental impairment, as defined. It is described using sensitivity and specificity, which are derived through a 2 × 2 table (Table 20).
| Assessment under evaluation | Reference ‘gold standard’ assessment | |
|---|---|---|
| Children with impairment | Children without impairment | |
| Tested positive for impairment | True positives | False positives |
| Tested negative for impairment | False negatives | True negatives |
Sensitivity is the proportion of children with impairment who were accurately identified as having impairment from the assessment under evaluation. It is calculated as:
Specificity is the proportion of children without impairment who were accurately identified by the assessment under evaluation and is calculated with the formula:
Sensitivity and specificity calculations are expressed as either proportions or percentages with corresponding 95% CIs. Values of < 0.7 (or 70%) were interpreted as low, values of 0.7 to 0.85 (70% to 85%) were interpreted as moderate and values of > 0.85 (> 85%) were interpreted as high. 259
Cohen’s kappa statistic was used to compare the agreement in classifying neurodevelopmental outcomes into the three categories of ‘no’, ‘mild–moderate’ and ‘severe’ impairment (ordinal data). The κ coefficient is a measure of the proportion of agreement above that is due to chance alone and is calculated by:
A κ value of 1 indicates perfect agreement and a value of 0 reflects agreement that is no better than by chance. Unweighted and weighted forms of κ coefficient were obtained. The purpose of the weighting was to derive a coefficient that provided a closer reflection of the clinical implications of disagreement between the ordinal categories. It is clinically more important to distinguish patients with impairments from those without impairment than to differentiate between the severity of ‘mild–moderate’ and ‘severe’ impairments. Hence, in the calculations for the weighted κ coefficient, discrepancy between ‘mild–moderate’ and ‘severe’ impairments was considered to be partial agreement.
The weighting matrix used is shown in Table 21.
| Level of impairment based on assessment Ia | Level of impairment based on assessment Ib | ||
|---|---|---|---|
| None | Mild–moderate | Severe | |
| None | 1 | 0 | 0 |
| Mild–moderate | 0 | 1 | 0.5 |
| Severe | 0 | 0.5 | 1 |
The κ values were interpreted according to the standards proposed by Landis and Koch: 0–0.4, slight to fair agreement; 0.4–0.6, moderate agreement; and 0.6–1, substantial to perfect agreement. 260
Sample size
A precision analysis for the estimated sensitivity of the NHS assessment in identifying children with Bayley-III scores of < –2 SDs in study 1 was used to calculate the target sample size for recruitment. The desired sensitivity of a developmental test is conventionally between 70% and 80%. The precisions (widths of CI) of the observed sensitivity and specificity of a test vary depending on sample size and the observed estimates. The aim was to achieve a precision of 95% CI half-width within 10% for the estimated sensitivity of identifying children with Bayley-III scores of < –2 SDs by the NHS assessment.
Based on the London Perinatal Networks 2008 Annual Report,261 it was estimated that approximately 500 children are born at < 30 weeks’ gestation and survive to discharge from the participating hospitals per year. Assuming that 10% of these children have Bayley-III scores of < –2 SDs, with an unstratified random sample, 650 participants would be required to achieve a CI half-width within 10% for an estimated sensitivity of 80%. We attempted to recruit a stratified sample to include higher proportions of children with medium and high risk for impairment to improve the precision of the study while maintaining a practical sample size262 (Table 22).
| Size of strata | Sample size | Estimated proportion with Bayley-III scores of < –2 SDs | Estimated sensitivity (%) | 95% CI half width (%) | |
|---|---|---|---|---|---|
| Unstratified sample | 650 | 10% | 80 | 9.7 | |
| Unstratified sample | 650 | 10% | 50 | 12.2 | |
| Higher risk | 200 | 500 | 25% | 80 | 8.9 |
| Medium risk | 200 | 15% | |||
| Lower risk | 100 | 0% | |||
| Higher risk | 200 | 500 | 25% | 50 | 11.2 |
| Medium risk | 200 | 15% | |||
| Lower risk | 100 | 0% | |||
| Higher risk | 100 | 300 | 25% | 80 | 11.4 |
| Medium risk | 150 | 15% | |||
| Lower risk | 50 | 0% | |||
| Higher risk | 100 | 300 | 25% | 50 | 14.2 |
| Medium risk | 150 | 15% | |||
| Lower risk | 50 | 0% | |||
Of the infants born at < 30 weeks’ gestation who survived to discharge in London in 2008, 20% were born at ≤ 25 weeks’ gestation (higher-risk group), 30% were born at 26 to 27 weeks’ gestation (medium-risk group) and 50% were born at 28 to 29 weeks’ gestation (lower-risk group). Assuming that 25% of higher-risk, 15% of medium-risk and 0% of lower-risk children achieve Bayley-III scores of < –2 SDs, and the sensitivity of identifying different severity of impairment is the same for all risk groups, Table 22 shows various sample size options and the resulting CI half-width for different sensitivity estimates. We aimed to recruit 500 children (i.e. 200 from the higher-risk group, 200 from the medium-risk group and 100 from the lower-risk group) over the 2-year recruitment period.
Representativeness of the study population
Neonatal and sociodemographic characteristics of study participants and non-participants were compared using data extracted from the NNRD.
Comparing classification of impairments
To estimate how comparable the three levels of impairment (i.e. none, mild–moderate and severe) based on the modified NPEU/Oxford criteria are to the three Bayley-III SD score groups of ‘> –1 SD’, ‘–1 to –2 SDs’ and ‘< –2 SDs’, using only the data obtained from the research assessment, the two sets of criteria were cross-tabulated and the unweighted and weighted Cohen’s κ coefficients were calculated. This was also performed for each neurodevelopmental domain.
Any participant with Bayley-III scores of < –1 SD was considered to have at least mild impairment. Taking the research assessment to be the reference ‘gold standard’, the sensitivity and specificity of the NHS data in identifying children with any impairment were calculated and the sensitivity and specificity of the severe impairment category in the NHS data for identifying children with Bayley-III scores of < –2 SDs were calculated.
To account for correlation clustering by study sites, robust standard errors were used to calculate the 95% CI for the estimated sensitivities and specificities. To examine the effect of correlated outcomes within multiple birth sets, analyses were repeated on all singleton births and one randomly selected child from each multiple birth set.
Weighted and unweighted κ coefficients were used to measure the concordance between the research and the NHS assessments, again matching the ‘no impairment’ category to Bayley-III scores of higher than –1 SD, ‘mild–moderate’ to Bayley-III scores of between –1 and –2 SDs and ‘severe’ to Bayley-III scores of lower than –2 SDs.
Defining question sets for identifying severe impairment
The NPEU/Oxford expert group suggested that a criterion of –3 SD scores be used to represent ‘severe cognitive (developmental) disability’ at the age of 2 years. 221 However, this cut-off point was not feasible because of the floor effect of the Bayley-III cognitive composite scores, which ranged between 55 and 145. A post hoc analysis was therefore performed to evaluate if applying broader criteria at the severe end of the impairment spectrum would improve the validity of NHS data in identifying children with Bayley-III scores of lower than –2 SDs. For this, impairment categories were re-defined as ‘none’, ‘mild’ and ‘moderate–severe’. Referring back to the ‘2-year’ questions on the EPR, participants who received a positive response to the following questions were recategorised into the ‘moderate–severe’ category (Table 23).
| Category | Question |
|---|---|
| D2 | Is this child’s development between 6 and 12 months behind corrected age? |
| RC1 | Does this child have difficulty with understanding outside of familiar context? |
| EC2 | Does this child have difficulty with speech (< 10 words/signs)? |
| FM2 | Does this child have difficulty with the use of both hands? |
| GM2 | Is this child’s gait non-fluent or abnormal, reducing mobility? |
| GM4 | Is this child unstable or needs to be supported when sitting? |
Participants who received a positive response to any of the other questions were classified as having mild impairment. The sensitivity and specificity of the ‘moderate–severe’ category in predicting children with Bayley-III scores of lower than –2 SDs was then calculated and the concordance of NHS data classified into these new categories with the Bayley-III SD score groups, matching ‘no impairment’ to Bayley-III scores of higher than –1 SD, ‘mild impairment’ to Bayley-III scores of between –1 to –2 SDs and ‘moderate–severe’ impairment to Bayley-III scores of lower than –2 SDs.
Variables associated with the validity of NHS neurodevelopmental data
The effect of the following factors on the validity of the NHS data were examined: gestation at birth, sex, supplemental oxygen requirement at 36 weeks corrected gestational age, IMD quintile at the time of assessment, English as the only language spoken at home, corrected age at NHS assessment, use of a standardised neurodevelopmental test or screening test during NHS follow-up, grade of NHS assessor, time interval between NHS and research appointments, behaviour during the research assessments as measured by the examiner-rated behavioural score, and whether or not the NHS assessor thought that the child was difficult to test during the NHS assessment. Cross-tabulations and the calculation of the sensitivities and specificities of NHS assessment, stratified by the factor under study, were performed for each domain of neurodevelopment.
Assessment of social communication and autistic traits in early childhood
For the purpose of assessing the applicability of the Q-CHAT for children who were born preterm, children with cerebral palsy and children with severe neurosensory impairments (defined as a hearing deficit not correctable with hearing aids or a visual deficit not correctable with glasses) were excluded from this analysis. Differences in characteristics between respondents and non-respondents, and between respondents and the ‘baseline population’, were compared to evaluate selection bias.
The overall and sex-specific Q-CHAT scores from the study population were compared with published scores from the general population [general population overall mean 26.7 (SD 7.8), mean for boys 27.5 (SD 7.8), mean for girls 25.8 (SD 7.7)]220 using the Student’s t-test. Differences in the distributions of item-specific scores between the study cohort and the general population in each category of autistic-like behaviour were examined by chi-squared tests. To overcome the chi-squared test restriction for low numbers, the proportions in adjacent score categories were combined to ensure that all expected values were larger than 5. 263
The correlation between the Q-CHAT scores and the Bayley-III cognitive, language and motor composite scores was explored using linear regression to determine if any observed differences in Q-CHAT scores between the study population and the general population were explained by delayed neurodevelopment in the preterm population. Post hoc analysis of the correlation between subcategorical Q-CHAT scores (total score from items within each category of autistic-like behaviour) and Bayley-III cognitive, language and motor composite scores was carried out with Bonferroni correction for multiple testing.
The following neonatal and sociodemographic factors were analysed for possible association with Q-CHAT scores: gestation at birth, birthweight z-score, sex, single versus multiple pregnancy, white versus non-white ethnicity, maternal age at birth, mode of delivery, length of mechanical ventilation, supplemental oxygen requirement at 36 weeks’ corrected gestational age and IMD quintile at the time of completion of the Q-CHAT. The current IMD quintile for participant was chosen rather than the birth quintile. Comparing the IMD quintiles at birth with those at the time of assessment, 177 (83.9%) participants continued to live within the same IMD quintile, 13 (9.2%) moved to a more deprived IMD quintile and 15 (10.6%) moved to a less deprived quintile. Linear regression models were created to determine the association between predictive variables and Q-CHAT scores. To account for correlated outcomes within multiple birth sets, cluster bootstrap analysis was used to estimate standard errors and the resultant 95% CI. Variables identified to be significant at a 5% level in univariable models were included in forward stepwise multivariable regression analyses to determine the independent effect of each factor on Q-CHAT scores. Post hoc analysis was conducted to explore possible interactions between ethnicity, Bayley-III language scores and IMD.
Using an arbitrary cut-off score of 2 SDs above the general population mean for Q-CHAT scores and 2 SDs below the standardised mean for Bayley-III Social-Emotional scores, participants were classified as ‘at risk for ASD’. A scatterplot was used to examine the relationship in score distribution between the Q-CHAT and Bayley-III Social-Emotional questionnaires and the agreement between the questionnaires in identifying children ‘at risk’ was measured using Cohen’s κ statistic.
Systematic literature review and meta-analysis
A systematic electronic literature search was conducted on MEDLINE for information on the early developmental outcomes and corresponding school-age cognitive outcomes of preterm children. The methods adopted in this review were based on recommendations outlined in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 264,265 Results are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. 265
Any cohort or matched-control studies published since 1 January 1990 on study populations of infants born ≤ 32 weeks’ gestation and/or who had a birthweight of < 1500 g (VLBW), in which at least two serial assessments, consisting of a neurodevelopmental assessment conducted between 1 and 3 years of age and a cognitive assessment at ≥ 5 years of age, were conducted and reported using validated standardised psychometric assessments [e.g. BSID, Griffiths Mental Development Scales (GMDS), Wechsler Preschool and Primary Scale of Intelligence] were considered for inclusion in the review. Assessments conducted before 1 year of age were not included because impairment, particularly if mild, may not be evident at this stage. Studies with populations that did not meet the gestation or birthweight criteria or reported outcomes using non-standardised assessments including measures of academic attainment were excluded. Studies that reported only outcomes in language or executive function (e.g. memory) were excluded as they would not reflect the overall cognitive function of the study populations. Case reports, narrative reviews, editorials, letters and comments on published articles were excluded.
The electronic search was conducted on MEDLINE through the PubMed interface on 13 April 2012, covering English-language literature published between 1 January 1990 and 31 March 2012. Search terms were selected a priori through a preliminary review of the literature. The following search terms were used both as keywords and as subject headings: (combinations of ‘preterm’ or ‘premature’ with ‘infant’ or ‘neonate’ or ‘children’) or (‘low birthweight’ or ‘extremely low birthweight’) and (‘cogniti*’ or ‘neurodevelopment*’ or ‘mental retardation’ or ‘disability’ or ‘intelligence’ or ‘IQ’). The ‘explode’ feature was used with subject headings to include articles categorised under more specific subheadings. The detailed search strategy was as follows:
((‘preterm children’[tiab] OR ‘premature children’[tiab]) OR (‘premature infant’[tiab] OR (‘preterm infant’[tiab]) OR (‘preterm neonate’[tiab] OR ‘premature neonate’[tiab]) OR (‘Infant, Premature’[MeSH]) OR (‘Infant, Very Low Birthweight’[MeSH]) OR (‘very low birthweight’[tiab] OR ‘very low birthweight’[tiab]) OR (‘extremely low birthweight’[tiab]) OR (‘extremely low birthweight’[tiab])) AND ((cogniti*[tiab]) OR (neurodevelopment*[tiab]) OR (mental retardation) OR (‘Developmental Disabilities’[Mesh] OR disability[tiab]) OR (intelligence[tiab] OR IQ[tiab])).
The electronic search was supplemented by a manual search of the reference lists of studies that met the inclusion criteria.
The titles and abstracts of studies retrieved from the literature search were screened to identify studies that reported developmental and/or cognitive outcomes among preterm children who were born before 32 weeks’ gestation and/or were VLBW. These were grouped into three categories: (1) studies that reported both early developmental outcomes between ages 1 and 3 years as well as school-age cognitive outcomes at ≥ 5 years, (2) studies that reported only early developmental outcomes and (3) studies that only reported school-age cognitive outcomes. The author lists for articles in groups (2) and (3) were matched to identify assessments and publications on the same population at different time points. Studies that satisfied the initial screening process were retrieved for full-text evaluation for final inclusion in the review.
The quality of included studies was assessed using a checklist adapted from the Quality of Diagnostic Accuracy Studies version 2 (QUADAS-2) appraisal tool. 266 The aim was to provide a qualitative judgement for the risk of bias and the applicability of each study to the review question. The QUADAS-2 tool uses ‘signalling questions’ to assess bias in four domains: patient selection, index test, reference standard, and flow of participants through the study and timing of the index test. The applicability of the study to the review question in the first three domains was also assessed. In the context of this review, the index tests referred to the early developmental assessments and the reference standards were the school-age cognitive assessments. An essential feature of QUADAS-2 was the tailoring of the signalling questions to enable review-specific appraisal. Table 24 lists the signalling questions and the quality standards set for this review. By appraising against the set standards, each study was given a rating of ‘low’, ‘high’ or ‘unclear’ for risk of bias and concerns regarding applicability in each domain. No summary ‘quality score’ was generated as such scores lack statistical justification and are not comparable across different scoring systems. 267 It was decided not to exclude any study on the basis of its quality, to achieve a review on the topic that was as comprehensive as possible.
| Domain | Patient selection | Index test (early developmental assessment) | Reference standard (school-age cognitive assessment) | Flow and timing |
|---|---|---|---|---|
| Signalling questions |
|
|
|
|
| High risk of bias | Non-consecutive or random sampling methods; additional inclusion criterion not based on birthweight or gestational age | Inappropriate test used for population under study | Inappropriate test used for population under study or assessors were not blinded to results of early developmental test | Participants received different assessments or dropout rates were > 30% |
| High concerns regarding applicability | Subcohort of infants (e.g. only IUGR infants were included) recruited. Infants born before 1990, as they would differ from the target population in terms of neonatal care received and severity/pattern of diseases experienced | Non-universal tests (e.g. only standardised in a specific population). Outdated versions of assessments (e.g. published before 1990) | Non-universal tests (e.g. only standardised in a specific population). Outdated versions of assessments (e.g. published before 1990) |
From each included study, the following data were extracted into a table (unpublished data were sought from study authors through e-mail requests): study characteristics (i.e. location, city, country); sampling method (i.e. single centre, multicentre, population based); inclusion and exclusion criteria; anticipation and/or follow-up rates (as percentage of eligible survivors); final sample size included in meta-analysis (i.e. number of participants who completed both early and school-age assessments); early developmental and school-age cognitive assessment tool used; and study population characteristics [i.e. year(s) of birth of participants, mean or median gestational age, mean or median birthweight, ages at assessment, mean test scores at assessment, data on the predictive validity of early developmental assessments].
For this review, mild–moderate deficit was defined as developmental or cognitive test scores of between 1 and 2 SDs below the means of the standardised or control groups. Severe deficit was defined as test scores of lower than 2 SDs below the means of the standardised or control groups. In studies for which a control group of children who were born at full term were recruited and assessed simultaneously, the mean and SD of the control group were used as the references for defining the presence of deficits. Data on the number of ‘true-positive’, ‘false-positive’, ‘false-negative’ and ‘true-negative’ cognitive deficits identified by early assessments were collated from each study. If serial assessments were performed at different time points, data obtained from participants at the oldest age were included in the meta-analysis. The estimated sensitivity and specificity with corresponding 95% CI for mild–moderate and severe deficits were calculated.
Meta-analysis
The goals of the meta-analysis were to evaluate the variation in the estimates of the diagnostic accuracy (sensitivity and specificity) of early developmental assessments between studies and to combine results from all studies to yield a more precise estimate than is possible from individual studies. Coupled forest plots were generated to depict the ranges of sensitivity and specificity derived from the studies. Homogeneity of the sensitivities and specificities from the studies were tested using chi-squared tests. It has been noted that, in meta-analyses of diagnostic tests, significant between-study heterogeneity often exists. One source of heterogeneity is attributable to variations in diagnostic threshold and the related ‘trade off’ between sensitivity and specificity. 264,268 This may occur even when the same diagnostic criterion was applied across the studies (as was in this review) because of, for example, inherent differences in the spectrum of impairments in the patient populations or interobserver interpretation of test performances. To examine this, a scatterplot of the true-positive rate (TPR) (or sensitivity) against the false-positive rate (or 1 – specificity) for each study was created and the Spearman correlation coefficient was computed. ‘Threshold effect’ was demonstrated when the points assume the shape of a ROC curve and the sensitivity and specificity were significantly correlated. In this circumstance, separate pooling of sensitivities and specificities that ignore the correlation between the two measures would lead to an underestimation of the diagnostic accuracy. 269 It is possible to combine estimates using the Moses–Littenberg method to generate a summary ROC curve. 268,270 However, this does not allow for between-study variation. Instead, the Rutter and Gatsonis approach was used to fit a hierarchical summary ROC (HSROC) curve of the data. 271 The HSROC model accounts for both sampling variation within study at a lower level and between-study heterogeneity at a higher level using random effects. It models the log-odds of a positive test result in each study and each impairment group as a function of the positivity threshold in each study and the true impairment status, with model parameters describing the accuracy and asymmetry of the ROC curves. The output includes a summary operating point (pooled values for sensitivity and specificity) with 95% confidence region and a 95% prediction region for a forecast of the true sensitivity and specificity in a future study. As this is a hierarchical model, the summary operating point represents an average of study effects rather than a common effect. Individual study effects may differ considerably because of heterogeneity, and this variation is represented by the 95% prediction region.
The possible association of the diagnostic validity with study-level variables that could account for the observed heterogeneity among studies was investigated using metaregression methods for continuous variables and subgroup analysis for categorical variables. The variables were gestational age, birthweight, age at early assessment, age at late assessment, time interval between assessments, year of birth of participants, prevalences of total and severe impairment, the developmental assessment tool used, and the inclusion/exclusion of neurosensory impaired participants. For categorical variables, couple forest plots stratified by the subgroups were generated to allow for visual assessment of the differences in diagnostic validity between subgroups.
For continuous variables, scatterplots of sensitivity and specificity against each study-level covariates were generated by taking the mean value for continuous variables within each study except for year of birth, when the earliest date was used, as the mean/median value was not available. Bivariate models272 were used to formally test whether or not sensitivity and specificity were associated with study-level covariates. Bivariate models are equivalent to HSROC models when no covariate is included. 273 When including covariates, the bivariate model measures the association with sensitivity and specificity (on the logit scale), whereas the HSROC model measures the association with the accuracy and threshold parameters; therefore, the former was chosen for ease of interpretation. For each study-level covariate, associations with sensitivity and specificity were tested separately; likelihood ratio test was then used to test both associations jointly. Results are reported as estimated ORs with associated 95% CI and p-values.
For the studies that reported data from multiple assessments at different time points, scatterplots were created of sensitivity and specificity against mean age at assessment to explore the stability of sensitivity and specificity estimates over time. For reviews of interventional trials, the funnel plot, a graphical display of the estimates of study effects plotted against their sample size or precision (standard error), is the recommended method for examining publication bias. Statistical tests, such as Egger’s regression test and Begg’s rank correlation, are used to test for funnel plot asymmetry, which would indicate the presence of publication bias and other sample size-related effects. The appropriate method for investigating publication bias for studies of diagnostic test accuracy is unclear. Funnel plots of the estimates of log-diagnostic odds ratio (DOR) against corresponding precision were proposed. 274 The DOR is a single statistic measure of diagnostic performance that is defined as:
Therefore, the larger the DOR, the more accurate the test is. In the Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0,275 the application of tests for funnel plot asymmetry designed for use in randomised trials, including the Egger and Begg tests, is specifically discouraged as these are associated with inflated type I error rates. Instead, a regressions test for the association between the log-DOR and the ‘effective sample size (ESS)’, developed by Deeks et al. ,276 was suggested. The ESS is a function of the number of non-diseased (n1) and diseased (n2) participants, in which:
Following the proposed methods outlined in the paper by Deeks et al. ,276 the possibility of publication and other sample size-related effects was investigated by developing funnel plots of log-DOR against 1/ESS1/2 and tested for plot asymmetry using linear regression of the two variables, weighted by ESS.
For this review, forest plots were generated using RevMan, version 5.2 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). All other analyses were performed using Stata statistical package, version 11.0, and SAS.
Results
Two-year neurodevelopmental outcomes
Two hundred and eight children were recruited from 13 hospitals (Addenbrooke’s, Cambridge; Queen’s, Romford; Chelsea and Westminster, London; Ealing Hospital, London; Hillingdon Hospital, London; Homerton University, London; Newham, London; North Middlesex University, London; Northwick Park, London; Royal London, London; St Thomas’, London; West Middlesex, London; Whipps Cross Hospital, London).
Figure 17 shows the flow of children through recruitment to the completion of research and NHS assessments. Two hundred and four children completed all the subtests of the Bayley-III assessment. One child with ataxic cerebral palsy could not be assessed for the cognitive and language scales, two children did not co-operate for the receptive communication assessment and one child did not co-operate for the gross motor assessment. Of the children who completed the research assessments, three did not attend their routine NHS follow-up visit. Data from the NHS assessment were not entered onto the EPR by the examining clinician in 15 cases and the overall category of impairment could not be assigned for nine children because of missing EPR data. The 190 children for whom both research and NHS data were available in at least one outcome domain formed the study cohort. A complete set of data in all outcome domains was available for 177 children. Although the original plan was to stratify recruitment based on the gestational ages of the eligible children, it became clear during the recruitment phase that the final study cohort would be smaller than the initial projection. Therefore, all children whose parents agreed to participate were recruited and assessed.
FIGURE 17.
Flow chart of children through research and NHS assessments to form the study population.
The characteristics of the study population were compared with the ‘baseline population’ (all infants born between 1 January 2008 and 31 December 2010, at gestational ages below 30 weeks and discharged from the participating hospitals) (Table 25). Participants received a shorter duration of mechanical ventilation and were less likely to be receiving oxygen therapy at 36 weeks postmenstrual age than the baseline population (p < 0.001). The study population was comparable to the baseline population in terms of gestational age, birthweight, sex, proportions of singletons, mode of delivery and maternal age, and consisted of larger proportions of children of white ethnicity and those born to mothers living in the least deprived IMD quintile. Nevertheless, a wide range of ethnic groups was represented, reflecting the diversity of the population living in London. Consequentially, 92 (48.4%) children were raised in a bilingual or multilingual environment.
| Characteristics | Population | p-value | |
|---|---|---|---|
| Study (N = 190) | Baseline (N = 1037) | ||
| Gestation (completed weeks) | |||
| Median (IQR), range | 27 (26–29), 23–29 | 27 (26–29), 22–29 | 0.25 |
| Birthweight (g) | |||
| Median (IQR), range | 965 (790–1140), 490–1720 | 1000 (812–1200), 455–1990 | 0.08 |
| Sex, n (%) | |||
| Girls | 99 (52.1) | 444 (42.8) | 0.19 |
| Boys | 91 (47.9) | 503 (48.5) | |
| Missing | 0 (0.0) | 90 (8.7) | |
| Ethnicity, n (%) | |||
| White | 88 (46.3) | 364 (35.1) | 0.03 |
| Black | 50 (26.3) | 287 (27.7) | |
| Asian | 41 (21.6) | 239 (23.1) | |
| Mixed | 0 (0.0) | 33 (3.2) | |
| Other | 11 (5.8) | 52 (5.0) | |
| Missing | 0 (0.0) | 62 (6.0) | |
| Pregnancy, n (%) | |||
| Singleton | 147 (77.4) | 690 (66.5) | 0.26 |
| Multiples | 43 (22.6) | 250 (24.1) | |
| Missing | 0 (0.0) | 97 (9.4) | |
| Mode of delivery, n (%) | |||
| Vaginal | 74 (39.0) | 475 (45.8) | 0.22 |
| Caesarean | 103 (54.2) | 540 (52.1) | |
| Missing | 13 (6.8) | 22 (2.1) | |
| Maternal age (years) | |||
| Mean (SD) | 31.9 (6.7) | 31.0 (6.4) | 0.08 |
| IMD quintile at birth, n (%) | |||
| One (least deprived) | 19 (10.0) | 43 (4.2) | 0.01 |
| Two | 20 (10.5) | 81 (7.8) | |
| Three | 26 (13.7) | 144 (13.9) | |
| Four | 52 (27.4) | 268 (25.8) | |
| Five (most deprived) | 73 (38.4) | 477 (46.0) | |
| Missing | 0 (0.0) | 24 (2.3) | |
| Length of mechanical ventilation (days) | |||
| Median (IQR), range | 0 (0–3), 0–54 | 4 (0–18), 0–444 | < 0.001 |
| Oxygen therapy at 36 weeks corrected age, n (%) | |||
| Yes | 54 (28.4) | 466 (44.9) | < 0.001 |
| No | 136 (71.6) | 574 (55.1) | |
The mean (SD) corrected age of the children at assessment was 24.8 (2.2) months. The research assessment took place at a median (IQR) interval of 8 (0–27) days after the children received their NHS assessment, with a range of between 89 days before and 82 days after the NHS assessment.
Based on information given by the parents, 30 (15.8%) children had a visual defect including reduced visual acuity and/or squints, although only 11 (5.8%) required glasses. A total of 16 (8.4%) children had a hearing impairment, of whom three (1.6%) wore hearing aids.
The children performed significantly worse than the normative population, in which Bayley-III scores were standardised in all domains other than fine motor skills (Table 26).
| Domain | Score | Mean (SD) Bayley-III scores | p-valuea |
|---|---|---|---|
| Cognitive | Cognitive composite (n = 189) | 92.65 (12.8) | < 0.001 |
| Language | Receptive communication scaled (n = 187) | 8.0 (2.4) | < 0.001 |
| Expressive communication scaled (n = 189) | 7.5 (2.5) | < 0.001 | |
| Language composite (n = 187) | 87.0 (13.6) | < 0.001 | |
| Motor | Fine motor scaled (n = 190) | 10.2 (2.5) | 0.23 |
| Gross motor scaled (n = 189) | 8.6 (2.3) | < 0.001 | |
| Motor composite (n = 189) | 96.7 (12.7) | < 0.001 |
Based on the worst score achieved in the cognitive, language and motor Bayley-III domains, 114 (61.3%) children were classified as having scores of higher than –1 SD from the standardised mean, 42 (22.6%) children had scores of between –1 and –2 SDs and 30 (16.1%) children had scores of lower than –2 SDs from the standardised mean.
In the cognitive domain, 156 (82.1%) children obtained Bayley-III scores of higher than –1 SD, 26 (13.7%) children had scores of between –1 and –2 SDs and seven (3.7%) children had scores of lower than –2 SDs from the standardised mean.
Nineteen (10.0%) children had specific expressive communication impairment (scaled score of < 7), with no impairment in receptive communication. Five of these children would have been classified as having no impairment based on the Bayley-III language composite score alone (language composite score of higher than –1 SD, i.e. ≥ 85) because of the compensation from the receptive communication subtest. Based on the worst SD score category from the receptive and expressive communication subtests and the language composite score, 120 (63.2%) children achieved scores of higher than –1 SD, 42 (22.1%) had scores of between –1 and –2 SDs and 25 (13.2%) had scores of lower than –2 SDs from the standardised mean.
Motor function was generally intact among the children, with only 11 children (5.8%) receiving scores of between –1 and –2 SDs and 11 (5.8%) children having scores of lower than –2 SDs from the standardised mean. The mean (SD) predicted BSID-II MDI for the study population was 77.9 (20.5) and was significantly lower than the mean Bayley-III cognitive and language composite scores (p < 0.001 for both). Figure 18 shows the proportions of children with scores of higher than –1 SD (≥ 85), scores of between –1 and –2 SDs (70–84), scores of between –2 and –3 SDs (55–69) and scores of lower than –3 SDs (< 55) from the standardised mean for the Bayley-III cognitive and language composite scores individually, when the lower of the two Bayley-III scores was used and for the predicted BSID-II MDI score. A post hoc analysis using McNemar’s test was performed to compare the proportions diagnosed with impairment using these cut-off scores. The proportions of children classified with impairment using a cut-off point of < 85 on the Bayley-III cognitive score (17.4%), language score (32.6%) and the lower of the cognitive and language scores (35.8%) were statistically dissimilar to the proportions with predicted BSID-II MDI < 70 (25.3%) (p < 0.001). However, the proportions of children with predicted BSID-II MDI of < 55 (11.6%) were similar to the proportions who scored < 70 (classified with severe impairment) on the Bayley-III language composite score (13.1%; p = 0.26) and the lower of the cognitive and language scores (13.7%; p = 0.16).
FIGURE 18.
Neurodevelopmental status by Bayley-III scores and predicted BSID-II MDI.
Classification using the modified NPEU/Oxford criteria showed that, for the communication domain, the assignment of outcome was heavily influenced by the presence of specific expressive communication impairment: 107 (56.3%) children were classified as having no language impairment, 55 (28.9%) children had mild–moderate language impairment and 27 (14.2%) children had severe language impairment. Thirteen (6.8%) children had isolated gross motor impairment with no fine motor difficulties, and only one (0.5%) child had specific fine motor impairment. The combined motor outcome was normal in 172 (90.5%) children. Nine (4.7%) children were classed as having mild–moderate motor impairment and nine (4.7%) children had severe motor impairment.
Evaluating the concordance in the classification of neurodevelopmental status by Bayley-III scores and NPEU/Oxford criteria, of the 187 children tested for their communication skills, 144 (77.0%) were classified in the same category. Of the other children, none differed by more than one category. The weighted kappa coefficient (κ) was 0.59 (95% CI 0.49 to 0.69), which indicated moderate agreement between the two criteria for the communication outcome. For the motor domain, 180 out of 189 (95.2%) children were classified in the same category. Classification differed by one category for eight children. One child who was assessed as having severe motor impairment by the Bayley-III was classified as having ‘no impairment’ on the NPEU/Oxford criteria. The weighted κ for concordance between the two methods in the motor domain was 0.76 (95% CI 0.62 to 0.93), which represented substantial agreement.
The per cent agreement across all assessed items was 97.2% (69/71 items in agreement) in the first session (midway) and 98.6% (69/70 items in agreement) in the second session end of study assessments).
Neurodevelopmental outcomes from NHS electronic patient record data
Children attended their NHS follow-up assessment at a mean (SD) corrected age of 24.4 (2.3) months. Data were entered on the EPR ‘2-year outcome’ screen by clinical consultants in 111 (58.4%) cases [36 (19.0%) by consultant neonatologists, 42 (22.1%) by hospital paediatrics consultants and 33 (17.4%) by community paediatrics consultants], trainee doctors in 15 (7.9%) cases, staff-grade doctors in 58 (30.5%) cases and administrative staff in six (3.2%) cases. Sixty-seven (35.3%) children received standardised neurodevelopmental assessment or screening tests during their NHS appointment [19 (10.0%) using the Schedule of Growing Scales, 44 (23.2%) using the GMDS and 4 (2.1%) using the Alberta Infant Motor Scale]. Table 27 shows the responses to each question on the electronic form and the classification of impairment for the developmental domains. The classification of overall neurodevelopmental outcome was possible in 181 children, of whom 124 (68.5%) had no impairments, 38 (21.0%) had mild–moderate impairments and 19 (10.5%) had severe impairments.
| Domain | Question | Response, n (%) | Classification of impairment, n (%) | ||
|---|---|---|---|---|---|
| No | Yes | Missing | |||
| Cognitive | D1 | 154 (81.1) | 35 (18.4) | 1 (0.5) |
None: 141 (74.2) Mild–moderate: 42 (22.1) Severe: 6 (3.2) Unknown: 1 (0.5) |
| D2 | 171 (90.0) | 19 (10.0) | 0 (0.0) | ||
| D3 | 183 (96.3) | 6 (3.2) | 1 (0.5) | ||
| Receptive communication | RC1 | 174 (91.6) | 13 (6.8) | 3 (1.6) |
None: 174 (91.6) Mild–moderate: 8 (4.2) Severe: 5 (2.6) Unknown: 3 (1.6) |
| RC2 | 183 (96.3) | 5 (2.6) | 2 (1.1) | ||
| Expressive communication | EC1 | 149 (78.4) | 40 (21.1) | 1 (0.5) |
None: 143 (75.3) Mild–moderate: 32 (16.8) Severe: 13 (6.8) Unknown: 2 (1.1) |
| EC2 | 153 (80.5) | 36 (18.9) | 1 (0.5) | ||
| EC3 | 176 (92.6) | 13 (6.8) | 1 (0.5) | ||
| Combined languagea |
None: 141 (74.2) Mild–moderate: 30 (15.8) Severe: 14 (7.4) Unknown: 5 (2.6) |
||||
| Fine motor | FM1 | 188 (98.9) | 2 (1.1) | 0 (0.0) |
None: 186 (97.9) Mild–moderate: 2 (1.1) Severe: 2 (1.1) Unknown: 0 (0.0) |
| FM2 | 189 (99.5) | 1 (0.5) | 0 (0.0) | ||
| FM3 | 188 (98.9) | 2 (1.1) | 0 (0.0) | ||
| Gross motor | GM1 | 178 (93.7) | 12 (6.3) | 0 (0.0) |
None: 174 (91.6) Mild–moderate: 5 (2.6) Severe: 8 (4.2) Unknown: 3 (1.6) |
| GM2 | 177 (93.2) | 9 (4.7) | 4 (2.1) | ||
| GM3 | 182 (95.8) | 8 (4.2) | 0 (0.0) | ||
| GM4 | 186 (97.9) | 4 (2.1) | 0 (0.0) | ||
| GM5 | 188 (98.9) | 2 (1.1) | 0 (0.0) | ||
| Combined motora |
None: 173 (91.1) Mild–moderate: 6 (3.2) Severe: 8 (4.2) Unknown: 3 (1.6) |
||||
| Overalla |
None: 124 (65.3) Mild–moderate: 38 (20.0) Severe: 19 (10.0) Unknown: 9 (4.7) |
||||
The proportions of children classified into each category of impairment by the research assessment (using Bayley-III scores and NPEU/Oxford criteria) and by the NHS assessment are displayed in Appendix 2, Figures 36–39.
Cross-tabulations to compare the agreement between the research and the NHS assessments for the classification of ‘any impairment’ and ‘severe impairment’ are displayed in Tables 28 and 29. The estimated sensitivities and specificities for NHS assessments in each developmental domain are presented taking the research assessment as the ‘gold standard’. The CIs for sensitivities and specificities were calculated using robust standard errors to account for clustering of data by study sites. Sensitivity analyses revealed that potential correlated outcomes from siblings did not affect the results. Therefore, the results presented included data from all participating children.
| Domain of developmenta | Method of classification of impairment for research assessment | Identification of impairment by NHS assessment against the ‘gold standard’ research assessment | |||||
|---|---|---|---|---|---|---|---|
| True positives, n (%) | False negatives, n (%) | False positives, n (%) | True negatives, n (%) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | ||
| Cognitive | Bayley-III scores (n = 188) | 23 (12.2) | 10 (5.3) | 25 (13.3) | 130 (69.1) | 69.7 (55.1 to 84.3) | 83.9 (75.6 to 92.1) |
| Receptive communication | Bayley-III scores (n = 184) | 9 (4.9) | 30 (16.3) | 4 (2.2) | 141 (76.6) | 23.1 (6.7 to 39.5) | 97.2 (94.6 to 99.9) |
| NPEU/Oxford (n = 186) | 8 (4.3) | 13 (7.0) | 5 (2.7) | 160 (86.0) | 38.1 (10.7 to 65.5) | 97.0 (94.8 to 99.2) | |
| Expressive communication | Bayley-III scores (n = 187) | 32 (17.1) | 22 (11.8) | 13 (7.0) | 120 (64.2) | 59.3 (46.5 to 72.0) | 90.2 (82.2 to 98.3) |
| NPEU/Oxford (n = 187) | 39 (20.9) | 41 (21.9) | 6 (3.2) | 101 (54.0) | 48.8 (33.9 to 63.6) | 94.4 (88.9 to 99.9) | |
| Combined language | Bayley-III scores (n = 182) | 33 (18.1) | 29 (15.9) | 11 (6.0) | 109 (59.9) | 53.2 (42.0 to 64.5) | 90.8 (83.5 to 98.2) |
| NPEU/Oxford (n = 184) | 38 (20.7) | 39 (21.2) | 6 (3.3) | 101 (54.9) | 49.4 (34.7 to 64.0) | 94.4 (88.9 to 99.9) | |
| Fine motor | Bayley-III scores (n = 190) | 3 (1.6) | 9 (4.7) | 1 (0.5) | 177 (93.2) | 25.0 (0.0 to 59.7) | 99.4 (98.3 to 100.0) |
| NPEU/Oxford (n = 190) | 4 (2.1) | 1 (0.5) | 0 (0.0) | 185 (97.4) | 80.0 (28.4 to 99.5) | 100.0 (98.0 to 100.0) | |
| Gross motor | Bayley-III scores (n = 186) | 12 (6.5) | 4 (2.2) | 1 (0.5) | 169 (90.9) | 75.0 (49.9 to 100.0) | 99.4 (98.1 to 100.0) |
| NPEU/Oxford (n = 187) | 11 (5.9) | 5 (2.7) | 2 (1.1) | 169 (90.4) | 68.8 (45.5 to 92.0) | 98.8 (97.1 to 100.0) | |
| Combined motor | Bayley-III scores (n = 186) | 13 (7.0) | 8 (4.3) | 1 (0.5) | 164 (88.2) | 61.9 (32.9 to 90.9) | 99.4 (98.1 to 100.0) |
| NPEU/Oxford (n = 187) | 12 (6.4) | 5 (2.7) | 2 (1.1) | 168 (89.8) | 70.6 (48.8 to 92.4) | 98.8 (97.0 to 100.0) | |
| Overall | Bayley-III scores (n = 177) | 40 (22.6) | 25 (14.1) | 16 (9.0) | 96 (54.2) | 61.5 (52.5 to 70.6) | 85.7 (77.4 to 94.0) |
| Domain of developmenta | Method of classification of impairment for research assessment | Identification of severe impairment by NHS assessment against the ‘gold standard’ research assessment | |||||
|---|---|---|---|---|---|---|---|
| True positives, n (%) | False negatives, n (%) | False positives, n (%) | True negatives, n (%) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | ||
| Cognitive | Bayley-III scores (n = 188) | 2 (1.1) | 5 (2.7) | 4 (2.1) | 177 (94.1) | 28.6 (5.0 to 52.2) | 97.8 (95.1 to 100.0) |
| Receptive communication | Bayley-III scores (n = 184) | 3 (1.6) | 8 (4.3) | 2 (1.1) | 171 (92.9) | 27.3 (0.0 to 62.9) | 98.8 (97.3 to 100.0) |
| NPEU/Oxford (n = 186) | 2 (1.1) | 1 (0.5) | 3 (1.6) | 180 (96.8) | 66.7 (4.9 to 100.0) | 98.4 (95.3 to 99.7) | |
| Expressive communication | Bayley-III scores (n = 187) | 7 (3.7) | 5 (2.7) | 6 (3.2) | 169 (90.4) | 58.3 (36.6 to 80.0) | 96.6 (92.7 to 98.7) |
| NPEU/Oxford (n = 187) | 9 (4.8) | 18 (9.6) | 4 (2.1) | 156 (83.4) | 33.3 (12.0 to 54.7) | 97.5 (93.7 to 99.3) | |
| Combined language | Bayley-III scores (n = 182) | 9 (4.9) | 12 (6.6) | 5 (2.7) | 156 (85.7) | 42.9 (14.2 to 71.5) | 96.9 (92.9 to 99.0) |
| NPEU/Oxford (n = 184) | 9 (4.9) | 15 (8.2) | 5 (2.7) | 155 (84.2) | 37.5 (15.4 to 59.6) | 96.9 (92.9 to 99.0) | |
| Fine motor | Bayley-III scores (n = 190) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 187 (98.4) | 50.0 (0.0 to 100.0) | 99.5 (97.1 to 100.0) |
| NPEU/Oxford (n = 190) | 1 (0.5) | 0 (0.0) | 1 (0.5) | 188 (98.9) | 100.0 (2.5 to 100.0) | 99.5 (97.1 to 100.0) | |
| Gross motor | Bayley-III scores (n = 186) | 8 (4.3) | 1 (0.5) | 0 (0.0) | 177 (95.2) | 88.9 (51.8 to 99.7) | 100.0 (97.9 to 100.0) |
| NPEU/Oxford (n = 187) | 8 (4.3) | 1 (0.5) | 0 (0.0) | 178 (95.2) | 88.9 (51.8 to 99.7) | 100.0 (97.9 to 100.0) | |
| Combined motor | Bayley-III scores (n = 186) | 8 (4.3) | 2 (1.1) | 0 (0.0) | 176 (94.6) | 80.0 (44.4 to 97.5) | 100.0 (97.9 to 100.0) |
| NPEU/Oxford (n = 187) | 8 (4.3) | 1 (0.5) | 0 (0.0) | 178 (95.2) | 88.9 (51.8 to 99.7) | 100.0 (97.9 to 100.0) | |
| Overall | Bayley-III scores (n = 177) | 13 (7.3) | 12 (6.8) | 5 (2.8) | 147 (83.1) | 52.0 (23.8 to 80.2) | 96.7 (92.5 to 99.9) |
The validity of the NHS assessments in identifying children with no impairments was high, with estimated specificities ranging between 83.9% and 100.0% for ‘any impairment’, and between 96.6% and 100.0% for ‘severe impairment’. However, the validity of the NHS and the research assessment in identifying and categorising children with impairments was variable. The sensitivities for identifying gross motor impairment were high, particularly when the impairment was severe. In the cognitive domain, the sensitivity for the identification of any impairment was 69.7% (95% CI 55.1% to 84.3%) but dropped to only 28.6% (95% CI 5.0% to 52.2%) for the identification of severe impairment. Of the seven children diagnosed with severe cognitive impairment through the research assessment, two were also classified as having impairment in the ‘severe’ category in the NHS data set, four were classified as having impairment ‘mild–moderate’ and one was classified as having ‘no’ impairment; hence, the disagreement in the classification occurred mainly between the ‘mild–moderate’ and ‘severe’ categories. Agreement between the NHS and research assessments was worst in the language domain, especially in receptive communication, where the sensitivity in identifying the presence of any impairment was only 23.1% (95% CI 6.7% to 39.5%). In the combined language domain, the 21 ‘false negatives’ for severe impairment, based on Bayley-III classification, were evenly distributed among the impairment categories in the NHS data (‘severe’, n = 9; ‘mild–moderate’, n = 6; ‘no’ impairments, n = 6). The sensitivities were estimated with low precision (wide CIs), particularly in the motor domains and with severe impairments when the prevalence of impairment was low.
Although the sensitivities in the receptive communication and fine motor domains appeared considerably higher when impairment was assigned using the NPEU/Oxford criteria than using Bayley-III scores for the research assessment, this was driven by the small numbers in the ‘false-negative’ cells and the estimated sensitivities were associated with wide and overlapping CIs, which suggested that the differences in sensitivities may not be statistically significant.
The sensitivities and specificities of NHS assessment in identifying cognitive deficit were 69.7% (95% CI 55.1% to 84.3%) and 83.9% (95% CI 75.6% to 92.1%) for the presence of any impairment and 28.6% (95% CI 5.0% to 52.2%) and 97.8% (95% CI 95.1% to 100.0%) for severe impairments (see Tables 28 and 29). The analyses were repeated using the predicted BSID-II MDI scores as the ‘gold standard’. Using the cut-off point of MDI < 85 (–1 SD) to define mild–moderate impairment and < 70 (–2 SDs) to define severe impairment, there was a reduction in the sensitivities and an increment in the corresponding specificities [sensitivity 39.3% (95% CI 30.2% to 49.0%) and specificity 94.6% (95% CI 86.7% to 98.5%) for any cognitive impairment; sensitivity 12.5% (95% CI 4.7% to 25.2%) and specificity 100% (95% CI 97.4% to 100%) for severe cognitive impairment]. However, if thresholds of MDI < 70 (–2 SDs) for mild–moderate and < 55 (–3 SDs) for severe impairments were used, the results were similar to the reported findings using the Bayley-III [sensitivity 64.6% (95% CI 49.5% to 77.8%), specificity 87.7% (95% CI 81.0% to 92.7%) for any impairment; sensitivity 18.2% (95% CI 5.1% to 40.3%), specificity 98.8% (95% CI 95.7% to 99.9%) for severe impairment].
The concordance of the research and the NHS assessments as measured by κ was consistent with the findings from the estimated sensitivities and specificities. The agreement between NHS and research assessment was substantial in the motor domain with weighted κ > 0.6. In the cognitive and communication domains, agreement was moderate at best.
Post hoc analysis of the validity of NHS assessments using a different question set to identify ‘moderate–severe’ impairment
The purpose of this post hoc analysis was to assess if, by applying a broader criterion to define ‘moderate–severe’ impairment, the validity of the NHS data in identifying children with Bayley-III scores of lower than –2 SDs could be improved. Children were reclassified as having moderate–severe impairment if they met the broader criterion in the NHS data. The results are displayed in Appendix 1, Table 58. The use of a broader category of moderate–severe impairment improved the sensitivity of the NHS data, although this was at a cost of a small reduction in specificity. The biggest increase in sensitivity was observed in the cognitive and expressive communication domains.
Variables affecting the validity of the NHS assessments
As the diagnostic validity of the NHS assessment did not differ between the use of Bayley-III scores and NPEU/Oxford criteria for classifying impairment, subgroup analyses were performed using only the results from Bayley-III assessments. Lower prevalence of impairment with higher gestational age at birth across all domains was observed, with apparent reduction in sensitivity but increased specificity of NHS assessments in identifying overall impairment with increasing gestational age. Sensitivity in identifying cognitive impairment was higher if a standardised neurodevelopmental test was used during NHS assessment. Accuracy in identifying impairment also appeared higher across all domains with increasing postnatal age at assessment. However, as the CIs for the estimated sensitivities and specificities overlapped widely, the observed effect of these factors on the diagnostic validity of NHS assessment can be conservatively considered statistically insignificant. 277 Similarly, there was no clear effect of the exposure to English language, the grade of the NHS assessor, IMD and the time interval between NHS and research assessments on the validity of NHS assessment.
Behaviour during assessments and the effect on study findings
The prevalence of impairment was significantly higher among children who were difficult to assess during the NHS assessment (86.7% vs. 31.7% for impairment in any domain; p < 0.001) or who had received lower examiner-rated behaviour scores (less positive behaviour) (73.8% for impairment in any domain among children with behaviour score of ≤ 22 vs. 28.5% among children with behaviour score of > 22; p < 0.001). However, challenging behaviour demonstrated during assessments did not appear to affect the test validity of the NHS assessment against the research assessment. The prevalence of impairment, sensitivity and specificity of NHS assessment did not differ by parent-rated behaviour scores.
Hammersmith Infant Neurological Examination and diagnosis of cerebral palsy
Forty-seven (24.7%) children had a suboptimal global score (< 73/78) on the HINE. In general, in the preterm population, although scores below 73 are suboptimal, those with scores of > 64 will walk independently by 2 years, those with scores of < 64 but > 52 will sit independently by 2 years and those with scores of < 52 will not be able to do either. The proportions of participants who achieved suboptimal score in each subsection were as follows: 30 (15.8%) for cranial nerve function, 39 (20.5%) for posture, 16 (8.4%) for movement, 36 (18.9%) for tone and 8 (4.2%) for reflexes.
Nine (4.7%) children were found to have cerebral palsy during the research assessment. The HINE scores for these children (median 53, IQR 38.5–59.5) were significantly lower than those without cerebral palsy (median 78, IQR 74–78; p < 0.001) and consistent with published data. 210 Two children had spastic quadriplegia, five had spastic diplegia, one had three-limb involvement and one had dyskinetic cerebral palsy. The gross motor function varied from GMFCS level 1 (walks without limitations) for one child with spastic diplegia to GMFCS level 5 (transported in manual wheelchair) for the child with dyskinetic cerebral palsy and one of the children with spastic quadriplegia. Most children with spastic diplegic cerebral palsy functioned at GMFCS level 2 (walks with limitations).
Two children with spastic diplegia were not identified to have cerebral palsy in the NHS data. The topographic classifications entered in the NHS data for all other children identified to have cerebral palsy were in agreement with the research assessment.
Early childhood social communication difficulties
The Quantitative Checklist for Autism in Toddlers (Q-CHAT) questionnaire was sent to the parents of all 208 children who attended the research assessment. Ten children were assessed to have major functional impairments (nine with cerebral palsy and one with severe hearing impairment) and were ineligible for this study. The parents of three children who declined to participate in the research assessment agreed to complete the Q-CHAT and Bayley-III Social-Emotional questionnaires. A total of 150 questionnaires, including eight from children who were ineligible, were returned. One questionnaire with seven missing responses was treated as a non-respondent and excluded, leaving 141 participants (70.1% of eligible participants) for the analyses.
Non-respondents were more likely to be parents of girls (66.7%; p = 0.02). Nonetheless, both boys and girls were equally represented in the respondent group. Respondents showed over-representation of children of white ethnicity who were born to mothers living in less deprived IMD quintiles, with significantly shorter duration of mechanical ventilation and who were less likely to have required supplemental oxygen therapy at 36 weeks corrected age (see Appendix 1, Table 59).
The mean corrected age of the respondents was 24.7 (SD 2.6, range 18.5–35.6) months at the time of completion of the questionnaires. The mean Bayley-III composite score of the 138 respondents who completed the assessment was 94.6 (SD 13.0) for the cognitive scale, 87.7 (SD 13.0) for the language scale and 98.0 (SD 10.1) for the motor scale.
The Q-CHAT scores of the study population (mean 33.7, SD 8.3, range 15–55) were normally distributed and significantly higher (less favourable) than the published general population scores (difference in means 7.0, 95% CI 5.6 to 8.3; p < 0.001) (Figure 19). In contrast with the higher scores described in boys in the general population, no sex differences in Q-CHAT scores were observed in the preterm population (p = 0.85).
FIGURE 19.
Histogram of Q-CHAT scores of the preterm study population with superimposed distribution of published Q-CHAT scores of unselected toddlers (general population).
The distribution of scores between the preterm study cohort and the general population differed significantly in 17 items. In all of these items, there were greater proportions of preterm children receiving higher scores, indicating greater social communication difficulties and autistic behaviour characteristics. The differences were most prominent in the categories of restricted, repetitive, stereotyped behaviour (seven out of nine items differ significantly), communication (three out of four items) and sensory abnormalities (all three items). Only four out of the nine items exploring social relatedness were scored differently in the preterm population.
On multivariable testing, cognitive and motor function did not appear to affect Q-CHAT scores (p = 0.18 for cognitive scores and p = 0.67 for motor scores). Bayley-III language composite scores independently predicted Q-CHAT scores in a linear fashion (correlation coefficient –0.51; p = 0.001) and accounted for 24.5% of the variance in Q-CHAT scores. The relationship between language and Q-CHAT scores was attributable to expressive communication ability (regression coefficient Bayley-III expressive communication subscale scores and Q-CHAT scores: –1.35, 95% CI –1.96 to –0.74, correlation coefficient –0.43; p < 0.001). There was no association between receptive communication ability and Q-CHAT scores (p = 0.22).
Non-white ethnicity and living in deprived areas were associated with higher Q-CHAT scores in univariable analyses. Although non-white children were more likely to live in areas of higher deprivation (test for trend p < 0.001), there was no interaction between ethnicity and IMD in the association with Q-CHAT scores (p = 0.72). As lower Bayley-III language scores were observed among non-white children (mean difference 7.31, 95% CI 3.07 to 11.5; p < 0.001) and children living in more deprived areas (mean decrease of 1.89, 95% CI 0.24 to 3.55; p = 0.03) points per IMD quintile increase in deprivation, language ability was considered to be a potential confounder in the relationship between ethnicity, IMD and Q-CHAT scores. There was no interaction between Bayley-III language scores and IMD quintiles (p = 0.88) or ethnicity (p = 0.51). The final multivariable regression model included all variables found to be statistically significant during univariable analysis (Bayley-III language composite score, ethnicity and IMD) and is displayed in Appendix 1, Table 60.
The Bayley-III Social-Emotional questionnaire was completed in 140 out of the 141 eligible respondents to the Q-CHAT questionnaires. The Bayley-III Social-Emotional score distribution of the preterm population (mean 97.8, SD 17.2, range 55–145) did not differ significantly from the standardised norm of mean 100 and SD of 15 (p = 0.12). Twenty-three (16.5%) children had Q-CHAT scores of higher than 2 SDs above the general population mean (i.e. > 42.3). Only five (3.6%) children scored lower than 2 SDs below the standardised mean (i.e. > 70) for the Bayley-III Social-Emotional scale. There was poor concordance between the questionnaires, with only three children classed to be ‘at risk’ for ASD by both questionnaires and a resulting Cohen’s κ coefficient of 0.17 (95% CI 0 to 0.36).
Systematic literature review and meta-analysis
The PRISMA flow diagram is shown in Figure 20. The electronic literature search yielded 3600 unique citations (one of which was a duplicate). Application of search limits excluded 343 non-English articles and 413 articles published before 1990. Two additional studies were identified through manual search and author correspondence. Sixty-eight studies were selected for full-text evaluation from the title/abstract screen and 44 met the eligibility criteria. By matching 375 articles that reported the conduct of early developmental assessments with 323 articles that reported school-age assessments, 10 additional studies (in 23 articles) were identified. Data required for the review and meta-analysis were extractable directly from six articles. The authors of 18 of the remaining 48 studies contributed unpublished data for this review. The list of included studies is in Appendix 1, Table 61. For simplicity of referencing, studies that are represented by more than one article are denoted by the first author and year of publication of the earliest article in all tables and figures.
FIGURE 20.
The PRISMA flow diagram depicting the literature search process.
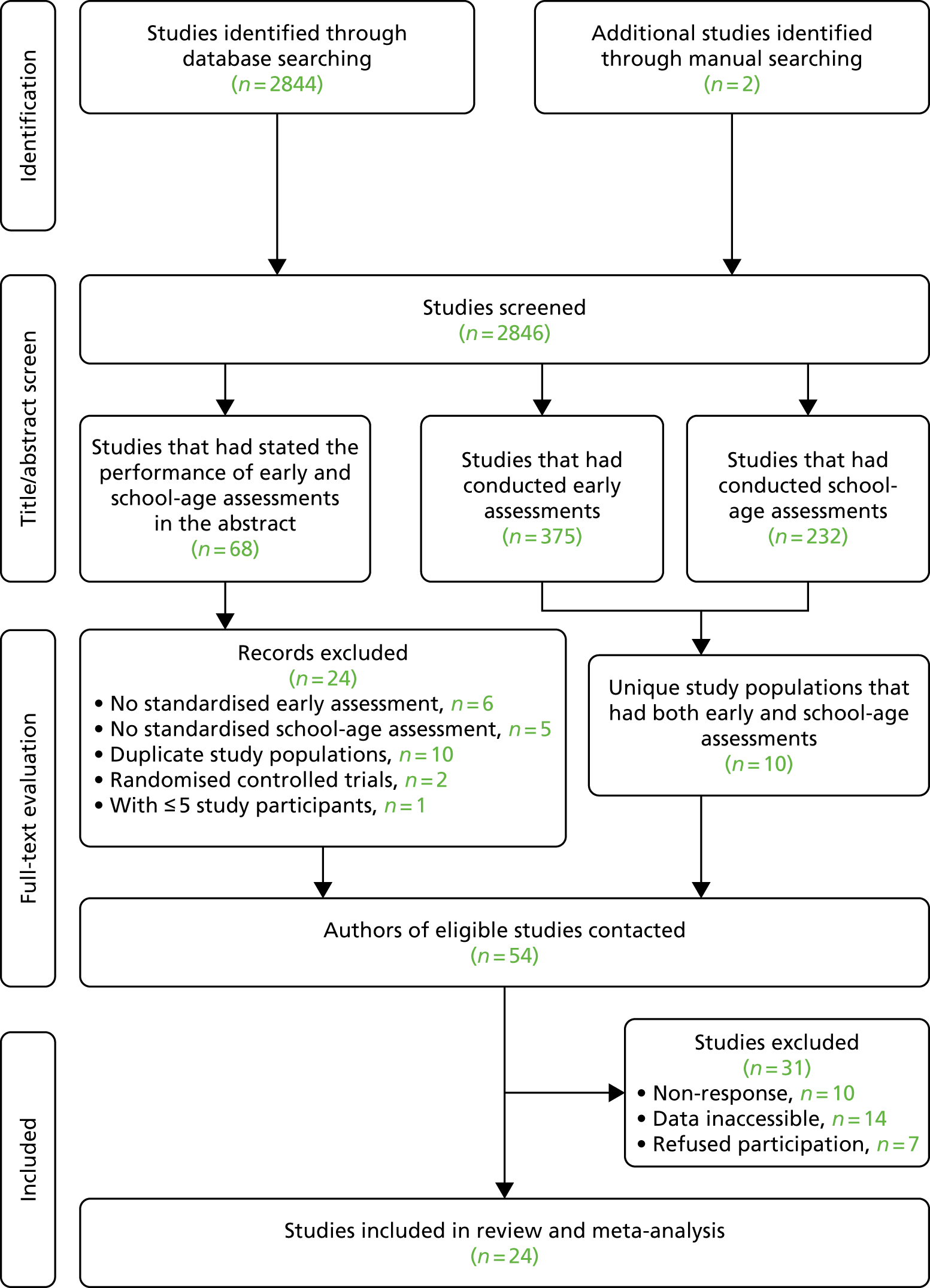
Description of included studies
The studies were conducted in Europe (12 studies), the USA (seven studies), Australia (three studies), New Zealand (one study) and Israel (one study). Sample sizes ranged from 11 to 313 participants. Most studies restricted the recruitment of participants to a single institution (15 studies), three studies were multicentre and six studies adopted a geographical population-based sampling method. The inclusion criteria were wholly based on birthweight in nine studies, and on gestational age in five studies and based on both birthweight and gestational age in five studies. For the other studies, additional inclusion criteria applied, including intrauterine growth restriction,278 spastic diplegia,279 specific neonatal diagnoses280,281 and low parental socioeconomic status. 282 The participants in six studies consisted of children who were born at > 32 weeks’ gestation and with a birthweight of > 1500 g, but the authors were able to provide relevant data limited to the subgroup that meet the criteria for this review. Tools used in each study and the ages of application are also listed in Appendix 1, Table 62. As the studies spanned a period of > 30 years, different editions of the same assessment tool were recorded.
Study populations
From these 24 studies, a total of 3133 children who were born at ≤ 32 weeks’ gestation and/or with a birthweight of < 1500 g received both early and school-age assessments. The mean gestational ages at birth ranged from 25.0 to 33.1 weeks and the mean birthweights were between 675 g and 1298 g. A total of 37.0% (1159 children) of the included populations were born in the years 1972–90, 49.6% (1555 children) in 1991–2000 and 13.4% (419 children) in 2000–5. Children with known genetic syndromes and congenital anomalies were excluded from the studies. Children with severe neurosensory (including blindness and deafness) and motor impairment were likely to be under-represented in the cohort, as 13 studies (contributing 48% of the final sample) excluded children who were unable to complete the assessments as a result of their physical disabilities. 161,278,281–291 The actual number of children excluded from the analysis for this reason is unknown, as not all studies provided this information. In the studies by Claas et al. 292 and Fedrizzi et al. ,279 no child was unable to complete the assessment because of the presence of physical disability. In studies that included participants who were ‘too physically disabled to be tested’,160,162,163,173,280,293,294 these children were assigned a nominal score that was equivalent to being more than 2–4 SDs below the population mean.
Developmental and cognitive assessments
Ten studies reported the results of developmental assessments conducted between 12 and 24 months corrected age and 11 studies reported the results at 24 months corrected age. In three of these studies,161,283,293 a repeat assessment was conducted at age 3 years. Fedrizzi et al. 279 reported results at 3 years and Smith et al. 282 reported results at 3.5 years chronological age.
The results of the school-age cognitive assessment were available at the ages of 5–6 years in 16 studies, 7–10 years in 11 studies and > 10 years in three studies. Cohen,286 Reuss et al. ,288 Marlow et al. ,225 Smith et al. ,282 and Wolke and Meyer173 conducted multiple school-age assessments at different time points for their study populations.
The proportion of children diagnosed with developmental impairment (test scores of > 1 SD below standardised or control group mean) varied widely among studies, ranging from 6.0%284 to 67.0%. 162 The reported prevalence of school-age cognitive deficit was between 5.0%286 and 67.4%225 for mild–moderate (1–2 SDs below the mean) and between 0.0%279,286 and 37.8%225 for severe impairment (> 2 SDs below the mean). In six studies,163,280,282,287,294 the categorisation of outcomes was based on the mean and SD of the scores achieved by concurrently recruited term-born controls. Wolke and Meyer173 used cohort-specific cut-off points derived from a normative sample representative of the total population of infants in the Bavarian region to categorise impairments. It should be noted that the study population in Smith et al. 282 was from middle to low socioeconomic groups and the mean test score achieved by the control group was about 0.5 SDs below the standardised mean. Using the results from the control group in this case could lead to an underestimation of the prevalence of impairment in this study. If the test standardised norm values were used, the prevalence of cognitive impairment diagnosed at 8 years of age would increase from 24.0% to 36.0% for mild–moderate and from 6.0% to 6.6% for severe impairment.
Quality of included studies: results of QUADAS-2 appraisal
Appendix 1, Table 62, shows the details of the quality of each included study based on the QUADAS-2 appraisal tool, and in Appendix 2, Figure 40, the proportions of studies that were considered at ‘low’, ‘high’ and ‘unclear’ risk for bias and applicability in each domain are displayed. The loss to follow-up of > 30% of the eligible birth cohort was a main source of selection bias in the included studies. Risk of information bias is low but may be introduced in three studies279,283,292 because of the lack of blinding of assessors performing the school-age assessments to the results of the early developmental tests. It was unclear if blinding occurred in the studies by Roberts et al. ,163 Reuss et al. ,288 Smith et al. 282 and Tommiska et al. 290 Although the overall risk of bias was low, there is high concern for the applicability of the results from the studies to our current population in > 50% of the studies. This is because many of the included studies were conducted more than 20 years ago and, therefore, the characteristics of the study populations would be different and the assessment tools have been superseded by newer versions.
Predictive validity of early developmental assessment
The results of the cross-tabulations and the estimated sensitivities and specificities of early assessments for identifying any cognitive deficit for each study, in the form of coupled forest plots ordered by the sample size of the study, are shown in Figure 21 with the same information for the diagnosis of severe cognitive impairment. In studies for which participants were examined at different time points, only the results from the assessment performed at the oldest age are presented. This gives a final sample size of 3060 children for the meta-analysis. There was significant heterogeneity in the reported sensitivities and specificities among studies (p < 0.001 for both). The estimated sensitivities of diagnosing any impairment ranged from 17.0% to 90.5% and the corresponding estimated specificities ranged from 46.8% to 98.4%. For the diagnosis of severe impairment, the range of sensitivities was 0.0% to 100.0% and the range of specificities was 70.8% to 100.0%. The sensitivity of detecting severe impairment could not be estimated in the studies by Cohen286 and Fedrizzi et al. 279 as no participant had severe impairment. There appears to be a wider range and poorer precision (wider CIs) in the estimated sensitivity than in the specificity across studies. This may reflect the presence of heterogeneity or more likely as a result of estimates of sensitivity being based on smaller samples than estimates of specificity. The estimated sensitivity of 0.0% for severe impairment was based on a denominator of 1283,284,290 and 10293 diagnosed cases at school-age assessments. In general, the larger the sample size, the more precise (the smaller the 95% CI) the sensitivity estimates. The precision of specificity estimates appears to be high with the CI half-widths in 10 studies being < 10.0%. 160,161,173,225,280–282,285,288,291
FIGURE 21.
Results of cross-tabulations and coupled forest plots of the estimated sensitivities and specificities of early developmental assessments in identifying the presence of (a) coupled forest plots for the identification of any cognitive impairment; and (b) coupled forest plots for the identification of severe cognitive impairment. Sensitivities and specificities are expressed as proportions.
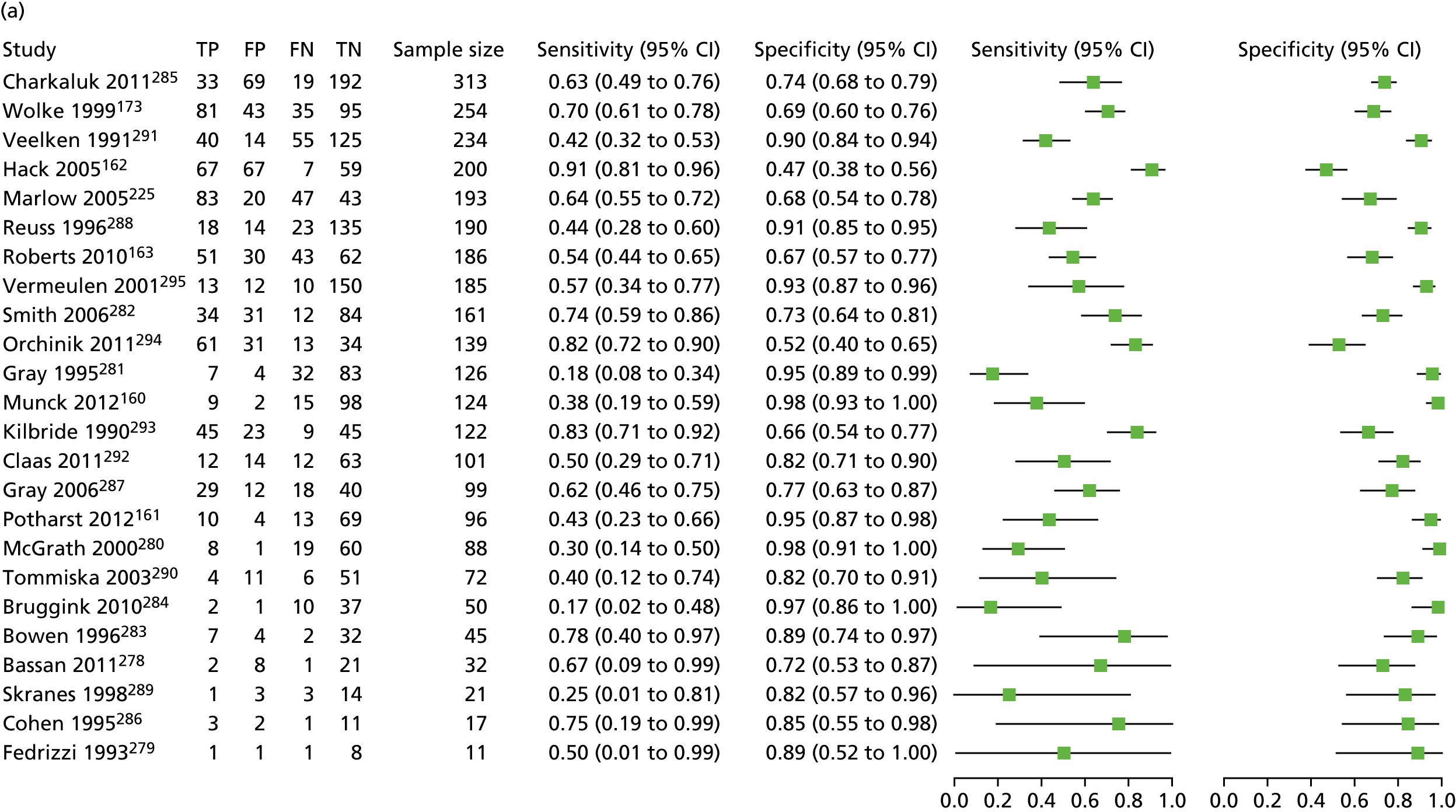
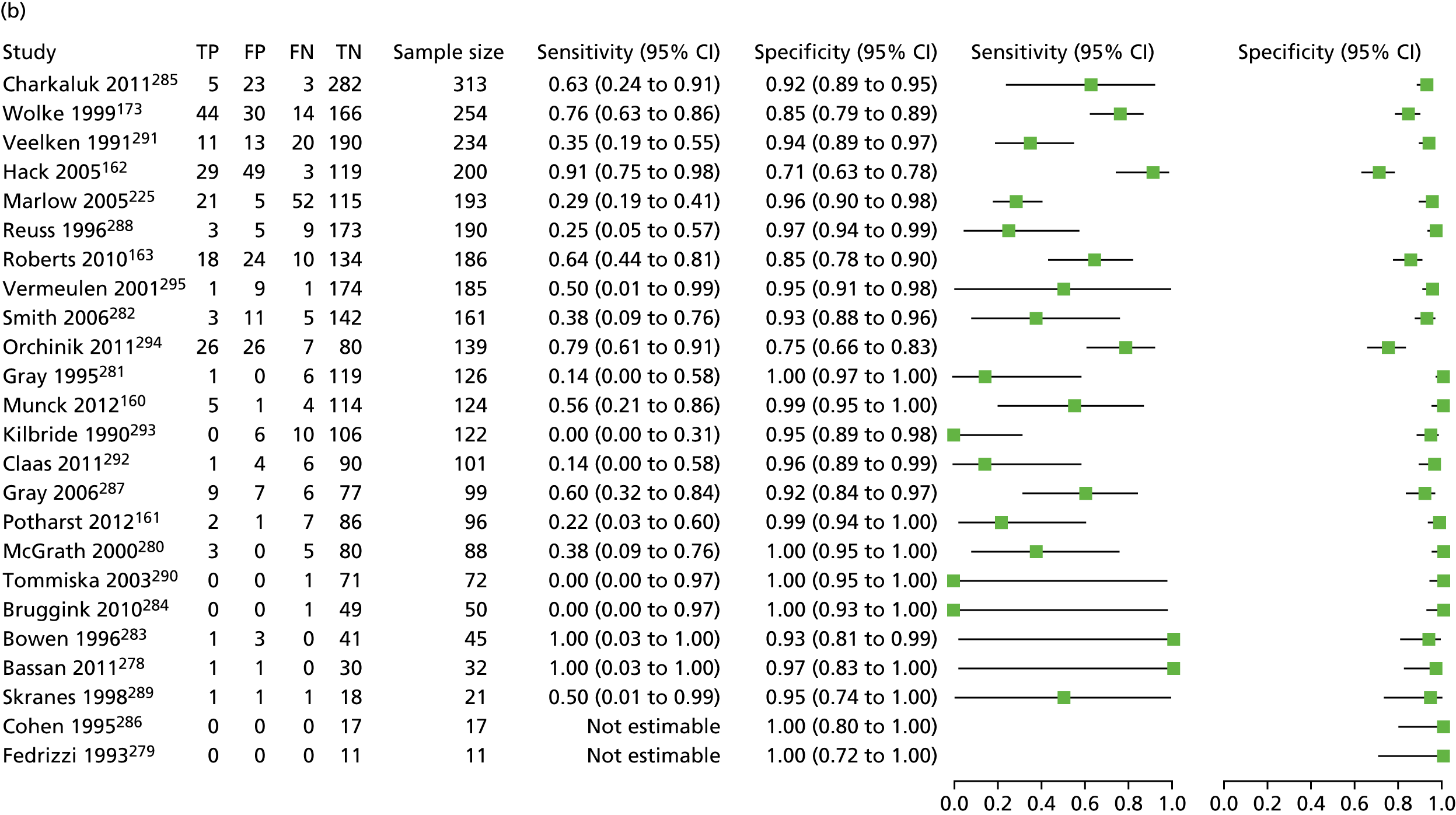
Meta-analytic pooled estimates of sensitivity and specificity
There was significant correlation between estimated sensitivities and specificities (see Appendix 2, Figure 41; Spearman’s rho –0.76; < 0.001). Therefore, the weighted averages of sensitivities and specificities were not computed separately. The pooled measures were estimated from the Rutter and Gatsonis HSROC curves that are presented in Appendix 2, Figure 42, for the presence of any impairment and severe impairments. The summary points and 95% CI regions are mapped out in the figures as well as the 95% prediction regions, which provide a forecast of the true sensitivity and specificity in a future study. The summary points corresponded to a pooled sensitivity of 55.0% (95% CI 45.7% to 63.9%) and pooled specificity of 84.1% (95% CI 77.5% to 89.1%) for the identification of any impairment. For the diagnosis of severe impairment, the pooled sensitivity was 39.2% (95% CI 26.8% to 53.3%) and pooled specificity was 95.1% (95% CI 92.3% to 97.0%).
Validity of early assessment assessed at different time points
In three studies,161,283,293 participants were assessed at two different time points for the early developmental assessments. In the five studies by Cohen,286 Reuss et al. ,288 Marlow et al. ,225 Smith et al. ,282 and Wolke and Meyer,173 participants received school-age cognitive assessments more than once. In Figure 22, the sensitivity and specificity for the identification of any impairment are plotted over the age at developmental assessment for the three studies that examined early assessment at two different time points. Figure 23 shows similar plots for the results obtained at serial school-age assessments in the five studies. It would appear, from these graphical displays, that the specificity of early assessment in excluding cognitive deficit remains relatively stable over time whereas no real correlation between sensitivity and age at assessment was apparent.
FIGURE 22.
Line graphs demonstrating the change in (a) sensitivity and (b) in specificity when early developmental assessments were repeated at different ages in three studies.

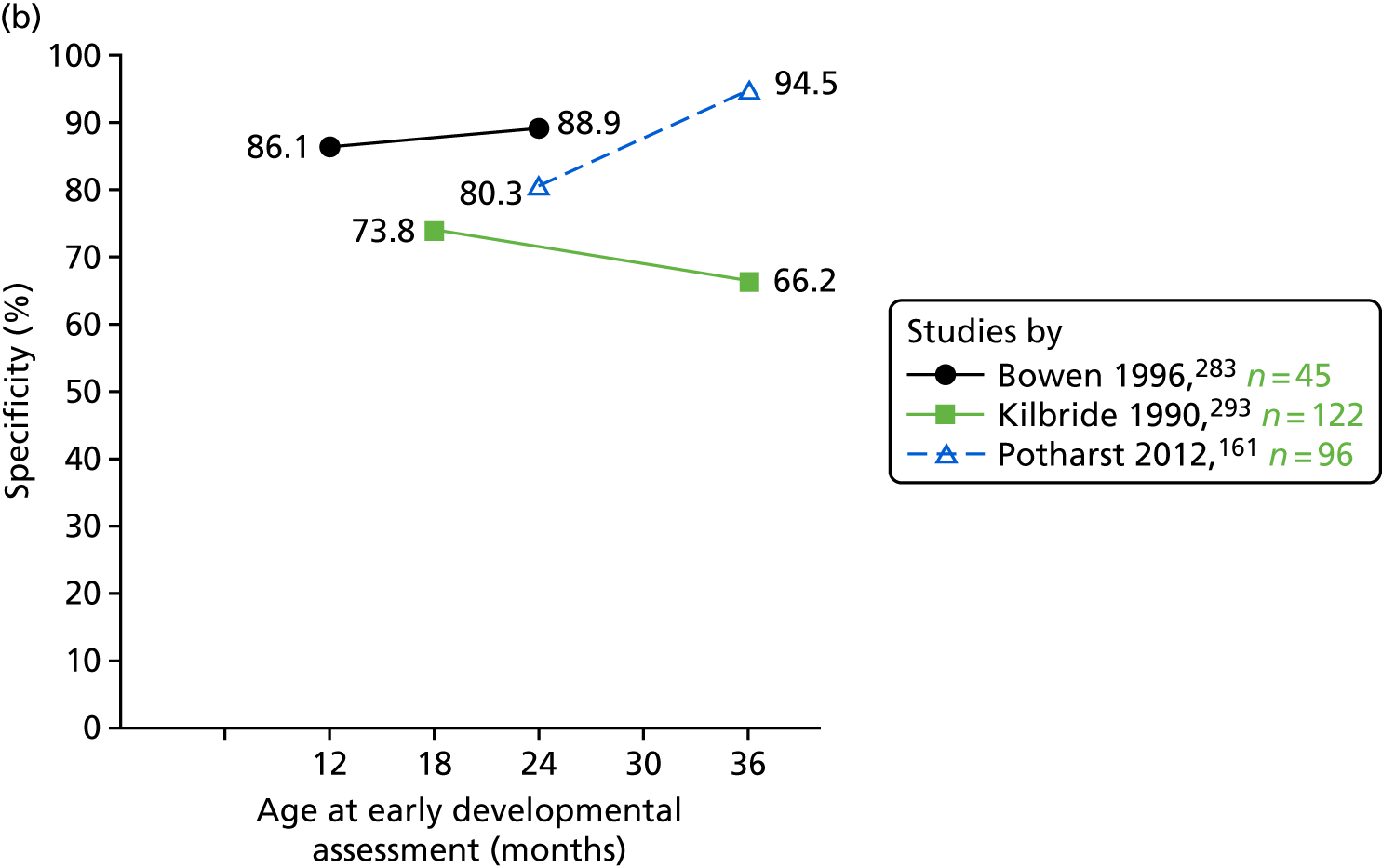
FIGURE 23.
