Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10340. The contractual start date was in September 2008. The final report began editorial review in September 2018 and was accepted for publication in January 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Story et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
This National Institute for Health Research (NIHR) programme grant was initiated because of the extreme challenges of controlling tuberculosis in socially complex groups that have great difficulty in accessing and using mainstream NHS tuberculosis services. The programme was ambitious and was working in highly challenging and complex settings. As researchers, we needed to adapt our methodologies in response to these challenges and we made these changes in discussion with the funders.
This study demonstrates that there are extremely high levels of latent tuberculosis infection and blood-borne viruses in people experiencing homelessness, prisoners and drug users; it also demonstrates the challenges of implementing screening programmes and ensuring that cases of infection are treated. It also shows that there are low levels of hepatitis B vaccination uptake in these groups. We show that, even at relatively low levels of treatment uptake, screening for latent tuberculosis infection in homeless groups is likely to be cost-effective at a willingness-to-pay threshold of £20,000–£30,000. This cost-effectiveness is likely to be improved by integrating with screening for blood-borne viruses and vaccination. Screening for latent tuberculosis infection and blood-borne viruses is now offered to patients experiencing homelessness screened by the mobile radiographic unit, along with vaccination for hepatitis B, influenza and Streptococcus pneumoniae.
Despite the NHS receiving ring-fenced funding to establish radiographic screening for tuberculosis in prisons and installing equipment in key prisons, none of the prisons had initiated and maintained routine radiographic screening. We provided a radiologist to support the pilot NHS programme and showed that screening was feasible and identified cases. Previous economic analyses have suggested that, if implemented, such screening would be cost-effective.
We have demonstrated that peer educators are no more or less effective than health-care workers in encouraging the uptake of mobile radiographic screening. We consider that the study findings may have been contaminated because the service already used peer educators to support its work. We consider that there are wider benefits of including peer educators as part of the Find&Treat team so that they can continue to work alongside health-care workers to encourage people to be screened and to support those identified with concerning radiographs to engage in further follow-up.
We overcame substantial technical difficulties to establish a polymerase chain reaction facility on the mobile radiographic unit. Although we were unable to fully evaluate this, the service has now been reinstated on the mobile radiographic unit and is used in situations where a strong need is identified, such as in response to tuberculosis outbreaks.
The most important component of our work is our trial of smartphone-enabled video-observed treatment (VOT). To our knowledge this is the first randomised controlled trial (RCT) evidence of this intervention to be published. We have demonstrated that the intervention can achieve high levels of adherence to daily treatment regimes in socially complex groups and is far superior to face-to-face, directly observed treatment (DOT) in ensuring treatment observation. The intervention is also cheaper than DOT. The technology has great potential to improve tuberculosis control internationally, particularly in preventing the development of drug-resistant tuberculosis and managing socially complex patients and those with multidrug-resistant tuberculosis. The London Find&Treat service has been commissioned to provide an NHS VOT service based on the model developed through this research. As part of this, the service provides support to the majority of London multidrug-resistant tuberculosis (MDRTB) cases. The service also provides VOT for selected cases nationally. Long-term funding arrangements for the VOT service, however, remain uncertain. VOT is now recommended in the World Health Organization (WHO) treatment guidelines1 and is being increasingly used internationally.
Although focused on tuberculosis, this research programme has implications for control of other infections and non-communicable diseases in socially complex groups. The emphasis on outreaching active case finding for tuberculosis and providing support for engagement in treatment is relevant across the spectrum of health conditions experienced by these groups.
Improving the management and control of tuberculosis in socially excluded groups: synopsis of work packages
This NIHR programme grant for applied research allowed us to investigate a series of approaches to improving the management and control of tuberculosis in socially excluded groups that have high rates of tuberculosis, delayed diagnosis, drug resistance, loss to follow-up, and often poor treatment adherence and high mortality. Key groups include people experiencing homelessness, prisoners and drug users. Through a series of work packages, we investigated (1) the value of screening for latent tuberculosis infection alongside blood-borne virus screening and radiographic screening for tuberculosis (work packages 1 and 2); (2) the use of a peer intervention to increase uptake of mobile radiographic screening for tuberculosis in hostels for people experiencing homelessness (work package 3); (3) the value of introducing rapid molecular tests for tuberculosis alongside mobile radiographic screening for active tuberculosis (work package 4); (4) the use of a smartphone-enabled approach to supporting adherence to tuberculosis treatment (VOT) compared with face-to-face DOT (work package 5); and (5) the cost-effectiveness of screening people experiencing homelessness for latent tuberculosis infection and of VOT. The key methodologies and results of these work packages are summarised below. Full results for most aspects of the work have been published elsewhere, as indicated in the text.
Evolution of the programme components
In view of these challenges, we designed a programme of research that was submitted to NIHR for consideration of funding. This included the following studies:
-
study 1 – cross-sectional survey on the prevalence of latent tuberculosis infection, human immunodeficiency virus (HIV), hepatitis B and hepatitis C in prisoners, drug users and people experiencing homelessness to inform a mathematical model (study 7) of the impact and cost-effectiveness of screening socially complex groups
-
study 2 – observational study of the effectiveness of a new prison radiographic screening service
-
study 3 – cluster randomised controlled trial (CRCT) evaluating interventions to increase the uptake of mobile radiographic screening in homeless populations
-
study 4 – establishment and evaluation of a rapid diagnostic pathway to reduce loss to follow-up prior to diagnosis
-
study 5 – individually randomised controlled trial of clinic-based DOT versus community-based DOT to inform optimal treatment delivery for socially complex groups
-
study 6 – evaluation of a specialist clinical service providing in-reach high-quality tuberculosis care in prison
-
study 7 – qualitative research to ensure that proposed interventions are acceptable and accessible to the target population and to inform understanding of barriers to services
-
study 8 – development of a dynamic transmission model to predict public health impact of the interventions and inform economic analysis comparing costs with the savings made through averting future cases.
Following peer review, the funders requested the following changes to this programme of work:
-
Removal of studies 6 and 7 in response to the programme being perceived as overambitious and the team having insufficient qualitative research expertise.
-
Simplification of study 3, which was originally conceived as a three-arm CRCT comparing usual practice with financial incentives and with peer educators as interventions to improve mobile radiographic screening uptake. We were requested to remove one study arm and, therefore, simplified this to a proposed trial of incentives versus usual practice.
-
Addressing concerns about the safety of conducting smear microscopy on the mobile radiographic unit. To address this issue, we proposed evaluating how the pan-London service would use a single NHS trust for rapid sputum smear microscopy.
-
Cost-effectiveness analyses – it was requested that the cost-effectiveness of the adherence interventions also included a societal perspective.
Subsequent to funding, a number of developments led to further modifications of the study proposal, which were agreed with funders at regular reviews. These are summarised below.
Surveys of latent tuberculosis infection
The scale of these surveys needed to be reduced because of the challenges of recruiting and consenting participants across homeless, drug user and prison settings, and of obtaining detailed baseline questionnaires and blood samples. The work was focused on homeless and prison settings, recognising that these settings would also capture many drug users. In view of the decreased sample size, the objective to measure progression rates to active tuberculosis, and the data linkage that this required, was removed.
Evaluation of the prison radiographic screening programme
Despite the NHS being funded to conduct a prison screening programme in key prisons, at the time of the study none of the selected prisons had implemented this. To address this, we provided radiological time to the study prison to enable a pilot of the screening programme to take place. This meant that the number of prisoners going through screening was considerably lower than anticipated in a routine comprehensive service. The objective of comparing cases identified through screening with cases that were identified through passive case finding also needed to be removed because of the small number of identified cases.
Cluster randomised controlled trial to increase the uptake of radiographic screening for tuberculosis in homeless hostels
Concerns about staff safety when regularly carrying and dispensing financial incentives were agreed to limit the feasibility of routine use of financial incentives to encourage uptake. The intervention was changed to peer education (which had originally been the third arm of the three-arm trial).
Substantial developments in the technology for rapid polymerase chain reaction (PCR)-based diagnostics led to the possibility of deploying highly compact PCR assays rather than smear microscopy. These technologies have been shown to be more sensitive than sputum smear microscopy, have the advantage of identifying rifampicin resistance (and are a good proxy for MDRTB) and require limited sample handling and lower levels of training than sputum microscopy. They also provided rapid turnaround times. The trust that had agreed to provide the rapid sputum microscopy service agreed to support the development and accreditation of the use of this technology on the mobile radiographic unit, including the development of valved sputum pots that removed the need for subsequent handling of sputum, safety standard operating procedures and staff training. A RCT was designed to evaluate the impact of this technology when used alongside mobile radiographic screening.
Randomised controlled trial to improve adherence to treatment
Although this was originally proposed as a trial of community-based DOT versus usual practice (clinic-based DOT), during the course of set-up, community-based DOT became much more commonly used in response to patients’ needs. This prevented the proposed randomisation. At the same time, Find&Treat had been developing in-house video-observed therapy with apparently high success. The University of San Diego had developed a smartphone application (app) to increase the simplicity and security of this approach. With the agreement of funders, we therefore changed this to a trial of smartphone-enabled VOT compared with face-to-face DOT (either clinic or community based). This required the need to overcome a range of technical and information governance challenges before the trial could commence. At the same time, the numbers of cases of tuberculosis in London had declined compared with when the original study was designed. In response, we extended recruitment to clinics outside London. It became apparent that cases randomised to DOT frequently did not take up this form of observation. In view of this and the slower than expected recruitment, the funders requested an interim analysis to inform the decision about whether or not the study should continue. Following publication of an interim analysis plan with agreed stopping rules, the trial was discontinued because of the overwhelming superiority of the VOT arm.
Cost-effectiveness analyses
During the course of the programme grant, a cost-effectiveness analysis of screening for latent tuberculosis infection and active tuberculosis in prisoners was conducted by the National Institute for Health and Care Excellence (NICE). In view of this new analysis, this aspect of the economic evaluation was removed. As the peer intervention to improve screening uptake showed no evidence of effectiveness, cost-effectiveness analyses were considered unnecessary. Similarly, as we were unable to demonstrate that the use of a rapid diagnostic approach improved outcomes, a cost-effectiveness analysis was deemed unnecessary. Finally, as the VOT intervention proved substantially more effective and cheaper than DOT, no cost-effectiveness analysis was required. This work package, therefore, focused on the cost-effectiveness of screening for latent tuberculosis infection alongside mobile radiographic screening in the homeless, and cost comparison of DOT and VOT.
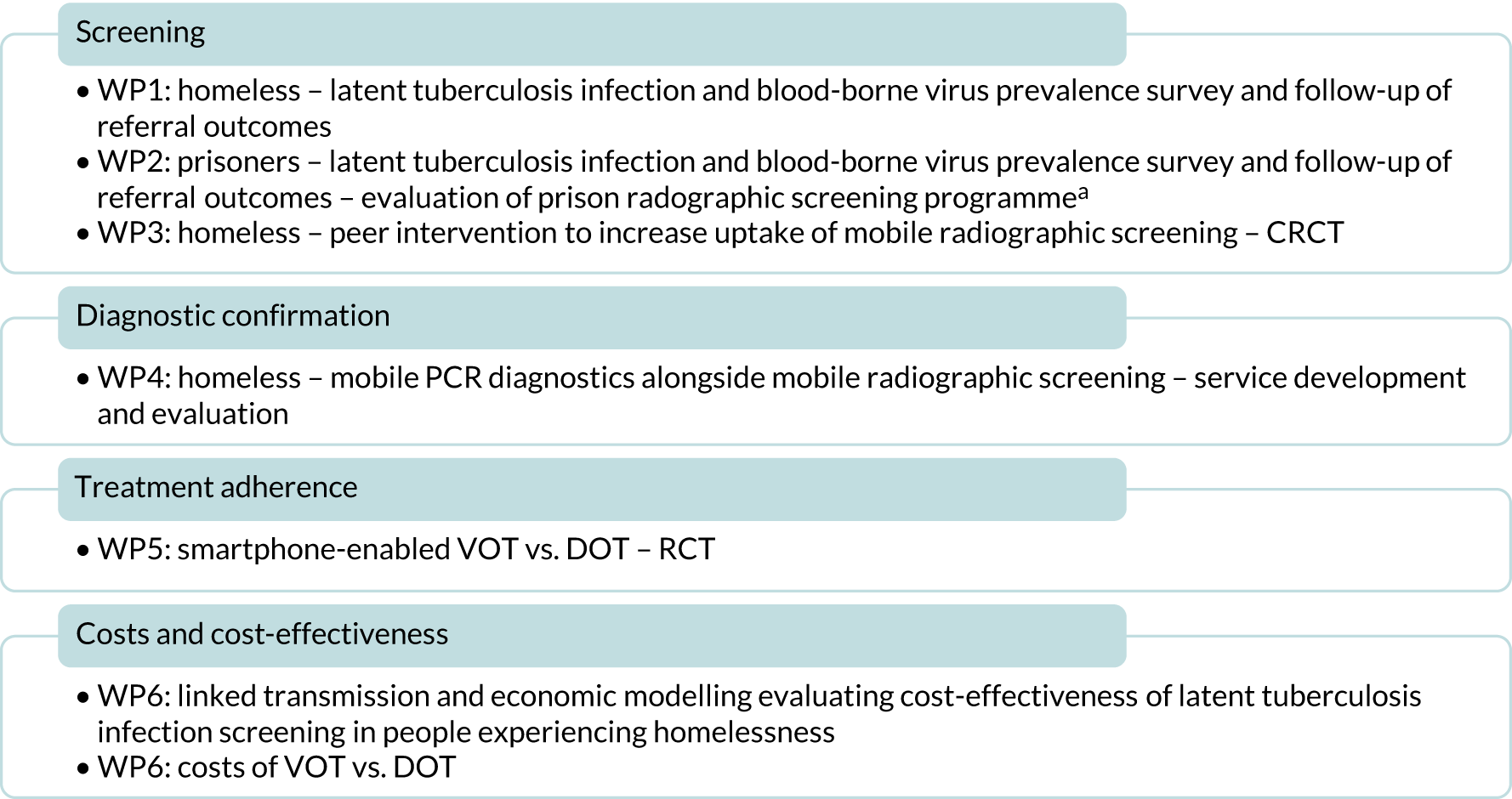
Following these changes, we divided the work into six work packages as outlined in Figure 1.
FIGURE 1.
Overview of work packages. a, Testing of prisoners for blood-borne viruses and latent tuberculosis infection was originally included in WP1 but for clarity and improved contextualisation of the results we have included the description of this activity in WP2. WP, work package.
Study data handling
Study nurses with relevant honorary NHS contracts had access to a look-up table between patient identifiers and study numbers to enable them to make referrals and follow up patients who had consented to this. This look-up table was held on University College London’s data safe haven. Anonymised data sets were used by other staff for analyses and were kept on secure servers with access to named individuals only.
Work package 1: latent tuberculosis infection and blood-borne virus prevalence in people experiencing homelessness in London
Our study findings relating to latent tuberculosis infection and blood-borne virus in homeless populations have previously been published. 2 Parts of this section have been reproduced or adapted from Aldridge et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
In our original application, work package 1 also included latent tuberculosis infection and blood-borne virus screening in prisoners but, for clarity, we present the prison results alongside the results of the evaluation of prison radiographic screening for tuberculosis in work package 2.
Introduction
Individuals experiencing homelessness have high rates of tuberculosis infection and often present late to health-care services. 3 Latent tuberculosis infection is common in homeless populations in low-burden countries,4,5 although limited data are available in the UK.
Homelessness and tuberculosis in homeless populations are both increasingly important problems in London. In 2014, it was estimated that 3.6% (89/2498) of tuberculosis cases with social risk factor information available had a history of homelessness. 6 The point prevalence of active tuberculosis in people experiencing homelessness in London has been estimated at 788 per 100,000, compared with 27 per 100,000 in the overall London population. 3
Developments in testing for latent tuberculosis infection and treatment of blood-borne viruses provide new opportunities to improve the health of people experiencing homelessness. 7,8 Poor adherence and potentially severe hepatotoxicity exacerbated by high rates of alcohol- or viral-related liver disease remain concerns for latent tuberculosis infection treatment in people experiencing homelessness. 9 Health services may not be well tailored to deliver care for identified infections in people experiencing homelessness, potentially limiting the value of screening.
We undertook a cross-sectional survey to estimate latent tuberculosis infection and blood-borne virus prevalence among individuals in homeless hostels in London and examined outcomes of referral to health-care services after 12 months.
Methods
Design
A cross-sectional survey of latent tuberculosis infection, hepatitis B, hepatitis C and HIV prevalence in residents of homeless hostels in London was carried out in May 2011 and June 2013, with 12-month follow-up of onward referrals.
Implementation
The study was conducted by NHS nurses employed by the study working alongside the NHS Find&Treat service. Find&Treat identifies cases of active tuberculosis using digital chest radiography and supports patients to complete treatment. 10 The eligibility criteria were aged ≥ 18 years, resident at a homeless hostel on the day of the Find&Treat screening, tuberculosis screening chest radiograph within the last 6 months (to help rule out active tuberculosis) and able to provide written informed consent. The study team collected sociodemographic and risk factor data using a paper questionnaire. In keeping with NICE guidance, up to March 2012 the study nurses offered individuals diagnosed with latent tuberculosis infection advice about tuberculosis symptoms. 11 After March 2012, we referred those with latent tuberculosis infection who were aged < 35 years to local health services, in line with revised NICE guidance for identifying and managing tuberculosis among hard-to-reach groups. 12 We referred individuals with current hepatitis B or hepatitis C infection and previously undiagnosed HIV infection to 14 local health services and the research team collected outcomes 12 months after referral by telephoning clinicians and nurses to whom the patients were referred.
The study received approval from the East of England – Essex National Research Ethics Service Committee (number 10/H0302/5).
Laboratory testing
We collected whole venous blood samples to test for latent tuberculosis infection and blood-borne viruses. We measured latent tuberculosis infection using the QuantiFERON-TB Gold gamma interferon release assay (Cellestis International Pty Ltd, Chadstone, VIC, Australia) following the manufacturer’s instructions for interpretation (Table 1).
| Infection (number screened) | Classification status | Definition | Number classified (%a) |
|---|---|---|---|
| Latent tuberculosis (n = 489)b | Positivec | Tuberculosis-specific antigen response > 0.35 IU/ml, and no evidence of active disease on clinical assessment | 81 (16.5) |
| Negative | Tuberculosis-specific antigen response < 0.35 IU/ml | 408 (83.1) | |
| Hepatitis B (n = 489)b | Current | HBsAg positive, anti-HBc negative, anti-HBs negative | 7 (1.4) |
| Past |
HBsAg negative, anti-HBc positive, anti-HBs positive (confirmed; n = 43) or HBsAg negative, anti-HBc positive, anti-HBs negative (probable past; n = 8) |
51 (10.4) | |
| Immune probably through vaccinationd | HBsAg negative, anti-HBc negative,e anti-HBs positive | 140 (28.7) | |
| Non-immune | HBsAg, anti-HBc, anti-HBs negative | 291 (59.5) | |
| Hepatitis C (n = 491) | Current | Anti-HCV positive and HCV RNA positive | 51 (10.4) |
| Past | Anti-HCV positive, HCV RNA negative, and RIBA positive | 13 (2.7) | |
| Uncertain past history | Anti-HCV positive or equivocal, HCV RNA negative and no RIBA or insufficient sample for testing | 3 (0.6) | |
| Negative | Anti-HCV and HCV RNA negative | 424 (86.4) | |
| HIV (n = 491) | Seropositive | Anti-HIV/p24 antigen positive | 5 (1.0) |
| Seronegative | Anti-HIV/p24 antigen positive | 486 (99.0) |
We used the Architect immunoassay (Abbott Diagnostics GmbH, Wiesbaden, Germany) to detect hepatitis B surface antigen (HBsAg), antibody to hepatitis B core antigen (anti-HBc) and antibody to hepatitis B surface antigen (anti-HBs). We classified hepatitis B infection as current in participants who tested positive for HBsAg at screening with confirmation by HBsAg neutralisation. We classified hepatitis B as ‘confirmed past’ in those who were HBsAg negative, anti-HBc positive and anti-HBs positive, and as ‘probable past’ in those who were HBsAg negative, anti-HBc positive and anti-HBs negative. We combined the confirmed and probable past groups into one group (referred to as ‘past hepatitis B infection’). We defined non-immune hepatitis B status by absence of all hepatitis B markers.
We detected antibodies to hepatitis C virus (anti-HCVs) using the Vitros chemiluminescence assay (Ortho Clinical Diagnostics, Raritan, NJ, USA). We measured hepatitis C ribonucleic acid (RNA) using either a real-time PCR assay based on the method described by Komurian-Pradel et al. ,14 or the Abbott M2000 RealTime hepatitis C assay (Abbott Laboratories, Abbott Park, IL, USA). Samples reactive for anti-HCV but with undetectable hepatitis C RNA underwent anti-HCV confirmation by the Recombinant ImmunoBlot Assay (RIBA) Chiron (Novartis Vaccines and Diagnostics, Inc, Cambridge, MA, USA) or the line immunoassay, Inno-Lia® (Fujirebio Europe N.V., Ghent, Belgium). Hepatitis C infection was classed as current in anti-HCV-positive participants who tested hepatitis C RNA positive, and past in those who showed undetectable hepatitis C RNA with confirmed anti-HCV positivity (see Table 1). We performed HIV screening using the Architect combined HIV antibody/p24 antigen chemiluminescence assay (Abbott Diagnostics).
Analysis
The primary outcome was the proportion of participants with a positive QuantiFERON-TB Gold assay result. Based on previous US studies,4,5,15 we expected a minimum of 10% of participants to test positive for latent tuberculosis infection. We required 500 participants to measure this within 95% confidence intervals (CIs) between 8% and 13%. The secondary outcomes were 12-month referral outcomes for hepatitis B, hepatitis C and HIV status. We undertook a descriptive analysis of baseline variables and their association with primary and secondary outcomes. We used logistic regression modelling to examine potential risk factors for latent tuberculosis infection (history of imprisonment, history of drug and alcohol use, history of homelessness and country of birth) and considered age, a priori, as a confounder. As those from high-incidence countries are already eligible for screening, we conducted a separate analysis of risk factors in those who were born in the UK. Data were analysed in Stata® version 14 (StataCorp LP, College Station, TX, USA).
