Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as award number RP-PG-1211-20011. The contractual start date was in September 2014. The draft manuscript began editorial review in February 2022 and was accepted for publication in January 2024. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Wells et al. This work was produced by Wells et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Wells et al.
Synopsis
Background
Cardiovascular disease and cardiac rehabilitation
Cardiovascular diseases (CVDs) are associated with approximately 25% (168,000) of all deaths each year in the UK. Survival rates are improving, with an estimated 7.64 million people in the UK living with heart or circulatory diseases. 1 Cardiac rehabilitation (CR) is recommended by the UK Department of Health and Social Care, the National Institute for Health and Care Excellence (NICE) and the British Association for Cardiovascular Prevention and Rehabilitation (BACPR) for eligible patients following a cardiovascular event. Components of CR focus on health behaviour change and education, lifestyle risk factor management and psychosocial management. 2 CR has been shown to be a cost-effective intervention that leads to a reduction in cardiovascular mortality and risk of hospital admissions, while also improving health-related quality of life. 3,4
Mental health provision in cardiac rehabilitation
The psychological impacts of CVD are considerable, with patients reporting high levels of anxiety and depression that have been linked to increased mortality, poorer quality of life, greater social problems and higher healthcare costs. In a recent analysis,5 19% of patients entering CR were classed as having borderline or clinical depression and 28% were classed as having borderline or clinical anxiety on the Hospital Anxiety and Depression Scale (HADS). An analysis of health records showed that 54.5% of CVD patients who reported consistently high levels of anxiety and depression were referred to a specialist or had active psychological management with a general practitioner (GP). 6 There is no standardised approach for psychological interventions in CR, and interventions can vary between stress management, counselling, relaxation, meditation and cognitive challenging of negative thoughts. Research on psychological interventions within CR is generally of low quality, with usually small reductions of psychological symptoms reported and limited improvement seen in anxiety, low mood and health-related quality of life. 7
Novel applications of metacognitive therapy: a translational approach
Metacognitive therapy (MCT)8 is a treatment approach based on the hypothesis that anxiety and depression are maintained by a common maladaptive thinking style, called cognitive attentional syndrome (CAS), of sustained, repetitive negative thinking, increased attention to threat and dysfunctional coping mechanisms. CAS is linked to biased metacognition, which is that part of cognition responsible for regulating thinking. Important components of metacognition are the beliefs a person holds about thinking, which in the metacognitive model can be defined as positive or negative. Positive metacognitive beliefs concern the usefulness of worry as a coping strategy (e.g. ‘worrying helps me find answers to my problems’), whereas negative metacognitive beliefs concern the uncontrollability and danger of thoughts and feelings (e.g. ‘I cannot stop worrying about the future’ or ‘thinking like this means I am losing my mind’). Such beliefs are considered to underlie unhelpful reactions to negative thoughts about life events, such as the thought ‘what if I have another heart attack?’. In comparison with other treatment approaches, MCT does not require in-depth analysis and challenging of the content of negative thoughts or worry, instead focusing on reducing unhelpful processing styles (e.g. reducing worry frequency and duration) in response to negative thoughts. 8 MCT has been demonstrated as highly effective in reducing symptoms of anxiety, depression and maladaptive metacognitions in people with mental health problems. 9–12 In a recent meta-analysis in mental health, MCT was found to be more effective in reducing anxiety and depression symptoms than other psychological therapies such as cognitive–behavioural therapies (CBT). 13
The chief investigator of this programme of research, Adrian Wells, is the originator of MCT and the director of the Metacognitive Therapy Institute. Therefore, it is important to draw attention to the steps taken throughout the PATHWAY research programme to maintain objectivity. These steps included masking to patient allocation, data management undertaken by a separate clinical trials unit, prespecified data analysis plans, pre-trial registration, the publication of trial protocols and project monitoring by an independent Trial Steering Committee (TSC).
The PATHWAY study
Current CR approaches vary considerably in the level and type of psychological interventions used, with many CR services offering little or no psychological input. Furthermore, trials investigating the efficacy of specific psychological interventions and techniques in heart disease are often of low quality. 7 In line with guidelines from BACPR, CR programmes offer a choice of treatment approaches in order to deliver a menu-based strategy to meet individual patient needs. 2 Currently, 75.4% of patients choose to undertake group-based CR, with 8.8% choosing home-based treatment, while 42.2% of patients engage with two or more modes of CR delivery. 5 Therefore, psychological interventions might offer similar variation in treatment delivery, providing the option for group- and home-based treatment to be integrated into existing CR and to maintain improved access to psychological treatment.
The PATHWAY programme aimed to improve access to more effective psychological interventions for patients attending CR services. This was approached through investigating the effects associated with introducing MCT alongside CR in group- and home-based formats.
Aims and objectives of PATHWAY
The primary aim of PATHWAY was to improve access to more effective psychological interventions for a range of heart disease patients attending CR services. We aimed to integrate two metacognitive interventions: a group intervention and a home-based intervention. The project set out to achieve the following objectives:
-
Conduct a pilot randomised controlled trial (RCT) of a group MCT (Group-MCT) for patients with depression and/or anxiety.
-
Establish evidence for the effectiveness and cost-effectiveness of Group-MCT in a full- scale RCT.
-
Produce a rigorous, well-specified Group-MCT package.
-
Develop a home-based metacognitive intervention (Home-MCT) for patients with depression and/or anxiety.
-
Establish the feasibility and acceptability of integrating Home-MCT into the CR pathway.
-
Establish provisional evidence of the effectiveness and cost-effectiveness of Home-MCT.
-
Develop a protocol and manual to inform a full-scale RCT of Home-MCT.
To meet these objectives, we developed a series of work streams (WSs) with integrated qualitative and health economic evaluations. Figure 1 shows the research pathway diagram and Table 1 gives an overview of the original programme objectives, WSs and outputs.
FIGURE 1.
Research pathway diagram.
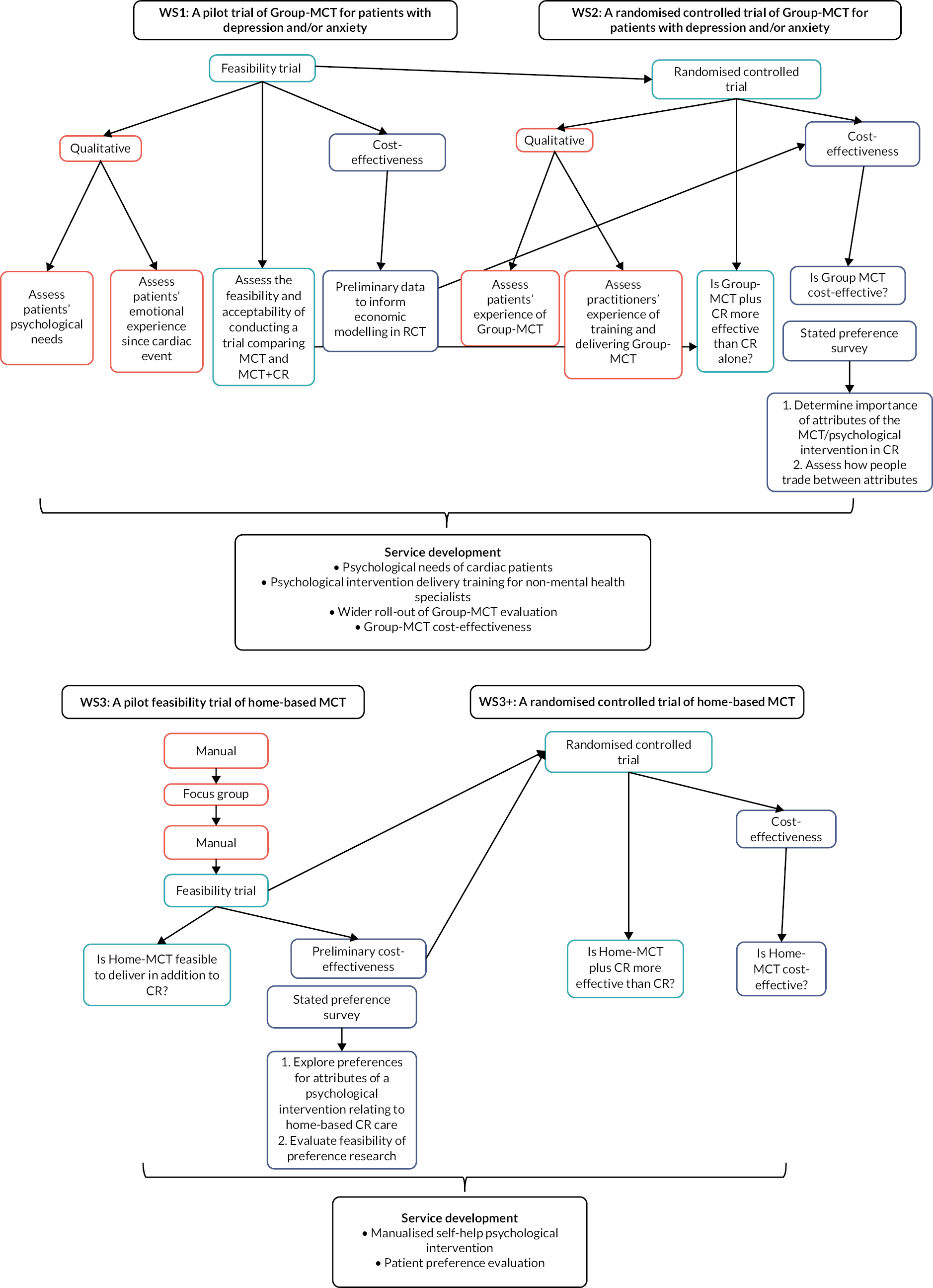
| Programme objectives | Research activity | Programme outputs |
|---|---|---|
| WS1: to conduct a pilot trial of Group-MCT for patients with depression and/or anxiety | Development of Group-MCT manual | Group-MCT intervention including treatment manual for practitioners, patient booklet and practitioner training |
| Qualitative interviews | McPhillips et al.1 McPhillips et al.15 |
|
| Pilot trial to assess the acceptability and feasibility of conducting a study in CR | Wells et al.16 Wells and Faija17 |
|
| WS2: to conduct a full-scale RCT to evaluate the effectiveness and cost-effectiveness of Group-MCT + usual CR compared with usual CR alone | RCT to assess the effects associated with and cost-effectiveness of Group-MCT + usual CR vs. usual CR alone Discrete choice experiment to investigate preferences for the delivery psychological therapy intervention in CR (clinic-based) PPI evaluation and framework |
Wells et al.18 Wells et al.19 Shields et al.3 Shields et al.20 Shields et al.21 McPhillips et al.;22 Anderson et al.23 Shields et al. (see Appendix 2) Wells et al.24 Wells et al.25 Shields et al.26 Capobianco et al.27 |
| WS3: to develop a home-based MCT intervention (Home-MCT) and then evaluate the acceptability and feasibility of integrating Home-MCT into the CR pathway in a feasibility trial | Development of Home-MCT manual | Home-MCT manual comprising six modules accompanied by three telephone support calls from MCT-trained CR staff |
| Feasibility trial to evaluate the acceptability and feasibility of Home-MCT Qualitative interview and focus groups |
Wells et al.28 Wells et al.29 Supplementary WS2/3 outputs: Faija et al.30,31 Capobianco et al.32 |
|
| Pilot discrete choice experiment to investigate preferences for delivery of psychological therapy intervention in CR (home-based) | Shields et al.33 | |
| WS3+: to conduct a full-scale RCT to evaluate the effectiveness and cost-effectiveness of Home-MCT + usual CR vs. usual CR alone | RCT to assess the effects associated with Home-MCT + usual CR vs. usual CR alone | Wells et al.34 |
Work stream 1 was a pilot trial of Group-MCT for patients with depression and/or anxiety. We undertook an initial small-scale pilot trial to establish the acceptability to CR patients and therapists (CR staff trained to deliver the manualised MCT treatment) of adding MCT to usual CR. The pilot also evaluated the feasibility of conducting a full-scale RCT of the intervention.
Work stream 2 was a full-scale RCT to evaluate the effectiveness and cost-effectiveness of Group-MCT plus usual CR compared with usual CR alone. Progression to WS2 depended on the findings of WS1 with regard to the acceptability and feasibility of delivering MCT and implementing a full-scale RCT.
Work stream 3 was to develop a home-based MCT intervention (Home-MCT) and then evaluate the acceptability and feasibility of integrating Home-MCT into the CR pathway in a feasibility trial.
We were able to fully meet the programme objectives:
-
WS1 demonstrated that a trial of Group-MCT added to usual CR was feasible and acceptable and confirmed our original sample size estimate.
-
The WS2 and WS3 trials recruited to target and had excellent retention.
-
Group-MCT + CR was found to be more effective than CR alone at 4- and 12-month follow-up.
-
Home-MCT + CR was found to be feasible and acceptable and was extended to a full-scale trial under a variation to contract (VTC) to utilise a study underspend.
-
The full-scale trial of Home-MCT demonstrated that the treatment was associated with significantly improved psychological outcomes when added to usual CR.
-
We co-designed the Home-MCT intervention with patients and clinicians.
-
We completed a cost-effectiveness analysis of group- and home-based MCT.
Summary of changes to original aims
Following the completion of WS3, we submitted a VTC on 29 January 2019 to progress WS3 from a feasibility trial to a full-scale RCT (WS3+). The VTC was awarded on 12 March 2019. The following was added as the aim of WS3+: to assess the effects associated with home-based MCT.
Work stream 1 a pilot trial of Group-MCT for patients with depression and/or anxiety
Some parts of the sections that follow have been reproduced with permission from Wells et al. 16 and McPhillips et al. 14,15 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Work stream 1 overview
Anxiety and depression are common among CR patients. However, existing psychological interventions used in CR produce only modest reductions in emotional distress. An alternative therapy currently not used in CR, MCT, has shown promising results in improving anxiety and depression in mental health settings and in patients with physical illnesses. WS1 aimed to evaluate the acceptability and feasibility of delivering Group-MCT to CR patients experiencing anxiety and depression.
Work stream 1 was a multicentre pilot feasibility study with 4- and 12-month follow-up comparing Group-MCT plus usual CR (intervention) with usual CR alone (control). The study was designed as an internal pilot of the full-scale RCT (WS2). Prespecified criteria for progression to a full RCT were (1) a mean recruitment rate of 8.7 per month, with a rate of 10 per month being desirable; (2) ≥ 65% of participants in the MCT arm attending at least four of the six Group-MCT sessions; and (3) 75% retention at 4-month follow-up. Additionally, the pooling of data collected under the pilot with those collected under the full RCT in the final analysis depended on no substantial changes being made to the trial procedures (e.g. patient eligibility criteria, follow-up schedule) or the trial instruments (e.g. outcome measures) as a result of the pilot. Both study progression and the pooling of data sets required the agreement of the TSC and NIHR as the funding body. The study was approved by the National Research Ethics Service of the NHS (reference 15/NW/0163) and registered with a clinical trial database (ISRCTN reference ISRCTN74643496).
Collaboration with our patient and public involvement (PPI) advisory group took place throughout the study, with PPI members involved at every stage. WS1 recruited 52 CR patients who had elevated anxiety and depression scores on the HADS. Patients were recruited from three NHS trusts in north-west England: University Hospital of South Manchester NHS Foundation Trust, Central Manchester University Hospitals NHS Foundation Trust and East Cheshire NHS Foundation Trust.
The results of the pilot study provided evidence that Group-MCT was acceptable and feasible to deliver within CR. With the agreement of the TSC and NIHR, we progressed directly to a full-scale randomised trial of adding Group-MCT to CR (WS2). The pilot study found that no substantial changes to the trial procedures or instruments were required; therefore, agreement was also given to merge the pilot and main trial data in the RCT analysis. The re-estimation of the full trial sample size based on the pilot data and available resources resulted in a decision to increase the total recruitment target to 332, providing 90% power to detect the desired 0.4 effect size.
Aims and objectives
-
Confirm procedures for recruitment, randomisation, intervention delivery and data collection prior to a full-scale trial.
-
Collect data on recruitment and retention rates, and variability and clustering, in outcome measures, to confirm the sample size calculation and timeline for the full-scale trial.
-
Obtain preliminary economic data to inform economic modelling in the full-scale trial.
-
Include outcome data in analysis of the full-scale trial if no changes are required to the key features of the trial following the pilot.
-
Interview Group-MCT patients, including those who declined to participate or dropped out, to assess their (1) emotional experience since the index event, (2) interaction of emotional state with clinical care, (3) reactions and expectations on being offered the intervention and (4) for those engaged, their perceptions of the intervention.
-
Interview control patients to assess their (1) emotional experience since the index event and (2) interaction of emotional state with clinical care.
Methods
Work stream 1 was delivered in accordance with the grant proposal and employed a randomised pilot feasibility study with 4- and 12-month follow-up comparing Group-MCT plus usual CR (intervention) with usual CR alone (control). The study was designed as an internal pilot of the full-scale RCT (WS2).
Fifty-two CR patients with elevated anxiety and/or depression were recruited to a single-blind randomised feasibility trial between July 2015 and February 2016 from three NHS trusts in north-west England (University Hospital of South Manchester NHS Foundation Trust, Central Manchester University Hospitals NHS Foundation Trust and East Cheshire NHS Foundation Trust). The target sample size was originally 50 patients (25 per arm), determined as sufficient to evaluate recruitment and retention rates for a full-scale trial as well as rates of completion of the intervention. This sample was also adequate for estimating variability in outcome measures for which samples of 40 are generally considered sufficient. However, parallel recruitment across sites meant that 52 patients had consented by the end of the recruitment period and were included in the sample.
After giving informed consent, patients were randomly allocated to a trial condition in a 1 : 1 ratio using a minimisation algorithm that incorporated a random component in order to maximise balance between the arms in sex distribution, HADS anxiety and depression scores, and hospital site. Randomisation was conducted via a telephone link to the Manchester University Clinical Trial Unit (Manchester CTU).
The acceptability and feasibility of adding Group-MCT to CR was evaluated with respect to recruitment rates; attrition by the primary end point of 4 months; number of MCT and CR sessions attended; completion of follow-up questionnaires; and ability of the outcome measures to discriminate between patients. The study was also used to re-estimate the required sample size for a full-scale trial. We also examined the extent to which non-specialists in mental health (i.e. CR health providers) adhered to the Group-MCT protocol. For details of the data collection method, see the protocol. 17
Trial population
The following inclusion criteria were applied:
-
Fulfilment of Department of Health and Social Care and/or BACPR CR eligibility criteria (acute coronary syndrome, revascularisation, stable heart failure, stable angina, implantation of cardioverter defibrillators/cardiac resynchronisation devices, heart valve repair/replacement, heart transplantation and ventricular assist devices, adult congenital heart disease, other atypical heart presentation)
-
A score of ≥ 8 on either the depression or the anxiety subscale of the HADS35
-
Age ≥ 18 years
-
A competent level of English-language skills (able to read, understand and complete questionnaires in English).
The following exclusion criteria were applied:
-
Cognitive impairment that precludes informed consent or ability to participate
-
Life expectancy of < 12 months
-
Acute suicidality
-
Active psychotic disorders
-
Current drug or alcohol abuse
-
Antidepressant or anxiolytic medications initiated in the previous 8 weeks
-
Concurrent psychological intervention for emotional distress.
Cardiac rehabilitation (treatment as usual)
Usual CR programmes comprise two components: exercise and educational sessions. CR programmes vary in content by site; however, all participating sites are offered core components (BACPR Standards and Core Components2) primarily using group-based delivery as part of outpatient provision in hospital or community settings supported by a multidisciplinary team. CR programmes across all sites ran weekly over 8–10 weeks. Exercise sessions were delivered in groups, with a therapist-to-patient ratio of 1 : 5 for low- and moderate-risk patients and 1 : 3 for high-risk patients. Educational seminars lasted 45–60 minutes and covered lifestyle and medical risk factor management. Additionally, all sites provided psychosocial intervention, including stress management and relaxation talks. Relaxation sessions at all sites included breathing techniques and progressive muscle relaxation. Two sites delivered psychoeducational talks on stress, while three sites included cognitive therapy methods for stress management (i.e. challenging negative thoughts, worry decision tree, behavioural activation). One site offered a 4-week stress management course as part of CR.
Group-metacognitive therapy
The MCT + CR intervention received group-based MCT in addition to the usual CR programme at their site. Group-MCT was delivered in six sessions lasting 60–90 minutes each, held once per week and facilitated by two CR professionals (i.e. physiotherapist, CR nurses and occupational therapists) or research nurses depending on the site. CR staff received basic training in implementing the treatment manual. Therapists completed a 2-day workshop delivered by the developer of MCT (AW). Training included didactic teaching, role-play, discussion and studying of the treatment manual. In addition, therapists delivered the intervention to a pilot group of volunteers along with an additional 1-day workshop that focused on enhancing initial skills. Therapists received ongoing supervision on an occasional basis while they were delivering the intervention.
Group-MCT focused on helping participants identify thoughts leading to the processes of worry, rumination and unhelpful coping behaviours. Participants were then guided through the practice of specific techniques to aid flexibility of and control over extended negative thinking patterns. Homework practice of the techniques was featured throughout the programme. At the end of treatment, patients received a ‘helpful behaviours’ prescription summarising what they had learned. Therapists’ adherence to the trial protocol was assessed through their completion at the end of each session of a checklist identifying the protocol components that had been implemented.
Data monitoring
Data monitoring, quality and handling were undertaken by the Manchester Clinical Trials unit and project oversight was conducted by an independent TSC.
Analysis
We assessed the feasibility and acceptability of adding Group-MCT to usual CR.
Feasibility outcomes included:
-
Completion of follow-up questionnaires (proportions of missing values, both overall and within-trial arms)
-
Ability of the outcome measures to discriminate between patients (range of scores, floor or ceiling effects)
-
Re-estimation of the required sample size based on the findings of this study (number of recruited patients required to detect an effect size of 0.4 on HADS total score at 80% power, controlling for baseline scores and allowing for attrition and clustering of patients within therapy groups)
-
Therapist adherence to study protocol
Acceptability outcomes included:
-
Study recruitment rate (number agreeing to participate out of those approached, and number recruited per month)
-
Withdrawal or drop-out by the primary end point of 4 months (attrition rate)
-
Numbers of MCT and CR sessions attended
-
Therapist adherence to study protocol
Results
Feasibility and acceptability of a trial of Group-MCT
The results of the feasibility study have been published. 16 Participants were recruited between July 2015 and February 2016, and 38% of eligible patients were consented and randomised to the study, resulting in a recruitment rate of approximately 6.5 patients per month. Fifty-two participants (33 male and 19 female) were recruited, with 23 participants allocated to Group-MCT + CR and 29 allocated to CR alone. The mean age of participants was 58.67 years (standard deviation 9.47 years, range 38–79 years).
