Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12015. The contractual start date was in June 2013. The final report began editorial review in March 2021 and was accepted for publication in June 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Murtagh et al. This work was produced by Murtagh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Murtagh et al.
SYNOPSIS
Background
The clinical challenge
People in the last year of life often suffer complex and multiple symptoms and distress because of their illness or impending death. 1–3 Functional status often deteriorates as the illness progresses, leading to increased dependency and greater care needs. 4 The families of those affected experience their own fears and losses. 5
This occurs across a wide range of different life-limiting illnesses. Symptoms, distress, functional trajectories and family support needs have been most widely studied in cancer, but other life-limiting conditions (e.g. advanced cardiac and respiratory diseases, progressive neurological conditions, dementia, end-stage kidney disease and advanced liver disease) bring equally challenging symptoms and support needs. 6,7 Family support is particularly important if bereavement is to be well negotiated8 and future psychological health maintained. 9
As the population age distribution changes, these needs are also extending over longer time periods as the length of time living with advanced illness increases. 10
Specialist palliative care: provision and inequities
Palliative care has developed to meet these complex needs of patients and families facing advanced progressive illness. It addresses physical and psychological symptoms and gives social, practical and spiritual support in the last months of life. 11 The UK ranked first in the 2015 Quality of Death Index, a measure of the quality of dying across 80 countries,12 with UK hospices leading the way internationally, providing a world-leading model of excellence in palliative care.
However, the work of UK hospices – which deliver both inpatient and home-based specialist palliative care – has often come from grass-roots and local initiatives, meaning that there are marked geographical inequities in provision across England. Much of hospice and specialist palliative care is funded from the charitable sector; in 2018, charities contributed £3 for every £1 from the NHS. 13 The balance between NHS and charitable funding varies markedly around the country;13 this and other factors has led to major inequities in palliative care provision.
A UK government-commissioned review into specialist palliative care14 found high levels of inequity in the provision of palliative and end-of-life care. There was diversity in the type and range of providers in any one geographical area15 and inequity in both the amount of funding (with money spent by the NHS in 2010 ranging from £186 to £6213 per person who died) and the source of funding (with the proportion funding coming from the NHS varying from 0% to 62%). 14 This could not be accounted for by variations in the populations served. 14
Even more marked variation in the commissioning of palliative services across England has been demonstrated more recently. 16 A ‘north–south’ divide has been identified,17 with substantial differences in the average time from referral to death between providers in the north and south of England. This is important: the benefits of specialist palliative care are notably greater if delivered earlier in the illness trajectory. 18
Older people and those with non-cancer diagnoses are also less likely to access specialist palliative care,19,20 often resulting in a poor match between individual needs, the resources provided to meet those needs and the health outcomes achieved.
Substantial differences in the approach to commissioning services suggests provision rather than population demographics account for a large component of the variation. 16
The research gap
One major challenge in improving our ability to better match resources to needs is the lack of research into what accounts for these apparent inequities in provision and – in particular – a lack of a standardised way to assess and monitor palliative care needs. With growing constraints on resources, it is becoming imperative for individual-level needs to be mapped accurately to reduce inequities, match resources to needs and improve the quality, consistency of provision, and efficiency of palliative care. This has been endorsed as a high priority nationally,14 but there is very limited evidence available to inform the best ways to match individual-level needs to resources. Most importantly, there is no ‘standard’ way to assess, capture and report the palliative care needs of individual people with advanced illness. This programme of research directly addresses this research gap.
What we already know
Illness in the last year of life places major resource burden on the NHS. Up to 20% of healthcare expenditure is spent on the last year of life. 21 Total NHS expenditure in 2017/18 was around £122B in England22 – roughly £2200 per person – indicating that around £25B per year is spent on health care in the last year of life. Much of this is on acute care, but it is estimated that palliative care is needed for about 75% of all those approaching death. 23
The amount of health and social care resources spent on those in the last year of their life is increasing and will increase further in coming years. Ageing populations, greater co-morbidity and longer chronic disease trajectories are all increasing this demand. 24,25 However, this is not a simple relationship; commonly used approximations of health, such as age or mortality, are not enough to capture the complex dynamics in healthcare requirements. 26 Population ageing particularly increases expenditures on acute and long-term care. 27 In this context, it is increasingly important to recognise palliative care needs and ensure they are effectively addressed with the best possible use of scarce resources.
Palliative care needs
Assessment of patients’ needs plays an important role in improving outcomes in palliative care. Patient-reported outcomes – where ‘outcome’ here is defined as a ‘change in current or future health status following intervention’28 – are a way of measuring the changes in patients’ health that they themselves perceive over time. These outcomes allow for assessment of intervention effectiveness. In terms of addressing needs, there is increasing evidence that home, hospital and inpatient specialist palliative care is effective and significantly improves patient outcomes, particularly for cancer patients with reduced pain and other symptoms, reduced anxiety, and reduced hospital admissions. 29 Similar evidence is emerging for patients with non-malignant disease. 30–32
Further systematic reviews,29,33,34 including meta-analyses and Cochrane reviews,35,36 and randomised controlled trials of specialist palliative care services30,37 provide consistent evidence of the effectiveness of palliative care services in improving symptoms, reducing family burden, improving satisfaction with care and preventing depression. There is wide acknowledgement that palliative care should be available to all, based on need not diagnosis and extending across settings,38,39 and these principles underpin the NHS End of Life Care Strategy. 40
Given the complexity of problems experienced in the last year of life, it is not surprising that a high proportion of healthcare resources is spent during this time: as much as 20% of all healthcare expenditure. 21 However, some of this may be poorly spent: hospital admissions may be prevented by better anticipatory symptom control, better family support and earlier facilitation of advance discussions about patient and family preferences for treatment and care. 41,42 Robust studies demonstrate improved patient outcomes and reduced costs when palliative care is provided early30,37,43 and a systematic review of trials of palliative care interventions shows greatest positive impact on quality of life when these interventions are provided early. 18
Casemix classifications: a potential way forward
Within health care, there is increasing use of casemix classifications to help gain a better understanding of how healthcare resources might be allocated in an equitable yet efficient way. Casemix classifications are based on patient-level criteria, which allow the grouping of patients into classes in terms of the resources needed to meet their needs. 44 Casemix is defined as ‘a means to classify patients into groups in order to provide a useful measure to make meaningful performance comparisons, to cost healthcare, or to fund it’ (information from NHS Digital, licenced under the current version of the Open Government Licence). 45 Casemix classifications are defined as ‘a system assigning each person [patient] into a hierarchical system of groups or classes, according to various individual casemix criteria. Each group or class is associated with higher or lower resource requirement to meet needs.’46
The USA developed such casemix classifications, including diagnosis-related groups (DRGs)47 and these have been used to develop prospective payment systems globally. 48 DRGs are a useful classification of healthcare needs driven by the diagnosis, but have been shown to be inappropriate for some areas of health care, such as mental health,49 primary care50 and palliative care. 46,51
Palliative care needs are not diagnosis-driven, but instead by factors such as functional status, physical symptoms and emotional burden. 51 Palliative care needs a consistent method of classifying types of patients with complexity of needs, treatment and costs, using casemix criteria. 52–54 Healthcare Resource Groups (HRGs) underpin the main casemix classification adopted in England. 44
It is necessary therefore to identify those with more complex palliative needs and requiring more resources. 55 An Australian casemix classification for palliative care was developed in 1997, empirically tested and progressively refined over time. 52–54 The Australian casemix classification consists of classes defined by five criteria most strongly predictive of resource use: Phase of Illness, problem severity, functional status and dependency, age and model of care. 55 Full class definition and categorizations are available at https://ahsri.uow.edu.au/pcoc/index.html (accessed 12 December 2021). Its implementation proved it was possible to consistently and routinely collect casemix data nationally;56 this has enabled consistent casemix adjustment in outcome measurement, with year-on-year improvement in outcomes at a national level and a funding model which matches patient’s needs. 51,57 Palliative care funding pilots in the UK have also suggested casemix data may be useful for these purposes. 58 However, it is unclear whether or not, and how, any existing palliative care classification can be easily applied to the UK to address unmet needs and reduce inequities.
What we do not know
Although casemix classifications have been widely used to manage resources across health care, they have rarely been applied to palliative care. Existing classifications (i.e. DRGs and HRGs) are based on diagnoses, but for patients receiving palliative care the priority is not on diagnosis but enhancement of well-being and quality of life, and the maintenance or maximisation of current health status in the face of advanced incurable illness. 52 A palliative care casemix classification must reflect these different goals. 53,54,57
Australia is the only country to have developed such a casemix classification for palliative care. 57 Diagnosis and procedure criteria were found to be ineffective in classifying the complexity of needs for those receiving palliative care. 53 Instead, palliative Phase of Illness and ‘problem severity’ were better indicators of increased complexity and consequent greater resource use. 56,59 Despite these advances, key questions remain unanswered:
-
How can the wide range of patient needs in palliative care provision best be classified across conditions and settings, so that services can be resourced to meet individual needs and deliver best outcomes? Without such a classification, it is difficult to ensure that sufficient resources can be matched to the right patient at the right time.
-
Within such a classification, how is complexity best understood/captured so that commissioners can commission, and providers can deliver, the optimal mix of services?
-
What are the best criteria for palliative care casemix? It is internationally acknowledged that diagnosis and procedures are not useful criteria in palliative care casemix categorisation53,57 and UK specialist palliative care providers seek a model more attuned to patient needs across wide-ranging levels of complexity.
-
What are the most useful patient-level data in palliative care? There is a lack of specific patient-level data in palliative care with which to model casemix and resource use. 14 This is especially true in community settings, where provision cuts across the NHS and voluntary sectors. 60,61
-
What evidence do we have to build on? Some work has been undertaken in recent palliative care funding pilots,58 but no evidence has been published from these to help determine which casemix criteria are optimal for use in England.
Rationale for our approach
This programme responds to these challenges by developing a palliative care casemix classification across conditions and settings. Recent UK initiatives to improve the provision of high-quality palliative care40,62 have not addressed the challenges of classifying needs and costs.
Worldwide, different palliative care funding models exist,63 but a formal palliative care casemix classification has been developed only in Australia. This Australian casemix classification incorporates functional status/dependency with problem severity and palliative Phase of Illness,57 and adopts a blended funding model. 64 There is no existing palliative care classification easily transferable to the UK, but the Australian model is a valuable starting point.
This programme leads directly to patient benefit through the improved matching of resources to needs at the individual patient-level, with corresponding outcome measurement, along with more effective and cost-effective use of resources through the following work:
-
the development of better patient-centred outcome measures that are relevant and meaningful for patients with advanced illness and their families
-
the development of a ‘standard’ way to assess, capture, and report the palliative care needs of individual people with advanced illness
-
the development of a casemix classification – casemix ‘classes’ based on individual-level criteria – which can predict the resources needed to address the symptoms/concerns of people with advanced illness needing specialist palliative care.
This approach has been directly recommended by the Palliative Care Funding Review,14 and we have liaised closely with NHS England in progressing this work.
Aims and objectives of the C-CHANGE programme
Aims
The aims of the C-CHANGE programme were to report the costs of specialist palliative care; develop and test a person-centred, nationally applicable casemix classification for adult specialist palliative care provision in England; accurately capture the complex needs of patients with advanced disease in last year of life; better quantify those needs; and support more equitable allocation of resources to meet them.
The programme also aimed to identify ways to measure the improvements in health status and well-being which patients and families experience following specialist palliative care so that the casemix classification could be developed, but also so that quality and effectiveness of services can be more readily demonstrated to patients, families, commissioners and services.
Objectives
The C-CHANGE programme had five objectives:
-
to refine, validate or test new and existing person-centred outcome measures to assess the main health status and symptoms/concerns of, and services received by, patients and families receiving specialist palliative care
-
to utilise the perspectives of key stakeholders (i.e. patients, families, professional caregivers, commissioners and policy-makers) on complexity in palliative care to inform subsequent casemix development
-
to understand the criteria which distinguish different models of palliative care to help inform how a casemix classification and per-patient funding models can best be utilised across different models of specialist palliative care
-
to develop a person-centred palliative care casemix classification, based on individual patient and family needs and costs of care, for adults with both cancer and non-cancer conditions in the last year of life
-
to test this person-centred palliative care casemix classification in terms of its ability to predict resource use in last year of life and to better understand transitions between services in order to improve care.
Overall design of the C-CHANGE programme
The C-CHANGE programme comprised six workstreams (see Figure 1):
-
Workstreams 1, 2, 3 and 4 ran consecutively.
-
Workstream 5 ran concurrently with workstreams 1, 2, 3 and 4 to integrate each workstream into the overall programme of work.
-
Workstream 6 also ran concurrently with workstreams 1, 2, 3 and 4, to maximise dissemination and outputs throughout the programme.
FIGURE 1.
Research pathway diagram for the C-CHANGE programme.

Changes to the overall programme design
We amended/expanded three components of the original workplan:
-
In workstream 1, emerging psychometric evidence changed the extent to which we needed to undertake validation of the different measures. We added a study of Phase of Illness, as new evidence56 confirmed Phase of Illness would potentially be a key casemix criteria.
-
During workstreams 1 and 2, as a direct result of input from our patient and public involvement (PPI) group, we added a patient experience measure and included an additional secondary analysis to understand the role of uncertainty in the care needs of patients and families with advanced illness.
-
Within workstreams 3 and 4, we studied the models of specialist palliative care operating at the participating sites in more detail; the need for this became apparent as we tried to characterise models of palliative care in the context of a rapidly changing healthcare environment.
Workstream 1: measures and training
Workstream 1 (‘measures and training’) was designed to meet objective 1: to refine, validate or test new and existing person-centred outcome measures to assess the main health status and symptoms/concerns of, and services received by, patients and families receiving specialist palliative care (for use in workstreams 3 and 4).
As planned, we undertook full validation of the Integrated Palliative care Outcome Scale (IPOS), which was the key measure of patients’ symptoms and other concerns in workstreams 3 and 4. We also undertook further testing of the palliative Phase of Illness measure, which had limited previous psychometric assessment, mostly of reliability. 59 After discussion with our PPI group, we also adapted and tested an experience measure: Views on Care (VoC).
The following other measures adopted for workstreams 3 and 4 already had published validation work (or this work emerged before the commencement of workstream 1):
-
the Client Service Receipt Inventory (CSRI),65 including adaption for palliative care66
-
the Zarit Caregiver Burden Interview – validity of the short version with 6 items,67 including in advanced illness68
-
the Medical Outcomes Study Short Form –12 (+ 4),69 including in advanced disease70
-
the Australian-modified Karnofsky Performance Scale (AKPS) – an adapted version of the widely-used Karnofsky Index to measure functional status and activity. 71
We also undertook training of all participating sites in the use of the measures for workstreams 3 and 4, as planned.
An added component of workstream 1 – at the request of our PPI group – was a secondary analysis of existing qualitative data to understand the role of uncertainty in assessing the care needs of patients and families with advanced progressive illness.
More details of the measures are available in Appendix 1.
Validation and testing of measures
Validation of the Integrated Palliative care Outcome Scale– cognitive testing
This work has also been published in Schildmann et al. 72 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Aim
Our aim was to explore patients’ views on the IPOS, with a focus on comprehensibility and acceptability, and to subsequently refine the questionnaire.
Methods
We carried out a cognitive interview study using ‘think aloud’ and verbal probing techniques. Interviews were audio-recorded, transcribed verbatim and analysed using thematic analysis. The IPOS was then refined according to findings. Purposively sampled patients were recruited from four palliative care teams across different settings.
Results
A total of 25 interviews were conducted. Overall, comprehension and acceptability of the IPOS were good. Identified difficulties comprised (1) comprehension problems with specific terms (e.g. ‘mouth problems’) and length of answer options; (2) judgement difficulties owing to, for example, the recall period; and (3) layout problems.
Validation of the Integrated Palliative care Outcome Scale: full validation study
This work has also been published in Murtagh et al. 73
Aim
Our aim was to validate the IPOS, a measure underpinned by early psychometric development, by evaluating its validity, reliability and responsiveness to change. 73
Design
We designed a validation study for both the patient self-report and staff proxy-report versions of the IPOS. We tested construct validity (factor analysis, known-group comparisons and correlational analysis), reliability (internal consistency, agreement and test–retest reliability) and responsiveness (through longitudinal evaluation of change).
Results
We recruited 376 adults receiving palliative care and 161 clinicians from a range of palliative care settings. We confirmed a three-factor structure (physical symptoms, emotional symptoms and communication/practical issues). 73 The IPOS showed a strong ability to distinguish between clinically relevant groups; total IPOS and IPOS subscale scores were higher (i.e. reflected more problems) in those patients with ‘unstable’ or ‘deteriorating’ versus ‘stable’ Phase of Illness (F = 15.1; p < 0.001). Good convergent and discriminant validity was found for hypothesised items and subscales of the Edmonton Symptom Assessment System and Functional Assessment of Cancer Therapy–General. 73 The IPOS showed good internal consistency (α = 0.77) and acceptable-to-good test–retest reliability (60% of items kw > 0.60). Longitudinal validity in the form of responsiveness to change was good.
To assess how palliative Phase of Illness related to the other measures
This work has also been published in Mather et al. 74
Aims
Our aim was to describe function, symptoms and other palliative care needs according to Phase of Illness, and to consider the strength of associations between these measures and Phase of Illness.
Design and setting
We performed a secondary analysis of patient-level data for a total of 1317 patients in three settings. Function was measured using the AKPS. Pain, other physical problems, psycho-spiritual problems and family and carer support needs were measured using the Palliative Care Problem Severity Scale (https://documents.uow.edu.au/content/groups/public/@web/@chsd/documents/doc/uow272193.pdf; accessed 21 August 2023).
Results
The AKPS and Palliative Care Problem Severity Scale items varied significantly by Phase of Illness. Mean function was highest in the stable phase [65.9, 95% confidence interval (CI) 63.4 to 68.3] and lowest in the dying phase (16.6, 95% CI 15.3 to 17.8). Mean pain was highest in the unstable phase (1.43, 95% CI 1.36 to 1.51). In multinomial regression, psycho-spiritual problems were not associated with Phase of Illness (χ2 = 2.940, df = 3; p = 0.401). Family and carer support needs were greater in the deteriorating phase than the unstable phase [odds ratio (deteriorating vs. unstable) 1.23, 95% CI 1.01 to 1.49]. Overall, 49% of variance in Phase of Illness is explained by the AKPS and Palliative Care Problem Severity Scale.
A patient experience measure: Views on Care
This work has also been published in Pinto et al. 75
Context
When patients face advanced illness, their experience of care is especially important. In palliative care, we often rely on the accounts of bereaved relatives to report the quality of end-of-life care, and there are no patient-reported measures of the experience of care. Our PPI group challenged us to consider and address this omission. We derived and tested a new questionnaire, called Views on Care (VoC), to address this gap.
Measure development
After research team, PPI group, and steering group discussions, VoC was derived from four questions selected from St Christopher’s Index of Patient Priorities76 that address patients’ evaluation of changes in experience of palliative services, and quality of life [the quality of life items are adapted from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – 15 items measure (EORTC QLQ-C15-PAL), which is well-validated in advanced illness]. 77 The St Christopher’s Index of Patient Priorities itself was considered too long.
Methods
We conducted a survey to examine patients’ views on care and the relationship between these views and changes in health status. Participants were adults receiving specialist palliative care in eight hospital, hospice inpatient and community settings across England. We collected demographic details, plus a patient-reported survey at baseline and follow-up. We reported VoC at follow-up, and change in health status (measured using the IPOS) between baseline and follow-up. Descriptive statistics characterise sample demographics and VoC responses, and the chi-squared statistic tests the association between VoC scores and IPOS change scores. IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA) was used throughout. Ethics approval was obtained from the Dulwich National Research Ethics Committee (REC), London, UK (reference number 124991).