Line graphs demonstrating the change in (a) sensitivity and (b) in specificity when school-age cognitive assessments were repeated at different ages in four studies.
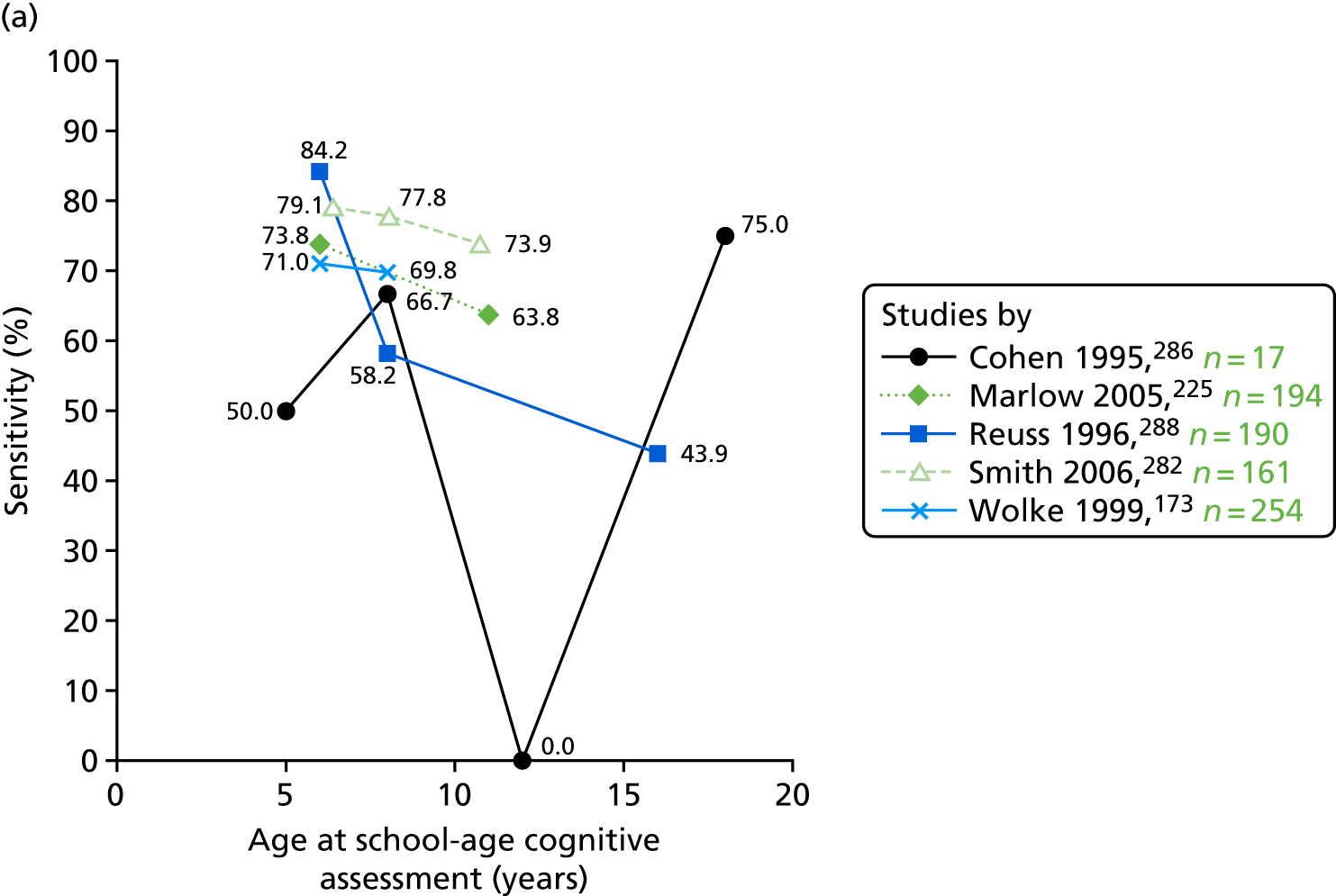
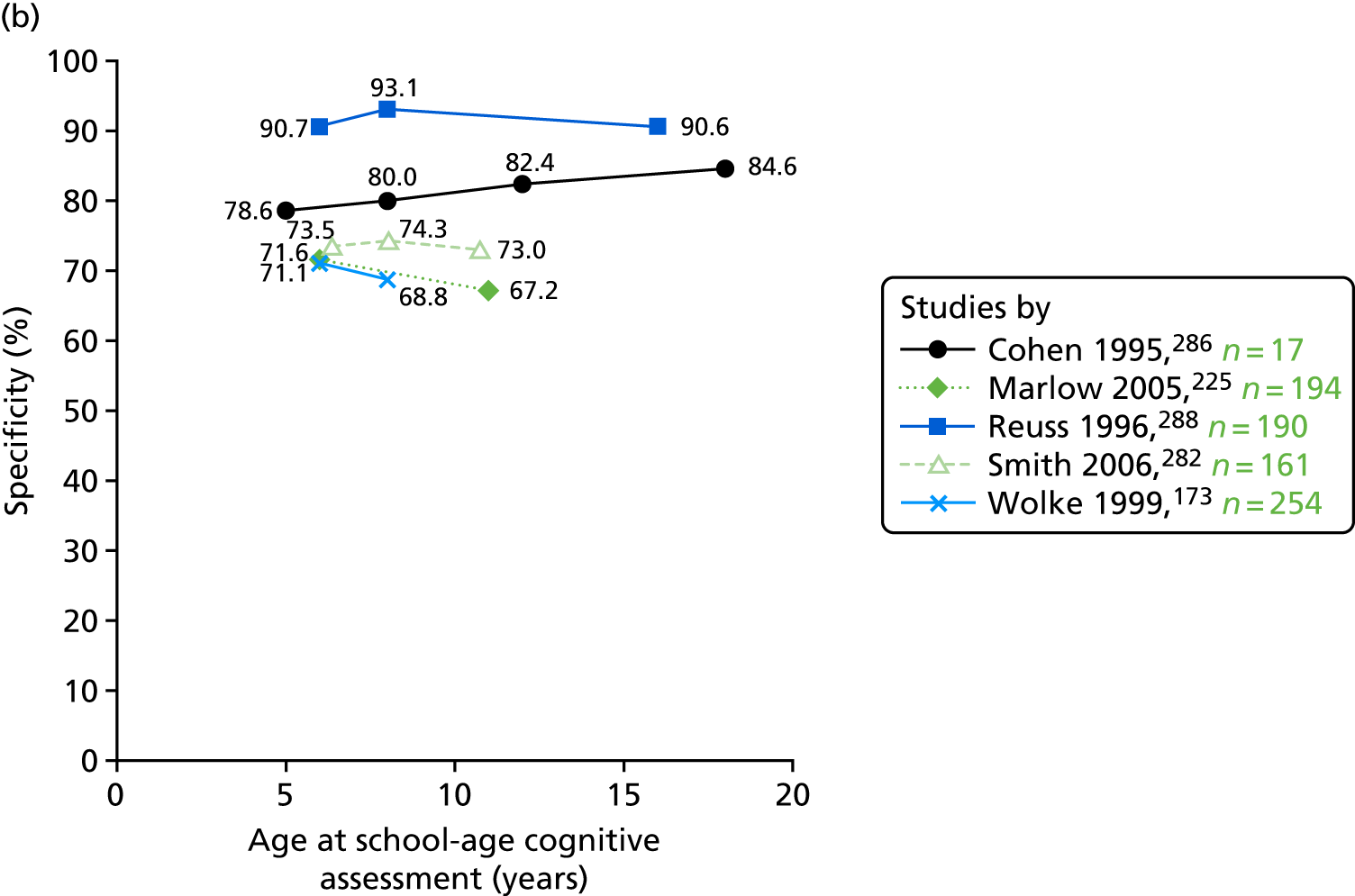
Metaregression: association of study-level variables with diagnostic validity
The ORs and 95% CIs, together with the corresponding p-values, for the association of study-level variables with sensitivity and specificity of identifying cognitive deficit by early developmental assessment are presented in Table 30. There was reduction in specificity with increased observed prevalence of impairment in the study population. For each 1% increase in cognitive impairment prevalence, the odds of identifying an additional ‘true-negative’ case among those with no cognitive impairment reduced by 3% (p = 0.01). The associations between mean gestational age and mean birthweight and specificity of identifying cognitive impairment reached borderline statistical significance (specificity increased with mean gestational age and mean birthweight of the study population). Post hoc analysis revealed no association between the prevalence of impairment reported in each study and the mean gestational age (p = 0.55) and mean birthweight (p = 0.95) of the study population; therefore, excluding the speculation that the observed association between specificity and mean gestational age and birthweight was mediated by the prevalence of impairment. The age at the assessments, the time interval between early and school-age assessments and the year of participant birth were not associated with sensitivity or specificity and, therefore, did not explain the heterogeneity present between studies.
| Study-level variable | Sensitivity | Specificity | p-value for joint test | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Mean gestational age (per 1-week increase) | 0.84 (0.68 to 1.04) | 0.11 | 1.29 (0.98 to 1.61) | 0.04 | 0.11 |
| Mean birthweight (per 100-g increase) | 0.86 (0.72 to 1.03) | 0.09 | 1.21 (1.00 to 1.48) | 0.05 | 0.14 |
| Mean age at early assessment (per 1-year increase) | 1.51 (0.77 to 2.98) | 0.22 | 0.79 (0.36 to 1.72) | 0.54 | 0.35 |
| Mean age at school-age assessment (per 1-year increase) | 0.98 (0.86 to 1.11) | 0.73 | 1.01 (0.88 to 1.17) | 0.86 | 0.90 |
| Mean time between assessments (per 1-year increase) | 0.97 (0.86 to 1.10) | 0.57 | 1.02 (0.89 to 1.17) | 0.78 | 0.82 |
| Year of birth (per 1-year increase) | 0.99 (0.97 to 1.01) | 0.291 | 0.99 (0.97 to 1.01) | 0.23 | 0.82 |
| Prevalence of impairment (per 1% increase) | 1.02 (0.99 to 1.04) | 0.16 | 0.97 (0.94 to 1.00) | 0.01 | 0.02 |
| Prevalence of severe impairment (per 1% increase) | 1.05 (0.99 to 1.12) | 0.12 | 0.93 (0.87 to 1.00) | 0.02 | 0.03 |
Funnel plot for sample size-related effects and publication bias
The funnel plot of the log-DOR against the inverse of the square root of the effect sample size is presented in Figure 24. Significance testing (ESS weighted regression test) confirmed that asymmetry was not present in the funnel plot (p = 0.22), indicating the absence of sample size-related effects in the meta-analysis.
FIGURE 24.
Funnel plot of the log-DOR against the inverse of the square root of the ESS, with pseudo-95% confidence limits.
Conclusions
Agreement between NHS and research-standard data
Among children who were born before 30 weeks’ gestation, the agreement in classifying neurodevelopmental status at the age of 2 years between data recorded during routine NHS assessments and those obtained through a research assessment was strong in the absence of neurodevelopment impairment. However, NHS assessments lack satisfactory sensitivity for identifying children with impairment, particularly in the cognitive and language domains. Using the Bayley-III scores as the ‘gold-standard’ tool, approximately 30% of children with at least mild cognitive impairments and nearly 50% with at least mild language impairments were falsely classified as having no impairment through NHS assessment. The discordance between NHS and research assessment remained, irrespective of the criteria used to categorise outcomes. This implies that the structural and content differences between the classification tools are unlikely to account for the discordances identified.
The strengths of the study include a single, carefully trained research assessor blinded to the results of the NHS assessment; the involvement of 13 hospitals serving patients from a wide range of ethnic, social, economic and cultural backgrounds; analyses that took into account the possibility of clustering by study sites and multiple births; and overestimation of abilities by the Bayley-III assessment. A limitation to the study was that the targeted sample size was not achieved. As the recruitment of participants occurred at a steady rate over the planned 2-year period, factors that may be relevant are the high population mobility in London, leading to loss to follow-up, and the large number of consultant and trainee doctors involved in outpatient follow-up clinics leading to missed opportunities to invite participation, because health professionals were unaware of the study. The sample size target was calculated with the desire to estimate the sensitivity of NHS assessment in correctly classifying children with severe impairment to a high precision, achieving a narrow 95% CI with half-widths within 10%. In addition to sample size, the precision was also dependent on the actual value of the sensitivity estimate: the lower the sensitivity, the wider the CI. As the estimated sensitivity for diagnosing severe overall impairment was low (52.0%), based on the 14.1% prevalence of severe impairment among the study participants, a sample of at least 680 children providing independent (unclustered) observations would be necessary to have achieved the intended precision. The increment in precision with increasing sample size followed the law of diminishing returns. It was therefore difficult to justify the continued provision of additional time and resources required to achieve the desired precision for the sensitivity estimate.
The study population differed from the population of very preterm children discharged from participating hospitals in that it consisted of proportionally more white children, who were less likely to have been mechanically ventilated, diagnosed with chronic lung disease (bronchopulmonary dysplasia) and/or were living in less deprived areas. It is possible, therefore, that the study population was at lower risk for neurodevelopmental impairment. The selection bias was introduced by attrition of children from routine NHS follow-up and the non-random recruitment method. In the literature, the proportions of VLBW children who were lost to follow-up or reviewed with difficulty in regional follow-up programmes were reported to be around 11–27% at 2 years296–298 and 25% at 5 years. 299 Characteristics associated with dropping out from follow-up included non-white ethnicity, young maternal age and low socioeconomic and maternal educational status. 297–300 Ideally, a random sample of participants selected from a known sampling frame (e.g. list of all children with scheduled follow-up appointments) would provide the most representative study cohort.
The presence of selection bias may have affected the accuracy of the estimated prevalence of impairment in the population. Traditionally, sensitivity and specificity are considered to be independent of disease prevalence. 301 Consequently, the adverse effect of selection bias on the validity of this study can be regarded as minimal. However, a number of studies have shown that variation in prevalence can result in either clinical or artefactual variation in test accuracy. 302,303 As it is probably easier to diagnose impairment in severe than in mild–moderate cases, a study population with a lower spectrum of impairment might have more false-negative or false-positive results.
Inter-rater variability in outcome assignment is likely to have been one of the main reasons for the disagreement between NHS assessments and research assessments. Clinical judgement is inevitably influenced by the assessor’s knowledge, experience, beliefs and preconceptions. Studies on behavioural psychology have shown that people tend to rely on judgemental heuristics (e.g. intuition), which are, by nature, unreliable, to simplify the complex task of assessing probabilities and predicting values to provide reasoning on the outcome of an event, such as the diagnosis of disability in a child. 304 The use of standardised assessment tools improves inter-rater agreement by establishing objective measures. Without using a standardised assessment, the judgement and interpretation of clinical findings may be highly variable. It is therefore unsurprising that, in a study comparing the diagnosis of cognitive impairment made using an intelligence test with judgements by paediatricians, the agreement was only fair (κ 0.39). Even if standardised assessments were used, the agreement between the different tools in classifying impairment was uncertain. Chaudhary et al. 305 reported that, at 22 months, children scored 5 points higher on the BSID-II MDI than on the Griffiths Scales developmental quotient. Furthermore, the interpretation and translation of the standardised assessment scores into the NPEU/Oxford classification instruction can still be inconsistent and subject to biases and errors.
Another difference between the conduct of the NHS assessments and research assessments was the reliance of the latter on parents’ reports on their child’s ability, particularly for language and cognitive skills. Parents are a valuable source of information in a time-restricted appointment, especially if the child does not engage in the assessment. However, studies investigating the level of agreement of neurodevelopmental status between parent and paediatrician evaluation have reported variable results. 244–246,248
Intrasubject (participant) variability in performance between assessments could also contribute to the discordance between NHS and research assessments. There are multiple factors, such as mood and ease of engagement of the participant, time of the day (meals/snacks or nap times) and environment, that can influence the children’s performance. Preterm children have been shown to be at risk of inattention/hyperactivity306,307 and social-emotional delays,308 which could manifest as inability to complete a task. The testing time is also generally longer for research assessments and it was not unusual for children to become tired during testing. These issues may not have been taken into account by the assessors and, specifically, the objective scoring of a standardised assessment would not have made allowance for underperformance because of these factors.
There are several possible reasons for the higher sensitivities for diagnosing motor impairment than for cognitive or language impairments at routine NHS assessments. Important motor developmental milestones (e.g. sitting and walking) are reached at a relatively young age and parents and health professionals place great emphasis on checking that children achieve these milestones. Cerebral palsy is the most commonly quoted morbidity of preterm birth; therefore, motor assessments are regularly performed at follow-up appointments. Cognitive and language skills can be difficult to ascertain in a single setting, particularly without the use of standardised assessment tools, and can be affected by the issues of judgement and reporting bias discussed above. Furthermore, in the modified NPEU/Oxford classification, the categorising of cognitive impairment by ‘number of months behind corrected age’ introduced another level of variability. In addition, in the electronic ‘2-year outcome’ form, the term ‘development’ was used as the heading for the cognitive domain. As a result, there was misinterpretation among the NHS assessors that the questions in that category applied to ‘overall development in all domains’ rather than being specific to cognitive function, potentially leading to misclassification.
The impact of inter-rater and intrasubject variability would be exacerbated by the classification of neurodevelopment skills, a continuous trait, into categories of ability or impairment. Levels of abilities or skills near the ‘cut-off’ point between categories are more difficult to discriminate and are at risk of being misclassified into higher or lower impairment categories.
Subgroup analyses were used to investigate whether or not the validity of the NHS assessment was affected by neonatal and sociodemographic factors, as well as factors related to the conduct of the assessments. However, given that the numbers of children with impairment (‘true positives’) within each subgroup were small, it was likely to that subgroup analyses were underpowered. Therefore, the possibility remains that the negative findings were a reflection of type II errors (‘false negatives’).
The Bayley-III was selected as the research assessment as it is the most commonly used assessment in neonatal outcome studies. However, the validity of the Bayley-III, particularly in identifying school-age outcomes, is unknown. Several studies have raised concerns that, when compared with the BSID-II, the Bayley-III was underestimating neurodevelopmental impairment. 202–205 It is, however, reassuring that validation studies of the Bayley-III showed that the scores are consistent with other revised ability tests such as the Wechsler Preschool and Primary Scale of Intelligence – Third Edition309 and the Preschool Language Scale – Fourth Edition. 201,310 In this study, more children were classified as being impaired using the predicted BSID-II MDI scores than using the Bayley-III scores if the same threshold were applied. Therefore, as expected, the sensitivities of the NHS assessment dropped when the predicted BSID-II MDI scores were used as the ‘gold standard’ instead of the Bayley-III scores.
Another issue that needs to be considered is the impact of administering the English-based Bayley-III assessment on cognitive and language scores in children whose primary language is not English. Although families who require interpretation for English were excluded, 52% of the study population was living in a bilingual environment. Studies that examined the effect of bilingualism on language acquisition have provided conflicting evidence,311–313 but testing bias cannot be ruled out. However, for a child who is functioning in the ‘severe impairment’ category, communication skills are assessed by the observation of gestures and the production of consonant and/or vowel sounds, which are not language specific, and, hence, the assessment of children with severe language impairment is likely to be valid. Testing bias can also occur in NHS assessments where there is likely to be greater reliance on parental reporting.
Social communication skills of children who were born very preterm
At 24 months corrected age, children who were born before 30 weeks’ gestation were rated by their parents on the Q-CHAT as having greater social communication difficulties and autistic traits than of the general population. The higher frequency in autistic traits was observed mainly in the areas of restricted, repetitive, stereotyped behaviour, communication and sensory abnormalities.
Previous studies have reported significantly higher odds of positive autism screening on the M-CHAT in children with motor, visual, hearing and cognitive impairments. 213,214 The Q-CHAT contains similar questions, leading to children with such disabilities receiving higher Q-CHAT scores. Therefore, it is likely that the distribution of Q-CHAT scores in a very preterm population would be even higher if children with cerebral palsy and severe neurosensory disabilities were included.
There was no sex difference in our study population. This may be due to insufficient statistical power, given that a sample size of 24,000 children would be required for the 0.3-point sex difference in Q-CHAT scores that we detected to be significantly different. Nevertheless, it has been suggested that the autistic phenotype seen in preterm children resembles more closely syndromic or medically explained autism, the sex ratio of which is closer to 1 : 1, than those with idiopathic autism,213 supporting the hypothesis that autism in preterm children, rather than being a primary deficit, represents part of a ‘preterm phenotype’ with different aetiology.
Preterm children experience difficulties across all aspects of autistic behaviour but particularly in the categories of restricted, repetitive, stereotyped behaviour, communication and sensory abnormalities. The presence of reduced language abilities among children who were born preterm is well described. 176,177 Dysfunction in sensory modulation in preterm children, characterised by either hyposensitivity or hypersensitivity to sensory input, is a problem anecdotally recognised by parents and clinicians. It is hypothesised that exposure to the stressful environment of the neonatal intensive care unit at a critical period of brain development in the third trimester interferes with the normal maturation of the sensory system. 314,315 Sensory modulation dysfunction is thought to be negatively associated with emotional development and can affect social interactive capabilities. 316
There is some evidence that restricted and repetitive behaviours are associated with cognitive status. 317,318 EPICure study investigators also concluded that cognitive deficits in their extremely preterm cohort accounted for the excess of repetitive and stereotyped behaviour when compared with the full-term controls. 319 Although there was no correlation between cognitive scores and subcategorical Q-CHAT scores in the restricted and repetitive behaviour domain, as the mean cognitive score of the preterm population was lower than would be expected in the general population. The potential association between cognition and restricted and repetitive behaviour could, in part, explain the higher Q-CHAT scores obtained by the participants in this category.
There were fewer differences between preterm children and the general population in response to items exploring social relatedness. Q-CHAT items exploring social relatedness may provide a higher degree of specificity for differentiating early autistic features from concurrent developmental delay in children without severe physical and neurosensory impairment compared with items in the other categories. Although parents reported a lower frequency of pretend play among the preterm children, development in joint attention (elucidated by questions on protodeclarative pointing and following a gaze) was similar in the general population. Focusing on elucidating social relatedness for autism screening in the preterm population may reduce the ‘false-positive’ screening rate associated with currently available screening tools.
The significant association between language ability at the age of 2 years and Q-CHAT scores was unsurprising, as four items on the Q-CHAT specifically examined language development. Furthermore, language ability was closely related to cognitive function, which, in turn, influenced performance on other Q-CHAT items. Separate cognitive and language scores were obtained from the Bayley-III assessment in this study. Language scores confounded and accounted for the association observed between cognitive scores and Q-CHAT scores.
This study also highlights the inter-relationship between ethnicity, area deprivation, language skills and Q-CHAT scores. Our findings suggest the possibility of an environmental impact of socioeconomic disadvantage on early social communication development.
The Q-CHAT, M-CHAT and the Bayley-III Social-Emotional are some of the developmental surveillance tools designed to identify toddlers at risk for developing ASD, with the aim of implementing timely intervention strategies to achieve better outcomes for these children. The M-CHAT has a sensitivity of 87% and specificity of 98% for ASD when applied in a mixed sample of children aged between 16 and 30 months. 211 The Bayley-III Social-Emotional questionnaire, using a scaled score of 6, reportedly had a sensitivity of 87.0% and specificity of 90.0% for the identification of ASD. 207 However, the predictive validity of these screening tools when applied to the preterm population has not been investigated. Furthermore, there is little understanding of the differences in properties of the available screening tools. Oosterling et al. 320 compared four instruments: the Early Screening of Autistic Traits Questionnaire;321–323 the Social Communication Questionnaire;323 the Communication and Symbolic Behaviour Scales-Developmental Profile, Infant-Toddler Checklist;216 and key items of the Checklist for Autism in Toddlers. 324 They found that no particular tool showed superior discriminating power for distinguishing children with ASD from those without.
Meta-analysis
The specificities of early neurodevelopmental assessment in predicting later school-age cognitive outcomes were generally high, especially for severe cognitive impairment, but sensitivities were inconsistent. Early neurodevelopmental assessment has low sensitivity and high specificity for identifying school-age cognitive deficit. This means that, when a neurodevelopmental impairment was diagnosed at ages 1–3 years, the likelihood of having cognitive deficit at school age was high (low false-positive rate, or 1 – specificity). However, it would not be possible to exclude later cognitive deficit even when an early assessment demonstrated normal neurodevelopmental outcomes (high false negative; or 1 – sensitivity). The results suggest that almost half the children who were thought to have normal neurodevelopmental function at ages 1–3 years will experience cognitive difficulties at school age. Even for cases of severe cognitive deficit, the accuracy in early detection was low (meta-analytic sensitivity of 39.2%). This finding is not unexpected. Cognitive function in infancy is a poor predictor of later IQ in the general population. 325 This may reflect changes in cognitive function during childhood, unveiling of deficits in complex task performance that are non-essential in early childhood, or the increasing effect of social and environmental influences on cognition over time. Other explanations may be the impact of behaviour and attention during testing at different ages, and differences in the contents and psychometric properties of early neurodevelopmental and later cognitive assessment tools.
The internal validity of this study is influenced by the quality of the data from the included studies as well as by the methods adopted. Data quality as appraised by the QUADAS-2 tool was good, with most studies considered to be at a low risk of bias. Nevertheless, the presence of missing data from participants who were lost to follow-up over time is a common problem affecting these longitudinal studies. Incomplete outcome ascertainment can distort the result in either direction. Another source of missing data arose from the exclusion of children with severe neurosensory and motor impairment who were unable to complete the assessments. If we assume that these children had stable diagnoses of severe neurodevelopmental and cognitive deficits throughout childhood, then the impact of excluding them from the study population would be an underestimation of the sensitivity of early neurodevelopmental assessments.
An additional bias that could affect accuracy, and which was not identified through the QUADAS-2 appraisal, is the experience of the assessors. Although all included studies employed trained assessors using standard assessment tools, interobserver differences are inevitable. Neurodevelopmental and cognitive abilities exist as a continuum but, for the purpose of the study, participants were dichotomised using a ‘cut-off’ score into groups ‘with impairment’ and ‘without impairment’. Interobserver variations around the ‘cut-off’ score would result in misclassification of outcomes. The effect of differential misclassification on the study results is difficult to predict but in general it can be expected to have a bigger impact on sensitivity, which is calculated using a small number of ‘positives’ in this condition of relative low prevalence, than on specificity, which is based on a large number of ‘negatives’.
Participants were included in the review if they fulfilled either the gestational age or the birthweight inclusion criterion. The birthweight criterion was used in order to capture all relevant studies, as it has been common for neonatal studies to base eligibility on birthweight rather than gestational age. However, the methodological bias in using a birthweight criterion is the inclusion of more mature but growth-restricted children. Notably, in the study by Bassan et al. 278 all the participants were small for gestational age (birthweight < 10th percentile for gestational age). Intrauterine growth restriction is a risk factor for poor neurodevelopmental outcome. 326 The QUADAS-2 appraisal highlighted the lack of applicability of older study populations and outdated assessment tools in more than half of the included studies and, hence, raises the question on the wider generalisability of the study findings. This is, of course, a reflection of the nature of all longitudinal studies but it is a significant limitation, particularly in the context of a rapidly advancing neonatal specialty. The past couple of decades have seen an overall reduction in the proportions of survivors of very preterm birth with adverse neurodevelopmental outcomes at the age of 2 years;231,327,328 therefore, we can expect the characteristics of the current preterm population to be different to those from past eras. Only 14 of the 24 included studies recruited participants born after 1990 and none was born in the previous 10 years (i.e. after 2004).
More importantly, the assessment tools used in the included studies, although validated and contemporary at the time of each study, have mostly been superseded by newer editions. Therefore, caution should be exercised when extrapolating results based on earlier versions of assessment tools to current practice. The timing and setting of the assessments also played a part in determining the external validity of the study findings. The early neurodevelopmental assessments were performed between 12 and 36 months and the timings matched common clinical practice. School-age assessments were mostly conducted between the ages of 5 and 8 years, when children were at the primary stages of schooling. Only three studies reported cognitive assessment during adolescence, one of which had only 20 participants. Therefore, the validity of early assessment in diagnosing cognitive deficit extending into adulthood could not be estimated from this study, although one could speculate that the sensitivity might be even poorer. As the sensitivity estimates from individual studies were based on a small number of participants with cognitive impairment, the corresponding 95% CIs were very wide. The use of a meta-analytic approach increases the sample size and improves the precision of the pooled estimate.
The review was restricted to English-language literature. There is concern that the English-language journals publish a skewed sample of studies that report positive and more noteworthy results. 329 Similarly, it is common for articles with negative or inconclusive findings to remain unpublished. The exclusion of grey literature, including abstracts and dissertations, could have led to the omission of essential and more recent information.
Heterogeneity between studies was investigated using metaregression. This method has a few drawbacks. The statistical power to detect associations between the study estimates and the explanatory variables is related to the magnitude of the relationship between them, and is typically considered low in metaregression. 330 This was compounded by the narrow range of values available for each of the explanatory variables under evaluation. For example, the mean gestational age of the included studies ranged only between 25.9 and 33.1 weeks. Hence, a type II error could not be excluded. More importantly, metaregression is subject to ecological fallacy (or aggregation bias) (i.e. the mistaken assumption that a statistical between-study relationship based on aggregated data reflects a within-study relationship). Therefore, in order to reliably identify factors that influence the validity of early developmental assessments, it would be necessary to obtain individual patient-level data.
In conclusion, early neurodevelopmental assessment has high specificity but low sensitivity in identifying later school-age cognitive deficit.
Implications of results
Clinical relevance of results
Routine NHS assessments have low sensitivity for identifying mild to moderate neurodevelopmental impairment. This has significant clinical implications. At an individual level, children with impairment may be missed. At a population level, current documentation of 2-year outcomes during routine NHS assessments, using the standardised EPR in its present format, will underestimate the proportion of children with impairment, compared with a research-standard Bayley-III assessment. Many neonatal networks and units rely on routine follow-up for impairment rates of their graduates. The results of this study question the validity of these practices.
The findings of higher Q-CHAT scores in the preterm population suggest that suboptimal development of social communication skills exists from early childhood. The 7-point right-shift in mean Q-CHAT score of the preterm population corresponds to nearly 1 SD difference. As ASD exists on a continuum, with autism representing the extreme end of the spectrum, the results also support the likelihood that a large proportion of preterm children experience clinically significant social communication difficulties below the diagnostic threshold for ASD from a young age, when early intervention may be possible. The findings draw attention to the need for better understanding and potentially early assessment of social communication skills in the preterm population.
The results from the systematic review and meta-analysis confirm that a significant proportion of children who were born very preterm and who are assessed as having normal neurodevelopment in early childhood go on to experience cognitive difficulties later in school. The implications of this finding on current clinical practice are considerable because neurodevelopmental assessment at 2 or 3 years of age is often used as the end point for post-discharge follow-up of very preterm infants. Outcome data used in discussions with parents during the antenatal and neonatal periods are commonly based on neurodevelopmental outcomes determined in early childhood. Given these findings, it is essential to discuss potential difficulties at school that children may face, even in the absence of obvious impairment or disability at the 2-year assessment.
Reassuringly, the false-positive rate for early diagnosis of impairment was low, indicating that children with more severe impairments, who would receive greater benefit from early intervention, will be correctly identified.
Implications for health care
There are advantages in the current practice of embedding neurodevelopmental follow-up of very preterm children with neonatal services. These include the continued involvement of health professionals known to the families and local flexibility in organisation. However, this also risks regional variation in follow-up criteria, reliability assessments and quality of data recording. Standardising the neurodevelopmental tool and ensuring that staff are trained in its use during follow-up assessment would be an obvious way of minimising some of this variability. Such a tool should have strong psychometric properties, be user friendly and, ideally, be adaptable for use in non-English-speaking patients. Since this study, the NICE guideline331 for developmental follow-up of children and young people born preterm has been published. The guideline recommends that the PARCA-R parent-completed questionnaire is used to identify children at risk of developmental delay. Misclassification occurs during categorisation of outcomes; hence, the strategy of presenting outcome data in categories should also be further considered. Categorical outcomes are easy to interpret and to communicate, and mirror clinical practice (e.g. referral of children below a certain threshold for further assessment or intervention). However, for an individual child, the labelling of ‘outcome category’ is unhelpful. Besides, as shown, categories of outcomes do not remain stable over time. It is, arguably, more valuable to present the distribution of standardised scores.
Under the UK Healthy Child Programme, all children receive health visitor-led developmental screening. There has been some interest in extending the roles of health visitors to capture developmental outcome data of children who were born preterm, assessed using developmental screening tools or through questions similar to those listed on the electronic ‘2-year outcome forms’ (NPEU/Oxford criteria). 332 The findings of this study indicate a need for caution in this approach. Even with the use of developmental screening tools, the false-negative rates (sensitivities) are unacceptably high. Other factors, such as shortage of health visitors, requirement for further training and lack of universal uptake of the screening programme, might further limit success.
Based on the findings of this study, a centralised approach to the assessment and recording of 2-year outcome data for children who were born very preterm is worthy of consideration. Typically, very preterm children are offered post-discharge appointments every 3 to 6 months; these visits might be conducted at hospitals where allied health professional support (e.g. dietetics, physiotherapy) can be sought if necessary. There may be advantages for the 2-year neurodevelopmental assessment to be organised at neonatal network level, as this could ensure that each child receives assessment by an appropriately trained team of health professionals using standardised tools, and could benefit from centralised, co-ordinated administrative support to trace and contact families.
In addition to being of high quality, the data recorded during clinical care should be complete to enable meaningful analysis. Currently, the utility of the routinely recorded electronic clinical data as a source of population-based outcome information is limited by poor data completeness. According to the National Neonatal Audit Programme report, 2-year outcome data were available from only 44% of all infants born before 30 weeks’ gestation in England and Wales between July 2010 and June 2011. Strategies to reduce missing data need to be aimed at clinician engagement.
Since 2007, the American Academy of Pediatrics (AAP) has recommended ASD-specific screening at 18 months for all children to facilitate early diagnosis and to prevent delay in the initiation of early intervention. 333 The UK National Screening Committee does not currently recommend universal screening, on the basis that none of the available screening tools has sufficient reliability in identifying children at risk for ASD when applied to the general population. 334 Regardless of an ASD diagnosis, toddlers who were born preterm experience problems in current functioning that may interfere with adaptive exploration and social engagement. It is important that clinicians recognise these difficulties and the impact that they have on families. Parents require information on the social communication difficulties that preterm children experience, particularly as some of these behaviours may be amenable to specific interventions, such as speech and language, occupation and sensory integration therapies, as well as educational programmes targeted at enhancing communication, social skills instruction and reducing interfering maladaptive behaviours. 335
Research recommendations
Improve the electronic ‘2-year outcome’ form
In the absence of the standardised use of a single assessment tool to allow comparison of outcomes between centres, improvements to routine data recording could be sought. On the electronic ‘2-year outcome’ form, the documentation of outcomes in the cognitive and language domains is more subjective than in the motor domains. It is possible that, by modifying the form to increase the objectivity of the items recorded, the validity of the data would be improved. For example, for the cognitive domain, it may be possible to identify a standardised set of cognitive test items, perhaps from the Bayley-III assessment or other tools that can be easily administered in a clinical setting. Language function can be ascertained by determining if a child can identify or say words from a list of commonly expressed words.
The NICE guidelines331 for developmental follow-up of children and young people born preterm recommend the use of the validated parent questionnaire PARCA-R to identify children at risk of global developmental delay, learning disability or language problems, and for the PARCA-R scores to be documented in the NNRD. The predictive validity of the PARCA-R at the age of 2 years in identifying impairments at a later age and special educational needs to be evaluated. The guidelines also recommend using different approaches, such as e-mails or text messages, to provide enhanced developmental support. The utility of these approaches in assessment and outcome data acquisition should also be explored.
Comprehensive behavioural assessment and identification of risk factors for ASD in the preterm population
As stated in Types of neurodevelopmental outcome measures, preterm children are at a higher risk of a range of behavioural problems, including ADHD and internalising behaviour, that were not examined in this study. The age at emergence of these behavioural difficulties is unclear and should be examined in future studies. Future studies would also benefit from an examination of a more comprehensive set of neonatal and environmental variables in order to identify potential moderators and mediators of risk for ASD and other behavioural difficulties. It would then be possible to develop risk scores or risk prediction models that could aid in early diagnosis and initiation of interventional therapies. Future studies will also need to focus on the challenges faced in the early assessment of behavioural features of children with major functional disabilities and of children in non-English-speaking groups.
Linkage with school-age outcome data
Currently, there is no process or provision in the UK for continuing formal follow-up assessment beyond early childhood. Long-term programmes require significant manpower and financial investment and the likelihood of high attrition rates will further jeopardise success. Therefore, it is worthwhile considering other sources of school-age outcome data, for example primary care or community child health records, or educational data. UK national structures provide a unique opportunity for data linkage. Future research could investigate the utility of these data sources through linkage of neonatal data with later outcomes.
Chapter 6 Using the National Neonatal Research Database to inform economic evaluations of neonatal interventions
Abstract
Background: Computerised record linkage with EPRs is increasingly considered a means of obtaining primary or complementary resource use data for the purposes of health economic evaluation and more broadly for health technology assessments. We addressed whether or not reliable trial-based economic evaluations can be conducted utilising data from the NNRD.
Methods: The Probiotic in Preterm babies Study (PiPS) (a multicentre, double-blind, placebo-controlled, randomised trial in infants born between 23+0 and 30+6 weeks gestational age) was used as the test bed. Health-care resource utilisation data were extracted from the PiPS trial case report forms (CRFs), the NNRD and a combined data source, and were primarily valued using national tariffs for 2012–13. 336 Differences in economic outcomes were estimated (1) within trial by data source, thereby allowing us to draw comparisons between the probiotic and the placebo, and (2) by pooling data between trial arms, thereby allowing comparisons between the alternative data sources.
Results: Within-trial comparisons of resource use and costs revealed no statistically significant differences between the trial comparators for any resource input or cost category, regardless of data source. Across-trial tests of concordance in resource use and costs between comparator data sources revealed high levels of agreement for the majority of categories of resource use or cost and the total cost of neonatal care. Comparisons of cost-effectiveness outcomes between data sources revealed low probabilities of miscoverage of incremental net monetary benefit between the alternative data sources when the NNRD acted as the sole source of information.
Conclusions: This empirical investigation demonstrates proof of principle for the potential of the NNRD as a data source for neonatal trial-based economic evaluations in the UK. This has potential to reduce costs and improve the efficiency of economic evaluations. Research assessing the utility of the NNRD across a wider range of trial-based economic evaluations and alternative study designs are logical and are the important next steps.
Background
Economic evaluation involves the comparative analysis of alternative programmes or interventions in terms of their costs and consequences. 337 In order to estimate the total cost for an individual patient included in single study-based economic evaluations, such as trial-based economic evaluations, the quantity of each resource item they use is multiplied by the unit cost of that item and the product calculated. The resources used by patients, such as hospital admissions, consultations and types and quantities of drugs administered, are normally recorded for each patient over the time horizon of the study. The categories of resource use that are included in the study are determined by the perspective of the analysis. The main alternatives are to confine the perspective to the health-care system (sometimes referred to as the ‘payer’) or to include broader societal costs. The former perspective typically covers direct medical care, comprising the intervention being evaluated, treatment of any side effects or complications of treatment, and follow-up care. It may also include medical care not directly associated with the underlying condition, although regression modelling may be required at the analytical stage to disentangle background ‘noise’ that often occurs when this is included. 338 The societal perspective also considers care provided by other sectors of the economy, costs incurred by patients, informal care provided by family and friends, and productivity losses from morbidity and premature death. Methodological guidelines for economic evaluation differ in their recommended perspective for the analysis. As a minimum, it is recommended that analysts adopt a health system perspective, which is currently considered to include the NHS and Personal Social Services in England and Wales. 339
In single study-based economic evaluations, such as trial-based economic evaluations, many resources used can normally be recorded on study CRFs with little or no additional burden, but sometimes additional information will be required from medical records, patient questionnaires and diaries, and other sources. 340 A recent trial-based economic evaluation of neonatal extracorporeal membrane oxygenation necessitated observational research to estimate resource use associated with complications, and parent-completed questionnaires to document post-neonatal discharge hospital and community health service use. 341 Increasingly, however, computerised record linkage with data from EPRs is being considered as a means of obtaining primary or complementary resource use data for the purposes of health economic evaluation and more broadly for the purposes of health technology assessment. In principle, the successful development of systems for extracting resource utilisation data from EPRs should reduce the complexity, time and cost of conduct of trial-based economic evaluations, and offer considerable additional utility for NHS commissioning and service management.
Aims
We aimed to assess whether or not reliable health service utilisation data can be obtained from the NNRD and if these data can be used to inform future trial-based economic evaluations of neonatal interventions.
Methods
Overview
The study population for this empirical investigation comprised infant participants in the Probiotic in Preterm babies Study (PiPS). For each study infant, health-care resource utilisation was measured using three primary data sources: (1) the PiPS trial CRFs, (2) the NNRD; and (3) a data source that combined information from both PiPS trial CRFs and the NNRD.
Resource inputs captured by each data source were primarily valued using national tariffs and expressed in Great British pounds (GBP) (2012/13 prices). In our empirical investigation we sought to estimate (1) the level of agreement for hospital resource utilisation and costs between the alternative data sources and (2) the level of precision of incremental cost-effectiveness for the probiotic evaluated in PiPS using alternative data sources.
PiPS trial: design
PiPS was a multicentre, double-blind, placebo-controlled, randomised trial of probiotic administration in infants born between 23+0 and 30+6 weeks gestational age. Infants were recruited within 48 hours of birth from 24 hospitals within 60 miles of London over a 37-month period from July 2010 onwards. They were randomised to either the probiotic (given in a daily oral dose of 8.3–8.8 log10 colony-forming units) or the placebo (provided as an identical powder in identical sachets, until 36 weeks postmenstrual age or discharge from hospital, if sooner). There were three primary outcomes: any episode of neonatal NEC Bell stage II or III;112 any positive blood culture of an organism not recognised as a skin commensal on a sample drawn > 72 hours after birth and < 46 weeks postmenstrual age or discharge if sooner (hereafter sepsis for brevity); and death before discharge from hospital. Secondary outcomes included a composite of the three primary outcomes. The trial was sized (n = 1300) to detect a 40% relative risk reduction from 15% to 9.1% for each of the primary outcomes at a two-sided significance level of 5% and with 90% power. PiPS was approved by a national research ethics committee and co-ordinated by the NPEU, University of Oxford. Further details about PiPS, sampling procedures, methodology, outcome measures and responses rates are reported in full elsewhere. 342
PiPS trial: measurement of resource use and costs
A comprehensive profile of resource inputs was integrated at the outset into the PiPS trial CRFs. There were four main trial CRFs: (1) form 1: entry, (2) form 2: daily data collection, (3) form 3: transfer/discharge and (4) form 4: abdominal pathology. The bulk of the relevant resource inputs were captured by the second and third of these trial CRFs. The forms captured a comprehensive profile of resource use by each infant, encompassing length of stay by intensity of care, surgeries, investigations, procedures, transfers and post-mortem examinations until final hospital discharge or death (whichever was earliest). Resource inputs were primarily valued based on data collated from secondary national tariff sets343,344 (Table 31). All costs were expressed in GBP and reflected values for the financial year 2012/13.
| Resource use variable | Unit cost | Source | Notes |
|---|---|---|---|
| Resource use variables in the PiPS data set | |||
| Vaginal birth – cephalic | 1337.31 | NHS Reference Costs 2012–13 343 | |
| Vaginal birth – breech | 2488.33 | NHS Reference Costs 2012–13 343 | |
| Vaginal birth – other presentation | 1958.75 | NHS Reference Costs 2012–13 343 | |
| Caesarean section before onset of labour | 2950.40 | NHS Reference Costs 2012–13 343 | |
| Caesarean section after onset of labour | 3690.41 | NHS Reference Costs 2012–13 343 | |
| Coroner/hospital | 649.66 | Birthplace report345 | Inflated to 2012/13 prices |
| Neonatal critical care transportation | 1370.37 | NHS Reference Costs 2012–13 343 | HRG code XA06Z |
| Cranial ultrasound scan | 53.84 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes RA23Z and RA24Z |
| ROP | 994.97 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA64A, PA64B and PA64C |
| ROP screen | 134.11 | Unit Costs of Health and Social Care 2012 344 | Assumed nurse input (20 minutes valued at £100 per hour) and consultant input (30 minutes valued at £201.55 per hour) |
| PDA | 2422.50 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA23A and PA23B |
| Repair of inguinal hernia (weighted average cost) | 1250.17 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA25A, PA25B, PA26A and PA26B |
| Insertion of ventricular reservoir | 2922.72 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes AA15C, AA15D and AA15E |
| NEC treatment, peritoneal drainage/laparotomy no enterostomy/laparotomy with enterostomy | 2458.01 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA25A and PA25B |
| Resource use variables in the NNRD data set | |||
| Emergency caesarean section – not in labour | 2784.30 | NHS Reference Costs 2012–13 343 | |
| Emergency caesarean section – in labour | 3269.02 | NHS Reference Costs 2012–13 343 | |
| Elective section – not in labour | 2784.30 | NHS Reference Costs 2012–13 343 | |
| Elective section – in labour | 3269.02 | NHS Reference Costs 2012–13 343 | |
| Vaginal – forceps assisted | 2248.64 | NHS Reference Costs 2012–13 343 | |
| Vaginal – spontaneous | 1337.31 | NHS Reference Costs 2012–13 343 | |
| Vaginal – ventouse assisted | 2248.64 | NHS Reference Costs 2012–13 343 | |
| Coroner/hospital | 649.66 | Birthplace report345 | |
| Neonatal critical care transportation | 1370.37 | NHS Reference Costs 2012–13 343 | |
| Cranial ultrasound scan | 53.84 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes RA23Z and RA24Z |
| ROP | 994.97 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA64A, PA64B and PA64C |
| ROP screen | 134.11 | Unit Costs of Health and Social Care 2012 344 | Assumed nurse input (20 minutes valued at £100 per hour) and consultant input (30 minutes valued at £201.55 per hour) |
| PDA | 2422.50 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA23A and PA23B |
| Insertion of ventriculoperitoneal shunt | 7563.54 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes AA15C, AA15D and AA15E |
| Inguinal herniotomy (bilateral) | 1250.17 | NHS Reference Costs 2012–13 343 | Weighted average of HRG codes PA25A, PA25B, PA26A and PA26B |
The total length of stay (total inpatient hospital days) was computed as the total number of hospital days until final discharge to home or death. Information was available on time spent in the neonatal unit by level of care (normal, transitional, special, high dependency or intensive). The cost of routine neonatal care was calculated for each infant by multiplying the length of stay by intensity (intensive, high dependency, special care, transitional) by the per diem cost of the respective level of care using data from the NHS Reference Costs trusts schedule 2012/13. 343 Non-routine investigations excluded from these per diem costs were valued using a combination of primary and secondary costs. The costs of surgeries were calculated by assignment of surgical procedures to relevant Healthcare Resource Group (HRG) codes and application of unit costs from national tariffs. Transfers were recorded whenever an infant was transported between specialist hospitals for neonatal critical care, and were valued using costs from the NHS Reference Costs trusts schedule 2010/11,336 and inflated using a health care specific pay and prices index to 2012/13 prices. Post-mortem costs were based on data from secondary sources. 345 Where costs of additional non-routine investigations excluded from per diem values for neonatal care were not available from national tariffs, clinicians were asked to identify the staff and material inputs required for these investigations. Staff time was valued using the Unit Costs of Health and Social Care 2012344 tariffs.
Linkage and data extraction from the National Neonatal Research Database
In order to compare resource use, cost and cost-effectiveness estimates based on data solely extracted from the PiPS trial CRFs and data solely extracted from the NNRD, a NNRD extract was created for infants participating in the PiPS trial. The NNRD has been created through the collaborative efforts of neonatal services across the country to be a national resource. The NNRD contains a defined set of data items (the Neonatal Data Set) that have been extracted from the Badger.net neonatal EPR of all admissions to NHS neonatal units. Badger.net is managed by Clevermed Ltd, an authorised NHS hosting company. The Neonatal Data Set is an approved NHS Information Standard (SCCI1575). Contributing neonatal units are known as the UK Neonatal Collaborative.
The trial co-ordinating centre for the PiPS trial, namely the NPEU at the University of Oxford, provided the NDAU at Imperial College with the final PiPS data set. This included data on 1310 of a total of 1315 infants recruited into the PiPS trial (five infants were excluded because of withdrawals from the study). Clevermed Ltd, the NHS hosting company, which separately receives data from individual neonatal units, was able to match Badger IDs to NHS numbers for 1280 (98%) of the 1310 infants (Figure 25). These 1280 infants had 2360 episodes of care which were linked to episodes in the NNRD using the Badger ID and hospital name. Episodes were renumbered on both databases as necessary to provide linkage. A total of 81 episodes that were effectively the second episode of care on the NNRD were renumbered as the first episode to match the PiPS episodes, because the infants were recruited into the PiPS trial after transfer from the neonatal unit at the hospital of birth. Similarly, 103 episodes of care that existed on the PiPS database but not on the NNRD were excluded from comparison; these largely occurred in non-Badger hospitals or wards. This resulted in the exclusion of all episodes of care from 22 infants, leaving 2257 episodes of care from 1258 infants eligible for our comparative analyses (see Figure 25).
FIGURE 25.
Linkage between PiPS trial data and the NNRD.
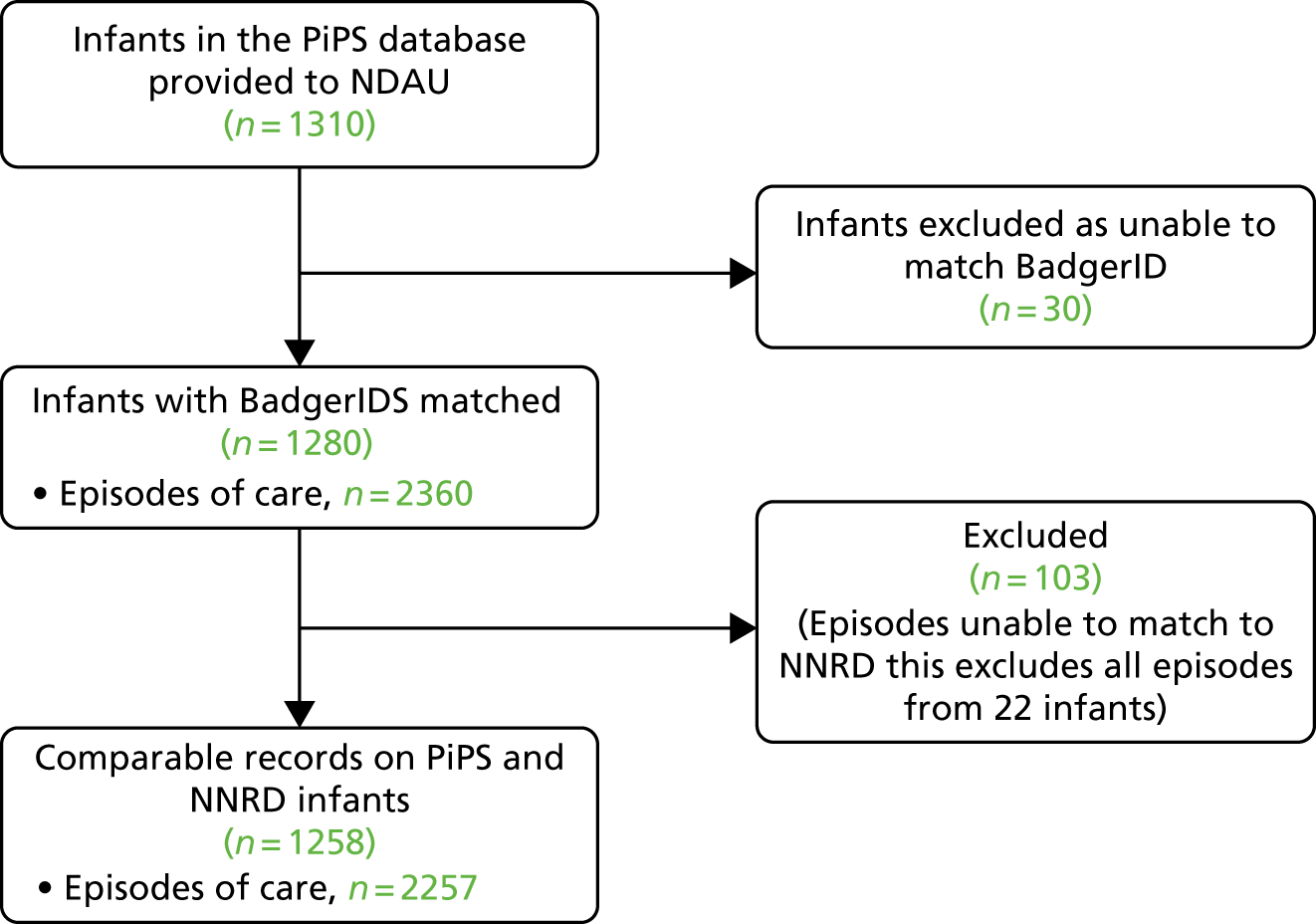
A comprehensive profile of resource use between randomisation into the PiPS trial until final hospital discharge or death (whichever was earliest) was compiled from the NNRD for the 1258 infants eligible for our comparative analyses. Our direct comparisons of resource use estimates between the PiPS and NNRD data sources required a further process of data manipulation to reconcile definitional and labelling differences for individual variables between the data sources, and coding differences by episodic and infant level. For example, information on surgery for PDA, medical treatment of PDA using ibuprofen or indometacin, and ROP treatment whether by laser or cryotherapy, can appear in multiple locations in the NNRD (e.g. discharge diagnoses, daily data, ad hoc forms). An overall summary of the interventions received during linkable episodes of care was generated on a ‘by infant’ rather than episodic level.
Statistical methods
A comprehensive statistical analysis plan was followed. All statistical analyses were performed using Stata or R (version 2.01).
The clinical and sociodemographic characteristics of the PiPS participants who were (n = 1258) and were not (n = 52) included in our comparative analyses of resource use, costs and cost-effectiveness were compared using the chi-squared test. Differences in resource use and costs, by category, were estimated (1) within trial by data source, thereby allowing us to draw comparisons between the probiotic and placebo arms, and (2) by pooling data between the trial arms, thereby allowing us to draw comparisons between the alternative data sources. In addition to data solely extracted from the PiPS trial CRFs and data solely extracted from the NNRD, we created a third data source for these comparative analyses. The third data source, hereafter termed the ‘combined’ data source for brevity, was constructed by selecting the preferred data source for each resource variable in terms of volume and granularity of information provided. It broadly followed the processes described in Chapter 4. The selection process for each resource variable was undertaken by the clinical investigators (KC, CB). For comparisons within trial by data source, differences in resource use and costs, by resource category, were tested using the independent-sample t-test for continuous variables, the chi-squared test for categorical variables and the Mann–Whitney U-test for medians. For comparisons between the alternative data sources, the levels of agreement in resource use and cost estimates, by category, for alternative combinations of data sources (PiPS vs. NNRD, PiPS vs. combined, NNRD vs. combined) were estimated using the Lin concordance correlation coefficient. 346 This statistic measures the agreement between two continuous variables obtained by two methods; the value of the statistic lies between 1 (perfect agreement) and –1 (perfect inverse agreement). A threshold 0.40 value for the statistic was adopted to indicate acceptable clinical or practical significance. 347 In addition, we estimated mean differences and 95% CIs to identify potential systematic biases, and the 95% limits of agreement, indicating random variation between individual measurements. 348
We additionally performed an economic evaluation of the probiotic. For comparisons within trial by data source, the economic evaluation took the form of an incremental cost-effectiveness analysis in which we estimated the incremental costs (ΔC) and incremental effects (ΔE) attributable to the probiotic in very preterm infants, with reference to the placebo. The results were primarily expressed each in terms of an incremental cost-effectiveness ratio (ICER) (ΔC/ΔE). Estimates of incremental cost-effectiveness were made for each of the three primary clinical outcomes (any episode of NEC Bell stage II or III, any case of sepsis, death before discharge from hospital), and for the composite secondary outcome. The economic evaluation was conducted from a health system perspective. 339 The time horizon for the economic evaluation was the period between trial randomisation and final hospital discharge or death, whichever was earlier. Non-parametric bootstrapping, involving 1000 bias-corrected replications of each of the ICERs, was used to calculate uncertainty around all cost-effectiveness estimates. This was represented on four quadrant cost-effectiveness planes. 349 Decision uncertainty was addressed by estimating net benefit statistics and constructing cost-effectiveness acceptability curves (CEACs) across cost-effectiveness threshold values (λ) of between £0 and £70,000 for the health outcomes of interest. The probability that the probiotic is less costly or more effective than the placebo was based on the proportion of bootstrap replicates that had negative incremental costs or positive incremental health benefits, respectively. A series of prespecified subgroup analyses repeated all analyses by selected subgroups (sex, birthweight, gestational age, colonisation status, randomisation age) for the primary and secondary cost-effectiveness outcomes.
For comparisons of cost-effectiveness outcomes between the alternative data sources, we estimated the overall probability of miscoverage of incremental net monetary benefit based on resource use data solely extracted from the NNRD and resource use data solely extracted from the PiPS trial CRFs. In order to estimate miscoverage for incremental net monetary benefit, the bootstrap replications of each of the ICERs were rearranged on a linear scale using the formula:
The miscoverage statistic was estimated as the percentage of bootstrap samples of incremental net monetary benefit that fell outside the CI for the reference data source. 350 For the purpose of these analyses, the combined data source acted as the referent, although we additionally assumed that the PiPS data source acted as a referent for analyses of data solely extracted from the NNRD. This was replicated for the primary and secondary outcomes of interest and for all prespecified subgroup analyses.
Finally, we performed sensitivity analyses that compared the key outputs of the economic evaluation using either resource use data and clinical outcomes extracted solely from the PiPS data set or resource use data and clinical outcomes extracted solely from the NNRD. These analyses were restricted to the two clinical outcomes used in the PiPS trial that were available in both data sources, namely (1) death before discharge from hospital and (2) sepsis. They acted as exemplars of the likely differences in economic outcomes that will be observed if we rely on the NNRD as a complete source of information (resource inputs, clinical outcomes) for an economic evaluation. The cost-effectiveness outcomes considered by these sensitivity analyses included estimates of incremental cost-effectiveness, probabilities of cost-effectiveness for the probiotic at alternative cost-effectiveness thresholds, and miscoverage of incremental net monetary benefit against the PiPS trial referent.
Results
Study population
A total of 1315 infants were recruited from 24 hospitals within 60 miles of London over 37 months, from July 2010 onwards. Data for 1310 infants were available for analysis in the PIPS trial. Of these 1310 infants, 52 infants were excluded from our empirical investigations of either because failure to match their Badger IDs to their NHS numbers or because they received part of their neonatal care in non-Badger hospitals or wards. A total of 1258 infants were therefore eligible for our comparative analyses. There were no significant differences between the baseline clinical and sociodemographic characteristics of the 1258 infants included in the analyses and the 52 infants excluded from the analyses, regardless of the use of NNRD or PiPS data (Table 32).
| Variable | Infants | ||
|---|---|---|---|
| Included | Excludeda (N = 52) | ||
| PiPS data (N = 1258) | NNRD data (N = 1258) | ||
| Gestational age (weeks), n (%) | |||
| < 28 | 602 (47.8) | 599 (47.6) | 32 (61.5) |
| ≥ 28 | 656 (52.1) | 658 (52.3) | 20 (38.5) |
| Birthweight (g) | |||
| Mean (SD) | 1043 (315.9) | 1042 (314.2) | 993.6 (268.6) |
| Birthweight of ≤ 1000 g, n (%) | 613 (48.7) | 613 (48.9) | 31 (59.6) |
| Birthweight of > 1000 g, n (%) | 645 (51.3) | 643 (51.1) | 21 (40.4) |
| Sex, n (%) | |||
| Boys | 709 (56.4) | 707 (56.2) | 34 (67.3) |
| Girls | 549 (43.6) | 549 (43.6) | 17 (33.7) |
| Unknown | 0 (0) | 1 (0.08) | 0 (0) |
| Multiplicity, n (%) | |||
| Singleton | 879 (69.8) | 885 (70.4) | 37 (71.2) |
| Multiple | 379 (30.2) | 372 (29.6) | 15 (28.8) |
| Apgar score at 5 minutes, n (%) | |||
| 0–3 | 38 (3.0) | 38 (3.0) | 2 (3.8) |
| 4–6 | 177 (14.1) | 169 (13.4) | 5 (9.6) |
| 7–10 | 1010 (80.3) | 987 (78.5) | 45 (86.5) |
| Missing | 33 (2.6) | 64 (5.1) | 0 (0.0) |
| Maternal age, years | |||
| Mean (SD) | 33.8 (12.5) | 33.4 (6.6) | 33.7 (7.8) |
| Maternal ethnicity, n (%) | |||
| White | 707 (56.2) | 684 (54.4) | 29 (55.7) |
| Indian | 58 (4.6) | 28 (2.2) | 3 (5.8) |
| Pakistani | 36 (2.9) | 54 (4.3) | 1 (1.9) |
| Bangladeshi | 57 (4.5) | 189 (15.0) | 5 (9.6) |
| Black African | 188 (15.0) | 71 (5.6) | 8 (15.4) |
| Black Caribbean | 62 (4.9) | 168 (13.3) | 1 (1.9) |
| Other | 140 (11.1) | 63 (5.0) | 5 (9.6) |
| Unknown | 10 (0.8) | 1 (0.08) | 0 |
| Membranes ruptured > 24 hours before birth, n (%) | |||
| Yes | 345 (27.4) | 1011 (80.4) | 13 (25.0) |
| No | 877 (69.7) | NA | 37 (71.2) |
| Unknown | 36 (2.9) | 247 (19.6) | 2 (3.8) |
| Maternal antenatal corticosteroid treatment, n (%) | |||
| Any | 816 (64.9) | 1147 991.2) | 36 (69.2) |
| Started < 24 hours before birth | 322 (25.6) | NA | 13 (25.0) |
| None | 111 (8.8) | 110 (8.7) | 3 (5.8) |
| Unknown | 9 (0.7) | 1 (0.08) | 0 (0.0) |
| Delivery by caesarean section, n (%) | |||
| Yes | 664 (52.9) | 652 (51.8) | 26 (50.0) |
| No | 593 (47.1) | 550 (43.7) | 26 (50.0) |
| Unknown | 1 (0.08) | 56 (4.5) | 0 (0.0) |
| Born in the recruiting hospital, n (%) | |||
| Yes | 1146 (91.1) | 1168 (92.8) | 46 (88.5) |
| No | 111 (8.8) | 11 (0.9) | 6 (11.5) |
| Missing | 1 (0.08) | 79 (62.8) | 0 (0.0) |
The key clinical and sociodemographic characteristics of the 1258 infants included in our empirical investigations are presented by trial arm in Table 32. There were no significant differences in these key characteristics between the trial arms. Furthermore, there was no evidence of clinical benefit associated with administration of the probiotic for any of the primary outcomes or the composite secondary outcome (Table 33).
| Characteristic | Trial arm, n (%) | p-valuea | |
|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | ||
| Male | 357 (55.96) | 352 (56.77) | 0.770 |
| Gestational age (weeks) | 0.883 | ||
| < 28 | 304 (47.65) | 298 (48.06) | |
| ≥ 28 | 334 (52.35) | 322 (52.94) | |
| Randomisation age | 0.881 | ||
| < 24 hours | 167 (26.18) | 160 (25.81) | |
| ≥ 24 hours | 471 (73.82) | 460 (74.19) | |
| Weight ≤ 100 g | 0.990 | ||
| No | 327 (51.25) | 318 (51.29) | |
| Yes | 311 (48.71) | 302 (48.71) | |
| Primary outcomes | |||
| Death before discharge homeb | 54 (8.46) | 51 (8.23) | 0.879 |
| Sepsisc | 72 (11.29) | 67 (10.81) | 0.787 |
| NEC | 63 (9.87) | 56 (9.03) | 0.610 |
| Secondary outcome | |||
| Composite of primary outcomes | 139 (21.79) | 133 (21.45) | 0.885 |
Resource use and cost estimates: comparisons within trial by data source
Resource use measures and their values between trial randomisation and final hospital discharge (or death) are summarised by trial arm in Tables 34 and 35 for the PiPS and NNRD data, respectively. Based on the PiPS data (see Table 33), the mean (SE) overall duration of hospitalisation was 75.49 (1.95) days for infants in the control arm, whereas the infants in the probiotic arm had a mean overall duration of hospitalisation of 76.60 (2.02) days. There were no statistically significant differences in the values for any resource input by trial arm. The results for the NNRD data (see Table 35) followed a similar pattern with no statistically significant differences in resource values by trial arm. However, the mean numbers of cranial ultrasound scans were higher in the PiPS data than in the NNRD data. Table 36 presents the resource use measures and their values by trial arm for the combined data set.
| Resource variable | Trial arm | p-valuea | |
|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | ||
| Mode of delivery, n (%) | 0.371 | ||
| Vaginal birth – cephalic | 229 (35.89) | 236 (38.06) | |
| Vaginal birth – breech | 64 (10.03) | 53 (8.55) | |
| Vaginal birth – other presentation | 6 (0.94) | 5 (0.81) | |
| Caesarean section before onset of labour | 197 (30.88) | 212 (34.19) | |
| Caesarean section after onset of labour | 141 (22.1) | 114 (18.39) | |
| Unknown | 1 (0.16) | 0 (–) | |
| Other | |||
| Post-mortems, n (%) | 10 (1.57) | 10 (1.61) | 0.9490 |
| Post-mortems, mean (SE) | 0.02 (0) | 0.02 (0.01) | 0.9486 |
| Hospital transfers, n (%) | 310 (48.59) | 312 (50.32) | 0.5390 |
| Hospital transfers, mean (SE) | 0.77 (0.04) | 0.80 (0.05) | 0.6648 |
| Special care, n (%) | 580 (90.91) | 565 (91.13) | 0.8920 |
| Length of special care stay (days), median (range) | 30 (0–203) | 30 (0–392) | 0.6979 |
| Length of special care stay (days), mean (SE) | 396.83 (68.15) | 416.28 (72.43) | 0.7849 |
| High-dependency care, n (%) | 570 (89.34) | 562 (90.65) | 0.4410 |
| Length of high-dependency care stay (days), median (range) | 17 (0–174) | 20 (0–195) | 0.1202 |
| Length of high-dependency care stay (days), mean (SE) | 22.34 (0.94) | 24 (0.99) | 0.2257 |
| Intensive care, n (%) | 604 (94.67) | 595 (95.97) | 0.2770 |
| Length of intensive care stay (days), median (range) | 11.5 (0–339) | 10 (0–378) | 0.9338 |
| Length of intensive care stay (days), mean (SD) | 22.20 (1.21) | 21.30 (1.07) | 0.5778 |
| Total length of stay care stay (days), median (range) | 64 (2–378) | 67 (2–547) | 0.3804 |
| Total length of stay (days), mean (SE) | 75.49 (1.95) | 76.60 (2.02) | 0.6914 |
| ROP screens, n (%) | 583 (91.38) | 571 (92.10) | 0.6440 |
| ROP screens, mean (SE) | 0.91 (0.01) | 0.92 (0.01) | 0.6442 |
| ROP treatment, n (%) | 9 (1.41) | 13 (2.10) | 0.3530 |
| ROP treatment, mean (SE) | 0.01 (0.005) | 0.02 (0.006) | 0.3551 |
| Cranial ultrasound scans, n (%) | 637 (99.84) | 619 (99.84) | 0.9840 |
| Cranial ultrasound scans, mean (SE) | 1.61 (0.04) | 1.63 (0.03) | 0.7718 |
| PDA surgery, n (%) | 26 (4.08) | 33 (5.32) | 0.2950 |
| PDA surgery, mean (SE) | 0.04 (0.01) | 0.05 (0.01) | 0.5574 |
| Hernia surgery, n (%) | 19 (2.98) | 18 (2.90) | 0.9370 |
| Hernia surgery, mean (SE) | 0.03 (0.01) | 0.03 (0.01) | 0.9306 |
| Reservoir surgery, n (%) | 0 | 1 (0.16) | 0.3100 |
| Reservoir surgery, mean (SE) | 0 (0) | 0 (0) | 0.3177 |
| VP shunt surgery, n (%) | 5 (0.78) | 4 (0.65) | 0.7710 |
| VP shunt surgery, mean (SE) | 0.01 (0) | 0.01 (0) | 0.7706 |
| NEC surgery, n (%) | 39 (6.11) | 34 (5.48) | 0.6330 |
| NEC surgery, mean (SE) | 0.06 (0.001) | 0.05 (0.001) | 0.6334 |
| Other procedures, n (%) | 16 (2.51) | 11 (1.77) | 0.3690 |
| Other procedures, mean (SE) | 0.03 (0.01) | 0.02 (0.01) | 0.2875 |
| Resource variable | Trial arm | p-valuea | |
|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | ||
| Mode of delivery, n (%) | 0.7000 | ||
| Vaginal – spontaneous | 281 (44.04) | 278 (44.84) | |
| Vaginal – forceps assisted | 11 (1.72) | 12 (1.94) | |
| Elective section – in labour | 5 (0.78) | 3 (0.48) | |
| Elective section – not in labour | 33 (5.17) | 32 (5.16) | |
| Emergency caesarean section – in labour | 139 (21.79) | 112 (18.06) | |
| Emergency caesarean section – not in labour | 158 (24.76) | 170 (27.42) | |
| Unknown | 11 (1.72) | 13 (2.1) | |
| Other | |||
| Post-mortems, n (%) | 4 (0.63) | 2 (0.32) | 0.4330 |
| Post-mortems, mean (SE) | 0.01 (0) | 0 (0) | 0.4317 |
| Hospital transfers, n (%) | 323 (50.63) | 323 (52.10) | 0.6020 |
| Hospital transfers, mean (SE) | 0.89 (0.05) | 0.92 (0.05) | 0.6858 |
| Normal care, n (%) | 185 (29.0) | 166 (26.77) | 0.3800 |
| Length of normal care stay (days), median (range) | 0 (0 to 9) | 0 (0 to 11) | 0.4195 |
| Length of normal care stay (days), mean (SE) | 0.55 (0.04) | 0.55 (0.05) | 0.9407 |
| Transitional care, n (%) | 13 (2.04) | 17 (2.74) | 0.6701 |
| Length of transitional care stay (days), median (range) | 0 (0 to 4) | 0 (0 to 3) | 0.4134 |
| Length of transitional care stay (days), mean (SE) | 0.03 (0.01) | 0.04 (0.01) | 0.5760 |
| Special care, n (%) | 589 (92.32) | 562 (90.65) | 0.2870 |
| Length of special care stay (days), median (range) | 31 (0–105) | 30.5 (0–87) | 0.7810 |
| Length of special care stay (days), mean (SE) | 31 (0.7) | 30.77 (0.72) | 0.8192 |
| High-dependency, n (%) | 583 (91.38) | 574 (92.58) | 0.4330 |
| Length of high-dependency care stay (days), median (range) | 16 (0–256) | 16 (0–175) | 0.6736 |
| Length of high-dependency care stay (days), mean (SE) | 24.45 (1.15) | 24.78 (1.09) | 0.8322 |
| Intensive care, n (%) | 574 (89.97) | 573 (92.42) | 0.1250 |
| Length of intensive care stay (days), median (range) | 11 (0–267) | 12 (0–166) | 0.5336 |
| Length of intensive care stay (days), mean (SD) | 19.11 (1.04) | 18.59 (0.84) | 0.6980 |
| Total length of stay care stay (days), median (range) | 65 (2–337) | 68 (2–277) | 0.4697 |
| Total length of stay (days), mean (SE) | 75.14 (1.87) | 74.73 (1.67) | 0.8697 |
| ROP screens, n (%) | 543 (85.11) | 530 (85.48) | 0.8510 |
| ROP screens, mean (SE) | 0.85 (0.01) | 0.85 (0.01) | 0.8515 |
| ROP treatment, n (%) | 26 (4.08) | 23 (3.71) | 0.7380 |
| ROP treatment, mean (SE) | 0.04 (0.001) | 0.04 (0.001) | 0.7377 |
| Cranial ultrasound scans, n (%) | 415 (65.05) | 393 (63.39) | 0.5390 |
| Cranial ultrasound scans, mean (SE) | 0.8 (0.03) | 0.80 (0.03) | 0.9876 |
| PDA surgery, n (%) | 22 (3.45) | 29 (4.68) | 0.2690 |
| PDA surgery, mean (SE) | 0.03 (0.001) | 0.05 (0.001) | 0.2705 |
| Hernia surgery, n (%) | 41 (6.43) | 40 (6.45) | 0.9850 |
| Hernia surgery, mean (SE) | 0.06 (0.01) | 0.06 (0.01) | 0.9854 |
| Reservoir surgery, n (%) | 0 | 0 | – |
| Reservoir surgery, mean (SE) | 0 (0) | 0 (0) | – |
| VP shunt surgery, n (%) | 5 (0.78) | 7 (1.13) | 0.5290 |
| VP shunt surgery, mean (SE) | 0.01 (0.003) | 0.01 (0.004) | 0.5301 |
| NEC surgery, n (%) | 34 (5.33) | 26 (4.19) | 0.3450 |
| NEC surgery, mean (SE) | 0.05 (0.01) | 0.04 (0.01) | 0.3443 |
| Other procedures, n (%) | 134 (21.0) | 108 (17.42) | 0.1070 |
| Other procedures, mean (SE) | 0.38 (0.04) | 0.40 (0.04) | 0.6858 |
| Resource variable | Source | Trial arm | p-valuea | |
|---|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | |||
| Mode of delivery, n (%) | 0.7000 | |||
| Vaginal – spontaneous | NNRD | 281 (44.04) | 278 (44.84) | |
| Vaginal – forceps assisted | NNRD | 11 (1.72) | 12 (1.94) | |
| Elective section – in labour | NNRD | 5 (0.78) | 3 (0.48) | |
| Elective section – not in labour | NNRD | 33 (5.17) | 32 (5.16) | |
| Emergency caesarean section – in labour | NNRD | 139 (21.79) | 112 (18.06) | |
| Emergency caesarean section – not in labour | NNRD | 158 (24.76) | 170 (27.42) | |
| Unknown | NNRD | 11 (1.72) | 13 (2.1) | |
| Other | ||||
| Post-mortems, n (%) | PiPS | 10 (1.57) | 10 (1.61) | 0.9490 |
| Post-mortems, mean (SE) | PiPS | 0.02 (0) | 0.02 (0.01) | 0.9486 |
| Hospital transfers, n (%) | PiPS | 310 (48.59) | 312 (50.32) | 0.5390 |
| Hospital transfers, mean (SE) | PiPS | 0.77 (0.04) | 0.80 (0.05) | 0.6648 |
| Normal care, n (%) | NNRD | 185 (29.0) | 166 (26.77) | 0.3800 |
| Length of normal care stay (days), median (range) | NNRD | 0 (0–9) | 0 (0–11) | 0.4195 |
| Length of normal care stay (days), mean (SE) | NNRD | 0.55 (0.04) | 0.55 (0.05) | 0.9407 |
| Transitional care, n (%) | NNRD | 13 (2.04) | 17 (2.74) | 0.6701 |
| Length of transitional care stay (days), median (range) | NNRD | 0 (0–4) | 0 (0–3) | 0.4134 |
| Length of transitional care stay (days), mean (SE) | NNRD | 0.03 (0.01) | 0.04 (0.01) | 0.5760 |
| Special care, n (%) | NNRD | 589 (92.32) | 562 (90.65) | 0.2870 |
| Length of special care stay (days), median (range) | NNRD | 31 (0–105) | 30.5 (0–87) | 0.7810 |
| Length of special care stay (days), mean (SE) | NNRD | 31 (0.7) | 30.77 (0.72) | 0.8192 |
| High-dependency care, n (%) | NNRD | 583 (91.38) | 574 (92.58) | 0.4330 |
| Length of high-dependency care stay (days), median (range) | NNRD | 16 (0–256) | 16 (0–175) | 0.6736 |
| Length of high-dependency care stay (days), mean (SE) | NNRD | 24.45 (1.15) | 24.78 (1.09) | 0.8322 |
| Intensive care, n (%) | NNRD | 574 (89.97) | 573 (92.42) | 0.1250 |
| Length of intensive care stay (days), median (range) | NNRD | 11 (0–267) | 12 (0–166) | 0.5336 |
| Length of intensive care stay (days), mean (SD) | NNRD | 19.11 (1.04) | 18.59 (0.84) | 0.6980 |
| Total length of stay care stay (days), median (range) | NNRD | 65 (2–337) | 68 (2–277) | 0.4697 |
| Total length of stay (days), mean (SE) | NNRD | 75.14 (1.87) | 74.73 (1.67) | 0.8697 |
| ROP screens, n (%) | PiPS | 583 (91.38) | 571 (92.10) | 0.6440 |
| ROP screens, mean (SE) | PiPS | 0.91 (0.01) | 0.92 (0.01) | 0.6442 |
| ROP treatment, n (%) | PiPS | 9 (1.41) | 13 (2.10) | 0.3530 |
| ROP treatment, mean (SE) | PiPS | 0.01 (0.005) | 0.02 (0.006) | 0.3551 |
| Cranial ultrasound scan, n (%) | PiPS | 637 (99.84) | 619 (99.84) | 0.9840 |
| Cranial ultrasound scans, mean (SE) | PiPS | 1.61 (0.04) | 1.63 (0.03) | 0.7718 |
| PDA surgery, n (%) | PiPS | 26 (4.08) | 33 (5.32) | 0.2950 |
| PDA surgery, mean (SE) | PiPS | 0.04 (0.01) | 0.05 (0.01) | 0.5574 |
| Hernia surgery, n (%) | PiPS | 19 (2.98) | 18 (2.90) | 0.9370 |
| Hernia surgery, mean (SE) | PiPS | 0.03 (0.01) | 0.03 (0.01) | 0.9306 |
| Reservoir surgery, n (%) | PiPS | 0 | 1 (0.16) | 0.3100 |
| Reservoir surgery, mean (SE) | PiPS | 0 (0) | 0 (0) | 0.3177 |
| VP shunt surgery, n (%) | NNRD | 5 (0.78) | 7 (1.13) | 0.5290 |
| VP shunt surgery, mean (SE) | NNRD | 0.01 (0.003) | 0.01 (0.004) | 0.5301 |
| NEC surgery, n (%) | PiPS | 39 (6.11) | 34 (5.48) | 0.6330 |
| NEC surgery, mean (SE) | PiPS | 0.06 (0.001) | 0.05 (0.001) | 0.6334 |
| Other procedures, n (%) | NNRD | 134 (21.0) | 108 (17.42) | 0.1070 |
| Other procedures, mean (SE) | NNRD | 0.38 (0.04) | 0.40 (0.04) | 0.6858 |
Costs
Cost measures and their values between trial randomisation and final hospital discharge (or death) are summarised by trial arm in Tables 37 and 38 for the PiPS and NNRD data, respectively. Based on the PiPS data (see Table 37), the mean (SE) total costs were estimated at £62,284 (£1876) for the control group compared with £62,799 (£1817) for the probiotic group. There were no significant differences across the cost categories by trial arm. The results for the NNRD data (see Table 38) followed a similar pattern with no statistically significant differences between the trial arms in cost estimates, overall and by cost category. Nevertheless, the mean total costs were lower (by > £1300 in the placebo arm and > £2000 in the probiotic arm) in the NNRD data. Table 39 presents the cost measures and their respective values for the combined data set.
