Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0608-10035. The contractual start date was in January 2010. The final report began editorial review in January 2017 and was accepted for publication in March 2018. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Stephen Morris is a member of the National Institute for Health Research (NIHR) Health Services and Delivery Research Board. Irwin Nazareth is a member of the NIHR Health Technology Assessment Commissioning Board. Sonia Saxena was funded by a NIHR Career Development fellowship (NIHR CDF-2011-04-048). Min Hae Park received grants from NIHR during the conduct of the study and received personal fees from Research Consultancy for Marie Stopes International (London, UK). Anthony S Kessel is the director of International Public Health at Public Health England.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Viner et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
This report details the methods and findings of the PROMISE programme grant, funded by the National Institute for Health Research (NIHR) and undertaken by the investigator team between March 2010 and September 2015.
Rationale
Obesity in children and young people (CYP) has been an enduring public health and clinical priority throughout the early 21st century. Growing concerns about a ‘childhood obesity epidemic’ in the first decade of this century prompted the undertaking of this research programme. 1–3 It remains a high priority in 2019/20; the Chief Medical Officer focused much of her 2012–13 annual report Our Children Deserve Better: Prevention Pays4 on prevention of childhood obesity. The UK government published Childhood Obesity: A Plan for Action in 2016. 5 Chapter 2 was published in 2018, with the aim of halving childhood obesity by 2030.
Since the start of this programme in 2010, there has been some evidence that child and adolescent obesity rates may be stabilising;6 however, the proportion of obese young people in the latest Health Survey for England7 remains worryingly high: 17% and 22% for 13- to 15-year-old boys and girls, respectively. The National Child Measurement Programme (NCMP) shows similar obesity rates, with 21% of girls and 17% of boys in Year 6 being classified as obese in 2013. 8
The rationale for concern about childhood obesity is supported by some key publications from this programme team. Childhood obesity is associated with increased adult morbidity and mortality. 9 Overweight and obesity also track strongly into adult life: ≈ 85% of females and ≈ 92% of males who were overweight or obese in childhood were also overweight or obese at two or more time points between 26 and 42 years of age. 10
It is neither appropriate nor necessary to give greater background information on the epidemiology, complications or manifestations, or the prevention or treatment, of child and adolescent obesity here. Further background information relevant to each substudy is given in the introduction to each chapter. The rationale for the programme and how this report will be structured are outlined here.
Development of the PROMISE programme
The National Institute for Health and Care Excellence (NICE) published its first guidance on management of childhood obesity in 2006, as part of wider guidance on the prevention and management of obesity issued in December 2006. 11 This guidance has been updated in several ways, with new guidance on identification and management of obesity in children and adults issued in November 2014,12 and new prevention guidance issued in March 2015. 13
The childhood obesity pathway, first outlined by NICE in 2006,11 has become the basis of childhood obesity prevention and management plans in most parts of England and Wales since that time, as developed by (initially) primary care trusts (PCTs) and their successor organisations, Clinical Commissioning Groups.
The 2006 NICE pathway11 commenced with assessment and guidance by front-line staff in primary care. The 2009/10 NHS Operating Framework14 directed that, from summer 2009, the NCMP feedback and parental responses to feedback (in terms of behaviour change and seeking help) would form the first step in identification, assessment and management for many obese children. However, the impact of NCMP feedback on parental behaviour and help-seeking, and the resultant burden on primary and secondary care, is unknown.
Optimal therapy was outlined by NICE as multicomponent lifestyle modification programmes, and anti-obesity drug (AOD) therapy and bariatric surgery were identified as appropriate for older adolescents with severe obesity and comorbidities.
Contemporary epidemiological data at the time suggested that 10–15% of children and adolescents were obese by the definition used by NICE [body mass index (BMI) ≥ 98th centile] and were therefore eligible for lifestyle modification treatment. It was estimated that of these, approximately one-third (i.e. 3–5%) would fail lifestyle modification and meet the NICE criteria for AOD therapy, with a further 0.5–1.0% of adolescents meeting the NICE criteria for bariatric surgery. It was noted that paediatric services in the UK were, at the time, poorly developed and unprepared to provide services to this number of children.
The development of the research questions in Paediatric Research in Obesity Multi-modal Intervention and Service Evaluation (PROMISE) was explicitly stimulated by gaps in the evidence for the NICE childhood obesity pathway. Areas were identified for which additional evidence was needed or for which the application of existing knowledge could support public health or NHS staff to provide the best care to overweight children. A programme of five linked substudies was created, which integrated public health and clinical perspectives, focusing on apparent gaps in evidence. These were as follows:
-
Study A investigated the impact of the NCMP on families and on health service use in obese children. The NCMP was very new at that time (the first wave of data collection occurred in 2006/7) and few data were available on its effect on families and behavioural change.
-
Study B developed a brief evidence-based electronic tool to improve management of obesity by front-line staff [e.g. general practitioners (GPs)]. At the time of programme development, GPs were not very involved in childhood obesity management and there were major concerns that identification of overweight and obese children through the new NCMP would impose a large new burden for primary care.
-
Study C tested a novel community-delivered lifestyle modification programme for obese adolescents. At the time of programme development, there were very few weight management programmes specifically aimed at adolescents, who have different developmental needs to children and to adults.
-
Study D investigated AOD use, safety and potential clinical effectiveness in children and adolescents in the NHS. The 2006 NICE guidance11 endorsed the use of AODs in those aged ≥ 12 years with significant obesity comorbidities. Data on safety and effectiveness in adolescents are very limited. There are no published data on acceptability and clincal effectiveness in NHS practice.
-
Study E investigated the safety and outcomes of bariatric surgery in adolescents in the NHS. The 2006 NICE guidelines11 endorsed bariatric surgery in adolescents within specialist centres, yet there were no available outcome data on adolescent bariatric surgery within the NHS.
A final implementation work package within the programme ensured the dissemination of findings to key audiences to maximise potential patient benefit within 3–5 years of the end of the programme.
Application for funding for PROMISE was made to NIHR in late 2008, with outline agreement for funding obtained in July 2009. The programme began in January 2010. The initial end of the programme of 30 March 2015 was extended to 15 December 2015.
Structure of this report
This report first describes each of the five studies and substudies in detail (see Chapters 2–6), including a brief background, methods, results and discussion section for each substudy. Chapter 7 describes the impact and dissemination, as well as the patient and public involvement (PPI) work undertaken for the whole programme. The implications of the programme for public health and policy, clinical practice and research, as well as the limitations and overall conclusions, are discussed in Chapter 8. Chapter 9 outlines future research recommendations.
Chapter 2 Study A: scoping the impact of the National Child Measurement Programme on the Childhood Obesity Pathway
Parts of this chapter were reproduced from (1) Falconer et al. 15 © Falconer et al. ; licensee BioMed Central Ltd, 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited; and (2) Syrad et al. 16 © 2014 The Authors. Journal of Human Nutrition and Dietetics published by John Wiley & Sons Ltd on behalf of British Dietetic Association. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License (https://creativecommons.org/licenses/by-nc-nd/3.0/), which permits use and distribution in any medium, provided the original work is properly cited, the use is noncommercial and no modifications or adaptations are made.
Abstract
The provision of feedback letters to parents about their child’s weight status following measurement for the NCMP has been recommended by the Department of Health and Social Care (DHSC) since 2008, with the aim of engaging the families of overweight children and supporting them to make healthy behaviour changes. This study aimed to assess the impact of NCMP feedback on parental recognition of childhood overweight, perceptions of the health risks associated with their child’s overweight status, and lifestyle behaviours and health service use. A longitudinal study was conducted of 1844 parents of children aged 4–5 and 10–11 years who received weight feedback as part of the 2010–11 NCMP in five PCTs in England. Self-completed questionnaires were administered before and after feedback. Positive effects of feedback on parental recognition of childhood overweight and its associated health risks were observed, but there were minimal effects on lifestyle behaviours (e.g. diet, physical activity, sedentary behaviour). More than one-third of parents of overweight or obese children sought further information regarding their child’s weight. There were no observable effects of feedback on child self-esteem or teasing. In qualitative interviews with a subsample of parents with overweight or obese children, perceptions of child health in terms of emotional well-being and physical health, irrespective of weight, were identified as a major barrier to behaviour change. There was evidence to suggest that ‘proactive’ feedback in the form of telephone calls or face-to-face meetings could have greater impact on changing parental perceptions than the letter alone, but there was no effect on behaviours, and parents preferred written feedback.
Key findings
At baseline (i.e. before NCMP feedback):
-
Three-quarters of parents of overweight and obese children do not recognise their child to be overweight. Before they received NCMP feedback, only 14% of parents with overweight children and 35% of parents with obese children perceived their child to be overweight. Parents were likely to classify their children as overweight only if their BMI was ≥ 99.7th centile (much higher than the standard 95th centile used by the NCMP).
-
Many parents do not consider their child’s overweight status to be a health risk: 41% of parents who acknowledged their child to be overweight did not perceive this to be a health risk. Interviews with parents suggested that parental definitions of health frequently did not include weight; some parents did not consider the NCMP result to be credible because it did not take into account their child’s background or lifestyle.
-
Cultural factors, as well as deprivation, may explain high levels of obesity among black and South Asian children in England. After accounting for deprivation and other sociodemographic characteristics, black and South Asian children were three times more likely to have an obesogenic lifestyle than white children. Qualitative work indicated that being overweight was not viewed negatively by some non-white parents.
After NCMP feedback:
-
The majority (87%) of parents found NCMP feedback to be helpful. More than one-fifth of parents of overweight children reported feeling upset, but only 1.8% of parents stated that they would withdraw their child from the NCMP in the future.
-
One-quarter of parents of overweight children and half of parents of obese children sought further information regarding their child’s weight: the most frequently reported sources of information were friends and family (reported by 14.4% of parents), the internet (9.9%), the GP (8.9%) and the school nurse (8.4%).
-
NCMP feedback has positive effects on parental knowledge, perceptions and intentions. After receiving NCMP feedback, parents’ general knowledge about the health risks associated with child overweight improved, particularly among non-white parents. The proportion of parents who recognised their child to be overweight nearly doubled after feedback, but remained low, at 38%. Nearly three-quarters of parents reported an intention to change lifestyle behaviours following NCMP feedback.
-
However, NCMP feedback has little impact on actual lifestyle behaviours. After feedback, there were no changes in reported dietary behaviours or screen time. There was a slight increase in the proportion of obese children meeting recommended levels for physical activity.
-
‘Proactive’ feedback may be more effective than letters alone. In areas where ‘proactive’ NCMP feedback (telephone call or face-to-face meeting) was given to parents of obese children in addition to the letter, there was a greater improvement in parental recognition of child overweight and health risks. However, telephonic feedback required additional resources (£9.50 vs. £1.24 per child) and most parents reported a preference for feedback by letter.
Background
The ‘Healthy Lives, Healthy People’17 cross-government strategy on obesity places strong emphasis on reducing the prevalence of childhood overweight and obesity in the UK, and highlights the instrumental role of primary and secondary prevention efforts in achieving this goal. In line with the strategy, the NCMP was established in 2006 by the DHSC to measure the height and weight of children in Reception (aged 4–5 years) and Year 6 (aged 10–11 years) at state-maintained schools in England. The aims of the NCMP are to gather data for population-level surveillance of childhood obesity levels and trends, and to inform local planning of services for children and families. Until March 2013, responsibility for delivery of the NCMP lay with PCTs, with guidance on implementation provided by the DHSC. From 1 April 2013, local authorities (LAs) became responsible for the collection of NCMP data, with guidance from Public Health England (PHE).
Since 2008, the DHSC and PHE have recommended the provision of NCMP results (weight feedback) to parents, with the aim of engaging families of overweight and obese children and supporting them to make healthy behaviour changes. Template letters for weight feedback have been developed by the DHSC and PHE, which contain the child’s weight, height and BMI centile category (i.e. underweight, healthy weight, overweight, very overweight). Some LAs (and previously PCTs) produce their own letters, and others additionally offer ‘proactive feedback’ in the form of telephone calls or face-to-face consultations for families of children with a BMI outside the healthy weight range.
More than half of parents of overweight and obese children underestimate their child’s weight status,18,19 and many parents who consider their child to be overweight are unconcerned about the health implications of excess weight. 20,21 Given that risk perception is associated with readiness to change health behaviours, widespread parental underestimation of childhood weight and health risk could compromise the success of childhood obesity interventions. 22,23 The provision of accurate information to parents in the form of weight feedback through the NCMP has been proposed as a way to increase parental recognition of childhood overweight and obesity, and to encourage families to make positive lifestyle changes. 24 However, to date there has been limited evidence that routine weight feedback of this nature leads to changes in parental perceptions or lifestyle behaviours. An early evaluation of NCMP feedback showed that one-third of parents planned to make lifestyle changes as a result of the written feedback,25 but this cross-sectional study did not assess effects on actual behaviour change and did not evaluate proactive forms of feedback. Small-scale studies have indicated that written feedback can improve parental awareness of their child’s overweight status,26 but benefits in terms of direct health outcomes have not been robustly demonstrated. A systematic review27 of the evidence concluded that there was a lack of data on the effectiveness of BMI screening for childhood overweight and obesity. In addition, the potential for harmful effects has not been fully explored; for example, there is a risk that weight feedback could lead to excessive weight concern or distress among families and precipitate unhealthy behaviours such as dietary restriction. Despite the lack of longitudinal evidence on the effects of population-based weight screening and feedback on parental perceptions and behaviours, over 1 million children are measured as part of the NCMP each year and their parents are provided with weight feedback. This study aimed to address this evidence gap and provide data that could inform future development of NCMP feedback and its delivery.
Objectives
The aim of this study was to scope the impact of the NCMP on families and on health service use in overweight and obese children, with four specific objectives:
-
to estimate the effects of NCMP feedback on parental recognition of childhood overweight, parental perceptions of the health risks associated with their child’s weight status, and weight-related lifestyle behaviours and health service use
-
to compare the effects of different forms of weight feedback (letter vs. proactive feedback)
-
to identify barriers to behaviour change and health service use, particularly among deprived and ethnic minority groups
-
to produce preliminary data on NHS costs associated with NCMP feedback.
Methods
A longitudinal study was conducted of parents of children taking part in the NCMP in five PCTs in England [Redbridge, Islington, West Essex, Bath and North East Somerset (BANES) and Sandwell], using a series of self-administered questionnaire surveys to follow up parents over 1 year (objectives 1 and 2). Qualitative interviews were also conducted with a subset of parents to explore barriers to health behaviour change (objective 3). Preliminary data on the costs of different types of NCMP feedback were generated as part of an economic evaluation (objective 4).
National Childhood Measurement Programme measurement and feedback
During the study, PCTs carried out their usual NCMP measurement and feedback procedures: PCTs sent letters to parents outlining the aims of the NCMP and measurement process, and provided an opportunity for parents to withdraw their child from the measurement. Eligible children had their height and weight measured at school by trained staff. Within 6 weeks of the measurement, written feedback was mailed to parents with information about their child’s BMI category, defined using centiles of the UK 1990 growth curves28 and cut-off points at the 2nd, 91st and 98th BMI centiles to define underweight, healthy weight, overweight and obese (described to parents as ‘very overweight’), respectively. Parents of underweight, overweight and obese children were provided with information about the health risks associated with their child’s weight status (see Appendix 1). Feedback also included information about healthy lifestyles from the DHSC’s Change4Life campaign,29 and information about local health and leisure services. A telephone number for the NCMP team was provided for parents who required further information and support. Redbridge, BANES and Sandwell PCTs supplemented the written feedback with proactive feedback in the form of telephone calls to parents of children identified as obese, during which parents were informed by a member of the measurement team (usually a school nurse) about their child’s result, and were able to get advice and support as required. Parents in Redbridge PCT were also offered a face-to-face appointment with a school nurse in a local clinic, during which families were provided with advice to support lifestyle change.
Survey questionnaires
Parents of all children participating in the NCMP in the five selected PCTs in 2010–11 (n = 18,000) were invited to participate in the study. Questionnaires were administered at baseline (approximately 6 weeks before parents received weight feedback), and at 1, 6 and 12 months after weight feedback (Figure 1). Baseline questionnaires were distributed through schools on the day of the NCMP measurements, between February and July 2011, and responses were returned to the study team in prepaid envelopes. The questionnaire assessed parental knowledge and perceptions of their child’s weight and health, and lifestyle behaviours (see Report Supplementary Material 1). The questionnaire was made available online also, and reminder postcards and e-mails were sent to parents via schools. To encourage participation, all respondents were entered into a free prize draw to win a Nintendo Wii (Nintendo Co. Ltd, Kyoto, Japan).
FIGURE 1.
Timeline for data collection in NCMP evaluation study, showing sample sizes and response rates. Figures in brackets show response rates as percentage of target population. Modified from Falconer et al. 15 © Falconer et al. ; licensee BioMed Central Ltd, 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
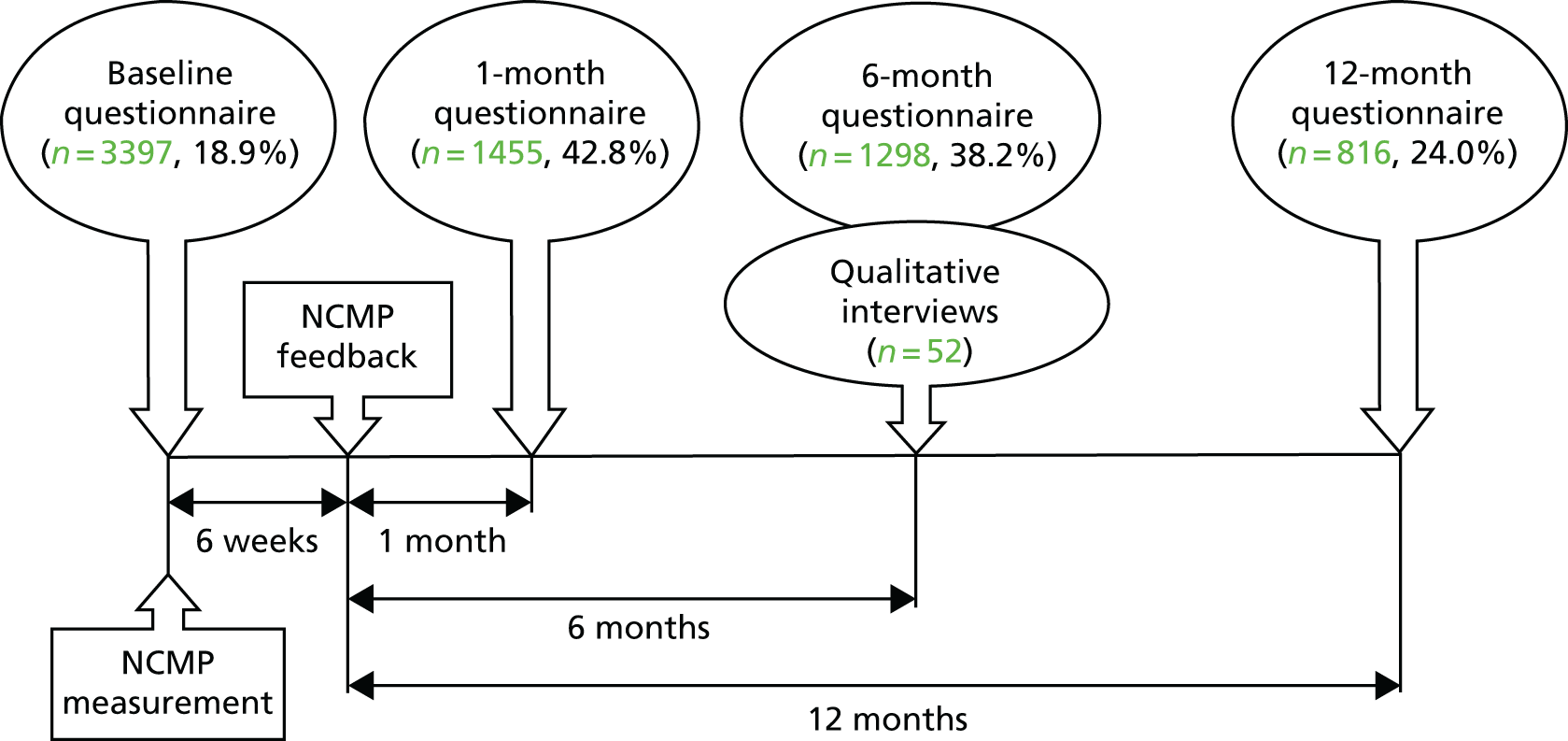
Follow-up questionnaires at 1, 3 and 12 months were mailed directly to parents who had completed a baseline questionnaire. In addition to the questions in the baseline questionnaire, follow-up questionnaires included questions to assess parents’ feelings and behaviours in response to NCMP feedback (see Report Supplementary Material 2). Up to two reminder contacts in the form of e-mails, text messages and telephone calls were made to non-responders. Questionnaire responses were linked to children’s NCMP data, including anthropometric measures, ethnicity and deprivation data [Index of Multiple Deprivation (IMD) score, a measure of local area deprivation based on a respondent’s postcode30] using a Microsoft Access® (Microsoft Corporation, Redmond, WA, USA) macro to match children based on their name and date of birth. Children who could not be matched automatically were identified using manual searches. The matching was carried out within the PCT and an anonymised linked data set was provided to the study team. All survey data were checked and entered electronically into a Stata®, release 12 (StataCorp LP, College Station, TX, USA) database.
Main outcome measures
The following outcomes were assessed at baseline and at 1, 6 and 12 months’ follow-up:
-
Parents’ general knowledge of childhood obesity as a health problem, assessed using the question ‘Do you think that being overweight increases a child’s future risk of any of the following: diabetes mellitus (DM), cancers, heart disease, high blood pressure, and arthritis?’. Parents who correctly identified four or more conditions were considered to have good knowledge.
-
The child’s diet, based on parent-reported frequency of consumption of fruits, vegetables, sugary drinks, sweet and savoury snacks (categories ranged from less than once a week to at least three times a day). 31 Each food category was assigned a score from 1 to 7, with a higher score indicating more frequent consumption of fruits and vegetables, and lower consumption of sugary drinks and snacks. A healthy eating score was derived as a mean of these subscores, with a score of ≥ 5 indicating a healthy diet.
-
The child’s daily physical activity, assessed with the question ‘On average, how many minutes of physical activity (described as any activity that increases heart rate and makes the child get out of breath) does your child do?’. Children who met the national physical activity recommendation of at least 1 hour per day were categorised as engaging in adequate physical activity.
-
The child’s daily screen time (the number of hours spent watching television or playing video games). Responses were categorised by whether or not children met screen time recommendations of < 2 hours per day. 32
The following outcomes were assessed in the parents of overweight and obese children only:
-
Parental recognition of their child’s overweight status (child described as ‘overweight’ or ‘very overweight’) in response to the question ‘How would you describe your child’s weight at the moment?’.
-
Parental perception of the health risks associated with their child’s overweight status (parent answered ‘yes’ to the question ‘Do you think your child’s current weight is a health risk?’).
-
Weight-related teasing, assessed using the Teasing/Marginalisation subscale from Sizing Them Up, a validated parent-proxy measure of obesity-specific health-related quality-of-life (HRQoL) scale. 33 A score of ≥ 50 represented frequent weight-related teasing.
-
The child’s self-esteem, assessed using the Emotional Functioning subscale of the Sizing Them Up scale. A score of ≥ 50 indicated frequent episodes of low self-esteem.
At follow-up, all parents were asked whether or not they had sought further information regarding their child’s weight, from sources including the school nurse, GP, pharmacist, or friends and family. Parents were also asked about the emotions that they experienced in response to the feedback (e.g. surprised, guilty, proud, pleased, upset, angry, ashamed, judged or indifferent).
Qualitative interviews
Qualitative interviews were conducted with a subsample of parents of overweight or obese children, who were purposively selected on the basis of their responses to the questionnaire surveys at follow-up; the sample was selected so that equal proportions did and did not perceive their child’s weight to pose a risk to their health. The sample was also selected to ensure that a range of socioeconomic and ethnic backgrounds were represented (around half of the sample were from deprived and ethnic minority households), and equal proportions of parents with children in Reception year and Year 6 were selected. Semistructured interviews were conducted by a researcher with considerable experience in conducting qualitative interviews with parents of school-aged children. Interviews were conducted either at the participant’s home or by telephone, at the parent’s request. The majority (81%) of interviews took place at the participant’s home. The interview schedule was developed to explore the barriers to behaviour change following receipt of the NCMP feedback, and consisted of open-ended questions with prompts as required (see Appendix 2). Each interview lasted ≈ 30 minutes on average. Interviews were audio-recorded using a digital voice-recorder and transcribed verbatim. A thematic analysis was carried out using the software package NVivo Version 9 (QSR International, Warrington, UK) to identify emergent themes: transcripts were read and reread, and initial codes were drawn from the data. With input from three experienced researchers, these initial codes were collated into themes and a coding frame was developed for data analysis.
Economic evaluation
The costs of delivering NCMP feedback were estimated using data on staff time and resource requirements associated with the feedback provided by PCTs. Staff costs were estimated using the NHS Agenda for Change 2012 pay rates. 34 Total costs for each PCT were divided by the number of children measured for the NCMP in the PCT, to derive a cost per child, and costs associated with different forms of feedback (written and proactive) were compared. The mean cost per child of providing routine written feedback across all PCTs was calculated to provide an England-wide estimate.
Statistical analysis
The characteristics of survey respondents were described using summary statistics (means and proportions), and compared with anonymised data on weight status, ethnicity, age and IMD score for all children participating in the NCMP in the five PCTs.
Parental perceptions were explored in analyses comparing parent-perceived and objectively derived assessments of child weight status in the study sample at baseline. A multinomial model of parental-perceived weight status was used, with three categories of perceived weight status: (1) underweight, (2) healthy weight and (3) overweight (including obese). A child’s BMI (calculated from measured weight and height), expressed as a z-score from the UK 1990 reference population,35 was the only independent variable included in the model. Using this model, cut-off scores were obtained that represented the points at which a child’s BMI z-score was equally likely to be classified by parents into two adjoining weight status categories, identifying BMI z-scores (expressed as centiles) at which parents were equally as likely to describe their child as underweight as healthy weight, or healthy weight as overweight. Analyses stratified by school year and sex produced little variability in the estimates across models, indicating that an unadjusted model was appropriate. To explore whether or not ethnic group, IMD quintile, child’s sex or school year might predict parental misclassification of child weight status (two outcomes: underestimation or overestimation), logistic regression was used. Standard population-level weight status cut-off points (underweight < 2nd, overweight > 85th, very overweight > 95th centile), rather than clinical cut-off points, were used for comparison with parent-reported categories, as parents are more likely to have been exposed to these population cut-off points before receiving NCMP feedback. Standard errors were adjusted for school-level clustering.
The main analyses (objective 1) were restricted to respondents with longitudinal data (data at baseline and at least one follow-up at 1 or 6 months). If parents had completed a questionnaire at the 1- and 6-month follow-ups, precedence was given to data at 1 month to reflect the immediate impact of feedback. A sensitivity analysis to assess the effect of including responses at 6 months (n = 452; 24.5%) showed no difference compared with restricting to responses at 1 month only; therefore, results for the combined data are presented. The proportions of parents reporting each outcome at baseline and follow-up were calculated. The difference between pre- and post-feedback proportions was assessed using McNemar’s test, and results are presented as the change in proportion between baseline and follow-up (a positive value indicates an increase from baseline to follow-up, and a negative value indicates a decrease).
Analyses of the differences were stratified by the following sociodemographic characteristics to assess potential effect modification: PCT, child’s sex, school year, ethnicity (categorised as white or non-white owing to small numbers from individual ethnic minority groups), deprivation (quintiles of IMD score) and child’s weight category (three categories: healthy weight and underweight combined, overweight or obese). Among parents of obese children, analyses were also stratified by type of feedback: letter only or letter plus proactive feedback (objective 2). Differences in the outcomes by sociodemographic characteristics and type of feedback were assessed using chi-squared tests. The potential effect of seasonality on outcomes, particularly physical activity and sedentary behaviour, was also explored by stratifying and comparing outcomes by season (March–May, June–August, September–November, December–February). To account for potential clustering by PCT, random-effects logistic regression models were fitted for each outcome, including PCT as a variable in the model. Interaction terms were included to assess potential modification of the main effect by feedback type, gender, ethnicity and deprivation. All analyses were conducted using the statistical software Stata, version 12.
Ethics
Ethics approval was obtained from the London School of Hygiene & Tropical Medicine Ethics Committee.
Results
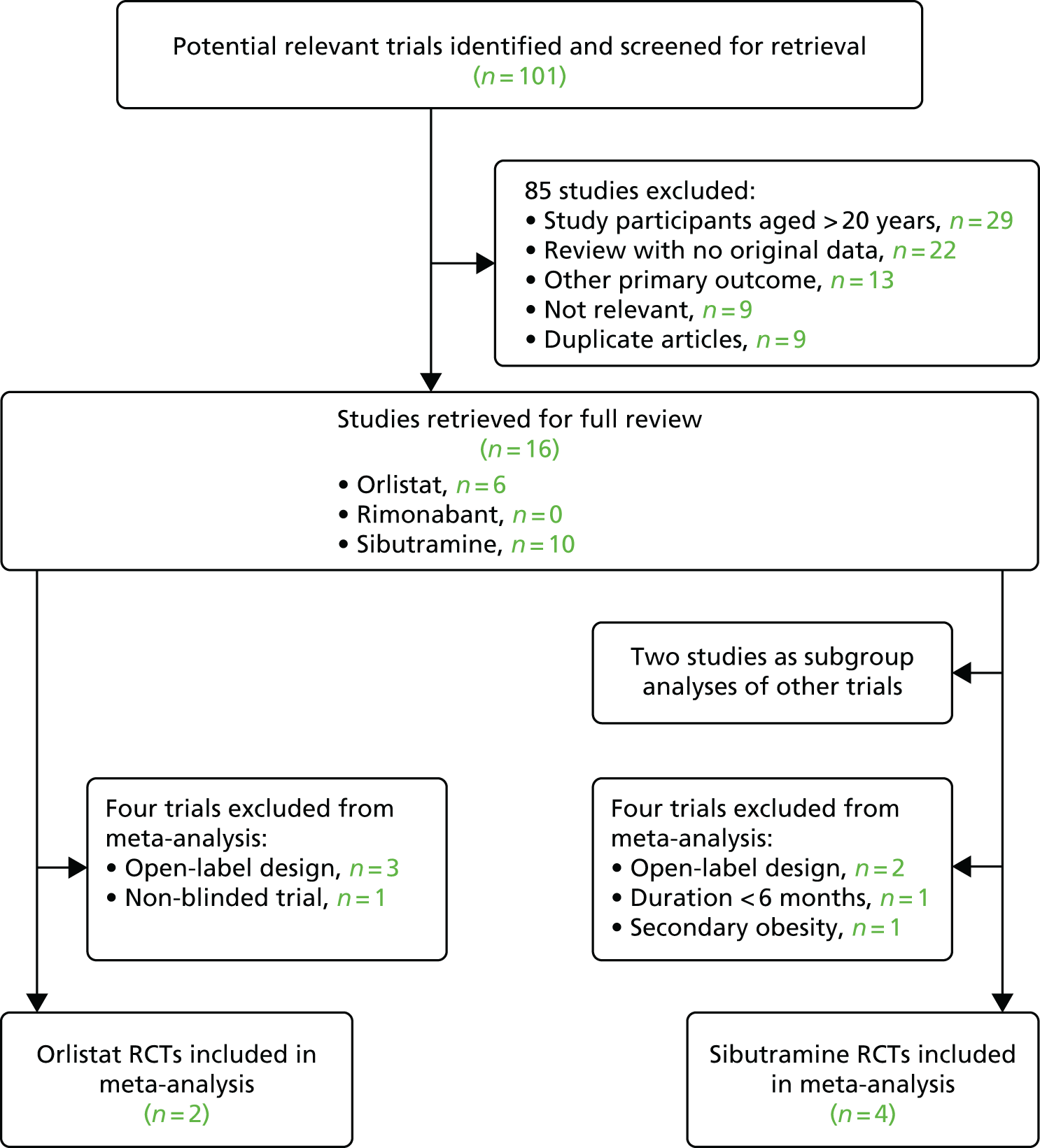
A total of 3397 parents responded to the baseline questionnaire (a response rate of 18.9%). Of these, 1844 parents completed at least one follow-up questionnaire at 1 month (n = 1455; a response rate of 42.8%) or 6 months (n = 1298; a response rate of 38.2%) and formed the sample for the main analyses. At 12 months, 816 participants (24.0%) returned a questionnaire; this was considered too small a sample for further analysis here. Among the parents of obese children (n = 105), 61.9% received proactive feedback in addition to the letter.
Comparison with target population
Compared with the whole population of children participating in the NCMP in the five PCTs, the study sample had lower proportions of overweight and obese children (15.4% vs. 22.1%; p-value from chi-squared tests for difference < 0.01), Year 6 children (44.5% vs. 50.9%; p < 0.01), parents from the most deprived areas (43.7% from the two most deprived quintiles vs. 49.1%; p < 0.01) and children from ethnic minority groups (34.0% vs. 45.5%; p < 0.01) (Table 1). Parents from these groups were also more likely to drop out of the study, resulting in greater differences at follow-up: at 6 months, 13.4% of children were overweight or obese, 40.2% were from the most deprived areas and 30.0% were from ethnic minority groups.
| Characteristic | Percentage | p-valuea | |
|---|---|---|---|
| Study sample (n = 1844) | NCMP population (n = 18,000) | ||
| Sex | |||
| Male | 49.7 | 48.4 | 0.29 |
| Female | 50.3 | 51.6 | |
| Ethnicity | |||
| White | 66.0 | 54.5 | < 0.01 |
| Asian | 5.5 | 10.8 | |
| Black | 15.7 | 21.2 | |
| Mixed/other | 12.8 | 13.5 | |
| School year | |||
| Reception (4–5 years) | 55.5 | 49.1 | < 0.01 |
| Year 6 (10–11 years) | 44.5 | 50.9 | |
| Weight status | |||
| Underweight | 1.9 | 1.4 | < 0.01 |
| Healthy weight | 82.8 | 76.5 | |
| Overweight | 9.7 | 12.5 | |
| Obese | 5.7 | 9.6 | |
| Deprivation quintileb | |||
| 1 | 19.1 | 20.3 | < 0.01 |
| 2 | 24.6 | 28.8 | |
| 3 | 19.9 | 21.6 | |
| 4 | 16.7 | 15.7 | |
| 5 | 19.7 | 13.7 | |
Of the 285 parents of overweight or obese children who had responded to the questionnaire survey, 108 were invited (by letter) to participate in the qualitative interviews. Interviews were carried out with 52 parents (43%). Theoretical saturation of the data was achieved with 40 interviews, with no new themes emerging in the final 12 interviews. The demographic characteristics of interview participants are presented in Table 2.
| Characteristic | % (n) (N = 52) |
|---|---|
| Parent sex | |
| Male | 17.0 (9) |
| Female | 83.0 (43) |
| Parent ethnicity | |
| White | 50.0 (26) |
| Non-white | 50.0 (26) |
| Socioeconomic deprivationa | |
| Deprived (IMD 1/2) | 44.0 (23) |
| Not deprived (IMD 3/4/5) | 56.0 (29) |
| PCT | |
| Islington | 15.4 (8) |
| Redbridge | 34.6 (18) |
| West Essex | 19.2 (10) |
| BANES | 13.5 (7) |
| Sandwell | 17.3 (9) |
| Child school year | |
| Reception (4–5 years) | 50.0 (26) |
| Year 6 (10–11 years) | 50.0 (26) |
| Child sex | |
| Male | 56.0 (29) |
| Female | 44.0 (23) |
| Child weight status | |
| Overweight | 37.0 (19) |
| Obese | 63.0 (33) |
| Parent perceives their child’s overweight status to pose a health risk | |
| Yes | 43.0 (22) |
| No | 57.0 (30) |
Parental knowledge, perceptions and lifestyle behaviours at baseline
At baseline, 72.3% of parents were able to identify the common health conditions associated with childhood overweight; this proportion was higher among parents with healthy weight and underweight children [74.8%, 95% confidence interval (CI) 72.5% to 77.1%] than among those with overweight (61.9%, 95% CI 54.2% to 69.7%) or obese (57.8%, 95% CI 47.4% to 68.2%) children. Parental knowledge also varied with ethnicity; just 59% of parents of children from non-white ethnic groups correctly identified common childhood obesity comorbidities, compared with 79% of parents of white children.
About half of the children were reported to meet dietary (48.7%) and screen time (53.4%) recommendations, and about one-third (36.3%) achieved recommended levels of physical activity. Compared with their healthy weight and underweight peers, levels of physical activity were lower and screen time was higher among overweight and obese children; the proportion achieving a healthy diet was lower among obese (but not overweight) children.
Among the parents of overweight and obese children (n = 285), the proportion who considered their child to be overweight was low: 14% of parents of overweight children and 35% of parents of obese children described their child as overweight. Similarly, 11.0% of parents of overweight children and 38.6% of parents of obese children considered their child’s weight status to pose a health risk. A small proportion of parents of overweight and obese children (8.9%; n = 10) reported frequent weight-related teasing, and 1.8% (n = 2) reported low self-esteem.
When parent-perceived weight cut-off points were estimated, the point at which a parent was equally likely to recognise underweight as healthy weight was when their child had a BMI at the 0.8th centile (95% CI 0.4 to 1.1 centile). A parent was more likely to classify their child as overweight rather than healthy weight when their child had a BMI of ≥ 99.7th centile (95% CI 99.3 to 99.9 centile). Based on population-based cut-off points for weight status, 915 (31%) parents underestimated and 25 (< 1%) overestimated their child’s weight status. Parents were more likely to underestimate their child’s weight status if they were black [reference group: white, odds ratio (OR) 1.5, 95% CI 1.1 to 2.1], south Asian (OR 1.6, 95% CI 1.3 to 2.0), male (OR 1.3, 95% CI 1.1 to 1.6) or in the older age group (OR 1.3, 95% CI 1.1 to 1.5). Parents from less deprived areas were less likely to underestimate their child’s weight status (OR for each quintile less deprived, OR 0.8, 95% CI 0.75 to 0.9). Overestimation of weight status was more likely for Year 6 than Reception children (OR 3.1, 95% CI 1.2 to 8.5). Ethnic group, deprivation and a child’s sex did not predict parental overestimation of weight status.
Objective 1: the impact of National Child Measurement Programme feedback
Parental knowledge
The proportion of parents with good general knowledge about the health conditions associated with childhood obesity increased following NCMP feedback (7.7%, 95% CI 5.1% to 10.3%). Variation in parental knowledge by child’s weight status and ethnicity remained, with parents of overweight and obese children and those from non-white ethnic groups having lower levels of knowledge. Detailed data by weight status can be seen in Table 3.
| Outcome | Weight status, % (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy weight and underweight (n = 1574) | Overweight (n = 180) | Obese (n = 105) | |||||||
| Baseline | Follow-up | Difference in proportiona | Baseline | Follow-up | Difference in proportiona | Baseline | Follow-up | Difference in proportiona | |
| Good parental knowledge of the health risks of child overweightb | 74.8 (72.5 to 77.1) | 81.9 (79.9 to 83.4) | 7.1 (4.6 to 9.6) | 61.9 (54.2 to 69.7) | 70.3 (63.1 to 77.6) | 8.4 (–0.4 to 17.2) | 57.8 (47.4 to 68.2) | 65.6 (55.5 to 75.6) | 7.8 (–4.5 to 20.1) |
| Parental recognition of child overweight | NA | NA | NA | 14.0 (8.8 to 19.3) | 25.1 (18.6 to 31.7) | 11.1 (4.0 to 18.3) | 35.3 (25.9 to 44.7) | 58.8 (49.1 to 68.5) | 23.5 (12.7 to 34.3) |
| Parental recognition of the health risks associated with child’s overweight | NA | NA | NA | 11.1 (6.4 to 15.9) | 18.1 (12.3 to 24.0) | 7.0 (1.4 to 12.6) | 38.0 (28.3 to 47.7) | 43.0 (33.1 to 52.9) | 5.0 (–6.9 to 16.9) |
| Child achieves a healthy dietc | 49.6 (47.0 to 52.2) | 48.9 (46.3 to 51.5) | –0.7 (–3.4 to 2.0) | 50.6 (42.8 to 58.4) | 46.3 (38.5 to 54.1) | –4.3 (–12.7 to 4.0) | 41.3 (31.1 to 51.6) | 41.3 (31.1 to 51.6) | 0 (–10.6 to 10.6) |
| Fruit and vegetable consumption (≥ 5 portions/day) | 32.5 (30.2 to 34.8) | 33.0 (30.6 to 35.3) | 0.5 (–2.0 to 2.8) | 27.8 (21.2 to 34.4) | 25.0 (18.6 to 31.4) | –2.8 (–10.4 to 4.7) | 22.9 (14.7 to 31.0) | 28.6 (19.8 to 37.4) | 5.7 (–3.5 to 14.9) |
| Sugar-sweetened beverage consumption (< 1/day) | 71.0 (68.7 to 73.3) | 70.0 (67.7 to 72.4) | –1.0 (–3.5 to 1.5) | 77.3 (71.0 to 83.5) | 75.6 (69.2 to 82.0) | –1.7 (–9.9 to 6.5) | 63.6 (54.0 to 73.3) | 66.7 (57.2 to 76.1) | 3.0 (–8.6 to 14.7) |
| Child achieves adequate physical activity (≥ 1 hour/day) | 38.1 (35.6 to 40.5) | 39.1 (36.7 to 41.5) | 1.0 (–1.6 to 3.6) | 27.9 (21.1 to 34.7) | 28.5 (21.7 to 35.3) | 0.6 (–6.1 to 7.3) | 25.2 (16.7 to 33.8) | 37.9 (28.3 to 47.4) | 12.6 (2.5 to 22.8) |
| Child achieves appropriate screen time behaviour (≤ 2 hours/day) | 55.4 (52.9 to 57.9) | 51.5 (48.9 to 54.0) | –4.0 (–6.6 to –1.4) | 45.5 (38.0 to 52.9) | 39.2 (31.9 to 46.5) | –6.3 (–14.2 to 17.3) | 41.6 (31.8 to 51.4) | 31.7 (22.5 to 40.9) | –9.9 (–20.6 to 0.8) |
| Weight-related teasingd | NA | NA | NA | 4.3 (–1.7 to 10.2) | 10.6 (1.5 to 19.8) | 6.4 (–2.7 to 15.5) | 19.0 (0.7 to 37.4) | 14.3 (–2.0 to 30.6) | –4.8 (–25.6 to 16.0) |
| Low self-esteeme | NA | NA | NA | 0 | 0 | 0 | 10.0 (–4.4 to 24.4) | 5 (–5.5 to 15.5) | –5.0 (–26.8 to 16.8) |
Parental perceptions
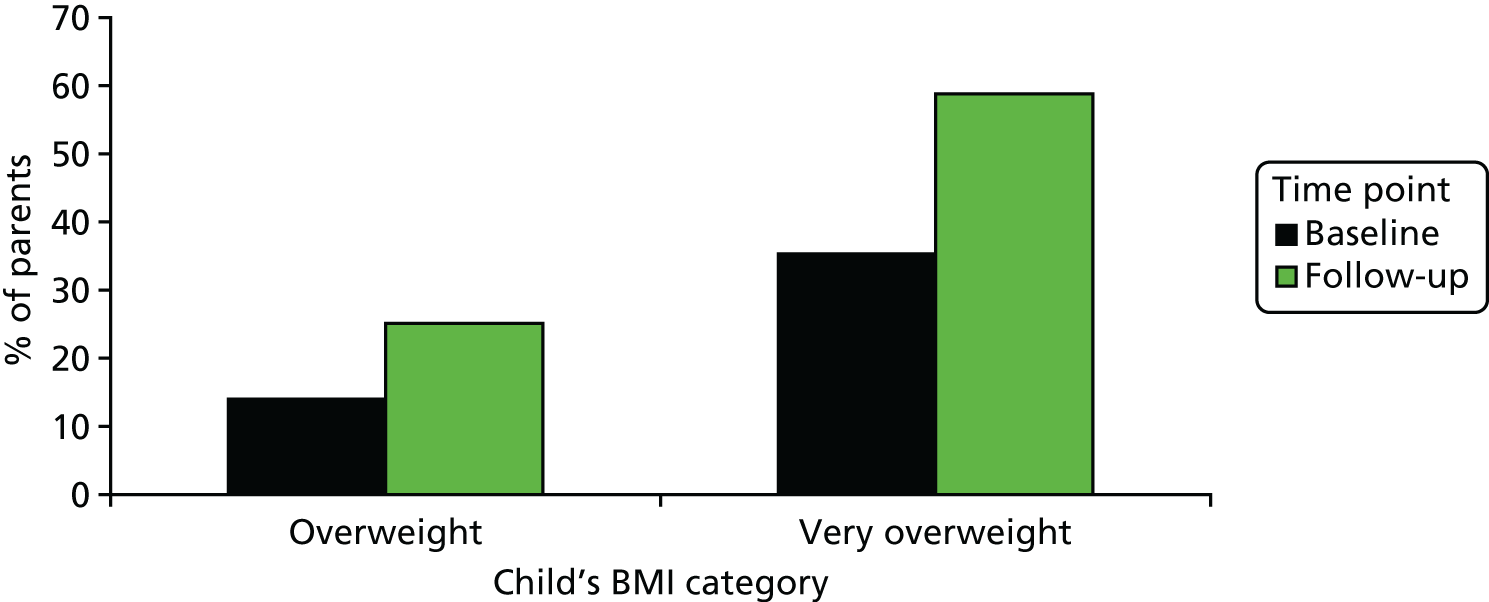
The proportion of parents of overweight and obese children who recognised their child’s overweight status increased post feedback (+15.8%, 95% CI 9.8% to 21.7%), with a greater increase among the parents of obese children (+23.5%) than among parents of overweight children (+11.1%) (Figure 2). However, 41% of parents of obese children and 75% of parents of overweight children continued to perceive their child to have a healthy weight after receiving NCMP feedback.
FIGURE 2.
The proportion of parents of overweight and obese (very overweight) children who considered their child to be overweight, before and after receiving NCMP feedback.
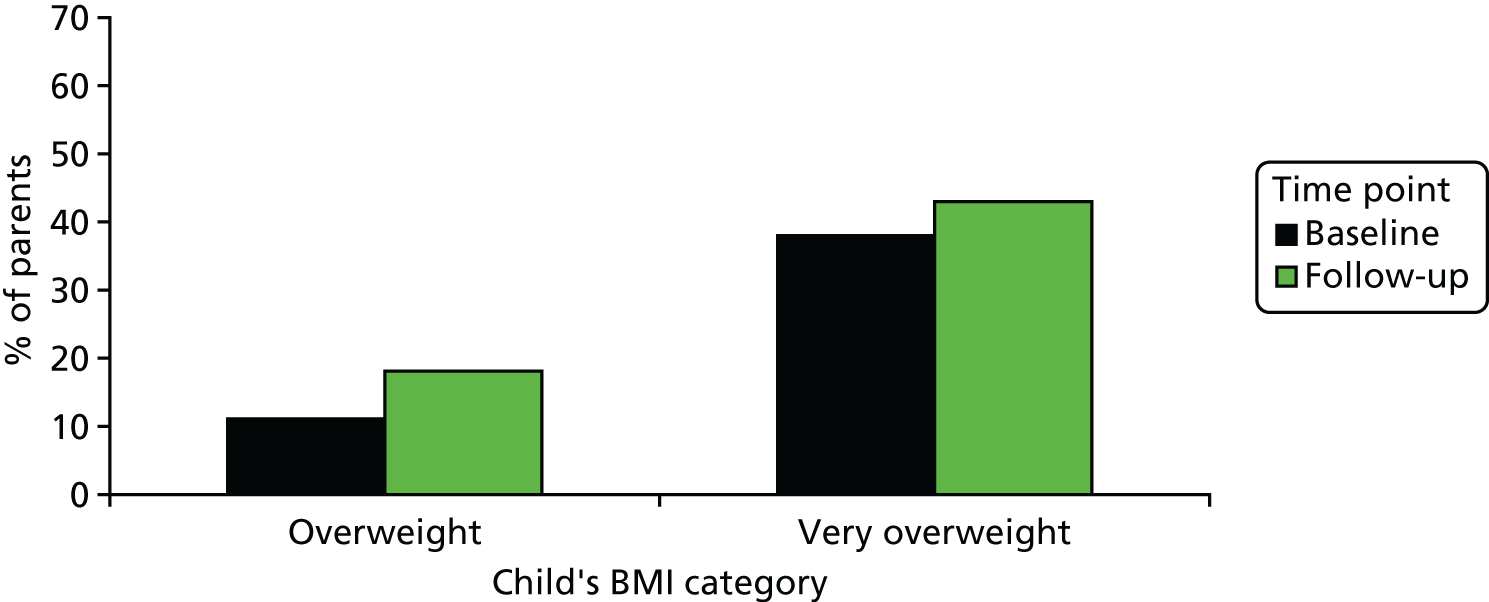
The increase in the proportion of parents who considered their child’s overweight status to pose a health risk was modest for both overweight (+7.0%, 95% CI 1.4% to 12.6%) and obese children (+5.0%, 95% CI –6.9% to 16.9%) (Figure 3).
FIGURE 3.
The proportion of parents of overweight and obese (very overweight) children who considered their child’s weight to pose a health risk, before and after receiving NCMP feedback.
Lifestyle behaviours
Neither consumption of fruit and vegetables nor consumption of sugar-sweetened beverages changed following feedback. Physical activity levels increased among obese children (+12.6%, 95% CI 2.5% to 22.8%), but not among children in other weight categories. The proportion of children engaging in recommended levels of screen time was lower after feedback (–4.6%, 95% CI –7.0% to –2.2%). There was no strong evidence for an effect of season on lifestyle behaviours. Detailed data by weight status can be seen in Table 3.
Health service use
One-quarter of parents of overweight children (26.9%) and half of parents of obese children (49.4%) sought further information or help regarding their child’s weight following receipt of the NCMP written feedback (compared with 12% of parents of healthy weight children). The most frequently reported sources of information were friends and family (reported by 14.4% of parents), the internet (9.9%), GPs (8.9%) and school nurses (8.4%). Attendance at a GP practice owing to concerns about their child’s weight was reported by 6% of parents of overweight children and 15% of parents of obese children.
Teasing and self-esteem
Frequent weight-related teasing was reported by 10% of parents of overweight and obese children at baseline, compared with 2% of parents of healthy weight children. Low child self-esteem was reported by 4% of parents of overweight and obese children and 1% of parents of healthy weight children. Both weight-related teasing and low self-esteem were more prevalent among obese children than among overweight children at both time points, but there was no apparent effect of NCMP feedback on either. The proportion of parents reporting weight-related teasing or low self-esteem did not change substantially after feedback.
Parents’ reactions to National Child Measurement Programme feedback
Parents reported experiencing a wide range of emotions in response to receiving NCMP feedback, which varied considerably by the child’s weight category. The most common reaction among parents of healthy weight children was ‘pleased’ (reported by 65.0% of parents) and 21.0% felt proud of their child’s result. Among the parents of overweight and obese children, the most commonly reported emotions were surprise (reported by 35.0%), upset (24.1%), guilt (15.4%) and shock or anger (14.8%).
Despite some negative emotional reactions to the feedback among the parents of overweight or obese children, the majority of parents across all child weight categories (87.2%) reported that they found the NCMP feedback to be either ‘somewhat helpful’ or ‘very helpful’. Parents of overweight children were most likely to find the feedback ‘not at all helpful’ (Figure 4). The majority of parents (70.0%) reported that they would encourage future participation in the NCMP for their child or child’s siblings. Only 1.8% said that they would withdraw their child from the programme in the future.
FIGURE 4.
The proportions of parents who found the NCMP feedback to be helpful, by child’s weight category.

Talking with children about National Child Measurement Programme feedback
Half of all parents (52%) had discussed their child’s weight status with their child. Parents of obese children were over two times more likely to have discussed the result with their child than parents of healthy weight children (adjusted OR 2.6, 95% CI 1.5 to 4.5). In all weight categories, the parents of children in Year 6 were more likely than parents of Reception-aged children to have discussed the result with their child (adjusted OR 4.4, 95% CI 3.4 to 5.8).
Among those parents who did discuss the result with their child, parents of underweight and healthy weight children mainly discussed the actual result, whereas parents of overweight and obese children mainly discussed making lifestyle changes or seeking help. Parents of overweight and obese children often chose not to discuss the weight result with their child, and during interviews some parents mentioned that this was because of a fear of inducing an eating disorder or low self-esteem.
Almost all parents of healthy weight children found talking to their child about the result to be easy (95.5%). More parents of overweight and obese children found talking to their child to be ‘a little difficult’ (30.4%) but very few reported it to be ‘difficult’ (3.2%). Among overweight and obese children, parents of boys more commonly reported finding the conversation to be easy.
Effects of sociodemographic characteristics on the impact of National Child Measurement Programme feedback
There was a larger increase in the proportion of parents who recognised the health risks associated with their child’s overweight status among those with children from non-white ethnic groups than those with white children (Table 4). In adjusted analyses, the parents of children from non-white ethnic groups were eight times more likely to have changed their recognition of the weight-related health risks (OR 8.6, 95% CI 1.9 to 39.8). The proportion of parents who reported an improvement in their child’s diet was larger among those with children in Year 6 than those with children in Reception year. There were no statistically significant effects of other sociodemographic characteristics (sex and deprivation) on any outcomes.
| Characteristic | Parental recognition of overweight | Parental recognition of health risks associated with child’s overweight | Child achieves a healthy diet | Child undertakes adequate physical activity | ||||
|---|---|---|---|---|---|---|---|---|
| Difference in proportion, % (95% CI)a | p-valueb | Difference in proportion, % (95% CI)a | p-valueb | Difference in proportion, % (95% CI)a | p-valueb | Difference in proportion, % (95% CI)a | p-valueb | |
| Ethnicity | ||||||||
| White | 12.6 (5.6 to 19.6) | 0.24 | –3.3 (–8.7 to 2.1) | < 0.01 | –2.9 (–10.9 to 5.1) | 0.98 | 7.3 (0.3 to 14.4) | 0.37 |
| Non-white | 19.3 (10.1 to 28.6) | 17.9 (8.8 to 27.1) | –2.7 (–12.0 to 6.6) | 2.5 (–5.7 to 10.6) | ||||
| Sex | ||||||||
| Female | 14.8 (6.4 to 23.3) | 0.75 | 5.8 (–2.3 to 14.1) | 0.88 | –3.9 (–12.2 to 4.4) | 0.71 | 2.2 (–3.7 to 8.1) | 0.27 |
| Male | 16.7 (9.2 to 24.2) | 6.7 (0.4 to 13.0) | –1.6 (–10.5 to 7.3) | 8.1 (–.07 to 16.7) | ||||
| School year | ||||||||
| Reception (age 4–5 years) | 16.9 (9.3 to 24.5) | 0.69 | 8.1 (1.2 to 15.1) | 0.48 | –10.5 (–18.9 to –2.1) | 0.01 | 5.9 (–1.5 to 13.4) | 0.76 |
| Year 6 (age 10–11 years) | 14.6 (6.3 to 22.9) | 4.4 (–3.3 to 12.1) | 4.6 (–4.0 to 13.2) | 4.3 (–3.2 to 11.8) | ||||
| Deprivation quintilec | ||||||||
| 1 | 13.3 (0.3 to 26.3) | 0.54 | 3.8 (–5.2 to 12.7) | 0.41 | 6.8 (–4.4 to 18.1) | 0.82 | 6.3 (–3.3 to 15.8) | 0.58 |
| 2 | 14.8 (4.3 to 25.4) | 5.6 (–3.0 to 14.1) | –5.9 (–16.1 to 4.3) | 10.9 (0.03 to 21.7) | ||||
| 3 | 26.4 (13.0 to 29.8) | 13.0 (–0.07 to 26.0) | –3.8 (–17.3 to 9.6) | –1.8 (–11.5 to 7.9) | ||||
| 4 | 13.6 (0.4 to 27.7) | 11.4 (–0.4 to 23.1) | –4.8 (–22.9 to 13.4) | 6.8 (–6.9 to 20.6) | ||||
| 5 | 11.1 (5.6 to 27.8) | –3.9 (–17.7 to 10.1) | –4.0 (–22.8 to 14.8) | 11.1 (–8.9 to 31.1) | ||||
Objective 2: the effects of different forms of weight feedback
Proactive feedback was implemented in Redbridge, BANES and Sandwell PCTs. In Redbridge PCT, approximately 60% of parents of obese children were successfully contacted by telephone and offered a face-to-face appointment; 69% of these parents accepted the appointment. Corresponding numbers for BANES and Sandwell PCTs could not be ascertained. There was some evidence to suggest that proactive forms of feedback may be more effective than written feedback alone in changing perceptions among the parents of obese children (Table 5). Parents who lived in PCTs offering proactive feedback demonstrated greater improvements in parental recognition of their child’s overweight status (32% vs. 10%) and greater recognition of the associated health risks (13% vs. –8%) than patients in all PCTs in the study taken as a whole. There was no effect of type of feedback on lifestyle behaviours or health service use.
| Outcome | Difference in proportion, % (95% CI)a | p-valueb | |
|---|---|---|---|
| Written feedback only | Proactive feedback | ||
| Parental recognition of overweight | 10.0 (–7.4 to 27.4) | 32.3 (20.2 to 44.2) | 0.03 |
| Parental recognition of health risks associated with child’s overweight | –7.9 (–27.2 to 11.4) | 12.9 (–0.5 to 26.3) | 0.07 |
| Child achieves a healthy diet | –8.8 (–24.6 to 6.9) | 5.2 (–7.3 to 17.7) | 0.17 |
| Child partakes in adequate levels of physical activity | 15.0 (–2.1 to 32.1) | 11.1 (–0.1 to 22.2) | 0.69 |
Parents expressed a strong preference for written feedback over other forms of feedback, with 84.5% of all parents preferring feedback by letter, whereas 3.0% preferred feedback by telephone. Among the parents of obese children, the type of feedback received during the study was not associated with preferences for future forms of weight feedback.
Objective 3: barriers to behaviour change and health service use
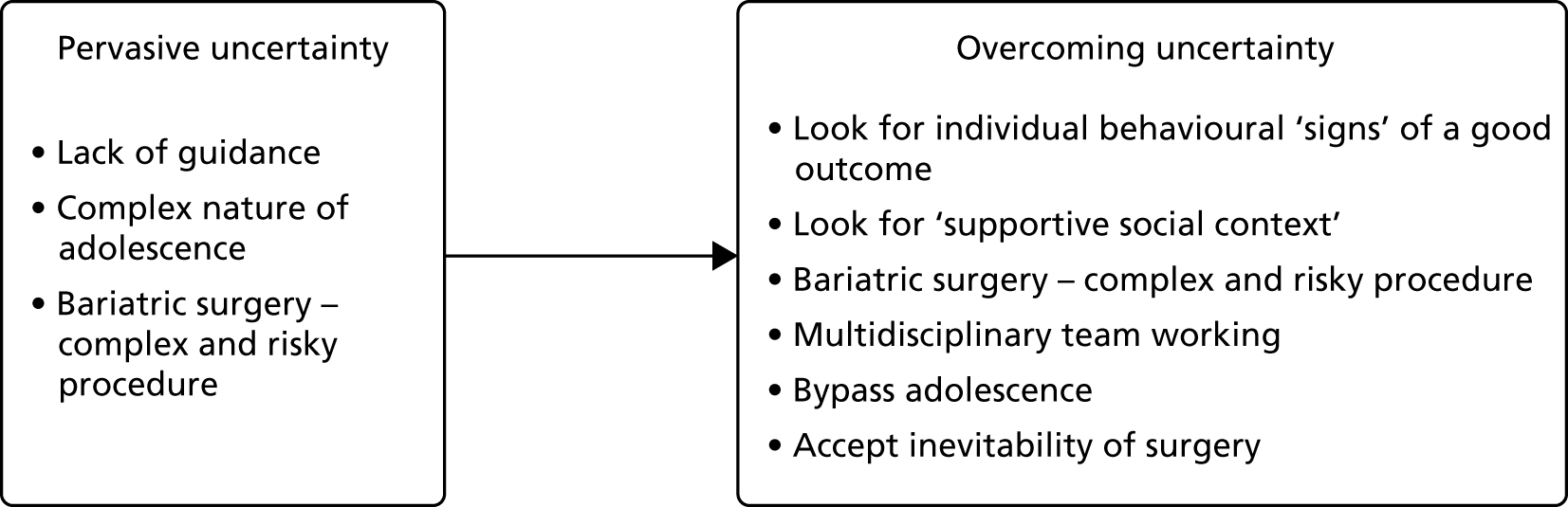
Qualitative interviews with parents of overweight and obese children identified several barriers to health-related behaviour change: (1) parental perceptions of their child’s health, weight and appearance, (2) cultural differences, (3) access to and availability of local services, (4) contradictory advice from health professionals, (5) relevance of lifestyle advice and (6) limits to parental control.
Barrier 1: parental perceptions of their child’s health, weight and appearance
Many parents did not consider the NCMP result and its associated health concerns to reflect their child’s situation. The predominant theme that arose during analysis was that parents had broad definitions of health, with a variety of features other than weight being considered to determine whether or not a child was healthy. Parents placed more importance on their child’s emotional and physical health than on weight per se:
No he’s a happy boy, he’s a proper happy little boy. He’s not concerned about anything . . . he doesn’t get picked on in any way by anybody.
Parent 20024; obese 4- to 5-year-old child
I think if he is happy with the way that he looks and he’s happy with himself, I think then that’s absolutely fine.
Parent 20031; obese 10- to 11-year-old child
Many parents considered a healthy lifestyle to be more important than weight as an indicator of health and well-being:
I never thought of the weight side particularly, I just looked at what the lifestyle was. He does exercise and he does eat well.
Parent 20022; obese 4- to 5-year-old child
If she’s not active I would be worried but she’s active, she runs, she do all of them things so I’m not worried about her health.
Parent 20021; obese 4- to 5-year-old child
For some parents, this discrepancy made the NCMP feedback less credible:
It only measures weight-to-height ratio and doesn’t take into account their lifestyle, their activity and things like that.
Parent 20010; overweight 10- to 11-year-old child
Another barrier to changing perceptions was that excess weight in their child was viewed by many parents to be puppy fat, which their child would grow out of as they got older:
I look at him and I see puppy fat, I don’t see overweight fat, I think they’re two different things.
Parent 30004; overweight 10- to 11-year-old child
It’s not a huge concern at the moment because I think we’ve got to get through puberty and see how she goes and how she grows.
Parent 30001; obese 4- to 5-year-old child
Some parents felt that their child was genetically predisposed to be heavy and, therefore, being overweight was not entirely within their control:
Some of it is down to poor diet from us but a lot of it has got to be to do with genetics.
Parent 20035; obese 4- to 5-year-old child
Many parents viewed their child’s weight status in comparison with that of other children and extreme examples of overweight, and, therefore, did not consider their child to look overweight:
I don’t think when you look at her you don’t look at her and think she’s overweight. She doesn’t have that overweight look feature-wise.
Parent 20001; obese 4- to 5-year-old child
He honestly doesn’t really look overweight to me so you see children in the street and they’re little round puddings. He’s not one of them.
Parent 20024; obese 4- to 5-year-old child
Barrier 2: cultural differences
Although results from the analysis of interview data were generally universal across parents (regardless of gender, age or ethnicity), there were some cultural influences that affected parents’ responses to the NCMP feedback. Some parents of non-white ethnicity described a disparity between the predominant ‘British’ views of overweight and those of their culture, in which overweight was not viewed negatively:
Back in my country, saying a child is overweight means it is a good thing.
Parent 20017; obese 4- to 5-year-old child
Barrier 3: access to and availability of local services
Around half of parents of obese children and three-quarters of parents of overweight children did not seek further help or information after receiving NCMP feedback. The most commonly reported barrier to seeking help was a lack of knowledge about where to go for help:
If there was literature sent out to parents with children with weight problems and they listed the organisations or the services as you say that are available, then that would give parents more ideas of where to go.
Parent 20006; obese 5- to 6-year-old child
Some parents described limited availability of services when they did follow up health advice:
The health visitor gave me the number of one of the sport centres and they offer 12 weeks’ activity but I put her name down and they haven’t . . . they said they don’t have enough people to do that course, so they haven’t let me know yet and it’s been 5–6 months now.
Parent 20008; overweight 11- to 12-year-old child
Others expressed concern about the perceived costs associated with services:
At the local sports centre, they have things going on but things are so expensive, to pay for that is a lot . . . I always keep thinking, ‘oh I want him to do another thing’, but we just don’t have the funds to do that.
Parent 20022; obese 6- to 7-year-old child
Barrier 4: contradictory advice from health professionals
Although not commonly reported, some parents who sought advice from a health professional were reassured that their child was not overweight, which undermined the NCMP feedback:
I took both children to the clinic at that time and [the] doctor checked both of them and said, ‘I can’t understand why they send them in the first place because they’re not overweight’. Maybe they sound overweight according to their age, but according to their structure, their height, they are not overweight . . . when a specialist like a doctor or someone tells something it takes more effect and she was a bit relieved that I’m not fat.
Parent 20007; overweight 10- to 11-year-old child
Barrier 5: relevance of lifestyle advice
Some parents dismissed the lifestyle advice provided in the NCMP feedback letter because they felt that they were already leading healthy lifestyles:
I felt it was talking to the person who feeds their child crisps every day and has fizzy drinks, and is going to, of course, just be feeding their child to a real budget and that’s really hard. I was thinking from my point of view this isn’t very helpful . . . I can’t remember what it said really ‘cause I felt it wasn’t even relevant to me.
Parent 20032; overweight 5- to 6-year-old child
Barrier 6: limits to parental control
Several parents described the difficulties of controlling all aspects of their child’s lifestyle, citing influences such as the child’s school, other family members and the child’s own preferences as obstacles to modifying lifestyle behaviours:
I don’t want to sound conceited, but I knew exactly how to eat healthily but we had a child who likes her sweet stuff and her snacks and her crisps.
Parent 30001; overweight 11- to 12-year-old child
I had always struggled with my partner about feeding our daughter. I felt he made inappropriate decisions regarding her portion sizes, and the amount of food that he allowed her to eat . . . the issue that I felt was the real impediment so any change was my partner, and I didn’t feel that I needed any advice as to how I could overcome . . . change his behaviour. I mean he’s going to behave the way he behaves and sadly he doesn’t really listen to me or anyone else to some extent.
Parent 20011; overweight, 6- to 7-year-old child
Objective 4: NHS costs associated with National Child Measurement Programme feedback
The cost of providing routine written feedback letters to parents was estimated at £1.24 per child, compared with £9.50 per child for telephone feedback and £41 per child for face-to-face appointments. The higher costs were attributable to the additional staff time required to deliver proactive feedback (Table 6). Based on these estimated costs and the national prevalence of obesity among children taking part in the NCMP, the mean cost per child of providing written feedback letters to all parents and a telephone call to the parents of all obese children was £2.03 (on average an additional £0.79 per child compared with written feedback only).
| Feedback type | Breakdown of tasks required | Staff involved | Cost per child (£) |
|---|---|---|---|
| Letter |
|
|
1.24 |
| Proactive telephone callsa |
|
|
9.50 |
| Proactive telephone calls plus face-to-face consultationa (Redbridge PCT) |
|
|
41.00 |
Discussion
We report findings from the first large-scale evaluation of the NCMP feedback and its impact on parental perceptions of their child’s weight status and health, and lifestyle behaviours and health service use. This study has shown that parents’ general knowledge about childhood obesity comorbidities increased following NCMP feedback, as did recognition of childhood overweight among parents of overweight and obese children. However, a sizable proportion of parents with obese children and the majority of parents of overweight children continued to perceive their child to have a healthy weight after receiving information to the contrary. Analysis of baseline data showed the sizeable discrepancy between parent-perceived and objectively assessed weight status cut-off points (99.7th centile compared with 91st centile used in NCMP feedback). The proportion of parents of overweight and obese children who perceived their child’s weight status to be a health risk increased after feedback, but remained low at follow-up; those parents who did change their perception of their child’s health may be more likely to engage with child weight issues and be prompted to make positive changes. 24,36 Parents of ethnic minority group children, boys and older children, and parents from more deprived areas, were more likely to underestimate their child’s weight at baseline. Research to understand how parental perceptions of their child’s weight status and health are formed in different groups may identify ways to more effectively communicate information to parents.
The parents of ethnic minority children demonstrated a larger change in recognition of the health risks than parents of white children. A similar effect has been observed in other studies, with ethnic minority groups reporting greater changes to lifestyle and plans for help-seeking following weight feedback. 37,38 Although this variation by ethnic group requires further investigation, these findings are encouraging, as many ethnic minority groups are at increased risk of obesity and its comorbidities,39 and typically have lower rates of participation in obesity prevention initiatives than white populations. 40,41 Qualitative interviews with parents also highlighted cultural influences on parental perceptions of child overweight, suggesting that there may be value in developing culturally specific feedback.
In this study, there was no observed effect of weight feedback on dietary behaviours, and a positive effect on physical activity was observed only among obese children. A previous evaluation of NCMP feedback showed that many parents plan to make changes to behaviour following feedback,25 but our study indicates that actual behaviour changes may be small and limited to certain groups. Given the low-intensity nature of routine weight feedback as an intervention, it may be expected that changes in behaviour would not be large. 42 In the case of screen time behaviour, fewer children met the screen time recommendations after feedback than before. A possible explanation for this unexpected finding is that the information provided with weight feedback does not make explicit recommendations about screen time; inclusion of screen time recommendations could be included in weight feedback in the future.
More than one-third of parents of overweight and obese children reported seeking further information regarding their child’s weight in response to the feedback. Informal sources of help (i.e. friends and family, the internet) were most commonly consulted; parents may feel more comfortable approaching these sources than more official sources of information, and interviews indicated that limited availability of services presented a barrier to seeking help. An implication of this finding is that the quality of information that parents receive may be difficult to monitor, and parents may be more likely to receive misinformation or advice; further work to explore the types of health information that parents access from informal sources may inform the development of resources for parents. Fifteen per cent of parents of obese children reported seeking help from a GP; in interviews, some parents described receiving advice from health professionals that contradicted the NCMP feedback. This finding highlights the importance of adequate training for primary care professionals to deal with childhood overweight and obesity. The capacity of local services to cope with increased help-seeking as a result of NCMP feedback also needs to be assessed, and taken into consideration when implementing the programme.
More than half of parents reported discussing the feedback with their child; however, we found no adverse effects on self-esteem and weight-related teasing in overweight and obese children. 43 The majority of parents found weight feedback to be helpful and reported that they would encourage future participation in the NCMP for their child. A small number of parents reported feeling upset or angry in response to the feedback. Consideration of the sensitive nature of weight issues should be a priority when devising feedback. 44
When written letters were supplemented by proactive weight feedback (i.e. telephone calls or face to face), there were greater improvements in parental recognition of their child’s overweight status and recognition of the associated health risks, but no effect on lifestyle behaviours or health service use. The success of proactive feedback may have been limited by relatively low contact rates; for example, in Redbridge PCT, just 60% of parents of obese children were contacted successfully by telephone, although the results reflect the logistical difficulties associated with this type of feedback. In general, more intensive behaviour change interventions have greater success and this may be applicable in the context of weight feedback provision. 42,45 However, the potential benefits of proactive feedback must be balanced against the higher costs, parents’ preference for written feedback over proactive modes of delivery and difficulties in contacting parents by telephone.
A major barrier to behaviour change among parents of overweight and obese children was that parents perceived their child’s health in terms of a variety of features other than weight, particularly emotional well-being and physical health. This was reflected in the low levels of parental recognition of overweight and the associated health risks in their children. Other barriers that were identified included limited availability and perceived high cost of local services, contradictory advice from health professionals and limits to parental influence over their children’s lifestyles. These barriers should be taken into consideration when developing weight feedback; an emphasis on healthy lifestyles and ways to encourage positive behaviours in all children, regardless of their weight status, may be a priority for future interventions, particularly in the context of low levels of recognition of the NCMP result among parents of overweight and obese children.
Strengths and limitations
This study was conducted in a large, population-based and sociodemographically diverse sample, which enabled examination of the effects of weight feedback in different weight and sociodemographic groups. To account for the lack of a comparison group, a short time interval was selected between baseline and follow-up questionnaires (≈ 3 months) to minimise potential changes in outcomes that may have occurred independently of the intervention.
The main limitation of this study was the low response rate and high attrition, which raise the possibility of a biased sample. It is plausible that parents who are more engaged with issues relating to their child’s health would be more likely to participate in the study and respond to feedback than parents who are not engaged with these issues, which would lead to an overestimation of the effect of weight feedback. Comparison of the study sample with all children taking part in the NCMP in the five PCTs revealed a slight under-representation of children from ethnic minorities, children from Year 6, children from deprived areas and overweight/obese children in our sample, which may limit the generalisability of the study findings to the UK NCMP population. However, the lifestyle behaviours of the study sample were similar to those previously observed in national surveys of primary school-aged children. 46–48 To keep the questionnaires concise, brief measures of behaviour and potential harms were used, not all of which are validated. Lifestyle behaviours are difficult to measure accurately,49 and self-reported measures of behaviours may be subject to misreporting. The reported lack of a change in lifestyle behaviours in this study argues against this being an issue;50 however, this does not discount the possibility of systematic social desirability bias, for example parents over-reporting fruit and vegetable consumption at both time points. The dietary measures used have been previously assessed using test–retest methods and found to be reasonably reliable. 31 In addition, parental perceptions of overweight and health risk were evaluated using questions that have been used in previous evaluations of weight feedback. 43 Questionnaires were not translated into other languages, as it was not standard practice to translate the feedback letters in any of the PCTs involved in the study. Consequently, the study does not reflect the impact of NCMP feedback on non-English-speaking households.
We did not collect data on parental weight as this would have only been by self-report, which is highly prone to bias. We did not collect data on disordered eating among children because of contraints of questionnaire length; however, this would have added to our understanding of the impact of NCMP feedback.
Conclusions
The NCMP feedback had a positive impact on parents’ general knowledge about childhood obesity, and parental recognition of overweight and the associated health risks in their own children. However, there were no improvements in children’s lifestyle behaviours following provision of feedback, suggesting that the impact on prevalence of childhood overweight and obesity is likely to be minimal. A number of barriers to behaviour change were identified in interviews with parents. A notable barrier was parents’ perceptions of health, which often did not take account of weight. However, the majority of parents found the feedback to be helpful and were happy to take part in the NCMP in the future. Proactive feedback appeared to offer some additional benefit in terms of changing parental perceptions, but was logistically complex to implement, and written feedback was preferred by parents. The mixed results of this study suggest that the NCMP may have a role in changing parental perceptions and health knowledge in the general population, but other initiatives may be required to precipitate lifestyle behaviour changes that will reduce childhood obesity prevalence. This includes provision and evaluation of online resources to support weight change for children and families.
Chapter 3 Study B: developing a new electronic tool to improve childhood obesity management in primary care
This chapter has been reproduced from Park et al. 51 Published by the BMJ Publishing Group Limited. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Abstract
Effective screening and management of childhood obesity in primary care is limited by time constraints and a lack of specialist training. The use of appropriate evidence-based technologies could improve treatment of overweight children in this setting. We developed a simple online tool, Computer-Assisted Treatment of CHildren (CATCH), to aid primary care practitioners in assessing and treating childhood obesity, and evaluated the feasibility and acceptability of implementing this tool in general practice. The tool incorporates risk estimation models, developed from analysis of large population-based data sets to identify children at risk of obesity-related comorbidities who may need referral to secondary care, and provides families with tailored, evidence-based lifestyle advice. An uncontrolled pilot study with integral process evaluation was conducted at three general practices in north-west London to evaluate the feasibility of using CATCH in primary care, and to assess the acceptability of tool-assisted consultations to families and practitioners. Families with concerns about excess weight in a child aged 5–18 years (n = 14 children) had a consultation with a GP or practice nurse using CATCH. Families and practitioners completed questionnaires to assess the acceptability and usefulness of the consultation, and participated in semistructured interviews that explored user experiences. Most families and practitioners were satisfied with the tool-assisted consultations, and reported that the tool was easy to use, the personalised lifestyle advice was useful and the use of visual aids was beneficial. Families and practitioners identified a need for practical, structured support for weight management following the consultation. However, CATCH was trialled in only three practices in one city and warrants investigation in different settings.
Key findings
-
Risk estimation models for cardiovascular and emotional/behavioural obesity-related comorbidities perform moderately well to stratify risk in overweight and obese CYP in primary care, although further work is needed to improve models.
-
A consultation using an online tool is acceptable to families presenting at a GP clinic with concerns about excess weight in a child: the majority of families (n = 12; 86%) were satisfied with the CATCH-assisted consultations, and all respondents found the personalised lifestyle advice useful or somewhat useful.
-
In interviews, parents described the consultation as informative, and several referred to the consultation as a ‘wake-up call’ that alerted them to the severity of their child’s weight problem.
-
Parents felt that the use of visual aids, such as the BMI chart, to show the child’s weight status and printed lifestyle advice reinforced the practitioner’s message. Similarly, practitioners felt that the tool could enhance the impact of their advice. It was suggested that the tool could be used as positive reinforcement for patients with BMIs in the healthy range and who had a healthy lifestyle.
-
Practitioners were satisfied with the tool-assisted consultation and reported that the tool was easy to use.
-
All practitioners were in agreement that the tool (or a version of it) would be something that they would continue to use in the future and would like to see integrated into their clinical software system.
-
Families and practitioners identified a need for practical, structured support for weight management following the consultation: several parents suggested that follow-up appointments for monitoring and guidance on weight management would be beneficial.
Background
Children who are overweight or obese are at an increased risk of developing cardiovascular abnormalities and other comorbidities. 52 For most children with such weight-related conditions, initial assessment of their health and access to treatment will be through primary care. However, primary care practitioners in the UK report that effective screening and management of overweight children is limited by time constraints, insufficient training and their concerns about raising weight-related issues with patients. 53–55 Assessing weight status in children is more complex than in adults, but formal training in child anthropometry is uncommon in general practice,56,57 and practitioners report a lack of expertise to treat childhood obesity and its comorbidities. 55 Primary care professionals are also concerned that the lack of medical treatment options for obesity could contradict patient expectations and, ultimately, be detrimental to the doctor–patient relationship. 54
Recent years have seen an increase in the development of electronic tools and other forms of information technology for health care. 58 Several studies indicate that the appropriate use of technology could assist practitioners in the identification and treatment of childhood obesity,59–63 but only a handful of electronic tools for use in primary care have been identified; in the USA, an electronic medical record upgrade was implemented in two Family Medicine Centers to allow the calculation of age- and-gender-specific BMI centiles for children,60 but the upgrade did not offer clinicians a way to assess individual health risk or to tailor advice to patients. The Bristol Online Obesity Screening Tool (BOOST) was developed in the UK to assess obese children and flag those with secondary causes of obesity or serious comorbidities based on blood pressure measurements, a urine sample and screening questions;61 BOOST was designed for obese (not overweight) children and did not provide lifestyle advice for patients. Another online tool, HeartSmartKidsTM (Boulder, CO, USA), provided computerised decision support to improve the implementation of clinical guidelines in the USA;62 it provided BMI centiles and charts, and semipersonalised lifestyle advice, but it did not enable assessment of individual health risk.
We developed an evidence-based online tool, CATCH, for the assessment and management of childhood obesity. CATCH incorporates national clinical guidelines for the assessment and management of childhood obesity (NICE guidance)11 and utilises risk stratification to identify overweight children who may require referral to secondary care. 64 The tool also enables practitioners to provide personalised, evidence-based lifestyle advice to children and their families. Following its development, we piloted the tool to evaluate the feasibility of implementing CATCH in primary care, and assessed families’ and health professionals’ satisfaction with consultations using the tool.
Objectives
The objectives of this study were to:
-
improve primary care management of childhood obesity through the development and pilot study of a brief evidence-based assessment and management tool
-
improve primary care decision-making about onwards referral through the development of evidence-based algorithms for identifying the risk of obesity comorbidities.
Methods
The study consisted of two main phases. The first phase involved the development of the CATCH tool and the second phase was a pilot study to evaluate the feasibility and acceptability of the tool.
Phase 1: development of the Computer-Assisted Treatment of CHildren tool
Overview
The CATCH tool was developed by a team of academic clinicians, in consultation with GPs and practice nurses unaffiliated with the research team. Throughout the development process, feedback from individual meetings and focus groups with practising clinicians informed the content and design of the tool. The tool was designed to guide a primary care practitioner through a consultation via three main steps:
-
calculation of a child’s BMI centile and weight status (overweight was defined as a BMI ≥ 91st centile of the UK 1990 reference;65 obese was defined as a BMI ≥ 98th centile)11
-
risk stratification and referral – estimation of a child’s risk of common obesity comorbidities and recommendation for referral when appropriate
-
lifestyle assessment – personalised lifestyle advice based on patient-reported information about diet, physical activity and sleep patterns.
Risk stratification and referral
The CATCH tool uses risk stratification to identify children who are at an increased risk of obesity comorbidities and may need referral to secondary care. Children needing referral were defined as (1) those children who probably had medical causes of obesity (for instance endocrine dysfunction) and (2) those children who were at risk of having other obesity comorbidities (most commonly cardiovascular abnormalities or mental health problems).
Children who may have medical causes of obesity are identified based on their height-for-age centile; low height for age is a characteristic associated with several endocrine and other medical causes of obesity in children. 66 A child whose height centile falls below their target centile range is identified as having a potential medical cause of obesity and flagged for referral. 67
Children at risk of obesity comorbidities are identified in a two-step risk stratification process. First, risk scores are calculated using risk estimation models. Second, the risk scores are classified into one of three risk groups: high, medium or low. We performed a systematic search that identified 26 comorbidities that have been shown to be independently associated with childhood obesity. Based on the prevalence and clinical value of referral for each condition, two broad categories of comorbidities warranting inclusion in the tool were identified: cardiovascular risk factors and mental health conditions (defined as emotional and behavioural difficulties). We developed two risk estimation models: one to estimate a child’s current risk of having one or more cardiovascular abnormalities, and one to estimate a child’s current risk of emotional and behavioural difficulties. The risk estimation models were derived from analysis of data from overweight and obese children in two large UK cohorts: Avon Longitudinal Study of Parents and Children (ALSPAC)68 and Research with East London Adolescents: Community Health Survey (RELACHS). 69 ALSPAC is a population-based cohort of the children of > 14,000 pregnant women who were enrolled in the study in Bristol in 1990–92. 68 The children in this cohort had nine clinical assessment visits between the ages of 4 weeks and 18 years. 68 The prevalence of overweight in ALSPAC was 15.9% and the prevalence of obesity was 5.2%. 70 The RELACHS data set contains information from an ethnically diverse (73% non-white) school-based cohort composed of nearly 2800 children aged 11–14 years from three deprived regional authorities in East London (Newham, Hackney and Tower Hamlets) collected in 2001–4. 69 The prevalence of overweight was 23% in boys and 21% in girls, and 14% of boys and 15% of girls were obese. 71
The cardiovascular risk model was developed in the ALSPAC data set owing to data availability. Because cardiovascular risk factors tend to cluster in children,72 the cardiovascular risk model estimated the risk of having one or more of the following abnormalities: elevated glucose levels (fasting glucose of ≥ 5.6 mmol/l73), elevated levels of low-density lipoprotein (low-density lipoprotein of ≥ 2.85 mmol/l74) or high blood pressure (systolic or diastolic blood pressure ≥ 95th centile for age, sex and height75). The emotional and behavioural difficulties model was developed in the RELACHS data set because of its rich data on mental health and well-being. Cases were assigned using responses to the Strengths and Difficulties Questionnaire (SDQ), a validated 25-item screening questionnaire widely used in the UK for assessing psychological distress in children and adolescents. 76,77 Children with emotional and behavioural difficulties were defined as those whose children’s SDQ score exceeded 17.5 points, a commonly used threshold indicating a raised probability of psychiatric disorder. 76,77
Stepwise logistic regression methods were used to identify risk factors to include in each risk prediction model, assessing the cross-sectional associations between predictors and outcomes in overweight and obese children. Basic characteristics (age, gender and BMI z-scores; BMIs of South Asian and black African-Caribbean children were adjusted to account for ethnic differences in adiposity at a given BMI78) were included in the models first, and additional variables that could be assessed in the primary care setting (including waist circumference, birth weight and family history of hypertension) were added or removed in a stepwise manner. The fit of the model was assessed after the inclusion of each variable using area under the curve (AUC) estimated from the receiver operating characteristic curve. Predictors that showed a statistically significant association (p < 0.05) with the outcome and an improvement in AUC were retained in the model. The only variable retained in the final cardiovascular risk estimation model was an age-, sex- and ethnicity-adjusted measure of BMI (ethnicity-adjusted BMI z-score) (p < 0.001); the risk of having cardiovascular abnormalities increased with increasing BMI z-score. For the emotional and behavioural difficulties model, the variables included in the final model were bullying status (p = 0.001), victimisation status (p = 0.003) and hours of sedentary behaviour (p = 0.002). The risk of emotional and behavioural difficulties rose with increasing hours of sedentary behaviour and if a child was bullied or victimised. Each risk estimation model estimated a risk score between 0 and 100:
The CATCH tool classifies a child’s risk score as low, medium or high by comparing the score to prespecified cut-off points. The range of risk scores that would classify a child as high risk for each comorbidity was chosen based on the prevalence of that outcome among overweight and obese children in the relevant data set. For example, if the prevalence of the outcome among overweight children was 10%, then the high-risk cut-off point was set so that the proportion of overweight children with scores that exceeded the cut-off point was 10%. The cut-off for low risk was chosen to maximise the negative predictive value of the model. Risk scores that fell between the low- and high-risk cut-off points were categorised as medium risk.
