Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0614-20009. The contractual start date was in March 2016. The final report began editorial review in September 2021 and was accepted for publication in June 2023. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the final manuscript document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Estcourt et al. This work was produced by Estcourt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Estcourt et al.
Synopsis
Structure of the Programme
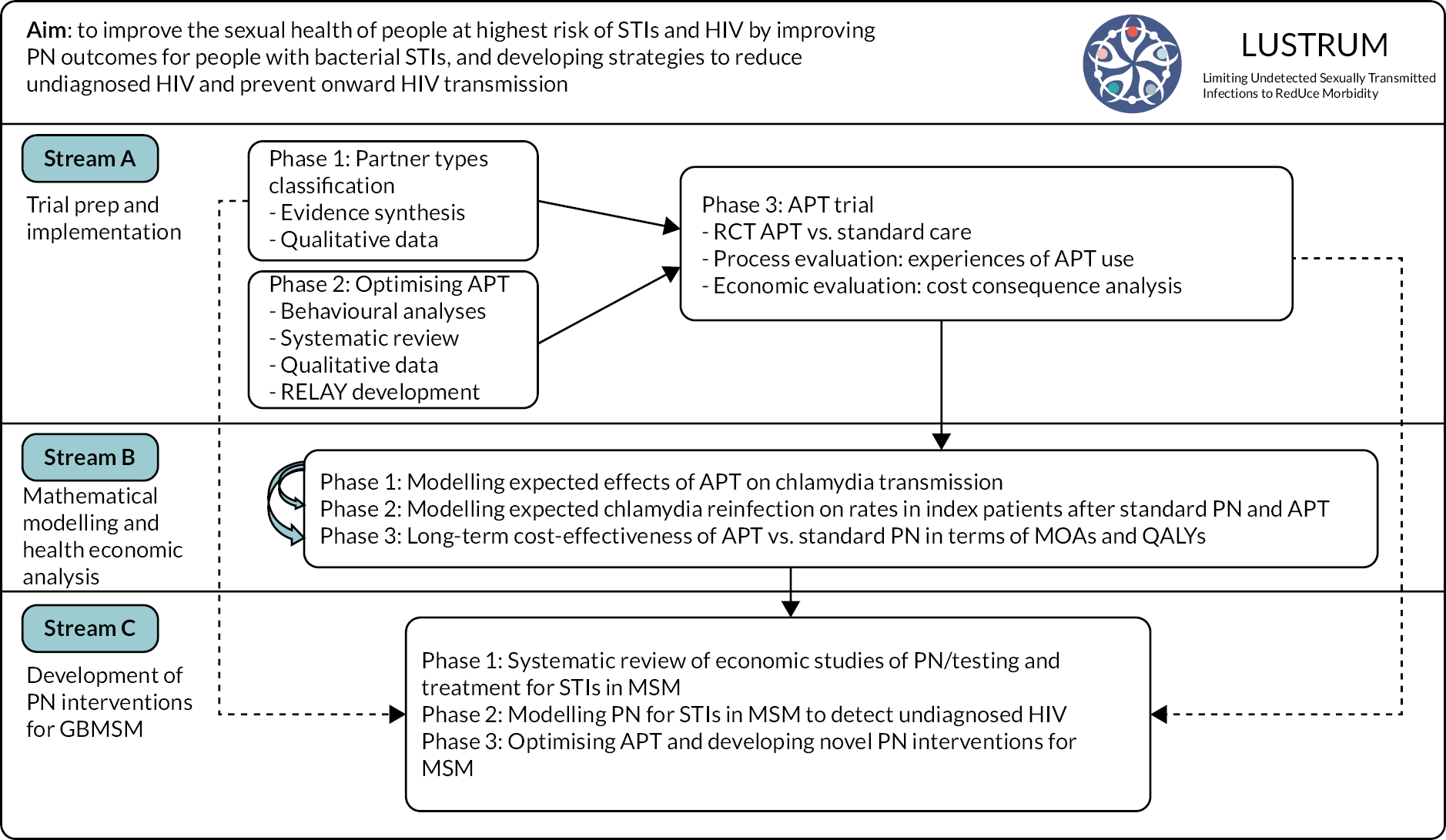
This Programme aims to improve the sexual health of people in Britain at highest risk of sexually transmitted infections (STIs) and human immunodeficiency virus (HIV) by improving partner notification (PN) outcomes for people with bacterial STIs through three inter-related research streams (see Research pathway diagram in Figure 1).
Research pathway diagram
FIGURE 1.
Research pathway diagram showing the inter-related workstreams within the LUSTRUM Research Programme. APT, accelerated partner therapy; GBMSM, gay and bisexual men who have sex with men; IP, index patient; MOAs, major outcomes averted; MSM, men who have sex with men; QALYs, quality-adjusted life-years; RCT, randomised controlled trial; RELAY, a clinical management and partner notification data collection system.
Changes to the Programme
To maximise utility and impact of the research, changes were made in areas as follows:
Firstly, we delayed the trial to avoid overlapping with another sexual health clinic-based National Institute for Health and Care Research (NIHR) funded trial. 1 The durations of intervention and control phases were extended by 2 months with a 2-week washout period instead of 1 month, to increase index patient (IP) recruitment. The target sample size was reduced from 5880 to 5440 to account for the inclusion of three additional clusters.
Secondly, the content of Stream C was updated to keep pace with scientific developments and in light of findings from the pre-trial phase (Stream A). Instead of a single online survey of one stakeholder group, we delivered a three-phased approach involving a wider spectrum of stakeholders. This included a literature review and stakeholder workshop, empirical qualitative work and intervention specification using programme theory. 2 We also switched to virtual rather than face-to-face focus groups with an additional group of men who have sex with men (MSM) instead of healthcare professionals (HCPs) due to COVID-19-related restrictions in April 2020. Although the online format worked well, it was more time-consuming and so we chose to focus on PN for bacterial STIs rather than including HIV. The issues for HIV PN are different and complex and therefore need separate discussion.
Finally, the Programme end date was extended by 4 months to mitigate the impact of COVID-19 on working patterns across the whole team.
This report is structured to reflect the way we conducted the Programme of research as shown in the Research pathway diagram, which differs slightly from some subheadings and numbering within the original bid document.
Stream A phase 1: qualitative studies – sex partner types and optimising accelerated partner therapy
Qualitative studies–developing a classification of sex partner types
Aim and objectives
To develop a classification of sex partner (SP) types for use in PN and other interventions to prevent STIs.
To conduct a synthesis of existing knowledge on partner types from published literature; establish contemporary evidence of public, patient and HCP staff beliefs about partner types; consult with multidisciplinary experts on the developing classification.
Methods for data collection and analysis
Firstly, we conducted an iterative synthesis of diverse sources of evidence to generate an initial comprehensive classification. Evidence sources included:
-
analysis of data from National Survey of Sexual Attitudes and Lifestyles (Natsal) 33
-
analysis of relationship types in 259 dating applications (apps)4
-
scoping review of social and health sciences literature on partner types (56 studies). 5
Secondly, we conducted qualitative interviews with public, patients and health professionals [57 patient and public participants (male n = 34; female n = 23)]. 6
Finally, we operationalised and sought external endorsement for the sex partnership classification through multidisciplinary clinical expert consultation at dedicated workshops facilitated by the British Association for Sexual Health and HIV (BASHH),7 later in the Programme, we piloted the revised classification in sexual health clinics during a randomised controlled trial (RCT). 8,9
Limitations
The diverse data types that were synthesised precluded a standardised approach to meta-analysis and relied on more qualitative approaches from meta-synthesis.
Key findings
We identified a spectrum of partner types and noted the malleability of partner types as relationships develop and across the life course.
We created a novel five-category classification, broadly predicated on duration of the relationship, likelihood of future sex and degree of emotional connection. 10 This was used successfully by sexual HCPs to record partner types within the RCT. 8
Stage 1: collating diverse evidence
We analysed data on partnership types from the Natsal-3 survey11 and used these findings within our review of published evidence (Evidence source 2).
Evidence source 1: We applied a systematic review approach to examine the top 500 downloaded dating apps in Britain to determine how they described and organised sexual relationships. After deduplication and full content screening, 259 apps were examined in detail. We extracted data on the architecture of dating apps, such as the options available to dating app users as they made connections with others. Most dating apps are designed for specific user populations, typically grouped according to sexual preferences, social commonalities or individual characteristics. We conducted a cluster analysis to explore if dating apps could be grouped along a range of shared denominators. Two clusters emerged – unfocused and ambiguous, and highly specific – catering to the different needs of possibly the same dating app users. We did not collect any information on dating app users, nor dating app designers. 4
Evidence source 2: Using a systematic search strategy, we examined 56 out of 15,592 papers which detailed types of sexual relationships and partnerships. Most studies were published within the 21st century in the USA and used a range of primarily quantitative designs. We extracted and detailed the partnership types described in each of the included papers. We found that studies tended to have a close focus on one kind of relationship/partner (e.g. ‘casual’) rather than explore a large range of diverse partnership types. Subsequently we synthesised the types of relationship/partner detailed within the literature by extracting and interpreting the commonalities and differences among reported partnership types. In this way, we were able to develop a spectrum of eight mutually exclusive categories of relationships. These categories were: (1) ‘married/committed’; (2) ‘main partner’/‘serious partner’/‘stable’ or ‘long term’; (3) ‘established’; (4) ‘girlfriend’/‘boyfriend’; (5) ‘dating’/‘going out’; (6) ‘friends with benefits’; (7) ‘**** buddies’ and ‘booty calls’; and finally (8) ‘super casual’, ‘hook-ups’, ‘meets and one-night stands’. 5
Evidence source 3: We conducted qualitative interviews with members of the public and sexual health clinic patients (male n = 34; female n = 23). After an initial thematic analysis, we subsequently explored our findings further in terms of resonance with key constructs from sexual script theory. 12 We showed how the ways people perceive and talk about their relationships relate to the changing and multilayered social organisation of their relationships. Our findings emphasise how recent, labile, sociocultural scenarios are shaping fluid and emerging interpersonal and intrapsychic sexual scripts leading to both new uncertainties and opportunities in the ways we relate to each other sexually. 6
Stage 2: integration and synthesis of diverse evidence
Using the constant comparative method and data visualisation, we synthesised these diverse findings to shape a pragmatic classification of partner types that could be useful within clinical practice and within the RCT itself. We identified five main partner types: ‘Established partner’, ‘New partner’, ‘Occasional partner’, ‘One-off partner’ and ‘Sex worker’. 10
Multidisciplinary clinical expert consultation to revise the classification – During a series of interactive workshops and meetings with diverse sexual health professionals and other experts, we iteratively tested and adapted the classification to ensure that it made sense, had face and ecological validity and could be used within clinical practice.
Piloting of the revised classification in sexual health clinics during a RCT of PN – We piloted the novel classification by implementing it into routine practice ahead of the trial and during the phase of stable data collection at the beginning of the RCT within a clinical management and PN data collection system (RELAY) (see Stream A phase 2: optimising accelerated partner therapy). Healthcare professionals were able to assign all SPs to a category without difficulty and no further changes were needed. 10
Interrelation with other parts of the Programme
The classification of partner types was used within the RCT to categorise SPs and thereby enabled determination of trial outcomes by partner type. The classification was also instrumental in shaping the intervention development focus of Stream C, in that it highlighted that APT was unlikely to be a useful approach with one-off partners and shifted our focus to alternative approaches.
Stream A phase 2: optimising accelerated partner therapy
Accelerated partner therapy barriers and facilitators and optimising accelerated partner therapy
Aim and objectives
To optimise APT and produce a manualised intervention, a complementary training package and a specific range of intervention resources.
To detail the behavioural system of APT; analyse videos of APT, conduct a synthesis of published evidence concerning the behavioural elements of APT and related interventions; establish in-depth behaviourally informed evidence concerning the barriers and facilitators to receiving/delivering APT including focused work with those likely to struggle with APT; specify the key components of APT and operationalise them within an APT manual, training package, a series of on-line videos (for staff and SPs using the self-sampling and treatment pack) and other intervention resources (optimised pack contents and laminated materials for HCPs and for IPs).
Methods for data collection and analysis
Insights from several contributing studies were combined to optimise APT. These included:
-
An analysis of the behavioural system of APT from videos of role play based on previous APT work. 13
-
A systematic review of PN intervention content (K = 14). 14
-
Qualitative interviews with public, patients and health professionals, including both heterosexuals and MSM with initial thematic analysis. 15
-
Application of the behaviour change wheel (BCW)16 and normalisation process theory (NPT)17 to specify the intervention in terms of its key components and associated mechanisms.
-
Further qualitative interviews and focus groups with people with mild to moderate learning disabilities addressed the intervention amongst those who may particularly struggle with APT. 18
-
Focus groups with diverse HCPs to explore the acceptability and pertinent barriers and facilitators to implementing APT.
-
We used the APEASE (acceptability, practicability, effectiveness, affordability, side-effects, equity) criteria19 to finalise the content of the intervention and operationalise it within the manual, the training packages, the videos and intervention materials.
-
Finally, we worked iteratively with the software development company (Epigenesys) to incorporate key stages of the APT intervention into RELAY (please see Stream A phase 3), which was used by HCPs during the RCT. 9
We adopted a user-centred approach with multiple pre-design and prototype testing phases to further develop the functionality and design of the bespoke web-based referral and data collection tool created and refined in previous studies. 20,21 RELAY supported the APT patient pathway, enabling rapid communication between the clinics and the research health advisers (RHAs) and pharmacy services.
Limitations
We relied only on qualitative data to optimise APT; participants for the interviews and focus groups chose to take part so may have different views to some end-users of APT.
Key findings
Building upon previous accelerated partner therapy work to detail the behavioural system of accelerated partner therapy through video analysis of accelerated partner therapy
We detailed the behavioural structure of APT across its key actors, the central behavioural domains addressed and the specific behaviours required for each of APTs key steps. 13,14
Systematic review of partner notification intervention content and behavioural steps
Fourteen studies met our inclusion criteria. Most focused on treating Chlamydia trachomatis through a series of sequential steps dependent on local context and policies resulting in relatively heterogeneous intervention steps although with considerable overlap. Analysis of intervention content showed commonly reported behaviour change techniques (BCTs) were ‘adding objects to the environment’, ‘credible source’ and ‘instruction on how to perform a behaviour’. Systematic review registration number: CRD42016051178. 14
Qualitative interviews with public, patients and health professionals to understand barriers and facilitators to delivery and uptake
There were diverse and varied barriers and facilitators for each step of APT and for each actor (e.g., HCP vs. IP or SP) and their distinct behavioural domains. Facilitators outnumbered reported barriers.
For IPs and SPs, many of the barriers related to psychosocial consequences of engaging in APT, confidence and skills needed to deliver and use the STI and HIV self-sampling pack and a lack of understanding of sexual health, and PN.
From the HCP perspectives, perceived barriers related to their own levels of knowledge and perceived competence in telephone consultations, perceived skills and beliefs (e.g. the safety of remote prescribing), and also the contexts in which they worked [e.g. need for dedicated space and time within busy sexual health services (SHSs)].
Optimising the intervention
To address the identified barriers, we systematically developed a comprehensive package of optimal intervention components. These components were all specified in terms of the causal mechanisms they moderated and the BCTs and intervention functions they employed. Analysis using the BCW showed that to support HCPs in delivering APT a combination of education, training and environmental restructuring would be particularly important.
Equally, for both IPs and SPs, enablement, education, persuasion and modelling were perceived to be particularly important. These key intervention functions were also detailed in terms of the BCTs they employed. Following the use of the APEASE criteria19 with expert team members, the intervention was specified and operationalised in the form of a manual for trial sites, training materials for face-to-face and on-line use, on-line videos for staff, IPs and SPs, and additional intervention materials such as laminated sheets to support both the HCP and the IP within APT. 13
Qualitative studies with people with mild to moderate learning disabilities
All participants found at least one element of the self-sampling pack challenging or impossible to use but welcomed the opportunity to undertake sexual health screening without attending a clinic. Reported barriers to correct use of the pack included perceived overly complex STI/blood-borne virus (BBV) information and instructions, feeling overwhelmed and the manual dexterity required for blood sampling. Many female participants struggled interpreting anatomical diagrams depicting vulvo-vaginal swabbing. Facilitators included pre-existing STI/BBV knowledge, familiarity with self-management, good social support and knowing that the service afforded privacy. 18
RELAY
RELAY functioned well as a web-based clinical management and PN data collection tool to support the APT patient pathway, and enabled rapid communication between the clinics, the supporting RHAs, and testing laboratory (for the IP repeat testing in the RCT). We are working with several trial clinics to explore options for retaining RELAY as a long-term clinic-wide PN system for all STIs.
Stable data collection and piloting of definitions
We successfully introduced RELAY into each clinical service for 2–4 weeks before the start of the trial for sexual HCPs to record PN consultations and outcomes of existing methods of PN and clinical data on SPs using the new SP classification. 10 Minor amendments were made to the webtool following user feedback.
Interrelation with other parts of the Programme
The optimised version of APT is central to the RCT. It is also important for understanding the specific focus of Stream Cs intervention development which focused on PN with ‘one-off partners’ as the work reported here showed viability of APT with ‘Established partner’, ‘New partner’, ‘Occasional partner’ amongst MSM.
Stream A phase 3: trial delivery, analyses and interpretation
Background
APT is a PN method whereby during the IP’s clinic attendance, HCPs assess SPs by telephone consultation, before sending out or giving the IP antibiotics and STI and HIV self-sampling kits to deliver to their SP(s). APT has shown promise in pilot trials. 20,21
Aim and objectives
Aim: to determine the effectiveness of APT in improving outcomes of PN for genital chlamydia in heterosexuals in a cluster crossover RCT.
Specific objectives are to determine:
-
The effect of APT on the proportion of IPs who test positive for chlamydia 12–16 weeks after the PN consultation (the gold standard outcome in PN trials).
-
The effect of APT on the proportion of SPs treated.
-
The effects of APT according to SP type.
-
The effect of APT on the proportion of SPs notified.
-
Whether APT is associated with faster treatment than standard PN.
Methods for data collection and analysis
Trial design: a cluster crossover RCT of APT offered as an additional PN method compared with standard PN alone. 9 The APT intervention was offered at the level of the sexual health clinic, with randomisation of each clinic to either intervention or control arm in the first phase of the trial.
The trial was accompanied by an economic evaluation, transmission dynamic modelling and a qualitative process evaluation.
Clusters were 17 sexual health clinics (publicly funded) in areas of Britain with contrasting patient demographics.
Participants were heterosexual women and men, aged ≥ 16 years with a positive test for C. trachomatis and/or clinical diagnosis of pelvic inflammatory disease (PID) or cervicitis (women) or non-gonococcal urethritis (NGU) or epididymo-orchitis (men) and reporting at least one contactable sexual partner in the past 6 months.
Recruitment: during the initial PN consultation with the IP, the HCP assessed eligibility for the study. As this was a low-risk health intervention, consent was provided at service level.
Intervention: APT offered as an additional PN method compared with standard PN alone (see the APT overview film22). The APT intervention was offered at the level of the sexual health clinic, with randomisation of each clinic to either intervention or control arm in the first phase of the trial (random permutation). Figure 2 shows an overview of the APT process.
FIGURE 2.
Diagram showing the steps of the APT process.