| Cost variable | Trial arm, mean (SE) | p-valuea | |
|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | ||
| Delivery cost | 2474.65 (37.02) | 2424.95 (36.87) | 0.3417 |
| Post-mortem cost | 10.18 (3.2) | 10.48 (3.29) | 0.9486 |
| Hospital transfers cost | 1058.92 (59.88) | 1096.3 (62.06) | 0.6648 |
| Cost of special care | 15,629.21 (387.46) | 15,809.23 (533.66) | 0.7849 |
| Cost of high-dependency care | 17,678.63 (746.1) | 18,992.10 (785.94) | 0.2257 |
| Cost of intensive care | 24,817.40 (1356.07) | 23,810.86 (1195.95) | 0.5778 |
| ROP screen cost | 122.55 (1.49) | 123.51 (1.45) | 0.6442 |
| ROP treatment cost | 7.82 (2.59) | 11.62 (3.19) | 0.3551 |
| Ultrasound scan costs | 86.84 (1.98) | 87.62 (1.84) | 0.7718 |
| PDA surgery cost | 98.70 (18.97) | 128.91 (21.85) | 0.2967 |
| Hernia surgery cost | 37.23 (8.42) | 38.31 (9.12) | 0.9306 |
| Reservoir surgery cost | 0 (0) | 4.71 (4.71) | 0.3177 |
| VP shunt surgery cost | 59.28 (26.43) | 48.80 (24.34) | 0.7706 |
| NEC surgery cost | 150.25 (23.33) | 134.79 (22.49) | 0.6334 |
| Other procedures cost | 52.13 (17.15) | 48.61 (26.18) | 0.9105 |
| Total cost | 62,283.80 (1875.53) | 62,799.06 (1816.75)b | 0.8436 |
| Cost variable | Trial arm, mean (SE) | p-valuea | |
|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | ||
| Delivery cost | 2199.15 (34.8) | 2156.65 (34.96) | 0.3890 |
| Post-mortem cost | 4.07 (2.03) | 2.10 (1.48) | 0.4317 |
| Hospital transfers cost | 1224.31 (69.55) | 1264.28 (70.1) | 0.6858 |
| Cost of normal care | 261.29 (20.41) | 259 (23.00) | 0.9407 |
| Cost of transitional care | 10.78 (3.47) | 13.56 (3.55) | 0.5760 |
| Cost of special care | 15,656.13 (352.45) | 15,540.38 (363.38) | 0.8192 |
| Cost of high-dependency care | 19,343.06 (911.54) | 19,608.54 (858.95) | 0.8322 |
| Cost of intensive care | 21,362.65 (1163.14) | 20,782.22 (940.36) | 0.6980 |
| ROP screen cost | 114.14 (1.89) | 114.64 (1.90) | 0.8515 |
| ROP treatment cost | 22.58 (4.34) | 20.55 (4.21) | 0.7377 |
| Ultrasound scan costs | 43.12 (1.61) | 43.16 (1.69) | 0.9876 |
| PDA surgery cost | 83.52 (17.51) | 113.29 (20.56) | 0.2705 |
| Hernia surgery cost | 80.33 (12.15) | 80.65 (12.34) | 0.9854 |
| Reservoir surgery cost | 0 (0) | 0 (0) | 0.3177 |
| VP shunt surgery cost | 59.28 (26.43) | 85.40 (32.12) | 0.5301 |
| NEC surgery cost | 130.99 (21.88) | 103.08 (19.80) | 0.3443 |
| Other procedures cost | 331.41 (51.17) | 344.02 (61.07) | 0.8743 |
| Total cost | 60,926.82 (1805.16) | 60,559.76 (1571.11)b | 0.8781 |
| Cost variable | Trial arm, mean (SE) | p-valuea | |
|---|---|---|---|
| Placebo (N = 638) | B. breve BBG (N = 620) | ||
| Delivery cost | 2199.15 (34.8) | 2156.65 (34.96) | 0.3890 |
| Post-mortem cost | 10.18 (3.2) | 10.48 (3.29) | 0.9486 |
| Hospital transfers cost | 1058.92 (59.88) | 1096.30 (62.06) | 0.6648 |
| Cost of normal care | 261.29 (20.41) | 259.00 (23.00) | 0.9407 |
| Cost of transitional care | 10.78 (3.47) | 13.56 (3.55) | 0.5760 |
| Cost of special care | 15,656.13 (352.45) | 15,540.38 (363.38) | 0.8192 |
| Cost of high-dependency care | 19,343.06 (911.54) | 19,608.54 (858.95) | 0.8322 |
| Cost of intensive care | 21,362.65 (1163.14) | 20,782.22 (940.36) | 0.6980 |
| ROP screen cost | 122.55 (1.49) | 123.51 (1.45) | 0.6442 |
| ROP treatment cost | 7.82 (2.59) | 11.62 (3.19) | 0.3551 |
| Ultrasound scan costs | 86.84 (1.98) | 87.62 (1.84) | 0.7718 |
| PDA surgery cost | 98.70 (18.97) | 128.91 (21.85) | 0.2967 |
| Hernia surgery cost | 37.23 (8.42) | 38.31 (9.12) | 0.9306 |
| Reservoir surgery cost | 0 (0) | 4.71 (4.71) | 0.3177 |
| VP shunt surgery cost | 59.28 (26.43) | 85.40 (32.12) | 0.5301 |
| NEC surgery cost | 150.25 (23.33) | 134.79 (22.49) | 0.6334 |
| Other procedures cost | 331.41 (51.17) | 344.02 (61.07) | 0.8743 |
| Total cost | 60,796.25 (1798.97) | 60,454.28 (1565.73)b | 0.8860 |
Resource use and cost estimates: comparisons across trial between data sources
Table 40 summarises the mean (SE) resource values for each resource category reported by the alternative data sources and the overall levels of agreement between combinations of data sources (Lin’s coefficient), denoted ρc. Table 41 summarises the mean (SE) cost values for each cost category reported by the alternative data sources and the overall levels of agreement between combinations of data sources. For these analyses, infants were pooled across trial arms. Agreement between data sources varied greatly by resource or cost category and by combination of data sources. When the PiPS and NNRD data were compared, agreement was relatively high for utilisation or cost of hospital stay by alternative levels of neonatal care, hospital transfers, ROP screens and treatment, forms of surgery [e.g. PDA, ventriculoperitoneal (VP), NEC], and total neonatal care. However, for post-mortem examinations, ultrasound scans and other procedures, the agreement levels fell below the 0.40 threshold indicating acceptable clinical or practical significance. 347 The 95% limits of agreement, exploring the amount of random variation between the PiPS and NNRD data sources, suggest that large individual differences are likely to be encountered for several categories of resource use and costs. Tables 40 and 41 also summarise the levels of agreement between the PiPS and combined data sources and between the NNRD and combined data sources for resource use and cost values, respectively. However, in the absence of an external gold standard for resource use and cost estimates, these analyses were constrained by the inclusion of values from either PiPS or the NNRD in the combined data source.
| Variable | Data set, mean (standard error) | Agreement between | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PiPS and NNRD data sets | PiPS and combined data sets | NNRD and combined data sets | |||||||
| PiPS | NNRD | Combined | ρc (95% CI)a | Mean difference (95% limits of agreementb) | ρc (95% CI)a | Mean difference (95% limits of agreementb) | ρ (95% CI) | Mean difference (95% limits of agreement) | |
| Post-mortem | 0.016 (0.004) | 0.005 (0.002) | 0.016 (0.004) | 0.148 (0.102 to 0.193) | 0.011 (–0.253 to 0.275) | 1 | 0 | 0.148 (0.102 to 0.193) | –0.011 (–0.275 to 0.253) |
| Hospital transfers | 0.78 6(0.031) | 0.908 (0.036) | 0.786 (0.031) | 0.93 (0.923 to 0.936) | –0.122 (–0.989 to 0.746) | 1 | 0 | 0.93 (0.923 to 0.936) | 0.122 (–0.746 to 0.989) |
| Length of intensive care stay (days) | 21.76 (0.81) | 18.857 (0.671) | 18.857 (0.671) | 0.81 (0.792 to 0.828) | 2.903 (–29.157 to 34.963) | 0.81 (0.792 to 0.828) | 2.903 (–29.157 to 34.963) | 1 | 0 |
| Length of high-dependency care stay (days) | 23.16 (0.684) | 24.61 (0.792) | 24.61 (0.792) | 0.842 (0.825 to 0.856) | –1.451 (–30.88 to 27.979) | 0.842 (0.825 to 0.856) | –1.451 (–30.88 to 27.979) | 1 | 0 |
| Length of special care stay (days) | 31.118 (0.65) | 30.882 (0.501) | 30.882 (0.501) | 0.531 (0.492 to 0.568) | 0.235 (–39.613 to 40.084) | 0.531 (0.492 to 0.568) | 0.235 (–39.613 to 40.084) | 1 | 0 |
| ROP screens | 0.917 (0.008) | 0.853 (0.01) | 0.917 (0.008) | 0.509 (0.469 to 0.546) | 0.064 (–0.558 to 0.687) | 1 | 0 | 0.509 (0.469 to 0.546) | –0.064 (–0.687 to 0.558) |
| ROP treatment | 0.017 (0.004) | 0.039 (0.005) | 0.017 (0.004) | 0.524 (0.487 to 0.559) | –0.021 (–0.343 to 0.3) | 1 | 0 | 0.524 (0.487 to 0.559) | 0.021 (–0.3 to 0.343) |
| Ultrasound scans | 1.62 (0.025) | 0.801 (0.022) | 1.62 (0.025) | 0.282 (0.247 to 0.316) | 0.819 (–0.973 to 2.61) | 1 | 0 | 0.282 (0.247 to 0.316) | –0.819 (–2.61 to 0.973) |
| PDA surgery | 0.047 (0.006) | 0.041 (0.006) | 0.047 (0.006) | 0.791 (0.769 to 0.811) | 0.006 (–0.258 to 0.271) | 1 | 0 | 0.791 (0.769 to 0.811) | –0.006 (–0.271 to 0.258) |
| Hernia surgery | 0.03 (0.005) | 0.064 (0.007) | 0.03 (0.005) | 0.526 (0.489 to 0.562) | –0.034 (–0.447 to 0.379) | 1 | 0 | 0.526 (0.489 to 0.562) | 0.034 (–0.379 to 0.447) |
| Reservoir surgery | 0.001 (0.001) | 0 | 0.001 (0.001) | NA | 0.001 (–0.056 to 0.057) | 1 | 0 | NA (NA, NA) | –0.001 (–0.057 to 0.056) |
| VP shunt surgery | 0.007 (0.002) | 0.01 (0.003) | 0.01 (0.003) | 0.76 (0.736 to 0.782) | –0.002 (–0.128 to 0.124) | 0.76 (0.736 to 0.782) | –0.002 (–0.128 to 0.124) | 1 | 0 |
| NEC surgery | 0.058 (0.007) | 0.048 (0.006) | 0.058 (0.007) | 0.722 (0.695 to 0.747) | 0.01 (–0.323 to 0.343) | 1 | 0 | 0.722 (0.695 to 0.747) | –0.01 (–0.343 to 0.323) |
| Other procedures | 0.025 (0.005) | 0.39 (0.029) | 0.39 (0.029) | 0.075 (0.059 to 0.091) | –0.365 (–2.391 to 1.661) | 0.075 (0.059 to 0.091) | –0.365 (–2.391 to 1.661) | 1 | 0 |
| Variable | Data set, mean (standard error) | Agreement between | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PiPS and NNRD data sets | PiPS and combined data sets | NNRD and combined data sets | |||||||
| PiPS | NNRD | Combined | ρc (95% CI)a | Mean difference (95% limits of agreementb) | ρc (95% CI)a | Mean difference (95% limits of agreementb) | ρc (95% CI)a | Mean difference (95% limits of agreement) | |
| Delivery cost | 2450.157 (26.125) | 2178.204 (24.662) | 2178.204 (24.662) | 0.793 (0.773 to 0.812) | 271.95 (–780.85 to 1324.755) | 0.793 (0.773 to 0.812) | 271.953 (–780.85 to 1324.755) | 1 | 0 |
| Post-mortem cost | 10.329 (2.292) | 3.099 (1.262) | 10.329 (2.292) | 0.148 (0.102 to 0.193) | 7.23 (–164.055 to 178.515) | 1 | 0 | 0.148 (0.102 to 0.193) | –7.23 (–178.515 to 164.055) |
| Hospital transfers cost | 1077.343 (43.09) | 1244.01 (49.357) | 1077.343 (43.09) | 0.93 (0.923 to 0.936) | –166.667 (–1355.215 to 1021.882) | 1 | 0 | 0.93 (0.923 to 0.936) | 166.667 (–1021.882 to 1355.215) |
| Cost of intensive care | 24,321.33 (905.508) | 21,076.591 (749.921) | 21,076.591 (749.921) | 0.81 (0.792 to 0.828) | 3244.739 (–32,588.886 to 39,078.365) | 0.81 (0.792 to 0.828) | 3244.739 (–32,588.886 to 39,078.365) | 1 | 0 |
| Cost of high-dependency care | 18,325.971 (541.596) | 19,473.899 (626.595) | 19,473.899 (626.595) | 0.842 (0.825 to 0.856) | –1147.929 (–24,434.902 to 22,139.045) | 0.842 (0.825 to 0.856) | –1147.929 (–24,434.902 to 22,139.045) | 1 | 0 |
| Cost of special care | 15,717.933 (328.192) | 15,599.083 (252.931) | 15,599.083 (252.931) | 0.531 (0.492 to 0.568) | 118.85 (–20,009.027 to 20,246.728) | 0.531 (0.492 to 0.568) | 118.85 (–20,009.027 to 20,246.728) | 1 | 0 |
| ROP screen cost | 123.023 (1.042) | 114.388 (1.34) | 123.023 (1.042) | 0.509 (0.469 to 0.546) | 8.635 (–74.852 to 92.122) | 1 | 0 | 0.509 (0.469 to 0.546) | –8.635 (–92.122 to 74.852) |
| ROP treatment cost | 9.688 (2.048) | 21.579 (3.023) | 9.688 (2.048) | 0.524 (0.487 to 0.559) | –11.89 (–189.834 to 166.053) | 1 | 0 | 0.524 (0.487 to 0.559) | 11.89 (–166.053 to 189.834) |
| Ultrasound scan costs | 87.223 (1.353) | 43.141 (1.163) | 87.223 (1.353) | 0.282 (0.247 to 0.316) | 44.082 (–52.378 to 140.542) | 1 | 0 | 0.282 (0.247 to 0.316) | –44.082 (–140.542 to 52.378) |
| PDA surgery cost | 113.591 (14.443) | 98.189 (13.473) | 113.591 (14.443) | 0.791 (0.769 to 0.811) | 15.402 (–624.694 to 655.498) | 1 | 0 | 0.791 (0.769 to 0.811) | –15.402 (–655.498 to 624.694) |
| Hernia surgery cost | 37.763 (6.197) | 80.485 (8.654) | 37.763 (6.197) | 0.526 (0.489 to 0.562) | –42.721 (–558.64 to 473.197) | 1 | 0 | 0.526 (0.489 to 0.562) | 42.721 (–473.197 to 558.64) |
| Reservoir surgery cost | 2.323 (2.323) | 0 | 2.323 (2.323) | NA (NA, NA) | 2.323 (–162.484 to 167.131) | 1 | 0 | NA (NA, NA) | –2.323 (–167.131 to 162.484) |
| VP shunt surgery cost | 54.111 (17.98) | 72.153 (20.737) | 72.153 (20.737) | 0.76 (0.736 to 0.782) | –18.041 (–971.458 to 935.375) | 0.76 (0.736 to 0.782) | –18.041 (–971.458 to 935.375) | 1 | 0 |
| NEC surgery cost | 142.634 (16.209) | 117.234 (14.775) | 142.634 (16.209) | 0.722 (0.695 to 0.747) | 25.401 (–793.334 to 844.135) | 1 | 0 | 0.722 (0.695 to 0.747) | –25.401 (–844.135 to 793.334) |
| Other procedures cost | 50.392 (15.557) | 337.626 (39.726) | 337.626 (39.726) | 0.216 (0.182 to 0.25) | –287.234 (–2952.95 to 2378.482) | 0.216 (0.182 to 0.25) | –287.234 (–2952.95 to 2378.482) | 1 | 0 |
| Total cost | 62,537.737 (1305.81) | 60,745.916 (1198.576) | 60,627.711 (1194.465) | 0.917 (0.908 to 0.925) | 1791.822 (–34,274.766 to 37,858.409) | 0.917 (0.907 to 0.925) | 1910.027 (–34,174.429 to 37,994.482) | 1 | 118.205 (–1428.536 to 1664.946) |
Cost-effectiveness: comparisons within trial by data source
The incremental cost-effectiveness of the probiotic is shown in Table 42 for the 1258 infants eligible for our comparative analyses, by clinical outcome and data source. Based on data collected from the PiPS trial CRFs, the average total cost was £62,799 in the probiotic group compared with £62,284 in the placebo group, generating a mean incremental cost of £515. The incremental cost-effectiveness of the probiotic was estimated at £216,369 per death avoided, £107,613 per episode of sepsis avoided, £61,170 per episode of NEC avoided and £153,703 per composite adverse outcome avoided. The mean ICERs fell in the north-east quadrant of the cost-effectiveness plane (see Figures 43–46). The corresponding CEACs (figures not shown) indicate that regardless of the clinical outcome measure of interest the probability that the probiotic is cost-effective varied between 40% and 50% depending on the value of the cost-effectiveness threshold. If decision-makers are willing to pay £30,000 to avoid an adverse perinatal outcome, the probability that the probiotic is cost-effective varied between 42.6% and 47.7%.
| Mean costs (95% CI) | Mean effects (95% CI) | ICER (£) | Probability B. breve BBG is (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. breve BBG (£) | Placebo (£) | Difference (£) | B. breve BBG | Placebo | Differencea | More effectiveb | Less costlyb | Cost-effectiveb,c | Cost-effectiveb,d | ||
| Deathe | |||||||||||
| PiPS data | 62,799.06 (59,231 to 66,367) | 62,283.79 (58,601 to 65,967) | 515.27 (–4611 to 5641) | 0.0823 (0.0606 to 0.1039) | 0.0846 (0.0629 to 0.1063) | 0.0024 (–0.0282 to 0.0330) | 216,369 | 58.8 | 42.0 | 42.8 | 44.2 |
| NNRD data | 60,559.76 (57,474 to 63,645) | 60,926.82 (57,382 to 64,472) | –367.07 (–5072 to 4338) | 0.0823 (0.0606 to 0.1039) | 0.0846 (0.0629 to 0.1063) | 0.0024 (–0.0282 to 0.0330) | Treatment dominates | 60.8 | 57.8 | 59.4 | 59.9 |
| Combined data | 60,454.28 (57,379 to 63,529) | 60,796.25 (57,264 to 64,329) | –341.98 (–5031 to 4347) | 0.0823 (0.0606 to 0.1039) | 0.0846 (0.0629 to 0.1063) | 0.0024 (–0.0282 to 0.0330) | Treatment dominates | 60.8 | 58.0 | 59.0 | 59.5 |
| Sepsisf | |||||||||||
| PiPS data | 62,799.06 (59,231 to 66,367) | 62,283.79 (58,601 to 65,967) | 515.27 (–4611 to 5641) | 0.1081 (0.0836 to 0.1326) | 0.1129 (0.0882 to 0.1375) | 0.0048 (–0.0299 to 0.0395) | 107,613 | 59.6 | 40.6 | 41.8 | 42.6 |
| NNRD data | 60,559.76 (57,474 to 63,645) | 60,926.82 (57,382 to 64,472) | –367.07 (–5072 to 4338) | 0.1081 (0.0836 to 0.1326) | 0.1129 (0.0882 to 0.1375) | 0.0048 (–0.0299 to 0.0395) | Treatment dominates | 60.9 | 55.0 | 56.7 | 56.8 |
| Combined data | 60,454.28 (57,379 to 63,529) | 60,796.25 (57,264 to 64,329) | –341.98 (–5031 to 4347) | 0.1081 (0.0836 to 0.1326) | 0.1129 (0.0882 to 0.1375) | 0.0048 (–0.0299 to 0.0395) | Treatment dominates | 63.2 | 58.0 | 58.4 | 58.5 |
| NEC | |||||||||||
| PiPS data | 62,799.06 (59,231 to 66,367) | 62,283.79 (58,601 to 65,967) | 515.27 (–4611 to 5641) | 0.0903 (0.0677 to 0.1129) | 0.0987 (0.0755 to 0.1220) | 0.0084 (–0.0240 to 0.0408) | 61,170 | 72.8 | 42.0 | 45.7 | 47.7 |
| NNRD data | 60,559.76 (57,474 to 63,645) | 60,926.82 (57,382 to 64,472) | –367.07 (–5072 to 4338) | 0.0903 (0.0677 to 0.1129) | 0.0987 (0.0755 to 0.1220) | 0.0084 (–0.0240 to 0.0408) | Treatment dominates | 69.3 | 57.8 | 60.3 | 60.5 |
| Combined data | 60,454.28 (57,379 to 63,529) | 60,796.25 (57,264 to 64,329) | –341.98 (–5031 to 4347) | 0.0903 (0.0677 to 0.1129) | 0.0987 (0.0755 to 0.1220) | 0.0084 (–0.0240 to 0.0408) | Treatment dominates | 69.3 | 58.0 | 59.8 | 60.3 |
| Compositeg | |||||||||||
| PiPS data | 62,799.06 (59,231 to 66,367) | 62,283.79 (58,601 to 65,967) | 515.27 (–4611 to 5641) | 0.2145 (0.1822 to 0.2468) | 0.2179 (0.1858 to 0.2499) | 0.0034 (–0.0421 to 0.0489) | 153,703 | 42 | 44.6 | 46.2 | |
| NNRD data | 60,559.76 (57,474 to 63,645) | 60,926.82 (57,382 to 64,472) | –367.07 (–5072 to 4338) | 0.2145 (0.1822 to 0.2468) | 0.2179 (0.1858 to 0.2499) | 0.0034 (–0.0421 to 0.0489) | Treatment dominates | 57.8 | 58.4 | 59.5 | |
| Combined data | 60,454.28 (57,379 to 63,529) | 60,796.25 (57,264 to 64,329) | –341.98 (–5031 to 4347) | 0.2145 (0.1822 to 0.2468) | 0.2179 (0.1858 to 0.2499) | 0.0034 (–0.0421 to 0.0489) | Treatment dominates | 57.9 | 58.3 | 59.1 | |
Based on data from the NNRD, the average total cost was £60,560 in the probiotic group, compared with £60,927 in the placebo group, generating a mean incremental saving of £367. Because the probiotic was, on average, more effective than the placebo regardless of clinical outcome, the mean ICERs fell in the south-east quadrant of the cost-effectiveness plane (see Figures 47–50), suggesting that the probiotic dominated the placebo in health economic terms. Regardless of the clinical outcome measure of interest, the probability that the probiotic is cost-effective varied between 50% and 60% depending on the value of the cost-effectiveness threshold. If decision-makers are willing to pay £30,000 to avoid an adverse perinatal outcome, the probability that the probiotic is cost-effective varied between 56.8% and 60.5%.
Based on the combined data, the average total cost was £60,454 in the probiotic group compared with £60,796 in the placebo group, generating a mean incremental saving of £342. Because the probiotic was, on average, more effective than the placebo regardless of clinical outcome, the mean ICERs fell in the south-east quadrant of the cost-effectiveness plane (see Figures 51–54), suggesting that the probiotic dominated the placebo in health economic terms. Regardless of the clinical outcome measure of interest, the probability that the probiotic is cost-effective varied between 55% and 60% depending on the value of the cost-effectiveness threshold. If decision-makers are willing to pay £30,000 to avoid an adverse perinatal outcome, the probability that the probiotic is cost-effective varied between 58.5% and 60.3%.
Our estimates of within-trial incremental cost-effectiveness were replicated for each of the prespecified subgroups, namely gender, birthweight, gestational age, colonisation status and randomisation age. Table 42 presents the cost-effectiveness outcomes by prespecified subgroup for the composite secondary outcome. Table 63 presents the cost-effectiveness outcomes by prespecified subgroup for the death primary outcome; Table 64 presents the cost-effectiveness outcomes by prespecified subgroup for the sepsis primary outcome; and Table 65 presents the cost-effectiveness outcomes by prespecified subgroup for the NEC primary outcome. The probability that the probiotic is cost-effective was notably higher for girls and for infants born at ≥ 1000 g, regardless of clinical outcome measure and data source. For the death primary outcome, the probability that the probiotic is cost-effective at a £30,000 cost-effectiveness threshold varied between 78.9% and 88.0% for girls, and between 62.7% and 93.0% for infants born at ≥ 1000 g, depending on data source (see Table 63). For the sepsis primary outcome, the probability that the probiotic is cost-effective at a £30,000 cost-effectiveness threshold varied between 73.4% and 81.2% for girls, and between 77.3% and 96.8% for infants born at ≥ 1000 g, depending on data source (see Table 64). For the NEC primary outcome, the probability that the probiotic is cost-effective at a £30,000 cost-effectiveness threshold varied between 74.2% and 82.3% for girls, and between 71.5% and 94.8% for infants born at ≥ 1000 g, depending on data source (see Table 65). Finally, for the composite secondary outcome, the probability that the probiotic is cost-effective at a £30,000 cost-effectiveness threshold varied between 77.1% and 85.1% for girls, and between 65.9% and 92.8% for infants born at ≥ 1000 g, depending on data source (see Table 66).
Comparisons of cost-effectiveness outcomes between data sources
To compare the discrepancy in the cost-effectiveness results between the different data sources, agreement statistics (namely the probability of miscoverage and two-sided probability values) were estimated using a double bootstrap strategy. A detailed description of methodology has been published. 350 Briefly, an estimate of the probability of miscoverage between the incremental net benefit generated by any two data sets was obtained as follows:
-
First, for any two data sets, one data set was designated as the reference data (referent) and the other as the test data. For the analyses reported here, the PiPS data set was initially designated as the referent; however, for completeness, we also report results where the combined data set was designated as the referent.
-
Second, bootstrapping was applied to the test data to generate 500 replicates of the test data.
-
Finally, the probability of miscoverage was obtained by counting the proportion of the 500 replicates in which the 95% CIs for the incremental net benefits (test data) did not contain the referent incremental net benefit estimate.
Tables 67 and 68 summarise the agreement statistics (two-sided p-values and probability estimates of miscoverage) between any two data sources obtained using the above strategy. The probability of miscoverage ranged from 3.9% at a cost-effectiveness threshold of £20,000 per case of sepsis avoided to 6.4% at a cost-effectiveness threshold of £30,000 per death avoided, when the source of outcomes data were the NNRD and the combined data sets, respectively. The p-values ranged from 0.387 (PiPS vs. NNRD data set at a cost-effectiveness threshold of £20,000 per death avoided) to 0.571 (PiPS vs. NNRD2 at a cost-effectiveness threshold of £30,000 per case of sepsis avoided). These p-values provide no evidence to suggest that the incremental net benefit estimated using one data set differs significantly from the incremental net benefit estimated from another data set. Separate analyses performed on the prespecified subgroups did not alter these findings.
Finally, sensitivity analyses that compared the key outputs of the economic evaluation using resource use and clinical outcomes data extracted solely from the NNRD are summarised in Table 69. These analyses were restricted to the two clinical outcomes used in the PiPS trial that were available in both data sources, namely (1) death before discharge from hospital and (2) sepsis. Notably, the mean ICER for the sepsis outcome moved from the south-east quadrant of the cost-effectiveness plane, denoting a less costly and more effective intervention when the NNRD acted as the sole source of resource use information (see Table 41) to the south-west quadrant of the cost-effectiveness plane, denoting a less costly and less effective intervention when the NNRD acted as the sole source of resource use and clinical outcomes.
Conclusions
This chapter outlined a study to assess whether or not reliable health service utilisation data can be obtained from the NNRD and can be used to inform future trial-based economic evaluations of neonatal interventions. The recently completed PiPS was used as the test bed for our empirical investigations. Health-care resource utilisation data were extracted from the PiPS trial CRFs, the NNRD and a combined data source, and primarily valued using national tariffs and expressed in GBP (2012/13 prices). Differences in economic outcomes were estimated (1) within trial by data source, thereby allowing us to draw comparisons between the probiotic and its comparator (placebo), and (2) by pooling data between the trial arms, thereby allowing us to draw comparisons between the alternative data sources. Within-trial comparisons of resource use and costs revealed no statistically significant differences between the trial comparators in the values for any resource input or cost category, regardless of data source. Across-trial tests of concordance in resource use and costs between comparator data sources revealed relatively high levels of agreement for the majority of categories of resource use or cost and notably for the total cost of neonatal care. Within-trial estimates of cost-effectiveness revealed relatively low probabilities of cost-effectiveness for the probiotic across a wide range of cost-effectiveness thresholds, regardless of data source. It was notable, however, that following subgroup analyses the probiotic had a high probability of cost-effectiveness for girls and for infants born at ≥ 1000 g, regardless of data source. Finally, comparisons of cost-effectiveness outcomes between data sources revealed low probability levels of miscoverage of incremental net monetary benefit sources when the NNRD acted as the sole source of resource use information. However, separate sensitivity analyses revealed that probability estimates of miscoverage for incremental net monetary benefit increased for both death and sepsis outcomes when the NNRD acted as the sole source of resource use information and clinical outcomes.
A number of caveats should be borne in mind when interpreting the results of this study. First, the third of our comparator data sources, the ‘combined’ data source, was constructed by selecting resource components from either the PiPS trial CRFs or the NNRD on the basis of volume and granularity of information provided. Second, there are a number of features of the economic evaluation, which although not directly impinging on our comparisons across data sources, they do constrain the conclusions we can draw about the cost-effectiveness of the probiotic. For example, by adopting the recommended health system perspective (NICE, 2013351), our study excluded broader costs, such as those borne by family members and informal carers, which are arguably of relevance to economic evaluations of neonatal interventions. However, given the absence of evidence of significant clinical effect for the probiotic, it is unlikely that incorporation of these broader societal costs into the analysis would have had an impact on our estimates of incremental cost-effectiveness. In addition, in the absence of validated multiattribute utility measures for use in early childhood, the effectiveness of the probiotic was not measured in terms of a preference-based outcome measure, such as the quality-adjusted life-year (QALY), which may have been more useful for cost-effectiveness comparative purposes and for which accepted threshold values are available. 339 Third, our comparisons of economic outcomes generated by the NNRD and the PiPS trial CRFs were based on a clinical trial in which there was no evidence of significant clinical effect for the intervention being evaluated. Many health economists have argued that reliance on traditional rules of statistical inference surrounding a single parameter, such as clinical effectiveness, is arbitrary, and may result in inferior health-care outcomes compared with basing decisions on expected cost-effectiveness. 352 Future research should consider the application of value of information techniques using NNRD data in order to quantify any economic costs associated with incorrect policy decisions around the adoption of neonatal interventions. 353 Under these circumstances, it is entirely appropriate to estimate the joint distribution of cost and effect differences, not either of these in isolation. Nevertheless, it will be important in future research to test the validity of our results in the context of other neonatal trials with disparate clinical and economic impacts.
Implications for health care
We have demonstrated proof of principle of the potential of the NNRD as a data source for neonatal trial-based economic evaluations in the UK context. This has potential to reduce the costs and improve the efficiency of economic evaluations conducted in relation to research studies. The results of our study have important implications for health economics research in the neonatal context. We have demonstrated that the bulk of hospital resource inputs incorporated into a rigorously designed economic evaluation of a neonatal intervention in a UK context can be successfully and accurately extracted from the NNRD. These include not only resource-generating events that contribute to national reference costs for relevant Healthcare Resource Groups for neonatal care, but resource-generating events that fall outside these per diem values, for example high-cost scans, tests and blood products, surgeries, transfers and post-mortem examinations.
Research recommendations
Further research is recommended to validate our results in the context of other trials and to assess the utility of the NNRD across a wider range of economic evaluations and alternative study designs. We would have ideally wished to triangulate the resource and cost profiles generated by the PiPS trial CRFs and the NNRD data against an external gold standard, such as data extracted directly from patient notes. However, such an exercise was not within the resources of this study and it remains an area for future research.
Chapter 7 Linking the National Neonatal Research Database to other NHS data sets; feasibility and birth cohort studies
Much of this chapter has already been published and is reproduced here with permission from Murray et al. 354 © 2014 Murray et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License International (CC by 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Abstract
Background: Linking routine NHS clinical and administrative data offers considerable potential for research and health service evaluations but is dependent on data completeness and quality.
Aims and methods: We examined HES and NNRD data and level of agreement. We linked data sets to create birth cohorts, ascertain mortality and identify individuals across time in relation to hospital admissions up to the age of 1 year for a key infant illness: bronchiolitis.
Results: One in ten neonates identified in HES represent admissions to neonatal specialised care as determined by a record in the NNRD. There is > 95% agreement for key items. Data quality and completeness are generally better in the NNRD than in HES. Approximately 20% of babies in HES have missing gestational age data and around 1.5% have a biologically implausible birthweight. The completeness of HES birth data varies substantially between hospitals but has improved over time. The higher mortality rates of extremely preterm babies extend throughout the first year. Most infants admitted to hospital with bronchiolitis in England are born at term and have no recognised predisposing risks.
Conclusions: Linkage between HES and the NNRD is feasible, enhances the quality and scope of birth records, and paves the way for ascertainment of lifelong health outcomes. Improved health data quality, as well as completeness, are important service goals. Reducing reliance on administrative data, promoting clinician involvement in data assurance and extracting data from electronic health records rather than recording them anew are measures that merit wider consideration.
Introduction
Potential of data set linkage
Data set linkage offers considerable potential to enlarge the scope, enhance the richness and improve the quality and completeness of information for clinical and health services research. Linked data sets provide the opportunity to address an extensive range of research questions, such as examining long-term health outcomes, identifying risk factors for re-admission, and quantifying mortality, morbidity and health-care utilisation following discharge from neonatal specialised care. Linkage also offers potential to develop cradle-to-grave data sets, a particularly useful resource for infant health research.
Information on long-term health outcomes at multiple points in time is important for evaluating interventions in pregnancy and in the neonatal period. However, although long-term assessment of outcomes is highly desirable, it is also complex and costly. Considerable demand is placed on participants when information on long-term outcomes is sought, and the costs of research-based follow-up are substantial. Most research is funded for a finite period, typically 3–5 years. Obtaining funding for long-term follow-up studies is extremely problematic and beyond the ability of any but a few large research organisations. As a consequence, much infant research is compromised by a focus on short-term outcomes.
Birth cohorts are an important resource for epidemiological research. They have usually involved recruitment at a specific age with repeated surveys of participants conducted at intervals of years. These longitudinal studies have typically contributed to examining societal change, for example in relation to family structures, educational attainments, equity, poverty and class. Inclusion of data on potential confounders, comorbidities and clinical outcomes, in addition to core baseline characteristics (e.g. gestational age, birthweight and sex), offer the opportunity to address causal relationships through the use of statistical techniques such as instrumental variable and multivariable analyses. This is particularly valuable when investigating exposures that are not amenable to randomisation.
Population-level clinical data sets can provide information that is highly generalisable, have power to detect small effect sizes and relate directly to real-life health-care practices. Electronic health records offer the potential to reduce the cost and complexity of data acquisition. The NHS in England, with near-universal national coverage, is potentially in a unique position to assess population-based outcomes following discharge of neonates from neonatal specialised care, using information from linked data sets. This would have major utility for neonatal clinical trials, health economic evaluations, post-marketing and other surveillance, and observational birth cohort and epidemiological studies, particularly those focused on aspects of health over the life course.
Hospital Episode Statistics
The administrative database, HES, contains details of patient diagnoses and procedures on all episodes of care in English NHS trusts (acute hospital, primary care and mental health trusts), going back to 1989. HES data were conceived in 1987, following a report on the collection and use of hospital activity information by a committee chaired by Dame Edith Körner (née Lowy), daughter of a maize miller, and a refugee who fled to England following the Nazi occupation of Czechoslovakia. She became an authority on health administration and the use of computers in the health service. In 1980, she was asked to chair a national review of NHS information. After 4 years of deliberation, the Körner Committee’s several recommendations were adopted, in what became the beginning of the computerisation of the NHS. For the following 20 years or so, NHS statistical information was known as ‘Körner Data’.
The HES data are submitted centrally and held by the Health and Social Care Information Centre (formerly the NHS Information Centre and now known as NHS Digital). HES data were initially collated subnationally by regional health authorities. Following the abolition of these bodies in 1996 the NHS-Wide Clearing Service took over until, in 2006, responsibility for HES data storage and management was taken over by the Secondary Uses Service, which was run by the Health and Social Care Information Centre and the National Programme for IT. HES data primarily serve administrative and financial purposes. Although not designed for these purposes, HES data are also widely used for research and health service evaluations.
The HES data are divided into financial years from 1 April to 31 March in the following year and cover admissions, outpatient appointments, and accident and emergency department attendances. HES data are recorded by clerical staff through patient administration or hospital information systems. They assign ICD-10 codes to clinical diagnoses recorded in medical notes. Data are a summary of each patient episode; they are not checked and there is no review of missing data or duplicate entries. The basic unit of measurement in HES is the ‘Finished Consultant Episode’, defined as an episode ‘where a patient completes a period of care under a consultant and is either transferred to another consultant or discharged’. HES data are stored as a collection of individual records for each period of care. A ‘patient key’ is derived from six HES fields (i.e. NHS number, date of birth, sex, postcode, provider code, local patient identifier). Each individual patient key is allocated to one unique pseudonymised HES identifier: the ‘HESID’. As some source fields can change, the HESID can be mapped to several different patient keys. The unique HESID and the discrete patient keys provide the means to uniquely identify a patient and track them in HES without the risk of revealing personal or sensitive information.
Hospital Episode Statistics maternity and birth data
The HES data are recorded on all births in NHS hospitals, non-NHS hospitals funded by the NHS and NHS home births in England. When a mother gives birth, her hospital admission record changes from a general inpatient admission record to a maternity record and is updated as such before it is submitted to HES. HES data contain two types of maternity record: the delivery and the birth record (both of which contain a ‘baby tail’ comprising an additional 19 fields). The delivery record relates to the mother and contains the same information as the general HES record, and the associated baby tail contains information about the delivery. The birth record relates to the baby and also contains general record information; the birth record contains the same information as the baby tail in the delivery record. Diagnoses and procedures recorded in the birth record refer to the baby and, conversely, diagnoses and procedures in the delivery record refer to the mother. For multiple births, separate tails for each baby appear in the delivery record, but each birth record contains only the individual baby tail.
Potential for linkage of National Neonatal Research Database with general practice records
It is estimated that > 98% of the UK population are registered with a general practitioner (GP), almost all of which use computerised record systems. During the Medicines for Neonates programme, several sources of GP records were identified and their utility for researching the health of neonates was explored. The largest and most comprehensive source of primary care data in the UK is the General Practice Research Database (GPRD),355 which has been widely used for research, from pharmacovigilance to risk score development, and is a rich source of longitudinal patient data. Information in GP records includes demographic data, coded clinical information including diagnoses, symptoms, preventative care and prescriptions. In the UK, a standardised hierarchical classification system of Read codes is used to record medical information in patient records. Alternative sources include The Health Improvement Network from similar and overlapping practices as well as directly accessing clinical records from smaller GP networks and individual practices. From 29 March 2012, GPRD became part of the Clinical Practice Research Datalink (CPRD), funded by the Medicines and Healthcare Regulatory Agency (MHRA) and the NIHR. The CPRD contains computerised clinical records from about 5 million active patients, 12 million patients overall, from 600 primary care practices across the UK, and is a nationally representative sample of around 8% of the UK population. The CPRD aims to maximise the way anonymised clinical data from the NHS can be used for observational research, using linkage to integrate data from primary care, secondary care and disease registries, with the aim of facilitating research that is beneficial to improving public health.
Our initial intention was to seek consent from parents during the hospital admission around the time of birth. However, we realised that that this was neither practicable nor necessary given alternatives whereby anonymised linkage between hospital and GP records were becoming available. To progress this avenue, we submitted a protocol for research ethics approval, which was approved by the Independent Scientific Advisory Committee for the MHRA, which reviews all research proposals for the use of the GPRD. Access to the data was granted free of charge under the previous Medical Research Council licence scheme with the GPRD. We focused our research on an exemplar project to demonstrate proof of concept that GP records could be used to build a birth cohort of infants with bronchiolitis, a common condition for which children are admitted to hospital. A cohort was created using medical records from the GPRD database and used to examine the natural history and management of bronchiolitis in the community setting. The main findings from this analysis of primary care data, conducted independently of the Medicines for Neonates programme, were that a cohort could be created and that data were available for research. By the end of the Medicines for Neonates programme we were, however, still awaiting access to linked hospital and general practice data and so were unable to address this area. After the Medicines for Neonates programme, data from around 50% of practices became available with linked hospitals records and practice records. We therefore recommend that this is a suitable area for future research.
Aims and objectives
We aimed to develop a continuous, longitudinal birth cohort through linkage between the NNRD and HES in order to provide a resource for observational and experimental studies of early exposures and interventions on later health outcomes in specific groups of newborn infants. Our objectives were to:
-
examine NNRD and HES data completeness and quality for key variables
-
link NNRD and HES data to create a birth cohort of infants admitted to neonatal units
-
examine level of agreement between NNRD and HES data
-
conduct a proof of concept study using linked data to ascertain health outcomes.
Methods
Approvals and time line
We received HES data at intervals over the duration of the Medicines for Neonates programme. Permission to receive NHS numbers was obtained later in the programme from the Confidentiality Advisory Group of the Health Research Authority Health Research Authority. Following approval we requested permission from all NHS Trusts in England that provide neonatal specialised care to receive infant identifiers, including the NHS number, for the purpose of data linkage. This workstream was undertaken by several research personnel over the course of the Medicines for Neonates programme.
Design
We conducted two methodological studies to assess the feasibility of using administrative hospital data to build birth cohorts for child health research. We used HES data covering the financial years 2005/6–2009/10 (study 1), and HES and NNRD data for the calendar year 2010 (study 2).
We engaged with stakeholders including health professionals within NW London CLAHRC and expert patients and parents of preterm babies to get their input into grant applications and design of studies. We held educational meetings, focus groups and dissemination events to obtain feedback at all stages of the study.
Birth episodes
We identified all individual birth episodes in HES. In study 1, we used the ‘admimeth’ variable, which contains a code recording how the patient was admitted to hospital. We used this field to select records with an admission method coded 82 (babies born in health-care provider) or 83 (babies born outside the health-care provider, except when born at home as intended). In study 2, we also used the ‘epitype’ variable, which contains a code defining the reason for which the patient was admitted to hospital. We used this field to select all records with an episode type coded 3 (birth episode) or 6 (other birth event).
Duplicate records
We identified duplicate birth episodes using the HESID in study 1 and the NHS number in study 2. If episodes were identical matches for all variables, only one record was retained. When records did not contain matching information, we retained the birth episode with the most diagnostic information (number of non-empty diagnosis fields).
In study 2, we excluded babies with a missing date of birth because it was not possible to verify if the records referred to a birth episode or a subsequent hospital readmission. We also excluded records with a missing NHS number. When single NHS numbers were associated with multiple HESIDs, we excluded them and their associated fields from the analysis because it was not possible to determine which record was correct.
Data management
Babies can have more than one episode of care within their birth admission; for example, if a baby receives specialist care from a different consultant or is transferred between hospitals. These additional episodes occur within the same birth admission but, where the initial birth event would have an ‘epiorder’ value of 1, subsequent episodes have an ‘epiorder’ value of > 1. To facilitate one-to-many linkage to subsequent hospital admission records, we developed a data set consisting of one row per individual. We incorporated key information, such as diagnostic codes, into the original birth episode and dropped subsequent episodes in the birth admission. Up to nine birth tails can be recorded for each delivery, allowing information from multiple births to appear in the mother’s delivery record. Identical baby tail information for each baby can be found in their mother’s delivery record. Therefore, if we found that a baby’s information was not recorded in the first field of a given variable, we condensed records to retain only one field for each variable. For example, if a baby was the second twin, their gestational age at birth (‘gestat’) may have appeared in the second field (‘gestat_2’) with the first field (‘gestat_1’) blank because in the mother’s delivery record this contained the first twin’s gestation. In this case, we transferred information for the gestation variable from ‘gestat_2’ into ‘gestat_1’ and then removed all additional fields (i.e. ‘gestat_2’ to ‘gestat_9’) for that variable. We identified stillbirths using the ‘birth status’ and ‘discharge method’ fields and removed these from the final cohort.
A range of exclusion criteria was developed to clean key variable fields and examine the quality of coding. The Care Quality Commission (CQC) conducted a review exploring quality indicator specifications used to assess the quality of HES maternity data from 2009 to 2010. We combined the criteria identified within the CQC review and HES inpatient cleaning rules and applied these to the HES birth fields to ensure that suspicious data and invalid records were removed. Fields validated by the CQC and related to maternity episodes were assessed using the following criteria: when values were ‘not known’, invalid or outside a specific range, the field was recorded as blank or ‘9’; dates of birth outside the birth admission were set as invalid; flags to determine finished and unfinished episodes were created.
Data completeness and quality
We examined the completeness of HES recording for baby tail fields over 5 years (2005/6–2009/10) and compared the total number of births with ONS birth registrations (study 1). We compared the proportion of missing data for each baby tail field in 2005/6 to 2009/10 using chi-squared tests. We compared the characteristics of hospitals using a cut-off point of 90% completeness of recording for key birth fields (gestational age and birthweight). To test for significant differences between hospitals with high versus low completeness of birth record fields, we used chi-squared tests to compare proportions and t-tests to compare mean values.
For calendar year 2010 (study 2), we analysed the completeness of recording in HES and the NNRD for the key variables (infant sex, gestational age, birthweight, multiple birth, LSOA, maternal age and ethnicity). We compared the proportion of complete data for each variable by gestational age group. We explored the distribution of birthweight by gestational age in both sources. We also examined the standardised distribution of birthweight by gestational age (birthweight z-score percentiles). We used the LMS growth Excel add-in program from the Medical Research Council, UK, based on the British 1990 growth reference356 to determine gestational age and sex-specific birthweight SDSs for both HES and the NNRD. We excluded observations for infants who were above (> 99.9th centile) or below (< 0.1st centile) 4 SDSs from the population mean.
Record linkage and agreement between Hospital Episode Statistics and the National Neonatal Research Database
We re-coded data when necessary to provide a common format for linkage. Non-informative characters and punctuation were removed from the diagnosis and procedure variables. We used a deterministic approach to link the NNRD and HES records using the NHS number as a common unique identifier. We performed a one-to-one merge of records from both sources. As we expected one unique record in the NNRD to be linked to one record in HES, we considered a successful one-to-one linkage a positive match. We created a new data set with single birth episodes and common variables from each source. Records in the NNRD that did not have a corresponding match in HES were retained separately. The data linkage rate was calculated by using the number of positive matches divided by the total number of records available for matching in the NNRD. We performed linkage in two stages. In the first stage, we utilised all records from both databases and, in the second stage, we excluded all values for birthweight by gestational age that were outside a predefined range. We compared infant sex, gestational age, birthweight, multiple birth, social deprivation, maternal age and ethnicity in linked and unlinked babies. Social deprivation was assigned using the IMD. This is based on 32,482 geographic LSOAs across England. Economic, social and housing indicators are combined to provide a score for each LSOA; a high score indicates greater deprivation. We split the birth population into IMD quintiles for comparison. We used one-way ANOVA to compare continuous variable means, and the chi-squared test to compare categorical variables. We calculated the percentage overall agreement and Cohen’s kappa, a measure of agreement adjusted for the proportion of agreement that would be expected on the basis of chance. We considered kappa values above 0.80 to indicate almost perfect agreement. 260,261 Analyses were carried out using SAS.
Health outcomes (based on the exemplar condition bronchiolitis)
We created a birth cohort from HES data for all infants born in English NHS hospitals and discharged during a 12-month period (from 1 April 2007 to 31 March 2008). We included only records from live births and excluded infants born in hospitals (85/156) with poor recording (< 90% complete) of key indicators (birthweight and gestational age) to enable us to group infants into term and preterm categories. We conducted sensitivity analyses based on number of maternity beds, annual number of births, geographic location and infant death rate to compare high- and low-recording hospitals. We linked birth records to subsequent hospital admission records up to a child’s first birthday, using the unique personal identifier (HESID). We identified deaths up to the age of 1 year, including out-of-hospital deaths, through linkage to ONS mortality records.
We used diagnostic information in individual birth records and any subsequent hospital admission records from the study year to group infants into categories of risk factors for severe respiratory syncytial virus (RSV) infection using ICD-10 codes, or larger subgroups using the Agency for Health Research and Quality’s Clinical Classification System (CCS):357
-
Immunodeficiency: CCS group 57 (immunity disorders – this includes ICD-10 codes D80, D81, D82, D83, D84 and D89).
-
Cystic fibrosis: CCS group 56 (cystic fibrosis – this includes ICD-10 codes under E84).
-
Chronic lung disease: ICD-10 codes P27 (chronic respiratory disease originating in the perinatal period) and P28 (other chronic respiratory diseases originating in the perinatal period).
-
Congenital heart diseases: CCS group 213 – this includes ICD-10 codes Q20, Q21, Q22, Q23, Q24, Q25, Q26, Q27 and Q28.
-
Nervous system congenital anomalies: CCS group 216 (this includes ICD-10 codes Q00 to Q07 which incorporate conditions such as spina bifida, anencephaly and other congenital malformations of the nervous system).
-
Other congenital anomalies and perinatal conditions: CCS groups 224 and 217 (this includes a broad range of congenital anomalies and perinatal conditions with ICD-10 P- and Q- codes, excluding those included within other definitions listed above, such as codes for chronic lung disease).
-
Down’s syndrome: ICD-10 code Q90.
-
Cerebral palsy: ICD-10 code G80.
If a birth record had no gestational age recorded (i.e. premature status was unknown), then the infant was assumed to be not preterm on the basis that they had similarly low intensive care unit admission rates and short length of stay at birth was with infants in the group known to be born at term.
We identified infants admitted with a primary diagnosis of acute bronchiolitis using the ‘J21’ ICD-10 codes (J210: acute bronchiolitis due to respiratory syncytial virus; J218: acute bronchiolitis due to other specified organisms; J219: acute bronchiolitis, unspecified). We grouped all bronchiolitis codes into a single category. We examined age at bronchiolitis admission and calculated the median length of stay for bronchiolitis admissions. We calculated the absolute risk of a bronchiolitis admission among infants with and without risk factors for severe infection. Infants without a particular risk factor condition were considered ‘healthy’. We used Poisson approximation to calculate 95% CI. We calculated the relative risk (RR) of a bronchiolitis admission, with associated 95% CI for infants in each individual risk group, by comparing them with the baseline group of infants without the particular risk factor. Infants may belong to more than one of these risk groups, so we controlled for this potential confounding using Poisson regression models to calculate the adjusted RR of bronchiolitis admission for infants in each risk group. To test for significant differences between proportions we used chi-squared tests and Mann–Whitney U-tests to compare median values for non-normal data. Data were analysed using the SAS 9.2 software package.
We have reported our findings in line with the reporting standards for observational research (RECORD statement). 358
Results
Completeness of Hospital Episode Statistics data (study 1)
The proportions of missing/unknown HES data by field for the period 2005/6 to 2009/10 are shown in Table 43. The proportion of missing or unknown data in key birth record fields decreased significantly over the 5-year period; for example, missing gestational age fell from 46.2% in 2005/6 to 18.1% in 2009/10, and birthweight from 43.9% in 2005/6 to 16.9% in 2009/10. Overall, the HES cohort captured 87% of all live births recorded by the ONS in England during the period 2005/6 to 2009/10.
| Baby tail fields in HES birth records (field name) | % missing or unknown | p-value | ||||
|---|---|---|---|---|---|---|
| 05/6 | 06/7 | 07/8 | 08/9 | 09/10 | ||
| Anaesthetic given during labour or delivery (delpren) | 41.9 | 41.8 | 44.8 | 29.6 | 16.5 | < 0.001 |
| Anaesthetic given post-labour or delivery (delposn) | 48.1 | 46.0 | 49.6 | 34.8 | 21.6 | < 0.001 |
| Antenatal days of stay (antedur) (derived from other HES fields) | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | < 0.001 |
| Baby’s age in days (neodur) (derived from other HES fields) | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | < 0.001 |
| Birth order (birorder) | 33.9 | 36.9 | 39.4 | 24.9 | 13.7 | < 0.001 |
| Birthweight (birweit) | 43.9 | 47.1 | 50.1 | 31.3 | 16.9 | < 0.001 |
| Delivery place change reason (delchang) | 45.2 | 45.8 | 47.4 | 34.6 | 21.7 | < 0.001 |
| Delivery method (delmeth) | 35.1 | 35.8 | 44.3 | 30.6 | 14.9 | < 0.001 |
| Delivery place (actual) (delplace) | 44.0 | 46.8 | 57.0 | 41.3 | 17.9 | < 0.001 |
| Delivery place (intended) (delinten) | 41.3 | 42.7 | 43.6 | 30.2 | 14.9 | < 0.001 |
| First antenatal assessment date (anasdate) | 41.7 | 44.3 | 44.6 | 34.7 | 20.4 | < 0.001 |
| Gestation in weeks at first antenatal assessment (anagest) | 54.5 | 63.9 | 55.2 | 45.6 | 28.3 | < 0.001 |
| Length of gestation (gestat) | 46.2 | 54.2 | 48.0 | 34.6 | 18.1 | < 0.001 |
| Birth status (birstat) | 43.9 | 47.0 | 48.0 | 32.9 | 16.2 | < 0.001 |
| Labour/delivery onset method (delonset) | 36.2 | 37.7 | 41.1 | 25.5 | 11.5 | < 0.001 |
| Mother’s age at delivery (matage) | 42.4 | 43.3 | 43.0 | 34.5 | 30.5 | < 0.001 |
| Neonatal level of care (neocare) | 16.1 | 16.0 | 17.1 | 18.4 | 12.4 | < 0.001 |
| Number of babies (numbaby) | 31.8 | 33.3 | 36.1 | 23.6 | 11.9 | < 0.001 |
| Resuscitation method (biresus) | 44.2 | 45.3 | 48.0 | 34.2 | 21.1 | < 0.001 |
| Status of person conducting delivery (delstat) | 38.9 | 42.6 | 48.4 | 33.9 | 19.1 | < 0.001 |
| Total number of births | 554 | 566 | 575 | 589 | 603 | – |
| Total number of birthsa | 521 | 749 | 493 | 684 | 786 | – |
We tested the effect of selecting birth records only from hospitals with high completeness of recording by creating a 2007/8 birth cohort comprising birth records only from hospitals where ≥ 90% of their birth records contained complete recording of the key variables, birthweight and gestational age. The resulting cohort included 296,618 babies born at 71 hospitals across England. Table 43 shows a comparison of characteristics of included (n = 71) and excluded (n = 85) hospitals. The mean numbers of births, maternity beds and access to neonatal intensive care (Table 44) were mostly similar among hospitals with high and low completeness of recording. The mean maternal age, the proportion of babies of non-white British ethnicity and the proportion of babies in the most deprived quintile were similar among the two groups of hospitals. Full details are provided in Murray et al. 360
| Hospital maternity factors | Hospitals with | p-value | |
|---|---|---|---|
| High completeness (n = 71) | Low completeness (n = 85) | ||
| Mean (SD) number of annual births | 3957 (2011) | 3465 (1997) | 0.13 |
| Mean (SD) number of maternity beds | 55.1 (30.3) | 55.3 (26.4) | 0.96 |
| Mean (SD) occupied maternity beds | 35.4 (21.4) | 35.2 (18.0) | 0.95 |
| Number (%) with neonatal intensive care | 52 (73) | 68 (80) | 0.30 |
| Mean (SD) number of neonatal intensive care cost | 10.6 (11.7) | 11.4 (10.9) | 0.66 |
| Mean maternal age (% missing data) | 28.9 (18.4) | 29.0 (70.1) | 0.39 |
| Proportion of births per hospital in most deprived deprivation score quintile (% missing the data) | 0.472 (69.4) | 0.435 (56.2) | 0.64 |
| Proportion of births of non-white British ethnicity (% missing the data) | 0.527 (7.1) | 0.564 (5.1) | 0.64 |
Completeness of National Neonatal Research Database and Hospital Episode Statistics data (study 2)
There were 66,403 records in the NNRD of admissions into NHS neonatal specialised care units for the period 1 January to 31 December 2010, of which 66,117 (99.6%) had complete recording of gestational age. For babies with a valid gestational age, all NNRD variables with the exception of maternal age were complete in > 90% of the records. NNRD records represented 9.7% of births identified in HES (683,556 records) for the same period. After removing duplicates and data cleaning, 651,073 babies remained. Of these, 528,671 (81.2%) had a complete recording for gestational age. For babies with a valid gestational age, HES records were complete in over 90% of cases (Table 45) with the exception of multiple birth and maternal age at delivery, for which completeness ranged from 84.4% to 91.7% and 77.9% to 87%, respectively, and LSOA, for which completeness was 0.01%.
| Key variables | HES | NNRD | ||||||
|---|---|---|---|---|---|---|---|---|
| Gestation weeks | Gestation weeks | |||||||
| ≤ 32 | 33–36 | 37–42 | ≥ 43 | ≤ 32 | 33–36 | 37–42 | ≥ 43 | |
| Infant sex | 99.8 | 99.9 | 99.9 | 99.9 | 100.0 | 100.0 | 99.9 | 100.0 |
| Birthweight | 93.9 | 91.5 | 91.2 | 97.1 | 100.0 | 100.0 | 100.0 | 100.0 |
| Number of babies | 91.7 | 86.4 | 88.2 | 84.4 | 99.9 | 99.8 | 99.8 | 100.0 |
| Maternal ethnicity | 95.8 | 95.0 | 94.4 | 94.5 | 96.6 | 97.4 | 94.8 | 92.5 |
| Maternal age | 87.0 | 78.3 | 77.9 | 79.1 | 66.8 | 72.6 | 66.5 | 50.0 |
| LSOA | 0.0 | 0.0 | 0.0 | 0.0 | 96.0 | 95.8 | 93.5 | 90.0 |
| Total | 13,883 | 28,648 | 48,3091 | 3025 | 10,533 | 19,300 | 36,248 | 40 |
In HES, the distribution of gestational age and birthweight was discordant with a large number of unlikely outlying combinations, especially for babies born preterm (Figure 26a). In the NNRD there were fewer outliers (Figure 26b). After excluding babies with values above and below 4 SDSs, the HES distribution approximated more closely to the NNRD (Figures 26c and 26d).
FIGURE 26.
Distribution of birthweights by gestational age in HES and the NNRD. (a) All babies HES; (b) NNRD; (c) after excluding birthweights above and below 4 SDS HES; and (d) NNRD.
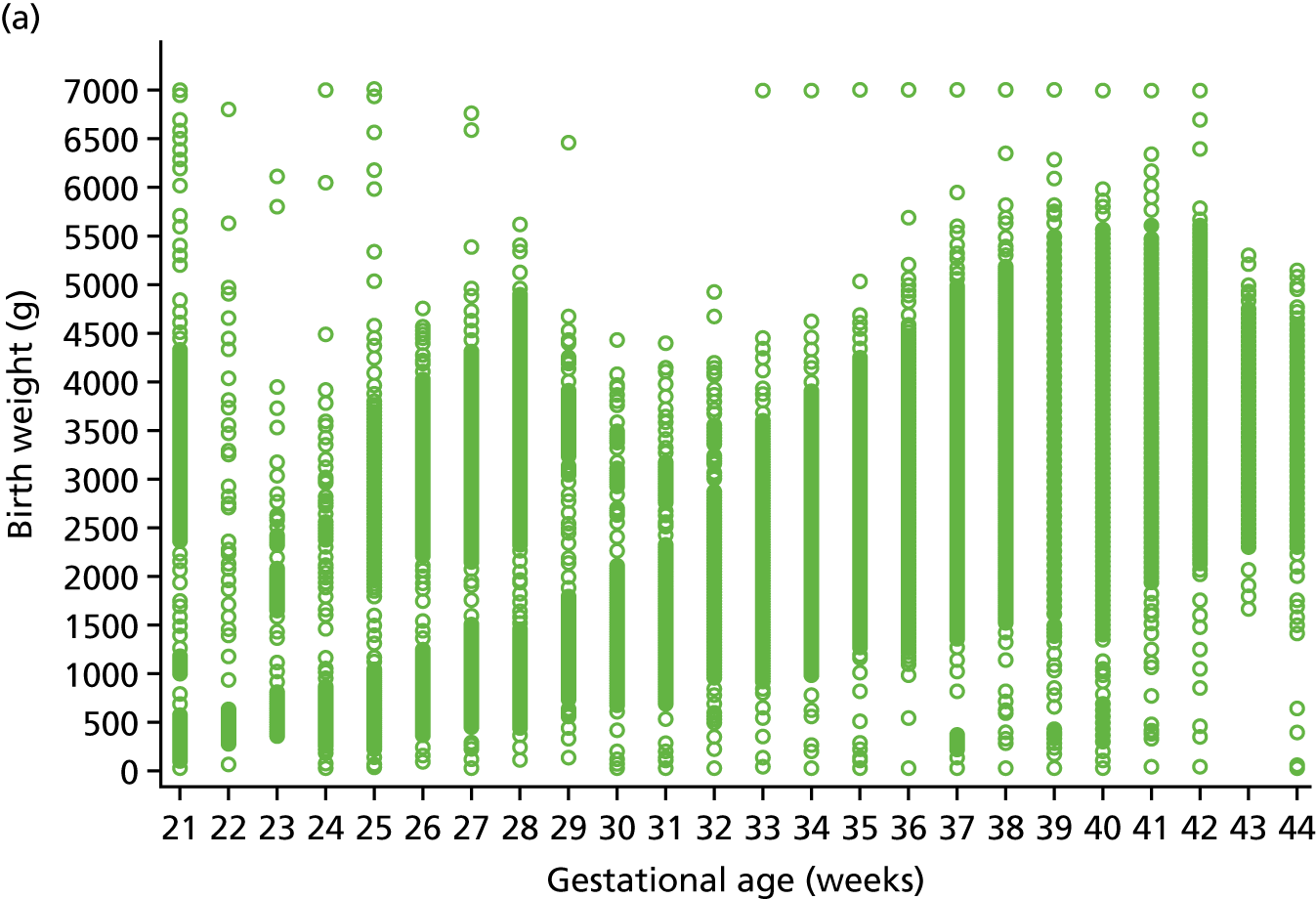
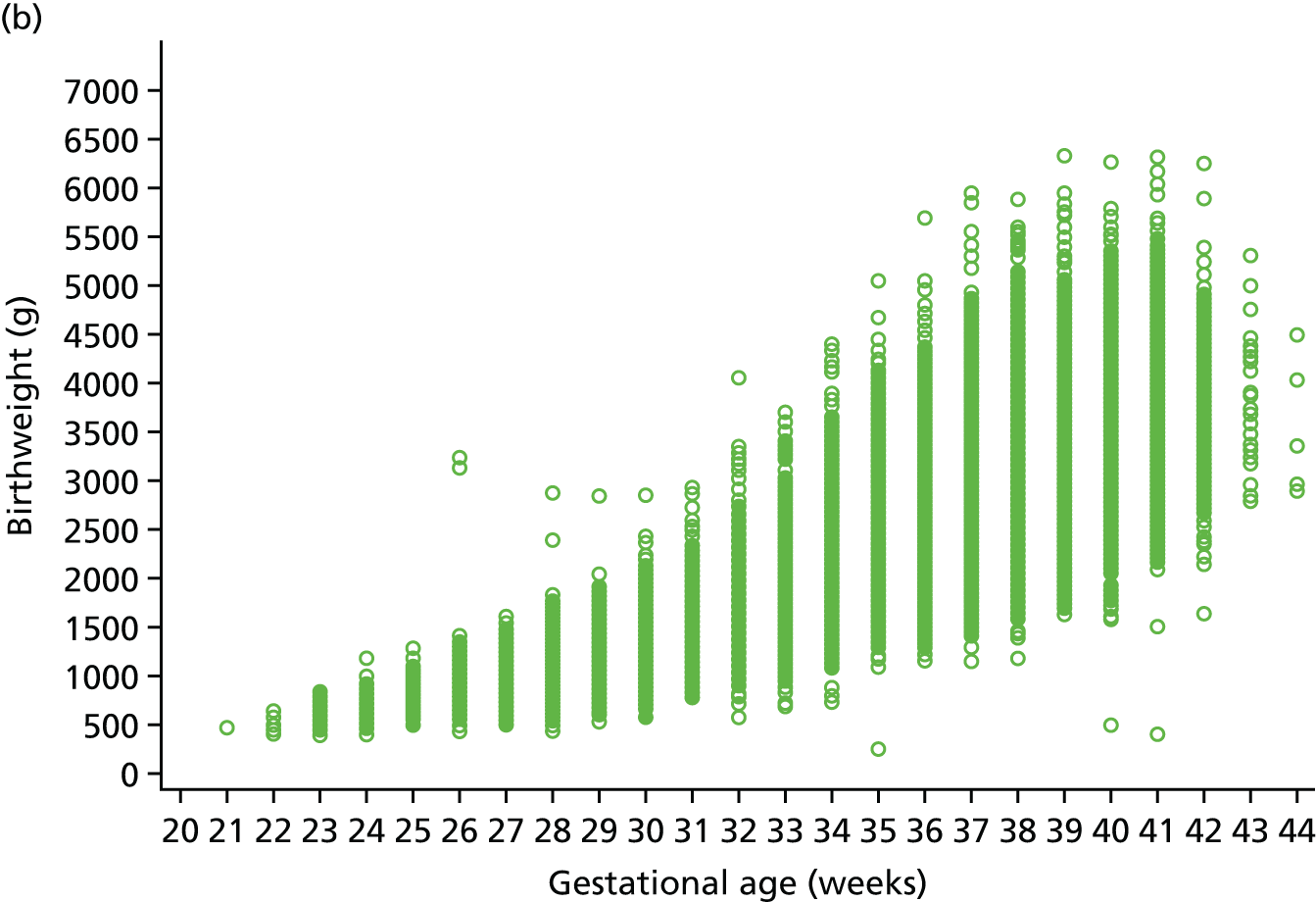


Record linkage, Hospital Episode Statistics and National Neonatal Research Database
We included only neonatal units from which permission had been obtained to receive identifier data at the time of study 2 (n = 159). When records from neonatal units that did not give permission to access NHS number were excluded from both sources, 47,345 and 650,301 babies remained eligible for record linkage in the NNRD and HES, respectively. Of 47,345 eligible NNRD records, 44,426 (93.8%) were successfully linked to HES (Figure 27). We combined information gained through record linkage by replacing missing values with information from either of the two data sources. After excluding babies with missing values (0.1%; step 2, see Figure 27) and babies with birthweight values above or below 4 SDSs (0.3%; step 3, see Figure 27), 44,271 records remained. Of these, 7463 (16.9%) were born before 32 weeks’ gestation, 13,569 (30.7%) between 33 and 36 weeks, 18,493 (41.7%) between 37 and 40 weeks, and 4746 (10.7%) ≥ 41 weeks’ gestation. In the second stage, we excluded babies with missing values and implausible birthweights before linkage (26.4% and 1.5% in HES; 0.1% and 0.2% in the NNRD), leaving 61.3% of babies in the NNRD successfully linked to HES.
FIGURE 27.
Flow diagram of record linkage.
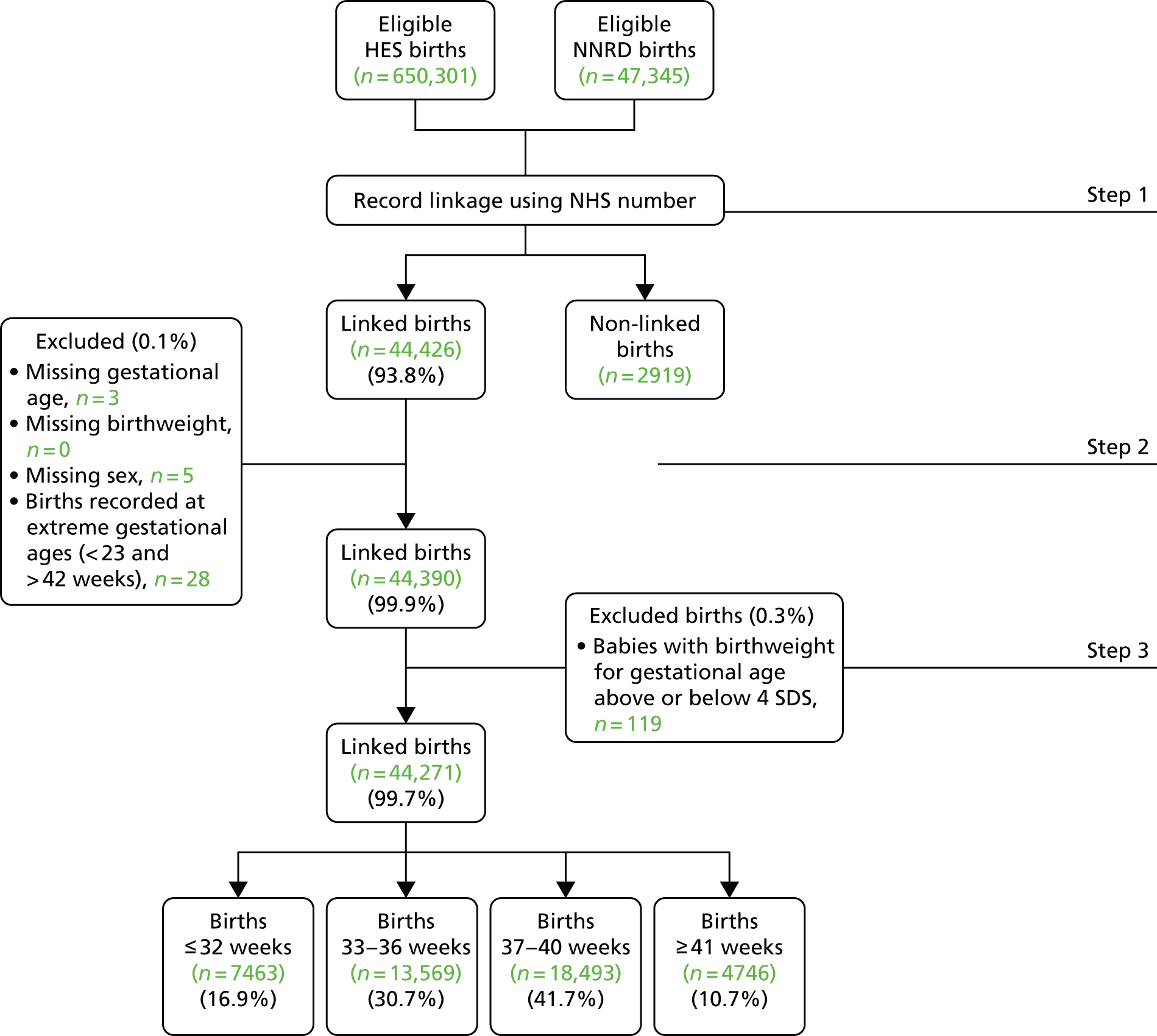
Table 46 shows the comparison of characteristics of babies linked (n = 44,426) and unlinked (n = 2919). Multiplicity at birth, birthweight and gestational age differed significantly between linked and unlinked babies. The unlinked group had more multiple births (unlinked 21.3%; linked 13.9%), extremely preterm births below 32 weeks’ gestational age (unlinked 24.1%; linked 16.8%), and lower birthweights (unlinked 2.527 kg; linked 2.682 kg). In a sensitivity analysis, restricted to singleton babies (unlinked 2290; linked 38,176), birthweight and gestational age remained significantly different between linked and unlinked groups.
| Fields | NNRD babies, n (%) | p-value | |
|---|---|---|---|
| Linked to HES | Not linked | ||
| Infant sex | 0.012 | ||
| Male | 25,182 (56.7) | 1584 (54.4) | |
| Female | 19,200 (43.3) | 1330 (45.6) | |
| Gestational age (weeks) | < 0.0001 | ||
| ≤ 32 | 7479 (16.8) | 702 (24.1) | |
| 33–36 | 13,602 (30.6) | 804 (27.6) | |
| 37–42 | 23,303 (52.5) | 1411 (48.4) | |
| ≥ 43 | 20 (0.1) | 0 (0) | |
| Birthweight, mean (SD) | 2681.5 (939.9) | 2527.4 (1024) | < 0.0001 |
| Multiple birth | < 0.0001 | ||
| Singleton | 38,208 (80.8) | 2294 (78. 7) | |
| Multiple births | 6185 (13.9) | 622 (21.3) | |
| Maternal age, mean (SD) | 29.4 (6.3) | 29.7 (6.4) | 0.018 |
| Maternal ethnicity | 0.75 | ||
| White British | 30,108 (72.5) | 1947 (72.2) | |
| Non-white British | 11,401 (27.5) | 748 (27.8) | |
| IMD score quintiles | 0.43 | ||
| 1 (most deprived) | 8568 (20.1) | 530 (19.2) | |
| 2 | 8552 (20.0) | 548 (19.9) | |
| 3 | 8568 (20.1) | 530 (19.2) | |
| 4 | 8523 (19.9) | 577 (20.9) | |
| 5 (least deprived) | 8527 (20.0) | 572 (20.8) | |
| Total | 44,426 | 2919 | |
Agreement between Hospital Episode Statistics and National Neonatal Research Database
Table 47 shows the level of agreement between HES and NNRD for the final linked birth cohort. For the key variables studied, overall agreement was > 95% (kappa coefficient 0.97 to 0.99) with the exception of gestational age (81.0%) and maternal ethnicity (86.1%) (kappa 0.71 and 0.79, respectively).
| Fields | Overall agreement (%) | Kappa coefficient | p-valuea |
|---|---|---|---|
| Infant sex | 99.5 | 0.99 | < 0.0001 |
| Gestational age | 81.0 | 0.79 | < 0.0001 |
| Birthweight | 98.1 | 0.98 | < 0.0001 |
| Number of babies | 98.2 | 0.92 | < 0.0001 |
| Maternal age | 99.3 | 0.99 | < 0.0001 |
| Maternal ethnicity | 86.1 | 0.71 | < 0.0001 |
| LSOA | 96.9 | 0.94 | < 0.0001 |
Admissions with bronchiolitis (study 1)
The birth cohort included 296,618 infants from 71 NHS hospitals in England; 410 infants in the cohort died during the study year; 51% (151,897/296,618) were boys, 1% (2,891) were multiple births and 7.5% (22,215) were born preterm before 37 weeks’ gestation. We identified 7189 admissions to hospital over the period 1 April 2007 to 31 March 2008 with a primary diagnosis of bronchiolitis up to the age of 1 year [admissions per 1000 infants 24.2 (95% CI 23.7 to 24.8)]. Of these, 2015 (28.0%) were specifically coded as being due to RSV and the remainder were coded ‘unspecific aetiology’. In total, 1529 (21.3%) infants had more than one bronchiolitis admission during their first year. The modal age for bronchiolitis admission was 1 month and the median age was 120 days (IQR 61 to 209 days). The median length of hospital stay was 1 day (IQR 0 to 3 days). The majority of infants admitted with bronchiolitis were not in recognised high-risk groups, with only 24% (1722/7189) having one or more recognised risk factors for severe infection.
Discussion
We conducted a series of analyses involving the NNRD containing point-of-care, clinician-entered health-care data, and HES maternity records containing administrative data. We found that the completeness of HES birth records varies substantially between hospitals but has improved over time. Data quality and completeness of recording were better in the NNRD than in HES for most key variables, including gestational age. We demonstrated the feasibility of record linkage between HES data and the NNRD. We also showed the feasibility of linkage of HES records across time to quantify and describe the burden of bronchiolitis, an important infectious disease of infancy. Our work provides proof of principle that routine NHS data sources may be utilised to create national longitudinal birth cohorts and that combining HES with the NNRD can substantially enhance the quality and scope of birth records. Our methods pave the way for future studies to support research, ascertainment of longer-term outcomes of babies admitted to neonatal units, and the delivery of neonatal specialised care, a high-cost, nationally commissioned clinical service.
We accept that our comparisons were limited to specific fields; the large number of missing data is a further consideration. The distribution of birthweight by gestational age revealed a large number of inconsistent values in HES compared with a more plausible distribution in the NNRD. Removal of implausible birthweights, prior linkage, resulted in a reduced NNRD to HES linkage rate comprising approximately two-thirds of the total number of babies. We performed only deterministic linkage, and probabilistic linkage is an alternative strategy. Linkage using a unique identifier, such as the NHS number, is considered highly acceptable with the greatest face validity, but combining probabilistic with deterministic linkage might have increased the linkage rate. Another limitation of our study is that we were unable to determine how accurately the HES identifier (the patient key) we used to link records across time is allocated to unique individuals.
We have shown that it is possible to identify infants up to the age of 1 year admitted to NHS hospitals and to report on the population burden of an important infant condition: bronchiolitis. Just over one-fifth of infants admitted with bronchiolitis had a further admission for the same condition during their first year. Our study has highlighted that the burden of bronchiolitis hospital admissions among infants in England predominantly affects those born at term, without any risk factors for severe infection, and the age at admission appears to be significantly lower now than previously reported. Although risk of admission is higher in known risk groups, 85% of infants admitted to hospital with bronchiolitis in England have no known predisposing risk factors. We also found that infants with Down’s syndrome, cerebral palsy and cystic fibrosis appear to be at higher risk of hospital admission. Our findings in study 2, namely that HES data were more likely to be missing for preterm babies, indicate that this was a weakness of our analysis. Other important limitations are that our case definition for bronchiolitis and comorbidity was dependent on the accuracy of clinical coding and recording in diagnosis fields in HES records. We combined RSV and unspecified bronchiolitis, presenting data on all bronchiolitis admissions. Only 28% of the bronchiolitis admissions were coded as being due to RSV, and the remainder had an unspecific bronchiolitis code. We found that the median length of stay for bronchiolitis admissions was only 1 day, suggesting that improved management in the community may reduce the need for admission.
Our study extends earlier work by investigating not only the completeness but also the quality of HES data. We found that about one-fifth of babies in HES have missing gestational age data,360–362 but a novel finding is that HES had 1.5% of recorded birthweights outside a biologically plausible range. Our analyses also showed that infants with missing HES data were more likely to have been very preterm (< 32 weeks’ gestation) and have a lower birthweight. A possible explanation is that infants born very preterm are more likely to be missed as a birth in HES registrations and coded as a new admission, as they are admitted directly to a neonatal unit.
Linkage of HES to other data sources has been explored in previous studies. Dattani et al. 361 linked maternity HES, birth registration data and NHS ‘numbers for babies’ to assess the quality and completeness of these sources, using the NHS number in combination with other information as identifiers, and achieved a similar linkage rate of > 90%. Hockley et al. 362 applied a variety of linkage methods for data from Scotland, Wales and England and concluded that the use of a probabilistic method for data from England would not have improved the linkage rate because of the poor completeness of HES data. This supports our choice of using a deterministic method with a single identifier for linkage.