The prevalence of emotional and behavioural difficulties among overweight and obese children in the RELACHS cohort was 11.0%, and 47.0% of overweight and obese children in the ALSPAC cohort had at least one cardiovascular abnormality. We used these prevalences to choose the high-risk cut-off points for the risk estimation models. The low-risk cut-off points identified 18.0% of children as being at low risk for cardiovascular risk factors, and 26.1% as being at low risk for emotional and behavioural difficulties. Using these cut-off point criteria, children with cardiovascular risk scores of > 45.8 were classified as being at high risk of having one or more cardiovascular risk factors, whereas children with scores of < 39.5 were classified as being at low risk of the same. Children with emotional risk scores of > 20 were categorised as being at high risk of emotional and behavioural difficulties, whereas children with scores of < 7 were classified as being at low risk. The performance of each risk estimation model (sensitivity, specificity, positive predictive value, negative predictive value and AUC) was assessed in the data set from which it was derived.
Providing personalised lifestyle advice
An algorithm to provide tailored healthy lifestyle advice for children and their families was developed for the CATCH tool. To develop the algorithm, a literature review was conducted to identify modifiable lifestyle behaviours associated with childhood overweight. We then assessed the generalisability of the findings from the literature review to UK children through analyses of population-based data.
In the early stages of the literature review we identified an existing high-quality review on the topic, published in late 2007 by Davis et al. 79 We updated this review by performing a review of abstracts using the same search criteria: we searched the MEDLINE electronic database for all articles investigating lifestyle predictors of childhood obesity published between September 2007 and October 2011, using the search terms presented in Table 7.
| Concept | Search terms |
|---|---|
| Obesity/overweight | exp obesity/or exp body mass index/or overweight.mp. or obes*.mp. or body mass index.mp. or BMI.mp. or adipos*.mp. or fat mass.mp. or ponderal index.mp. or weight for height.mp. or skinfold thickness.mp. or weight gain.mp. or weight loss.mp. |
| Children | exp child/or exp adolescent/or juvenile/or exp pediatrics/or child*.mp. or adolescent.mp. or pre-adolescent.mp. or teen*.mp. or youth.mp. or juvenile.mp. or paediatric.mp. or pediatric.mp. |
| Physical activity | Exp physical exertion/or exp television/or exp computers/or exp sleep/or physical activity.ti,ab. or sedentary lifestyle. ti,ab. or television. ti,ab. or computer. ti,ab. or video games. ti,ab. or sleep. ti,ab. |
| Diet | exp caloric intake/or exp calorie/or exp fruit/or exp vegetable/or exp fast food/or exp meal/or energy intake.mp. or energy dense.mp. or calorie.mp. or fruit.mp. or vegetables.mp. or healthy eating.mp. or healthy diet.mp. or fast food.mp. or junk food.mp. or take away.mp. or vending machine.mp. or breakfast.mp. or portion size.mp. or serving size.mp. or snack*.mp. |
| Beverages | (exp beverage/or exp carbonated beverage/or exp water/or exp milk/or exp soy milk/or exp tea/or (drink or drinks).af. or juice$.af. or soda$.af. or lemonade$.af. or milk.af. or water.af.) |
A total of 7711 abstracts were screened and, of these, 225 were selected for review and data extraction. The following six lifestyle behaviours were consistently associated with childhood overweight and/or obesity: (1) skipping breakfast, (2) eating meals away from home (e.g. fast food and takeaways), (3) consuming sugar-sweetened drinks, (4) limited physical activity, (5) short sleep duration and (6) sedentary behaviour (most often assessed as daily hours of television or computer screen time).
To assess the generalisability of the literature review findings, lifestyle behaviours in the ALSPAC data set were analysed. The ALSPAC data set contained information on 17 lifestyle variables related to physical activity and diet in children aged 11 years, which was used to develop a multivariable linear regression model for BMI. All six of the lifestyle behaviours identified by the literature review were represented among the 17 variables used in modelling. Analyses were restricted to overweight and obese children. Regression models with 5–10 lifestyle variables were developed using stepwise methods to maximise R2 improvement and include variables with p < 0.05. The R2 values for models with 5–10 explanatory variables were compared with the R2 value of a model that included all 17 lifestyle variables. The final model was chosen to minimise the number of variables included, to maintain a R2 value close to the model including all lifestyle variables, and to include variables that could be measured in a primary care setting and are conducive to behaviour modification. Results from linear regression analyses were consistent with the findings of the abstract review; therefore, the six behaviours identified above (i.e. skipping breakfast, eating meals away from home, consuming sugar-sweetened drinks, limited physical activity, short sleep duration and sedentary behaviour) were included in the lifestyle advice generated by the tool.
For each lifestyle behaviour, an algorithm was generated to compare patient-reported information with recommended levels (recommendations from national guidelines or expert consensus80). If a child meets the target level, encouraging feedback is provided; if the recommended level is not met, the behaviour is identified as an area for improvement and lifestyle modification suggestions are provided. Information on local weight management services is also provided. The advice can be printed directly from the CATCH tool and given to the child and his or her family.
Online application development
The web-based CATCH tool was written using Ruby on Rails, an open-source web application framework for the Ruby programming language. It was designed to be accessed through standard internet browsers and incorporates secure tunnel technologies to allow secure data transfer and storage. The CATCH tool was designed to follow the standard flow of a clinical consultation and guide a health-care professional to record the child’s demographic information (e.g. age, sex, ethnicity) and take height and weight measurements. The tool computes the child’s BMI and age- and sex-specific BMI centile, displays a sex-specific BMI chart and the child’s weight status, identifies children for referral and provides lifestyle advice. Figures 5–7 show screenshots from a consultation using the CATCH tool to illustrate the main stages and user interface. The functionality and usability of the CATCH tool were pretested with anticipated end-users (primary care clinicians) during the web development stage using a paper-based version of the application.
FIGURE 5.
Screenshot of the BMI and height-for-age features of the CATCH tool, for a 12-year-old boy with a BMI of 24.9 kg/m2. Reproduced from Park et al. 51 Published by the BMJ Publishing Group Limited. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

FIGURE 6.
Screenshot of the risk assessment results for a 12-year-old boy with a BMI of 24.9 kg/m2, who is at high risk of cardiovascular comorbidity and medium risk of emotional and behavioural difficulties. Sources: unpublished data from the PROMISE programme; Dr Min Hae Park from the Department of Non-Communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK, personal communication; and reproduced from Park et al. 51 Park et al. 51 is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
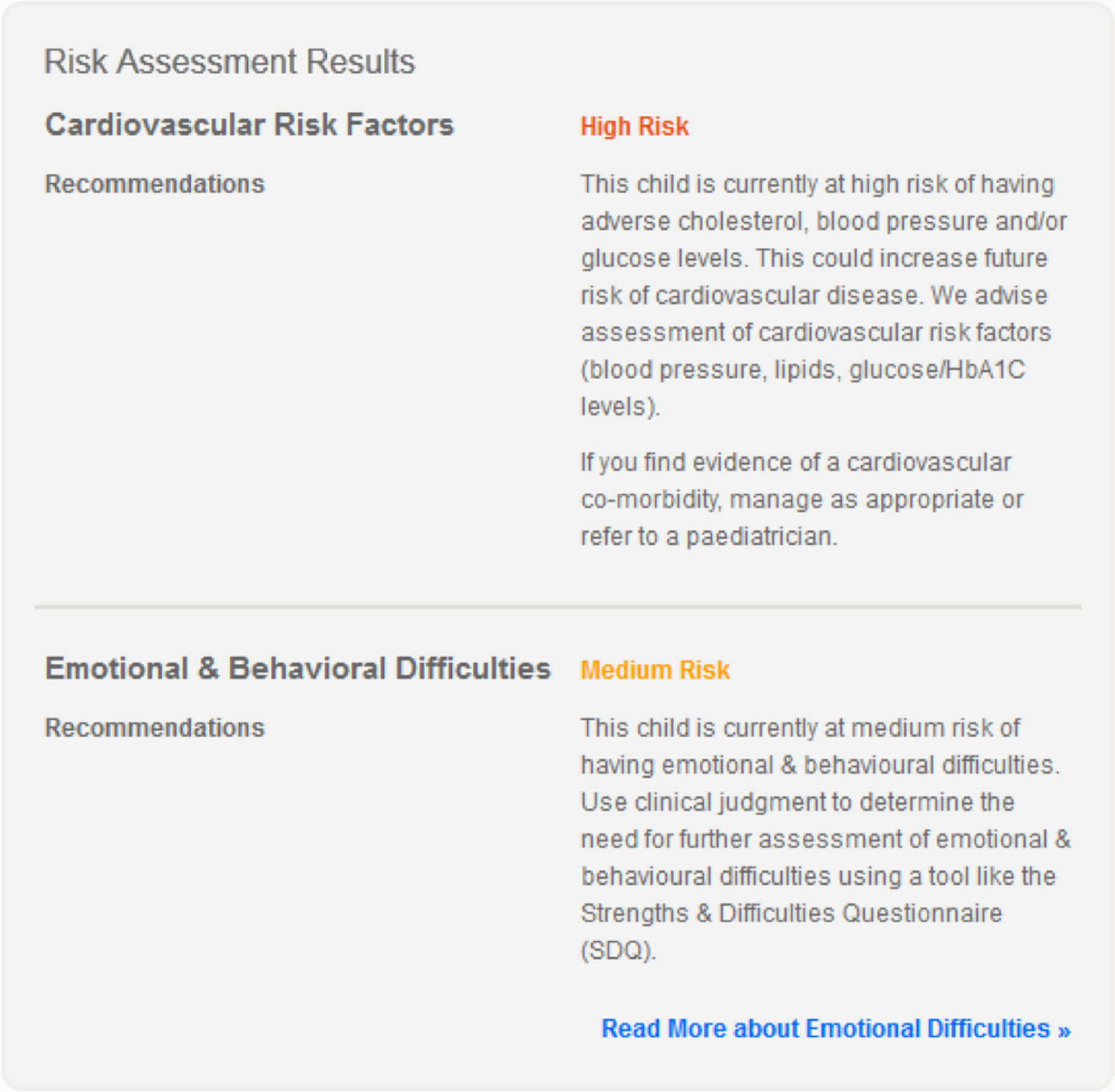
FIGURE 7.
Screenshot of an excerpt of the lifestyle assessment results for a 12-year-old boy who reports that he eats breakfast every day, eats meals away from home four times per week and drinks 22 sugary drinks each week. Sources: unpublished data from the PROMISE programme; Dr Min Hae Park from the Department of Non-Communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK, personal communication; and reproduced from Park et al. 51 Park et al. 51 is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Phase 2: evaluation of the Computer-Assisted Treatment of CHildren tool
Study design and recruitment
To evaluate the acceptability and feasibility of implementing the CATCH tool in primary care, we conducted an uncontrolled pilot intervention study with an integral process evaluation at three GP practices in north-west London. Practices mailed letters to the parents of all children aged 5–18 years on their registration lists (≈ 2600 children) inviting them to participate in the study. The study was approved by the National Research Ethics Service Committee of West London (reference number 11/LO/2049), the West London Primary Care Consortium and the London School of Hygiene & Tropical Medicine Research Ethics Committee (reference number 6220).
Participants
Families were eligible to participate if they presented at one of the three practices during the study period (November 2012–April 2013) with concerns about excess weight in a child aged 5–18 years. The child did not have to be clinically overweight or obese to take part. Families in which either the parents or child could not read English and families of children already receiving care for weight management were excluded. Of the 15 young people who attended the practices, only one did not meet the inclusion criteria (aged > 18 years) and did not participate in the study.
Intervention
The intervention was a single consultation with a general practice doctor (n = 2) or nurse (n = 2) using the CATCH tool. As described above, the main steps in the consultation were assessment of the child’s BMI centile and weight status, estimation of cardiovascular risk and emotional and behavioural difficulties, and lifestyle assessment and personalised advice.
Outcomes
The main outcomes of interest were family satisfaction with the CATCH-assisted consultation, practitioners’ satisfaction with the consultation and acceptability and usefulness of the intervention to families and practitioners.
Data collection
Practitioners were interviewed before the trial period to collect information on existing childhood obesity care, before implementation of the tool. Topics covered in the interviews included the number of families attending with weight concerns, normal practice in a consultation with an overweight child, treatment options and barriers to management. After the consultation, parents completed a self-administered 13-item questionnaire (see Report Supplementary Material 3). The questionnaire asked about the respondents’ satisfaction with the consultation and acceptability of the tool. Parents were also asked about the usefulness of the risk assessment and personalised lifestyle advice, and the quality of the care that they received. Practitioners completed an online questionnaire after each consultation. They rated the usefulness and ease of using the tool, and were asked whether or not the tool saved time and improved their ability to provide care, and whether or not they would recommend it to other health-care professionals. All questionnaire items were scored using four- or five-point Likert scales.
All participating families and practitioners were invited to take part in semistructured, face-to-face interviews that explored user experiences with the tool. Interviews with practitioners lasted, on average, 12 minutes (range 7–36 minutes), and were conducted at the general practice surgeries. Interviews with families lasted, on average, 12.5 minutes (range 9–17 minutes) and took place in participants’ homes. Interviewers used open questions and probes to explore the main themes in the questionnaires (i.e. acceptability, satisfaction, usefulness) and to capture emergent themes. Interview topic guides are provided in Appendix 3. All interviews were audio-recorded and transcribed verbatim.
Analysis
Questionnaire data were summarised using frequency counts and proportions. Family satisfaction was defined as the proportion of parents who were ‘very satisfied’ or ‘extremely satisfied’, and acceptability was defined as the proportion of parents who were ‘comfortable’ or ‘very comfortable’ with the consultation. Practitioners’ responses from their first completed questionnaire were analysed. We report the proportions that found the tool useful, were satisfied with the consultation and found the tool easy to use.
Qualitative data were analysed using NVivo software, version 10.0. The framework analysis approach was adopted,81 using both deductive and inductive methods. Transcripts were read and reread to identify a priori and emerging themes to be used as coding categories. Matrix-based thematic frameworks were developed (one for practitioners and one for families), into which the textual data were indexed and summarised by frequency for the purpose of interpretation.
Results
Performance of risk estimation models
Table 8 provides the risk scores (between 0 and 100) that correspond to each risk category and the performance measures for each risk estimation model, by risk category.
| Model outcome | Risk cut-off value | Risk category | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|
| One or more cardiovascular risk factors | 45.8a | High | 52.7 | 58.9 | 53.2 | 58.4 | 0.585 |
| 39.5b | Low | 85.5 | 21.9 | 49.3 | 63.1 | ||
| Emotional and behavioural difficulties | 20a | High | 23.3 | 94.9 | 36.2 | 90.8 | 0.712 |
| 7b | Low | 89.0 | 28.0 | 13.4 | 95.3 |
When tested in the databases from which they were derived, both risk prediction models had high sensitivity for identifying children (see Table 8) who are at risk of cardiovascular disease and are emotional and having behavioural difficulties. When used to identify high-risk children, the sensitivity of the models was lower: 23.3% of children who had emotional and behavioural difficulties were detected by the model and classed as being at high risk, and the cardiovascular risk factor model classified 52.7% of children with at least one cardiovascular risk factor as being at high risk. The positive predictive value of the cardiovascular risk factor model was moderate: just over half of children whom the model identified as being at high risk actually had one or more cardiovascular risk factors. The emotional and behavioural difficulties model had high negative predictive values for both risk categories, meaning that the vast majority (95.3%) of children whom the model identified as being at medium or high risk did have emotional and behavioural difficulties, and > 90% of children who were classified as not being at high risk did not have emotional and behavioural difficulties.
Pilot study
In total, 14 children from 12 families (including two sibling pairs) received the intervention. A family questionnaire was completed after each consultation (n = 14), and nine families (n = 11 children) were interviewed. One family did not consent to be interviewed, another could not be contacted and another missed two scheduled interviews and was assumed to no longer wish to participate. With the exception of one interview in which both parents participated, interviews were conducted in the presence of the mother and child; in both families with sibling pair participants, only one child was present during the interview. The characteristics of participating children are shown in Table 9.
| Characteristic | Mean (SD) or % (n) |
|---|---|
| Age (years), mean (SD) | 10.7 (2.6) |
| Sex: female, % (n) | 50 (7) |
| Ethnicity, % (n) | |
| White | 7.1 (1) |
| Asian | 64.3 (9) |
| Black | 28.6 (4) |
| Height (cm), mean (SD) | 148.6 (14.3) |
| Weight (kg), mean (SD) | 54.0 (12.5) |
| BMI (kg/m2), mean (SD) | 24.1 (2.3) |
| BMI z-score, mean (SD) | 2.25 (0.6) |
| Weight status,a % (n) | |
| Healthy weight | 7.1 (1) |
| Overweight | 25.7 (5) |
| Obese | 57.1 (8) |
Baseline interviews with practitioners
Five practitioners were interviewed before the trial period (three doctors and two nurses; one doctor who was interviewed at baseline did not take part in intervention delivery). Respondents reported that they saw very few children presenting with overweight or obesity as a primary health concern; numbers ranged from one or two children a month to 25 children each year. All practitioners reported that they did not normally measure BMI or conduct other tests, and they would give general advice about diet and physical activity rather than any specific treatment. However, uptake of lifestyle advice among patients was seen to be poor, and none of the practitioners routinely engaged in proactive follow-up because of the lack of response from patients.
Family questionnaire
Satisfaction and acceptability
Among respondents to the family questionnaire, 86% (n = 12 out of 14) were satisfied with the tool-aided consultation. The remaining respondents were ‘somewhat satisfied’. All parents reported that they and their child felt comfortable with the consultation. All parents were comfortable being asked about their child’s lifestyle and medical history, but one parent was ‘slightly uncomfortable’ during assessment of emotional and behavioural difficulties when asked about whether or not their child had been teased or bullied.
Usefulness and quality of care
The majority of respondents (79%, n = 11) found it useful to receive personalised feedback on weight management; 21% (n = 3) found it ‘somewhat useful’. All parents reported that their child was (1) asked questions about his/her health habits, (2) helped to set goals to improve lifestyle and (3) given a copy of their treatment plan. All parents agreed that they were treated with care and concern, their child’s care was well organised and they had confidence and trust in their practitioner.
Family interviews
Table 10 shows the frequency of the main themes in the analytical framework. In line with the questionnaire responses, family interviews revealed that experiences with the CATCH tool were generally positive.
| Themes | Number of participants |
|---|---|
| Reasons for consultation | |
| Parents’ concern about child’s weight | 6 |
| Child’s concern about her/his weight | 1 |
| GP/nurse advised them to | 2 |
| Expectation of free practical support (with weight issues) | 1 |
| Acceptability | |
| Parents responded positively to tool-based consultation (e.g. with interest) | 10 |
| Children responded with apprehension to tool’s outputs | 1 |
| Problems with the risk assessment feature | 0 |
| Reasons for perceived usefulness | |
| Generally useful | 12 |
| Informative | 5 |
| Advice is instructive | 5 |
| Impact of lifestyle advice | |
| Dietary changes | 4 |
| More physical activity | 3 |
| Weight loss | 1 |
| Go to sleep earlier | 1 |
| Lifestyle changes for whole family | 2 |
| Satisfaction | |
| Overall satisfied | 12 |
| Would recommend it to others | 8 |
| Reasons for (dis)satisfaction | |
| Generally satisfied | 12 |
| Revelatory: ‘wake-up call’ | 1 |
| Questions’ format is sermonic in places | 1 |
| Delivery of advice could have been more assertive | 1 |
| Recommendations | |
| Have follow-up consultations/practical support (including monthly weight targets) | 5 |
| Address psychological issues | 1 |
| More tailored question format | 1 |
Parents described the consultation as informative, non-judgemental and non-intrusive:
I think the questions were very good because there are a lot of things that we were doing and we didn’t know that it was good or bad. It was good. The whole consultation was very beneficial.
Mother of boys aged 10 (obese) and 12 (overweight) years
All interviewees found the tool’s outputs useful. Even though most parents had been concerned about their child’s weight previously, several referred to the consultation as a ‘wake-up call’ that alerted them to the severity of the problem:
. . . I would know that [my daughter was] putting on weight but I would not say, I didn’t expect that it was as bad as it was and especially when they weighed her and they found she was 61 [kg] when she’s 9. It was a wake-up call. It was good.
Mother of girl aged 9 years, obese
However, two mothers described that the results of the consultation had generated some anxiety in their child:
. . . when she saw what was on the computer, that she was on the red line, and they explained to her what it means for her health . . . she really [got] scared.
Mother of girl aged 9 years, obese
[My son] was a little bit worried [when hearing the results of the consultation] like, ‘Oh what’s going to happen next?’. [But in the end] everything was fine.
Mother of boy aged 9 years, obese
All of the respondents found the lifestyle advice informative and instructive. In particular, specific advice on diet was highlighted as being useful:
[I found the lifestyle advice] very, very helpful because I was buying some stuff, like, I wanted them to eat breakfast, they eat breakfast every morning, but I was buying them wrong breakfast, the cereals . . . You know the sugary stuff so now . . . I’ve cut down on those things and the drinks that I was buying, I thought they are orange juice and stuff but [it’s] not 100% juice . . .
Mother of boys aged 10 (obese) and 12 (overweight) years
The use of visual aids, such as the BMI chart and the printed lifestyle advice, was described as reinforcing the advice given:
It just confirmed everything that we already knew, but you know, when it’s on paper, it’s sort of a bit more . . . I wouldn’t say serious, but it’s a bit more in your face.
Mother of boy aged 7 years, overweight
Five interviewees said that they had made lifestyle changes following the tool-based consultation. In at least two cases, changes had been introduced for the whole family. A theme that emerged was the need for practical support following the consultation. Five parents suggested that follow-up appointments for monitoring and guidance would be beneficial:
I would probably say that now, as a stepping-forward thing, although the study thing may be over, maybe to follow up and do like a plan on how to maintain or reduce the weight or some kind of thing like that . . . To be honest, I don’t mind doing it for myself but then there’s no monitoring or anything like that through the GP, which might be beneficial for them as well so they can keep an eye on whether we’re doing it correctly and there’s no other side effects or anything like that.
Mother of boy aged 7 years, overweight
Practitioner questionnaire
Satisfaction
All practitioners (n = 4) were satisfied with the consultation. Three practitioners indicated that they would recommend the tool to other health professionals; one was not sure whether or not they would recommend it.
Usefulness and ease of using the tool
Two respondents reported that the tool was useful during the consultation; two found it ‘somewhat useful’. All practitioners reported that the tool was easy to use. Three respondents agreed that using the tool saved them time; one respondent ‘slightly agreed’. The same three respondents also agreed that using the tool improved their ability to care for the child.
Quality of care
All respondents reported that they felt confident of their skills and knowledge during the consultation. All practitioners felt that they provided the patient with appropriate treatment, had contributed to patient well-being and had provided well-organised care.
Practitioner interviews
The analytical framework used for practitioner interviews is shown in Table 11.
| Themes | Number of participants |
|---|---|
| Feasibility | |
| Overall electronic consultation delivery: unproblematic | 4 |
| Output interpretation: unproblematic | 4 |
| Acceptability | |
| Patients’ overall response to tool | |
| Patients responded positively to tool-based consultation (e.g. with interest) | 3 |
| Children responded with indifference to tool’s outputs | 1 |
| Left wanting for more detailed advice or tangible/practical support | 2 |
| Parents showed more concern than children | 1 |
| Patients’ responses to individual features | |
| Found BMI chart revelatory | 2 |
| Found lifestyle advice helpful/informative | 3 |
| Found lifestyle advice sermonic | 1 |
| Parents seemed uncomfortable with emotional risk assessment questions | 2 |
| Reasons for (dis)satisfaction | |
| Generally satisfied | 4 |
| Provides BMI chart tailored to children | 2 |
| Provides printouts with lifestyle advice | 3 |
| Gives opportunity to discuss weight issues and lifestyle choices | 3 |
| Provides enquiries not normally covered in routine consultations | 2 |
| Provides ‘authoritative’ information (empowering to staff and parents) | 1 |
| Question format can be perceived as overly sermonic | 1 |
| Recommendations | |
| Integrate tool into clinical software system | 4 |
| Have follow-up consultations/practical support (including monthly weight targets) | 3 |
| Redesign lifestyle advice to speak to youth | 1 |
| More concise question format | 1 |
Overall, practitioners described satisfaction with the tool. A theme that emerged was that the tool could enhance the impact of practitioners’ advice by adding authority to the message:
. . . I thought that was really good. We talked about it all the time. And because patients can take it away with them, it is quite good for them. I literally go over what the [lifestyle advice] told them: what you should eat [etc.]. And they got something in writing from the computer. They see ‘I’m not just saying that’ . . . that [there is truth] to it.
Nurse, practice 3
All practitioners found the tool straightforward to use, and none reported having problems interpreting the tool’s outputs. One respondent (doctor, practice 1) felt that children reacted to the consultation with indifference, but others felt that patients reacted positively. The BMI chart and lifestyle advice were highlighted as generating the strongest responses from children and parents:
I think for some people they probably underestimated the time that their kids were spending watching TV or playing games, or computer games or whatever. So that was good. The thing about sleep was good. How much sleep that they need on average, was good . . . The other thing was about fruit juices. Sugar. I think that was a revelation to a lot of people. I think, you know, there was nothing negative about it [the tool].
Doctor, practice 2
Although none of the practitioners described serious problems with the tool, two respondents described that it can be uncomfortable to broach the subject of a child’s weight-related health risk with parents:
[The risk assessment component] was tricky as far as the parent’s concerned . . . The parents found it uncomfortable. Maybe it opened something up for them to say, you know, . . . ‘Is this going on [with my child]? Am I not being told?’
Nurse, practice 2
All four respondents were in agreement that the tool (or a version of it) would be something that they would continue to use in the future and would like to see integrated into their clinical software system. In particular, the child-specific BMI chart was seen to be desirable, as current tools on the system allow calculation of only adult weight status. Three of the four respondents suggested that the tool-based consultations would benefit from the addition of more tangible support, such as a structured programme of activities and follow-up consultations to monitor weight management.
Discussion
The CATCH tool is an online tool that was developed to facilitate the treatment of overweight and obese children in primary care. Using risk estimation and stratification methods to identify children likely to need referral to secondary care and algorithms that enable clinicians to provide families with tailored lifestyle advice, the CATCH tool can support clinicians in making evidence-based referral decisions and managing overweight children in primary care. Compared with other electronic tools for treating overweight children in primary care, the CATCH tool has the additional features of predicting individual risk of common comorbidities and providing personalised lifestyle advice (Table 12).
| Tool technology/type | Risk prediction models? | Referral feature? | Tailored lifestyle advice? |
|---|---|---|---|
| Electronic medical record with BMI percentile calculator (40) | N | N | N |
| Bristol Online Obesity Screening Tool (41) | N | Y | N |
| HeartSmartKidsTM (42) | N | N | Y |
| CATCH tool | Y | Y | Y |
The findings from our evaluation study indicate that CATCH can feasibly be implemented in primary care. Overall, family and practitioner experiences with the tool-assisted consultations were positive. The majority of families were satisfied with their consultation and found the personalised weight management advice useful, particularly in relation to diet. Practitioners reported that they did not routinely assess or treat childhood obesity pre intervention; they all found the tool easy to use and time-saving, and most reported that they would recommend it to other health professionals. Further development to integrate the tool into current clinical software systems may be desirable.
A common theme that was identified by families and practitioners was the increased impact of practitioners’ advice due to the use of visual aids and the perceived authority of the tool. This is consistent with previous studies that have indicated that appropriate visuals can help individuals to understand health risks. 82 In addition, personalised estimates of risk have been shown to be effective tools for increasing knowledge and improving risk perception among patients. 83 Most parents made lifestyle changes for their child following the tool-based consultation, and in some cases extended these changes to the whole family, pointing to potential health benefits of the tool beyond those for the overweight child.
Parents and practitioners highlighted the need for follow-up and structured support for children identified as overweight or obese. Follow-up care is likely to be an important factor in successful weight management; therefore, adequate care pathways need to be in place before assessment tools are implemented on a large scale, and further work is needed to establish the impact on health outcomes. 63 Concerns about the tool were related to anxiety in children, and practitioners feeling uncomfortable discussing weight-related health problems with parents, which point to the importance of training for practitioners to deliver sensitive information in a supportive and non-judgemental manner. Despite these concerns, all parents reported that their child felt comfortable with the consultation and most practitioners felt that the tool improved their ability to care for the child.
Strengths and limitations
The development of the CATCH tool was informed by the evidence base: risk prediction models were based on analyses of large, well-established, population-based cohorts, whereas the lifestyle behaviours for which healthy recommendations were provided were identified through comprehensive literature reviews, and the generalisability of findings was confirmed in analyses of a UK data set. The tool was also extensively piloted among future user groups in order to obtain feedback relevant to analysis, design and development. Limitations of the CATCH tool include features of the data sets in which the risk estimation models were created: the cardiovascular risk factor model was developed within the ALSPAC data set, which comprises data from a relatively healthy population with a low prevalence of overweight and ethnic minority groups. Similarly, the emotional and behavioural difficulties model was developed in the RELACHS data set, which comprised mainly deprived children of ethnic minorities. For both data sets, data were available for adolescents only; therefore, the validity of the prediction models in younger children is unknown. AUC values indicated that the cardiovascular risk factor model was not good at correctly classifying those with and without elevated risk. However, when compared with similar measures for algorithms designed to detect children at risk of glucose intolerance84 or having atherosclerotic lesions,85 specificity was comparable and positive predictive values were higher, although sensitivity was lower. Further validation of the models in larger, more diverse data sets is required. Statistical analyses of lifestyle behaviours in the ALSPAC data set showed that, even though the six chosen behaviours explained a similar amount of variation as a model with all 17 lifestyle behaviours, lifestyle behaviours in general explained very little (7–10%) of the observed variations in BMI among overweight and obese children. This could indicate that most of the variation in the degree of overweight is actually due to unknown or unmeasured factors.
The generalisability of findings from the pilot study is limited by its small sample, which is not representative of the wider target population. In particular, owing to the ethnically diverse population in north-west London, the majority of participants in our study were from Asian or black ethnic groups (in the boroughs of Harrow and Brent, around two-thirds of the population are from black and minority ethnic communities, predominantly South Asian groups86). However, children from these ethnic groups are at increased risk of obesity87 and associated health problems39 and may therefore be priority groups for obesity interventions. Parents and practitioners who participated in the study are likely to be those who are engaged with weight-related health issues; experience of the tool-based consultation may be different if delivered by practitioners, or administered to families, with less interest in these issues. We did not collect data on parental weight and, thus, could not comment on implications of parental weight for management of child obesity. We did not collect data on disordered eating behaviour in this small pilot; this would be useful in a future larger evaluation.
Despite these limitations, the evaluation has identified key themes relating to the feasibility, acceptability and usefulness of an online tool for the assessment and management of overweight children in primary care. The responses from families and practitioners are promising and provide a basis for further intervention development and evaluation in a larger study.
Conclusions
The CATCH tool is a simple online tool that provides primary care providers with evidence-based guidance on how to treat and appropriately refer children who are overweight. A pilot evaluation has indicated that the tool can feasibly be implemented in primary care and that tool-assisted consultations are acceptable to the families of overweight children and practitioners. Further work is required to evaluate the utility of the tool for practitioners and its effectiveness in prompting behaviour change. It will be important to examine whether or not the tool has utility in wider community primary care beyond general practice, including with school nurses. Further evaluation should take the form of a randomised pilot trial to understand potential effect sizes and inform any future full trial.
Chapter 4 Study C: the Healthy Eating and Lifestyle Programme randomised clinical trial
Some parts of this chapter have been reproduced from Christie et al. 88 © Christie et al. ; licensee BioMed Central Ltd, 2011. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Healthy Eating and Lifestyle Programme trial outcomes summary
A community-based motivational personalised lifestyle intervention to reduce body mass index in obese adolescents: results from the Healthy Eating and Lifestyle Programme randomised controlled trial
The trial was conceptualised by Deborah Christie and Russell M Viner. The intervention was developed by Deborah Christie with input from Russell M Viner. Russell M Viner and Deborah Christie developed the trial design, with input from Sanjay Kinra, Ian C Wong, Irwin Nazareth and Tim J Cole. Tim J Cole and Ulla Sovio developed the statistical plan, with input from Russell M Viner and JG. Anthony S Kessel, Anne Mathiot and Lee Hudson contributed to the trial design and writing of the protocol. Steve Morris and MP contributed to the trial design and undertook the economic analysis. RH supervised the providers and analysed the fidelity data and process evaluation. All authors read and approved the final manuscript.
Abstract
Background
Approximately 7% of CYP aged 5–15 years in the UK have obesity at a level likely to be associated with comorbidities. The majority of multicomponent lifestyle modification programmes, as recommended by NICE for childhood obesity, have limited applicability and generalisability for British adolescents. The Healthy Eating and Lifestyle Programme (HELP) was developed as a specific adolescent-focused intervention, designed for obese 12- to 18-year-olds seeking help to manage their weight. Rather than focusing only on weight, HELP uses motivation to change techniques, developing self-efficacy and self-esteem.
Methods
A randomised controlled trial (RCT) of HELP was undertaken, consistent with a Phase III trial in the Medical Research Council (MRC)’s guidance on complex interventions. 89 Participants were individually randomised to receive either the 12-session HELP intervention or defined standard care for 6 months. The primary outcome was the difference in mean BMI (kg/m2) between groups at the end of the intervention (week 26), adjusted for baseline BMI, age and sex.
Results
A total of 174 subjects were randomised (87 in each arm), of whom 145 (83%) provided primary outcome data at week 26. The median number of HELP sessions attended was 10 out of 12. Sixty-nine young people (79%) in the intervention arm met the criteria for compliance. Fidelity of delivery across all sessions was 2.7 out of 3.0, with 76% of sessions rated as good, 22% as adequate and 2% as poor. At week 26, there were no significant effects of the intervention on BMI (mean change in BMI was 0.18 kg/m2 for the intervention arm and 0.25 kg/m2 for the control arm; the adjusted difference between groups was –0.11 kg/m2, 95% CI –0.62 to 0.40 kg/m2; p = 0.7). At weeks 26 and 52, there were no significant differences between groups in any secondary outcomes, nor at 52 weeks when adjusted for baseline characteristics.
Conclusion
The HELP intervention was no more effective than a single educational session for reducing BMI in a community sample of obese adolescents. This may reflect a mismatch between the intervention, based on evidence developed largely from affluent white populations, and the ethnically diverse and highly deprived community sample recruited, in terms of both content and intensity. The study has significant implications in terms of informing public health interventions to tackle childhood obesity. The trial registration number is ISRCTN99840111.
Background
Approximately 7% of CYP aged 5–15 years in the UK have obesity at a level likely to be associated with comorbidities. 1 Over 1% of adolescents have extreme obesity, with a BMI of > 3 standard deviations (SDs) above the mean, equivalent to a BMI of approximately 35 kg/m2 in adults. 90 The treatments for childhood obesity recommended by NICE are multicomponent lifestyle modification programmes. 11 The majority of published programmes are, however, aimed at primary school children, with existing adolescent studies largely carried out in non-UK academic tertiary care centres with highly specialised staff involving white, middle-class, motivated families. 42,91 The applicability and generalisability of these studies for British adolescents, therefore, is limited, and reviews42,91 conclude that there is a need to conduct adequately powered high-quality trials in representative populations with integral process evaluation and appropriate lifestyle tools.
Major cognitive and psychosocial changes during adolescence mean that children’s family-centred weight management programmes are inappropriate for adolescents. During adolescence, young people spend more time outside the home, start making their own choices about food and exercise, and develop diet and activity patterns that will stay with them into adult life. Issues related to body image and self-esteem become important, and behaviour change depends as much on individual motivation as parental authority. Yet few weight management programmes recognise these issues, as programmes are generally designed for younger children or older adults.
The Healthy Eating and Lifestyle Programme was developed as a specific adolescent-focused intervention, designed for obese 12- to 18-year-olds seeking help to manage their weight. HELP was developed from best practice,11 and is a multicomponent intervention focused on enhancing motivation to change and developing self-efficacy and self-esteem (rather than focusing on weight only). Unpublished pilot data from application of HELP by psychologists in a clinical setting for 20 subjects aged 13–17 years showed a mean BMI reduction of 1.7 kg/m2 over 6 months, equivalent to a 0.4 SD effect size. We report a RCT of HELP in a community sample of obese adolescents. The primary aim was to assess whether or not HELP delivered by graduate mental health workers in the community was more effective in reducing BMI in obese adolescents than enhanced standard care. Secondary aims were to assess the cost-effectiveness of HELP and its impact on cardiometabolic risk and psychological function.
Methods
Design
A randomised efficacy trial of HELP was undertaken, consistent with a Phase III trial in the MRC guidance on complex interventions. 92 Subjects were individually randomised to receive either the HELP intervention or enhanced standard care for 6 months. Details of the HELP trial methodology are available in the published protocol. 88
Intervention
The Healthy Eating and Lifestyle Programme is a family-based weight management programme for adolescents. Young people and at least one parent were invited to attend 12 fortnightly sessions, each lasting approximately 60 minutes, delivered over 6 months. A key aspect of the programme was the use of motivational interviewing93 and solution-focused approaches94 to increase engagement and concordance. The programme consists of an introductory session plus four modules of 2–4 sessions each:
-
‘where and how we eat’ (two sessions) – modifying eating behaviours and encouraging regular eating patterns
-
‘what we do’ (two sessions) – decreasing sedentary behaviour and increasing lifestyle and programme activity
-
‘what we eat’ (four sessions) – reducing intake of energy-dense foods, and increasing healthy nutritional choices
-
‘why we eat’ (three sessions) – addressing emotional eating triggers.
Young people were invited to choose the order in which the modules were delivered. The intervention was delivered by psychology graduates (i.e. those graduates who had completed an undergraduate degree in psychology) who had completed a 5-day training programme that included information on obesity, good clinical practice, motivational interviewing techniques and solution-focused questioning used in the programme. A manual written by Deborah Christie was used to enable delivery in a standardised manner by all the providers. The intervention was delivered in community settings local to the young people.
Standard care
Control arm participants were offered enhanced standard care, defined here as one standardised educational session on obesity self-management delivered to young people by a primary care nurse within 3 months of recruitment. The session was designed to last for 40–60 minutes and incorporated standard DHSC guidance and published information on obesity. The session provided information that addressed eating behaviours, healthy activity levels and healthy eating patterns. No training in delivery style was offered. The session was delivered in the participant’s general practice or an alternative community setting. Where a practice nurse was not available, a trained nurse practitioner was provided by the study team to deliver the session. Each nurse was required to return a brief questionnaire to ensure fidelity of information-giving and to monitor whether or not practitioners used motivational or other techniques that were not part of the control session.
Sample and recruitment
Recruitment began in January 2011 and finished in July 2013. The eligibility criteria were initially defined as obese young people (i.e. aged 13–17 years) with a BMI > 98th centile for age and sex in accordance with the UK 1990 growth reference,35 recruited from primary care and community settings within the Greater London area. Exclusion criteria were as follows:
-
patients with diagnosed significant mental health problems or undergoing mental health treatment
-
patients with chronic illness (apart from asthma, unless it was severe and required excessive doses of regular steroids), known secondary obesity or monogenic obesity syndrome or using medications known to promote obesity, and those with a BMI of ≥ 40 kg/m2, who were considered likely to need a more clinical intervention
-
patients with a significant learning disability or those with lack of command of English sufficient to render them unable to participate effectively in the planned intervention
-
patients who had participated in formal behavioural weight management programmes in the previous 12 months.
Owing to slow recruitment, the criteria were widened in March 2012 to a revised definition of obesity, that is a BMI > 95th BMI centile for age and sex based on the UK 1990 growth reference, and to accept those with a BMI < 45 kg/m2. The age range was also widened to 12–19 years to facilitate recontacting young people who were previously declined participation. These protocol changes were agreed by the ethics committee and registered with the International Standard Randomised Controlled Trial Number (ISRCTN) registry.
Recruitment was conducted through a range of mechanisms:
-
General practices, contacted through the local Primary Care Research Network (PCRN), particularly in areas with a known high prevalence of obesity. We asked GPs to identify and refer eligible subjects from existing databases; GPs sent study information packs asking potential participants to contact the research team if they were interested in participating.
-
Other professionals, including paediatricians, school nurses, pharmacists and dietitians, were asked to refer any eligible subjects presenting to them during the study period.
-
Advertising through community media, newsletters, community youth groups, secondary schools and colleges.
-
Advertising through social media and the study website.
Interested young people and families contacted via these routes were provided with age-appropriate information sheets, approved by the ethics committee, and the same information was used in advertising.
Those interested in entering the study and judged potentially eligible were sent an information pack and invited to attend with a parent for a baseline assessment when written consent was obtained. Baseline assessments were undertaken before randomisation at NIHR Great Ormond Street Hospital Clinical Research Facility (CRF) using a standardised protocol by a paediatrician (LH) and CRF nurses. Eligibility status was also checked.
Participants who enrolled were given a £20 iTunes (Apple Inc., Cupertino, CA, USA) or high-street store voucher at entry and again at completion, and were reimbursed for travel costs.
Randomisation
Participants were randomised after baseline assessment into the intervention or control arms independently of the investigators by the Health Services Research Unit at the University of Aberdeen. Allocation was 1 : 1 to the two arms and balanced for sex.
Outcomes
Outcomes were measured for all participants at baseline (week 0), mid-treatment (week 13), end of the intervention (week 26) and 6 months post intervention (week 52) (Table 13 presents details on data collected at each time point). Outcomes were assessed by clinical assessment, venepuncture and completion of psychological questionnaires by young people and parents. The primary outcome (i.e. BMI, calculated from measured height and weight) was assessed by trained CRF nurses who were blinded to the treatment status of the participant.
| Data | Time point | |||
|---|---|---|---|---|
| Week 0 | Week 13 | Week 26 | Week 52 | |
| Anthopometry | ✓ | ✓ | ✓ | ✓ |
| Motivation | ✓ | ✓ | ✓ | |
| QoL measure | ✓ | ✓ | ✓ | |
| Blood pressure | ✓ | ✓ | ✓ | ✓ |
| Venepuncture | ✓ | ✓ | ||
| Psychological function | ✓ | ✓ | ✓ | |
| Accelerometry | ✓ | ✓ | ||
Primary outcome
The primary outcome was the difference in mean BMI (kg/m2) between groups at the end of the intervention (week 26), adjusted for baseline BMI, age and sex.
Secondary outcomes
-
Anthropometric measures:
-
BMI at week 52.
-
BMI SD score (zBMI) at weeks 0, 26 and 52.
-
Waist circumference at weeks 0, 26 and 52.
-
Non-invasive measurement of fat mass and fat mass percentage using Tanita 418 bioimpedance scales (Tanita Europe B.V., Amsterdam, the Netherlands). Fat mass (kg) was calculated from the impedance value using the formula validated in obese adolescents,95 and fat mass percentage was calculated by dividing fat mass by body mass.
-
-
Health-related quality of life was assessed using the Impact of Weight on Quality of Life (IWQoL)-Kids,96 a 27-item instrument consisting of four scales: (1) physical comfort (six items), (2) body esteem (nine items), (3) social life (six items) and (4) family relations (six items). 96 Young people and parents completed separate IWQoL questionnaires referring to the young person. Initially, it was also planned to use the Pediatric Quality of Life Inventory™ (PedsQL), as outlined in the protocol, but this was removed after early piloting to reduce the number of questionnaires and decrease client and researcher burden.
-
Psychological factors were measured using:
-
Eating Attitudes Test, a validated self-report 26-item checklist of issues relating to eating disorder and body image. 97 It provides a total score over three subscales: (1) dieting, (2) bulimia and food preoccupation and (3) oral control.
-
Rosenberg Self-Esteem Scale – the most widely used measure of global self-esteem in adolescents. 98
-
Psychological health was measured using the online version of the Development and Well-being Assessment (DAWBA) interview, which generates likely psychiatric diagnosis at the > 50% likely level. 99 It was intended to be completed online confidentially before or during the assessment. Although it was collected reasonably well at baseline, data were missing at follow-up in > 50% of cases. For this reason, the effect of the intervention on DAWBA outcomes could not be assessed.
-
-
Physical activity: physical activity was assessed using an ActiGraph (ActiGraph, LCC, Pensacola, FL, USA) accelerometer 7-day measurement.
-
Cardiometabolic risk factors. Young people underwent a clinical examination and venepuncture after an 8-hour fast, which measured:
-
fasting insulin (mU/l) and glucose (mmol/l)
-
fasting lipids (mmol/l) – high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides
-
peripheral, seated blood pressure measured using an electronic DINAMAP (GE Healthcare, Chicago, IL, USA) after 20 minutes’ rest.
-
-
Health economic outcomes. Data were obtained on preference-based HRQoL, resource utilisation and costs to inform a cost-effectiveness analysis, which will be reported elsewhere.
Demographic and clinical data were collected:
-
Deprivation – socioeconomic status was assessed using the 2010 IMD for the participant’s postcode. 100 This is a standard small-area measure of deprivation. Scores were ranked into IMD quintiles allowing comparison with the whole of England. 30
-
Data were also collected on ethnicity, medical history, pubertal status (self-report) and weight management history.
Data collection
Outcome data were collected during attendance at the CRF. When subjects were unable or unwilling to attend, a standardised protocol was followed to offer home or telephone assessments. The aim of the home visit was to prioritise the collection of primary outcome data (i.e. BMI). The visits were completed by researchers trained to assess height and weight using portable instruments [Seca 875 Flat Scales for Mobile Use (Seca GmbH & Co. KG, Hamburg, Germany) and Seca Leicester Height Measure (Seca GmbH & Co. KG)]. Sagittal abdominal diameter was measured using a Holtain Kahn Abdominal Calliper (Seritex Sprl, Wavre, Belgium). Blood pressure was measured, and young people were invited to provide morning and evening salivary cortisol samples, a urine sample and self-report pubertal staging. They were also asked to complete the psychological questionnaires, the health-care usage questionnaire and a brief smoking questionnaire. Serious adverse events were monitored by the research team and reported to the steering committee.
Power and sample size
Our pilot data identified a likely effect size of a 1.7 kg/m2 reduction in BMI. Lifestyle modification programmes in obese children result in improvements in HRQoL of 0.3–0.5 SDs. 101,102 It was calculated that a sample size of 126 subjects would be required to detect a reduction in BMI of ≈ 1 kg/m2 (equivalent to CD 0.5) with 80% power at 5% significance. Initally, the sample size was inflated to account for clustering as a result of persistent therapist effects,103 assuming a therapist cluster size of 10.0 in both arms and an interclass correlation coefficient of 0.025, inflating the size to 126 × {1 + [(10 – 1) × 0.025]} = 155. To allow for 20% dropout, the initial aim was to recruit 200 subjects.
In June 2012, the required sample size was recalculated using within-study data on therapist cluster size and dropout rates. The mean therapist cluster size was < 4.0 in the intervention arm and all participants bar one had a different nurse provider in the control arm. The dropout rate had been 14%. Using conservative cluster sizes of 4.5 for the intervention arm and 2.0 for the control arm and a dropout rate of 14%, it was planned to recruit at least 155 subjects in order to retain 80% power at a 5% significance threshold. These changes were agreed with our Data Monitoring and Ethics Committee, Trial Steering Committee and funder.
Fidelity and compliance
Programme compliance was predefined as attendance at the first session, ‘setting the focus’, plus five or more of the 12 HELP sessions. Adherence to the programme by intervention providers was measured by self-assessment checklists, live observations and audio-recordings of a quasi-random subsample of sessions. As part of the training and supervision process, a qualified clinical psychologist observed each provider when delivering session 1 of the intervention with their first client. The remaining 11 sessions for each provider’s first client were then rated from an audio-recording. For providers still delivering the intervention after completion of their first client (at 6 months), a second set of ratings for a further participant was completed. A Fidelity Adherence Scale based on the Motivational Interviewing Supervision and Training Scale (MISTS)104 was used to assess fidelity to delivery of session components and adherence to the psychological model. Each session was rated by the observer on competence and frequency of delivery of desired motivational language and methods from 1 (poor) to 3 (high).
Participant experience
Twenty-three young people agreed to take part in a semistructured interview after they had completed their 26-week medical assessment. These participants were sampled to include a range of participants (male and female across three different age categories: 12–14 years, 15–16 years and ≥ 17 years). Participants were selected to reflect the number of young people in each of these age categories across the sample as a whole. Interviews took place at the young person’s home, at Great Ormond Street Hospital or at their local community health clinic. If a parent was available at the interview, they were also invited to take part. Fifteen parents/carers took part in the interviews.
Semistructured interview guides with open-ended questions were constructed in order to understand and explore the young person’s and the parent/carer’s experience of HELP. Questions were guided by an evaluation framework in order to elicit information about the structure, process and outcome issues. The interviews were conducted by independent trained researchers. Informed consent was sought from both the young people and their parents. Each interview lasted ≈ 20 minutes. The interviews were audio-recorded and transcribed verbatim.
Analysis
Analyses were by intention to treat (ITT). For the primary outcome, BMI at 26 weeks was compared between treatment groups using a linear regression model adjusted for age, sex and baseline BMI. A random intercept was fitted at the therapist level to allow for persistent therapist effects. In order to adjust for biases caused by missing data, the primary analysis used multiple imputation with chained equations to impute missing BMI measurements at 6 or 12 months, under the assumption that data were missing at random. An imputation model with 20 imputations containing age, sex, baseline BMI, treatment group and the number of attempts to contact the participant was used. Secondary analyses included only participants with available outcome data at baseline and 6 or 12 months (see Table 14).
To estimate the difference in mean outcomes among compliers, a complier-average causal effect model was used. 105 This inflates estimates from ITT analyses to adjust for non-compliance, thereby avoiding problems caused by patient selection in per-protocol or on-treatment analyses. Compliance was defined as above. Secondary outcomes were analysed using similar methodology. When outcomes were binary or ordinal, logistic or ordinal logistic regression models were used. For highly skewed continuous variables, robust standard errors were used. All analyses were performed using Stata, version 13.0.
Process evaluation
A thematic framework method was conducted on all transcripts. Data were entered into the NVivo qualitative software package. The thematic analysis followed three phases. Phase 1 involved combing through each interview for key words and concepts by the primary coder. Phase 2 involved collating the separate interviews and joining all the highlighted quotes and phrases together into categories, key words and concepts. These key words and concepts were further developed in discussions between two researchers who independently conducted their own reading of the data and discussed the analysis with the intention of creating a solid inter-rater reliability. Phase 3 involved choosing key quotations that were considered to be the best examples of each subtheme. These data were then used to develop a number of subthemes and a number of key overarching subthemes. The analysis aimed to identify aspects of structure process and outcome across all participants.
Ethics
The study was approved by National Research Ethics Service, West London Research Ethics Committee 3, on 27 August 2010 (reference number 10/H0706/54).
Results
There were 519 contacts from young people or their families interested in participating in the trial, of whom 352 completed a telephone screening interview and the remainder were unwilling to participate further, were not eligible or could not be contacted futher after the first contact (Figure 8). Of those young people who completed the telephone screen, 210 were considered potentially eligible and invited to attend a baseline assessment. A total of 174 young people (88%) were randomised into the trial, with 87 in each arm. Primary outcome data on BMI at 26 weeks were collected on 145 (83%) participants (Figure 9): 119 at the hospital and 26 at home. At study entry, mean age was 15 years and mean BMI was 32.0 kg/m2; 63% of the participants were female, and participants were from a range of ethnic groups (Table 14). The two arms were well balanced with respect to baseline BMI and other baseline characteristics.
FIGURE 8.
The CONSORT recruitment flow diagram.
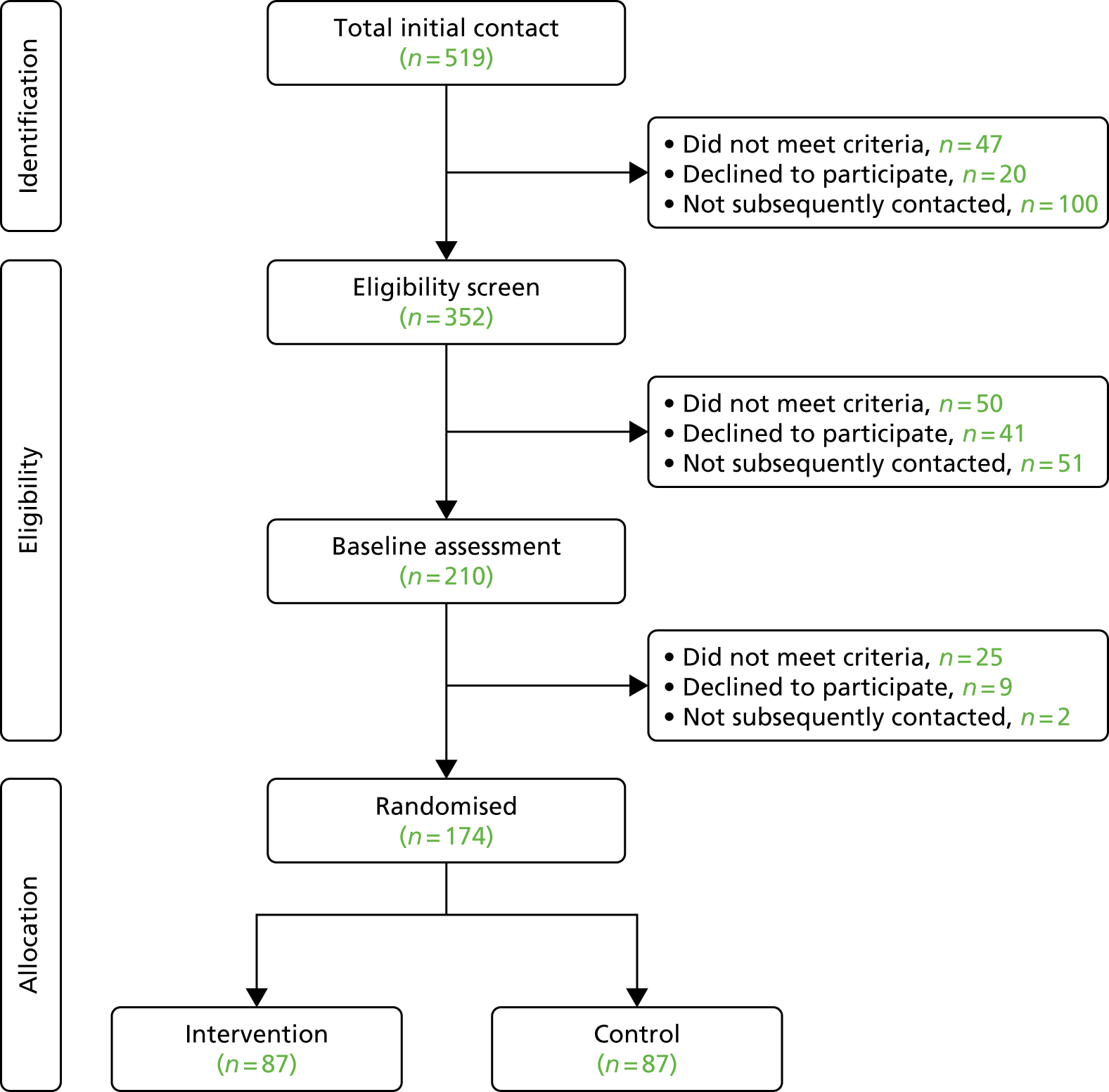
FIGURE 9.
The CONSORT flow chart of follow-up of the 174 participants randomised in the HELP study.
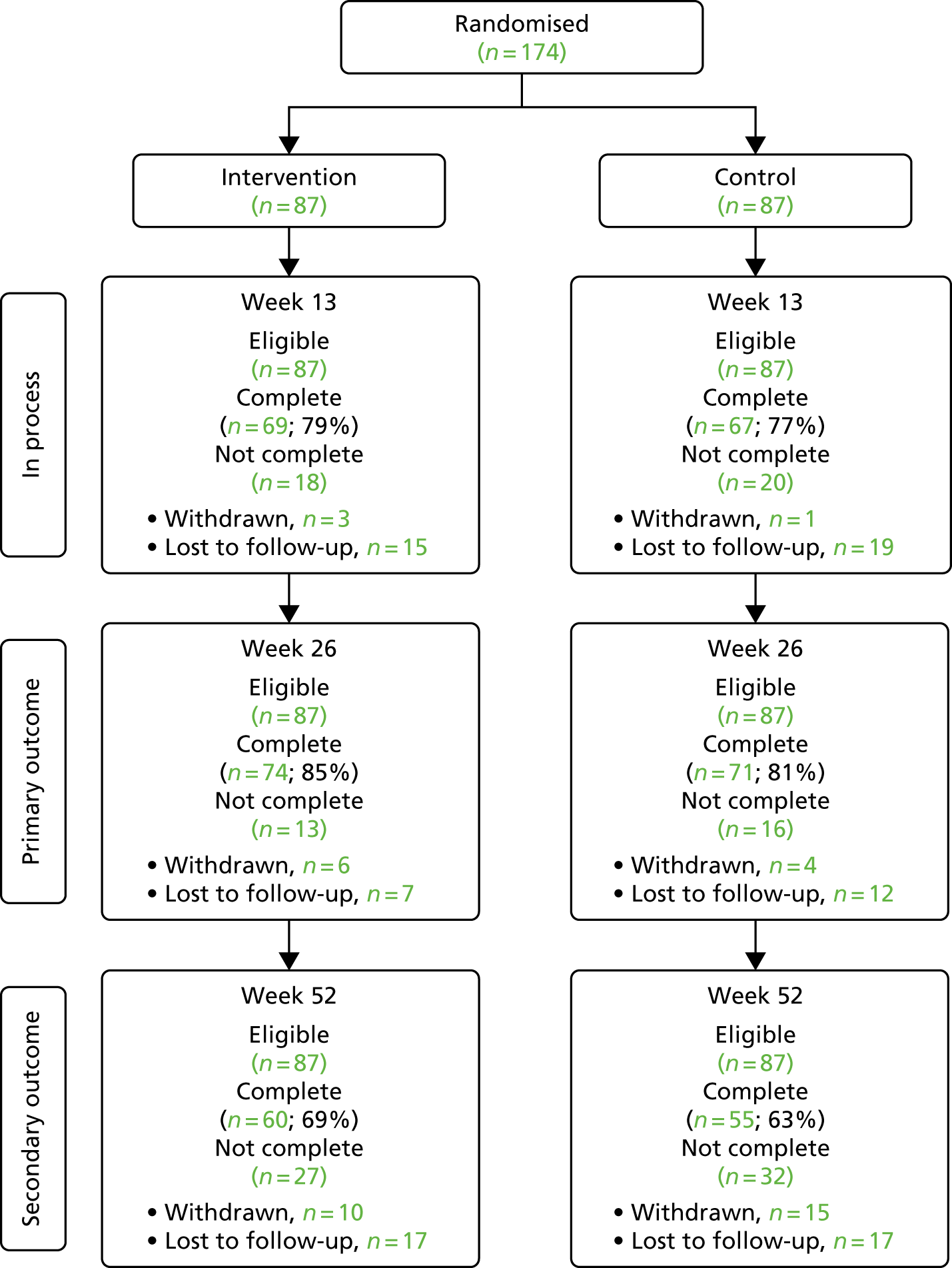
| Characteristic | Treatment group (N = 87) | |
|---|---|---|
| Control | Intervention | |
| Age (years), mean (SD) | 15.3 (2.0) | 15.0 (2.3) |
| Female, n (%) | 55 (63) | 54 (62) |
| Ethnicity, n (%) | ||
| Black | 34 (41) | 18 (22) |
| Asian | 13 (16) | 21 (25) |
| White or mixed | 36 (43) | 44 (53) |
| Unknown | 4 (5) | 4 (5) |
| Anthropometry, mean (SD) | ||
| BMI (kg/m2) | 32.0 (4.1) | 32.0 (4.6) |
| Weight (kg) | 88.9 (14.6) | 84.4 (14.9) |
| Waist circumference (cm) | 99 (11) | 98 (12) |
| Estimated fat percentage | 44 (8) | 43 (8) |
| Psychological scales, n (%) | ||
| EAT-26 | ||
| Eating attitude score (0–39) (n = 173) | 11 (8) | 10 (8) |
| Dieting scale score (0–39) (n = 173) | 7 (5) | 7 (6) |
| IWQoL-Kids | ||
| Participant-reported global score (0–100) (n = 171) | 74 (15) | 77 (17) |
| Parent-reported global score (0–100) (n = 156) | 74 (16) | 70 (17) |
| Rosenberg global score (0–30) (n = 165) | 18 (5) | 18 (6) |
| Cardiometabolic risk factors, mean (SD) | ||
| Blood glucose (mmol/l) (n = 173) | 4.4 (0.5) | 4.5 (0.4) |
| Insulin (IU/l) | 11.7 (8.0) | 14.2 (13.0) |
| LDL cholesterol (mmol/l) (n = 171) | 2.5 (0.8) | 2.7 (0.8) |
| HDL cholesterol (mmol/l) (n = 171) | 1.1 (0.3) | 1.1 (0.3) |
| Triglycerides (mmol/l) (n = 173) | 1.0 (0.4) | 1.0 (0.7) |
On average, participants in the intervention arm attended 10 of the 12 HELP sessions; 27 young people (31%) attended all 12 sessions and 69 (79%) met the criteria for compliance. Of observed sessions, 76% were rated as good, 22% as adequate and only 2% as poor.
Body mass index at 26 weeks was available for 74 (85%) participants in the intervention arm and 71 (82%) participants in the control arm (Table 15). The mean BMI at 26 weeks was 32.9 kg/m2 in the intervention arm and 32.4 kg/m2 in the control arm, representing an increase in BMI of 0.18 kg/m2 and 0.25 kg/m2, respectively, compared with baseline (Table 16). The effect of the intervention on BMI at 26 weeks adjusted for baseline age, sex and BMI was –0.11 kg/m2 (95% CI –0.62 to 0.40 kg/m2; p = 0.7), indicating that the effect of the intervention was non-significant (Table 17). The effect among compliers was –0.14 kg/m2 (95% CI –0.78 to 0.51 kg/m2; p = 0.7) and the distributions of BMI change at 26 weeks in the intervention and control arms were very similar (Figure 10).
| Variable | Number with both baseline and outcome data, control/intervention | Mean change in control group | Mean change in intervention group | Adjusted difference between intervention and control groups (95% CI)a | p-value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| BMI at 26 weeks (kg/m2) | 71/74 | 0.25 | 0.18 | –0.11 (–0.62 to 0.40) | 0.7 |
| Secondary outcomes (at 26 weeks unless specified) | |||||
| BMI at 52 weeks (kg/m2) | 55/60 | 0.80 | 0.50 | –0.22 (–1.05 to 0.61) | 0.6 |
| Waist circumference (cm) | 61/64 | 0.16 | –0.11 | –0.72 (–3.28 to 1.84) | 0.6 |
| Weight (kg) | 71/74 | 1.77 | 2.00 | 0.07 (–1.51 to 1.65) | 0.9 |
| Fat percentage | 55/60 | 1.25 | 0.71 | –0.21 (–1.57 to 1.14) | 0.8 |
| Supine systolic blood pressure (mmHg) | 68/73 | 2.19 | 3.04 | 0.01 (–3.24 to 3.26) | > 0.9 |
| Supine diastolic blood pressure (mmHg) | 68/73 | 3.41 | 2.08 | –1.21 (–4.63 to 2.20) | 0.5 |
| EAT-26 score (0–39) | |||||
| Eating attitude score | 65/69 | –0.72 | –0.84 | 0.15 (–1.24 to 1.54) | 0.8 |
| Dieting scale score | 65/69 | –1.03 | –1.26 | –0.09 (–2.32 to 2.14) | 0.9 |
| IWQoL global score (0–100) | |||||
| Participant reported | 63/69 | 6.22 | 5.94 | 0.14 (–3.53 to 3.81) | 0.9 |
| Parent reported | 52/50 | 4.37 | 7.46 | 0.51 (–3.91 to 4.93) | 0.8 |
| Rosenberg global score | 61/64 | 1.77 | 1.45 | –0.25 (–1.70 to 1.20) | 0.7 |
| Insulin (IU/l) | 56/61 | 4.31 | 3.90 | 1.51 (–1.46 to 4.48) | 0.3 |
| LDL cholesterol (mmol/l) | 53/61 | –0.20 | –0.13 | 0.09 (–0.07 to 0.26) | 0.3 |
| HDL cholesterol (mmol/l) | 53/61 | –0.08 | –0.04 | 0.02 (–0.06 to 0.09) | 0.7 |
| Triglycerides (mmol/l) | 55/61 | –0.01 | –0.07 | 0.08 (–0.08 to 0.24) | 0.3 |
| Characteristic | Group | n (mean) | |
|---|---|---|---|
| Week 26 | Week 52 | ||
| BMI (kg/m2) | Control | 71 (32.4) | 55 (32.5) |
| Intervention | 74 (32.9) | 60 (33.2) | |
| Weight (kg) | Control | 71 (91.2) | 55 (91.6) |
| Intervention | 74 (90.0) | 60 (91.1) | |
| Waist circumference (cm) | Control | 61 (99.1) | 55 (97.4) |
| Intervention | 64 (100.2) | 60 (99.4) | |
| Estimated fat percentage | Control | 55 (43.3) | 52 (43.6) |
| Intervention | 62 (44.2) | 50 (43.9) | |
| EAT-26 score (0–39) | |||
| Dieting scale | Control | 65 (6.8) | 54 (6.1) |
| Intervention | 70 (7.0) | 59 (6.6) | |
| Eating attitude | Control | 65 (11.1) | 54 (9.8) |
| Intervention | 70 (10.7) | 59 (10.3) | |
| IWQoL global score (0–100) | |||
| Participant reported | Control | 65 (80.9) | 53 (78.5) |
| Intervention | 70 (79.8) | 59 (82.5) | |
| Parent reported | Control | 54 (77.6) | 34 (76.4) |
| Intervention | 53 (73.6) | 41 (75.9) | |
| Rosenberg global score (0–30) | Control | 64 (19.9) | 53 (20.4) |
| Intervention | 66 (20.0) | 56 (20.4) | |
| LDL cholesterol (mmol/l) | Control | 55 (2.5) | |
| Intervention | 61 (2.6) | ||
| HDL cholesterol (mmol/l) | Control | 55 (1.1) | |
| Intervention | 61 (1.1) | ||
| Glucose (mmol/l) | Control | 55 (4.5) | |
| Intervention | 61 (4.6) | ||
| Insulin (IU/l) | Control | 56 (17.3) | |
| Intervention | 61 (20.7) | ||
| Triglycerides (mmol/l) | Control | 56 (1.0) | |
| Treatment | 61 (1.2) | ||
| Variable | Number with both baseline and outcome data, control/intervention | Mean change in control group | Mean change in intervention group | Adjusted difference between intervention and control groups (95% CI)a | p-value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| BMI at 26 weeks (kg/m2) | 71/74 | 0.25 | 0.18 | –0.08 (–0.56 to 0.40) | 0.7 |
| Secondary outcomes (at 26 weeks unless specified) | |||||
| BMI at 52 weeks (kg/m2) | 55/60 | 0.80 | 0.50 | –0.45 (–1.31 to 0.41) | 0.3 |
| Waist circumference (cm) | 61/64 | 0.16 | –0.11 | –0.35 (–2.92 to 2.22) | 0.8 |
| Weight (kg) | 71/74 | 1.77 | 2.00 | 0.18 (–1.32 to 1.68) | 0.8 |
| Fat percentage | 55/60 | 1.25 | 0.71 | –0.22 (–1.50 to 1.07) | 0.7 |
| Supine systolic blood pressure (mmHg) | 68/73 | 2.19 | 3.04 | 0.03 (–3.14 to 3.20) | > 0.9 |
| Supine diastolic blood pressure (mmHg) | 68/73 | 3.41 | 2.08 | –1.17 (–4.54 to 2.21) | 0.5 |
| EAT-26 score (0–39) | |||||
| Dieting scale | 65/69 | –0.72 | –0.84 | –0.05 (–1.25 to 1.15) | 0.9 |
| Eating attitude | 65/69 | –1.03 | –1.26 | –0.36 (–2.45 to 1.72) | 0.7 |
| IWQoL global score (0–100) | |||||
| Participant reported | 63/69 | 6.22 | 5.94 | –0.45 (–3.97 to 3.07) | 0.8 |
| Parent reported | 52/50 | 4.37 | 7.46 | 1.52 (–2.75 to 5.78) | 0.5 |
| Rosenberg global score | 61/64 | 1.77 | 1.45 | –0.31 (–1.70 to 1.07) | 0.7 |
| Insulin (IU/l) | 56/61 | 4.31 | 3.90 | 0.76 (–1.99 to 3.52) | 0.6 |
| LDL cholesterol (mmol/l) | 53/61 | –0.20 | –0.13 | 0.08 (–0.09 to 0.24) | 0.4 |
| HDL cholesterol (mmol/l) | 53/61 | –0.08 | –0.04 | 0.00 (–0.07 to 0.08) | 0.9 |
| Triglycerides (mmol/l) | 55/61 | –0.01 | –0.07 | 0.06 (–0.08 to 0.20) | 0.4 |
FIGURE 10.
Distribution of changes in BMI from baseline to 26 weeks in control and treatment groups among 145 participants with measurements at both time points. Reproduced from Christie et al. ,88 licensee BioMed Central Ltd, 2011. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
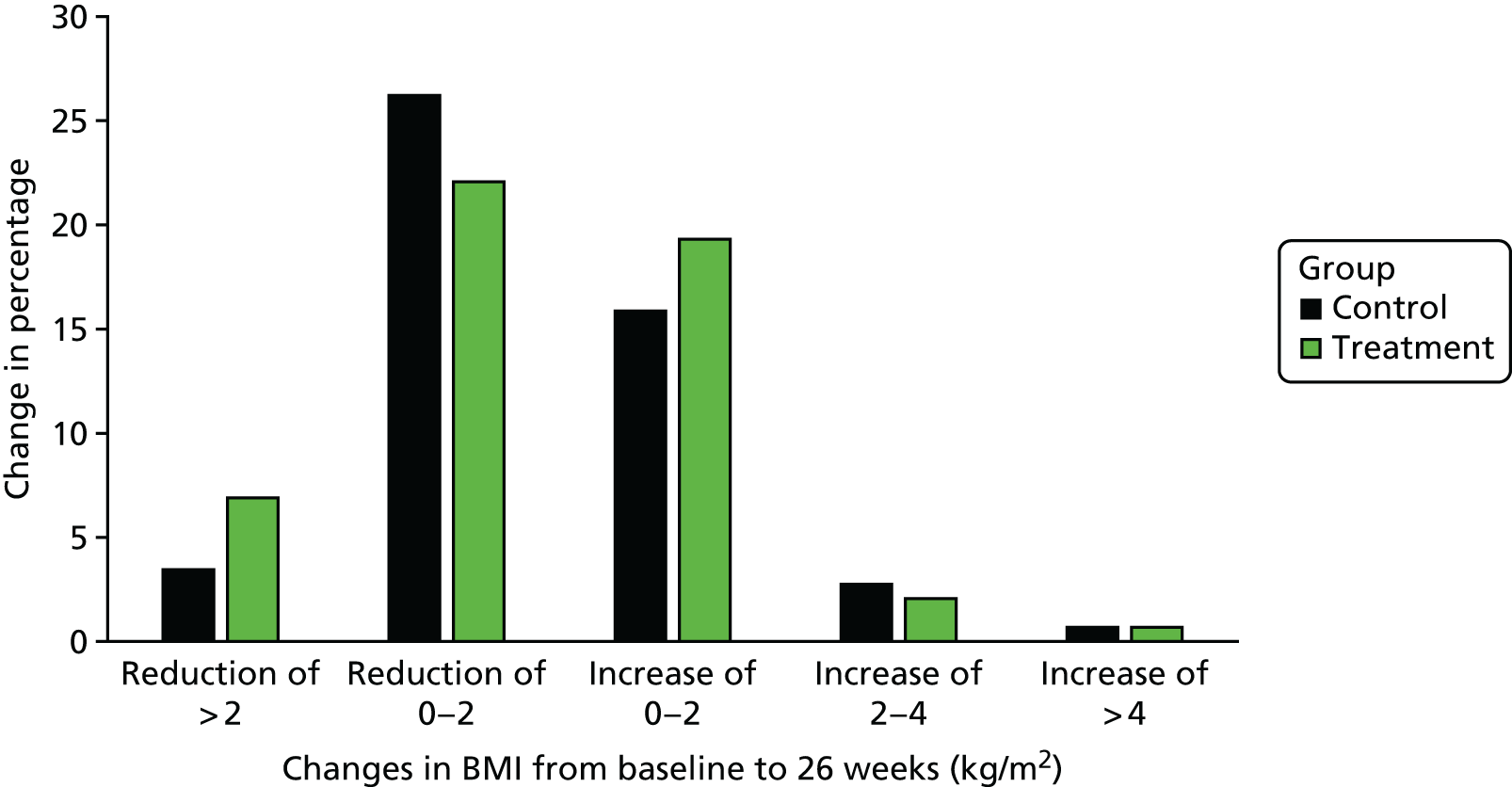
Secondary outcome analyses
There were also no significant differences between arms for a range of other measures of adiposity, including BMI, at 52 weeks (effect –0.22 kg/m2, 95% CI –1.05 to 0.61 kg/m2), weight (–0.72 kg, 95% CI –3.28 to 1.84 kg), waist circumference (0.07 cm, 95% CI –1.51 to 1.65 cm) and fat percentage (–0.21%, 95% CI –1.57% to 1.14%) (see Table 14). In addition, there were no significant differences for blood pressure, psychological function or measures of lipid and glucose metabolism.
Safety
There were no serious adverse events reported that could have been related to participation in the study.
Process evaluation results
The themes and subthemes generated are illustrated in Table 18.
| Question area | Themes | Subthemes |
|---|---|---|
| Reasons for joining the study | Desire for weight loss | |
| To gain knowledge | ||
| Practicalities | Factors that promoted attendance | Suitable/flexible timings |
| Close proximity of the venue | ||
| Factors that prevented attendance | School work | |
| Other commitments | ||
| Ideas for improvement | Desire for physical activity component | |
| Desire for meal plan | ||
| Experience of HELP overall | Comfortable to talk | |
| Individualised | ||
| Style of delivery | Easy to understand | |
| Collaborative vs. instruction | ||
| Behavioural outcomes | Increased activity levels | Changes in lifestyle activity |
| Engagement in programme activities | ||
| Changes in eating behaviours | Choosing healthier options | |
| Introduction of regular eating | ||
| Changing eating habits outside the home | ||
| Psychological outcomes | Increased confidence | Talking with others |
| Confidence related to behaviour change | ||
| Increased motivation | ||
| Cognitive outcomes | Increased awareness | |
| Increased knowledge | Knowledge related to regular eating | |
| Knowledge related to portion size |
Reasons for joining the study
Two themes were connected to a choice to participate in the study, namely a desire to lose weight and the desire for more knowledge:
Mainly I wanted to lose weight . . . I wanted to see what it was about and get more information and knowledge on how to lose weight.
Male, 16 years
Practicalities
Participants were asked about their experience of the practical aspects of the programme, for example the venue, location and timing of the sessions. Factors that both prevented and promoted attendance were identified as themes.
The timing of the sessions, usually after school, was well accepted by the young people. Participants spoke positively about the flexibility of the timings. For example, the following participant explained that session times were adjusted to fit with school holidays:
It varied because we went during the summertime so it used to be quite, it used to be Mondays after school, but then I went to summer holidays and then that time was when we’d done, so my HELP provider said that if it was easier for me, she could go earlier, so she changed it. It varied on what would be easier for me.
Female, 14 years
Young people described how competing demands of school and other commitments made it difficult to attend.
One young person described how other commitments ultimately led to her withdrawing from the intervention sessions:
Yeah, I mentioned when I stopped it, it was mainly literally because of time, because I used to commute to uni[versity] and I had work as well and then I worked on Saturdays as well; so my week was sort of just fully loaded and I didn’t want to keep postponing the sessions.
Female, 17 years
Ideas for improvements
Participants were asked about their ideas for any changes or improvements. The two main themes to emerge were desire for physical activity and desire for a meal plan.
Several young people explained that they would have liked the session to include a physical component:
What would have improved it if, there’s more exercise, but there was hardly any exercise, it’s more of a communication . . . . Yeah, activities and stuff.
Male, 16 years
A further suggestion offered by a number of young people was the request for a meal plan:
I think I would have like, like you know some kind um, you know, what we eat module something what you could everyday as like an example, kind of like a diet plan . . . Like, maybe you could try and follow for a week, ‘cause I found it a bit difficult.
Female, 18 years
Experience of the Healthy Eating and Lifestyle Programme overall
Participants were asked general open-ended questions about their experience of the HELP intervention sessions.
Young people spoke about how comfortable they felt talking during the HELP sessions. Participants varied in their ideas of which factors influenced their ability to talk in the sessions. For one person, it was related to feeling that she could be herself:
Just that it’s made me feel comfortable, welcome and I felt I could be myself here and I don’t need to be anyone that someone wants me to be, I can just be me and it just helped.
Female, 13 years
. . . and she always seemed to be interested in what I had to say, if you know what I mean. And yeah, I was glad I got her, she was really nice . . . I was really comfortable with talking to her.
Female, 15 years
One parent explained how having someone outside the family was an important factor in making her daughter feel comfortable about talking about overweight:
It enabled her to talk about overweight in a way she hadn’t been able to previously.
Parent
The following young person spoke about how, despite having worries, she felt that the session provided a safe place for her to talk:
Because obviously at my age weight isn’t something you would want to talk about, but she made me open to it and she didn’t make me feel scared because obviously, like, if I was to have questions or I felt scared, or I was worried about it, I wouldn’t really want to ask, because I would feel insecure, but she made me know it was a safe place to talk and I think what she done was actually really cool because not many people do that.
Female, 14 years
Young people described how they had experienced the programme as individualised for them:
So the thing I really liked about it was the fact that it was, the setting could be changed to pinpoint what I need and although it was there, it was kind of customised just for me and I thought that was really helpful.
Female, 14 years
Yeah and they break down our weeks and come up with things we can do, so it’s more personal about me, it’s not kind of, they’re not telling me to do stuff. It’s all about me and how I can do it.
Female, 15 years
Young people reported that the information was easy to understand. They valued the collaborative style of the providers, preferring this style to one of didactic teaching:
I liked the fact that the way she displayed things to me was slightly different from actually teaching me about why we gained weight, what can we do to lose it.
Male, 16 years
Young people spoke about how the information was clearly explained:
If I didn’t understand something, yeah she would explain it to me properly, so I could understand.
Female, 12 years
Participants valued the collaborative approach, preferring this style to one of didactic teaching:
And it was good to be, to kind of just talk about it, instead of being instructed to do this, or to do that.
Female, 16 years
One young person explained how the collaborative, supportive style of HELP was very different from her experience of Weight Watchers® (New York City, NY, USA) previously:
It’s better because it was more, a lot more, I wasn’t told exactly what to do. I didn’t feel like ‘oh gosh I’m disobeying this, I’m disobeying that’. I was just allowed to kind of accept the terms and use what I wanted to. Like, learn everything, but use what I wanted to use in the sessions.
Female, 16 years
Cognitive outcomes
Participants were asked about any perceived changes in the way that they thought about overweight.
Increased awareness emerged as a main theme. A subtheme was an awareness of the factors that influenced eating:
Well, to be honest I just thought because I, the reason I thought I was overweight is because I eat too much and I don’t exercise but then I realised it’s because the reason why I eat so much – to be honest I am losing weight. The reason why I eat too much is linked to how the days has been, or, what’s it called, my mood on the day.
Female, 14 years
It did help, talking about why we eat, ‘cause then she realised sometimes that she was eating out of boredom.
Parent
I know she’s become a bit more, kind of like, I remember one time, she was going to eat cheese or something and we said no ‘cause it’s got a lot of fat in it, so she’s become kind of aware.
Parent
One participant described a self-awareness that had developed through the programme:
But I came here and it’s something totally different and something that I did not expect but I’ve benefited from it because I know who I am now and that’s something I didn’t know before.
Female, 16 years
Young people reported that participation led to increased knowledge. Within this theme, two subthemes were identified: (1) knowledge in relation to portion size and (2) knowledge about regular eating.
Like, made me understand what kind of portions I have and should I have a smaller or bigger, or um should I have one scoop, two scoops, it just helped me to understand . . . I don’t need to have lot to feel hungry; I just need to have a little bit to feel good like, it helps.
Female, 13 years
Others spoke about gaining knowledge about how to structure meal times:
I think it was interesting to find out, like, that you can eat what you like, but obviously in moderate sizes and portions yeah . . . Um, the 3 + 2 thing was really helpful . . . the balanced diet, so three main meals and then you have two snacks in between . . . That was quite good . . . you don’t have to feel hungry, like, all day.
Female, 18 years
Some participants spoke about the way this increased knowledge was gained across the whole programme:
Yeah, I learnt just like overall, I don’t know specifically, but overall I just learnt how it can affect me, why I eat and what I should do like, how to space everything out and how to, it’s not about cutting stuff out; it’s more about portion sizes, stuff like that . . . And I just learnt that, it’s the way I think about food, not just ‘cause I’m greedy, but because it’s the way, like how things change and how I am that affects the way I eat.
Female, 14 years
One young person explained that with this knowledge came an increase in self-efficacy:
It just generally expanded my knowledge of how overweight, you can conquer it and I can do things that I didn’t know I used [to be able] to do.
Female, 13 years
Although many participants reported increased knowledge, two participants alluded to how an increase in knowledge had not translated into behaviour change:
I know what to do and not do, but I don’t do it.
Female, 15 years
No, I’m still the same old me, with more knowledge.
Male, 12 years
Behavioural outcomes
Young people spoke about how they had incorporated changes into their daily activities:
Oh yeah, like I said, I try and take public transport as much as I can rather than the car.
Female, 18 years
He’s not just staying and don’t do nothing, now he’s started going to school walking and not use bus. His brother is a bit better and now they walking together with his brother and coming back, walking and he’s going basketball.
Parent
Other young people mentioned that they had joined activities including sports such as swimming, basketball and football. Several young people also spoke about how they had been inspired to join the gym:
I started going to the gym in, yeah . . . running, that’s it, more running and swimming.
Male, 13 years
I’ve been doing a lot more exercise, like been going to the gym and been going jogging.
Female, 17 years
Participants reported changes in their eating behaviours. Several young people explained how they were actively choosing ‘healthier options’. This was often linked with an increase in the consumption of fruit and vegetables:
And she doesn’t have to clear her plate. If she’s full, she’s full. She doesn’t make herself eat it and that kind of thing, isn’t it. I mean she still goes through bad days where YP [young person] can’t resist, but, generally speaking, most of the time she’ll go for the healthy more often than not.
Parent
The choices she makes, like instead of going for chocolate, she’ll go for an apple, or she’ll say ‘oh no, I’ll wait till dinner’, which is a big change.
Parent
After that session, she [mother] tries to like, when we have dinner, she’s tried to keep it to a lower portion size, or less than we, less than we used to have before.
Male, 13 years
Several young people described how they were eating more regularly. In particular, they spoke about introducing breakfast and the positive impact that this had generated:
Better, oh I started having breakfast as well ‘cause I don’t normally have breakfast and it like, it made me feel really, like, energetic.
Female, 17 years
Changes were also reported in eating habits outside the home. Several young people spoke about how they are now taking food from home to school, rather than buying food, for example at the shop or in the school canteen. There was a belief that this had a positive impact as it increased both the sense of control and the likelihood of eating a ‘healthier option’:
. . . ‘cause I bring pack lunch into school now instead of, like, eating what’s there so I can control, like, the sort of food that it is.
Female, 17 years
Psychological outcomes
Participants were also asked about whether or not there had been any changes in the way that they feel about themselves. The two main themes were increased confidence and changes in motivation.
Participants described increased confidence and the positive impact that this had on their behaviour:
She used to not come to the shopping centres or some other crowded places with us, she’d rather stay indoors, but now, she can go with her sister look, shopping city and all this go and walk around.
Parent
Made me more confident, I don’t need to be someone they want me to be, I can, like at school I would usually hide myself . . . I don’t hide away and go ‘OK, I’m this, I’m that’. I say to them ‘no, this is not me, why are you talking to me’ and I would feel much better about myself.
Female, 13 years
One young person explained how his increased confidence had changed his experience of physical education in school:
. . . like in PE [physical education] last year, I would, I don’t know, I would hide behind the door until it was over. So wouldn’t do it and with badminton I would do it, but I wouldn’t put that much effort into it and now it’s, I don’t know, like something must of snapped or something, but I’ve started doing it now.
Male, 12 years
Participants spoke about how their ability to talk with others about overweight had changed in a positive way. The following participant described how her response to advice from others had changed:
I find them more easy to talk about, so like if someone was to tell me you know, ‘you’re eating too much’, I wouldn’t be offended by it, I’d be like ‘oh yeah that’s true, I might as well cut down because what I’m doing is obviously not going to help me’. That’s . . . I’m not as, um, I can take like, I can take opinions more now. Yeah, family, like before if I was eating too much they would tell me and I’d be offended, but now, I’ll understand and, yeah.
Female, 14 years
One participant spoke about how her feelings of shame had reduced and the effect this had on her social interactions with others:
. . . like, before I was really ashamed to talk, I was ashamed to like talk about myself, so I wouldn’t participate; I would just kind of ignore it and walk off or something and feel quite awkward, but now I think, I can handle that a bit better, you know like, I can like joke around about it . . . And people can joke around me.
Female, 18 years
Um, it’s like before I didn’t used to be bothered at all so now, sometimes, most of the time, I am bothered and, like, I do try hard and think and I don’t think it’s useless.
Female, 13 years
The following participant explained that realising the skills she has helped with this motivation:
. . . like HELP, it made me realise the different skills I have and, like, it taught me that I can be motivated, that if I wanted to do something, I would do it.
Female, 16 years
Discussion
It was found that HELP, a multicomponent adolescent-specific weight management intervention based on published best practice and designed to enhance motivation to change and develop self-efficacy and self-esteem, did not reduce BMI or improve psychological or cardiometabolic function when compared with a single educational session in a community sample of obese adolescents. There was no evidence of harm from the intervention.
A high-quality randomised trial of a complex intervention, previously shown to be promising in clinical practice, was conducted. The HELP intervention was based on published best practice and was designed specifically for adolescents struggling to manage their weight; it included parents as well as young people and included an element of personalisation. The primary outcome was a reproducible quantitative measure recorded by blinded observers. ‘Raw’ BMI was used rather than BMI SD score (zBMI), as the skewness in the BMI distribution means that the same change in kg/m2 will produce a smaller change in zBMI the more obese the subject is. 106 A community sample of obese young people was recruited and the intervention was tested against a standardised educational session. Randomisation was conducted independently of the research team. Attrition was low compared with other paediatric obesity studies,107 and the recruited sample was sufficient to identify the desired effect size with high power. Fidelity of delivery of the intervention was good and ≈ 80% of participants received at least the intended ‘dose’ of the intervention, which is good for complex interventions.
