Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0609-10107. The contractual start date was in March 2011. The final report began editorial review in April 2018 and was accepted for publication in June 2019. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lelia Duley reports National Institute for Health Research (NIHR) Clinical Trials Unit support funding. Jon Dorling reports grants from NIHR during the conduct of the study, grants from NIHR and grants from Nutrinia Ltd (Ramat Gran, Israel) outside the submitted work. He also reports membership to the Health Technology Assessment (HTA) Efficient Study Designs 2015–16, HTA General Board 2016–19, HTA Maternal, Neonatal and Child Health (MNCH) Methods Group 2014–18, HTA MNCH Panel 2013–18, and HTA Post Board Funding Teleconference 2015–18. Andrew Weeks reports grants from Liverpool Women’s Hospital Charity during the conduct of the study. Gill Gyte reports personal fees from Cochrane Pregnancy and Childbirth Group outside the submitted work. Jim Thornton reports membership of HTA Clinical Trials Board 2010–15, HTA and EME Editorial Board 2016–18, Rapid Trials and Add on Studies 2012, CPR Decision-Making Committee. David Field reports HTA Commissioning Board 2013–2018, HTA and Efficacy and Mechanism Evaluation (EME) Editorial Board 2012–18. William McGuire reports grants from NIHR during the conduct of the study, and membership of the HTA Commissioning Board 2013–present and the HTA and EME Editorial Board 2012–present.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Duley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
SYNOPSIS
Background
Being born too early (preterm birth) has a major impact on survival and quality of life for the child, on psychosocial and emotional stress on the family, and on costs for health services and society. 1–3 Mortality is highest for infants born very preterm, before 32 weeks’ gestation. In the UK, infant mortality (deaths in the first year of life) for babies born very preterm is 144 deaths per 1000 live births, compared with 1.8 deaths per 1000 live births at term. 4 Although only 1.4% of live births in the UK are very preterm, these babies account for 51% of infant deaths. 4
The costs of neonatal care for infants born very preterm are high. For those born before 28 weeks’ gestation, duration of hospital stay is 85 times that for term births and hospital inpatient costs are £15,000 higher. For those born at 28 to 31 weeks, duration of hospital stay is 16 times that for term births and hospital inpatient costs are £12,000 higher. 5 Morbidity among children born very preterm who survive is also higher than those born at term. 3 Of very preterm infants who survive, around 5% develop cerebral palsy and those without severe disability have a twofold or greater increased risk for developmental, cognitive and behavioural difficulties. 1,2 These impairments may persist into adolescence and early adulthood. 6,7 Even modest improvements in outcome would be of substantial benefit to the children, their families and the health services.
Aims and objectives
The aims of this programme were to improve the quality of immediate care at very preterm birth, enhance family-centred care, and improve outcome for infants born very premature and their families.
Specific objectives were to:
-
Develop a James Lind Alliance (JLA) Priority Setting Partnership (PSP) between service users and clinicians to identify and prioritise treatment uncertainties relevant to preterm birth (work package 1; Figure 1) and to:
-
identify and prioritise research gaps for preterm birth, using the methods developed by the JLA
-
describe how service users and clinicians interact when making collective decisions about research priorities, and how they communicate when deciding research priorities together.
-
-
Develop strategies for providing initial neonatal care at birth beside the mother, rather than away from the mother, for preterm or sick babies by:
-
conducting a survey of current practice for initial neonatal care with the cord intact at NHS hospitals (work package 3.1)
-
describing parents’ experiences and views of care at very preterm birth, and developing a questionnaire tool to assess their views of care at very preterm birth (work package 3.2)
-
improving understanding of the physiology of transition from fetal to neonatal circulation by measuring umbilical flow at preterm birth and assessing how it varies with gestation (work package 3.3)
-
conducting systematic reviews and overviews (umbrella review) to assess the evidence for delivery room transitional assessment and support, and to identify research gaps (work package 2.1)
-
developing a mobile Bedside Assessment, Stabilisation and Initial Circulatory Support (BASICS) trolley to support providing newborn life support beside the mother, and with the cord intact (work package 3.4)
-
describing parents’ and clinicians’ experiences and views of neonatal care at birth beside the mother, and of the BASICS trolley (work package 3.5).
-
-
Generate information that will inform the design of a high-quality large multicentre UK trial comparing a policy for very preterm births of deferred cord clamping and initial neonatal care with umbilical cord intact, against immediate clamping and initial neonatal care after cord clamping, by:
-
conducting a narrative systematic review (framework synthesis) to identify the ethical challenges and their potential solutions in consent to recruitment of preterm or sick infants to clinical trials (work package 2.2)
-
conducting a pilot randomised trial comparing deferred cord clamping and initial neonatal care with the cord intact, against immediate clamping and initial neonatal care after cord clamping for very preterm births, including follow-up of the women for 1 year and until 2 years of age (corrected for gestation at birth) for the children (work package 4)
-
establishing a collaborative group to conduct a prospective meta-analysis of trials evaluating alternative strategies to influence placental transfusion at preterm birth, and developing the protocol for this analysis (work package 5).
-
FIGURE 1.
Overview of work packages. WP, work package.
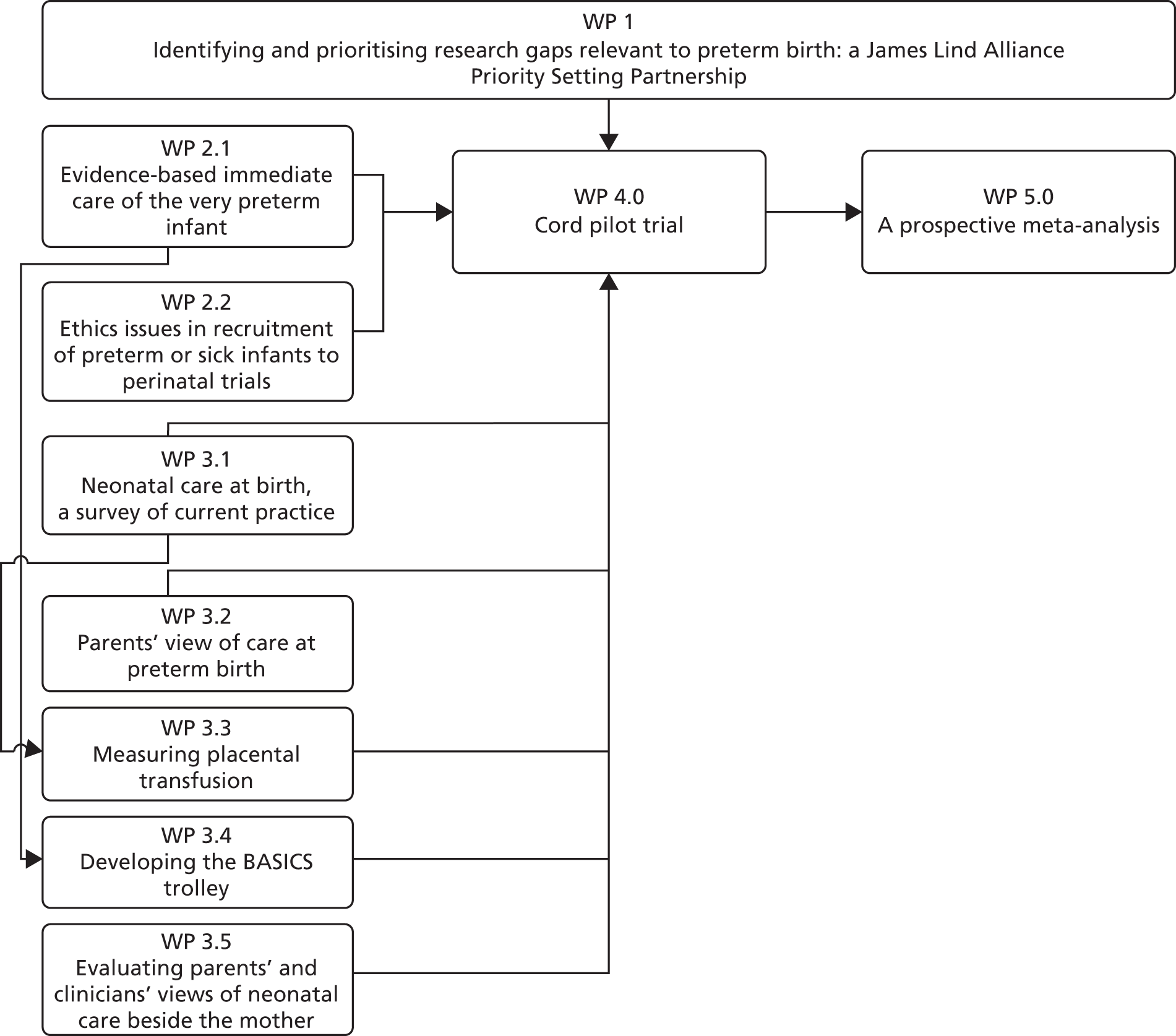
The prioritisation process ran in parallel to other work packages. It was added to the programme in response to feedback from the funding board at stage 1 of the grant application process. Hence, other work packages were not dependent on its outcomes. Although cord clamping did emerge as a top priority, we had already identified this as a priority via an informal process.
Priority setting for future preterm birth research
In the past, the health-care research agenda was determined primarily by researchers, and the processes for priority setting in research lacked transparency. 8 Often, research does not address the questions about treatments that are of greatest importance to patients, their carers and practising clinicians. 9,10 Many research questions have not been investigated and for many more the existing evidence is incomplete. The JLA (www.lindalliance.org/; accessed 24 January 2019) has developed methods for bringing patients and clinicians together to establish PSPs that then identify and prioritise ‘treatment uncertainties’ to inform publicly funded research. 11 This approach has been used successfully for a wide range of topics including asthma,12 urinary incontinence,13 vitiligo,14 prostate cancer15,16 and schizophrenia. 17 For urinary incontinence, of the top 10 priorities, five came from clinicians, four from patients and one from researchers. 13
When uncertainty about the effects of treatments relates to preterm birth, it seems particularly pertinent that research should address the most important priorities, and the most pressing needs, of these vulnerable children and their families and clinicians. Failure to identify and prioritise these uncertainties may result in suffering and death. 18 Examples in perinatal care where this failure to identify and prioritise important uncertainties has happened include the use of caffeine, which was shown to reduce the risk of cerebral palsy and developmental delay, and so was widely taken up in clinical practice, but only 30 years after being suggested for prevention of apnoea in premature babies. 19,20 In addition, the use of magnesium sulphate, which, after 60 years of controversy, was finally shown to be better for women with eclampsia than either diazepam or phenytoin. 21–23 Although the importance of women and their clinicians contributing to the perinatal research agenda is well established,8 we proposed the first PSP in the perinatal field.
To establish a PSP required bringing together representatives of women and their families and of clinicians and other health-care workers. Understanding of how people interact and make collective decisions in groups or committees with members from diverse organisations comes from health research, and from social psychology and business administration. 24 Larger groups may increase membership diversity,25 although this may be offset by a reduction in reliability of decision-making. The chairperson is crucial for establishing inclusiveness, openness and trust in the discussion. 25–28 If time is short, less knowledge is shared and evaluated and then decisions result more from judgement based on prior preferences than problem-solving,29 which may mean that they are more influenced by an individual’s status within the group. 25
When we planned the Preterm Birth PSP, evidence about how service users and clinicians make joint decisions on research priorities was lacking. 24 Improved understanding of how such partnerships work (e.g. if expertise based on qualifications, experience or problem-solving skills influences decisions30 or if the way arguments are framed changes as consensus develops) may offer insight into how the process could be improved.
The Preterm Birth PSP published a list of the top 15 research priorities,31 which is being used by the National Institute for Health Research (NIHR) and by researchers in planning new research. Study of the groups’ working processes has also led to recommendations about how decision-making might be improved.
Parents’ views and experiences of very preterm birth
Very preterm birth and subsequent hospitalisation of the baby can be an extremely distressing time for parents. 1,32 There has been little research into parents’ experiences at the time of very preterm birth, or their satisfaction with their care during the birth. In addition, previous work has often failed to include fathers. Women’s views and experiences during labour and childbirth are increasingly important to health-care providers and policy-makers,33 and may have an impact on subsequent health and well-being for the woman and her baby. 34,35
To ensure a strong parent focus throughout our programme, we planned qualitative interviews to explore parents’ experiences of very preterm birth and their first moments with their baby. 36–38 Regular multidisciplinary project meetings to plan these interviews, and to discuss the emerging themes and verbatim transcripts, were immensely valuable for subsequent work packages. These were particularly valuable for developing strategies for neonatal care beside the mother, and for planning the pilot trial. Our experience in conducting these interviews also contributed to further qualitative work exploring the views of both parents and clinicians of neonatal care at birth beside the mother,39,40 and of the new two-stage consent pathway in our pilot trial. 41,42
A number of instruments have been developed to assess women’s satisfaction with care during childbirth. When planning our programme we were not aware that any of these were designed to be used following very preterm birth, which is usually a different experience from giving birth to a healthy term baby. To confirm this we conducted a systematic review of available measures to assess parents’ satisfaction with care during labour and birth. 43 Having demonstrated there was not a suitable tool for very preterm birth, we developed a 20-item questionnaire called Preterm Birth experience and Satisfaction Scale (P-BESS) for use in this situation. 44 We used this questionnaire for the follow-up of women recruited to our pilot trial. It has been translated into Spanish and Portuguese.
Placental transfusion and neonatal transition
At birth, if the umbilical cord is not clamped then blood flow between the baby and the placenta may continue for several minutes. 45–48 This umbilical flow is part of the physiological transition from fetal to neonatal circulation, which, for very preterm infants, may improve resilience during this transition. 49–51 ‘Placental transfusion’ refers to the net transfer of blood to the baby between birth and cord clamping.
Cord clamping before umbilical flow ceases may restrict neonatal blood volume and red cell mass, and/or disrupt transition from fetal to neonatal circulation. For term births, umbilical flow usually continues for 2 minutes, but may continue for over 5 minutes. 46,48 The mean volume of placental transfusion at term is 100 ml, which is 29 ml/kg birthweight and 36% of neonatal blood volume at birth. 48 For preterm births, umbilical flow may continue for longer than for term births52 and is incomplete if the cord is clamped in 30–90 seconds. 53 This corresponds with development during gestation; at term, two-thirds of the fetoplacental circulation is in the infant, whereas for those born below 30 weeks’ gestation, a greater proportion of the fetoplacental circulation is in the placenta. 45 In addition, the preterm umbilical vein is smaller than at term, and uterine contraction less efficient; therefore, transition from the fetal to the neonatal circulation may be slower. Cord milking or ‘stripping’ seems to be an attractive option for preterm births, as potentially it increases neonatal blood volume without the need to defer cord clamping. 54 However, cord milking over-rides the infant’s physiological control of its own blood volume and blood pressure, and it interrupts transition to the neonatal circulation. 55
To improve understanding of the physiology of placental transfusion and assess when might be the best time to clamp the cord for preterm births, we measured umbilical flow at preterm birth. Although this proved to be more challenging than we had anticipated, the resulting data helped to inform the decision to wait at least 2 minutes before clamping the cord in the pilot trial.
Neonatal stabilisation and resuscitation at birth beside the mother
In the UK, about one-third of all newborn babies are attended at birth by neonatal resuscitation staff. For most, all that happens is an assessment, stimulation, thermal care and simple airway management. However, around 15% of these babies receive active stabilisation and/or resuscitation at birth, such as mask ventilation, intubation, cardiac massage or drug administration.
Transition to the neonatal circulation begins at birth when pulmonary vascular resistance falls as the lungs expand and fill with air, pulmonary blood flow increases and ductal blood flow declines as peripheral resistance falls. 56 Stabilisation at very preterm birth aims to assist this transition, and recommendations for newborn life support for very preterm infants are to prioritise establishment of respiration and a resting lung volume. 57 Traditionally, this was facilitated by immediate cord clamping, allowing the baby to be transferred quickly away from the mother for interventions such as airway opening manoeuvres, continuous positive airways pressure (CPAP) and tracheal intubation with or without prophylactic surfactant administration.
Providing initial neonatal stabilisation and resuscitation for very preterm or sick infants beside the mother would potentially allow newborn life support to be provided with the cord intact, facilitating a longer period before cord clamping than was possible at the time when we were planning this programme. It would also allow the woman and her partner to share the first moments of their child’s life, a more family-centred approach to care at birth. 58,59 Family-centred care in neonatal units, with improved communication and involvement of parents in their baby’s care, appears to benefit babies, is welcomed by parents60 and has been prioritised by the NHS. 61 Providing initial neonatal care beside the mother also has parallels with family presence during resuscitation of adults and children, an approach that is preferred by families and clinicians, and that appears to be beneficial. 62–65 Should the baby subsequently die, this time spent with the parents may prove to be an important factor in their experience of the life of their baby, and could potentially be of benefit in bereavement.
At the start of our programme, information about neonatal care beside the mother within the UK was anecdotal. 53,66 Therefore, we began by describing current practice at that time for care at birth67,68 and by assessing the evidence from randomised trials for delivery room neonatal interventions. We then developed and piloted strategies for providing initial neonatal care beside the mother, and assessed whether or not this was acceptable to parents and clinicians. This included both developing a new mobile trolley designed specifically for this purpose69 and adapting the existing equipment. 70 Our work demonstrating that providing newborn life support beside the mother is valued by parents and clinicians,39,40 and that care with the cord intact is feasible, contributed to the success of the Cord pilot trial. 71 Providing neonatal care beside the mother, with the cord intact, compared with after clamping and cutting the cord, has growing interest nationally and internationally. 72,73
Ethics issues in recruitment of preterm or sick infants to perinatal trials
Recruitment of preterm or sick infants to clinical trials requires approaching parents at a particularly difficult time, often with a tight time scale for making a decision. This raises challenges for obtaining informed consent to such research, especially issues regarding competence for consent, understanding of complex issues, insufficient time for parents to consider participation, and voluntariness if parents have a sense of obligation or feeling of debt to the clinician-researcher who is caring for their child. 74 On the other hand, if the problem of consent is not successfully addressed, this risks becoming an ‘orphan’ area of research. That is, if ethically permissible research cannot be designed, then this area of medical research will be abandoned.
Earlier work has explored these difficulties, specifically in the neonatal context. 74 Discussion of both the nature and the importance of consent, as well as empirical work on methods of obtaining consent and parental experience of those methods, has continued. 75–77 Hence, when we planned our programme it seemed timely to review understanding of these ethics issues in the light of this expanding literature, and of both the changing regulation for clinical trials and the increased public expectations of the conduct of research. Our overall aim was to identify a way of conducting ethical neonatal research in those circumstances where obtaining valid consent from parents has proved to be a significant challenge. The goal was to identify both the challenges to an ethically defensible consent process and their potential solutions. 78,79 This led to the development of a two-stage pathway for consent used and evaluated in the Cord pilot trial. This pathway accounted for almost one-third of recruitment to the trial, and was viewed positively by both parents and clinicians. 41,42 It was rapidly included in updated guidance for intrapartum research from the Royal College of Obstetricians and Gynaecologists. 80,81 The same two-stage approach has also been adapted for use in an acute stroke trial, for which randomisation is within 8 hours of the stroke. 82
Systematic review of timing of cord clamping at very preterm birth
Thirty years ago, it was first suggested that immediate cord clamping for preterm babies might increase the risk of intraventricular haemorrhage (IVH). 83 Postulated mechanisms for this increase were hypovolaemia or increased fluctuation in blood pressure during the abrupt transition from fetal to neonatal circulation.
At the time that we planned this programme, the Cochrane review of timing of cord clamping and other strategies for influencing placental transfusion at preterm birth included 10 trials, with 454 mother–infant pairs largely recruited before 33 weeks’ gestation. 84,85 In these trials, deferred cord clamping ranged from 31 to 120 seconds and immediate cord clamping ranged from 5 to 20 seconds. Many outcomes were reported by only a few studies, with potential for reporting bias. Immediate clamping was associated with an increased risk of transfusion for anaemia [relative risk (RR) 1.57, 95% confidence interval (CI) 1.14 to 2.16; four trials, 183 infants] or hypotension (RR 1.94, 95% CI 1.06 to 3.54; 3 trials, 90 infants) compared with deferred clamping. The risk of IVH on ultrasound scan was also higher for babies allocated immediate clamping (RR 1.90, 95% CI 1.27 to 2.84; seven trials, 329 infants), but for severe IVH (grade 3 or 4), a more reliable predictor of long-term outcome, there was no clear difference (RR 1.17, 95% CI 0.27 to 5.02; five trials, 269 infants). There was no clear difference between the groups in temperature on admission to a neonatal unit, but only three trials (143 infants) reported this outcome. One study had reported follow-up at a median age of 7 months for 58 out of 67 surviving infants, with no clear differences between the groups. 86
Alternative policies for timing of cord clamping at very preterm birth
In previous trials for deferred clamping, the decision about when to clamp the cord was usually a balance between allowing some umbilical flow and what was perceived as an acceptable delay in transferring the baby to the neonatal team. As standard practice was for the neonatal team to be located either at the side of the room or in a room nearby, this necessitated early clamping and cutting of the cord, particularly for infants requiring stabilisation or resuscitation at birth. Therefore, providing initial neonatal care at birth with the cord intact would make it feasible to defer cord clamping for longer than had previously been possible, including for high-risk infants who have the most potential for benefit.
Our programme to improve the quality of care and outcome at very preterm birth focused on care at the time of birth. We reviewed evidence from systematic reviews 36–38,67,68,70,78,79,87 relevant to delivery room neonatal care, surveyed current practice, described parent experiences, measured umbilical blood flow, developed strategies for providing newborn life support beside the mother, and reviewed ethics issues in recruitment of preterm and sick babies to clinical trials. These work packages all contributed to the design and conduct of the Cord pilot trial, a pilot randomised trial to assess the feasibility of conducting a large UK trial comparing alternative strategies for cord clamping. To provide adequate power for long-term follow-up of the children and inform the design of a large definitive trial, we planned a prospective meta-analysis of similar studies.
This focus on the time of birth and cord clamping was identified as a priority by informal discussion with parent representatives,88 and parent representatives worked in partnership with clinicians and researchers to develop the application and conduct the research. The topic had also been identified as a research priority by researchers,58,84,85,89 obstetricians,90 midwives,90 neonatologists (Duley L, Farrar D, McGuire W, Oddie S. Survey of the Extended Neonatal Network to Assess Views on Timing of Cord Clamping and Placental Transfusion: Report Prepared for the Extended Neonatal Network. 2009. Unpublished), the National Institute for Care Excellence (NICE),91,92 and the Royal College of Obstetricians and Gynaecologists. 59 Therefore, it was unsurprising that this uncertainty was included in the list of top priorities from our JLA Preterm Birth PSP. 31
Changes from the original programme plan
The phrase ‘preterm birth’ is broad. Our programme had a specific focus on care around the time of birth, and our intention was that this would be reflected in the scope of our JLA PSP (work package 1). However, from the initial scoping meeting, it was clear that both service users and clinicians had a different view and the discussion ranged from risk factors to prognosis and the grandparent perspective. This continued at the first meeting of the partnership steering group. As the partnership was between service users and clinicians, the researchers agreed that their views should determine scope. The challenge of too wide a scope led to delays in progressing the priority setting, and the process progressed only once it was agreed that this should be narrowed. This delay and the resource demands of the priority setting process meant that it was not possible to conduct the planned work on outcomes.
Although our sampling frame for the qualitative interviews with parents (work package 3.2) included one hospital where deferred cord clamping was in routine practice for very preterm birth, no parents interviewed had experienced neonatal care beside the mother. It was, therefore, not possible at that time to assess their experiences of this type of care. Instead, we did this by assessing parent and clinician experiences of neonatal care beside the mother using semistructured interviews, rather than the planned focus groups (work package 3.5). This was in order to gain more detailed accounts of the events and experiences than would be available in a focus group. In addition, because in focus groups individuals might be reluctant to disagree with the dominant view, you get a socially constructed view rather than people’s individual views and experiences.
Measuring placental transfusion at preterm birth proved to be far more challenging than our earlier work at term birth. 48 This was primarily because of the unpredictability of preterm birth, which made it difficult for the research team to be present and with the equipment set up in time for the birth. As we secured funding to assess feasibility of a similar trial in low and middle income countries, this study was replicated successfully in India. 47
The Cord pilot trial was conducted as planned. Added value of this multidisciplinary programme led to several additional elements. First, innovative methods for consent to participate in emergency perinatal trials was identified as a research gap by the ethics framework review. This led to the development of a two-stage oral assent pathway used in the trial when birth was imminent, which boosted recruitment. Second, to evaluate this pathway we used our experience of semistructured interviews with parents and clinicians (work packages 3.2 and 3.5) and of the framework analysis of ethics issues (work package 2.2) to design and conduct qualitative interviews with women and clinicians who had experience of consent in the Cord pilot trial. Finally, excellent recruitment contributed to the Trial Steering Committee (TSC) assessment that feasibility had been demonstrated and that we should seek funding to progress to the definitive trial. The TSC also recommended that recruitment continue while funding was sought, to avoid ‘stop/start’. Hence, recruitment was extended and only closed when the funding application was rejected. To maximise the value of this additional recruitment, follow-up of both women and children was also extended and data for all those randomised are presented here.
For the prospective meta-analysis, the first cycle of analysis had been planned within the programme. However, as data from the two largest studies (Cord pilot trial93 and the Australian Placental Transfusion Study94) were not available within the time scale of the programme, this analysis has been postponed. In addition, the collaborative group of triallists has agreed to expand the protocol to a retrospective individual participant meta-analysis with nested prospective meta-analysis.
Programme management
The co-applicants formed a programme steering group to oversee implementation of the research programme as well as integration and timely delivery of the work packages. This group met every 4 months for the first 3 years, and every 6 months thereafter. A project management group of coinvestigators supported each work package. In addition, the JLA Preterm Birth Priority Setting Partnership (PSP) formed a steering group from membership organisations and stakeholders, and independent oversight of the Cord pilot trial was by an independent TSC and Data Monitoring Committee.