Retention at both 4- and 12-month follow-up was reasonable. At 4-month follow-up, 72.4% of patients in the control arm returned follow-up questionnaires; one (3.5%) participant withdrew from the study, six (20.7%) participants did not return the questionnaires and one (3.5%) questionnaire pack was lost in the post. The return rate of the intervention arm questionnaires was 69.6%; four (17.4%) participants formally withdrew from the study, two (8.8%) participants did not return the questionnaires and one (4.4%) questionnaire pack was lost in the post.
All questionnaires demonstrated a good range of observed scores, covering the majority of the possible score range, and with little in the way of floor or ceiling effects.
The trial did not negatively impact on attendance at usual CR, which was much the same in both trial arms. Participants attended a median of six sessions, with 58.6% of the CR-alone arm and 52.2% of the Group-MCT + CR attending at least six sessions of CR.
Among those allocated to the Group-MCT intervention, 56.5% of patients (n = 13) attended at least four of the six sessions, 21.7% (n = 5) attended one or two sessions and 21.7% (n = 5) did not attend any sessions. Therapist adherence to the MCT treatment protocol was monitored using an adherence checklist. Therapists were asked to indicate if specific components of the intervention had been completed at each session. Adherence was high at an average rating of 98.2% across all sites, with all sites deviating from the protocol only once.
Progression to a full randomised controlled trial
The decision to progress or not to a full RCT was made based on the data available at the time of submission of the study milestone report. At this point all patients had been recruited but only 18 had reached the 4-month follow-up point. The results at this point differed somewhat from those given above for the full study sample. The study’s overall recruitment rate of 6.5 patients per month reflected a slow start, followed by an average of nine patients recruited per month over the final 3 months. Seventeen patients (94%) had returned the 4-month follow-up questionnaire. Of 10 patients in the Group-MCT + CR arm, 6 (60%) had attended at least four treatment sessions: although slightly below the target of 65%, the small sample made this figure subject to large uncertainty. On the basis of these results and other evidence for acceptability and feasibility, including the qualitative work with patients and therapists, the NIHR as funder agreed that the research could progress to a full-scale RCT. To address the shortfall due to slow early recruitment, the study was expanded to include an additional two sites.
Sample size
Under assumptions of 25% attrition, a correlation of 0.5 between baseline and follow-up outcome scores, mean therapy group size of 5.75, and intracluster correlation coefficient (ICC) of 0.05, we originally estimated that a total recruitment sample of 230 patients for the main trial would provide 80% power to detect a treatment effect size of 0.4. As no substantial changes were made to the trial procedures or instruments following the pilot, with the consent of our TSC and NIHR as the funder the decision was taken to merge the pilot data with those of the main trial. Considering the updated parameters from WS1 [a 35% attrition rate, correlation of 0.5 (unchanged), mean group size of 3 and ICC of 0.05 (assumed)] and available resources, we revised the total recruitment target to 332 to give the full study 90% power to detect the desired 0.4 effect size.
Conclusion
The results suggested that a full-scale trial of Group-MCT within CR was feasible and acceptable to deliver.
Qualitative evaluations
Study participants (intervention and control) were interviewed in semi-structured qualitative interviews to explore the potential enablers of/barriers to recruitment/retention at several levels: patient (e.g. attitudes to emotional needs and support), intervention (e.g. comprehensibility) and service (e.g. practices or staff communications that contradict or support the intervention).
Some parts of these sections have been reproduced with permission from McPhillips et al. 14 and McPhillips et al. 15 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Ethics approval was obtained from NRES Committee Northwest REC (reference 15/NW/0163). All participants provided written informed consent prior to being interviewed. Qualitative interviews were audio-recorded, transcribed verbatim and pseudonymised. Analysis varied depending on the research question.
Analysis of the data to determine CR patients’ emotional distress and psychological needs followed a constant comparative approach that occurred in parallel with interviews to develop a thematic framework. The framework was further developed with subsequent interview transcripts. 14 The analysis was evaluated based on its ‘catalytic’ and ‘theoretical’ validity, whereby findings had practical implications and connected with broader theory. The analysis was inductive in that they presented features of patients’ accounts based on emerging data/transcripts rather than on the significance for a priori theories. Theoretical frameworks were drawn on after the analysis was complete to consider the implications of the findings. 14,36
Analysis of qualitative interviews also aimed to understand patient distress from the perspectives of CBT and MCT and comprised three stages: inductive analysis followed by a constant comparative approach, and, finally, reviewing transcripts combining deductive and inductive elements. 15 The exploration of patients’ experience of MCT used thematic analysis. Using a systematic approach, codes were produced by grouping similar concepts together to identify key themes.
Psychological experiences and psychological needs of cardiac rehabilitation patients
We conducted a qualitative study using semi-structured interviews of 46 CR patients who had elevated symptoms of anxiety and/or depression. 14 The study aims included:
-
Understanding how distressed cardiac patients describe their emotional needs
-
Understanding how CR patients described and understood their distress
-
Exploring patients’ thoughts about how well they thought their current CR and routine care addressed their psychological needs and their views on the role of formal psychological interventions
Patients often described their emotional experience since their cardiac event as negative, reporting how they felt low in mood and often engaged in worrying about and dwelling on a range of concerns, including ones that were unrelated to their health and predated their cardiac event. 14 We did not find differences in accounts between men and women or between patients from different centres.
While patients were found to worry about and dwell on a range of topics, which is in line with findings of previous studies,36,37 they also described how they believed that worrying was uncontrollable and harmful, and they worried about worry (a process known as metaworry). The concerns CR patients have about worry (i.e. metaworry) have not been described previously; however, they are central to the metacognitive model38–40 and clarify how CR patients’ distress might be better addressed.
Patients described how they wanted to ‘get back to normal’ and stop worrying. 14 They felt that they lacked a way to achieve this other than waiting for time to pass, and when they did seek support, they sought reassurance from staff and peers to check that they were responding ‘normally’. 14 However, the effects of reassurance were generally transient and, consistent with previous findings, appeared to have little benefit for patients with cardiac symptoms. 41,42 A new and potentially important finding was that, despite wanting reassurance, most patients were reluctant to talk about their worries in the context of CR unless they had been previously socialised into psychological interventions. 1 Furthermore, despite being troubled by worry, most were dismissive of stress management and guided relaxation techniques offered in the context of existing CR. These techniques seemed superficial and difficult to apply in real life and needed more practice than CR provided. Patients also noted that they associated CR primarily with exercise classes and physical rehabilitation, which is in line with previous research, which may be a barrier to using CR as a setting to support mental health. 43
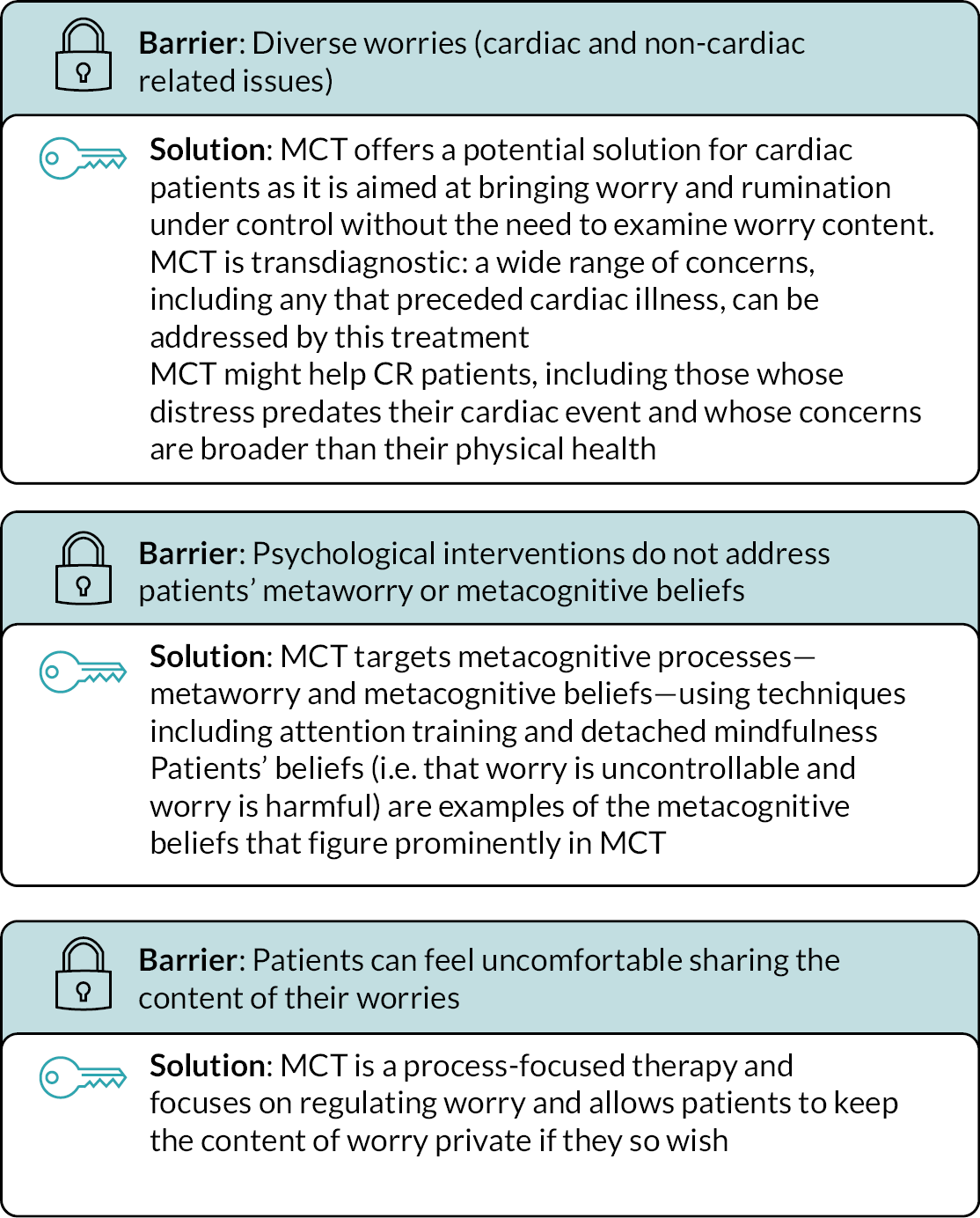
Using MCT may overcome some of the barriers associated with current psychological approaches in CR, as summarised in Figure 2.
FIGURE 2.
Barriers faced in current CR psychological treatment and solutions provided by MCT: a visual summary of discussion in our qualitative study. 14
Assessing the ‘fit’ of the metacognitive model versus the cognitive–behavioural model in dealing with anxiety and depression in cardiac rehabilitation
The acceptability of the metacognitive model for CR patients was assessed both alone and comparatively with a model more frequently used in NHS services, CBT. 15 Although CBT achieves moderate effects in CR for patients experiencing anxiety and depression,7 it may be that a psychological theory that does not focus on the content of patients’ negative thoughts (e.g. ‘I might have another heart attack’) but instead focuses on regulating worry and rumination may be better able to moderate the diverse range of concerns linked to anxiety and depression. By comparing patients’ perspectives via an interview guide designed to assess their concerns and causes of distress, it became apparent that worry and rumination exacerbated distress and that this perseverative negative thinking often began with a realistic negative thought, for example ‘I’ll never get back to full fitness, I’ll never live a full life in the same way’. Although it is evidently the case that CR patients will experience such thoughts, it is not always the case that they will continue to think those thoughts. CBT seeks to challenge such realistic thoughts, which is frequently unachievable. MCT would enable patients not to further engage with such thoughts, thereby limiting the time spent ruminating and having the positive effect of reducing the extent of negative thinking. Findings from the study15 illustrated that MCT may have a better fit with the experiences of CR patients; this overall conclusion was based on a sample of 49 patients who took part in a thematic interview. 15 This group of patients reported a diverse range of worries but were reluctant to discuss them, offering the ideal opportunity for MCT to be used to overcome the distress of these patients as with this approach there is no need to discuss the content of worries. Conceptualising patients’ distress from the perspective of CBT involved applying many distinct categories to describe specific details of patients’ talk, particularly the diversity of their concerns and the multiple types of cognitive distortion. It also required distinction between realistic and unrealistic thoughts, which was difficult when thoughts were associated with the risk or consequences of cardiac events. From the perspective of MCT, a single category – perseverative negative thinking – was sufficient to understand all this talk, regardless of whether it indicated realistic or unrealistic thoughts, and could also be applied to some talk that did not seem relevant from a CBT perspective.
Work stream 2 a randomised controlled trial of Group-MCT for patients with depression and/or anxiety
Some parts of these sections have been reproduced with permission from Wells et al. 19 This is an Open Access article, distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Work stream 2 overview
A full-scale, two-arm, single-blind RCT with a nested qualitative study was conducted to assess the clinical effectiveness and cost-effectiveness associated with the Group-MCT intervention plus usual CR (MCT + CR). CR services from five NHS trusts across north-west England (University Hospital of South Manchester, Central Manchester University Hospitals NHS Foundation Trust, East Cheshire NHS Trust, Stockport NHS Foundation Trust and Pennine Acute Hospitals NHS Trust) recruited 332 patients attending CR with symptoms of anxiety and/or depression. Participants were randomly assigned to receive MCT + CR or usual CR alone. Patients assigned to receive MCT + CR attended six additional weekly sessions of Group-MCT led by two CR staff members, with each session lasting 60–90 minutes. The primary outcome was level of anxiety/depression as measured by total HADS score at 4-month follow-up. Secondary outcomes included HADS score at 12 months plus scores on the Impact of Events Scale Revised (IES-R), Metacognitive Beliefs Questionnaire-30 (MCQ-30), EuroQol-5 Dimensions, five-level version (EQ-5D-5L) and Cognitive Attentional Syndrome Scale-1 Revised (CAS-1R) at 4- and 12-month follow-up. At 4 months, patients in the MCT + CR arm had significantly reduced total HADS scores compared with those in the CR-alone arm. At 12 months, the group difference was reduced but still statistically significant (p < 0.01). The results for most secondary outcomes also favoured the MCT + CR arm at both 4 and 12 months. The protocol of this trial and the principal results have been published. 17,19
A qualitative interview study found Group-MCT to be effective, positive and beneficial. Patients identified advantages of the group format linked to non-specific supportive factors, and they valued the techniques used in treatment and supported delivery of the intervention by non-mental health specialists. The full qualitative results have been published. 22
Work stream 2 aims
-
Evaluate the effectiveness of MCT + CR compared with usual CR alone in alleviating depression and/or anxiety in patients attending CR.
-
Evaluate the impact of Group-MCT on secondary outcomes including post-traumatic stress, metacognitive beliefs, health status, adherence to CR, health and social care utilisation and work resumption.
-
Assess the durability of treatment outcomes at 4- and 12-month follow-up.
-
Obtain patient qualitative data to help interpret evidence of effectiveness, including processes that might underpin or compromise effectiveness or explain heterogeneity in effectiveness.
-
Obtain practitioner qualitative data to evaluate practitioner experience of Group-MCT delivery and understanding of patients’ emotional needs and identify potential enablers of/barriers to the recruitment and retention of patients.
-
Obtain data from a stated preferences survey about participants’ relative preferences, utility and willingness to pay (WTP) for components of Group-MCT to inform future policy and commissioning decisions.
-
Establish the cost-effectiveness of Group-MCT.
Methods
We conducted a multicentre, two-arm, single-blind RCT with 4- and 12-month follow-up comparing Group-MCT plus usual CR (MCT + CR) with usual CR alone.
Participants were recruited from CR centres across five NHS trusts in north-west England (University Hospital of South Manchester, Central Manchester University Hospitals NHS Foundation Trust, East Cheshire NHS Trust, Stockport NHS Foundation Trust and Pennine Acute Hospitals NHS Trust). No changes were made to the study eligibility criteria following WS1; see WS1 for a full description. For details of patient recruitment and study eligibility, see the published trial protocol. 17 Patients were randomly allocated to the trial arms by Manchester Academic Health Science Centre Clinical Trials Co-ordination Unit using a computer in a 1 : 1 ratio using a minimisation algorithm to balance the trial arms with respect to hospital site, sex and HADS scores. Patients were informed of their trial arm allocation by a member of the research team. The trial chief investigator, trial statistician and research assistants collecting assessment data were masked to treatment allocation.
Group-MCT intervention (MCT + CR)
No changes were made to the Group-MCT intervention following WS1 (pilot feasibility study); see WS1 for a description of the intervention.
Usual CR
Usual CR was delivered as described above.
Data collection and outcomes
Data collection and outcomes mirrored the pilot study; see the trial protocol for details. 17
Analysis
Analysis was conducted in accordance with a prespecified analysis plan specifying the analytical models, primary and secondary outcomes, choice of covariates, sensitivity analyses and other key aspects of the analysis. Prior to data analysis or unmasking, the analysis plan was finalised and approved by the TSC.
The primary outcome was:
-
HADS total score at 4-month follow-up (after treatment).
The secondary outcomes included:
-
HADS total score at 12-month follow-up
-
Post-traumatic stress symptoms measured on the IES-R at 4- and 12-month follow-up
-
Metacognitive beliefs measured on the MCQ-30 total and uncontrollability and danger subscale at 4- and 12-month follow-up
-
Health status measured on the EQ-5D-5L at 4- and 12-month follow-up
-
Repetitive negative thinking and coping mechanisms measured on the CAS-1R at 4- and 12-month follow-up
-
Adverse events related or unrelated to the study
The primary analyses used intention-to-treat principles. A linear mixed-effects regression model was applied for continuous outcomes, incorporating all three time points (baseline, 4 months and 12 months). The prespecified covariates used were randomisation factors (hospital site, sex, baseline total HADS score), age and medication for depression or anxiety (never taken/currently taking/taken in the past). All other potential covariates were below predefined imbalance criteria for sensitivity testing [standardised mean difference (SMD) > 0.25 or category difference of > 10% between arms]. We applied hierarchical regression models with random effects at the levels of the patient and the CR (or MCT + CR) course attended. The covariance matrix for the model was chosen as either unstructured or first-order autoregressive depending on whichever gave the lower Bayesian information criteria score.
The effects associated with the intervention at 4- and 12-month follow-up were examined using the treatment-group-by-time-point interaction terms from the mixed-effects model analysis, where time point was a categorical variable to provide independent tests of effect at 4 and 12 months. No adjustments for multiple testing were applied, and an alpha value of 5% was used throughout. Sensitivity analysis was conducted using multiple imputation (MI) to assess the robustness of the results against missing values. There were very few missing values at baseline (one missing outcome value and a maximum of three missing values on any covariate); therefore, these were imputed by simple regression imputation using all available variables at baseline but excluding trial arm. MI was then used to impute missing outcome values at 4 and 12 months using the full set of variables and including the interaction term between trial arm and time point (for consistency with the analysis model). The chained-equations MI procedure was used and 20 MI data sets.
A mixed-effects logistic regression was conducted to assess the differences between the arms in engagement in economic activity (as a binary outcome) at 4- and 12-month follow-up. Covariates in the model were the same as for the continuous outcome measures.
All outcome measures demonstrated skewness and kurtosis below the threshold of 1.0 specified in the analysis plan and so sensitivity against non-normality was not assessed. The trial eligibility criteria allowed the inclusion of participants without clinically relevant anxiety provided they had at least mild depression, and vice versa; 23% and 40% of participants respectively fell into these categories at baseline, closely balanced between the arms. To determine how this might have impacted on analysis results for HADS anxiety and depression as separate outcomes we conducted sensitivity analyses excluding these individuals. Analyses were conducted using Stata version 14 (StataCorp LP, College Station, TX, USA).
Results
Participants: overview
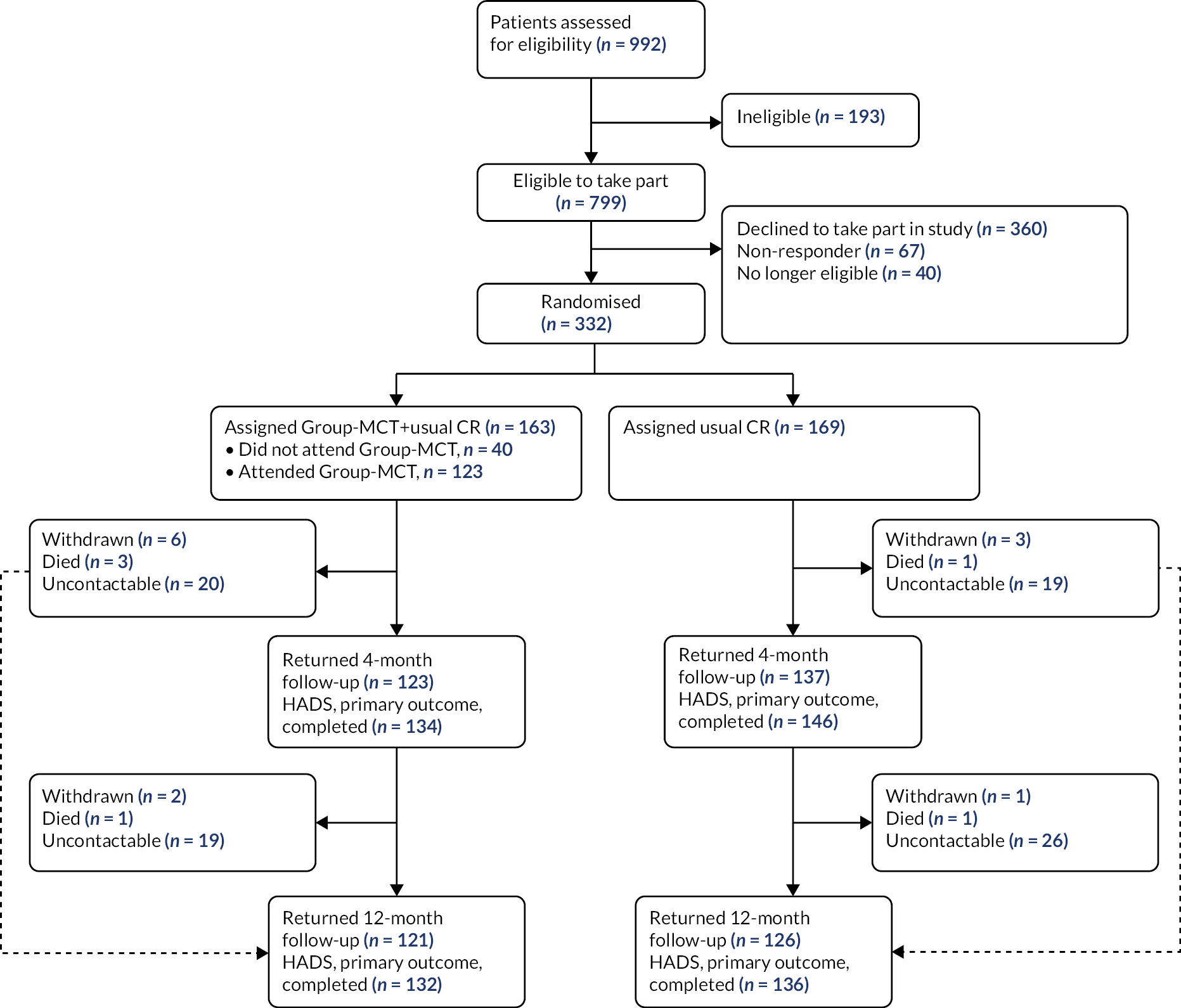
Between July 2015 and January 2018, 3808 patients were referred to CR across all five sites. A total of 992 patients had a score of ≥ 8 on the HADS subscales; of these, 332 were consented to the trial following eligibility screening and initial contact. One hundred and sixty-three patients were randomly allocated to MCT + CR and 169 patients were randomly allocated to usual CR alone (see Figure 3). For further details, see Wells et al. 19
FIGURE 3.