Results
A total of 212 participants were recruited, with a mean age of 65.84 years [standard deviation (SD) 13.5 years]; 137 participants completed both baseline and follow-up surveys. Responses to VoC items 1, 3 and 4 were reasonably normally distributed. Responses to VoC item 2 were positively skewed with most participants indicating that palliative care was giving positive benefit. Participants reporting that ‘things had got better’ (item 1) were more likely to have improved overall outcomes (reduction in IPOS total score: χ2 = 6.057; p = 0.48). With regard to IPOS subscales, there was significant positive association between those reporting that ‘things had got better’ (item 1) and improved outcomes on the IPOS physical symptoms subscale (χ2 = 11.254; p = 0.004). Patients reporting benefit from palliative services (item 2) were more likely to have improved scores on the IPOS communication/practical issues subscale (χ2 4.743; p = 0.051).
Exploring uncertainty in relation to palliative care needs
This section has been reproduced with permission from Etkind et al. 78 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/3.0. The text below includes minor additions and formatting changes to the original text.
Aim
Our aim was to understand patient experiences of uncertainty in advanced illness and develop a typology of patients’ responses and preferences to inform subsequent research in this programme.
Design
We performed a secondary analysis of qualitative interview transcripts. 78 Studies were assessed for inclusion and interviews were sampled using maximum variation sampling. Analysis used a thematic approach with 10% of coding cross-checked to enhance reliability. Qualitative interviews from six studies were analysed, comprising patients with advanced heart failure, end-stage chronic obstructive pulmonary disease, end-stage renal disease, advanced cancer and advanced liver failure. 78
Results
A total of 30 transcripts were analysed. The median patient age was 75 years (range 43–95 years) and 12 patients were women. The impact of uncertainty was frequently discussed: the main related themes were engagement with illness, information preferences, patient priorities and the period of time that patients focused their attention on (temporal focus). A typology of patient responses to uncertainty was developed from these themes (see Figure 2). 78
FIGURE 2.
Two different examples of patient responses to uncertainty, dependent on level of engagement, information preferences and temporal focus. Reproduced with permission from Etkind et al. 78 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/3.0. The figure includes minor additions and formatting changes to the original figure.

Workstream 2: stakeholders’ perspectives on measuring complexity
This work has also been published in Pask et al. 79
Workstream 2 (‘stakeholders’ perspectives on measuring complexity’) was planned to meet objective 2: to utilise the perspectives of key stakeholders (i.e. patients, families, professional caregivers, commissioners and policy-makers) on complexity in palliative care to inform subsequent casemix development.
Aim
Our aim was to explore the perspectives of key stakeholders on complexity in palliative care to inform subsequent casemix development.
Methods
Design
We designed a qualitative study using semistructured interviews with key stakeholders in specialist palliative care.
Recruitment and consent
We undertook audio-recorded, individual interviews with various stakeholders across specialist palliative care. Participants were either recruited from one of the C-CHANGE participating sites for workstreams 3 and 4 or – for policy and national leads – sought out by the wider research team at the Cicely Saunders Institute (the leading UK research institute for palliative care). Within this frame, participants were sampled purposively, by personal and/or professional background, geographical location and experiences of settings of care (hospital, hospice and community).
Data collection
A topic guide was developed from a review of evidence on complexity, potential criteria for casemix already used46 or proposed,58 existing casemix classifications in palliative care57 and predictors of resource use in the last year of life. It was refined by our PPI group, by the research team, and through discussion with and feedback from the Programme Steering Committee.
Face-to-face, semistructured interviews were conducted by C-CHANGE researchers in the participant’s preferred setting. To increase the credibility of the data, interviewers summarised the interview back to each respondent, to allow the participant to verify the data and clarify any misconceptions or add additional information. All interviews were digitally audio-recorded, anonymised and transcribed verbatim to ensure confidentiality.
Analysis
Interviews were analysed independently by two researchers from the C-CHANGE team using the five analytical steps of framework analysis: (1) familiarisation, (2) identifying a thematic framework, (3) indexing, (4) charting and (5) mapping and interpretation. Framework analysis was considered the optimal approach to allow both an inductive and deductive approach, which would facilitate comparison across stakeholder groups and support the service delivery and policy focus of this research. Emerging themes were discussed with the whole C-CHANGE research team to improve the confirmability and dependability of the findings. Charts were created for each theme, grouped by stakeholder type, and were used to explore stakeholder assonance and dissonance among perspectives on each theme and subtheme. Analysis was managed using NVivo version 10 (QSR International, Warrington, UK). The framework was presented to the PPI advisory group, the Project Steering Committee and other qualitative research experts to refine and improve the presentation of the developed framework.
Ethics approval
Ethics approval was gained from the King’s College London REC (BDM/14/15–2).
Results
Sixty-five participants – including patients and families – were recruited and interviewed (see Pask et al. 79 for full details of participant characteristics). Participants provided valuable insights into how complexity might best be understood. They largely understood and valued any use of individual person-level criteria to determine complexity and had nuanced perspectives on how this might be undertaken.
Based on these qualitative findings, we developed a theoretical framework – adapted from Bronfenbrenner’s Ecological Systems Theory80 – directly using the interview data provided by patient, family and professional participants to help understand complexity in specialist palliative care (see Figure 3). This framework emphasises that considering physical, psychological, social and spiritual domains (a classic approach in specialist palliative care) is not enough to characterise complexity. Other aspects – such as ‘pre-existing’ (often social), ‘cumulative’ and ‘invisible’ (such as unrecognised depression in the context of physical illness) complexity – are important too, yet frequently overlooked.
FIGURE 3.
A theoretical framework of complexity in the palliative care context. Reproduced with permission from Pask et al. 79 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0. The figure includes minor additions and formatting changes to the original figure.
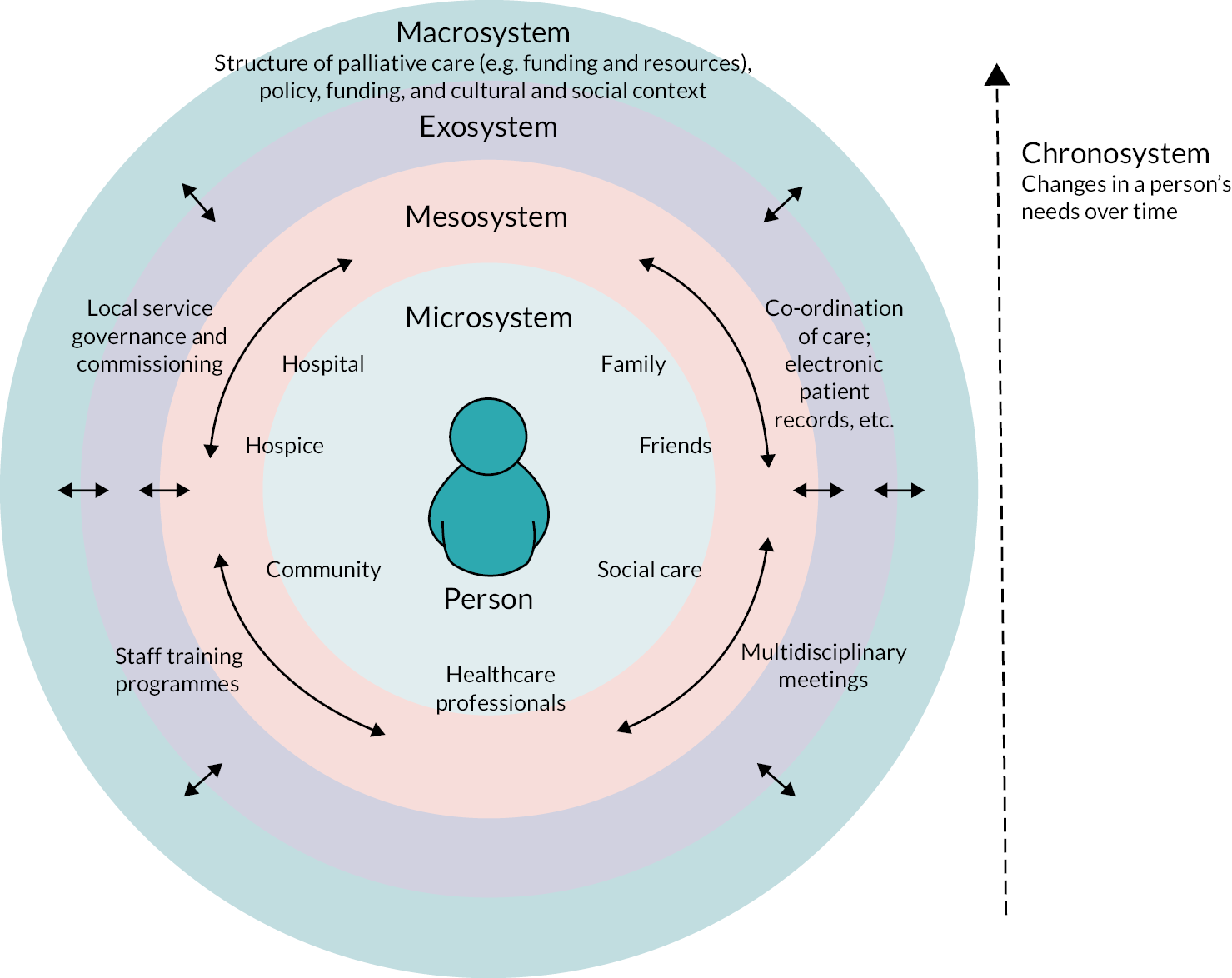
The way in which professionals and services interact with people and their families was also considered to be crucial to assessing, understanding and responding appropriately to complexity. Number, severity, range and temporality of needs – as well as ‘hidden’ or overlooked aspects of complexity, as noted above – all needed to be considered in the development of a meaningful casemix classification for specialist palliative care.
Workstream 2: models of specialist palliative care
This work has also been published in Firth et al. 81
This work in workstream 2 (‘models of specialist palliative care’) was extended from that proposed in our original bid to meet objective 3: to understand the criteria which distinguish different models of palliative care, to help inform how a casemix classification and per-patient funding models can best be utilised across different models of specialist palliative care.
The originally planned work needed to be expanded to understand and describe the different models of palliative care, which were rapidly evolving in a changing healthcare environment (especially in community settings). This change was fully supported by the Programme Steering Committee and PPI group.
Aim
We aimed to understand the criteria which characterise and distinguish different models of palliative care. 81
Methods
Design
We developed a mixed-methods study with (1) semistructured interviews to identify criteria for models of care, (2) a two-round Delphi study to rank/refine these criteria and (3) structured interviews to test the acceptability and feasibility of these criteria. 81
Semistructured interviews
A rapid scoping review was conducted to identify literature related to models of palliative care. Original papers and reviews were examined for possible criteria which could help define models of specialist palliative care and a topic guide was created covering the 28 preliminary criteria identified from this literature. Semistructured interviews using a pre-specified topic guide were conducted with a range of palliative care service leads across organisations. 81
Delphi study
We selected Delphi survey methods for this second stage because it enabled us to present potential criteria derived from the semistructured interviews to all respondents, allowed them time to absorb this complex information at their own pace and enabled us to sample a wide range of views in a way that gave all opinions equal weight. A two-round Delphi survey of UK clinical, policy or PPI leads were invited from the Outcome Assessment and Complexity Collaborative network (a multidisciplinary network of professionals engaged in the implementation of outcome measures in specialist palliative care in England), and the national C-CHANGE sites. Participants were advised that we were aiming to establish a list of key criteria to describe and compare models of care. The Delphi survey was conducted to refine the criteria from the semistructured interviews, identify any additional criteria, achieve consensus on how each criterion was defined and rank the criteria in terms of importance. 81 CREDES (Conducting and REporting DElphi Studies in palliative care) guidelines were followed. 82
An online survey was developed using Bristol Online Survey (BOS) v1.0 Bristol, UK. 83 The survey was piloted for face validity prior to going live.
In the first Delphi round, panel members were presented with a list of 34 criteria. Participants were asked to state whether or not they agreed with the inclusion of each criterion as an important criterion for describing and comparing models of specialist palliative care (answering ‘yes’, ‘no’ or ‘don’t know’) and their reasons for this. They were also asked to comment on the phrasing and clarity of the criterion, as well as the answer options listed. Finally, participants were asked to suggest any additional criteria they thought should be included. 81
Each criterion was retained if at least 75% participants answered ‘yes’. Free-text comments were analysed using content analysis and used to refine and expand the set of criteria.
In round 2 of the Delphi process, participants received anonymised feedback from round 1 and the amended list of criteria for further refinement and ranking. Participants were asked to rate the importance of each criterion for characterising and comparing different models of care on a five-point Likert scale (1 = not at all important; 2 = not very important; 3 = important; 4 = very important; 5 = extremely important). In addition to the rating scales, participants were also given the opportunity to add additional free-text comments to help refine criteria and answer options. 81
Responses were analysed to capture both central tendency (median rating) and dispersion [interquartile range (IQR)]. Consensus was deemed to have been reached for criteria that received aggregated responses with an IQR of ≤ 1 and a median of 4 or 5. Both methods are considered to offer robust measurements for Delphi surveys. 84,85 Criteria reaching this consensus were then included in the final set.
Ranking responses were collated and analysed using IBM SPSS Statistics version 22. Free-text responses underwent content analysis and were used to refine the criteria and response options.
Structured interviews to test for acceptability and feasibility of the criteria
The criteria developed from the Delphi survey component were then tested with clinical leads from three different specialist palliative care settings (hospice inpatient care, hospital advisory teams and community-based care) using structured interviews. Participants consented to be interviewed and audio-recorded. The data from these interviews were classified according to the criteria used, and entered into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) to identify whether or not the criteria could discriminate between services. 81
Ethics approval
Prior ethics approval for all three components was gained from the King’s College London REC (LRS-15/16–2449).
Results
Semistructured interviews
Semistructured interviews were conducted with 14 service leads from eight organisations, discussing 12 settings of care (five hospice inpatient units, two hospital advisory teams and five community teams).
An early finding was that the clinical leads struggled to know at which level within the organisation to describe their models of care. 81 It was often confusing when an organisation covered multiple settings of care (i.e. hospice inpatient, community, hospital inpatient and day services) and also provided multiple services within each setting, which often overlapped. For example, a hospice may have inpatient hospice, home care and ambulatory settings. Within any one of these settings, multiple services or teams were often operating. Within the day services there may be a physiotherapy clinic, a lymphoedema service and a day service, all operating with different models of care.
After all interviews were completed, out of the 28 criteria in the topic guide, 11 were not reported as useful and were removed; 17 criteria were refined; and a further 17 criteria were created. This resulted in 34 criteria to take forward to the Delphi survey. 81
Delphi survey
A total of 190 participants were invited to take part in the Delphi survey. Of the 190 clinical, policy and PPI leads contacted, 54 agreed to participate (response rate 28.4%).
Results of Delphi round 1
Out of thirty-four criteria, six were removed due to not reaching the 75% consensus rate, and one removed due to poor comprehension. Three new criteria were added:
-
How many referrals are accepted and seen annually by this service/team? (to reflect the size of service)
-
Does this service/team accept patient or family self-referrals? (to reflect the approach to self-referral)
-
Who undertakes the first assessment? (to reflect whether the model of care was doctor-led, nurse-led or another kind of model).
The out-of-hours criteria were heavily refined to improve comprehension and four new criteria relating to ‘out-of-hours’ were created. This resulted in a refined list of 34 criteria. 81
Results of Delphi round 2
Thirty participants (out of 54 in round 1) completed round 2 (60% response rate). In round 2, the 34 revised criteria from round 1 were ranked and rated, and criteria not meeting the predetermined consensus level were excluded. Sixteen criteria reached consensus (see Box 1).
-
Setting of care (inpatient hospital, inpatient hospice, home based, etc.)
-
Type of care delivered (‘hands on’ or advisory)
-
Size of service (measured by number of referrals accepted annually)
-
Number of disciplines delivering the care
-
Mode of care (face-to-face, telephone or other remote delivery)
-
Number of interventions available
-
Whether or not out-of-hours referrals are accepted
-
Whether or not out-of-hours care is available to patients already known to the service
-
Time when out-of-hours care available
-
Out-of-hours mode (face to face or advisory)
-
Type of out-of-hours provision (‘hands on’ or advisory)
-
Extent of education/training provided to external professionals
-
Whether or not outcome and experience measures are used in the service
-
Whether or not standard bereavement follow-up is provided
-
Whether or not complex grief follow-up is provided
-
The primary diagnosis of those patients receiving care (cancer/non-cancer)
-
Is the service a publicly funded or voluntary funded service?
-
Whether or not there are patient or family self-referrals
-
Whether or not there are standard discharge criteria
-
Purpose of care provided.
Copyright © 2023 Murtagh et al.
Structured interviews
Interviews were conducted with 21 service leads from 19 different services (six hospice inpatient, four hospital advisory and nine community settings). The responses to each criterion were compared to see if the criteria could distinguish and discriminate effectively between services. A further four criteria relating to context were also added (see Box 1); these were reported by the clinical leads as providing important context for the practical application of the criteria. These four contextual criteria were the purpose of the team, who funds/manages the team, the ability to self-refer and the discharging of patients. 81
Workstream 3: development of the casemix classification
The protocol for this study has been published55 was planned to meet objective 4: to develop a patient-centred palliative care casemix classification, based on individual patient and family needs, for adults with both cancer and non-cancer conditions in the last year of life.
Aim
Our aim was to develop a casemix classification for UK specialist palliative care, for use in hospital-based palliative care, inpatient hospices (palliative care units) and home-based palliative care.
Methods
Design
We designed a multicentre prospective cohort study, following patients during episodes of specialist palliative care and reported according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement. 86
Definitions
We defined an episode of care as a ‘period of contact between a patient and palliative care service provider or team of providers that occurs in one setting’. 87 A new referral into palliative care or change of setting (e.g. re-location into an inpatient setting from home/care home, or vice versa) signalled the start of an episode of palliative care. Discharge from palliative care, change of setting or death signalled the end of an episode of care. We defined the setting of care as one of three types: hospital advisory (hospital-based specialist palliative care teams providing an advisory or consultant service), inpatient hospice (where patients are admitted to a hospice or specialist palliative care unit for an overnight stay of one or more days) or community-based specialist palliative care (where the patient receives care in their usual place of residence, either at home or in a care home).
Population and settings
Patients were recruited from 14 organisations providing specialist palliative care services in England: four hospital advisory services, five inpatient hospice services and seven community-based services (some organisations provided more than one setting of care). Sites were selected to ensure our study sample was representative of UK palliative care patients and services in terms of participant age, ethnic background, service size, proportion of cancer/non-cancer patients and urban–rural balance. We included more community services than hospital or inpatient hospital services.
Inclusion/exclusion criteria
We included consecutive adult patients (≥ 18 years) receiving specialist palliative care at all participating sites. Exclusion criteria comprised patients aged < 18 years, those who declined participation and/or those who previously expressed a wish not to participate in research.
Data collection and primary outcome
Data were collected from clinicians between July 2015 and October 2016, with completion of follow-up at the end of January 2017; no data were collected directly from patients. Collected data included demographic and clinical variables, episode start and end dates, potential casemix variables, and data on patient-level and other costs of providing specialist palliative care. All participants received the usual specialist palliative care at that site, including a multidisciplinary team with specialist training delivering holistic care focused on physical and psychological symptom management, social/family support, planning ahead around priorities and preferences, and care into the dying phase including post-death care of the family, where relevant.
Potential casemix variables were selected based on (1) being patient-level attributes and (2) existing evidence of association with casemix/complexity. 88 The key casemix variables included were age, sex, ethnicity, living circumstances, need for interpreter, primary diagnosis, palliative Phase of Illness, functional status, dependency and symptoms/problem severity.
Details of the measures used are presented in Appendix 1.
Our primary outcome was the cost of specialist palliative care per day. We adopted a (palliative) provider perspective for costs. The costs of acute hospital care, primary care, generic end-of-life care (i.e. provided by non-specialist teams), and informal care costs were excluded, not because these are unimportant, but because we sought casemix criteria relevant to specialist palliative care. A broader perspective on costs is planned for future work.