Results
Study population
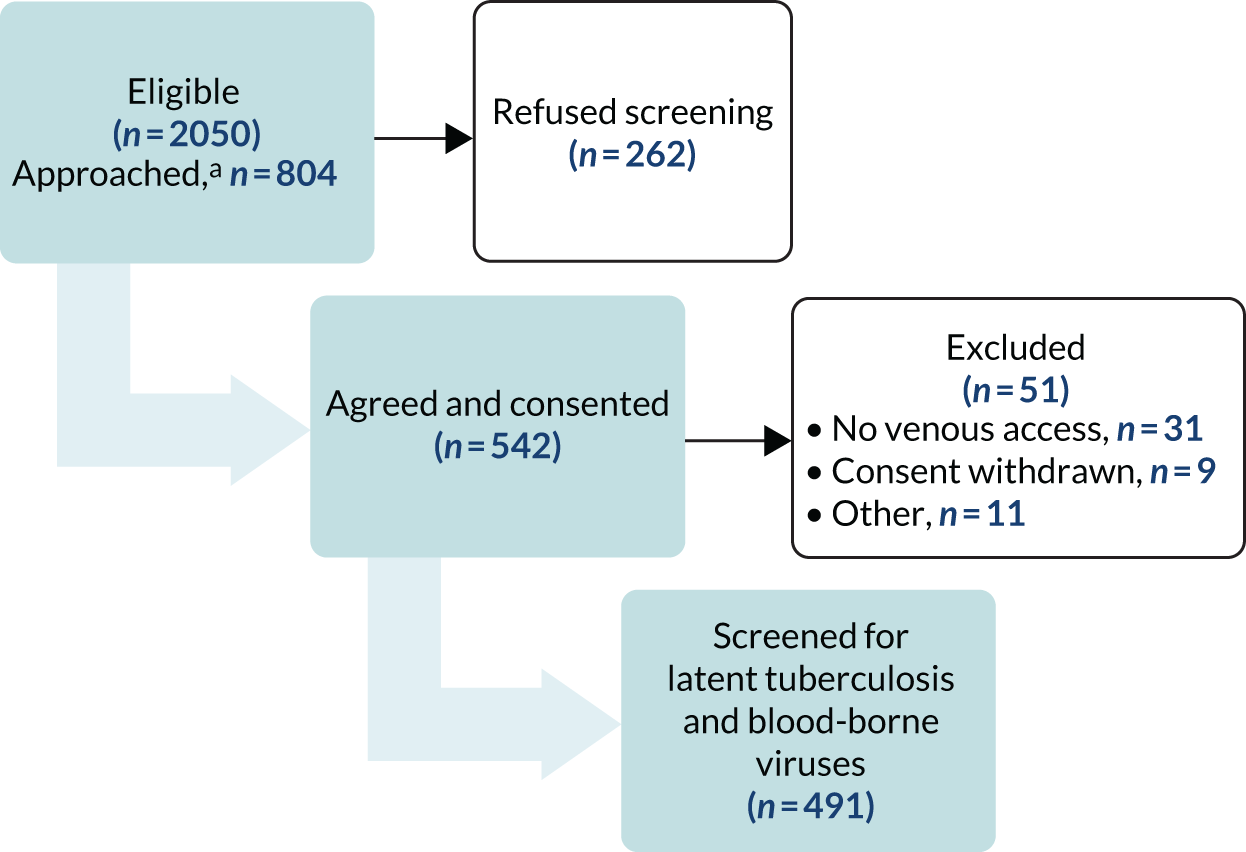
We invited 804 individuals to participate following Find&Treat mobile radiographic screening. A total of 542 out of 804 (67.4%) individuals consented to take part (Figure 2). We subsequently excluded 51 (9.4%) individuals, mainly because of a lack of venous access for blood sampling (n = 31); therefore, we included 491 individuals in the analysis.
FIGURE 2.
Recruitment flow chart for work package 1. a, It was operationally extremely intensive to collect data on the number of individuals who were eligible, approached and refused screening, therefore these data were collected only at the start of the study for the first 474 eligible patients. The numbers presented are estimates based on this initial sample.
Most participants were men (437/491, 89.0%) aged 30–49 years (257/491, 52.3%) who were born in the UK (305/491, 62.1%), were current tobacco smokers (394/491, 80.2%) and reported being homeless for ≥ 1 year (443/491, 90.2%). Of those not born in the UK, 45 were from other Western European countries, 18 were from Central Europe, Eastern Europe or Central Asia, 23 were from the Eastern Mediterranean, Middle East, North, East or West Africa, 41 were from Central or sub-Saharan Africa, 25 were from South or South East Asia or the Indian subcontinent, five were from East Asia, 18 were from Latin America or the Caribbean and 11 were from other regions. Just over half (263/481, 54.7%) had spent time in prison. Drug use was common, with 107 out of 491 (21.8%) having ever smoked heroin or crack cocaine and 86 out of 491 (17.5%) having ever injected either crack cocaine or heroin. A high proportion (202/477, 42.3%) had ever been concerned about their drinking, or had had a health worker express concern about this. Latent tuberculosis infection and blood-borne virus results are shown in Tables 1 and 2.
| Characteristic | All | QuantiFERON positive | Hepatitis B positivea | Hepatitis C positiveb | |||
|---|---|---|---|---|---|---|---|
| n | n | % | n | % | n | % | |
| All | 491 | 81 | 16.5 | 58 | 11.9 | 64 | 13.0 |
| Age (years) | |||||||
| 18–29 | 69 | 8 | 11.6 | 6 | 8.7 | 3 | 4.3 |
| 30–49 | 257 | 39 | 15.2 | 28 | 10.9 | 43 | 16.7 |
| ≥ 50 | 165 | 34 | 20.6 | 24 | 14.5 | 18 | 10.9 |
| Sex | |||||||
| Female | 54 | 4 | 7.4 | 5 | 9.3 | 3 | 5.6 |
| Male | 437 | 77 | 17.6 | 53 | 12.1 | 61 | 14.0 |
| Born in the UK | |||||||
| Yes | 305 | 29 | 9.5 | 29 | 9.5 | 50 | 16.4 |
| No | 186 | 52 | 28.0 | 29 | 15.6 | 14 | 7.5 |
| Total time spent homeless | |||||||
| < 1 year | 48 | 8 | 16.7 | 6 | 12.5 | 4 | 8.3 |
| 1 year | 135 | 18 | 13.3 | 16 | 11.9 | 13 | 9.6 |
| 2–3 years | 141 | 28 | 19.9 | 19 | 13.5 | 11 | 7.8 |
| > 3 years | 167 | 27 | 16.2 | 17 | 10.2 | 36 | 21.6 |
| Has ever spent time in prison | |||||||
| No | 218 | 35 | 16.1 | 27 | 12.4 | 12 | 5.5 |
| Yes | 263 | 45 | 17.1 | 30 | 11.4 | 50 | 19.0 |
| Missing | 10 | 1 | 1 | 2 | |||
| Illicit drug use | |||||||
| Neither smoked nor injected | 298 | 44 | 14.8 | 27 | 9.1 | 13 | 4.4 |
| Has ever smoked heroin/crack cocaine | 107 | 20 | 18.7 | 14 | 13.1 | 5 | 4.7 |
| Has ever injected drugs | 86 | 17 | 19.8 | 17 | 19.8 | 46 | 53.5 |
| Currently smokes cigarettes | |||||||
| No | 97 | 18 | 18.6 | 10 | 10.3 | 2 | 2.1 |
| Yes | 394 | 63 | 16.0 | 48 | 12.2 | 62 | 15.7 |
| Participant or health worker ever been concerned about drinking | |||||||
| No | 275 | 51 | 18.5 | 30 | 10.9 | 24 | 8.7 |
| Yes | 202 | 28 | 13.9 | 25 | 12.4 | 36 | 17.8 |
| Missing | 14 | 2 | 3 | 4 | |||
Latent tuberculosis infection
We estimated latent tuberculosis infection prevalence at 16.5% (81/491) (95% CI 13.2% to 19.8%). Prevalence was higher in the foreign-born patients (52/186, 28.0%, 95% CI 21.4% to 34.4%) than in the UK-born patients (29/305, 9.5%, 95% CI 6.2% to 12.8%). In the UK-born patients, there was evidence that a history of imprisonment was associated with an increased risk of latent tuberculosis infection [odds ratio (OR) 3.49, 95% CI 1.10 to 11.04; p = 0.018] after adjusting for age, length of time spent homeless and any illicit drug use (Table 3).
| Risk factor | Univariable OR (95% CI) | Multivariable OR (95% CI) | p-valuea |
|---|---|---|---|
| Age (years) | |||
| < 30 | 1.0 | 1.0 | |
| 30–49 | 1.36 (0.61 to 3.07) | 0.69 (0.14 to 3.51) | |
| ≥ 50 | 1.98 (0.86 to 4.53) | 2.04 (0.41 to 10.05) | 0.07 |
| Total time spent homeless | |||
| < 1 year | 1.0 | 1.0 | |
| 1 year | 0.77 (0.31 to 1.91) | 0.32 (0.06 to 1.79) | |
| 2–3 years | 1.24 (0.52 to 2.94) | 0.79 (0.18 to 3.44) | |
| > 3 years | 0.96 (0.41 to 2.29) | 0.82 (0.20 to 3.32) | 0.43 |
| Has ever been to prison | |||
| No | 1.0 | 1.0 | |
| Yes | 1.08 (0.67 to 1.75) | 3.49 (1.10 to 11.04) | 0.018 |
| Illicit drug use | |||
| Neither smoked nor injected | 1.0 | 1.0 | |
| Has ever smoked heroin/crack cocaine | 1.33 (0.74 to 2.37) | 1.44 (0.49 to 4.22) | |
| Has ever injected drugs | 1.42 (0.77 to 2.64) | 2.65 (0.92 to 7.62) | 0.20 |
Blood-borne viruses
Seven out of 489 patients (1.4%, 95% CI 0.4% to 2.5%) had current hepatitis B infection as confirmed by HBsAg neutralisation. In addition, 10.4% (51/489) (95% CI 7.7% to 13.1%) of patients had evidence of past hepatitis B infection. A total of 59.5% (291/489) (95% CI 55.1% to 63.9%) of patients were non-immune to hepatitis B; this was lower for those who had ever injected drugs (27.1%, 23/85) (95% CI 17.4% to 36.7%; Figure 3). Most non-immune individuals (77.7%, 226/291) did not recall whether or not they had been previously vaccinated for hepatitis B, and 29 out of 291 (10.0%) patients reported having never received vaccination. A total of 41.2% of these non-immune individuals (120/291) had spent time in a UK prison. Only four non-immune individuals reported being vaccinated against hepatitis B more than once.
FIGURE 3.
Work package 2 flow diagram. BBV, blood-borne virus; CXR, chest X-ray; LTBI, latent tuberculosis infection.
Among a total of 64 out of 491 (13.0%, 95% CI 10.0% to 16.0%) participants with anti-HCV seropositivity, 51 (10.4%, 95% CI 7.8% to 13.1%) tested positive for hepatitis C RNA, indicating current infection. The remaining 13 participants (2.7%, 95% CI 1.2% to 4.1%) showed confirmed anti-HCV reactivity in the absence of hepatitis C RNA, indicating a resolved infection. Individuals who had ever injected drugs had a much higher prevalence of hepatitis C (46/86, 53.4%, 95% CI 42.4% to 64.3%) than those who reported no injecting history (12/405, 3.0%, 95% CI 1.3% to 4.6%). In those diagnosed with latent tuberculosis infection, the frequency of co-infection with either hepatitis B or hepatitis C (past or current) was 37.0% (95% CI 26.3% to 47.8%), and co-infection with both hepatitis B and hepatitis C (past or current) was 16.2% (95% CI 9.7% to 24.7%).
The prevalence of HIV seropositivity was 1.02% (95% CI 0.1% to 1.9%); all cases were due to HIV-1 and all participants were previously aware of their diagnosis.
Clinical management and outcome
There were 81 individuals who had a positive latent tuberculosis infection test result, none of whom was co-infected with HIV. Three individuals who were diagnosed with latent tuberculosis infection, after March 2012 and the introduction of updated NICE treatment guidelines, were referred to local health services for chemoprophylaxis (Table 4). One participant declined referral, and, at 12 months’ follow-up, the remaining two had disengaged with services and had not started treatment.
| Outcome at 12 months | LTBI positive, n (%) | HBV positive, n (%) | HCV positive, n (%) |
|---|---|---|---|
| Diagnosed and eligible for referral | 3 (100.0) | 7 (100.0) | 51 (100.0) |
| Treatment started | |||
| On treatment | 0 (0) | 0 (0) | 1 (2.0) |
| Completed treatment | 0 (0) | 0 (0) | 1 (2.0) |
| Incomplete treatment | 0 (0) | 0 (0) | 0 (0) |
| Engaged with services, no treatment | |||
| Seen, discharged, no treatment required | 0 (0) | 6 (85.7) | 0 (0) |
| Under review, no treatment at present | 0 (0) | 0 (0) | 19 (29.4) |
| No engagement with services | |||
| DNA, discharged/LFU | 2 (66.6) | 1 (14.3) | 28 (49.0) |
| Declined referral | 1 (33.3) | 0 (0) | 2 (3.9) |
Among participants with a current hepatitis B infection, all seven accepted a referral; six out of seven were seen at least once in specialist services, none of whom was deemed to require immediate antiviral therapy over 12 months following diagnosis.
A total of 96.1% (49/51) of those with current hepatitis C accepted a referral to specialist services but only two initiated treatment (interferon based), with one having completed treatment and one still on treatment at 12 months’ follow-up. A further 19 (37.3%; 19/51) participants were seen at least once over 12 months of follow-up and remained under review in the absence of treatment; 28 (54.9%; 28/51) individuals were lost to follow-up after referral.
Discussion
This study demonstrates a high burden of latent tuberculosis infection and blood-borne virus infections in a London homeless population at levels that are substantially higher than in the general population. For example, the prevalence of latent tuberculosis infection in inflammatory bowel disease patients screened for latent tuberculosis infection before the initiation of anti-tumour necrosis factor alpha (anti-TNFα) therapy in the UK is 1.6% (95% CI 0.2% to 5.7%)16 and the prevalence of hepatitis C infection in the general population is 0.4%. 17
Although the highest latent tuberculosis infection prevalence was in foreign-born participants, around 10% of UK-born patients experiencing homelessness were infected. Prison history increased the risk threefold in UK-born participants. During the study, referral rates for treatment for latent tuberculosis infection were low because of the criteria in operation at the time. Under the most recent (2016) NICE guidelines,11 all those with a positive test aged up to 65 years would be eligible for referral for treatment; therefore, instead of three people (4%; 3/81) being referred, 76 (93.8%; 76/81) would now be eligible.
Significantly higher levels of current and past hepatitis B infection were seen in this study than in the general population (1.4% and 10.4%, respectively). Hepatitis B vaccination was higher in those reporting injecting drug use, possibly as a result of targeted vaccination in this population, but there remained a substantial proportion of this homeless population who were non-immune and who would benefit from vaccination. No patients were initiated on treatment; however, this is not necessarily unexpected given the prolonged clinical assessment (typically two or three appointments spaced out by a few months) that was required before treatment initiation for hepatitis B.
Hepatitis C prevalence was high (13%). This was mainly among those who reported injecting drug use, but even those without such a history had higher levels than the general population. Engagement with health services was poor in those diagnosed with current hepatitis C infection, with just over half of those referred either not attending appointments or being lost to follow-up. In only a minority of those referred was antiviral therapy initiated within 12 months. Until recently, hepatitis C care in general has been characterised by a small number of treatment initiations relative to the number of people needing and accessing care. 17 With the introduction of interferon-free regimens of short duration (typically 12 weeks), there is a new emphasis on increasing treatment coverage, but the impact on this vulnerable population has not yet been formally investigated. In individuals diagnosed with latent tuberculosis infection, co-infection with either hepatitis B or hepatitis C (past or current) was high, at 37.0%, as was co-infection with both hepatitis B and hepatitis C, at 16.2%. The implications of this for latent tuberculosis infection treatment and risk of hepatotoxicity need to be carefully considered.
We used convenience sampling as it was not possible to use a formal sampling framework in a study conducted alongside a very-high-throughput NHS clinical service dealing with complex groups. The requirement for individuals to be able to consent meant that our results do not include individuals who were intoxicated (by drugs or alcohol) and, therefore, are likely to under-represent those at highest risk of blood-borne virus infection. Despite this, the homeless population in this study included a high proportion of previous rough sleepers, people with either current or previous high-risk drug use, and people with harmful and hazardous alcohol use. The population sampled is broadly demographically comparable to homeless populations nationally according to the Find&Treat data collected from extensive screening outside London, and Homeless Link’s (London, UK) health needs audit. 18
To our knowledge, this study provides the first measure of latent tuberculosis infection and blood-borne virus prevalence in people experiencing homelessness in the UK. Previous studies in other high-income countries (including Italy, Japan, South Korea and the USA) have reported latent tuberculosis infection prevalence in homeless populations and found rates varying from 16% to 75.9%. 5,19–22
A meta-analysis of active tuberculosis and blood-borne virus prevalence in homeless populations internationally found that hepatitis C virus (HCV) infection ranged from 3.9% to 36.2%, and HIV infection ranged from 0.3% to 21.1%. 23 None of the studies testing for HIV was conducted in the UK, but one hepatitis C study, which recruited individuals experiencing homelessness from shelters, special projects and medical centres in Oxford, found 26.5% of individuals to be HCV infection positive using oral fluid testing. 24
Low levels of hepatitis B immunity indicate inadequate access to vaccination in this high-risk group. This finding is consistent with our previous work demonstrating the inverse care law with respect to influenza vaccination. 25 Our data demonstrated that the eligibility of people experiencing homelessness for influenza vaccination as a result of clinical risk factors was 38.9%, compared with 13.0% in the general population, but only 23.7% of those eligible were vaccinated compared with national levels of 53.2%. Given the unmet need for hepatitis B vaccination, there is a strong rationale for offering universal provision of hepatitis B vaccination to people experiencing homelessness through existing services engaged with this group. 26 The work also indicates the need to strengthen the prison hepatitis B vaccination programme and vaccination alongside drug treatment services. Individuals who tested HBsAg positive generally maintained links with services after referral, whereas those diagnosed with hepatitis C infection showed suboptimal retention by care services. Further studies are required to determine whether or not expanded availability of interferon-free regimens of short duration will increase engagement in this population.
Since 2010, the number of people seen rough sleeping has doubled nationally. 27,28 People experiencing homelessness represent the extreme end of health inequalities in high-income countries and they experience a high burden of preventable morbidity and mortality from infectious and non-infectious disease. 29,30 We demonstrated the high prevalence of undiagnosed latent tuberculosis infection, hepatitis B infection and hepatitis C infection in homeless populations in the UK, and a large unmet need for hepatitis B vaccination. We also demonstrated the need for intensive case management and ongoing support to ensure that testing can translate into treatment opportunities. High rates of co-infection highlight the importance of service integration through combined testing and treatment pathways. 31 NICE now recommends that persons accessing targeted mobile radiology should be offered tests for blood-borne viruses11 and our data provide the basis to estimate the cost-effectiveness of this approach. The recent national collaborative tuberculosis strategy32 commits to new investment in a national outreach service in line with the proven Find&Treat outreach model. 10
Deviations from the protocol
The protocol for work package 1 and other studies as submitted to NIHR are provided in Report Supplementary Material 1. The key deviation from the protocol was in the numbers of patients screened for latent tuberculosis infection and blood-borne viruses. The original target had been 3000 (1500 in prison and 1500 in homeless hostels). This target number had been based on power calculations that would enable the progression rates from latent to active tuberculosis to be measured with accuracy through data linkage to national surveillance data. We were able to recruit only one-third of this target (491 individuals in homeless hostels and 515 individuals in prison). In the homeless populations, we conducted the screening alongside the mobile radiographic unit, which has a high turnover. It was not possible to approach, consent and take blood samples from a high proportion of eligible participants under this arrangement. As completion of radiographic screening (to help rule out active disease in those with evidence of latent tuberculosis infection) was an eligibility criterion, this limited the recruitment. In addition, it often proved difficult to identify private spaces in which to take blood. Similarly, in the prison we were restricted to screening those who had undergone radiographic screening. This greatly limited numbers as the prison radiographic screening that was due to be delivered by the NHS had major implementation problems, as described in work package 2. As the number of recruits was lower than anticipated, the recruitment number was renegotiated with NIHR as it was agreed that the lower number would still enable accurate measurement of latent tuberculosis infection and blood-borne viruses; however, the second objective of measuring rates of progression through data linkage was abandoned because of insufficient numbers.
Successes/added value
Findings from this study contributed to the NICE public health programme guidelines Identifying and Managing Tuberculosis Among Hard-to-Reach Groups. 33 Citing high rates of infection has, in part, influenced the recommendation to screen people experiencing homelessness for tuberculosis and to refer latent tuberculosis infection cases for further clinical investigation if the individual is aged < 35 years. Our research showing high levels of latent tuberculosis infection in those screened on the mobile radiographic ray unit has led to latent tuberculosis infection screening being commissioned on the mobile radiographic unit to migrants experiencing homelessness across London who are from high-incidence countries and to the commissioning of hepatitis B vaccination on the mobile radiographic unit.
Further work, alongside this study, identified a high unmet need for influenza vaccination among people experiencing homelessness. 25 The mobile radiographic unit has now been commissioned to provide influenza vaccination. Work carried out alongside this study also showed the high prevalence of chronic disease including cardiovascular disease in people experiencing homelessness screened by the mobile radiographic unit. 29,34 This has recently provided support for a successful application for a NIHR programme development grant on Cardiovascular Disease in People Experiencing Homelessness (Chief Investigator – Ami Banerjee). The work also led to a Department of Health and Social Care policy research programme, the HALT Hepatitis Study, a randomised control trial of the use of peers to support full diagnosis and treatment completion for hepatitis B and hepatitis C among socially complex groups (URL: www.isrctn.com/ISRCTN24707359; accessed 25 September 2020). The charity Groundswell (London, UK) is now commissioned in parts of London to support patients experiencing homelessness to attend HCV appointments.
The study also contributed to the successful procurement of a European Union-funded grant on hepatitis C screening in Europe, which is funding hepatitis C screening and portable liver FibroScan® (Echosens, Paris, France) screening alongside the mobile radiographic unit to assess disease severity. 35
Although the objective of data linkage was dropped from this study, the work was used to fund the development of probabilistic data linkage methodologies at PHE. The algorithms developed have been validated by members of our team. 36 They have also been used to link data from the mobile radiographic unit to national tuberculosis surveillance in order to confirm which patients who were screened went on to develop active tuberculosis. This has enabled members of our group to measure the sensitivity and specificity of mobile radiographic screening for tuberculosis26 and to evaluate the accuracy of computer-aided detection of tuberculosis from radiographic images. 37 The Find&Treat team is currently purchasing a licence to allow the use of computer-aided diagnostics technology on its radiographic database. The linkage has also been used to link data from the pre-entry migrant screening programme to national tuberculosis surveillance data, enabling the measurement of the incidence of tuberculosis post migration. This was published in The Lancet38 and the lead author (RW Aldridge) based a successful Wellcome Trust (London, UK) postdoctoral fellowship application on this approach.
Work package 2: evaluation of an NHS prison screening programme for active tuberculosis, and survey of latent tuberculosis infection and blood-borne virus prevalence in prisoners
Our study findings relating to hepatitis C in prisoners have previously been published. 39
Background/rationale
Prison populations are at an increased risk of infectious diseases including tuberculosis and blood-borne viruses. 40 In London, a large continued outbreak of drug-resistant tuberculosis has had many cases that are linked to imprisonment and substance misuse,41 and research using a mobile radiographic unit for intermittent screening in prisons found a rate of tuberculosis of > 208.4 per 100,000 prisoners. 3 Prisons have disproportionally large numbers of people who are infected with HIV,42 involved in substance misuse,42–44 homeless,45 and with known clinical and social risk factors for infection and progression to active disease. Prisoners with tuberculosis are also more likely to have infectious pulmonary disease, drug resistance and poor treatment outcomes. 46 The prevalence of HIV and hepatitis C among prisoners is also higher than in the general population, and prisoners are among the most vulnerable population to contract hepatitis B virus (HBV) in the UK. 47 In the UK, unlinked anonymous blood-borne virus monitoring showed that 10.1% of those in specialist drug services and 9.4% of those in prisons tested positive for hepatitis C, compared with 2.2% overall in primary care in 2013. 48 Of all hepatitis C diagnoses, 90% are associated with injecting drug use, and 40% of those who are injecting drugs are estimated to be living with hepatitis C infection. 49 The prevalence of HIV in those injecting drugs remains low, at 1.1%, and HBV prevalence has been continuously decreasing (now 17%). This has been attributed to the HBV vaccination programme in specialist drug services and in prisons, where 75% of individuals report having received HBV vaccination.
