Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as award number RP-PG-1212-20009. The contractual start date was in September 2015. The draft manuscript began editorial review in December 2020 and was accepted for publication in February 2023. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Sarkany et al. This work was produced by Sarkany et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Sarkany et al.
Synopsis
Background
Xeroderma pigmentosum (XP) is a rare autosomal recessive inherited condition caused by defective nucleotide excision repair. The incidence is 2.3 per million live births in Western Europe. 1 Patients may develop skin cancers from childhood onwards, ocular damage and neurological deterioration,2 and many patients suffer abnormal, severe and rapid sunburn reactions. 3 The phenotype is variable and strongly dependent on the complementation group and on the mutations. 2 Lifespan varies between countries and, in the USA, the median age at death is 32 years; the main cause of death is malignant melanoma. 4 The clinical management of XP relies on minimising exposure of the skin to ultraviolet radiation (UVR), to prevent skin cancer and eye disease. Reduction in UVR exposure is achieved by a combination of lowering overall exposure (i.e. staying indoors as much as realistically possible) and rigorously photoprotecting when outdoors. This involves wearing protective clothing (e.g. face visor, hat, glasses, scarf, long sleeves and trousers) and application of factor 50 sunscreen with high ultraviolet A (UVA) protection to any exposed skin. 5 Adherence to photoprotection is poor in non-XP survivors of malignant melanoma6 and anecdotal evidence from clinicians caring for patients with XP suggests that this group also varies widely in the degree to which they photoprotect. ‘Usual care’ in the UK comprises all patients being cared for by the National Clinical Xeroderma Pigmentosum Service, based in London, which provides the following components of care: photoprotection advice and instruction from XP specialist nurses including NHS prescription of sunscreens and provision of ultraviolet (UV) meters for home use; multidisciplinary clinic appointments with dermatologist, ophthalmologist, neurologist, geneticist, neuropsychologist and paediatrician; outreach visits by specialist nurses.
A recent review of photoprotection in immunosuppressed patients highlighted the gap between knowledge of photoprotection recommendations and behaviour. 7 Since information provision is not enough to change adherence behaviour in chronic conditions,8 recent research has focused on identifying modifiable psychosocial determinants of behaviour. As no prior research had been undertaken to investigate either the level of adherence to photoprotection or its psychosocial determinants in XP, this was a key aim of this project, with a view to developing and evaluating an evidence-based intervention to improve adherence.
Programme aims and objectives
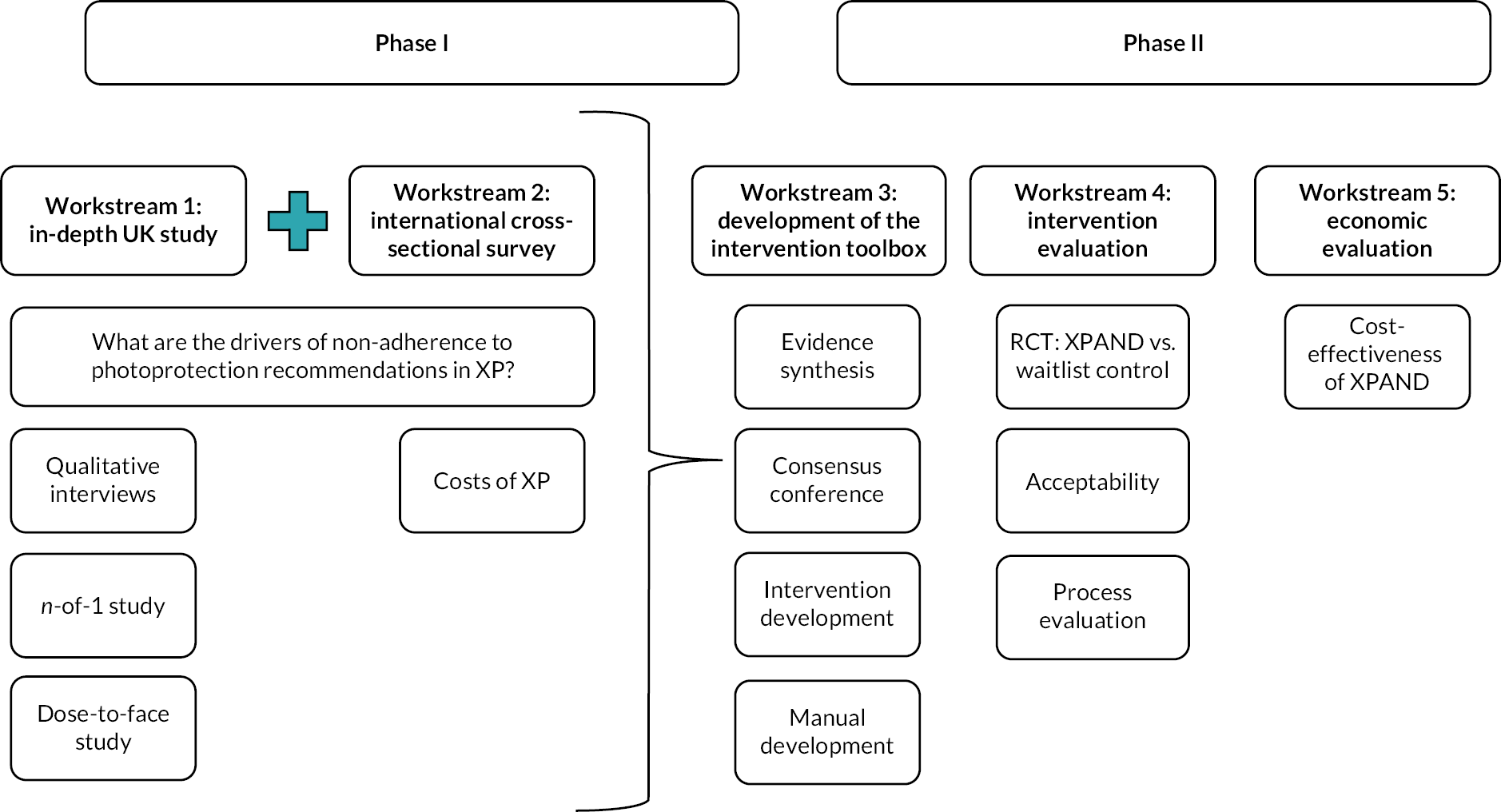
This programme of work comprises five interconnecting workstreams, across two phases of research (Phase I: formative research → Phase II: intervention design and evaluation).
Consistent with the Medical Research Council Guidelines for Good Clinical Practice, the XP Project Steering Committee and XP Trial Steering Committee were formed to govern the conduct of the project and randomised controlled trial (RCT).
Phase I of the programme was designed to gain a comprehensive understanding of photoprotection behaviour and its modifiable biopsychosocial predictors, which could then inform a personalised intervention [Enhancing Xeroderma Pigmentosum Photoprotection Activities – New Directions (‘XPAND’)] to improve photoprotection and clinical outcomes in XP.
-
Workstream 1: Detailed mixed-methods research including (a) qualitative interviews; (b) objective measurement of UVR exposure, photoprotection behaviour and sunscreen use, n-of-1 study, socio-demographic and clinical data, and cognitive testing of 45 individuals diagnosed with XP.
-
Workstream 2: A larger cross-sectional survey with international recruitment (UK, Germany, France, USA, Tunisia and Japan) to further explore determinants of photoprotection and UVR exposure to the face. It also assessed levels and predictors of service use and costs associated with XP in each country.
-
Workstream 3: Consensus conference to select modifiable targets for intervention arising from the Phase I studies; development of a personalised intervention to improve photoprotection in XP; and development of a facilitator manual to guide intervention delivery.
-
Workstream 4: RCT with mixed-methods process evaluation to test the efficacy and potential mechanisms of impact of the developed intervention (‘XPAND’).
-
Workstream 5: Economic evaluation of the XPAND trial.
The interconnection of the workstreams can be seen in Figure 1. Protocols for the Phase I formative research9 and the Phase II XPAND trial10 have been published.
FIGURE 1.
Research pathway diagram.
Alterations to the programme’s original aims and design
We were not able to complete the following cross-cultural objective that was planned as part of the international cross-sectional survey (Workstream 2): To examine differences between three regions (Western Europe + USA, Tunisia and Japan) to assess the effects of culture and climate on photoprotection behaviour in XP patients and the psychosocial factors affecting photoprotection.
Despite extending the recruitment period, numbers in Japan (n = 41, target n = 140) and Tunisia (n = 20, target n = 150) fell significantly short of their targets. Staff sickness and political unrest contributed to the recruitment challenges in Tunisia. The data collected has contributed to the first description of the service use and costs of XP in an international survey of the XP population (Workstream 5).
Workstream 1: in-depth UK study
Workstreams 1 and 2 involved differing methodologies to identify the extent and drivers of inadequate photoprotection in people diagnosed with XP. As recruitment and some data collection methods were in common, an overview of those methods is provided first, followed by the specific details pertaining to each study.
Participants and recruitment
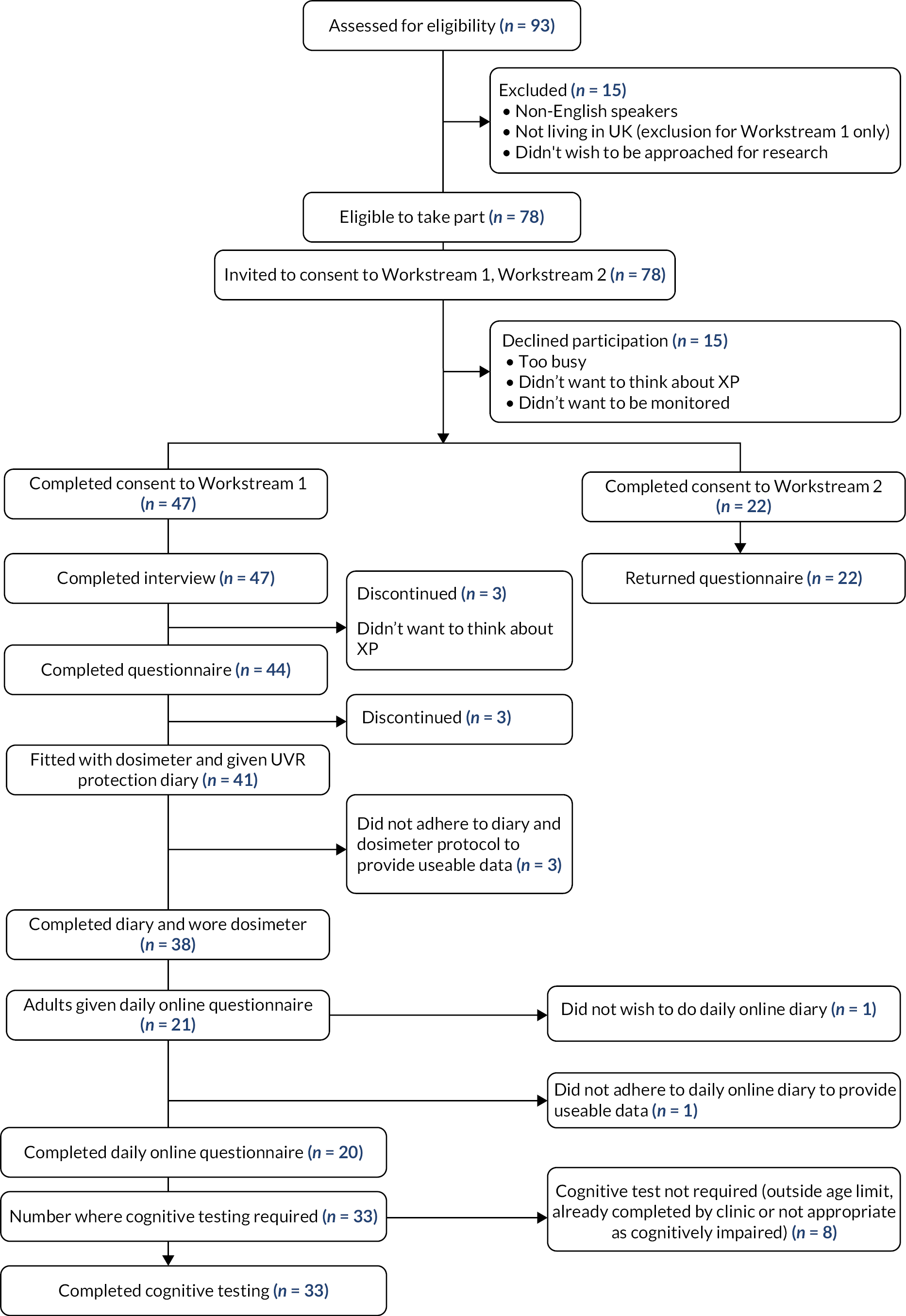
As detailed in our protocol reference,9 UK-based participants were recruited from the 93 known cases of XP in the UK attending the National Xeroderma Pigmentosum Service at Guy’s and St Thomas’ NHS Foundation Trust, which cares for all XP patients in the UK, aiming to recruit 25 adults (> 16 years) responsible for their own photoprotection and 20 younger people (< 16 years) and adults with cognitive impairment, for whom a parent/carer was responsible for photoprotection (the ‘cared for’ sample). Details including inclusion and exclusion criteria and rationale for recruitment are detailed in protocol reference. 9 Figure 2 shows an overview of recruitment and study completion for the UK sample.
FIGURE 2.
Overview of recruitment for Workstreams 1 and 2 (UK sample only).
Procedure
The research nurse’s recruitment and consenting procedure is detailed in protocol reference. 9
Data collection was conducted in 2016 across three home visits to each patient: qualitative interview visit 1 (Workstream 1a); UVR dosimetry and n-of-1 studies, visits 2 and 3 (Workstreams 1b and 2). Methodological details are in Walburn et al. 9
Workstream 1a: qualitative interview study
The full methods and results of the qualitative interviews have been published. 11–14
Objectives
-
To qualitatively explore individuals’ experiences of XP.
-
To identify the range of factors that influence their photoprotection behaviours.
Methods
Inclusion criteria
Patients > 16 years, without cognitive impairment, and parents/caregivers of children (< 16 years) (including the child in the interview) were interviewed. Interviews were guided by a topic guide informed by relevant literature, and expert input.
Recorded interviews were entered in QSR NVivo v.11 and analysed using thematic framework analysis. Validity of emerging explanations and categories was examined through triangulation, based on discussion groups held with clinical staff of the XP service. Full methodology (interview details, topic guide and data analysis) is published separately. 12
Limitations
The main limitation of this study relates to the rare nature of XP and low patient numbers in the UK, and the fact that the recruited sample were also participating in other phase I studies. Consequently, concerns about participant burden meant that it was not feasible to undertake follow-up interviews to further explore emerging issues or to conduct participant validation of the findings. In a very rare disease, confidentiality and maintaining participant anonymity are also concerns, as standard reporting of demographic and disease-related information may well lead to individual participants being identifiable. For this reason, some details have been withheld and analysis by characteristics such as sex, XP subtype and age at diagnosis were not possible.
Key findings
Thirty-seven interviews were conducted with 25 adults (17 men, 8 women; aged 16–63 years) and 12 parent-child dyads (children: 7 female, 5 male; aged 5–13 years).
Three modes of adjustment to photoprotection were identified among the adult sample (n = 25). 12
-
Dominated by photoprotection: This group (n = 4) achieved a very high level of photoprotection, with the demands of time and planning activities dominating their lives. Drivers for photoprotection were the immediate effects of UVR involving risks of skin reddening and painful and unpleasant sunburn; concerns about pigmentation; risks of cancerous lesions and treatment scars to their face; and recognition of the risks of a reduced length of life. This group described considerable social and psychological costs of their high level of photoprotection.
-
Resistant to photoprotection: This group (n = 11) acknowledged lacking routines for photoprotection and described limited practices. A fundamental driver for some was the desire to resist the self-identity of a person with a chronic condition, which was made visible by photoprotection practices. This group was reluctant to disclose their XP to friends and often responded negatively to family help. Another reason for resistance was their view that XP was only one of many risks in life and they preferred to ‘live for today’. They questioned the importance of protection on cloudy days, explaining that skin lesions still occurred despite photoprotection, and a dislike of sunscreen as greasy and not comfortable when thickly applied.
-
Integrating photoprotection: This group (n = 10) had generally accommodated photoprotection requirements into their lives as habitual without a major practical or emotional burden. Two forms of adaptation were identified: (1) staying indoors, which they now preferred; (2) accommodating photoprotection as part of a normal life, facilitated by social support and, for older people, by changing expectations and circumstances.
XP-related stigma was experienced as a potential barrier to photoprotection, with variations across the lifespan. Bullying often occurred in childhood, affecting internalised feelings of stigma. Resilience and rejection of feelings of stigma increased with age, although the ‘resistant’ group had the greatest worries and felt less likely to be able to reject stigma.
Support from family and friends included photoprotection assistance (e.g. reminders to wear a hat) and adjustment of daily activities to take account of the needs of the person with XP. Response to support, however, differed; for some, it facilitated their photoprotection, whereas for others, it was experienced as annoying and regarded as inappropriate because it conflicted with their aim of maintaining normality through resistance. Disclosure was absent from those finding support unhelpful, as to disclose would be contrary to the goal of not being different.
Parents/caregivers of children with XP were highly adherent to the photoprotection regime but did this in different ways. Some attempted to keep their lives similar to those of healthy children by doing outdoor activities with strict photoprotection to achieve normalisation on a day-to-day basis. Others were more restrictive with their child staying indoors whenever possible, with the aim of giving their child a normal life in the future.
Conclusions
The qualitative study provided a detailed understanding of the burden of XP and why adherence to rigorous photoprotection can be so difficult for some patients. The trade-off between protecting well and living life in a way that was ‘normal’ was a salient theme throughout. The influence of the social context in terms of stigma and social support was also important.
Inter-relationship with other parts of the programme
Preliminary themes emerging from the qualitative analysis and field notes were considered when selecting the constructs for the n-of-1 study (Workstream 1b). This led to the inclusion of questions to assess perception of social support, how self-conscious participants felt when outdoors, and how satisfied they were with their daily level of protection.
The three-way categorisation from the adult analysis was used to inform the behavioural options for improving protection in XPAND, as well as the pathways via which change may occur. ‘Resistant’ and ‘integrated’ participants were eligible for the XPAND intervention due to suboptimal adherence.
The findings also suggested several targets for intervention, including the need for social skills training to help people increase their confidence and ability to manage XP in various contexts (e.g. the workplace). Individual-level qualitative data also contributed to the ‘psychological profile’ for each eligible intervention participant, which was used to make decisions about the delivery of personalised content throughout XPAND.
Workstream 1b-i: a series of n-of-1 observational studies with ecological momentary assessment
The methods and results of a series of n-of-1 observational studies have been published. 15
Objectives
-
To understand and categorise the within-individual variability in photoprotection activities used to protect the face from UVR.
-
To identify the environmental, contextual and psychological predictors of within-individual variation in behaviours over time.
-
To identify individual needs for interventions to improve photoprotection.
Methods
Detailed methodology of the n-of-1 study is published separately. 15 In summary, participants with XP over the age of 16 provided 50 days of data using an ecological momentary assessment (EMA) methodology via mobile phone and also using the paper diary detailed in Workstream 1b-ii.
The mobile phone survey was designed to assess self-reported protection and a range of putative predictors.
Data from the paper-based daily UVR protection diary from Workstream 1b-ii were also analysed along with the mobile phone data as part of this study. The following methodological details are published and discussed in full separately:15 details of the mobile phone questionnaire, the development of the Daily Photoprotection Scale (DPS) used to rank and categorise the relative protection afforded by each photoprotection behaviour/combination (see DPS in Table 1), and all statistical methods used to analyse the relationships between DPS category and other variables.
| Photoprotection behaviour | Protection category |
|---|---|
| 1. No protection | None |
| 2. Hoodie or scarf/buff or glasses | Very poor |
| 3. Glasses + scarf/buff | Poor |
| 4. Glasses + hoodie | |
| 5. Scarf/buff + hoodie | |
| 6. Hat | Moderate |
| 7. Hat + hoodie | |
| 8. Glasses + scarf/buff + hoodie | |
| 9. Hat + glasses | Good |
| 10. Hat + glasses + hoodie | |
| 11. Hat + scarf/buff | |
| 12. Hat + scarf/buff + hoodie | |
| 13. Hat + glasses + scarf/buff | Very good |
| 14. Hat + glasses + scarf/buff + hoodie | |
| 15. Face visor | Excellent |
Limitations
The lack of previous research in XP and unclear applicability of sun protection research for a rare and high-risk group, plus the issue of patient burden in a longitudinal design, meant that important predictors of behaviour may have been missed. Similarly, factors that are stable but hold predictive value (e.g. age of diagnosis) are not suitable for intra-individual study.
The optimal parameters in n-of-1 designs are not known and are dependent on variability in behaviour and predictors. A 50-day period was selected, but for participants who stayed indoors a lot, insufficient observations were produced to conduct analysis.
Key findings
Twenty-one adults agreed to complete the n-of-1 study; one stopped responding to the survey after 5 days and was excluded from analysis. The final sample (n = 20) consisted of 14 men and 6 women, with a mean age of 40.7 years [standard deviation (SD) = 15.7, range = 16–63].
Behaviour
The number of times participants went outside ranged from 5 to 153 and the median time spent outside ranged from 15 minutes to 2 hours (maximum = 2–14 hours). Most participants failed to protect their face from UVR on at least some of the occasions they went outside; 13 participants were using ‘very poor’ or no protection during at least 20% of all outdoor time (≥ 97% in 4 participants). Three participants achieved ‘very poor’ or ‘poor’ protection; four achieved ‘moderate’ protection. Only four participants reported ever wearing a visor (8–86% of outdoor time). Ten participants reported at least ‘good’ protection 50% or more of outdoor time (Figure 3).
FIGURE 3.
Summary of photoprotection (DPS category) used across all outdoor occasions.
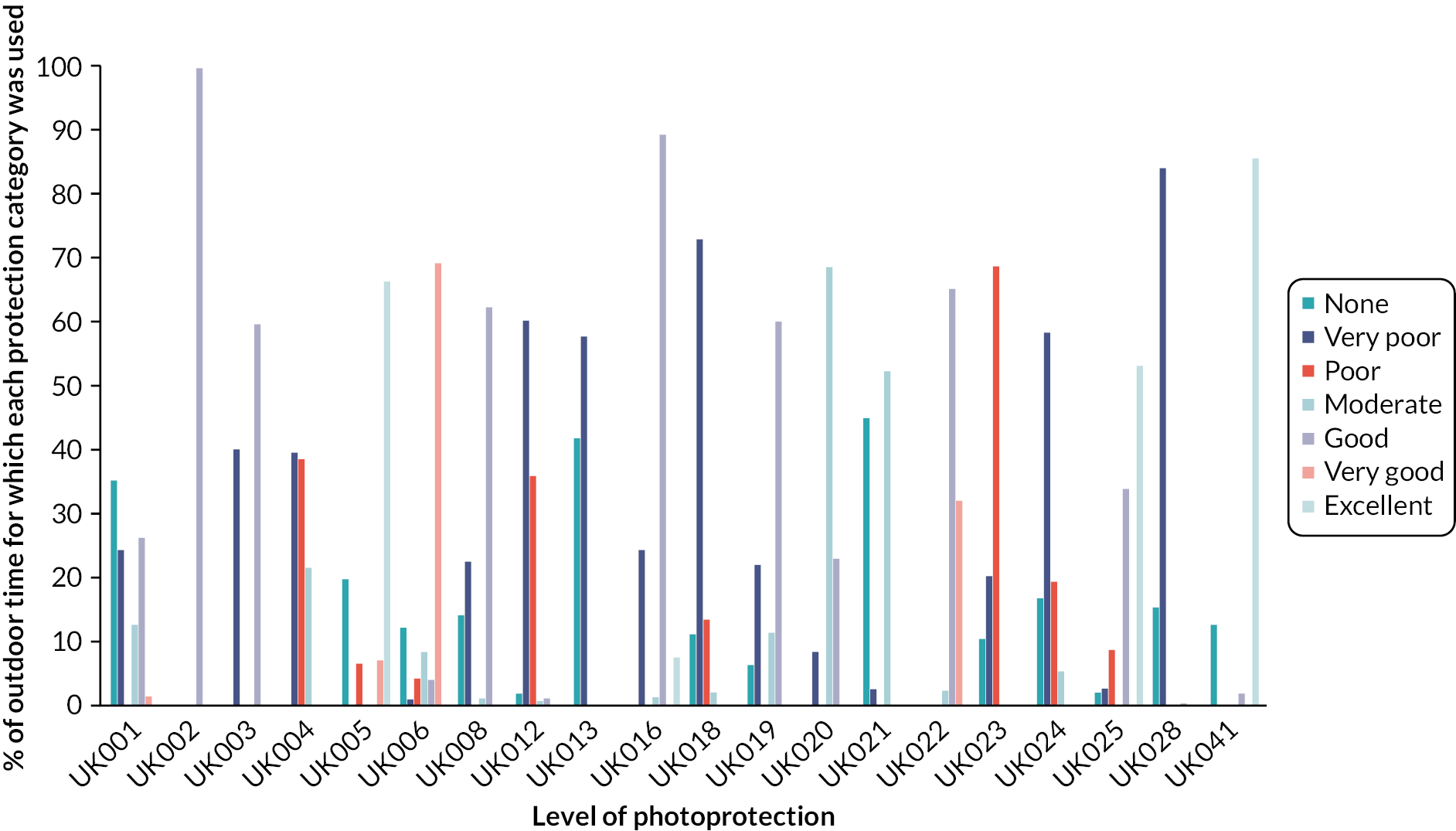
Sunscreen protection was used for 0–89% of outdoor time (median = 57%). Sunscreen was sometimes not applied at all or not applied frequently enough, given the duration of the outdoor occasion. Despite reasonable protection when they first went outside (initiation), this was not always maintained; more instances of changing protection resulted in a worsening of protection. Participants showed varying levels of awareness of their photoprotection behaviour and varying levels of satisfaction with their protection, which did not always correspond with good protection.
Three patterns of photoprotection were identified: (1) protecting predominantly by staying indoors; (2) more frequent outdoor occasions but with stable photoprotection behaviours (whether good or poor, the latter warranting intervention); and (3) frequently going outside and using a range of photoprotection behaviours (warranting intervention to target the times when lesser protection was used).
Predictors of behaviour
A different number and pattern of predictors was significant for each participant:
-
fluctuations in behaviour were associated with the time of day (11 a.m. to 3 p.m., classed as ‘high-risk’ time, was related to better protection); and weekday versus weekend (the direction of the relationship differed across individuals)
-
protection was higher if the weather was perceived as sunnier and perceived risk was greater
-
protection was higher when experienced symptoms were attributed to UVR exposure
-
self-regulatory constructs (greater planning, effort) and psychological factors (fewer negative thoughts, less mental exhaustion, higher arousal) were positively associated with protection
-
stress, negative mood and feeling self-conscious showed different relationships with protection for different individuals and are likely to be bidirectional.
Conclusions
The n-of-1 study indicated that photoprotection was inadequate in most individuals. The large degree of between-individual variability in behaviour and predictors supported the usefulness of the single-case approach for planning a personalised intervention strategy.
Inter-relationship with other parts of the programme
The daily UVR protection diary data were also used in the calculation of UVR dose-to-face, the primary outcome for the dose-to-face study (Workstream 1b-ii).
The n-of-1 findings provided guidance on the range of behavioural targets to achieve adequate protection in the Phase II intervention. For example, balancing staying indoors with good protection when outdoors, which may include combining behaviours in novel ways, adding new forms of protection and generalising higher-level protection being used only sometimes to situations and contexts where lesser protection is being used. Personalised feedback, derived from the n-of-1 behavioural data, was presented to the participants in session 1 of XPAND, with the above behavioural options used as the basis for goal setting to improve protection. The categories from the DPS were the basis for the two goal-setting tools used in intervention delivery.