Work stream 2 trial profile. Figure reproduced with permission from Wells et al. 19 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Effects associated with Group-MCT
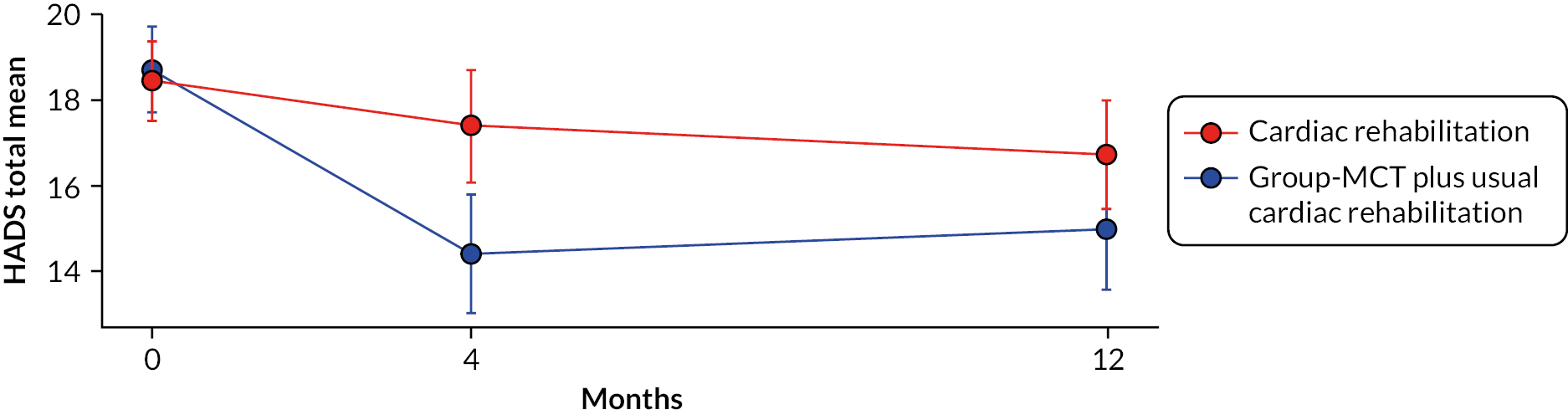
Mean HADS total scores and 95% confidence intervals (CIs) for each trial arm at pre- and post-treatment assessment points are presented in Figure 4. The mean HADS total score under CR alone declined gradually over time in an almost linear fashion, compared with a large reduction over the first 4 months under MCT + CR followed by a plateauing of scores. On the primary outcome – HADS total at 4 months – the results significantly favoured MCT + CR [adjusted mean difference (AMD) −3.24, 95% CI −4.67 to −1.81, p < 0.001; SMD 0.52]. The between-group difference in HADS remained significant at 12 months, albeit at a lower level (AMD −2.19, 95% CI −3.72 to −0.66, p = 0.005; SMD 0.33). Mean HADS anxiety was significantly lower in patients in the MCT + CR arm at 4 months (AMD −1.67, 95% CI −2.54 to −0.81, p < 0.001; SMD 0.44) and 12 months (AMD −1.35, 95% CI −2.22 to −0.48, p = 0.002; SMD 0.34). MCT + CR patients achieved a lower HADS depression mean score at 4 months (AMD −1.58, 95% CI −2.37 to −0.79, p < 0.001; SMD 0.47) but not at 12 months (AMD −0.85, 95% CI −1.75 to 0.05, p = 0.065; SMD 0.23).
FIGURE 4.
Unadjusted mean total HADS scores at baseline and at 4- and 12-month follow-up. Note: bars are 95% CIs. Figure reproduced with permission from Wells et al. 19 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Most other outcomes also favoured the MCT intervention: adjusted mean IES-R scores were lower with MCT + CR at 4 months (AMD −4.92, 95% CI −9.04 to −0.81; p = 0.019) but not at 12 months (AMD −3.28, 95% CI −7.92 to 1.36; p = 0.166); MCQ-30 total scores were lower at both 4 and 12 months (AMD −8.57, 95% CI −11.95 to −5.18, p < 0.001; AMD −7.37, 95% CI −11.24 to −3.50, p < 0.001, respectively); MCQ-30 negative beliefs subscale scores were lower at both time points (AMD −3.15, 95% CI −4.16 to −2.14, p < 0.001; AMD −2.35, 95% CI −3.43 to −1.26, p < 0.001) and the CAS-1R was also lower with MCT + CR at both 4 and 12 months (AMD −126.25, 95% CI −165.83 to −86.67, p < 0.001; AMD −116.29, 95% CI −159.13 to −73.45, p < 0.001). EQ-5D-5L utility scores showed no statistically significant group difference at 4 months (AMD 0.03, 95% CI −0.02 to 0.09; p = 0.200) or 12 months (AMD 0.03, 95% CI −0.02 to 0.10; p = 0.201); the difference on the EQ-5D-VAS was not significant at either time point (4 months: AMD 4.62, 95% CI −0.10 to 9.34, p = 0.055; 12 months: AMD 0.66, 95% CI −4.12 to 5.45, p = 0.786). Sensitivity analysis using MI changed the statistical significance of one secondary outcome, the IES-R at 4 months, which ceased to be statistically significant (p > 0.05). There was no significant difference between the arms in engagement in economic activity at 4-month (odds ratio 0.94, 95% CI 0.26 to 3.47; p = 0.93) or 12-month follow-up (odds ratio 1.10, 95% CI 0.24 to 4.99; p = 0.90).
To aid in the interpretation of the clinical impact of findings, the Reliable Change Index (RCI)44 was computed for the primary outcome. The RCI represents the difference between two measurements made in a single individual that would be statistically significant at a p-value of < 0.05. It was computed for the HADS total score at the primary 4-month follow-up. Using the control sample, we calculated a Cronbach’s alpha of 0.91 at 4 months for the HADS to estimate reliability for the usual CR population. Based on this, a reduction of 6 points in an individual’s score was defined as statistically reliable improvement, while an increase in 6 points was defined as a reliable worsening of symptoms. Calculations of the HADS total score at 4-month follow-up showed that 21% of patients in the CR-alone arm reliably improved compared with 33% of patients in the MCT + CR arm. The proportion of patients exhibiting psychological deterioration was 15% in the CR-alone arm compared with 4% in the MCT + CR arm.
Qualitative evaluation: overview
From the intervention arm of the trial, 32 patients took part in qualitative interviews prior to starting Group-MCT but during CR [time point 1 (T1)]. Patients who attended four or more Group-MCT sessions were defined a priori as having completed a minimal dose of the intervention likely to produce benefit. Among intervention patients who consented to take part in qualitative interviews, 22 completed the intervention, with 20 completing time point 2 (T2) interviews. Ten did not complete the intervention but five completed T2 interviews; four of these patients attended two or more Group-MCT sessions and their interviews were included in the analysis, and one patient interviewed did not attend any Group-MCT sessions due to work commitments and therefore was excluded from the analysis.
Interviews were conducted at T1 and T2 and were conversational in nature. Topic guides with a mixture of open and closed questions with open-ended prompts were used to encourage patients to share experiences and probe specific points. T1 interviews are discussed in WS1. Data gathered in T1 interviews were used to inform T2 interviews, which explored patients’ emotional experiences since T1, their views and experiences of Group-MCT, and their engagement with techniques from the intervention. Interviews were tailored to the individual, drawing on specifics from T1 interviews. Interview guides were modified iteratively as the interviews and analysis proceeded so that developing ideas could be tested. The interviews lasted an average of 52 minutes (range 14–88 minutes). The interviews were audio-recorded, transcribed verbatim and pseudonymised. Full detailed results have been published. 22
Ten CR staff delivering MCT were interviewed about their experiences of training in and delivery of Group-MCT. Group-MCT was initially delivered by seven CR practitioners across three CR services participating in PATHWAY. Six of these practitioners were interviewed before training, with one practitioner declining to be interviewed at this point. However, all seven were interviewed during training and after they had delivered Group-MCT, as the practitioner who had originally declined later contacted the research team to take part. Two more CR services later joined the study. Two CR practitioners from one of these services and two clinical research nurses (CRNs) were trained in Group-MCT. The two CR practitioners provided written informed consent and were interviewed during training and after they had delivered Group-MCT. One CRN was interviewed during training only, as she left the study shortly afterwards, and the other CRN declined to take part in the study.
Qualitative studies received ethics approval from NRES Committee Northwest REC (reference 15/NW/0163). All participants provided written informed consent prior to being interviewed.
Qualitative data analysis
Inductive thematic analysis was used. Data in each transcript were coded to explore patients’ experiences and understanding of Group-MCT. Generated codes were discussed within the research team and discrepancies were resolved during discussion. Coded data were reviewed and collated into candidate themes. Candidate themes were discussed by the research team and on agreement semantic themes were identified.
Patients experience of Group-MCT
Two main themes were identified in patient experience of Group-MCT: general therapy factors and MCT-specific factors. The first theme concerned general therapy factors central to positive experiences of treatment, with subthemes of interaction with other CR patients and CR staff’s delivery of the intervention. Interaction with other CR patients was an important factor to most patients, providing reassurance, normalisation of feelings in a positive environment and facilitation of the intervention. Patients who were in small groups found that this negatively impacted their experience of Group-MCT, as small groups affected the delivery of the therapy. This highlighted the importance of having a minimum of three or four patients in a group to optimise patient experience. The delivery of the therapy by CR staff was generally received positively by patients as it enabled a positive and relaxing environment. For some patients, CR staff’s specific knowledge and experience of cardiology was important to their delivery of the therapy. However, a minority of patients criticised the staff’s delivery by because of a perceived lack of knowledge and the style of delivery. Patients’ perceptions of CR staff’s delivery were overall positive and demonstrated that CR staff, who were not mental health specialists, established and maintained a therapeutic alliance valued by patients.
The second theme related to MCT-specific factors, with subthemes of patients’ perceptions and understanding of the aims, experiences of individual techniques and perceptions of effectiveness of Group-MCT. Accounts of the aims of Group-MCT varied from those consistent with the model to those that were ‘off-model’. Patients who did not complete Group-MCT had negative perceptions of the aims of the therapy. Patients who correctly understood the aims of the therapy appeared to demonstrate a greater flexibility in their reaction to worry. All patients who completed the intervention were positive about the techniques introduced in MCT. Most patients were able to use these techniques in a manner consistent with the therapy model. However, in some cases techniques were used in a manner not consistent with the model. The results suggest that the techniques used in Group-MCT are largely understood and beneficial; however, therapists should be mindful of patients’ potential misinterpretation and inappropriate use of the techniques based on these narratives.
Practitioners experience of training and delivery of Group-MCT
Aim
To assess CR staff’s experience of learning and delivering MCT.
Methodology
Cardiac rehabilitation staff were interviewed at three time points: before Group-MCT training, in the middle of training, and after delivering Group-MCT. A topic guide was developed and used to guide the interviews.
Nine CR staff delivering MCT were interviewed about their experiences of training in and delivery of Group-MCT. See Appendix 1, Table 4, for an overview of therapist demographic characteristics.
For the results, see Appendix 1.
Cost-effectiveness of group-metacognitive therapy
Aims
A within-trial economic evaluation aimed to compare the cost-effectiveness of MCT plus usual care (CR) with that of CR alone from a health and social care perspective in the UK. The protocol of the cost-effectiveness evaluation has been published21 and details of the findings of the evaluation are reported in Appendix 2.
Methods
The economic evaluation used intention-to-treat and estimates total costs and quality-adjusted life-years (QALYs) for the trial follow-up (cost–utility analysis). An NHS and social care perspective was taken. The time horizon of the primary analysis was 12 months to incorporate sufficient time for any impact of MCT on service use and health status. Unit costs are reported in Appendix 3.
Analysis
Quality-adjusted life-years were estimated using the EQ-5D-5L, which was collected at baseline and at 4- and 12-month follow-up. Data on health and social care use were collected using an economic patient questionnaire adapted from other trials. This captured secondary, primary, community and social care use. Unit costs of NHS and social care services were taken from national average unit cost data, and the price year was 2019. 45,46 Single imputation was used to impute missing baseline variables, with MI used to impute values missing at follow-up. Costs were imputed by category and utility by individual EQ-5D-5L domain to use all available data. The primary outcome of interest is the incremental cost-effectiveness ratio (ICER). Regression analysis was used to estimate net costs and net QALYs and these estimates were bootstrapped to generate 10,000 net pairs of costs and QALYs to inform the probability of cost-effectiveness. Sensitivity analyses tested the impact of the study design and assumptions on the ICER and cost-effectiveness acceptability analysis. See Appendix 2, Table 5, for the sensitivity analysis rationale.
Results
In the primary cost-effectiveness analysis, the MCT intervention is dominant, meaning it is both cost saving (net cost −£219, 95% CI −£1446 to £1007) and health increasing (net QALY 0.015, 95% CI −0.015 to 0.045). However, the CIs are wide and overlap zero, indicating high level of variability in the data and uncertainty in the estimates. The primary analysis found that at a willingness-to-pay threshold (WTPT) of £30,000 per QALY the MCT intervention is around 76% likely to be cost-effective, again reflecting uncertainty. See Appendix 2, Tables 5–7, for further details; see Appendix 2, Figure 9, for the cost-effectiveness plane; and see Appendix 2, Figure 10, for the cost-effectiveness acceptability curve. Sensitivity analysis demonstrated that the results at 4-month follow-up were similar to those of the primary analysis, and the complete-case analysis or the use of different assumptions around the cost of MCT did not affect conclusions. In these sensitivity analyses MCT remained dominant but with CIs wide and overlapping zero, demonstrating significant uncertainty.
Conclusion
Although the primary cost-effectiveness analysis and the majority of sensitivity analysis indicate that the MCT intervention may be cost saving and health increasing, or below typically accepted thresholds, the wide CIs that overlap zero indicate a high level of variability and uncertainty in the estimates. Further research should aim to reduce the uncertainty in the findings, for example with larger sample sizes and alternative measures used to produce utilities. In addition, research should explore how cost-effectiveness differs according to the implementation of MCT within CR.
Stated preferences survey
Aims
A discrete choice experiment (DCE) aimed to explore preferences for different characteristics of a clinic-based psychological intervention added to CR. The PPI work to develop the stated preference study has been published as a case study,20 and the analysis of the survey has been published. 33 An example of survey materials is included in Appendix 4.
Methods
A DCE was conducted and recruited a general population sample and a trial sample. DCE attributes included the modality (group or individual), the healthcare professional providing care, information provided prior to therapy, the location, and the cost to the NHS. Participants were asked to choose between two hypothetical designs of therapy, with a separate opt-out included. A mixed logit model was used to analyse preferences. The cost to the NHS was used to estimate the WTP for aspects of the intervention design/delivery. The study recruited a range of participants, including members of the UK general public aged ≥ 18 years, recruited via a commercial survey sample provider, as well as Group-MCT trial participants.
Analysis
The DCE was analysed using individual choice responses as the dependent variable in the model. 47 Owing to the presence of potential scale and preference heterogeneity (confirmed by a Swait and Louviere plot) a mixed logit model was used for analysis; this model assumes that parameters vary between individuals and accounts for heterogeneity across samples. Random utility theory assumes that a participant chooses between two options by interpreting the information described as a set of characteristics and selecting the one that provides the highest overall utility or value to them. Therefore, characteristic coefficients indicate the direction of preference. Marginal rates of substitution for each attribute were estimated by dividing the coefficient for that characteristic by the inverse of the NHS cost coefficient.
Results
Three hundred and four participants completed the DCE, the majority of whom were a sample of the general public (n = 262). The general population appeared to favour individual therapy (WTP £213, 95% CI £160 to £266) delivered by a CR professional (WTP £48, 95% CI £4 to £93) and at a lower cost to the NHS (β = −0.002; p = 0.000). Participants preferred to avoid options where no information was received prior to starting therapy (WTP −£106, 95% CI −£153 to −£59). The results for the location attribute were variable and challenging to interpret.
Conclusion
The study demonstrates a preference for psychological therapy as part of a programme of CR, as participants were more likely to opt in to therapy than they were to opt out. The results indicate that some aspects of the delivery that may be important to participants can be used to design a tailored psychological therapy that reflects preferences. However, preference heterogeneity is an issue that may prevent a ‘one-size-fits-all’ approach to psychological therapy delivery in CR. The COVID-19 pandemic (during which recruitment took place) is likely to have affected the DCE.
Work stream 3: the development and evaluation of Home-MCT
Work stream 3 overview
BACPR suggests that CR should employ a menu-based approach to CR, allowing a choice of home-based programmes. 2 Although CR offers home-based CR programmes, this has not been applied to psychological support, which has predominantly been delivered and evaluated in face-to-face formats. 48
Home-based psychological support may increase access to psychological help, especially for CR patients who may not be able or willing to attend face-to-face treatment or may be returning to work. Current self-help psychological therapies for cardiac patients are focused on applying relaxation techniques and CBT7 and are limited in efficacy. 48–50 As current home-based psychological options have variable effects, there is room to develop more effective alternatives.
While MCT has demonstrated efficacy in face-to-face delivery, a self-help version of MCT has yet to be evaluated. We therefore set out to develop and evaluate the feasibility and acceptability of home-based MCT.
Work stream 3 aims
-
Develop a home-based metacognitive intervention (Home-MCT) for CR patients with depression and/or anxiety.
-
Establish the acceptability and feasibility of integrating Home-MCT into the CR pathway.
-
Establish provisional evidence of the effectiveness and cost-effectiveness of Home-MCT.
-
Obtain qualitative data to help refine the presentation and delivery or Home-MCT for a full-scale trial.
-
Obtain data from a stated preferences survey about participants’ relative preferences, utility and WTP for components of the intervention to inform the design of a full-scale trial.
-
Collect data on patient variables and outcome measures to inform the design of a full-scale trial.
Methods
The PATHWAY Home-MCT feasibility study is a multicentre RCT with 4- and 12-month follow-up comparing home-based MCT plus usual CR (intervention) with usual CR alone (control). The trial received full ethics approval from the Northwest – Greater Manchester West Research Ethics Committee (reference 16/NW/0786, IRAS ID 186990) and was registered with a clinical trials database (ClinicalTrials.gov, identifier number NCT03129282). Participants were recruited from two NHS CR services (Bolton NHS Foundation Trust and Aintree University Hospitals NHS Foundation Trust). For further details of the trial protocol, including participant assessment and recruitment, see Wells et al. 28
Trial population
Inclusion criteria were as follows:
-
Fulfil Department of Health and Social Care and/or BACPR CR eligibility criteria. Thus, the patient was to have at least one of the following: acute coronary syndrome, revascularisation, stable heart failure, stable angina, implantation of cardioverter defibrillators/cardiac resynchronisation devices, heart valve repair/replacement, heart transplantation and ventricular assist devices, adult congenital heart disease, other atypical heart presentation
-
A score of ≥ 8 on the anxiety and/or depression subscales of the HADS (screening HADS)35
-
Minimum of 18 years old
-
A competent level of English-language skills (able to read, understand and complete questionnaires in English)
Participants were excluded if they met any of the following criteria:
-
Cognitive impairment that precludes informed consent or ability to participate
-
Acute suicidality
-
Active psychotic disorders (i.e. two or more of the following: delusions, hallucinations, disorganised speech, grossly disorganised or catatonic behaviour, negative symptoms)
-
Current drug/alcohol abuse (a maladaptive pattern of drinking, leading to clinically significant impairment or distress)
-
Concurrent psychological intervention for emotional distress that is not part of usual care
-
Antidepressant or anxiolytic medications initiated in the previous 8 weeks
-
Life expectancy of < 12 months
Self-help metacognitive therapy (Home-MCT)
Home-MCT is a self-help paper manual that consists of six modules. Modules focus on developing a case formulation, developing new strategies to regulate worry and rumination, and challenging metacognitive beliefs that maintain maladaptive patterns of thinking. In addition, participants received three telephone support calls lasting up to 30 minutes each. Call 1 was introductory; during this call, the manual and format were explained to patients and calls 2 and 3 were scheduled. Calls 2 and 3 were made after the completion of modules 2 and 4 and focused on reviewing the key learning points and providing support and guidance on the modules and implementing MCT strategies. Support calls followed a structured script, and staff were reminded that their role was to provide support and guidance on completing Home-MCT. Home-MCT was offered in addition to usual CR.
Development of Home-MCT
The treatment manual was developed by the PATHWAY chief investigator (Adrian Wells) and based on the Group-MCT manual. Before the Home-MCT manual was used in the feasibility trial, the manual and telephone support calls were tested with our PPI group, and after this, focus groups were conducted to review the manual and calls.
During the focus group, PPI members noted that the manual was easy to use and modules were easily to follow and flexible and encouraged adherence to the intervention. However, they felt that the size of the manual may deter patients from using it.
Patient and public involvement feedback on individual modules was as described below.
Piloting the manual with the PPI group provided important insight into patient experience of the intervention and the timing and content of telephone support calls prior to participant recruitment. This resulted in alterations to the manual, including adding information on what to expect from the intervention and what to do when the manual was received by post, emphasising that patients could complete the different modules at their own pace, increasing simplicity of instructions for completing the homework sections, and making a clearer differentiation between anxiety and stress. Amendments were also made to the telephone support scripts. Feedback from the PPI group was addressed prior to trialling Home-MCT, and PPI members were informed of how all the changes were addressed.