The effectiveness of routine health record linkage in adults has been demonstrated in Australia and Canada, where it has improved both data quality and utility. 363–366 Birth records have been successfully linked to hospital discharge data in Australia, with matching rates of 99%. 367 In several regions of the USA, data from birth certificates have been linked to hospital discharge records to examine maternal outcomes. 368,369 In Scandinavian countries, the assignment of unique personal identification numbers permits linkage between civil registration systems and enables the development of population-based cohorts. These have facilitated a broad array of epidemiological studies such as investigations of the impact of place of birth, familial risk factors for autism370 and the association between prenatal exposures and ADHD in childhood. 371 In Wales, the Secure Anonymised Information Linkage databank brings together anonymised person-based electronic health and social care data. This is being combined to establish an anonymised Wales-wide Electronic Cohort for Children. 372,373 This databank has been used successfully to identify potential clinical trial participants from primary care data. 372–374 The Scottish Health Informatics Programme (SHIP) is an example of a complex database of linked EPRs, providing health and social care information from birth through to death. 375 To date, SHIP data have primarily been used to conduct pharmacovigilance and diabetes epidemiology research. 375,376 Another UK cohort is the Oxford Record Linkage Study, established in 1963 and comprising > 10 million records on around 5 million people. 377 This has been used in longitudinal research studies to identify maternal and perinatal risk factors for conditions such as inflammatory bowel disease,378 asthma379 and coeliac disease. 380
Other aspects of routine NHS data are worth mentioning. Stand-alone maternity systems in around 20 hospitals are not linked to their patient administration system, from which HES data are obtained. 380–382 Some hospitals return data on birth or delivery episodes but not both, and stillbirths are neither reliably recorded in every hospital nor allocated a NHS number. 382,383 We suggest that future studies involving HES records are likely to benefit from steps to check data quality as well as completeness. Improvement in administrative data quality and completeness are important health services goals. Reducing reliance on administrative data, promoting clinician involvement in data assurance and, as is the case with the NNRD, extracting maternity data from electronic health records are measures that also merit wider consideration.
Implications for health care
We have shown that EPR data can be used to create UK birth cohorts. The sole use of the NHS administrative database, HES, to build birth cohorts will result in the inclusion of many babies with missing gestational age data and implausible birthweights. However, linking the NNRD to HES substantially enhances the quality and scope of UK birth records. Improvements in administrative and clinical data quality and completeness are important health services goals.
Research recommendations
Researchers who are planning to study the association of gestational age with specific outcomes in childhood may find it helpful to use the NNRD. If HES data are used, we recommend removing implausible birthweights for gestational age from the study cohort in order to improve the validity of the conclusions.
More exploration is needed to exploit the use of the NNRD linked to HES to understand the epidemiology and health-care resource utilisation of conditions, such as bronchiolitis in healthy infants and infants with multiple comorbidities, and to examine the long-term independent impacts of preterm births and such conditions on health in childhood.
Chapter 8 Parent involvement in the National Neonatal Research Database
Abstract
Background: Parents (or legal guardians) have a primary responsibility for contributing to the current debate on the use of clinical data in research on behalf of their infants.
Aims: We aimed to establish a PPI group of parents with experience of a baby in neonatal care. We aimed to co-design with this group a survey for parents of infants admitted to neonatal units in England in order to obtain their views.
Methods: We undertook a review of the literature on public understanding of the use of clinical data for research purposes and identified parameters of relevance to the intended survey. We established and supported a PPI group to co-design a survey. Research nurses at each of 28 participating hospitals approached potential parent participants to explain the study, provide written information (available in eight languages) and seek consent (see Appendix 5).
Results: The survey was completed by 1319 parents or primary carers. Overall, there was a very high level of support for the use of health data for research purposes, with parents of babies who had experienced higher intensity care more likely to say ‘yes’. Over 80% and 85% of respondents respectively were very or fairly confident about data security and accuracy. We identified a high level of altruism. Nearly two-thirds agreed with ‘opt out’ as the default position for data-sharing.
Conclusions: There is strong parent support for sharing health data for research. The identification of effective and efficient methods to improve knowledge of potential benefits, processes and regulation are important to secure trust and confidence in the use of clinical data in research.
Background
The use of routinely recorded clinical data for research purposes is a key concern of contemporary e-health policy, research governance and public debate. 384 For infants and young children, without autonomous decision-making capacity, it is their parents (or legal guardians) whose voices represent their contribution to the debate on the values, benefits, risks and uncertainties of permitting use of their clinical data for research. In the case of the neonatal care population, at the time of the commencement of the Medicines for Neonates programme, the attitudes of parents were unknown as we had been unable to identify any prior study in this specific clinical care context. 385 Influences on the diversity of parental attitudes, the acceptability of data use and what, if anything, might be particular to the neonatal care context were unexplored.
Aims and objectives
We aimed to conduct a survey of attitudes in relation to the use of personal data in research of parents whose children were in receipt of neonatal care services across NHS sites in England. Our objectives were to:
-
explore the relevance, in the newborn context, of dimensions of public attitudes to data used for research purposes that have been previously identified from the literature
-
identify additional issues and concerns from the perspective of parents that are relevant to the newborn context
-
identify advantages, disadvantages and preferences for communication of information and knowledge about the use of data for research purposes.
Methods
Survey instrument design, and patient (parent) and public involvement
Two strands of work informed the design of the survey instrument that was to be completed by parents (and/or primary carers) who had a baby or babies in neonatal care.
First, we undertook an initial review of literature concerning public understanding of health data use for research purposes and contemporary e-health policy and identified 10 parameters of relevance to the survey design:385
-
being specific about what counts as routinely collected health/patient/clinical data in the context of the investigation
-
the influence of the digital and e-format of data on patient and public understanding and attitudes
-
data use in the context of protection, promotion and prevention
-
informed consent in relation to identifiable versus de-identified data
-
personal benefit, indirect benefit and altruism
-
the framing of routine data use for research purposes within professional discourse(s)
-
informed choice may not be synonymous with informed consent
-
privacy and confidentiality are distinct but related
-
rational and emotional approaches to decision-making
-
the balance of rights and responsibilities.
Second, we established a PPI group (10 mothers and one father) with previous experience of a child in neonatal care to be supported to co-design the survey instrument. Parents were recruited following response to an advert sent to community groups, online support groups and through Bliss networks. We specifically targeted two geographical areas, in the North West and the West Midlands, where we could reasonably assume a diverse population and which had well-established Bliss support groups. Advertising and recruitment and information materials were made available in eight languages in addition to English (Urdu, Punjabi, Bangla, Mandarin, Somali, Polish, French and Spanish). The two groups met separately on the first two occasions, then jointly for the subsequent three meetings. Each meeting lasted 4 hours with suitable breaks facilitated by one of the research team.
The study was approved by the University of Manchester Research Ethics Committee (reference 10/H1013/35). The aims of the parent groups were to:
-
inform the research team, based on personal experience, of the key questions, benefits and concerns associated with the routine use of babies’ clinical data for research purposes and thus contribute to the content of the survey items
-
provide guidance to the research team on the format of the survey including how to ask questions, in which order and why to maximise uptake, increase clarity and minimise any potential distress to parents completing the survey.
The research team’s responsibility was conceived of as:
-
facilitating information and experience sharing relevant to the study, in a manner that supported all who were involved
-
equipping parents with additional knowledge skills, should they not already possess them, that would enable them to fully participate in the instrument design process supported by the researcher
-
creating an approach to co-design that enabled confident challenge of any pre-existing assumptions, respected differences of opinion and valued equally a wide range of contributions.
The approach to parent involvement in research design was, therefore, based on a participatory model that was not merely consultation on what had been pre-designed, but rather involved an active contribution to both process and output from those involved. 385 That said, the overall aims and objectives of the research study were already set and this group of parents had not been involved at the outset. The topics covered in the meetings were (1) introductions, personal experiences and initial thoughts on data-sharing, (2) introduction to research methods, research design and the role of the research nurse, (3) asking questions and creating questions in a written format, (4) testing out the draft questionnaire and recruitment materials, and (5) evaluation of the process, outcomes and future plans.
From the parent groups’ perspective, there were seven key issues that influenced the final content, format and design of the questionnaire that was used:
-
The use of a personal ‘voice’ throughout the questionnaire – this meant that the questionnaire was written using ‘we’ in the instruction sections and there was an explicit commentary throughout, which spoke directly to the person who would be filling in the questionnaire. For example: ‘In this section we want to hear about your attitudes towards . . .’ and ‘The first three questions might seem very similar, but they are looking at slightly different circumstances so please answer all 3.’
-
Producing the questionnaire as a booklet – it was considered important that parents were not confronted with something that looked like a form because they would be so used to filling out lots of similar items in their stay in hospital. The questionnaire had to look different and less official.
-
The use of colour – colours were considered important not just so that the questionnaire looked attractive but also that the colours should be muted and gentle to create a soft impression. In addition, each of the four sections was assigned its own colour and the answers were marked against the coloured background.
-
Order of questions – the group recommended that potentially the most distressing questions should be left to the end, such as those concerning previous miscarriages or infant deaths. Furthermore, they recommended that such questions were explicitly marked ‘sensitive’ so that a parent would be warned in advance and could choose not to complete them if they wished.
-
Font – the font that was chosen for the questionnaire was one that was regarded as less formal looking (Comic Sans MS) to create a more welcoming feel to the questionnaire.
-
Options for completion – the parent group felt it was important to emphasise that there was not one right answer to anything and not one way to complete the questionnaire that was preferable to another. Therefore, throughout the questionnaire there were occasional reminders that there were no right or wrong answers and plenty of spaces for any additional comments. In addition, parents could choose to complete the questionnaire anonymously or leave their contact details, they could request it in a written language other than English, they could request an interpreter to complete it with them or they could state that a family member had assisted them.
-
Clarity about words and phrases – the parent group was particularly helpful in spotting jargon and suggesting simpler alternatives as well as making sure that key terms were well defined within with the questionnaire; for example:
-
‘Data’ refers to all sorts of information that is collected, from birthweight to drugs administered, to the progress your baby is making and so on. This might be recorded on a database or in paper notes. We are asking about information only, not tissue samples, etc.
-
‘Research’ refers to the process of collecting, ordering and evaluating information in order to provide further understanding, new knowledge and/or a basis for decision-making and action or change. This might include research on the frequency of disease in babies (epidemiology), on the safety of drugs prescribed to babies (drug safety), on the impact of drugs or treatments on babies’ health (clinical effectiveness), or to identify babies with certain specific diseases for inclusion in research studies.
-
The survey content and design was tested with the parent group at each stage of design.
Survey distribution and recruitment
With the support of the Greater Manchester, Lancashire and South Cumbria Medicines for Children Research Network, 29 NHS hospitals in England with neonatal care units were recruited as research sites (three of which were in London). One hospital dropped out of the study as it was unable to recruit a research nurse to assist with the study; therefore, data are presented from 28 hospitals.
Research ethics approval was obtained from the National Research Ethics Committee North West Cheshire (REC reference 11/NW/0765; UK Clinical Research Network Portfolio ID: 11960).
Research nurses at each site were responsible for approaching parents in person while they were still on the ward to explain the study, provide written information (available in English, Urdu, Punjabi, Bangla, Cantonese and Polish and on request any other additional language) and/or go through the information in person or through a translator (to overcome any potential literacy difficulties). They were also responsible for taking consent and distributing the survey subsequently and arranging any language support required to complete it. The principal inclusion criterion was that a parent had a child in any level of neonatal care at any of the 28 participating hospitals. The survey questionnaire could be completed by the mother and/or the father or other principal carer (i.e. someone who was not the child’s other biological parent but who would play a significant role in their care and upbringing). This could be a partner of the same sex or a grandparent, for example. Participants were invited to complete the questionnaire alone, or with their partner (other carer) and/or through an interpreter if required. The research nurse was available to clarify any questions and to provide support if requested. Parents could complete the questionnaire on the ward before they left or take it home and send it in later.
The majority of the questionnaires were completed in hospital (n = 1090). A total of 1225 participants (92.9%) did the questionnaire on their own; 80 (6.1%) reported that they had not filled it in on their own, and there were missing data in questionnaires from 14 people (1.1%). Of those who reported that they did not fill the questionnaire in on their own, the majority (n = 63) reported that they had filled it in with their partner, 16 stated that a research nurse/midwife had helped them, and one person said that they had done it with an interpreter. When asked if an interpreter had assisted with the questionnaire, four people responded (three had completed it with a Polish/English interpreter and one had completed it with an Urdu/English interpreter).
Sample size
The target sample size was 1300, allowing a percentage to be estimated with a margin of error ≤ 2.5% for 95% CI. Written consent to take part in the study was provided by 1722 people and 1319 completed questionnaires were received (return rate = 76.6%). The discrepancy between number of consents and number of questionnaires completed is largely explained by those parents who gave consent while in hospital but did not complete the questionnaire before discharge. A breakdown of participating sites and completed questionnaires is shown in Table 48.
| Site number | Neonatal unit designationa | Questionnaires received | Consent forms received | Recruitment target | Neonatal admissions 1 November 2011–30 September 2012 |
|---|---|---|---|---|---|
| 1 | LNU | 52 | 60 | 55 | 168 |
| 2 | – | 28 | 41 | 25 | Data not available |
| 3 | SCBU + LNU | 12 | 12 | 15 | 869 |
| 4 | LNU | 55 | 77 | 50 | 234 |
| 5 | NICU | 53 | 74 | 50 | 546 |
| 6 | NICU | 70 | 103 | 70 | 483 |
| 7 | LNU | 23 | 30 | 20 | 182 |
| 8 | LNU | 80 | 81 | 70 | 404 |
| 9 | NICU | 48 | 99 | 55 | 437 |
| 10 | LNU | 48 | 52 | 50 | 415 |
| 11 | NICU | 58 | 79 | 60 | 545 |
| 12 | NICU | 97 | 152 | 95 | 724 |
| 13 | LNU | 30 | 30 | 30 | 226 |
| 14 | NICU | 66 | 73 | 30 | 1069 |
| 15 | LNU | 20 | 22 | 30 | 119 |
| 16 | NICU | 71 | 72 | 55 | 669 |
| 17 | NICU | 58 | 75 | 55 | 471 |
| 18 | SCBU + LNU | 47 | 57 | 30 | 200 |
| 19 | NICU | 20 | 52 | 20 | 352 |
| 20 | SCBU + LNU | 28 | 30 | 20 | 435 |
| 21 | NICU | 65 | 108 | 60 | 425 |
| 22 | NICU | 14 | 14 | 25 | 446 |
| 23 | LNU | 20 | 26 | 20 | 223 |
| 24 | LNU | 35 | 36 | 30 | 242 |
| 25 | LNU | 86 | 87 | 70 | 297 |
| 26b | – | 0 | 0 | 25 | Site withdrew |
| 27 | LNU | 82 | 98 | 80 | 206 |
| 28 | NICU | 21 | 28 | 30 | 303 |
| 29 | LNU | 32 | 54 | 30 | 293 |
| Total | – | 1319 | 1722 | 1255 | 10,983 |
Of the sites (n = 27) where neonatal unit admissions data are available for the recruitment period (November 2011 to September 2012), total admissions were 10,983 and the sample size total was 1291 (11.75% of all admissions). The sample included three special care baby units (SCBUs) (level 1), 15 local neonatal units (LNUs) (level 2) and 12 neonatal intensive care units (NICUs) (level 3).
Sample characteristics
Of the 1319 parents or carers who completed a questionnaire, 930 (70.5%) were mothers, 370 (28%) were fathers and 12 were others who identified themselves as having a primary care responsibility for the baby who was in neonatal care (including 10 who were grandparents). Data were missing in seven cases. The median age of the mothers in our sample was 30 years (range 15–52 years). This compares favourably with all mothers in the 28 sample sites (n = 10,983; median age 30 years; range 13–55 years) and all mothers recorded in the NNRD encompassing 167 neonatal units in England during the period 1 November 2011 to 30 September 2012 (n = 55,731; median age 31 years; range 12–59 years). The median age of fathers in our sample was 32 years (range 15–52 years). Of the 1273 returns for which data are available, the baby in neonatal care was the first child for 45.6% (n = 601) of participants and the second child for 31.6% (n = 417) of participants.
The vast majority of participants described their ethnic group as white British (82.5%; 1088/1290). Over half of the sample described themselves as Christian (56%; 732/1306), with an additional one-third preferring to state that they had no religion (33%; 433/1306). Ninety-seven participants stated that they had not been born in the UK with over half (n = 50) arriving in this country between 2005 and 2011. A comparison of mothers’ ethnicity in the sample with data from the 28 sites overall and that of the NNRD overall for the study period (1 November 2011 to 30 September 2012) reveals some differences in representativeness. The study sample has a greater proportion of mothers who are ‘white British’ (81.4%) than either the 28 sites overall (56.5%) or the NNRD (65.3%); mothers of Asian ethnicity (7.4%) are slightly under-represented, as are those who are black (2.3%), in comparison both with the 28 sites and with the NNRD (see Table 70).
Most participants described themselves as married, in a civil partnership or in a relationship (93.3%; 1209/1296). The proportion that were married or in a civil partnership (42.3%, 393/930) is consistent with the proportion recorded in NNRD for the same time period of the study (40.6%, 22,643/55,731). Two-thirds of participants were in employment either full time or part time (65.8%; 868/1316). Of the 1218 who were prepared to provide information on their educational qualifications, one-fifth (21%; 251/1218) had no qualifications beyond O level (ordinary level)/GCSE (General Certificate of Secondary Education), with five of those declaring no qualifications whatsoever. Nearly one-third of participants had a university or other higher degree (386/1218; 32%).
At the time of completing the questionnaire, the amount of time that participants’ babies had been in neonatal care ranged from 1 to 217 days (mean 20.4 days; median 11 days). Parents were asked to state the highest level of care that their babies had received at any point. Just under half (609/1288; 47.5%) had experience of level 3 (intensive care), 15.5% (199) of level 2 (high-dependency care) and 30% (387/1288) of level 1 (special care). A total of 93 (7.2%) participants reported that they did not know what level of care their baby had received.
Participants displayed very high levels of satisfaction with their experiences of neonatal care; on an ordinal scale of 1 (least satisfied) to 7 (most satisfied), 84.8% (n = 1119) responded either 6 or 7. When asked if they thought that their level of satisfaction with care had influenced how they had responded to the survey questions about the routine use of their baby’s data for research purposes, opinion was divided, with a slight majority responding ‘no’ (391 responded ‘yes’; 511 responded ‘no’; 326 responded ‘possibly’; 59 responded ‘don’t know’).
Participants were also asked about how ‘included’ they felt in their baby’s care and scored feelings of inclusion on an ordinal scale of 1 (least included) to 7 (most included). Of 1297 available sets of data, the majority of parents (638; 49.2%) reported that they felt most included (scoring 7). The mean and median scores were 6.4 and 7.0, respectively. The question was also one that the parent group had requested to be added, because they identified this feeling of inclusion as a key marker of quality provision at the time when their baby was still on the neonatal unit.
Summary
-
The sample constitutes 11.75% of admissions to the neonatal units in the study during the recruitment period (total admissions = 10,983, sample size total = 1291).
-
The sample includes three SCBUs (level 1), 15 LNUs (level 2) and 12 NICUs (level 3).
-
The age of mothers in the sample is consistent with mothers overall for the 28 neonatal units and for all units within the NNRD.
-
One-fifth (21%; 251) of the sample had no qualifications beyond O level/GCSE and nearly one-third of participants had a university or other higher degree (386/1218; 32%).
-
The study sample has a greater proportion of mothers who are ‘white British’ than either the 28 sites overall or those in the NNRD; mothers of Asian ethnicity are slightly under-represented, as are those who are black, in comparison both with the 28 sites and with the NNRD.
-
The vast majority of participants felt positively included in their baby’s care and displayed very high levels of satisfaction with their experience of neonatal care, but were divided on whether or not these experiences would influence their attitudes to routine use of their baby’s data for research purposes.
Results
Willingness for data-sharing for research purposes
Parents were asked about their willingness for their baby’s data to be used for research purposes in three different conditions: (1) in general, (2) if identifying information was removed, and (3) if explicit permission was asked on each occasion. Overall, there was a very high level of support for the routine use of health data for research purposes (69.4%), increasing to nearly 80% ‘yes’ responses if identifying information were removed. If permission was asked each time, the percentage agreement was 77%; 847 participants (68.9%) responded ‘yes’ to all three questions (Table 49).
| Participants’ willingness for their baby’s data to be used for research purposes | Participants’ willingness for their baby’s data to be used for research purposes, n (%) | Missing data, n (%) | |||
|---|---|---|---|---|---|
| Yes | No | Possibly | Don’t know | ||
| In general | 915 (69.4) | 84 (6.4) | 262 (19.9) | 41 (3.1) | 17 (1.3) |
| If identifying information was removed | 1052 (79.8) | 59 (4.5) | 163 (12.4) | 30 (2.3) | 15 (1.1) |
| If permission was asked each time | 1015 (77) | 50 (3.8) | 217 (12.4) | 31 (2.4) | 6 (0.5) |
A statistically significant association was found between participants’ responses when asked about data-sharing in the different conditions offered: willingness in general and willingness if identifying information was removed; willingness in general and willingness if permission were asked; willingness for non-identifiable data to be used and willingness if permission were asked. In each case, the association was significant (p < 0.001), with each of the kappa values approaching 0.60.
A comparison of individual participants’ responses to each question reveals that, if identifying information were removed, 128 participants changed their response from ‘possibly’ in the general condition to ‘yes’ in the non-identifiable condition, and 25 participants changed from ‘no’ to ‘yes’. If permission were asked, 115 changed their response from ‘possibly’ to ‘yes’ in comparison with the general condition. However, of those who said ‘yes’ if identifying information were removed, 89 said ‘possibly’ instead to the condition of permission being asked each time.
The association between participants’ highest level of qualification and their willingness for their babies’ data to be used for research if identifying information were to be removed was found to be significant (χ2trend = 7.625; p = 0.022). Participants who said ‘yes’ to using de-identified information about their baby for research were likely to be those whose highest qualification was a university or higher degree. Those with O levels or GCSEs selected ‘possibly’ more than those in the two other groups. Overall, 83.3% responded ‘yes’ to willingness for de-identified data to be shared, 12.6% responded ‘possibly’ and 4.2% responded ‘no’ (see Table 71).
In addition, the association between participants’ levels of qualification and their awareness of electronic health records prior to the study was found to be significant (χ1t = 119.26, df = 2; p = 0.000). Those with O levels or GCSEs were more likely to say ‘no’ (66.2%) (they had not heard of electronic records prior to participation) than those in the other two groups, and those with degrees or higher degrees were more likely to say ‘yes’ (76.6%) (see Table 72).
Of those who reported that they had heard of electronic health records, the NHS was the most common source of this information (n = 374), followed by the media (n = 271).
We investigated whether or not there was an association between willingness for data to be used for research purposes (defined as ‘yes’, ‘possibly’, ‘no’) and whether or not parents had one child (the one in neonatal care) or more than one child (the youngest being in neonatal care) (see Table 73).
A chi-squared trend test showed that the association between having one child or more than one child was significant with regard to participants’ willingness for their babies’ data to be used for research (χ1trend = 9.32, df = 1; p = 0.002). More people with more than one child (n = 496) (i.e. the child in neonatal care was not their first child) said that they would be willing for their baby’s data to be used for research compared to those who had only one child (n = 395).
In the case of willingness for data to be used for research purposes if they were de-identified (see Table 74), participants who said ‘yes’ were more likely to be those who had more than one child (n = 555) (i.e. the child in neonatal care was not their only child). This association was significant (χ1trend = 4.14, df = 1; p = 0.042).
We investigated whether or not there was an association between the highest level of neonatal care experienced and willingness for data to be used for research purposes. Level of care refers to the highest level of care a baby had experienced at any point. It does not necessarily refer to the duration of that care or to the current level of care at the point of completing the questionnaire (Table 50).
| Statement | Highest level of care experienced | Willingness, frequency (%) | Total | ||
|---|---|---|---|---|---|
| Yes | Possibly | No | |||
| Willingness for baby’s data to be used for health research | 1 | 264 (71.5) | 74 (20.1) | 31 (8.4) | 369 |
| 2 | 131 (67.2) | 51 (26.2) | 13 (6.7) | 195 | |
| 3 | 443 (76.2) | 111 (19.1) | 27 (4.6) | 581 | |
| Total | 838 (73.2) | 236 (20.6) | 71 (6.2) | 1145 | |
| Willingness for baby’s de-identified data to be used for health research | 1 | 289 (77.1) | 65 (17.3) | 21 (5.6) | 375 |
| 2 | 155 (79.9) | 28 (14.4) | 11 (5.7) | 194 | |
| 3 | 515 (87.7) | 55 (9.4) | 17 (2.9) | 587 | |
| Total | 959 (83.0) | 148 (12.8) | 49 (4.2) | 1156 | |
| Willingness for baby’s data to be used for research if asked for permission | 1 | 284 (75.3) | 73 (19.4) | 20 (5.3) | 377 |
| 2 | 152 (77.9) | 38 (19.5) | 5 (2.6) | 195 | |
| 3 | 488 (83.0) | 84 (14.3) | 16 (2.7) | 588 | |
| Total | 924 (79.7) | 195 (16.8) | 41 (3.5) | 1160 | |
According to the results of a Kruskal–Wallis test, the association between the level of a baby’s care experienced and the parents’ willingness for their baby’s data to be used for research was found to be significant (χ2trend = 7.218; p = 0.027). Participants whose babies had experienced level 1 care were more likely to say ‘no’ than those who had experienced levels 2 or 3, and those with babies who had experienced level 3 care were more likely to say ‘yes’ than those whose babies had not. With regard to participants’ willingness for their baby’s de-identified data to be used for research, the level of care was found to be significant (χ2trend = 19.963; p = 0.000). Participants who had babies in level 3 care were more willing to say ‘yes’ if any identifying information were removed, whereas those who had babies in level 1 were more likely to say ‘no’. The association of the level of care with participants’ willingness for their baby’s data to be used if asked permission was also found to be significant (χ2trend = 9.111; p = 0.011); those with babies who had experienced level 3 care said ‘yes’ more than those with babies with experience of levels 1 and 2.
We also investigated willingness for babies’ data to be used for research purposes in the case of participants who had lost a baby previously through termination, miscarriage or stillbirth. The question referred specifically to data that might be associated with the lost child. Of the 611 parents who responded, 315 (51.5%) said ‘yes’, 121 (19.8%) said ‘no’, 140 (22.9%) said ‘possibly’ and 35 (5.7%) said they did not know.
Consent
Opt-out system
Participants expressed strong support for an ‘opt-out system’, described as ‘your baby’s data would be used for research unless you actively said you didn’t want this to happen’. Almost two-thirds thought that this was a ‘good idea’ (802/1307; 61.4%), with an additional 15.6% (203/1307) thinking that it was ‘possibly’ a good idea and fewer than one-fifth clearly saying ‘no’ (229/1307; 17.5%). Some participants expressed concern that, if an opt-out system were in place, parents might not fully understand that their data would be used unless they explicitly opted out, or that distressed parents might tick an ‘opt-out’ option without really understanding what it implied.
Associations between qualification-based groups and their responses regarding whether or not it would be a good idea to use an opt-out system were found to be significant (χ4 = 18.768; p = 0.001). Participants who had university or other higher degrees were more likely to say ‘no’ (25.1%) to an opt-out system, whereas participants who had O levels or GCSEs as their highest qualification were more likely to say ‘possibly’ (19.8%). Those with advanced levels (A levels) or vocational qualifications as their highest qualification were more likely to say ‘yes’ (70%) to an opt-out system (see Table 75 and Figure 55).
No significant differences in response to the question concerning the opt-out system were found in relation to the number of children, the highest level of baby’s care or the relationship status.
Despite strong support for an opt-out system, when asked ‘Are there any occasions when it would be OK to use a baby’s data for research without asking parents?’, nearly two-thirds (806/1301; 62%) responded that there was no occasion on which this would be acceptable, although just over one-third responded ‘yes’ (211/1301; 16.2%) or ‘possibly’ (237/1301; 17.4%), with the remainder responding ‘don’t know’ (57/1301; 4.4%).
If specific permission were requested
Participants were also asked about their preferences if, instead of an opt-out system, parents were to be asked specific permission (i.e. consent for the use of their baby’s data) (see Table 76). When asked whether the way in which permission might be sought (rather than what they personally might prefer) would make a difference to whether or not they consented to the use of their baby’s data for research purposes, there was no strong trend: 398 participants (30.2%) said ‘yes’, 486 participants (36.8%) said ‘no’, 356 participants (27%) said ‘possibly’, and the rest [79 (6%)] said that they did ‘not know’ or they did not answer.
If asked specifically for consent (rather than an opt-out system), half of them would prefer to be asked in writing (658/1299; 50.7%) and around one-quarter would prefer to be asked in person (349/1299; 26.9%). Some people commented that being asked in person is more personal and presents opportunities for clarification, whereas others said that being asked in writing could be useful as a record and would give them time to digest the information.
The association between the highest level of care experienced and participants’ preferences (in person or in writing) if permission were requested was found to be significant (χ2trend = 8.84; p = 0.012). Overall, more participants preferred to be asked in writing than in person, but as their experience of level of care increased, there was a significant rise in the percentage requesting to be asked in person (χ2trend = 8.83, df = 1; p = 0.003) (see Table 76).
If an opt-out system were not in place, participants were asked whether or not they would be influenced if the person who was directly involved in their baby’s care was the one who asked them to give permission for their baby’s data to be used for research. Over half said ‘yes’ (668/1300; 51.4%) and a further quarter said that it would ‘possibly’ influence them (324/1300; 25%), whereas just under one-fifth said that it definitely would not (228/1300; 17.5%).
Influence was regarded as both positive and negative by the participants. Some participants felt that being asked by their direct carer would have made a difference because of the trust that had been built up already, but others raised concerns that it might mean that they would have less choice and they would be worried that saying ‘no’ would affect the care. In addition, some participants said that it would not make any difference as long as the right person asked them (e.g. the person would need to have knowledge and understanding of the research).
We investigated the response rate for each qualification-based group regarding their likeliness to agree to share their baby’s data for research if the person who asked them was directly involved in their baby’s care. The association here was significant (χ2trend = 6.060; p = 0.048). Participants who said ‘yes’ (n = 548) were more likely to be those with lower levels of academic qualifications (see Table 77).
Data access for research purposes
Participants were told that sometimes researchers need to identify which babies would be suitable to take part in medical research and one of the ways to do this is for non-medical staff to access their baby’s data. There was little objection to this occurring. Over 50% of participants (703/1306; 53.8%) reported that they would be happy for this to happen, with a further 25% saying ‘possibly’ (337/1306; 25.8%). One-fifth said ‘no’ (200/1306; 15.3%) or ‘don’t know’ (66/1306; 5.1%).
Data security
Participants displayed very high levels of confidence about the security and accuracy of patient data held within neonatal services. Over 80% were very or fairly confident about its security (Table 51) and over 85% were very or fairly confident about its accuracy (Table 52). However, participants’ qualification levels were found to be significantly associated with their responses regarding their levels of confidence about the security of the data (χ2trend = 27.07, df = 2; p = 0.000) and the accuracy of the data (χ2trend = 20.95, df = 2; p = 0.000). Those with degrees/higher degrees were less likely to be confident about both the security of data and the accuracy of data.
| Highest qualification | Level of confidence, n (%) | Total | ||||
|---|---|---|---|---|---|---|
| Very confident | Fairly confident | Neither confident nor unconfident | Fairly unconfident | Very unconfident | ||
| O levels/GCSEs | 82 (35.3) | 112 (48.3) | 35 (15.1) | 3 (1.3) | 0 (0) | 232 |
| A levels/vocational qualification | 140 (37.5) | 169 (45.3) | 55 (14.7) | 7 (1.9) | 2 (0.5) | 373 |
| Degree/higher degree | 75 (20.9) | 194 (54.2) | 73 (20.4) | 14 (39) | 2 (0.6) | 358 |
| Total | 297 (30.8) | 475 (49.3) | 163 (16.9) | 24 (2.5) | 4 (0.4) | 963 |
| Highest qualification | Level of confidence, n (%) | Total | ||||
|---|---|---|---|---|---|---|
| Very confident | Fairly confident | Neither confident nor unconfident | Fairly unconfident | Very unconfident | ||
| O levels/GCSEs | 81 (35.7) | 114 (50.2) | 28 (12.3) | 4 (1.8) | 0 (0) | 227 |
| A levels/vocational qualification | 125 (33.9) | 196 (53.1) | 40 (10.8) | 8 (2.2) | 0 (0) | 369 |
| Degree/higher degree | 71 (19.9) | 220 (61.8) | 55 (15.4) | 10 (2.8) | 0 (0) | 356 |
| Total | 277 (29.1) | 530 (55.7) | 123 (12.9) | 22 (2.3) | 0 (0) | 952 |
Subsequent contact as a result of research findings
Participants were asked to rate how important it would be for them to have feedback on any research for which their baby’s data were used (1 being the least important and 7 being the most important). The majority of participants (n = 629; 47.7%) thought that it was very important to have feedback. The mean and median of the importance ratings were 5.7 and 6.0, respectively.
When asked if they would want to be contacted if new information were discovered about their baby’s condition as a result of research that used their baby’s data, the majority of participants said ‘yes’ (1037/1299; 79.8%). A further 15.3% (199/1299) said ‘possibly’ and only 3.4% (44/1299) said ‘no’, with 1.5% (19/1299) responding that they did not know.
Research benefit for others
Participants were asked about the use of their baby’s data for research that may benefit other babies in the future, but may not have any direct benefits for their own baby. On a scale of 1 (least happy) to 7 (most happy), the overwhelming majority of parents responded positively, with 65.3% (845/1294) scoring 7 and a further 10.7% (203/1294) scoring 6. The mean and median scores were 6.32 and 7, respectively. When parents’ responses were investigated by the highest level of care they had experienced, the result remained consistent: there were high levels of support for research that might benefit others but not themselves.
Conclusions
This is the largest sample to date of parents with experience of babies in neonatal care who have been consulted on the issue of the use of clinical data for research purposes. Our sample constitutes 11.8% of admissions to participating neonatal units at the time of conducting the survey. The survey for parents was co-designed with parents of babies in neonatal care and represents participatory preparatory work that proved effective in gathering high numbers of participants. The commitment of the children’s clinical local research network to a non-medicines study was crucial in the mobilisation of adequate numbers of research nurses and the oversight of the process of recruitment and consent. The age of those participating is consistent with broader national data, but black mothers and those of Asian ethnicity are slightly under-represented in the sample in terms of both the neonatal units from where data were collected and the national figures.
The parents sampled displayed very high levels of satisfaction with the care they experienced. This result should be considered in the context that the majority of parents completed the questionnaire when their baby was still in hospital; it is unknown whether or not, when asked the same question at a later point and after having returned home, the result would remain the same. Although parents were explicitly reminded as part of the consent procedure that participation in the survey would not affect their baby’s care, this may have been an influencing factor on high expressed levels of satisfaction. It is of note that the participants themselves were equivocal about whether or not their level of satisfaction with the care received might be a source of bias in their responses.
In broad terms, we find strong support for the sharing of routinely recorded health data for research purposes among parents with children in neonatal care, with over two-thirds of participants responding positively. The possibility of de-identified data-sharing or sharing only if explicit permission were asked raised the percentage of those saying ‘yes’ by around 10%, but most of those who had said ‘no’ in general terms remained opposed despite the introduction of these additional conditions. This conclusion is supported by results from questions that explored ‘opt out’ as the default position for data-sharing; nearly two-thirds agreed that this was a good idea, with less than one-fifth definitely saying ‘no’.
This headline result is moderated by several factors. We also explored the bias inherent in one-third of the sample having a degree or higher degree qualification in the conclusions we draw. Key among those is highest level of educational qualifications. This was found to be statistically significant in respect of whether or not electronic records had been heard of in the first place; the group with the lowest levels of educational qualifications were the least likely to be aware of them. Educational background also had a statistically significant effect on willingness for de-identified data to be used. Those with a degree/higher degree were more likely to agree and those with the lowest qualifications were more likely than others to respond ‘possibly’, which indicated some element of uncertainty perhaps through limited understanding, given their limited awareness of electronic records. In addition, in the case of the acceptability of an opt-out system, despite strong support, educational background was found to have a statistically significant relationship with the responses; those with a degree/higher degree were more likely to say ‘no’ than those with lowest qualifications, and those with lowest qualifications were more likely to say ‘possibly’.
Overall, these results point to the need to ensure that those who are less well educated are afforded every opportunity to understand the new digital NHS in order to make informed choices about the use of data and/or the implications of systems, such as opt out, to which they may be asked to subscribe. This conclusion is lent modest support by the statistically significant relationship between educational background and parents’ willingness to consent to data-sharing if asked by an individual directly involved in their baby’s care. Parents with the lowest level of educational qualifications were more likely than those in the other qualification groups to say yes to data-sharing if asked by someone they know who cared for their baby. Direct knowledge and understanding through an individual contact with a trusted person, rather than knowledge and understanding in the abstract, is, for this group, more influential.
Whether or not the child in neonatal care was the parents’ only child also had a statistically significant effect on responses to questions about data-sharing. Parents with more than one child often said that they would be willing for their baby’s data to be used for research purposes. Parents whose child in neonatal care was not their first child were more likely to say ‘yes’ to routine data-sharing both in general terms and if data were de-identified. These results point perhaps to the role of maternal experience in moderating attitudes. More experienced parents were less concerned about any potential difficulties caused by agreeing to the use of their baby’s data and/or were more appreciative of the potential advantages of doing so.
The level of care a baby had experienced was also found to have a statistically significant effect on parental attitudes. (Level of care refers to the highest level of care experienced at any point. It does not refer to the duration of care at that level, nor the current level of care at the time of responses.) Parents whose children had experienced level 3 care (the highest level), were in general terms, more likely to say ‘yes’ in all three conditions associated with willingness to share data than those who had not, if data were de-identified and if permission were obtained. These three conditions were not treated in the research as alternatives, but rather attitudes to all three were sought in their own right without seeking an expressed preference between them. Overall, these results demonstrate that the greater the level of concern or the more complex the level of care experienced by parents, the stronger their willingness to permit the routine sharing of data for research purposes, regardless of how that is framed.
The data revealed a strong orientation towards the assistance of others through routine use of health data for research, even if such data-sharing would not have direct beneficial effects for the parent or their baby. When asked this question directly, over three-quarters responded in the two most positive categories of willingness for their babies’ data to be used. In addition, parents who had lost a child through miscarriage, termination or stillbirth, were asked specifically about data that might be associated with that child. Over half of them expressed willingness for data to be shared for research purposes and, additionally, nearly one-quarter said ‘possibly’. These results suggest that a greater emphasis could be placed on the contribution that parents’ willingness to share data makes to the benefit of others. This contribution can be cast in terms of altruism, because the motivation clearly is disengaged from direct benefit to self.
There are some contradictions in the results that may be an artefact, in part, of how the questions were asked. Responses are elicited to seemingly unconnected questions in the layout of the survey, but when results are placed side by side the contradiction is revealed. However, the contradictions revealed may be real for individuals. It is perfectly possible to hold one attitude alongside another seemingly contradictory one and to be unaware of the conflict between them until prompted to consider both attitudes at the same time. For example, participants strongly supported the notion of being contacted again if new information were discovered about their baby’s condition as a result of research that used their baby’s data. Yet the majority of those who said ‘yes’ to this question were also those who said ‘yes’ to data-sharing if the data were de-identified. The survey asked for their attitude to de-identified data unconnected to their attitude to being contacted again as a result of discoveries linked to their willingness to share their baby’s data. As the possibilities of e-health, digital records and digital data mining become even greater in the future, these will inevitably create dilemmas and contradictions in attitude and approach at an individual patient level. It is hard to think through the likely consequences of a personal response in the fast-changing world of digital health, when the possibilities of that world are unknown or ill-understood. Hence, seeming contradictions will appear in attitude and response. Moving forward, it will be important to be mindful of new dilemmas that EPRs and data-sharing for research might provoke for individuals and how to support individuals in those circumstances.
We find that there is strong support for the routine sharing of babies’ data for research purposes, particularly among those whose babies have experienced more complex levels of neonatal care and are more experienced parents. The differences in degrees of willingness to share data are small, but those saying ‘no’ are likely to remain opposed regardless of whether data are de-identified or individual permission is sought. More experienced parents were less concerned about any potential difficulties caused by agreeing to the use of their baby’s data and/or appreciated more the potential advantages of doing so. The support for routine sharing of babies’ data for research purposes is overall underpinned by a strong altruistic motivation from parents to support the benefit of others regardless of benefit to self.
We acknowledge that a limitation in fully understanding the influences underlying the observed trends and associations is the lack of follow-up exploratory, qualitative inquiry with a subsample of those who participated, which might have illuminated further aspects of the conclusions drawn.
Implications for health care
The positive result in support of routine data-sharing should be tempered by a concern to ensure that those with lower educational backgrounds are afforded greater opportunities to understand the significance of digital records and data-sharing, and the possibilities such as opt out, in order to make informed decisions. Our finding that, in this group, the provision of information through individual contact with a trusted person is more influential than the provision in the abstract, indicates the important role of clinical staff in explaining the way in which clinical data may be used in research. This suggests the importance of doctors and nurses being aware of these issues, being trained in conveying information to parents, and having sufficient time for explanation.
Research recommendations
The identification of effective and efficient methods to engage the public in debate and improve their knowledge of potential benefits, processes and regulation are important to secure their trust and confidence in the use of clinical data in research.
Chapter 9 Conclusions
What we found
We established the Medicines for Neonates programme on the principle that it should be possible to employ EPR data to support, improve and advance patient care and health services. We obtained multiple approvals, including from the Caldicott Guardians of every NHS trust providing NHS neonatal services. We showed that it is possible to create a national data resource, the NNRD, from extractions from EPRs, with the support of the neonatal clinical community.
We conducted formal assessment of the utility of the NNRD in population research into neonatal NEC, mortality of very preterm babies admitted to NHS neonatal units, and in trial-based economic evaluations. We compared NNRD data against research-standard data from a NIHR multicentre clinical trial, determined the validity of 2-year neurodevelopmental status recorded in the EPR against a research assessment by a single examiner, and demonstrated that it is possible to link the NNRD to HES data to create a longitudinal patient record. We examined parent attitudes to the use of personal clinical data in research and identified both support and strong altruism.
We developed standard operating procedures to assure the quality and completeness of data held in the NNRD. These include internal consistency, logic, range and completeness checks and identification of duplicate entries. We linked multiple episodes of care across different hospitals to create a single record for each infant. We conducted a comparison of NNRD and HES data, also extracted from hospital systems. We show that for key newborn variables, rates of both missing and potentially erroneous data are substantially lower in the NNRD. Other important differences are that the NNRD contains a far wider range of data items and we have defined each data item clearly, with a comprehensive metadata set available.
We show that clinical data from EPRs held in the NNRD can be used to create UK birth cohorts. We also show that the sole use of the NHS administrative database, HES, to build birth cohorts will result in the inclusion of many babies with missing gestational age data and implausible birthweights. However, linking the NNRD to HES substantially enhances the quality and scope of data.
We performed a formal comparison of NNRD data against clinical trial data. This showed that for economic evaluations and baseline information, data in the NNRD perform as well as trial data. We identified strong parent support underpinned by altruism for the routine sharing of babies’ data for research purposes.
Our overall conclusion is that we have established proof of principle that EPR data may be employed successfully to support patient care and clinical services through research and a range of health service evaluations. We show that the potential of EPR to serve as the source of data for secondary uses is substantial. In addition to the National Neonatal Audit Programme, the NNRD is now used for a growing range of outputs by NHS England, Public Health England, Department of Health and Social Care and other organisations.
Implications for health care
We have demonstrated proof of principle of the potential of the NNRD as a data source for neonatal trial-based economic evaluations in the UK context. This has potential to reduce the costs and improve the efficiency of economic evaluations conducted in relation to neonatal research studies. The creation of a national data resource from EPR data minimises the burden placed on busy clinical teams by providing a single national data source to service multiple outputs, eliminating the need for multiple individual collections, with repetitive capture of many commonly required data items, and reduces the risk of transcription errors and other errors that arise from repeated data recording.
The reasons for the differences between NNRD and HES data merit consideration. HES data are administrative, with entry by coding clerks and no clinical oversight. Data quality checks are minimal and in effect restricted to ensuring that the format of the NHS number is valid. There is no feedback to clinical teams. In contrast, the use of EPR data de facto ensures close clinical involvement. However, we do not believe that clinician involvement in assuring data quality and completeness should be taken for granted. Considerable effort is required to secure clinician engagement. In the case of the NNRD, the pivotal factor was its use for national clinical audit that involved publication of data completeness, detailed analyses and outlier status for named neonatal units and networks. We maintain close engagement with the clinical community through regular newsletters, national meetings and a ‘hot-line’ for staff, for one-to-one responses to queries. Neonatal units have been important stakeholders and collaborators in outputs to date, including Department of Health and Social Care reports and peer-reviewed publications.
To assist clinical teams, we developed a web portal that enables users from individual neonatal units to view data items identified as missing or potentially erroneous so that they could make corrections in the real-time infant EPRs. We developed this feedback loop initially to assist users in ensuring only reliable complete data were used in analyses for the National Neonatal Audit Programme; however, this has been extended (e.g. for annual case-mix mortality analyses conducted by the NDAU) and the intention is to further develop this approach. The strength of this data quality feedback loop is that both the clinical records and the NNRD are updated. The traditional model involving separate databases for research or other evaluations has meant that, although the research database might be corrected, the original record used clinically remained uncorrected, with potential detriment to patient care. 387
It is worth noting that, although improvement in completeness and accuracy is required for some key clinical outcomes, the extent of agreement we identified, even though there had been no prior notification to clinical teams that a comparison would be made, is an encouraging indication of EPR data quality. It is equally worth noting that we acted on the assumption that trial data represented the gold standard but this may not in fact be the case. A potential advantage of using the NNRD for clinical research is that the EPR system has a clear audit trail so that source data verification is assured.
The high degree of parent support that we identified for use of EPR data for research is in sharp contrast with the experience of high-profile projects, such as care.data, where public distrust has been marked. It may be of relevance that we found that more experienced parents were less concerned about any possible difficulties caused by agreeing to the use of their baby’s data and evidenced greater appreciation of the potential advantages. The implication for health care of our findings of strong parent support for data-sharing, but also that provision of information through individual contact with a trusted person is more influential than the provision in the abstract, is that doctors and nurses with adequate knowledge and training in conveying such information to parents and patients from a wide range of backgrounds need sufficient time for explanation.
Research recommendations
We suggest that future research might test the roadmap that we have established to create research databases from EPR by other specialties. More work is also needed to develop and evaluate such secondary databases if they are to be reliable sources of data for research. Current regulatory processes for data linkage are challenging. We would therefore welcome initiatives to develop regulatory frameworks that are clear and straightforward to navigate. The NNRD has been developed and is currently maintained through academic endeavour; an operational challenge for health-care services is how best to develop and maintain such databases as long-term national resources.
Improvements in administrative and clinical data quality and completeness are important health services goals. To our best knowledge, there are no national processes to evaluate health services administrative and clinical data formally and systematically, or to improve quality and completeness. These are also important issues for future research.
The potential of EPRs as data sources for secondary purposes requires further research and development. At present, using a real-time system directly for health services analytics such as benchmarking, or as a source of data without further processing, would result in several difficulties. In a real-time system, data change from second to second, hence the same request, conducted again at a different point in time, is very likely to yield a different result. Data in a real-time system have not undergone any quality assurance and, in the case of the neonatal EPR, contain duplicate, erroneous and missing entries. Users are able to access only data relating to patients in their hospital, with access to data from other providers only possible with specific regulatory approval. When attempting to make comparisons across neonatal units, variation between users in the application of algorithms will lead to outputs that are not necessarily comparable (e.g. selecting on < 1500 g instead of ≤ 1500 g will produce different results). Complex algorithms are particularly problematic (e.g. ROP screening criteria that are based on birthweight, gestational age, postnatal age, postmenstrual age and age at discharge). Thus a real-time platform, although excellent in enabling the rapid sharing of data between providers and facilitating a move away from paper medical records, is not an appropriate vehicle for even simple health services evaluations and research or for providing data without further processing. Future research, for example to flag missing entries, embed prompts and alerts, and range and internal consistency checks into the EPR, might assist users in improving data accuracy and completeness.
The development of methods to improve clinician and NHS trust engagement in data quality assurance, such as incentives, and mandates might also have utility. The identification of effective and efficient methods to involve parents in helping to assure the accuracy and completeness of their babies’ data and to improve their knowledge of processes, regulatory safeguards and potential benefits might assist in securing continuing trust and confidence in the use of clinical data in research.
We have shown that screening neurodevelopment assessments of very preterm children at the age of 2 years, carried out by health-care staff with a wide range of training and experience, is insufficient to identify neurocognitive impairment, hence approaches to improve NHS assessments of impairment following preterm birth require to be identified. The complete national coverage of the NNRD of all admissions to neonatal units with no gestational age, birthweight or other restrictions, offers the opportunity to acquire reliable estimates of the prevalence of conditions likely to lead to neurodisability and other impairment. Such population data would have wide utility (e.g. to examine time-trends and national variation, conduct natural history of disease research, and epidemiological surveillance of rare conditions, as we illustrated in our study of NEC).
Acknowledgements
We wish to thank Professor Michael Goldacre, Emeritus Professor of Public Health at the University of Oxford the independent chairperson, and Professor Andrew Wilkinson, Emeritus Professor of Paediatrics at the University of Oxford, the independent deputy chairperson of the programme Steering Committee. We are also grateful to Dr Matthew Hyde for assisting in the preparation of the final report and Mr Richard Colquhoun for administrative support.
Contributions of authors
Neena Modi (Professor of Neonatal Medicine and Honorary Consultant) was the lead applicant; was the lead investigator; conceived study and led grant application; was responsible for the overall programme co-ordination and delivery, report writing and organisation of programme committees; was the lead for Chapter 1; and was the lead supervisor of the research conducted for Chapters 2, 3 and 5.
Deborah Ashby (Professor of Medical Statistics and Clinical Trials) was a co-applicant and supplied statistical supervision.
Cheryl Battersby (Clinical Research Fellow) conducted the research reported in Chapters 2 and 4.
Peter Brocklehurst (Professor of Women’s Health and Director, Birmingham Clinical Trials Unit) was a co-applicant and supplied methodological expertise.
Zoe Chivers (Head of Services, Bliss) was a co-applicant and supplied parent–public engagement expertise.
Kate Costeloe (Professor of Paediatrics) was a co-applicant and the lead supervisor for research conducted for Chapter 4.
Elizabeth S Draper (Professor of Perinatal and Paediatric Epidemiology) took over the co-applicant role from David Field and supplied methodological expertise.
Victoria Foster (Senior Lecturer in Social Sciences) conducted research reported in Chapter 8.
Jacquie Kemp (National Programme of Care Senior Manager) was a co-applicant and supplied health services expertise.
Azeem Majeed (Professor of Primary Care and Public Health) was a co-applicant and the senior supervisor of research conducted for Chapter 7.
Joanna Murray (PhD Student) conducted the research reported in Chapter 7.
Stavros Petrou (Professor of Health Economics) was a co-applicant and the lead for research conducted for Chapter 6.
Katherine Rogers (Research Fellow) conducted research reported in Chapter 8.
Shalini Santhakumaran (Statistician) supplied statistical support and conducted research reported in Chapter 3.
Sonia Saxena (Clinical Professor of Primary Care) was the supervisor of research conducted for Chapter 7.
Yevgeniy Statnikov (Data Manager) conducted data management; conducted research for Chapter 1; assisted with the work reported in Chapter 4; and assisted with work reported in Chapter 7.
Hilary Wong (Clinical Research Fellow) conducted the research reported in Chapter 5.
Alys Young (Professor of Nursing, Midwifery and Social Work) was a co-applicant and the lead supervisor for research conducted for Chapter 8.
Contributions of others
Felix Achana (Research Fellow) assisted with work reported in Chapter 6.
Richard Colquhoun (Programme Manager) supplied administrative support.
Buthaina Ibrahim (Research Assistant) contributed to work reported in Chapter 7.
Kamran Khan (Research Associate) assisted with work reported in Chapter 6.
Sam Watson (PhD student) assisted with work reported in Chapter 6: Using the National Neonatal Research Database to inform economic evaluations of neonatal interventions.
Publications
Foster V, Young A, Modi N, Brocklehurst P, Abbott J, Costeloe K, et al. The use of routinely collected patient data for research: a critical review. Health 2012;16:448–63.
Gale C, Santhakumaran S, Nagarajan S, Statnikov Y, Modi N, on behalf of the Neonatal Data Analysis Unit and the Medicines for Neonates Investigator Group. The impact of introducing managed clinical networks on neonatal care in England: a population-based study. BMJ 2012;344:e2105.
Blencowe H, Lee ACC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Beyond newborn survival: preterm birth associated impairment estimates at regional and global level for 2010. Pediatr Res 2013;74:17–23.
Foster V, Young A. Reflecting on participatory methodologies: research with parents of babies requiring neonatal care. Int J Social Res Methodol 2013;18:91–104.
Murray J, Saxena S, Modi N, Majeed A, Aylin P, Bottle A, Medicines for Neonates Investigator Group. Quality of routine hospital birth records and the feasibility of their use for creating birth cohorts. J Public Health 2013;35:298–307.
Spencer A, Modi N. National neonatal data to support specialist care and improve infant outcomes. Arch Dis Child Fetal Neonatal Ed 2013;98:F175–80.
Battersby C, Santhakumaran S, Upton M, Radbone L, Birch J, Modi N, East of England Perinatal Networks. The impact of a regional care bundle on maternal breast milk use in preterm infants: outcomes of the East of England quality improvement programme. Arch Dis Child Fetal Neonatal Ed 2014;99:F395–401.
Cole TJ, Statnikov Y, Santhakumaran S, Pan H, Modi N, on behalf of the Neonatal Data Analysis Unit and the Preterm Growth Investigator Group. Birth weight and longitudinal growth in infants below 32 weeks’ gestation: a UK population study. Arch Dis Child Fetal Neonatal Ed 2014;99:F34–4.
Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, et al. Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PLOS ONE 2014;9:e89186.
Shah PK, Lee SK, Lui K, Sjörs G, Mori R, Reichman B, et al. The International Network for Evaluating Outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr 2014;14:110.
Watson SI, Arulampalam W, Petrou S, Marlow N, Morgan AS, Draper ES, et al. The effects of designation and volume of neonatal care on mortality and morbidity outcomes of very preterm infants in England: retrospective population-based cohort study. BMJ Open 2014;4:e004856.
Wong HS, Huertas-Ceballos A, Cowan FM, Modi N, on behalf of the Medicines for Neonates Investigator Group. Evaluation of early childhood social-communication difficulties in children born preterm using the Quantitative Checklist of Autism in Toddlers. J Pediatr 2014;164:26–33.
Wong HS, Santhakumaran S, Statnikov Y, Grey D, Watkinson M, Modi N, the UK Neonatal Collaborative. Retinopathy of prematurity in English neonatal units: a national population-based analysis using NHS operational data. Arch Dis Child Fetal Neonatal Ed 2014;99:F196–202.
Gale C, Modi N; WHEAT trial development group. Neonatal randomised point-of-care trials are feasible and acceptable in the UK: results from two national surveys. Arch Dis Child Fetal Neonatal Ed 2016;101:F86–7.
Gale C, Morris I, Neonatal Data Analysis Unit (NDAU) Steering Board. The UK National Neonatal Research Database: using neonatal data for research, quality improvement and more. Arch Dis Child Educ Pract Ed 2016;101:216–8.
Gemmell L, Martin L, Murphy KE, Modi N, Håkansson S, Reichman B, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks’ gestation. J Perinatol 2016;36:1067–72.
Martin LJ, Reichman B, Darlow BA, Morisaki N, Modi N, Bassler D, et al. Country-specific vs. common birthweight-for-gestational age references to identify small for gestational age infants born at 24–28 weeks: an international study. Paediatr Perinat Epidemiol 2016;30:450–61.
Seaton S, Barker L, Draper ES, Abrams KR, Modi N, Manktelow BN, on behalf of the UK Neonatal Collaborative. Modelling neonatal care pathways for babies born preterm: an application of multistate modelling. PLOS ONE 2016;1:e0165202.
Shah PK, Lui K, Sjörs G, Mirea L, Reichman B, Modi N, et al. Neonatal outcomes of very low birthweight and very preterm neonates: an international comparison. J Pediatr 2016;177:144–52.
Springett A, Mann JP, Statnikov E, Modi N, Johnson N, Morris JK. Management and outcomes of neonates with Down syndrome admitted to neonatal units. Birth Defects Res A Clin Mol Teratol 2016;106:468–74.
Watson SI, Arulampalam W, Petrou S, Marlow N, Morgan AS, Draper ES, Modi N. The effects of a one-to-one nurse to patient ratio on the mortality rate in neonatal intensive care: a retrospective, longitudinal, population-based study. Arch Dis Child Fetal Neonatal Ed 2016;101:F195–200. (Ranked first of the top 10 most read papers published in ADC FNN in 2016.)
Wong HS, Santhakumaran S, Cowan FM, Modi N. Developmental assessments in preterm children: a meta-analysis. Pediatrics 2016;138:e20160251.
Battersby C, Longford N, Costeloe K, Modi N, for UK Neonatal Collaborative Necrotising Enterocolitis Study Group. Development of a gestational age-specific case-definition for neonatal Necrotising Enterocolitis. JAMA Pediatr 2017;171:256–63.
Battersby C, Longford N, Mandalia S, Costeloe K, Modi N and the UK Neonatal Collaborative Necrotising Enterocolitis (UKNC-NEC) study group. Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis in England 2012–13: a two-year, population surveillance study. Lancet Gastroenterol Hepatol 2017;2:43–51.
Darlow BA, Lui K, Kusuda S, Reichman B, Gagliardi L, Håkansson S, et al. International variations and trends in the treatment for retinopathy of prematurity. Br J Opthalmol 2017;101:1399–1404.
Gale C, Hyde MJ, Modi N, on behalf of the WHEAT trial development group. Research Ethics Committee decision-making in relation to an efficient neonatal trial. Arch Dis Child Fetal Neonatal Ed 2017;102:F291–8.
Helenius K, Sjörs G, Shah PS, Modi N, Reichman B, Morisaki N, et al. Survival in very preterm infants: an international comparison of 10 national neonatal networks pediatrics. Pediatrics 2017;240:e20172264.
Hines D, Modi N, Lee SK, Isayama T, Sjörs G, Gagliardi L, et al. Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr 2017;106:366–74.
Statnikov Y, Ibrahim B, Modi N. A systematic review of administrative and clinical databases of infants admitted to neonatal units. Arch Dis Child 2017;102:F270–6.
Achana F, Petrou S, Khan K, Gaye A, Modi N, on behalf of the Medicines for Neonates Investigators. A methodological framework for assessing agreement between cost-effectiveness outcomes estimated using alternative sources of data on treatment costs and effects for trial-based economic evaluations. Eur J Health Econ 2018;19:75–86.
Adams G, Williams C, Modi N, Xing W, Bunce C, UK Retinopathy of Prematurity Special Interest Group, Dahlmann-Noor A. Can we reduce the burden of the current UK guidelines for retinopathy of prematurity screening. Eye 2018;32:235–7.
Santhakumaran S, Statnikov Y, Gray D, Battersby C, Ashby D, Modi N, on behalf of the Medicines for Neonates Investigator Group. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed 2018;103:F208–215.
Wong HS, Cowan FM, Modi N, Medicines for Neonates Investigator Group. Validity of neurodevelopmental outcomes of children born very preterm assessed during routine clinical follow-up in England. Arch Dis Child Fetal Neonatal Ed 2018;103:F479–84.
Data-sharing statement
Requests for access to data should be addressed to the corresponding author in the first instance who will convey this to the relevant lead investigator.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Department of Health and Social Care . Best Research for Best Health: A New National Health Research Strategy 2006. www.gov.uk/government/publications/best-research-for-best-health-a-new-national-health-research-strategy (accessed 8 November 2018).
- Department of Health and Social Care . The Power of Information: Putting All of Us in Control of the Health and Care Information We Need 2012. https://webarchive.nationalarchives.gov.uk/20130802094648/http://informationstrategy.dh.gov.uk/ (accessed 29 August 2018).
- Department of Health and Social Care . Delivering 21st Century IT Support for the NHS: National Strategic Programme 2002. https://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4008227 (accessed 29 August 2018).
- British Association of Perinatal Medicine . The BAPM Neonatal Dataset for the Annual Reporting of Data by Neonatal Intensive Care Units 1997. www.bapm.org/publications/documents/general/neonatal_dataset.pdf (accessed 8 November 2018).
- World Health Organization . Classifications n.d. www.who.int/classifications/icd/icdonlineversions/en/ (accessed 8 November 2018).
- Donovan LE, Boyle SL, McNeil DA, Pedersen SD, Dean SR, Wood S, et al. Label of gestational diabetes mellitus affects caesarean section and neonatal intensive care unit admission without conventional indications. Can J Diabetes 2012;6:58-63. https://doi.org/10.1016/j.jcjd.2012.01.003.
- Kirkby S, Genen L, Turenne W, Dysart K. Outcomes and milestone achievement differences for very low-birth-weight multiples compared with singleton infants. Am J Perinatol 2010;27:439-44. https://doi.org/10.1055/s-0030-1247597.
- Joy S, Istwan N, Rhea D, Desch C, Stanziano G. The impact of maternal obesity on the incidence of adverse pregnancy outcomes in high-risk term pregnancies. Am J Perinatol 2009;26:345-9. https://doi.org/10.1055/s-0028-1110084.
- Moore PD, Bay RC, Balcazar H, Coonrod DV, Brady J, Russ R. Use of home visit and developmental clinic services by high risk Mexican-American and white non-Hispanic infants. Matern Child Health J 2005;9:35-47. https://doi.org/10.1007/s10995-005-2449-1.
- Wariki WM, Mori R, Boo NY, Cheah IG, Fujimura M, Lee J, et al. Risk factors associated with outcomes of very low birthweight infants in four Asian countries. J Paediatr Child Health 2013;49:E23-7. https://doi.org/10.1111/jpc.12054.
- Vendittelli F, Rivière O, Neveu B, Lémery D. Does induction of labor for constitutionally large-for-gestational-age fetuses identified in utero reduce maternal morbidity?. BMC Pregnancy Childb 2014;14. https://doi.org/10.1186/1471-2393-14-156.
- Lee QY, Quek WS, Chow S, Lui K. A population study of demographic changes and outcomes of very premature multiple births infants admitted to nicu in australia and new zealand. J Paediatr Child Health 2013;49.
- Fleming N, Ng N, Osborne C, Biederman S, Yasseen AS, Dy J, et al. Adolescent pregnancy outcomes in the province of Ontario: a cohort study. J Obstet Gynaecol Can 2013;35:234-45. https://doi.org/10.1016/S1701-2163(15)30995-6.
- Merritt TA, Goldstein M, Philips R, Peverini R, Iwakoshi J, Rodriguez A, et al. Impact of ART on pregnancies in California: an analysis of maternity outcomes and insights into the added burden of neonatal intensive care. J Perinatol 2014;34:345-50. https://doi.org/10.1038/jp.2014.17.
- Guner YS, Friedlich P, Wee CP, Dorey F, Camerini V, Upperman JS. State-based analysis of necrotizing enterocolitis outcomes. J Surg Res 2009;157:21-9. https://doi.org/10.1016/j.jss.2008.11.008.
- Kastenberg ZJ, Lee HC, Profit J, Gould JB, Sylvester KG. Effect of deregionalized care on mortality in very low-birth-weight infants with necrotizing enterocolitis. JAMA Pediatr 2015;169:26-32. https://doi.org/10.1001/jamapediatrics.2014.2085.
- Vigod SN, Kurdyak PA, Dennis CL, Gruneir A, Newman A, Seeman MV, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG 2014;121:566-74. https://doi.org/10.1111/1471-0528.12567.
- Ballantyne M, Sauve R, Creighton D, Saigal S, Asztalos E, Couture E, et al. Preterm infant journeys in a canadian regionalized health services context. J Paediatr Child Health (Canada) 2014;19. https://doi.org/10.1093/pch/19.6.e35-176.
- Mirea L, Yang J, Paterson AD, Shah V, Bassil KL, Lee SK, et al. Canadian Neonatal Network . Relationship of mode of conception and sex concordance with mortality/morbidity in preterm twins. Twin Res Hum Genet 2013;16:985-93. https://doi.org/10.1017/thg.2013.61.
- Baird R, Puligandla P, Skarsgard E, Laberge JM. Canadian Pediatric Surgical Network . Infectious complications in the management of gastroschisis. Pediatr Surg Int 2012;28:399-404. https://doi.org/10.1007/s00383-011-3038-6.
- Grover TR, Brozanski BS, Barry J, Zaniletti I, Asselin JM, Durand DJ, et al. High surgical burden for infants with severe chronic lung disease (sCLD). J Pediatr Surg 2014;49:1202-5. https://doi.org/10.1016/j.jpedsurg.2014.02.087.
- Tabano DC, Schroeder A, Sullivan K, Vaidya N. Impact of Assisted Reproductive Therapy (Art) on infant health and health care cost outcomes. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.1621.
- de Jongh BE, Locke R, Paul DA, Hoffman M. The differential effects of maternal age, race/ethnicity and insurance on neonatal intensive care unit admission rates. BMC Pregnancy Childb 2012;12. https://doi.org/10.1186/1471-2393-12-97.
- Gasparović V, Gornik I, Ivanović D. Sepsis syndrome in Croatian intensive care units: piloting a national comparative clinical database. Croat Med J 2006;47:404-9.
- Engelbrechtsen L, Nielsen EH, Perin T, Oldenburg A, Tabor A, Skibsted L. Danish Fetal Medicine Study Group . Cesarean section for the second twin: a population-based study of occurrence and outcome. Birth 2013;40:10-6. https://doi.org/10.1111/birt.12023.
- Andersson S, Petersen JP, Henriksen TB, Ebbesen F. The Danish neonatal clinical database is valuable for epidemiologic research in respiratory disease in preterm infants. BMC Pediatr 2014;14. https://doi.org/10.1186/1471-2431-14-47.
- Corvaglia L, Fantini MP, Aceti A, Gibertoni D, Rucci P, Baronciani D, et al. ‘Emilia Romagna Perinatal Network’ . Predictors of full enteral feeding achievement in very low birth weight infants. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0092235.
- Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012;345. https://doi.org/10.1136/bmj.e7961.
- Sengupta S, Carrion V, Shelton J, Wynn RJ, Ryan RM, Singhal K, et al. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr 2013;167:1053-9. https://doi.org/10.1001/jamapediatrics.2013.2581.
- Hummler H, Lang K, Azpeitia A, Valls ISA. Short-term outcome of very low birth weight infants (VLBWI) requiring cardiopulmonary resuscitation in the delivery room. Monatsschr Kinderheilkd 2014;162:1-103.
- Doyle TJ, Goodin K, Hamilton JJ. Maternal and neonatal outcomes among pregnant women with 2009 pandemic influenza A(H1N1) illness in Florida, 2009–2010: a population-based cohort study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0079040.
- Christensen RD, Lambert DK, Henry E, Wiedmeier SE, Snow GL, Baer VL, et al. Is ‘transfusion-associated necrotizing enterocolitis’ an authentic pathogenic entity?. Transfusion 2010;50:1106-12. https://doi.org/10.1111/j.1537-2995.2009.02542.x.
- Kugelman A, Reichman B, Chistyakov I, Boyko V, Levitski O, Lerner-Geva L, et al. Postdischarge infant mortality among very low birth weight infants: a population-based study. Pediatrics 2007;120:e788-94. https://doi.org/10.1542/peds.2006-3765.
- Imaizumi Y, Hayakawa K. Infant mortality among singletons and twins in Japan during 1999–2008 on the basis of risk factors. Twin Res Hum Genet 2013;16:639-44. https://doi.org/10.1017/thg.2012.156.
- Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics 2011;128:e1155-63. https://doi.org/10.1542/peds.2010-3464.
- Lorch SA, Baiocchi M, Silber JH, Even-Shoshan O, Escobar GJ, Small DS. The role of outpatient facilities in explaining variations in risk-adjusted readmission rates between hospitals. Health Serv Res 2010;45:24-41. https://doi.org/10.1111/j.1475-6773.2009.01043.x.
- Morriss FH. Increased risk of death among uninsured neonates. Health Serv Res 2013;48:1232-55. https://doi.org/10.1111/1475-6773.12042.
- Pandey A, Ranjan R. Hypertensive disorders in pregnancy requiring emergency (108) transportation in the state of Gujarat (India): an epidemiological study. J Clin Diagn Res 2010;4:2017-22.
- Wilson K, Nagy A, Green C, Boyd D, Ratnavel N, Mohinuddin S. Factors influencing early neonatal mortality in retrieved extreme preterm neonates. Arch Dis Child 2012;97. https://doi.org/10.1136/archdischild-2012-302724.0799.
- Clements KM, Barfield WD, Ayadi MF, Wilber N. Preterm birth-associated cost of early intervention services: an analysis by gestational age. Pediatrics 2007;119:e866-74. https://doi.org/10.1542/peds.2006-1729.
- Merewood A, Brooks D, Bauchner H, MacAuley L, Mehta SD. Maternal birthplace and breastfeeding initiation among term and preterm infants: a statewide assessment for Massachusetts. Pediatrics 2006;118:e1048-54. https://doi.org/10.1542/peds.2005-2637.
- Boo NY, Cheah IG. Malaysian National Neonatal Registry . Risk factors associated with pneumothorax in Malaysian neonatal intensive care units. J Paediatr Child Health 2011;47:183-90. https://doi.org/10.1111/j.1440-1754.2010.01944.x.
- Lorch SA, Passarella M, Zeigler A. Challenges to measuring variation in readmission rates of neonatal intensive care patients. Acad Pediatr 2014;14:47-53. https://doi.org/10.1016/j.acap.2014.06.010.
- Galyean AM, Lagrew DC, Bush MC, Kurtzman JT. Previous cesarean section and the risk of postpartum maternal complications and adverse neonatal outcomes in future pregnancies. J Perinatol 2009;29:726-30. https://doi.org/10.1038/jp.2009.108.
- Xu X, Grigorescu V, Siefert KA, Lori JR, Ransom SB. Cost of racial disparity in preterm birth: evidence from Michigan. J Health Care Poor Underserved 2009;20:729-47. https://doi.org/10.1353/hpu.0.0180.
- Wingate MS, Alexander GR. Racial and ethnic differences in perinatal mortality: the role of fetal death. Ann Epidemiol 2006;16:485-91. https://doi.org/10.1016/j.annepidem.2005.04.001.
- Yunis KA, Khawaja M, Beydoun H, Nassif Y, Khogali M, Tamim H. National Collaborative Perinatal Neonatal Network (NCPNN) . Intrauterine growth standards in a developing country: a study of singleton livebirths at 28–42 weeks’ gestation. Paediatr Perinat Epidemiol 2007;21:387-96. https://doi.org/10.1111/j.1365-3016.2007.00827.x.
- Luoto R, Matomäki J, Isolauri E, Lehtonen L. Incidence of necrotizing enterocolitis in very-low-birth-weight infants related to the use of Lactobacillus GG. Acta Paediatr 2010;99:1135-8. https://doi.org/10.1111/j.1651-2227.2010.01795.x.
- Moro M, Pérez-Rodriguez J, Figueras-Aloy J, Fernández C, Doménech E, Jiménez R, et al. Predischarge morbidities in extremely and very low-birth-weight infants in Spanish neonatal units. Am J Perinatol 2009;26:335-43. https://doi.org/10.1055/s-0028-1110083.
- Agarwal R, Jain A, Deorari AK, Paul VK. Post-resuscitation management of asphyxiated neonates. Indian J Pediatr 2008;75:175-80. https://doi.org/10.1007/s12098-008-0026-5.
- Watson SI, Arulampalam W, Petrou S, Marlow N, Morgan AS, Draper ES, et al. The effects of designation and volume of neonatal care on mortality and morbidity outcomes of very preterm infants in England: retrospective population-based cohort study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-004856.
- Johnson J, Anderson B, Raker C, Wenstrom K. Elective inductions at term and adverse neonatal outcomes. Am J Obstet Gynecol 2009;1:S124-S5. https://doi.org/10.1016/j.ajog.2009.10.326.
- Tomazevic T, Ban-Frangez H, Ribic-Pucelj M, Premru-Srsen T, Verdenik I. Small uterine septum is an important risk variable for preterm birth. Eur J Obstet Gynecol Reprod Biol 2007;135:154-7. https://doi.org/10.1016/j.ejogrb.2006.12.001.
- van Heesch MM, Evers JL, Dumoulin JC, van der Hoeven MA, van Beijsterveldt CE, Bonsel GJ, et al. A comparison of perinatal outcomes in singletons and multiples born after in vitro fertilization or intracytoplasmic sperm injection stratified for neonatal risk criteria. Acta Obstet Gynecol Scand 2014;93:277-86. https://doi.org/10.1111/aogs.12328.
- Laws PJ, Tracy SK, Sullivan EA. Perinatal outcomes of women intending to give birth in birth centers in Australia. Birth 2010;37:28-36. https://doi.org/10.1111/j.1523-536X.2009.00375.x.
- Tunescu M, Olariu G, Man O, Olariu S, Olariu L. Chorioamnionitis and multisystem impairment to a premature with GA under 32 weeks. J Mater-Fetal Neo M 2014;27.
- Doran J, McGowan JE, Alderdice F, McCall E, Craig S, Jenkins J. Regional follow up of late preterm neonatal intensive care graduates. Nurse Res 2012;19:37-43. https://doi.org/10.7748/nr2012.07.19.4.37.c9223.
- Kusuda S, Fujimura M, Uchiyama A, Totsu S, Matsunami K. Neonatal Research Network, Japan . Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res 2012;72:531-8. https://doi.org/10.1038/pr.2012.114.
- De Los Santos-Garate AM, Villa-Guillen M, Villanueva-García D, Vallejos-Ruíz ML, Murguía-Peniche MT. NEOSANO’s Network . Perinatal morbidity and mortality in late-term and post-term pregnancy: NEOSANO perinatal network’s experience in Mexico. J Perinatol 2011;31:789-93. https://doi.org/10.1038/jp.2011.43.
- Potti S, Jain NJ, Mastrogiannis DS, Dandolu V. Obstetric outcomes in pregnant women with diabetes versus hypertensive disorders versus both. J Matern Fetal Neonatal Med 2012;25:385-8. https://doi.org/10.3109/14767058.2011.580403.
- Maheshwari R, Luig M. An audit of respiratory management and outcomes of outborn extremely preterm neonates retrieved on the first day of life. Journal of Paediatrics and Child Health 2012;48.
- Lipkind HS, Duzyj C, Rosenberg TJ, Funai EF, Chavkin W, Chiasson MA. Disparities in cesarean delivery rates and associated adverse neonatal outcomes in New York City hospitals. Obstet Gynecol 2009;113:1239-47. https://doi.org/10.1097/AOG.0b013e3181a4c3e5.
- Crane JM, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG 2011;118:865-71. https://doi.org/10.1111/j.1471-0528.2011.02941.x.
- Morriss FH, Saha S, Bell EF, Colaizy TT, Stoll BJ, Hintz SR, et al. Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr 2014;168:746-54. https://doi.org/10.1001/jamapediatrics.2014.307.
- Nili F, McLeod L, O’Connell C, Sutton E, McMillan D. Outcomes of pregnancies in women with suspected antiphospholipid syndrome. J Neonatal Perinatal Med 2013;6:225-30. https://doi.org/10.3233/NPM-1370113.
- Abdel-Latif ME, Kecskés Z, Bajuk B. NSW and the ACT Neonatal Intensive Care Audit Group . Actuarial day-by-day survival rates of preterm infants admitted to neonatal intensive care in New South Wales and the Australian Capital Territory. Arch Dis Child Fetal Neonatal Ed 2013;98:F212-7. https://doi.org/10.1136/adc.2011.210856.
- Spitzer AR, Ellsbury DL, Handler D, Clark RH. The pediatrix babySteps (R) data warehouse and the pediatrix qualitySteps improvement project system-tools for ‘meaningful use’ in continuous quality improvement. Clin Perinatol 2010;37:49-70. https://doi.org/10.1016/j.clp.2010.01.016.
- Vogtmann C, Koch R, Gmyrek D, Kaiser A, Friedrich A. Risk-adjusted intraventricular hemorrhage rates in very premature infants: towards quality assurance between neonatal units. Dtsch Arztebl Int 2012;109:527-33. https://doi.org/10.3238/arztebl.2012.0527.
- Brown HK, Speechley KN, Macnab J, Natale R, Campbell MK. Neonatal morbidity associated with late preterm and early term birth: the roles of gestational age and biological determinants of preterm birth. Int J Epidemiol 2014;43:802-14. https://doi.org/10.1093/ije/dyt251.
- Anderberg E, Källén K, Berntorp K. The impact of gestational diabetes mellitus on pregnancy outcome comparing different cut-off criteria for abnormal glucose tolerance. Acta Obstet Gynecol Scand 2010;89:1532-7. https://doi.org/10.3109/00016349.2010.526186.
- Lisonkova S, Sheps SB, Janssen PA, Lee SK, Dahlgren L. Effect of older maternal age on birth outcomes in twin pregnancies: a population-based study. J Perinatol 2011;31:85-91. https://doi.org/10.1038/jp.2010.114.
- Heaman M, Kingston D, Brownell M, Helewa M. Predictors of prenatal and postpartum psychological distress: a population-based study in Manitoba, Canada. Reproductive Sciences 2014;1.
- Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ 2012;344. https://doi.org/10.1136/bmj.e2838.
- Jasso-Gutiérrez L, Durán-Arenas L, Flores-Huerta S, Cortés-Gallo G. Recommendations to improve healthcare of neonates with respiratory insufficiency beneficiaries of Seguro Popular. Salud Publica Mex 2012;54:57-64. https://doi.org/10.1590/S0036-36342012000700008.
- Tiblad E, Kublickas M, Ajne G, Bui, TH, Ek S, Karisson A, et al. Procedure-related complications and perinatal outcome after intrauterine transfusions in red cell alloimmunization in Stockholm. Fetal Diagn Ther 2011;30:266-73. https://doi.org/10.1159/000328683.
- Emilsson L, Lindahl B, Köster M, Lambe M, Ludvigsson JF. Review of 103 Swedish Healthcare Quality Registries. J Intern Med 2015;277:94-136. https://doi.org/10.1111/joim.12303.
- Rüegger C, Hegglin M, Adams M, Bucher HU. Swiss Neonatal Network . Population based trends in mortality, morbidity and treatment for very preterm- and very low birth weight infants over 12 years. BMC Pediatr 2012;12. https://doi.org/10.1186/1471-2431-12-17.
- Tsai WH, Hwang YS, Hung TY, Weng SF, Lin SJ, Chang WT. Association between mechanical ventilation and neurodevelopmental disorders in a nationwide cohort of extremely low birth weight infants. Res Dev Disabil 2014;35:1544-50. https://doi.org/10.1016/j.ridd.2014.03.048.
- Herrod HG, Chang CF, Steinberg SS. Variations in costs for the care of low-birth-weight infants among academic hospitals. Clin Pediatr 2010;49:443-9. https://doi.org/10.1177/0009922809341750.
- van Dommelen P, Mohangoo AD, Verkerk PH, van der Ploeg CP, van Straaten HL. Dutch NICU Neonatal Hearing Screening Working Group . Risk indicators for hearing loss in infants treated in different neonatal intensive care units. Acta Paediatr 2010;99:344-9. https://doi.org/10.1111/j.1651-2227.2009.01614.x.
- Manktelow BN, Seaton SE, Field DJ, Draper ES. Population-based estimates of in-unit survival for very preterm infants. Pediatrics 2013;131:e425-32. https://doi.org/10.1542/peds.2012-2189.
- Vogel JP, Holloway E, Cuesta C, Carroli G, Souza JP, Barrett J. Outcomes of non-vertex second twins, following vertex vaginal delivery of first twin: a secondary analysis of the WHO Global Survey on maternal and perinatal health. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-55.
- Shah A, Faundes A, Machoki M, Bataglia V, Amokrane F, Donner A, et al. Methodological considerations in implementing the WHO Global Survey for Monitoring Maternal and Perinatal Health. Bull World Health Organ 2008;86:126-31. https://doi.org/10.2471/BLT.06.039842.