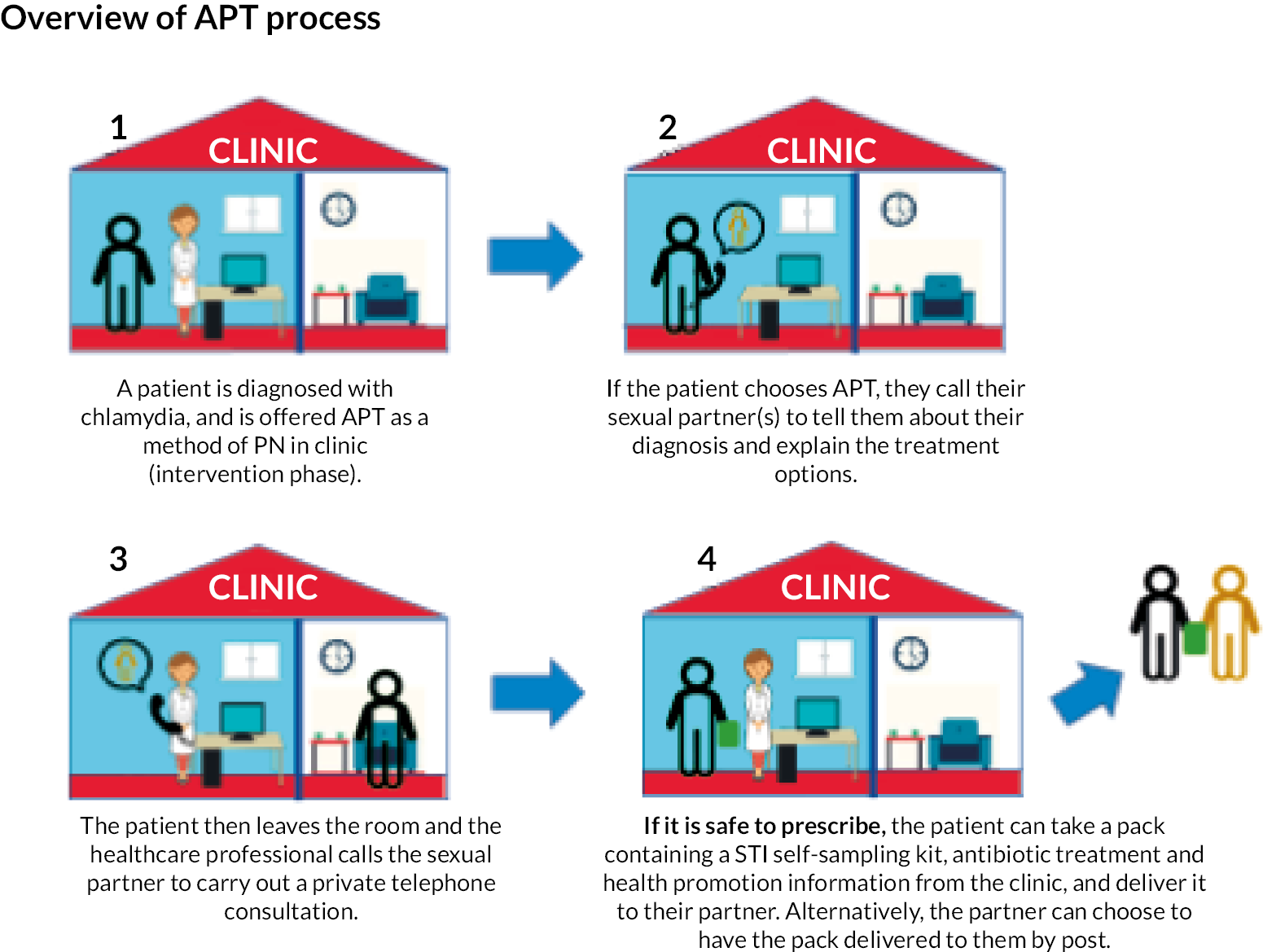
Control: standard PN, which was enhanced patient referral in which HCPs asked the IP to inform their SP(s) of the need for testing and treatment, supplemented by written or website information.
Trial periods: there was a 4-month run-in period (July–October 2018), consisting of rolling clinic set-up including training for HCPs and a period of at least 2 weeks of baseline data collection when HCPs used RELAY to record standard PN data. Then nine clinics entered intervention phase while eight entered control phase, according to the randomisation schedule. At the end of the first 6-month trial phase (November 2018 to April 2019), there was a 2-week washout period where clinics did not offer APT to patients and followed their standard PN procedures. Then clinics crossed over to the opposite arm (intervention or control) for phase two (for 6 months, May–November 2019). The total duration of the trial was 19 months, allowing for a 3-month follow-up period to enable outcome data collection to be completed for all patients in the second trial phase. Clinics which did not start phase one of the trial in November 2018 completed recruitment in November 2019 and trial phases were condensed.
The primary outcome was the proportion of IPs testing positive for C. trachomatis 12–24 weeks after the PN consultation. Secondary outcomes included the proportion of SPs treated; the proportion of SPs notified; cost effectiveness; model-predicted chlamydia prevalence; SP and HCP experiences of APT.
The primary outcome analysis was by intention to treat, fitting random-effects logistic regression models that account for clustering of IPs within clinics and trial periods. The statistician carried out analysis of the primary outcome blinded to allocation.
Trial registration: This trial is registered as ISRCTN 15996256.
Development of APT web tool: we adopted a user-centred approach to further develop the functionality and design of RELAY created and refined in previous studies. 20,21 Clinic HCPs entered clinical data and any data collected for research purposes directly onto RELAY during the PN consultation. RELAY supported the APT patient pathway, enabling rapid communication between the clinics and the supporting RHAs. A new function enabled a clinical summary to be downloaded and incorporated into the patients’ clinic electronic patient records.
We undertook an extensive pre-design (product testing) phase and rounds of iterative development with health professionals and sexual health clinic health advisers to ensure that the tool met their needs, current activity, and work habits.
We also ‘stress tested’ RELAY and performed a dummy data retrieval exercise, in which an independent company comprehensively tested the system for errors and its ability to extract all relevant data variables needed for the statistical analysis plan for the trial.
RELAY had been developed to exceed all contemporary NHS data storage and transfer standards and NHS information governance compliance with strict adherence to the Caldicott principles of confidentiality, as outlined in the Caldicott report 1997. 23 However, the process for research and development (R&D) and Information Governance approval in several of our trial sites was extremely arduous, created substantial delays in some cases and almost prevented participation in one site.
Ethical approval and the General Data Protection Regulation paradox: Ethical approval for the trial was provided by London – Chelsea Research Ethics Committee (18/LO/0773) and approved us to seek consent for trial participation from lead clinicians at participating clinics (service-level) rather than seeking individual informed consent from IPs other than for the process evaluation studies. Following Weijer et al. ,24 we believed that APT is a complex, ‘low-risk’ healthcare delivery intervention. APT is offered in addition to standard PN and operationalised as a supplement to usual care; thus, IPs have the choice of taking up APT or not. It is widely accepted that individual consent may not be essential in such trials,25 in which the situation is analogous to the introduction of changed processes in routine services. 26 This was important for the RCT as individual-level consent is thought to have contributed to low recruitment numbers in a previous study of APT. 21
However, the newly introduced General Data Protection Regulations (GDPR) required that individuals have the option to choose whether their data may be used for research. This presents a challenge when consent has been given by the clinical service and not by individual service users. We developed a pragmatic opt-out solution to this consent paradox. 27 Our approach supported the individual’s right to withhold their data from trial analysis while routinely offering the same care to all patients.
Selection of clinics: we selected 17 NHS (publicly funded, free to access) specialist sexual health clinics (clusters) across England and Scotland from those expressing interest. Selection was based on numbers of reported chlamydia diagnoses data in the Public Health England Genitourinary Medicine Clinical Activity Dataset for STI surveillance28 (England) and geographical diversity (Scotland) to create three strata: London, non-London metropolitan ‘cities’ and non-London urban ‘towns’. A full list of study sites is included in the published study protocol. 9
Training of clinic staff and site set-up and support: multidisciplinary members of the trial team (researchers, research heath advisers and clinicians) made a study initiation visit to each clinic prior to randomisation to train staff in intervention delivery, the use of RELAY and the novel classification of SP types needed for data collection. The training consisted of (1) a whole clinic presentation explaining an overview of the trial, (2) small group interactive APT training using observed and participatory role plays and use of the intervention manual, (3) training and quizzes on use of the new partner type classification and (4) one-to-one or small group training in use of RELAY.
In addition, we created a series of training videos which enabled those who were unable to attend, new staff who joined during the study period, staff from clinics which entered intervention phase second to learn/refresh their skills and knowledge. We have subsequently made all resources available on our website and YouTube channel.
The trial team liaised with and supported clinics extensively. Typically, this included a weekly telephone call with each site lead, refresher training either on site or remotely when clinics switched from control phase to intervention phase and ad hoc as requested by the clinics or if recruitment appeared to slow.
Stable data collection: we introduced RELAY into each clinic 1 month before the start of the trial. Clinic health advisers used RELAY as their routine method of recording PN consultations and outcomes of existing methods of PN and clinical data on SPs using the new SP classification.
Limitations
Overall enrolment and follow-up were lower than expected20,21 and statistical power was lower than assumed. APT uptake itself was not a part of the power calculations, but we expected more IPs to choose it. We were unable to determine whether APT was associated with faster treatment because only small number of IPs knew when, as opposed to whether, their SPs had been treated.
The pragmatic trial design was intended to ensure that the effectiveness of APT would be evaluated under real-life clinical conditions. However, trial procedures meant that APT required additional data collection regardless of whether the patient accepted the APT intervention. This, together with wider operational factors (see Stream A phase 3: process evaluation), meant that it was seldom offered routinely. 29
Key findings
Figure 3 provides an overview of the trial.
FIGURE 3.
Flow diagram of enrolment by clinic randomisation status and period. a, Administrative service data on all chlamydia diagnoses within trial period in non-MSM patients aged ≥ 16 years not attending as PN contact; b, All potentially eligible SPs treated prior to clinic consultation of IP.
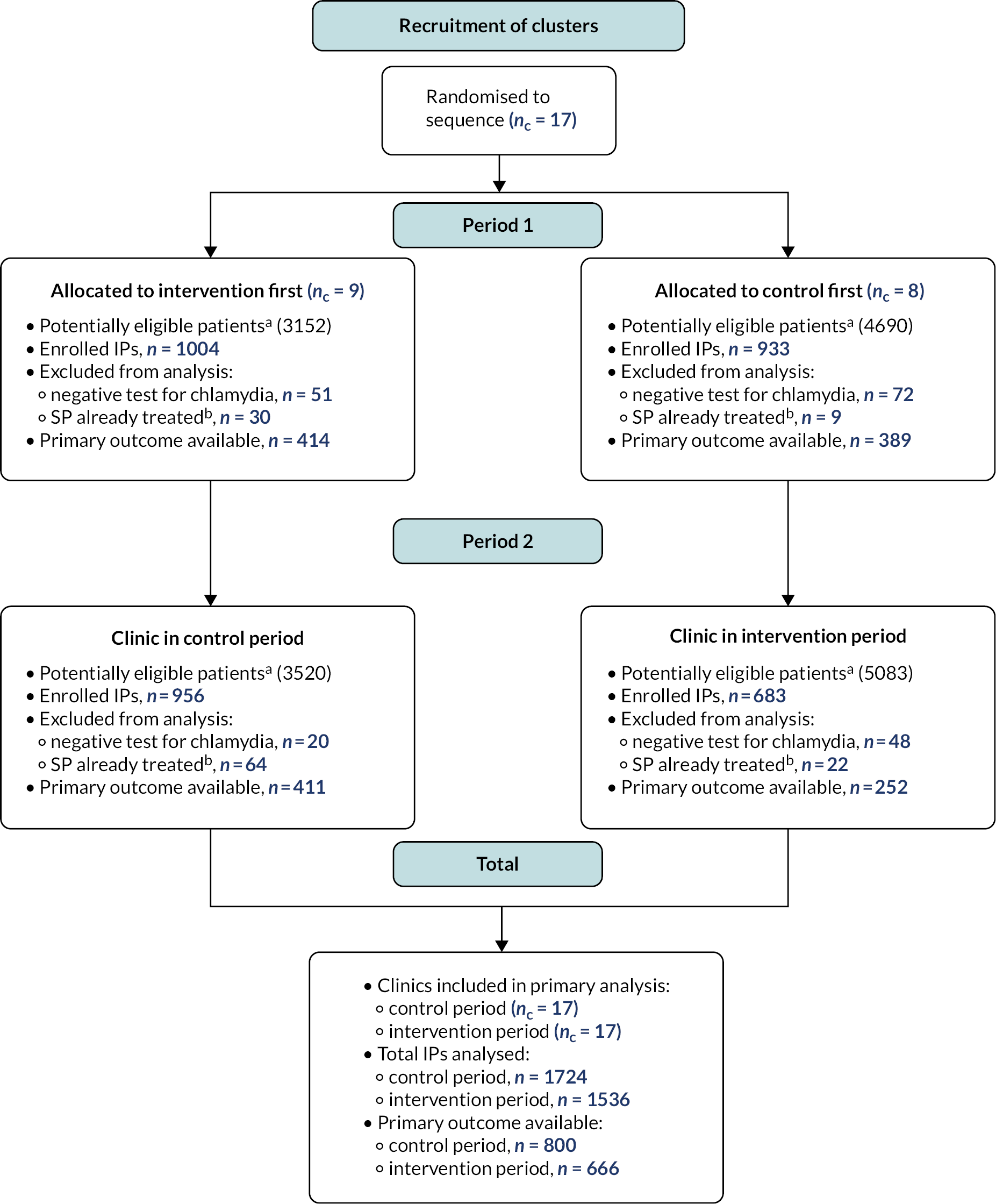
All clinics completed both periods. One thousand five hundred and thirty-six and 1724 IPs provided data in intervention and control phases. In intervention and control phases, 666 (43.4%) and 800 (46.4%) IPs were tested for C. trachomatis; 31 (4.7%) and 53 (6.6%) were positive, adjusted odds ratio (aOR) 0.66 [95% confidence interval (CI) 0.41 to 1.04; p = 0.07]. In total, 4807 SPs were reported, of whom 1636 (34.0%) were committed/established partners. Characteristics of index cases and partners were balanced. Overall, 293/1536 (19.1%) of IPs chose APT for a total of 305 partners, of whom 248 accepted. The proportion of IPs with one or more SPs notified was 1123/1150 (97.7%) in the intervention phase and 1185/1218 (97.3%) in the control phase (aOR 1.18, 95% CI 0.70 to 2.00; p = 0.54), while the proportion of all partners notified was 95% in both phases (aOR 0.80, 95% CI 0.49 to 1.29; p = 0.35). The proportion with ≥ 1 SP treated was 775/881 (88.0%) in intervention and 760/898 (84.6%) in the control phase, aOR 1.27 (95% CI 0.96 to 1.68; p = 0.10).
One thousand five hundred and thirty-six IPs with 2218 partners were enrolled in APT intervention phases, but APT could not be offered by the clinic in 81/2218 of these. The IP selected APT for 305/2137 (14.3%) partners when available. Of these, 166/305 (54.4%) were committed/established, 85/305 (27.9%) were new, 45/305 (14.8%) were occasional and 9/305 (3.0%) were one-off partners. Common index reasons for declining APT included: preference for face-to-face conversation 400/1832 (21.8%), partner already in clinic 388/1832 (21.2%), unwilling to engage with partner 206/1832 (11.2%), preferring partner to attend clinic 202/1832 (11.0%), partner overseas 150/1832 (8.2%). Of 241 partners sent APT packs, 120/241 (49.8%) returned chlamydia and gonorrhoea testing samples, of which 78/119 (65.5%) were positive for chlamydia (no result obtained for one returned sample), but only 60/241 (24.9%) HIV and syphilis samples (all negative). Of 106 IPs offered APT, which was accepted ≥ 1 partners, and tested for chlamydia at 12–24 weeks, only 2 (1.9%) were positive. This contrasts with 6.6% (53/800) in the control arm and 5.2% (29/560) in IPs not selecting APT or whose partners refused. There were seven adverse events reported (see Report Supplementary Material 1), all deemed to be of low severity and managed through discussion with the Trial Steering Group and Trial Management Group.
Tables 1–8 show the trial data and outcomes.
