Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0609-10171. The contractual start date was in June 2011. The final report began editorial review in May 2017 and was accepted for publication in May 2019. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Karl Claxton reports personal fees from F. Hoffmann-La Roche Ltd (Basel, Switzerland) and personal fees from HERON (Parexcel, Walton, MA, USA), outside the submitted work. Nicky Cullum reports non-financial support from Kinetic Concepts, Inc. (KCI; San Antonio, TX, USA), outside the submitted work. David Torgerson reports grants from the National Institute for Health Research (NIHR), outside the submitted work, and is director of a clinical trial unit funded by NIHR. Nicky Welton reports grants from NIHR, during the conduct of the study, and she is principal investigator for a research grant jointly funded by the Medical Research Council and Pfizer Inc. (New York, NY, USA), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Chetter et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
Introduction
More than 10 million surgical operations are conducted every year in the NHS, with the majority of these involving a wound created by surgical incision. 1 Most incised surgical wounds heal by primary intention, when the wound edges are closely apposed and sutured, clipped or glued together. However, not all surgical wounds heal in this way; some wounds are left open after surgery and heal from the bottom up by the formation of granulation tissue (known as healing by secondary intention). The surgical wounds healing by secondary intention (SWHSIs) may be planned (e.g. after significant tissue loss for which primary closure is not possible, or in the presence of infection). Alternatively, healing by secondary intention may be unplanned when a primarily closed wound opens or ‘dehisces’ (full or partial separation of wound edges). These SWHSIs, also called ‘open surgical wounds’, may remain open for many months, are prone to infection, may prolong hospital stays, and necessitate further hospital admissions and surgeries (delayed primary closure) or skin grafting,2 all adversely affecting patients’ quality of life and incurring substantial NHS costs.
Prevalence of SWHSIs
Although SWHSIs are thought to be relatively common, when we planned this research there were few national or international data regarding their epidemiology. Two published studies estimated that SWHSIs constituted approximately 28% of all prevalent surgical wounds. 3,4 From data collected in Hull and East Yorkshire, UK, Srinivasaiah et al. 4 reported 136 open surgical wounds and 74 dehisced surgical wounds, from a total population of 590,000 (a point prevalence of 0.36/1000 population). Further UK cross-sectional data from Bradford, UK, reported 111 wounds that were classed by the study authors as open surgical wounds and a further 88 that were classed by the study authors as having broken down post surgery. 3 However, the size of the underlying population and, hence, point prevalence for these wounds was not reported. A population-based estimate of the prevalence of SWHSIs alongside in-depth investigation of their origin, natural history, typical treatments and impact on patients and health services was needed.
Details of patient and wound characteristics
Previous research on wound prevalence by Vowden and Vowden3 and Srinivasaiah et al. 4 focused on all wounds, rather than specific wound types. To the best of our knowledge, when we began this research, there was no high-quality information (national or international) describing patients with a SWHSI (age, sex, comorbidities, concomitant medications, surgical speciality); wound characteristics (site, size location, aetiology, whether or not the wounds were planned to be left open before surgery, frequency of wound infection); SWHSI treatments or healing rates in general and for specific patient groups (e.g. those with diabetes mellitus). Robust time to healing data were also limited and reports frequently presented inaccurately analysed data. 5–10 The generalisability of the existing research was also limited, as many trials reported time to healing rates in relation to subpopulations of SWHSI patients and treatments: one trial5 of perineal wounds and foam dressing, two trials7,8 of pilonidal sinus wounds and silastic foam dressing, and one trial10 of below-knee amputation wounds and plaster cast with silicone sleeve or elastic compression. The lack of systematic data describing SWHSIs and their treatment and outcomes was matched by a lack of knowledge about the impact of SWHSIs on patients’ quality of life or daily functioning, as well as the impact of these wounds on the use of health-care resources. This, in combination, made it almost impossible to assess the impact of SWHSIs and their treatments on patients, or to plan new research studies to assess the clinical effectiveness and cost-effectiveness of potential new or alternative treatments or interventions.
The treatment of SWHSIs
Management of SWHSIs often involves daily or more frequent dressing changes, sometimes with packing of the wound cavity. Many different dressing options are available, from simple dressings such as gauze (which can be painful to remove) to more modern options such as foam, hydrocolloid and alginate dressings. Wounds may also be treated by debridement (the removal of foreign material and devitalised tissue) or by skin grafting. When this research was conceived, the type and frequency of treatments being used for SWHSIs was not known. Randomised controlled trials (RCTs) in SWHSI patients are infrequent and often poorly designed, underpowered, utilise surrogate outcomes and often report limited or incorrectly analysed healing data, making interpretation of these data incredibly difficult. For example, a multicentre RCT comparing the effects of zinc oxide with placebo mesh on secondary-healing pilonidal wounds was underpowered, recruiting only 64 participants (n = 33 zinc and n = 31 placebo), and although it did not detect a difference in median healing times (54 days, interquartile range 42–71 days for zinc; 62 days, interquartile range 55–82 days for placebo; p = 0.32), this may have been due to the lack of power. In the same study, fewer postoperative antibiotics (a surrogate marker of wound infection) were prescribed in the zinc oxide group (n = 3) than in the placebo group (n = 12) (p = 0.005). 11
In terms of evidence of effectiveness of different treatment options, Vermeulen et al. 12 explored the relative effects of dressings and topical agents for SWHSIs in a systematic review. They found only 13 RCTs, all of which were small, of poor quality and often conducted > 10 years previously. There was no evidence to indicate that the choice of dressing or topical treatment offered any benefit in terms of wound healing time, but the data were sparse. A systematic review, conducted to inform National Institute for Health and Care Excellence (NICE) clinical guidelines on surgical site infection, investigated the effects of topical antiseptics and antibiotics (including impregnated dressings) on the risk of wound infection in SWHSIs. 13 The four included studies were small and provided generally uncertain evidence on a range of uncommon treatment options. The guidelines recommend that chlorinated lime and boric acid [Edinburgh University Solution of Lime (EUSOL)] and gauze, moist cotton gauze or mercuric antiseptic solutions should not be used to prevent surgical site infection in the management of SWHSIs. 13
Increasingly, more advanced and costly wound treatments have been extensively adopted into clinical practice, with inadequate supporting evidence. One example is negative-pressure wound therapy (NPWT), a device that applies a carefully controlled negative pressure (or vacuum) to the dressing, moving tissue fluid away from the treated wound area into a disposable canister. 14 The canister is removed and replaced either when it becomes full or at least once per week. The device is generally used as part of the SWHSI treatment pathway, rather than to the point of healing, and can be administered and removed by both nurses and surgeons.
Cost of treating SWHSIs
Surgical wounds healing by secondary intention can be large and may produce a significant volume of exudate, which can be difficult to manage, and frequent, time-consuming, dressing changes are common. In addition, SWHSIs must also be protected from infection and trauma during healing. It has been suggested that SWHSIs are at high risk of postoperative infection, with the wound providing an ideal environment for bacterial proliferation, which may be associated with delayed healing. 15 These factors, and the possible need for further surgery, suggest that the costs of treating patients with SWHSIs could be substantial. The lack of available epidemiological evidence in relation to SWHSIs had, however, made it difficult to ascertain the health and social care costs of treating these wounds.
SWHSIs: the patients’ perspective
The lack of systematic data describing SWHSIs, their treatment and outcomes was matched by a scarcity of knowledge about the impact of SWHSIs on patients’ quality of life. Little was known regarding patients’ perspectives and experiences of living with a SWHSI, or their views, expectations and concerns regarding management and outcomes.
SWHSIs: the clinicians’ perspective
A variety of clinicians, including surgeons, tissue viability nurses and general practice nurses, are all commonly involved in the management of patients with SWHSIs. As previously stated, there is a dearth of high-level evidence to guide the management of these patients, particularly regarding the clinical effectiveness and cost-effectiveness of different treatments or interventions. Greater clarity is required regarding assessment of these wounds and the factors that influence management decisions.
Summary
In summary, this research programme was undertaken recognising that SWHSIs may be common and costly but that there was a need for further exploration of the extent, nature, outcomes and impact of these wounds. The lack of international epidemiological data regarding these patients seriously impairs the development of new research studies in this area. There was also huge uncertainty regarding SWHSI management. Options ranged from inexpensive simple dressings to the more complex and costly NPWT, but accurate data regarding the frequency of use of specific treatments was non-existent. In addition, all treatment options for SWHSIs lacked robust, supportive evidence bases in terms of clinical effectiveness and cost-effectiveness, and the impact on patients’ quality of life. As a result, clinicians and patients were uninformed about how to care for SWHSIs and, specifically, which treatment options were most effective and offered the best value for money. The need for research to fill these evidence gaps was also highlighted by the NICE guidance. 13
Given the number of treatment options available, it is important that patients, carers and health professionals are involved in trial design, including the selection of significant outcome measures, and that research is conducted through sufficiently powered trials to detect significant differences in healing rates between treatments. However, the enormous cost of conducting the trials required to fill the evidence gaps means that a rush to undertake such studies, before obtaining additional key intelligence, would be hugely inefficient. There were several important unknowns: information about the natural history and epidemiology of SWHSIs; which treatments were being used most commonly and, thus, should be evaluated; whether or not the decision uncertainty associated with various treatments justifies an expensive trial; and whether or not a trial is feasible. In addition, little was known about which outcomes matter to patients with SWHSIs and what the desirable treatment characteristics are from the perspective of the staff treating them. We have undertaken research to generate vital data regarding an important, costly and hugely under-researched health problem. The research findings will aid in planning service delivery and future research, including economic modelling and primary studies.
Aims
We aimed to characterise and quantify SWHSIs, their outcomes and impact, and to begin to identify effective SWHSI treatments. The core aims of the research were achieved via four interdependent workstreams. The links between the four workstreams are shown in Figure 1.
FIGURE 1.
Overview of the whole research programme and its inter-relationships.

Workstream 1
Workstream 1 aimed to describe the number, characteristics, current treatments, impacts and health outcomes of patients with SWHSIs. This was achieved using an initial prevalence survey and a subsequent inception cohort of patients with a SWHSI to collect naturalistic data regarding treatments used, rates of healing, adverse events and quality-of-life changes.
Workstream 2
Workstream 2 aimed to use all available research data to estimate the cost-effectiveness of current treatments for SWHSIs, as identified in workstream 1, and was completed using evidence synthesis and decision-analytic modelling. If assessments of cost-effectiveness suggested that there was decision uncertainty (i.e. uncertainty about which treatment offers best value to the NHS), we would extend analyses to assess whether or not investment in future research on treatments for SWHSIs was likely to be worthwhile and, if so, what research would offer best value for money.
Workstream 3
Workstream 3 aimed to determine the impact of SWHSIs on patients, specifically their perspectives and experiences of living with a SWHSI, of wound management and relevant wound outcomes. In addition, this workstream aimed to determine NHS professionals’ views about SWHSI treatments and important outcomes to measure treatment effects. This was achieved through qualitative interviews with both patients and NHS professionals.
Workstream 4
Workstream 4 aimed to determine the feasibility of conducting further primary research on SWHSIs through a pilot, feasibility RCT.
This synopsis describes the results of our findings from all four workstreams. Publications arising from this research programme are listed in the Acknowledgements.
Patient and carer involvement
Involvement of patients and carers was identified at the outset as a priority for this research.
In workstream 1, two patient representatives contributed to the design of documentation for the cohort study and provided their perspective on study processes, questions and data interpretation through attendance at associated workstream meetings. A dedicated section of the meeting was devoted to patient feedback and the meeting was followed by an informal discussion between the research lead, a study research nurse and the patient representatives. When possible, any comments received from the patient representatives were incorporated into relevant study documentation or procedures. A newsletter to inform participants about the progress of the research was initiated by the patient representatives who assisted in the design and review of this document.
In workstream 3, the input of patient and carer representatives was crucial in the design and piloting of the interview topic guide, and the interpretation of the findings arising from the qualitative interviews, including review of the main study findings and input into the writing of the results. A summary of the results of the qualitative work was subsequently sent to participants, following review by the patient representatives.
Patient and carer involvement for this programme of work culminated in the development of a ‘Patient User Group’, convened at the start of workstream 4. This group was developed, following experiences in workstream 1, to increase the number of patient representatives involved and so reduce any burden on the two initial representatives involved. Ten patients or carers with experience of SWHSIs were invited to attend the inaugural meeting in March 2015, with five representatives subsequently attending. This resulted in a diverse group, with a mix of age, sex, SWHSI history and previous research experience. A further two meetings were convened during workstream 4 to provide comment on patient-facing documentation and study procedures and to discuss the interpretation of the results of the pilot, feasibility RCT. As in workstream 1, when possible, any comments received from the patient representatives were incorporated into study documentation or procedures. One member of the group was subsequently recruited to act as a patient and public representative for the workstream 4 Management Group meetings. The member provided a patient perspective on the trial processes and documentation, and made substantial contributions. This patient representative also contributed to and reviewed the summary of workstream 4 for this report and the associated papers arising from this workstream. In addition, a lay representative was also included in the workstream 4 Data Monitoring and Ethics Committee to provide patient input on data quality and patient safety and to advise on any issues arising during the trial.
The inclusion of patient and carer representatives has been instrumental in shaping this research programme and will undoubtedly be beneficial in shaping future research in this area. The mix of patients and carers providing input to the individual workstreams and in the Patient User Group provided the research programme team with a diverse range of experiences to draw on and consider when making decisions in relation to this research programme. Given the number of patient and public members involved in the various stages of this programme, there was naturally a range of views noted regarding patient and carer experiences, study procedures and documentation; however, in the vast majority of cases there was a consensus of opinion regarding progression of this research.
Workstream 1: cross-sectional survey and cohort study of SWHSIs
The dearth of national and international clinical and research information regarding the prevalence, patient characteristics and treatments for SWHSIs meant that we did not know the extent and nature of these wounds, how and where they were managed or the associated outcomes. To enable accurate assessment of time to healing and treatment use, we planned an inception cohort study to address key uncertainties. An inception cohort approach was essential to allow accurate assessment of SWHSI duration. Planning the cohort study was difficult, however, as there were no data regarding where most SWHSIs occurred. For example, were most SWHSIs left open in theatre or did they subsequently break down in hospital or in the community? We needed basic information regarding the numbers of patients with SWHSIs and their location in order to put adequate resources in place for the inception cohort study. We therefore preceded the cohort study with a cross-sectional survey. This approach facilitated the gathering of initial intelligence to support subsequent work and, in addition, the use of a cross-sectional design allowed us to estimate a point prevalence estimate for SWHSIs. The cross-sectional survey has been published (see Appendix 1). 16 The cohort study has also been published (see Appendix 2). 17
Cross-sectional survey
Objectives
The cross-sectional survey aimed to capture information to aid the design of the cohort study. This included the:
-
number of patients with a SWHSI (point prevalence), their characteristics and the NHS settings where they were receiving treatment
-
proportions of SWHSIs planned prior to surgery and those that occurred as a result of wound breakdown (dehisced) after surgery.
Information was also collected on typical durations of SWHSIs, the types of surgery preceding the SWHSI and the treatments received by patients.
Methods
The cross-sectional survey was conducted over a 2-week period (spring 2012) in primary, secondary and community care settings in Hull and the East Riding of Yorkshire, UK. We asked health-care professionals to identify patients on their case load who had at least one SWHSI (i.e. a wound deliberately left open owing to infection, swelling, contamination or empty tissue space, a wound initially closed but subsequently dehisced or a wound arising from surgical debridement). When patients had multiple SWHSIs, the largest wound was deemed to be the ‘reference SWHSI’. Detailed data were collected, including patient and wound characteristics, health-care provider, clinical and surgery details, and treatment events (see Appendix 3). As this survey was limited to secondary use of routinely collected information, Research Ethics Committee approval was not required. 18 Approval was, however, obtained from associated NHS trusts prior to commencement of data collection.
Data analysis was conducted using IBM SPSS Statistics, version 21 (IBM Corporation, Armonk, NY, USA). Categorical variables were summarised as frequencies and proportions, whereas continuous variables were summarised as medians and associated 95% confidence intervals (CIs) and ranges. The usual resident regional population aged ≥ 20 years, as taken from the 2011 census, was used as the denominator when calculating point prevalence. 19
Results
Following removal of duplicate cases (identified through age, ethnicity and wound characteristics), data were analysed for 187 patients with a SWHSI. In total, 62% of patients were male, 95.2% were of white British ethnicity and the median age was 58.0 years (range 19–90 years), as detailed in Table 1.
| Variable | Patients (N = 187) |
|---|---|
| Sex, n/N (%) | |
| Male | 116/187 (62.0) |
| Female | 62/187 (33.2) |
| Age (years) | |
| Median (95% CI) | 58.0 (55 to 61) |
| Minimum–maximum | 19.0–90.0 |
| Missing, n/N (%) | 3/187 (1.6) |
| Ethnicity, n/N (%) | |
| White British | 178/187 (95.2) |
| Asian Indian, Asian other, black other, other mixed background, white other, white and Asian and not specified | 7/187 (3.7) |
| Missing | 2/187 (1.1) |
| Number of SWHSI per patient, n/N (%) | |
| 1 | 164/187 (87.7) |
| 2 | 16/187 (8.6) |
| 3 | 4/187 (2.1) |
| 4 | 1/187 (0.5) |
| Missing | 2/187 (1.1) |
We estimated the point prevalence of treated SWHSIs as 4.1 per 10,000 population (95% CI 3.5 to 4.7 per 10,000 population), using the resident regional population value as the denominator. 19 The observed maximum number of SWHSIs per patient was four (1/187, 0.5%), with the majority of patients (164/187, 87.7%) having only one SWHSI. Patients were more frequently treated in community than in secondary care settings (109/187, 58.3% vs. 56/187, 29.9%, respectively), with the remaining patients treated within primary care (8/187, 4.3%) or other care settings (2/187, 1.1%). There were 12 patients (12/187, 6.4%) for whom treatment location was not recorded.
We found that almost half of the SWHSIs were planned to heal by secondary intention (n = 89, 47.6%) and 77 (41.2%) were primarily closed wounds that had subsequently dehisced. A further six wounds (3.2%) were surgically opened, six (3.2%) arose for other reasons [surgical debridement (n = 2, 1.1%), sutures removed and left open to heal (n = 1, 0.5%), skin graft failure (n = 1, 0.5%), non-healing wound (n = 1, 0.5%) and necrotising fasciitis requiring debridement (n = 1, 0.5%)] and for nine patients (4.8%) the information was not known or was missing. The median time from surgery to wound breakdown was 9 (95% CI 7 to 10) days.
In addition, we found that the median duration of wounds at the point of survey was 28 days (95% CI 21 to 35 days). Fully dehisced SWHSIs had the longest median duration (35 days, 95% CI 15 to 150 days), and those with long wound durations were most frequently treated within primary care (median duration 112 days, 95% CI 21 to 469 days) and community settings (median duration 35 days, 95% CI 28 to 56 days) (Table 2).
| Wound duration (days)a | Treatment setting | ||
|---|---|---|---|
| Community | Secondary care | Primary care | |
| n/N | 89/109 | 56/56 | 7/8 |
| Median (95% CI) | 35.0 (28 to 56) | 14.5 (6 to 21) | 112.0 (21 to 469) |
| Minimum–maximum | 1–560 | 1–238 | 21–896 |
| Missing, n (%) | 20 (18.3) | 0 (0) | 1 (12.5) |
In total, we identified 43 different surgical procedures that preceded the development of a SWHSI. The most common surgical procedures were for pilonidal sinuses/abscesses (28/187, 15.0%), lower-limb amputations (19/187, 10.2%) and laparotomy with bowel resection (19/187, 10.2%). As detailed in Table 3, the most common surgical specialties associated with SWHSIs were colorectal (80/187, 42.8%), plastic (24/187, 12.8%) and vascular (22/187, 11.8%) surgery.
| Surgical specialty | Wound category (n) | Total (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Planned | Partially dehisceda | Fully dehisceda | Surgically openeda | Other | Not known | Missing | ||
| Colorectal | 51 | 16 | 8 | 1 | 1 | 2 | 1 | 80 |
| Plastics | 5 | 10 | 5 | 4 | 24 | |||
| Vascular | 14 | 3 | 4 | 1 | 22 | |||
| Orthopaedic | 4 | 3 | 2 | 2 | 1 | 2 | 14 | |
| Upper GI tract | 2 | 7 | 2 | 1 | 1 | 13 | ||
| Cardiothoracic | 2 | 6 | 1 | 1 | 10 | |||
| Urology | 3 | 3 | 6 | |||||
| Obstetrics and gynaecology | 2 | 2 | 1 | 5 | ||||
| Otherb | 5 | 1 | 6 | |||||
| Breast | 2 | 2 | ||||||
| Missing | 4 | 1 | 5 | |||||
At the time of the survey, most patients (184/187, 98.4%) were receiving active treatment for their wound. Dressings were the most common single treatment, being received by 169 (93.4%) patients. Eleven (6.1%) patients were receiving NPWT (10 patients in secondary care and one patient in the community setting). One patient (0.5%) was receiving larval therapy.
Conclusions
This cross-sectional study is the first to characterise SWHSI patients and their wound origin, duration and treatment.
This study provided initial data on wound and treatment characteristics, for which there were limited data when the research programme was conceived. Identifying common surgical specialties (within secondary care) that lead to SWHSI, that almost 50% of SWHSIs were planned and that the most common treatment location was the community helped us to plan recruitment strategies (i.e. identification of planned SWHSIs in advance of surgery) and time frames for both the cohort study and the subsequent pilot, feasibility RCT (workstream 4), and will also help to inform research studies in the future.
A total of 43 surgical procedures preceding SWHSIs were identified, with common associated surgical specialties being colorectal, plastic and vascular surgeries. SWHSIs occur in a wide range of specialties; however, there are specific populations in whom SWHSIs more frequently occur and so these may be appropriate as the focus of further research.
Data collected in relation to wound duration at the point of the survey have enabled us to estimate the duration of follow-up required to capture healing events. Again, this information helped to inform our design of the cohort study and pilot, feasibility RCT (workstream 4).
When this research programme commenced, we had identified the need for a rigorous population-based estimate of SWHSI prevalence. This study has provided a population-based estimate of treated SWHSI prevalence, which has suggested that these wounds are relatively common. Given the prevalence observed, this therefore supports the need for further research into SWHSI epidemiology and treatments.
Cohort study
Objectives
Following the cross-sectional survey, we conducted a prospective, inception cohort study with the following objectives:
-
to investigate the clinical characteristics of patients with SWHSIs from the point of inception of the wound
-
to clearly delineate the clinical outcomes of patients with SWHSIs over the lifetime of their wound, with a particular focus on time to wound healing and associated determinants
-
to describe the types of treatments received by those with a SWHSI
-
to assess the measurable impact SWHSIs have on patients’ quality of life.
Methods
The cohort study was conducted over a 21-month period (18 February 2013 to 30 November 2014) in primary, secondary and community care settings at eight study sites across Yorkshire and the Humber, UK. Prior to commencement of the study, approval was obtained from Yorkshire and Humber – Humber Bridge Research Ethics Committee (reference 12/YH/0350) and from the associated NHS trusts.
Patients were eligible for inclusion if they had a SWHSI, defined as ‘an acute (< 3 weeks’ duration) open wound, resulting from surgery and requiring treatment, which was healing from the bottom up by the formation of granulation tissue’. When patients had multiple SWHSIs, the largest wound was deemed to be the ‘reference SWHSI’ and detailed data were collected for this one wound. Health-care professionals in primary, secondary and community care settings initially identified and screened patients for eligibility. Clinical areas identified as having a high prevalence of SWHSIs were targeted for recruitment, including colorectal and vascular surgical wards. Patients meeting the eligibility criteria were approached for informed consent by a research nurse before any data collection. A formal sample size calculation was not required for this study, although it was anticipated that 443 participants could be recruited over a 6-month period.
Data, including patient, surgery and wound information, quality of life and a wound photograph (used to assess wound features), were collected at baseline. Participants were followed up every 1–2 weeks for a minimum of 12 months and a maximum of 21 months to collect key clinical outcome data [including healing through either participant self-reporting or clinician observation, defined as ‘complete epithelial cover in the absence of a scab (eschar)’]; key clinical events such as surgical site infection (in accordance with Public Health England guidance on surgical site infection surveillance) or hospital admission;20 SWHSI treatments received; and any changes to a participant’s study involvement, including death. To ensure accurate data collection, healing status was further verified through a home visit to a sample of patients (10–20%). Participants also completed quality-of-life [Short Form questionnaire-12 item (SF-12)21 and EuroQol-5 Dimensions, three-level version (EQ-5D-3L)22] and pain [Brief Pain Inventory (BPI)23] assessments via postal questionnaires at 3-monthly intervals for a minimum of 12 months and a maximum of 21 months.
Data analysis was conducted using Stata® version 13 (StataCorp LP, College Station, TX, USA). Summary statistics for all variables were generated. Association with healing was examined for prespecified variables and multivariate logistic regression analyses conducted. Kaplan–Meier curves and Cox proportional hazards model analyses were used to examine time to event data. SF-12,21 EQ-5D-3L22 and BPI23 data were summarised, with SF-1221 data subsequently analysed using linear mixed models.
Results
A total of 396 patients were recruited to the cohort study from primary and acute care settings in Yorkshire, UK, with 393 participants included in data analysis (three patients were found to be ineligible following recruitment and so were excluded from the analysis). Just over half of participants were male (n = 222, 56.5%), participants had a median age of 55 (range 19–95) years and 69.5% of the study population were classed as overweight or obese (31.3% and 38.17%, respectively). Current smokers made up 28.5% of participants (i.e. were currently smoking or had quit within the last year).