- Soll RF, Edwards EM, Badger GJ, Kenny MJ, Morrow KA, Buzas JS, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics 2013;132:222-8. https://doi.org/10.1542/peds.2013-0501.
- Doyle LW. Victorian Infant Collaborative Study Group . Evaluation of neonatal intensive care for extremely low birth weight infants in Victoria over two decades: I. Effectiveness. Pediatrics 2004;113:505-9. https://doi.org/10.1542/peds.113.3.505.
- El-Sheikh A, Francis A, Gardosi J. Comparative analysis of length of stay in neonatal intensive care after 34 weeks in singleton babies with and without intrauterine growth restriction. Arch Dis Child 2011;96. https://doi.org/10.1136/adc.2011.300161.59.
- Van Dijk JW, Anderko L, Stetzer F. The impact of Prenatal Care Coordination on birth outcomes. J Obstet Gynecol Neonatal Nurs 2011;40:98-108. https://doi.org/10.1111/j.1552-6909.2010.01206.x.
- Heller G, Günster C, Misselwitz B, Feller A, Schmidt S. Annual patient volume and survival of very low birth weight infants (VLBWs) in Germany: a nationwide analysis based on administrative data. Z Geburtshilfe Neonatol 2007;211:123-31. https://doi.org/10.1055/s-2007-960747.
- Gleissner MW, Spantzel T, Bücker-Nott HJ, Jorch G. Risk factors of retinopathy of prematurity in infants 32 to 36 weeks gestational age. Z Geburtshilfe Neonatol 2003;207:24-8. https://doi.org/10.1055/s-2003-37841.
- Hummler HD, Poets C, Vochem M, Hentschel R, Linderkamp O. Mortality and morbidity of very premature infants in Baden-Württemberg depending on hospital size: is the current degree of regionalization adequate?. Z Geburtshilfe Neonatol 2006;210:6-11. https://doi.org/10.1055/s-2006-931508.
- Gay S, Ferdinus C, Sagot P, Gouyon JB. What are the neonatal risks in low risk pregnancies: place of paediatric organisation in birth centers development?. Arch Pediatr 2007;14:1174-7. https://doi.org/10.1016/j.arcped.2007.06.028.
- Marcoux MO, Denizot S, Dassieu G, Picaud JC, Cristini C, Arnaud C, et al. Evidence versus experience in neonatal practice: the example of extremely premature infants. Arch Pediatr 2009;16:49-55. https://doi.org/10.1016/S0929-693X(09)75301-1.
- Schlößer RL, Frey G, Zemlin M, Misselwitz B. Mortality of very low birth weight infants during a 24 year period in Hesse a province of Germany: impact of variation in registration. Z Geburtshilfe Neonatol 2014;218:100-5. https://doi.org/10.1055/s-0034-1376992.
- Research Ethics Service . Standard Operating Procedures for Research Ethics Committees: Version 7.4 2019.
- Council for Science and Technology . Better Use of Personal Information: Opportunities and Risks 2005. http://webarchive.nationalarchives.gov.uk/20130705054945/www.bis.gov.uk/assets/cst/docs/files/cst-reports/05-2177-better-use-personal-information.pdf (accessed 19 December 2015).
- Evans TW. Best research for best health: a new national health research strategy. Clin Med 2006;6:435-7. https://doi.org/10.7861/clinmedicine.6-5-435.
- The Academy of Medical Sciences . Personal Data for Public Good: Using Health Information in Medical Research 2013. www.acmedsci.ac.uk/policy/policy-projects/personal-data (accessed 10 October 2015).
- Department of Health and Social Care . Toolkit for High Quality Neonatal Services 2009. https://webarchive.nationalarchives.gov.uk/20130123200735/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_107845 (accessed 8 November 2018).
- Wang W, Krishnan E. Big data and clinicians: a review on the state of the science. JMIR Med Inform 2014;2. https://doi.org/10.2196/medinform.2913.
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. https://doi.org/10.1056/NEJMra1005408.
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271-83. https://doi.org/10.1016/S0140-6736(06)69525-1.
- Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 1991;119:630-8. https://doi.org/10.1016/S0022-3476(05)82418-7.
- Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. National Institute of Child Health and Human Development Neonatal Research Network . Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2006;117:e137-42. https://doi.org/10.1542/peds.2005-1543.
- Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003;23:278-85. https://doi.org/10.1038/sj.jp.7210892.
- Kamitsuka MD, Horton MK, Williams MA. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics 2000;105:379-84. https://doi.org/10.1542/peds.105.2.379.
- Ballard RA, Ballard PL. Antenatal hormone therapy for improving the outcome of the preterm infant. J Perinatol 1996;16:390-6.
- Bauer CR, Morrison JC, Poole WK, Korones SB, Boehm JJ, Rigatto H, et al. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics 1984;73:682-8.
- Halac E, Halac J, Begue EF, . Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. J Pediatr 1990;117:132-8. https://doi.org/10.1016/S0022-3476(05)72461-6.
- Smith LM, Qureshi N, Chao CR. Effects of single and multiple courses of antenatal glucocorticoids in preterm newborns less than 30 weeks’ gestation. J Matern Fetal Med 2000;9:131-5. https://doi.org/10.1002/(SICI)1520-6661(200003/04)9:2<131::AID-MFM9>3.0.CO;2-M.
- Bajwa NM, Berner M, Worley S, Pfister RE. Swiss Neonatal Network . Population based age stratified morbidities of premature infants in Switzerland. Swiss Med Wkly 2011;141. https://doi.org/10.4414/smw.2011.13212.
- Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987;17:213-88. https://doi.org/10.1016/0045-9380(87)90031-4.
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1-7. https://doi.org/10.1097/00000658-197801000-00001.
- Pan H, Cole T. LMS Growth, a Microsoft Excel Add-in to Access Growth References Based on LMS Method 2014. wwwhealthforallchildrencom/ (accessed 8 November 2018).
- Breslow N, Day N. Statistical Methods in Cancer Research: IARC Scientific Publications No. 32. Lyon: International Agency for Research on Cancer (IARC); 1994.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289-300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
- Battersby C, Santhakumaran S, Upton M, Radbone L, Birch J, Modi N. East of England Perinatal Networks . The impact of a regional care bundle on maternal breast milk use in preterm infants: outcomes of the East of England quality improvement programme. Arch Dis Child Fetal Neonatal Ed 2014;99:F395-401. https://doi.org/10.1136/archdischild-2013-305475.
- Palmer SR, Biffin A, Gamsu HR. Outcome of neonatal necrotising enterocolitis: results of the BAPM/CDSC surveillance study, 1981–84. Arch Dis Child 1989;64:388-94. https://doi.org/10.1136/adc.64.3.388.
- Rees CM, Eaton S, Pierro A. National prospective surveillance study of necrotizing enterocolitis in neonatal intensive care units. J Pediatr Surg 2010;45:1391-7. https://doi.org/10.1016/j.jpedsurg.2009.12.002.
- Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria?. J Perinatol 2007;27:661-71. https://doi.org/10.1038/sj.jp.7211782.
- Llanos AR, Moss ME, Pinzòn MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol 2002;16:342-9. https://doi.org/10.1046/j.1365-3016.2002.00445.x.
- Stoll BJ. Epidemiology of necrotizing enterocolitis. Clin Perinatol 1994;21:205-18. https://doi.org/10.1016/S0095-5108(18)30341-5.
- Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Eunice Kennedy Shriver NICHD Neonatal Research Network . Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 2008;122:e573-82. https://doi.org/10.1542/peds.2007-3449.
- Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health 1997;87:2026-31. https://doi.org/10.2105/AJPH.87.12.2026.
- Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol 2006;20:498-506. https://doi.org/10.1111/j.1365-3016.2006.00756.x.
- Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K, Lagercrantz H, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009;301:2225-33. https://doi.org/10.1001/jama.2009.771.
- Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115:696-703. https://doi.org/10.1542/peds.2004-0569.
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345. https://doi.org/10.1136/bmj.e7976.
- Sankaran K, Puckett B, Lee DS, Seshia M, Boulton J, Qiu Z, et al. Canadian Neonatal Network . Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr 2004;39:366-72. https://doi.org/10.1097/00005176-200410000-00012.
- Luig M, Lui K. NSW. ACT NICUS Group . Epidemiology of necrotizing enterocolitis: Part I – changing regional trends in extremely preterm infants over 14 years. J Paediatr Child Health 2005;41:169-73. https://doi.org/10.1111/j.1440-1754.2005.00582.x.
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443-56. https://doi.org/10.1542/peds.2009-2959.
- Battersby C, Longford N, Mandalia S, Costeloe K, Modi N. UK Neonatal Collaborative Necrotising Enterocolitis (UKNC-NEC) study group . Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012–13: a whole-population surveillance study. Lancet Gastroenterol Hepatol 2017;2:43-51. https://doi.org/10.1016/S2468-1253(16)30117-0.
- Royal College of Paediatrics and Child Health . UK-WHO Growth Charts: Neonatal and Infant Close Monitoring (NICM) n.d. www.rcpch.ac.uk/resources/uk-who-growth-charts-neonatal-infant-close-monitoring-nicm (accessed 8 November 2018).
- McLennan D, Barnes H, Noble M, Davies J, Garratt E, Dibben C. The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- NHS Digital . Linked HES–ONS Mortality Data 2018. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/linked-hes-ons-mortality-data (accessed 8 November 2018).
- Cole TJ, Hey E, Richmond S. The PREM score: a graphical tool for predicting survival in very preterm births. Arch Dis Child Fetal Neonatal Ed 2010;95:F14-9. https://doi.org/10.1136/adc.2009.164533.
- Office for National Statistics 2018. www.ons.gov.uk/ (accessed 8 November 2018).
- Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000;106:659-71. https://doi.org/10.1542/peds.106.4.659.
- Esteve J. Statistical Methods in Cancer Research: Volume IV – Descriptive Epidemiology. New York, NY: Oxford University Press; 1994.
- Kiely JL. What is the population-based risk of preterm birth among twins and other multiples?. Clin Obstet Gynecol 1998;41:3-11. https://doi.org/10.1097/00003081-199803000-00005.
- Buekens P, Wilcox A. Why do small twins have a lower mortality rate than small singletons?. Am J Obstet Gynecol 1993;168:937-41. https://doi.org/10.1016/S0002-9378(12)90849-2.
- Jones HE, Ohlssen DI, Spiegelhalter DJ. Use of the false discovery rate when comparing multiple health care providers. J Clin Epidemiol 2008;61:232-40. https://doi.org/10.1016/j.jclinepi.2007.04.017.
- Bolisetty S, Legge N, Bajuk B, Lui K. New South Wales and the Australian capital territory neonatal intensive care units’ data collection . Preterm infant outcomes in New South Wales and the Australian Capital Territory. J Paediatr Child Health 2015;51:713-21. https://doi.org/10.1111/jpc.12848.
- Costeloe KL, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20660.
- Levene MI. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 1981;56:900-4. https://doi.org/10.1136/adc.56.12.900.
- Agresti A, Coull BA. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Stat 1998;52:119-26. https://doi.org/10.1080/00031305.1998.10480550.
- Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci 2001;16:101-17. https://doi.org/10.1214/ss/1009213286.
- Rogers W. Regression standard errors in clustered samples. Stata Tech Bull 1994;3.
- Royal College of Paediatrics and Child Health . Annual Reports 2009, 2010, 2012, 2013 2014.
- Köpcke F, Lubgan D, Fietkau R, Scholler A, Nau C, Stürzl M, et al. Evaluating predictive modeling algorithms to assess patient eligibility for clinical trials from routine data. BMC Med Inform Decis Mak 2013;13. https://doi.org/10.1186/1472-6947-13-134.
- De Moor G, Sundgren M, Kalra D, Schmidt A, Dugas M, Claerhout B, et al. Using electronic health records for clinical research: the case of the EHR4CR project. J Biomed Inform 2015;53:162-73. https://doi.org/10.1016/j.jbi.2014.10.006.
- Newsham AC, Johnston C, Hall G, Leahy MG, Smith AB, Vikram A, et al. Development of an advanced database for clinical trials integrated with an electronic patient record system. Comput Biol Med 2011;41:575-86. https://doi.org/10.1016/j.compbiomed.2011.04.014.
- Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 2010;362:1959-69. https://doi.org/10.1056/NEJMoa0911781.
- Horbar JD, Carpenter JH, Buzas J, Soll RF, Suresh G, Bracken MB, et al. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ 2004;329. https://doi.org/10.1136/bmj.329.7473.1004.
- Vohra S, Reilly M, Rac VE, Bhaloo Z, Zayack D, Wimmer J, et al. Study protocol for multicentre randomized controlled trial of HeLP (Heat Loss Prevention) in the delivery room. Contemp Clin Trials 2013;36:54-60. https://doi.org/10.1016/j.cct.2013.06.001.
- Milligan DW. Outcomes of children born very preterm in Europe. Arch Dis Child Fetal Neonatal Ed 2010;95:F234-40. https://doi.org/10.1136/adc.2008.143685.
- Hille ET, Weisglas-Kuperus N, van Goudoever JB, Jacobusse GW, Ens-Dokkum MH, de Groot L, et al. Functional outcomes and participation in young adulthood for very preterm and very low birth weight infants: the Dutch project on preterm and small for gestational age infants at 19 years of age. Pediatrics 2007;120:e587-95. https://doi.org/10.1542/peds.2006-2407.
- Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics 2010;126:342-51. https://doi.org/10.1542/peds.2010-0710.
- Pedersen SJ, Sommerfelt K, Markestad T. Early motor development of premature infants with birthweight less than 2000 grams. Acta Paediatr 2000;89:1456-61. https://doi.org/10.1111/j.1651-2227.2000.tb02776.x.
- Paneth N, Qiu H, Rosenbaum P, Saigal S, Bishai S, Jetton J, et al. Reliability of classification of cerebral palsy in low-birthweight children in four countries. Dev Med Child Neurol 2003;45:628-33. https://doi.org/10.1111/j.1469-8749.2003.tb00968.x.
- Munck P, Niemi P, Lapinleimu H, Lehtonen L, Haataja L. PIPARI Study Group . Stability of cognitive outcome from 2 to 5 years of age in very low birth weight children. Pediatrics 2012;129:503-8. https://doi.org/10.1542/peds.2011-1566.
- Potharst ES, Houtzager BA, van Sonderen L, Tamminga P, Kok JH, Last BF, et al. Prediction of cognitive abilities at the age of 5 years using developmental follow-up assessments at the age of 2 and 3 years in very preterm children. Dev Med Child Neurol 2012;54:240-6. https://doi.org/10.1111/j.1469-8749.2011.04181.x.
- Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley scales of infant development for cognitive function of extremely low birth weight children at school age. Pediatrics 2005;116:333-41. https://doi.org/10.1542/peds.2005-0173.
- Roberts G, Anderson PJ, Doyle LW. Victorian Infant Collaborative Study Group . The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Arch Dis Child 2010;95:786-90. https://doi.org/10.1136/adc.2009.160283.
- Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Ment Retard Dev Disabil Res Rev 2002;8:241-8. https://doi.org/10.1002/mrdd.10049.
- Pallás Alonso CR, de La Cruz Bértolo J, Medina López MC, Orbea Gallardo C, Gómez Castillo E, Simón De Las Heras R. Cerebral palsy and age of sitting and walking in children weighing less than 1500 g at birth. An Esp Pediatr 2000;53:48-52. https://doi.org/10.1016/S1695-4033(00)77413-3.
- de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA 2009;302:2235-42. https://doi.org/10.1001/jama.2009.1708.
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth: EPICure Study Group. N Engl J Med 2000;343:378-84. https://doi.org/10.1056/NEJM200008103430601.
- Stoelhorst GM, Rijken M, Martens SE, van Zwieten PH, Feenstra J, Zwinderman AH, et al. Developmental outcome at 18 and 24 months of age in very preterm children: a cohort study from 1996 to 1997. Early Hum Dev 2003;72:83-95. https://doi.org/10.1016/S0378-3782(03)00011-2.
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002;288:728-37. https://doi.org/10.1001/jama.288.6.728.
- Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. Victorian Infant Collaborative Study Group . School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 2013;131:e1053-61. https://doi.org/10.1542/peds.2012-2311.
- Jansson-Verkasalo E, Valkama M, Vainionpää L, Pääkkö E, Ilkko E, Lehtihalmes M. Language development in very low birth weight preterm children: a follow-up study. Folia Phoniatr Logop 2004;56:108-19. https://doi.org/10.1159/000076062.
- Sansavini A, Guarini A, Alessandroni R, Faldella G, Giovanelli G, Salvioli G. Are early grammatical and phonological working memory abilities affected by preterm birth?. J Commun Disord 2007;40:239-56. https://doi.org/10.1016/j.jcomdis.2006.06.009.
- Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev Med Child Neurol 1999;41:94-109. https://doi.org/10.1017/S0012162299000201.
- Pietz J, Peter J, Graf R, Rauterberg-Ruland I, Rupp A, Sontheimer D, et al. Physical growth and neurodevelopmental outcome of nonhandicapped low-risk children born preterm. Early Hum Dev 2004;79:131-43. https://doi.org/10.1016/j.earlhumdev.2004.05.001.
- Vohr B. Speech and language outcomes of very preterm infants. Semin Fetal Neonatal Med 2014;19:78-83. https://doi.org/10.1016/j.siny.2013.10.007.
- Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr 2011;158:766-74.e1. https://doi.org/10.1016/j.jpeds.2010.10.032.
- van Noort-van der Spek IL, Franken MC, Weisglas-Kuperus N. Language functions in preterm-born children: a systematic review and meta-analysis. Pediatrics 2012;129:745-54. https://doi.org/10.1542/peds.2011-1728.
- Synnes AR, Anson S, Baum J, Usher L. Incidence and pattern of hearing impairment in children with ≤ 800 g birthweight in British Columbia, Canada. Acta Paediatr 2012;101:e48-54. https://doi.org/10.1111/j.1651-2227.2011.02437.x.
- D’Amore A, Broster S, Le Fort W, Curley A. East Anglian Very Low Birthweight Project . Two-year outcomes from very low birthweight infants in a geographically defined population across 10 years, 1993–2002: comparing 1993–1997 with 1998–2002. Arch Dis Child Fetal Neonatal Ed 2011;96:F178-85. https://doi.org/10.1136/adc.2009.171876.
- Ari-Even Roth D, Hildesheimer M, Maayan-Metzger A, Muchnik C, Hamburger A, Mazkeret R, et al. Low prevalence of hearing impairment among very low birthweight infants as detected by universal neonatal hearing screening. Arch Dis Child Fetal Neonatal Ed 2006;91:F257-62. https://doi.org/10.1136/adc.2005.074476.
- Veen S, Sassen ML, Schreuder AM, Ens-Dokkum MH, Verloove-Vanhorick SP, Brand R, et al. Hearing loss in very preterm and very low birthweight infants at the age of 5 years in a nationwide cohort. Int J Pediatr Otorhinolaryngol 1993;26:11-28. https://doi.org/10.1016/0165-5876(93)90192-6.
- Bliss . UK Retinopathy of Prematurity Guideline May 2008 2018. www.rcophth.ac.uk/wp-content/uploads/2014/12/2008-SCI-021-Guidelines-Retinopathy-of-Prematurity.pdf (accessed 8 November 2018).
- Dhaliwal C, Fleck B, Wright E, Graham C, McIntosh N. Incidence of retinopathy of prematurity in Lothian, Scotland, from 1990 to 2004. Arch Dis Child Fetal Neonatal Ed 2008;93:F422-6. https://doi.org/10.1136/adc.2007.134791.
- Rahi JS, Cable N. British Childhood Visual Impairment Study Group . Severe visual impairment and blindness in children in the UK. Lancet 2003;362:1359-65. https://doi.org/10.1016/S0140-6736(03)14631-4.
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk AM. Psychiatric symptoms and disorders in adolescents with low birth weight. Arch Dis Child Fetal Neonatal Ed 2004;89:F445-50. https://doi.org/10.1136/adc.2003.038943.
- Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Mental health and social competencies of 10- to 12-year-old children born at 23 to 25 weeks of gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics 2007;120:118-33. https://doi.org/10.1542/peds.2006-2988.
- Elgen I, Sommerfelt K, Markestad T. Population based, controlled study of behavioural problems and psychiatric disorders in low birthweight children at 11 years of age. Arch Dis Child Fetal Neonatal Ed 2002;87:F128-32. https://doi.org/10.1136/fn.87.2.F128.
- Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res 2011;69:11R-8R. https://doi.org/10.1203/PDR.0b013e318212faa0.
- Pinto-Martin JA, Levy SE, Feldman JF, Lorenz JM, Paneth N, Whitaker AH. Prevalence of autism spectrum disorder in adolescents born weighing < 2000 grams. Pediatrics 2011;128:883-91. https://doi.org/10.1542/peds.2010-2846.
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr 2010;156:525-31.e2. https://doi.org/10.1016/j.jpeds.2009.10.041.
- Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child 2006;91:8-15. https://doi.org/10.1136/adc.2004.062083.
- Centers for Disease Control and Prevention (CDC) . Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, United States, 2006. MMWR Surveill Summ 2009;58:1-20.
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124:717-28. https://doi.org/10.1542/peds.2008-2816.
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry 2010;49:453-63.e1. https://doi.org/10.1016/j.jaac.2010.02.002.
- Johnson S, Marlow N. Developmental screen or developmental testing?. Early Hum Dev 2006;82:173-83. https://doi.org/10.1016/j.earlhumdev.2006.01.008.
- Flynn JR. Searching for justice: the discovery of IQ gains over time. Am Psychol 1999;54:5-20. https://doi.org/10.1037/0003-066X.54.1.5.
- Aylward GP, Aylward BS. The changing yardstick in measurement of cognitive abilities in infancy. J Dev Behav Pediatr 2011;32:465-8. https://doi.org/10.1097/DBP.0b013e3182202eb3.
- Shribman S, Billingham K. Healthy child programme-pregnancy and the first five years. Department of Health and Social Care; 2009.
- The Victorian Infant Collaborative Study Group . Improved outcome into the 1990s for infants weighing 500–999 g at birth: The Victorian Infant Collaborative Study Group. Arch Dis Child Fetal Neonatal Ed 1997;77:F91-4. https://doi.org/10.1136/fn.77.2.F91.
- Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics 2004;113:781-9. https://doi.org/10.1542/peds.113.4.781.
- Bayley N. Technical manual for the Bayley Scales of Infant and Toddler Development (Third Edition). San Antonio, TX: Harcourt Assessment; 2006.
- Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Victorian Infant Collaborative Group . Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med 2010;164:352-6. https://doi.org/10.1001/archpediatrics.2010.20.
- Lowe JR, Erickson SJ, Schrader R, Duncan AF. Comparison of the Bayley II Mental Developmental Index and the Bayley III Cognitive Scale: are we measuring the same thing?. Acta Paediatr 2012;101:e55-8. https://doi.org/10.1111/j.1651-2227.2011.02517.x.
- Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr 2012;160:553-8. https://doi.org/10.1016/j.jpeds.2011.09.047.
- Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr 2012;161:222-8.e3. https://doi.org/10.1016/j.jpeds.2012.01.057.
- Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used?. Pediatr Res 2014;75:670-4. https://doi.org/10.1038/pr.2014.10.
- Casenhiser D, Breinbauer C, Greenspan S. Evaluating Greenspan’s Social Emotional Growth Scale Chart As a Screening for Autism n.d.
- Vohr BR, Msall ME, Wilson D, Wright LL, McDonald S, Poole WK. Spectrum of gross motor function in extremely low birth weight children with cerebral palsy at 18 months of age. Pediatrics 2005;116:123-9. https://doi.org/10.1542/peds.2004-1810.
- Haataja L, Mercuri E, Regev R, Cowan F, Rutherford M, Dubowitz V, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr 1999;135:153-61. https://doi.org/10.1016/S0022-3476(99)70016-8.
- Frisone MF, Mercuri E, Laroche S, Foglia C, Maalouf EF, Haataja L, et al. Prognostic value of the neurologic optimality score at 9 and 18 months in preterm infants born before 31 weeks’ gestation. J Pediatr 2002;140:57-60. https://doi.org/10.1067/mpd.2002.119626.
- Robins DL, Fein D, Barton ML, Green JA. The modified checklist for autism in toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord 2001;31:131-44. https://doi.org/10.1023/A:1010738829569.
- Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Moore M, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics 2008;121:758-65. https://doi.org/10.1542/peds.2007-2158.
- Kuban KC, O’Shea TM, Allred EN, Tager-Flusberg H, Goldstein DJ, Leviton A. Positive screening on the modified checklist for autism in toddlers (M-CHAT) in extremely low gestational age newborns. J Pediatr 2009;154:535-40.e1. https://doi.org/10.1016/j.jpeds.2008.10.011.
- Moore T, Johnson S, Hennessy E, Marlow N. Screening for autism in extremely preterm infants: problems in interpretation. Dev Med Child Neurol 2012;54:514-20. https://doi.org/10.1111/j.1469-8749.2012.04265.x.
- Luyster RJ, Kuban KC, O’Shea TM, Paneth N, Allred EN, Leviton A. ELGAN Study investigators . The modified checklist for autism in toddlers in extremely low gestational age newborns: individual items associated with motor, cognitive, vision and hearing limitations. Paediatr Perinat Epidemiol 2011;25:366-76. https://doi.org/10.1111/j.1365-3016.2010.01187.x.
- Wetherby AM, Prizant BM. Communication and Symbolic Behavior Scales Developmental Profile. Baltimore, MD: Paul H Brookes Publishing; 2002.
- Dunn W. Infant/Toddler Sensory Profile. San Antonio, TX: Harcourt Assessment; 2002.
- Stephens BE, Bann CM, Watson VE, Sheinkopf SJ, Peralta-Carcelen M, Bodnar A, et al. Screening for autism spectrum disorders in extremely preterm infants. J Dev Behav Pediatr 2012;33:535-41. https://doi.org/10.1097/DBP.0b013e31825fd0af.
- Dudova I, Kasparova M, Markova D, Zemankova J, Beranova S, Urbanek T, et al. Screening for autism in preterm children with extremely low and very low birth weight. Neuropsychiatr Dis Treat 2014;10:277-82. https://doi.org/10.2147/NDT.S57057.
- Allison C, Baron-Cohen S, Wheelwright S, Charman T, Richler J, Pasco G, et al. The Q-CHAT (Quantitative CHecklist for Autism in Toddlers): a normally distributed quantitative measure of autistic traits at 18-24 months of age – preliminary report. J Autism Dev Disord 2008;38:1414-25. https://doi.org/10.1007/s10803-007-0509-7.
- National Perinatal Epidemiology Unit (NPEU) . Disability and Perinatal Care: Measurement of Health Status at Two Years: A Report of Two Working Groups Convened by the National Perinatal Epidemiology Unit and the Former Oxford Regional Health Authority 1994.
- British Association of Perinatal Medicine . Classification of Health Status at 2 Years As a Perinatal Outcome 2008. www.networks.nhs.uk/nhs-networks/staffordshire-shropshire-and-black-country-newborn/documents/2_year_Outcome_BAPM_WG_report_v6_Jan08.pdf (accessed 8 November 2018).
- Jones HP, Guildea ZE, Stewart JH, Cartlidge PH. The health status questionnaire: achieving concordance with published disability criteria. Arch Dis Child 2002;86:15-20. https://doi.org/10.1136/adc.86.1.15.
- Bohin S, Draper ES, Field DJ. Health status of a population of infants born before 26 weeks gestation derived from routine data collected between 21 and 27 months post-delivery. Early Hum Dev 1999;55:9-18. https://doi.org/10.1016/S0378-3782(99)00003-1.
- Marlow N, Wolke D, Bracewell MA, Samara M. EPICure Study Group . Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005;352:9-19. https://doi.org/10.1056/NEJMoa041367.
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214-23. https://doi.org/10.1111/j.1469-8749.1997.tb07414.x.
- Jahnsen R, Aamodt G, Rosenbaum P. Gross motor function classification system used in adults with cerebral palsy: agreement of self-reported versus professional rating. Dev Med Child Neurol 2006;48:734-8. https://doi.org/10.1017/S0012162206001575.
- Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol 2006;48:424-8. https://doi.org/10.1017/S0012162206000934.
- Wood E, Rosenbaum P. The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Dev Med Child Neurol 2000;42:292-6. https://doi.org/10.1017/S0012162200000529.
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, Ohrvall AM, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 2006;48:549-54. https://doi.org/10.1017/S0012162206001162.
- Salt A, D’Amore A, Ahluwalia J, Seward A, Kaptoge S, Halliday S, et al. East Anglian Very Low Birthweight Project Group . Outcome at 2 years for very low birthweight infants in a geographical population: risk factors, cost, and impact of congenital anomalies. Early Hum Dev 2006;82:125-33. https://doi.org/10.1016/j.earlhumdev.2005.10.016.
- Dorling JS, Field DJ. Follow up of infants following discharge from the neonatal unit: structure and process. Early Hum Dev 2006;82:151-6. https://doi.org/10.1016/j.earlhumdev.2006.01.006.
- Dawson C, Perkins M, Draper E, Johnson A, Field D. Are outcome data regarding the survivors of neonatal care available from routine sources?. Arch Dis Child Fetal Neonatal Ed 1997;77:F206-10. https://doi.org/10.1136/fn.77.3.F206.
- Johnson A, King R. Can routine information systems be used to monitor serious disability?. Arch Dis Child 1999;80:63-6. https://doi.org/10.1136/adc.80.1.63.
- Modi N, Carpenter T. Fetal growth and coronary heart disease. Lancet 1997;349:286-7. https://doi.org/10.1016/S0140-6736(05)64900-8.
- House of Commons Health Committee Session 1991–2 . Maternity Services Vol. 1: Report Together With Appendices and the Proceedings of the Committee 1992.
- Audit Commission . Children First: A Study of Hospital Services 1993. https://webarchive.nationalarchives.gov.uk/20150423154441/http://archive.audit-commission.gov.uk/auditcommission/aboutus/publications/pages/national-reports-and-studies-archive.aspx.html (accessed 9 July 2019).
- Clinical Standards Advisory Group . Neonatal Intensive Care 1993.
- Cumberlege J. Changing Childbirth: Part I – Report of the Expert Maternity Group. Winterton Report 1993.
- British Association of Perinatal Medicine . Standards for Hospitals Providing Neonatal Intensive and High Dependency Care and Categories of Babies Requiring Neonatal Care 2001.
- The National Audit Office . Caring for Vulnerable Babies: The Reorganisation of Neonatal Services in England 2007.
- National Institute for Health and Care Excellence (NICE) . Neonatal Specialist Care: Quality Standard 2010.
- Fooks J. Four key questions that identify severe disability. Arch Dis Child 1999;80:67-8. https://doi.org/10.1136/adc.80.1.67.
- Kim MM, O’Connor KS, McLean J, Robson A, Chance G. Do parents and professionals agree on the developmental status of high-risk infants?. Pediatrics 1996;97:676-81.
- Bortolus R, Parazzini F, Trevisanuto D, Cipriani S, Ferrarese P, Zanardo V. Gruppo di Studio Metodologie nei Follow-up Pediatrici . Developmental assessment of preterm and term children at 18 months: reproducibility and validity of a postal questionnaire to parents. Acta Paediatr 2002;91:1101-7. https://doi.org/10.1111/j.1651-2227.2002.tb00106.x.
- Johnson S, Marlow N, Wolke D, Davidson L, Marston L, O’Hare A, et al. Validation of a parent report measure of cognitive development in very preterm infants. Dev Med Child Neurol 2004;46:389-97. https://doi.org/10.1017/S0012162204000635.
- Johnson S, Wolke D, Marlow N. Preterm Infant Parenting Study Group . Developmental assessment of preterm infants at 2 years: validity of parent reports. Dev Med Child Neurol 2008;50:58-62. https://doi.org/10.1111/j.1469-8749.2007.02010.x.
- Pritchard MA, Colditz PB, Beller EM. Queensland Optimising Preterm Infant Outcomes Group . Parents’ evaluation of developmental status in children born with a birthweight of 1250 g or less. J Paediatr Child Health 2005;41:191-6. https://doi.org/10.1111/j.1440-1754.2005.00586.x.
- Da Costa D, Bann CM, Hansen NI, Shankaran S, Delaney-Black V. National Institute of Child Health and Human Development Neonatal Research Network . Validation of the Functional Status II questionnaire in the assessment of extremely-low-birthweight infants. Dev Med Child Neurol 2009;51:536-44. https://doi.org/10.1111/j.1469-8749.2009.03318.x.
- Skellern CY, Rogers Y, O’Callaghan MJ. A parent-completed developmental questionnaire: follow up of ex-premature infants. J Paediatr Child Health 2001;37:125-9. https://doi.org/10.1046/j.1440-1754.2001.00604.x.
- Fooks J, Mutch L, Yudkin P, Johnson A, Elbourne D. Comparing two methods of follow up in a multicentre randomised trial. Arch Dis Child 1997;76:369-76. https://doi.org/10.1136/adc.76.4.369.
- Martin AJ, Darlow BA, Salt A, Hague W, Sebastian L, McNeill N, et al. Performance of the Parent Report of Children’s Abilities-Revised (PARCA-R) versus the Bayley Scales of Infant Development III. Arch Dis Child 2013;98:955-8. https://doi.org/10.1136/archdischild-2012-303288.
- Marlow N, Greenough A, Peacock JL, Marston L, Limb ES, Johnson AH, et al. Randomised trial of high frequency oscillatory ventilation or conventional ventilation in babies of gestational age 28 weeks or less: respiratory and neurological outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed 2006;91:F320-6. https://doi.org/10.1136/adc.2005.079632.
- Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, Haque K, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 2011;365:1201-11. https://doi.org/10.1056/NEJMoa1100441.
- Cummings SM, Savitz LA, Konrad TR. Reported response rates to mailed physician questionnaires. Health Serv Res 2001;35:1347-55.
- Field D, Draper ES, Gompels MJ, Green C, Johnson A, Shortland D, et al. Measuring later health status of high risk infants: randomised comparison of two simple methods of data collection. BMJ 2001;323:1276-81. https://doi.org/10.1136/bmj.323.7324.1276.
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Gross Motor Function Classification System (GMFCS) n.d. www.motorgrowth.canchild.ca/en/GMFCS/resources/GMFCS_English.pdf (accessed 8 July 2014).
- Department for Communities and Local Government . English Indices of Deprivation 2010 2011. www.communities.gov.uk/documents/statistics/pdf/1871208.pdf (accessed 29 August 2018).
- National Council on Measurement in Education . Standards for Educational and Psychological Testing 1999.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- London Perinatal Group . London Perinatal Networks Annual Report 2008. www.neonatal.org.uk/documents/4391.pdf (accessed 29 August 2018).
- Obuchowski NA, Zhou XH. Prospective studies of diagnostic test accuracy when disease prevalence is low. Biostatistics 2002;3:477-92. https://doi.org/10.1093/biostatistics/3.4.477.
- Cochran WG. Some methods for strengthening the common Chi2 tests. Biometrics 1954;10:417-51. https://doi.org/10.2307/3001616.
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Cochrane diagnostic test accuracy working group systematic reviews of diagnostic test accuracy group. Ann Intern Med 2008;149:889-97.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 2005;5. https://doi.org/10.1186/1471-2288-5-19.
- Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-316. https://doi.org/10.1002/sim.4780121403.
- Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-62. https://doi.org/10.1136/bmj.323.7305.157.
- Littenberg B, Moses LE. Estimating diagnostic accuracy from multiple conflicting reports: a new meta-analytic method. Med Decis Making 1993;13:313-21. https://doi.org/10.1177/0272989X9301300408.
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865-84. https://doi.org/10.1002/sim.942.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. https://doi.org/10.1093/biostatistics/kxl004.
- Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol 2002;31:88-95. https://doi.org/10.1093/ije/31.1.88.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0. The Cochrane Collaboration; 2011.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. https://doi.org/10.1016/j.jclinepi.2005.01.016.
- Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat 2001;55:182-6. https://doi.org/10.1198/000313001317097960.
- Bassan H, Stolar O, Geva R, Eshel R, Fattal-Valevski A, Leitner Y, et al. Intrauterine growth-restricted neonates born at term or preterm: how different?. Pediatr Neurol 2011;44:122-30. https://doi.org/10.1016/j.pediatrneurol.2010.09.012.
- Fedrizzi E, Inverno M, Botteon G, Anderloni A, Filippini G, Farinotti M. The cognitive development of children born preterm and affected by spastic diplegia. Brain Dev 1993;15:428-32. https://doi.org/10.1016/0387-7604(93)90082-J.
- McGrath MM, Sullivan MC, Lester BM, Oh W. Longitudinal neurologic follow-up in neonatal intensive care unit survivors with various neonatal morbidities. Pediatrics 2000;106:1397-405. https://doi.org/10.1542/peds.106.6.1397.
- Gray PH, Burns YR, Mohay HA, O’Callaghan MJ, Tudehope DI. Neurodevelopmental outcome of preterm infants with bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed 1995;73:F128-34. https://doi.org/10.1136/fn.73.3.F128.
- Smith KE, Landry SH, Swank PR. The role of early maternal responsiveness in supporting school-aged cognitive development for children who vary in birth status. Pediatrics 2006;117:1608-17. https://doi.org/10.1542/peds.2005-1284.
- Bowen JR, Gibson FL, Leslie GI, Arnold JD, Ma PJ, Starte DR. Predictive value of the Griffiths assessment in extremely low birthweight infants. J Paediatr Child Health 1996;32:25-30. https://doi.org/10.1111/j.1440-1754.1996.tb01536.x.
- Bruggink JL, Van Braeckel KN, Bos AF. The early motor repertoire of children born preterm is associated with intelligence at school age. Pediatrics 2010;125:e1356-63. https://doi.org/10.1542/peds.2009-2117.
- Charkaluk ML, Truffert P, Marchand-Martin L, Mur S, Kaminski M, Ancel PY, et al. Epipage study group . Very preterm children free of disability or delay at age 2: predictors of schooling at age 8 – a population-based longitudinal study. Early Hum Dev 2011;87:297-302. https://doi.org/10.1016/j.earlhumdev.2011.01.033.
- Cohen SE. Biosocial factors in early infancy as predictors of competence in adolescents who were born prematurely. J Dev Behav Pediatr 1995;16:36-41. https://doi.org/10.1097/00004703-199502000-00006.
- Gray D, Woodward LJ, Spencer C, Inder TE, Austin NC. Health service utilisation of a regional cohort of very preterm infants over the first 2 years of life. J Paediatr Child Health 2006;42:377-83. https://doi.org/10.1111/j.1440-1754.2006.00876.x.
- Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med 1996;334:821-7. https://doi.org/10.1056/NEJM199603283341303.
- Skranes J, Vik T, Nilsen G, Smevik O, Andersson HW, Brubakk AM. Can cerebral MRI at age 1 year predict motor and intellectual outcomes in very-low-birthweight children?. Dev Med Child Neurol 1998;40:256-62. https://doi.org/10.1111/j.1469-8749.1998.tb15458.x.
- Tommiska V, Heinonen K, Kero P, Pokela ML, Tammela O, Järvenpää AL, et al. A national two year follow up study of extremely low birthweight infants born in 1996–1997. Arch Dis Child Fetal Neonatal Ed 2003;88:F29-35. https://doi.org/10.1136/fn.88.1.F29.
- Veelken N, Stollhoff K, Claussen M. Development of very low birth weight infants: a regional study of 371 survivors. Eur J Pediatr 1991;150:815-20. https://doi.org/10.1007/BF02026720.
- Claas MJ, de Vries LS, Bruinse HW, van Haastert IC, Uniken Venema MM, Peelen LM, et al. Neurodevelopmental outcome over time of preterm born children ≤ 750 g at birth. Early Hum Dev 2011;87:183-91. https://doi.org/10.1016/j.earlhumdev.2010.12.002.
- Kilbride HW, Daily DK, Claflin K, Hall RT, Maulik D, Grundy HO. Improved survival and neurodevelopmental outcome for infants less than 801 grams birthweight. Am J Perinatol 1990;7:160-5. https://doi.org/10.1055/s-2007-999471.
- Orchinik LJ, Taylor HG, Espy KA, Minich N, Klein N, Sheffield T, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc 2011;17:1067-79. https://doi.org/10.1017/S135561771100107X.
- Vermeulen GM, Bruinse HW, de Vries LS. Perinatal risk factors for adverse neurodevelopmental outcome after spontaneous preterm birth. Eur J Obstet Gynecol Reprod Biol 2001;99:207-12. https://doi.org/10.1016/S0301-2115(01)00383-9.
- Tin W, Fritz S, Wariyar U, Hey E. Outcome of very preterm birth: children reviewed with ease at 2 years differ from those followed up with difficulty. Arch Dis Child Fetal Neonatal Ed 1998;79:F83-7. https://doi.org/10.1136/fn.79.2.F83.
- Campbell MK, Halinda E, Carlyle MJ, Fox AM, Turner LA, Chance GW. Factors predictive of follow-up clinic attendance and developmental outcome in a regional cohort of very low birth weight infants. Am J Epidemiol 1993;138:704-13. https://doi.org/10.1093/oxfordjournals.aje.a116908.
- Catlett AT, Thompson RJ, Johndrow DA, Boshkoff MR. Risk status for dropping out of developmental followup for very low birth weight infants. Public Health Rep 1993;108:589-94.
- Callanan C, Doyle L, Rickards A, Kelly E, Ford G, Davis N. Children followed with difficulty: how do they differ?. J Paediatr Child Health 2001;37:152-6. https://doi.org/10.1046/j.1440-1754.2001.00621.x.
- Wolke D, Söhne B, Ohrt B, Riegel K. Follow-up of preterm children: important to document dropouts. Lancet 1995;345. https://doi.org/10.1016/S0140-6736(95)90425-5.
- Gordis L. Epidemiology. Philadelphia, PA: Elsevier; 2014.
- Leeflang MM, Bossuyt PM, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. J Clin Epidemiol 2009;62:5-12. https://doi.org/10.1016/j.jclinepi.2008.04.007.
- Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med 1997;16:981-91. https://doi.org/10.1002/(SICI)1097-0258(19970515)16:9<981::AID-SIM510>3.0.CO;2-N.
- Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science 1974;185:1124-31. https://doi.org/10.1126/science.185.4157.1124.
- Chaudhary T, Walch E, Herold B, Metze B, Lejeune A, Burkhardt F, et al. Predictive and concurrent validity of standardized neurodevelopmental examinations by the Griffiths scales and Bayley scales of infant development II. Klin Padiatr 2013;225:8-12. https://doi.org/10.1055/s-0032-1331169.
- Brogan E, Cragg L, Gilmore C, Marlow N, Simms V, Johnson S. Inattention in very preterm children: implications for screening and detection. Arch Dis Child 2014;99:834-9. https://doi.org/10.1136/archdischild-2013-305532.
- Wilson-Ching M, Molloy CS, Anderson VA, Burnett A, Roberts G, Cheong JL, et al. Attention difficulties in a contemporary geographic cohort of adolescents born extremely preterm/extremely low birth weight. J Int Neuropsychol Soc 2013;19:1097-108. https://doi.org/10.1017/S1355617713001057.
- Boyd LA, Msall ME, O’Shea TM, Allred EN, Hounshell G, Leviton A. Social-emotional delays at 2 years in extremely low gestational age survivors: correlates of impaired orientation/engagement and emotional regulation. Early Hum Dev 2013;89:925-30. https://doi.org/10.1016/j.earlhumdev.2013.09.019.
- Wechsler D. Wechlser Preschool and Primary Scale of Intelligence for Children: Third Edition. San Antonio, TX: The Psychological Corporation; 2002.
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale: Fourth Edition. San Antonio, TX: The Psychological Corporation; 2002.
- Páez MM, Tabors PO, López LM. Dual language and literacy development of Spanish-speaking preschool children. J Appl Dev Psychol 2007;28:85-102. https://doi.org/10.1016/j.appdev.2006.12.007.
- Bialystok E, Martin MM. Attention and inhibition in bilingual children: evidence from the dimensional change card sort task. Dev Sci 2004;7:325-39. https://doi.org/10.1111/j.1467-7687.2004.00351.x.
- Walch E, Chaudhary T, Herold B, Obladen M. Parental bilingualism is associated with slower cognitive development in very low birth weight infants. Early Hum Dev 2009;85:449-54. https://doi.org/10.1016/j.earlhumdev.2009.03.002.
- Als H. A synactive model of neonatal behavioral organization: framework for the assessment and support of the neurobehavioral development of the premature infant and his parents in the environment of the neoantal intensive care unit. Phys Occup Ther Pediatr 1986;6:3-55. https://doi.org/10.1080/J006v06n03_02.
- Bar-Shalita T, Vatine JJ, Parush S. Sensory modulation disorder: a risk factor for participation in daily life activities. Dev Med Child Neurol 2008;50:932-7. https://doi.org/10.1111/j.1469-8749.2008.03095.x.
- Bart O, Shayevits S, Gabis LV, Morag I. Prediction of participation and sensory modulation of late preterm infants at 12 months: a prospective study. Res Dev Disabil 2011;32:2732-8. https://doi.org/10.1016/j.ridd.2011.05.037.
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychol 2006;12:247-67. https://doi.org/10.1080/09297040600630288.
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism 2008;12:457-72. https://doi.org/10.1177/1362361308096402.
- Johnson S, Marlow N. Positive screening results on the modified checklist for autism in toddlers: implications for very preterm populations. J Pediatr 2009;154:478-80. https://doi.org/10.1016/j.jpeds.2008.11.028.
- Oosterling IJ, Swinkels SH, van der Gaag RJ, Visser JC, Dietz C, Buitelaar JK. Comparative analysis of three screening instruments for autism spectrum disorder in toddlers at high risk. J Autism Dev Disord 2009;39:897-909. https://doi.org/10.1007/s10803-009-0692-9.
- Dietz C, Swinkels S, van Daalen E, van Engeland H, Buitelaar JK. Screening for autistic spectrum disorder in children aged 14–15 months: II – population screening with the Early Screening of Autistic Traits Questionnaire (ESAT): Design and general findings. J Autism Dev Disord 2006;36:713-22. https://doi.org/10.1007/s10803-006-0114-1.
- Swinkels SH, Dietz C, van Daalen E, Kerkhof IH, van Engeland H, Buitelaar JK. Screening for autistic spectrum in children aged 14 to 15 months. I: the development of the Early Screening of Autistic Traits Questionnaire (ESAT). J Autism Dev Disord 2006;36:723-32. https://doi.org/10.1007/s10803-006-0115-0.
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Cox A, Baird G, Charman T, Swettenham J, et al. Early identification of autism by the CHecklist for Autism in Toddlers (CHAT). J R Soc Med 2000;93:521-5. https://doi.org/10.1177/014107680009301007.
- Aylward GP. Prediction of function from infancy to early childhood: implications for pediatric psychology. J Pediatr Psychol 2004;29:555-64. https://doi.org/10.1093/jpepsy/jsh057.
- Gutbrod T, Wolke D, Soehne B, Ohrt B, Riegel K. Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison. Arch Dis Child Fetal Neonatal Ed 2000;82:F208-14. https://doi.org/10.1136/fn.82.3.F208.
- Wilson-Costello D, Friedman H, Minich N, Siner B, Taylor G, Schluchter M, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000–2002. Pediatrics 2007;119:37-45. https://doi.org/10.1542/peds.2006-1416.
- Doyle LW, Roberts G, Anderson PJ. Victorian Infant Collaborative Study Group . Changing long-term outcomes for infants 500–999 g birth weight in Victoria, 1979–2005. Arch Dis Child Fetal Neonatal Ed 2011;96:F443-7. https://doi.org/10.1136/adc.2010.200576.
- Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 2012;28:138-44. https://doi.org/10.1017/S0266462312000086.
- Bossuyt P, Davenport C, Deeks J, Hyde C, Leeflang M, Scholten R, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 09. London: The Cochrane Collaboration; 2013.
- National Institute for Health and Care Excellence (NICE) . Developmental Follow-up of Children and Young People Born Preterm [NG72] 2017.
- Amess P, Young T, Burley H, Khan Y. Developmental outcome of very preterm babies using an assessment tool deliverable by health visitors. Eur J Paediatr Neurol 2010;14:219-23. https://doi.org/10.1016/j.ejpn.2009.06.005.
- American Academy of Pediatrics . Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006;118:405-20. https://doi.org/10.1542/peds.2006-1231.
- UK National Screening Commitee . The UK NSC Policy on Autism Screening in Children 2009. www.screening.nhs.uk/autism (accessed 8 November 2018).
- Myers SM, Johnson CP. American Academy of Pediatrics Council on Children With Disabilities . Management of children with autism spectrum disorders. Pediatrics 2007;120:1162-82. https://doi.org/10.1542/peds.2007-2362.
- UK Government . NHS Reference Costs 2010–11 n.d. https://data.gov.uk/dataset/7fa41b07-a296-47c7-b74b-40ec3a102e6a/nhs-reference-costs-2010-11 (accessed 11 July 2019).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Simon J, Gray A, Duley L. Magpie Trial Collaborative Group . Cost-effectiveness of prophylactic magnesium sulphate for 9996 women with pre-eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG 2006;113:144-51. https://doi.org/10.1111/j.1471-0528.2005.00785.x.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 9 July 2019).
- Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011;342. https://doi.org/10.1136/bmj.d1548.
- Petrou S, Edwards L. UK Collaborative ECMO Trial . Cost effectiveness analysis of neonatal extracorporeal membrane oxygenation based on four year results from the UK Collaborative ECMO Trial. Arch Dis Child Fetal Neonatal Ed 2004;89:F263-8. https://doi.org/10.1136/adc.2002.025635.
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Probiotics in Preterm Infants Study Collaborative Group . Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387:649-60. https://doi.org/10.1016/S0140-6736(15)01027-2.
- Department of Health and Social Care . NHS Reference Costs 2012–13 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/261154/nhs_reference_costs_2012-13_acc.pdf (accessed 9 July 2019).
- Curtis L. Unit Costs of Health and Social Care 2012. Personal Social Services Research Unit; n.d.
- Schroeder E, Petrou S, Patel N, Hollowell J, Puddicombe D, Redshaw M, et al. Birthplace in England Collaborative Group . Cost effectiveness of alternative planned places of birth in woman at low risk of complications: evidence from the Birthplace in England national prospective cohort study. BMJ 2012;344. https://doi.org/10.1136/bmj.e2292.
- Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45:255-68. https://doi.org/10.2307/2532051.
- Byford S, Leese M, Knapp M, Seivewright H, Cameron S, Jones V, et al. Comparison of alternative methods of collection of service use data for the economic evaluation of health care interventions. Health Econ 2007;16:531-6. https://doi.org/10.1002/hec.1175.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. https://doi.org/10.1016/S0140-6736(86)90837-8.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
- Achana FA, Petrou S, Khan K, Gaye A, Modi N. A methodological framework for assessing agreement between cost-effectiveness outcomes estimated using alternative sources of data on treatment costs and effects for trial-based economic evaluations. Eur J Health Econ 2018;19:75-86. https://doi.org/10.1007/s10198-017-0868-8.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 9 July 2019).
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. https://doi.org/10.1016/S0167-6296(98)00039-3.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8310.
- Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, et al. Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0089186.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-39.
- Pan H, Cole T. LmsGrowth, A Microsoft Excel Add-in to Access Growth References Based on the LMS Method 2009. www.healthforallchildren.com/ (accessed 9 July 2019).
- (AHRQ) AfHRaQ . Clinical Classifications Software for ICD-10 Data 2003. www.ahrqgov/data/hcup/icd10usrgdhtm (accessed June 2012).
- Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001885.
- Murray JC. The Clinical Burden of Respiratory Syncytial Virus (RSV) Bronchiolitis Among Infants in the United Kingdom (UK) 2013.
- Murray J, Saxena S, Modi N, Majeed A, Aylin P, Bottle A. Medicines for Neonates Investigator Group . Quality of routine hospital birth records and the feasibility of their use for creating birth cohorts. J Public Health 2013;35:298-307. https://doi.org/10.1093/pubmed/fds077.
- Dattani N, Datta-Nemdharry P, Macfarlane A. Linking maternity data for England, 2005–06: methods and data quality. Health Stat Q 2011;49:53-79. https://doi.org/10.1057/hsq.2011.3.
- Hockley C, Quigley MA, Hughes G, Calderwood L, Joshi H, Davidson LL. Linking Millennium Cohort data to birth registration and hospital episode records. Paediatr Perinat Epidemiol 2008;22:99-109. https://doi.org/10.1111/j.1365-3016.2007.00902.x.
- Field K, Kosmider S, Johns J, Farrugia H, Hastie I, Croxford M, et al. Linking data from hospital and cancer registry databases: should this be standard practice?. Intern Med J 2010;40:566-73. https://doi.org/10.1111/j.1445-5994.2009.01984.x.
- Chamberlayne R, Green B, Barer ML, Hertzman C, Lawrence WJ, Sheps SB. Creating a population-based linked health database: a new resource for health services research. Can J Public Health 1998;89:270-3.
- Roos LL, Brownell M, Lix L, Roos NP, Walld R, MacWilliam L. From health research to social research: privacy, methods, approaches. Soc Sci Med 2008;66:117-29. https://doi.org/10.1016/j.socscimed.2007.08.017.
- Taylor LK, Travis S, Pym M, Olive E, Henderson-Smart DJ. How useful are hospital morbidity data for monitoring conditions occurring in the perinatal period?. Aust N Z J Obstet Gynaecol 2005;45:36-41. https://doi.org/10.1111/j.1479-828X.2005.00339.x.
- Ford JB, Roberts CL, Taylor LK. Characteristics of unmatched maternal and baby records in linked birth records and hospital discharge data. Paediatr Perinat Epidemiol 2006;20:329-37. https://doi.org/10.1111/j.1365-3016.2006.00715.x.
- Bradford HM, Cárdenas V, Camacho-Carr K, Lydon-Rochelle MT. Accuracy of birth certificate and hospital discharge data: a certified nurse-midwife and physician comparison. Matern Child Health J 2007;11:540-8. https://doi.org/10.1007/s10995-007-0178-3.
- Lydon-Rochelle MT, Holt VL, Nelson JC, Cárdenas V, Gardella C, Easterling TR, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol 2005;19:460-71. https://doi.org/10.1111/j.1365-3016.2005.00682.x.
- Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry 2005;46:963-71. https://doi.org/10.1111/j.1469-7610.2004.00391.x.
- Linnet KM, Wisborg K, Secher NJ, Thomsen PH, Obel C, Dalsgaard S, et al. Coffee consumption during pregnancy and the risk of hyperkinetic disorder and ADHD: a prospective cohort study. Acta Paediatr 2009;98:173-9. https://doi.org/10.1111/j.1651-2227.2008.00980.x.
- Lyons RA, Jones KH, John G, Brooks CJ, Verplancke JP, Ford DV, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak 2009;9. https://doi.org/10.1186/1472-6947-9-3.
- Welsh Electronic Cohort for Children (WECC) n.d. www.swan.ac.uk/ils/research/chiral/methodologies/healthinformatics/wecc/ (accessed October 2012).
- McGregor J, Brooks C, Chalasani P, Chukwuma J, Hutchings H, Lyons RA, et al. The Health Informatics Trial Enhancement Project (HITE): using routinely collected primary care data to identify potential participants for a depression trial. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-39.
- Programme SHI n.d. www.scot-ship.ac.uk (accessed October 2011).
- Govan L, Wu O, Briggs A, Colhoun HM, McKnight JA, Morris AD, et al. Inpatient costs for people with type 1 and type 2 diabetes in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2011;54:2000-8. https://doi.org/10.1007/s00125-011-2176-7.
- Gill L, Goldacre M, Simmons H, Bettley G, Griffith M. Computerised linking of medical records: methodological guidelines. J Epidemiol Community Health 1993;47:316-19. https://doi.org/10.1136/jech.47.4.316.
- Roberts SE, Wotton CJ, Williams JG, Griffith M, Goldacre MJ. Perinatal and early life risk factors for inflammatory bowel disease. World J Gastroenterol 2011;17:743-9. https://doi.org/10.3748/wjg.v17.i6.743.
- Davidson R, Roberts SE, Wotton CJ, Goldacre MJ. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med 2010;10. https://doi.org/10.1186/1471-2466-10-14.
- Roberts SE, Williams JG, Meddings D, Davidson R, Goldacre MJ. Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment Pharmacol Ther 2009;29:222-31. https://doi.org/10.1111/j.1365-2036.2008.03871.x.
- NHS Digital . Hospital Episode Statistics n.d. www.hesonline.nhs.uk (accessed 8 November 2018).
- Abrahams C, Davy K. Linking HES maternity records with ONS birth records. Health Stat Q 2002;13:22-30.
- Dezateux CHC, Johnson J, Joshi H, Quigley M, Rosenburg R. Millenium Cohort study: Birth registration and maternity Hospital Episode Statistics Linkage. Centre for Longitudinal Studies Report 2006.
- UK Clinical Research Collaboration (UKCRC) . UK Clinical Research Collaboration Research and Development Advisory Group to Connecting for Health: Report of Research Simulations 2007. www.ukcrc.org/wp-content/uploads/2014/06/CfH-report-June-07-full.pdf (accessed 11 July 2019).
- Foster V, Young A, Modi N, Brocklehurst P, Abbott J, Costeloe K, et al. The use of routinely collected patient data for research: a critical review. Health 2012;16:448-63. https://doi.org/10.1177/1363459311425513.
- British Association of Perinatal Medicine . Service Standards for Hospitals Providing Neonatal Care (3rd Edition) 2010. www.bapm.org/resources/service-standards-hospitals-providing-neonatal-care-3rd-edition-2010 (accessed 9 July 2019).
- Adams WG, Mann AM, Bauchner H. Use of an electronic medical record improves the quality of urban pediatric primary care. Pediatrics 2003;111:626-32. https://doi.org/10.1542/peds.111.3.626.
- Royal College of Obstetricians and Gynaecologists . Antenatal Corticosteroids to Reduce Neonatal Morbidity and Mortality: Green–top Guideline No. 7 2010. www.glowm.com/pdf/Antenatal%20Corticosteroids%20to%20Reduce%20Neonatal%20Morbidity.pdf (accessed 9 July 2019).
- National Institute for Health and Care Excellence (NICE) . Antenatal Care for Uncomplicated Pregnancies Clinical Guideline [CG62] 2008. www.nice.org.uk/guidance/cg62/chapter/guidance#fetal-growth-and-wellbeing (accessed 9 July 2019).
- National Institute for Health and Care Excellence (NICE) . BNF for Children 2019. https://bnfc.nice.org.uk (accessed 19 July 2019).
