Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1211-20012. The contractual start date was in May 2014. The final report began editorial review in March 2020 and was accepted for publication in August 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Price et al. This work was produced by Price et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Price et al.
SYNOPSIS
Content and changes during the programme
The programme had two work packages (WPs) with matching objectives.
Work package 1
The aim was to determine the content, clinical effectiveness and cost-effectiveness of an enhanced Paramedic Acute Stroke Treatment Assessment (PASTA) trial to facilitate emergency stroke treatment.
The objectives were to:
-
develop an enhanced paramedic role for assessment of patients with acute stroke symptoms by a review of relevant literature and qualitative assessment of factors influencing the role from public and professional perspectives
-
examine the paramedic intervention by a cluster randomised trial of cost-effectiveness and qualitative process evaluation of professional and public experiences
-
report a within-trial economic evaluation of the enhanced role compared with standard care.
Work package 2
The aim was to determine the clinical effectiveness, costs, cost-effectiveness and affordability of delivering intra-arterial therapy (IAT) for acute ischaemic stroke patients in England.
The objectives were to:
-
develop a conceptual model of potential care pathways for IAT patients across NHS services, including pre-hospital, secondary and tertiary care settings
-
convert the conceptual model into a mathematical model, identify the evidence for parameterising key decision points and estimate outcomes
-
understand patient, public and relevant professional groups’ views on possible IAT service designs
-
estimate the effectiveness, incremental cost per quality-adjusted life-year (QALY) and other outcomes from an NHS and societal perspective of a national IAT service for stroke
-
develop an implementation plan for IAT in English stroke services that optimises access.
During the programme, there were a number of changes made in response to emerging evidence for treatment effectiveness, evolution of clinical services and challenges for trial recruitment. These are summarised below.
Changes to work package 1
-
The original proposal included a short phase to test the feasibility of delivering the intervention and data collection in a clinical setting. However, because of the logistical and training challenges created by designing and delivering a separate pilot study within part of a participating ambulance service, the objectives were incorporated into the main clinical trial as an internal pilot phase. This was approved by the Trial Steering Committee (TSC). Recruitment began in December 2015 and the pre-set pilot criteria were confirmed by the TSC as achieved in April 2016.
-
During the main PASTA trial phase examining the cost-effectiveness of an enhanced ambulance stroke pathway, there were delays in training sufficient numbers of intervention paramedics to achieve the planned recruitment target. In April 2017, it was agreed with the funder that the primary outcome should change from a health outcome [i.e. 3-month modified Rankin Scale (mRS) score] to a process outcome (i.e. administration of thrombolysis), as the latter measured the intended impact of the intervention, but required fewer patients to show a clinically important impact. The original sample size of 3640 patients was replaced by a new estimate of 1297. A major amendment was approved by NHS Ethics in October 2017.
-
During the parallel process evaluation, it quickly became evident that patients who had recently experience acute stroke were unable to provide views on the PASTA intervention that might inform its acceptability. After discussion with the Programme Steering Committee, it was agreed that attempts to identify patients for this purpose should cease, and notification was given to NHS Ethics.
Changes to work package 2
There were no significant changes to the planned model purpose or development, but the following aspects were altered in response to events outside the programme:
-
The systematic review of IAT effectiveness also included a trial sequential analysis to understand the impact of the most recent trials. 1–3
-
NHS England issued guidance4 for commissioners during the programme (featuring early work undertaken in the programme), which pre-empted part of the intended dissemination activity. The dissemination focus changed to providing commissioners with directly relevant information about choices for their area via an online configurable tool: Interface for Thrombectomy Economic Modelling and outcomeS in stroke (ITEMS).
These changes were agreed by the Programme Steering Committee. There were no implications for research permissions.
Background
Stroke is the single largest cause of adult disability and the third leading cause of death in England, but outcomes are significantly improved when patients are quickly admitted to specialist care for time-critical treatments and multidisciplinary care. 5,6 Acute stroke management across the NHS improved substantially following the publication of a National Stroke Strategy in 2007 and National Institute for Health and Care Excellence (NICE) guidance in 2008,7,8 but emergency provision of the only licensed emergency drug treatment has remained variable and below aspirational targets. Known as ‘intravenous thrombolysis’, effective treatment requires administration of intravenous recombinant tissue plasminogen activator to selected ischaemic stroke cases within 4.5 hours of symptom onset, thereby promoting breakdown of any thrombus responsible for a sudden reduction in cerebral blood flow. Earlier treatment is more likely to reduce future dependency, but there is also a 3% risk of deterioration as a result of symptomatic intracranial haemorrhage. 9 As well as requiring rapid assessment, individual patients must be carefully selected based on a combination of clinical and brain-imaging information, which provides an indication of their potential to benefit from treatment.
At a service level, thrombolysis delivery is challenging because both brain imaging and specialist assessment must be rapidly available to confirm treatment eligibility, achieve optimal treatment outcomes and avoid harm. Despite wide dissemination of the National Strategy, NICE guidelines and corresponding national clinical guidelines,10 and significant reorganisation of services in some regions,11,12 the national audit has continued to show large variations in the rate and speed of thrombolysis delivery between services and diurnal variations within services. 6,13 At the start of the programme in 2014, only 11% of total stroke admissions in the NHS were being treated against an aspirational target of 20%, with a median door-to-treatment time of 54 minutes, despite a target of < 40 minutes. 6 This largely remained unchanged by the end of the programme in 2019, implying that further improvements are unlikely to be achieved by focusing solely on the process of care delivery within hospitals. Even if stroke patients are unsuitable for thrombolysis after rapid processing, they might still benefit from other aspects of early specialist management to avoid complications, such as intravenous blood pressure lowering or reversal of anticoagulation medication, to reduce the risk of intracerebral haematoma expansion. 10
Ambulance stroke assessment
The NHS ambulance assessment of suspected stroke patients consists of initial symptom recognition using the Face, Arm, Speech Test (FAST),14 exclusion of hypoglycaemia and urgent transfer with pre-notification to the nearest hyperacute stroke unit (HASU) if onset is believed to be within 4 hours. 10 Local pathway variations exist according to the location of the specialist HASU, but the role of the paramedic has fundamentally remained the same for 15 years. Despite proximity to the patient and audit data showing scope for improvement in overall service delivery, the pre-hospital content of the emergency stroke pathway and related training has not been further optimised for thrombolysis decision-making. There have been reports that additional pre-hospital-phase interventions can facilitate thrombolysis treatment, including multiprofessional workforce training,15 raising the service priority level for suspected stroke16 and personalised feedback to paramedics about care quality. 17 However, studies were setting specific and/or observational, and generally described short-term improvements in thrombolysis-naive services.
In other specialties, evidence is increasing that imposing a structure on interactions within multidisciplinary teams at specific points along a clinical pathway has a major bearing on the efficiency of care delivery. Simple tools can standardise communication of key information and confirm whether or not essential tasks have been undertaken, including structured formats for paramedic handover to emergency department (ED) staff18,19 and multidisciplinary care process checklists for pre- or post-care delivery. 20,21 Enhanced handover and team checklists might, therefore, be valuable during the specific scenario of assessment for thrombolysis eligibility, as well as improving access to other stroke treatments and organised stroke care.
In view of the potential for ambulance personnel to play a more significant role during the initial assessment of suspected stroke patients who may be suitable for thrombolysis, WP1 developed and evaluated an enhanced PASTA pathway.
Intra-arterial thrombectomy
Although clinical services were seeking effective implementation of thrombolysis provision, an evidence base was rapidly developing for a powerful additional treatment suitable for selected patients with moderate to severe ischaemic stroke as a result of large artery occlusion (LAO), known as IAT. Although thrombolysis reduces long-term disability, restoration of cerebral blood flow occurs in only 50% of patients and in only 10% with LAO,22 thereby limiting its effectiveness. During IAT, an arterial catheter is guided into the cerebral circulation by a trained interventionist to extract the thrombus directly, thereby achieving greater success in restoring the blood supply. To reduce disability, this must also be performed as soon as possible, usually within 6 hours of symptom onset and following initial treatment with thrombolysis. 23,24
As the IAT procedure requires interventionists and facilities currently only available at regional neuroscience centres, the clinical pathway is more complicated than thrombolysis and the majority of IAT-eligible patients require rapid secondary transfer to the centre following initial assessment at a local HASU. 25,26 Advanced symptom checklists for ambulance personnel have been developed in an attempt to identify patients who are more likely to have LAO for selective redirection, but, so far, these have not shown acceptable levels of accuracy for widespread clinical deployment. 27,28
At the start of this programme, in 2014, there was no commissioned provision of IAT across > 120 HASUs. Critical issues were still unclear, including how many patients were suitable for IAT based on emerging trial data, the optimal configuration of HASU and IAT centres, and stakeholder preferences regarding the inevitable trade-off between possible health gains at a central site relative to additional travel distance and displacement. This information was essential for services and commissioners to prepare for IAT implementation, but required presentation in a format facilitating comparison of options in a local service context. Hence, WP2 sought to determine the clinical effectiveness, costs, cost-effectiveness and affordability of delivering IAT for acute ischaemic stroke patients.
Work package 1
Development of the Paramedic Acute Stroke Treatment Assessment
Material throughout this section has been reproduced with permission from Flynn et al. 29 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Systematic literature review
The completed review has been published. 29
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-compliant protocol30 was registered online as PROSPERO CRD42014010785.
To develop an enhanced paramedic assessment capable of supporting hospital thrombolysis of appropriately selected stroke patients, it was first necessary to understand challenges and successes reported during pre-hospital roles for any condition with known time-sensitive outcomes (i.e. trauma, myocardial infarction and stroke).
Therefore, an electronic search of published literature (from January 1990 to September 2016) was conducted across eight bibliographic databases that focused on (1) generic or specific structured handovers between ambulance and hospital personnel and (2) paramedic-initiated care processes at handover or post handover.
A narrative review of 36 studies shortlisted at the full-text stage indicated that (1) enhanced paramedic skills might supplement handover information as there would be a greater shared understanding of what information is important for the receiving medical team; (2) structured handover tools and feedback on performance can improve clinical communication during emergency transfer of patient care; and (3) enhanced paramedic roles following arrival at hospital were limited to ‘direct transportation’ of patients to imaging/specialist care facilities, and there were no examples of paramedics continuing to assist with patient care after handover. No descriptions were identified for pre-hospital thrombolysis-specific information collection tools or stroke-specific handover formats, but the review provided general support for the development of an enhanced paramedic role containing these elements.
Stakeholder engagement
To develop an intervention that was likely to be acceptable and feasible within UK ambulance and stroke services, relevant stakeholders were formally engaged in the design process. Fifteen focus groups and interviews were undertaken over the first 12 months of the programme in north-east England, north-west England and Wales, involving patient representatives (n = 20) and health-care professionals (n = 103), comprising paramedics and ED and HASU clinicians. 31 Digital recordings were transcribed, anonymised and analysed using open and then focused coding with constant comparison. 32,33 During four iterative rounds of data collection, themes were developed to understand barriers to and facilitators of the adoption of the paramedic intervention and the developing enhanced role/PASTA pathway material was amended accordingly.
In summary, paramedics, hospital clinicians and patients welcomed the use of enhanced skills during pre-hospital stroke assessment. Paramedics believed that they were capable of undertaking more detailed clinical assessments aimed at thrombolysis eligibility, but were unsure if their experience and skills would be recognised by hospital teams. Both professional groups strongly supported the use of a standardised handover format to enable new skills to be more effective and minimise the possibility of thrombolysis potential not being recognised by hospital triage staff receiving the patient. To encourage a joint working approach, there was general support for paramedics providing reminders about key time targets for brain imaging and treatment administration during handover.
All participants were uncertain about the feasibility of paramedics spending extra time in the hospital to assist the clinical team because of the wider implications for ambulance service response times. However, as there could be times when few hospital staff were available for initial care processes post handover, they agreed that it may be beneficial to assist with practical tasks as part of the intervention for up to 15 minutes (the standard service target interval between ambulance handover and departure). There was no system in place for paramedics to routinely receive feedback about their assessment process, but all professional groups were interested in the evidence showing that simple individual feedback could improve pre-hospital stroke care quality,17 and were enthusiastic for this to be incorporated.
During the fourth round of interviews, there were no additional changes to the proposed PASTA pathway or concerns from public representatives, and the Programme Steering Committee agreed that WP1 should progress towards delivery of the main trial.
The Paramedic Acute Stroke Treatment Assessment intervention
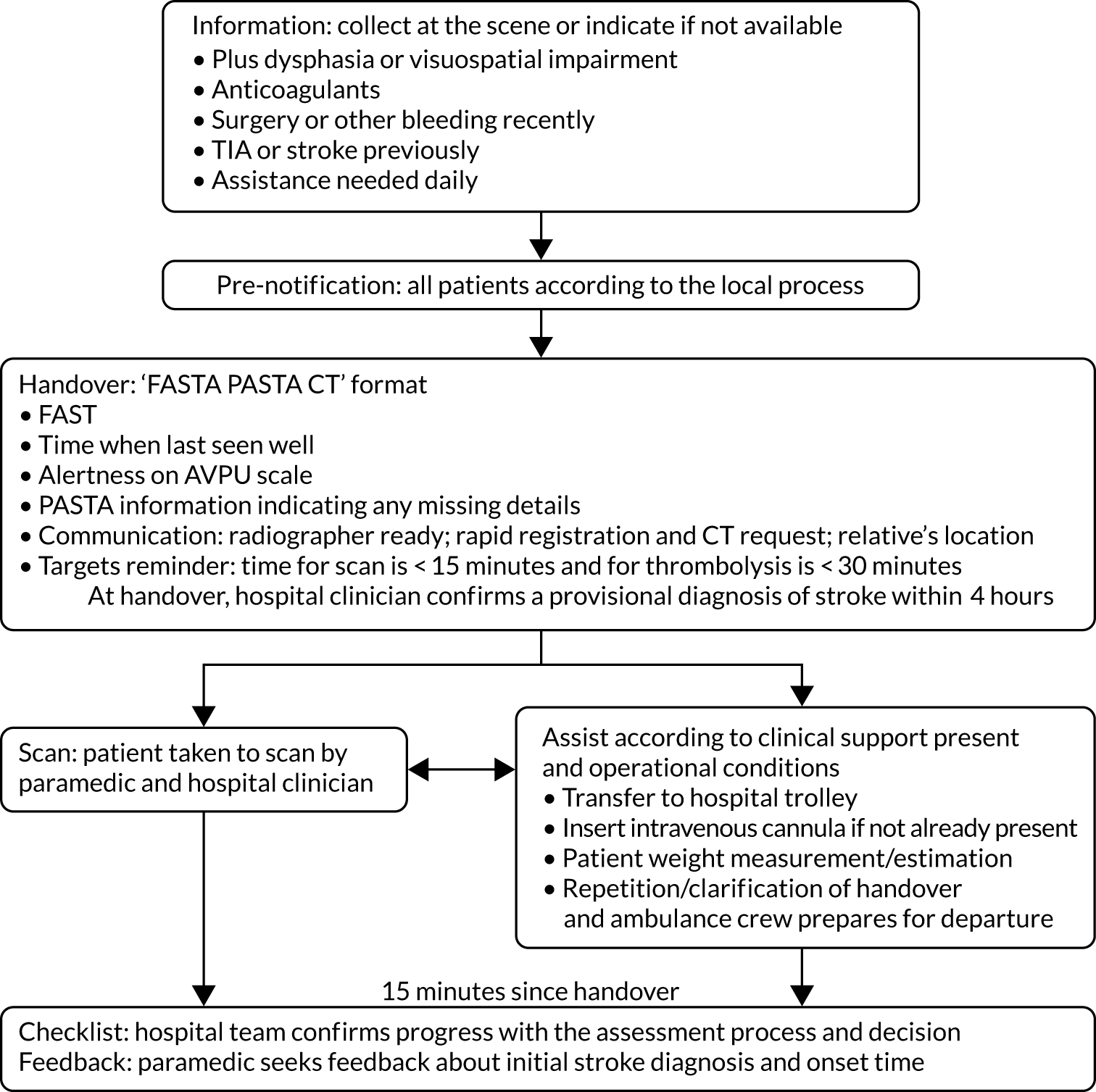
The PASTA pathway consisted of the following components (Figure 1):
-
Information – the paramedic seeks additional information at the scene, which is routinely considered during thrombolysis treatment decisions but typically is not obtained until after hospital admission (e.g. prescription of anticoagulant medication).