| Variable | Control period, n (%) or median (IQR) | Intervention period, n (%) or median (IQR) | |
|---|---|---|---|
| Number of IPs | 1724 | 1536 | |
| Sociodemographic factors | |||
| Age | Years [median (IQR, range)] | 24 (21–28, 17–62) | 24 (21–28, 16–72) |
| IP sex at birtha | Male | 547 (32) | 522 (34) |
| Female | 1177 (68) | 1014 (66) | |
| Other | 0 (0) | 0 (0) | |
| Enrolment of IP based on | Diagnosed chlamydia | 1678 (97) | 1506 (98) |
| PID | 7 (0.4) | 1 (0.06) | |
| Cervicitis | 0 (0) | 0 (0) | |
| NGU | 37 (2.1) | 27 (1.7) | |
| Epididymo-orchitis | 2 (0.12) | 2 (0.13) | |
| Ethnicity | White British or Irish | 829 (48) | 707 (46) |
| White other | 199 (12) | 181 (12) | |
| Black/Black British | 368 (21) | 377 (25) | |
| Asian/British Asian | 100 (6) | 92 (6) | |
| Mixed ethnicity | 193 (11) | 134 (9) | |
| Other ethnicity | 35 (2) | 45 (3) | |
| SPs per IP | |||
| SPs last 12 months | Count [median (IQR, range)] | 2 (1–3, 1–100) | 2 (1–4, 1–60) |
| New SPs last 12 months | Count [median (IQR, range)] | 2 (1–3, 0–99) | 1 (1–3, 0–50) |
| SPs in 1/3/6-month look-backb | Count [median (IQR, range)] | 2 (1–2, 1–25) | 1 (1–2, 1–39) |
| SPs included in analysis | Count [median (IQR, range)] | 1 (1–2, 1–20) | 1 (1–2, 1–10) |
| Variable | Control period, n (%) | Intervention period, n (%) | |
|---|---|---|---|
| Total number of SPs | 2589 | 2218 | |
| Sociodemographic factors | |||
| Gender identitya | Male | 1699 (66) | 1419 (64) |
| Female | 890 (34) | 799 (36) | |
| Partner typeb | Committed/established | 880 (34) | 756 (34) |
| New relationship | 342 (13) | 343 (15) | |
| Occasional partner | 687 (27) | 610 (28) | |
| One-off partner | 680 (26) | 509 (23) | |
| Condom use with this partner | Always | 293 (11) | 202 (9) |
| Sometimes | 870 (34) | 800 (36) | |
| Never | 1426 (55) | 1216 (55) | |
| Likelihood of future sex with this partner | No | 1066 (41) | 844 (38) |
| Not sure | 614 (24) | 458 (21) | |
| Yes | 909 (35) | 916 (42) | |
| Control period | Intervention period | |||
|---|---|---|---|---|
| Outcome measures | n (%)a | n (%)a | OR (95% CI); p-value | aOR (95% CI); p-value |
| Number of IPs | 1724 | 1536 | ||
| Primary outcome | ||||
| IP chlamydia test 12–24 weeks (observed data) | ||||
| Positive | 53 (6.6) | 31 (4.7) | 0.67 (0.42 to 1.06); 0.08 | 0.66 (0.41 to 1.04); 0.07 |
| Negative | 747 (93.4) | 635 (95.3) | – | – |
| No testb | 924 | 870 | Excludedb | Excludedb |
| IP chlamydia test 12–24 weeks (MAR MI) | ||||
| Positive | 116 (6.7) | 73 (4.8) | 0.67 (0.40 to 1.14); 0.14 | 0.67 (0.39 to 1.14); 0.14 |
| Negative | 1608 (93.3) | 1463 (95.2) | – | – |
| IP chlamydia test 12–24 weeks [MNAR MI: δ = loge(0.5)] | ||||
| Positive | 154 (8.9) | 86 (5.6) | 0.58 (0.36 to 0.92); 0.02 | 0.58 (0.36 to 0.92); 0.02 |
| Negative | 1570 (91.1) | 1450 (94.4) | – | – |
| IP chlamydia test 12–24 weeks [MNAR MI: δ = loge(2)] | ||||
| Positive | 98 (5.7) | 55 (3.6) | 0.57 (0.38 to 0.88); 0.01 | 0.57 (0.37 to 0.88); 0.01 |
| Negative | 1626 (94.3) | 1481 (96.4) | – | – |
| Secondary outcome | ||||
| ≥ 1 SP treated for chlamydia (observed data) | ||||
| Yesc | 760 (84.6) | 775 (88.0) | 1.25 (0.94 to 1.64); 0.12 | 1.27 (0.96 to 1.68); 0.10 |
| Nod | 138 (15.4) | 106 (12.0) | – | – |
| Not knownb | 826 | 655 | Excludedb | Excludedb |
| ≥ 1 SP treated for chlamydia (MAR MI) | ||||
| Yes | 1452 (84.2) | 1344 (87.5) | 1.29 (0.94 to 1.77); 0.12 | 1.30 (0.94 to 1.81); 0.12 |
| Noe | 272 (15.8) | 192 (12.5) | – | – |
| ≥ 1 SP notified (observed data) | ||||
| Yesc | 1185 (97.3) | 1123 (97.7) | 1.17 (0.69 to 1.97); 0.56 | 1.18 (0.70 to 2.00); 0.54 |
| Nod | 33 (2.7) | 27 (2.3) | – | – |
| Not knownb | 506 | 386 | Excludedb | Excludedb |
| Control period | Intervention period | |||
|---|---|---|---|---|
| Outcome measures | n (%) | n (%) | OR (95% CI); p-value | aOR (95% CI); p-value |
| Total number of SPs | 2589 | 2218 | ||
| Treated at 2 weeks (observed data) | ||||
| Yesa | 859 (79.6) | 842 (83.6) | 1.31 (0.94 to 1.83); 0.11 | 1.25 (0.88 to 1.77); 0.20 |
| No | 220 (20.4) | 165 (16.4) | – | – |
| Not known by IPb | 699 | 538 | Excludedb | Excludedb |
| Follow-up not recordedb | 811 | 673 | Excludedb | Excludedb |
| Known to be treated at 2 weeks | ||||
| Yesa | 859 (33.2) | 842 (38.0) | 1.50 (1.08 to 2.10); 0.01 | 1.27 (0.99 to 1.65); 0.06 |
| No | 1730 (66.8) | 1376 (62.0) | – | – |
| Notified at 2 weeks (observed data) | ||||
| Yes | 1700 (95.0) | 1514 (95.0) | 0.93 (0.58-1.47); 0.75 | 0.80 (0.49-1.29); 0.35 |
| No | 89 (5.0) | 79 (5.0) | – | – |
| Follow-up not recordedb | 800 | 625 | Excludedb | Excludedb |
| With stratification by relationship type | ||||
| Treated at 2 weeks (observed data) | ||||
| Yesa: committed establishedc | 400/478 (83.7) (n = 880) | 400/447 (89.5) (n = 756) | 1.74 (1.04 to 2.91); 0.04 | 1.65 (0.96 to 2.82); 0.07 |
| Yesa: new relationshipc | 151/176 (85.8) (n = 342) | 182/200 (91.0) (n = 343) | 1.83 (0.79 to 4.24); 0.16 | 1.72 (0.72 to 4.14); 0.22 |
| Yesa: occasional partnerc | 175/232 (75.4) (n = 687) | 162/207 (78.3) (n = 610) | 1.19 (0.62 to 2.28); 0.59 | 1.16 (0.59 to 2.29); 0.66 |
| Yesa: one-off partnerc | 133/193 (68.9) (n = 680) | 98/153 (64.1) (n = 509) | 0.64 (0.32 to 1.27); 0.20 | 0.65 (0.32 to 1.32); 0.23 |
| n/N (%) | ||
|---|---|---|
| Per IP summary | ||
| APT uptake | Total IP s in intervention period | 1536 |
| APT not selected for any partner | 1243 (80.9) | |
| APT selected by IP for ≥ 1 partner | 293 (19.1) | |
| APT accepted by ≥ 1 partner | 244 (15.9) | |
| Per SP summary | ||
| APT pathway | Total SPs in intervention period | 2218 |
| APT not offered by clinic | 81/2218 (3.7) | |
| Staffing limitations | 68/81 (84.0) | |
| Drug supply issues | 13/81 (16.0) | |
| APT not selected by IP | 1832/2137 (85.7) | |
| IP prefers to have the conversation with the partner face-to-face | 400/1832 (21.8) | |
| Partner is in clinic to be treateda | 388/1832 (21.2) | |
| IP doesn’t want to talk or see partner | 206/1832 (11.2) | |
| IP prefers the partner to visit the clinic | 202/1832 (11.0) | |
| Partner is overseas | 150/1832 (8.2) | |
| IP doesn’t have partner’s phone number | 59/1832 (3.2) | |
| IP is worried about partner’s reaction | 57/1832 (3.1) | |
| IP does not understand how APT works | 1/1832 (0.1) | |
| Other/missing | 369/1832 (20.1) | |
| APT selected by IP | 305/2137 (14.3) | |
| No answer to phone call | 49/305 (16.1) | |
| SP declined APT | 8/305 (2.6) | |
| APT accepted | 248/305 (81.3) | |
| APT not clinically appropriate | 7/248 (2.8) | |
| Receipt of APT pack | ||
| Not known | 36/241 (14.9) | |
| Confirmedb | 205/241 (85.1) | |
| STI and HIV testing | ||
| Chlamydia | Test returnedc | 120/241 (49.8) |
| Positive | 78/120 (65.0) | |
| No result obtained | 1/120 (0.8) | |
| Gonorrhoea | Test returnedc | 120/241 (49.8) |
| Positive | 1/120 (0.8) | |
| No result obtained | 1/120 (0.8) | |
| Syphilis | Test returnedc | 60/241 (24.9) |
| Positive | 0/60 (0) | |
| No result obtained | 0/60 (0) | |
| HIV | Test returnedc | 60/241 (24.9) |
| Positive | 0/60 (0) | |
| No result obtained | 0/60 (0) | |
| Control period | Intervention period | |||
|---|---|---|---|---|
| Outcome measures | n (%) | n (%) | OR (95% CI); p-value | aOR (95% CI); p-value |
| Number of IPs | 828 | 586 | ||
| Primary outcome | ||||
| IP chlamydia test 12–24 weeks (observed data) | ||||
| Positive | 30 (7.8) | 12 (5.0) | 0.59 (0.29 to 1.18); 0.12 | 0.56 (0.28 to 1.13); 0.09 |
| Negative | 357 (92.2) | 229 (95.0) | – | – |
| Not knowna | 441 | 345 | Excludeda | Excludeda |
| Secondary outcome | ||||
| ≥ 1 SP treated for chlamydia (observed data) | ||||
| Yesb | 342 (83.8) | 318 (90.1) | 1.72 (1.09 to 2.73); 0.02 | 1.72 (1.08 to 2.72); 0.02 |
| Noc | 66 (16.2) | 35 (9.9) | – | – |
| Not knowna | 420 | 233 | Excludeda | Excludeda |
| C. trachomatis test result at 12–24 weeks, n (%) | ||||
|---|---|---|---|---|
| Variable | Negative result | Positive result | Missing | |
| Sociodemographic factors of IP | ||||
| Ethnicity | White British or Irish | 655 (42.6) | 34 (2.2) | 847 (55.1) |
| White other | 173 (45.5) | 8 (2.1) | 199 (52.4) | |
| Black/Black British | 313 (42.0) | 25 (3.4) | 407 (54.6) | |
| Asian/British Asian | 69 (35.9) | 6 (3.1) | 117 (60.9) | |
| Mixed ethnicity | 134 (41.0) | 9 (2.8) | 184 (56.3) | |
| Other ethnicity | 38 (47.5) | 2 (2.5) | 40 (50.0) | |
| Age (years) | 16–20 | 259 (39.8) | 23 (3.5) | 369 (56.7) |
| 21–24 | 475 (42.6) | 27 (2.4) | 613 (55.0) | |
| 25–29 | 351 (42.3) | 19 (2.3) | 460 (55.4) | |
| 30+ | 297 (44.7) | 15 (2.3) | 352 (53.0) | |
| APT selected for one or more SP, n (%) | |||
|---|---|---|---|
| Variable | No | Yes | |
| Sociodemographic factors of IP | |||
| Ethnicity | White British or Irish | 543 (76.8) | 164 (23.2) |
| White other | 155 (85.6) | 26 (14.4) | |
| Black/Black British | 321 (85.2) | 56 (14.9) | |
| Asian/British Asian | 81 (88.0) | 11 (12.0) | |
| Mixed ethnicity | 105 (78.4) | 29 (21.6) | |
| Other ethnicity | 38 (84.4) | 7 (15.6) | |
| Age (years) | 16–20 | 248 (78.2) | 69 (21.8) |
| 21–24 | 422 (80.7) | 101 (19.3) | |
| 25–29 | 315 (84.5) | 58 (15.6) | |
| 30+ | 258 (79.9) | 65 (20.1) | |
Conclusions
APT is a safe, feasible and effective way of clinically managing SPs of people with chlamydia as part of a menu of contact tracing and management options. While APT uptake was low among patients assessed for eligibility, it was associated with a small reduction in chlamydia positivity in IPs at 4 months and a higher number of partners treated. In almost all instances where APT was accepted, this was for established/committed relationships, while one-off partnerships made up only 1 in 30 APT decisions, although these amounted to 1 in 5 partnerships in the intervention period.
More work is needed to increase engagement of the SPs with self-sampling for STIs including syphilis and HIV so that opportunities for screening are not lost. The trial was not powered to evaluate differences in APT uptake according to ethnicity or age, but data from the trial indicate that this may be lower in ethnic minority groups.
Accelerated partner therapy processes could be adapted for use in other groups such as MSM, trans and transgender people, but they are only feasible for infections routinely treated by oral medication. The first-line therapies for both gonorrhoea and syphilis are currently parenteral in many countries. 30 More broadly, we need to consider the partners who will not be reached by APT (one-off partners with whom future sex is not anticipated). Although not a risk to the IP, they are likely to make an important contribution to community transmission. New interventions are needed to directly target this group.
Interrelation with other parts of the Programme
Earlier intervention optimisation and associated studies provided the trial with a new classification of SP types used in collection of outcome data, an optimised APT intervention within a fully manualised intervention and HCP training package, RELAY, a bespoke data collection and clinical management webtool, to manage IPs and SPs during the trial. Trial data informed the health economics evaluation and the mathematical model (Stream B). New knowledge gained from the prospective logic model and its retrospective application to assist interpretation of trial and Programme findings caused us to move away from basing our novel PN intervention for MSM with one-off sexual partners on APT. The finding that APT appealed to people in relationships with a greater degree of emotional connection but much less so within one-off or short duration partnerships paved the way for a different approach in Stream C.
Stream A phase 3: process evaluation
Aim and objectives
To conduct a qualitative process evaluation to understand IPs’, SPs’ and HCPs’ experiences of APT, the trial and its key contexts.
Specific objectives were:
-
to use programme theory to detail assumptions and expectations about how APT would work within SHSs and the wider context before data collection and analysis
-
to use qualitative analyses from multiple stakeholders to explore the relative role of the context, issues of intervention fidelity and the actual contribution of varied putative intervention mechanisms in shaping intervention outcomes, both intended and unintended.
Methods for data collection and analysis
Data collection
Qualitative data: collected through six focus groups and individual interviews (n = 10) with purposively sampled HCPs (n = 34 from 14 sites), IPs (n = 15) and SPs who received APT (n = 17). 29
Data analysis
Qualitative process evaluation study: we developed initial programme theory iteratively combining results of the pre-trial studies of video analysis of APT and the systematic review of PN interventions with input from the wider interdisciplinary trial team. We created a narrative account and visualised it within logic models. We conducted subsequent analyses as data became available and these were independent of trial results.
We undertook deductive thematic analysis15 to focus on the key elements of programme theory. These primary thematic structures addressed questions of context, context dependency in relation to local implementation, fidelity and adaptions, experiences and perceptions of the relative functioning of putative intervention mechanisms as well as perceived outcomes (both anticipated and unanticipated). Within these primary thematic structures, more inductive themes were identified driven by the data. PF and FM conducted all analyses and audited each other’s work, discussed the findings with the wider team and came to agreement about the final coding. Finally, we used these analyses to illustrate the dynamic functioning of the programme theory using colour-coded visualisations within the logic model.
Limitations
Limitations relate to the inherent biases within the sampling (e.g. self-selected). Furthermore, if we had been able to collect data within both trial arms (rather than via single retrospective recall), it may have been possible to delineate trial burden from intervention more clearly.
Key findings
We developed initial programme theory and an accompanying logic model to describe how APT was imagined to work and we detailed various intervention mechanisms using behavioural and implementation science. 29 Preliminary work showed that APT was anticipated to primarily change key interactions and SHS organisation to accommodate accelerated and safe remote care to lead to reductions in IP reinfection. We theorised these mechanisms at various levels, drawing on behaviour change perspectives, implementation science and systems perspectives.
Subsequently, using the deductive thematic analysis, we used programme theory to create an evidence-based, theoretically informed overview of how APT worked dynamically within the context of the trial and within British SHSs. This is visualised in the colour-coded overview logic model (see Figure 4). We found APT training and resources (especially RELAY) transformed key interactions as anticipated. Overall intervention fidelity was good, and APT was well-liked by those who delivered and received it. Equally putative intervention mechanisms worked mostly as expected, although those concerned with local implementation sometimes worked counter to expectations because of contextual interdependencies. The trial struggled to be implemented at scale across all sites. Considerable external pressures drove all services to constantly adapt to achieve efficiencies. In some services, APT was perceived as time-consuming and without palpable impact. This seemed to be because APT did not visibly reduce patients ‘in the waiting room’. As such, the ‘invisibility’ of the effectiveness of APT curtailed the establishment of positive feedback loops driving normalisation within services.
FIGURE 4.
An overview of findings from the qualitative process evaluation shown through a colour-coded logic model of APT.
Text in light blue shows where our initial programme theory was supported through process evaluation data. Text in dark blue shows mixed findings in relation to supporting our initial programme theory. Text in red shows where our initial programme theory was not supported through process evaluation data.
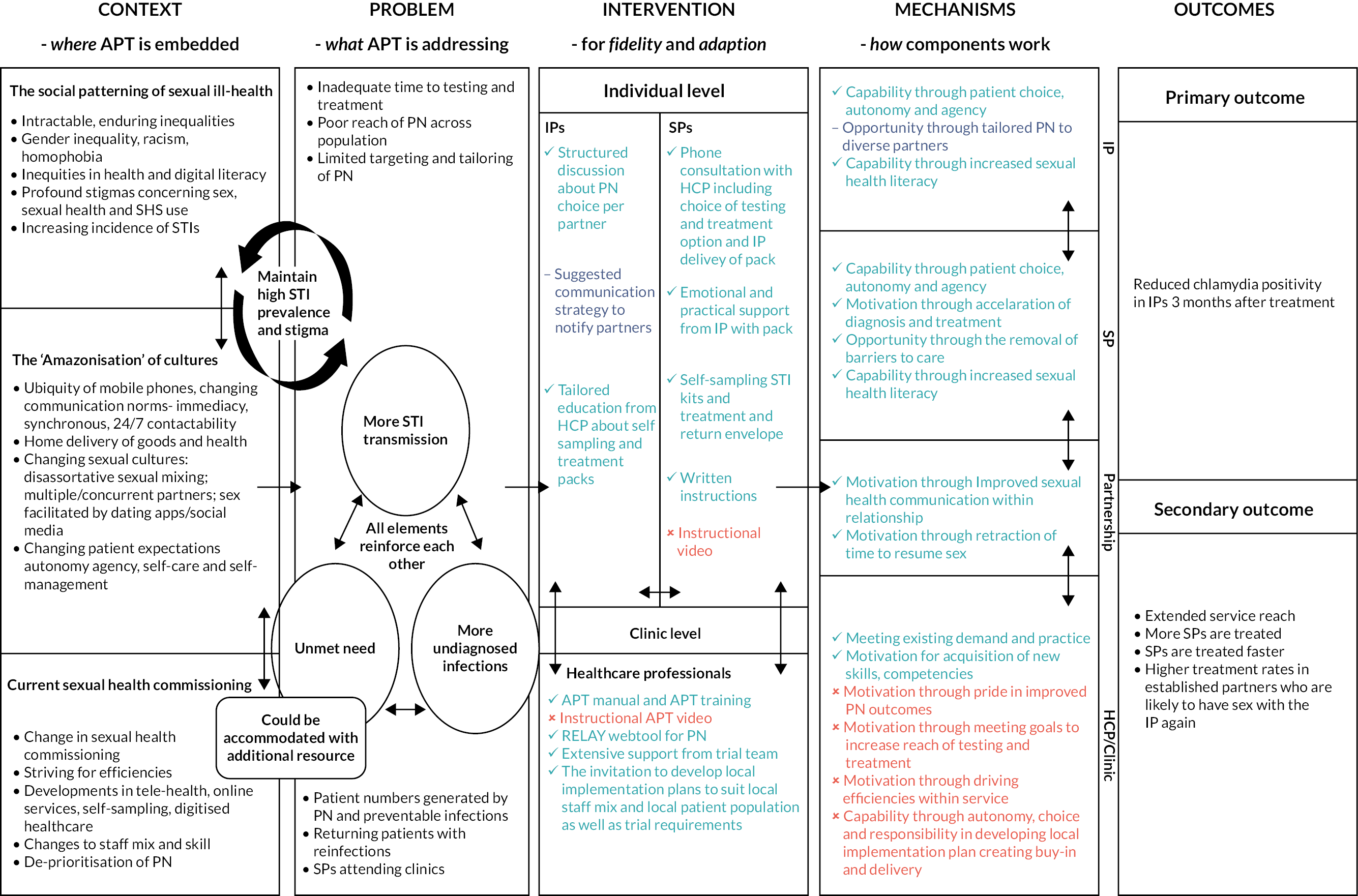
Index patients and SPs who used APT primarily did so within established relationships. APT was particularly beneficial when one partner experienced barriers to attending face-to-face sexual health care (such as STI stigma, work constraints). Despite including a consultation with an appropriate HCP, APT necessitates a shift in responsibilities away from staff within services and onto patients and their partners. This worked well for some who felt more empowered and in control of their care, but others struggled with the burden of information and new processes. For example, some SPs took the treatment immediately but waited until treatment was completed to use the self-sampling kits as a ‘test of cure’ and others reported difficulties in doing finger-prick blood sampling. There was also confusion about the rationale for testing for STIs other than chlamydia despite provision of verbal and written explanations. The intended support to mitigate some of these aspects of APT was disrupted by NHS data communication constraints, which prevented HCPs from sending direct links to short YouTube videos we had created to assist engagement with APT processes. Overall, we found a mixed picture of a well-liked, intuitive, coherent intervention struggling to gain traction within already pressured services.
Interrelation with other parts of the Programme
These analyses helped us understand and contextualise the RCT findings and were informative for the intervention development work with MSM (Stream C). Notably, findings illustrated the key pressures that SHSs were experiencing at the time of the RCT and the apparent systemic deprioritisation of PN activities. As a result, this led to the team reconsidering their approach to intervention development with MSM and the decision to avoid an intervention which relied upon the work and activities of SHSs alone. Drawing upon concepts from systems science, we decided to conceptualise a future PN intervention for MSM in a more distributed way, including the activities of wider stakeholders such as MSM themselves, community-based organisations and those who provide dating apps/dating sites to facilitate sexual mixing. In this way, with a range of agents distributed across the system driving poor PN amongst MSM, it was feasible to avoid intervention components which risked placing undue burden on SHSs.
Stream A phase 3: health economics
Background
Economic evaluations are typically conducted to inform decisions on the best intervention among a given set of alternatives. 31 Most infections are asymptomatic leading to a delay in detection and treatment and onward transmission. Untreated chlamydia can cause reproductive health complications. 32 Chlamydia imposes a considerable economic burden on healthcare systems mostly attributed to complications of non-treatment and re-infections. 33 In Britain, the economic burden of chlamydia to the NHS is approximately £100 million per annum. 33
We conducted a cost-consequence analysis (CCA) alongside the trial. We considered a CCA as an appropriate method because the predetermined trial outcome of ‘cases of reinfection avoided for the index patient’ is deemed as an intermediate outcome for health economics since the full impact of infection, for both the individual and the population, given the infectious nature of the disease, cannot be fully assessed based on this outcome. The longer-term impact and the related cost effectiveness of the APT intervention are evaluated using appropriate methods34 as part of the LUSTRUM Programme and are reported elsewhere.
Aim and objectives
To compare the costs and outcomes of APT versus standard PN for avoiding reinfections in IPs with chlamydia.
Methods for data collection and analysis
The CCA adopted the perspective of the NHS; hence, only direct healthcare costs were considered. We collected data on costs and resource use prospectively during the trial – from the initial consultation up until 24 weeks post intervention. We drew unit costs primarily from the trial and the Personal Social Services Research Unit (PSSRU) costs35 and we applied weighted averages to these costs where appropriate. All costs were reported in 2019–20 British pounds. We inflated Sterling values and cost estimates from previous years using the NHS cost Inflation Index (NHSCII). 35
Resource use data were recorded on RELAY by various cadres of HCPs. The key categories of resource use data for the IPs were:
-
Initial consultations: this included data on HCPs’ pay grade (bands) and the length of the consultation. A precise measurement of the initial consultation duration within the trial was not feasible; hence, assumptions were made based on clinical advice and estimates from a convenience sample of the trial HCPs. All estimates included time for trial-related tasks but did not include any telephone consultations with the SPs.
-
Two week follow-up calls: to determine IP reported PN outcomes, follow-up calls were made 2 weeks after initial consultations by two RHAs. Cost estimates included the average wage/hour 6 length of call and additional time for administrative tasks.
-
Retesting: to provide the primary outcome, IPs were retested for chlamydia at 12–24 weeks. This was either self-testing (using a self-sampling kit posted to them) or testing in the clinic. The costs of the retest pack were obtained from the trial. For retest in a clinic, we assumed standard practice for staff band and test duration. 36
-
Text message: we used text messaging service costs for a large London hospital trust as a proxy. 37
The key categories of resource use data for the SPs who accepted APT were:
-
Telephone Consultation: trial data provided the average duration of a telephone consultation. The cost of a phone call was sourced from a National UK telephone Company (BT). In estimating costs, additional time used by the HCP for patient-related administrative work was included.