Analysis
With a view to the potential for the study to act as an internal pilot for a subsequent definitive trial (i.e. the data would be combined with subsequent data if the trial were extended and no changes were made to the methods), data analysis was restricted so that it was primarily descriptive, with no between-group analysis of outcome measures, thereby ensuring that masking to treatment allocation would be maintained if the trial were extended.
Acceptability of adding Home-MCT to usual CR was assessed by:
-
Recruitment into the study (number of patients agreeing to participate out of those approached, and number recruited per month)
-
Withdrawal or drop-out by the primary end point of 4 months and by 12-month follow-up (attrition rates)
-
Numbers of MCT modules completed (including time spent on each module)
-
Number of CR sessions attended
The feasibility of conducting a full trial was assessed by:
-
Completion of follow-up questionnaires (proportions of missing values)
-
Ability of the outcome measures to discriminate between patients (range of scores; floor or ceiling effects)
-
Re-estimation of the sample size for a definitive trial based on the findings of this study
Unlike the WS1 pilot, specific thresholds for progression to a full RCT were not defined for WS3, as our original protocol was not designed to continue to a full RCT within the research programme. However, we found ourselves in the position of being able to progress to a full-scale evaluation at no extra cost and did so following consultation with the TSC and NIHR under a ‘variation to contract’. The results of the full trial (WS3+) are reported later in this report.
Results (work stream 3)
Participant overview
Between 1 April 2017 and 26 February 2018, 632 patients were referred to CR services, of whom 200 (31.6%) were eligible to take part. One hundred and eight patients (69 male and 39 female) agreed to participate and were consented and randomised to the study. Patients had a mean age of 59.9 years (standard deviation 9.7 years, range 40–84 years).
Acceptability and feasibility trial of Home-MCT
The study achieved a recruitment rate of approximately 10.8 patients per month.
Retention at 4-month follow-up was > 80% for both arms. In the control arm, 52 (96.3%) patients returned 4-month follow-up data, 2 patients died and no patients withdrew from the study. In the intervention arm, 45 (83.3%) patients returned follow-up data, 4 patients withdrew, 4 patients did not return data, and 1 patient died. Attrition was therefore higher in the MCT + CR arm, at 16.7% compared with 3.7% in the CR-alone arm. Retention remained high at 12 months, but with a similar difference between the arms, with 90.7% of control and 81.5% of intervention participants returning follow-up data.
All questionnaires demonstrated a good range of observed scores, covering the majority of the possible score range, with little in the way of floor or ceiling effects.
Attendance at CR was high in both arms; 89% of CR-alone patients attended CR exercise classes and 85% attended educational seminars on health-related topics. Only 11% did not attend any CR exercise sessions and 15% did not attend any educational seminars. In the Home-MCT + CR arm, 80% of patients attended CR exercise classes and 78% of patients attended educational seminars. A higher proportion of intervention group participants did not attend exercise sessions, at 20%, and 22% did not attend educational seminars.
Information on engagement with the Home-MCT manual was collected via an end-of-study questionnaire mailed to participants, which had a 69% response rate. In total, 72.7% of patients who returned the end-of-study questionnaire completed four or more of the six modules, although owing to attrition these were just 45.3% of all Home-MCT patients still alive at 4 months. Although most patients reported completing a module in 60 minutes, individual times for doing this varied, ranging from 40 to 105 minutes.
Among those who returned the questionnaire, Home-MCT demonstrated high credibility. After completing the manual, patients were assessed on how user-friendly they found Home-MCT. Home-MCT was rated highly, with patients stating that they found the manual easy to use and understand (median rating of 80 out of 100), that the homework was easy to follow (median rating of 85 out of 100), and that the exercise SpACE was easy to use (median rating of 90 out of 100). When patients were asked if they found that they needed the telephone support calls, results were mixed: 40% said they did not need the support calls, while 40% stated they did. No adverse events were reported.
Verification of estimated sample size
Under assumptions of 20% attrition and a 0.5 correlation between baseline and 4-month follow-up, we provisionally estimated that a total recruitment sample of 246 patients would be required for a definitive trial, subject to revision based on the findings of this feasibility study. In the event, this feasibility study had an overall attrition rate of 10% at 4 months and a baseline-to-follow-up correlation of 0.58. However, there was evidence of greater attrition in the Home-MCT group at 17%, so for conservative reasons we chose to retain our original sample size estimate for a main trial.
Summary of findings of work stream 3
Overall, Home-MCT was found to be an acceptable and feasible addition to CR. No adverse events were reported in either trial arm. These results suggested that we could progress to a full-scale RCT to evaluate the efficacy of Home-MCT. The full trial would also provide a more definitive evaluation of the tendencies seen in the pilot for retainment in the trial and attendance at usual care to be somewhat lower among those offered Home-MCT. The success of the feasibility study supported a VTC to extend recruitment to a full-scale RCT.
Stated preference survey
Introduction
A pilot stated preference survey, using a DCE design, was conducted to explore the preferences of participants in the Home-MCT feasibility study for attributes of a psychological therapy intervention, relevant to home-based care, delivered in CR. The objectives were to evaluate the feasibility of preference research, estimate the sample size needed for a full study and explore preliminary preferences for included attributes. The paper has been published. 33
Methods
Following a review of qualitative feedback, PPI feedback and iterative discussion with the trial team, attributes and levels were selected for the DCE. A fractional factorial design was chosen, using a published design catalogue (http://neilsloane.com/oadir/oa.16.5.4.2.txt) and modulo arithmetic. 47,51 Participants were asked to choose their preferred scenario from two hypothetical options, and then whether they would choose this scenario or no psychological therapy (opt-out). Data were analysed using individual choice responses as the dependent variable in the model with a conditional logit using maximum likelihood estimation. 47 Results were used to estimate the sample size that would be required to calculate significant preference coefficients in a full study, generated for D-efficient and Bayesian designs in the experimental design software Ngene (Choice-Metrics, Sydney, NSW, Australia). 52
Results
The survey had a 39% response rate (n = 35/89). The conditional logistic regression identified significant results for two factors: participants disliked having no information about the therapy before it started and favoured lower costs to the NHS. Participants appeared to favour home-based therapy, with reduced waiting times, and online or smartphone-assisted therapy, although these results were not statistically significant. Significant positive constants for therapy options suggest that participants highly valued receiving therapy (compared with receiving no therapy). It was estimated that a sample size of around 370 would be needed to identify significant coefficients for most attributes.
Conclusions
The pilot study demonstrates the feasibility of a DCE in this group; it identifies potential attributes and levels and estimates the sample sizes needed for a full study. Preliminary evidence indicates that sampled participants tended to prefer home-based psychological therapy in CR and wanted to receive information before initiating therapy. Limitations include the sample size and the lack of diversity in the sample, as well as the potential impact of the COVID-19 pandemic, which may have influenced participant responses and subsequently the elicited preferences. Results are limited owing to the pilot design, and further research is needed to increase the robustness of the findings.
Work stream 3+ (variation to contract): a randomised controlled trial of Home-MCT
Overview
The PATHWAY study was granted a VTC to undertake a full RCT of the effectiveness of Home-MCT. The results of the full home-based trial have been published. 34
Aims
We aimed to evaluate if the addition of Home-MCT to usual CR improved anxiety and depression outcomes in comparison with usual care alone.
Methods
A multicentre, two-arm, single-blind, RCT with 4-month follow-up comparing Home-MCT plus usual CR (Home MCT + CR) with usual CR alone was conducted between April 2017 and March 2020. Patients were recruited from CR services at five NHS hospital trusts (Aintree University Hospitals NHS Foundation Trust, Bolton NHS Foundation Trust, Manchester University NHS Foundation Trust, East Cheshire NHS Trust and Pennine Acute Hospitals NHS Trust) across north-west England. The study followed the published protocol. 28 Participant eligibility criteria were not changed from earlier WSs.
The trial was designed to detect a SMD between the trial arms of 0.4 in HADS total score at 4-month follow-up, with 90% power, where 0.4 is in the middle of the range of effect sizes reported for other forms of psychological interventions for depression. 53 We assumed a 0.5 correlation between HADS at baseline and 4-month follow-up and 20% attrition. This indicated a total recruitment target of 246 (123 per arm). The first 108 patients constituted a pilot study (WS3) for ascertaining the feasibility of recruitment and retention and for confirming the sample size for the main trial.
Analyses were conducted in accordance with a prespecified analysis plan approved by the TSC and used intention-to-treat principles. A linear mixed-effects regression model was applied including both time points (baseline and 4 months). Prespecified covariates in the model were the randomisation factors (hospital site, sex, baseline total HADS score), plus age and medication for depression or anxiety (never taken/currently taking/taken in the past).
The RCI,44 which represents the difference between two measurements made in a single individual that would be statistically significant at a p-value of < 0.05, was computed for the HADS total score at 4-month follow-up. Using the control sample, we calculated a Cronbach’s alpha of 0.91 at 4 months for the HADS in order to estimate reliability for the usual CR population. Based on this, a reduction of 7 points in an individual’s score was defined as a statistically reliable improvement, and an increase in 7 points was defined as a reliable worsening of symptoms.
The RCI can be a conservative calculation of recovery; as such, we repeated this process using a minimally clinically important difference (MCID) of a 2-point change in the HADS anxiety and depression subscale. 54 For the HADS total we used an MCID of 3 points, requiring at least one subscale to change by ≥ 2 points.
Results
Two hundred and forty patients were consented to the trial, of whom 118 (49.2%) were randomly allocated to Home-MCT plus CR and 122 (50.8%) were randomly allocated to CR alone. Due to the COVID-19 pandemic, recruitment ended before the intended sample size target had been reached (n = 246), which was approved by the TSC. As the study had a high rate of retention at 4-month follow-up, it was deemed that ending recruitment ahead of target would have minimal impact on the analyses.
Demographic and clinical data for participants at baseline are provided in Wells et al. 34
Attendance at usual CR was high in both trial arms. Patients in the CR-alone arm attended a median of 5 [interquartile range (IQR) 2–6] CR exercise sessions. Similarly, the MCT + CR patients attended a median of 5 (IQR 2–7) CR exercise session; there was no significant difference between the arms in CR exercise attendance (p = 0.951).
Attendance at CR educational sessions was lower [medians: CR 4 (IQR 1 to 6), MCT + CR 4 (IQR 0–6)] and did not differ between the arms (p = 0.657).
At 4-month follow-up, the adjusted group difference on the HADS total (primary outcome) significantly favoured the MCT + CR arm (AMD −2.64, 95% CI −4.49 to −0.78, p = 0.005; SMD 0.38, 95% CI 0.11 to 0.64). Figure 5 presents the mean HADS total scores and 95% CIs for each trial arm at each assessment point, for complete cases only. The CR-alone arm mean score demonstrated little change, in comparison with a notable reduction in the mean for the Home-MCT + CR arm.
FIGURE 5.
Mean HADS total scores and 95% CIs for each trial arm at each assessment point, for complete cases only. Reproduced with permission from Wells et al. 34 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
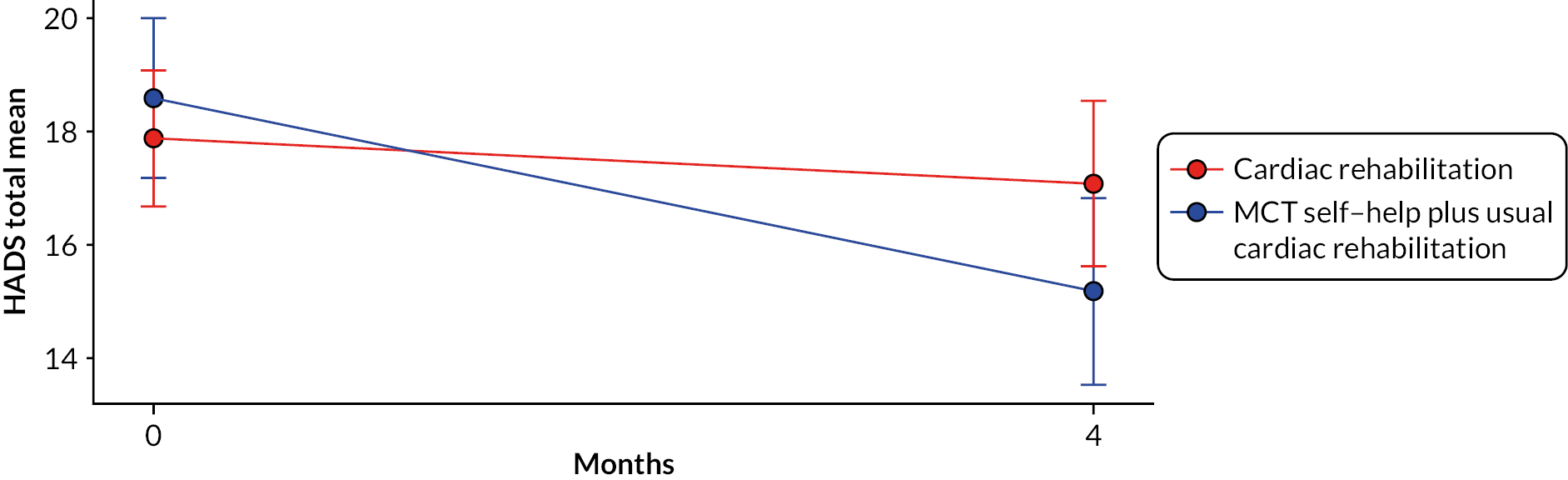
Patients in the MCT + CR arm achieved a significantly lower mean HADS anxiety subscale score (AMD −1.18, 95% CI −2.26 to −0.10, p = 0.032; SMD 0.29), plus a lower HADS depression subscale mean score (AMD −1.46, 95% CI −2.48 to −0.45, p = 0.005; SMD 0.39). Most other secondary outcomes also favoured the MCT intervention. 34
The percentages of patients reaching the threshold for a reliable improvement on HADS total and clinical improvement based on the MCID are presented in Table 2.
| Home-MCT + CR, n (%) | CR alone, n (%) | Total, N (%) | |
|---|---|---|---|
| Reliable change | |||
| No change | 52 (54.7) | 66 (55.9) | 118 (55.4) |
| Improveda | 34 (35.8) | 30 (25.4) | 64 (30.1) |
| Deterioratedb | 9 (9.5) | 22 (18.6) | 31 (14.6) |
| Minimum clinically important difference | |||
| No change | 24 (25.3) | 39 (33.1) | 63 (29.6) |
| Improvedc | 56 (59.0) | 43 (36.4) | 99 (46.5) |
| Deterioratedd | 15 (15.8) | 36 (30.5) | 51 (23.9) |
Figure 6 outlines the patient flow through Home-MCT. Approximately 76% of participants in the Home-MCT arm entering treatment completed four or more Home-MCT modules, which is our criterion for a minimal clinically effective exposure.
FIGURE 6.
Home-MCT patient flow.
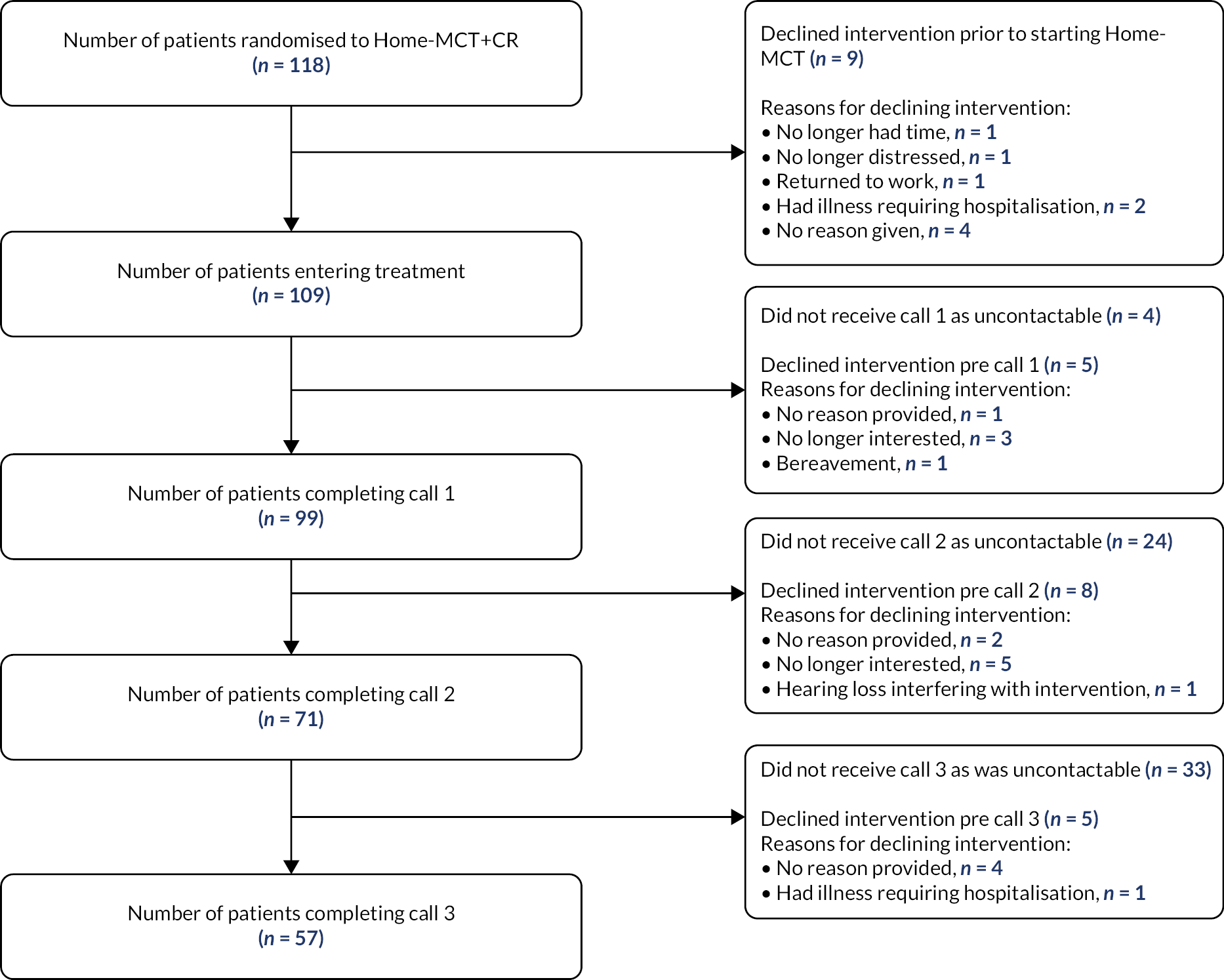
Conclusion
The addition of Home-MCT to CR was associated with significantly better symptoms of anxiety and depression than CR alone. The tendency seen in the pilot study for attendance at CR sessions to be lower for patients in the Home-MCT + CR arm was not borne out in the larger RCT, which found no impact of trial arm on CR attendance. However, attrition from the trial was greater under the MCT + CR condition. Sensitivity analyses indicated it highly unlikely that the results of the trial in favour of the MCT + CR condition could be accounted for by the group differences in attrition. The study demonstrated for the first time that a home-based intervention of MCT in addition to CR is associated with improvements in symptoms of anxiety and depression beyond the effects of CR alone. Home-based MCT might be offered as a psychological intervention option for CVD patients in addition to or as an alternative to group-based therapist-led MCT.
Cost-effectiveness of Home-MCT
The economic component of the Home-MCT trial originally aimed to establish provisional evidence for the cost-effectiveness of Home-MCT, utilising health status (EQ-5D-5L) and service use data collected prospectively collected from participants in the pilot trial. A request to NHS Digital was planned, with the aim of capturing comprehensive service use from participants using electronic records, which has the benefit of reducing participant burden and minimising missing data. A reduced economic patient questionnaire that captured primary, community and social care was administered to collect data that could not be accessed or linked using NHS Digital. However, the NHS Digital request could not go ahead as planned due to difficulties requesting the data, which included information and technology requirements as well as timeframe and budget constraints. Subsequently, the economic evaluation component was left with insufficient data on healthcare service use and could not robustly make conclusions about cost-effectiveness. Furthermore, there were no statistically significant differences in EQ-5D-5L values (utility) between the arms at follow-up, and mortality rates were similar, and so a difference in QALYs would not be expected. 34
Patient and public involvement
Overview
This section provides an overview of PPI in PATHWAY, demonstrating how PPI was integrated throughout the study. A detailed overview of our PPI framework and how this compared with NIHR PPI standards has been published. 27
Involvement in grant development
Although the study idea was generated by members of the research team, prior to submitting the grant proposal, links were formed with local charity organisations such as the Ticker Club at Wythenshawe Hospital. In developing the grant application, 30 service users across two cardiac service groups were consulted on the preliminary research ideas, the acceptability and feasibility of the proposed research within services and plans for PPI.
Forming the advisory group
To ensure that our patient advisory group comprised a range of service users with varying experiences, we recruited from multiple patient networks, including Salford Citizen Scientist, the Ticker Club and Salford Heart Care, and asked that they had experience of one or more of the following:
-
Heart disease
-
Psychological distress (anxiety and/or depression)
-
Caring for someone with heart disease and/or psychological distress, including professional carers
Sixteen individuals expressed an interest in joining the group; 13 of these attended an initial meeting, and 10 went on to form our patient advisory group. Table 3 gives the demographic characteristics of our PPI members.
| ID number | Gender | Age at beginning of study (years) | Ethnicity | Marital status | Qualification | Are you a CR service user? | Do you have experience in research? | Do you have experience as a PPI member in other research studies? |
|---|---|---|---|---|---|---|---|---|
| 01 | M | Not disclosed | Not disclosed | Married | Not disclosed | Y | N | N |
| 02 | F | 70 | Black African | Divorced | Degree | N (mental health nurse) | Y | Y |
| 03 | F | 53 | Black | Divorced | Degree | Y | Y | Y |
| 04 | M | 67 | White background | Married | Vocational qualification | Y | Y | N |
| 05 | M | 69 | White British | Married | Vocational qualification | Y | N | N |
| 06 | M | 61 | White British | Married | Diploma | Y | N | N |
| 07 | F | 65 | White British | Married | Degree | Y | Y | No |
| 08 | F | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Y (carer) | Y | Y |
| 09 | M | 63 | White British | Married | Degree | Y | N | N |
| 10 | M | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Not disclosed |
Over the next 5 years, three members left, the most common reason being ill-health. When consulted, the advisory group felt that this was to be expected due to the length of the study and the health of the membership and highlighted that that it would not be helpful to re-recruit to the group due to the complex nature and the duration of the project.