The most common comorbidities we observed were cardiovascular disease (n = 151, 38.4%), followed by diabetes mellitus (n = 103, 26.2%) and peripheral vascular disease (n = 57, 14.5%). The majority of participants had no previous history of SWHSIs (n = 93, 72.0%) and had only one SWHSI (n = 358, 91.0%), and their surgical wound had been planned to heal by secondary intention (n = 236, 60.1%). The median area of the reference SWHSI at baseline was 6 cm2 (range 0.01–1200 cm2). SWHSI location varied; however, the most common sites were the abdomen (n = 132, 33.6%), foot (n = 59, 15.0%) and leg (n = 58, 14.8%), linking the commonly represented surgical specialities of colorectal (n = 136, 39.7%) and vascular (n = 82, 20.9%). At baseline, 164 patients (41.7%) were receiving hydrofibre dressings and 89 patients (22.7%) were receiving NPWT. Further details of baseline demographics (patient, wound and surgery) and treatments received are presented in Tables 4–7.
| Variable | Patients (N = 393) |
|---|---|
| Age (years), median (range) | 55.0 (19.0–95.3) |
| Sex, n (%) | |
| Male | 222 (56.5) |
| Female | 171 (43.5) |
| BMI (kg/m2), mean (SD) | 28.9 (6.6) |
| History of SWHSI, n (%) | |
| Yes | 93 (23.7) |
| No | 283 (72.0) |
| Do not know | 17 (4.3) |
| Tobacco use, n (%) | |
| None in last 10 years | 219 (55.7) |
| None current, but in last 10 years | 62 (15.8) |
| Current (less than one pack/day) or quit in last year | 84 (21.4) |
| Current (more than one pack/day) | 28 (7.1) |
| Baseline comorbidities | Patients (N = 286), n (%)a |
| Cardiovascular disease | 151 (38.4) |
| Diabetes mellitus | 103 (26.2) |
| Airways (e.g. asthma) | 69 (17.6) |
| Arthritis | 65 (16.5) |
| Peripheral vascular disease | 57 (14.5) |
| Cancer | 51 (13.0) |
| Orthopaedic (e.g. fractures) | 27 (6.9) |
| Stroke | 20 (5.1) |
| Autoimmune | 19 (4.8) |
| Neurological | 11 (2.8) |
| Other | 31 (7.9) |
| Medications used | Patients (N = 272), n (%)b |
| Anticoagulants/antiplatelets | 196 (49.9) |
| Vasodilator | 111 (28.2) |
| NSAIDs | 66 (16.8) |
| Corticosteroids | 11 (2.8) |
| Immunosuppressant | 9 (2.3) |
| Cytotoxic | 5 (1.3) |
| Variable | Patients (N = 393) |
|---|---|
| Number of SWHSIs, median (range) | 1 (1–6) |
| Area (cm2), median (range) | 6 (0.01–1200) |
| SWHSI location, n (%) | |
| Abdomen | 132 (33.6) |
| Foot | 59 (15.0) |
| Leg | 58 (14.8) |
| Perianal | 34 (8.7) |
| Back | 19 (4.8) |
| Natal cleft | 16 (4.1) |
| Buttocks | 16 (4.1) |
| Breast | 7 (1.8) |
| Arm | 5 (1.3) |
| Perineum | 5 (1.3) |
| Head | 3 (0.8) |
| Hand | 2 (0.5) |
| Neck | 2 (0.5) |
| Missing | 35 (8.9) |
| Aetiology, n (%) | |
| Planned | 236 (60.0) |
| Dehisced | 141 (35.9) |
| Surgically opened | 16 (4.1) |
| Tissue involvement, n (%) | |
| Full thickness | 235 (59.8) |
| Muscle, tendon or bone exposed | 120 (30.5) |
| Organ exposed | 1 (0.3) |
| Unsure | 35 (8.9) |
| Missing | 2 (0.5) |
| Infection at baseline, n (%) | |
| Yes | 79 (20.1) |
| No | 314 (79.9) |
| Antibiotics used, n (%) | |
| Yes | 182 (46.3) |
| No | 211 (53.7) |
| Dressing, n (%) | |
| Hydrofibre/spun hydrocolloid | 164 (41.7) |
| Other | 129 (32.8) |
| Wound contact | 114 (29.0) |
| NPWT | 89 (22.7) |
| Foam | 36 (9.2) |
| Alginate | 27 (6.9) |
| Silver containing | 23 (5.9) |
| Iodine | 19 (4.8) |
| Soft polymer | 19 (4.8) |
| Hydrocolloid | 10 (2.5) |
| Superabsorbent | 7 (1.8) |
| Cavity foam | 4 (1.0) |
| Hydrogel | 1 (0.3) |
| Silver sulfadiazine | 1 (0.3) |
| Treatment environment, n (%) | |
| Hospital inpatient | 229 (58.3) |
| Home | 88 (22.4) |
| GP | 55 (14.0) |
| Hospital outpatient | 11 (2.8) |
| Other | 8 (2.0) |
| Missing | 2 (0.5) |
| Variable | Patients (N = 393), n (%) |
|---|---|
| Subspecialty | |
| Colorectal | 156 (39.7) |
| Vascular | 82 (20.9) |
| Other | 50 (12.7) |
| Plastics | 33 (8.4) |
| Orthopaedic | 17 (4.3) |
| Obstetrics and gynaecology | 13 (3.3) |
| Surgical debridement | 11 (2.8) |
| Upper GI tract | 7 (1.8) |
| Urology | 7 (1.8) |
| Cardiothoracic | 7 (1.8) |
| Neurosurgery | 3 (0.8) |
| Thoracic | 3 (0.8) |
| Breast | 2 (0.5) |
| Trauma | 1 (0.3) |
| Oral and maxillofacial | 1 (0.3) |
| Surgery type | |
| Emergency | 236 (60.1) |
| Elective | 135 (34.4) |
| Missing | 22 (5.6) |
| Contamination level | |
| Dirty | 247 (62.9) |
| Contaminated | 65 (16.5) |
| Clean-contaminated | 51 (13.0) |
| Clean | 26 (6.6) |
| Missing | 4 (1.0) |
| Treatment dressings received (at any time) | Patients (N = 393), n (%)a |
|---|---|
| Hydrofibre | 259 (65.9) |
| Basic wound contact dressing | 212 (53.9) |
| Other | 196 (49.9) |
| NPWT | 115 (29.3) |
| Foam | 113 (28.8) |
| Silver-containing dressing | 89 (22.7) |
| Soft polymer dressing | 73 (18.6) |
| Iodine-containing dressing | 71 (18.1) |
| Alginate dressing | 49 (12.5) |
| Hydrogel | 23 (5.9) |
| Hydrocolloid | 22 (5.6) |
| Superabsorbent | 21 (5.3) |
| Cavity | 12 (3.1) |
| Polyhexamethylene biguanide dressing | 8 (2.0) |
The mean length of participant follow-up was 499 days [standard deviation (SD) 127.4 days], with a range of 13–651 days (median 528 days). Sixty-six participants (16.8%) were not followed up for the entire study duration (31 withdrew, 29 died and six were lost to follow-up). When the reference SWHSI was situated on a limb, this was amputated for 3.3% of participants (n = 13), of whom 61.5% had experienced an infection during follow-up (n = 8) and 76.9% had diabetes mellitus (n = 10). Infections were experienced by 32.1% of study participants (n = 126). Hospital admissions were reported for 97 participants (24.7%), of which 66 participants (68.0%) returned to the operating theatre. Thirty-six cases (38.1%) of hospital admissions were related to SWHSIs.
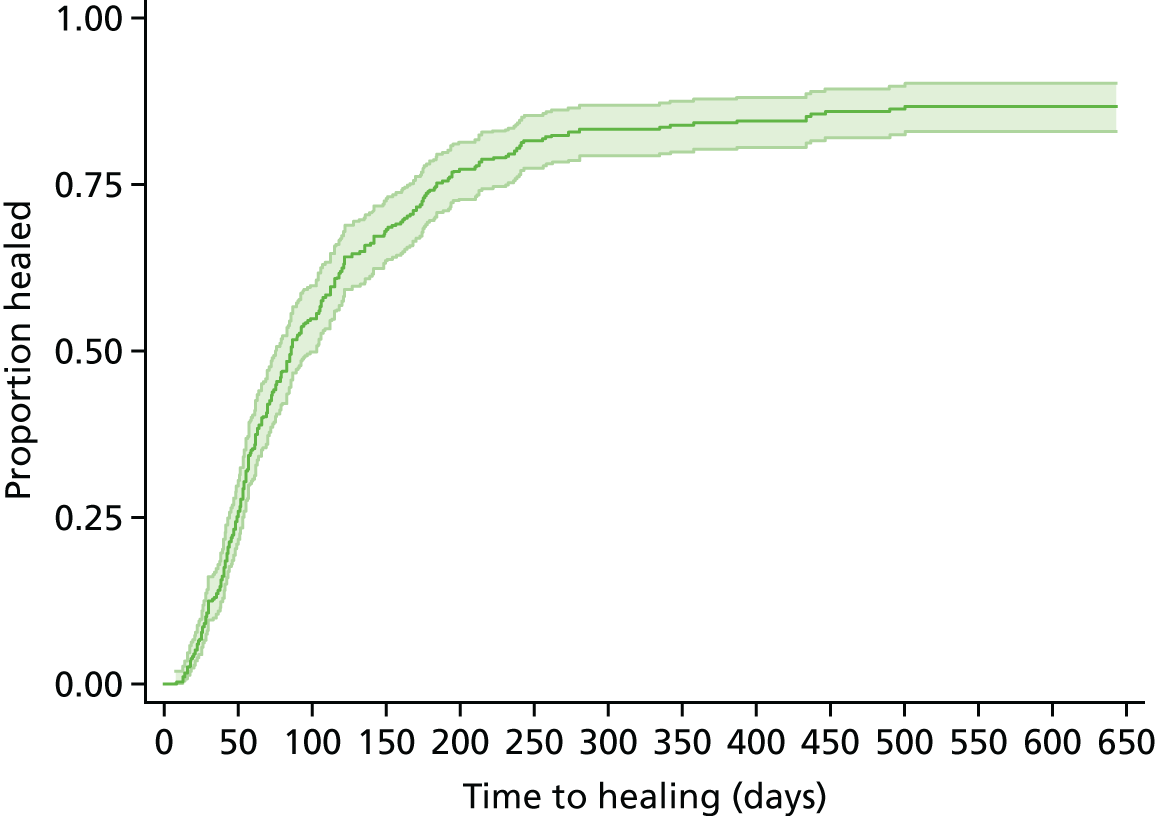
Data indicated that the reference SWHSI healed for 81.4% of participants (n = 320) during the study. Agreement between patient self-report and nurse assessment of healing status verification was relatively high (77.8% at 6 months and 74.2% at 12 months). Disagreements were present for four participants: three patients had healing confirmed during a visit but paperwork was not completed to this effect and one participant was not healed at the verification visit but healed shortly afterwards. The median time to healing for all cohort participants was 86 (95% CI 75 to 103) days, as presented in Figure 2. When we assessed time to healing against baseline participant and wound characteristics in a Cox proportional hazards model, baseline wound area above median (p < 0.01) and surgical wound contamination level as determined at the point of surgery (p = 0.04) were significant predictors of prolonged time to healing (see Appendix 4). Infection at any point was also found to be a significant predictor of prolonged time to healing (p < 0.01).
FIGURE 2.
Cohort study: Kaplan–Meier curve of time to healing in days.
The most commonly received treatment throughout the study was hydrofibre dressings, with 65.9% (n = 259) of participants receiving this treatment at least once. Nearly one-third (n = 115, 29.3%) of participants received NPWT at some point during the study.
Health utility scores captured by the EuroQol-5 Dimensions (EQ-5D) measure22 (in which 0 = death and 1 = perfect health) were a mean of 0.5 (SD 0.4) at baseline, increasing to a mean of 0.6 (SD 0.4) at 6 months and 0.6 (SD 0.4) at 12 months. Further details are provided in Table 8.
| Time point | Overall | Healing status | p-value | |
|---|---|---|---|---|
| Healed | Not healed | |||
| Baseline, n | 383 | 311 | 72 | |
| Mean (SD) | 0.5 (0.4) | 0.5 (0.37) | 0.4 (0.42) | 0.07 |
| 3 months, n | 258 | 223 | 35 | |
| Mean (SD) | 0.6 (0.4) | 0.6 (0.38) | 0.6 (0.33) | 0.58 |
| 6 months, n | 219 | 191 | 28 | |
| Mean (SD) | 0.6 (0.4) | 0.6 (0.37) | 0.5 (0.34) | 0.08 |
| 12 months, n | 185 | 166 | 19 | |
| Mean (SD) | 0.6 (0.4) | 0.6 (0.35) | 0.6 (0.38) | 0.31 |
Pain, measured as BPI scores for pain severity (in which 0 = no pain and 10 = pain as bad as you can imagine) and pain interference (in which 0 = does not interfere and 10 = completely interferes)23 reduced slightly over time. Mean pain severity reduced from 3.7 (SD 2.54) at baseline to 3.0 (SD 3.0) by 15 months; similarly, mean pain interference scores reduced from 4.2 (SD 2.54) at baseline to 3.1 (SD 3.17) at 15 months. Further details are provided in Table 9.
| Time point | BPI | |||||||
|---|---|---|---|---|---|---|---|---|
| Pain severity | Interference | |||||||
| Overall | Healing status | p-value | Overall | Healing status | p-value | |||
| Healed | Not healed | Healed | Not healed | |||||
| Baseline, n | 385 | 313 | 72 | 390 | 318 | 72 | ||
| Mean (SD) | 3.7 (2.54) | 3.6 (2.53) | 4.0 (2.53) | 0.28 | 4.2 (3.12) | 4.2 (3.14) | 4.4 (3.05) | 0.63 |
| 3 months, n | 257 | 220 | 37 | 260 | 223 | 37 | ||
| Mean (SD) | 3.3 (2.63) | 3.3 (2.70) | 3.4 (2.17) | 0.83 | 3.8 (3.12) | 3.7 (3.13) | 4.3 (3.08) | 0.30 |
| 6 months, n | 210 | 182 | 28 | 214 | 186 | 28 | ||
| Mean (SD) | 3.4 (2.67) | 3.3 (2.71) | 3.9 (2.38) | 0.22 | 3.8 (3.18) | 3.7 (3.20) | 4.6 (2.97) | 0.13 |
| 12 months, n | 179 | 161 | 18 | 179 | 161 | 18 | ||
| Mean (SD) | 3.4 (2.66) | 3.3 (2.70) | 4.0 (2.32) | 0.29 | 3.6 (3.18) | 3.5 (3.20) | 4.3 (2.97) | 0.36 |
Quality of life was measured using the SF-12 and presented as SF-12 physical component scores (PCSs) and mental component scores (MCSs). 21 At baseline, the mean PCS was 33.1 (SD 10.7) and the mean MCS was 42.2 (SD 12.35). At 15 months’ follow-up, the mean PCS was 40.8 (SD 13.46) and the mean MCS was 48.3 (SD 10.73). Further details are provided in Table 10. A linear mixed model, adjusting for duration of SWHSI, baseline SWHSI area, location, participant age and baseline SF-12 subscale score, found follow-up time point, baseline score, age and SWHSI duration to be significant predictors of PCS (p < 0.001 in all cases) and MCS (p = 0.03 for time point and p < 0.01 for age, baseline score and SWHSI duration).
| Time point | SF-12 dimensions (overall population) | |
|---|---|---|
| PCS | MCS | |
| Baseline, n | 390 | 390 |
| Mean (SD) | 33.1 (10.17) | 42.2 (12.35) |
| 3 months, n | 255 | 255 |
| Mean (SD) | 37.7 (13.15) | 45.1 (11.69) |
| 6 months, n | 223 | 223 |
| Mean (SD) | 39.2 (12.91) | 45.1 (12.13) |
| 9 months, n | 210 | 210 |
| Mean (SD) | 39.4 (12.89) | 46.4 (11.28) |
| 12 months, n | 187 | 187 |
| Mean (SD) | 38.8 (13.11) | 47.2 (11.58) |
| 15 months, n | 147 | 147 |
| Mean (SD) | 40.8 (13.46) | 48.3 (10.73) |
| 18 months, n | 78 | 78 |
| Mean (SD) | 39.4 (13.52) | 47.3 (11.12) |
Conclusions
This prospective inception cohort study in patients with SWHSIs is the first we have identified. Broad patient groups (patients undergoing abdominal colorectal surgery, lower-limb vascular surgery and a mixed group of other procedures and specialities) were identified, which were similar to those identified in our cross-sectional survey. This information flags populations of clinical relevance, as well as those likely to be the focus of further research.
The median time to healing for these wounds, of approximately 3 months, is new information, which highlights the extended period of time that patients might expect to cope with open surgical wounds. There was high variability, with time to healing ranging from 8 days to 1 year and 4 months (prior to censoring) and from 5 days to 1 year and 7 months (following censoring). The chronicity of these wounds is crucial for patients and carers to appreciate, in order for them to have realistic expectations regarding wound healing. The cohort also highlights the large number of adverse events that occur in SWHSI patients, with wound infection and readmission to hospital (with the associated care costs) being particularly common. This cohort study data clearly demonstrated that SWHSIs pose a distinctive management challenge that was previously unsupported by high-level evidence.
Adjusted analyses provide further initial insights into possible patient and wound ‘risk’ factors that might have a detrimental impact on healing, including wound infection at any point, baseline wound area and higher levels of surgical wound contamination, as determined at the point of surgery. The predictive baseline factors may be important for the stratification of randomisation or adjustment for prognostic factors in future trials; however, it should be noted that there are many other covariates that are associated and correlate with these predictors. The SF-12 data indicate that, at baseline, SWHSI patients have quality-of-life limitations comparable to patients with congestive cardiac failure; however, they were significantly younger. 24 This therefore demonstrates the potential impact that SWHSIs have on patient quality of life. However, as the improvements in SF-12 scores during the study demonstrate, quality of life may be improved by time or wound healing. The research to date is therefore building a picture of a relatively common wound type that takes a prolonged time to heal, negatively impacts quality of life and puts patients at high risk of infection and other adverse events.
The common treatments for SWHSIs, in the centres involved in this cohort that were treating SWHSI patients across Yorkshire and the Humber, UK, were dressings and NPWT. There is currently limited and generally low-quality evidence on how effective dressings are in terms of wound management and promoting optimal clinical outcomes in a cost-effective way. 14 A recent Cochrane systematic review found only two RCTs, with a total of 69 participants, that had investigated the relative effectiveness of NPWT on SWHSIs, both reporting limited outcome data, which prevents any firm conclusions to be made. 14 Given that all SWHSI patients in this cohort were receiving dressings and/or NPWT, further research to explore clinical effectiveness and cost-effectiveness of these treatments in one or more of the common surgical groups identified seems warranted, and so was considered appropriate for further assessment in workstream 2.
The prospective and observational design of this study allowed us to follow patients over time and to collect the important natural history, treatment and outcome data presented here. Although we tried to maintain a representative sample, the need for consent meant that the subset recruited might differ in some systematic way from the wider SWHSI patient population. Comparison of the cohort and the cross-sectional survey data may be useful here, as the survey data were captured away from the patient’s bedside, did not require consent and thus may provide a more epidemiologically complete overview of the SWHSI population. In terms of patient, epidemiological, wound and surgery data, the populations in the survey and cohort appear similar; however, the findings from the survey may have influenced specific areas for focused recruitment into the cohort study (e.g. subspecialty-specific hospital wards). Some patient groups for whom wound problems are recognised nationally seem under-represented in the cohort study (e.g. post-caesarean wounds, for which dehiscence rates as high as 6% have been reported). 25
The cohort study was conducted in a single geographical area (two large centres in Yorkshire, UK), and therefore the patient groups and the treatment availability observed may not be reflective of national or international patient populations or treatment availability. However, we have no reason to suspect that there are huge differences between these surgical patients and those elsewhere, particularly given the breadth of our inclusion criteria.
We also note that our analyses exploring the impact of factors on time to healing are based on our observational data set and thus these cannot be assumed to be causal relationships. Thus, firm conclusions regarding factors that may affect healing cannot be drawn. However, we are able to report the associations found between observed covariates and healing, and reflect on them. From the outset of the study, there was careful consideration regarding measured study variables, with the aim of capturing important data, including factors that had the potential to be prognostic in relation to healing. We consulted widely with clinical experts and extensively scoped the literature, with the aim of providing the most comprehensive analysis possible.
The further research conducted as part of this research programme (workstreams 2–4) focused on further investigation of interventions that may offer clinical effectiveness and cost-effective treatment options for SWHSIs (workstream 2), as well as more detailed exploration of patients’ experiences of living with SWHSIs and health professionals’ views of delivering care to patients with these challenging wounds.
Workstream 2: using evidence from observational research to estimate the value of negative-pressure wound therapy in SWHSIs
Following characterisation of SWHSI patients and their treatment in workstream 1, the evidence gap regarding the cost-effectiveness of SWHSI treatments remained. Data collected in workstream 1 indicated that a variety of interventions are used when treating and managing SWHSIs. Although the majority of participants in the cohort study received dressings throughout follow-up, 29.3% (115/393) received a significantly more expensive technology: NPWT.
Negative-pressure wound therapy devices apply a carefully controlled negative pressure (or vacuum) to a dressing, creating an air-tight seal and removing tissue fluid from the treated wound area into a disposable canister. 14 The canister is removed and replaced either when it becomes full or at least once a week. The device is generally used as part of the SWHSI treatment pathway, rather than to the point of healing, and can be administered and removed by both nurses and surgeons. It has been claimed that NPWT speeds up wound healing by removing excess fluid, increasing tissue perfusion and removing bacteria. 26 However, although a recent Cochrane systematic review identified two RCTs investigating the relative effectiveness of NPWT on SWHSIs,14 it could not draw any conclusions, as the outcome data reported in both studies were very limited.
Given the current level of clinical use on SWHSIs, the cohort data collected in workstream 1 have been used here to evaluate the cost-effectiveness of NPWT for the healing of SWHSIs (the clinical outcome of wound treatments most valued by patients)27 and the value of a future RCT. From this work, a paper highlighting methodological challenges of analysing observational evidence in this context has been published (see Appendix 5). The current chapter presents the clinical implications of the research.
Objectives
-
To estimate the cost-effectiveness of NPWT compared with wound dressings for the healing of SWHSIs, using cohort data from workstream 1.
-
To assess whether or not investment in further research on treatments for SWHSIs is likely to be worthwhile.
Methods
In this workstream, we evaluated if, after adjustment, NPWT was clinically effective and cost-effective when compared with standard dressings. For clinical effectiveness, we used wound healing as the outcome; for cost-effectiveness, we quantified the impact of differences in time to healing on quality-adjusted life-years (QALYs). This approach assumed that no mortality differences existed and, thus, any improvements in health-related quality of life (HRQoL) were associated with accelerated time to healing only. We also noted that any additional HRQoL improvements that may have arisen from treatment (independent of healing status) were expected to be transient and not to have a significant impact on HRQoL (which is a multidimensional concept that includes domains related to physical, mental, emotional and social functioning). For this reason, such effects were not considered here.
We hypothesised that NPWT could be cost-effective by reducing healing time, improving HRQoL and reducing treatment costs (via reduced healing time). We envisaged that if the total costs of treatment were higher with NPWT than for comparator treatments, NPWT would only be cost-effective if the incremental costs to QALY gains fell below the standard NICE £20,000–30,000 per QALY threshold.
Specifically, the cost-effectiveness analysis used cost per QALY gained as the cost-effectiveness outcome. A 1 year time horizon was considered and a NHS perspective was adopted. Formally, we aimed to quantify the impact of NPWT in expected time to healing. The difference in QALYs between the interventions was derived from the expected time to healing and the EQ-5D index score while healed/unhealed, the latter estimated using the multilevel model (described in supplementary material in Appendix 5). Incremental disease costs were calculated in an analogous way to incremental QALYs, making use of the cost estimates derived in the multilevel two-part modelling approach (see Appendix 5). Treatment-related costs considered that patients received some form of treatment up to healing. The daily costs associated with the ‘non-NPWT’ group were based on average costs of dressings in participants who had received other treatments and were calculated by multiplying this cost by the difference in time to healing. For patients receiving NPWT, an average daily cost of NPWT was applied to the mean time on NPWT treatment in the cohort study. In the remaining time, patients were assumed to use dressings, with a daily cost equal to patients in the ‘non-NPWT’ group.
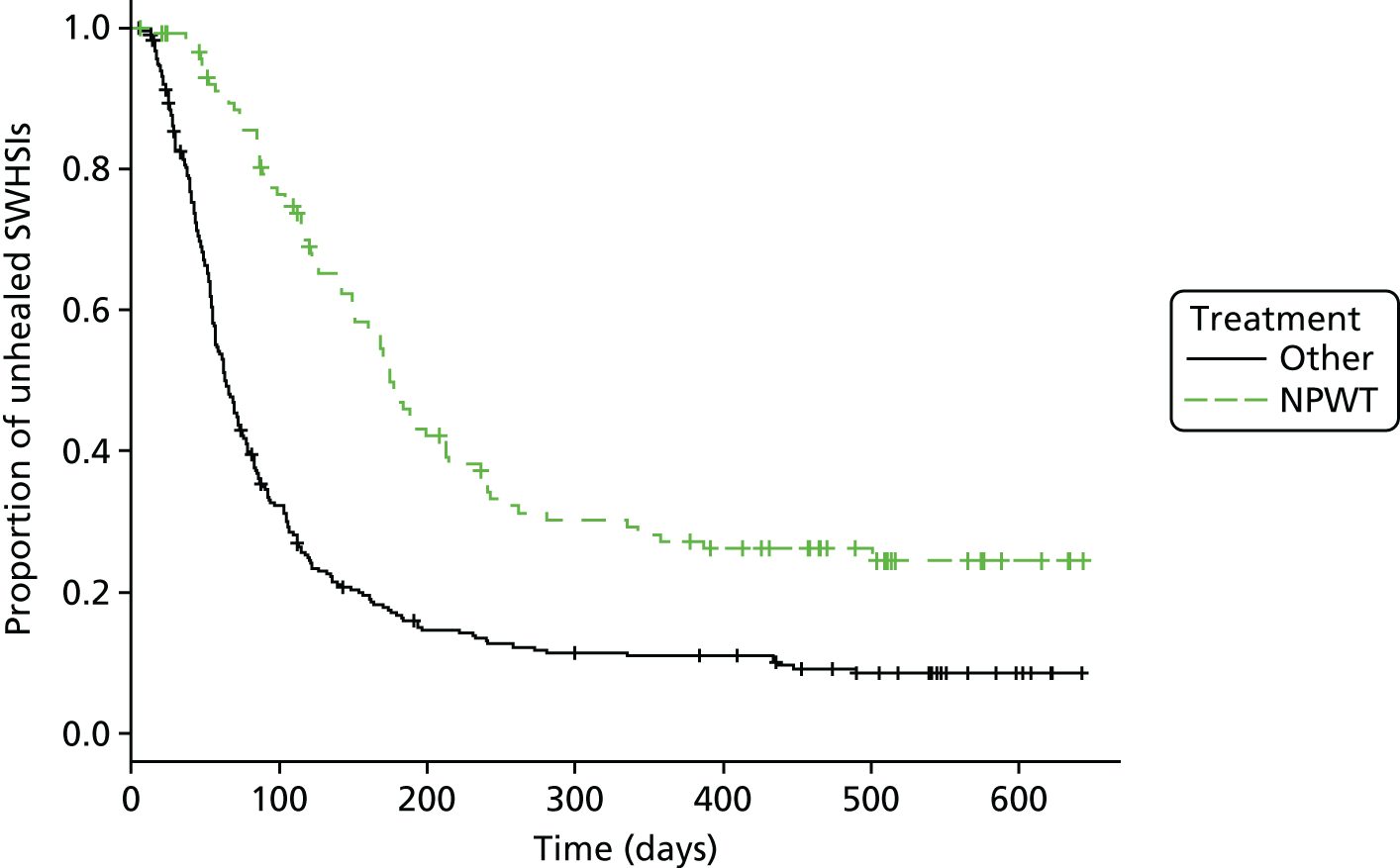
The cohort data were analysed for evidence regarding the various aspects of treatment and wound healing, including the impact of treatment (NPWT vs. dressings) on the time to healing and the impact of this SWHSI healing on disease-specific costs and HRQoL, as is presented in Figure 3. To estimate the effect of treatment on healing, we attempted to control the confounding (and selection bias) inherent when using observational data in this context. We adjusted for observed confounding (i.e. for characteristics observed within the cohort study sample that determine time to healing and may have differed between the groups). We also attempted to account for any potential confounding from unobserved sources.
FIGURE 3.
Time to healing: descriptive Kaplan–Meier curve. Reproduced with permission from Saramago et al. 28 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Health-related quality of life was assessed in the cohort study using the EQ-5D index score, obtained through a self-reported questionnaire, comprising five domains (mobility, self-care, usual activities, pain and discomfort, and anxiety and depression). 22 Participants were also asked to complete a resource use questionnaire quarterly. This questionnaire was developed for this study to allow costing of NHS resources specifically attributable to the SWHSIs and included collection of number of contacts with general practitioners (GPs), nurses and doctors in NHS institutions or the patient’s home, as well as the number of nights spent as an inpatient between study follow-up time points. The costing used national sources and 2014 prices. 29
Analyses required the application of econometric models to the cohort data. A Bayesian approach to inferences was utilised, computed using Markov chain Monte Carlo simulation, using WinBUGS version 1.4.3 (Medical Research Council Biostatistics Unit, Cambridge, UK). 30
Identification of external evidence to supplement cohort data
To evaluate the existence of any external evidence to complement the cohort data, we also conducted the following range of systematic literature searches.
We conducted Cochrane systematic reviews of RCTs evaluating the treatment of SWHSIs, with any recognised form of NPWT. 14,31
Using the general review methods and with the support of an information specialist and an expert systematic reviewer, we conducted systematic searches and identification of the following studies:
-
any cohort studies evaluating the use of NPWT for SWHSIs
-
any EQ-5D or related health utility data for people with SWHSIs
-
any previous modelling work for the treatments of SWHSIs that might inform the planned work.
Details of the search strategies used are detailed in Appendix 6.
EuroQol-5 Dimensions index scores and disease-specific costs
A multilevel (or panel data) approach was used to model both EQ-5D index scores and quarterly costs that accounted not just for between-participant variation but also for within-participant variation across the quarterly observations. A time-dependent indicator of whether the participant had healed or not at each time point was used to capture the effect of healing. Changes from baseline EQ-5D index scores were modelled using a standard multilevel model based on the normal distribution (random intercept). For cost data, due to a high proportion of zero observations and the typical skewness associated with these data, a two-part model was used: the first part was a multilevel logistic regression (to explain the probability of observing zero costs over time) and the second was mixed gamma model with log-transformed costs (to evaluate the expected value of the non-zero observations). Both parts were multilevel models, to account for the repeated observations per participant.
We calculated the average daily cost of NPWT as £31.78 (according to centre-specific costings). For dressings, a unit cost of £2.39 per day was calculated (based on the mix of dressings used within the cohort data). The duration of NPWT treatment was evaluated from the cohort data (mean 37 days, SD 64.6 days) and was assumed to be independent of time to wound healing, as this treatment is rarely used up to the point of wound healing. NPWT patients were assumed to receive only dressings for the remainder of time unhealed.
Results indicated that healing was associated with a small (but statistically significant) increase in EQ-5D index score [mean 0.055, standard error (SE) 0.02]. Cost data, as presented in Table 11, indicated that healing significantly reduced quarterly wound-related costs (mean £865, SE £112).
| Model | Mean | 95% CrI |
|---|---|---|
| Expected EQ-5D (unhealed) | 0.091 | 0.036 to 0.146 |
| Expected EQ-5D (healed) | 0.091 + 0.055 = 0.146 | 0.098 to 0.193 |
| Difference in EQ-5D attributed to healing | 0.055 | 0.015 to 0.094 |
| Expected costs (£) (unhealed) | 1124.45 | 916.9 to 1359.1 |
| Expected costs (£) (healed) | 258.91 | 208.1 to 317.7 |
| Difference in costs (£) attributed to healing | 865 | 674.2 to 1126.5 |
Relative effectiveness (time to healing) of negative-pressure wound therapy and dressing-treated wounds
Based on the cohort data, we took two different approaches to modelling the time to healing and assessed robustness of our conclusions to the approach taken.