Appendix 1 Supplementary tables
Chapter 2
| Characteristic | Number of infants born before 32 weeks’ gestation, n (%) (N = 14,678) | p-value | |
|---|---|---|---|
| No severe NEC (n = 14,216) | Severe NEC (n = 462) | ||
| Gestational age (weeks) (mean ± SD) | 28.5 (2.27) | 26.2 (2.16) | < 0.001 |
| Birthweight (g) (mean ± SD) | 1217.7 (378.9) | 884.2 (310.6) | < 0.001 |
| Birthweight SDS, n (%) | < 0.001 | ||
| Missing | 39 (0.3) | 1 (0.2) | |
| –4 SD | 6 (0.04) | 0 (0) | |
| –3 SD | 153 (1.1) | 16 (3.5) | |
| –2 SD | 931 (6.6) | 46 (10.0) | |
| –1 SD | 2998 (21.1) | 108 (23.4) | |
| 1 SD | 3404 (23.9) | 81 (17.5) | |
| 2 SD | 497 (3.5) | 11 (2.4) | |
| 3 SD | 63 (0.4) | 1 (0.2) | |
| 4 SD | 5 (0.04) | 0 (0) | |
| Average | 6120 (43.1) | 198 (42.9) | |
| Sex, n (%) | 0.7074 | ||
| Missing | 8 (0.06) | 0 | |
| Male | 7807 (54.9) | 261 (56.5) | |
| Fetus number | 0.0006 | ||
| Missing | 1 (0.01) | 1 (0.2) | |
| 1 | 10,505 (73.9) | 344 (74.5) | |
| 2 | 3345 (23.5) | 111 (24.0) | |
| ≥ 3 | 365 (2.6) | 6 (1.3) | |
| Antenatal steroids | 0.8068 | ||
| Missing | 155 (1.1) | 5 (1.1) | |
| Yes | 12,595 (88.6) | 405 (87.7) | |
| Maternal factors | |||
| Chorioamnionitis | 552 (3.9) | 21 (4.6) | 0.4632 |
| Maternal infectiona | 725 (5.1) | 21 (4.6) | 0.6003 |
| Received antibiotics in labour | 3460 (23.3) | 112 (24.3) | < 0.001 |
| Pyrexia above 38 °C in labour | 674 (4.7) | 29 (6.3) | 0.2615 |
| Mode of delivery | < 0.001 | ||
| Unknown | 1068 (7.5) | 40 (8.7) | |
| Emergency caesarean (not in labour) | 4326 (30.4) | 106 (22.9) | |
| Emergency caesarean (in labour) | 2445 (17.2) | 64 (13.9) | |
| Elective section (not in labour) | 794 (5.6) | 19 (4.1) | |
| Elective section (in labour) | 97 (0.7) | 3 (0.7) | |
| Vaginal | 5486 (38.6) | 230 (49.8) | |
| Variable | Unadjusted OR for severe NEC relative to reference category | 95% CI | p-value | Adjusted OR for severe NEC relative to reference category | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Gestation in weeks(+days) | ||||||
| 22 to 25+6 | 13.4 | 10.2 to 17.9 | < 0.001 | 13.8a | 10.5 to 18.5 | < 0.001 |
| 26+0 to 28+6 | 5.8 | 4.4 to 7.7 | 5.6 | 4.3 to 7.5 | < 0.001 | |
| 29+0–31+6 | Reference | Reference | ||||
| Birthweight SDS | ||||||
| Missing | 0.8 | 0.05 to 3.7 | < 0.001 | 0.5b | 0.03 to 2.7 | 0.6 |
| –4 SD | 0.0 | – | 0.0 | – | – | |
| –3 SD | 3.2 | 1.8 to 5.4 | 4.1 | 2.3 to 7.0 | < 0.001 | |
| –2 SD | 1.5 | 1.1 to 2.1 | 1.9 | 1.3 to 2.6 | < 0.001 | |
| –1 SD | 1.1 | 0.9 to 1.4 | 1.1 | 0.9 to 1.4 | 0.3 | |
| 1 SD | 0.7 | 0.6 to 0.9 | 0.9 | 0.7 to 1.2 | 0.5 | |
| 2 SD | 0.7 | 0.6 to 1.0 | 1.0 | 0.5 to 1.8 | 1.0 | |
| 3 SD | 0.5 | 0.3 to 1.2 | 0.9 | 0.05 to 4.3 | 0.9 | |
| 4 SD | 0.0 | – | – | – | ||
| Average | Reference | Reference | ||||
| Any steroids given | ||||||
| Missing | 0.9 | 0.7 to 1.3 | 0.8 | 1.1c | 0.4 to 2.6 | 0.9 |
| Yes | 0.9 | 0.3 to 2.1 | 1.0 | 0.7 to 1.3 | 0.8 | |
| No | Reference | Reference | ||||
| Network of booking | Unadjusted OR of severe NEC relative to reference network | 95% CI of OR | Bonferroni adjusted p-value | Adjusted OR of severe NEC relative to reference networka | 95% CI of OR | Bonferroni adjusted p-value |
|---|---|---|---|---|---|---|
| Bedfordshire and Hertfordshire | 0.69 | 0.29 to 1.4 | 0.9 | 0.71 | 0.30 to 1.52 | 0.9 |
| Cheshire and Merseyside | 1.29 | 0.74 to 2.22 | 0.9 | 1.54 | 0.87 to 2.68 | 0.9 |
| Eastern | 1.59 | 0.98 to 2.6 | 0.9 | 1.93 | 1.17 to 3.21 | 0.2 |
| Greater Manchester | 1.37 | 0.84 to 2.27 | 0.9 | 1.47 | 0.89 to 2.46 | 0.9 |
| Kent | 1.08 | 0.56 to 2.00 | 0.9 | 1.30 | 0.67 to 2.44 | 0.9 |
| Lancashire and South Cumbria | 0.91 | 0.43 to 1.77 | 0.9 | 1.07 | 0.50 to 2.12 | 0.9 |
| London North Central | 0.72 | 0.32 to 1.46 | 0.9 | 0.71 | 0.31 to 1.47 | 0.9 |
| London North West | 1.40 | 0.84 to 2.35 | 0.9 | 1.65 | 0.98 to 2.80 | 0.9 |
| London South East | 1.31 | 0.75 to 2.27 | 0.9 | 1.33 | 0.75 to 2.33 | 0.9 |
| London South West | 1.05 | 0.53 to 2.00 | 0.9 | 1.20 | 0.59 to 2.31 | 0.9 |
| Midlands Central | 1.63 | 1.00 to 2.69 | 0.9 | 1.90 | 1.15 to 3.16 | 0.3 |
| Midlands South West | 0.72 | 0.38 to 1.29 | 0.9 | 0.76 | 0.40 to 1.39 | 0.9 |
| Midlands North | 0.94 | 0.51 to 1.68 | 0.9 | 1.05 | 0.57 to 1.91 | 0.9 |
| North Trent | 1.09 | 0.60 to 1.92 | 0.9 | 1.41 | 0.77 to 2.52 | 0.9 |
| Northern | 0.83 | 0.45 to 1.47 | 0.9 | 0.85 | 0.46 to 1.53 | 0.9 |
| Peninsula South West | 0.68 | 0.27 to 1.48 | 0.9 | 0.80 | 0.32 to 1.76 | 0.9 |
| South Central (North) | 1.03 | 0.56 to 1.85 | 0.9 | 1.15 | 0.62 to 2.08 | 0.9 |
| South Central (South) | 0.74 | 0.38 to 1.37 | 0.9 | 0.81 | 0.41 to 1.53 | 0.9 |
| Surrey and Sussex | 1.51 | 0.88 to 2.58 | 0.9 | 1.63 | 0.93 to 2.82 | 0.9 |
| Trent | 0.97 | 0.51 to 1.80 | 0.9 | 1.09 | 0.56 to 2.03 | 0.9 |
| Western | 0.66 | 0.33 to 1.25 | 0.9 | 0.76 | 0.38 to 1.45 | 0.9 |
| Yorkshire | 0.79 | 0.45 to 1.38 | 0.9 | 0.98 | 0.56 to 1.73 | 0.9 |
| London North East (Reference network) | Reference network | – | Reference network | – |
Chapter 3
| Variable | Coefficient (SE) | p-value |
|---|---|---|
| Intercept | –4.058 (0.297) | |
| Male | 0.315 (0.036) | < 0.001 |
| Multiple pregnancy | –0.122 (0.062) | 0.048 |
| Antenatal steroids given | –0.726 (0.047) | < 0.001 |
| Gestational age spline terms (/week) | ||
| GA1 | –1.193 (0.117) | < 0.001 |
| GA2 | 0.95 (0.212) | < 0.001 |
| GA3 | –2.961 (1.071) | 0.006 |
| GA4 | 7.434 (3.329) | 0.026 |
| Birthweight (BWT) (/100 g) | ||
| BWT | –0.09 (0.052) | 0.081 |
| BWT2 | 0.045 (0.002) | < 0.001 |
| Interactions | ||
| GA1*BWT | –0.17 (0.026) | < 0.001 |
| GA2*BWT | 0.012 (0.039) | 0.753 |
| GA3*BWT | 0.309 (0.22) | 0.159 |
| GA4*BWT | –0.657 (0.73) | 0.368 |
| GA1*BWT2 | –0.008 (0.001) | < 0.001 |
| GA*multiple pregnancy | 0.007 (0.028) | 0.788 |
| BWT*multiple pregnancy | –0.083 (0.022) | < 0.001 |
| Survived to discharge, n (%) | Missing, n | p-value | Survived to 28 days, n (%) | Missing, n | p-value | |
|---|---|---|---|---|---|---|
| Gestational age (weeks+days) | ||||||
| 22+0 to 23+6 | 452 (35) | 57 | 646 (48.4) | 13 | ||
| 24+0 to 25+6 | 3555 (66.7) | 311 | 4240 (76) | 59 | ||
| 26+0 to 27+6 | 7324 (86.2) | 392 | p < 0.001 | 7943 (90.2) | 87 | p < 0.001 |
| 28+0 to 29+6 | 12,155 (94.2) | 443 | 12,609 (95.6) | 163 | ||
| 30+0 to 31+6 | 19,958 (97.8) | 487 | 20,224 (98.2) | 281 | ||
| Birthweight (g) | ||||||
| < 500 | 127 (34.8) | 17 | 192 (50.7) | 3 | ||
| 500 to 999 | 11,748 (76.8) | 772 | 13,256 (83.4) | 167 | ||
| 1000 to 1499 | 19,918 (95.6) | 613 | p < 0.001 | 20,431 (96.4) | 259 | p < 0.001 |
| 1500 to 1999 | 10,913 (97.9) | 262 | 11,031 (98.1) | 158 | ||
| ≥ 2000 | 738 (94.4) | 26 | 752 (94.9) | 16 | ||
| SGA | ||||||
| No | 37,309 (90.4) | 1406 | p < 0.001 | 38,985 (92.5) | 538 | p < 0.001 |
| Yes | 6135 (85.9) | 284 | 6677 (90.7) | 65 | ||
| Sex | ||||||
| Female | 20,190 (90.6) | 732 | p < 0.001 | 21,090 (92.8) | 284 | p < 0.001 |
| Male | 23,254 (88.9) | 958 | 24,572 (91.7) | 319 | ||
| Multiplicity of pregnancy | ||||||
| Singleton | 31,845 (89.7) | 1225 | p < 0.001 | 33,506 (92.3) | 417 | p < 0.001 |
| Twins | 10,472 (89.3) | 433 | 10,992 (91.7) | 172 | ||
| Triplets or more | 1127 (93.1) | 32 | 1164 (94.8) | 14 | ||
| Any antenatal steroids given | ||||||
| No | 4421 (82.1) | 233 | p < 0.001 | 4711 (85) | 72 | p < 0.001 |
| Yes | 38,327 (90.8) | 1369 | 40,196 (93.2) | 485 | ||
| Mode of delivery | ||||||
| Vaginal | 16,346 (85.9) | 546 | p < 0.001 | 17,275 (89.1) | 190 | p < 0.001 |
| Caesarean | 23,473 (93) | 665 | 24,367 (94.9) | 227 | ||
| Maternal age (years) | ||||||
| < 20 | 3143 (88.3) | 147 | p < 0.001 | 3326 (90.9) | 51 | p < 0.001 |
| 20 to 24 | 7639 (88.5) | 308 | 8063 (91.3) | 108 | ||
| 25 to 29 | 11,268 (90.3) | 395 | 11,821 (92.7) | 122 | ||
| 30 to 34 | 11,890 (90) | 421 | 12,460 (92.5) | 157 | ||
| 35 to 40 | 7171 (90.4) | 246 | 7505 (92.8) | 89 | ||
| > 40 | 2105 (90.7) | 67 | 2198 (93) | 23 | ||
| Maternal ethnicity | ||||||
| British, Irish, other white | 29,511 (90.2) | 1004 | 30,810 (92.4) | 364 | ||
| Mixed | 670 (92.2) | 21 | 696 (94.2) | 9 | ||
| Indian, Pakistani, Bangladeshi, other Asian | 5036 (89.5) | 131 | p < 0.001 | 5255 (92.1) | 48 | p < 0.001 |
| Black Caribbean, Black African, other black | 4205 (88.7) | 106 | 4479 (92.8) | 21 | ||
| Chinese | 161 (90.4) | 7 | 173 (95.1) | 3 | ||
| Other | 672 (87.7) | 20 | 709 (90.8) | 5 | ||
| Smoking | ||||||
| No | 28,210 (90.6) | 764 | p = 0.001 | 29,446 (93) | 233 | p < 0.001 |
| Yes | 7560 (89.4) | 224 | 7912 (91.9) | 71 | ||
| IMD quintile | ||||||
| 1 (most deprived) | 12,957 (89.2) | 511 | 13,664 (91.8) | 159 | ||
| 2 | 9638 (89.2) | 342 | 10,146 (91.9) | 111 | ||
| 3 | 7427 (90.5) | 248 | p < 0.001 | 7785 (93) | 82 | p < 0.001 |
| 4 | 6033 (90.7) | 217 | 6299 (92.8) | 80 | ||
| 5 (least deprived) | 5288 (90.7) | 184 | 5511 (92.7) | 72 | ||
| Birth year | ||||||
| 2008 | 4993 (88) | 426 | 5364 (91.4) | 233 | ||
| 2009 | 5509 (88.9) | 289 | 5823 (91.8) | 143 | ||
| 2010 | 6384 (89.5) | 255 | 6704 (92.1) | 108 | ||
| 2011 | 6776 (89.5) | 159 | p < 0.001 | 7059 (91.7) | 39 | p < 0.001 |
| 2012 | 6728 (89.7) | 170 | 7032 (92.2) | 36 | ||
| 2013 | 6547 (90.7) | 151 | 6812 (92.7) | 17 | ||
| 2014 | 6507 (91.3) | 240 | 6868 (93.5) | 27 | ||
Chapter 5
| Domain of development | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| Cognitive | 71.4 (14.4 to 100.0) | 91.7 (85.6 to 97.8) |
| Receptive communication | 36.4 (0.0 to 79.8) | 94.8 (93.1 to 96.5) |
| Expressive communication | 91.7 (72.9 to 100.0) | 85.2 (78.4 to 92.1) |
| Combined language | 66.7 (39.7 to 93.6) | 85.8 (78.2 to 93.4) |
| Fine motor | 50.0 (30.2 to 100.0) | 99.5 (98.4 to 100.0) |
| Gross motor | 88.9 (63.8 to 100.0) | 98.3 (95.6 to 100.0) |
| Combined motor | 80.0 (51.3 to 100.0) | 98.3 (95.6 to 100.0) |
| Overall | 72.0 (47.9 to 96.1) | 86.3 (77.8 to 94.8) |
| Characteristics | Respondents (N = 141) | Non-respondents (N = 60) | ‘Baseline’ population (N = 1037) | p-value | |
|---|---|---|---|---|---|
| Respondents vs. non-respondents | Respondents vs. ‘baseline’ population | ||||
| Gestation (completed weeks), median (IQR), range | 27 (26–29), 23–29 | 27 (26–28), 23–29 | 27 (26–29), 22–29 | 0.15 | 0.58 |
| Birthweight (g), median (IQR), range | 958 (810–1167), 490–1720 | 920 (740–1082), 560–1400 | 1000 (812–1200), 455–1990 | 0.07 | 0.44 |
| Sex, n (%) | 0.02 | 0.09 | |||
| Female | 68 (48.2) | 40 (66.7) | 444 (42.8) | ||
| Male | 73 (51.8) | 20 (33.3) | 503 (48.5) | ||
| Missing | 0 (0.0) | 0 (0.0) | 90 (8.7) | ||
| Ethnicity, n (%) | 0.12 | 0.03 | |||
| White | 66 (46.8) | 21 (35.0) | 364 (35.1) | ||
| Non-white | 75 (52.2) | 39 (65.0) | 611 (58.9) | ||
| Missing | 0 (0.0) | 0 (0.0) | 62 (6.0) | ||
| Pregnancy, n (%) | 0.67 | 0.25 | |||
| Singleton | 110 (78.0) | 48 (80.0) | 690 (66.5) | ||
| Multiples | 31 (22.0) | 12 (20.0) | 250 (24.1) | ||
| Missing | 0 (0.0) | 0 (0.0) | 97 (9.4) | ||
| Mode of delivery, n (%) | 0.34 | 0.84 | |||
| Vaginal | 61 (43.3) | 22 (36.7) | 475 (45.8) | ||
| Caesarean | 71 (50.4) | 32 (53.3) | 540 (52.1) | ||
| Missing | 9 (6.4) | 6 (10.0) | 22 (2.1) | ||
| Maternal age (years), mean (SD) | 31.5 (6.0) | 31.9 (7.8) | 31.0 (6.4) | 0.68 | 0.35 |
| IMD quintile at birth, n (%) | 0.77 | 0.05 | |||
| One | 13 (9.2) | 6 (10.0) | 43 (4.2) | ||
| Two | 13 (9.2) | 5 (8.3) | 81 (7.8) | ||
| Three | 20 (14.2) | 5 (8.3) | 144 (13.9) | ||
| Four | 42 (29.8) | 17 (28.3) | 268 (25.8) | ||
| Five | 53 (37.6) | 27 (45.0) | 477 (46.0) | ||
| Missing | 0 (0.0) | 0 (0.0) | 24 (2.3) | ||
| Length of mechanical ventilation (days), median (IQR), range | 1 (0–3), 0–54 | 1 (0–7), 0–61 | 4 (0–18), 0–444 | 0.13 | < 0.001 |
| Oxygen therapy at 36 weeks’ corrected age, n (%) | 0.11 | < 0.001 | |||
| Yes | 38 (27.0) | 23 (38.3) | 466 (44.9) | ||
| No | 103 (73.1) | 37 (61.7) | 571 (55.1) | ||
| Variable | Q-CHAT score coefficient (95% CI) | p-value |
|---|---|---|
| Bayley-III language composite score (per point) | –0.23 (–0.33 to –1.39) | < 0.001 |
| White ethnicity | –5.30 (–7.92 to –2.67) | < 0.001 |
| IMD quintile (per quintile increase in deprivation) | 0.96 (–2.00 to 0.08) | 0.07 |
| Study | Characteristic of study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Population | Years of birth | Sampling method | Sample size | Mean (SD) or median (IQR) GA (weeks) | Mean (SD) or median (IQR) BW (grams) | Early or school age | Ages at assessments (month for early, years for school-age) | Assessment tools used | Mean (SD) assessment scoresa | |
| Bassan 2011278 | Israel | BW < 10th percentile for GAb | 1992–7 | SC | 32 | 33.1 (2.2) | 1182 (229) | Early | 24 | BSID-II | 95.8 (19.1) |
| School-age | 6 | WPPSI-R | 103.4 (17.7) | ||||||||
| Bowen 1996283 | Australia | BW < 1000 g | 1985–8 | SC | 45 | 27.6 (2.3) | 864 (90) | Early | 12, 36 | GMDS | – |
| School-age | 5 | S-B-IV | 94.4 (11.2) | ||||||||
| Bruggink 2010284 | The Netherlands | ‘Preterm’ | 1992–7 | SC | 50 | 30.0 (1.9) | 1184 (292) | Early | 19 | BSID-II | 100.5 (11.2) |
| School-age | 8 | WISC-III | 92.2 (10.6) | ||||||||
| Charkaluk 2011285 | France | GA < 33 weeks | 1997 | PB | 313 | 29.8 (2.1) | 1355 (406) | Early | 24 | Brunet-Lezine Revised | 96.7 (12.7) |
| School-age | 5 | KABC | 94.7 (18.7) | ||||||||
| Claas 2011292 | The Netherlands | BW ≤ 750 g and GA ≥ 24 weeks | 1996–2005 | SC | 101 | 28.0 (24.8–34.4)c | 675 (480–750)c | Early | 24 | BSID-II/GMDS | – |
| School-age | 5.5 | WPPSI/RAKIT/SON-R | – | ||||||||
| Cohen 1995286 | USA | ‘Preterm’b | 1972–4 | SC | 20 | 28.1 (2.1) | 1111 (187) | Early | 24 | BSID | 103.9 (21.1) |
| School-age | 5, 8, 12, 18 | S-B-III/WISC-R/WAIS-R | 101.8 (19.0) | ||||||||
| Fedrizzi 1993279 | Italy | Spastic diplegia | 1984–1991 | SC | 11 | 29.6 (1.6) | 1474 (321) | Early | 36 | GMDS | 72.6 (14.5) |
| School-age | 6 | WPPSI | 76.4 (18.9) | ||||||||
| Gray 1995281 | Australia | GA 23–33 weeks with diagnosis of BPD | 1989–1990 | SC | 126 | 28.2 | 1065 | Early | 24 | GMDS | 108.5 |
| School-age | 8 | WISC-III | 90.5 | ||||||||
| Gray 2006287 | New Zealand | GA < 32 weeks or BW < 1500 g | 1998–2000 | SC | 99 | 27.8 (2.4) | 1065 (321) | Early | 24 | BSID-II | 86.1 (17.3) |
| School-age | 6 | WPPSI-R | 95.4 (15.2) | ||||||||
| Hack 2005162 | USA | BW < 1000 g | 1992–5 | SC | 200 | 26.4 (2) | 811 (125) | Early | 20 | BSID-II | 75.6 (16.0) |
| School-age | 8 | KABC | 87.8 (19.0) | ||||||||
| Kilbride 1990293 | USA | BW < 801 g | 1983–1990 | MC | 129 | 25.9 (1.6) | 698 (82) | Early | 12–24, 36 | BSID/S-B-III | 84.4 (10.0) |
| School-age | 5 | S-B-III | 85.7 (11.6) | ||||||||
| Marlow 2005225 | UK | GA < 26 weeks | 1995 | PB | 212 | 25.0 (0.7) | 748 (116) | Early | 30 | BSID-II | 81.7 (14.5) |
| School-age | 6, 11 | KABC | 83.8 (18.0) | ||||||||
| McGrath 2000280 | USA | BW < 1850 g with neonatal diagnosesb | 1985–9 | SC | 88 | 29.6 (2.2) | 1200 (285) | Early | 18 | BSID-II | 105.2 (19.0) |
| School-age | 8 | WISC-III | 96.3 (18.4) | ||||||||
| Munck 2012160 | Finland | BW < 1500 g | 2001–4 | SC | 124 | 28.7 (2.8) | 1061 (260) | Early | 24 | BSID-II | 101.2 (16.3) |
| School-age | 5 | WPPSI-R | 99.3 (17.7) | ||||||||
| Orchinik 2011294 | USA | GA < 28 weeks or BW < 1000 g | 2001–3 | SC | 139 | 25.9 (1.6) | 818 (174) | Early | 20 | BSID-II | 77.2 (17.3) |
| School-age | 6 | BIA | 86.3 (21.1) | ||||||||
| Potharst 2012161 | The Netherlands | GA < 30 weeks or BW < 1000 g | 2003–4 | SC | 100 | 28.7 (1.6) | 1040 (253) | Early | 24, 36 | BSID-II | 102.0 (14.0) |
| School-age | 5 | WPPSI-III | 93.0 (17.0) | ||||||||
| Reuss 1996288 | USA | BW 501–2000 gb | 1984–7 | MC | 231 | 29.2 (2.9) | 1142 (223) | Early | 24 | BSID/S-B-III | – |
| School-age | 6, 9, 16 | S-B-IV/WISC-III/WASI | – | ||||||||
| Roberts 2010163 | Australia | GA 22–27 weeks or BW 500–999 g | 1997 | PB | 186 | 26.5 (2.0) | 832 (164) | Early | 24 | BSID-II | – |
| School-age | 8 | WISC-R | 94.4 (14.2) | ||||||||
| Skranes 1998289 | Norway | BW < 1500 g | 1988 | PB | 21 | 29.0 (2.0) | 1218 (193) | Early | 12 | BSID | 99.0 (18.3) |
| School-age | 6 | WPPSI | 96.0 (16.4) | ||||||||
| Smith 2006282 | USA | BW < 1500 g from lower socioeconomic groups | 1990–2 | MC | 161 | 29.7 (2.5) | 1114 (267) | Early | 40 | S-B-IV | 86.2 (10.6) |
| School-age | 6, 8, 10 | S-B-IV | 85.1 (12.4) | ||||||||
| Tommiska 2003290 | Finland | BW < 1000 g | 1996–7 | SC | 72 | 27.1 | 778 | Early | 24 | BSID-II | 95.5 |
| School-age | 5 | WPPSI-R | 101.0 | ||||||||
| Veelken 1991291 | Germany | BW < 1500 g | 1983–6 | PB | 234 | 29.9 (2.8) | 1196 (211) | Early | 18–20 | GMDS | 97.3 (15.9) |
| School-age | 9 | KABC | 88.3 (17.6) | ||||||||
| Vermeulen 2001295 | The Netherlands | GA ≤ 32 weeks or BW < 1500 g | 1991–3 | SC | 185 | 29.2 (2.1) | 1183 (313) | Early | 18 | GMDS | 99.0 (13.9) |
| School-age | 7–10 | WISC-R | 100.6 (14.0) | ||||||||
| Wolke 1999173 | Germany | GA < 32 weeks | 1985–6 | PB | 254 | 29.6 (1.5) | 1298 (340) | Early | 20 | GMDS | 90.8 (22.8) |
| School-age | 6, 8 | KABC | 88.2 (18.6) | ||||||||
| Study | Risk of bias | Applicability concerns | Reasons for being considered high risk for bias or applicability concerns, as judged against the standards set, with statements being numbered according to the domain it is applied to | |||||
|---|---|---|---|---|---|---|---|---|
| Patient selection [1] | Index test [2] | Reference standard [3] | Flow and timing [4] | Patient selection [5] | Index test [6] | Reference standard [7] | ||
| Bassan 2011278 | ↑ | ↔ | ↔ | ↑ | ↑ | ↔ | ↑ |
[1] Inclusion criteria: birthweight below 10th percentile for gestational age [4] Final cohort represents < 30% of eligible population [5] Study population restricted to children with birthweight below 10th percentile for gestational age [7] Outdated assessment tool (WPPSI-R, 1989) used |
| Bowen 1996283 | ↔ | ↔ | ↑ | ↔ | ↑ | ↑ | ↑ |
[3] Assessors not blinded to results of developmental assessment [5] Study population was born before 1990 [6] Outdated assessment tool (GMDS, 1970) used [7] Outdated assessment tool (S-B-IV, 1986) used |
| Bruggink 2010284 | ? | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ |
[1] Recruitment/sampling method not stated [4] Final cohort represents < 30% of eligible population |
| Charkaluk 2011285 | ↔ | ↔ | ↔ | ↑ | ↔ | ↑ | ↑ |
[4] Final cohort represents < 30% of eligible population [6] Non-universal assessment tool (Brunet-Lezine Revised, a French psychometric test) used [7] Outdated assessment tool (KABC, 1983) used |
| Claas 2011292 | ↔ | ↔ | ↑ | ↔ | ↔ | ↑ | ↑ |
[3] Assessors not blinded to results of developmental assessment [6] Outdated assessment tool (GMDS, 1984) used [7] Non-universal assessment tools (RAKIT and SON-R, Dutch psychometric tests) used |
| Cohen 1995286 | ↔ | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ |
[5] Study population was born before 1990 [6] Outdated assessment tool (BSID, 1969) used [7] Outdated assessment tools (S-B-III, 1973; WISC-R, 1974 and WAIS-R, 1981) used |
| Fedrizzi 1993279 | ↑ | ↔ | ↑ | ↑ | ↑ | ↑ | ↑ |
[1] Inclusion criteria: diagnosis of spastic diplegia [3] Assessors not blinded to results of developmental assessment [4] Final cohort represents < 30% of eligible population [5] Study population restricted to children with spastic diplegia [6] Outdated assessment tool (GMDS, 1970) used [7] Outdated assessment tool (WPPSI, 1967) used |
| Gray 1995281 | ↑ | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ |
[1] Inclusion criteria: diagnosis of bronchopulmonary dysplasia [5] Study population restricted to children with bronchopulmonary dysplasia [6] Outdated assessment tool (GMDS, 1970) used [7] Outdated assessment tool (WPPSI-R, 1989) used |
| Gray 2006287 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Hack 2005162 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | [7] Outdated assessment tool (KABC, 1983) used |
| Marlow 2005225 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | [7] Outdated assessment tool (KABC, 1983) used |
| Kilbride 1990293 | ↔ | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ |
[6] Outdated assessment tool (BSID, 1969) used [7] Outdated assessment (S-B-III, 1973) used |
| McGrath 2000280 | ↑ | ↔ | ↔ | ↑ | ↑ | ↔ | ↔ |
[1] Inclusion criteria: meets a priori medical criterion (not specified) [4] Final cohort represents < 30% of eligible population [5] Study population was born before 1990 |
| Munck 2012160 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | [7] Outdated assessment (WPPSI-R, 1989) used |
| Orchinik 2011294 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Potharst 2012161 | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | [4] Final cohort represents < 30% of eligible population |
| Reuss 1996288 | ↔ | ↔ | ? | ↑ | ↑ | ↑ | ↔ |
[3] Blinding of assessors not stated [4] Final cohort represents < 30% of eligible population [5] Study population was born before 1990 [6] Outdated assessment tool (BSID, 1969 and S-B-III, 1973) used |
| Roberts 2010163 | ↔ | ↔ | ? | ↔ | ↔ | ↔ | ↔ | [3] Blinding of assessors not stated |
| Skranes 1998289 | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ | ↑ |
[4] Final cohort represents < 30% of eligible population [5] Study population was born before 1990 [6] Outdated assessment tool (BSID, 1969 and S-B-III, 1973) used [7] Outdated assessment tool (WPPSI, 1967) used |
| Smith 2006282 | ↑ | ↔ | ? | ↑ | ↑ | ↑ | ↑ |
[1] Inclusion criteria: from middle to lower socioeconomic groups [3] Blinding of assessors not stated [4] Final cohort represents < 30% of eligible population [5] Study population restricted to children from middle to lower socioeconomic groups [6] Outdated assessment tool (S-B-IV, 1986) used [7] Outdated assessment tool (S-B-IV, 1986) used |
| Tommiska 2003290 | ↔ | ↔ | ? | ↔ | ↔ | ↔ | ↑ |
[3] Blinding of assessors not stated [7] Outdated assessment tool (WPPSI-R, 1989) used |
| Veelken 1991291 | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ | ↑ |
[4] Final cohort represents < 30% of eligible population [5] Study population was born before 1990 [6] Outdated assessment tool (GMDS, 1970) used [7] Outdated assessment tool (KABC, 1983) used |
| Vermeulen 2001295 | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | ↑ |
[6] Assessment tool developed before 1990 (GMDS, 1970) used [7] Assessment tool developed before 1990 (WISC-R, 1974) used |
| Wolke 1999173 | ↔ | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ |
[5] Study population was born before 1990 [6] Outdated assessment tool (GMDS, 1970) used [7] Outdated assessment tool (KABC, 1983) used |
Chapter 6
| Subgroup variable | Mean costs (£) (95% CI) | Mean effects (95% CI) | ICER (£) | Probability (%) B. breve BBG is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. breve BBG | Placebo | Difference | B. breve BBG | Placebo | Differencea | More effectiveb | Less costlyb | Cost-effectiveb,c | Cost-effectiveb,d | ||
| PiPS data set | |||||||||||
| Colonisation (yes) | 61,354.61 (57,739.13 to 64,970.09) | 55,071.11 (49,211.64 to 60,930.58) | 6283.5 (–601.64 to 13,168.63) | 0.029 (0.014 to 0.0439) | 0.0327 (0.0089 to 0.0565) | 0.0037 (–0.0244 to 0.0319) | 1,686,947.4 | 60.6 | 4.9 | 5.5 | 5.7 |
| Colonisation (no) | 86,147.5 (72,490.12 to 99,804.87) | 69,272.01 (64,240.99 to 74,303.03) | 16,875.49 (2320.93 to 31,430.04) | 0.1463 (0.0698 to 0.2228) | 0.0523 (0.0294 to 0.0753) | –0.094 (–0.1739 to –0.0141) | –179,526.72 | 0.3 | 0.1 | 0.1 | 0.0 |
| Gestational age < 28 years | 85,643.61 (79,893.44 to 91,393.79) | 85,485.04 (79,268.07 to 91,702) | 158.58 (–8309.9 to 8627.06) | 0.1309 (0.0926 to 0.1692) | 0.1711 (0.1287 to 0.2134) | 0.0402 (–0.0169 to 0.0973) | 3946.6786 | 90.6 | 50.0 | 56.4 | 61.3 |
| Gestational age ≥ 28 years | 42,427.22 (39,409.85 to 45,444.6) | 41,882.82 (39,068 to 44,697.65) | 544.4 (–3582.07 to 4670.87) | 0.0373 (0.0166 to 0.058) | 0.006 (–0.0023 to 0.0143) | –0.0313 (–0.0536 to –0.009) | –17,404.544 | 0.0 | 39.0 | 28.8 | 23.8 |
| Randomisation age ≤ 24 hours | 60,552.92 (54,496.09 to 66,609.75) | 59,907.42 (53,228.36 to 66,586.49) | 645.5 (–8370.89 to 9661.88) | 0.0625 (0.025 to 0.1) | 0.0838 (0.0418 to 0.1259) | 0.0213 (–0.035 to 0.0777) | 30,259.012 | 77.6 | 41.9 | 44.7 | 45.7 |
| Randomisation age > 24 hours | 64,119.34 (59,745.71 to 68,492.97) | 63,634.34 (59,196.12 to 68,072.56) | 485 (–5746.09 to 6716.08) | 0.0891 (0.0631 to 0.1152) | 0.0849 (0.0597 to 0.1101) | –0.0042 (–0.0404 to 0.032) | –115,345.2 | 39.0 | 44.7 | 43.7 | 43.3 |
| Sex (male) | 64,813.38 (60,104.75 to 69,522.02) | 62,142.4 (57,119.77 to 67,165.02) | 2670.99 (–4213.63 to 9555.61) | 0.0938 (0.0633 to 0.1242) | 0.0784 (0.0505 to 0.1063) | –0.0153 (–0.0566 to 0.026) | –174,361.95 | 22.9 | 21.1 | 18.0 | 17.1 |
| Sex (female) | 61,078.55 (55,495.49 to 66,661.62) | 63,314.87 (57,795.57 to 68,834.17) | –2236.32 (–10,087.01 to 5614.37) | 0.0672 (0.0372 to 0.0971) | 0.0925 (0.0586 to 0.1264) | 0.0254 (–0.0199 to 0.0706) | –88,174.182 | 86.6 | 71.8 | 76.6 | 78.9 |
| Weight < 1000 g (yes) | 87,432.57 (81,654.37 to 93,210.78) | 85,122.31 (78,960.42 to 91,284.21) | 2310.26 (–6137.03 to 10,757.54) | 0.1424 (0.103 to 0.1818) | 0.164 (0.1228 to 0.2051) | 0.0216 (–0.0354 to 0.0786) | 106,941.31 | 76.8 | 28.6 | 31.5 | 32.9 |
| Weight < 1000 g (no) | 40,184.67 (37,715.64 to 42,653.71) | 41,294.42 (38,612.23 to 43,976.61) | –1109.74 (–4755.32 to 2535.84) | 0.0252 (0.0079 to 0.0424) | 0.0092 (–0.0012 to 0.0195) | –0.016 (–0.0361 to 0.0041) | 69,433.126 | 5.5 | 71.4 | 65.6 | 62.7 |
| NNRD data set | |||||||||||
| Colonisation (yes) | 59,017.58 (55,765.98 to 62,269.17) | 53,138.61 (47,903.19 to 58,374.03) | 5878.97 (–284.03 to 12,041.96) | 0.029 (0.014 to 0.0439) | 0.0327 (0.0089 to 0.0565) | 0.0037 (–0.0244 to 0.0319) | 1,578,342.3 | 58.4 | 2.9 | 3.2 | 3.3 |
| Colonisation (no) | 81,452.58 (72,626.84 to 90,278.33) | 67,767.97 (62,941.77 to 72,594.18) | 13,684.61 (3625.48 to 23,743.74) | 0.1463 (0.0698 to 0.2228) | 0.0523 (0.0294 to 0.0753) | –0.094 (–0.1739 to –0.0141) | –145,581.17 | 0.5 | 0.7 | 0.3 | 0.1 |
| Gestational age < 28 years | 81,834.95 (77,038.62 to 86,631.28) | 81,626.86 (75,675.75 to 87,577.97) | 208.08 (–7435.24 to 7851.41) | 0.1309 (0.0926 to 0.1692) | 0.1711 (0.1287 to 0.2134) | 0.0402 (–0.0169 to 0.0973) | 5178.8003 | 93 | 48.4 | 55.8 | 59.7 |
| Gestational age ≥ 28 years | 41,194.96 (38,659.84 to 43,730.09) | 42,334.79 (39,428.07 to 45,241.5) | –1139.82 (–4996.75 to 2717.1) | 0.0373 (0.0166 to 0.058) | 0.006 (–0.0023 to 0.0143) | –0.0313 (–0.0536 to –0.009) | 36,440.48 | 0.2 | 70.1 | 59.1 | 53.4 |
| Randomisation age ≤ 24 hours | 59,180.96 (53,370.47 to 64,991.46) | 58,124.43 (51,774.27 to 64,474.58) | 1056.54 (–7550.8 to 9663.88) | 0.0625 (0.025 to 0.1) | 0.0838 (0.0418 to 0.1259) | 0.0213 (–0.035 to 0.0777) | 49,527.488 | 78.6 | 38.7 | 42.1 | 43.6 |
| Randomisation age > 24 hours | 61,266.6 (57,611.07 to 64,922.13) | 62,096.83 (57,835.16 to 66,358.49) | –830.22 (–6444.9 to 4784.46) | 0.0891 (0.0631 to 0.1152) | 0.0849 (0.0597 to 0.1101) | –0.0042 (–0.0404 to 0.032) | 197,448.99 | 42.4 | 60.3 | 59.6 | 59.5 |
| Sex (male) | 63,399.49 (58,920.37 to 67,878.61) | 61,730.88 (56,698.18 to 66,763.58) | 1668.6 (–5068.65 to 8405.86) | 0.0938 (0.0633 to 0.1242) | 0.0784 (0.0505 to 0.1063) | –0.0153 (–0.0566 to 0.026) | –108,926.51 | 23.8 | 33.0 | 30.1 | 28.6 |
| Sex (female) | 57,220.04 (53,157.04 to 61,283.04) | 60,200.92 (55,254.06 to 65,147.77) | –2980.88 (–9382.39 to 3420.63) | 0.0672 (0.0372 to 0.0971) | 0.0925 (0.0586 to 0.1264) | 0.0254 (–0.0199 to 0.0706) | –117,530.82 | 86.2 | 81.4 | 86.2 | 88.0 |
| Weight < 1000 g (yes) | 83,083.85 (78,193.05 to 87,974.64) | 80,400.47 (74,646.03 to 86,154.91) | 2683.38 (–4868.67 to 10,235.43) | 0.1424 (0.103 to 0.1818) | 0.164 (0.1228 to 0.2051) | 0.0216 (–0.0354 to 0.0786) | 124,213.13 | 75.7 | 25.9 | 29.6 | 31.3 |
| Weight < 1000 g (no) | 39,497.7 (37,562.52 to 41,432.88) | 42,660.06 (39,475.43 to 45,844.69) | –3162.35 (–6888.86 to 564.15) | 0.0252 (0.0079 to 0.0424) | 0.0092 (–0.0012 to 0.0195) | –0.016 (–0.0361 to 0.0041) | 197,858.35 | 5.9 | 95.5 | 93.8 | 92.3 |
| Combined data set | |||||||||||
| Colonisation (yes) | 59,082.53 (55,813.78 to 62,351.27) | 53,064.15 (47,856.46 to 58,271.83) | 6018.38 (–130.17 to 12,166.93) | 0.029 (0.014 to 0.0439) | 0.0327 (0.0089 to 0.0565) | 0.0037 (–0.0244 to 0.0319) | 1,615,770.8 | 58.4 | 2.5 | 2.7 | 2.8 |
| Colonisation (no) | 82,009.26 (73,172.95 to 90,845.58) | 67,938.27 (63,094.89 to 72,781.64) | 14,071 (3994.36 to 24,147.64) | 0.1463 (0.0698 to 0.2228) | 0.0523 (0.0294 to 0.0753) | –0.094 (–0.1739 to –0.0141) | –149,691.66 | 0.5 | 0.6 | 0.3 | 0.1 |
| Gestational age < 28 years | 82,110.69 (77,277.46 to 86,943.93) | 81,796.8 (75,847.03 to 87,746.58) | 313.89 (–7351.61 to 7979.4) | 0.1309 (0.0926 to 0.1692) | 0.1711 (0.1287 to 0.2134) | 0.0402 (–0.0169 to 0.0973) | 7812.1565 | 93.0 | 46.9 | 54.4 | 59.2 |
| Gestational age ≥ 28 years | 41,205.5 (38,669.57 to 43,741.43) | 42,375.67 (39,459.53 to 45,291.81) | –1170.17 (–5034.72 to 2694.39) | 0.0373 (0.0166 to 0.058) | 0.006 (–0.0023 to 0.0143) | –0.0313 (–0.0536 to –0.009) | 37,410.579 | 0.2 | 71.0 | 59.7 | 53.6 |
| Randomisation age ≤ 24 hours | 59,476.77 (53,591.8 to 65,361.74) | 58,194.19 (51,854.17 to 64,534.22) | 1282.58 (–7367.79 to 9932.94) | 0.0625 (0.025 to 0.1) | 0.0838 (0.0418 to 0.1259) | 0.0213 (–0.035 to 0.0777) | 60,123.538 | 78.6 | 37 | 40.3 | 41.9 |
| Randomisation age > 24 hours | 61,349.73 (57,679.36 to 65,020.1) | 62,210.76 (57,941.56 to 66,479.97) | –861.04 (–6491.11 to 4769.04) | 0.0891 (0.0631 to 0.1152) | 0.0849 (0.0597 to 0.1101) | –0.0042 (–0.0404 to 0.032) | 204,777.36 | 42.4 | 60.6 | 59.7 | 59.7 |
| Sex (male) | 63,431.42 (58,926.17 to 67,936.66) | 61,714.56 (56,686.93 to 66,742.19) | 1716.86 (–5034.01 to 8467.73) | 0.0938 (0.0633 to 0.1242) | 0.0784 (0.0505 to 0.1063) | –0.0153 (–0.0566 to 0.026) | –112,076.61 | 23.8 | 32.4 | 29.5 | 27.8 |
| Sex (female) | 57,497.39 (53,404.44 to 61,590.33) | 60,454.11 (55,484.46 to 65,423.76) | –2956.72 (–9394.86 to 3481.42) | 0.0672 (0.0372 to 0.0971) | 0.0925 (0.0586 to 0.1264) | 0.0254 (–0.0199 to 0.0706) | –116,578.36 | 86.2 | 80.6 | 85.7 | 87.8 |
| Weight < 1000 g (yes) | 83,364.15 (78,439.38 to 88,288.91) | 80,535.46 (74,783.56 to 86,287.36) | 2828.68 (–4743.48 to 10,400.85) | 0.1424 (0.103 to 0.1818) | 0.164 (0.1228 to 0.2051) | 0.0216 (–0.0354 to 0.0786) | 130,939.25 | 75.7 | 24.7 | 27.8 | 29.3 |
| Weight < 1000 g (no) | 39,500.58 (37,565.42 to 41,435.75) | 42,731.41 (39,531.03 to 45,931.79) | –3230.83 (–6970.78 to 509.13) | 0.0252 (0.0079 to 0.0424) | 0.0092 (–0.0012 to 0.0195) | –0.016 (–0.0361 to 0.0041) | 202,142.56 | 5.9 | 95.8 | 94.3 | 93.0 |
| Subgroup variable | Mean costs (£) (95% CI) | Mean effects (95% CI) | ICER (£) | Probability (%) B. breve BBG is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. breve BBG | Placebo | Difference | B. breve BBG | Placebo | Differencea | More effectiveb | Less costlyb | Cost-effectiveb,c | Cost-effectiveb,d | ||
| PiPS data set | |||||||||||
| Colonisation (yes) | 61,354.61 (57,739.13 to 64,970.09) | 55,071.11 (49,211.64 to 60,930.58) | 6283.5 (–601.64 to 13,168.63) | 0.087 (0.0618 to 0.1121) | 0.0981 (0.0583 to 0.138) | 0.0112 (–0.0359 to 0.0583) | 562,315.79 | 69.9 | 4.9 | 6.2 | 7.8 |
| Colonisation (no) | 86,147.5 (72,490.12 to 99,804.87) | 69,272.01 (64,240.99 to 74,303.03) | 16,875.49 (2320.93 to 31,430.04) | 0.2073 (0.1196 to 0.2951) | 0.1212 (0.0876 to 0.1548) | –0.0861 (–0.1801 to 0.0078) | –195,987.42 | 3.2 | 0.1 | 0.0 | 0.0 |
| Gestational age < 28 years | 85,643.61 (79,893.44 to 91,393.79) | 85,485.04 (79,268.07 to 91,702) | 158.58 (–8309.9 to 8627.06) | 0.1913 (0.1466 to 0.2359) | 0.1743 (0.1317 to 0.217) | –0.0169 (–0.0787 to 0.0448) | –9365.0001 | 29.7 | 50.0 | 46.5 | 45.4 |
| Gestational age ≥ 28 years | 42,427.22 (39,409.85 to 45,444.6) | 41,882.82 (39,068 to 44,697.65) | 544.4 (–3582.07 to 4670.87) | 0.0311 (0.0121 to 0.05) | 0.0569 (0.032 to 0.0817) | 0.0258 (–0.0054 to 0.0571) | 21,075.912 | 94.9 | 39.0 | 47.5 | 52.2 |
| Randomisation age ≤ 24 hours | 60,552.92 (54,496.09 to 66,609.75) | 59,907.42 (53,228.36 to 66,586.49) | 645.5 –8370.89 to 9661.88) | 0.075 (0.0342 to 0.1158) | 0.1377 (0.0855 to 0.19) | 0.0627 (–0.0036 to 0.129) | 10,290.953 | 97.3 | 41.9 | 51.4 | 57.9 |
| Randomisation age > 24 hours | 64,119.34 (59,745.71 to 68,492.97) | 63,634.34 (59,196.12 to 68,072.56) | 485 (–5746.09 to 6716.08) | 0.1196 (0.0899 to 0.1492) | 0.104 (0.0765 to 0.1316) | –0.0155 (–0.056 to 0.025) | –31,227.186 | 23.1 | 44.7 | 41.6 | 40.3 |
| Sex (male) | 64,813.38 (60,104.75 to 69,522.02) | 62,142.4 (57,119.77 to 67,165.02) | 2670.99 (–4213.63 to 9555.61) | 0.1165 (0.083 to 0.15) | 0.1176 (0.0842 to 0.1511) | 0.0012 (–0.0462 to 0.0485) | 2,283,311.2 | 51.6 | 21.1 | 24.4 | 25.2 |
| Sex (female) | 61,078.55 (55,495.49 to 66,661.62) | 63,314.87 (57,795.57 to 68,834.17) | –2236.32 (–10,087.01 to 5614.37) | 0.097 (0.0616 to 0.1325) | 0.1068 (0.0707 to 0.1429) | 0.0097 (–0.0408 to 0.0603) | –229,445.08 | 63.8 | 71.8 | 73.5 | 73.4 |
| Weight < 1000 g (yes) | 87,432.57 (81,654.37 to 93,210.78) | 85,122.31 (78,960.42 to 91,284.21) | 2310.26 (–6137.03 to 10,757.54) | 0.1887 (0.1446 to 0.2329) | 0.1833 (0.1403 to 0.2263) | –0.0055 (–0.0671 to 0.0562) | –422,970.59 | 42.1 | 28.6 | 27.5 | 27.0 |
| Weight < 1000 g (no) | 40,184.67 (37,715.64 to 42,653.71) | 41,294.42 (38,612.23 to 43,976.61) | –1109.74 (–4755.32 to 2535.84) | 0.0314 (0.0123 to 0.0506) | 0.0459 (0.0232 to 0.0685) | 0.0144 (–0.0153 to 0.0441) | –76,931.903 | 82.0 | 71.4 | 75.3 | 77.3 |
| NNRD data set | |||||||||||
| Colonisation (yes) | 59,017.58 (55,765.98 to 62,269.17) | 53,138.61 (47,903.19 to 58,374.03) | 5878.97 (–284.03 to 12,041.96) | 0.087 (0.0618 to 0.1121) | 0.0981 (0.0583 to 0.138) | 0.0112 (–0.0359 to 0.0583) | 526,114.09 | 65.6 | 2.9 | 4.9 | 5.4 |
| Colonisation (no) | 81,452.58 (72,626.84 to 90,278.33) | 67,767.97 (62,941.77 to 72,594.18) | 13,684.61 (3625.48 to 23,743.74) | 0.2073 (0.1196 to 0.2951) | 0.1212 (0.0876 to 0.1548) | –0.0861 (–0.1801 to 0.0078) | –158,929.43 | 2.5 | 0.7 | 0.3 | 0.3 |
| Gestational age < 28 years | 81,834.95 (77,038.62 to 86,631.28) | 81,626.86 (75,675.75 to 87,577.97) | 208.08 (–7435.24 to 7851.41) | 0.1913 (0.1466 to 0.2359) | 0.1743 (0.1317 to 0.217) | –0.0169 (–0.0787 to 0.0448) | –12,288.679 | 31.5 | 48.4 | 45.3 | 44.4 |
| Gestational age ≥ 28 years | 41,194.96 (38,659.84 to 43,730.09) | 42,334.79 (39,428.07 to 45,241.5) | –1139.82 (–4996.75 to 2717.1) | 0.0311 (0.0121 to 0.05) | 0.0569 (0.032 to 0.0817) | 0.0258 (–0.0054 to 0.0571) | –44,127.348 | 94.9 | 70.1 | 76.7 | 79.3 |
| Randomisation age ≤ 24 hours | 59,180.96 (53,370.47 to 64,991.46) | 58,124.43 (51,774.27 to 64,474.58) | 1056.54 (–7550.8 to 9663.88) | 0.075 (0.0342 to 0.1158) | 0.1377 (0.0855 to 0.19) | 0.0627 (–0.0036 to 0.129) | 16,844.074 | 97.0 | 38.7 | 48.6 | 53.4 |
| Randomisation age > 24 hours | 61,266.6 (57,611.07 to 64,922.13) | 62,096.83 (57,835.16 to 66,358.49) | –830.22 (–6444.9 to 4784.46) | 0.1196 (0.0899 to 0.1492) | 0.104 (0.0765 to 0.1316) | –0.0155 (–0.056 to 0.025) | 53,454.985 | 23.9 | 60.3 | 56.4 | 54.9 |
| Sex (male) | 63,399.49 (58,920.37 to 67,878.61) | 61,730.88 (56,698.18 to 66,763.58) | 1668.6 (–5068.65 to 8405.86) | 0.1165 (0.083 to 0.15) | 0.1176 (0.0842 to 0.1511) | 0.0012 (–0.0462 to 0.0485) | 1,426,418.6 | 50.5 | 33.0 | 33.8 | 33.9 |
| Sex (female) | 57,220.04 (53,157.04 to 61,283.04) | 60,200.92 (55,254.06 to 65,147.77) | –2980.88 (–9382.39 to 3420.63) | 0.097 (0.0616 to 0.1325) | 0.1068 (0.0707 to 0.1429) | 0.0097 (–0.0408 to 0.0603) | –305,836.34 | 65.9 | 81.4 | 81.8 | 81.2 |
| Weight < 1000 g (yes) | 83,083.85 (78,193.05 to 87,974.64) | 80,400.47 (74,646.03 to 86,154.91) | 2683.38 (–4868.67 to 10,235.43) | 0.1887 (0.1446 to 0.2329) | 0.1833 (0.1403 to 0.2263) | –0.0055 (–0.0671 to 0.0562) | –491,283.52 | 44.6 | 25.9 | 25.6 | 25.5 |
| Weight < 1000 g (no) | 39,497.7 (37,562.52 to 41,432.88) | 42,660.06 (39,475.43 to 45,844.69) | –3162.35 (–6888.86 to 564.15) | 0.0314 (0.0123 to 0.0506) | 0.0459 (0.0232 to 0.0685) | 0.0144 (–0.0153 to 0.0441) | –219,227.05 | 83.8 | 95.5 | 96.2 | 96.6 |
| Combined data set | |||||||||||
| Colonisation (yes) | 59,082.53 (55,813.78 to 62,351.27) | 53,064.15 (47,856.46 to 58,271.83) | 6018.38 (–130.17 to 12,166.93) | 0.087 (0.0618 to 0.1121) | 0.0981 (0.0583 to 0.138) | 0.0112 (–0.0359 to 0.0583) | 538,590.27 | 65.6 | 2.5 | 4.6 | 5.0 |
| Colonisation (no) | 82,009.26 (73,172.95 to 90,845.58) | 67,938.27 (63,094.89 to 72,781.64) | 14,071 (3994.36 to 24,147.64) | 0.2073 (0.1196 to 0.2951) | 0.1212 (0.0876 to 0.1548) | –0.0861 (–0.1801 to 0.0078) | –163,416.8 | 2.5 | 0.6 | 0.3 | 0.3 |
| Gestational age < 28 years | 82,110.69 (77,277.46 to 86,943.93) | 81,796.8 (75,847.03 to 87,746.58) | 313.89 (–7351.61 to 7979.4) | 0.1913 (0.1466 to 0.2359) | 0.1743 (0.1317 to 0.217) | –0.0169 (–0.0787 to 0.0448) | –18,537.321 | 31.5 | 46.9 | 44.3 | 43.3 |
| Gestational age ≥ 28 years | 41,205.5 (38,669.57 to 43,741.43) | 42,375.67 (39,459.53 to 45,291.81) | –1170.17 (–5034.72 to 2694.39) | 0.0311 (0.0121 to 0.05) | 0.0569 (0.032 to 0.0817) | 0.0258 (–0.0054 to 0.0571) | –45,302.084 | 94.9 | 71.0 | 77.5 | 80.1 |
| Randomisation age ≤ 24 hours | 59,476.77 (53,591.8 to 65,361.74) | 58,194.19 (51,854.17 to 64,534.22) | 1282.58 (–7367.79 to 9932.94) | 0.075 (0.0342 to 0.1158) | 0.1377 (0.0855 to 0.19) | 0.0627 (–0.0036 to 0.129) | 20,447.743 | 97.0 | 37.0 | 46.5 | 51.7 |
| Randomisation age > 24 hours | 61,349.73 (57,679.36 to 65,020.1) | 62,210.76 (57,941.56 to 66,479.97) | –861.04 (–6491.11 to 4769.04) | 0.1196 (0.0899 to 0.1492) | 0.104 (0.0765 to 0.1316) | –0.0155 (–0.056 to 0.025) | 55,438.983 | 23.9 | 60.6 | 57.7 | 55.4 |
| Sex (male) | 63,431.42 (58,926.17 to 67,936.66) | 61,714.56 (56,686.93 to 66,742.19) | 1716.86 (–5034.01 to 8467.73) | 0.1165 (0.083 to 0.15) | 0.1176 (0.0842 to 0.1511) | 0.0012 (–0.0462 to 0.0485) | 1,467,669.9 | 50.5 | 32.4 | 33.7 | 33.9 |
| Sex (female) | 57,497.39 (53,404.44 to 61,590.33) | 60,454.11 (55,484.46 to 65,423.76) | –2956.72 (–9394.86 to 3481.42) | 0.097 (0.0616 to 0.1325) | 0.1068 (0.0707 to 0.1429) | 0.0097 (–0.0408 to 0.0603) | –303,357.87 | 65.9 | 80.6 | 81.5 | 81.2 |
| Weight < 1000 g (yes) | 83,364.15 (78,439.38 to 88,288.91) | 80,535.46 (74,783.56 to 86,287.36) | 2828.68(–4743.48 to 10,400.85) | 0.1887 (0.1446 to 0.2329) | 0.1833 (0.1403 to 0.2263) | –0.0055 (–0.0671 to 0.0562) | –517,886.44 | 44.6 | 24.7 | 25.0 | 24.8 |
| Weight < 1000 g (no) | 39,500.58 (37,565.42 to 41,435.75) | 42,731.41 (39,531.03 to 45,931.79) | –3230.83 (–6970.78 to 509.13) | 0.0314 (0.0123 to 0.0506) | 0.0459 (0.0232 to 0.0685) | 0.0144 (–0.0153 to 0.0441) | –223,973.96 | 83.8 | 95.8 | 96.6 | 96.8 |
| Subgroup variable | Mean costs (£) (95% CI) | Mean effects (95% CI) | ICER (£) | Probability (%) B. breve BBG is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. breve BBG | Placebo | Difference | B. breve BBG | Placebo | Differencea | More effectiveb | Less costlyb | Cost-effectiveb,c | Cost-effectiveb,d | ||
| PiPS data set | |||||||||||
| Colonisation (yes) | 61,354.61 (57,739.13 to 64,970.09) | 55,071.11 (49,211.64 to 60,930.58) | 6283.5 (–601.64 to 13,168.63) | 0.0663 (0.0441 to 0.0884) | 0.0514 (0.0218 to 0.081) | –0.0149 (–0.0518 to 0.0221) | –423,110.58 | 22.7 | 4.9 | 4.7 | 4.7 |
| Colonisation (no) | 86,147.5 (72,490.12 to 99,804.87) | 69,272.01 (64,240.99 to 74,303.03) | 16,875.49 (2320.93 to 31,430.04) | 0.2073 (0.1196 to 0.2951) | 0.1102 (0.078 to 0.1424) | –0.0971 (–0.1906 to –0.0037) | –173,751.56 | 1.7 | 0.1 | 0.0 | 0.0 |
| Gestational age < 28 years | 85,643.61 (79,893.44 to 91,393.79) | 85,485.04 (79,268.07 to 91,702) | 158.58 (–8309.9 to 8627.06) | 0.1443 (0.1044 to 0.1842) | 0.1645 (0.1228 to 0.2061) | 0.0202 (–0.0375 to 0.0779) | 7858.813 | 76.2 | 50.0 | 51.7 | 53.3 |
| Gestational age ≥ 28 years | 42,427.22 (39,409.85 to 45,444.6) | 41,882.82 (39,068 to 44,697.65) | 544.4 (–3582.07 to 4670.87) | 0.0404 (0.0189 to 0.0619) | 0.0389 (0.0182 to 0.0597) | –0.0015 (–0.0313 to 0.0284) | –375,313.36 | 44.4 | 39.0 | 38.5 | 38.4 |
| Randomisation age ≤ 24 hours | 60,552.92 (54,496.09 to 66,609.75) | 59,907.42 (53,228.36 to 66,586.49) | 645.5 (–8370.89 to 9661.88) | 0.0812 (0.0389 to 0.1236) | 0.0599 (0.0239 to 0.0959) | –0.0214 (–0.0769 to 0.0342) | –30,206.019 | 23.4 | 41.9 | 39.2 | 37.8 |
| Randomisation age > 24 hours | 64,119.34 (59,745.71 to 68,492.97) | 63,634.34 (59,196.12 to 68,072.56) | 485 (–5746.09 to 6716.08) | 0.0935 (0.0669 to 0.1201) | 0.1125 (0.084 to 0.1411) | 0.019 (–0.02 to 0.0581) | 25,461.469 | 81.5 | 44.7 | 49.7 | 51.6 |
| Sex (male) | 64,813.38 (60,104.75 to 69,522.02) | 62,142.4 (57,119.77 to 67,165.02) | 2670.99 (–4213.63 to 9555.61) | 0.0938 (0.0633 to 0.1242) | 0.098 (0.0672 to 0.1289) | 0.0043 (–0.0391 to 0.0476) | 622,721.24 | 56.3 | 21.1 | 24.2 | 24.6 |
| Sex (female) | 61,078.55 (55,495.49 to 66,661.62) | 63,314.87 (57,795.57 to 68,834.17) | –2236.32 (–10,087.01 to 5614.37) | 0.0858 (0.0523 to 0.1194) | 0.0996 (0.0646 to 0.1347) | 0.0138 (–0.0347 to 0.0623) | –161,779.72 | 72.6 | 71.8 | 74.0 | 74.2 |
| Weight < 1000 g (yes) | 87,432.57 (81,654.37 to 93,210.78) | 85,122.31 (78,960.42 to 91,284.21) | 2310.26 (–6137.03 to 10,757.54) | 0.149 (0.1088 to 0.1892) | 0.1608 (0.1199 to 0.2016) | 0.0118 (–0.0455 to 0.069) | 196,365.53 | 66.1 | 28.6 | 31.3 | 32.2 |
| Weight < 1000 g (no) | 40,184.67 (37,715.64 to 42,653.71) | 41,294.42 (38,612.23 to 43,976.61) | –1109.74 (–4755.32 to 2535.84) | 0.0346 (0.0145 to 0.0547) | 0.0398 (0.0186 to 0.0609) | 0.0052 (–0.024 to 0.0344) | –214,893.58 | 63.7 | 71.4 | 71.8 | 71.5 |
| NNRD data set | |||||||||||
| Colonisation (yes) | 59,017.58 (55,765.98 to 62,269.17) | 53,138.61 (47,903.19 to 58,374.03) | 5878.97 (–284.03 to 12,041.96) | 0.0663 (0.0441 to 0.0884) | 0.0514 (0.0218 to 0.081) | –0.0149 (–0.0518 to 0.0221) | –395,870.86 | 21.8 | 2.9 | 2.4 | 2.6 |
| Colonisation (no) | 81,452.58 (72,626.84 to 90,278.33) | 67,767.97 (62,941.77 to 72,594.18) | 13,684.61 (3625.48 to 23,743.74) | 0.2073 (0.1196 to 0.2951) | 0.1102 (0.078 to 0.1424) | –0.0971 (–0.1906 to –0.0037) | –140,898 | 2.4 | 0.7 | 0.4 | 0.4 |
| Gestational age < 28 years | 81,834.95 (77,038.62 to 86,631.28) | 81,626.86 (75,675.75 to 87,577.97) | 208.08 (–7435.24 to 7851.41) | 0.1443 (0.1044 to 0.1842) | 0.1645 (0.1228 to 0.2061) | 0.0202 (–0.0375 to 0.0779) | 10,312.272 | 75.3 | 48.4 | 51.5 | 53.1 |
| Gestational age ≥ 28 years | 41,194.96 (38,659.84 to 43,730.09) | 42,334.79 (39,428.07 to 45,241.5) | –1139.82 (–4996.75 to 2717.1) | 0.0404 (0.0189 to 0.0619) | 0.0389 (0.0182 to 0.0597) | –0.0015 (–0.0313 to 0.0284) | 785,806.24 | 46.7 | 70.1 | 68.7 | 67.8 |
| Randomisation age ≤ 24 hours | 59,180.96 (53,370.47 to 64,991.46) | 58,124.43 (51,774.27 to 64,474.58) | 1056.54 (–7550.8 to 9663.88) | 0.0812 (0.0389 to 0.1236) | 0.0599 (0.0239 to 0.0959) | –0.0214 (–0.0769 to 0.0342) | –49,440.75 | 22.1 | 38.7 | 36.1 | 34.9 |
| Randomisation age > 24 hours | 61,266.6 (57,611.07 to 64,922.13) | 62,096.83 (57,835.16 to 66,358.49) | –830.22 (–6444.9 to 4784.46) | 0.0935 (0.0669 to 0.1201) | 0.1125 (0.084 to 0.1411) | 0.019 (–0.02 to 0.0581) | –43,585.177 | 84.7 | 60.3 | 64.8 | 66.3 |
| Sex (male) | 63,399.49 (58,920.37 to 67,878.61) | 61,730.88 (56,698.18 to 66,763.58) | 1668.6 (–5068.65 to 8405.86) | 0.0938 (0.0633 to 0.1242) | 0.098 (0.0672 to 0.1289) | 0.0043 (–0.0391 to 0.0476) | 389,023.25 | 55.5 | 33.0 | 34.8 | 36.2 |
| Sex (female) | 57,220.04 (53,157.04 to 61,283.04) | 60,200.92 (55,254.06 to 65,147.77) | –2980.88 (–9382.39, 3420.63) | 0.0858 (0.0523 to 0.1194) | 0.0996 (0.0646 to 0.1347) | 0.0138 (–0.0347 to 0.0623) | –215,642.53 | 73.2 | 81.4 | 81.8 | 82.3 |
| Weight < 1000 g (yes) | 83,083.85 (78,193.05 to 87,974.64) | 80,400.47 (74,646.03 to 86,154.91) | 2683.38 (–4868.67 to 10,235.43) | 0.149 (0.1088 to 0.1892) | 0.1608 (0.1199 to 0.2016) | 0.0118 (–0.0455 to 0.069) | 228,080.04 | 64.9 | 25.9 | 28.6 | 30.3 |
| Weight < 1000 g (no) | 39,497.7 (37,562.52 to 41,432.88) | 42,660.06 (39,475.43 to 45,844.69) | –3162.35 (–6888.86 to 564.15) | 0.0346 (0.0145 to 0.0547) | 0.0398 (0.0186 to 0.0609) | 0.0052 (–0.024 to 0.0344) | –612,366.06 | 66.1 | 95.5 | 95.3 | 94.6 |
| Combined data set | |||||||||||
| Colonisation (yes) | 59,082.53 (55,813.78 to 62,351.27) | 53,064.15 (47,856.46 to 58,271.83) | 6018.38 (–130.17 to 12,166.93) | 0.0663 (0.0441 to 0.0884) | 0.0514 (0.0218 to 0.081) | –0.0149 (–0.0518 to 0.0221) | –405,258.48 | 21.8 | 2.5 | 2.1 | 2.3 |
| Colonisation (no) | 82,009.26 (73,172.95 to 90,845.58) | 67,938.27 (63,094.89 to 72,781.64) | 14,071 (3994.36 to 24,147.64) | 0.2073 (0.1196 to 0.2951) | 0.1102 (0.078 to 0.1424) | –0.0971 (–0.1906 to –0.0037) | –144,876.26 | 2.4 | 0.6 | 0.4 | 0.4 |
| Gestational age < 28 years | 82,110.69 (77,277.46 to 86,943.93) | 81,796.8 (75,847.03 to 87,746.58) | 313.89(–7351.61 to 7979.4) | 0.1443 (0.1044 to 0.1842) | 0.1645 (0.1228 to 0.2061) | 0.0202 (–0.0375 to 0.0779) | 15,555.935 | 75.3 | 46.9 | 50.8 | 52.6 |
| Gestational age ≥ 28 years | 41,205.5 (38,669.57 to 43,741.43) | 42,375.67 (39,459.53 to 45,291.81) | –1170.17 (–5034.72 to 2694.39) | 0.0404 (0.0189 to 0.0619) | 0.0389 (0.0182 to 0.0597) | –0.0015 (–0.0313 to 0.0284) | 806,725.57 | 46.7 | 71 | 69.1 | 67.8 |
| Randomisation age ≤ 24 hours | 59,476.77 (53,591.8 to 65,361.74) | 58,194.19 (51,854.17 to 64,534.22) | 1282.58 (–7367.79 to 9932.94) | 0.0812 (0.0389 to 0.1236) | 0.0599 (0.0239 to 0.0959) | –0.0214 (–0.0769 to 0.0342) | –60,018.243 | 22.1 | 37 | 34.7 | 33.4 |
| Randomisation age > 24 hours | 61,349.73 (57,679.36 to 65,020.1) | 62,210.76 (57,941.56 to 66,479.97) | –861.04 (–6491.11 to 4769.04) | 0.0935 (0.0669 to 0.1201) | 0.1125 (0.084 to 0.1411) | 0.019 (–0.02 to 0.0581) | –45,202.854 | 84.7 | 60.6 | 65.3 | 66.8 |
| Sex (male) | 63,431.42 (58,926.17 to 67,936.66) | 61,714.56 (56,686.93 to 66,742.19) | 1716.86 (–5034.01 to 8467.73) | 0.0938 (0.0633 to 0.1242) | 0.098 (0.0672 to 0.1289) | 0.0043 (–0.0391 to 0.0476) | 400,273.6 | 55.5 | 32.4 | 34.3 | 35.3 |
| Sex (female) | 57,497.39 (53,404.44 to 61,590.33) | 60,454.11 (55,484.46 to 65,423.76) | –2956.72 (–9394.86 to 3481.42) | 0.0858 (0.0523 to 0.1194) | 0.0996 (0.0646 to 0.1347) | 0.0138 (–0.0347 to 0.0623) | –213,894.98 | 73.2 | 80.6 | 81.6 | 82.1 |
| Weight < 1000 g (yes) | 83,364.15 (78,439.38 to 88,288.91) | 80,535.46 (74,783.56 to 86,287.36) | 2828.68 (–4743.48 to 10,400.85) | 0.149 (0.1088 to 0.1892) | 0.1608 (0.1199 to 0.2016) | 0.0118 (–0.0455 to 0.069) | 240,430.54 | 64.9 | 24.7 | 27.3 | 28.9 |
| Weight < 1000 g (no) | 39,500.58 (37,565.42 to 41,435.75) | 42,731.41 (39,531.03 to 45,931.79) | –3230.83 (–6970.78 to 509.13) | 0.0346 (0.0145 to 0.0547) | 0.0398 (0.0186 to 0.0609) | 0.0052 (–0.024 to 0.0344) | –625,625.58 | 66.1 | 95.8 | 95.4 | 94.8 |
| Subgroup variable | Mean costs (£) (95% CI) | Mean effects (95% CI) | ICER (£) | Probability (%) B. breve BBG is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. breve BBG | Placebo | Difference | B. breve BBG | Placebo | Differencea | More effectiveb | Less costlyb | Cost-effectiveb,c | Cost-effectiveb,d | ||
| PiPS data set | |||||||||||
| Colonisation (yes) | 61,354.61 (57,739.13 to 64,970.09) | 55,071.11 (49,211.64 to 60,930.58) | 6283.5 (–601.64 to 13,168.63) | 0.1408 (0.1098 to 0.1718) | 0.1449 (0.0977 to 0.192) | 0.0041 (–0.0524 to 0.0605) | 1,542,695.3 | 60.1 | 4.9 | 6.5 | 8.2 |
| Colonisation (no) | 86,147.5 (72,490.12 to 99,804.87) | 69,272.01 (64,240.99 to 74,303.03) | 16,875.49 (2320.93 to 31,430.04) | 0.4024 (0.2963 to 0.5086) | 0.2011 (0.1599 to 0.2423) | –0.2013 (–0.3152 to –0.0875) | –83,817.078 | 0.0 | 0.1 | 0.0 | 0.0 |
| Gestational age < 28 years | 85,643.61 (79,893.44 to 91,393.79) | 85,485.04 (79,268.07 to 91,702) | 158.58 (–8309.9 to 8627.06) | 0.3423 (0.2884 to 0.3962) | 0.3651 (0.311 to 0.4193) | 0.0228 (–0.0535 to 0.0992) | 6940.0532 | 70.3 | 50 | 52.7 | 54.4 |
| Gestational age ≥ 28 years | 42,427.22 (39,409.85 to 45,444.6) | 41,882.82 (39,068 to 44,697.65) | 544.4 (–3582.07 to 4670.87) | 0.0963 (0.0641 to 0.1285) | 0.0838 (0.0541 to 0.1136) | –0.0124 (–0.0563 to 0.0314) | –43,758.509 | 28.5 | 39 | 35.7 | 34.2 |
| Randomisation age ≤ 24 hours | 60,552.92 (54,496.09 to 66,609.75) | 59,907.42 (53,228.36 to 66,586.49) | 645.5 (–8370.89 to 9661.88) | 0.175 (0.1161 to 0.2339) | 0.2275 (0.164 to 0.2911) | 0.0525 (–0.0341 to 0.1392) | 12,284.642 | 89.2 | 41.9 | 49.7 | 54.7 |
| Randomisation age > 24 hours | 64,119.34 (59,745.71 to 68,492.97) | 63,634.34 (59,196.12 to 68,072.56) | 485 (–5746.09 to 6716.08) | 0.2283 (0.1899 to 0.2666) | 0.2144 (0.1774 to 0.2515) | –0.0138 (–0.0672 to 0.0395) | –35,084.968 | 29 | 44.7 | 42.2 | 41.3 |
| Sex (male) | 64,813.38 (60,104.75 to 69,522.02) | 62,142.4 (57,119.77 to 67,165.02) | 2670.99 (–4213.63 to 9555.61) | 0.2301 (0.1861 to 0.2741) | 0.2157 (0.173 to 0.2584) | –0.0144 (–0.0757 to 0.0468) | –185,133.34 | 30.6 | 21.1 | 21.0 | 20.3 |
| Sex (female) | 61,078.55 (55,495.49 to 66,661.62) | 63,314.87 (57,795.57 to 68,834.17) | –2236.32 (–10,087.01 to 5614.37) | 0.194 (0.1467 to 0.2414) | 0.2206 (0.1722 to 0.2691) | 0.0266 (–0.0412 to 0.0944) | –84,038.267 | 76.7 | 71.8 | 75.7 | 77.1 |
| Weight < 1000 g (yes) | 87,432.57 (81,654.37 to 93,210.78) | 85,122.31 (78,960.42 to 91,284.21) | 2310.26 (–6137.03 to 10,757.54) | 0.3543 (0.3004 to 0.4083) | 0.3666 (0.313 to 0.4201) | 0.0123 (–0.0638 to 0.0883) | 188,517.73 | 62 | 28.6 | 30.3 | 32.1 |
| Weight < 1000 g (no) | 40,184.67 (37,715.64 to 42,653.71) | 41,294.42 (38,612.23 to 43,976.61) | –1109.74 (–4755.32 to 2535.84) | 0.0818 (0.0516 to 0.1119) | 0.0765 (0.0477 to 0.1053) | –0.0053 (–0.047 to 0.0364) | 209,054.08 | 36.3 | 71.4 | 68.4 | 65.9 |
| NNRD data set | |||||||||||
| Colonisation (yes) | 59,017.58 (55,765.98 to 62,269.17) | 53,138.61 (47,903.19 to 58,374.03) | 5878.97 (–284.03 to 12,041.96) | 0.1408 (0.1098 to 0.1718) | 0.1449 (0.0977 to 0.192) | 0.0041 (–0.0524 to 0.0605) | 1,443,377.1 | 52.2 | 2.9 | 4.7 | 5.7 |
| Colonisation (no) | 81,452.58 (72,626.84 to 90,278.33) | 67,767.97 (62,941.77 to 72,594.18) | 13,684.61 (3625.48 to 23,743.74) | 0.4024 (0.2963 to 0.5086) | 0.2011 (0.1599 to 0.2423) | –0.2013 (–0.3152 to –0.0875) | –67,968.65 | 0.0 | 0.7 | 0.2 | 0.2 |
| Gestational age < 28 years | 81,834.95 (77,038.62 to 86,631.28) | 81,626.86 (75,675.75 to 87,577.97) | 208.08 (–7435.24 to 7851.41) | 0.3423 (0.2884 to 0.3962) | 0.3651 (0.311 to 0.4193) | 0.0228 (–0.0535 to 0.0992) | 9106.6827 | 73.9 | 48.4 | 51.5 | 55.0 |
| Gestational age ≥ 28 years | 41,194.96 (38,659.84 to 43,730.09) | 42,334.79 (39,428.07 to 45,241.5) | –1139.82 (–4996.75 to 2717.1) | 0.0963 (0.0641 to 0.1285) | 0.0838 (0.0541 to 0.1136) | –0.0124 (–0.0563 to 0.0314) | 91,618.665 | 26.7 | 70.1 | 65.0 | 61.8 |
| Randomisation age ≤ 24 hours | 59,180.96 (53,370.47 to 64,991.46) | 58,124.43 (51,774.27 to 64,474.58) | 1056.54 (–7550.8 to 9663.88) | 0.175 (0.1161 to 0.2339) | 0.2275 (0.164 to 0.2911) | 0.0525 (–0.0341 to 0.1392) | 20,107.313 | 90.8 | 38.7 | 46.6 | 51.7 |
| Randomisation age > 24 hours | 61,266.6 (57,611.07 to 64,922.13) | 62,096.83 (57,835.16 to 66,358.49) | –830.22 (–6444.9 to 4784.46) | 0.2283 (0.1899 to 0.2666) | 0.2144 (0.1774 to 0.2515) | –0.0138 (–0.0672 to 0.0395) | 60,058.773 | 31.3 | 60.3 | 57.3 | 55.5 |
| Sex (male) | 63,399.49 (58,920.37 to 67,878.61) | 61,730.88 (56,698.18 to 66,763.58) | 1668.6 (–5068.65 to 8405.86) | 0.2301 (0.1861 to 0.2741) | 0.2157 (0.173 to 0.2584) | –0.0144 (–0.0757 to 0.0468) | –115,655.56 | 31.5 | 33.0 | 31.9 | 30.8 |
| Sex (female) | 57,220.04 (53,157.04 to 61,283.04) | 60,200.92 (55,254.06 to 65,147.77) | –2980.88 (–9382.39 to 3420.63) | 0.194 (0.1467 to 0.2414) | 0.2206 (0.1722 to 0.2691) | 0.0266 (–0.0412 to 0.0944) | –112,017.9 | 79.9 | 81.4 | 84.4 | 85.1 |
| Weight < 1000 g (yes) | 83,083.85 (78,193.05 to 87,974.64) | 80,400.47 (74,646.03 to 86,154.91) | 2683.38 (–4868.67 to 10,235.43) | 0.3543 (0.3004 to 0.4083) | 0.3666 (0.313 to 0.4201) | 0.0123 (–0.0638 to 0.0883) | 218,964.77 | 63.6 | 25.9 | 28.5 | 30.7 |
| Weight < 1000 g (no) | 39,497.7 (37,562.52 to 41,432.88) | 42,660.06 (39,475.43 to 45,844.69) | –3162.35 (–6888.86 to 564.15) | 0.0818 (0.0516 to 0.1119) | 0.0765 (0.0477 to 0.1053) | –0.0053 (–0.047 to 0.0364) | 595,725.68 | 42.7 | 95.5 | 93.9 | 92.2 |
| Combined data set | |||||||||||
| Colonisation (yes) | 59,082.53 (55,813.78 to 62,351.27) | 53,064.15 (47,856.46 to 58,271.83) | 6018.38 (–130.17 to 12,166.93) | 0.1408 (0.1098 to 0.1718) | 0.1449 (0.0977 to 0.192) | 0.0041 (–0.0524 to 0.0605) | 1,477,605.1 | 52.2 | 2.5 | 4.1 | 5.3 |
| Colonisation (no) | 82,009.26 (73,172.95 to 90,845.58) | 67,938.27 (63,094.89 to 72,781.64) | 14,071 (3994.36 to 24,147.64) | 0.4024 (0.2963 to 0.5086) | 0.2011 (0.1599 to 0.2423) | –0.2013 (–0.3152 to –0.0875) | –69,887.747 | 0.0 | 0.6 | 0.2 | 0.1 |
| Gestational age < 28 years | 82,110.69 (77,277.46 to 86,943.93) | 81,796.8 (75,847.03 to 87,746.58) | 313.89 (–7351.61 to 7979.4) | 0.3423 (0.2884 to 0.3962) | 0.3651 (0.311 to 0.4193) | 0.0228 (–0.0535 to 0.0992) | 13,737.319 | 73.9 | 46.9 | 51.1 | 53.8 |
| Gestational age ≥ 28 years | 41,205.5 (38,669.57 to 43,741.43) | 42,375.67 (39,459.53 to 45,291.81) | –1170.17 (–5034.72 to 2694.39) | 0.0963 (0.0641 to 0.1285) | 0.0838 (0.0541 to 0.1136) | –0.0124 (–0.0563 to 0.0314) | 94,057.689 | 26.7 | 71.0 | 65.2 | 61.6 |
| Randomisation age ≤ 24 hours | 59,476.77 (53,591.8 to 65,361.74) | 58,194.19 (51,854.17 to 64,534.22) | 1282.58 (–7367.79 to 9932.94) | 0.175 (0.1161 to 0.2339) | 0.2275 (0.164 to 0.2911) | 0.0525 (–0.0341 to 0.1392) | 24,409.129 | 90.8 | 37.0 | 45.4 | 49.9 |
| Randomisation age > 24 hours | 61,349.73 (57,679.36 to 65,020.1) | 62,210.76 (57,941.56 to 66,479.97) | –861.04 (–6491.11 to 4769.04) | 0.2283 (0.1899 to 0.2666) | 0.2144 (0.1774 to 0.2515) | –0.0138 (–0.0672 to 0.0395) | 62,287.872 | 31.3 | 60.6 | 58.0 | 56.1 |
| Sex (male) | 63,431.42 (58,926.17 to 67,936.66) | 61,714.56 (56,686.93 to 66,742.19) | 1716.86 (–5034.01 to 8467.73) | 0.2301 (0.1861 to 0.2741) | 0.2157 (0.173 to 0.2584) | –0.0144 (–0.0757 to 0.0468) | –119,000.26 | 31.5 | 32.4 | 31.0 | 30.3 |
| Sex (female) | 57,497.39 (53,404.44 to 61,590.33) | 60,454.11 (55,484.46 to 65,423.76) | –2956.72 (–9394.86 to 3481.42) | 0.194 (0.1467 to 0.2414) | 0.2206 (0.1722 to 0.2691) | 0.0266 (–0.0412 to 0.0944) | –111,110.12 | 79.9 | 80.6 | 83.5 | 84.7 |
| Weight < 1000 g (yes) | 83,364.15 (78,439.38 to 88,288.91) | 80,535.46 (74,783.56 to 86,287.36) | 2828.68 (–4743.48 to 10,400.85) | 0.3543 (0.3004 to 0.4083) | 0.3666 (0.313 to 0.4201) | 0.0123 (–0.0638 to 0.0883) | 230,821.67 | 63.6 | 24.7 | 27.9 | 29.3 |
| Weight < 1000 g (no) | 39,500.58 (37,565.42 to 41,435.75) | 42,731.41 (39,531.03 to 45,931.79) | –3230.83 (–6970.78 to 509.13) | 0.0818 (0.0516 to 0.1119) | 0.0765 (0.0477 to 0.1053) | –0.0053 (–0.047 to 0.0364) | 608,624.88 | 42.7 | 95.8 | 94.4 | 92.8 |
| Comparator data sets | Outcome | Data source, mean net benefit (95% CI) | Agreement statistics | |||
|---|---|---|---|---|---|---|
| PiPS vs. NNRDa | PiPS | NNRDa | Mean difference (95% CI)b | p-valuec | % miscoveraged | |
| Death | –468 (–5574 to 4638) | 415 (–4259 to 5089) | 882 (–1118 to 2883) | 0.387 | 0.061 | |
| Sepsis | –420 (–5776 to 4937) | 463 (–4472 to 5397) | 882 (–1118 to 2883) | 0.387 | 0.064 | |
| NEC | –347 (–5673 to 4979) | 536 (–4383 to 5454) | 882 (–1118 to 2883) | 0.387 | 0.06 | |
| Composite | –448 (–5860 to 4964) | 434 (–4561 to 5429) | 882 (–1118 to 2883) | 0.387 | 0.058 | |
| Combined vs. PiPS | Outcome | Combined | PiPS | Agreement statistics | ||
| Mean difference (95% CI)b | p-valuec | % miscoveraged | ||||
| Death | 390 (–4269 to 5048) | –468 (–5574 to 4638) | –857 (–2858 to 1144) | 0.401 | 0.047 | |
| Sepsis | 438 (–4481 to 5357) | –420 (–5776 to 4937) | –857 (–2858 to 1144) | 0.401 | 0.044 | |
| NEC | 510 (–4393 to 5414) | –347 (–5673 to 4979) | –857 (–2858 to 1144) | 0.401 | 0.049 | |
| Composite | 409 (–4571 to 5389) | –448 (–5860 to 4964) | –857 (–2858 to 1144) | 0.401 | 0.049 | |
| Combined vs. NNRDa | Outcome | Combined | NNRDa | Agreement statistics | ||
| Mean difference (95% CI)b | p-valuec | % miscoveraged | ||||
| Death | 390 (–4269 to 5048) | 415 (–4259 to 5089) | 25 (–1976 to 1144) | 0.401 | 0.039 | |
| Sepsis | 438 (–4481 to 5357) | 463 (–4472 to 5397) | 25 (–1976 to 1144) | 0.401 | 0.048 | |
| NEC | 510 (–4393 to 5414) | 536 (–4383 to 5454) | 25 (–1976 to 1144) | 0.401 | 0.045 | |
| Composite | 409 (–4571 to 5389) | 434 (–4561 to 5429) | 25 (–1976 to 1144) | 0.401 | 0.049 | |
| PiPS vs. NNRDe | Outcome | PiPS | NNRDe | Agreement statistics | ||
| Mean difference (95% CI)b | p-valuec | % miscoveraged | ||||
| Death | –468 (–5574 to 4638) | 415 (–4259 to 5089) | 882 (–1118 to 2883) | 0.387 | 0.061 | |
| Sepsis | –420 (–5776 to 4937) | 301 (–4542 to 5145) | 721 (–1382 to 2824) | 0.502 | 0.056 | |
| Comparator data sets | Outcome | Data source, mean net benefit (95% CI) | Agreement statistics | |||
|---|---|---|---|---|---|---|
| PiPS vs. NNRDa | PiPS | NNRDa | Mean difference (95% CI)b | p-valuec | % miscoveraged | |
| Death | –444 (–5571 to 4684) | 439 (–4257 to 5134) | 882 (–1118 to 2883) | 0.387 | 0.057 | |
| Sepsis | –372 (–5876 to 5133) | 511 (–4577 to 5599) | 882 (–1118 to 2883) | 0.387 | 0.06 | |
| NEC | –263 (–5719 to 5194) | 620 (–4440 to 5680) | 882 (–1118 to 2883) | 0.387 | 0.059 | |
| Composite | –415 (–6024 to 5194) | 468 (–4733 to 5668) | 882 (–1118 to 2883) | 0.387 | 0.056 | |
| Combined vs. PiPS | Outcome | Combined | PiPS | Agreement statistics | ||
| Mean difference (95% CI)b | p-valuec | % miscoveraged | ||||
| Death | 413 (–4267 to 5094) | –444 (–5571 to 4684) | –857 (–2858 to 1144) | 0.401 | 0.049 | |
| Sepsis | 486 (–4587 to 5558) | –372 (–5876 to 5133) | –857 (–2858 to 1144) | 0.401 | 0.049 | |
| NEC | 595 (–4451 to 5640) | –263 (–5719 to 5194) | –857 (–2858 to 1144) | 0.401 | 0.048 | |
| Composite | 443 (–4744 to 5629) | –415 (–6024 to 5194) | –857 (–2858 to 1144) | 0.401 | 0.049 | |
| Combined vs. NNRDa | Outcome | Combined | NNRDa | Agreement statistics | ||
| Mean difference (95% CI)b | p-valuec | % miscoveraged | ||||
| Death | 413 (–4267 to 5094) | 439 (–4257 to 5134) | 25 (–1976 to 1144) | 0.401 | 0.043 | |
| Sepsis | 486 (–4587 to 5558) | 511 (–4577 to 5599) | 25 (–1976 to 1144) | 0.401 | 0.046 | |
| NEC | 595 (–4451 to 5640) | 620 (–4440 to 5680) | 25 (–1976 to 1144) | 0.401 | 0.042 | |
| Composite | 443 (–4744 to 5629) | 468 (–4733 to 5668) | 25 (–1976 to 1144) | 0.401 | 0.052 | |
| PiPS vs. NNRDe | Outcome | Combined | NNRDa | Agreement statistics | ||
| Mean difference (95% CI)b | p-valuec | % miscoveraged | ||||
| Death | –444 (–5571 to 4684) | 439 (–4257 to 5134) | 882 (–1118 to 2883) | 0.387 | 0.057 | |
| Sepsis | –372 (–5876 to 5133) | 268 (–4671 to 5207) | 640 (–1573 to 2853) | 0.571 | 0.051 | |
| NNRD clinical outcome | Mean costs (£) (95% CI) | Mean effects (95% CI) | ICER (£) | Probability (%) B. breve BBG is | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. breve BBG | Placebo | Difference | B. breve BBG | Placebo | Differencea | More effectiveb | Less costlyb | Cost-effectiveb,c | Cost-effectiveb,d | ||
| Deathe | 60,559.76 (57,480.38 to 63,639.13) | 60,926.82 (57,388.7 to 64,464.94) | –367.07 (–5057.57 to 4323.44) | 0.0823 (0.0606 to 0.1039) | 0.0846 (0.063 to 0.1062) | 0.0024 (–0.0282 to 0.0329) | –154,137.2 (SE) | 60.8 | 57.8 | 59.4 | 59.9 |
| Sepsisf | 60,559.76 (57,480.38 to 63,639.13) | 60,926.82 (57,388.7 to 64,464.94) | –367.07 (–5057.57 to 4323.44) | 0.0613 (0.0424 to 0.0802) | 0.058 (0.0399 to 0.0761) | –0.0033 (–0.0295 to 0.0229) | 111,347.5 (SW) | 39.3 | 57.8 | 56.9 | 56.1 |
Chapter 8
| Mothers’ ethnicity | Participating mothers, n (%) (n = 930) | NNRD, n (%) | |
|---|---|---|---|
| 28 sites (n = 10,983) | All neonatal units (n = 55,731) | ||
| Do not wish to answer | 19 (2%) | – | 1 (0.0%) |
| White: British | 757 (81.4%) | 6202 (56.5%) | 36,409 (65.3%) |
| White: Irish | 6 (0.6%) | 38 (0.3%) | 253 (0.5%) |
| White: Gypsy or Irish traveller | 3 (0.3%) | 0 (0%) | 0 (0%) |
| White: any other white background | 29 (3.1%) | 532 (4.8%) | 4164 (7.5%) |
| White and black Caribbean | 15 (1.6%) | 43 (0.4%) | 246 (0.4%) |
| White and black African | 2 (0.2%) | 30 (0.3%) | 101 (0.2%) |
| White and Asian | 6 (0.6%) | 34 (0.3%) | 129 (0.2%) |
| Any other mixed background | 3 (0.3%) | 33 (0.3%) | 209 (0.4%) |
| Asian/Asian British: Indian | 18 (1.9%) | 247 (2.2%) | 1978 (3.5%) |
| Asian/Asian British: Pakistani | 30 (3.2%) | 857 (7.8%) | 2337 (4.2%) |
| Asian/Asian British: Bangladeshi | 7 (0.8%) | 293 (2.7%) | 821 (1.5%) |
| Asian/Asian British: Chinese | 1 (0.1%) | 46 (0.4%) | 249 (0.4%) |
| Any other Asian background | 5 (0.5%) | 184 (1.7%) | 1317 (2.4%) |
| Black: African | 7 (0.8%) | 308 (2.8%) | 2157 (3.9%) |
| Black: Caribbean | 13 (1.4%) | 71 (0.6%) | 749 (1.3%) |
| Any other black background | 1 (0.1%) | 53 (0.5%) | 340 (0.6%) |
| Arab | 2 (0.2%) | – | – |
| Any other | 2 (0.2%) | 158 (1.4%) | 816 (1.5%) |
| Not stated | – | 1479 (13.5%) | 2120 (3.8%) |
| Missing data | 4 (0.4%) | 375 (3.4%) | 1335 (2.4%) |
| Total | 930 | 10,983 | 55,731 |
| Highest level of qualification | Willingness, n (%) | Total, n | ||
|---|---|---|---|---|
| Yes | Possibly | No | ||
| O levels/GCSEs | 191 (80.6%) | 34 (14.3%) | 12 (5.1%) | 237 |
| A levels/vocational qualification | 319 (81%) | 54 (13.7%) | 21 (5.3%) | 394 |
| Degree/higher degree | 331 (87.3%) | 39 (10.3%) | 9 (2.4%) | 379 |
| Total | 841 (83.3%) | 127 (12.6%) | 42 (4.2%) | 1010 |
| Highest level of qualification | Awareness, n (%) | Total, n | |
|---|---|---|---|
| Yes | No | ||
| O levels/GCSEs | 77 (33.8%) | 151 (66.2%) | 228 |
| A levels/vocational qualification | 185 (47.6%) | 204 (52.4%) | 389 |
| Degree/higher degree | 281 (76.6%) | 86 (23.4%) | 367 |
| Total | 543 (55.2%) | 441 (44.8%) | 984 |
| Group | Willingness, n (%) | Total, n | ||
|---|---|---|---|---|
| Yes | Possibly | No | ||
| More than one child | 496 (76.7%) | 118 (18.2%) | 33 (5.1%) | 647 |
| Only one child | 395 (68.8%) | 135 (23.7%) | 43 (7.5%) | 570 |
| Total | 888 (73%) | 253 (20.8%) | 76 (6.2%) | 1217 |
| Group | Willingness, n (%) | Total, n | ||
|---|---|---|---|---|
| Yes | Possibly | No | ||
| More than one child | 555 (85.1%) | 71 (10.9%) | 26 (4%) | 652 |
| Only one child | 463 (80.2%) | 85 (14.7%) | 29 (5%) | 577 |
| Total | 1018 (82.8%) | 156 (12.7%) | 55 (4.5%) | 1229 |
| Highest level of qualification | Acceptability, n (%) | Total, n | ||
|---|---|---|---|---|
| Yes | Possibly | No | ||
| O levels/GCSEs | 154 (66.4%) | 46 (19.8%) | 32 (13.8%) | 232 |
| A levels/vocational qualification | 268 (70%) | 51 (13.3%) | 64 (16.7%) | 383 |
| Degree/higher degree | 217 (59.3%) | 57 (15.6%) | 92 (25.1%) | 366 |
| Total | 639 (65.1%) | 154 (15.7%) | 188 (19.2%) | 981 |
| Level of carea | Expressed preference, n (%) | Total, n | |
|---|---|---|---|
| In person | In writing | ||
| 1 | 88 (28.9%) | 216 (71.1%) | 304 |
| 2 | 54 (34.6%) | 102 (65.4%) | 156 |
| 3 | 175 (39.5%) | 268 (60.5%) | 443 |
| Total | 317 (35.1%) | 586 (64.9%) | 903 |
| Highest level of qualification | Willingness, n (%) | Total, n | ||
|---|---|---|---|---|
| Yes | Possibly | No | ||
| O levels/GCSEs | 143 (62.2%) | 58 (25.2%) | 29 (12.6%) | 230 |
| A levels/vocational qualification | 215 (56%) | 102 (26.6%) | 67 (17.4%) | 384 |
| Degree/higher degree | 190 (53.1%) | 97 (27.1%) | 71 (19.8%) | 358 |
| Total | 548 (56.4%) | 257 (26.4%) | 167 (17.2%) | 972 |
Appendix 2 Supplementary figures
Chapter 3
FIGURE 28.
Isosurv plots for survival prediction: survival probability and birthweight centiles (singleton birth girls, antenatal steroids not received).

FIGURE 29.
Isosurv plots for survival prediction: survival probability and birthweight centiles (singleton birth girls, antenatal steroids received).
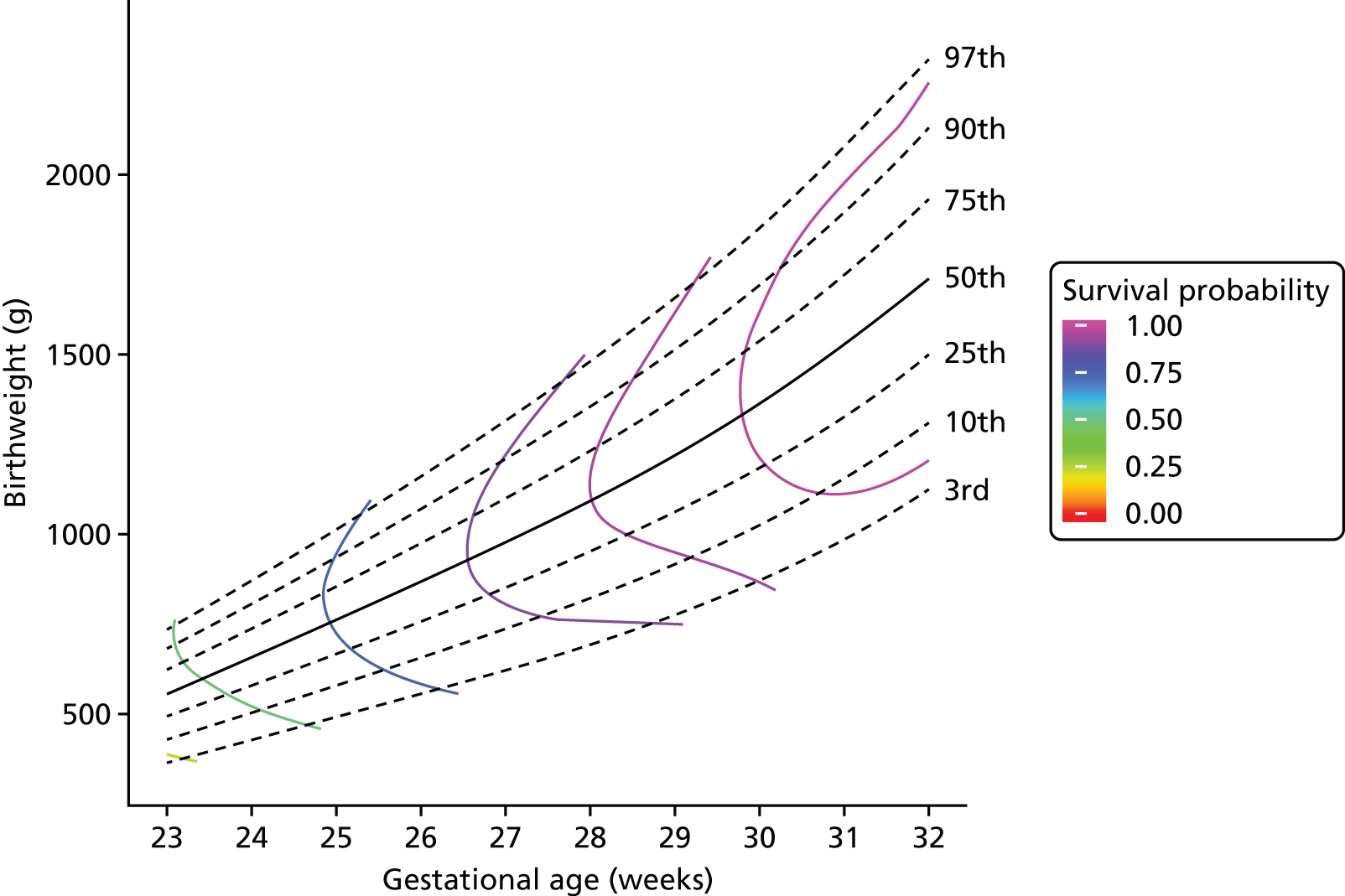
FIGURE 30.
Isosurv plots for survival prediction: survival probability and birthweight centiles (multiple birth girls, antenatal steroids not received).
FIGURE 31.
Isosurv plots for survival prediction: survival probability and birthweight centiles (multiple birth girls, antenatal steroids received).
FIGURE 32.
Isosurv plots for survival prediction: survival probability and birthweight centiles (singleton birth boys, antenatal steroids not received).
FIGURE 33.
Isosurv plots for survival prediction: survival probability and birthweight centiles (singleton birth boys, antenatal steroids received).
FIGURE 34.
Isosurv plots for survival prediction: survival probability and birthweight centiles (multiple birth boys, antenatal steroids not received).
FIGURE 35.
Isosurv plots for survival prediction: survival probability and birthweight centiles (multiple birth boys, antenatal steroids received).
Chapter 5
FIGURE 36.
Classification of the severity of cognitive impairment of the children by research and NHS assessments.

FIGURE 37.
Classification of the severity of receptive communication, expressive communication and overall language impairment of the participants based on the Bayley-III and modified NPEU/Oxford classification by research assessment, and by NHS assessments.

FIGURE 38.
Classification of the severity of fine motor, gross motor and overall motor impairment of the participants based on the Bayley-III and modified NPEU/Oxford classification by research assessment, and by NHS assessments.