-
Pre-notification – although this is an expected component of standard care, the PASTA paramedics were specifically reminded to pre-alert the destination hospital.
-
Handover – on arrival at the hospital, the paramedic provided a standardised handover of stroke-specific details to the hospital team, including FAST, onset time, patient alertness as measured using the Alert, Verbal, Pain, Unresponsive (AVPU) scale34 and PASTA information.
-
Scan – if the computed tomography (CT) scanner was immediately available, the paramedic assisted with patient transfer to radiology.
-
Assist – up until 15 minutes after arrival, the paramedic undertook the following tasks as required: insertion of an intravenous cannula, obtaining the patient’s weight and repetition/clarification of clinical information for the arriving stroke team members.
-
Checklist – at 15 minutes after handover, the paramedic asked a member of the hospital team to confirm progress with key tasks (e.g. status of the scan request).
-
Feedback – the paramedic requested feedback from a hospital clinician about the accuracy of their provisional stroke diagnosis and onset time estimation.
FIGURE 1.
The PASTA pathway intervention. FASTA PASTA CT, Face, Arm, Speech, Time, Alertness Plus Anticoagulants Surgery TIA Assistance Communication Targets; TIA, transient ischaemic attack.
Examination of the Paramedic Acute Stroke Treatment Assessment pathway intervention clinical effectiveness: a cluster randomised trial
The published study protocol35 and the main study report36 have been published.
Material throughout this section has been reproduced with permission from Price et al. 35 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Material throughout this section has been reproduced with permission from Flynn et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 1.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/1.0. The text below includes minor additions and formatting changes to the original text.
The PASTA trial objectives were to:
-
determine whether or not the PASTA pathway increased the proportion of patients receiving thrombolysis (the primary outcome)
-
describe the impact of the PASTA pathway on key time intervals during delivery of care
-
describe the number and subsequent diagnoses of suspected stroke patients who travelled to the hospital with a study paramedic but, following assessment at hospital, were not given a diagnosis of stroke (‘stroke mimics’).
Methods
A pragmatic multicentre cluster randomised controlled trial (RCT) design was chosen to reduce contamination of standard care by the intervention and avoid potential delays in care due to individual randomisation. Ethics approval was granted by the National Research Ethics Committee North East – Newcastle and North Tyneside 1 (reference 15/NE/0309).
The study was hosted by three ambulance services (i.e. north-east England, north-west England and Wales) with similar clinical pathways for standard care that reflected national clinical guidelines. 10 These served 15 study hospital sites. Important acute activity and workforce characteristics are shown in Appendix 1, Table 6. Clusters were individual paramedics based within pre-randomised ambulance stations stratified by service, size and distance of station from the nearest study hospital. Paramedics who were based at stations randomised to the PASTA trial only became involved following successful completion of study-specific training (i.e. an online video and knowledge assessment). Paramedics based at standard care stations were simply informed that their clinical record entries would be supporting a study of pre-hospital assessment. Patients received the PASTA intervention or standard care according to which paramedic attended to them. Participating hospitals were not randomised and received both PASTA and standard care patients.
Participants
Patients were identified and recruited after completion of the thrombolysis assessment in participating hospitals if the following criteria were met:
-
they travelled to hospital with a study paramedic
-
they were aged ≥ 18 years
-
they received a diagnosis of stroke from a hospital specialist
-
they were within 4 hours of stroke onset (onset time determined by the hospital stroke team) when assessed by the study paramedic.
Intervention
Trained paramedics were requested to provide the PASTA pathway intervention (see Figure 1) to patients who they suspected were suffering a stroke and were within 4 hours of symptom onset. Initial paramedic stroke identification processes were unchanged. A study-specific ambulance data collection form was completed to record delivery of the different PASTA components. The patients attended by paramedics who were randomised to the standard care group received routine assessment and treatment. 10
Outcome measures
The primary outcome was the proportion of patients receiving thrombolysis. The secondary outcomes included key time intervals during assessment and thrombolysis treatment, stroke severity 24 hours after thrombolysis [as measured on the National Institutes of Health Stroke Scale (NIHSS)],37 delivery of other components of acute care, day 90 death or dependency (mRS) score38,39 and complications after thrombolysis. 40
Statistical analysis
Based on the effects reported by previous studies and our eligibility criteria, the sample size estimation considered that a change from 43% to 53% of study-eligible patients receiving thrombolysis would be clinically important. At 90% power, a 5% significance, an average cluster size (patients per paramedic) of five patients, an intracluster correlation coefficient of 0.02, an imbalance of two control patients per intervention patient (reflecting delays in the PASTA training uptake) and attrition of 1%, it was calculated that 1297 patients were required (standard care, n = 865; PASTA intervention, n = 432). However, the study protocol allowed for the final recruitment target to be kept under review and adjusted to reflect any changes in the underlying assumptions. The final required number of patients was 1149 based on a cluster size of three and a standard care-to-PASTA group imbalance of 8 : 5 patients.
Analysis was by ‘treatment allocated’ (i.e. the study group allocation of the station base for the attending paramedic). Imputation was used for missing NIHSS scores and day 90 mRS scores.
The primary analysis used logistic regression allowing for clustering by paramedic, with adjustment for clinically important and statistically significant covariates and factors to estimate an adjusted odds ratio (aOR) for the proportion of all patients receiving thrombolysis. The mRS was dichotomised into ‘favourable outcome’ (mRS score 0–2) or ‘poor outcome’ (mRS score 3–6) and an aOR of a ‘poor outcome’ was calculated. Other comparisons used odds ratios (ORs) by logistic regression and t-tests as appropriate. Cox proportional hazard regression estimated a hazard ratio for the combined impact of the intervention on thrombolysis and time to treatment since the emergency call.
A post hoc analysis considered whether or not routine hospital specialist availability for thrombolysis decision-making had any bearing on the treatment received in each study group. Workforce information reported in the 2016 Sentinel Stroke National Audit Programme (SSNAP) Acute Organisational Audit6 was used to categorise hospitals as compliant or non-compliant with the current national standard regarding hospital provision of a specialist thrombolysis service [i.e. there should be a minimum of six specialists trained in emergency stroke care providing a continuous rota without input from non-specialists, so that all treatment decisions are made by a stroke specialist (see Appendix 1, Table 6)].
Results
At 62 PASTA stations, 453 of 817 (55%) paramedics completed training. At 59 standard care stations, 700 of 723 (97%) paramedics agreed to assist. Between 10 December 2015 and 31 July 2018, 11,478 stroke patients travelling by ambulance were screened, 1391 fulfilled the eligibility criteria and were approached, and 1214 patients were enrolled. Of these, 500 were assessed by 242 PASTA paramedics (2.1 patients per paramedic) and 714 were assessed by 355 standard care paramedics (2.0 patients per paramedic). The follow-up is shown as per Consolidated Standards of Reporting Trials (CONSORT) reporting recommendations in Figure 2. Primary outcome data were available for all patients.
FIGURE 2.
Trial profile. a, Eight paramedics allocated to standard care completed the PASTA training during the study.

Demographic and clinical characteristics were very similar in the two study groups for all patients (see Appendix 1, Table 7). The mean age was 74.7 [standard deviation (SD) 13.2] years, women comprised 48% of the patient group and the median/mean admission NIHSS score was 9.0/11.4. Appendix 1, Table 8, shows the demographics and clinical characteristics according to the study group and receipt of thrombolysis.
The PASTA paramedics took an average of 13.4 minutes longer [95% confidence interval (CI) 9.4 to 17.4 minutes longer; p < 0.001] than the standard care paramedics to complete patient care episodes (i.e. ‘clear’ a patient), mainly because an additional 8.8 minutes was spent in the hospital (95% CI 6.5 to 11.0 additional minutes; p < 0.001). There was no significant difference between the groups for paramedic time spent on scene (PASTA intervention, 26.0 minutes; standard care, 24.2 minutes; difference 1.61 minutes, 95% CI –0.2 to 3.4; p = 0.08). There was no evidence of other differences between time intervals (see Appendix 1, Table 9).
There was no significant difference in the proportion of patients who received thrombolysis (Table 1) in the PASTA [197/500 (39.4%)] and standard care groups [319/714 (44.7%)], but there was a possible trend in the opposite direction to that of the anticipated intervention effect (aOR 0.81, 95% CI 0.61 to 1.08; p = 0.15; intracluster correlation coefficient 0.00). Among thrombolysis-treated patients, a PASTA paramedic assessment to assign a patient to thrombolysis was longer by an average of 8.5 minutes (95% CI 2.1 to 13.9 minutes longer; p = 0.01) than that of a standard care paramedic. The Cox regression analysis of time from the 999 call to treatment for the PASTA intervention group compared with standard care group reported an adjusted hazard ratio of 0.85 (95% CI 0.71 to 1.02; coefficient –0.17; p = 0.07), indicating that thrombolysis in the PASTA intervention group was less likely at any time point after the start of the emergency care pathway. After thrombolysis, there were no significant differences evident between groups for reduction in stroke severity or any treatment complication, but the number of events was small (see Table 1). No evidence for significant differences was observed for other individual acute care processes delivered to all patients (see Appendix 1, Table 10).
| Group | Analysis | |||
|---|---|---|---|---|
| PASTA intervention | Standard care | Unadjusted OR | Adjusted OR | |
| Thrombolysis treatment, n/N (%) | ||||
| All patients | 197/500 (39.4) | 319/714 (44.7) | 0.81 (95% CI 0.64 to 1.02; p = 0.07) | 0.81 (95% CI 0.61 to 1.08; p = 0.15) |
| Ischaemic stroke only | 196a/409 (47.9) | 319/607 (52.6) | 0.83 (95% CI 0.65 to 1.07; p = 0.15) | 0.84 (95% CI 0.60 to 1.17; p = 0.30) |
| Times | N = 197 | N = 319 | Difference in mean PASTA minus standard care | |
| Onset to treatment time (minutes) | ||||
| Mean (SD) | 154.4 (55.3) | 149.9 (51.7) | 4.47 (95% CI –4.97 to 13.93; p = 0.35) | – |
| Median (IQR) | 146 (110–194) | 137 (110–190) | – | |
| Paramedic assessment to treatment time (minutes) | N = 194 | N = 315 | ||
| Mean (SD) | 98.1 (37.6) | 89.6 (31.1) | 8.50 (95% CI 2.10 to 14.80; p = 0.01) | – |
| Median (IQR) | 90 (72–114) | 86 (68–107) | – | |
| Hospital arrival to treatment time (minutes) | N = 176 | N = 286 | ||
| Mean (SD) | 58.9 (33.4) | 54.2 (26.9) | 4.69 (95% CI –1.20 to 10.55; p = 0.12) | – |
| Median (IQR) | 48.5 (35–75) | 48.5 (36–65) | – | |
| Stroke severity (NIHSS) | ||||
| After treatment (24–48 hours) | N = 193 | N = 307 | ||
| Mean (SD) | 8.5 (9.0) | 9.6 (9.3) | –1.12 (95% CI –2.7 to 0.54; p = 0.19) | – |
| Median (IQR) | 5 (1–14) | 6 (2–15) | – | |
| Reduction after treatment | ||||
| Mean (SD) | 3.7 (6.5) | 2.8 (7.2) | 0.90 (95% CI –0.35 to 2.2; p = 0.16) | – |
| Median (IQR) | 4 (0–7) | 3 (0–7) | – | |
| Complications, n (%) | N = 196 | N = 319 | ||
| Symptomatic intracranial haemorrhage | 4 (2.0) | 10 (3.1) | 0.64 (95% CI 0.20 to 2.10; p = 0.46) | – |
| Extracranial haemorrhage | 6 (3.1) | 6 (1.9) | 1.65 (95% CI 0.52 to 5.20; p = 0.39) | – |
| Angiooedema | 2 (1.0) | 7 (2.2) | 0.46 (95% CI 0.10 to 2.24; p = 0.32) | – |
| Other complication | 2 (1.0) | 1 (0.3) | 3.30 (95% CI 0.30 to 36.40; p = 0.56) | – |
| Any complication | 13 (6.6) | 24 (7.5) | 0.87 (95% CI 0.43 to 1.76; p = 0.70) | – |
At day 90, there was no significant difference between groups for mortality [the PASTA intervention, 140/499 (28.1%); standard care, 199/712 (27.9%); OR 1.00 (95% CI 0.78 to 1.30); p = 0.97)]. Figure 3 shows the distribution of mRS score values at day 90. Although the CIs were wide enough to include clinically important differences favouring either group, there was an unexpected non-significant trend towards fewer poor outcomes (i.e. a mRS score ≥ 3) at day 90 among the PASTA intervention patients [PASTA intervention, 313/489 (64.0%); standard care, 461/690 (66.8%); aOR 0.86 (95% CI 0.60 to 1.2); p = 0.39], which was also seen among those who received thrombolysis [PASTA intervention, 108/193 (56.0%); standard care, 191/312 (61.2%); aOR 0.78 (95% CI 0.47 to 1.30); p = 0.34].
FIGURE 3.
Distribution of day 90 mRS scores for all patients.
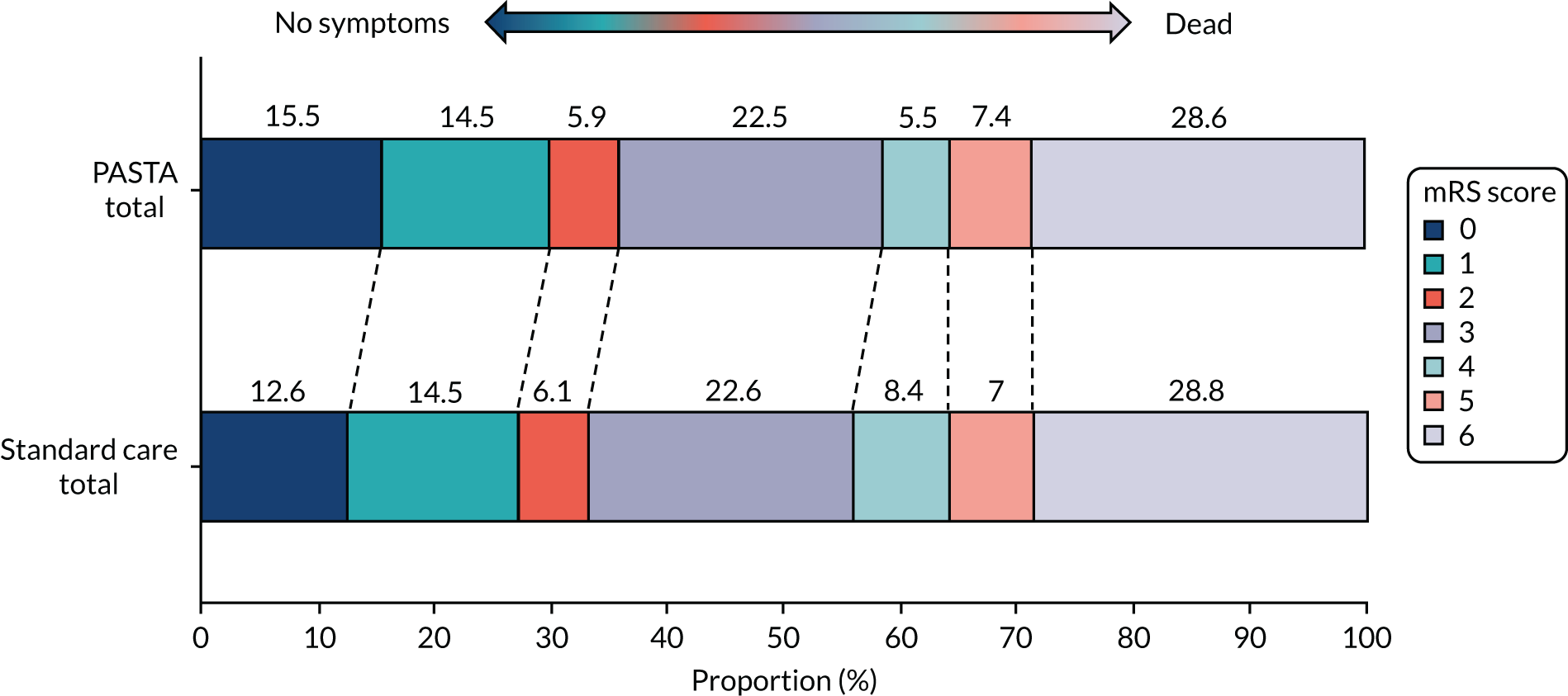
Similar day 90 mRS score distributions for thrombolysis and non-thrombolysis groups are shown in Appendix 1, Figures 14 and 15. The serious adverse events were recorded from 81 PASTA patients (16%; total of 94 events) and 136 standard care patients (19%; total of 161 events). None of the serious adverse events had a causal link to the study intervention.