Modelling strategy A: ordinary least squares with imputation
Modelling strategy A used ordinary least squares (OLS) (or linear regression) to describe time to healing. OLS methods do not deal with censoring and 73 participants did not heal within the study follow-up period. To generate a complete data set on time to healing, the censored observations on time to wound healing were imputed, assuming their follow-up time plus an additional quantity, either 1 day (i.e. participants were conservatively assumed to have healed the day after they were censored) or expected healing time (data elicited from three experienced clinicians and pooled) (see Appendix 5). Note that by attributing an additional time to healing, elicited by clinical experts, to patients who have been censored in the study, we are also extrapolating beyond the study follow-up. Such extrapolation is required to allow achieving an unrestricted estimate of the effects of NPWT on mean time to healing, required for cost-effectiveness analysis.
Modelling strategy B: two-stage model
A second, more complex, modelling strategy (modelling strategy B) was also implemented, which explicitly considered the unhealed participants. Instead of OLS, it used a two-step modelling approach: a logistic regression estimated the probability of healing within the follow-up for each treatment, followed by linear regression, which estimated the expected time to healing for the population that healed within follow-up. This strategy reflected the possibility of there being a group of ‘long-term non-healers’ and allowed exploration of different determinants of the probability of healing and of time to wound healing, conditional on having healed in the adjustment for observables.
In terms of adjustment for observed confounding, and in the absence of evidence on determinants of healing in this patient group, a comprehensive variable selection process was undertaken using the cohort data sample. It aimed to identify predictors of treatment assignment with NPWT, predictors of healing and treatment effect modifiers. The selection criterion was based on goodness of fit (using the deviance information criteria at a widely used cut-off point of 5 deviance information criterion points). 32 All the predictors identified were used as adjustment covariates in the regression model for time to healing.
To account for unobservable confounding, instrumental variable (IV) regression was used. This method uses a variable (the instrument) that is associated with treatment assignment but not with outcome (except for any indirect effect on treatment assignment) to adjust for unobservable confounding. A range of instruments focused on the decisions of health professionals to use NPWT, that is use of NPWT on previous patient treated, perceptions of NPWT as effective treatment, likelihood of NPWT use for patients, desire to use NPWT more frequently, perceptions of NPWT as affordable and value for money, and company representative input, were considered (see Appendix 5). The selection of instruments was based on frequentist tests aimed at assessing aspects of validity and relevance (see Appendix 5). Previous treatment used by the treating health professional was the best-performing instrument.
The IV model used two regressions (two stages). The first stage regressed treatment allocation by the instrument and the set of relevant predictors of outcome (logistic regression) to predict the probability of each individual participant being assigned to NPWT. The predictions were then used as a covariate in the second-stage regression(s), which, again, conditions on the set of relevant predictors of outcome. Inferences were obtained using a Bayesian framework, so that uncertainty over predictions of treatment allocation was fully considered. Any external evidence located (from previous research) was to have been used as prior evidence to update the data obtained from the cohort.
Cost-effectiveness of negative-pressure wound therapy
The cost-effectiveness analysis uses cost per QALY gained as the cost-effectiveness outcome. A 1-year time horizon was considered and a NHS perspective was adopted. Formally, we aimed to quantify the impact of NPWT in expected time to healing. The difference in QALYs between the interventions was derived from the expected time to healing and the EQ-5D index score while healed or unhealed, the latter estimated by the multilevel model (described in the supplementary material in Appendix 5). Incremental disease costs were calculated in an analogous way to incremental QALYs, making use of the cost estimates derived in the multilevel two-part modelling approach described above (see Appendix 5). Treatment-related costs considered that patients received some form of treatment up to healing. The daily costs associated with the ‘non-NPWT’ group were based on average costs of dressings in participants who had received other treatments and were calculated by multiplying this cost by the difference in time to healing. For patients receiving NPWT, an average daily cost of NPWT was applied to the mean time on NPWT treatment in the cohort study. In the remaining time, patients were assumed to use dressings, with a daily cost equal to that for patients in the ‘non-NPWT’ group.
Results
Identification of external evidence to supplement cohort data
We screened 1477 citations from the literature searches and found no existing health utility data collected from patients with SWHSIs that could be used. Neither did we find any relevant published models exploring either the clinical effectiveness or the cost-effectiveness of treatments for SWHSIs. In terms of relative effects for NPWT compared with alternative treatments on healing, we located four potentially relevant RCTs33–36 and no observational data.
Of the four trials, two explored the use of NPWT compared with standard dressings to treat foot wounds in patients with diabetes mellitus. 33,34 One trial33 included 162 adult patients who had undergone a transmetatarsal amputation of the foot. It was not clear from the study whether or not the wounds had been left open following surgery. A second trial34 included 342 adult patients who had undergone debridement of a foot wound and were randomised to receive NPWT or dressings (predominantly hydrogels and alginates). Although it was not clear whether or not the debridement of these wounds was surgical, we were advised by local clinicians that it was likely to be. Both trials of NPWT in foot wounds reported time to healing data; however, the data appeared to include wounds that had been closed by surgery following treatment with NPWT or dressings. The two trials were considered to be at high risk of performance bias, as unblinded health professionals had made key decisions regarding the treatment of wounds, such as closure surgery. This issue has been noted previously and the validity of combining wounds closed by secondary intention and those closed by surgery has been questioned. 37 The potential for high risk of bias in these studies, combined with the fact that the data from these trials related to only a subset of the heterogeneous group of patients of interest, meant that we felt that the relative healing data could not be reasonably combined with the adjusted estimates of the cohort data.
Two further trials were identified. 35,36 The first study,35 with 20 participants, compared NPWT with an alginate dressing in the treatment of open, infected groin wounds. The second study,36 with 49 participants, compared NPWT with a silicone dressing in the treatment of excised pilonidal sinus. No useable healing data were reported in either trial. For this reason, these studies did not provide relevant evidence to complement the cohort data.
Effectiveness results when adjusting for observables
Modelling strategy A: ordinary least squares with imputation
Relevant associations found with predictors of treatment assignment with NPWT were wound area > 25 cm2, skin and subcutaneous tissue loss (a measure of tissue involvement) and inpatient (vs. outpatient) management. Relevant associations found with predictors of healing were inpatient (vs. outpatient) management and previous history of a SWHSI. The data also suggested that history of a SWHSI could be a relevant treatment effect modifier. Predictors of treatment assignment and predictors of healing were used to adjust all regressions within modelling strategy A.
In the model in which treatment effect modifiers were not considered, and censored observations were assumed to heal 1 day after follow-up, participants treated with NPWT were expected to take longer to heal than those who did not receive NPWT, on average 73 days longer [95% credible interval (CrI) 33.8 to 112.8 days longer] (Table 12). The addition of the interaction term between treatment and SWHSI history indicated that NPWT was still expected to increase time to healing compared with the use of dressings alone, but the magnitude of effect differed between the groups (181 days for participants with history of SWHSIs vs. 42 days for patients without history of a SWHSI). Results were similar when elicited expert opinion was used to impute censored healing times; however, the point estimates were even less favourable for NPWT (requiring, on average, 114 days additional days to wound healing compared with the use of standard dressings) and uncertainty was significantly increased.
| Model ID | Model | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Instrument used, z | None | None | None | Previous treatment | Previous treatment with interaction |
| Equation | TTH | TTH | TTH | (Stage 2) TTH | (Stage 2) TTH |
| Adjustment covariates | None | Observables | Observables + interaction | Observables | Observables + interaction |
| Area (> 25 cm2), x1 | 18.0 (95% CrI –23.1 to 58.0) | 21.1 (95% CrI –20.3 to 62.1) | 23.8 (95% CrI 29.8 to 43.7) | 36.2 (95% CrI –23.7 to 56.3) | |
| Treatment location,a x2 | 45.3 (95% CrI 11.3 to 79.2) | 43.8 (95% CrI 9.9 to 76.8) | 48.9 (95% CrI 3.0 to 63.8) | 55.3 (95% CrI 10.8 to 70.8) | |
| Tissue involvement,b x3 | 31.9 (95% CrI –1.5 to 65.0) | 26.6 (95% CrI –5.3 to 58.7) | 33.8 (95% CrI –8.0 to 73.1) | 37.2 (95% CrI –2.0 to 50.6) | |
| History, w | 7.5 (95% CrI –28.4 to 42.9) | –29.3 (95% CrI –71.5 to 12.1) | 7.2 (95% CrI –29.5 to 44.6) | –10.3 (95% CrI –61.4 to 7.4) | |
| NPWT, t | 108.0 (95% CrI 73.6 to 142.2) | 73.2 (95% CrI 33.8 to 112.8) | 41.9 (95% CrI –1.1 to 84.1) | 56.9 (95% CrI –71.7 to 192.8) | 9.1 (95% CrI –120.2 to 55.9) |
| NPWT × history, w.t | 138.7 (95% CrI 56.9 to 221.8) | 69.4 (95% CrI –67.5 to 118.0) | |||
| Constant | 116.8 (95% CrI 97.9 to 135.2) | 81.4 (95% CrI 53.3 to 110.0) | 92.7 (95% CrI 64.5 to 120.8) | 81.1 (95% CrI 52.3 to 109.1) | 86.7 (95% CrI 56.7 to 97.0) |
| Observations | 393 | 372 | 372 | 372 | 372 |
Modelling strategy B: two-stage model
This modelling approach explicitly modelled the probability of healing within follow-up and, in addition to the predictors of treatment assignment mentioned in Modelling strategy A: ordinary least squares with imputation, it identified associations with body mass index (BMI) and wound location being the foot (vs. other locations). For those healing within follow-up, time to healing was associated with surgery type (colorectal vs. other surgeries), wound duration and diabetic foot wounds. No evidence of relevant interaction terms was found. As detailed in Table 13, results indicated that participants treated with NPWT were estimated to have lower probability of healing than those who did not receive NPWT [odds ratio (OR) of healing with NPWT was 0.59 (95% CrI 0.28 to 1.12)]. The expected time to healing of NPWT-treated participants who healed compared with participants treated with dressings alone was an additional 46 days (95% CrI 19.6 to 72.5 days).
| Model ID | Model | |||
|---|---|---|---|---|
| b0 | b1 | |||
| Equation | (Stage 2A) OR for healing | (Stage 2B) TTH | (Stage 2A) OR for healing | (Stage 2B) TTH |
| Type of regression model | Logit | OLS | IV, logit | IV, OLS |
| Adjustment | None | None | Adjustment set | Adjustment set |
| Area (> 25 cm2), x1 | 0.52 (95% CrI 0.24 to 0.99) | 27.7 (95% CrI 0.6 to 54.5) | ||
| Treatment location,a x2 | 0.52 (95% CrI 0.22 to 1.04) | 30.9 (95% CrI 9.2 to 52.3) | ||
| Tissue involvement,b x3 | 0.50 (95% CrI 0.25 to 0.91) | 11.4 (95% CrI –8.1 to 30.9) | ||
| BMI (kg/m3), v1 | 1.04 (95% CrI 1.00 to 1.09) | |||
| Location: foot, v2 | 0.34 (95% CrI 0.16 to 0.64) | 30.1 (95% CrI 9.2 to 52.3) | ||
| Surgery type, v3 | 13.1 (95% CrI –6.1 to 32.4) | |||
| SWHSI duration (days), v4 | 2.3 (95% CrI 0.6 to 4.0) | |||
| Diabetic feet, v5 | 42.8 (95% CrI 9.1 to 76.3) | |||
| NPWT, t | 0.35 (95% CrI 0.20 to 0.57) | 69.0 (95% CrI 48.2 to 89.1) | 0.59 (95% CrI 0.28 to 1.12) | 46.0 (95% CrI 19.6 to 72.5) |
| Constant, γ1 | 6.56 (95% CrI 4.70 to 9.37) | 82.0 (95% CrI 71.9 to 92.1) | 6.98 (95% CrI 1.62 to 29.55) | 36.9 (95% CrI 12.4 to 61.3) |
| Observations | 393 | 354 | ||
Effectiveness results when adjusting for observables and unobservables
Overall, conclusions were similar using modelling strategies A and B, and treatment effect estimates adjusted for unobservables were broadly consistent with the results we observed when adjusting only for observables. Participants treated with NPWT were predicted to take longer to heal, albeit with a high level of uncertainty in the estimates [e.g. modelling strategy B, second-stage regression: 46 days longer (95% CrI 19.6 to 72.5 days longer)].
Cost-effectiveness of negative-pressure wound therapy
The results for healing, utilities and costs were combined to generate cost-effectiveness estimates for NPWT. Evidence relating to the additional days until healing after censoring for unhealed patients within the follow-up period was elicited from three experts and used in an extended analysis, the results of which are shown in Table 14. Irrespective of the adjustment made (on either observables or unobservables), the results indicated that treatment with NPWT was expected to be less effective and a more costly use of NHS resources than treatment with standard dressings alone: associated incremental QALYs of –0.012 (SE 0.005) (model A, observables), –0.008 (SE 0.011) (model A, unobservables), –0.007 (SE 0.004) (model B, observables) and –0.027 (SE 0.017) (model B, unobservables). There was little decision uncertainty in this result. When losses arising from the displaced health-care resources were added to the direct health effects (by valuing 1 QALY at £20,000), the health consequences of using NPWT were evaluated at –0.111 (model A, observables), –0.095 (model A, unobservables), –0.087 (model B, observables) and –0.183 (model B, unobservables).
| Elicited | Fitted values for parameters of a gamma distribution | ||||||
|---|---|---|---|---|---|---|---|
| 50th percentile (days) | 90th percentile (days) | Shape, first and third quartiles | Scale, first and third quartiles | Mean (days), first and third quartiles | 50th percentile (days), first and third quartiles | 90th percentile, (days), first and third quartiles | |
| Expert 1 | 1044 | 3234 | 1.12 | 1290 | 1445 | 1031 | 3322 |
| Expert 2 | 496 | 679 | 15.3 | 33.1 | 506 | 495 | 683 |
| Expert 3 | 180 | 3650 | 0.23 | 5222 | 1201 | 176 | 3176 |
| Pooled using formal synthesis (95% CrI) | 1.79 (0.03 to 54.5) | 615 (241 to 1615) | 1038 (29 to 39,065) | 559 (0 to 28,011) | 1820 (15 to 37,161) | ||
Data and analyses of the cohort study indicate, with little uncertainty, that treatment with NPWT is less effective and more costly than treatment with standard dressings alone.
Value of a future randomised controlled trial
Although there is uncertainty in the incremental cost and QALY estimates, our analyses consistently indicate that there is little uncertainty in the optimal decision (i.e. the probability of treatment with NPWT being considered cost-effective to the UK NHS was close to zero). This means that conducting a future study in order to reduce the associated uncertainty (in the costs and QALYs) would not change the optimal decision and therefore there is no value in conducting such a study for this purpose. For this reason, the value of information analyses originally planned were redundant: the value of information is zero.
Negative-pressure wound therapy is currently widely used in clinical practice. The results from the observational study may contradict beliefs with regards the effectiveness of NPWT, but, given that our findings have been derived from observational research, there is the potential for unresolved confounding to affect the results and reduce confidence in them and the associated conclusions drawn. This lack of confidence may lead decision-makers and funders to justify calls for definitive evidence from a RCT. The value of conducting such research, to obtain definitive evidence, will be looked at next.
The value of a RCT to promote implementation was calculated here using a ‘value of implementation’ approach. 38 This assumes that the results of our research provide the best available evidence on the clinical effectiveness and cost-effectiveness of NPWT, and that the adjustments made for confounding returned unbiased estimates. Data from the cross-sectional survey conducted within this research programme (workstream 1) indicated a prevalence of treated SWHSIs of 4.1 per 10,000 population (95% CI 3.5 to 4.7 per 10,000 population). Using the observed median duration of wounds (28 days), the daily incidence of SWHSIs was estimated to be approximately 0.0146. Therefore, across England and Wales (population of 57,408,654, as per the Office for National Statistics’ 2014 mid-year estimates),39 we would expect 305,000 patients with a SWHSI per year. Cohort study data indicated that 29.3% (115/393) of incident patients received NPWT and so we would expect > 89,000 patients with a SWHSI receiving NPWT per year. Using this information, the value of implementation approach indicated, should definitive RCT evidence correspond to the findings of the modelling of observational data, that successful cessation of NPWT in clinical practice would have direct health benefits that would sum to between 719 and 2427 QALYs. When displaced health-care benefits were included (by valuing 1 QALY at £20,000), the value of a definitive trial increased to approximately 8500–16,400 QALYs per year (conditional on the findings of a future trial and their successful implementation).
Conclusions
This work compared the clinical effectiveness and cost-effectiveness of NPWT in healing of SWHSIs, relative to dressings, using advanced methods of adjustment for the potential selection effects expected with observational data. The published paper from this stream of work (see Appendix 5) covers methodological aspects of this research; thus, here we aimed to discuss the clinical implications of our results.
Results show that NPWT users in the cohort took longer to heal and this result (which did not reach conventional levels of statistical significance) sustains even after adjustment for differences in potential prognostic factors between the groups. A number of limitations of the data and methods of analyses were also tested (e.g. alternative assumptions on censoring), with none leading to different conclusions. Across all analyses, treatment with NPWT for SWHSIs was not clinically effective or cost-effective and there was little uncertainty over this result. Our treatment effect findings are derived from modelling of observational data and our results (and our interpretation of these) assume that inferences obtained (after adjustment) are unbiased. However, even extensive adjustment of observational data does not negate the potential for unresolved confounding to affect the results and the risk of this can reduce confidence in conclusions drawn from observational data. Definitive evidence from a RCT may be the only way to overcome this lack of confidence.
It is also important to emphasise that we evaluated the causal effects of NPWT on the healing of SWHSIs. Wound infection at baseline was explored in our analyses and was not found to be associated with either treatment allocation or wound healing. However, NPWT may have further direct effects on HRQoL (independent of healing), for example in prevention of wound infection or assisting with wound management. These other effects are likely to be less significant in magnitude and in duration, as NPWT is typically used for only a short duration of time. If future research further explores the effectiveness of NPWT on infection (prevention or treatment), it is important that this research is designed to also explore effects on healing.
There are other important clinical conclusions to be drawn from our analyses. First, patients treated with NPWT in the cohort study differ from those who were not; that is, there was significant selection into treatment with NPWT in the cohort study. Trying to understand how clinicians allocate treatment with NPWT was, however, difficult. Wound area, level of tissue involvement and being an inpatient (rather than outpatient) seemed to have contributed to decisions on whether or not to use NPWT, but do not fully explain these treatment decisions.
Second, in adjusting for selection effects, it was important to consider relevant predictors of healing. However, given the absence of epidemiological research, there was no a priori evidence on what could predict healing in this patient group. The associations found within our work used a predefined process of model selection and indicated that being an inpatient (rather than outpatient) and having a history of a SWHSI may be relevant prognostic characteristics for healing. However, the sample of patients included in the cohort was very heterogeneous. For example, there was a range of anatomical sites for SWHSIs, with the most frequent being the abdomen (33.6%) and foot and leg wounds (30%); there was an approximately equal share of dehisced versus planned open wounds (60% vs. 40%, respectively) and an approximately equal share of wounds with superficial versus deeper tissue involvement (59% with skin involvement only vs. 41% with skin and subcutaneous tissue involvement). If determinants of healing differed across different subpopulations, then a more homogeneous sample would have meant that it was easier to identify these.
Third, our analyses of the cohort data suggest the existence of a subpopulation of ‘hard-to-heal’ patients: time to healing for those who healed (mean 99 days) was very different from the mean follow-up for those who did not heal (mean 416 days). Those who did not heal within follow-up also differed from those who did and our explorations indicate that BMI and foot wound location are associated with a lower probability of healing. Further research could continue to explore characteristics of ‘hard-to-heal’ patients who present with the biggest burden of disease and thus have a larger capacity to benefit from treatments.
Finally, our research also suggests that individuals with a history of SWHSIs treated with NPWT are expected to take even longer to heal than those who did not have a history of SWHSIs. Although we are not clear of the clinical plausibility of such an effect, future research on NPWT could explore the evidence for such treatment effect interaction.
The findings from this workstream have informed the final phase of work in our research programme: a pilot, feasibility RCT assessing NPWT compared with standard dressings.
Workstream 3: qualitative interviews to explore patient and clinician perspectives regarding SWHSIs
The absence of available literature meant that there was limited knowledge about patient experience of SWHSIs or their impact when we began this programme. There was also a lack of research about patient and professional opinions regarding wound management, treatment options and relevant outcomes in relation to measuring treatment effectiveness. These key elements were explored through qualitative interviews with both patients and health professionals (surgeons and nurses). A paper detailing findings from the patient interviews has been published (see Appendix 7). 40 A paper detailing findings from the clinician interviews has been published (see Appendix 8).
Objectives
To explore, using qualitative interviews:
-
patients’ perspectives and experiences of living with a SWHSI and to elicit their views regarding management and desirable outcomes
-
NHS professionals’ views and experiences concerning treatment choices for SWHSIs.
Methods
The purpose of this qualitative research was to explore the daily, lived experiences of patients with a SWHSI and to understand health-care professionals’ perspectives of managing these wounds. The topic guide developed for use with patients (see Appendix 9) comprised a few key questions to encourage in-depth exploration of how a SWHSI might affect every aspect of patients’ lives. Patient advisors were asked to comment on the content and clarity of the patient topic guide prior to its use. During interview, individual patients were encouraged to identify and speak freely about any issue related to their SWHSI that affected them. The clinicians’ topic guide (see Appendix 10) similarly encouraged clinicians to fully express their own perspectives, with the topic guide acting as a flexible tool to guide the discussion.
Patients and clinicians were recruited from both acute and community nursing services in two settings in the north of England: one was a large city with an economically and ethnically diverse population and high levels of deprivation and the other was a smaller city, also with high levels of deprivation and ethnic diversity.
Patients were eligible to participate if they had a surgical wound considered to be healing by secondary intention by the referring clinician, were aged ≥ 18 years and able to give informed consent. Patients were purposively sampled to include those who had SWHSIs that were slow to heal or non-healing, and to ensure representation across sex, age, duration of wound and surgical discipline (e.g. general, vascular, orthopaedic surgery). We aimed for ethnic diversity within the patient sample and for a range of patients from both acute and community treatment settings. Potentially eligible patients were identified and approached regarding study participation by a member of their usual health-care team. Clinicians were purposively sampled from among those with responsibility for caring for patients with SWHSIs, and a range of clinicians from community and acute settings were selected for participation.
The backgrounds of the two researchers involved in the study differed: one was a registered nurse with extensive experience of applied health services research, whereas the other had a background in social sciences research. The two researchers, who were co-located in the same office, held regular, informal meetings during data collection to discuss any differences and ensure consistency of approach. No significant differences were identified in the data collected by the two individuals.
Semistructured interviews were conducted in patients’ homes and at the clinicians’ place of work. Interviews with patients lasted approximately 1 hour (interviews with surgeons ranged from 30 to 45 minutes and those with nurses ranged from 60 to 75 minutes). All interviews were audio-recorded and fully transcribed before being analysed for thematic content using the ‘framework’ approach (see Appendix 11). 41 Interviews continued until no new information was forthcoming. 42 After familiarisation with the data set and initial identification of themes, a coding framework was developed to allow for linkage between participants and their responses and comparison across the full data set. This coding framework was modified during the initial phases of data analysis to accommodate new responses from patients. Interpretation relied on the abilities of the analysts to determine meaning, salience and connection. 43 The input of patient advisors was sought during the analytic process and advisors were asked to contribute to reflections on the meaning and interpretation of the data arising from the interviews.
The study received research ethics approval from Yorkshire and the Humber – Leeds Central Research Ethics Committee (reference 11/YH/0313) and written, informed consent was obtained from all patients prior to participation in the interviews.
Results
Patient interviews
Twenty patients with SWHSIs participated in the qualitative interviews. The mean age of patients was 53 (range 19–76) years, with an almost equal split in sex (11 women and nine men); 19 patients were of white British ethnicity and one was of South Asian ethnicity. The surgical procedure preceding the SWHSI was abdominal surgery (11 patients), vascular surgery (five patients), orthopaedic surgery (two patients) and excision of pilonidal sinus and drainage of abscess (one patient each). The median duration of SWHSIs was 5.5 (range 1.5–60) months. Further details of the sample are presented in Table 15.
| ID | Sex | Age (years) | Employment status | Wound type | Wound duration (months) | Comorbidities (self-reported) |
|---|---|---|---|---|---|---|
| P1 | Female | 58 | Unemployed; registered disabled | Dehisced abdominal wound; had had multiple operations | 6 | Diabetes mellitus; Crohn’s disease |
| P2 | Female | 73 | Retired | Dehisced wound on upper thigh after bypass graft | 2 | Diabetes mellitus |
| P3 | Female | 19 | Unemployed since surgery | Dehisced wound on leg after open fracture | 3 | |
| P4 | Female | 65 | Retired | Open surgical wound following amputation of toes | 12 | Diabetes mellitus |
| P5 | Male | 30 | Employed | Dehisced wound in groin after infection | 3 | |
| P6 | Male | 32 | Unemployed since surgery | Open surgical wound after abdominal surgery | 2 | |
| P7 | Female | 44 | Employed | Open surgical wound after drainage of abscess in axilla | 1.5 | |
| P8 | Male | 52 | Unemployed since surgery | Dehisced abdominal wound | 24 | |
| P9 | Male | 76 | Retired | Non-healing abdominal wound following surgery for bowel cancer | 60 | |
| P10 | Male | 64 | Early retirement due to work injury | Open surgical wound following amputation of toes; had undergone skin graft | 5 | |
| P11 | Male | 45 | Unemployed since surgery | Dehisced wound due to infection after surgery for Crohn’s disease | 3 | Crohn’s disease; necrotising fasciitis; pyoderma |
| P12 | Female | 72 | Retired | Dehisced abdominal wound | 8 | |
| P13 | Female | 54 | Employed | Dehisced abdominal wound | 1.5 | |
| P14 | Female | 47 | Unemployed since surgery | Dehisced abdominal wound | 4 | |
| P15 | Female | 46 | Unemployed since surgery | Dehisced abdominal wound | 9 | Crohn’s disease |
| P16 | Female | 76 | Retired | Dehisced abdominal wound | 10 | |
| P17 | Male | 44 | Unemployed | Open surgical wound following partial amputation of foot | 24 | Diabetes mellitus |
| P18 | Male | 65 | Employed | Non-healing abdominal wound | 2 | |
| P19 | Male | 25 | Employed | Slow to heal wound following treatment for pilonidal sinus | 30 | |
| P20 | Female | 71 | Retired | Dehisced wound following spinal surgery | 9 | Rheumatoid arthritis |
Six emerging themes were identified through the interviews: (1) initial reactions, (2) wound symptoms, (3) expectations of wound healing, (4) psychosocial impact, (5) service provision and (6) SWHSI treatment.
Initial reaction
Unexpected SWHSIs (i.e. following emergency surgery or a dehisced wound) resulted in feelings of alarm, shock, disbelief and disgust among patients. This was most significant in patients whose SWHSIs resulted in the exposure of their internal organs. These patients frequently reported that they had presumed that their wound would be stitched closed and they were shocked and confused when this had not happened. When wounds were extensive, patients indicated their fear that the wound ‘would never heal’, to the extent that they could often recall the specific dimensions of their wound at various points in time.
In contrast, elective SWHSIs (e.g. from an elective partial foot amputation in a diabetic patient) resulted in less shock and alarm in relation to the initial appearance and immediate impact of the wound. These patients frequently viewed surgical intervention as a means of respite from pain, as well as offering a reduced risk of limb loss. Given the planned nature of these wounds, preoperative discussion with clinicians regarding their postoperative surgical wound was much more common in this group of patients, which is likely to have helped to manage patient expectations.
Wound-related symptoms
Wound-related symptoms were reported as having a negative impact on daily life, physical and psychosocial functioning, and sense of well-being. Frequently reported symptoms included pain and reduced mobility; leakage; smell; difficulties with personal hygiene; reduced appetite; disrupted sleep; lack of energy and low mood; skin problems; and side effects from wound-related medication (such as analgesia).
The limited physical mobility (and especially being unable to drive) was particularly frustrating for patients and disruptive of normal activities. In addition, patients felt unable to socialise, as they believed that the wound smell and exudate might be offensive to others.
Healing expectations
The majority of patients with open abdominal wounds feared that their wound would never heal. Patients reported frequently feeling disappointed, dismayed and frustrated with the slow healing process of their SWHSI and lack of, or delayed, healing also resulted in increased anxiety about future surgery for some patients. These feelings were intensified when patients had experienced healing delays due to infection. Many of those who had experienced previous SWHSIs expected healing to be a slow process, whereas patients without a previous SWHSI often had unrealistic expectations of time to healing. Patients’ expectations often conflicted with information provided by health-care professionals. Comments from health-care professionals regarding the wound and its prospects of healing often had profound implications for patients. Positive remarks were a morale boost, whereas negative remarks frequently adversely affected the patients’ general outlook. When conflicting information was provided, this was identified as a cause of confusion and consternation.
Patients were ever-hopeful that a new or untried treatment might accelerate or achieve healing of the wound, and they often looked to tissue viability nurses, regarded as experts in their field, to provide a solution for their unhealed wound. There was a willingness among patients to undergo almost any procedure or treatment if it might promise accelerated healing, even if it was unpleasant.