The n-of-1 study also contributed possible targets for intervention. These included the important roles of planning and overcoming barriers, the incorrect use of symptoms and changeable environmental cues to prompt protection, and the complex interactions that exist between emotions and protection. Finally, the EMA data contributed to the unique ‘psychological profile’, which was used to make personalised suggestions for intervention participants.
Workstream 1b-ii: calculation and predictors of ultraviolet radiation dose reaching the face
The results of Workstream 1b-ii are published. 16
Objectives
-
To objectively measure UVR exposure and, by adjusting for facial photoprotection behaviours, to calculate the UVR dose reaching the face.
-
To determine the feasibility of UVR ‘dose-to-face’ as the primary outcome for the Phase II intervention.
-
To provide initial evidence of the validity and reliability of the methodology that we developed to estimate UVR dose-to-face.
-
As a benchmark to identify how the UV exposure of XP patients compared to healthy individuals.
Methods
Participants
Any patient with XP who participated in the in-depth UK studies could contribute data to this study. The rationale for studying the healthy adults was as a ‘benchmark’ for the new technique to see how the behaviour of the least adherent patients compared to this healthy group.
The healthy sample comprised employees at King’s College London.
Design and procedure
All methodological details for this workstream are published and discussed in detail in Sarkany et al. (2021, 2022). Twenty-one days of data were collected for objective assessment of UVR from a wrist-worn electronic dosimeter and combined with self-report data from a paper-based daily UVR protection diary to calculate the level of environmental UVR dose that reaches the face, after accounting for photoprotection used. The face is the most clinically relevant site in XP, as it is both the hardest region to protect and the area where most skin cancers occur.
The following details are published in full in Sarkany et al. (2021): the UVR dosimeter for objective measurement of UVR exposure, and daily UVR protection diary.
Cognitive testing was completed in patients > 6 years old, without cognitive impairment, and where testing had not already been completed for clinical reasons: The Vocabulary and Matrix Reasoning subtests from the Weschler Abbreviated Scales of Intelligence (2nd edn)17 assessed general intellectual functioning (participants > 6 years). The Verbal Fluency (phonemic and semantic) and Tower of London tasks from the Delis–Kaplan Executive Function System18 assessed response generativity, planning and monitoring (participants > 16 years). Testing was conducted at the third home visit. Clinical data were collected from medical notes held at the XP clinical service.
Cross-sectional survey: psychosocial predictors were taken from the UK cross-sectional survey, which included necessity and concerns19 and automaticity of photoprotection,20 self-efficacy,21 satisfaction with social support22 and emotional well-being. 23
Clinical and sociodemographic data were collected from medical records: XP complementation group, skin cancer and lesion history, age at diagnosis.
Data synthesis
The detailed methodology to calculate the dose of UVR to the face [in Standard Erythemal Doses (SEDs)] and the average daily photoprotection, from the UVR exposure recorded by the dosimeter and the record of photoprotection behaviours in the daily UVR protection diary, is published. 16
The analysis of the association between clinical and psychosocial factors (from the cross-sectional survey) and the average daily UVR dose-to-face was done with mixed-effects longitudinal models: methodological details are published in Sarkany et al. (2022).
Limitations
Despite the high number of recorded observations and the recruitment of nearly half the known cases of XP in the UK, caution is needed as our sample was small for capturing statistical significances. Also, despite being an advance on previous approaches measuring adherence to photoprotection purely by self-report, there are limitations. The dose of UVR reaching the face is an estimation – not a direct objective measure. The dosimeter measures the environmental UVR level, taking no account of what proportion of the environmental UVR penetrates the method of face photoprotection being used to reach the skin of the face. The activity diary provides the information about the proportion of the environmental UVR which reaches the skin of the face, with the conversion factor being the ‘face protection factor’ associated with the protection method recorded by the patient in the activity diary for that period. The dosimeter is subject to measurement error since a dosimeter, worn on the wrist, might under-estimate exposure by missing times when covered by a sleeve. To reduce this risk, participants were trained to roll up the sleeve slightly or put the watch over it. The activity diary is subject to self-presentation and recall bias. To reduce these risks, patients were reassured that the clinical team would not have access to their data and were encouraged to complete the diary daily.
Key findings
In the healthy group:
-
A total of 491 days of data (mean 19.6 days) was collected where the dosimeter was worn, and diary completed.
-
Intra-individual variation across days (SD = 131.3) was larger than inter-individual variation in this group (SD = 89.9).
-
Large variation in behaviour across the group [SD of the UVR dose (wrist) = 1.24, compared to a mean of 0.58].
-
The mean daily dose (wrist) on weekdays was much lower (0.41 SED, SD = 0.05) than at weekends (0.99 SED, SD = 0.14), with intra-individual variation in dose across days (SD = 1.11) larger than inter-individual variation (SD = 0.55).
-
Most healthy participants, 15/25 (60%), had a mean daily UVR dose (wrist) > 1.5 SED on at least 'day.
In the XP patient group:
Forty-one patients (21 adults, 20 ‘cared for’) were fitted with dosimeters and completed the daily UVR protection diary.
-
Across 509 useable days (median = 20 days/person), the mean daily UVR dose for adults (n = 21) was 0.24 SED (unadjusted for protection). The ‘cared for’ sample received a higher mean daily dose of 0.50 SED (266 useable days).
-
The mean daily UVR dose (unadjusted for protection) for the healthy sample was 0.58 SED, which was significantly higher than the adult XP sample (p < 0.001).
-
There was a wide variation in UVR dose in the XP group (< 0.01–0.48 SED).
-
Adjusting for photoprotection, mean daily UVR dose-to-face was similar for adults (0.13) and the ‘cared for’ sample (0.12). Although those with carer reports were exposed to a higher level of environmental UVR, their photoprotection was also higher, shielding them from 66% of UVR dose-to-face, compared to 43% for adults.
-
Average daily facial photoprotection correlated highly with self-reported and clinician-reported photoprotection adherence (r = 0.66 and 0.49, respectively); compared with UVR dose-to-face, self-report had high sensitivity but low specificity.
-
Cross-sectional predictors of lower UVR dose-to-face included:
-
Greater perceived necessity of photoprotection [risk ratio (RR) 0.86, 90% confidence interval (CI) 0.65 to 1.13].
-
Stronger concerns about photoprotection (RR 0.84, CI 0.59 to 1.19).
-
Greater automaticity of photoprotection (RR 0.72, CI 0.55 to 0.95).
-
Greater self-efficacy (RR 0.67, CI 0.53 to 0.84).
-
Lower satisfaction with social support (RR 1.26, CI 0.92 to 1.72).
-
Greater negative emotional impact of XP (RR 0.76, CI 0.58 to 0.99).
-
The protocol was considered acceptable to patients, with just 3% of days excluded from the analysis due to non-adherence to the protocol. Of the three people totally excluded from the UVR dose-to-face analysis, two were excluded due to dosimeter failure and one due to low adherence to the dosimeter protocol.
Clinical variables associated with lower UVR dose-to-face:
-
being younger chronologically
-
being younger when diagnosed
-
fairer skin type
-
experiencing a severe XP sunburn response.
Conclusions
The feasibility of estimating UVR dose-to-face was demonstrated by combining measurement of objective UVR exposure and self-report daily diary recording of photoprotection behaviour. UVR dose-to-face was similar for adults and children and, although children received more UVR exposure, they were also better protected when outside. Psychosocial variables influenced both exposure and dose-to-face but had a larger impact on overall exposure. The healthy group acted as a benchmark for the methodology and for the XP group. The worse protecting patients had a dose to the face similar to the mean in the healthy group.
Inter-relationship with other parts of the programme
The calculation of UVR dose-to-face was a key objective for Phase I, with demonstration of its feasibility being necessary to justify its use as the primary outcome for the Phase II RCT. The observation of worse protection in adults with XP reinforced the decision to target adults in the Phase II intervention. The confirmation that overall exposure and protection behaviour when outside were important determinants of risk led to the decision to include both as behavioural targets in the XPAND intervention. Individual-level data concerning dose-to-face and overall exposure were included in the personalised behavioural feedback given to XPAND participants in session 1, with both also contributing to the decision of eligibility for the intervention.
Several modifiable psychosocial predictors of UVR dose-to-face were also identified as targets for intervention. The finding of an inverse association between better protection and greater negative emotions suggests the existence of an emotional trade-off. An important consideration for the intervention was, therefore, ensuring that the emotional burden of protection was acknowledged and supporting patients to protect their emotional well-being in the pursuit of increased protection.
Workstream 2: international cross-sectional survey
Methods and results of the international cross-sectional survey (Western sample)21 and the development of a self-reported adherence to photoprotection measure24 have been published.
Objectives
-
To further test the relevance of the factors (identified by Workstream 1) to photoprotection behaviour in a larger questionnaire study of international XP patients (as statistical power in Workstream 1 was limited by the small number of patients in the UK).
Methods
Recruitment and participants
Four Western countries (UK, France, Germany and USA) were involved. The target sample for the Western sample size was 193. The only inclusion criterion was that the participant had a clinical diagnosis of XP.
Adults with XP (> 16 years) who were responsible for their own protection completed the survey themselves; parents/caregivers of children (< 16 years) and adults with cognitive impairment completed the survey on behalf of the person diagnosed with XP (‘cared for’ sample). Details of recruitment and consent procedures are published. 21
Measures
The primary outcome was a self-reported measure of adherence to photoprotection,24 in which 24 items were analysed using an algorithm to produce protection scores for the protection of the face and body on sunny and cloudy days.
Avoidance of going outside during daylight was also measured, again for sunny and cloudy days separately, using a five-point scale (higher score indicates more avoidance).
Two versions of the survey were created: the adult, self-completed version, and the ‘cared for’ version. The only difference was in the framing of the questions (i.e. self vs. person with XP).
Additional questionnaires assessed psychological predictors:
including perceptions about the need for photoprotection, effectiveness of photoprotection, perceived consequences, timeline, personal control of XP, photoprotection control of XP, treatment control, identity, negative emotional representation (referred to as XP-related distress) and perceived understanding, intention to photoprotect, self-efficacy to photoprotect, automaticity of photoprotection, level and degree of satisfaction with support received in relation to UVR protection and to assess clinical characteristics. Further details of measures used and language translation methodology are published. 21
Data synthesis
Univariate ordinal logistic regression analyses identified associations between psychological variables and adherence to photoprotection. Hierarchical ordinal logistic regression analyses determined the strength of association of variables with standardised odds ratio (OR) ≥ 1.40 for at least one photoprotection outcome, with the photoprotection outcomes. The amount of variance was calculated for each photoprotection outcome that was explained by the psychological variables after accounting for demographic and clinical factors presented as OR and 95% CI. See published paper for further details. 21
Key findings
A total of 156 patients with XP (mean age = 24.8, SD = 19.5; 50.6% male) from the UK (n = 66), France (n = 58), Germany (n = 15) and USA (n = 17) completed the survey. The overall recruitment rate in the Western sample was 57.3% (UK: 84.6%, France: 70.7%, Germany: 72%, USA: 40.5%). Although small, this number represents more than half of the known cases of XP in the three European countries.
The sample was comprised of 71 adults responsible for their own photoprotection and 85 patients (children, and adults with cognitive impairment) for whom a caregiver was responsible for photoprotection (‘cared for’ sample). The mean age at diagnosis was 10.9 years (SD = 14.7); 30% were classified as having an extreme sunburn reaction upon UVR exposure (‘burners’); 45% had been diagnosed with a skin cancer; 22.4% had XP-related neurological problems; and 72% had XP-related eye problems.
Level of photoprotection
-
One-third (35.3%) reported suboptimal adherence to face photoprotection, which was higher on sunny (M = 4.27, SD = 1.04) compared to cloudy days [M = 4.01, SD = 1.28, F (4149) = 140.5, p < 0.001], with the largest weather-dependent difference for sunscreen use (47.4% used on cloudy, 59.6% on sunny days).
-
The ‘cared for’ sample were better protected than the adults on the face (M = 4.19, SD = 0.73 vs. M = 2.89, SD = 1.22) and body (M = 4.65, SD = 0.63 vs. M = 3.70, SD = 1.10). A minority of adults wore a visor (32.4%), whereas a large proportion of the ‘cared for’ sample used it on sunny days (85.9%).
Perceptions of xeroderma pigmentosum and beliefs about photoprotection
Participants perceived XP to have serious consequences, to be a chronic condition that could be effectively managed by treatment, and with a moderate negative emotional response. Overall, current photoprotection was perceived to be an effective barrier from UVR, with 66.2% reporting they were ‘completely’ or ‘very well’ protected. Beliefs about the necessity of photoprotection were high (M = 4.41 out of 5, SD = 0.72) and there was less concern about having to photoprotect (M = 2.98, SD = 0.99). Participants reported strong intention to photoprotect (M = 5.10 out of 7, SD = 1.19) and were generally confident that they could carry out photoprotection (M = 5.20, SD = 1.21).
Correlates of photoprotection
-
Variables associated with increased likelihood of better facial photoprotection were:
-
Greater perceived personal control over XP (OR 1.72, CI 1.20 to 2.45; OR 1.63, CI 1.15 to 2.30).
-
Greater perceived photoprotection control of XP (OR 1.64, CI 1.19 to 2.26).
-
Stronger necessity beliefs (OR 1.88, CI 1.34 to 2.64).
-
Stronger belief in the effectiveness of protection against UVR (OR .22, CI 1.53 to 3.24).
-
Stronger intentions to photoprotect (OR 1.83, CI 1.28 to 2.61).
-
Greater self-efficacy to photoprotect (OR 1.83, CI 1.28 to 2.61).
-
Greater automaticity (OR 2.16, CI 1.47 to 3.19).
-
-
The same variables were associated with greater body protection:
-
Greater perceptions of personal control over XP (OR 1.63, CI 1.15 to 2.30).
-
Greater perceived photoprotection control of XP (OR 1.39, CI 1.02 to 1.90).
-
Stronger necessity beliefs (OR 2.09, CI 1.46 to 2.99).
-
Stronger belief in the effectiveness of protection against UVR (OR 2.09, CI 1.39 to 2.76).
-
Stronger intentions to photoprotect (OR 1.59, CI 1.14 to 2.20).
-
Greater self-efficacy to photoprotect (OR 1.68, CI 1.20 to 2.12).
-
Greater automaticity (OR 2.20, CI 1.52 to 3.18).
-
Additionally, stronger belief in the serious consequences of XP was related to better body protection (OR 1.42, CI 1.01 to 1.99).
-
-
The first five of the above variables (personal control, photoprotection control, necessity beliefs, effectiveness beliefs and intention) were not associated with avoidance of going outside whereas self-efficacy (OR 2.20, CI 1.61 to 3.00) and automaticity (OR 2.52, CI 1.82 to 3.49) were. Additional variables associated with avoidance of going outside were:
-
Higher XP-related distress (OR 2.11, CI 1.54 to 2.89).
-
Greater XP-related concerns (OR 1.65, CI 1.20 to 2.25).
-
Stronger belief in the serious consequences of XP (OR 1.47, CI 1.09 to 1.99).
-
Greater concerns about photoprotection (OR 1.80, CI 1.32 to 2.46).
-
-
Social support was not related to any of the photoprotection outcomes.
-
Demographic and clinical variables (sex, age, skin type, country, age at diagnosis, burning type and history of skin cancer) accounted for 8% of the variance in adherence to facial protection, 5% of the variance in body protection and 1% of the variance in avoidance of going outside.
-
The addition of the psychological variables to the above regression models increased the amount of variance accounted for to 22% for both facial and body protection, and 6% for avoidance of going outside.
Limitations
The main limitations of this study were the cross-sectional design and the reliance on self-report. Due to concerns about over-burdening participants, shortened versions of most questionnaires were used which may explain the relatively low amount of variance accounted for failure to recruit to the target (n = 193), reduced the power of analysis and prevented between country comparisons.
Conclusions
Approximately half of all known cases across three European countries participated and one-third did not achieve optimal face photoprotection. After controlling for demographic and clinical factors, stronger beliefs about the efficacy of photoprotection and about the necessity of protecting, higher intention, self-efficacy and automaticity were related to better photoprotection while outside. Identified modifiable predictors of photoprotection were used to inform the design of the XPAND intervention.
Inter-relationship with other parts of the programme
The psychosocial data from the cross-sectional survey were also used as predictors of UVR dose-to-face, as reported in Workstream 1b-ii.
The findings from the cross-sectional survey were used to inform the selection of targets for intervention in XPAND. Specifically, this led to the inclusion of content to boost necessity and effectiveness beliefs, reduce concerns about photoprotection and increase intentions, self-efficacy and personal control, and the automaticity of photoprotection behaviour. Similar to the identified predictors of UVR dose-to-face (Workstream 1b), the finding that greater XP-related distress was related to avoidance of going outside confirmed the need to be sensitive to the emotional impact of improved protection and ensure that behavioural improvements were not at the cost of reduced well-being.
Workstream 2: economic costs of xeroderma pigmentosum
Objectives
-
Describe service use for patients in the four Western countries (France, Germany, USA and the UK) and two non-Western countries (Japan and Tunisia).
-
Calculate and describe service costs.
-
Identify demographic and clinical predictors of total service costs.
-
Measure and report health-related quality of life (HRQoL).
Methods
An adapted Client Service Receipt Inventory (CSRI)25 was used to record the use of services during the previous 6 months. Participants reported the number of times they had used specific health and social care services. Costs were calculated by combining service use with appropriate unit costs. Ideally, we would have country-specific unit costs that reflect different ways of providing services in each setting. However, that was not feasible within the study and so we started by obtaining unit cost information for services provided in the UK and then adjusted these to reflect differences in the cost of living using purchasing power parity rates.
To identify predictors of service costs, we used a series of generalised linear models with a gamma distribution and log link. Total service cost was used as the dependent variable, and independent variables included age, gender, age at diagnosis, whether they had had skin cancer, skin colour, eye colour and XP complementation group.
Health-related quality of life was measured with the EuroQol-5 Dimensions, five-level version (EQ-5D-5L). 26
Key findings
In France, all but two of the respondents had seen a dermatologist during the costing period, and on average nearly three contacts were made (Table 2). Around three-quarters had contacts with ophthalmologists. Other specialist outpatient contacts were used by substantially fewer respondents. Surgery for skin problems was performed for one-third, but on more than seven occasions. Just over half of the French group had general practitioner (GP) contacts. Most other services were used by relatively few respondents, although over 40% had blood tests. The most expensive service was skin-related surgery followed by inpatient stays (even though this was used by very few).
| France (n = 59) | Germany (n = 16) | USA (n = 17) | UK (n = 66) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) contacts |
Mean (SD) cost Euro |
N (%) | Mean (SD) contacts |
Mean (SD) cost Euro |
N (%) | Mean (SD) contacts |
Mean (SD) cost USD |
N (%) | Mean (SD) contacts |
Mean (SD) cost £s |
|
| Outpatient appointments | ||||||||||||
| Dermatology | 57 (97) | 2.9 (2.9) | 363 (372) | 16 (100) | 4.0 (4.0) | 531 (530) | 4 (24) | 3.5 (1.3) | 175 (347) | 49 (74) | 2.1 (1.6) | 171 (185) |
| Ophthalmology | 46 (78) | 1.9 (1.9) | 171 (208) | 10 (63) | 2.7 (3.3) | 196 (335) | 3 (18) | 1.7 (0.6) | 55 (129) | 35 (53) | 1.4 (1.2) | 71 (108) |
| Neurology | 3 (5) | 1.0 (0.0) | 10 (43) | 5 (31) | 2.6 (1.9) | 161 (317) | 0 (0) | – | 0 (0) | 26 (39) | 1.0 (0.2) | 68 (88) |
| Paediatrics | 9 (15) | 3.6 (3.5) | 133 (448) | 2 (13) | 2.0 (1.4) | 62 (193) | 0 (0) | – | 0 (0) | 9 (14) | 1.3 (0.5) | 38 (104) |
| Plastic surgery | 10 (17) | 3.1 (1.9) | 65 (173) | 3 (19) | 2.3 (1.2) | 55 (130) | 0 (0) | – | 0 (0) | 9 (14) | 2.0 (1.0) | 29 (83) |
| Psychology | 9 (15) | 8.3 (10.3) | 78 (299) | 5 (31) | 2.4 (0.9) | 47 (78) | 0 (0) | – | 0 (0) | 24 (36) | 1.3 (1.0) | 24 (46) |
| Other | 16 (27) | 12.1 (26.5) | 444 (1962) | 3 (19) | 2.0 (1.0) | 51 (119) | 0 (0) | – | 0 (0) | 12 (18) | 3.3 (3.3) | 80 (252) |
| Surgery | ||||||||||||
| Skin | 20 (34) | 7.4 (13.1) | 1956 (6463) | 11 (69) | 3.0 (1.7) | 1610 (1548) | 3 (18) | 1.7 (0.6) | 377 (880) | 19 (29) | 2.4 (1.9) | 462 (990) |
| Eyes | 5 (8) | 2.8 (2.0) | 222 (890) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 3 (5) | 1.3 (0.6) | 49 (240) |
| Other | 2 (3) | 1.0 (0.0) | 51 (276) | 1 (6) | 1.0 (−) | 96 (386) | 0 (0) | – | 0 (0) | 2 (3) | 1.0 (0.0) | 40 (225) |
| Professional contacts | ||||||||||||
| GP | 32 (54) | 5.1 (17.4) | 120 (562) | 12 (75) | 7.0 (7.3) | 232 (310) | 3 (18) | 2.0 (1.7) | 25 (71) | 37 (56) | 2.4 (2.0) | 49 (71) |
| Nurse | 6 (10) | 13.7 (21.9) | 179 (985) | 0 (0) | – | 0 (0) | 2 (12) | 3.0 (2.8) | 75 (260) | 24 (36) | 2.0 (1.4) | 79 (139) |
| Physiotherapist | 4 (7) | 21.0 (21.6) | 89 (454) | 5 (31) | 10.8 (8.6) | 216 (436) | 0 (0) | – | 0 (0) | 7 (11) | 4.7 (4.4) | 27 (107) |
| Talking therapy | 9 (15) | 2.9 (2.8) | 27 (92) | 5 (31) | 2.6 (0.5) | 51 (80) | 1 (6) | 1.0 (−) | 6 (25) | 13 (20) | 2.2 (1.8) | 22 (61) |
| Complementary therapist | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 1 (6) | 2.0 (−) | 17 (72) | 1 (2) | 5.0 (−) | 6 (48) |
| Occupational therapist | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 5 (8) | 3.4 (2.4) | 20 (85) |
| Social worker | 2 (3) | 3.5 (2.1) | 8 (49) | 1 (6) | 3.0 (−) | 13 (53) | 0 (0) | – | 0 (0) | 7 (11) | 2.4 (2.0) | 15 (58) |
| Home care worker | 1 (2) | 25.0 (−) | 12 (95) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 3 (5) | 81.0 (92.3) | 93 (593) |
| Support group | 1 (2) | 3.0 (−) | 5 (31) | 1.2 (0.4) | 0 (0) | – | 1 (2) | 1.0 (−) | ||||
| Tests | ||||||||||||
| MRI | 7 (12) | 1.4 (0.5) | 13 (35) | 3 (19) | 2.3 (2.3) | 30 (87) | 1 (6) | 1.0 (−) | 7 (27) | 8 (12) | 1.0 (0.0) | 9 (21) |
| Audiometry | 3 (41) | 1.7 (1.2) | 11 (47) | 1 (6) | 1.0 (0.0) | 6 (25) | 1 (6) | 1.0 (−) | 10 (40) | 9 (14) | 1.1 (0.3) | 13 (35) |
| Nerve testing | 0 (0) | – | 0 (0) | 2 (13) | 1.0 (0.0) | 25 (167) | 1 (6) | 1.0 (−) | 19 (78) | 8 (12) | 1.1 (0.4) | 23 (65) |
| Blood test | 24 (41) | 2.2 (2.8) | 3 (7) | 10 (63) | 4.3 (5.9) | 10 (18) | 2 (12) | 1.0 (0.0) | 1 (2) | 31 (47) | 1.3 (0.7) | 2 (2) |
| Other tests | 9 (15) | 1.6 (0.5) | 45 (100) | 7 (44) | 1.9 (1.5) | 130 (212) | 1 (6) | 1.0 (−) | 15 (63) | 14 (21) | 1.6 (0.8) | 246 (1128) |
| Inpatient stays | 5 (8) | 25.9 (52.1) | 1594 (11,259) | 5 (31) | 1.8 (0.8) | 417 (714) | 0 (0) | – | 0 (0) | 3 (5) | 5.3 (7.5) | 152 (1082) |
| Total | 59 | 4379 (8929) | 16 | 3951 (3445) | 17 | 782 (1770) | 66 | 1758 (2399) | ||||
| 95% CI (total costs) | 2052.27 to 6706.28 | 2115.20 to 5786.62 | −127.79 to 1692.25 | 1167.88 to 2347.21 | ||||||||
In the German sample, all had contacts with dermatologists and around two-thirds with ophthalmologists. Two-thirds also had skin-related surgery and three-quarters had GP contacts. One-third of the group had talking therapy and one-third received physiotherapy. Inpatient care was received also by around one-third, but on average for < 2 days. The most expensive service was skin-related surgery followed by dermatologist outpatient contacts and inpatient stays.
In the USA, only one-quarter had dermatologist contacts and less than one-fifth had skin-related surgery. These were the most expensive services used by this group.
Respondents from the UK were likely to have dermatologist and ophthalmologist contacts. Around one-third had contacts with psychologists and neurologists, and a relatively high number had skin-related surgery on more than two occasions on average. Over half had GP contacts and about one-third had nurse contacts. The most expensive service was skin-related surgery followed by ‘other tests’, dermatologist contacts and inpatient stays.