Eight hospitals were not fully compliant with the national standard for local specialist availability (see Appendix 1, Table 6). In the post hoc analysis, these non-compliant services showed a statistically significant 9.8% absolute reduction in the PASTA thrombolysis treatment rate compared with the standard care thrombolysis treatment rate [PASTA intervention, 99/276 (35.9%); standard care, 105/230 (45.7%); unadjusted OR 0.67 (95% CI 0.47 to 0.95); p = 0.03], whereas there was no difference at the seven compliant hospitals [PASTA intervention, 98/224 (43.8%); standard care, 214/484 (44.2%); unadjusted OR 0.98 (95% CI 0.71 to 1.35); p = 0.91].
Study-specific ambulance data collection forms recording delivery of the PASTA pathway were located for 227 out of 500 (45.5%) intervention patients. Use of the structured handover was recorded for 59.0% and use of the individual components of the checklist ranged from 57.3% to 90.3%. Full data are shown in Appendix 1, Table 11.
Although patients with symptoms mimicking stroke were not enrolled in the trial, hospital research support staff recorded that 1596 such patients were transferred by a study paramedic. The most common non-stroke diagnoses were transient ischaemic attack (TIA) (34.5%), headache/neurological (13.1%), epilepsy/seizure (10.3%), infection/sepsis (9.0%), syncope/circulation (6.1%), functional (4.1%), brain imaging diagnosis [e.g. tumour (3.5%)] and metabolic disturbances (3.3%).
Discussion
This multisite pragmatic trial showed that a paramedic-initiated thrombolysis-focused emergency stroke assessment that extended beyond hospital handover did not increase thrombolysis rates. Instead, there was a trend towards less thrombolysis administration. Although there was a longer initial paramedic assessment process, it is unlikely that the PASTA intervention resulted in patients simply ‘timing out’ of treatment as this was, proportionally, a minor extension of the whole emergency pathway, and the Cox regression analysis indicated that intervention thrombolysis was probably less likely at any time point since the emergency call.
It may be surprising that these results show that the PASTA pathway did not improve thrombolysis delivery when simpler pre-hospital interventions have increased treatment rates (e.g. raising the ambulance priority level for suspected stroke)16 and reduced hospital treatment delays (e.g. pre-notification),41 but the service context of each report is likely to be relevant. Previously, additional thrombolysis activity was observed at four out of six US centres following a multilevel intervention comprising public awareness activities, a paramedic symptom checklist and competitive benchmarking. 15 The two unchanged centres had high baseline treatment rates and may have already achieved optimal performance. A similar ceiling effect may explain the lack of effect among the PASTA sites, which were already established thrombolysis providers. A multisite Scandinavian trial16 randomised 942 suspected stroke/TIA patients to a higher and standard response level after multidisciplinary training. The study reported a thrombolysis rate of 24%, compared with 10% among controls. Like the PASTA intervention, there was no significant change in door-to-needle time, suggesting that delays following admission relate to logistical factors such as scan capacity, image reporting and specialist availability.
Despite the intervention group showing a surprising trend towards fewer thrombolysis treatments, outcomes were not adversely affected and there was a counter-intuitive trend towards better health. If indicative of a genuine effect, one possible hypothesis is an influence on case selection, that is structured communication of directly relevant and timely information by the PASTA paramedics might increase clinician confidence about withholding treatment when there is borderline benefit, higher than average risk or uncertainty about key details, such as onset time. The post hoc analysis showed that intervention group thrombolysis was significantly less likely across services with specialist availability below the level recommended by national guidelines. 6 Relatively inexperienced clinicians under time pressure may tend towards overtreatment rather than undertreatment of borderline cases, which could be moderated by the PASTA handover and/or checklist, whereas services with greater specialist continuity may already apply a more systematic approach to case selection. Being an unexpected finding, we had not collected the required data describing clinical and radiological quality of individual treatment decisions to confirm this hypothesis. However, previous ED studies have reported that, typically, less than half of pertinent items of information are shared during standard handover of mixed patient groups,42 with significant variation due to the level of experience of the clinicians involved. 18,43 The relevance and clarity of handover can be improved by introduction of simple generic formats19,29 while multidisciplinary team checklists make care safer through clarification of information and reinforcement of important standards. 20,21
The distribution of mimic conditions observed was typical of previous ambulance studies and clinical reports,44 providing reassurance that the suspected stroke cohort underpinning the trial was representative of the wider clinical population.
Examination of the Paramedic Acute Stroke Treatment Assessment pathway intervention cost-effectiveness
The main study report has been published. 45
Material throughout this section has been reproduced with permission from Bhattarai et al. 45 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
A within-trial health economic analysis estimated the cost-effectiveness of the PASTA intervention, compared with standard care, over 90 days of follow-up. As the trial post hoc analysis showed that thrombolysis varied according to specialist availability, a sensitivity analysis was undertaken to determine if costs and cost-effectiveness varied by site-level specialist availability. The economic evaluation was reported following best practice guidelines conforming to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS).
Methods
Outcomes were QALYs and cost per participant reported in 2017/18 Great British pounds from an NHS and social service perspective. QALYs were based on health utility scores generated from mapping discharge and day 90 mRS scores to EuroQol-5 Dimensions, three-level version, values. 46 These were converted into QALYs using the area under the curve method, controlling for pre-stroke disability, age and sex. Costs were derived from prospectively captured resource utilisation data, including paramedic time, acute medical treatments, bed-days, post-discharge rehabilitation, social services involvement (paid carers at home and in social care settings) and hospital re-admissions. Standard unit costs were used in calculations. 47,48 As the time horizon was 90 days, discounting of costs and outcomes was not required.
A complete-case data set and an imputed data set were analysed. Missing cost and utility data were imputed using predictive mean matching within the multiple imputation generated by chained equations. Generalised linear model regressions with gamma family link function estimated marginal costs while controlling for age, sex and pre-stroke disability clustered by site. 49 Stochastic sensitivity analysis used non-parametric bootstrapping to quantify and explore the impact of statistical imprecision surrounding the point estimates of costs, QALYs and cost-effectiveness. The likelihood that the PASTA intervention would be cost-effective, compared with standard care, was reported over a range of willingness-to-pay (WTP) values.
Results
The unadjusted differences for complete-case mean mRS scores, utility, QALYs and total cost estimates between the PASTA intervention and standard care are shown in Appendix 2, Table 12. Over the 90-day follow-up period, there was no evidence of QALY differences between groups in either complete-case (0.007, 95% CI –0.003 to 0.018) or imputed data (0.005, 95% CI –0.004 to 0.015) (Table 2). There were lower total costs in the PASTA intervention group for both complete-case (–£1473, 95% CI –£2736 to –£219) and imputed data sets (–£1086, 95% CI –£2236 to –£13).
| Outcome measure | Data, mean (95% CI) | |||
|---|---|---|---|---|
| Complete case | Imputation | |||
| Standard care | PASTA intervention | Standard care | PASTA intervention | |
| QALYs | 0.100 (0.093 to 0.108) | 0.108 (0.099 to 0.116) | 0.104 (0.097 to 0.110) | 0.109 (0.102 to 0.117) |
| ΔQALY | 0.007 (–0.003 to 0.018) | 0.005 (–0.004 to 0.015) | ||
| Total costs (£) | 13,103 (12,292 to 14,019) | 11,630 (10,702 to 12,586) | 13,106 (12,421 to 13,904) | 12,019 (11,223 to 12,865) |
| ΔTotal costs (£) | –1473 (–2736 to –219) | –1086 (–2236 to –13) | ||
| ICER (ΔCost/ΔQALY) | Dominant | Dominant | ||
| Probability of being cost-effective at £20,000 WTP for a QALY (%) | 1 | 99 | 1.9 | 98.1 |
| Probability of being cost-effective at £30,000 WTP for a QALY (%) | 0.3 | 99.7 | 1.7 | 98.3 |
Detailed descriptive costs from the complete-case data set are shown in Appendix 2, Table 13. Although there was a greater mean cost for paramedic training time and longer patient episode duration in the PASTA intervention group than in the standard care group, there were savings from less use of thrombolysis medication, reductions in length of hospital stay and reductions in the duration of rehabilitation and provision of social care support. The costs for other acute stroke treatments were slightly higher among the PASTA patients.
A plot of bootstrapped differences in mean costs and QALYs for the imputed data set showed that for most iterations (91.3%), the PASTA intervention was less costly and more effective than standard care (Figure 4).
FIGURE 4.
Cost-effectiveness plane for imputed data.
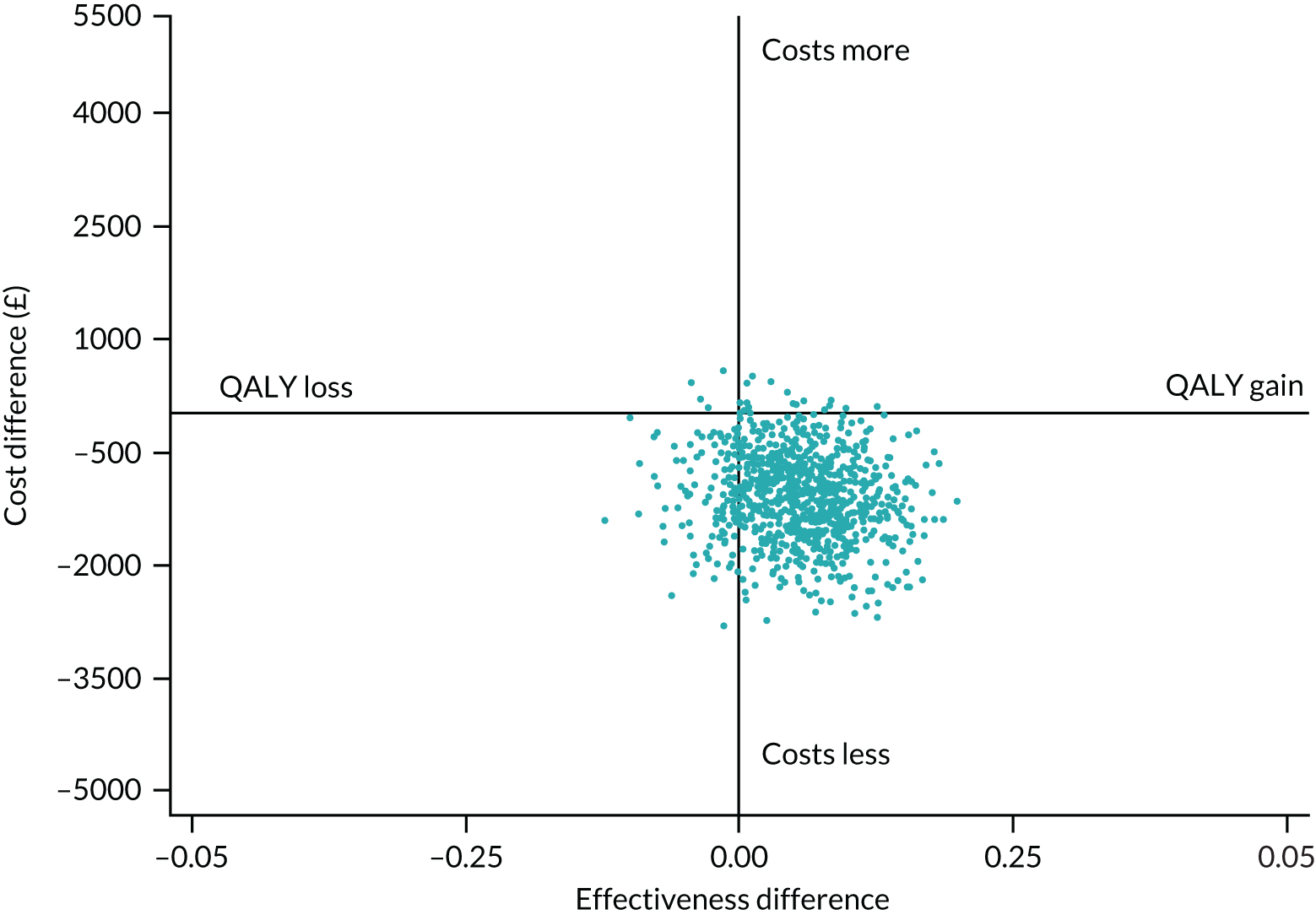
Over the range of values for society’s WTP for 1 QALY, there was a > 97.5% chance that the PASTA intervention group would be considered cost-effective (see Appendix 2, Figure 16).
The post hoc economic sensitivity analysis results are shown in Appendix 2, Table 14. The seven compliant hospitals where there was no evidence of a thrombolysis rate difference between the PASTA and standard care groups showed no evidence of a difference in QALYs (0.005, 95% CI –0.008 to 0.018) or costs (–£423, 95% CI –£2220 to £1362). The eight non-compliant hospitals that together had a thrombolysis reduction in the PASTA intervention group provided no evidence of a difference in QALYs (0.009, 95% CI –0.008 to 0.025), but costs were lower for the PASTA patients than for standard care patients (–£2952, 95% CI –£4988 to –£917)]. Cost-effectiveness planes for the compliant and non-compliant hospitals are shown in Appendix 2, Figures 17 and 18.
Discussion
Although the paramedic-led thrombolysis-focused emergency stroke assessment used in the PASTA trial did not increase thrombolysis rates, the economic evaluation showed a cost saving associated with the PASTA intervention group. The QALY differences were, on average, small and there was no evidence of differences, but, overall, there was a very high chance that the PASTA intervention would be cost-effective across all WTP threshold values.
Although it is not surprising that fewer thrombolysis treatments resulted in lower costs, it was unexpected to observe the PASTA intervention group savings in other aspects of care, including length of hospital stay, rehabilitation and social care. Although these data require cautious interpretation, patients with better health following emergency assessment would require lower costs for each of these resources in turn. 50 However, it is important to acknowledge that the health difference was non-significant, small and short term.
Although the economic results are not intuitive, previous triallists have proposed that costs of care can be more sensitive indicators of all consequences (expected and unexpected) of a complex intervention in a pragmatic trial than a pre-chosen primary end point that focuses on one anticipated impact only. 51 This proposal is consistent with the idea that the PASTA intervention could have a mixed effect on patient care.
In the post hoc analysis, a reduction in costs up until 90 days was particularly evident for the PASTA patients across services with specialist availability below the level recommended by national guidelines. This is consistent with the earlier hypothesis (see Examination of the Paramedic Acute Stroke Treatment Assessment pathway intervention clinical effectiveness: a cluster randomised trial, Discussion) that relative inexperience normally leads to overtreatment rather than undertreatment of borderline cases by non-specialists. Better clinical acumen would not only save costs by preventing futile thrombolysis, but could also avoid a longer length of stay associated with essential monitoring after treatment and rehabilitation following harmful complications.
Impact of the Paramedic Acute Stroke Treatment Assessment intervention on ambulance response times
During funding of the programme and development of the PASTA intervention (see Development of the Paramedic Acute Stroke Treatment Assessment, Stakeholder engagement), concerns were raised by reviewers and ambulance personnel regarding any negative impact from the additional time that intervention paramedics could spend in the hospital post handover, rather than becoming ‘clear’ again for another emergency call. To identify any effect on ambulance service responsiveness as a result of the PASTA intervention delivery, we undertook a retrospective observational study of the North East Ambulance Service (NEAS)’s emergency performance in parallel with the PASTA trial.