Psychosocial impact
Surgical wounds healing by secondary intention were frequently found to have a devastating effect on the lives of patients and their families, particularly following emergency surgery or if associated with a large cavity. Patients reported feeling uncertain and anxious following their surgery, particularly in relation to wound healing, physical health, financial concerns and relationships with others.
Patients felt that they had become isolated, withdrawn from society and confined to their homes while their SWHSI was healing. Patients receiving NPWT as a SWHSI treatment reported feeling unable to leave the house due to the pump and tubes associated with the device and concerns about their self-image in public. Feelings of isolation were particularly acute in patients who were incapable of driving after surgery, especially if they were the only car driver in the family. Disruption of established roles and responsibilities within the family unit was common and patients often reported feeling burdensome and dependent. Anxiety was also extremely common and often multifaceted.
The financial impact associated with SWHSIs was severe for some patients and their families, causing high levels of stress. The uncertainties and restrictions associated with SWHSIs appeared particularly difficult for younger male participants who were often the main earner in the family. Family members were also affected as they tried to balance maintaining the household income and acting as a carer for the patient. Out-of-pocket expenses in relation to transport to clinic appointments were troublesome for several patients, particularly for those who were unwilling to use public transport to attend appointments due to concerns about wound odour and who preferred to pay for private taxis.
Most participants described support from partners, family members, friends and neighbours as crucial, whether that support was practical (e.g. with transport and shopping) or emotional (helping them to adapt and adjust to living with their wound). Some older patients were, however, reluctant to ask their offspring for assistance, preferring to try to manage independently if they could.
Living with a SWHSI had a profound impact on patients’ mental well-being. Almost one-quarter of patients indicated that they had become depressed as a result of factors associated with their SWHSI. This was sometimes associated with acceptance of the diagnosis of the condition (e.g. Crohn’s disease) that had resulted in their SWHSI. Other patients did not report feeling depressed, but did acknowledge periods of low mood as a result of their experience of living with a SWHSI.
Service provision
Patients were managed in a variety of settings, including acute hospital and community settings, where district, community or general practice nurses predominantly gave patient care. Reported impressions of care were wide ranging, from ‘brilliant’ to ‘rubbish’.
Prolonged or multiple hospital admissions were common. Duration of hospital admission ranged from an overnight stay (abscess drainage) to 9 months (spinal surgery). Patients indicated that timely provision of information, and regular contact with surgeons (especially those who had performed the operation) and nurses during this time, was important and valued. Comments from surgeons regarding general progress and wound healing were highly important to patients. Positive comments generated reassurance, whereas brusque and hurried interactions led to patients feeling unheard, dismissed and angry.
Several patients reported that their wound dressings had not been changed as frequently as they thought necessary while in hospital and a majority reported that their care on the wards was less than satisfactory. Significantly, many patients reported feeling dissatisfied and unsupported at the point of hospital discharge, due to a lack of available information relating to follow-up care and risks, symptoms and management of infection. Following discharge from hospital, however, only a minority of patients felt unsupported.
All patients expressed views about the district, community and general practice nursing service, with the majority having received home visits by district, community or general practice nurses during their healing pathway. It was widely perceived that the district, community or general practice nurses had limited time to devote to visits, leaving patients feeling that visits were rushed, although there was acknowledgement that the nurses had tried to attend to all needs during the visit. Patients also indicated that the unpredictability of visit timings caused substantial frustration and inconvenience, and for three patients, care via a general practice or treatment centre nurse was preferred, due to convenience and ongoing continuity of care.
With specific regard to district, community or general practice nursing services, patients were particularly troubled by a lack of continuity of care, with some patients claiming that they would ‘never see the same nurse twice in a row’. Patients became anxious when they received conflicting information regarding the condition and likely healing and management of their wound, particularly if the information given was at variance to that provided by their surgeon.
SWHSI treatment
The main wound treatments experienced by study participants were NPWT, debridement, dressings and skin grafting.
Eleven patients had experience of NPWT, which they generally viewed as an effective treatment. The majority of patients receiving NPWT had undergone bowel or abdominal surgeries; however, patients also experienced NPWT for foot and orthopaedic surgical wounds. Perceived advantages of NPWT included reduced dressing change frequency, compared with ‘traditional’ dressings, and a reduced risk of wound infection. Patients did, however, note that NPWT dressing changes were painful when sponge or foam, rather than gauze, were used. Patients with prior experience of NPWT reported that some district, community and general practice nurses lacked appropriate knowledge, experience and expertise in NPWT and, in some instances, required guidance from the patient in its application. Perceptions concerning lack of nursing expertise in the use of this therapy were linked to feelings of anxiety by some patients. Several patients reported that they were disinclined to go out of the home with NPWT in situ, due to it being cumbersome or due to embarrassment about the equipment, and when they did leave the house they felt a need to somehow disguise the device. Patients also reported delay in discharge from hospital due to difficulty associated with obtaining NPWT within the community setting and they expressed the view that this treatment was more easily administered within a hospital environment as opposed to community settings.
Six patients had undergone wound debridement and described this as an extremely painful procedure. The rationale for and potential effectiveness of debridement were often poorly communicated to patients.
Three patients underwent skin grafting as a method of promoting healing, with a further two participants having discussed the possibility of a skin graft for their SWHSI. Views regarding the desirability of skin grafting were mixed, due to fears of infection and/or graft failure.
Patients reported that they would have liked more information from clinicians about the rationale for using different treatment methods and approaches, including different dressings, and they described nurses as rarely ‘singing from the same hymn sheet’ when selecting dressings.
Clinician interviews
Five surgeons and seven nurses participated in the qualitative interviews: two general surgeons, two vascular surgeons, one plastic surgeon, three tissue viability nurses, one general surgery nurse, two district nurses and one community staff nurse. Further details of the sample are presented in Tables 16 and 17.
| ID | Sex | Age (years) | Qualifications | Role | Specialist training |
|---|---|---|---|---|---|
| 1 | Female | 49 | RN; BA (Hons) nursing practice; postgraduate certificate nursing studies; community/specialist practitioner | Tissue viability nurse specialist (specialist assessment; care planning; evaluation; prescribing; liaison with surgeons; supporting nurses) | Diploma in wound care and ulcer management |
| 2 | Female | 46 | RN; BSc (Hons) health and social care; diploma in nursing | Clinical nurse specialist (education; training; support for colleagues) | No specialist training. Experience of working within the tissue viability team |
| 3 | Female | 42 | RN; BSc (Hons); district nursing qualification | Tissue viability nurse specialist (assessment of complex surgical wounds; instigation and monitoring of NPWT) | No specialist training. Experience of reading relevant research; ‘peer shadowing’ |
| 4 | Female | 44 | RN | Senior nurse working on acute general surgery ward caring for patients with abscess/fistulas/wound dehiscence | Training from employees of commercial company in use of NPWT |
| 5 | Female | 39 | RN; district nursing qualification | Senior treatment room nurse (seven treatment rooms city wide) | Study days; experience; leg ulcer management course |
| 6 | Female | 38 | RN; district nurse | District nurse; link nurse tissue viability | Link nurse tissue viability and related study sessions |
| 7 | Female | 52 | RN | Community staff nurse; general wound care | Wound management course |
| Surgeon | Specialty | Study site |
|---|---|---|
| 1 | General surgeon | 1 |
| 2 | Vascular surgeon | 1 |
| 3 | General surgeon | 2 |
| 4 | Plastic surgeon | 1 |
| 5 | Vascular surgeon | 2 |
Six themes were identified in the interview data: (1) factors influencing SWHSI development, (2) devolving care to nurses, (3) multifaceted assessment, (4) NPWT for specific types of SWHSI, (5) presumed cost-effectiveness and (6) dressing selection by nurses.
Factors influencing SWHSI development
Surgeons and nurses identified that the main types of SWHSIs encountered were extensive cavity wounds (e.g. from abdominal or pilonidal sinus surgery), open wounds on the feet of diabetic patients and wounds associated with lymph node removal (e.g. in the axilla and groin). Abdominal surgery wounds were regarded as being most likely to heal slowly or not to heal and were thought to be most likely to dehisce.
Both surgeons and nurses broadly agreed that there were a number of factors that predicted slow or no healing. These included surgical factors (e.g. emergency surgery, reason for surgery, type of procedure or presence of a foreign body), patient comorbidities or factors (e.g. obesity, diabetes mellitus, cancer, arterial and/or vascular disease, impaired immune system or Crohn’s disease, nutritional status, smoking status and age), infection, medication, restricted mobility and lack of treatment compliance. It was, however, noted that in many instances it was difficult to identify any specific reason for impaired wound healing.
Wound healing was sometimes viewed as completely unpredictable: a wound was said to sometimes progress well, stop healing for a period, with no obvious explanation, and then recommence healing. It was suggested that a pause in healing might occur following removal of NPWT. Overgranulation was also mentioned as a factor that could delay the final stages of wound healing.
Devolving care to nurses versus ‘looking after our own’
Surgeon involvement with the wound management of individual patients was related to wound-related factors, as well as the patient’s general condition and the nature of the surgery. The two general surgeons included in the study indicated that usually their patients’ wound care was passed on to nurses following discharge. However, the two vascular and one plastic surgeon interviewed said that they were more likely to continue to have personal involvement in wound care for ‘their’ patients through follow-up in specialist clinics, which they described as ‘looking after our own’.
Surgeons and/or tissue viability nurse specialists generally initiated the decisions to use NPWT. Ward, district and community nurses indicated that there was limited knowledge and expertise about use of NPWT among general nurses working in both acute and community nursing settings. Tissue viability nurses reported that they were often regarded as the first port of call for expert advice in relation to complex non-healing wounds and use of NPWT.
Assessment is multifaceted and complex
Factors cited by the surgeons as associated with wound healing were the size of the wound; presence of wound infection, slough and/or granulation tissue; whether or not the wound appears to have healed superficially, but remains unhealed at a deeper level; level of exudate; the condition of the wound edges; the patients’ general condition, including nutritional state and ability to mobilise; signs of overgranulation; whether the wound seems ‘static’ and/or appears to be colonised; and blood supply at the wound site. Nurses described similar factors as affecting wound healing. They also emphasised psychosocial and practical issues that might have an impact on patients’ quality of life, such as level of family support and patients’ potential for self-care of the wound. Tissue viability nurses, for example, commented that they would adopt a holistic approach to assessment, by gathering background information about the history of the wound, as well as other relevant details about the patient’s physical, social and psychological status.
Only one nurse mentioned using a wound-specific assessment tool – the TIME (Tissue, Infection, Moisture and wound Edge) tool – during the initial wound assessment. 45
Perceived advantages and disadvantages of negative-pressure wound therapy
Negative-pressure wound therapy was favoured for a variety of reasons. Surgeons reported that the use of NPWT was increasing and that complex cavity wounds (e.g. extensive abdominal wounds) were ideally suited to benefit from NPWT. It was perceived that NPWT controlled exudate, contained and limited contamination (reducing infection risk), supported resolution of infection, promoted growth of granulation tissue, hastened wound healing and was liked by patients because it was convenient, promoted mobility, enhanced quality of life, shortened hospital stay and reduced the number of required dressing changes. Clinicians generally perceived NPWT as a cost-effective and transformative treatment, particularly for patients with hard-to-heal wounds.
One tissue viability nurse did, however, express strong reservations about the perceived ‘espoused universal benefit’ of NPWT, noting that it was not necessarily ‘the answer to everything’ and inappropriate for some patient groups. This view was supported by some surgeons and nurses who indicated that NPWT was an inappropriate treatment for some types of wound, particularly in patients following abdominal surgery who might be at risk of developing fistulae, as well as some patients who were unable to cope with the device. The same tissue viability nurse felt that NPWT was sometimes ‘oversold’ to patients, raising expectations unrealistically. This nurse also commented that longer-term healing of SWHSIs could be impeded by the use of NPWT due to the use of foam, rather than gauze dressings, which, they suggested, could result in the formation of less ‘robust’ granulation tissue. Further disadvantages, perceived by surgeons, included its lack of suitability for some patients who might be unable to cope with the equipment due to frailty or impairment, limited availability in the community, high cost, and the need for staff (predominantly nurse) training in its application. Disadvantages from a patient perspective mentioned by nurses were anxiety and disruption to sleep, and restrictions, even with ‘portable’ devices, when mobility increased in the time following surgery.
Presumed cost-effectiveness
Three surgeons noted the lack of research evidence relating to NPWT, but felt that their own and their colleagues’ experiences supported its use. These surgeons perceived NPWT use as increasingly widespread and safe. They viewed NPWT as a cost-effective treatment option on the basis that patients with complex wounds could be discharged from hospital sooner, required less input from district, community or general practice nurses (in terms of dressing changes), once back in the community, and could return to work more quickly.
One surgeon was of the opinion that there was currently sufficient evidence to support the use of NPWT and referred to various studies (small scale, mainly case series) apparently demonstrating its effectiveness. This surgeon, the strongest advocate of NPWT among those interviewed, asserted that the advent of NPWT had ‘changed practice considerably’.
Dressing selection usually devolved to nurses by surgeons in the study
Surgeons’ knowledge concerning dressings was, by their own admission, limited, resulting in reliance on nurses to make appropriate dressing choices. The nurses described how wound, patient and general factors influenced wound management decisions. Nurses felt that they had adequate information to support wound management decisions for most routine SWHSIs, with choices determined by local guidance (formularies and protocols), that purported to be evidence based, alongside an appreciation of the need for cost-effective treatment decisions, patient preference and likely concordance, and inclusive of the views of colleagues.
Nurses also commented that if they were uncertain about how to manage a complex, non-healing open surgical wound, they would contact tissue viability nurse specialists for advice and guidance. The tissue viability nurses noted that in these situations, they were able to draw on each other’s knowledge and experience in dealing with difficult-to-heal wounds, through their involvement in close-knit clinical teams and other wound-focused clinical networks.
Similarities and differences between responses from surgeons and nurses
Surgeons and nurses were in broad agreement about the types of wounds likely to be slow to heal/non-healing and their symptom burden. During interview, tissue viability nurses focused, more than surgeons, on describing the possible psychosocial and financial consequences for patients of experiencing a SWHSI. Tissue viability nurses explained that they had more time than surgeons to explore the range of impacts of a SWHSI on the lives of individual patients and their family members; in their interviews, surgeons tended to focus mainly, though not exclusively, on clinical aspects of patients’ surgical wounds and healing.
Tissue viability nurses reported their own first-hand experiences of the challenges of managing patients’ hopes and expectations for healing. Nurses suggested that sometimes patients appeared ill-informed about likely healing projections after discharge from hospital and they said that sometimes there appeared to be a ‘mismatch’ between surgeons’ and patients’ expectations for healing (with patients holding unrealistic expectations).
Six of the seven nurses interviewed were in agreement with the study surgeons’ views that NPWT is effective in the management of SWHSIs, particularly in the management of wound exudates, due to the good dressing seal that can be obtained and the potential for reduced likelihood of infection, resulting in perceived speedier healing of the wound. Reservations expressed by the seventh nurse related to the use of gauze rather than foam in the application of NPWT (she thought use of gauze caused less pain to patients) and the possibility of patients with an abdominal SWHSI developing an intestinal fistula connected to use of NPWT, a concern also noted by the general surgeons. Surgeons and nurses both commented that NPWT was not a suitable treatment for the very frail (who could find the equipment difficult to manage) and/or for patients with cognitive impairment.
During interview, surgeons were more likely than nurses to talk about perceived cost-effectiveness of NPWT in relation to length of hospital stay, reporting that use of NPWT allowed patients to be discharged from hospital sooner than with use of conventional dressings; surgeons also pointed to a reduced need for dressing changes by community-based nurses, which they perceived as beneficial in terms of reducing these nurses’ workload and accrual of savings to the NHS. Variability in community and district nurses’ expertise in application of NPWT, and the need for more widespread training in its application, were reported more frequently by nurses than surgeons, who appeared to be less aware of these issues.
Conclusions
To the best of our knowledge, this is the first study investigating patients’ and clinicians’ views about the management and treatment of SWHSIs, and patients’ experiences of living with this condition. Findings from our study therefore lay the groundwork for further research in this important aspect of health care about which so little is known. For example, understanding more about what matters to patients helps to ensure that appropriate outcomes are captured in future trials.
Patients’ initial reactions to their SWHSIs were of shock and disbelief, and the visual reminder following surgery of a ‘violated’ body intensified these feelings, particularly for patients with large abdominal cavity wounds. Having a SWHSI had a substantial impact on daily living and this impact was particularly important for those patients with children or in employment, who constitute a younger demographic than seen in previous wound research associated with other wound types. As healing progressed, patients were concerned with both their own reaction to the wound and the reactions of others. The long healing process for many patients, characterised by a reduced quality of life and a constant need for treatment and care, concurs with previous evidence in relation to patients with other wound types or ulceration. 46–53 It is worth emphasising, however, that SWHSI patients are typically much younger than patients with leg or pressure ulcers and frequently have major caring and employment responsibilities: Cullum et al. 27 reported a mean age of 79.65 (SD 11.32) years for venous leg ulcer patients, a difference of 25 years when compared with the mean age observed in workstream 1 [54.1 (SD 18.2) years]. Wound care experiences among patients varied greatly, but most patients were willing to try any treatment that promised healing. Understanding the ‘lived experience’ of patients is crucial for clinicians to provide effective management and improve patient quality of life. Establishing a therapeutic relationship and listening to patient concerns is vital to meeting patient’s physical, emotional and psychosocial needs during the wound healing process. The provision of additional information for patients in relation to the rationale of treatment choices is also an important element for consideration with regards future treatment delivery.
Clinicians had varying levels of knowledge in relation to wound care treatment options. Nurses were frequently responsible for dressing choice decisions, made on the basis of wound condition, clinical experience and recommendations. A treatment frequently favoured by clinicians was NPWT. The lack of robust evidence for this treatment option was noted by several of the study clinicians; however, for many, individual experiences of its use were deemed to sufficiently demonstrate the effectiveness of this treatment. 14
The use of purposive sampling enabled us to obtain a diverse range of views from both patients and clinicians. It is noted, however, that only a limited number of interviews were conducted with clinicians. Therefore, further research may be warranted to build on these findings.
Generalisability in qualitative research involves thinking through what kind of relationship the study findings have with other populations and settings, and unpacking exactly what inferences can be drawn from the data analysis. 41 Our study yielded ‘rich’ data that captured a wide-ranging, yet in-depth, picture of significant issues relating to patients’ and clinicians’ views and experiences of SWHSIs. The study findings, therefore, promote and enhance understanding of the impact of a SWHSI on patients’ lives, as well as describing the challenges faced by clinicians in managing these wounds, thereby adding to the evidence base by ‘sensitising’ readers, including the research community, to new ways of thinking about SWHSIs. Our study findings may be considered transferable to the ‘parent populations’ of patients with SWHSIs and the clinicians looking after them; however, decisions concerning broader extrapolation of the findings must take into account any study-specific contextual factors that may limit applicability. 41
Workstreams 1–3: overall conclusions
Workstreams 1–3 have substantially contributed to the evidence base and understanding of SWHSIs, providing data about patient characteristics, wound origins, durations and treatments, alongside patient and clinician views of living with and treating SWHSIs.
Workstream 1 has identified the prevalence of treated SWHSIs and has provided extensive data on populations of clinical relevance for inclusion in further research. The median time to healing we observed in this workstream highlights the extended period of time that patients might expect to cope with open surgical wounds. This workstream has also highlighted the distinctive management challenge posed by SWHSIs.
Workstream 2 has identified that NPWT is not a clinically effective or cost-effective treatment for SWHSIs. However, because this uses observational research, even with adjustment, there is still the potential for unresolved confounding to affect the results and so reduce confidence in them. This lack of confidence may justify calls for definitive evidence from a RCT, which would be more likely to effect a change in clinical practice should the evidence support one.
Workstream 3 has provided the first evidence of clinicians’ and patients’ views about the management and treatment of SWHSIs, and patients’ experiences of living with this condition. The impact of SWHSI on patients was evident in all interviews and the understanding of patient experiences will assist in providing effective management of this condition. The work conducted in workstream 3 has provided the necessary groundwork for further research in this area.
Interviews with clinicians identified increasing use of NPWT and illustrated the strength of support for NPWT among clinical staff, despite the lack of research. Surgeons and nurses perceived NPWT as safe and cost-effective; however, we have been unable to identify existing, or derive new, evidence to support this. Given the increasing use and perceived effectiveness of this treatment, it is crucial that further, robust evidence is obtained to support, or refute, the estimates derived from observational data in workstream 2.
In addition to the need to establish clinical effectiveness and cost-effectiveness of treatments, such as NPWT, the findings from workstreams 1–3 suggested that further research is necessary to understand the epidemiology of SWHSIs, prognostic factors for healing and the clinical effectiveness and cost-effectiveness of other treatment options. The findings of workstreams 1–3 helped formalise key components of the design of workstream 4 for this research programme, which comprised a pilot, feasibility RCT, required to assess feasibility of RCT assessment of NPWT compared with usual care (no NPWT), prior to a larger study being conducted.
Workstream 4: a pilot, feasibility randomised controlled trial of negative-pressure wound therapy compared with usual care
The results of workstream 2 indicated that NPWT was, with little uncertainty, not clinically effective or cost-effective. This conclusion was, however, based solely on observational data, which, even with extensive adjustment, may be subject to unresolved confounding, leading to a lack of confidence in the finding. A RCT may therefore be justified, to enable definitive, high-level evidence regarding the clinical effectiveness and cost-effectiveness of NPWT to be generated.
Given the difficulties in recruitment identified in previous studies of NPWT,54,55 we conducted a pilot, feasibility study to assess the feasibility of conducting a larger RCT of this treatment. The study was registered as ISRCTN12761776 and the protocol has been published (see Appendix 12). 56 The results of this study have been published (see Appendix 13)57 and the findings relating to nurse participation in the pilot, feasibility study have been published (see Appendix 14).
Objectives
-
To test the methods and feasibility of a full RCT of NPWT compared with usual care (no NPWT) for SWHSI.
-
Specifically, to determine:
-
likely recruitment rate, including potential participants’ willingness to be randomised and if this depends on wound location and other factors
-
clinicians’ willingness and ability to recruit and randomise participants
-
testing of inclusion and exclusion criteria
-
fitness for purpose of data collection methods, including across and between care settings
-
ability of sites and clinicians to supply NPWT to participants in a timely fashion, irrespective of care settings, and assess any training requirements
-
ability of community staff to manage participants randomised to NPWT
-
suitability of method of outcome ascertainment
-
adequacy of duration of follow-up
-
rates of withdrawal from treatment, response rates to questionnaires, attrition from the trial and likely rates of missing data for outcomes
-
other methodological issues, including feasibility of blinded outcome assessment and whether or not trial documentation is acceptable to NHS nurses collecting data
-
feasibility of collecting data for and conducting of economic analyses in a larger trial (through exploratory analyses).
-
Methods
The pilot, feasibility RCT was conducted over a 10-month period (20 November 2015 to 30 September 2016: recruitment 20 November 2015 to 30 June 2016 and follow-up 20 February 2016 to 30 September 2016) in acute and community settings at three study sites in Leeds and Hull, Yorkshire, UK. Prior to study activity commencing, approval was obtained from Yorkshire and the Humber – Leeds East Research Ethics Committee (reference 15-YH-0307) and from the associated NHS trusts.
Patients were eligible for inclusion if they were aged ≥ 18 years and able to give full informed consent; were receiving care from Hull and East Yorkshire Hospitals NHS Trust (now Hull University Teaching Hospitals NHS Trust), Leeds Teaching Hospitals NHS Trust or Leeds Community Healthcare NHS Trust; had a SWHSI that could be reasonably treated with NPWT and wound dressings; had a SWHSI that was considered ready for NPWT (i.e. minimum 80% viable tissue or thin layer of slough requiring no further debridement); and were receiving adequate nutrition (as assessed by the senior nurse responsible for nursing care). Patients were ineligible for inclusion if they had limited life expectancy; active systemic infection; inadequate haemostasis or risk of bleeding; chronic wounds (e.g. pressure or foot ulcers) that were non-surgical in origin but had been surgically debrided; or had wound characteristics that precluded the use of NPWT (e.g. unclear undermining, necrotic or malignant tissue, eschar, exposed blood vessels, organs, anastomotic sites or nerves). Patients were also excluded if they had previously been or were currently receiving NPWT on their SWHSIs (including applications while in theatre for the surgery resulting in the SWHSI), if the wound was located where a vacuum seal could not be obtained, if they had participated in a research study in the previous 4 weeks, or if they were unwilling to have wound photographs taken.
Health-care professionals initially identified and screened patients for eligibility. Those meeting eligibility criteria were approached with further details of the study by a clinical or research nurse (subject to patient consent to approach), prior to study consent being obtained. As the main aim of this study was to determine the feasibility of conducting a larger trial, rather than to detect a treatment effect, a formal sample size calculation was not required. We aimed to randomise 50 patients over a 7-month recruitment period.
Baseline data were collected, including participant, surgery and wound information, wound dimensions (measurements and wound tracing) and a photograph, pain assessment [using a visual analogue scale (VAS) and the BPI]23 and quality-of-life data (SF-12 and EQ-5D-3L). 21,22 As this was a feasibility trial, a primary outcome was not defined; rather, a range of feasibility (e.g. recruitment rate, time to intervention delivery) and clinical (e.g. time to wound healing) outcomes were collected to inform a future definitive trial. Participants were followed up for a maximum of 3 months, unless they withdrew consent, died or became lost to follow-up. Clinical outcomes were collected prospectively on a weekly to fortnightly basis at follow-up assessment visits conducted by the research nurses, including wound dimensions and photographs, healing status [defined as ‘complete epithelial cover in the absence of a scab (eschar)’], infection, hospital admission, return to operating theatre and treatment changes. Participants also completed self-report postal questionnaires (EQ-5D-3L,22 BPI,23 SF-1221 and resource use and pain via a VAS) at 2 weeks, 1 month and 3 months post randomisation. When consent was provided, participants were also contacted by text message on a weekly basis and asked to provide a numeric rating of their wound pain in the previous week. When participants failed to respond, one further message was sent before text message data collection discontinued. When participants’ wounds were reported to have healed during the study, text messaging data collection ceased at the point of wound healing.
Information concerning acceptability of study participation was collected from participants using Likert scales. Data relating to acceptability of documentation and training requirements for NPWT were initially collected from research nurses, using a Likert scale. Qualitative interviews were then completed with the research nurses to ascertain additional information regarding acceptability of the study processes, documentation and delivery.
Participants were allocated (1 : 1) to the intervention (NPWT) or usual care (no NPWT), using random permuted blocks, with stratification by wound area (< 28 cm2, ≥ 28 cm2). Allocations were concealed through a central randomisation service at the University of York.
The study intervention (NPWT) was delivered using NPWT devices that were in use in the acute and community services in Hull and Leeds, which included V.A.C® (KCI Medical Asia Pte Ltd, Singapore), Renasys® (Smith & Nephew, London, UK) and PICO® (Smith & Nephew, London, UK) devices. The NPWT treatment delivery (e.g. device type, pressure cycles, dressing change and canister emptying frequency, application and removal of the device and treatment duration) was as per the standard practice at the time to ensure that the pragmatic nature of the pilot feasibility study was maximised. This was therefore decided by the clinical care team in conjunction with the participant and nurse, with the only stipulation for NPWT being that use must be clinically appropriate.
The control group participants received usual care (wound dressings, no NPWT), with dressing type and frequency of change left to the discretion of the clinical care team. As there is no evidence to suggest that any one dressing is more clinically effective or cost-effective than another,12 types of dressings were not stipulated for the trial.
Owing to the nature of the intervention, it was not possible to blind participants or health-care professionals to trial treatment; however, the feasibility of blinded outcome assessment of healing was assessed within the trial using study photographs. Initially, it was proposed that blinded review of healing would be completed for every participant photograph obtained, but this was reduced during the study to review of only the final photograph, due to time and resource constraints. Assessment of blinding to treatment allocation was conducted through review of photographs taken at the week 1 follow-up.
Data analysis was conducted using Stata, using the principles of intention to treat and two-sided statistical tests at the 5% significance level, when appropriate. Summary statistics for all variables were generated by trial arm and overall. Time to healing was defined as the number of days from randomisation to date of healing. Data were right censored at the date of the final assessment visit for participants not deemed to have healed. Time to healing was assessed using Kaplan–Meier curves, recognising that the study was not powered to detect a treatment effect. A Cox proportional hazards regression was used to investigate the impact of covariates (contamination level of surgery, wound infection, wound size, SWHSI history and location). Cost-effectiveness was explored using total mean resource use costs and mean QALYs per trial arm.
To understand improvements to trial processes, to support research nurses in conducting a larger RCT in this area, qualitative interviews with eight research nurses were completed. Interviews were audio-recorded and fully transcribed before being analysed for thematic content using a ‘framework’ approach. 41,58 Interpretation of the findings enabled linkage between individual nurses and their responses to account for variation between the type of study site (acute or community).