Patient and public involvement
Throughout this report, we use the term ‘service users’ to describe patient and public involvement. Because having a baby is a physiological event, pregnant women and parents are not ‘patients’. The topic of when to clamp the umbilical cord at very preterm birth was identified through discussion with parent representatives, including Gill Gyte, offering perspectives from the National Childbirth Trust (NCT). Consultation with parents, through Bliss, quickly made clear that parents viewed change in practice at this difficult time with considerable caution. A strong parent perspective throughout the planning and conduct of our research was clearly essential to ensure relevance, quality and a timely delivery. This was achieved through partnership with representatives of the NCT (https://www.nct.org.uk/) (GG) and Bliss (https://www.bliss.org.uk/) (Jane Abbott and Zoe Chivers). Their input included being co-applicants in the grant application, membership of the programme steering group, membership of most project management groups, co-authorship of many programme publications, and active dissemination of outputs through their organisations. Success of this collaboration was reflected in a Bliss ‘Advancing care through research’ award for the programme.
Additional patient and public involvement contributed to the JLA Preterm Birth PSP, which involved both service user organisations and service users (parents and adults born premature). A personal reflection from one service user member of the steering group demonstrates both the value and the challenges of such involvement (Box 1). In addition, the TSC for the Cord pilot trial included two independent parent representatives, and parents commented on information for participants.
In the early part of 2012, I received an e-mail in my work capacity at the charity KIDS. Attached to the introductory e-mail was a survey about preterm birth research, which I circulated to my caseload of parents in the London borough of Camden. I then completed the survey myself, as I also happen to be a parent of a daughter who was born 11 weeks prematurely.
I was at the time just completing a Master’s degree and my research dissertation regarded an aspect of preterm birth and the development of Cerebral Palsy. With this added interest, I contacted the researcher who had sent out the survey in order to discuss the research further. During our meeting, she mentioned that a fundamental part of this project was for service users and health-care professionals to come together to set priorities and that my involvement, as a parent, within the steering group would be most welcome.
The journey that followed was both fascinating and rewarding. There was a steep learning curve about a process like this – which saw the identification of unanswered questions about causes, care and treatment of preterm birth through to the prioritisation by service users, clinicians and researchers of the Top 15 issues for future research. There was also great validation in being a respected and involved participant of the steering group throughout the process, culminating in my presentation of the parent perspective on this piece of work at the European Congress of Perinatal Medicine in Florence (where our work won a prize in the top 6)!
Having felt incredibly held and supported during steering group meetings where my contribution was encouraged and valued, there were times, particularly during group conference calls or in the e-mail rounds, when I wondered whether my input was necessary or appropriate and I often felt quite overwhelmed by the scientific complexity of the overall process and project. This seems pertinent to acknowledge in a field that has patients at its centre, but often has to go beyond their sphere of understanding or comfort.
Initially it felt that my value was to bring in my personal experience, and I was often called on to reflect my perspective as someone who had been impacted by preterm birth. However, at times it was hard to know how to pitch this within the high level of theoretical rhetoric, both from a medical and a research position. I did often wonder at how the personal voice can be lost in the higher level of complexities and detail.
All of that said, the overarching feeling has been one of being embraced and encouraged, with the reminder that this sort of collaborative work brings together individuals with very different skills and experience for a reason. The process as a whole felt empowering and the knowledge that my personal experience was playing a vital part in bringing fresh understanding was validating.
Bev Chambers
Reproduced with permission from Bev Chambers.
Work package 1: identifying and prioritising research gaps relevant to preterm birth – a James Lind Alliance Priority Setting Partnership
See Appendices 2–4 for the published and unpublished reports of this work.
Research aims
Our aims were to:
-
identify and prioritise research gaps relevant to preterm birth that are most important to people affected by preterm birth and health-care practitioners in the UK
-
describe how service users and clinicians interact when making collective decisions about research priorities, and how they communicate when deciding research priorities together.
Identifying and prioritising research gaps relevant to preterm birth
Prioritisation process
Using methods established by the JLA,95 we first identified unanswered questions about the prevention and treatment of preterm birth from people affected by preterm birth, clinicians and researchers. Then we prioritised those questions that people affected by preterm birth and clinicians agreed are the most important (Figure 2).
FIGURE 2.
Flow chart of the JLA Preterm Birth PSP. HP, health professional; PaPB, people affected by preterm birth.

Initiation of the partnership
The Preterm Birth PSP was initiated in November 2011, following an earlier introductory meeting of potential stakeholders. The 29 participating organisations were asked to complete a declaration of interests, and a steering group was convened that included members from nine of these organisations. At the introductory workshop it was clear that many participants felt that the scope of the partnership should be wider than was initially envisaged, for example including uncertainties about the causes of preterm birth, the prognosis following being born preterm, and interventions long before birth.
Consultation
As widening the scope too far risked making the prioritisation unachievable, the steering group restricted the scope to uncertainties about treatments and to interventions during pregnancy and around the time of birth or shortly afterwards (taken up to the time of hospital discharge for the baby after birth).
Research questions were gathered from people affected by preterm birth, clinicians and researchers using methods developed by the JLA. 95 This included a survey completed online by 349 people, and in paper format by 37 women attending specialist preterm birth antenatal clinics at two tertiary level hospitals and parents visiting two neonatal intensive care units. These 386 responses contained 593 potential research questions. In addition, 540 potentially relevant research questions were identified from systematic reviews of existing research and from national UK clinical guidelines. 96–107
Collation
All questions were screened to identify those sufficiently similar to either merge or group into broader questions and to remove any that were out of scope, unclear or being answered by a subsequent or in-progress randomised trial. This left 70 unanswered questions from the survey, 28 from systematic reviews and 24 from clinical guidelines remaining in the process. Of these 122 questions, 18 overlapped with other questions and were merged to give a final ‘long list’ of 104 unanswered research questions.
Prioritisation
This was a two-stage process. First, the long list of 104 questions was sent out for voting on the top 10 questions, online and in paper format, using a modified Delphi survey. The steering group reviewed ranking by the 507 people who voted, overall and by stakeholder group. They removed remaining overlap or repetition between questions, before agreeing the shortlist of 30 questions to go forward to the second stage: a prioritisation workshop. This workshop had 34 participants, including representatives from people affected by preterm birth and clinician organisations as well as parents of babies born preterm and adults who were born preterm. At the workshop, nominal group technique95 was used to achieve consensus on ranking the 30 questions.
Key findings
We identified 104 unanswered research questions (Oliver S, Duley L, Uhm S, Crowe S, David A, James CP, et al. Top Research Priorities for Preterm Birth: Results of a Prioritisation Partnership Between People Affected by Preterm Birth and Healthcare Professionals. Unpublished). Consensus was not achieved on a top 10, and so a top 15 research priorities was agreed (Box 2). 31 This top 15 had significant differences to the ranking following public voting. The most noticeable were two questions ranked 18 (How do stress, trauma and physical workload contribute to the risk of preterm birth, are there effective ways to reduce those risks and does modifying those risks alter outcome?) and 26 (What treatments can predict reliably the likelihood of subsequent infants being preterm?) at the workshop. These were outside the top 15 but had been ranked 3 and 4, respectively, in the public vote.
-
Which interventions are most effective to predict or prevent preterm birth?
-
How can infection in preterm babies be better prevented?
-
Which interventions are most effective to prevent necrotising enterocolitis in premature babies?
-
What is the best treatment for lung damage in premature babies?
-
What should be included in packages of care to support parents and families/carers when a premature baby is discharged from hospital?
-
What is the optimum milk-feeding strategy and guidance (including quantity and speed of feeding and use of donor and formula milk) for the best long-term outcomes of premature babies?
-
What is the best way to judge whether or not a premature baby is feeling pain (for example, by their face, behaviours or brain activities)?
-
Which treatments are most effective to prevent early-onset pre-eclampsia?
-
What emotional and practical support improves attachment and bonding, and does the provision of such support improve outcomes for premature babies and their families?
-
Which treatments are most effective for preterm premature rupture of membranes?
-
When is the best time to clamp the umbilical cord in preterm birth?
-
What type of support is most effective at improving breast feeding for premature babies?
-
Which interventions are most effective to treat necrotising enterocolitis in premature babies?
-
Does specialist antenatal care for women at risk of preterm birth improve outcomes for mother and baby?
-
What are the best ways to optimise the environment (such as light and noise) in order to improve outcomes for premature babies?
Reprinted from The Lancet, Vol. 383, Duley et al. ,31 Top 15 UK research priorities for preterm birth, pp. 2041–2, © 2014, with permission from Elsevier.
Describing how service users and health-care professionals interact when making collective decisions about research priorities
Methods for data collection
The study sample comprised attendees at 13 meetings of the Preterm Birth PSP: 12 steering group meetings (three conference calls, nine face to face) and the final prioritisation workshop. These were all recorded and transcribed. An ethnographical approach108 was adopted with participant observation109 and discourse analysis110 of the recordings, field notes and analysis of documentary records of meetings and steering group activities.
Analysis
Transcribed data were coded using an analytical framework based on the Elaboration Likelihood Model111–113 using NVivo 10 (QSR International, Warrington, UK) software. We coded discussion as using either a central route (rational argument with evidence) for persuasion or a peripheral route (relying on emotional responses to cues such as authority, commitment, consistency, liking, reciprocation, social proof or scarcity). For example, prioritising a treatment because the speaker ‘wanted to know if there would be better outcomes’ was coded as ‘central’ because of its rational approach. Asserting ‘I think this topic has to be number one because that is sort of nice’ was coded as ‘peripheral’ with a ‘liking’ cue.
To investigate decision-making based on values, transcripts were coded into three types of discussion:114 informational (facilitator encourages participants to speak, defers controversy and lets participants know their ideas will not be evaluated), problematical (participants consider the information and/or values needed to address the issue intelligently) and reflexive (participants discuss their own discussion to learn from the process).
To understand the process of consensus development, the JLA approach was compared with the ‘Group Development Model’115 (Figure 3), which argues that every group experiences these five stages before becoming a self-reliant unit. At each stage, the dynamics of the group change from periods of inefficiency and uneasiness through to a period of high performance.
FIGURE 3.
Group Development Model versus JLA stages of partnership working.

Key findings
Throughout their meetings, steering group members used a central route pathway (281/502, 56%) more often than a peripheral route (221/502, 44%), and the peripheral cues they used most were ‘commitment’ and ‘consistency’. During the final workshop, ‘social proof’ (i.e. peer pressure, ‘we do this in our group’) was used most frequently. Health-care professionals used the central route (n = 33) more than service users (n = 15), and service users used the peripheral route (n = 23) more often than health-care professionals (n = 17).
The main types of discussion were ‘informational’ and ‘problematical’, with ‘problematical’ increasing over time. For the first 18 months (up to compiling the long list of uncertainties) there were no ‘reflexive’ discussions, and these remained less frequent (n = 9) than ‘informational’ (n = 104) and ‘problematical’ (n = 169). For both ‘informational’ and ‘problematical’ discussion, speakers used central routes more often than peripheral ones. When they used peripheral routes, for ‘informational’ discussion they used all the peripheral cues. For ‘problematical’ discussion, cues used the most were ‘consistency’ during steering group meetings, and ‘social proof’ during the final workshop. ‘Scarcity’ was more common later in the process when there was more time pressure. Participants were less likely to accept peripheral route messages, although when supported by a central route message from another speaker it became more persuasive.
Four questions ranked in the top 10 after public voting were outside the final top 15. These were stress and physical workload, preventing subsequent preterm birth, screening in the first trimester and multiple birth. The last three moved down the ranking based on the argument that they were included in the overarching top ranked question ‘Which interventions are most effective to predict or prevent preterm birth?’. Arguments against ‘stress and physical workload’ were that it was similar to another question and that it is not a conventional treatment and would be difficult to define or change.
There were similarities between the Group Development Model stages and the JLA PSP process, in particular that ‘forming’ (or team building) was comparable with ‘initiation’, and ‘adjourning’116 was comparable with ‘reporting’. At ‘storming’, team members were comfortable expressing discontent and challenging other opinions, and at ‘norming’ they had a common goal and shared responsibility for achieving it. In the PSP, these two stages were difficult to distinguish because the group repeated ‘storming’ after ‘norming’. At ‘performing’, team members were competent, autonomous and able to handle the decision-making process, which was similar to ‘prioritisation’ in the PSP. However, when new members joined for the final workshop, this returned the group to an early development stage (‘forming’), as they needed to get to know each other and define their roles and tasks. Only then could the group begin to ‘perform’ and prioritise the research questions. This discrepancy in terms of group development between steering group members and the new participants may have influenced the quality of the final consensus.
Strengths
Strengths of this Preterm Birth PSP include the large numbers of participants in the process and the range of stakeholders involved. Although several of the top priorities are already well recognised as important, such as what is the optimum milk-feeding regimen for preterm infants, others are indicative of areas previously under-represented in research (e.g. packages of care to support families after discharge and the role of stress, trauma and physical workload in the risk of preterm birth). This is in keeping with findings from previous JLA partnerships and highlights the value of shared decision-making. 117
Challenges
Preterm birth is associated with factors such as lower socioeconomic status, ethnicity and maternal age. 118 Despite implementing strategies to reach a representative population, our respondents remained primarily white and with a relatively high proportion of homeowners and so were not representative of those most affected by preterm birth. This may limit the relevance of these priorities to other populations.
The JLA PSP process uses a modified Delphi with individual voting, followed by a face-to-face workshop using Nominal Group Technique. Combining these two methods aims to maximise the advantages of both while minimising their disadvantages. The ‘lost priorities’ demonstrate that merging the two methods may, in some instances, weaken the benefits of each method. Large changes in ranking between individual voting and the final workshop appeared to be related to difficulty in the perspective of people affected by preterm birth being heard in the large group session, and a difference in the priorities of two key groups of health professionals (neonatologists and obstetricians).
Maintaining balanced representation between people affected by preterm birth and the different groups of health professionals for the final prioritisation workshop was challenging, and may have influenced the final ranking. The difficulty in achieving consensus underlines the complexity of priority setting for research, particularly for preterm birth. Pregnancy is not an illness or disease, and it involves at least two people (mother and child); preterm birth can have lifelong consequences for them and their families, as well as for the health services and society. This complexity and the differing priorities of different stakeholders make it important to consider the top 30 list, and the long list of 104 questions (Oliver et al. , unpublished), as well as the top 15 priorities, when planning and funding new research. 31
Implications for future research
These 15 top priorities provide guidance for researchers and funding bodies to ensure that future preterm birth research addresses questions that are important both to service users and to clinicians. Although people affected by preterm birth and health-care professionals had many shared priorities, they had different perspectives on some questions. Priorities may also change over time and in different settings. Therefore, when planning and funding research it is important to consider not only the top 15 priorities but also the top 30 ranked by those affected by preterm birth and the top 30 ranked by health-care professionals.
Future prioritisation processes, particularly those with a similar wide range of health-care professionals, should endeavour to anticipate potential different perspectives and mitigate any imbalance where possible, and should report voting separately by ‘service users’ and health-care professionals. Health-care professionals who are also researchers should declare this potential conflict before participating in the prioritisation workshop, so that it can be taken into account.
Work package 2.1: evidence-based immediate care of the very preterm infant
Newborn infants who have delay in establishing independent respiratory effort after birth may require transition support in the delivery room. To support development of guidance for providing initial neonatal care beside the mother, and also to provide a context for determining how deferred cord clamping might be integrated with evidence-based transitional assessment and support practices in the Cord pilot trial, we conducted an overview or ‘umbrella review’ of relevant Cochrane reviews.
See Appendix 5 for the unpublished report of this work.
Research aims
Our aims were to identify Cochrane reviews of delivery room transition support interventions, appraise review quality and identify important gaps in the evidence.
Methods for data collection
We undertook a systematic overview (umbrella review) using the standard methods of the Cochrane Collaboration and the Centre for Reviews and Dissemination. 119,120 We registered the overview on PROSPERO (registration number CRD42012003038). We searched the Cochrane Database of Systematic Reviews121 (Issue 6, 2015) for reviews evaluating any intervention for delivery room support of newborn infants. We excluded reviews of interventions that are more usually or feasibly delivered following admission to the neonatal unit, and those administered to all infants as part of routine practice.
Analysis
For each review, two reviewers independently extracted information on review quality characteristics, and on the participants, treatment and control interventions, and outcomes. Review quality was assessed using the 11-item AMSTAR (A MeaSurement Tool to Assess systematic reviews) tool. 122,123
Key findings
Eighteen Cochrane reviews were identified. Broadly, these reviews assessed delivery room interventions for airway management, respiratory or circulatory support (four reviews); surfactant replacement therapy for preterm infants with or at risk of respiratory distress syndrome (eight reviews); supplemental oxygen or other drugs for infants compromised at birth (five reviews); and strategies for influencing placental transfusion (one review). The overall quality of reviews was good, but methodological quality of the included trials varied greatly. Four reviews had no included trials. The most commonly prespecified primary outcomes were death, incidence of chronic lung disease and neurodisability. Two reviews prespecified surrogate outcomes, such as physiological measures, rather than clinically important primary outcomes. There are few data on long-term neurodevelopmental outcomes.
Of the four reviews that assessed interventions to support the infant airway and breathing, two did not find any eligible trials and one included only a single small trial. 124–126 The fourth review assessed delivery room airway support for infants at risk of meconium aspiration (four trials, 2884 participants) and concluded that there is no evidence that routine endotracheal intubation reduces mortality or morbidity in vigorous term babies with meconium staining compared with standard resuscitation. 127
The strongest evidence supported type and timing of surfactant administration for preterm infants. Two reviews, originally published in 1997, provided strong evidence that for very preterm infants, surfactant replacement reduced the risk of death by about 40%. 128,129 A related review concludes that natural surfactant is more effective than synthetic surfactant for reducing the risk of death. 130 These reviews are now regarded as ‘complete’, as further trials would be unlikely to change their conclusions. Subsequent reviews found evidence that early surfactant administration with brief ventilation reduces the need for mechanical ventilation and associated morbidity, but that prophylactic (delivery room) administration is not more effective than delayed selective administration when infants have prophylactic nasal continuous positive airway pressure support. 131,132 Uncertainty remains about the comparative effects of newer synthetic surfactants that contain surfactant protein mimics, and of novel non-invasive routes for administering these. 133,134
Five reviews assessed supplemental oxygen or other drugs for infants compromised at birth. One compared using air rather than 100% oxygen for newborn resuscitation at birth. 135 Five trials were identified (three were quasi-randomised), but the review concluded that there was insufficient evidence to support a recommendation for using either intervention. Three reviews assessed other drug interventions. 136–138 Of these, two assessed adrenaline or sodium bicarbonate during resuscitation and found insufficient evidence for reliable conclusions. 136,137 The third review assessed naloxone for infants exposed in utero to opiates and identified nine trials, but none assessed clinically important outcomes. 138 The fifth review examined interventions to prevent hypothermia in newborn very preterm infants,139 and it concluded that various measures, including plastic wraps or bags and warming mattresses, reduce the risk of delivery room hypothermia, but found insufficient data to assess effects on infant morbidity and mortality.
One review assessed timing of cord clamping and other strategies for influencing placental transfusion at preterm birth. 140 This review found evidence that deferring cord clamping for 30–120 seconds, rather than clamping before 30 seconds, probably reduced the need for blood transfusion and possibly reduced the risk of IVH. Data from 13 out of the 15 included trials did not identify a statistically significant effect on risk of death. Long-term neurodevelopmental outcomes were not reported.
Implications for research
Some reviews identified key evidence gaps. These were mainly related to pharmacological intervention for transition support or resuscitation of newborn infants, and the effectiveness of new, less invasive forms of airway management (and related issues regarding surfactant delivery).
Work package 2.2: ethics issues in recruitment of preterm or sick infants to perinatal trials
The recruitment of very preterm or sick infants to clinical trials requires approaching parents at a difficult time, often with a tight time scale for making a decision. This raises challenges for obtaining valid informed consent to such research.
See Appendices 6 and 7 for the published reports of this work.
Research aims
We aimed to synthesise:
-
Observational and qualitative studies that explored the process of recruitment and consent, and reported parents’ and clinicians’ views and experiences.
-
Analytical (or philosophical) studies that have examined the pertinent ethical questions. These concern the validity of consent, the proper understanding of the parental role in giving or withholding consent, varied possible methods of seeking consent, the best interests of those involved, issues of risk, and the parallels between consent processes in relevant research and clinical contexts.
The goal was to identify both the challenges to an ethically defensible consent process and their potential solutions. Ideally, these solutions would include strategies that we could implement to improve the consent process in the Cord pilot trial.
Methods for data collection
The methods used for this review conformed to those set out for a framework synthesis. 141 The first stage was the development of a tentative initial conceptual framework based on prior knowledge of the existing philosophical literature on informed consent, including in neonatal research. 142,143 This initial framework informed the criteria for including studies and it suggested terms for the literature searches. After refining the framework in the light of the literature from the first searches, the second searches were developed. All searches were updated (for examples of search strategies, see Appendices 6 and 7).
Analysis
Empirical studies
For the empirical studies, the conceptual framework was modified to focus on specific questions prioritised by the authors, and further searches devised, guided by bioethics review methods studies. 144,145 We screened abstracts of the papers from identified citations for inclusion, using the five criteria from the modified framework:
-
parents’ views of neonatal trials
-
clinicians’ views of neonatal trials
-
parents’ and clinicians’ views of parental consent/decision-making in clinical practice if the articles concerned the validity of the consent in an emergency situation, or during or soon after labour
-
validity of consent
-
other options for gaining consent.
Analytical studies
For the analytical studies, discussion of the initial themes that were identified confirmed the conceptual framework. Further searches were informed by this framework and by bioethics review methods studies. 144–146 We screened abstracts of papers from identified citations for inclusion, using the five criteria from the confirmed framework:
-
consent, participation or recruitment for neonatal research (relevant to clinical trials)
-
parental decision-making for treatment of, or research with, sick or preterm neonates
-
parental decision-making for birth and/or labour
-
methodology in emergency/urgent neonatal research
-
alternative ways of gaining consent for neonatal research.
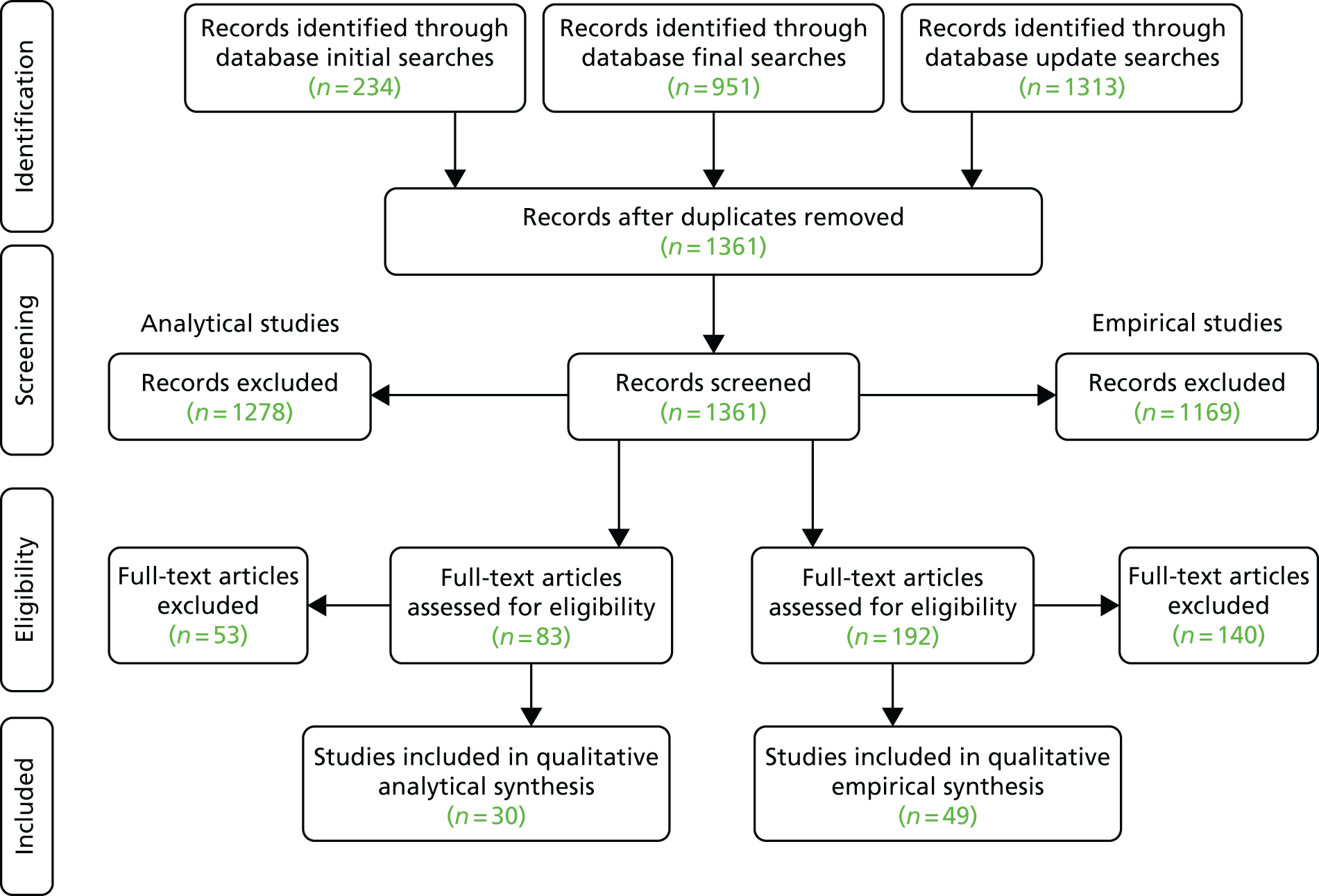
Overall, we ‘included’ 49 empirical papers and 30 analytical papers (Figure 4). All studies met the quality assessment criteria. For the empirical papers, we identified five themes: (1) attitudes of parents, (2) attitudes of clinicians, (3) validity of consent, (4) different consent processes and (5) miscellaneous topics. For the analytical papers we identified four themes: (1) ethical basis of parental informed consent for neonatal research, (2) validity of parental consent, (3) other options for gaining consent, and (4) risk and the double standard between consent for treatment and consent for research. We coded articles and tabulated them against these themes.