Palliative Phase of Illness was assessed daily for people receiving inpatient (hospital or inpatient unit) care and at every contact for those receiving community-based care. Each change in Phase of Illness (or end of episode) triggered the collection of the AKPS, IPOS or Palliative Care Problem Severity Score (PCPSS), and the Barthel Index. All staff involved in patient care recorded the time spent delivering care to participants at the patient level using the staff activity matrix.
We collected data from participating sites on the costs of delivering their services and patient-level resource use data from the staff activity matrix to derive actual patient-level costs according to a standard costing methodology based on current NHS costing principles. 89 Note that costs captured for the hospital advisory and community-based settings represented the additional or ‘top-up’ costs for adding palliative care support to the hospital or community setting (and thus are reasonably compared). In contrast, the costs for inpatient hospices represented all the costs of inpatient care (and so are more reasonably compared with the costs of acute hospital admission). The full costing methodology (how costs were collected, classified and compiled) is available from the corresponding author on request.
Sample size
Based on standard recommendations for fitting multivariate models, a minimum of 50 + 8 × m cases for testing multiple correlation (where m is the number of predictors) are required to test the null hypothesis that the population multiple correlation equals zero with a power of 80%, α = 5% and a medium effect size for the regression analysis (R2 = 0.13). 90,91 The unit of analysis was episodes within sites; therefore, 10 predictors required 130 episodes per site. Allowing an additional 15% for episodes with missing data and 20% for cost outliers, we estimated that a target of 2674 episodes of care (191 episodes × 14 sites) was required.
Data handling
Data were collected prospectively at each site, recorded on an electronic database, cleaned and checked. Checked data were transferred to statistical software [Stata® standard edition (SE) V.12 (StataCorp LP, College Station, TX, USA) and MATLAB® 8.2 (The MathWorks, Inc., Natick, MA, USA)] for analysis.
Analysis
An exploratory data analysis was undertaken first, examining variables of interest one at a time. Descriptive statistics (means, standard deviations, medians, ranges and correlations) were calculated. Comparative box plots were constructed to investigate the differences between sites, episodes and phases. Cost of care was used as the response variable, measured as the total cost per episode.
The aim of the analysis was to form distinct groups within the data, such that patients within each group were similar to each other, but different from patients in the other groups.
To discover which baseline casemix variables could best predict the cost of a particular episode of care, the following nine steps were then undertaken:
-
We removed incomplete episodes, retaining only the complete episodes of care.
-
Following a previously adopted approach,46 high- and low-cost outliers were identified and removed using a trimming algorithm based on the IQR with the upper trim point at Q3 + 1.5 IQR and the lower trim point at Q1 – 1.5 IQR (where Q1 is first quartile and Q3 is third quartile). 46 The trimming algorithm was applied to each setting separately.
-
We examined the distribution of costs of specialist palliative care, by setting.
-
Then, following the same approach as the development of the Australian casemix classification,88 we developed and validated a cost-predictive model using classification and regression tree (CART) analysis, which constructs decision rules in a hierarchical manner to form a branching classification.
-
We used CART analysis to enable the more complex interactions between the predictor variables (both categorical and continuous) to be explored. CART has the advantage of being non-parametric and is not significantly impacted by outliers in the input variables. It enables the use of each variable more than once, if required for the optimal regression tree.
-
Explanatory variables were compared to find the one which could best split the data into two homogeneous groups that were as different from one another as possible. These two groups would then be further split, using the same or another explanatory variable. Successive binary splits were performed on the data until there was no further improvement to be made and the best possible classification solution was reached.
-
The best CART was deemed to be that which accounted for the largest proportion of variation in the cost of care (the response variable). The criterion used to compare the different ‘trees’ was the proportion of the variance of the response variable that could be explained by the selected groups.
-
Costs were log-transformed for better modelling and back-transformed for providing mean costs per class or at each terminal node. Decisions about rules for splitting were informed by clinical utility (for instance, allowing branches that made clinical – as well as statistical – sense), as well as statistical performance as outlined in 7. We selected a maximum of four branches and a minimum of 30 cases per branch, for reasons of clinical utility. 10–fold cross-validation was used to prevent overfitting of the developed classification. No recalibration was undertaken. The analysis was done in R 3.5 (The R Foundation for Statistical Computing, Vienna, Austria) using the rpart, caret and (for bagging the trees) RWeka packages.
-
For each setting, we reported the variance explained by the CART model and the root-mean-squared error (RMSE) (i.e. the square root of the variance of the residuals). The RMSE indicates the absolute (rather than relative, as with R2) fit of the model to the data.
Ethics approval
The trial registration number is ISRCTN90752212. Ethics approval was received from the Camberwell St Giles National Research Ethics Service REC on 2 July 2015 [REC Reference: 15/LO/0887, Integrated Research Application System (IRAS) Project ID:172938].
Results
Subject characteristics
A total of 2469 patients were recruited, providing data on 2968 complete episodes of specialist palliative care (12 incomplete episodes were removed prior to analysis); 2087 participants contributed one episode of care, 283 participants contributed two episodes and 99 contributed three or more episodes. Demographic and clinical characteristics for the 2469 participants are reported in Table 1. No participants withdrew after recruitment. Further details are available in Appendix 2.
| Characteristic | n (%) |
|---|---|
| Socio-demographic details | |
| Age | |
| Mean (SD) | 71.6 (13.9) |
| Median (range) | 73 (20–104) |
| < 65 years | 740 (30.0) |
| ≥ 65 years | 1729 (70.0) |
| Missing | 0 (0.0) |
| Sex | |
| Male | 1258 (51.0) |
| Female | 1205 (48.8) |
| Missing | 6 (0.2) |
| Ethnicity | |
| White | 1825 (73.9) |
| Black African or Black Caribbean | 217 (8.8) |
| Asian | 151 (6.1) |
| Mixed ethnic background | 118 (4.8) |
| Other | 32 (1.3) |
| Missing | 126 (5.1) |
| Living alone | |
| Yes | 548 (22.2) |
| No | 1921 (77.8) |
| Missing | 0 (0.0) |
| Interpreter needed | |
| Yes | 22 (0.9) |
| No | 2447 (99.1) |
| Missing | 0 (0.0) |
| Primary diagnosis | |
| Cancer | 1857 (75.2) |
| Lip, oral cavity and pharynx | 83 (3.4) |
| Digestive organs | 392 (15.9) |
| Liver and biliary | 73 (3.0) |
| Pancreas | 119 (4.8) |
| Respiratory and intrathoracic | 349 (14.1) |
| Bone, skin and mesothelial | 93 (3.8) |
| Breast | 150 (6.1) |
| Female genital organs | 116 (4.7) |
| Male genital organs, including prostate | 142 (5.7) |
| Urinary tract | 75 (3.0) |
| Brain, eye and other central nervous system | 72 (2.9) |
| Unknown primary | 52 (2.1) |
| Lymphoid and haematopoietic | 131 (5.3) |
| Independent multiple sites | 10 (0.4) |
| Non-cancer | 612 (24.8) |
| HIV/AIDS | 13 (0.5) |
| Motor neurone disease/ALS | 9 (0.4) |
| Dementia, including Alzheimer’s | 45 (1.8) |
| Neurological (excluding MND) | 7 (0.3) |
| Diabetes mellitus | 6 (0.2) |
| Heart failure | 17 (0.8) |
| Stroke, infarction or haemorrhagic | 3 (0.1) |
| Other heart or circulatory | 12 (0.5) |
| Chronic respiratory including COPD | 39 (1.6) |
| Liver failure or chronic liver disease | 12 (0.5) |
| Renal failure | 8 (0.3) |
| All other non-cancer conditions | 433 (17.5) |
| Multiple non-cancer conditions | 8 (0.3) |
| Missing | 0 (0.0) |
Episode characteristics
Details of the 2968 complete episodes of care and related casemix variables are reported in Table 2.
| Episode characteristics | n (%) |
|---|---|
| Setting | |
| Hospital advisory | 767 (25.9) |
| Inpatient hospice | 764 (25.7) |
| Community | 1437 (48.4) |
| Total | 2968 (100.0) |
| Palliative Phase of Illness at episode start | |
| Stable | 451 (15.2) |
| Unstable | 1422 (47.9) |
| Deteriorating | 834 (28.1) |
| Dying | 261 (8.8) |
| Missing | 0 (0.0) |
| AKPS score at episode start | |
| Mean (SD) [range] | 45.9 (19.9) [10–100] |
| 0–50 | 1759 (59.2) |
| 60–100 | 934 (31.5) |
| Missing | 275 (9.3) |
| Modified Barthel Index score at episode start | |
| Mean (SD) [range] | 8.28 (6.6) [0–20] |
| Missing | 1395/2469 (56.5)a |
| PCPSS at episode start | |
| Pain | |
| Mean score (SD) [range] | 1.5 (1.06) [0–3] |
| Absent | 615 (20.7) |
| Mild | 703 (23.7) |
| Moderate | 724 (24.4) |
| Severe | 515 (17.4) |
| Missing | 411 (13.8) |
| Other physical symptoms | |
| Mean score (SD) [range] | 1.9 (0.88) [0–3] |
| Absent | 188 (7.0) |
| Mild | 630 (23.4) |
| Moderate | 1096 (40.6) |
| Severe | 659 (24.4) |
| Missing | 125 (4.6) |
| Psychological symptoms | |
| Mean score (SD) [range] | 1.8 (0.92) [0–3] |
| Absent | 335 (11.3) |
| Mild | 827 (27.9) |
| Moderate | 932 (31.4) |
| Severe | 423 (14.2) |
| Missing | 451 (15.2) |
| Family concerns | |
| Mean score (SD) [range] | 1.8 (0.92) [0–3] |
| Absent | 273 (9.2) |
| Mild | 592 (20.0) |
| Moderate | 1057 (35.6) |
| Severe | 552 (18.6) |
| Missing | 494 (16.6) |
| Length of episode (days) | |
| Hospital advisory | |
| Mean (SD) | 19.3 (39.01) |
| Median (range) | 8 (1–402) |
| Inpatient hospice | |
| Mean (SD) | 15.6 (15.77) |
| Median (range) | 12 (1–140) |
| Community | |
| Mean (SD) | 50.4 (53.95) |
| Median (range) | 30 (1–313) |
Outliers
Using the trimming algorithm described in Methods, Analysis, 123 (16.0%) hospital advisory episodes, 185 (24.2%) inpatient hospice episodes and 305 (21.2%) community episodes were removed to ensure that the principal cost and classification reporting was not based on outliers (a common challenge in costing studies).
Costs of specialist palliative care
The distribution of the total cost of specialist palliative care episodes, derived from the trimmed data set, is shown in Table 3; costs are shown (1) by day, (2) by day, broken down by Phase of Illness, and (3) by episode of care, together with details of length of episodes.
| Setting of care | n | % |
|---|---|---|
| Hospital advisory | 644 | 27.3 |
| Inpatient hospice | 579 | 24.6 |
| Community | 1132 | 48.1 |
| Total | 2335 | 100.0 |
| Mean (SD) | Median | |
| Hospital advisory | ||
| Cost per day: all episodes (£) | 72.65 (57.18) | 56.15 (31.22–100.03) |
| Cost per day by Phase of Illness (£)a | ||
| Stable | 60.03 (49.54) | 48.11 (24.91–78.34) |
| Unstable | 76.41 (58.71) | 58.61 (35.20–105.71) |
| Deteriorating | 68.55 (52.85) | 53.87 (31.54–95.77) |
| Dying | 81.28 (63.88) | 61.76 (33.00–119.90) |
| Length of episode: all episodes (days) | 15.18 (32.32) | 7 (3–15) |
| Length of episode, by Phase of Illness (days)a | ||
| Stable | 26.35 (59.22) | 7 (2–18) |
| Unstable | 16.47 (30.91) | 8 (4–17) |
| Deteriorating | 10.90 (24.35) | 6 (3–11.5) |
| Dying | 7.58 (16.83) | 3 (1–6) |
| Cost per episode: all episodes (£) | 507.36 (446.38) | 385.84 (176.15–698.85) |
| Cost per episode, by Phase of Illness (£)a | ||
| Stable | 387.57 (415.61) | 244.67 (116.38–508.32) |
| Unstable | 585.26 (268.11) | 458.01 (229.71–839.04) |
| Deteriorating | 431.33 (416.19) | 307.33 (146.68–558.59) |
| Dying | 335.53 (299.38) | 206.89 (106.59–521.89) |
| Inpatient hospice | ||
| Cost per day: all episodes (£) | 716.38 (765.04) | 434.33 (365.72–664.50) |
| Cost per day, by Phase of Illness (£)a | ||
| Stable | 669.55 (783.49) | 407.69 (292.81–588.00) |
| Unstable | 690.28 (726.03) | 428.38 (388.53–527.13) |
| Deteriorating | 832.60 (879.18) | 458.01 (353.11–967.66) |
| Dying | 645.08 (647.98) | 453.44 (364.51–606.52) |
| Length of episode: all episodes (days) | 14.74 (15.69) | 11 (5–19) |
| Length of episode, by Phase of Illness (days)a | ||
| Stable | 19.55 (22.70) | 12 (6–22.5) |
| Unstable | 16.68 (16.25) | 13 (6–22) |
| Deteriorating | 12.29 (10.02) | 10 (6–16) |
| Dying | 9.17 (17.66) | 6 (2–11) |
| Cost per episode: all episodes (£) | 7202.25 (7679.24) | 4428.28 (1601.00–10,533.93) |
| Cost per episode, by Phase of Illness (£)a | ||
| Stable | 8001.18 (9082.44) | 4179.83 (2054.33–9346.56) |
| Unstable | 7731.80 (7644.43 | 5345.43 (2069.59–10,654.10) |
| Deteriorating | 7654.97 (7839.31) | 4623.73 (1716.47–11,947.12) |
| Dying | 3119.63 (4948.30) | 1021.32 (463.39–2957.72) |
| Community | ||
| Cost per day: all episodes (£) | 35.76 (40.49) | 21.37 (6.23–49.13) |
| Cost per day, by Phase of Illness (£)a | ||
| Stable | 23.20 (32.60) | 10.58 (3.21–28.35) |
| Unstable | 33.97 (38.13) | 21.52 (6.23–47.03) |
| Deteriorating | 40.02 (43.28) | 24.01 (8.14–54.22) |
| Dying | 61.86 (44.84) | 55.87 (24.91–88.46) |
| Length of episode: all episodes (days) | 49.45 (51.53) | 30.5 (12–68) |
| Length of episode, by Phase of Illness (days)a | ||
| Stable | 65.03 (58.02) | 46.5 (20.5–90) |
| Unstable | 50.80 (50.91) | 32 (15–70) |
| Deteriorating | 45.42 (47.15) | 28 (12–61.5) |
| Dying | 16.67 (33.48) | 5 (2–19) |
| Cost per episode: all episodes (£) | 858.43 (780.77) | 624.18 (264.18–1230.44) |
| Cost per episode, by Phase of Illness (£)a | ||
| Stable | 818.91 (798.19) | 569.97 (207.24–1187.43) |
| Unstable | 879.57 (782.01) | 607.70 (282.47–1257.20) |
| Deteriorating | 870.39 (781.96) | 641.70 (274.30–1215.15) |
| Dying | 806.66 (725.88) | 577.93 (224.56–1230.57) |
Classification and regression tree analysis
Figures 4–6 show the CARTs for each setting. The per cent variance explained (and RMSE) were 20% (RMSE = 0.30), 51% (RMSE = 0.51) and 27% (RMSE = 0.36), for hospital advisory, inpatient hospice and community episodes, respectively.
FIGURE 4.
Classification tree of casemix criteria for hospital advisory episodes of specialist palliative care (costs per day reported for each class).
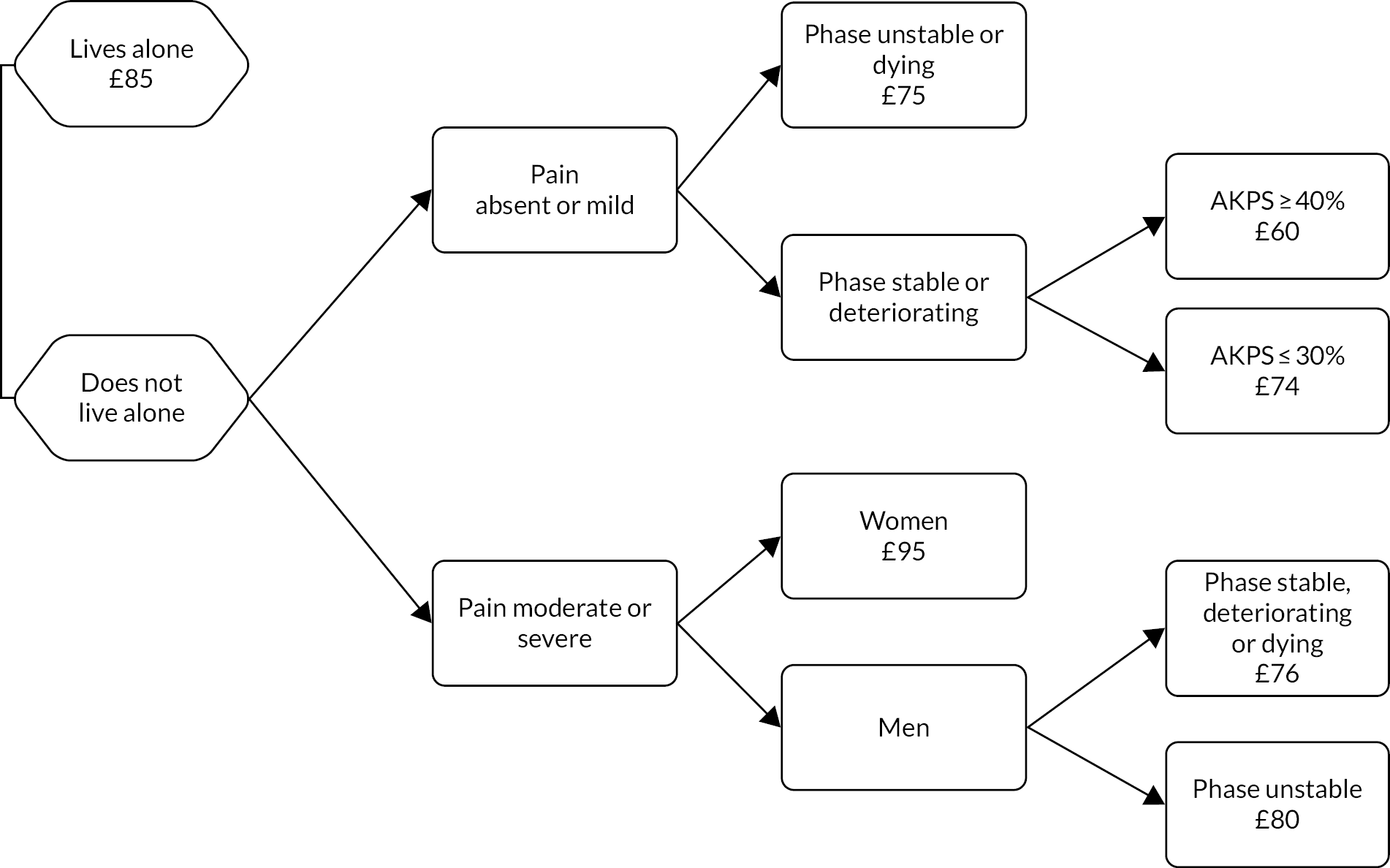
FIGURE 5.
Classification tree of casemix criteria for inpatient hospice episodes of specialist palliative care (costs per day reported for each class).