Japan followed the pattern of the Western countries (except the USA) with high use of dermatologists (Table 3). Over one-quarter had ophthalmologist contacts and neurology contacts. GPs were seen by one-third of participants, and none had inpatient stays. Nurse contacts were the most expensive service due to the high number of contacts among the few who used this. The next most expensive services were outpatient contacts (dermatology, ‘other’ and paediatrics).
| Japan (n = 41) | Tunisia (n = 22) | |||||
|---|---|---|---|---|---|---|
| N (%) | Mean (SD) contacts |
Mean (SD) cost Yen |
N (%) | Mean (SD) contacts |
Mean (SD) cost Dinar |
|
| Outpatient appointments | ||||||
| Dermatology | 32 (78) | 2.36 (1.9) | 29,030 (30,652) | 17 (77) | 2.1 (1.2) | 33 (28) |
| Ophthalmology | 11 (27) | 2.0 (1.8) | 7446 (17,580) | 12 (55) | 2.8 (3.4) | 28 (51) |
| Neurology | 11 (27) | 2.5 (3.2) | 15,604 (46,071) | 0 (0) | - | 0 (0) |
| Paediatrics | 14 (34) | 2.7 (2.0) | 27,674 (51,453) | 1 (5) | 1.0 (–) | 2 (8) |
| Plastic surgery | 8 (20) | 3.3 (3.6) | 9788 (30,582) | 0 (0) | - | 0 (0) |
| Psychology | 3 (7) | 1.3 (0.6) | 733 (2812) | 1 (5) | 1.0 (–) | < 1 (2) |
| Other | 15 (37) | 4.1 (3.8) | 28,460 (56,925) | 3 (14) | 1.0 (0.0) | 18 (47) |
| Surgery | ||||||
| Skin | 4 (10) | 1.5 (1.0) | 13,893 (50,059) | 3 (14) | 1.3 (0.6) | 22 (61) |
| Eyes | 0 (0) | - | 0 (0) | 2 (9) | 1.0 (0.0) | 13 (43) |
| Other | 0 (0) | - | 0 (0) | 0 (0) | - | 0 (0) |
| Contacts | ||||||
| GP | 13 (32) | 7.2 (7.6) | 12,150 (28,544) | 7 (32) | 1.9 (1.2) | 4 (8) |
| Nurse | 4 (10) | 46.1 (70.5) | 70,776 (373,683) | 3 (14) | 1.7 (0.6) | 5 (12) |
| Physiotherapist | 12 (29) | 8.0 (6.3) | 17,916 (37,858) | 0 (0) | - | 0 (0) |
| Talking therapy | 4 (10) | 1.8 (1.0) | 1282 (4412) | 1 (5) | 1.0 (–) | < 1 (2) |
| Complementary therapist | 2 (5) | 19.0 (19.8) | 10,178 (57,028) | 0 (0) | - | 0 (0) |
| OT | 5 (12) | 4.0 (1.9) | 5391 (16,039) | 0 (0) | - | 0 (0) |
| Social worker | 0 (0) | - | 0 (0) | 0 (0) | - | 0 (0) |
| Home care worker | 1 (2) | 180.0 (–) | 15,707 (100,575) | 0 (0) | - | 0 (0) |
| Support group | 1 (2) | 30.0 (–) | 0 (0) | 3 (14) | 2.3 (1.5) | 0 (0) |
| Tests | ||||||
| MRI | 1 (2) | 1.0 (–) | 401 (1792) | 1 (5) | 2.0 (0.0) | 1 (5) |
| Audiometry | 13 (32) | 2.2 (2.6) | 8962 (21,667) | 0 (0) | - | 0 (0) |
| Nerve testing | 2 (5) | 1.0 (0.0) | 1154 (5160) | 0 (0) | - | 0 (0) |
| Blood test | 8 (20) | 3.6 (3.3) | 413 (911) | 0 (0) | - | 0 (0) |
| Other tests | 2 (5) | 1.5 (0.4) | 3499 (9743) | 2 (9) | 1.0 (0.0) | 2 (7) |
| Inpatient stays | 0 (0) | - | 0 (0) | 0 (0) | - | 0 (0) |
| Total | 41 | 379,622 (830,623) | 22 | 133 (156) | ||
| 95% CI (total costs) | 117,445.30 to 641,798.30 | 63.98 to 202.0393 | ||||
In Tunisia, dermatology was used by most (about three-quarters), with over half having ophthalmologist contacts. About one-third had GP contacts. Other services were used by relatively few respondents. Interestingly, in both Japan and Tunisia, there was a relatively low level of skin-related surgery.
Total service costs in each country were: France €4379, Germany €3951, USA $782, UK £1758, Japan ¥379,622, Tunisia 133 DT.
In Tables 4–9 we show unadjusted regression results (where each variable was entered separately) and results adjusted for all other variables. The analysis on the USA sample was confined to unadjusted models due to the small sample.
In the UK, age and age at diagnosis were both significant predictors of costs, with every extra year related to a 1% increase in costs (see Table 4). Having light brown skin colour was associated with lower costs (by 51%) compared to fair or light-coloured skin. Lower costs were also revealed for those with Afro-Caribbean skin colour, by 84% compared to fair or light-coloured skin. Having blue eyes was associated with costs that were 117% higher than for brown eyes. In the adjusted model, age at diagnosis, having had skin cancer and having eyes that were not brown in colour were all associated with higher costs.
| Unadjusted (95% CI) | Adjusted (95% CI) | |
|---|---|---|
| Age of participant | 1.01 (1.00 to 1.03) | 0.99 (0.97 to 1.01) |
| Gender of participant | ||
| Male | 1.12 (0.59 to 2.11) | 1.03 (0.57 to 1.87) |
| Female | ||
| Age of diagnosis | 1.01 (1.00 to 1.03) | 1.02 (1.00 to 1.05) |
| Skin cancer | ||
| Yes | 1.47 (0.76 to 2.87) | 1.97 (1.02 to 3.79) |
| No | ||
| Skin colour | ||
| Fair or light-coloured | ||
| Asian | 0.57 (0.22 to 1.50) | 1.06 (0.50 to 2.22) |
| Light brown | 0.49 (0.25 to 0.95) | 0.60 (0.25 to 1.42) |
| Afro-Caribbean | 0.16 (0.09 to 0.28) | 0.41 (0.13 to 1.27) |
| Dark brown | 0.83 (0.22 to 3.12) | 1.98 (0.68 to 5.72) |
| Eye colour | ||
| Brown | ||
| Blue | 2.17 (1.10 to 4.29) | 2.47 (1.37 to 4.46) |
| Green, other | 1.98 (0.75 to 5.23) | 4.26 (1.56 to 11.65) |
| XP complementation | ||
| Group | ||
| A | ||
| C | 0.54 (0.20 to 1.44) | 0.48 (0.20 to 1.14) |
| D | 1.62 (0.55 to 4.84) | 2.26 (0.82 to 6.20) |
| V | 0.83 (0.30 to 2.26) | 0.50 (0.19 to 1.27) |
| Miscellaneous (B, E, F, G) | 1.18 (0.32 to 4.28) | 1.08 (0.43 to 2.70) |
In Germany, older age and age at diagnosis were associated with increased costs when other variables were not adjusted for (Table 5). In the adjusted model, only age was significantly associated with cost. In France, older age was associated with higher costs, and having brown eyes was associated with lower costs than if eyes were blue (Table 6). In the adjusted analyses, having green or brown eyes was associated with lower costs. No variables were significantly associated with cost in the USA sample (Table 7).
| Unadjusted (95% CI) | Adjusted (95% CI) | |
|---|---|---|
| Age of participant | 1.03 (1.01 to 1.06) | 1.04 (1.01 to 1.08) |
| Gender of participant | ||
| Male | 1.17 (0.54 to 2.51) | 1.75 (0.83 to 3.71) |
| Female | ||
| Age of diagnosis | 1.02 (1.00 to 1.04) | 0.97 (0.91 to 1.05) |
| Skin cancer | ||
| Yes | 2.55 (0.86 to 7.52) | 2.06 (0.24 to 17.37) |
| No | ||
| Skin colour | ||
| Fair or light-coloured | 0.80 (0.35 to 1.85) | 0.18 (0.08 to 3.39) |
| Light brown, dark brown | ||
| Eye colour | ||
| Brown | 0.79 (0.34 to 1.85) | 0.36 (0.03 to 3.78) |
| Green, blue | ||
| Unadjusted (95% CI) | Adjusted (95% CI)a | |
|---|---|---|
| Age of participant | 1.03 (1.00 to 1.05) | 1.02 (0.97 to 1.06) |
| Gender of participant | ||
| Male | 2.20 (0.91 to 5.36) | 0.72 (0.29 to 1.81) |
| Female | ||
| Age of diagnosis | 1.02 (0.99 to 1.05) | 1.02 (0.92 to 1.12) |
| Skin cancer | ||
| Yes | 2.08 (0.77 to 5.64) | 1.52 (0.72 to 3.21) |
| No | ||
| Skin colour | ||
| Fair or light-coloured | ||
| Light brown | 1.03 (0.34 to 2.88) | 1.79 (0.94 to 3.40) |
| Afro-Caribbean, dark brown | 1.53 (0.33 to 7.13) | 0.73 (0.22 to 2.45) |
| Eye colour | ||
| Blue | ||
| Green | 0.76 (0.16 to 3.48) | 0.13 (0.03 to 0.56) |
| Brown | 0.16 (0.06 to 0.42) | 0.15 (0.04 to 0.56) |
| Other | 0.67 (0.15 to 3.07) | 0.39 (0.10 to 1.56) |
| XP complementation | ||
| Group | ||
| C | ||
| V | 2.79 (0.56 to 13.83) | 0.59 (0.10 to 3.67) |
| Miscellaneous (A, B, D, E) | 2.51 (0.93 to 6.76) | 3.81 (0.84 to 12.27) |
| Unadjusted (95% CI) | |
|---|---|
| Age of participant | 1.44 (0.74 to 2.81) |
| Gender of participant | |
| Male | 0.40 (0.04 to 3.70) |
| Female | |
| Age of diagnosis | 1.15 (0.86 to 1.53) |
| Eye colour | |
| Blue | 0.20 (0.02 to 1.83) |
| (green, brown, other) | |
| XP complementation | |
| Group | |
| C | 0.81 (0.09 to 7.59) |
| Miscellaneous (A, D) | |
In Tunisia, the unadjusted analyses showed that older age was associated with higher costs (Table 8). Compared to XP complementation groups A and V, being in group C was associated with increased costs. The adjusted analyses showed that costs were higher for older patients but lower for older patients at the age of diagnosis. If patients in Tunisia had had skin cancer, then costs were lower than if they had not had skin cancer.
| Unadjusted (95% CI) | Adjusted (95% CI)a | |
|---|---|---|
| Age of participant | 1.12 (1.05 to 1.19) | 1.61 (1.32 to 1.96) |
| Gender of participant | ||
| Male | 0.35 (0.14 to 0.85) | 5.80 (0.85 to 39.66) |
| Female | ||
| Age of diagnosis | 0.89 (0.73 to 1.08) | 0.88 (0.83 to 0.93) |
| Skin cancer | ||
| Yes | 1.20 (0.43 to 3.37) | 0.15 (0.10 to 0.23) |
| No | ||
| Skin colour | ||
| Fair or light-coloured | ||
| Asian | ||
| Light brown | 0.69 (0.23 to 2.13) | 0.36 (0.11 to 1.16) |
| Afro-Caribbean | ||
| Dark brown | ||
| XP complementation | ||
| Group | ||
| C | 4.31 (1.96 to 9.51) | 0.70 (0.45 to 1.08) |
| Miscellaneous (A, V) | ||
Finally, in Japan, unadjusted analyses revealed lower costs associated with being in complementation groups other than A (Table 9). Adjusted analyses also showed this, and also lower costs associated with having had skin cancer and higher costs associated with older age.
| Unadjusted (95% CI) | Adjusted (95% CI)a | |
|---|---|---|
| Age of participant | 1.02 (0.99 to 1.04) | 1.05 (1.01 to 1.10) |
| Gender of participant | ||
| Male | 1.50 (0.41 to 5.44) | 2.28 (0.91 to 5.71) |
| Female | ||
| Age of diagnosis | 0.99 (0.95 to 1.04) | 1.02 (0.95 to 1.10) |
| Skin cancer | ||
| Yes | 1.77 (0.48 to 6.61) | 0.12 (0.03 to 0.44) |
| No | ||
| Skin colour | ||
| Asian | 0.91 (0.20 to 4.12) | 0.62 (0.23 to 1.64) |
| (fair or light-coloured, light brown) | ||
| Eye colour | ||
| Brown | 1.16 (0.31 to 4.38) | 0.68 (0.33 to 1.41) |
| Green, other | ||
| XP complementation | ||
| Group | ||
| A | ||
| V | 0.13 (0.04 to 0.39) | 0.03 (0.01 to 0.10) |
| Miscellaneous (C, D) | 0.06 (0.02 to 0.19) | 0.03 (0.01 to 0.15) |
Mean HRQoL weights were as follows: France 0.85, Germany 0.85, USA 0.84, UK 0.75, Japan 0.70, Tunisia 0.89.
Limitations
These data were collected via participant self-report and recall may not have been fully accurate. The costs that were calculated were based on unit costs adjusted from UK figures. While this should reflect cost of living differences, it does not reflect differences in how each country organises and structures services. A further limitation is that the sample size in some countries was too small to enable extensive multivariable analyses of costs to be carried out.
Inter-relationship with other parts of the programme
This study informed the economic evaluation of the intervention in Workstream 5.
Workstream 3: consensus conference, intervention design and manual development
The XPAND intervention development process, the consensus conference27 and the personalisation process28 have been published.
Objectives
-
To achieve consensus regarding the targets for intervention in Phase II.
-
To design an evidence-based, clinically relevant set of intervention strategies for delivery and evaluation in Workstream 4.
-
To develop a facilitator manual to guide intervention delivery.
Methods
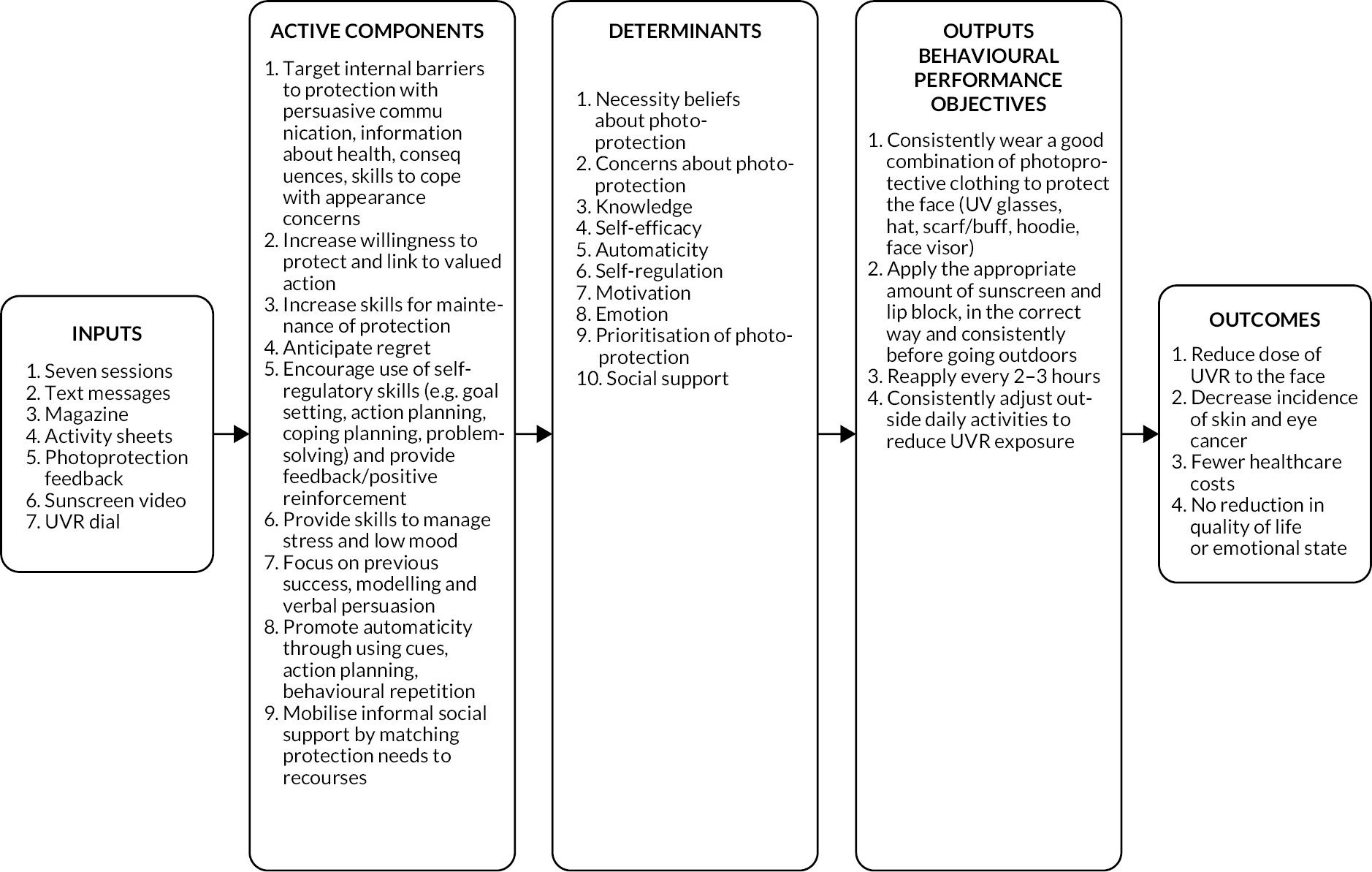
An adapted version of the consensus methodology, based on Nominal Group Theory29 which was used to agree changes to a diabetes self-management package,30,31 was used to guide the consensus conference. Evidence statements were presented by research teams, and recommendations were approved, rejected or amended. Researchers (n = 10), the patient and public involvement (PPI) panel (n = 3) and the XP clinical team (n = 5) attended. The PPI and clinical teams defined their priorities for intervention. The agreed recommendations (Table 10) informed the intervention design. The design process involved specifying the ‘behavioural objectives’ required for improved photoprotection and the ‘change objectives’ needed to achieve behavioural changes, captured in a series of intervention matrices (see Appendix 1). From here, logic models of (1) the problem (Figure 4) and (2) the process of change (Figure 5) were derived. Change objectives were mapped to behaviour change methods derived from several taxonomies32,33 and clinical approaches (e.g. acceptance and commitment therapy,34 motivational interviewing35).
| 1 | Recommendations related to photoprotection behaviour To gain largest reduction in UVR dose to the face, the intervention should include tools to promote better sunscreen use, greater use of protective clothing, and target lifestyle adjustments such as time of day and duration of time spent outdoors |
| 2 | To improve photoprotection, the intervention should include tools to assess the extent and nature of changes in the level of protection within an individual. Where change results in worse protection, maintenance of better protection across different contexts and situations will be targeted. |
| 3 | To improve photoprotection, the intervention should include tools to increase awareness and insight into photoprotection behaviour. |
| 4 | Recommendations related to beliefs To improve photoprotection, the intervention should include tools to elicit and challenge doubts about the necessity of photoprotection related to negative health consequences. |
| 5 | To improve photoprotection, the intervention should include tools to elicit and challenge doubts about the effectiveness of photoprotection and emphasise that the best way to protect is to combine all the different ways to protect. |
| 6 | To improve photoprotection, the intervention should include tools to target the perception of low personal control over health consequences related to XP. |
| 7 | To improve photoprotection, the intervention should include tools to elicit the extent and nature of any concerns about photoprotection practices and include tools to manage any such concerns. |
| 8 | Recommendations related to risk perception To improve photoprotection, the intervention should include tools to target perceptions of low UVR risk in relation to time, weather and season. |
| 9 | To improve photoprotection, the intervention should include tools to counteract the belief that an absence of noticeable physical symptoms (in both burners and non-burners) means photoprotection is not required. It should sever the link between symptom experience and photoprotection behaviour and encourage photoprotection regardless of symptoms. |
| 10 | Recommendation related to acceptance To improve photoprotection in patients who are resistant to the XP identity, the intervention should include tools to promote illness acceptance. |
| 11 | Recommendations related to motivation and habit To improve photoprotection, the intervention should include tools to increase and reinforce reflective motivation to photoprotect. |
| 12 | To improve photoprotection, the intervention should include tools to target low prioritisation of photoprotection and reinforce the priority in the context of competing daily priorities. |
| 13 | To improve photoprotection, the intervention should include tools to target self-efficacy for photoprotection in the presence of personally relevant barriers. |
| 14 | To improve photoprotection, the intervention should include tools to establish routines and habits. |
| 15 | Recommendations related to social context To increase the likelihood that new photoprotection behaviours will be maintained, the intervention should include tools to manage any experience of receiving (or perceiving) negative reactions from others (enacted stigma). |
| 16 | To improve photoprotection, the intervention should include tools to encourage participants to appropriately and skilfully disclose about XP, when it is acknowledged by the patient to be a barrier to photoprotection. The level of disclosure will be decided by the patient. |
| 17 | To improve photoprotection, the intervention should include tools to enhance informal social support from family and friends (e.g. adjustment of daily activities, reminders to photoprotect), if lack of support is a barrier to photoprotection. |
| 18 | Recommendations related to psychological impact of photoprotection To improve photoprotection, the intervention should include tools to target general negative low mood. |
| 19 | To improve photoprotection, the intervention should include tools to elicit the extent and nature of the relationship between emotional experiences (in the moment) and photoprotection (e.g. feeling stressed, worried, mentally exhausted) and include tools to reduce/manage the negative impact of any such emotional experiences on photoprotection. |
FIGURE 4.
The logic model of poor photoprotection.
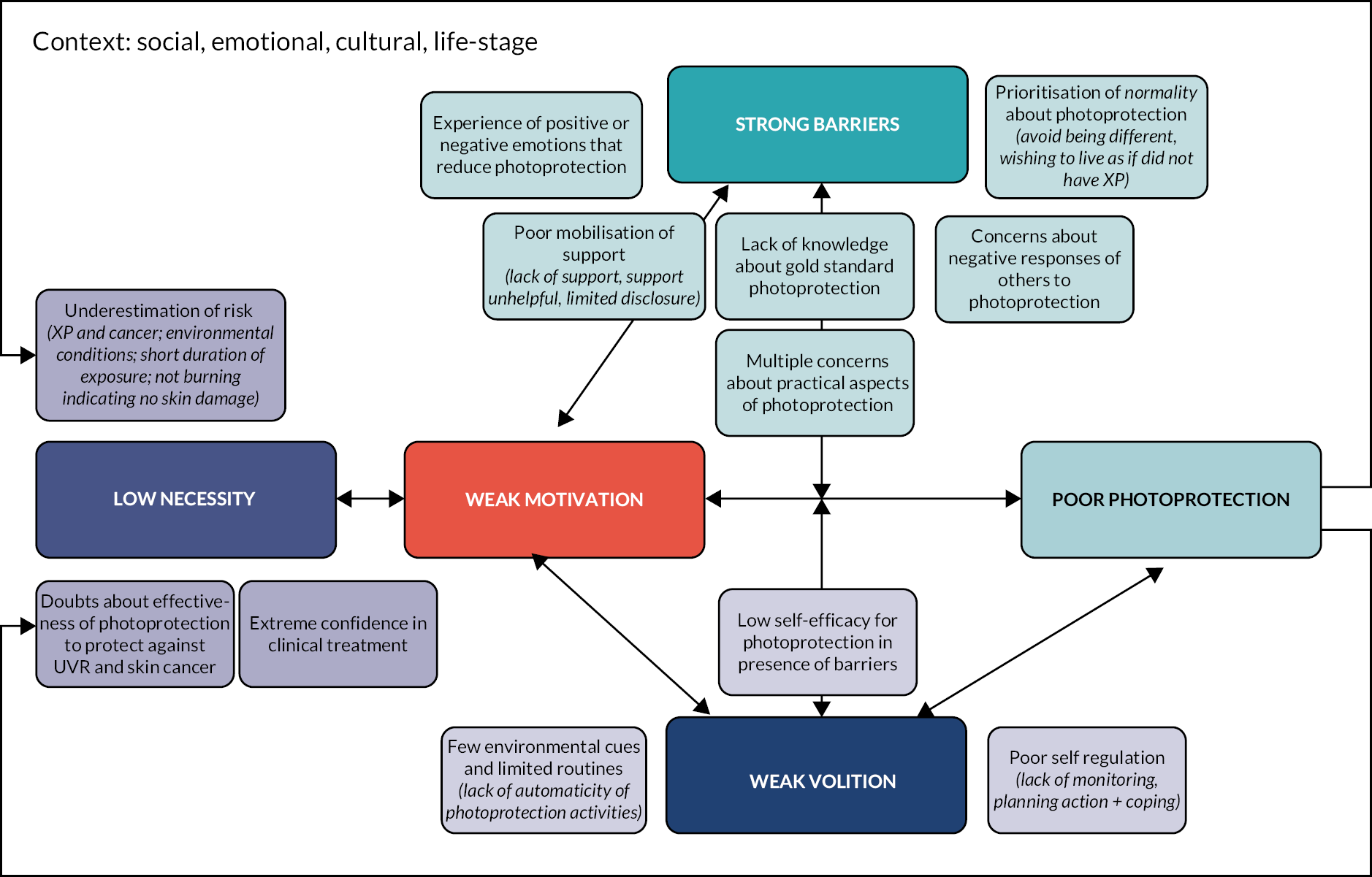
FIGURE 5.
The logic model of change.
Intervention piloting was not possible due to small numbers in a rare disease. Instead, the intervention was reviewed by the PPI team and several XP patients with excellent photoprotection, who were ineligible for participation in the trial.
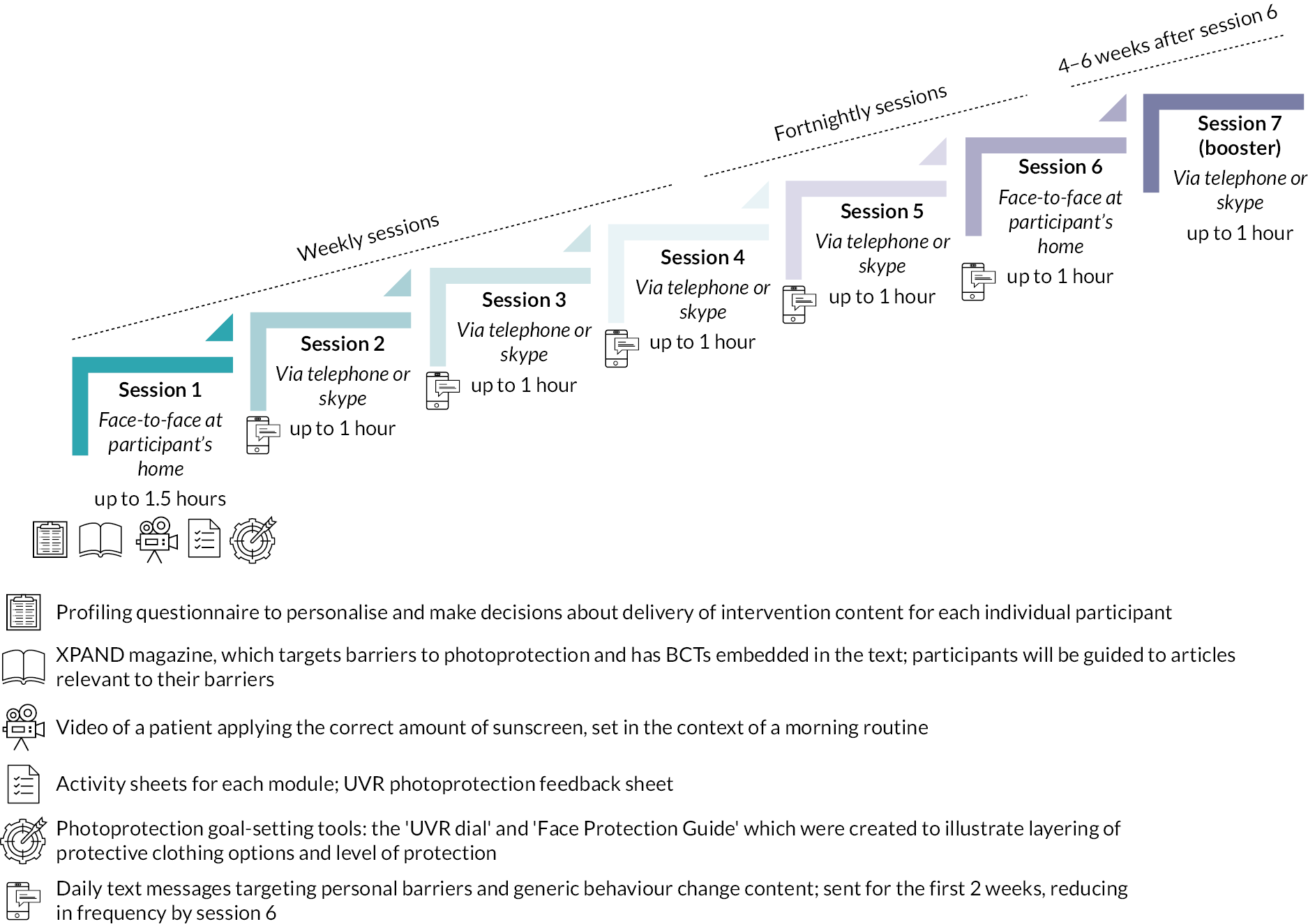
Intervention description
XPAND is a seven-session behaviour change programme, containing both core and personalised elements. It was designed to be delivered by non-psychologists. Facilitators in the trial were trained in how to deliver using a manual and received supervision during the trial. Sessions 1, 6 and 7 focused on the development of motivational self-regulatory and habit formation processes, and relapse prevention strategies. All subsequent sessions commenced with goal review and identification of barriers and facilitators to goal achievement over the past week, and close with respecification of the weekly goal.