FIGURE 4.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for the framework synthesis. Derived from Wilman et al. 79 [© Wilman et al. , 2015. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated] and Megone et al. 78 [© The Authors, 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated].
Key findings
Empirical research
Our review found that the stated motivations for parents to consent were altruistic: the benefit entering the trial might bring to others; the possibility of some benefit for their baby, or themselves; or the trial bringing some hope in a hopeless situation. 79 Motivations to decline participation in research were inconvenience to parents, burden to their child, or worries about risks, particularly for the baby. Some parents felt that they did not have enough time to decide. Severity of illness of the infant did not appear to affect trial participation.
Parents felt that formal consent for research was necessary and protected their child. Most parents felt that they ought to make the decision about whether or not their child participated in research. They wanted to feel informed and involved, and considered it their responsibility to make this sort of decision. However, many parents also wanted input from others before making the decision, including family and their doctor. Some parents did not want to make the decision. Others felt that being approached about a trial added to their stress and anxiety, particularly if they were approached at an inappropriate time.
Clinicians respected parental authority and largely felt that parental consent was necessary for trials. Reasons for this were that it respects ‘parental rights’, parents are best placed to act in the best interests of their child, and parents must live with the long-term outcomes of their decision. On the other hand, some clinicians felt that clinicians are the best decision-makers for sick babies, and some wanted to spare parents the burden of making this decision.
Clinicians felt that consent forms protected researchers (by providing confirmation that information was given), and aided communication with parents. However, clinicians also worried that too much information added to parents’ burden, and noted barriers to effective communication (e.g. intimidation of parents, and lack of care and support for them). Clinicians raised concerns about balancing their responsibility to the trial with their responsibilities to the parents. Some considered these ‘equal responsibilities’ and felt that it was possible to discharge both. Others saw the possibility of ‘divided responsibilities’ causing anxiety to clinicians, or saw the need for ‘prioritised responsibility’ in which clinicians put parents’ interests before the interests of the trial.
Interviews with parents who had given consent for neonatal trials suggested that this consent was valid for only 59% in terms of voluntariness, competence and informedness. Some parents reported feeling pressure to participate, whereas others felt no pressure. Competence, or capacity, to give valid consent may be affected by factors such as emotional state, understanding and time available to decide. Parents reported being calm when they made the decision, but some felt that they had been anxious or stressed. Some parents reported not making a proper decision because of the pain or anxiety, others reported making considered decisions despite pain, time pressure or anxiety. Some parents had clear understanding of a trial, but others reported problems with understanding a trial about which they were asked for consent. Some parents felt that they could make a genuine decision even with suboptimal understanding. Communication skills of the clinician affected understanding. Although parents largely felt that they had adequate time, in the circumstances, to make the decision, a significant minority did not. Parents made rapid decisions regardless of how much time was available, and they needed more time if there was a greater risk. Parents largely received satisfactory information; however, some parents reported problems with the information that they were given. The information sheet was often unread, although in some studies parents remembered being given the sheet and used it when making their decision.
In general, mothers acknowledged the difficulties for researchers in finding the ‘right time’ to approach them for consent for perinatal research. Some parents would prefer antenatal consent rather than consent during, or after, labour, and would like information earlier in pregnancy even if not recruited then. However, parents were not completely comfortable with antenatal consent as they reported not seeing the relevance of the trial at that time, and felt greater anxiety if told about the trial earlier in pregnancy. Some parents were comfortable with consent in labour, but some were not. Parents were not comfortable with waived consent.
Opting out is when consent is presumed unless the parent explicitly opts out of the trial. Although some parents were comfortable with opting out, others were not. For continuous consent, there is initial agreement to participate with further discussion and information after recruitment. Validity of the later ‘continued’ consent improves when discussion continues after recruitment. Some parents approve of continuous consent, but some have concerns about receiving further information at a later stage when that might have affected their original decision. One study reported a staged consent process in which consent (oral or written) is sought antenatally and the consent is sought again at the point of intervention. However, at present this is only reported, not discussed.
The main conclusions are that:
-
there is widely held agreement that it is important that parents do give or decline consent for neonatal participation in trials, but that
-
there is evidence that existing consent procedures are unsatisfactory, and that
-
none of the proposed alternative consent processes reviewed by the research is satisfactory
-
there are some significant gaps in the empirical research in this area.
Analytical research
The analytical research addressed the justification for seeking consent from a parent (or parents) to neonatal or perinatal trials. 78 This issue arises because it may be thought that it is not sensible to seek consent when the most affected party is unable to give consent, in which case the focus should simply be on that individual’s best interests. Hence, it is relevant to query the basis for consent being given, or declined, by the parent. Justification for parental consent includes the importance of autonomy, such as parental autonomy, or the parents’ own rights to make decisions about their child. A similar claim holds that parenting decisions are part of deciding how to conduct one’s own life, while another defence suggests that fetal rights are part of maternal autonomy. However, others have rejected any defence of parental consent that appeals to autonomy, arguing that a parent’s interest in autonomous parenting may not outweigh the child’s interests, or that parents do not ‘own’ their children.
A second claim in defence of parental consent is that parents should be seen as surrogate or proxy decision-makers, where ‘proxy’ means not simply someone who acts on behalf of another but one who represents another’s views. Some suggest that there might be reason for a parent to give consent even if an appeal to autonomy or rights is rejected. Thus, it is held that it is not appropriate to think of the ‘autonomy’ of a neonate and, therefore, it is not appropriate to think of ‘informed consent’ for a child. Another line of argument is that consent in the neonatal context is a case of ‘family decision-making’, because it is not appropriate to consider the child’s decision in isolation. In addition, it is suggested that parents should give consent because they will bear the consequences of the decision. However, some claim that the requirement for parental consent rests on the value of beneficence; as the purpose of informed consent is protection of the best interests of the child, it is the responsibility of parents to make decisions as a way of promoting their child’s best interests, so they should give consent. Some have argued that parents should be allowed to make the decision only when it is in the best interests of their child. In reply to this, it is argued that the protection of the child’s best interests should not rest entirely or even mainly on informed consent. It is the responsibility of the researcher or the ethics review process to protect the participant’s best interests (therefore, it is inappropriate to defend parental consent by an appeal to the protection of those interests). In addition, the concept of parental consent is a misnomer; for neonates, what should be discussed is parental permission (or authorisation).
An important theme was whether or not, in the neonatal and perinatal context, parental consent is valid, and to what extent validity matters. Potential barriers to obtaining valid consent in these circumstances include time limitations, which adversely affect the amount and quality of the information given; stress, anxiety or pain of the parents; and the mother’s sedation. However, some writers have claimed that these features can also contribute (positively) to an autonomous decision process. Parental desperation or fear may affect the voluntariness of any consent given. Parents may also see the researcher as a figure of authority, which may affect the voluntariness of their decision. A more indirect problem for gaining valid consent is that the consent process itself increases parental anxiety.
Barriers to informed consent in this context have implications for respect for the principle of autonomy, if that is the basis for seeking parental consent. Some argue that parental autonomy will be violated if there is a defect in the consent process, whereas others suggest that parents are being used as a means to an end in a consent process that may be flawed. A further argument is that the principle of beneficence (acting for the benefit of the child and/or mother) becomes a more important ethical principle in research with neonates because informed consent is not possible. Another response to the difficulties with and barriers to consent is that we must strive for improvements in what we do to seek consent, and attempt to get the best possible consent even when perfect informed consent is not possible.
The analytical research has examined a cluster of issues around risk in medical research and clinical treatment. There are disagreements about the nature of the risks involved in research. One claim is that if the context for the clinical trial is a potentially life-threatening condition, and the outcome of the intervention is unknown, then the trial intervention should itself be viewed as a significant risk for the participant. An opposing claim in this situation is that this is a risk of the disease and/or the situation, and not of the trial, so it should not be viewed as a risk of the trial intervention. Another view is that fully informed consent is not possible for clinical trials because the very information needed for fully informed consent is that which is uncertain and under investigation.
Other ways of addressing the consent requirement include a claim that defends the idea of a waiver of consent in emergencies. The suggestion is that consent can be waived, but that provisional assent must be given at the time of being invited to participate, and there should be community involvement at the design stage. The aim is to make research possible for the benefit of patients, so this waiver should not be used to make research easier for researchers. Another approach defends a method by which consent is achieved through giving women antenatal notification of the intended clinical trial, and then seeking consent if they meet the criteria for trial entry. A third option discussed is that of deferred or continuing consent. This is a process in which, if the parents are absent or affected by situational incapacity, they are assumed to give initial consent and then provide consent when they are capable of taking in the information and making the decision. However, this leaves open the possibility that consent is not given, in which case the trial intervention would have constituted assault. Retrospective consent is argued to be ‘logically incongruent’ and, if consent is retrospectively withheld, the researcher is left in the position of never having had consent. Another option is the Zelen method, in which parents are not informed if the novel ‘intervention’ will not be offered to the child. This final suggestion is the opt-out method for giving consent. This may lessen the burden on parents, increase recruitment and increase understanding, but autonomy may be overridden, for example if a participant fails to exercise their opt-out right by default rather than opting out autonomously. All of these approaches involve a parent giving (or waiving) consent, but a final alternative is to have a consent process in which an independent proxy gives consent for the child.
These results reveal five key points about the consent process and highlight one important gap in the research. The key points are as follows:
-
There is a variety of possible defences for seeking parental ‘consent’ to neonatal and/or perinatal trials, and these are consistent with the strongly and widely held view that it is important that parents do give (or decline) consent for such research, as found in the empirical literature.
-
In giving parental ‘consent’ in a perinatal context, parents are authorising infant participation, not giving ‘proxy consent’.
-
There are philosophical reasons for supposing that at least some parents will fail to give valid consent in a neonatal context. These support concerns about the consent process raised in the empirical literature.
-
None of the existing consent processes reviewed by the research is satisfactory. This matches the findings of the empirical research.
-
There are reasons for giving weight to both parental ‘consent’ and the infant’s best interests in both research and clinical treatment, but also reasons to treat these factors differently in the two contexts, and this may be partly attributable to the differing relevance of risk in each case.
The significant gap in the philosophical literature is the lack of any detailed discussion of a process of emergency and/or urgent assent followed by later full consent. This matches a gap found in the empirical literature.
Successes
This is the first review focusing on the ethics issues around consent to neonatal or perinatal research. Other reviews have focused on methods for increasing recruitment or improving how information is conveyed, but none has focused directly on the ethics issues. 61,147–151
Challenges
The key challenge lay in this project being a relatively novel departure for systematic review methods, as it involved reviewing systematically across the disciplinary boundaries between philosophy, social science and medicine. To address this, the multidisciplinary team included expertise in philosophy, clinical practice, social science, information science and advocacy for parents. For the philosophers, the systematic review methods were somewhat different from those that are conventionally adopted in work on ethics. The notion of a systematic review is uncommon, indeed almost unknown, as a method for conducting research in philosophy, both in philosophy as a whole and in philosophical medical ethics in particular. 144,152 On the other hand, for the social scientists and clinicians the standard philosophical means for resolving ethics problems were unconventional. As a result, the whole process of developing a systematic review in ethics through interdisciplinary collaboration has given rise to considerable reflection in its own right.
Implications for future research
We identified three important gaps relevant to consent for perinatal trials:
-
Studies on a new process for obtaining urgent or emergency consent in perinatal research where there is little time in which to seek consent to participation. This process involves seeking ‘assent’ to participation in the trial from parents, accepting that there is insufficient information for fully informed consent at that point, but then providing further information over time which allows the parents to give full consent (or an informed withdrawal).
-
Studies on fetal–maternal research, that is, trials in which both the mother and the fetus or neonate are participants and thus in which both fetal/neonatal interests and maternal interests are in question.
-
Empirical studies that report the views of bereaved parents on the consent process.
This review provided part of the rationale and justification for including a two-stage consent pathway in our Cord pilot trial (see Work Package 4), as part of the feasibility assessment for a large trial. Participation by parent representatives (members of NCT and Bliss) in the ethics review, in the development of the pilot trial protocol and in the programme steering group contributed to robust discussion about development of this pathway. They focused on how it should be presented to women and their partners and how it should be evaluated. Endorsement of the trial protocol from these organisations was important in securing ethics committee approval and in communication with parents. Hence, this programme has contributed to filling the first two research gaps identified in our review.
At the time of our research, empirical studies had almost completely excluded views of parents whose sick child participated in research and died. Since then, however, the Bereavement and RAndomised ControlLEd Trials (BRACELET) study addressing this gap has been published. 153
Work package 3.1: neonatal care at birth, a survey of current practice
There are guidelines for initial resuscitation and stabilisation of preterm or sick babies at birth. To assess compliance with these guidelines and determine if any units were providing initial neonatal care beside the mother, we conducted a survey and interviewed selected sites.
See Appendices 8 and 9 for published reports of this work.
Research aims
This study aimed to describe current practice for providing neonatal care in the delivery room, and for providing neonatal care at birth beside the mother.
Methods for data collection
We surveyed all neonatal units in the UK using a short online questionnaire to ask about neonatal care in the delivery room at very preterm births. Units reporting they were using deferred cord clamping or cord milking were selected, using purposive sampling, to participate in semistructured interviews. Two researchers interviewed experienced practitioners either in person or by telephone. Interviews were recorded and transcribed.
Analysis
Overall, 197 survey responses were received from 199 hospitals, of which 186 (94%) were fully completed. Seven units participated in the semistructured interviews: five tertiary hospitals, and one large and one small district hospital. Overall, 33 staff members were interviewed: seven midwives, seven neonatologists, two paediatricians, six neonatal nurses, seven obstetricians and four managers. Constant comparative analysis identified five core themes: (1) variability in guidelines and practice, (2) assessing eligibility, (3) competing priorities, (4) anxiety about timing and (5) persisting uncertainty.
Key findings
There was variation in delivery room stabilisation practice for infants born very premature, and in tertiary units the care provided was more consistent with current international guidance than in non-tertiary units. 68 For example, tertiary units administered more surfactant in the delivery room (93% vs. 78%), were more likely to provide CPAP (77% vs. 50%) or positive end-expiratory pressure in the delivery room (91% vs. 69%), and were more likely to start resuscitation in air or blended oxygen (91% vs. 78%) than non-tertiary units. Routine out of hours consultant attendance at very preterm birth was also more common in tertiary units (82% vs. 55%).
Our interviews suggested that variation in how deferred cord clamping and other strategies to influence placental transfusion at very preterm birth were being implemented, both between units and within the same unit. 67 For example, there was variation in when the cord was clamped, position of the baby with the cord intact, timing of the prophylactic uterotonic drug, and which babies were seen as eligible for this intervention. Deferring cord clamping was felt to require multidisciplinary agreement because of the perceived conflict between waiting to allow placental transfusion and a wish to ‘get on’ with keeping the baby warm and providing newborn life support interventions. In some units, whether or not this happened depended on which staff members were present. No unit was providing neonatal care with the cord intact, and there was staff anxiety associated with this practice.
Implementation of deferred cord clamping, or cord milking, appeared most likely to be successful if there was strong local multidisciplinary support, with agreement on a single technique and the eligibility criteria. Clinical leadership and training in the practical techniques, combined with audit, also appeared to be helpful.
Successes
Use of telephone reminders for the online survey helped us achieve a high response rate. A strength of our interviews was the inclusion of all the relevant professional groups, and that the prior survey allowed us to identify units with relevant experience.
Challenges
Both the survey and the interviews relied on reported practice rather than direct observation. The interviews were based on a small sample and, as at that time deferred cord clamping was not widely practised, may not be representative of all UK units.
One of the reasons this study was included in the programme was to identify UK units where neonatal care was being provided with the cord intact, so that we could learn from their experience. No such unit was identified, although introducing neonatal care with the cord intact was being discussed at one unit.
Implications for future research
Since this work was completed,154 both the timing of cord clamping for very preterm births and providing neonatal care with the cord intact have become increasingly topical. This has been fuelled by new research, including the work conducted within this programme. A change in culture is leading to a shift away from the previous practice of immediate cord clamping at very preterm birth. In the UK, waiting at least 30 seconds, but no longer than 3 minutes, before clamping the cord of preterm babies is now recommended if the mother and baby are stable. 155 Hence it would be timely to repeat a survey of practice for timing of cord clamping and immediate neonatal care.
Work package 3.2: parents’ views of care at preterm birth
There has been little research looking at parents’ initial experiences and reactions to very preterm birth, or into their experiences and satisfaction with care during very preterm birth.
See Appendices 10–12 for the published reports of this work.
Research aims
The aims of this work package were to:
-
Explore parents’ experiences of very preterm birth, and their first moments with their baby, through three separate analyses to explore:
-
mothers’ and fathers’ initial experiences of the birth of their very preterm baby
-
parents’ experiences and satisfaction with their care during very preterm birth, and to identify the domains associated with positive and negative experiences of care
-
parents’ views and experiences of the care for their very premature baby on a neonatal intensive care unit (NICU).
-
-
Systematically review available measures of parents’ satisfaction with care during labour and giving birth.
-
Develop a questionnaire to measure parents’ satisfaction with care during very preterm birth (P-BESS).
Methods for data collection
Parents whose baby was born before 32 weeks’ gestation during a 6-month period at three NHS hospitals in the south of England were sent letters of invitation to participate in the qualitative interviews (see Appendices 10 and 11 for full details). Of 123 eligible parents, 39 (32%) agreed to be interviewed (32 mothers and 7 fathers). 36 Two babies died shortly after birth. Interviews contained 13 open-ended questions and lasted about 45 minutes. They were conducted by one psychologist and took place at the participants’ home, or in a private hospital room. Interviews were recorded and transcribed. Reporting complied with COREQ (Consolidated criteria for reporting qualitative research). 154
For the comparative review, studies were included if they reported use of a questionnaire that was a multi-item scale of satisfaction with care during labour and birth, and provided psychometric information (about questionnaire construction, reliability or validity) for the satisfaction measure. 43 To identify potentially eligible studies we used the search terms (Birth or Childbirth or Lab*r or Intrapart*) AND (Satisfaction or Perception or Evaluation) AND (Questionnaire or Measure* or Scale or Instrument). We searched Scopus, PsycArticles, PsycINFO, PubMed and Web of Science from inception to 30 July 2011, and checked reference lists in reports of included studies for additional studies. Duplicate citations were removed. Finally, Web of Knowledge and Scopus were searched for all reports that cited the final questionnaire measures; no new citations were identified. Data extraction was by two reviewers.
Initially we developed the questionnaire using data from the interviews and studies identified in the comparative review. We identified seven areas of satisfaction with care during preterm birth: (1) information and explanations, (2) emotional support, (3) encouragement and reassurance, (4) staff being confident and in control, (5) staff being calm in a crisis, (6) involvement of the partner and (7) birth environment. Thirty questions were included, both positively and negatively phrased, and responses scored on a Likert scale; we made minor changes following feedback from nine parent representatives. The questionnaire was then posted to parents of babies born very preterm during the previous 12 months at five tertiary care centres in England.
Analysis
We used inductive systematic thematic analysis to identify themes across interviews. Data were managed using NVivo software. For the systematic review, we assessed psychometric quality of each questionnaire using questionnaire construction (item generation, pilot study), reliability (internal consistency, test retest reliability) and validity (content, face, criterion and construct).
For the questionnaire, a factor analysis was conducted to explore whether questions could be combined into subscales that represent different aspects of satisfaction with care during very preterm birth. Three questions asked about partner’s involvement so, as they were not relevant for all women, they were excluded from the initial analysis. Hence, 27 questions entered into the factor analysis. The number of factors to be retained was determined using the scree plot and eigenvalues > 1. Questions that loaded on a factor at > 0.4 were considered significant and were retained. Questions that loaded on more than one factor ≥ 0.3 were removed and the analysis was rerun. To check whether questions and subscales for the women were applicable to partners, we conducted a confirmatory factor analysis.
Content validity is evident through the systematic series of steps taken when designing the questionnaire. Convergent validity was explored by examining the relationship between the total score (and associated subscales) with two questions assessing overall satisfaction with care during the birth, and reliability by looking at indicators of internal consistency.
Key findings
Parents’ experiences of very preterm birth and their first moments with their baby
Following very preterm birth, almost half of parents had difficulty remembering aspects of the birth. 36 Two-thirds saw their baby at birth and one-third saw their baby for the first time in the neonatal unit. The anticipation before seeing their baby for the first time was characterised by contrasting emotions, with some parents eager and excited, whereas others, while wanting to see their baby, nevertheless felt scared and even dreaded the experience. For example, one father (2, caesarean section, delayed card clamping) said ‘They rang down and said “do you want to come up and see little one?” We went ”yeah course we do, you know, brilliant”’, one mother (27, caesarean section, delayed card clamping) said ‘I was very scared of seeing him’, and another mother (24, vaginal birth, delayed cord clamping) said ‘I thought I’ll go onto the ward and, thoughts running through my mind of what I was, what I was gonna find, how many tubes was he gonna have, was he gonna be OK, what colour was he gonna be, did he have everything, 10 fingers 10 toes, and I found myself sitting by the incubator counting and making sure he had 10 fingers and had 10 toes’.
Similar contrasting emotions were described about touching their baby, for example one mother saying ‘You don’t want to hurt them. You’re so on edge, and you want to care for them and touch them if you can, or whatever, but also you just feel terrible if you think you’ve done something wrong’.
First contact with their baby was characterised by turbulent emotions, described as a ‘rollercoaster of emotions’. Parents spoke about the confusion of feeling both elated and devastated, and others felt guilt about the preterm birth. One mother said ‘. . . I was all prepared, arms out and they give her to me, and it was wonderful, absolutely wonderful’, and another commented ‘you just feel guilt, the guilt is overwhelming, you know you do kind of go through aah, not feeling sorry for yourself, it’s just the guilt, it’s not “oh why’s this happened to me?” it’s “why’s it happened to her?”‘. Half of parents who talked about touching and holding their babies described immediate bonding with the first touch. 37 Visiting NICU was initially overpowering, especially for those who had not been before or who were seeing their baby for the first time. This was described as ‘a little hidden world, full of poorly babies’. 38 Parents described awkwardness and exclusion felt by fathers, particularly during emergency caesarean section, one comment being ‘It’s different being a man . . .’. Nevertheless, fathers often saw their baby first, and typically experienced this alone.
Overall, the parents’ experiences of care during the birth were positive. 36 Our study identified four determinants of parents’ experiences of care during very preterm birth: (1) staff professionalism, (2) staff empathy, (3) involvement of the father and (4) the birth environment. These are consistent with previous research on term births. However, two factors unique to very preterm birth were the importance of the staff appearing calm during the birth, and staff taking control during the birth. Parents felt that care could have been improved in two areas: staff could listen more to what women said, and believe them; and the father could be more involved in the birth.
Comparative review of measures of parents’ satisfaction with care during labour and birth
Nine questionnaires measuring satisfaction with care during labour and birth were identified (Figure 5). For seven of these questionnaires, how the items were selected was described. Eight of these questionnaires had tests of internal consistency, but only one reported test–retest reliability. 43 At least one aspect of validity was reported for all questionnaires, but none reported criterion validity.
FIGURE 5.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart for the comparative review. Reproduced from Sawyer et al. 43 © Sawyer et al. ; licensee BioMed Central Ltd 2013. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Only two questionnaires assessed satisfaction with different aspects of care, as well as the perceived importance of these aspects of care. Three questionnaires were designed for particular types of births, two for operative births and one for uncomplicated vaginal birth. None of the nine questionnaires evaluated care for specific populations, such as parents of sick or preterm babies, or whose baby was stillborn. Parent experiences in these situations may be substantially different from giving birth to a healthy, term baby.
Preterm Birth experience and Satisfaction Scale questionnaire to measure parents’ satisfaction with care during very preterm birth
Based on the qualitative interviews with parents, the review and discussion with relevant experts, we identified 97 potential questions in seven domains. Following screening by two experts, 30 items were chosen (27 in the maternal section and three in the partner section) for the draft questionnaire. 44 To check face validity, content validity and ease of comprehension the P-BESS was sent to nine parent representatives, following which minor changes were made to the wording.
We posted this 30-item questionnaire to 458 couples/single parents, and 147 were returned completed (32% of couples/single parents, 147 women and 107 partners). Of these 24 were excluded, largely as they were completed by partners who were not present at the birth, leaving 145 women and 85 partners. Initial screening removed three questions that were not performing well. Another three were removed because they did not significantly correlate with other questions. The remaining 21 questions in the maternal section were entered into the factor analysis and a further four questions were removed (see Appendix 12). The final factor analysis identified three factors with 17 questions for the maternal section: ‘staff professionalism and empathy’ [seven questions, mean 29.2, standard deviation (SD) 5.1], ‘information and explanations’ (seven questions, mean 27.9, SD 5.7), and ‘confidence in staff’ (three questions, mean 12.4, SD 2.5) (Table 1). The mean score for the total scale was 69.5 (SD 11.6), out of a possible range of 17–85. Rerunning the factor analysis with the addition of partner involvement questions confirmed that the three factors remained, with the addition of a fourth factor with the partner involvement questions.
| During the birth | Strongly agree | Agree | Neutral | Disagree | Strongly disagree | |
|---|---|---|---|---|---|---|
| 1 | The staff explained everything really well | |||||
| 2 | There was a pleasant atmosphere in the room | |||||
| 3 | The staff made me feel cared for as an individual | |||||
| 4 | The staff took control of the situation | |||||
| 5 | I was given all the information I needed | |||||
| 6 | The staff put me at ease | |||||
| 7 | The staff were encouraging | |||||
| 8 | I understood what was happening | |||||
| 9 | The staff were reassuring | |||||
| 10 | I did not have confidence in the staff | |||||
| 11 | The staff explained to me what would happen during the birth | |||||
| 12 | The staff did not listen to what I had to say | |||||
| 13 | The staff kept me informed of what was happening | |||||
| 14 | The staff did not understand how I was feeling | |||||
| 15 | The staff explained to me what would happen to my baby when he/she was born | |||||
| 16 | There were occasions when no one explained to me what was going on | |||||
| 17 | The staff were warm and friendly | |||||
| 18 | The staff encouraged my partner’s/my involvement | |||||
| 19 | The staff involved my partner/me in what was going on | |||||
The total scale and subscales had good reliability and individual items correlated well with the total scale. Reliability for the ‘partner involvement’ subscale was 0.72 but this increased to 0.91 with deletion of one question, which was therefore removed. Convergent validity was explored by comparing the scales with the two questions measuring overall satisfaction with care and the need for improvement. The total scale and three subscales were all moderately to strongly correlated with these items. A confirmatory factor analysis to check applicability to fathers showed that the scale was reliable (α = 0.93), but the three subscales in women’s responses were not applicable to partners and the three factor solution did not fit the partner’s data well. One possible explanation for this is that fathers’ experiences of preterm birth differ from mothers’. 44 We recommend that only the total score on satisfaction with care is used for partners. Total scores were related to higher levels of overall satisfaction and less need for improvements, indicating convergent validity for partners.