-
The content of APT packs: we estimated the costs of the APT packs based on information collected during the trial. A breakdown of these costs is provided.
For SPs during the control phase, we sought data on the number of contactable SPs, clinic attendance for consultations and tests, and test outcome when available and made assumptions if data were not available.
A CCA is typically the first step in an economic evaluation in which costs and outcomes are assessed in a disaggregated manner to see if any intervention shows clear dominance. The result of the CCA analysis is presented as the total costs for PN. We conducted a further analysis of the SPs. We did not apply discounting to either the costs or outcomes due to the short duration of the trial. The reporting is in line with the consolidated health economic evaluation reporting standards (CHEERS) statement. 38 We performed statistical analyses using STATA 15.0®39 or Excel Spreadsheet.
We conducted one-way deterministic sensitivity analyses (see Appendix, Table 11) to assess the impact of changes to the base-case assumptions and included variations in:
-
pay band/grade of the staff for the initial consultation
-
duration of the initial consultation
-
follow-up calls.
Limitations
The CCA benefited from the robustness of the main analyses as shown by the sensitivity analyses. We were unable to use data from RELAY on the duration of initial consultations due to wide variations in data quality between sites. However, we made assumptions based on trial evidence and hence were able to conduct analyses with practical values.
A CCA provides a breakdown of costs and outcomes only – it can sometimes be used to inform decision-making, if the decision-maker can make judgements on the ranges of cost and outcome presented. 40,41 However, in this case, we caution against any such interpretation.
This is because the CCA is based on an intermediate outcome only, and the full impact of the intervention on the transmission of the STIs across the population and its impact on sequelae associated with the disease can only be assessed by modelling the impact on the transmission flow using a transmission dynamic model. 33,34
Key findings
We estimated the costs and outcomes of APT versus standard PN in avoiding reinfection based on negative tests at trial end. The primary outcome was available for 809 IPs in the control phase and 671 IPs in the intervention phase inclusive of 125 patients that selected APT for their SPs. Amongst these, 747 (92%) patients in the control phase and 513 (94%) patients without APT and 122 (98%) (with APT) in the intervention phase had a negative test result, an indication that re-infection was avoided. The total costs of PN for the IPs were estimated as £71.26 for the control phase, and as £91.23 and £74.83 for the intervention phase, with and without APT, respectively. The total cost of PN was £33.17 for SPs who utilised APT and £39.58 for the SPs in the control phase. The sensitivity analyses showed that for all scenarios explored, the results made no substantial difference to the base-case results.
The CCA provides preliminary results only, hence at this stage, no judgement can be made on the cost effectiveness of the intervention. The outcome (reinfections avoided) is an intermediate outcome since it is impossible to know how the outcome would impact on the final outcome and the ultimate sequelae caused by the infection.
The preliminary results show that the APT intervention was more costly than the standard PN (£91.23 vs. £75.21). The intervention with APT accepted avoided re-infections in 98% of patients, compared with 92% for standard PN. The findings suggest that APT could provide an effective addition to the current standard PN practice in Britain.
Interrelation with other parts of the Programme
The findings of the economic evaluation will provide costs and resource use input for the health economic analysis of Stream B which will evaluate the long-term effects of APT versus standard PN.
Stream B: mathematical modelling and health economics
We used mathematical modelling and health economic analyses to investigate (1) the expected effects of APT on chlamydia transmission, (2) the expected rates of chlamydia reinfection in index cases after standard PN and APT, (3) the long-term cost effectiveness of APT versus standard PN in terms of major outcomes averted (MOA) and quality-adjusted life-years (QALYs) and (4) whether improving outcomes of PN for gonorrhoea in MSM could reduce undiagnosed HIV. The four related studies are described in more detail below.
Stream B phase 1: modelling the effects of accelerated partner therapy on chlamydia transmission
Aim and objectives
While the direct effects of standard PN and APT on the identification of new chlamydia infections are well-documented42 and can be observed in the RCT, the indirect population-level effects on incidence and prevalence of chlamydia are less clear. 43 In order to better understand and interpret the outcomes of the RCT, we estimated the expected proportions of chlamydia positivity in partners of people with diagnosed chlamydia (index cases) and quantified the effects of APT on chlamydia prevalence compared with standard PN in Britain.
Methods for data collection and analysis
We developed a novel deterministic, population-based chlamydia transmission model (see Figure 5). 44 A dedicated PN module allowed us to track the most recent partners of index cases and to identify the index–partner combinations that result in the largest effect of PN on reducing chlamydia prevalence. We considered a population aged 16–34 years and calibrated the model to sexual behaviour data between people of the opposite sex and chlamydia prevalence data reported by 3671 participants in Britain’s third (Natsal-3, 2010–12)3,45 using approximate Bayesian computation (ABC). In different scenarios, we calibrated the model to sex- and activity group-specific prevalence in the presence (current situation) and absence of control interventions. We simulated the effects of APT on chlamydia transmission by (1) increasing the number of treated partners by 5%, 10%, 15%, 20% and 25%, and (2) reducing the time to partner treatment by 1, 2 and 3 days compared to standard PN. We then calculated the relative reduction in prevalence 5 years after the implementation of APT.
FIGURE 5.
Schematic illustration of chlamydia transmission model.
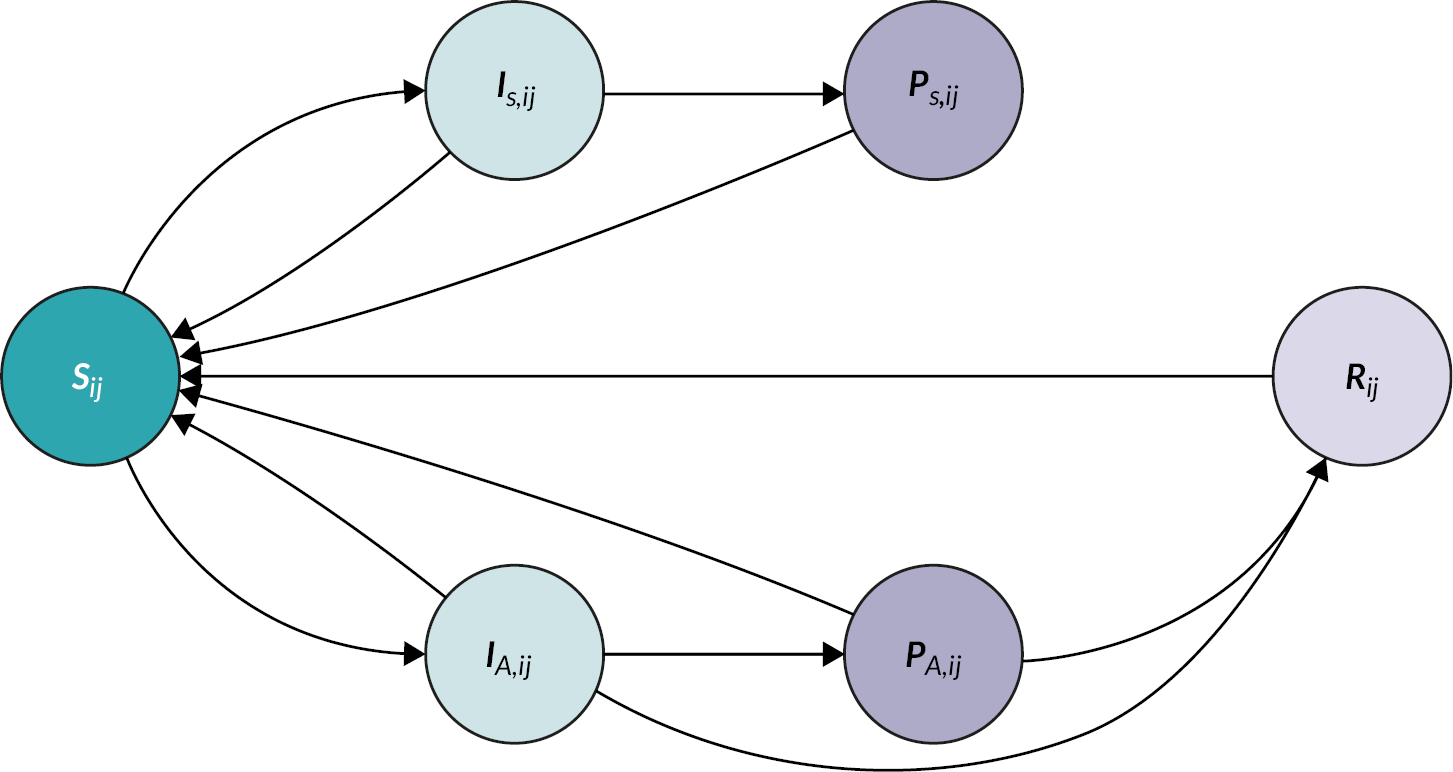
Susceptible individuals Sij can become symptomatically and asymptomatically infected (IS,ij and IA,ij). Infected individuals can then become notified (PS,ij and PA,ij) by their partners. All infected individuals can receive treatment to become susceptible again, or acquire temporary immunity (Rij) through spontaneous clearance of the infection. Movement of individuals into and out of the population is omitted in the scheme. Subscripts i and j denote sex and sexual activity groups, respectively. A more detailed description of the model structure is given in Althaus et al. 44
Limitations
First, we considered notification of the index case’s most recent partner only. This was a necessary simplification of our modelling framework. As the average number of notified partners is typically below one, we expect that including notification of additional partners in our model would not substantially affect our results. Second, we did not consider reinfection of index cases by untreated partners. This aspect was investigated in a separate study (study 2 of Stream B). Finally, the model does not include data from the RCT as the studies were run in parallel.
Key findings
We found that chlamydia positivity is highest in partners of symptomatic index cases with low sexual activity, whereas the infected partners are typically asymptomatic and highly sexually active. Conducting PN for this particular index–partner combination will thus be most effective for preventing further transmission. Increasing the number of treated partners from current levels in Britain [0.51, 95% credible interval (CrI) 0.21 to 0.80] by 25% would reduce chlamydia prevalence by 18% (95% CrI 5% to 44%) in both women and men within 5 years (see Figure 5). In contrast, reducing the time to partner treatment alone had a minor effect on reducing prevalence. Together, these results suggest that PN typically identifies sexual partners that are likely to further transmit chlamydia, and that APT in particular has the potential to further reduce prevalence through an increase in PN uptake.
Interrelation with other parts of the Programme
First, the results of this study on chlamydia positivity in partners of index cases help to better interpret the outcomes of the RCT. Second, simulated data from the model were used as input parameters for modelling reinfection with chlamydia (study 2 in Stream B) and the cost-effectiveness analysis (study 3 in Stream B).
The projected effect of APT on chlamydia prevalence after 5 years is shown in Figure 6.
FIGURE 6.
Projected effect of APT on chlamydia prevalence after 5 years.
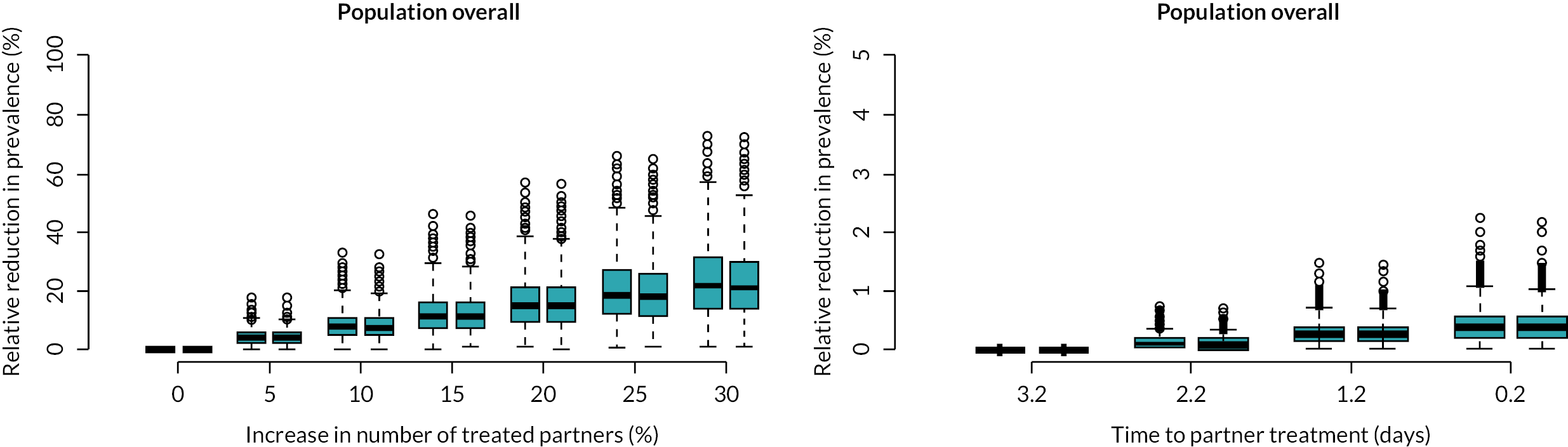
APT is modelled as an increase in the number of treated partners (left) or a reduction in the time to partner treatment (right). Changes in prevalence are given for females (red) and males (blue). Note the difference in scales of the axes between the left and right panels.
Stream B phase 2: modelling reinfection with chlamydia after standard partner notification and accelerated partner therapy
Aim and objectives
The expected effects of APT on the reinfection of treated index cases by untreated partners with chlamydia remain unclear. We did not consider reinfection of treated index cases in the transmission model (study 1 of Stream B). Here, we analysed data from the RCT using another mathematical model and quantified the effects of offering APT on the probability of successful partner treatment.
Methods for data collection and analysis
We extended a previously developed mathematical model42 to compute the probability of chlamydia reinfection of index cases by their untreated partners with chlamydia. We fitted the model to data from the RCT and estimated the probability of successful treatment of the partner of index cases in a Bayesian framework.
Limitations
The model does not distinguish between reinfection in women and men and considers reinfection by a single partner only. Furthermore, the remaining uncertainty in some key parameters together with the relatively small numbers in the primary outcome of the RCT result in considerable uncertainty of the modelling results.
Key findings
We estimated the median probability of reinfection at 16.2% (50% CrI 12.7 to 20.0%) without partner treatment and 2.3% (50% CrI 1.7% to 3.6%) when partner treatment is 100% successful. The observed rates of reinfection in the RCT correspond to a median probability of successful partner treatment of 63% (50% CrI 47% to 75%) during the control period and 77% (50% CrI 64% to 87%) during the intervention period, where APT was offered in addition to standard PN. Hence, the study suggests that the observed reduction in reinfection with chlamydia when offering APT is consistent with a higher probability of successful partner treatment.
Interrelation with other parts of the Programme
The results of this modelling study help to better interpret the effect of offering APT on the primary outcome of the RCT (Stream A).
Stream B Phase 3: cost-effectiveness analysis of accelerated partner therapy versus standard partner notification
Aims and objectives
We estimated the cost effectiveness of APT compared with standard PN in terms of MOA and QALYs gained to assess the long-term impact of APT on chlamydia and its sequelae at the population level.
Methods of data collection and analysis
We developed a static spreadsheet-based model using output from the chlamydia PN model (Stream B phase 1) to estimate the impact of APT on healthcare costs and numerous health outcomes: mild and severe PID, ectopic pregnancy, tubal factor infertility, chronic pelvic pain, epididymitis and QALYs in a population of 100,000 adults aged 16–34. 46 Estimates of resource use and unit costs were drawn from the Stream A within-trial CCA and suitable published secondary sources. Probability values relating to the complication were drawn from suitable published secondary sources. Utility values informing QALYs were obtained from a primary study (for female complications) and published literature (for epididymitis). Our base-case analysis assumed that APT increased the number of partners treated from current levels by 25%.
We calculated incremental cost-effectiveness ratios (ICERs) for APT versus standard PN in terms of cost per MOA and cost per QALY gained. We then conducted extensive deterministic sensitivity analyses and a probabilistic sensitivity analysis to assess parameter uncertainty. Lastly, we conducted scenario analyses whereby the increase in the number of partners treated by APT was lowered to 15%, 10% and 5%, respectively.
Limitations
Firstly, the analysis did not consider the effect that repeat or persistent chlamydia/PID would have on tubal damage. Secondly, only an IP’s most recent partner was considered in the analysis. Thirdly, due to a lack of availability, robust utility values were not used for epididymitis. Lastly, the analysis made no comparisons for different forms of APT (e.g. APT Pharmacy,20 which was considered by a previous CCA36).
Key findings
The base-case results, which assume that APT increases the number of partners treated by 25%, showed that APT is less costly and more effective in terms of MOA and QALYs than standard PN, hence is cost-saving. Deterministic sensitivity analyses found that APT remained either cost-saving or cost-effective, the latter with ICERs that were very low and well within acceptable thresholds. APT remained cost-effective when the increase in the number of partners treated by APT was lowered to 15% and 5%, respectively; however, it was more costly than standard PN.
Interrelation with other parts of the Programme
The health economic analysis of Stream B models the long-term effects of APT versus standard PN and thereby complements the trial and CCA from Stream A that measure the short-term effects. It additionally draws estimates of resource use and unit costs from the Stream A CCA. The cost-effectiveness model relies on simulated data about the long-term effects of standard PN and APT from the dynamic transmission model (Stream B phase 1). 44
Stream C: development of optimal partner notification interventions for men who have sex with men with bacterial sexually transmitted infections
Men who have sex with men are disproportionately affected by STIs and HIV. Patterns of sexual partnership for MSM tend to differ from heterosexual patterns; MSM tend to report higher numbers of SPs and a greater proportion of one-off partners who contribute disproportionately to onward transmission.
Little research has focused on PN amongst MSM possibly because of the challenges associated with reaching one-off partners. Different PN strategies which appeal to MSM, and their one-off partners are needed. This has become particularly important in recent years given the emergence of increasing antimicrobial resistance to Neisseria gonorrhoea, the causative agent of gonorrhoea for which the majority of British cases are reported in MSM. Furthermore, more effective PN for MSM with a bacterial STI could identify MSM at particularly high risk of HIV acquisition because patterns of infection overlap. The ability to identify MSM at HIV risk provides opportunities for targeted HIV prevention and health promotion in the form of STI and HIV testing, appropriate vaccinations and HIV pre-exposure prophylaxis (PrEP).
Stream C phase 1: identifying and evaluating existing economic studies about partner notification and/or testing and treatment for sexually transmitted infections/human immunodeficiency virus
Economic research on PN has typically focused on heterosexuals, with a lack of evidence on effectiveness in MSM. Novel PN interventions for MSM need to be grounded in economic reality.
Aim and objectives
We conducted a systematic review of economic studies of PN interventions for STIs in MSM. PN often involves testing and treatment of SPs; hence, to ensure a comprehensive inclusion of all PN-related interventions, we also explored studies associated with testing and treatment strategies.
Methods for data collection and analysis
We undertook a systematic review according to the guidelines of the UK‘s Centre for Reviews and Dissemination (CRD)47 and reported this following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 48 A search strategy was developed using the population, intervention, comparison and outcomes (PICO) framework. 49 A scoping search was carried out on Google Scholar and MEDLINE. This was followed by a search on six electronic databases including MEDLINE, EMBASE, Web of Science, NHS Economic Evaluation Database (NHS EED), HIMC and cumulative index to nursing and allied health literature (CINAHL), up to June 2020. The reference lists of potentially key papers were hand-searched to identify additional papers. Search results were entered into the endnote database manager,50 to exclude irrelevant studies and code relevant studies.
Inclusion and exclusion criteria: formal economic evaluation and cost-analysis studies were included if participants were MSM who had any STI and/or HIV and the intervention was related to PN, testing or treatment. Studies were excluded if they were editorials, reviews or reports on the use of technology for interventions not related to PN, such as health promotion and education.
Study selection: a two-stage process was used to screen studies for inclusion using published methods. 51,52 Studies were categorised independently by two reviewers. A formal quality appraisal was not conducted because the review’s objectives required a description of all economic evidence but not a methodological assessment.
Data extraction and synthesis: a bespoke data extraction form was developed based on the study objectives and subsequent planned analysis. Data were extracted and checked for consistency by two reviewers. Relevant information was tabulated to facilitate comparison across studies and evidence was summarised using a narrative synthesis.
Limitations
The systematic review benefited from a comprehensive search using best practices. The review provides useful information on the cost of PN for STIs/HIV in MSM that could potentially be used as model inputs for any future model-based analyses. We had anticipated that evidence from this review would be used to develop a preliminary decision-analytic model to explore the cost effectiveness of alternate pathways developed in the Programme. However, this was possible because we did not identify any consistent pathways to evaluate. Furthermore, we identified no studies focusing on the costs and outcomes associated with digital technologies for PN.