FIGURE 39.
Classification of the neurodevelopmental outcome of participants by the severity of the worst impairment in the cognitive, language and motor domains through research and NHS assessments.

FIGURE 40.
Proportions of studies with low, high or unclear risk of bias and concerns regarding applicability. Bar charts are annotated with the number of studies in each category.

FIGURE 41.
Scatterplot of the true-positive rate (sensitivity) against the false-positive rate (1 – specificity). The true-positive rate and the false-positive rate are expressed as proportions; each marker represents the result from one study. Spearman’s rho –0.76; p < 0.001.
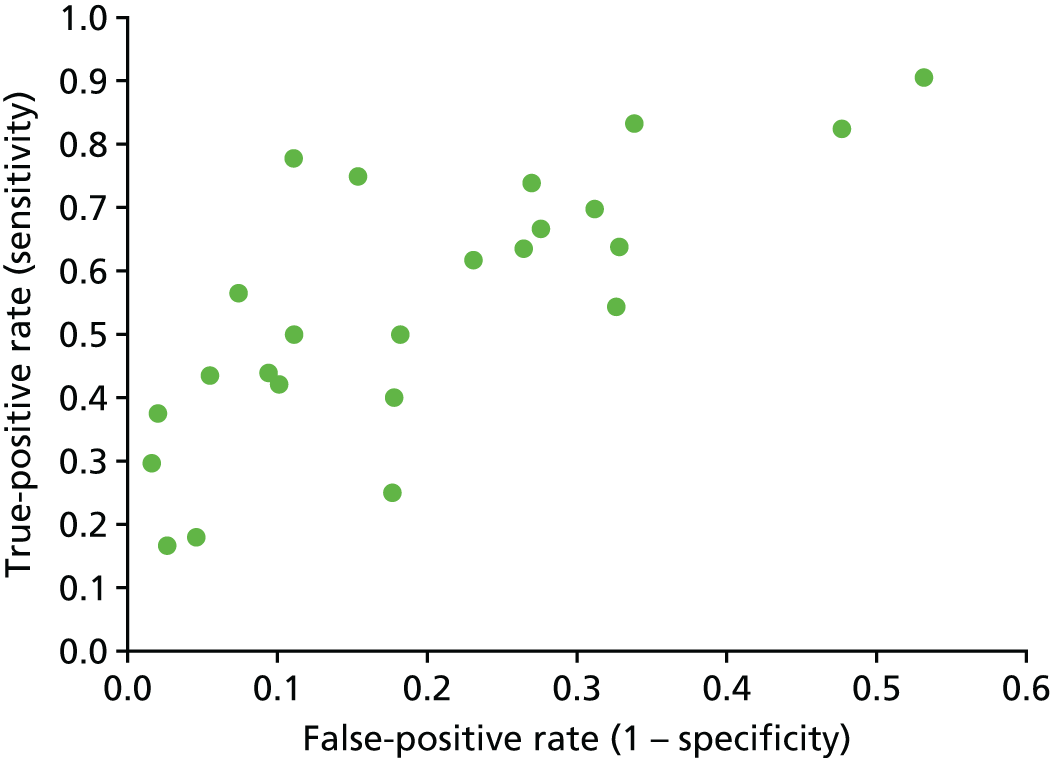
FIGURE 42.
Hierarchical summary receiver operator characteristic (HSROC) curves for the pooled sensitivity and specificity of early developmental assessment in identifying (a) any impairment and (b) severe impairment. Each marker display the study estimates from one included study and is scaled according to the sample size of the study.
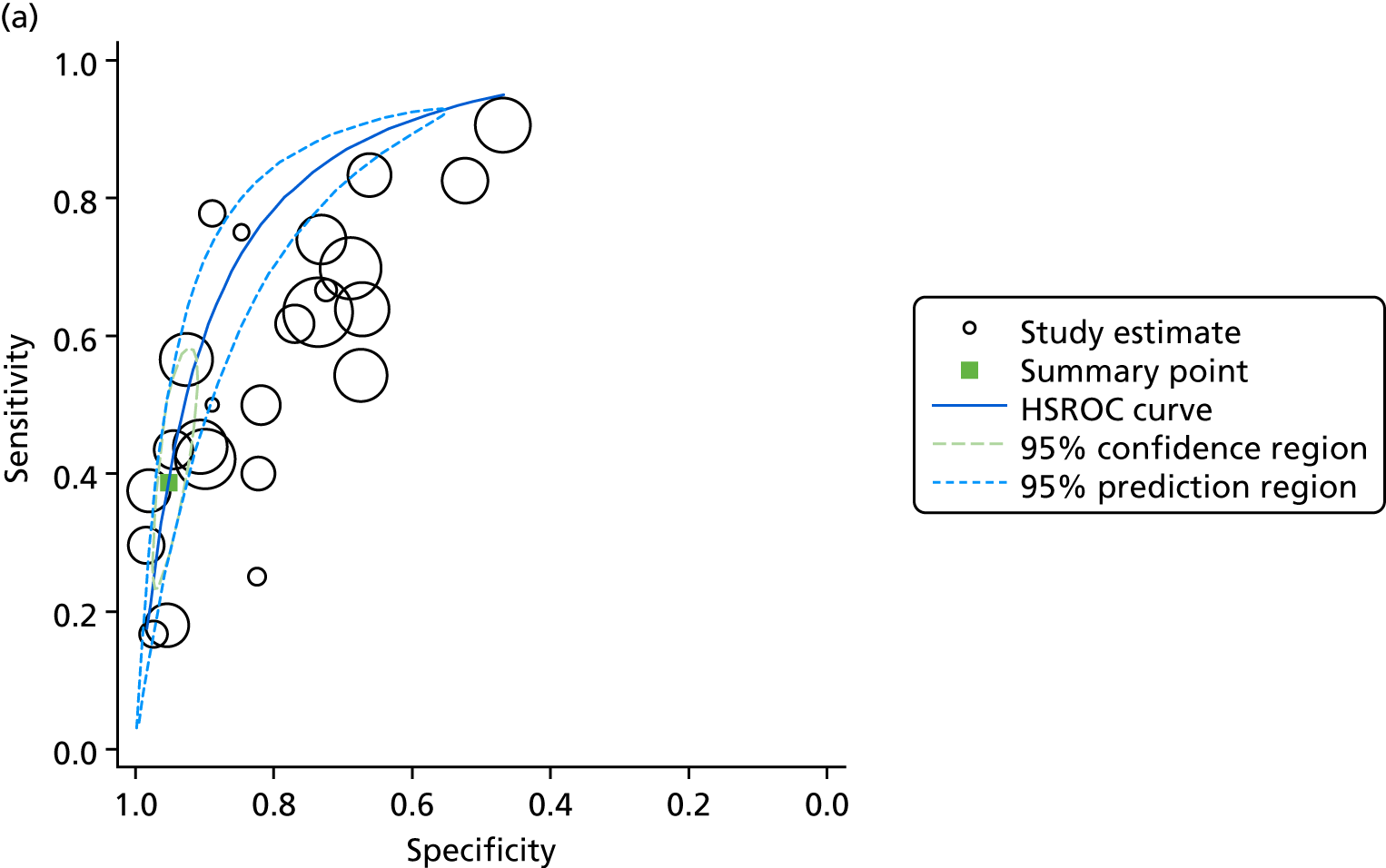
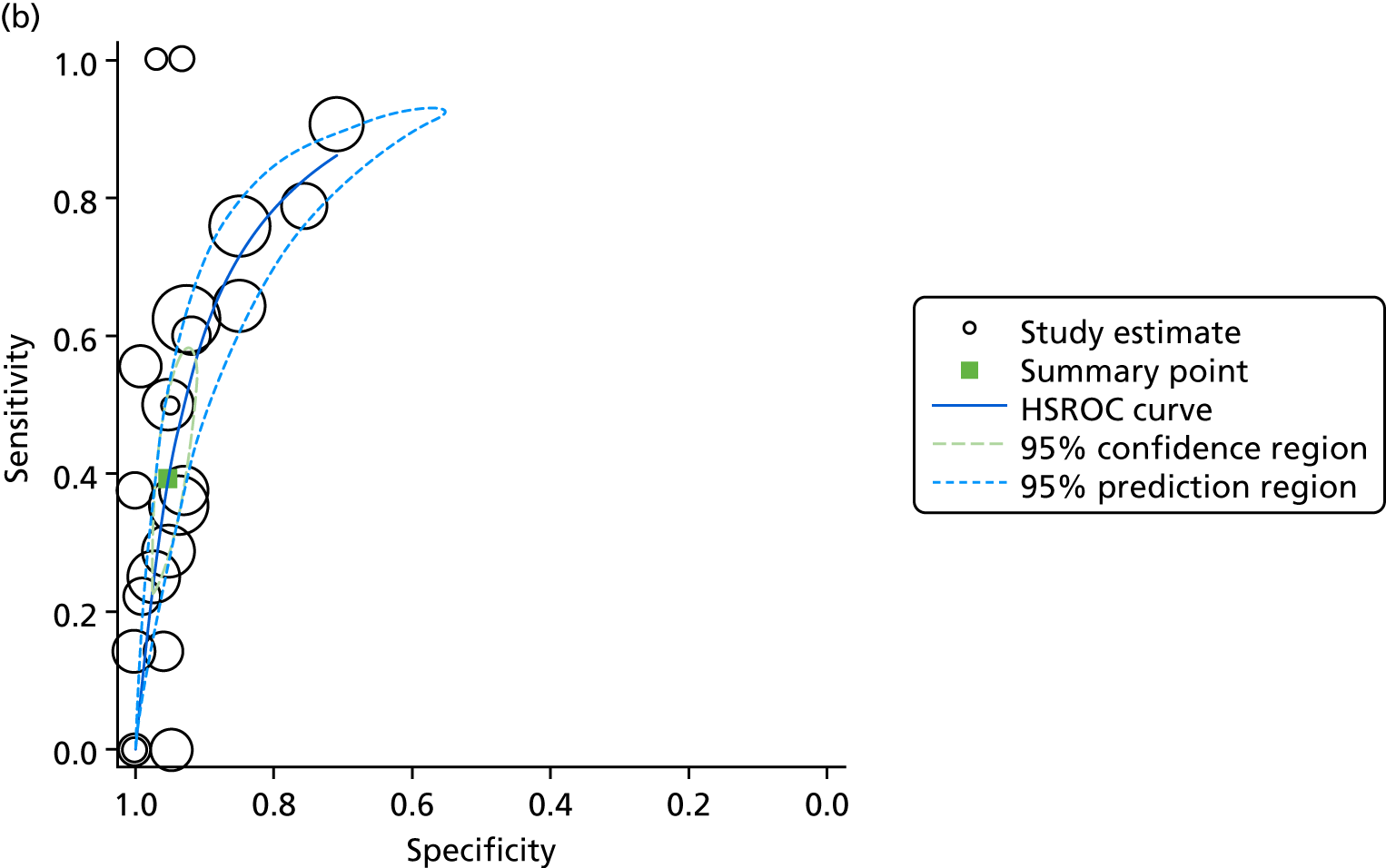
Chapter 6
FIGURE 43.
Cost-effectiveness plane: death as primary outcome – PiPS data.
FIGURE 44.
Cost-effectiveness plane: sepsis as primary outcome – PiPS data.
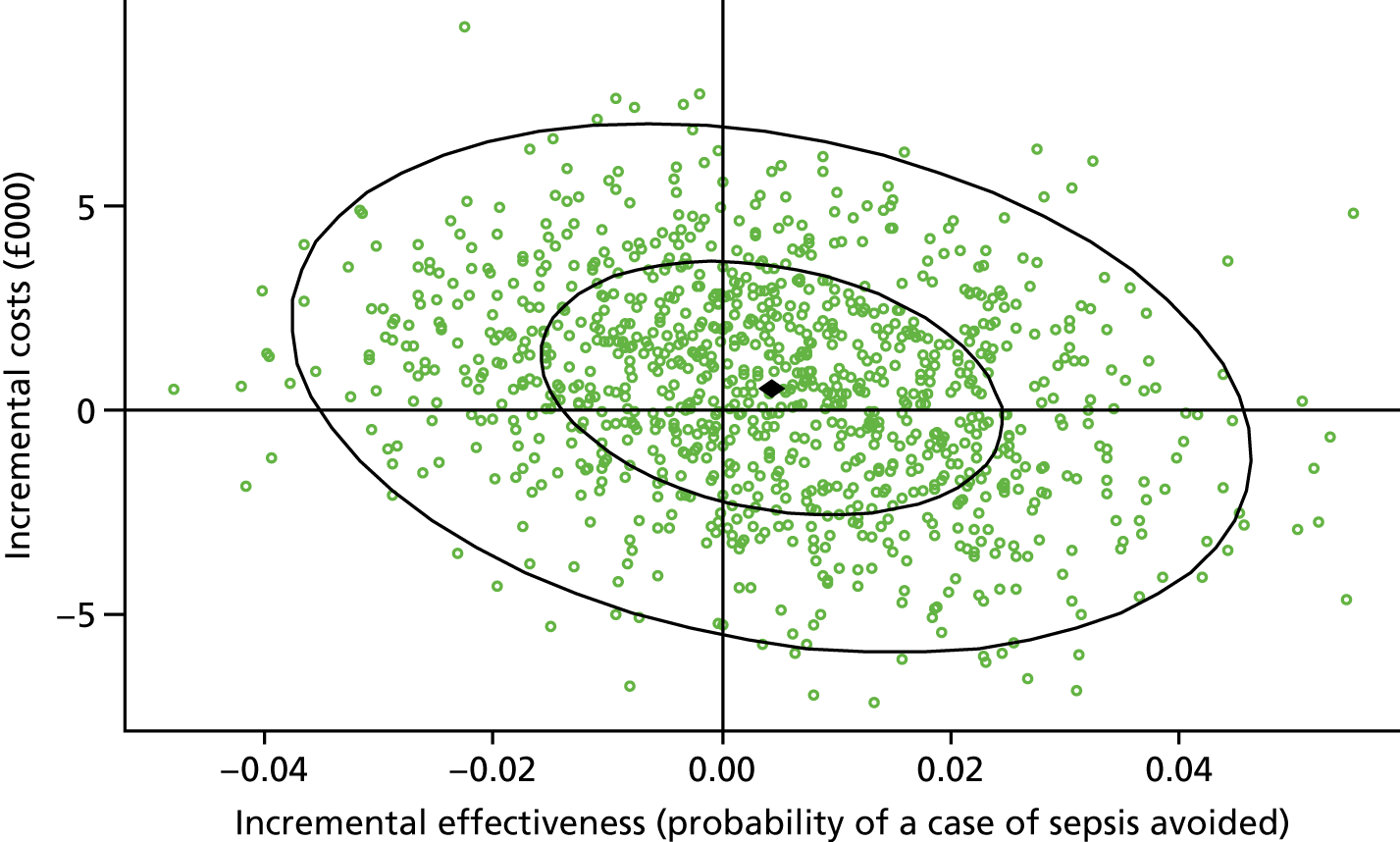
FIGURE 45.
Cost-effectiveness plane: NEC as primary outcome – PiPS data.
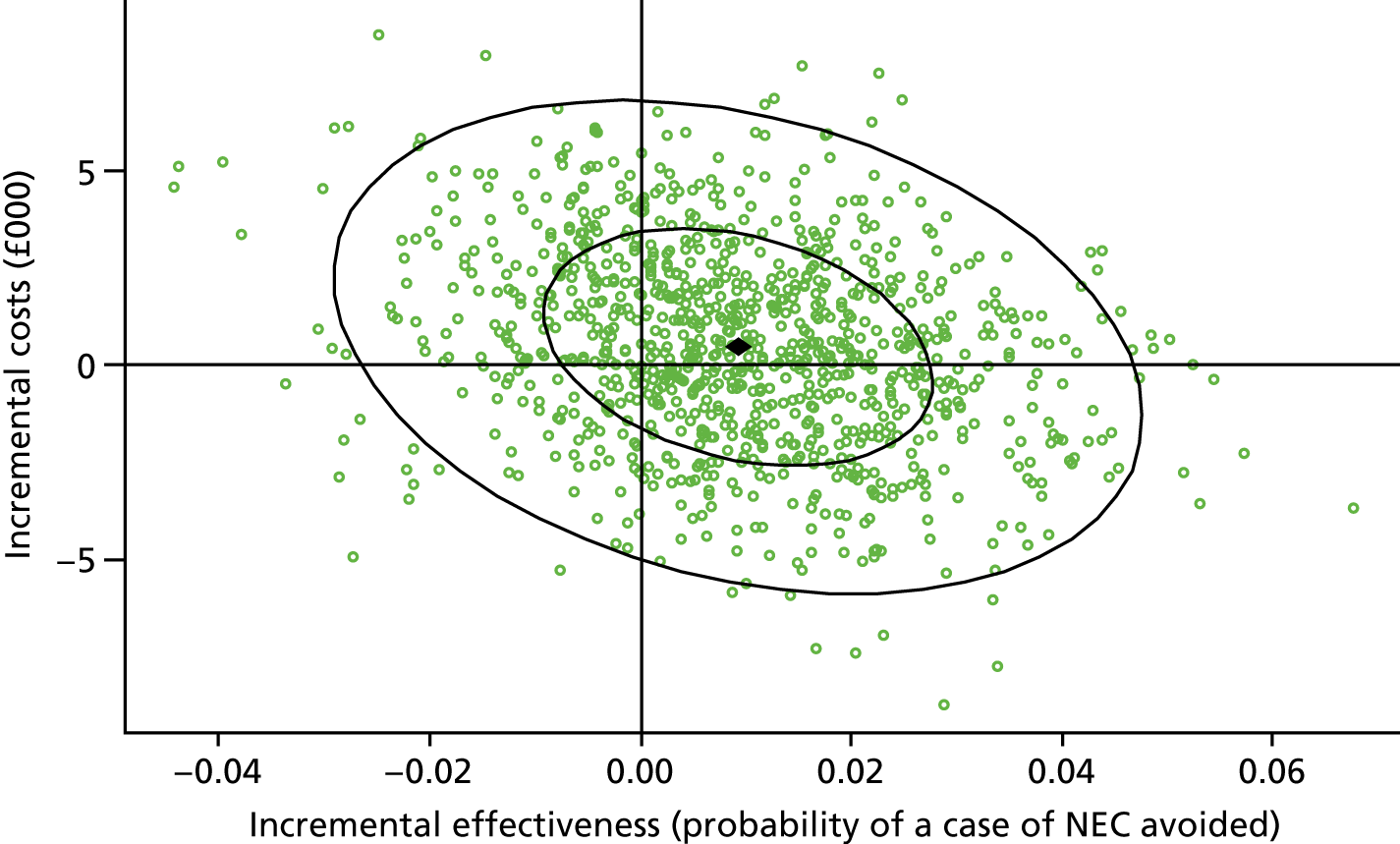
FIGURE 46.
Cost-effectiveness plane: composite secondary outcome – PiPS data.
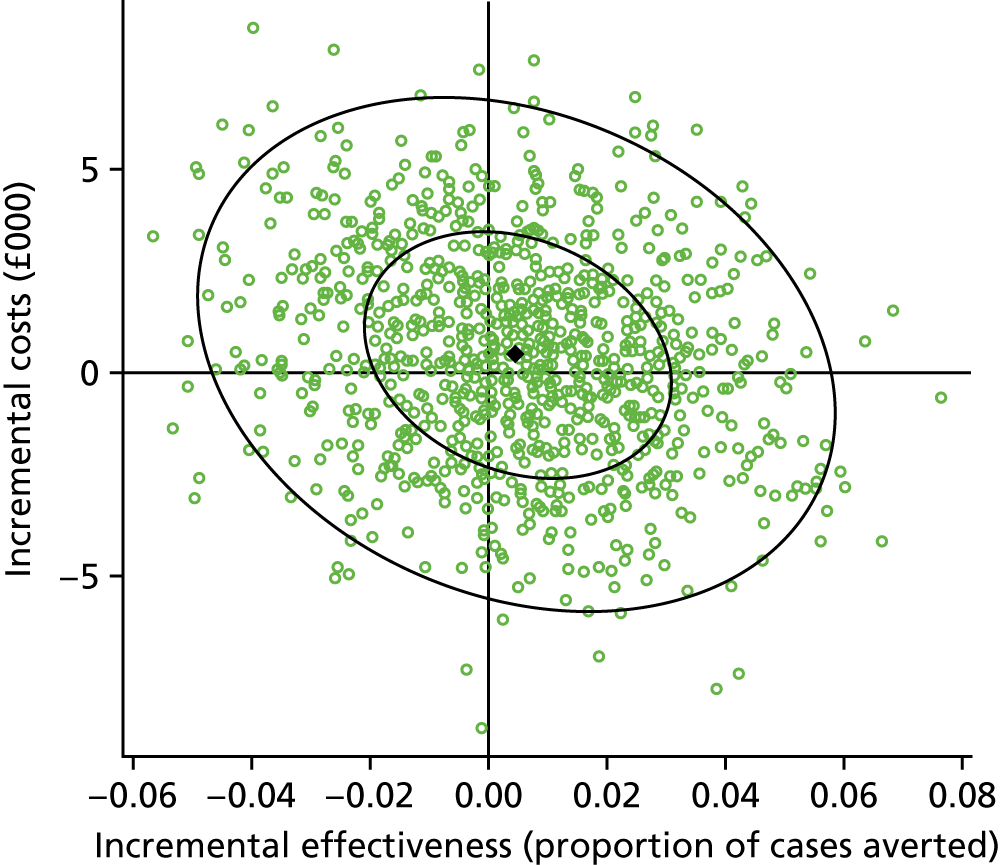
FIGURE 47.
Cost-effectiveness plane: death as primary outcome – NNRD data.

FIGURE 48.
Cost-effectiveness plane: sepsis as primary outcome – NNRD data.
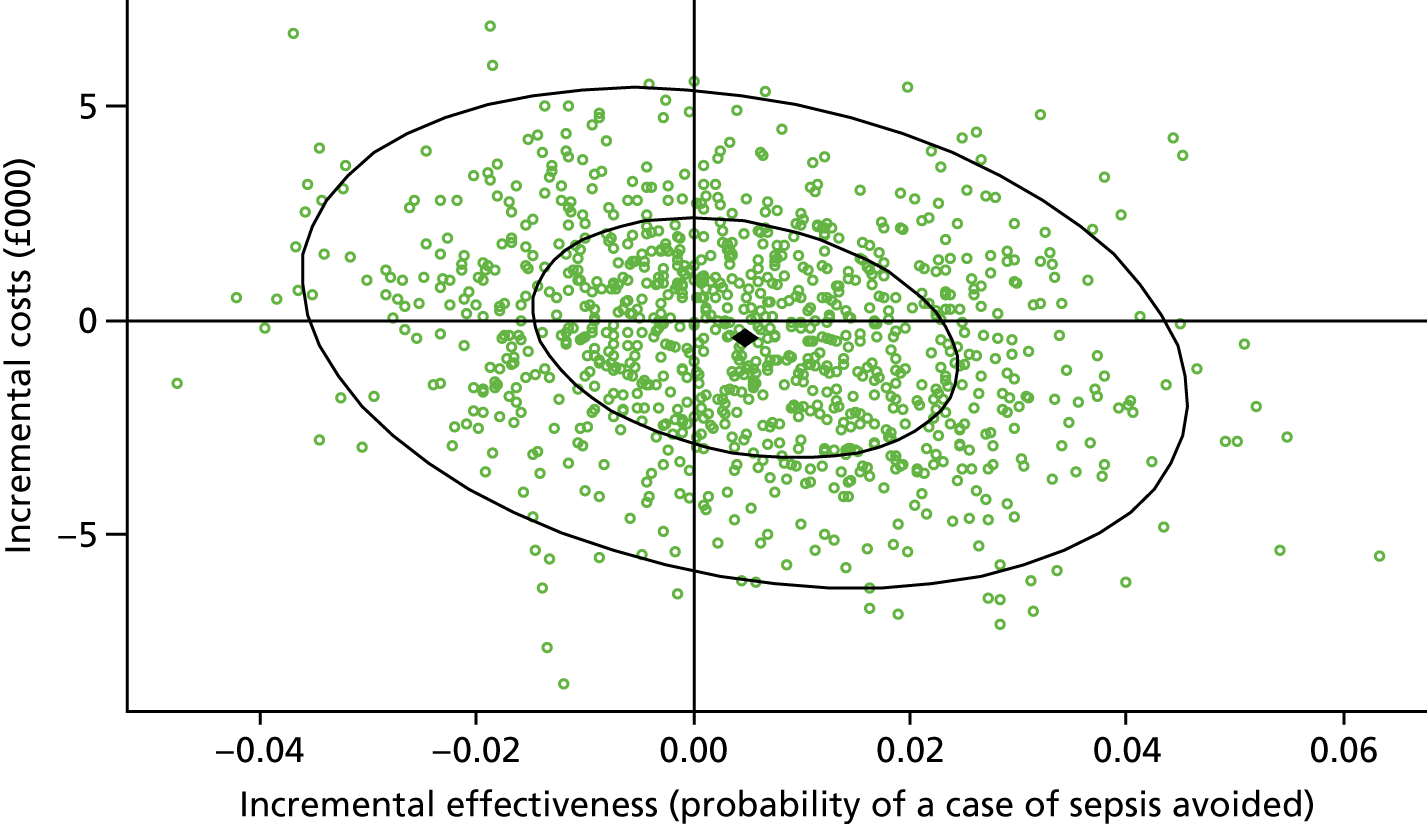
FIGURE 49.
Cost-effectiveness plane: NEC as primary outcome – NNRD data.

FIGURE 50.
Cost-effectiveness plane: composite secondary outcome – NNRD data.
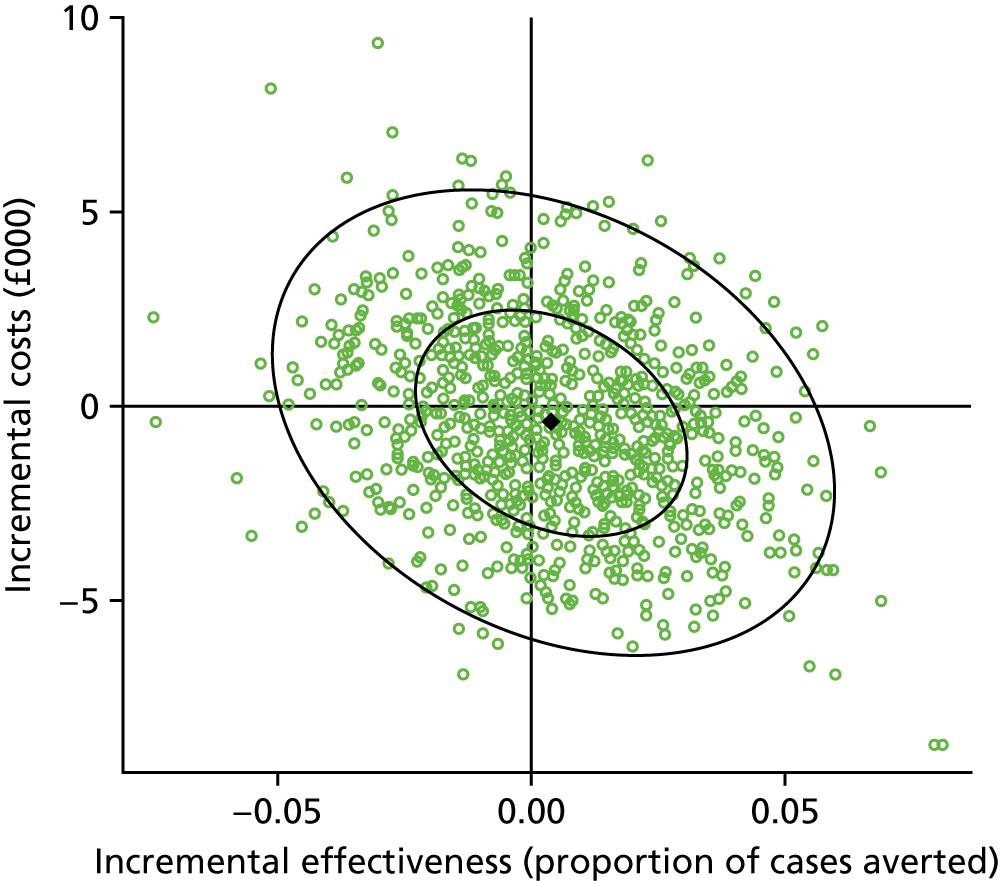
FIGURE 51.
Cost-effectiveness plane: death as primary outcome – combined data.
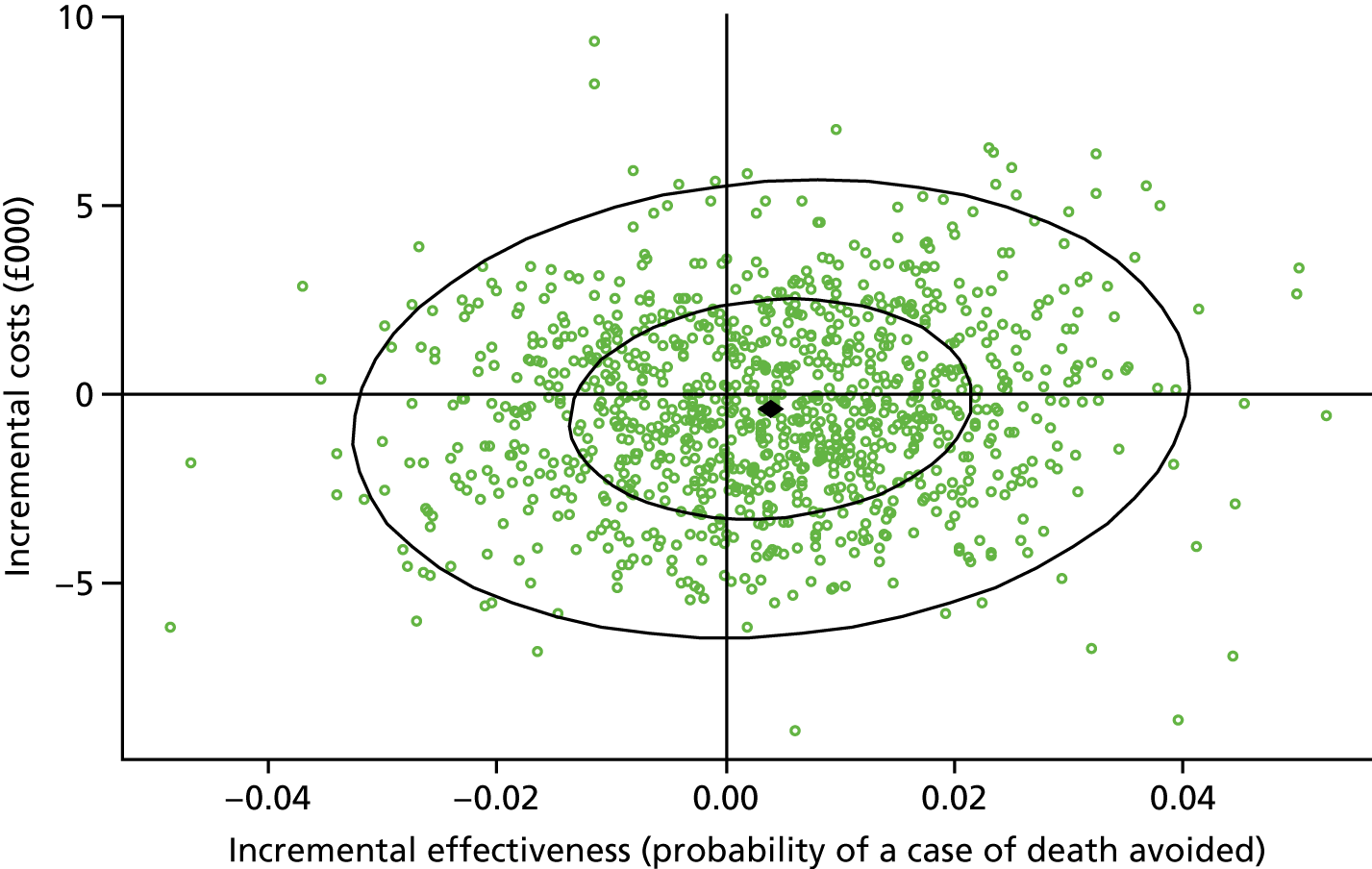
FIGURE 52.
Cost-effectiveness plane: sepsis as primary outcome – combined data.
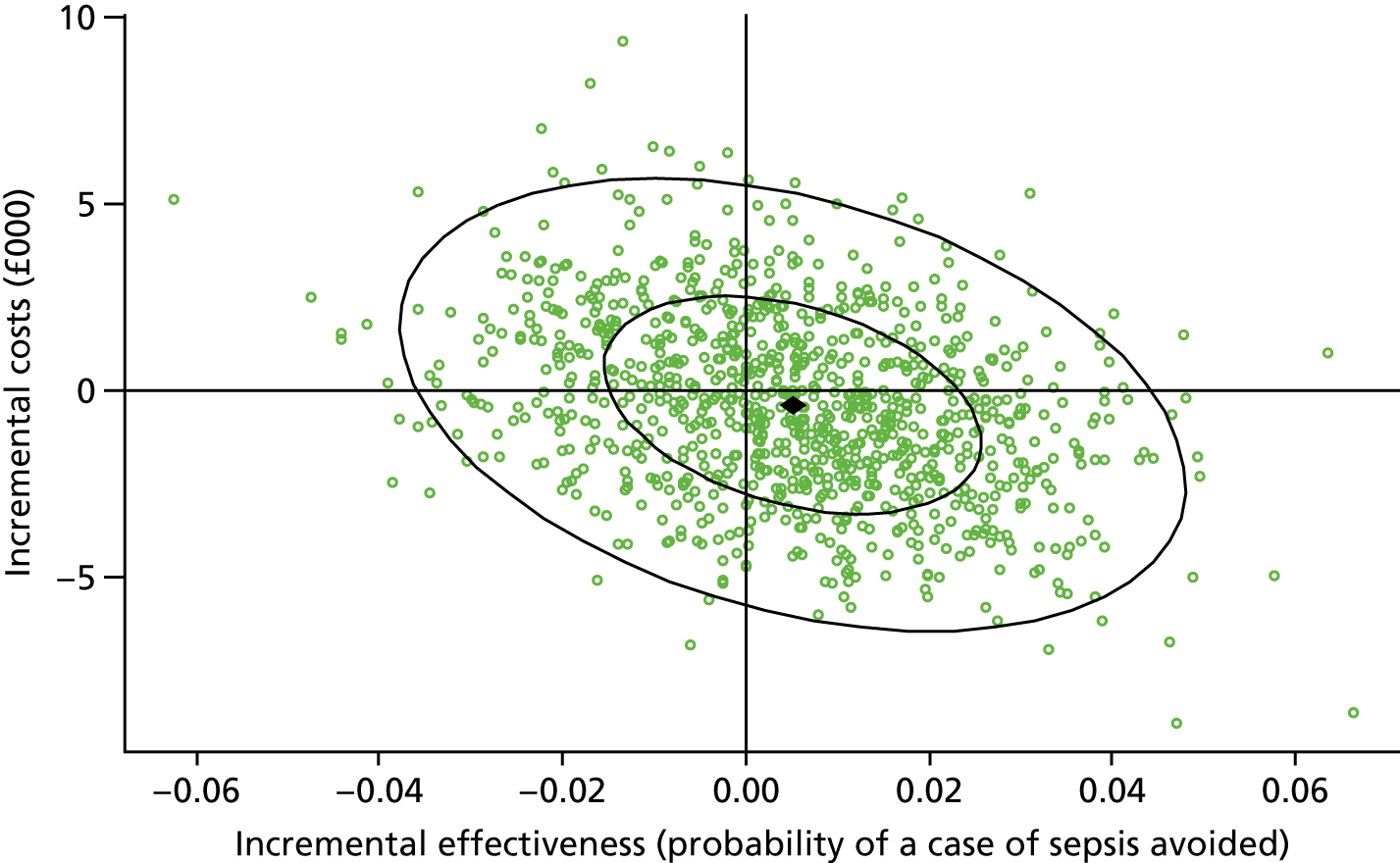
FIGURE 53.
Cost-effectiveness plane: NEC as primary outcome – combined data.
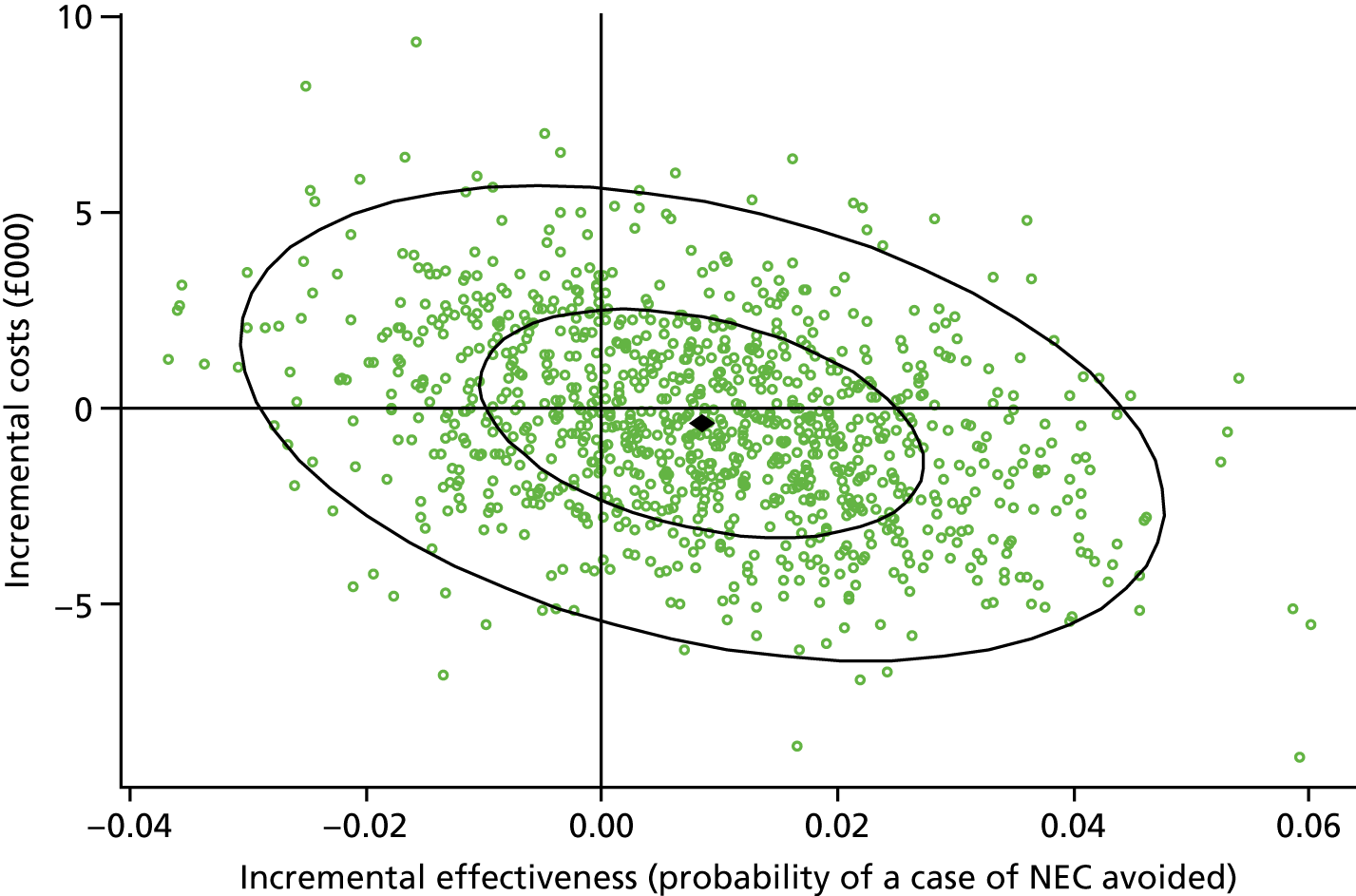
FIGURE 54.
Cost-effectiveness plane: composite secondary outcome – combined data.
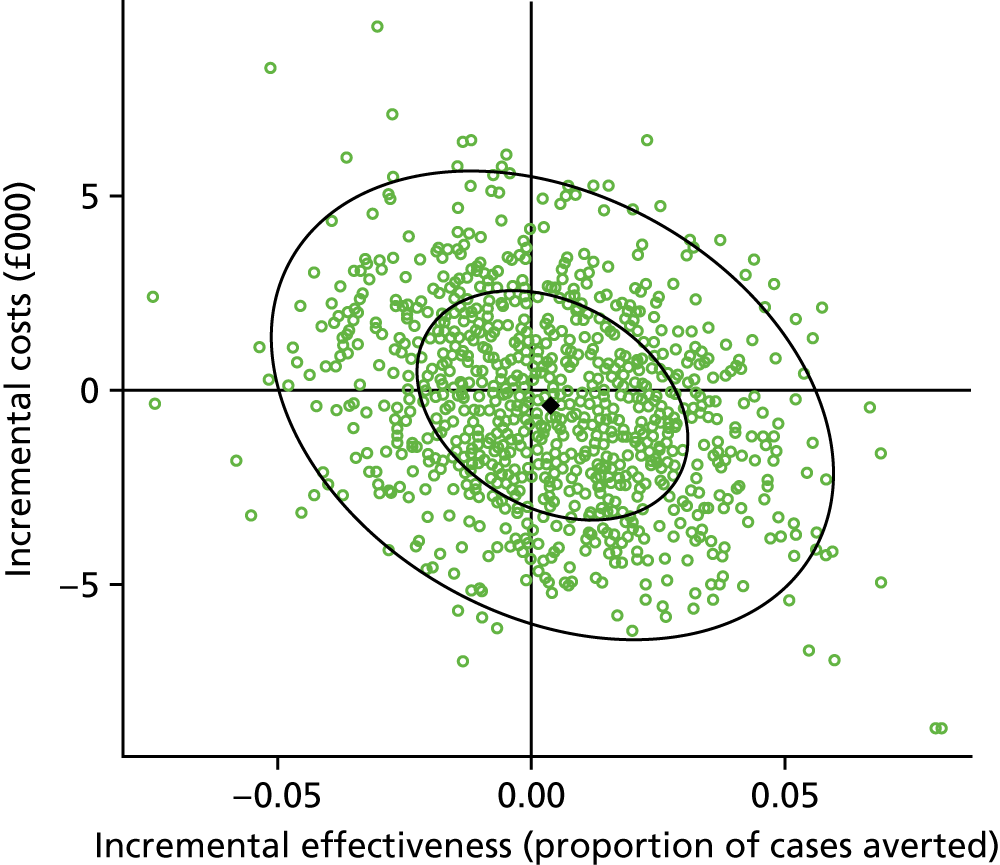
Chapter 8
FIGURE 55.
Acceptability of opt-out system by highest educational qualification. Acceptability of an opt-out system to use routinely collected health data for research purposes by highest educational qualification.
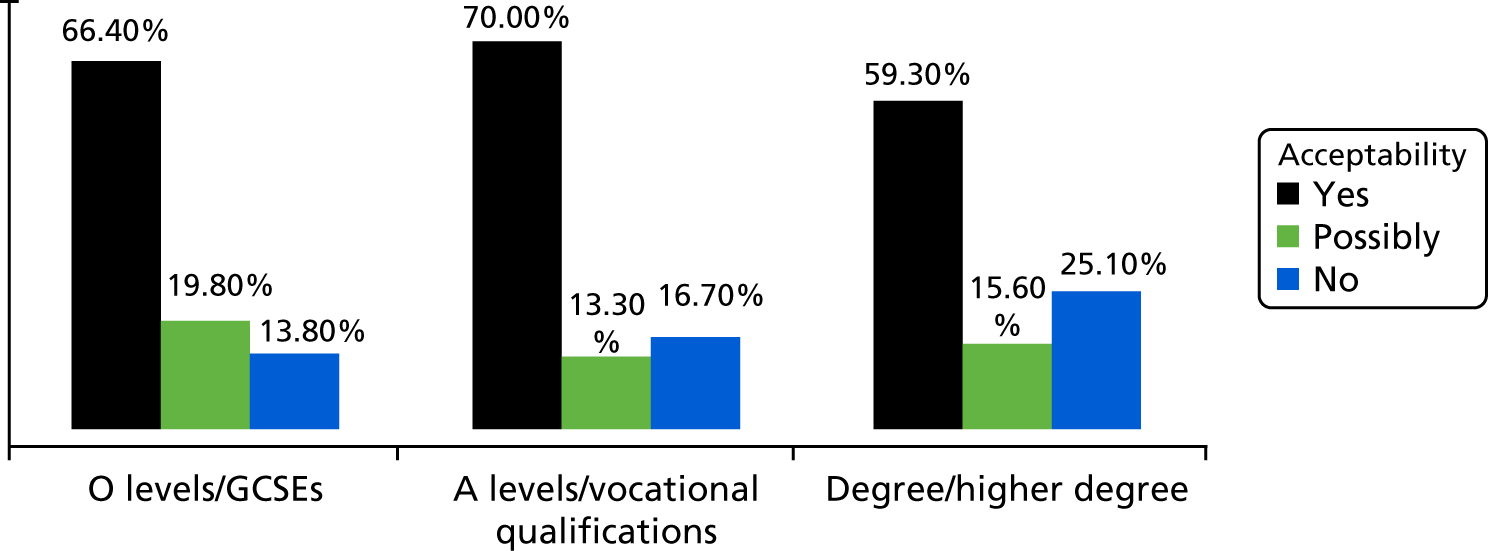
Appendix 3 Neonatal Data Set ISB1595 release 1 version 22
| Category | Category detail | Item name | Field ID | Data dictionary item status | NHS data dictionary item name | Format/length | Coding/allowed values | Description | Purpose |
|---|---|---|---|---|---|---|---|---|---|
| NNUEpisodes | Demographics and birth information (baby) | National identification baby | NationalIDBaby | R | NHS NUMBER (BABY) | n10 | England and Wales – NHS number format | Baby’s unique national identifier in a neonatal episode | Used to identify infant |
| NNUEpisodes | Demographics and birth information (baby) | National identification baby | NationalIDBaby (ENCRYPTED) | R | n/a | n10 | England and Wales – NHS number format ENCRYPTED | Baby’s unique national identifier in a neonatal episode encrypted using MD5 | Used to identify infant |
| NNUEpisodes | Demographics and birth information (baby) | National identification baby status | NationalIDBabyStatus | M | NHS NUMBER STATUS INDICATOR CODE (BABY) | n2 |
01 number present and verified 02 number present but not traced 03 trace required 04 trace attempted – no match or multiple match found 05 trace needs to be resolved – (NHS number or PATIENT detail conflict) 06 trace in progress 07 number not present and trace not required 08 trace postponed (baby < 6 weeks old) |
Whether or not the NHS number of the baby has been verified. Can be derived from patient demographic system by system provider | Used to identify whether the baby’s NHS number has been verified by the NHS care records service |
| NNUEpisodes | Demographics and birth information (baby) | Scotland CHI number | BabyCHINumber | R | COMMUNITY HEALTH INDEX NUMBER (BABY) | n10 | Scotland – CHI number format | Baby’s unique national identifier in a neonatal episode | Used to identify infant |
| NNUEpisodes | Demographics and birth information (baby) | Scotland CHI number | BabyCHINumber (ENCRYPTED) | R | n/a | n10 | Scotland – CHI Number format ENCRYPTED | Baby’s unique national identifier in a neonatal episode encrypted using MD5 | Used to identify infant |
| NNUEpisodes | Demographics and birth information (baby) | Northern Ireland H&C number | BabyHCNumber | R | HEALTH AND CARE NUMBER (BABY) | n10 | Northern Ireland – H&C number format | Baby’s unique national identifier in a neonatal episode | Used to identify infant |
| NNUEpisodes | Demographics and birth information (baby) | Northern Ireland H&C number | BabyHCNumber (ENCRYPTED)) | R | n/a | n10 | Northern Ireland – H&C number format ENCRYPTED | Baby’s unique national identifier in a neonatal episode encrypted using MD5 | Used to identify infant |
| NNUEpisodes | Demographics and birth information (baby) | Unique system identification | AnonPatientID | M | BABY LOCAL PATIENT IDENTIFIER (NATIONAL NEONATAL DATA SET) | an50 | ID utilises capital letters of the English alphabet only | A unique ID that will only identify the baby if used by a user with permission to see the record of that baby | Used to identify infant in locations of care for the purpose of communication where patient identifiers are not included |
| NNUEpisodes | Demographics and birth information (baby) | Date and time of birth | DateTimeofBirth | M | DATE TIME OF BIRTH (BABY) | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The calendar date and time of birth of the baby in co-ordinated universal time | Used for calculating anonymised times in minutes, identify verification of baby, and secondary data linkages |
| NNUEpisodes | Demographics and birth information (baby) | Place of birth NHS code (location of baby’s birth) | PlaceofBirthNHSCode | M | SITE CODE (OF ACTUAL PLACE OF DELIVERY) or ORGANISATION CODE (OF ACTUAL PLACE OF DELIVERY) | an20 | Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) | Place at which the birth took place as recorded | Used to conduct data analysis on the organisation |
| NNUEpisodes | Demographics and birth information (baby) | Birthweight (g) | Birthweight | R | BIRTHWEIGHT | n4 |
Accepted range is between 001–9998 9999 unknown |
Birthweight at the time of delivery in grams | Used to identify risk factor on admission to neonatal care |
| NNUEpisodes | Demographics and birth information (baby) | Birth length (cm) | Birthlength | O | BIRTH LENGTH | nn.n | 99.9 unknown | Length measured just after birth | Used to assess growth development |
| NNUEpisodes | Demographics and birth information (baby) | Birth head circumference | BirthHeadCircumference | O | BIRTH HEAD CIRCUMFERENCE | nn.n | 99.9 unknown | Occipitofrontal circumference measured at birth, in centimetres to one decimal place | Used to monitor outcomes according to head circumference |
| NNUEpisodes | Demographics and birth information (baby) | Gestation age in weeks | GestationWeeks | R | GESTATION LENGTH (AT DELIVERY) | n2 | 10–49 | The best obstetric estimate at the time of delivery in weeks. This will normally be based on the postmenstrual age but, if appropriate, may be modified on the basis of antenatal ultrasound. Where gestation at delivery is not known, this is based on the postnatal estimate of maturity | Used to identify risk factor on admission to neonatal care |
| NNUEpisodes | Demographics and birth information (baby) | Gestation age days | GestationDays | R | GESTATION LENGTH (REMAINING DAYS AT DELIVERY) | n | 0–6 9 unknown | Specify, if known, the number of days between whole weeks in the gestation period | Used to identify risk factor on admission to neonatal care |
| NNUEpisodes | Demographics and birth information (baby) | Sex of the baby (phenotypic) | SexPhenotype | R | PERSON PHENTOTYPIC SEX | n1 |
1 male 2 female 9 indeterminate/intersex |
The sex of the baby. ‘Not known’ is an option if information is missing or not recorded. ‘Not specified’ is an option for instances where the sex cannot be determined at birth. This option can be changed later if the chromosomal sex of the baby has been determined as follows: male (XY) or female (XX) or remain ‘not specified’ if the genotypic sex is not defined as XX/XY or is still not known | Used to aggregate by sex |
| NNUEpisodes | Demographics and birth information (baby) | Sex of the baby (genotypic) | SexGenotype | P | PERSON GENOTYPIC SEX (NATIONAL NEONATAL DATA SET) | n1 |
1 male 2 female 9 indeterminate/intersex X genotypic sex Unknown |
Specify the genotypic sex of the infant when the phenotypic sex of the baby is indeterminate and requires genetic testing. Male (XY) or female (XX) or remain ‘not specified’ if the genotypic sex is not defined as XX/XY or is still not known | Used to aggregate by sex |
| NNUEpisodes | Demographics and birth information (baby) | Baby’s blood group | BabyBloodGroup | O | BLOOD GROUP (BABY) | an2 |
National codes: A blood group A B blood group B AB blood group AB O blood group O 77 baby not tested 99 not known |
The blood group of a baby established as a result of a clinical investigation using the ABO classification system for human blood | Used to monitor implementation of anti-D prophylaxis guidance and outcomes for mothers who are rhesus negative and their babies |
| NNUEpisodes | Demographics and birth information (baby) | Baby’s rhesus factor | BabyRhesusFactor | O | RHESUS GROUP (BABY) – note default codes are 777 and 999 not 077 and 099 | an3 |
POS RhD-positive NEG RhD-negative 777 Baby not tested 999 not known |
An indication of whether or not a baby has the rhesus factor (or RhD antigen) on the surface of their red blood cells, using the Rh system. This is indicated in association with the baby’s blood group, established as a result of a clinical investigation, by RhD-positive (does have the RhD antigen) or RhD-negative (does not have the antigen) | Used to monitor implementation of anti-D prophylaxis guidance and outcomes for mothers who are rhesus negative and their babies |
| NNUEpisodes | Demographics and birth information (baby) | Worst base deficit within 12 hours after birth (mmol/l) | WorstBaseWithin12 | R | BASE DEFICIT CONCENTRATION (WORST WITHIN 12 HOURS AFTER BIRTH) | nn.n | 99.9 unknown | The worst base deficit concentration, an amount added to 1 l of the baby’s blood at 40 mmHg pCO2 to return the pH to normal, recorded within 12 hours of birth | Used to monitor outcomes according to base excess |
| NNUEpisodes | Parents | National identification mother | NHSNumberMother | R | NHS NUMBER (MOTHER) | n10 | England and Wales – NHS number format | Mother’s unique national identifier in England and Wales | Used to link children with pregnancy and mother |
| NNUEpisodes | Parents | National identification mother | NHSNumberMother (ENCRYPTED) | R | n/a | n10 | England and Wales – NHS number format ENCRYPTED | Mother’s unique national identifier in England and Wales encrypted using MD5 | Used to link children with pregnancy and mother |
| NNUEpisodes | Parents | National identification mother status | NHSNumberMotheStatus | M | NHS NUMBER STATUS INDICATOR CODE (MOTHER) | n2 |
01 number present and verified 02 number present but not traced 03 trace required 04 trace attempted – no match or multiple match found 05 trace needs to be resolved – NHS number or patient detail conflict 06 trace in progress 07 number not present and trace not required 08 trace postponed (baby < 6 weeks old) |
Whether or not the NHS number of the mother has been verified. Can be derived from patient demographic system by system provider | Used to identify whether or not the baby’s NHS number has been verified by the NHS care records service |
| NNUEpisodes | Parents | Scotland CHI number for mother | MotherCHINumber | R | COMMUNITY HEALTH INDEX NUMBER (MOTHER) | n10 | Scotland – CHI number format | Mother’s unique national identifier in Scotland | Used to link children with pregnancy and mother |
| NNUEpisodes | Parents | Scotland CHI number for mother | MotherCHINumber (ENCRYPTED) | R | COMMUNITY HEALTH INDEX NUMBER (MOTHER) | n10 | Scotland – CHI number format ENCRYPTED | Mother’s unique national identifier in Scotland encrypted using MD5 | Used to link children with pregnancy and mother |
| NNUEpisodes | Parents | Northern Ireland H&C number for mother | MotherHCNumber | R | HEALTH AND CARE NUMBER (MOTHER) | n10 | Northern Ireland – H&C number format | Mother’s unique national identifier in Northern Ireland | Used to link children with pregnancy and mother |
| NNUEpisodes | Parents | Northern Ireland H&C number for mother | MotherHCNumber (ENCRYPTED) | R | HEALTH AND CARE NUMBER (MOTHER) | n10 | Northern Ireland – H&C number format ENCRYPTED | Mother’s unique national identifier in Northern Ireland. Encrypted using MD5 | Used to link children with pregnancy and mother |
| NNUEpisodes | Parents | Birth year mother | BirthYearMother | R | YEAR OF BIRTH (MOTHER) | n4 | Year of date | The calendar year of mother’s birth from the mother’s date of birth | Used to derive ages for comparison |
| NNUEpisodes | Parents | Postcode mother | PostCodeMother | R | POSTCODE OF USUAL ADDRESS (MOTHER) | an8 |
No-use NHS defaults: ZZ99 3VZ No fixed abode ZZ99 3WZ At sea ZZ99 3WZ In the air ZZ99 3WZ Inadequately described/specified ZZ99 3WZ Information refused ZZ99 3WZ Not collected ZZ99 3WZ Not known ZZ99 3WZ Not stated/specified |
A UK postcode of the mother’s residence at time of delivery | Used to derive PCT and other geographical areas, including Sure Start areas, for aggregation to compare outcomes and plan services |
| NNUEpisodes | Parents | Postcode mother (LSOA) | PostCodeMotherLSOA | R | n/a (derived on receipt) | an8 | Derived on mother’s postcode. LSOA-equivalent of mother’s postcode | An LSOA-equivalent of UK postcode of the mother’s residence at time of delivery | Used to derive PCT and other geographical areas, including Sure Start areas, for aggregation to compare outcomes and plan services |
| NNUEpisodes | Parents | Mother’s education | MumEducation | P | QUALIFICATION ATTAINMENT LEVEL MOTHER (NATIONAL NEONATAL DATA SET) | n2 |
00 No qualifications 01 1–4 O levels/CSEs/GCSEs (any grades), Entry Level, Foundation Diploma 02 NVQ Level 1, Foundation GNVQ, Basic Skills 03 5 + O levels(passes)/CSE (grade 1)/GCSEs (grades A*–C), School Certificate, 1A level/2–3 AS levels/VCEs, Higher Diplomas 04 NVQ Level 2, Intermediate GNVQ, City and Guilds Craft, BTEC First/General Diploma, RSA Diploma 05 2+ A levels/VCEs, 4+ AS levels, Higher School Certificate, Progression/Advanced Diploma 06 NVQ Level 3, Advanced GNVQ, City and Guilds Advanced Craft, ONC, OND, BTEC National, RSA Advanced Diploma 07 Degree (for example BA, BSc), higher degree (for example MA, PhD, PGCE) 08 NVQ Level 4–5, HNC, HND, RSA Higher Diploma, BTEC Higher Level 09 Professional qualifications (for example teaching, nursing, accountancy) 10 Other vocational/work-related qualifications 11 Foreign qualifications 99 unknown |
Specify the current educational attainment of the mother | Used as a factor in socioeconomic analysis |
| NNUEpisodes | Parents | Mother’s occupation | MumOccupation | O | OCCUPATION MOTHER (SNOMED CT) | n18 | Snomed CT for Concept ID 14679004 | Mother’s description of her occupation | Used to derive mother’s occupational category |
| NNUEpisodes | Parents | Mother’s ethnicity | MumEthnicity | R | ETHNIC CATEGORY (MOTHER) | An2 | White A, British B, Irish C, any other white background mixed D, white and black Caribbean E, white and black African F, white and Asian G, any other mixed background Asian or Asian British H, Indian J, Pakistani K, Bangladeshi L, any other Asian background black or black British M, Caribbean N, African P, any other black background, Other ethnic groups R, Chinese S, any other ethnic group Z, not stated, 99 not known | Mother’s declared ethnicity based on the NHS (England) standard codes for ethnic group | Used to compare outcomes according to ethnicity |
| NNUEpisodes | Parents | GPPractisecode | GPPractiseCode | R | GENERAL MEDICAL PRACTICE CODE (PATIENT REGISTRATION (MOTHER)) | an8 |
NHS organisation code V81997 – no registered GP practice V81998 – GP practice code not applicable V81999 – GP practice code not known |
Please specify mother’s GP at time of delivery | Required for aggregation by GP/area |
| NNUEpisodes | Parents | Birth year father | BirthYearDad | R | YEAR OF BIRTH (FATHER) | n4 | Year of date | The calendar year of father’s birth from the father’s date of birth | Used to derive ages for comparison |
| NNUEpisodes | Parents | Father’s ethnicity | DadEthnicity | R | ETHNIC CATEGORY (FATHER) | An2 | White A, British B, Irish C, any other white background mixed D, white and black Caribbean E, white and black African F, white and Asian G, any other mixed background Asian or Asian British H, Indian J, Pakistani K, Bangladeshi L, any other Asian background black or black British M, Caribbean N, African P, any other black background other ethnic groups R, Chinese S, any other ethnic group Z, not stated 99, not known | Biological father’s declared ethnicity based on the NHS (England) standard codes for ethnic group of father | Used to compare outcomes according to ethnicity |
| NNUEpisodes | Parents | Parents consanguineous | ParentsConsaguinous | R | PARENTS CONSANGUINOUS INDICATOR | an1 |
N no Y yes 9 unknown |
Records if parents are consanguineous – first cousins | Used to determine association with congenital anomaly |
| NNUEpisodes | Antenatal (pregnancy details) | Mother antenatally booked indicator | BookingIndicator | P | MOTHER ANTENATALLY BOOKED INDICATOR | an1 |
N no Y yes |
Specify if the mother was booked for delivery of the infant | Used to assess the use of default codes in site code |
| NNUEpisodes | Antenatal (pregnancy details) | Intended place of delivery NHS code | BookingNHSCode | M | SITE CODE (OF INTENDED PLACE OF DELIVERY) or ORGANISATION CODE (OF INTENDED PLACE OF DELIVERY) | an9 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
Place at which mother was first booked for her confinement. The first intended place of delivery by the health-care professional in consultation with the woman | Used to aggregate by geographical area |
| NNUEpisodes | Pregnancy, labour and delivery | Mother’s number of previous pregnancies | NumberOfPreviousPregnancies | R | PREGNANCY TOTAL PREVIOUS PREGNANCIES | n2 | 0–29 99 unknown | Number of known pregnancies for the mother previous to the current pregnancy | Used to monitor outcome |
| NNUEpisodes | Labour and delivery | Birth order | BirthOrder | R | BIRTH ORDER (MATERNITY SERVICES SECONDARY USES) | n2 | NN UU unknown | The numbered order in which babies are delivered in a multiple pregnancy independent of ‘numbering’ before delivery | Used to monitor outcomes comparing singleton and multiple pregnancies |
| NNUEpisodes | Labour and delivery | Fetus total | FetusTotal | R | NUMBER OF FETUSES (NOTED DURING PREGNANCY EPISODE) | n2 | 99 unknown | Total number of fetuses noted at any time in the pregnancy which resulted in delivery of a live or stillborn baby. This excludes fetus papyraceous and fetuses reabsorbed in utero and not delivered | Used to monitor outcomes comparing singleton and multiple pregnancies |
| NNUEpisodes | Antenatal (pregnancy details) | Medical problems prior to pregnancy of mother | ProblemsMedicalMother | R | MATERNITY COMPLICATING MEDICAL DIAGNOSIS TYPE (NATIONAL NEONATAL DATA SET) | an60 |
01 hypertension 02 cardiac disease 03 renal disease 04 mental health disease 06 haematological disease 07 central nervous system disease 08 diabetes 09 autoimmune disease 10 cancer 12 infection disease: hepatitis A 13 infection disease: hepatitis B 14 infection disease: hepatitis C 16 endocrine disease 17 respiratory disease 18 gastrointestinal disease 19 musculoskeletal disease 0 gynaecological problems |
List of maternal problems that were present prior to this pregnancy | Used to monitor different targets for complicated/uncomplicated pregnancies |
| NNUEpisodes | Antenatal (pregnancy details) | Problems (obstetric) during pregnancy with mother | ProblemsObstPregnancyMother | P | MATERNITY OBSTETRIC DIAGNOSIS TYPE (CURRENT PREGNANCY) | an60 |
01 pre-eclampsia 02 haemolytic anaemia 03 eclampsia 05 liver cholestasis of pregnancy 06 gestational diabetes mellitus 07 gestational hypertension 08 gestational proteinuria 09 antepartum haemorrhage 11 feto-maternal haemorrhage 19 placenta praevia 20 severe pre-eclampsia |
List of maternal obstetric problems encountered relating to this pregnancy | To monitor outcomes for mothers and babies where complicating or risk factors are present |
| NNUEpisodes | Antenatal (pregnancy details) | Problems (infectious or medical condition) during pregnancy with mother | ProblemsInfctPregnancyMother | R | MATERNITY MEDICAL DIAGNOSIS TYPE (CURRENT PREGNANCY) | n2 |
01 rubella 02 varicella 03 Group B Streptococcus 04 asymptomatic bacteriuria 05 toxoplasmosis 08 ruberculosis 09 cytomegalovirus 10 parvovirus 11 malaria 13 cardiac disease 14 renal disease 15 mental health disorder 16 thromboembolic disorder 17 haematological disorder 18 central nervous system (CNS) disorder 19 diabetes 20 autoimmune disease 21 cancer 22 infectious hepatitis A 23 hepatitis B 24 hepatitis C 25 endocrine disorder 26 respiratory disease 27 gastrointestinal disorder 28 musculoskeletal disorder 29 gynaecological problems |
The infections disease or medical condition diagnosed within this pregnancy | To monitor outcomes for mothers and babies where complicating or risk factors are present |
| NNUEpisodes | Antenatal (pregnancy details) | Mother’s blood group | MumBloodGroup | R | BLOOD GROUP (MOTHER) | an2 |
A blood group A B blood group B AB blood Group AB O blood Group O 77 mother not tested 99 not known |
The blood group of a mother established as a result of a clinical investigation using the ABO classification system for human blood | Used to monitor implementation of anti-D prophylaxis guidance and outcomes for mothers who are rhesus negative and their babies |
| NNUEpisodes | Antenatal (pregnancy details) | Mother’s rhesus factor | MumBloodRhesus | R | RHESUS GROUP (MOTHER) | an3 |
POS RhD-positive NEG RhD-negative 777 mother not tested 999 not known |
An indication of whether or not a mother has the rhesus factor (or RhD antigen) on the surface of their red blood cells, using the Rh system. This is indicated in association with the mother’s blood group, established as a result of a clinical investigation, by: RhD-positive (does have the RhD antigen) or RhD-negative (does not have the antigen) | Same as for blood group |
| NNUEpisodes | Antenatal (pregnancy details) | Mother’s haemoglobinopathology status | MumHaemoglobinopathy | O | HAEMOGLOBINOPATHY INVESTIGATION RESULT CODE FOR NATIONAL NEONATAL DATA SET (MOTHER) | an250 |
1 sickle cell disease 2 sickle cell trait 3 sickle cell C disease 4 thalassemia major 5 thalassemia minor 9 unknown |
Presence of known problem with a haemoglobinopathy in mother | Used to determine screening protocols for sickle cell disease and Thalassemia |
| NNUEpisodes | Antenatal (pregnancy details) | Smoking in pregnancy | SmokingInPregnancy | R | MOTHER CURRENT SMOKER AT BOOKING INDICATOR | an1 |
N no Y yes 9 unknown |
Mother’s smoking at the time of booking in this pregnancy | Used to compare outcomes for babies of mothers who smoke |
| NNUEpisodes | Antenatal (pregnancy details) | Number of cigarettes mother smoked during pregnancy | NoCigarettes | O | GIGARETTES PER DAY (MOTHER AT BOOKING) | n3 | 0–999 | The number of cigarettes that the mother smoked on average, per day, at the time of booking for this pregnancy | Used to compare outcomes for babies of mothers who smoke |
| NNUEpisodes | Antenatal (pregnancy details) | Were steroids given during pregnancy? | SteroidsAntenatalGiven | R | STEROIDS GIVEN DURING PREGNANCY TO MATURE FETAL LUNGS INDICATOR | an1 |
Derived N no Y yes 9 unknown |
Administration of any dose of steroid to mother (dexamethasone or betamethasone), at any time during pregnancy, with the intention of maturing fetal lungs | Used to compare outcomes for babies |
| NNUEpisodes | Antenatal (pregnancy details) | Number of antenatal steroid courses given | SteroidsAntenatalCourses | R | ANTENATAL STEROID COURSE COMPLETION STATUS CODE | n1 |
1 – complete: a full course of steroids at any time during pregnancy with the intention of maturing the fetal lungs 2 – incomplete: at least one injection of steroids given at any time during pregnancy with the intention of maturing the fetal lungs 3 – not given 9 – unknown |
A complete course of steroids is defined by the RCOG guideline388 as two 12 mg doses of betamethasone, given intramuscularly, 24 hours apart. Some units may use another regimen, including a different steroid. A complete course is one which complies with the local protocol. The time between the course of steroids and delivery of the baby does not matter. Enter the course as complete if the mother received the requisite course of steroids at any time, or in any unit, during the pregnancy. An incomplete course is where mother has received at least one injection of steroids but has not gone on to complete the course as defined by the local protocol | Used to compare outcomes for babies |
| NNUEpisodes | Antenatal (pregnancy details) | If any, which steroids were given? | SteroidsAntenatalDrug | O | STEROID TYPE GIVEN TO MOTHER (SNOMED CT DM+D) | n18 |
1 betamethasone 2 dexamethasone 3 other dm+d selection |
The name of the steroid given to mother presenting in preterm labour | Used to compare outcomes for babies |
| NNUEpisodes | Antenatal (pregnancy details) | Mother’s rubella antibody status | MumRubellaStatus | O | INVESTIGATION RESULT CODE (MOTHER RUBELLA SCREENING) | n1 |
01 Rubella antibodies detected (> 10 IU/ml) 02 Rubella susceptible (< 10 ul/mol) 77 Not tested 99 Unknown |
Result of test on mother for rubella antibody | Used to monitor implementation of screening guidelines and take up of services, and outcomes for babies |
| NNUEpisodes | Antenatal (pregnancy details) | Mother’s date of last menstrual period | MumLMP | M | LAST MENSTRUAL PERIOD DATE | an10 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
Date of the first day of the mother’s last menstrual period in this pregnancy in co-ordinated universal time | Used as a guide for calculation of timing of tests and other interventions, and gestational age at birth for those requiring critical care |
| NNUEpisodes | Antenatal (pregnancy details) | Mother’s estimated date of delivery | MumEDD | M | ESTIMATED DATE OF DELIVERY (AGREED) | an10 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
Mother’s agreed estimated date of delivery for this pregnancy is the last menstrual date if the dating ultrasound scan (performed in accordance with NICE guidelines389) agrees within 7 days, or if the difference is > 7 days, the date calculated from the dating ultrasound scan will be taken. If the dating ultrasound scan is unavailable then the last menstrual period date is used and if that is unavailable then a clinical assessment date is used. In co-ordinated universal time | Used as a guide for calculation of timing of tests and other interventions, and gestational age at birth for those requiring critical care |
| NNUEpisodes | Labour and delivery | Meconium stained liquor | MeconiumStainedLiquor | O | MECONIUM PRESENT IN LIQUOR INDICATOR | an1 |
N no Y yes 9 unknown |
Confirm if there was presence of meconium in the liquor following rupture of the membranes or at delivery | Used to monitor the incidence of complications of delivery |
| NNUEpisodes | Labour and delivery | Medications administered during labour | DrugsInLabour | O | MEDICATION GIVEN DURING LABOUR (SNOMED CT DM+D) | n18 | dm+d code for any drug | List of drugs given to mother during labour | Used to compare outcomes according to methods employed to induce labour |
| NNUEpisodes | Labour and delivery | Mother’s onset of labour | Onsetoflabour | R | LABOUR OR DELIVERY ONSET METHOD CODE (NATIONAL NEONATAL DATA SET) | an2 |
01 spontaneous (where the labour or delivery onset method is ‘Spontaneous’) 02 induced (where the labour or delivery onset method is ‘Surgical induction’, ‘Medical induction’, or ‘Combination of surgical induction and medical induction’) 03 none (where the labour or delivery onset method is ‘Caesarean section carried out before the onset of labour or a planned elective caesarean section carried out immediately following onset of labour’) 09 not known |
Specify the status of mother’s labour | Used to monitor delays in delivery and outcomes for mothers and babies |
| NNUEpisodes | Labour and delivery | Date and time of rupture of membranes | DateROM | R | RUPTURE OF MEMBRANES DATE TIME | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The date and time when membranes were ruptured in this pregnancy in co-ordinated universal time | Used to monitor implementation of guidance |
| NNUEpisodes | Labour and delivery | Maternal pyrexia in labour > 38C | MaternalPyrexiaInLabour38c | O | SIGNIFICANT MATERNAL PYREXIA IN LABOUR INDICATOR | an1 |
N no Y yes 9 unknown |
Details the development of significant pyrexia by the mother during labour | Used to monitor the incidence of complications of delivery |
| NNUEpisodes | Labour and delivery | Intrapartum antibiotics given | IntrapartumAntibioticsGiven | O | INTRAPARTUM ANTIBIOTICS GIVEN INDICATOR | an1 |
N no Y yes 9 unknown |
Details if mother was given antibiotics during labour | Used to monitor the incidence of complications of delivery |
| NNUEpisodes | Labour and delivery | Presentation of fetus at delivery | PresentationOfFetusAtDelivery | R | PRESENTATION AT DELIVERY | an2 |
01 cephalic 02 breech 03 transverse/oblique 04 not known XX other |
Presentation of the fetus at delivery | Used to monitor changes in intended plan of care |
| NNUEpisodes | Labour and delivery | Mode of delivery | ModeOfDelivery | R | MODE OF DELIVERY | an1 |
1 emergency caesarean section 2 elective caesarean section 3 vaginal – instrument assisted 4 vaginal – spontaneous 9 not known |
Specify the mode of delivery | Used to compare outcomes and variance in practice |
| NNUEpisodes | Labour and delivery | Mother’s labour status at time of caesarean | ModeofDelivery Caesarean | P | IN LABOUR BEFORE CAESAREAN SECTION INDICATOR | n1 |
Y mother in labour before caesarean delivery N mother not in labour before caesarean delivery |
An indication of whether or not the mother had established labour onset before delivery of the baby by caesarean section. If the mode of delivery is caesarean then this item is required | Used to compare outcomes and variance in practice |
| NNUEpisodes | Labour and delivery | Mother’s mode of delivery instrument | ModeofDeliveryInstrument | P | DELIVERY INSTRUMENT TYPE | an1 |
1 forceps 2 ventouse 3 other |
Specify the instrument used during delivery of the infant | Used to monitor delays in delivery and outcomes for mothers and babies |
| NNUEpisodes | Labour and delivery | Spontaneous respiration time of onset | SpontaneousRespirationTime | O | TIME BETWEEN DELIVERY AND SPONTANEOUS RESPIRATION CODE | n1 |
1 < 1 minutes 2 1–1.5 minutes 3 1.6–2 minutes 4 2.1–3 minutes 5 3.1–4 minutes 6 4.1–5 minutes 7 > 5 minutes |
Recorded time at which the infant’s first gasp was observed following birth in the delivery suite | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | APGAR at 1 minute | apgar_1min | R | APGAR SCORE (1 MINUTE) | n2 |
0–10 Apgar score 99 unknown |
Apgar score at 1 minute of age as determined by immediate examination of the baby following delivery for appearance, pulse, grimace, activity, and respiration. Each of these five criteria is scored between a 0 and 2, with each score contributing to a cumulative total from 0 to 10 | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | APGAR at 5 minutes | apgar_5min | R | APGAR SCORE (5 MINUTES) | n2 |
0–10 Apgar score 99 unknown |
Apgar score at 5 minute of age as determined by immediate examination of the baby following delivery for appearance, pulse, grimace, activity, and respiration. Each of these five criteria is scored between a 0 and 2, with each score contributing to a cumulative total from 0 to 10 | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | APGAR at 10 minutes | apgar_10min | R | APGAR SCORE (10 MINUTES) | n2 |
0–10 Apgar score 99 unknown |
Apgar score at 10 minute of age as determined by immediate examination of the baby following delivery for appearance, pulse, grimace, activity, and respiration. Each of these five criteria is scored between a 0 and 2, with each score contributing to a cumulative total from 0 to 10 | Used to monitor neonatal outcomes including cooling |
| NNUEpisodes | Labour and delivery | Methods of resuscitation | MethodsOfResuscitation | R | NEONATAL RESUSCITATION METHOD (NATIONAL NEONATAL DATA SET) | an3 |
00 none 10 stimulation 11 positioning managing airways 12 oxygen 13 suction 14 bag and face mask IPPV 15 intubation 16 cardiac massage |
Interventions used during resuscitation or stabilisation immediately after delivery of the baby | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Drugs used during resuscitation | DrugsforResuscitation | O | NEONATAL RESUSCITATION DRUG (SNOMED CT DM+D) | n18 | dm+d code for any drug | If medication was administered at resuscitation please select relevant medications | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Admission: time of cord clamping | CordClamp | O | UMBILICAL CORD CLAMPED IMMEDIATELY AFTER BIRTH INDICATOR | an1 |
N no Y yes 9 unknown |
Indicate if the cord was clamped immediately after birth | |
| NNUEpisodes | Labour and delivery | Admission: time of cord clamping | TimeofCordClamp | O | TIME BETWEEN DELIVERY AND UMBILICAL CORD CLAMPING | n4 |
0–3600 seconds 9999 unknown |
Please indicate the time of blood cord clamping in seconds from birth | |
| NNUEpisodes | Labour and delivery | Admission: stripping of blood from cord | StripBloodCord | O | UMBILICAL CORD MILKING PERFORMED INDICATOR | an1 |
N no Y yes U unknown |
Indicate if the umbilical cord was stripped or milked of blood to enhance placental-infant transfusion at birth | |
| NNUEpisodes | Labour and delivery | Admission: cord artery pH | CordPhArterial | O | UMBILICAL CORD BLOOD PH LEVEL (ARTERIAL) | n.nn |
6.00–8.00 9.99 unknown |
The pH of cord arterial blood taken after delivery | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Admission: cord venous pH | CordVenousPH | O | UMBILICAL CORD BLOOD PH LEVEL (VENOUS) | n.nn |
6.00–8.00 9.99 unknown |
The pH of cord venous blood taken after delivery | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Admission: cord artery pCO2 | CordArterialPCO2 | O | UMBILICAL CORD BLOOD PARTIAL PRESSURE CARBON DIOXIDE (ARTERIAL) | n.nn |
5.0–8.50 KPa 9.99 unknown |
The partial pressure of CO2 value of the blood taken from the umbilical cord artery at birth | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Admission: cord lactate | CordlLactate | O | UMBILICAL CORD BLOOD LACTATE LEVEL | nn.nn |
mmol/l 99 99 unknown |
The lactate results of the umbilical lactate value of the blood taken from the umbilical cord at birth | Used for cooling intervention |
| NNUEpisodes | Labour and delivery | Admission: cord artery base excess | CordArterialBaseExcess | O | UMBILICAL CORD BLOOD BASE EXCESS CONCENTRATION (ARTERIAL) | an3 |
–30 – 30 mmol 99 unknown |
Base excess concentration of arterial cord blood taken after the delivery | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Admission: cord venous base excess | CordVenousBaseExcess | O | UMBILICAL CORD BLOOD BASE EXCESS CONCENTRATION (VENOUS) | an3 |
–30 – 30 mmol 99 unknown |
Base excess concentration of venous cord blood taken after the delivery | Used to monitor neonatal outcomes |
| NNUEpisodes | Labour and delivery | Was surfactant given during resuscitation? | SurfactantGivenResuscitation | R | SURFACTANT GIVEN INDICATOR (DURING RESUSCITATION) | an1 |
N no Y yes 9 unknown |
Surfactant given during resuscitation | Used to monitor neonatal outcomes |
| NNUEpisodes | Admission details | Admission: date and time | AdmitTime | M | CRITICAL CARE START DATE AND TIME | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The calendar date and time, co-ordinated universal time, on which an inpatient stay commences an episode of care in a neonatal unit | Used for the NCCMDS, calculate the anonymised daily dates |
| NNUEpisodes | Admission details | Episode of care | CriticalCareIdentifier | R | EPISODE NUMBER (NEONATAL CRITICAL CARE SPELL) | n2 | The number of this episode of care for this baby | The EPISODE NUMBER (NEONATAL CRITICAL CARE SPELL) is used to sequentially identify each CRITICAL CARE PERIOD within a Neonatal Critical Care Spell. The first CRITICAL CARE PERIOD identifier commences at 1; subsequent CRITICAL CARE PERIODS during the same period of care (within the same or different Health-Care Providers) are then incremented by 1. For example, a Neonate is admitted to the Neonatal Intensive Care Unit at Trust A, starting a CRITICAL CARE PERIOD and generating EPISODE NUMBER (NEONATAL CRITICAL CARE SPELL) 1. The Neonate is then transferred to a different Health-Care Provider, Trust B (ending the CRITICAL CARE PERIOD at Trust A), which generates EPISODE NUMBER (NEONATAL CRITICAL CARE SPELL) 2. The Neonate may then return to Trust A (ending the CRITICAL CARE PERIOD at Trust B), generating EPISODE NUMBER (NEONATAL CRITICAL CARE SPELL) 3 | Used to ascribe outcomes |
| NNUEpisodes | Admission details | Hospital baby admitted to | ProviderNHSCode | M | SITE CODE (OF ADMITTING NEONATAL UNIT) or ORGANISATION CODE (OF ADMITTING NEONATAL UNIT) | an20 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
This is the code for the hospital recording information on this patient. It is a code that identifies an organisation uniquely. For NHS organisations it is a code that is managed by the Corporate Data Administration section of the Department of Health and Social Care to identify most organisations that exchange information within the NHS or return information to the Centre. Examples of organisations that can be identified this way are NHS Trusts and Health Authorities | Used to ascribe outcomes |
| NNUEpisodes | Admission details | Location baby admitted from | AdmitFromNHSCode | R | SITE CODE (ADMITTED FROM TO NEONATAL UNIT) or ORGANISATION CODE (ADMITTED FROM TO NEONATAL UNIT) | an20 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
The place from which a baby was admitted into this episode of care. If the baby is admitted to the neonatal unit from its own local labour ward or theatres, then the value entered is the NHS code of this hospital | Used to analyse transfer of patients |
| NNUEpisodes | Admission details | Hospital baby admitted from location detail | AdmissionSource | O | LOCATION IN HOSPITAL TYPE (BABY ADMITTED FROM) | an2 |
1 labour and delivery ward 2 operating theatre 3 children’s ward 4 postnatal ward 5 neonatal intensive care unit/special care baby unit 6 other |
The exact location at the hospital from which a baby was admitted into this episode of care. Specialist care baby Unit. Neonatal unit | Used to analyse transfer of patients |
| NNUEpisodes | Admission details | Admission: reason for admit | ReasonForAdmit | R | PRIMARY CATEGORY OF CARE REQUIRED ON ADMISSION TO NEONATAL CRITICAL CARE | n2 |
10 medical intensive care 11 medical high-dependency care 12 medical special care 13 surgical care 14 cardiac care 15 tertiary specialist investigation 16 back transfer for continuing medical intensive care 17 back transfer for continuing medical high-dependency care 18 back transfer for continuing medical special care 19 social care 20 transitional care 99 unknown |
Specify the type of clinical service the infant is being admitted to receive, including if the service is part of back transfer (returning to hospital from which an infant was transferred for care to location of the previous episode of care). Type of care is identified using BAMP (www.BAPM.org) classification | Used to analyse transfer of patients |
| NNUEpisodes | Admission details | Admission temperature status | AdmitTempStatus | M | TEMPERATURE RECORDED AFTER ADMISSION TO NEONATAL CRITICAL CARE INDICATOR | an1 |
N no Y yes 9 unknown |
Specify the temperature measured after admission. A prompt to verify that an admission temperature was recorded after admission | Used in the National Neonatal Audit Programme |
| NNUEpisodes | Admission details | Temperature at admission | AdmitTemperature | M | TEMPERATURE (ON ADMISSION TO NEONATAL CRITICAL CARE) | nn.n | 77.7 not recordable | Baby’s axillary/skin temperature in degrees Celsius measured within 60 minutes of admission to this episode of care | Used in the National Neonatal Audit Programme |
| NNUEpisodes | Admission details | Admission temperature date and time | AdmissionTempDateTime | M | OBSERVATION DATE AND TIME (TEMPERATURE) | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The date and time, co-ordinated universal time, at which the admission temperature was measured | Used in the National Neonatal Audit Programme |
| NNUEpisodes | Admission details | Admission blood pressure | AdmitBP | R | MEAN ARTERIAL BLOOD PRESSURE (ON ADMISSION TO NEONATAL CRITICAL CARE) | n3 | 10–150 999 unknown | Specify the mean blood pressure of the baby on admission to this episode of care (in mmHg) | Used to assess clinical condition of infant on admission to care as well as transfers |
| NNUEpisodes | Admission details | Admission heart rate | AdmitHR | R | HEART RATE (ON ADMISSION TO NEONATAL CRITICAL CARE) | n3 | 50–350 999 unknown | Specify the heart rate of the baby on admission to this episode of care (per minute) | Used to assess clinical condition of infant on admission to care as well as transfers |
| NNUEpisodes | Admission details | Respiratory rate at admission | AdmissionRR | O | RESPIRATORY RATE (ON ADMISSION TO NEONATAL CRITICAL CARE) | n3 | 10–200 999 unknown | Specify the respiratory rate of the baby on admission to this episode of care (per minute) | Used to assess clinical condition of infant on admission to care as well as transfers |
| NNUEpisodes | Admission details | Oxygen saturation at admission | AdmissionOxygenSaturation | O | OXYGEN SATURATION (ON ADMISSION TO NEONATAL CRITICAL CARE) | n3 | 10–100 999 unknown | Specify the oxygen saturation of the baby on admission to this episode of care (in %) | Used to assess clinical condition of infant on admission to care as well as transfers |
| NNUEpisodes | Admission details | Blood glucose concentration (mmol/l) at admission | BloodGlucose | O | BLOOD GLUCOSE CONCENTRATION (ON ADMISSION TO NEONATAL CRITICAL CARE) | nn.n | 0.0–50.0 99.9 unknown | Specify the blood glucose concentration of the baby on admission to this episode of care (in mmol/l) | Used to assess clinical condition of infant on admission to care as well as transfers |
| NNUEpisodes | Admission details | Clinical diagnosis at admission | DiagnosisAtAdmission | R | DIAGNOSIS (ICD ON ADMISSION TO NEONATAL CRITICAL CARE) and/or DIAGNOSIS (SNOMED CT ON ADMISSION TO NEONATAL CRITICAL CARE) | an6/n18 | ICD-10 and/or Snomed CT | Specify the clinical reasons for admission of the baby from the list of ICD | Used to compare outcomes for babies |
| NNUEpisodes | Admission details | Was Vitamin K permission given | VitaminKPermission | O | PARENTAL CONSENT TO ADMINISTER VITAMIN K INDICATOR | an1 |
Y parental consent to administer vitamin K given N parental consent to administer vitamin K not given 9 not known if parental consent given |
An indication of whether parental consent was given to administer vitamin K to the baby | Used to monitor the uptake of vitamin K prophylaxis |
| NNUEpisodes | Admission details | Was vitamin K indicator | VitaminKIndicator | O | VITAMIN K ADMINISTERED INDICATOR | an1 |
Y vitamin K given N vitamin K not given |
Specify if the baby had received any dose of vitamin K following delivery | Used to monitor the uptake of vitamin K prophylaxis |
| NNUEpisodes | Admission details | Route of administration of Vitamin K | VitaminKRoute | O | VITAMIN K ROUTE OF ADMINISTRATION | n |
1 intramuscular injection 2 intravenous injection 3 oral administration 9 Route of administration unknown |
Specify the route of administration of the first dose of vitamin K given following delivery | Used to monitor the uptake of vitamin K prophylaxis |
| NNUEpisodes | Admission details | Admission: designation of member of staff completing admission form | AdmissionStaffDes | O | CARE PRFESSIONAL JOB ROLE CODE (COMPLETING NEONATAL INTENSIVE CARE UNIT ADMISSION FORM) | an5 | NHS Data Dictionary Codes from JOB ROLE Codes | The professional designation of the person completing the admission form | Used to monitor data quality |
| NNUEpisodes | Admission details | Consultation with parents | ConsultationWithParents | M | PARENTS SEEN BY SENIOR STAFF MEMBER WITHIN 24 HOURS INDICATOR | an1 |
N no Y yes 9 unknown |
Parents seen by senior member of staff within 24 hours of admission of the baby to the neonatal unit in this episode of care | Used in the National Neonatal Audit Programme |
| NNUEpisodes | Discharge details | Discharge date and time | DischDateTime | M | CRITICAL CARE DISCHARGE DATE AND TIME | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The date and time, co-ordinated universal time, on which an inpatient completes this episode of care, either because of death, transfer to another ward or hospital, or because of discharge home | Used to measure length of stay and to calculate the anonymised version of this field |
| NNUEpisodes | Discharge details | Discharge destination | DischargeDestination | M | DESTINATION ON DISCHARGE FROM NEONATAL CRITICAL CARE | n2 |
1 home with parent(s) 2 ward in same hospital 3 died 4 social/foster care 5 transferred to another hospital 6 hospice |
The destination of the baby at discharge from this episode of care | Used to compare outcomes for babies |
| NNUEpisodes | Discharge details | Discharge reason | DischargeReason | R | TRANSFERRED FOR FURTHER CARE TYPE (NATIONAL NEONATAL DATA SET) | an2 |
10 transferred to another hospital for continuing care 11 transferred to another hospital for specialist care 12 transferred to another hospital for surgical care 13 transferred to another hospital for cardiac care 99 unknown |
The destination of the baby at discharge from this episode of care. If the discharge destination is a transfer to another location then this item is required | Used to compare outcomes for babies |
| NNUEpisodes | Discharge details | Discharge destination ward | DischargeWard | O | WARD TYPE DISCHARGED TO (NATIONAL NEONATAL DATA SET) | an1 |
1 postnatal ward 2 transitional care 3 other neonatal unit 4 paediatric Intensive care unit |
Specify the type or ward the baby will be discharged to | Used to compare outcomes for babies, a National Neonatal Audit Programme filter |
| NNUEpisodes | Discharge details | Discharge hospital NHS code | DischargeHospitalNHSCode | M | SITE CODE (RECEIVING) or ORGANISATION CODE (RECEIVING) | an20 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
The hospital to which a baby is being transferred from this episode of care. Record if discharge reason is specified | Used to compare outcomes for babies |
| NNUEpisodes | Discharge details | Date of death and time | DateofDeath | R | PERSON DEATH DATE AND TIME (DURING NEONATAL CRITICAL CARE PERIOD | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The date and time, co-ordinated universal time, on which an inpatient had died in this episode of care as stated on the death certificate. If the discharge destination indicates the infant that died this item is required | Used in survival analyses and to calculate anonymised dates |
| NNUEpisodes | Discharge details | Cause of death | Causeofdeath | R | DEATH CAUSE ICD CODE (DURING NEONATAL CRITICAL CARE PERIOD) | an6 | ICD-10 | Specify the major reasons for death of the baby from the list of ICD as corresponding with death certificate | Used to compare outcomes for babies |
| NNUEpisodes | Discharge details | Discharge: If baby died was a post-mortem done? | IfPostMortemDone | O | POST-MORTEM CARRIED OUT INDICATOR | an1 |
N no Y yes 9 unknown |
Specify if a post-mortem was done | Used to monitor neonatal death |
| NNUEpisodes | Discharge details | Discharge: post-mortem, was consent sought? | PostMortemConsent | O | PARENTAL CONSENT TO POST-MORTEM INDICATOR | an1 |
N no Y yes 9 unknown |
Confirm if consent was obtained from the parents for the post-mortem | Used to monitor neonatal death |
| NNUEpisodes | Discharge details | Discharge: if NEC diagnosed, did post-mortem confirm this? | PostmortemConfirmation | O | POST-MORTEM CONFIRMED-NECROTISING ENTEROCOLITIS INDICATOR | an1 |
N no Y yes 9 unknown |
If a NEC diagnosis was made at any point at admission, specify if the post-mortem confirmed it | Used to monitor neonatal death |
| NNUEpisodes | Discharge details | Discharge: Oxygen | DischargeOxygen | O | RECEIVING OXYGEN THERAPY ON DISCHARGE INDICATOR | n |
Derived from daily data item N no Y yes |
Item specifies if the baby is receiving and is dependent on oxygen therapy on discharge home | Used to compare outcomes for babies |
| NNUEpisodes | Discharge details | Unit responsible for 2 year follow-up | Locationforfollowup | O | SITE CODE (TWO YEAR NEONATAL OUTCOMES ASSESSMENT RESPONSIBILITY) or ORGANISATION CODE (TWO YEAR NEONATAL OUTCOMES ASSESSMENT RESPONSIBILITY) | an20 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
Specify the name of the hospital that is responsible for undertaking the 2-year follow-up of this baby after discharge from neonatal care | Used to plan 2-year follow-up |
| NNUEpisodes | Discharge details | Clinical diagnoses at discharge | DiagnosesAtDischarge | R | DIAGNOSIS (ICD RECORDED ON DISCHARGE FROM NEONATAL CRITICAL CARE) and/or DIAGNOSIS (SNOMED CT ON DISCHARGE FROM NEONATAL CRITICAL CARE) | an16/n18 | ICD-10 and/or Snomed CT can be derived from daily records | Specify all the applicable diagnoses for this baby if not already recorded in the daily diagnoses | Used to monitor infant care, evaluate health status and outcomes |
| NNUEpisodes | Discharge details | Procedures performed | ProcedureAtDischarge | R | PROCEDURE (OPCS RECORDED ON DISCHARGE FROM NEONATAL CRITICAL CARE) and/or PROCEDURE (SNOMED CT RECORDED ON DISCHARGE FROM NEONATAL CRITICAL CARE) | an4/n18 | OPCS coded and/or Snomed CT can be derived from daily records | Specify the procedures performed in this episode of neonatal care if not already recorded in the daily procedures | Used to monitor infant care, evaluate health status and outcomes |
| NNUEpisodes | Discharge details | Date of procedures performed | ProcedureAtDischargeDate | R | PROCEDURE DATE AND TIME (DURING NEONATAL CRITICAL CARE PERIOD) | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) Can be derived from daily records |
The date and time, co-ordinated universal time, on which an inpatient had a surgical procedure in this episode of care | Used in survival analyses and calculate anonymised dates |
| NNUEpisodes | Clinical trials | This episode of care: research study enrolment | ResearchStudyEnrol | O | CLINICAL TRIAL NAME | an250 | Free–text entry | If this baby was enrolled in one or more research studies, in this episode of care, specify the study. Do not record any patient identifiable details such as contact details, address, and patient names | Used to monitor participation in research studies where applicable |
| NNUEpisodes | Clinical trials | This episode of care: research study medication received | ResearchDrugs | O | CLINICAL TRIAL MEDICATION ADMINISTERED NAME | an250 | Free–text entry | Specify the medications the baby has received as part of the research study. Do not record any patient identifiable details such as contact details, address, and patient names | Used to monitor participation in research studies where applicable |
| Daily | General information | General information: date of day of care | ActiveDate | M | NEONATAL CRITICAL CARE DAILY CARE DATE | an19 |
DateTime coding (e.g. 1997–07–16T19:20:30) Date (an10 CCYY-MM-DD) Time (an8 HH:MM:SS) |
The clinical data for a 24-hour period in a neonatal unit; a new day of care begins at midnight following the previous day of neonatal care | Used in commissioning and to determine level of care |
| Daily | General information | General information: day provider NHS code | DayProviderNHSCode | M | SITE CODE (OF ADMITTING NEONATAL UNIT) or ORGANISATION CODE (OF ADMITTING NEONATAL UNIT) | an20 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
The code for the hospital recording information on this day and episode for the patient. Can be derived by system provider | Used in commissioning to ascribe daily activity |
| Daily | General information | General information: weight today | DayWorkingWeight | R | PERSON WEIGHT IN GRAMS | n4 | 001–9998 g 9999 unknown | The weight, in grams, of the baby on this day of care | Used to monitor development of infant |
| Daily | General information | General information: head circumference today | DayHeadCirc | O | PERSON HEAD CIRCUMFERENCE IN CENTIMETRES | nn.n | 99.9 unknown | Head circumference is in centimetres to one decimal | Used to monitor development of infant |
| Daily | General information | General information: length today | DayLength | O | PERSON LENGTH IN CENTIMETRES | nn.n | 99.9 unknown | Length measured, in centimetrers, on this day of care | Used to monitor development of infant |
| Daily | General information | General information: location of care | LocationofCare | R | LOCATION OF HIGHEST LEVEL OF CARE | n2 |
01 neonatal unit 02 transitional care 11 postnatal ward 12 other area |
This is the ‘highest’ location of care on this day | Used in the National Neonatal Audit Programme |
| Daily | General information | General information: receiving 1 : 1 nursing | OneToOneNursing | R | PATIENT RECEIVING ONE TO ONE NURSING CARE INDICATOR | an1 |
N no Y yes |
Specify if the baby received 1 : 1 nursing on this day of care | Used to determine level of care |
| Daily | General information | General information: carer resident | CarerResident | R | CARER RESIDENT INDICATION CODE | n |
1 carer not resident 2 carer resident – not caring for baby 3 carer resident – caring for baby |
Specify the detail of the carer resident | Used to determine level of care |
| Daily | General information | Clinical diagnoses on day of care | DiagnosesDaily | R | DIAGNOSIS (ICD ON NEONATAL CRITICAL CARE DAILY CARE DATE) and/or DIAGNOSIS (SNOMED CT ON NEONATAL CRITICAL CARE DAILY DATE) | an16/n18 | ICD-10 and/or Snomed CT | Specify all the applicable diagnoses for this baby | Used to monitor infant care and evaluate health status and outcomes |
| Daily | General information | General information: any surgical procedures | SurgicalProcedure | R | PROCEDURE (OPCS DURING NEONATAL CRITICAL CARE PERIOD) or PROCEDURE (SNOMED CT DURING NEONATAL CRITICAL CARE PERIOD) | an4/n18 | OPCS coded and/or Snomed CT | Surgical procedure on the date and time specified | Used to monitor health status and outcomes, and calculate anonymised versions |
| Daily | General information | General information: baby transported | TransportedDay | R | PERSON ACCOMPANYING TRANSPORTED PATIENT | n1 |
1 with nurse (non-ANNP) 2 with nurse (ANNP) 3 with doctor 4 with paramedics 5 with parent |
If baby has been transported today, specify with whom the transport took place. ANNP is an Advanced Neonatal Nurse Practitioner | Used in neonatal transport data set |
| Daily | Respiratory | Respiration: respiratory support device | RespiratorySupport | R | RESPIRATORY SUPPORT DEVICE TYPE (NATIONAL NEONATAL DATA SET) | n1 |
1 endotracheal tube 2 tracheostomy 3 nasal cannula 4 nasopharyngeal cannula 5 face mask |
Type of respiratory support device any time during the 24-hour period (00.00–23.59) | Used to determine level of care |
| Daily | Respiratory | Respiration: respiratory support mode | ModeofRespiratorySupport | R | RESPIRATORY SUPPORT MODE (NATIONAL NEONATAL DATA SET) | n1 |
1 positive pressure ventilation 2 high frequency oscillatory ventilation 3 high frequency jet ventilation 4 CPAP 5 BiPAP/SiPAP 6 high flow |
Mode of respiratory support via endotracheal tube. Conventional ventilation includes intermittent mandatory ventilation, synchronised intermittent mandatory ventilation, assist/control ventilation, pressure support ventilation, pressure targeted, volume targeted, hybrid | Used to determine level of care |
| Daily | Respiratory | Respiration: nitric oxide given | PulmonaryVasodilator | R | NITRIC OXIDE GIVEN INDICATOR | an1 |
N no Y yes |
Specify if the baby is receiving nitric oxide on this day of care | Used to determine level of care |
| Daily | Respiratory | Respiration: chest drain present | ChestDrain | R | CHEST DRAIN IN SITU INDICATOR | an1 |
N no Y yes |
Specify if the baby has a chest drain present on this day of care | Used to determine level of care |