The National Institute for Health and Care Excellence recommends that prisons and immigration removal centres should have a tuberculosis liaison lead and a tuberculosis policy that is developed in conjunction with tuberculosis services and Public Health England. In prisons with Department of Health and Social Care-funded static digital radiographic facilities for tuberculosis screening, all new prisoners, detainees and transfers should have a chest radiograph within 48 hours of arrival unless they have had a chest radiograph in the last 6 months. 33
In addition, NICE advises that prisons in high-incidence areas, or which receive prisoners from high-incidence areas, offer latent tuberculosis screening [interferon gamma release assay (using IGRA)] to prisoners who are aged < 35 years and in regular contact with support services, and where continued support is provided on release, including directly observed preventative treatment (DOPT). 33
This part of the guidance has not been widely implemented. Little is known about the prevalence of latent tuberculosis infection in prisons. No such studies have been conducted in the UK, although a Cochrane Review found that the average incidence rate of latent tuberculosis infection in prisons in Brazil and the USA was 26.4 times that of the general population. 40 The co-infection rate with latent tuberculosis infection and blood-borne viruses also needs to be established because of the potentially increased risk of hepatotoxicity in the treatment of latent tuberculosis infection with pre-existing liver disease. Furthermore, DOT is currently provided to, on average, only 60% of eligible patients (in London) with active tuberculosis,50 and the impact on health-care services of adding DOPT for prisoners who are released on preventative treatment needs to be assessed.
This study aimed to evaluate the effectiveness of a new prison radiographic screening programme using a teleradiology network of static digital radiographic units to reduce the risk from tuberculosis in prisons, and to determine the prevalence of latent tuberculosis infection and co-infection with blood-borne viruses as well as outcomes of referrals for onward care.
Methods
The primary objectives were to evaluate the effectiveness of a new prison radiographic screening programme using a teleradiology network of static digital radiographic units to reduce the risk from tuberculosis to those in prisons, and to determine the prevalence of latent tuberculosis, HIV, hepatitis B and hepatitis C infection among prisoners in London, with clinical outcomes recorded for participants referred to health-care services. Secondary objectives were to determine interobserver reliability for the interpretation of chest radiographs, and the prevalence of co-infection with latent tuberculosis, HIV, and hepatitis B and hepatitis C in prisoners in London and how this varied according to demographics and lifestyle risk factors such as alcohol and drug use.
Study design
This was an evaluation of a chest radiographic tuberculosis screening programme, and a survey of infection with latent tuberculosis, hepatitis B, hepatitis C and HIV among participants who had undergone chest radiographic screening. The study was conducted over a 6-month period between 7 January 2013 and 28 June 2013.
Ethics approval was obtained from the Offender Health and East of England – Essex National Research Ethics Service (committee number 10/H0302/51).
Setting
The setting was a male London prison with a static digital radiographic facility for tuberculosis screening. The operational capacity was 1200 prisoners, and there was a high turnover rate. The radiography machine was situated on the admissions wing, where most prisoners, with the exception of substance misusers, initially stay when they arrive in the prison, and it was used for routine radiography but not for tuberculosis screening. To assist the start of the tuberculosis screening programme, three alternating radiographers from the local NHS trust were engaged to perform radiography. The screening was conducted as 3-hour sessions 5 days per week, either in the morning or in the afternoon.
Participants for evaluation of chest radiographic screening
All new prisoners who had not had a chest radiograph in the last 6 months were eligible to participate. Other prisoners on the admissions wing were asked when possible. New prisoners arrived in the evening, and some were screened on arrival. Most new prisoners were identified from the daily admissions lists the following day. Those located on the admissions wing who were available and agreed to be screened were escorted to the radiography room by the study team. Prisoners on the substance misuse wing had to be escorted to the radiography room by a prison officer. Other prisoners were approached and offered radiography by the study team when possible.
Participants for evaluation of survey of latent tuberculosis infection and blood-borne viruses
All prisoners who had received a chest radiograph with the tuberculosis screening programme were eligible to participate in the latent tuberculosis infection/blood-borne virus screening. Following the radiography, interest in participating was noted on the radiography list. Prisoners located on the admissions wing were consented and screened immediately, when possible, or encouraged to present themselves to the study clinic during free-flow (free time). The study team also knocked on cell doors on the admissions wing and on the substance misuse wing and requested that officers unlock the cells of interested prisoners to allow them to participate. Some participants were booked into a weekly phlebotomy clinic and were escorted by prison officers.
Evaluation of chest radiographic screening
Chest radiographs were reviewed by a team of radiologists at a local NHS trust, which did not use a standard approach for reporting or categorising radiographs. Reports were e-mailed to the prison health-care medical administrator within 24 hours, printed out by the medical secretary at the prison and given to the general practitioner (GP) for review. A list of all chest radiographs with results that were indicated as negative (i.e. no abnormality detected) or positive (i.e. abnormality detected) was also sent to the research team on a monthly basis. Those prisoners with radiographs that were reported or interpreted by the prison GP as needing follow-up were offered appointments in the prison GP clinic, or were referred for advice or review as decided by the GP.
Individual consent was not sought for the study evaluating the NHS radiographic programme because anonymised data were provided for analysis. For prisoners with an abnormal chest radiograph who required follow-up, additional data on outcomes and actions taken by the prison health-care team while the individuals were inmates were provided.
As part of the evaluation of the chest radiographic tuberculosis screening and to facilitate the classification of chest radiographs, a second reading of all chest radiographs was subsequently performed by an independent consultant chest physician with extensive experience in chest radiographic screening. No clinical information was available and the reviewer was blinded to previous reports and repeat chest radiography. All personal identifiers were removed for the second reading and radiographs were reviewed using the prisoner number and XRIS (radiography) number only. The chest radiographs were categorised using the American Thoracic Society (ATS) classification system, with abnormalities defined as typically associated with active or inactive tuberculosis. This classification is currently used for the reporting of chest radiographs by the pan-London Find&Treat mobile radiographic unit tuberculosis screening service.
The reports from the second reading were then compared with the initial reports to assess interobserver reliability. A list of chest radiographs initially reported as normal or not requiring follow-up but subsequently thought to be suggestive of active tuberculosis by the second reader was sent to the prison health service with template letters to participants and their GPs requesting a medical review to exclude active tuberculosis. Participants with previous consent from the latent tuberculosis infection screening were referred directly to NHS health services by the study team when possible. The participants were also referred to the Find&Treat tuberculosis outreach service to encourage attendance.
Survey of latent tuberculosis infection and blood-borne viruses
All prisoners who had received a chest radiograph were approached to test for latent tuberculosis infection and blood-borne viruses. Informed consent was requested and, if provided, a questionnaire with sociodemographic information and self-reported risk factors for tuberculosis was completed by a study nurse. These were developed in conjunction with prisoners, and piloted and modified at the start of the study following feedback from participants. Data collected included self-reported age, country of birth, history and length of imprisonment, current and past history of substance misuse, smoking and homelessness. Those with a planned release date within 2 weeks of the screening were asked to provide contact details in the community. A whole venous blood sample was then collected for latent tuberculosis (QuantiFERON-TB Gold In-Tube gamma interferon releasing assay), HIV-1 and HIV-2 antibodies, hepatitis C (HCV-IgG, HCV-RNA) and hepatitis B (HBsAg, anti-HBs, anti-HBc – see work package 1 for further details). A phlebotomy clinic was run weekly for consented prisoners where a blood sample could not be taken on the day, and, as needed, on the substance misuse wing to maximise uptake. A link was set up with the prison information technology system and latent tuberculosis and blood-borne virus results were fed into the prisoner’s medical record. Results were also sent to the study team via e-mail. Positive blood-borne virus results were telephoned through directly to the study team by the virology department. Positive results were given in person to prisoners within 2 weeks by the study team for latent tuberculosis infection, or by the prison GP for blood-borne viruses, together with a letter explaining the results. Negative results were fed back only on request.
Based on previous studies of the prevalence of latent tuberculosis infection in high-income countries’ prison populations, we expected a minimum of 15% of participants to test positive for latent tuberculosis infection;51–55 therefore, we powered the study to measure a 15% prevalence of latent infection with 95% CIs of 12% to 18%, which required 500 participants.
For participants with a positive IGRA test who were aged < 35 years, a referral was made to the local tuberculosis clinic for prophylactic treatment as per NICE guidance. Those with HIV and latent tuberculosis infection co-infection were referred directly to joint local HIV/tuberculosis services, irrespective of age. Participants with hepatitis B, hepatitis C and HIV infection were referred to local health services depending on existing local arrangements, or asked to contact their GP to make a referral upon their release (hepatitis B and hepatitis C only).
Referral and treatment outcomes for positive cases were collated from local NHS services by study nurses at 1 year post the screening date. All study data were collected on paper forms, and then entered onto Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) 2010 spreadsheets and Microsoft Access® (Microsoft Corporation, Redmond, WA, USA) databases by the research database manager. The final data set was cleaned by the study statistician. Interobserver reliability between the hospital trust radiology department and the secondary reader for the radiographs was evaluated using Cohen’s kappa statistic. For the latent tuberculosis infection prevalence study, results were analysed using Stata version 13. A descriptive analysis of baseline variables and their association with primary and secondary outcomes was performed. Infection prevalence is presented according to exposure variables, including history of homelessness, history of drug and alcohol use and country of birth.
Results
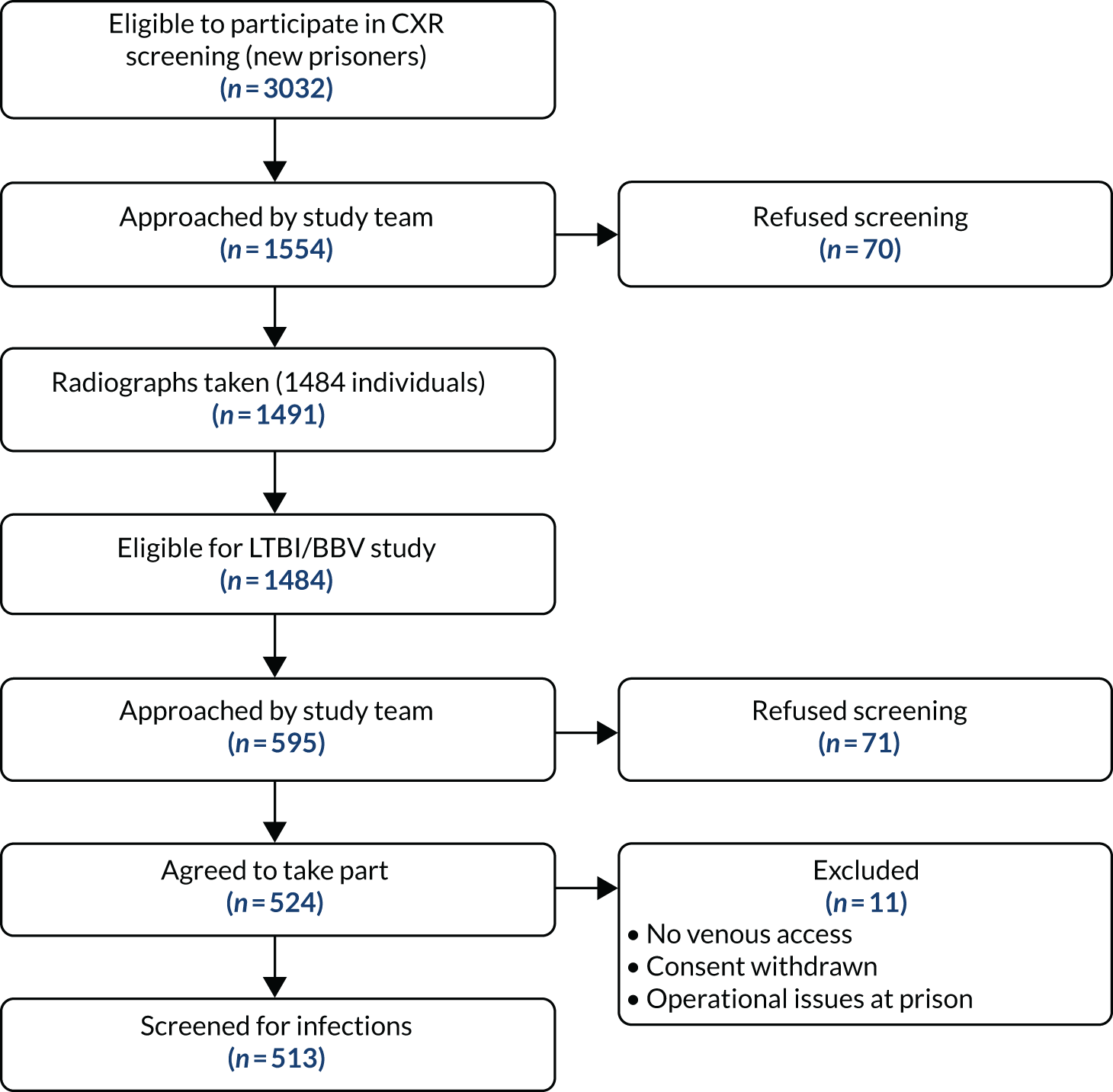
A total of 1491 chest radiographs were taken for 1484 individuals. There were 3032 new arrivals during the study, of whom 618 (20%) arrived in the substance misuse wing. The overall screening coverage of new prisoners was 43% (1302/3032). The vast majority of radiographs that were taken were from new arrivals (87%), 1242 (84%) were from prisoners on the admission wing, 158 (11%) were from the substance misuse wing and 91 (6%) were from other wings. The total number of active screening days was 112, with an average of 13 radiographs per day.
From the initial reporting by the NHS trust radiology department, 141 out of 1491 (9%) radiographs showed some kind of abnormality (see Figure 3). Ninety-seven (69%) of these were acted on by the prison health-care team, including 29 prisoners (29/141, 20%) who had further investigations for tuberculosis. Of the 29 suspected tuberculosis cases, 15 (52%) had one or more sputum sent off for acid-fast bacillus smear and culture and sensitivity. Of these, two had a positive smear result for acid-fast bacillus; none was culture positive for Mycobacterium tuberculosis but one prisoner started tuberculosis treatment and completed a full course of antibiotics under DOT in the prison (see Figure 3). This translates into a tuberculosis rate of 67 per 100,000 prisoners. Of the other 14 cases, one was referred to the tuberculosis clinic for prophylactic treatment, three were reviewed and discharged by the prison GP, three had a computerised tomography scan (from which two cases did not suggest tuberculosis and were discharged and one case was referred to the chronic obstructive pulmonary disease nurse), three had a history of tuberculosis or atypical tuberculosis and required no action, two were lost to follow-up and two had no interventions initiated.
From the secondary reporting, 56 chest radiographs (49 individuals) were reported as suggestive of active tuberculosis. When compared with the initial reporting, 14 of the 56 radiographs (or 49 individuals) had been identified as suggestive of tuberculosis in both reports and 42 radiographs (or 35 individuals) had not been identified as suggestive of tuberculosis in the initial reporting (Table 5). The NHS trust radiology reporters classified 1.9% of chest radiographs as potentially indicating active tuberculosis, whereas the Find&Treat reporter classified 3.8% as active tuberculosis. The inter-rater reliability for suspicion of active tuberculosis was fair (κ = 0.312, 95% CI 0.181 to 0.442).
| Result of second reading | NHS trust radiology – reading suggestive of active tuberculosis? | ||
|---|---|---|---|
| Yes | No | ||
| Suggestive of active tuberculosis? | Yes | 14 | 42 |
| No | 15 | 1420 | |
A total of 15 of the 35 individuals who had not initially been identified as having concerning radiographs had also consented to and participated in the latent tuberculosis infection screening and were referred to local chest clinics. Of these individuals, six were discharged following a review or repeat chest radiography showing no changes, seven had an unknown outcome and two were lost to follow-up. For the 15 participants who were to be contacted by the prison health-care team, no outcome data were available.
Of the 1484 prisoners screened with a chest radiograph, 595 prisoners were approached by the research team and 88% of them consented to participate in the study. The final analysis included 513 individuals (see Figure 3 for full details).
All participants were male, and the majority were born in the UK (336/513, 66%) and aged 16–39 years (404/513, 79%) (Table 6). Just over half (274/506, 54%) of participants had a history of homelessness, slept rough, sofa surfed, squatted or stayed in a homeless hostel, and 16% (81/513) had slept at a place where people used illicit drugs. The vast majority (408/513, 80%) were current smokers and around one-third (170/513, 31%) had smoked crack cocaine or heroin [with crack cocaine more commonly used (160/513, 31%) than heroin (99/513, 19%)]. Injecting drug use was less frequent (40/513, 8%), and heroin use (39/513, 8%) was higher than crack cocaine use (26/513, 5%). Four per cent of participants (21/513) reported having shared needles.
| Demographic information and social risk factors | Totala | QuantiFERON result | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| N | n | % | n | % | |
| All | 513 | 454 | 88.5 | 59 | 11.5 |
| Age category (years) | |||||
| 16–29 | 263 | 242 | 92 | 21 | 8 |
| 30–39 | 141 | 122 | 86.5 | 19 | 13.5 |
| 40–49 | 79 | 68 | 86.1 | 11 | 13.9 |
| 50–59 | 24 | 17 | 70.8 | 7 | 29.2 |
| ≥ 60 | 6 | 5 | 83.3 | 1 | 16.7 |
| Total | 511 | 454 | 88.5 | 59 | 11.5 |
| Born in the UK | |||||
| UK | 336 | 315 | 93.8 | 21 | 6.3 |
| Non-UK | 177 | 139 | 78.5 | 38 | 21.5 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has spent time living in a hostel | |||||
| No | 347 | 307 | 88.5 | 40 | 11.5 |
| Yes | 163 | 144 | 88.3 | 19 | 11.7 |
| Total | 510 | 451 | 88.4 | 59 | 11.6 |
| Participant has spent time squatting or sofa surfing | |||||
| No | 341 | 298 | 87.4 | 43 | 12.6 |
| Yes | 170 | 154 | 90.6 | 16 | 9.4 |
| Total | 511 | 452 | 88.5 | 59 | 11.5 |
| Participant has slept in a location where people purchase or use drugs | |||||
| No | 432 | 381 | 88.2 | 51 | 11.8 |
| Yes | 81 | 73 | 90.1 | 8 | 9.9 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has ever been homeless (slept rough/sofa/squat/hostel) | |||||
| No | 232 | 205 | 88.4 | 27 | 11.6 |
| Yes | 274 | 244 | 89.1 | 30 | 10.9 |
| Total | 506 | 449 | 88.7 | 57 | 11.3 |
| Participant spent time in prison in the UK | |||||
| No | 2 | 2 | 100 | 0 | 0 |
| Yes | 511 | 452 | 88.5 | 59 | 11.5 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant spent time in prison outside the UK | |||||
| No | 482 | 428 | 88.8 | 54 | 11.2 |
| Yes | 28 | 23 | 82.1 | 5 | 17.9 |
| Total | 510 | 451 | 88.4 | 59 | 11.6 |
| Participant has ever spent time in prison | |||||
| No | 1 | 1 | 100 | 0 | 0 |
| Yes | 511 | 452 | 88.5 | 59 | 11.5 |
| Total | 512 | 453 | 88.5 | 59 | 11.5 |
| Participant has injected heroin | |||||
| No | 474 | 420 | 88.6 | 54 | 11.4 |
| Yes | 39 | 34 | 87.2 | 5 | 12.8 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has injected crack cocaine | |||||
| No | 487 | 430 | 88.3 | 57 | 11.7 |
| Yes | 26 | 24 | 92.3 | 2 | 7.7 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has smoked heroin | |||||
| No | 414 | 368 | 88.9 | 46 | 11.1 |
| Yes | 99 | 86 | 86.9 | 13 | 13.1 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has smoked crack cocaine | |||||
| No | 353 | 311 | 88.1 | 42 | 11.9 |
| Yes | 160 | 143 | 89.4 | 17 | 10.6 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has shared needles | |||||
| No | 492 | 434 | 88.2 | 58 | 11.8 |
| Yes | 21 | 20 | 95.2 | 1 | 4.8 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has ever injected drugs | |||||
| No | 473 | 419 | 88.6 | 54 | 11.4 |
| Yes | 40 | 35 | 87.5 | 5 | 12.5 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has smoked heroin or crack cocaine | |||||
| No | 343 | 303 | 88.3 | 40 | 11.7 |
| Yes | 170 | 151 | 88.8 | 19 | 11.2 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant has slept in a location where people purchase or use drugs and if so frequency | |||||
| Never | 432 | 381 | 88.2 | 51 | 11.8 |
| 1–9 times | 27 | 26 | 96.3 | 1 | 3.7 |
| ≥ 10 times | 54 | 47 | 87 | 7 | 13 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant takes drugs | |||||
| No drugs | 341 | 302 | 88.6 | 39 | 11.4 |
| Smokes hard drugs (does not inject) | 132 | 117 | 88.6 | 15 | 11.4 |
| Injects | 40 | 35 | 87.5 | 5 | 12.5 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant smokes cigarettes | |||||
| No | 105 | 90 | 85.7 | 15 | 14.3 |
| Yes | 408 | 364 | 89.2 | 44 | 10.8 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
| Participant or a health worker have ever been concerned about participant’s drinking or suggested they cut down | |||||
| No | 396 | 347 | 87.6 | 49 | 12.4 |
| Yes | 112 | 102 | 91.1 | 10 | 8.9 |
| Total | 508 | 449 | 88.4 | 59 | 11.6 |
| Participant has undertaken a treatment programme for alcohol | |||||
| No | 466 | 414 | 88.8 | 52 | 11.2 |
| Yes | 47 | 40 | 85.1 | 7 | 14.9 |
| Total | 513 | 454 | 88.5 | 59 | 11.5 |
The estimated overall prevalence of latent tuberculosis infection was 13% (65/513); among this group 5% (3/65) of participants were co-infected with HCV and 6% (4/65) were co-infected with HBV. Univariable analysis demonstrated higher rates of latent tuberculosis infection in non-UK-born participants than in UK-born participants (21.5% vs. 6.3%), and in those aged 30–39 years than in those aged 16–29 years (13.5% vs. 8%).
Thirty-seven participants with a positive IGRA result were eligible for preventative treatment and referred to the local hospital chest clinic. A total of 40.5% (15/37) of participants started preventative treatment, of whom 60% (9/15) completed their treatment course and 40% (6/15) had incomplete treatment. The remaining 22/37 (59.5%) were lost to follow-up or discharged as they did not attend appointments. Prevalence of current hepatitis C was 4% (22 individuals), which in 45% of cases (10/22) was a new infection. None of these individuals was referred to a hepatitis clinic, half (5/10) were seen by a prison or community GP and the other half were lost to follow-up. Of the 12 participants with known hepatitis C infection, the majority (58%) were lost to follow-up or discharged because of non-attendance, and 5 out of 12 (42%) were under medical review by their GP or the hepatitis clinic. None of the 22 participants started hepatitis C treatment.
Prevalence of current hepatitis B was low (2%), and 70% (7/10) of the individuals with current hepatitis B had new or previously unknown infections, of whom 43% were seen by prison GPs or community GPs and 57% were lost to follow-up or discharged because of non-attendance. Of the three individuals with known HBV infection, one was referred to a hepatitis clinic, one was deported before referral and one had no interventions. Sixty-five per cent of all participants had insufficient or no immunity to HBV, and none had HIV infection.
Discussion
Our study of the tuberculosis radiographic screening programme found a tuberculosis rate of 67 per 100,000 prisoners but, as this is based on a single case, CIs are wide. In combination with the high prevalence of latent tuberculosis, and compared with the London rate of 35.5 per 100,000 Londoners,57 this indicates that prisoners remain at high risk of tuberculosis in the UK.
The lack of classification system in the initial reporting meant that radiographs were reported in a variety of ways by the different radiologists from the NHS trust. As a consequence of this, we changed a secondary objective from looking at the outcome of radiographs reported as suggestive of tuberculosis to looking at the outcome of radiographs where investigations for tuberculosis were instigated by the prison.
Prisoners were out of their cells for 3.5 hours per day and screening competed with paid prison work, attending courses that could lead to transfer to a lower-category prison or earlier release on probation, exercise and social visits. Access outside these times was very limited. During lockdown (security alert or staff meetings), no movement among prisoners was allowed, which had a further impact on the screening uptake; this, and restricted operational capacity within the research team, meant that we were unable to approach all eligible participants in the studies. Owing to the study setting, a sampling framework was not possible for recruiting patients, and we used convenience sampling.