Methods
The NEAS was selected because all hospitals within the boundary of the service were trial sites, and the likelihood of finding any effect would, therefore, be higher than in regions with only partial coverage (i.e. north-west England and Wales). Until October 2017, for each patient consented to the trial, NEAS provided audit compliance information regarding any ‘Red 1’ (suspected cardiac arrest requiring an 8-minute response) and ‘Red 2’ calls (any other serious emergency requiring an 8-minute response time) occurring in the service 1 hour before the trial patient was attended by a study paramedic, and hourly afterwards for the next 3 hours. This allowed examination of whether or not the PASTA intervention had a wider impact on the service response by comparing, between the PASTA and standard groups, whether or not national audit targets for any parallel Red 1 and Red 2 calls were achieved (i.e. an appropriate ambulance vehicle arrived for those patients within 8 minutes of the 999 call). It was not possible to continue this evaluation after October 2017 as national ambulance metrics changed and data were no longer available.
Results
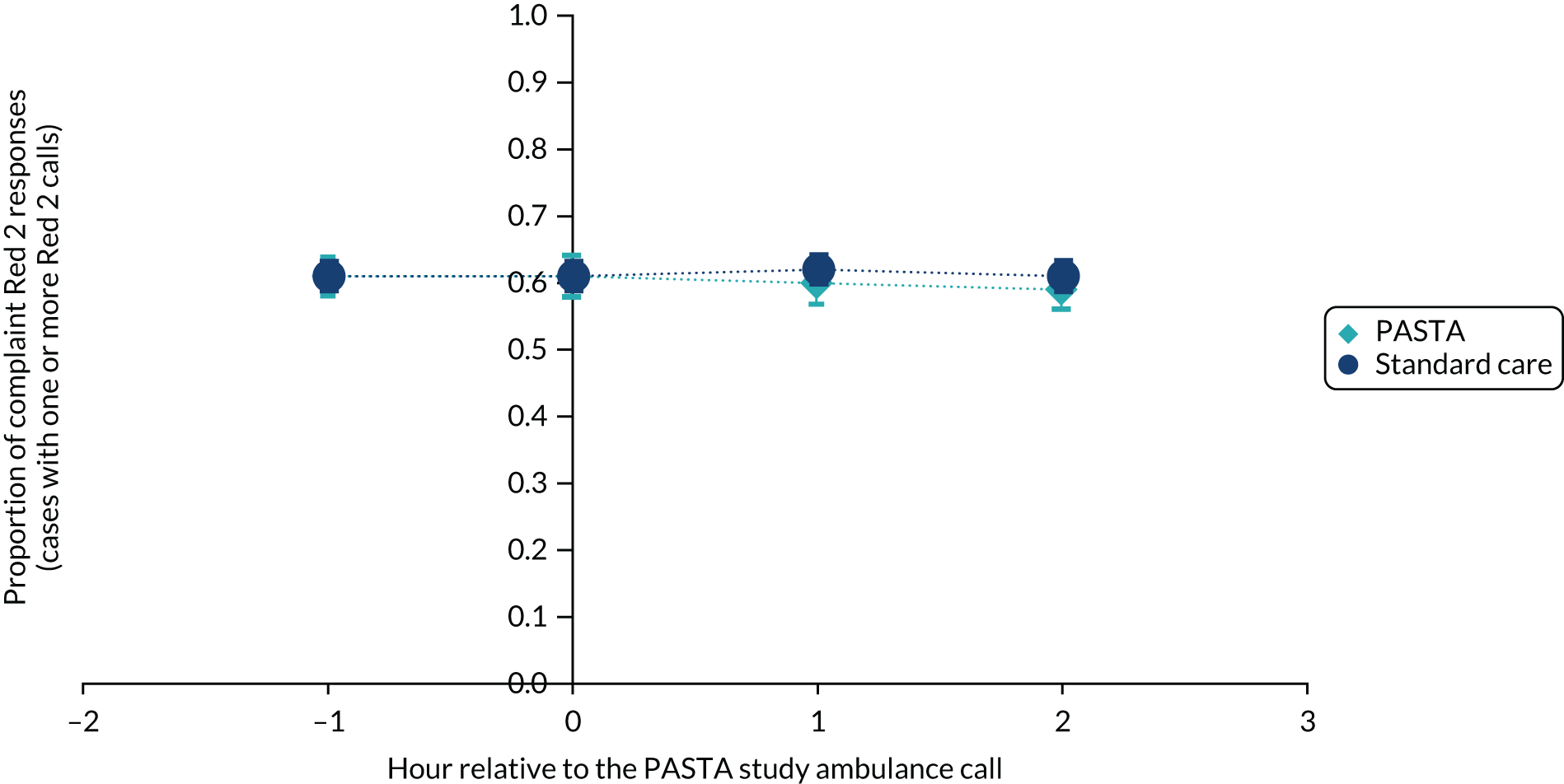
Between December 2015 and October 2017 (i.e. for 23 months), in the 1 hour before and 3 hours after any enrolled trial patient being attended by a study paramedic, there were a total of 831 and 2533 Red 1 calls, and 12,155 and 38,708 Red 2 calls, respectively (see Appendix 3, Tables 15 and 16). The average compliance across all patients was 68.7% for Red 1 and 61.1% for Red 2. As shown in Figures 5 and 6, after removal of hours when Red calls did not occur, there were no significant differences between the PASTA and standard care groups in the proportion of compliant Red 1 or Red 2 calls achieved by the service at any hourly time point in relation to an enrolled patient being attended by a study paramedic.
FIGURE 5.
Proportion of compliant Red 1 ambulance responses in the 1 hour before and 3 hours after a study patient was attended by a paramedic. The dark blue circles show the standard care and the light blue diamonds show the PASTA study groups.
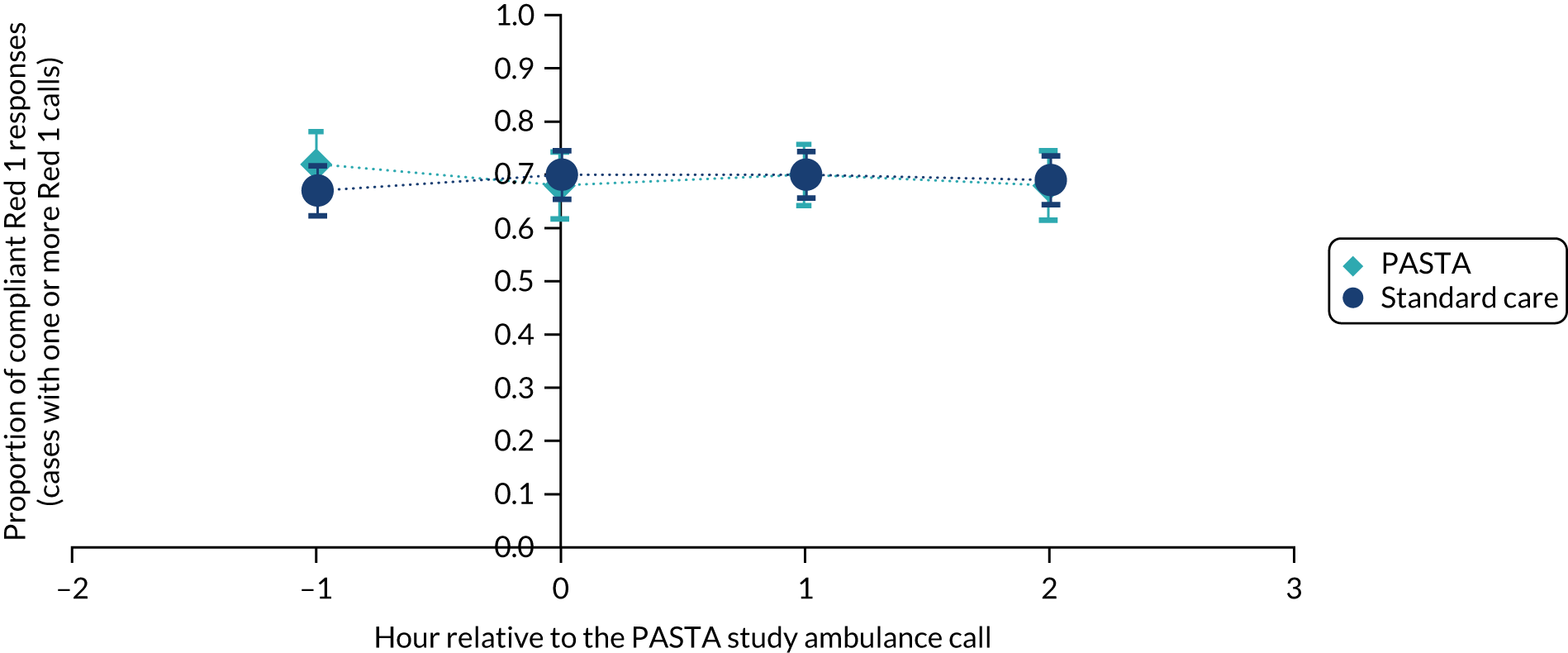
FIGURE 6.
Proportion of compliant Red 2 ambulance responses in the 1 hour before and 3 hours after a study patient was attended by a paramedic. The dark blue circles show the standard care and the light blue diamonds show the PASTA study groups.
Discussion
The results provide reassurance that, despite engaging paramedics for an average of 13.4 minutes longer with trial patients, the PASTA intervention did not cause decompensation of the ambulance service’s ability to respond to its highest priority calls. This is not surprising as this time interval fell within the 15 minutes’ tolerance for ambulance departure after hospital handover, and suspected stroke is a relatively small proportion (approximately 3%) of ambulance service activity, so should not create a service-wide capacity issue. It is important to recognise that these data reflect calls related to patients who were consented to the trial and not all suspected stroke patients who were attended by study paramedics, but the results support the ongoing hosting of stroke research by ambulance services.
Process evaluation of the Paramedic Acute Stroke Treatment Assessment intervention
The main study report has been published. 52
Material throughout this section has been reproduced with permission from Lally et al. 52 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
There were three groups involved in a process evaluation that intended to describe the acceptability and feasibility of the PASTA pathway in the clinical setting: intervention paramedics, hospital clinicians and intervention group patients.
Methods
Participants were invited to semistructured interviews (in person or over the telephone). Hospital research support staff sought medically stable patients who were attended by a NEAS intervention paramedic within the last 7 days. Interviews were audio-recorded, anonymised and transcribed. Data collection and analysis was an iterative process, following the combined principles of the constant comparative32 and thematic analysis. 33 Two researchers coded transcripts independently, which were then compared and further analysed during data sessions. Approval was given by the National Health Service Research Ethics Committee Newcastle and North Tyneside (reference 15/NE/0309).
Results: intervention paramedics
In total, 26 interviews were conducted across the three ambulance services (north-east England, n = 11; north-west England, n = 10; and Wales, n = 5). Participants’ length of service ranged from 14 months to 27 years, with qualifications up to postgraduate degree level.
Iterative data analysis identified four key themes, which reflected paramedics’ experiences at different stages of the intervention care pathway:
-
Enhanced assessment at scene – paramedics reported that the PASTA intervention complemented their skill set and confidence, and the trial training allowed them to feel well prepared. Illustrative quotations are shown in Appendix 4, Box 1.
-
The pre-alert to the hospital – when local standard care pathways permitted conveyance of additional patient details, pre-notification contained more detailed and appropriate information as a result of the PASTA enhanced assessment. Illustrative quotations are shown in Appendix 4, Box 2.
-
Handover to hospital team – the standard ‘scripted’ format for handover of thrombolysis-specific information was viewed as the primary benefit of the PASTA pathway. Paramedics felt more confident during communication with the hospital team, and felt that they were less likely to forget important details. Illustrative quotations are shown in Appendix 4, Box 3.
-
Assisting in the hospital and feedback – owing to traditional professional boundaries, paramedics found these aspects harder to achieve, although feedback from the clinical team was valued when available. Illustrative quotations are shown in Appendix 4, Box 4.
Results: hospital clinicians
Seven focus groups and one telephone interview were conducted across the three study regions. A total of 25 staff participated, including stroke specialist nurses, ED nurses and stroke consultants.
The following main themes were identified, also reflecting key stages of the pathway:
-
Enhanced paramedic role – hospital staff perceived that paramedics generally seemed to enjoy the role and receiving feedback (see Appendix 4, Box 5: quotations 1 and 2). They reported that there were not as many paramedics trained as they had expected.
-
Handover value – staff who had directly experienced the handover viewed it positively. They reported that the PASTA paramedics required fewer prompts from the stroke or ED team to get the details they needed, and the information was provided in the appropriate order (see Appendix 4, Box 5: quotations 3 and 4).
-
Post-handover uncertainty – in a few settings, it was valued that the PASTA paramedics assisted with practical tasks after handover, such as taking the patient for urgent brain imaging, as this freed up the hospital staff to prepare for the possibility of thrombolysis treatment (see Appendix 4, Box 5: quotation 5). However, this assistance was not always required by the hospital staff or consistently offered by the paramedics, who may have found it difficult to initiate (see Appendix 4, Box 5: quotation 6). Even if extra assistance was not needed, feedback to paramedics was viewed positively (see Appendix 4, Box 5: quotation 7).
Results: intervention patients
Six patients (five male and one female) were interviewed, after which a decision was made by the TSC to stop further patient recruitment because of the limited information being obtained about the PASTA intervention. Some patients could not remember any specific details, and those who could provide an account of their admission to hospital could not recall any specific details of paramedic actions. This may reflect the nature of acute stroke on perception or memory and the emotional state of patients at the time, but it is also likely that patients had no prior knowledge of paramedic care processes that would have enabled them to identify any actions that were related to the intervention.
Discussion
Even though there was overlap with existing practice, participating paramedics and hospital staff valued the structure of the PASTA pathway. The pathway was considered to enhance paramedic confidence and shared care, although there was less value post handover because help was not always required and traditional boundaries were harder to overcome.
Strong support was expressed for the structured patient assessment and corresponding information handover. A review of 12 studies examining structured patient assessment frameworks found evidence of improved documentation,53 but no examples from pre-hospital care. The general need to improve emergency handover has been highlighted by a review of 21 studies, which identified concerns about communication in the chaotic ED environment, exacerbated by a lack of time and resources. 18 More recently, there have been reports in support of structured generic handovers19 and a version for trauma care,54–56 but there have been no previous descriptions of a stroke-specific handover. 29
On completion of the PASTA pathway checklist, paramedics welcomed the opportunity to receive immediate feedback, which hospital staff were happy to provide. Previous interviews with Canadian paramedics also found positive perceptions of feedback, but this was described as informal and opportunistic. 57 Feedback to individual paramedics has been shown to encourage adherence to standard stroke care assessment, but was not in given real time and did not allow clarification by the recipient. 17
Work package 2
Baseline characteristics of intra-arterial thrombectomy service provision in England
A full report has been published and parts of this section have been reproduced from the report. 58 Flynn D, Ford GA, McMeekin PJ, White P. International Journal of Stroke (volume 11, issue 8), pp. NP85–85, copyright © 2016 by World Stroke Organization. Reprinted by permission of SAGE Publications.
Results from randomised trials demonstrating that rapid IAT treatment reduced dependency for selected stroke patients became available in 201523,24 and led to interventional procedure guidance being issued by NICE in March 2016,59 but very few UK hospitals were able to provide this service. Most treatments were opportunistic in the absence of clearly defined clinical pathways, and there was no information about how interventional neuroradiologists (INRs) performing the procedure were interpreting the evidence. Understanding baseline service provision and patient selection processes was an essential first step towards modelling optimal NHS service provision.
Methods
In November/December 2014, a survey was sent to clinical leads in all 24 regional INR services in England to obtain data on current provision of IAT, IAT patient selection and diagnostic imaging criteria, and centre opinions on future IAT service provision.