Structures and processes
Patient and public involvement meetings took place two or three times per year to provide feedback on and insight into various aspects of the trial. Initially meetings were face to face but during the COVID-19 pandemic these were changed to online meetings using Zoom (Zoom Video Communications, San Jose, CA, USA). Group members were provided with additional opportunities to contribute throughout the study by e-mail or telephone, allowing choice and flexibility of approach and opportunity.
The PPI lead developed and oversaw all aspects of involvement and worked in collaboration with the advisory group and research team to ensure a shared understanding and that everyone’s needs were met.
The advisory group included a chairperson to represent the group and to provide an additional point of contact for any concerns they wanted to raise. The chairperson attended the executive committee along with the PPI lead.
A PPI member independent of the research team and advisory group was also appointed as a member of the TSC.
All service users were reimbursed for their time and travel expenses, in line with INVOLVE guidelines.
Service user involvement throughout the study
Patient and public involvement was integrated throughout the lifecycle of the study and in all WSs, including the stated preference survey, with the degree of involvement and contribution altering throughout the research process. Figure 7 shows an overview of PPI throughout the study.
FIGURE 7.
Overview of PPI throughout the PATHWAY trial. Reproduced with permission from Capobianco et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
The advisory group’s insights ensured that the language in our patient-facing documents was appropriate and that any likely concerns or questions had been addressed. For example, the group said that an explanation was needed of what to expect from the home-based manual and what to do when it arrived. They also suggested we give a simpler explanation of how to complete the homework sections and emphasise that the modules should be worked through at the patient’s own pace.
Asking group members to pilot the psychological therapy (home-based manual) not only allowed us to modify the timing and content of phone calls, but also provided them with greater knowledge of the intervention and participant experience that they used to inform dissemination activities. For example, advisory group members noted that it would be interesting and engaging to try to incorporate some the techniques in the manual into dissemination workshops and presentations.
The group’s suggestion of a newsletter to provide updates on the study, remind participants of its importance and highlight the impact of returning questionnaires was an important factor in improving our rate of return on follow-up questionnaires.
The group’s input also informed the designs of the stated preference studies, including choosing and defining attribute and levels, and revising survey materials. For further details of PPI involvement in the stated preference survey, see Shields et al. 20 The PPI activities were used as a case study on a paper exploring PPI involvement in stated preference research.
Our PPI group also co-developed the dissemination and impact plan and dissemination materials prior to the COVID-19 pandemic. Our PPI group helped us to modify our dissemination and impact plan, which was reduced because of the pandemic. This included identifying key messages, audiences and channels for dissemination, and co-creating dissemination materials (i.e. videos and blogs about personal experiences). They also suggested that we ask members of the Ticker Club to evaluate our first patient-facing presentation and suggest other potential audiences for dissemination (see Appendix 5).
Our PPI group was involved in co-delivering the dissemination activities. For example, on 15 July 2021, we held an online dissemination event for patients, members of the public and clinicians. The dissemination event was developed in collaboration with our PPI group, and one of our PPI members was involved in discussing their experience along with a study participant, which provided a patient voice and highlighted the study’s importance and benefit.
Impact on the advisory group
The advisory group members each completed a questionnaire to assess the personal impact of their involvement (see Report Supplementary Material 1). Members agreed that taking part had given them an opportunity to use their skills and make a difference and meet people who had experienced similar things, given them more hope that people will be supported in the future, increased their understanding of how research works, and improved their understanding of coping with anxiety and depression and their own ability to cope with mental health needs. One advisory group member reported:
I think it’s great that psychological, the emotional side is now being sort of trying to be managed because it’s not been there … I didn’t know until we started getting the paperwork what it was [anxiety and depression] … and now I know why I didn’t want to go out of the house for 6 months. I never ever suffered with depression before, but I now know why.
PPI member 3
They concluded that it had been an enjoyable experience, it had increased their trust in research and researchers, and it had made them more likely to discuss research with friends and family. Comments included:
I have gained a better understanding of all the difficult decisions that researchers have to make … The opportunity for the future looks promising and will certainly help more participants to cope.
PPI member 5
It has been a fulfilling experience. Too often patients’ views are not taken into account. I felt that we were listened to at every stage of the research and our suggestions were treated with respect.
PPI member 7
Although no negative impacts of participation were reported, one member had been unaware that they were eligible for transport to the venue by taxi and undertook quite a challenging journey until the research team became aware of the situation.
INVOLVE standards and suggestions for future patient and public involvement
The advisory group’s regular evaluation of our PPI approach, including their review of our approach against the INVOLVE national standards (see Figure 8), highlighted various challenges in achieving the standards. Below we discuss the main challenges and solutions, and the advisory group’s suggestions for the future.
FIGURE 8.
Overview of PATHWAY trial implementing INVOLVE PPI standards.
Understanding of research
Although the advisory group ultimately felt that sufficient information had been given, at times confusion about some of the processes and protocols and unfamiliarity with the psychological intervention in question (MCT) had resulted in feelings of being underused.
For example, members suggested that the wording of the standardised questionnaires could have been contributed to low return rates and noted that they had not been consulted on this before the questionnaires were sent out. They were also keen to encourage the public and funder to promote the implementation of MCT in CR programmes, which was a premature suggestion as they were unaware that further research into this area would be needed first. The belated explanation of MCT was also highlighted as a barrier in the early stages of involvement. One member commented:
When we first started, most, possible all of us didn’t understand the nature of the actual treatment. It was quite a long way down the line before we saw the materials … and understood more about how the treatment would work. I think some training earlier on in the process would have put us in a position where we were better informed to contribute.
PPI member 6
The group agreed that a workshop on the wider research process at the start of the project would have been helpful. We suggest including (1) what can and cannot be changed in a study and why (2) setting realistic expectations for roll-out and study impact, and (3) a clear explanation of the intervention, including a chance to trial or watch a demonstration of it.
Working together
Towards the end of the study the group felt that the varying types of communication (group-based, one-to-one, e-mails, telephone calls) was sufficient and the level of language and approach was effective:
What has been done well overall is that communication has been excellent.
PPI member 8
However, it was suggested that further consideration be given to supporting those who find group settings challenging; for example, some might feel pressure to agree with the majority of the group or have concerns around re-joining the group after missing meetings. One member recalled that on returning after missing meetings they felt like things had changed, leaving them overwhelmed and unsure of their role.
The group suggested that a returning PPI member and the PPI lead meet to discuss changes and progress prior to the next meeting, along with the returning member being reminded that they could contribute ideas on a one-to-one basis before or after the meeting.
Conclusion
Patient and public involvement had a positive impact throughout the lifecycle of our study and across the varying components. It also had a positive impact on the advisory group members who took part.
Our approach to PPI was felt to be effective by our advisory group and was aided by the process of ongoing evaluation and adjustment. Several key learning points have emerged that will inform our practice in the future.
General discussion
We used a multi-methods approach across three research WSs with the goals to better understand the psychological needs and preferences for psychological therapy in CR patients with anxiety and depression symptoms, and to conduct two separate randomised feasibility trials followed by two full-scale RCTs of MCT.
We were able to meet almost all our study objectives as outlined under the primary funded proposal. However, we did not complete a preliminary cost-effectiveness analysis around the home-based intervention. Nevertheless, we did accomplish a full-scale RCT extension of the home-based intervention (WS3) under the same VTC.
We found both Group-MCT and Home-MCT to be feasible and acceptable treatments to deliver and evaluate within CR, and both treatments were associated with better overall depression and anxiety at 4 months. Group-MCT was also evaluated at 12 months, at which point outcomes remained improved compared with CR alone. However, the long-term impact of Home-MCT remains to be evaluated.
Challenges and limitations
A number of challenges were faced throughout the grant, and below we summarise the main challenges and limitations.
The studies reported here did not include a comparison or control group involving the addition of an alternative psychological treatment to CR. We are therefore unable to attribute the improved outcomes in the MCT conditions to the effect of MCT solely, rather than to the provision of extra support and contact time this entailed. As previous trials in mental health have demonstrated that MCT can produce superior outcomes to other psychological treatments in anxiety and depression,13 we chose a more pragmatic research question here: can we improve outcomes when we add MCT to CR?
One of the main challenges faced in WS1 and WS2 was the delivery of MCT by CR staff who had no previous training in mental health treatment. During the feasibility study,14 it was not possible to assess the quality of therapy delivered because of limitations of obtaining consent for audio-recording the sessions. As a result, we were reliant on staff feedback on challenges they encountered during Group-MCT delivery. As the treatment was delivered by CR staff who are not mental health specialists, this may mean that Group-MCT was not delivered optimally. To understand the delivery of MCT further, we interviewed patients to assess their understanding and experience of Group-MCT. 22 Despite therapists receiving limited training in MCT, patients did understand the aims of MCT and its techniques and found the therapy beneficial. However, we were unable to gain a thorough understanding of why some patients did not complete Group-MCT, as only four patients who withdrew from Group-MCT were interviewed. This is an important consideration for any future roll-out of Group-MCT and could provide information to support the transferability of MCT across the CR context.
The study did not include a measure specific to quality of life, and further research should explore the relationship between anxiety and depression outcomes and broader quality of life in the CR population.
In the economic evaluation components, the key challenges and limitations concerned the availability of data. For WS3+ in particular, the inability to access service use data to support costing because of information and technology requirements, as well as time frame and budget constraints, prevented an economic evaluation. For WS2, the economic evaluation was also affected by the sample size and large amount of missing data.
In the stated preference survey,26 one of the limitations was sample recruitment as the sample size for the study was limited and lacked diversity. The sample that responded to the survey was homogeneous, with most respondents being male, over the age of 55 years and retired. This limited the ability to investigate which patient characteristics were tied to delivery preferences. Recruitment for the stated preference survey occurred during the COVID-19 pandemic, which may have influenced the response rate and the responses, which in turn limits generalisability. We discussed the results with our PPI group, who noted that patient preference indicated in the survey may have been affected by local and national lockdown restrictions, which might have strengthened preferences for home-based interventions.
Implications for practice
Improving psychological support within cardiac services is imperative, as elevated anxiety and depression in CVD patients is associated with increased mortality and morbidity, poorer quality of life, greater social problems and higher healthcare costs. The PATHWAY programme has demonstrated that adding Group-MCT to CR was associated with significantly improved anxiety and depression and a wider range of psychological gains that could be sustained over 12 months. Furthermore, a home-based version of MCT when added to CR was also related to improved outcomes when assessed over 4 months. The effect sizes of treatment compare favourably with those of previous studies of psychological interventions, and the treatments could potentially be delivered by CR staff with minimal additional training.
The implications for clinical practice can be summarised as follows:
-
Evidence of positive outcomes associated with a recent psychological treatment (MCT) when used in the context of CR
-
Greater integration of physical and mental health care in heart disease
-
Two formats of psychological treatment (group based and home based) that contribute towards a menu-based approach to CR that is sensitive to patient needs and improves access
-
A better understanding of the mental health needs of CR patients and their preferences for psychological intervention delivery in the NHS that will support service planning, evaluation and research
-
Manualised interventions that could potentially be incorporated in routine CR as a first-line approach for patients with anxiety/depression, with more complex specialist delivered mental health interventions offered to non-responders or those requiring additional help
The results of qualitative analysis coupled with trial outcomes on primary and secondary (psychological mechanism) variables are supportive of MCT as particularly well suited to CVD in comparison with other treatments. It may offer improvements over interventions that target reality-testing of thoughts and relaxation as it offers a good fit with patients’ needs and experiences. The results add to the growing body of studies suggesting that MCT, which targets metacognitions may be suited to alleviating anxiety and depression in patients with a range of physical health conditions. 22,23,31,32
If used in practice, the expectation would be that MCT should be adopted as an option in the National Audit of Cardiac Rehabilitation database so that its use can continue to be assessed. Further training and supervision in the delivery of MCT would be required.
Recommendations for future research
There are a wide range of recommendations for future research that stem from the current series of studies. The relative effects of MCT in comparison with other therapies or additional contact time remains a major question for future research to examine. Further important recommendations in order of priority are summarised as follows:
-
To help realise the clinical potential of MCT in CR, future research should examine its implementation in the NHS and examine the barriers to and enablers of its adoption and roll-out across CR services.
-
Evaluation of the longer-term (12-month) follow-up effects associated with home-based MCT is recommended and should be undertaken as a matter of priority.
-
Health-economic impacts of the inclusion of MCT in CR should be part of future large-scale evaluations. Further research should aim to reduce the uncertainty in the findings for Group-MCT related to cost-effectiveness, for example with larger sample sizes. Additional research exploring how cost-effectiveness differs according to the implementation of MCT within CR and how cost-effectiveness differs by setting would be useful for decision-makers in other settings. Finally, an economic evaluation to establish the cost-effectiveness of Home-MCT is needed.
-
Our stated preference studies demonstrated that there is likely to be heterogeneity across populations with respect to their preferences for the delivery of psychological therapy in CR. Further research with larger and more varied samples should assess how preferences differ by group.
-
We were able to interview only a small number of patients who did not complete treatment and future research is required to examine the reasons that patients drop out of therapy.
-
Given that MCT is based on a model of specific causal psychological mechanisms that are directly targeted in treatment, mechanism-focused research is clearly indicated. Such research can test mechanisms of anxiety and depression and mechanisms of recovery, helping to refine and strengthen interventions.
Conclusions
The data suggest that currently the psychological needs of patients in CR are not being met, as evidenced by the rates of anxiety and depression among this group, by the lack of standard provision of psychological therapy and by the results of our qualitative studies on patient needs and preferences for therapy. An analysis of psychological needs appeared to fit the objectives of MCT better than the objectives of cognitive–behavioural approaches. Moreover, CR patients did not particularly value current techniques such as relaxation methods used in CR.
We found that both Group-MCT and Home-MCT were feasible and acceptable treatments to deliver and evaluate within CR for the treatment of anxiety and depression. Both treatments were associated with significant reductions in overall depression and anxiety symptoms at 4 months when added to CR that exceeded the effects observed with usual CR alone. Group-MCT was also evaluated at 12 months, at which point anxiety and depression outcomes remained better than with usual CR alone. The long-term impact of Home-MCT remains to be evaluated. The economic evaluation suggests that Group-MCT may be a cost-effective treatment to deliver within CR. However, sample size, missing data and variability in the data led to considerable uncertainty about the cost-effectiveness.
The DCEs demonstrate that participants (including the general public and trial participants) would value making psychological treatment available. For face-to-face therapy there was a preference for individual treatment, while people appear to favour online/smartphone-assisted therapy for home-based therapy, but this was not significant and might have been impacted by the COVID-19 pandemic restrictions.
It is important to highlight the context of this research programme, namely that the chief investigator (AW) is the originator of MCT and the director of the Metacognitive Therapy Institute. He has received funding as chief investigator on the subsequent study ‘Implementing Group Metacognitive Therapy in Cardiac Rehabilitation Services (PATHWAY-Beacons; NIHR29567)’ and is chief investigator on the projects NIHR201495 and NIHR35997 and also co-chief investigator on NIHR203634. Steps were taken throughout the research programme to maintain objectivity; these have been highlighted in this report and included the masking of patient treatment allocation from Adrian Wells, the trial statistician and the research assistants, data being managed by a separate clinical trials unit, data analysis following a prespecified plan and project monitoring and support being provided by an independent TSC.
In conclusion, MCT appeared to be an acceptable addition to CR that fit well with patients’ underlying needs in addressing symptoms of depression and anxiety. MCT was associated with improved psychological outcomes, with the implication that it could contribute to effective treatment offered in CR services within the NHS.
Additional information
Acknowledgements
We would first like to thank all of our patients who took part in the trial. Thank you for your time and dedication to the project, without which this study would not have been possible. We would also like to thank the PATHWAY research assistants, Dr Rebecca McPhillips, Ms Helen Morley, Dr Hannah Gaffney, Ms Rebecca Anderson, Dr Cintia Faija, Mr Avinash Atwal, Ms Zara Husain and Ms Bethany Cooper, and PATHWAY administrator Wendy Clarke, who contributed to patient recruitment and study organisation. We would like to thank Professor Bob Lewin for his contributions to the PATHWAY design. We would like to thank Dr Peter Salmon for his contribution to the qualitative aspects of the PATHWAY trial. We would like to thank Dr Stuart Wright for his contribution to the stated preference research. We would like to thank Dr Elizabeth Camacho for her contribution to the economic evaluation work. We would also like to thank Dr Kirsten McNicoll for assisting in grant preparation. We are most grateful for and thank the members of the Trial Steering Committee chaired by Professor Kate Jolly and including Professor Gill Lancaster, Professor Gerry Humphris and Mr Chris Houston. We would also like to thank our patient and public involvement group who advised throughout the entirety of the project: Christine Barker, Jerry Benjamin, Stephen Clegg, Jim Collins, Chris Cooper and Marie Holmes.
Contributions of authors
Adrian Wells (https://orcid.org/0000-0001-7713-1592). Professor of Clinical & Experimental Psychopathology. Conceived the study and obtained CLAHRC funding to develop the initial grant proposal and assemble the team; designed the study programme and obtained the funding; had overall responsibility for grant development and programme implementation; developed the MCT interventions, wrote the treatment manuals and clinically trained staff in delivering MCT; designed the study programme.
David Reeves (https://orcid.org/0000-0001-6377-6859). Professor of Biostatistics. Contributed to and oversaw the methodological and statistical design of the trials and contributed to study design, was responsible for the analysis of the feasibility trials and RCTs, and designed the study programme and obtained the study funding.
Peter Fisher (https://orcid.org/0000-0002-7388-720X). Reader in Clinical Psychology. Supported the qualitative methodology, analysis and dissemination and designed the study programme and obtained the study funding.
Linda Davies (https://orcid.org/0000-0001-8801-3559). Professor of Health Economics. Contributed to the health economics study design, conducted the health economic evaluation, and designed the study programme and obtained the study funding.
Gemma Shields (https://orcid.org/0000-0003-4869-7524). Lecturer in Health Economics. Conducted the health economic evaluation.
Patrick Joseph Doherty (https://orcid.org/0000-0002-1887-0237). Professor of Cardiovascular Health. Consulted on the structure of CR and supported study dissemination.
Anthony Heagerty (https://orcid.org/0000-0002-9043-2119). Professor of Medicine. Provided medical oversight and supported study dissemination.
Calvin Heal (https://orcid.org/0000-0002-6445-1551). Research Fellow. Was responsible for the analysis of the feasibility trials and RCTs.
Lindsey Brown (https://orcid.org/0009-0004-7332-1320). PPI Contributor. Provided PPI oversight and input.
Lora Capobianco (https://orcid.org/0000-0001-6877-8650). Senior Research Fellow. Contributed to the management of the study, data collection and report writing and co-ordinated PPI contributions to the studies.
Adrian Wells and Lora Capobianco compiled the first draft of this report with contributions from David Reeves, Calvin Heal, Peter Fisher, Gemma Shields, Lindsey Brown and Linda Davies.
All named authors reviewed and commented on drafts of the report. Investigators making specific contributions are listed in Acknowledgements.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/TMJA2644.
Primary conflicts of interest: Adrian Wells is chief investigator of the current research funded by the National Institute for Health Research (NIHR) (RP-PG-1211-20011). Adrian Wells is the originator of metacognitive therapy and director of the Metacognitive Therapy Institute. Adrian Wells received funding as chief investigator for a subsequent study, ‘Implementing Group Metacognitive Therapy in Cardiac Rehabilitation Services’ (PATHWAY-Beacons; NIHR29567), and is chief investigator on projects NIHR201495 and NIHR35997 and co-chief investigator on project NIHR203634. David Reeves was a co-investigator and received funding from the NIHR Programme Grants for Applied Research (PGfAR) programme for a subsequent study ‘Implementing Group Metacognitive Therapy in Cardiac Rehabilitation Services’ (PATHWAY-Beacons; reference number 29567).
Linda Davies reports grants from NIHR (NIHR132269, NIHR200460, RP-PG-0218-20006, 17/80/09, 16/167/76, 17/31/05) and Trial Steering Committee/Data Monitoring Committee membership for various NIHR studies (16/111/91; 16/116/82). Linda Davies also reports membership of advisory boards for Public Health England and is a member of the NIHR Health Technology Assessment Clinical Evaluation and Trials Committee (2010–14). Linda Davies has been a member of a core group of methodological experts for the NIHR PGfAR programme (2011–15). Linda Davies also reports membership of the editorial board for BMC Psychiatry. Gemma Shields is co-investigator for other NIHR studies (NIHR134702, NIHR132690, NIHR201495, NIHR132622, NIHR203300, NIHR201482, NIHR203474, NIHR203507, NIHR132269, NIHR201093) unrelated to the MCT Pathway programme of work. Lora Capobianco is a co-chief investigator for the NIHR study NIHR203634 and a co-investigator for the following NIHR studies: NIHR29567, NIHR201495 and NIHR35997.
The NIHR PGfAR programme funded the work that this manuscript reports and a subsequent (ongoing) study to evaluate the implementation of group-metacognitive therapy within cardiac rehabilitation services.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
Work stream 1 (WS1) was approved by the National Research Ethics Service of the UK’s NHS (reference 15/NW/0163) and registered with a clinical trial database (ISRCTN74643496). Ethical approval was sought from NRES Committee North West REC reference (15/NW/0163) for associated qualitative work.
Work stream 2 (WS2) was obtained from the Preston Research Ethics Committee (REC Reference 15/NW/0163) along with site-specific approval. The trial is registered with the ISCRTN registry, No. ISRCTN74643496. Associated Qualitative work received ethical approval from NRES Committee Northwest REC reference (15/NW/0163) on 6 March 2015.
Work stream 3 (WS3) received full ethical approval from the Northwest – Greater Manchester West Research Ethics Committee (Reference 16/NW/0786, IRAS ID 186990) and was registered with a clinical trials database (NCT03129282) on 11 Nov 2016.
Work stream 3+ (WS3+) obtained ethical approval from the Northwest – Greater Manchester West Research Ethics Committee (REC Reference 16/NW/0786) on 03 January 2017, along with site-specific approval.