Successes
We achieved a good response to the invitation to be interviewed. The use of qualitative methods provides an in-depth insight into the experiences of parents who have had a very premature baby. The inclusion of fathers and bereaved parents also provides a valuable and unique perspective. Our data underline the importance of listening to women during preterm labour and of encouraging fathers to feel involved during the birth. Whenever possible, parents should be encouraged to visit the NICU before birth. If this is not possible, parents could be provided with a photograph of their baby in the neonatal unit before they visit.
Parents who are worried about touching their baby should be reassured and taught to recognise infant behaviour in response to touch.
Having identified only two existing questionnaire measures of satisfaction with care during labour and childbirth, both designed for term birth, we developed a new tool for use by both parents following very preterm birth. This tool is the first specific to preterm birth. The total score may be useful to compare satisfaction with care during very preterm birth across hospitals and differing practices, and individual aspects of care can be evaluated using the separate subscales. We used this tool to measure satisfaction with care in the Cord pilot trial (see Work Package 4), and it has been translated into Spanish and Portuguese.
Challenges
Although the response rate for our qualitative interviews was good for this type of study, we received responses from parents whose baby was born only from two of the three hospitals, reducing the generalisablity of our data. In addition, participants were mainly white, married women, which is not typical for very preterm birth. In common with other studies of satisfaction, parents may have been reluctant to criticise the professionals who took care of them and their preterm baby. This ‘halo effect’ may be even more pronounced for parents of premature babies, as the staff have been looking after their baby for many weeks. 44 Similarly, if women do not know what care during birth should be like they may just evaluate the status quo. 156,157 Our study used in-depth interviews by a researcher not associated with the hospital, which should have helped to pick up relevant negative experiences.
The response rate for development of the questionnaire was relatively low, although again this is a good response for studies of this kind. The sample size was relatively small for a factor analysis, which limits the validation process. In addition, the sample was not representative of all parents who have a very preterm birth, as it included largely white, highly educated, married/cohabiting women and their partners. Finally, as the same factor structure was not identified in partners as in women, only the total score is recommended for use with partners, which means that the individual factors of care cannot be explored for them.
Implications for future research
Further research is needed to replicate our findings about parent experiences at very preterm birth, and to explore whether or not there is variation for parents from different backgrounds. For the P-BESS, further studies are needed to test the refined instrument in a larger, more representative sample of parents. Fathers of preterm or sick babies are a difficult group to recruit into research. 158 Our work highlights the importance of including them in research studies to ensure that their perspective is represented.
Our experience in conducting semistructured interviews with parents following very preterm birth also contributed to the design and conduct of an evaluation of parent and clinician experiences of neonatal care beside the mother (see Work Package 3.5), and of women and clinicians’ experiences of the two-stage consent pathway in the Cord pilot trial (see Work Package 4).
Work package 3.3: measuring placental transfusion
Previous research into the physiology of placental transfusion has largely involved term births. It remains unclear how the volume and the duration of placental transfusion vary with gestation at birth, and what the optimal timing is for umbilical cord clamping for preterm births.
See Appendix 13 for the unpublished report of this work.
Research aims
The aim of this work package was to measure the volume and the duration of placental transfusion for preterm births by weighing babies with the umbilical cord intact.
Methods for data collection
Recruitment was at three maternity units in England. Women likely to give birth to a live baby before 36 completed weeks’ gestation were offered participation. At each site, research staff undertook an initial training phase recruiting women giving birth at term. At birth, the baby was placed on the weighing platform and wrapped in warm towels with the umbilical cord intact. We used high-quality pharmacy scales (Excellence XS Precision Balance Model XS8001L, Mettler Toledo, Im Langacher, Switzerland), which calculated an average weight twice every second, with data stored in a linked computer. Before the birth, scales were zeroed to allow for towels, probes and any other equipment. To ensure that the baby was no higher than the level of the woman’s abdomen, the woman’s bed was raised or lowered if necessary. The baby was monitored throughout weighing using saturation monitors.
Analysis
Characteristics of the women, events during labour and mode of birth were described for all women. Placental transfusion was assessed by change in weight over time, as 1 ml of blood weighs 1.05 g. For each birth, two authors (JD and SO) independently assessed the weight change by inspecting the graphs of weight against time, using information on the time of cord clamping and when the baby was touched. Differences were resolved by discussion. Owing to the small numbers recruited, data are described only as summary statistics were inappropriate.
Key findings
Of 97 women approached, 33 gave consent, of whom six had their baby weighed. Reasons why the baby was not weighed for the remaining 27 with consent were: term birth (n = 10), research staff not available as out of working hours (n = 6), birth too rapid (n = 5), cord too short (n = = 2), consent withdrawn (n = 2), hardware problem (n = 1) and clinician decision (n = 1). For the six babies who were weighed, gestation was from 34+4 weeks to 36+5 weeks (Figure 6). Three were vaginal births and three were caesarean. The time of cord clamping ranged from 2 minutes to 3 minutes 57 seconds. For two babies, drying and applying probes led to a poor recording of weight for the first minute. An initial 10 seconds ‘hands-off’ period to give a baseline weight was therefore adopted. Weight change ranged from a 20-g decline to a 128-g increase. For one baby, weight change was continuing when the cord was clamped at 120 seconds; for another, weight change ceased at 2 minutes, and for the remaining four babies weight change appeared to continue for at least 3 minutes.
FIGURE 6.
Caesarean section at 35+6 weeks, weight gain estimated at 51 g.
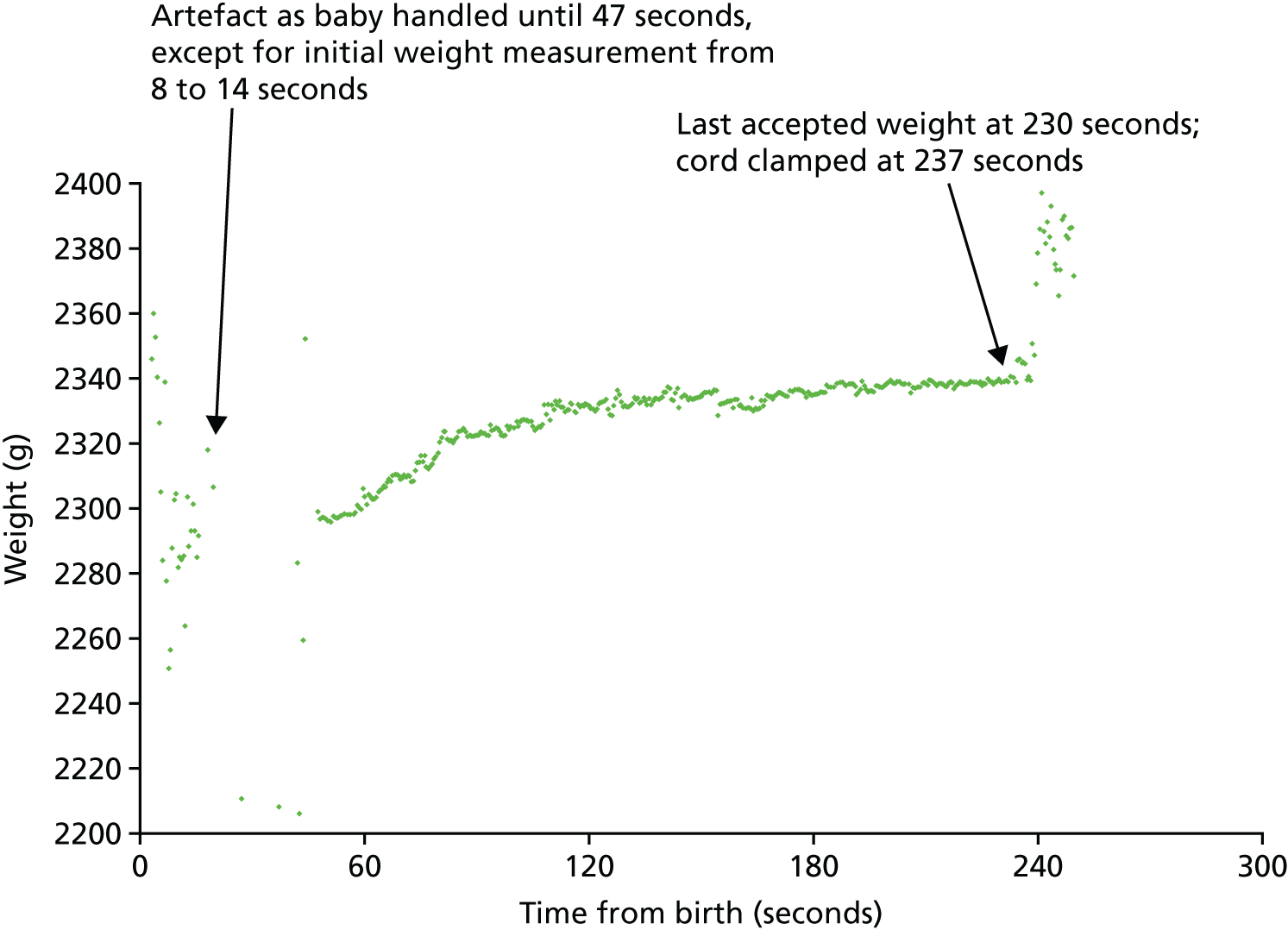
This study is small; nevertheless, as with term births, there appears to be variation in the volume and duration of placental transfusion and, for some, net flow is to the placenta rather than the baby. For preterm births, umbilical flow may continue for more than 3 minutes. As placental transfusion may have a role in stabilising the cardiorespiratory circulation during transition from the fetal to neonatal circulation, the duration of time that the cord is left unclamped and umbilical flow continues may be as, or possibly more, relevant than the volume of any net flow. 49,159
Challenges
Major challenges were recruitment and, when appropriate women were recruited, having enough time for research staff to arrive and prepare the equipment, made even more challenging as births were often out of hours. Despite considerable effort at each site, these challenges were only partly overcome.
Implications for future research
Despite the small numbers, these data contributed to evidence supporting the decision to wait at least 2 minutes before clamping the cord for the intervention arm in the Cord pilot trial (see Work Package 4). These data also suggest that the effect of delayed cord clamping may not simply be to allow more blood to reach the baby from the placenta, but rather to allow the changes from an in utero placental circulation to an ex utero lung one, to occur more gradually. Improved understanding of the physiology of placental transfusion at very preterm birth would help identify the optimal strategy for cord clamping. Better methods for assessing placental transfusion are required. 46,72
Work package 3.4: developing a Bedside Assessment, Stabilisation and Initial Circulatory Support trolley
Newborn resuscitation with an intact cord had been described previously,53,160 but with no agreed strategy for how to do this and little apparent uptake in clinical practice. 67 In 2010, a 1-day meeting led to the idea for a small mobile resuscitation trolley, the ‘Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support’ (BASICS) trolley,69 extending the concept of a simple platform. 53 This work package describes its subsequent development into a commercially available medical device.
See Appendix 14 for the published report of this work.
Research aims
The aims of this work package were to develop a mobile trolley to provide newborn life support beside the mother, and with the umbilical cord intact, and to identify a commercial partner so that the trolley could be made more widely available.
Methods
A multidisciplinary team including clinical engineers, neonatologists, obstetricians, midwives and parent representatives met regularly to discuss and develop the design, using a prototype device based on an overbed hospital table with piped gasses, suction and a timer. Two simulated resuscitations provided assessment of functionality and practicality for both vaginal and caesarean births, following which the prototype was modified further.
A ‘second-generation’ prototype was developed in collaboration with Inditherm Medical (Rotherham, UK), a company specialising in the development and sale of neonatal equipment and devices. Following a third simulation to refine the design further, and certification with the Conformité Européenne (CE) logo, this was subsequently marketed as LifeStart™.
Key findings
The first prototype trolley was adapted from an overbed hospital table, which has a long base directly below the platform that slides under the bed, providing stability. However, modern operating tables and delivery beds have pedestal bases with little room beneath them. Therefore, the next prototype used a circular base, with larger wheels and enough weight to provide stability. This then led to the first commercially available trolley (Figure 7). The design struck a balance between trolley stability and reach of the platform. The platform is widest at the proximal end, allowing space for the baby’s shoulders and to access the head. The distal end is narrower, allowing the platform to get close to the mother. The baby is kept warm with an electric heated mattress produced by Inditherm; the company already produced the CosyTherm© (Inditherm Medical) mattress for neonatal cots, and this was reshaped to fit the trolley platform. To allow the platform height to be adjusted to be level with the mother, the central pillar adjusts up and down via an electronic mechanism. The trolley also has a battery driven digital timer with alerts at 1 minute and at 5 minutes, for use during resuscitation.
FIGURE 7.
First commercially available LifeStart trolley, October 2012. Reproduced from Thomas et al. 161 © 2014 The Authors, licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0 (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

Medical gases for newborn resuscitation (air or oxygen) usually come from wall sockets; hoses connecting these sockets to the usual resuscitation equipment are short and tuck in against the wall. For a mobile trolley, long hoses connected to wall sockets create a trip hazard. Using small gas cylinders attached to the trolley was not possible at the time we developed the trolley, as the smallest size available for medical air was too large to attach to the trolley. Gasses are blended and the pressures are regulated using a Tom Thumb Infant Resuscitator© (Viamed, Keighley, UK) and an oxygen blender (Inspiration Health Care Ltd, Leicestershire, UK) attached to the back of the trolley, along with a suction bottle (Oxylitre Ltd, Manchester, UK). Other equipment is attached, as required, using two medical equipment rails on the central pillar.
Successes
This is the first mobile neonatal resuscitation unit designed to facilitate newborn resuscitation beside the mother, and with the umbilical cord intact. Our initial prototype was entered into the Medical Futures Innovation Awards in 2011 (www.medicalfutures.co.uk/) and awarded ‘Best Redesign in Cardiovascular Medicine’. Further development has led to a successful commercial product, LifeStart, marketed in the UK and internationally, and cheaper than the usual equipment (around half to one-third of the cost). Regardless of timing of cord clamping, the trolley has a role in supporting neonatal care beside the mother and allowing her to share her baby’s first moments.
Challenges
Feedback to the manufacturer led to further modifications. For example, the platform was lengthened to extend its reach, an important development as premature babies tend to have shorter cords than those born at term.
The trip hazard caused by piped gas from wall sockets has reduced acceptability of the trolley in clinical care. A medical air gas cylinder small enough for the trolley is now available and can be used following a local risk assessment (as it is not covered by the CE mark). A disadvantage of this solution is that the cylinder requires frequent refilling. Another less practical option is to put the large cylinder on a second trolley and move this to the bedside with the resuscitation trolley.
Resuscitation can be provided without a pressurised gas supply, using a bag, valve and mask system, and room air. Disadvantages of this method for preterm resuscitation are the inability to control inspiratory time or provide positive end expiratory pressure, and also that oxygen would not be available for those babies who fail to respond to resuscitation with air.
Implications for future research
Once a trolley was available for use, we introduced it into clinical service and conducted a service evaluation161 followed by qualitative interviews to explore the experiences and views of parents and clinicians of neonatal care at birth beside the mother, and of their experiences and views of the trolley (see Work Package 3.5). 39,40 We also developed strategies for providing neonatal care beside the mother using the usual resuscitation equipment. 162 As discussed in Work package 3.5, further research is needed to evaluate the clinical and psychological impact of neonatal care beside the mother and of providing care with the cord intact.
Work package 3.5: evaluating parents’ and clinicians’ views of neonatal care beside the mother
See Appendices 15 and 16 for the published reports of this work.
Research aims
Our aims were to provide proof of concept that the trolley could be used in clinical practice, then to assess whether or not the trolley could be used as part of routine care within a busy tertiary care hospital, and whether or not it was acceptable to parents and to clinicians. Therefore, we aimed to conduct:
-
a case study of first use of the trolley in clinical practice
-
a questionnaire-based service evaluation
-
qualitative semistructured interviews with parents and clinicians about their experiences.
Methods for data collection
First use of the trolley in clinical practice was for a baby born by caesarean section at 37 weeks’ gestation who required an EXIT (Ex utero Intrapartum Treatment) procedure163 at Liverpool Women’s hospital. We delivered the baby’s head and shoulders, while the chest and abdomen stayed inside the uterus, and administered tocolytics to maintain placental perfusion. Once a safe airway was achieved (by endotracheal intubation), the baby was born and the umbilical cord cut.
Following this the LifeStart trolley was introduced into clinical service at Liverpool. We conducted a service evaluation from March 2012 to October 2013. 161 The trolley was available at births for which hospital policy required attendance of an advanced neonatal nurse practitioner or a paediatrician. As this was the first time that the trolley was available for routine clinical practice because its use was initially restricted to ‘low-risk’ births. This was extended to high-risk births after review of data from the first 20 births, which was satisfactory. Babies were assessed and resuscitated at birth according to hospital guidelines. Overall, 78 births were included in the evaluation. Data were from the clinical notes, including demographics, temperature of the baby after resuscitation, care provided on the trolley, whether or not there was a need to move the baby to a standard resuscitation platform, and any problems experienced. For 61 of these births, clinicians completed a questionnaire asking their views of care beside the mother and of using the trolley, and any views expressed by the women or their partners. This questionnaire was not completed for 17 births, as the women were recruited into the Cord pilot trial.
For the qualitative interviews, we recruited parents and clinicians between November 2012 and January 2014. Interviews were conducted by a female psychologist or one of three midwives trained in qualitative methods, using a standard schedule of open-ended questions. They were recorded and transcribed, and reporting complied with COREQ. 154
Fifty-six women and their birth partners who had initial neonatal care beside the mother were invited to participate, of whom 30 were interviewed (19 mothers, 10 partners and one grandmother). 39 We conducted 19 interviews, as 11 women chose to be interviewed with their birth partner. Five of the babies required advanced neonatal resuscitation at birth. If their baby had not received intervention at birth, it was explained to parents what this might involve, and they were asked about how they might feel about being so close if this had been necessary. Interviews lasted approximately 30 minutes, and took place either in a private hospital room (17 women) or at the woman’s home (two women). We extracted demographic data from the clinical notes.
Using purposive sampling, 26 clinicians who were present at a birth where initial neonatal care was beside the mother were invited to participate. All initially agreed to be interviewed; as six later declined, 20 were interviewed. 40 Most were neonatal specialist doctors or nurses (Table 2). Five had provided or observed advanced resuscitation beside the mother. Interviews lasted approximately 20 minutes, and took place in a private room on the neonatal unit.
| Characteristics | n (%) (N = 20) |
|---|---|
| Profession | |
| Advanced neonatal practitioner | 10 (50) |
| Consultant obstetrician | 1 (5) |
| Consultant neonatologist | 2 (10) |
| Neonatal nurse | 1 (5) |
| Senior house officer (paediatrics) | 4 (20) |
| Midwife | 1 (5) |
| Senior registrar (paediatrics) | 1 (5) |
| Role in providing neonatal care at birth | |
| Observer | 2 (10) |
| Contributor | 18 (90) |
| Trolley experiencea | |
| Used once | 9 (50) |
| Used more than once | 9 (50) |
Analysis
For the service evaluation, we assessed usability of the trolley based on the range of resuscitation procedures performed while babies were on it; the feasibility of resuscitation with the cord intact, based on the proportion who received this; and the acceptability to clinicians based on questionnaire responses. The only anticipated safety concern was hypothermia. We monitored unexpected safety issues using the Hospital Incident Reporting System.
The qualitative interviews ended when we achieved data saturation. We used inductive systematic thematic analysis to identify themes across interviews. Data were managed using NVivo software. We identified five themes in the parent interviews: (1) reassurance, (2) involvement of the family, (3) staff communication, (4) reservations and (5) experiences of the trolley. For the clinician interviews, we also identified five themes: (1) parents’ involvement, (2) reservations about neonatal care at birth beside the mother, (3) practical challenges in providing neonatal care beside the mother, (4) comparison of the trolley with usual resuscitation equipment, and (5) training and integration into clinical routine.
Key findings
Case report of the first use in clinical practice
Endotracheal intubation was at 7 minutes of age; at 8 minutes, the baby was born, the cord was clamped and cut and the baby was transferred to the trolley. At 15 minutes of age, the baby was moved to the standard resuscitation equipment and at 16 minutes the temperature was normal.
Service evaluation
Common delivery room resuscitation procedures were performed successfully for babies on the trolley, and none was moved to the standard resuscitation platform. 161 All resuscitation interventions were performed with an intact cord, apart from umbilical venous catheterisation, which requires division of the cord. Immediate neonatal care with an intact umbilical cord was attempted for 61 births and was achieved for 43 (70%). For 18 births (30%), the cord was too short to allow the baby to reach the trolley. Births for which the cord was judged to be too short were no different in gestation or mode of birth to those with the baby on the trolley with the cord intact; two-thirds of these births took place during the first half of the study, which included fewer very low-birthweight babies. As experience increased, the proportion of babies able to receive care on the trolley with an intact cord also increased.
Only one baby had a temperature of < 36 °C and this baby was born at 30 weeks’ gestation and their temperature was 36.4 °C at 10 minutes of age on the trolley; therefore, (s)he probably got cold during transfer to the neonatal unit. No serious adverse events related to the trolley were reported. A reported practical difficulty was the trip hazard due to hoses connecting the trolley to gas sockets on the wall. A partial solution was to attach a small size oxygen cylinder to the trolley, reducing the number of gas hoses.
Compared with conventional resuscitation equipment, clinicians rated the trolley as ‘the same’, ‘better’ or ‘much better’ for most aspects of care, and none rated it ‘much worse’. Some rated the trolley as ‘worse’ than conventional resuscitation equipment for ease of access to the baby (15% of clinicians), ease of assessing the baby (10%) and ease of access to resuscitation equipment (18%). These issues seemed largely due to difficulty in getting sufficiently close to the operating table at caesarean section. There were also concerns about maintenance of the sterile field, about the sterile drapes covering the trolley obstructing the view of the airway management equipment, and that preparing the trolley for use in theatre was time-consuming. Two-thirds of clinicians rated the trolley as ‘better’ or ‘much better’ for ease of communication with parents, with a similar proportion rating the overall experience for parents as ‘better’ or ‘much better’.
Parents’ experiences of immediate neonatal care beside the mother
Parents reported that they liked having their baby close by and described feeling reassured by knowing what was going on. 39 At some births the woman was unable to see her baby, but the father (or other birth partner) could and relayed information about the baby, which reassured her. Parents reported feeling involved as a family, as they could see and sometimes touch their baby. By watching, parents felt that they understood what was happening to their baby, felt that they could see that staff were doing the best that they could, and felt part of their baby’s care. One mother reported that having her baby so close gave some ‘normality’ and ‘goodness’ to a birth she otherwise experienced as unnatural. Some parents felt that seeing the initial neonatal care helped staff to communicate with them. Others said that they would have liked more explanation about what was going on and why, as this might have allayed some of their fears.
Parents whose babies had only low intensity interventions at birth, such as drying or receiving oxygen by mask, reported no reservations about watching. However, half of them said that they might have reservations if their baby needed more intensive intervention. No parent whose baby received intensive intervention, such as intubation or cardiac massage, expressed regrets about watching. These parents reported feeling scared or finding the intervention unpleasant to watch, but three out of five stated that it was ‘fine’ or ‘nice’. A few parents suggested that they should be asked beforehand whether or not they would like to watch their baby’s care at birth. Two were concerned that watching might have an impact on the staff in terms of adding pressure or distraction.
Of 11 parents who gave an overall opinion of the trolley, nine were favourable and two were unsure. The standard resuscitation equipment was also in the room, and some parents thought that this equipment looked ‘scary’, clinical or like their baby needed a lot of help or would be taken away. Two parents felt that the standard equipment looked ‘more advanced’.
Clinicians’ experiences of immediate neonatal care beside the mother
Eighteen clinicians mentioned that initial neonatal care at birth beside the mother allowed parents to see and touch their baby, and to see what the clinical team was doing. 40 They felt that this was especially important for babies subsequently admitted to the neonatal unit, in contrast with usual care, where the woman might not have been able to see her baby until she visited the neonatal unit. Some clinicians reported that parents were not able to see their baby at caesarean section because of the screen, or at an assisted vaginal birth when the trolley was positioned close to the perineum.
Almost half of the clinicians reported positive comments from parents about being close to their baby, which included one woman whose baby died soon after birth. None mentioned negative comments from parents. Four clinicians said that they felt that being near to the parents aided or increased their communication with them, three clinicians felt that being close made no difference, but one clinician said that communication was an issue of practice, not equipment. One clinician felt that when advanced resuscitation was required, a member of staff should be assigned to support parents.
Most clinicians had no reservations about being watched by parents, but five thought that staff with less experience might feel insecure about this. Clinicians varied in their views about the potential impact on parents of watching neonatal care at birth: five felt that it would be beneficial, four were unsure and two felt that seeing advanced resuscitation might be upsetting. Two clinicians suggested that parents are asked beforehand whether or not they wanted to watch.
Practical challenges at caesarean births were that parents were sometimes unable to see their baby and that scrubbing to enter the sterile field was perceived as a burden that took time out of already busy work days. Clinicians also noted that the trolley switches and controls were covered by the sterile drapes, making their use awkward. Other practical issues were: (1) for admission to the neonatal unit, the baby had to be moved to the standard equipment, and (2) the small platform size limited space for equipment. Problems with integration into clinical care included not having the trolley routinely available at a birth, or it not being set up in time. A common concern was that the trolley interfered with other staff members’ space beside the mother. However, three clinicians reported no problems with space for other staff. Eighteen clinicians gave an overall evaluation of initial neonatal care beside the mother and using the trolley as positive or conditionally positive.