We consider that the sample size obtained in both studies was a strength in our research. The prison population is often considered challenging to engage with, and our research nurses had extensive experience of working with prisoners as well as substance misusers. We found that both chest radiography and latent tuberculosis infection/blood-borne virus screening were highly acceptable among prisoners. We were also able to collect individual biological data, including information on self-reported clinical and social risk factors for tuberculosis, on all participants in the latent tuberculosis infection screening. These questionnaires were developed with prisoners, piloted and altered following user feedback at the start of the study. A limitation of the research was that the questionnaires were not validated for risk factors for prisoners. We used robust data collection methods, and obtained a high level of clinical outcome data.
The levels of hepatitis C and hepatitis B were lower than expected. This is probably explained by the small number of substance misusers (those with the highest risk of tuberculosis) who participated in the study as a result of the remote location of the chest radiography equipment, which was a prerequisite for the latent tuberculosis infection/blood-borne virus screening.
Many other studies have reported rates of tuberculosis in prisons. In London, sporadic radiographic screening in prisons found a rate of 208 per 100,000 prisoners. A survey from the WHO European region58 found a median notification rate of 232 (range 0–17,808) per 100,000 prisoners. Although the type and timing of screening varied, most countries performed radiographic screening at prison entry. 58 The cost-effectiveness of chest radiographic screening for this high-risk population has also been demonstrated. 59–61
We are not aware of any published data on tuberculosis and blood-borne virus co-infection among UK prisoners. A study of tuberculosis and blood-borne viruses in a male prison in Pakistan demonstrated higher prevalence of HIV, HBV and hepatitis C (2%, 5.9% and 15.2%, respectively), but also a higher prevalence of illicit drug use and slightly more injecting drug use. 62 Other prevalence studies on sexually transmitted infections and blood-borne viruses found rates of hepatitis C between 4.9% and 29.7%, HIV of 0% and 6.6% and HBV of 2.4% and 25.2%. 63–65
Other studies on latent tuberculosis infection prevalence in prisons have been carried out in various countries, although, to our knowledge, not in a UK setting. Latent tuberculosis infection rates vary considerably: 17% in the USA,15 40.3–53.3% in Spain,66 48% in Pakistan,67 49% in Brazil and 87.6–88.8% in Malaysia. 68,69 Comparisons with our study results are challenging because of the heterogeneity between the studies including the different tests used for latent tuberculosis infection, varying background rates of tuberculosis, prevalence of HIV infection, eligibility criteria and levels of uptake. Chan et al. 70 used QuantiFERON IGRA to assess the lifelong effect of bacillus Calmette–Guérin (BCG) vaccination and found a latent tuberculosis infection prevalence of 25% in Taiwan.
We found a continued high rate of active tuberculosis (albeit with wide CIs). Although lower than previous London estimates, this still supports the use of systematic screening for active tuberculosis among prisoners, although how this should be arranged needs further development to maximise uptake including in the most vulnerable groups, such as substance misusers. Funding for training and allocation of prison staff to carry out regular radiographic screening need to be provided, and the timing of radiography considered. Screening prisoners immediately on arrival may maximise uptake before prisoners are allocated to different wings. Another option would be to offer chest radiographic screening as an opt-out part of the ‘next-day’ health screening offered to all new prisoners. For future large-scale tuberculosis chest radiographic screenings in a prison setting, we would recommend using an established classification for the reporting of chest radiographs, such as the American Thoracic Society or the Public Health England pre-departure tuberculosis screening, to enable follow-up, analysis and comparison with other studies.
Our study also demonstrated previously unknown high levels of latent tuberculosis infection and blood-borne virus, as well as low levels of HBV vaccination despite a target vaccination programme. This indicates a high level of unmet need and highlights the inequalities in health seen within this population. The prison setting with DOT facilities provides an excellent opportunity to treat individuals with latent tuberculosis who have social risk factors, such as homelessness and substance misuse, that frequently prevent them from being offered latent tuberculosis infection treatment in the community. However, our study demonstrates extremely poor outcomes across all three infections for those referred to NHS health services following a positive result. This may partly be explained by the nature of prison services: a high and fast turnover of prisoners with limited notice, complicated follow-up and onward referral to local NHS services, and incomplete contact details in health-care records, which meant that some prisoners did not receive their results and missed out on onward care upon release. New and better performance indicators are required to improve patient pathways through the prison; however, improved co-ordination of services and follow-up support in the community is also essential to ensure continued care and treatment completion upon release. Alternative models using multidisciplinary teams and appointment sites acceptable to patients have been trialled for hepatitis C71–73 and have improved outcomes for tuberculosis in London, which has been recognised by NICE. 10,26 Further research and health and social care service development are required to improve health outcomes and address the wider determinants of health in this population.
Deviations from the protocol
The planned work had been based on the assumption that the prison would start a routine radiographic screening service; as this did not happen, we provided radiographic resources to the prison to undertake screening. This reduced the numbers going through screening, preventing full evaluation. Owing to the small numbers, the planned linkage to surveillance data was abandoned as only one active tuberculosis case was identified. Participants were recruited into the study of latent tuberculosis infection and blood-borne viruses only if they had undergone radiographic screening, which also limited recruitment.
Work package 3: peer educators to increase uptake of mobile radiographic screening for tuberculosis in homeless hostels
Our study findings relating to the trial of peer educators to increase the uptake of mobile radiographic screening have previously been published. 74 Parts of this section have been reproduced or adapted from Aldridge et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0. The text includes minor additions and formatting changes to the original text.
Introduction
Tuberculosis rates in London are among the highest in Europe, and account for nearly 40% of UK cases. 75,76 Rates are highest in so-called ‘hard-to-reach’ groups (people experiencing homelessness, prisoners and drug users),3 many of whom have infectious tuberculosis, delays to diagnosis, poor adherence to treatment and high levels of loss to follow-up before treatment completion. 3 Congregate living and reluctance to engage with services complicate attempts to identify active and latent tuberculosis in these populations. 77
The pan-London Find&Treat tuberculosis service includes a mobile digital radiographic unit and has been shown to be cost-effective among hard-to-reach groups (people experiencing homelessness, substance misusers and prisoners). 10 Mobile radiographic unit screening can detect tuberculosis early, often before people become infectious. 26,78 Historical data (Dr Alistair Story, Find&Treat, 2019, personal communication) show low uptake rates in eligible individuals resident in homeless hostels (around 50%).
Increased screening uptake has the potential to increase cost-effectiveness, reduce the transmission of tuberculosis and improve the health of this vulnerable population. Peer educators (with lived experience of homelessness, problem drug or alcohol use and tuberculosis) may be able to engage more effectively with those experiencing these issues than health service staff. Peer educators have been shown to improve knowledge about health conditions and increase the use of health services in several areas including HIV, smoking and condom use. 79,80 Previous qualitative research has found that volunteer peers working alongside tuberculosis services derive value from their involvement with the service and the opportunities this provides for meaningful, structured activity. 81
Using a CRCT, we aimed to compare current practice in central London (where Find&Treat and hostel staff encourage people experiencing homelessness to be screened for tuberculosis) with the use of peer educators who have direct experience of tuberculosis and homelessness.
Methods
Setting
The setting was London hostels for people experiencing homelessness targeted for mobile radiographic screening by Find&Treat between February 2012 and October 2013.
Inclusion criteria
All homeless hostels in London taking part in mobile radiographic unit screening for active pulmonary tuberculosis run by Find&Treat were eligible for inclusion in the study if they had taken part in two previous screening sessions and had not been screened in the 6 months prior to the scheduled mobile radiographic unit screening session.
Exclusion criteria
People experiencing homelessness screened at projects such as day centres and drug and alcohol recovery projects, and street populations accessing soup kitchens (where the denominator population is unknown) were excluded. Hostels with a previous uptake of > 80% were excluded as it was felt that peers would be unlikely to further improve screening uptake.
Usual practice
Find&Treat staff were present on the mobile radiographic unit to encourage uptake and manage onward referrals for suspected cases of active tuberculosis (usual practice for Find&Treat).
Intervention
Peer educators were recruited via tuberculosis clinics in London or from the Find&Treat service where staff identified, or were approached by, interested service users. Training was provided through a 3-day training session run by Groundswell, the research team and Find&Treat. Training covered tuberculosis transmission, tuberculosis risk groups, tuberculosis treatment, the importance of screening, how to maximise screening uptake (based on the past experiences of Find&Treat staff) and the additional support available for those undergoing screening. Peer educators also underwent a period of shadowing an existing peer educator to learn how to increase screening uptake. Ongoing support for peers was provided by Groundswell, a registered charity that exists to enable homeless and vulnerable people to take more control of their lives, have a greater influence on services and play a fuller role in the community. 82
On the day of screening, peers introduced themselves to the hostel staff and agreed a work plan. Peers then moved around the hostel according to the agreed plan of work, knocked on residents’ doors (in conjunction with hostel staff), spoke to residents in all communal areas and those available close to the hostel location (next to the mobile radiographic unit) to encourage them to take up the offer of screening. Mobile radiographic unit staff were available in the mobile radiographic unit to encourage uptake as per usual practice.
Additional interventions
Hostel managers were invited to a meeting hosted by Find&Treat to explain the purpose of the study and obtain their agreement and consent for participation in the study. Hostel managers who were unable to attend the meeting were contacted by e-mail and telephoned when necessary. Hostel managers were reassured that the study was evaluating a peer intervention and not individual hostel performance.
Primary outcome
The primary outcome was the proportion of those resident at the hostel who were screened for active tuberculosis in the mobile radiographic unit. The number of residents eligible for screening at each hostel was determined from bed lists at each site, which took account of the number of residents actually staying at a venue overnight and excluded persons who were absent on the day of screening as a result of hospital admission, arrest or their whereabouts being unknown. Outcome data were collected at an aggregate (hostel) level, with no collection of individual-level data.
Power
The intracluster correlation coefficient for this study was estimated at 0.08 based on previous service data. This produced an inflation factor of 6.52, assuming an average hostel size of 70 residents. Subsequent to funding and prior to trial initiation, a decrease in average hostel size across London reduced the average size to around 50 residents. Consequently, the inflation factor was recalculated as 4.84. To detect a 15% difference in screening uptake in the two groups (60% vs. 75%) in an individually randomised controlled trial with 90% power at the 5% significance level would require 216 individuals in each arm. Applying the 4.84-fold inflation factor for the clustered randomised design required at least 1045 individuals, or approximately 21 hostels in each arm.
Randomisation
The unit of randomisation was hostel screening venue. A cluster randomised design was chosen because the intervention was aimed at the hostel sites rather than at individual clients. Randomisation was carried out by the study research team using a master list of hostels. Sites were randomised to the intervention group or the control group using the internet-based service Sealed Envelope™ (Sealed Envelope Ltd, London, UK), which ensured allocation concealment until the interventions were assigned. To ensure comparability between the intervention arm and the control arm, hostels were stratified on the basis of their size (binary variable indicating whether or not hostels had > 43 beds – the median size) and previous screening uptake level (binary variable indicating whether or not hostels had > 50% historical uptake).
Blinding
Blinding of participants and observers was not possible. The study statistician (RWA) conducted analysis blind to the allocation of intervention arm or control arm.
Poisson regression analysis was used to analyse outcome events at screening hostels with robust standard errors to account for clustering at the hostel level. Bed occupancy level was included as the exposure variable, screening uptake as the outcome (or indicator) variable, and hostel venue as a random effect to account for clustering at each site. The analysis was adjusted by inclusion of the randomisation stratification factors of historical uptake rates and bed numbers.
Data were analysed in Stata version 12.
The study had ethics approval from East of England – Essex National Research Ethics Service (committee number 10/H0302/5). The study trial registration number is ISRCTN17270334.
Results
There were 59 hostels considered; 12 were excluded because of uptake rates of > 80% and one was excluded because it would not allow peers into the venue. Forty-six hostels with a total of 2342 residents were randomised, 1192 in control hostels and 1150 in intervention hostels (Figure 4). Randomisation appeared well balanced (chi-squared p-value > 0.47 for all baseline characteristics; Table 7).
FIGURE 4.
Work package 3 flow diagram. ITT, intention to treat. Reproduced from Aldridge et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
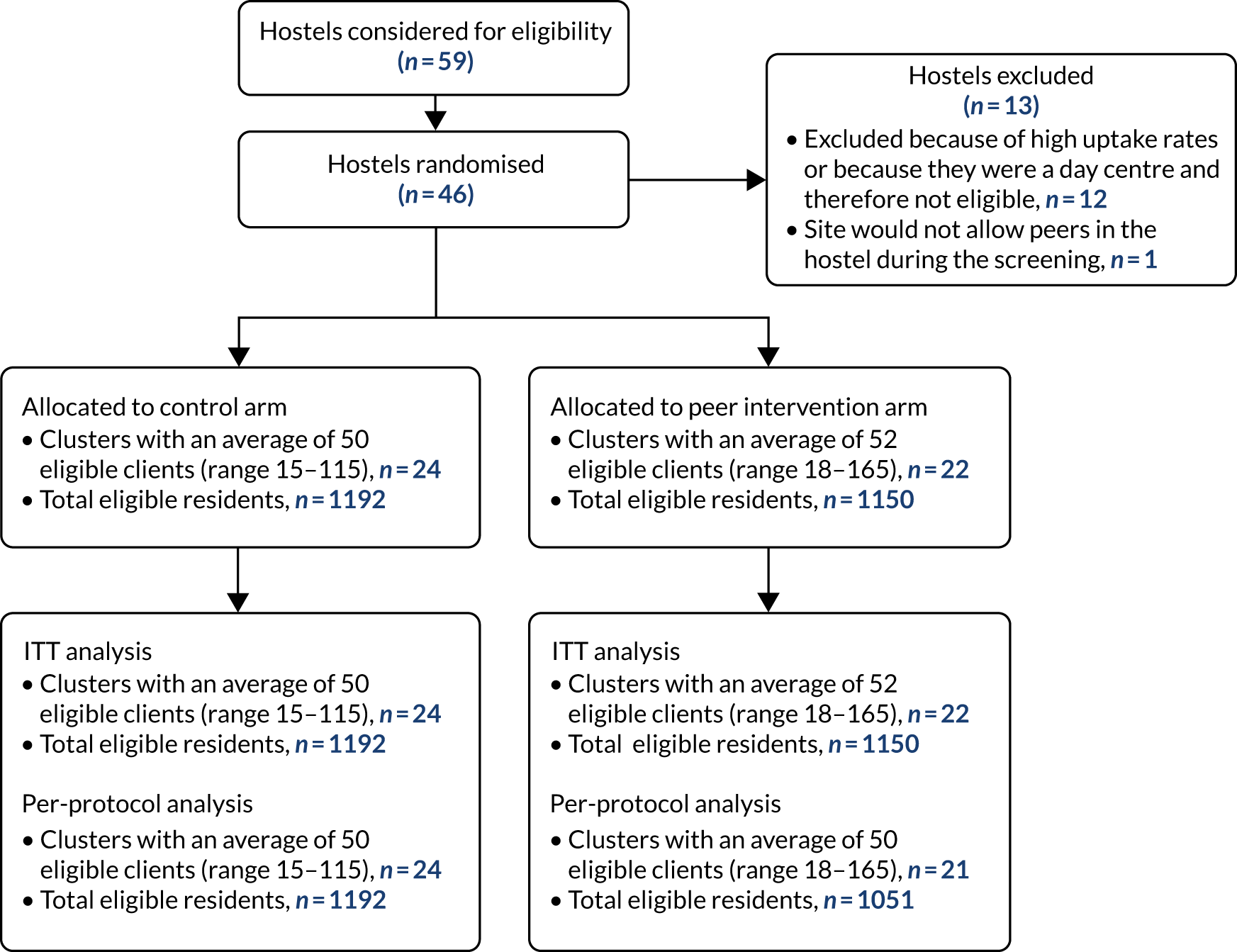
| Hostel characteristic | Control | Intervention | ||
|---|---|---|---|---|
| n | % | n | % | |
| London tuberculosis sectora | ||||
| North Central | 7 | 29 | 4 | 18 |
| North East | 6 | 25 | 7 | 32 |
| North West | 5 | 21 | 8 | 36 |
| South East | 6 | 25 | 3 | 14 |
| Hostel size | ||||
| ≤ 43 beds | 13 | 54 | 12 | 55 |
| > 43 beds | 11 | 46 | 10 | 45 |
| Historical screening uptake | ||||
| ≤ 50% | 15 | 63 | 12 | 55 |
| > 50% | 9 | 38 | 10 | 45 |
| Incentives provided for screeningb | ||||
| No | 15 | 63 | 15 | 68 |
| Yes | 9 | 38 | 6 | 27 |
| Unknown | 0 | 0 | 1 | 5 |
Median screening uptake was 44% [interquartile range (IQR) 26–59%)]: 45% (IQR 33–55%) in control hostels and 40% (IQR 25–61%) in intervention hostels. Using Poisson regression to account for the clustered study design, size of hostel and previous screening uptake, there was no evidence of peer educators affecting the uptake of screening, with an adjusted risk ratio of 0.98 (95% CI 0.80 to 1.20) (Table 8). These results did not differ significantly in secondary analyses examining the effect by hostel size, historical screening uptake and intervention fidelity (Table 9).
| Variable | Control (n) | Intervention (n) | Total | Unadjusteda,b intervention group risk ratio (95% CI) | Adjusteda,c intervention group risk ratio (95% CI) |
|---|---|---|---|---|---|
| Number of individuals eligible for screening | 1192 | 1150 | 2342 | – | – |
| Number of individuals eligible for screening per hostela | 35 (27–71) | 36 (27–52) | 35 (27–70) | – | – |
| Number of individuals screened | 503 | 468 | 29 (13–38) | 0.96 (0.76 to 1.23) | 0.98 (0.79 to 1.21) |
| Hostel engagement and characteristics | Control: eligible (number screened) | Intervention: eligible (number screened) | Total | Unadjusted intervention group risk ratio (95% CI) | Adjusteda intervention group risk ratio (95% CI) |
|---|---|---|---|---|---|
| Per protocol – peers attended intervention hostel on day of screening | 1192 (503) | 1051 (432) | 2243 (935) | 0.97 (0.75 to 1.26) | 0.97 (0.78 to 1.22) |
| Hostel did not participate effectivelyb | 748 (267) | 444 (137) | 1192 (404) | 0.86 (0.67 to 1.11) | 0.88 (0.67 to 1.14) |
| Hostel size | |||||
| ≤ 43 beds | 362 (176) | 338 (134) | 700 (310) | 0.82 (0.60 to 1.11) | 0.80 (0.60 to 1.06) |
| > 43 beds | 830 (327) | 812 (334) | 1642 (661) | 1.04 (0.76 to 1.43) | 1.08 (0.82 to 1.42) |
| Historical screening uptake | |||||
| ≤ 50% | 694 (272) | 718 (241) | 1412 (513) | 0.86 (0.64 to 1.14) | 0.86 (0.65 to 1.14) |
| > 50% | 498 (231) | 432 (227) | 930 (458) | 1.13 (0.85 to 1.51) | 1.13 (0.85 to 1.51) |
The study team noted no adverse events.
Discussion
This CRCT showed no evidence that peer educators increased the uptake of screening.
The study included most homeless hostels being screened by Find&Treat, a project with broad geographical coverage across London. Cluster randomisation was used as individual randomisation would not have been possible. The study was not powered to detect a difference in tuberculosis cases identified by the two arms as this would require a considerably larger sample. We did not collect individual data as part of the study as this would have required individual consent and would have interrupted the flow of screening and caused unacceptable delays for service users.
Evidence of the effectiveness of peer education interventions is mixed. One review found evidence for peer education increasing physical activity, decreasing smoking and increasing condom use, but no evidence for breastfeeding, medication adherence, women’s health and participation in general activities. 79 A further review of the use of peers educators in HIV also found mixed evidence of effects. 80 This same review attempted to examine what implementation factors, including peer educator recruitment, supervision and training, improved effectiveness, but found a lack of evidence to support any of these approaches, partly as a result of low sample size.
Several factors may have influenced results. Most sites were not naive to the peer intervention as a result of the increased use of peers alongside Find&Treat in the time between the study design and its conduct; thus, at several sites we, in effect, withdrew the ‘intervention’ from the control arm. The prior use of peers may have led to an increased awareness among hostel staff and residents, reducing any effect during the trial. The primary outcome of screening uptake does not take account of peers’ previously reported ability81 to engage the more difficult-to-reach and vulnerable cases, which could be assessed only by the collection of individual-level data. Such an effect (if it exists) could ultimately lead to an increase in the rate of detection of active tuberculosis. Having peers available at the time of screening may also help with the engagement of those who are screened and require further health-care management. Peers are then able to accompany people to follow-up appointments, based on a relationship that began on the mobile radiographic unit during the peer education work. Finally, the presence of peers may have reduced the intensiveness with which Find&Treat staff promoted uptake but may have freed them to focus on other activities. Peers in this study were associated with minimal costs as they were volunteers and may benefit from involvement in structured, meaningful activity as suggested by previous qualitative studies. 81
We found no evidence for an increased uptake of screening in this study; however, peer educators may have contributed to other, unmeasured factors in the screening process. Additionally, involvement of peers in the screening is likely to have directly benefited the peers as a result of the training and skills that were learnt during the research process, factors that were not measured during this study.
Acknowledgements
We would like to acknowledge the invaluable help and support of Groundswell and all the peers who volunteered for this trial.
Deviations from the protocol
With the exception of the revised power calculations that were required because of the change in average hostel size and the exclusion of one hostel as a result of the refusal of the hostel to allow peer workers on site, there were no recorded protocol violations.
Added value
Find&Treat and Groundswell continue to highly value the role of peers; however, partly as a result of this study, Find&Treat has evolved the peer model, increasingly moving volunteer peers into paid roles providing support across the diagnostic and treatment pathway. There is an emphasis on matching peers to clients’ backgrounds to support their care. There is also an emphasis on training peers to undertake clinical activities, such as obtaining oral swabs, conducting near-patient tests for blood-borne viruses and undertaking liver fibroscanning. Partly as a result of our involvement in this study, we are now collaborating on a NIHR Research for Patient Benefit grant assessing the effectiveness of the Groundswell peer advocacy model (Chief Investigator Lucy Platt). A Wellcome Trust Clinical Research Fellow (Neha Pathak) is also using Groundswell peer educators in a survey of cervical cancer screening uptake and sexually transmitted infection prevalence among women experiencing homelessness.
Work package 4: evaluating the impact of using polymerase chain reaction, Cepheid Xpert MTB/RIF as a point-of-care diagnostic alongside mobile radiographic screening for tuberculosis
Our findings relating to technical details of safe collection and processing of sputum for testing outside a laboratory have previously been published. 83 Parts of this section have been reproduced or adapted from Gliddon et al. 83 Copyright © ERS 2019. This article is open access and distributed under the terms of the Creative Commons Attribution Non-Commercial Licence 4.0. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
Experience of tuberculosis screening among socially complex groups and other tuberculosis patients by the pan-London Find&Treat tuberculosis outreach service has shown that current service provision in the UK rarely achieves same-day diagnosis in smear-positive pulmonary cases. In the UK, patients often have to wait for a follow-up appointment to be given the results of smear microscopy. Patients who are smear negative and clinically well often have to wait several weeks for the results of the culture prior to commencing treatment and there is further delay in the availability of drug sensitivity tests to inform clinical management. This carries an important risk of loss to follow-up care, especially among socially complex groups, and inappropriate arrangements for isolation and infection control in hospital and prison settings. The use of PCR-based molecular technologies allows a high proportion of smear-negative cases (later confirmed by culture) to be diagnosed within 48 hours. 84 In addition, these technologies enable the identification of mutations specific to rifampicin drug resistance (a key marker of multidrug resistance). The reagents used in the test are effective in sterilising samples, making them suitable for testing outside category 3 laboratories and suggesting a potential role as a point-of-care test. 85 Although these tests are available on request in several London laboratories, there is a paucity of evidence to support their use as a near-patient test in field conditions.
Methods
Setting
The setting was a rapid diagnostic service within the mobile radiographic screening unit run by the Find&Treat service, an NHS pan-London specialist community outreach team.