Sessions 2–5 were personalised, depending on preferences and identification of individual-level determinants.
Personalised topics included:
-
Stress and photoprotection.
-
Mood and photoprotection.
-
Appearance concerns.
-
Social support (practical and emotional), including an optional submodule on disclosure.
-
Necessity of photoprotection:
-
Values clarification as motivation for protection (extension of the motivation content in session 1) and acceptance.
-
Willingness for protection.
Intervention materials
-
Intervention facilitator delivery manual.
-
Reflection sheet completed by the facilitator at the end of each session.
-
Worksheets for each session/module, referred to throughout the sessions and completed by patient and/or facilitator.
-
Personalised UVR feedback form with a risk-ruler:
-
Goal-setting tools: risk-ruler and UVR dial both used to visually communicate the different levels of photoprotection and the improvement of protection via layering of clothing options.
-
Video demonstrating the correct application of sunscreen and process of habit formation via a morning sunscreen routine.
-
Patient-facing XPAND magazine contains articles focused on key topics and barriers to protection identified in the Phase I studies. See Figure 6 for a summary of the structure of XPAND.
FIGURE 6.
Summary of the structure of XPAND.
See supplementary documents for examples of intervention materials.
Personalisation process
Decisions about which content to deliver to each XPAND participant in sessions 2–5 were informed by (1) individual-level Phase I data, which were summarised into an individualised pattern of drivers of protection; (2) the ‘personalisation profiling questionnaire’, completed by all RCT participants at baseline; and (3) identification of barriers throughout XPAND delivery.
Inter-relationship with other parts of the programme
The resultant XPAND intervention was tested by RCT in Phase II, Workstream 4.
Workstream 4: randomised controlled trial
The protocol for the XPAND RCT is published. 10
The results of the XPAND RCT are in preparation for publication.
Objectives
The primary objective of this RCT was to investigate whether the average daily UVR D-to-F (SED), across 21 consecutive days in June–July 2018, was reduced after receipt of XPAND compared to delayed intervention control. We also assessed whether change was maintained across 21 consecutive days in August 2018, and explored intervention-related changes across outcomes from baseline in the delayed intervention control group, who received the intervention 1 year later.
Methods
Design
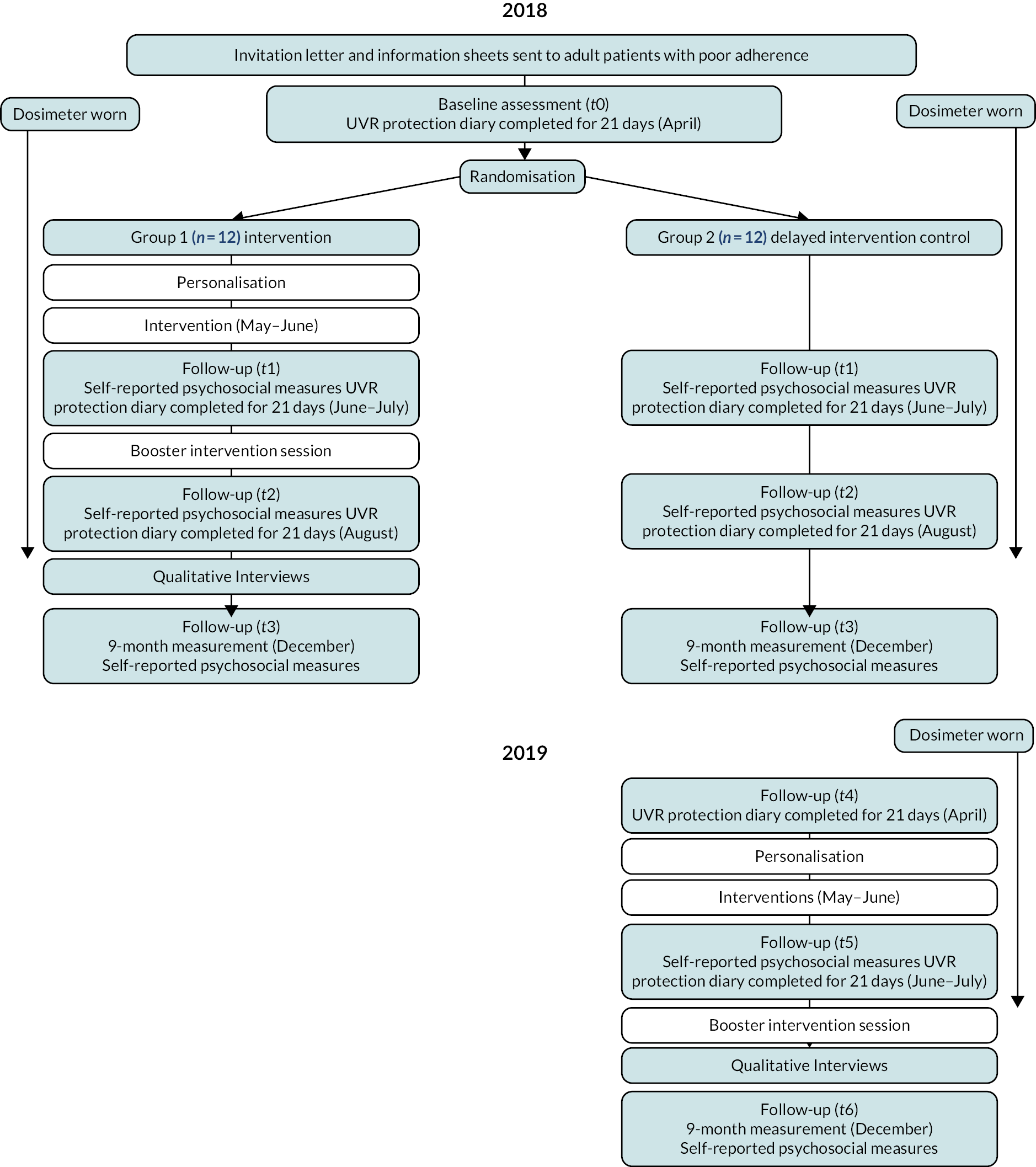
A Phase II, assessor blind, two-armed parallel group RCT compared the efficacy of XPAND between participants who received the XPAND intervention May–June 2018 (+ routine care) and a delayed intervention treatment as usual (TAU) control group who received routine clinical care in 2018, and XPAND a year later (May–June 2019). The intervention and measurement periods were fixed for all participants to control for seasonal differences in environmental UVR. See Figure 7 for a flow diagram of the trial design.
FIGURE 7.
Flow diagram of the trial design.
Treatment as usual involves multidisciplinary clinics for patients at intervals dependent on the clinical severity of the disease, in which patients are treated and assessed over a whole day appointment by consultants in the fields of dermatology, dermatological surgery, ophthalmology, neurology, clinical genetics and neuropsychology. Any skin or eye cancers identified during these appointments are surgically treated either on the day or, if the surgery is more complex, on a later date. Patients and their families also receive outreach care remotely or in the patient’s home by the specialist XP nursing team. In terms of photoprotection, patients are given a portable UVR meter, instruction in applying sunscreen, UV visors and all the other methods of photoprotection, and they are prescribed SPF50 high UVA protection sunscreens which are funded by the NHS for this purpose. At each clinic appointment the patients are asked what photoprotection measures they are taking in detail by the clinical team, who discuss photoprotection and its importance in XP and explain what measures need to be taken if photoprotection needs to be improved.
Eligibility
Adults with a confirmed diagnosis of XP, no cognitive impairment and sufficient English language skills were eligible if they had suboptimal adherence to photoprotection recommendations when outdoors, as identified from data held in medical notes or by the research team from data collected during Phase I.
Hypothesis
The intervention group will have a lower mean daily UVR dose-to-face (SED) compared to the delayed intervention control group at post intervention (June–July 2018) and follow-up (August 2018).
Randomisation and masking
Participants were 1 : 1 randomised to receive XPAND in 2018 (intervention group) or in 2019 (control group). The delayed intervention control group acted as the control in the 2018 analysis of the primary outcome. Equal allocation to both groups employed a random allocation sequence for all participants together, using a computer programme with fixed block sizes of four stratified by burning type to balance those with a genetic complementation group associated with an extreme versus normal skin burning response. Group contamination between two participants from the same family was avoided by randomising related participants as a cluster. The trial statistician and the XP clinical team were blinded to group allocation.
Procedure
Participants completed baseline assessments for 21 days in April 2018 (t0), which were repeated for 21 days in June–July 2018 (t1) after the intervention or control, and after an XPAND booster session in August 2018 (t2). This involved completion of a daily photoprotection diary with daily ratings of psychological. Participants wore the UVR dosimeter on the wrist (SunSaver 3, Bispebjerg Hospital, Copenhagen, Denmark) continuously from the start of the first assessment period (t0) until the end of the August assessment period (t2). Participants completed additional self-report measures at the start of each 21-day period and 6 months after XPAND delivery in December 2018 (t3). The delayed intervention control group followed a similar protocol of assessments and measurements in 2019, when they were treated with XPAND [dosimetry and daily UVR protection diary in April 2019 (t4) and June–July 2019 (t5), and self-report measures in December 2019 (t6)].
Intervention participants received ‘XPAND’ over 12 weeks in spring to summer 2018. Sessions 1 and 6 were delivered in the participants’ homes; the remaining sessions were delivered via Skype. Sessions 1–4 were conducted weekly, reducing to bi-weekly for sessions 5–6; session 7 (the ‘booster’ session) was delivered following a 4- to 6-week break, during which time the post-intervention measures were taken. The same procedure was followed in spring to summer 2019 for the delayed intervention control group.
Outcome measures
Primary outcome: Average daily UVR dose to the face (dose-to-face) (SED) across 21 consecutive days between June and July 2018 (t1).
The UVR D-to-F was calculated as the product of the dose of UVR recorded at the wrist by the dosimeter, and the ‘protection factor’ of the facial photoprotection behaviours recorded in the daily UVR protection diary.
Secondary outcomes
Secondary outcomes measured daily during the reporting periods were:
-
Average daily UVR D-to-F across 21 consecutive days in August 2018 (t2).
-
Average daily total UVR exposure during t1, t2.
-
Average daily total time outside during daytime.
-
Average daily total time outside when UVR levels are highest (11 a.m. to 3 p.m.).
-
Average daily proportion of time spent outside during which face photoprotection using clothing was considered to be either ‘very good’ or ‘excellent’ (t1, t2).
-
Average daily number of times sunscreen was applied irrespective of time outside during each of the 21-day periods (t1, t2).
-
Average daily measures of psychological factors measured using single items on the UVR photoprotection diary, rated on 0–10 scale where higher scores are more favourable (t1, t2): (1) mood, (2) extent to which photoprotection activities are done without having to think about it consciously (‘automaticity’), (3) self-efficacy to manage barriers to photoprotection (‘confidence’) and (4) prioritisation of photoprotection (‘importance’).
The secondary outcomes measured on a single occasion at each assessment are:
-
Health-related quality of life using the EQ-5D-5L. 26
-
Emotional well-being measured by the Short-form Warwick Edinburgh Mental Well-Being scale (SWEMWBS). 23 Reliability in the sample was good (α = 0.75).
-
Automaticity of photoprotection activities assessed using the four-item Self-report Behavioural Automaticity Index (SRBAI),20 adapted to photoprotection (α = 0.98).
-
Self-efficacy to photoprotect assessed by a 21-item scale (photoprotection self-efficacy questionnaire; PhotoSEQ) developed for this study. This assesses includes clothing (α = 0.88) and sunscreen subscales (α = 0.93). 21
-
Photoprotection activities were measured using the brief photoprotection adherence questionnaire (BPAQ) designed for this study. This five-item scale assesses duration of time outdoors and photoprotection used when outdoors during the previous 7 days. Items of the BPAQ were analysed individually. 10
Statistical analysis
Data regarding daily UVR exposure and daily self-report assessments were analysed, following a modified intention-to-treat framework, using linear mixed-effects models with patient as a random effect to account for repeated observations within individuals. This included all daily assessments during the June 2018 and August 2018 assessment periods (maximum 42 assessments per participant). Treatment group and assessment period were included as dummy coded variables. Group by period interaction terms allowed for the estimate of the treatment effect to vary across time points. Average daily UVR dose-to-face during the baseline period, burning type (randomisation stratification factor) and daily environmental UVR from the nearest Public Health England monitoring station were included as covariates. A first-order autoregressive structure was specified to further account for anticipated relation within residuals over time. Given anticipated issues with heteroscedasticity of the residuals, heteroscedasticity robust standard errors were estimated using the Huber–White sandwich estimator. On days where either data were not available for the dosimeter or diary assessments were not reported, they were not included in the model, under the assumption that these data are missing at random. Days where the dosimeter was likely not worn, but a diary entry was completed indicating that the participant had not gone outside that day, were included in the analysis. Sensitivity analyses for the missing at random assumption for the primary outcome imputed missing days with the mean of the participants’ daily assessments across the assessment period. Given the small sample size, planned exploratory analyses were undertaken for the delayed intervention control group. This was based on comparing the June 2019 assessments for the delayed intervention control group to their June 2018 assessments using a similar analytical approach.
Fidelity
Four independent assessors used a bespoke treatment fidelity checklist to check the adherence of facilitators to the intervention manual. Forty (39%) audio tapes, (all session 1s, all session 6s and a random selection of follow-up sessions) were evaluated.
Key findings
Recruitment and attrition
Between February and March 2018, a total of 37 patients were screened and 16 (43%) consented and were randomised to receive either XPAND (N = 8) or to the delayed intervention control arm (N = 8) (Figure 8). Recruitment was lower than the target sample size (n = 24) but was deemed adequate to power the study to detect a similar reduction in SED (0.12). The Programme Steering Committee and the Trial Steering Committee were also consulted and agreed for the trial to proceed.
FIGURE 8.
Participant flow through the study.
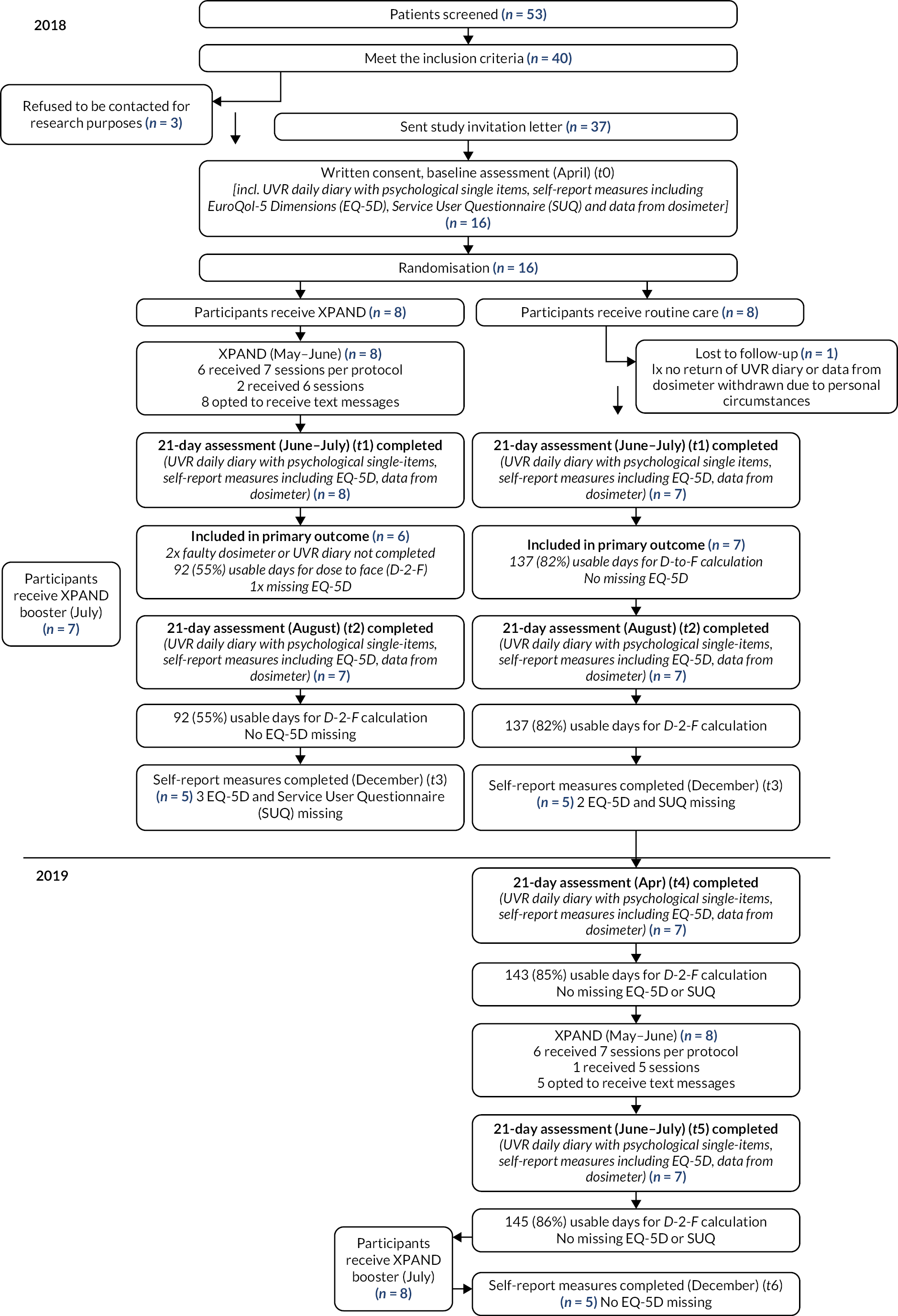
Attrition was minimal, with one participant from the delayed intervention control withdrawing after the baseline assessment. All eight patients randomised to the intervention arm received treatment. No further participants were lost to follow-up by the end of t2 (August 2019). However, dosimeter failure and non-adherence meant a further two individuals (from the intervention group) could not be included in the analyses for the primary outcome. One participant reported not wearing the dosimeter and failed to provide any diary data. The other returned the dosimeter which on inspection was found not to have been worn. For this participant, the diary data raised a number of concerns and were also excluded. Total number of days with diary completion and dosimeter worn are given in Table 11.
| Participant | Baseline | June 18 | August 18 | April 19 | June 19 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diary | Diary + UV | Diary | Diary + UV | Diary | Diary + UV | Diary | Diary + UV | Diary | Diary + UV | |
| C1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C2a | 21 | 15 | 19 | 19 | 21 | 21 | 21 | 21 | 21 | 21 |
| C3a | 21 | 19 | 21 | 21 | 21 | 21 | 20 | 20 | 21 | 21 |
| C4a | 21 | 21 | 21 | 15 | 21 | 21 | 21 | 19 | 21 | 21 |
| C5a | 21 | 21 | 21 | 19 | 21 | 20 | 21 | 21 | 21 | 21 |
| C6a | 21 | 21 | 20 | 20 | 20 | 20 | 20 | 20 | 19 | 19 |
| C7a | 21 | 21 | 21 | 21 | 20 | 20 | 21 | 21 | 21 | 21 |
| C8a | 21 | 21 | 21 | 21 | 20 | 20 | 21 | 21 | 21 | 21 |
| I1 | 0 | 0 | 21 | 0 | 20 | 0 | ||||
| I2 | 21 | 17 | 7 | 0 | 21 | 0 | ||||
| I3a | 21 | 0 | 21 | 21 | 21 | 19 | ||||
| I4a | 21 | 8 | 21 | 13 | 21 | 0 | ||||
| I5a | 14 | 20 | 21 | 0 | 21 | 21 | ||||
| I6a | 21 | 16 | 21 | 16 | 21 | 16 | ||||
| I7a | 20 | 21 | 21 | 21 | 21 | 21 | ||||
| I8a | 21 | 21 | 21 | 21 | 21 | 21 | ||||
| Median | 21 | 20 | 21 | 19 | 21 | 20 | 21 | 21 | 21 | 21 |
| Percentage completed | 85 | 72 | 89 | 68 | 93 | 72 | 86 | 85 | 86 | 86 |
The final analysis sample for the primary outcome using dosimetry data involved 13 participants, for whom a total of 492 useable days (dosimetry and daily UVR protection diary) were recorded across periods t1 and t2 (90% complete across included patients). Where analyses relied on the diary only, the analysis sample included 15 participants providing a total of 540 useable days (86% complete across included patients) (see Figure 9 for an example of dosimeter data for one participant).
FIGURE 9.
Example of dosimeter data for one participant. Note that the dosimeter was not worn on day 15.
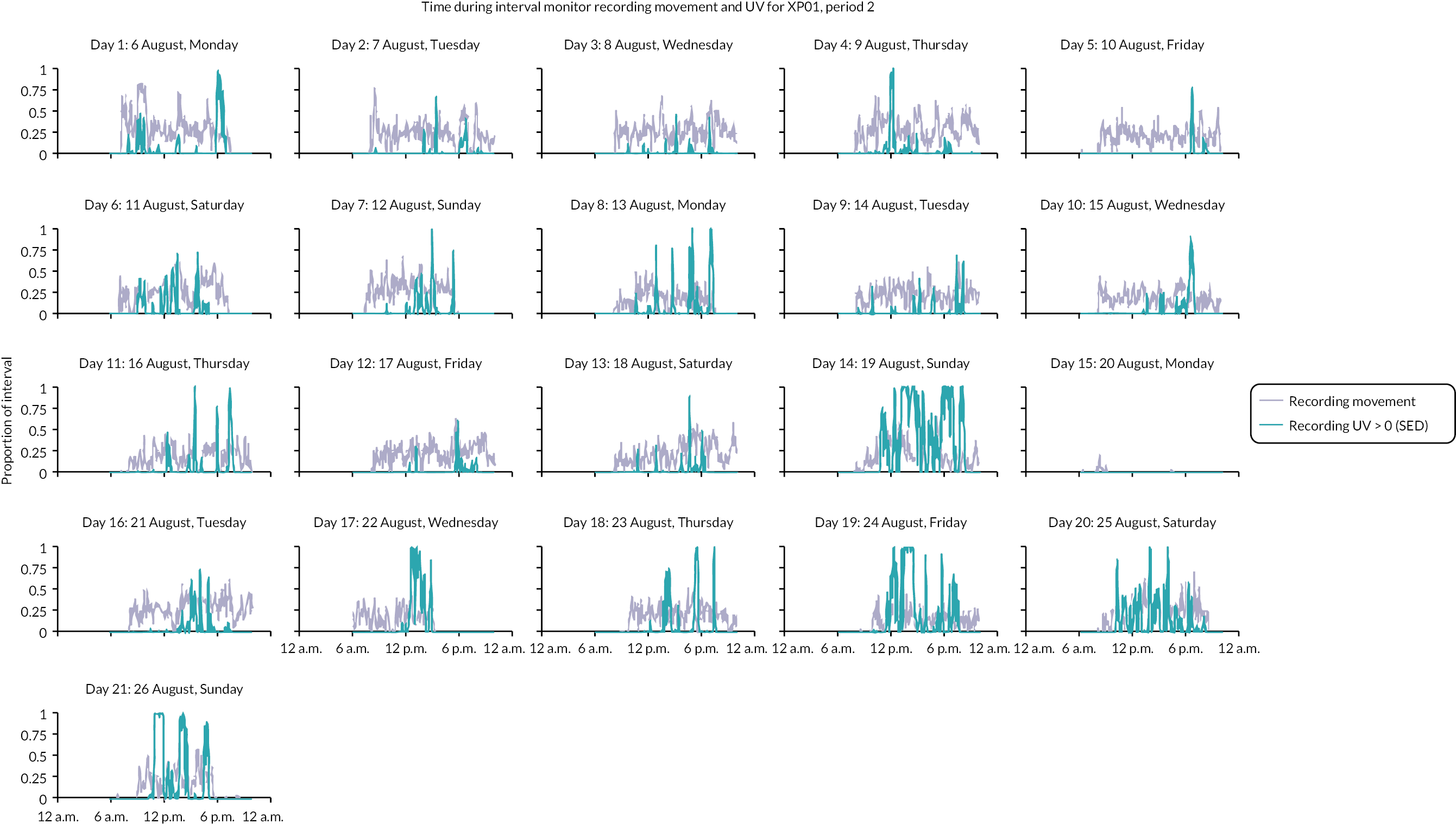
Baseline demographic and clinical characteristics of the sample by group are shown in Table 3. Randomisation did not achieve good balance between the groups on several key variables at baseline (Table 12). The intervention group tended to be younger at the time of the study, younger at diagnosis and with a broader spread of complementation groups than those randomised to the delayed intervention control group. Importantly, those in the intervention were observed to have lower SED exposure and have better photoprotection during the t0 baseline observation period. Differences were also observed in some of the psychological characteristics related to adherence to photoprotection advice: overall they described protection as more automatic, were more confident that they could achieve good protection and thought protection was more important than those in the delayed intervention control group.
| XPAND intervention arm (n = 8) | Delayed intervention control arm (n = 8) | Total | |
|---|---|---|---|
| Demographic factors | |||
| Gender, N (%) | |||
| Female | 3 (37.5) | 3 (37.5) | 6 (37.5) |
| Male | 5 (62.5) | 5 (62.5) | 10 (62.5) |
| Age, M (SD) | 39.9 (15.3) | 48.8 (15.9) | 44.3 (15.7) |
| Ethnicity, N (%) | |||
| Caucasian | 5 (62.5) | 5 (62.5) | 10 (62.5) |
| Asiana | 3 (37.5) | 3 (37.5) | 6 (37.5) |
| Clinical factors and quality of life | |||
| Self-reported age at diagnosis, M (SD) | 16.1 (17.0) | 38.1 (7.4) | 27.1 (17.0) |
| Age of lab molecular diagnosis from medical notes, M (SD) | 36.1 (14.9) | 44.5 (15.3) | 40.3 (15.2) |
| Propensity to burn, N (%) | |||
| Burner | 3 (37.5) | 3 (37.5) | 6 (37.5) |
| Non-burner | 5 (62.5) | 5 (62.5) | 10 (62.5) |
| History of previous cancer, N (%) | |||
| Yes | 5 (62.5) | 5 (62.5) | 10 (62.5) |
| No | 3 (37.5) | 3 (37.5) | 6 (37.5) |
| XP complementation group, N (%) | |||
| A | 1 (12.5) | 3 (37.5) | 4 (25.0) |
| C | 3 (37.5) | 0 | 3 (18.8) |
| E | 1 (12.5) | 2 (25.0) | 3 (18.8) |
| F | 2 (25.0) | 0 | 2 (12.5) |
| V | 1 (12.5) | 3 (37.5) | 4 (25.0) |
| Quality of life (EQ-5D-5L), M (SD) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) |
Treatment effect on primary outcome – mean daily dose-to-face (t1)
Observed means and adjusted mean differences between groups for the primary outcome are shown in Table 13 with a forest plot of standardised mean differences displayed in Figure 12. Box plots indicating observed medians and ranges of the primary outcome for each group at each period are displayed in Figures 10 and 11. Mean differences between the groups were estimated for the June–July 2018 period 201(t1) and August 2018 period (t2) using a linear mixed-effect model. This included daily UVR dose-to-face recordings across each period (i.e. a maximum of 42 observations per patient) with a random effect to account for repeated observations within participants and controlled for mean daily UVR dose to the face during the baseline reporting period, propensity to burn and total environmental UVR recording from the nearest recording station to the patients home for that day.