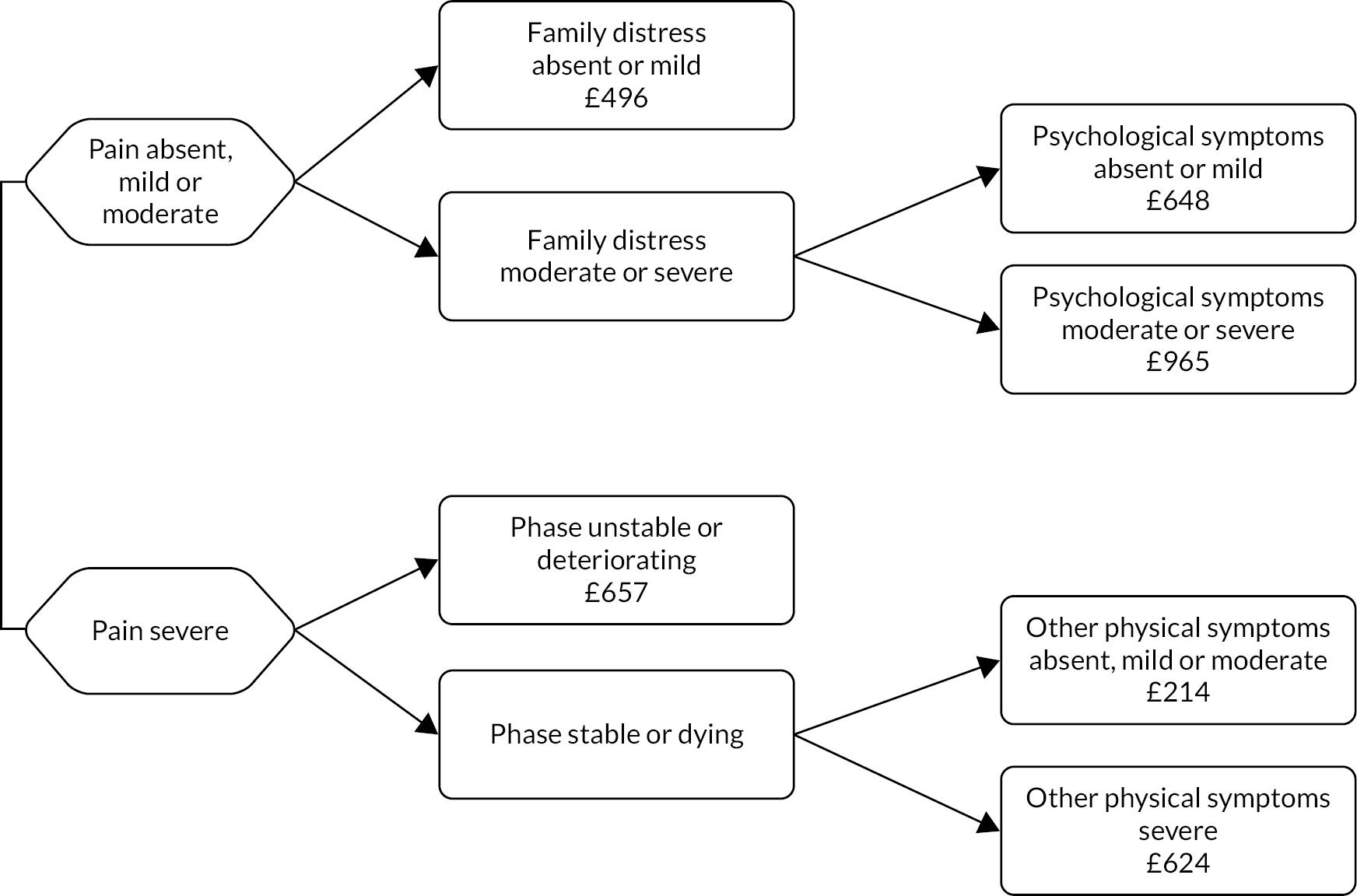
FIGURE 6.
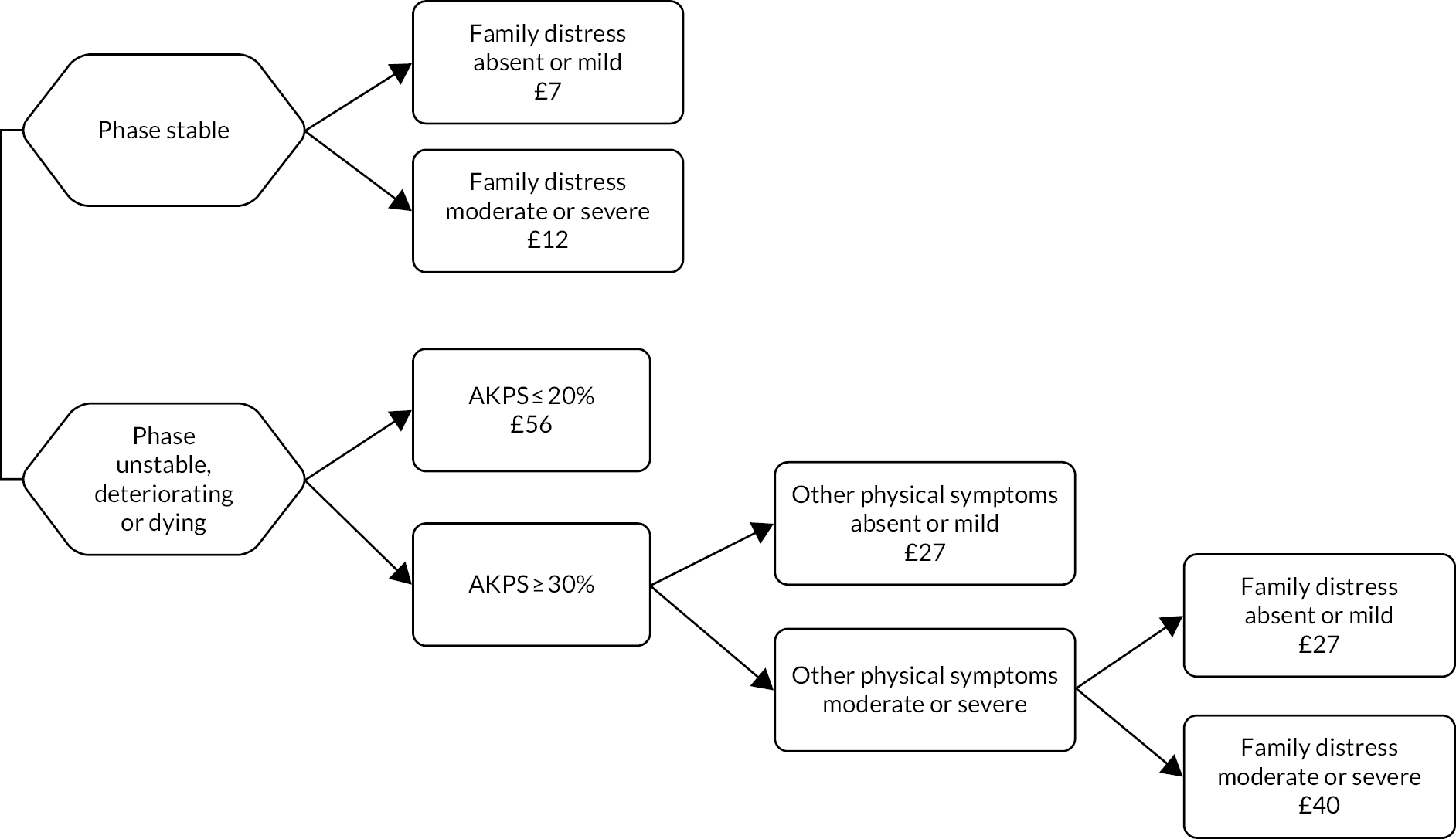
Classification tree of casemix criteria for community episodes of specialist palliative care (costs per day reported for each class).
Seven different casemix variables provide the optimal combination to develop classes for each of the settings. Table 4 shows which variables were used and how they were combined to constitute the casemix classes, including cost weights.
| Class | Living situation | Pain | Functional status | Palliative Phase of Illness | Family distress | Physical symptoms other than pain | Psychological symptoms | Sex | Class cost per day (£) | Cost weighta |
|---|---|---|---|---|---|---|---|---|---|---|
| Classes for hospital advisory episodes of care | ||||||||||
| A | Lives alone | – | – | – | – | – | – | – | 85 | 1.4 |
| B | Does not live alone | Absent or mild | – | Unstable or dying | – | – | – | – | 75 | 1.3 |
| C | Does not live alone | Absent or mild | AKPS ≥ 40% | Stable or deteriorating | – | – | – | – | 60 | 1.0 |
| D | Does not live alone | Absent or mild | AKPS ≤ 30% | Stable or deteriorating | – | – | – | – | 74 | 1.2 |
| E | Does not live alone | Moderate or severe | – | – | – | – | – | Female | 95 | 1.6 |
| F | Does not live alone | Moderate or severe | – | Stable, deteriorating or dying | – | – | – | Male | 76 | 1.3 |
| G | Does not live alone | Moderate or severe | – | Unstable | – | – | – | Male | 80 | 1.3 |
| Classes for inpatient hospice episodes of care | ||||||||||
| A | – | Absent, mild or moderate | – | – | Absent or mild | – | – | – | 496 | 2.3 |
| B | – | Absent, mild or moderate | – | – | Moderate or severe | – | Absent or mild | – | 648 | 3.0 |
| C | – | Absent, mild or moderate | – | – | Moderate or severe | – | Moderate or severe | – | 965 | 4.5 |
| D | – | Severe | – | Unstable or deteriorating | – | – | – | – | 657 | 3.1 |
| E | – | Severe | – | Stable or dying | – | Absent, mild or moderate | – | – | 214 | 1.0 |
| F | – | Severe | – | Stable or dying | – | Severe | – | – | 624 | 2.9 |
| Classes for community episodes of care | ||||||||||
| A | – | – | – | Stable | Absent or mild | – | – | – | 20 | 1.0 |
| B | – | – | – | Stable | Moderate or severe | – | – | – | 24 | 1.2 |
| C | – | – | AKPS ≤ 20% | Unstable, deteriorating or dying | – | – | – | – | 56 | 2.8 |
| D | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | – | Absent or mild | – | – | 27 | 1.4 |
| E | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | Absent or mild | Moderate or severe | – | – | 27 | 1.4 |
| F | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | Moderate or severe | Moderate or severe | – | – | 40 | 2.0 |
Workstream 4: testing of the casemix classification
This work has also been published in Guo et al. 92
Workstream 4 (testing the casemix classification) was planned to meet objective 5: to test this person-centred palliative care casemix classification in terms of its ability to predict resource use in the last year of life, and to better understand transitions between services in order to improve care.
Aim
Our aim was to test the palliative care casemix classification developed in workstream 3 in terms of its ability to predict resource use for patients receiving episodes of specialist palliative care, and to explore the experience of transitions between care settings for those receiving specialist palliative care.
Methods
Design
We designed a multicentre prospective cohort study, following patients during episodes of specialist palliative care, with a qualitative nested component (interviews with a subsample of participants to better understand the experience of transitions between care settings).
Definitions
We defined both an episode of care and the setting of care as in Workstream 3: development of the casemix classification, Definitions.
Population and settings
Patients were recruited from 12 organisations providing specialist palliative care services in England, comprising three hospital advisory services, eight inpatient hospice services and five community-based services (some organisations provided more than one setting of care). Sites were selected for diversity in terms of participant age, ethnic background, service size, proportion of cancer/non-cancer patients and urban–rural balance
Inclusion/exclusion criteria
The inclusion criteria comprised adult patients aged ≥ 18 years who were able to consent and receiving specialist palliative care at any of the participating sites. The exclusion criteria were patients aged < 18 years and those unable to consent.
Data collection and primary outcome
Data were collected from patient participants and clinicians between December 2016 and May 2018. Collected data included demographic and clinical variables, episode start and end dates, casemix variables as required for the casemix classification developed in workstream 3, and data on patient-level and other costs of providing specialist palliative care. All participants received the usual specialist palliative care at that site, including a multidisciplinary team with specialist training delivering holistic care focused on physical and psychological symptom management, social/family support, planning ahead around priorities and preferences, and care into the dying phase including post-death care of the family, where relevant. Details of the measures used are presented in Appendix 1.
Palliative Phase of Illness was assessed daily for people receiving inpatient (hospital or inpatient unit) care and at every contact for those receiving community-based care. Each change in Phase of Illness (or end of episode) triggered the collection of the AKPS, IPOS or PCPSS and Barthel Index score. All staff involved in patient care recorded the time spent delivering care to patient participants at a patient level using the staff activity matrix.
We also collected data from participating sites on the costs of delivering their services, plus patient-level resource use data, as in workstream 3.
A subsample of participants who had experienced at least two transitions between care settings were invited for interview. A purposive sampling approach was used to include participants from a range of age groups, sex, diagnoses, types of transitions in either direction and geographical areas.
Data handling
Quantitative data were collected prospectively at each site, recorded on an electronic database, cleaned and checked. Checked data were transferred to statistical software (Stata SE V.12) for analysis. Qualitative data were recorded, transcribed verbatim and handled using NVivo version 12.
Analysis
For the quantitative data, the casemix classes developed in Workstream 3 were applied to predict costs for episodes of care and this was contrasted with the actual costs captured for each episode of care. For the qualitative data we adopted a similar approach to Pinnock et al.’s qualitative study,93 undertaking a thematic94 and narrative analysis of interviews, exploring how perspectives on transitions evolve over time, with detailed attention to patient and family perspectives on their experience of care in each setting and during transitions, including their experience of interventions that potentially influenced changes in settings of care.
Ethics
The trial registration number is ISRCTN90752212. Written or oral witnessed consent was taken and documented for each participant, and continuing consent was confirmed at follow-up. Ethics approval was received from Bromley REC on 5 September 2016 (REC Reference: 16/LO/1021, IRAS Project ID: 204926).
Quantitative results
Subject characteristics
A total of 309 patients were recruited, providing data on 751 episodes of specialist palliative care. Of these participants, 309 contributed one episode of care, 177 (57%) contributed a second episode of care and 119 (39%) contributed a third episode of care. Only 63 (20%) participants contributed four or more episodes of care. Demographic and clinical characteristics for the 309 participants are reported in Table 5. Just over three-quarters (76%) of participants had cancer, but we were able to recruit one-fifth with a range of different non-cancer conditions.
| Characteristic | n (%) |
|---|---|
| Socio-demographic details a | |
| Age | |
| Mean (SD) | 66.9 (13.15) |
| Median (range) | 68 (18–96) |
| < 65 years | 117 (37.9) |
| ≥ 65 years | 183 (59.2) |
| Missing or prefer not to say | 9 (2.9) |
| Sex | |
| Male | 134 (43.4) |
| Female | 170 (55.0) |
| Missing or prefer not to say | 5 (1.6) |
| Ethnicity | |
| White | 265 (85.8) |
| Black African or Black Caribbean | 9 (2.9) |
| Asian | 8 (2.6) |
| Mixed ethnic background | 9 (2.9) |
| Other | 11 (3.6) |
| Missing or prefer not to say | 7 (2.2) |
| Marital status | |
| Married or partner | 165 (53.4) |
| Separated or divorced | 43 (13.9) |
| Widowed | 46 (14.9) |
| Single | 46 (14.9) |
| Missing or prefer not to say | 9 (2.9) |
| Living alone | |
| Yes | 107 (34.6) |
| No | 191 (61.8) |
| Missing or prefer not to say | 11 (3.6) |
| Interpreter neededb | |
| Yes | 2 (0.6) |
| No | 302 (97.8) |
| Missing | 5 (1.6) |
| Primary diagnosis b | |
| Cancer | 237 (76.7) |
| Lip, oral cavity and pharynx | 1 (0.3) |
| Digestive organs | 55 (17.8) |
| Liver and biliary | 8 (2.6) |
| Pancreas | 10 (3.2) |
| Respiratory and intrathoracic | 46 (14.9) |
| Bone, skin and mesothelial | 11 (3.6) |
| Breast | 28 (9.1) |
| Female genital organs | 16 (5.1) |
| Male genital organs including prostate | 25 (8.1) |
| Urinary tract | 11 (3.6) |
| Brain, eye and other central nervous system | 4 (1.3) |
| Unknown primary | 7 (2.3) |
| Lymphoid and haematopoietic | 15 (4.8) |
| Independent multiple sites | 0 (0.0) |
| Non-cancer | 59 (19.1) |
| HIV/AIDS | 1 (0.3) |
| Motor neurone disease/ALS | 11 (3.6) |
| Dementia including Alzheimer’s | 0 (0.0) |
| Neurological (excluding MND) | 8 (2.6) |
| Diabetes mellitus | 0 (0.0) |
| Heart failure | 5 (1.6) |
| Stroke, infarction or haemorrhagic | 0 (0.0) |
| Other heart or circulatory | 0 (0.0) |
| Chronic respiratory including COPD | 28 (9.1) |
| Liver failure or chronic liver disease | 1 (0.3) |
| Renal failure | 1 (0.3) |
| All other or multiple non-cancer conditions | 4 (1.3) |
| Missing | 13 (4.2) |
Episode characteristics
Details of the episodes of care are reported in Table 6. Seventy-seven episodes of care (10% of all episodes) occurred at sites not participating in the study; although we had endeavoured to include all specialist palliative care sites in each geographical area, inevitably some episodes of care (especially in hospital) were outside of our C-CHANGE sites. Tables 7 and 8 report the duration of episodes of care, by setting and by episode number, respectively. As expected, the median duration of episode was shortest for hospital advisory episodes (10 days) and longest for community episodes (26 days). Note that the absence of the dying Phase of Illness strongly affects these data; those first seen in the dying phase had the shortest length of stay yet are effectively excluded from these data because of the requirement for consent.
| Episode of care | Setting, n | Total, n | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Community | Hospice inpatient | Hospital inpatient (advisory) | Non-C-CHANGE site: communitya | Non-C-CHANGE site: hospice inpatienta | Non-C-CHANGE site: hospital inpatient (advisory)a | Other | Unknown | ||
| 1st | 136 | 64 | 107 | 2 | 309 | ||||
| 2nd | 88 | 33 | 13 | 13 | 1 | 25 | 2 | 2 | 177 |
| 3rd | 40 | 43 | 19 | 2 | 1 | 12 | 1 | 1 | 119 |
| 4th | 21 | 24 | 4 | 2 | 10 | 2 | 63 | ||
| 5th | 13 | 12 | 4 | 5 | 34 | ||||
| 6th | 10 | 8 | 2 | 2 | 22 | ||||
| 7th | 5 | 4 | 2 | 11 | |||||
| 8th | 2 | 4 | 2 | 8 | |||||
| 9th | 3 | 2 | 5 | ||||||
| 10th | 1 | 1 | |||||||
| 11th | 1 | 1 | |||||||
| 12th | 1 | 1 | |||||||
| Total | 319 | 195 | 152 | 19 | 2 | 56 | 5 | 5 | 751 |
| Duration of episode of care (days)a | ||||
|---|---|---|---|---|
| Community | Hospice inpatient | Hospital inpatient (advisory) | All settings | |
| Mean (SD) | 52.67 (67.17) | 25.31 (29.07) | 18.38 (29.18) | 36.48 (53.84) |
| Median (range) | 26 (1–365) | 15.5 (1–168) | 10 (1–250) | 17 (1–365) |
| Missing, n | 24 | 3 | 4 | 35b |
| Duration of episode of care (days)a | ||||
|---|---|---|---|---|
| Episode 1 (n = 307) | Episode 2 (n = 134) | Episode 3 (n = 102) | All (n = 543) | |
| Mean (SD) | 41.18 (56.73) | 34.86 (55.74) | 28.24 (39.76) | 36.48 (53.84) |
| Median (range) | 20 (1–304) | 15 (1–365) | 16 (1–250) | 17 (1–365) |
| Missing, n | 23 | 3 | 9 | 35 |
Casemix variables
Details of the casemix variables are reported in Table 9. Apart from the dying Phase of Illness, the casemix variables extended across the full range of categories or scores.