Development of the point-of-care diagnostic service
The use of the Cepheid Xpert MTB/RIF test as a point-of-care test in this setting was approved by the Royal Free London NHS Foundation Trust point-of-care testing committee. Training and competence assessment to ensure the safe handling of specimens was performed on the mobile radiographic unit by the Royal Free Microbiology Department staff.
Considerable work went into establishing the Cepheid technology as a point-of-care test. Major hurdles that needed to be addressed were the installation of the Cepheid Unit on the mobile radiographic unit, acquisition and installation of an uninterruptable power supply to enable the tests to continue while the mobile radiographic unit was in transit, development of specialist sputum pots to enable safe handling and aliquoting of sputum samples into Cepheid-reagent tubes, laboratory accreditation of the mobile radiographic unit as a diagnostic facility and training of mobile radiographic unit staff in conducting the assay. Further details are provided in Report Supplementary Material 2.
Participants and eligibility
The eligibility criteria were any socially complex patient, male or female, aged ≥ 16 years with an abnormal chest radiograph that was suggestive of pulmonary tuberculosis identified through mobile radiographic unit screening for active pulmonary tuberculosis. The patient needed to be willing and able to provide informed consent.
Study description
Staff on the mobile radiographic unit aimed to provide eligible participants with written information, explain the differences in the diagnostic approach and care pathway for those in the intervention arm and the control arm, answer any questions that participants may have and obtain written consent. Those who consented were then randomised using a text messaging randomisation service provided by Sealed Envelope. Baseline data and follow-up information were collected on all participants.
Intervention arm
Those randomised to the intervention arm were asked to produce a sputum sample for immediate analysis using the Cepheid Xpert MTB/RIF test by staff working on the mobile radiographic unit. An additional sample was also submitted for routine sputum microscopy and culture in the hospital laboratory. Patients with a positive point-of-care Cepheid Xpert MTB/RIF test result were referred immediately to a local hospital for clinical assessment, isolation and to commence tuberculosis treatment. Patients with a negative test result but with clinical symptoms of haemoptysis, night sweats or weight loss were also referred as above. Intervention arm participants who were negative according to Cepheid Xpert MTB/RIF and who did not have symptoms (e.g. haemoptysis, night sweats or weight loss) were followed up in the community with two further sputum samples (including one early-morning specimen for microscopy and culture) and clinic referral if these subsequent tests were positive. Patients with three negative microscopy and culture results were offered a repeat chest radiograph on the mobile radiographic unit 3 months from the initial radiograph.
Control arm
Those randomised to the control arm were managed according to standard practice (i.e. patients were accompanied directly to a hospital and, if possible, a sputum sample was collected for routine analysis in a hospital laboratory). Further investigations at the clinic included collection of additional samples for microscopy and culture.
Primary outcome
The primary outcome was the number of clinic visits needed for the exclusion or confirmation of tuberculosis diagnosis.
Secondary outcome
The secondary outcomes were the proportion of patients completing the investigations required to exclude active tuberculosis (three sputa, including one early-morning sputum, for smear microscopy and culture when possible), time to onset of appropriate treatment, time to isolation for infectious cases, loss to follow-up, and how many patients developed active tuberculosis from the time of the initial radiograph on the mobile radiographic unit.
Analysis
We aimed to recruit 40 participants in each arm based on an ability to detect a decreased number of visits until diagnostic conclusion in the intervention arm compared with the control arm {mean 2.3 visits [standard deviation (SD) 1.1 visits] vs. mean 1.5 visits (SD 1.1 visits), 90% power, 5% significance level}.
Results
During the study period, 95 patients met the inclusion criteria for the study, 37 of whom were recruited (Figure 5). In the 18 patients in whom Cepheid was used, 14 had negative results, two were positive for Mycobacterium tuberculosis and two had indeterminate results. Six of these patients were ultimately diagnosed with active tuberculosis, 10 were classified as not having tuberculosis and two were lost to follow-up. In the control arm, of the 19 patients identified, five were ultimately diagnosed with active tuberculosis, two were lost to follow-up and 12 were classified as not having active tuberculosis. It did not prove possible to obtain more detailed primary and secondary outcome measures from the NHS services. Owing to low recruitment and difficulties collecting primary and secondary outcome data (see Challenges and deviations from the protocol) and following discussion with the funders, the trial was abandoned. The intention was to continue the evaluation as an observational study of the technology on the mobile radiographic unit, but the mobile radiographic unit stopped using the technology soon after trial abandonment.
FIGURE 5.
Work package 4: Cepheid recruitment update/outcomes – from August 2013 to July 2015. CXR, chest X-ray; LFU, lost to follow-up; MXU, mobile X-ray unit; PHE, Public Health England; TB, tuberculosis.
Challenges and deviations from the protocol
This study, which sought to evaluate the use of molecular diagnostics on a mobile radiographic unit screening highly complex patients, was subject to many challenges. Optimising the Cepheid technology to function in transit was a major challenge. After initial installation, it became apparent that brief interruptions of power supply disrupted its use, requiring the installation of a custom-made back-up battery. We overcame practical issues in implementation, which included the development of sputum pots with one-way valves to allow the safe handling of sputum specimens outside a category 3 laboratory. We also trained non-specialist staff in the use of the equipment and developed procedures and permissions for the local microbiology service to accredit the mobile diagnostic service. Recruitment commenced but became very slow as the mobile radiographic unit was a highly pressured area for the consenting and randomisation of participants alongside routine screening plus other screening tests being offered in the unit that had an important impact on the recruitment rate. Staff reported that it was extremely challenging to explain the randomised nature of the study to patients in a highly pressurised clinical environment. Conducting the test interrupted their other clinical activities. They also reported that the test took too long to produce results (up to 1 hour), which made it hard for patients to wait for results. In addition, in many cases the mobile unit’s schedule meant that it needed to move to another screening location before test results were completed. In light of these challenges, it was decided to terminate the study. Although it was planned to establish this as a service evaluation study rather than a RCT, we found that, following cessation of the trial, the Cepheid system was used only twice (both results were negative). We conclude that routine use of this diagnostic technology has limited utility in the current context. The Find&Treat team now uses the technology in selected cases and during outbreak investigations. More technologically simple and rapid confirmatory tests are required.
Work package 5: a randomised controlled trial comparing smartphone-enabled video-observed treatment with face-to-face directly observed treatment
Our study findings relating to this trial have previously been published. 86 Parts of this section have been reproduced or adapted from Story et al. 86 © 2019 Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
The trial was a multicentre, randomised, superiority study comparing the efficacy of VOT versus DOT in supporting adherence in patients with active tuberculosis. The trial registration number is ISRCTN26184967.
Introduction
Directly observed treatment has been the standard of care for tuberculosis since the early 1990s. 87,88 DOT was developed following observations that irregular treatment could threaten clinical and public health outcomes through the generation of drug resistance, relapse and transmission of infection. 89 DOT is recommended by WHO1 and the American Thoracic Society. 90 In England, DOT is recommended for patients at high risk of poor adherence. 11 This includes those with clinically complex disease, MDRTB, mental health problems, previous tuberculosis treatment or previous poor adherence. 11 It also includes socially complex groups (persons with a history of homelessness, imprisonment, drug use or alcohol problems).
Directly observed treatment can be administered in clinic, community or home settings but is a substantial inconvenience to patients and service providers. Regimens of three times per week have also been approved with DOT and are used in England,11 although they are not currently recommended by WHO owing to increased risk of treatment failure. 1 Seven-day regimens are, therefore, generally administered through DOT 5 days per week and through self-administered treatment at the weekend.
Advances in technology have raised the possibility of remote VOT. 91 Initially, this required a live video call (synchronous VOT). 92–97 More recently, apps have been developed that enable video clips to be recorded and forwarded for later viewing (asynchronous VOT). 98 Asynchronous VOT is currently used by some clinics in the USA and has high reported levels of patient acceptability, decreased costs and programmatic evidence of effectiveness. 98–102 The WHO, therefore, recently conditionally recommended VOT as an alternative to DOT, but the evidence was graded as weak because of a lack of RCTs. 1 In addition, VOT has yet to be assessed in patients from socially complex groups.
Methods
Trial design
This was a multicentre, analyst-blinded, randomised controlled, superiority trial to compare asynchronous VOT with clinic-, community- or home-based DOT for supporting treatment adherence in patients with active tuberculosis in England.
The full trial protocol is published on the International Standard Randomised Controlled Trial Number Registry (study ISRCTN26184967, doi 10.1186/ISRCTN26184967). Ethics approval was granted by the National Research Ethics Service Committee East of England – Essex on 20 March 2014 (reference 10/H0302/51). All patients provided written informed consent to participate in the study.
Setting
Patients were recruited from clinics in London (17 sites), Birmingham (three sites), Coventry (one site) and Leicester (one site). Case managers at each participating clinic identified eligible patients and referred them to the study team, which provided further information.
Inclusion criteria
The inclusion criteria were patients aged ≥16 years with active pulmonary or non-pulmonary tuberculosis who were eligible for DOT according to national guidance. 11 Patients were invited to participate regardless of whether or not they had previously agreed to treatment observation.
Exclusion criteria
The exclusion criteria were lack of access to facilities to charge a smartphone and < 2 months remaining on their treatment regimen (the primary outcome required measurement of adherence over 2 months). Patients with MDRTB were also excluded because they require twice-daily treatment and it was considered that DOT was not practicable under these circumstances. (These patients were offered VOT under a separate non-randomised study.)
Randomisation
Randomisation was provided by Sealed Envelope, a telephone and online software application for randomising patients into clinical trials. The system used randomisation by minimisation103 to ensure balance across study sites and stage of treatment at the time of enrolment (within the first 2 months of treatment and after the first 2 months of treatment).
Directly observed treatment was delivered according to usual clinical practice (observation of treatment in clinic, at home or in community settings three to five times per week by a health-care or lay worker, with the remaining daily doses being self-administered).
Video-observed treatment was provided by a centralised service in London. Patients were trained to record and send videos of every dose ingested 7 days per week using an app developed by researchers at the University of California, San Diego. 98 Trained treatment observers viewed these videos through a password-protected website. Patients were also encouraged to report adverse drug events on the videos. Smartphones and data plans (including UK calls and texts) were provided free of charge.
Directly observed treatment or VOT observation records were completed by observers until treatment end or study end.
Primary outcome
The primary outcome was successful completion of ≥ 80% of scheduled treatment observations in the 2 months following enrolment.
Secondary outcomes
The main secondary outcome was the proportion of scheduled observations (measured on a continuous scale) successfully completed in the 2 months following enrolment, and throughout treatment.
Other secondary outcomes collected were sputum culture results at 2 months post treatment initiation, 9- or 12-month treatment outcomes, side effects, number of hospitalisations, staff time spent observing or travelling to observe patients, estimated costs and patient satisfaction.
Power
We determined that a sample of 200 patients per arm would provide a power of 90% to detect a 15% difference in the proportion of patients with the primary outcome (60% vs. 75%). This was based on a two-sided significance level of 5%.
Study interruption
Following a review of study progress by an external committee, funders requested an interim analysis, the plan for which was published on the International Standard Randomised Controlled Trial Number Registry prior to analysis (study ISRCTN26184967, doi 10.1186/ISRCTN26184967). It included a stopping rule using the Haybittle–Peto boundary of 0.001 for the primary outcome. As the interim analysis plan showed overwhelming evidence of superiority, the committee advised that the trial should be terminated early.
Analysis
Video-observed treatment observations were classified as successfully completed if ingestion of all medicines had been observed, or if video clips had been received but were not viewable as a result of a technical complication (as patients had no control over whether or not videos were corrupted).
The intention-to-treat (ITT) analysis included all patients, analysed according to the arm to which they were originally randomised. The restricted analysis excluded patients with < 1 week of observation in the allocated arm. This was designed to include only those patients who had, at least initially, engaged with the allocated intervention.
If patients had two episodes of non-adherence (see Report Supplementary Material 1 for definitions), they were switched into the other trial arm. Four patients crossed over from VOT to DOT, and five crossed over from DOT to VOT. For the purposes of this analysis, all doses following crossover were considered unobserved.
We used logistic regression to analyse the primary outcome and linear regression to analyse the main secondary outcome. The time elapsed since the start of treatment, age and sex were considered a priori as potential confounders and were included in all models. For the restricted analysis, we also considered covariates that may have affected initial engagement with the allocated intervention (homelessness, imprisonment, drug use, alcohol problems, immigration concerns, mental health problems, previous loss to follow-up and no recourse to public funds). All analyses accounted for clustering at the level of the clinic using robust standard errors. 104 Analyses were conducted in Stata (version 14) and R (The R Foundation for Statistical Computing, Vienna, Austria) (version 3.3.2) software.
Results
Trial population
Recruitment began on 1 September 2014 and continued until 1 October 2016, when the study’s independent Trial Steering Committee advised that recruitment should be stopped based on interim analysis results. Follow-up continued until 31 December 2016. Flow through the study is summarised in Figure 6.
FIGURE 6.
Enrolment and randomisation. a, The most common ‘other reason’ for failing to enrol patients (32 out of 45) was that the clinic staff considered that the patient needed intensive face-to-face support owing to emotional, medical or physical reasons or because of imminent risk of loss to follow-up. Reproduced from Story et al. 86 © 2019 Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.

The ITT analyses included 114 patients randomised to DOT and 112 patients randomised to VOT. Baseline characteristics of patients are shown in Table 10. Patients were mainly young adults born outside the UK. A high proportion (131 out of 226 patients; 58%) had a history of homelessness, imprisonment, drug use, alcohol problems or mental health problems. The baseline characteristics were similar in the two arms.
| Characteristic | DOT | VOT | ||
|---|---|---|---|---|
| n | % | n | % | |
| Total | 114 | 112 | ||
| Age group (years) | ||||
| 16–34 | 61 | 53.5 | 64 | 57.1 |
| 35–54 | 45 | 39.5 | 35 | 31.3 |
| ≥ 55 | 8 | 7.0 | 13 | 11.6 |
| Sex | ||||
| Male | 83 | 72.8 | 82 | 73.2 |
| Female | 31 | 27.2 | 30 | 26.8 |
| Born in UK | ||||
| No | 83 | 72.8 | 93 | 83.0 |
| Yes | 31 | 27.2 | 19 | 17.0 |
| Previous tuberculosis | ||||
| No | 82 | 71.9 | 85 | 75.9 |
| Yes | 30 | 26.3 | 27 | 24.1 |
| Pulmonary tuberculosis | ||||
| Yes | 73 | 64.0 | 69 | 61.6 |
| No | 41 | 36.0 | 43 | 38.4 |
| Social risk factora | ||||
| Never | 48 | 42.1 | 47 | 42.0 |
| > 5 years ago | 19 | 16.7 | 19 | 17.0 |
| Within 5 years | 47 | 41.2 | 46 | 41.1 |
| Homeless | ||||
| Never | 77 | 67.5 | 70 | 62.5 |
| > 5 years ago | 14 | 12.3 | 16 | 14.3 |
| Within 5 years | 23 | 20.2 | 24 | 21.4 |
| Prison | ||||
| Never | 93 | 81.6 | 97 | 86.6 |
| > 5 years ago | 9 | 7.9 | 8 | 7.1 |
| Within 5 years | 11 | 9.6 | 7 | 6.3 |
| Drug use | ||||
| Never | 96 | 84.2 | 89 | 79.5 |
| > 5 years ago | 2 | 1.8 | 4 | 3.6 |
| Within 5 years | 15 | 13.2 | 18 | 16.1 |
| Alcohol problems | ||||
| No | 91 | 79.8 | 92 | 82.1 |
| Yes | 21 | 18.4 | 17 | 15.2 |
| Mental health problems | ||||
| No | 94 | 82.5 | 94 | 83.9 |
| Yes | 18 | 15.8 | 14 | 12.5 |
In the ITT analysis, 78 out of 122 (70%) VOT patients successfully achieved the primary outcome (≥ 80% scheduled observations successfully completed during the first 2 months), compared with 35 out of 114 (31%) DOT patients [adjusted odds ratio (aOR) 5.48, 95% CI 3.10 to 9.68; p < 0.0001]. In the restricted analysis, the proportions with the primary outcome were 78 out of 101 patients (77%) for VOT and 35 out of 56 patients (63%) for DOT (aOR 2.52, 95% CI 1.17 to 5.47; p = 0.019).
High observation rates were maintained in the VOT arm, but they rapidly declined in the DOT arm. Over the full follow-up period (up to 6 months), 12,422 out of 16,230 (77%) scheduled observations were completed in the VOT arm, compared with 3884 out of 9882 (39%) scheduled observations in the DOT arm (p < 0.0001). In the restricted analysis over the full follow-up period, 12,422 out of 14,907 (83%) scheduled observations were completed in the VOT arm, compared with 3884 out of 6351 (61%) scheduled observations in the DOT arm (p < 0.0001). Observation completion rates were higher for VOT than DOT in all subgroups.
There were no significant differences in positive sputum cultures at 2 months following treatment onset, treatment completion and loss to follow-up or numbers of hospital admissions between trial arms.
Average staff time per dose observed was 56 (SD 54) minutes for community-based DOT (including travel time), 15 (SD 12) minutes for clinic-based DOT and 3.2 (SD 0.5) minutes for VOT. Patients on DOT spent a mean of 29 (SD 48) minutes per week on treatment observation (including travelling to/from clinics, waiting for appointments and appointment time). Those on VOT spent a mean of 1.8 (SD 2.2) minutes setting up and recording each video.
Discussion
Video-observed treatment was substantially more effective than DOT at ensuring that scheduled doses were observed. A large part of this effect was due to patients failing to engage in DOT over the first week of treatment. After restricting analyses to those patients who engaged with observation in the first week, VOT remained significantly superior at ensuring that scheduled observations took place. This effect was maintained throughout treatment. VOT observations involved substantially less health-care worker time and patient time than DOT.
Systematic reviews and meta-analyses draw differing conclusions about the effectiveness of DOT;105,106 however, a recent review showed that DOT increased treatment success, adherence and 2-month sputum conversion, and decreased loss to follow-up and acquired drug resistance compared with self-administered treatment. 1 Community DOT was more effective than hospital-based or clinic-based DOT, demonstrating the importance of making DOT convenient for patients. The review also showed that DOT was more effective when delivered by health staff or lay workers than when delivered by family members. 1 VOT provides the technology to support professional treatment observation that is potentially more convenient and cheaper than in-person DOT.
Video-observed treatment, as used in this study, has a wide range of components beyond convenience of observation. The intervention included personal support: patients met the VOT observer for training and received regular personalised messages as reminders, confirmation of receipt of video clips or follow-up when clips were not received. The observers also supported onward referral to deal with reported adverse events. Patients were provided with a smartphone with a data plan and free domestic calls and text messages. This acted both as an enabler (as it facilitated easy communication and improved access to care providers) and as a material incentive that was valued by patients. Phones were reused throughout the trial.
The trial had a number of limitations. It was not possible to blind patients or treatment observers to the intervention, although the investigators and analysts were blinded. We could not distinguish between doses that were taken but were not observed and doses that were not taken.
The primary outcome (observing 80% of scheduled doses) could be considered to be biased towards DOT; it required substantially more VOT doses (scheduled 7 days per week) to be observed than DOT doses (scheduled three or five times per week). The restricted analysis further favoured DOT as it included only the subset of patients allocated to DOT who were willing to be observed.
The study was not powered to detect differences in culture conversion rate, treatment completion, loss to follow-up, relapse or development of drug resistance; nevertheless, it is reasonable to assume that improved adherence might improve all of these outcomes.
Another limitation was the exclusion of MDRTB patients from the randomised trial. They were excluded because they had a range of treatment regimes, some of which included multiple scheduled doses to be observed per day. It would be impractical for all of these observations to be made through face-to-face DOT and we therefore did not consider there to be equipoise between VOT and DOT for MDRTB patients; however, 26 MDRTB patients were offered VOT in a non-randomised arm of the trial. In this arm, it was harder to be certain about which doses were scheduled for VOT because of the higher frequency of dosing, the fact that some doses (particularly injectable doses) continued to be administered at regular clinic visits and the higher rates of hospitalisation. Nevertheless, all but one of the 26 MDRTB patients accepted VOT and 16 MDRTB patients had > 80% doses observed in the first 2 months. There was also evidence that the intervention could be sustained for the long-term regimens required for treatment completion in MDRTB; VOT was maintained for a maximum of 796 days.
There is an urgent global need for more effective and cheaper alternatives to DOT to enable effective ambulatory care of both drug-sensitive tuberculosis and MDRTB. In particular, it is critical that new opportunities for shorter regimens for MDRTB are not lost as a result of insufficient attention to adherence. 107
The WHO now recommends that VOT can be a ‘suitable alternative’ to DOT and has published guidance on its implementation. 1 The results of this trial may allow WHO to make firmer recommendations about the use of DOT and VOT.
Deviations from the protocol
The full trial protocol and analysis plan are presented in Report Supplementary Material 3 and Report Supplementary Material 4. Some eligible patients (45/548 patients assessed for eligibility) were not recruited for a range of reasons that were not within the original exclusion criteria (see Figure 8). With the exception of the trial being terminated following an interim analysis requested by the funder, there were no clear protocol violations. No adverse effects were reported.
Challenges
In our original submission to NIHR, we proposed a trial comparing community DOT with clinic DOT; however, in the time between study conception and delivery, the use of community DOT in routine practice had increased considerably. We also found it very challenging to recruit community DOT providers for this work; therefore, with the permission of NIHR, we redesigned the trial to assess VOT. This followed a small pilot by Find&Treat that had found the approach to be acceptable to patients. Considerable delays were introduced by the multicentre aspect of the study, which required research and development approvals across 22 sites in four cities. Local principal investigators were required in each site and, frequently, these principal investigators (primarily specialist tuberculosis nurses) had not previously been involved in research and needed to undertake training in Good Clinical Practice before the trial could start. When sites were up and running, we found recruitment to be slower than anticipated. This was, in part, due to the decreasing numbers of tuberculosis cases in London and, in part, due to a reluctance by staff in clinics to randomise people who, despite being eligible for DOT, they thought would not accept DOT or for whom they thought that it would be difficult to arrange. In view of these delays, NIHR requested an interim analysis and, following this, based on overwhelming evidence of the superiority of one intervention arm, the external steering group recommended cessation of the trial.
Added value
At the time of writing, the VOT trial has already had considerable impact. The work has led to a collaboration with the Behavioural Insights Team to undertake a trial of video-observed therapy for tuberculosis in Moldova, which is now complete and being analysed. Discussion of the findings at meetings of the WHO Digital Health for the End Tuberculosis Strategy (for which the co-chairperson and co-applicant is A Story) has contributed to WHO recommending VOT as a suitable alternative to DOT in countries with suitable technological infrastructure. The London Clinical Commissioning Groups have commissioned Find&Treat to provide a VOT service for London, and other parts of the country also commission VOT from Find&Treat on an ad hoc basis.
Work package 6: cost-effectiveness analysis
The latent tuberculosis infection screening and VOT intervention demonstrated clear clinical effectiveness and are, hence, considered for this analysis. This section includes separate reporting of the cost-effectiveness analyses for these two interventions.
Latent tuberculosis infection screening in the homeless: transmission dynamic and health economic analysis
Introduction
We analysed the cost-effectiveness of adding screening and treatment for latent tuberculosis infection to screening and treatment for active tuberculosis in people experiencing homelessness in London, using an integrated transmission dynamic and health economic model. 108 It is an individual-based model in which individuals are ‘followed’ while they are homeless and after re-entering the general population to capture the long-term health consequences of tuberculosis infection, case finding and treatment while homeless; infection acquired while homeless can result in active tuberculosis disease after re-entering the general population (which causes QALY loss and incurs treatment cost), and treatment of latent tuberculosis infection while homeless may avert this.