We also showed that the usual resuscitation equipment can easily be adapted to do this. 70,162 Some clinicians, particularly those with less experience, may find it challenging to conduct neonatal resuscitation in such close proximity to parents. Although many will appreciate the opportunity this gives to communicate with parents about their baby, others may prefer to be away from parental scrutiny. Thus, successful introduction of neonatal care at birth beside the mother requires adequate training and support, and a multidisciplinary team approach.
Successes
As far as we are aware, the work reported here describes experiences at the first hospital to provide initial neonatal care beside the mother within routine clinical service. We have shown that all common resuscitation procedures can be provided this way, with reassurance that this is also safe. Although we used the mobile trolley designed for this purpose, the same care can be provided using the standard resuscitation equipment, as happened in the Cord pilot trial. 70 Although larger than the trolley, standard resuscitation equipment is easily moved alongside the mother, the side panel dropped to allow the mattress on which the baby is placed to be moved closer to the mother, and the height adjusted. Using standard equipment has the advantages of being already available in the room or operating theatre, having the usual radiant heater, and requiring no equipment-specific training as staff are already familiar with its use. Data presented here on initial neonatal care beside the mother are therefore generalisable to settings in which care is provided using standard equipment, with the exception of specific practical issues with the trolley.
Although this requires further investigation, the positive view of a woman whose baby had care beside her and died shortly afterwards has important implications for bereavement.
Challenges
A practical concern about the trolley was the potential trip hazard, especially in the operating theatre, associated with the hose required for gas supply. Replacing one hose with a small oxygen cylinder fixed to the trolley has reduced this hazard. Removing the second hose requires a small air cylinder, which was not commercially available at the time. It is now available, but is not included in the CE mark. A concern about providing care with the cord intact at caesarean section is the potential to compromise the sterile field. Maintaining the sterile field and negotiation about space for the neonatal team at the operating table remain a challenge at caesarean births, especially in an emergency. Our experience is that this becomes easier as staff become familiar with providing this care.
Generalisability of these data is limited by recruitment of both parents and clinicians from a single hospital (where the trolley had been developed) with a particular interest in care beside the mother. Other limitations of the interviews with parents were that participants were not necessarily representative of the wider population, as they were primarily white, they were either married or living with their partner, the babies were all alive at the time of interview, and most babies had not required advanced resuscitation at birth. Parents may have been reluctant to criticise their care, but we mitigated this risk by having an interviewer who was not directly involved in their care or care of their baby.
Implications for future research
The work presented here should be repeated in other hospitals and should include using the standard resuscitation equipment as well as the mobile trolley to assess whether or not outcomes, experiences and costs are similar. Further research should include parents from more diverse backgrounds, those whose baby required advanced resuscitation and those whose baby died. Further research should also include follow-up of both parents and infants to assess any long-term impact.
The optimal study design for further evaluation would be a randomised trial comparing outcome for parents who are offered initial neonatal care beside the mother with the outcome for those offered traditional care only.
Work package 4: Cord pilot trial, a randomised trial of deferred cord clamping and initial neonatal care with cord intact versus immediate cord clamping and initial neonatal care after clamping for very preterm births
This work package was planned as an assessment of the feasibility of a large randomised trial based on recruitment for 1 year. As recruitment was extended for a further 11 months, we present outcomes by allocated group for all participants at hospital discharge and follow-up at 1 year for women and at the age of 2 years (corrected for gestational age at birth) for the children. In addition, parent and clinician experiences of the two consent pathways are described.
See Appendices 17–23 for the unpublished and published reports of this work.
Research aims
The aim of the Cord pilot trial was to assess the feasibility of conducting a large multicentre randomised trial in the UK comparing deferred cord clamping and immediate neonatal care (if needed) with the cord intact, with immediate cord clamping and with immediate neonatal care after cord clamping for very preterm births. Recruitment was planned for 1 year and feasibility was assessed based on prespecified process measures, such as recruitment, compliance, completeness of data collection and participants’ views.
At an interim review, the independent TSC agreed that feasibility criteria had been met and advised that recruitment continue while funding was sought for the full trial. Recruitment closed in February 2015, when this funding application was unsuccessful. For the planned full trial, joint primary outcomes were death before discharge and IVH (all grades), hence these outcomes were considered the primary outcomes for the extended pilot trial. As the two-stage consent pathway was an important addition to the trial protocol, we also conducted a qualitative evaluation of the alternative consent pathways.
Methods for data collection
Recruitment to this pragmatic trial was at eight UK tertiary maternity units: five had contributed to earlier work in the programme and three had not been involved and so were more typical of UK sites. Three parent representatives were coinvestigators, involved in identifying the research question, securing funding, and designing and conducting the study. Target recruitment for assessment of feasibility was 100–110 women over 1 year, based on target accrual of 16–18% of eligible births. As we planned this as a pilot, there was no formal power calculation.
Women expected to have a live birth before 32 weeks’ gestation were potentially eligible. The intervention was umbilical cord clamping after at least 2 minutes and neonatal stabilisation and resuscitation, if needed, with the cord intact. Babies were placed onto a firm surface with access to resuscitation equipment; six sites used their usual resuscitation equipment moved alongside the woman’s bed162 (153 women recruited) and two sites used the mobile trolley (108 women). 69 The control was cord clamping within 20 seconds and neonatal stabilisation and resuscitation, if needed, after clamping. For both groups, neonatal care was based on local unit policy and consistent with newborn life support guidelines. 57,164
For the assessment of feasibility we prespecified measures of feasibility. For the planned main trial, joint primary outcomes were death before discharge and IVH (all grades);165 therefore, these were the main outcomes for the extended pilot trial. We adjudicated diagnosis of IVH. Secondary outcomes for the babies included severe IVH (grade 3 or 4),165 a range of measures of neonatal morbidity and neurodevelopment in early childhood. Secondary outcomes for the women included complications of the third stage of labour, well-being and satisfaction with care at birth. Follow-up for women was by self-completed questionnaire at 2–3 months and again at 1 year, and for the children at the age of 2 years (corrected for gestation at birth) by parent-completed Ages and Stages Questionnaire (ASQ)166 and an assessment at home using the Bayley Scales of Infant Development III (Bayley-III). 167 If no ASQ or Bayley-III data were available, we asked the site to complete an outcome form using routine clinical data. We agreed the statistical analysis plan for the extended study before unblinding the data.
Whenever possible, we offered women the usual written consent process (Figure 8). However, if birth was imminent and the attending clinician considered it appropriate, we offered women a brief explanation of the study and randomised those who said ‘yes’ (i.e. they gave oral assent). Before discharge from hospital, these women had an opportunity to discuss the study in detail and to give written consent for participation in follow-up. This two-stage process was developed in consultation with the NCT and Bliss.
FIGURE 8.
The two alternative consent pathways. a, Women approached to give oral assent in established labour or at emergency caesarean section only if the attending clinicians considered it to be appropriate. Women were not approached if there was insufficient time to give a brief verbal summary of the trial or if they did not speak fluent English and no translator was available. How long was required for oral assent depended on factors such as how much the woman already knew about the study, and her knowledge and wishes about care during the third stage. If recruitment was after oral assent, (1) women were approached before discharge to give written consent to participation in follow-up and (2) the chief investigator was notified of this within 15 days, and this recruitment was monitored by the TSC. Derived from Pushpa-Rajah et al. 168 © Pushpa-Rajah et al. 2014. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
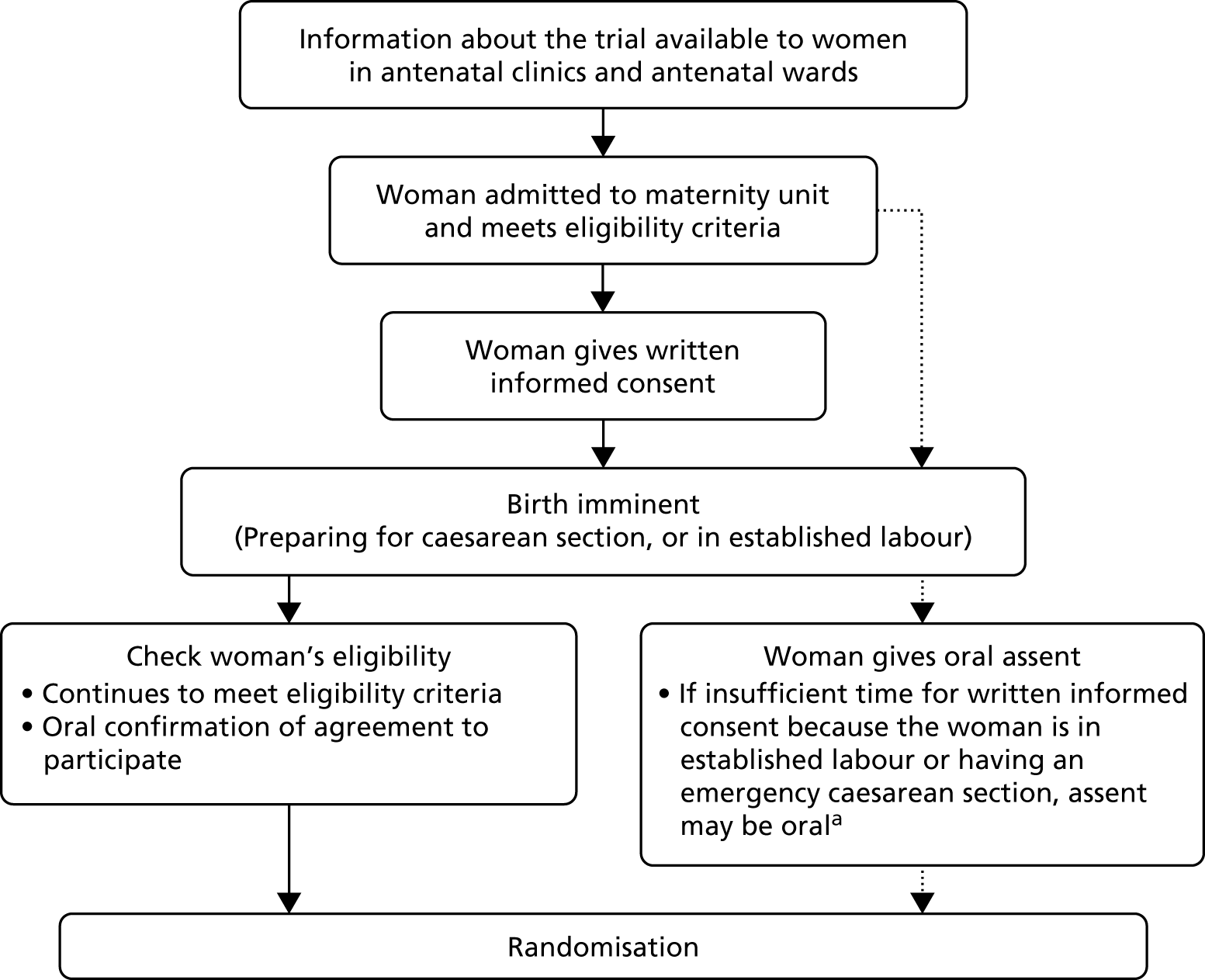
Randomisation was by the attending clinicians, using sealed consecutively numbered opaque envelopes. Clinical staff were trained, with regular study-specific training provided at each site to as many neonatal staff, midwives, obstetricians and theatre staff as possible (see Appendix 17). We supported site training with film clips of simulations for providing immediate neonatal care with the cord intact, and of role-playing to offer consent via the two pathways. Multidisciplinary teamworking on site was essential for successful trial conduct.
We sent 179 women in the trial an invitation to participate in semistructured interviews. A total of 23 were interviewed (usual consent pathway, n = 18; two-stage pathway, n = 5). The interview schedule consisted of open-ended questions42 to explore the women’s views and experiences of being offered participation. Interviews lasted approximately 30 minutes and took place either at their home or by telephone. We sent 20 clinicians who recruited women invitations for semistructured interviews, and interviewed 17 from seven sites. The interview schedule consisted of open-ended questions, to explore clinicians’ views and experiences of offering participation. 41 Interviews took place either in a private hospital room or by telephone. They lasted 20–30 minutes and were audio-recorded and transcribed. Reporting complied with COREQ. 154
Analysis
For the assessment of feasibility of a large trial, the prespecified outcomes are presented based on recruitment at 1 year. For participants in the extended pilot, analyses were based on the groups as randomly allocated (intention to treat). Women who gave birth after 35+6 weeks were not included in the analyses, as outcomes for these babies are different from those born very preterm. Where appropriate, results are presented as RR or risk difference with 95% CIs.
For the qualitative interviews, we used inductive thematic analysis using NVivo version 10 software.
Key findings
Feasibility of a large multicentre trial, based on recruitment for 1 year
Overall, the feasibility objectives were met. During the feasibility phase, we randomised 22% of women who gave birth before 32 weeks’ gestation (varying from 9% to 43% between sites) (see Appendix 17). Factors in this variation included availability of the mobile trolley and of trained clinical staff. Sites already involved in the programme randomised their first participant more quickly than new sites; most sites randomised the first woman within 1 month of opening, whereas new sites took up to 5 months. Of the 125 women randomised, over one-quarter (29%, 36/125) were recruited using the two-stage oral assent pathway. Time from randomisation to birth was within half an hour for over one-third of women, and within 1 hour for over half; three-quarters of women gave birth within 2 hours of randomisation and only 6% gave birth > 1 day after randomisation. Recruitment was across the range of gestational age with approximately one-third before 28 weeks’ gestation, one-third at 28–29 weeks’ gestation and one-third at 30–31 weeks’ gestation.
Compliance with the allocated intervention was good. In the intervention group, median time to cord clamping was 120 seconds (interquartile range 30–135 seconds), compared with 10 seconds (10–15 seconds) in the control group. Overall, 82% (111/135) of babies in the intervention arm had later cord clamping than the control arm (< 20 seconds). In the intervention group, the main reason for cord clamping before 2 minutes was the cord being too short. There were no obvious differences in compliance according to the mode of birth, or whether the site used usual equipment or the mobile trolley for neonatal care.
Fifteen babies (11%) died before discharge; this included three stillbirths all of whom were extremely premature and resuscitation at birth was attempted. Nine (60%) deaths (including one stillbirth) were of babies born < 26 weeks’ gestation. Of liveborn babies, 51 (40%) had an IVH, of whom this was severe for eight (6%).
Results by allocated group, for all women randomised
Of 945 women approached, 472 (50%) gave consent and 261 (28%) were randomised (Figure 9). 71 One-third of women were randomised before 28 weeks’ gestation and two-thirds of women were randomised before 30 weeks. Just over half were women in their first pregnancy and a similar proportion had a caesarean birth. Compliance was good, with median time to cord clamping 120 seconds for women allocated to cord clamping after at least 2 minutes, and 11 seconds for those allocated to cord clamping within 20 seconds. Neonatal care was comparable between the two allocated groups.
FIGURE 9.
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram for outcome to discharge from hospital. a, Woman changed her mind (n = 2), intrauterine death (n = 2), equipment failure (n = 1), study closed (n = 1), randomisation website failed (n = 1). b, Compliance reported by baby. c, Birth at ≥ 36 weeks’ gestation (all singleton pregnancies). d, One woman and her baby withdrew, data reported for mortality only. Reproduced from Duley et al. 71 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
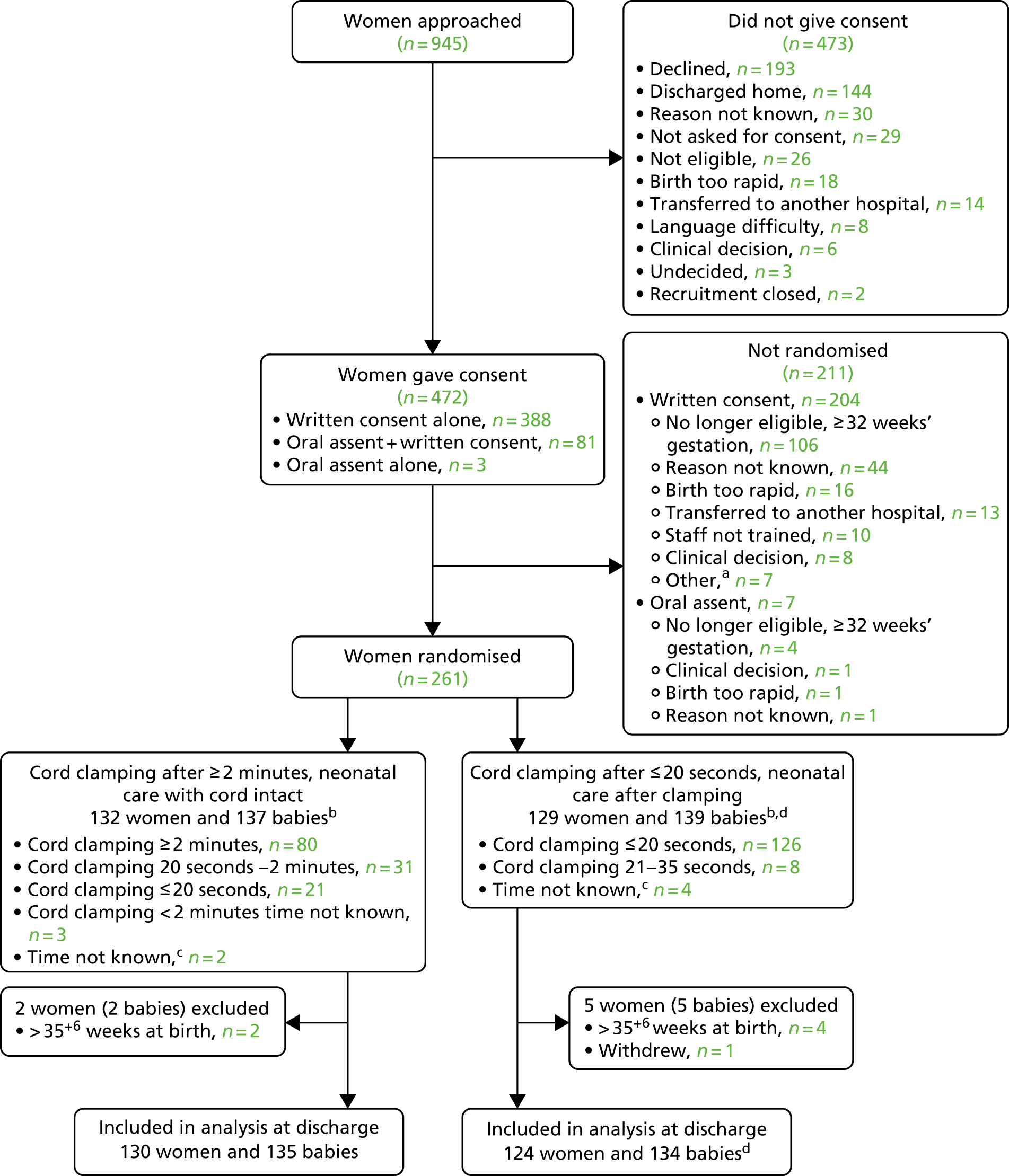
There were no clear differences between the allocated groups in either of the primary outcomes (Table 3). Three-quarters of infants who died were born before 28 weeks’ gestation. The three stillborn babies were all born before 28 weeks’ gestation and resuscitation was attempted at birth. There were no clear differences between the allocated groups for any other outcome for the baby, or in any outcome for the mother.
| Clamp ≥ 2 minutes + neonatal care with cord intact, n (n = 135) | Clamp ≤ 20 seconds + neonatal care after clamping, n (n = 135)a | Risk ratio (95% CI) | |
|---|---|---|---|
| Death | 7 (5%) | 15 (11%) | 0.47 (0.20 to 1.11)b |
| Stillbirth | 1 | 2 | |
| Early neonatal death | 3 | 7 | |
| Late neonatal death | 2 | 5 | |
| Post neonatal death | 1 | 1 | |
| For babies who died, gestation at birth (weeks) | |||
| 30 – 31+6 | – | 1c | |
| 28 – 29+6 | 1 | 3 | |
| 26 – 27+6 | – | 4 | |
| < 26 | 6 | 7 | |
| Any IVH (grade 1–4)d,e | 43 (32%) | 47 (36%) | 0.90 (0.64 to 1.26) |
| Alive at discharge | 38 | 42 | |
| Severe IVH (grade 3–4)d | 6 (4%) | 7 (5%) | |
Qualitative interviews about the consent pathways
Women’s experiences were similar regardless of the consent pathway (see Appendix 20). Few showed a good understanding of randomisation, but how staff spoke to them when offering participation was important to women. They appreciated a calm manner, clear information with time to consider participation, and staff being warm and friendly and not making them feel pressured. Those recruited following oral assent reported being given less information about the trial, but felt that it was sufficient to make a decision regarding participation. Irrespective of the consent pathway, there were gaps in women’s understanding of the trial. Unsurprisingly, the trial was a minor event in comparison to the birth of their baby and, once women had agreed to take part, often they did not give it further thought. Common reasons for agreeing to participate were contributing to research, that the trial might help their baby, and that it appeared to be low risk.
Overall, clinicians were supportive of the two-stage consent pathway (see Appendix 19). Over half discussed the importance of a team approach to inviting participation, regardless of which consent pathway was used. Clinicians were aware of the tension between the signed consent form as a record of the consent process and as a legal document proving consent was given. They viewed consent as a continual process and thought that different consent pathways could be appropriate for different trials. Some clinicians felt that in time-critical situations, oral assent had advantages over the usual consent process, as information was provided on a ‘need-to-know’ basis. There was some concern about the balance between time, information and understanding, for example how much information should be given for oral assent and how this is understood by women when birth is imminent. However, issues about the validity of consent applied to both pathways.
Follow-up of women up to 1 year
Response to the women’s questionnaires was 76% (186/244) at 2–3 months, which dropped to 55% (133/242) at 1 year (see Appendices 21 and 22). 169 There were no clear differences between the groups for any responses, and responses were similar at the two time frames. Scores on the Hospital Anxiety and Depression Scale170 were lower for depression than for anxiety on both questionnaires, and satisfaction with care at birth was high. Women were largely positive about their experiences of participation, with 92% answering ‘definitely yes’169 or ‘probably yes’ at 1 year to a question about whether or not they would participate again in the trial. 171 Things women liked about the trial were good information about the study, caring staff and that their baby or others in the future might benefit from the research. Suggestions about what could have been improved in the trial including approaching women earlier in labour, and better communication about the study from staff.
Follow-up of children at the age of 2 years (corrected for gestation at birth)
Overall, data were available for 83% (218/262) of the children included in the follow-up (see Appendix 23). Children born to women allocated to cord clamping after at least 2 minutes and neonatal care, if required, with the cord intact had a lower risk of death or adverse neurodevelopmental outcome at the age of 2 years (corrected for gestation) (RR 0.61, 95% CI 0.39 to 0.96). However, although the response rate for follow-up was higher among those in the intervention group, neonatal morbidity at hospital discharge was higher for children with no follow-up data available than for those with outcome data. Hence, this difference no longer achieved statistical significance after adjusting for missing data using multiple imputation (RR 0.69, 95% CI 0.44 to 1.09).
Successes
We conducted a substantial and successful pilot trial in a difficult clinical area, and demonstrated feasibility of conducting a large multicentre study. As the study was conducted within existing NHS clinical services, results are generalisable to similar settings.
A key limitation of previous trials is that they largely excluded babies who were likely to need immediate resuscitation at birth. 172 We developed two successful strategies for recruiting such high-risk infants. First, for women allocated deferred clamping, neonatal stabilisation and resuscitation was available with the cord intact. This had the additional advantage that stabilisation was equivalent in the two intervention groups. Second, if birth was imminent, we could offer women the opportunity to participate using the two-stage consent pathway. We recruited one-quarter of women using this pathway – women and babies it would not otherwise have been possible to recruit. Qualitative interviews and questionnaire responses from the women suggest the two-stage consent pathway was acceptable in the trial.
Design and conduct of the Cord pilot trial drew on outputs from earlier parts of the programme, and the experience we developed of working as a multidisciplinary team. In particular, the strong patient and public involvement in the programme and in the trial was essential for changing the way neonatal care was provided for very preterm births, and for developing the two-stage consent pathway.
Our study is also the first cord clamping trial to use independent adjudication of cranial ultrasound scans, thereby reducing potential for bias and improved reliability in ascertainment of IVH. 173 Other successes were being the one of the first deferred cord clamping versus early cord clamping trials at preterm birth to report outcome for the women, and outcome for the children at 2 years of age (corrected for gestation at birth).
Challenges
Although we demonstrated feasibility and had strong support for the main trial, both from the independent oversight committees and from clinical sites keen to participate, we were not able to secure the necessary funding. The reason given by the funding board was that they had concerns over the timing of the study given other relevant trials that had yet to conclude.
When an external pilot trial is successful, transforming it into an internal pilot by continuing into the full trial may maximise efficiency and value for money, but our experience demonstrates it is a challenge to achieve. 174 Nevertheless, data from our trial will contribute to the design of any future trials,72 in particular through the planned prospective meta-analysis and the experience gained in delivering the intervention.
As for many perinatal trials, long-term follow-up of the children was challenging and it required a range of strategies. The importance of complete follow-up to minimise potential bias is underlined by the apparent difference between the allocated groups in response to follow-up and that children without follow-up data in the intervention group were higher risk than those for whom data were available.
Implications for future research
With appropriate planning and training, large multicentre trials of interventions around the time of preterm birth are feasible within existing maternity and neonatal services in the NHS. To improve the opportunity for participation, such trials should consider including the two-stage consent pathway. Nevertheless, this two-stage pathway merits further evaluation in a wider range of studies. Providing neonatal care beside the mother so that she can see and, if she wishes, touch her baby, also merits further evaluation in a wider range of clinical settings, and with follow-up to assess any long-term effects for parents or baby.
Since our trial was planned, new research has emerged that should be considered in planning new trials of timing of cord clamping. This includes work with pregnant sheep which has improved understanding of the physiology of transition from the fetal to neonatal circulation51,159,175,176 and the publication of other relevant trials including a large international trial. 177 The design of any future trial, including the interventions to be compared, should therefore be informed by the systematic review and meta-analysis based on individual participant data (IPD) planned in our final work package.