Information governance statement
Greater Manchester Mental Health NHS Foundation Trust is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, Greater Manchester Mental Health NHS Foundation Trust is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here (https://www.gmmh.nhs.uk/gdpr-in-research)
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the PGfAR programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publications
Articles
-
Wells A, Faija C. Metacognitive Therapy for anxiety and depression in cardiac rehabilitation: commentary on the UK National Institute of Health Research funded PATHWAY programme. J Cardiol Cardiovasc Sci 2018;2:10–14. https://doi.org/10.29245/2578-3025/2018/3.1131
-
Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Metacognitive therapy home-based self-help for cardiac rehabilitation patients experiencing anxiety and depressive symptoms: study protocol for a feasibility randomised controlled trial (PATHWAY Home-MCT). Trials 2018;19. https://doi.org/10.1186/s13063-018-2826-x
-
Shields G, Wells A, Doherty P, Heagerty A, Buck D, Davies L. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart 2018;104:1403–10. https://doi.org/10.1136/heartjnl-2017-312809
-
Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group-based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2593-8
-
McPhillips R, Salmon P, Wells A, Fisher P. Cardiac rehabilitation patients’ accounts of their emotional distress and psychological needs: a qualitative study. J Am Heart Assoc 2019;8. https://doi.org/10.1161/JAHA.118.011117
-
McPhillips R, Salmon P, Wells A, Fisher P. Qualitative analysis of emotional distress in cardiac patients from the perspectives of cognitive behavioral and metacognitive theories: why might cognitive behavioral therapy have limited benefit, and might metacognitive therapy be more effective? Front Psychol 2019;9. https://doi.org/10.3389/fpsyg.2018.02288
-
Faija C, Reeves D, Heal C, Capobianco L, Anderson R, Wells A. Measuring the cognitive attentional syndrome in cardiac patients with anxiety and depression symptoms: psychometric properties of the CAS-1R. Front Psychol 2019;10. https://doi.org/10.3389/fpsyg.2019.02109
-
Anderson R, Capobianco L, Fisher P, Reeves D, Heal C, Faija C, et al. Testing relationships between metacognitive beliefs, anxiety, and depression in cardiac and cancer patients: are they transdiagnostic? J Psychosom Res 2019;124:109738. https://doi.org/10.1016/j.jpsychores.2019.109738
-
Shields G, Wells A, Doherty P, Reeves D, Capobianco L, Heagerty A, et al. Protocol for the economic evaluation of metacognitive therapy for cardiac rehabilitation participants with symptoms of anxiety and/or depression. BMJ Open 2020;10:e035552. https://doi.org/10.1136/bmjopen-2019-035552.
-
Wells A, Reeves D, Heal C, Fisher P, Davies L, Heagerty A, et al. Establishing the feasibility of group metacognitive therapy for anxiety and depression in cardiac rehabilitation: a single-blind randomized pilot study. Front Psychiatry 2020;11. https://doi.org/10.3389/fpsyt.2020.00582
-
Faija C, Reeves D, Heal C, Wells A. Metacognition in cardiac patients with anxiety and depression: psychometric performance of the metacognitions questionnaire 30 (MCQ-30). Front Psychol 2020;11. https://doi.org/10.3389/fpsyg.2020.01064
-
Shields G, Brown L, Wells A, Capobianco L, Vass C. Utilising patient and public involvement in stated preference research in health: learning from the existing literature and a case study. The Patient – Patient-Centered Outcomes Research 2020;14:399–412. https://doi.org/10.1007/s40271-020-00439-2
-
Capobianco L, Faija C, Husain Z, Wells A. Metacognitive beliefs and their relationship with anxiety and depression in physical illnesses: a systematic review. PLOS ONE 2020;15:e0238457. https://doi.org/10.1371/journal.pone.0238457
-
Shields G, Wright S, Wells A, Doherty P, Capobianco L, Davies L. Delivery preferences for psychological intervention in cardiac rehabilitation: a pilot discrete choice experiment. Open Heart 2021;8:e001747. https://doi.org/10.1136/openhrt-2021-001747
-
McPhillips R, Capobianco L, Cooper B, Husain Z, Wells A. Cardiac rehabilitation patients experiences and understanding of group metacognitive therapy: a qualitative study. Open Heart 2021;8:e001708. https://doi.org/10.1136/openhrt-2021-001708
-
Wells A, Reeves D, Capobianco L, Heal C, Davies L, Heagerty A et al. Improving the effectiveness of psychological interventions for depression and anxiety in cardiac rehabilitation: PATHWAY – a single-blind, parallel, randomized, controlled trial of group metacognitive therapy. Circulation 2021;144:23–33. https://doi.org/10.1161/CIRCULATIONAHA.120.052428
-
Wells A, Reeves D, Heal C, Davies LM, Shields GE, Hagerty A, et al. Evaluating metacognitive therapy to improve treatment of anxiety and depression in cardiovascular disease: the NIHR funded PATHWAY research programme. Front Psychiatry 2022;13:886407. https://doi.org/10.3389/fpsyt.2022.886407
-
Wells A, Reeves D, Heal C, Fisher P, Doherty P, Davies L, et al. Metacognitive therapy home-based self-help for anxiety and depression in cardiovascular disease patients in the UK: a single-blind randomised controlled trial. PLOS Med 2023;20:e1004161. https://doi.org/10.1371/journal.pmed.100416
Conference abstracts
-
Anderson R, Fisher P, Gaffney H, Morley H, Wells A. Are Metacognitive Beliefs Transdiagnostic: A Comparison of Cancer and Cardiac Patients Experiencing Anxiety and Depression. The 3rd International Conference of Metacognitive Therapy, 8–9 April 2016, Milan, Italy, symposium 9, abstract 2.
-
Atwal A, Capobianco L, Wells A. Project Management in Clinical Research: A Case Study of the MCT PATHWAY Trial. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, poster 6.
-
Capobianco L, Faija C, Wells A. Metacognitive Beliefs in Physical Illnesses: A Systematic Review and Meta-Analysis. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, symposium 1, abstract 1.
-
Cooper B, Capobianco L, Heal C, Doherty P, Wells A. Exploring Sociodemographic and Psychological Predictors of Attendance at Cardiac Rehabilitation. British Association for Cardiovascular Prevention and Rehabilitation Conference, virtual/online, October 2021.
-
Faija C, Reeves D, Heal C, Wells A. Assessing Metacognitions in Cardiac Patients with Co-Morbid Anxiety and/or Depression. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, symposium 1, abstract 2.
-
Faija C, Reeves D, Heal C, Capobianco L, Anderson R, Wells A. The Cognitive Attentional Syndrome in Cardiac Patients Experiencing Anxiety and/or Depression. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, poster 1.
-
Gaffney H, Anderson R, Morley H, Shields G, Fisher P, Salmon P, et al. Key Considerations in the Development, Implementation and Evaluation of a Metacognitive Therapy Intervention for Anxiety and Depression in the Cardiac Rehabilitation Pathway: The PATHWAY Trial. The 3rd International Conference of Metacognitive Therapy, 8–9 April 2016, Milan, Italy, symposium 9, abstract 3.
-
Husain Z, Capobianco L, Brown L, Wells A. Patient and Public Involvement within the MCT Cardiac Rehabilitation Pathway. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, poster 5.
-
McPhillips R, Fisher P, Salmon P, Wells A. Cardiac Rehabilitation Patients’ Views, and Experience of Group Metacognitive Therapy: A Nested Longitudinal Study. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, symposium 2, abstract 1.
-
McPhillips R, Fisher P, Salmon P, Wells A. Cardiac Rehabilitation Practitioners’ Views and Experiences of Being Trained in, and Delivering, Group Metacognitive Therapy: A Nested Longitudinal Study. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, symposium 2, abstract 2.
-
McPhillips R, Salmon P, Fisher P, Wells A. Integrating Group-Metacognitive Therapy into Cardiac Rehabilitation Pathways: Practitioner Perspectives. The 3rd International Conference of Metacognitive Therapy, 8–9 April 2016, Milan, Italy, symposium 10, abstract 7.
-
McPhillips R, Salmon P, Wells A, Fisher P. ‘It’s kind of like, life changing for me’: A Qualitative Investigation of Cardiac Patients’ Experiences of Group-Metacognitive Therapy as Part of The PATHWAY Trial. The 3rd International Conference of Metacognitive Therapy, 8–9 April 2016, Milan, Italy, symposium 9, abstract 1.
-
McPhillips R, Salmon P, Wells A, Fisher P. The Psychological Needs of Emotionally Distressed Cardiac Rehabilitation Patients: A Qualitative Study of How Well Cardiac Rehabilitation Addresses Them. British Association for Cardiovascular Prevention and Rehabilitation Conference. Cardiff, UK, October 2016.
-
Shields GE, Davies LM, Doherty P, Reeves D, Capobianco L, Heagerty A, et al. Exploring the Costs and Cost-Effectiveness of Metacognitive Therapy for Cardiac Rehabilitation Patients with Symptoms of Anxiety and/or Depression. British Association for Cardiovascular Prevention and Rehabilitation Conference, virtual/online, October 2021.
-
Shields GE, Davies LM, Doherty P, Capobianco L, Buck D, Wells A. Attendance at Cardiac Rehabilitation Sessions in a Group with Symptoms of Anxiety and/or Depression. Health Services Research UK, virtual/online, July 2020.
-
Shields GE, Wells A, Capobianco L, Davies LM. Factors Associated with Symptoms of Depression and/or Anxiety, Health Status and Metacognitive Beliefs in a Population Undergoing Cardiac Rehabilitation. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, symposium 1, abstract 3.
-
Shields GE, Wells A, Capobianco L, Davies LM. Costing Metacognitive Therapy in the UK. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, symposium 2, abstract 3.
-
Shields GE, Wells A, Capobianco L, Davies LM. Patient and Public Involvement: Feedback on Important Characteristics of MCT Therapy Included in the Cardiac Rehabilitation Pathway. 4th International Conference of Metacognitive Therapy, 30 April–2 May 2019, Prague, Czechia, poster 3.
-
Shields GE, Buck D, Wells A, Doherty P, Heagerty T Davies LM. A Systematic Literature Review of the Cost-effectiveness of Cardiac Rehabilitation Interventions. British Association for Cardiovascular Prevention and Rehabilitation Conference. Cardiff, UK, October 2016.
-
Wells A, Reeves D, Capobianco L, Heal C, Davies L, Heagerty A, et al. Improving the Effectiveness of Psychological Interventions for Depression and Anxiety in Cardiac Rehabilitation: PATHWAY – A Single Blind, Parallel, Randomized, Controlled Trial of Group Metacognitive Therapy. British Association for Cardiovascular Prevention and Rehabilitation Conference, virtual/online, October 2021.
-
Wells A, Reeves D, Fisher P, Heal C, Davies L, Heagerty A, et al. Home-based Metacognitive Therapy for Anxiety and Depression: A Single Blind Randomised Feasibility Trial of Assisted Self-help in Patients with Cardiovascular Disease. British Association for Cardiovascular Prevention and Rehabilitation Conference, virtual/online, October 2021.
Blog posts and newsletters
-
Barker C. Me, my heart, my mind. ADePT-RU News, 2021. URL: https://adept-ru.com/me-my-heart-my-mind/ (accessed 14 July 2021).
-
Brown L. The PPI balance: benefits to research and those who take part. ADePT-RU News, 2021. URL: https://adept-ru.com/the-ppi-balance-benefits-to-research-and-those-who-take-part/ (accessed 10 August 2021).
-
Franklin S. Patient and Public Involvement in Stated Preference Research in Health. BMH Patient and Public Involvement and Engagement; 2020. URL: https://blogs.manchester.ac.uk/bmh-ppie/2020/12/01/patient-and-public-involvement-in-stated-preference-research-in-health/ (accessed 1 December 2020).
-
Manchester Centre for Health Economics. Metacognitive Therapy in Cardiac Rehabilitation. Newsletter, autumn 2021. Manchester: University of Manchester; 2021, p. 3.
-
The Ticker Club. Pathway: Taking Part in Cardiac Research. Newsletter, autumn 2021. Manchester: The Ticker Club; 2021, p. 4.
Community engagement
-
‘The PATHWAY Study Results Event’, delivered on Microsoft Teams. 15 July 2021.
-
‘Improving the effectiveness of psychological interventions for anxiety and depression in cardiac rehabilitation’ interactive presentation delivered at the Ticker Club Annual Patient Support Visitors’ Seminar. 25 June 2019.
-
‘Sharing perspectives; PPI in the MCT PATHWAY Trial’ presentation delivered at Greater Manchester Mental Health Trust, Climbing the Ladder of Co–Production: The Involvement of Service Users and Carers in Clinical Research event for service users and staff. 17 May 2019.
-
Research and Innovation (R&I) Staff Engagement Event: Equality and Diversity, ‘Accessibility and disability in research – not every disability is visible’. 18 May 2018.
-
Holy Cross College Presentation. 2016. Two senior research assistants from the PATHWAY programme attended a Sixth Form Careers Day at Holy Cross College, Bury, to talk about the PATHWAY programme, their roles as senior research assistants, and their careers in psychology to date. The PATHWAY staff gave a short presentation, following which they answered questions and later spoke with students in more detail at a lunchtime event. Staff from the college reported that students enjoyed learning about how research in psychology is done in practice, and that this had encouraged students to consider different careers in psychology.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- British Heart Foundation (BHF) . BHF UK Factsheet 2021 2021. www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications (accessed 14 December 2021).
- BACPR . The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017, 3rd Edition 2017. www.bacpr.com/resources/AC6_BACPRStandards&CoreComponents2017.pdf (accessed 14 December 2021).
- Shields G, Wells A, Doherty P, Heagerty A, Buck D, Davies L. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart 2018;104:1403-10. https://doi.org/10.1136/heartjnl-2017-312809.
- Anderson L, Oldridge N, Thompson D, Zwisler A, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. J Am Coll Cardiol 2016;67:1-12. https://doi.org/10.1016/j.jacc.2015.10.044.
- National Audit of Cardiac Rehabilitation . National Audit of Cardiac Rehabilitation Annual Report 2019 2019. www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2019 (accessed 14 December 2021).
- Palacios J, Khondoker M, Mann A, Tylee A, Hotopf M. Depression, and anxiety symptom trajectories in coronary heart disease: associations with measures of disability and impact on 3-year health care costs. J Psychosom Res 2018;104:1-8. https://doi.org/10.1016/j.jpsychores.2017.10.015.
- Richards S, Anderson L, Jenkinson C, Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev 2017;4. https://doi.org/10.1002/14651858.CD002902.pub4.
- Wells A. Metacognitive Therapy for Anxiety and Depression. New York, NY: Guilford Press; 2009.
- Normann N, van Emmerik AA, Morina N. The efficacy of metacognitive therapy for anxiety and depression: a meta-analytic review. Depress Anxiety 2014;31:402-11. https://doi.org/10.1002/da.22273.
- Callesen P, Reeves D, Heal C, Wells A. Metacognitive therapy versus cognitive behaviour therapy in adults with major depression: a parallel single-blind randomised trial. Sci Rep 2020;10. https://doi.org/10.1038/s41598-020-64577-1.
- Nordahl HM, Borkovec TD, Hagen R, Kennair LEO, Hjemdal O, Solem S, et al. Metacognitive therapy versus cognitive-behavioural therapy in adults with generalised anxiety disorder. BJPsych Open 2018;4:393-400. https://doi.org/10.1192/bjo.2018.54.
- Wells A, Walton D, Lovell K, Proctor D. Metacognitive therapy versus prolonged exposure in adults with chronic post-traumatic stress disorder: a parallel randomized controlled trial. Cognit Ther Res 2015;39:70-8. https://doi.org/10.1007/s10608-014-9636-6.
- Normann N, Morina N. The efficacy of metacognitive therapy: a systematic review and meta-analysis. Front Psychol 2018;9. https://doi.org/10.3389/fpsyg.2018.02211.
- McPhillips R, Salmon P, Wells A, Fisher P. Cardiac rehabilitation patients’ accounts of their emotional distress and psychological needs: a qualitative study. J Am Heart Assoc 2019;8. https://doi.org/10.1161/JAHA.118.011117.
- McPhillips R, Salmon P, Wells A, Fisher P. Qualitative analysis of emotional distress in cardiac patients from the perspectives of cognitive behavioral and metacognitive theories: why might cognitive behavioral therapy have limited benefit, and might metacognitive therapy be more effective?. Front Psychol 2019;9. https://doi.org/10.3389/fpsyg.2018.02288.
- Wells A, Reeves D, Heal C, Fisher P, Davies L, Heagerty A, et al. Establishing the feasibility of group metacognitive therapy for anxiety and depression in cardiac rehabilitation: a single-blind randomized pilot study. Front Psychiatry 2020;11. https://doi.org/10.3389/fpsyt.2020.00582.
- Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group-based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2593-8.
- Wells A, Faija C. Metacognitive therapy for anxiety and depression in cardiac rehabilitation: commentary on the UK National Institute of Health Research funded PATHWAY programme. J Cardiol Cardiovasc Sci 2018;2:10-4. https://doi.org/10.29245/2578-3025/2018/3.1131.
- Wells A, Reeves D, Capobianco L, Heal C, Davies L, Heagerty A, et al. Improving the effectiveness of psychological interventions for depression and anxiety in cardiac rehabilitation: PATHWAY—a single-blind, parallel, randomized, controlled trial of group metacognitive therapy. Circulation 2021;144:23-3. https://doi.org/10.1161/CIRCULATIONAHA.120.052428.
- Shields G, Brown L, Wells A, Capobianco L, Vass C. Utilising patient and public involvement in stated preference research in health: learning from the existing literature and a case study. Patient Centered Outcomes Res 2020;14:399-412. https://doi.org/10.1007/s40271-020-00439-2.
- Shields G, Wells A, Doherty P, Reeves D, Capobianco L, Heagerty A, et al. Protocol for the economic evaluation of metacognitive therapy for cardiac rehabilitation participants with symptoms of anxiety and/or depression. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-035552.
- McPhillips R, Capobianco L, Cooper B, Husain Z, Wells A. Cardiac rehabilitation patients experiences and understanding of group metacognitive therapy: a qualitative study. Open Heart 2021;8. https://doi.org/10.1136/openhrt-2021-001708.
- Anderson R, Capobianco L, Fisher P, Reeves D, Heal C, Faija C, et al. Testing relationships between metacognitive beliefs, anxiety, and depression in cardiac and cancer patients: are they transdiagnostic?. J Psychosom Res 2019;124. https://doi.org/10.1016/j.jpsychores.2019.109738.
- Wells A, Reeves D, Heal C, Davies LM, Shields GE, Heagerty A, et al. Evaluating metacognitive therapy to improve treatment of anxiety and depression in cardiovascular disease: the NIHR funded PATHWAY research programme. Front Psychiatry 2022;13. https://doi.org/10.3389/fpsyt.2022.886407.
- Wells A, Heal C, Reeves D, Capobianco L. Prevalence of post-traumatic stress and tests of metacognition as a PTSD risk marker in patients with coronary heart disease and elevated HADS scores: analysis of data from the PATHWAY RCT’s in UK cardiac rehabilitation. Front Psychiatry 2023;14. https://doi.org/10.3389/fpsyt.2023.1198202.
- Shields G, Wright S, Wells A, Doherty P, Capobianco L, Davies L. Delivery preferences for psychological intervention in cardiac rehabilitation: a pilot discrete choice experiment. Open Heart 2021;8. https://doi.org/10.1136/openhrt-2021-001747.
- Capobianco L, Faija C, Cooper B, Brown L, McPhillips R, Shields G, et al. A framework for implementing patient and public involvement in mental health research: the PATHWAY research programme benchmarked against NIHR standards. Health Expect 2023;26:640-50. https://doi.org/10.1111/hex.13676.
- Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Metacognitive therapy home-based self-help for cardiac rehabilitation patients experiencing anxiety and depressive symptoms: study protocol for a feasibility randomised controlled trial (PATHWAY Home-MCT). Trials 2018;19. https://doi.org/10.1186/s13063-018-2826-x.
- Wells A, Reeves D, Heal C, Fisher P, Doherty P, Davies L, et al. Metacognitive therapy self-help for anxiety-depression: single-blind randomized feasibility trial in cardiovascular disease. Health Psychol 2022;41:366-77. https://doi.org/10.1037/hea0001168.
- Faija C, Reeves D, Heal C, Capobianco L, Anderson R, Wells A. Measuring the cognitive attentional syndrome in cardiac patients with anxiety and depression symptoms: psychometric properties of the CAS-1R. Front Psychol 2019;10. https://doi.org/10.3389/fpsyg.2019.02109.
- Faija C, Reeves D, Heal C, Wells A. Metacognition in cardiac patients with anxiety and depression: psychometric performance of the metacognitions questionnaire 30 (MCQ-30). Front Psychol 2020;11. https://doi.org/10.3389/fpsyg.2020.01064.
- Capobianco L, Faija C, Husain Z, Wells A. Metacognitive beliefs and their relationship with anxiety and depression in physical illnesses: a systematic review. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0238457.
- Shields GE, Wells A, Wright S, Vass CM, Doherty PJ, Capobianco L, et al. Discrete choice experiment to investigate preferences for psychological intervention in cardiac rehabilitation. BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2022-062503.
- Wells A, Reeves D, Heal C, Fisher P, Doherty P, Davies L, et al. Metacognitive therapy home-based self-help for anxiety and depression in cardiovascular disease patients in the UK: a single-blind randomised controlled trial. PLOS Med 2023;20. https://doi.org/10.1371/journal.pmed.1004161.
- Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- DeJean D, Giacomini M, Vanstone M, Brundisini F. Patient experiences of depression and anxiety with chronic disease: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser 2013;13:1-33.
- Dekker R. Patient perspectives about depressive symptoms in heart failure. J Cardiovasc Nurs 2014;29:E9-15. https://doi.org/10.1097/jcn.0b013e318273a5d6.
- Wells A, Matthews G. Attention and Emotion: A Clinical Perspective. Hove: Erlbaum; 1994.
- Wells A, Matthews G. Modelling cognition in emotional disorder: the S-REF model. Behav Res Ther 1996;34:881-8. https://doi.org/10.1016/s0005-7967(96)00050-2.
- Wells A. Breaking the cybernetic code: understanding and treating the human metacognitive control system to enhance mental health. Front Psychol 2019;10. https://doi.org/10.3389/fpsyg.2019.02621.
- McDonald I, Daly J, Jelinek V, Panetta F, Gutman J. Opening Pandora’s box: the unpredictability of reassurance by a normal test result. BMJ 1996;313:329-32. https://doi.org/10.1136/bmj.313.7053.329.
- Robertson N, Javed N, Samani N, Khunti K. Psychological morbidity, and illness appraisals of patients with cardiac and non-cardiac chest pain attending a rapid access chest pain clinic: a longitudinal cohort study. Heart 2008;94:e12-e12. https://doi.org/10.1136/hrt.2006.100537.
- Clark A, Barbour R, White M, MacIntyre P. Promoting participation in cardiac rehabilitation: patient choices and experiences. J Adv Nurs 2004;47:5-14. https://doi.org/10.1111/j.1365-2648.2004.03060.x.
- Jacobson N, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12-9. https://doi.org/10.1037/0022-006x.59.1.12.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent 2019. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ (accessed 14 December 2021).
- NHS England and NHS Improvement . 2018/19/National/Cost/Collection/Data/Publication 2021. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 14 December 2021).