Analysis
In the model, each individual is in one of a number of health states: uninfected (naive), uninfected (recovered), latently infected (fast progressing or slow progressing), with active tuberculosis disease (sputum smear negative or positive), on treatment for latent tuberculosis infection, or on treatment for active tuberculosis (see Appendix 1, Figure 7). Uninfected individuals who become infected move into the latent infection state. Individuals with tuberculosis infection history (recovered) are assumed to have partial immunity and, therefore, have a reduced probability of (re-)acquiring tuberculosis infection compared with naive individuals. Individuals in the latent state can progress to active tuberculosis disease, which is infectious and can be diagnosed through active or passive case finding, resulting in individuals being placed on tuberculosis treatment. Active case finding for active tuberculosis takes place through chest radiography in the mobile radiographic unit, with cases of abnormal radiology being referred to hospital for tuberculosis diagnosis. Passive case finding for active tuberculosis takes place through individuals presenting for care. In scenarios where latent tuberculosis infection screening occurs, a proportion of diagnosed individuals are treated. Patients who are successfully treated for latent tuberculosis infection or active tuberculosis enter the recovered state. Patients who are not successfully treated return to their former state of latent tuberculosis infection or active tuberculosis disease. Individuals whose IGRA test was false positive remain in the naive or recovered state regardless of whether they are treated or not, but costs are incurred for those who are treated. Active tuberculosis disease causes QALY loss through morbidity (which is reduced by treatment) and mortality.
Economic analysis
The economic analysis is from an NHS perspective. In the baseline (current practice) scenario, a mobile radiographic unit provides screening for active tuberculosis to people experiencing homelessness; individuals with abnormal radiographs are referred to hospital for diagnosis and those with tuberculosis are treated. Individuals with active tuberculosis can also be found passively, when they present for care.
We compared the baseline scenario with intervention scenarios in which people experiencing homelessness are offered testing for latent tuberculosis infection by IGRA, and offered treatment if they test positive. As treatment was not offered in the empirical study, there is uncertainty regarding the proportion of IGRA-positive patients who would accept and complete latent tuberculosis infection treatment, so we compared scenarios in which this proportion varied (i.e. 25%, 50% and 75%). The intervention is applied for 10 years, and the model considers the lifetime of the population cohort. Analysis was from the perspective of the NHS. The total net costs incurred (including staff time, drugs and diagnostic tests) and QALYs accrued by the patient cohort are calculated. Cost-effectiveness of different options is compared using incremental cost-effectiveness ratios (ICERs) relative to current practice. Each scenario (baseline and interventions) was run 1000 times. Costs and health utilities are discounted at 3.5% per annum. Parameter values were obtained primarily from sources used to inform NICE guidance11,33 or this study, as appropriate (see Appendix 1, Tables 13 and 14).
Results
In our analysis, screening for and treating latent tuberculosis infection had a net cost, as the intervention cost was greater than the averted costs of active tuberculosis disease; however, the intervention was beneficial to health, with a QALY gain.
Whether or not the intervention would be considered cost-effective depends on the value of 1 QALY and the proportion of IGRA-positive individuals who are treated for latent tuberculosis infection (see Figures 8 and 9, and Appendix 1). The higher the value of 1 QALY, and the greater the proportion of IGRA-positive patients who are treated for latent tuberculosis infection, the more likely it is that the intervention would be considered to be cost-effective.
In the three scenarios that we considered regarding the proportion of IGRA-positive patients who are treated for latent tuberculosis infection, we find that if this proportion was 25% then introducing latent tuberculosis infection screening and treatment would be cost-effective when 1 QALY was valued at £30,000 (see Appendix 1, Figure 8). If the proportion was 50% or 75%, then introducing latent tuberculosis infection screening and treatment would be cost-effective if 1 QALY was valued at £20,000; however, there is substantial uncertainty about the cost-effectiveness (see Appendix 1, Table 15).
Discussion
Successful treatment of latent tuberculosis infection prevents progression to infectious active tuberculosis disease and thereby averts transmission, which benefits population health and averts future costs of treatment in others, as well as benefiting the individual patient. However, not all patients with latent tuberculosis infection would progress to active tuberculosis disease without treatment and, therefore, several patients with latent tuberculosis infection have to be treated to avert a case of active tuberculosis disease. We find that the cost-effectiveness of adding screening and treatment for latent tuberculosis infection to screening and treatment for active tuberculosis in people experiencing homelessness depends on the proportion of IGRA-positive patients who accept and complete latent tuberculosis infection treatment, and that the greater this proportion the more likely it is that the intervention would be considered cost-effective. As treatment for latent tuberculosis infection was not recommended at the time of the study, it was not offered to patients, and, therefore, we do not know what proportion of patients would accept and complete treatment. As this is a key determinant of cost-effectiveness, further empirical study is required to determine if this intervention should be recommended.
The probability that latent tuberculosis infection screening is cost-effective would be increased if the cost of latent tuberculosis infection testing were lower than in our analysis. With latent tuberculosis infection screening and treatment for immigrants from high-burden countries now recommended, bulk procurement of test kits may enable cost savings that would make latent tuberculosis infection screening and treatment of people experiencing homelessness more likely to be cost-effective.
Economic analysis of video-observed treatment versus directly observed treatment
Parts of this section have been reproduced or adapted from Story et al. 86 © 2019 Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
The trial showed that VOT has superior outcomes to DOT. Here, we present a comparison of costs of VOT versus costs of DOT.
Analysis
Taking the perspective of the NHS, DOT involving observations three times weekly costs £570 per patient per month based on costs calculated by White et al. 109 and inflated to 2015–16 prices using the Hospital and Community Health Service index. 109 Observation five times weekly costs £950 per patient per month. All of the costs of DOT are unit costs and, therefore, the cost of DOT per patient is determined only by the duration of treatment.
Costs of VOT are summarised in Table 11. For VOT, there is an initial information technology infrastructure set-up cost of £2000 and a monthly cost of cloud data storage, software licences and system maintenance of £3270. Providing VOT requires a band 7 nurse to lead the service, conduct face-to-face training with patients and liaise with tuberculosis clinics. The monthly (combined) cost of a 100% full-time equivalent band 5 and band 7 nurse in inner London is £4425.
| Component | Cost | Source |
|---|---|---|
| Information technology set-up: one-off cost | £2000 | This study |
| Cloud data storage, software licences, maintenance: monthly cost | £3270 | This study |
| Band 7 nurse to manage the service: monthly cost | £4425 | Agenda for Change pay scale |
| Mobile phone: one-off cost per patient | £49 | This study |
| Data charges and insurance for mobile phone: monthly cost per patient | £26 | This study |
| Band 5 nurse to observe videos: monthly cost per patient | £79 | This study, Agenda for Change pay scale |
Observation of videos and patient support is provided by band 5 nurses. A patient creates seven videos per week. With 20% of videos being checked for quality assurance, each patient requires 8.4 observations per week. A nurse can observe 65 videos per day (based on this study), which is 325 videos per week, and, therefore, each full-time equivalent nurse can manage 38 patients at once (i.e. each patient requires 2.6% of a full-time equivalent nurse). The monthly cost of a 100% full-time equivalent band 5 nurse in inner London is £3045, of which 2.6% is £79.
Results
The monthly cost per patient of DOT depends on the frequency of observation, but the minimum of three observations per week costs £2850 per patient for 5 months of treatment, £3420 for 6 months of treatment and £3990 for 7 months of treatment. The per-patient cost is not affected by the number of patients.
In contrast, the per-patient cost of VOT depends on the number of patients, owing to the fixed costs of setting up the service and the fixed monthly cost of a band 7 nurse to manage the service. If only 10 patients were managed by VOT then the cost per patient would be higher than if DOT were used three times per week, although it would still be cheaper than DOT five times per week (except for the longest treatment durations) and VOT provides seven observations per week. If 25 patients were managed by the VOT service then it would be cheaper than DOT three times per week, and with greater numbers of patients the cost per patient of VOT falls substantially (Table 12).
| Treatment support | Costs (£) by duration (months) | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 12 | 15a | 24a | |
| DOT costs | |||||||
| DOT (3 observations/week) | 2850 | 3420 | 3990 | 4560 | 6840 | 8490 | 13,440 |
| DOT (5 observations/week) | 4750 | 5700 | 6650 | 7600 | 11,400 | 13,050 | 18,000 |
| VOT costs including initial set-up cost | |||||||
| VOT: 10 patients | 4620 | 5500 | 6370 | 7245 | 10,745 | 13,280 | 20,875 |
| VOT: 25 patients | 2195 | 2610 | 3020 | 3435 | 5085 | 6280 | 9870 |
| VOT: 50 patients | 1385 | 1645 | 1900 | 2160 | 3200 | 3950 | 6200 |
| VOT: 100 patients | 980 | 1160 | 1345 | 1525 | 2255 | 2780 | 4365 |
| VOT: 200 patients | 780 | 920 | 1065 | 1210 | 1785 | 2200 | 3445 |
| VOT costs excluding initial set-up cost | |||||||
| VOT: 10 patients | 4420 | 5300 | 6170 | 7045 | 10,545 | 13,080 | 20,675 |
| VOT: 25 patients | 2115 | 2530 | 2940 | 3355 | 5005 | 6200 | 9790 |
| VOT: 50 patients | 1345 | 1605 | 1860 | 2120 | 3160 | 3910 | 6160 |
| VOT: 100 patients | 960 | 1140 | 1325 | 1510 | 2235 | 2760 | 4345 |
| VOT: 200 patients | 770 | 910 | 1055 | 1200 | 1775 | 2190 | 3435 |
Conclusion
Video-observed treatment is cheaper than DOT except if small numbers of patients are managed. In settings with sufficient demand for tuberculosis treatment support, VOT is, therefore, cheaper for the NHS than DOT and has superior outcomes, so VOT dominates DOT. VOT has the additional benefits of being cheaper for patients and more convenient than DOT, as there are fewer appointments to be attended in person. The economies of scale offered by VOT are particularly beneficial in the context of tuberculosis control, where, historically, the distribution of resources has often not reflected the distribution of need. 110
Deviation from the protocol
The protocol specified economic analyses of effective interventions identified in the programme. Prior economic analysis of radiographic and latent tuberculosis infection screening had already been conducted for NICE Public Health Guidance 3712 so were not undertaken.
Patient and public involvement
Public involvement has been vital to the implementation of this research. As part of the grant development process, we consulted with user representatives including Groundswell and its tuberculosis peer educator project, and TBAlert (Brighton, UK) (an advocacy charity for people with tuberculosis). Through collaboration, these organisations have been involved in different aspects of the project. TBAlert is enlisted on our participant information sheets as a point of contact for independent information on tuberculosis and participation in our research. The peer educators (people who have personal experience of tuberculosis, homelessness and/or drug and alcohol problems) have been extensively consulted throughout the development of the research project and are part of the intervention in substudy 3 (improving mobile radiographic unit uptake). They are trained and highly supported by Groundswell, a professional service user involvement organisation, to use their experience to increase screening uptake, increase adherence to treatment and raise awareness of tuberculosis among socially complex groups accessing homelessness and/or drug and alcohol services, as well as to train health professionals working with the targeted group. Links with community drug and alcohol services as well as local blood-borne virus screening groups have also been an integral part of executing substudy 1 (latent tuberculosis infection screening in the community setting).
The peer educators from our study have been actively involved in patient and public involvement work, such as undergraduate teaching sessions and a number of radio and television programmes highlighting their work (including an edition of the BBC programme ‘A day in the life of the NHS’ and Radio 4’s ‘You and Yours’ programme). One peer educator has also contributed to the NICE Public Health Guidance Identification and Management of Tuberculosis in Socially Complex Groups as a patient representative and another of our peer educators is a patient representative on the current NICE guideline development committee for ‘Clinical and Public Health Guidance for Tuberculosis Management and Control’. 33
Discussion
Through a series of work packages, we have investigated a range of approaches that could improve the management and control of tuberculosis in socially excluded groups. In work package 1, we identified very high levels of latent tuberculosis infection and hepatitis C, HIV and hepatitis B in people experiencing homelessness in London and evidence of inadequate levels of hepatitis B vaccination. We have shown that, provided treatment uptake is > 50%, screening for latent tuberculosis infection among people experiencing homelessness would be cost-effective at a willingness-to-pay threshold of £20,000. This has led to NHS-funded commissioning of screening for latent tuberculosis in people experiencing homelessness alongside the Find&Treat service. The service is also screening for hepatitis C and offering influenza B vaccination, although at present this is largely funded through other research proposals and clear commissioning approaches need to be developed. In work package 2, we showed similar high rates of latent tuberculosis in prisoners but lower levels of blood-borne virus infection. Our evaluation of routine radiographic screening in a London prison demonstrated feasibility but showed that there are many barriers to implementing this approach in prisons. Within both homeless populations and prison populations, injecting drug users are a particularly high-risk group for tuberculosis and blood-borne viruses. In work package 3, we found that peer educators with experience of homelessness and tuberculosis were no better or worse than health-care workers at encouraging the uptake of screening. Peer educators continue to play an important role within the Find&Treat service, including encouraging the uptake of screening, engaging those identified with radiographs potentially indicating tuberculosis or other abnormal results and supporting the management of socially complex cases. In work package 4, we developed the capability to undertake rapid PCR-based investigation on the mobile radiographic unit to investigate those with radiographs potentially indicating tuberculosis. Although we were not able to deliver a RCT of effectiveness in this challenging high-throughput setting, the technology is now used selectively to support the work of the Find&Treat screening and outbreak investigation work. In work package 5, we undertook, to our knowledge, the world’s first RCT of smartphone-enabled VOT, showing it to be considerably more effective and cheaper in ensuring that treatment observation takes place over the course of treatment than the current standard of care for tuberculosis. This work has led to the service being commissioned across London and in some other parts of the UK. The work has also supported WHO to recommend the use of VOT as an alternative to DOT. In work package 6, we showed that VOT is cheaper to deliver than DOT.
We faced a series of difficulties in undertaking this research, which primarily involved challenges in recruiting socially excluded groups for consented research as well as logistical difficulties in conducting work outside mainstream NHS settings. These difficulties, to a large extent, mirror the challenges that socially excluded groups experience in accessing health services. Nevertheless, we were able to identify important approaches to improving management and control that have already influenced practice, demonstrating the value of funding research in these groups despite the challenges. Although focused in London, the results of our work are likely to be generalisable to other major urban settings where socially excluded groups, such as people experiencing homelessness, drug users and prisoners, have high levels of tuberculosis and poor outcomes. The VOT intervention also shows very wide potential applicability for improving adherence to tuberculosis treatment in settings across the world.
Recommendations for future research
-
How can the uptake of treatment of latent tuberculosis infection be maximised in homeless populations?
-
How can radiographic and latent tuberculosis infection screening programmes in prisons best be operationalised?
-
How can identification and treatment of homeless populations, prisoners and drug users for hepatitis C best be maximised?
-
How can prison and community tuberculosis care best be integrated to reduce loss to follow-up and treatment interruption?
-
What is the effectiveness of VOT in high-incidence countries?
-
Can VOT be used to support adherence in other conditions in socially complex groups (e.g. hepatitis C and mental health management)?
Acknowledgements
We wish to acknowledge the support of the Find&Treat service – University College London Hospitals, without which the work could not have happened.
We wish to acknowledge the many specialist tuberculosis nurses, clinical teams and diagnostic services across London, Leicester, Coventry and Birmingham who contributed to the VOT trial (participating sites: Royal Free Hospital; Edgware Community Hospital; Whipps Cross University Hospital; Mile End Hospital; Newham University Hospital; Chelsea and Westminster Hospital; Whittington Hospital, Birmingham – Heart of England NHS Foundation Trust; Hammersmith Hospital; St Mary’s Hospital; Charing Cross Hospital, Leicester; Glenfield Hospital, Coventry; George Eliot Hospital; North Middlesex University Hospital, Birmingham; Sandwell General Hospital; Birmingham City Hospital; Croydon University Hospital; Northwick Park Hospital; Central Middlesex Hospital; Guy’s Hospital and St Thomas’ Hospital).
We wish to acknowledge the VOT trial independent steering group: Andrew Nunn (chairperson), Mark Woodhead (clinical representative), Anne Chapman (clinical representative), Claire Stephenson (tuberculosis nurse advisor) and Josie Mavromatis (Pathway representative).
We also wish to acknowledge the many services across London that supported the study, including the Groundswell peer advocacy charity, Pathway (London, UK), homeless hostels across London and London prisons.
Finally, we wish to acknowledge the patients who participated in the studies despite having often complex lives.
Contributions of authors
Alistair Story (https://orcid.org/0000-0003-3853-1715) (Honorary Senior Lecturer and Find&Treat Clinical Lead) contributed to the co-design of the programme grant, was co-lead for study implementation, and contributed to analysis and interpretation.
Elizabeth Garber (https://orcid.org/0000-0002-5962-3232) (Project Manager) contributed to the analysis, interpretation and drafting of reports.
Robert W Aldridge (https://orcid.org/0000-0003-0542-0816) (Research Fellow) contributed to the study implementation, was the lead for analysis, and contributed to analysis and interpretation.
Catherine M Smith (https://orcid.org/0000-0003-3959-8479) (Research Associate) contributed to the analysis of trial-based data and the compilation of the final report.
Joe Hall (https://orcid.org/0000-0002-7681-6124) (Research Associate) contributed to data collection and study implementation, and to the analysis and interpretation.
Gloria Ferenando (https://orcid.org/0000-0001-8580-3037) (Research Nurse) contributed to data collection and the VOT trial implementation, and to the analysis and interpretation.
Lucia Possas (https://orcid.org/0000-0001-9758-3449) (Study Data Manager) contributed to the study implementation, analysis and interpretation.
Sara Hemming (https://orcid.org/0000-0003-3487-0517) (Research Nurse) contributed to the study implementation, analysis and interpretation.
Fatima Wurie (https://orcid.org/0000-0003-4802-2308) (Research Assistant) contributed to the study implementation, analysis and interpretation.
Serena Luchenski (https://orcid.org/0000-0002-6260-4606) (Research Fellow) contributed to the study analysis and interpretation.
Ibrahim Abubakar (https://orcid.org/0000-0002-0370-1430) (Professor of Infectious Disease Epidemiology) contributed to the study design, implementation, analysis and interpretation.
Timothy D McHugh (https://orcid.org/0000-0003-4658-8594) (Professor of Microbiology) contributed to the study design, implementation, analysis and interpretation.
Peter J White (https://orcid.org/0000-0002-6644-3512) (Reader) contributed to the transmission modelling and economics.
John M Watson (https://orcid.org/0000-0002-2595-405X) (Honorary Professor of Epidemiology) contributed to the study design, implementation, analysis and interpretation.
Marc Lipman (https://orcid.org/0000-0001-7501-4448) (Consultant in Respiratory Medicine) contributed to the study design, implementation, analysis and interpretation, and was the clinical lead.
Richard Garfein (https://orcid.org/0000-0003-3663-7153) (Professor of Global Health) contributed to the VOT study design, implementation, analysis and interpretation.
Andrew C Hayward (https://orcid.org/0000-0002-3549-6232) (Professor of Infectious Disease Epidemiology and Inclusion Health) was the chief investigator, contributed to the co-design of the programme grant and was co-lead for study implementation, contributed to analysis and interpretation, and collation of the final report.
Publications
The following publications have arisen directly or indirectly (e.g. as a result of methodological developments and collaborations that would not have otherwise happened) from this programme grant.
Work arising directly from work packages 1 and 2 (screening for latent tuberculosis and blood-borne viruses)
Aldridge RW, Yates S, Hemming S, Possas L, Garber E, Lipman EM, et al. Latent TB infection and bloodborne viruses in a London prison: a cross sectional survey. Int J Epidemiol 2015;44:i247.
Aisyah DN, Shallcross L, Hayward A, Aldridge RW, Hemming S, Yates S, et al. Hepatitis C virus infection in vulnerable populations: a seroprevalence study of homeless, people who inject drugs and prisoners in London, UK. Lancet 2017;390:S18.
Aisyah DN, Shallcross L, Hayward A, Aldridge RW, Hemming S, Yates S, et al. Hepatitis C among vulnerable populations: a seroprevalence study of homeless, people who inject drugs and prisoners in London. J Viral Hepat 2018;25:1260–9.
Aldridge RW, Hayward AC, Hemming S, Yates SK, Ferenando G, Possas L, et al. High prevalence of latent tuberculosis and bloodborne virus infection in a homeless population. Thorax 2018;73:557–64.
Work arising from the health survey conducted alongside work package 1
Story A, Aldridge RW, Gray T, Burridge S, Hayward AC. Influenza vaccination, inverse care and homelessness: cross-sectional survey of eligibility and uptake during the 2011/12 season in London. BMC Public Health 2014;14:44.
Aldridge RW, Hayward A, Story A. Homelessness and quality adjusted life years: slopes and cliffs in health inequalities a cross-sectional survey. Int J Epidemiol 2015;44:80–81.
Work arising directly from work package 3 (effectiveness of peer educators)
Aldridge RW, Hayward AC, Hemming S, Possas L, Ferenando G, Garber E, et al. Effectiveness of peer educators on the uptake of mobile X-ray tuberculosis screening at homeless hostels: a cluster randomised controlled trial. BMJ Open 2015;5:e008050.
Work conducted alongside work package 3
Croft LA, Hayward AC, Story A. Tuberculosis peer educators: personal experiences of working with socially excluded communities in London. Int J Tuberc Lung Dis 2013;17(Suppl. 10):36–40.
Work arising directly from work package 4 (rapid diagnosis)
Gliddon HD, Shorten RJ, Hayward AC, Story A. A sputum sample processing method for community and mobile tuberculosis diagnosis using the Xpert MTB/RIF assay. ERJ Open Res 2019;5:00165–2018.
Work arising directly from work package 5 (randomised controlled trial of video-observed therapy)
Story A, Aldridge RW, Smith CM, Garber E, Hall J, Ferenando G, et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet 2019;393:1216–24.
Work arising indirectly from work package 5
Falzon D, Timimi H, Kurosinski P, Migliori GB, Van Gemert W, Denkinger C, et al. Digital health for the End TB Strategy: developing priority products and making them work. Eur Respir J 2016;48:29–45.
Story A, Garfein RS, Hayward A, Rusovich V, Dadu A, Soltan V, et al. Monitoring therapy compliance of tuberculosis patients by using video-enabled electronic devices. Emerging Infect Dis 2016;22:538–40.
Studies using the linkage algorithm developed for the programme grant
Aldridge RW, Shaji K, Hayward AC, Abubakar I. Accuracy of probabilistic linkage using the enhanced matching system for public health and epidemiological studies. PLOS ONE 2015;10:e0136179.
Aldridge RW, Zenner D, White PJ, Williamson EJ, Muzyamba MC, Dhavan P, et al. Tuberculosis in migrants moving from high-incidence to low-incidence countries: a population-based cohort study of 519,955 migrants screened before entry to England, Wales and Northern Ireland. Lancet 2016;388:2510–18.
Publications on tuberculosis and social exclusion arising from collaborations fostered by the programme grant
van Hest NA, Aldridge RW, de Vries G, Sandgren A, Hauer B, Hayward A, et al. Tuberculosis control in big cities and urban risk groups in the European Union: a consensus statement. Euro Surveill 2014;19:20728.
Anderson C, Anderson SR, Maguire H, Hayward AC, Story A. Tuberculosis in London: the convergence of clinical and social complexity. Eur Respir J 2016;48:1233–6.
Publications on health and social exclusion arising from collaborations fostered by the programme grant
Aldridge RW, Story A, Hwang SW, Nordentoft M, Luchenski SA, Hartwell G, et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet 2017;391:241–50.
Blackburn RM, Hayward A, Cornes M, McKee M, Lewer D, Whiteford M, et al. Outcomes of specialist discharge coordination and intermediate care schemes for patients who are homeless: analysis protocol for a population-based historical cohort. BMJ Open 2017;7:e019282.
Luchenski S, Maguire N, Aldridge RW, Hayward A, Story A, Perri P, et al. What works in inclusion health: overview of effective interventions for marginalised and excluded populations. Lancet 2018;391:266–80.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- WHO . WHO Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care (2017 Update) 2017.