Work package 5: a prospective meta-analysis
Providing really reliable data on long-term safety and disability-free survival for children born very premature requires very large numbers. To achieve this, international collaboration is required and, therefore, we included planning a prospective meta-analysis as the final element of this programme. Our plan was that the first cycle of this analysis would inform the design of the main trial, should this go ahead. Although the time scale for this first cycle of analysis has been delayed beyond the end of this programme, nevertheless it will inform the design of any future large trial.
See Appendices 24 and 25 for the unpublished reports of this work.
Research aims
The aims were to establish a collaborative group of triallists to conduct an IPD meta-analysis to assess whether timing of cord clamping and other strategies to alter placental transfusion at preterm birth influence (1) the composite outcome of death or serious morbidity at discharge from hospital and (2) disability-free survival in early childhood (aged 2–3 years). This work is referred to as Cord Clamping and Placental Transfusion at Preterm birth (CCPTP). The unexpected rapid increase in small randomised trials being published led to agreement by the CCPTP collaboration that, if sufficient funding is available, the planned prospective meta-analysis should be expanded to a retrospective IPD meta-analysis, with a nested prospective meta-analysis.
Methods for data collection
The CCPTP collaborative group of triallists was established and the protocol (see Appendix 24) discussed at the annual face-to-face meeting. The PROSPERO registration identifier is CRD42013004405. Following quarterly searches of trial registries, the chief investigators of studies potentially eligible for the prospective meta-analysis are contacted and invited to join the collaboration. Studies are included if they are randomised trials meeting the eligibility criteria and the investigator(s) are blind to outcome data by intervention group at the time that they agree to the CCPTP protocol.
Eligibility criteria are that participants gave birth (if women were randomised) or were born (if baby was randomised) before 37 completed weeks’ gestation. Interventions are comparisons of alternative policies for timing of cord clamping, and other strategies to influence umbilical flow (e.g. lowering the baby below the level of the placenta, use of uterotonic drugs and umbilical cord milking or stripping). Studies are included if they compare strategies to maintain ‘physiological’ umbilical flow (i.e. none or minimal intervention) with strategies that aim to alter umbilical blood flow (e.g. using gravity by lowering the baby, cord milking or stripping). Studies comparing alternative strategies for influencing umbilical flow without a ‘timing of cord clamping’ arm will also be included.
Planned comparisons are:
-
Early cord clamping (short delay) versus deferred cord clamping (long delay).
-
Early cord clamping (short delay) versus umbilical cord milking or stripping.
-
Deferred cord clamping (long delay) versus umbilical cord milking or stripping.
-
Deferred cord clamping (long delay) plus umbilical cord milking or stripping versus early cord clamping (short delay).
As there is no consensus about the definition of ‘early’ and ‘deferred’ cord clamping, early clamping is defined whenever possible as within 30 seconds, and deferred clamping as after at least 1 minute. Trials that use different definitions will be included in the analysis.
As outcomes for babies born very preterm (< 32 weeks’ gestation) are different for those born moderately preterm (32 to 37 weeks’ gestation), separate analyses are planned for these two groups of infants. For those born < 32 weeks’ gestation, primary outcomes are (1) death or serious morbidity at hospital discharge (serious morbidity is defined as one or more of severe IVH, necrotising enterocolitis, late onset sepsis, chronic lung disease and retinopathy of prematurity); (2) chronic lung disease; and (3) neurodevelopmental delay in early childhood. Secondary outcomes include individual items in the composite outcome and measures of neonatal morbidity and adverse neurodevelopmental outcome in early childhood.
For infants born at or after 32 weeks’ gestation, the primary outcome is admission to NICU. Secondary outcomes are measures of neonatal morbidity, iron status in infancy, and adverse neurodevelopmental outcome in early childhood.
For the women, outcomes are complications of the third stage of labour, postpartum infection, breastfeeding and postnatal depression.
Analysis
The detailed statistical analysis plan will be developed and agreed before any analysis begins. Planned subgroup analyses are based on:
-
participant-level characteristics – gestation at birth, whether singleton or multiple pregnancy, mode of birth and whether or not spontaneous onset of labour
-
hospital-level characteristics – highest level of neonatal unit available at site, timing of uterotonic drug, timing of cord clamping, planned position of the baby relative to the placenta with the cord intact, and whether or not babies needing immediate resuscitation at birth were recruited.
To assess whether or not the results are robust to trial quality and different methods of analysis, the following sensitivity analyses are planned for the primary outcomes, if there are sufficient data: excluding studies with a high risk of bias; for comparison of early with deferred clamping, analysis weighted by observed between-arm difference in mean timing between the groups; analysis weighted by degree of separation in haemoglobin between the groups.
If a policy of deferred cord clamping appears to be beneficial, additional analyses will therefore explore the effects based on duration of allocated deferred cord clamping and, if the allocation was clamping after 20 to 30 seconds, whether or not neonatal care, if needed, was provided with the cord intact.
Successes
Currently we know of 53 trials, involving 11,811 infants (3020 for long-term outcomes). Our success in establishing the CCPTP collaboration emphasises the international interest in this topic. Annual collaborators meetings, held alongside neonatal conferences to minimise cost, have facilitated collaboration and biannual newsletters provide regular updates.
Success of the collaboration between the Nottingham Clinical Trials Unit and the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney, which jointly support the secretariat for CCPTP, means that the first cycle of analysis is now planned for 2020 and funding has been secured to expand this to include a retrospective meta-analysis based on IPD, with a nested prospective meta-analysis (see Appendix 25). The collaboration also changed its name to systematic review and network meta-analysis with individual participant data on Cord Management at Preterm birth (iCoMP).
Challenges
The main challenge for this project is common to all planned prospective meta-analyses: the time scale for analysis is unpredictable. Results for the large study planned for inclusion in CCPTP, the Australian Placental Transfusion Trial, were published later than anticipated,177 as were results for the Cord pilot trial. Hence, the first cycle of analysis was not possible within the programme time scale. The number of new trials under way is accelerating, with 29 registered in the past 2 years. Many of these are relatively small and data analysis may have begun before the investigators agree the CCPTP protocol. Growing numbers being published risked undermining the relevance of a standalone prospective meta-analysis. Hence, we expanded the protocol to allow inclusion of a retrospective IPD meta-analysis, with network meta-analysis. Work commenced on this IPD in 2019.
Implications for future research
Placental transfusion at preterm birth is a current ‘hot topic’, with wide variation in clinical practice. This has led to a plethora of recent trials largely undertaken in isolation, with little standardisation of the intervention(s) being tested or the outcomes being collected. Value of these existing trials will be substantially enhanced by combining and fully utilising the IPD, as planned in CCPTP. Provision of individual participant data by each trial investigator will provide the required statistical power and enable reliable subgroup analyses to be undertaken.
Results of these analyses based on IPD from each trial will inform clinical practice and will identify the most promising intervention(s) for further evaluation. Co-ordinating international efforts in this way will also help achieve consensus on the most important clinical outcomes to assess in any future trials.
Conclusions
Our programme aimed to improve the quality of immediate care at very preterm birth, enhance family-centred care at birth and improve outcome for infants and their families. To do this, we conducted 10 projects grouped into five work packages. First, we identified and ranked the top research gaps for preterm birth. Then, to develop strategies for providing initial neonatal care at birth beside the mother at very preterm birth, we conducted a survey of practice, assessed parents’ views of initial neonatal care beside the mother, improved understanding of the physiology of transition from fetal to neonatal circulation, systematically reviewed strategies for initial care and assessment at preterm birth, and developed a mobile newborn life support trolley. Finally, to generate the information to enable conduct of a large NHS trial comparing alternative policies for cord clamping at preterm births, we systematically reviewed ethics issues in recruiting preterm or sick infants to trials, conducted a feasibility pilot trial and developed the protocol for a prospective meta-analysis.
The prioritisation process ran in parallel to other work packages, as it was added in response to feedback from the funding board at stage 1 of the grant application process. Hence, the other work packages were not dependent on its outcomes. Although cord clamping did emerge as a top priority, we had already identified this as a priority via an informal process.
Central to the success of our programme was that service user representatives were partners throughout its planning and conduct. What worked well was being equal partners, rooted in a service user perspective, and being guided throughout by service user representatives who shared responsibility for the research. This allowed us to tackle emotive issues, such as immediate neonatal care at very preterm birth, and consent for participation in a clinical trial evaluating an intervention during labour at very preterm birth. Our service users came with an understanding of different research methods, but this should not be a prerequisite as good training could also be included. Our research team included experience and expertise in working with service users and their representatives. Vital to equal partnership is the support from those leading the research to specifically include service users who bring their personal experience to the project. The time commitment required did not prove a problem with this particular programme of research, but may well be a problem generally. In addition, our qualitative interviews with parents who had experienced very preterm birth were conducted early in the programme. The experiences these parents described informed our work, helping to ensure that it remained parent-focused throughout.
Providing care for the mother, her baby and other family members involves complex multidisciplinary teams. A substantive output from our programme has been to demonstrate that providing initial neonatal care at birth beside the mother is both feasible within the NHS and acceptable to parents and to clinicians. Our work is contributing to a shift in culture, for example the World Health Organization now recommends that ventilation can be started before cutting the umbilical cord, if the attending clinicians have experience in providing effective positive-pressure ventilation with the cord intact. 73 Although further evaluation is required, our data suggest that, with adequate training, preparation and support, parents and clinicians prefer care beside the mother rather than moving the baby away from the mother for assessment, stabilisation and resuscitation at birth.
Although this might seem to be a relatively simple change in practice, achieving it required a change in culture for care at the time of very preterm birth. When we planned this work, usual practice was to quickly clamp the umbilical cord and then move the baby either to the side of the room or to another room nearby. This requires little communication between the obstetric and the midwifery team responsible for the mother and the birth and the neonatal team responsible for the newborn infant. In contrast, providing immediate neonatal care beside the mother requires discussion in advance and negotiation about where to place the resuscitation equipment, who will stand where and who will support the mother and her partner so they understand what is happening. Parents and clinicians felt that this improved communication between them. Anecdotally, clinicians involved in the programme also felt that this multidisciplinary working enhanced how they interacted with other clinical colleagues on the delivery suite, with wider benefits in terms of improved quality of care.
Another example of this change in culture is the change in attitude to the role of the mobile trolley. When we were planning the programme, the neonatologists we consulted were unanimous that developing this trolley, or a similar device, was essential if they were to provide neonatal care beside the mother. However, following consultation with the necessary disciplines about providing care beside the mother and with the umbilical cord intact, and having demonstrated that this is possible in clinical practice, opinion shifted. Working as a multidisciplinary team, we showed that it was also possible to adapt the existing equipment to provide neonatal care beside the mother. 70 This has the advantages of not needing to purchase new equipment and that staff are already familiar with its use. For the pilot trial, six out of the eight hospitals used existing equipment to provide the intervention and two hospitals chose to purchase the new mobile trolley.
Very preterm birth is a difficult time for parents and their experiences of care may differ from those having a term birth. Our P-BESS questionnaire may be useful to compare views and experiences across hospitals and differing practices for very preterm birth, whereas individual aspects of the care environment can be evaluated using the separate subscales.
Conducting randomised trials of interventions around the time of very preterm birth is particularly challenging, and in the past infants at highest risk have largely been excluded from trials. 55 To help ensure that recruitment to the Cord pilot trial was representative of the total population of very preterm births, we developed a two-stage consent pathway that allowed women for whom birth was imminent the opportunity to consider participation. This strategy is now recommended for use in other intrapartum trials by the Royal College of Obstetricians and Gynaecologists. 81 The current informed consent process is laborious and a major barrier to conducting research on important, but time-sensitive, topics. The two-stage consent process should help to open neonatology and perinatology to making research participation more like the everyday clinical care. A similar approach may also be relevant in other emergency trials,178 or in those for which recruitment is time critical. For example, it has been adapted for use in an acute stroke trial. 82
Part of the rationale for including a prospective meta-analysis in our programme was that a large trial was under way in Australia. That study compared cord clamping after at least 60 seconds and lowering the baby with clamping within 10 seconds, and results have just been reported for the 1634 babies randomised. 177 There was no clear difference in the composite primary outcome of death or serious morbidity at 36 weeks postmenstrual age, although fewer babies in the intervention group died (6.4% in delayed clamping group versus 9.0% in immediate clamping). 177 In the light of these new data, remaining research questions to address in future trials include whether or not stabilisation and resuscitation can safely be left until after cord clamping, even for those with apnoea and the very premature, and what is the optimal time for cord clamping at very preterm birth. 72,179
Our prioritisation process for preterm birth research is already influencing the UK research agenda. For example, the NIHR Health Technology Assessment programme has funded studies addressing topics in the top priorities, and Bliss, the charity for babies born premature or sick, has included the priorities in their strategy for research they support.
Limitations of our programme
Preterm birth is associated with factors such as lower socioeconomic status, ethnicity and maternal age. For our prioritisation process, despite implementing strategies to reach a representative population, respondents were primarily white and a relatively high proportion of respondents were homeowners and were therefore not representative of those most affected by preterm birth. This may limit relevance of the ranked priorities to other populations.
For the qualitative work on very preterm birth, limitations of our data were that participants in the interviews came from two hospitals in the south of England and they were mainly white married women; therefore, not typical for vey preterm birth. For development of the P-BESS questionnaire, the sample size was relatively small for a factor analysis and, again, was unrepresentative.
Generalisability of our findings on neonatal care beside the mother is limited by recruitment of parents and clinicians from a single hospital with a particular interest in care beside the mother, and where the trolley had been developed. In addition, we were again not successful in recruiting parents for interview who were representative of the wider population with very preterm birth. Furthermore, the babies were all alive at the time of interview and most had not required advanced resuscitation at birth.
Finally, although out pilot trial demonstrated feasibility and had strong support for progression to the main trial, we were not able to secure the necessary funding. Hence, we have not been able to conduct a definitive trial.
Implications for health care
Providing initial neonatal care at birth beside the mother is feasible within the NHS, acceptable to parents and to clinicians, and both parents and clinicians felt that it improved communication. Although further evaluation is required, our data suggest that, with adequate training, preparation and support, parents and clinicians prefer care beside the mother rather than moving the baby away from the mother for assessment, stabilisation and resuscitation at birth. This has major implications for maternity services. Although this is a relatively simple change in practice, implementation of neonatal care beside the mother at birth requires a change in culture to multidisciplinary teamworking. Care beside the mother can be done either by adapting the usual resuscitation equipment or by using a mobile trolley specifically designed for the purpose.
The Cord pilot trial demonstrates that neonatal stabilisation and resuscitation can be provided with the umbilical cord intact, providing a practical and generalisable strategy for supporting continued umbilical flow at birth. Results of this trial add to the growing body of data from randomised trials suggesting that there is no need to rush to clamp the cord at very preterm birth. Although the optimal policy for cord clamping at very preterm birth remains unclear, waiting 20 to 30 seconds seems prudent.
Only a small proportion of births are very preterm, and parents’ experiences of care during the birth can differ from those having a term birth. The P-BESS questionnaire was developed to assess experiences and satisfaction with care during very preterm birth and can be used by hospitals to assess their care, potentially identifying areas where care can be improved. A total score can be used to compare across hospitals and differing practices, whereas individual aspects of care can be evaluated using the subscales.
Recommendations for future research
-
The 15 top research priorities should guide researchers and funding bodies when planning new research.
-
When planning and funding preterm birth research, it may be relevant to consider not only the top 15 priorities but also the full list of 104 unanswered questions, the top 30 ranked by those affected by preterm birth and the top 30 ranked by health-care professionals.
-
Future prioritisation processes, particularly those with a similar wide range of health-care professionals, should anticipate potential different perspectives and mitigate any imbalance where possible, and should report voting separately by ‘service users’ and health-care professionals. Health-care professionals who are also researchers should declare this potential conflict before participating in the prioritisation workshop, so that it can be taken into account.
-
Important ‘evidence gaps’ for neonatal care at birth include pharmacological intervention for transition support or resuscitation of newborn infants, and the effectiveness of new, less invasive forms of airway management (and related issues regarding surfactant delivery).
-
Since our programme started, both timing of cord clamping for very preterm births and providing neonatal care with the cord intact have become increasingly topical. New research has emerged, including the work conducted within this programme, which is changing opinion and clinical practice. Therefore, it would be timely to repeat a survey of practice.
-
Further research is needed to replicate our findings about parent experiences at very preterm birth, and to explore whether or not there is variation for parents from different backgrounds.
-
Further studies are needed to test the P-BESS questionnaire in a larger, more representative sample of parents.
-
Fathers of preterm or sick babies are a difficult group to recruit into research. Our work highlights the importance of including them in research studies to ensure that their perspective is represented.
-
Further research is needed to evaluate clinical outcomes, parent experiences and the costs of neonatal care beside the mother and of care with the cord intact. This should include a wider range of settings and using the standard resuscitation equipment as well as the mobile trolley. It should involve parents from more diverse backgrounds, those whose baby required advanced resuscitation, and those whose baby died. Future studies should also include follow-up of parents and infants.
-
The optimal study design would be a randomised trial to compare outcomes (for children and parents) for parents offered initial neonatal care beside the mother, with outcomes for those offered traditional care.
-
With appropriate planning and training, large multicentre trials of interventions around the time of preterm birth are feasible within existing maternity and neonatal services in the NHS.
-
The two-stage consent pathway should be included in future perinatal trials to increase participation when there is little time in which to seek consent to participation. It also has potential to increase participation in other emergency or time-critical trials, but this merits further evaluation.
-
Design of future trials comparing alternative policies for cord management should take account of results from our trial and the planned meta-analysis based on individual participant data from all relevant trials.
Acknowledgements
Our thanks to all the women and their families who participated in this research, and to the members of the public, clinicians and researchers who have contributed. This report is based on a programme of work that included multiple contributions from many authors and contributors.
Programme steering group
Lelia Duley (chairperson), Susan Ayers, Zoe Chivers, Jon Dorling, David Field, Gill Gyte, William McGuire, Chris Megone, Sam Oddie, Sandy Oliver, Jim Thornton, Andrew Weeks and Charles William Yoxall.
Programme management team
Lelia Duley, Charlotte Lloyd (2011–13), Virginia Portillo (2014–15), Sarah Somerset (2016–17), Sarah Smith (2011–13) and Ruth Fletcher (2014–16).
Thanks to Lindsay Armstrong-Buisseret for assistance with compiling this report.
Work package 1
James Lind Alliance Preterm Birth Priority Setting Partnership
This work would not have been possible without the organisations that contributed to the partnership, the people who responded to the survey and who voted on the priorities, and the participants in the final workshop. For the qualitative work on consensus development, thanks to Lizzie Oliver and Sarah Thum-Bonanno for transcribing the data, Sergio Grazio for technical support, members of the steering group, and delegates who attended the final workshop.
Ethics approval
Research Ethics Committee approval for the whole priority setting exercise was obtained from the Institute of Education (reference FCL 318), and for distribution of paper versions of the survey from the Liverpool Research Ethics Committee (reference 12/WA/0286).
Steering group
Sally Crowe (chairperson), Fiona Alderdice, Bev Chambers, Zoe Chivers, Anna L David, Sanjeev Deshpande, Irene Dowling, Lelia Duley, Chris Gale, Gill Gyte, Catherine P James, Jenny McNeill, Sandy Oliver, Andrew Shennan, Mark Turner and Seilin Uhm.
Consensus development for tackling technical and emotive challenges
Seilin Uhn and Sandy Oliver.
Work package 2.1
Evidence-based immediate care of the very preterm infant
This systematic overview is registered with PROSPERO CRD42012003038. It was conducted by Thirimon Moe-Byrne, Jennifer VE Brown, Mark Corbett and William McGuire.
Work package 2.2
Ethical issues in recruitment of preterm or sick infants to perinatal trials
This framework synthesis was conducted by Chris Megone, Eleanor Wilman, Sandy Oliver, Lelia Duley and Gill Gyte and Judith Wright.
Work package 3.1
Neonatal care at birth, a survey of current practice
This research would not have been possible without the neonatal units and staff who responded to the survey, and the participants who agreed to be interviewed. Thanks also to Richard A Parker from the Centre for Applied Medical Statistics, University of Cambridge, for statistical support for the survey.
Ethics approval
The survey was approved by the local research and development department at Bradford Teaching Hospitals NHS Foundation Trust but did not require ethics committee approval. The interview study was approved by the Rotherham sitting of National Research Ethics Committee 11/YH/0302.
Project team
Sam Oddie, Penny Rhodes and Yogen Singh.
Work package 3.2
Parents’ views of care at preterm birth
This research would not have been possible without the parents who agreed to be interviewed and who so generously shared their experiences.
Ethics approval
The study received approval from the National Research Ethics Committee South East Coast – Kent. Reference: 11/LO/0143.
Project team
Susan Ayers, Jane Abbott, Leah Arnold, Lelia Duley, Gill Gyte, Heike Rabe, Gillian Russell and Alexandra Sawyer.
Work package 3.3
Measuring placental transfusion
This research would not have been possible without the women who agreed to participate, and to the clinical staff who made it possible. Particular thanks to Rebecca Palethorpe, Naseeba Azmat, Natalie Batey, Dush Batra, Yvette Davis, Chris Day, Claire Dinsdale, Nicky Grace, Yvonne Hooton, Waheeda Hussein, Gill Perkins, Carys Smith, Kumar Swamy, Jim Thornton, Derek Tuffnell and Kelly Young for their support. Thanks also to Boliang Guo and Min Yang for statistical advice.
Ethics approval
The study received ethics approval from the London Riverside National Research Ethics Service Committee.
Project team
Jon Dorling, Sam Oddie, Bernard Schoonakker and Lelia Duley.
Work package 3.4
Developing a Bedside Assessment, Stabilisation and Initial Circulatory Support trolley
This research would not have been possible without contributions from a large number of people: a group who met at Worcestershire Royal Hospital to discuss ways of bedside resuscitation consisting of Susan Bewley (who coined the term ‘BASICS’), Amanda Burleigh, Lelia Duley, Andrew Gallagher, Ann Marie Heuchan, David Hutchon, Rabia Imtiaz, David Odd and Andrew Weeks. David Hutchon, who convened the initial meeting, was a member of the team for the Medical Futures award in 2010, and provided input into the design of the BASICS trolley prototype, and identified Inditherm as a commercial partner. Inditherm and their managing director Nick Bettles for their work in developing the fully functional CE marked mobile resuscitaire from the initial prototype. Gill Gyte who attended the Liverpool design meetings and provided important input into the trolley design. Tony Fisher, Head of Department of Clinical Engineering at the Royal Liverpool University Teaching Hospital, who provided important advice about intellectual property as well as agreeing to develop the prototype in his department at a discounted cost.
Ethics approval
The commercially produced trolley (with CE mark) was subsequently introduced into clinical service at Liverpool Women’s Hospital following approval from the Medical Devices Committee, the head of Research and Development, and the Clinical Director for the Neonatology Directorate.
Project team
Andrew Weeks, Peter Watt, Charles William Yoxall and Lelia Duley.
Work package 3.5
Evaluating parents’ and clinicians’ views of neonatal care beside the mother
This research would not have been possible without the clinciains who completed the service evaluation forms, and the parents and clinical staff who generously agreed to be interviewed and shared their experiences. Particular thanks to Louise Goodman, Gill Houghton, Heather Longworth and Angela Pascall for conducting the qualitative interviews.
Ethics approval
The service evaluation was approved by Trust governance procedures during the introduction of the trolley into clinical practise in Liverpool Women’s Hospital. Ethics approval for the qualitative interviews was from the Yorkshire and Humber Research Ethics Committee (reference 12/YH/0321).
Project team
Charles William Yoxall, Andrew Weeks, Susan Ayers, Alex Sawyer, Sophia Bertullies, Lelia Duley and Margaret Thomas.
Work package 4
Cord pilot trial
This research would not have been possible without the women who participated in the trial, and their families, and the clinical and research staff at the sites. Particlar thanks to Diane Whitham and Gill Bumphrey for preparation of the randomisation envelopes, and to Alec Whitham for making the mail boxes.
This study is registered as ISRCTN21456601.
Ethics approval
Ethics approval was from Nottingham REC 2 (National Research Ethics Service reference 12/EM/0283).
Cord Pilot Trial Collaborative Group
Nottingham Clinical Trials Unit
Lelia Duley, Lindsay Armstrong-Buisseret (from November 2015), Brian Barnes, Lucy Bradshaw, Natalie Hutchings, Eleanor Mitchell, Angela Pushpa-Rajah (May 2013–July 2015) and Keith Whittaker.
Trial Management Group
Lucy Bradshaw, Jon Dorling, Lelia Duley, Eleanor Mitchell and Angela Pushpa-Rajah (chairperson).
Trial Steering Committee
Mike Clarke (chairperson), Lucy Bradshaw, Kate Branchett, Richard Cooke, Jon Dorling, Lelia Duley, Liz Goddard, Sara Kenyon, Angela Pushpa-Rajah and Philip Steer.
Data Monitoring Committee
Douglas Altman (chairperson), Declan Devane, Andrew Shennan and Ben Stenson.
Patient and public involvement
Jane Abbott (until June 2014), Zoe Chivers and Gill Gyte.
Cranial ultrasound adjudication
Lindsay Armstrong-Buisseret, Lucy Bradshaw, Robert Dineen, Jon Dorling, Lelia Duley and Eleanor Mitchell.
Clinical advisors
David Field, Jim Thornton and William Tarnow-Mordi.
Women’s and clinicians’ views of the consent pathways
Celine Chhoa, Alexandra Sawyer, Susan Ayers, Angela Pushpa-Rajah and Lelia Duley.
Cord Pilot Trial Cranial Ultrasound Adjudication Collaborative Group
Working group
Lindsay Armstrong-Buisseret, Lucy Bradshaw, Robert Dineen, Jon Dorling, Lelia Duley and Eleanor Mitchell.
Nottingham Clinical Trials Unit
Lelia Duley, Lindsay Armstrong-Buisseret, Brian Barnes, Lucy Bradshaw, Eleanor Mitchell and Keith Whitaker.
Cranial ultrasound adjudication
Narendra Aladangady, Dushyant Batra, Robert Dineen, Jon Dorling, Joe Fawke, Louise Hattingh, Shoaib Khan, Bernard Schoonakker and Kiran Yajamanyam.