The study was subject to a number of limitations. Recruitment was difficult and the eligibility criteria were widened to include adolescents with morbid obesity. However, it is unlikely that this influenced the results, as only nine participants (4.6%) had a baseline BMI of between 40 and 45 kg/m2. Attrition was significant by 52 weeks; although the smaller sample size reduced the power to identify an effect of the intervention, it is likely that the direction of bias from attrition would have been to preferentially retain those in whom the intervention had been effective and inflate any identified effect. Although full data on the primary outcome were available in all who attended for follow-up, it was not possible to collect sufficient planned follow-up data on physical activity and psychological morbidity. However, given the absence of any significant effects on other secondary outcomes, it is unlikely that the intervention would have had an effect on physical activity and psychological morbidity.
Comparison with the literature
Systematic reviews show that the outcomes of programmes that include adolescents are more variable than those limited to earlier childhood. 42,91,108 Many published trials are of low quality, with a 2015 systematic review108 reporting that 28 out of 31 included studies had an unclear or high risk of bias due to the lack of information about or lack of procedures to ensure adequate randomisation and blinding of outcome assessments. The findings of this study of no effect for HELP are similar to a number of other weight management trials in the literature. Recent meta-analyses91,108 suggest that lifestyle modification programmes in children are associated with a reduction in BMI of 1.15 kg/m2 (see Peirson et al. 108) to 1.3 kg/m2 (see Ho et al. 91). Systematic reviews91,108 suggest that lifestyle modification interventions improve quality of life (QoL)108 and may have small but discernible effects on lipids and insulin. 91 In contrast, this study found no effect of HELP on BMI, lipids or QoL.
Qualitative findings
These accounts offer an important insight into the experiences of the young people attending the HELP intervention sessions. Young people were attracted to the study by the desire to lose weight and gain more knowledge of how to do this. Access to the programme was widely accepted in terms of the practical aspects, such as the location, flexibility and timing of the sessions. However, competing demands on the young person’s schedules, such as school work and other activities, had an impact on attendance. Young people felt that the programme was individualised for their specific needs. The sessions were experienced as collaborative and the participants felt comfortable talking with the HELP provider. In relation to outcome, young people reported a general increase in knowledge and awareness of healthy eating. This knowledge was translated into behaviour change, with young people reporting improved choices, regular eating, reduced portion sizes and changes in eating habits outside the home. Increased activity levels, improved self confidence and changes to motivation were also reported.
There are a number of possible explanations for the null findings, relating to the methods, the population or the intervention. First, it is possible that the trial did not provide an adequate test of the intervention; however, it was adequately powered and methodologically robust, as described earlier.
We found that the control arm achieved similar levels of BMI change to the intervention group, raising the possibility that the comparator intervention had some effect. This is supported by the observation that a large proportion of those in the control arm achieved a small BMI reduction.
Second, it is possible that the population was different from those paediatric obesity interventions that have identified an effect. A community sample of young people was recruited from London and the surrounding counties. The sample was highly deprived, with ≈ 40% of participants in the most deprived IMD quintile (20% was expected) and around half were from black or minority ethnic groups. Related to this, mental health problems were identified in 34% of the sample at baseline, despite excluding young people with previously known mental health conditions. This is considerably higher than in the general population,109 although the prevalence of mental health problems in obese groups is not well established. In contrast, most published paediatric obesity studies have been undertaken in hospital settings,91 particularly academic centres in the USA, where study populations are dominated by more affluent white families. 42 There is evidence that childhood obesity interventions based on education and personal behavioural change are less likely to be effective in more deprived populations,110 as poorer families may face structural and cultural barriers to lifestyle change. These may include financial barriers and fewer family and community resources for young people wishing to change.
Third, the results may be explained by intervention issues, including delivery, content and intensity factors. The pilot data were obtained from a small clinical programme delivered by highly trained clinical psychologists. However, given that use of clinical psychologists is not generalisable to larger community settings owing to issues of cost and lack of available workforce, provision in this trial was modified to delivery by less well-qualified graduate psychologists. Providers were given 5 days of intensive training in intervention delivery, together with regular supervision; fidelity, as judged by a clinical psychologist, was good. However, the reduction in the therapist skill level may have contributed to the null results.
The HELP intervention was based on best practice as defined by NICE,12 but sessions focused on addressing key adolescent issues in weight self-management, including self-esteem, emotional eating and self-regulation relating to food, rather than focusing on calorie restriction or provision of exercise sessions. Systematic reviews suggest that programme features associated with efficacy of lifestyle interventions include involvement of parents, weekly or more frequent contact with participants, inclusion of a structured exercise component and intervention duration of > 6 months. 91,108 HELP included involvement of parents but did not include the last three elements.
Conclusions
The HELP intervention was no more efficacious than standard care of a single educational session for reducing BMI in a community sample of obese adolescents. This adds to the existing evidence base of lack of effectiveness of weight management trials for children and adolescents, despite HELP being based on best practice and designed for adolescent development needs. Further work is needed to understand how weight management programmes can be delivered effectively to young people from diverse and often deprived backgrounds in which childhood obesity is common. At minimum, this study reinforces the need for higher-level, structured interventions to tackle the growing public health burden of obesity in the UK and internationally.
Healthy Eating and Lifestyle Programme trial cost-effectiveness: cost–utility analysis alongside a randomised controlled trial
Abstract
Background
The HELP trial was a RCT in which 174 obese adolescents were randomly allocated to receive a motivational multicomponent lifestyle modification intervention in a community setting or enhanced standard care, and followed up for 12 months.
Aims
The costs and cost-effectiveness of HELP versus enhanced standard care from the perspective of the UK NHS were compared.
Methods
A cost–utility analysis was carried out to estimate the mean costs and quality-adjusted life-years (QALYs) per subject over a 1-year period, using resource use data and utility values collected during the trial.
Results
Mean intervention costs per subject were £918 for HELP and £68 for enhanced standard care. There were no significant differences between the two groups in mean resource use per subject for any type of health-care contact. Adjusted costs were significantly higher in the intervention group (mean incremental costs for HELP vs. enhanced standard care £1003, 95% CI £837 to £1168). There were no differences in adjusted QALYs between groups (mean QALYs gained 0.008, 95% CI –0.031 to 0.046 QALYs).
Conclusions
No evidence was found that a motivational multicomponent lifestyle modification intervention delivered in the community was more effective than a single educational session in improving QoL in a sample of obese adolescents. HELP was associated with higher costs, mainly due to the extra costs of delivering the intervention.
Introduction
Childhood obesity has increasingly become one of the most serious global public health challenges. Globally, an estimated 42 million children aged < 5 years were overweight or obese in 2010. 111
The prevalence of obesity in England remains high. The NCMP found in 2014 that 9.5% of children in the Reception year at school were obese and a further 13% were overweight; 19% of children aged 10–11 years were obese and 14% were overweight. 112 Figures from the Health Survey for England (HSE) for 2012 show that ≈ 28% of children aged 2 to 15 years were overweight or obese. 113,114
Childhood obesity has both immediate and long-term effects on health and well-being. Obese children are more likely to have risk factors for cardiovascular disease, such as high cholesterol or high blood pressure,115 pre-DM,116 bone and joint problems,117 sleep apnoea118 and social and psychological problems (such as stigmatisation and poor self-esteem). 119,120
More than half of children who are obese will grow up to be obese as adults121 and are therefore more at risk of adult health problems such as heart disease, type 2 DM (T2DM), stroke, some cancers and musculoskeletal and psychosocial problems. 122–125
As well as having a substantial impact on health, obesity is also associated with significant health expenditures. In England, the costs of overweight and obesity to the health system have been calculated to be £4.2B per annum. 126 Obese individuals have been found to have medical costs ≈ 30% greater than their peers of normal weight. 127 In terms of non-health-care costs, productivity losses due to obesity have been estimated to be £15.8B. 126 Childhood obesity in particular imposes a substantial cost to the health system, with estimated costs of ≈ US$14B per annum in the USA. 128
There are few economic evaluations of childhood lifestyle obesity interventions and, among those that have been conducted, differences in the type of lifestyle intervention, age of participants, cost components included, country of setting, outcomes of interest and methodologies mean that it is difficult to draw conclusions about whether or not such interventions are cost-effective. Table 19 shows findings from a literature review of economic evaluations of studies in childhood obesity.
| Study | Country | Intervention | Age of participants | Authors’ conclusion |
|---|---|---|---|---|
| Hayes et al.129 | Australia | The intervention consisted of eight home visits by specially trained community nurses, including one visit at 30–36 weeks’ gestational age, and seven visits at 1, 3, 5, 9, 12, 15 and 24 months after birth. These visits included one-on-one consultations of 1 hour duration at which age-appropriate education and advice on feeding, nutrition and physical activity were provided. Both control and intervention participants received the usual childhood nursing service from their local area health service, consisting of one home visit by a community nurse within 1 month of birth plus visits to the local clinic | 1 month; followed up until 2 years of age | ‘Healthy Beginnings’ is a moderately priced intervention with demonstrated effectiveness that offers similar or better value for money than existing obesity prevention or treatment interventions targeted at older children |
| The control group also received home safety information sent by mail at 1, 3, 5, 9 and 18 months | ||||
| Moodie et al.130 | Australia | The interventions targeted evidence-based behaviour change: reduction in television viewing, reduced consumption of sugar-sweetened drinks and increased water consumption, reduced consumption of energy-dense snacks and increased consumption of fruit and vegetables, increased active play after school and at weekends and increased active transport to school | 4–12 years | Be Active Eat Well Program (BAEW) was affordable and cost-effective, and generated substantial spin-offs in terms of activity beyond funding levels. Elements fundamental to its success and any potential cost efficiencies associated with scaling up now require identification |
| Current practice covered any initiatives to address concerns about healthy eating, physical activity or childhood obesity | ||||
| McAuley et al.131 | New Zealand | The main intervention was the provision of community activity co-ordinators to encourage all children to be a little more physically active every day by increasing the variety of and opportunities for physical activity at interval, lunchtime and after school beyond what was currently provided. Nutrition-based interventions included supplying intervention schools with a cooled water filter and the provision of free fruit for a 6-month period. Several nutrition resources were developed, targeting reductions in sugary drinks and increased fruit and vegetable intakes, including ‘APPLE Bites’ (a community-based resource highlighting ideas, recipes, hints and tips for being more active and eating well at home), science lessons at school and an innovative card game simulating completing a triathlon | 5–12 years | The relatively simple intervention approach employed by the APPLE project was successful in significantly reducing the rate of excessive weight gain in children, with implementation costs of NZ$664–1708 per kg of weight gain prevented over 4 years |
| Kalavainen et al.132 | Finland | The routine programme consisted of two individual appointments for the children by school nurses; it was modified from the current counselling practice for obese children in school health care in the region. The group programme consisted of 14 evening sessions held separately for parents and children, and of one joint session of making healthy snacks | 7–9 years | Family-based group treatment is more costly than individual routine counselling |
| Wake et al.133 | Australia | The intervention consisted of primary care screening, followed by a brief, structured secondary prevention programme, which consisted of four standard consultations over 12 weeks with the aim of changing nutrition, physical activity and sedentary behaviour. The comparator was usual care | 5–10 years | Primary care screening followed by brief counselling did not improve BMI, physical activity, or nutrition in overweight or mildly obese 5- to 10-year-olds, and it would be very costly if universally implemented |
| Goldfield et al.134 | USA | Families were randomised to one of two groups: mixed treatment whereby subjects received a mixture of individualised plus group treatment (mixed), and group treatment that did not involve individual therapy (group) | 8–12 years | A family-based, behavioural intervention employing group treatment alone is a more cost-effective approach to treating paediatric obesity than a mixed group plus individual format |
| Hollinghurst et al.135 | UK | The treatments were (1) a hospital clinic (control in both trials), comprising a MDT of consultant, dietitian and exercise specialist, (2) a nurse-led primary care clinic replicating the service provided by the hospital and (3) an intensive intervention using Mandometer® (AB Mando, Huddinge, Sweden), a behaviour modification tool aimed at encouraging slower eating and better recognition of satiety | 5–16 years | Intensive management using the Mandometer was effective but costly (£432 per 0.1 reduction in BMI SDS) compared with conventional care (range £153–173). A total of 26% of children receiving conventional care achieved a clinically meaningful reduction in BMI SDS; however, use of Mandometer training may be justified in children not responding to conventional lifestyle interventions |
| Janicke et al.136 | USA | For both the family-based and parent-only interventions, 90-minute group sessions were held weekly for the first 8 weeks, then biweekly for the next 8 weeks. Child and parent participants in both treatments monitored dietary intake and physical activity. Families were taught to categorise foods as red, yellow and green based on a modified version of the Stoplight programme. Increased physical activity was promoted through a pedometer-based step programme. Families and group leaders worked together to set daily dietary and physical activity goals at the end of each group session | 8–14 years | Parent-only interventions may be a cost-effective alternative treatment for paediatric obesity, especially for families in medically underserved settings |
In an accompanying paper,138 the efficacy of a community-delivered motivational multicomponent intervention compared with enhanced standard care for obese adolescents was evaluated. The aim of this paper was to assess the costs and cost-effectiveness of the intervention from the perspective of the UK NHS.
Methods
Overview of economic evaluation
A cost–utility analysis was undertaken to compare the costs and outcomes associated with HELP versus those associated with enhanced standard care. The outcome measure was QALYs, which combine length of life and quality of life, and are consistent with NICE recommendations. 139 The analysis took a UK NHS perspective; costs from a Personal Social Services perspective were likely to be negligible and so were excluded. Costs were calculated in 2013/14 Great British pounds. The time horizon was 1 year, reflecting follow-up in the trial. Extrapolation beyond the end of the trial using decision-analytical modelling was not undertaken because the within-trial analysis found no evidence of significant differences in benefits between the intervention and control groups, and costs were significantly higher in the intervention group; the 1-year time horizon was long enough to reflect all important differences in costs or outcomes between the two treatments. As the time horizon was 1 year, discounting was unnecessary. Given the findings, incremental costs and QALYs were presented separately.
Resource use and costs
For every subject, the cost of the lifestyle intervention and the cost of follow-up, using resource use data collected retrospectively in the trial, were calculated via questionnaires sent to subjects at baseline and at 6 and 12 months post randomisation.
The cost of HELP included providers’ training, learning materials for providers and subjects and time spent by providers/an experienced psychologist in each follow-up session with subjects.
There were 21 providers who received training to deliver HELP (each trained for 5 days for 7 hours per day) from psychologists experienced in specific motivational interviewing techniques and in the use of solution-focused questioning in the intervention group. In the enhanced standard-care group, the nurses did not receive any training in delivery style.
In the HELP group, the 1.5 hours spent by the provider to deliver the intervention and room rental for each session were accounted for, as well as the 1.5 hours spent by the experienced psychologist to supervise the delivery of session 1 of the intervention with the provider’s first subject. In the enhanced standard-care group, the 1 hour spent by the nurse to deliver the single educational session and room rental were accounted for.
Providers’ travel time (1.5 hours) to and from session venues and travel costs were accounted for in the HELP group. Travel time (2 hours) to and from session venues was accounted for in the enhanced standard-care group only if the intervention was delivered by a nurse provided by the study team (n = 36), as other standard-care sessions were provided by local practice nurses in their own practices.
Educational materials (manuals/leaflets) were supplied to both providers and subjects in the HELP group. Leaflets were supplied to subjects in the enhanced standard-care group.
Follow-up resource use included GP surgery consultations, GP telephone consultations, GP home consultations, contacts with the practice nurse, referrals to secondary care services (e.g. dietitian, physiotherapist, osteopath, chiropractor, psychologist, counsellor, dentist, radiologist, community pharmacist), hospital inpatient admissions, hospital day-cases and hospital outpatient visits.
Unit costs were obtained from published sources140,141 and local costs, and were inflated when appropriate.
Utilities and quality-adjusted life-years
Subjects’ generic health state was assessed using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), descriptive system142,143 at baseline and at 6 and 12 months post randomisation. The EQ-5D-3L is a validated, generic health-related, preference-based measure comprising five dimensions (mobility, self-care, usual activities, pain, anxiety/depression) with three levels in each item. Every EQ-5D-3L health state was converted into a single summary index (utility value) using a formula that attaches weights to each of the levels in each dimension based on valuations by general population samples. Given the perspective of this analysis, a value set for the UK population was used to calculate utility values at each time point for every subject. 144 Utility values at each point in time were calculated. The values lie on a scale between 0 (equivalent to death) and 1 (equivalent to full health); negative values are possible, representing states worse than death. A utility profile was created for each subject assuming a straight-line relation between their utility values at each measurement point in time. QALYs for each subject from baseline to 12 months were calculated as the area under the utility profile.
Dealing with missing data
Multiple imputation was used to impute missing data for the costs of primary and secondary care contacts, total cost, utility values at every time point and total QALYs. The cost variables were unit costs multiplied by resource use. Age, gender and treatment allocation were included in the imputation models as additional explanatory variables.
The imputation was based on a multivariate normal regression, and generated 20 imputed data sets.
Statistical methods
Mean resource use, costs, utility values and QALYs were compared between all subjects on an ITT basis.
Differences in mean costs and QALYs between groups were calculated using regression analysis, regressing individual QALYs and costs against treatment allocation in the trial controlling for other factors. QALYs gained were adjusted for age, gender and baseline utility values. Incremental costs were adjusted for age, gender and costs in the 6-month period prior to baseline. To analyse QALYs gained, a linear regression model was used. To account for skewness of the cost data, a generalised linear model with gamma family and log-link was used;145 using log-normal, Gaussian, inverse Gaussian and negative binomial distributions was also considered, but the gamma model gave the best fit in terms of residual plots and the Akaike Information Criterion. Standard errors were corrected to account for uncertainty in the imputed values. Analyses were carried out using Stata, version 13.1.
Results
Subject characteristics
Subjects in the clinical trial were well balanced in terms of age, gender and baseline BMI and EQ-5D values, although there were slightly fewer black subjects and slightly larger numbers of white and Asian subjects in the HELP arm. 138
Resource use and costs
During the 12-month follow-up, there were no significant differences in any component of health-care resource use between the HELP and enhanced standard-care arms (Table 20). The most common types of contact were GP surgery visits for both groups, with a mean of 1.57 (SD 2.31) visits in the HELP group (n = 58) and 1.46 (SD 1.80) visits in the enhanced standard-care group (n = 50), followed by outpatient visits in the HELP group [mean 0.64 (SD 1.22), n = 58] and counsellor visits in the enhanced standard-care group [mean 0.30 (SD 1.48), n = 53]. Any other health-care visits comprised visits with a dentist, an orthodontist or an optician.
| Type of resource use (unit) | Unit costs (£) | Trial arm | Difference | p-value | |||
|---|---|---|---|---|---|---|---|
| HELP | Enhanced standard care | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Hospital | |||||||
| Inpatient (admission) | 1758.36 [23] | 59 | 0.05 (0.29) | 53 | 0.06 (0.41) | –0.01 | 0.93 |
| Day case (attendance) | 693.00 [23] | 59 | 0.15 (0.81) | 52 | 0.04 (0.19) | 0.11 | 0.32 |
| Outpatient (visit) | 108.22 [23] | 58 | 0.64 (1.22) | 52 | 0.29 (0.70) | 0.35 | 0.07 |
| A&E (visits) | 115.00 [23] | 58 | 0.29 (0.56) | 53 | 0.23 (0.54) | 0.07 | 0.53 |
| GP | |||||||
| Surgery (consultation) | 45.00 [24] | 58 | 1.57 (2.31) | 50 | 1.46 (1.80) | 0.11 | 0.79 |
| Home (visit) | 114.00 [24] | 59 | 0.00 (0.00) | 53 | 0.00 (0.00) | 0.00 | 0.00 |
| Telephone (consultation) | 27.00 [24] | 59 | 0.27 (0.69) | 53 | 0.32 (0.94) | –0.05 | 0.75 |
| Nurse | |||||||
| Home (visit) | 70.00 [24] | 59 | 0.02 (0.13) | 52 | 0.00 (0.00) | 0.02 | 0.35 |
| Surgery (visit) | 13.43 [24] | 58 | 0.26 (0.78) | 53 | 0.25 (0.78) | 0.01 | 0.93 |
| Telephone (consultation) | 13.43 [24] | 58 | 0.02 (0.13) | 52 | 0.00 (0.00) | 0.02 | 0.35 |
| NHS dietitian (visit) | 35.00 [24] | 58 | 0.09 (0.34) | 52 | 0.04 (0.19) | 0.05 | 0.37 |
| Community pharmacist (contact) | 56.00 [24] | 58 | 0.31 (0.78) | 52 | 0.15 (0.54) | 0.16 | 0.23 |
| NHS physiotherapist (visit) | 34.00 [24] | 58 | 0.14 (0.71) | 53 | 0.19 (1.00) | –0.05 | 0.76 |
| Private physiotherapist (visit) | 77.00 [24] | 58 | 0.03 (0.26) | 53 | 0.06 (0.41) | –0.02 | 0.73 |
| Osteopath (visit) | 34.00 [24] | 58 | 0.03 (0.26) | 53 | 0.00 (0.00) | 0.03 | 0.34 |
| Chiropractor (visit) | 34.00 [24] | 58 | 0.00 (0.00) | 53 | 0.00 (0.00) | 0.00 | 0.00 |
| Psychologist (visit) | 59.00 [24] | 58 | 0.31 (1.88) | 53 | 0.06 (0.41) | 0.25 | 0.34 |
| Counsellor (visit) | 58.00 [24] | 58 | 0.09 (0.66) | 53 | 0.30 (1.48) | –0.22 | 0.31 |
| Any other health care (contact) | 81.00 [23, 24] | 57 | 0.46 (1.31) | 53 | 0.45 (1.25) | 0.01 | 0.99 |
In the complete-case analysis, the mean total cost of health-care resource use per subject was £363 (95% CI £221 to £505) in the HELP group (n = 55) and £357 (95% CI £114 to £600) in the enhanced standard-care group (n = 48) (Table 21). Accounting for missing data using multiple imputation, the values were £320 (95% CI £212 to £428) for the HELP group and £462 (95% CI £268 to £657) for the enhanced standard-care group.
| Type of cost | Trial arm (£) | HELP vs. enhanced standard care (£) | |
|---|---|---|---|
| HELP | Enhanced standard care | ||
| Training, mean (SD) | |||
| Provider/nurse | 130 (0) | 0 (0) | 130 |
| Psychologist | 46 (0) | 0 (0) | 46 |
| Intervention, mean (SD) | |||
| Provider/nurse | 201 (90) | 33 (17) | 168 |
| Psychologist | 134 (60) | 0 (0) | 134 |
| Room rental | 114 (51) | 13 (6) | 101 |
| Travel, mean (SD) | |||
| Provider/nurse | 279 (124) | 22 (26) | 257 |
| Materials, mean (SD) | |||
| Manual for provider | 3 (0) | 0 (0) | 3 |
| Manual/leaflets for subjects | 5 (0) | 1 (0) | 4 |
| Other materials for subjects | 5 (0) | 0 (0) | 5 |
| Intervention costs per subject, mean (SD) | 918 (201) | 68 (22) | 850 |
| Mean costs (95% CI) of health-care resource use during follow-up | |||
| Complete cases | 363 (221 to 505) (n = 55) | 357 (114 to 600) (n = 48) | 6 (–264 to 275) (n = 103) |
| With imputation | 320 (212 to 428) | 462 (268 to 657) | –142 (–363 to 79) |
| Total costs (95% CI) per subject (intervention cost plus health-care resource use during follow-up) | |||
| Complete cases | 1281 (1246 to 1527) (n = 55) | 425 (188 to 675) (n = 48) | 855 (685 to 1224) (n = 103) |
| With imputation | 1238 (1237 to 1452) | 530 (343 to 732) | 708 (587 to 1028) |
A total of 758 sessions were delivered in the intervention group and 71 sessions were delivered in the control group. The total intervention cost per subject was £918 (95% CI £875 to £960) for the delivery of the HELP intervention and £68 (95% CI £63 to £73) (see Table 21) for enhanced standard care.
Accounting for missing data using multiple imputation, the mean total cost per subject was £1238 (95% CI £1237 to £1452) in the intervention group (n = 87) and £530 (95% CI £343 to £732) in the control group (n = 87) (see Table 20). Values for complete-case analysis followed the same trend (see Table 20). The difference in intervention costs between the intervention and control groups was driven by the costs of the delivery of the HELP intervention and providers’ travel.
Utilities and quality-adjusted life-years
Mean utility values per subject at each follow-up point were similar for both groups and did not vary significantly over time in either group (Table 22).
| Utility values | Trial arm, mean (95% CI) | |
|---|---|---|
| HELP | Enhanced standard care | |
| Complete cases | ||
| Baseline | 0.793 (0.735 to 0.850) (n = 83) | 0.833 (0.792 to 0.875) (n = 85) |
| 6 months | 0.852 (0.803 to 0.902) (n = 68) | 0.881 (0.844 to 0.918) (n = 63) |
| 12 months | 0.851 (0.799 to 0.902) (n = 59) | 0.871 (0.812 to 0.930) (n = 52) |
| QALYs | 0.837 (0.787 to 0.887) (n = 54) | 0.867 (0.828 to 0.907) (n = 50) |
| With imputation | ||
| Baseline | 0.792 (0.736 to 0.849) | 0.829 (0.788 to 0.870) |
| 6 months | 0.848 (0.806 to 0.890) | 0.891 (0.858 to 0.924) |
| 12 months | 0.849 (0.807 to 0.891) | 0.847 (0.805 to 0.889) |
| QALYs | 0.855 (0.816 to 0.895) | 0.881 (0.851 to 0.921) |
Accounting for missing data, mean utility values per subject increased from 0.792 (95% CI 0.736 to 0.849) at baseline to 0.848 (95% CI 0.806 to 0.890) at 6 months and then remained unchanged at 0.849 (95% CI 0.807 to 0.891) at 12 months in the intervention group. In the control group, the mean utility values per subject increased from 0.829 (95% CI 0.788 to 0.870) at baseline to 0.891 (95% CI 0.858 to 0.924) at 6 months and declined to 0.847 (95% CI 0.805 to 0.889) at 12 months. The mean total QALYs per subject were 0.855 (95% CI 0.816 to 0.895) in the intervention group and 0.881 (95% CI 0.851 to 0.912) in control group. Utility values and QALYs were similar for complete cases.
Cost–utility analysis
In the base-case analysis, accounting for missing data using multiple imputation and baseline characteristics, there were no significant differences in costs or QALYs between the two groups: the mean incremental cost of HELP versus enhanced standard care was £1003 (95% CI £837 to £1168) and the mean QALYs gained were 0.008 (95% CI –0.031 to 0.046) (Table 23). The incremental costs and QALYs gained for HELP versus enhanced standard care remained not significantly different from zero when rerunning the base-case analysis without adjustment, and using complete cases.
| Mean (95% CI) | ||
|---|---|---|
| Incremental cost (£) | QALYs gained | |
| Base casea | 1003 (837 to 1168) | 0.008 (–0.031 to 0.046) |
| No adjustmentb | 861 (713 to 1010) | –0.037 (–0.100 to 0.027) |
| Complete-case analysisc | 1022 (673 to 1372) | 0.018 (–0.021 to 0.058) |
| Complete-case analysis with no adjustmentd | 955 (432 to 1477) | –0.030 (–0.093 to 0.033) |
Discussion
The economic analysis of the HELP trial showed that the intervention had similar outcomes but higher costs when compared with enhanced standard care. Resource use during follow-up was similar between the trial arms; therefore, the cost of the HELP intervention was the major driver of the cost difference.
Given that there were no differences in outcomes or resource use during follow-up between groups, and given the higher intervention costs, these findings indicate that the HELP intervention is not cost-effective.
As noted, there are few evaluations of childhood obesity interventions that include an economic evaluation with which to compare these results; however, differences between studies in terms of age of participants, country, components of the interventions and outcome measures make comparisons with other studies difficult.
Implications for policy
The health risks and health-care costs associated with overweight and obesity are considerable. A public health approach to develop lifestyle interventions in adolescents should target factors contributing to obesity and barriers to lifestyle change at personal, environmental and socioeconomic levels. 146
The HELP intervention was no more efficacious than standard care of a single educational session for reducing BMI in a community sample of obese adolescents. This adds to a large literature on negative weight management trials for children and adolescents. Further work to understand how weight management programmes can be delivered effectively to young people should be considered. Such research must also consider the cost implications of weight management programmes.
Strengths and weaknesses
The HELP intervention was designed specifically for adolescents who have difficulty losing weight and encouraged parents to participate and support their children. The major strengths of the study are that it is based on data from a RCT and that best practice methods have been used in the economic evaluation. The use of subject-level data allowed a comprehensive and accurate assessment of all health-care costs, which was more reliable and less biased than use of self-reported data. Moreover, the numbers of control and intervention subjects in the economic evaluation were balanced and their baseline characteristics were similar, although there were slightly fewer black subjects and slightly larger numbers of white and Asian subjects in the HELP arm.
A limitation of the study was the difficulties in recruiting obese adolescents; therefore, the eligibility criteria were widened to include adolescents with morbid obesity. This represented only 4.6% of subjects,138 making it unlikely to influence the outcomes.
The economic analysis had a time horizon of only 1 year, reflecting the duration of follow-up in the trial. Although this is a limited duration, given that no differences in utility values, QALYs or resource use during follow-up were found between groups up to 12 months, then measuring costs and benefits after 1 year using modelling was unlikely to change the results.
Only NHS costs were included in the evaluation and not, for example, costs to subjects and families. Given that there were no differences in health-care resource use during follow-up or utility values between groups, then including these costs is unlikely to change the conclusions. These costs may make the intervention appear less cost-effective if the HELP intervention costs were to increase when incorporating time and travel costs borne by subjects and families to participate in the intervention.
Implications for future research
Future research is needed to identify the most cost-effective lifestyle intervention for obese adolescents, including its length and intensity as well as long-term sustainability into adulthood.
Summary
The HELP trial showed that changes in BMI in obese adolescents who received the intervention are similar to those seen in adolescents who received enhanced standard care. The complementary economic analysis has further shown that there were no differences in HRQoL and health-care resource use during 12 months’ follow-up between the two groups. The HELP intervention was more costly than the control because of higher intervention costs, and so is unlikely to be cost-effective.
Key findings across study C
-
Across all young people in the study, approximately one-third reduced their BMI, one-third increased their BMI and one-third retained the same BMI at 6 and 12 months after starting the study.
-
Recruitment to the trial was difficult, with a large degree of work required to recruit obese young people into the trial.
-
No difference was found in the BMI of young people between the HELP group and the control group at 6 or 12 months. This means that HELP and the single nurse-delivered session had very similar effects on the BMI of young people and their cardiovascular risk over the 6 and 12 months of the study.
-
Regarding secondary outcomes, there was no difference in young people’s waist circumferences, blood pressure and other cardiovascular risk markers (e.g. cholesterol, glucose and insulin), measures of QoL, self-esteem or problem eating behaviours at 6 or 12 months between those young people who received HELP and those who received the nurse session.
-
Regardless of trial group, young people and their families reported that joining the trial had helped them make positive changes to their diet, activity levels and motivation.
-
Mean intervention costs per subject were £918 for HELP and £68 for enhanced standard care. Adjusted costs were significantly higher in the intervention group [mean incremental costs for HELP vs. enhanced standard care £1003 (95% CI £837 to £1168)]. There were no differences in adjusted QALYs between HELP and the nurse session (standard care).
Chapter 5 Study D: evaluation of anti-obesity drug treatment in children and adolescents
Abstract
Background
Three drugs were approved for obesity treatment in the UK between 1998 and 2006 (although the last two were subsequently withdrawn from the market): orlistat (Alli®; GlaxoSmithKline plc, Brentford, UK), sibutramine (Meridia®; Abbott Laboratories, Abbott Park, IL, USA) and rimonabant (Acomplia®; Sanofi, Paris, France). In 2014, the revised NICE guidelines recommended that orlistat was appropriate for obesity management in children and adolescents aged ≥ 12 years in certain circumstances. 12 The aims of this study were to (1) gain a better understanding of young people’s and families’/carers’ experiences of using AODs, (2) use this improved understanding to develop interventions that optimise AOD usage in young people and (3) analyse GP prescribing patterns of AODs and compare their prescribing to current recommendations.
Methods
To assess the aims, a series of studies were conducted: (1) a systematic review and meta-analysis evaluating the efficacy and safety of AODs from published RCTs in children and adolescents; (2) cohort studies to investigate prescribing patterns [for orlistat, sibutramine, metformin (Glucophage®; Merck Serono, Darmstadt, Germany)] to young people in primary care settings; (3) a survey study to determine current practice in prescribing these drugs to young people for obesity treatment, with examining GP-completed questionnaires across UK general practices (in England, Scotland, Wales and Northern Ireland); and (4) semistructured interviews with young people (i.e. aged 13–18 years) and their parents from three specialist obesity clinics, to understand children’s and families’ perspectives of treatments.
Key results
Evidence from published RCTs showed that orlistat and sibutramine (in combination with behavioural therapy) significantly reduced BMI compared with placebo in young people.
Prescribing of AODs (orlistat, sibutramine) to children and adolescents increased 15-fold between 1999 and 2006, but approximately 45% of orlistat and 25% of sibutramine prescriptions were discontinued after 1 month of treatment, and before patients could see any weight benefits.
There was also a steady increase in metformin prescribing to obese and overweight young people, particularly in girls aged 16–18 years.
In 121 returned questionnaires (80% response rate), 61% of GPs reported issuing the drugs to their patients without secondary or tertiary care team advice. Despite comprehensive guidance provided in the NICE guideline, GPs expressed a need to develop a new guide for prescribing AODs to young people.
Young people and parents described side effects as a significant experience and few adhered to prescribed regimens, independently changing lifestyle and dosage to tolerate medications.
Conclusions
General practitioners require more support from a specialist in managing the treatment of obesity in children in the community. Pharmacological treatment is unlikely to be effective without further assistance, such as lifestyle modification or psychological support. There should be further research to develop a collaborative, prospective, obesity management surveillance network for children and adolescents across other specialist centres in the UK. This could examine effectiveness, safety and prescribing patterns of medications along with other interventions (e.g. psychological intervention, bariatric surgery) for weight management in clinical practice.
Key findings across study D
-
Evidence from published RCTs has shown that orlistat and sibutramine significantly reduce BMI compared with placebo in young people: orlistat together with behavioural therapy reduced BMI by 0.83 kg/m2, with a large number of gastrointestinal adverse drug reactions. Sibutramine with behavioural therapy reduced BMI by 2.20 kg/m2. The pooled analysis based on 12 RCTs showed a BMI reduction of 0.64 kg/m2 with metformin treatment compared with placebo after 6 months of treatment.
-
The majority of AOD prescriptions were rapidly discontinued, before patients could see any weight benefits: prescribing of AODs (e.g. orlistat, sibutramine) to children and adolescents increased 15-fold between 1999 and 2006. However, approximately 45% of orlistat and 25% of sibutramine prescriptions were discontinued after 1 month of treatment. This indicates that these drugs are poorly used in the general population.
-
There was a steady increase in metformin prescribing to obese and overweight young people: the use of metformin increased fivefold between 2000 and 2010 in primary care, particularly in girls aged 16–18 years.
-
Use of AODs in primary care is rare, particularly in males and those aged < 16 years. High rates of discontinuation were seen, primarily in those prescribed orlistat. Over half of GPs who initiated pharmacological treatment to obese and overweight young people did not consult a specialist for advice. Rates of compliance with NICE guidance for orlistat were low and GPs report low confidence in the use of AODs in this age group. Improved training and support for GPs is needed to guide AOD use in primary care, for both current and future generations of drugs.
-
Use of AODs is challenging and complex for many adolescents, and few young people describe positive experiences. Few adhered to prescribed regimens, independently changing lifestyle and dosage to tolerate medications. Improved clinician–patient partnership in decision-making and better patient education and subsequent support are likely to improve medication concordance and maximise efficacy.
Background
There has been a dramatic rise in the prescription of AODs in children and adolescents147 in response to the global epidemic of childhood obesity. It is estimated that the global prevalence of child and adolescent obesity in 2006, using conservative definitions by World Health Organization (WHO) region, varied from 3% in South-East Asia to 8% in Europe, rising to 13% in the Americas. 1 Clear evidence of high levels of current and future comorbidity associated with childhood obesity has driven a search for effective treatments of both childhood obesity and related comorbidities, including problems of glucose-insulin homeostasis, dyslipidaemia, hypertension and other metabolic and psychological problems. 148,149 Although attention has focused appropriately on lifestyle modification interventions, a role has been identified for AODs in the treatment of older children and adolescents. 150 In both the USA151 and the UK,11 AODs are recommended for those in whom lifestyle modification has failed or who have significant obesity-related comorbidities. There are few data on the scale of AOD use in children and adolescents.
At the time of planning the PROMISE programme, there were two medications internationally approved for obesity treatment in adults: orlistat (a gastric and pancreatic lipase inhibitor) and sibutramine (a serotonin and noradrenergic reuptake inhibitor). Rimonabant, a selective cannabinoid receptor 1 (CB1) antagonist, was withdrawn worldwide in 2008 because of severe psychiatric side effects.
The US Food and Drug Administration approved the use of orlistat in obese adolescents aged ≥ 12 years, and sibutramine in obese young people aged ≥ 16 years. In the UK, NICE guidance11 identified orlistat and sibutramine as appropriate second-line treatments for those aged ≥ 12 years with significant obesity comorbidities. In 2007, orlistat was approved for over-the-counter (OTC) use in the USA, and in October 2008, the European Medicines Agency (EMA) recommended that orlistat be made available OTC in Europe as well. 152 However, each of these recommendations was based on a limited evidence base, with a small number of RCTs for each drug. Two recent reviews42,153 of obesity treatment in children and adolescents did not adequately address pharmacotherapy for paediatric obesity.
Prior work by this research team had shown that prescribing of AODs for children and adolescents aged ≤ 18 years rose 15-fold in the UK between 1999 and 2006. 154 Notably, this occurred largely before the publication of the NICE guidance in 2006, which endorsed the use of AODs in children and adolescents for the first time. There is a need to have compelling evidence to support AOD use in children. 150
The overall aims of this study were to (1) gain a better understanding of young people’s and families’/carers’ experiences of using AODs, (2) use this improved understanding to help inform future interventions that optimise AOD usage in young people and (3) analyse GP prescribing patterns of AODs and compare their prescribing to current recommendations.
Subsequent to the commencement of PROMISE, sibutramine was withdrawn in Europe (in January 2010) and the USA (in October 2010) as a result of concerns about cardiovascular side effects. Because of this, the use of metformin for non-DM uses in children and adolescents was included as part of this study. Metformin is the most commonly used drug in the management of childhood obesity, although it is not classed as an AOD because it is used as an insulin-sensitising agent in DM.
Substudy 5.1: systematic review and meta-analysis to evaluate the efficacy and safety of anti-obesity drugs
This work was reproduced and published as Russell M. Viner, Yingfen Hsia, T. Tomsic, and Ian C. K. Wong. ‘Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis’. Obesity Reviews, 2010;11:593–602. https://doi.org/10.1111/j.1467-789X.2009.00651.x. 155
Licence to reproduce number (John Wiley & Sons): 4675940164204.
As part of the preparation for the anti-obesity drug (AOD) workstream of PROMISE, we undertook a systematic review and meta-analysis of AOD use in children and adolescents. This was conducted and submitted after the award of the programme, and we therefore include it in the programme outputs. The findings reported here are substantially those reported in the published paper.
Aim
The aim of this substudy was to systematically review the literature and investigate the efficacy and safety on AOD in children and adolescents.
Methods
Literature search
Search strategies drew on published optimal search strategies for drug trials. 156,157 The following databases were search between January 1996 and July 2008 for clinical trials investigating AOD and body weight reduction: MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL). We also searched the trial registers: the metaRegister of Controlled Trials (www.controlled-trials.com), WHO clinical trial registered (www.who.int/ctrp/en/) and the Clinical trials government (www.clinicaltrials.gov/). Hand-searching was also carried out to examine the reference lists of identified studies.
Eligibility criteria
The inclusion criteria for systematic review were published double-blind randomised placebo-controlled clinical trials investigating the effects and safety of AOD (orlistat, sibutramine, rimonabant) for body mass index (BMI) reduction in children and adolescents aged < 20 years, with duration ≥ 6 months. We excluded quasirandomised, open-label crossover trials and studies published only in abstract form. There was no restriction on language. Note that, given that a range of definitions of childhood obesity exist, we included any trials which used an established definition of overweight or obesity (BMI ≥ 85th, 95th or 98th centile; BMI > International Obesity Taskforce definitions). 158 Given our age range, we examined BMI reduction rather than weight loss as the primary outcome, as growing children with a stable weight may exhibit reduced BMI.
Data extraction and quality assessment
Two reviewers (YH, TT) performed the electronic searches and screened the articles independently. Articles that clearly did not meet eligibility criteria were rejected on initial review. Articles marked for potential inclusion were then obtained electronically or in paper copy, and assessed again for inclusion. Disagreement was resolved by consensus.
Those included studies deemed to meet inclusion criteria by both reviewers were appraised. A standardised form was used to record all details of the papers reviewed. 159 The standard form included study design, blinding status, trial duration, mean age of participants, gender, number of participants in treatment and placebo group, interventions and the assessment of intention-to-treat (ITT) analysis. The Quality of Reporting of Meta-analyses (QUOROM) guideline was used for reporting our review. 106
Statistical analysis
We expressed the primary outcome as change in raw BMI rather than in BMI standard deviation score (SDS), as use of BMI SDS masks significant loss of body mass in the very obese during adolescence. 106 We calculated weighted mean differences for continuous outcomes (e.g. BMI) and risk difference for dichotomous outcomes at the end of study follow-up. The meta-analysis used a random-effects model with RevMan 5.0.16 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). The primary outcome analysis (BMI) was based on ITT data from the completion of the randomised trial, prior to any crossover or open-label extension. However, data on secondary outcomes and adverse events were taken from the same trial end point as the BMI data, using the highest-quality data reported in each trial (whether the ITT population or for completers). Where SDs were not reported, these were obtained from standard errors, confidence intervals (CI), t-values or p-values that relate to the differences between means in two groups. The DerSimonian and Laird Q-test was performed to assess the degree of heterogeneity between studies, and the I2-statistic was used to describe the percentage of total variation across studies due to heterogeneity. Owing to the small number of studies, we were unable to assess publication bias by inspection of Funnel plots. Secondary outcomes were included in the meta-analysis if each outcome was reported in more than two studies for each drug. Findings were reported in accordance with the QUOROM consensus statement. 106
Results
The review flow chart is shown in Figure 11. The initial search identified 101 studies, of which 85 were excluded after reviewing the abstracts. The most common reasons for exclusion were study participants were aged > 20 years, review article or ineligible primary outcome. Sixteen studies were appraised in detail and assessed for inclusion in the meta-analysis.
Two were identified as subgroup analyses and excluded. Details of the remaining 14 studies are shown in Table 24. Of eight appraised studies of sibutramine, four were excluded from the meta-analysis: two were open-label studies without a control (placebo) group,160,161 one randomised controlled trial had a study duration of only 3 months162 and one randomised controlled trial excluded subjects with primary or nutritional obesity. 163 Of six studies of orlistat, four were excluded: three were open-label uncontrolled studies,164–166 and one was an open-label non-blinded randomised controlled trial. 167 Two studies of orlistat and four of sibutramine were identified as eligible for meta-analysis (Table 24).
| Study and year of publication | Location | Design | Duration | Age (years) | Sample case: placebo | BMI (standard deviation) pre-treatment | Co-intervention | Dose | Ethnic group | Included for meta-analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Sibutramine RCTs | ||||||||||
| Berkowitz et al., 2003171 | USA | RCT, followed by OL | Phase 1: 6 months RCT | 13–17 | 43:39 | 37.8 ± 3.8 | Behavioural protocol; dietary counselling, encouraged exercise | 15 mg q.d. | White: 54.9% | Yes |
| Black: 41.5% | ||||||||||
| Others: 3.6% | ||||||||||
| Godoy-Matos et al., 2005169 | Brazil | RCT | Phase 2: 6 months OL 6 months | 14–17 | 30:30 | Case | 500 kcal/day deficit diet; dietary counselling; encouraged exercise | 10 mg q.d. | Brazilian: 100% | Yes |
| Female: 37.5 ± 3.8 | ||||||||||
| Male: 37.6 ± 4.3 | ||||||||||
| Placebo | ||||||||||
| Female: 35.8 ± 4.2 | ||||||||||
| Male: 37.4 ± 1.9 | ||||||||||
| Berkowitz et al., 2006170 | USA | RCT | 12 months | 12–16 | 368:130 | Case: 36.1 ± 3.8 | 500 kcal/day deficit diet; behaviour protocol; encouraged exercise | 10 mg q.d. (15 mg q.d. if did not lose ≥ 10% BMI from baseline) | White: 57% | Yes |
| Placebo: 35.9 ± 4.1 | Black: 21% | |||||||||
| Hispanic: 16% | ||||||||||
| García-Morales et al., 2006168 | Mexico | RCT | 6 months | 14–18 | 23:23 | Case: 35.1 ± 5.3 | Dietary recommendations | 10 mg q.d. | Mexican | Yes |
| Placebo: 36.6 ± 5.2 | ||||||||||
| Van Mil et al., 2007162 | The Netherlands | RCT | 3 months | 12–18 | 12:12 | Case: 30.1 ± 4.5 | Dietary and exercise advice | 10 mg q.d. | No | |
| Placebo: 33.3 ± 5.0 | ||||||||||
| Danielsson et al., 2007163 | Sweden | Crossover RCT, followed by OL | 20 weeks + 20 weeks crossover; by 6 months OL | 7–20 | 50 | BMI SDS (range): 2.9–9.7 | Lifestyle modification | Phase 1: 10 mg q.d.; At 8 weeks, 15 mg if loss < 4 kg within 8. Phase 2: sibutramine 10 mg or 15 mg | Scandinavian | No |
| Sibutramine open-label trials | ||||||||||
| Reisler et al., 2006160 | Israel | OL | 12 months | 13–18 | 20 | 40 ± 5.6 | Calorie-restricted diet; encouraged exercise | 10 mg q.d. | White | No |
| Violante-Ortíz et al., 2005161 | Mexico | OL | 6 months | 12–18 | 67 | 34.2 ± 6.0 | Diet; aerobic physical activity | 10 mg q.d. | Mexican | No |
| Orlistat RCTs | ||||||||||
| Chanoine et al., 2005172 | USA, Canada | RCT | 54 weeks | 12–16 | 352:181 | Case: 35.7 ± 4.2 | Diet; behavioural modification; exercise counselling | 120 mg t.i.d. | White: 405 | Yes |
| Placebo: 35.4 ± 4.1 | Black: 90 | |||||||||
| Other: 38 | ||||||||||
| Maahs et al., 2006173 | USA | RCT | 6 months | 4–18 | 20:20 | Case: 39.2 ± 1.2 | Dietary counselling; exercise counselling | 120 mg t.i.d. | White | Yes |
| Placebo: 41.7 ± 2.6 | ||||||||||
| Orlistat open-label trials | ||||||||||
| McDuffie et al., 2004164 | USA | OL | 3 months | 12–17 | 20 | 44.1 ± 12.6 | Diet; behavioural programme | 120 mg t.i.d. | White: 10 | No |
| African–American: 10 | ||||||||||
| Norgren et al., 2003166 | Sweden | OL | 12 months | 7–12 | 11 | 33.3 | Diet | 120 mg t.i.d or q.i.d. | Scandinavian | No |
| McDuffie et al., 2002165 | USA | OL | 6 months | 12–17 | 20 | White: 36.2 ± 1.2 | Diet; behavioural programme | 120 mg t.i.d. | Caucasian: 10 | No |
| African–American: 50.3 ± 1.3 | African–American: | |||||||||
| Ozkan et al., 2004167 | Turkey | OC | 5–15 months | 10–16 | 22:20 | Case: 32.5 | 20% reduction in daily calories based on age and gender; increased activity level | 120 mg t.i.d. | White Turkish | No |
| Placebo: 31.2 | ||||||||||
Subjects and co-interventions
Subjects across all trials included in the meta-analysis had similar demographic profiles: the majority were aged 12–18 years, mean BMI was between 30 and 40 kg/m2 and subjects were predominantly white or Hispanic. In each trial, subjects with secondary causes of obesity were excluded, as were those with DM. All trials included a standardised low-fat, low-energy diet and encouragement to exercise, with a variable element of behavioural modification in some trials.
Methodological quality
Studies were all of similar quality. All studies included an ITT analysis, reported eligibility criteria, and co-interventions were similar in intervention and control arms. The main limitation to quality was moderately high attrition rates, averaging 19% for sibutramine studies and 25% for orlistat studies. Most studies did not describe the randomisation process nor comment on allocation concealment or blinding of outcome assessors. Because there was little variation in quality, we did not perform sensitivity analyses according to study quality. Secondary end points were reported inconsistently, and frequently in a subgroup of patients or not in an extractable fashion.
Orlistat
Two studies fulfilled criteria to be included in the meta-analysis, with a total sample size for BMI outcomes of 573 adolescents (see Table 24). One ran for 6 months and one for 12 months. Each used the recommended dose of 120 mg three times daily, and participants also received behavioural, dietary and exercise counselling. Participants in both studies also received multivitamin supplements.
Figure 12 shows the pooled estimate of mean BMI change with orlistat was a reduction of 0.83 kg/m2 (95% CI 0.47 to 1.19 kg/m2) compared with placebo. There was no evidence of heterogeneity. We were unable to undertake an analysis of proportions achieving 5% and 10% reductions in BMI or weight as this was reported in only one study.
FIGURE 12.
Mean reduction in body mass index (kg/m2) with sibutramine and orlistat. IV, instrumental variable. Source: Viner et al. 155
Secondary outcomes for orlistat compared with placebo are shown in Table 25. There were no significant differences in fasting lipids, glucose or insulin between the orlistat and placebo groups. As waist circumference, body fat and blood pressure were each reported in only a single study, these outcomes were not included in the meta-analysis. The effect of orlistat and placebo on changes in vitamin A, D and E levels was not included in the meta-analysis as the dose of multivitamin used in each trial was not specified; however, neither study reported any significant difference in levels of each vitamin between groups during the trial. We were unable to undertake sensitivity analyses for orlistat due to the low study numbers.
| Secondary outcomes (fasting) | Number of studies (sample size: orlistat, placebo) | Weighted mean difference (95% CI) |
|---|---|---|
| Triglycerides (mmol/l) | 2 (567: 368, 199) | 0.00 (–0.17 to 0.18) |
| Cholesterol Total (mmol/l) | 2 (520: 339, 181) | 0.03 (–0.17 to 0.23) |
| HDL | 2 (520: 339, 181) | 0.00 (–0.02 to 0.03) |
| LDL | 2 (518: 338, 180) | –0.05 (–0.11 to 0.01) |
| Glucose (mmol/l) | 2 (452: 298, 154) | 0.02 (–0.25 to 0.28) |
| Insulin (mU/l) | 2 (437: 287, 150) | –0.41 (–4.83 to 4.01) |
| Adverse reactions | Number of studies (sample size: orlistat, placebo) | Risk difference (95% CI) |
| Fatty/oily stool | 2 (467: 368, 199) | 0.53 (0.27 to 0.79) |
| Oily spotting | 2 (467: 368, 199) | 0.49 (0.00 to 0.99) |
| Oily evacuation | 2 (467: 368, 199) | 0.51 (–0.08 to 1.10) |
| Faecal urgency | 2 (467: 368, 199) | 0.10 (0.04 to 0.16) |
| Flatus with discharge | 2 (467: 368, 199) | 0.17 (0.12 to 0.21) |
| Flatulence | 2 (467: 368, 199) | 0.05 (0.01 to 0.09) |
| Faecal incontinence | 2 (467: 368, 199) | 0.08 (0.05 to 0.11) |
Adverse reactions for orlistat compared with placebo are shown in Table 25. Those taking orlistat were significantly more likely to experience a range of gastrointestinal side effects. It was not possible to assess the risk of any gastrointestinal event or study discontinuation due to gastrointestinal side effects.
Sibutramine
Data from four randomised controlled trials were included in the meta-analysis, with a total sample size for BMI outcomes of 686 adolescents (see Table 24). Three studies ran for 6 months and one study for 12 months. Two studies used a dose of 10 mg sibutramine per day,168,169 one study used 10 mg per day for the first 6 months, increasing the dose to 15 mg per day for the second 6 months if subjects had failed to lose ≥ 10% of initial BMI,170 and one study used a dose which increased from 5 to 15 mg over the first 7 weeks. 171 Figure 12 shows that the pooled estimate of mean change in BMI for sibutramine was a reduction of 2.20 kg/m2 (95% CI –2.83 to –1.57 kg/m2). There was no significant heterogeneity. Secondary outcomes for sibutramine compared with placebo are shown in Table 3. Sibutramine treatment increased the absolute percentage of 5% and 10% BMI responders by 45% and 39% respectively, and decreased waist circumference by nearly 6 cm on average, compared with placebo.
Sibutramine was associated with significant improvements in triglycerides and high-density lipoprotein (HDL)-cholesterol compared with placebo (two studies each). Data on the effect of sibutramine on body composition (e.g. fat mass loss) were unavailable. Sensitivity analyses for sibutramine: we conducted sensitivity analyses for study duration (6 or 12 months) and for the use of a behaviour therapy (BT) programme as a co-intervention. A BT co-intervention was used for all study participants in two170,171 of the four sibutramine studies. Sibutramine plus BT co-intervention produced a mean BMI reduction of 2.23 kg/m2 (95% CI –3.12 to –1.34 kg/m2) compared with placebo and BT. Sibutramine without BT produced a mean BMI reduction of 2.04 kg/m2 (95% CI –3.50 to –0.58 kg/m2). The ITT analysis was undertaken at 6 months in three studies and at 12 months in one study. Sibutramine produced a mean BMI reduction of 2.60 kg/m2 (95% CI –3.16 to –2.04 kg/m2) in the single 12-month study32 and 1.95 kg/m2 (95% CI –2.81 to –1.08 kg/m2) in the three 6-month studies. We repeated the 6-month analyses with the addition of intermediate non-ITT data from 6-month assessments in the single 12-month study; mean BMI reduction across the four studies at 6 months was largely unchanged: –2.02 kg/m2 (95% CI –2.49 to –1.55 kg/m2). It was not possible to undertake analyses by sibutramine dose. Adverse reactions for sibutramine compared with placebo are shown in Table 26. Those receiving sibutramine had higher systolic (1.4 mmHg) and diastolic (1.7 mmHg) blood pressure and heart rate (4.7 beats per minute). As hypertension was not an exclusionary condition in all studies, and because of variable data presentation, we were unable to assess trial withdrawal due to hypertension across the studies. Those taking sibutramine were also significantly more likely to experience dry mouth but no other adverse events.
| Secondary outcomes | Number of studies (sample size: sibutramine, placebo) | Risk difference (95% CI) |
|---|---|---|
| Loss of ≥ 5% of initial BMI | 4 (548: 377, 171) | 0.45 (0.32 to 0.59) |
| Loss of ≥ 10% of initial BMI | 4 (548: 377, 171) | 0.39 (0.31 to 0.65) |
| Waist circumference (cm) | 4 (542: 375, 167) | Weighted mean difference (95% CI) |
| Triglycerides (mmo/l) | 2 (395: 299, 96) | –5.78 (–7.03 to –4.52) |
| Cholesterol total (mmo/l) | 2 (84: 46, 38) | –0.31 (–0.39 to –.0.23) |
| HDL | 2 (395: 299, 96) | –0.02 (–0.72 to 0.69) |
| LDL | 2 (84: 46, 38) | 0.09 (0.05 to 0.13) |
| Glucose (mmo/l) | 2 (84: 46, 38) | –0.18 (–0.62 to 0.25) |
| Insulin (mU/l) | 2 (395: 300, 95) | –4.21 (–9.79 to 1.38) |
| Systolic blood pressure (mmHg) | 4 (658: 453, 205) | 1.38 (0.13 to 2.63) |
| Diastolic blood pressure (mmHg) | 4 (658: 453, 205) | 1.73 (1.01 to 2.46) |
| Heart rate (beats per minute) | 4 (658: 453, 205) | 4.70 (1.65 to 7.76) |
| Adverse reactions | Number of studies (sample size: sibutramine, placebo) | Risk difference (95% CI) |
| Headache | 3 (598: 419, 179) | –0.08 (–0.24 to 0.08) |
| Dry mouth | 3 (598: 419, 179) | 0.06 (0.01 to 0.11) |
| Dizziness | 2 (558: 398, 160) | 0.02 (–0.02 to 0.06) |
| Abdominal pain | 2 (558: 398, 160) | 0.00 (–0.05 to 0.06) |
| Constipation | 2 (558: 398, 160) | 0.14 (–0.12 to 0.40) |
| Flu-like symptoms | 2 (558: 398, 160) | 0.01 (–0.04 to 0.05) |
Discussion
Meta-analysis of randomised controlled trials of AODs in children and adolescents with primary obesity showed that both orlistat and sibutramine result in significant BMI reduction compared with placebo over 6–12 months: 0.83 kg/m2 for orlistat and 2.20 kg/m2 for sibutramine. As the SD for BMI in overweight and obese adolescents is approximately 3.5 kg/m2 in US and UK populations,65,174 for sibutramine this therefore equates to an 0.63-SD reduction in BMI. This is a clinically meaningful effect size; recent longitudinal epidemiological data suggest that each additional BMI SD at age 13 years increases risk of non-fatal cardiovascular events by 11–17% and of fatal cardiovascular events by 23–24%. 148 Sibutramine also increased the absolute percentage of those achieving a ≥ 10% BMI loss by approximately 40%, reduced waist circumference by a mean of 5.8 cm compared with placebo (an effect size of approximately 0.6 SDs)175 and minimally improved HDL-cholesterol. Sensitivity analyses suggested that the addition of BT programmes to sibutramine minimally increased mean BMI loss (by approximately 0.2 kg/m2), and that a longer duration of sibutramine use may increase BMI loss by approximately 0.6 kg/m2. Adverse reactions with sibutramine included significant but small increases in systolic and diastolic blood pressure and heart rate. Sibutramine did not increase the risk of other adverse events except for dry mouth.
The effect size for orlistat was smaller and of borderline clinical significance at 0.24-SD reduction in BMI. Orlistat had no beneficial or adverse effects on metabolic outcomes. Orlistat was associated with an approximately 50% increase in minor gastrointestinal adverse events, such as oily spotting, and an 8–17% increase in the absolute incidence of more major gastrointestinal events, such as flatus with discharge and faecal incontinence.
Comparison with the literature
Our findings are similar to another meta-analysis of AODs in children and adolescents, by McGovern et al. ,153 who reviewed drug trials as part of a wider systematic review of childhood obesity treatment. They reported a mean BMI reduction for sibutramine of 2.4 kg/m2 from three randomised controlled trials, similar to our finding of 2.2 kg/m2, and a mean BMI reduction for orlistat of 0.7 kg/m2, again similar to our finding of 0.83 kg/m2. However, this review included unblinded studies, failed to identify eligible trials, included sibutramine and orlistat as subcategories within a larger random-effects meta-analysis and did not undertake sensitivity analyses or examine secondary outcomes in detail. A second (Cochrane) systematic review by Oude Luttikhuis et al. 42 reported similar findings for orlistat (mean reduction: 0.76 kg/m2). This review included only two small studies in the meta-analysis for sibutramine, reporting a mean reduction of 1.66 kg/m2, which is considerably lower than our estimate. Neither of these studies undertook a meta-analysis of adverse events with orlistat or sibutramine. Our findings also align with an update of the Cochrane review in November 2016,176 which again reported similar findings for orlistat but noted the poor overall quality of the literature.
We are unable to directly compare our findings with adult studies of AOD, either in terms of absolute BMI loss or proportions who lost ≥ 5% or ≥ 10% of initial weight. We did not include weight loss in our review, as BMI reduction is the goal of treatment in childhood and adolescence,177 growing children may lose BMI while gaining weight, and BMI centiles continue to shift in later adolescence even after height growth has ceased. 65 However, approximate comparisons can be made for older adolescents who are at or near final height. Meta-analyses in adults show that weight loss from sibutramine and orlistat are limited to 3–4 kg over 12 months. 178 In contrast, the weight loss corresponding to a loss of 2.2 kg/m2 related to sibutramine use in 14- and 15-year-old adolescents with a height on the 50th centile for sex is approximately 6 kg for both boys and girls, a relationship that holds true across the obese BMI range. The corresponding weight loss for orlistat is approximate 2.3 kg. These figures suggest the possibility that sibutramine therapy may be more effective in obese adolescents than in adults. The reasons for this are unclear. This may be an artefact of the paucity of data on adolescents and the lack of long-term data. Alternatively, this may suggest that sibutramine is more potent in suppressing appetite in adolescents, possibly because of developmental immaturity in hypothalamic appetite control systems.
We found sibutramine to have modest beneficial effects on triglycerides and HDL-cholesterol, similar to findings in adults. 178 However, we found no evidence of beneficial metabolic effects associated with orlistat use, in contrast to meta-analysis in adults, which suggests that orlistat has small beneficial effects on LDL and total cholesterol. 178 The reasons for this difference are unclear but may relate to the modest BMI loss seen with orlistat in adolescents and the small number of studies.
Study quality was relatively high; attrition rates (19% for sibutramine and 25% for orlistat) were moderately high, but lower than those reported in a meta-analysis of adult trials of these drugs (30% for orlistat, 40% for sibutramine). 178 However, the number of studies of each drug in adolescents is small, and trials have all been undertaken in secondary care settings, limiting the generalisability of findings. We identified no published studies with a duration ≥ 12 months, so we were unable to examine long-term maintenance of BMI loss.
Our findings apply to young people with simple or primary obesity. However, we note that our BMI effect size finding for sibutramine is similar to the 0.7-SD BMI reduction reported by Danielsson et al. 163 in a randomised controlled trial of sibutramine for 20 weeks in adolescents with secondary or monogenic obesity that was not eligible for our meta-analysis.
Safety
The safety profile of orlistat was similar to that noted in adults (i.e. a marked increase in unpleasant and anti-social gastrointestinal experiences but there was little evidence of significant health risk). Theoretical concerns about fatsoluble vitamin deficiencies were not supported although subjects in both trials were given multivitamins. In contrast, sibutramine was generally well tolerated by subjects but was associated with small rises in systolic and diastolic blood pressure and resting heart rate. The magnitude of these changes is highly similar to that seen in adult studies; a recent meta-analysis found that sibutramine increased adult systolic blood pressure by 1.7 mmHg, diastolic blood pressure by 2.4 mmHg and heart rate by 4.5 beats/minute. 178 Even small increments in blood pressure can have an adverse impact on cardiovascular risk in the long term, particularly in at-risk groups, such as obese adolescents, who often have high blood pressure compared with peers.
Authorities note that the long-term safety of anorectic agents has not been established in children and adolescents. 150 However, the clinical significance of small blood pressure increments over a short treatment period remains unclear, particularly when balanced against the beneficial cardiovascular effects of successful weight reduction.
Strengths and limitations
We undertook a rigorous systematic review and meta-analysis using independent reviewers adhering to the established Cochrane Collaboration methodology. In contrast to adult studies,178 studies in our review included a range of non-white ethnic groups.
However, our findings have several limitations which need to be considered. First, all published studies have demonstrated efficacy of BMI and body weight reduction in both orlistat and sibutramine. This suggests the possibility of publication bias; however, there were too few studies for either drug to warrant the generation of funnel plots to assess publication bias. Second, there was moderate but non-significant statistical heterogeneity between studies in BMI outcomes from sibutramine use. This was addressed by using a random-effects meta-analysis. It is likely that this heterogeneity is the result of differences in co-interventions, study duration and study populations. We included studies of differing length in the meta-analysis, as it was not possible to standardise duration owing to the differing timings of the ITT analysis. We did not have access to individual patient data to investigate the cause of this heterogeneity. Third, for sibutramine, we excluded one trial from the meta-analysis as study duration was only 3 months and an ITT analysis was not performed. 162 Repeating the meta-analysis for BMI including this study reduced the estimate of BMI reduction to –1.8 kg/m2 (95% CI –2.65 to –0.95 kg/m2). Fourth, all included studies were conducted in specialist environments, and the generalisability of these findings to more general populations of obese adolescents is unclear. Finally, our analyses included only data that were extractable from studies, which may be a source of bias as studies may publish only secondary outcomes that differed significantly from placebo. This was the case for the largest included trial of sibutramine, by Berkowitz et al. ,170 which published only metabolic outcomes that differed significantly between sibutramine and placebo, and we were unable to include this study’s data on other not significantly different secondary outcomes in our analyses. However, we believe that this is unlikely to have been important, as we found no significant mean difference for any of these secondary outcomes in the studies that were included in meta-analyses for these outcomes.
Conclusion
Sibutramine together with behavioural support in obese adolescents produces a clinically meaningful reduction in BMI of 0.6–0.8 SDs and is well tolerated. In contrast, orlistat together with behavioural support has limited utility as a weight reduction treatment in adolescents, producing a small effect (0.24 to 0.3 SDs) with frequent gastrointestinal side effects. Further studies of the effectiveness of sibutramine in a range of clinical populations of young people are needed to assess effectiveness and longer-term maintenance of BMI loss.
Substudy 5.2: current practice in the prescription of anti-obesity drugs
The unlicensed use of metformin in children and adolescents in the UK
This work was reproduced and published as Hsia Y, Dawoud D, Sutcliffe AG, Viner RM, Kinra S, Wong IC. Unlicensed use of metformin in children and adolescents in the UK. Br J Clin Pharmacol 2012;73:135–9. https://doi.org/10.1111/j.1365-2125.2011.04063.x. 179
Licence to reproduce number (John Wiley & Sons): 4675940922641.
Background
Metformin is the most commonly prescribed oral antidiabetic drug for DM in children and adolescents in the UK. 180 As metformin has been shown to be effective in reducing testosterone concentrations and improving irregular menstrual cycles,181 it has also been prescribed for the treatment of polycystic ovarian syndrome (PCOS) in women of reproductive age. 182,183 However, there is still controversy regarding metformin use in PCOS. Two large randomised controlled trials (RCTs) did not show metformin to be more efficacious than placebo in adult women with PCOS. 184,185 In adolescents, RCTs have shown the effectiveness of metformin treatment in girls with PCOS. 186–188 In contrast to these findings, a recent RCT did not show benefit from metformin treatment along with lifestyle modification in adolescents with PCOS. 189 Despite the controversy, metformin is still recommended as one of the therapeutic options for PCOS in teenage girls. 181 In addition to PCOS, metformin is also effective as an anti-obesity drug due to its effect on insulin resistance. 190 Metformin may also have other effects on weight loss, as it reduces hepatic glucose production, inhibits fat cell lipogenesis, increases peripheral insulin sensitivity and may reduce food intake. 150 Studies have shown that metformin is associated with moderate BMI reduction in obese non-diabetic adolescents. 191,192 In the UK, metformin is licensed for children over the age of 10 years with T2DM who have failed strict dieting. 193 At present, metformin is not licensed for the treatment of PCOS and obesity in adults or children in the UK. 193,194 Little is known about the extent to which this drug has been used in young people in UK primary care.
Aims
To examine metformin prescribing patterns of general practitioners (GPs) for children and adolescents in the UK primary care setting.
Methods
A retrospective cohort study was conducted using a primary care database, the IMS Disease Analyzer (IMS DA) database. This database contains approximately two million anonymous patient records and over 95 million prescriptions from about 125 general practices and more than 500 GPs. 195 In the UK, virtually all patient care is managed by GPs in primary care. When patients are seen in secondary care (e.g. hospital), consultants or specialists will make the diagnosis and initiate treatment, and GPs will usually continue to monitor patients and issue prescriptions. Electronic medical records are routinely used in UK general practice. GPs usually enter diagnosis and prescription information into the electronic medical records to assist them in patient management. 196
Information held on the database includes patient demographics, indications for treatment and prescription details. Prescribed drugs are coded based on the Anatomical Therapeutic Chemical classification issued by the European Pharmaceutical Market Research Association,197 and medical diagnoses are coded in accordance with the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), codes. 198 The database has been shown to be of high quality and is widely used in paediatric pharmacoepidemiological studies. 199–201 This study consisted of children and adolescents aged 0–18 years registered with a GP who contributed data to the IMS DA between January 2000 and December 2010. All subjects needed to have a minimum of 6 months’ valid data in the database. Age bands were based on the modification of the International Conference of Harmonisation as follows: < 2, 2–11, 12–15 and 16–18 years. 202 Prevalence was calculated as the total number of subjects with at least one prescription of metformin during each year of investigation divided by the total number of study subjects registered on the database in the same year, stratified by age and gender. Annual prevalence of metformin prescribing was calculated using Poisson distribution with a 95% confidence interval (CI). A chi-squared test (Cochran–Armitage test for trend) was used to examine the yearly trend of metformin prescribing. As IMS DA directly links prescriptions to medical indications, the following indications were examined for metformin prescriptions: DM (ICD-10 codes E10–E14), PCOS (ICD-10 code E282) and obesity (ICD-10 code E66). 198 Analyses were carried out using Stata, version 11.0.
This study protocol was approved by the IMS Independent Scientific and Ethical Advisory Committee.
The PRISMA guidelines were followed in undertaking the review and reporting.
Results
A total number of 2674 metformin prescriptions were issued to 337 children and adolescents between 2000 and 2010, 80% of whom were female (n = 270).There were no metformin prescriptions issued to children aged < 2 years. The majority of children were taking metformin for DM treatment were aged 2–11 years. The metformin prescribing started at age 5 years for DM treatment. The number of female adolescents aged 12–18 years who received metformin treatment steadily increased over time (Table 27). The annual prevalence of metformin prescribing increased from 0.03 per 1000 person-years (95% CI 0.02 to 0.05 per 1000 person-years) to 0.16 per 1000 person-years (95% CI 0.12 to 0.20 per 1000 person-years; p = 0.001) (Figure 13).
| Year | Age (years) | Total | Person-time (person-years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 2 | 2–11 | 12–15 | 16–18 | |||||||||
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Total | ||
| 2000 | 0 | 0 | 2 | 4 | 0 | 3 | 1 | 10 | 20 |
266 537.2 |
257 100.8 |
523 638.0 |
| 2001 | 0 | 0 | 1 | 9 | 1 | 3 | 5 | 18 | 38 |
265 419.0 |
256 182.8 |
521 601.7 |
| 2002 | 0 | 0 | 4 | 5 | 2 | 7 | 1 | 18 | 37 |
263 778.0 |
254 243.0 |
518 020.9 |
| 2003 | 0 | 0 | 7 | 5 | 7 | 10 | 3 | 29 | 61 |
258 312.5 |
248 746.9 |
507 059.4 |
| 2004 | 0 | 0 | 5 | 7 | 6 | 15 | 2 | 38 | 73 |
244 468.2 |
235 088.5 |
479 556.7 |
| 2005 | 0 | 0 | 5 | 6 | 5 | 9 | 5 | 34 | 64 |
233 251.9 |
223 886.7 |
457 138.6 |
| 2006 | 0 | 0 | 7 | 8 | 5 | 9 | 5 | 41 | 75 |
226 924.2 |
218 008.5 |
444 932.7 |
| 2007 | 0 | 0 | 1 | 6 | 4 | 11 | 3 | 41 | 66 |
219 137.0 |
210 379.6 |
429 516.6 |
| 2008 | 0 | 0 | 4 | 6 | 1 | 15 | 10 | 29 | 65 |
211 939.1 |
203 246.9 |
415 186.0 |
| 2009 | 0 | 0 | 2 | 4 | 3 | 14 | 7 | 28 | 58 |
201 208.5 |
192 740.3 |
393 948.8 |
| 2010 | 0 | 0 | 3 | 3 | 3 | 13 | 5 | 34 | 61 |
191 773.4 |
183 644.6 |
375 418.0 |
FIGURE 13.
Overall prevalence of metformin prescribing in children and adolescents (age: 0–18 years), between 2000 and 2010. Source: Hsia et al. 179
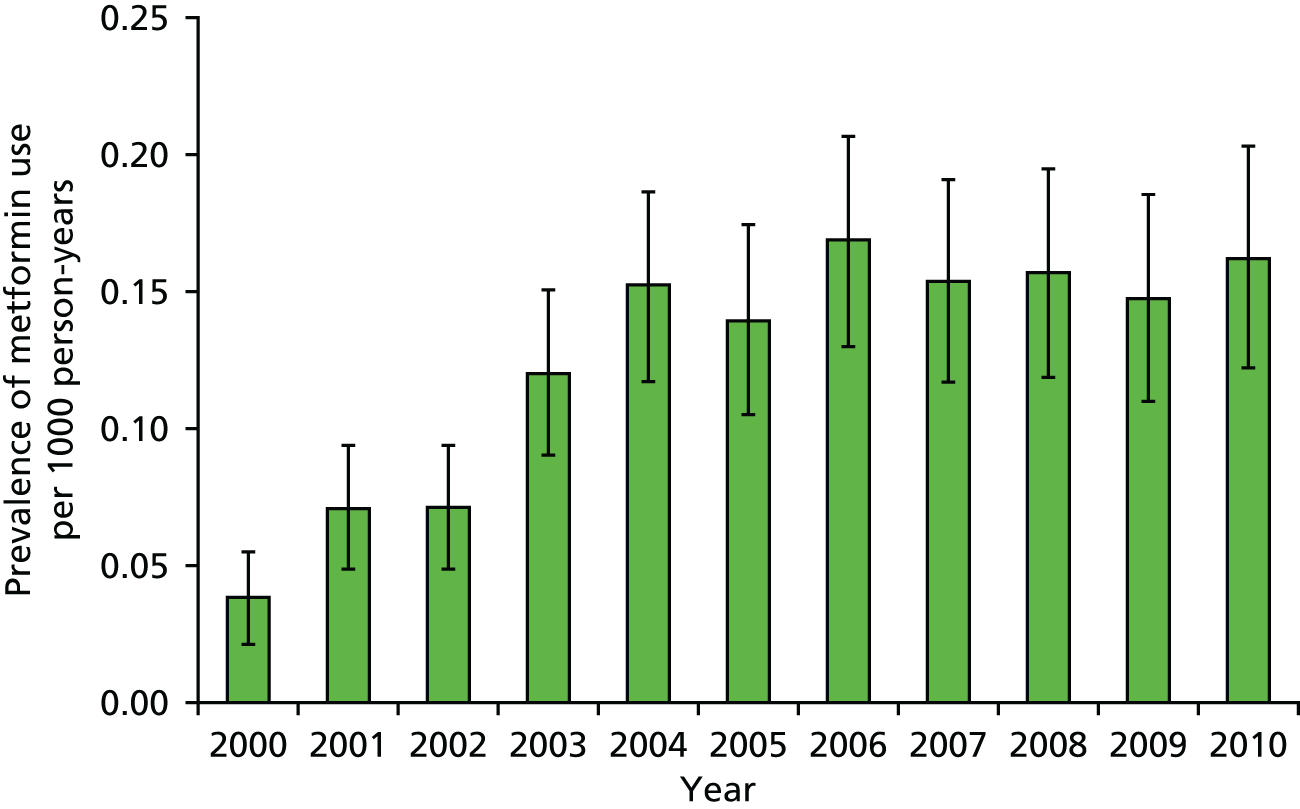
There were a total of 290 patients with at least one diagnosis of DM, PCOS or obesity in their medical records, of whom 235 patients were female (81%) (Table 28). Of 290 patients, 120 female patients were prescribed metformin for the treatment of PCOS and obesity. There were 23 female patients with DM, PCOS and obesity diagnoses who received metformin treatment during the study period. There were 22 patients (7.6%, 22/290) who were prescribed metformin for obesity treatment alone. A total of 47 patients were prescribed metformin without specific relevant diagnosis. After scrutinising their medical records, the most common diagnosis for prescribing ‘unknown and unspecified causes of morbidity’. As IMS DA contains data only from GPs, there is no hospital record in the database to verify diagnoses for these prescriptions.
| Diagnosis | Number of patients | ||
|---|---|---|---|
| Boys | Girls | Total | |
| DM only | 48 | 66 | 114 |
| Obesity only | 4 | 18 | 22 |
| PCOS and obesity | NA | 120 | 120 |
| DM and obesity | 3 | 8 | 11 |
| DM, PCOS and obesity | NA | 23 | 23 |
Discussion
Our study showed that the use of metformin in the paediatric population increased markedly between 2000 and 2010 in primary care, with prescribing prevalence increasing from 0.03 to 0.16 per 1000 person-years. This increase was particularly marked among girls aged 12–18 years.
There are limited data on paediatric metformin prescribing patterns in the UK. As some prescribing databases do not have links with indications for prescribing, an added strength of this study was that we were able to identify the disease indication for metformin therapy. However, our findings are subject to some limitations. First, the IMS DA records only prescriptions issued in primary care, excluding prescriptions dispensed from hospitals. It is possible that our data did not include a small number of initial hospital prescriptions. While the great majority of these would have been continued in primary care, unfortunately there are no data to investigate the extent of metformin prescribing in hospitals. Second, we were unable to identify whether subjects were treated with lifestyle modification along with metformin, as healthy diet and exercise are mainstays of the treatment for obesity, PCOS and T2DM. 181,183 Third, we have no information on diagnostic criteria used for any of the conditions under study as diagnoses are often made in secondary care and as the data set does not record criteria for those made in primary care. While diagnostic criteria for T2DM are internationally accepted, a number of different definitions exist for obesity and PCOS. Fourth, the IMS DA does not contain data on ethnicity and socioeconomic status and thus their impact on prescribing patterns remains unstudied.
Our finding that PCOS was the main indication for metformin prescribing in female adolescents was unexpected. This indicates that the use of metformin for PCOS in teenage girls is increasing in general practice. It has been well documented that adolescent obesity is increasing in population-based studies in the USA203 and UK. 147 In addition, a previous study found an increased prevalence of T2DM in adolescents aged 12–18 years in the UK. 180 Therefore, it is possible that the prevalence of PCOS in adolescents may also have increased. Although metformin has been shown to be of benefit in teenage girls with PCOS in a number of studies,186–188 the current evidence is limited and inconsistent. The efficacy of metformin treatment in adolescents with PCOS remains to be confirmed by well-designed RCTs. In addition, to extrapolate treatment from adults to adolescents with PCOS is questionable since the risks and benefits are unclear. There were a small number of patients who received metformin for obesity treatment in our study. This prescribing trend may increase in the next few years. In January 2010, the European Medicines Agency recommended removing sibutramine from all markets in the European Union because of the risk of developing cardiovascular events in adults. 204 Consequently orlistat is the only licensed AOD in the European Union. As there is limited drug choice for obesity treatment, metformin is likely to gain in popularity for obesity treatment. In addition, a systematic review on economic consequences of obesity treatments found that metformin appeared to be a cost-saving treatment for obese patients with T2DM,205 To date, metformin has not yet received a paediatric licence for PCOS or obesity treatment. As it is mainly prescribed for these unlicensed indications in the paediatric population, more studies are needed to demonstrate its efficacy and safety in this population.
In conclusion, metformin prescribing in children and adolescents has increased substantially in the past decade. An increased number of teenage girls are receiving metformin for PCOS treatment in general practice. As metformin is not licensed for PCOS and obesity treatment in the paediatric population, further studies are required to investigate its long-term efficacy and safety for these conditions.
Anti-obesity drug prescribing for obese children and young people in the UK
Adapted from White et al. 206 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Abstract
Aims
Primary care databases show rising prevalence of AOD prescribing to CYP, but these prescriptions are frequently short-lived.
Methods
A primary care database (with 5.7% UK population coverage), The Health Improvement Network (THIN) Additional Information Service (AIS), was used to identify CYP aged ≤ 18 years who were prescribed an AOD between 31 May 2010 and 31 May 2012. A mailed questionnaire to GPs was used to ascertain patient demographics and comorbidities, indications for treatment, and outcomes. We audited against NICE guidance.
Results
A total of 151 patients were identified as receiving orlistat or metformin during the study period. The response rate was 78.9% (119/151). Ninety-four per cent of subjects were eligible for this study. Of these, 86.2% (n = 81) were female. In terms of AODs, 46.8% were prescribed metformin, 58.5% were prescribed orlistat and 5.3% were prescribed both drugs.
Of all orlistat prescriptions, 89.1% (49/55) were initiated in primary care, independent of specialist advice; for metformin, this was 27.3% (12/44). Orlistat was largely prescribed in those aged > 16 years without physical comorbidities. Metformin was initiated for treatment of PCOS (70.5%), insulin resistance (25.0%) and impaired glucose tolerance/impaired fasting glucose (9.1%).
Drug cessation was high, with 5% of orlistat and 57% of metformin prescriptions active at the time of the survey. Median supply of metformin was 10.5 months [interquartile range (IQR) 4.0–18.5 months], compared with 2.0 months (IQR 1.0–4.0 months) for orlistat (p = ≤ 0.001). The proportion of all orlistat treatment lasting a single prescription was 45.5%; none lasted more than 1 year. The majority of all drug terminations were due to families not requesting repeat prescriptions (96.2% for orlistat and 89.5% for metformin) rather than medically led terminations.
Adherence to NICE paediatric guidance for prescribing of orlistat was low: 17% of prescriptions were initiated in the specialist setting and 56% had evidence of obesity-related comorbidity.
General practitioners reported lower confidence in prescribing AODs to CYP than to adults (10-point Likert score median 3 vs. 8; p < 0.0001). GPs requested additional support in the management of childhood obesity, both lifestyle interventions and drugs.
Conclusions
Prescribing of AODs in childhood is rare, with the majority of orlistat prescribing ocurring in those aged > 16 years. GPs requested more support in managing obesity in this group.
Introduction
Little is known about the use of medication for obesity in children and adolescents in the UK, particularly use in primary care. Orlistat is currently the only licensed AOD in the UK since sibutramine was withdrawn owing to concerns about cardiovascular safety. 207 However, the most commonly used AOD in CYP is metformin, an anti-diabetes drug used off-licence to treat the metabolic sequelae of obesity in CYP,192,208 although not formally an AOD. Both orlistat and metformin appear to offer small benefits for BMI reduction in CYP; systematic reviews show small reductions in BMI, compared with placebo, for orlistat (by 0.83 kg/m2)155 and metformin (by 1.4 kg/m2). 191 Fewer data exist for the cardiometabolic benefits of these drugs, with only half of the studies of metformin in the systematic review reporting cardiometabolic outcomes.
In the UK, NICE12 recommends community-based lifestyle modification programmes as the first tier of weight management for childhood obesity, with pharmacotherapy as a second-line treatment. NICE guidance covers only usage of orlistat, which should be prescribed only in exceptional circumstances for those with obesity-related comorbidities (life-threatening in those aged < 12 years) and prescribed only by teams with expertise in these conditions.
Randomised trial data on orlistat and metformin come from specialist clinical settings and largely outside the UK. Very little is known about how these AODs are prescribed and used in actual practice. Pharmacoepidemiology studies of AOD prescribing in primary care in the UK show increasing use of AODs, but also high levels of drug discontinuation, with approximately half of the prescriptions of orlistat not being continued beyond 1 month. 155 The one qualitative study209 examining adolescent use of AODs showed frequent cessation by families independent of their doctors, usually because the perceived advantages did not outweigh the side effects, often with minimal professional support. These data suggest that the effectiveness of AODs in real-life settings may be considerably less than shown in trials, and suggest a need for further research in order to identify strategies to improve the effectiveness of AODs for CYP.
A questionnaire survey of GPs prescribing AODs was undertaken to better understand the use of AODs in primary care in the UK. The aims were to characterise patient demographics, quantify adherence to NICE guidance and identify primary care perceptions of AODs with the long-term aim of optimising AOD prescribing and efficacy.
Methods
Routinely collected primary care data from the THIN database were used to identify general practices that prescribed orlistat or metformin to CYP aged ≤ 18 years between 31 May 2010 and 31 May 2012. THIN covers ≈ 5.7% of the UK population, with 3.6 million active patients from 587 general practices using the Vision General Practice System (In Practice Systems, London, UK). 210 These practices are broadly representative of practices in the UK in terms of patients’ demographics and characteristics. 211 Questionnaire administration was undertaken by THIN AIS, an independent research organisation affiliated with THIN, with data protection firewalls. Patients prescribed metformin for T2DM were excluded.
A paper questionnaire was sent to all identified general practices. The questionnaire was designed by a multidisciplinary study team comprising an academic GP, two paediatricians, a pharmacist and a GP representative. The questionnaire contained questions regarding patient demographics and comorbidities, outcomes and GP experiences and opinions related to AOD usage in children (see Report Supplementary Material 4 for full questionnaire). GPs prescribing AODs were contacted up to three times over 3 months until the questionnaire was returned. GPs received a £35 payment for each completed and returned questionnaire. THIN AIS anonymised questionnaires prior to analysis by the study team.
A patient’s year of birth, practice identification and practice region were provided by THIN AIS. All other data were provided by individual GPs within prescribing practices. BMI was calculated from GP-derived height and weight measurements when available, and zBMI was calculated using the least mean squares (LMS) method and UK reference data. 65 AOD termination was assumed if no prescription had been issued within 3 months of the survey. Age at first prescription was calculated from the mid-point of birth year, as month and day of birth were not provided owing to data protection restrictions. Orlistat use was audited against NICE 2006 recommendations,11 which remain unchanged in the 2014 update,12 bar some text clarifications. For audits against NICE, an assumed birth date of 1 January was used to ensure that no subjects were misclassified as children if they were aged 18 years.
Analyses
Analyses were conducted using Stata 11.0. Simple descriptive statistics were used for the majority of data, including chi-squared test and t-test (paired and independent) for parametric data, and Wilcoxon–Mann–Whitney and Wilcoxon-signed rank tests for unpaired and paired non-parametric data. Thematic analysis was used to analyse qualitative responses. 212
Governance
The study was reviewed and approved by the National Research Ethics Service (NRES) Committee London – Surrey Borders [Research Ethics Committee (REC) reference number 11/LO/1020].