- Aldridge RW, Hayward AC, Hemming S, Yates SK, Ferenando G, Possas L, et al. High prevalence of latent tuberculosis and bloodborne virus infection in a homeless population. Thorax 2018;73:557-64. https://doi.org/10.1136/thoraxjnl-2016-209579.
- Story A, Murad S, Roberts W, Verheyen M, Hayward AC. London Tuberculosis Nurses Network . Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax 2007;62:667-71. https://doi.org/10.1136/thx.2006.065409.
- Gelberg L, Panarites CJ, Morgenstern H, Leake B, Andersen RM, Koegel P. Tuberculosis skin testing among homeless adults. J Gen Intern Med 1997;12:25-33. https://doi.org/10.1007/s11606-006-0004-4.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006;6. https://doi.org/10.1186/1471-2334-6-47.
- Public Health England . Tuberculosis (TB): Regional and Devolved Administration Reports 2016. www.gov.uk/government/publications/tuberculosis-tb-regional-reports (accessed 3 May 2019).
- Menzies D, Al Jahdali H, Al Otaibi B. Recent developments in treatment of latent tuberculosis infection. Indian J Med Res 2011;133:257-66.
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011;365:2155-66. https://doi.org/10.1056/NEJMoa1104875.
- Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006;174:935-52. https://doi.org/10.1164/rccm.200510-1666ST.
- Jit M, Stagg HR, Aldridge RW, White PJ, Abubakar I. Find and Treat Evaluation Team . Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. BMJ 2011;343. https://doi.org/10.1136/bmj.d5376.
- NICE . Clinical Diagnosis and Management of Tuberculosis and Measures for Its Prevention and Control 2016. www.nice.org.uk/guidance/ng33 (accessed 3 May 2019).
- NICE . Tuberculosis: Identification and Management in Under-Served Groups. Public Health Guideline (PH37) 2012. www.nice.org.uk/guidance/ph37 (accessed 3 May 2019).
- Qiagen . Interpretation Criteria for QuantiFERON-TB Gold Plus (Table 4) 2016. www.quantiferon.com/wp-content/uploads/2017/04/English_QFTPlus_ELISA_R04_022016.pdf (accessed 3 May 2019).
- Komurian-Pradel F, Perret M, Deiman B, Sodoyer M, Lotteau V, Paranhos-Baccalà G, et al. Strand specific quantitative real-time PCR to study replication of hepatitis C virus genome. J Virol Methods 2004;116:103-6. https://doi.org/10.1016/j.jviromet.2003.10.004.
- Lobato MN, Leary LS, Simone PM. Treatment for latent TB in correctional facilities: a challenge for TB elimination. Am J Prev Med 2003;24:249-53. https://doi.org/10.1016/S0749-3797(02)00583-4.
- Greveson K, Goodhand J, Capocci S, Woodward S, Murray C, Cropley I, et al. Yield and cost effectiveness of mycobacterial infection detection using a simple IGRA-based protocol in UK subjects with inflammatory bowel disease suitable for anti-TNFα therapy. J Crohns Colitis 2013;7:412-18. https://doi.org/10.1016/j.crohns.2012.08.010.
- Public Health England . Hepatitis C in England and the UK n.d. www.gov.uk/government/publications/hepatitis-c-in-the-uk (accessed 3 May 2019).
- Homeless Link . Health Needs Audit – Explore the Data n.d. www.homeless.org.uk/facts/homelessness-in-numbers/health-needs-audit-explore-data (accessed 3 May 2019).
- Laurenti P, Bruno S, Quaranta G, La Torre G, Cairo AG, Nardella P, et al. Tuberculosis in sheltered homeless population of Rome: an integrated model of recruitment for risk management. Scientific World Journal 2012;2012. https://doi.org/10.1100/2012/396302.
- Lee CH, Jeong YJ, Heo EY, Park JS, Lee JS, Lee BJ, et al. Active pulmonary tuberculosis and latent tuberculosis infection among homeless people in Seoul, South Korea: a cross-sectional study. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-720.
- McAdam JM, Bucher SJ, Brickner PW, Vincent RL, Lascher S. Latent tuberculosis and active tuberculosis disease rates among the homeless, New York, New York, USA, 1992–2006. Emerging Infect Dis 2009;15:1109-11. https://doi.org/10.3201/eid1507.080410.
- Tabuchi T, Takatorige T, Hirayama Y, Nakata N, Harihara S, Shimouchi A, et al. Tuberculosis infection among homeless persons and caregivers in a high-tuberculosis-prevalence area in Japan: a cross-sectional study. BMC Infect Dis 2011;11. https://doi.org/10.1186/1471-2334-11-22.
- Beijer U, Wolf A, Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:859-70. https://doi.org/10.1016/S1473-3099(12)70177-9.
- Sherriff LC, Mayon-White RT. A survey of hepatitis C prevalence amongst the homeless community of Oxford. J Public Health Med 2003;25:358-61. https://doi.org/10.1093/pubmed/fdg088.
- Story A, Aldridge RW, Gray T, Burridge S, Hayward AC. Influenza vaccination, inverse care and homelessness: cross-sectional survey of eligibility and uptake during the 2011/12 season in London. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-44.
- Story A, Aldridge RW, Abubakar I, Stagg HR, Lipman M, Watson JM, et al. Active case finding for pulmonary tuberculosis using mobile digital chest radiography: an observational study. Int J Tuberc Lung Dis 2012;16:1461-7. https://doi.org/10.5588/ijtld.11.0773.
- GOV.UK . Statutory Homelessness January to March 2016 and Homelessness Prevention and Relief 2015 to 2016: England n.d. www.gov.uk/government/statistics/statutory-homelessness-january-to-march-2016-and-homelessness-prevention-and-relief-2015-to-2016-england (accessed 3 May 2019).
- GOV.UK . Rough Sleeping in England: Autumn 2015 n.d. www.gov.uk/government/statistics/rough-sleeping-in-england-autumn-2015 (accessed 3 May 2019).
- Story A. Slopes and cliffs in health inequalities: comparative morbidity of housed and homeless people. Lancet 2013;382. https://doi.org/10.1016/S0140-6736(13)62518-0.
- Morrison DS. Homelessness as an independent risk factor for mortality: results from a retrospective cohort study. Int J Epidemiol 2009;38:877-83. https://doi.org/10.1093/ije/dyp160.
- Fenton KA, Aquino GA, Dean HD. Program collaboration and service integration in the prevention and control of HIV infection, viral hepatitis, STDs, and tuberculosis in the U.S.: lessons learned from the field. Public Health Rep 2014;129:1-4. https://doi.org/10.1177/00333549141291S101.
- Public Health England and NHS England . Tuberculosis (TB): Collaborative Strategy for England n.d. www.gov.uk/government/publications/collaborative-tuberculosis-strategy-for-england (accessed 3 May 2019).
- NICE . Identifying and Managing Tuberculosis Among Hard-to-Reach Groups. NICE Public Health Guidance 37 2012. www.nice.org.uk/guidance/ng33/evidence/appendix-n-ph37-80851860866 (accessed 16 October 2020).
- Aldridge RW. Homelessness and quality adjusted life years: slopes and cliffs in health inequalities a cross-sectional survey. Int J Epidemiol 2015;44:i80-1. https://doi.org/10.1093/ije/dyv097.295.
- Swan D, Cullen W, Macias J, Oprea C, Story A, Surey J, et al. Hepcare Europe – bridging the gap in the treatment of hepatitis C: study protocol. Expert Rev Gastroenterol Hepatol 2018;12:303-14. https://doi.org/10.1080/17474124.2018.1424541.
- Aldridge RW, Shaji K, Hayward AC, Abubakar I. Accuracy of probabilistic linkage using the enhanced matching system for public health and epidemiological studies. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0136179.
- Melendez J, Hogeweg L, Sánchez CI, Philipsen RHHM, Aldridge RW, Hayward AC, et al. Accuracy of an automated system for tuberculosis detection on chest radiographs in high-risk screening. Int J Tuberc Lung Dis 2018;22:567-71. https://doi.org/10.5588/ijtld.17.0492.
- Aldridge RW, Zenner D, White PJ, Williamson EJ, Muzyamba MC, Dhavan P, et al. Tuberculosis in migrants moving from high-incidence to low-incidence countries: a population-based cohort study of 519,955 migrants screened before entry to England, Wales, and Northern Ireland. Lancet 2016;388:2510-18. https://doi.org/10.1016/S0140-6736(16)31008-X.
- Aisyah DN, Shallcross L, Hayward A, Aldridge RW, Hemming S, Yates S, et al. Hepatitis C among vulnerable populations: a seroprevalence study of homeless, people who inject drugs and prisoners in London. J Viral Hepat 2018;25:1260-9. https://doi.org/10.1111/jvh.12936.
- Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLOS Med 2010;7. https://doi.org/10.1371/journal.pmed.1000381.
- Ruddy MC, Davies AP, Yates MD, Yates S, Balasegaram S, Drabu Y, et al. Outbreak of isoniazid resistant tuberculosis in north London. Thorax 2004;59:279-85. https://doi.org/10.1136/thx.2003.010405.
- Winetsky DE, Almukhamedov O, Pulatov D, Vezhnina N, Dooronbekova A, Zhussupov B. Prevalence, risk factors and social context of active pulmonary tuberculosis among prison inmates in Tajikistan. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0086046.
- Hanau-Berçot B, Grémy I, Raskine L, Bizet J, Gutierrez MC, Boyer-Mariotte S, et al. A one-year prospective study (1994–1995) for a first evaluation of tuberculosis transmission in French prisons. Int J Tuberc Lung Dis 2000;4:853-9.
- March F, Coll P, Guerrero RA, Busquets E, Caylà JA, Prats G. Predictors of tuberculosis transmission in prisons: an analysis using conventional and molecular methods. AIDS 2000;14:525-35. https://doi.org/10.1097/00002030-200003310-00008.
- Williams K, Poyser J, Hopkins K. Research Summary 3 12. Accommodation, Homelessness and Reoffending of Prisoners: Results from the Surveying Prisoner Crime Reduction (SPCR) Survey 2012. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/278806/homelessness-reoffending-prisoners.pdf (accessed 3 May 2019).
- Anderson C, Story A, Brown T, Drobniewski F, Abubakar I. Tuberculosis in UK prisoners: a challenge for control. J Epidemiol Community Health 2010;64:373-6. https://doi.org/10.1136/jech.2009.094375.
- Department of Health and National AIDS Trust . Tackling Blood-Borne Viruses in Prison. A Framework for Best Practice in the UK 2011. www.nat.org.uk/publication/tackling-blood-borne-viruses-prisons-framework-best-practice-uk (accessed 3 May 2019).
- Public Health England . Unlinked Anonymous HIV and Viral Hepatitis Monitoring Among PWID: 2014 Report 2014. www.gov.uk/government/publications/people-who-inject-drugs-hiv-and-viral-hepatitis-monitoring (accessed 3 May 2019).
- Public Health England, Health Protection Scotland, Public Health Wales and Public Health Agency . Shooting Up. Infections Among People Who Inject Drugs in the United Kingdom 2013. An Update November 2014 2014. www.gov.uk/government/publications/shooting-up-infections-among-people-who-inject-drugs-in-the-uk (accessed 3 May 2019).
- Tamne S. The Use of Directly Observed Therapy in Tuberculosis. A Survey by the London TB Workforce. London: London TB Workforce; 2017.
- Sagnelli E, Starnini G, Sagnelli C, Monarca R, Zumbo G, Pontali E, et al. Blood born viral infections, sexually transmitted diseases and latent tuberculosis in Italian prisons: a preliminary report of a large multicenter study. Eur Rev Med Pharmacol Sci 2012;16:2142-6.
- Ritter C, Elger BS. Prevalence of positive tuberculosis skin tests during 5 years of screening in a Swiss remand prison. Int J Tuberc Lung Dis 2012;16:65-9. https://doi.org/10.5588/ijtld.11.0159.
- Marco A, Solé N, Orcau A, Escribano M, del Baño L, Quintero S, et al. Prevalence of latent tuberculosis infection in inmates recently incarcerated in a men’s prison in Barcelona. Int J Tuberc Lung Dis 2012;16:60-4. https://doi.org/10.5588/ijtld.11.0007.
- García-Guerrero J, Marco Mouriño A, Sáiz de la Hoya Zamácola P, Vera-Remartínez EJ. Grupo de estudio PREVALHEP de prisiones . Multi-centre study of the prevalence of latent tuberculosis infection amongst inmates in Spanish prisons. Rev Esp Sanid Penit 2010;12:79-85. https://doi.org/10.4321/S1575-06202010000300003.
- Baillargeon J, Black SA, Leach CT, Jenson H, Pulvino J, Bradshaw P, et al. The infectious disease profile of Texas prison inmates. Prev Med 2004;38:607-12. https://doi.org/10.1016/j.ypmed.2003.11.020.
- Cohen JA. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37-46. https://doi.org/10.1177/001316446002000104.
- Public Health England . Tuberculosis in the UK. 2014 Report 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/360335/TB_Annual_report__4_0_300914.pdf (accessed 3 May 2019).
- Aerts A, Hauer B, Wanlin M, Veen J. Tuberculosis and tuberculosis control in European prisons. Int J Tuberc Lung Dis 2006;10:1215-23.
- Puisis M, Feinglass J, Lidow E, Mansour M. Radiographic screening for tuberculosis in a large urban county jail. Public Health Rep 1996;111:330-4.
- Jones TF, Schaffner W. Miniature chest radiograph screening for tuberculosis in jails: a cost-effectiveness analysis. Am J Respir Crit Care Med 2001;164:77-81. https://doi.org/10.1164/ajrccm.164.1.2010108.
- Leung CC, Chan CK, Tam CM, Yew WW, Kam KM, Au KF, et al. Chest radiograph screening for tuberculosis in a Hong Kong prison. Int J Tuberc Lung Dis 2005;9:627-32.
- Kazi AM, Shah SA, Jenkins CA, Shepherd BE, Vermund SH. Risk factors and prevalence of tuberculosis, human immunodeficiency virus, syphilis, hepatitis B virus, and hepatitis C virus among prisoners in Pakistan. Int J Infect Dis 2010;14:e60-6. https://doi.org/10.1016/j.ijid.2009.11.012.
- Verneuil L, Vidal JS, Ze Bekolo R, Vabret A, Petitjean J, Leclercq R, et al. Prevalence and risk factors of the whole spectrum of sexually transmitted diseases in male incoming prisoners in France. Eur J Clin Microbiol Infect Dis 2009;28:409-13. https://doi.org/10.1007/s10096-008-0642-z.
- Fialho M, Messias M, Page-Shafer K, Farre L, Schmalb M, Pedral-Sampaio D, et al. Prevalence and risk of blood-borne and sexually transmitted viral infections in incarcerated youth in Salvador, Brazil: opportunity and obligation for intervention. AIDS Behav 2008;12:17-24. https://doi.org/10.1007/s10461-008-9409-x.
- Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health 2004;81:25-37. https://doi.org/10.1093/jurban/jth085.
- Solé N, Marco A, Escribano M, Orcau A, Quintero S, Del Baño L, et al. Prevalence of latent tuberculosis infection amongst immigrants entering prison. Rev Esp Sanid Penit 2012;14:12-8. https://doi.org/10.1590/S1575-06202012000100003.
- Hussain H, Akhtar S, Nanan D. Prevalence of and risk factors associated with Mycobacterium tuberculosis infection in prisoners, North West Frontier Province, Pakistan. Int J Epidemiol 2003;32:794-9. https://doi.org/10.1093/ije/dyg247.
- Margolis B, Al-Darraji HA, Wickersham JA, Kamarulzaman A, Altice FL. Prevalence of tuberculosis symptoms and latent tuberculous infection among prisoners in northeastern Malaysia. Int J Tuberc Lung Dis 2013;17:1538-44. https://doi.org/10.5588/ijtld.13.0193.
- Al-Darraji HA, Kamarulzaman A, Altice FL. Latent tuberculosis infection in a Malaysian prison: implications for a comprehensive integrated control program in prisons. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-22.
- Chan PC, Yang CH, Chang LY, Wang KF, Kuo YC, Lin CJ, et al. Lower prevalence of tuberculosis infection in BCG vaccinees: a cross-sectional study in adult prison inmates. Thorax 2013;68:263-8. https://doi.org/10.1136/thoraxjnl-2012-202208.
- Tait JM, McIntyre PG, McLeod S, Nathwani D, Dillon JF. The impact of a managed care network on attendance, follow-up and treatment at a hepatitis C specialist centre. J Viral Hepat 2010;17:698-704. https://doi.org/10.1111/j.1365-2893.2009.01227.x.
- Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction 2008;103:905-18. https://doi.org/10.1111/j.1360-0443.2008.02188.x.
- Knott A, Dieperink E, Willenbring ML, Heit S, Durfee JM, Wingert M, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol 2006;101:2254-62. https://doi.org/10.1111/j.1572-0241.2006.00731.x.
- Aldridge RW, Hayward AC, Hemming S, Possas L, Ferenando G, Garber E, et al. Effectiveness of peer educators on the uptake of mobile X-ray tuberculosis screening at homeless hostels: a cluster randomised controlled trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008050.
- Public Health England . Tuberculosis in the UK: 2013 Report 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/325632/TB_in_the_UK.pdf (accessed 3 May 2019).
- de Vries G, Aldridge RW, Cayla JA, Haas WH, Sandgren A, van Hest NA, et al. Tuberculosis in European Union Big Cities Working Group . Epidemiology of tuberculosis in big cities of the European Union and European Economic Area countries. Euro Surveill 2014;19. https://doi.org/10.2807/1560-7917.es2014.19.9.20726.
- de Vries G, van Hest RA. From contact investigation to tuberculosis screening of drug addicts and homeless persons in Rotterdam. Eur J Public Health 2006;16:133-6. https://doi.org/10.1093/eurpub/cki203.
- Paquette K, Cheng MP, Kadatz MJ, Cook VJ, Chen W, Johnston JC. Chest radiography for active tuberculosis case finding in the homeless: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2014;18:1231-6. https://doi.org/10.5588/ijtld.14.0105.
- Webel AR, Okonsky J, Trompeta J, Holzemer WL. A systematic review of the effectiveness of peer-based interventions on health-related behaviors in adults. Am J Public Health 2010;100:247-53. https://doi.org/10.2105/AJPH.2008.149419.
- Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Educ Prev 2009;21:181-206. https://doi.org/10.1521/aeap.2009.21.3.181.
- Croft LA, Hayward AC, Story A. Tuberculosis peer educators: personal experiences of working with socially excluded communities in London. Int J Tuberc Lung Dis 2013;17:36-40. https://doi.org/10.5588/ijtld.13.0309.
- Groundswell . Groundswell 2014. www.groundswell.org.uk/ (accessed 3 May 2019).
- Gliddon HD, Shorten RJ, Hayward AC, Story A. A sputum sample processing method for community and mobile tuberculosis diagnosis using the Xpert MTB/RIF assay. ERJ Open Res 2019;5:00165-2018. https://doi.org/10.1183/23120541.00165-2018.
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15. https://doi.org/10.1056/NEJMoa0907847.
- Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, Fennelly K, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol 2010;48:3551-7. https://doi.org/10.1128/JCM.01053-10.
- Story A, Aldridge RW, Smith CM, Garber E, Hall J, Ferenando G, et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet 2019;393:1216-24. https://doi.org/10.1016/S0140-6736(18)32993-3.
- Bayer R, Wilkinson D. Directly observed therapy for tuberculosis: history of an idea. Lancet 1995;345:1545-8. https://doi.org/10.1016/S0140-6736(95)91090-5.
- WHO TB Control Programme . WHO Report on the Tuberculosis Epidemic: Stop TB at the Source 1996.
- Fox W. The problem of self-administration of drugs; with particular reference to pulmonary tuberculosis. Tubercle 1958;39:269-74. https://doi.org/10.1016/s0041-3879(58)80088-4.
- Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016;63:e147-95. https://doi.org/10.1093/cid/ciw376.
- Story A, Garfein RS, Hayward A, Rusovich V, Dadu A, Soltan V, et al. Monitoring therapy compliance of tuberculosis patients by using video-enabled electronic devices. Emerging Infect Dis 2016;22:538-40. https://doi.org/10.3201/eid2203.151620.
- Krueger K, Ruby D, Cooley P, Montoya B, Exarchos A, Djojonegoro BM, et al. Videophone utilization as an alternative to directly observed therapy for tuberculosis. Int J Tuberc Lung Dis 2010;14:779-81.
- Wade VA, Karnon J, Eliott JA, Hiller JE. Home videophones improve direct observation in tuberculosis treatment: a mixed methods evaluation. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0050155.
- Mirsaeidi M, Farshidpour M, Banks-Tripp D, Hashmi S, Kujoth C, Schraufnagel D. Video directly observed therapy for treatment of tuberculosis is patient-oriented and cost-effective. Eur Respir J 2015;46:871-4. https://doi.org/10.1183/09031936.00011015.
- Holzschuh EL, Province S, Johnson K, Walls C, Shemwell C, Martin G, et al. Use of video directly observed therapy for treatment of latent tuberculosis infection - Johnson County, Kansas, 2015. MMWR Morb Mortal Wkly Rep 2017;66:387-9. https://doi.org/10.15585/mmwr.mm6614a3.
- Gassanov MA, Feldman LJ, Sebastian A, Kraguljac MJ, Rea E, Yaffe B. The use of videophone for directly observed therapy for the treatment of tuberculosis. Can J Public Health 2013;104. https://doi.org/10.17269/cjph.104.3869.
- Chuck C, Robinson E, Macaraig M, Alexander M, Burzynski J. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int J Tuberc Lung Dis 2016;20:588-93. https://doi.org/10.5588/ijtld.15.0738.
- Garfein RS, Collins K, Muñoz F, Moser K, Cerecer-Callu P, Raab F, et al. Feasibility of tuberculosis treatment monitoring by video directly observed therapy: a binational pilot study. Int J Tuberc Lung Dis 2015;19:1057-64. https://doi.org/10.5588/ijtld.14.0923.
- Sinkou H, Hurevich H, Rusovich V, Zhylevich L, Falzon D, de Colombani P, et al. Video-observed treatment for tuberculosis patients in Belarus: findings from the first programmatic experience. Eur Respir J 2017;49. https://doi.org/10.1183/13993003.02049-2016.
- Hoffman JA, Cunningham JR, Suleh AJ, Sundsmo A, Dekker D, Vago F, et al. Mobile direct observation treatment for tuberculosis patients: a technical feasibility pilot using mobile phones in Nairobi, Kenya. Am J Prev Med 2010;39:78-80. https://doi.org/10.1016/j.amepre.2010.02.018.
- Nguyen TA, Pham MT, Nguyen TL, Nguyen VN, Pham DC, Nguyen BH, et al. Video directly observed therapy to support adherence with treatment for tuberculosis in Vietnam: a prospective cohort study. Int J Infect Dis 2017;65:85-9. https://doi.org/10.1016/j.ijid.2017.09.029.
- Olano-Soler H, Thomas D, Joglar O, Rios K, Torres-Rodríguez M, Duran-Guzman G, et al. Notes from the field: use of asynchronous video directly observed therapy for treatment of tuberculosis and latent tuberculosis infection in a long-term-care facility – Puerto Rico, 2016–17. MMWR Morb Mortal Wkly Rep 2017;66:1386-7. https://doi.org/10.15585/mmwr.mm6650a5.
- Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974;15:443-53. https://doi.org/10.1002/cpt1974155443.
- Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull 1993;13:19-23.
- Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD003343.pub3.
- Tian JH, Lu ZX, Bachmann MO, Song FJ. Effectiveness of directly observed treatment of tuberculosis: a systematic review of controlled studies. Int J Tuberc Lung Dis 2014;18:1092-8. https://doi.org/10.5588/ijtld.13.0867.
- Moodley R, Godec TR. STREAM Trial Team . Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur Respir Rev 2016;25:29-35. https://doi.org/10.1183/16000617.0080-2015.