Key findings
The systematic review selected 26 studies out of a possible 1909. Overall, 11 studies included PN strategies in the assessment, while 15 studies focused on testing and/or treatment, 16 papers focused on MSM, but only 3 of these were on PN, indicating a paucity of PN studies in this population. The review did not identify any PN studies on MSM for curable STIs, including chlamydia. However, two studies on HIV that reported on PN in this population were identified. Few studies reported on patients’ characteristics and settings.
The studies (22) were mostly formal economic evaluations, with only four cost analyses. The majority of the economic evaluations were cost-utility analyses with outcomes reported as QALYs which were derived from studies on heterosexual people due to a lack of data on MSM. These may not be directly relevant to MSM given the different patterns of SPs reported by these two groups. Few studies reported cost components or types of resource use to identify the costs none of these cost studies was relevant to digital PN. The studies mostly derived their data from secondary sources and only six used data from primary sources. Information on partner types or digital PN was not available within the selected studies. There was also little information on using a digital tool for PN, with just one paper53 reporting the use of an online PN tool. The lack of evidence on efficient approaches for MSM supports the need for new interventions with parallel economic evaluation.
Interrelation with other parts of the Programme
The dearth of appropriate economic evidence highlighted by this review supports a call for future research in this area to embed economic evaluation and associated appropriate data collection so that the process evaluation as outlined in Stream C can be used to develop models to explore a novel PN approach for MSM with STIs and aid decision-making.
Stream C phase 2: investigating partner notification for bacterial sexually transmitted infection to increase detection of undiagnosed human immunodeficiency virus in men who have sex with men
Aim and objectives
The original aim was to investigate the effects of improved PN interventions in MSM with gonorrhoea on identifying sexual partners that are infected with Neisseria gonorrhoea, HIV or both. In recent years, the research questions with respect to PN in MSM have changed due to the introduction of HIV PrEP which has become an accepted biomedical HIV prevention intervention. 54 We aimed to develop a gonorrhoea/HIV co-infection model for MSM.
Furthermore, we summarise the recent modelling literature on PN and PrEP in MSM to identify future research questions.
Methods of data collection and analysis
We attempted to extend the modelling framework from Stream B (phase 1) to include gonorrhoea/HIV co-infection and fit it to incidence and prevalence of gonorrhoea and HIV. We searched the literature for novel mathematical modelling studies on bacterial STIs, HIV, PN and PrEP.
Limitations
The development of the gonorrhoea/HIV co-infection model did not extend beyond an experimental phase. First, fitting the model to data about both infections, using the same method as in Stream B (phase 1), did not result in convergence of posterior parameter distributions. Second, the PN module could not be easily extended to co-infections within the time frame of the project. The existing literature on modelling PN in MSM focuses on expedited partner therapy (EPT)55–57 as carried out in the USA, in which an IP is given additional (oral) antibiotics to deliver to SPs. The findings are thus not generalisable because current treatment for gonorrhoea in many countries is parenteral due to the concerns about the efficacy of oral cefixime treatment.
Key findings
We identified several challenges when attempting to fit the compartmental gonorrhoea/HIV co-infection model to data. The issues we experienced with parameter identifiability need to be addressed in future modelling studies that aim to provide quantitative estimates of interventions in MSM. However, the results from existing modelling studies shed light on the potential effects of PN and PrEP in MSM. First, a network-based model of HIV/gonorrhoea/chlamydia found that PrEP in combination with HIV/STI screening recommendations could result in a significant decline of gonorrhoea and chlamydia in MSM. 58 Second, an agent-based simulation model of HIV/HIV/gonorrhoea/chlamydia Neisseria gonorrhoea/Chlamydia trachomatis illustrated that targeted delivery of PrEP to people diagnosed with gonorrhoea and chlamydia could increase the effectiveness of PrEP and reduce incidence of all STIs. 59 Finally, a network-based model highlighted the potential of EPT in MSM to reduce gonorrhoea and chlamydia infections but raised concerns about a possible increase in antimicrobial resistance and missed opportunities for HIV prevention. In summary, the recent modelling studies underline the importance of PrEP for prevention of HIV as well as bacterial STIs. Future modelling studies should consider PN interventions in presence of PrEP.
Interrelation with other parts of the Programme
The reported findings inform the development of appropriate PN interventions for MSM.
Stream C phase 3: optimising accelerated partner therapy and developing a novel partner notification intervention amongst men who have sex with men
Aim and objectives
To enhance patient choice and improve public health by developing acceptable, theoretically informed, evidence-based, PN interventions (tailored according to SP type)
(1) To optimise APT for MSM; (2) to examine psychosocial aspects of PN amongst MSM; (3) to respond directly to emerging insights from the wider research Programme (i.e. the need to tailor PN interventions by SP type) and develop a novel, multilevel, multistakeholder intervention that focuses on improving PN with ‘one-off’ partners – a relationship type known to be particularly challenging for effective PN; to detail intervention content ready for evaluability assessment within further research.
Methods of data collection and analysis
Following new guidance relating to intervention development for complex interventions,2,60,61 we moved away from our initial intention to use exploratory social science (e.g., the use of interpretative phenomenological analysis and cross-sectional surveys). Instead, we used a range of methodological approaches, including stakeholder workshops, in-depth qualitative data through focus groups and in-depth interviews, consultation with PN experts and the application of theories and conceptual tools from behavioural and systems science.
-
Optimising APT amongst MSM: a full account of our methodological and analytic approach and findings generated is presented under Stream A phase 2. Briefly, 14 MSM took part in the wider Programme of work optimising APT. 13
-
We incorporated study of the psychosocial aspects of PN amongst MSM into the wider process of intervention development outlined below in (3). This meant that in addition to exploring these psychosocial aspects we also focused closely on the development of intervention ideas outlined through stakeholder engagement (see details below).
-
In relation to our intervention development work, directed by what we were learning from the wider Programme about the importance of partner type in shaping PN choices,10 we engaged in a multistaged, programmatic approach to intervention development. 62
Partner notification for MSM and their one-off partners is challenging. Findings from earlier parts of the Programme suggested that APT best suits IPs (including MSM) and their more emotionally connected SPs. In order to address the particular challenges of PN solutions for one-off partners, we drew upon ideas from systems science63 to explore novel ways of understanding and responding to the drivers of these poor PN outcomes.
Systems science invites us to consider the complexity of upstream and interdependent drivers of health outcomes and to engage with distal and distributed levers of change. For (3) this meant not assuming that the best place for intervening was within a SHS or through engaging MSM individually in behaviour change. Instead, it meant exploring the wider system which drives PN to understand and detail potential ‘hot spots’ where the simultaneous implementation of diverse future intervention elements across the whole system could make the biggest difference by changing the system itself.
Stage 1: we conducted a stakeholder event with 45 diverse participants from across Britain including MSM, public health experts, service commissioners, multidisciplinary sexual HCPs, non-governmental organisations (NGOs), academics and dating app providers (dating apps are often used by MSM to find one-off SPs). We focused on exploring the diverse and multilevelled social, cultural and healthcare system-level context shaping poor PN outcomes for one-off partners amongst MSM (see Table 9). We were careful not to prematurely privilege any profession (e.g. health advisors), community (e.g. HIV positive men), service (e.g. NGO STI testing sites) or stakeholder (e.g. dating app providers) as particularly responsible for delivering future intervention content.
| The distal drivers of contact tracing |
|---|
| The proximal drivers of contact tracing |
| Negative drivers (factors limiting contact tracing with one-off partners) |
| The socioeconomic context of SHS provision |
| Overstretched SHSs |
| The business models of dating apps |
| Elements of contemporary and historical culture |
| The on-going impact of heterosexism on MSM |
| The on-going impact of homophobia on MSM |
| History of pathologising and blaming MSM |
| Legacy of inadequate sex and relationship education |
| High levels of sexual- and STI-related stigma |
| Positive drivers (factors facilitating contact tracing with one-off partners) |
| A distributed approach to improving the drivers of contact tracing that did not over burden any single stakeholder |
| The agency and continued involvement of MSM throughout intervention development |
| Collective and co-ordinated efforts across the wider system |
| Saturate the system with clear signals about the positive value of contact tracing |
| Working with communities of MSM to enhance the existing salutogenic aspects of MSM cultures |
| Use existing assets: consolidate the presence and history of existing norms about contact tracing, community resilience in relation to HIV |
| Harness peer interactions to drive improved contact tracing |
| Use all available infrastructures (e.g. websites, dating apps, broad range of health services to saturate the system with positive messages about contact tracing) |
| Compensatory sexual health education |
| New cultural norms about contact tracing |
| New cultural expectations about contact sharing |
| Co-produce mass and social media intervention elements to change MSM cultures and norms |
| Ensure dating apps promote and endorse norms to facilitate contact tracing |
| Change dating app functionality and features to facilitate contact tracing |
| Negative drivers (factors limiting contact tracing with one-off partners) |
| SHSs experienced as unwelcoming to MSM |
| Interactions between HCPs and GBMSM experienced as judgemental and unhelpful |
| Positive drivers (factors facilitating contact tracing with one-off partners) |
| Opportunities for HCPs to reflect on current and optimal practice |
| Data-driven approaches to understand current success and failure |
| Opportunities to share good practice within and across services |
| Training to ensure HCP cultural competence in relation to MSM |
| Scripts for HCPs to motivate MSM to engage in contact tracing |
| Work with MSM to anticipate and prepare for contact tracing interactions within SHSs |
| Enhanced use of digital technology within contact tracing interactions (smart phones, dating apps) |
| Work with key people organising sex parties, sex clubs for MSM |
| Work around contact tracing within public sex environments |
We recorded detailed notes and visualisations and analysed them thematically to develop an initial set of ideas for potentially useful future intervention content.
Stage 2: taking the initial ideas for intervention content generated in Stage 1, we collected and analysed much more detailed data to (1) gauge the acceptability of these ideas, (2) further specify and optimise them drawing on participants’ expertise and insights and (3) using tools from behavioural science, to specify potential intervention content at a highly granular level in relation to content, theory, mechanism of action and BCTs.
Given our whole-system focus, it was important to encompass multiple and diverse perspectives on these initial intervention ideas each addressing different hot spots within the system. We aimed to recruit diverse MSMs, diverse HCPs and those involved within the dating app industry.
However, the COVID-19 pandemic impacted on our recruitment plans. We changed our approach to encompass online, in addition to in-person, data collection and completed five focus groups with MSM. Three took place in person (Glasgow, London and Leeds) and two online (n = 28). We systematically explored the psychosocial aspects of PN amongst MSM; intervention ideas relating to interactions between MSM; interactions between MSM with HCPs and SHSs; and MSM’s perspectives on intervention ideas relating to dating app functionality and dating app provider responsibilities.
Despite our best efforts, we only managed to recruit three dating app providers with whom we conducted one-to-one telephone interviews. We systematically explored intervention ideas relating to dating app functionality and dating app provider responsibilities, the potential of dating app provider partnerships with HCPs and public health teams, intervention ideas relating to interactions between MSM; and finally, interactions between MSM with HCPs and SHSs. Our planned focus groups with HCPs were unable to take place due to the national suspension of a range of research activities during the COVID-19 pandemic.
We analysed data from Stage 2 initially using deductive thematic analysis63 and subsequently using the BCW64 to suggest granular intervention content. In this way, Stage 1 had oriented us to where to intervene within the wider system, and Phase 2 showed us exactly how we might do so.
Stage 3: COVID-19 restrictions on HCP participation in research precluded our final planned stakeholder event.
Instead, we held four smaller virtual stakeholder engagement events involving a broad range of participants from NGOs (n = 6) and PN experts drawn from the wider LUSTRUM team (n = 5 including health advisors, nurses, and doctors). Participants appraised the detailed intervention suggestions outlined within Stage 2 and using the APEASE criteria,19 discussed potential for implementation across the whole system. The outline of our systemic intervention is illustrated in Figure 7.
FIGURE 7.
The agreed focal points to be operationalised within future interventions.
Light blue elements – consensus of intervention content to be included within future interventions. Orange elements – some support for intervention content in this area within future interventions. White elements – intervention content developed in Stage 1 and 2 but rejected in Stage 3. Light blue arrows – factors associated with facilitating effective contract tracing.
Orange arrows – factors associated with constraining effective contact tracing.
Limitations
We did not collect quantitative data; however, recent European work on PN had been conducted through European MSM Internet Survey (EMIS),65 and this was incorporated into the initial stakeholder event. The COVID-19 pandemic meant that it was not possible to include HCPs within Stage 2 of the intervention development process and although we could compensate in part by asking MSM about their interactions with HCPs, and through our inclusion of HCP experts in the final round of stakeholder engagement, the lack of systematic data and analysis of HCP perspectives on PN with one-off partners remains a limitation.
Key findings
Although we had envisaged that we would focus on optimising APT for the specific needs and preferences of MSM, and that a modified form of APT would be our developed MSM intervention, Programme findings about the importance of tailoring PN interventions to different partner types meant that in addition to optimising APT for MSM (and their more emotionally connected partners), we also developed recommendations for a multilevel, multi-stakeholder intervention targeting MSM with one-off partners, for whom PN is known to be particularly challenging.
Key areas for a multilevel, multistakeholder intervention that could simultaneously address various aspects of the system that drives poor PN outcomes amongst MSM included acknowledging everyone has a part to play; modifying the cultural and social context of MSM communities; improving skills, practice and service organisation of HCPs and their services to better accommodate PN for one-off partners; working with dating app providers to explore possible digital PN options through dating apps, or stand-alone online PN platforms.
Close partnership working between MSM, NGOs, HCPs and dating app providers across the system was perceived to be important. A series of coterminous and interdependent intervention components together may change the whole system that drives poor PN outcomes (i.e., push the system to ‘a tipping point’).
Multiple reinforcing positive feedback loops may be possible that cascade change effectively without over-burdening any particular part of the system (e.g. HCPs).
Key intervention elements included a coordinated and co-produced mass and social media intervention that changes the norms and beliefs of MSM to challenge STI-related stigma and other barriers to notification; NGO’s leading funded peer-led work reducing stigma and persuading MSM to actively participate in notification to protect others and their communities, stressing the value and importance of PN (e.g. using key opinion leaders in MSM communities online and off); HCPs and their services to prime MSM to prepare for a PN interaction at the point of diagnosis (e.g. by thinking through the types of sexual partner they have had and their contact details and the kinds of notification that is appropriate), changing national audits and monitoring systems to directly address one-off partner PN outcomes, bespoke training to HCPs and NGO staff on issues for one-off partners and the social and cultural context of MSM ensuring they demonstrate cultural competency and reinforce the importance of PN; dating app providers promoting appropriate PN messaging and being active collaborators throughout. More detailed intervention elements include public health messages and materials to educate MSM on PN, virtual or real-world training opportunities for MSM modelling how to contact partners after a diagnosis (e.g. suggestions for wording of how to tell a one-off partner), how to respond to being notified of increased risk (suggestions for wording), targeted, punchy messaging about contact tracing and management for one-off partners. Stakeholders suggested an intervention could be branded with 4T’s: ‘Test, Treat, Trace, Tell’ with messaging branded by reputable organisations endorsing notification. Intervention content should also be co-produced with and being visibly inclusive of a diverse range of MSM.
Interrelation with other parts of the Programme
These elements are closely related to Stream A, ‘optimisation of APT’ and build very clearly on the results of the main trial and the findings relating to the importance of partner type in the choices of PN approach. They also touch upon the problems of implementing APT outlined within the process evaluation and have oriented future solutions to PN outside SHSs rather than adding to the existing service burden. 66
Equality, diversity and inclusion
Which groups of people were represented in our research, and how did we involve them?
The LUSTRUM Programme was funded and designed to improve the sexual health of people most impacted by STIs and HIV by preventing transmission and reducing undiagnosed infections. National data show that young heterosexual people and MSM are most likely to be diagnosed with STIs. In line with the data, the LUSTRUM Programme involved people who identify as either a young heterosexual person or a MSM. We were aware that these two groups are very broad, so we identified people from different sexual health clinics and community-based SHSs throughout England and Scotland. By including people from London, metropolitan ‘cities’ and non-London urban ‘towns’, we gained representation from broad geographic areas and different levels of social poverty. In addition to broad geographic representation amongst the people who took part in our studies (participants), we achieved representation from men, women, people who identify as heterosexual, MSM, plus a wide range of ages, with the average being 24 and ethnicities (including White British/Irish/other, black/Black British, mixed ethnicity and other ethnicities) within these. We also worked with 25 people with mild learning disabilities, 56 members of the public and patients and 30 HCPs to help us improve APT so that it was suitable for as many different types of people as possible.
The focus of the Programme was on groups that national data show experience an unequal burden of Chlamydia infection. In the future, we would continue to review the most up-to-date data, about groups most affected by STIs, with particular attention to those not included in this Programme following any changes in the epidemiology of STIs. This is so that we can explore and address any PN and management needs of other groups that could benefit from innovations in PN.
Inclusion and accessibility of participant and public engagement materials
Throughout the Programme, we aimed to include public perspectives in the design, delivery and dissemination of the research with varied audiences. We did this to increase the likelihood of our research being relevant, acceptable, effective and impactful. We considered the needs of current and future users of sexual health care and those of the HCPs that deliver SHSs. To do this, we created a virtual patient and public involvement and engagement (PPIE) panel and consulted regularly with wider stakeholders, including people with recent experience of STI testing and diagnosis.
All public-facing, text-based and image-based documents were reviewed by representatives from our PPIE panel before being published or shared more widely. This included recruitment materials, website text, explanations of the trial design and programme branding. Their suggestions and questions helped us improve the language, accessibility and understanding of our outputs (including the Plain language summary of this report).
Following a recommendation from a lay contributor, we produced explainer videos and instruction videos to support trial participants in taking their own STI samples. We worked with PPIE members to develop the video scripts and concepts. These videos presented information in a short, engaging and informative way. In each video, the main character was either from a racially marginalised community and/or had a lesser-heard regional accent. The public-facing explainer video received 670 views and, in total, all public-facing videos received over 4100 views. In our final video summarising the trial findings, we received feedback from a member of the public that an animation style may work better – we took this on board. In the animation video, we intentionally showed a range of genders, ethnicities, sexual orientations, partnership types and religions on screen to make the video more accessible to a wide audience. We also used simple, lay-friendly language.
Diversity and inclusion among the LUSTRUM research team and associates
The LUSTRUM Programme team is a diverse group. We ranged in age from early 20s to 60s and included a range of genders, ethnicities, sexual orientations, cultural identities. The team also had both personal and professional experiences of STI. Importantly many team members have intersectional identifies, broad life experiences and non-traditional career paths. The research has benefited enormously from the perspectives offered by our truly diverse team that is able and enthusiastic about engaging in challenging discussions concerning inequalities, wider determinants of health and reflecting critically on our research approaches.
The ‘whole systems’ and progressive nature of this research programme required a multidisciplinary research team which included clinicians, health psychologists, epidemiologists, social scientists, statisticians, public health professionals, economists and mathematical modellers. The team was geographically diverse, including members from urban metropolitan settings in London, Birmingham and Glasgow and rural areas such as Wessex and further afield, colleagues in Bern, Switzerland. This geographical diversity complemented the multidisciplinary team and helped us achieve true diversity of thought. We engaged in reflective meetings where we questioned underlying assumptions of different disciplines and health systems to help our understanding. This breadth of knowledge, networks and professional interests supported external multidisciplinary expert consultation, including within Stream C, where we explored diverse social, cultural and healthcare system levels.
Team members reflected different levels of seniority, across and within disciplines, which supported peer-to-peer learning, mentoring, reverse-mentoring and career development opportunities. For example, the key post-doctoral role holder on the LUSTRUM Programme has now secured a management role on a new programme grant, a research assistant from a minority ethnic background developed an interest in and is now pursuing an academic career and another team member is using her research knowledge in an industry-specific role. One of the HCPs that worked closely with the research team has also been inspired to explore career opportunities in research.
Development opportunities were also provided to those outside of the core staff group. In the first year of the programme, we provided research experience placements for medical students, the majority of whom were from non-white ethnic backgrounds. Further, over several years, we provided 3-month placements to three students on University College London (UCL) Behavioural Psychology Master’s programme.
Both the research team members, the programme of research and the service users and members of the public involved in this study have gained enormously from a considered approach to equity, equality, diversity and inclusion and are grateful for the opportunity that NIHR is providing for us to share our learnings and achievements. In future work, we would continue to consider the protected characteristics, as a baseline for our work, plus any other factors which may support/challenge engagement with and progression within research.
Patient and public involvement and engagement
Aims
-
To include public perspectives in research design, conduct and dissemination.