Results
Eighteen centres (75%) responded that provided INR services to a population of ≈ 43 million. No centres reported delivery by non-INRs. Ten centres (56%) had formal IAT protocols and six (33%) had protocols for interhospital transfers. A median of 10 [interquartile range (IQR) 16] stroke patients underwent IAT per centre during the previous year. One centre had 24 hours per day, 7 days per week (24/7) IAT provision, two centres had 7-day provision during normal hours, 12 delivered IAT on weekdays and three had no regular provision at all. There was substantial variation in the patient selection criteria and protocols for provision of IAT (Table 3). For future IAT services, there was clear support for centralisation of provision into large HASUs at neuroscience centres (89%) with ‘drip and ship’ interhospital transfers across a formal network (94%).
| Question | Number of patients (%) |
|---|---|
| Time window for INR accepting anterior circulation stroke for IAT | |
| < 4.5 hours | 13 (72) |
| 4.5 to < 6 hours | 3 (17) |
| > 6 hours | 0 (0) |
| Not time based | 2 (12) |
| Patient category considered for IAT | |
| Perform IAT only when thrombolysis contraindicated | 1 (6) |
| Perform IAT only in accordance with the 2013 NICE guidelines | 7 (39) |
| Will perform IAT outside 2013 NICE criteria | 8 (44) |
| No response | 2 (11) |
| Referral source | |
| Stroke physician/neurologist (own hospital) | 8 (44) |
| Stroke physician/neurologist (all hospitals) | 8 (44) |
| Referrals from wider range of specialties/grades | 1 (6) |
| No response | 1 (6) |
| Evidence for arterial occlusion before accepting the patient | |
| Accept patients with clinically/plain CT-suspected LAO | 8 (44) |
| Only accept patients for IAT where LAO is confirmed by CT angiography/MRA | 8 (44) |
| No response | 2 (11) |
| Anaesthetic technique | |
| General anaesthesia | 4 (22) |
| Conscious sedation with or without general anaesthesia | 10 (55) |
| No response | 4 (22) |
| Local primary IAT therapeutic strategy | |
| ‘Stentriever’ device alone | 8 (44) |
| ‘Stentriever’ device with balloon guide catheter | 3 (17) |
| Direct aspiration alone | 1 (6) |
| Varies between operators | 5 (28) |
| No response | 1 (6) |
Conclusions
Most centres in England in 2014 were limited to weekday ad hoc provision of IAT. There was considerable variation across centres in imaging and patient selection for IAT, but the responses to questions about the organisation of future service provision for IAT in England showed a degree of consensus.
Updated estimates of certainty for intra-arterial thrombectomy effectiveness and safety
The full systematic review has been published and parts of this section have been reproduced from the review. 60 Flynn D, Francis R, Halvorsrud K, Gonzalo-Almorox E, Craig D, Robalino S, et al. European Stroke Journal (volume 2, issue 4), pp. 308–18, copyright © 2017 by European Stroke Organization. Reprinted by permission of SAGE Publications.
The PRISMA-compliant protocol61 was registered online as PROSPERO CRD42015016649.
Soon after the programme started, several meta-analyses of IAT RCTs were published,23,24,62,63 each of which had taken a slightly different approach, but all found that IAT is an effective treatment. The most robust of these was the academic collaborative triallists (the HERMES group) individual patient record meta-analysis. 23 However, the evidence base to define the safety and effectiveness of IAT had expanded by ≈ 45%, with THERAPY (The Randomized, Concurrent Controlled Trial to Assess the Penumbra System’s Safety and Effectiveness in the Treatment of Acute Stroke),1 THRACE (The Contribution of Intra-arterial Thrombectomy in Acute Ischemic Stroke in Patients Treated With Intravenous Thrombolysis)2 and PISTE (Pragmatic Ischaemic Stroke Thrombectomy Evaluation)3 trials all reporting later in 2016. Therefore, an updated evidence synthesis was warranted. A systematic review and meta-analysis with trial sequential analysis (TSA) was conducted to understand the impact of trials reporting in 2016 on the magnitude/certainty of the estimates for effectiveness and safety of IAT.
In parallel with this systematic review looking at efficacy and safety, a detailed narrative review was undertaken of IAT complications. 64
Methods
The search strategy is shown in Appendix 5. Random-effects models were conducted of RCTs comparing IAT with/without adjuvant thrombolysis against thrombolysis and other forms of best medical care in the treatment of acute ischaemic stroke. The TSA established the strength of the evidence derived from the meta-analyses.
Results
Patients treated with IAT were significantly more likely to be functionally independent (mRS score of 0–2) at 90 days’ follow-up (OR 2.39, 95% CI 1.88 to 3.04). As shown in Figure 7, the impact of the three 2016 trials1–3 was a slightly decreased pooled effect size, but increased certainty of the mid-point estimate (OR 2.07, 95% CI 1.70 to 2.51).
FIGURE 7.
Forest plot for functional independence. M–H, Mantel–Haenszel. Reproduced with permission from Flynn et al. 60 Flynn D, Francis R, Halvorsrud K, Gonzalo-Almoroz E, Craig D, Robalino S, et al. , European Stroke Journal (Volume 2, Issue 4), pp. 308–18, copyright © 2017 by SAGE Publications Ltd. Reprinted by permission of SAGE Publications Ltd.
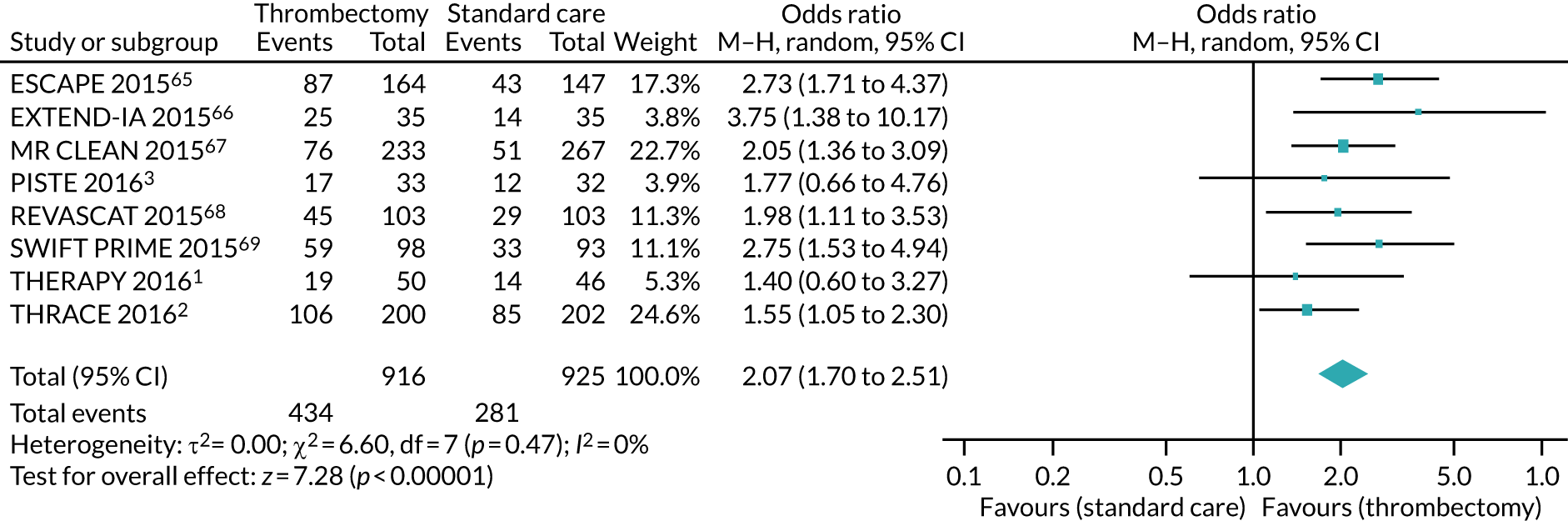
Intra-arterial thrombectomy, compared with best medical care, did not show any effect on mortality or symptomatic intracerebral haemorrhage at 90-days’ follow-up. Results of the TSA satisfied the criterion for ‘sufficient evidence’ on effectiveness; however, uncertainty remains as to whether IAT is associated with lower mortality or increased risk of symptomatic intracerebral haemorrhage.
Conclusions
The expanded evidence base for IAT yielded a more precise assessment of effectiveness, but uncertainty remained as to the net effect of IAT on mortality and symptomatic intracerebral haemorrhage.
Expert consensus on preferred implementation option for intra-arterial thrombectomy services
A full description is available in Halvorsrud et al. 70 Parts of this text have been reproduced with permission from Halvorsrud et al. 70 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
Although there was evidence supporting IAT therapeutically, there was no consensus on how it should be made available within clinical services. The aim was to establish consensus on options for future organisation of IAT services, based on agreement among physicians with clinical expertise in LAO stroke management.
Methods
A survey questionnaire was developed with 12 options (propositions) for future organisation of thrombectomy services in England (see Appendix 6, Box 6). The British Association of Stroke Physicians (BASP) facilitated recruitment of panellists to provide representative ratings of options (by location and experience). Consensus was defined as ≥ 75% of ratings for each option falling within three categories (i.e. approve, quite strongly approve or very strongly approve) on a seven-point Likert scale. Wider BASP membership and members of the British Society of Neuroradiologists (BSNR) then ranked those propositions on a seven-point Likert scale, reaching consensus following two initial assessment rounds. Data was collected from November 2015 to March 2016, and participation was pseudo-anonymous.
Results
Eleven respondents completed two rounds. 71 Three options achieved consensus:
-
selective transfer to nearest neuroscience centre for INR-delivered IAT (100% approve)
-
local imaging then transfer to nearest neuroscience centre for INR-delivered IAT (91% approve)
-
local imaging then transfer to nearest neuroscience centre for advanced imaging and INR-delivered IAT (82% approve).
Subsequently, the wider group of BASP and BSNR members (n = 64) assigned the highest approval ranking for transferring LAO stroke patients to the nearest neuroscience centre for thrombectomy based on the results of local CT/computed tomography angiography (CTA) (option 2).
Conclusions
The Delphi exercise by clinical and imaging stroke experts in England established consensus on a ‘simple’ imaging-driven option, which advocates the secondary transfer of patients (‘drip and ship’) with LAO stroke for thrombectomy based on local CT/CTA alone.
Patient and public preferences on attributes of intra-arterial thrombectomy service organisation
To integrate the opinions of stroke survivors/relatives/carers and the public into the outputs of a health economic model for IAT in England, their preferences were elicited regarding attributes of IAT service provision, including thresholds for additional travel time in the context of this time-critical emergency.
Methods
The research programme patient and public involvement (PPI) representative facilitated recruitment of 14 stroke survivors and their relatives/carers from a stroke PPI panel to engage in an iterative process to develop an inclusive and accessible ‘aphasia-friendly’ survey. Interactive meetings were convened to obtain feedback on an (1) initial draft of the survey and (2) updated version with graphics and textual presentation that adhered to guidance on developing resources for people with aphasia. 72 Subsequent testing was undertaken with 10 stroke survivors/carers and local PPI panels. The resulting anonymous survey was hosted and advertised on the Stroke Association website73 (from January 2017 to May 2017) with information on IAT, including the time sensitive nature of outcomes, limited number of specialist facilities and delays that can occur during secondary transfer from a local stroke unit.
Results
Responses were received from 147 individuals [mean age 49 (SD 16) years; 61% female]: 27 stroke survivors (18%), 51 relatives/carers of stroke survivors (35%) and 69 other members of the public (47%). The majority of stroke survivors were male and the majority of the other groups were female. Respondents were spread across England (50% were resident in north-east England). Full details of respondents are in Appendix 7, Table 17. The survey results are show in Table 4. Differences in proportions for each survey item as a function of participant type were not statistically significant (p > 0.05), indicating that experience of stroke did not influence responses.
| Question | Number of respondents (%) |
|---|---|
| Thrombectomy can be delivered only at specialist centres. Would you agree to be transferred from your local hospital to such a centre to undergo thrombectomy? (N = 142) | |
| Yes | 138 (97) |
| No | 4 (3) |
| How long would you be prepared to travel via an emergency (999) ambulance for thrombectomy? (N = 145) | |
| 1: up to 20 miles/29 minutes | 36 (25) |
| 2: up to 30 miles/41 minutes | 46 (32) |
| 3: up to 40 miles/53 minutes | 17 (12) |
| 4: up to 50 miles/65 minutes | 46 (32) |
| How long would you be prepared to stay at the specialist centre for thrombectomy before you are returned to your local centre/hospital? (N = 144) | |
| 24 hours | 8 (6) |
| 48 hours | 26 (18) |
| > 48 hours | 110 (76) |
| Should a thrombectomy service be made available in your local stroke unit, even if this meant that thrombectomy would be carried out by a less experienced stroke team? (N = 144) | |
| Yes | 33 (23) |
| No | 57 (40) |
| Uncertain | 54 (38) |
Conclusions
In agreement with expert views favouring centralised provision of IAT with secondary transfer as appropriate, 97% of respondents would accept hospital transfer and 75% would be prepared to travel up to 30 miles to access IAT.
Establishing the number of stroke patients eligible for intra-arterial thrombectomy
A full report has been published. 74
Material throughout this section has been reproduced with permission from McMeekin et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
To model optimal national service configurations, it was necessary to first estimate the proportion of UK stroke patients eligible for IAT, as this had not previously been established at a population level.
Methods
Using national registry data from the SSNAP for England, Wales and Northern Ireland,6 adjusted for Scotland using data from the Scottish Stroke Care Audit,75 a decision tree with 14 nodes (A–N) was constructed from published trials74 depicting eligibility for IAT, regardless of geographical or service constraints. An updated version in 201876 included two new reports describing IAT efficacy among late presenters. 77,78
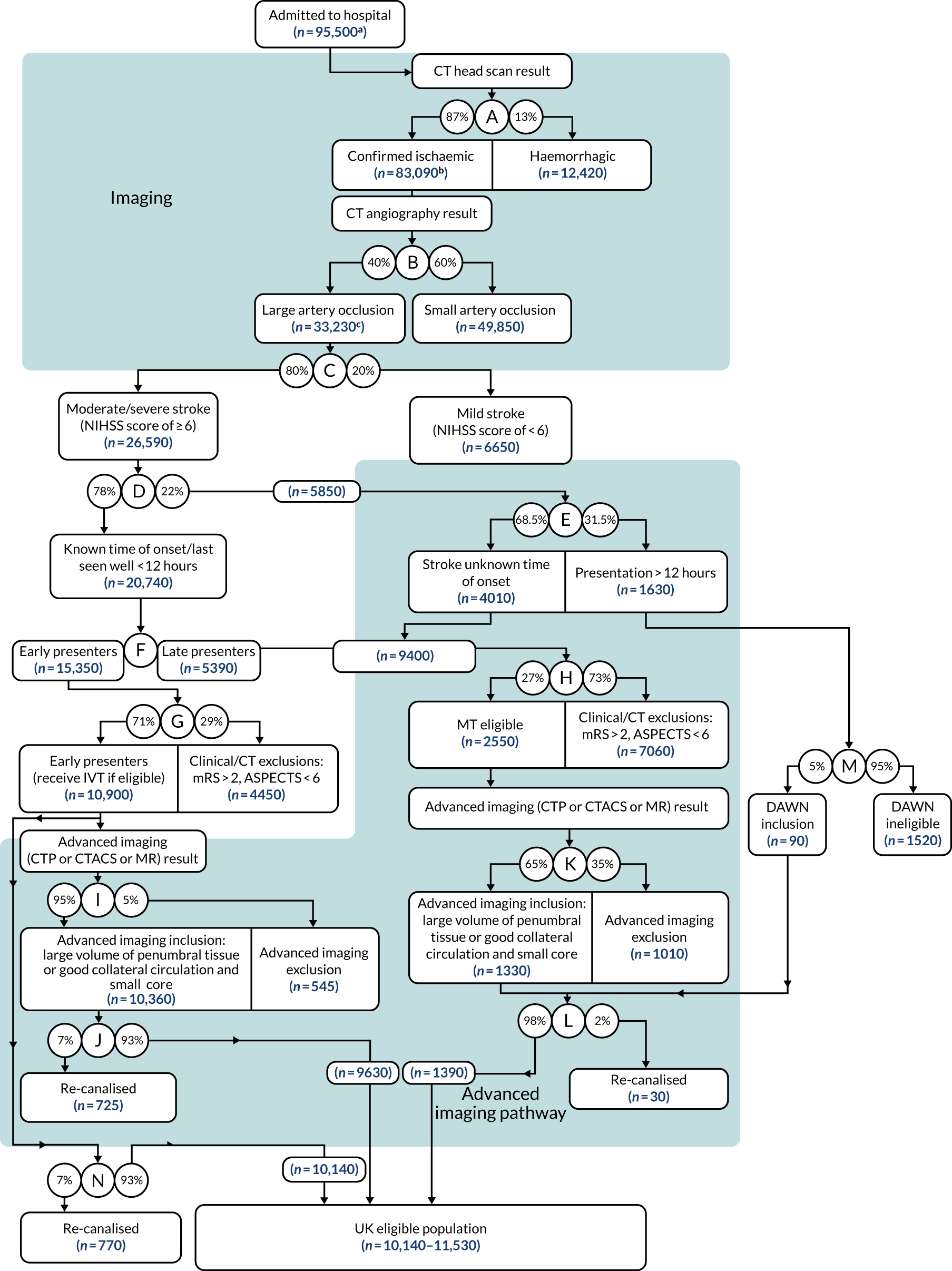
Results
The updated decision tree is presented in Figure 8. 74 The eligible population includes (1) the total UK population, even if currently geographically inaccessible; (2) patients with a confirmed infarct, excluding patients with unconfirmed status (≈ 2%); and (3) patients with basilar artery occlusions eligible for treatment. Patients in the large, lower blue-shaded box are selected by advanced imaging. It was originally estimated that between 9620 and 10,920 UK stroke patients would be eligible for treatment. The revised estimate based on new evidence was that an additional 490 early-presenting and 205 late-presenting patients would be eligible for IAT (i.e. between 10,140 and 11,530 patients). The largest increase was a result of new evidence of IAT benefit for patients with Alberta Stroke Program Early Computed Tomography Score (ASPECTS) of ≥ 5,80 with a smaller contribution from the treatment of patients presenting between 12 and 24 hours. 77,78
FIGURE 8.