Follow-up of the women postnatally and at 1 year
Lindsay Armstrong-Buisseret, Lucy Bradsaw, Lelia Duley, Eleanor Mitchell, Alexandra Sawyer and Susan Ayers.
Follow-up of the children at 2 years of age
Lindsay Armstrong-Buisseret, Lucy Bradsaw, Jon Dorling, Lelia Duley, Samantha Johnson, Eleanor Mitchell, Rob Dineen and Kathryn Powers.
Work package 5
A prospective meta-analysis
This systematic review and prospective meta-analysis is registered with PROSPERO CRD42013004405.
This research would not have been possible without the triallists who agreed to participate in the collaborative group. Particular thanks to Kylie Hunter for advice on the search strategy for ongoing trials.
Cord Clamping and Placental Transfusion at Preterm Birth Collaborative Group
Secretariat
Lelia Duley (chairperson), Lisa Askie, Alan Montgomery (from 2013), Min Yang (to 2016), Charlotte Lloyd (2011–13), Virginia Portillo (2014–15) and Sarah Somerset (2016–17).
Trialists and collaborators
Krishna Agarwal, Ola Andersson, John Bauer, Melchor Carbonell Socias, Sangkae Chamnanvanakij, Vikram Datta, Eugene Dempsey, Catalina De Paco Matallana, Monica Domingo Puiggros, Walid El-Naggar, Karen Fairchild, Neil Finer, Meena Garg, Alejandra Gurzman, Shigeharu Hosono, Omar Kamlin, Anup Katheria, John Kattwinkel, Justin Josephsen, Judy Mercer, G Ram Mohan John Simes, William Tarnow-Mordi, Tanai Trongkamonthum, Sundaram Venkataseshan and Danielle Young.
Contributions of authors
Lelia Duley (Professor of Clinical Trials, University of Nottingham) substantially contributed to the design, methodology, data collection, analysis and reporting for all of the work packages. She was the grant holder, the chief investigator for the Cord pilot trial and chaired the programme steering group. She led the writing, revision and approval of the final report.
Jon Dorling (Associate Professor of Neonatology, University of Nottingham) substantially contributed to the design, methodology, data collection, analysis and reporting for the measuring placental transfusion work package (3.3), and the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Susan Ayers (Professor of Maternal Health, City, University of London) led on the qualitative aspects of the programme. She substantially contributed to the design, methodology, data collection, analysis and reporting for the parents’ views of care at preterm birth work package (3.2), and the evaluating parents’ and clinicians’ views of neonatal care beside the mother work package (3.5). She also led on the qualitative aspects of the Cord pilot trial work package (4). She was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Sandy Oliver (Professor of Public Policy, University College London) led the preterm birth priority setting work package (1) including supervising the doctor of philosophy (PhD) student, and substantially contributed to the design, methodology, data collection, analysis and reporting for the framework synthesis of ethics issues in recruitment of preterm or sick infants to perinatal trials work package (2.2). She was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Charles William Yoxall (Consultant Neonatologist, Liverpool Women’s Hospital) substantially contributed to design and development of the BASICS trolley work package (3.4), and the design, methodology, data collection, analysis and reporting for the evaluating parents’ and clinicians’ views of neonatal care beside the mother work package (3.5) and the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Andrew Weeks (Professor of International Maternal Health Care, University of Liverpool) substantially contributed to design and development of the BASICS trolley work package (3.4), and the design, methodology, data collection, analysis and reporting for the evaluating parents’ and clinicians’ views of neonatal care beside the mother work package (3.5) and the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Chris Megone (Professor of Philosophy, University of Leeds) led the design, methodology, data collection, analysis and reporting for the framework synthesis of ethics issues in recruitment of preterm or sick infants to perinatal trials work package (2.2), and advised on development of the two-stage oral assent consent pathway in the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Sam Oddie (Consultant Neonatologist, University of York) substantially contributed to the design, methodology, data collection, analysis and reporting for the neonatal care at birth work package (3.1), the measuring placental transfusion work package (3.3), and the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Gill Gyte (NCT) provided patient and public involvement expertise across all work packages. In particular she substantially contributed to the design, methodology, data collection, analysis and reporting for the preterm birth priority setting work package (1), the framework synthesis of ethics issues in recruitment of preterm or sick infants to perinatal trials work package (2.2), the parents’ views of care at preterm birth work package (3.2), design and development of the BASICS trolley work package (3.4), and the Cord pilot trial work package (4). She was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Zoe Chivers (Bliss) provided patient and public involvement expertise across all work packages. In particular she substantially contributed to the design, methodology, data collection, analysis and reporting for the preterm birth priority setting work package (1), and the Cord pilot trial work package (4). She was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Jim Thornton (Professor of Obstetrics and Gynaecology, University of Nottingham) supported the measuring placental transfusion work package (3.3) and substantially contributed to the design, methodology, data collection, analysis and reporting for the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
David Field (Professor of Neonatal Medicine, University of Leicester) substantially contributed to the design, methodology, data collection, analysis and reporting for the Cord pilot trial work package (4). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Alexandra Sawyer (Research Fellow, University of Brighton) substantially contributed to the design, methodology, data collection, analysis and reporting for the parents’ views of care at preterm birth work package (3.2), the evaluating parents’ and clinicians’ views of neonatal care beside the mother work package (3.5), and the qualitative aspects of the Cord pilot trial work package (4).
William McGuire (Professor of Child Health, University of York) substantially contributed to the design, methodology, data collection, analysis and reporting for the evidence-based immediate care of the very preterm infant work package (2.1). He was a member of the programme steering group and contributed to the writing, revision and approval of the final report.
Publications
Uhm S, Liabo K, Stewart R, Rees R, Oliver S. Patient and public perspectives shaping scientific and medical research: panels for data, discussions, and decisions. Patient Intell 2012;4:1–10.
Arnold L, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Qualitative Collaborative Group. Parents’ first moments with their very preterm babies: a qualitative study. BMJ Open 2013;3:e002487.
Sawyer A, Ayers S, Abbott J, Gyte G, Rabe H, Duley L. Measures of satisfaction with care during labour and birth: a comparative review. BMC Pregnancy Childbirth 2013;13:108.
Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Qualitative Collaborative Group. Parents’ experiences and satisfaction with care during the birth of their very preterm baby: a qualitative study. BJOG 2013;120:637–43.
Duley L, Uhm S, Oliver S, Preterm Birth Priority Setting Partnership Steering Group. Top 15 UK research priorities for preterm birth. Lancet 2014;383:2041–2.
Oddie S, Rhodes P, Very Preterm Birth Qualitative Collaborative Group. Barriers to deferred cord clamping in preterm infants. Arch Dis Child Fetal Neonatal Ed 2014;99:F391–4.
Pushpa-Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, Duley L, Cord Pilot Trial Collaborative Group. Cord pilot trial – immediate versus deferred cord clamping for very preterm birth (before 32 weeks gestation): study protocol for a randomized controlled trial. Trials 2014;15:258.
Russell G, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Qualitative Collaborative Group. Parents’ views on care of their very premature babies in neonatal intensive care units: a qualitative study. BMC Pediatr 2014;14:230.
Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Collaborative Group. Measuring parents’ experiences and satisfaction with care during very preterm birth: a questionnaire development study. BJOG 2014;121:1294–301.
Singh Y, Oddie S. Marked variation in delivery room management in very preterm infants. Resuscitation 2013;84:1558–61.
Thomas MR, Yoxall CW, Weeks AD, Duley L. Providing newborn resuscitation at the mother’s bedside: assessing the safety, usability and acceptability of a mobile trolley. BMC Pediatr 2014;14:135.
Bradshaw LE, Pushpa-Rajah A, Dorling J, Mitchell E, Duley L. Cord pilot trial: update to randomised trial protocol. Trials 2015;16:407.
Sawyer A, Ayers S, Bertullies S, Thomas M, Weeks AD, Yoxall CW, Duley L. Providing immediate neonatal care and resuscitation at birth beside the mother: parents’ views, a qualitative study. BMJ Open 2015;5:e008495.
Wilman E, Megone C, Oliver S, Duley L, Gyte G, Wright JM. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the empirical research. Trials 2015;16:502.
Yoxall CW, Ayers S, Sawyer A, Bertullies S, Thomas M, D Weeks A, Duley L. Providing immediate neonatal care and resuscitation at birth beside the mother: clinicians’ views, a qualitative study. BMJ Open 2015;5:e008494.
Corbett MS, Moe-Byrne T, Oddie S, McGuire W. Randomisation methods in emergency setting trials: a descriptive review. Res Synth Methods 2016;1:46–54.
Megone C, Wilman E, Oliver S, Duley L, Gyte G, Wright J. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the analytical (theoretical/philosophical) research. Trials 2016;17:443.
Batey N, Yoxall CW, Fawke JA, Duley L, Dorling J. Fifteen-minute consultation: stabilisation of the high-risk newborn infant beside the mother. Arch Dis Child Educ Pract Ed 2017;102:235–8.
Chhoa CY, Sawyer A, Ayers S, Pushpa-Rajah A, Duley L. Clinicians’ views and experiences of offering two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials 2017;18:196.
Sawyer A, Chhoa C, Ayers S, Pushpa-Rajah A, Duley L. Women’s views and experiences of two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials 2017;18:422.
Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed 2018;103:F6–F14.
Bradshaw L, Sawyer A, Armstrong-Buisseret L, Mitchell E, Ayers S, Duley L. Cord pilot trial, comparing alternative policies for timing of cord clamping before 32 weeks gestation: follow-up for women up to one year. BMC Pregnancy Childbirth 2019;19:78.
Bradshaw L, Sawyer A, Mitchell E, Armstrong-Buisseret L, Ayers S, Duley L. Women’s experiences of participating in a randomised trial comparing alternative policies for timing of cord clamping at very preterm birth: a questionnaire study. Trials 2019;20:225.
Armstrong-Buisseret L, Powers K, Dorling J, Bradshaw L, Johnson S, Mitchell E, Duley L. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years. [published online ahead of print August 1 2019]. Arch Dis Child Fetal Neonatal Ed 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261-9. https://doi.org/10.1016/S0140-6736(08)60136-1.
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002;288:728-37. https://doi.org/10.1001/jama.288.6.728.
- Zeitlin J, Draper ES, Kollée L, Milligan D, Boerch K, Agostino R, et al. Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics 2008;121:e936-44. https://doi.org/10.1542/peds.2007-1620.
- Moser K, Macfarlane A, Chow YH, Hilder L, Dattani N. Introducing new data on gestation-specific infant mortality among babies born in 2005 in England and Wales. Health Stat Q 2007;35:13-27.
- Petrou S, Mehta Z, Hockley C, Cook-Mozaffari P, Henderson J, Goldacre M. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics 2003;112:1290-7. https://doi.org/10.1542/peds.112.6.1290.
- Anderson P, Doyle LW. Victorian Infant Collaborative Study Group . Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003;289:3264-72. https://doi.org/10.1001/jama.289.24.3264.
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124:717-28. https://doi.org/10.1542/peds.2008-2816.
- Chalmers I. The perinatal research agenda: whose priorities?. Birth 1991;18:137-41. https://doi.org/10.1111/j.1523-536X.1991.tb00083.x.
- Chalmers I. Well informed uncertainties about the effects of treatments: how should clinicians and patients respond?. BMJ 2004;328:475-6. https://doi.org/10.1136/bmj.328.7438.475.
- Partridge N, Scadding J. The James Lind Alliance: patients and clinicians should jointly identify their priorities for clinical trials. Lancet 2004;364:1923-4. https://doi.org/10.1016/S0140-6736(04)17494-1.
- The James Lind Alliance . The James Lind Alliance Guidebook. Version 6 2016. www.jla.nihr.ac.uk/jla-guidebook/ (accessed 14 December 2016).
- James Lind Alliance . Research Priorities in Asthma: Description of a Workshop to Set Priorities for Treatment Uncertainty Research in Asthma, March 2007 n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/asthma/downloads/Final-workshop-report-for-Asthma-PSP-March-2007.pdf (accessed 2 May 2019).
- Buckley BS, Grant AM, Tincello DG, Wagg AS, Firkins L. Prioritizing research: patients, carers, and clinicians working together to identify and prioritize important clinical uncertainties in urinary incontinence. Neurourol Urodyn 2009;29:708-14. https://doi.org/10.1002/nau.20816.
- Eleftheriadou V, Whitton ME, Gawkrodger DJ, Batchelor J, Corne J, Lamb B, et al. Future research into the treatment of vitiligo: where should our priorities lie? Results of the vitiligo priority setting partnership. Br J Dermatol 2011;164:530-6. https://doi.org/10.1111/j.1365-2133.2010.10160.x.
- Tyndale-Biscoe S, Malcolm E, Gnanapragasam V. Setting priorities for prostate cancer research. Trends Urol Men’s Health 2012;3:31-3. https://doi.org/10.1002/tre.244.
- Lophatananon A, Tyndale-Biscoe S, Malcolm E, Rippon HJ, Holmes K, Firkins LA, et al. The James Lind Alliance approach to priority setting for prostate cancer research: an integrative methodology based on patient and clinician participation. BJU Int 2011;108:1040-3. https://doi.org/10.1111/j.1464-410X.2011.10609.x.
- Lloyd K, White J. Democratizing clinical research. Nature 2011;474:277-8. https://doi.org/10.1038/474277a.
- Chalmers I. Confronting therapeutic ignorance. BMJ 2008;337. https://doi.org/10.1136/bmj.39555.392627.80.
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 2007;357:1893-902. https://doi.org/10.1056/NEJMoa073679.
- Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, Davis PG, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr 2017;171:564-72. https://doi.org/10.1001/jamapediatrics.2017.0238.
- Eclampsia Trial Collaborative Group . Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 1995;345:1455-63. https://doi.org/10.1016/S0140-6736(95)91034-4.
- Chalmers I, Grant A. Salutary lessons from the Collaborative Eclampsia Trial. Evid Based Med 1996;1:39-40.
- Neilson JP. Magnesium sulphate: the drug of choice in eclampsia. BMJ 1995;311:702-3. https://doi.org/10.1136/bmj.311.7007.702.
- Oliver S HK, Briner R. Effectiveness and Efficiency of Committee Work: A Rapid Systematic Review for NICE by Its Research Support Unit – Report of the Research Support Unit for the National Institute for Health and Care Excellence 2015.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2030.
- Jonsdottir T. The Impact of Gender Demography on Male and Female Role Interpretations and Contributions [Electronic Resource]: A Qualitative Study of Non-executive Directors of Icelandic Boards. Cranfield University; 2010.
- Bonetti D, Johnston M, Pitts NB, Deery C, Ricketts I, Bahrami M, et al. Can psychological models bridge the gap between clinical guidelines and clinicians’ behaviour? A randomised controlled trial of an intervention to influence dentists’ intention to implement evidence-based practice. Br Dent J 2003;195:403-7. https://doi.org/10.1038/sj.bdj.4810565.
- Sajadi HS MM, Ravaghi H, Hadi M, Hasanzadeh H, Tahmasby B. The indicators of board evaluation in healthcare organizations: a review of evidence. Life Sci J 2013;10:92-8.
- Brodbeck FC KR, Mojzisch A, Schulz-Hardt S. Group decision making under conditions of distributed knowledge: the information asymmetries mode. Acad Manage Rev 2007;32:459-79. https://doi.org/10.5465/amr.2007.24351441.
- Collins HM, Evans R. The third wave of science studies. Soc Stud Sci 2002;32:235-96. https://doi.org/10.1177/0306312702032002003.
- Duley L, Uhm S, Oliver S. Preterm Birth Priority Setting Partnership Steering Group . Top 15 UK research priorities for preterm birth. Lancet 2014;383:2041-2. https://doi.org/10.1016/S0140-6736(14)60989-2.
- Siegel R, Gardner SL, Merenstein GB, Merenstein GB, Gardner SL. Handbook of Neonatal Intensive Care. Maryland Heights, MO: Mosby; 2002.
- Redshaw M. Women as consumers of maternity care: measuring ‘satisfaction’ or ‘dissatisfaction’?. Birth 2008;35:73-6. https://doi.org/10.1111/j.1523-536X.2007.00215.x.
- Michels A, Kruske S, Thompson R. Women’s postnatal psychological functioning: the role of satisfaction with intrapartum care and the birth experience. J Reprod Infant Psychol 2013;31:172-82. https://doi.org/10.1080/02646838.2013.791921.
- Simkin P. Just another day in a woman’s life? Women’s long-term perceptions of their first birth experience: part I. Birth 1991;18:203-10. https://doi.org/10.1111/j.1523-536X.1991.tb00103.x.
- Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S. Very Preterm Birth Qualitative Collaborative Group . Parents’ experiences and satisfaction with care during the birth of their very preterm baby: a qualitative study. BJOG 2013;120:637-43. https://doi.org/10.1111/1471-0528.12104.
- Arnold L, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, et al. Very Preterm Birth Qualitative Collaborative Group. Parents’ first moments with their very preterm babies: a qualitative study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002487.
- Russell G, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, et al. Very Preterm Birth Qualitative Collaborative Group. Parents’ views on care of their very premature babies in neonatal intensive care units: a qualitative study. BMC Pediatr 2014;14. https://doi.org/10.1186/1471-2431-14-230.
- Sawyer A, Ayers S, Bertullies S, Thomas M, Weeks AD, Yoxall CW, et al. Providing immediate neonatal care and resuscitation at birth beside the mother: parents’ views, a qualitative study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008495.
- Yoxall CW, Ayers S, Sawyer A, Bertullies S, Thomas M, D Weeks A, et al. Providing immediate neonatal care and resuscitation at birth beside the mother: clinicians’ views, a qualitative study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008494.
- Chhoa CY, Sawyer A, Ayers S, Pushpa-Rajah A, Duley L. Clinicians’ views and experiences of offering two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials 2017;18. https://doi.org/10.1186/s13063-017-1940-5.
- Sawyer A, Chhoa C, Ayers S, Pushpa-Rajah A, Duley L. Women’s views and experiences of two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials 2017;18. https://doi.org/10.1186/s13063-017-2149-3.
- Sawyer A, Ayers S, Abbott J, Gyte G, Rabe H, Duley L. Measures of satisfaction with care during labour and birth: a comparative review. BMC Pregnancy Childbirth 2013;13. https://doi.org/10.1186/1471-2393-13-108.
- Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S. Very Preterm Birth Collaborative Group . Measuring parents’ experiences and satisfaction with care during very preterm birth: a questionnaire development study. BJOG 2014;121:1294-301. https://doi.org/10.1111/1471-0528.12925.
- Dawes GS. Foetal and Neonatal Physiology: A Comparative Study of the Changes at Birth [by] Geoffrey S. Dawes – Chapter 13. Chicago, IL: Year Book Medical Publishers; 1968.
- Boere I, Roest AA, Wallace E, Ten Harkel AD, Haak MC, Morley CJ, et al. Umbilical blood flow patterns directly after birth before delayed cord clamping. Arch Dis Child Fetal Neonatal Ed 2015;100:F121-5. https://doi.org/10.1136/archdischild-2014-307144.
- Vijayaselvi R, Abraham A, Kumar M, Kuruvilla A, Mathews J, Duley L. Measuring Umbilical Flow and Placental Transfusion for Preterm Births: Weighing Babies at 33–36 Weeks Gestation With Cord Intact. Budapest, Hungary: First Congress of Joint European Neonatal Societies; 2015.
- Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG 2011;118:70-5. https://doi.org/10.1111/j.1471-0528.2010.02781.x.
- Gunther M. The transfer of blood between baby and placenta in the minutes after birth. Lancet 1957;272:1277-80. https://doi.org/10.1016/S0140-6736(57)92302-4.
- American College of Obstetricians and Gynecologists . Committee Opinion No.543: timing of umbilical cord clamping after birth. Obstet Gynecol 2012;120:1522-6. https://doi.org/10.1097/01.AOG.0000423817.47165.48.
- Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol 2013;591:2113-26. https://doi.org/10.1113/jphysiol.2012.250084.
- Saigal S, O’Neill A, Surainder Y, Chua LB, Usher R. Placental transfusion and hyperbilirubinemia in the premature. Pediatrics 1972;49:406-19.
- Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics 2006;117:93-8. https://doi.org/10.1542/peds.2004-1773.
- Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks’ gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2008;93:F14-9. https://doi.org/10.1136/adc.2006.108902.
- Tarnow-Mordi WO, Duley L, Field D, Marlow N, Morris J, Newnham J, et al. Timing of cord clamping in very preterm infants: more evidence is needed. Am J Obstet Gynecol 2014;211:118-23. https://doi.org/10.1016/j.ajog.2014.03.055.
- Hooper SB, Polglase GR, te Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Arch Dis Child Fetal Neonatal Ed 2015;100:F355-60. https://doi.org/10.1136/archdischild-2013-305703.
- Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: Neonatal resuscitation – 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010;122:516-38. https://doi.org/10.1161/CIRCULATIONAHA.110.971127.
- Wyllie J, Niermeyer S. The role of resuscitation drugs and placental transfusion in the delivery room management of newborn infants. Semin Fetal Neonatal Med 2008;13:416-23. https://doi.org/10.1016/j.siny.2008.04.017.
- RCOG Scientific Advisory Committee . Clamping of the Umbilical Cord and Placental Transfusion 2009.
- POPPY Steering Group . Family-Centred Care in Neonatal Units: A Summary of Research Results and Recommendations from the POPPY Project 2009.
- Department of Health and Social Care . Toolkit for High Quality Neonatal Services n.d. www.londonneonatalnetwork.org.uk/wp-content/uploads/2015/09/Toolkit-2009.pdf (accessed 2 May 2019).
- Critchell CD, Marik PE. Should family members be present during cardiopulmonary resuscitation? A review of the literature. Am J Hosp Palliat Care 2007;24:311-17. https://doi.org/10.1177/1049909107304554.
- Resuscitation Council . Should Relatives Witness Resuscitation? 1996.
- Shaw K, Ritchie D, Adams G. Does witnessing resuscitation help parents come to terms with the death of their child? A review of the literature. Intensive Crit Care Nurs 2011;27:253-62. https://doi.org/10.1016/j.iccn.2011.05.001.
- Howell E, Graham C. Parents’ Experiences of Neonatal Care: A Report on the Findings from a National Survey 2011.
- Hutchon D. A view on why immediate cord clamping must cease in routine obstetric delivery. Obstet Gynaecol 2008;10:112-16. https://doi.org/10.1576/toag.10.2.112.27400.
- Oddie S, Rhodes P. Very Preterm Birth Qualitative Collaborative Group . Barriers to deferred cord clamping in preterm infants. Arch Dis Child Fetal Neonatal Ed 2014;99:F391-4. https://doi.org/10.1136/archdischild-2014-305968.
- Singh Y, Oddie S. Marked variation in delivery room management in very preterm infants. Resuscitation 2013;84:1558-61. https://doi.org/10.1016/j.resuscitation.2013.06.026.
- Weeks AD, Watt P, Yoxall CW, Gallagher A, Burleigh A, Bewley S, et al. Innovation in immediate neonatal care: development of the Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support (BASICS) trolley. BMJ Innov 2015;1:53-8. https://doi.org/10.1136/bmjinnov-2014-000017.
- Batey N, Yoxall CW, Fawke JA, Duley L, Dorling J. Fifteen-minute consultation: stabilisation of the high-risk newborn infant beside the mother. Arch Dis Child Educ Pract Ed 2017;102:235-8. https://doi.org/10.1136/archdischild-2016-312276.
- Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed 2018;103:F6-F14. https://doi.org/10.1136/archdischild-2016-312567.
- Te Pas AB. Timing is everything. Arch Dis Child Fetal Neonatal Ed 2018;103:F2-F3. https://doi.org/10.1136/archdischild-2017-313416.
- World Health Organization (WHO) . Guideline: Delayed Umbilical Cord Clamping for Improved Maternal and Infant Health and Nutrition Outcomes 2014.
- Mason SA, Megone C. European Neonatal Research: Consent, Ethics Committees and Law. Aldershot: Ashgate; 2001.
- Manson N, O’Neill O. Rethinking Informed Consent in Bioethics. Cambridge: Cambridge University Press; 2007.
- Maclean A. Autonomy, Informed Consent and Medical Law: A Relational Challenge. Cambridge: Cambridge University Press; 2009.
- de Melo-Martín I, Ho A. Beyond informed consent: the therapeutic misconception and trust. J Med Ethics 2008;34:202-5. https://doi.org/10.1136/jme.2006.019406.
- Megone C, Wilman E, Oliver S, Duley L, Gyte G, Wright J. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the analytical (theoretical/philosophical) research. Trials 2016;17. https://doi.org/10.1186/s13063-016-1562-3.
- Wilman E, Megone C, Oliver S, Duley L, Gyte G, Wright JM. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the empirical research. Trials 2015;16. https://doi.org/10.1186/s13063-015-0957-x.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Obtaining Valid Consent for Research While in Labour: Clinical Governance Advice No. 6a 2010.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Obtaining Valid Consent to Participate in Perinatal Research Where Consent Is Time Critical. Clinical Governance Advice No. 6a 2016.
- Sprigg N, Robson K, Bath P, Dineen R, Roberts I, Robinson T, et al. Intravenous tranexamic acid for hyperacute primary intracerebral hemorrhage: protocol for a randomized, placebo-controlled trial. Int J Stroke 2016;11:683-94. https://doi.org/10.1177/1747493016641960.
- Hofmeyr GJ, Bex PJ, Skapinker R, Delahunt T. Hasty clamping of the umbilical cord may initiate neonatal intraventricular hemorrhage. Med Hypotheses 1989;29:5-6. https://doi.org/10.1016/0306-9877(89)90157-6.
- Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev 2004;4. https://doi.org/10.1002/14651858.CD003248.pub2.
- Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology 2008;93:138-44. https://doi.org/10.1159/000108764.
- Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W. Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. J Perinatol 2010;30:11-6. https://doi.org/10.1038/jp.2009.170.
- Weeks AD, Peake M, Yoxall W. The Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support (BASICS) Trolley: Enabling Neonatal Resuscitation With an Intact Cord n.d.
- Gyte G. NCT evidence based briefing: third stage of labour – part 2: active management of third stage. New Digest 2006:22-8.