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. PharmacoEconomics 2008;26:661-77. https://doi.org/10.2165/00019053-200826080-00004.
- Whalley B, Thompson D, Taylor R. Psychological interventions for coronary heart disease: Cochrane systematic review and meta-analysis. Int J Behav Med 2012;21:109-21. https://doi.org/10.1007/s12529-012-9282-x.
- Dickens C, Cherrington A, Adeyemi I, Roughley K, Bower P, Garrett C, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease. Psychosom Med 2013;75:211-21. https://doi.org/10.1097/psy.0b013e31827ac009.
- Reavell J, Hopkinson M, Clarkesmith D, Lane D. Effectiveness of cognitive behavioral therapy for depression and anxiety in patients with cardiovascular disease: a systematic review and meta-analysis. Psychosom Med 2018;80:742-53. https://doi.org/10.1097/psy.0000000000000626.
- Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health 2013;16:3-13. https://doi.org/10.1016/j.jval.2012.08.2223.
- Choicemetrics . Ngene 2019. http://www.choice-metrics.com/index.html (accessed 14 December 2021).
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev 2011;12. https://doi.org/10.1002/14651858.CD008012.pub3.
- Lemay K, Tulloch H, Pipe A, Reed J. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 2019;39:E6-11. https://doi.org/10.1097/HCR.0000000000000379.
- Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart 2006;92:62-7. https://doi.org/10.1136/hrt.2004.052787.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 14 December 2021).
- Van Hout B, Janssen M, Feng Y, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- McCabe C, Claxton K, Culyer A. The NICE cost-effectiveness threshold. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- Bertelsen J, Dehbarez N, Refsgaard J, Kanstrup H, Johnsen S, Qvist I, et al. Shared care versus hospital-based cardiac rehabilitation: a cost-utility analysis based on a randomised controlled trial. Open Heart 2018;5. https://doi.org/10.1136/openhrt-2016-000584.
- Hwang R, Morris N, Mandrusiak A, Bruning J, Peters R, Korczyk D, et al. Cost-utility analysis of home-based telerehabilitation compared with centre-based rehabilitation in patients with heart failure. Heart Lung Circ 2019;28:1795-803. https://doi.org/10.1016/j.hlc.2018.11.010.
- Taylor R, Sadler S, Dalal H, Warren F, Jolly K, Davis R, et al. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: a decision model-based analysis. Eur J Prev Cardiol 2019;26:1252-61. https://doi.org/10.1177/2047487319833507.
- Leggett L, Khadaroo R, Holroyd-Leduc J, Lorenzetti D, Hanson H, Wagg A, et al. Measuring resource utilization. Medicine 2016;95. https://doi.org/10.1097/MD.00000000000027.
- NHS England and NHS Improvement . 2017/18/National/Cost/Collection/Data/Publication 2018. webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 14 December 2021).
Appendix 1 Practitioners’ experience of training and delivering Group-MCT
Aims
To assess CR staff’s experience of learning and delivering MCT.
Methodology
Cardiac rehabilitation staff were interviewed at three time points: before Group-MCT training, in the middle of training, and after delivering Group-MCT. A topic guide was developed and used to guide the interviews.
Nine CR staff delivering MCT were interviewed regarding their experience of training and delivery of Group-MCT. Table 4 gives the CR staff characteristics.
Group-MCT was initially delivered by seven CR practitioners across three CR services participating in PATHWAY. Six of these practitioners were interviewed before training, with one practitioner declining to be interviewed at this point. However, all seven were interviewed during training and after they had delivered Group-MCT, as the practitioner who originally declined subsequently contacted the research team in order to take part. Two more CR services later joined the study. Two CR practitioners from one of these services and two CRNs were trained in Group-MCT. The two CR practitioners provided written informed consent and were interviewed during training and after they had delivered Group-MCT. One CRN was interviewed during training only, as she left the study shortly afterwards, and the other CRN declined to take part in the study. See Table 4 for an overview of the therapists included in interviews.
The study received ethics approval from NRES Committee North West REC (reference 15/NW/0163). All participants provided written informed consent prior to being interviewed. Qualitative interviews were audio-recorded, transcribed verbatim, pseudonymised and analysed using thematic analysis.
Extracts representative of participants’ responses are used to illustrate the main findings. Practitioners are identified by participant numbers, which are prefaced with ‘BT’ when extracts are taken from interviews conducted before Group-MCT training, with ‘DT’ when extracts are taken from interviews during training, and with ‘AD’ when extracts are taken from interviews after practitioners had delivered Group-MCT as part of the RCT. Ellipses indicate omitted talk and square brackets are used for explanatory comments.
Results
Group-MCT training
Before and during training
All of the practitioners were enthusiastic about MCT and anticipated that it would be of benefit to patients; however, they felt that they faced a number of dilemmas regarding the delivery of the intervention. These included language use, engagement with patients’ concerns, seeing MCT as part of a toolkit and feeling conflicted in their role between being a teacher as a CR practitioner and a therapist in MCT.
| ID | Site | Occupation | Length of time in role at time of first interview | Previous training and/or experience of psychology | Number of interviews completed |
|---|---|---|---|---|---|
| 01 | CM | CR physiotherapist | 4 years | On-the-job training from an occupational therapist; attendance at a Manchester City Council-delivered course to deal with the emotional and psychological needs of patients | 3 |
| 02 | M | CR nurse practitioner | 15 years | MSc in CR – including one module on the psychological aspects of CR (covered misconceptions around CHD; psychological concerns that patients have – did not teach any techniques or interventions) | 3 |
| 03 | SM | Occupational therapist in CR | 6 months | BSc in occupational therapy; CBT certification; currently completing MSc in health care; previously worked in a psychiatric setting) | 3 |
| 04 | M | Exercise and healthy lifestyle facilitator in CR | 3 years | BACPR training | 3 |
| 05 | SM | Cardiology nurse (does not deliver CR) | 2 years | BACPR training; courses in relaxation training; helping people change training | 3 |
| 06 | SM | Cardiac nurse; co-ordinator of community cardiac programme | 24 years | BACPR training (draws on training and experience of community nursing and MSc in public health when talking about training to deal with psychological issues) | 3 |
| 07 | CM | CR nurse practitioner | 1 month (previously a coronary care nurse) | On-the-job training for CR role | 2 (did not complete pre-training interview) |
| 08 | P | Cardiology nurse consultant | 10 years | MSc in health research; MSc modules in ‘advanced practice’; modules in ‘practitioner with a special interest in cardiology’ | 2 (did not complete pre-training interview) |
| 09 | P | Cardiology specialist nurse | 12 years (8 years in a different cardiac setting) | BACPR training (advanced) | 2 (did not complete pre-training interview) |
Group-MCT followed a structured manual, and practitioners wanted to be able to deliver Group-MCT using their own words so that the delivery did not appear scripted. However, they also noted that they wanted to maintain treatment fidelity and adherence to the treatment protocol by ensuring that they were delivering the correct message. This led some practitioners to create their own crib-sheets and summarise the manual in their own words.
Practitioners also felt conflicted about the fact that MCT lacked engagement with the content of various patients’ concerns. Practitioners had previously described supporting patients by having in-depth conversations about what was causing patients to worry, and described how using MCT caused them to feel dismissive of patients’ worries.
However, Group-MCT presented others with an opportunity to deal with patients’ distress more efficiently and effectively.
Two more dilemmas were identified that were implicit in practitioners’ talk. The first concerned their ‘toolkit’: some saw Group-MCT as simply fitting in with the psychological interventions they already delivered, while others saw it as being at odds with these interventions:
I would say other areas of the care – we sit and listen to people’s problems and offer solutions and metacognitive therapy is not really looking at the ins and outs of a problem, it is just looking at ways of directing your thoughts … I think you need to have both really.
DT01
A further dilemma concerned their role as teacher in CR or therapist in MCT. Practitioners’ usual roles saw them teaching CBT techniques such as the worry tree and discussing the content of patients’ thoughts, but therapists noted how this approach opposes what is taught in MCT:
If they are supposed to be having standard treatment then the MCT sessions are on top … what we normally teach in our stress sessions is if you’ve got a problem, is there anything you can do about it? If it’s a yes problem solve it, if it’s a no, like use the decision tree, decide if you need to worry about it, use these techniques to help control stress and worry, that is completely the opposite to metacognitive therapy.
DT01
Interviews after delivering Group-MCT
Following the delivery of Group-MCT sessions, practitioners remained enthusiastic about Group-MCT and reported believing that patients had benefited.
Therapists also noted that it had changed their own beliefs and discussed how they had applied MCT in their personal lives:
I couldn’t do worry postponement personally.
DT01
Their understanding of (and confidence in) MCT had improved over time, with practice, but some misunderstandings remained.
They also noted that previous dilemmas such as language use and feeling dismissive of patients’ worries had been resolved with further practice. One therapist noted how the role-playing in supervision was particularly important in learning MCT, noting:
In pretending to be a patient, and see how we would respond … you got to know your patients and what their thought patterns were and things … So you could anticipate what they were gonna ask and then quite often, I’d say fifty per cent of the time, they did come out with those things in the session … So, then you were prepared for what to say.
01AD
Therapists also noted that their views on MCT forming part of their toolkit had changed, saying that with practice they had further understood the different components of MCT:
I think I understand the difference between different elements of it [MCT] more now … Something might work better for one, one person at a particular time than another … it’s actually lots of little things that you can try.
DT01
Appendix 2 The cost-effectiveness of Group-MCT plus usual care, versus usual care alone, for cardiac rehabilitation participants with symptoms of anxiety and/or depression
Aim
The within-trial economic evaluation compares the cost-effectiveness of MCT plus usual care with that of usual care from the perspective of health and social care in the UK.
Methodology
A protocol reporting the design of the economic evaluation has been published separately. 21
In brief, the measure of health benefit used was the QALY; this was estimated using the EQ-5D-5L, which was collected at baseline and at 4- and 12-month follow-up. The EQ-5D-5L has been validated in the population and recommended by NICE. 55,56 In line with current NICE recommendations, the crosswalk algorithm was used to estimate utility values from the EQ-5D-5L. 57
Data on health and social care use were collected using an economic patient questionnaire (capturing inpatient, outpatient, day case, accident, and emergency, primary, community and social care use). Unit costs of NHS and social care services were derived from national average unit cost data, and the price year was 2019.
Cardiac rehabilitation sessions (both education and exercise) were costed as £48 per participant per session. 46 In the primary analysis, MCT costs included staff time for preparation and delivery, and the costs associated with providing a manual and CD (compact disc). The cost of manual and CD was negligible (£3.55). Staff costs were estimated using the mean of a range of staff at band 6 and band 7, including community nurses, hospital-based physiotherapists and occupational therapists. 45 Two healthcare practitioners were costed to deliver sessions, with 2 hours assumed to cover preparation and delivery. A cost per participant was calculated using the average group size from the trial. This resulted in a mean cost per metacognitive therapy session per participant of £54, which was multiplied by the number of sessions attended.
Single imputation was used to impute missing baseline variables, with MI used to impute values missing at follow-up. The primary measure of interest is the ICER. Regression analysis was used to estimate net costs and net QALYs, and these estimates were bootstrapped to generate 10,000 net pairs of costs and QALYs to inform the probability of cost-effectiveness. Regression analyses adjusted for participant characteristics (covariates). Covariates for costs and QALYs included age, gender, hospital site, baseline HADs score, medication for depression or anxiety, body mass index, smoking status, alcohol units consumed per month and number of comorbidities. Net monetary benefit statistics were produced for each pair of simulated net costs and net benefits. The monetary value of simulated QALYs were varied from £0 to £30,000 to reflect a range of hypothetical WTPT. Key sensitivity analyses were used to test the impact of the study design on the results of the cost-effectiveness acceptability analysis.
Data manipulation and analysis were conducted in SPSS version 25 (IBM Corporation, Armonk, NY, USA) and Stata version 14. Details of the methods for the economic evaluation can be found in the protocol. 21
It was planned that a de novo economic model would be constructed with the aims of (1) exploring the cost-effectiveness of MCT over a longer time horizon and (2) exploring the cost-effectiveness of MCT in different populations/settings. However, during the model design, discussions highlighted that without additional evidence becoming available the economic model would not be useful and robust. In particular, high-quality data generalisable to the UK are needed to support the rates of relapse and remission of depression and/or anxiety symptoms and mortality rates specific to the CR population, as is evidence to support the long-term effectiveness of MCT for this population. The protocol noted that subgroup analysis would be conducted if participant numbers/completion allowed; however, owing to the existing limitations of sample size/missingness, these were not explored.
Results
Baseline participant demographics are reported in the trial publication by Wells et al. with no significant differences identified in any of the measured variables across groups. 19 Cost and QALY data were complete for 179 participants (54%; 91 control, 88 intervention). Three hundred and thirty-one participants had complete EQ-5D-5L data at baseline, 260 (78%) had complete data at 4-month follow-up and 245 (74%) had complete data at 12-month follow-up. A total of 262 (79%) participants at baseline, 203 (61%) participants at 4-month follow-up and 211 (64%) participants at 12-month follow-up had sufficient data from the service use questionnaire to estimate baseline costs. Table 5 reports the mean utility value at each assessment for participants with complete cost and QALY data. Table 6 reports a breakdown by cost category for these cases; wide 95% CIs indicate a high level of variation.
Table 7 reports the key results. In primary analyses and the majority of sensitivity analysis, MCT intervention is dominant (cost saving and health increasing). However, the CIs are wide and overlap zero, indicating a high level of variability/uncertainty in the estimates.
Figure 9 displays the uncertainty in the analysis as demonstrated as the net cost/QALY pairs are spread across each of the four quadrants. Figure 10 shows that at a commonly discussed threshold (£30,000 per QALY), the MCT intervention is around 70% likely to be cost-effective.
Discussion
Although the primary cost-effectiveness analysis and the majority of sensitivity analyses indicate that MCT intervention may be cost saving and health increasing, the wide CIs that overlap zero indicate a high level of variability and uncertainty in the estimates. In the primary analysis the probability of cost-effectiveness ranges from 59% at a threshold of £0 per QALY to 76% at a threshold of £30,000 per QALY. Even so, given the uncertainty in the estimates, it cannot be concluded that there is evidence to suggest that MCT is or is not cost-effective.
Regarding the sensitivity analysis, the results at 4-month follow-up are very similar to the primary analysis (12 months). The complete-case analysis does not affect conclusions and uncertainty remains. As would be expected, different assumptions around the cost of MCT affect the probability of cost-effectiveness (e.g. larger group size). Although the decrease in HADS score is significant, it is left to decision-makers to decide how much they would be prepared to pay for a reduction in this measure. In two of the sensitivity analyses, MCT was associated with a net cost increase (not significant). Both of these analyses restricted the participant sample. The first focused on those who met the HADS cut-off point for depression and/or anxiety at baseline, excluding those who no longer met the criteria. The second restricted the MCT arm to the participants assigned to intervention who attended one or more sessions of MCT. Although the ICERs estimated for these analyses were under commonly discussed thresholds, the level of uncertainty is vast, as demonstrated by the CIs and probability of cost-effectiveness. These highly explorative analyses again highlight the need to consider how the implementation of MCT in CR will have an impact on cost-effectiveness. For example, if there is a substantial wait time for therapy in reality, this will have a knock-on effect on the cost-effectiveness.
| Time point | Usual care (n = 91) | MCT plus usual care (n = 88) | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Baseline | 0.642 | 0.019 | 0.607 | 0.025 |
| 4 months | 0.643 | 0.024 | 0.665 | 0.030 |
| 12 months | 0.642 | 0.027 | 0.645 | 0.029 |
| Cost category | Usual care (n = 91) | MCT plus usual care (n = 88) | ||
|---|---|---|---|---|
| Mean (SE) | 95% CI | Mean (SE) | 95% CI | |
| Inpatient | ||||
| Pre-baseline | 4659 (800) | 3069 to 6248 | 5372 (770) | 3842 to 6901 |
| 4 months | 1105 (539) | 34 to 2177 | 566 (206) | 157 to 975 |
| 12 months | 1110 (323) | 468 to 1753 | 718 (258) | 205 to 1232 |
| Outpatient | ||||
| Pre-baseline | 174 (24) | 126 to 222 | 197 (31) | 136 to 259 |
| 4 months | 154 (20) | 114 to 194 | 223 (35) | 152 to 293 |
| 12 months | 216 (37) | 142 to 289 | 310 (51) | 208 to 411 |
| Day case | ||||
| Pre-baseline | 288 (81) | 127 to 449 | 99 (32) | 36 to 163 |
| 4 months | 53 (28) | 2 to 109 | 103 (47) | 10 to 197 |
| 12 months | 78 (32) | 15 to 141 | 235 (84) | 68 to 403 |
| Accident and emergency | ||||
| Pre-baseline | 189 (22) | 145 to 234 | 219 (21) | 178 to 260 |
| 4 months | 55 (14) | 26 to 84 | 53 (15) | 23 to 83 |
| 12 months | 84 (20) | 45 to 123 | 81 (20) | 41 to 120 |
| Primary, community and social care | ||||
| Pre-baseline | 149 (23) | 103 to 195 | 140 (12) | 117 to 163 |
| 4 months | 140 (17) | 107 to 173 | 185 (27) | 131 to 239 |
| 12 months | 239 (39) | 162 to 316 | 215 (34) | 147 to 282 |
| Usual care and MCT | ||||
| MCT intervention | NA | NA | 238 (12) | 213 to 263 |
| CR | 520 (29) | 463 to 577 | 599 (27) | 545 to 654 |
| Analysisa | Net cost (95% CI) (£)b | Net QALY (95% CI) | ICER (£/QALY)b | Probability that MCT is cost-effective vs. usual care if WTPT is £30,000/QALY (%) |
|---|---|---|---|---|
| Primary (n = 332) | −219 (−1446 to 1007) | 0.015 (−0.015 to 0.045) | Dominant | 76 |
| Sensitivity analysis | ||||
| Complete case (n = 179) | −1 (−1387 to 1385) | 0.035 (−0.004 to 0.074) | Dominant | 83 |
| Participants with anxiety and/or depression confirmed by HADS at baseline (n = 284) | 75 (−1090 to 1241) | 0.013 (−0.020 to 0.045) | 5901 | 60 |
| Treatment received rather than intention-to-treat (n = 292) | 133 (−1166 to 1432) | 0.015 (−0.018 to 0.049) | 8618 | 58 |
| MCT costs (inclusive of training and supervision) (n = 332) | −9 (−1225 to 1207) | 0.015 (−0.015 to 0.045) | Dominant | 67 |
| MCT costs (larger group size) (n = 332) | −356 (−1604 to 891) | 0.015 (−0.015 to 0.045) | Dominant | 82 |
| Alternative measure of benefit (HADS) (n = 332) | −219 (−1446 to 1007) | −1.999 (−3.537 to −0.61) | Dominant | 99c |
| Time horizon (4-month follow-up) (n = 332) | −175 (−£832 to 482) | 0.005 (−0.008 to 0.018) | Dominant | 74 |
FIGURE 9.
Cost-effectiveness plane.
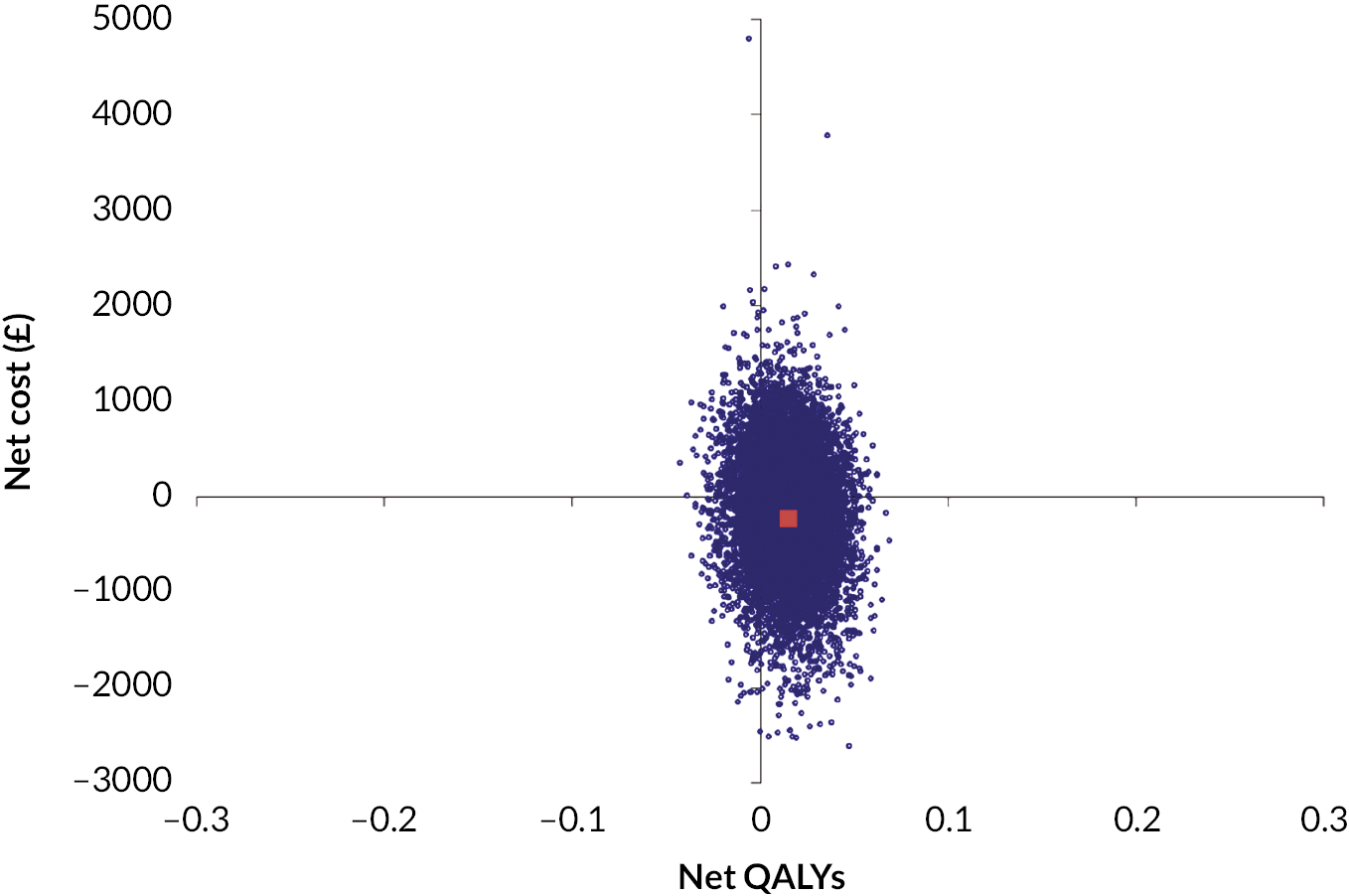
FIGURE 10.