- White PJ, Abubakar I. Improving control of tuberculosis in low-burden countries: insights from mathematical modeling. Front Microbiol 2016;7. https://doi.org/10.3389/fmicb.2016.00394.
- White P, Jit M, Stagg H, Pimpin L, Choi Y, Mugwagwa T. Economic Analysis of Identifying and Managing Tuberculosis in Hard to Reach Groups: Homeless and Prison Populations 2011. www.nice.org.uk/guidance/ph37/documents/economic-analysis-of-identifying-and-managing-tuberculosis-in-hard-to-reach-groups-homeless-and-prison-populations-2 (accessed 3 May 2019).
- Pareek M, Abubakar I, White PJ, Garnett GP, Lalvani A. Tuberculosis screening of migrants to low-burden nations: insights from evaluation of UK practice. Eur Respir J 2011;37:1175-82. https://doi.org/10.1183/09031936.00105810.
- Greater London Authority . CHAIN Annual Report 2016–17 2018. https://data.london.gov.uk/dataset/chain-reports (accessed 25 September 2020).
- Cabinet Office . Rough Sleeping: Report by the Social Exclusion Unit (Command Paper) 1998.
- Salomon JA, Lloyd-Smith JO, Getz WM, Resch S, Sánchez MS, Porco TC, et al. Prospects for advancing tuberculosis control efforts through novel therapies. PLOS Med 2006;3. https://doi.org/10.1371/journal.pmed.0030273.
- The National Tuberculosis Institute B . Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull World Health Organ 1974;51:473-88.
- Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 2010;137:952-68. https://doi.org/10.1378/chest.09-2350.
- White PJ. Imperial College – LTBI Treatment Report 2015.
- Cudahy P, Shenoi SV. Diagnostics for pulmonary tuberculosis. Postgrad Med J 2016;92:187-93. https://doi.org/10.1136/postgradmedj-2015-133278.
- Taegtmeyer M, Beeching NJ, Scott J, Seddon K, Jamieson S, Squire SB, et al. The clinical impact of nucleic acid amplification tests on the diagnosis and management of tuberculosis in a British hospital. Thorax 2008;63:317-21. https://doi.org/10.1136/thx.2007.083816.
- Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;1. https://doi.org/10.1002/14651858.CD009593.pub3.
- Abubakar I, White P, Jit M, Pimpin L, Aldridge R, Tamne S, et al. Evaluation of the Find and Treat Service for the Control of Tuberculosis Amongst Hard to Reach Groups. London: Department of Health; 2011.
- Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Dye C, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA 2009;106:13980-5. https://doi.org/10.1073/pnas.0901720106.
- NICE . Developing NICE Guidelines: The Manual. Process and Methods Guide 2014.
- Pareek M, Bond M, Shorey J, Seneviratne S, Guy M, White P, et al. Community-based evaluation of immigrant tuberculosis screening using interferon γ release assays and tuberculin skin testing: observational study and economic analysis. Thorax 2013;68:230-9. https://doi.org/10.1136/thoraxjnl-2011-201542.
- Drobniewski F, Cooke M, Jordan J, Casali N, Mugwagwa T, Broda A, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19340.
- NHS England . Guide to the Enhanced Tariff Option for 2015 16 2016. https://webarchive.nationalarchives.gov.uk/20161103233943tf_/https://www.england.nhs.uk/resources/pay-syst/tariff-guide/ (accessed 3 May 2019).
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. https://doi.org/10.1136/bmj.316.7133.736.
- Kruijshaar ME, Lipman M, Essink-Bot ML, Lozewicz S, Creer D, Dart S, et al. Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis 2010;14:296-302. https://doi.org/10.1164/ajrccm-conference.2009.179.1_MeetingAbstracts.A1416.
Appendix 1 Latent tuberculosis infection screening in the homeless: transmission dynamic health economic analysis
Introduction
We analysed the cost-effectiveness of adding screening and treatment for latent tuberculosis infection to screening and treatment for active tuberculosis in people experiencing homelessness in London, using an integrated transmission dynamic and health economic model. 108 Dynamic transmission models are preferred over health-state transition models as they can take account of onward transmission chains more effectively. The model used is an individual-based model in which individuals are ‘followed’ while they are homeless and after re-entering the general population, to capture the long-term health consequences of tuberculosis infection, for case finding and for treatment while homeless; infection acquired while homeless can result in active tuberculosis disease after re-entering the general population (which causes QALY loss and incurs treatment cost), and the treatment of latent tuberculosis infection while homeless may avert this.
Analysis
In the model, each individual is in one of a number of health states: uninfected (naive), uninfected (recovered), latently infected (fast progressing or slow progressing), with active tuberculosis disease (sputum smear negative or positive), on treatment for latent tuberculosis infection or on treatment for active tuberculosis. Figure 7 shows transitions between states that can occur in the model. Uninfected individuals who become infected move into the latent infection state. Individuals with tuberculosis infection history (recovered) are assumed to have partial immunity and, therefore, have a reduced probability of (re-)acquiring tuberculosis infection compared with naive individuals. Individuals in the latent state can progress to active tuberculosis disease, which is infectious and can be diagnosed through active or passive case finding, resulting in individuals being placed on tuberculosis treatment. Active case finding for active tuberculosis occurs through chest radiography on the mobile radiographic unit, with cases of abnormal radiology being referred to hospital for tuberculosis diagnosis. Passive case finding for active tuberculosis occurs when individuals present for care. In scenarios in which latent tuberculosis infection screening occurs, a proportion of diagnosed individuals are treated. Patients who are successfully treated for latent tuberculosis infection or for active tuberculosis enter the recovered state. Patients who are not successfully treated return to their former state of latent tuberculosis infection or active tuberculosis disease. Individuals whose IGRA test was false positive remain in the naive or recovered state regardless of whether they are treated or not, but costs are incurred for those who are treated. Active tuberculosis disease causes QALY loss through morbidity (which is reduced by treatment) and mortality.
FIGURE 7.
Flow diagram showing health states of and treatment outcomes for patients in the model of tuberculosis. ‘Untreated smear positive’ and ‘Untreated smear negative’ compartments denote infectious health states. Different lines represent different transitions: infection (dark-blue lines), disease progression (mid-blue lines), tuberculosis treatment initiation (orange lines), tuberculosis treatment failure (dashed grey lines) and recovery from tuberculosis disease (light-blue lines). LTBI, latent tuberculosis infection; TB, tuberculosis.

Economic analysis
The economic analysis is from an NHS perspective. In the baseline (current practice) scenario, a mobile radiographic unit provides people experiencing homelessness with screening for active tuberculosis; individuals with abnormal radiographs are referred to hospital for diagnosis and those with tuberculosis are treated. Individuals with active tuberculosis can also be found passively, when they present for care.
We compared the baseline scenario with intervention scenarios in which people experiencing homelessness are offered testing for latent tuberculosis infection by IGRA, and offered treatment if they test positive. As treatment was not offered in the empirical study, there is uncertainty regarding the proportion of IGRA-positive patients who would accept and complete latent tuberculosis infection treatment, so we compared scenarios in which this proportion varied (i.e. 25%, 50% and 75%). The intervention is applied for 10 years, and the model considers the lifetime of the population cohort. Analysis was from the perspective of the NHS. The total net costs incurred (including staff time, drugs and diagnostic tests) and the QALYs accrued by the patient cohort are calculated. The cost-effectiveness of different options is compared using incremental ICERs relative to current practice. Each scenario (baseline and interventions) was run 1000 times. Costs and health utilities are discounted at 3.5% per annum. Parameter values were obtained primarily from sources used to inform NICE guidance11,33 or this study, as appropriate (Tables 13 and 14).
| Parameter description | Value | Source/reference |
|---|---|---|
| Population characteristics | ||
| Size of homeless population | 20,000 | Greater London Authority111 |
| Average duration of homelessness | 7 years | Cabinet Office112 |
| Prevalence of latent tuberculosis infection | 18% | This study |
| Tuberculosis natural history | ||
| Proportion of incident infections that are slow progressing | 0.72 | Fitted |
| Per-capita rate of slow progression to active tuberculosis disease | 1.13 × 10–4 per annum | Salomon et al.113 |
| Per-capita rate of fast progression to active tuberculosis disease | 0.79 per annum | Salomon et al.113 |
| Proportion of incident active tuberculosis disease that is smear positive | 0.6 | Salomon et al.113 |
| Per-capita mortality rate of untreated active disease | 0.23 per annum | National Tuberculosis Institute114 |
| Per-capita rate of conversion from smear negative to smear positive | 0.015 per annum | Salomon et al.113 |
| Per-capita rate of self-cure: natural reversion from active disease to latent infection | 0.21 per annum | National Tuberculosis Institute114 |
| Screening and treatment | ||
| Number of latent tuberculosis infection screening events per year | 4500 | This study |
| IGRA sensitivity for latent tuberculosis infection | 0.84 | Diel et al.115 |
| IGRA specificity for latent tuberculosis infection | 0.99 | Diel et al.115 |
| Proportion of IGRA-positive individuals treated for latent tuberculosis infection | 25–75% | Varied in scenarios |
| Mean duration of successful treatment for latent tuberculosis infection | 90 days | White and Jit116 |
| Mean duration of unsuccessful treatment for latent tuberculosis infection | 30 days | Assumed |
| Number of mobile radiographic unit screening events per year | 7500 | This study |
| Per-capita rate of passive case finding for active tuberculosis disease | 2.35 per annum | Jit et al.10 |
| Proportion of homeless active tuberculosis cases treated successfully | 0.65 | Jit et al.10 |
| Chest radiograph specificity for active tuberculosis disease | 0.63 | Cudahy and Shenoi117 |
| Chest radiograph sensitivity for active tuberculosis disease | 0.73 | Cudahy and Shenoi117 |
| Sputum smear microscopy specificity for active tuberculosis disease | 0.77 | Taegtmeyer et al.118 |
| Sputum smear microscopy sensitivity for active tuberculosis disease | 0.54 | Taegtmeyer et al.118 |
| Average time to culture result | 28 days | Steingart et al.119 |
| Mean duration of successful treatment for active tuberculosis disease | 0.34 years (125 days) | Abubakar et al.120 |
| Mean duration of unsuccessful treatment for active tuberculosis disease | 0.16 years (2 months) | Abubakar et al.120 |
| Per-capita mortality rate of unsuccessfully treated active tuberculosis disease | 0.077 per annum | Salomon et al.113 |
| Relative infectivity of unsuccessfully treated active tuberculosis disease | 0.75 | Fitted |
| Transmission | ||
| Relative infectivity of smear negatives (vs. smear positives) | 0.22 | Abu-Raddad et al.121 |
| Relative infectivity of unsuccessfully treated with appropriate regimen (vs. untreated) | 0.25 | Salomon et al.113 |
| Relative susceptibility of latent (slow) and recovered patients (vs. naive) | 0.25 | Salomon et al.113 |
| Parameter | Value | Source/reference |
|---|---|---|
| Discount rate | 3.5% per annum | NICE122 |
| IGRA testing cost | £56 | Pareek et al.123 |
| Cost of hospital-based active tuberculosis diagnosis | £206 | Drobniewski et al.124 |
| Cost of active tuberculosis treatment (homeless) | £10,530 | NHS125 and Jit et al. 201110 |
| Cost of latent tuberculosis treatment (homeless) | £1800 | White and Jit116 |
| Cost of active tuberculosis treatment (general population) | £5270 | NHS125 and Jit et al.10 |
| Cost of latent tuberculosis treatment (general population) | £900 | White and Jit116 |
| Quality-of-life weight for healthy individuals | 0.87 | Kind et al.126 |
| Quality-of-life weight for active tuberculosis (untreated) | 0.68 | Kruijshaar et al.127 |
| Quality-of-life weight for active tuberculosis (on treatment) | 0.81 | Kruijshaar et al.127 |
Results
In our analysis, screening for and treating latent tuberculosis infection had a net cost, as the intervention cost was greater than the averted costs of active tuberculosis disease; however, the intervention was beneficial to health, with a QALY gain.
Whether or not the intervention would be considered cost-effective depends on the value of 1 QALY and the proportion of IGRA-positive individuals who are treated for latent tuberculosis infection (Figures 8 and 9). The higher the value of 1 QALY, and the greater the proportion of IGRA-positive patients who are treated for latent tuberculosis infection, the more likely it is that the intervention would be considered cost-effective.
FIGURE 8.
Cost-effectiveness plane for latent tuberculosis infection screening and treatment compared with current practice over a 10-year intervention, with discounting at 3.5% per annum. LTBI, latent tuberculosis infection.
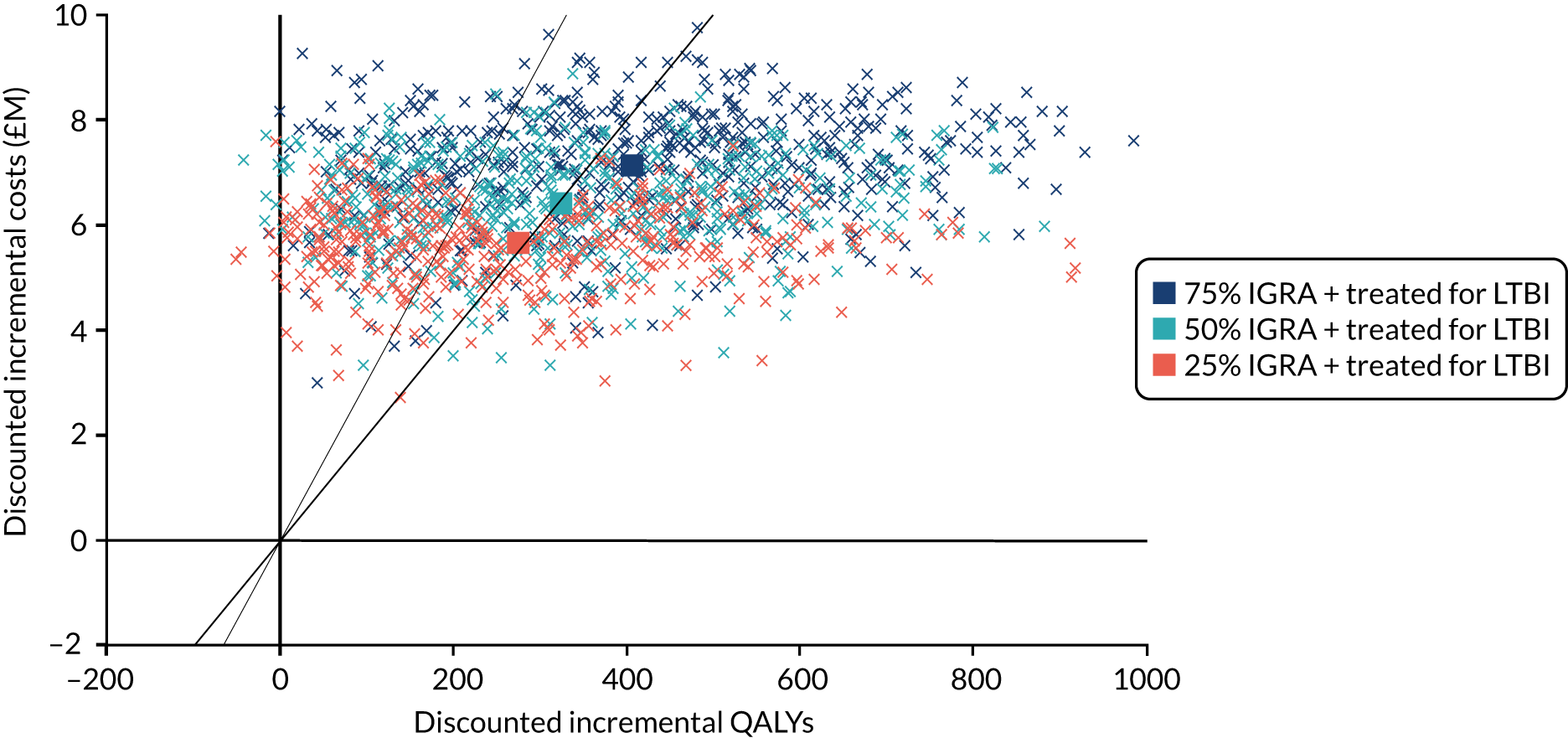
FIGURE 9.
Cost-effectiveness acceptability curves for latent tuberculosis infection screening and treatment compared with current practice for a 10-year intervention, with discounting at 3.5% per annum. Three different proportions (25%, 50% and 75%) of IGRA-positive individuals being treated for latent tuberculosis infection are considered. LTBI, latent tuberculosis infection.
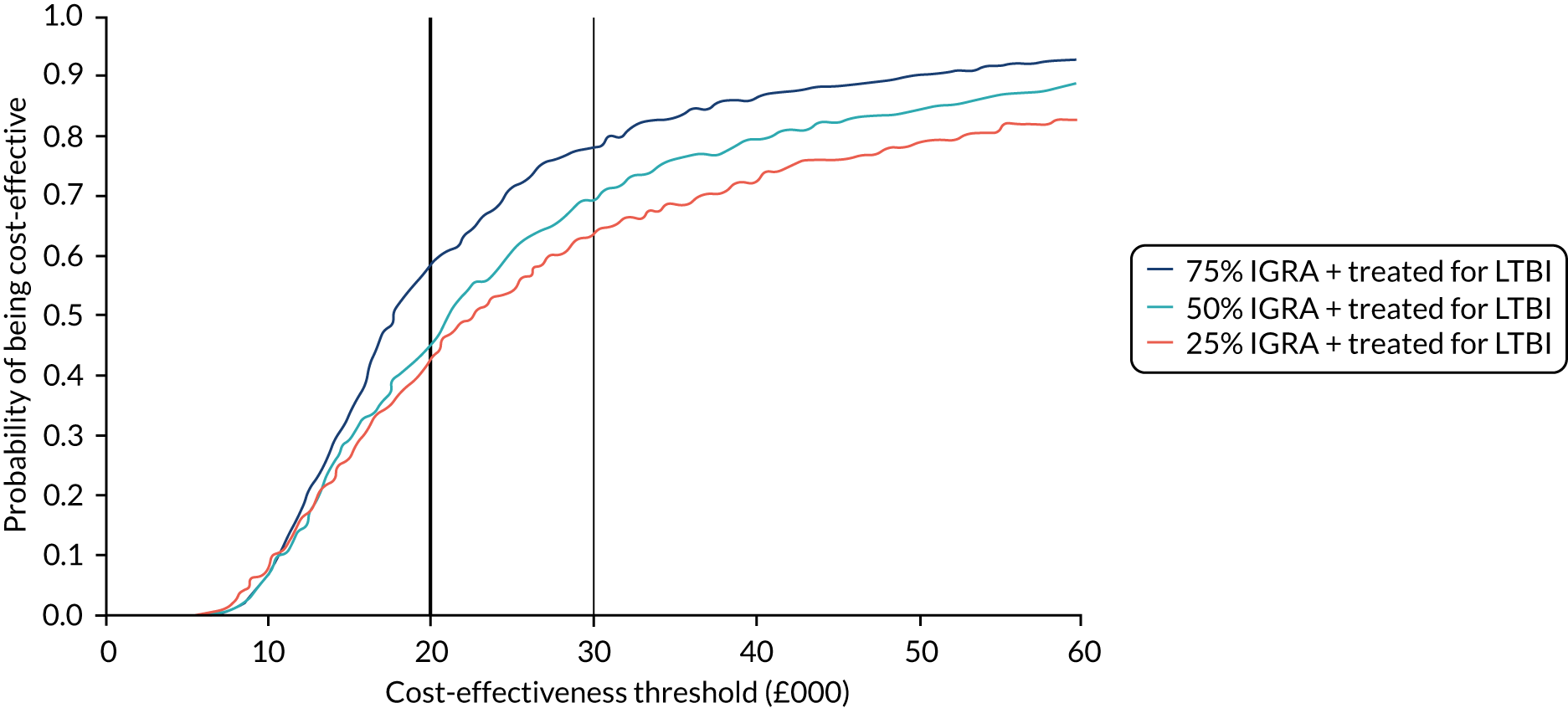
In the three scenarios that we considered regarding the proportion of IGRA-positive patients who are treated for latent tuberculosis infection, we found that if this proportion was 25% then introducing latent tuberculosis infection screening and treatment would be cost-effective when 1 QALY was valued at £30,000 (see Figure 9). If the proportion was 50% or 75%, then introducing latent tuberculosis infection screening and treatment would be cost-effective if 1 QALY was valued at £20,000; however, there is substantial uncertainty about the cost-effectiveness (Table 15).
| Scenario | Cost (£M) | Total QALYs accrued | Compared with baseline | ||
|---|---|---|---|---|---|
| Incremental cost (£M) | Incremental QALYs | ICER (£/QALY) | |||
| Baseline | 16.3 | 204,490 | – | – | – |
| 25% of IGRA + treated | 21.9 | 204,770 | 5.7 | 275 | 20,600 |
| 50% of IGRA + treated | 22.7 | 204,970 | 6.4 | 326 | 19,720 |
| 75% of IGRA + treated | 23.4 | 205,000 | 7.1 | 406 | 17,550 |
The points in Figure 8 show incremental costs and incremental QALYs from individual model realisations comparing the effect of latent tuberculosis infection screening and treatment with current practice for different proportions (25%, 50% and 75%) of IGRA-positive individuals being treated for latent tuberculosis infection. Square markers show the mean for each scenario. The diagonal lines in Figure 8 show the cost-effectiveness thresholds if 1 QALY is valued at £30,000 (thin line) or £20,000 (bold line).
Limitations
There are limited sensitivity analyses conducted and estimates of uncertainty are not presented. We do not report cost per case averted. We do not consider the adverse effects of latent tuberculosis infection and tuberculosis treatment.
Discussion
Successful treatment of latent tuberculosis infection prevents progression to infectious active tuberculosis disease and thereby averts transmission, which benefits population health and averts future costs of treatment in others, as well as benefiting the individual patient. However, not all patients with latent tuberculosis infection would progress to active tuberculosis disease without treatment and, therefore, several patients with latent tuberculosis infection have to be treated to avert a case of active tuberculosis disease. We find that the cost-effectiveness of adding screening and treatment for latent tuberculosis infection to screening and treatment for active tuberculosis in people experiencing homelessness depends on the proportion of IGRA-positive patients who accept and complete latent tuberculosis infection treatment, and that the greater this proportion is, the more likely that the intervention would be considered cost-effective. As treatment for latent tuberculosis infection was not recommended at the time of the study, it was not offered to patients and, therefore, we do not know what proportion of patients would accept and complete treatment. As this is a key determinant of cost-effectiveness, further empirical study is required to determine if this intervention should be recommended.
The probability that latent tuberculosis infection screening is cost-effective would be increased if the cost of latent tuberculosis infection testing were lower than in our analysis. With latent tuberculosis infection screening and treatment for immigrants from high-burden countries now recommended, bulk procurement of test kits may enable cost savings that would make latent tuberculosis infection screening and treatment in the homeless more likely to be cost-effective.
List of abbreviations
- anti-HBc
- antibody to hepatitis B core antigen
- anti-HBs
- antibody to hepatitis B surface antigen
- anti-HCV
- antibody to hepatitis C virus
- aOR
- adjusted odds ratio
- app
- smartphone application
- CI
- confidence interval
- CRCT
- cluster randomised controlled trial
- DOPT
- directly observed preventative treatment
- DOT
- directly observed treatment
- GP
- general practitioner
- HBsAg
- hepatitis B surface antigen
- HBV
- hepatitis B virus
- HCV
- hepatitis C virus
- HIV
- human immunodeficiency virus
- ICER
- incremental cost-effectiveness ratio
- IGRA
- interferon gamma release assay
- ITT
- intention to treat
- MDRTB
- multidrug-resistant tuberculosis
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCR
- polymerase chain reaction
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RNA
- ribonucleic acid
- SD
- standard deviation
- VOT
- video-observed treatment
- WHO
- World Health Organization
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar08090).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