Prior to analysis, wound tracings were measured using Mouseyes software (R Taylor, Manchester, UK) to obtain perimeter and area estimates. 59 Both adjusted (length × width × Π)60 and unadjusted (length × width) wound area (cm2) measurements were calculated using ruler measurements obtained at clinical follow-up visits.
Results
Recruitment and participation
Recruitment to the study commenced on 20 November 2015 and continued until 30 June 2016. Delays in study set up, beyond the control of the research team, had an impact on recruitment rate in the early stages of the trial; there was only one recruiting site active until the two remaining sites were opened to recruitment in February and March 2016, respectively. The overall target recruitment rate for the study was seven participants per month and, following successful opening of all three recruitment sites, the study achieved this target in the final 4 months of recruitment (Figure 4). The flow of participants through the trial is shown in Figure 5.
FIGURE 4.
Cumulative target and actual participant recruitment.
FIGURE 5.
Pilot feasibility RCT: Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, Reasons not mutually exclusive.
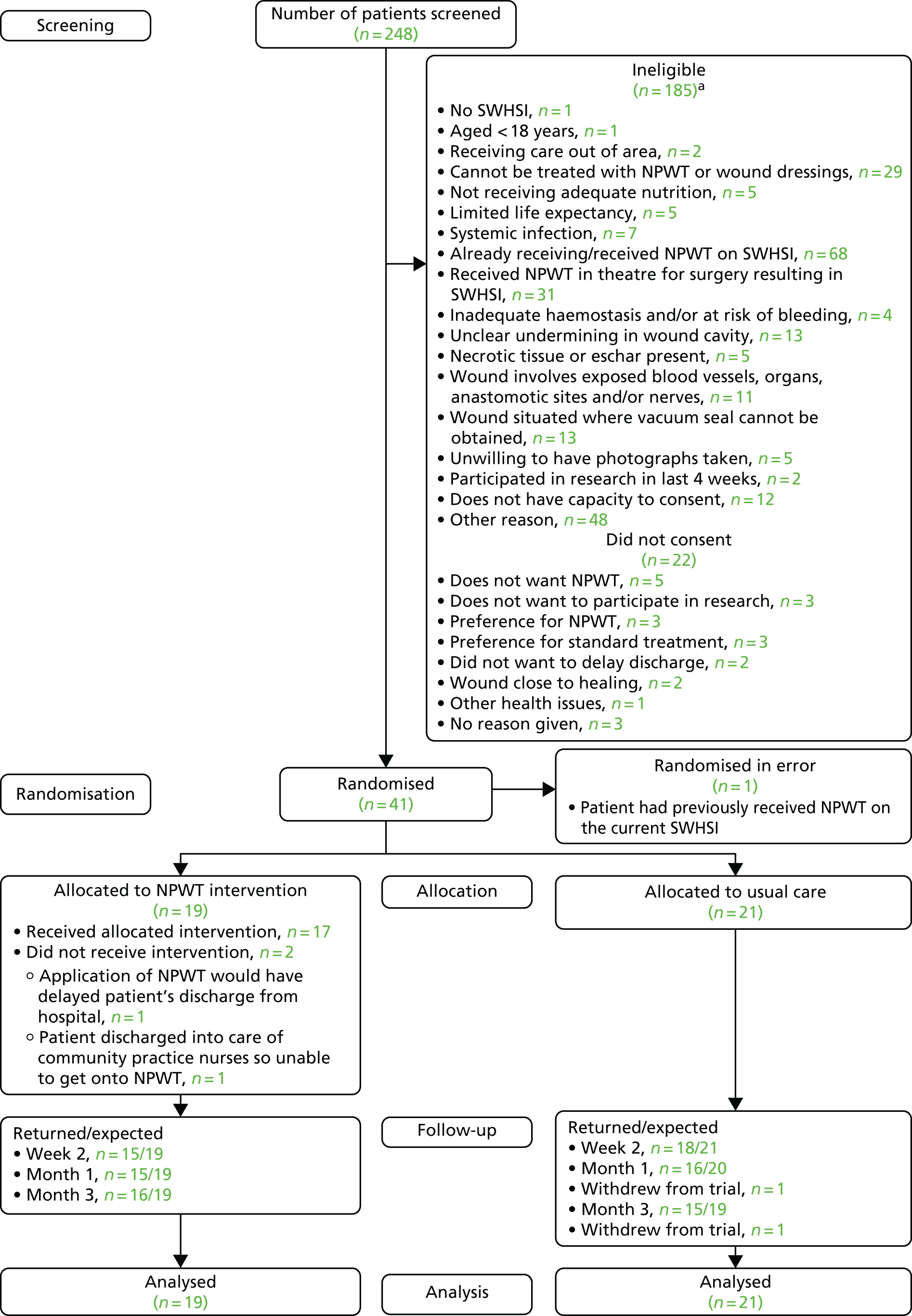
Of 248 patients screened for eligibility, 75.0% (n = 186) were ineligible (including one participant found to be randomised in error). The most common reason for ineligibility was that the patient was receiving or had previously received NPWT on their current SWHSI (45/248, 18.1%), had NPWT applied while in theatre (n = 7/248, 2.8%) or both (24/248, 9.7%). Twenty-two participants were eligible but did not wish to consent to study participation; the most common reason for declination was not wishing to receive NPWT (5/22, 22.7%). Reasons for ineligibility were largely distributed across the observed wound locations, with the exception of ‘receiving inadequate nutrition’, which was only reported for five patients with abdominal wounds, and ‘presence of exposed vessels or organs’, which was reported for 11 abdominal and two leg wounds only.
Forty-one of the 248 screened patients (16.5%) were randomised to the pilot feasibility RCT, with 40 participants included in the study analyses (one patient was found to be ineligible following randomisation and so was withdrawn from study data collection and analysis). Of those correctly randomised, 19 out of 40 patients (47.5%) were allocated to NPWT and 21 out of 40 patients (52.5%) were allocated to usual care.
The mean age of participants was 57.8 (SD 14.4) years and just over half (n = 22, 55.0%) were male. Participants were, on average, overweight [mean BMI 29.1 (SD 5.7) kg/m2], 60% (n = 24) were diabetic and 10 (25.0%) reported being a current smoker. The most common location of the wounds in recruited participants was the foot (n = 24, 60.0%), of which 19 out of 24 (79.2%) were due to toe or foot amputation, two (8.3%) were due to incision and drainage, two (8.3%) occurred following debridement (of amputation site, n = 1; of non-chronic ulcer, n = 1) and the cause of the remaining foot wound was unknown. Usual-care participants had their wound for a median of 12 days and over one-third (8/21, 38.1%) had a wound infection at baseline. NPWT participants had their wound for a median of 7 days and 26.3% (5/19) of participants had a wound infection at baseline. Baseline characteristics are summarised in Table 18.
| Variable | Treatment group | Total (N = 40) | |
|---|---|---|---|
| NPWT (N = 19) | Usual care (N = 21) | ||
| Age (years) | |||
| Mean (SD) | 58.8 (15.1) | 56.9 (13.9) | 57.8 (14.4) |
| Median (minimum, maximum) | 62 (33, 88) | 55 (35, 86) | 58.5 (33, 88) |
| Sex, n (%) | |||
| Male | 11 (57.9) | 11 (52.4) | 22 (55.0) |
| Female | 8 (42.1) | 10 (47.6) | 18 (45.0) |
| BMI (kg/m2) | |||
| Mean (SD) | 29.2 (5.6) | 29.0 (6.0) | 29.1 (5.7) |
| Median (minimum, maximum) | 28.6 (20.9, 39.9) | 29.3 (13.7, 39.9) | 28.7 (13.7, 39.9) |
| Diabetic, n (%) | |||
| Yes | 12 (63.2) | 12 (57.1) | 24 (60.0) |
| Peripheral vascular disease, n (%) | |||
| Yes | 7 (36.8) | 7 (33.3) | 14 (35.0) |
| Tobacco use, n (%) | |||
| None | 13 (68.4) | 10 (47.6) | 23 (57.5) |
| Ex-smoker | 1 (5.3) | 6 (28.6) | 7 (17.5) |
| Current (less than or equal to one pack a day) | 2 (10.5) | 4 (19.1) | 6 (15.0) |
| Current (more than one pack a day) | 3 (15.8) | 1 (4.8) | 4 (10.0) |
| History of SWHSI, n (%) | |||
| Yes | 5 (26.3) | 5 (23.8) | 10 (25.0) |
| No | 11 (57.9) | 15 (71.4) | 26 (65.0) |
| Do not know | 3 (15.8) | 1 (4.8) | 4 (10.0) |
| Wound area (cm2) | |||
| < 28 cm2, n (%) | 10 (52.6) | 11 (52.4) | 21 (52.5) |
| ≥ 28 cm2, n (%) | 9 (47.4) | 10 (47.6) | 19 (47.5) |
| Median (minimum, maximum) | 18.2 (0.2, 122.5) | 20.4 (2.3, 93.2) | 19.3 (0.2, 122.5) |
| Wound duration (days) | |||
| Median (minimum, maximum) | 7 (0, 27) | 12 (1, 142) | 7 (0, 142) |
| Wound infected, n (%) | |||
| Yes | 5 (26.3) | 8 (38.1) | 13 (32.5) |
| No | 14 (73.7) | 13 (61.9) | 27 (67.5) |
| Wound location, n (%) | |||
| Foot | 11 (57.9) | 13 (61.9) | 24 (60.0) |
| Abdomen | 3 (15.8) | 3 (14.3) | 6 (15.0) |
| Leg | 3 (15.8) | 1 (4.8) | 4 (10.0) |
| Breast | 1 (5.3) | 1 (4.8) | 2 (5.0) |
| Groin | 0 (0.0) | 2 (9.5) | 2 (5.0) |
| Buttocks | 0 (0.0) | 1 (4.8) | 1 (2.5) |
| Perianal area | 1 (5.3) | 0 (0.0) | 1 (2.5) |
| Type of surgery, n (%)a | |||
| Elective | 12 (66.7) | 18 (85.7) | 30 (76.9) |
| Emergency | 6 (33.3) | 3 (14.3) | 9 (23.1) |
| Contamination level of surgery, n (%) | |||
| Clean | 6 (31.6) | 2 (9.5) | 8 (20.0) |
| Clean/contaminated | 9 (47.4) | 12 (57.1) | 21 (52.5) |
| Contaminated | 4 (21.1) | 7 (33.3) | 11 (27.5) |
Data collection methods
Twenty-six participants (65.0%) consented to data collection via text messaging; 20 participants (76.9%) subsequently provided at least one response (NPWT, n = 10; usual care, n = 10). Response rates to text messaging varied throughout the trial, ranging from 57.7% of those receiving a text in the week following randomisation to 100% of those receiving a text in week 12. Participants who responded to text messaging tended to be younger than those who did not respond (mean age 51 years vs. 60 years, respectively) and non-responders were predominantly male (85.7%). All non-responders had a foot wound, had at least one comorbidity and over half of those who did not respond to text messaging had been allocated to the control arm (57.1%). A range of wound locations were observed in those participants who did respond to text messages (e.g. foot wounds accounted for 36.8%) and 78.9% of participants had one or more comorbidities. Participants who responded to text messaging were almost evenly split between the intervention and control groups (52.6% vs. 47.4%, respectively).
Wound area (cm2) was collected by ruler measurement and wound tracing at each follow-up visit. Ruler measurements were found to overestimate the true area as obtained using wound tracings (mean difference 7.0 cm2, SD 9.1 cm2), even when an adjustment of ‘Π/4’ was used. Wound area decreased in both treatment groups throughout the trial.
The movement of patients from acute to community settings did not substantially affect follow-up completion. In the majority of cases, follow-up continued as planned; however, qualitative interviews with the study nurses showed that, on occasion, it was impossible to assess the wound at follow-up visits. This was primarily due to community health-care professionals (e.g. district, community or general practice nurses) either having already attended and redressed the wound or not attending at the agreed time for the visit, meaning that research data could not be collected in full.
Negative-pressure wound therapy supply
Nineteen participants (of 40, 47.5%) were allocated to receive NPWT; however, two participants (of 19, 10.5%) did not receive the allocated intervention (one because NPWT would have caused delay in discharge and the other because all NPWT devices were with other patients in use in the community at that time). Approximately three-quarters of allocated participants (14/19, 73.7%) received NPWT within 48 hours of randomisation. Seven participants (of 19, 36.9%) did not receive NPWT within 24 hours of randomisation, with the primary reason for delay being lack of device availability (6/7, 85.7%). Participants received NPWT for a median of 18 (range 0–72) days, with eight participants discontinuing NPWT due to wound improvement. One participant recommenced NPWT following their initial cessation. Two participants (of 19, 10.5%) ceased NPWT therapy on the day they received it due to failure to maintain a seal. Twenty-one participants (of 40, 52.5%) were allocated to usual care, of whom more than half were initially treated with a hydrocolloid dressing (11/21, 52.4%), nine with a silver-containing (n = 3, 14.3%), iodine-containing (n = 3, 14.3%) or basic dressing (n = 3, 14.3%), and one with a soft polymer dressing (4.8%). Five usual-care participants (23.8%) switched to NPWT during the trial at a median of 4 (range 0–17) days after randomisation and subsequently remained on NPWT for a median of 31 (range 19–36) days. The most common reason for treatment crossover was wound deterioration (2/5, 40.0%).
Withdrawal and retention
During the trial, two participants (of 40, 5.0%) were fully withdrawn from the study due to amputation of the limb on which the reference wound was situated. The study protocol was revised following these withdrawals to enable continued data collection from participants after amputation, provided that they continued to give consent for this study. However, in the event, no further participants underwent amputation.
Participant questionnaire response rates were positive, achieving ≥ 77.5% at all time points, and 291 follow-up clinic visits were completed (NPWT, median 8; usual care, median 7) at intervals of between 4 and 33 days after randomisation (NPWT, median 7.5 days; usual care, median 9 days).
Blinded outcome assessment
In total, 308 photographs were taken as part of the study (38 at baseline, 270 at follow-up visits). Assessments of the final photographs for healing indicated that there was agreement between two reviewers for 29 (of 40, 72.5%) participants (NPWT, n = 15, 79.0%; usual care, n = 14, 66.7%), increasing to 38 participants (95.0%) following a third review. When healing status was recorded by one or more reviewers as ‘unsure’, this was due to poor photograph quality in 50.0% (4/8) of cases. For two participants (of 40, 5.0%), blinded outcome assessment of healing provided no consensus, with each reviewer recording a different healing status. The blinded assessors classed eight (of 40, 20.0%) wounds as healed (NPWT, n = 3, 15.8%; usual care, n = 5, 23.8%), of which seven (87.5%) were also recorded as healed by the unblinded investigators. Blinded assessors were unsure of healing status, or there was discordance between reviewers, for a further three wounds judged to have healed by the unblinded investigators.
The feasibility of blinding to treatment allocation was assessed using photographs taken at week 1, as the final photograph would not necessarily reflect the originally allocated treatment (given that NPWT is not necessarily used to the point of wound healing). In terms of blinding success, the assessors correctly identified treatment allocation for 15 participants (of 40, 37.5%): nine participants allocated to NPWT and six participants allocated to usual care. Allocation to NPWT was incorrectly identified in six cases (15.0%).
Documentation acceptability: nurses
Half of the eight research nurses who responded (n = 4, 50.0%) indicated that they found that the questionnaires asked all relevant questions and were straightforward to complete. There were mixed opinions on the ease of participant identification, with three nurses agreeing (agree or strongly agree), two nurses remaining impartial (neither agree nor disagree) and three strongly disagreeing. Half of the nurses (n = 4, 50%) indicated that trial processes were clear and the randomisation process was straightforward and just over half (n = 5, 62.5%) found that the assessments were straightforward to complete.
Qualitative interviews were subsequently conducted to ascertain further information on responses provided. Five face-to-face and three telephone interviews were conducted with research nurses across the three study sites, which identified key points of feedback on the trial procedures and processes:
-
Trial documentation was found to be acceptable, straightforward and in keeping with expectations based on nurses’ prior research experience. Amendments to clarify consistency in interpretation of eligibility criteria, processes for recording of wound depth and general minor amendments to study documentation were, however, suggested. The nurses acknowledged that the majority of issues encountered with the implementation of this pilot, feasibility RCT were not dissimilar to other new studies.
-
Nurses based at the secondary care sites highlighted issues with engagement of consultants, due to concerns that randomisation could interfere with their preferred wound management plan. Nurses suggested that direct principal investigator involvement enabled more patients to be successfully screened at the preoperative stage. Nurses based within the community settings noted that the majority of patients were identified in the acute settings. Randomisation was regarded as an easy process, although availability of a 24-hour randomisation service would have been preferred.
-
Given the geographical spread of participants at each study site, arranging visits to coincide with a patient’s clinic appointment was the most efficient approach, although this was not always viable and often time was wasted waiting for district, community or general practice nurses at home visits. The frequency of follow-up visits was noted as having an impact on time available to recruit and the value of continued follow-up beyond wound healing (up to three visits completed post healing) was queried. Access to equipment was generally better in the acute setting than in the community setting, although there were occasions when equipment needed to be borrowed by the trial team across both acute and community settings to facilitate patient treatment.
-
There was mixed feedback regarding nurses and consultants indirectly involved with the study. At one site, there was a perception that if NPWT was involved, some nurses would avoid a study due to a lack of confidence or training in its application and also the potential for additional work involved. At another site, it was reported that negative pressure is widely used and therefore there was a potential bias towards use of negative pressure. It was suggested that additional principal investigator involvement to promote the study, particularly during the set-up phase, would be helpful.
-
All research nurses received training in the application of NPWT but, as a result of randomisation, not all nurses had the opportunity to apply it. Generally, there was confidence in the use of NPWT; however, staff turnover and levels of junior staffing were noted to highlight gaps in training. Nurses reported that for patient safety, patient lifestyle, in particular mobility requirements, should be taken into account during recruitment.
Study acceptability: patients
Feedback from participants indicated that taking part in the study had matched expectations (30/31, 96.8%), and that completing questionnaires had been straightforward (28/31, 90.3%) and manageable (29/31, 93.6%).
Clinical outcomes
Healing
Participants were followed for a median of 84 days after randomisation (range 13 to 105 days) to assess wound healing. During the study, 10 wounds (of 40, 25.0%) were deemed to have healed based on assessment at weekly follow-up [six in the NPWT group (31.8%) and four in the usual-care group (19.1%)]. Time to healing was summarised using Kaplan–Meier curves (Figure 6). Adjusted Cox proportional hazards models indicated that wound size (p = 0.03) and duration of wound (p < 0.001) were predictors of time to wound healing, with smaller and longer duration of wounds associated with increased time to healing (see Appendix 13).
FIGURE 6.
Pilot feasibility RCT: Kaplan–Meier curve – time from randomisation to healing.
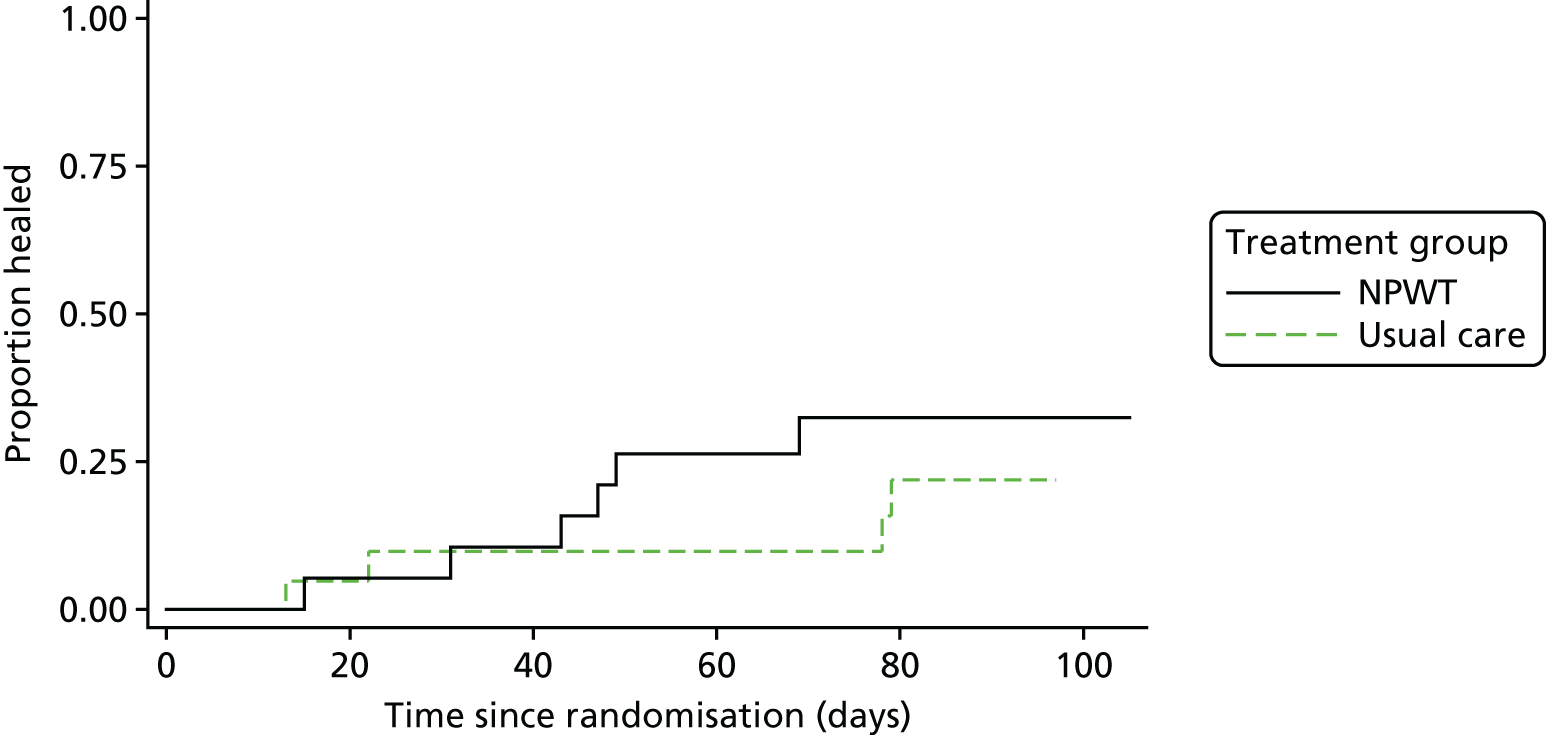
Infection
During the trial, 17 participants (NPWT, n = 6, 31.6%; usual care, n = 11, 52.4%) experienced an infection. Of these, nine experienced infection at baseline only (NPWT, n = 3, 15.8%; usual care, n = 6, 28.6%), four during follow-up only (NPWT, n = 1, 5.3%; usual care, n = 3, 14.3%) and four both at baseline and during follow-up (NPWT, n = 2, 10.5%; usual care, n = 2, 9.5%).
Hospitalisation
Participants in the NPWT group spent a median of 29 nights in hospital during the trial and participants in the usual-care group spent a median of 10 nights in hospital.
There were five reported hospitalisations during the trial, all of which were associated with serious adverse events (as Adverse events). In four instances (of five, 80.0%) the participants were subsequently discharged and in one instance (20.0%) the participant was withdrawn from the study (due to amputation) and subsequently died, and so further information regarding hospital discharge was not collected.
Patient-reported outcomes
Participant-completed questionnaires indicated that pain severity (BPI),23 interference and VAS wound pain reduced (improved outcome) in the NPWT group between baseline and month 1, but increased again (worse outcome) slightly at month 3 of follow-up. In the usual-care group, pain interference followed the same pattern as in the NPWT group, but VAS wound pain reduced consistently over time and BPI interference fluctuated between the follow-up time points. There was lower reported pain severity (improved outcome), interference scores (BPI)23 and wound pain (VAS) for usual-care participants at all time points relative to the NPWT group (Table 19). A total of 20 participants provided at least one weekly text message providing a pain score (NPWT, n = 10; usual care, n = 10). Mean scores reduced between week 1 and month 3 (week 1 4.0, SD 3.0; week 12 1.6, SD 2.0); however, there was some fluctuation observed in scores during this time (see Appendix 15).
| Treatment group | Total (N = 40) | |||||
|---|---|---|---|---|---|---|
| NPWT (N = 19) | Usual care (N = 21) | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| BPI severity | ||||||
| Baseline | 16 | 4.4 (2.8) | 20 | 2.7 (2.8) | 36 | 3.5 (2.9) |
| Week 2 | 15 | 3.7 (2.8) | 17 | 2.5 (2.5) | 32 | 3.0 (2.7) |
| Month 1 | 14 | 3.5 (3.4) | 15 | 1.9 (2.6) | 29 | 2.7 (3.1) |
| Month 3 | 14 | 4.3 (2.9) | 13 | 2.1 (2.6) | 27 | 3.2 (2.9) |
| BPI interference | ||||||
| Baseline | 15 | 5.0 (3.5) | 16 | 2.8 (3.6) | 31 | 3.8 (3.6) |
| Week 2 | 15 | 4.5 (3.5) | 17 | 3.3 (3.3) | 32 | 3.9 (3.4) |
| Month 1 | 14 | 4.2 (3.7) | 15 | 2.3 (2.8) | 29 | 3.2 (3.4) |
| Month 3 | 13 | 5.3 (2.8) | 15 | 2.7 (3.4) | 28 | 3.9 (3.4) |
| VAS wound pain | ||||||
| Baseline | 15 | 51.2 (32.1) | 18 | 34.8 (32.7) | 33 | 42.2 (33.0) |
| Week 2 | 13 | 32.4 (33.4) | 17 | 26.5 (29.3) | 30 | 29.1 (30.7) |
| Month 1 | 13 | 27.1 (31.1) | 14 | 20.7 (26.7) | 27 | 23.8 (28.5) |
| Month 3 | 12 | 30.8 (27.2) | 15 | 13.5 (23.1) | 27 | 21.1 (26.0) |
| VAS pain at dressing changesa | ||||||
| Week 2 | 13 | 22.3 (25.5) | 15 | 22.6 (24.5) | 28 | 22.5 (24.5) |
| Month 1 | 13 | 26.5 (32.8) | 14 | 16.4 (24.2) | 27 | 21.2 (28.5) |
| Month 3 | 11 | 18.1 (20.2) | 15 | 12.5 (27.0) | 26 | 14.8 (24.1) |
There was improvement (i.e. better health status) in the mean SF-12 PCS over time in both groups. MCSs reduced in the NPWT group over time, but improved initially in the usual-care group (between baseline and 1 month) before declining slightly at the month 3 follow-up visit. Further detail regarding these results is presented in Table 20 (see also Appendix 16, Figures 7 and 8).
| SF-12 | Treatment group | Total (N = 40) | |
|---|---|---|---|
| NPWT (N = 19) | Usual care (N = 21) | ||
| PCS | |||
| Baseline | |||
| n | 16 | 19 | 35 |
| Mean (SD) | 16, 28.9 (10.6) | 19, 34.9 (11.4) | 35, 32.1 (11.3) |
| Median (minimum, maximum) | 28.0 (12.2, 47.9) | 32.7 (15.5, 53.2) | 32.4 (12.2, 53.2) |
| Month 1 | |||
| n | 14 | 15 | 29 |
| Mean (SD) | 29.8 (10.1) | 38.8 (12.1) | 34.5 (11.9) |
| Median (minimum, maximum) | 28.0 (14.9, 48.9) | 39.8 (21.4, 55.1) | 33.2 (14.9, 55.1) |
| Month 3 | |||
| n | 14 | 14 | 28 |
| Mean (SD) | 30.4 (11.9) | 42.8 (8.3) | 36.6 (11.9) |
| Median (minimum, maximum) | 26.6 (17.4, 51.0) | 40.8 (30.5, 57.9) | 37.5 (17.4, 57.9) |
| MCS | |||
| Baseline | |||
| n | 16 | 19 | 35 |
| Mean (SD) | 44.7 (12.6) | 42.2 (11.2) | 43.3 (11.8) |
| Median (minimum, maximum) | 44.2 (19.0, 62.4) | 42.5 (18.8, 56.6) | 43.4 (18.8, 62.4) |
| Month 1 | |||
| n | 14 | 15 | 29 |
| Mean (SD) | 42.7 (13.8) | 48.6 (11.7) | 45.8 (12.9) |
| Median (minimum, maximum) | 40.8 (21.8, 61.7) | 46.8 (31.7, 67.5) | 45.0 (21.8, 67.5) |
| Month 3 | |||
| n | 14 | 14 | 28 |
| Mean (SD) | 41.5 (10.9) | 47.1 (12.1) | 44.3 (11.6) |
| Median (minimum, maximum) | 41.5 (24.8, 62.3) | 46.6 (29.8, 64.7) | 42.1 (24.8, 64.7) |
Adverse events
Adverse event data collection throughout the study identified 28 non-serious and five serious adverse events. Two serious adverse events were reported in the NPWT group from one participant and three in the usual care group from two participants. All five events were hospitalisations and only one (usual-care group) was classed as related to the reference wound. Three events (of five, 60.0%) were said to be possibly or probably related to treatment, all of which were recorded as expected. There were no suspected unexpected serious adverse reactions. The majority of non-serious adverse events related to the reference wound (25/28, 89.3%) and were reported as definitely related to treatment (19/28, 67.9%). The most common event type was ‘other’ (12/28, 42.9%), which included events such as skin irritation (3/12, 25.0%) and dressings falling off (3/12, 25.0%), followed by ‘pump failure’ (8/12, 28.6%).