- Reynolds GJ. Beyond sweetness and warmth: transition of the preterm infant. Arch Dis Child Fetal Neonatal Ed 2008;93:F2-3. https://doi.org/10.1136/adc.2007.120790.
- Farrar D, Tuffnell D, Airey R, Duley L. Care during the third stage of labour: a postal survey of UK midwives and obstetricians. BMC Pregnancy Childbirth 2010;10. https://doi.org/10.1186/1471-2393-10-23.
- Bick D. Caesarean Section. Clinical Guideline: National Collaborating Centre for Women’s and Children’s Health – Commissioned by the National Institute for Clinical Excellence. London: RCOG Press; 2004.
- National Institute for Health and Care Excellence (NICE) . Intrapartum Care: Care Of Healthy Women and Their Babies During Childbirth 2007.
- Pushpa-Rajah A, Duley L. CORD Pilot Trial. Immediate Cord Clamping Versus Deferred Cord Clamping for Preterm Birth Before 32 Weeks Gestation: A Pilot Randomised Trial ISRCTN21456601 n.d. www.controlled-trials.com/ISRCTN21456601 (accessed 14 March 2019).
- Tarnow-Mordi W. The Australian Placental Transfusion Study (APTS): Should Very Pre Term Babies Receive a Placental Blood Transfusion at Birth via Deferring Cord Clamping Versus Standard Cord Clamping Procedures? n.d. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335752 (accessed 2 May 2019).
- Cowan K, Oliver S. The James Lind Alliance Guidebook. Southampton: James Lind Alliance; 2012.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Antenatal Corticosteroids to Reduce Neonatal Morbidity and Mortality. Green-Top Guideline No. 7 2012.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Preterm Labour, Tocolytic Drugs. Green-Top Guideline No. 1B 2011.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Placenta Praevia, Placenta Praevia Accreta and Vasa Praevia: Diagnosis and Management. Green-Top Guideline No. 27 2011.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Prevention of Early-Onset Neonatal Group B Streptococcal Disease. Green-Top Guideline No. 36 2012.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Birth After Previous Caesarean Birth. Green-Top Guideline No. 45 2007.
- Royal College of Obstetricians and Gynaecologists (RCOG) . Cervical Cerclage. Green-Top Guideline No. 60 2011.
- National Institute for Health and Care Excellence (NICE) . Inducing Labour. Clinical Guideline [CG70] 2008.
- National Institute for Health and Care Excellence (NICE) . Antenatal Care for Uncomplicated Pregnancies. Clinical Guideline [CG62] 2008.
- National Institute for Health and Care Excellence (NICE) . Hypertension in Pregnancy: Diagnosis and Management. Clinical Guideline [CG107] 2010.
- National Institute for Health and Care Excellence (NICE) . Drainage, Irrigation and Fibrinolytic Therapy (DRIFT) for Post-Haemorrhagic Hydrocephalus in Preterm Infants. Interventional Procedures Guidance [IPG412] 2011.
- National Institute for Health and Care Excellence (NICE) . Multiple Pregnancy: Antenatal Care for Twin and Triplet Pregnancies. Clinical Guideline [CG129] 2011.
- National Institute for Health and Care Excellence (NICE) . Antibiotics for Early-Onset Neonatal Infection. Clinical Guideline [CG149] 2012.
- Hammersley M, Atkinson P. Ethnography: Principles in Practice. London: Routledge; 1995.
- Becker HS. Problems of inference and proof in participant observation. Am Sociol Rev 1958;23:652-60. https://doi.org/10.2307/2089053.
- Keller R. Doing Discourse Research: An Introduction for Social Scientists. London: SAGE; 2012.
- Cacioppo JT, Petty RE. Perspectives in Cardiovascular Psychophysiology. New York, NY: Guilford Press; 1982.
- Petty RE, Cacioppo JT. Communication and Persuasion: Central and Peripheral Routes to Attitude Change. New York, NY: Springer-Verlag; 1986.
- Cialdini RB. Influence: The Psychology of Persuasion. New York, NY: Morrow; 1993.
- Kurfiss JG. Critical Thinking: Theory, Research, Practice and Possibilities. ASHE-ERIC Higher Education Report, no.2 1988. https://files.eric.ed.gov/fulltext/ED304041.pdf (accessed 2 May 2019).
- Tuckman B. Developmental sequence in small groups. Psychol Bull 1965;63:384-99. https://doi.org/10.1037/h0022100.
- Tuckman BW, Jensen MAC. Stages of small group development revisited. Group Organ Stud 1977;2:419-27. https://doi.org/10.1177/105960117700200404.
- Crowe S, Fenton M, Hall M, Cowan K, Chalmers I. Patients’, clinicians’ and the research communities’ priorities for treatment research: there is an important mismatch. Res Involv Engagem 2015;1. https://doi.org/10.1186/s40900-015-0003-x.
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75-84. https://doi.org/10.1016/S0140-6736(08)60074-4.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions n.d. http://handbook-5-1.cochrane.org/ (accessed 2 May 2019).
- CRD’s Guidance for Undertaking Reviews in Healthcare: Systematic Reviews. York: CRD, University of York; 2008.
- Cochrane Library . Reviews n.d. www.cochranelibrary.com/cdsr/reviews (accessed 21 June 2015).
- Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLOS ONE 2007;2. https://doi.org/10.1371/journal.pone.0001350.
- Popovich I, Windsor B, Jordan V, Showell M, Shea B, Farquhar CM. Methodological quality of systematic reviews in subfertility: a comparison of two different approaches. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0050403.
- Schmölzer GM, Morley CJ, Davis PG. Respiratory function monitoring to reduce mortality and morbidity in newborn infants receiving resuscitation. Cochrane Database Syst Rev 2010;9. https://doi.org/10.1002/14651858.CD008437.pub2.
- Grein AJ, Weiner GM. Laryngeal mask airway versus bag-mask ventilation or endotracheal intubation for neonatal resuscitation. Cochrane Database Syst Rev 2005;2. https://doi.org/10.1002/14651858.CD003314.pub2.
- O’Donnell C, Davis P, Morley C. Positive end-expiratory pressure for resuscitation of newborn infants at birth. Cochrane Database Syst Rev 2004;4. https://doi.org/10.1002/14651858.CD004341.pub2.
- Halliday HL. Endotracheal intubation at birth for preventing morbidity and mortality in vigorous, meconium-stained infants born at term. Cochrane Database Syst Rev 2001;1. https://doi.org/10.1002/14651858.CD000500.
- Soll R, Ozek E. Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2010;1. https://doi.org/10.1002/14651858.CD001079.pub2.
- Soll R, Özek E. Prophylactic animal derived surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 1997;4. https://doi.org/10.1002/14651858.CD000511.
- Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 2001;2. https://doi.org/10.1002/14651858.CD000144.
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD003063.pub3.
- Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD000510.pub2.
- Pfister RH, Soll R, Wiswell TE. Protein-containing synthetic surfactant versus protein-free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD006180.pub2.
- Pfister RH, Soll RF, Wiswell T. Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD006069.pub3.
- Tan A, Schulze A, O’Donnell CP, Davis PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev 2005;2. https://doi.org/10.1002/14651858.CD002273.pub3.
- Ziino AJ, Davies MW, Davis PG. Epinephrine for the resuscitation of apparently stillborn or extremely bradycardic newborn infants. Cochrane Database Syst Rev 2003;2. https://doi.org/10.1002/14651858.CD003849.
- Beveridge CJ, Wilkinson AR. Sodium bicarbonate infusion during resuscitation of infants at birth. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD004864.pub2.
- Moe-Byrne T, Brown JV, McGuire W. Naloxone for opiate-exposed newborn infants. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD003483.pub2.
- McCall EM, Alderdice F, Halliday HL, Jenkins JG, Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev 2010;3. https://doi.org/10.1002/14651858.CD004210.pub4.
- Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD003248.pub3.
- Thomas J, Harden A, Newton M, Gough E, Oliver S, Thomas J. An Introduction to Systematic Reviews. London: SAGE Publications Ltd; 2012.
- Faden RR, Beauchamp TL, King NM. A History and Theory of Informed Consent. New York, NY: Oxford University Press; 1986.
- Mason SA, Megone C. European Neonatal Research: Consent, Ethics Committees and Law. Aldershot: Ashgate Publishing Ltd; 2001.
- Strech D, Synofzik M, Marckmann G. Systematic reviews of empirical bioethics. J Med Ethics 2008;34:472-7. https://doi.org/10.1136/jme.2007.021709.
- Droste S, Dintsios CM, Gerber A. Information on ethical issues in health technology assessment: how and where to find them. Int J Technol Assess Health Care 2010;26:441-9. https://doi.org/10.1017/S0266462310000954.
- Fangerau H. Finding European bioethical literature: an evaluation of the leading abstracting and indexing services. J Med Ethics 2004;30:299-303. https://doi.org/10.1136/jme.2003.003269.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002360.
- Farrell EH, Whistance RN, Phillips K, Morgan B, Savage K, Lewis V, et al. Systematic review and meta-analysis of audio-visual information aids for informed consent for invasive healthcare procedures in clinical practice. Patient Educ Couns 2014;94:20-32. https://doi.org/10.1016/j.pec.2013.08.019.
- Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-28.
- Caldwell PH, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLOS Med 2010;7. https://doi.org/10.1371/journal.pmed.1000368.
- Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making 2011;31:151-73. https://doi.org/10.1177/0272989X10364247.
- Coverdale JH, McCullough LB, Chervenak FA. The ethics of randomized placebo-controlled trials of antidepressants with pregnant women: a systematic review. Obstet Gynecol 2008;112:1361-8. https://doi.org/10.1097/AOG.0b013e31818c2a27.
- Snowdon C, Brocklehurst P, Tasker R, Ward Platt M, Harvey S, Elbourne D. Death, bereavement and randomised controlled trials (BRACELET): a methodological study of policy and practice in neonatal and paediatric intensive care trials. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18420.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- National Institute for Health and Care Excellence (NICE) . Preterm Labour and Birth: NICE Guideline 2015.
- van Teijlingen ER, Hundley V, Rennie AM, Graham W, Fitzmaurice A. Maternity satisfaction studies and their limitations: ‘What is, must still be best’. Birth 2003;30:75-82. https://doi.org/10.1046/j.1523-536X.2003.00224.x.
- Sandin-Bojö AK, Larsson BW, Hall-Lord ML. Women’s perception of intrapartal care in relation to WHO recommendations. J Clin Nurs 2008;17:2993-300. https://doi.org/10.1111/j.1365-2702.2007.02123.x.
- Sloan K, Rowe J, Jones L. Stress and coping in fathers following the birth of a preterm infant. J Neonatal Nurs 2008;14:108-15. https://doi.org/10.1016/j.jnn.2007.12.009.
- Polglase GR, Blank DA, Barton SK, Miller SL, Stojanovska V, Kluckow M, et al. Physiologically based cord clamping stabilises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch Dis Child Fetal Neonatal Ed 2017:F1-F9.
- Hutchon DJ, Thakur I. Resuscitate with the placental circulation intact. Arch Dis Child 2008;93.
- Thomas M, Yoxall C, Weeks A, Duley L. Providing newborn resuscitation at the mother’s bedside: assessing the safety, usability and acceptability of a mobile trolley. BMC Pediatr 2014;14. https://doi.org/10.1186/1471-2431-14-135.
- Schoonakker BDJ, Oddie S, Batra D, Grace N, Duley L. Bedside Resuscitation of Preterm Infants With Cord Intact Is Achievable Using Standard Resuscitaire 2013.
- Abraham RJ, Sau A, Maxwell D. A review of the EXIT (Ex utero Intrapartum Treatment) procedure. J Obstet Gynaecol 2010;30:1-5. https://doi.org/10.3109/01443610903281656.
- Richmond S, Wyllie J. European Resuscitation Council Guidelines for Resuscitation 2010 Section 7: resuscitation of babies at birth. Resuscitation 2010;81:1389-99. https://doi.org/10.1016/j.resuscitation.2010.08.018.
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529-34. https://doi.org/10.1016/S0022-3476(78)80282-0.
- Squires J, Bricker D. Ages & Stages Questionnaires®, Third Edition (ASQ-3™): A Parent-Completed Child Monitoring System. Baltimore, MD: Brookes Publishing; 2009.
- Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III). San Antonio, TX: Pearson Assessment; 2005.
- Pushpa-Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, et al. Cord Pilot Trial Collaborative Group . Trials 2014;15. https://doi.org/10.1186/1745-6215-15-258.
- Bradshaw L, Sawyer A, Armstrong-Buisseret L, Mitchell E, Ayers S, Duley L. Cord pilot trial, comparing alternative policies for timing of cord clamping before 32 weeks gestation: follow-up for women up to one year. BMC Pregnancy Childbirth 2019;19. https://doi.org/10.1186/s12884-019-2223-9.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Bradshaw L, Sawyer A, Mitchell EJ, Armstrong-Buisseret L, Ayers S, Duley L. Womens’ experiences of participating in a trial, comparing alternative policies for timing of cord clamping at very preterm birth: a questionnaire study. Trials 2019;20.
- Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7 – resuscitation and support of transition of babies at birth. Resuscitation 2015;95:249-63. https://doi.org/10.1016/j.resuscitation.2015.07.029.
- Dorling J, Duley L, Bradshaw L, Armstrong-Buisseret L, Fawke J, Schoonakker B, et al. Impact of Independent Adjudication of Neonatal Cranial Ultrasound Scans in a Randomised Trial 2016.
- Duley L, Pushpa-Rajah A, Bradshaw L, Dorling J, Mitchell E. When an external pilot is successful, should it be possible to transform it into an internal pilot by continuing recruitment into the full trial is ready? A case study of the Cord pilot trial. Trials 2015;16. https://doi.org/10.1186/1745-6215-16-s2-p15.
- Hooper SB, Crossley KJ, Zahra VA, van Vonderen J, Moxham A, Gill AW, et al. Effect of body position and ventilation on umbilical artery and venous blood flows during delayed umbilical cord clamping in preterm lambs. Arch Dis Child Fetal Neonatal Ed 2017;102:F312-F319. https://doi.org/10.1136/archdischild-2016-311159.
- Blank DA, Polglase GR, Kluckow M, Gill AW, Crossley KJ, Moxham A, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed 2017:F1-8.
- Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med 2017;377:2445-55. https://doi.org/10.1056/NEJMoa1711281.
- Woolfall K, Frith L, Dawson A, Gamble C, Lyttle MD, Young B. CONNECT advisory group . Fifteen-minute consultation: an evidence-based approach to research without prior consent (deferred consent) in neonatal and paediatric critical care trials. Arch Dis Child Educ Pract Ed 2016;101:49-53. https://doi.org/10.1136/archdischild-2015-309245.
- O’Donnell CPF. The timing of cord clamping for preterm infants. N Engl J Med 2017;377:2488-9. https://doi.org/10.1056/NEJMe1714522.
- Bradshaw L, Sawyer A, Mitchell E, Armstrong-Buisseret L, Ayers S, Duley L. Women’s experiences of participating in a randomised trial comparing alternative policies for timing of cord clamping at very preterm birth: a questionnaire study. Trials 2019;20. https://doi.org/10.1186/s13063-019-3325-4.
- Armstrong-Buisseret, Powers K, Dorling J, Bradshaw L, Johnson S, Mitchell E, et al. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years. [published online ahead of print August 1 2019]. Arch Dis Child Fetal Neonatal Ed 2019. https://doi.org/10.1136/archdischild-2019-316912.
Appendix 1 List of programme journal publications, with status and whether or not included in appendices
Work package 1
Duley L, Uhm S, Oliver S, Preterm Birth Priority Setting Partnership Steering Group. Top 15 UK research priorities for preterm birth. Lancet 2014;383:2041–2. See Appendix 2.
Oliver S, Uhm S, Duley L, Crowe S, David A, James CP, et al. Top research priorities for preterm birth: results of a prioritisation partnership between people affected by preterm birth and healthcare professionals. Submitted, in peer review. See Appendix 3.
Uhm S, Liabo K, Stewart R, Rees R, Oliver S. Patient and public perspectives shaping scientific and medical research: panels for data, discussions, and decisions. Patient Intell 2012;4:1–10.
Work package 2.1
Corbett MS, Moe-Byrne T, Oddie S, McGuire W. Randomisation methods in emergency setting trials: a descriptive review. Res Synth Methods 2016;1:46–54.
Moe-Byrne T, Brown JV, McGuire W. Naloxone for opiate-exposed newborn infants. Cochrane Database Syst Rev 2013;2:CD003483.
Work package 2.2
Wilman E, Megone C, Oliver S, Duley L, Gyte G, Wright JM. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the empirical research. Trials 2015;16:502. See Appendix 6.
Megone C, Wilman E, Oliver S, Duley L, Gyte G, Wright J. The ethical issues regarding consent to clinical trials with pre-term or sick neonates: a systematic review (framework synthesis) of the analytical (theoretical/philosophical) research. Trials 2016;17:443. See Appendix 7.
Work package 3.1
Singh Y, Oddie S. Marked variation in delivery room management in very preterm infants. Resuscitation 2013;84:1558–61. See Appendix 8.
Oddie S, Rhodes P, Very Preterm Birth Qualitative Collaborative Group. Barriers to deferred cord clamping in preterm infants. Arch Dis Child Fetal Neonatal Ed 2014;99:F391–4. See Appendix 9.
Work package 3.2
Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Qualitative Collaborative Group. Parents’ experiences and satisfaction with care during the birth of their very preterm baby: a qualitative study. BJOG 2013;120:637–43. See Appendix 10.
Arnold L, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Qualitative Collaborative Group. Parents’ first moments with their very preterm babies: a qualitative study. BMJ Open 2013;3:e002487. See Appendix 11.
Russell G, Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Qualitative Collaborative Group. Parents’ views on care of their very premature babies in neonatal intensive care units: a qualitative study. BMC Pediatr 2014;14:230.
Sawyer A, Ayers S, Abbott J, Gyte G, Rabe H, Duley L. Measures of satisfaction with care during labour and birth: a comparative review. BMC Pregnancy Childbirth 2013;13:108.
Sawyer A, Rabe H, Abbott J, Gyte G, Duley L, Ayers S, Very Preterm Birth Collaborative Group. Measuring parents’ experiences and satisfaction with care during very preterm birth: a questionnaire development study. BJOG 2014;121:1294–301. See Appendix 12.
Work package 3.4
Weeks AD, Watt P, Yoxall CW, Gallagher A, Burleigh A, Bewley S, et al. Innovation in immediate neonatal care: development of the Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support (BASICS) trolley. BMJ Innov 2015;1:53–8. See Appendix 14.
Work package 3.5
Sawyer A, Ayers S, Bertullies S, Thomas M, Weeks AD, Yoxall CW, Duley L. Providing immediate neonatal care and resuscitation at birth beside the mother: parents’ views, a qualitative study. BMJ Open 2015;5:e008495. See Appendix 15.
Yoxall CW, Ayers S, Sawyer A, Bertullies S, Thomas M, D Weeks A, Duley L. Providing immediate neonatal care and resuscitation at birth beside the mother: clinicians’ views, a qualitative study. BMJ Open 2015;5:e008494. See Appendix 16.
Thomas MR, Yoxall CW, Weeks AD, Duley L. Providing newborn resuscitation at the mother’s bedside: assessing the safety, usability and acceptability of a mobile trolley. BMC Pediatr 2014;14:135.
Work package 4
Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed 2018;103:F6–F14. See Appendix 18.
Chhoa CY, Sawyer A, Ayers S, Pushpa-Rajah A, Duley L. Clinicians’ views and experiences of offering two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials 2017;18:196. See Appendix 19.
Sawyer A, Chhoa C, Ayers S, Pushpa-Rajah A, Duley L. Women’s views and experiences of two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials 2017;18:422. See Appendix 20.
Bradshaw L, Sawyer A, Armstrong-Buisseret L, Mitchell E, Ayers S, Duley L. Cord pilot trial, comparing alternative policies for timing of cord clamping before 32 weeks gestation: follow-up for women up to one year. BMC Pregnancy Childbirth 2019;19:78. See Appendix 21.
Bradshaw L, Sawyer A, Mitchell E, Armstrong-Buisseret L, Ayers S, Duley L. Women’s experiences of participating in a randomised trial comparing alternative policies for timing of cord clamping at very preterm birth: a questionnaire study. Trials 2019;20:225. See Appendix 22.
Armstrong-Buisseret L, Powers K, Dorling J, Bradshaw L, Johnson S, Mitchell E, Duley L. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years [published online ahead of print August 1 2019]. Arch Dis Child Fetal Neonatal Ed 2019. See Appendix 23.
Bradshaw LE, Pushpa-Rajah A, Dorling J, Mitchell E, Duley L. On behalf of the ‘Cord Pilot Trial Collaborative Group’. Cord pilot trial: update to randomised trial protocol. Trials 2015;16:407.
Pushpa-Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, Duley L, Cord Pilot Trial Collaborative Group. Cord pilot trial – immediate versus deferred cord clamping for very preterm birth (before 32 weeks gestation): study protocol for a randomized controlled trial. Trials 2014;15:258.
Batey N, Yoxall CW, Fawke JA, Duley L, Dorling J. Fifteen-minute consultation: stabilisation of the high-risk newborn infant beside the mother. Arch Dis Child Educ Pract Ed 2017;102:235–8.
Work package 5
Duley L, Askie L, Montgomery A, Hunter K, Barba A, Seidler AL, et al. Systematic review and network meta-analysis with individual participant data on Cord Management at Preterm Birth (iCoMP): study protocol. Draft prepared for submission. See Appendix 25.
Appendix 2 Top 15 UK research priorities for preterm birth
Duley et al. 31 https://doi.org/10.1016/S0140-6736(14)60989-2
Appendix 3 Top research priorities for preterm birth: results of a prioritisation partnership between people affected by preterm birth and health-care professionals
Appendix 4 Consensus development for tackling technical and emotive challenges: a case study of the James Lind Alliance Preterm Birth Priority Setting Partnership
Appendix 5 Delivery room transition support for newborn infants: an overview of Cochrane reviews
Appendix 6 The ethical issues regarding consent to clinical trials with preterm or sick neonates: a systematic review (framework synthesis) of the empirical research
See Wilman et al. 79 https://doi.org/10.1186/s13063-015-0957-x
Appendix 7 The ethical issues regarding consent to clinical trials with preterm or sick neonates: a systematic review (framework synthesis) of the analytical (theoretical/philosophical) research
See Megone et al. 78 https://doi.org/10.1186/s13063-016-1562-3
Appendix 8 Marked variation in delivery room management in very preterm infants
See Singh and Oddie. 68 https://doi.org/10.1016/j.resuscitation.2013.06.026
Appendix 9 Barriers to deferred cord clamping in preterm infants
See Oddie et al. 67 https://doi.org/10.1136/archdischild-2014-305968
Appendix 10 Parents’ experiences and satisfaction with care during the birth of their very preterm baby: a qualitative study
See Sawyer et al. 36 https://doi.org/10.1111/1471-0528.12104
Appendix 11 Parents’ first moments with their very preterm babies: a qualitative study
See Arnold et al. 37 https://doi.org/10.1136/bmjopen-2012-002487
Appendix 12 Measuring parents’ experiences and satisfaction with care during very preterm birth: a questionnaire development study
See Sawyer et al. 44 https://doi.org/10.1111/1471-0528.12925
Appendix 13 Measuring placental transfusion at preterm birth
Appendix 14 Innovation in immediate neonatal care: development of the Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support trolley
See Weeks et al. 69 https://doi.org/10.1136/bmjinnov-2014-000017
Appendix 15 Providing immediate neonatal care and resuscitation at birth beside the mother: parents’ views, a qualitative study
See Sawyer et al. 39 https://doi.org/10.1136/bmjopen-2015-008495
Appendix 16 Providing immediate neonatal care and resuscitation at birth beside the mother: clinicians’ views, a qualitative study
See Yoxall et al. 40 https://doi.org/10.1136/bmjopen-2015-008494
Appendix 17 Cord pilot trial: assessment of feasibility of a large trial
Appendix 18 Randomised trial of cord clamping and initial stabilisation at very preterm birth
See Duley et al. 71 https://doi.org/10.1136/archdischild-2016-312567
Appendix 19 Clinicians’ views and experiences of offering two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study
See Chhoa et al. 41 https://doi.org/10.1186/s13063-017-1940-5
Appendix 20 Women’s views and experiences of two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study
See Sawyer et al. 42 https://doi.org/10.1186/s13063-017-2149-3
Appendix 21 Cord pilot trial, comparing alternative policies for timing of cord clamping before 32 weeks’ gestation: follow-up for women up to 1 year
See Bradshaw et al. 169 https://doi.org/10.1186/s12884-019-2223-9
Appendix 22 Womens’ experiences of participating in a randomised trial comparing alternative policies for timing of cord clamping at very preterm birth: a questionnaire study
See Bradshaw et al. 180 https://doi.org/10.1186/s13063-019-3325-4
Appendix 23 Randomised trial of cord clamping at very preterm birth: outcomes at 2 years
See Armstrong-Buisseret, et al. 181 https://doi.org/10.1136/archdischild-2019-316912
Appendix 24 Preterm cord clamping and placental transfusion prospective meta-analysis: protocol v1.0, dated 28 January 2013
Appendix 25 Systematic review and network meta-analysis with individual participant data on cord management at preterm birth: study protocol
Glossary
- Bliss
- The special care baby charity.
List of abbreviations
- ASQ
- Ages and Stages Questionnaire
- BASICS
- Bedside Assessment, Stabilisation and Initial Circulatory Support
- Bayley-III
- Bayley Scales of Infant Development III
- CCPTP
- Cord Clamping and Placental Transfusion at Preterm birth
- CE
- Conformité Européenne
- CI
- confidence interval
- COREQ
- Consolidated Criteria for Reporting Qualitative Research
- CPAP
- continuous positive airways pressure
- IPD
- individual participant data
- IVH
- intraventricular haemorrhage
- JLA
- James Lind Alliance
- NCT
- National Childbirth Trust
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- P-BESS
- Preterm Birth experience and Satisfaction Scale
- PhD
- doctor of philosophy
- PSP
- Priority Setting Partnership
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- TSC
- Trial Steering Committee