| Episode characteristics | Episodes, n (%)a |
|---|---|
| Setting of care | |
| Hospital advisory | 139 (25.6) |
| Inpatient hospice | 140 (25.8) |
| Community | 264 (48.6) |
| Total | 543 (100.0) |
| Palliative Phase of Illness at episode start | |
| Stable | 193 (35.6) |
| Unstable | 195 (35.9) |
| Deteriorating | 148 (27.2) |
| Dying | 0 (0.0) |
| Missing | 7 (1.3) |
| AKPS score at episode start | |
| Mean (SD) [range] | 53.5 (14.4) [20–90] |
| 0–50 | 260 (47.9) |
| 60–100 | 271 (49.8) |
| Missing | 12 (2.3) |
| Modified Barthel Index score at episode start | |
| Mean (SD) [range] | 15.00 (5.2) [1–20] |
| Missing | 16 (2.9) |
| PCPSS at episode start | |
| Pain | |
| Mean score (SD) [range] | 1.52 (1.06) [0–3] |
| Absent | 118 (21.8) |
| Mild | 140 (25.7) |
| Moderate | 162 (29.8) |
| Severe | 118 (21.8) |
| Missing | 5 (0.9) |
| Other physical symptoms | |
| Mean score (SD) [range] | 1.03 (0.63) [0–3] |
| Absent | 83 (15.2) |
| Mild | 358 (66.0) |
| Moderate | 80 (14.7) |
| Severe | 12 (2.2) |
| Missing | 10 (1.9) |
| Psychological symptoms | |
| Mean score (SD) [range] | 1.62 (0.88) [0–3] |
| Absent | 49 (9.0) |
| Mild | 186 (34.3) |
| Moderate | 186 (34.3) |
| Severe | 91 (16.7) |
| Missing | 31 (5.7) |
| Family concerns | |
| Mean score (SD) [range] | 2.03 (0.99) [0–3] |
| Absent | 53 (9.8) |
| Mild | 70 (12.9) |
| Moderate | 169 (31.1) |
| Severe | 192 (35.3) |
| Missing | 59 (10.9) |
Actual and predicted costs per day
Table 10 presents the actual costs per day, whereas Table 11 compares the predicted class costs with the actual costs per day. The actual cost of inpatient hospice care are consistently higher than the predicted class cost, perhaps because of the 2-year interval between the collection of costs for workstreams 3 and 4 (no uplift was applied) or possibly because of improvements in the application of our costing methodology (the same costing methodology was used in both workstreams, but the teams were more familiar with how to apply it).
| Setting of care | n | % |
|---|---|---|
| Hospital advisory | 139 | 25.6 |
| Inpatient hospice | 140 | 25.8 |
| Community | 264 | 48.6 |
| Total | 543 | 100.0 |
| Mean (SD) | Median | |
| Hospital advisory | ||
| Cost per day (£)a | 50.83 (10.88) | 51.45 (38.17–57.84) |
| Length of episode (days) | 18.38 (29.18) | 10 (5.5–15) |
| Inpatient hospice | ||
| Cost per day (£)a | 602.02 (257.73) | 699.61 (536.90–772.66) |
| Length of episode (days) | 25.31 (29.07) | 15.5 (7.75–24.25) |
| Community | ||
| Cost per day (£)a | 34.56 (8.22) | 32.83 (25.75–40.79) |
| Length of episode (days) | 52.67 (67.17) | 26 (23–207) |
| Class | Living situation | Pain | Functional status | Palliative phase of illness | Family distress | Physical symptoms other than pain | Psychological symptoms | Sex | Predicted class cost (£) | Actual cost (£), median |
|---|---|---|---|---|---|---|---|---|---|---|
| Classes for hospital advisory episodes of care | ||||||||||
| A | Lives alone | – | – | – | – | – | – | – | 85 | 86.44 (75.99–88.94) |
| B | Does not live alone | Absent or mild | – | Unstable or dying | – | – | – | – | 75 | 76.87 (70.99–86.33) |
| C | Does not live alone | Absent or mild | AKPS ≥ 40% | Stable or deteriorating | – | – | – | – | 60 | 62.12 (53.79–73.61) |
| D | Does not live alone | Absent or mild | AKPS ≤ 30% | Stable or deteriorating | – | – | – | – | 74 | 78.22 (70.72–84.51) |
| E | Does not live alone | Moderate or severe | – | – | – | – | – | Female | 95 | 96.55 (89.68–98.44) |
| F | Does not live alone | Moderate or severe | – | Stable, deteriorating or dying | – | – | – | Male | 76 | 74.47 (69.01–80.37) |
| G | Does not live alone | Moderate or severe | – | Unstable | – | – | – | Male | 80 | 81.55 (69.87–89.99) |
| Classes for inpatient hospice episodes of care | ||||||||||
| A | – | Absent, mild or moderate | – | – | Absent or mild | – | – | – | 496 | 597.18 (531.80–642.03) |
| B | – | Absent, mild or moderate | – | – | Moderate or severe | – | Absent or mild | – | 648 | – |
| C | – | Absent, mild or moderate | – | – | Moderate or severe | – | Moderate or severe | – | 965 | 1003.67 (931.49–1088.67) |
| D | – | Severe | – | Unstable or deteriorating | – | – | – | – | 657 | 757.07 (710.46–823.62) |
| E | – | Severe | – | Stable or dying | – | Absent, mild or moderate | – | – | 214 | 351.34 (279.67–386.68) |
| F | – | Severe | – | Stable or dying | – | Severe | – | – | 624 | – |
| Classes for community episodes of care | ||||||||||
| A | – | – | – | Stable | Absent or mild | – | – | – | 20 | 24.80 (21.76–30.56) |
| B | – | – | – | Stable | Moderate or severe | – | – | – | 24 | 25.82 (24.88–28.96) |
| C | – | – | AKPS ≤ 20% | Unstable, deteriorating or dying | – | – | – | – | 56 | – |
| D | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | – | Absent or mild | – | – | 27 | 29.57 (27.92–31.33) |
| E | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | Absent or mild | Moderate or severe | – | – | 27 | 26.90 (25.66–28.92) |
| F | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | Moderate or severe | Moderate or severe | – | – | 40 | 40.17 (38.67–41.67) |
Qualitative results
A total of 20 interviews with 26 participants were conducted. Participants’ ages ranged from 36 to 91 years (mean 68 years). Of these 20 interviews, 14 were conducted with patients and family caregivers separately and six jointly.
Four main themes and various subthemes were identified from the data analysis.
Theme 1: uncertainty about the new care setting
Lack of information about the new setting of care and patients’ uncertainty regarding their discharge plan predominated in interviews.
Many participants commented on the ‘bad’ timing of a transition in care setting (e.g. emergency admissions during out of hours) and uncertainty around access to care.
Participants highlighted the fact that clear and effective communication is as important as the quality of care delivered; receiving the right information in the right place at the right time helped them to gain a better understanding of their health status and care plan, which reduced their anxiety and worries.
Theme 2: biographical disruption
Sense of identity was considered important but participants felt that transitions often compromised one’s sense of self, which linked to an inevitable loss of control and independence.
Theme 3: importance of continuity of care
Both family and patients praised clinicians who provided continuity and got to know them as individuals. They appreciated not having to retell their stories and recognised the difference in care when staff knew them and their stories.
Participants highlighted the importance of the correct and timely transfer of patient information, medical notes and medication prescriptions; when things were disjointed, patients and their families felt unsafe and that lack of continuity could lead to medical mistakes.
Theme 4: need for emotional and practical support
Family members often act as advocates in the healthcare setting and the majority of participants specified a great need for support from family and friends not only in practical matters, such as arranging transport or coordinating care, but also in the emotional aspect of transitions in care.
Patient and public involvement
Over the course of the programme we have followed our plan for PPI at three levels, as originally proposed:
-
through our dedicated C-CHANGE PPI group
-
through patient/carer participation in the Programme Steering Committee
-
through engagement with a wider Consumer Panel at the Cicely Saunders Institute, King’s College London, where the research team is based.
The C-CHANGE Patient and Public Involvement Group
Throughout the programme, we have been strongly supported by a committed and active C-CHANGE PPI Group. Initially this consisted of four members, then expanded to seven members from 2015. The Group supported the work of the programme in a variety of ways: through regular face-to-face quarterly meetings; through Skype (Microsoft Corporation, Redmond, WA, USA) and e-mail consultations about specific elements of the work; through contributing to test surveys and interviews prior to data collection from patient participants in our studies; through the attendance of one or two delegated members at Ethics Review meetings; and through the active involvement of the whole group in providing invaluable feedback on the extent to which our work was patient and family centred (or not), and the effectiveness of the programme in delivering relevant evidence.
The PPI group sought to widen and improve its ethnic diversity, and this was addressed by recruiting six new members from diverse ethnic backgrounds from October 2015 onwards. The group changed again in the final year of the programme when two members chose to leave owing to deteriorating health. The group was kept informed of the programme and provided advice through regular contact by telephone, e-mail and Skype, and in face-to-face meetings: the Group has been an integral part of the team. There were also numerous individual or smaller group meetings by telephone and Skype, and at home (according to preference and to accommodate those less well). Payment for involvement and related expenses were in accordance with the recommendations of the NIHR.
Engagement with the Consumer Panel
We also worked with a more diverse group, the Consumer Panel (a network of patients, families and the public developed and sustained at the Cicely Saunders Institute; see Brighton et al. 95 for further details), which enabled engagement with a wider and more diverse group constituency. The following individuals and organisations especially contributed in this respect: Carolyn Morris and Kirstie Newson (who worked with us on our Dissemination and Engagement Group at the Cicely Saunders Institute); our Macmillan Information & Support Centre (which was located in the Cicely Saunders Institute, provided information and support to patients and public, and hosted several patient/family support groups who were able to contribute); and Hospice UK (London, UK) (a national organisation which supports palliative care providers).
We engaged with the Consumer Panel throughout the programme, largely by phone, Skype and e-mail but also through several smaller face-to-face group meetings (according to need) to discuss issues that emerged. The focus of our work with the Consumer Panel was on considering and discussing individual measures, considering which measures could be used together, how the measures met the priorities and domains of concern of patients/families with advanced illness, how the measures work both individually and together for this population, and how a casemix classification might best support good quality care and reduce inequities in access and care. Through the Consumer Panel we were able to test the training materials for the use of measures, and review and refine participant and other materials for workstreams 3 and 4. The Consumer Panel was kept informed through our quarterly newsletter, although we also met with a number of individuals on the Consumer Panel for specific pieces of work, as the programme required.
Patient participation in the Programme Steering Committee
Mr Jonathan Hope and Mrs Sue Farr (co-leads for PPI) regularly attended the meetings of the C-CHANGE Programme Steering Committee, where they were updated on the programme’s progress, provided feedback from the PPI group, informed the planning of next steps, addressed challenges that arose and helped the committee to better consider patient and family perspectives. We owe them both a great debt for their wisdom, their contributions, their ability to challenge us, and the insight they brought from extensive experience of both serious illness and providing care.
Innovation and evolution in our patient and public involvement and engagement
Patient and public involvement in palliative care is challenging because of the severity of illness and short survival time of those affected (some of our PPI partners, for example, were very seriously ill), the substantial caring demands on families, and the impact of bereavement often experienced. However, we linked to Collaborations for Leadership in Applied Health Research and Care (CLAHRC) South London to help us develop and sustain a sufficient ‘critical mass’ within the C-CHANGE PPI group to support effective PPI engagement over the years of the programme.
In addition to the PPI described above, over the course of the programme we held three PPI workshops across the Cicely Saunders Institute (14/06/2016, 15/11/2016 and 10/04/2017) to improve the quality and relevance of the research and maximise its impact on improving care. Both the C-CHANGE researchers and our PPI C-CHANGE group members were actively involved and engaged in this work. These workshops focused on increasing dialogue and collaboration, listening to our PPI partners, facing challenges, and seeking ideas and solutions together.
Impact of patient and public involvement to date
Involvement in identifying the research topic, prioritising the research questions and preparing the application
Patients and the public contributed to (1) the prioritisation of the research topic and questions, (2) the development of the research design and (3) the preparation of the application. The research topic was identified and prioritised with the involvement of the Consumer Panel of the National Cancer Research Institute collaborative in supportive and palliative care as part of our Programme Development Grant bid. There was universal agreement from our patient and public partners that patient and family needs towards end of life are often poorly matched to resources, leading to inequity and, for some, major gaps in provision.
We made changes to the protocols and ethics submissions as a result of PPI feedback. In addition, we had encouragement from our PPI partners throughout, which was very supportive of the team.
Developing and refining research resources (i.e. participant information sheets and topic guides)
All patient-facing materials within the programme were reviewed, amended and/or written by our PPI group.
In workstream 2, the PPI group recommended that certain groups were represented within the stakeholders’ interviews (e.g. spiritual leads and social workers) to diversify the sample. Workstream 2 was extended to include patient and family stakeholders on the recommendations of our PPI group and with agreement from our Programme Steering Committee. We therefore increased the number of interviews from the planned 40–50 to 65. This allowed us to identify the most important domains for patients and carers across a range of diagnoses and backgrounds and ensure they were captured in workstreams 3 and 4.
We continued to engage with and consult members of our PPI group on the design and data collection for workstreams 3 and 4. This included enlisting support for drafting the questionnaires, developing the interview topic guide and refining our methods for collecting data. The PPI group suggested that we shorten the patient questionnaires to reduce overall research burden and we have had direct discussion on how best to do this.
Modification of research plans
As a consequence of PPI feedback, carer questions were added to workstreams 3 and 4 to further assess the strain or burden for the main family caregiver of caring for their relative. This question was derived from the Zarit Carer Interview. 96 The PPI group also suggested that we include a question to measure whether or not the care received is sensitive to religious beliefs and cultural needs, based on their own direct experiences of caring for family members at the end of life. The PPI group encouraged the team to include pre- and post-bereavement written materials for patients, families and carers, especially regarding the needs of bereaved carers.
Secondary analysis on complexity
We were urged to further consider the role of uncertainty in the complexity of care and domains relevant for patients and families. As a result of this dialogue, a secondary analysis was conducted that examined the main priorities and concerns of patients and carers in advanced illness and how uncertainty shaped their experience. This work was published in Palliative Medicine in 2017. 78
Dissemination
As a way to disseminate the C-CHANGE project and the PPI work, our PPI group developed a video diary, taking a ‘reflections and responses’ approach. The aim was to capture PPI perspectives on video and enable the research team to consider and respond to the evolution of the research. In total, our PPI group, alongside a C-CHANGE researcher, produced six videos (see Table 12). The process for creating the videos was as follows:
-
The PPI group suggested a topic for a video log.
-
A PPI member co-wrote a script with a C-CHANGE researcher.
-
A PPI member and C-CHANGE researcher edited and refined the script and filmed the video.
-
The PPI member and/or group provided feedback on how best to edit the video.
| Video title | URL |
|---|---|
| The C-CHANGE research project: A Carer’s Experience – How can we improve coordination of care? 1/3 | https://youtu.be/RnOYg0myU0M |
| The C-CHANGE project: A Carer’s Experience – Supporting families when the news is difficult 2/3 | https://youtu.be/v_NxKMlGNgc |
| The C-CHANGE research project: A Carer’s Experience – Bereavement care 3/3 | https://youtu.be/0Bt67hIOHMc |
| How are patients’ needs supported by C-CHANGE? | https://youtu.be/SFuArqcpkEU |
| Patient and Public Involvement impact on developing study questionnaires | https://youtu.be/Ho87sRWn-Xg |
| How the C-CHANGE project responds to patient distress | https://www.youtube.com/watch?v=1-6Po1aeOuA |
The main topics raised in the videos comprised: (1) identifying different cultural needs, (2) the individual concerns of people with advanced illness, (3) capturing emotional distress, (4) thinking about goal-based outcomes, (5) the experiences of patients alone and (6) the safety of patients and their family members.
Contribution to dissemination documents (i.e. lay summaries and newsletters)
Throughout the course of the programme, we enlisted the help of our PPI members to provide feedback on lay summaries of our published academic papers. In addition, the PPI group contributed to and reviewed quarterly C-CHANGE newsletters that have been circulated to research sites, local community groups and organisations of interest.
Summary
Throughout the programme we attempted to achieve a careful balance between incorporating and reflecting the invaluable insights from our PPI group, which we were very keen to adopt, and yet working within the constraints of our existing timelines and resources. The Programme Steering Committee was very helpful in advising on this and we believe we successfully achieved this balance.
Successes and limitations in the C-CHANGE programme
One of our major successes in workstream 1 was the refinement and validation of the IPOS. This represents a major step forward internationally for palliative care outcome measurement, for several reasons. First, we have demonstrated that this is a valid and reliable outcome measure, both in patient self-report and staff proxy-report versions. There are very few measures to assess well-being and health status in advanced illness which include both patient-report and proxy-report versions, with detailed evidence on how these correlate. 73 Proxy-report is often required in palliative care as patients become too ill or fatigued to self-report. Second, although there are pre-existing psychometrically robust symptom measures (such as the Edmonton Symptom Assessment Scale97 and the Memorial Symptom Assessment Scale98) and quality of life measures (such as EORTC QLQ-C15-PAL77,99) for palliative care, there are no measures for advanced illness which capture the full range of symptoms/concerns which affect people with advanced illness. The IPOS reflects not just their physical or psychological symptoms, but also their information needs, family distress and support needs, and practical concerns. Third, our IPOS validation study included a high proportion of people with poor functional status, strengthening our conclusions for the advanced illness population. These are often the most difficult participants to recruit into studies, but our close working with participating sites helped to maximise recruitment and ensure that we included participants across the full range of functional status.
The IPOS is now freely available on our measures webpage: https://pos-pal.org/. It is already widely used both nationally and internationally; we have a network of UK-based palliative care services who link into an Extension of Community Healthcare Outcomes (ECHO) webinar network hosted by Hospice UK to support implementation and use, and there are already 12 translated versions of the IPOS (following our manual for translation and cultural adaption) in Czech, Estonian, French, German, Greek, Hindi, Italian, Korean, Polish, Portuguese, Swedish and Turkish, for use both in the UK and internationally. At least seven further translations are in process.
In addition, we were able to complete further work on the palliative Phase of Illness measure during Workstream 1, which was essential for the later casemix work. Preliminary work in the UK100 and Australia59 had suggested that palliative Phase of Illness is important in determining casemix within palliative care. However, little psychometric or clinical testing had been undertaken; we were able to show that Phase of Illness has clinical utility as a measure of overall palliative need, capturing additional information beyond other measures such as the AKPS and PCPSS. 74 A limitation of this work was that we did not have any scope to analyse how Phase of Illness and the IPOS relate to each other; it was too early in validation of the IPOS to use the measure in this preliminary work. Nevertheless, our work confirmed the importance of including palliative Phase of Illness in workstreams 3 and 4 and that it reflected a distinct (and different) dimension of palliative care needs.
A further achievement was being able to derive and test a patient experience measure. VoC is brief and easy to use on a large scale with patients receiving palliative care across different settings. 75 Patient, PPI and clinical feedback on this measure has been good; it has been clinically adopted across a range of palliative care services (e.g. St Christopher’s Hospice) and it too is freely available at https://pos-pal.org/.
Through discussion with our PPI group, it became much clearer to us how uncertainty influences patient experience during advanced illness by affecting patients’ information needs, preferences and future priorities for care. Assessment of these three factors is a useful starting point to guide clinical assessment and shared decision-making, and we considered how to include them in workstreams 3 and 4. However, it proved difficult to incorporate these findings into workstreams 3 and 4; this work was already complicated, and finding a workable way to add this dimension to our data capture (such that it would help inform the casemix classification) proved unfeasible.
In workstream 2, we conducted and analysed a large number and diverse range of interviews exploring stakeholders’ perspectives on measuring complexity. A major success from this workstream was the production of a novel framework for understanding complexity in palliative care. The findings from this piece of work were very well received by clinicians and researchers working in palliative care. The Field-Weighted Citation Impact in Scopus (Elsevier, Amsterdam, the Netherlands) is already 4.16 (as of 1 August 2020), indicating 316% more citations than average for the field.
We had several challenges with recruitment in workstream 2, particularly with recruiting a representative sample of the various stakeholder groups, but we overcame these to achieve a balanced sample by approaching additional sites and participants. Our study was able to represent views from stakeholder groups that are usually under-represented in complexity research, including patients and families, allied health professionals and national leads. To develop a well-integrated and meaningful theoretical framework to understand complexity, we presented the findings to internal and external experts at different stages of our data analysis. This meant going through several iterations to develop and refine a comprehensive and meaningful framework. Although this required more time and effort, the resulting output was a more integrated and useful framework to describe complexity in palliative care. In terms of informing workstreams 3 and 4, this work was invaluable in helping to ensure we measured psychological symptoms, social concerns, information needs (reported as highly relevant for pre-existing complexity), and practical concerns were identified.