Economic outcomes
Usual-care participants reported that they received a mean of 8.0 (SD 14.8) nurse visits at home and had a mean of 12.41 (SD 15.04) visits at the GP surgery for their SWHSIs. NPWT participants reported a limited number of nurse visits at the GP surgery (mean 1.14, SD 2.24), but additional nurse visits at home (mean 6.78, SD 9.82) and substantial mean inpatient hospital stays (mean 17.5, SD 19.8) and GP visits (mean 1.35, SD 2.70) (Table 21).
| Type of resource use | Treatment group | |||
|---|---|---|---|---|
| NPWT (N = 19) | Usual care (N = 21) | |||
| Mean (SD) | Missing, n (%) | Mean (SD) | Missing, n (%) | |
| Doctor: hospital outpatient clinic | ||||
| Wound related | 2.73 (3.23) | 5 (25.0) | 0.76 (1.16) | 8 (38.0) |
| Not wound related | 1.70 (2.00) | 10 (50.0) | 0.20 (0.63) | 11 (52.0) |
| Nurse: hospital outpatient clinic | ||||
| Wound related | 6.66 (10.35) | 5 (25.0) | 4.07 (10.07) | 8 (38.0) |
| Not wound related | 1.45 (2.39) | 9 (45.0) | 0 (0) | 11 (52.0) |
| Day-case visit to the hospital | ||||
| Wound related | 0.33 (0.88) | 8 (40.0) | 0.21 (0.80) | 7 (33.3) |
| Not wound related | 0.09 (0.30) | 9 (45.0) | 0 (0) | 9 (43.0) |
| Nights stayed as hospital inpatient | ||||
| Wound related | 17.5 (19.8) | 6 (30.0) | 0.90 (3.01) | 10 (48.0) |
| Not wound related | 14 (22.01) | 10 (50.0) | 0.54 (1.80) | 10 (48.0) |
| GP visit at general practice | ||||
| Wound related | 1.35 (2.70) | 6 (30.0) | 0.69 (1.31) | 8 (38.0) |
| Not wound related | 0.54 (1.03) | 9 (45.0) | 0.83 (1.19) | 9 (43.0) |
| GP visit at home | ||||
| Wound related | 0.28 (0.61) | 6 (30.0) | 0 (0) | 9 (43.0) |
| Not wound related | 0.15 (0.37) | 7 (35.0) | 0.08 (0.29) | 9 (43.0) |
| Nurse visit at general practice | ||||
| Wound related | 1.14 (2.24) | 6 (30.0) | 8.00 (14.8) | 9 (43.0) |
| Not wound related | 0.31 (0.75) | 7 (35.0) | 0.09 (0.30) | 10 (48.0) |
| Nurse visit at home | ||||
| Wound related | 6.78 (9.82) | 6 (30.0) | 12.41 (15.04) | 9 (43.0) |
| Not wound related | 1.91 (3.87) | 8 (40.0) | 0 (0) | 11 (52.0) |
Costs associated with NHS and health-care resource use were calculated on a per-contact basis, using NHS Reference Costs61 and Unit Costs of Health and Social Care62 (see Appendix 17). The associated NHS and health-care costs were higher for the NPWT group: the mean total cost for the NPWT intervention was £9490 (SD £7346), compared with £1153 (SD £1806) for patients receiving usual care (Table 22).
| Type of resource use | Treatment group, total mean cost (£) (SD) [n]a | Incremental cost (£) (NPWT – usual care) (95% CI)a | |
|---|---|---|---|
| NPWT | Usual care | ||
| Combined resource use | 9490.00 (7346.00) | 1153.00 (1806.00) | 8333.00 (3311.13 to 13,362.96) |
| Doctor: hospital outpatient clinic | 360.77 (427.59) [15] | 101.53 (153.86) [13] | 259.24 (1.63 to 516.84) |
| Nurse: hospital outpatient clinic | 472.46 (733.90) [15] | 288.93 (713.67) [13] | 183.53 (–380.89 to 747.96) |
| Day-case visit to the hospital | 240.26 (639.78) [12] | 154.45 (577.90) [14] | 85.80 (–407.07 to 578.69) |
| Nights stayed as hospital inpatient | 6284.77 (7128.17) [14] | 326.48 (1082.81) [11] | 5958.29 (1452.14 to 10,464.44) |
| GP visit at general practice | 59.71(119.07) [14] | 30.46 (57.88) [13] | 29.25 (–45.92 to 104.43) |
| GP visit at home | 25.40 (54.35) [14] | 0 (0) [13] | 25.40 (–5.68 to 56.49) |
| Nurse visit at general practice | 12.69 (24.97) [14] | 88.88 (165.46) [12] | –76.19 (–168.35 to 15.98) |
| Nurse visit at home | 158.31 (229.16) [14] | 289.68 (350.93) [12] | –131.37 (–367.93 to 105.19) |
Negative-pressure wound therapy participants reported a mean baseline EQ-5D-3L score of 0.34 (SD 0.10) and usual-care participants reported a mean baseline score of 0.54 (SD 0.08). At 3 months, following adjustment for baseline utility, mean scores had improved over time [NPWT participants 0.49 (SD 0.35) and usual-care participants 0.77 (SD 0.23)] (Table 23, see also Appendices 18 and 19).
| Time point | Treatment group | Mean difference (NPWT – usual care) (95% CI) | ||||
|---|---|---|---|---|---|---|
| NPWT (N = 19) | Usual care (N = 21) | Unadjusted | Adjusteda | |||
| n | Mean (SD) | n | Mean (SD) | |||
| Baseline | 16 | 0.34 (0.10) | 21 | 0.54 (0.08) | –0.20 (–0.46 to 0.05) | |
| 3 months | 15 | 0.49 (0.35) | 15 | 0.77 (0.23) | –0.28 (–0.50 to –0.06) | –0.05 (–0.31 to 0.20) |
The results show that NPWT had higher total NHS and health-care costs and also achieved lower QALY gains than usual care (complete-case analysis –0.007, 95% CI –0.04 to 0.02; multiple imputation analysis –0.004, 95% CI –0.03 to –0.02).
During the study, 1135 dressing changes were completed (NPWT, n = 518; usual care, n = 617). The average number of dressing changes completed was 27.26 for NPWT participants and 29.38 for usual-care participants. The majority of dressing changes were completed by district, community or general practice nurses (NPWT, n = 253; usual care, n = 272) or the participant’s clinic nurse (NPWT, n = 107; usual care, n = 167). The distribution of dressing changes by group corresponds to the distribution by group of nursing visits received or attended by participants: nursing visits at home or at the GP clinic were higher for usual-care participants than those allocated to NPWT.
Conclusions
This pilot, feasibility RCT provides invaluable information on the feasibility of conducting a large-scale trial of NPWT for treatment of SWHSIs and identifies areas of key consideration in the planning of future research.
Recruitment, participation and retention
The consistent rate of recruitment observed following the opening of all sites to recruitment, demonstrates that it is possible to recruit participants to a trial of NPWT compared with usual care. A full trial would, however, need to ensure that an adequate number of recruiting sites are identified. On the basis of the recruitment rate observed (two patients per site per month), to recruit 400 participants to a similar trial over an 18-month recruitment period, 11 study sites would need to be opened to recruitment. This example is based on hypothetical detail and would therefore be subject to revision following full sample size calculations for a larger trial. A larger trial should consider the impact of, and account for in associated sample size calculations, the rates of participants randomised to, but subsequently not receiving, NPWT (10.5% of participants in the pilot feasibility RCT) and rates of crossover to NPWT (23.8% of usual-care participants in the pilot feasibility RCT).
Qualitative interviews indicated that nurses perceived the majority of SWHSI patients to be identified within acute care settings, highlighting that the primary source of recruitment in a future RCT may be best achieved in acute care settings. This distribution may, however, differ between individual localities and so work would be required prior to recruitment to ensure that appropriate and effective recruitment strategies and pathways were implemented at individual sites.
Having previously received, or currently receiving, NPWT for their SWHSI was found to be the most frequent reason for patients’ ineligibility to take part in the study. Prior to undertaking a full RCT, it would be necessary to undertake some promotional work with clinical colleagues and research groups in order to fully engage surgeons and nurses within the study site, and to ensure consistent and continued referrals into the study. In addition, a future trial should recruit study sites where there is sufficient equipoise with regard to treatment of patients with SWHSIs, thus thorough evaluation of potential trial sites will be essential.
Work to engage clinical colleagues and research groups prior to recruitment commencement would also be crucial to allay resistance to trial recruitment from certain clinical groups. Anecdotal evidence from the study sites indicated some resistance to trial recruitment participation from colorectal surgeons, either because NPWT was primarily used for wound management rather than healing or because a surgeon’s prior experience of NPWT had led them to feel that this treatment was inappropriate for use in this patient group. Given the small number of sites involved in this pilot feasibility study, it is impossible to draw firm conclusions on the likely resistance among specific clinical groups, which may be observed in a larger RCT. Promotional work with clinicians across potential research sites in advance of study commencement should, however, help to minimise any resistance to recruitment in a larger RCT. Where necessary, promotional work may need to be targeted to ensure that clinicians from a cross-section of surgical disciplines are aware of, and open to, recruitment for a larger trial.
Participant-reported questionnaire response rates in excess of 75% and the limited number of withdrawals observed also demonstrate that it is possible to retain participants in a RCT of NPWT compared with usual care. During the study, only two (5.0%) participants were withdrawn from the trial due to amputation of the limb on which the reference wound was situated. Data collection did not continue for these participants. The trial team subsequently amended the study protocol to reflect that clinical data collection would cease at the point of amputation, but participant-reported data could continue to be collected, subject to continued consent. A future trial should ensure that the process for continued data collection following amputation is discussed and is clearly defined prior to study recruitment commencing.
Data collection methods
Assessment of wound measurements indicated that ruler measurements, both with and without adjustment, overestimate wound size, compared with wound tracings. Wound tracings should therefore be chosen over ruler measurement in any future RCT.
We have shown how wound pain data can be successfully collected on a weekly basis using text messaging; however, there were limited numbers of participants who responded to this method of data collection and rates of response varied considerably throughout the study. Exploration of participant characteristics in relation to text message respondents and non-respondents indicated that collection of data using this method may be most effective in younger populations; those who routinely responded were, on average, almost 10 years younger than those who did not respond routinely. The use of text messaging data collection in a larger study should therefore be considered carefully, before implementation, to ensure that use of this method will provide sufficient data for analysis.
Negative-pressure wound therapy supply
Negative-pressure wound therapy delivery was largely completed within 48 hours; however, three participants did not receive NPWT within this time frame. Furthermore, two participants did not receive NPWT at all, due to delays to discharge and lack of intervention availability. A future RCT would therefore need to establish the availability of NPWT devices with sites from the outset, to ensure timely intervention delivery.
Nurses involved in the study reported that they had received sufficient training within their individual institutions to confidently apply NPWT. It was, however, noted that nursing colleagues were not always confident in using NPWT, which corresponds with findings from workstream 3 of this research programme. A future trial would therefore need to invest sufficient resource to ensure that NPWT training was provided to all staff who may be providing trial treatment to a participant.
Blinded outcome assessment
This pilot feasibility trial explored the feasibility of blinded outcome assessment in the context of NPWT for SWHSI healing. Blinded and unblinded investigator assessment in relation to wound healing agreed 87.5% of the time and so blinded outcome assessment can be deemed to be feasible and appropriate within a future RCT. Blinded assessment of healing was, however, often hampered by the quality of the wound photograph. A robust photography protocol would therefore be required for a full RCT to facilitate consistency of imaging throughout the study and accurate blinded healing assessment.
It was not possible within this study to blindly assess time to healing due to difficulties in transferring the necessary numbers of photographs required to complete this activity. Dependent on the outcomes included in a larger study, this may warrant further exploration and a robust and tested process for transfer of photographs to blinded assessors for review should be established prior to the study commencing.
It is important to note that assessors correctly identified treatment allocation in approximately one-third (37.5%) of cases when reviewing photographs taken in the first week of the study. Therefore, assessment of time to healing in a larger study may become biased through identification of treatment allocation and so inclusion of this assessment should be carefully considered before implementation.
Documentation acceptability
Participant questionnaires were largely well received and so limited changes would be required for data collection in a larger RCT. The variation in opinions from research nurses involved in the study regarding study documentation and study processes, especially frequency and method of follow-up visits, indicates that further work should be conducted during the design of a larger trial to develop and test data collection processes to ensure fitness for purpose.
Clinical events
Given the limited number of participants involved in this study, there were insufficient data to enable any true treatment effect of NPWT to be identified; and this was not the purpose of this study. A full RCT is therefore required to fully assess the clinical effectiveness and cost-effectiveness of NPWT as a treatment for patients with SWHSIs.
The cohort study undertaken before this pilot, feasibility RCT (workstream 1) estimated that a follow-up duration of 90 days should be sufficient to observe wound healing. However, in this pilot feasibility study, only 25% of participants healed, which meant that a median time to healing could not be calculated. As a result, a 3-month follow-up may be inadequate to reliably investigate and compare healing rate; therefore, a longer follow-up should be considered for future trials. The difference in time to healing between this pilot feasibility study and our cohort study may be explained by the greater proportion of participants with foot and leg wounds in the pilot feasibility study (usually secondary to diabetes mellitus). The complexities associated with wound healing in patients with diabetes mellitus63 may explain these lower healing rates.
Regional arrangements for the supply of NPWT in community settings may have resulted in between-group differences in relation to nights spent as an inpatient. It is, however, worth noting that this may be due to specific regional arrangements present in the participating sites, rather than being an issue that would manifest in a larger national study. To ensure that inpatient stay is not distorted by regional arrangements, it is imperative to assess provisions for treatment delivery (e.g. local discharge arrangements and availability of NPWT within community settings) prior to commencing research activity at any study site. Associated with NPWT supply is the aforementioned requirement for training. Nurses noted that clinical colleagues were not always confident in using NPWT and so sufficient resource would be required to ensure full coverage of training across all those who may provide treatment to a participant.
This pilot feasibility study has clearly demonstrated the feasibility of completing a full RCT to provide definitive evidence in relation to NPWT effectiveness as a treatment for patients with SWHSIs. The pilot feasibility study has also identified key elements of, and recommendations for, recruitment, data collection, outcome measurement, trial design and safety reporting, which would need to be considered during the design of a larger RCT to investigate the clinical effectiveness and cost-effectiveness of NPWT compared with usual care.
Conclusions and recommendations
What do we now know about the SWHSI patient population?
This research programme emerged from clinical uncertainty about the best way of managing SWHSIs. To the best of our knowledge, there has been very little previous research on open surgical wounds, and what small amount there has been has focused only on dehisced surgical wounds;64,65 we have now shown that these constitute approximately half of all SWHSIs. We have studied both dehisced surgical wounds and those intentionally left open after surgery because they present similar challenges for clinical management. We planned this research at a time (2009) when the use of a costly intervention, NPWT, was increasing; however, access to it varied hugely, based on clinical setting, local policies and resources. Prior to this research, little was known about the frequency of SWHSIs, the characteristics of the patients who experience them and longer-term outcomes. Without basic information about the frequency and prognosis of a health condition and how patients are affected by it, it is impossible to plan research on treatments. 66 Initially, we undertook a cross-sectional survey of the prevalence of treated SWHSIs, which had the dual purpose of deriving the first population-based estimate of prevalence, as well as identifying where and when SWHSIs occurred, to aid the planning of an inception cohort. The cross-sectional survey (conducted in 2012) was anonymised, completed away from the patient and did not require consent in order to optimise data capture. To the best of our knowledge, this survey provides the first estimate of the prevalence of treated SWHSIs, which we estimate at 4.1 per 10,000 of the population (95% CI 3.5 to 4.7). 16 There are several things worth noting about this estimate: the prevalence is similar to best estimates of the UK prevalence of leg ulcers (4.4 per 10,000),27 almost half of SWHSIs (48%) were planned (before surgery) to be left open, the wounds had been present for a median of 28 days, the patients were mainly receiving care in primary and community care settings and 93% of patients were receiving wound dressings (6% had NPWT). Colorectal surgery was the most frequent type of surgery to precede the SWHSI, although plastic surgery and vascular surgery were also common.
This survey, although rather basic in design (lacking independent case ascertainment, for example, due to resourcing), provided crucial intelligence for planning the cohort study. Our median time from surgery to wound breakdown (in those whose wounds were not intentionally left open) of 9 days accords with previous studies of dehiscence. 64,67
Our inception cohort collected data from 393 patients with SWHSIs and, to the best of our knowledge, is the first detailed analysis of this patient population, their wound treatments and healing trajectories. A notable difference between this and the cross-sectional survey (apart from the longitudinal follow-up) was the requirement for informed consent, which may have influenced participation. In total, 57% of the cohort were men, the overall median age was 55 years, 70% of the cohort were overweight or obese (higher than the 2015 national average of 63%)68 and 29% were smokers or had quit in the last 12 months (compared with 19% of the adult population in Great Britain). 69 Common comorbidities were cardiovascular disease (in 38%), diabetes mellitus (in 26%) and peripheral vascular disease (in 14.5%). The wounds of 60% of the cohort participants were planned to be left open before surgery, higher than for the cross-sectional survey in which 48% were planned. This probably reflects the relative ease of recruitment of participants to the cohort, when it was known in advance that their wound would be open after surgery.
Our research illustrates, for the first time, the heterogeneous nature of the population of people with SWHSIs and of their wounds. Although it was anticipated that SWHSIs would stem from a range of surgical specialties, the distribution of SWHSI sites is noteworthy (34% abdominal, 15% feet, 15% leg) and anatomical site is very likely to influence the impact of the wound on quality of life (if it influences mobility or ability to drive, for example). The clinical specialty under whose auspices the preceding surgery took place was most commonly colorectal (40%), but also commonly vascular (21%). This information is vital to the planning of future research to ensure that the studies are carried out in the populations most affected. Moreover, these subpopulations of people with SWHSIs are likely to demonstrate very different healing trajectories from one another (an open wound on the foot in somebody with peripheral vascular disease and/or diabetes mellitus is likely to heal differently from a partially dehisced abdominal wound post appendectomy). Those conducting future trials in this area will need to make decisions about trading off ease of recruitment of a heterogeneous population (with consequent ‘noisy’ healing data) with recruitment from smaller, focused patient populations. This is illustrated by our pilot trial, to which we recruited 40 eligible participants, 60% of whom had a SWHSI on the foot (compared with 15% in the cohort study). The nature of those recruited to the pilot trial meant that the time to healing was much longer than for the cohort study and far fewer healing events were observed (only 25% healed), despite the 90-day follow-up period. The reasons for the excess of patients with foot wounds in the pilot trial are unclear, but it is likely that they were more readily recruited for some reason. It is possible that the current enthusiasm for NPWT as a treatment for SWHSIs meant that some health professionals were not in equipoise and were unwilling to randomise, thus making it difficult to recruit specific patient subgroups.
Future trials will therefore need to be carefully planned, with a desired population in mind and appropriate targeted recruitment. The cohort study time to healing data will be invaluable to the planning of new research, particularly in relation to appropriate follow-up time frames.
Exploratory analysis of predictors of healing within the cohort identified larger-area surgical wound contamination classification (as judged at the point of surgery)70 and infection at any time point as predictors of slower healing. The median time to healing of 86 (95% CI 75 to 103) days is very similar to that for venous leg ulcers (84 days in typical venous ulcer patients in a recent trial). 71 The typical healing trajectory for people with a SWHSI is not smooth, with episodes of infection and hospital readmission commonplace. The relatively young age of SWHSI patients compared with other patient groups with chronic wounds (e.g. leg and pressure ulcers) is particularly noteworthy. The wide-ranging impact on quality of life was detectable using the generic EQ-5D and SF-12 instruments, and was both physical and mental. 21,22 We heard directly from the patients themselves of the often devastating impact on their lives. Patients often felt isolated and confined to the home with disruption of working and caring responsibilities, causing feelings of dependency and burden. There were frequently financial impacts of the SWHSIs on the person and their family. To the best of our knowledge, the cost to the NHS of a SWHSI had not previously been estimated. We estimated that the mean cost of a SWHSI to health services was £1060 before the inclusion of treatment costs. Use of NPWT was estimated to cost, on average, an additional £1323 per month and use of dressings was estimated to cost, on average, an additional £441 per month.
What do we now know about treating SWHSIs?
Prior to this research, we had no information about treatments that were being used for SWHSIs. From our research we have learned that SWHSI treatments fall into three main categories: dressings, NPWT and further surgery. We observed a plethora of treatments being used for SWHSIs; however, hydrofibre dressings were the most commonly used (66% of patients received these at some time), alongside a high use of NPWT. Although the cross-sectional survey identified NPWT use in only 6% of patients, the cohort study indicated that a much larger proportion (29%) were treated with NPWT at some time.
Dressings are the cornerstone of treatment for complex wounds and there are many categories available, ranging from simple, basic wound contact dressings to ‘advanced’ dressings containing ingredients such as silver. 29 However, the evidence for the effectiveness of specific dressings and NPWT for SWHSIs is sparse and of low quality. 12 The most recent systematic review of the effects of dressings for open surgical wounds highlighted the paucity of evidence and a review of the effects of NPWT on open surgical wounds (search date June 2015) similarly concluded that there is currently no rigorous evidence available and therefore the benefits and harms are unclear. 14 There is an increasing amount of research regarding NPWT for the primary prevention of surgical site infections in closed surgical wounds; however, its effects in this context also remain unclear. 72,73 There is some evidence of potential benefit of NPWT for foot wounds in diabetes mellitus; however, this evidence is of low certainty due to its low quality. 31
We planned to construct a cost-effectiveness model for NPWT compared with dressings for open surgical wounds using a combination of published and cohort study data. We were unable to populate this model with published data as there were no data reporting time to healing as an outcome in RCTs of NPWT in this patient population. Using the cohort data alone to compare the effects of NPWT and dressings on time to healing effectiveness, and after using different approaches to adjust for confounding, we concluded that NPWT is less effective and more costly than wound dressings for SWHSIs. We had originally intended to conduct a value of information analysis to determine the potential impact of uncertainty on the decision regarding NPWT to treat SWHSIs. This analysis would assess the health and cost implications of the wrong decision being made and thus inform investments in future research to mitigate these uncertainties. However, our analyses showed that uncertainty around the decision not to use NPWT was very small. Even with the use of advanced adjustment techniques, such as those applied here, observational data may still affect the conclusions. Meta-analyses comparing the findings of RCTs and cohorts designed to answer the same clinical question give an OR of 1.04 (95% CI 0.89 to 1.21), highlighting the potential for agreement between the designs and the associated uncertainty. 74 The current economic evaluation uses all available evidence on the clinical effectiveness and cost-effectiveness of NPWT; thus, we argue that it should be seriously considered. However, the potential for unresolved confounding may limit confidence in the associated conclusions and therefore this may lead to justified calls for definitive RCT evidence to be obtained.
Interviews with patients and health professionals provided further insights into the use of, and views about, treatments for SWHSIs. The wide range of dressings used and inconsistency of use was noted by patients. Sharp, sometimes painful, debridement of SWHSIs was also reported by patients, despite the lack of high-quality evidence to support its use. 75 However, debridement was not measured explicitly as a treatment in the cohort study, so further data are needed on how widespread sharp debridement is as a treatment for SWHSIs. Finally, although our study participants generally viewed NPWT as an effective treatment, they reported various drawbacks, including pain during dressing changes. Some study participants reported feeling too embarrassed to go outside the home with NPWT in situ, due to the bulky appearance of the equipment and their fears that the exudate and odour might be detected by other people. Nurses’ varying levels of expertise in use of NPWT appeared to provoke anxiety in patients. These findings mirror those from other small qualitative interview studies with patients receiving NPWT. 76–78
What do we now know about living with a SWHSI?
The interviews with patients, together with the cohort HRQoL data, show the devastating effect that open surgical wounds can have on those affected. For those who did not expect to have an open wound after surgery, there were feelings of shock, fear and anxiety; these are feelings akin to those reported by people who have undergone life-changing trauma. 79 Significantly, the presence of an open wound resulted in the withdrawal of many people from normal employment, leisure and social activities. During interviews, participants indicated that their physical and mental resources had become depleted through the dominance of the wound in their lives.
We note the low baseline mean SF-12 PCSs and MCSs for SWHSI patients [mean PCS 33.1 (SD 10.17); mean MCS 42.2 (SD 12.35)]. These values, for people who have recently undergone surgery and who have an open wound, are lower than the mean PCS reported for an, on average, older venous leg ulcer population [mean PCS 38.4 (SD 11.2); mean MCS 48.6 (SD 12.0)],71 and are also lower than mean scores for other patient groups, such as those with coronary heart disease and arthritis. 80,81 However, the SWHSI cohort mean scores are similar to postoperative SF-12 data from 85 participants with mainly closed wounds [mean PCS 35.1 (SD 7.8); mean MCS 43.5 (SD 12.4) 30 days post surgery]. 82 Thus, it is difficult to separate the impact of surgery from the impact of an open wound on HRQoL. In the SWHSI cohort, we noted a significant improvement over time in both PCS and MCS, suggesting that the SF-12 is responsive to changes in HRQoL in this population. Improved quality of life was likely to be the result of recovery from surgery, changes in the underlying condition the surgery aimed to ameliorate or resolve and wound healing. The baseline EQ-5D index score was found to be, on average, 0.43 (SD 0.45). Modelling results indicated that wound healing was associated with a small (but statistically significant) increase in EQ-5D index score (mean 0.055, SE 0.02). This level of improvement is smaller than what has been detected for the healing of other wound types,71 but this may be due to different estimation methods. This value is also within the range of minimal clinically important differences evaluated across a wide range of conditions. 83,84
Although baseline HRQoL was relatively poor in this SWHSI cohort, pain severity and interference scores of 4 (SD 3) (measured using the BPI)23 were indicative of relatively mild pain when compared with, for example, the mean pain severity score of 7.0 (SD 1.8) and pain interference score of 7.6 (SD 2.0) reported in 440 patients with known chronic, non-malignant pain. 85 In addition, for the SWHSI cohort, mean pain scores reduced, on average, by only 1 point, to 3 points, by 15 months, follow-up. In interviews, some patients discussed pain and its impact, but it did not seem to be a key topic of concern and was not an obvious source for the overall poor HRQoL observed in those with a SWHSI.
Our findings, from exploring living with a SWHSI with patients, illuminate the significance of psychosocial effects, due to diminished sense of self, inability to fulfil social roles and obligations, and curtailment of normal social activities, especially when patients’ hopes for healing are not met. Our study also highlights the impact of financial pressures on younger patients whose wound rendered them unfit for work, and their concerns about consequences for their family and implications for employment in the longer term.
From a patient perspective, the aspiration for complete wound healing was a major focus of all those interviewed. Healing was largely linked to return to some semblance of life prior to surgery, and was considered a sign that the body was returning to ‘normality’. This focus on healing as a key outcome agrees with previous findings from interviews with 33 patients with a range of complex wounds, including leg ulcers, foot ulcers and surgical wounds. 27 Here, most people, again, said that being healed or ‘cured’ was their main goal, as did eight participants with complex wounds who, when responding to the question ‘what did you most want from treatment?’, reported that they most wanted the wound to heal. 46
Health professionals’ management of patient expectations is crucial to the patient experience. We know from our cohort study that, although the median time to healing was around 3 months, some people would live with a SWHSI for significantly longer than this. We heard how comments from health-care professionals regarding healing and the condition of their wound had profound impacts on patients. Positive feedback was a boost for morale and sustained the patient’s hope for healing, whereas negative remarks adversely affected the patient’s general outlook. Conflicting information led to feelings of confusion and consternation in patients. Even quite casual remarks from health-care professionals could have a profound impact, which has been noted in other recent research on patients with complex wounds. 86 Patients’ expectations for wound healing may often appear unrealistic, yet clinicians need to manage these expectations within a framework that allows patients some measure of hope for healing, while avoiding the arousal of ‘false hope’, which may result in feelings of disappointment, disillusionment and distrust, which undermine professional–patient relationships. 46 Open, non-healing wounds that endure, such as SWHSIs, are associated with the ‘forever healing’ process, characterised by a constant need for treatment and care and a diminished quality of life, as described in patients with venous, diabetic and pressure ulceration. 47–51,53,87 This is challenging for clinicians, who must communicate uncertainty regarding wound progress and likely treatment outcomes without raising false hope or inducing a ‘spiral of hopelessness’ in patients. 51
Given the severity of impact that these wounds have on patients’ well-being, it is not clear whether or not psychological interventions may be useful in helping patients with hard-to-heal wounds to accept and adapt to their non-healing wound. Our findings also point to the need for improvements in communication with health-care professionals concerning the slow healing processes associated with SWHSIs; further research is required to investigate how, when and by whom information should be conveyed to patients with these types of wounds. Study (nurse) participants also highlighted the need for patients to receive written, as well as verbal, information, including information about signs and symptoms of infection and what action to take should these occur.