-
To increase relevance, acceptability, effectiveness and impact of the programme.
-
To address the needs of current and potential users of sexual health care and the HCPs that deliver them.
Approach and methods
Traditional PPIE approaches are less acceptable within sexual health research. Stigma can mean that people avoid discussing experiences of STI care in public fora and the transience of many STIs means that service users do not tend to form enduring links with clinical services. We adopted an innovative, virtual approach by maintaining and expanding ‘Barts Sexual Health Public Voice Research Group’, composed of 27 diverse lay people interested in/users of SHSs.
Communication was mostly by e-mail and our website. 67 Members fed back directly to the Programme Manager instead of through collective discussion, enabling sharing of opinions whilst preserving anonymity. A PPIE representative(s), prepared/debriefed by a researcher, attended all levels of Programme meetings as appropriate.
Key outcomes
-
Better decision-making, informed by public views on research design and conduct.
-
Improved language, accessibility and understanding of our outputs (including the Plain language summary of this report).
-
Feedback on study documentation, including lay explanation of the trial design and programme branding.
-
Advice on research outputs, including, the novel classification of SP types.
-
Directly informed ethical considerations, underpinning our decision to pursue service level consent in the RCT.
-
Co-development of a GDPR-compliant solution for permission for research data use and contribution to a related scientific manuscript. 27
-
Joined expert workshop discussions with the BASHH and co-produced the first national recommendations for STI and BBV self-sampling packs and processes. 7
-
Shared lay views at a national British HIV Association/BASHH conference panel discussion.
-
Assisted production of a video summarising the RCT findings (over 200 views).
The PPIE Group and our lay representatives have also been invaluable in achieving the Programme’s research aims:
Using a dedicated YouTube channel,68 we increased engagement with a broader audience including:
-
A video series of ‘explainer’ clips about APT (the public-facing video received three times the views of the professional-facing video).
-
Videos demonstrating how to use STI self-sampling kits, initially intended for RCT use, were also used by the public (over 3000 views).
Patient and public involvement and engagement increased stakeholder engagement, using Twitter and highlighted driven traffic to our key outputs and created interest in dissemination events including conference presentations and scientific-lay webinars.
Discussion and reflections
Through our innovative virtual PPIE approach, we maximised the impact and relevance of the LUSTRUM Programme in academic, service delivery and non-research settings, within a stigmatised health area. In future, we will work with our PPIE group to explore ways of facilitating group-based discussion in a way that is acceptable to lay contributors so that the benefits of collective discussion can be realised. Furthermore, although we captured some demographic information from PPIE members, we would attempt to do this systematically, if acceptable, to better enable us to assess and fill any gaps in diversity of backgrounds and experiences.
Programme successes and things to improve
We engaged key stakeholders early, including BASHH, English Sexual Health and HIV Commissioning Group, Public Health Scotland, and service users. Through these relationships, end beneficiaries, providers, commissioners and policy-makers were continuously engaged with the research. This ensured alignment with clinical and service user priorities and provided opportunities to help shape national policy and practice through leadership of new national BASHH STI PN outcomes,69 a new classification of SP types,10 and national recommendations for self-sampling kits and processes,70,71 and involvement of co-investigators in England’s forthcoming National Sexual Health Strategy, Scotland’s COVID-19 Recovery Planning and providing specialist advice to SARS-Cov-2 contact tracing Programmes.
In addition to our scientific outputs (publication and outputs list, table 10), we sustained a rigorous research Programme despite the challenges presented by the COVID-19 pandemic, created a novel solution to a GDPR paradox27 within service-level consent clinical trials, developed a web-based data collection tool, RELAY, to support PN, which many clinical staff found superior to existing clinic systems.
We established new ways of communicating sexual health research to a broad range of audiences including: (1) using video media as a tool in focus groups and to summarise trial findings (2) sustaining a social media following of over 500 followers and having the top tweeted paper from BMJ STI 2020/2021,10 (3) conceptualising, producing and disseminating four explainer videos68 (over 2000 views), (4) hosting a webinar series exploring our trial methodology and new frontiers for contact tracing–attended by approximately 70 people and with over 100 views on YouTube70 to date.
We strengthened our team while developing an accessible research culture through: (1) interdisciplinary and cross-institutional working to enhance creativity and avoid ‘silo’ studies, (2) supporting staff development and preparing them for their future careers, (3) providing work experience to undergraduate and postgraduate summer interns, (4) sustaining engagement with patient and public representatives.
Critical to our success have been close working with our host institution and a highly engaged Programme Steering Committee (PSC) which we expanded to include an independent researcher from all disciplines within the Programme.
Conclusions
Partner notification is a highly complex intervention with multiple, interacting agents (IP, SP, HCPs) undertaking specific roles and involving actions and interactions which may be emotionally challenging and require behaviour change. This makes it hard to achieve good outcomes. Current clinical practice implicitly prioritises the individual benefits of PN (prevention of reinfection and associated health sequelae) for the IP and their more emotionally connected SPs over the public health benefits of control of infection at population level through PN among ‘one-off’ partners who contribute disproportionately to transmission and are harder to reach.
The impact of PN on reducing transmission is likely to be limited by this narrow focus. There is a need for cost-effective interventions for established SPs and novel interventions which prioritise reaching one-off partners so that both the individual and public health aims of PN are addressed. The intervention development work in Stream C, Phase 3, for example, suggested that intervening ‘up-stream’ by working with communities of MSM to change PN norms and community values provides a complementary focus to working within SHSs. However, long-standing pressures on clinical services, limitations with existing PN outcome measures and models of funding which do not appropriately resource effort with one-off partners will need to be resolved to enable this strategy to be embraced. Over the duration of the Programme, prompted by a greater awareness of antibiotic stewardship, immediate antibiotic treatment for all SPs has shifted to a more nuanced approach in which SPs might be given the option of waiting for their (positive) test result before being prescribed antibiotics. The impact of this is unknown.
The LUSTRUM Programme has addressed some of these challenges. At the same time, it has drawn attention to gaps in research that can improve PN outcomes for one-off and other partnerships where outcomes are particularly poor, and potential for onward STI transmission highest. The process evaluation of APT and the work in Stream 3 identify a need to develop this beyond SHSs.
Recommendations for future research
Research is needed on how to increase the uptake of APT and optimise the reach and uptake of STI and BBV self-sampling in partners managed through APT with a focus on health inequalities. A key focus should be people likely to struggle with self-management, such as those with mild learning disabilities. This can be facilitated by adoption of the RELAY system or equivalent into clinical electronic patient record systems. The use of immediate versus deferred (until test results are known) antibiotic treatment for SPs needs to be explored.
Research is required to identify PN interventions for one-off partners, as IPs rarely chose APT for these partners who are hard to reach by all standard approaches.
Research is required on how to provide real-time or fast feedback on the impact of interventions whose value is not obvious at the level of the practitioner when making a choice on which PN methods to offer. To improve research and normalisation, different levels of feedback (individual, service) may be effective.
Research is required to understand how services can acceptably and effectively use partnership-type information to improve reach for all modalities of PN especially for hard-to-reach groups.
Research is needed to develop and evaluate a system intervention to optimise readiness in MSM for and engagement with PN for bacterial STIs. This should focus on one-off partnerships, address QALYs and partnership types relevant to this population and explore whether more effective PN for bacterial STIs could identify MSM with undiagnosed co-infections such as HIV.
Implications and lessons learnt for health services
-
APT is safe, feasible and effective, and patients using it had a reduction in chlamydia test positivity and partners treated at 4 months. However, it was not taken up by most IPs, and notably very rarely by one-off partners. 8 APT also has health economic advantages. This finding has implications for further research (please see below).
-
APT is well received by sexual health clinic practitioners. However, the invisibility of its impact on outcomes and its need for flexibility within clinic workflow (to accommodate immediate SP remote consultation during the IP’s appointment) limited normalisation within the service. The health economics analysis shows that higher uptake of APT beyond 5% enables further health economic gains. The implication of this is that ongoing efforts will be required to support staff in understanding the value of APT to patients and normalise its offer in services. In the British context, these are likely to include local and national audits, and education of staff on evidence for APT will be required to maximise its reach and effectiveness. This finding also has implications for research.
-
Collection in routine practice of new evidence-based SP types is acceptable, useful to practitioners and feasible in clinical practice both within and without the operation of a trial. 8,10 This was achieved. The implication is that more nuanced partnership type can be collected in routine sexual health clinic practice. This can inform choice and targeting of PN approaches and be used to audit outcomes.
-
The cost effectiveness of APT is reduced but not eliminated by inclusion of syphilis/HIV sampling kits and is increased by an uptake over 5%. The implication is that syphilis and HIV testing should be included, and efforts made to maintain uptake above 5% to maximise cost effectiveness. Together, these ensure parity with the offer to partners who attend in person in accordance with current clinical guidelines,72 and those managed through APT. This finding also has implications for research.
-
People with mild to moderate learning disability without exception found one or more elements of the APT STI and HIV self-sampling and treatment pack challenging or impossible. 18 The implication is that care must be taken when establishing novel pathways such as APT to meet the needs of people with learning disability, whether IPs or partners. The requirements of self-sampling place people with learning disability at particular risk of failing to access services or using tests ineffectively.
-
High sexual activity partners of IPs should be priorities for PN in order to reduce transmission. The implication is that partnerships likely to fall into this group, based on information collected about partnership type,10 should be prioritised for PN using all approaches. This also has implications for research.
-
Time to PN (treatment of partner) did not impact on transmission as much as reaching additional partners. The implication is that priority and efforts to complete PN through any modality should be informed by partnership type, and not by whether swift action can be taken.
-
The development of interventions to improve PN for one-off partnerships among MSM needs to include multiple stakeholders and cannot be limited to innovations within SHSs where capacity for PN is limited. This has implications for research.
-
There is a lack of evidence on all aspects of PN for treatable STIs in MSM, including the contribution of partnership type to outcomes and QALYS for treatable STIs in this population.
Acknowledgements
The LUSTRUM Programme was a true team effort which extended far beyond the core co-investigators. We are hugely appreciative, thankful and humbled by the support, engagement and wide-ranging inputs we have received from everyone who has joined us on our journey.
We would like to thank all of the participants who took part in qualitative interviews and focus group discussions for the partner types’ work, optimising APT, process evaluation and developing PN interventions for MSM and generated such high-quality data. Similarly, to all of the patients and SPs who are included in our trial data set, thank you for enabling us to collect robust data about APT.
We are indebted to every HCP working in our trial sites to deliver the LUSTRUM trial of APT. We know it was not easy, particularly at a time that SHSs are being asked to do more and more, but we also know that as individuals and within your teams you made an incredible difference to many patients and their SPs. Thank you to the PIs of each site: Barking, Havering and Redbridge University Hospitals NHS Trust (Avan Umaipalan), Barts Health NHS Trust (Vanessa Apea), Buckinghamshire Healthcare NHS Trust (Jackie Sherrard), Chelsea and Westminster Hospital NHS Foundation Trust (Ceri Evans), Croydon Health Services NHS Trust (David Phillips), Manchester University NHS Foundation Trust (Gabriel Schembri), Midlands Partnership NHS Foundation Trust (Joti Dhar), NHS Greater Glasgow and Clyde (Rak Nandwani), Northamptonshire Healthcare NHS Foundation Trust (Sophie Herbert), Royal Berkshire NHS Foundation Trust (Fabian Chen), Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust (Kate Schroeder), Solent NHS Trust (Raj Patel), University Hospitals Birmingham NHS Foundation Trust (Jonathon Ross).
A big thank you to our virtual ‘Research voices’ PPIE group for their many and varied inputs from the very start. We have really valued your perspectives, ideas, feedback, critical engagement and commitment to making LUSTRUM a better, more considered and less jargon-loaded study.
LUSTRUM has benefitted from the commitment and clarity provided by the Programme Steering Committee throughout the past 5 years. Your support and guidance with strategic decision-making were critical to our success despite the many uncertainties and unpredictable circumstances this study has weathered. PSC: Simon Barton (chair), Gill Bell, Andrew Copas, David Crundwell, Robbie Currie, Claudia Estcourt, Alison Howarth, Artemis Koukounari, Lynis Lewis, Fiona Mapp, Alec Miners, Emmanuel Rollings-Kamara, Saima Saddiqui, Rachel Shaw, Rebecca Turner, Melvina Woode Owusu.
The PSC also kindly performed the function of the Trial Steering Committee for the duration of the trial and some members were co-opted on to the Data Monitoring Committee along with Oliver Stirrup and Anna Tostevin.
The outstanding research support provided by Noclor on behalf of CNWL has helped with the smooth management of co-investigator institution contracts, finances, aspects relating to sponsorship as well as navigating IRAS, HRA and R&D approvals. Thanks go to Keji Dalemo, Nick Portman, Shantao McQueen-Ryan, Elayitha Mathiaparanam, Rukayat Adewoye, Rameez Subhan, Atote Hameso and in particular Emmanuel Rollings-Kamara and Lynis Lewis for unwavering good humour, top quality problem-solving and the most enjoyable budget meetings we could have asked for! Similarly, we are incredibly grateful to Marc Lawlor at UCL for his pragmatism and knowledge of UCL systems and processes to enable us to get on with the research. We have also been supported by three NIHR Programme managers: Vasileios Kontogiannis, Emma Thomson and Saima Saddiqui who have been responsive to our queries and helped us understand NIHR reporting requirements.
We have been fortunate to work with some brilliantly talented and creative individuals who have helped shape the course of specific LUSTRUM studies as well as our overall look. Thank you to EpiGenesys especially Mike Brown, Shuo Chen, Chrissie Elliott, Chris Murray, and Anthony Nettleship for creating such a good-looking and easy to use website as well as all of your patient explanations of technical details, multiple iterations and last-minute changes on RELAY. Jane Shepherd, you took our somewhat vague ideas for the LUSTRUM logo and turned them into a stunning branding design which has had huge impact from the start. Thanks go to Will Gardner and Bundu media for all of your hard work and quick turnaround on the explainer films and to Florian Nonnenmacher for editing the films for our focus groups. The partner typology graphic was greatly improved through working with Lock Cheung at Diva Creative and our APT packs were enhanced by the z cards from Alpha cards. Thank you also to Sealed envelope and TDL, namely Niamh Sherry, for helping us get the trial up and running successfully.
It is the fantastic group of staff who have really brought LUSTRUM ideas to life whether working short term, in for the long haul or somewhere in between. All of your contributions have come together to make for a wonderfully successful Programme, and it has been a real joy working with you all: Matt Smith for the dating apps study, Karen Pickering for the initial economic evaluation work, Sangita Patel, Morag Whitefield, Lynn McHugh, Kay Musonda and Jacqueline Gray for admin support, Makeda Gerressu, Alan Middleton, Rebecca Laidlow and Tamsin McKinnon for your help with papers and Lucy Cullen for your support with qualitative data collection. Thank you to our ‘friends of the Programme’ Soazig Clifton for a bit of IRAS magic, Jo Gibbs for the many, many informal consults and Pam Sonnenberg for your timely pearls of wisdom. We would also like to thank all of the Programme volunteers, interns and students who have worked with us: Selma Stearns, Astrid Nikiel, Darcey Hookway, Morgan Williamson, Tavishi Kanwar, Krish Patel, Noemie Levy; Amir Palermo, Mario Valencia, Oluwatomilayo Ejedenawe, Stephanie Dankyi, Sally-Rae Attah; Summer A.
Finally thank you to the NIHR for funding this Programme of research.
Contributions of authors
Claudia S Estcourt (https://orcid.org/0000-0001-5523-5630), Professor of Sexual Health & HIV, was the Chief Investigator. She led programme design and development, funding acquisition, governance and overall programme, budget and staff management. She led the RCT, contributed to all studies, conducting data analysis and interpretation, and was responsible for PPIE, stakeholder engagement and management, writing reports and leading/contributing to publications and all dissemination activities across the Programme.
Fiona Mapp (https://orcid.org/0000-0003-0733-6036), Senior Research Fellow, was the trial manager and mixed-methods researcher across the Programme. She co-ordinated all aspect of trial planning, contributed to RELAY design, trial and process evaluation analysis and interpretation, contributed to and co-ordinated the drafting of the final report and other publications across the Programme.
Melvina Woode Owusu (https://orcid.org/0000-0003-2102-3802), Honorary Senior Research Fellow in Behavioural Epidemiology, was the Programme Manager. She supported programme delivery including budget, risk, time and resource management. She contributed to PPIE, stakeholder engagement and management, writing reports and to publications and dissemination activities throughout the programme.
Nicola Low (https://orcid.org/0000-0003-4817-8986), Professor of Epidemiology & Public Health, was a co-investigator. She contributed to programme design and development, funding acquisition, and staff management. She led the mathematical modelling studies, contributed to the development and interpretation of the RCT, and to writing of reports and publications.
Paul Flowers (https://orcid.org/0000-0001-6239-5616), Professor of Health Change, was a co-investigator. He led the design and delivery of the quantitative research and behavioural and implementation science, and contributed to funding acquisition and staff management. He led the intervention development and manualisation of APT, led the process evaluation, and the intervention development and coproduction for PN interventions amongst gay men. He also contributed to writing reports and leading/contributing to publications and dissemination activities across the programme.
Andrew Copas (https://orcid.org/0000-0001-8968-5963), Professor of Trials in Global health, was a co-investigator and the overall statistical lead. He led the statistical aspects of funding acquisition and of the design of the RCT. He oversaw the analysis of the RCT data and contributed to the interpretation of the findings, and the writing of RCT-related publications and the RCT sections of the final report.
Tracy E Roberts (https://orcid.org/0000-0002-0624-0537), Professor of Health Economics, was a co-investigator. She contributed to the overall programme design and development. She led all the Health Economic components (systematic review, data collection, economic analysis and modelling) of the programme, overseeing data analysis and interpretation, and contributing to writing and editing the relevant reports for publications and associated dissemination activities relating to health economics and other sections relating to the delivery of the programme.
Catherine H Mercer (https://orcid.org/0000-0002-4220-5034), Professor of Sexual Health Science, was a co-investigator. She contributed to the overall programme design and development and funding acquisition. She predominantly advised on the work around developing a clinically useful SP classification, including leading the publication resulting from this, as well as contributing to the writing of reports and contributing to other publications resulting from the programme.
John Saunders (https://orcid.org/0000-0001-9658-7798), Senior Clinical Researcher, was a co-investigator and contributed to the overall design of the programme, delivery of the RCT, writing of reports and other publications resulting from the programme, and contributed to dissemination of findings.
Rak Nandwani (https://orcid.org/0000-0002-4611-3786), Consultant Physician and Honorary Clinical Associate Professor in Sexual Health & HIV, was a co-investigator. He contributed to the programme design and development, funding acquisition, governance, data analysis and interpretation, patient and public engagement, writing reports, contributing to publications and dissemination activities, including drafting and review of the final report.
Christian L Althaus (https://orcid.org/0000-0002-5230-6760), Senior Research Fellow in Infectious Disease Modelling, conducted the mathematical modelling and was responsible for writing publications. He contributed to writing Stream B (phase 1 and 2) and Stream C (phase 2) and reviewed the final report.
Oliver Stirrup (https://orcid.org/0000-0002-8705-3281), Research Associate, was the statistician for the RCT. He drafted the statistical analysis plan, based on the Protocol working with the Senior Statistician, and conducted the data analysis for the trial. He contributed to drafting of the methods and results sections regarding the trial for the primary journal publication and the programme grant final report.
Merle Symonds (https://orcid.org/0000-0003-4632-5655), Lead Health Sexual Health Adviser, was a co-investigator and provided clinical advice and support on pathway development, development of webtool, training of participating site staff, management of Research Health Advisers and reviewed the final report.
Alison R Howarth (https://orcid.org/0000-0002-0597-6614), Senior Research Associate in Sexual Health & HIV, was the trial manager (maternity cover). She contributed to data collection and the delivery of the RCT, and reviewed the final report.
Anne Johnson (https://orcid.org/0000-0003-1330-7100), Professor of Infectious Disease Epidemiology, contributed to the overall design of the programme, funding acquisition and reviewed the final report.
Chidubem Okeke Ogwulu (https://orcid.org/0000-0002-8133-7021), Research Fellow in Health Economics, conducted the within-trial economic evaluation, carried out the systematic review of economic studies, prepared the findings for publication including the final report.
Maria Pothoulaki (https://orcid.org/0000-0003-4785-1446), Research Fellow in Health Psychology, contributed to the design of qualitative studies, co-ordinated the implementation of qualitative studies, conducted data collection, data analysis and interpretation, and contributed to dissemination activities, paper and report writing.