| Variable | Period | XPAND | Control | Adjusted mean difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | Mean diff | SE | p | 95%ll | 95%ul | Hedge’s g | ||
| Daily dose-to-face (SED) | April18 | 6 | 0.03 | 0.03 | 7 | 0.26 | 0.17 | ||||||
| June 18 | 5 | 0.03 | 0.02 | 7 | 0.43 | 0.17 | −0.25a | 0.05 | 0.000 | −0.35 | −0.15 | −2.21 | |
| August 18 | 5 | 0.04 | 0.03 | 7 | 0.33 | 0.20 | −0.20a | 0.05 | 0.000 | −0.29 | −0.10 | −1.40 | |
| April 19 | 0 | 7 | 0.14 | 0.07 | |||||||||
| June 19 | 0 | 7 | 0.41 | 0.27 | −0.05b | 0.08 | 0.542 | −0.19 | 0.10 | −0.12 | |||
| Daily total (SED) | April 18 | 6 | 0.05 | 0.05 | 7 | 0.36 | 0.21 | ||||||
| June 18 | 5 | 0.07 | 0.05 | 7 | 0.58 | 0.19 | −0.30a | 0.06 | 0.000 | −0.42 | −0.18 | −2.01 | |
| August 18 | 5 | 0.08 | 0.05 | 7 | 0.42 | 0.26 | −0.24a | 0.06 | 0.000 | −0.36 | −0.11 | −1.20 | |
| April 19 | 0 | 7 | 0.21 | 0.07 | |||||||||
| June 19 | 0 | 7 | 0.56 | 0.32 | −0.05b | 0.09 | 0.588 | −0.22 | 0.13 | −0.10 | |||
| Daily minutes outside (daylight hours) | April 18 | 6 | 75.95 | 42.93 | 7 | 356.43 | 163.48 | ||||||
| June 18 | 5 | 105.57 | 65.83 | 7 | 274.41 | 150.86 | −51.11a | 81.03 | 0.528 | −209.92 | 107.70 | 0.33 | |
| August 18 | 5 | 123.57 | 38.28 | 7 | 280.20 | 177.17 | −43.52a | 73.90 | 0.556 | −88.36 | 101.311 | 0.32 | |
| April 19 | 0 | 7 | 237.65 | 124.03 | |||||||||
| June 19 | 0 | 7 | 227.65 | 131.92 | −69.90b | 48.31 | 0.148 | −164.59 | 24.78 | −0.28 | |||
| Daily high-risk minutes outside (11 a.m.–3 p.m.) | April 18 | 6 | 27.26 | 15.65 | 7 | 127.52 | 68.92 | ||||||
| June 18 | 5 | 21.86 | 18.84 | 7 | 71.29 | 45.18 | −23.80a | 23.15 | 0.304 | −69.18 | 21.58 | −0.53 | |
| August 18 | 5 | 41.05 | 14.05 | 7 | 87.82 | 66.20 | −27.34a | 24.54 | 0.265 | −75.43 | 20.75 | −0.54 | |
| April 19 | 0 | 7 | 76.43 | 49.57 | |||||||||
| June 19 | 0 | 7 | 68.50 | 48.06 | −21.32b | 16.26 | 0.190 | −53.19 | 10.54 | −0.20 | |||
| Daily proportion time outside photoprotection very good/excellent | April 18 | 6 | 0.68 | 0.32 | 7 | 0.29 | 0.41 | ||||||
| June 18 | 6 | 0.67 | 0.38 | 7 | 0.34 | 0.35 | 0.06a | 0.11 | 0.546 | −0.27 | 0.15 | 0.11 | |
| August 18 | 8 | 0.68 | 0.34 | 7 | 0.34 | 0.43 | 0.01a | 0.10 | 0.897 | −0.21 | 0.18 | 0.02 | |
| April 19 | 0 | 7 | 0.31 | 0.44 | |||||||||
| June 19 | 0 | 7 | 0.61 | 0.37 | 0.23b | 0.13 | 0.065 | −0.01 | 0.48 | 0.45 | |||
| Daily number times sunscreen applied | April 18 | 6 | 1.03 | 0.51 | 7 | 1.32 | 0.37 | ||||||
| June 18 | 7 | 0.98 | 0.65 | 7 | 1.40 | 0.56 | −0.33a | 0.27 | 0.213 | −0.86 | 0.19 | −0.34 | |
| August 18 | 8 | 1.04 | 0.62 | 7 | 1.24 | 0.24 | −0.18a | 0.21 | 0.389 | −0.59 | 0.23 | −0.23 | |
| April 19 | 0 | 7 | 1.04 | 0.46 | |||||||||
| June 19 | 0 | 7 | 1.66 | 0.54 | 0.27b | 0.20 | 0.161 | −0.11 | 0.66 | 0.28 | |||
| Mood | April 18 | 6 | 7.81 | 0.89 | 7 | 6.77 | 1.47 | ||||||
| June 18 | 7 | 8.47 | 1.47 | 7 | 7.23 | 1.40 | 0.20a | 0.49 | 0.686 | −0.77 | 1.17 | 0.09 | |
| August 18 | 8 | 8.38 | 1.50 | 7 | 7.38 | 1.33 | 0.01a | 0.56 | 0.984 | −1.09 | 1.11 | 0.00 | |
| April 19 | 0 | 7 | 6.88 | 1.78 | |||||||||
| June 19 | 0 | 7 | 8.23 | 1.21 | 0.80b | 0.28 | 0.005 | 0.25 | 1.35 | 0.40 | |||
| Automaticity of protection | April 18 | 6 | 8.29 | 1.36 | 7 | 6.30 | 1.97 | ||||||
| June 18 | 7 | 8.07 | 2.62 | 7 | 6.86 | 2.01 | −0.93a | 0.95 | 0.329 | −2.79 | 0.94 | −0.24 | |
| August 18 | 8 | 7.51 | 3.28 | 7 | 7.18 | 1.89 | −1.71a | 1.13 | 0.130 | −3.94 | 0.51 | −0.38 | |
| April 19 | 0 | 7 | 6.51 | 2.60 | |||||||||
| June 19 | 0 | 7 | 7.88 | 1.58 | 0.55b | 0.18 | 0.003 | 0.19 | 0.90 | 0.21 | |||
| Confidence in protection | April 18 | 6 | 7.21 | 3.07 | 7 | 6.21 | 1.83 | ||||||
| June 18 | 7 | 8.36 | 1.93 | 7 | 6.86 | 1.95 | 0.76a | 0.56 | 0.175 | −0.34 | 1.86 | 0.25 | |
| August 18 | 8 | 8.11 | 2.19 | 7 | 7.21 | 1.81 | 0.12a | 0.47 | 0.790 | −0.80 | 1.04 | 0.04 | |
| April 19 | 0 | 7 | 6.42 | 2.43 | |||||||||
| June 19 | 0 | 7 | 7.99 | 1.29 | 0.58b | 0.28 | 0.041 | 0.02 | 1.13 | 0.23 | |||
| Importance of protection | April 18 | 6 | 9.06 | 0.77 | 7 | 6.88 | 1.87 | ||||||
| June 18 | 7 | 8.82 | 1.64 | 7 | 7.11 | 1.87 | −0.24a | 0.69 | 0.727 | −1.59 | 1.11 | −0.09 | |
| August 18 | 8 | 8.60 | 1.80 | 7 | 7.40 | 1.71 | −0.65a | 0.77 | 0.395 | −2.15 | 0.85 | −0.23 | |
| April19 | 0 | 7 | 6.57 | 2.52 | |||||||||
| June 19 | 0 | 7 | 8.27 | 1.42 | 0.78b | 0.28 | 0.006 | 0.22 | 1.33 | 0.32 | |||
FIGURE 10.
Box plots indicating distribution of primary and secondary photoprotection outcomes for each group at each time point.
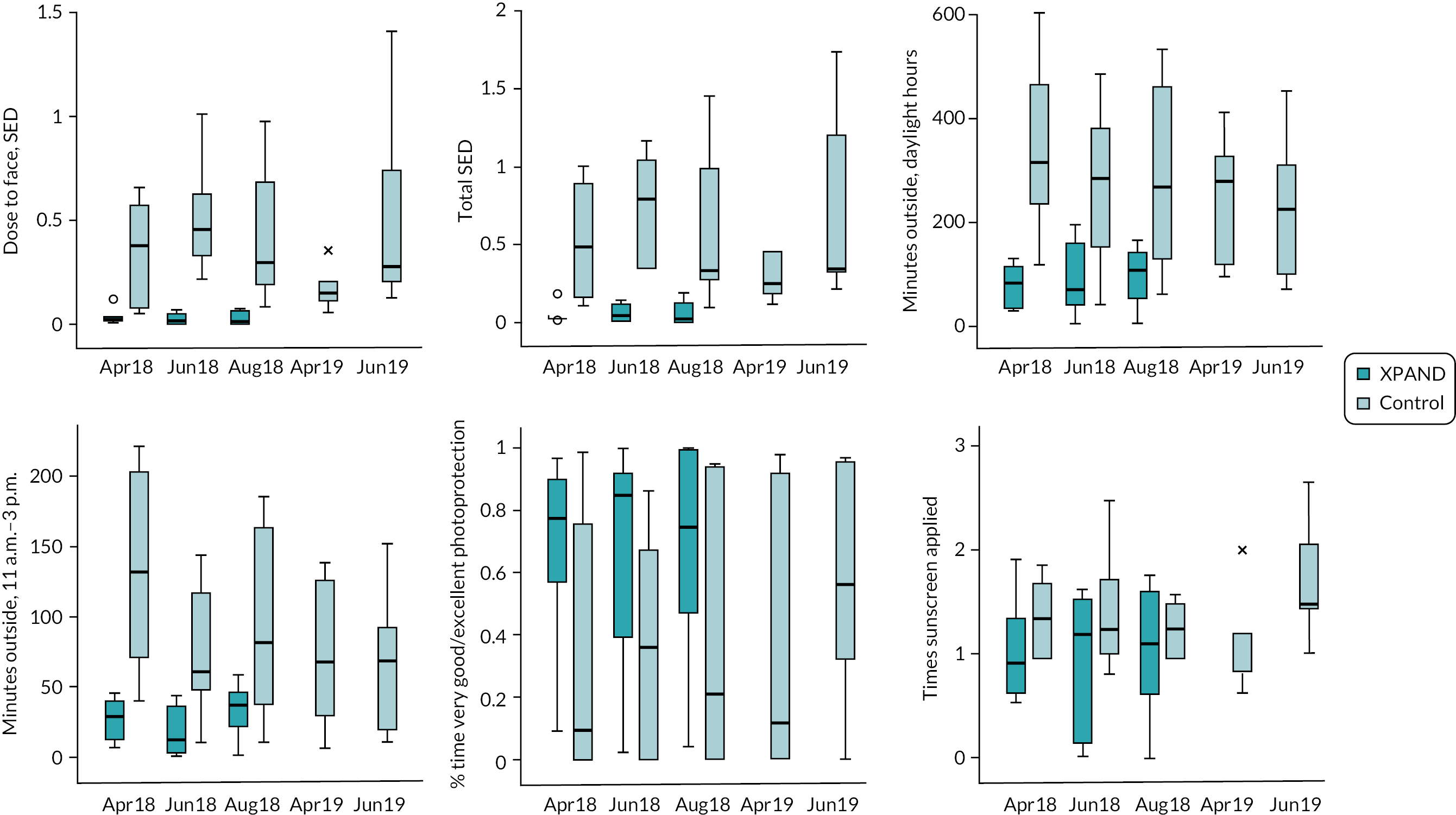
FIGURE 11.
Box plots indicating distribution of secondary psychological outcomes measured daily for each group at each time point.
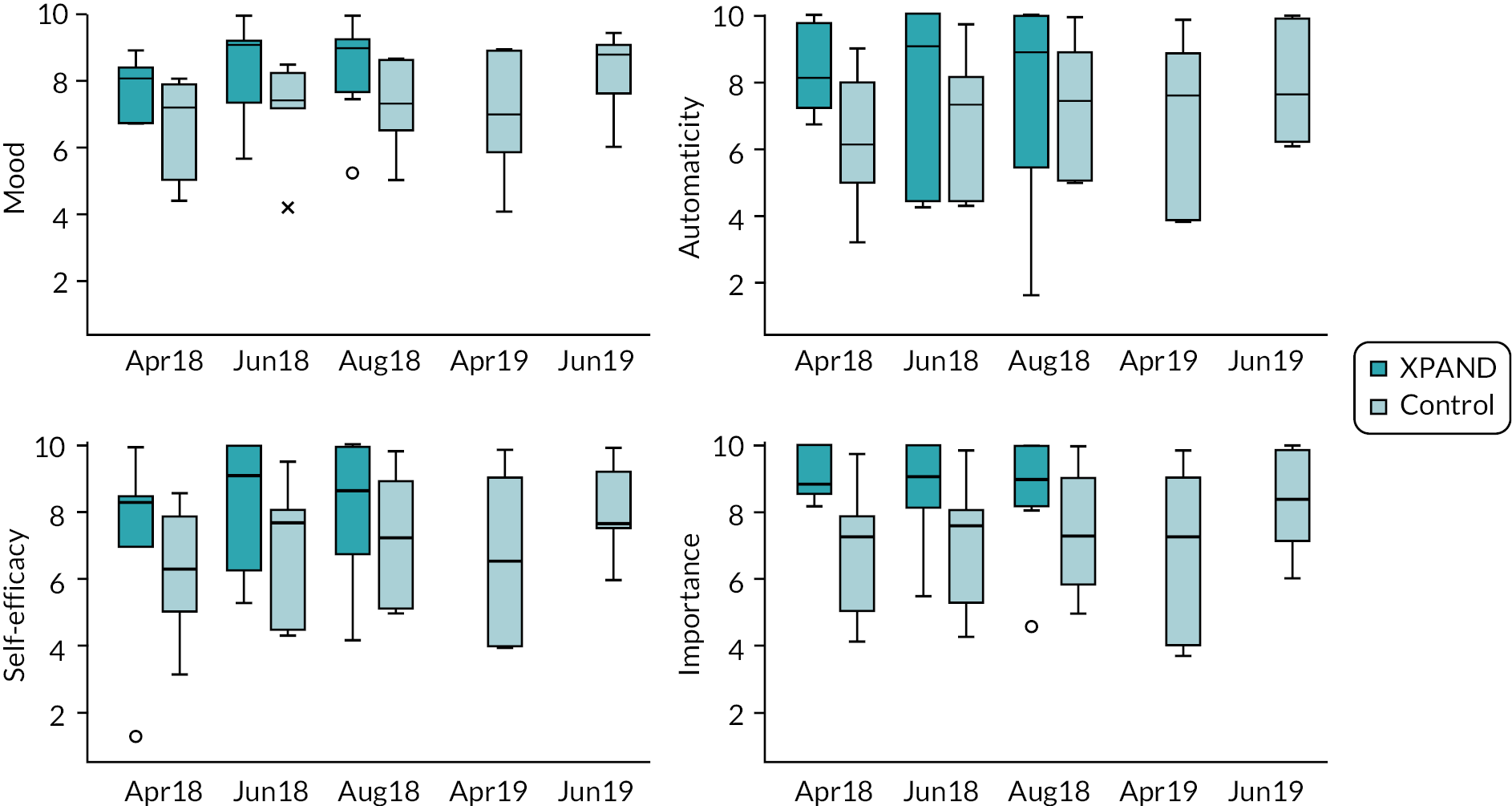
FIGURE 12.
Adjusted mean daily dose-to-face (SED) for the (primary outcome) at assessment t1 and t2, with 95% confidence interval.
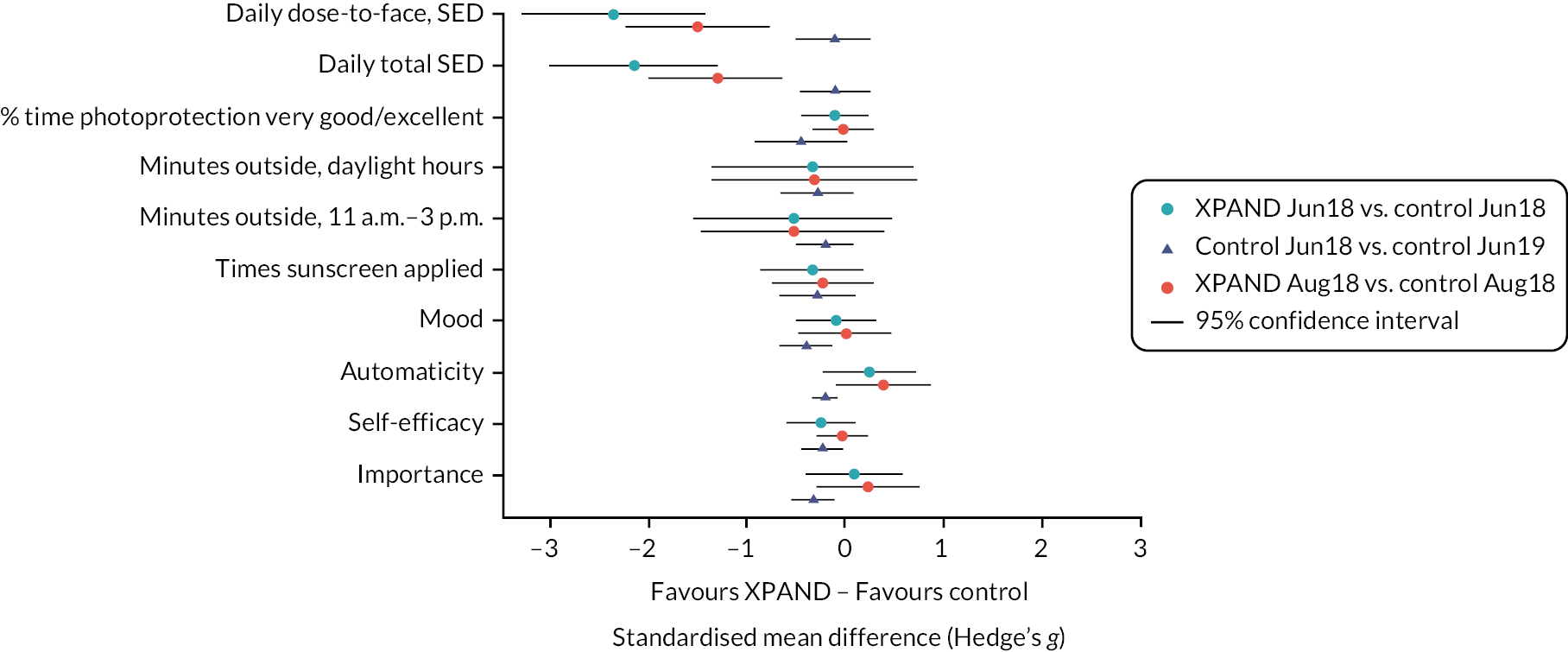
Based on 492 daily observations from 13 of the 16 randomised patients, the XPAND intervention group had significantly lower mean daily UVR dose-to-face (M = 0.03, SD = 0.02) compared to the control group at the June 2018 (primary outcome) post-intervention assessment (n = 7; M = 0.36, SD = 0.16; adjusted difference = −0.25 SED, p < 0.001). The effect size was very large with those in the control group receiving a mean daily UVR dose to the face an order of magnitude higher than the control group (Hedge’s g = 2.2). This difference was maintained at the August 2018 follow-up (intervention: M = 0.04, SD = 0.03; control: M = 0.28, SE = 0.08; adjusted difference = −0.19 SED, p < 0.001; g = −1.4). Given that large differences in the mean daily UVR dose-to-face were observed in the baseline reporting period, there is a concern that, even controlling for this imbalance between groups, the differences observed may be an overestimate of the true treatment effect.
Treatment effect on secondary outcomes
A linear mixed-effects model indicated total UVR exposure was also lower in the intervention group at the June 2018 post-intervention assessment (intervention: M = 0.07, SD = 0.05; control: M = 0.58, SD = 0.19; adjusted difference = −0.30 SED, p < 0.001) and at the August 2018 follow-up (intervention: M = 0.08, SD = 0.05; control: M = 0.42, SD = 0.26; adjusted difference = −0.30, p < 0.001). Again, baseline imbalances in total UVR exposure led to concerns that the differences observed are an overestimate of the treatment effect.
Based on the same data using 492 observations from 13 of the 16 randomised patients, there were no significant differences between the two groups in the time spent outside during the daytime, time outside during the highest risk period or percentage of outdoor time better protected by clothing, with effect sizes tending to be small to medium and favouring the intervention.
Based on 540 daily observations from the diary only for 15 of the 16 randomised patients (Table 13), there were also no significant differences observed for average daily frequency of sunscreen application, and average daily ratings of confidence in the ability to photoprotect, automaticity of photoprotection and perceived importance of photoprotection, or mood, with effect sizes tending to be small but favouring the intervention (see Table 13 and Figure 12).
Additional analyses were undertaken considering patient-reported outcomes using standardised scales completed once at the end of each reporting period. These analyses used linear mixed-effects models including 29 observations for 15 of the 16 randomised patients from the June and August 2018 reporting periods. Observed means and estimated mean differences are shown in Table 14. Due to the small sample size, to maximise power, the adjusted difference was estimated combining across both these reporting periods. The differences observed between groups for either of the quality-of-life (QoL) measures, self-efficacy for photoprotection and automaticity of photoprotection were small and non-significant, except for self-efficacy for applying sunscreen. Differences for the adherence subscales ranged from small to medium in favour of the intervention but again were only significant for the item regarding adherence to sunscreen application.
| Variable | Time | XPAND | Control | Adjusted mean difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | Difference | SE | p | SMD | ||
| Quality of life (EQ-5D-5L) | t0 | 8 | 0.8 | 0.09 | 8 | 0.9 | 0.14 | ||||
| t1 | 8 | 0.9 | 0.11 | 6 | 0.9 | 0.11 | 0.004a | 0.04 | 0.920 | 0.03 | |
| t2 | 8 | 0.9 | 0.12 | 7 | 0.9 | 0.12 | |||||
| Emotional well-being (SWEMWBS metric) | t0 | 8 | 25.0 | 3.50 | 8 | 24.9 | 3.76 | ||||
| t1 | 8 | 23.7 | 3.47 | 6 | 23.1 | 1.66 | −0.02a | 0.93 | 0.980 | −0.01 | |
| t2 | 8 | 23.7 | 3.48 | 7 | 23.9 | 4.23 | |||||
| Automaticity of photoprotection activities (SRBAI) | t0 | 8 | 4.8 | 2.34 | 8 | 4.0 | 2.22 | ||||
| t1 | 8 | 5.4 | 1.67 | 6 | 5.0 | 1.58 | −0.31a | 0.21 | 0.143 | −0.14 | |
| t2 | 8 | 5.1 | 1.92 | 7 | 5.3 | 1.88 | |||||
| Self-efficacy to photoprotect (PhotoSEQ) | |||||||||||
| Self-efficacy to photoprotect using clothing (PhotoSEQ clothing subscale) |
t0 | 8 | 6.7 | 1.62 | 8 | 6.6 | 1.88 | ||||
| t1 | 8 | 7.5 | 1.80 | 6 | 6.7 | 2.27 | −0.09a | 0.30 | 0.762 | −0.05 | |
| t2 | 8 | 7.4 | 1.95 | 7 | 7.1 | 1.85 | |||||
| Self-efficacy to photoprotect using sunscreen (PhotoSEQ sunscreen subscale) |
t0 | 8 | 6.4 | 2.48 | 8 | 6.4 | 2.22 | ||||
| t1 | 8 | 8.3 | 2.28 | 6 | 7.3 | 1.91 | −0.46a | 0.20 | 0.019 | −0.20 | |
| t2 | 8 | 7.8 | 2.53 | 7 | 7.8 | 1.61 | |||||
| Photoprotection adherence (BPAQ) | |||||||||||
| Photoprotection adherence (BPAQ): Item 1- When you went outside, how often did you protect your face against UVR using protective clothing? | t0 | 8 | 5.5 | 3.30 | 8 | 7.4 | 2.97 | ||||
| t1 | 8 | 7.6 | 3.38 | 6 | 7.7 | 2.25 | −0.38a | 0.71 | 0.596 | −0.12 | |
| t2 | 8 | 7.3 | 3.77 | 7 | 6.7 | 3.77 | |||||
| Photoprotection adherence (BPAQ): Item 2- How many days did you apply sunscreen on your face when getting ready in the morning? | t0 | 8 | 5.0 | 2.45 | 8 | 4.8 | 2.55 | ||||
| t1 | 8 | 6.0 | 1.77 | 6 | 6.3 | 1.03 | −0.38a | 0.36 | 0.302 | −0.16 | |
| t2 | 8 | 5.6 | 2.45 | 7 | 6.3 | 0.95 | |||||
| Photoprotection adherence (BPAQ): Item 3- When you went outside for longer periods, how often did you reapply sunscreen on your face? | t0 | 8 | 3.1 | 3.40 | 8 | 5.3 | 3.41 | ||||
| t1 | 8 | 6.8 | 3.73 | 6 | 5.2 | 4.12 | −1.25a | 0.54 | 0.021 | −0.36 | |
| t2 | 8 | 5.5 | 3.59 | 7 | 5.4 | 3.74 | |||||
| Photoprotection adherence (BPAQ): Item 4- On average, how many hours did you spend outside per day between 11 a.m. and 3 p.m.? | t0 | 8 | 11.0 | 13.42 | 8 | 14.4 | 12.00 | ||||
| t1 | 8 | 16.1 | 14.25 | 7 | 17.8 | 13.38 | −7.13a | 4.32 | 0.099 | −0.57 | |
| t2 | 8 | 10.6 | 12.65 | 7 | 19.4 | 13.87 | |||||
| Photoprotection adherence (BPAQ): Item 5- On average, how many hours did you spend outside per day between 7 a.m. and 7 p.m.? | t0 | 8 | 17.2 | 10.74 | 6 | 24.9 | 14.44 | ||||
| t1 | 7 | 19.1 | 9.90 | 7 | 26.4 | 13.56 | −8.46a | 4.62 | 0.067 | −0.67 | |
| t2 | 8 | 16.8 | 14.44 | 6 | 25.7 | 11.11 | |||||
Planned exploratory delayed intervention control group outcomes
Differences between the June 2018 (t1) and June 2019 (t4) periods for the delayed intervention control group were estimated using linear mixed-effects models including observations from all periods for this groups with a random effect to account for repeated observations within periods. The difference between the model expected means for each period was then estimated using the delta method to calculate the standard error of the difference and thus 95% CIs and p-values. Although effect sizes generally favoured the intervention, no statistically significant differences were observed between the two periods for the delayed intervention group for the data collection using the combination of dosimeter and diary. However, statistically significant differences were observed in favour of the intervention for daily self-reported ratings of mood, automaticity of photoprotection, confidence in the ability to photoprotect and the importance of photoprotection, with effect sizes observed to be small to medium.
Fidelity
Adherence to the XPAND intervention by the therapists was high, with 85% treatment fidelity achieved across sessions (S) (S1 92%, 95% CI 90 to 94; S2–5 78%, 95% CI 73 to 83, S6 86%, 95% CI 83 to 90; Overall: 83%, 95% CI 81 to 85). Inter-rater agreement between the two coders rating the fidelity of delivery for each session was observed to be good (Gwet’s agreement coefficients of 0.91, 0.76, and 0.84, respectively, for each session).
Limitations
The primary limitation of the study was the failure of randomisation to balance baseline differences in dose-to-face between the intervention and control groups. The control group had significantly higher UVR dose-to-face, total UVR exposure and time spent outside (total and high risk) than the intervention group at baseline. Although statistical analysis adjusted for baseline levels, the difference was too large to ascertain the true treatment effect size. Failure of randomisation in small samples is a known pitfall of rare disease trial design. 36
Conclusions
-
Rigorous photoprotection is the only means of preventing skin and eye cancers and increasing lifespan in XP. Participants who received XPAND, a theory-based complex intervention targeting personalised psychological determinants of poor photoprotection, had a significantly lower UVR D-to-F and lower exposure to UVR compared to controls. The size of the effect was large, indicating tangible clinical benefits associated with less DNA damage, although interpretation requires caution due to the failure of randomisation to balance baseline group differences. There was no difference between the groups on secondary outcome measures of mood and ratings of photoprotection, automaticity, confidence and importance. The planned exploratory analysis within the delayed intervention control group gives us some reassurance that XPAND was effective, as it showed improvement in photoprotection activities when outdoors and in the psychological process variables.