Updated estimate of the number of stroke patients eligible for IAT. ASPECTS,79 Alberta Stroke Program Early Computed Tomography Score; CTP, computed tomography perfusion; CTACS, computed tomography angiography collateral scoring; DAWN,78 DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neutrointervention with Trevo; IVT, intravenous thrombolysis; MR, magnetic resonance; MT, mechanical thrombectomy. a, Total UK population, including those deemed to be geographically inaccessible; b, confirmed infarcts, excluding ≈ 2% of patients whose status is unconfirmed. Note that totals between levels in the tree may differ slightly due to decimal rounding. Reproduced with permission from McMeekin et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Conclusion
Up to 12% (11,530/95,500) of UK stroke admissions are eligible for IAT based on treatment criteria from randomised clinical trials.
Helicopter Emergency Medical Services survey
While undertaking the survey of INR centres (see Baseline characteristics of intra-arterial thrombectomy service provision in England), it became apparent that some parts of the population live remotely from a hospital able to provide IAT. However, many hospitals are served by the air ambulance network, which could provide an approach to improve access to treatment. A survey of Helicopter Emergency Medical Services (HEMS) was undertaken to parameterise a health economic model for the cost-effectiveness of HEMS compared with ground-based ambulances providing secondary transfer of stroke patients for IAT.
Methods
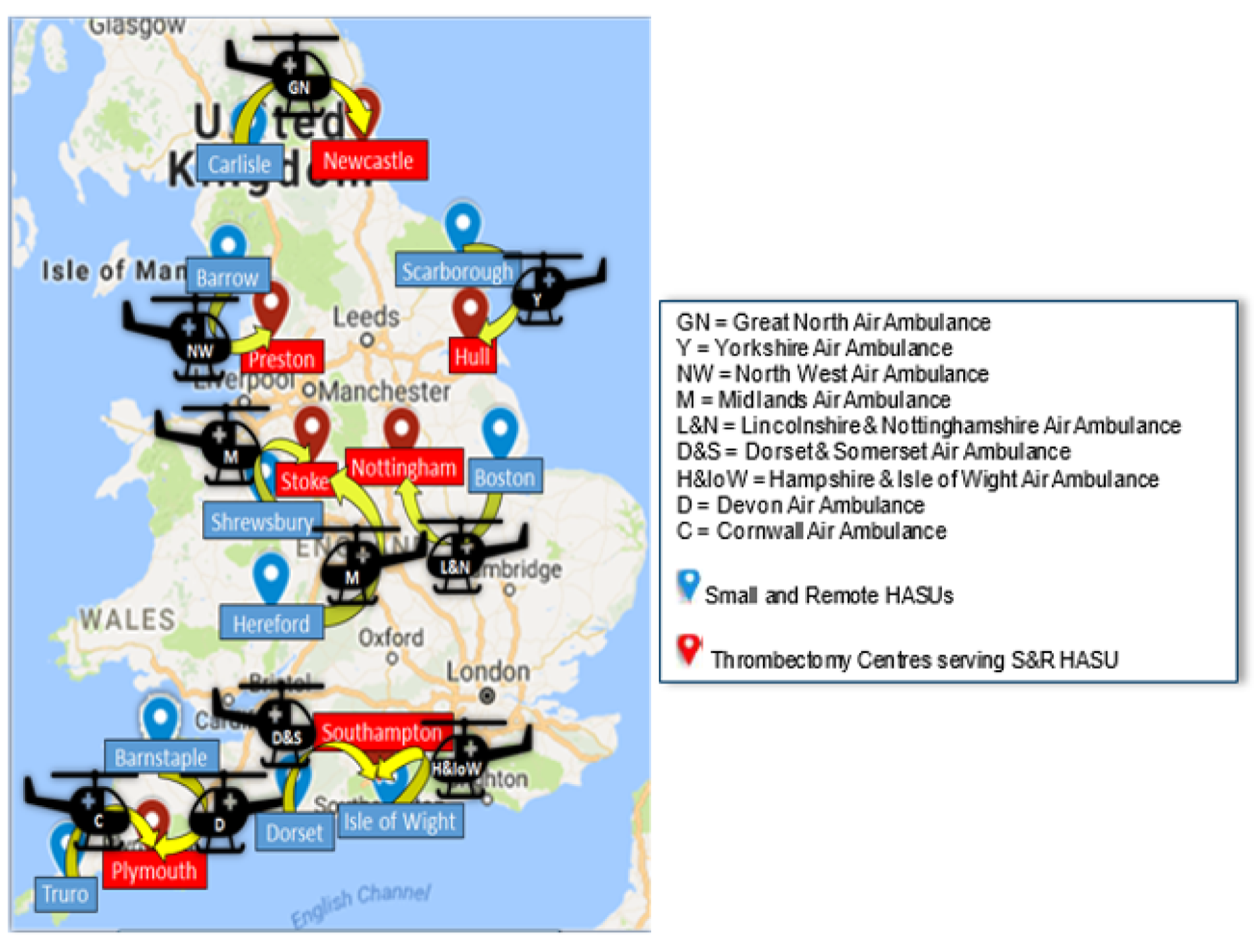
An online survey was sent to the clinical leads of nine HEMS serving ‘unavoidably small and remote’ hospitals (NHS England definition: < 200,000 population and > 1 hour of travel from nearest major hospital)81 (Figure 9).
FIGURE 9.
Unavoidably small and remote hospitals in England with HEMS. Reproduced with permission from Google Maps (Google Inc., Mountain View, CA, USA). Map data © 2021 Google GeoBasis-DE/BKG (© 2009).
The survey gathered data on the number of helicopters, time in operation, number of hospitals served, average travel times by air, fit for purpose helipads, availability (09.00 to 17.00, 24/7 or other) and conditions under which flight is permitted.
Results
Responses were received from all nine HEMS (Table 5).
| Characteristic | Median | Range | IQR |
|---|---|---|---|
| Annual number of stroke transfers | 2.5 | 11 | 5.5 |
| Number of helicopters | 2 | 1–3 | 1.5 |
| Operational hours (per day) | 14 | 7 | 6.5 |
| Response time (minutes) | 4 | 3 | 1.5 |
| Cost per mission (£) | 2750 | 750 | 375 |
All HEMS were willing to provide secondary transfers for IAT. HEMS operated a median of 14 hours per day, with extensions to operational hours being planned in two HEMS. The median response time from notification to take-off was 4 minutes and the cost per mission was £2750 (range £2500–3500). Most HEMS (eight of the nine) were Instrument Flight Rules-approved82 for flying in low-visibility conditions. To deliver transfer for IAT robustly, three of the nine HEMS indicated additional funding and/or organisational changes would be required.
Microcosting study to establish true cost of intra-arterial thrombectomy
The full report has been published and parts of this section have been reproduced from this report. 83 Republished with permission of Royal College of Physicians, from The cost of providing mechanical thrombectomy in the UK NHS: a micro-costing study, Balami JS, Coughlan D, White PM, McMeekin P, Flynn D, Roffe C, et al. , volume 20, edition 3, 2020; permission conveyed through Copyright Clearance Center, Inc.
To estimate cost-effectiveness of national implementation, it was first necessary to establish the cost of providing IAT within the first 72 hours since stroke onset and to explore resource and costs variations across UK centres.
Methods
Microcosting methods were used to enable a precise assessment of the costs of IAT from an NHS perspective. Data were derived from the five nearest neuroscience centres from 2015 to 2018. The resources used and the costs were collected on patients admitted by secondary transfer (i.e. ‘drip and ship’ patients) and directly to centres (i.e. mothership patients).
Results
Data were abstracted directly from clinical records of 310 patients treated with IAT. The mean total per-patient cost of providing IAT and inpatient care within 24 hours of stroke onset was £10,846. The main driver of cost was IAT procedure costs, accounting for 73% (i.e. £7930) of the total 24-hour cost. Total mean cost within 72 hours of stroke onset was £12,440. Costs were higher for patients treated under general anaesthesia than for those treated under local anaesthesia, with a mean difference of £1070 (95% CI £381 to £1759; p = 0.003); the admission to an intensive care unit (ICU)/high-dependency unit (HDU) mean difference was £8210 (95% CI £4833 to £11,588; p < 0001). There was no statistically significant difference in 24-hour costs between ‘mothership’ and secondary ‘drip and ship’ patients: mean difference –£368 (95% CI –£1016 to £279; p = 0.26).
Conclusions
The major factors contributing to the costs of IAT for stroke include consumables and staff for the intervention, the use of general anaesthesia and admission of patients to ICU/HDU, and inter-hospital transport and repatriation. These findings may inform the reimbursement, provision and strategic planning of stroke services.
Estimating the effectiveness and cost-effectiveness of establishing intra-arterial thrombectomy: a discrete-event simulation
The full report for modelling study one has been published,84 as has the full report for modelling study two. 85
Material throughout this section has been reproduced with permission from McMeekin et al. 84 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
Material throughout this section has been reproduced with permission from Coughlan et al. 85 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The evidence that IAT for acute ischaemic stroke is both a highly effective and cost-effective treatment is unequivocal. Less certain is how NHS providers might maximise the benefits of IAT and how services should be extended across the NHS beyond its currently low level of provision. To support decision-makers, we developed an economic modelling framework to estimate the health and financial consequences of alternative models of service provision. In keeping with best practice,86 the model included uncertainty and discounting, but capital expenditure and other costs associated with establishing new health-care infrastructure were not included.
Model structure
There were two stages incorporated in to the model: (1) short-term predictions of the mRS at 90 days based on characteristics of stroke patients at presentation and (2) long-term, lifetime projections based on 90-day mRS patient characteristics. Resource consequences (financial costs) and health outcomes were derived from morbidity (mRS) and mortality. The ‘code base’ of the discrete-event simulation (DES) originates from a previous Programme Grants for Applied Research (PGfAR) [Development and Assessment of Services for Hyper-acute Stroke (DASH); RP-PG-0606-1241], which involved building a model to examine the consequences of pre-hospital redirection of patients eligible for thrombolysis. 87 It was extended to include the secondary transfer of patients eligible for mechanical thrombectomy.
The DES was iterated 2000 times for each person in the population who would be affected by the change in service configuration. The mean outcomes were aggregated to estimate the marginal effects before and after proposed service change.
Key assumptions
The model assumes that the patients included in our simulation had the same properties as those included in the HERMES analysis of time to treatment. 23 Although we varied the age of patients within our simulation, this had an effect on post 90-day survival and deterioration only.
Data sources
Short-term outcomes (up to 270 minutes) for patients treated with IAT were defined by mRS using results of the HERMES meta-analysis,23 which identified the relationship between time to treatment and groin puncture.
Long-term mortality in the model was derived from the Oxford Vascular Study (OxVasc) study,88 data from the Lothian stroke register89 about the increased mortality associated with stroke survivors and repeated random draws from national life tables90 for patients alive at censor. Using a knotted spline regression technique to extrapolate survival in each simulation run generated a different set of extrapolated parametric survival curves for each iteration of the simulation, from which the time to death could be calculated from the uniform randomly drawn probability of death. 84,91 To allow for improvements in survival since the Lothian Stroke Register88 data were collected, we applied a reduction in mortality of 25% from year 6 onwards. 84,92 The simulation was implemented in the R statistical package (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Two scenarios were modelled with the DES framework, reflecting current issues facing commissioners of IAT services in England. The first considered the effectiveness and cost-effectiveness of increasing current IAT provision from 24 to 30 centres, and the second considered the effectiveness and cost-effectiveness of secondary transfer of eligible patients from remote hospitals to current IAT centres.
Modelling study one: increasing the numbers of centres providing intra-arterial thrombectomy
To achieve the objectives of the National Commissioning Policy for IAT in a geographically equitable way would require the creation of new centres. Collaborating with colleagues from the National Institute for Health and Care Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South West Peninsula, it had been previously ascertained that the optimal number of neuroscience/comprehensive stroke centres (CSCs) providing endovascular thrombectomy (IAT) in England would be 30 (net six new centres), subject to geographical and service level constraints. 93 The DES was used to estimate the relative effectiveness and cost-effectiveness of increasing the number of centres from current provision at 24 centres to 30 centres. Using the parameter values (see Appendix 8, Table 18, the DES estimated effectiveness and lifetime cost-effectiveness (from a payer perspective) for 1 year of incidence of stroke in England.
Of the estimated 80,800 patients admitted to hospital in England with acute stroke per year, 21,740 were modelled to have their acute care affected by the proposed reconfiguration. The median time to treatment for eligible early presenters (< 270 minutes since onset) would reduce from a median of 195 (IQR 155–249) minutes to 165 (IQR 105–224) minutes. The model predicted that the reconfiguration would mean an additional 33 independent patients (mRS 0–1), and 30 fewer dependent patients dying (mRS 3–6) per year. The addition of six centres generates 190 QALYs (95% CI –6 to 399 QALYs) and results in savings to the health-care system of £1,864,000 per year (95% CI –£1,204,000 to £5,017,000 per year). The estimated budget impact was a saving of £980,000 in year 1 and £7.07M across years 2–5. We concluded that changes in acute stroke service configuration would produce clinical and cost impacts that were highly likely to result in cost saving. Over 5 years, there would be a return on capital investment of £8M, but it is important to acknowledge that capital expenditure, workforce expansion and other infrastructure costs are not included and are likely to be substantial.
Modelling study two: using helicopters to transfer eligible patients from geographically remote hospitals
Although England faces fewer geographical challenges to the provision of acute and emergency care than other parts of the UK, there are populations for whom transfer times from their local hospital to a major centre may compromise care. NHS England defines an ‘unavoidably small and remote’ hospital as one serving a population of < 200,000 people who are domiciled > 60 minutes’ travel by road from the nearest (major acute) hospital. 81 We aimed to estimate the marginal cost-effectiveness of secondary transfer by air ambulance compared with ground-based ambulances for patients attending such a hospital.