Results
Patient demographics
Figure 14 summarises patient sampling. A total of 151 patients were identified by THIN as having received orlistat or metformin during the study period. GP response rate was 78.8% (119/151). GPs identified 21 out of 119 patients as being ineligible for the study. An additional four subjects were excluded after inspection of questionnaires, owing to duplicate questionnaires received for the same drug with inconsistent responses (n = 1), missing patient demographics and drug details (n = 2), and an empty questionnaire (n = 1).
FIGURE 14.
Patient sampling. Adapted from White et al. 206 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
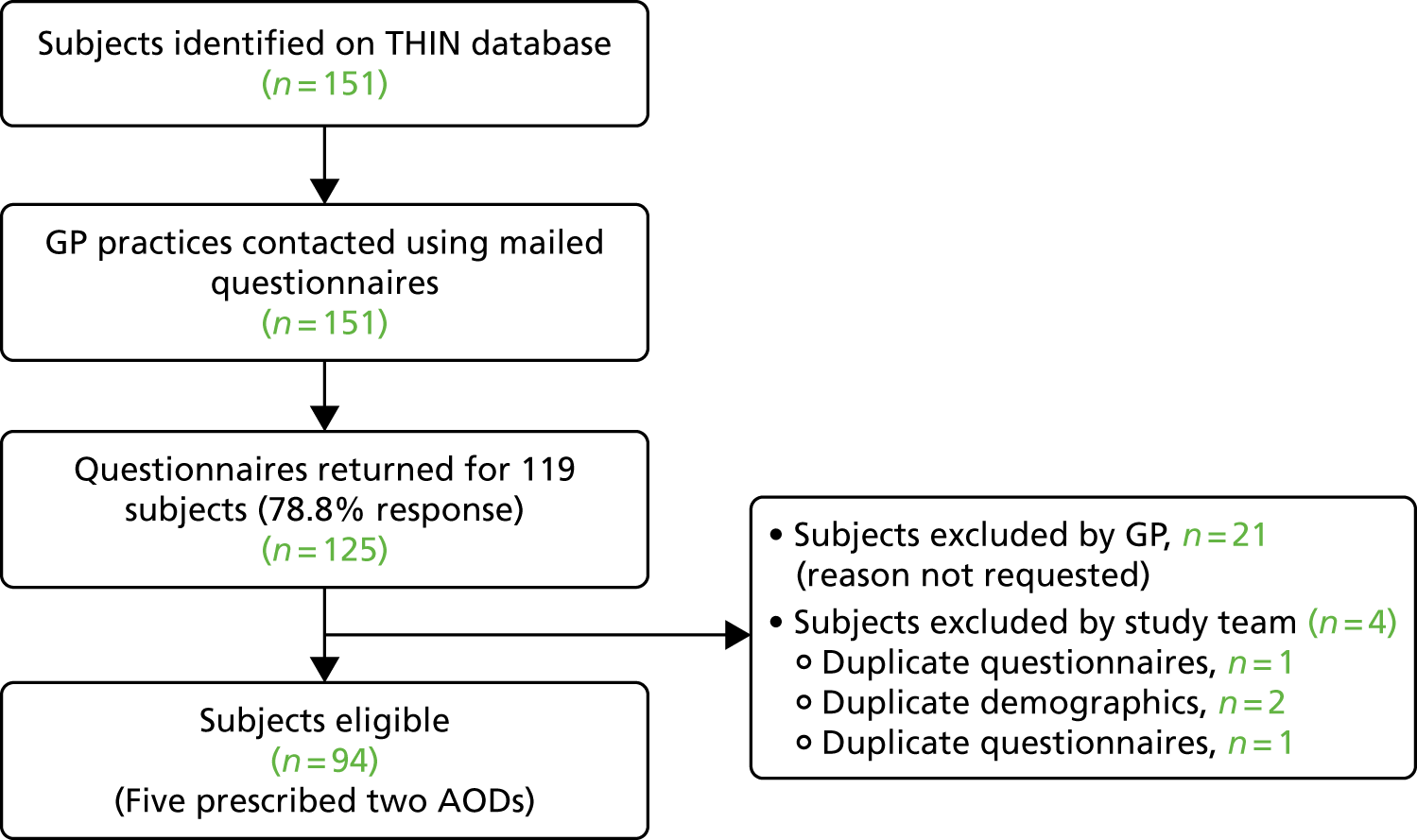
A total of 94 subjects were eligible for this study, of whom 86.2% (n = 81) were female. GPs identified ethnicity as British for 44.7% of patients (29 white, three Pakistani, one mixed, one British-African, one mixed African-white British and seven British), white for 30.9% (n = 29), Asian for 4.3% (n = 4) and other for 4.3% (n = 1 each from the Caribbean, Turkey, Iran and Afro-Caribbean/white mixed); 16.0% (15/94) were of unknown ethnicity. The majority of subjects came from England (78.7%, n = 74); the remainder came from Wales (11.7%, n = 11), Scotland (7.4%, n = 7) and Northern Ireland (2.1%, n = 2).
A total of 99 AOD initiations occurred in the 94 subjects (five subjects were prescribed both orlistat and metformin), consisting of 44 metformin (46.8% of sample) and 55 orlistat (58.5%) prescriptions. Drugs were initiated in 68 practices, with 46 practices prescribing one drug each, 15 practices prescribing two drugs each, six practices prescribing three drugs each and one practice prescribing five drugs.
Table 29 summarises baseline demographic and comorbidities by drug. Comorbidities appeared higher in those taking metformin. Sufficient data were provided to calculate BMI and zBMI during the study period for 90.9% (40/44) of those prescribed metformin and 89.1% (49/55) of those prescribed orlistat. All had a BMI above the 98th centile (> 2 SDs). Of the remaining 10, one had no recorded measurements, seven had no height data and two had no drug start date provided by GPs.
| Demographics and comorbidities | AOD | |
|---|---|---|
| Metformin (N = 44) | Orlistat (N = 55) | |
| Demographics | ||
| Female, % (n) | 90.9 (40) | 83.6 (46) |
| BMI (kg/m2), mean (SD) | 35.9 (6.1) | 37.6 (6.5) |
| zBMI (kg/m2), mean (SD) | 3.2 (0.7) | 3.2 (0.6) |
| Age (years), median (range) | 15.7 (6.5–19.2) | 17.3 (13.8–18.8) |
| < 12 (n) | 5 | 0 |
| 12–15.9 (n) | 17 | 9 |
| 16–17.9 (n) | 16 | 29 |
| ≥ 18 (n) | 4 | 17 |
| Missing age (n) | 2 | 0 |
| Comorbidities, n (%) | ||
| Hypertension | 1 (2.3) | 0 (0) |
| Hyperinsulism/insulin resistance | 13 (29.5) | 0 (0) |
| T2DM | 0 (0) | 3 (5.5) |
| Dyslipidaemia | 1 (2.3) | 3 (5.5) |
| Emotional distress | 12 (27.3) | 17 (30.9) |
| Sleep apnoea | 0 (0) | 1 (1.8) |
| PCOS | 32 (72.7) | 6 (10.9) |
| Orthopaedic issues | 3 (6.8) | 3 (5.5) |
| Pervasive developmental disorder | 3 (6.8) | 0 (0) |
| Hypothyroidism | 1 (2.3) | 3 (5.5) |
Drug initiation
Figure 15 summarises the frequency of drug prescription by age and drug initiator.
FIGURE 15.
Bar graph summarising age at AOD initiation, by drug and prescriber. Adapted from White et al. 206 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

Of 55 orlistat prescriptions, 89.1% (n = 49) were initiated in primary care, independent of specialist advice. Of this 89.1% of prescriptions, 66.7% were to those aged 12–15.9 years, 93.1% were to those aged 16–17.9 years and 94.1% were to those aged > 18 years. Orlistat was not prescribed to any patient aged < 12 years. In the case of orlistat treatment initiated in primary care on specialist recommendation, the recommending specialist was a paediatrician in three cases (50%) and an adult physician, a lipid clinic and a dietitian in one case each (16.7%).
Of 44 metformin prescriptions, 27.3% (n = 12) were initiated in primary care, independent of specialist advice. Of these, none was for those aged < 12 years, 23.5% were for those aged 12–15.9 years, 25% were for those aged 16–17.9 years and 51.5% were for those aged > 18 years. When metformin was initiated in primary care on recommendation of a specialist, 59.4%(19/32) of recommendations were from a paediatrician, 21.9% (7/32) were from a gynaecologist, 12.5% (4/32) were from an adult physician and 3.1% (1/32) were from an endocrinologist (some had multiple/joint consultations).
Indications for metformin initiation were obesity together with (1) PCOS (70.5%, 31/44), (2) insulin resistance (25.0%, 11/44), (3) impaired glucose tolerance/impaired fasting glucose (9.1%, 4/44) and (4) obesity without known comorbidity (6.8%, 3/44).
Drug monitoring
Of the 61 prescriptions initiated independently by a GP, 67.2% (n = 41) were subsequently reviewed by a GP (75% of metformin prescriptions and 65.3% of orlistat prescriptions), including three that were additionally reviewed by their practice nurse. One was reviewed by a gynaecologist, but not the GP, after drug initiation to confirm appropriateness of the drug prescription.
Of the 38 prescriptions initiated by a GP on specialist recommendation, 26.3% (n = 13) of patients had a GP consultation related to the drug for ‘supervision’ (n = 1), weighing (n = 2), psychological support (n = 1), prescribing (n = 2), answering queries (n = 1) and to discuss efficacy (n = 6). No patients discussed side effects with their GP. GPs were aware of monitoring by a clinician in 84.2% (32/38) of cases, of whom 81.8% (18/22) were followed up by paediatricians and 40% (2/5) were followed up by adult physicians.
General practitioners were made aware of adverse drug effects by two patients, both prescribed metformin: one had diarrhoea and the other had nausea.
Drug duration and termination
Duration of drug prescription is summarised in Figure 16.
FIGURE 16.
Kaplan–Meier survival curve demonstrating treatment duration of metformin and orlistat. Figure shows proportion still actively prescribed AOD for orlistat and metformin by time since initiation, beginning from time 0 (100% active prescriptions) out to 85 months (longest active prescription). Adapted from White et al. 206 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
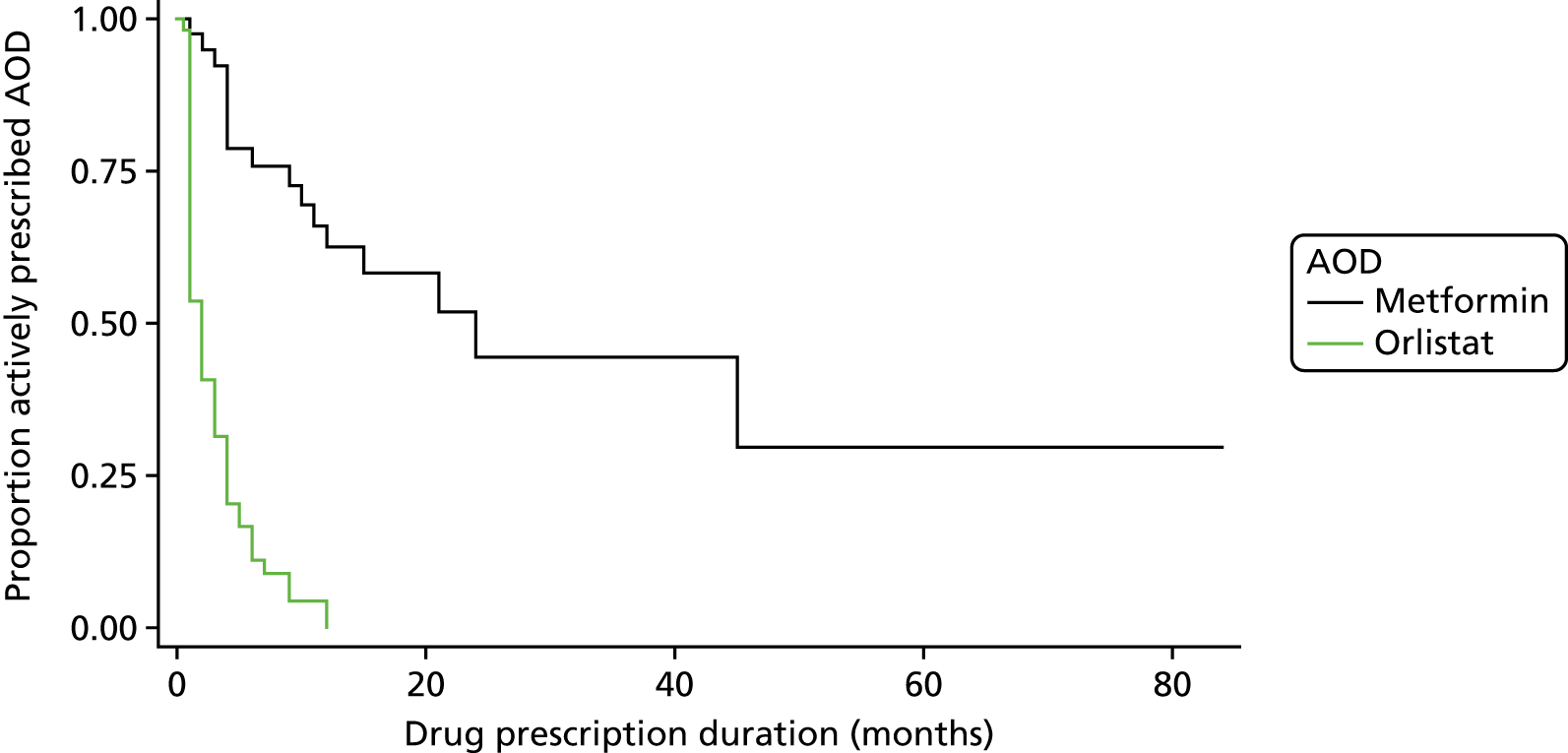
Over half of metformin prescriptions (25/44), but only 5.5% of orlistat prescriptions (3/55), were active at the time of the survey; an active prescription was defined as a new prescription issued within the preceding 3 months. Twenty-seven patients had only a single prescription issued from primary care, being 45.5% (25/55) of all orlistat and 4.5% (2/44) of metformin treatments. The median duration for supply of metformin was 10.5 months (IQR 4.0–18.5 months), compared with 2.0 months (IQR 1.0–4.0 months) for orlistat (p ≤ 0.001). None of these single prescriptions was issued in the 3 months prior to the survey, and all were given a maximum of 1 month’s supply, making ongoing use highly unlikely. There was a disparity between the reported length of drug prescription and the amount of drug prescribed, suggesting non-continuous use at the prescribed dose.
The majority of all drug terminations were due to families not requesting repeat prescriptions (96.2% of all orlistat terminations and 89.5% of metformin terminations), rather than medically led terminations. GPs reported that in three cases orlistat cessation may have been due to a lack of drug supply in pharmacies. Of four prescriptions actively terminated by a doctor (n = 2 for metformin and n = 2 for orlistat), two were caused by lack of efficacy, one by lack of drug adherence and the other two for reasons unknown.
Adherence to the National Institute for Health and Care Excellence’s guidance
The compliance analysis by NICE12 was restricted to recommendations for children (Box 1). Using 1 January as their assumed birthday, 23 subjects were definitely aged < 18 years at drug initiation (different age criteria to analyses above).
1.8.4 – Drug treatment is not generally recommended for children younger than 12 years.
1.8.5 – In children younger than 12 years, drug treatment may be used only in exceptional circumstances, if severe comorbidities are present. Prescribing should be started and monitored only in specialist paediatric settings.
1.8.6 – In children aged 12 years and older, treatment with orlistat is recommended only if physical comorbidities (such as orthopaedic problems or sleep apnoea) or severe psychological comorbidities are present. Treatment should be started in a specialist paediatric setting, by multidisciplinary teams with experience of prescribing in this age group.
1.8.7 – Do not give orlistat to children for obesity unless prescribed by a multidisciplinary team with expertise in: drug monitoring, psychological support, behavioural interventions, interventions to increase physical activity interventions to improve diet.
1.8.8 – Drug treatment may be continued in primary care for example with a shared care protocol if local circumstances and/or licensing allow.
1.9.2 – Adults and children: If there is concern about micronutrient intake adequacy, a supplement providing the reference nutrient intake for all vitamins and minerals should be considered, particularly for vulnerable groups, such as older people (who may be at risk of malnutrition) and young people (who need vitamins and minerals for growth and development).
1.9.11 – If orlistat is prescribed for children, a 6- to 12-month trial is recommended, with regular review to assess effectiveness, adverse effects and adherence.
Source
© NICE (2014) Obesity: Identification, Assessment and Management. NICE Clinical Guideline 189 [CG189]. Available from www.nice.org.uk/guidance/cg189. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this publication. 12
The following criteria were fully met. First, all subjects were aged > 12 years (recommendation 1.8.4). Second, no participants were prescribed orlistat for more than 12 months (1.9.11).
The following criteria were partially met. Four (17.4%) patients were prescribed orlistat following specialist advice (recommendation 1.8.5; see Box 3). Recommending specialists were paediatricians (n = 3) and an adult physician, with one known to be part of a specialist multidisciplinary team (MDT) (1.8.7). All prescriptions recommended by specialists were continued in primary care (1.8.8).
Comorbidities were reported in 56.5% of the sample (13/23), despite NICE requiring comorbidities to be present (recommendation 1.8.6; see Box 3). Comorbidities were emotional distress (7/23), hypothyroidism (3/23), T2DM (1/23), medulloblastoma (1/23), PCOS (1/23) and worsening of another chronic disease secondary to obesity (1/23). No patients had sleep apneoa. Low levels of comorbidity screening in primary care were reported, suggesting that higher numbers of comorbidities may have existed (6/23 were screened for psychosocial distress, five were screened for hypertension, two each for T2DM and dyslipidaemia, and one each and none for sleep apnoea).
No patient was prescribed a multivitamin (1.9.2). Screening for micronutrient intake was not assessed, nor was risk of vitamin deficiencies.
Improving prescribing in primary care
General practitioners reported their confidence in prescribing AODs using a 10-point Likert scale (10 = highest). Confidence was higher for prescribing to adults (median 8, IQR 8–9) than to children (median 3, IQR 1–5; p < 0.0001). There was no difference in confidence between prescribing orlistat or metformin in children (p = 0.8).
The most popular resources used by GPs to support prescribing were NICE guidance12 (orlistat, n = 20; metformin, n = 7), British National Formulary193,194 (orlistat, n = 17; metformin, n = 8), local prescribing guidelines (orlistat, n = 8; metformin, n = 11) and specialist guidance (orlistat, n = 1; metformin, n = 7).
General practitioners perceived that 27.3% (n = 12) of patients prescribed metformin and 12.7% (n = 7) prescribed orlistat benefited from the drug; half (50% of those prescribed metformin and 52.7% of those prescribed orlistat) reported not knowing if there had been any benefits for the patient.
Thirty-five GPs provided free-text reflections of their experiences of prescribing orlistat (n = 20) and metformin (n = 14). Three main themes arose. First, metformin was mostly prescribed to PCOS rather than as a weight loss drug. One GP stated that (s)he ‘wouldn’t normally prescribe this just for weight loss’.
Second, there was controversy about whether or not AODs should be prescribed in primary care in this age group, with one GP saying (s)he would ‘usually not prescribe for children’ and another saying (s)he avoided orlistat ‘where possible’. Either metformin was issued ‘on advice of specialist only’ or the patient received specialist follow-up after initiation.
Third, GPs noted concern about the efficacy of these drugs. ‘Inadequate counselling’, lack of drug availability and patient compliance (‘clearly patient was not able to comply’) were hypothesised reasons for ineffectiveness.
Sixty-two GPs wanted improved support, primarily split into two main themes. First, they requested improved age-related guidance for prescribing AODs that is ‘realistic’, with ‘clear [and] concise’ advice including ‘flow diagrams’ and ‘stepwise advice’. This would include instructions on assessment prior to initiation, indications, contraindications, monitoring, safety advice, duration, targets and indications for stopping treatment. Second, they wanted improved guidance for managing patients with obesity, namely advice about lifestyle management and details of available interventions. GPs requested details of ‘non-drug treatments’ including ‘community support for adolescents’ and ‘special clinics for monitoring and support of patients.’ One GP highlighted that ‘non-drug treatments need to be key alongside drug treatment.’
Discussion
This is the first detailed study of primary care prescribing of AODs in CYP at individual patient level. Small numbers of prescriptions were issued in this age group, with most practices surveyed having just a single child or young person on an AOD. However, clear patterns are seen that can help guide prescribing of current and future generations of AODs.
Recipients of AODs were mostly female. Prescribing to those aged < 12 years was rare, with AOD prescribing rates increasing with age. Two-thirds (65%) of the sample was aged ≥ 16 years, including 84% of those prescribed orlistat.
Metformin was mainly prescribed by specialists to those CYP who were obese and had either PCOS or insulin resistance. In contrast, orlistat was mostly prescribed in primary care, independent of specialist advice, to patients with few physical comorbidities.
Drug cessation was high, with only 1 in 18 patients prescribed orlistat continuing to take it at the time of survey, and half requesting only a single prescription. Metformin treatment was more long-standing, with half continuing at the time of survey. Despite well-described side effects associated with these drugs,209 GPs were aware of side effects in only two cases, despite high rates of follow-up. Cessation was almost universally patient led via non-request of repeat prescriptions, independent of medical discussion or decisions. Similar findings have been previously reported. 147,209
Comparison with NICE orlistat guidelines showed low prevalence of comorbidities and drug initiation without specialist advice. Given that most orlistat prescriptions were for those aged > 16 years, it could be hypothesised that patients were treated as adults, with drugs prescribed in line with adult guidelines that do not necessitate the presence of comorbidities.
General practitioners reported low confidence in prescribing AODs to CYP, despite high levels of confidence when prescribing to adults. They reported a desire for improved guidance not only on drug initiation and monitoring, but also on lifestyle interventions, suggesting possible low overall confidence in managing obesity in children. This fits with findings from this study’s paired qualitative study exploring patients’ experience, which found unease within primary care and pharmacy about AOD usage in CYP, which can result in heightened familial concerns about the use of these drugs. 209
Some GPs did not consider metformin an AOD despite it treating the metabolic sequelae of obesity. Low levels of comorbidity screening were found, suggesting that more patients may have benefited from the metabolic benefits of metformin.
Use of AODs in males was low, in keeping with uptake in other obesity interventions. Reasons may include access to primary care, gender differences in acceptance of obesity and other gender norms.
Limitations
This study relied on retrospective, notes-based recall by GPs, increasing the likelihood of missing data. Questionnaire completion rates were variable, with some having only a few questions answered. It was assumed that unanswered questions implied lack of evidence to support the questions. As a result, rates of comorbidities and screening may have been higher than reported. Sequential BMI measurements were sparse, and measurement of BMI changes associated with these drugs was not attempted.
Free-text answers were very brief. Given this, caution was exercised in the analysis of the responses, and themes were kept simple. Future interview studies could allow a better understanding of GP experiences.
The exact age of subjects could not be ascertained, resulting in risk of misclassification bias. Given this, caution was exercised in analysing compliance with NICE guidelines, and it is likely that the number of subjects aged < 18 years who were prescribed orlistat was underestimated.
General practitioners were not asked to justify exclusion of patients from this study. Given some free-text comments about metformin not being an AOD, it is hypothesised that some patients may have been inappropriately excluded.
The research team are unable to comment on specialist prescribing recommendations, as a result of sampling methods. Further research is needed to understand the prescribing practices of specialists and their use of these drugs.
Conclusion
Use of AODs in primary care is rare, particularly in males and those aged < 16 years. High rates of discontinuation were seen, primarily in those prescribed orlistat. Rates of compliance with NICE guidance for orlistat were low and GPs report low confidence in the use of AODs in this age group. Improved training and support for GPs is needed to guide AOD use in primary care, for both current and future generations of drugs.
Subsudy 5.3: understanding young people’s experiences of anti-obesity drugs
This work was reproduced and published as White B, Jamieson L, Clifford S, Shield JP, Christie D, Smith F, et al. Adolescent experiences of anti-obesity drugs. Clin Obes 2015;5:116–26. https://doi.org/10.1111/cob.12101. 209
Licence to reproduce number (John Wiley & Sons): 4675941463435.
Background
The role of medications in the management of obesity in children and young people is unclear. In the UK, NICE suggests that AODs may have a role in treatment of young people over 12 years of age with very high body mass index (BMI) or obesity comorbidities. 11 Currently only one drug, orlistat, is licensed in the UK as an AOD in children. In addition, metformin is used off-licence predominantly in obese subjects with insulin resistance. 191 There are no current data to compare relative usage of these two drugs; however, it is likely that metformin is more widely prescribed, especially by endocrinologists and gynaecologists in subjects with T2DM, insulin resistance and polycystic ovarian syndrome (PCOS). Systematic reviews of metformin and orlistat show small reductions in BMI: orlistat by 0.83 kg/m2 (see Viner et al. 155) and metformin by 1.4 kg/m2 (see Park et al. 191). While these clinical trials suggest the benefits of AODs may be very small, even small reductions in BMI can be important in growing children and adolescents. Primary care prescribing of these drugs increased 15-fold in the UK between 1998 and 2007. 147
Despite encouraging trial results which have evaluated the safety and efficacy of AOD, pharmacoepidemiological studies show that medication discontinuation rates outside the trial environment are very high in both children147 and adults. 213 Analysis of a national primary care prescribing database found 45% of orlistat prescriptions were discontinued after 1 month, and approximately 10% of children and young people remained on the drug for 6 months after initiation. 147 The reasons for these high rates of discontinuation are unclear. Metformin and orlistat have high rates of gastrointestinal side effects, which may limit their use. 165,214 However, there are no published data regarding patient experience of AODs in young people. Qualitative investigation of young people’s experiences allows the generation of hypotheses regarding reasons for early discontinuation.
Aims
The aim of this substudy was to investigate the experiences associated with AOD prescribing and use in adolescents in the UK, in order to inform potential strategies to improve AOD use and, therefore, efficacy in young people.
Methods
We used a qualitative design utilising an in-depth, semistructured interview schedule for young people and parent/carers developed by a multidisciplinary team (two psychologists, two paediatricians, a pharmacist and a patient representative). The schedule contained questions regarding decision processes to take the AOD, expectations of AOD outcomes, experiences of AOD usage, understanding of mechanism of drug action, outcomes of AOD usage and suggestions for improved outcomes. The study was reviewed and approved by the NRES Committee London – Surrey Borders REC (reference number 11/LO/1020).
Recruitment and sample
Young people aged 12–18 years were eligible if they had BMI ≥ 98th centile215 and had been prescribed orlistat or metformin for weight control within the last 3 years. Exclusion criteria were (1) use of metformin for management of T2DM, pre-DM or PCOS in non-obese young people, (2) inability to participate in a face-to-face interview or (3) insufficient mastery of English language to participate. Young people were recruited from three paediatric obesity clinics in England (London, Bristol and Liverpool) through the Medicines for Children Research Network. We anticipated requiring approximately 10–20 families to achieve theme saturation. 212 Based on our previous difficulties recruiting participants into obesity studies, all 109 subjects fulfilling eligibility criteria were invited by their hospital doctor to enrol in the study. Those consenting were invited to interview, together with one parent, and each given a £10 gift voucher as participation compensation.
Face-to-face interviews were conducted in the participant’s own home by one researcher (LJ) experienced in interviewing young people. Parent/young person dyads were independently interviewed unless either of the dyad opted out or they wished to be interviewed together. Written assent for under-16-year-olds and consent for those over 16 were obtained from both the young person and parent/carer. Interviews were audio-recorded and field notes written immediately after the interview.
Analysis
Audio recordings from each interview were transcribed verbatim and anonymised by LJ. Transcripts and field notes were read and coded independently by LJ, and a subsample coded by BW using a general thematic coding methodology. 216 Memos were written to summarise and synthesise emerging themes. This initial coding framework was used to code the subsequent transcripts and new codes were added as they emerged using a constant comparative technique to compare new and previously collected data to understand emerging themes. To ensure reliability, BW read all transcripts and reviewed the coding. BW and LJ developed models through an iterative process, in which the initial model was reviewed using constant comparison techniques (in which successive items of data are appraised and compared to ensure that the code is reflective of all) and the models revised accordingly. The qualitative analysis was facilitated by the use of NVivo software [QSR International (UK) Limited, Southport, UK].
Results
The interviews took place between January and May 2013; each lasted between 24 and 95 minutes. Theme saturation was reached after views from 16 families were collected. There were 13 parent and young person dyads, two young people without parents (one carer was not available and one did not speak English) and one parent alone (the young person did not wish to be interviewed). Four families (25%) were recruited from Bristol, one (6%) from Liverpool and 11 (69%) from London. Young people were aged 13 to 18 years, 12 (75%) were female, and 12 identified themselves as white British (the remaining identified themselves as White Jewish, Caribbean, British Bangladeshi, and British African). All carers interviewed were female. Ten participants (63%) were prescribed metformin only; eight continue to take metformin. Four were prescribed (25%) orlistat only; none continue on the drug. Two (13%) were prescribed both metformin and orlistat; two continue on metformin and one continues on orlistat. Self-reported weight change ranged from no change to 12.7 kg loss. Participant-reported comorbidities included insulin resistance, T2DM, asthma, hypothyroidism, epilepsy, androgen excess, obsessive–compulsive disorder, depression and hypertension. Participant demographics are summarised in Table 30.
| ID | Interviewees | Age (years) | Sex | Ethnicity | Self-reported comorbidities | AOD + duration | Self-reported weight change |
|---|---|---|---|---|---|---|---|
| 1 | YP + Mother (together) | 18 | F | White British | Underactive thyroid | Metformin (4 years: ongoing) | 2 stone (12.7 kg) |
| Borderline DM | Sibutramine (1 year: stopped) | ||||||
| Polycystic ovaries | Orlistat (1 month: stopped) | ||||||
| 2 | YP + Mother (separately) | 17 | F | White British | None | Orlistat (9 months: stopped) | 1.5 stone (9.5 kg) |
| 3 | YP + Mother (separately) | 16 | F | White British | Underactive thyroid | Orlistat (3 months: stopped) | None |
| Suspected polycystic ovaries | |||||||
| 4 | YP + Mother (separately) | 16 | F | White British | None | Orlistat (6 months: stopped) | None |
| 5 | YP + Mother (separately) | 13 | M | White British | None | Metformin (2 years: stopped) | None |
| 6 | YP + Mother (separately) | 14 | F | White British | Genetic disorder (leptin receptor missing) | Sibutramine (1 year: stopped) | Maintenance only |
| Orlistat (2 months: stopped) | |||||||
| 7 | YP + Mother (together) | 14 | M | White British | Raised blood sugars | Metformin (3–4 months: ongoing) | Unknown |
| 8 | YP only | 16 | F | British Bangladeshi | T2DM | Metformin (4 years: ongoing) | Dropped a clothes size |
| High level of male hormone | |||||||
| 9 | YP + Mother (separately) | 17 | F | White British | Glucose intolerant | Metformin (4 years: ongoing) | None |
| Depression | |||||||
| 10 | YP + Mother (separately) | 14 | F | White Jewish | Underactive thyroid | Metformin (15 months: ongoing) | Weight maintenance |
| Insulin resistant | |||||||
| 11 | YP + Mother (together) | 14 | F | White British | Osgood–Schlatter | Metformin (18–24 months: ongoing) | No weight loss but possibly clothes size |
| Insulin dependent [not on insulin] | |||||||
| 12 | YP only | 16 | F | Caribbean | Asthma | Metformin (2 years: ongoing) | About a quarter of a stone (1.6 kg) |
| Need to regulate insulin levels | |||||||
| 13 | YP + Mother (separately) | 13 | F | African/British | Complex medical conditions including seizures and insulin resistance | Metformin (9 years with 4 year intermission: ongoing) | Weight maintenance/small amount of weight loss at times |
| Orlistat (18 months then 15 months: ongoing) | |||||||
| 14 | Mother only | 17 | F | White British | Bone condition | Metformin (6 months then 7 years: ongoing) | 7–8 pounds (3.2–3.6 kg) |
| 15 | YP + Mother (separately) | 15 | M | White British | Periodic fevers | Metformin (1 month: stopped) | None |
| Anaemia | |||||||
| 16 | YP + Mother (separately) | 14 | M | White British | High blood pressure | Metformin (4 months: ongoing) | 4 kg |
Three conceptual models were developed from the emerging themes and are summarised in Figure 17. Models were (a) the factors influencing the commencement of an AOD, (b) the management of side effects and (c) decision to terminate the drug including balancing efficacy and side effects. Below we relate the emergent themes within each model.
Model 1: factors influencing why young people commenced on an anti-obesity drug
Six themes fed into the decision by young people to take the AOD: passive acceptance of the AOD, enthusiasm and relief at the prospect of a drug treatment, medication as a last resort, fear as a motivating factor, AOD as a way out of obesity and their own perceived uniqueness (Figure 17).
Theme 1: passive acceptance of medication
In all cases the doctor suggested an AOD to the young people. Young people’s views were mixed; many had reservations about initiation but there was a general feeling that they should follow the doctor’s advice. Passive acceptance was especially likely when the doctor medicalised obesity by highlighting the increased risk of comorbidities such as T2DM, and where patients were younger:
At the time I was thinking, well if it is stopping her from becoming totally diabetic, then the best thing for her to do is to take it, I suppose.
Parent 9, young girl
I remember them saying to me ‘you haven’t lost any weight, you have gone up, so let’s use this’ and I am like 12/13 years old, what am I supposed to do at this age?
Young person 12, girl aged 16 years
Theme 2: enthusiasm and relief at the prospect of a pharmaceutical treatment
Many participants wanted a novel solution for obesity control and described the potential of a medicine helping them with weight control as ‘awesome’ (young person 12, girl aged 16 years) and ‘exciting’ (young person 8, girl aged 16 years). One participant described how the doctor ‘made it sound like a miracle cure – he made it sound like it was going to fix all of my problems’ (young person 9, girl aged 17 years). However, some young people felt disappointed that they were not able to lose weight without medication:
I felt incredibly relieved that there was something that could help her.
Parent 14, young girl
Theme 3: medication as a last resort
Timing of drug initiation was an important factor, both in terms of timing in relation to other treatments and in relation to readiness to control obesity. Many viewed an AOD as a ‘last resort’ in obesity management. Some had tried all other treatment modalities with insufficient long-lasting benefits, including changes in diet and exercise, participating in programmes such as Slimming World® (Alfreton, UK) and Weight Watchers (WW International, New York, NY, USA), and ‘gastric band hypnotherapy’ (young person 5, boy aged 13 years):
I think I was at my wits end so anything was better than nothing.
Parent 13, young girl
In contrast, others felt that they were put on the medication before they had had a chance to fully explore other treatment modalities, including management of eating disorders:
. . . if I wasn’t binge eating I think there would have been an impact [on my weight].
Young person 6, girl aged 14 years
Families saw an AOD as an alternative to, rather than part of, a treatment package that included lifestyle changes:
. . . and it was just about like, well, OK then, if this is the last resort, but in some ways I don’t think it was.
Young person 12, girl aged 16 years
The importance of controlling weight at the time of drug initiation varied. Some believed that they would be able to control weight in the future without a drug whereas others wanted additional support immediately:
. . . if I was referred to it a year later or 2 years later I don’t think it would make too much difference.
Young person 15, boy aged 15 years
Theme 4: fear as a motivating factor
Three types of health-related fears were associated with initiation of AOD treatment. Firstly, parents and young people were both concerned and confused by discussions with clinicians about DM; any mention of DM increased their acceptance of the AOD. Young people’s concerns were amplified if their parents or grand-parents had obesity-related conditions:
It is because I am concerned that [my son] does not set up health problems for himself in later life and if we can avoid diabetes that to me seems a very good thing to do indeed while he is struggling to get his weight down, not only for diabetes itself, but all the related things that come in its wake. So that seemed to be absolutely excellent.
Parent 15, young boy
. . . I know that I have to get the weight off for health reasons, because like family members in the past have had lots of health things, especially on my mum’s side and being overweight will affect those things and make them worse or make me more susceptible to them which I don’t want.
Young person 9, girl aged 17 years
Secondly, some young people described feeling threatened by their doctor, reporting that they were told that they would have to undergo bariatric surgery if weight loss was not achieved; they saw surgery as a ‘last resort’ (young person 6, girl aged 14 years) or ‘final straw’ (young person 9, girl aged 17 years) which they wished to avoid.
Thirdly, participants described a fear of being told off or patronised by clinicians, particularly dietitians, in regard to either continued weight gain, lack of exercise, or diet:
I didn’t really find her [the dietitian] very helpful. I think I need guidelines. And it was just oh, you need to eat healthily. I didn’t really find her useful . . . [she] ask(ed) me what I eat and it made me feel guilty then. I know that that is kinda what the aim is but do that as well as help me. Not just make me feel bad.
Young person 3, girl aged 16 years
For the first 2 years they wanted me to see the dietitian and all you ever got were these really skinny bitches (mind my language) who just patronised you and said eat healthily and I am, like I have been coming here 2 years already, I already know what I am meant to do, and no matter how hard I try nothing is happening and you are not helping.
Young person 9, girl aged 17 years
Theme 5: anti-obesity drug as a way out of obesity
For some young people, an AOD was seen as a ‘way out’ of obesity. Young people had their own personal reasons for wanting to lose weight. Some were emotional, and saw the AOD as a way out of being bullied, feeling self-conscious or a way to alleviate their feeling of ‘desperation’ (young person 9, aged 17 years). Others had lifestyle reasons for wanting to lose weight, which included improved fitness and ability to wear certain clothes:
Just to help me lose weight because at the time I was feeling really self-conscious.
Young person 13, girl aged 13 years
I want to just fit into a medium [sized clothes].
Young person 8, girl aged 16 years
Theme 6: perceived uniqueness
Some young people considered themselves to be ‘unique’ because they had a complex medical history or a genetic tendency to obesity and thus felt they needed specialised treatment to help them with their weight. Some believed that they were unlike other overweight young people, in that they did not have a sedentary lifestyle or poor diet, but were incorrectly judged by others as doing so:
It is not that I eat a lot, it is just because of my . . . I can’t remember what it is called, they think it has something to do with my complex medical issues ‘cos I don’t eat that much.
Young person 13, girl aged 13 years
Model 2: management of side effects
The overall themes in model 2 can be seen in Figure 18.
Side effects from AOD were a key issue for many young people, although a minority did not experience any. Side effects of both metformin and orlistat were usually gastrointestinal, particularly abdominal cramps and diarrhoea. For some, this involved spending ‘many, many hours on the loo’ (young person 15, boy aged 15 years, using metformin), and ‘really weird, intense pain’ (young person 1, girl aged 18 years, using metformin). Some were taken aback by the severity of the side effects; one young person reported ‘I have never seen diarrhoea like it’ (young person 6, girl aged 14 years, using orlistat), while another said ‘we were told that there could be mild stomach upsets or whatever but I didn’t expect it to be as uncomfortable as it was’ (young person 15, boy aged 15 years, using metformin). A few young people attributed unusual symptoms to metformin, including hand tremor, headaches and change in moods.
Parents expressed concern about their children having to experience such unpleasant side effects. One mother felt that it was ‘totally wrong’ for her daughter to have such side effects at her age (parent 6, young girl using orlistat):
It was awful and he would do it [faecal incontinence] in his trousers and he would phone me up and say Mum, I need to come home.
Parent 5, young boy
Nearly all young people were prepared to endure some side effects as their doctor had forewarned them. However, there was personal variation as to how much each individual tolerated these side effects; some continued taking the medication despite episodes of faecal incontinence while others stopped with much milder side effects. Some young people were more resilient than others in coping with side effects.
Some initiated self-devised lifestyle strategies or changes in drug regimen to minimise side effects; we identified a number of key likely mediators that influenced how these side effects were managed.
Regimen change to minimise the side effects
Regimen changes were devised and initiated by both doctors and families. Some young people used orlistat flexibly and omitted doses to minimise side effects at predetermined important times. Firstly, this allowed them dietary freedom at special occasions such as birthdays and, secondly, it minimised side effects in certain environments or events, such as school time or during exams:
I stopped [the medication] because I didn’t want to take them during the exams in case I had a bad stomach it would take time off [the exam] . . .
Young person 2, girl aged 17 years
At times, doctors recommended changes in formulation, dosage and frequency to minimise side effects:
They [doctors] changed her metformin dose and changed it to slow release. Then after that they changed the time she was taking it. It would help her a bit more.
Parent 1, young girl using metformin
With the higher [initial] dosage I was vomiting more . . . by breaking it down to two in the morning and two in the evening, I think it [vomiting] is a lot better.
Young person 8, girl aged 16 years, using metformin
In contrast, some young people reported discussing side effects with their doctors but were told that their symptoms would improve if they continued with the current regimen:
. . . [I was] just told to take it and get on with it really.
Young person 5, boy aged 13 years
Alternative self-initiated strategies to manage side effects
Families reported a range of self-initiated strategies to cope with the side effects, particularly diarrhoea and faecal incontinence. These included taking spare clothes to school in case of incontinence, not leaving the house or taking additional medication to counteract the effects.
. . . she would start taking loperamide [anti-diarrhoeal medication] to counteract the effects of it . . . a few times because she was like, I have got to go to school, I have got the runs and I can’t keep going out of the lessons, so it was a bit difficult that one.
Parent, young girl using orlistat
I was asked to bring in spare things because she kept having accidents [faecal incontinence]. She had [already taken] a few herself which she had taken in her bag.
Parent, young girl using orlistat
It made me stop going out for a while as I was worried that it might come over me and I might have to dash off and it would be embarrassing.
Young person, boy aged 16 years, using orlistat
Only a minority of young people taking orlistat recognised that the ‘side effects’ they were experiencing were the result of the fat they had consumed and changed their diet:
I went back to the really healthy stuff.
Young person 13, girl aged 13 years, using orlistat
Mediators in dealing with side effects
Young people identified certain mediators which influenced how side effects were managed.
Reluctance to discuss side effects with clinicians
Despite reporting trust in their doctors, some participants were disinclined to talk to them at planned appointments or initiate additional interim appointments. Some did not feel their GP had sufficient expertise to support AOD usage:
I probably would have liked more support – however, my consultant is very good and I do prefer her to the local GP. She [consultant] sees loads of people with the same condition. Helping them to change their ways and this and that. But at the GPs they do loads of different things . . .
Young person 10, girl aged 14 years
Few turned to other health-care professionals, such as pharmacists, for support, and sometimes these interactions heightened familial concerns, particularly if the professional questioned the appropriateness of the medication:
. . . when I picked up his prescription the pharmacist said ‘the child is only 13’ and I thought oh, is there a reason . . . He just thought it was unusual, it was normally for older people. He thought it was a bit odd and he said maybe you should ask that question. And he also said it would be helpful for me to give him some feedback after he takes them.
Parent 7, young boy
Understanding mechanism of action
Understanding of food content, in particular fat content, was also variable, and some young people reported not having received any dietetic advice prior to commencement of the AOD. This included patients prescribed orlistat. Many had familial experience of AOD usage that increased their own understanding; this was often grandparents taking metformin for control of T2DM or mothers who had taken orlistat for weight control.
Some perceived their drug-related symptoms as ‘side effects’ while others realised that they were a consequence of high fat intake. The majority of participants who correctly understood the mechanism modified either their diet or drug regimen:
. . . if I was eating the fat, I would have to go to the toilet.
Young person 3, girl aged 16 years, using orlistat
Age
Young people reported that if they had been early adolescents at the time the AOD was introduced, they did not listen to the information given by the doctor, preferring to leave understanding to the parent:
I was at that age where I don’t need to know.
Young person 6, girl aged 14 years
Concerns about safety
A few had concerns about the safety of the medicine because of the side effects while others felt that the medicines must be safe because a doctor had prescribed them:
I thought that it couldn’t be, like, safe if it was keeping me awake all night and making me like go a lot.
Young person 5, boy aged 13 years, using metformin
Environmental influences
Many had heightened awareness of toilet facilities, particularly their proximity and the impact of sharing toilets. This was driven by concerns about faecal urgency, risk of incontinence and the embarrassment related to the staining of toilets with oily faeces:
She felt she couldn’t be comfortable taking them at school and college, because she just couldn’t rush out, and when she said she did, it was like an orangey/yellow oil that goes into the toilet and it doesn’t flush away. So that is very embarrassing if you are out somewhere.
Parent 1, young girl using orlistat
Model 3: drug continuation – efficacy versus side effects
The overall model for the decision to continue or stop taking the prescribed AOD is shown in Figure 19.
FIGURE 19.
Model 3: patient-led decision process regarding drug continuation. Source: White et al. 209

Participants continued with the medication for between 1 month and 8 years. Nine out of 12 were prescribed metformin, and one of six participants prescribed orlistat continued beyond 6 months. Seven young people discontinued an AOD. The decision to either continue with, or stop, the AOD was frequently based on a decisional balance between the efficacy of the AOD and ongoing side effects. The decision to terminate treatment was frequently described as a balance between the perceived benefits of the AOD and its side effects. Various mediators influenced this decision, including perceived benefits and expectations, lack of support and understanding of drug action:
I don’t want to take something that I don’t think was working and making me ill.
Young person 6, girl aged 14 years
I just thought what is the point of taking it if it is not working and I am not eating the rubbish foods, there is no point as it weren’t really doing anything.
Young person 3, girl aged 16 years
Participants described efficacy in terms of body weight, body shape and metabolic parameters. Individual goals varied from going down a clothes size to weight stabilisation. Most young people had expectations of weight control that were aligned with published outcomes, although some hoped for outcomes that were faster, more extreme or more guaranteed. Weight stabilisation was acceptable for some participants, and they continued to take it, fearing that their weight would increase faster if they stopped taking the medicine:
He [clinician] said that they may help me lose weight and my mind crossed out the word ‘may’ and replaced it with the word ‘will’.
Young person 9, aged 17 years
It is keeping her weight not going up.
Parent 10, young girl
There was significant variation in the understanding of drug action, in terms of mechanism and efficacy. Some perceived that the AOD required a restricted diet and increased exercise to be effective, while others believed that lifestyle changes were not necessary. These views seemed to be unrelated to the drug prescribed. Some described the futility of taking an AOD as they were unable to undertake healthy behaviours, and subsequently stopped the drug:
. . . they said this [the medicine] is not a miracle worker it doesn’t help you lose weight. You help yourself to lose weight and it just gives you a little pat on the back every so often to help you carry on what you need to do . . .
Young person 10, girl aged 14 years
I don’t know whether the tablet actually makes you lose weight or just because it makes you stop eating, it makes you lose weight.
Young person 2, girl aged 17 years
Drug termination was an active decision by young people and their families, and not by their doctor. The decision was taken by the young person alone, or with the advice and support of their parent. Very few young people or parents reported adequate drug monitoring and support from the obesity specialist; this influenced their decision to independently stop the drug:
She [Mum] advised me not to take them because it wasn’t very nice for me experiencing this.
Young person 6, girl aged 14 years
I just stopped taking them, went cold turkey.
What put you off ringing up the clinic to discuss it?
I just didn’t think it was important.
Young person 15, boy aged 15 years
Support was a theme that spanned across all three models. One mother described the period taking the AOD as a ‘lonely’ time. Few reported adequate support from their obesity specialist, primary care physician or pharmacist. Two participants described disheartenment after being discharged from a specialist service due to inadequate progress, and reported subsequent weight gain. General practitioners mostly only issued repeat prescriptions. Many young people said that they would be happy to be monitored by their GP if they could not get an appointment at the specialist clinic, yet others felt that GPs had insufficient experience to support them. Emotional support mainly came from friends and family. Parental supervision, usually from a mother, ensured that younger adolescents took the medication. As young people matured, parents were more likely to step back and let the young person take responsibility for their medication:
I think there were times when she tried skipping it, but she had a dragon as a mother. So as long as I am aware it happens, and what I do now is put it all out in individual pill boxes for the day.
Parent 13, young girl
Discussion
These are the first published qualitative data on adolescent experiences of AOD use. In this sample, AOD prescriptions were uniformly suggested and initiated by specialist paediatricians, with passive acceptance by young people and families. After initiation, families mostly described receiving minimal support from the specialist prescriber as well as from local clinicians including GPs and pharmacists. There was a wide variation in the experience and tolerance of side effects, which were largely managed by families independently of clinicians using self-directed strategies. Although doctors made the decision to start the drug, we saw that patients decided to terminate the drug, usually because of insufficient benefit to justify the side effects.
Participants had a range of comorbidities, including depression and hypothyroidism, which may have impacted on their experience of AOD usage. Owing to the wide range of comorbidities and small numbers of each, it was not possible to explore more fully the interaction between these individual conditions and AOD usage.
Similar findings have been demonstrated in the adult studies exploring AOD usage. Qualitative study participants from three primary care practices reported that doctors initiated AOD, giving patients little choice in the decision and inadequate information about the drugs and related lifestyle changes. 216 Similar patterns of use have been reported in adults. Two previous studies showed that side effects were a major factor influencing adherence, and many adults report using the medication flexibly to fit in with their lifestyles, and minimise side effects at inappropriate times. 217,218 The highly visual side effects also encouraged some adults to consider their behaviour as a cause of their obesity and to adopt a healthier diet. 218 Similar themes for drug discontinuation were reported in these previous studies; participants who benefited from the drug continued with, or adapted, the medication, and those participants who did not lose weight abandoned it. 217 Similar themes have also been demonstrated in the adolescent adherence literature, with insufficient clinician support, embarrassment, insufficient belief in drug efficacy, interference with usual activities and side effects all being reported as barriers to medication adherence in other chronic conditions. 219
Results from this study offer insight into the experiences of young people who are taking AOD, and offer potential targets for change that could potentially improve drug adherence and outcomes in this patient population. They suggest that more careful approaches are needed to improve drug initiation and ongoing support. Potential strategies are summarised in Box 2.
-
Ensure that families accept the need for medication, with alternative options discussed, and drug initiated only when it fits in with the families’ own treatment ladder.
-
Initiate the medication at a time that is right for the family, with consideration of the school day, week and year, and avoidance of periods where side effects may be problematic (e.g. examinations).
-
Ensure that those prescribed orlistat are fully informed and understand the difference between treatment effects and side effects. This could include dietetic input to enable its use as an educational tool to identify high-fat foods, and subsequently enable a low-fat diet.
-
Provide sufficient information about side effects at initiation, with written advice related to side effect management strategies.
-
Provide sufficient ongoing support from specialist services, particularly related to management of side effects. Active monitoring of non-weight related benefits, e.g. cardiometabolic risk factors, may reduce drug cessation.
Source: White et al. 209
Obesity is likely to be a lifelong disease for this cohort, given that current treatments have modest efficacy. Long-term drug use is likely to be an integral treatment modality in addition to behaviour modification strategies at both the individual and population level. Effective prescribing habits are needed to support both current and future generations of anti-obesity medications.
Limitations
Participants were recruited from three hospital clinics in England, with the majority from one hospital in which two of the authors are clinicians. This has the potential of limiting generalisability and introducing bias. However, AODs in young people are largely initiated in specialist centres, and those centres included in this study were among the largest of a very small number of specialist paediatric obesity clinics in the UK. To minimise bias, all data were collected by an independent researcher not part of any clinical team and responses were anonymised before analysis. Delays in the study may have led to problems with recall for those patients who stopped the medication some time previously. As with all qualitative studies, the researcher’s presence during interviews could have affected the subjects’ responses; every effort was made to reassure the participants that the researcher was both non-judgemental and not part of the clinical team, and their responses would be fully anonymised prior to analysis by the team. We aimed to include patients who were prescribed an AOD but never took it. However, no such young people responded to recruitment invitations. We are therefore unable to comment on those participants who were prescribed an AOD but who never initiated medication. Only a small minority of eligible subjects enrolled in the study, despite thorough attempts to contact them using clinical research nurses. It is highly possible that those with negative experiences felt more motivated to participate in the study. Largely negative responses indicate that participants are likely to have felt reassured about their anonymity, and did not fear reprisal from their clinicians.
Conclusion
Use of AOD is challenging and complex for many adolescents, and few young people in our study described positive experiences. Multiple factors were identified that could be targeted to improve medication concordance and maximise efficacy, including improved clinician–patient partnership in decision-making, and better patient education and subsequent support. Many of these are not unique to the current generation of AOD, and are likely to be relevant to novel drugs.
Chapter 6 Study E: evaluation of acceptability and early outcomes of adolescent bariatric surgery in the UK
Key findings across study E
-
Adolescent bariatric surgery in a NHS service compares favourably to international outcomes, and shows promise as a safe, effective treatment for severe obesity. Approximately 1 in 10 young people has a surgical complication. Further work is needed to improve patient selection to better match patient need to operative type and to reduce pre- and post-surgical attrition.
-
Decisions about bariatric surgery differ between clinicians and young people. For clinicians, there is a predominant theme of ‘uncertainty’ about whether or not surgery is appropriate for young people. Young people also share this uncertainty, but use a number of strategies to ‘bracket away’ these dilemmas in the hope of a better future. Both clinicians and young people see bariatric surgery as a ‘last resort’.
-
Systematic review evidence shows that QoL and depressive symptomatology improve after bariatric surgery, although not in all patients. Improvement appears to peak at 6–12 months. Improvements in psychological function appear to be associated with reduction in BMI rather than with baseline psychological function.
-
There is weak evidence that psychological function predicts BMI outcomes after surgery; those with higher baseline levels of loss of control eating and family conflict appear to have poorer BMI outcomes.
-
Bariatric surgery in severely obese adolescents is a cost-effective alternative to no surgery over a lifetime in obese adolescents, taking the perspective of the UK NHS.
-
The full third National Bariatric Surgery Register report will be released in 2020. This will involve an updated data set – adding patient identifiers (i.e. NHS numbers), glycosylated haemoglobin (HbA1c) levels and EuroQol-5 Dimensions quality-of-life measurements – which will help with making outcomes more patient centred (which also fits with the Healthcare Quality Improvement Partnership mandate).
Background
Bariatric surgery in adults is accepted to be a highly clinically effective and cost-effective intervention for moderately to severely obese individuals. 12 Compared with non-surgical treatment of obesity, bariatric surgery leads to greater body weight loss and higher remission rates of T2DM and metabolic syndrome. 220 In 2014, the UK National Bariatric Surgery Registry reported that ≈ 6000 bariatric procedures are carried out annually on adults. 221 There are, however, no routinely collected data or published outcome data on bariatric surgery in children and adolescents in the UK, with available data limited to case series. 222
At the time of development of the PROMISE programme, NICE guidelines12 had endorsed bariatric surgery in adolescents as an appropriate treatment in exceptional circumstances if undertaken within specialist centres. Yet there were no available outcome data on adolescent bariatric surgery in the NHS. A series of linked studies was undertaken to (1) examine the outcomes of adolescent bariatric surgery in one centre in the UK, (2) understand clinicians’ and young people’s decision-making about bariatric surgery, (3) investigate factors influencing outcomes in bariatric surgery and (4) examine the cost-effectiveness of bariatric surgery for adolescents. In addition, work was conducted to form a national network of clinicians undertaking adolescent bariatric surgery.
Substudy 6.1: characteristics and outcomes of the first 50 patients entering an adolescent bariatric surgery programme at University College Hospital
Parts of this section are reproduced from White et al. 223
This work was reproduced and published as White B, Doyle J, Colville S, Nicholls D, Viner RM, Christie D. Systematic review of psychological and social outcomes of adolescents undergoing bariatric surgery, and predictors of success. Clin Obes 2015;5:312–24. https://doi.org/10.1111/cob.12119. 269
Licence to reproduce number (John Wiley & Sons): 4675960442600.
Background
Bariatric surgery is currently the only evidence-based intervention resulting in highly meaningful weight loss for obese children and young people. 224 In the UK, national guidelines for adolescent bariatric surgery are contained within the most recent NICE obesity guideline12 summarised in Box 3. It recommends that bariatric surgery is considered only in exceptional circumstances, and only if young people have achieved or nearly achieved physiological maturity.
1.10.12 Surgical intervention is not generally recommended in children or young people. [2006]
1.10.13 Bariatric surgery may be considered for young people only in exceptional circumstances, and if they have achieved or nearly achieved physiological maturity. [2006]
1.10.14 Surgery for obesity should be undertaken only by a multidisciplinary team that can provide paediatric expertise in:
-
preoperative assessment, including a risk-benefit analysis that includes preventing complications of obesity, and specialist assessment for eating disorder(s)
-
information on the different procedures, including potential weight loss and associated risks
-
regular postoperative assessment, including specialist dietetic and surgical follow up
-
management of comorbidities
-
psychological support before and after surgery
-
information on or access to plastic surgery (such as apronectomy) when appropriate
-
access to suitable equipment, including scales, theatre tables, Zimmer frames, commodes, hoists, bed frames, pressure-relieving mattresses and seating suitable for children and young people undergoing bariatric surgery, and staff trained to use them. [2006]
1.10.15 Coordinate surgical care and follow up around the child or young person and their family’s needs. Comply with the approaches outlined in the Department of Heath [and Social Care]’s [Healthy Lives, Healthy People:] A Call to Action on Obesity in England.[225] [2006, amended 2014]
1.10.16 Ensure all young people have had a comprehensive psychological, educational, family and social assessment before undergoing bariatric surgery. [2006, amended 2014]
1.10.17 Perform a full medical evaluation, including genetic screening or assessment before surgery to exclude rare, treatable causes of obesity. [2006]
Source
© NICE (2014) Obesity: Identification, Assessment and Management. NICE Clinical Guideline 189 [CG189]. Available from www.nice.org.uk/guidance/cg189. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this publication. 12
Published adolescent data have largely focused on weight outcomes of surgery. 1 The largest and most complete data sets have come from the USA155 and Sweden,226 with only very small case reports published from the UK. 222,227,228 A recent systematic review of adolescent bariatric surgery showed mean loss of 13.5 kg/m2 at 1 year post surgery with greatest change seen in those undergoing the Roux-en-Y gastric bypass (RYGB), followed by sleeve gastrectomy (SG) and then adjustable gastric band (AGB). 224 Although data were drawn largely from observational studies, the magnitude of effects in these were similar to those reported in the only randomised controlled trial undertaken in adolescents. Such changes are very much greater than those seen with drug treatments, such as metformin (mean loss 1.4 kg/m2) and229 orlistat (–0.8 kg/m2),10 or with lifestyle interventions (–1.25 kg/m2). 224
Psychosocial outcomes after surgery have been less rigorously studied, with four large cohorts reporting outcomes for up to 2 years after surgery. 226,230–232 However, there are no published outcomes of eating disorders, and only one study exploring predictors of success after surgery.
Furthermore, outcome studies have universally focused on those people who received surgery,224 with no studies of patient pathways into adolescent bariatric surgery programmes. It is unclear (1) how many young people who are referred for surgery actually receive it and (2) the reasons for not receiving it in those referred. Such data are important for planning and evaluating adolescent bariatric surgery programmes, particularly within state-funded systems.
Aim
The aim of this substudy was to describe the characteristics and outcomes of patients referred to an adolescent bariatric surgery programme within the NHS in England.
Methods
All patients referred to an adolescent weight management service for consideration of bariatric surgery between its inception in 2007 and 27 January 2014 were included. Subjects were referred by their GP orlocal paediatrician, or from within our medical weight management service. Patients were identified by means of an ongoing database. Body weight was measured to the nearest 0.1 kg using a Tanita BC-418MA scale (Tanita, Europe, Amsterdam, the Netherlands) or SECA 645 scale (Seca GmbH, Hamburg Germany) where body weight exceeded 200 kg. Height was measured to the nearest millimetre using a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Patients selected the ethnicity with which they most associated, based on a predetermined list used by the hospital trust. This list included only one category for British, and did not differentiate between white British, black, Asian or other ethnic groupings. Socioeconomic status was derived from the patient’s home postcode using the 2007 Index of Multiple Deprivation30 (IMD) score and rank (England only). The 32,482 IMD ranks were split into 10 groups to allow us to rank patients by deprivation deciles (decile 10 being the most deprived). This study fulfilled National Research Ethics Service (NRES) criteria for service evaluation, and was registered in the UCLH Research & Development Department.
Cardiometabolic health
A two-tiered screening process was employed. Baseline cardiometabolic screening was employed in patients completing the initial evaluation process. Those eligible for surgery had a more complete assessment, including screening for sleep apnoea and polycystic ovarian syndrome (PCOS) if history and examination were suggestive.
All patients without previously diagnosed DM underwent a standard oral glucose tolerance test (OGTT) (1.75 g/kg of body weight, up to 75 kg). Plasma glucose and insulin concentrations were measured at 0, 30, 60, 90 and 120 minutes after a 12-hour overnight fast. In an attempt to simplify the investigation pathway, we subsequently measured only baseline HbA1c, fasting glucose and insulin levels, and an OGTT was undertaken only in those with a HbA1c level of > 6.5%. 230 Plasma insulin was measured by the Abbott AxSYM method. Blood pressure was measured using automated blood pressure monitor (Datascope Accutor® PLUS; Mindray (UK) Ltd, Huntingdon, UK) with an appropriately sized cuff for arm circumference. Lipid levels were stratified in accordance with American Heart Association guidelines. 231 Metabolic syndrome was diagnosed using International Diabetes Federation criteria. 229 A sleep study was undertaken in patients with loud snoring together with clinical signs of apnoea. 233
Psychosocial status
Generic quality of life was measured using PedsQL 2.0 questionnaire234,235 and weight-specific quality of life using IWQoL-kids. 96 Psychological distress was measured using the SDQ,77,236 and depressive symptomatology was assessed using the Short Moods and Feelings questionnaire (SMFQ). 237,238 Self-esteem was measured using the Rosenberg Self-Esteem Scale (RSE). 239,240 Eating behaviours were assessed using the Dutch Eating Behaviour Questionnaire (DEB-Q),241,242 and disordered eating was assessed using the Eating Disorders Examination Questionnaire (EDE-Q). 243,244
We assumed that participants elected not to proceed if they missed two successive appointments and we were unable to contact them by telephone and letter. Completeness of comorbidity data varied by length of time in bariatric pathway; some of those participants who exited the pathway early did not have full assessment. Assessments of eligibility for surgery depended in part on comorbidities; some participants exited the pathway after the first consultation and did not have comorbidities assessed.
Statistical analyses
We first used descriptive statistics to describe patients and comorbidities before and after surgery. Questionnaire scores were compared with normative data (where available) using one-tailed Student t-tests. We derived BMI trajectories of each patient undergoing surgery to understand trajectories associated with bariatric surgery. For this, multilevel models were developed using XTMIXED command in Stata, version 12. We modelled BMI trajectories using demographic predictors, and fitted the model using maximum likelihood with unstructured variances and covariances. Association between demographic characteristics and surgery type, timing and outcomes was assessed using linear or logistic regression. To allow retrospective comparison with published outcomes, we compared BMI at 1 year. One-year outcomes were defined as BMI recorded between 9 and 15 months after surgery.
Results
Patient flow and characteristics
The patient flow is summarised in Figure 20. Fifty-two patients were referred for consideration of bariatric surgery. Two young people had bariatric surgery in the private sector while waiting for their initial assessment, and so were not eligible for the pathway and are not included here.
Fifty young people were assessed for eligibility for bariatric surgery between 26 July 2007 and 27 January 2014. Demographics are summarised in Table 31. Thirty (60%) were aged 16 years or over at their first assessment. As of 27 January 2014, 42 had completed the preoperative pathway and eight were still undergoing assessment and/or preparation for surgery.
| Characterisitc | Whole cohort (N = 50) | Completed | |
|---|---|---|---|
| Surgery (N = 20) | No surgery (N = 22) | ||
| Female, n (%) | 29 (58) | 11 (55) | 11 (50) |
| Mean (SD) age (years) at baseline | 16.0 (1.3) | 16.4 (1.1) | 15.6 (1.3) |
| Age (years) range | 12.8–18.5 | 13.3–17.8 | 12.8–17.2 |
| Mean (SD) baseline BMI (kg/m2) | 51.2 (7.5) | 51.8 (8.7) | 50.8 (7.5) |
| BMI range (kg/m2) | 37.5–69.6 | 38.2–69.6 | 37.5–64.8 |
| High–normal blood pressure, n/N (%) | 10/48 (20.8) | 3/20 (15) | 4/20 (20) |
| High blood pressure, n/N (%) | 16/48 (33.3) | 8/20 (40) | 6/20 (30) |
| High–normal lipids, n/N (%) | 25/43 (58.1) | 10/20 (50) | 9/15 (60) |
| High lipids, n/N (%) | 17/43 (39.5) | 9/20 (45) | 6/15 (40) |
| T2DM, n/N (%) | 6/50 (12) | 2/20 (10) | 3/21 (14.3) |
| Ethnicity, n (%) | |||
| British | 29 (58) | 9 (45) | 14 (70) |
| African/Caribbean | 8 (16) | 4 (20) | 3 (15) |
| Asian | 5 (10) | 4 (20) | 1 (5) |
| Other | 8 (16) | 3 (15) | 2 (10) |
| Deprivation decile,a n (%) | 18 (36) | 6 (30) | 8 (40) |
Of the 42 young people, six (14.3%) were deemed not eligible for surgery and were discharged from the pathway, two after the initial assessment and four during the preparation phase. The principal reasons for exclusion were (1) resident outside the UK, which the clinical team deemed to be incompatible with safe postoperative monitoring (n = 1); (2) inability to consent owing to learning difficulties (n = 2); (3) severe needle phobia (n = 1); (4) inconsistent desire to undergo bariatric surgery (n = 1); and (5) complex behavioural difficulties (n = 1). No patients were excluded because of psychiatric disorders. A further 15 participants (35.7%) did not proceed along the bariatric surgery pathway, due to either active withdrawal after receiving further information about surgery (n = 8) or lack of engagement (n = 7). Two (5%) patients were lost in the transfer between adolescent and adult bariatric surgery services within the same hospital. We have been unable to ascertain if they underwent surgery at a different centre. Twenty (47.6%) patients underwent bariatric surgery during the study period, and their demographics can be seen in Table 31.
Secondary obesity
Three young people had previously undergone treatment of hypothalamic–pituitary tumours (two craniopharyngioma and one optic glioma), including surgery and radiotherapy. All developed hypothalamic–pituitary dysfunction with resultant severe obesity. One developed significant additional comorbidities related to treatment, most notably moyamoya, a disease of small cerebral vessels probably caused by radiotherapy, which had resulted in multiple previous cerebrovascular events (CVEs). Both patients previously treated for craniopharyngioma underwent surgery with poor outcomes. Their BMIs at surgery were 76.6 and 62.8 kg/m2. The patient with pre-existing cerebrovascular disease had a further CVE in the peri-operative period resulting in hemiplegia, expressive and receptive dysphasia and loss of thirst. He required prolonged neurorehabilitation with residual loss of function. The second patient had no complications, but regained all lost weight in the second year after surgery. Given our outcomes, and those in published cohorts, we decided not to offer bariatric surgery to the third patient with hypothalamic–pituitary dysfunction (she had already decided against surgery so was not formally refused for surgery).
No patient had obesity secondary to hypothyroidism, medication or any obesity syndrome. No patients had any identified obesity-promoting monogenic genetic mutations, nor were they excluded for having variants that were likely to have poor response to bariatric surgery.
Obesity-related comorbidities
Of the total sample, 47 (94%) patients underwent baseline cardiometabolic screening. Of the remaining three patients, two only attended a single assessment and did not wish to pursue further investigations or assessment. One other had known T2DM and exited the pathway before further investigations were done.
Four (8%) patients were known to have T2DM when entering the pathway. Of the remaining 46 patients, 44 underwent baseline screening using either HbA1c (n = 43) and/or OGTT (n = 38), and no further cases were identified. Those patients not screened by us had previously undergone screening, with no DM detected. Ten per cent of patients undergoing surgery had T2DM, with a HbA1c level of 9.0% (multiple treatments including multiple daily injections of insulin, exenatide) and 6.8% (metformin only).
Forty-eight (96%) patients had baseline blood pressure data available. Of those, 41% (20/48) had high–normal blood pressure (systolic or diastolic > 1.33 SDs and < 2 SDs) and 33.3% (16/48) had high blood pressure (systolic or diastolic > 2 SDs) for their age. Of the 20 patients undergoing surgery, 30% (6/20) and 45% (9/20) of patients had baseline blood pressure data available. One patient was on antihypertensive medication.
Forty-three out of 47 patients had baseline lipid levels assessed, of whom 39.5% had levels of either high-density or low-density lipoprotein that were ‘significantly abnormal’; one was on a lipid-lowering medication (statin). Preoperative lipid counts were available for all 20 patients undergoing surgery: 45% had levels of either high-density or low-density lipoprotein that were ‘significantly high’; one patient was on medication.
All patients put forward for surgery (n = 20) completed a full medical assessment for weight-related comorbidities. Eighteen per cent (2/11) of females fulfilled the diagnostic criteria for PCOS; 10% (2/20) had mild obstructive sleep apnoea (OSA) not requiring CPAP, with none having severe OSA; and 10% (2/20) had non-alcohol fatty liver disease, as indicated by raised levels of ALT. One patient had achondroplasia with a final height of 128 cm and a BMI of 69.9 kg/m2, with severe mobility difficulties requiring both crutches and a wheelchair.
Bariatric surgery
All patients fulfilled criteria specified by NICE. All patients had BMIs greater than 40 kg/m2 at the time of surgery planning. Deprivation data were available for all 39 participants from England. Graphically, it appears that patients from deprived populations were less likely to get to surgery (Figure 21); however, there was no difference in access to surgery between those patients in the most deprived quintile and the remainder of the cohort (p = 0.4).
FIGURE 21.
Distribution of socioeconomic status in those patients completing the bariatric pathway. Source: White et al. 223
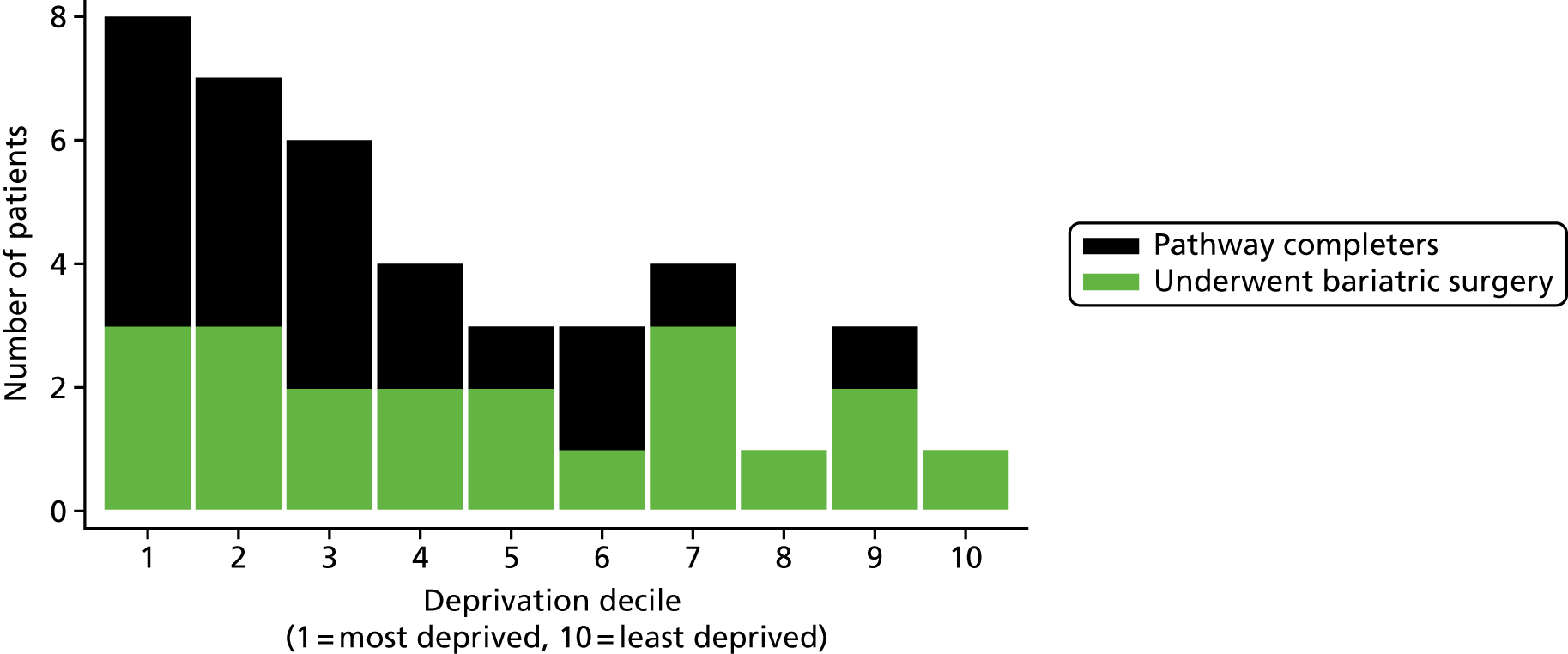
The mean time between first appointment and surgery was 1.8 years (SD 0.7 years) (range 0.5–3.7 years). Those patients with higher BMIs had a longer time to surgery (0.04 years per kg/m2, 95% CI 0.00 to 0.07 years per kg/m2; p = 0.08) after adjustment for age, although deprivation and duration of the programme did not influence time.
Patients underwent RYGB and SG in equal numbers (10 each), with no patients having LGB. The mean BMI in those patients undergoing SG was higher than in those undergoing RYGB [57.6 (SD 10.2) vs. 49.6 (SD 6.9) kg/m2, respectively; p = 0.06]. There was no difference in age between those undergoing each procedure.
Complications
Surgical complications
One patient out of 20 (5%) had a gastric perforation identified in the immediate postoperative period after RYGB, which was successfully repaired by laparotomy with no further sequalae. Two out of 20 (10%) patients had a gastrojejunal stricture. Both were successfully treated, one after one endoscopic dilatation and one after two endoscopic dilatations.
Perioperative complications
The patient with pre-existing cerebrovascular disease had a further CVE in the perioperative period resulting in hemiplegia, expressive and receptive dysphasia, and loss of thirst. He required prolonged neurorehabilitation with residual loss of function.
Micronutrient deficiencies
No patients experienced clinical manifestations of nutrient deficiencies secondary to bariatric surgery.
Body mass index trajectories
Figure 22 shows the BMI trajectory of each patient undergoing surgery in the adolescent pathway and the change in BMI relative to day of surgery. A substantial variability in preoperative BMI trajectories was seen across the subjects, with up to 10 kg/m2 weight gain seen in the time before surgery for some, and minimal visible change for others. Varying changes in BMI were seen during the 2- to 4-week preoperative hypocaloric diet phase, with weight change varying from minimal change to over 5 kg/m2.
Similarly, a substantial variability in BMI outcomes was seen after surgery, with only limited data after 2 years. All patients rapidly lost weight in the first 3 months after surgery at a similar rate. The majority of patients lost weight rapidly in the first 6 months, with subsequent deceleration in the rate of weight loss in the next 6 months and weight stability in the second year. The majority lost between 10 and 20 kg/m2 in this time period, with subsequent maintenance of weight loss. One patient lost over 30 kg/m2 in the first year after surgery although this magnitude is probably exaggerated by his short stature secondary to achondroplasia. Two patients regained all weight lost after bariatric surgery in the second year after surgery.
The two patients with hypothalamic obesity secondary to craniopharyngioma had differing outcomes. One regained all weight lost in the second year after surgery and the other regained substantial weight, although not back to presurgical levels.
Nine patients had reached 1-year follow-up at our centre at time of analysis. The mean change in BMI at 1 year was –15.2 kg/m2 (SD 9.3 kg/m2). There was no evidence of difference in means between the two surgical procedures [mean RYGB –12.7 kg/m2 (SD 2.7 kg/m2), SG –17.2 kg/m2 (SD 12.6 kg/m2); p = 0.5]. Greater variation in BMI change at 1 year was seen in patients undergoing SG than in those undergoing RYGB, with post-SG change ranging from −33.4 to 0.2 kg/m2, compared with −16.3 to –10.4 kg/m2 post RYBG.
Data were sufficient to develop a multilevel model of BMI change associated with surgery, including 249 data points on the 20 patients who had surgery (Table 32 and Figure 23). The best model included a linear term describing the period before surgery and linear and quadratic terms for the period after surgery. Figure 24 shows that the mean BMI trajectory postoperatively was very rapid loss in the first 3–6 months with a nadir at 12 months and regain in BMI thereafter. Owing to limited data we did not extend the model beyond 18 months or include predictors in the model.
| Model | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Fixed terms | No change | Time (years) | Time (years) model 2 + surgery | Model 3 + surgery × time (years) | Model 4 + surgery × time2 (years2) | Model 5 + surgery × time3 (years3) |
| Random terms | No change | Time (years) | Model 2 + surgery | Model 3 + surgery × time (years) | Model 4 + surgery × time2 (years2) | Model 5 |
| Fixed effects | ||||||
| Composite model | ||||||
| Intercept | 50.53 (46.69, 54.38) | 55.26 (51.14, 59.14) | 54.43 (50.49, 58.37) | 52.21 (48.46, 55.95) | 52.05 (48.35, 55.74) | 52.06 (48.35, 55.78) |
| Time (linear) | 3.09 (–4.39, –1.79) | –0.24 (–1.59, 1.11) | 2.48 (1.40, 3.55) | 2.60 (1.67, 3.53) | 2.60 (1.66, 3.54) | |
| Surgery | –7.37 (–9.02, –5.73) | –6.98 (–8.09, –5.88) | –4.94 (–5.80, –4.07) | –4.58 (–5.43, –3.74) | ||
| Surgery × time | –12.22 (–17.09, –7.37) | –26.87 (–34.47, –19.27) | –29.68 (–36.58, –22.78) | |||
| Surgery × time2 | 10.71 (6.72, 14.70) | 14.07 (10.44, 17.71) | ||||
| Surgery × time3 | –1.22 (–1.72, –0.72) | |||||
| Variance intercept | ||||||
| SD (intercept) | 8.59 (6.22, 11.86) | 8.97 (6.42, 12.55) | 8.64 (6.20, 12.02) | 8.35 (6.05, 11.52) | 8.32 (6.06, 11.42) | 8.38 (6.11, 11.49) |
| Years in bariatric | ||||||
| SD (years in bariatric) | 2.33 (1.49, 3.65) | 2.20 (1.40, 3.45) | 1.61 (0.91, 2.83) | 1.64 (1.05, 2.58) | 1.66 (1.07, 2.58) | |
| Correlation with baseline | –0.14 (–0.59, 0.39) | –0.26 (–0.66, 0.25) | –0.17 (–0.63, 0.39) | –0.06 (–0.53, 0.44) | –0.12 (–0.57, 0.38) | |
| Correlation with surgery × time | –0.29 (–0.73, 0.33) | –0.24 (–0.68, 0.34) | –0.14 (–0.62, 0.42) | |||
| Correlation with surgery × time2 | 0.49 (–0.32, 0.89) | 0.29 (–0.48, 0.81) | ||||
| Surgery × time | ||||||
| SD | 9.15 (6.04, 11.87) | 15.22 (10.56, 21.95) | 13.43 (9.18, 19.67) | |||
| Correlation with baseline | –0.31 (0.70, 0.22) | –0.50 (–0.79, –0.01) | –0.51 (–0.80, –0.04) | |||
| Correlation with surgery × time2 | –0.95 (–0.99, –0.77) | –0.94 (–0.99, –0.74) | ||||
| Surgery × time2 | ||||||
| SD | 7.60 (4.66, 12.38) | 6.04 (3.65, 10.00) | ||||
| Correlation with baseline | 0.44 (–0.22, 0.82) | 0.55 (–0.11, 0.87) | ||||
| Residual SD | 6.20 (5.70, 6.74) | 4.93 (4.48, 5.42) | 4.27 (3.88, 4.69) | 2.70 (2.44, 2.98) | 1.93 (1.74, 2.14) | 1.85 (1.66, 2.05) |
| Number of observations | 291 | 249 | 249 | 249 | 249 | 249 |
| Log-likelihood | –676.53 | –798.99 | –765.62 | –679.26 | –621.85 | –611.26 |
| AIC | 1959.06 | 1609.98 | 1545.24 | 1380.71 | 1275.7 | 1256.52 |
| BIC | 1970.08 | 1631.08 | 1569.96 | 1419.40 | 1331.98 | 1316.32 |
FIGURE 23.
Superimposed BMI trajectories of patients undergoing bariatric surgery, by type of surgery. Source: White et al. 223
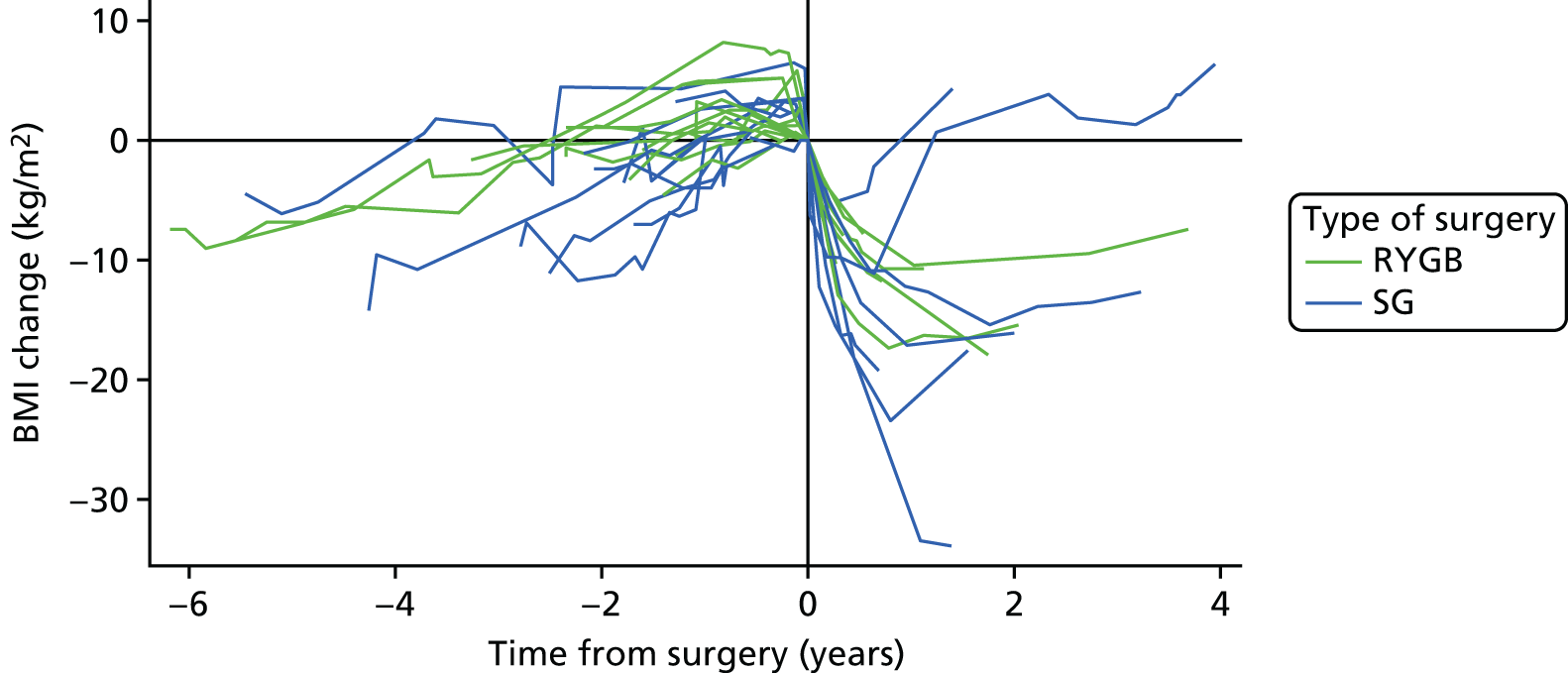
FIGURE 24.
Modelled BMI trajectory for the average adolescent patient, with surgery 1.8 years after first assessment.
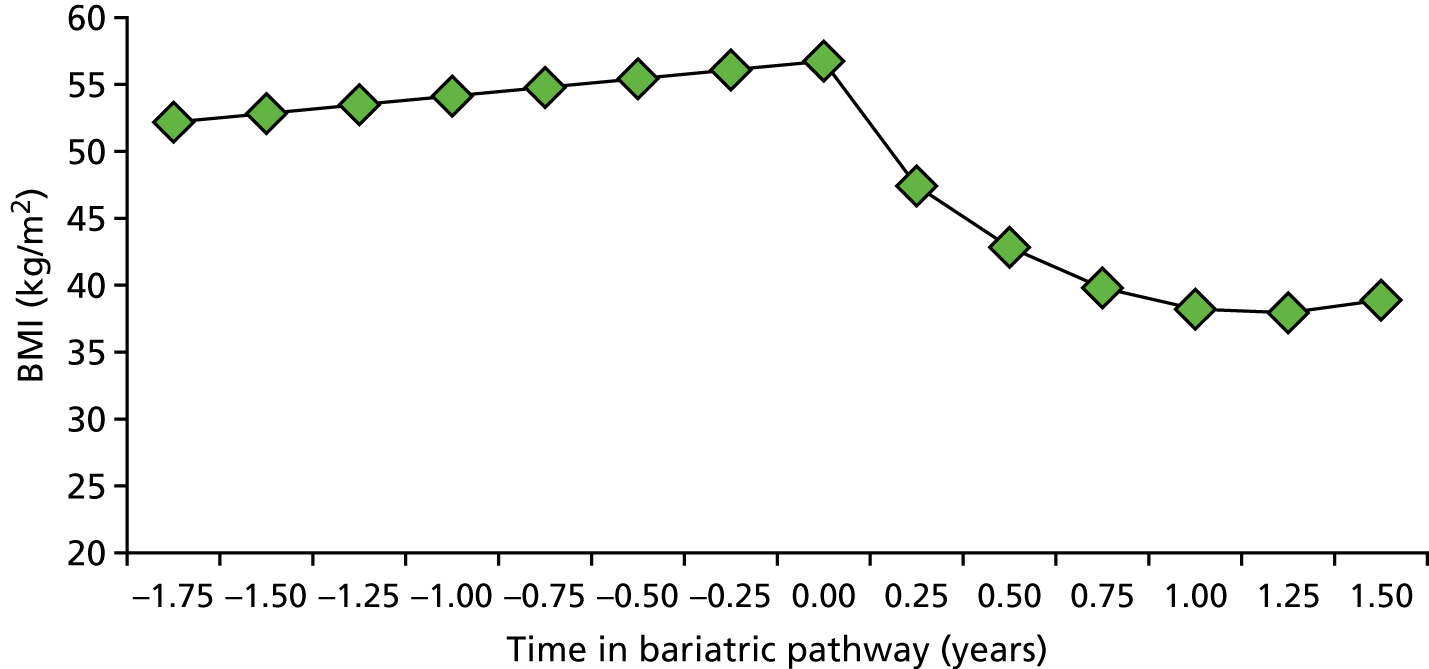
Follow-up
Follow-up post surgery was highly inconsistent. Seven patients (35%) dropped out of routine follow-up after surgery, at follow-up points varying from 8 weeks to 2 years. Three (15%) were last seen within 3 months of surgery. Some patients chose to attend follow-up with the surgical but not the paediatric team, limiting data on resolution of comorbidities. Data were insufficient to allow detail analysis of resolution of comorbidities postoperatively, with the exception of DM; DM control improved in both patients with T2DM post surgery and one was able to stop hypoglycaemic agents.