-
Receipt of XPAND improved photoprotection without diminishing emotional well-being. Moreover, the delayed intervention group reported an improvement in daily ratings of mood after completing the programme. This outcome was of significance as lowering mood was considered to be a potential adverse event of XPAND.
-
Improvements in perceptions of importance, self-efficacy and automaticity were associated with receipt of XPAND (albeit in the delayed intervention group only), suggesting that these variables are potential mechanisms of change underlying improvements in photoprotection.
-
We recommend testing XPAND in an international XP population or another more common at-risk group and extending the duration of follow-up to investigate maintenance of improvements.
Workstream 4b: process evaluation
The results of the XPAND process evaluation has been published in Walburn et al. (see Publications).
Following best practice guidelines for the testing of complex interventions,37 we conducted a qualitative process evaluation alongside a RCT to evaluate the impact of XPAND on adherence to photoprotection. 10
Objectives
-
To explore the acceptability of XPAND to patients.
-
To explore the influence on changes in photoprotection behaviours among people with XP.
Methods
Semistructured interviews were conducted with each intervention participant following delivery of XPAND in 2018 (immediate group) or in 2019 (delayed group). Interviews were conducted by a health psychologist and research nurse, both of whom were involved in intervention design and delivery. Interviewers did not work with participants to whom they had delivered the intervention. A topic guide explored acceptability, changes in photoprotection and reported influences on behavioural changes.
Interviews were recorded and transcribed verbatim. The analysis was based on a framework approach38 with an initial thematic analysis followed by a constant comparative approach based on mapping and charting to identify patterns and relationships.
Results
We interviewed nine male and six female participants from white (n = 10) and Asian (n = 5) backgrounds. Three were aged in their 20s, eight in their 30s and 40s, and five were aged between 50 and 73 years. The length of time they had lived with XP varied; five were diagnosed as children and others as adults. Twelve participants had experienced skin cancers.
Acceptability of XPAND
Participants were generally very positive about both the content and delivery of the intervention with the quality of the separate components of the intervention being well rated in terms of both the delivery and content. These included the XPAND magazine, sunscreen application video, text messages and other tools (e.g. UVR dial). Participants viewed the texts messages as informative, enjoyable and supportive. Although the magazine was liked by participants, few read it in its entirety. The video was the least personalised part of XPAND and, although helpful to some, it was viewed as less useful for those who had lived with XP for years.
The one-to-one sessions were the central and most personalised component of XPAND and were both enjoyed and regarded as particularly supportive. Patients mainly preferred the face-to-face sessions compared with the online sessions, as they felt it was easier to connect with the facilitator, although the mix provided was acceptable. The content of sessions was generally viewed very favourably as participants liked interacting with the facilitator and identified many aspects of personal relevance, including understanding more about UVR, being assisted with developing habits and using advance planning, and they felt that they had gained greater confidence and control. Responses to the value-based ‘carrot and stick’ tool were however varied with younger people often describing this very positively whereas some older participants felt this was childlike and unnecessary.
Changes in photoprotection behaviours
Every participant reported improvements to at least one face photoprotection activity and almost two-thirds (9/15) reported positive change for multiple photoprotection (> 3) activities. Individuals were most successful in making changes to sunscreen application whereas wearing the face buff was particularly challenging. Few participants reduced the duration of time outdoors or altered when they went outside.
Influences on photoprotection behaviours
Changes involved influences grouped into those increasing motivation; increasing confidence in managing psychosocial barriers; and enhancing enactment of photoprotection which mainly occurred through the development of habit. The participants who achieved change in at least three photoprotection activities described influences across each of the three categories, although the specific influences and modes of delivery perceived as most effective varied between individuals and in relation to the type of activity.
-
Sunscreen application, hats and glasses: Increased automaticity and habit was the main reason for improvement in sunscreen application. This was due to content in the magazine, sessions and text messages. Other influences were messages that challenged their misconceptions about the nature of UVR.
-
Face buff: Greater confidence to overcome the psychosocial barrier of worry about looking different, addressed during the sessions, influenced change in the face buff.
-
Time and duration outdoors: XPAND was least successful in changing this behaviour, as participants felt it was beyond their control. Where it was helpful, this was attributed to strategies to enhance communication skills and disclosure.
Using SMART goals, general confidence and feeling more at ease with living with XP were cited as reasons for positive change across photoprotection activities.
Limitations
The study was cross-sectional, and we therefore do not know if the improvements in photoprotection were sustained.
Conclusions
The positive findings of behavioural changes support the trial outcomes and suggest that the intervention could be successfully implemented for other groups at high risk of the effects of UVR. Results highlighted the importance of a complex intervention with various modes of delivery presenting content in different formats. People responded differently to the same intervention content, and dynamic tailoring of content to individual participants in the context of their own lives, priorities and circumstances was a key influence on engagement and feelings of support. These results provide detailed insight into the likely change mechanisms of XPAND and justify its planned clinical rollout within the National Xeroderma Pigmentosum Service.
Workstream 5: economic analysis of the intervention
Objectives
-
To compare the service use and costs for those receiving the XPAND intervention with those on the waitlist control.
-
To compare the cost-effectiveness of the two groups.
-
To model the long-term cost-effectiveness of the intervention.
Methods
A health and social care perspective was adopted. Intervention costs were calculated based on therapist time and unit costs of psychologists and nurses. Other service use was measured using CSRI25 which recorded contacts with health and social care services over the previous 6 months. Costs of visits to the XP clinic at St Thomas’ Hospital were based on budget data for the clinic with one daylong attendance including as required the six specialist appointments, skin surgery and any other required skin or eye surgical interventions – investigations were estimated to cost £7721. Other costs were calculated by combining the service use data with appropriate unit cost information.
Quality-adjusted life-years (QALYs) accrued over the period from baseline to t3 were derived from the EQ-5D-5L26 combined with appropriate tariffs and using area under the curve methods. 39,40 An incremental cost-effectiveness ratio (ICER) was computed and compared to the National Institute for Health and Care Excellence (NICE) threshold of £20,000–30,000 per QALY. Cost and QALY differences were analysed using regression model adjusting for baseline values and with CIs calculated using non-parametric bootstrapping.
This trial does not measure the longer-term impacts of reduced UVR exposure as a result of the intervention. However, if reduced exposure is assumed to lead to fewer cases of skin cancer, then we would expect there to be economic consequences. To investigate this, a simple decision model was produced using the probability of patients achieving photoprotection following the intervention or control and then the likelihood of cancer occurring (Figure 13). The model inputs are provided in Table 15.
| Parameter | Mean | Source |
|---|---|---|
| Probabilities | ||
| Intervention | ||
| Patient achieves photoprotection | 0.78 | Trial results (Workstream 5) |
| Photoprotection is not achieved | 0.22 | Trial results (Workstream 5) |
| Usual care | ||
| Patient achieves photoprotection | 0.47 | Trial results (Workstream 5) |
| Photoprotection is not achieved | 0.53 | Trial results (Workstream 5) |
| Patient develops cancer having achieved photoprotection | Increasing to 0.25 by year 15 | Assumption |
| Patient develops cancer without photoprotection | Increasing to 0.5 by year 15 | Assumption |
| Costs | ||
| Intervention | 1200 | Trial results (Workstream 5) |
| NHS/Personal Social Services | 1758 | Workstream 2 |
| Photoprotection private costs | 167 | Workstream 2 |
| Non-photoprotection private costs | £42 | One quarter of above |
| Skin cancer | 1736 | Vallejo-Torres et al. (2014)41 |
| Utilities | ||
| Cancer | 0.71 | Derived from Philipp-Dormston et al. (2018)42 and Beusterien et al. (2009),43 adjusting for population norms |
| Cancer free | 0.75 | Survey data |
| Time horizon | 15 years | |
| Discount rate for costs | 3.5% | |
| Discount rate for utilities | 3.5% | |
FIGURE 13.
Decision tree structure.
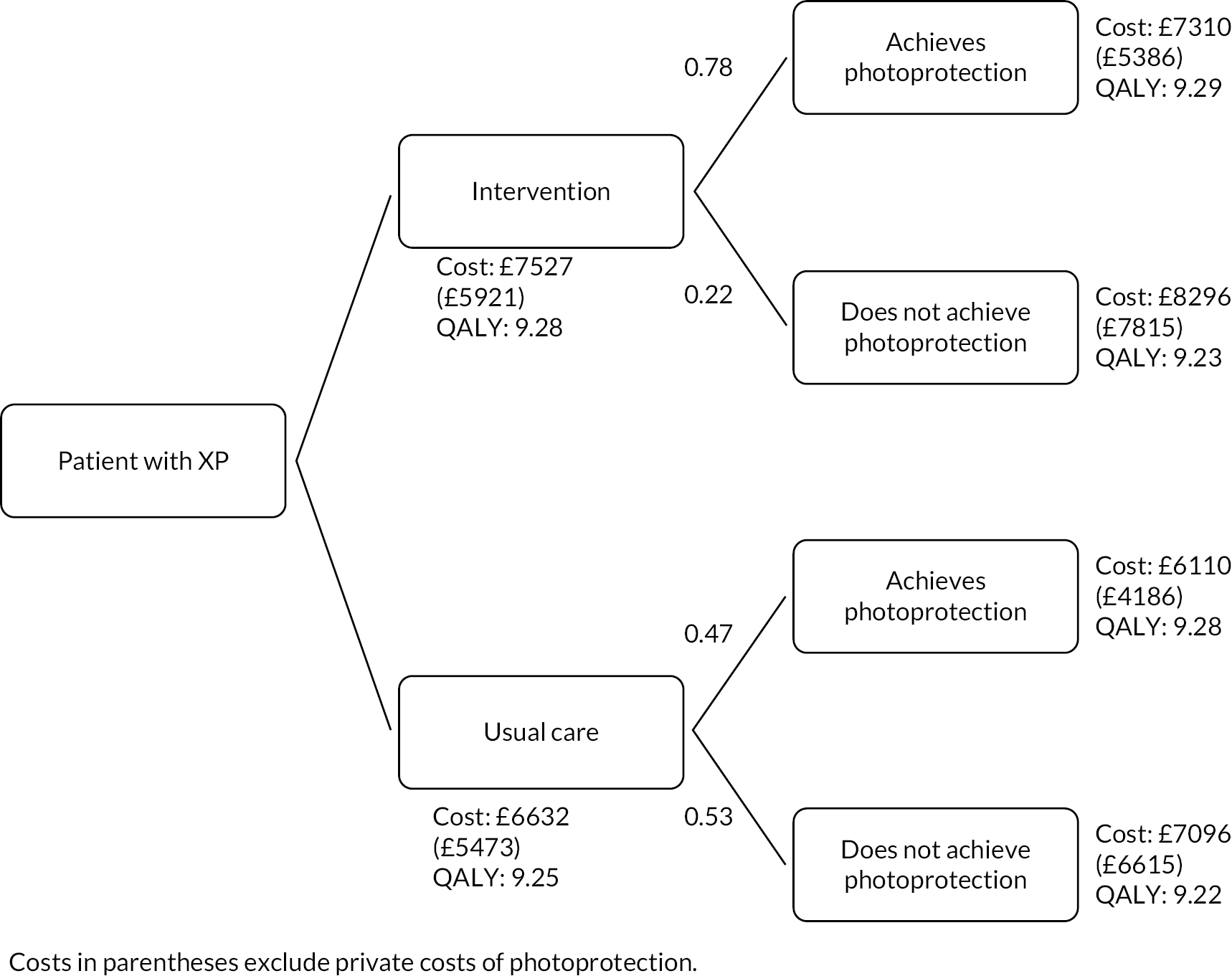
The probability of achieving photoprotection was taken from the results of the trial. The probability that the patient develops cancer having achieved photoprotection was based on clinical expertise within the research team that the use of photoprotection would reduce the incidence of skin cancer by 50% by year 15. The probability that the patient develops cancer having not achieved photoprotection was assumed to be 0.5% at year 15 for without photoprotection, following incremental probabilities of 0.033, and 0.25 at year 15 with photoprotection, following incremental probabilities of 0.0165.
Key findings
The use of services at baseline and follow-up in both groups is shown in Table 16. At baseline, the delayed intervention group made more use of the XP clinic, and this has a major impact on costs. This continued at follow-up. Services contacts outside of the XP clinic were less common. At baseline, the intervention group had costs that were on average £4924 lower than for TAU (95% CI −£13,485 to £3938). The intervention was estimated to cost £1200 per participant. Total mean health and social care costs during follow-up in the intervention group were £3715, and in the control group they were £8072. After controlling for baseline, the intervention group had costs that were £2642 less than the control group (95% CI −£8715 to £3873).
| t0 (baseline) (n = 16) | t3 (9 months after baseline) (n = 16) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 intervention (n = 8) | Group 2 (delayed intervention control) (n = 8) | Group 1 intervention (n = 8) | Group 2 (delayed intervention control) (n = 8) | |||||||||
| N (%) using | Mean contactsa | Mean costs (SD)b | N (%) using | Mean contacts | Mean costs (SD) | N (%) using | Mean contacts | Mean costs (SD) | N (%) using | Mean contacts | Mean costs (SD) | |
| Visit(s) to the XP clinic | 3 (38) | 1.67 (0.58) | 4825.63 (7073.40) | 6 (75) | 1.5 (0.84) | 8686.13 (7651.75) | 2 (25) | 1.0 (0.0) | 1930.25 (3574.13) | 5 (63) | 1.2 (0.4) | 5790.75 (559.57) |
| Did you have to spend the night away from home in order to visit the clinic? | ||||||||||||
| Yes, I stayed in hospital | 0 (0) | – | – | 0 (0) | – | – | 0 (0) | – | – | 0 (0) | – | – |
| Yes, but not in hospital | 0 (0) | – | – | 4 (50) | – | – | 0 (0) | – | – | 2 (25) | – | – |
| No | 8 (100) | – | – | 4 (50) | – | – | 8 (100) | – | – | 6 (75) | – | – |
| Which specialists do you recall seeing on your visit(s) to the XP clinic? | ||||||||||||
| Dermatologist | 3 (38) | 1.7 (0.6) | – | 6 (75) | 1.5 (0.8) | – | 2 (25) | 1.0 (0.0) | – | 5 (63) | 1.2 (0.5) | – |
| Ophthalmologist | 2 (25) | 1.5 (0.7) | – | 6 (75) | 1.5 (0.8) | – | 2 (25) | 1.0 (0.0) | – | 5 (63) | 1.2 (0.5) | – |
| Neurologist | 2 (25) | 1.5 (0.7) | – | 4 (50) | 1.8 (1.0) | – | 1 (13) | 1.0 (–) | – | 4 (50) | 1.3 (0.5) | – |
| Geneticist | 2 (25) | 1.5 (0.7) | – | 1 (13) | 1.0 (–) | – | 0 (0) | 0 (0) | – | 1 (13) | 1.0 (–) | – |
| Psychologist | 3 (38) | 1.7 (0.6) | – | 6 (75) | 1.5 (0.8) | – | 2 (25) | 1.0 (–) | – | 5 (63) | 1.2 (0.5) | – |
| Specialist nurse | 3 (38) | 1.7 (0.6) | – | 3 (38) | 2.0 (1.0) | – | 2 (25) | 1.0 (–) | – | 4 (50) | 1.3 (0.5) | – |
| Plastic surgeon | 2 (25) | 2.0 (0.0) | – | 1 (13) | 2.0 (–) | – | 0 (0) | 0.0 (0.0) | – | 2 (25) | 1.5 (0.7) | – |
| Hospital outpatient appointments | ||||||||||||
| Dermatologist | 1 (13) | 1.0 (–) | 13.91 (39.34) | 4 (50) | 1.67 (1.15) | 69.54 (118.01) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 4 (50) | 2.0 (1.15) | 111.26 (145.67) |
| Ophthalmologist | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 2.0 (–) | 24.48 (69.25) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 12.24 (34.63) |
| Neurologist | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 20.90 (59.12) |
| Geneticist | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Psychologist | 1 (13) | 1.0 (–) | 6.63 (18.74) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 6.63 (18.74) |
| Plastic surgeon | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Accident and Emergency | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 15.0 (42.43) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Surgery | ||||||||||||
| Biopsy | 2 (25) | 1.0 (0.0) | 167.5 (310.15) | 3 (38) | 2.67 (1.53) | 670 (1074.4) | 1 (13) | 1.0 (–) | 83.75 (236.88) | 3 (38) | 3.33 (0.58) | 837.50 (1174.21) |
| Skin | 3 (38) | 1.0 (0.0) | 251.25 (346.76) | 2 (25) | 3.5 (2.12) | 586.25 (1211.17) | 2 (25) | 1.0 (0) | 167.5 (310.15) | 3 (38) | 2.67 (1.15) | 670 (1012.95) |
| Eyes | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Other | 1 (13) | 1.0 (–) | 163 (461.03) | 3 (38) | 1.67 (0.58) | 815 (1194.63) | 1 (13) | 1.0 (–) | 163 (461.03) | 1 (13) | 2.0 (–) | 326 (922.07) |
| Inpatient stays (mean days) | 1 (13) | 1.0 (–) | 78.25 (221.32) | 1 (13) | 2.0 (–) | 78.25 (221.32) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 2.0 (–) | 78.25 (221.32) |
| Community contacts | ||||||||||||
| GP (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| GP (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 10.0 (–) | 46.75 (132.23) |
| Nurse (surgery/clinic) | 4 (50) | 12.0 (18.81) | 540 (1249.86) | 2 (25) | 1.5 (0.71) | 33.75 (66.96) | 2 (25) | 4.5 (4.95) | 101.25 (251.99) | 1 (13) | 7.0 (–) | 78.75 (222.74) |
| Nurse (home) | 2 (25) | 1.0 (0.0) | 22.50 (41.66) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 11.25 (31.82) |
| Physiotherapist (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Physiotherapist (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Psychologist/counsellor/ psychotherapist (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Psychologist/ counsellor/ psychotherapist (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 6.63 (18.74) |
| Alternative medicine/complementary therapist (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Alternative medicine/complementary therapist (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| OT (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| OT (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Social worker (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Social worker (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Home care worker (surgery/clinic) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Home care worker (home) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Support group (surgery/clinic) (assumed free) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Support group (home) (assumed free) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Tests | ||||||||||||
| Magnetic resonance image brain scan | 1 (13) | 1.0 (–) | 7.25 (20.51) | 1 (13) | 1.0 (–) | 7.25 (20.51) | 2 (25) | 1.0 (0.0) | 14.5 (26.85) | 2 (25) | 1.0 (0.0) | 14.5 (26.85) |
| Audiometry | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 10.75 (30.41) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Nerve testing (nerve conduction or EMG test) | 1 (13) | 1.0 (–) | 20.88 (59.04) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 1.0 (–) | 20.88 (59.04) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Blood test | 1 (13) | 2.0 (–) | 0.75 (2.12) | 3 (38) | 1.0 (0.0) | 1.13 (1.55) | 2 (25) | 3.0 (2.83) | 2.25 (5.26) | 3 (38) | 1.0 (0.0) | 1.13 (1.55) |
| Other investigations/tests | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 2 (25) | 1.5 (0.71) | 50.63 (100.44) | 1 (13) | 1.0 (–) | 16.88 (47.73) | 1 (13) | 2.0 (–) | 33.75 (95.46) |
| Help from friends or relatives (informal care) | ||||||||||||
| Child care | 0 (0) | 0.0 (0.0) | – | 0 (0) | 0.0 (0.0) | – | 0 (0) | 0.0 (0.0) | – | 1 (13) | Missing | – |
| Personal care | 1 (13) | Missing | – | 0 (0) | 0.0 (0.0) | – | 0 (0) | Missing | – | 0 (0) | 0.0 (0.0) | – |
| Help in the house | 1 (13) | Missing | – | 0 (0) | 0.0 (0.0) | – | 1 (13) | 0.0 (0.0) | – | 0.0 (0.0) | 0.0 (0.0) | – |
| Help outside the house | 1 (13) | Missing | – | 2 (25) | 0.14 (0.38) | – | 0.0 (0.0) | 0.0 (0.0) | – | 0.0 (0.0) | 0.0 (0.0) | – |
| Going with you to medical appointment | 2 (25) | Missing | – | 2 (25) | 1.13 (2.80) | – | 0.0 (0.0) | 0.0 (0.0) | – | 2 (25) | 1.5 (0.71) | – |
| Employment and education – helper(s) | N (%) using | Days off work | Costs (SD) | N (%) using | Days off work | Costs (SD) | N (%) using | Days off work | Costs (SD) | N (%) using | Days off work | Costs (SD) |
| Days off work (helper) | 2 (25) | 2.0 (0.0) | 56.9 (105.36) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Days taken off education (helper) | 0 (0) | 0.0 (0.0) | – | 0 (0) | 0.0 (0.0) | – | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Employment status | ||||||||||||
| Paid employment – full time | 1 (13) | – | – | – | – | – | 1 (13) | – | – | 4 (50) | – | – |
| Paid employment – part time | 3 (38) | – | – | 4 (50) | – | – | 0 (0) | – | – | 0 (0) | – | – |
| Student | 1 (13) | – | – | 1 (13) | – | – | 0 (0) | – | – | 0 (0) | – | – |
| Unpaid voluntary work | – | – | – | 2 (25) | – | – | 1 (13) | – | – | 2 (25) | – | – |
| Unpaid voluntary work/retired | 1 (13) | – | – | – | – | – | 1 (13) | – | – | 0 (0) | – | – |
| Unemployed | 0 (0) | – | – | 0 (0) | – | – | 0 (0) | 1 (13) | ||||
| Employment and education | N (%) using | Days off work | Costs (SD) | N (%) using | Days off work | Costs (SD) | N (%) using | Days off work | Costs (SD) | N (%) using | Days off work | Costs (SD) |
| Days taken off education | 1 (13) | 2.0 (–) | – | 0 (0) | 0.0 (0.0) | – | 0 (0) | 0.0 (0.0) | – | 0 (0) | 0.0 (0.0) | – |
| Days off work | 1 (13) | 1.0 (–) | 14.23 (40.23) | 2 (25) | 4.0 (1.41) | 113.8 (219.32) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 182.5 (–) | 2596.06 (7342.77) |
| Impact of XP on household finances | ||||||||||||
| Large | 0 (0) | – | – | 1 (13) | – | – | 0 (0) | – | – | 1 (13) | – | – |
| Moderate | 1 (13) | – | – | 0 (0) | – | – | 1 (13) | – | – | 0 (0) | – | – |
| Small | 1 (13) | – | – | 3 (38) | – | – | 1 (13) | – | – | 2 (25) | – | – |
| Very small | 5 (63) | – | – | 3 (38) | – | – | 2 (25) | – | – | 3 (38) | – | – |
| Additional costs | ||||||||||||
| Sunscreen creams (6 months) | 3 (38) | 33.33 (15.28) | 12.5 (19.09) | 3 (38) | 43.33 (5.77) | 16.25 (22.64) | 1 (13) | 20.0 (–) | 2.5 (7.07) | 2 (25) | 27.0 (18.38) | 6.75 (14.30) |
| Sunscreen creams (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 4 (50) | 10.75 (5.06) | 5.38 (6.63) | 1 (13) | 15.0 (–) | 1.88 (5.30) | 4 (50) | 25.24 (23.69) | 12.62 (20.56) |
| UV protective window films (6 months) | 1 (13) | 200 (–) | 25.0 (70.71) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| UV protective window films (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 750 (–) | 93.75 (265.17) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Other house/car adaptations (6 months) | 1 (13) | 200 (–) | 25.0 (70.71) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 1 (13) | 800.0 (–) | 100 (282.84) |
| Other house/car adaptations UV (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| UV visor (6 months) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| UV visor (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| UV protective clothes (6 months) | 3 (38) | 93.33 (92.92) | 35.0 (69.28) | 1 (13) | 70 (–) | 8.75 (24.75) | 2 (25) | 6.0 (5.66) | 1.5 (3.51) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| UV protective clothes (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 2 (25) | 32.5 (17.68) | 8.13 (16.46) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Glasses (6 months) | 1 (13) | 15.0 (–) | 1.88 (5.30) | 1 (13) | 10 (–) | 1.25 (3.54) | 1 (13) | 100.0 (–) | 12.5 (35.36) | 2 (25) | 78.0 (101.82) | 19.5 (52.77) |
| Glasses (one-off cost) | 1 (13) | 110 (–) | 13.75 (38.89) | 2 (25) | 126.67 (87.37) | 47.5 (80.49) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Vitamin D tablets | 2 (25) | 13.0 (4.24) | 3.25 (6.23) | 2 (25) | 3.5 (2.12) | 0.88 (1.81) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 2 (25) | 7.0 (7.07) | 1.75 (4.20) |
| Vitamin D tablets (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 2 (25) | 15.67 (4.04) | 5.88 (8.39) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 2 (25) | 18.5 (4.95) | 4.63 (8.77) |
| UV meter (6 months) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| UV meter (one-off cost) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0 (0) | 0.0 (0.0) | 0.0 (0.0) |
| Medication | 26.24 (65.82) | 14.29 (17.60) | 0.0 (0.0) | 40.78 (56.53) | ||||||||
After adjusting for baseline utility, the intervention group resulted in 0.0141 fewer QALYs (95% CI −0.0369 to 0.0028). Therefore, at follow-up, the intervention resulted in lower costs and fewer QALYs. The ICER was £2642.
Over a 15-year period, it was estimated that the intervention would result in fewer cases of cancer with cost savings and more QALYs. The estimated long-term ICER is £13,455 per QALY.
Limitations
The small sample size means we have to be cautious about both costs and QALYs, and in the short term we probably would not expect QALY differences given the long-term aims of photoprotection. Furthermore, the HRQoL scores as measured with the EQ-5D-5L were high and most likely indicate that the measure is not sensitive in this area. The long-term model was overly simplistic. This was inevitable given the paucity of data relating to the expected incidence of cancer. We were not able to factor in mortality rates, and so if reduced cancer likelihood results in differences in mortality, then our results may be under-estimates of treatment effects. However, it does indicate that a measure that can increase photoprotection does have the potential to be cost-effective.
Conclusions
This economic evaluation has shown that HRQoL and hence QALYs are similar between the two groups, although slightly lower in the intervention group. Costs are lower in the intervention group. However, neither difference is statistically significant. If we focus on the difference in mean costs and QALYs (adjusted for baseline), then we would conclude that the intervention was the most cost-effective option. This is not because we have demonstrated it to be more effective in terms of QALYs, but because it was associated with much lower costs. The lower costs are sufficient for the ICER to fall below the £20,000 threshold line on a cost-effectiveness plane, but we need to be cautious with the findings. The lower costs for the intervention were due to lower service use and this may simply have been because of an imbalance between the groups given the small sample size. The model showed that the intervention resulted in higher costs and more QALYs over the longer term and the ICER was under the NICE threshold, again indicating value for money.
Inter-relationship with other parts of the programme
This component complements the clinical results from the trial and provides information on value for money of the intervention.
Involvement of patients and/or the public
The PPI panel played an extensive and active role throughout the entire duration of the XP project, ensuring that the research has remained patient-centred and acceptable to the target population. This was particularly important given low numbers in a rare disease, where study burden was a key consideration because the same participant pool was relied on for all Phase I studies and was to be the target population for the developed intervention, and given our plans to integrate XPAND into the existing specialist XP clinical service following demonstration of its efficacy and acceptability.