Gabriele Vojt (https://orcid.org/0000-0002-9135-0684), Research Associate in Psychology, assisted with qualitative studies. She contributed to the design of research materials, recruitment and data collection and assisted with data analysis and interpretation. and led the analysis and interpretation on the structure of dating apps and contributed to publications and dissemination activities.
Sonali Wayal (https://orcid.org/0000-0002-5878-7665), Honorary Senior Research Fellow at University College London, was a co-investigator. She contributed towards seeking funding and Programme design, and contributed to reviewing publications and report writing.
Susie Brice (https://orcid.org/0000-0001-5096-4041) Senior Research Health Adviser, All East Sexual Health (Barts NHS Trust), led follow-up of patients recruited into the RCT, contributed to the design of the RELAY system, contributed to supporting/training of clinicians in RCT sites and reviewed the final report.
Alex Comer-Schwartz (https://orcid.org/0000-0002-4446-0389), Research Health Adviser, All East Sexual Health (Barts NHS Trust), followed up patients recruited into the RCT, contributed to the design of the RELAY system, contributed to supporting/training of clinicians in RCT sites and reviewed the final report.
Anna Tostevin (https://orcid.org/0000-0002-5520-8496), Data Manager in HIV & Sexual Health, contributed to data management and reviewed the final report.
Eleanor Williams (https://orcid.org/0000-0003-4641-2409), Research Associate in Health Economics, conducted the model-based economic evaluation, and prepared the health economic findings for publication and reviewed the final report.
Sarah Lasoye (https://orcid.org/0000-0002-2408-3555) was Programme Administrator for the LUSTRUM Programme, led web dissemination of findings, contributed to and supported co-ordination of the final report.
Jean McQueen (https://orcid.org/0000-0001-5330-3564). In collaboration with Paul Flowers planned and completed interviews with index patients and partners (Stream B), planned, contributed, analysed data from stakeholder engagement (Stream C), ran focus groups and contributed to data analysis for behaviour change taxonomy, assisted with drafting of publications and reports.
Zainab Abdali (https://orcid.org/0000-0002-2736-5427), Research Fellow in Health Economics, contributed to the systematic review of economic evidence on partner notification of STI in MSM and acted as the second reviewer in the systematic review, contributed to and reviewed the final report.
Jackie A Cassell (https://orcid.org/0000-0003-0777-0385), Professor of Primary Care Epidemiology and Honorary Consultant in Health Protection, was a co-investigator and co-led programme design and development, funding acquisition, governance and overall programme. She contributed to all studies, conducting data analysis and interpretation, writing and reviewing publications and reports and variously leading and contributing to publications and all dissemination activities across the programme.
Publications
Peer-reviewed papers
Mercer CH, Jones KG, Johnson AM, Lewis R, Mitchell KR, Gravningen K, et al. How can we objectively categorise partnership type? A novel classification of population survey data to inform epidemiological research and clinical practice. Sex Transm Infect. 2017;93(2):129–36. http://doi.org/10.1136/sextrans-2016-052646
Saunders J, Mapp F, Wayal S, Pothoulaki M, Estcourt CS. Response to van Aar et al. STIs in sex partners notified for chlamydia exposure: implications for expedited partner therapy. Sex Transm Infect. 2018;94:619–21. http://doi.org/10.1136/sextrans-2017-053364
Estcourt CS, Howarth AR, Copas A, Low N, Mapp F, Owusu MW, et al. Accelerated partner therapy (APT) partner notification for people with Chlamydia trachomatis: protocol for the Limiting Undetected Sexually Transmitted infections to RedUce Morbidity (LUSTRUM) APT cross-over cluster randomised controlled trial. BMJ Open. 2020;10(3):e034806. http://doi.org/10.1136/bmjopen-2019-034806
Middleton A, Pothoulaki M, Owusu MW, Flowers P, Mapp F, Vojt G, et al. How can we make self-sampling packs for sexually transmitted infections and bloodborne viruses more inclusive? A qualitative study with people with mild learning disabilities and low health literacy. Sex Transm Infect. 2021;97(4):276–81. http://doi.org/10.1136/sextrans-2020-054869
Estcourt CS, Flowers P, Cassell JA, Pothoulaki M, Vojt G, Mapp F, et al. Going beyond ‘regular and casual’: development of a classification of sexual partner types to enhance partner notification for STIs. Sex Transm Infect. 2022;98(2):108–14. http://doi.org/10.1136/sextrans-2020-054846
Estcourt CS, Stirrup O, Copas A, Low N, Mapp F, Saunders J, et al. Accelerated partner therapy contact tracing for people with chlamydia (LUSTRUM): a crossover cluster-randomised controlled trial. Lancet Public Health 2022;7(10):e853–65. https://doi.org/10.1016/S2468-2667(22)00204-3
Flowers P, Pothoulaki M, Vojt G, Mapp F, Owusu MW, Estcourt C, et al. Understanding the barriers and facilitators to using self-sampling packs for sexually transmitted infections and blood borne viruses: thematic analyses supporting intervention optimisation. Br J Health Psychol. 2022;28(1):156–73. https://doi.org/10.1111/bjhp.12617
Flowers P, Pothoulaki M, Vojt G, Mapp F, Owusu MW, Estcourt C, et al. Using the behaviour change wheel approach to optimise self-sampling packs for sexually transmitted infection and blood borne viruses. Br J Health Psychol. 2022;27(4):1382–97. https://doi.org/10.1111/bjhp.12607
Howarth AR, Estcourt CS, Ashcroft R, Cassell J. Building an opt-out model for service-level consent in the context of new data regulations. Public Health Ethics 2022;15:175–80. https://doi.org/10.1093/phe/phab030
Wayal S, Estcourt CS, Mercer CH, Saunders J, Low N, McKinnon T, Symonds M, Cassell JA. Optimising partner notification outcomes for bacterial sexually transmitted infections: a deliberative process and consensus, United Kingdom, 2019. Euro Surveill. 2022;27(3):2001895. https://doi.org/10.2807/1560-7917.ES.2022.27.3.2001895
Pre-prints
(Published on pre-print servers and submitted/planned submission to peer reviewed journals)
Vojt G, Smith M, Owusu MW, et al. How do Dating Apps reflect the social organisation of sexual relationships? A review of dating apps and their key features. SocArXiv; 2021. URL: https://osf.io/preprints/socarxiv/wf3vd/
Vojt G, Pothoulaki M, Laidlaw R, Flowers P. What do we know about types of intimate relationship and associated sexual partners – A scoping review of interdisciplinary literature. SocArXiv; 2020. URL: https://osf.io/preprints/socarxiv/fvmqw/
Pothoulaki M, Vojt G, Mapp F, Owusu MW, Mercer CH, Estcourt C, et al. The social organisation of sexual relationships: an exploratory qualitative study. SocArXiv; 2020. URL: https://osf.io/preprints/socarxiv/m4fgb/
Pothoulaki M, Vojt G, Mapp F, Owusu MW, Symonds M, Estcourt C, Flowers P. Accelerated partner therapy: optimising an inter-actional contact tracing intervention to reduce chlamydia reinfection. SocArXiv; 2021. https://doi.org/10.31235/osf.io/zf8y7
Flowers P, Mapp F, McQueen J, The LUSTRUM programme, Estcourt C. Accelerated partner therapy contact tracing intervention for people with chlamydia: the LUSTRUM process evaluation using programme theory. MedRxiv; 2021. https://doi.org/10.1101/2021.08.07.21261736
Althaus CL, Mercer CH, Cassell JA, Estcourt CS, Low N. Investigating the effects of accelerated partner therapy (APT) on chlamydia transmission in Britain: a mathematical modelling study. MedRxiv; 2020. https://doi.org/10.1101/2020.12.07.20245142
Althaus CL, Mercer CH, Cassell JA, Estcourt CS, Low N. Estimating the effects of accelerated partner therapy (APT) on reinfection of index cases with chlamydia: a mathematical modelling study. MedRxiv; 2021. https://doi.org/10.1101/2021.08.11.21261692
Williams EV, Ogwulu CBO, Estcourt CS, Howarth AR, Copas A, Low N, et al. . The cost-effectiveness of accelerated partner therapy (APT) compared to standard contact tracing for people with chlamydia: an economic evaluation based on the LUSTRUM population-based chlamydia transmission model. MedRxiv; 2021. https://doi.org/10.1101/2021.07.27.21261128
Okeke Ogwulu CB, Abdali Z, Williams EV, Estcourt CS, Howarth AR, Copas A, Mapp F, et al. Systematic review of Economic studies of Partner Notification and management interventions for sexually transmitted infections including HIV in men who have sex with men. MedRxiv; 2021. https://doi.org/10.1101/2021.06.08.21258534
McQueen JM, Woode Owusu M, Mapp F, Estcourt CS, Symonds M, Howarth AR, et al. Intervention Development Protocol for a novel, co-produced, sexually transmitted infection partner notification intervention for men who have sex with men. MedRxiv; 2020. https://doi.org/10.1101/2020.10.21.20209049
Flowers P, Gerressu M, McLeod J, McQueen J, Vojt G, Symonds M, et al. . How can we increase notification of exposure to STIs between gay and bisexual men? Intervention development using stakeholder engagement, qualitative methods and behavioural science. MedRxiv; 2021. https://doi.org/10.1101/2021.05.27.21257903
Flowers P, Lasoye S, McQueen J, Woode Owusu M, Symonds M, Estcourt C. Developing a co-produced, systems-informed, sexually transmitted infection contact tracing intervention for gay and bisexual men who have sex with men and their ‘one-off’ sexual partners. MedRxiv; 2021. https://doi.org/10.1101/2021.07.07.21260064
| LUSTRUM output | Link |
|---|---|
| Conference abstracts and plenary presentations | |
| Estcourt CS. British Association of Sexual Health and HIV annual conference 2016: Partner Notification: Aiming higher (and smarter) | www.bashh.org/events-education/conferences/annual-conference-2016/ |
| Estcourt CS. HIV Scotland annual meeting – policy seminar 2017: Partner Notification in the context of HIV: Why is a fresh look needed and why now? | No URL available |
| Vojt G, et al. British Association of Sexual Health and HIV annual conference 2017: How can sexual history taking for sexually transmitted infection partner notification be improved? | https://www.bashh.org/events-education/conferences/annual-conference-2017/ |
| Vojt G, et al. British Association of Sexual Health and HIV annual conference 2017: The Functionality of Dating Applications in Sexual Relationships and Sexual Health | https://www.bashh.org/events-education/conferences/annual-conference-2017/ |
| Pothoulaki M, et al. British Association of Sexual Health and HIV annual conference 2017: The lexicon of love – Understanding types of relationships as primary contexts of STI transmission | https://www.bashh.org/events-education/conferences/annual-conference-2017/ |
| Mapp F, et al. British Association of Sexual Health and HIV annual conference 2017: What makes expedited partner therapy (EPT) and accelerated partner therapy (APT) work for partner notification for bacterial STIs? A systematic review of interventions | https://www.bashh.org/events-education/conferences/annual-conference-2017/ |
| British Association of Sexual Health and HIV annual conference 2017: LUSTRUM breakfast symposium | www.bashh.org/events-education/conferences/annual-conference-2017/ |
| Estcourt C, et al. International Union against Sexually Transmitted Infections annual conference 2018: Going beyond ‘regular’ and ‘casual’: targeting and tailoring PN approaches by partner type | https://dv4.mediasite.com/mediasite/Play/573f10d8e27c4c57bfbbee98139292c01d?catalog=d89afbadab6142908b8fc3766cd595d721 |
| Estcourt C, et al. International Union against Sexually Transmitted Infections annual conference 2018: Effectiveness and cost-effectiveness of Accelerated Partner Therapy partner notification for people with Chlamydia trachomatis infection: A Randomised Controlled Trial in the LUSTRUM Programme | https://www.morressier.com/o/event/5aea03d31dd164001d5ef1b3/article/5af060631dd164001d5ef375 |
| Pothoulaki M, et al. Society for Social Medicine annual scientific meeting 2018: Towards understanding the ‘partner’ in partner notification for sexually transmitted infection healthcare: moving beyond the dichotomy of ‘regular’ and ‘casual’ partners | http://doi.org/10.1136/jech-2018-SSMabstracts.31 |
| Howarth et al. British Association of Sexual Health and HIV annual conference 2019: Squaring the circle in the GDPR era: How can we inform patients about non-consented use of their data for research? Achieving good practice in the LUSTRUM chlamydia partner notification RCT | https://doi.org/10.1177/0956462419853210 |
| Middleton A, et al. International Union against Sexually Transmitted Infections annual conference 2019: How can we maximise the accessibility of STI/BBV self-sampling for people with mild learning disabilities? | www.conference-expert.eu/en/iusti2019/en/abstract-book/1/O-26 |
| Flowers P, et al. STI&HIV World Congress 2019: Improving ‘home-based’ STI/HIV self-sampling and boosting sample return rates | http://doi.org/10.1136/sextrans-2019-sti.253 |
| Flowers P, et al. STI&HIV World Congress 2019: Using theory and evidence to optimise an accelerated partner therapy intervention in the context of a chlamydia partner notification trial | http://doi.org/10.1136/sextrans-2019-sti.276 |
| Wayal S, et al. STI&HIV World Congress 2019: Developing partner notification outcomes for bacterial sexually transmitted infections by sex-partner type: international perspectives | http://doi.org/10.1136/sextrans-2019-sti.384 |
| Althaus C, et al. STI&HIV World Congress 2019: Investigating the effects of accelerated partner therapy on chlamydia transmission in Britain: a mathematical modelling study | http://doi.org/10.1136/sextrans-2019-sti.277 |
| Mapp F, et al. British Association of Sexual Health and HIV annual conference 2020: P143 Using programme theory to evaluate complex sexual health interventions: evidence from the process evaluation of the LUSTRUM trial of accelerated partner therapy (APT) | https://doi.org/10.1177/0956462420967532 |
| Estcourt CS. British Association of Sexual Health and HIV annual conference 2020 Harrison lecture: STI partner notification in the modern age | www.bashh.org/events-education/conferences/virtual-annual-conference-2020/ |
| Estcourt CS. Joint Australasian HIV & AIDS and Sexual Health Conference 2020: eSexual Health – where do we go from here? | No URL available |
| Estcourt CS. STI&HIV World Congress 2021: O18.2 Does Accelerated partner therapy improve partner notification outcomes for people with chlamydia? The LUSTRUM cluster crossover randomised control trial | http://doi.org/10.1136/sextrans-2021-sti.153 |
| Estcourt CS. STI&HIV World Congress 2021: O18.3 Characteristics and outcomes of people who used Accelerated Partner Therapy for chlamydia in the LUSTRUM cluster crossover randomised control trial | http://doi.org/10.1136/sextrans-2021-sti.154 |
| Mapp F, et al. STI&HIV World Congress 2021: P281 Explaining experiences of Accelerated Partner Therapy partner notification for people with chlamydia in the LUSTRUM randomised control trial: Process evaluation | http://doi.org/10.1136/sextrans-2021-sti.348 |
| Films (YouTube Channel: LUSTRUM PROGRAMME) | |
| LUSTRUM APT teaser | www.youtube.com/watch?v=471U0Wydv58 |
| APT explainer for HCPs | www.youtube.com/watch?v=3k1MgLGdOGU |
| APT explainer for the public | www.youtube.com/watch?v=Z0_oSlfl7y0 |
| 60 second APT explainer | www.youtube.com/watch?v=rGQH0Yzymzk |
| 30 second APT explainer | www.youtube.com/watch?v=ZlYGkzBXeEg |
| LUSTRUM APT teaser | www.youtube.com/watch?v=471U0Wydv58 |
| LUSTRUM test and treat: welcome | www.youtube.com/watch?v=YO1igKmfJaY |
| LUSTRUM test and treat: how to take your urine sample | www.youtube.com/watch?v=q8hIW7yBkD8 |
| LUSTRUM test and treat: how to take your vulvo-vaginal swab sample | www.youtube.com/watch?v=DE-4m0vW2KA |
| LUSTRUM test and treat: how to take your blood sample | www.youtube.com/watch?v=oJL9zR8K6dc |
| LUSTRUM re-test: welcome | www.youtube.com/watch?v=xcPEUDOE5-g |
| LUSTRUM re-test: how to take your urine sample | www.youtube.com/watch?v=_Cq9zTEWJF0 |
| LUSTRUM re-test: how to take your vulvo-vaginal swab sample | www.youtube.com/watch?v=PL39k9dsyIg |
| Webinar–COVID-19: New Frontiers in Contact-Tracing | www.youtube.com/watch?v=rpZ7XPVR6aU |
| Webinar–Trial methodology: Pushing the boundaries in cluster RCTs | www.youtube.com/watch?v=YV5ikIi0FoM |
| LUSTRUM: Improving partner notification with Accelerated Partner Therapy | www.youtube.com/watch?v=mVLLih8XbSA |
Data-sharing statement
All trial data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Qualitative study data generated are not suitable for sharing beyond that contained within the report. Further information can be obtained from the corresponding author.
Ethical approval
Ethical approval for the trial was provided by London – Chelsea Research Ethics Committee (18/LO/0773) and approved us to seek consent for trial participation from lead clinicians at participating clinics (service-level) rather than seeking individual informed consent from IPs other than for the process evaluation studies.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the Programme Grants for Applied Research Programme or the Department of Health and Social Care.
References
- London School of Hygiene and Tropical Medicine . Safetxt Study n.d. https://safetxt.lshtm.ac.uk/ (accessed 12 August 2021).
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9.
- Erens B, Phelps A, Clifton S, Mercer CH, Tanton C, Hussey D, et al. Methodology of the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Sex Transm Infect 2014;90:84-9.
- Vojt G, Smith M, Owusu MW, Mapp F, Pothoulaki M, Estcourt C, et al. How do Dating Apps reflect the social organisation of sexual relationships? A review of dating apps and their key features. SocArXiv 2021. https://osf.io/preprints/socarxiv/wf3vd/ (accessed 12 August 2021).
- Vojt G, Pothoulaki M, Laidlaw R, Flowers P. What do we know about types of intimate relationship and associated sexual partners – a scoping review of interdisciplinary literature. SocArXiv 2020. https://osf.io/preprints/socarxiv/fvmqw/ (accessed 12 August 2021).
- Pothoulaki M, Vojt G, Mapp F, Owusu MW, Mercer C, Estcourt C, et al. The social organisation of sexual relationships: an exploratory qualitative study. SocArXiv 2020. https://osf.io/preprints/socarxiv/m4fgb/ (accessed 12 August 2021).
- BASHH . British Association for Sexual Health and HIV 2021. https://bashh.org/ (accessed 12 August 2021).
- Estcourt CS, Stirrup O, Copas A, Low N, Mapp F, Saunders J, et al. Accelerated partner therapy contact tracing for people with chlamydia (LUSTRUM): a crossover cluster-randomised controlled trial. Lancet Public Health 2022;7:e853-65. https://doi.org/10.1016/S2468-2667(22)00204-3.
- Estcourt CS, Howarth AR, Copas A, Low N, Mapp F, Owusu MW, et al. Accelerated partner therapy (APT) partner notification for people with Chlamydia trachomatis: protocol for the Limiting Undetected Sexually Transmitted infections to RedUce Morbidity (LUSTRUM) APT cross-over cluster randomised controlled trial. BMJ Open 2020;10.
- Estcourt CS, Flowers P, Cassell JA, Pothoulaki M, Vojt G, Mapp F, et al. Going beyond ‘regular and casual’: development of a classification of sexual partner types to enhance partner notification for STIs. Sex Transm Infect 2022;98:108-14.
- Mercer CH, Jones KG, Johnson AM, Lewis R, Mitchell KR, Gravningen K, et al. How can we objectively categorise partnership type? A novel classification of population survey data to inform epidemiological research and clinical practice. Sex Transm Infect 2017;93:129-36. http://doi.org/10.1136/sextrans-2016-052646.
- Simon W, Gagnon JH. Sexual scripts: permanence and change. Arch Sex Behav 1986;15:97-120.
- Pothoulaki M, Vojt G, Mapp F, Owusu MW, Symonds M, Estcourt C, et al. Accelerated Partner Therapy: optimising an inter-actional contact tracing intervention to reduce chlamydia reinfection. SocArXiv 2021. https://osf.io/preprints/socarxiv/zf8y7/ (accessed 12 August 2021).
- Mapp F, Pothoulaki M, Flowers P, Vojt G, Woode Owusu M, Wayal S, et al. Implementation of expedited partner therapy (EPT) interventions for sexually transmitted infections (STIs): a systematic review. PROSPERO 2016. https://crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016051178&ID=CRD42016051178 (accessed 12 August 2021).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8:1-11.