Discussion
This is the first study describing the characteristics and outcomes of adolescent patients entering an adolescent bariatric surgical programme in the NHS. We found that half of adolescents completing the assessment were both eligible and interested in surgery. The main reasons for non-eligibility were lack of ability to consent, behavioural difficulties and inconsistent desire for surgery. Patients had relatively few comorbidities and surgery was undertaken mainly for weight loss rather than control of obesity-related conditions.
Change in BMI in our patients (15 kg/m2 loss) compared well with that described in a recent systematic review (13.5 kg/m2). 224 There was no operative mortality and surgery complication rates were within the range (4–33%) described for adolescent SG and RYGB in other centres internationally. 224 Patients undergoing SG had a higher baseline BMI and greater variation in BMI change after surgery compared with those having a RYGB. High rates of attrition to follow-up were seen after surgery, despite earlier careful identification and support of medical and psychological comorbidities, and previous good adherence to the preoperative preparation programme.
The model for BMI changes we presented is consistent with clinical experience in adult bariatric surgery, which is one of early dramatic weight loss in the first 3 months, slowing weight loss thereafter and then a nadir of weight reached some time between 6 and 24 months after surgery. After adult bariatric surgery, many individuals put back on significant weight after this nadir. The model developed by us is useful to estimate the speed of BMI loss in the first 6 months.
These data raise important issues. First, we were referred low numbers of adolescents with weight-related comorbidities that respond well to bariatric surgery, such as T2DM, sleep apnoea and idiopathic intracranial hypertension. The reasons for this are not clear, and we believe that raised awareness of the safety and efficacy of bariatric surgery to control these conditions will increase referrals. Subsequent to this cohort, we have seen an increase in patient referrals, particularly of those with T2DM.
Second, the mean age at surgery was 18 years and the youngest patient was 15.7 years old. This is higher than a mean age of 16.5 years in a large Swedish cohort. 227 This probably reflects reluctance to refer younger patients in the NHS, probably influenced by NICE guidance that limits surgery to those who have ‘nearly reached physiological maturity’, a recommendation that is largely based on theoretical concerns about the impact of surgery on bone density. Earlier referrals may help reduce the age at surgery, and change the BMI trajectory of this population. If surgery was undertaken on younger adolescents, it would be important to ensure that monitoring of bone density, pubertal progression and other nutritional parameters were embedded in follow-up programmes.
Third, a systematic review undertaken by these authors has shown that there is a very limited evidence base on psychosocial predictors of outcome after surgery in adolescence, with only one study comparing baseline psychosocial variables and no studies looking at physiological predictors. 209,246 We encountered various dilemmas about eligibility provision for surgery, particularly for those who perceived that surgery was the only remaining treatment option for them. We reviewed three patients with previous pituitary–hypothalamic tumours. Two underwent a bariatric procedure (SG) with disappointing results. A subsequently published small case series suggests improved outcomes after RYGB compared with SG in this cohort;31 however, small numbers and the non-randomised methodology limit its generalisability. A registry has since been started to monitor the outcomes of those with obesity due to hypothalamic dysfunction undergoing surgery (Professor Thomas Inge, Director if Adolescent Bariatric Surgery, Akers Endowed Chair in Pediatric Surgery, Chief of Pediatric Surgery, School of Medicine, University of Colorado, personal communication).
Fourth, there was significant attrition from our pathway both before and after surgery. Further work is needed to understand this, particularly for those not attending routine postoperative care and who are subsequently at risk of nutritional deficiencies. Our data suggested a non-significant trend for those with lower socioeconomic status to be more likely to drop out pre surgery, and it will be important to monitor and facilitate access of deprived groups to surgery over time.
Limitations
We present data from a small number of young people having surgery at a single site and follow-up data were very limited. Our study is subject to the common limitations of service evaluation studies (e.g. data were not collected at standardised time points and collection of comorbidity data at follow-up was also not standardised, limiting data availability). We did not routinely collect data on quality of life or psychosocial functioning. Significant loss to follow-up postoperatively is a potential source of bias, suggesting that our findings may underestimate or overestimate BMI loss and complications. It is possible that those with poorer outcomes were less likely to return for follow-up. However, our clinical experience was that those with poorer BMI outcomes were often keen attenders at follow-up (to work with us on increasing weight loss). The majority of those who decided against surgery did not remain within our service, and we are unable to compare their outcomes with the outcomes of those who had surgery.
Conclusions
In conclusion, adolescent bariatric surgery in a NHS service compares favourably to international cohorts, and shows promise as a safe, effective treatment for severe obesity. Further work is needed to improve patient selection, better match patient need to operative type and reduce pre- and post-surgical attrition.
Substudy 6.2: clinicians’ decision-making for adolescent bariatric surgery
This work was reproduced and published as Doyle J, Colville S, Brown P, Christie D. ‘For the cases we’ve had . . . I don’t think anybody has had enormous confidence’ – exploring ‘uncertainty’ in adolescent bariatric teams: an interpretative phenomenological analysis. Clin Obes 2014;4:45–52. https://doi.org/10.1111/cob.12039. 247
Licence to reproduce number (John Wiley & Sons): 4675951183573.
Aim
The aim of this substudy was to investigate the process of clinicians’ decision-making around bariatric surgery for adolescents, and understand how professionals manage in the context of limited research and guidelines for bariatric surgery for young people, in order to reach a decision as to which adolescents should be offered surgery and which should not.
Methods
Participants
Three leading NHS centres offering bariatric surgery to adolescents were approached and two of these agreed to participate. The third centre declined because of its own research commitments. All surgeons, paediatricians, nurses and psychologists actively working in the two centres were approached to participate. Nine clinicians out of the 10 approached agreed to participate, with one declining because of work commitments. These clinicians represented a variety of different professional backgrounds, including surgeons, physicians, psychologists, psychiatrists, nurses and dietitians.
Procedure
Potential participants were sent a copy of an information sheet outlining the details of the study and requesting that they take part in a research interview. Informed written consent was obtained from all participants prior to interview. It was anticipated that the experiences of clinicians with regard to adolescent bariatric surgery were likely to be complex and varied. A qualitative methodology was chosen to avoid imposing a set of preconceived ideas or variables. A semistructured interview was designed to allow participants to ‘tell their own story’ and for the research to follow up on any areas of complexity (see Appendix 4 for the interview guide). This interview schedule has not been used in any other studies. The development of key themes within this schedule was informed by the existing literature on decision-making around bariatric surgery as well as a more general social science literature on decision-making within conditions of uncertainty.
Interviews with nine participants were completed at both sites and lasted approximately 45 minutes (range 25–75 minutes). Questions focused on the following areas of discussion: background (e.g. history of service, current pathway for adolescents); how the team makes decisions; issues that influence the decision that surgery would or would not be appropriate; how evidence and experience are drawn on when making decisions; how the NICE guidelines are interpreted; ways the team supports a young person to make a decision; and developments that the team would like to see for adolescents considering surgery. All interviews were digitally recorded and subsequently transcribed. At this stage digital recordings were destroyed.
Analysis
Investigators PB, SC and JD analysed the nine written transcripts using interpretative phenomenological analysis (IPA), following procedures outlined by Smith et al. 248 In line with IPA, the thematic analysis was especially focused on the various ways in which participants sought to make sense of, and construct meaning within, the situations being described, developing an ‘insider’s perspective’248 of how these professionals experienced the phenomena being described. Initial parallel analyses were completed by JD and SC and a combined preliminary outline of themes was produced. Investigator PB completed secondary analysis of transcripts and added to the conceptual framework to produce a final thematic analysis of the data.
Results
Following analysis of the data, extracts have been taken from various interviews, which are characteristic of the dominant themes emerging within the data analysis (Figure 25).
In order to protect the anonymity of participants, names are not stated. Clinicians will be referred to by a number indicating the order in which interviews were completed (C1–C9).
Pervasive uncertainty
A predominant theme of ‘uncertainty’ emerged from the interviews, as reflected in these extracts:
It’s not like a blood test with a plus or minus answer . . . it’s a judgement call.
Clinician C7
. . . I guess you do have a bad feeling about some people and it’s non-specific you just . . . it’s just a very woolly thing.
Clinician C9
The sources of uncertainty were numerous, as outlined below.
Uncertainty around a lack of data/guidance
Clinicians agreed that bariatric surgery is an effective treatment for weight loss but that research investigating the effect of surgery on psychosocial outcomes is lacking, and without this evidence it was difficult for teams to decide who might benefit from surgery:
I think the reality is that we’re doing surgery with young people without really knowing . . . you know there aren’t good long-term data on outcome and what constitutes who is likely to have a good prognosis so we’re to some extent going in blind.
Clinician C6
. . . we know where it is indicated and it is not indicated roughly, but there are no clear guidelines . . . for adolescents.
Clinician C3
Well, I mean the evidence is that it produces good weight loss and weight loss maintenance, it improves metabolic status of individuals and so on. Does it make them happier? Don’t know. Does it make them more productive socially? Do not know.
Clinician C1
Bariatric surgery as a medical treatment like no other
The apparent discomfort in relation to offering bariatric surgery to young people appeared to be exacerbated by the perception of bariatric surgery as a complex procedure, unlike other treatments:
. . . normally a surgeon does an operation to remove something that shouldn’t be there or to untwist something that got twisted or to put right something that went wrong. Bariatric surgery is quite the reverse. The surgeon is going in there to actually create an abnormality . . . an abnormal physiology and structure for a greater good. So I do think that that is conceptually quite a different surgery.
Clinician C1
The complex nature of adolescence
There was also speculation about the extent to which young people have the capacity to make long-term treatment decisions. Much of this speculation arose from clinicians’ concerns about the relative influence of ‘adolescence’, with the majority perceiving adolescence as a potential obstacle to good decision-making:
. . . they’re physically mature but do they really understand? . . . the whole concept of ‘future self’ is something people have to be aware of.
Clinician C7
One of the concerns is at what age do you have the consent that you are having this not to feel better today, but for a greater good over the next 20 years? . . . do adolescents take out life insurance? No they don’t. You know, do adolescents drive cars very slowly because they think of their life ahead? No they don’t. Do adolescents engage in safe sex? No they don’t. I mean that is not an age where usually you are thinking about your life ahead of you.
Clinician C1
. . . it’s quite challenging for them to make these life changing decisions that are going to affect them for the rest of their lives at a time when they are already in quite a turbulent period of their lives . . .
Clinician C2
Adolescent bariatric surgery as a ‘risky’ business
Bariatric surgery was considered a novel and relatively controversial treatment for obesity in adolescence, especially in the public domain, which reinforced a sense of risk and uncertainty around offering this treatment to some:
Yeah. I mean so far I think it’s been fairly contentious. We’ve been on the front page of the press on a couple of occasions . . .
Clinician C8
. . . the younger we consider them the more uncomfortable it becomes. You can argue 16–17 are nearly adults erm . . . both physically and psychologically. But 9 years old it’s a different ball game and I’m not sure if the UK is ready for it.
Clinician C3
Participants appeared to use a range of strategies to overcome these multiple layers of uncertainty, although some of these strategies were in themselves a source of uncertainty.
Overcoming uncertainty?
Look for individual behavioural ‘signs’ of a good outcome: ‘insight’ and weight targets
One potential solution to uncertainty involved looking for particular ‘signs’ from the individual that they were appropriate for surgery as a way of ensuring a good outcome. For some clinicians, the adolescent’s capacity for ‘insight’ helped guide the decision-making process:
. . . it worries me if a patient isn’t able to tell me why they have a weight problem so if they can’t describe what it is that’s made them overweight then it’s hard to see how surgery will help change that.
Clinician C2
Well, one of the questions I ask all my patients, be they adults or children, is ‘why are you overweight?’ and I like it when they say ‘because I eat too much’. About two-thirds of them do say that and at least they’ve got a grasp of why they’re overweight. About 30–40% of them look at me and say ‘dunno doctor, it’s my glands doctor, it’s this doctor, it’s that doctor’ and you just think ‘ooeurggh’ you know, they’re more difficult to work with when they do that. So yeah, I’d like to see a grasp of a) why they’re overweight and b) the fact that this surgery isn’t a magic wand, it makes them eat less, and that’s how they lose weight. So as long as they’ve grasped that then that’s fine.
Clinician C9
Some participants described feeling reassured when young people made preoperative behavioural changes to diet and weight, considering this an indication of engagement with treatment. However, the majority of participants perceived preoperative weight targets as flawed and illogical. This debate is outlined below:
. . . if they’ve changed the ways that they eat before surgery they should technically lose some weight! So I guess it’s as an indicator of how well they’ve taken that information on board and the fact that they are able to make some of those changes.
Clinician C5
I wouldn’t expect them to have been losing weight before we’ve offered it because I think that’s a bit futile really. By definition, it’s a last resort. If they’re losing weight then why operate on them?
Clinician C8
Looking for a ‘supportive social context’
Other participants considered the role of the family or wider social context as significant in considering who will/will not do well after surgery. This ranged from looking for signs that family members would not sabotage success and an active avoidance of adolescents coming from more complex social backgrounds:
I think the adolescent is going to be a lot more successful if the whole family is engaged rather than just them. It’s going to make it difficult for them if their family isn’t engaged in what they’re doing . . .
Clinician C5
Where the family seem reasonably coherent and cohesive. Erm . . . going back to things that would ring alarm bells – children who have been ‘looked after’ children, you know where there are safeguarding issues. They’re often children of high need but you’re just not entirely convinced that we have the decision-making basis there, given the numbers we’ve done in paediatrics so far . . .
Clinician C8
Multidisciplinary team working
The clinicians described using MDT meetings to discuss patients and make joint decisions. There were mixed views as to the usefulness of the MDT process. Some participants valued the role of the MDT in moderating uncertainty, as is outlined below:
MDT is a core process. It’s nearly as important as surgery. Erm . . . you will assess the patient only from one perspective – psychological, surgical, purely medical – and you might miss key pieces of information if you just assess the problem from one angle. You need different angles.
Clinician C3
. . . it’s good to have a multidisciplinary focus because from the surgeon’s perspective they see very much the surgical problem that they can fix with surgery and then I think the dietitians will see an eating behaviour that they want to help fix and we all see different things.
Clinician C2
. . . the MDT has to be unanimous for this sort of surgery because it is to a certain extent still contentious. Unless everyone feels it’s appropriate we probably would say ‘well this is probably not the right thing, let’s review it in 6 months/a year’.
Clinician C8
Other participants questioned the value of the MDT and for some this was an area of additional uncertainty, particularly as regards the issue of clinical responsibility:
Now, at the adolescent meetings they have been quite large because what we also had of course is adolescent psychology services, adolescent social work services, sometimes paediatric, sometimes adolescent psychiatry . . . so there have been at times quite large numbers of people, which of course doesn’t always mean that decision-making is good.
Clinician C1
. . . some of these decisions are very, very subjective and it’s your own feeling or view and it’s quite difficult when you draw out a care pathway, you know do you have X, Y, Z. And personally I’m not overly keen on that because I think it takes away that element of ‘I’m just not sure that’s the right thing to be doing’ which can sometimes, and that’s what our MDT is a little bit about people . . . it’s more of the ‘hmmm what do you think? Hmmm just not sure.
Clinician C8
Some saw clinical responsibility as residing with the surgeon, while other saw the surgeon as a technician, the person who carries out the MDT decision:
So . . . [the surgeon] is the one who physically erm . . . does the operation even though it is a team decision; it requires a lot of ground work. So in the event that things don’t go as planned it is . . . [the surgeon] who will be blamed . . .
Clinician C3
Because ultimately it’s the surgeon’s decision whether he wants to take the risk. And so it feels as if it’s the surgeon’s decision but the patients are under the care of [paediatrician] so there is I think a little bit of a how does one reconcile and negotiate that and whose decision is it?
Clinician C4
. . . in many ways it’s other people making the decisions as to who is appropriate to refer to . . . [the surgeon] and then obviously . . . [the surgeons] make decisions about which operation and that sort of thing.
Clinician C9
Other participants also questioned the role of the psychologist and psychiatrists within the MDT and the potential role as ‘gatekeeper’ to the provision of surgery, and others questioned what kind of psychological professional is best suited to participate in the decision-making:
. . . traditionally, psychology has been thought of as the gatekeeper to whether people have surgery or not.
Clinician C6
. . . the role of the psychologist is the role of a psychologist so it’s always controversial. So it depends on if you are taking a mostly biological view or psychological view . . .
Clinician C3
I think there is always a question about what the role of a psychiatrist is, as opposed to a psychologist or another type of therapist . . .
Clinician C4
Bypass adolescence
One major source of the dilemma around adolescent bariatric surgery is related to the perceived nature of the developmental period of adolescence. One solution generated to overcome this dilemma was to treat people under the age of 18 years as adults, or to bypass adolescence completely:
. . . we just don’t treat them any differently from how we treat the adult patients . . . we don’t do anything different.
Clinician C9
I’m not saying this is something we do tomorrow, but I actually as a . . . if you like a hypothesis, would argue that actually it may be far easier for a child of 8 or 9 to cope, adapt and be supported through surgery than a child of 14 or 15 in the throes of a ghastly adolescence.
Clinician C1
The NICE guidelines specify that bariatric surgery should be performed only in young people who have reached physiological maturity. However, clinicians here have argued that this particular developmental stage is fraught with emotional turmoil and that psychologically this may not be the most appropriate time for surgery. This has led them to suggest that surgery might best be postponed until adulthood or be undertaken prior to adolescence. To our knowledge, the suggestion of performing bariatric surgery in childhood was hypothetical and the authors are not aware of surgery being performed in the UK in individuals as young as 8 or 9 years. All participants spoke only of surgery performed in the UK with adolescents who had indeed met physiological maturity.
Accepting the inevitability of surgery
Although participants made reference to debates around the type of procedure, and agreement that weight loss surgery should be considered a ‘last resort’ treatment for young people, there was also a perceived inevitability of surgery. Many commented on the potential of bariatric surgery to allow the young person to live a ‘normal life’, given the apparent ineffectiveness of other treatments for obesity:
And you know if you say well ‘we’re not sure about A, B, C or D’, the surgeon may say ‘well what else have you got to offer them?’.
Clinician C1
. . . every piece of research that shows the ineffectiveness of other interventions is another nail in the coffin I think for anything other than bariatric surgery.
Clinician C
. . . I fully admit that it is in my opinion a hundred per cent psychological . . . or ninety per cent psychological. Having said that, when they are massive I personally think that the surgery is pretty much the only thing that works.
Clinician C9
But I do I think that if you can help somebody change at that point in their life before they get all the comorbidities that come with that . . . like diabetes for example, and give them a shot at having a normal life as an adult.
Clinician C2
. . . these young people they haven’t really started adult life yet. So you can actually make them start a proper life, a proper healthy life.
Clinician C3
Discussion
The paper by Doyle et al. 247 demonstrates that making decisions about bariatric surgery for young people is a challenge for health-care professionals in bariatric surgery teams across the UK, characterised by multiple uncertainties. To our knowledge there has been only one other study investigating the views of medical professionals on this topic. Bailey et al. 249 conducted a qualitative study to understand Canadian physicians’ attitudes to the treatment of paediatric obesity. Physicians were more accepting of medical treatment and lifestyle management of obesity in young people than of surgical treatment and showed scepticism for this in the absence of long-term data. In our UK study, the challenges were described as coming from the perception of adolescent bariatric surgery as a controversial subject for the public, as a complex intervention unlike other medical treatments, with limited outcome data to guide decision-making. This is further complicated by a view of adolescence as being a turbulent developmental period, with some professionals querying whether adolescents are capable of future-orientated thinking that is thought to be necessary for good postoperative outcomes.
Social scientific research into decision-making amid uncertainty suggests that a range of different ‘tools’ are drawn on to manage uncertainty. 250 These include more rational-calculative approaches, based on empirically ‘proven’ knowledge or non-rational strategies (e.g. decisions based on beliefs, hopes or emotions). Trust, as placed in either the patient, other colleagues or self-judgement, enables uncertainty to be ‘bracketed away’ and complexity reduced, thus facilitating decisions and action. 251 In this study, professionals are unable to draw on the preferred method of making decisions based on empirically derived knowledge. In the absence of this, professionals manage uncertainty by looking for other knowledge (i.e. behavioural information and signs of an adolescent’s ability to make preoperative changes), signs that appear to make intuitive sense to the professional, building his/her confidence that it is safe to proceed to surgery. Trust in the workings of the multidisciplinary process is also one way in which the dilemmas around surgery are bracketed away, although this too is a source of uncertainty, with a number of professionals questioning the value of a consensus decision, doubt around the roles of different professionals and who ultimately has legal responsibility for the decision. Some professionals therefore have looked for other ways of managing uncertainty, arguing that adolescence should be bypassed completely. Furthermore, viewing surgery as inevitable and a treatment that can ‘normalise’ allowed teams to dispel many of the uncertainties connected to the provision of a treatment still relatively controversial to the public.
Current NICE guidelines with regard to bariatric surgery emphasise the need for a multidisciplinary approach to decision-making. However, up until now there has been very little written about the nuances and complexity of these interactions with adolescents and health-care professionals. At present, professional decisions appear to be driven by intuition and ‘judgement calls’ rather than empirical data. This is not to suggest that empirically derived knowledge is superior or that patients are deliberately being misled, however, it is argued that this process needs to be made transparent. Revealing the ways in which clinicians work through, and reach, decisions amid uncertainty facilitates greater awareness of difficulties and a more reflective practice as a result. Adolescents too ‘bracket away’ uncertainty regarding bariatric surgery. 252 A ‘shared decision-making model’ is one means of reaching an agreement regarding appropriateness of surgery,253 yet this approach potentially becomes more problematic when dealing with adolescents in terms of heightened power asymmetries. It is also potentially complicated by the active process of bracketing away uncertainty by both professionals and adolescents.
This study has made use of IPA as an insightful analytical framework for exploring participants’ experiences of the phenomenon in question, while recognising that this involves interpretation by the onlooker. However, many have argued that language is not merely a means to express reality but instead that it prescribes what we can feel and think (i.e. that language both reflects and shapes reality). 254 One medium by which professionals moderate the dilemma around surgery for young people is to accept the inevitability of surgery. Surgery is seen as something that can have profound effects to ‘normalise’ and there is an implicit acceptance that offering something (surgery) is possibly better than doing nothing. It is argued here that these assumptions need to be interrogated and questions need to be asked as to how this way of thinking came to be taken for granted. Brown255 has argued that that examining these interactions has implications for policy and practice in ‘underlining for practitioners the significant interpretive scope to which their actions/utterances are liable’. These questions are essential as the discourses around the inevitability of surgery for a normal life are ones that adolescents themselves take up and, therefore, within this context, the option not to choose surgery if it is offered is potentially rendered invisible. It is argued that the uncertainty that professionals experience, around what is known and not known, needs to be shared with potential patients. However, professionals must also question the social context that informs their thinking, such that, if surgery is offered, accepting and not accepting surgery are both regarded as legitimate decisions.
Moves towards a shared decision-making model could usefully include the following developments:
-
professional agreement about the limits of knowledge as regards adolescent bariatric surgery and support for staff to communicate this information, in a context where patients often believe that ‘doctors know best’
-
parent- and adolescent-friendly information leaflets outlining what is known and not known about adolescent bariatric surgery
-
adolescent-specific support groups and web-based resources for young people battling overweight, such that adolescents are in a position to become more equal partners in medical consultations around managing overweight and considering the pros and cons of surgery.
There are a number of limitations to this study that need consideration. The research has taken place in the UK, where adolescent bariatric surgery is an emerging field. Therefore, the views expressed herein may not be representative of those of professionals where surgery for young people is more readily available, for example in the USA and Australia. Investigating whether these countries have been through a similar process in the early stages of the availability of bariatric surgery, and how this has been managed, would be of interest.
Substudy 6.3: how adolescents decide on bariatric surgery
Background
In 2010, just over one-quarter of adults in England were classified as obese, with a BMI of ≥ 30 kg/m2. A NIHR Health Technology Assessment report256 concluded that bariatric surgery is a clinically effective and cost-effective intervention for moderately to severely obese individuals, in comparison with non-surgical treatments. The NICE guidelines11 indicate that bariatric surgery is not generally recommended for children or young people and should be offered only in ‘exceptional circumstances’. There is no national database to record bariatric surgery performed specifically in adolescents, although anecdotal evidence suggests that this, too, is increasing.
Adolescent bariatric surgery is an area that is fraught with many ethical dilemmas. 257 Although it might seem logical to postpone surgery as long as possible, there is evidence that weight loss surgery may be less effective the longer it is delayed and the more the health risks of obesity increase. Despite this, many clinicians do not agree that bariatric surgery performed in adolescence is an appropriate intervention. One survey of > 300 family physicians and paediatricians based in the USA revealed that 48% would not ever consider referring an adolescent for bariatric surgery, and the most frequently endorsed age at which the doctors would consider referring was 18 years. 258 Furthermore, this research team’s study of bariatric surgery teams based in the UK revealed a great deal of uncertainty around bariatric surgery for young people and how to decide which adolescents should be put forward for surgery, in the absence of a firm evidence base or specific guidelines in this area. 247 Professional uncertainty about weight loss surgery for adolescents is likely to be compounded by the fact that adolescents who are delayed from having surgery often change their minds about proceeding.
Ogden et al. 259 conducted interviews with 15 adults who had bariatric surgery. The authors reported on the ‘paradox of control’ in that, although many turned to bariatric surgery because they no longer had faith that they could lose weight without medical assistance, the imposed restriction of surgery seemed to enhance the sense of self-efficacy and sense of control. A recent qualitative study260 explored attitudes and beliefs regarding bariatric surgery for young people among obesity specialists, morbidly obese adolescents and parents. These authors found that views on the aetiology of obesity (i.e. whether it should be considered primarily a medical condition or more a psychosocial problem) seemed to affect decision-making around bariatric surgery. Parents and adolescents who saw obesity as something that they could influence themselves were more in favour of non-surgical treatment, and vice versa. Apart from these two studies, however, there has been very little in-depth research on how adolescents make decisions about the appropriateness of bariatric surgery.
Aim
This study aimed to investigate adolescents’ decision-making around bariatric surgery, to understand how adolescents conceptualise bariatric surgery and decide whether or not this is the right intervention for them, in a context characterised by limited research and guidelines around bariatric surgery for young people.
Methods
Participants
All adolescents were recruited from one NHS centre offering bariatric surgery to young people. Twenty-five people who (1) had already undergone bariatric surgery, (2) had been assessed for surgery but had declined or (3) were in the process of being assessed for surgery were approached. Sixteen people declined to take part, with a range of reasons being cited, leaving nine young people who took part in the study.
Participants were all aged 17–20 years and comprised four males and five females. Five participants had undergone surgery (at ages 16–18 years) and four participants were in the process of being assessed for surgery.
Procedure
Potential participants were sent a copy of an information sheet outlining the details of the study and requesting that they take part in a research interview. Informed written consent was obtained from all participants prior to the interview. Parental consent was not obtained as all participants were aged ≥ 16 years. A qualitative methodology was chosen to avoid imposing a set of preconceived ideas or variables, especially given the limited existing research regarding adolescent decision-making. A semistructured interview was designed to allow participants to ‘tell their own story’ and for the researcher to follow up on any areas of complexity. This interview guide has not been used in any other studies and can be seen in Appendix 5. The development of key themes within this schedule was informed by the existing literature on decision-making around bariatric surgery, as well as a more general social science literature on decision-making in conditions of uncertainty.
Interviews with nine participants, five of whom had already had surgery, were completed and lasted ≈ 1 hour. Questions focused on various considerations of the young people in deciding whether or not surgery was an appropriate option for them, and how they arrived at the decision to proceed. One of the participants who was interviewed declined to have surgery and was interviewed in relation to that decision. All interviews were digitally recorded and subsequently transcribed verbatim. Digital recordings were destroyed after all interviews were transcribed.
Analysis
Investigators PB, SC and JD analysed the nine written transcripts using IPA, following procedures outlined by Smith et al. 248 In line with IPA, the thematic analysis was especially focused on the various ways in which participants sought to make sense of and construct meaning within the situations being described, developing an ‘insider’s perspective’248 of how these professionals experienced the phenomena being described. Initial parallel analyses were completed by JD and SC and a combined preliminary outline of themes was produced. Investigator PB completed secondary analysis of transcripts and added to the conceptual framework to produce a final thematic analysis of the data. Critical discussions of coding and the development of themes took place between the researchers, as a means of enhancing reliability of coding and the construct validity of the themes emerging in the analysis.
Results
Following data analysis, extracts have been taken from various interviews that are characteristic of the emerging themes and subthemes (Figure 26), as understood in terms of both repetition and salience within and across participants’ accounts. 261 To protect their anonymity, participants are referred to by a number indicating the order in which interviews were completed [participant (P) 1–P9].
FIGURE 26.
Main themes and subthemes emerging from adolescents’ interviews.
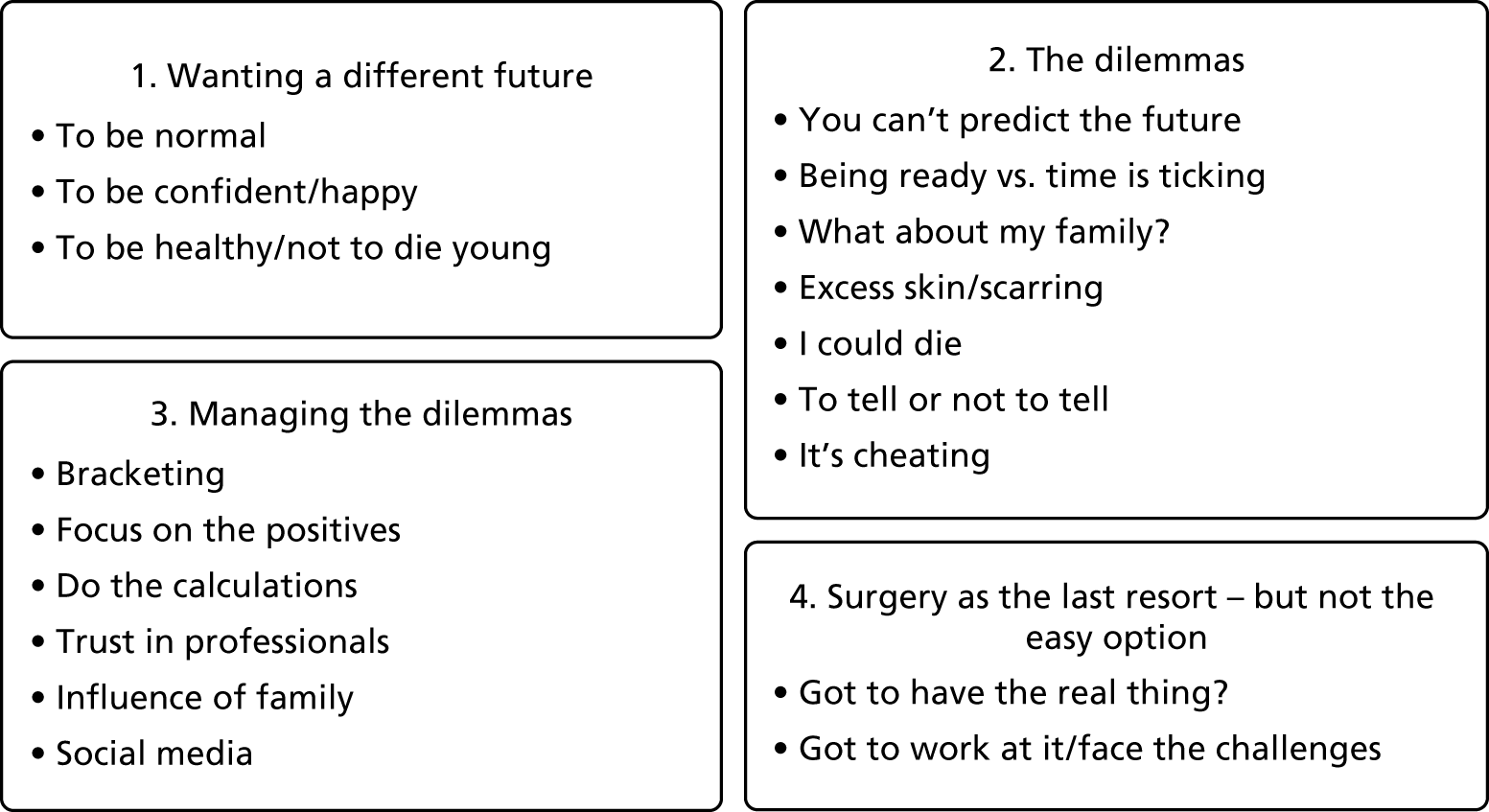
Wanting a different future
For a number of the participants, bariatric surgery represented a chance to change the course of their lives:
Yeah . . . basically I want to live life and I think if I just try . . . not doing something about it then I’ll just be home all the time.
Adolescent P9
A fresh start: I wanted to, like, start all over, start from fresh.
Adolescent P1
That’s when I thought to myself ‘I wanna actually do it’, ‘cos I knew if I didn’t do it, I’d carry on . . . I’d be the same person I am now. Now I’ve had it and I’m just different now.
Adolescent P1
To be ‘normal’ and do normal things (e.g. go shopping)
For others, the decision to have surgery was based on a desire to be normal:
I struggle to do basic things sometimes and that’s frustrating. So things like that. You can find clothes easier; you don’t have to avoid doing certain things in public . . . you can be . . . I don’t want to say normal but yeah, you can be normal!
Adolescent P6
Feeling good about myself and losing weight, going out to go shopping and buying clothes for like a size 12 not a size 18, feeling welcome . . . like, not an outcast.
Adolescent P4
It will be a lot easier just to go out shopping and be able to buy clothes and things like that. And theme parks, I can’t fit on the rides there.
Adolescent P8
Just the thought that, ‘yes I’m going to lose some weight and go shopping’. They’re the things that excited me.
Adolescent P2
To be confident/happier
The desire to be confident was mentioned by a number of participants:
I actually wanted to do it when I started college. ‘Cos, you know when you start college, like, you meet new people and then I didn’t have any confidence like that so . . . and I was, like, very shy; I still am shy but not as much as I was back then so . . . I wanted to have, like, confidence in me, like, yeah, I could approach people.
Adolescent P1
I think it’ll just give me more confidence, I feel like I stick out a bit at the moment and it’s just . . . I think it’ll just be nice.
Adolescent P8
To be healthy (and not die young)
Health was a motivating factor when considering surgery for some of the participants:
. . . when I came to about Year 11 [age 15–16 years], that’s when I thought like it . . . and then when the surgeon said to my parents that if I carry on the way I am then I won’t see past 30-something, and then I really thought, like, it really hit me.
Adolescent P1
It’s not exactly what they said, but it seemed they’d sort of say ‘you have to have it or you’re going to die’ . . .
Adolescent P3
Overall, a range of hopes for the future were referred to as central motivating factors that encouraged them to consider surgery. As is implicit within the previous two quotations, professionals’ communication and interpretations of this communication were partially influential on the structure of participants’ hopes.
Dilemmas
Alongside the apparent benefits of surgery and the positively hopeful perspectives expressed by the participants, the surgical course of action was described by most participants as giving rise to a number of dilemmas. The most common and salient concerns are outlined in the following sections.
Uncertainty regarding efficacy
The innate ‘unknowableness’ of the future was referred to as problematic, especially regarding the longer-term efficacy of the intervention:
Obviously, it’s always in the back of my head that it could go wrong, something could go wrong in the future. It could not work as effectively as I wanted it to and I could just . . . I don’t know, maybe I’ll find out it’s not for me and it’ll be too late because I’ve had it already . . .
Adolescent P6
Balancing being ‘ready’ and ‘mature’ enough versus being too old for surgery
A number of the participants discussed the need for maturity in order to proceed to surgery, while simultaneously considering that there could come a time when one is too old for the surgery to make a difference. As with the shaping of hopes by others, referred to previously, the concerns and dilemmas regarding age and maturity expressed by participants implicitly reflected the arbitrary delineations (around 18 years of age) made by policy and professionals:
There are no really cons about it, as long as you are mature enough to choose it and it’s for you then go for it.
Adolescent P4
When I was 16 I was obviously . . . you’re not really . . . when you’re still in that immaturity stage, I was not quite sure.
Adolescent P4
I think from the age of 18 [years] they should offer it, you’ve matured then, you have your own mind. At 16 [years], you don’t really have your own mind, you’re still . . . childhood footsteps if that’s a way to put it.
Adolescent P4
I’m not saying it’s now or never but it will come to a stage where you’re too old to have the operation or you can’t have the operation if you’re not in a specific category or whatever. I just think it’s better if you have it younger.
Adolescent P2
What about my family?
Some of the participants considered the impact that having surgery could have not only on them, but on the family as a whole:
They explained to my family as well that when you go out for meals and all that, like, they don’t have to feel bad that I am not eating as much and all that . . . my mum’s the one that found it hard that I wasn’t eating as much but then we . . . then everyone got used to it and now she’s fine with it. At first . . . it used to kind of bother me because everyone used to be upset and all that but now I’m happy with it and they’re happy with it as well.
Adolescent P1
Trade-offs between being overweight and having excess skin
A number of participants were aware of the possibility, or had already experienced, that weight loss can lead to loose or excess skin postoperatively. A number of participants engaged in weighing up the pros and cons of this compared with being overweight for life:
I didn’t like showing my arms but now my arms aren’t fat, but they are saggy. I’ve got skin but I don’t even care because it’s better than having some hefty arm than a little bit of sag. So it doesn’t even matter I think. Like people ask me, they say like ‘what’s your skin like?’ but I just say ‘I’d prefer to have saggy skin than be ginormous’ sort of thing.
Adolescent P2
At the end of the day, you’d rather have scars and be smaller in size that not have scars and be bigger.
Adolescent P4
I’d rather lose weight and have loose skin than be overweight and my skin being tight . . . My skin is quite droopy around my stomach and stuff but I still wouldn’t change the decision I’ve made.
Adolescent P5
Not really . . . I think, no matter how much skin I’m going to have, it’ll still mean less weight. I’d rather care about my health than how I look. Even though how I look affects me, my health affects me more. You can’t exactly have it all your own way.
Adolescent P9
What is noticeable here, as emerged elsewhere in the findings, was a general congruence between concerns and value attributions between those who were considering surgical interventions and those who had already undergone such interventions.
Concern over mortality risks
Some of the participants debated the importance of the mortality risks of surgery, although these were assessed in relation to the positive outcomes hoped for and were, therefore, largely discounted:
Like, I knew that with every surgery there is a death risk but with the band it’s like 1/2000 and a sleeve 1/200 but that didn’t change my mind wanting the sleeve.
Adolescent P9
I’m OK with it all but deep down you are worried about surgery, dying . . . and the challenge after. But I think . . . the way I’ve wanted for so many years, I’ve basically wanted to take on [the] challenge.
Adolescent P9
To tell or not to tell? Disclosure/impact on social circles
Participants varied in their desire to discuss surgery with friends and relatives for reasons that were often diverse or hard to articulate. A number of the participants preferred not to talk about their operation beyond their most intimate social circles:
I had a gastric bypass so I never kept it a secret, I don’t think it’s something to be embarrassed about.
Adolescent P2
I don’t like telling people because it kind of makes me look like I’m trying to get some attention or something, so I like keeping myself to myself at college.
Adolescent P1
I’m very close with my friends and stuff but for some reason I didn’t want to tell them. I don’t know why, even my best friend, like, I’d tell everything and I still sometimes do feel . . . not guilty but just like ‘why didn’t I tell her?’ but I don’t know, it was just something I wanted my mum and dad and my brother and sister [to know].
Adolescent P5
Nah. It’s not . . . you don’t really talk about things like that. Nah, nah, nah.
Adolescent P6
Concern about disclosing
Arguably, part of the reason for concerns about disclosing the surgery pertains to stigma about body weight and associations linking obesity to limited self-control and autonomy. A number of participants discussed the perception of bariatric surgery as an ‘easy solution’ to weight loss and how this would be seen by others:
You haven’t done anything yourself, it’s nothing to be proud of. You’re not gonna get praised like ‘well done, you look really great’ – well, you’ve just had surgery done, what do you expect?
Adolescent P7
I think it’s an easy option . . . an easy way out.
Adolescent P7
I think some think it’s just an easy way out and things like that.
Adolescent P8
If you’ve tried every way possible, then you can’t exactly say that’s the easy way out.
Adolescent P9
This last quotation emphasises what many participants referred to, that this decision was not an easy option and, as this section has noted, there were many difficult dilemmas and concerns to be worked through. Nevertheless, broader societal discourses regarding body weight, obesity and autonomy seemed to implicitly frame aspects of the participants’ decisions.
Managing the dilemmas
The participants used a variety of strategies to manage the dilemmas, as described in the following sections.
‘Put it out of your head’: bracketing away the dilemma
Some of the participants discussed actively ‘bracketing away’ the dilemmas associated with surgery. In other words, they described ‘looking past’ various concerns or uncertainties by focusing on more positive outcomes:
When I was thinking . . . like, it would come up every now and then in my head, like they said the risks and they said that possibly there is a chance of someone actually not making it through the surgery and that’s what I was thinking about most, but then I didn’t really want it to get to me so I’d always just pull it out of my head if you say, then just . . . I didn’t really want to think about it. I just didn’t let it all build up ‘cos then that would’ve probably . . . I could have just said at the last minute I don’t want to do it and just backed out, but I didn’t want that because I was, like, waiting that long for the surgery and everyone was supporting me, and I didn’t want to let everyone else down and I didn’t want to let myself down ‘cos then I knew I’d go and say, ‘I wish I had it done’.
Adolescent P1
Yeah the different options and all the information about what they [the surgical interventions] did and everything, but I didn’t read it . . . I just looked at the pictures.
Adolescent P2
Do you know what, there was never actually a time . . . I just think I was so determined to have the operation that none of that even crossed my mind. There was never a time where I thought, ‘no I don’t think I should have the surgery’.
Adolescent P2
I didn’t really think about it too much . . . I had to have it done, everyone was saying ‘you need it’.
Adolescent P3
Focus on the positives
Some participants actively emphasised the positive aspects of surgery as a way of dealing with the dilemmas:
And also that if they’re scared that they might not make it through the operation, like, don’t let it get to you, think about the positives and stuff.
Adolescent P1
It made me think more of the negative because even though I try to block out the negative, it’s always still there. I suppose if I think about the negative, it brings more positives.
Adolescent P9
Probabilistic approaches to uncertainty
In line with much of the literature on lay interpretations of probabilistic expressions, the participants referred at times to specific risk-related probabilities; these were ‘explained away’ through various techniques, including normalising the risks, seemingly equating low probabilities with ‘no risk’, or choosing the less likely option:
Yeah, they [the professionals] did [talk about risks] but I didn’t really want to think about it too much. I didn’t want to like, . . . ‘cos it would worry me too much and they did say that there’s a chance but it’s like 99.9% or something like that so it didn’t really bother me too much, so that’s why I probably didn’t think about it too much as well. They . . . there is . . . I always had it in my head that there is that 1% but thank god that it’s not.
Adolescent P1
But they said don’t panic because there’s a risk in everything, even if you cross the road there’s a risk in you dying and they gave all the statistics and said sometimes you can have internal bleeding or an infection and they did tell me . . . and I won’t lie, I did get a bit worried because you don’t think it but I asked, ‘how many times does it happen to people?’. They’d tell me ‘we’re not going to tell you false information; we’re going to tell you the facts’. I wanted to know but I didn’t want to know, in a way.
Adolescent P5
Yeah, because the sleeve, there is risk during the operation . . . so it’s right there. So it’s not continuous risk like the band. So I see it as with the band more things could go wrong but with the sleeve, obviously it’s still touch and go, but it takes more effect on you.
Adolescent P9
These excerpts from participants’ accounts point to a more general tendency to refer to risk through probabilistic expressions, while simultaneously looking past these risks in various ways. This means of coping was seemingly related to the limited choice participants experienced to do otherwise. Their experienced need to have the operation, alongside the need to cope and to minimise worry, rendered this manner of dealing with risk practically rational,255 even though it might not fit standard perceptions of procedural rationality in assessing risk through probability.
Trust in professionals
Thus far, various ways in which problems of uncertainty were negotiated by participants, through focusing on hopes, interpretation of probabilities and so on, have been considered. One further fundamental manner of coping with uncertainty was to trust in the competence and care of health-care professionals:
At first I was having thoughts ‘am I not going to do it? Should I do it?’ and then I made the decision that I wanted to do it, so I done the operation that the surgeon recommended.
Adolescent P1
Anything that would come into my head, I would just ask him and he’d tell me straight away. He was reassuring that nothing bad would happen.
Adolescent P1
My surgeon, he was the one who wrote the information pack so I was happy that I was following something that was properly done, not just made up.
Adolescent P1
You kind of really trust the professors because they know what they’re talking about.
Adolescent P4
‘Are you scared?’ and I was like ‘no, I’m feeling OK about it’ because they did explain everything to me. I didn’t know anything so I knew whatever was right, the doctors would know better than me.
Adolescent P5
In these various excerpts, deference to the expertise of the doctors as an important way of dealing with uncertainty and developing positive expectations is seen.
Influence of family experiences
Family views or experiences of surgery also aided decision-making:
My dad and my mum and my older brother, they used to come with me to the surgery appointments. They knew about what kind of procedure and all of that. They were willing for me to have it as well, they said ‘it’s entirely up to you whether you have it done, we’re not going to force you’ and all of that, so they were happy with it as well and then there wasn’t any like . . . none of them were saying like ‘no we don’t want you to do it’, they wanted me to go and do it.
Adolescent P1
My auntie got it done a few years back when I was younger but I’ve always known about it from TV and that, yeah.
Adolescent P6
I think that the fact mum had an operation was a big part of it.
Adolescent P2
In various senses, these indirect experiences of the intervention through social circles rendered the surgical option more familiar and less alien, thus reducing the uncertainty in these health-care contexts.
Social media
One further means of developing meaning and familiarity in regard to these interventions was through televisual and social media. These enabled an awareness and attribution of certain meanings to the intervention and postoperative outcomes:
Basically, I was watching people and how surgery changed them and I was researching on, like, the period of time and how long it will take to heal and what kind of procedures are involved after it, and I’ve seen the video on how they actually perform the surgery as well.
Adolescent P1
I went on YouTube [YouTube, LLC, San Bruno, CA, USA] and I typed it in and I saw the guy just cutting up loads of things . . .
Adolescent P1
Yeah there’s loads on YouTube about people who’ve had operations and everything, that was interesting and that just got me more excited.
Adolescent P2
. . . I know there was something on the television, Channel 5, but that was a couple of months before I had it. So then, when I saw it, I was really happy because it was on television.
Adolescent P3
Reducing complexity through limiting options
Despite all the mechanisms for dealing with uncertainty listed previously, uncertainty continued to linger in the experiences and perspectives of many of the participants. One further, but rather distinct, manner of dealing with uncertainty was to emphasise the lack of alternative options. Thus, reducing complexity regarding choices, even of uncertainty itself, could not be fully overcome.
Surgery as the only option
Surgery was depicted as a last resort for many and the degree of agency that the young people felt determined whether or not surgery was the right option for them:
Erm . . . Because I’ve tried it all before, basically. I’ve been trying to lose weight forever, so I thought weight loss surgery would be more helpful than these diets and things that are going.
Adolescent P6
It’s not the extremist, but it is kind of the last resort in a way. You’re obviously only going to go to surgery if you’re like ‘what else can I do?’.
Adolescent P5
Obviously, being bullied made me want to lose weight even more, so yeah, being bullied. Knowing I couldn’t do it on my own, I knew that I’m lazy! I am so lazy! And I knew I couldn’t do it on my own, no matter how hard I tried, I know that I would not have been able to do it on my own.
Adolescent P2
I just think I could do it on my own. With enough help from family and friends, and confidence and strictness on myself, I think I could just do it on my own . . .
Adolescent P7
However, feelings of ‘last resort’ raised further dilemmas for some of the participants:
And then I suppose the risk that it just could not work. I don’t know . . . it does sort of feel like it is the last resort . . . It’s sort of worrying. If it doesn’t work, what do I do next? I don’t really know what to do if this doesn’t work.
Adolescent P8
Choose the best option for permanent change
The idea of surgery as a last resort, in the absence of confidence and agency around one’s own ability to lose weight, led to much discussion about which procedures were most appropriate. Some of the participants debated which was the most ‘proper’ surgical solution to change habits ‘once and for all’. In most cases, the gastric band was not seen as the most appropriate procedure because this was seen as the intervention that was the least permanent solution and the one that was least likely to change the ‘mindset’:
I preferred the bypass because the band will just mess you up in the way you think about food. I wanted the bypass more because I wanted to know more about portion sizes and about how much I can eat. It’s like a lesson to you sort of thing.
Adolescent P4
It’s the more permanent one out of them, and I just think that would suit me more because, I think if I had the band, which you can adjust, I might just get into the mind ‘well, if I stretch it, it’s alright because I can just go back and get it adjusted’.
Adolescent P8
Because that was ‘the one’! That’s the one that is going to make you . . . It was between the sleeve or the bypass but I thought if I’m going to have this operation I may as well have the one that’s actually going to work. Because I was like chocolate, crisps and fizzy drinks, once that’s in my mouth, it can go straight through the band, so it’s not like I was just eating normal food but lots of it, I was eating crisps, chocolate, fizzy drinks. They were my main things. So it was between the sleeve and the bypass and I was just like, yeah, if I’m gonna have an operation I want the best one so I know I’m actually going to lose weight.
Adolescent P2
I think, obviously I know people’s biggest issue with it being reversed is why would you want it reversed when you could go back to your original self? I think once you’ve been in this state once, you’re not going to go do it again.
Adolescent P7
However, the gastric bypass was not favoured by some because of the need for lifelong postoperative supplementation and in terms of what it might mean for the long-term future:
. . . I’d really hate it. If I’ve got to take medication for the rest of my life, I’ll be . . . not tense but a bit . . . not fully free.
Adolescent P5
If you think about it, for the rest of my life I have to have these tablets; what if I miss it? And they said if I miss it, then it will make my health really serious – not if I missed a day but if I stopped . . . obviously when you’re 30 or something maybe you’re not going to remember ‘oh I’ve still go to have these tablets’ when you had the surgery, like, 20 years ago.
Adolescent P5
‘Work at it!’
Despite bariatric surgery being the last resort, and one that had the potential to ‘force’ behaviour change, a number discussed the need to work with it:
Everyone just thinks ‘oh yeah, I’ll just shed a few pounds’, but it takes a lot more than that, you’ve got to change your whole mind, the way you see things and the way you do things . . .
Adolescent P4
I knew if I had it, I would lose weight substantially, but obviously it’s on me as well to work hard at it. It don’t just come to you, obviously.
Adolescent P6
When I was thinking, like, how much of a challenge it would be and that it isn’t a cure, it’s just a way forward, then it was like, well, I don’t exactly want a cure, I just want to be healthy. I will work at it because if I know that I’ve got to change, then I’ve got it in my head that I will try my best.
Adolescent P9
These last responses, as with those of other participants, reflect the earlier assertion that participants did not perceive surgery as an easy option. Indeed, they regularly emphasised the necessity of change in their eating and lifestyle behaviours. However, although surgery was seen as a difficult option, by also framing this as the only option, the dilemmas referred to above were, in various ways, overcome.
Discussion
There have been very few qualitative studies of adolescents considering bariatric surgery. This research demonstrates that young people have a range of motivations for choosing surgery, including wanting to be normal, confident and healthy, as well as not wanting to die young. Surgery is seen as the last option when people have exhausted all other options; therefore, choosing the right method and ‘working at it’ is viewed as vital. Despite the apparent advantages of surgery, the young people interviewed had several reservations and faced multiple uncertainties. The dilemmas were partly personal in nature, involving the relative importance of weight loss versus having excess skin, the mortality risks and also dilemmas around personal maturity. These and other dilemmas were also social in nature, as some young people worried about the impact of changes in eating behaviour on their relationship with family, the views of others about bariatric surgery and the perception of it as a form of cheating. These findings cast doubt on the assertion that adolescents are perhaps not sufficiently future orientated enough to decide about such an intervention. 247 Adolescents, however, do appear to actively bracket away these concerns, by focusing on the positives (hope), referring to and (re)interpreting probabilistic expressions of risk, trusting professionals and developing knowledge and familiarity through the influence of family and social media.
Puhl and Heuer262 have clearly documented negative attitudes towards obesity and the pervasive nature of weight bias in Western society. The apparent perception of obesity as a ‘lifestyle choice’ is prevalent, irrespective of growing evidence of strong genetic influences on adiposity despite the obesogenic environment. 263 It has also been hypothesised that weight stigma is stressful and that this ultimately begets weight gain through a complex relationship between physiological responses (e.g. increased cortisol production) and behavioural responses, such as increased eating behaviour. 264 Rees et al. 265 conducted a review of 28 qualitative studies that reported on the perspectives of UK children aged 4–11 years on obesity or body size, shape or weight. The authors demonstrated that, instead of a focus on health, children emphasised the social impact of body size, describing experiences and awareness of abuse and isolation for children with a greater weight. A second review266 among those aged 12–18 years pointed to a similar trend. Regardless of their own size, young people were judgemental of individuals who were overweight, and those who had experience of obesity described an environment that contained multiple barriers to weight loss. The authors concluded that the studies revealed a ‘picture of a stigmatising and abusive social world’. 266 Interestingly, this is more implicit than explicit in the accounts presented in this report. In this study, young people alluded to such attitudes in their accounts of their desire for surgery to help ‘normalise’, that is, in the words of one participant, surgery was seen as a way of ‘feeling welcome . . . like not an outcast’. It is, perhaps, in this context that the need or desire to ‘bracket’ away uncertainty about surgery should be viewed.
In terms of the strategies used, the young people do not appear to follow procedurally rational approaches to manage uncertainty. They do reference probabilistic information, but, although this is decidedly useful in planning and predicting across large numbers of cases, the impossibility of knowing whether any one person is the one or the 99 leaves residual uncertainty that still has to be overcome. 267 This remaining uncertainty, along with anxiety about the unknowable future, still needs to be bridged over or ‘bracketed away’. 268 The young people, therefore, make use of other methods, such as trusting in professionals, media and family, in order to cope with both aspects of the dilemma. Alongside uncertainty pertaining to the future, the complexity pertaining to various different outcomes, and the need to decide between these, may be further neutralised by viewing surgery as the last and only resort. In the context of these young people having tried many other options that have failed to bring about lasting and sustained weight loss, perceiving a particular format of surgical intervention as the only option provides a very useful way forward in reaching a decision while minimising anxiety. 255
There are a number of implications of this study. In order to consent for treatment in their own right, the treating clinician has to ascertain that the adolescent is competent; is able to understand and retain the information pertinent to the decision, the purpose and possible consequences of treatment and the consequences of not having treatment; and is able to use this information to consider whether or not they should consent to the intervention offered. This study demonstrates that the process of ‘informed consent’ around weight loss surgery is a complex one. In the eyes of the young people interviewed, it makes sense to have surgery, lose weight and be ‘normal’ because all other non-surgical options have failed. Individual change rather than social change appears obvious and the effects of weight bias and ubiquitous discrimination are not interrogated. This is despite growing evidence of strong genetic influences on adiposity, despite the obesogenic environment. 263 There are multiple consequences of not having weight loss surgery, not just in terms of health, but also in terms of having to face the difficulties of living in a social world that is less than tolerant of obesity. The stakes are high and the option not to have surgery is invisible. It is argued that clinicians working with young people deciding on weight loss surgery need to make these processes visible in supporting them to make their decisions.
Substudy 6.4: systematic review of psychological and social outcomes of adolescents undergoing bariatric surgery, and predictors of success
This work was reproduced and published as White B, Doyle J, Colville S, Nicholls D, Viner RM, Christie D. Systematic review of psychological and social outcomes of adolescents undergoing bariatric surgery, and predictors of success. Clin Obes 2015;5:312–24. https://doi.org/10.1111/cob.12119. 269
Licence to reproduce number (John Wiley & Sons): 4675960442600.
Abstract
The psychological and social outcomes of bariatric surgery in adolescents, together with psychological and social predictors of success, were systematically reviewed. PubMed, EMBASE, Web of Science and PsycINFO were searched to July 2014. Existing data were sparse; 15 studies were suitable for qualitative review and six were suitable for meta-analysis (four quality-of-life studies and two depression studies). One study was a randomised control trial. A total of 139 subjects underwent RYGB, 202 underwent adjustable gastric band and 64 underwent SG. Overall quality of life improved after bariatric surgery, regardless of surgical type, with peak improvement at 6–12 months. Meta-analysis of four studies showed changed in overall QoL at latest follow-up of 2.80 SDs (95% CI 1.23 to 4.37 SDs). Depression improved across all studies, regardless of procedure [effect size –0.47 SDs (95% CI –0.76 to –0.18 SDs) at 4–6 months]. Two cohorts reported changes in both overall QoL and depression following a quadratic trajectory, with overall improvement over 2 years and deterioration in the second postoperative year. There were limited data on other psychological and social outcomes. There were insufficient data on psychosocial predictors of outcome to form evidence-based recommendations for patient selection for bariatric surgery at this time.
Introduction
Bariatric surgery in adults is the most effective treatment option for long-term weight loss, and has been shown to also be an effective treatment of medical comorbidities with resulting improvement in psychological and social functioning,220,270–273 Increasing numbers of obese adults are turning to bariatric surgery, with smaller numbers of adolescents following suit. 274,275 In the UK, NICE advises that bariatric surgery in adolescents is a potential treatment for young people where BMI is > 40 kg/m2, or > 35kg/m2 together with other significant disease that could be improved by weight loss, and where multidisciplinary lifestyle interventions lasting at least 6 months have failed. 11
Evidence supporting bariatric surgery in adolescents is limited. Adolescence is a complex period of biological, psychological and social development276 and outcomes of bariatric surgery in adolescents are potentially different from those in adults. Bariatric surgery for adolescents is potentially fraught with medical and ethical dilemmas. 277 Optimal timing of surgery is hotly debated, with some arguing that surgery should be postponed until adulthood, and others suggesting that delay results in poorer outcomes and increased health risks. 224,278 Systematic reviews have identified significant benefits for BMI loss in adolescence but have not thoroughly addressed psychological, social or QoL outcomes after surgery. 224,279 To date, only two systematic reviews have evaluated the psychosocial outcomes of surgery; one was limited to outcomes of gastric banding, included only six studies with psychosocial data and did not include the other more common weight loss procedures currently performed in adolescents,279 and the other only evaluated QoL. 224
Obese adolescents seeking bariatric surgery report impaired QoL and significant levels of psychological morbidity,226,280 and these problems are regarded as part of the rationale for surgery. However, we know little about the benefits of surgery for psychological function and QoL in adolescence and existing evidence has not been systematically reviewed across all surgery types. Furthermore, it has been suggested that certain psychological problems may be contraindications for bariatric surgery, although this is controversial. Again, existing evidence on psychological predictors of outcomes of bariatric surgery in adolescents, whether weight-related or psychological and social outcomes, has not been systematically examined. Knowledge of both the psychological and social outcomes of surgery and of psychological predictors of outcome is necessary to improve the effectiveness of bariatric surgery by allowing clinicians to offer surgery to those for whom the intervention is likely to be most effective, and protect those unlikely to benefit.
We undertook a systematic review of both of these issues, reviewing existing literature on children and adolescents up to the age of 21 years who have undergone bariatric surgery to answer the questions:
-
What psychological and QoL factors are associated with outcomes of bariatric surgery?
-
What are the psychological and QoL outcomes of surgery in adolescence?
Methods
We report methods in accordance with the PRISMA statement. 281
Eligibility criteria
Studies were eligible if they fulfilled the following criteria:
-
Subjects were children, adolescents or young adults aged up to 21 years undergoing bariatric surgery of any type.
-
The study reported either:
-
psychological outcomes of bariatric surgery, including QoL
-
psychological or social predictors of outcomes of surgery. Outcomes were defined in the broadest sense, and included changes in weight and medical comorbidities as well as psychological outcomes.
-
-
The study used either:
-
validated questionnaires before and after surgery, or questionnaires that compared postoperative with preoperative state or qualitative studies
-
a validated diagnostic system to detect prevalence of mental health disorders.
-
Study type
All study types were included with the exception of non-sequential case series and studies with fewer than 10 cases, with the aim of minimising selection bias.
Publication status
We included only studies published in peer-reviewed journals. Conference abstracts and letters to editors were excluded.
Search strategy
Sources
Searches were initially undertaken on 6 February 2013 and repeated on 28 July 2014. Electronic searches were conducted in PubMed, EMBASE, Web of Science and PsycINFO using the PubMed search terms presented in the list in Synthesis of results (mapped and exploded terms used). Similar terms were used in other databases. No date limits were set during the search. The reference lists of all included studies and reviews were searched for additional studies. Only papers written in English were retrieved. Results of the searches are shown in Table 33. The same procedure was repeated in July 2014, covering the publications released between February 2013 and 28 July 2014.
| First author and year | Cohort number | Cohort location | Cohort notes | Type of study/data analysis | Bariatric procedure | % female | Number at start (number completing questionnaire) | Number at latest follow-up (number completing questionnaire) | Baseline mean (SD) age (years) | Baseline age range (years) | Baseline mean (SD) BMI (kg/m2) | QoL questionnaire | Depression questionnaire | Other questionnaire | Outcome analysis at uniform time points | Uniform time points (months) | Other time points (months) | Eligible for meta-analsyis? | Psychosocial assessment reporting issues |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Brien et al., 2010282 | 1 | Melbourne Children’s Hospital, Australia | RCT: AGB vs. lifestyle | RCT | AGB | 68 | AGB: 25 (25) Control: 25 ( 25) | AGB: 24 (24) Control: 18 (18) | AGB: 16.5 (1.4) Control: 16.6 (1.2) | 14.0–18.0 |
AGB: 42.3 (6.1) Control: 40.4 (3.1) |
CHQ CF-50 | Nil | Nil | Yes | 0, 24 | No | No – no summative QOL scores | No CF-50 summative score results published |
| Silberhumer et al., 2006283 | 2 | Salzburg and Vienna, Austria | NR | AGB | 62 | 50 (NR) | 50 (50) | 17.1 (2.2) | 9.0–19.0 | 45.2 (7.6) | M–A | Nil | Nil | No | N/A | Mean 34.7; SD 17.5; range 3.6–85 | Yes | ||
| Silberhumer et al., 2011284 | 2 | Salzburg and Vienna, Austria | NR | AGB | NR | 50 (NR) | 45 (NR) | 17.1 (2.2) | 9.0–19.0 | 45.2 (7.6) | M–A | Nil | NIl | No | N/A | Two waves:
|
No – inadequate baseline data | ||
| Järvholm et al., 2012285 | 3 | AMOS (multicentre), Sweden | AMOS subgroup only (defined time period) | Prospective | RYGB | 68 | 37 (37) | 37 (37) | 16.6 (1.3) | 14.5–18.6 | 46.5 (5.9) | Nil | BDI, BYI-D | BYI | Yes | 0, 4 | No | Yes – depression | |
| Olbers et al., 2012227 | 3 | AMOS (multicentre), Sweden | Full AMOS cohort | Prospective with matched controls | RYGB | 65 | 81 (NR) | 81 (NR) | 16.5 (1.2) | 13–18 | 45.5 (6) | SF-36 | Nil | Nil | Yes | 0, 12, 24 | No | No – no summative data not quantified | |
| Loux et al., 2008286 | 4 | Birmingham Children’s Hospital, AL, USA | NR | RYGB | NR | 16 (13) | 12 (9) | 18.6 (1.7) | 14.0–20.0 | 54.1 (7.6) |
SF-36 IWQoL |
Nil | Nil | No | N/A | Mean 17; SD 12; range 1–39 | Yes | Incomplete data set | |
| Ratcliffe et al., 2012287 | 5 | Cincinnati Children’s Hospital, OH, USA | Consecutive subgroup within limited time frame | Prospective | RYGB | 69 | 16 (16) | NR | 16.3 (1.2) | NR | 66.2 (12.0) | IWQoL | Nil | SFRS, SPPA | Yes | 0, 6, 12 | No | No – no QoL summative score | Full data collected for 14/16 |
| Zeller et al., 2009288 | 5 | Cincinnati Children’s Hospital, OH, USA | Full cohort |
Prospective Linear mixed models |
RYGB | 63 | 31 (31) | 29 (28–29) | 16.4 (1.4) | 13.7–18.4 | 63.5 (10.6) | PedsQL IWQoL | BDI | Nil | Yes | 0, 6, 12 | No | No – Zeller 2011 has longer follow-up | |
| Zeller et al., 2011289 | 5 | Cincinnati Children’s Hospital, OH, USA | First 16 subjects with data at 18 and 24 months |
Prospective Hierarchial Linear Modelling |
RYGB | 65 | 16 (16) | 14 (14) | 16.2 (1.4) | N/A | 59.9 (8.7) | PedsQL IWQoL | BDI | SPPA | Yes | 0, 6, 12, 18, 24 | No | Yes – QoL + Depression | |
| Sysko et al., 2012290 | 6 | Columbia, NY, USA |
Prospective Latent curve analysis |
AGB | 72 | 101 (NR) | NR (NR) | 15.8 (1.1) | 14–18 | 47.2 (0.89)a | PedsQL | BDI |
EDE-Q FES |
Yes | 0, 1, 3, 6, 9, 12, 15 | No | No – modelled outcomes published only | . | |
| Collins et al., 2007291 | 7 | Pittsburgh Hospital, PA, USA | Retrospective | RYGB | 64 | 11 (N/A) | 9 (NR) | 16.5 (0.2) | 15.0–18.0 | 50.5 (2.0) | M–A | Nil | Nil | No | N/A | Mean 11.5; SD 2.8; range 3–32 | No – no summative score | ||
| Holterman et al., 2007292 | 8 | University of Illinois, Chicago, IL, USA | First 10 with 9-month data | Prospective | AGB | 100 | 10 (10) | 10 (10) | 16.0 (0.8) | 15.0–17.0 | 50.0 (13.0) | PedsQL | BDI | Nil | Yes | 0, 3, 6, 9 | No | No – only results of those with impaired function published | Not clear if sequential patients |
| Holterman et al., 2010293 | 8 | University of Illinois, Chicago, IL, USA | Full cohort, except for n = 1 who developed a comorditity, and n = 5 who did not attend follow-up | Prospective | AGB | 75 | 26 (20) | 12 (12) | 16.0 (1) | 14.0–17.0 | 50.0 (10.0) | PedsQL | Nil | NIl | Yes | 0, 6, 12, 18 | No | No – inadequate data | Limited results
|
| Aldaqal et al., 2013294 | 9 | Jeddah, Saudi Arabia | 32 young people, with intrafamilial controls (not weight matched) | Prospective | SG | 68 | SG: 32 (32) Control: 32 | 32 (32) | 15.2 (1.2) | 13–17 | 49.6 (4.9) | PedsQL | No | RSE | Yes | 0, 12 | No | Yes – QoL | Excluded those with any past or family history of psychiatric disorder |
| Raziel et al., 2014295 | 10 | Tel Aviv, Israel | Prospective | SG | 63 | 32 (N/A) | 31 (22) | 16.75 | 14–18 | 43.23 | M–A | No | No | No | N/A | Range: 1–2 years | No – no summative scores |
Study selection
The electronic search was performed by Billy White and Silvia Costa. The initial exclusion by title and abstract was performed independently by Billy White and Silvia Costa and any disagreements were discussed. Full-text review was carried out if reviewers could not agree based on information in the title and abstract. Papers selected for full-text review were reviewed by both Billy White and either Silvia Costa, Deborah Christie or Russell M Viner. Provisionally eligible papers were then independently reviewed by two authors (BW and RMV) and eligibility was agreed. The reviewers were not blinded to study authors, affiliations or journal name. 296
Data collection process
Data were extracted from eligible studies independently by two authors (BW and SC), with differences harmonised. Where inadequate data were available, authors were contacted by e-mail and any further data were included in this review where appropriate.
Summary measures
The majority of studies were uncontrolled pre–post studies and used a variety of validated questionnaires to measure QoL and mental health. To allow synthesis of results across studies, we followed guidance from the Cochrane Collaboration and calculated Cohen’s d standardised mean difference for each questionnaire outcome, defined as difference in mean pre test to post test divided by the pre-test standard deviation. 297,298 We calculated estimates of the standard error (SE) of the SMD using published formulas299 as a result of insufficient detail in included studies. Given that correlations between pre and post scores were not provided by any included study, we followed guidance from the Cochrane Collaboration in assuming a correlation of 0.5. 300,301 Where questionnaires gave two separate summative scores [e.g. Short Form questionnaire-36 (SF-36) physical component and mental component scores], the more conservative outcome was used in the meta-analysis. We have defined a large effect as ≥ 0.8 (four-fifths of a SD), a medium effect as 0.5 (half a SD) and a small effect as 0.2 (one-fifth of a SD). 302
Synthesis of results
Meta-analyses were conducted using the metan commands in Stata, version 12,303 specifying a DerSimonian and Laird random-effects model. 304 Heterogeneity between studies was measured using the I2 measure in addition to Cochran’s Q-test, as the latter has been shown to be a poor test of heterogeneity, especially in meta-analyses with small numbers. 305
We present results in two sections, first for psychosocial outcomes and second for psychosocial predictors of postoperative outcomes. Psychosocial outcomes were divided in three sections, namely quality of life, depression and other psychological and social outcomes. Findings are first presented in each section descriptively and then data were assessed for suitability for meta-analysis.
These are the search terms for MEDLINE (via Ovid SP). Equivalent terms and MeSH terms used for other EMBASE and PsycINFO (via OVID SP) and Web of Science:
-
exp Obesity/
-
obes*.mp
-
exp overweight/
-
overweight.mp
-
exp weight loss/
-
weight loss.mp
-
overweight*.mp
-
exp Body Mass Index/
-
body mass index.mp
-
body mass.mp
-
bmi.mp
-
obese,mp
-
exp body fat distribution
-
exp Child/
-
child*.mp
-
exp Adolescent
-
adol*.mp
-
adolescen*.mp
-
pediatr*.mp
-
paediatr*.mp
-
exp Bariatrics/
-
exp Bariatric Surgery/
-
bariatric*.mp
-
lagb*.mp
-
gastric band.mp
-
lap band*.mp
-
lap-band*.mp
-
Gastric Bypass/
-
Gastric bypass*.mp
-
Roux-en-y*.mp
-
Exp Jejunoilieal Bypass
-
Jejuno-ileal bypass*.mp
-
Malabsorptive surgery.mp
-
Weight loss surgery.mp
-
Weight reductions surgery.mp
-
Exp Biliopancreatic Diversion/
-
Bilipancreatic bypass.mp
-
Exp Gastric Balloon/
-
Gastric balloon.mp
-
Exp Quality of Life/
-
Quality of life.mp
-
Exp Depression/
-
Depression.mp
-
Exp Mental Health
-
Mental health.mp
-
Psychosocial.mp
-
Exp Anxiety
-
Anxiety.mp
-
Exp Self Concept/
-
Self concept.mp
-
Exp Anger/
-
Anger.mp
-
Exp Eating Disorders/
-
Eating disorder.mp
-
Exp Bulimia Nervosa or exp Bulimia/
-
Bulimia.mp
-
Ednos.mp
-
Eating disorder not otherwise specified.mp
-
Exp Anorexia Nervosa
-
Anorexia nervosa.mp
-
Or/1-13
-
Or/14-20
-
Or/21-39
-
Or/40-60
-
And/ 61-64
This is a list of the search terms used in the search strategy for indexed reference libraries.
Results
A total of 1049 records were identified in the original search; 778 unique citations fulfilled the search criteria, and a further six records were identified from other sources, including papers identified from the references of full-text papers reviewed. A total of 674 records were excluded on abstract review. A total of 110 full-text papers were reviewed and 97 were excluded; 68 did not contain separate data for subjects aged < 21 years, five were case studies or case series with fewer than 10 cases, 13 did not include measures of psychosocial status, five were reviews and six did not use validated methodology for measuring psychosocial status. Thirteen papers were eligible for qualitative synthesis.
The second search using the same search criteria identified 231 records with 175 unique citations. A total of 161 were excluded on abstract review. Fourteen full-text papers were reviewed and 12 were excluded; four did not contain separate data for subjects aged < 21 years, three did not include measures of psychosocial status, two did not use validated methodology for measuring psychosocial status, two were not original studies and one reported data that that had previously been published and identified in the original search. Two additional papers were eligible for qualitative synthesis.
A total of 15 papers were included in qualitative synthesis and six were included in quantitative synthesis (meta-analysis). A PRISMA flow diagram is shown in Figure 27.
Study characteristics
A total of 15 papers from 10 different patient cohorts met the inclusion criteria, and are summarised in Table 33. Four cohorts published outcomes at multiple time points across separate publications. Five cohorts were based in the USA, and one each in Austria, Australia, Israel, Saudi Arabia and Sweden. Two studies used control groups as comparators; one was a RCT282 and the other used both matched adolescents undergoing non-surgical treatment and adults undergoing similar surgery. 227 Four cohorts published high-quality prospective longitudinal outcomes, using well-validated measures at uniform time points with clear reporting and high retention rates. 227,282,285,287–290 The remaining cohorts used poorly validated outcome measures,283,284,291,295 reported incomplete data292,293 or reported outcomes at non-uniform time points. 283,284,286,291,295 A variety of different measures were used, at different time points, making comparison and meta-analysis difficult. Sufficient data were available to perform meta-analysis for change in QoL (four studies) and change in depressive symptoms (two studies).
Outcomes of 405 patients undergoing bariatric surgery from 10 cohorts were evaluated (median 32, range 11–101). In terms of surgical type, 139 underwent RYGB in four cohorts, 202 underwent adjustable gastric band (AGB) in four cohorts, and 64 underwent sleeve gastrectomy (SG) in two cohorts. The ages of participants were 9–20 years. Baseline BMI appeared higher in studies of RYGB (RYGB mean 50.9 kg/m2, AGB 46.5 kg/m2, SG 46.4 kg/m2) with similar mean age (16.2 vs. 16.7 years, respectively). Change in mean BMI in studies of RYGB ranged from –9.3 kg/m2 at 4 months285 to –24.1 kg/m2 at 12 months of follow-up. 288 The mean BMI change in studies of AGB ranged from –8.0 kg/m2 at 9 months292 to –12.7 kg/m2 at 24 months. 282 The mean BMI change was only reported in one study evaluating SG, with a difference of –20.3 kg/m2 at 1 year. 294
Psychological and social outcomes of surgery
Quality of life
A total of 405 adolescents undergoing bariatric surgery and 138 adolescent controls (25 randomised, 103 matched) were included in studies that measured QoL outcomes using validated questionnaires. One study was a RCT. QoL was measured using five validated tools. Four cohorts used the PedsQL,235 three used the Moorehead–Ardelt Quality of Life Questionnaire,306,307 two used the SF-36,308 one used the Child Health Questionnaire – Child Form (CHQ CF-50)309 and two used the IWQoL-Kids. 96
In the only RCT, O’Brien et al. 282 compared AGB with intensive lifestyle intervention and reported QoL outcomes at 2 years using CHQ CF-50 subscores. CF-50 summative scores were neither reported nor available from the study authors and so were not included in the meta-analysis. Compared with the intensive lifestyle control arm, physical functioning and change in health scores were significantly improved 2 years after AGB surgery, but there were no significant between-group differences for self-esteem, mental health, family activities, family cohesion, general behaviour or general health scores. Subjects undergoing AGB surgery showed significant improvements (within-group differences) in QoL related to family activities (p = 0.006), general health (p = 0.003), physical functioning (p ≤ 0.001), self-esteem (p = 0.01) and change in health (p < 0.001) but not general behaviour, family cohesion or mental health.
Using reported data, we then calculated Cohen’s d standardised mean difference between baseline and 2-year data across individual QoL domains in those who underwent AGB surgery, allowing us to compare magnitude of change across different QoL domains. The AGB group showed statistically significant improvements 2 years after surgery across five domains (largest improvement shown first): change in health (2.38 SDs, 95% CI 1.61 to 3.14 SDs), physical functioning (1.18 SDs, 95% CI 0.67 to 1.69 SDs), general health (1.05 SDs, 95% CI 0.56 to 1.54 SDs), self-esteem (0.80 SDs, 95% CI 0.35 to 1.25 SDs) and family activities (0.66 SDs, 95% CI 0.22 to 1.09 SDs). No significant changes were seen for general behaviour, family cohesion or mental health (standardised mean difference CIs crossed zero).
Sysko et al. used latent curve modelling to examine changes in QoL over the first 15 months after AGB surgery for 101 adolescents aged 14 to 18 years. 290 PedsQL modelled mean total score improved from 77.22 (SE 3.33) pre surgery to 86.36 (SE 4.96) at 12 months, with a linear model best fitting the data. All PedsQL mean subscores improved over the duration of the study. Linear models provided the best fit for all QoL subscales with the exception of emotional functioning score, where a quadratic model provided a better fit, indicating a gradual deceleration in improvement over time. There was a significant variance in baseline emotional functioning subscores across the subjects, but improvement in PedsQL emotional function over time was independent of baseline scores.
Silberhumer et al. 283,284 reported outcomes for 50 adolescents after AGB surgery using the Moorehead–Ardelt scale. To date they have first published outcomes at a mean follow-up duration of 34.7 months, and subsequently outcomes at 3 and 5 years for the 45 adolescents remaining under surveillance. The mean scores improved from 0.8 (SD 0.3) at baseline to 2.11 (SD 0.8) at a mean follow-up duration of 34.7 months. No change was seen between 3- and 5-year scores [mean 2.13 (SD 0.8) and 2.11 (SD 0.8), respectively]. There are no published normative data on the Moorehead–Ardelt scale to allow further interpretation of these data. The changes in mean (SD) scores at a mean follow-up duration of 34.7 months are included in the meta-analysis, as baseline scores were not available for the cohort who reached 5-year follow-up.
Holterman et al. 292,310 reported outcomes for 26 adolescents who underwent AGB surgery. To date, they have published 9-month follow-up data for the first 10 adolescents292 and subsequently data for 20 adolescents who reached 12-month follow-up and 12 adolescents who reached 18-month follow-up. 310 At baseline 75% of adolescent-reported and 80% of parent-proxy PedsQL scores were impaired, compared with two-thirds of adolescent-reported and half of parent-proxy at latest follow-up, although numerator and denominator were not reported. Holterman et al. 292,310 reported mean (SD) baseline and follow-up scores of only the subgroup with impaired baseline scores but not the whole group and, as a result, these scores could not be included in the meta-analysis.
Zeller et al. 288,289 reported prospective PedsQL outcomes from a very obese population (mean BMI 63.5 kg/m2) undergoing RYGB. To date they have published 1-year follow-up data for 31 young people288 and, subsequently, data for the first 16 who have had 2-year assessments. 289 One-year data were analysed using linear mixed models for repeated measures data and 2-year data using hierarchical linear modelling.
Both linear and log-linear models were applied to 0-, 6- and 12-month data from the two generic QoL scores (PedsQL: Physical Health and Psychosocial Health) and three IWQoL subscores (IWQoL: Physical Comfort, Body Esteem, Social Life). 288 All HRQoL measures improved after surgery and, with the exception of IWQoL: Social life, log-linear models provided the best fit, indicating a reduction in rate of improvement over time. Change in social life was linear, and showed no deceleration over time. PedsQL physical health summative scores improved at a faster rate compared with psychosocial health [Slope (SE) 12.10 (1.11) vs. 7.62 (1.15)], and IWQoL physical comfort scores were faster than body esteem [20.58 (1.38) vs. 11.75 (1.41)]. Changes in PedsQL summative scores were deemed to be clinically meaningful over the first 6 months post surgery, but not the subsequent 6 months (defined as the smallest change in score that patients perceive to be beneficial); the authors suggest that these changes coincide with maximal weight loss in the first 6 months and subsequent deceleration in the next 6 months.
Both linear and quadratic models were applied to 0-, 6-, 12-, 18- and 24-month data from the two generic QoL scores (PedsQL: Physical Health and Psychosocial Health) and IWQoL subscores. 289 Quadratic models provided a better fit for both PedsQL subscores and three IWQoL subscores (IWQoL: Physical Comfort, Body Esteem, Social Life), indicating initial improvement in QoL, with subsequent deceleration in improvement and then decline over the 2-year period (physical health p ≤ 0.0001, psychosocial health p = 0.013, social life p = 0.002, body esteem p = 0.0007, physical comfort p ≤ 0.0001, total score p = 0.0001). The authors suggested that these changes again coincide with weight loss in the first year and subsequent minor weight regain in the second year. Clinically meaningful improvements were seen from baseline to 6 months for both PedsQL subscores and three IWQoL subscores (IWQoL: Physical Comfort, Body Esteem, Social Life), and for weight-related IWQoL scores only from 6 to 12 months. No clinically meaningful improvements were perceived between 12 and 18 months or between 18 and 24 months after surgery. Changes in QoL scores from baseline to 24 months are included in the meta-analysis.
Olbers et al. 227 reported prospective outcomes for 81 young people at 12 and 24 months post RYGB surgery, and compared them with matched adolescents undergoing non-surgical treatment and adults undergoing similar surgery. Only QoL outcomes of the adolescent surgery group have been reported to date. Compared with baseline, SF-36 physical component summary score and all four physical health domains were improved at 1 year and 2 years. Changes in mental health summary scores were not reported, suggesting that they did not reach statistical significance. Compared with baseline, three of four mental health subscales were improved at 1 year (vitality, social functioning, and mental health but not role–emotional), and two of four subscales were improved at 2 years (vitality and social functioning, but not role–emotional and mental health). Minor decreases were detected between 12 and 24 months, but these changes did not reach statistical significance. Summative data were not quantified and, therefore, could not be included in the meta-analysis.
Loux et al. 286 reported QoL outcomes of 16 patients undergoing RYGB, with follow-up data collected at variable follow-up points. Four of the 13 patients who completed preoperative questionnaires were lost to follow-up, and follow-up data included those who did not have baseline assessments. The authors reported significant changes between preoperative and postoperative means for all QoL domains (p < 0.001). The review authors have undertaken post hoc analysis using Cohen’s d standardised mean differences to allow comparison of magnitudes of change. Analyses showed largest effect size changes in general health (2.68 SDs, 95% CI 1.47 to 3.89 SDs), vitality (2.01 SDs, 95% CI 1.03 to 2.99 SDs), role–physical (1.65 SDs, 95% CI 0.78 to 2.52 SDs) and physical functioning (1.65 SDs, 95% CI 0.78 to 2.52 SDs) and with least change in role limitations due to emotional health (0.99 SE, 95% CI 0.30 to 1.68 SE), social function (1.01 SDs, 95% CI 0.31 to 1.70 SDs), bodily pain (1.07 SDs, 95% CI 0.36 to 1.78 SDs) and mental health (1.11 SDs, 95% CI 0.39 to 1.83 SDs).
Collins et al. 291 reported outcomes of 9 out of 11 adolescents undergoing RYGB surgery at a mean follow-up duration of 11.5 ± 2.8 months. Only the results of three of the five Moorehead–Ardelt questions were reported; all nine reported physical function, social interactions and function at work as ‘improved’ or ‘greatly improved’ compared with before surgery but no further details were published.
Only two cohorts have reported outcomes for the adolescents undergoing SG. 294,295 Aldaqal et al. 294 reported 1-year outcomes for 32 adolescents. QoL improved across all PedsQL domains and changes in 1-year total score have been included in the meta-analysis.
Raziel et al. 295 reported 1- to 2-year outcomes for 22 of the 31 young people (71%) undergoing SG using four of the five Moorehead–Ardelt questions (question related to sexual activity was omitted). The mean scores for each question were general feeling 5.0 (SD 0.0), exercise satisfaction 2.6 (SD 1.3), social activity 4.8 (SD 0.4), work capability 4.8 (SD 0.3) and food perception 3.8 (SD 1.1) using a five-point Likert scale (5 = highest score).
Data on patient-reported mean change in overall QoL at latest follow-up were available for meta-analysis from four cohorts (total population 110 subjects). The mean duration of follow-up ranged from 12 to 34.7 months. The mean change in QoL scores was 2.52 SDs (95% CI 2.10 to 2.94 SDs) with an I2 of 92.6%, indicating a ‘large’ effect302 with high heterogeneity between studies. 305 The results are shown in the forest plot in Figure 28. We undertook further subgroup analysis, investigating the effect of including only studies that utilitised questionnaires with a single summative score, and found mean change in QoL scores of 3.40 SDs (95% CI 1.99 to 4.82 SDs) with an I2 of 86.0%.
FIGURE 28.