Strengths of the research
A key strength of this research is the new information that has been captured and reported on this SWHSI patient population. Prior to this research, we did not know the prevalence of SWHSIs, the surgeries involved, how many SWHSIs were planned compared with how many formed spontaneously, how costly these wounds are to the health service, how best to treat these wounds and how best we might direct future research on SWHSIs. There was also an absence of rigorous data regarding SWHSI management in the UK, how long they took to heal and what the impact of these wounds is on patients and their families. This research has provided this information for one geographical area of the UK (Yorkshire and the Humber).
We present data and conclusions that address many of the uncertainties that were faced at the start of the work, which we aimed to address using the methodologies described here. This work should provide a solid foundation for the design and planning of further research in the field.
We have employed robust epidemiological, analytical and qualitative methodologies, as required. By conducting an inception cohort study (as opposed to a prevalence cohort study), we were able to obtain accurate estimates of time to event data on outcomes for this SWHSI patient group; we also obtained a full picture of treatments received over the natural history of the wound. Although a prevalence cohort would have allowed higher recruitment figures, it would have provided far less clarity on crucial outcomes, such as time to healing. We were unable to rely on retrospective extraction of routinely collected data on wound treatments and outcomes, as systematically collected data, especially in the community, are limited. Thus, obtaining robust data, such as we have, requires data collection to be embedded in an appropriately designed primary study from the inception of the wound.
Limitations of the research
Our cohort study was conducted in only two centres in the UK and we therefore acknowledge that practice may vary across different centres and that the prevalence rates observed may not reflect those observed across the UK.
We have noted previously that the generalisability of findings of the cohort study may have been reduced by the need for consent, which removes some of the patient group from the study group. Our cross-sectional survey findings reassured us, however, as there were no major differences in key demographic features, although more wounds in cohort participants were planned to be left open before surgery. Those patients treated with NPWT in the cohort study were found to differ from those who were not, therefore indicating potential treatment selection. Interviews with clinicians helped to explore how NPWT may be allocated; however, this did not provide a definitive explanation for treatment decisions.
The nature of the cross-sectional survey may have resulted in some cases of SWHSIs not being reported, resulting in an underestimation of treated SWHSI prevalence. The number of responses received was as we expected, and so we are confident that the number of missed wounds was low and not systematic, probably due to publicity of the survey and support provided for nurses to complete study activity.
Within the cohort study, 73 wounds (18.6%) did not heal by the end of the study follow-up period. Time to healing was used as the primary outcome, given that this is an important and recommended outcome in wounds research. However, when using this outcome, it is acknowledged that there will inevitably be some participants who do not heal by the end of the follow-up time point. We acknowledge that the lack of healing data for these participants may have had an impact on the robustness of the findings; however, we expect this to be minimal, given the small number of participants this affected.
To meet the objective of our research programme, we opted to take a broad approach and collected data on a wide range of SWHSIs. This means that we have captured data on the heterogeneity of the wound type, but this also results in more limited data with which to investigate subgroups of SWHSIs in more detail, and this may be the focus of future work.
We present findings on the relative treatment effects of NPWT using observational data, given that there was a lack of RCT data available on relative effects of NPWT on SWHSIs. It is widely acknowledged that experimental data with groups formed by randomisation provide the least biased estimates of treatment effects. We implemented use of advanced adjustment approaches to try and reduce bias as much as possible, for both known (e.g. factors identified as prognostic for wound healing such as wound size, previous wound history or level of tissue involvement) and unknown confounders. However, even with adjustment, there is still the potential for unresolved confounding to affect the results and reduces confidence in them, leading to calls for definitive evidence from a RCT.
The relative treatment effects of NPWT were evaluated only on the healing of SWHSIs and so could not account for other direct effects on HRQoL (e.g. wound management). Again, these limitations may benefit from further assessment in a RCT.
The qualitative interviews conducted as part of this research programme identified a diverse range of views from both patients and clinicians. It is, however, noted that only a limited number of interviews were conducted with both clinicians (12 clinicians comprising seven nurses and five surgeons) and patients, which may limit the conclusions drawn. Interviews with both patients and clinicians continued until data saturation was achieved, thus ensuring comprehensive data. Further qualitative research may, however, be warranted to build on these findings.
Implications for practice
Findings from this research indicate that SWHSIs are relatively common, at least within the Yorkshire and the Humber region, UK (4.1 per 10,000 population), and such wounds frequently arise following colorectal, plastic or vascular surgery.
Data suggest that wounds will demonstrate different healing trajectories. Wounds with a large surface area, contaminated wounds (at the point of surgery) and infection at any time were found to predict slow healing. Furthermore, wound location and patient comorbidity may have an impact on wound healing trajectory.
This research has identified the vast range of treatments in use for SWHSIs and has identified increasing use of NPWT as a treatment during the wound healing pathway (rising from 6% to 29% over the course of approximately 3 years). Despite increasing use of this treatment within the NHS, RCT evidence supporting the use of this treatment for SWHSIs is sparse and of low quality. Our results indicate that NPWT has a negative impact on healing and is not cost-effective. However, as our results are based solely on observational data and, even after thorough adjustment, there may be unresolved confounding, the potential for which may limit confidence in the research findings.
Implications for research
This research signals the importance of, scope of, feasibility of and opportunity for further research on SWHSIs. As is the case across the wound care field, there are few good data on the prognostic factors for healing and on which factors may be amenable to treatment to promote better outcomes. NPWT, as with other medical devices in wound care, has been adopted widely, with relatively little evidence to support its use. If decision-makers and funders call for definitive evidence derived from a RCT, the design of such a RCT would need to be carefully considered, based on data presented here. The overall population of those with SWHSIs is too heterogeneous for inclusion into a single trial and we would suggest that future research should focus on specific groups. Although people with open surgical wounds after colorectal surgery formed our largest subpopulation, careful engagement with colorectal surgeons and patients would be required for a full trial in this patient population given the difficulties and barriers outlined in the pilot trial.
Careful consideration also needs to be given to the outcomes measured in a full trial. We focused in this research on healing, and maintain, based on the findings of patient interviews, that this is a key outcome for future research. However, it is important to note that if NPWT is not cost-effective for healing, it may still offer worthwhile advantages for patients and health services, which need to be balanced against disbenefits and costs. Impacts of NPWT on outcomes such as infection and reoperation should also be considered, as should patients’ view of the treatment. The type of patient group recruited and the outcomes of interest will all influence the duration of follow-up of any planned study.
In terms of how NPWT is evaluated, investigators must carefully consider the level of pragmatism that will be employed in any future trial. 88 There is a range of NPWT machines available for use, including those that use gauze dressings, foam dressings, single-use machines and repeat-use devices. There is no good evidence of any differences in the clinical effectiveness or cost-effectiveness of different machines, thus the trial design choice of whether to mandate use of a specific machine or allow sites to use their current NPWT provision will be down to the investigator. Although use of a single device will reduce the ‘noise’ in an evaluation, it is likely to limit the appeal of involvement to potential sites, as well as the potential generalisability of data. Thus, a pragmatic approach will arguably reflect effectiveness findings as they might be seen in the NHS and also make a large study more feasible. The same is true in terms of NPWT usage protocols (e.g. levels of pressure and cycle length), as well as how NPWT is used as part of the treatment pathway. The more prescriptive a future study is, the less pragmatic and feasible it may become but there would be less ‘noise’ in the findings.
Similar intervention issues also need to be considered in terms of the comparator in any future study. We know that SWHSI patients receive multiple dressings and there is no high-quality evidence that one dressing type is better than another for SWHSIs or any other complex wound type. 89 Use of a specific dressing type, such as hydrofibre, could be explored in a trial, but large deviations from use of a single dressing over the duration of a patient’s SWHSI would be expected.
Blinded outcome assessment is crucial for studies with subjective outcomes, such as healing and infection. 90 We have demonstrated here that blinded outcome assessment in a NPWT trial in people with SWHSIs is possible, but protocols and equipment that ensure high-quality images are required. We also note the importance of taking photographs beyond the point at which the unblinded investigator considers a wound healed, so that a later healing date can be recorded by a blinded assessor should they disagree with the unblinded assessment.
Careful consideration should be given to use of data collection via text message in future trials investigating treatments for SWHSIs. Although this method has been identified as a simple, inexpensive, valid and acceptable method of data collection in previous studies,91–93 our pilot, feasibility RCT suggested that data collection via text messaging was more successful in younger patients (respondents were, on average, almost 10 years younger than non-respondents). Other studies, which have successfully used text messaging for data collection have also included younger populations ranging from 22.0 (SD 1.47) years93 to 44.0 (SD 13.4) years of age. 91 Future trials of SWHSI treatments should therefore consider the demographics of the target patient population prior to inclusion of such methods, to ensure that if utilised, data collection is likely to be successful and so provide sufficient data for analysis.
Questions for future research
We consider key research questions raised by this programme of research to be:
-
Which treatments are clinically effective and cost-effective for SWHSIs for all patients or for particular patient subgroups?
-
Can particular prognostic factors predict time to healing of SWHSIs?
-
Do psychosocial interventions have the potential to improve quality of life in people with hard-to-heal SWHSIs?
-
What are the current care pathways for people with SWHSIs, how do they vary nationally and can they be optimised to improve efficiencies and outcomes?
-
Is the development of evidence-based practice guidelines for the management of SWHSI patients possible, in order to benefit and inform patients, clinicians and NHS commissioning?
Acknowledgements
This research would not have been possible without the participating patients, their relatives and clinical staff. We are grateful to all of the patients and relatives who took part in this research programme and to the nurses and clinicians in Leeds, Hull, Humber and the East Riding of Yorkshire for their help in recruiting patients to the various components of this research programme.
We are particularly grateful to all of the patient and public involvement representatives involved in this research programme, but specifically Stephen Dixon, Barbara Piggot and Pauline Stabler, who have provided indispensable input across the programme of work.
The contribution of Claire Acey to the co-ordination of all elements of this programme is also gratefully acknowledged.
Finally, we would like to thank the members of the Data Monitoring and Ethics Committee for workstream 4: Peter Vowden, Robert Hinchliffe, Sarah Brown and Christine Gratus.
Contributions of authors
Ian Chetter (Professor, Vascular Surgery) was the chief investigator; led the research programme and chaired the Programme Management Group; and led workstreams 1 and 4.
Catherine Arundel (Research Fellow, Health Research and Trials) contributed to the design, conduct, analysis, interpretation and reporting of workstream 4.
Kerry Bell (Statistician, Health Research and Trials) undertook the statistical analysis and interpretation for workstream 1.
Hannah Buckley (Statistician, Health Research and Trials) undertook the statistical analysis and interpretation and advised on conduct for workstream 1, and advised on the design, conduct and statistical analysis for workstream 4.
Karl Claxton (Professor, Economics) oversaw all aspects of workstream 2 (design, conduct, analysis, interpretation and reporting).
Belen Corbacho Martin (Health Economist, Health Research and Trials) undertook the economic analysis and interpretation for workstream 4.
Nicky Cullum (Professor, Nursing) contributed to the conception, design, conduct, analysis and reporting of all workstreams and provided input to the programme management and strategy.
Jo Dumville (Senior Lecturer, Health Sciences) contributed to the design, conduct, analysis and reporting of all workstreams.
Caroline Fairhurst (Statistician, Health Research and Trials) undertook the statistical analysis for workstream 4.
Eileen Henderson (Assistant to Medical Director, NHS Needs) ensured that all workstreams met NHS needs and were relevant to NHS decision uncertainty.
Karen Lamb (Research Co-ordinator, Wound Research) contributed to the design and conduct of workstream 1 and the design, conduct, analysis and reporting of workstream 4.
Judith Long (Project Manager, Vascular Surgery Research) led and undertook qualitative data collection, analysis and reporting for workstream 4.
Dorothy McCaughan (Research Fellow, Health Research) led and undertook qualitative data collection, analysis and reporting for workstream 3.
Elizabeth McGinnis (Research Fellow, Wound Research) contributed to the design, conduct, analysis and reporting of workstreams 1 and 4.
Angela Oswald (Tissue Viability Matron, Wound Care and Research) provided clinical leadership and expertise in relation to NPWT.
Pedro Saramago Goncalves (Research Fellow, Health Economics) undertook the analyses in workstream 2 and contributed to the design, conduct, interpretation and reporting of workstream 2.
Laura Sheard (Senior Research Fellow, Health Research) undertook qualitative data collection and analysis for workstream 3.
Marta O Soares (Senior Research Fellow, Health Economics) supervised all aspects of workstream 2 (design, conduct, analysis, interpretation and reporting) and contributed to the design, conduct, analysis and reporting of workstreams 1 and 4.
Nikki Stubbs (Clinical Pathway Lead, Wound Research) contributed to the design, conduct, analysis and reporting of workstreams 1 and 4.
David Torgerson (Professor, Health Research and Trials) contributed to the management of the programme of work and the design, conduct, analysis, interpretation and reporting of workstreams 1 and 4.
Nicky Welton (Reader, Statistics and Health Economic Modelling) oversaw the design, interpretation and reporting of the analyses in workstream 2.
Publications
Workstream 1
Chetter C, Oswald A, Fletcher M, Dumville J, Cullum N. A survey of patients with surgical wounds healing by secondary intention; an assessment of prevalence, aetiology, duration and management. J Tissue Viability 2017;26:103–7.
Chetter I, Oswald AV, McGinnis E, Stubbs N, Arundel C, Buckley H, et al. Patients with surgical wounds healing by secondary intention: a prospective, cohort study. Int J Nurs Stud 2019;89:62–71.
Workstream 2
Saramago P, Claxton K, Welton NJ, Soares M. Bayesian econometric modelling of observational data for cost-effectiveness analysis: establishing the value of negative pressure wound therapy in the healing of open surgical wounds. J R Statist Soc A 2020; in press.
Workstream 3
McCaughan D, Sheard L, Dumville J, Cullum N, Chetter I. Patient’s perspectives and experiences of living with a surgical wound healing by secondary intention: an in-depth qualitative study. Int J Nurs Stud 2018;77:29–38.
McCaughan D, Sheard L, Dumville J, Cullum N. Nurses’ and surgeons’ views and experiences of surgical wounds healing by secondary intention: a qualitative study. J Clin Nurs 2020;29:2557–71.
Workstream 4
Arundel C, Buckley H, Clarke E, Cullum N, Dixon S, Dumville J, et al. Negative pressure wound therapy versus usual care for surgical wounds healing by secondary intention (SWHSI trial): study protocol for a randomised controlled pilot trial. Trials 2016;17:535.
Arundel C, Fairhurst C, Corbacho-Martin B, Buckley H, Clarke E, Cullum N, et al. Pilot, feasibility randomized clinical trial of negative pressure wound therapy versus usual care in patients with surgical wounds healing by secondary intention. BJS Open 2018;2:99–111.
Long J, Meethan K, Arundel C, Clarke E, Firth A, Sylvester M, Chetter I. Exploring feedback from research nurses in relation to the design and conduct of a randomised controlled trial of wound care treatments: a sequential, dependent, mixed-methods study. J Tissue Viability 2020; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- NHS Confederation . Key Statistics on the NHS 2016 n.d. www.nhsconfed.org/resources/key-statistics-on-the-nhs (accessed 11 June 2016).
- Norman G, Dumville JC, Mohapatra DP, Owens GL, Crosbie EJ. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD011712.pub2.
- Vowden KR, Vowden P. A survey of wound care provision within one English health care district. J Tissue Viability 2009;18:2-6. https://doi.org/10.1016/j.jtv.2008.11.003.
- Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north-east England. J Wound Care 2007;16:413-16. https://doi.org/10.12968/jowc.2007.16.10.27910.
- Macfie J, McMahon MJ. The management of the open perineal wound using a foam elastomer dressing: a prospective clinical trial. Br J Surg 1980;67:85-9. https://doi.org/10.1002/bjs.1800670205.
- Williams RH, Wood RA, Mason MC, Edwards M, Goodall P. Multicentre prospective trial of Silastic foam dressing in management of open granulating wounds. Br Med J 1981;282:21-2. https://doi.org/10.1136/bmj.282.6257.21.
- Walker AJ, Shouler PJ, Leicester RJ. Comparison between Eusol and Silastic foam dressing in the postoperative management of pilonidal sinus. J R Coll Surg Edinb 1991;36:105-6.
- Eldrup J. Silastic foam dressing compared with mèche-treatment in open management following excision of a pilonidal cyst. Ugeskr Laeg 1985;147:408-9.
- Schmidt JM, Greenspoon JS. Aloe vera dermal wound gel is associated with a delay in wound healing. Obstet Gynecol 1991;78:115-17.
- Vigier S, Casillas JM, Dulieu V, Rouhier-Marcer I, D’Athis P, Didier JP. Healing of open stump wounds after vascular below-knee amputation: plaster cast socket with silicone sleeve versus elastic compression. Arch Phys Med Rehabil 1999;80:1327-30. https://doi.org/10.1016/S0003-9993(99)90038-2.
- Agren MS, Ostenfeld U, Kallehave F, Gong Y, Raffn K, Crawford ME, et al. A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair Regen 2006;14:526-35. https://doi.org/10.1111/j.1743-6109.2006.00159.x.
- Vermeulen H, Ubbink DT, Goossens A, de Vos R, Legemate DA. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg 2005;92:665-72. https://doi.org/10.1002/bjs.5055.
- National Institute for Health and Care Excellence (NICE) . Surgical Site Infections: Prevention and Treatment. Clinical Guideline: CG74 2008.
- Dumville JC, Owens GL, Crosbie EJ, Peinemann F, Liu Z. Negative pressure wound therapy for treating surgical wounds healing by secondary intention. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD011278.pub2.
- Sussman C, Bates-Jensen BM. Wound Care: A Collaborative Practice Manual for Health Professionals. Philadelphia, PA: Lippincott Williams and Wilkins; 2011.
- Chetter IC, Oswald AV, Fletcher M, Dumville JC, Cullum NA. A survey of patients with surgical wounds healing by secondary intention; an assessment of prevalence, aetiology, duration and management. J Tissue Viability 2017;26:103-7. https://doi.org/10.1016/j.jtv.2016.12.004.
- Chetter I, Oswald AV, McGinnis E, Stubbs N, Arundel C, Buckley H, et al. Patients with surgical wounds healing by secondary intention: a prospective, cohort study. Int J Nurs Stud 2019;89:62-71. https://doi.org/10.1016/j.ijnurstu.2018.09.011.
- National Research Ethics Service . Does My Project Require Review by a Research Ethics Committee? 2011.
- Office for National Statistics . UK 2011 Census: Usual Resident Population by Five Year Age Group, Local Authorities in the United Kingdom 2011.
- Public Health England . Protocol for the Surveillance of Surgical Site Infection 2013.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Brooks R, Rabin R, De Charro F. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2003.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 1994;23:129-38.
- Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart 2006;92:62-7. https://doi.org/10.1136/hrt.2004.052787.
- Mackeen AD, Berghella V, Larsen ML. Techniques and materials for skin closure in caesarean section. Cochrane Database Syst Rev 2012;11. https://doi.org/10.1002/14651858.CD003577.pub3.
- Banwell PE, Musgrave M. Topical negative pressure therapy: mechanisms and indications. Int Wound J 2004;1:95-106. https://doi.org/10.1111/j.1742-4801.2004.00031.x.
- Cullum N, Buckley H, Dumville JC, Hall J, Lamb K, Madden M, et al. Wounds research for patient benefit: a 5-year programme of research. Programme Grants Appl Res 2016;4. https://doi.org/10.3310/pgfar04130.
- Saramago P, Claxton K, Welton NJ, Soares M. Bayesian econometric modelling of observational data for cost-effectiveness analysis: establishing the value of negative pressure wound therapy in the healing of open surgical wounds. J R Statist Soc A 2020. https://doi.org/10.1111/rssa.12596.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Lunn D. The BUGS Book: A Practical Introduction to Bayesian Analysis. Boca Raton, FL: CRC Press Taylor and Francis Group; 2013.
- Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD010318.pub2.
- Spiegelhalter DJ, Best NG, Carlin BP, Van der Linde A. The deviance information criterion: 12 years on. J R Stat Soc Series B Stat Methodol 2014;64:583-616. https://doi.org/10.1111/1467-9868.00353.
- Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg 2008;195:782-8. https://doi.org/10.1016/j.amjsurg.2007.06.023.
- Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631-6. https://doi.org/10.2337/dc07-2196.
- Monsen C, Wann-Hansson C, Wictorsson C, Acosta S. Vacuum-assisted wound closure versus alginate for the treatment of deep perivascular wound infections in the groin after vascular surgery. J Vasc Surg 2014;59:145-51. https://doi.org/10.1016/j.jvs.2013.06.073.
- Biter LU, Beck GM, Mannaerts GH, Stok MM, van der Ham AC, Grotenhuis BA. The use of negative-pressure wound therapy in pilonidal sinus disease: a randomized controlled trial comparing negative-pressure wound therapy versus standard open wound care after surgical excision. Dis Colon Rectum 2014;57:1406-11. https://doi.org/10.1097/DCR.0000000000000240.
- Medical Advisory Secretariat . Negative pressure wound therapy: an evidence-based analysis. Ont Health Technol Assess Ser 2006;6:1-38.
- Fenwick E, Claxton K, Sculpher M. The value of implementation and the value of information: combined and uneven development. Med Decis Making 2008;28:21-32. https://doi.org/10.1177/0272989X07308751.
- Office for National Statistics . Annual Mid Year Population Estimates: 2014 2015.
- McCaughan D, Sheard L, Cullum N, Dumville J, Chetter I. Patient’s perceptions and experiences of living with a surgical wound healing by secondary intention: a qualitative study. Int J Nurs Stud 2018;77:29-38. https://doi.org/10.1016/j.ijnurstu.2017.09.015.
- Green J, Thorogood N. Qualitative Methods for Health Research. London: Sage; 2014.
- Baker SE, Edwards R. How Many Qualitative Interviews Is Enough 2012. http://eprints.ncrm.ac.uk/2273/ (accessed 7 November 2016).
- Ritchie J, Spencer L, Bryman A. Analyzing Qualitative Data. London: Routledge; 1994.
- McCaughan D, Sheard L, Dumville J, Cullum N. Nurses’ and surgeons’ views and experiences of surgical wounds healing by secondary intention: a qualitative study. J Clin Nurs 2020;29:2557-71. https://doi.org/10.1111/jocn.15279.
- Watret L. World Wide Wounds – Teaching Wound Management: A Collaborative Model for Future Education 2005. www.worldwidewounds.com/2005/november/Watret/Teaching-Wound-Mgt-Collaborative-Model.html (accessed 7 November 2016).
- Chase SK, Melloni M, Savage A. A forever healing: the lived experience of venous ulcer disease. J Vasc Nurs 1997;15:73-8. https://doi.org/10.1016/S1062-0303(97)90004-2.
- Moffat C, Vowden P, Calne S. European Wound Management Association Position Document. London, UK: Medical Education Partnership; 2008.
- Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10-7. https://doi.org/10.1111/j.1742-481x.2004.00007.x.
- Kinmond K, McGee P, Gough S, Ashford R. ‘Loss of self’: a psychosocial study of the quality of life of adults with diabetic foot ulceration. J Tissue Viability 2003;13:6-8. https://doi.org/10.1016/S0965-206X(03)80025-6.
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 2009;57:1175-83. https://doi.org/10.1111/j.1532-5415.2009.02307.x.
- Briggs M, Flemming K. Living with leg ulceration: a synthesis of qualitative research. J Adv Nurs 2007;59:319-28. https://doi.org/10.1111/j.1365-2648.2007.04348.x.
- Persoon A, Heinen MM, Van der Vleuten CJ, De Rooij MJ, Van der Kerkhof PC, Van Achterberg T. Leg ulcers: a review of their impact on daily life: financial, social and psychologic implications. J Am Acad Dematol 1994;31:49-53. https://doi.org/10.1016/S0190-9622(94)70134-2.
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-44.
- Ashby RL, Dumville JC, Soares MO, McGinnis E, Stubbs N, Torgerson DJ, et al. A pilot randomised controlled trial of negative pressure wound therapy to treat grade III/IV pressure ulcers [ISRCTN69032034]. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-119.
- ClinicalTrials.gov . Negative Pressure Wound Therapy for the Treatment of Chronic Pressure Wounds n.d. http://clinicaltrials.gov/ct2/show/NCT00691821 (accessed 1 December 2016).
- Arundel C, Buckley H, Clarke E, Cullum N, Dixon S, Dumville J, et al. Negative pressure wound therapy versus usual care for surgical wounds healing by secondary intention (SWHSI trial): study protocol for a randomised controlled pilot trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1661-1.
- Arundel C, Fairhurst C, Corbacho-Martin B, Buckley H, Clarke E, Cullum N, et al. Pilot, feasibility randomized clinical trial of negative pressure wound therapy versus usual care in patients with surgical wounds healing by secondary intention. BJS Open 2018;2:99-111. https://doi.org/10.1002/bjs5.49.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Taylor RJ. ‘Mouseyes’: an aid to wound measurement using a computer. J Wound Care 1997;6:123-6. https://doi.org/10.12968/jowc.1997.6.3.123.
- Kundin JI. A new way to size up a wound. Am J Nurs 1989;89:206-7. https://doi.org/10.1097/00000446-198902000-00020.
- Department of Health and Social Care . NHS Reference Costs 2014 15 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 7 November 16).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2014 2015. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2014/ (accessed 7 November 2016).
- Armstrong DG, Lavery LA. Diabetic Foot Study Consortium . Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704-10. https://doi.org/10.1016/S0140-6736(05)67695-7.
- van Ramshorst GH, Nieuwenhuizen J, Hop WC, Arends P, Boom J, Jeekel J, et al. Abdominal wound dehiscence in adults: development and validation of a risk model. World J Surg 2010;34:20-7. https://doi.org/10.1007/s00268-009-0277-y.
- Sandy-Hodgetts K, Carville K, Leslie GD. Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J 2015;12:265-75. https://doi.org/10.1111/iwj.12088.
- Patient-Centered Outcomes Research Institute Methodology Committee . The PCORI Methodology Report 2013. www.pcori.org/sites/default/files/PCORI-Methodology-Report-November2013.pdf (accessed 7 August 2019).
- Spiliotis J, Tsiveriotis K, Datsis AD, Vaxevanidou A, Zacharis G, Giafis K, et al. Wound dehiscence: is still a problem in the 21th century: a retrospective study. World J Emerg Surg 2009;4. https://doi.org/10.1186/1749-7922-4-12.
- Public Health England . About Adult Obesity: UK and Ireland Prevalence and Trends 2017.
- Office for National Statistics . Adult Smoking Habits in Great Britain: 2014 2014.
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250-78. https://doi.org/10.1086/501620.
- Ashby RL, Gabe R, Ali S, Saramago P, Chuang LH, Adderley U, et al. VenUS IV (Venous leg Ulcer Study IV) – compression hosiery compared with compression bandaging in the treatment of venous leg ulcers: a randomised controlled trial, mixed-treatment comparison and decision-analytic model. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18570.
- Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. Closed incision negative-pressure therapy is associated with decreased surgical-site infections: a meta-analysis. Plast Reconstr Surg 2015;136:592-60. https://doi.org/10.1097/PRS.0000000000001519.
- Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev 2014;10. https://doi.org/10.1002/14651858.CD009261.pub3.
- Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.MR000034.pub2.
- Smith F, Dryburgh N, Donaldson J, Mitchell M. Debridement for surgical wounds. Cochrane Database Syst Rev 2011;5. https://doi.org/10.1002/14651858.CD006214.pub3.
- Bolas N, Holloway S. Negative pressure wound therapy: a study on patient perspectives. Br J Community Nurs 2012;17:S30-5. https://doi.org/10.12968/bjcn.2012.17.Sup3.S30.
- Abbotts J. Patients’ views on topical negative pressure: ‘effective but smelly’. Br J Nurs 2010;19:S37-41. https://doi.org/10.12968/bjon.2010.19.Sup10.79692.
- Moffatt CJ, Mapplebeck L, Murray S, Morgan PA. The experience of patients with complex wounds and the use of NPWT in a home-care setting. J Wound Care 2011;20:512-27. https://doi.org/10.12968/jowc.2011.20.11.512.
- Sleney J, Christie N, Earthy S, Lyons RA, Kendrick D, Towner E. Improving recovery-learning from patients’ experiences after injury: a qualitative study. Injury 2014;45:312-19. https://doi.org/10.1016/j.injury.2012.12.025.
- Müller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart 2004;90:523-7. https://doi.org/10.1136/hrt.2003.013995.
- Hill CL, Gill T, Taylor AW, Daly A, Grande ED, Adams RJ. Psychological factors and quality of life in arthritis: a population-based study. Clin Rheumatol 2007;26:1049-54. https://doi.org/10.1007/s10067-006-0439-3.
- Vilagut G, Forero CG, Pinto-Meza A, Haro JM, de Graaf R, Bruffaerts R, et al. The mental component of the short-form 12 health survey (SF-12) as a measure of depressive disorders in the general population: results with three alternative scoring methods. Value Health 2013;16:564-73. https://doi.org/10.1016/j.jval.2013.01.006.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care 2010;48:365-71. https://doi.org/10.1097/MLR.0b013e3181c162a2.