The panel was comprised of two parents of patients diagnosed with XP (one of whom was the founder of the XP support group), an adult patient diagnosed with XP, and a teacher who taught in a school where three children diagnosed with XP attended (for list of names see Report Supplementary Materials 1–18). Regular contact was maintained via e-mail, telephone and face-to-face meetings. We were honoured when the NIHR recognised the value of our PPI work and has since used it as an exemplar for other research teams.
The following is a summary of the involvement of the PPI panel (see annual progress reports for further detail of PPI feedback and changes made in response to that feedback):
-
Reviewed the content and design of the daily diary (paper-based UVR protection diary and mobile phone survey) completed as part of the in-depth study of photoprotection behaviour (Workstream 1).
-
Reviewed the content of the questionnaires to be included in the international cross-sectional survey study (Workstream 2), as well as advised on the length, clarity and relevance, and any missing constructs.
-
Input into the format and nature of participant feedback following Phase I participation (with recommendation for group-level rather than individual-level feedback).
-
Reviewed the qualitative interview findings.
-
Assistance with the organisation of, and attendance at, two participant feedback and thank-you events (one at Cadbury World in Birmingham, attended by children and their families, and one at a restaurant in London, attended by adults and their partners/families).
-
Attended the consensus conference, participated in discussion of the intervention recommendation statements, and put forward priorities and guidelines for intervention development (including intervention targets, modes of delivery and contact, materials, etc.).
-
Reviewed the intervention materials including personalised intervention topics and strategies to target these, worksheets, goal-setting tools, magazine articles and layout, at several points throughout the development process. Several extra patients, who had participated in Phase I but were not eligible for participation in the trial as they had excellent adherence already, were also consulted at this point.
-
Contribution to the content of the XPAND magazine, including giving interviews and providing photos (again, including several extra patients).
-
Input into the script and editing of the sunscreen application video; one patient also attended the filming day and acted as a consultant to the actors portraying people diagnosed with XP.
The following are recent reflections from several members of the PPI panel, gathered for the purposes of this report:
Reflections (by a patient member of the patient and public involvement panel)
I can’t give a scientific or even a very objective contribution at this point at the end of my time on the Patients’ Panel for the XPAND research. My input for the most part has been pretty subjective … however as part of the Panel, I was constantly struck by the value of the work being done. I have been delighted to sit in on the research and to watch the intricate, careful and conscientious and hugely imaginative and creative work being done by the researchers and designers of the study. My abiding feeling is that patients should be included so that we can see the extraordinary value of the research that is being done.
Reflections on what was and what was not successful in the programme
The XP programme has been successful in many areas. Key achievements related to recruitment and retention in the Phase I and Phase II Workstreams based in UK; use of a variety of mixed research methodologies and validation of a novel outcome measure; and collaboration with the clinical team and PPI panel.
-
Poor recruitment was the main anticipated threat to the programme of research which, excluding Workstream 2 (international), did not materialise [e.g. recruited above target in Workstream 1 and Workstream 2 (n = 47/n = 45); n = 66/n = 62]. This was in part due to the foresight of the team who recruited a research nurse with extensive experience of working with patients with a rare disease, continuous involvement of the PPI panel to ensure that awareness of participant burden was at the fore and promotion of the research by the XP charity – the XP support group. Although recruitment was lower in Phase II (Workstream 4), it was sufficient to power the study for the primary analyses. Once recruited, participants were committed to the research and attrition was minimal. We speculate that this was due to the lack of existing patient-centred research in XP which prompted patients to support this novel project. We also showed our appreciation to the study participants by running thank-you events and maintaining communication throughout the programme (e.g. by sending programme updates).
-
The programme featured novel innovative mixed methods to gain a comprehensive understanding of the lived experience of XP patients (Workstream 1), as well as insights into determinants of photoprotection between (Workstream 2) and within individuals (n-of-1, Workstream 1). Moreover, we used an innovative approach to measure a clinically relevant outcome (i.e. dose-to-face) that combined objective assessment of UVR from a wrist-worn electronic dosimeter with self-report data from a daily photoprotection time-use diary. This was a significant advance considering that previous research had relied on either self-report questionnaires or dosimetry – we integrated both to create a new outcome measure with usability that extends to the wider photo-dermatology field. It was challenging and exciting to bring together experts from different disciplines.
-
It is doubtful whether the programme would have achieved these successes without the collaboration and support from the clinical team and the PPI panel. The team were aware that they would be critical to the programme’s success and were determined to involve these stakeholders at every phase of the research (i.e. research design, questionnaire selection and development, intervention design and content development). We are indebted to their enthusiasm and support, which highlights that stakeholder involvement is pivotal in rare disease research.
Limitations relating to the method or execution of the research
There were a number of important limitations across the different aspects of the programme, and these are indicated in the summaries of the different studies and phases outlined above. Many of these limitations arose from the rarity of XP and the very small pool of potential participants for each aspect of the programme, particularly the RCT. The key limitations were as follows:
-
Lack of piloting of the intervention.
It was not possible to pilot the intervention on a sample of XP patients with poor UVR protection as this would have further reduced the potential sample of participants for the RCT. Although we received extensive feedback from the PPI group on the planned intervention content, it would have been advisable to have obtained feedback from those with poorer protection. Also, we could have obtained detailed feedback from the first intervention group, but this may have resulted in changes for the delayed (control) group intervention content, resulting in lack of comparability.
-
Imbalance in baseline measures of UVR protection between the intervention and control groups.
The major limitation of the RCT was the failure of randomisation to balance baseline group differences which constrained the evaluation of the efficacy of XPAND in Workstream 4. Despite the use of a robust randomisation protocol, there was a substantial difference between the intervention and control groups in their level of UVR exposure and dose-to-face at baseline. Although the statistical team used standard methods to adjust for baseline levels, the difference was too large to be confident that XPAND alone was responsible for the large effect size. Moreover, the lack of baseline balance between the two arms of the study is very likely to have had effects on the health economic analysis (i.e. the lower costs for the intervention were due to lower service use and this may simply have been because of imbalance between the groups given the small sample size). With the benefit of hindsight, it would have been sensible to include dose-to-face as a factor in the randomisation. However, it was not possible to calculate this before randomisation occurred due to the rigid time frame imposed on the trial design. That is, XPAND needed to be delivered before the summer solstice, when environmental levels in UVR are highest and the risk to XP patients is greatest. Overall, we see the failure of randomisation as a reflection of the challenges of research in a very rare disease.
-
Lack of direct measure of UVR dose-to-face.
The primary outcome measure of UVR dose to the face was an innovative approach, combining objective and self-reported indices, used by Workstreams 1b and 4. However, it was an estimation of dose to the face and not a direct measure. We aimed to limit the impact of bias both in the objective capture of dose of UVR reaching the wrist (i.e. sleeve covering the dosimeter) by instructing patients to wear the dosimeter over their sleeves and in the subjective recording of photoprotection activities (i.e. self-presentation bias and inaccurate recall) by reassurance about anonymity in study participation and strategies to reduce recall problems. However, we consider exposure to these biases to be unsystematic and therefore unlikely to significantly influence findings.
-
Lack of blinding in the intervention.
As with any behavioural intervention, it was not possible to blind the participants as to which arm of the study they had been allocated to. Although we were able to avoid selection bias by allocating patients to the groups (i.e. intervention vs. waiting list/delayed intervention) after they had agreed to be in the study, it was not possible to control for or estimate the magnitude of any expectancy and related effects. Many factors can impact the magnitude of bias due to lack of blinding which makes it very difficult to estimate the extent to which this may have contributed to the intervention effect in our study. If there were expectancy effects, then these are likely to have had a larger effect on the patient-rated (i.e. subjective) outcome measures, including the QoL measures and diary data. However, as we also collected objective data on our primary outcome (UV exposure) from the dosimeters, we were able to see that these were broadly aligned with the patient-rated (i.e. daily diary) estimates of UVR exposure. The very small pool of potential participants meant that it was not possible to have an additional attention-placebo control group, which could have provided additional insights into the magnitude of the actual treatment effect.
-
Duration of the intervention effect.
From the RCT outcome data, it is difficult to estimate the duration of the intervention effect. Comparing the first and second follow-up findings in the intervention group indicates that the effect is reasonably robust over 3 months, but additional follow-up data in the next summer period would have provided more definitive evidence. Reviews of other UVR photoprotection interventions in at-risk and general population samples indicate that the effects may be quite durable, but this may not necessarily be the case in XP, where there is a significant range of restrictive behavioural demands. There is a clear need to assess the duration of the effects of XPAND, and to establish whether further personalisation and booster sessions are needed across individuals.
-
Selection of participants for the RCT.
The XPAND intervention was only offered to adults with no cognitive impairment, sufficient English language skills and suboptimal adherence to photoprotection, which raises questions about its generalisability. It will be important to establish whether it could be effective either in those with better protection or for parents/carers who play the key role in ensuring photoprotection in their children or cognitively impaired adult relatives.
-
Low recruitment in Workstream 2.
The international cross-sectional survey was limited by poor recruitment. Despite repeated efforts to support collaborating researchers based abroad (e.g. by extending the recruitment window), it was not possible to collect sufficient data to conduct between-country analysis. Future research needs to focus on obtaining larger data sets across countries to have a better understanding of the factors affecting UVR protection, and how these might be related to different climate patterns, cultural influences and healthcare contexts. However, given the complexity of our mixed-methods Phase I research, this limitation needs to be seen in the context of an otherwise successful programme of research.
-
Dosimeter failure.
Although the dosimeters provided vital detailed data about daily UV exposure, it should be noted that they failed in two participants in the intervention arm of the RCT, thereby further limiting the sample size and power. Future work is needed to consolidate their reliability and useability since they are a crucial source of objective assessment in studies of UVR protection and intervention evaluation.
Conclusions from the whole programme
The XP programme grant fulfilled its primary objective to develop a psychological intervention that improves photoprotection in adults with XP. In doing so, it filled gaps in our understanding of how people photoprotect, the impact of different photoprotection activities on the level of UVR reaching the face, the modifiable psychological determinants of photoprotection, as well as an in-depth understanding of the impact of photoprotection on patients’ lives. We demonstrated that it was possible to synthesise this complex data set into a theory-based intervention which showed positive impacts on adherence and reduced the dose of UVR reaching participants’ faces. As well as being a ‘research’ success, we have shown that a complex intervention can be valued by patients and staff and integrated into standard NHS care pathways.
Key conclusions from Phase I:
-
The dose of UVR reaching the face was determined not just by the extent of protection worn when outdoors but also, to an extent not previously suspected, by the duration of time outdoors.
-
Levels of photoprotection (including time outdoors) varied considerably between individuals, and some adults protected poorly and were receiving a larger dose of UVR to the face than the average for healthy individuals.
-
This non-adherence was likely to have clinical implications and confirmed that intervention in the adult population was needed.
-
The negative impact of such a rigorous photoprotection regime on participants’ QoL and emotional well-being was considerable. For some the cost was too high and they resisted the photoprotection regime, preferring to protect less well in order to live a ‘normal life’. Others integrated photoprotection more easily in their daily life. Mixed-methods studies showed a trade-off between skin protection and emotional health, as higher doses of UVR were associated with worse emotional well-being.
-
Inadequate photoprotection was determined by a variety of factors that could be modified by a psychological intervention. These included doubts about the necessity of photoprotection, experience of negative and positive emotions, lack of self-efficacy, practical concerns, worries about the reaction of others to appearance changes, overall resistance to the regime and a lack of helpful social support. Automaticity was a key driver of good photoprotection, highlighting the importance of habit as an adherence enabler.
Key conclusions from Phase II:
-
A complex personalised adherence intervention improved photoprotection and reduced the dose of UVR reaching the face in patients with XP. This was achieved without reducing emotional well-being.
-
The magnitude, duration, cost-effectiveness and generalisability of the intervention are difficult to evaluate. Cost-effectiveness results did not reach statistical significance.
-
Robust measurement of dose-to-face showed that comprehensive UVR protection required reduction in the degree of exposure alongside photoprotection during exposure. Future adaptations of XPAND need to focus on strategies to reduce time out of doors during higher risk times of the day, which is challenging given that this is influenced by factors potentially outside the individual’s control.
-
It is challenging to apply a RCT design to rare diseases, and other n-of-1 approaches need to be considered to avoid methodological limitations (i.e. failure of randomisation) associated with small sample sizes.
-
The highly personalised nature of XPAND alongside the complex multicomponent design contributed to patients feeling supported and engaged with the intervention.
-
Greater automaticity was a potential mechanism of the intervention identified by the process evaluation.
-
Specific ‘active ingredients’ of XPAND varied between individuals, highlighting the importance of personalisation and the interactive nature of behaviour change mechanisms – dependent on the content provided and the response of the recipient.
Recommendations for future research
Following on from the findings and limitations of the studies in the programme, the following topics are prioritised for future research in (1) patients with XP and (2) other at-risk and general population groups:
-
Future behavioural research in XP.
-
To ascertain whether the XPAND intervention can be effective either for XP patients with better protection or for parents/carers who play the key role in ensuring photoprotection in their children or cognitively impaired adult relatives.
-
To evaluate the duration of the positive effects of the XPAND intervention and the potential for both booster sessions and re-personalisation to maintain the changes in UVR protection behaviour over time.
-
To evaluate whether specialist nurses can deliver XPAND with intervention fidelity and efficacy in routine clinical settings.
-
To investigate the determinants and nature of UVR protection in adequately sized samples of XP patients across different countries – in order to have a better understanding of these factors, and how they are related to different climate patterns, cultural influences and healthcare contexts.
-
To adapt and evaluate the XPAND intervention for a web-based digital delivery which could be accessed by an international XP population.
-
To test future interventions using n-of-1 ‘micro’ trial designs, which can evaluate efficacy and heterogenous responses in individual patients.
-
-
In other at-risk groups
-
vii. To adapt and evaluate the XPAND intervention for use with other groups of adults at higher risk of non-malignant skin cancers.
This is a larger population which should avoid the methodological challenges of using RCT in rare disease.
-
viii. To investigate and evaluate novel intervention methods to tackle ‘when’ and for ‘how long’ patients are outdoors.
This was the less successful part of the intervention, as participants found it difficult to alter their overall exposure. It would benefit from further enhancement.
-
To develop and evaluate habit-based interventions for sunscreen application which could be appropriate to prevent UVR damage in the healthy population.
-
Implications for practice and any lessons learnt
Implications for practice
The XPAND intervention improved adherence to photoprotection by each participant. Therefore, the clinical team at the National Xeroderma Pigmentosum Service, Guy’s and St. Thomas’ Hospital NHS Foundation Trust will be adopting the XPAND intervention into routine care for patients with adherence challenges. To ensure that staff are equipped with the skills and confidence to deliver the intervention, the XPAND team have adapted the intervention manual and supporting materials for use by the clinic. We have developed a training package composed of four workshops and videos of key techniques. We are also supporting the team to identify how to integrate XPAND into current pathways. We are ensuring that XPAND is integrated in a step-by-step approach that allows the team to systematically build their confidence. Firstly, to follow-up those adults who participated in the trial to offer a booster session which will explore levels of adherence and barriers to photoprotection. The team will continue to personalise XPAND to the barriers currently impacting photoprotection. These sessions will follow the structure of trial conversations that focused on the maintenance of photoprotection. Secondly, to contact adult patients who were eligible for the trial but did not wish to participate. Thirdly, to adapt the existing home visit carried out for all new patients, to include exploration of potential barriers to photoprotection and increase awareness of tools available to support photoprotection. We will continue to provide supervision to support the clinical team as they start to use XPAND in practice. For the future, if XPAND is shown to improve photoprotection in adults at higher risk of developing non-malignant skin cancers without XP, this training package can easily be adapted to this new setting.
Studies in both phases of our research highlighted the importance of the time and duration of exposure to UVR in determining the dose reaching the face. This suggests that clinicians caring for people with XP should place greater emphasis on – recommendations to limit exposure, as is given to photoprotection worn or sunscreen applied during exposure. We acknowledge that this is more likely when there is a greater understanding of how to intervene to successfully modify exposure.
Additional information
Acknowledgements
We would like to thank all research staff who have contributed to this programme. Without exception, team members and co-investigators have been dedicated, enthusiastic and productive. We appreciate the efforts of research nurse, Lesley Foster, who was instrumental in recruitment, retention and communication with participants and their families. We are grateful to Tess van Leeuwen, programme administrator, for her excellent organisational skills and events management. We wish to acknowledge the staff who contributed to the development of the XPAND materials. Thanks also to the independent members of our steering committees for their guidance and support. We would like to thank the students for their research assistance, especially in entering daily diary data.
This project would not have succeeded without the input of our stakeholders. We thank the PPI panel (Ben Fowler, Ros Tobin and Sandra Webb) for their continued support and constructive comments. The authors thank the XP clinical team at Guy’s and St Thomas’ NHS Foundation Trust, especially Isabel Garrood, Hiva Fassihi, Tanya Henshaw, Alan Lehmann and Sally Turner.
We are indebted to the participants for their commitment to this programme of research.
This involved giving up their time, as well as completing the daily diaries, questionnaires and wearing the dosimeters. We appreciate the willingness of those individuals who participated in the XPAND trial to try different approaches to managing their photoprotection.
Contributions of authors
Robert Sarkany (https://orcid.org/0000-0003-1067-3973) (Consultant Dermatologist, Guy’s and St Thomas’ NHS Foundation Trust) was the joint principal investigator and supervised all aspects of the research programme. This included the conception of the research project, the development of protocols, application for funding, supervision of the research team, organisation of the patient recruitment, as well as intellectual input into all areas of the programme. Dr Sarkany was particularly involved in the dermatological aspects of the programme and dose-to-face measurement in Workstream 1. He contributed to nearly all the published peer-reviewed journal articles.
Jessica Walburn (https://orcid.org/0000-0001-7396-0182) (Senior Lecturer, Health Psychology, King’s College London) was the research fellow and was responsible for the implementation of the research programme. This included contributing to the development of protocols, management of the research team and intellectual input into the health psychology areas of the programme. In particular, she was the lead researcher for the intervention development, running of the RCT in Phase II and was an intervention facilitator. She was responsible for liaising between the different stakeholders, running all steering group meetings and submitting annual reports for NIHR. She contributed to all the published peer-reviewed journal articles and is the lead author of the report.
Rebecca Anderson (https://orcid.org/0000-0002-7095-8914) (Postdoc, Kingston University) was the qualitative research assistant for Workstream 1. She contributed to all aspects of the qualitative study, in particular analysis related to experiences of stigma in XP patients.
Vera Araujo-Soares (https://orcid.org/0000-0003-4044-2527) (Professor of Health Psychology, University of Twente and Co-applicant) lead the n-of-1 study (Workstream 1). She was responsible for the n-of-1 study protocol and she supervised the running of the study, analysis and paper write-up. In addition, she contributed to the development of the intervention and other health psychology aspects of the programme.
Janette Boadu (https://orcid.org/0000-0001-7012-3514) [Health Economist (assistant), King’s College London] supported the economic analyses studies (Workstream 2 and Workstream 5) and contributed to the report. This included data entry, analysis and write-up.
Martha Canfield (https://orcid.org/0000-0002-4494-8237) (Postdoc Researcher, King’s College London) supported the lead statistician to analyse data in Workstreams 1, 2 and 4. Dr Canfield led on the development of a self-report measure of adherence to photoprotection activity. She contributed to numerous papers (excluding n-of-1 and qualitative studies).
Lesley Foster (Research Nurse, Guy’s and St Thomas’ NHS Foundation Trust) was responsible for patient recruitment and retention, and all liaison with XP patients. She organised participant events, kept participants updated with research progress, managed the relationship between the research and XP clinical teams, as well as providing research assistant support to the research fellow. She was part of the intervention development team and was an intervention facilitator.
Paul McCrone (https://orcid.org/0000-0001-7001-4502) (Professor of Healthcare Economics, University of Greenwich and Co-applicant) led all health economic elements of the project (Workstream 2 and Workstream 5).
Myfanwy Morgan (https://orcid.org/0000-0001-5532-8941) (Professor of Medical Sociology, King’s College London and Co-applicant) led all the qualitative studies in the programme (Workstream 1 and Workstream 4). This included developing study design, managing the development of topic guides, supervising the running of the qualitative studies and leading the analyses. She led or supervised the qualitative publications and other papers from the programme.
Sam Norton (https://orcid.org/0000-0003-1714-9963) (Senior Lecturer, Research Methods and Statistics, King’s College London and Co-applicant) led all the quantitative analysis in the research programme (excluding the n-of-1 study). He contributed to numerous peer-reviewed publications.
Kirby Sainsbury (https://orcid.org/0000-0002-6716-8305) (Research Fellow, Newcastle University) was responsible for running the n-of-1 study (Workstream 1). She led or contributed to the n-of-1 peer-reviewed publications and other papers from the programme. She was a co-contributor to the development of the intervention and was also a facilitator.
John Weinman (https://orcid.org/0000-0002-6786-0166) (Professor of Psychology as Applied to Medicines, King’s College London) was the joint principal investigator and supervised all aspects of the research programme. This included the conception of the research project, the development of protocols, application for funding, supervision of the research team, as well as intellectual input into all areas of the programme. Professor Weinman supervised the health psychology aspects, including the intervention development. He contributed to nearly all the published peer-reviewed journal articles.
Contributions of our co-investigators:
Mark Berneburg (Universitatsklinikum Regensburg); Meriem Jones (Hopital Charles Nicolle De Tunis); Chikako Nishigori (Kobe University); and Alain Sarasin (Gustave Roussy Institute) contributed to Workstream 2. They informed the transcreation of the questionnaire to suit their respective countries, organised patient recruitment and contributed to write-up for peer-reviewed publications.
Hiva Fassihi (Consultant Dermatologist, Guy’s and St Thomas’ NHS Foundation Trust) and Isabel Garrood (Clinical Psychologist, Guy’s and St Thomas’ NHS Foundation Trust) contributed to aspects of the study involving XP patients.
Kate Johnstone (Research Assistant, King’s College London) collected the dose-to-face data for our healthy sample.
Alan Lehmann (University of Sussex) advised on DNA repair, clinical aspects of the study and chaired our internal project steering committee.
Jakob Heydenreich and Hans Christian Wulf (Bispebjerg Hospital and Co-applicant) provided the dosimeters and advised on function and measurement.
Adrian Mander (Cardiff University and Co-applicant) advised on the design of the RCT.
Falko F Sniehotta (Professor of Health Psychology, University of Twente and Co-applicant) advised on the n-of-1 study design and aspects of the project related to health psychology.
Jonathan Spencer (Junior Health Economist, King’s College London) contributed to economic analyses studies (Workstream 2).
Antony Young (King’s College London and Co-applicant) advised on photodermatological aspects of the programme and measurement of dose-to-face.
Tess van Leeuwen (King’s College London) was the programme administrator and provided organisational support detailed in the acknowledgements.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/PZCW1478.
Primary conflicts of interest: Jessica Walburn is employed by IQVIA (as of August 2020) and has completed consultancy for Sprout Behaviour Change Limited. John Weinman reports personal fees from Atlantis Healthcare, outside the submitted work.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
The Chief Investigator will act as custodian for the research data and materials. All data requests should be submitted to the corresponding author for consideration. Study data will be made available to the scientific community with as few restrictions as feasible.
Ethics Statement
The phase 1 research was approved by Camden and King’s Cross Research Ethics Committee 15/LO/1395, 2016. The phase 2 research was approved by West London & GTAC REC 17/LO/2110, 2018.
Information Governance Statement
Guy’s & St Thomas’ Foundation NHS Trust is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, Guy’s & St Thomas’ Foundation NHS Trust is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.guysandstthomas.nhs.uk/about-us/your-health-records/managing-your-data
Department of Health and Social Care Disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the Programme Grants for Applied Research or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publications
Anderson R, Walburn J, Morgan M. Experiences of stigma over the lifetime of people with xeroderma pigmentosum: a qualitative interview study in the United Kingdom. J Health Psychol 2017;24:2031–41. https://doi.org/10.1177/1359105317714643
Sainsbury K, Walburn J, Araujo-Soares V, Weinman J. Challenges and proposed solutions for formative research to inform systematic intervention development in rare and unstudied conditions: the case example of xeroderma pigmentosum. Br J Health Psychol 2017;23:229–37. https://doi.org/10.1111/bjhp.12287
Walburn J, Sarkany R, Norton S, Foster L, Morgan M, Sainsbury K, et al. An investigation of the predictors of photoprotection and UVR dose to the face in patients with XP: a protocol using observational mixed methods. BMJ Open 2017;7:e018364. http://doi.org/10.1136/bmjopen-2017-018364
Sainsbury K, Vieira R, Walburn J, Sniehotta F, Sarkany R, Weinman J, Araujo-Soares V. Understanding and predicting a complex behaviour using n-of-1 methods: photoprotection in xeroderma pigmentosum. Health Psychol 2018;37:1145–58. https://doi.org/10.1037/hea0000673
Anderson R, Walburn J, Morgan M. Approaches to photoprotection and normalization in highly adherent families of children with xeroderma pigmentosum in the United Kingdom. Qual Health Res 2019;30:1275–86. https://doi.org/10.1177/1049732319826561
Canfield M, Norton S, Walburn J, Morrison-Bowen N, Sainsbury K, Araujo-Soares V, et al. Facial photoprotection in xeroderma pigmentosum (XP) patients: validation of a new self-reported questionnaire of adherence. Photodermatol Photoimmunol Photomed 2019;36:118–25. https://doi.org/10.1111/phpp.12519
Morgan M, Anderson R, Walburn J, Weinman J, Sarkany R. The influence of perceived medical risks and psychosocial concerns on photoprotection behaviours among adults with xeroderma pigmentosum: a qualitative interview study in the UK. BMJ Open 2019;9. http://doi.org/10.1136/bmjopen-2018-024445
Walburn J, Anderson R, Morgan M. Forms, interactions, and responses to social support: a qualitative study of support and adherence to photoprotection amongst patients with Xeroderma Pigmentosum. Br J Health Psychol 2019;25:89–106. https://doi.org/10.1111/bjhp.12396
Walburn J, Canfield M, Norton S, Sainsbury K, Araújo-Soares V, Foster L, et al. Psychological correlates of adherence to photoprotection in a rare disease: international survey of people with Xeroderma Pigmentosum. Br J Health Psychol 2019;24:668–86. https://doi.org/10.1111/bjhp.12375
Walburn J, Norton S, Sarkany R, Sainsbury K, Araújo-Soares V, Morgan M, et al. Evaluation of a personalised adherence intervention to improve photoprotection in adults with Xeroderma Pigmentosum (XP): Protocol for the trial of XPAND. BMJ Open 2019;9:e028577. http://doi.org/10.1136/bmjopen-2018-028577
Sainsbury K, Walburn J, Foster L, Morgan M, Sarkany R, Weinman J, Araujo-Soares V. Improving photoprotection in adults with xeroderma pigmentosum: personalisation and tailoring in the ‘XPAND’intervention. Health Psychol Behav Med 2020;8:543–72. https://doi.org/10.1080/21642850.2020.1840379
Walburn J, Sainsbury K, Foster L, Weinman J, Morgan M, Norton S, et al. Why? What? How? Using an Intervention Mapping approach to develop a personalised intervention to improve adherence to photoprotection in patients with Xeroderma Pigmentosum. Health Psychol Behav Med 2020;8:475–500. https://doi.org/10.1080/21642850.2020.1819287
Sarkany RPE, Canfield M, Morgan M, Foster L, Johnstone K, Sainsbury K, et al. Ultraviolet exposure to the face in patients with xeroderma pigmentosum and healthy controls: applying a novel methodology to define photoprotection behaviour. Br J Derm 2021. https://doi.org/10.1111/bjd.20899
Sarkany R, Norton S, Canfield M, Morgan M, Foster L, Sainsbury K, et al. Identifying the psychosocial predictors of ultraviolet exposure to the face in patients with xeroderma pigmentosum: a study of the behavioural factors affecting clinical outcomes in this genetic disease. J Med Genet 2022;59:1095–103. http://doi.org/10.1136/jmedgenet-2021-108323
Walburn J, Foster L, Araújo‐Soares V, Sarkany R, Weinman J, Sainsbury K., Morgan M. Acceptability and influence of a complex personalized intervention on changes in photoprotection behaviours among people with xeroderma pigmentosum. Br J Health Psychol 2023;28(4): 1113–31. https://doi.org/10.1111/bjhp.12675
In preparation:
Walburn J, Norton S, Sarkany R, Sainsbury K, et al. A personalised adherence intervention improves photoprotection in adults with Xeroderma Pigmentosum (XP): Results of the XPAND trial.