Ten hospitals in England were identified that served a combined population of two million, including 512,875 domiciled in remote locations. It was calculated that up to 501 early-presenting stroke patients per annum would benefit from secondary transfer to IAT using an air ambulance compared with using ground-based ambulances. The mean probability of living independently at 90 days increased when using an air ambulance (0.57) compared with using ground-based ambulances (0.53). Using an air ambulance as a secondary transportation strategy that enabled patients to receive IAT 60 minutes earlier resulted in greater QALYs (0.14) over a lifetime horizon, but was more costly (£3785). The incremental cost-effectiveness ratio was £28,027 per QALY gained.
Discussion
As with all models reporting ordinal outcome measures, the DES under-reports gains because small improvements as a result of speedier treatment are not counted. However, the DES was used successfully to model two important issues facing commissioners considering how to meet the goals of the National Commissioning Policy for IAT. Both scenarios modelled showed a small benefit from increasing provision and speeding up access to treatment.
Patient, carer and public preferences
It is important that public views are included during modelling and commissioning decisions for the provision of a new service. A survey approach established the preferences and trade-offs related to localised versus centralised IAT services from the perspective of stroke survivors, their relatives/carers and the public.
Methods
Best–worst scaling (BWS) is a preference elicitation technique where respondents select their ‘best’ (most preferred) and ‘worst’ (least preferred) items across a range of subsets. A co-design process involving stroke survivors and their relatives/carers established the form and content of the BWS survey (maximising readability and accessibility), informed by best evidence on presentation of probabilities (numerical and graphical)94 and design of information for people with aphasia. 72 Online testing was conducted with stroke survivors/carers and researchers with expertise in choice experiments.
The BWS survey was hosted on the Stroke Association website, and a link to the survey was distributed to all Healthwatch services in England and in the June 2019 NHS ‘In Touch’ newsletter.
Respondents stated their best and worst preferred options for four attributes (with two levels each – derived from the survey in Patient and public preferences on attributes of intra-arterial thrombectomy service organisation) in part 1 (i.e. service organisation), and three attributes (with two levels each) in part 2 (i.e. modelled outcomes for 24 vs. 30 IAT centres). Examples are shown in Appendix 9, Figures 19 and 20. Individual respondents were required to answer 2 sets of 8 (total of 16) BWS questions. The BWS responses were transformed into standardised scores with 95% CIs [ranging from –1 to +1, which represented the salience (best or worst) of attribute levels].
Results
One hundred and five respondents fully completed the survey [mean age 37 (range 18–86) years; 70% female; 47% from north-east England; 18% urban, 56% suburban, 26% rural]. The respondent types were as follows: stroke survivors, 18% (10% with aphasia); relative or carer of a stroke survivor, 32%; and members of the public, 50%.
Standardised scores for service organisation (Figure 10) showed that experienced medical teams (rather than a local service) and local services with less experienced medical teams were the most and least preferred attributes, respectively. Secondary transfer (present or not) was associated with positive (best) preferences. Length of stay of < 48 hours was associated with negative (worst) preferences. Travel times of > 45 minutes were associated with negative preferences.
FIGURE 10.
Standardised scores for service design attributes. Note that overlapping 95% CIs indicate that the difference is not significant. LOS, length of stay.

Standardised scores for modelled outcomes (Figure 11) showed that maximal effectiveness (30 centres) and costs (both levels) were the most and least preferred attributes, respectively.
FIGURE 11.
Standardised scores for outcomes. Note that overlapping 95% CIs indicate that the difference is not significant. MRI, magnetic resonance imaging.
Equity was associated with positive preferences, but the differences between levels were marginal. There were no statistically significant differences between standardised scores stratified in terms of stroke survivor/relative status compared with the public for service organisation or outcomes.
Conclusions
Greater expertise/experience of medical teams (compared with local services with less experienced teams) was the most preferred service organisation attribute. Secondary transfers with travel times of up to 45 minutes are acceptable to stroke patients/carers and the public. Respondents preferred effectiveness over equity (and both were valued more than costs).
A commissioning decision support tool: the Interface for Thrombectomy Economic Modelling and outcomeS in stroke
To assist commissioners and other stakeholders in understanding the health, financial and equity (proximity to treatment) implications of alternative geographical locations of CSCs (where IAT is available) and primary stroke centres (thrombolysis but no IAT available), a web platform was created using the model described in Estimating the effectiveness and cost-effectiveness of establishing intra-arterial thrombectomy: a discrete-event simulation.
Methods
The Interface for Thrombectomy Economic Modelling and outcomeS in stroke is a web-based application based on the R statistical package. Like the DES, the ITEMS software repeatedly models individual patient lifetimes based on random draws from the probabilities of relevant events occurring. The conceptual process for running the DES and saving the outputs is shown in Appendix 10, Figure 21.
The input is by a graphical interface, which restricts the parameters that can be altered in any simulation, but allows the user to try a large combination of values for the main factors determining service configuration and performance (Figure 12). Owing to expert and public preferences for secondary transportation to receive IAT if appropriate, ITEMS allows users to specify the initial distribution of the population sampled from ‘urban’, ‘suburban’ or ‘rural’, and considers uncertainty around inputs resulting from changes in journey times.
FIGURE 12.
The ITEMS online user interface.
The output displays a cost-effectiveness plane, and estimates marginal costs and health outcomes (either as individual mRS scores at 90 days or as lifetime QALYs). In addition to the DES described previously, ITEMS presents outputs that describe how individual patients might be affected by mean changes in outcomes on a cost-effectiveness plane (Figure 13). By trading off complexity for performance, ITEMS enables commissioners and other stakeholders to undertake a rapid review of alternative regional IAT service configurations, including economic impact.
FIGURE 13.
The ITEMS interface output display.
Patient and public involvement and engagement
Individual public representatives were integrated into programme committees as follows:
-
Peter Dodds (stroke survivor; co-applicant and Programme Steering Committee member)
-
Bill Laing (stroke survivor; PASTA TSC member)
-
Melissa Roberts (carer; PASTA TSC member)
-
David Burgess (carer; WP2 group member).
They attended project management meetings and contributed to the design and interpretation of all aspects of the work. They facilitated recruitment of participants for the public engagement work to design the PASTA intervention (see Work package 1, Development of the Paramedic Acute Stroke Treatment Assessment), preferences for IAT provision (see Work package 2, Patient and public preferences on attributes of intra-arterial thrombectomy service organisation) and BWS survey (see Work package 2, Patient, carer and public preferences). There was a strong connection to the regional NIHR Clinical Research Network North-East Stroke Patient and Carer Research Panel, which provided views on the priority order of research questions, public materials and information sheets to be used in the programme. These individual representatives and groups are acknowledged in the programme publications. 31,36,83–85,95 In addition, David Burgess is a co-author on several WP2 outputs83–85,95 and shared the results at several public stakeholder groups including the National Stroke Assembly. Feedback from public dissemination activities was fed back into the WP2 objectives, including the development of the ITEMS online tool.
Across the programme, there was comprehensive engagement with patients, carers and relevant public stakeholders through the research designs employed. Twenty patient representatives assisted with the development of the PASTA intervention in an iterative co-production process in groups with hospital and ambulance practitioners. This increased the probability that the content of the enhanced paramedic role and stroke care pathway would be acceptable to patients and the purpose of the intervention was meaningful. Over 147 members of the public responded to our questionnaire on stroke service configuration to ensure that their views were incorporated into modelling options, and 105 members of the public participated in the BWS exercise, which was developed out of the information and learning from the preceding survey. A public summary of results was distributed to participants who provided contact details. The findings were important as they demonstrated broad agreement between the views of professionals and stroke survivors, their carers and the wider public on critical attributes related to thrombectomy service organisation.
Reflections
The main objectives were achieved in both WP1 and WP2, but, as described in Content and changes during the programme, some changes were required in response to factors that were difficult to predict.
Work package 1
Objective 1
We did not find any previous published studies of enhanced paramedic roles in the context of time-critical care provision to address the main aim of the systematic review,29 but reports of simple innovations (e.g. a generic structure for handover formats) provided useful material for discussion with a wide range of stakeholders to develop the PASTA intervention. Service and public engagement at this stage was essential for the success of the trial.
Objective 2
As described in Content and changes during the programme, owing to slow uptake of the intervention training, the primary outcome was changed to the proportion of patients receiving thrombolysis, which required far fewer patients to show an important effect than a health outcome would have required. Future ambulance trials could plan timelines that reflect the challenge of pre-hospital research training and choose a primary outcome that will provide direct evidence of the intervention effect. In the trial process evaluation, we had not anticipated that patients within 7 days of stroke would be unable to provide views about the intervention, and future studies may have to consider whether or not another approach (e.g. video data) should be used to address this question. The use of ambulance audit data to show the impact of the PASTA intervention on response targets was a novel approach that could be used in other trials to guide future implantation decisions.
Objective 3
The PASTA trial generated an unexpected cost-effectiveness result, which has raised questions about the quality of standard care decisions for thrombolysis in sites with lower levels of specialist availability. However, as this outcome had not been anticipated and there were limited research delivery resources, we did not collect data from individual patient records to prove this hypothesis and masking of outcome assessors was not possible.
Work package 2
Objectives 1, 2 and 4
There was very productive engagement with collaborators outside the programme (notably the NIHR CLAHRC South West Peninsula) to co-ordinate development of a high-impact model of IAT cost-effectiveness. This used existing data sets and a DES model developed in our previous PGfAR study,87 but it was adapted to respond to the changing landscape of IAT evidence and service provision.
Objective 3
The incorporation of public views into the modelling was a novel approach, particularly the use of BWS. Although online data capture was the only realistic method and we hoped for a larger number of responses, these were consistent enough to provide confidence in the development of pathway configurations.
Objective 5
It was beyond the remit of the programme to formulate an IAT implementation plan across the NHS, but outputs fed directly into NHS Commissioning Guidance 20184 and The NHS Long Term Plan. 96 However, the programme lead, WP2 lead and an external collaborator (Professor Martin James) developed and published online an implementation guide aimed at commissioners and stroke care providers. 25 We have also developed an online tool aimed at health-care commissioners/providers (i.e. ITEMS) to facilitate commissioning decisions by providing readily adjustable inputs of key variables to suit the local context.
Implications for practice
The PASTA trial provides evidence that incremental improvements in thrombolysis delivery are unlikely to be achieved through isolated use of more sophisticated pre-hospital assessments. It is more likely that large scale co-ordinated approaches across service boundaries will achieve a step change in performance.
The unexpected combination of thrombolysis, health and economic outcomes observed among PASTA patients led us to consider whether structured handover of additional information and/or a multidisciplinary checklist could improve the selection of patients for thrombolysis, particularly in hospitals with lower levels of specialist availability. As no harm was observed through the PASTA intervention, implementation should be considered in stroke services where there is an unavoidably low level of specialist availability for thrombolysis decision-making.
The PASTA trial process evaluation suggested that ongoing assistance with patient care by paramedics post handover did not make a significant difference to emergency stroke treatment. Although this was valued personally by some paramedic interviewees and the extra time did not cause decompensation of ambulance service response times, it was the hardest part of the pathway to initiate and there was little hospital enthusiasm because only limited actions were possible. However, both paramedics and hospital staff supported provision of feedback, which could be readily incorporated into the exchange of information during a structured handover.
Modelling outputs from WP2 informed widely disseminated recommendations for services and clinicians produced by NHS England,4 NICE97 and Oxford Academic Health Science Network. 25 These strongly support the evidence that IAT should be routinely delivered, and that the largest increase in provision in the short term will be through a ‘drip and ship’ service configuration. An increase in IAT provider centres could enable more efficient delivery of treatment, and, for the most remote populations, air ambulance transport appears to be a viable approach, although three of the nine HEMS indicated that additional funding and/or organisational changes would be required. Although clinical effectiveness was considered paramount, where travel time would be > 45 minutes, participants in our BWS study were willing to forego some health benefits to access a less expert service locally. Commissioning decisions should probably, therefore, not be made solely based on changes to maximise health gains from IAT.
Limitations
All findings must be considered in the context of potential limitations related to the research process itself. The most important ones are summarised below.
Work package 1
-
Owing to the nature of the emergency pathway developed, specific training was required for the intervention paramedics, but uptake was approximately 55% (i.e. 453/817). This self-selection may have influenced intervention delivery and the views expressed during the process evaluation. It was not possible within the remit of the programme to explore any implications for interpretation of the results or to understand the reasons for the reluctance to train.
-
Challenging operational conditions impeded objective confirmation of intervention fidelity and approximately half of the study ambulance data forms were not returned, despite efforts to encourage completion. It is unclear whether these forms were completed and accidentally lost within clinical services or not completed, and reasons for the possibility of them not being completed have not been explored.
-
As the PASTA intervention sought to directly influence clinician behaviour, it was not possible to mask group allocation. However, the lack of imbalance in baseline characteristics makes selection bias an unlikely explanation for the results.
-
It is important to recognise that the post hoc association found between the PASTA intervention and specialist availability was hypothesis-generating and mechanisms remain unclear for any influence on treatment decisions, health and economic outcomes.
-
The main limitation of the economic analysis is that utility values were estimated using published algorithms for mapping mRS scores on to the EuroQol-5 Dimensions, rather than being based on responses collected directly from participants. Although the QALY difference found was small and, therefore, potentially prone to measurement error, the value reflects the entire trial population, whereas only a proportion of patients received thrombolysis, which is a treatment that benefits or harms only a proportion of those who are treated.
-
It was not possible to obtain views from patients receiving the PASTA pathway intervention; however, the WP2 surveys strongly indicated that rapid specialist care is valued from the start of the emergency stroke pathway.
Work package 2
-
Delphi exercises and surveys reflect the views of the individuals involved only. In particular, only 150 respondents completed the BWS survey, although it is reassuring that the responses agreed with the earlier simpler survey of 103 volunteers.
-
Simulation models assumed that populations were consistent with published meta-analyses,23,24 included limited parameters reflecting underlying data sets and did not consider capital costs for setting up new services. Consequently, the results may not be completely replicated post implementation.
-
The modelling work also cannot account for unforeseen developments in future services or technologies. For example, currently, there are few published data about how IAT treatment effect varies for older patients, which could influence future stakeholder views about changes to the emergency stroke pathway.
Overall conclusions
Optimal provision of emergency stroke care is challenging because of the multiprofessional involvement, complex clinical pathways and limited time windows for effective treatment, but further gains are possible by improving the delivery of thrombolysis and by implementing the routine provision of IAT. The Promoting Effective And Rapid Stroke Care (PEARS) programme has increased our understanding of how care can be improved both within and across services in ways that are likely to be acceptable to professionals, services and the public.
In 2018/19, IAT was performed on only 1.4% of stroke admissions nationally. 6 Although rates continue to increase annually, the provision of IAT lags significantly behind thrombolysis, with marked regional variations. The key for successful NHS implementation of IAT is to take a whole-pathway approach, including ambulance call, initial assessment, initial thrombolysis, imaging, transfer for IAT procedure as appropriate and subsequent repatriation to local rehabilitation services if required. Across the programme, we addressed the optimal means of delivering stroke reperfusion therapies and improving patient outcomes and/or improving cost-effectiveness of services from initial paramedic contact and a thrombolysis treatment decision (WP1) through to selection, routes and transfer mechanisms for IAT (WP2). It has recently been suggested that paramedics could also include thrombectomy eligibility in their routine assessment,98 and there is at least one ongoing trial of paramedic-initiated identification of patients with LAO symptoms,99 both of which could lead to pre-hospital redirection to a CSC. If this were to happen on a large scale, with corresponding movement of specialist resources to hubs to match the growth in central activity, the PASTA intervention might be valuable in the remaining peripheral smaller HASUs. These would then receive only smaller volumes of patients suitable for thrombolysis, but the enhanced paramedic information collection and communication could help to promote good-quality clinical decisions, despite a reduction in local expertise.