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5:133-7. https://doi.org/10.1016/j.jpain.2003.12.005.
- Kelly J, Clifton E, Fearns N, Harbour J, Heller-Murphy S, Herbert P, et al. Edinburgh: Healthcare Improvement Scotland; 2015.
- Green J, Jester R, McKinley R, Pooler A. The impact of chronic venous leg ulcers: a systematic review. J Wound Care 2014;23:601-12. https://doi.org/10.12968/jowc.2014.23.12.601.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- National Institute for Health and Care Excellence (NICE) . Chronic Wounds: Advanced Wound Dressings and Antimicrobial Dressings: Evidence Summary 2016.
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012;344. https://doi.org/10.1136/bmj.e1119.
- Richmond SJ, Keding A, Hover M, Gabe R, Cross B, Torgerson D, et al. Feasibility, acceptability and validity of SMS text messaging for measuring change in depression during a randomised controlled trial. BMC Psychiatry 2015;15. https://doi.org/10.1186/s12888-015-0456-3.
- Brabyn S, Adamson J, MacPherson H, Tilbrook H, Torgerson DJ. Short message service text messaging was feasible as a tool for data collection in a trial of treatment for irritable bowel syndrome. J Clin Epidemiol 2014;67:993-1000. https://doi.org/10.1016/j.jclinepi.2014.05.004.
- Kew S. Text messaging: an innovative method of data collection in medical research. BMC Res Notes 2010;3. https://doi.org/10.1186/1756-0500-3-342.
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167-82. https://doi.org/10.1111/1467-9566.ep11339939.
- Charmaz K. Loss of self: a fundamental form of suffering in the chronically ill. Sociol Health Illn 1983;5:168-95. https://doi.org/10.1111/1467-9566.ep10491512.
- Long J, Meethan K, Arundel C, Clarke E, Firth A, Sylvester M, et al. Exploring feedback from research nurses in relation to the design and conduct of a randomised controlled trial of wound care treatments: a sequential, dependent, mixed-methods study. J Tissue Viability 2020. https://doi.org/10.1016/j.jtv.2020.07.007.
Appendix 1 Cross-sectional survey publication
Chetter C, Oswald AV, Dumville JC, Cullum NA. A survey of patients with surgical wounds healing by secondary intention; an assessment of prevalence, aetiology, duration and management. J Tissue Viability 2017;26:103–7. 16
Appendix 2 Cohort study publication
Chetter I, Oswald AV, McGinnis E, Stubbs N, Arundel C, Buckley H, et al. Patients with surgical wounds healing by secondary intention: a prospective, cohort study. Int J Nurs Stud 2019;89:62–71. 17
Appendix 3 Cross-sectional survey data collection instrument
Appendix 4 Cohort results: adjusted Cox proportional hazards model results for time to wound healing
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| Diabetes mellitus present (vs. not) | 0.81 (0.61 to 1.08) | 0.16 |
| Female (vs. male) | 0.97 (0.77 to 1.22) | 0.80 |
| CVD present (vs. not) | 1.03 (0.81 to 1.22) | 0.79 |
| Infection at any point (vs. not) | 0.65 (0.51 to 0.84) | < 0.01 |
| Baseline area above median (vs. below) | 0.46 (0.36 to 0.59) | < 0.01 |
| Reason for SWHSIa | ||
| Planned due to infection | 1.35 (0.96 to 1.89) | 0.29 |
| Planned for other reason | 0.43 (0.10 to 1.76) | |
| Dehisced | 1.16 (0.68 to 1.97) | |
| Partially dehisced | 1.24 (0.89 to 1.75) | |
| Surgically opened | 0.86 (0.46 to 1.61) | |
| Contamination level of surgeryb | ||
| Clean-contaminated | 0.75 (0.46 to 1.25) | 0.04 |
| Contaminated | 0.52 (0.32 to 0.86) | |
| Dirty | 0.56 (0.36 to 0.89) | |
Appendix 5 Cost-effectiveness study publication
Saramago P, Claxton K, Welton NJ, Soares M. Bayesian econometric modelling of observational data for cost-effectiveness analysis: establishing the value of negative pressure wound therapy in the healing of open surgical wounds. J R Statist Soc A 2020; in press. 28
Appendix 6 Search strategies for identification of external evidence to supplement cohort data
Search strategy: dressings
We searched the following electronic databases:
-
Cochrane Wounds Group Specialised Register (date range searched: inception to 10 January 2012)
-
Cochrane Central Register of Controlled Trials (date range searched: inception to The Cochrane Library, 2012, Issue 1)
-
Ovid MEDLINE (date range searched: 1946 to 10 January 2012)
-
Ovid EMBASE (date range searched: 1974 to 10 January 2012)
-
EBSCOhost Cumulative Index to Nursing and Allied Health Literature (CINAHL) (date range searched: 1982 to 10 January 2012).
Date searched: 10 January 2012.
Ovid MEDLINE search strategy
-
MeSH descriptor: [Bandages] explode all trees
-
MeSH descriptor: [Hydrogels] explode all trees
-
MeSH descriptor: [Alginates] explode all trees
-
(dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non-adherent or non adherent):ti,ab,kw (Word variations have been searched)
-
or/1-4
-
exp Surgical Wound Infection/
-
exp Surgical Wound Dehiscence/
-
(surg* adj5 infect*).tw.
-
(surg* adj5 wound*).tw.
-
(surg* adj5 site*).tw.
-
(surg* adj5 incision*).tw.
-
(surg* adj5 dehisc*).tw.
-
(wound* adj5 dehisc*).tw.
-
(wound* adj5 infect*).tw.
-
(wound adj5 disrupt*).tw.
-
wound complication*.tw.
-
or/6-16
-
(intent* or second* or heal* or complic*).tw.
-
((open* or clos*) adj5 wound*).tw.
-
25 or 26
-
24 and 27
-
12 and 28
-
randomised controlled trial.pt.
-
controlled clinical trial.pt.
-
randomi?ed.ab.
-
placebo.ab.
-
clinical trials as topic.sh.
-
randomly.ab.
-
trial.ti.
-
exp animals/ not humans.sh.
-
29 and 37
Search strategy: cohort studies
We searched the following electronic databases:
-
Ovid MEDLINE (date range searched: 1946 to 31 December 2013)
-
Ovid EMBASE (date range searched: 1974 to 31 December 2013)
-
EBSCOhost CINAHL (date range searched: 1982 to 31 December 2013).
Date searched: 31 December 2013.
Ovid MEDLINE search strategy
-
exp Surgical Wound/
-
exp Surgical Wound Dehiscence/
-
(surg* adj5 wound*).ti,ab.
-
(surg* adj5 site*).ti,ab.
-
(surg* adj5 incision*).ti,ab.
-
(surg* adj5 dehisc*).ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6
-
(intent* or second*).ti,ab.
-
((open* or clos*) adj5 wound*).ti,ab.
-
8 or 9
-
7 and 10
-
SWHSI.ti,ab.
-
11 or 12
-
epidemiologic studies/
-
exp Case-Control Studies/
-
exp Cohort Studies/
-
case control.ti,ab.
-
(cohort adj (study or studies)).ti,ab.
-
Cohort analy$.ti,ab.
-
(Follow up adj (study or studies)).ti,ab.
-
(observational adj (study or studies)).ti,ab.
-
Longitudinal.ti,ab.
-
Retrospective.ti,ab.
-
Cross sectional.ti,ab.
-
Cross-sectional studies/
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
-
13 and 26
-
exp Wound Healing/
-
(wound* adj3 heal*).ti,ab.
-
28 or 29
-
27 and 30
-
limit 27 to ed=19460101-20131231
-
limit 31 to ed=19460101-20131231
Search strategy: economic and health utility studies
We searched the following electronic databases:
-
Ovid MEDLINE (date range searched: 1946 to 31 December 2013)
-
Ovid EMBASE (date range searched: 1974 to 31 December 2013)
-
EBSCOhost CINAHL (date range searched: 1982 to 31 December 2013)
-
NHS Economic Evaluation Database (date range searched: inception to 2013, Issue 12).
Date searched: 31 December 2013.
Ovid MEDLINE search strategy
-
exp Surgical Wound/
-
exp Surgical Wound Dehiscence/
-
(surg* adj5 wound*).ti,ab.
-
(surg* adj5 site*).ti,ab.
-
(surg* adj5 incision*).ti,ab.
-
(surg* adj5 dehisc*).ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6
-
(intent* or second*).ti,ab.
-
((open* or clos*) adj5 wound*).ti,ab.
-
8 or 9
-
7 and 10
-
SWHSI.ti,ab.
-
11 or 12
-
ECONOMICS/
-
“Costs and Cost Analysis”/
-
“Cost Allocation”/
-
Cost-Benefit Analysis/
-
“Cost Control”/
-
“Cost Savings”/
-
“Cost of Illness”/
-
“Cost Sharing”/
-
“Deductibles and Coinsurance”/
-
Medical Savings Accounts/
-
Health Care Costs/
-
Direct Service Costs/
-
Drug Costs/
-
Employer Health Costs/
-
Hospital Costs/
-
Health Expenditures/
-
Capital Expenditures/
-
“Value of Life”/
-
exp ECONOMICS, HOSPITAL/
-
exp Economics, Medical/
-
Economics, Nursing/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp BUDGETS/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 4
-
13 and 46
Appendix 7 Patient interviews paper
McCaughan D, Sheard L, Cullum N, Dumville J, Chetter I. Patient’s perceptions and experiences of living with a surgical wound healing by secondary intention: a qualitative study. Int J Nurs Stud 2018;77:29–38. 40
Appendix 8 Nurses’ and surgeons’ views and experiences of SWHSIs: a qualitative study
McCaughan D, Sheard L, Dumville J, Cullum N. Nurses’ and surgeons’ views and experiences of surgical wounds healing by secondary intention: a qualitative study. J Clin Nurs 2020;29:2557–71. 44
Appendix 9 Patient topic guide for qualitative interviews
-
Ask for brief details about age, occupation, ethnicity, partner, children, etc., to frame the interview.
-
Can you tell me a little bit about yourself and how you came to have this wound? (Explore important aspects further.)
-
How has this wound impacted on your daily life?
-
From answer to above question, what factors have affected you most and why? (Explore important aspects further.)
-
What effect has the wound had on your relationship with your immediate family/partner/friends?
-
How do you feel about the treatment you have received in connection with your wound? How could this have been improved?
-
How do you feel about the interactions you have had with health-care professionals? How could this have been improved?
-
Any other aspects that have not already been discussed or you would like to expand on?
Appendix 10 Health-care professional topic guide for qualitative interviews
-
Could you please tell me about your clinical role in the care of patients with surgical wounds that are difficult or slow to heal?
-
What are your experiences of caring for patients with wounds which are difficult or slow to heal?
(Probe: approximately how often do you see patients with surgical wounds which are slow to heal? Why do you think wounds do not heal or heal very slowly in some patients? What are the main issues for you as a clinician? Who looks after these patients after discharge from hospital?)
-
What are the aspects of wound management that need to be considered when choosing treatment options for patients with hard-to-heal surgical wounds?
(Probe: management of pain and exudates; frequency of dressing changes; availability of clinical staff with appropriate skills and training; patient needs and convenience. )
-
What would you consider to be the desirable performance characteristics of treatment options?
(Probe: symptom management; wound cleaning/debridement; signs of healing and sustainability; patient acceptability; cost-effectiveness. )
-
What kinds of outcomes might be achieved for patients with surgical wounds that are difficult to heal, and how might these vary for different types of wounds and patient subgroups?
-
From your perspective as a health-care professional, what are the challenges of looking after a patient with a surgical wound that is difficult to heal?
(Probe: patient adherence to treatment; dealing with patients’ feelings about their wound; own feelings as a health-care professional when wound is not healing.)
-
Are there any other aspects that have not already been discussed or that you would like to expand on?
Appendix 11 Analysis of data using the framework approach
Appendix 12 Pilot feasibility randomised controlled trial: protocol paper
Arundel C, Buckley H, Clarke E, Cullum N, Dixon S, Dumville J, et al. Negative pressure wound therapy versus usual care for surgical wounds healing by secondary intention (SWHSI trial): study protocol for a randomised controlled pilot trial. Trials 2016;17:535. 56
Appendix 13 Pilot feasibility randomised controlled trial: results paper
Arundel C, Fairhurst C, Corbacho-Martin B, Buckley H, Clarke E, Cullum N, et al. Pilot feasibility randomized clinical trial of negative-pressure wound therapy versus usual care in patients with surgical wounds healing by secondary intention. BJS Open 2018;2:99–111. 57
Appendix 14 Pilot feasibility randomised controlled trial: nurse participation in the SWHSI pilot feasibility trial
Long J, Meethan K, Arundel C, Clarke E, Firth A, Sylvester M, Chetter I. Exploring feedback from research nurses in relation to the design and conduct of a randomised controlled trial of wound care treatments: a sequential, dependent, mixed-methods study. J Tissue Viability 2020; in press. 96
Appendix 15 Pilot and feasibility randomised controlled trial: wound pain (text message) by treatment group and time point
| Time point | Weekly text message pain scores | Treatment group | Total (N = 20) | |
|---|---|---|---|---|
| NPWT (N = 10) | Usual care (N = 10) | |||
| Week 1 | n received/n sent (%) | 6/13 (46.2) | 9/13 (69.2) | 15/26 (57.7) |
| Mean (SD) | 5.0 (1.9) | 3.3 (3.4) | 4.0 (3.0) | |
| Median (minimum, maximum) | 5 (3, 8) | 4 (0, 8) | 5 (0, 8) | |
| Week 2 | n received/n sent (%) | 10/13 (76.9) | 9/12 (75.0) | 19/25 (76.0) |
| Mean (SD) | 4.9 (3.2) | 3.0 (3.0) | 4.0 (3.2) | |
| Median (minimum, maximum) | 6 (0, 9) | 3 (0, 9) | 3 (0, 9) | |
| Week 3 | n received/n sent (%) | 9/12 (75.0) | 8/10 (80.0) | 17/22 (77.3) |
| Mean (SD) | 3.4 (2.8) | 2.6 (3.0) | 3.1 (2.8) | |
| Median (minimum, maximum) | 3 (0, 8) | 1.5 (0, 7) | 2 (0, 8) | |
| Week 4 | n received/n sent (%) | 9/11 (81.8) | 9/9 (100.0) | 18/20 (90.0) |
| Mean (SD) | 3.9 (3.0) | 2.2 (2.4) | 3.1 (2.8) | |
| Median (minimum, maximum) | 3 (0, 8) | 1 (0, 6) | 3 (0, 8) | |
| Week 5 | n received/n sent (%) | 10/11 (90.9) | 6/8 (75.0) | 16/19 (84.2) |
| Mean (SD) | 3.6 (3.2) | 2.2 (2.5) | 3.1 (3.0) | |
| Median (minimum, maximum) | 2.5 (0, 9) | 1.5 (0, 7) | 2 (0, 9) | |
| Week 6 | n received/n sent (%) | 9/11 (81.8) | 7/8 (87.5) | 16/19 (84.2) |
| Mean (SD) | 2.6 (3.5) | 2.7 (2.9) | 2.6 (3.1) | |
| Median (minimum, maximum) | 0 (0, 8) | 1 (0, 8) | 1 (0, 8) | |
| Week 7 | n received/n sent (%) | 7/8 (87.5) | 7/7 (100.0) | 14/15 (93.3) |
| Mean (SD) | 3.6 (3.9) | 2.1 (2.7) | 2.9 (3.3) | |
| Median (minimum, maximum) | 2 (0, 8) | 1 (0, 6) | 1 (0, 8) | |
| Week 8 | n received/n sent (%) | 6/7 (85.7) | 5/7 (71.4) | 11/14 (78.6) |
| Mean (SD) | 3.7 (3.5) | 1.4 (2.1) | 2.6 (3.0) | |
| Median (minimum, maximum) | 3.5 (0, 8) | 1 (0, 5) | 1 (0, 8) | |
| Week 9 | n received/n sent (%) | 5/7 (71.4) | 7/7 (100.0) | 12/14 (85.7) |
| Mean (SD) | 2.8 (2.6) | 1.4 (1.9) | 2.0 (2.2) | |
| Median (minimum, maximum) | 2 (0, 6) | 1 (0, 5) | 1 (0, 6) | |
| Week 10 | n received/n sent (%) | 5/7 (71.4) | 6/6 (100.0) | 11/13 (84.6) |
| Mean (SD) | 3.0 (2.1) | 1.2 (1.6) | 2.0 (0.2) | |
| Median (minimum, maximum) | 3 (0, 5) | 0.5 (0, 4) | 2 (0, 5) | |
| Week 11 | n received/n sent (%) | 5/5 (100.0) | 7/7 (100.0) | 12/12 (100.0) |
| Mean (SD) | 3.2 (2.4) | 0.9 (1.2) | 1.8 (2.1) | |
| Median (minimum, maximum) | 3 (0, 6) | 0 (0, 3) | 1.5 (0, 6) | |
| Week 12 | n received/n sent (%) | 5/5 (100.0) | 6/6 (100.0) | 11/11 (100.0) |
| Mean (SD) | 2.4 (2.4) | 1.0 (1.5) | 1.6 (2.0) | |
| Median (minimum, maximum) | 1 (0, 5) | 0.5 (0, 4) | 1 (0, 5) | |
Appendix 16 Pilot feasibility randomised controlled trial: physical and mental composite scale scores derived from the Short Form questionnaire-12 items by treatment group and time point
FIGURE 7.
Physical composite scale scores derived from the SF-12 by randomised group across time.
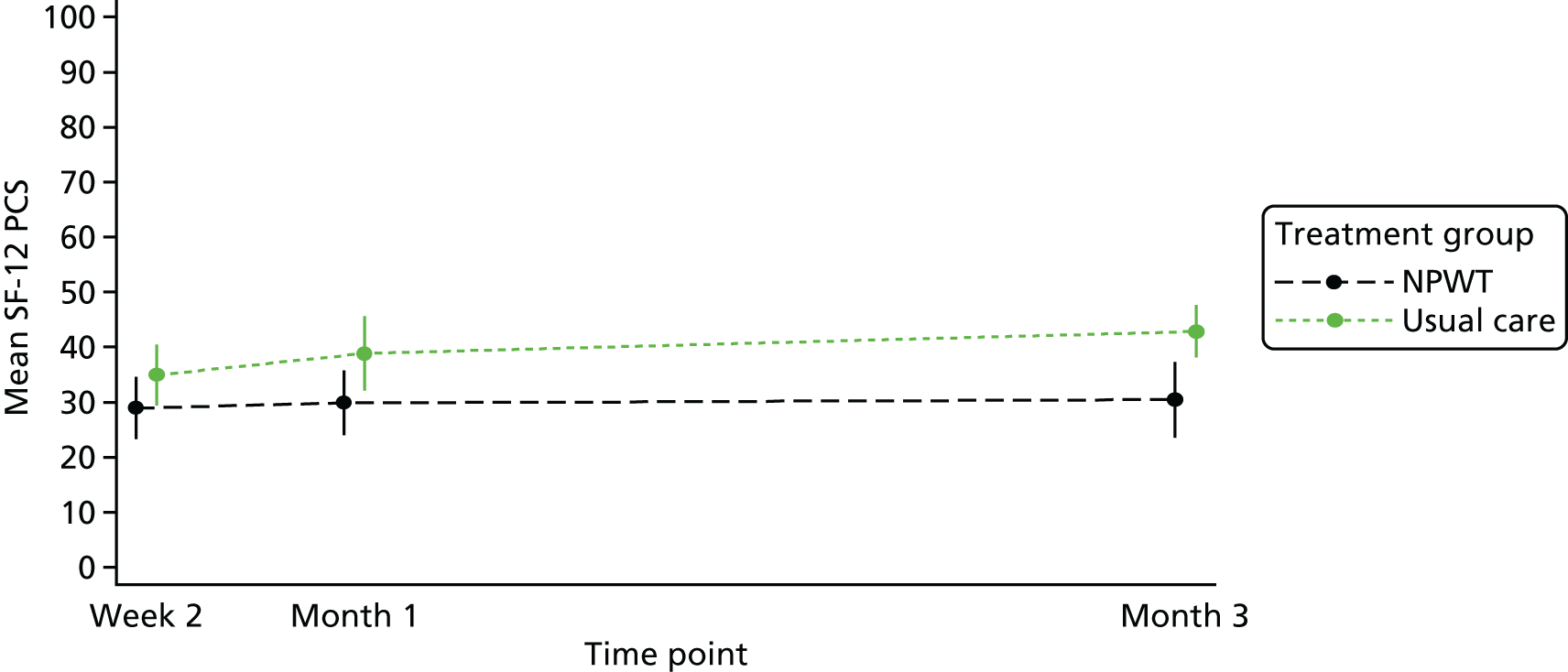
FIGURE 8.
Mental composite scale scores derived from the SF-12 by randomised group across time.
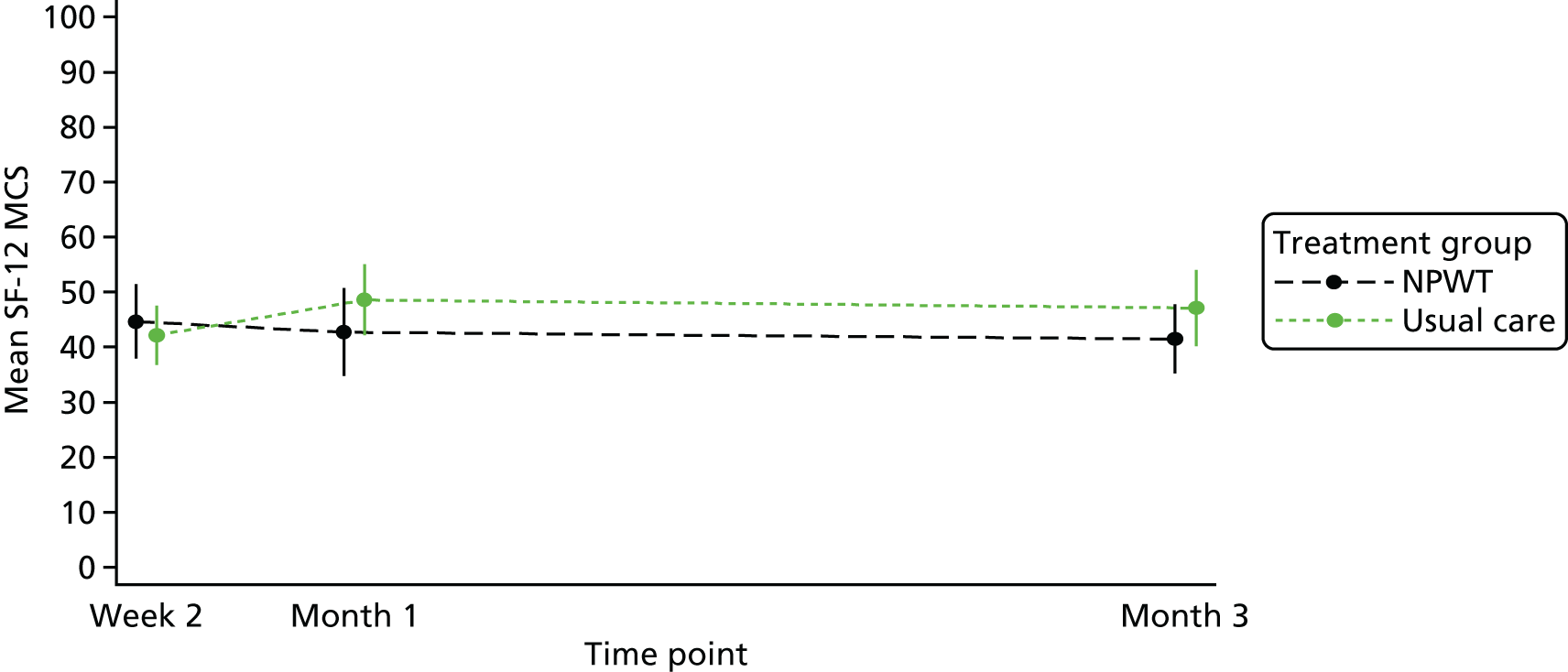
Appendix 17 Unit costs of health-care services
| Item | Unit of measurement | Unit cost (£) | Additional notes | Source |
|---|---|---|---|---|
| Hospital outpatient (doctor) | Per clinic visit | 131.99 | Based on average of total outpatient attendances | Department of Health and Social Care61 |
| Hospital outpatient (nurse) | Per clinic visit | 70.87 | Department of Health and Social Care61 | |
| Day case | Per admission | 720.78 | Based on average of total day-case attendances | Department of Health and Social Care61 |
| GP visit at general practice | Per patient contact (surgery), lasting 11.7 minutes | 44.00 | Curtis and Burns62 | |
| GP visit at home | Per home visit (11.4 minutes) plus 12 minutes of travel time | 88.92 | Curtis and Burns62 | |
| Nurse visit at general practice | Per 15.5-minute appointment (based on £43.00 per hour) | 11.11 | Curtis and Burns62 | |
| Nurse visit at home | Per home visit (lasting 25 minutes) plus 12 minutes of travel time | 23.33 | Based on average of most commonly reported | Department of Health and Social Care;61 Curtis and Burns62 |
| Inpatient admission | Per night | 359.13 | Based on elective inpatient excess bed-days | Curtis and Burns62 |
Appendix 18 Completion and missingness of EQ-5D questionnaires
| Time point | Completed EQ-5D, n (%) | Missing EQ-5D (≥ 1 dimension missing), n (%) | ||
|---|---|---|---|---|
| NPWT (N = 19) | Usual care (N = 21) | NPWT (N = 19) | Usual care (N = 21) | |
| Baseline | 15 (78.9) | 21 (100.0) | 4 (21.1) | 0 (0.0) |
| 3 months | 14 (73.7) | 15 (71.4) | 5 (26.3) | 6 (28.6) |
Appendix 19 Proportion reporting EQ-5D-3L levels 1–3 by dimension, treatment group and time point
| EQ-5D scale | Health state severitya | Time point | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | ||||||||
| NPWTb | Usual carec | NPWTb | Usual carec | ||||||
| n | % | n | % | n | % | n | % | ||
| Mobility | No problems | 5 | 31.25 | 6 | 28.57 | 3 | 20.00 | 6 | 40.00 |
| Some problems | 8 | 50.00 | 14 | 66.67 | 12 | 80.00 | 9 | 60.00 | |
| Unable/extreme problems | 3 | 18.75 | 1 | 4.76 | 0 | 0.00 | 0 | 0.00 | |
| Number reporting any problems | 11 | 68.75 | 15 | 71.43 | 12 | 80 | 9 | 60.00 | |
| Self-care | No problems | 9 | 56.25 | 17 | 80.95 | 8 | 53.33 | 11 | 73.33 |
| Some problems | 7 | 43.75 | 4 | 19.05 | 7 | 46.67 | 4 | 26.67 | |
| Unable/extreme problems | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Number reporting any problems | 7 | 43.75 | 4 | 19.05 | 7 | 46.7 | 4 | 26.67 | |
| Usual activities | No problems | 3 | 18.75 | 6 | 28.57 | 2 | 13.33 | 8 | 53.33 |
| Some problems | 4 | 25.00 | 11 | 52.38 | 10 | 66.67 | 6 | 40.00 | |
| Unable/extreme problems | 9 | 56.25 | 4 | 19.05 | 3 | 20.0 | 1 | 6.67 | |
| Number reporting any problems | 13 | 81.25 | 15 | 71.43 | 13 | 86.7 | 7 | 46.67 | |
| Pain | No problems | 4 | 25.00 | 5 | 23.81 | 2 | 13.33 | 10 | 66.67 |
| Some problems | 7 | 43.75 | 13 | 61.90 | 10 | 66.67 | 5 | 33.33 | |
| Unable/extreme problems | 5 | 31.25 | 3 | 14.29 | 3 | 20.0 | 0 | 0.00 | |
| Number reporting any problems | 12 | 75 | 16 | 76.19 | 13 | 86.7 | 5 | 33.33 | |
| Anxiety | No problems | 11 | 68.75 | 14 | 66.67 | 9 | 60.00 | 12 | 80.00 |
| Some problems | 4 | 25.00 | 5 | 23.81 | 5 | 33.33 | 2 | 13.33 | |
| Unable/extreme problems | 1 | 6.25 | 2 | 9.52 | 1 | 6.67 | 1 | 6.67 | |
| Number reporting any problems | 5 | 31.25 | 7 | 33.33 | 6 | 40 | 3 | 20.00 | |
List of abbreviations
- BMI
- body mass index
- BPI
- Brief Pain Inventory
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CrI
- credible interval
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- HRQoL
- health-related quality of life
- IV
- instrumental variable
- MCS
- mental component score
- NICE
- National Institute for Health and Care Excellence
- NPWT
- negative-pressure wound therapy
- OLS
- ordinary least squares
- OR
- odds ratio
- PCS
- physical component score
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SWHSI
- surgical wound healing by secondary intention
- VAS
- visual analogue scale