Our work on models of specialist palliative care was, to the best of our knowledge, one of the first attempts at deriving empirical criteria to distinguish different specialist palliative care models. Using mixed methods in a sequential approach, we developed a set of criteria from these primary data to characterise and distinguish different specialist palliative care services in the UK. These criteria comprised setting, type of care, size of service, diagnoses accepted, disciplines, mode of care, types of interventions, out-of-hours characteristics, external education provision, use of outcome/experience measures, and bereavement provision, plus the purpose of the team, who funds/manages the team, ability to self-refer and discharge processes. These criteria capture the key differentiating components between different models of specialist palliative care across settings (i.e. hospice inpatient care, hospital-based care and community-based care) and, to our knowledge, enable these different models of care to be described and compared accurately for clinical and commissioning purposes for the first time. This study also provides the foundational work for improved research on which components of a model of care are most effective. A strength of this work is that it sought expert consensus from ‘real world’ professionals to identify the key criteria to characterise and differentiate these highly varied models of specialist palliative care. 81 Our criteria, taken together, provide a defined and workable way to characterise and distinguish different models of specialist palliative care. These criteria are not exhaustive and they are not intended to be; however, they do help to discriminate between different models. We recognise that they do not reflect all models of palliative care across England but they provide a starting point in terms of evidence on which to build. In recent years, and particularly since this programme began, there has been a growing number of different models of palliative care developing,101–103 especially in community settings;104 distinguishing them in both practice and research is important.
In workstreams 3 and 4 we have for the first time, to our knowledge, demonstrated robustly which casemix variables are associated with higher or lower costs for specialist palliative care across different settings of care, and tested these classes. A casemix classification was developed for each of the three settings: hospital advisory, inpatient hospice and community specialist palliative care. Casemix classes are presented in the casemix classification and are based on the clinical criteria of pain, other physical symptoms, psychological symptoms, family distress, palliative Phase of Illness, functional status and living situation. This is a major achievement. Some work was previously undertaken on this by NHS England and Public Health England using similar casemix criteria,58 but there was limited evidence of how the classes were derived, and classification and regression tree analyses were not applied.
In our work on the casemix classification, it became clear that each participating site had very different models of care. A challenge was attempting to use the multiple criteria meaningfully in the work to develop the casemix classification. There were too many models of care and too many variables to describe them for any attempt to stratify or adjust for the different models. To some extent, we allowed for differing models of care in our sample size calculation (and we achieved our sample size), but the wide range of different models of care we encountered across participating sites was unexpected. When fitting ‘site’ as a variable into the CARTs to explain costs for inpatient hospice and community episodes of specialist palliative care, we found that site (as a proxy variable for model of care) explained a notable amount of variance in the outcome and more than the patient-level complexity factors. In all settings, there seemed to be a clustering of sites offering more traditional models of care versus sites with innovative/new care models. Owing to the limit on the number of sites per model of care in our design, however, we did not explore this independent variable in the trees and deliberately restricted the CART to patient–level complexity levels only. In some ways, this was helpful; the casemix classification is derived from patient-level variables alone; it is not the model of care which dictates the casemix class. However, clearly the model of specialist palliative care delivered does – at some level – influence costs. We need far more evidence about which models of care (when properly characterised) are most cost-effective.
Lastly, two factors had a major impact on the completion of the final report and the preparation of the remaining two main papers for publication; the Chief Investigator left the programme to take up a new position – with a part-time transition period during 2018 and 2019 – and the COVID-19 pandemic interrupted and severely delayed the completion of this report.
Conclusions
In workstream 1, we were able to refine, validate and test new and existing patient-centred outcome measures to assess the main symptoms and concerns of patients and families receiving specialist palliative care, for use in workstreams 3 and 4. In addition – at the request of our PPI group – we completed a secondary analysis of existing qualitative data to understand the role of uncertainty in assessing the care needs of patients and families with advanced progressive illness.
The IPOS is a valid and reliable outcome measure, both in its patient self-report and staff proxy-report versions. 73 It can assess and monitor symptoms and concerns in advanced illness, determine the impact of healthcare interventions and demonstrate quality of care. This represents a major step forward internationally for palliative care outcome measurement. 73
We demonstrated that palliative Phase of Illness has value as a clinical measure of overall palliative need, capturing additional information beyond the AKPS and PCPSS. In addition, we have shown that VoC (a measure of quality of life and experience of care) is brief and easy to use on a large scale with patients receiving palliative care across different settings. It is brief and easy enough to use for ill patients receiving palliative care, which, to the best of our knowledge, makes VoC uniquely able to provide patient-level feedback in real time when compared with the institutional-level indicators that are often used to assess the quality of healthcare services.
Uncertainty influences patient experience in advanced illness through affecting patients’ information needs, preferences and future priorities for care. 78 Our typology aids understanding of how patients with advanced illness respond to uncertainty. Assessment of these three factors may be a useful starting point to guide clinical assessment and shared decision-making.
To the best of our knowledge, workstream 2 provided for the first time in palliative care a structured and evidence-based framework to conceptualise and consider the complex palliative care needs of those with advanced illness and their families. It also enabled us to characterise more fully what needed to be measured for workstreams 3 and 4 (the development of the casemix classification). Overall, participants reported that they thought it acceptable to measure complexity at the individual patient-level, but that any system to do so needed to incorporate the key dimensions of complexity included in the framework.
To our knowledge, until now there has not been a clear set of criteria to define models of UK specialist palliative care, making it challenging to compare different models of care provided by services. This component of the programme identified the criteria needed to characterise and differentiate models of specialist palliative care, a major paradigm shift to enable accurate reporting and comparison in practice and research.
Our detailed evidence on specialist palliative care costs and our casemix classification for specialist palliative care deliver a major advance for the sector. Each person needing specialist palliative care is different, with varying degrees of complex needs. We now have the means to understand this, systematically and at scale, and for practice, policy (including resourcing of palliative care) and research. The casemix classes show cost weight variations of up to 60% in hospital advisory care, up to 4.5-fold in inpatient hospices, and up to almost threefold in community care. The needs of each person are varied – not fixed – and require different resources to deliver care effectively. Understanding the casemix of those needing care, how this affects what outcomes can be achieved, how this varies across services and regions, and how this changes over time has the potential to help address inequities and provide more equitable specialist palliative care to all who need it.
Finally, our exploration of the impact of transitions between settings of care has clearly highlighted the human cost of poor communication and information-giving, the sometimes lack of continuity of care and the need for emotional and practical support for patients and families to ensure that moves between settings are better negotiated.
Recommendations for future research
The research recommendations from this programme fall into three areas: (1) research recommendations about the measures themselves, (2) research recommendations on the models of palliative care and (3) research recommendations about the casemix classification.
Research recommendations about the measures
Implementation research using the IPOS – particularly on the best ways to implement it into clinical services and how to assess which is the best version for use (patient-reported or proxy-reported) – is much needed. Some of this research has been subsequently undertaken105 and more research is already in process (see Bradshaw et al. 106 and www.hyms.ac.uk/research/research-centres-and-groups/wolfson/resolve).
In addition, the IPOS needs to be adapted for specific advanced disease populations, for example those with end-stage kidney disease107 and heart failure108 – this work is developing rapidly.
Research recommendations about the models of palliative care
Our theoretical framework to understand the complexity of palliative care needs is a useful advance for both clinical practice and research, but we need to study more fully certain aspects of complexity within the exo-system (services/systems) and macro-system (societal influences). These areas are much less studied than the micro- and meso-levels, and yet they are crucial if integrated care (i.e. care that cuts across service and community boundaries) is to become a reality. It is important to determine and explore socioeconomic status, and how this affects palliative care needs. ‘Cumulative’ and ‘invisible’ complexity may interact with socioeconomic status and so influence some of the known inequities in provision according to socioeconomic position. 109
The research on models of care needs to be extended and externally validated beyond the C-CHANGE sites, which – by nature of their willingness to participate – may not reflect all models of specialist palliative care.
Although a systematic review of the components of models of palliative care has been undertaken,110 it is not yet clear which components are used in different models of care, let alone whether or not they are clinically effective and cost-effective. Brereton et al. 111 identified the importance of defining and describing the components of models of care to differentiate them in both practice and research, and to truly understand clinical effectiveness and cost-effectiveness. We have progressed work on defining models of care (see Workstream 2: models of specialist palliative care),81 but much more is needed. There is also a major need to understand how primary and community services work with specialist palliative care; some of our data suggested that specialist palliative care models were significantly adapted according to the primary- and community-based services for end-of-life care available, and especially in relation to COVID-19.
Research recommendations about the casemix classification
The casemix classification for specialist palliative care provides a standard way to measure complexity of needs, enable services to compare workload between teams and determine whether or not outcomes are achieved as expected for different levels of complexity. The classification could also – if used judiciously – allow teams and services to make a better case for sufficient resources, and – in the longer term – support casemix-adjusted outcome measurement. There are a range of implications for research.
First, these casemix classes should be applied within research studies to better characterise study populations and therefore improve understanding of generalisability of research findings.
Second, whether or not these casemix classes work across the full range of non-cancer conditions should be tested, especially as the proportion of people with multiple long-term conditions increases.
Third, further research should determine how these casemix classes hold over time as the population age distribution changes and the patterns of end-of-life conditions changes. The classes developed within this programme are similar to those developed in the UK by the Palliative Care Funding Pilots58 and to the Australian casemix classification,57 with some of the same criteria found to predict variance in resource use, notably symptoms, palliative Phase of Illness, functional status and family distress. The combination of criteria within each class is, however, somewhat different in this programme than in these earlier studies. These differences may reflect a changing palliative care population since the Australian classification and UK Funding Pilots were completed. The proportion of those with non-cancer conditions receiving specialist palliative care, for instance, is increasing steadily112 and 20% of participants recruited had non-cancer conditions to reflect this change.
Fourth, it is important that these casemix classes and related costs are tested across a wider range of NHS and non-NHS services. This study provides reasonably current patient-level cost data for episodes of specialist palliative care. NHS unit costs for specialist palliative care do exist,113 but their reliability is unclear and they do not reflect non-NHS services (which includes most specialist palliative care in the UK). A recent systematic review of the cost of UK palliative care114 found very limited cost evidence (only 10 studies over 20 years), with most studies combining estimates of resource use with potentially unreliable unit cost data. Compared with the 2018 NHS unit costs collected in 2017113 (i.e. at the same time as the collection of cost data for this study), we found actual costs of specialist palliative care – at least in hospital and community settings – to be lower: hospital advisory care cost an average of £73 per day (compared with £201 in NHS unit costs113), and community care was £36 per day (compared with the unit cost for a specialist nurse of £64113). In part, this may reflect the challenges of collecting accurate cost data, particularly in community settings, but it may also reflect the range of staff providing care and the frequency of visits (i.e. less visits under cost constraints leading to reduced costs per day). Models of specialist palliative care are also increasingly diverse,81 especially in the community,110 and workforce shortages are leading to new models, for instance with senior staff supporting less experienced staff with skilled work, a reduced frequency of visits and new models for older people with multimorbidity. 100 By contrast, we found that at an average of £716 per day, inpatient palliative care was notably more costly than the 2018 unit cost of £404 per day;113 this may reflect the increasingly complex needs of those needing inpatient specialist palliative care.
Implications for decision-makers
Our theoretical framework developed in workstream 2 provides a structured and comprehensive way of considering complexity in palliative care. This understanding of complexity can help move both clinicians and policy-makers towards a more meaningful response to complexity, highlighting as it does the need for services to move away from ‘standard’ and ‘one size fits all’ care, and towards more individualised and tailored delivery.
Stakeholders – including decision-makers – highlighted the importance of having a shared understanding of complexity across different specialist palliative care providers and settings. Frameworks that accurately capture patient-level complexity can allow us to communicate the specialist palliative care provider role more clearly to other specialists and generalists providing palliative care to patients and families.
An important consideration is that these criteria should not be used to inform a ‘baseline’ level of specialist palliative care service; by the very nature of this study, we have identified criteria which differentiate between existing models. It follows therefore, that – inevitably – some specialist palliative care services will provide some elements and not others; this is to be expected, given the purpose and methodology of our work. Other characteristics, such as holistic care, training in specialist palliative care and the use of multidisciplinary teams in delivery of care, are considered to be ‘core’ to the definition of specialist palliative care11 and so are not included in these differentiating criteria.
The casemix classes enable providers of specialist palliative care to determine the likely resources needed for care, at the individual patient level. If the specified criteria are measured and combined into these classes at the start of an episode of care, they provide systematic insight into the level of complexity of needs and the probable resources required to meet those needs. The Pricing Team at NHS England and Improvement are currently working on a Community Currency Development Project, work somewhat delayed by the COVID-19 pandemic, and propose introducing a currency for last year of life (www.england.nhs.uk/pay-syst/development/palliative-care-development-currency/; accessed 21 August 2023). This work will help inform this project.
Managers and senior leads should reconsider communication and information-giving in relation to transitions, in particular what information and preparation is provided when and in how much detail. Continuity of care and emotional and practical support for patients and families as they make necessary transitions between settings needs to be well resourced to prevent future distress and difficulties.
Acknowledgements
We would like to acknowledge the major contributions of participants in all workstreams; we especially thank those with advanced illness and their families, who were often highly committed to contributing whatever they could despite their advancing illness. We also thank all the staff at participating sites who worked hard to ensure that the data collection within this programme was possible, including the often overlooked ‘back office’ staff; administrators, finance officers, accountants.
Contributions of authors
Fliss EM Murtagh (https://orcid.org/0000-0003-1289-3726) was the joint lead investigator. She wrote the study protocols, analysed the quantitative data, undertook the qualitative data analyses and drafted the report manuscript. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content. Fliss Murtagh is the guarantor of the data.
Ping Guo (https://orcid.org/0000-0003-0979-7047) wrote the study protocols, liaised with sites, undertook data collection and undertook the qualitative data analyses. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Alice M Firth (https://orcid.org/0000-0003-0726-0502) had critical input in the study protocols, liaised with sites, undertook data collection and undertook the qualitative data analyses. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Ka Man Yip (https://orcid.org/0000-0002-1577-3431) had critical input in the study protocols and analysed the quantitative data. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Christina Ramsenthaler (https://orcid.org/0000-0002-9996-1818) had critical input in the study protocols and analysed the quantitative data. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Abdel Douiri (https://orcid.org/0000-0002-4354-4433) had critical input in the study protocols and analysed the quantitative data. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Cathryn Pinto (https://orcid.org/0000-0001-7607-7192) had critical input in the study protocols, liaised with sites, undertook data collection and undertook the qualitative data analyses. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Sophie Pask (https://orcid.org/0000-0001-6152-1940) had critical input in the study protocols, liaised with sites, undertook data collection and undertook the qualitative data analyses. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Mendwas Dzingina (https://orcid.org/0000-0002-6237-9250) had critical input in the study protocols and analysed the quantitative data. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Joanna M Davies (https://orcid.org/0000-0002-6375-0023) had critical input in the study protocols and analysed the quantitative data. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Suzanne O’Brien (https://orcid.org/0000-0001-8267-0881) had critical input in the study protocols, liaised with sites, undertook data collection and undertook the qualitative data analyses. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Beth Edwards (https://orcid.org/0000-0001-7742-4432) had critical input in the study protocols, liaised with sites, undertook data collection and undertook the qualitative data analyses. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Esther I Groeneveld (https://orcid.org/0000-0003-4367-8543) wrote the study protocols, liaised with sites and undertook data collection. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Mevhibe Hocaoglu (https://orcid.org/0000-0003-1417-7117) had critical input in the study protocols, liaised with sites and undertook data collection. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Claudia Bausewein (https://orcid.org/0000-0002-0958-3041) had critical input in the study protocols and analysed the quantitative data. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Irene J Higginson (https://orcid.org/0000-0002-3687-1313) was the joint lead investigator and wrote the study protocols. They contributed to the analysis plan and provided critical revision of the manuscript for important intellectual content.
Publications
Collins ES, Witt J, Bausewein C, Daveson BA, Higginson IJ, Murtagh FE. A systematic review of the use of the Palliative care Outcome Scale and the Support Team Assessment Schedule in palliative care. J Pain Symptom Manage 2015;50:842–53. https://doi.org/10.1016/j.jpainsymman.2015.07.015
Etkind SN, Daveson BA, Kwok W, Witt J, Bausewein C, Higginson IJ, et al. Capture, transfer and feedback of patient-centred outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 2015;49:611–24. https://doi.org/10.1016/j.jpainsymman.2014.07.010
Schildmann EK, Groeneveld I, Denzel J, Brown A, Bernhardt F, Bailey K, et al. Discovering the hidden benefits of cognitive interviewing in two languages: the first phase of a validation study of the Integrated Palliative Outcome Scale (IPOS). Palliat Med 2016;30:599–610. https://doi.org/10.1177/0269216315608348
Etkind SN, Bristowe K, Bailey K, Selman LE, Murtagh FE. How does uncertainty shape patient experience in advanced illness? A secondary analysis of qualitative data. Palliat Med 2017;31:171–80. https://doi.org/10.1177/0269216316647610
Groeneveld EI, Cassel JB, Bausewein C, Csikos A, Krajnik M, Ryan K, et al. Funding models in palliative care: lessons from international experience. Palliat Med 2017;31:296–305. https://doi.org/10.1177%2F0269216316689015
Guo P, Dzingina M, Firth AM, Davies JM, Douiri A, O’Brien SM, et al. Development and validation of a casemix classification to best predict costs of palliative care provision across inpatient, community and outpatient settings in the UK: a study protocol. BMJ Open 2018;8:e020071. https://doi.org/10.1136/bmjopen-2017-020071
Mather H, Guo P, Firth AM, Davies JM, Sykes N, Landon A, et al. Phase of illness in palliative care: cross-sectional analysis of clinical data from community, hospital and hospice patients. Palliat Med 2018;32:404–12. https://doi.org/10.1177/0269216317727157
Pask S, Pinto C, Bristowe K, van Vliet L, Nicholson C, Evans CJ, et al. A framework for complexity in palliative care: a qualitative study with patients, family carers and professionals. Palliat Med 2018;32:1078–90. https://doi.org/10.1177/0269216318757622
Pinto C, Bristowe K, Witt J, Davies JM, de Wolf-Linder S, Dawkins M, et al. Perspectives of patients, family caregivers and health professionals on the use of outcome measures in palliative care and lessons for implementation: a multi-method qualitative study. Ann Palliat Med 2018;7(Suppl. 3):S137–50. https://doi.org/10.21037/apm.2018.09.02
Murtagh FEM, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, et al. A brief, patient- and proxy-reported outcome measure in advanced illness: validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med 2019;33:1045–57. https://doi.org/10.1177/0269216319854264
de Wolf-Linder S, Dawkins M, Wicks F, Pask S, Eagar K, Evans CJ, et al. Which outcome domains are important in palliative care and when? An international expert consensus workshop, using the nominal group technique. Palliat Med 2019;33:1058–68. https://doi.org/10.1177/0269216319854154
Firth AM, O’Brien SM, Guo P, Seymour J, Richardson H, Bridges C, et al. Establishing key criteria to define and compare models of specialist palliative care: a mixed-methods study using qualitative interviews and Delphi survey. Palliat Med 2019;33:1114–24. https://doi.org/10.1177/0269216319858237
Pinto C, Firth AM, Groeneveld EI, Guo P, Sykes N, Murtagh FE. Patients’ views on care and their association with outcomes in palliative care. Palliat Med 2019;33:467–9. https://doi.org/10.1177/0269216319831383
Guo P, Pinto C, Edwards B, Pask S, Firth A, O’Brien S, Murtagh FEM. Experiences of transitioning between settings of care from the perspectives of patients with advanced illness receiving specialist palliative care and their family caregivers: a qualitative interview study. Palliat Med 2022;36:124–34. https://doi.org/10.1177/02692163211043371
Murtagh FEM, Firth A, Guo P, Man Yip K, Ramsenthaler C, Douiri A, et al. Developing a casemix classification for specialist palliative care: a multi-centre cohort study to develop a multi-variable prediction model for the cost of specialist palliative care using classification and regression tree analysis. (submitted and review comments received.)