| Daily | Respiratory | Respiration: tracheostomy tube present | DayTracheostomyTube | R | TRACHEOSTOMY TUBE IN SITU INDICATOR | an1 |
N no Y yes |
Specify if the baby has a tracheostomy tube insert present on this day of care | Used to determine level of care |
| Daily | Respiratory | Respiration: repogle tube in situ | ReplogleTube | R | REPLOGLE TUBE IN SITU INDICATOR | an1 |
N no Y yes |
Specify if the baby has a repogle tube insert present on this day of care | Used to determine level of care |
| Daily | Respiratory | Respiration: surfactant given today | DaySurfactantGiven | R | SURFACTANT GIVEN INDICATOR (ON NEONATAL CRITICAL CARE DAILY CARE DATE) | an1 |
N no Y yes |
Records if baby received any dose of surfactant in this day while in the neonatal unit. Surfactant given at delivery/resuscitation is a data item collected separately | Used to determine health status of infant |
| Daily | Respiratory | Respiration: maximum oxygen supplementation today | OygenPerc | P | FRACTION OF INSPIRED OXYGEN PERCENTAGE | N3 | Percentage fraction of inspired oxygen | Specify the maximum fraction of inspired oxygen percentage that the baby received on this day of care | Used to monitor oxygen dependency |
| Daily | Cardiovascular | Cardiovascular: i.v. infusion pulmonary vasodilator | PulmonaryVasodilator | R | CONTINUOUS INFUSION OF PULMONARY VASODILATOR RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if the baby is receiving a continuous infusion of a pulmonary vasodilator on this day of care | Used to determine level of care |
| Daily | Cardiovascular | Cardiovascular: Inotropes given | Inotropesgiven | R | INOTROPE INFUSION RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby is receiving an inotrope infusion on this day of care. For list of inotropic drugs refer to the British National Formulary390 | Used to determine level of care |
| Daily | Cardiovascular | Cardiovascular: prostaglandin infusion | Prostaglandin | R | PROSTAGLANDIN INFUSION RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby is receiving an inotrope infusion on this day of care. For list of inotropic drugs refer to the British National Formulary390 | Used to determine level of care |
| Daily | Cardiovascular | Cardiovascular: treatment for PDA | SurgeryforPDA | R | TREATMENT TYPE FOR PATENT DUCTUS ARTERIOSUS | n |
1 indometacin/indomethacin 2 ibuprofen 3 surgery 9 not applicable |
If baby has PDA, specify treatment a baby has had for PDA on this day of care | Used to determine level of care |
| Daily | Gastrointestinal | Gastrointestinal: peritoneal dialysis | PeritonealDialysis | R | PERITONEAL DIALYSIS OR HAEMOFILTRATION RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby is receiving peritoneal dialysis or haemofiltration on this day of care | Used to determine level of care |
| Daily | Gastrointestinal | Gastrointestinal: haemofiltration | Haemofiltration | R | PERITONEAL DIALYSIS OR HAEMOFILTRATION RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby is receiving peritoneal dialysis or haemofiltration on this day of care | Used to determine level of care |
| Daily | Gastrointestinal | Gastrointestinal: treatment for NEC | DayNEC | R | TREATMENT TYPE FOR NECROTISING ENTEROCOLITIS | n |
Derived 1 medical 2 surgical 9 not applicable – no treatment given |
Specify if a baby received NEC treatment on this day of care. This item can be derived from the surgical procedures item | Used to determine level of care |
| Daily | Gastrointestinal | Gastrointestinal: rectal washouts (> 3/day) | Rectalwashout | R | MORE THAN THREE RECTAL WASHOUTS RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby has had more than three rectal washout procedures on this day of care | Used to determine level of care |
| Daily | Gastrointestinal | Gastrointestinal: stoma in situ | StomaInSitu | R | STOMA PRESENT INDICATOR | an1 |
N no Y yes |
Specify if a baby has any stoma in place on this day of care | Used to determine level of care |
| Daily | Blood transfusions | Transfusions: type of transfusion today | DayTransfusion | R | BLOOD TRANSFUSION TYPE | n1 |
1 partial (dilutional) exchange transfusion 2 full exchange transfusion today |
If a baby has received a transfusion on this day of care, specify type of transfusion | Used to determine level of care |
| Daily | Blood transfusions | Transfusions: blood products | BloodProductsTrans | R | BLOOD TRANSFUSION PRODUCT TYPE | n1 |
1 packed red cells or whole blood transfusion 2 fresh-frozen plasma 3 cryoprecipitate 4 platelets 5 albumin |
Specify the blood products used in the transfusion procedure on this day of care | Used to determine level of care |
| Daily | Neurology | Neurology: central tone | Centraltone | R | CENTRAL TONE STATUS | n1 |
1 normal 2 increased 3 decreased |
Specify if any changes were observed in central tone by monitoring the baby’s neuromotor functions during this 24-hour period (00.00–23.59), for example Floppy limbs would mean decreased central tone | Used in the National Neonatal Audit Programme |
| Daily | Neurology | Neurology: consciousness status | Consciousness | R | NEONATAL CONSCIOUSNESS STATUS | n1 |
0 normal 1 hyper aler 2 lethargic 3 comatose |
The consciousness status of the baby during this 24-hour period (00.00–23.59) | Used in the National Neonatal Audit Programme |
| Daily | Neurology | Neurology: convulsion today | Convulsion | R | SIEIZURE OCCURRED INDICATOR | an1 |
N no Y yes |
This records if the baby had any seizures (clinical or noted on erg/cam monitoring) on this day | Used in the National Neonatal Audit Programme |
| Daily | Neurology | Neurology: neonatal abstinence syndrome | NeonatalAbstinenceSyndrome | R | NEONATAL ABSTINENCE SYNDROME OBSERVED INDICATOR | an1 |
N no Y yes |
Specify is neonatal abstinence syndrome is observed on this day of care | Used to determine level of care |
| Daily | Neurology | Neurology: surgery for ventricular- peritoneal shunt | SurgeryVPShunt | R | n/a | n1 |
Derived 0 no 1 yes |
Derived item from surgical procedures today; ‘yes’ is if a VP shunt procedure is found in the field and ‘no’ if it has not | Used to determine level of care |
| Daily | Neurology | Neurology: EEG/CFM | EEGCFM | R | BRAIN ACTIVITY SCAN PERFORMED INDICATOR | an1 |
N no Y yes |
Specify if this baby has had a brain activity scan on this day of care using either eithelectroencephalogram or bedside with a cerebral function monitor | Used to determine level of care |
| Daily | Neurology | Neurology: therapeutic hypothermia | Therapeutichypothermia | R | THERAPEUTIC HYPOTHERMIC INDUCED INDICATOR | an1 |
N no Y yes |
This records if the baby was being cooled today as part of management of suspected hypoxic ischaemic damage to the brain. Includes both passive and active cooling | Used in the National Neonatal Audit Programme |
| Daily | Neurology | Neurology: hypoxic ischaemic encephalopathy diagnosis | HIEDiagnosis | R | HYPOXIC ISCHAEMIC ENCEPHALOPATHY GRADE (HIGHEST ON NEONATAL CRITICAL CARE DAILY CARE DATE) | n |
0 none 1 1-mild 2 2-moderate 3 3-severe |
The highest Hypoxic Ischaemia Encephalopathy diagnosis on this day of care | Used to determine level of care |
| Daily | Ophthalmology | ROP screen | ROPScreen | R | RETINOPATHY OF PREMATURITY SCREENING PERFORMED INDICATOR | an1 |
N no Y yes |
Specify if the baby has had a screen for ROP on this day of care | Used in the National Neonatal Audit Programme |
| Daily | Ophthalmology | ROP surgery | ROPSurgery | R | Derived from procedures | n2 |
Derived 0 no 1 yes |
Derived item from any major surgery today; ‘yes’ if a ROP surgical procedure is recorded and ‘no’ if not | Used in the National Neonatal Audit Programme |
| Daily | Fluids and feeding | Fluids and feeding: vascular lines in situ | LinesIn | R | VASCULAR LINE TYPE IN SITU | n2 |
1 peripheral arterial line 2 umbilical arterial line 3 umbilical venous line 4 percutaneous central venous line (long line) 5 surgically inserted central venous line 6 peripheral venous line 9 not applicable/no lines in situ |
Specify any line that is in situ for any time during this day | Used to determine level of care |
| Daily | Fluids and feeding | Fluids and feeding: parenteral nutrition today (partial or total) | ParenteralNutrition | R | PARENTERAL NUTRITION RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby has received any parenteral nutrition on this day of care | Used to determine level of care |
| Daily | Fluids and feeding | Fluids and feeding: intravenous glucose and electrolyte solutions | GlucoseElectrolytes | R | INTRAVENOUS INFUSION OF GLUCOSE AND ELECTROLYTE SOLUTION RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if a baby has received an intravenous infusion of a glucose and electrolyte solution on this day of care | Used to determine level of care |
| Daily | Fluids and feeding | Fluids and feeding: enteral feeding given | DayEnteralFeed | R | ENTERAL FEED TYPE GIVEN | an250 |
1 suckling at the breast 2 mother’s fresh expressed breast milk 3 mother’s frozen expressed breast milk 4 donor expressed breast milk 5 breast milk fortifier 6 formula 9 not applicable (nil by mouth) |
Types of milk given during this 24-hour period (00.00–23.59). Record all the types of milk given during the day. Nil by mouth is single choice and only selected if no enteral feed for the whole of the 24-hour period | Used in the National Neonatal Audit Programme |
| Daily | Fluids and feeding | Fluids and feeding: formula type | DayFormulaTypedmd | R | FORMULA MILK TYPE (SNOMED CT DM+D) | max n18 | dm+d virtual therapeutic moiety listing | If enteral feeding is formula, specify the name of the formula. List will be maintained by d-dm+d | Used to monitor development of infant |
| Daily | Fluids and feeding | Fluids and feeding: Formula type | DayFormulaType | R | FORMULA MILK TYPE (bespoke) | an2 |
10 Nutriprem 1 11 Nutriprem 2 12 Neocate 13 Nutramigen 14 Pepti Junior 15 Infatrini 16 SMA High energy 17 Aptamil First milk 18 Cow & Gate First infant milk 19 SMA First infant milk 20 Enfamil A.R. 21 Aptamil Preterm 22 SMA Gold Prem 1 23 SMA Gold Prem 2 24 Aptamil Pepti 1 25 Aptamil Pepti 2 26 Pregestimil 27 Caprilon 28 Wysoy 29 Infasoy 88 Other |
If enteral feeding is formula, specify the name of the formula. This is a bespoke list used instead of dm+d listing if it is unavailable. Multiple selections allowed | Used to monitor development of infant |
| Daily | Fluids and feeding | Fluids and feeding: measured volume milk | Totalvolume | R | TOTAL VOLUME OF MILK RECEIVED | an4 | 000.0–999.9 | The volume of milk a baby has been fed on this day of care in ml | Used to monitor development of infant |
| Daily | Fluids and feeding | Fluids and feeding: method of feeding | Methodfeeding | O | ENTERAL FEEDING METHOD | n2 |
1 breast 2 bottle 3 cup 4 nasogastric tube 5 orogastric tube 6 gastrostomy 7 nasojejunal tube 8 other |
The method of feeding at this 24-hour period (00.00–23.59). Record all methods of feeding given during the day. No enteral feeds is single choice and only selected if no milk given for the whole of the 24-hour period | Used in the National Neonatal Audit Programme |
| Daily | Infections | Infection: suspected sepsis | DaySuspectedSepsis | R | SEPSIS SUSPECTED INDICATOR | an1 |
N no Y yes |
Specify if you believe the baby may have a blood stream infection on this day of care | Used in the National Neonatal Audit Programme |
| Daily | Jaundice | Jaundice: phototherapy | Phototherapy | R | PHOTOTHERAPY RECEIVED INDICATOR | an1 |
N no Y yes |
Specify if this baby has received a phototherapy treatment for jaundice on this day of care | Used to determine level of care |
| Daily | General information | General information: level of care (2001 definition) | LevelOfCare2001(derived) | O | Derived based on the BAPM categories of care 2001 | n1 |
Derived 1 intensive care 2 high-dependency care 3 special care |
Applying the BAPM 2001 categories of care definition | Used in commissioning |
| Daily | General information | General Information: level of care (2011 definition) | LevelOfCare2011(derived) | O | Derived based on the BAPM categories of care 2011 | n1 |
Derived 1 intensive care 2 high-dependency care 3 special care 4 normal care |
Applying the BAPM 2011 categories of care definition | Used in commissioning |
| Daily | Medication | Drugs given: medications given on this day | DailyDrugs | R | MEDICATION GIVEN DURING NEONATAL CRITICAL CARE DAILY CARE DATE (SNOMED CT DM+D) | n18 | dm+d code for any drug | Specify the exact medications the baby has received on this day of care | Used for multiple purposes including National Neonatal Audit Programme, monitor outcomes, and derive items for level of care where necessary |
| NNUAdhoc | Infection cultures | Culture indicator – blood | CultureIndicatorBLD | P | INFECTION CULTURE TEST INDICATOR (BLOOD) | an1 |
N no Y yes |
Specify if at least one blood culture was taken in this episode of care | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Culture indicator – CSF | CultureIndicatorCSF | P | INFECTION CULTURE TEST INDICATOR (CEREBROSPINAL FLUID) | an1 |
N no Y yes |
Specify if at least one CSF culture was taken in this episode of care | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Culture indicator – urine | CultureIndicatorURN | P | INFECTION CULTURE TEST INDICATOR (URINE) | an1 |
N no Y yes |
Specify if at least one urine culture was taken in this episode of care | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Date and time future sample taken – year | CultureDateTimeYear | R | SAMPLE COLLECTION YEAR AND MONTH | n4 | Derived year | Derived from the date and time variable. Date and time variable is identified by prefix to year in the Field ID | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Type of culture sample taken | Sampletype | R | SAMPLE TYPE (NATIONAL NEONATAL DATA SET) | n |
1 blood 2 urine (suprapubic) 3 urine (catheterisation) 4 urine (clean catch) 5 cerebrospinal fluid |
Specify the type of culture samples taken at the relevant date and time | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Clinical signs at time of culture | Clinicalsigns | R | CLINICAL SIGN OBSERVED AT SAMPLE COLLECTION | an250 |
01 increased oxygen requirement or ventilator support 02 lethargy/irritability/poor handling 03 temperature instability 04 ileus/onset of poor feed tolerance 05 fall in urine output 06 impaired peripheral perfusion (capillary refill time > 3 seconds/pallor/mottling/core-peripheral temperature gap > 2 °C) 07 increase in apnoea/bradycardia 08 hypotension 09 glucose intolerance 10 metabolic acidosis/base deficit < 10 mmol/l |
Specify the clinical signs observed when the culture sample was taken at the relevant date and time | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Result of culture | Pathogen | R | SAMPLE TEST RESULT ORGANISM TYPE (SNOMED CT) | n18 | Organisms list in Snomed CT (Concept ID 410607006) | Specify the culture results if there was growth or no growth by selecting the relevant options in the list of codes related to this item | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Infection cultures | Sensitivity of culture | Sensitivity | O | SAMPLE ANTIBIOTIC SENSITIVITY RESULT (SNOMED CT DM+D) | n18 | dm+d virtual therapeutic moiety listing | The sensitivity results obtained from the microbiology report of the culture sample taken at the relevant date and time | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Abdominal X-rays | Abdominal X-ray indicator | ADXIndicator | P | ABDOMINAL X-RAY PERFORMED INDICATOR | an1 |
N no Y yes |
Specify if at least one abdominal X-ray was performed in this episode of care | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | Date and time of abdominal X-ray – year | AbdominalXrayDateTimeYear | R | PROCEDURE YEAR AND MONTH (ABDOMINAL X-RAY) | n4 | Derived year | Derived from the date and time variable. Date and time variable is identified by prefix to year in the Field ID | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | Was X-ray performed to investigate abdominal signs? | InvestigateAbdsigns | R | ABDOMINAL X-RAY PERFORMED TO INVESTIGATE ABDOMINAL SIGNS INDICATOR | an1 |
N no Y yes |
Specify if the X-ray was performed to investigate abdominal signs | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | X-ray appearance? | XrayAppearance | R | CONDITION SEEN IN ABDOMEN DURING X-RAY | an50 |
1 pneumatosis 2 air in the liver 3 pneumoperitoneum 4 fixed loop 5 gasless 9 none of the above |
Specify if any of these appear in this abdomen X-ray | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | Clinical findings abdominal X-ray | abdominalxrayfindings | R | ABDOMINAL X-RAY PERFORMED REASON | an50 |
01 abdominal distension 02 abdominal tenderness 03 increased/bilious aspirates 04 abdominal discolouration 05 abdominal mass 06 bloody stools 07 mucusy stools 09 none of the above |
Specify the clinical reasons that resulted in this abdominal X-ray taking place | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | This episode of care: Baby transferred out for management of NEC | TransferredOutManagementNEC | R | TRANSFERRED FROM NEONATAL INTENSIVE CARE UNIT FOR NECROTISING ENTEROCOLITIS MANAGEMENT INDICATOR | an1 |
N no Y yes |
Specify if the baby was transferred out of the neonatal unit for further management of NEC following this abdominal X-ray | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | Was laparotomy for NEC required? | necLaparotomy | R | LAPAROTOMY FOR NECROTISING ENTERCOLITIS INDICATION CODE | n |
0 laparotomy not required 1 laparotomy required but patient too ill to carry it out 2 laparotomy required and carried out |
Specify if a laparotomy for NEC was required based on this abdominal X-ray | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | If laparotomy done, did visual inspection confirm NEC | laparotomyConfirm | R | VISUAL INSPECTION CONFIRMED NECROTISING ENTEROCOLITIS DURING LAPAROTOMY INDICATOR | an1 |
N no Y yes |
Specify if a visual inspection confirmed NEC from a laparotomy carried out following this abdominal X-ray | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | Was NEC histology confirmed? | necHistologyConfirmed | R | HISTOLOGY-CONFIRMED NECROTISING ENTEROCOLITIS FOLLOWING LAPAROTOMY INDICATOR | n |
0 not confirmed 1 yes confirmed 9 no histological inspection/not applicable |
Specify if histology confirmed NEC based on the laparotomy carried out following this abdominal X-ray | Used to monitor health status and outcomes |
| NNUAdhoc | Abdominal X-rays | Was peritoneal drain inserted after abdominal X-ray? | WasPeritonealdrainInserted | R | PERITONEAL DRAIN INSERTED FOLLOWING ABDOMINAL X-RAY INDICATOR | an1 |
N no Y yes |
Specify if a peritoneal drain was inserted after this abdominal X-ray | Used to monitor health status and outcomes |
| NNUAdhoc | ROP screening | ROP indicator | ROPindicator | M | RETINOPATHY OF PREMATURITY SCREENING PERFORMED INDICATOR | an1 |
N no Y yes |
Specify if a ROP screening was performed in this episode of care | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | Date and time of ROP test – year | ROPtestDateTimeYear | R | PROCEDURE YEAR AND MONTH (RETINOPATHY OF PREMATURITY SCREENING) | n4 | Derived year | Derived from the date and time variable. Date and time variable is identified by prefix to year in the Field ID | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | Hospital performing test | HospitalPerforminTest | R | SITE CODE (OF RETINOPATHY OF PREMATURITY SCREENING) or ORGANISATION CODE (OF RETINOPATHY OF PREMATURITY SCREENING) | an20 |
Use organisation code and site code ZZ201 – not applicable (intended to deliver at home) ZZ888 – not applicable (intended to deliver at non-NHS organisation) ZZ203 – not known (intended place of delivery not known) |
The code for the hospital that is recording the ROP screening at this event in this episode of care | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP stage – left eye | ROPStageLeft | R | RETINOPATHY OF PREMATURITY STAGE (LEFT EYE) | an1 |
0 no ROP 1 stage 1 ROP 2 stage 2 ROP 3 stage 3 ROP 4 stage 4 ROP 5 stage 5 ROP A aggressive posterior ROP |
The ROP stage for the left eye at the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP stage – right eye | ROPStageRight | R | RETINOPATHY OF PREMATURITY STAGE (RIGHT EYE) | an1 |
0 no ROP 1 stage 1 ROP 2 stage 2 ROP 3 stage 3 ROP 4 stage 4 ROP 5 stage 5 ROP A aggressive posterior ROP |
The ROP stage for the right eye at the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP clock hours max stage – left eye | ROPClockHourLeft | R | RETINOPATHY OF PREMATURITY CLOCK HOURS MAXIMUM STAGE (LEFT EYE) | n2 | 0–12 | Number of clock hours affected by maximum stage of ROP in left eye – shown as number from 0 to 12 | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP clock hours max stage – right eye | ROPClockHourRight | R | RETINOPATHY OF PREMATURITY CLOCK HOURS MAXIMUM STAGE (RIGHT EYE) | n2 | 0–12 | Number of clock hours affected by maximum stage of ROP in right eye – shown as number from 0 to 12 | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP maximum zone – left eye | ROPMaxZoneLeft | R | RETINOPATHY OF PREMATURITY CLOCK HOURS MAXIMUM ZONE (LEFT EYE) | n |
0 no ROP 1 zone 1 2 zone 2 3 zone 3 |
The ROP maximum zone for the left eye at the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP maximum zone – right eye | ROPMaxZoneRight | R | RETINOPATHY OF PREMATURITY CLOCK HOURS MAXIMUM ZONE (RIGHT EYE) | n |
0 no ROP 1 zone 1 2 zone 2 3 zone 3 |
The ROP maximum zone for the right eye at the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP plus disease – left eye | ROPPlusDiseaseLeft | R | RETINOPATHY OF PREMATURITY PLUS DISEASE STATUS (LEFT EYE) | n |
0 no plus disease 1 pre-plus disease 2 plus disease |
The plus disease status of the left eye of the baby at the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP plus disease – right eye | ROPPlusDiseaseRight | R | RETINOPATHY OF PREMATURITY PLUS DISEASE STATUS (RIGHT EYE) | n |
0 no plus disease 1 pre-plus disease 2 plus disease |
The plus disease status of the right eye of the baby at the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | ROP screening | ROP outcome | ROPOutcome | R | RETINOPATHY OF PREMATURITY SCREENING OUTCOME STATUS CODE | n |
0 No ROP 1 ROP Follow-up screening required 2 ROP diagnosed, treatment needed 3 ROP diagnosed, transferred out of neonatal unit for treatment |
Specify the outcome of the relevant ROP screening | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Cranial ultrasound scan | Cranial ultrasound scan indicator | CranialUSSIndicator | P | CRANIAL ULTRASOUND SCAN INDICATOR | an1 |
N no Y yes |
Specify if a cranial ultrasound scan test was performed in this episode of care | Used in the National Neonatal Audit Programme |
| NNUAdhoc | Cranial ultrasound scan | Date and time of cranial ultrasound scan test – year | CarnialUSSDateTimeYear | R | PROCEDURE YEAR AND MONTH (CRANIAL ULTRASOUND SCAN) | n4 | Derived year | Derived from the date and time variable. Date and time variable is identified by prefix to year in the Field ID | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings (left): IVH | LeftIVH | O | INTRAVENTRICULAR HAEMORRHAGE GRADE (LEFT SIDE) | n2 |
0 no IVH seen 1 grade 1 (germinal matrix) IVH 2 grade 2 IVH 3 grade 3 IVH 4 grade 4 IVH |
Most severe grade of intraventricular haemorrhage seen on left side | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings (left): porencephalic cyst(s) | LeftPorencephalic | O | PORENCEPHALIC CYST VISIBLE DURING CRANIAL ULTRASOUND SCAN INDICATOR (LEFT SIDE) | an1 |
Y porencephalic cyst visible on left side N no porencephalic cyst on left side |
Records if there was a porencephalic cyst visible on left side | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings (left): ventricular dilatation | LeftDilation | O | VENTRICULAR DILATATION DIAGNOSED DURING CRANIAL ULTRASOUND SCAN INDICATOR (LEFT SIDE) | an1 |
Y yes ventricular dilatation on right side N no, ventricle on right side not dilated |
Records if clinical diagnosis made of ventricular dilatation on left side | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings (right): IVH | RightIVH | O | INTRAVENTRICULAR HAEMORRHAGE GRADE (RIGHT SIDE) | n1 |
0 no IVH seen 1 grade 1 (germinal matrix) IVH 2 grade 2 IVH 3 grade 3 IVH 4 grade 4 IVH |
Most severe grade of intraventricular haemorrhage seen on right side | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings (right): Porencephalic cyst(s) | RightPorencephalic | O | PORENCEPHALIC CYST VISIBLE DURING CRANIAL ULTRASOUND SCAN INDICATOR (RIGHT SIDE) | an2 |
Y porencephalic cyst visible on right side N no porencephalic cyst on right side |
Records if there was a porencephalic cyst visible on right side | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings (right): ventricular dilatation | RightDilation | O | VENTRICULAR DILATATION DIAGNOSED DURING CRANIAL ULTRASOUND SCAN INDICATOR (RIGHT SIDE) | an2 |
Y yes ventricular dilatation on right side N No, ventricle on right side not dilated |
Records if clinical diagnosis made of ventricular dilatation on right side | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings: cystic PVL | PVL | O | CYSTIC PERIVENTRICULAR LEUKOMALACIA OBSERVED DURING CRANIAL ULTRASOUND SCAN INDICATOR | an2 |
Y yes, cystic PVL on scan N no cystic PVL seen on scan |
Records if there is any evidence of cystic PVL on the scan | Used to monitor health status and outcomes |
| NNUAdhoc | Cranial ultrasound scan | Cranial scan findings: post haemorrhagic hydrocephalus | hydorcephalus | O | POST HAEMORRHAGIC HYDROCEPHALUS OBSERVED DURING CRANIAL ULTRASOUND SCAN INDICATOR | an2 |
Y yes, post haemorrhagic hydrocephalus on scan N no post haemorrhagic hydrocephalus seen on scan |
Records if there is post haemorrhagic hydrocephalus evident on scan | Used to monitor health status and outcomes |
| NNUAdhoc | Biochemical screening | Blood spot test indicator | BloodSpotTestIndicator | M | NEWBORN BLOOD SPOT TEST PERFORMED INDICATOR | an1 |
N no Y yes |
Specify if a blood spot test was performed | |
| NNUAdhoc | Biochemical screening | Date of blood spot test – year | BloodSpotTestYear | R | BLOOD SPOT CARD COMPLETION YEAR AND MONTH | n4 | Derived year | Derived from the date and time variable. Date and time variable is identified by prefix to year in the Field ID | Used to monitor health status and outcomes |
| NNUAdhoc | Newborn hearing screening | Hearing test indicator | HearingTestIndicator | P | NEWBORN HEARING SCREENING PERFORMED INDICATOR | an1 |
N no Y yes |
Specify if a hearing test was performed in this episode of care | Used to monitor health status and outcomes |
| NNUAdhoc | Newborn hearing screening | Date and time of hearing test – year | HearingtestDateTimeYear | R | PROCEDURE YEAR AND MONTH (NEWBORN HEARING SCREENING) | n4 | Derived year | Derived from the date and time variable. Date and time variable is identified by prefix to year in the Field ID | Used to monitor health status and outcomes |
| NNUAdhoc | Newborn hearing screening | This episode of care: hearing screen result (left ear) | HearingTestLeft | O | NEWBORN HEARING SCREENING OUTCOME LEFT EAR (NATIONAL NEONATAL DATA SET) | n |
1 passed 2 fail 9 not done (default) |
Specify the result of the hearing screening for the left ear | Used to monitor health status and outcomes |
| NNUAdhoc | Newborn hearing screening | This episode of care: hearing screen result (right ear) | HearingTestRight | O | NEWBORN HEARING SCREENING OUTCOME RIGHT EAR (NATIONAL NEONATAL DATA SET) | n |
1 passed 2 fail 9 not done (default) |
Specify the result of the hearing screening for the right ear | Used to monitor health status and outcomes |
| NNUAdhoc | Two Year Follow-up | Special questions – why was child difficult to test? | SpecialQuestionsDifficultToTestReason | R | CHILD DIFFICULT TO TEST REASON CODE | an1 |
A child was tired B poor attention C difficult to engage D other reason |
Specify the reason the child was difficult to test | Use in assessing two year health outcomes following discharge from neonatal care |
Appendix 4 National Information Governance Board Confidentiality Advisory Group Approval
Appendix 5 Patient information leaflets
Appendix 6 Research ethics committee approvals
Appendix 7 Presentations arising from the Medicine for Neonates Programme
Battersby C. Improving the Quality of Routinely Collected Electronic Data. Bristol: Neonatal Nutrition Network meeting; September 2011.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. London: King’s College Hospital London; September 2011.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. Taunton: Western Neonatal Network meeting; November 2011.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. Newcastle: Yorkshire Neonatal Network meeting; February 2012.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. London: NNAP/NDAU collaborators meeting; January 2012.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. Basildon: Basildon Hospital; February 2012.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. London: Neonatal Nutrition Network meeting; May 2012.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. Cambridge: East of England Neonatal Network meeting; May 2012.
Battersby C Improving the Quality of Routinely Collected Electronic Data. London: SIGNEC (Specialist Interest Group NEC) meeting; June 2012.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. Birmingham: West Midlands Neonatal Network research meeting; November 2012.
Battersby CW, Santhakumaran S, Modi N. The UK Neonatal Collaborative Necrotising Enterocolitis Study: A Prospective Population-based Study Using the National Neonatal Research Database. Scottish Informatics Programme Conference; April 2013.
Battersby C. Improving the Quality of Routinely Collected Electronic Data. London: 1st International SIGNEC conference (Specialist Interest Group NEC); July 2013.
Battersby C. UK Neonatal Collaborative Necrotising Enterocolitis Study: A Prospective Population-based Study Using the National Neonatal Research Database. St Andrews: SHIP conference; August 2013.
Battersby C. UK Neonatal Collaborative Necrotising enterocolitis study: Feeding Practices in Babies Born Less than 33 Weeks in England. Windsor: 21st European Neonatal Workshop; 17 September 2013.
Battersby C. UK Neonatal Collaborative Necrotising Enterocolitis Study. Cambridge: East of England Neonatal Network meeting; September 2013.
Battersby C. Enteral Feed Exposures in Babies Born Less than 32 Weeks’ Gestation. London: Neonatal Society Autumn meeting; November 2013.
Battersby C. The UKNC-NEC Study: Interim Results. Medway Maritime Hospital; July 2014.
Foster V. ‘We Just Want to Give Something Back . . .’ Altruism and Data-sharing in Neonatal Services. British Sociological Association Medical Sociology Annual Conference. Leicester: University of Leicester; September 2012.
Ibrahim B, Statnikov E, Gray D, Modi N, Saxena S. Linking Electronic Records to Create a Birth Cohort of Infants Admitted to Neonatal Units in England. London: Neonatal Society Autumn Meeting; November 2014.
Modi N. Improving the Quality of Routinely Collected Electronic Data. London: Neonatal Nutrition Network meeting; September 2011.
Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, Saxena S. Changes in the Severity and Age of RSV Bronchiolitis Hospital Admission Among Infants in England: A Population-based Birth Cohort Study. London: Neonatal Society Autumn Meeting; November 2011.
Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, Saxena S. The Burden of RSV Bronchiolitis Among Infants in England: a Cohort Study. London: European Society for Paediatric Infectious Diseases; June 2011.
Murray J, Saxena S, Majeed A, Modi N, Bottle A, Aylin P. Creating a Birth Cohort to Examine RSV Bronchiolitis Hospital Admission Rates Among Term and Preterm Infants in England. RCPCH Annual Meeting; May 2012.
Rodgers K. Data-sharing in Neonatal Services Annual Stakeholder Meeting for MCRN. Manchester: University of Manchester; June 2013.
Santhakumaran S. Evaluating Mortality Rates for Neonatal Units Using Multiple Membership Models. London: International Society of Clinical Biostatistics; September 2012.
Santhakumaran S. The Neonatal Survival Prediction Tool: A New Resource for Clinicians, Managers, and Parents. London: NNAP/NDAU collaborators meeting; February 2013.
Santhakumaran S. Survival of Preterm Infants Admitted to Neonatal Care in England: A Population-based Study Using NHS Electronic Clinical Records. York: RCPCH meeting; June 2013.
Santhakumaran S. The NEC Care Bundle: Statistical Findings and Outcomes. UK Neonatal Collaborative Necrotising Enterocolitis Study. Cambridge: East of England Neonatal Network meeting; September 2013.
Santhakumaran S. A Regional Care Bundle Approach to Increase Maternal Breast Milk Use in Preterm Infants: Outcomes of the East of England Network Quality Improvement Project. London: Neonatal Society Autumn Meeting; November 2013.
Statnikov Y, Santhakumaran S, Manktelow B, Modi N. Intensive Care Provided by Non-level 3 Neonatal Units in England. London: Neonatal Society Autumn Meeting; November 2009.
Statnikov Y, Santhakumaran S, Manktelow B, Modi N. Surveillance of Necrotising Enterocolitis in England. London: Neonatal Society Summer Meeting; June 2010.
Statnikov Y, Wong HS, Gray DR, Santhakumaran S, Modi N. Screening for Retinopathy of Prematurity in English Neonatal Units. London: BAPM Annual meeting; September 2012.
Statnikov Y. National Neonatal Research Database British Intestinal Failure Working Group Meeting. London: November 2012.
Statnikov Y. A UK Neonatal Collaborative Online Portal NNAP/NDAU Collaborators Meeting. London: February 2013.
Statnikov Y, Modi N. Establishing a National Neonatal Research Database from Operational NHS Electronic Records. Edinburgh Scottish Informatics Programme; August 2013.
Wong H, Huertas-Ceballos A, Cowan FM, Modi N. Comparison of Two Parent-completed Questionnaires for the Identification of Children at Risk for Autism Spectrum Disorder in the Preterm Population. Newcastle: Annual Meeting of the European Society for Paediatric Research; 2011.
Wong HS, Huertas-Ceballos A, Cowan FM, Modi N. Evaluation of Early Childhood Social-communication Difficulties in Children Born Preterm Using the Quantitative Checklist for Autism In Toddlers (Awarded Prize for Best Presentation by a Trainee). London: Neonatal Society Spring Meeting; April 2012.
Wong HS, Huertas-Ceballos A, Cowan FM, Modi N. Sociodemographic and Neonatal Factors Associated with Early Childhood Social-communication Difficulties in Children Born Preterm. Canterbury: Neonatal Society Summer Meeting; June 2012.
Wong HS, Huertas-Ceballos A, Cowan FM, Modi N. Sociodemographic and Neonatal Factors Associated with Early Childhood Social-communication Difficulties in Children Born Preterm. Istanbul: Fourth Congress of the European Academy of Pediatric Societies; October 2012.
Wong HS, Santhakumaran S, Cowan FM, Modi N. Predictive Validity of Early Developmental Assessments in Identifying School-age Cognitive Deficits in Children Born Preterm or Very Low Birthweight: Systematic Review and Meta-analysis. Barcelona: Fifth Congress of the European Academy of Paediatric Societies; October 2014.
Appendix 8 Higher degrees awarded relating to the Medicines for Neonates Programme
Murray JC. The Clinical Burden of Respiratory Syncytial Virus Bronchiolitis Among Infants in the United Kingdom. PhD thesis. London: Imperial College London; 2013.
Watson S. Economic and Healthcare Related Determinants of Infant Health at Birth. PhD thesis. Coventry: University of Warwick; 2015.
Wong H. Neurodevelopmental Outcomes of Children Born Preterm: Analyses into the Validity of Data Collection and Outcome Reports. PhD thesis. London: Imperial College London; 2016.
Battersby C. The UK Neonatal Collaborative Necrotising Enterocolitis (NEC) Study: Testing the Utility of Operational Clinical Data to Conduct Population Surveillance, Develop an Evidence-based Case-definition and Identify Risk Factors Associated with NEC. PhD thesis. London: Imperial College London; 2017.
Santhakumaran S. Statistical Implications of Centralised Care for Estimating Neonatal Unit Mortality Rates. PhD thesis. London: Imperial College London; 2017.
Appendix 9 Studies and organisations using the National Neonatal Research Database
British Association of Perinatal Medicine
Revision of neonatal level of care definitions.
Royal College of Paediatrics and Child Health
National Neonatal Audit Programme Annual Reports 2008, 2009, 2010, 2011, 2012, 2013; 2014 in preparation (www.rcpch.ac.uk/improving-child-health/quality-improvement-and-clinical-audit/national-neonatal-audit-programme-nnap).
Department of Health and Social Care
Atlas of Variation in Healthcare for Children and Young People, 2012, 2013 (www.chimat.org.uk/variation#cmoreport).
Reducing Perinatal Brain Injury (from 2016).
HM Government
Data for Parliamentary questions (2013, 2014).
Health and Social Care Information Centre
Admissions with neonatal jaundice (from 2015).
NHS England
Impact of Greater Manchester Perinatal Services Re-configuration (2014).
Patient Safety Domain (Full Term Admissions to Newborn Care, from 2014; Neonatal Umbilical Venous Catheter insertions, 2014).
National Neonatal Clinical Reference Group (neonatal admissions annual mortality reports, from 2014).
Public Health England
Neonatal specialised activity metrics (from 2014).
Smoking in pregnancy (2015).
London Operational Delivery Network
Quarterly activity, clinical outcomes and benchmarking analyses for the London Neonatal Networks (from 2014).
World Health Organization
Data to inform the Every Newborn Action Plan (from 2014).
National and International Benchmarking and Quality Improvement Programmes
International Network for Evaluation of Outcomes of Neonates (iNeo): a quality improvement project based on collaborative comparisons of population-based international health care for neonates led by the University of Toronto (http://ineonetwork.org) (from 2013).
East of England Neonatal Networks regional care bundle to improve maternal breast milk use in preterm infants (www.unicef.org.uk/BabyFriendly/News-and-Research/Research/Neonatal/Impact-of-a-regional-care-bundle-on-maternal-breast-milk-use-in-preterm-infants) (2013–14).
Each Baby Counts: a national quality improvement programme led by the Royal College of Obstetricians and Gynaecologists to reduce the number of babies who die or are left severely disabled as a result of incidents occurring during term labour; cross-validation data will be provided from the NNRD (www.rcog.org.uk/eachbabycounts) (from 2014).
eNewborn: a pan-European preterm benchmarking platform led from Saint-Pierre University Hospital, Brussels (from 2015).
Medicines for Neonates Applied Health Research Programme
Lead institutions: Imperial College London, University of Manchester and NPEU (2009–15).
Downs in Neonates
Lead institutions: Queen Mary University of London and Hinchingbrooke Hospital NHS Trust (2012–15).
Neonatal Economics, Staffing and Clinical Outcomes Project
Lead institutions: University College London, University of Warwick, Imperial College London and University of Leicester (2012–15).
The right cot, at the right time, at the right place: providing a national demand/capacity model for neonatal care in England
Lead institutions: Peninsula Collaboration for Health Operational Research and Development, NIHR CLAHRC South West Peninsula and University of Exeter (2015–17).
Modelling care pathways in neonatal care: costs and consequences for the future
Lead institution: University of Leicester (2014–17).
PREVenting infection using antibiotic impregnated long lines (PreVail)
Lead institutions: Public Health England and Bradford Teaching Hospitals NHS Foundation Trust (2014–18).
Gentamicin, genetic variation and deafness in preterm children: the Mitogent Study
Lead institution: University College London (2015).
Medical Research Council
Preterm birth and neuropsychiatric genetic risk.
Lead institution: University of Cardiff (2016).
Clinician Science Award.
Lead institution: Imperial College London (2016).
National Institute for Health Research
Optimising service provision for preterm babies.
Lead institution: University of Leicester (2017).
Appendix 10 List of participating NHS trusts in England, and Neonatal Clinical Leads
-
Airedale General Hospital, Airedale NHS Trust
Dr Matthew Babirecki
-
Alexandra Hospital, Worcestershire Acute Hospitals NHS Trust
Dr Liza Harry
-
Arrowe Park Hospital, Wirral University Teaching Hospital NHS Foundation Trust
Dr Oliver Rackham
-
Barnet Hospital, Royal Free London NHS Foundation Trust
Dr Tim Wickham
-
Barnsley District General Hospital, Barnsley Hospital NHS Foundation Trust
Dr Sanaa Hamdan
-
Basildon Hospital, Basildon and Thurrock University Hospitals NHS Trust
Dr Aashish Gupta
-
Basingstoke and North Hampshire Hospital, Hampshire Hospitals NHS Foundation Trust
Dr Ruth Wigfield
-
Bassetlaw District General Hospital, Doncaster and Bassetlaw Hospitals NHS Foundation Trust
Dr L M Wong
-
Bedford Hospital, Bedford Hospital NHS Trust
Dr Anita Mittal
-
Birmingham City Hospital, Sandwell and West Birmingham Hospitals NHS Trust
Dr Julie Nycyk
-
Birmingham Heartlands Hospital, Heart of England NHS Foundation Trust
Dr Phil Simmons
-
Birmingham Women’s Hospital, Birmingham Women’s NHS Foundation Trust
Dr Alison Bedford-Russell
-
Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust
Dr Sunita Seal
-
Broomfield Hospital, Chelmsford, Mid Essex Hospital Services NHS Trust
Dr Ahmed Hassan
-
Calderdale Royal Hospital, Calderdale and Huddersfield NHS Foundation Trust
Dr Karin Schwarz
-
Chelsea and Westminster Hospital, Chelsea and Westminster Hospital NHS Foundation Trust
Dr Mark Thomas
-
Chesterfield & North Derbyshire Hospital, Chesterfield Royal Hospital NHS Foundation Trust
Dr Aiwyne Foo
-
Colchester General Hospital, Colchester Hospital NHS Foundation Trust
Dr Karen Moss
-
Conquest Hospital, East Sussex Hospitals NHS Trust
Dr Jayaram Pai
-
Countess of Chester Hospital, Countess of Chester Hospital NHS Foundation Trust
Dr Stephen Brearey
-
Croydon University Hospital, Croydon Health Services
Dr John Chang
-
Cumberland Infirmary, North Cumbria University Hospitals NHS Trust
Dr Khairy Gad
-
Darent Valley Hospital, Dartford and Gravesham NHS Trust
Dr Abdul Hasib
-
Darlington Memorial Hospital, County Durham and Darlington NHS Foundation Trust
Dr Mehdi Garbash
-
Derriford Hospital, Plymouth Hospitals NHS Trust
Dr Nicci Maxwell
-
Dewsbury and District Hospital, Mid Yorkshire Hospitals NHS Trust
Dr David Gibson
-
Diana Princess of Wales Hospital, Northern Lincolnshire and Goole Hospitals NHS Foundation Trust
Dr Pauline Adiotomre
-
Doncaster Royal Infirmary, Doncaster and Bassetlaw Hospitals NHS Foundation Trust
Dr Jamal S Ahmed
-
Dorset County Hospital, Dorset County Hospital NHS Foundation Trust
Dr Abby Deketelaere
-
Ealing Hospital, London North West Health Care NHS Trust
Dr Ramnik Mathur
-
East Surrey Hospital, Surrey and Sussex Healthcare NHS Trust
Dr K Abdul Khader
-
Epsom General Hospital, Epsom and St Helier University Hospitals NHS Trust
Dr Ruth Shephard
-
Frimley Park Hospital, Frimley Park Hospital NHS Foundation Trust
Dr Abdus Mallik
-
Furness General Hospital, University Hospitals of Morecambe Bay NHS Trust
Dr Belal Abuzgia
-
George Eliot Hospital, George Eliot Hospital NHS Trust
Dr Mukta Jain
-
Gloucester Royal Hospital, Gloucestershire Hospitals NHS Foundation Trust
Dr Simon Pirie
-
Good Hope Hospital, Heart of England NHS Foundation Trust
Dr Phil Simmons
-
Great Western Hospital, Great Western Hospitals NHS Foundation Trust
Dr Stanley Zengeya
-
Guy’s & St Thomas’ Hospital, Guy’s and St Thomas’ NHS Foundation Trust
Dr Timothy Watts
-
Harrogate District Hospital, Harrogate and District NHS Foundation Trust
Dr C Jampala
-
Hereford County Hospital, Wye Valley NHS Trust
Dr Cath Seagrave
-
Hillingdon Hospital, The Hillingdon Hospital NHS Trust
Dr Michele Cruwys
-
Hinchingbrooke Hospital, Cambridgeshire Community Services NHS Trust
Dr Hilary Dixon
-
Homerton Hospital, Homerton University Hospital NHS Foundation Trust
Dr Narendra Aladangady
-
Hull Royal Infirmary, Hull and East Yorkshire Hospitals NHS Trust
Dr Peter Pairaudeau
-
Ipswich Hospital, Ipswich Hospital NHS Trust
Dr Matthew James
-
James Cook University Hospital, South Tees Hospitals NHS Trust
Dr M Lal
-
James Paget Hospital, James Paget University Hospitals NHS Foundation Trust
Dr Ambadkar
-
Kettering General Hospital, Kettering General Hospital NHS Foundation Trust
Dr Patti Rao
-
King George Hospital, Barking, Havering and Redbridge University Hospitals NHS Trust
Dr Khalid Mannan
-
King’s College Hospital, King’s College Hospital NHS Foundation Trust
Dr Ann Hickey
-
King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust
Dr Vibert Noble
-
Kingston Hospital, Kingston Hospital NHS Trust
Dr Nader Elgharably
-
Lancashire Women and Newborn Centre, East Lancashire Hospitals NHS Trust
Dr Meera Lama
-
Leeds Neonatal Service, Leeds Teaching Hospitals NHS Trust
Dr Lawrence Miall
-
Leicester General Hospital, University Hospitals of Leicester NHS Trust
Dr Jonathan Cusack
-
Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust
Dr Venkatesh Kairamkonda
-
Leighton Hospital, Mid Cheshire Hospitals NHS Foundation Trust
Dr Jayachandran
-
Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust
Dr Kollipara
-
Lister Hospital, East and North Hertfordshire NHS Trust
Dr J Kefas
-
Liverpool Women’s Hospital, Liverpool Women’s NHS Foundation Trust
Dr Bill Yoxall
-
Luton and Dunstable Hospital, Luton and Dunstable Hospital NHS Foundation Trust
Dr Sarah Skinner
-
Macclesfield District General Hospital, East Cheshire NHS Trust
Dr Gail Whitehead
-
Manor Hospital, Walsall Hospitals NHS Trust
Dr Bashir Jan Muhammad
-
Medway Maritime Hospital, Medway NHS Foundation Trust
Dr Aung Soe
-
Milton Keynes General Hospital, Milton Keynes Hospital NHS Foundation Trust
Dr I Misra
-
New Cross Hospital, The Royal Wolverhampton Hospitals NHS Trust
Dr Tilly Pillay
-
Newham General Hospital, Barts Health
Dr Imdad Ali
-
Norfolk & Norwich University Hospital, Norfolk and Norwich University Hospitals NHS Foundation Trust
Dr Mark Dyke
-
North Devon District Hospital, North Devon Healthcare NHS Trust
Dr Michael Selter
-
North Manchester General Hospital, The Pennine Acute Hospitals NHS Trust
Dr Nagesh Panasa
-
North Middlesex University Hospital, North Middlesex University Hospital NHS Trust
Dr Lesley Alsford
-
Northampton General Hospital, Northampton General Hospital NHS Trust
Dr Subodh Gupta
-
Northwick Park Hospital, London North West Health Care NHS Trust
Dr Richard Nicholl
-
Nottingham City Hospital, Nottingham University Hospitals NHS Trust
Dr Steven Wardle
-
Ormskirk District General Hospital, Southport and Ormskirk Hospital NHS Trust
Dr Tim McBride
-
Oxford University Hospitals, Horton Hospital, Oxford University Hospitals NHS Trust
Dr Naveen Shettihalli
-
Oxford University Hospitals, John Radcliffe Hospital, Oxford University Hospitals NHS Trust
Dr Eleri Adams
-
Peterborough City Hospital, Peterborough and Stamford NHS Foundation Trust
Dr Seif Babiker
-
Pilgrim Hospital, United Lincolnshire Hospitals NHS Trust
Dr Margaret Crawford
-
Pinderfields General Hospital, Mid Yorkshire Hospitals NHS Trust
Dr David Gibson
-
Poole General Hospital, Poole Hospital NHS Foundation Trust
Dr Minesh Khashu
-
Princess Alexandra Hospital, The Princess Alexandra Hospital NHS Trust
Dr Caitlin Toh
-
Princess Anne Hospital, Southampton University Hospitals NHS Trust
Dr M Hall
-
Princess Royal Hospital, Brighton and Sussex University Hospitals NHS Trust
Dr P Amess
-
Princess Royal University Hospital, King’s College Hospital NHS Foundation Trust
Dr Elizabeth Sleight
-
Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust
Dr Charlotte Groves
-
Queen Charlotte’s Hospital, Imperial College Healthcare NHS Trust
Dr Sunit Godambe
-
Queen Elizabeth Hospital, Gateshead, Gateshead Health NHS Foundation Trust
Dr Dennis Bosman
-
Queen Elizabeth Hospital, The Queen Elizabeth Hospital King’s Lynn NHS Trust
Dr Susan Rubin
-
Queen Elizabeth Hospital, Woolwich, Lewisham and Greenwich NHS Trust
Dr Banjoko
-
Queen Elizabeth the Queen Mother Hospital, East Kent Hospitals University NHS Trust
Dr Rfidah
-
Queen’s Hospital, Burton on Trent, Burton Hospitals NHS Foundation Trust
Dr A Manzoor
-
Queen’s Hospital, Romford, Barking, Havering and Redbridge University Hospitals NHS Trust
Dr Khalid Mannan
-
Rosie Maternity Hospital, Addenbrookes Cambridge University Hospitals NHS Foundation Trust
Dr Angela D’Amore
-
Rotherham District General Hospital, Rotherham NHS Foundation Trust
Dr MacFarlane
-
Royal Albert Edward Infirmary, Wrightington, Wigan and Leigh NHS Foundation Trust
Dr Vibha Sharma
-
Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trust
Dr Peter De Halpert
-
Royal Bolton Hospital, Royal Bolton Hospital NHS Foundation Trust
Dr Paul Settle
-
Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust
Dr Paul Munyard
-
Royal Derby Hospital, Derby Teaching Hospitals NHS Foundation Trust
Dr Gitika Joshi
-
Royal Devon & Exeter Hospital, Royal Devon and Exeter NHS Foundation Trust
Dr Vaughan Lewis
-
Royal Hampshire County Hospital, Hampshire Hospitals NHS Foundation Trust
Dr D Schapira
-
Royal Lancaster Infirmary, University Hospitals of Morecambe Bay NHS Trust
Dr Joanne Fedee
-
Royal Oldham Hospital, The Pennine Acute Hospitals NHS Trust
Dr Natasha Maddock
-
Royal Preston Hospital, Lancashire Teaching Hospitals NHS Foundation Trust
Dr Richa Gupta
-
Royal Shrewsbury Hospital, Shrewsbury and Telford Hospital NHS Trust
Dr Deshpande
-
Royal Surrey County Hospital, The Royal Surrey County Hospital NHS Trust
Dr Charles Godden
-
Royal Sussex County Hospital, Brighton and Sussex University Hospitals NHS Trust
Dr P Amess
-
Royal United Hospital, Royal United Hospital Bath NHS Trust
Dr Stephen Jones
-
Royal Victoria Infirmary, Newcastle Upon Tyne Hospitals Foundation Trust
Dr Alan Fenton
-
Russells Hall Hospital, Dudley Group of Hospitals NHS Foundation Trust
Dr Mahadevan
-
Salisbury District Hospital, Salisbury NHS Foundation Trust
Dr Nick Brown
-
Scarborough General Hospital, York Teaching Hospitals NHS Foundation Trust
Dr Kirsten Mack
-
Scunthorpe General Hospital, Northern Lincolnshire and Goole Hospitals NHS Foundation Trust
Dr Pauline Adiotomre
-
South Tyneside District Hospital, South Tyneside NHS Foundation Trust
Dr Rob Bolton
-
Southend Hospital, Southend University Hospital NHS Foundation Trust
Dr A Khan
-
Southmead Hospital, North Bristol NHS Trust
Dr Paul Mannix
-
St George’s Hospital, St George’s Healthcare NHS Trust
Dr Charlotte Huddy
-
St Helier Hospital, Epsom and St Helier University Hospitals NHS Trust
Dr Salim Yasin
-
St Mary’s Hospital, Isle of Wight Healthcare NHS Trust
Dr Sian Butterworth
-
St Mary’s Hospital, London, Imperial College Healthcare NHS Trust
Dr Sunit Godambe
-
St Mary’s Hospital, Manchester, Central Manchester University Hospitals NHS Foundation Trust
Dr Ngozi Edi-Osagie
-
St Michael’s Hospital, University Hospitals Bristol NHS Foundation Trust
Dr David Harding
-
St Peter’s Hospital, Ashford and St Peter’s Hospitals NHS Trust
Dr Peter Reynolds
-
St Richard’s Hospital, Western Sussex Hospitals NHS Trust
Dr Nick Brennan
-
Stepping Hill Hospital, Stockport NHS Foundation Trust
Dr Carrie Heal
-
Stoke Mandeville Hospital, Buckinghamshire Hospitals NHS Trust
Dr Sanjay Salgia
-
Sunderland Royal Hospital, City Hospitals Sunderland NHS Foundation Trust
Dr Majd Abu-Harb
-
Tameside General Hospital, Tameside Hospital NHS Foundation Trust
Dr Jacqeline Birch
-
Taunton and Somerset Hospital, Taunton and Somerset NHS Foundation Trust
Dr Chris Knight
-
The Jessop Wing, Sheffield, Sheffield Teaching Hospitals NHS Foundation Trust
Dr Simon Clark
-
The Royal Free Hospital, Royal Free London NHS Foundation Trust
Dr Vivienne Van Sommen
-
The Royal London Hospital, Constance Green, Barts Health
Dr Nandiran Ratnavel
-
Torbay Hospital, South Devon Healthcare NHS Foundation Trust
Dr Mala Raman
-
Tunbridge Wells Hospital, Maidstone and Tunbridge Wells NHS Trust
Dr Hamudi Kisat
-
University College Hospital, University College London Hospitals NHS Foundation Trust
Dr Sara Watkin
-
University Hospital Coventry, University Hospitals Coventry and Warwickshire NHS Trust
Dr Kate Blake
-
University Hospital Lewisham, Lewisham and Greenwich NHS Trust
Dr Jauro Kuna
-
University Hospital of North Durham, County Durham and Darlington NHS Foundation Trust
Dr Mehdi Garbash
-
University Hospital of North Staffordshire, University Hospitals of North Midlands NHS Trust
Dr Kate Palmer
-
University Hospital of North Tees, North Tees and Hartlepool NHS Foundation Trust
Dr B Reichert
-
University Hospital of South Manchester, University Hospital of South Manchester NHS Foundation Trust
Dr Gopi Vemuri
-
Victoria Hospital, Blackpool, Fylde and Wyre Hospitals NHS Foundation Trust
Dr Chris Rawlingson
-
Wansbeck General Hospital, Northumbria Healthcare NHS Trust
Dr Alan Fenton
-
Warrington Hospital, Warrington and Halton Hospitals NHS Foundation Trust
Dr Delyth Webb
-
Warwick Hospital, South Warwickshire General Hospitals NHS Trust
Dr Semeer Kallaroth
-
Watford General Hospital, West Hertfordshire Hospitals NHS Trust
Dr Sankara Narayanan
-
West Cumberland Hospital, North Cumbria University Hospitals NHS Trust
Dr Mithun Urs
-
West Middlesex University Hospital, West Middlesex University Hospital NHS Trust
Dr Elizabeth Eyre
-
West Suffolk Hospital, West Suffolk Hospital NHS Trust
Dr Ian Evans
-
Wexham Park Hospital, Heatherwood and Wexham Park Hospitals NHS Foundation Trust
Dr Rekha Sanghavi
-
Whipps Cross University Hospital, Barts Health
Dr Caroline Sullivan
-
Whiston Hospital, St Helens and Knowsley Teaching Hospitals NHS Trust
Dr Laweh Amegavie
-
Whittington Hospital, The Whittington Hospital NHS Trust
Dr Wynne Leith
-
William Harvey Hospital, East Kent Hospitals University NHS Trust
Dr Vimal Vasu
-
Worcestershire Royal Hospital, Worcestershire Acute Hospitals NHS Trust
Dr Andrew Gallagher
-
Worthing Hospital, Western Sussex Hospitals NHS Trust
Dr Katia Vamvakiti
-
Yeovil District Hospital, Yeovil District Hospital NHS Foundation Trust
Dr Megan Eaton
-
York District Hospital, York Teaching Hospitals NHS Foundation Trust
Dr Guy Millman
Appendix 11 Medicines for Neonates Steering Committee
Professor Michael Goldacre (chairperson): independent member.
Professor Andrew Wilkinson (deputy chairperson): independent member.
Mrs Jane Abbott (later Ms Zoe Chivers): investigator.
Professor Deborah Ashby: investigator.
Professor Peter Brocklehurst: investigator.
Professor Kate Costeloe: investigator.
Professor Elizabeth Draper: investigator.
Mrs Jacquie Kemp: investigator.
Professor Azeem Majeed: investigator.
Professor Neena Modi: lead investigator.
Professor Stavros Petrou: investigator.
Professor Alys Young: investigator.
Appendix 12 Other funding sources contributing to this research
Unrestricted donations from
Abbott Laboratories (Maidenhead, UK): £35,000.
Nutricia Research Foundation (Schiphol, the Netherlands): £15,000.
GE Healthcare (Amersham, UK): £1000.
Grant to support the use of routinely collected, standardised, electronic clinical data for audit, management and multidisciplinary feedback in neonatal medicine.
Department of Health and Social Care: £135,494.
List of abbreviations
- ADHD
- attention deficit hyperactivity disorder
- ASD
- autism spectrum disorder
- AUC
- area under the receiver operating characteristic curve
- BAPM
- British Association of Perinatal Medicine
- Bayley-III
- Bayley Scales of Infant and Toddler Development, third edition
- BSID
- Bayley Scales of Infant Development
- BSID-II
- Bayley Scales of Infant Development, second edition
- CI
- confidence interval
- CQUIN
- Commissioning for Quality and Innovation
- CRF
- case report form
- DOR
- diagnostic odds ratio
- DQ
- developmental quotients
- EPR
- electronic patient record
- ESS
- effective sample size
- GBP
- Great British pounds
- GMDS
- Griffiths Mental Development Scales
- GMFCS
- Gross Motor Function Classification System
- HES
- Hospital Episode Statistics
- HINE
- Hammersmith Infant Neurological Examination
- HSROC
- hierarchical summary receiver operator characteristic curve
- ICD-9
- International Classification of Diseases, Ninth Edition
- ICD-10
- International Classification of Diseases, Tenth Edition
- IMD
- Index of Multiple Deprivation
- IQ
- intelligence quotient
- IQR
- interquartile range
- LSOA
- lower-layer super output area
- M-CHAT
- Modified Checklist for Autism in Toddlers
- MDI
- Mental Development Index
- NDAU
- Neonatal Data Analysis Unit
- NEC
- necrotising enterocolitis
- NICHD
- National Institute of Child Health and Human Development
- NNRD
- National Neonatal Research Database
- NPEU
- National Perinatal Epidemiology Unit
- ODN
- Operation Delivery Network
- ONS
- Office for National Statistics
- OR
- odds ratio
- PARCA-R
- Parent Report of Children’s Abilities-Revised
- PiPS
- Probiotic in Preterm infants Study
- PPI
- patient and public involvement
- PPV
- positive predictive values
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- Q-CHAT
- Quantitative Checklist for Autism in Toddlers
- QUADAS-2
- Quality of Diagnostic Accuracy Studies version 2
- ROC
- receiver operating characteristic
- ROP
- retinopathy of prematurity
- RSV
- respiratory syncytial virus
- SD
- standard deviation
- SDS
- standard deviation score
- TN
- true negative
- TP
- true positive
- VLBW
- very low birthweight
- VP
- ventriculoperitoneal
- WHO
- World Health Organization