Forest plot showing pooled standardised mean difference in quality of life at latest follow-up. Source: White et al. 269
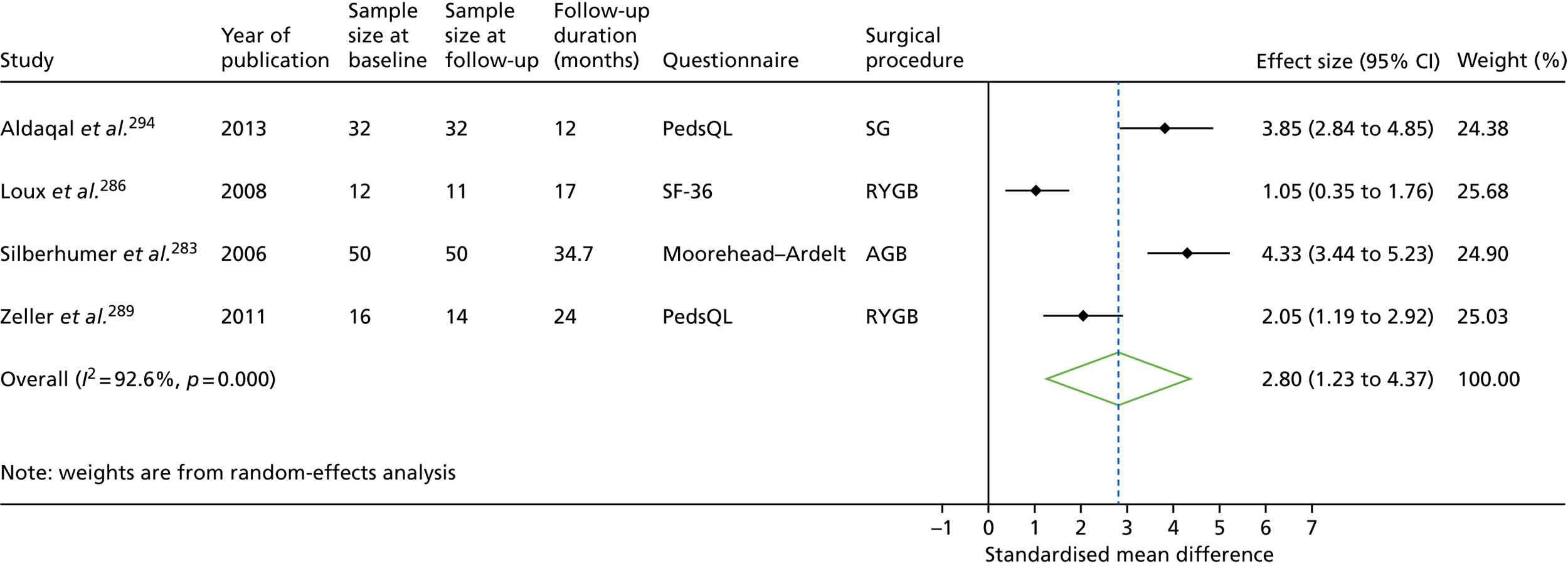
Depression
Data on change in depressive symptoms following bariatric surgery were available for 180 young people in five papers reporting outcomes of four prospective cohorts. 285,288,290,292,310 All five studies used the Beck Depression Inventory (BDI); one of these additionally used the Beck Youth Inventory™ (BYI) depression subscale. No data were available for confirmed diagnoses of depression.
Sysko et al. 290 used latent curve modelling to examine changes in depression scores in the first 15 months after AGB surgery. Linear and quadratic models were tested on the data. A quadratic model best fitted changes in BDI score, indicating that BDI scores improved after surgery (i.e. less depressive symptomatology), but with a deceleration in improvement over time, and subsequent decline from around 9 months post surgery. There was significant variance in baseline BDI score, but it was the change in BMI after surgery rather than baseline BDI that predicted the change in BDI after surgery. Only modelled BDI scores, and not measured scores, were reported and, as such, they could not be included in the meta-analysis.
Holterman et al. 292 have published depression data on the first 10 subjects (of 26) who completed 9 months of follow-up after AGB surgery, and further data have not been published in subsequent reports of this cohort. 293 Data were published for the 3 out of 10 adolescents who had BDI scores in the clinical range at baseline (suggestive of a diagnosis of depression); at 9 months, none of the three had depression scores in the clinical range and mean scores in this subgroup had fallen from 25.3 (SD 7.8) at baseline to 2.2 (SD 2.7) at 9 months. The before-and-after surgery BDI mean scores were available only for the three patients with abnormal scores, and, therefore, these scores could not be included in the meta-analysis.
Zeller et al. 288 reported firstly outcomes of 31 young people at 12 months after RYGB, and, subsequently, outcomes of the first 16 who reached 2-year follow-up. 289 At baseline, 12 out of 31 adolescents (38.7% of cohort, 45% of females, 27.3% of males) had BDI scores above a threshold from normative data indicating likely clinical depression. 311 At 12 months post RYGB, 2 out of 31 (6.5%, both females) had scores within the clinical depression range. BDI data were available for 16 patients at 2 years postoperatively; of these, 10 out of 16 (62.5%) had scores in the clinical range at baseline whereas only 2 out of 16 (12.5%) had levels in the clinical range at 2 years.
One-year data were analysed using linear mixed models,288 and 2-year data using hierarchical linear modelling289 as described above. Log-linear models provided the best fit for 1-year data, indicating a deceleration in improvement over time [intercept 15.11 (SE 2.14), slope –3.87 (0.78); p < 0.001]. Quadratic models rather than linear models best fitted data for the first 2 years post RYGB [t(1,15) = 3.38; p = 0.004], indicating an overall improvement over time, but with a deceleration in improvement by the end of the first year and a slight deterioration in the second year. Six-month outcomes have been included in the meta-analysis.
Järvholm et al. 285 reported 4-month outcomes in 37 patients following RYGB using the BYI depression subscale. The mean scores fell from a mean of 15.8 (SD 10.3) at baseline to a mean of 12.3 (SD 9.5) at 4 months post surgery (p = 0.02). At baseline, 27% of adolescents (10/37) had scores above a threshold at which everyday life is substantially affected (> 90th centile of normative data311); at 4 months, this figure was 11% (4/37), although significance of change was not reported. Four out of 37 adolescents (11%) had substantially more depressive symptoms on the BYI depression subscale at 4 months than at baseline (defined as falling outside the 95% CI of the baseline score), and were reported as impaired compared with baseline, whereas the rest (33/37, 89%) had unchanged or improved scores. Four-month outcomes have been included in the meta-analysis.
Data on adolescent-reported mean change in depressive symptoms were available for meta-analysis from two cohorts (total population 68 subjects). The latest follow-up period ranged from 4 to 24 months, but only 4- to 6-month data were meta-analysed to improve homogeneity of studies. The forest plot in Figure 29 shows that mean depressive symptoms improved by –0.47 SDs (95% CI –0.76 to –0.18 SDs; I2 = 23.7%), indicating a medium effect with low heterogeneity.
FIGURE 29.
Forest plot showing pooled standardised mean difference in depression scores at 4–6 months post surgery. Source: White et al. 269
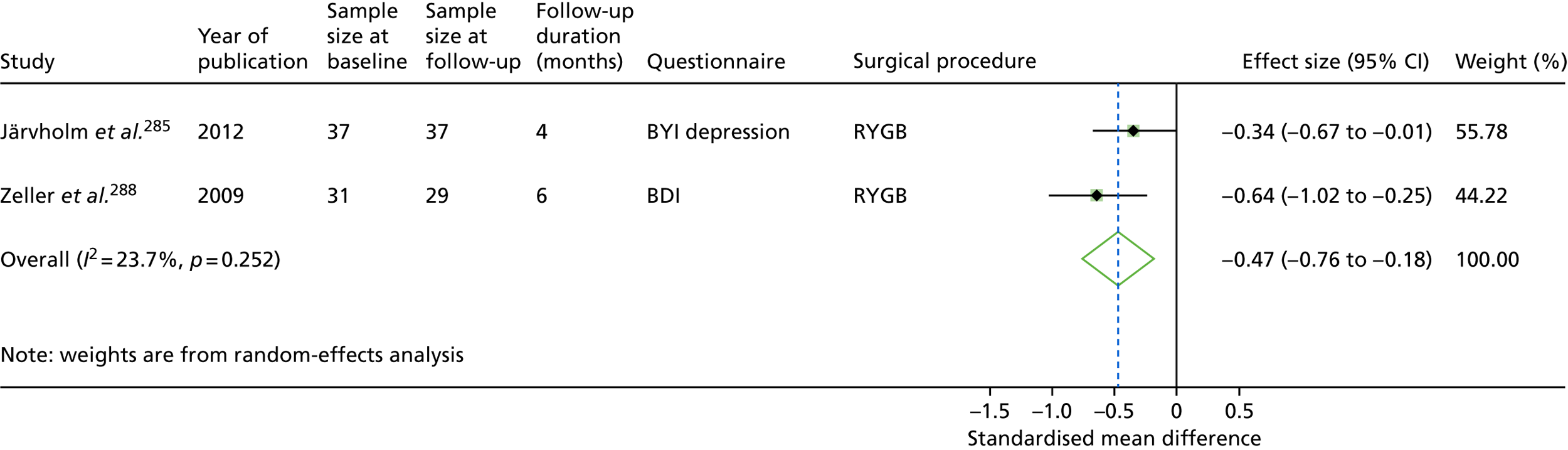
Other psychological and social outcomes
Outcomes for other psychological and social outcomes in adolescents are sparse, and largely derived from three studies: one that assessed a range of psychological outcomes using the BYI,285 one assessing body image dissatisfaction using the Stunkard Figure Rating Scale287 and another that assessed changes in self-concept using Harter’s Self-Perception Profile for adolescents. 312
Järvholm et al. 285 reported baseline and 4-month postoperative data for a subgroup of participants in the ongoing AMOS study. Individual BYI youth scores were compared with gender-specific national normative data, and scores > 90th centile (< 10th for self-concept) were interpreted as highly elevated and likely to substantially interfere with daily life. A 4-month score outside the 95% CI of the baseline score was interpreted as a clinically significant change. Eight out of 37 (21.6%) had a clinically significant BYI anxiety score at baseline; at 4 months, 4 out of 37 (10.8%) had a clinically significant score and 4 out of 37 (10.8%) had significantly increased anxiety scores. Eight out of 37 (21.6%) had a clinically significant BYI anger score at baseline; at 4 months 5 out of 37 (13.5%) had significantly increased anger scores (incidence of raised scores at 4 months not reported). Five out of 37 (13.5%) had a clinically significant BYI disruptive behaviour score at baseline, with 7 out of 37 (18.9%) having significantly increased disruptive behaviour scores (incidence of raised scores at 4 months not reported). Seven out of 37 (18.9%) had impaired self-concept scores at baseline; at 4 months 3 out of 37 (8.1%) had impaired scores, with 6 out of 37 (16.2%) reporting significantly reduced (i.e. worse) self-concept scores. Improvements in mean domain scores between before and after bariatric surgery were significant for BYI anxiety (p = 0.004, Student’s t-test), BYI depression (0.02) and BYI self-concept (p = 0.004).
Zeller et al. 289 analysed changes in self-concept subscores over the 2-year period following RYGB using hierarchical linear modelling as previously described. Quadratic models best fitted social acceptance [t(1,69) = –2.42; p = 0.02], physical appearance [t(1,69) = –3.19; p = 0.02] and close friendship scores [t(1,30) = –2.76; p = 0.008]. Substantial improvement was seen over time across these domains, but with deceleration towards the end of the first year, and deterioration in the second postoperative year. Linear models best fitted global self-esteem [t(1,69) = 2.98; p = 0.004], athletic competence [t(1,69) = 2.37; p = 0.02], job competence [t(1,69) = 2.24; p = 0.03] and romantic appeal scores [t(1,69) = 2.98; p = 0.004), indicating continued improvement over 24 months postoperatively, with no evidence of deceleration. Changes in scholastic and behavioural conduct were not reported.
Ratcliff et al. 287 analysed changes in body image dissatisfaction (BID) in the first year following RYGB. BID, defined as the difference between perceived and desired body shape, fell in the first 6 months post surgery but not in the subsequent 6 months. This coincides with the fact that weight loss was greatest in the first 6 months (mean loss 22.4 kg/m2) and much lower in the subsequent 6 months (4.7 kg/m2). However, the authors did not report the statistical significance of this association. Change in perceived body shape was correlated with change in BMI. Discrepancy between current and ideal body size, rather than current body size alone, was negatively correlated with weight-related QoL, indicating that the difference between the actual body weight and the ideal body weight most influences QoL, and those with the smallest discrepancy have the best weight-related QoL. No other correlations were detected between current body size or body image dissatisfaction and IWQoL, body esteem or physical appearance attitudes as measured by the SPPA.
Aldaqal et al. 294 analysed changes in self-esteem over the first year follow SG using the Rosenberg Self-Esteem Scale (RSE). The mean group scores significantly increased, from 16.01 (SD 2.7) preoperatively to 24.66 (SD 2.36) at the end of the first year, indicating improved self-esteem.
Psychological and social predictors of success after bariatric surgery
Body mass index outcomes
We identified only two studies that analysed associations between preoperative psychological, QoL or social measures and weight-related outcomes after surgery. Both cohorts have been described earlier. Sysko et al. examined baseline predictors of both initial and postoperative BMI trajectory over the first year post surgery including gender, age, race/ethnicity, median household income, distance from treatment centre, ‘clinically significant symptoms’ (self-injurious behaviours, loss of control eating, BDI and PedsQL scores), family factors (involvement with social services, Family Environment Scale scores), DSM-IV diagnoses, and current and past psychiatric treatment290). Only higher levels of loss of control eating (as measured by the EDE-Q) and family conflict (as measured by the conflict subscale of the Family Environment Scale) were significant predictors of rate of weight change over time, and both predicted a lower rate of change over time. Järvholm et al. 285 found no significant relationship between changes in BMI in the 4 months post RYGB and any baseline psychological parameter.
Psychological and social outcomes
Loux et al. 286 reported a correlation between preoperative QoL scores and change in QoL after surgery [Physical Component Score (PCS), r = –0.94, p < 0.001; Mental Component Score (MCS), r = –0.9, p < 0.005], indicating that those participants with increased impairment at baseline benefited the most from surgery. In a larger and more complete data set, Sysko et al. 290 found no correlation between baseline QoL or depression scores and change in QoL. Importantly, after controlling for baseline BMI and PedsQL score, improvement in PedsQL was significantly correlated with change in BMI after surgery. A similar pattern was seen for BDI scores.
Discussion
This is the first systematic review of psychological and social outcomes of all types of bariatric surgery in adolescents. We found that data on the psychosocial outcomes of adolescent bariatric surgery are limited, particularly for outcomes beyond 24 months. Relative to other outcomes such as BMI and cardiometabolic comorbidities, psychological and social outcomes have been poorly measured and reported. Multiple publications, including one reporting 1-year outcomes of 890 adolescents, were not eligible because they did not provide adequate measures of psychological function. 313 Only one study provided RCT-level evidence, and three ongoing cohorts reported high-quality prospective data.
We found moderate but consistent evidence that bariatric surgery in adolescents resulted in overall improvement in QoL and in depressive symptoms in the first 2 years.
Overall QoL improved from before to after bariatric surgery in all studies identified in this review, regardless of surgical operation performed. In meta-analysis, overall QoL improved by 2.8 SDs at latest follow-up, although only four studies were eligible for inclusion. Improvement in QoL was greater in the domains relating to physical function than in those relating to emotional/mental function. In the only RCT, adolescents who underwent AGB had superior self-reported physical function and overall health than control group adolescents who underwent intensive lifestyle intervention. Post hoc analysis of QoL domains across studies shows QoL improvement predominantly associated with the physical benefits of weight loss, notably general health and mobility.
Depressive symptomatology improved across all studies in the first year after bariatric surgery, and meta-analysis showed a 0.52-SD improvement in depression scores 4–6 months after surgery. Depressive symptoms appear to follow a similar quadratic pattern to QoL, with significant improvement in the first 6 postoperative months, substantial deceleration by 12 months and suggestions of moderate deterioration at 24 months. 289 Depression scores in the clinical range were seen in up to 38.7% of cohort subjects at baseline; only 2 out of 12 (16.7%) patients in this cohort had clinical range symptoms at last follow-up. 288
A consistent theme emerged that QoL and depressive symptoms improved most in the first 6–9 months, with little further improvement and even some deterioration. One study reported that changes in QoL scores were meaningful only in the first 6 months, but not in the subsequent 18 months. Given that weight loss after bariatric surgery is most dramatic in the first 6 months, and is generally complete by 12–24 months postoperatively, this suggests that QoL improvement may be linked with timing and degree of weight loss postoperatively. Little is understood about this association. Only one study formally examined this association and reported that change in QoL and depression scores post surgery was associated with change in BMI, rather than baseline score, and another suggests that desired weight, and the mismatch between actual and desired weight, is an important factor in changes in QoL.
Limited outcome data for other psychosocial measures were available. Four-month outcomes of the AMOS cohort show improvement in mean anxiety and self-concept scores, but not disruptive behaviour or anger. 285 Improvements were seen across all self-perception domains over 2 years in one study, with mean levels across all domains except for athletic competence lying within normative ranges at 2 years. As seen in QoL and depression scores, some domains showed a quadratic trend, notably social acceptance, appearance and close friendship. 289 No outcome data were found for eating disorders in adolescents.
We found only one study to report baseline psychosocial predictors of BMI or psychosocial outcomes of surgery. 290 Multiple factors were measured, and only loss of control eating and family conflict were predictors of BMI outcome; both predicted a reduced rate of change of BMI.
Current data regarding the psychological impact of surgery are sparse, with inconsistent and incomplete reporting. Compared with other outcomes, particularly BMI and metabolic parameters, psychosocial outcomes are both poorly measured and poorly reported. There are no data describing the impact of surgery on disordered eating behaviours and cognitions in this age group.
Adolescents presenting for bariatric surgery have severely impaired QoL with high rates of depression and disordered eating. 280 Adolescents have not usually accumulated the medical comorbidities seen in adults undergoing BAS and indications for surgery are likely to be predominantly related to psychosocial distress. This review shows that for the average subject undergoing bariatric surgery, QoL, depressive and anxiety symptoms improve after surgery, with peak improvement at 6 months after surgery. There was some evidence that psychological function deteriorated thereafter.
The outcomes of the only RCT are interesting and merit attention. 282 Although the group undergoing AGB lost 31.6 kg more than controls, QoL changes in many domains (notably general behaviour, family cohesion and mental health) were not significant either within or between groups. This is potentially due to the small sample size (only 25 in each group), and larger RCTs are needed to determine if surgery in adolescence results in superior QoL change compared with intensive lifestyle intervention.
The QoL meta-analysis showed high levels of heterogeneity between studies. This is not surprising for two reasons. First, the studies used different time points and the review has shown a clear temporal change in QoL over time. Second, different measures were used across studies with differing validity in this population. Despite their heterogeneity, they all show large magnitudes of change in QoL after surgery.
The domains with significant improvement after surgery are those closely related to the physical and physiological effects of substantial weight loss, namely change in physical health and physical functioning. These outcomes are seen across several studies and we hypothesise that improvement in QoL is most closely linked to the physical and physiological benefits of substantial and prolonged weight loss, currently achieved only by bariatric surgery. 224,314
Many questions remain unanswered, particularly the long-term outcomes, optimal time for undergoing surgery and psychosocial predictors. There are currently insufficient data to predict which patients will benefit most from surgery and, therefore, no evidence-based recommendations can be made following this review. Some questions will be answered by ongoing studies but additional research is needed into this important patient group.
Limitations
Only a small number of studies were eligible for inclusion in the meta-analysis, for two reasons: (1) lack of reporting of summative scores where summative score is available and (2) use of questionnaires that do not include a summative score. Generally, psychosocial outcomes were poorly reported across all studies, requiring use of accepted measures for calculating unreported study parameters (e.g. no study reported correlation between baseline and post-surgery individual scores). A wide range of questionnaires were used, with varying quality, making comparison between different studies and procedures difficult. We chose to use the SMD approach to allow comparison across these different instruments. High heterogeneity observed in our meta-analyses is likely to reflect differences in instruments used as well as populations. Furthermore, SMD is influenced by the baseline variance, which differs between studies. Our inclusion of follow-up data from different time points, made necessary by insufficiency of data and lack of consistency across studies, together with some studies reporting outcomes only at latest follow-up is likely to obscure the time trends described earlier. 226 Additionally, psychosocial outcomes were frequently poorly reported, and this may have resulted in misinterpretation of results.
Conclusions
High-quality psychosocial outcome data for adolescent bariatric surgery are lacking, with data restricted to 2 years post surgery. These data suggest that mean QoL and depressive symptomatology improve after bariatric surgery, although not in all patients. Improvements in psychological function appear to be associated with reduction in BMI rather than baseline psychological function. Improvement appears to peak at 6–12 months. There is weak evidence that psychological function predicts BMI outcomes after surgery; those with higher baseline levels of loss of control eating and family conflict appear to have poorer BMI outcomes. There are insufficient data to predict which young people are likely to benefit psychologically from surgery, and this is an important area for future study.
Substudy 6.5: cost-effectiveness of bariatric surgery in severely obese adolescents in the UK
Parts of this section (including tables and figures) are reproduced from Panca et al. 315
This work was reproduced and published as Panca M, Viner RM, White B, Pandya T, Melo H, Adamo M, et al. Cost-effectiveness of bariatric surgery in adolescents with severe obesity in the UK. Clin Obes 2018;8:105–13. https://doi.org/10.1111/cob.12232. 315
Licence to reproduce number (John Wiley and Sons): 4675960973678.
Abstract
Background
Severe obesity presents an increasing source of clinical and economic strain to health-care providers. The economic burden of obesity is substantial. In England, the costs of overweight and obesity to the health system have been calculated to be £4.2B per annum.
Aims
To develop a decision-analytic model that quantifies the lifetime health and economic consequences of bariatric surgery compared with no surgery in severely obese adolescents in the UK.
Methods
Eighteen severely obese adolescents (BMI of 40 kg/m2 and over) who underwent bariatric surgery between January 2008 and December 2013 were included. Bariatric surgery costs included operative costs and 24 months of postoperative care for two types of surgery, laparoscopic RYGB and laparoscopic SG. We used published sources to estimate utilities for obese people, utility decreases in BMI and utilities associated with obesity-related comorbidities.
We used a Markov cohort model to compare the lifetime expected costs and QALYs between those participants who underwent bariatric surgery and those participants who had no surgery. The starting age of the cohort was 18 years.
We then estimated the incremental costs per QALY of bariatric surgery compared with not having surgery from the UK NHS perspective.
Results
The mean total RYGB costs per male were £14,660 (95% CI £14,183 to £14,836) and per female were £14,102 (95% CI £13,925 to £14,279). The mean total SG costs per male were £14,815 (95% CI £14,637 to £14,994) and per female were £14,217 (95% £14,038 to £14.396).
For RYGB intervention, the difference in costs between bariatric surgery and no surgery was £11,245 (95% CI £10,777 to £11,413) in males and £11,343 (95% CI £11,172 to £11,514) in females per person per year. Surgery translated into an increase in QoL equivalent to 5.57 QALYs (95% CI 5.55 to 5.59 QALYs) gained over a lifetime in males and 5.66 QALYs (95% CI 5.64 to 5.67 QALYs) in females.
For SG, the difference in costs between bariatric surgery and no surgery was £10,877 (95% CI £10,707 to £11,046) in males and £10,954 (95% CI £10,782 to £11,125) in females per person per year. This translated in to a lifetime gain in QoL equivalent to 5.50 QALYs (95% CI 5.48 to 5.52 QALYs) in males and 5.64 QALYs (95% CI 5.63 to 5.65 QALYs) in females.
Conclusions
We found that bariatric surgery in severely obese adolescents is a cost-effective alternative to not having surgery over a lifetime from the UK NHS perspective. Bariatric surgery was more costly than no surgery; however, it markedly improved QoL.
Background
Severe obesity presents an increasing source of clinical and economic strain to health-care providers. The National Child Measurement Programme (NCMP) in England data show that, in 2012/13, severe obesity (BMI ≥ UK90 99.86th centile) equivalent to the adult bariatric threshold of 35 kg/m2 was found in 1.2% of girls and 1.5% of boys aged 10–11 years. 90
The economic burden of obesity is substantial. In England, the costs of overweight and obesity to the health system have been calculated to be £4.2B per annum. 316 Much of this burden is driven by a number of comorbidities, which occur at a higher prevalence among obese patients including T2DM, hyperlipidaemia, hypertension and sleep apnoea, as well as significant psychosocial consequences and the increased likelihood of becoming obese adults. 9,317,318 Thus, the clinical, psychological and economic consequences of obesity represent a significant challenge to health-care systems.
Conservative treatment of severe obesity largely produces poor long-term results, whereas surgery for obesity usually results in significant permanent weight loss. After obesity surgery, most patients experience improved health and psychological functioning,319 with bariatric surgery considered cost-effective in adults. 256
In contrast, data on bariatric surgery for adolescents are extremely limited. Systematic reviews suggest that surgery is highly effective for short-term BMI reduction224 and improves QoL. 269 However, there are very few published data on the cost-effectiveness of bariatric surgery in adolescence, and none from the UK. In the UK, NICE guidance12 notes that bariatric surgery is not generally recommended for CYP (recommendation 1.10.12) but may be considered in exceptional circumstances in those who have reached physiological maturity (recommendation 1.10.13) and have BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with significant comorbidities that would be improved if they lost weight (recommendation 1.10.1).
We assessed the lifetime cost-effectiveness of bariatric surgery (RYGB or SG) compared with no intervention, modelling data obtained from a cohort of 18 severely obese adolescents undergoing surgery in one NHS centre in the UK.
Methods
Framework of economic model
We conducted a cost–utility analysis using lifetime expected costs and QALYs that compared bariatric surgery with no surgery.
A Markov model was used to project lifetime costs, BMI and QALYs. The Markov transition model comprised five health states [‘no comorbidity’, ‘DM’, ‘coronary health disease (CHD)’, ‘stroke’ and ‘colon cancer’] and death. A diagrammatic presentation of the Markov model is presented in Figure 30.
Severely obese adolescents (BMI of 40 kg/m2 and over) entered the model in the ‘no comorbidity’ health state. The model consisted of yearly cycles. At the end of each 1-year period, a proportion of the cohort can move from one health state to another or stay in the same health state. We assume that adolescents remain in those health states they have entered until they die (age-specific death or obesity-related comorbidity death). The disease transition probabilities are based on disease progression with age, gender, BMI and cycle number. The model is run over 82 years to estimate the lifetime costs and effects of the intervention. Participants entered the model at the starting age of 18 years.
The higher an individual’s BMI, the more likely they are to develop related comorbidities, such as DM, CHD, stroke, sleep apnoea and some forms of cancers. Owing to lack of accurate data on other risk factors, the model focused on the increased risk of developing DM, CHD, stroke and colon cancer, for which there are good data. There is a complex relationship between the degree of obesity and the four comorbidities included in the model, as well as a lack of accurate data on correlations between comorbidities. However, for simplicity, the possibility of having combinations of comorbidities at the same time was not incorporated in the model. This assumption therefore potentially underestimates the burden associated with obesity-related comorbidities.
In the model, a hypothetical cohort of 1000 severely obese adolescents underwent bariatric surgery (RYGB or SG) or no surgery. Effects of surgery were modelled as a reduction in BMI and development of DM, CHD, stroke and colon cancer. Decline in BMI was taken from the cohort of 18 adolescents undergoing surgery at University College London Hospitals (UCLH) but included an annual increment (obesity drift) to update an adolescent’s BMI over time. Published literature shows that BMI is predicted to increase by 0.12 kg/m2 per year in patients aged < 45 years,320 0.07 kg/m2 per year for those aged between 45 and 65 years and –0.14 kg/m2 per year for those aged ≥ 65 years. 321 Our assumption in the no surgery group was for BMI to stay constant with no incremental drift; this potentially underestimates lifetime BMI in the no surgery group.
Parameter estimates and data sources
Parameter values for the model were taken from a variety of secondary sources.
The correlation between BMI and annual risk of developing DM, CHD, stroke and colon cancer was calculated based on estimates from the DYNAMO-HIA project. 322 The document provides estimates of the relative risks of defined diseases according to BMI status [given as per-unit increase from BMI 22 kg/m2 = 1.0; we then adjusted this estimate for the mean BMI of the adult UK population (BMI 27 kg/m2 = 1.0)]. The risk of comorbidities was adjusted according to age, gender and prevalence of DM, CHD, stroke and colon cancer based on information provided by a large representative population health survey, Health Survey for England – 2013,7 complemented by a published source for colon cancer. 323
Mortality due to comorbidities was taken from the National Life Tables, UK 2011 to 13 for DM, CHD, stroke and colon cancer. 324 The yearly probability of DM, CHD, stroke and colon cancer were obtained from the actual number of deaths324 and disease disability. 7 Information on length of life associated with each health state was taken from published literature,325–328 interpolated to reflect the remaining length of life after each cycle. Mortality rates of severely obese adolescents in the ‘no comorbidity’ health state are assumed to be equivalent to those observed in the general population.
Health-related quality of life
The incremental cost per additional QALY gained for bariatric surgery compared with no surgery was calculated.
We used three published sources to estimate utilities for obese people329 (which explored the relationship between BMI and HRQoL, measured using the EQ-5D for men and women within a national population sample), utilities associated with decreases in BMI (changes due to impact of a 1-unit decrease in BMI over 1-year period associated with a 0.0170 gain in utility)330 and utilities associated with comorbidities associated with obesity331 (multipliers were applied to the utility weights for adolescents with comorbidities included in the model using a catalogue of EQ-5D scores for a variety of health conditions).
From these, further calculations were undertaken to estimate the QALYs gained per year.
Costs
Data on resource use and costs associated with bariatric surgery were obtained from the UCLH’s finance department and included operative costs and costs for 24 months of postoperative care. Costs were based on the average costs for each intervention type in the Healthcare Resource Groups costing system.
Costs in the surgery group were those direct costs of surgery and additional follow-up costs. The direct medical costs for the surgery group were those associated with the intervention and surgical follow-up for 1 year. Other costs included the pre-surgical preparation vists (up to 2 years before surgery) and follow-up costs (dietetic, physician, psychology and nursing follow-up) until year 5 post surgery. From year 6 post surgery, annual medical costs related to weight control equivalent to one GP visit were applied.
The direct medical costs for the no surgery group were assumed to be the same as those incurred pre intervention in the surgical group, plus regular follow-up for the first year. After 1 year, no medical costs related to weight control were applied.
The costs of comorbidities were considered when adolescents moved to the Markov model for the second year. The cost of DM, CHD, stroke332 and colon cancer333 were obtained from published sources.
Costs owing to loss of productivity caused by obesity and its comorbidities were not included in the analysis. Also, medication costs were not incorporated in the model.
Cost-effectiveness analysis
The main result of the study was the incremental cost-effectiveness ratio (ICER) of bariatric surgery versus no surgery. The ICER was calculated by dividing the incremental costs (difference in costs between the two groups) by incremental QALYs (difference in QALYs between the two groups).
The analysis took the UK NHS perspective. Costs were calculated in 2013/14 UK pounds, inflated where appropriate using the consumer price index. 334
A discount rate of 3.5% was applied for costs and effects. 335
The model was developed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) software.
Sensitivity analysis
In one-way sensitivity analyses, ICERs were recalculated when individual parameters were varied, including the gain in QoL per unit of BMI, the intervention costs, the initial weight and weight reduction after intervention and the discount rate.
The parameter values and their ranges used in sensitivity analyses are shown in Table 34.
| Parameter | Base case | Standard error | Uncertainty ranges | Uncertainty distribution | Sources for input parameters | |
|---|---|---|---|---|---|---|
| Cost of RYGB | £7100 | £32 | £5619 | £8023 | Gammaa | UCLH data |
| Cost of RYGB follow-up: first year | £2524 | £601 | £315 | £4982 | Gammab | Assumption |
| Cost of RYGB follow-up: second to fifth years | £445 | £106 | £56 | £879 | Gammab | Assumption |
| Cost of RYGB follow-up: from year 6 | £46 | £5 | £41 | £51 | Gamma | PSSRU |
| Cost of SG | £7312 | £134 | £5787 | £8263 | Gammaa | UCLH data |
| Cost of SG follow-up: first year | £2168 | £512 | £575 | £5030 | Gammab | Assumption |
| Cost of SG follow-up: second to fifth years | £383 | £90 | £102 | £888 | Gammab | Assumption |
| Cost of SG follow-up: from year 6 | £46 | £5 | £41 | £51 | Gamma | PSSRU |
| Cost of DM per year | £1006 | £101 | £906 | £1107 | Gamma | Anokye et al. |
| Cost of CHD per year | £498 | £50 | £448 | £548 | Gamma | Anokye et al. |
| Cost of stroke per year | £2475 | £247 | £2227 | £2722 | Gamma | Anokye et al. |
| Cost of colon cancer per year | £8229 | £823 | £7406 | £9052 | Gamma | Cancer Research UK |
| Utility male (BMI 30–39.9 kg/m2) | 0.82 | 0.082 | 0.74 | 0.90 | Beta | Macran S |
| Utility female (BMI 30–39.9 kg/m2) | 0.78 | 0.078 | 0.70 | 0.86 | Beta | Macran S |
| Utility male (BMI 40–49.9 kg/m2) | 0.56 | 0.056 | 0.50 | 0.62 | Beta | Assumption |
| Utility female (BMI 40–49.9 kg/m2) | 0.52 | 0.052 | 0.47 | 0.57 | Beta | Assumption |
| Utility male (BMI ≥ 50 kg/m2) | 0.30 | 0.030 | 0.27 | 0.33 | Beta | Assumption |
| Utility female (BMI ≥ 50 kg/m2) | 0.26 | 0.026 | 0.24 | 0.29 | Beta | Assumption |
| Utility gain for 1-unit BMI reduction | 0.017 | 0.0017 | 0.015 | 0.019 | Beta | Hakim Z et al. |
| Disutility score: age | –0.0003 | 0.0002 | –0.0003 | –0.0003 | Gamma | Sullivan PW et al. |
| Disutility score: DM | –0.0714 | 0.0048 | –0.0643 | –0.0785 | Gamma | Sullivan PW et al. |
| Disutility score: CHD | –0.0627 | 0.0131 | –0.0564 | –0.0690 | Gamma | Sullivan PW et al. |
| Disutility score: stroke | –0.0330 | 0.0223 | –0.0297 | –0.0363 | Gamma | Sullivan PW et al. |
| Disutility score: colon cancer | –0.0674 | 0.0172 | –0.0607 | –0.0741 | Gamma | Sullivan PW et al. |
| Obesity drift: up to 45 years | 0.12 | 0.012 | 0.11 | 0.13 | Log-normal | Baum CL et al. |
| Obesity drift: 45–65 years | 0.07 | 0.007 | 0.06 | 0.08 | Log-normal | Borg S et al. |
| Obesity drift: over 65 years | –0.17 | –0.017 | –0.15 | –0.19 | Log-normal | Borg S et al. |
| Mean BMI at 1 year: RYGB | 34.07 | 3.54 | 30.58 | 38.86 | Log-normalb | UCLH data |
| Mean BMI at 1 year: SG | 43.88 | 4.13 | 30.92 | 54.83 | Log-normalb | UCLH data |
| Discount rate for cost (%) | 3.5 | 0.0 | 5.0 | NICE | ||
| Discount rate for outcomes (%) | 3.5 | 0.0 | 5.0 | NICE | ||
We conducted probabilistic sensitivity analyses336 with 1000 bootstraps replicates to assess the robustness of our results to input parameters’ uncertainty. The probabilistic sensitivity analyses were based on a gamma distribution for intervention, medical costs and disutility scores, a beta distribution for the QoL gain associated with BMI reduction, a log-normal distribution for post-surgical weight loss and obesity drift. The results of the probabilistic sensitivity analyses are presented on the cost-effectiveness acceptability curve (Figure 31). 337
FIGURE 31.
Cost-effectiveness acceptability curves.
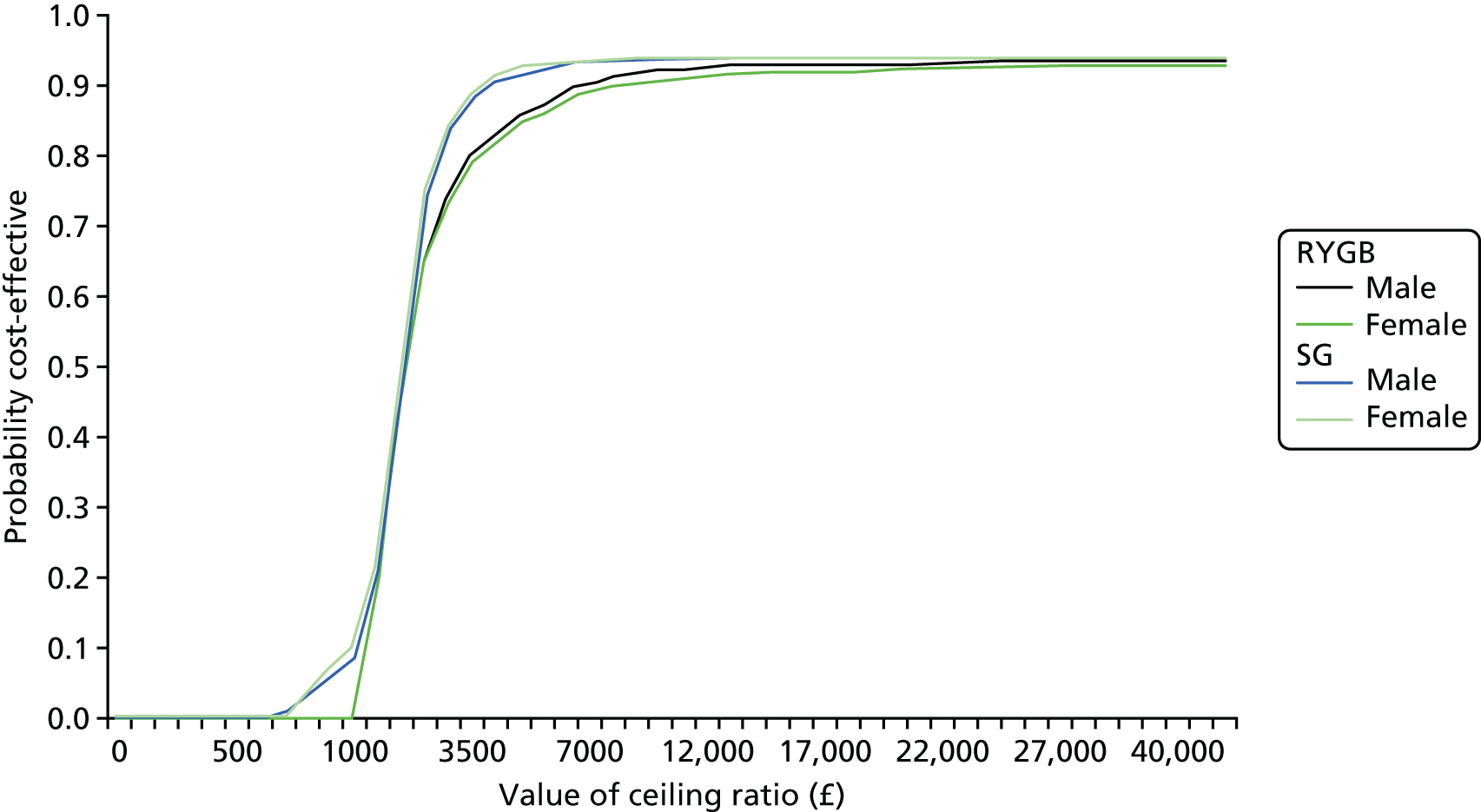
Results
Data from 18 adolescents who underwent bariatric surgery were analysed. Nine adolescents underwent RYBG and nine adolescents underwent SG. The mean age of adolescents who underwent RYGB was 17.8 years and of those who underwent SG was 18.7 years. The mean BMI at baseline for those who underwent RYBG was 48.3 (SD 6.2) kg/m2 and for those who underwent SG was 60.0 (SD 9.3) kg/m2 (Table 35).
| Clinical variable | Surgery | |
|---|---|---|
| RYGB (n = 9) | SG (n = 9) | |
| Female (%) | 56 | 44 |
| Age (years) | ||
| Mean | 17.8 | 18.7 |
| SD | 1.2 | 1.2 |
| Age range | 16–19 | 17–20 |
| BMI at baseline (kg/m2) | ||
| Mean | 48.3 | 60.0 |
| SD | 6.2 | 9.3 |
| Weight at baseline (kg) | ||
| Mean | 142.2 | 170.3 |
| SD | 21.1 | 42.3 |
| Height at baseline (cm) | ||
| Mean | 171.3 | 168.0 |
| SD | 7.3 | 16.3 |
Base-case analysis
At the 1-year follow-up, the mean reduction in BMI was 12.28 kg/m2 (SD 2.98 kg/m2) for adolescents who had undergone RYGB and 16.16 kg/m2 (SD 9.96 kg/m2) for adolescents who had undergone SG.
The mean differences in costs and effects between bariatric surgery and no surgery are presented in Table 36. Bariatric surgery resulted in improved QoL equivalent to a difference of 5.57 (95% CI 5.55 to 5.59) QALYs per person for males and 5.66 (95% CI 5.64 to 5.67) QALYs per person for females who underwent RYBG. For SG this was a difference of 5.50 (95% CI 5.48 to 5.52) QALYs per person for males and 5.64 (95% CI 5.63 to 5.65) QALYs per person for females. Compared with no surgery, the lifetime incremental cost of bariatric surgery was higher for both RYGB [£11,245 (95% CI £10,777 to £11,413) for males; £11,343 (95% CI £11,172 to £11,514) for females] and SG [£10,877 (95% CI £10,707 to £11,046) for males; £10,954 (95% CI £10,782 to £11,125) for females].
| 35A. RYGB compared with no surgery | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RYGB | |||||||||||
| Surgery | No comorbidities | DM | CHD | Stroke | Colon cancer | Total cost | QALY | ||||
| Male | |||||||||||
| Mean | £7100 | £4825 | £1421 | £206 | £706 | £402 | £14,660 | 18.84 | |||
| 95% CI | £7037 to £7162 | £4765 to £4886 | £1417 to £1424 | £205 to £206 | £704 to £708 | £401 to £403 | £14,183 to £14,836 | 18.79 to 18.89 | |||
| Female | |||||||||||
| Mean | £7100 | £4879 | £1025 | £150 | £616 | £333 | £14,102 | 18.30 | |||
| 95% CI | £7049 to £7576 | £4819 to £4939 | £1022 to £1027 | £150 to £151 | £614 to £617 | £332 to £334 | £13,925 to £14,279 | 18.25 to 18.34 | |||
| No surgery | Differences | ICER | |||||||||
| Surgery | No comorbidities | DM | CHD | Stroke | Colon cancer | Total cost | QALY | Total cost | QALY | ||
| Male | |||||||||||
| Mean | £0 | £96 | £1912 | £238 | £752 | £417 | £3414 | 13.27 | £11,245 | 5.57 | £2018 |
| 95% CI | £93 to £96 | £1908 to £1916 | £237 to £239 | £750 to £754 | £416 to £418 | £3406 to £3423 | 13.24 to 13.30 | £10,777 to £11,413 | 5.55 to 5.59 | £1942 to £2042 | |
| Female | |||||||||||
| Mean | £0 | £96 | £1469 | £184 | £675 | £336 | £2759 | 12.64 | £11,343 | 5.66 | £2005 |
| 95% CI | £93 to £98 | £1466 to £1472 | £183 to £184 | £673 to £677 | £335 to £337 | £2753 to £2765 | 12.61 to 12.67 | £11,172 to £11,514 | 5.64 to 5.67 | £1974 to £2031 | |
| 35B. SG compared with no surgery | |||||||||||
| SG | |||||||||||
| Surgery | No comorbidities | DM | CHD | Stroke | Colon cancer | Total cost | QALY | ||||
| Male | |||||||||||
| Mean | £7312 | £4232 | £1869 | £236 | £749 | £416 | £14,815 | 12.47 | |||
| 95% CI | £7037 to £7362 | £4181 to £4284 | £1865 to £1873 | £235 to £237 | £747 to £751 | £415 to £417 | £14,637 to £14,994 | 12.44 to 12.51 | |||
| Female | |||||||||||
| Mean | £7312 | £4287 | £1430 | £181 | £670 | £336 | £14,217 | 11.82 | |||
| 95% CI | £7049 to £7576 | £4236 to £4339 | £1427 to £1433 | £181 to £182 | £669 to £672 | £335 to £337 | £14,038 to £14,396 | 11.79 to 11.85 | |||
| No surgery | Differences | ICER | |||||||||
| Surgery | No comorbidities | DM | CHD | Stroke | Colon cancer | Total cost | QALY | Total cost | QALY | ||
| Male | |||||||||||
| Mean | £0 | £96 | £2357 | £267 | £794 | £426 | £3939 | 6.98 | £10,877 | 5.50 | £1978 |
| 95% CI | £93 to £98 | £2351 to £2362 | £266 to £267 | £791 to £796 | £425 to £428 | £3930 to £3948 | 6.96 to 6.99 | £10,707 to £11,046 | 5.48 to 5.52 | £1954 to £2002 | |
| Female | |||||||||||
| Mean | £0 | £96 | £1894 | £211 | £727 | £337 | £3264 | 6.18 | £10,954 | 5.64 | £1941 |
| 95% CI | £93 to £98 | £1890 to £1898 | £210 to £211 | £725 to £729 | £336 to £338 | £3256 to £3271 | 6.16 to 6.20 | £10,782 to £11,125 | 5.63 to 5.65 | £1915 to £1969 | |
Repeated by surgical type, the mean incremental cost of RYGB versus no surgery was £2018 (95% CI £1942 to £2042) for males and £2005 (95% CI £1974 to £2031) for females. The mean incremental cost of SG versus no surgery was £1978 (95% CI £1954 to £2002) for males and £1941 (95% CI £1915 to £1969) for females. Most of the costs in the surgery group were associated with the intervention costs; other health-care costs were reduced due to loss of weight.
Sensitivity analysis
The results were shown to be robust in sensitivity analyses. Bariatric surgery remained cost-effective for both types of procedures when the parameter values for intervention cost and utility value were varied.
For the RBYG group, variation in BMI at baseline for both the male population and the female population seemed to have an effect on results. No surgery was less costly and more effective than RYGB if the BMI at baseline was 39 kg/m2 or lower (Table 37).
| Scenario | Costs | Δ Costs | QALY | Δ QALY | ICER |
|---|---|---|---|---|---|
| Males | |||||
| Base case | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £14,660 | £11,245 | 18.84 | 5.57 | £2018 |
| Surgery costs | |||||
| Upper values | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £15,583 | £12,168 | 18.84 | 5.57 | £2183 |
| Lower values | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £13,179 | £9765 | 18.84 | 5.57 | £1752 |
| Utility weight of BMI 30–39.9 kg/m2 | |||||
| Upper values | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £14,660 | £11,245 | 20.84 | 7.57 | £1486 |
| Lower values | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £14,660 | £11,245 | 16.85 | 3.58 | £3141 |
| Utility weight of BMI 40–49.9 kg/m2 | |||||
| Upper values | |||||
| No surgery | £3414 | 14.36 | |||
| Surgery | £14,660 | £11,245 | 18.84 | 4.21 | £2669 |
| Lower values | |||||
| No surgery | £3414 | 11.91 | |||
| Surgery | £14,660 | £11,245 | 18.84 | 6.93 | £1622 |
| BMI at baseline (39–57 kg/m2) | |||||
| 39 kg/m2 | |||||
| No surgery | £2938 | 19.63 | |||
| Surgery | £14,660 | £11,721 | 18.84 | –0.79 | –£14,863 |
| 57 kg/m2 | |||||
| No surgery | £3817 | 6.99 | |||
| Surgery | £14,660 | £10,843 | 18.84 | 11.86 | £914 |
| Reduction in BMI (20–37%) after surgery | |||||
| 20% reduction | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £14,923 | £11,509 | 18.81 | 5.55 | £2075 |
| 37% reduction | |||||
| No surgery | £3414 | 13.27 | |||
| Surgery | £14,455 | £11,040 | 18.86 | 5.60 | £1973 |
| Costs | |||||
| Discount rate 0% | |||||
| No surgery | £18,787 | 13.27 | |||
| Surgery | £29,488 | £10,701 | 18.84 | 5.57 | £1920 |
| Discount rate 5% | |||||
| No surgery | £1799 | 13.27 | |||
| Surgery | £12,977 | £11,177 | 18.84 | 5.57 | £2006 |
| Outcomes | |||||
| Discount rate 0% | |||||
| No surgery | £3414 | 31.24 | |||
| Surgery | £14,660 | £11,245 | 44.30 | 13.06 | £861 |
| Discount rate 5% | |||||
| No surgery | £3414 | 10.18 | |||
| Surgery | £14,660 | £11,245 | 14.49 | 4.31 | £2609 |
| Females | |||||
| Base case | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £14,102 | £11,343 | 18.30 | 5.66 | £2005 |
| Surgery costs | |||||
| Upper values | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £15,025 | £12,266 | 18.30 | 5.66 | £2168 |
| Lower values | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £12,621 | £9862 | 18.30 | 5.66 | £1743 |
| Utility weight of BMI 30–39.9 kg/m2 | |||||
| Upper values | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £14,102 | £11,343 | 20.24 | 7.60 | £1493 |
| Lower values | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £14,102 | £11,343 | 16.36 | 3.72 | £3049 |
| Utility weight of BMI 40–49.9 kg/m2 | |||||
| Upper values | |||||
| No surgery | £2759 | 13.93 | |||
| Surgery | £14,102 | £11,343 | 18.30 | 4.36 | £2599 |
| Lower values | |||||
| No surgery | £2759 | 11.35 | |||
| Surgery | £14,102 | £11,343 | 18.30 | 6.95 | £1632 |
| BMI at baseline (39–57 kg/m2) | |||||
| 39 kg/m2 | |||||
| No surgery | £2320 | 19.11 | |||
| Surgery | £14,102 | £11,782 | 18.30 | –0.81 | –£14,538 |
| 57 kg/m2 | |||||
| No surgery | £3145 | 6.19 | |||
| Surgery | £14,102 | £10,957 | 18.30 | 12.11 | £905 |
| Reduction in BMI (20–37%) after surgery | |||||
| 20% reduction | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £14,341 | £11,582 | 18.27 | 5.63 | £2057 |
| 37% reduction | |||||
| No surgery | £2759 | 12.64 | |||
| Surgery | £13,920 | £11,161 | 18.32 | 5.68 | £1965 |
| Costs | |||||
| Discount rate 0% | |||||
| No surgery | £16,278 | 12.64 | |||
| Surgery | £14,102 | –£2176 | 18.30 | 5.66 | –£385 |
| Discount rate 5% | |||||
| No surgery | £1427 | 12.64 | |||
| Surgery | £14,102 | £12,675 | 18.30 | 5.66 | £2240 |
| Outcomes | |||||
| Discount rate 0% | |||||
| No surgery | £2759 | 30.84 | |||
| Surgery | £27,080 | £24,321 | 44.65 | 13.81 | £1761 |
| Discount rate 5% | |||||
| No surgery | £2759 | 9.62 | |||
| Surgery | £12,674 | £9915 | 13.96 | 4.34 | £2283 |
In the SG group, results appeared to be affected by variation in BMI at baseline, reduction in BMI after intervention for both the males and the females and discount rates for costs for the females only. No surgery was less costly and more effective than SG if the BMI at baseline was 49kg/m2 or lower, or the reduction of BMI following surgery was less than 9%. In the female population, no surgery intervention was less costly and more effective than SG if costs were not discounted (0%) (Table 38).
| Scenario | Costs | Δ Costs | QALY | Δ QALY | ICER |
|---|---|---|---|---|---|
| Males | |||||
| Base case | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £14,815 | £10,877 | 12.47 | 5.50 | £1978 |
| Surgery costs | |||||
| Upper values | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £15,766 | £11,827 | 12.47 | 5.50 | £2151 |
| Lower values | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £13,290 | £9351 | 12.47 | 5.50 | £1701 |
| Utility weight of BMI 40–49.9 kg/m2 | |||||
| Upper values | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £14,815 | £10,877 | 13.83 | 6.86 | £1586 |
| Lower values | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £14,815 | £10,877 | 11.11 | 4.14 | £2628 |
| Utility weight of BMI ≥ 50 kg/m2 | |||||
| Upper values | |||||
| No surgery | £3939 | 7.71 | |||
| Surgery | £14,815 | £10,877 | 12.47 | 4.76 | £2283 |
| Lower values | |||||
| No surgery | £3939 | 6.24 | |||
| Surgery | £14,815 | £10,877 | 12.47 | 6.23 | £1745 |
| BMI at baseline (49–77 kg/m2) | |||||
| 49 kg/m2 | |||||
| No surgery | £3462 | 13.26 | |||
| Surgery | £14,815 | £11,353 | 12.47 | –0.79 | –£14,374 |
| 77 kg/m2 | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £14,815 | £10,877 | 12.47 | 5.50 | £1978 |
| Reduction in BMI (9–48%) after surgery | |||||
| 9% reduction | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £15,279 | £11,341 | 6.19 | –0.79 | –£14,345 |
| 48% reduction | |||||
| No surgery | £3939 | 6.98 | |||
| Surgery | £14,120 | £10,181 | 18.86 | 11.89 | £857 |
| Costs | |||||
| Discount rate 0% | |||||
| No surgery | £20,869 | 6.98 | |||
| Surgery | £31,306 | £10,437 | 12.47 | 5.50 | £1898 |
| Discount rate 5% | |||||
| No surgery | £2106 | 6.98 | |||
| Surgery | £12,926 | £10,820 | 12.47 | 5.50 | £1968 |
| Outcomes | |||||
| Discount rate 0% | |||||
| No surgery | £3939 | 15.99 | |||
| Surgery | £14,815 | £10,877 | 28.80 | 12.82 | £849 |
| Discount rate 5% | |||||
| No surgery | £3939 | 5.39 | |||
| Surgery | £14,815 | £10,877 | 9.65 | 4.26 | £2554 |
| Females | |||||
| Base case | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £14,217 | £10,954 | 11.82 | 5.64 | £1941 |
| Surgery costs | |||||
| Upper values | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £15,168 | £11,904 | 11.82 | 5.64 | £2109 |
| Lower values | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £12,692 | £9428 | 11.82 | 5.64 | £1670 |
| Utility weight of BMI 40–49.9 kg/m2 | |||||
| Upper values | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £14,217 | £10,954 | 13.12 | 6.94 | £1579 |
| Lower values | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £14,217 | £10,954 | 10.53 | 4.35 | £2518 |
| Utility weight of BMI ≥ 50 kg/m2 | |||||
| Upper values | |||||
| No surgery | £3264 | 6.83 | |||
| Surgery | £14,217 | £10,954 | 11.82 | 4.99 | £2194 |
| Lower values | |||||
| No surgery | £3264 | 5.53 | |||
| Surgery | £14,217 | £10,954 | 11.82 | 6.30 | £1740 |
| BMI at baseline (49–77 kg/m2) | |||||
| 49 kg/m2 | |||||
| No surgery | £2804 | 12.64 | |||
| Surgery | £14,217 | £11,413 | 11.82 | –0.81 | –£14,046 |
| 77 kg/m2 | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £14,217 | £10,954 | 11.82 | 5.64 | £1941 |
| Reduction in BMI (9–48%) after surgery | |||||
| 9% reduction | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £14,664 | £11,401 | 5.37 | –0.81 | –£14,007 |
| 48% reduction | |||||
| No surgery | £3264 | 6.18 | |||
| Surgery | £13,582 | £10,318 | 18.32 | 12.14 | £850 |
| Costs | |||||
| Discount rate 0% | |||||
| No surgery | £18,609 | 6.18 | |||
| Surgery | £14,217 | –£4392 | 11.82 | 5.64 | –£778 |
| Discount rate 5% | |||||
| No surgery | £1706 | 6.18 | |||
| Surgery | £14,217 | £12,511 | 11.82 | 5.64 | £2216 |
| Outcomes | |||||
| Discount rate 0% | |||||
| No surgery | £3264 | 14.61 | |||
| Surgery | £29,022 | £25,758 | 28.29 | 13.68 | £1883 |
| Discount rate 5% | |||||
| No surgery | £3264 | 4.74 | |||
| Surgery | £12,588 | £9325 | 9.08 | 4.34 | £2150 |
The results of the probabilistic cost-effectiveness analysis are presented in Table 39. The cost-effectiveness acceptability curves for bariatric surgery compared with no surgery indicate the probability of bariatric surgery being more cost-effective than no surgery for a range of values a decision-maker is willing to pay for an additional unit of health gain.
| Gender | Intervention | Differences | ICER | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RYGB | No surgery | |||||||||||||
| Mean cost | 95% CI | QALY | 95% CI | Mean cost | 95% CI | QALY | 95% CI | Mean cost | 95% CI | QALY | 95% CI | Mean | 95% CI | |
| Male | £14,706 | £14,664 to £14,747 | 19.10 | 18.96 to 19.24 | £3410 | £3397 to £3422 | 13.97 | 13.89 to 14.06 | £11,296 | £11,255 to £11,337 | 5.13 | 4.97 to 5.29 | £2201 | £10 to £4393 |
| Female | £14,123 | £14,083 to £14,163 | 18.62 | 18.48 to 18.76 | £2754 | £2744 to £2764 | 13.28 | 13.20 to 13.36 | £11,369 | £11,329 to £11,408 | 5.34 | 5.18 to 5.50 | £2129 | £1186 to £3071 |
| Gender | SG | No surgery | Differences | ICER | ||||||||||
| Mean cost | 95% CI | QALY | 95% CI | Mean cost | 95% CI | QALY | 95% CI | Mean cost | 95% CI | QALY | 95% CI | Mean | 95% CI | |
| Male | £14,819 | £14,782 to £14,855 | 13.49 | 13.29 to 13.70 | £3926 | £3910 to £3942 | 7.70 | 7.66 to 7.75 | £10,893 | £10,859 to £10,928 | 5.79 | 5.58 to 6.00 | £1882 | £1637 to £2126 |
| Female | £14,229 | £14,193 to £14,265 | 13.01 | 12.81 to 13.21 | £3268 | £3255 to £3281 | 6.87 | 6.83 to 6.91 | £10,961 | £10,927 to £10,996 | 6.14 | 5.94 to 6.35 | £1784 | £1580 to £1989 |
At the NICE-recommended threshold of £20,000 per QALY gained,337 the probability of bariatric surgery being cost-effective is > 90%.
Discussion
Our data show that bariatric surgery in severely obese adolescents is highly cost-effective over the long term and also improves QoL. Surgery is significantly more costly than non-surgical treatment; however, the benefits of improved QoL results in surgery being highly cost-effective.
Few studies of bariatric surgery in adolescents that include an economic evaluation have been published, and all are from Australia or the USA. Ours is the first published from the perspective of a national health service. Caution is needed when comparing results of cost-effectiveness studies owing to variations in type of surgery, population of young people, perspective of the study, time horizon, QoL instrument and discount rate used. A meta-analysis of bariatric surgery published in 2011338 identified one Australian study that undertook economic modelling, which reported that bariatric surgery in the form of a laparoscopic adjustable gastric band (LAGB) was cost-effective in an Australian adolescent population. Another more recent study of American adolescents from a single centre224 concluded that bariatric surgery (RYGB) may be cost-effective when evaluated over a long period of time. These findings are comparable to those reported for adults. A systematic review of bariatric surgery in adults concluded that bariatric surgery was cost-effective in comparison with non-surgical interventions for those populations with a higher range of BMIs. 256
Strengths and weaknesses
We used an unselected cohort from the largest UK centre undertaking adolescent bariatric surgery, together with modelled data from nationally representative surveys and authoritative data sources. Our estimates of cost-effectiveness are likely to be conservative. We accounted for likely upwards BMI drift after surgery using data taken from studies of obese adults after bariatric surgery, but did not include drift in the non-surgery group, although this is likely. QoL estimates were taken from samples of morbidly obese adults. We included costs of follow-up after surgery out to 5 years but did not include any costs for the non-surgery group past 1 year. Obesity is recognised as being a risk factor for comorbidities other than those included in the model (i.e. DM, CHD, stroke and colon cancer) and thus our model probably underestimates the impact of obesity. Together these suggest that our findings probably underestimate the cost-effectiveness of surgery. We undertook a long-term evaluation of bariatric surgery and used sensitivity analyses. Findings were further strengthened by sensitivity analyses showing that large changes in cost or utility estimates did not change the main conclusions.
Our findings are subject to a number of limitations. Our model captures only direct costs. This is a conservative assumption as many of the costs associated with obesity may fall on individuals as well as society. Although we had a precise estimate of the costs of bariatric surgery at UCLH, the lack of empirical QoL data from patients who underwent bariatric surgery could cause uncertainty in the model. Future evaluation is needed to measure and incorporate changes in QoL from the adolescent population undergoing bariatric surgery. Costs for procedures were provided by a single centre (UCLH), which has high costs because it is a central London teaching hospital. The costs shown here therefore may be higher than in other hospitals; however, this would lead to an underestimate of the cost-effectiveness of surgery. Furthermore, our assumptions regarding postoperative resource use may be excessively high. Again, this suggests that the model provides a conservative estimate of the cost-effectiveness of the bariatric surgery and potentially overestimates the overall impact of obesity.
Conclusions
Bariatric surgery of severely obese adolescents is a cost-effective alternative to no surgery over a lifetime in obese adolescents, taking the perspective of the UK NHS.
Substudy 6.6: formation of a national network for adolescent bariatric surgery
As part of study 6, the Institute of Child Health research team, led by Professor Russell Viner, hosted a series of meetings aimed at setting up a national UK network of providers undertaking adolescent bariatric surgery.
Through clinical contacts, four NHS providers were identified that met NICE criteria and undertook any bariatric surgery in adolescents:
-
University College London Hospitals NHS Foundation Trust, led by Professor Russell Viner and Dr Billy White, with bariatric surgeon Mr Marco Adamo
-
Bristol Royal Hospital for Children, led by Professor Julian Shield
-
Sheffield Children’s Hospital, led initially by Dr Jerry Wales and later by Dr Nick Wright, with bariatric surgeon Mr Roger Ackroyd
-
King’s College Hospital, London, led by Dr Martha Adams, Dr Simon Chapman and paediatric surgeon Mr Ashish Desai.
The first meeting took place in early 2010, with a subsequent meeting in 2011.
The group agreed at that point (i.e. 2011 meeting) that it would regard itself as a national working group/network on adolescent bariatric surgery, given that it contained all the NHS services undertaking this work. It agreed that the most logical workplan at that time was to (1) share protocols and patient pathways and (2) inform development of the service specifications for adolescent bariatric surgery for NHS England. NHS England had been formed in 2012/13 and Clinical Reference Groups (CRGs) were quickly set up, one being on morbid obesity. The initial CRG was focused on only adults but an adolescent subgroup was set up in 2013. A service specification was developed together with commissioners and the lead of the obesity CRG, Dr Julian Barth. 339
Subsequent meetings focused on the development of a national database and data collection. Meetings took place in May 2013 and December 2014, with colleagues from this bariatric group being part of wider programme dissemination meetings during 2015. A final meeting was held in January 2016. All meetings were held in London, with travel funding provided by the programme.
Agreement was reached in December 2015 to host data from the four centres on adolescent bariatric surgery with the UK National Bariatric Registry. 221 The full third National Bariatric Surgery Register report will be released in 2020. This will involve an updated data set – adding patient identifiers (i.e. NHS numbers), glycosylated haemoglobin (HbA1c) levels and EuroQol-5 Dimensions quality-of-life measurements – which will help with making outcomes more patient centred (which also fits with the Healthcare Quality Improvement Partnership mandate). Subsequent to this, data on adolescent bariatric surgery will be routinely entered into the national registry.
Chapter 7 Impact and dissemination
The dissemination work package had two main strands: (1) dissemination through policy and practitioner fora and through academic publications and presentations, and (2) patient and public dissemination.
Policy and academic dissemination
The programme results were disseminated to key stakeholders, the academic community and the wider public through a series of publications and conference presentations, as well as five events organised by the research team, to which targeted audiences were invited.
The team presented the results and outcomes of the programme at several local, national and international conferences, including the 19th European Congress on Obesity (Lyon, France, 2012), the Royal College of Paediatrics and Child Health Annual Meeting (Glasgow, UK, 2012; Birmingham, UK, 2015), Association for the Study of Obesity: Lightening the Load in Primary Care (London, UK, 2012), Society for Adolescent Health and Medicine (Los Angeles, CA, USA, 2015), the International Congress on Obesity (Kuala Lumpur, Malaysia, 2014), and the 13th and 14th Meetings of the International Society for Behavioral Nutrition and Physical Activity (San Diego, CA, USA, 2014; Edinburgh, UK, 2015). See Appendix 6 for a full list of presentations.
In addition, the results of the programme have been published (or accepted for publication) in national and international peer-reviewed journals, accounting for a total of 25 articles (at time of writing). The results of some of these articles were also picked up and advertised to the wider public via the mass media. For example, the study by Black et al. 340 had articles about its results in several print and online press outlets, such as the BBC News and ITV News websites, The Telegraph, The Guardian, The Times Health News, Huffington Post, as well as an interview on BBC Radio 4’s Today show. The article by Black et al. 340 was also among the top 10 most read articles in the British Journal of General Practice for 2015. 341
The complete list of publications from the PROMISE programme (by study) can be found in the Acknowledgements.
The research team also organised five events to present the programme results to selected audiences. The first four events were organised to disseminate the results of each study individually (see event list in Appendix 7):
-
Sharing the Outcomes of Adolescent Bariatric Surgery in England (17 December 2014, UCL Institute of Child Health) was a 1-day, invitation-only seminar in London, where the outcomes of the evaluation of the adolescent bariatric surgery at University College Hospital (study E) were shared, and discussions about next steps for adolescent bariatric surgery in England began. The four main NHS centres performing adolescent bariatric surgeries in England were represented on the day – University College Hospital, London; Sheffield Children’s Hospital; Bristol Royal Hospital for Children; and King’s College Hospital NHS Foundation Trust. The chairperson (Dr Julian Barth) and the NHS commissioner for the Severe and Complex Obesity Clinical Reference Group were also present on the day. A follow-up meeting was held on 3 June 2015, with the same invited participants.
-
Evaluation of the National Child Measurement Programme Feedback: Implications for Policy and Action (21 January 2015, London School of Hygiene & Tropical Medicine) was a half-day seminar, open to the public, organised by the London School of Hygiene & Tropical Medicine. The event brought together researchers and policy experts to report the main findings of study A and to discuss their implications for the future development of the NCMP feedback, programme implementation and wider policy relating to child obesity. The seminar was led by Professor Anthony Kessel, Director of International Public Health at PHE, and speakers included Dr Sanjay Kinra; Co-Dr Harry Rutter, Founder Director of the National Obesity Observatory; and Pamela Naylor, PHE Lead for Child Obesity.
-
Outcomes of the HELP Trial and Next Steps for the Adolescent Lifestyle Interventions for Obesity (6 February 2015, Friends House) was a 1-day, invitation-only seminar, organised to present the outcomes of the trial and discuss the next possible steps for adolescent lifestyle interventions for obesity. The main speakers were the two principal investigators – Professors Russell Viner and Deborah Christie. The morning session presented details about the intervention (e.g. recruitment process, intervention delivery and results), and ended with a question-and-answer session led by Dr Rebecca Holt with some of the participating young people and parents. The afternoon session was chaired by Dr Ann Hoskins, Director of Children, Young People and Families at PHE, and included individual group discussions on three topics, (1) what are the implications for policies locally and nationally?, (2) what are the implications for obesity interventions for children and adolescents? and (3) what should the future direction be for obesity research?, before a joint discussion plenary session.
-
How can GPs Help Prevent and Manage Obesity in Children? (4 March 2015, Royal College of General Practitioners) was an invitation-only, 2-hour event targeted mainly at GPs and health-care practitioners, to raise awareness among GPs of the research examining what we can do to play a greater role in the prevention and management of obesity in children. Data were presented on what parents were saying about the feedback, and difficulties in raising concerns about children’s weight with parents and demonstrated tools that are available to guide discussion and engage families. In an open forum, it was then discussed how we can achieve this together. The line-up of speakers included Dr Sonia Saxena, GP and Child Health Researcher at Imperial College London, who chaired the panel discussion; James Cracknell, double Olympic gold medallist and Senior Fellow at the Health Policy Exchange; and Dr Rachel Pryke, Royal College of General Practitioners Champion for Nutrition (GP magazine: www.gponline.com/gps-urged-play-greater-role-preventing-child-obesity/obesity/article/1337358). 342
The final event, Findings from the PROMISE Programme and Next Steps for Treatments of Obesity in Children and Young People (17 June 2015, UCL Institute of Child Health) was a national-level, half-day symposium examining the findings of the PROMISE programme and what they mean for UK actions on child and adolescent obesity, particularly looking at implications for policy, local authorities and public health, as well as clinical pathways in primary and secondary care. Researchers, policy-makers, practitioners and young people were present and shared their views during the event. In addition to presentations from the co-principal investigators (Professors Russell Viner and Dr Sanjay Kinra), there were oral presentations from several academics, clinicians and key stakeholders, including Professor Anthony Kessel, Director of International Public Health at PHE; Dr Dasha Nicholls, Consultant Child & Adolescent Psychiatrist at Great Ormond Street Hospital for Children; Councillor Gillian Ford, London Borough of Havering and Lead member for the Community Wellbeing Board at the Local Government Association; and Professor Edna Roche, Head of Paediatrics at Trinity College Dublin. The Association for Young People’s Health (AYPH) presented the outcomes of a consultation with young people and their parents through an online survey and focus groups, and four young people were also present on the day to share their experiences (see Patient and public dissemination for further details).
Members of the PROMISE research team were also invited to several meetings and round tables to discuss the programme findings with policy-makers: Round Table on Obesity organised by the Chief Medical Officer, Professor Dame Sally Davies (Professor Russell Viner, June 2015), NCMP Reference Group meeting (October 14, Dr Sanjay Kinra and Ms Helen Croker; DHSC NCMP workshops (autumn 2012, Dr Catherine Falconer); and DHSC, Obesity and Food Policy Branch (July 2012, Dr Catherine Falconer). Professor Russell Viner was invited to become a NCMP board member from July 2015.
Patient and public dissemination
The AYPH343 was commissioned to undertake a novel piece of work that both disseminated findings to young people and families and involved young people directly in the dissemination events above. Note that this was separate from the PPI strand across the whole programme (provided by membership of the Programme Board by Tam Fry on behalf of the Child Growth Foundation) and individual PPI elements within some of the substudies.
The participation dissemination work had three strands: (1) a workshop with young people who had taken part in PROMISE, (2) a focus group for parents of young people who had been involved and (3) an online survey about obesity that was aimed at a wider audience of young people.
There were two main outputs. First, findings were presented at the national dissemination event for PROMISE at the Institute of Child Health in London, in June 2015. Three young people who had been involved in PROMISE were supported to put questions to the researchers. AYPH ran a social media campaign before, during and after the conference, widely disseminating the findings from the young people’s participation work. Second, the AYPH is preparing a summary of findings from the PROMISE programme for young people, using appropriate language and communication styles.
How young people and family voices were heard
Young people from the HELP study (study C) were recruited to take part in a workshop designed and facilitated by the AYPH. A total of seven young people attended on the day (four males and three females, aged 12–19 years). The workshop ran in central London for 2.5 hours with three members of the AYPH team. Parents and young people jointly heard the findings of PROMISE. The groups were then split for the feedback sessions, with parents in one room and young people in another. Young people’s comments and feedback were anonymous. The analyses and report were undertaken by the AYPH, and findings were reported by order of questions, using participants’ quotations to illustrate the main themes/opinions that emerged.
What young people told us
The young people were asked a set of trigger questions, some individually, some in the whole group and then some in two smaller groups, split by age. The young people and parents were clearly familiar with the HELP project in which they had been involved, but none were aware that this was part of the bigger PROMISE research programme of five separate studies.
1: Taking part in research
(a) How did young people rate the experience?
Young people were asked individually about their experience of taking part in HELP. Six out of seven of the young people rated it at the top end of the ‘Happy’ scale and one rated it in the middle category. Similarly, six out of seven said that they would recommend involvement in the project to a friend.
(b) How did young people hear about the study?
There was a group discussion about recruitment to the project. A variety of methods were reported. Two young people had been sent a letter by their GP, one had seen a poster in a college toilet and three had been prompted by their parents.
(c) Why did young people choose to get involved?
The group was asked why they chose to get involved. Broadly, the young people wanted to lose weight and most had already tried other ways. They saw this as something different that was a good opportunity and had come along at ‘the right time’ for them. Some also thought that losing weight would boost their confidence.
(d) What could make the research better for future young people?
The need for a variety of activities for different age groups was a key concern. Older teenagers did not want to do ‘the vegetable A–Z’ and some wanted more action and exercise and less education. They suggested incentives, such as a gym pass. They would have liked to meet up with other young people for a shared experience and to create online groups on social media platforms such as Facebook (www.facebook.com; Facebook, Inc., Menlo Park, CA, USA). They all liked the idea of having a way to monitor progress over time and suggested methods including apps.
2: What health issues do obese young people face?
The group was split into two age groups (11–14 years and 15–19 years) and each was asked what health problems they thought an overweight or obese young person might face. They identified a range of physical health problems from tired legs to heart disease and also suggested a range of mental health issues from anxiety to suicidal thoughts. The younger age group focused more on physical ailments, although they did also mention anxiety, being worried and fear of bullying. The older age group named more mental health issues including depression, mood swings and suicidal thoughts. See Appendix 8 for the full list and responses from the two age groups.
3: What were seen as the barriers to getting help?
The two groups, split by age, were asked to suggest what barriers they thought obese young people might face.
Both age groups said that they were worried about parents finding out, with concerns ranging from ‘not wanting to worry them’ to ‘fear of what action they might take’. Similarly, both groups said that they were worried about friends finding out, in case it led to loss of friendships or bullying/increased bullying. Health professionals were also perceived as a barrier, with comments including ‘being judged’, ‘having your problems dismissed’ or feeling that they were ‘wasting the GP’s time’. The older age group also cited denial and thinking that ‘nothing would help’.
4: What are the solutions to getting help?
The groups were asked to come up with solutions for getting help. Their suggestions included practical ideas, such as schools advertising weight loss activities or NHS 111 phone lines covering the issues. They said that parents should be ‘more honest’ and less ‘protective’ and GPs should also tackle weight problems. The 15- to 19-year-olds highlighted the need for confidential services and ‘feeling safe’ to open up to someone. Knowing what weight loss services or counselling facilities were available and doing things in pairs or groups were additional ideas. See Appendix 9 for a full list.
5: Parents’ feedback
The parents discussed their experiences of the research project and highlighted the factors that they felt would help future work, but they all felt that ‘the time needed to be right’ for young people to engage voluntarily. It was not something that could be imposed on them.
All of them said that there needed to be a focus on the emotional well-being of a young person, with counselling and mental health support being an integral part of any weight loss programme. They felt that a ‘whole-person approach’ was necessary and that practitioners had to understand young people’s lives and have effective communication skills to work with that age group. Links needed to be made between obesity, stress and bullying and those issues needed to be addressed. Services needed to support young people’s aspiration.
Practical help was important. Parents said that they wanted accessible information, assessment tools to help them make decisions and incentives such as vouchers or gym membership. Classes on nutrition and weight would be useful, led by staff who could offer information and support. The need to have safe places to play was also highlighted.
In terms of services, they favoured a non-medical approach, including venues and non-medical language, and said that one-off medical advice was not helpful. Parents suggested prevention work through schools and said that there needed to be support for families around the NCMP results. Finally, they agreed that stigma around obesity prevented parents from seeking help for their children.
Online survey
An online survey for young people was designed, based on the PROMISE findings, which ran for 6 weeks from mid-May 2015. It was publicised to young people’s networks through social media and attracted 66 responses. The age breakdown was as follows: 6% were aged 10–14 years, 45% were aged 15–19 years, 35% were aged 20–24 years and 14% were aged ≥ 25 years; 66% were female, 30% were male and 4% described themselves as ‘other’ or did not want to say.
Figure 32 shows how the respondents described their weight. The majority (56%) said that they were ‘normal’, with 27% ‘overweight’, 6% ‘very overweight’ and 5% ‘obese’. The ‘underweight’ category accounted for 6% of respondents.
FIGURE 32.
Young people’s answers to ‘how would you describe your weight?’.
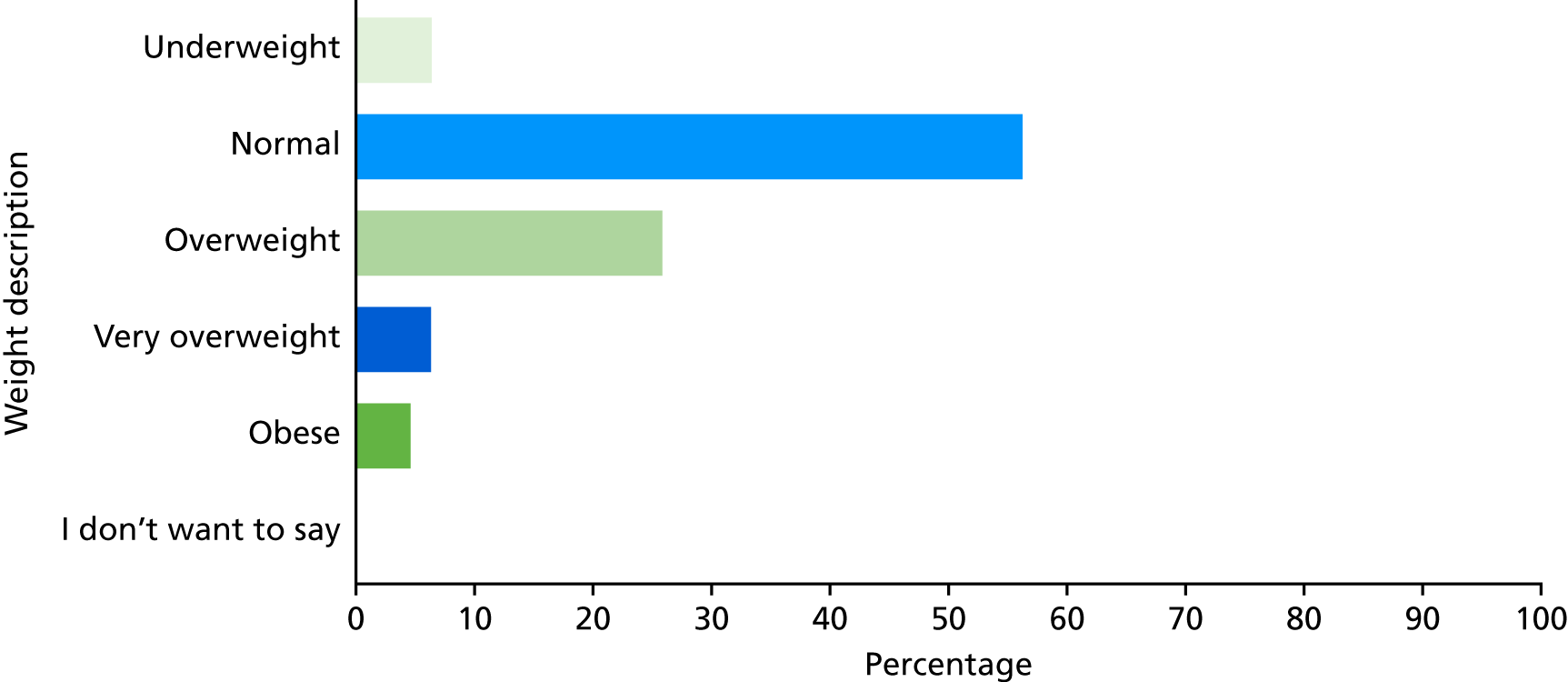
Figure 33 shows how they would identify a weight problem: 75% could work it out themselves, 8% would get weighed and measured and 6% said that they would identify it by being told by a health professional. A total of 11% said that ‘tighter clothes’, ‘bullying’ and comments from others would indicate weight gain.
FIGURE 33.
Young people’s answers to ‘how would you know if you were getting overweight?’.
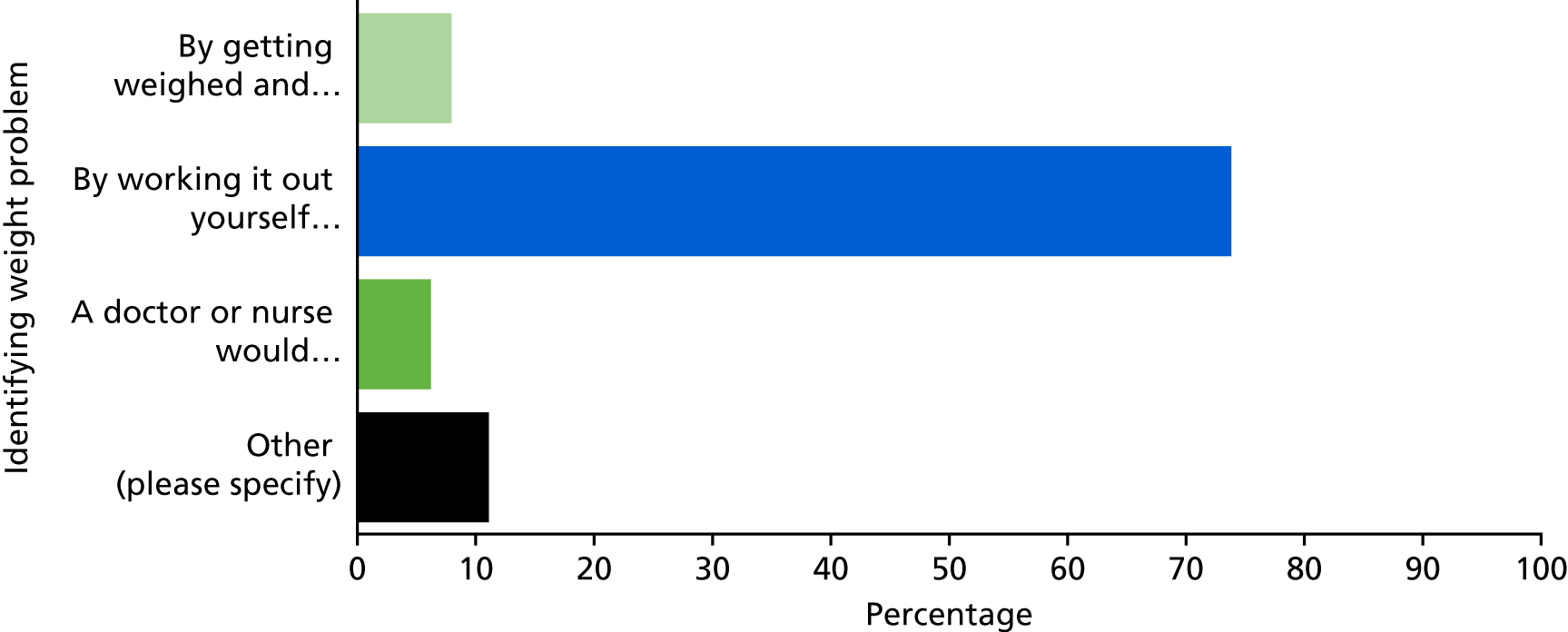
The online survey asked what information was required by young people with weight issues. The responses were fairly evenly split between medical advice, diet and exercise information, a choice of weight loss methods, advice on local services and information on who they could talk to.
Respondents were asked to rank on a scale of 1–5 who should give young people information about their weight. The results (Figure 34 – aggregated score after ranking preferences) show that family and carer come first, followed by a doctor or nurse, and then school or college.
FIGURE 34.
Young people’s answers to ‘who should give young people information about their weight?’.

When asked what they thought about young people being very overweight or obese, 89% said that they thought it was ‘a problem the UK needs to look at’. No-one said that it was ‘not a problem’, 8% said that it was up to young people to decide about their own bodies and 3% opted for ‘other’, with comments including that fitness was more important than weight and parents might encourage unhealthy lifestyles. A total of 67% would be interested in taking part in research to lose weight if they were very overweight or obese. Eight per cent said that they would not, 20% said ‘maybe’ and 6% did not know.
The survey asked two open questions.
The first open question was ‘Have you a message for the Government about young people becoming very overweight or obese?’. Answers included:
I myself have been obese since I was 11. I have tried many methods to lose weight; these are all very expensive or aimed at older people.
Sports classes, etc. are usually full of much fitter people, so it is intimidating to go to these.
If my emotional problems and addictions had been sorted out when I was a lot younger, say 11 or 12, I wouldn’t still be obese and putting a strain on the NHS.
A full list of responses for this question can be seen in Appendix 9.
The second open question was ‘Please tell us anything else you want to about young people and weight management’. This also drew a range of interesting responses, including the following:
It’s not as simple as eating right and exercise. Eating is a very emotional issue and should be treated with respect and dignity.
There needs to be more education for young people around weight gain, weight loss and being healthy. Lots of young people aren’t aware of facts surrounding how to safely lose weight and the impact diets can have on their health.
There were also personal stories, such as:
I have found it very difficult to lose weight as my parents were overweight and were not totally aware of how to reduce the risk of childhood obesity, which means that I have had the wrong building box to begin with. I have been going to the gym regularly and have been with a weight loss group for the last 3 years but cannot keep my focus and seem to be putting weight on faster than I lose it.
All responses for this second question can be seen in Appendix 9.
Social media messages [Twitter (www.twitter.com; Twitter, Inc., San Francisco, CA, USA)]
In addition to disseminating the results of the workshop at the national event, the AYPH also tweeted daily messages from the PROMISE participation findings for 30 days in the run-up to the national event disseminating the PROMISE programme results, on the day of the event and in the follow-up. The hashtag #promiseresearch was used, as agreed with the PROMISE team. A sample of these tweets can be seen in Appendix 10.
Summary of findings from young people’s and parents’ participation
The findings from the young people’s and parent’s participation work and the online survey showed that weight loss programmes needed to acknowledge young people’s emotional and mental health needs. There also needs to be support for parents and families in terms of information and advice.
It emerged that there needed to be recognition of the barriers to seeking help among obese and very overweight young people. These included the fear of being judged, not wanting to worry parents or to bother health professionals and not wanting to tell friends because they worried that they would lose them or suffer bullying.
The implications for service delivery included the need for better publicity for weight loss programmes (including social media), more options in non-medical settings and support around results from the NCMP. There was a call for a choice of weight loss methods as well as age-appropriate activities for this age group. Group or peer support was identified as being important for both young people and their parents.
The above findings are being prepared as a programme report for young people and families (being prepared at time of writing).
Chapter 8 Modelling the burden of child and adolescent obesity on the health services in England
Parts of this chapter (specifically tables) were reproduced from Viner et al. 344
Licence to reproduce number (BMJ Publishing Group Ltd): 4675970161737.