Data-sharing statement
Access to anonymised data may be granted following review. In particular, applications for the use of workstream 3 and workstream 4 data will be considered on a case-by-case basis. All requests for data access should be addressed to the corresponding author, Fliss Murtagh, at fliss.murtagh@hyms.ac.uk.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Fitch MI. Needs of patients living with advanced disease. Can Oncol Nurs J 2005;15:230-42. https://doi.org/10.5737/1181912x154230235.
- Higginson IJ, Shipman C, Gysels M, White P, Barclay S, Forrest S, et al. Scoping Exercise on Generalist Services for Adults at the End of Life: Research, Knowledge, Policy and Future Research Needs. London: National Coordinating Centre for Service Delivery and Organisation; 2007.
- Currow DC, Abernethy AP, Fazekas BS. Specialist palliative care needs of whole populations: a feasibility study using a novel approach. Palliat Med 2004;18:239-47. https://doi.org/10.1191/0269216304pm873oa.
- Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med 2010;362:1173-80. https://doi.org/10.1056/NEJMoa0909087.
- Grande G, Stajduhar K, Aoun S, Toye C, Funk L, Addington-Hall J, et al. Supporting lay carers in end of life care: current gaps and future priorities. Palliat Med 2009;23:339-44. https://doi.org/10.1177/0269216309104875.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006;31:58-69.
- Moens K, Higginson IJ, Harding R. EURO IMPACT . Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage 2014;48:660-77. https://doi.org/10.1016/j.jpainsymman.2013.11.009.
- Grande GE, Austin L, Ewing G, O’Leary N, Roberts C. Assessing the impact of a Carer Support Needs Assessment Tool (CSNAT) intervention in palliative home care: a stepped wedge cluster trial. BMJ Support Palliat Care 2017;7:326-34. https://doi.org/10.1136/bmjspcare-2014-000829.
- Burns E, Prigerson HG, Quinn SJ, Abernethy AP, Currow DC. Moving on: factors associated with caregivers’ bereavement adjustment using a random population-based face-to-face survey. Palliat Med 2018;32:257-67. https://doi.org/10.1177/0269216317717370.
- Kingston A, Wohland P, Wittenberg R, Robinson L, Brayne C, Matthews FE, et al. Is late-life dependency increasing or not? A comparison of the Cognitive Function and Ageing Studies (CFAS). Lancet 2017;390:1676-84.
- World Health Organization . Palliative Care n.d. http://who.int/cancer/palliative/definition/en/ (accessed 12 December 2029).
- The Economist Intelligence Unit . The 2015 Quality of Death Index: Ranking Palliative Care Across the World 2015.
- Hospice UK. Hospice Accounts: Analysis of the Accounts of UK Charitable Hospices for the Year Ended 31 March 2017. London: Hospice UK; 2018.
- Hughes-Hallett T, Craft A, Davies C, Mackay I, Nielsson T. Funding the Right Care and Support for Everyone: Creating a Fair and Transparent Funding System; The Final Report of the Palliative Care Funding Review 2011.
- Tebbit P. Population-based Needs Assessment for Palliative and End of Life Care. London: National Council for Palliative Care; 2008.
- Lancaster H, Finlay I, Downman M, Dumas J. Commissioning of specialist palliative care services in England. BMJ Support Palliat Care 2018;8:93-101. https://doi.org/10.1136/bmjspcare-2016-001119.
- Allsop MJ, Ziegler LE, Mulvey MR, Russell S, Taylor R, Bennett MI. Duration and determinants of hospice-based specialist palliative care: a national retrospective cohort study. Palliat Med 2018;32:1322-33. https://doi.org/10.1177/0269216318781417.
- Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357. https://doi.org/10.1136/bmj.j2925.
- Sleeman KE, Davies JM, Verne J, Gao W, Higginson IJ. The changing demographics of inpatient hospice death: population-based cross-sectional study in England, 1993-2012. Palliat Med 2016;30:45-53. https://doi.org/10.1177/0269216315585064.
- Rosenwax L, Spilsbury K, McNamara BA, Semmens JB. A retrospective population based cohort study of access to specialist palliative care in the last year of life: who is still missing out a decade on?. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0119-2.
- Roos NP, Montgomery P, Roos LL. Health care utilization in the years prior to death. Milbank Q 1987;65:231-54.
- Full Fact Team . What Is the NHS Budget? n.d. https://fullfact.org/health/what-is-the-nhs-budget/ (accessed 1 March 2019).
- Murtagh FE, Bausewein C, Verne J, Groeneveld EI, Kaloki YE, Higginson IJ. How many people need palliative care? A study developing and comparing methods for population-based estimates. Palliat Med 2014;28:49-58. https://doi.org/10.1177/0269216313489367.
- Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0860-2.
- Bone AE, Gomes B, Etkind SN, Verne J, Murtagh FEM, Evans CJ, et al. What is the impact of population ageing on the future provision of end-of-life care? Population-based projections of place of death. Palliat Med 2018;32:329-36. https://doi.org/10.1177/0269216317734435.
- de Meijer C, Wouterse B, Polder J, Koopmanschap M. The effect of population aging on health expenditure growth: a critical review. Eur J Ageing 2013;10:353-61. https://doi.org/10.1007/s10433-013-0280-x.
- Martikainen P, Murphy M, Metsä-Simola N, Häkkinen U, Moustgaard H. Seven-year hospital and nursing home care use according to age and proximity to death: variations by cause of death and socio-demographic position. J Epidemiol Community Health 2012;66:1152-8. https://doi.org/10.1136/jech-2011-200756.
- Donabedian A. The quality of care. How can it be assessed?. JAMA 1988;260:1743-8.
- Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families?. Cancer J 2010;16:423-35.
- Higginson IJ, McCrone P, Hart SR, Burman R, Silber E, Edmonds PM. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009;38:816-26. https://doi.org/10.1016/j.jpainsymman.2009.07.002.
- Edmonds PM, Hart SR, Wei G, Vivat B, Burman R, Silber E, et al. Palliative care for people severely affected by multiple sclerosis: evaluation of a novel palliative care service. Mult Scler 2010;16:627-36.
- Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164:83-91. https://doi.org/10.1001/archinte.164.1.83.
- Finlay IG, Higginson IJ, Goodwin DM, Cook AM, Edwards AG, Hood K, et al. Palliative care in hospital, hospice, at home: results from a systematic review. Ann Oncol 2002;13:257-64. https://doi.org/10.1093/annonc/mdf668.
- Goodwin DM, Higginson IJ, Edwards AG, Finlay IG, Cook AM, Hood K, et al. An evaluation of systematic reviews of palliative care services. J Palliat Care 2002;18:77-83.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD007760.pub2.
- Rayner L, Price A, Evans A, Valsraj K, Hotopf M, Higginson IJ. Antidepressants for the treatment of depression in palliative care: systematic review and meta-analysis. Palliat Med 2011;25:36-51. https://doi.org/10.1177/0269216310380764.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. https://doi.org/10.1056/NEJMoa1000678.
- Royal College of Physicians . Palliative Care Services: Meeting the Needs of Patients: Report of a Working Party 2007.
- Field D, Addington-Hall J. Extending specialist palliative care to all?. Soc Sci Med 1999;48:1271-80.
- Department of Health and Social Care (DHSC) . End of Life Care Strategy 2008.
- Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, et al. Evidence for overuse of medical services around the world. Lancet 2017;390:156-68.
- Glasziou P, Straus S, Brownlee S, Trevena L, Dans L, Guyatt G, et al. Evidence for underuse of effective medical services around the world. Lancet 2017;390:169-77.
- Morrison RS. Models of palliative care delivery in the United States. Curr Opin Support Palliat Care 2013;7:201-6. https://doi.org/10.1097/SPC.0b013e32836103e5.
- National Casemix Office and Health and Social Care Information Centre . Casemix Companion 2016.
- NHS Digital . Definition of Casemix 2018 n.d. https://digital.nhs.uk/services/national-casemix-office/our-definitions-remit-and-deliverables (accessed 28 August 2020).
- Eagar K, Gordon R, Hodkinson A, Green J, Eagar L, Erven J, et al. The Australian National Sub-Acute and Non-Acute Casemix Classification (AN-SNAP): Report of the National Sub-Acute and Non-Acute Casemix Classification Study 1997.
- Averill RF. The evolution of casemix measurement using DRGs: past, present and future. Stud Health Technol Inform 1994;14:75-83.
- Roger France FH. Casemix use in 25 countries: a migration success but international comparisons failure. Int J Med Inform 2003;70:215-9.
- Wilberforce M, Tucker S, Brand C, Abendstern M, Jasper R, Stewart K, et al. Community mental health teams for older people: variations in casemix and service receipt (II). Int J Geriatr Psychiatry 2015;30:605-13. https://doi.org/10.1002/gps.4190.
- Halter M, Joly L, de Lusignan S, Grant RL, Gage H, Drennan VM. Capturing complexity in clinician casemix: classification system development using GP and physician associate data. BJGP Open 2018;2. https://doi.org/10.3399/bjgpopen18X101277.
- Eagar K, Gordon R, Green J, Smith M. An Australian casemix classification for palliative care: lessons and policy implications of a national study. Palliat Med 2004;18:227-33. https://doi.org/10.1191/0269216304pm876oa.
- Lee LA, Eagar KM, Smith MC. Subacute and non-acute casemix in Australia. Med J Aust 1998;169:S22-5.
- Eagar K, Cromwell D, Kennedy C, Lee L. Classifying sub-acute and non-acute patients: results of the New South Wales Casemix Area Network study. Aust Health Rev 1997;20:26-42. https://doi.org/10.1071/ah970026.
- Turner-Stokes L, Sutch S, Dredge R, Eagar K. International casemix and funding models: lessons for rehabilitation. Clin Rehabil 2012;26:195-208. https://doi.org/10.1177/0269215511417468.
- Guo P, Dzingina M, Firth AM, Davies JM, Douiri A, O’Brien SM, et al. Development and validation of a casemix classification to predict costs of specialist palliative care provision across inpatient hospice, hospital and community settings in the UK: a study protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020071.
- Currow DC, Allingham S, Yates P, Johnson C, Clark K, Eagar K. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer 2015;23:307-15. https://doi.org/10.1007/s00520-014-2351-8.
- Eagar K, Green J, Gordon R. An Australian casemix classification for palliative care: technical development and results. Palliat Med 2004;18:217-26. https://doi.org/10.1191/0269216304pm875oa.
- Sheers D. Palliative Care Funding Pilots Update. London: Department of Health and Social Care; 2014.
- Masso M, Allingham SF, Banfield M, Johnson CE, Pidgeon T, Yates P, et al. Palliative Care Phase: inter-rater reliability and acceptability in a national study. Palliat Med 2015;29:22-30. https://doi.org/10.1177/0269216314551814.
- Gill A. Development of Healthcare Resource Groups for Cancer in England 2005.
- Turner-Stokes L. Politics, policy and payment – facilitators or barriers to person-centred rehabilitation?. Disabil Rehabil 2007;29:1575-82.
- National Institute for Clinical Evidence . Improving Supportive and Palliative Care for Adults With Cancer. Cancer Service Guideline [CSG4] 2004.
- Department of Health and Social Care (DHSC) . Palliative Care Service Delivery Framework and Funding Model Review Literature Review 2008.
- Poulos CJ, Eagar K. Determining appropriateness for rehabilitation or other subacute care: is there a role for utilisation review?. Aust New Zealand Health Policy 2007;4.
- Knapp M, Beecham J. Costing mental health services. Psychol Med 1990;20:893-908. https://doi.org/10.1017/s003329170003659x.
- Gomes B, McCrone P, Hall S, Koffman J, Higginson IJ. Variations in the quality and costs of end-of-life care, preferences and palliative outcomes for cancer patients by place of death: the QUALYCARE study. BMC Cancer 2010;10. https://doi.org/10.1186/1471-2407-10-400.
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist 2001;41:652-7. https://doi.org/10.1093/geront/41.5.652.
- Higginson IJ, Gao W, Jackson D, Murray J, Harding R. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol 2010;63:535-42. https://doi.org/10.1016/j.jclinepi.2009.06.014.
- Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res 2009;18:727-35. https://doi.org/10.1007/s11136-009-9483-1.
- Tan SB, Williams AF, Kelly D. Effectiveness of multidisciplinary interventions to improve the quality of life for people with Parkinson’s disease: a systematic review. Int J Nurs Stud 2014;51:166-74. https://doi.org/10.1016/j.ijnurstu.2013.03.009.
- Abernethy AP, Shelby-James T, Fazekas BS, Woods D, Currow DC. The Australian modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat Care 2005;4. https://doi.org/10.1186/1472-684x-4-7.
- Schildmann EK, Groeneveld EI, Denzel J, Brown A, Bernhardt F, Bailey K, et al. Discovering the hidden benefits of cognitive interviewing in two languages: the first phase of a validation study of the Integrated Palliative care Outcome Scale. Palliat Med 2016;30:599-610. https://doi.org/10.1177/0269216315608348.
- Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, et al. A brief, patient- and proxy-reported outcome measure in advanced illness: validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med 2019;33:1045-57. https://doi.org/10.1177/0269216319854264.
- Mather H, Guo P, Firth A, Davies JM, Sykes N, Landon A, et al. Phase of Illness in palliative care: cross-sectional analysis of clinical data from community, hospital and hospice patients. Palliat Med 2018;32:404-12. https://doi.org/10.1177/0269216317727157.
- Pinto C, Firth AM, Groeneveld EI, Guo P, Sykes N, Murtagh FE. Patients’ views on care and their association with outcomes in palliative care. Palliat Med 2019;33:467-9. https://doi.org/10.1177/0269216319831383.
- Addington-Hall J, Hunt K, Rowsell A, Heal R, Hansford P, Monroe B, et al. Development and initial validation of a new outcome measure for hospice and palliative care: the St Christopher’s Index of Patient Priorities (SKIPP). BMJ Support Palliat Care 2014;4:175-81. https://doi.org/10.1136/bmjspcare-2012-000352.
- Groenvold M, Petersen MA, Aaronson NK, Arraras JI, Blazeby JM, Bottomley A, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 2006;42:55-64.
- Etkind SN, Bristowe K, Bailey K, Selman LE, Murtagh FE. How does uncertainty shape patient experience in advanced illness? A secondary analysis of qualitative data. Palliat Med 2017;31:171-80. https://doi.org/10.1177/0269216316647610.
- Pask S, Pinto C, Bristowe K, van Vliet L, Nicholson C, Evans CJ, et al. A framework for complexity in palliative care: a qualitative study with patients, family carers and professionals. Palliat Med 2018;32:1078-90. https://doi.org/10.1177/0269216318757622.
- Bronfenbrenner U. Readings on the Development of Children. New York, NY: W. H. Freeman and Company; 1994.
- Firth AM, O’Brien SM, Guo P, Seymour J, Richardson H, Bridges C, et al. Establishing key criteria to define and compare models of specialist palliative care: a mixed-methods study using qualitative interviews and Delphi survey. Palliat Med 2019;33:1114-24. https://doi.org/10.1177/0269216319858237.
- Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684-706. https://doi.org/10.1177/0269216317690685.
- Cleeland CS, Reyes-Gibby CC. When is it justified to treat symptoms? Measuring symptom burden. Oncology (Williston Park) 2002;16:64-70.
- von der Gracht HA. Consensus measurement in Delphi studies: review and implications for future quality assurance. Technol Forecast Soc Change 2012;79:1525-36. https://doi.org/10.1016/j.techfore.2012.04.013.
- Trevelyan EG, Robinson PN. Delphi methodology in health research: how to do it?. Eur J Integr Med 2015;7:423-8. https://doi.org/10.1016/j.eujim.2015.07.002.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350. https://doi.org/10.1136/bmj.g7594.
- NHS England and Finance/Strategic Finance/Pricing Team . Developing a New Approach to Palliative Care Funding 2015.
- Eagar K. The Australian National Sub-Acute and Non-Acute Patient casemix classification. Aust Health Rev 1999;22:180-96. https://doi.org/10.1071/ah990180.
- Monitor . Approved Costing Guidance: Updated February 2016 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/498792/ACG_february_2016_final_with_cover.pdf (accessed 28 August 2020).
- Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res 1991;26:499-510. https://doi.org/10.1207/s15327906mbr2603_7.
- Wilson VanVoorhis CR, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol 2007;3:43-50.
- Guo P, Pinto C, Edwards B, Pask S, Firth A, O’Brien S, et al. Experiences of transitioning between settings of care from the perspectives of patients with advanced illness receiving specialist palliative care and their family caregivers: a qualitative interview study. Palliat Med 2022;36:124-34. https://doi.org/10.1177/02692163211043371.
- Pinnock H, Kendall M, Murray SA, Worth A, Levack P, Porter M, et al. Living and dying with severe chronic obstructive pulmonary disease: multi-perspective longitudinal qualitative study. BMJ 2011;342. https://doi.org/10.1136/bmj.d142.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Brighton LJ, Pask S, Benalia H, Bailey S, Sumerfield M, Witt J, et al. Taking patient and public involvement online: qualitative evaluation of an online forum for palliative care and rehabilitation research. Res Involv Engagem 2018;4. https://doi.org/10.1186/s40900-018-0097-z.
- Zarit S, Reever K, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55.
- Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88:2164-71.
- Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT. The memorial symptom assessment scale short form (MSAS-SF). Cancer 2000;89:1162-71.
- Groenvold M, Petersen MA, Aaronson NK, Arraras JI, Blazeby JM, Bottomley A, et al. EORTC QLQ-C15-PAL: the new standard in the assessment of health-related quality of life in advanced cancer?. Palliat Med 2006;20:59-61. https://doi.org/10.1191/0269216306pm1133xx.
- Nicholson C, Davies JM, George R, Smith B, Pace V, Harris L, et al. What are the main palliative care symptoms and concerns of older people with multimorbidity? – a comparative cross-sectional study using routinely collected Phase of Illness, Australia-modified Karnofsky Performance Status and Integrated Palliative Care Outcome Scale data. Ann Palliat Med 2018;7:S164-75. https://doi.org/10.21037/apm.2018.06.07.
- Finn L, Malhotra S. The development of pathways in palliative medicine: definition, models, cost and quality impact. Healthcare 2019;7.
- Saygili M, Çelik Y. An evaluation of the cost-effectiveness of the different palliative care models available to cancer patients in Turkey. Eur J Cancer Care 2019;28. https://doi.org/10.1111/ecc.13110.
- Cassel JB, Albrecht TA. Emerging models of providing oncology palliative care. Semin Oncol Nurs 2018;34:202-14.
- Beasley A, Bakitas MA, Edwards R, Kavalieratos D. Models of non-hospice palliative care: a review. Ann Palliat Med 2019;8:S15-S21. https://doi.org/10.21037/apm.2018.03.11.
- Pinto C, Bristowe K, Witt J, Davies JM, de Wolf-Linder S, Dawkins M, et al. Perspectives of patients, family caregivers and health professionals on the use of outcome measures in palliative care and lessons for implementation: a multi-method qualitative study. Ann Palliat Med 2018;7:S137-50. https://doi.org/10.21037/apm.2018.09.02.
- Bradshaw A, Santarelli M, Mulderrig M, Khamis A, Sartain K, Boland JW, et al. Implementing person-centred outcome measures in palliative care: an exploratory qualitative study using Normalisation Process Theory to understand processes and context. Palliat Med 2021;35:397-40. https://doi.org/10.1177/0269216320972049.
- Raj R, Ahuja K, Frandsen M, Murtagh FEM, Jose M. Validation of the IPOS-renal symptom survey in advanced kidney disease: a cross-sectional study. J Pain Symptom Manage 2018;56:281-7.