Other relevant outputs:
Sainsbury K, Walburn J, Vieira R, Sniehotta F, Sarkany R, Weinman J, Araujo-Soares V. Using N-of-1 Methodology to Inform the Development of Individualised, Evidence-based Interventions for Patients with Xeroderma Pigmentosum (International Symposium: Systematic Approaches to Designing Effective Behaviour Change Interventions to Impact Health). International Congress of Behavioural Medicine (ICBM), Melbourne, Australia, December 2016.
Sainsbury K, Vieira R, Sniehotta F, Walburn J, Sarkany R, Weinman J, Araujo-Soares V. Using n-of-1 Methods to Understand a Complex Behaviour: Photoprotection in Patients with Xeroderma Pigmentosum. EHPS conference, Padua, Italy, September 2017.
Sainsbury K, Walburn J, Sarkany R, Weinman J, Araujo-Soares V. Challenges and Proposed Framework for Formative Research to Inform Systematic Intervention Development in Rare and Unstudied Conditions: The Case Example of Xeroderma Pigmentosum. DHP, British Psychological Society, Cardiff, UK, September 2017.
Sarkany R. The NIHR Xeroderma Pigmentosum (XP) Study: Key Findings from Phase 1. Detailed Investigation of Photoprotection Behaviours in XP Patients, and of the Psychosocial Factors Causing These Behaviours. European Academy of Dermatology and Venerology (EADV), Geneva, 2017.
Norton S, Walburn J, Canfield M, Sainsbury K, Araujo-Soares V, Foster L, et al. Predictors of Ultraviolet Radiation Exposure in Patients with Xeroderma Pigmentosum: Prospective Daily Diary Study with Objective Measurement of Ultraviolet Radiation. DHP, British Psychological Society, Cardiff, UK, September 2017.
Walburn J, Morgan M, Anderson R, Weinman J, Sarkany R. Self-management Goals and Response to Social Support: A Qualitative Study of Patients with Xeroderma Pigmentosum. EHPS conference, Padua, Italy, 2017.
Norton S, Walburn J, Canfield M, Sainsbury K, Araujo-Soares V, Foster L, et al. Ultraviolet Radiation Exposure in People with Xeroderma Pigmentosum: Daily Diary Study with Objective Measurement of Ultraviolet Radiation. EHPS, Galway, Ireland, August 2018.
Sainsbury K, Walburn J, Sniehotta F, Vieira R, Foster L, Sarkany R, et al. Intervention Development and Tailoring Using N-of-1 Data: Improving Photoprotection in Patients with Xeroderma Pigmentosum. EHPS, Galway, Ireland, August 2018.
Sainsbury K, Walburn J, Foster L, Morgan M, Sarkany R, Weinman J, Araujo-Soares V. Levels and Sources of Personalisation in a Complex Intervention to Improve Photoprotection in Patients with Xeroderma Pigmentosum (XP). ASBHM, Christchurch, New Zealand, February 2019.
Sarkany R. The NIHR Xeroderma Pigmentosum (XP) Study: Detailed Investigation of Photoprotection Behaviours in XP Patients, and of the Psychosocial Factors Causing These Behaviours. British Association of Dermatologists, Liverpool, July 2019.
Morgan M, Walburn J, Anderson R, Weinman J, Sarkany R. Seeking ‘Normality’: Parents’ Management of Photoprotection for Children with a Rare Skin Condition. European Health Psychology Society, Dubrovnik, Croatia, September 2019.
Walburn J, Chadwick P, Weinman J, Sainsbury K, Araújo-Soares V, Canfield M, et al. Using Consensus Conference methodology to bridge the gap between mixed-methods findings and complex intervention design. ASBHM, Christchurch, New Zealand, February 2019.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, et al. Incidence of DNA repair deficiency disorders in western Europe: xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744-50. https://doi.org/10.1016/j.dnarep.2008.01.014.
- Fassihi H, Sethi M, Fawcett H, Wing J, Chandler N, Mohammed S, et al. Deep phenotyping of 89 xeroderma pigmentosum patients reveals unexpected heterogeneity dependent on the precise molecular defect. Proc Natl Acad Sci USA 2016;113:E1236-45. https://doi.org/10.1073/pnas.1519444113.
- Sethi M, Lehmann A, Fawcett H, Stefanini M, Jaspers N, Mullard K, et al. Patients with xeroderma pigmentosum complementation groups C, E and V do not have abnormal sunburn reactions. Br J Dermatol 2013;169:1279-87. https://doi.org/10.1111/bjd.12523.
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet 2011;48:168-76. https://doi.org/10.1136/jmg.2010.083022.
- Tamura D, DiGiovanna JJ, Khan SG, Kraemer KH. Living with xeroderma pigmentosum: comprehensive photoprotection for highly photosensitive patients. Photodermatol Photoimmunol Photomed 2014;30:146-52. https://doi.org/10.1111/phpp.12108.
- Idorn L, Datta P, Heydenreich J, Philipsen P, Wulf H. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma. JAMA Dermatology 2014;150:163-8. https://doi.org/10.1001/jamadermatol.2013.5098.
- Surber C, Ulrich C, Hinrichs B, Stockfleth E. Photoprotection in immunocompetent and immunocompromised people. Br J Dermatol 2012;167:85-93. https://doi.org/10.1111/j.1365-2133.2012.11093.x.
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008. https://doi.org/10.1002/14651858.CD000011.pub3.
- Walburn J, Sarkany R, Norton S, Foster L, Morgan M, Sainsbury K, et al. An investigation of the predictors of photoprotection and UVR dose to the face in patients with XP: a protocol using observational mixed methods. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2018-028577.
- Walburn J, Norton S, Sarkany R, Sainsbury K, Araujo-Soares V, Morgan M, et al. Evaluation of a personalised adherence intervention to improve photoprotection in adults with xeroderma pigmentosum (XP): protocol for the trial of XPAND. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028577.
- Anderson R, Walburn J, Morgan M. Approaches to photoprotection and normalization in highly adherent families of children with xeroderma pigmentosum in the United Kingdom. Qual Health Res 2020;30:1275-86. https://doi.org/10.1177/1049732319826561.
- Morgan M, Anderson R, Walburn J, Weinman J, Sarkany R. The influence of perceived medical risks and psychosocial concerns on photoprotection behaviours among adults with xeroderma pigmentosum: a qualitative interview study in the UK. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-024445.
- Walburn J, Anderson R, Morgan M. Forms, interactions, and responses to social support: a qualitative study of support and adherence to photoprotection amongst patients with xeroderma pigmentosum. Br J Health Psychol 2019;25:89-106. https://doi.org/10.1111/bjhp.12396.
- Anderson R, Walburn J, Morgan M. Approaches to photoprotection and normalization in highly adherent families of children with xeroderma pigmentosum in the United Kingdom. Qual Health Res 2019;30:1275-86. https://doi.org/10.1177/1049732319826561.
- Sainsbury K, Vieira R, Walburn J, Sniehotta F, Sarkany R, Weinman J, et al. Understanding and predicting a complex behaviour using n-of-1 methods: Photoprotection in xeroderma pigmentosum. Health Psychol 2018;37:1145-58. https://doi.org/10.1037/hea0000673.
- Sarkany R, Norton S, Canfield M, Morgan M, Foster L, Sainsbury K, et al. Identifying the psychosocial predictors of ultraviolet exposure to the face in patients with xeroderma pigmentosum: a study of the behavioural factors affecting clinical outcomes in this genetic disease. J Med Genet 2022;59:1095-103. http://doi.org/10.1136/jmedgenet-2021-108323.
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999.
- Delis D, Kramer J, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan executive function system: an update. J Int Neuropsychol Soc 2004;10:301-3. https://doi.org/10.1017/S1355617704102191.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24. https://doi.org/10.1080/08870449 908407311.
- Gardner B, Abraham C, Lally P, de Bruijn GJ. Towards parsimony in habit measurement: testing the convergent and predictive validity of an automaticity subscale of the Self-Report Habit Index. Int J Behav Nutr Phys Act 2012;9. https://doi.org/10.1186/1479-5868-9-102.
- Walburn J, Canfield M, Norton S, Sainsbury K, Weinman J. Psychological correlates of adherence to photoprotection in a rare disease: international survey of people with xeroderma pigmentosum. Br J Health Psychol 2019;24:668-86. https://doi.org/10.1111/bjhp.12375.
- Sarason IG, Levine HM, Basham RB, Sarason BR. Assessing social support: the social support questionnaire. J Pers Soc Psychol 1983;44:127-39. https://doi.org/10.1037/0022-3514.44.1.127.
- Stewart-Brown S, Tennant A, Tennant R, Platt S, Parkinson J, Weich S. Internal construct validity of the Warwick-Edinburgh mental well-being scale (WEMWBS): a Rasch analysis using data from the Scottish health education population survey. Health Qual Life Out 2009;7. https://doi.org/10.1186/1477-7525-7-15.
- Canfield M, Norton S, Walburn J, Morrison-Bowen N, Sainsbury K, Araujo-Soares V, et al. Facial photoprotection in xeroderma pigmentosum (XP) patients: validation of a new self-reported questionnaire of adherence. Photodermatol Photo 2019;36:118-25. https://doi.org/10.1111/phpp.12519.
- Beecham J, Knapp M. Costing Psychiatric Interventions. London: Gaskell; 2001.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Walburn J, Sainsbury K, Foster L, Weinman J, Morgan M, Norton S, et al. Why? What? How? Using an intervention mapping approach to develop a personalised intervention to improve adherence to photoprotection in patients with Xeroderma Pigmentosum. Health Psychol Behav Med 2020;8:475-500. https://doi.org/10.1080/21642850.2020.1819287.
- Sainsbury K, Walburn J, Foster L, Morgan M, Sarkany R, Weinman J, et al. Improving photoprotection in adults with xeroderma pigmentosum: personalisation and tailoring in the ‘XPAND’ intervention. Health Psychol Behav Med 2020;8:543-72. https://doi.org/10.1080/21642850.2020.1840379.
- Bartunek JM, Murninghan JK. The nominal group technique: expanding the basic procedure and underlying assumptions. Group Organ Manage 1984;9:417-32. https://doi.org/10.1177/105960118400900307.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. Br Med J (Clin Res Ed) 2002;325. https://doi.org/10.1136/bmj.325.7367.746.
- Dennick K, Stanton-Fay S, Chadwick P, Heller S, Coates L, Gianfrancesco C, et al. From DAFNE to DAFNE Plus: Applying the Nominal Group Technique to Optimise a Diabetes Self-management Programme 2016.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Kok G, Gottlieb NH, Peters GJ, Mullen PD, Parcel GS, Ruiter RA, et al. A taxonomy of behaviour change methods: an intervention mapping approach. Health Psychol Rev 2016;10:297-312. https://doi.org/10.1080/17437199.2015.1077155.
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther 2006;44:1-25. https://doi.org/10.1016/j.brat.2005.06.006.
- Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychoth 2009;37:129-40. https://doi.org/10.1017/S1352465809005128.
- Hilgers RD, König F, Molenberghs G, Senn S. Design and analysis of clinical trials for small rare disease populations. J Rare Dis Res Treat 2016;1:53-60. https://doi.org/10.29245/2572-9411/2016/3.1054.
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029954.
- Richie J, Spencer L, O’Connor W. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Vallejo-Torres L, Morris S, Kinge JM, Poirier V, Verne J. Measuring current and future cost of skin cancer in England. J Public Health (Oxf) 2014;36:140-8. https://doi.org/10.1093/pubmed/fdt032.
- Philipp-Dormston WG, Müller K, Novak B, Strömer K, Termeer C, Hammann U, et al. NMSC-QoL Study Group. Patient-reported health outcomes in patients with non-melanoma skin cancer and actinic keratosis: results from a large-scale observational study analysing effects of diagnoses and disease progression. J Eur Acad Dermatol Venereol 2018;32:1138-46. https://doi.org/10.1111/jdv.14703.
- Beusterien K, Szabo S, Kotapati S, Mukherjee J, Hoos A, Hersey P, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer 2009;101:387-9. https://doi.org/10.1038/sj.bjc.6605187.
Appendix 1 Excerpt from intervention mapping matrices
| Change objectives | Behaviour-change strategies mapped to taxonomies [intervention mapping taxonomy of behaviour change techniques (V1)] | Key theory or framework | One-to-one session (summary of content included in the intervention manual) | Magazine | Text messages | Video showing sunscreen application | Other materials (determinant-specific activity sheets) |
|---|---|---|---|---|---|---|---|
| 1. Reduce concerns about looking different while photoprotecting | IM: belief selectiona; tailoringa; modellinga; planning coping responsesa; persuasive communicationa; reinforcementa; selfmonitoring of behaviourb; reattribution trainingb; provide opportunities for social comparisonc BCTv1: Problem-solving (1.2); instruction on how to perform the behaviour (4.1); reattribution (4.3); behavioural experiments (4.4); information about emotional consequences (5.6); social comparison (6.2); credible source (9.1); pros and cons (9.2) |
TDF (beliefs about consequences; skills) NCF (concerns) CBT (attention training; social skills and coping strategies) |
The aim is to affirm appearance concerns and acknowledge that they can be an important part of the daily burden of having XP. It provides practical strategies to manage unwanted attention involving diversion of attention in the moment, choosing types of protection that are more likely to blend in and boosts general social skills. Content adapted from existing manual. Manual module/s: Concerns about appearance when photoprotecting fac |
Article on managing barriers to photoprotection includes key strategies to manage appearance worries – ‘What’s stopping you getting the UVR protection you need?’ | X | X | Activity sheet reiterates that concerns about appearance are natural; summarises tips to manage staring; gives examples relevant to photoprotection |
| 2. Minimise impact of positive or negative emotions that reduce photoprotection | IM: tailoringa; planning coping responsesa; reinforcementa; selfmonitoring of behaviourb; Improving physical and emotional statesb; Anticipated regret.d BCTv1: Problem-solving (1.2); Monitoring of emotional consequences (5.4); Anticipated regret (5.5); Information about emotional consequences (5.6); Pros and cons (9.2); Reducing negative emotion (11.2) |
TDF (skills; emotions) CBT (stress management) |
The aim is to modify emotion if it has a negative impact on photoprotection. The relationship between emotions (positive and negative) and photoprotection will be explored (i.e. not wishing to wear face-buff when feeling happy in case it lowers mood). Facilitators will provide cognitive, emotional and behavioural strategies to manage fluctuations in mood and stress, to minimise influence on protection and improve emotional stability in the long term. Manual module/s: Mood and photoprotection Stress and photoprotection |
Article on managing barriers to photoprotection includes positive and negative emotions as a barrier – ‘What’s stopping you getting the UVR protection you need?’ | Feeling good today and don’t want protection to bring you down? Remind yourself how protecting now can help you to achieve the things you want in the future | X | Activity sheets reinforce skills and concepts discussed in the session. Content related to pleasant activity scheduling adapted from Getselfhelp.co.uk. Symptoms of low mood adapted from www.nhs.uk/conditions/stress-anxiety-depression/low-mood-and-depression/ |
| 3. Reduce concerns about looking different while photoprotecting | IM: belief selectiona; tailoringa; modellinga; planning coping responsesa; persuasive communicationa; reinforcementa; self- monitoring of behaviourb; reattribution trainingc; provide opportunities for social comparisonc (Bartholomew Eldredge et al., 2016) BCTv1: Problem-solving (1.2); instruction on how to perform the behaviour (4.1); reattribution (4.3); behavioural experiments (4.4); Information about emotional consequences (5.6); social comparison (6.2); credible source (9.1); pros and cons (9.2) |
TDF (beliefs about consequences; skills) NCF (concerns) CBT (attention training; social skills and coping strategies) |
The aim is to affirm appearance concerns and acknowledge that they can be an important part of the daily burden of having XP. It provides practical strategies to manage unwanted attention involving diversion of attention in the moment, choosing types of protection that are more likely to blend in and boosts general social skills. Content adapted from existing manual Manual module/s: Concerns about appearance when photoprotecting |
Article on managing barriers to photoprotection includes key strategies to manage appearance worries – ‘What’s stopping you getting the UVR protection you need?’ | X | X | Activity sheet reiterates that concerns about appearance are natural; summarises tips to manage staring; gives examples relevant to photoprotection |
Appendix 2 Synopsis of manuscript to be submitted to British Journal of Dermatology in September 2022
A personalised adherence intervention improves photoprotection in adults with xeroderma pigmentosum: results of the XPAND trial
Jessica Walburn, Sam Norton, Robert Sarkany, Martha Canfield, Kirby Sainsbury, Paul McCrone, Myfanwy Morgan, Vera Araujo-Soares, Janette Boadu, Lesley Foster, Jakob Heydenreich, Martin Nemec, Adrian Mander, Falko F Sniehotta, Hans Christian Wulf, John Weinman
Abstract
Since poor adherence to photoprotection for people with the rare condition of XP can be fatal, this study was designed to test a personalised intervention (XPAND) to reduce the UVR D-to-F by improving photoprotection in adults with XP. It was a two-armed parallel groups RCT, randomising 16 patients to receive XPAND or a delayed intervention control. Due to the rarity of XP, a novel approach with continuous UVR assessment ensured sufficient statistical power. XPAND involved seven one-to-one sessions using techniques targeting barriers to photoprotection. Primary outcome, average daily UVR D-to-F across 21 days in June to July 2018, was calculated by combining UVR exposure with a photoprotection daily diary. Secondary outcomes were D-to-F across 21 days in August 2018, time spent outside, photoprotection while outside, and daily ratings of mood and photoprotection automaticity, confidence and importance.
The XPAND group had lower average daily UVR D-to-F during the June to July 2018 assessment period [0.03 (SD 0.02) SED] compared to control [0.36 SED (SD 0.16)] (adjusted difference = −0.25, p < 0.001). Similar difference was observed for average daily total UVR exposure. Exploratory analysis of the delayed intervention control group also showed improvements in D-to-F, time outdoors and photoprotection while outdoors between June 2018 and June 2019.
Introduction
In XP, DNA damage caused by UVR cannot be repaired, which substantially increases the chances of skin and eye cancers. The average life expectancy is 32 years, with 60% of the premature deaths resulting from metastatic cutaneous malignancies. Photoprotection, in all weathers and seasons, is the only means of preventing the cancers. The intervention named ‘XPAND: Enhancing XP Photoprotection Activities – New Directions’ was systematically constructed to target modifiable psychological determinants of poor photoprotection.
The primary objective of this RCT was to investigate whether the average daily UVR D-to-F (SED), across 21 consecutive days, was reduced after receipt of XPAND compared to delayed intervention control. We also assessed whether change was maintained across 21 consecutive days in August 2018, investigated changes in relevant psychological variables and explored intervention-related changes in the delayed intervention control group. A cost-utility analysis was conducted to evaluate the economic viability of implementing XPAND into routine care.
Methods
A Phase II, assessor blind, two-armed parallel group RCT compared the efficacy of XPAND between participants who received the XPAND intervention May to June 2018 (plus routine care) and a delayed intervention control group who received XPAND a year later (May to June 2019).
Participants were recruited from the National Xeroderma Pigmentosum Service at Guy’s and St Thomas’ NHS Foundation Trust. Eligible patients were ≥ 16 years with a diagnosis of XP confirmed on DNA repair assay and whose adherence to photoprotection had previously been identified as being poor.
Participants completed baseline assessments for 21 days in April 2018 which were repeated for 21 days in June to July 2018 after the intervention or control, and after an XPAND booster session in August 2018. This involved completion of a daily photoprotection diary with daily ratings of psychological factors (i.e. mood, and photoprotection automaticity, confidence and importance). Participants wore the UVR dosimeter on their wrist continuously from the start of the first assessment period until the end of the August assessment period. The delayed intervention control group followed a similar protocol of assessments and measurements in 2019.
XPAND was delivered by one of two psychologists or a trained research nurse following a manual. Each patient received seven sessions of personalised and core behaviour change content (i.e. self-regulation, habit formation and motivation), using a range of behaviour change techniques.
Outcomes
Primary outcome: Average daily UVR dose to the face (D-to-F) (SED) across 21 consecutive days between June and July 2018. The UVR D-to-F was calculated as the product of the dose of UVR recorded at the wrist by the dosimeter, and the ‘protection factor’ of the facial photoprotection behaviours recorded in the daily UVR protection diary.
Secondary outcomes: Included a range of other measures of UVR exposure, combined measures of UVR protection behaviours, and psychological factors influencing UVR protection, mood and quality of life.
Health economic outcome: QALYs accrued over the period from baseline to t3 were derived from the EQ-5D-5L combined with tariffs. Cost and QALY differences between the two groups at t3 were estimated using regression models with baseline cost or EQ-5D-5L score used as an independent variable along with the group identifier.
Fidelity: Four independent assessors used a bespoke treatment fidelity measure developed for this trial.
Statistical analysis
Data were analysed using Stata version 16.1 statistical software. Data regarding daily UVR exposure and daily self-report assessments were analysed, following a modified intention-to-treat framework, using linear mixed-effects models with patient as a random effect to account for repeated observations within individuals. A similar approach using linear mixed-effects models was used to estimate between group differences in June 2018 and August 2018 for the self-report measures assessed once at the beginning of each period. Given the small sample size, planned exploratory analyses were undertaken for the delayed intervention control group. This was based on comparing the June 2019 assessments for the delayed intervention control group to their June 2018 assessments using a similar analytical approach described above.
Results
The final analysis sample for the primary outcome involved 13 participants providing a total of 492 useable days where both dosimetry was available and daily UVR protection diary were recorded (90% complete). Where analyses relied on the diary only, the analysis sample included 15 participants providing a total of 540 useable days (86%).
The patients were predominantly white (62.5%) and male (62.5%) with a mean age of 44.3 years (SD = 15.7). Most of the participants belonged to the three XP complementation groups (C, E, V)21 (62.5%) that do not cause abnormal sunburn responses and most had already had a skin cancer (62.5%). EQ-5D scores were above norm for UK adults (M = 0.9, SD = 0.1). Baseline levels of daily D-to-F were lower in the intervention group compared to the control (M = 0.04, SE = 0.02; M = 0.27, SE = 0.03) and both groups self-reported their mood as moderate. Those randomised to the intervention group differed from those randomised to the delayed intervention control group in some of the psychological characteristics related to adherence to photoprotection advice.
The XPAND intervention group had significantly lower mean daily UVR dose-to-face (M = 0.03, SD = 0.02) compared to the control group at the June 2018 (primary outcome) post-intervention assessment (n = 7; M = 0.36, SD = 0.16; adjusted difference = −0.25 SED, p < 0.001). This difference was maintained at the August 2018 follow-up (intervention: M = 0.04, SD = 0.03; control: M = 0.28, SE = 0.08; adjusted difference = −0.19 SED, p < 0.001).
Total UVR exposure was also lower in the intervention group at the June 2018 post-intervention assessment and at the August 2018 follow-up (intervention: M = 0.08, SD = 0.05; control: M = 0.42, SD = 0.26; adjusted difference = −0.30, p < 0.001). There were no significant differences between the two groups on the time spent outside during the daytime, time outside during the highest-risk period, percentage of outdoor time better protected by clothing, frequency of sunscreen application, average daily measure of confidence, automaticity, importance of photoprotection or mood.
The delayed intervention control group received the XPAND intervention in May 2019. Although effect sizes favoured the intervention, no statistically significant differences were observed between the two periods for the mean daily UVR dose-to-face, total UVR exposure, time outside, use of better photoprotection while outside or the number of times sunscreen was applied. Statistically significant differences were observed in favour of the intervention for daily self-reported ratings of mood, automaticity, confidence and importance of photoprotection.
The intervention group accrued on average 0.714 QALYs over the period from baseline to t3 compared with 0.699 for the control group. After adjusting for baseline quality of life, the intervention group accrued 0.014 fewer QALYs (95% CI, -0.037 to 0.003). The ICER was £187,376 for treatment as usual compared to the intervention.
Discussion
Participants who received XPAND had a significantly lower UVR D-to-F and lower exposure to UVR compared to controls. The size of the effect was large, indicating tangible clinical benefits associated with less DNA damage, although interpretation requires caution due to the failure of randomisation to balance baseline group differences in these outcomes.
Receipt of XPAND had a positive impact on D-to-F without diminishing emotional well-being. Moreover, the delayed intervention group reported an improvement in daily ratings of mood after completing the programme. This suggests that XPAND enabled participants to better manage photoprotection challenges that often elicited distress, such as coping with negative reactions from strangers when looking different while protecting.
This economic evaluation has shown that HRQoL and hence QALYs are similar between the two groups although slightly lower in the intervention group. Costs are much lower in the intervention group. However, neither difference is statistically significant. The sample size was small, and we cannot assume that the results are robust.
Strengths and limitations
The primary limitation of the study was the failure of randomisation to balance baseline differences in D-to-F between the intervention and control groups, because of the small size of the study. Although statistical analysis adjusted for baseline levels, the difference was too large to ascertain the true treatment effect size. By having a delayed control arm we had adapted the design to the rare disease context, which aided interpretation of the between-groups findings and increased confidence that XPAND was potentially effective.
Conclusion
Despite the challenges of evaluating an intervention in an extremely rare disease, our findings show that receipt of XPAND was associated with a lower UVR D-to-F, supported by exploratory analysis showing improved photoprotection activities and insight into psychological mechanisms contributing to these changes. That these benefits were achieved by a cost-effective intervention without a reduction in emotional well-being, justifies clinical rollout. Robust measurement of D-to-F showed that comprehensive UVR protection required reduction in the level of exposure alongside better photoprotection during exposure. Future adaptations of XPAND need to focus on strategies to reduce time out of doors during higher-risk times of the day, which is challenging given that this is influenced by factors potentially outside the individual’s control.
List of abbreviations
- BPAQ
- brief photoprotection adherence questionnaire
- CSRI
- Client Service Receipt Inventory
- DPS
- Daily Photoprotection Scale
- EMA
- ecological momentary assessment
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HRQL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- NICE
- National Institute for Health and Care Excellence
- PhotoSEQ
- photoprotection self-efficacy questionnaire
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SED
- Standard Erythemal Dose
- SRBAI
- Self-report Behavioural Automaticity Index
- SWEMWBS
- Short-form Warwick Edinburgh Mental Well-Being scale
- TAU
- treatment as usual
- UV
- ultraviolet
- UVR
- ultraviolet radiation
- XP
- xeroderma pigmentosum
- XPAND
- Enhancing Xeroderma Pigmentosum Photoprotection Activities – New Directions
Notes
-
The UVR protection diary
-
Example of personalised feedback form which includes the face protection guide, risk-ruler (goal-setting tool one)
-
Ultraviolet radiation dial (goal-setting tool two)
-
XPAND magazine front cover
-
Daily UVR protection diary used in trial (including daily questions)
-
XPAND interview topic guide
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/PZCW1478).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