- Middleton A, Pothoulaki M, Owusu MW, Flowers P, Mapp F, Vojt G, et al. How can we make self-sampling packs for sexually transmitted infections and bloodborne viruses more inclusive? A qualitative study with people with mild learning disabilities and low health literacy. Sex Transm Infect 2021;97:276-81.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. London, UK: Silverback Publishing; 2014.
- Estcourt C, Sutcliffe L, Cassell J, Mercer CH, Copas A, James L, et al. Can we improve partner notification rates through expedited partner therapy in the UK? Findings from an exploratory trial of Accelerated Partner Therapy (APT). Sex Transm Infect 2012;88:21-6.
- Estcourt CS, Sutcliffe LJ, Copas A, Mercer CH, Roberts TE, Jackson LJ, et al. Developing and testing accelerated partner therapy for partner notification for people with genital Chlamydia trachomatis diagnosed in primary care: a pilot randomised controlled trial. Sex Transm Infect 2015;91:548-54.
- LUSTRUM Programme . 60 Second Overview of APT 2018. https://youtube.com/watch?v=rGQH0Yzymzk (accessed 12 August 2021).
- The Caldicott Committee . Report on the Review of Patient-Identifiable Information 1997.
- Weijer C, Grimshaw JM, Taljaard M, Binik A, Boruch R, Brehaut JC, et al. Ethical issues posed by cluster randomized trials in health research. Trials 2011;12.
- Cassell J, Young A. Why we should not seek individual informed consent for participation in health services research. J Med Ethics 2002;28:313-7.
- Campbell MK, Weijer C, Goldstein CE, Edwards SJL. Do doctors have a duty to take part in pragmatic randomised trials?. BMJ 2017;357.
- Howarth A, Nandwani R, Ashcroft R, Rosen D, Mapp F, Estcourt C, et al. Building an opt-out model for service-level consent in the context of new data regulations. Press Public Health Ethics 2021. https://rps.ucl.ac.uk/viewobject.html?id=1863507&cid=1 (accessed 12 August 2021).
- Public Health England . GUMCAD STI Surveillance System n.d. https://gov.uk/guidance/gumcad-sti-surveillance-system#history (accessed 12 August 2021).
- Flowers P, Mapp F, McQueen J, Nandwani R, Estcourt C. The LUSTRUM programme . Accelerated Partner Therapy contact tracing intervention for people with chlamydia: the LUSTRUM process evaluation using programme theory. MedRxiv 2021. https://medrxiv.org/content/10.1101/2021.08.07.21261736v1 (accessed 12 August 2021).
- World Health Organization . Sexually Transmitted Infections (STIs) Fact Sheet 2021. https://who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed 12 August 2021).
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford, UK: Oxford University Press; 2015.
- Lanjouw E, Ouburg S, de Vries H, Stary A, Radcliffe K, Unemo M. 2015 European guideline on the management of Chlamydia trachomatis infections. Int J STD AIDS 2015;27:333-48. https://doi.org/101177/0956462415618837.
- Roberts TE, Robinson S, Barton PM, Bryan S, McCarthy A, Macleod J, et al. Cost effectiveness of home based population screening for Chlamydia trachomatis in the UK: economic evaluation of chlamydia screening studies (ClaSS) project. BMJ 2007;335.
- Roberts TE, Robinson S, Barton P, Bryan S, Low N. Chlamydia Screening Studies (ClaSS) Group . Screening for Chlamydia trachomatis: a systematic review of the economic evaluations and modelling. Sex Transm Infect 2006;82:193-200.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Kent, UK: Personal Social Services Research Unit, University of Kent; 2019.
- Roberts TE, Tsourapas A, Sutcliffe L, Cassell J, Estcourt C. Is Accelerated Partner Therapy (APT) a cost-effective alternative to routine patient referral partner notification in the UK? Preliminary cost–consequence analysis of an exploratory trial. Sex Transm Infect 2012;88:16-20.
- Estcourt C, Sutcliffe L, Mercer C, Copas A, Saunders J, Roberts T, et al. The Ballseye programme: a mixed-methods programme of research in traditional sexual health and alternative community settings to improve the sexual health of men in the UK. Programme Grants Appl Res 2016;4.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ 2013;346.
- StataCorp LP . Stata Statistical Software: Release 15.0 2017. https://stata.com/products/ (accessed 12 August 2021).
- Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford, UK: Oxford University Press; 2017.
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford, UK: Oxford University Press; 2011.
- Low N, Heijne JCM, Herzog SA, Althaus CL. Reinfection by untreated partners of people treated for Chlamydia trachomatis and Neisseria gonorrhoeae: mathematical modelling study. Sex Transm Infect 2014;90:254-6.
- Althaus C, Heijne J, Herzog S, Roellin A, Low N. Individual and population level effects of partner notification for Chlamydia trachomatis. PLOS ONE 2012;7.
- Althaus CL, Mercer CH, Cassell JA, Estcourt CS, Low N. Investigating the effects of accelerated partner therapy (APT) on chlamydia transmission in Britain: a mathematical modelling study. MedRxiv 2020. https://medrxiv.org/content/10.1101/2020.12.07.20245142v1 (accessed 12 August 2021).
- Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Survey of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013;382:1795-806.
- Williams E, Okeke Ogwulu C, Estcourt C, Low N, Althaus C, Mapp F, et al. The cost-effectiveness of accelerated partner therapy (APT) compared to standard partner notification (PN) for people with Chlamydia trachomatis: an economic evaluation based on the LUSTRUM population-based chlamydia transmission model. MedRXiv 2021. https://medrxiv.org/content/10.1101/2021.07.27.21261128v1 (accessed 12 August 2021).
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2008.
- Moher D, Liberati A, Tetzlaff J, Altman D. The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6.
- Booth A, Fry-Smith A. Health Technology Assessment (HTA) Information Resources. Bethsseda, MD: National Information Center on Health Services and Health Care Technology; 2003.
- EndNote . EndNote. Clarivate Analytics 2021. https://endnote.com/ (accessed 12 August 21).
- Roberts T, Henderson J, Mugford M, Bricker L, Neilson J, Garcia J. Antenatal ultrasound screening for fetal abnormalities: a systematic review of studies of cost and cost effectiveness. BJOG An Int J Obstet Gynaecol 2002;109:44-56.
- Ogwulu C, Jackson L, Kinghorn P, Roberts T. A systematic review of the techniques used to value temporary health states. Value Heal 2017;20:1180-97.
- Nichols BE, Götz HM, van Gorp ECM, Verbon A, Rokx C, Boucher CAB, et al. Partner notification for reduction of HIV-1 transmission and related costs among men who have sex with men: a mathematical modeling study. PLOS ONE 2015;10.
- McCormack S, Dunn D, Desai M, Dolling D, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53-60.
- Golden MR, Whittington WLH, Handsfield HH, Hughes JP, Stamm WE, Hogben M, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005;352:676-85.
- Golden MR, Hughes JP, Brewer DD, Holmes KK, Whittington WLH, Hogben M, et al. Evaluation of a population-based program of expedited partner therapy for gonorrhea and chlamydial infection. Sex Transm Dis 2007;34:598-603.
- Golden MR, Kerani RP, Stenger M, Hughes JP, Aubin M, Malinski C, et al. Uptake and population-level impact of expedited partner therapy (EPT) on Chlamydia trachomatis and Neisseria gonorrhoeae: the Washington State community-level randomized trial of EPT. PLOS Med 2015;12.
- Jenness S, Weiss K, Goodreau S, Gift T, Chesson H, Hoover K, et al. Incidence of Gonorrhea and Chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis 2017;65:712-8.
- Kasaie P, Schumacher CM, Jennings JM, Berry SA, Tuddenham SA, Shah MS, et al. Gonorrhoea and Chlamydia diagnosis as an entry point for HIV pre-exposure prophylaxis: a modelling study. BMJ Open 2019;9.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions. Medical Research Council; 2006.
- Duncan E, O’Cathain A, Rousseau N, Croot L, Sworn K, Turner KM, et al. Guidance for reporting intervention development studies in health research (GUIDED): an evidence-based consensus study. BMJ Open 2020;10.
- McQueen JM, Owusu MW, Mapp F, Estcourt CS, Symonds M, Howarth AR, et al. Intervention Development Protocol for a novel, co-produced, sexually transmitted infection partner notification intervention for men who have sex with men. MedRxiv 2020. https://medrxiv.org/content/10.1101/2020.10.21.20209049v1 (accessed 12 August 2021).
- Rutter H, Savona N, Glonti K, Bibby J, Cummins S, Finegood DT, et al. The need for a complex systems model of evidence for public health. Lancet 2017;390:2602-4.
- Flowers P, Gerressu M, McLeod J, McQueen J, Vojt G, Symonds M, et al. How can we increase notification of exposure to STIs between gay and bisexual men?: intervention development using stakeholder engagement, qualitative methods and behavioural science. MedRxiv 2021. https://medrxiv.org/content/10.1101/2021.05.27.21257903v1 (accessed 12 August 2021).
- The EMIS Network . The European Men-Who-Have-Sex-With-Men Internet Survey. Key Findings from 50 Countries 2019.
- Robertson R, Wenzel L, Thompson J, Charles A. Understanding NHS Financial Pressures: How Are They Affecting Patient Care?. London, England: The King’s Fund; 2017.
- The LUSTRUM Programme . The LUSTRUM Research Programme 2016. www.lustrum.org.uk (accessed 12 August 2021).
- The LUSTRUM Programme . LUSTRUM Programme YouTube Channel n.d. https://youtube.com/channel/UCBV8smLmkOQVT9D0OR-md1g/featured (accessed 12 August 2021).
- Wayal S, Estcourt C, Mercer C, Saunders J, Low N, McKinnon T, et al. Optimising Partner Notification Outcomes for Bacterial Sexually Transmitted Infections – A Deliberative Process and Consensus 2021. https://discovery.ucl.ac.uk/id/eprint/10137905/ (accessed 12 August 2021).
- Estcourt C, Cassell J, Gibbs J, Woode Owusu M, Saunders J, Mapp F, et al. BASHH Guidance for the Design of Self-Sampling Packs and Associated Support for Self-Sampling Processes Within Sexually Transmitted Infection and Blood Borne Virus Testing 2021. https://bashh.org/news/news/postal-self-sampling-kits-guidelines-and-report/ (accessed 12 August 2021).
- STI self-sampling working group on behalf of BASHH and LUSTRUM . BASHH Recommendations for Self-Sampling Processes 16.02.21 2021. https://bashh.org/news/news/postal-self-sampling-kits-guidelines-and-report/ (accessed 12 August 2021).
- Coleman H, Samuel I, Soni S, Murchie M, Clarke M, Williams A, et al. BASHH Summary Guidance on Testing for Sexually Transmitted Infections, 2023. British Association for Sexual Health and HIV n.d. www.bashhguidelines.org (accessed 3 March 2024).
Appendix
| Sensitivity analyses | Control | Intervention without APT | Intervention with APT |
|---|---|---|---|
| Total cost | Total cost | Total cost | |
| 1. Pay band/grade of the staff for the initial consultation | 74.30 | 78.47 | 89.70 |
| 2. Duration of the initial consultation | 71.26 | 74.83 | 86.83 |
| 3. Follow-up calls | 87.42 | 90.92 | 112.94 |
Previous published content
LUSTRUM published papers (peer-reviewed journals and pre-prints).
This list of papers covers all of the research work within the LUSTRUM Programme, arranged by workstream.
| Study | Short title | Lead author | Citation (with URL/DOI hyperlink) | |
|---|---|---|---|---|
| Stream A | Phase 1: partner types classification | Natsal-3 partner types | Cath Mercer | Mercer CH, Jones KG, Johnson AM, Lewis R, Mitchell KR, Gravningen K, et al. How can we objectively categorise partnership type? A novel classification of population survey data to inform epidemiological research and clinical practice. Sex Transm Infect 2017;93(2):129–36. |
| Dating apps review | Gaby Vojt | Vojt G, Smith M, Owusu MW, Mapp F, Pothoulaki M, Estcourt C, Flowers P. How do Dating Apps reflect the social organisation of sexual relationships? A review of dating apps and their key features. SocArXiv 2021 | ||
| Partnership types review | Gaby Vojt | Vojt G, Pothoulaki M, Laidlaw R, Flowers P. What do we know about types of intimate relationship and associated sexual partners – a scoping review of interdisciplinary literature. SocArXiv 2020. | ||
| Qualitative work – social organisation of sexual relationships | Maria Pothoulaki | Pothoulaki M, Vojt G, Mapp F, Owusu MW, Mercer C, Estcourt C, et al. The social organisation of sexual relationships: an exploratory qualitative study. SocArXiv 2020. | ||
| LUSTRUM partner typology | Claudia Estcourt | Estcourt CS, Flowers P, Cassell JA, Pothoulaki M, Vojt G, Mapp F, et al. Going beyond ‘regular and casual’: development of a classification of sexual partner types to enhance partner notification for STIs. Sex Transm Infect 2021;0:1–7. | ||
| Phase 2: optimising APT | APT/EPT/PDPT review | Fiona Mapp | Mapp F, Pothoulaki M, Flowers P, Vojt G, Woode Owusu M, Wayal S, et al. Implementation of expedited partner therapy (EPT) interventions for sexually transmitted infections (STIs): a systematic review. PROSPERO 2016 [cited 2020 Aug 14];CRD4201605. | |
| Qualitative work – APT packs | Paul Flowers | Flowers P, Pothoulaki M, Vojt G, Mapp F, Owusu MW, Estcourt C, et al. Understanding the barriers and facilitators to using self-sampling packs for sexually transmitted infections and blood borne viruses: thematic analyses supporting intervention optimisation. MedRxiv 2020 | ||
| BCW analysis of APT | Paul Flowers | Flowers P, Pothoulaki M, Vojt G, Mapp F, Owusu MW, Estcourt C, et al. Using the behaviour change wheel approach to optimise self-sampling packs for sexually transmitted infection and blood borne viruses. MedRxiv 2021 | ||
| Inclusive self-sampling | Alan Middleton | Middleton A, Pothoulaki M, Owusu MW, Flowers P, Mapp F, Vojt G, et al. How can we make self-sampling packs for sexually transmitted infections and bloodborne viruses more inclusive? A qualitative study with people with mild learning disabilities and low health literacy. Sex Transm Infect 2021;97(4):276–81. | ||
| Intervention manual development – optimising APT | Maria Pothoulaki | Pothoulaki M, Vojt G, Mapp F, Owusu MW, Symonds M, Estcourt C, et al. Accelerated Partner Therapy: optimising an inter-actional contact tracing intervention to reduce chlamydia reinfection. SocArXiv 2021 | ||
| Phase 3: APT trial | Trial protocol | Claudia Estcourt | Estcourt CS, Howarth AR, Copas A, Low N, Mapp F, Owusu MW, et al. Accelerated partner therapy (APT) partner notification for people with Chlamydia trachomatis: protocol for the Limiting Undetected Sexually Transmitted infections to RedUce Morbidity (LUSTRUM) APT cross-over cluster randomised controlled trial. BMJ Open 2020;10(3):e034806 | |
| Main trial results including cost- consequence analysis | Claudia Estcourt | Estcourt CS, Copas A, Low N, Mapp F, Stirrup O, et al.; The LUSTRUM research Programme. Accelerated partner therapy contact tracing for people with chlamydia: The LUSTRUM cluster cross-over randomised controlled trial. MedRxiv 2021 | ||
| Process evaluation | Paul Flowers | Flowers P, Mapp F, McQueen J, Nandwani R, programme TL, Estcourt C. Accelerated Partner Therapy contact tracing intervention for people with chlamydia: the LUSTRUM process evaluation using programme theory. medRxiv 2021;2021.08.07.21261736 | ||
| GDPR and service-level consent | Alison Howarth | Howarth A, Nandwani R, Ashcroft R, Rosen D, Mapp F, Estcourt C, et al. Building an opt-out model for service-level consent in the context of new data regulations. Press Public Health Ethics 2021 | ||
| Stream B | Phase 1: modelling the effects of APT on chlamydia transmission | Chlamydia transmission model | Christian Althaus | Althaus CL, Mercer CH, Cassell JA, Estcourt CS, Low N. Investigating the effects of accelerated partner therapy (APT) on chlamydia transmission in Britain: a mathematical modelling study. medRxiv. 2020;2020.12.07.20245142 |
| Phase 2: modelling reinfection with chlamydia after standard PN and APT | Chlamydia reinfection model | Christian Althaus | Althaus CL, Mercer CH, Cassell JA, Estcourt CS, Low N. Estimating the effects of accelerated partner therapy (APT) on reinfection of index cases with chlamydia: a mathematical modelling study. medRxiv. 2021.08.11.21261692 | |
| Phase 3: cost-effectiveness analysis of APT vs. standard PN | Long-term cost- effectiveness MOA and QALYs | Eleanor Williams | Williams EV, Ogwulu CBO, Estcourt CS, Howarth AR, Copas A, Low N, et al. The cost-effectiveness of accelerated partner therapy (APT) compared to standard contact tracing for people with chlamydia: an economic evaluation based on the LUSTRUM population-based chlamydia transmission model. MedRxiv. 2021 | |
| Stream C | Phase 1: identifying and evaluating existing economic studies about PN and/or testing and treatment for STIs/HIV | Economics PN review | Chidebum Okeke Ogwulu | Okeke Ogwulu CB, Abdali Z, Williams EV, Estcourt CS, Howarth AR, Copas A, et al. Systematic review of Economic studies of Partner Notification and management interventions for sexually transmitted infections including HIV in men who have sex with men. MedRxiv. 2021 |
| Phase3: investigating PN for bacterial STI to increase detection of undiagnosed HIV in MSM | Intervention development protocol | Jean McQueen | McQueen JM, Woode Owusu M, Mapp F, Estcourt CS, Symonds M, Howarth AR, et al.. Intervention Development Protocol for a novel, co-produced, sexually transmitted infection partner notification intervention for men who have sex with men. MedRxiv. 2020 | |
| Phase 3: optimising APT and developing a novel PN intervention amongst MSM | Barriers and facilitators to PN for MSM | Paul Flowers | Flowers P, Gerressu M, McLeod J, McQueen J, Vojt G, Symonds M, et al. How can we increase notification of exposure to STIs between gay and bisexual men?: intervention development using stakeholder engagement, qualitative methods and behavioural science. medRxiv. 2021. 2021.05.27.21257903. | |
| Intervention development | Paul Flowers | Flowers P, Lasoye S, McQueen J, Owusu MW, Symonds M, Estcourt C. Developing a co-produced, systems-informed, sexually transmitted infection contact tracing intervention for gay and bisexual men who have sex with men and their ‘one-off’ sexual partners. medRxiv. 2021;2021.07.07.21260064 | ||
| PN outcomes | Sonali Wayal | Cassell J, Wayal S, Estcourt C, Mercer C, Saunders J, Low N, et al. Optimising partner notification outcomes for bacterial sexually transmitted infections – a deliberative process and consensus, United Kingdom, 2019. Euro Surveill. 2022;27(3):pii=2001895 | ||
| Response to van Aar et al. 25.01.2018 | John Saunders | Saunders J, Mapp F, Wayal S, Pothoulaki M, Estcourt CS. Response to van Aar et al. STIs in sex partners notified for chlamydia exposure: implications for expedited partner therapy. Sex Transm Infect 2018;94:619–21 |
List of abbreviations
- aOR
- adjusted odds ratio
- APEASE
- acceptability, practicability, effectiveness, affordability, side-effects, equity
- apps
- dating applications
- APT
- accelerated partner therapy
- BASHH
- British Association for Sexual Health and HIV
- BBV
- blood-borne virus
- BCT
- behaviour change technique
- CCA
- cost-consequence analysis
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- EPT
- expedited partner therapy
- GBMSM
- gay and bisexual men who have sex with men
- GDPR
- General Data Protection Regulations
- HCP
- healthcare professional
- HIV
- human immunodeficiency virus
- ICER
- incremental cost-effectiveness ratio
- IP
- index patient
- LUSTRUM
- Limiting Undetected Sexually Transmitted infections to RedUce Morbidity
- MOA
- major outcomes averted
- MSM
- men who have sex with men
- Natsal
- National Survey of Sexual Attitudes and Lifestyles
- NGO
- non-governmental organisation
- NGU
- non-gonococcal urethritis
- NHS
- National Health Service
- NIHR
- National Institute for Health and Care Research
- PID
- pelvic inflammatory disease
- PN
- partner notification
- PPIE
- patient and public involvement and engagement
- PrEP
- pre-exposure prophylaxis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RELAY
- a clinical management and partner notification data collection system
- RHA
- research health adviser
- SHS
- sexual health service
- SP
- sex partner
- STI
- sexually transmitted infection
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/TRQW3886).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