Cost-effectiveness acceptability curve.
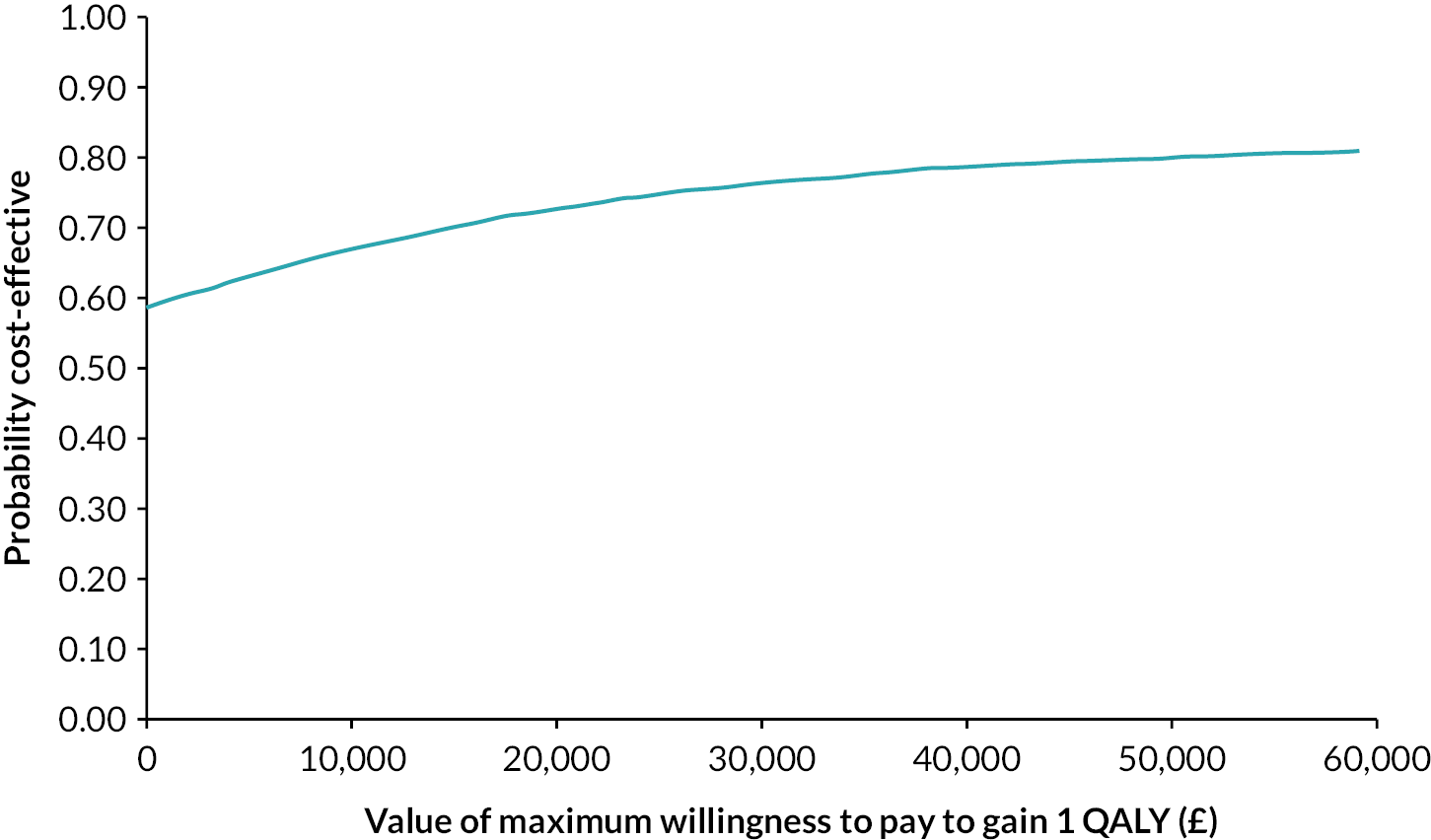
To the authors’ knowledge, as well as expanding the evidence base for psychological therapy in CR, this is the first economic of MCT (for any population group).
The economic evaluation shared the strengths and limitations of the trial. 19 Although the trial achieved a high rate of follow-up at the primary time horizon, there was a relatively large number of missing data for economic measures at the final follow-up. Overall, 54% of participants had complete cost and utility data at both baseline and follow-up. Larger numbers of missing data reduce the robustness of imputation. Data were imputed by category of cost and EQ-5D-5L domain to make best use of all available data and a complete-case analysis was conducted for comparison. However, given the number of missing data, the results should be interpreted with caution. The number of missing data is similar to that in other trials that have collected self-report data using a similar questionnaire in mental health populations. 58–61 The sample size and missing data limited the potential for subgroup analyses. Health and social care service use was self-reported. While this is a valid approach to data collection, especially in the UK where access to electronic data is associated with hurdles in terms of time and budget, it is open to recall bias and missing data. 62 Service use data are often variable and the sample size of the study and data completeness limits conclusions. Unit costs (especially related to cardiac inpatient admissions) can be substantial. Further research should investigate how the addition of psychological therapy impacts the categories of service use and the interactions between these categories, to more robustly determine how intervention may affect net costs across health and social care. The Recovering Quality of Life (ReQoL) measure is now available, which is a generic self-report measure for use with people experiencing mental health concerns. In comparison with the EQ-5D-5L, this has more focus on mental health and quality of life and also allows for the estimation of utilities for use in economic evaluation. Subsequently, in future research, the exploration of different measures is recommended, as the EQ-5D-5L cannot reflect all aspects of health for all diseases and all patients. The results may not be generalisable to other settings; cardiac services (including the type of CR offered) and populations vary by area. 2,5
Appendix 3 Economic evaluation unit costs
| Service type | Unit cost (£) | Reference (unit measure) |
|---|---|---|
| Inpatient staysa,b,c | ||
| Angiogram | 760 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Angioplasty elective | 1819 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Angioplasty | 1086 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Blood disorder non-elective | 495 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Bowel cancer elective | 1480 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Breast surgery elective | 2835 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Broken shoulder non-elective | 523 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Bypass elective | 2299 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Bypass | 1853 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Cardiology elective | 1116 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Cardiology non-elective | 632 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Catheter procedures | 760 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Coronary elective | 2261 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Coronary non-elective | 1199 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Gastroenterology elective | 3207 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| General admission elective | 1480 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| General admission non-elective | 523 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| ICU | 1501 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Kidney non-elective | 461 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Neurology non-elective | 1038 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Respiratory infection elective | 601 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Shoulder operation elective | 2871 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Stroke non-elective | 466 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Sturnem rewire elective | 1116 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Transplant elective | 2381 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Vascular non-elective | 519 | NHS reference costs 2017/18 63 updated to 2019 prices (per day) |
| Outpatient visits | ||
| Ambulatory care unit | 167 | NHS reference costs 2018/19 46 (per visit) |
| Anticoagulation | 37 | NHS reference costs 2018/19 46 (per visit) |
| Cardiology | 139 | NHS reference costs 2018/19 46 (per visit) |
| Cardiothoracic surgery | 238 | NHS reference costs 2018/19 46 (per visit) |
| Clinical immunology | 286 | NHS reference costs 2018/19 46 (per visit) |
| Clinical oncology | 143 | NHS reference costs 2018/19 46 (per visit) |
| Clinical oncology (previously radiotherapy) | 143 | NHS reference costs 2018/19 46 (per visit) |
| Clinical psychology | 199 | NHS reference costs 2018/19 46 (per visit) |
| Colorectal surgery | 121 | NHS reference costs 2018/19 46 (per visit) |
| Dental medicine | 138 | NHS reference costs 2018/19 46 (per visit) |
| Dermatology | 113 | NHS reference costs 2018/19 46 (per visit) |
| Diabetic medicine | 142 | NHS reference costs 2018/19 46 (per visit) |
| Diagnostic imaging | 32 | NHS reference costs 2018/19 46 (per visit) |
| Dietetics | 85 | NHS reference costs 2018/19 46 (per visit) |
| Endocrinology | 161 | NHS reference costs 2018/19 46 (per visit) |
| ENT | 107 | NHS reference costs 2018/19 46 (per visit) |
| Gastroenterology | 141 | NHS reference costs 2018/19 46 (per visit) |
| General medicine | 167 | NHS reference costs 2018/19 46 (per visit) |
| General surgery | 134 | NHS reference costs 2018/19 46 (per visit) |
| Gynaecology | 141 | NHS reference costs 2018/19 46 (per visit) |
| Haematology | 167 | NHS reference costs 2018/19 46 (per visit) |
| Hepatology | 196 | NHS reference costs 2018/19 46 (per visit) |
| Hernia procedures | 219 | NHS reference costs 2018/19 46 (per visit) |
| Index outpatient | 127 | NHS reference costs 2018/19 46 (per visit) |
| Infectious diseases | 291 | NHS reference costs 2018/19 46 (per visit) |
| Interventional radiology | 93 | NHS reference costs 2018/19 46 (per visit) |
| Liaison psychiatry | 210 | NHS reference costs 2018/19 46 (per visit) |
| Maxillo-facial surgery | 124 | NHS reference costs 2018/19 46 (per visit) |
| Nephrology | 164 | NHS reference costs 2018/19 46 (per visit) |
| Neurology | 177 | NHS reference costs 2018/19 46 (per visit) |
| Oncology | 143 | NHS reference costs 2018/19 46 (per visit) |
| Ophthalmology | 98 | NHS reference costs 2018/19 46 (per visit) |
| Pain management | 157 | NHS reference costs 2018/19 46 (per visit) |
| Phlebotomy | 4 | NHS reference costs 2018/19 46 (per visit) |
| Physiotherapy | 58 | NHS reference costs 2018/19 46 (per visit) |
| Plastic surgery | 107 | NHS reference costs 2018/19 46 (per visit) |
| Podiatry | 54 | NHS reference costs 2018/19 46 (per visit) |
| Respiratory medicine | 157 | NHS reference costs 2018/19 46 (per visit) |
| Respiratory physiology | 120 | NHS reference costs 2018/19 46 (per visit) |
| Respiratory sleep study | 85 | NHS reference costs 2018/19 46 (per visit) |
| Rheumatology | 147 | NHS reference costs 2018/19 46 (per visit) |
| Smoking cessation support | 143 | NHS reference costs 2018/19 46 (per visit) |
| Stroke medicine | 197 | NHS reference costs 2018/19 46 (per visit) |
| Trauma and orthopaedics | 120 | NHS reference costs 2018/19 46 (per visit) |
| Urology | 108 | NHS reference costs 2018/19 46 (per visit) |
| Day cases | ||
| Angioplasty | 1970 | NHS reference costs 2018/19 46 (per case) |
| Biopsy | 607 | NHS reference costs 2018/19 46 (per case) |
| Cardiac catheterisation | 1092 | NHS reference costs 2018/19 46 (per case) |
| Cardiac | 1052 | NHS reference costs 2018/19 46 (per case) |
| Cataract | 914 | NHS reference costs 2018/19 46 (per case) |
| Chemotherapy | 110 | NHS reference costs 2018/19 46 (per case) |
| Colonoscopy | 608 | NHS reference costs 2018/19 46 (per case) |
| Defibrillator | 2336 | NHS reference costs 2018/19 46 (per case) |
| Dermatology | 706 | NHS reference costs 2018/19 46 (per case) |
| Diabetic | 744 | NHS reference costs 2018/19 46 (per case) |
| Diagnostic imaging | 342 | NHS reference costs 2018/19 46 (per case) |
| Dialysis | 964 | NHS reference costs 2018/19 46 (per case) |
| Echocardiogram | 614 | NHS reference costs 2018/19 46 (per case) |
| Endoscopy | 621 | NHS reference costs 2018/19 46 (per case) |
| ENT | 423 | NHS reference costs 2018/19 46 (per case) |
| Eye procedures | 401 | NHS reference costs 2018/19 46 (per case) |
| Foot procedures | 1646 | NHS reference costs 2018/19 46 (per case) |
| Gastroenterology | 524 | NHS reference costs 2018/19 46 (per case) |
| Hand procedure | 1547 | NHS reference costs 2018/19 46 (per case) |
| Implant defibrillator | 2336 | NHS reference costs 2018/19 46 (per case) |
| Index cost | 752 | NHS reference costs 2018/19 46 (per case) |
| Kidney | 1003 | NHS reference costs 2018/19 46 (per case) |
| Multiple stent | 1328 | NHS reference costs 2018/19 46 (per case) |
| Pacemaker | 1953 | NHS reference costs 2018/19 46 (per case) |
| Respiratory | 563 | NHS reference costs 2018/19 46 (per case) |
| Shoulder procedure | 2232 | NHS reference costs 2018/19 46 (per case) |
| Sigmoidoscopy | 443 | NHS reference costs 2018/19 46 (per case) |
| Stent | 1075 | NHS reference costs 2018/19 46 (per case) |
| Urology | 440 | NHS reference costs 2018/19 46 (per case) |
| Accident and emergency | ||
| No hospital admission | 144 | NHS reference costs 2018/19 46 (per attendance) |
| With hospital admission | 261 | NHS reference costs 2018/19 46 (per attendance) |
| Primary, community and social care | ||
| Anticoagulant service | 37 | NHS reference costs 2018/19 46 (per visit) |
| Asthma nursing | 91 | NHS reference costs 2018/19 46 (per visit) |
| Blood test | 4 | NHS reference costs 2018/19 46 (per visit) |
| Breast clinic | 32 | NHS reference costs 2018/19 46 (per visit) |
| Cardiac nursing | 84 | NHS reference costs 2018/19 46 (per visit) |
| Care worker | 12 | |
| Community or primary care based cardiac unit | 84 | NHS reference costs 2018/19 46 (per visit) |
| Counsellor or mental health worker | 35 | PSSRU 2019 46 |
| Dentist | 17 | PSSRU 2019 46 (cost per hour used and 10-minute appointment assumed) |
| Diabetic nursing | 72 | NHS reference costs 2018/19 46 (per visit) |
| District nurse | 40 | NHS reference costs 2018/19 46 (per visit) |
| Drug and alcohol | 91 | NHS reference costs 2018/19 46 (per visit) |
| GP (at the surgery/practice) | 33 | PSSRU 2019 46 |
| GP (at home) | 85 | PSSRU 2019 46 |
| GP (telephone call) | 16 | PSSRU 2019 46 |
| Health visitor | 57 | NHS reference costs 2018/19 46 (per visit) |
| Home care worker/home help | 12 | PSSRU 2019 46 (cost per hour used and assumed 30 minutes of support provided as the majority of visits lasted < 30 minutes) |
| Influenza vaccination | 17 | PSSRU 2019 46 (for nurse administration) and BNF |
| Mental health nurse | 70 | NHS reference costs 2018/19 46 (per visit) |
| Nurse (at home) | 40 | NHS reference costs 2018/19 46 (per visit) |
| Occupational therapist | 86 | NHS reference costs 2018/19 46 (per visit) |
| Ophthalmology | 98 | NHS reference costs 2018/19 46 (per visit) |
| Physiotherapist | 62 | NHS reference costs 2018/19 46 (per visit) |
| Podiatrist | 43 | NHS reference costs 2018/19 46 (per visit) |
| Practice nurse (at the surgery) | 6 | PSSRU 2019 46 (cost per hour) and PSSRU 2015 (average duration of contact) |
| Social worker | 45 | PSSRU 2019 |
| Stroke rehabilitation | 92 | NHS reference costs 2018/19 46 (per visit) |
| Urology | 108 | NHS reference costs 2018/19 46 (per visit) |
| Walk-in centre | 33 | PSSRU 2019 46 |
Appendix 4 Stated preference survey example materials
Survey materials provided to the general public sample are provided as an example below.
Section C: hypothetical psychological therapy alternatives
The next section of questions asks you to compare possible descriptions of different psychological therapies and to choose which you prefer by ticking a box to indicate your choice. Following your choice, you can indicate whether you would take part in your choice or whether you would actually opt out of partaking in psychological therapy. There are 16 of these questions. There are no right or wrong answers. But if you are unsure or have problems answering these questions, please do feel free to contact the research team for help with the questionnaire. Contact details are provided on the instructions. Please try to answer all questions.
C1. Two potential psychological therapies are described below. Remember these would be received in addition to the standard CR package. The statements on the left describe different delivery of the therapy. The statements on the right describe the different options. Imagine that you are offered the choice between therapy A and B. Taking everything into account which therapy would you prefer? Choose which therapy you prefer by ticking the box under therapy A and therapy B. There are no right or wrong answers; it is your view that is important.
| Delivery | Therapy A | Therapy B |
|---|---|---|
| Psychological intervention to be received alongside your standard CR programme | Peer group support that provides general support and advice | Group psychological therapy where you are not required to share detailed information about personal concerns/experiences |
| The person who provides the psychological therapy | Occupational therapist trained to deliver psychological therapy | CR professional trained in delivery of psychological therapy |
| The information given to you prior to accepting and starting treatment that gives you an idea of what to expect from the therapy | No information provided | A printed leaflet of information |
| Location you need to visit to attend psychological therapy sessions | Primary care (GP surgery) | Community care (NHS clinic in the community) |
| Additional cost to the NHS | £0 | £500 |
| C1.1. Of the options presented above which do you like most (Tick one) | □ | □ |
| C1.2. If you had to choose from the option that you like the most, or no psychological therapy included in your cardiac rehabilitation pathway, which would you choose? (Tick one) | ||
| The option I chose above. | □ | |
| No psychological therapy | □ | |
C2. Two potential psychological therapies are described below. Remember these would be received in addition to the standard CR package. The statements on the left describe different delivery of the therapy. The statements on the right describe the different options. Imagine that you are offered the choice between therapy A and B. Taking everything into account which therapy would you prefer? Choose which therapy you prefer by ticking the box under therapy A and therapy B. There are no right or wrong answers; it is your view that is important.
| Delivery | Therapy A | Therapy B |
|---|---|---|
| Psychological intervention to be received alongside your standard CR programme | Peer group support that provides general support and advice | Group psychological therapy where you are not required to share detailed information about personal concerns/experiences |
| The person who provides the psychological therapy | CR professional trained in delivery of psychological therapy | Healthcare professional trained in delivery of psychological intervention, no background in CR or psychology |
| The information given to you prior to accepting and starting treatment that gives you an idea of what to expect from the therapy | A printed leaflet of information | An overview of the therapy from a healthcare provider with a chance to ask questions |
| Location you need to visit to attend psychological therapy sessions | Community care (NHS clinic in the community) | Outpatient (clinic at a hospital) |
| Additional cost to the NHS | £500 | £1000 |
| C2.1. Of the options presented above which do you like most (Tick one) | □ | □ |
| C2.2. If you had to choose from the option that you like the most, or no psychological therapy included in your cardiac rehabilitation pathway, which would you choose? (Tick one) | ||
| The option I chose above. | □ | |
Appendix 5 Ticker Club evaluation
-
1. Please score our presentation:
| Very poor | Poor | Average | Good | Very good | |
|---|---|---|---|---|---|
| Level of information | |||||
| Style of delivery |
-
2. Could you give us some comments about what you enjoyed, found helpful or how we could improve?
_______ _______ _______ ____________ __________ _________ ________ _______ _______ _________ _________ _______ _____ ____________ _________ ______ ________ ______ _______ ______ _______ _______ ___________ _______ _______ _______ ______ ______ ______ _______ _______ _______ _______ _______ _______ _________
-
3. Our advisory group identified messages for us to share with the patients, carers, volunteers, and the public. Please could you let us know which of the following messages came across in our presentation by scoring the following statements?
I feel that this presentation would raise awareness of:
| Strongly agree | Agree | No opinion | Disagree | Strongly disagree | |
|---|---|---|---|---|---|
| What anxiety and depression are and how to recognise the signs that someone may be experiencing these psychological effects | |||||
| What it is like to experience anxiety and depression after a cardiac event, how common this is and that those who experience this are not alone | |||||
| The impact this has on friends, family and carers | |||||
| What MCT is and what it is like to do it |
Once our results are in, we intend to add the following messages:
-
How taking part in MCT as part of the cardiac rehab programme helped patients (both with stats and patient stories).
-
The cost benefits of implementing MCT into rehabilitation pathways.
-
That MCT in cardiac rehab is not currently available and what the next stages of roll out are including how the public can encourage trusts to become involved in this.
We are planning to use the following ways of promoting our messages:
-
Presentations (similar to today’s).
-
Articles for newsletters (to be sent to patient groups, PPI groups, cardio groups, etc.).
-
Information/articles/blogs suitable for websites used by patients/public/families/carers.
-
Facebook/Twitter posts/emails to groups followed by patients/public/families/carers.
-
TV/radio watched by patients/public/families/carers (talk shows, news shows, etc.).
-
Fun but informative activities/workshops at events aimed at patients, family, carers, support groups, PPI groups, the public and volunteers.
-
Newspaper articles targeted at public/patient audience.
-
Screen in waiting rooms (focused on raising awareness of A&D following a cardiac event and the need to speak to their GP).
-
Links from Google and other websites used by patients/public/families/carers to our website.
-
4. Do you have any suggestions to help us with this? For example: names of newsletters, newspapers, TV or radio programmes, websites, groups, or events that we could contact?
_______ _______ _______ ____________ __________ _________ ________ _______ _______ _________ _________ _______ _____ ____________ _________ ______ ________ ______ _______ ______ _______ _______ _______ ____ _______ _______ _______ ______ ______ ______ _______ _______ _______ _______ _______ _______ _______ __
List of abbreviations
- AMD
- adjusted mean difference
- BACPR
- British Association for Cardiovascular Prevention and Rehabilitation
- CAS
- cognitive attentional syndrome
- CAS-1R
- Cognitive Attentional Syndrome Scale-1 Revised
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CR
- cardiac rehabilitation
- CRN
- clinical research nurse
- CVD
- cardiovascular disease
- DCE
- discrete choice experiment
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HADS
- Hospital Anxiety and Depression Scale
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IES-R
- Impact of Events Scale Revised
- IQR
- interquartile range
- MCID
- minimally clinically important difference
- MCT
- metacognitive therapy
- MCQ-30
- Metacognitive Beliefs Questionnaire-30
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCI
- Reliable Change Index
- RCT
- randomised controlled trial
- SMD
- standardised mean difference
- T1
- time point 1
- T2
- time point 2
- TSC
- Trial Steering Committee
- VTC
- variation to contract
- WS
- work stream
- WTP
- willingness to pay
- WTPT
- willingness-to-pay threshold
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/TMJA2644).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