As part of examining the implications of PROMISE findings across the childhood obesity pathway, we searched for data describing the burden of obesity for the health services. We identified no systematic study of the burden of childhood obesity for the health services, so undertook the analyses described in this chapter.
Background
Estimates of the burden of child and adolescent obesity on the health services have focused on future burden due to obesity-related diseases. 316 However, the contemporary burden in terms of the numbers of obese CYP and proportions with current comorbidities who potentially require assessment and/or treatment is unclear. This is essential for resource allocation and to plan services.
Community-based estimates of the prevalence of child and adolescent obesity in England are based largely on the NCMP for children aged 4–5 and 10–11 years and estimates from the annual Health Surveys for England for children aged 2–18 years. NCMP estimates use population thresholds for obesity (BMI ≥ 95th centile on the UK 1990 growth reference) rather than the threshold of the 98th centile for BMI used clinically on growth charts and recommended for clinical use in guidance from NICE. 345 The childhood obesity pathway outlined by NICE extends from assessment in primary care, and lifestyle modification management in the community and steps up to assessment in secondary or tertiary care for more specialised interventions including anti-obesity drugs or bariatric surgery (see Figure 35). 12
Surveillance estimates such as those from the NCMP are essential for planning preventative responses, but do not allow mapping of burden to various levels of the health service, particularly as eligibility for second-line treatments and specialist care for obese CYP rest on the presence of obesity comorbidities and/or more extreme levels of obesity.
It is unclear how many obese CYP are currently seen in primary or secondary care in the UK.
Prevalence estimates do not reflect burden on primary care, as help-seeking among families with obese CYP is low. Limited qualitative data from the UK suggest that parents of obese children often seek help late and can be reluctant to seek help from GPs because of concerns about being blamed for their child’s obesity or the consultation having a negative impact on their child’s mental well-being. 346 Parents also differ from health professionals in their perception of their child’s weight status. 44 The only available estimates come from our large-scale evaluation of the NCMP, which found that attendance at a GP practice owing to concerns about their child’s weight was reported by 6% of parents of overweight children and 15% of parents of obese children. 340 A recent study using the large Clinical Practice Research Datalink suggested that the prevalence of obesity among CYP measured in primary care from 1994 to 2013 was similar to the NCMP and HSE estimates,15 yet this study provided no information as to why children were measured, and was therefore uninformative about the proportion of obese CYP consulting for obesity.
Routine data on use of secondary care by obese CYP are limited to data on hospital admissions. A study of inpatient activity in England related to obesity showed a fourfold increase in admissions from 2000 to 2009, with approximately 79 per million admissions with obesity as the primary cause and 335 per million as a secondary cause among 15- to 19-year-olds in 2009. 347 During the same period the number of bariatric surgery operations for obesity in children aged ≤ 19 years in NHS practice increased from 1 per year to 31. 347 It is likely that numbers of CYP eligible for bariatric surgery are much higher than this, as has been shown for adults in a recent modelling study using Health Survey for England data. 348 Yet, given that most obese CYP are healthy and that obesity comorbidities are largely treatable in outpatients, inpatient data will quantify only a small proportion of the burden of obesity for secondary and tertiary care services. However, diagnostic coding of routine outpatient visits in England is currently insufficient to assess this burden.
Our aim was to assess the numbers of obese CYP eligible for assessment and management at each stage of the childhood obesity pathway in England and the proportions with different comorbidities known to be associated with obesity, using criteria from national guidance (Figure 35) and data from the nationally representative Health Survey for England (HSE) for the years 2006 to 2013. A secondary aim was to examine the sociodemographic profile of eligible CYP. Our hypothesis was that there is a major mismatch between current use of health services and those potentially eligible for obesity treatment, and our target population comprised only obese CYP.
FIGURE 35.
The NICE pathway: managing children and young people who are overweight or obese.
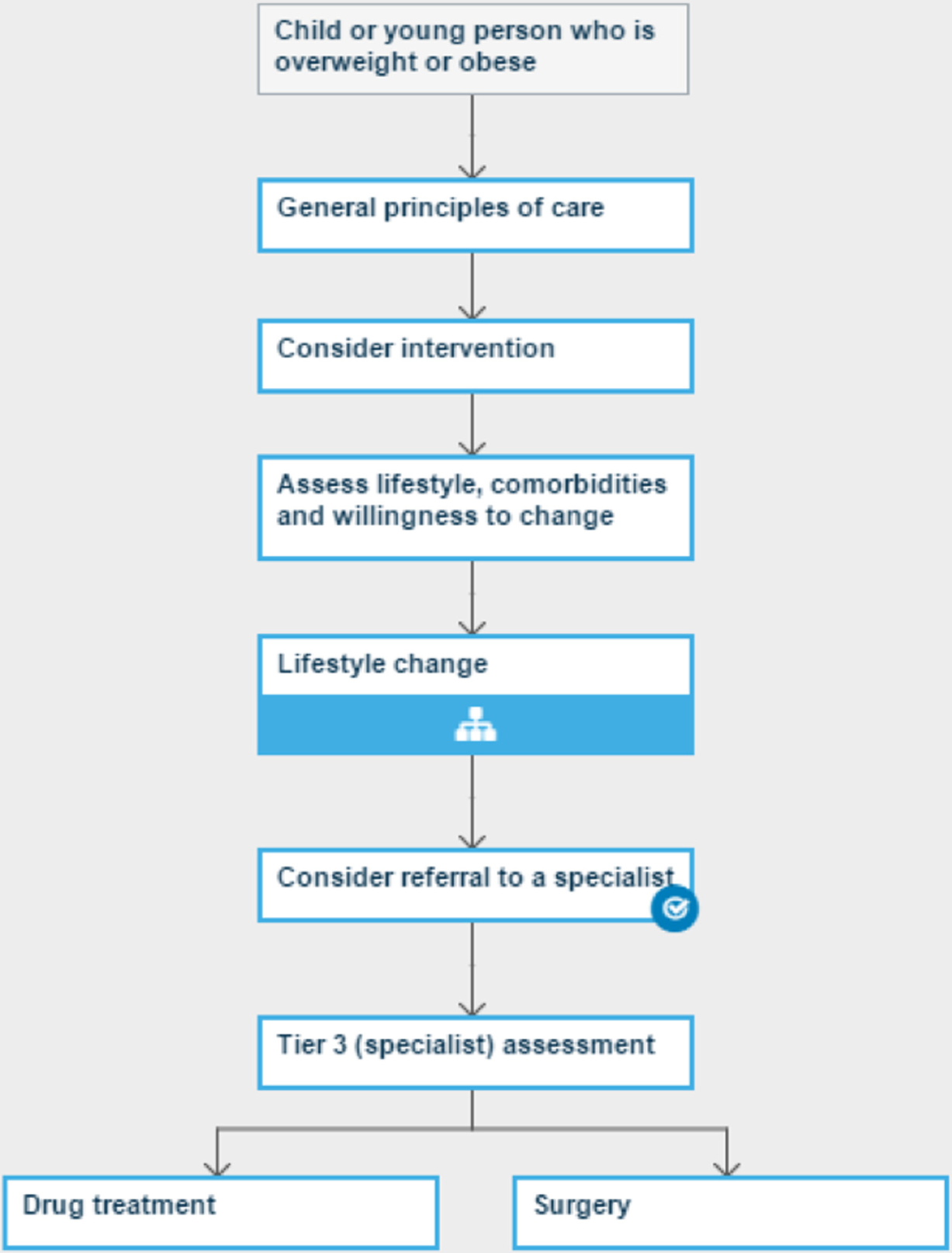
Methods
We created a model of the UK child population to identify the potential burden on different tiers of NHS services if all children who were eligible were assessed and treated under current guidance for best practice. We used the two main sources of guidance used by UK health services: (1) NICE guidance,12 the main source of guidance on the assessment and management of obesity in children and adolescents in the UK; and (2) an expert consensus document on the assessment of childhood obesity in secondary care, produced in 2012 by the Obesity Services for Children and Adolescents (OSCA) group349 allied to the Royal College of Paediatrics and Child Health.
Our main outcomes were estimates of proportions of the total CYP population eligible for obesity assessment or treatment at each service tier and resultant numbers of eligible obese children when applied to the population of England. To assess variation in burden, which may have implications for health services planning, we also present prevalences by deprivation quintile, ethnicity and region.
Data on obesity and comorbidities were obtained from the HSE for each year from 2006 to 2013 (the latest available at time of access), downloaded from the UK Data Service on 26 November 2015. 350 The sequential years 2006–13 of the survey were analysed together, taking account of survey design effects including weighting, stratification and clustering for each year, as undertaken previously. 233 Each survey year provided a sample of approximately 2500 CYP aged 2–18 years, with BMI available from measured height and weight. Each year had a different focus (e.g. 2006 focused on cardiovascular disease) and data were collected using a different mix of interviews, nurse assessments and venepuncture in each year; therefore, data availability for comorbidities varied between years. Venesection and, therefore, data on dyslipidaemia and high HbA1c levels are available only in 16- to 18-year-olds. Blood pressure was measured in 15- to 18-year-olds. Table 40 shows data availability for BMI, comorbidities and family history by year.
| Characteristics | Presentation to general practice | Referral to secondary care | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Psychological distress | Family history of CVD or T2DM | Either distress or family history | Hypertension | Musculoskeletal problems | Any comorbidity/family history | Any comorbidity or extreme obesity | ||||||||
| Total number | 7714 | 18,383 | 6505 | 7965 | 5917 | 11,205 | 11,205 | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Age (years) | ||||||||||||||
| 2–9 | 0.5 | 0.3 to 0.7 | 0.9 | 0.7 to 1.1 | 1.1 | 0.6 to 2.2 | 3.1 | 2.5 to 3.9 | 4.6 | 3.8 to 5.6 | ||||
| 10–14 | 3 | 2.0 to 4.4 | 1.2 | 0.9 to 1.6 | 3.1 | 2.1 to 4.7 | 1.6 | 1.2 to 2.1 | 3 | 1.7 to 5.0 | 4.2 | 3.3 to 5.3 | 5.5 | 4.4 to 6.8 |
| 15–18 | 2.5 | 1.8 to 3.4 | 1.3 | 1.0 to 1.7 | 2.4 | 1.7 to 3.3 | 1.2 | 0.9 to 1.7 | 1.3 | 0.8 to 1.9 | 3.8 | 3.0 to 4.8 | 5.2 | 4.2 to 6.4 |
| Total | 2.6 | 2.0 to 3.4 | 0.9 | 0.8 to 1.1 | 2.6 | 2.0 to 3.4 | 1.2 | 1.0 to 1.4 | 1.5 | 1.1 to 2.1 | 3.7 | 3.2 to 4.3 | 5.1 | 4.5 to 5.8 |
| Deprivation quintile | ||||||||||||||
| 5 (least deprived) | 1.8 | 1.0 to 3.1 | 0.6 | 0.3 to 0.9 | 1.3 | 0.7 to 2.4 | 0.7 | 0.5 to 1.1 | 1.6 | 0.9 to 2.9 | 2.5 | 1.8 to 3.6 | 3.2 | 2.3 to 4.5 |
| 4 | 2.3 | 1.3 to 4.3 | 0.7 | 0.4 to 1.0 | 2.1 | 1.2 to 3.8 | 0.9 | 0.6 to 1.4 | 0.7 | 0.4 to 1.4 | 2.7 | 1.9 to 3.8 | 3.6 | 2.6 to 5.0 |
| 3 | 2.3 | 1.2 to 4.1 | 0.6 | 0.4 to 1.1 | 1.7 | 0.9 to 3.2 | 1 | 0.7 to 1.6 | 1.3 | 0.5 to 3.1 | 3.3 | 2.4 to 4.6 | 4.8 | 3.7 to 6.3 |
| 2 | 3.5 | 2.0 to 5.9 | 1.2 | 0.9 to 1.8 | 3.7 | 2.2 to 6.1 | 1.5 | 1.1 to 2.2 | 1.8 | 1.0 to 3.2 | 4.9 | 3.7 to 6.4 | 6.6 | 5.2 to 8.5 |
| 1 (most deprived) | 3.4 | 2.0 to 5.5 | 1.5 | 1.0 to 2.1 | 4.1 | 2.4 to 6.9 | 1.6 | 1.2 to 2.2 | 2.2 | 1.2 to 4.1 | 5.3 | 4.0 to 6.9 | 7.5 | 5.9 to 9.4 |
| Total | 2.6 | 2.0 to 3.4 | 0.9 | 0.8 to 1.1 | 2.6 | 2.0 to 3.4 | 1.2 | 1.0 to 1.4 | 1.5 | 1.1 to 2.1 | 3.7 | 3.2 to 4.3 | 5.1 | 4.5 to 5.8 |
| Ethnicity | ||||||||||||||
| White | 2.7 | 2.1 to 3.6 | 0.9 | 0.7 to 1.1 | 2.4 | 1.8 to 3.2 | 1.2 | 1.0 to 1.5 | 1.6 | 1.1 to 2.2 | 3.6 | 3.1 to 4.3 | 5 | 4.4 to 5.8 |
| South Asian | 1 | 0.3 to 3.4 | 1.4 | 0.8 to 2.5 | 3.3 | 1.6 to 6.7 | 1.1 | 0.6 to 1.9 | 1 | 0.3 to 2.9 | 4 | 2.7 to 6.0 | 5.4 | 3.6 to 8.0 |
| Black | 3.2 | 1.3 to 7.5 | 0.7 | 0.3 to 1.8 | 4.7 | 1.9 to 11.2 | 1.1 | 0.5 to 2.3 | 0 | 3.5 | 1.9 to 6.5 | 5.7 | 3.4 to 9.5 | |
| Mixed ethnicity | 5.1 | 1.6 to 14.8 | 1.1 | 0.4 to 3.0 | 4.3 | 1.0 to 17.0 | 0.7 | 0.3 to 1.7 | 3.3 | 0.9 to 10.9 | 4.5 | 2.3 to 8.5 | 5.7 | 3.1 to 10.1 |
| Other | 0 | 1.2 | 0.3 to 5.1 | 2.5 | 0.2 to 24.8 | 1.3 | 0.3 to 6.2 | 2 | 0.1 to 27.7 | 4.2 | 1.1 to 14.2 | 4.2 | 1.1 to 14.2 | |
| Total | 2.6 | 2.0 to 3.4 | 0.9 | 0.8 to 1.1 | 2.6 | 2.0 to 3.4 | 1.2 | 1.0 to 1.4 | 1.5 | 1.1 to 2.1 | 3.7 | 3.2 to 4.3 | 5.1 | 4.5 to 5.8 |
| Region | ||||||||||||||
| North East | 2.1 | 0.8 to 5.4 | 0.8 | 0.4 to 1.7 | 1.8 | 0.5 to 6.1 | 1.3 | 0.8 to 2.2 | 1.5 | 0.5 to 4.5 | 3.6 | 2.3 to 5.5 | 6.4 | 4.2 to 9.6 |
| North West | 3.2 | 1.6 to 6.2 | 1 | 0.6 to 1.6 | 2.4 | 1.3 to 4.4 | 1.2 | 0.7 to 1.9 | 1.3 | 0.6 to 3.0 | 3.6 | 2.5 to 5.3 | 5.4 | 3.8 to 7.5 |
| Yorkshire and Humber | 0.9 | 0.3 to 2.6 | 1.2 | 0.8 to 2.0 | 1.4 | 0.6 to 3.1 | 2 | 1.3 to 3.1 | 2.5 | 0.9 to 6.7 | 5 | 3.3 to 7.4 | 7 | 4.8 to 9.9 |
| East Midlands | 2.9 | 1.4 to 6.2 | 0.8 | 0.4 to 1.6 | 1.2 | 0.4 to 3.3 | 1.1 | 0.6 to 1.8 | 1.3 | 0.4 to 4.2 | 2.9 | 1.7 to 4.8 | 3.5 | 2.1 to 5.5 |
| West Midlands | 2.5 | 1.2 to 5.3 | 1.1 | 0.6 to 1.9 | 4.2 | 2.2 to 7.8 | 1.1 | 0.6 to 1.9 | 1.7 | 0.9 to 3.3 | 3.9 | 2.6 to 5.8 | 5.4 | 3.7 to 7.8 |
| East of England | 4.5 | 2.3 to 8.5 | 1 | 0.6 to 1.8 | 4.4 | 2.3 to 8.2 | 1.1 | 0.6 to 2.0 | 1.7 | 0.7 to 3.6 | 4.2 | 2.8 to 6.4 | 5.6 | 3.7 to 8.3 |
| London | 2.8 | 1.4 to 5.5 | 1.1 | 0.6 to 1.9 | 3.3 | 1.5 to 7.2 | 0.7 | 0.4 to 1.3 | 1.1 | 0.4 to 3.0 | 3 | 2.0 to 4.5 | 4.4 | 3.1 to 6.1 |
| South East | 2.4 | 1.3 to 4.6 | 0.5 | 0.3 to 1.0 | 1.6 | 0.7 to 3.9 | 1.1 | 0.6 to 1.8 | 1.5 | 0.7 to 3.2 | 3.4 | 2.2 to 5.1 | 4 | 2.9 to 5.6 |
| South West | 2.1 | 1.0 to 4.3 | 0.8 | 0.4 to 1.4 | 2.6 | 1.3 to 5.1 | 1.3 | 0.8 to 2.0 | 1.4 | 1.0 to 2.0 | 3.8 | 2.5 to 5.6 | 5.3 | 3.8 to 7.4 |
| Total | 2.6 | 2.0 to 3.4 | 0.9 | 0.8 to 1.1 | 2.6 | 2.0 to 3.4 | 1.2 | 1.0 to 1.4 | 1.5 | 1.1 to 2.1 | 3.7 | 3.2 to 4.3 | 5.1 | 4.5 to 5.8 |
Anthropometry
Height and BMI data were obtained from data reported as ‘valid’ in each HSE survey after implausible values were removed. Sample sizes are shown in Table 2. Overall, the proportion with valid BMI was 80%. Age- and sex-standardised z-scores were obtained using the zanthro command in Stata, which uses Cole’s LMS method65 to calculate z-scores using the UK 1990 growth reference. As age was given only to integer year, z-scores were calculated based on mid-year of age.
Obesity was defined as clinical obesity (BMI ≥ 98th centile),345 obesity according to the International Obesity Taskforce threshold (IOTF) (equivalent to a BMI of 30 kg/m2 at age 18 years) and two more extreme categories. We followed Ells et al. 90 in defining extreme obesity (BMI ≥ 99.86th centile, i.e. a BMI of ≥ 3 SDs, equivalent to an adult BMI of ≥ 35 kg/m2, so-called class 2 obesity) and morbid obesity (BMI ≥ 99.98th centile, i.e. a BMI of ≥ 3.5 SDs, equivalent to adult class 3 obesity, i.e. a BMI of ≥ 40 kg/m2). Table 41 has a summary of these categories. Note that each category includes more extreme categories of obesity [e.g. extreme (class 2) obesity includes those with morbid (class 3) obesity].
| Type of obesity | BMI centile | Adult equivalent at age 18 years or older |
|---|---|---|
| Clinical obesity | BMI ≥ 98th centile | N/A |
| IOTF obesity | BMI over IOTF threshold. Equivalent to 99th centile | Class 1 obesity, i.e. a BMI of ≥ 30 kg/m2 |
| Extreme obesity | BMI ≥ 99.86th centile, i.e. 3 SDs | Class 2 obesity, i.e. a BMI of ≥ 35 kg/m2 |
| Morbid obesity | BMI ≥ 99.98th centile, i.e. 3.5 SDs | Class 3 obesity, i.e. a BMI of ≥ 40 kg/m2 |
Other measures
The majority of the following were assessed in most but not all years from 2006 to 2013.
Family history of cardiovascular disease or type 2 diabetes mellitus
This was obtained from self-report from adults (> 18 years) sharing the same household code as the child. Any report of cardiovascular disease (self-report of ‘stroke’, ‘heart attack or angina’ or ‘other heart problem’) or T2DM (self-report of DM either diagnosed over age 35 years or not treated with insulin) in a household member was taken to be a proxy for family history, given that most children live with family members.
Obesity comorbidities
-
Psychological distress: measured in those aged ≥ 13 years by self-report using the General Health Questionnaire (GHQ), a commonly used measure of current psychological distress. High scorers were defined using the standard threshold of a score of ≥ 4 indicating high risk for psychological caseness. 350
-
Musculoskeletal or mobility problems: self-report of significant musculoskeletal or mobility problems.
-
Hypertension: blood pressure was measured by nurses using a standardised protocol and an electronic instrument (OMRON). Systolic blood pressure only was used in these analyses. Hypertension was defined as a systolic blood pressure ≥ 95th centile for age and sex using the US National Heart, Lung and Blood Institute norms. 351
-
Dyslipidaemia: this was defined from available lipid data (total cholesterol and HDL cholesterol) on those aged 16–18 years who underwent venesection. Dyslipidaemia was defined as either high total cholesterol (≥ 95th centile) or low HDL cholesterol (≤ 5th centile) for age and sex from the American Academy of Pediatrics Clinical Report on Lipid Screeening in Children. 75
-
Elevated HbA1c: data on elevated HbA1c levels, defined as ≥ 6.0% and, therefore, consistent with pre-DM or DM, were available only in 16- to 18-year-olds. 352
-
Self-report of cardiovascular disease: self-report of suffering from angina, heart attack, heart disease or stroke.
Each of the above comorbidities had a different sample size depending on whether data were drawn from interviews, psychological scales or measurements (e.g. blood pressure) or blood samples, and because different outcomes were assessed in different years. Because of this, to calculate the proportion of CYP with any comorbidity, we include only those with a maximum of missing data on one comorbidity.
Findings
Potential burden for the health services
Table 42 shows proportions of CYP with clinical obesity (BMI ≥ 98th centile) and significant obesity (IOTF) by age, deprivation and ethnicity for 2010–13. Overall 11% of CYP had clinical obesity and 7% had significant obesity, both numbers increasing with age and showing dramatic social gradients.
| Characteristics | Clinical obesity: BMI ≥ 98th centile | IOTF threshold of obesity (approximately 99th centile) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Age (years) | ||||||||||||
| 2 (n = 474) | 5.9 | 2.9 to 11.6 | 3.6 | 1.7 to 7.5 | 4.8 | 2.8 to 8.1 | 3.8 | 1.6 to 8.8 | 3.1 | 1.4 to 6.9 | 3.5 | 1.9 to 6.4 |
| 3 (n = 445) | 5.5 | 2.8 to 10.6 | 8.7 | 3.7 to 19.4 | 7.2 | 3.8 to 12.9 | 5.1 | 2.4 to 10.5 | 4.8 | 2.9 to 7.8 | 5 | 3.1 to 7.8 |
| 4 (n = 494) | 6.2 | 3.4 to 11.0 | 4.8 | 2.5 to 8.8 | 5.5 | 3.6 to 8.2 | 4 | 1.8 to 8.5 | 3.8 | 1.8 to 7.9 | 3.9 | 2.3 to 6.5 |
| 5 (n = 451) | 7 | 3.3 to 14.1 | 6.5 | 4.1 to 10.2 | 6.8 | 4.3 to 10.5 | 3.9 | 1.7 to 8.6 | 6.2 | 3.9 to 9.7 | 5 | 3.3 to 7.6 |
| 6 (n = 449) | 12.5 | 8.1 to 18.9 | 8.4 | 4.8 to 14.5 | 10.6 | 7.4 to 14.9 | 8 | 4.5 to 13.9 | 8.4 | 4.8 to 14.5 | 8.2 | 5.4 to 12.3 |
| 7 (n = 474) | 13 | 8.2 to 19.9 | 12.6 | 8.3 to 18.6 | 12.8 | 9.2 to 17.4 | 9 | 5.0 to 15.7 | 12.2 | 8.0 to 18.2 | 10.6 | 7.3 to 15.0 |
| 8 (n = 428) | 10.7 | 6.9 to 16.2 | 6.6 | 3.6 to 11.5 | 8.8 | 6.0 to 12.5 | 5.3 | 2.9 to 9.5 | 6 | 3.2 to 10.8 | 5.6 | 3.6 to 8.7 |
| 9 (n = 429) | 13.9 | 9.1 to 20.7 | 14.2 | 9.2 to 21.3 | 14.1 | 10.6 to 18.5 | 7.1 | 3.6 to 13.6 | 11.3 | 6.8 to 18.4 | 9.2 | 6.3 to 13.3 |
| 10 (n = 445) | 9.9 | 6.4 to 15.1 | 13.3 | 8.2 to 21.0 | 11.6 | 8.2 to 16.2 | 5.2 | 2.8 to 9.5 | 6.7 | 3.3 to 12.9 | 5.9 | 3.6 to 9.6 |
| 11 (n = 457) | 16.8 | 12.1 to 23.0 | 7.4 | 4.5 to 11.9 | 11.9 | 8.8 to 15.8 | 7.8 | 4.6 to 13.0 | 4.4 | 2.4 to 8.0 | 6 | 4.0 to 9.1 |
| 12 (n = 428) | 19.1 | 12.9 to 27.4 | 16.9 | 11.2 to 24.7 | 18 | 13.4 to 23.7 | 7.7 | 4.5 to 13.1 | 11.3 | 6.8 to 18.1 | 9.6 | 6.4 to 14.0 |
| 13 (n = 452) | 18.4 | 12.2 to 26.8 | 13.4 | 8.8 to 19.9 | 15.9 | 11.4 to 21.6 | 8.9 | 4.9 to 15.6 | 8.1 | 4.8 to 13.4 | 8.5 | 5.6 to 12.7 |
| 14 (n = 464) | 11 | 6.9 to 17.1 | 12 | 6.8 to 20.4 | 11.5 | 7.9 to 16.3 | 7.4 | 4.0 to 13.2 | 8.1 | 3.9 to 16.3 | 7.7 | 4.7 to 12.4 |
| 15 (n = 496) | 11.7 | 7.6 to 17.5 | 11 | 7.0 to 16.9 | 11.4 | 8.1 to 15.7 | 6.7 | 3.5 to 12.7 | 5.5 | 2.9 to 10.2 | 6.2 | 3.8 to 10.0 |
| 16 (n = 498) | 13.5 | 8.5 to 20.6 | 10.1 | 6.0 to 16.7 | 12 | 8.3 to 16.9 | 7.6 | 3.9 to 14.3 | 5.8 | 2.7 to 12.0 | 6.8 | 4.0 to 11.3 |
| 17 (n = 449) | 10.7 | 6.3 to 17.5 | 13.9 | 8.0 to 23.0 | 12.3 | 8.3 to 17.9 | 6.9 | 3.6 to 12.9 | 11.3 | 5.8 to 20.8 | 9.1 | 5.6 to 14.6 |
| 18 (n = 440) | 13 | 7.7 to 21.2 | 12.6 | 8.0 to 19.3 | 12.9 | 8.9 to 18.2 | 10.1 | 5.7 to 17.4 | 10 | 6.2 to 15.9 | 10.1 | 6.8 to 14.8 |
| Total (n = 7773) | 11.8 | 10.5 to 13.3 | 10.6 | 9.2 to 12.0 | 11.2 | 10.2 to 12.3 | 6.9 | 5.9 to 8.1 | 7.6 | 6.5 to 8.8 | 7.2 | 6.4 to 8.1 |
| Deprivation quintile | ||||||||||||
| 5 (least deprived) (n = 1708) | 9.1 | 6.6 to 12.4 | 5.9 | 4.2 to 8.3 | 7.6 | 6.0 to 9.7 | 5.6 | 3.6 to 8.6 | 3.9 | 2.5 to 6.0 | 4.8 | 3.5 to 6.7 |
| 4 (n = 1522) | 8.5 | 6.2 to 11.7 | 8 | 5.7 to 11.3 | 8.3 | 6.4 to 10.6 | 4.8 | 3.1 to 7.6 | 5.3 | 3.4 to 8.1 | 5.1 | 3.6 to 7.0 |
| 3 (n = 1472) | 11.6 | 8.9 to 15.0 | 10.9 | 8.3 to 14.2 | 11.2 | 9.2 to 13.7 | 6.6 | 4.6 to 9.3 | 7.8 | 5.7 to 10.6 | 7.2 | 5.6 to 9.2 |
| 2 (n = 1483) | 12.4 | 9.5 to 16.0 | 14 | 10.6 to 18.2 | 13.2 | 10.8 to 15.9 | 6.6 | 4.5 to 9.5 | 11.1 | 8.1 to 15.0 | 8.7 | 6.8 to 11.2 |
| 1 (most deprived) (n = 1588) | 17.6 | 14.2 to 21.5 | 13.9 | 10.7 to 17.9 | 15.8 | 13.2 to 18.7 | 10.9 | 8.3 to 14.1 | 10 | 7.5 to 13.0 | 10.4 | 8.5 to 12.7 |
| Total (n = 7773) | 11.8 | 10.5 to 13.3 | 10.6 | 9.2 to 12.0 | 11.2 | 10.2 to 12.3 | 6.9 | 5.9 to 8.1 | 7.6 | 6.5 to 8.8 | 7.2 | 6.4 to 8.1 |
| Ethnicity | ||||||||||||
| White (n = 6299) | 11.5 | 10.1 to 13.2 | 9.9 | 8.5 to 11.5 | 10.8 | 9.7 to 11.9 | 7.2 | 6.0 to 8.6 | 6.8 | 5.7 to 8.1 | 7 | 6.1 to 8.0 |
| South Asian (n = 713) | 9.9 | 6.3 to 15.2 | 11.2 | 7.4 to 16.7 | 10.6 | 7.5 to 14.7 | 3.7 | 2.1 to 6.4 | 9.7 | 6.2 to 14.9 | 6.8 | 4.5 to 10.1 |
| Black (n = 324) | 15.6 | 10.3 to 23.0 | 13 | 7.6 to 21.3 | 14.2 | 10.0 to 19.9 | 7.2 | 3.6 to 13.9 | 7.6 | 4.4 to 12.8 | 7.4 | 4.6 to 11.6 |
| Mixed ethnicity (n = 354) | 12.8 | 7.4 to 21.4 | 14.9 | 8.0 to 26.2 | 13.9 | 8.9 to 20.9 | 6.9 | 3.4 to 13.6 | 13.8 | 7.0 to 25.5 | 10.4 | 6.1 to 17.2 |
| Other (n = 78) | 30.4 | 12.9 to 56.3 | 21.5 | 12.1 to 35.3 | 26.2 | 14.7 to 42.2 | 15.8 | 5.2 to 39.1 | 17.2 | 10.4 to 27.2 | 16.5 | 9.3 to 27.6 |
| Total (n = 7768) | 11.8 | 10.5 to 13.3 | 10.6 | 9.2 to 12.1 | 11.2 | 10.2 to 12.3 | 6.9 | 5.9 to 8.1 | 7.6 | 6.5 to 8.8 | 7.2 | 6.4 to 8.1 |
| Region | ||||||||||||
| North East (n = 602) | 16.4 | 11.1 to 23.5 | 12.4 | 6.7 to 21.8 | 14.6 | 10.3 to 20.2 | 8.4 | 4.8 to 14.3 | 11.3 | 5.8 to 20.9 | 9.7 | 6.2 to 15.0 |
| North West (n = 1075) | 14.3 | 10.3 to 19.5 | 10.9 | 7.8 to 15.1 | 12.6 | 9.8 to 16.1 | 9.9 | 6.4 to 14.9 | 7.2 | 4.7 to 10.9 | 8.5 | 6.1 to 11.8 |
| Yorkshire and The Humber (n = 751) | 11.9 | 8.2 to 16.9 | 11 | 7.2 to 16.4 | 11.5 | 8.4 to 15.4 | 7.6 | 4.6 to 12.5 | 9.8 | 6.4 to 14.8 | 8.6 | 6.0 to 12.3 |
| East Midlands (n = 711) | 13.6 | 9.5 to 19.1 | 7.6 | 4.5 to 12.5 | 10.7 | 7.8 to 14.6 | 6.3 | 3.8 to 10.3 | 5.8 | 3.2 to 10.2 | 6.1 | 4.0 to 9.1 |
| West Midlands (n = 815) | 9.5 | 6.4 to 13.9 | 9.8 | 6.7 to 13.9 | 9.6 | 7.3 to 12.6 | 7.1 | 4.5 to 11.0 | 6.2 | 4.1 to 9.2 | 6.7 | 4.9 to 9.1 |
| East of England (n = 850) | 11.3 | 7.8 to 16.1 | 13.4 | 9.1 to 19.2 | 12.3 | 9.3 to 16.2 | 7.5 | 4.7 to 11.6 | 8.3 | 5.0 to 13.4 | 7.9 | 5.6 to 11.0 |
| London (n = 1032) | 12.3 | 9.0 to 16.6 | 10.2 | 7.0 to 14.5 | 11.2 | 8.9 to 13.8 | 5.3 | 3.3 to 8.5 | 6.1 | 4.2 to 8.6 | 5.7 | 4.2 to 7.7 |
| South East (n = 1238) | 7.7 | 5.3 to 10.9 | 9.8 | 7.1 to 13.4 | 8.7 | 6.8 to 11.1 | 3.2 | 1.8 to 5.5 | 8.4 | 5.8 to 11.9 | 5.8 | 4.2 to 7.9 |
| South West (n = 699) | 13.6 | 9.5 to 19.2 | 11.1 | 6.8 to 17.6 | 12.5 | 9.4 to 16.4 | 9.2 | 5.9 to 14.2 | 8.2 | 4.5 to 14.5 | 8.8 | 6.0 to 12.6 |
| Total (n = 7773) | 11.8 | 10.5 to 13.3 | 10.6 | 9.2 to 12.0 | 11.2 | 10.2 to 12.3 | 6.9 | 5.9 to 8.1 | 7.6 | 6.5 to 8.8 | 7.2 | 6.4 to 8.1 |
Burden for primary care
The NICE guidance12 suggests that CYP who are overweight (i.e. with a BMI ≥ 91st centile) or clinically obese (i.e. with a BMI ≥ 98th centile) can be assessed and have management planned in primary care (NICE 2014, recommendation 1.2.13). Those with clinical obesity should have obesity comorbidities assessed (NICE 2014, recommendation 1.3.8).
The OSCA guidance suggests that it is appropriate for GPs to assess CYP with moderate obesity (i.e. with a BMI ≥ 98th centile) where there are significant concerns, including psychological distress or long-term conditions such as asthma. 349
Evidence suggests that families and young people themselves are most likely to present to GPs when they have concerns about their health relating to obesity, particularly when there is a family history of obesity-related conditions (e.g. stroke, heart disease or T2DM), and when parents are concerned that children are suffering significant distress related to obesity. 346 There is some evidence that parental concerns about medical causes or consequences of obesity arise when children have BMIs ≥ 85th centile,353 suggesting that such concerns are frequent in those with clinical obesity.
Table 40 shows proportions with clinical obesity (BMI ≥ 98th centile) plus either significant psychological distress (GHQ score above threshold) or a family history of cardiovascular disease or T2DM. Proportions in these categories are shown by age group, deprivation quintile, ethnicity and region. Some 2.6% of the CYP population aged ≥ 13 years have obesity and psychological distress, 0.9% have obesity and a positive family history and overall 2.6% have obesity and either distress or a family history.
Community lifestyle modification programmes
The National Institute for Health and Care Excellence354 recommends that multicomponent lifestyle modification programmes are the gold standard for management of obesity in CYP (recommendation 1.4.1), but provides no more restrictive eligibility criteria other than obesity. These programmes are widely provided in the community catering for 5- to 12-year-olds or 13- to 18-year-olds, with younger children thought to be better suited to parenting programmes rather than weight management per se. Those eligible for such programmes are those obese at either the clinical (98th centile) level (i.e. 10–12% of those over 5 years) or 7–9% using the IOTF threshold.
Referral to secondary care
The National Institute for Health and Care Excellence recommends that assessment of physical, psychological and social comorbidities should be considered in obese children in primary care but provides no detail as to which should be referred onwards to secondary care. OSCA guidance suggests that obese CYP suitable for referral to secondary care are either (1) those with clinical obesity plus known comorbidities; (2) those with clinical obesity and potential red flags for possible secondary causes of obesity); (3) those with clinical obesity and a strong family history of cardiovascular disease or T2DM; or (4) those with extreme obesity, regardless of other factors.
A wide range of comorbid conditions are related to obesity, from those easily assessed in primary care (hypertension, orthopaedic or mobility problems, a history of significant bullying or psychological distress) to those unlikely to be assessed by most GPs (dyslipidaemia, hyperinsulinaemia, high HbA1c levels, abnormal liver function, which all require venesection and interpretation of results according to age- and sex-specific centiles). The degree to which hypertension is assessed in primary care is unclear, given that children’s blood pressure measurement requires smaller cuffs and interpretation against age and sex centiles.
The prevalence of clinical obesity together with various obesity comorbidities is shown in Table 40. In addition to psychological distress as discussed above, the table also shows prevalence of obesity plus hypertension, musculoskeletal problems and mobility problems. The group eligible for referral to secondary care (i.e. those with obesity and any comorbidity likely to be detected in primary care or who have a family history of obesity-related disorders) make up 3.7% of the CYP population, highest in the most deprived quintile (5.3%).
Those with extreme or morbid obesity are eligible to be referred to secondary care paediatrics regardless of comorbidities or family history. Table 43 shows proportions with extreme obesity and morbid obesity (BMI ≥ 99.98th centile) by age group, deprivation, ethnicity and region, again for 2006–13. Overall, 2.7% had extreme obesity and 0.9% had morbid obesity. Again, prevalence was highest in the most deprived quintile (3.6% and 1.2%, respectively), in South Asian ethnicity (4.3% and 1.1%) and in London and North East and North West regions.
| Characteristics | Class 2 obesity BMI ≥ 99.86th centile | Class 3 obesity BMI ≥ 99.98th centile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Age (years) | ||||||||||||
| 2–9 (n = 8227) | 3.4 | 2.8 to 4.2 | 2.5 | 2.0 to 3.2 | 3.0 | 2.5 to 3.5 | 1.6 | 1.1 to 2.2 | 0.9 | 0.5 to 1.3 | 1.2 | 0.9 to 1.6 |
| 10–14 (n = 5659) | 2.3 | 1.6 to 3.2 | 2.5 | 1.8 to 3.5 | 2.4 | 1.9 to 3.1 | 0.4 | 0.2 to 0.8 | 0.4 | 0.2 to 0.9 | 0.4 | 0.3 to 0.7 |
| 15–18 (n = 2724) | 2.7 | 1.9 to 3.8 | 2.6 | 1.8 to 3.7 | 2.6 | 2.0 to 3.4 | 0.5 | 0.2 to 1.1 | 0.7 | 0.3 to 1.3 | 0.6 | 0.3 to 1.0 |
| Total (n = 16,610) | 2.9 | 2.5 to 3.4 | 2.5 | 2.1 to 3.0 | 2.7 | 2.4 to 3.1 | 0.9 | 0.7 to 1.2 | 0.7 | 0.5 to 0.9 | 0.8 | 0.6 to 1.0 |
| IMD score quintile | ||||||||||||
| 5 (least deprived) (n = 6990) | 1.9 | 1.3 to 3.0 | 1.6 | 1.0 to 2.5 | 1.8 | 1.3 to 2.5 | 0.6 | 0.3 to 1.3 | 0.4 | 0.2 to 1.0 | 0.5 | 0.3 to 1.0 |
| 4 (n = 6346) | 1.9 | 1.1 to 3.0 | 2.1 | 1.4 to 3.4 | 2.0 | 1.4 to 2.8 | 0.4 | 0.2 to 1.2 | 0.4 | 0.1 to 1.3 | 0.4 | 0.2 to 0.9 |
| 3 (n = 6383) | 3.0 | 2.2 to 4.2 | 2.2 | 1.4 to 3.3 | 2.6 | 2.0 to 3.4 | 1.0 | 0.6 to 1.8 | 0.8 | 0.4 to 1.5 | 0.9 | 0.6 to 1.4 |
| 2 (n = 6300) | 3.1 | 2.2 to 4.3 | 4 | 2.8 to 5.6 | 3.5 | 2.7 to 4.5 | 1.0 | 0.6 to 1.7 | 0.8 | 0.4 to 1.7 | 0.9 | 0.6 to 1.4 |
| 1 (most deprived) (n = 6897) | 4.4 | 3.3 to 5.8 | 2.7 | 2.0 to 3.7 | 3.6 | 2.9 to 4.4 | 1.5 | 0.9 to 2.4 | 0.9 | 0.5 to 1.7 | 1.2 | 0.8 to 1.8 |
| Total (n = 32,916) | 2.9 | 2.5 to 3.4 | 2.5 | 2.1 to 3.0 | 2.7 | 2.4 to 3.1 | 0.9 | 0.7 to 1.2 | 0.7 | 0.5 to 0.9 | 0.8 | 0.6 to 1.0 |
| Ethnicity | ||||||||||||
| White (n = 27,345) | 2.8 | 2.4 to 3.4 | 2.4 | 1.9 to 2.9 | 2.6 | 2.3 to 3.0 | 1 | 0.7 to 1.3 | 0.7 | 0.4 to 1.0 | 0.8 | 0.6 to 1.0 |
| South Asian (n = 2735) | 2.6 | 1.6 to 4.3 | 2.9 | 1.6 to 5.1 | 2.8 | 1.9 to 4.0 | 0.5 | 0.2 to 1.4 | 0.7 | 0.2 to 2.1 | 0.6 | 0.3 to 1.4 |
| Black (n = 1171) | 5.3 | 2.8 to 9.8 | 3.3 | 1.7 to 6.3 | 4.3 | 2.6 to 6.9 | 0.8 | 0.2 to 3.3 | 1.5 | 0.5 to 4.3 | 1.1 | 0.5 to 2.7 |
| Mixed ethnicity (n = 1372) | 3 | 1.6 to 5.3 | 4 | 2.1 to 7.4 | 3.5 | 2.2 to 5.4 | 1.3 | 0.6 to 3.0 | 0.4 | 0.1 to 1.8 | 0.9 | 0.4 to 1.8 |
| Other (n = 277) | 0.7 | 0.1 to 5.0 | 1.6 | 0.6 to 4.0 | 1.1 | 0.4 to 2.8 | 0 | 0 | 0 | |||
| Total (n = 32,900) | 2.9 | 2.5 to 3.4 | 2.5 | 2.1 to 3.0 | 2.7 | 2.4 to 3.1 | 0.9 | 0.7 to 1.2 | 0.7 | 0.5 to 0.9 | 0.8 | 0.6 to 1.0 |
| Region | ||||||||||||
| North East (n = 1851) | 4.0 | 2.2 to 7.3 | 3.1 | 1.7 to 5.7 | 3.6 | 2.3 to 5.6 | 1.1 | 0.4 to 3.0 | 1.1 | 0.3 to 4.0 | 1.1 | 0.5 to 2.6 |
| North West (n = 4742) | 3.7 | 2.6 to 5.3 | 2.2 | 1.4 to 3.6 | 3.0 | 2.2 to 4.0 | 1.5 | 0.9 to 2.5 | 0.8 | 0.4 to 1.7 | 1.1 | 0.7 to 1.8 |
| Yorkshire and The Humber (n = 3506) | 2.9 | 1.6 to 5.0 | 2.2 | 1.2 to 3.9 | 2.6 | 1.7 to 4.0 | 0.3 | 0.1 to 1.1 | 0.5 | 0.2 to 1.4 | 0.4 | 0.2 to 0.9 |
| East Midlands (n = 3168) | 2.7 | 1.6 to 4.6 | 2.3 | 1.3 to 3.8 | 2.5 | 1.7 to 3.7 | 1.0 | 0.5 to 2.2 | 0.4 | 0.1 to 1.1 | 0.7 | 0.4 to 1.4 |
| West Midlands (n = 3435) | 2.2 | 1.4 to 3.7 | 2.3 | 1.4 to 3.8 | 2.3 | 1.5 to 3.3 | 0.8 | 0.4 to 1.8 | 0.7 | 0.2 to 2.0 | 0.7 | 0.4 to 1.5 |
| East of England (n = 3557) | 2.6 | 1.7 to 4.0 | 3.3 | 1.9 to 5.8 | 2.9 | 2.0 to 4.2 | 1.0 | 0.5 to 2.3 | 0.2 | 0.1 to 1.1 | 0.7 | 0.3 to 1.4 |
| London (n = 4080) | 3.5 | 2.3 to 5.4 | 2.6 | 1.7 to 4.2 | 3.1 | 2.2 to 4.2 | 0.9 | 0.4 to 2.1 | 0.9 | 0.4 to 2.0 | 0.9 | 0.5 to 1.6 |
| South East (n = 4363) | 1.8 | 1.1 to 3.0 | 2.4 | 1.4 to 3.8 | 2.1 | 1.5 to 3.0 | 0.8 | 0.3 to 1.8 | 0.8 | 0.3 to 2.0 | 0.8 | 0.4 to 1.5 |
| South West (n = 4214) | 2.9 | 1.8 to 4.6 | 2.7 | 1.6 to 4.6 | 2.8 | 2.0 to 4.0 | 0.9 | 0.4 to 2.2 | 0.6 | 0.3 to 1.3 | 0.8 | 0.4 to 1.4 |
| Total (n = 32,916) | 2.9 | 2.5 to 3.4 | 2.5 | 2.1 to 3.0 | 2.7 | 2.4 to 3.1 | 0.9 | 0.7 to 1.2 | 0.7 | 0.5 to 0.9 | 0.8 | 0.6 to 1.0 |
Those with extreme obesity are likely to be a large subgroup of those with lesser degrees of obesity (i.e. clinical obesity) and primary care detectable comorbidities/family history. Combining these two groups [i.e. those who are extremely obese (or greater) or are obese and have primary care-detectable comorbidities/family history], we find that 5.3% of the CYP population are eligible for referral to secondary care (see Table 43). In the most deprived quintile this group makes up 7.5% of the population.
Anti-obesity drug treatment
The NICE criteria355 for starting orlistat, the only currently licensed anti-obesity drug, are that it is appropriate for those aged ≥ 12 years with significant physical or psychological comorbidities (recommendation 1.8.6), with initiation to be undertaken by a paediatric team with specialist weight management experience. Those eligible for orlistat are therefore those with clinical obesity together with comorbidities, both those comorbidities likely to be detected in primary care, discussed above, and comorbidities likely to be identified only in secondary care.
Comorbidities such as dyslipidaemia and abnormal glucose homeostasis (i.e. elevated HbA1c levels suggesting pre-DM or DM) are available for only 16- to 18-year-olds in a subset of HSE years. Table 44 shows the prevalence of the latter (dyslipidaemia, elevated HbA1c levels and T2DM) together with obesity in 16- to 18-year-olds by deprivation quintile (numbers were insufficient to show by ethnicity or region). Of those who were obese, 29.7% (95% CI 16.0% to 48.5%) had high cholesterol (≥ 95th centile), 23.2% (95% CI 6.6% to 53.1%) had low HDL (≤ 5th centile) and 48.8% (95% CI 31.7% to 66.1%) had either or both of these (defined as dyslipidaemia). Elevated HbA1c levels (≥ 6%) were found in 1.6% (95% CI 0.1% to 22.0%) of the obese group, although none of them reported having T2DM. Some 0.8% (95% CI 0.4% to 1.6%) of those who were obese also gave a history of cardiovascular disease (stroke or heart disease).
| Characteristics | Secondary care – detectable comorbidities | Any cardiometabolic comorbiditya (n = 1182) | Any comorbidity (n = 1381) | |||||
|---|---|---|---|---|---|---|---|---|
| Abnormal lipids levels (n = 1149) | High HbA1c level (n = 1134) | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Deprivation quintile | ||||||||
| 5 (least deprived) | 8.1 | 1.9 to 29.1 | 0.5 | 0.0 to 41.4 | 8.5 | 2.5 to 24.9 | 9.1 | 3.8 to 20.2 |
| 4 | 4.4 | 2.0 to 9.5 | 0 | 4.6 | 2.1 to 9.8 | 3.8 | 1.7 to 8.5 | |
| 3 | 5.9 | 2.2 to 14.7 | 0.3 | 0.2 to 0.4 | 7.3 | 2.9 to 17.1 | 7.4 | 3.7 to 14.4 |
| 2 | 3.7 | 1.4 to 9.3 | 0 | 4 | 1.2 to 12.3 | 4.5 | 1.4 to 13.9 | |
| 1 (most deprived) | 12.5 | 4.4 to 30.7 | 0.3 | 0.0 to 31.2 | 14.4 | 6.7 to 28.4 | 15.4 | 8.6 to 26.1 |
| Ethnicity | ||||||||
| White | 7.2 | 4.5 to 11.4 | 7.6 | 5.2 to 10.9 | ||||
| South Asian | 11.7 | 5.9 to 21.8 | 10.5 | 4.8 to 21.6 | ||||
| Black | 15.1 | 0.6 to 83.6 | 14 | 1.1 to 69.9 | ||||
| Mixed ethnicity | 3.9 | 2.2 to 6.8 | 9 | 0.5 to 64.0 | ||||
| Other | 11.4 | 6.8 to 18.6 | 9.4 | 5.9 to 14.6 | ||||
| Total | 7 | 4.1 to 11.8 | 0.2 | 0.0 to 3.4 | 7.9 | 5.1 to 11.9 | 8.2 | 5.8 to 11.4 |
Table 44 shows that 7.9% of the 16- to 18-year-olds had clinical obesity together with any cardiometabolic comorbidity (hypertension, dyslipidaemia, elevated HbA1c levels or self-report of cardiovascular disease). Prevalence was highest in the most deprived quintile, although the CIs overlapped. The prevalence of obesity with any physical or psychological comorbidity (i.e. the group eligible for anti-obesity drug treatment) was 8.2% overall, rising to 15.4% in the most deprived quintile.
Bariatric surgery
The NICE guidance12 notes that bariatric surgery is not generally recommended for CYP (recommendation 1.10.12) but it may be considered in exceptional circumstances in those who have reached physiological maturity (recommendation 1.10.13). Table 45 shows estimates of the population of CYP eligible for each stage of the obesity pathway. Criteria are otherwise as for adults [i.e. surgery is appropriate in those with BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with significant comorbidities that would be improved if they lost weight (recommendation 1.10.1)].
| Age group | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2- to 18-year-olds | 13- to 18-year-olds | |||||||
| Age group | Population 2–18 years mid-2014 | Eligible for primary care or community management | Likely to present to primary care | Eligible for referral to secondary care | Age group | Population 13–18 years mid-2014 | Eligible for anti-obesity drug therapy | Eligible for bariatric surgery |
| Obesity (BMI ≥ 98th centile) | Obesity + distress or family history | Obesity + comorbidities or extreme obesity | Obesity + any cardiometabolic or other comorbidity | Morbid obesity or extreme obesity plus comorbidities | ||||
| 2–9 years | 5,356,436 | 514,473 | 139,267 | 246,396 | ||||
| 10–14 years | 2,973,055 | 390,389 | 92,165 | 163,518 | 13–15 years | 1,828,135 | 149,907 | 45,703 |
| 15–18 years | 2,571,077 | 310,963 | 61,706 | 133,696 | 16–18 years | 1,943,898 | 159,400 | 44,710 |
| Total | 10,900,568 | 1,220,055 | 283,415 | 555,929 | Total | 3,772,033 | 309,307 | 90,529 |
Table 46 shows these criteria operationalised to identify young people aged 13–18 years who are eligible for bariatric surgery [i.e. those with (1) BMI ≥ 40 kg/m2 if aged 18 years or BMI ≥ 99.98th centile, i.e. equivalent to BMI ≥ 40 kg/m2 at age 18 years (morbid obesity); (2) those with BMI 35–40 kg/m2 if aged 18 years or BMI between 99.86th and 99.98th centile with any comorbidities; and (3) all those eligible for bariatric surgery (both 1 and 2)]. Owing to dyslipidaemia and HbA1c data being available for only 16- to 18-year-olds, comorbidities here are limited to psychological or musculoskeletal problems or hypertension.
| Characteristics | Morbid obesity (BMI ≥ 40 kg/m2 or BMI ≥ 99.98th centile) (n = 10,079) | Extreme obesity (BMI 35–39.99 kg/m2 or BMI 99.86–99.98th centile) with comorbidities (n = 10,079) | Both extreme obesity with comorbidities or morbid obesity (n = 10,079) | |||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Age (years) | ||||||
| 13–15 | 0.3 | 0.2 to 0.6 | 2.2 | 1.6 to 3.1 | 2.5 | 1.8 to 3.4 |
| 16–18 | 0.7 | 0.4 to 1.2 | 1.6 | 1.1 to 2.3 | 2.3 | 1.7 to 3.1 |
| Total | 0.5 | 0.3 to 0.8 | 1.9 | 1.4 to 2.5 | 2.4 | 1.9 to 3.0 |
| Deprivation quintile | ||||||
| 5 (least deprived) | 0.7 | 0.3 to 1.6 | 1.3 | 0.7 to 2.1 | 1.9 | 1.2 to 3.0 |
| 4 | 0.4 | 0.1 to 1.4 | 1.5 | 0.8 to 2.6 | 1.9 | 1.1 to 3.2 |
| 3 | 0.6 | 0.3 to 1.3 | 1.2 | 0.6 to 2.4 | 1.8 | 1.1 to 3.1 |
| 2 | 0.3 | 0.1 to 1.3 | 2.9 | 1.8 to 4.8 | 3.2 | 2.0 to 5.1 |
| 1 (most deprived) | 0.6 | 0.3 to 1.3 | 2.5 | 1.6 to 4.0 | 3.1 | 2.1 to 4.7 |
| Total | 0.5 | 0.3 to 0.8 | 1.9 | 1.4 to 2.4 | 2.4 | 1.9 to 3.0 |
| Ethnicity | ||||||
| White | 0.6 | 0.4 to 0.9 | 1.8 | 1.4 to 2.4 | 2.4 | 1.9 to 3.0 |
| South Asian | 0.4 | 0.1 to 1.2 | 1.9 | 0.7 to 4.8 | 2.2 | 1.0 to 5.0 |
| Black | 0.4 | 0.0 to 2.6 | 1.6 | 0.6 to 4.5 | 2 | 0.8 to 4.9 |
| Mixed ethnicity | 0 | 3.7 | 1.6 to 8.3 | 3.7 | 1.6 to 8.3 | |
| Other | 0 | 1.4 | 0.3 to 5.7 | 1.4 | 0.3 to 5.7 | |
| Region | ||||||
| North East | 1.9 | 0.7 to 4.8 | 3 | 1.5 to 6.0 | 4.9 | 2.8 to 8.5 |
| North West | 0.3 | 0.1 to 1.1 | 2.2 | 1.2 to 4.2 | 2.5 | 1.4 to 4.5 |
| Yorkshire and Humber | 0.2 | 0.0 to 1.1 | 2.3 | 1.1 to 4.8 | 2.4 | 1.2 to 4.9 |
| East Midlands | 0.6 | 0.1 to 2.5 | 1.7 | 0.8 to 4.0 | 2.3 | 1.1 to 4.8 |
| West Midlands | 0.9 | 0.3 to 2.4 | 0.9 | 0.4 to 1.9 | 1.8 | 0.9 to 3.6 |
| East of England | 0.6 | 0.2 to 1.7 | 2.6 | 1.3 to 5.1 | 3.2 | 1.8 to 5.7 |
| London | 0.2 | 0.0 to 0.7 | 1.7 | 0.8 to 3.6 | 1.9 | 0.9 to 3.7 |
| South East | 0.5 | 0.2 to 1.7 | 1.4 | 0.8 to 2.7 | 2 | 1.1 to 3.4 |
| South West | 0.6 | 0.2 to 2.2 | 1.5 | 0.6 to 3.6 | 2.2 | 1.1 to 4.4 |
| Total | 0.5 | 0.3 to 0.8 | 1.9 | 1.4 to 2.4 | 2.4 | 1.9 to 3.0 |
In total, 2.4% of young people aged 13–18 years are potentially eligible for bariatric surgery.
Repeating this analysis in 16- to 18-year-olds and including dyslipidaemia and high HbA1c levels among comorbidities made minimal difference to this estimate: the eligible group was 2.4% (95% CI 1.7% to 3.6%).
Summary findings
The 2014 population estimate for CYP aged 2–18 years totalled 10.9 million. Table 45 summarises estimates of numbers of CYP in England eligible for assessment and treatment of obesity in primary, secondary and tertiary care. Approximately 1.2 million CYP across England are eligible for assessment and treatment in primary or community care, with approximately 0.5 million eligible for assessment in secondary care. Large numbers are likely to be eligible for anti-obesity drug therapy (approximately 300,000), with around 90,000 CYP potentially eligible for bariatric surgery.
Discussion
This is the first paper to quantify the potential current burden of CYP obesity in terms of numbers of CYP eligible for assessment and treatment at each stage of the NICE clinical pathway.
Of the nearly 11 million CYP aged 2–18 years in England, approximately 11.2% (i.e. 1.2 million) are clinically obese (BMI ≥ 98th centile) and, therefore, eligible for primary care assessment or community management. We estimate that 2.6% of the population (i.e. 283,000) are likely to attend because of distress related to obesity or a concerning family history. However, we have previously noted that only 15% of parents of obese children reported having taken their child to the GP for their obesity,340 which would suggest a lighter burden for primary care of 1.6% of the population (i.e. 174,000). This appears to confirm suggestions that help-seeking is low among obese CYP and that many who would benefit from assessment and treatment are not seeking it.
Some 46% of the 1.2 million English CYP who are clinically obese (i.e. 556,000 young people) would potentially warrant referral to secondary care for their obesity because of comorbidities or extreme obesity. No data are available on the numbers who are seen in secondary care for obesity, however this will be only a subset of the 174,000 who are likely to present to primary care. Of the 3.8 million young people in England aged 13–18 years, some 8.2% or 309,000 have obesity and any comorbidity that would make them eligible for anti-obesity drug use. Of this age group, 2.4% are potentially eligible for bariatric surgery, amounting to 90,000 young people in 2014. This is many orders of magnitude higher than the estimates of those who undergo bariatric surgery,347 although there are no recent data on surgery in < 19-year-olds.
Deprived CYP appear to make up a greater proportion of those eligible for treatment at each step, although this has not been formally tested. There are no consistent differences by age or ethnicity. Burden appears highest in the North East and North West regions, although again this has not been formally tested.
We are not aware of previous studies estimating burden in this manner in the UK or elsewhere. In adults, a similar study focusing only on eligibility for bariatric surgery found that many more adults (over 2 million) were eligible for surgery than received it, and that those eligible were more likely to be of lower socioeconomic status. 348
Limitations
We used nationally representative data from eight consecutive Health Surveys for England to estimate pathway burden based on authoritative guidance. Obesity measures were based on calculated BMI standard deviation scores derived from measured height and weight, and estimates of comorbidity predominantly drawn from validated measures (e.g. psychological distress) or age- and sex-standardised objectively measured cardiovascular risk factors. Blood samples and blood pressure were assessed using validated replicable methods suitable for large-scale surveys. Implausible values for BMI, blood pressure and lipids were removed by the survey team. Burden estimates were calculated by reference to 2014 population estimates for England, the year following the last HSE survey.
Our data are subject to a number of limitations. Age was recorded only to the nearest year, making the obesity definitions less precise. Mapping eligibility for each stage of the pathway required operationalisation of guidance against CYP characteristics (e.g. degree of obesity, presence of comorbidities), which may be subject to error or disagreement. We had data on psychological distress, a well-described comorbidity of obesity; however, psychological distress in those obese in this sample was not necessarily due to obesity as we did not have data on obesity-related distress. Characterisation of population eligibility with respect to comorbidities was restricted by data not being available on certain comorbidities (e.g. sleep apnoea) and by restricted age ranges for other comorbidities. Thus, it is likely that our estimates of burden are underestimates. Family history and a number of comorbidities were derived from self-report data (e.g. musculoskeletal or mobility problems), possibly introducing bias. For family history, we only had history from household members and thus our variable probably underestimated the prevalence of a positive family history of cardiovascular disease or T2DM. Sample sizes did not allow examination of sexes separately aside from the prevalence of obesity by age. Our estimate of those eligible for bariatric surgery may be an underestimate owing to missing data on some comorbidities. However, we had no data on those ineligible for surgery because of anaesthetic issues and we note that young people with severe learning difficulties or severe mental health problems may be unsuitable for surgery, thus suggesting that our findings overestimate those who are eligible. We had no data on the proportion of those eligible for surgery who would wish to have it.
Some of our estimates assume a staged approach (i.e. treatment is matched to severity), rather than a stepped care approach, in which only non-responders to the previous level of care progress to the next level of intervention. Which approach is most effective at a population level is unclear as yet.
Implications
We found that 1.2 million CYP aged 2–18 years in England in 2014 were clinically obese and potentially eligible for assessment and treatment in primary care, of whom 500,000 were probably eligible for assessment in secondary care. These data will be important for commissioning services and ensuring equitable access for obese young people to services.
Chapter 9 Future research recommendations
Recommendations for future research directions are made in each chapter and substudy, and are summarised here.
Study A: scoping the impact of the National Child Measurement Programme on the childhood obesity pathway
It was found that NCMP feedback has a role in changing parental perceptions and health knowledge in the general population, but improvements or other initiatives may be required to precipitate lifestyle behaviour changes that will reduce childhood obesity prevalence.
First, in terms of methods, future research should seek to evaluate behaviour change and BMI change in the NCMP more accurately, for example through assessment and measurement of children before and after feedback. This would allow more accurate assessment of the ability of the NCMP to precipitate change.
Second, further work is needed to understand how best to address the marked and persistent discrepancy found between NCMP-reported weight status and parental perceptions. This appeared to have a key mediating role in whether or not parents made changes as a result of NCMP feedback. This work should include a better understanding of the causes of inaccurate perception and development of potential strategies to redress this. Consideration of inequalities will be essential in this, given the findings that deprivation and ethnicity were strongly associated with accuracy of weight perception, and also with likelihood to make changes after feedback.
Third, given the findings that parents report inconsistent and sometimes contradictory advice from health professionals regarding NCMP feedback, work is needed to understand primary care health professionals’ awareness and understanding of NCMP feedback and to understand how they deal with NCMP feedback in managing childhood obesity. Both quantitative and qualitative work are needed to identify barriers for health professionals to helping parents respond to NCMP feedback and to help professionals initiate difficult conversations about weight with children.
Finally, there is wide scope for investigation of potential improvements to delivery of NCMP feedback to parents. This clearly depends on collaboration with local authorities and PHE. The findings suggest a number of potential further avenues to explore in terms of modifying feedback to parents. Key among these would be investigation of how feedback could be more motivational and whether or not methods of feedback have utility in improving parental awareness of overweight and acceptance of NCMP findings, for example use of more graded systems for representing weight status feedback.
Study B: developing a new electronic tool to improve childhood obesity management in primary care
The key lines for further research in this study relate to further development and evaluation of the tool. The first stages in the recognised pathway for development of a complex intervention were undertaken, namely modelling, development and a feasibility pilot study. A larger pilot was aimed for; however, this did not prove feasible.
The logical next steps are a randomised pilot study of CATCH and then, potentially, an efficacy trial. The randomised pilot should be in a different and broader geographical area than the small number of practices in London used in this study. To reduce time and redundancy, the pilot could be an internal pilot with direct relation to the larger trial.
However, further developmental work of the tool is needed as well. It is believed that further work should be done to improve the accuracy of the risk assessment tool by updating risk assessment analyses using additional cohorts, if data are available. Second, there should be consideration of inclusion of training for professionals in the tool, given that practitioners feeling uncomfortable discussing weight-related health problems with parents was found to be a concern.
Study C: the Healthy Eating and Lifestyle Programme randomised clinical trial
It is unlikely that there is further work to be done on the model of the HELP intervention delivered in this trial, given the negative findings. However, given that HELP was developed as a intervention delivered by clinical psychologists, and that there were promising pilot data relating to its use in that setting, it would be useful to further evaluate the delivery of HELP in a more clinical setting with more expert providers. This evaluation should take the form of a randomised pilot, potentially followed by an efficacy trial.
Given the negative findings in this study, and similar negative findings by many other programmes, as noted in Chapter 4, Discussion, further work is needed to understand how weight management programmes can be delivered effectively to young people from diverse and often deprived backgrounds in which childhood obesity is common. This should consider the barriers to and facilitators of effective lifestyle weight management in deprived children and young people. Furthermore, given the finding of very high levels of psychological distress and disorder in this trial population, work should be undertaken to examine how standard lifestyle modification weight management can be tailored and delivered effectively to young people with significant psychological distress or disorder.
Study D: evaluation of anti-obesity drug treatment in children and adolescents
Study D findings highlighted a number of directions for future research. Although AOD treatment options are currently limited for young people (and adults), future new drugs are likely to share many of the characteristics and issues found with current drugs.
In terms of the findings that use of, persistence with and confidence in prescribing AODs were each poor to very poor, future research needs to consider how to improve the prescribing of and experience of AODs for young people. First, studies should consider combining AOD use with formal behavioural lifestyle modification programmes. There is some suggestion from the adult literature that such combinations are likely to be most effective, but such trials have not been carried out in adolescents. Second, for orlistat, the findings suggest that some of the reasons for cessation related to poor preparation of patients (in terms of awareness of effects and side effects) and training of prescribing teams (in preparing patients). Work should examine how both of these barriers can be overcome, with support for patients being provided within a behavioural framework, as outlined above. Furthermore, for orlistat, consideration should be given to study of its utility as a diet training aid, that is in providing feedback on levels of fat in the diet, rather than as a weight loss drug per se. Development and evaluation of training for health professionals should be undertaken to improve utility of AODs, both for current and future generations of drugs.
In terms of the evidence base for AODs in adolescence, this is largely limited to short-term use; there is a need for long-term data on AOD interventions in adolescence, in terms of both efficacy and adverse events. AOD trial populations also need to be broadened to young people with specific comorbidities, for example those with learning disabilities, those with psychological disorders and those with T2DM, as these are currently excluded from AOD trials, yet are groups with a high obesity burden.
Study E: evaluation of acceptability and early outcomes of adolescent bariatric surgery in the UK
The range of studies on the very new area of adolescent bariatric surgery highlighted a range of future avenues for research, perhaps not surprising given the newness of the field and the paucity of existing research.
In terms of surgery itself, future work should move past individual hospital series and collect comparable consistent data across all centres nationally. These data should include routine collection of psychological, health economic and QoL data, to enable routine monitoring of outcomes over the shorter and longer term. The need for longer-term data is particularly acute. Psychological and QoL data are necessary to understand which young people benefit most from surgery and, thus, potentially allow clinicians to make more informed decisions about which young people are most likely to benefit from surgery. Routine collection of data on metabolic comorbidities and bone density are also necessary to inform studies of the long-term impacts of surgery in young people.
Further work is needed to help clinicians move towards a shared decision-making model with young people and families. This could take the form of an implementation science study building on the findings of the present decision-making study. As part of this, it would be important to investigate and evaluate mechanisms to reduce dropout from follow-up after surgery and in long-term follow-up, and whether or not this dropout is related to involvement in decision-making pre surgery.
Other work should examine whether or not the evidence of cost-effectiveness identified here for adolescent bariatric surgery within the NHS is also found in other health systems.
Acknowledgements
The HELP research team acknowledges the support of NIHR, through the Primary Care Research Network.
We are grateful for the contributions of the Trial Steering Committee [independent members: Professor Carolyn Summerbell (Durham University), Dr Edna Roche (University of Dublin), Dr Mike Robling (Cardiff University) and Ms Sandra Wharton (Independent Adviser, Mathematics, Functional Skills and Whole School Improvement)], the Data Monitoring Committee [independent members: Professor John W Gregory (Chairperson) (Wales School of Medicine) and Professor Mark Shevlin (Statistician) (University of Ulster)] and the Ethics Committee (NRES Committee London – Riverside, NRES, Bristol Research Ethics Committee Centre).
We would like to thank Saffron Karlsen for her input into the trial design and economic analysis.
We are grateful to all investigators and collaborators who assisted in undertaking the work across the 5 years of the PROMISE programme. We particularly thank the young people and families who participated in the various elements of each substudy. We are also very grateful to Tam Fry and to the AYPH, who led the PPI element of the programme.
Russell Viner and Sanjay Kinra co-led the programme, with support from coinvestigators Deborah Christie, Ian C Wong, Tim J Cole, Anthony S Kessel, Steve Morris, Irwin Nazareth, Dasha Nicholls, Sonia Saxena and Jane Wardle (sadly, our colleague Jane Wardle died in 2015); from the programme manager Anne Mathiot; and from collaborators Billy White, Lee Hudson, Barry Taylor, Min Hae Park, Marco Adamo, P Brown, S Colville, Silvia Costa, Helen Croker, J Doyle, John Gregson, Yingfen Hsia, R Holt, A Melo, Monica Panca, T Pandya and U Sovio.
The PROMISE investigators
PROMISE was a collaboration of UCL [Russell Viner (co-principal investigator), Dr Dasha Nicholls and Professor Tim J Cole from the UCL Institute of Child Health; Professor Ian Wong from the UCL School of Pharmacy (and Hong Kong University); Professor Steve Morris, Dr Helen Croker and Professor Jane Wardle from the UCL Institute of Epidemiology & Health Care; and Professor Irwin Nazareth from the UCL Department of Primary Care); and Dr Sanjay Kinra (co-principal investigator) from the London School of Hygiene & Tropical Medicine]; University College London Hospitals NHS Foundation Trust (Dr Deborah Christie), the author works at University College Hospital London; Imperial College London (Dr Sonia Saxena); and PHE (Professor Anthony Kessel). Patient and public representation on the investigator group was provided by Tam Fry from the Child Growth Foundation.
Contributions of authors
Publications
Journal articles
Viner RM, Hsia Y, Tomsic T, Wong ICK. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes Rev 2010;11:593–602.
Christie D, Hudson L, Mathiot A, Cole TJ, Karlsen S, Kessel A, et al. Assessing the efficacy of the healthy eating and lifestyle programme (HELP) compared with enhanced standard care of the obese adolescent in the community: study protocol for a randomized controlled trial. Trials 2011;12:242.
Falconer C, Park M, Skow A, Black J, Sovio U, Saxena S, et al. Scoping the impact of the national child measurement programme feedback on the child obesity pathway: study protocol. BMC Public Health 2012;12:783.
Hsia Y, Dawoud D, Sutcliffe AG, Viner R, Kinra S, Wong ICK. Unlicensed use of metformin in children and adolescents in UK. Br J Clin Pharmacol 2012;73:135–9.
White B, Finer N, Adamo M, Duke E, Kingett H, Christie D, et al. Outcomes of an adolescent bariatric service. Arch Dis Child 2012;97:A63.
Black JA, White B, Viner RM, Simmons RK. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev 2013;14:634–44.
Hudson L, Christie D, Kinra S, Mathiot A, Wong IC, Nazareth I, et al. Low levels of metabolic syndrome (MetS) in a community based obesity trial. Turk Pediatri Ars 2013;48(Suppl. 2):104.
Jones Nielsen JD, Laverty AA, Millett C, Mainous Iii AG, Majeed A, Saxena S. Rising obesity-related hospital admissions among children and young people in England: national time trends study. PLoS ONE 2013;8:e65764.
Park MH, Falconer CL, Saxena S, Kessel AS, Croker H, Skow Á, et al. Perceptions of health risk among parents of overweight children: a cross-sectional study within a cohort. Prev Med 2013;57:55–9.
Sovio U, Skow A, Falconer C, Park MH, Viner RM, Kinra S. Improving prediction algorithms for cardiometabolic risk in children and adolescents. J Obes 2013;2013:684782.
Doyle J, Colville S, Brown P, Christie D. ‘For the cases we’ve had . . . I don’t think anybody has had enormous confidence’ – exploring ‘uncertainty’ in adolescent bariatric teams: an interpretative phenomenological analysis. Clin Obes 2014;4:45–52.
Falconer C, Park M, Croker H, Skow A, Black J, Saxena S, et al. The benefits and harms of providing parents with weight feedback as part of the national child measurement programme: a prospective cohort study. BMC Public Health 2014;14:e549.
Falconer CL, Park MH, Croker H, Kessel AS, Saxena S, Viner RM, et al. Can the relationship between ethnicity and obesity-related behaviours among school-aged children be explained by deprivation? A cross-sectional study. BMJ Open 2014;4:e003949.
Karlsen S, Morris S, Kinra S, Vallejo-Torres L, Viner RM. Ethnic variations in overweight and obesity among children over time: findings from analyses of the Health Surveys for England 1998–2009. Paediatr Obes 2014;9:186–96.
Park MH, Falconer CL, Croker H, Saxena S, Kessel AS, Viner RM, et al. Predictors of health-related behaviour change in parents of overweight children in England. Prev Med 2014;62:20–4.
Syrad H, Falconer C, Cooke L, Saxena S, Kessel AS, Viner R, et al. ‘Health and happiness is more important than weight’: a qualitative investigation of the views of parents receiving written feedback on their child’s weight as part of the National Child Measurement Programme. J Hum Nutr Diet 2014;28:47–55.
Black JA, Park M, Gregson J, Falconer CL, White B, Kessel AS, et al. Child obesity cut-offs as derived from parental perceptions: cross-sectional questionnaire. Br J Gen Pract 2015;65:e234–9.
Christie D, Hudson L, Costa S, Mathiot A, Holt R, Kinra S, et al. RCT of a motivational lifestyle intervention (the Healthy Eating and Lifestyle Programme (HELP)) for obese young people. Arch Dis Child 2015;100(Suppl. 3):A2.
Christie D, Hudson LD, Kinra S, Nazareth I, Holt R, Kessel A, et al. 35. Does a motivational lifestyle intervention (the Healthy Eating and Lifestyle Programme (HELP)) work for obese young people. J Adolesc Health 2015;56(Suppl. 1):S19.
Ells LJ, Hancock C, Copley VR, Mead E, Dinsdale H, Kinra S, et al. Prevalence of severe childhood obesity in England: 2006–2013. Arch Dis Child 2015;100:631–6.
Ells LJ, Mead E, Atkinson G, Corpeleijn E, Roberts K, Viner R, et al. Surgery for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev 2015;6:CD011740.
Park MH, Skow Á, Puradiredja DI, Lucas A, Syrad H, Sovio U, et al. Development and evaluation of an online tool for management of overweight children in primary care: a pilot study. BMJ Open 2015;5:e007326.
Skow A, Park MH, De Matteis S, Kessel AS, Saxena S, Viner RM, et al. Adiposity and carotid-intima media thickness in children and adolescents: a systematic review. BMC Pediatrics 2015;15:161.
White B, Doyle J, Colville S, Nicholls D, Viner RM, Christie D. Systematic review of psychological and social outcomes of adolescents undergoing bariatric surgery, and predictors of success. Clin Obes 2015;5:312–24.
White B, Jamieson L, Clifford S, Shield JPH, Christie D, Smith F, et al. Adolescent experiences of anti-obesity drugs. Clin Obes 2015;5:116–26.
White B, Jamieson L, Clifford S, Shield JPH, Christie D, Smith F, et al. Understanding young people’s experiences of anti-obesity drugs. Clin Obes 2015.
Christie D, Hudson LD, Kinra S, Wong ICK, Nazareth I, Cole TJ, et al. A community-based motivational personalised lifestyle intervention to reduce BMI in obese adolescents: results from the Healthy Eating and Lifestyle Programme (HELP) randomised controlled trial. Arch Dis Child 2017;102:695–701.
White B, Doyle J, Matschull K, Adamo M, Christie D, Nicholls D, et al. Outcomes of 50 patients entering an adolescent bariatric surgery programme [published online ahead of print August 9 2017]. Arch Dis Child 2017.
White B, Hsia Y, Kinra S, Saxena S, Christie D, Viner RM, Wong ICK. Survey of anti-obesity drug prescribing for obese children and young people in UK primary care. BMJ Paediatr Open 2017;1:e000104.
Panca M, Christie D, Cole TJ, Costa S, Gregson J, Holt R, et al. Cost-effectiveness of a community-delivered multicomponent intervention compared with enhanced standard care of obese adolescents: cost–utility analysis alongside a randomised controlled trial (the HELP trial). BMJ Open 2018;8:e018640.
Panca M, Viner RM, White B, Pandya T, Melo H, Adamo M, et al. Cost-effectiveness of bariatric surgery in adolescents with severe obesity in the UK. Clin Obes 2018;8:105–13.
Viner RM, Kinra S, Nicholls D, Cole T, Kessel A, Christie D, et al. Burden of child and adolescent obesity on health services in England. Arch Dis Child 2018;103:247–54.
Presentations
Falconer C. Can Parents Tell Whether their Child is a Healthy Weight or Have we Become Accustomed to Child Obesity? Oral presentation. Department of Health and Social Care, Obesity and Food Policy Branch, July 2012.
Falconer C. National Child Measurement Evaluation. Oral presentation. Obesity in Mothers and Children. What are the Links and What Can we do About Them?, London School of Hygiene & Tropical Medicine, London, UK, November 2012.
Falconer C, Skow A, Black JA, Croker H, Sovio U, Kessel A, et al. The Majority of Parents of Overweight and very Overweight Children Underestimate their Child’s Weight Status and Weight-Related Health Risk. Oral presentation. Department of Health and Social Care, Obesity and Food Policy Branch, July 2012.
Falconer C, Skow A, Park MH, Sovio U, Croker H, Kessel A, et al. Does BMI Feedback Change Parental Perceptions about the Health Risk Associated with their Child’s BMI? Poster presentation. The 19th European Congress on Obesity, Lyon, France, 9–12 May 2012.
Falconer C. The Findings from the LSHTM Evaluation of NCMP Feedback. Oral presentation at a series of Department of Health and Social Care NCMP workshops, Autumn 2012.
Skow A, Black JA, Falconer C, Sovio U, White B, Kessel A, et al. Development of an Electronic Tool to Estimate Health Risks and Provide Personalised Weight Management Advice for Overweight Children. Poster presentation. The 19th European Congress on Obesity, Lyon, France, 9–12 May 2012.
White B, Finer N, Adamo M, Duke E, Kingett H, Christie D, et al. Outcomes of an Adolescent Bariatric Service. Oral presentation. The Royal College of Paediatrics and Child Health Annual Conference, Glasgow, UK, 22–24 May 2012.
Costa S, Hudson LD, Christie D, Kinra S, Mathiot A, Wong I, et al. Fitness May be More Important for Cardiovascular Health than Adiposity in Adolescent Girls. Poster presentation. 2014 International Congress on Obesity, Kuala Lumpur, Malaysia, 17–20 March 2014.
Costa S, Hudson LD, Kessel AS, Mathiot A, Wong IC, Nazareth I, et al. Eating Behaviours and their Relationship with Adiposity in a Sample of Obese Adolescents. Poster presentation. 2014 International Society for Behavioral Nutrition and Physical Activity Annual Meeting, San Diego, CA, USA, 21–24 May 2014.
Hudson LD, Costa S, Viner RM, Kinra S, Wong I, Nazareth I, et al. Associations of Physiological and Life-event Measures of Stress with Adiposity in Obese Adolescents. Poster presentation. 2014 International Congress on Obesity, Kuala Lumpur, Malaysia, 17–20 March 2014.
White B, Jamieson E, Clifford S, Smith F, Wong I, Viner RM. Understanding Young People’s Experiences of Anti-obesity drugs. Poster presentation. 2014 International Congress on Obesity, Kuala Lumpur, Malaysia, 17–20 March 2014.
Christie D. Does a Motivational Lifestyle Intervention (the Healthy Eeating and Lifestyle Programme (HELP)) Work for Obese Young People? Oral presentation. The Society for Adolescent Health and Medicine 2015 Annual Meeting, Los Angeles, CA, USA, 18–21 March 2015.
Christie D. RCT of a Motivational Lifestyle Intervention (the Healthy Eating and Lifestyle Programme (HELP)) for Obese Young People. Oral presentation. The 2015 Royal College of Paediatrics and Child Health Annual Conference, Birmingham, UK, 28–30 April 2015.
Costa S, Hudson LD, Kessel A, Mathiot A, Wong IC, Nazareth I, et al. Objectively Measured Physical Activity and Sedentary Behaviour in Obese Adolescents, and their Relationship with Fatness and Metabolic Outcomes. Poster presentation. 2015 International Society for Behavioral Nutrition and Physical Activity Annual Meeting, Edinburgh, UK, 3–6 June 2015.
PhD thesis
White WJ. Novel Approaches in Adolescent Obesity Management. PhD thesis. London: University College London; 2017.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Lobstein T, Leach RJ. Tackling Obesities: Future Choices – International Comparisons of Obesity Trends, Determinants and Responses – Evidence Review. 2 Children. London: Government Office for Science; 2007.
- Department of Health and Social Care . Healthy Lives, Brighter Futures – The Strategy for Children and Young People’s Health 2009.
- Department of Children, Schools and Families . Healthy Weight, Healthy Lives: A Cross-Government Strategy for England 2008.
- Chief Medical Officer . Annual Report of the Chief Medical Officer 2012. Our Children Deserve Better: Prevention Pays 2013.
- HM Government . Childhood Obesity: A Plan for Action 2016. www.gov.uk/government/publications/childhood-obesity-a-plan-for-action (accessed 18 September 2019).
- NHS Digital . Statistics on Obesity, Physical Activity and Diet 2015.
- NHS Digital . Health Survey for England – 2013 2013. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2013 (accessed 19 August 2019).
- NHS Digital . National Child Measurement Programme – England, 2013–14 2014.
- Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012;13:985-1000. https://doi.org/10.1111/j.1467-789X.2012.01015.x.
- Costa S, Johnson W, Viner RM. Additive influences of maternal and paternal body mass index on weight status trajectories from childhood to mid-adulthood in the 1970 British Cohort Study. Longit Life Course Stud 2015;6:147-72. https://doi.org/10.14301/llcs.v6i2.301.
- National Institute for Health and Care Excellence (NICE) . Obesity Prevention. NICE Clinical Guideline 43 [CG43] 2006. www.nice.org.uk/guidance/CG43 (accessed 19 August 2019).
- National Institute for Health and Care Excellence (NICE) . Obesity: Identification, Assessment and Management. NICE Clinical Guideline 189 [CG189] 2014.
- National Institute for Health and Care Excellence (NICE) . Preventing Excess Weight Gain. NICE Guideline 67 [NG7] 2015.
- NHS Digital . Quality and Outcomes Framework – 2009–10, England Level 2010. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-data/quality-and-outcomes-framework-2009-10-england-level (accessed 18 September 2019).
- Falconer CL, Park MH, Croker H, Skow A, Black J, Saxena S, et al. The benefits and harms of providing parents with weight feedback as part of the NCMP: a prospective cohort study. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-549.
- Syrad H, Falconer C, Cooke L, Saxena S, Kessel AS, Viner R, et al. ‘Health and happiness is more important than weight’: a qualitative investigation of the views of parents receiving written feedback on their child’s weight as part of the National Child Measurement Programme. J Hum Nutr Diet 2015;28:47-55. https://doi.org/10.1111/jhn.12217.
- Department of Health and Social Care . Healthy Lives, Healthy People: A Call to Action on Obesity in England 2011.
- Lundahl A, Kidwell KM, Nelson TD. Parental underestimates of child weight: a meta-analysis. Pediatrics 2014;133:e689-703. https://doi.org/10.1542/peds.2013-2690.
- Rietmeijer-Mentink M, Paulis WD, van Middelkoop M, Bindels PJ, van der Wouden JC. Difference between parental perception and actual weight status of children: a systematic review. Matern Child Nutr 2013;9:3-22. https://doi.org/10.1111/j.1740-8709.2012.00462.x.
- Crawford D, Timperio A, Telford A, Salmon J. Parental concerns about childhood obesity and the strategies employed to prevent unhealthy weight gain in children. Public Health Nutr 2006;9:889-95. https://doi.org/10.1017/PHN2005917.
- Lampard AM, Byrne SM, Zubrick SR, Davis EA. Parents’ concern about their children’s weight. Int J Pediatr Obes 2008;3:84-92. https://doi.org/10.1080/17477160701832552.
- Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol 2007;26:136-45. https://doi.org/10.1037/0278-6133.26.2.136.
- Wee CC, Davis RB, Phillips RS. Stage of readiness to control weight and adopt weight control behaviors in primary care. J Gen Intern Med 2005;20:410-15. https://doi.org/10.1111/j.1525-1497.2005.0074.x.
- Rhee KE, De Lago CW, Arscott-Mills T, Mehta SD, Davis RK. Factors associated with parental readiness to make changes for overweight children. Pediatrics 2005;116:e94-101. https://doi.org/10.1542/peds.2004-2479.
- Mooney A, Statham J, Boddy J, Smith M. The National Child Measurement Programme: Early Experiences of Routine Feedback to Parents of Children’s Height and Weight. London: Institute of Education; 2010.
- Chomitz VR, Collins J, Kim J, Kramer E, McGowan R. Promoting healthy weight among elementary school children via a health report card approach. Arch Pediatr Adolesc Med 2003;157:765-72. https://doi.org/10.1001/archpedi.157.8.765.
- Westwood M, Fayter D, Hartley S, Rithalia A, Butler G, Glasziou P, et al. Childhood obesity: should primary school children be routinely screened? A systematic review and discussion of the evidence. Arch Dis Child 2007;92:416-22. https://doi.org/10.1136/adc.2006.113589.
- Cole TJ. Growth monitoring with the British 1990 growth reference. Arch Dis Child 1997;76:47-9. https://doi.org/10.1136/adc.76.1.47.
- Public Health England . Change4life n.d. www.nhs.uk/change4life (accessed 18 September 2019).
- Department for Communities and Local Government . English Indices of Deprivation 2010 2011. www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (accessed 19 August 2019).
- Croker H, Lucas R, Wardle J. Cluster-randomised trial to evaluate the ‘Change for Life’ mass media/social marketing campaign in the UK. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-404.
- American Academy of Pediatrics . Where We Stand: TV Viewing Time 2015. www.healthychildren.org/English/family-life/Media/Pages/Where-We-Stand-TV-Viewing-Time.aspx.
- Modi AC, Zeller MH. Validation of a parent-proxy, obesity-specific quality-of-life measure: sizing them up. Obesity 2008;16:2624-33. https://doi.org/10.1038/oby.2008.416.
- Royal College of Nursing . NHS Agenda for Change Pay Scales – 2012 2013 2012. www.nsft.nhs.uk/About-us/Documents/Pay_Scales_2012_Final270312.pdf (accessed 18 September 2019).
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407-29. https://doi.org/10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L.