- Oriani A, Guo P, Gadoud A, Dunleavy L, Kane P, Murtagh FEM. What are the main symptoms and concerns reported by patients with advanced chronic heart failure? – a secondary analysis of the Palliative care Outcome Scale (POS) and Integrated Palliative care Outcome Scale (IPOS). Ann Palliat Med 2019;8:775-80. https://doi.org/10.21037/apm.2019.08.10.
- Davies JM, Sleeman KE, Leniz J, Wilson R, Higginson IJ, Verne J, et al. Socioeconomic position and use of healthcare in the last year of life: a systematic review and meta-analysis. PLOS Med 2019;16. https://doi.org/10.1371/journal.pmed.1002782.
- Bainbridge D, Seow H, Sussman J. Common components of efficacious in-home end-of-life care programs: a review of systematic reviews. J Am Geriatr Soc 2016;64:632-9. https://doi.org/10.1111/jgs.14025.
- Brereton L, Clark J, Ingleton C, Gardiner C, Preston L, Ryan T, et al. What do we know about different models of providing palliative care? Findings from a systematic review of reviews. Palliat Med 2017;31:781-97. https://doi.org/10.1177/0269216317701890.
- The National Council for Palliative Care . National Survey of Patient Activity Data for Specialist Palliative Care Services 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Gardiner C, Ryan T, Gott M. What is the cost of palliative care in the UK? A systematic review. BMJ Support Palliat Care 2018;8:250-7. https://doi.org/10.1136/bmjspcare-2018-001519.
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965;14:61-5.
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. Int Disabil Stud 1988;10:64-7.
- Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 1999;8:219-27. https://doi.org/10.1136/qshc.8.4.219.
- Murphy EL, Murtagh FE, Carey I, Sheerin NS. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract 2009;111:c74-80. https://doi.org/10.1159/000183177.
- Bausewein C, Le Grice C, Simon S, Higginson I. PRISMA . The use of two common palliative outcome measures in clinical care and research: a systematic review of POS and STAS. Palliat Med 2011;25:304-13. https://doi.org/10.1177/0269216310395984.
- Collins ES, Witt J, Bausewein C, Daveson BA, Higginson IJ, Murtagh FE. A systematic review of the use of the Palliative Care Outcome Scale (POS) and the Support Team Assessment Schedule (STAS) in palliative care. J Pain Symptom Manage 2015;50:842-53.e19. https://doi.org/10.1016/j.jpainsymman.2015.07.015.
- Eagar K, Watters P, Currow DC, Aoun SM, Yates P. The Australian Palliative Care Outcomes Collaboration (PCOC) – measuring the quality and outcomes of palliative care on a routine basis. Aust Health Rev 2010;34:186-92. https://doi.org/10.1071/AH08718.
- Masso M, Allingham SF, Johnson CE, Pidgeon T, Yates P, Currow D, et al. Palliative Care Problem Severity Score: reliability and acceptability in a national study. Palliat Med 2016;30:479-85. https://doi.org/10.1177/0269216315613904.
- Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014;2:979-87. https://doi.org/10.1016/S2213-2600(14)70226-7.
- Selman L, Speck P, Gysels M, Agupio G, Dinat N, Downing J, et al. ‘Peace’ and ‘life worthwhile’ as measures of spiritual well-being in African palliative care: a mixed-methods study. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-94.
- Witt J, Murtagh FEM, de Wolf-Linder S, Higginson IJ, Daveson BA. Introducing the Outcome Assessment and Complexity Collaborative (OACC) Suite of Measures. London: King’s College London; 2014.
- Johnson MJ, Allgar V, Chen H, Dunn L, Macleod U, Currow DC. The complex relationship between household income of family caregivers, access to palliative care services and place of death: a national household population survey. Palliat Med 2018;32:357-65. https://doi.org/10.1177/0269216317711825.
- Dumont S, Jacobs P, Turcotte V, Anderson D, Harel F. The trajectory of palliative care costs over the last 5 months of life: a Canadian longitudinal study. Palliat Med 2010;24:630-40. https://doi.org/10.1177/0269216310368453.
- Culyer AJ. Need: the idea won’t do – but we still need it. Soc Sci Med 1995;40:727-30.
- Feldhaus I, Mathauer I. Effects of mixed provider payment systems and aligned cost sharing practices on expenditure growth management, efficiency, and equity: a structured review of the literature. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-3779-1.
- Quentin W, Geissler A, Wittenbecher F, Ballinger G, Berenson R, Bloor K, et al. Paying hospital specialists: experiences and lessons from eight high-income countries. Health Policy 2018;122:473-84.
Appendix 1 Measures
Table 13 provides details of the measures used in workstreams 3 and 4.
| Measure | Details of measure | Background, validation and source |
|---|---|---|
| Palliative Phase of Illness | A single staff-reported item collected daily (inpatient hospice or hospital) or at each contact (community) to provide the context of the current palliative Phase of Illness, with five categories: stable, unstable, deteriorating, dying and deceased | Palliative Phase of Illness categorises seriously ill patients according to the urgency of the care plan to address palliative care needs, and has been used in the Australian casemix classification. It shows good inter-rater reliability and clinical utility in populations with advanced progressive illness59,100 |
| AKPS | A single score between 0% and 100% (in 10% increments) based on a patient’s ability to perform common tasks relating to activity, work and self-care. A score of 10% signfies the patient is ‘comatose or barely rousable, unable to care for self’, whereas 100% signifies ‘normal physical abilities with no evidence of disease’ | The AKPS is based on the Karnofsky Performance Status, but is adapted for advanced illness. It has been validated in both cancer and non-cancer conditions71 |
| Modified Barthel Index | The modified Barthel Index is a measure of a person’s ability to perform 10 common activities of daily living relating to toileting, mobility and eating. Each item is scored 0, 1 or 2. A total score is produced by adding up the scores; the highest possible summary score is 20, indicating complete independence, and the lowest score is 0, indicating complete dependence | The Barthel Index of Activities of Daily Living was first developed by Mahoney and Barthel115 as an index of independence to monitor improvement in the rehabilitation of patients with long term conditions. Wade and Collin116 proposed a modified version that includes the original items but with a simplified scoring system |
| IPOS | The IPOS combines the items from the Palliative care Outcome Scale (POS) and those from its symptom module Palliative care Outcome Scale-symptom module (POS-S) into one integrated measure. There are two versions of the IPOS: a patient self-reported and staff proxy-reported version. Both consist of 17 scorable items on physical, psychological, spiritual problems, communication needs including with family, and practical support, scored on a 5-point Likert scale from 0 (best) to 4 (worst). The IPOS total score ranges from 0–68 and is an overall measure of how symptoms and concerns are affecting the individual. The full IPOS measure is available at https://pos-pal.org; see The Integrated Palliative care Outcome Scale for additional information | The original POS included 10 items covering the domains most important to patients with advanced illness.117 Following patient and staff feedback, a symptom module (POS-S) was added.118 This was recently combined into the 19-item IPOS which is valid, reliable and responsive to change in a palliative care population.73 The POS and IPOS have also been translated, culturally adapted and validated for use in a range of different languages and are widely used internationally119,120 |
| PCPSS | The PCPSS has four items: pain, other symptoms, psychological/spiritual concerns and family/carer concerns. Each item is scored 0 (absent), 1 (mild), 2 (moderate) or 3 (severe) | PCPSS is used by the Australian Palliative Care Outcomes Collaborative,121 and has been shown to have good reliability and acceptability122 |
The Integrated Palliative care Outcome Scale
The IPOS is a person-centred assessment and outcome measure for palliative care (for the full measure, see https://pos-pal.org/).
The IPOS questionnaire measures the symptomatic, psychological and spiritual needs of palliative care patients over 3 or 7 days in a variety of care settings. There are currently four different versions of the IPOS measure, which have been designed for use by staff and patients in different settings:
-
IPOS Patient version, 3-day recall period – for use by patients in inpatient settings
-
IPOS Patient version, 1-week recall period – for use by patients in community settings
-
IPOS Staff version, 3-day recall period – for use by staff in inpatient settings
-
IPOS Staff version, 1-week recall period – for use by staff in community settings.
This clinical and research measure supports the assessment of the physical symptoms, psychological symptoms, spiritual considerations, practical concerns, emotional concerns and psychosocial needs of patients in a hospice, at home, in hospital and in other community settings. When repeated, it provides individual-level outcomes data that reflect the most common symptoms and concerns reported by people with advanced illness.
The Integrated Palliative care Outcome Measure short version
For the full measure, see https://pos-pal.org.
The Integrated Palliative care Outcome Scale short version (IPOS-5) is a briefer version of the full IPOS. It contains the five items considered most important to patients and families (such as pain, breathlessness, anxiety and information needs) but that also relate to their healthcare provision (e.g. breathlessness is one of the commonest reasons for emergency admission123). It also includes the ‘at peace’ question, as this globally reflects psychological and existential domains across diverse backgrounds, cultures and beliefs. 124
Owing to its shortened content, the IPOS-5 has increased the usability and adaptability of the IPOS while continuing to capture the needs and concerns that are most important to patients and families.
Views on Care measure
The VoC measure is an assessment of patient quality of life and experience of care (for the full measure, see https://pos-pal.org).
Views on Care focuses on the patient’s quality of life and perceived impact of the palliative care service, and provides staff with an indication of whether or not they are having a positive impact on patients’ lives. 125
Appendix 2 Summary of casemix classification paper: developing a casemix classification for specialist palliative care – a multicentre cohort study to develop a multivariable prediction model for the cost of specialist palliative care using classification and regression tree analysis
Introduction
People with advanced illness suffer multiple physical and psychological symptoms, plus family/social concerns,6,7 because of their illness and impending death. 3 Their families often provide day-to-day care, as well as being affected by their own distress and imminent losses. 126 These issues bring increased need for health services’ support, which escalates in the weeks and months before death. 127 Palliative care needs – with ‘need’ defined as ‘the ability to benefit’ from care128 – are met by a range of services. For individuals approaching the end of life and their families, the level and complexity of their palliative care needs vary widely, yet this complexity has rarely been quantified or measured. ‘Casemix’ is a method to classify patients with similar needs and/or resource consumption into clinically meaningful groups, using patient-level criteria. An Australian casemix classification for palliative care was developed in 1997,88 empirically tested,46 and progressively refined over time. 51,57 This classification consists of classes defined by patient factors including palliative Phase of Illness,59 problem severity,122 functional status,71 and dependency. 46 However, it is unclear whether this classification can be directly applied in other countries. We therefore aimed to develop a casemix classification for UK specialist palliative care, for use across settings.
Methods
Design
We designed a multicentre prospective cohort study, following patients during episodes of specialist palliative care and reported according to the TRIPOD statement. 86 The study was registered with a International Standard Randomised Controlled Trial Number on 2 March 2017 (https://doi.org/10.1186/ISRCTN90752212).
Definitions
We defined an ‘episode of care’ as a ‘period of contact between a patient and palliative care service provider or team of providers that occurs in one setting’. 87 We defined the ‘setting of care’ as: hospital advisory (specialist palliative care teams providing an advisory or consultation service within hospitals), inpatient hospice (patients admitted to a hospice or specialist palliative care unit for an overnight stay of one or more days) or community-based specialist palliative care (when the patient receives care in their usual place of residence, i.e. at home or in a care home).
Population and settings
Patients were recruited from 14 organisations providing specialist palliative care services in England: four hospital advisory services, five inpatient hospice services and seven community-based services (some organisations provided more than one setting of care). We included consecutive adult patients (≥ 18 years) receiving specialist palliative care at all participating sites. The exclusion criteria were being aged < 18 years, declining participation and/or previously expressing a wish not to participate in research.
Data collection and primary outcome
Collected data included demographic and clinical variables, episode start and end dates, potential casemix variables, and data on patient-level and other costs in providing specialist palliative care. Potential casemix variables were selected based on (1) being patient-level attributes and (2) prior evidence of association with casemix/complexity. 88 The key casemix variables included were age, sex, ethnicity, living circumstances, need for interpreter, primary diagnosis, palliative Phase of Illness, functional status, dependency and symptoms/problem severity. Our primary outcome was the cost of specialist palliative care per day, measured over an episode of care.
Sample size
Based on standard recommendations for fitting multivariate models, a minimum of 50 + 8 × m cases for testing multiple correlation (where m is the number of predictors) were required to test the null hypothesis that the population multiple correlation equals zero with a power of 80%, α = 5% and a medium effect size for the regression analysis (R2 = 0.13). 90,91 The unit of analysis was episodes within sites, therefore, 10 predictors required 130 episodes per site. Allowing an additional 15% for episodes with missing data and 20% for cost outliers, we estimated a target of 2674 episodes of care (191 episodes × 14 sites) was required.
Analysis
To discover which baseline casemix variables could predict the cost of that episode of care, the following steps were undertaken:
-
We removed incomplete episodes, retaining only the complete episodes of care.
-
Following a previously adopted approach,88 high and low cost outliers were identified and removed using a trimming algorithm based on the interquartile range (IQR) with the upper trim point at Q3 + 1.5 IQR and the lower trim point at Q1–1.5 IQR (where Q1 is first quartile and Q3 is third quartile). 57
-
We examined the distribution of costs of specialist palliative care by setting.
-
Following the same approach as in the development of the Australian casemix classification,88 we developed and validated a cost-predictive model using CART analysis, which constructs decision rules in a hierarchical manner to form a branching classification.
Results
Subject characteristics
A total of 2469 patients were recruited, providing data on 2968 complete episodes of specialist palliative care (12 incomplete episodes were removed prior to analysis). Demographic and clinical characteristics of the 2469 participants, and details of the 2968 episodes of care and related casemix variables are reported in Workstream 3: development of the casemix classification, Results, with 767 episodes in the hospital advisory setting, 764 episodes in the inpatient hospice setting and 1437 episodes in the community setting.
Costs of specialist palliative care
The distribution of the total cost of specialist palliative care episodes, derived from the trimmed data set, is shown in Table 3; costs are reported (1) per day, (2) per day, broken down by Phase of Illness, and (3) by episode of care, together with details of length of episodes.
Classification and regression tree analysis
Seven different casemix variables provided the optimal combination to deliver classes in each of the settings. Table 14 shows these variables and how they are combined to constitute the casemix classes, including cost weights.
| Class | Living situation | Pain | Functional status | Palliative Phase of Illness | Family distress | Physical symptoms other than pain | Psychological symptoms | Sex | Class cost per day (£) | Cost weighta |
|---|---|---|---|---|---|---|---|---|---|---|
| Classes for hospital advisory episodes of care | ||||||||||
| A | Lives alone | – | – | – | – | – | – | – | 85 | 1.4 |
| B | Does not live alone | Absent or mild | – | Unstable or dying | – | – | – | – | 75 | 1.3 |
| C | Does not live alone | Absent or mild | AKPS ≥ 40% | Stable or deteriorating | – | – | – | – | 60 | 1.0 |
| D | Does not live alone | Absent or mild | AKPS ≤ 30% | Stable or deteriorating | – | – | – | – | 74 | 1.2 |
| E | Does not live alone | Moderate or severe | – | – | – | – | – | Women | 95 | 1.6 |
| F | Does not live alone | Moderate or severe | – | Stable, deteriorating or dying | – | – | – | Men | 76 | 1.3 |
| G | Does not live alone | Moderate or severe | – | Unstable | – | – | – | Men | 80 | 1.3 |
| Classes for inpatient hospice episodes of care | ||||||||||
| A | – | Absent, mild or moderate | – | – | Absent or mild | – | – | – | 496 | 2.3 |
| B | – | Absent, mild or moderate | – | – | Moderate or severe | – | Absent or mild | – | 648 | 3.0 |
| C | – | Absent, mild or moderate | – | – | Moderate or severe | – | Moderate or severe | – | 965 | 4.5 |
| D | – | Severe | – | Unstable or deteriorating | – | – | – | – | 657 | 3.1 |
| E | – | Severe | – | Stable or dying | – | Absent, mild or moderate | – | – | 214 | 1.0 |
| F | – | Severe | – | Stable or dying | – | Severe | – | – | 624 | 2.9 |
| Classes for community episodes of care | ||||||||||
| A | – | – | – | Stable | Absent or mild | – | – | – | 20 | 1.0 |
| B | – | – | – | Stable | Moderate or severe | – | – | – | 24 | 1.2 |
| C | – | – | AKPS ≤ 20% | Unstable, deteriorating or dying | – | – | – | – | 56 | 2.8 |
| D | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | – | Absent or mild | – | – | 27 | 1.4 |
| E | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | Absent or mild | Moderate or severe | – | – | 27 | 1.4 |
| F | – | – | AKPS ≥ 30% | Unstable, deteriorating or dying | Moderate or severe | Moderate or severe | – | – | 40 | 2.0 |
Discussion
To our knowledge we have for the first time, and in different settings of care, demonstrated robustly which casemix variables are associated with higher or lower costs for specialist palliative care. A casemix classification was developed for each of the three settings: hospital advisory, inpatient hospice and community specialist palliative care.
These casemix classes enable providers of specialist palliative care to determine the likely resources needed for care, at the individual patient level. If the specified criteria are measured and combined into these classes at the start of an episode of care, they provide systematic insight into the level of complexity of needs and the probable resources required to meet those needs.
For instance, a patient receiving care from a hospital advisory palliative team is likely to need 40% more daily resources (largely staff time) if they live alone (compared with the lowest cost class), and between 30–60% more daily resources if they have moderate/severe pain. In contrast, within an inpatient hospice setting, a patient needs 4.5-fold more resources if there are moderate/severe psychological symptoms and moderate/severe family distress (again, as compared with the lowest cost class). Pain, other physical symptoms and Phase of Illness also drive costs in this setting, with unstable or deteriorating phase and severe pain plus severe other physical symptoms associated with threefold higher costs. In the community setting, almost threefold more resources are needed if the patient is in the unstable, deteriorating or dying phase with poor functional status (AKPS ≤ 20%). It is important to note that costs in the hospital advisory and community settings are simply the extra costs of the specialist palliative care (the costs of primary care and other community services are not included). In contrast, the inpatient hospice costs include all the costs of care, including the cost of occupying a hospice inpatient bed.
This study also provides reasonably current patient-level cost data for episodes of specialist palliative care. NHS unit costs for specialist palliative care do exist,113 but their reliability is unclear and they do not reflect non-NHS services (which includes most specialist palliative care in the UK). A recent systematic review of the cost of UK palliative care114 found very limited cost evidence (only 10 studies over 20 years), with most combining estimates of resource use with potentially unreliable unit cost data.
Some limitations need consideration. First, participating sites had different models of care; to some extent, we allowed for this in our sample size and in oversampling community episodes, where there is the greatest diversity of models of care. Second, the casemix variables accounted for a limited amount of variance in costs when using a bias-corrected bootstrapped estimation of R² values in all three settings. This is perhaps unsurprising, given that patient-level variables explain only an additional amount of variance over the total length of an episode. The number of days of each episode of care is the bigger determinant of the overall cost of the episode of care, hence our focus on costs per day and the acknowledged need for blended payment models (which combine per day, per episode and outcome-based elements). 129,130
Conclusions
Together, our detailed evidence on specialist palliative care costs and our casemix classification deliver a major advance. Each person needing specialist palliative care is different, with varying degrees of complex needs. We now have the means to understand this, systematically and at scale, for practice, policy and research. This delivers the potential to help address inequities and provide more equitable specialist palliative care to all who need it.
List of abbreviations
- AKPS
- Australia-modified Karnofsky Performance Status
- CART
- classification and regression tree
- C-CHANGE
- the name of this programme of research
- CI
- confidence interval
- CREDES
- Conducting and REporting DElphi Studies in palliative care
- DRG
- diagnosis-related group
- EORTC QLQ-C15-PAL
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – 15 items measure
- HRG
- Healthcare Resource Group
- IPOS
- Integrated Palliative care Outcomes Scale
- IPOS-5
- Integrated Palliative care Outcome Measure short version
- IQR
- interquartile range
- PCPSS
- Palliative Care Problem Severity Score
- PPI
- patient and public involvement
- REC
- Research Ethics Committee
- RMSE
- root-mean-squared error
- SD
- standard deviation
- TRIPOD
- Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis
- VoC
- Views on Care