- Park MH, Falconer CL, Croker H, Saxena S, Kessel AS, Viner RM, et al. Predictors of health-related behaviour change in parents of overweight children in England. Prev Med 2014;62:20-4. https://doi.org/10.1016/j.ypmed.2014.02.002.
- Kubik MY, Fulkerson JA, Story M, Rieland G. Parents of elementary school students weigh in on height, weight, and body mass index screening at school. J Sch Health 2006;76:496-501. https://doi.org/10.1111/j.1746-1561.2006.00147.x.
- Johnson SB, Pilkington LL, Lamp C, He J, Deeb LC. Parent reactions to a school-based body mass index screening program. J Sch Health 2009;79:216-23. https://doi.org/10.1111/j.1746-1561.2009.00401.x.
- Ehtisham S, Crabtree N, Clark P, Shaw N, Barrett T. Ethnic differences in insulin resistance and body composition in United Kingdom adolescents. J Clin Endocrinol Metab 2005;90:3963-9. https://doi.org/10.1210/jc.2004-2001.
- Maynard MJ, Baker G, Rawlins E, Anderson A, Harding S. Developing obesity prevention interventions among minority ethnic children in schools and places of worship: the DEAL (DiEt and Active Living) study. BMC Public Health 2009;9. https://doi.org/10.1186/1471-2458-9-480.
- Szczepura A. Access to health care for ethnic minority populations. Postgrad Med J 2005;81:141-7. https://doi.org/10.1136/pgmj.2004.026237.
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD001872.pub2.
- Grimmett C, Croker H, Carnell S, Wardle J. Telling parents their child’s weight status: psychological impact of a weight-screening program. J Pediatr 2008;122:e682-8. https://doi.org/10.1542/peds.2007-3526.
- Turner KM, Salisbury C, Shield JP. Parents’ views and experiences of childhood obesity management in primary care: a qualitative study. Fam Pract 2012;29:476-81. https://doi.org/10.1093/fampra/cmr111.
- Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev 2011;12. https://doi.org/10.1002/14651858.CD001871.pub3.
- Jago R, Fox KR, Page AS, Brockman R, Thompson JL. Parent and child physical activity and sedentary time: do active parents foster active children?. BMC Public Health 2010;10. https://doi.org/10.1186/1471-2458-10-194.
- Ransley JK, Greenwood DC, Cade JE, Blenkinsop S, Schagen I, Teeman D, et al. Does the school fruit and vegetable scheme improve children’s diet? A non-randomised controlled trial. J Epidemiol Community Health 2007;61:699-703. https://doi.org/10.1136/jech.2006.052696.
- Craig R, Shelton N, Craig R, Shelton N. Health Survey for England – 2007. 1. Leeds: NHS Digital; 2008.
- Burrows T, Golley RK, Khambalia A, McNaughton SA, Magarey A, Rosenkranz RR, et al. The quality of dietary intake methodology and reporting in child and adolescent obesity intervention trials: a systematic review. Obes Rev 2012;13:1125-38. https://doi.org/10.1111/j.1467-789X.2012.01022.x.
- Kohl HW, Fulton JE, Caspersen CJ. Assessment of physical activity among children and adolescents: a review and synthesis. Prev Med 2000;31:S54-76. https://doi.org/10.1006/pmed.1999.0542.
- Park MH, Skow Á, Puradiredja DI, Lucas A, Syrad H, Sovio U, et al. Development and evaluation of an online tool for management of overweight children in primary care: a pilot study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007326.
- Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e4759.
- Walker O, Strong M, Atchinson R, Saunders J, Abbott J. A qualitative study of primary care clinicians’ views of treating childhood obesity. BMC Fam Pract 2007;8. https://doi.org/10.1186/1471-2296-8-50.
- Epstein L, Ogden J. A qualitative study of GPs’ views of treating obesity. Br J Gen Pract 2005;55:750-4.
- Turner KM, Shield JP, Salisbury C. Practitioners’ views on managing childhood obesity in primary care: a qualitative study. Br J Gen Pract 2009;59:856-62. https://doi.org/10.3399/bjgp09X472269.
- Gerner B, McCallum Z, Sheehan J, Harris C, Wake M. Are general practitioners equipped to detect child overweight/obesity? Survey and audit. J Paediatr Child Health 2006;42:206-11. https://doi.org/10.1111/j.1440-1754.2006.00831.x.
- Jochemsen-van der Leeuw HG, van Dijk N, Wieringa-de Waard M. Attitudes towards obesity treatment in GP training practices: a focus group study. Fam Pract 2011;28:422-9. https://doi.org/10.1093/fampra/cmq110.
- Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:742-52. https://doi.org/10.7326/0003-4819-144-10-200605160-00125.
- Rattay KT, Ramakrishnan M, Atkinson A, Gilson M, Drayton V. Use of an electronic medical record system to support primary care recommendations to prevent, identify, and manage childhood obesity. Pediatrics 2009;123:100-7. https://doi.org/10.1542/peds.2008-1755J.
- Keehbauch J, Miguel GS, Drapiza L, Pepe J, Bogue R, Smith-Dixon A. Increased documentation and management of pediatric obesity following implementation of an EMR upgrade and education. Clin Pediatr 2012;51:31-8. https://doi.org/10.1177/0009922811417293.
- Owen SE, Sharp DJ, Shield JP. The Bristol Online Obesity Screening Tool: experience of using a screening tool for assessing obese children in primary care. Prim Health Care Res Dev 2011;12:293-300. https://doi.org/10.1017/S1463423611000132.
- Gance-Cleveland B, Gilbert LH, Kopanos T, Gilbert KC. Evaluation of technology to identify and assess overweight children and adolescents. J Spec Pediatr Nurs 2010;15:72-83. https://doi.org/10.1111/j.1744-6155.2009.00220.x.
- Smith AJ, Skow Á, Bodurtha J, Kinra S. Health information technology in screening and treatment of child obesity: a systematic review. Pediatrics 2013;131:e894-902. https://doi.org/10.1542/peds.2012-2011.
- Sovio U, Skow A, Falconer C, Park MH, Viner RM, Kinra S. Improving prediction algorithms for cardiometabolic risk in children and adolescents. J Obes 2013;2013. https://doi.org/10.1155/2013/684782.
- Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child 1995;73:25-9. https://doi.org/10.1136/adc.73.1.25.
- Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305-19. https://doi.org/10.1002/sim.4780111005.
- Butler G, Kirk J. Paediatric Endocrinology and Diabetes. Oxford: Oxford University Press; 2011.
- Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: the ‘children of the 90s’ – the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111-27. https://doi.org/10.1093/ije/dys064.
- Stansfeld S, Haines M, Booy R, Taylor SJ, Viner R, Head J, et al. Health of Young People in East London: The RELACHS Study 2001. London: The Stationery Office; 2003.
- Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J 2010;31:3063-72. https://doi.org/10.1093/eurheartj/ehq355.
- Viner RM, Haines MM, Taylor SJ, Head J, Booy R, Stansfeld S. Body mass, weight control behaviours, weight perception and emotional well being in a multiethnic sample of early adolescents. Int J Obes 2006;30:1514-21. https://doi.org/10.1038/sj.ijo.0803352.
- Andersen LB, Wedderkopp N, Hansen HS, Cooper AR, Froberg K. Biological cardiovascular risk factors cluster in Danish children and adolescents: the European Youth Heart Study. Prev Med 2003;37:363-7. https://doi.org/10.1016/S0091-7435(03)00145-2.
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160-7. https://doi.org/10.2337/diacare.26.11.3160.
- Jolliffe CJ, Janssen I. Distribution of lipoproteins by age and gender in adolescents. Circulation 2006;114:1056-62. https://doi.org/10.1161/CIRCULATIONAHA.106.620864.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. J Pediatr 2004;114:555-76. https://doi.org/10.1542/peds.114.2.S2.555.
- Goodman R, Ford T, Simmons H, Gatward R, Meltzer H. Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry 2000;177:534-9. https://doi.org/10.1192/bjp.177.6.534.
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry 2001;40:1337-45. https://doi.org/10.1097/00004583-200111000-00015.
- Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart And health Study in England (CHASE Study). Int J Epidemiol 2011;40:33-44. https://doi.org/10.1093/ije/dyq180.
- Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics 2007;120:229-53. https://doi.org/10.1542/peds.2007-2329E.
- Barlow SE. Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120:164-92. https://doi.org/10.1542/peds.2007-2329C.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage Publications; 2003.
- Garcia-Retamero R, Okan Y, Cokely ET. Using visual aids to improve communication of risks about health: a review. Scientific World J 2012;2012. https://doi.org/10.1100/2012/562637.
- Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD001865.pub3.
- Ehtisham S, Shaw N, Kirk J, Barrett T. Development of an assessment tool for screening children for glucose intolerance by oral glucose tolerance test. Diabetes Care 2004;27:280-1. https://doi.org/10.2337/diacare.27.1.280.
- McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE, et al. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med 2005;165:883-90. https://doi.org/10.1001/archinte.165.8.883.
- Office for National Statistics . 2011 Census: Ethnic Group, Local Authorities in England and Wales 2012.
- Saxena S, Ambler G, Cole TJ, Majeed A. Ethnic group differences in overweight and obese children and young people in England: cross sectional survey. Arch Dis Child 2004;89:30-6.
- Christie D, Hudson L, Mathiot A, Cole TJ, Karlsen S, Kessel A, et al. Assessing the efficacy of the Healthy Eating and Lifestyle Programme (HELP) compared with enhanced standard care of the obese adolescent in the community: study protocol for a randomized controlled trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-242.
- Skivington K, Matthews L, Craig P, Simpson S, Moore L. Developing and evaluating complex interventions: updating Medical Research Council guidance to take account of new methodological and theoretical approaches. Lancet 2018;392. https://doi.org/10.1016/S0140-6736(18)32865-4.
- Ells LJ, Hancock C, Copley VR, Mead E, Dinsdale H, Kinra S, et al. Prevalence of severe childhood obesity in England: 2006–2013. Arch Dis Child 2015;100:631-6. https://doi.org/10.1136/archdischild-2014-307036.
- Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics 2012;130:e1647-71. https://doi.org/10.1542/peds.2012-1176.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2014;16:381-7. https://doi.org/10.1111/dom.12195.
- George E, Iveson C, Ratner H. Problem to Solution: Brief Therapy with Individuals and Families. London: BT Press; 1999.
- Haroun D, Croker H, Viner RM, Williams JE, Darch TS, Fewtrell MS, et al. Validation of BIA in obese children and adolescents and re-evaluation in a longitudinal study. Obesity 2009;17:2245-50. https://doi.org/10.1038/oby.2009.98.
- Kolotkin RL, Zeller M, Modi AC, Samsa GP, Quinlan NP, Yanovski JA, et al. Assessing weight-related quality of life in adolescents. Obesity 2006;14:448-57. https://doi.org/10.1038/oby.2006.59.
- Garner D. Eating Disorder Inventory-2: Professional Manual. Lutz, FL: Psychological Assessment Resources; 1991.
- Rosenberg M. Society and the Adolescent Self-image. Middletown, CT: Wesleyan University Press; 1989.
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000;41:645-55. https://doi.org/10.1111/j.1469-7610.2000.tb02345.x.
- Greater London Authority . The English Indices of Deprivation 2010: Local Authority District Summaries 2010. https://data.london.gov.uk/download/indices-deprivation-2010/9300b2b1-8071-464a-9420-e2fed284f93e/indices-of-deprivation-2010.xls (accessed 18 September 2019).
- Wille N, Erhart M, Petersen C, Ravens-Sieberer U. The impact of overweight and obesity on health-related quality of life in childhood – results from an intervention study. BMC Public Health 2008;8. https://doi.org/10.1186/1471-2458-8-421.
- Robertson W, Friede T, Blissett J, Rudolf MC, Wallis M, Stewart-Brown S. Pilot of ‘Families for Health’: community-based family intervention for obesity. Arch Dis Child 2008;93:921-6. https://doi.org/10.1136/adc.2008.139162.
- Lee KJ, Thompson SG. Clustering by health professional in individually randomised trials. BMJ 2005;330:142-4. https://doi.org/10.1136/bmj.330.7483.142.
- Madson MB, Campbell TC, Barrett DE, Brondino MJ, Melchert TP. Development of the Motivational Interviewing Supervision and Training Scale. Psychol Addict Behav 2005;19:303-10. https://doi.org/10.1037/0893-164X.19.3.303.
- Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444-55. https://doi.org/10.1080/01621459.1996.10476902.
- Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile?. Eur J Clin Nutr 2005;59:419-25. https://doi.org/10.1038/sj.ejcn.1602090.
- Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev 2011;12:e273-81. https://doi.org/10.1111/j.1467-789X.2010.00803.x.
- Peirson L, Fitzpatrick-Lewis D, Morrison K, Warren R, Usman Ali M, Raina P. Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis. CMAJ Open 2015;3:E35-46. https://doi.org/10.9778/cmajo.20140047.
- Meltzer H, Gatward R, Goodman R, Ford T. Mental health of children and adolescents in Great Britain. Int Rev Psychiatry 2003;15:185-7. https://doi.org/10.1080/0954026021000046155.
- Beauchamp A, Backholer K, Magliano D, Peeters A. The effect of obesity prevention interventions according to socioeconomic position: a systematic review. Obes Rev 2014;15:541-54. https://doi.org/10.1111/obr.12161.
- National Obesity Observatory (Public Health England) . International Comparisons n.d. www.noo.org.uk/NOO_about_obesity/child_obesity/international (accessed 20 January 2015).
- NHS Digital . National Child Measurement Programme – England, 2013–14 2014.
- British Paediatric Surveillance Unit (BPSU) . Eighth Annual Report 1993 1994.
- NHS Digital . Health Survey for England – 2012 2013. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2012 (accessed 18 September 2019).
- Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007;150:12-17.e2. https://doi.org/10.1016/j.jpeds.2006.08.042.
- Khaodhiar L, Cummings S, Apovian CM. Treating diabetes and prediabetes by focusing on obesity management. Curr Diab Rep 2009;9:348-54. https://doi.org/10.1007/s11892-009-0055-0.
- Paulis WD, Silva S, Koes BW, van Middelkoop M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: a systematic review. Obes Rev 2014;15:52-67. https://doi.org/10.1111/obr.12067.
- Narang I, Mathew JL. Childhood obesity and obstructive sleep apnea. J Nutr Metab 2012;2012. https://doi.org/10.1155/2012/134202.
- Xavier S, Mandal S. The psychosocial impacts of obesity in children and young people: a future health perspective. J Public Health Community Med 2005;6:23-7.
- Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes 2010;5:282-304. https://doi.org/10.3109/17477160903473697.
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med 1993;22:167-77. https://doi.org/10.1006/pmed.1993.1014.
- Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327:1350-5. https://doi.org/10.1056/NEJM199211053271904.
- Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med 1993;329:1008-12. https://doi.org/10.1056/NEJM199309303291406.
- Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metab Clin Exp 1996;45:235-40. https://doi.org/10.1016/S0026-0495(96)90060-8.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. https://doi.org/10.1016/S0140-6736(08)60269-X.
- National Obesity Observatory (Public Health England) . The Economic Burden of Obesity 2010. www.noo.org.uk/uploads/doc/vid_8575_Burdenofobesity151110MG.pdf (accessed 21 January 2015).
- Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 2011;12:131-41. https://doi.org/10.1111/j.1467-789X.2009.00712.x.
- National Collaborative on Childhood Obesity Research (NCCOR) . Childhood Obesity in the United States 2009. www.nccor.org/downloads/ChildhoodObesity_020509.pdf (accessed 21 January 2015).
- Hayes A, Lung T, Wen LM, Baur L, Rissel C, Howard K. Economic evaluation of ‘Healthy Beginnings’ an early childhood intervention to prevent obesity. Obesity 2014;22:1709-15. https://doi.org/10.1002/oby.20747.
- Moodie ML, Herbert JK, de Silva-Sanigorski AM, Mavoa HM, Keating CL, Carter RC, et al. The cost-effectiveness of a successful community-based obesity prevention program: the Be Active Eat Well Program. Obesity 2013;21:2072-80. https://doi.org/10.1002/oby.20472.
- McAuley KA, Taylor RW, Farmer VL, Hansen P, Williams SM, Booker CS, et al. Economic evaluation of a community-based obesity prevention program in children: the APPLE project. Obesity 2010;18:131-6. https://doi.org/10.1038/oby.2009.148.
- Kalavainen M, Karjalainen S, Martikainen J, Korppi M, Linnosmaa I, Nuutinen O. Cost-effectiveness of routine and group programs for treatment of obese children. Pediatr Int 2009;51:606-11. https://doi.org/10.1111/j.1442-200X.2009.02810.x.
- Wake M, Baur LA, Gerner B, Gibbons K, Gold L, Gunn J, et al. Outcomes and costs of primary care surveillance and intervention for overweight or obese children: the LEAP 2 randomised controlled trial. BMJ 2009;339. https://doi.org/10.1136/bmj.b3308.
- Goldfield GS, Epstein LH, Kilanowski CK, Paluch RA, Kogut-Bossler B. Cost-effectiveness of group and mixed family-based treatment for childhood obesity. Int J Obes Relat Metab Disord 2001;25:1843-9. https://doi.org/10.1038/sj.ijo.0801838.
- Hollinghurst S, Hunt LP, Banks J, Sharp DJ, Shield JP. Cost and effectiveness of treatment options for childhood obesity. Pediatr Obes 2014;9:e26-34. https://doi.org/10.1111/j.2047-6310.2013.00150.x.
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. Comparison of program costs for parent-only and family-based interventions for paediatric obesity in medically underserved rural settings. J Rural Health 2009;25:326-30. https://doi.org/10.1111/j.1748-0361.2009.00238.x.
- Panca M, Christie D, Cole TJ, Costa S, Gregson J, Holt R, et al. Cost-effectiveness of a community-delivered multicomponent intervention compared with enhanced standard care of obese adolescents: cost–utility analysis alongside a randomised controlled trial (the HELP trial). BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018640.
- Christie D, Hudson LD, Kinra S, Wong ICK, Nazareth I, Cole T, et al. A community-based motivational personalised lifestyle intervention to reduce BMI in obese adolescents: results from the Healthy Eating and Lifestyle Programme (HELP) randomized controlled trial. Arch Dis Child 2017;102:695-701. https://doi.org/10.1136/archdischild-2016-311586.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Department of Health and Social Care . NHS Reference Costs 2012 to 2013 2014.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Williams A. EuroQol – a new facility for the measurement of health-related quality-of-life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. https://doi.org/10.1258/1355819042250249.
- Chan RS, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health 2010;7:765-83. https://doi.org/10.3390/ijerph7030765.
- Viner RM, Hsia Y, Neubert A, Wong IC. Rise in antiobesity drug prescribing for children and adolescents in the UK: a population-based study. Br J Clin Pharmacol 2009;68:844-51. https://doi.org/10.1111/j.1365-2125.2009.03528.x.
- Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007;357:2329-37. https://doi.org/10.1056/NEJMoa072515.
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Arch Dis Child 2003;88:748-52. https://doi.org/10.1136/adc.88.9.748.
- Freemark M. Pharmacotherapy of childhood obesity: an evidence-based, conceptual approach. Diabetes Care 2007;30:395-402. https://doi.org/10.2337/dc06-1569.
- August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576-99. https://doi.org/10.1210/jc.2007-2458.
- European Medicines Agency . European Medicines Agency Recommends First Switch from Prescription-Only to Non-Prescription for a Centrally Authorised Medicine 2008.
- McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab 2008;93:4600-5. https://doi.org/10.1210/jc.2006-2409.
- Hsia Y, Neubert AC, Viner RM, Wong ICK. Dramatic rise in anti-obesity drug prescribing in children and adolescents: a population-based study in the UK. Pharmacoepidemiol Drug Saf 2008;17.
- Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes Rev 2010;11:593-602. https://doi.org/10.1111/j.1467-789X.2009.00651.x.
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286-91. https://doi.org/10.1136/bmj.309.6964.1286.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions V 5.0.1. Chichester: John Wiley & Sons Ltd; 2008.
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240-3. https://doi.org/10.1136/bmj.320.7244.1240.
- Egger M, Smith GD, Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Books; 2001.
- Reisler G, Tauber T, Afriat R, Bortnik O, Goldman M. Sibutramine as an adjuvant therapy in adolescents suffering from morbid obesity. Isr Med Assoc J 2006;8:30-2.
- Violante-Ortìz R, Del-Rio-Navarro BE, Lara-Esqueda A, Pèrez P, Fanghänel G, Madero A, et al. Use of sibutramine in obese Hispanic adolescents. Adv Ther 2005;22:642-9. https://doi.org/10.1007/BF02849958.
- Van Mil EG, Westerterp KR, Kester AD, Delemarre-van de Waal HA, Gerver WJ, Saris WH. The effect of sibutramine on energy expenditure and body composition in obese adolescents. J Clin Endocrinol Metab 2007;92:1409-14. https://doi.org/10.1210/jc.2006-0264.
- Danielsson P, Janson A, Norgren S, Marcus C. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab 2007;92:4101-6. https://doi.org/10.1210/jc.2007-0826.
- McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Frazer TE, et al. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab 2004;17:307-19. https://doi.org/10.1515/JPEM.2004.17.3.307.
- McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Hubbard VS, et al. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res 2002;10:642-50. https://doi.org/10.1038/oby.2002.87.
- Norgren S, Danielsson P, Jurold R, Lötborn M, Marcus C. Orlistat treatment in obese prepubertal children: a pilot study. Acta Paediatr 2003;92:666-70. https://doi.org/10.1111/j.1651-2227.2003.tb00596.x.
- Ozkan B, Bereket A, Turan S, Keskin S. Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr 2004;163:738-41. https://doi.org/10.1007/s00431-004-1534-6.
- García-Morales LM, Berber A, Macias-Lara CC, Lucio-Ortiz C, Del-Rio-Navarro BE, Dorantes-Alvárez LM. Use of sibutramine in obese mexican adolescents: a 6-month, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2006;28:770-82. https://doi.org/10.1016/j.clinthera.2006.05.008.
- Godoy-Matos A, Carraro L, Vieira A, Oliveira J, Guedes EP, Mattos L, et al. Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab 2005;90:1460-5. https://doi.org/10.1210/jc.2004-0263.
- Berkowitz RI, Fujioka K, Daniels SR, Hoppin AG, Owen S, Perry AC, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med 2006;145:81-90. https://doi.org/10.7326/0003-4819-145-2-200607180-00005.
- Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA 2003;289:1805-12. https://doi.org/10.1001/jama.289.14.1805.
- Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 2005;293:2873-83. https://doi.org/10.1001/jama.293.23.2873.
- Maahs D, Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract 2006;12:18-2. https://doi.org/10.4158/EP.12.1.18.
- Centers for Disease Control and Prevention (CDC) . National Center for Health Statistics: Growth Charts 2009. www.cdc.gov/GrowthCharts/ (accessed 19 August 2019).
- McCarthy HD, Ellis SM, Cole TJ. Central overweight and obesity in British youth aged 11–16 years: cross sectional surveys of waist circumference. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7390.624.
- Mead E, Atkinson G, Richter B, Metzendorf MI, Baur L, Finer N, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD012436.
- Viner R, Bryant-Waugh R, Nicholls D, Christie D. Childhood obesity. Aim should be weight maintenance, not loss. BMJ 2000;320.
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 2007;335:1194-9. https://doi.org/10.1136/bmj.39385.413113.25.
- Hsia Y, Dawoud D, Sutcliffe AG, Viner RM, Kinra S, Wong IC. Unlicensed use of metformin in children and adolescents in the UK. Br J Clin Pharmacol 2012;73:135-9. https://doi.org/10.1111/j.1365-2125.2011.04063.x.
- Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: results from prescription data from a UK general practice database. Br J Clin Pharmacol 2009;67:242-9. https://doi.org/10.1111/j.1365-2125.2008.03347.x.
- Harwood K, Vuguin P, DiMartino-Nardi J. Current approaches to the diagnosis and treatment of polycystic ovarian syndrome in youth. Horm Res 2007;68:209-17. https://doi.org/10.1159/000101538.
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223-36. https://doi.org/10.1056/NEJMra041536.
- Mastorakos G, Lambrinoudaki I, Creatsas G. Polycystic ovary syndrome in adolescents: current and future treatment options. Paediatr Drugs 2006;8:311-18. https://doi.org/10.2165/00148581-200608050-00004.
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007;356:551-66. https://doi.org/10.1056/NEJMoa063971.
- Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ 2006;332. https://doi.org/10.1136/bmj.38867.631551.55.
- Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med 2006;160:241-6. https://doi.org/10.1001/archpedi.160.3.241.
- De Leo V, Musacchio MC, Morgante G, Piomboni P, Petraglia F. Metformin treatment is effective in obese teenage girls with PCOS. Hum Reprod 2006;21:2252-6. https://doi.org/10.1093/humrep/del185.
- Ibáñez L, Ferrer A, Ong K, Amin R, Dunger D, de Zegher F. Insulin sensitization early after menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr 2004;144:23-9. https://doi.org/10.1016/j.jpeds.2003.08.015.
- Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab 2008;93:4299-306. https://doi.org/10.1210/jc.2008-0461.
- Wald AB, Uli NK. Pharmacotherapy in pediatric obesity: current agents and future directions. Rev Endocr Metab Disord 2009;10:205-14. https://doi.org/10.1007/s11154-009-9111-y.
- Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743-5. https://doi.org/10.2337/dc09-0258.
- Rogovik AL, Chanoine JP, Goldman RD. Pharmacotherapy and weight-loss supplements for treatment of paediatric obesity. Drugs 2010;70:335-46. https://doi.org/10.2165/11319210-000000000-00000.
- British National Formulary For Children. London: BMJ Group and Pharmaceutical Press; 2009.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2009.
- Wong IC, Murray ML. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol 2005;59:750-5. https://doi.org/10.1111/j.1365-2125.2005.02450.x.
- Stergachis A, Saunders KW, Davis RL, Strom BL. Textbook of Pharmacoepidemiology. Chichester: John Wiley & Son Ltd; 2006.
- European Pharmaceutical Market Research Association (EphMRA) . The EphMRA Classification System 2011. www.whocc.no/atc_ddd_methodology/the_ephmra_classification_system/ (accessed 9 October 2019).
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems: 10th Revision 1992.
- Hsia Y, Neubert A, Sturkenboom MC, Murray ML, Verhamme KM, Sen F, et al. Comparison of antiepileptic drug prescribing in children in three European countries. Epilepsia 2010;51:789-96. https://doi.org/10.1111/j.1528-1167.2009.02331.x.
- Murray ML, Thompson M, Santosh PJ, Wong IC. Effects of the Committee on Safety of Medicines advice on antidepressant prescribing to children and adolescents in the UK. Drug Saf 2005;28:1151-7. https://doi.org/10.2165/00002018-200528120-00009.
- Neubert A, Verhamme K, Murray ML, Picelli G, Hsia Y, Sen FE, et al. The prescribing of analgesics and non-steroidal anti-inflammatory drugs in paediatric primary care in the UK, Italy and the Netherlands. Pharmacol Res 2010;62:243-8. https://doi.org/10.1016/j.phrs.2010.04.006.
- Rose K, Stötter H, Rose K, van den Anker JN. Guide to Paediatric Clinical Research. Basel: Karger AG; 2007.
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 2002;288:1728-32. https://doi.org/10.1001/jama.288.14.1728.
- European Medicines Agency (EMA) . Questions and Answers on the Suspension of Medicines Containing Sibutramine 2006. www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Sibutramine_107/WC500094238.pdf (accessed 9 August 2019).
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8210.
- White B, Hsia Y, Kinra S, Saxena S, Christie D, Viner RM, et al. Survey of antiobesity drug prescribing for obese children and young people in UK primary care. BMJ Paediatrics Open 2017;1. https://doi.org/10.1136/bmjpo-2017-000104.
- Scheen AJ. Sibutramine on cardiovascular outcome. Diabetes Care 2011;34:114-19. https://doi.org/10.2337/dc11-s205.
- Petkar R, Wright N. Pharmacological management of obese child. Arch Dis Child Educ Pract Ed 2013;98:108-12. https://doi.org/10.1136/archdischild-2011-301127.
- White B, Jamieson L, Clifford S, Shield JP, Christie D, Smith F, et al. Adolescent experiences of anti-obesity drugs. Clin Obes 2015;5:116-26. https://doi.org/10.1111/cob.12101.
- IMS Health . IMS Health – Statistics n.d. http://csdmruk.cegedim.com/our-data/statistics.shtml (accessed 9 August 2019).
- Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251-5. https://doi.org/10.14236/jhi.v19i4.820.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Padwal R, Kezouh A, Levine M, Etminan M. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int J Obes 2007;31:1567-70. https://doi.org/10.1038/sj.ijo.0803631.
- Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247-56. https://doi.org/10.1056/NEJMoa1109333.
- Guest G, Bunce A, Johnson L. How many interviews are enough?: an experiment with data saturation and variability. Field Methods 2006;18:59-82. https://doi.org/10.1177/1525822X05279903.
- Psaroua A, Brown I. Patients’ experiences of prescribed anti-obesity drugs and perceptions of support from primary care: a qualitative study. Prim Health Care Res Dev 2010;11:250-9. https://doi.org/10.1017/S1463423610000083.
- Hollywood A, Ogden J. Taking orlistat: predicting weight loss over 6 months. J Obes 2011;2011. https://doi.org/10.1155/2011/806896.
- Ogden J, Sidhu S. Adherence, behavior change, and visualization: a qualitative study of the experiences of taking an obesity medication. J Psychosom Res 2006;61:545-52. https://doi.org/10.1016/j.jpsychores.2006.04.017.
- Hanghøj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: a systematic review. J Adolesc Health 2014;54:121-38. https://doi.org/10.1016/j.jadohealth.2013.08.009.
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347. https://doi.org/10.1136/bmj.f5934.
- Wellbourn R, Small P, Finlay I, Sareela A, Somers S, Mahawar K. The UK National Bariatric Surgery Registry Second Registry Report 2014.
- Sachdev P, Makaya T, Marven SS, Ackroyd R, Wales JK, Wright NP. Bariatric surgery in severely obese adolescents: a single-centre experience. Arch Dis Child 2014;99:894-8. https://doi.org/10.1136/archdischild-2013-305583.
- White B, Doyle J, Matschull K, Adamo M, Christie D, Nicholls D, et al. Outcomes of 50 patients entering an adolescent bariatric surgery programme [published online ahead of print August 9 2017]. Arch Dis Child 2017. https://doi.org/10.1136/archdischild-2017-312670.
- Black JA, White B, Viner RM, Simmons RK. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev 2013;14:634-44. https://doi.org/10.1111/obr.12037.
- Department of Health and Social Care . Healthy Lives, Healthy People: A Call to Action on Obesity in England 2011. https://www.gov.uk/government/publications/healthy-lives-healthy-people-a-call-to-action-on-obesity-in-england (accessed 18 October 2019).
- Sysko R, Zakarin EB, Devlin MJ, Bush J, Walsh BT. A latent class analysis of psychiatric symptoms among 125 adolescents in a bariatric surgery program. Int J Pediatr Obes 2011;6:289-97. https://doi.org/10.3109/17477166.2010.545411.
- Olbers T, Gronowitz E, Werling M, Mårlid S, Flodmark CE, Peltonen M, et al. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish nationwide Study (AMOS). Int J Obes 2012;36:1388-95. https://doi.org/10.1038/ijo.2012.160.
- White B, Finer N, Adamo M, Duke E, Kingett H, Christie D, et al. Outcomes of an adolescent bariatric service. Arch Dis Child 2012;97. https://doi.org/10.1136/archdischild-2012-301885.152.
- Ford-Adams M, Mortimer H, Bevan D, Datta S, Lax-Pericall M, Desai A. G98 multidisciplinary assessment for bariatric surgery in adolescents: a pilot project from a national referral service. Arch Dis Child 2013;98:A47-8. https://doi.org/10.1136/archdischild-2013-304107.110.
- American Diabetes Association . Standards of medical care in diabetes – 2010. Diabetes Care 2010;33:S11-61. https://doi.org/10.2337/dc10-S011.
- McCrindle BW, Urbina EM, Dennison BA, Jacobson MS, Steinberger J, Rocchini AP, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation 2007;115:1948-67. https://doi.org/10.1161/CIRCULATIONAHA.107.181946.
- Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes 2007;8:299-306. https://doi.org/10.1111/j.1399-5448.2007.00271.x.
- Viner RM, White B, Barrett T, Candy DC, Gibson P, Gregory JW, et al. Assessment of childhood obesity in secondary care: OSCA consensus statement. Arch Dis Child Educ Pract Ed 2012;97:98-105. https://doi.org/10.1136/edpract-2011-301426.
- Upton P, Eiser C, Cheung I, Hutchings HA, Jenney M, Maddocks A, et al. Measurement properties of the UK-English version of the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes 2005;3. https://doi.org/10.1186/1477-7525-3-22.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. https://doi.org/10.1097/00005650-200108000-00006.
- Goodman E. SDQ: SDQ Frequency Distribution for British 11–15 Year Olds, Both Sexes. London: youth in mind; 2001.
- Angold A, Costello E, Messer C. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res 1995;5:237-49.
- Thapar A, McGuffin P. Validity of the shortened Mood and Feelings Questionnaire in a community sample of children and adolescents: a preliminary research note. Psychiatry Res 1998;81:259-68. https://doi.org/10.1016/S0165-1781(98)00073-0.
- Bagley C, Mallick K. Normative data and mental health construct validity for the Rosenberg Self-Esteem Scale in British adolescents. Int J Adolesc Youth 2001;9:117-26. https://doi.org/10.1080/02673843.2001.9747871.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord 1986;5:295-31. https://doi.org/10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T.
- Wardle J, Marsland L, Sheikh Y, Quinn M, Fedoroff I, Ogden J. Eating style and eating behaviour in adolescents. Appetite 1992;18:167-83. https://doi.org/10.1016/0195-6663(92)90195-C.
- Carter JC, Stewart DA, Fairburn CG. Eating disorder examination questionnaire: norms for young adolescent girls. Behav Res Ther 2001;39:625-32. https://doi.org/10.1016/S0005-7967(00)00033-4.
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire?. Int J Eat Disord 1994;16:363-70.
- White WJ. Novel Approaches in Adolescent Obesity Management 2017.
- Weismann D, Pelka T, Bender G, Jurowich C, Fassnacht M, Thalheimer A, et al. Bariatric surgery for morbid obesity in craniopharyngioma. Clin Endocrinol 2013;78:385-90. https://doi.org/10.1111/j.1365-2265.2012.04409.x.
- Doyle J, Colville S, Brown P, Christie D. ‘For the cases we’ve had . . . I don’t think anybody has had enormous confidence’ – exploring ‘uncertainty’ in adolescent bariatric teams: an interpretative phenomenological analysis. Clin Obes 2014;4:45-52. https://doi.org/10.1111/cob.12039.
- Smith J, Jarman M, Osborne M. Doing Interpretative Phenomenological Analysis. Qualitative Health Psychology. London: SAGE Publications Ltd; 1999.
- Bailey K, Otal D, Pemberton J. Understanding Clinician Attitudes Towards Medical and Surgical Treatment of Childhood Obesity Across Canada: A Descriptive Qualitative Approach. Quebéc City, QC: The Canadian Association of Paediatric Surgeons; 2012.
- Zinn JO. Heading into the unknown: everyday strategies for managing risk and uncertainty. Health Risk Soc 2008;10:439-50. https://doi.org/10.1080/13698570802380891.
- Brown P, Calnan M. Trusting on the Edge: Managing Uncertainty and Vulnerability in The Midst of Serious Mental Health Problems. Bristol: Policy Press; 2012.
- Doyle J, Colville S, Brown P, Christie D. How adolescents make decisions about bariatric surgery: an interpretative phenomenological analysis. Clin Obes 2018;8:114-21. https://doi.org/10.1111/cob.12236.
- Politi MC, Clark MA, Ombao H, Légaré F. The impact of physicians’ reactions to uncertainty on patients’ decision satisfaction. J Eval Clin Pract 2011;17:575-8. https://doi.org/10.1111/j.1365-2753.2010.01520.x.
- Burr V, Burr V. An Introduction to Social Constructionism. London: Routledge; 1995.
- Brown PR. The phenomenology of trust: a Schutzian analysis of the social construction of knowledge by gynae-oncology patients. Health Risk Soc 2009;11:391-407. https://doi.org/10.1080/13698570903180455.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13410.
- Hofmann B. Bariatric surgery for obese children and adolescents: a review of the moral challenges. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-18.
- Woolford SJ, Clark SJ, Gebremariam A, Davis MM, Freed GL. To cut or not to cut: physicians’ perspectives on referring adolescents for bariatric surgery. Obes Surg 2010;20:937-42. https://doi.org/10.1007/s11695-010-0152-9.
- Ogden J, Clementi C, Aylwin S. The impact of obesity surgery and the paradox of control: a qualitative study. Psychol Health 2006;21:273-93. https://doi.org/10.1080/14768320500129064.
- van Geelen SM, Bolt IL, van der Baan-Slootweg OH, van Summeren MJ. The controversy over pediatric bariatric surgery: an explorative study on attitudes and normative beliefs of specialists, parents, and adolescents with obesity. J Bioeth Inq 2013;10:227-37. https://doi.org/10.1007/s11673-013-9440-0.
- Bryman A. Social Research Methods. Oxford: Oxford University Press; 2008.
- Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity 2009;17:941-64. https://doi.org/10.1038/oby.2008.636.
- Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 2008;87:398-404. https://doi.org/10.1093/ajcn/87.2.398.
- Tomiyama AJ. Weight stigma is stressful. A review of evidence for the cyclic obesity/weight-based stigma model. Appetite 2014;82:8-15. https://doi.org/10.1016/j.appet.2014.06.108.
- Rees R, Oliver K, Woodman J, Thomas J. The views of young children in the UK about obesity, body size, shape and weight: a systematic review. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-188.
- Rees RW, Caird J, Dickson K, Vigurs C, Thomas J. ‘It’s on your conscience all the time’: a systematic review of qualitative studies examining views on obesity among young people aged 12–18 years in the UK. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004404.
- Heyman B, Shaw M, Alaszewski A, Titterton M. Risk, Safety and Clinical Practice: Health Care Through the Lens of Risk. Oxford: Oxford University Press; 2010.
- Brown P, de Graaf S. Considering a future which may not exist: the construction of time and expectations amidst advanced-stage cancer. Health Risk Soc 2013;15:543-60. https://doi.org/10.1080/13698575.2013.830081.
- White B, Doyle J, Colville S, Nicholls D, Viner RM, Christie D. Systematic review of psychological and social outcomes of adolescents undergoing bariatric surgery, and predictors of success. Clin Obes 2015;5:312-24. https://doi.org/10.1111/cob.12119.
- Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, et al. Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med 2011;26:1183-94. https://doi.org/10.1007/s11606-011-1721-x.
- Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, et al. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes Rev 2011;12:602-21. https://doi.org/10.1111/j.1467-789X.2011.00866.x.
- Sjostrom L. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56-65. https://doi.org/10.1001/jama.2011.1914.
- Herpertz S, Kielmann R, Wolf AM, Langkafel M, Senf W, Hebebrand J. Does obesity surgery improve psychosocial functioning? A systematic review. Int J Obes Relat Metab Disord 2003;27:1300-14. https://doi.org/10.1038/sj.ijo.0802410.
- Ali SM, Lindström M. Socioeconomic, psychosocial, behavioural, and psychological determinants of BMI among young women: differing patterns for underweight and overweight/obesity. Eur J Public Health 2006;16:325-31. https://doi.org/10.1093/eurpub/cki187.
- Pallati P, Buettner S, Simorov A, Meyer A, Shaligram A, Oleynikov D. Trends in adolescent bariatric surgery evaluated by UHC database collection. Surg Endosc 2012;26:3077-81. https://doi.org/10.1007/s00464-012-2318-0.
- Christie D, Viner R. Adolescent development. BMJ 2005;330:301-4. https://doi.org/10.1136/bmj.330.7486.301.
- Caniano DA. Ethical issues in pediatric bariatric surgery. Semin Pediatr Surg 2009;18:186-92. https://doi.org/10.1053/j.sempedsurg.2009.04.009.
- Browne AF, Inge T. How young for bariatric surgery in children?. Semin Pediatr Surg 2009;18:176-85. https://doi.org/10.1053/j.sempedsurg.2009.04.008.
- Willcox K, Brennan L. Biopsychosocial outcomes of laparoscopic adjustable gastric banding in adolescents: a systematic review of the literature. Obes Surg 2014;24:1510-19. https://doi.org/10.1007/s11695-014-1273-3.
- Zeller MH, Roehrig HR, Modi AC, Daniels SR, Inge TH. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics 2006;117:1155-61. https://doi.org/10.1542/peds.2005-1141.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. https://doi.org/10.1136/bmj.b2700.
- O’Brien PE, Sawyer SM, Laurie C, Brown WA, Skinner S, Veit F, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA 2010;303:519-26. https://doi.org/10.1001/jama.2010.81.
- Silberhumer GR, Miller K, Kriwanek S, Widhalm K, Pump A, Prager G. Laparoscopic adjustable gastric banding in adolescents: the Austrian experience. Obes Surg 2006;16:1062-7. https://doi.org/10.1381/096089206778026262.
- Silberhumer GR, Miller K, Pump A, Kriwanek S, Widhalm K, Gyoeri G, et al. Long-term results after laparoscopic adjustable gastric banding in adolescent patients: follow-up of the Austrian experience. Surg Endosc 2011;25:2993-9. https://doi.org/10.1007/s00464-011-1658-5.
- Järvholm K, Olbers T, Marcus C, Mårild S, Gronowitz E, Friberg P, et al. Short-term psychological outcomes in severely obese adolescents after bariatric surgery. Obesity 2012;20:318-23. https://doi.org/10.1038/oby.2011.310.
- Loux TJ, Haricharan RN, Clements RH, Kolotkin RL, Bledsoe SE, Haynes B, et al. Health-related quality of life before and after bariatric surgery in adolescents. J Pediatr Surg 2008;43:1275-9. https://doi.org/10.1016/j.jpedsurg.2008.02.078.
- Ratcliff M, Eshleman K, Reiter-Purtill J, Zeller M. Prospective changes in body image dissatisfaction among adolescent bariatric patients: the importance of body size estimation. Surg Obes Relat Dis 2012;8:470-75. https://doi.org/10.1016/j.soard.2011.10.017.
- Zeller MH, Modi AC, Noll JG, Long JD, Inge TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity 2009;17:985-90. https://doi.org/10.1038/oby.2008.644.
- Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis 2011;7:727-32. https://doi.org/10.1016/j.soard.2011.01.034.
- Sysko R, Devlin MJ, Hildebrandt TB, Brewer SK, Zitsman JL, Walsh BT. Psychological outcomes and predictors of initial weight loss outcomes among severely obese adolescents receiving laparoscopic adjustable gastric banding. J Clin Psychiatry 2012;73:1351-7. https://doi.org/10.4088/JCP.12m07690.
- Collins J, Mattar S, Qureshi F, Warman J, Ramanathan R, Schauer P, et al. Initial outcomes of laparoscopic Roux-en-Y gastric bypass in morbidly obese adolescents. Surg Obes Relat Dis 2007;3:147-52. https://doi.org/10.1016/j.soard.2006.12.002.
- Holterman AX, Browne A, Dillard BE, Tussing L, Gorodner V, Stahl C, et al. Short-term outcome in the first 10 morbidly obese adolescent patients in the FDA-approved trial for laparoscopic adjustable gastric banding. J Pediatr Gastroenterol Nutr 2007;45:465-73. https://doi.org/10.1097/MPG.0b013e318063eef6.
- Holterman AX, Browne A, Tussing L, Gomez S, Phipps A, Browne N, et al. A prospective trial for laparoscopic adjustable gastric banding in morbidly obese adolescents: an interim report of weight loss, metabolic and quality of life outcomes. J Pediatr Surg 2010;45:74-8. https://doi.org/10.1016/j.jpedsurg.2009.10.013.
- Aldaqal SM, Sehlo MG. Self-esteem and quality of life in adolescents with extreme obesity in Saudi Arabia: the effect of weight loss after laparoscopic sleeve gastrectomy. Gen Hosp Psychiatry 2013;35:259-64. https://doi.org/10.1016/j.genhosppsych.2012.12.011.
- Raziel A, Sakran N, Szold A, Teshuva O, Krakovsky M, Rabau O, et al. Mid-term follow-up after laparoscopic sleeve gastrectomy in obese adolescents. Isr Med Assoc J 2014;16:37-41.
- Berlin JA. Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet 1997;350:185-6. https://doi.org/10.1016/S0140-6736(05)62352-5.
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods 1996;1. https://doi.org/10.1037/1082-989X.1.2.170.
- The Cochrane Collaboration . Meta-Analysis of Continuous Data: Measuring the Effect of Treatment Online Learning: Cochrane Collaboration 2002. www.iecs.org.ar/cochrane/guias/Handbook_4-2-2.pdf (accessed 19 August 2019).
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester: John Wiley & Sons, Ltd; 2009.
- Cochrane Heart . Preferred Method for Handling Continuous Variables n.d. https://heart.cochrane.org/sites/heart.cochrane.org/files/uploads/Handling continuous variables.pdf (accessed 19 August 2019).
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769-73. https://doi.org/10.1016/0895-4356(92)90054-Q.
- Sedgwick P. Effect sizes. BMJ 2012;345. https://doi.org/10.1136/bmj.e7370.
- Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. Metan: fixed- and random-effects meta-analysis. Stata J 2008;8:3-28. https://doi.org/10.1177/1536867X0800800102.
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105-14. https://doi.org/10.1016/j.cct.2006.04.004.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Moorehead MK, Ardelt-Gattinger E, Lechner H, Oria HE. The validation of the Moorehead–Ardelt Quality of Life Questionnaire II. Obes Surg 2003;13:684-92. https://doi.org/10.1381/096089203322509237.
- Oria HE, Moorehead MK. Bariatric analysis and reporting outcome system (BAROS). Obes Surg 1998;8:487-99. https://doi.org/10.1381/096089298765554043.
- Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ 2009;338. https://doi.org/10.1136/bmj.a3006.
- Waters E, Salmon L, Wake M. The parent-form Child Health Questionnaire in Australia: comparison of reliability, validity, structure, and norms. J Pediatr Psychol 2000;25:381-91. https://doi.org/10.1093/jpepsy/25.6.381.
- Holterman AX, Holterman M, Browne A, Henriques S, Guzman G, Fantuzzi G. Patterns of surgical weight loss and resolution of metabolic abnormalities in superobese bariatric adolescents. J Pediatr Surg 2012;47:1633-9. https://doi.org/10.1016/j.jpedsurg.2012.02.002.
- Beck JS, Beck AT, Jolly JB, Tideman E, Näswall K. Beck Ungdomsskalor: Manual: Svensk Version: [Bedomning av Emotionell och Social Problematik hos barn och Ungdomar.]. Stockholm: Harcourt Assessment; 2007.
- Zeller MH, Modi AC. Development and initial validation of an obesity-specific quality-of-life measure for children: sizing me up. Obesity 2009;17:1171-7. https://doi.org/10.1038/oby.2009.47.
- Messiah SE, Lopez-Mitnik G, Winegar D, Sherif B, Arheart KL, Reichard KW, et al. Changes in weight and co-morbidities among adolescents undergoing bariatric surgery: 1-year results from the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2013;9:503-13. https://doi.org/10.1016/j.soard.2012.03.007.
- Oude HL, Baur L, Jansen H, Shrewsbury VA, O’ Malley C, Stolk RP, et al. Cochrane review: interventions for treating obesity in children. Evid Based Child Health 2009;4:1571-729. https://doi.org/10.1002/ebch.462.
- Panca M, Viner RM, White B, Pandya T, Melo H, Adamo M, et al. Cost-effectiveness of bariatric surgery in adolescents with severe obesity in the UK. Clin Obes 2018;8:105-13. https://doi.org/10.1111/cob.12232.
- Butland B, Jebb SA, Kopelman PG, McPherson K, Thomas S, Mardell J, et al. Foresight – Tackling Obesities: Future Choices – Project Report 2007.
- Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 2016;17:95-107. https://doi.org/10.1111/obr.12334.
- Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev 2016;17:56-67. https://doi.org/10.1111/obr.12316.
- Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA 2014;312:934-42. https://doi.org/10.1001/jama.2014.10706.
- Baum CL, Ruhm CJ. Age, socioeconomic status and obesity growth. J Health Econ 2009;28:635-48. https://doi.org/10.1016/j.jhealeco.2009.01.004.
- Borg S, Näslund I, Persson U, Odegaard K. Budget impact analysis of surgical treatment for obesity in Sweden. Scand J Surg 2012;101:190-7. https://doi.org/10.1177/145749691210100309.
- National Institute for Public Health and the Environment . DYNAMO-HIA: Generic Software to Quantify the Health Impact of Policies n.d. www.dynamo-hia.eu/en (accessed 9 August 2019).
- Maddams J, Parkin DM, Darby SC. The cancer burden in the United Kingdom in 2007 due to radiotherapy. Int J Cancer 2011;129:2885-93. https://doi.org/10.1002/ijc.26240.
- Office for National Statistics (ONS) . National Life Tables, UK: 2011 to 2013 2014. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014-09-25 (accessed 9 October 2019).
- Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59. https://doi.org/10.1007/s00125-004-1527-z.
- Tsevat J, Weinstein MC, Williams LW, Tosteson AN, Goldman L. Expected gains in life expectancy from various coronary heart disease risk factor modifications. Circulation 1991;83:1194-201. https://doi.org/10.1161/01.CIR.83.4.1194.
- Boysen G, Marott JL, Grønbaek M, Hassanpour H, Truelsen T. Long-term survival after stroke: 30 years of follow-up in a cohort, the Copenhagen City Heart Study. Neuroepidemiology 2009;33:254-60. https://doi.org/10.1159/000229780.
- Soerjomataram I, Thong MS, Ezzati M, Lamont EB, Nusselder WJ, van de Poll-Franse LV. Most colorectal cancer survivors live a large proportion of their remaining life in good health. Cancer Causes Control 2012;23:1421-8. https://doi.org/10.1007/s10552-012-0010-2.
- Macran S. The Relationship Between Body Mass Index and Health-Related Quality Of Life. York: Centre for Health Economics, University of York; 2004.
- Hakim Z, Wolf A, Garrison LP. Estimating the effect of changes in body mass index on health state preferences. PharmacoEconomics 2002;20:393-404. https://doi.org/10.2165/00019053-200220060-00004.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Anokye N, Lord J, Fox-Rushby J. Economic Modelling of Brief Advice on Physical Activity for Adults in Primary Care. London: The National Institute for Health and Care Excellence Centre for Public Health Excellence; 2012.
- Incisive Health . Saving Lives, Averting Costs: An Analysis of the Financial Implications of Achieving Earlier Diagnosis of Colorectal, Lung and Ovarian Cancer 2014.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Methods for the Development of NICE Public Health Guidance. London: NICE; 2012.
- Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics 2000;17:479-500. https://doi.org/10.2165/00019053-200017050-00006.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- National Institute for Health and Care Excellence (NICE) . Social Value Judgements: Principles for the Development of NICE Guidance 2008.
- NHS England . Revisions to Clinical Reference Groups in Specialised Commissioning: Engagement Period Outcome Report 2016. www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2014/01/crg-revisions-outcome-report.pdf (accessed October 2019).
- Black JA, Park M, Gregson J, Falconer CL, White B, Kessel AS, et al. Child obesity cut-offs as derived from parental perceptions: cross-sectional questionnaire. Br J Gen Pract 2015;65:e234-9. https://doi.org/10.3399/bjgp15X684385.
- British Journal of General Practice Life . Top 10 Most Read BJGP Research Articles Published in 2015 n.d. http://bjgplife.com/2016/01/26/top-10-most-read-bjgp-research-articles-published-in-2015/ (accessed 3 December 2018).
- Chan S. GPS urged to play greater role in preventing child obesity. GP Online 2015. www.gponline.com/gps-urged-play-greater-role-preventing-child-obesity/obesity/article/1337358 (accessed October 2019).
- Association for Young People’s Health . Home n.d. www.ayph.org.uk (accessed 3 December 2018).
- Viner RM, Kinra S, Nicholls D, Cole T, Kessel A, Christie D, et al. Burden of child and adolescent obesity on health services in England. Arch Dis Child 2018;103:247-54.
- Bairdain S, Samnaliev M. Cost-effectiveness of adolescent bariatric surgery. Cureus 2015;7. https://doi.org/10.7759/cureus.248.
- Scientific Advisory Committee on Nutrition (SACN) and Royal College of Paediatrics and Child Health (RCPCH) . Consideration of Issues Around the Use of BMI Centile Thresholds for Defining Underweight, Overweight and Obesity in Children Aged 2–18 Years in the UK 2012.
- van Jaarsveld CH, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population-based cohort study. Arch Dis Child 2015;100:214-19. https://doi.org/10.1136/archdischild-2014-307151.
- Jones Nielsen JD, Laverty AA, Millett C, Mainous AG, Majeed A, Saxena S. Rising obesity-related hospital admissions among children and young people in England: national time trends study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0065764.
- Ahmad A, Laverty AA, Aasheim E, Majeed A, Millett C, Saxena S. Eligibility for bariatric surgery among adults in England: analysis of a national cross-sectional survey. JRSM Open 2014;5. https://doi.org/10.1177/2042533313512479.
- Higgins V, Marshall A. Health Survey for England Time Series Dataset, 1991–2009 2012.
- Goldberg D, Williams P. The Users’ Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Daniels SR, Greer FR. Committee on Nutrition . Lipid screening and cardiovascular health in childhood. Pediatrics 2008;122:198-20. https://doi.org/10.1542/peds.2008-1349.
- John WG. UK Department of Health Advisory Committee on Diabetes . Use of HbA1c in the diagnosis of diabetes mellitus in the UK. The implementation of World Health Organization guidance 2011. Diabet Med 2012;29:1350-7. https://doi.org/10.1111/j.1464-5491.2012.03762.x.
- National Institute For Health and Care Excellence (NICE) . Mental Health Problems in People With Learning Disabilities. Quality Standards and Indicators: Briefing Paper 2016. www.nice.org.uk/guidance/qs142/documents/briefing-paper (accessed October 2019).
- National Institute For Health and Care Excellence (NICE) . Depression in Children and Young People. Quality Standards and Indicators: Briefing Paper 2013. www.nice.org.uk/guidance/qs142/documents/briefing-paper (accessed October 2019).
Appendix 1 Wording from specimen National Child Measurement Programme result letters to parents and carers, by child’s weight category (study A)
| Child’s weight category | Summary paragraph from specimen NCMP letters |
|---|---|
| Underweight (BMI lower than second centile of UK 1990 reference) |
Your child’s result is in the underweight range We wanted to let you know your child’s result because it is an important way of checking how your child is growing Many underweight children are perfectly healthy, but sometimes it can mean they have a health problem Some parents find it helps to re-check their child’s BMI after a few months, to see if they have moved into the healthy range as they grow. You can do this using the Healthy Weight tool at: www.nhs.uk/tools/pages/healthyweightcalculator.aspx If you would like to speak to us about your child’s result, please phone the number at the top of this letter |
| Healthy weight (BMI between the second and 90th centiles) |
Your child’s result is in the healthy range We wanted to let you know your child’s result because it is an important way of checking how your child is growing Children of a healthy weight are more likely to grow into healthy adults. To keep growing healthily, it is important that your child eats well and is active Some parents find it helpful to re-check their child’s BMI after a few months, to see if they remain in the healthy range as they grow. You can do this using the Healthy Weight tool at: www.nhs.uk/tools/pages/healthyweightcalculator.aspx Many parents have found the tips in the enclosed leaflet and at www.nhs.uk/change4life useful in helping them make changes to help their child grow healthily. If you would like more advice about your child’s eating or activity, visit www.nhs.uk/change4life or phone the number at the top of this letter |
| Overweight (BMI between the 91st and 97th centiles) |
You may be surprised that your child’s result is in the overweight range It can sometimes be difficult to tell if your child is overweight as they may look similar to other children of their age, but more children are overweight than ever before Research shows that if your child is overweight now, they are more likely to grow up to be overweight as an adult. This can lead to health problems. So this measurement is an important way of checking how your child is growing Many parents have found the tips in the enclosed leaflet and at www.nhs.uk/change4life useful in helping them make small lifestyle changes to keep their child in the healthy weight range Some parents also find it helpful to re-check their child’s BMI after a few months, to see if they remain in the healthy range as they grow. You can do this using the Healthy Weight tool at: www.nhs.uk/tools/pages/healthyweightcalculator.aspx If you are concerned about the result and would like further information and to find out about local activities, please phone us on the number at the top of this letter |
| Very overweight (obese, BMI of more than the 98th centile) |
Your child’s result is in the very overweight range. Doctors call this clinically obese. We wanted to let you know your child’s result because it is an important way of checking how your child is growing Children who are very overweight are more likely to have health problems at a young age, such as high blood pressure, early signs of type two diabetes and low self-confidence. Later in life, they are more likely to have illnesses like heart disease and some types of cancer Small lifestyle changes started now can help your child to grow healthily. Many parents have found the tips in the enclosed leaflet and at www.nhs.uk/change4life useful in helping them make changes to help their child grow healthily Some parents also find it helpful to re-check their child’s BMI after a few months, to see if they have moved towards the healthy range as they grow. You can do this using the Healthy Weight tool at: www.nhs.uk/tools/pages/healthyweightcalculator.aspx If you are concerned about the result and would like further information, please phone us on the number at the top of this letter |
Appendix 2 Interview schedule for parents (study A)
Background
-
Could you tell me a bit about your family?
-
Who lives at home.
-
Ages of children.
-
Feedback
-
Can you remember what your NCMP feedback said and how it made you feel?
-
Do you agree/disagree with the feedback?
-
If agree – why do you think (child) is overweight?
-
If disagree – why do you think feedback states (child) as overweight?
-
-
How do you judge whether (child) is healthy weight or not?
-
Actions/behaviour changes
-
What was your reaction to the feedback?
-
What did you do when you got the feedback?
Prompt: spoke with (child)? Their response
Prompt: changes to your family’s diet/lifestyle after receiving the feedback? (What changes? How these were implemented: specific to child/as a family. Anything that made this difficult? Anything that helped you to make these changes?)
* If no changes: have you wanted to make any? Was there anything that has prevented this or made this difficult?
-
Anything that would help you to make changes to your child’s diet/to other parents?
Prompt: Anything about feedback that helped/could be improved? Format preference?
Service use
-
Have you ever contacted any services e.g. GP, school nurse about your child’s weight? If yes:
-
When?
-
What was the outcome?
If no: Have you wanted to? Is there anything that has prevented this/made this difficult?
-
-
Are you aware of all the different services available to you locally/nationally if you were worried about your child’s weight/wanted to make lifestyle changes?
-
Is there anything that might help you/other parents to seek help from services?
Risk perception
-
How do you think being overweight might affect children now/in future?
Prompts: health, physically, emotionally
-
Are any of these concerns you have for your child?
Prompts: now/in the future
-
If yes were these before or after feedback?
-
If not why not?
-
Overall experience
-
Has getting this feedback influenced whether you would:
-
Withdraw/allow sibling to take part in NCMP (if applicable)
-
Advise friends/family members to let their child take part
Why?
-
-
Are there any changes you would like made to the NCMP?
Prompts: measurements, feedback – anything that would be useful for parents?
Appendix 3 Interview topic guides (study B)
Appendix 4 Clinician interview guide for study D, substudy 6.2
Adolescent bariatric surgery: clinician interview
Introductions
[Explain research and confidentiality again. If happy to proceed, acquire signatures of consent.]
Understanding the context
-
How long have you worked with this service and what is your role?
-
Could you outline the current bariatric surgery pathway for adolescents from referral onwards?
Relevant issues in adolescent bariatric surgery
-
What issues might you consider relevant when making a decision about bariatric surgery for an adolescent?
-
In what situations might you think that a person should not have bariatric surgery, despite their obesity?
Prompt: remember to explore meanings of words (e.g. ‘readiness’ or ‘suitability’ for surgery). For example, when you say X, what are you looking for? What are the indicators of X would you say?
-
What informs your thinking about which young people should or should not have surgery?
Prompt: are there specific guidelines that you adhere to (e.g. NICE guidelines) or is there specific research to inform your thinking? Are these guidelines of use and if not, why not and in what ways?
Differences between clinicians
-
You mentioned that you look for . . . in considering whether a young person should have surgery. Do you think these factors are commonly viewed as important across the service?
-
Are there factors you think others might consider that differ from your own?
-
Have there been times where you have felt uncertain about supporting a young person to have surgery? Why?
-
Have your colleagues had the same uncertainty or have there been differences of opinion?
Differences between adolescents and adults
[Check whether clinician also works with adults having bariatric surgery.]
-
Are there any specific adaptations made for adolescents in comparison to adults having bariatric surgery?
Prompt: for example adaptations made to information given, the way in which information is given or who is involved in the decision.
[If yes] – what are they and why do you think this is important?
The future of adolescent bariatric surgery
-
Have you noticed any changes in the service for adolescents?
-
What do you believe drove these changes?
-
What has been the result of these changes?
-
Are there any developments you’d like to see incorporated into the service for adolescents and why?
Closing the interview
-
We are coming to a close now; are there any questions you thought would be relevant to ask in this interview that I have not yet asked?
-
Do you have any questions for me?
[Explain again what happens now i.e. anonymised interview will be transcribed and then interview recording will be destroyed, etc.]
End.
Appendix 5 Adolescent interview guide for study E, substudy 6.3
Adolescent bariatric surgery: adolescent interview
| Themes | Intention | Possible questions |
|---|---|---|
| 1. Orientation |
|
|
| 2. Process |
|
|
| 3. Decision-making |
|
|
| 4. Expectations and risks |
|
|
| 5. Ending | What have we not considered? | Are there any questions you were expecting to be asked that we haven’t [asked]? |
Appendix 6 Presentations at meetings and congresses
Study A
Falconer C. The Findings from the LSHTM Evaluation of NCMP Feedback. Oral presentation at a series of Department of Health and Social Care NCMP workshops, Autumn 2012.
Falconer C. National Child Measurement Evaluation. Oral presentation. Obesity in Mothers and Children. What are the Links and What Can we do About Them? London School of Hygiene & Tropical Medicine, November 2012.
Falconer C. Can Parents Tell Whether their Child is a Healthy Weight or Have we Become Accustomed to Child Obesity? Oral presentation. Department of Health and Social Care, Obesity and Food Policy Branch, July 2012.
Falconer C, Skow A, Black JA, Croker H, Sovio U, Kessel A, et al. The Majority of Parents of Overweight and very Overweight Children Underestimate their Child’s Weight Status and Weight-Related Health Risk. Oral presentation. Department of Health and Social Care, Obesity and Food Policy Branch, July 2012.
Falconer C, Skow A, Park MH, Sovio U, Croker H, Kessel A, et al. Does BMI Feedback Change Parental Perceptions about the Health Risk Associated with their Child’s BMI? Poster presentation. The 19th European Congress on Obesity, Lyon, France, 9–12 May 2012.
Study B
Skow A, Black JA, Falconer C, Sovio U, White B, Kessel A, et al. Development of an Electronic Tool to Estimate Health Risks and Provide Personalised Weight Management Advice for Overweight Children. Poster presentation. The 19th European Congress on Obesity, Lyon, France, 9–12 May 2012.
Study C
Costa S, Hudson LD, Kessel A, Mathiot A, Wong IC, Nazareth I, et al. Objectively Measured Physical Activity and Sedentary Behaviour in Obese Adolescents, and their Relationship with Fatness and Metabolic Outcomes. Poster presentation. 2015 International Society for Behavioral Nutrition and Physical Activity Annual Meeting, Edinburgh, UK, 3–6 June 2015.
Christie D. RCT of a Motivational Lifestyle Intervention (the Healthy Eating and Lifestyle Programme (HELP)) for Obese Young People. Oral presentation. The 2015 Royal College of Paediatrics and Child Health Annual Conference, Birmingham, UK, 28–30 April 2015.
Christie D. Does a Motivational Lifestyle Intervention (the Healthy Eeating and Lifestyle Programme (HELP)) Work for Obese Young People? Oral presentation. The Society for Adolescent Health and Medicine 2015 Annual Meeting, Los Angeles, CA, USA, 18–21 March 2015.
Costa S, Hudson LD, Kessel AS, Mathiot A, Wong IC, Nazareth I, et al. Eating Behaviours and their Relationship with Adiposity in a Sample of Obese Adolescents. Poster presentation. 2014 International Society for Behavioral Nutrition and Physical Activity Annual Meeting, San Diego, CA, USA, 21–24 May 2014.
Costa S, Hudson LD, Christie D, Kinra S, Mathiot A, Wong I, et al. Fitness May be More Important for Cardiovascular Health than Adiposity in Adolescent Girls. Poster presentation. 2014 International Congress on Obesity, Kuala Lumpur, Malaysia, 17–20 March 2014.
Hudson LD, Costa S, Viner RM, Kinra S, Wong I, Nazareth I, et al. Associations of Physiological and Life-event Measures of Stress with Adiposity in Obese Adolescents. Poster presentation. 2014 International Congress on Obesity, Kuala Lumpur, Malaysia, 17–20 March 2014.
Study D
White B, Jamieson E, Clifford S, Smith F, Wong I, Viner RM. Understanding Young People’s Experiences of Anti-obesity drugs. Poster presentation. 2014 International Congress on Obesity, Kuala Lumpur, Malaysia, 17–20 March 2014.
Study E
White B, Finer N, Adamo M, Duke E, Kingett H, Christie D, et al. Outcomes of an Adolescent Bariatric Service. Oral presentation. The Royal College of Paediatrics and Child Health Annual Conference, Glasgow, UK, 22–24 May 2012.
Appendix 7 Dissemination events organised
Full programme
Findings from the PROMISE Programme and Next Steps for Treatments of Obesity in Children and Young People.
17 June 2015, 1.00–5.20 p.m.
Kennedy Lecture Theatre, UCL Institute of Child Health, London
A national symposium that examined the findings of the PROMISE programme, their implications for public health and policy, clinical practice, and the research community, and, in particular, what they mean for UK actions on child and adolescent obesity. Researchers, policy-makers, practitioners and young people presented and shared their views during the event.
Study A
Evaluation of the National Child Measurement Programme Feedback: Implications for Policy and Action.
21 January 2015, 2.00–4.30 p.m.
Manson Lecture Theatre, London School of Hygiene & Tropical Medicine, London
This event brought together researchers and policy experts to report the main findings of study A, and to discuss the implications of these findings for future development of NCMP feedback, programme implementation and wider policy relating to child obesity.
Studies B and D
How Can GPs Help Prevent and Manage Obesity in Children?
4 March 2015, 6.00–8.00 p.m.
Royal College of General Practitioners, London
This event presented results of the evaluation of the NCMP, what parents were saying about the feedback, and difficulties in raising concerns about children’s weight with parents; in addition, tools that are available to guide discussion and engage families were demonstrated. The aim was to raise awareness among GPs of study B’s and E’s research examining what can be done to play a greater role in the prevention and management of childhood obesity, including a discussion on how this can be achieved together in an open forum.
Study C
Outcomes of the HELP Trial and Next Steps for the Adolescent Lifestyle Interventions for Obesity.
6 February 2015, 10.00 a.m.–4.00 p.m.
Friends House, London
This event highlighted the study design, results and process evaluation. There were group discussions looking at the implication of study results and next possible steps for adolescent lifestyle interventions for obesity. The event was directed at academics, policy-makers, local government and NHS representatives.
Study E
Sharing the Outcomes of Adolescent Bariatric Surgery in England.
17 December 2014, 10.00 a.m.–4.00 p.m.
Levinsky Room, UCL Institute of Child Health, London
This was an invitation-only seminar to share the outcomes of study E’s evaluation of adolescent bariatric surgery at University College Hospital, London, including testimonies of young people who have undergone surgery and discussion about next steps for adolescent bariatric surgery in England. The event was targeted at the four main NHS centres performing adolescent bariatric surgeries in England, with each of them being represented on the day.
Appendix 8 Young people’s responses in the PROMISE focus group
Q1. What health issues might an overweight/obese young person have?
Responses from 11- to 14-year-olds
-
Depressed.
-
Unhappy.
-
Might commit suicide.
-
Might self-harm.
-
Back pain.
-
Tired legs.
-
High cholesterol levels.
-
Specific illnesses, for example cystic fibrosis/sickle cell.
-
Brain damaged.
-
Unconfident.
-
Tired.
-
Craving junk food.
-
Do not want to leave the house.
-
Heart disease.
-
DM.
-
Worried.
-
Anxious.
-
Upset.
-
Being bullied.
-
Not excited about break time because their friends might run around but they cannot . . .
Responses from 15- to 19-year-olds
-
Anxiety.
-
Shutting yourself away.
-
Back pain.
-
Bingeing/starving (disordered eating).
-
Cyber-bullying.
-
Depression.
-
Peer-pressure.
-
Breathing problems.
-
Mood swings.
-
Inactivity.
-
Suicidal thoughts.
-
Self-conscious.
-
Society (creates unreal expectations).
-
Substance misuse.
-
Heart pain.
-
Bloated.
-
Ankle problems.
-
Unprotected sex.
-
Temptation.
Q2. What are the barriers to getting help for overweight/obese young people?
Responses from 11- to 14-year-olds
-
Parents: do not want to say anything to their parents/parents might be unkind.
-
Denial/might not think there is a problem.
-
Lack of opportunities.
-
Worried about bullying getting worse.
-
Hard to get out of school.
-
Fear of being judged.
-
Why they got a weight issue in the first place.
-
Might not want to tell anyone about their issue.
-
Other people . . . Makes them do something they do not want to do.
-
Friends gossip about the problem.
-
Do not want people to worry about you so you hide it.
-
Worried about wasting doctor’s time.
-
GPs only want to hear one problem at a time . . .
Responses from 15- to 19-year-olds
-
Unsure of what the help would be.
-
Fear of consequences.
-
No one believing you.
-
Fear of taking the wrong decision.
-
Getting judged by professionals.
-
Not wanting help.
-
Believing nothing will help.
-
Having your problems dismissed.
-
Losing friends.
-
The media.
-
Ashamed.
-
Parents finding out.
-
People thinking you are different.
-
Not understanding it is a problem.
-
Fear of how people will react.
-
Facing people.
Q3. What are the solutions to getting help?
Responses from 11- to 14-year-olds
-
Childline to expand to cover these issues.
-
111 telephone line to help.
-
Schools could advertise services.
-
Admitting the problem.
-
More research programmes.
-
Parents’ groups.
-
Friends – if they care for you they will help you.
-
Parents to be more honest with their kids and not ‘protect’ them.
-
The GP can send people who are overweight to the gym with a pass.
-
Sports clubs should reinvest profits into more services for young people.
-
The council can make people aware of what they are offering.
-
GPs need to inform parents if their child has weight problems.
-
GPs should not rely on hospitals all the time (and should do more at their practice).
Responses from 15- to 19-year-olds
-
Research and time to ask questions.
-
Feeling safe opening up to someone.
-
Finding someone who has gone through it before.
-
Being informed of all the options.
-
Finding a specialist.
-
Counselling/someone to talk to.
-
Range of different options for help.
-
Doing things in pairs/groups/not alone.
-
Finding friends who support you in what you do and do not do.
-
Stop buying magazines/be critical of media.
-
Opening up to friends.
-
Services being confidential.
-
GPs knowing more about services for young people.
-
Go to centres/be with people who are like you/do the same things.
-
Getting a good first response.
-
Going out with friends/family.
Appendix 9 Responses to the online survey questions
Responses to ‘have you a message for the government about young people becoming very overweight or obese?’
The message that is being sent out by the government is a high carb, low fat, low protein diet which has been scientifically proven to cause weight increase and health issues. The government blamed the obesity epidemic on saturated and unsaturated fats but when studied along with a high protein diet this is show an increase in fat loss on a calorie-controlled diet. The government send mixed messages around this issue but if they were to send out the correct message the government would save millions on the NHS and the health issue associated with obesity.
Can u help us with weight problems, it will save change our lives.
They need to make it easier to make healthy choices as all the fast food etc is just too cheap and available and much easier to have than healthier options.
We don’t have enough education . . . Equally what about those of us that are post 16 that never received that education?!
Try to add taxes on fast food so it is less affordable.
Whatever needs to be done has to be done no matter what method is chosen.
Please provide more opportunities for young people such as free gym and free classes such as Aerobics/Boxing. Also I think young people become very overweight and obese because they finish school so late and this is why they eat junk and fast foods.
Do not pressure people. We don’t need a rise in other eating disorders too!
More needs to be done to stop people becoming overweight in the first place – there is a trend of it being acceptable to be overweight but there are obvious health concerns. I think that there is a link between parents being unhealthy and giving these habits to their children.
I myself have been obese since I was 11, I have tried many methods to lose weight, these are all very expensive or aimed at older people. Sports classes etc are usually full of much fitter people so it is intimidating to go to these. If my emotional problems and addictions had been sorted out when I was a lot younger say 11 or 12 I wouldn’t still be obese and putting a strain on the NHS.
Educate people. Being fat is not healthy, and can severely impact people’s lives. Shortening their life expectancy, reducing their ability to do simple tasks and increasing mental and physical health problems. It’s something that should be looked to reduce through means of education.
It’s clearly a very current and problematic situation that needs to be dealt with more seriously than it currently is.
This issue needs to be looked into and preventative measures need to be taken. Promoting healthy lifestyle especially.
If young people are obese and unhappy, then they must have access to advice and support to help motivate them to live a healthier lifestyle. We can’t tell people when and how to lose weight, we can just make sure healthy living is promoted and encouraged where possible.
Obesity is a problem that is gripping my generation in a chokehold. To an extent, I can see this as the result of powerful trans-national corporations and the hold they have over the British Government. However, once the blame of the problem is solely laid onto another party it is easier for the overweight individual not to see their personal influence in their lifestyle choices. I think that the Government should certainly take a harder line against the TNCs [transnational corporations] that are causing the obesity crisis but individuals also need to be responsible for making their own decisions. The cost on the NHS resulting from obese people is huge, and it is tragic to think that such consequences could be avoided if preventative measures were taken earlier.
People need to feel comfortable being at a healthy weight, when there is too much pressure for young people to be thin people lose sight of what is healthy and unhealthy.
Tax unhealthy foods, make exercise mandatory twice a week through school and college.
Need to educate young people about the health factors of being overweight and underweight rather than letting the media tell young people how they should look, need to stop fat/thin shaming as they are both important.
I feel as though this issue is just getting bigger and bigger – if you’ll pardon the pun. It is one that we, as a country, need to address. Young people need to be educated on what negative impacts their lifestyle decisions that are causing them to be overweight or obese can have, not only to them now, but also in the future, and then encouraged to change that lifestyle into a better one!
Help prevent by having restrictions on places like McDonalds, and make it easier and cheaper to access fitness activities such as gyms.
How could you let this happen It is an abuse of trust.
There are some misconceptions about weight. But some people can change the position that they put themselves in. Being strong and healthy should be enough motivation for people to stay at a balanced weight.
That more campaigns are needed. The government need to be more proactive by increasing the diversity of sport within school – as private [schools] benefit from more equipment and more information on health and nutrition.
It’s a product of young people repeatedly being told that it’s fine to be any shape or size you like. It’s completely OK to be curvy or carry a bit of excess weight, but if you’re big enough to be considered obese then it’s definitely a problem.
If people are unhealthy educate them, do not promote the idea of you are fine as you are in all cases, some obese sleb’s [celebrities] are routinely proclaiming you are fine as you are, when they are actually promoting an unhealthy lifestyle.
Council gyms at low prices!
Need encouragement about eating healthier and educated on what bad food could affect.
Responses to ‘please tell us anything else you want to about young people and weight management’
There should be better awareness of the obesity issue in the UK. It’s costing the government millions and it’s the tax payer who has to pay the bill.’
There is so much pressure on girls and boys to look a certain way – it makes for lots of stress and worry.
I am disabled which makes it difficult for me to lose weight one of my conditions PCOS piles on weight but my Colitis makes me lose weight drastically so I yoyo all the time . . . Nutritional advice would benefit me greatly as well as others in my situation who perhaps aren’t as physically mobile as others our age . . . It’s about finding the things we can do and supporting us in that not about lecturing us on our weight and poor lifestyle choices which we can’t do anything about to a certain extent . . . i.e.: I’m not allowed to do contact sports, cycle, yoga . . . the only ‘safe’ exercise is walking, swimming and hydrotherapy/physiotherapy – but I have no support – no budget or signposting on achievable goals I can reach.
I’ve described myself as overweight but I know I’m not. I’m average body weight if not skinny however I feel massive and worry about my weight all the time. I used to be an obese child between the ages of 9 and 19 however I lost 5 stone at 19. I never worried about weight then but the fear of getting fat is worse. I’m psychologically worse now as a healthy looking young woman than I was before. I suffer with binge eating disorder but everyone just laughs it off if I’m having a bad day as I’m slim. I find it difficult to speak to people about it and even my boyfriend doesn’t know that I binge quite a lot. I can’t talk to anyone and I feel that as I’m in a professional job it will show weakness by admitting that I suffer with this problem.
I hope you appreciate that underweight young people are just as big of a problem as overweight young people.
Young people do have their own responsibly but only know how to make the best choices when educated correctly.
We should be focusing on fitness and not weight.
I think at the moment we have quite an ‘all or nothing’ approach to health, there are the people that are super fit, and then everyone else. I think small adjustments to diet or exercise should be encouraged and rewarded more so than a focus on complete change of lifestyle as they are much more achievable and sustainable so people would be more likely to try.
It’s not as simple as eating right and exercise. Eating is a very emotional issue and should be treated with respect and dignity.
The parents are the biggest problem, they often influence their kids from a young age.
There needs to be more education for young people around weight gain, weight loss and being healthy. Lots of young people aren’t aware of facts surrounding how to safely lose weight and the impact diets can have on their health.
I feel as though those young people that are obese/overweight should not be shamed for their body image, instead encouraged and helped to change it – assuming that is what they wish. If you encourage people to lose weight by themselves and give them the motivation and education on how to do so, it is far more likely to have a positive effect and have that individual keep the weight off! Younger people should not be given a choice to lose weight via ways other than healthy eating and lots of exercise!
I have found it very difficult to lose weight as my parents were overweight and were not totally aware of how to reduce the risk of childhood obesity, which means that I have had the wrong building box to begin with. I have been going to the gym regularly and have been with a weight loss group for the last 3 years but cannot keep my focus and seem to be putting weight on faster than I lose it.
No idea!
Too many people are lazy.
Coming from someone who has lost circa 20 kg in the past year I have found that it is surprisingly easy to lose weight (without any formal diet/calorie counting) once you start exercising properly. From my own experiences, encouraging people to do more exercise is a lot more effective than constant negative messages about eating!
It’s not always healthy for people to have information forced down their throats. People aren’t always underweight when they struggle with eating disorders and more care needs to be taken in helping both ends of the spectrum. Information should be readily available for everyone with regards to diet and exercise. Keeping our bodies healthy should be as much of a priority as education.
Institutions such as schools colleges military employers etc need to provide healthier food. Employers such as the NHS should look at their work force and consider if they are setting a good example. The number of obese nurses and teachers is concerning. Likewise the number of obese/smoker nursing students is also something that needs to be addressed.
Appendix 10 Social media messages by the Association for Young People’s Health
The following are the Twitter messages by AYPH:
@AYPHcharity #promiseresearch

Findings from #promiseresearch on obesity treatment for young people now being launched. Major 5 year study involving @UCL @UCL_ICH funded by @NIHR.
Findings from @ucl Paediatric Research in Obesity Multi-Modal Intervention and Service Evaluation Programme (PROMISE) will be tweeted under #promiseresearch.
#promiseresearch was major 5 year programme with 5 strands. www.ucl.ac.uk/promise We’ve been working with young people involved to hear their views.
Young people involved in #promiseresearch into obesity treatment said they enjoyed taking part and would recommend research opportunities to other young people.
GPs and parents encouraged young people to take part in #promiseresearch but it should’ve been advertised in social media, said participants.
Young people took part in #promiseresearch as they ‘needed to lose weight’ and it came along at the right time for them.
#promiseresearch was a ‘good opportunity’ to lose weight, but needed self-motivation, said young people who took part. #obesity.
Age appropriate activities needed for children and teens, said young people involved in #promiseresearch – 16 year olds don’t want to do ‘Vegetable A-Z’.
Could obesity research involve more action and less education? Q from young people involved in #promiseresearch.
1Teens involved in #promiseresearch called for group work opportunities and chance to meet others going through same experience.
Young people in #promiseresearch said weight loss programmes should include counselling to address social and emotional health issues.
Obesity treatment for young people should include ‘range of options’ to lose weight, said participants on #promiseresearch programme @UCL @UCL_ICH @NIHR.
Incentives such as vouchers encouraged young people to take part in #promiseresearch but some would’ve liked a gym pass too!
‘Why did parents allow us to get overweight in the first place?’ ask young people involved in #promiseresearch.
‘Parents need to be less protective and more honest with children who are overweight’ said young people in #promiseresearch @UCL @UCL_ICH @NIHR.
#promiseresearch found parents were not good at identifying overweight and obesity in their children.
Taking part in #promiseresearch needed the whole family’s commitment as parents had to buy certain food and some lost weight too, said teens.
‘I wanted to make my family proud’ said young person in #promiseresearch to lose weight. Each had different motivations.
Young people who took part in #promiseresearch said teens with weight issues face range of physical and mental health problems.
Suicidal thoughts were identified as one issue facing young obese people, along with bullying, fear, depression and anxiety. #promiseresearch.
Overweight and obese young people didn’t want to tell parents as they ‘feared consequences’ and ‘didn’t want to worry them’ #promiseresearch #barrierstohelp.
Young people with weight issues didn’t know where to get help and didn’t want to ‘waste GPs’ time’ #promiseresearch #barrierstohelp.
Young people with weight problems sometimes feel ‘nothing will help’ so don’t seek help. #promiseresearch.
2Denial and not realising there’s a problem were seen as reasons for not getting weight loss help #promiseresearch.
Stigma and fear of ‘being judged’ by health professionals stopped young people seeking help with overweight and obesity #promiseresearch.
Losing friends and being bullied were reasons to not get help for obesity, said young people involved in #promiseresearch @UCL @UCL_ICH @NIHR.
Gyms ‘full of fit people’ are intimidating to teens who are overweight, said young people in #promiseresearch.
Helplines for overweight teens might help, said young people in #promiseresearch – eg Childline or NHS 111.
Councils should make sports centres free/affordable to young people with weight issues, said focus group in #promiseresearch.
Weight loss services need to be confidential and easily available, said young people in #promiseresearch.
Glossary
- Complier-average causal effect
- The average impact of an intervention on those who comply with their treatment assignment.
- EuroQol-5 Dimensions, three-level version
- A descriptive system of health-related quality of life states consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each taking one of three responses according to level of severity: (1) no problems; (2) some or moderate problems; and (3) extreme problems within a particular EuroQol-5 Dimension.
- Interpretative phenomenological analysis
- An approach to psychological qualitative research with an idiographic focus, aiming to offer detail on how a given person, in a given context, makes sense of a given phenomenon.
- Medical Research Council Phase III efficacy randomised clinical trial
- A randomised clinical trial comparing a fully defined complex intervention with an appropriate alternative, using a protocol that is theoretically defensible, reproducible and adequately controlled, with appropriate statistical power.
- National Child Measurement Programme
- A nationally mandated public health programme in which the weight and height of children in Reception class (aged 4–5 years) and Year 6 (aged 10–11 years) are measured, to assess overweight and obesity levels within primary schools. The resulting data can be used at a national level to support local public health initiatives and inform the local planning and delivery of services for children.
- National Institute for Health and Care Excellence
- An entity responsible for producing evidence-based national guidance and advice to improve health and social care; developing quality standards and performance metrics for those providing and commissioning health, public health and social care services; and sourcing a range of information services for commissioners, practitioners and managers across the spectrum of health and social care.
- Outcome
- The measure used to assess change following an intervention.
List of abbreviations
- AGB
- adjustable gastric band
- AIS
- Additional Information Service
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- AOD
- anti-obesity drug
- AUC
- area under the curve
- AYPH
- Association for Young People’s Health
- BANES
- Bath and North East Somerset
- BMI
- body mass index
- BOOST
- Bristol Online Obesity Screening Tool
- BT
- behaviour therapy
- BYI
- Beck Youth Inventory™
- CATCH
- Computer-Assisted Treatment of CHildren
- CI
- confidence interval
- CRF
- clinical research facility
- CVE
- cerebrovascular event
- CYP
- children and young people
- DAWBA
- Development and Well-being Assessment
- DHSC
- Department of Health and Social Care
- DM
- diabetes mellitus
- EDE-Q
- Eating Disorders Examination Questionnaire
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- HELP
- Healthy Eating and Lifestyle Programme
- HRQoL
- health-related quality of life
- HSE
- Health Survey for England
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IMD
- Index of Multiple Deprivation
- IMS DA
- IMS® Disease Analyzer
- IOTF
- International Obesity Task Force
- IPA
- interpretative phenomenological analysis
- IQR
- interquartile range
- ITT
- intention to treat
- IWQoL
- Impact of Weight on Quality of Life
- LA
- local authority
- LMS
- least mean squares
- MDT
- multidisciplinary team
- MRC
- Medical Research Council
- NCMP
- National Childhood Measurement Programme
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- OSA
- obstructive sleep apnoea
- OTC
- over the counter
- P
- participant
- PCOS
- polycystic ovarian syndrome
- PCT
- primary care trust
- PedsQL
- Pediatric Quality of Life Inventory
- PHE
- Public Health England
- PPI
- patient and public involvement
- PROMISE
- Paediatric Research in Obesity Multi-modal Intervention and Service Evaluation
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUOROM
- Quality of Reporting of Meta-analyses
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RELACHS
- Research with East London Adolescents: Community Health Survey
- RSE
- Rosenberg Self-Esteem Scale
- RYGB
- Roux-en-Y gastric bypass
- SD
- standard deviation
- SDQ
- Strengths and Difficulties Questionnaire
- SDS
- standard deviation score
- SG
- sleeve gastrectomy
- T2DM
- type 2 diabetes mellitus
- THIN
- The Health Improvement Network
- UCL
- University College London
- WHO
- World Health Organization
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar08030).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.