Work package 1 highlighted the importance of evaluating a complex intervention (an enhanced paramedic assessment) using a pragmatic RCT design, as the result was not consistent with the previous positive evaluations of pre-hospital stroke interventions. 15,16,41 As the PASTA pathway was associated with lower thrombolysis rates but more dominant cost-effectiveness at hospitals with less specialist availability, the structured information collection and communication components of the intervention may have led to better-informed non-specialist treatment decisions. This is a novel hypothesis regarding clinician behaviour under specific circumstances and requires further evaluation, but is consistent with wider evidence that care delivery is improved by structured handovers and checklists. As most IAT patients also initially receive thrombolysis, the PASTA intervention has direct relevance to the delivery of this more complex emergency care pathway defined during WP2. As IAT becomes more available, it would be logical to evaluate whether or not further enhancement of pre-hospital information collection and communication can also facilitate this treatment decision, especially in the absence of a portable diagnostic test.
As might be expected from earlier publications,23,24,62,63 the WP2 systematic review and TSA60 confirmed that IAT for acute ischaemic stroke is both a highly effective and safe treatment for up to 12% of UK stroke admissions,74 which is also unequivocally cost-effective. 84 The magnitude of UK costs per treatment is similar to that described in other settings with greater volumes. These findings have had a significant impact on NHS England policy and commissioning guidance, with initial implementation at existing regional neuroscience/CSCs. When we modelled an increase from 24 to 30 CSCs to provide more equitable access to IAT, there was a return on capital investment of £8M over 5 years because of the additional volume and speed of treatment. In the absence of an acceptable pre-hospital selective redirection protocol or technology, a mixed model of Stroke Units and Thrombectomy Centres (with ‘drip and ship’ transfers when required) remains the only viable option for thrombectomy delivery currently. For the furthest (primary) HASU from the CSC, air ambulance secondary transfer for thrombectomy provides a viable cost-effective option if a 45-minute reduction in journey time is achieved compared with ground-based ambulance transport. Public views generally showed support for scenarios where additional travel time was needed to access emergency stroke treatments.
To our knowledge, ITEMS is the first online economic stroke modelling tool designed to support commissioning decisions. Being web based, it will be updated as new evidence and data emerge to promote maximal cost-effectiveness under different geographical and system conditions. Presentation of configurable service options based on region-specific information will promote changes to help realise the The NHS Long Term Plan96 goal of a 10-fold increase in the proportion of patients who receive IAT by 2022.
Recommendations for research
There are a number of research questions generated by the PASTA trial because of the unexpected combination of primary and secondary outcomes:
-
To explain the cost-effectiveness results, we have proposed a novel mechanism whereby clinicians used additional pre-hospital information to moderate their treatment threshold during difficult decisions, but proving this would require a different study design, confirming guideline-compliant treatment for individual patients.
-
We also considered whether or not the paramedic’s pre-departure checklist may have generally reinforced adherence to acute care guidelines. This approach has been adopted in other clinical settings and could be further assessed as an additional quality improvement tool for stroke and other emergency conditions.
-
Future studies could consider the value of immediate and structured paramedic feedback to improve performance of specific actions during pre-hospital assessment.
-
A wider aim would be to understand the reasons why a significant proportion of paramedics randomised to the intervention group did not engage with study training.
As more information becomes available from trials and audit reports about how IAT effectiveness and efficiency varies between patient groups and service configurations, the WP2 DES/ITEMS model can be further improved by:
-
retrospective validation of the model output after a planned service reconfiguration, which would help to define limits of uncertainty for cost-effectiveness
-
building in ambulance parameters that reflect resource availability and a potential redirection bias towards CSCs
-
feedback from stakeholders using the ITEMS interface to understand barriers to and facilitators of wider implementation
-
adding new parameters that might have a significant impact on pathways, such as the introduction of a new point-of-care diagnostic for LAO.
The programme outputs reflect the multilevel interactions between health-care monitoring systems, service configurations, clinician behaviours and evidence for specific treatments. These require mapping and parallel evaluation to maximise our understanding of the real-world consequences following introduction of complex interventions. It will be crucial that all future research in this area and any resulting care recommendations consider the whole emergency stroke pathway and the wider population of suspected stroke patients, so that specific gains do not inadvertently lead to a clinical or economic disadvantage that is unacceptable to services or patients.
Acknowledgements
We are grateful to the following individuals and groups:
-
Peter Dodds (stroke survivor; co-applicant and Programme Steering Committee member).
-
Bill Laing (stroke survivor; PASTA TSC member).
-
Melissa Roberts (carer; PASTA TSC member).
-
David Burgess (carer; WP2 group member).
-
The NIHR Clinical Research Network North-East Stroke Patient and Carer Research Panel.
-
The National Lay Member Stroke Research Network.
-
All of the patients who took part in the PASTA trial, stakeholder engagement and surveys.
The following ambulance services assisted with the development and evaluation of the PASTA intervention: NEAS NHS Foundation Trust, North West Ambulance Service NHS Foundation Trust, and Welsh Ambulance Service NHS Trust. We would particularly like to thank Chris Moore (Welsh Ambulance Service) and Andy Mawson (Great North Air Ambulance).
We also thank the external Data Monitoring Committee members [Tom Robinson (chairperson), Caroline Watkins, Tom Quinn and Chris Sutton] and TSC members [Charles Wolfe (chairperson), Martin James, Niro Siriwardena, Amanda Farrin, Olivia Wu, Christine Roffe, Damian Jenkinson and Michael Speed].
We are grateful for the administration support provided by Liam Dale, Deborah Jones, Anne Harrison and Clare Bowes.
We also want to acknowledge the assistance provided by the following individuals:
-
Amy Crowder (NIHR Clinical Research Network North East and North Cumbria).
-
Dominic Brand (The Stroke Association, London, UK).
-
Terry Flynn (TF Choices Ltd, Nottingham, UK).
-
Tomos Robinson (Newcastle University).
-
Kai Wai Lee (Newcastle University).
-
Stephen Rice (Newcastle University).
-
Daniel Nesbitt (Newcastle University).
Contributions of others
Gary A Ford, Christopher I Price, Phil White, Luke Vale, Peter McMeekin, Helen Snooks, Ian Russell (retired), Catherine Exley, Pippa Tyrrell (retired), Paul Fell and Peter Dodd were responsible for the original proposal and for securing funding.
The papers described in this report and listed as outputs have author lists that reflect the contributions of individuals funded by the programme and external collaborators. Those individuals who are not report authors but are co-authors on a programme output are thanked and acknowledged below.
Work package 1
Richard Francis, Saiful Islam, Medhi Javanbakht, Rachel Lakey, Graham McClelland, Shannon Robalino, Helen Rodgers and Lou Sutcliffe.
Work package 2
Michael Allen, Ajay Bhalla, Alastair Buchan, Jayan Chembala, Adela Cora, Dawn Craig, Anand Dixit, Peter Flynn, Eduardo Gonzalo-Almorox, Alastair Gray, Martin James, Nader Khandanpour, Hannah Lumley, Indira Natarajan, Sanjeev Nayak, Stephen Rice, Shannon Robalino, Christine Roffe, Yezen Sammaraiee, Rob Simister, Don Sims, Ken Stein (NIHR Journals Library Editor-in-Chief) and Ivan Wiggam.
Contributions of authors
Christopher I Price (https://orcid.org/0000-0003-3566-3157) was a co-applicant and lead for WP1, was the chief investigator for the PASTA trial and provided design and data interpretation assistance for WP2.
Phil White (https://orcid.org/0000-0001-6007-6013) was a co-applicant, was a lead for WP2 and contributed to the design, analysis and reporting of all relevant studies.
Joyce Balami (https://orcid.org/0000-0001-9968-3179) led the thrombectomy safety review and the costing data collection, analysis and interpretation for WP2.
Nawaraj Bhattarai (https://orcid.org/0000-0002-1894-2499) assisted with the economic analysis design, interpretation and reporting for the PASTA trial.
Diarmuid Coughlan (https://orcid.org/0000-0002-5348-3750) assisted with the design, analysis and reporting of the helicopter survey, public survey and economics modelling in WP2.
Catherine Exley (https://orcid.org/0000-0002-5348-3750) was a co-applicant, and designed and supervised the PASTA trial process evaluation. She also provided guidance on data interpretation.
Darren Flynn (https://orcid.org/0000-0001-7390-632X) led on the development work for the PASTA intervention and the PPI components of WP2, and contributed to the design, analysis and reporting of all studies.
Kristoffer Halvorsrud (https://orcid.org/0000-0002-8813-0939) assisted with the design, data collection, analysis and interpretation of the Delphi exercise and systematic review in WP2.
Joanne Lally (https://orcid.org/0000-0002-8468-1397) assisted with the design, data analysis, interpretation and reporting of the process evaluation during the PASTA trial.
Peter McMeekin (https://orcid.org/0000-0003-0946-7224) was a co-applicant and provided methodological expertise, data analysis and interpretation for the economic modelling in WP2. He also oversaw the web tool development and assisted with the economic analysis design and interpretation for the PASTA trial.
Lisa Shaw (https://orcid.org/0000-0002-3435-9519) co-designed the PASTA trial, prepared the protocol, developed the intervention, led the trial delivery team, provided trial reports, and assisted with data verification and interpretation.
Helen Snooks (https://orcid.org/0000-0003-0173-8843) was co-applicant, provided methodological expertise for the PASTA trial and assisted with data interpretation.
Luke Vale (https://orcid.org/0000-0001-8574-8429) was co-applicant, provided oversight of the economic analysis for the PASTA trial and provided methodological expertise for all aspects of economic evaluation.
Alan Watkins (https://orcid.org/0000-0003-3804-1943) provided oversight of the statistical analysis and provided methodological expertise for the PASTA trial.
Gary A Ford (https://orcid.org/0000-0001-8719-4968) was lead applicant for the PEARS programme and contributed to the design, analysis and reporting of all studies.
All authors have provided substantial contributions to the conception and design of the PEARS programme and interpretation of data, and had input into drafting the report and/or revising it critically for important intellectual content. All authors have given final approval of the version to be published.
Publications
Work package 1 publications
Flynn D, Francis R, Robalino S, Lally J, Snooks H, Rodgers H, et al. A Systematic Review of Paramedics in Hospital for Acute Stroke, Acute Myocardial Infarction and Trauma Patients. PROSPERO 2014 CRD42014010785. URL: www.crd.york.ac.uk/prospero/display_record.php?ID%20=%20CRD42014010785 (accessed 8 May 2021).
Flynn D, Francis R, Robalino S, Lally J, Snooks H, Rodgers H, et al. A review of enhanced paramedic roles during and after hospital handover of stroke, myocardial infarction and trauma patients. BMC Emerg Med 2017;17:5. https://doi.org/10.1186/s12873-017-0118-5
Price CI, Shaw S, Dodd P, Exley C, Flynn D, Francis R, et al. Paramedic Acute Stroke Treatment Assessment (PASTA): study protocol for a randomised controlled trial. Trials 2019;20:121. https://doi.org/10.1186/s13063-018-3144-z
Price CI, Shaw L, Islam S, Javanbakht M, Watkins A, McMeekin P, et al. The effect of an enhanced Paramedic Acute Stroke Treatment Assessment (PASTA) on thrombolysis delivery during emergency stroke care: a cluster randomised controlled trial. JAMA Neurol 2020;77:840–8. https://doi.org/10.1001/jamaneurol.2020.0611
Lally J, Vaittinen A, McClelland G, Price CI, Ford GA, Shaw L, et al. Paramedic experiences of using an enhanced stroke assessment during a cluster randomised trial: a qualitative thematic analysis. EMJ 2020;37:480–5. https://doi.org/10.1136/emermed-2019-209392
Bhattarai N, Price CI, McMeekin P, Javanbakht M, Vale L, Ford GA, Shaw L. Cost-effectiveness of an enhanced Paramedic Acute Stroke Treatment Assessment (PASTA) during emergency stroke care: economic results from a pragmatic cluster randomized trial. Int J Stroke 2021;7:17474930211006302. https://doi.org/10.1177/17474930211006302
Work package 1 conference abstracts
Lally J, Flynn D, Ford GA, Price CI, Exley C. Barriers and facilitators to further development of the paramedic role during assessment of suspected stroke patients. Int J Stroke 2015;10:50–1.
Lally J, McClelland G, Exley C, Ford GA, Price CI. Stakeholder engagement in the design of a novel pre-hospital acute stroke assessment. Emerg MedJ 2016;33:e9–10. https://doi.org/10.1136/emermed-2016-206139.31
Work package 2 publications
Flynn D, Francis R, McMeekin P, Price C, Ford G, Robalino S, et al. Intra-arterial Mechanical Thrombectomy in the Treatment of Acute Ischaemic Stroke: A Systematic Review and Meta-analysis. PROSPERO 2015 CRD42015016649. URL: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID%20=%20CRD42015016649 (accessed 8 May 2021).
Flynn D, Ford GA, McMeekin PJ, White P. Characteristics of intra-arterial thrombectomy service provision in England. Int J Stroke 2016;11:NP83–5. https://doi.org/10.1177/1747493016655363
Flynn D, Francis R, Halvorsrud K, Gonzalo-Almorox E, Craig D, Robalino S, et al. Intra-arterial mechanical thrombectomy in the treatment of acute ischaemic stroke: a systematic review and meta-analysis. Eur Stroke J 2017;2:308–18. https://doi.org/10.1177/2396987317719362
McMeekin P, White P, James MA, Price CI, Flynn D, Ford GA. Estimating the numbers of stroke patients in the UK that are eligible for treatment with mechanical thrombectomy. Eur Stroke J 2017;2:319–26. https://doi.org/10.1177/2396987317733343
Balami J, White P, McMeekin P, Ford GA, Buchan AM. Complications of endovascular treatment for acute ischaemic stroke: prevention and management. Int J Stroke 2018;13:348–61. https://doi.org/10.1177/1747493017743051
Halvorsrud K, Flynn D, Ford GA, McMeekin P, Bhalla A, Balami J, et al. Future delivery of thrombectomy services in England: a Delphi study and ranking exercise with clinicians. BMC Health Serv Res 2018;18:135. https://doi.org/10.1186/s12913-018-2922-3
McMeekin P, Flynn D, Allen M, Coughlan D, Ford GA, Lumley H, et al. Estimating the effectiveness and cost-effectiveness of establishing additional endovascular thrombectomy stroke centres in England: a discrete event simulation. BMC Health Serv Res 2019;19:821. https://doi.org/10.1186/s12913-019-4678-9
Oxford Academic Health Science Network. Mechanical Thrombectomy for Acute Ischaemic Stroke: An Implementation Guide for the UK. Ford G, James M, White P, editors. 2019. URL: www.oxfordahsn.org/our-work/adopting-innovation/cardiovascular-disease/mt-guide/ (accessed 6 July 2021).
Ford GA, James M, White P. Mechanical Thrombectomy for Acute Ischaemic Stroke: Challenges and Opportunities. Specialised Commissioning. November 2019. URL: www.specialisedmedicine.co.uk/articles/mechanical-thrombectomy-for-acute-ischaemic-stroke-challenges-and-opportunities/ (accessed 8 May 2021).
Balami JS, Coughlan D, White PM, McMeekin P, Flynn D, Roffe C, et al. The cost of providing mechanical thrombectomy in the UK National Health Service: a micro-costing study. Clin Med 2020;2:e40–5. https://doi.org/10.7861/clinmed.2019-0413
Coughlan D, McMeekin P, Flynn D, Ford GA, Lumley H, Burgess D, et al. Secondary transfer of emergency stroke patients eligible for mechanical thrombectomy by air in rural England: economic evaluation and considerations. Emerg Med J 2021;38:33–9. https://doi.org/10.1136/emermed-2019-209039
Work package 2 conference abstracts
Flynn D, Ford G, McMeekin P, Halvorsrud K, Bhalla A, Balami J, White P. Future delivery of thrombectomy services in England: a Delphi study with stroke physicians. Int J Stroke 2016;11:S6.
Balami JS, McMeekin P, White PM, Flynn D, Roffe C, Wiggam I, et al. Case costing of mechanical thrombectomy for acute ischaemic stroke in routine clinical setting: cost differences between mothership vs drip and ship. Int J Stroke 2018;13(Suppl. 3):10–65.
Lumley H, Flynn D, Coughlan D, McMeekin P, Ford GA, Craig D, et al. Secondary transfers by helicopter emergency services for thrombectomy in rural England: a feasibility study. Int J Stroke 2018;13:10–65. https://doi.org/10.1136/emermed-2019-209039
McMeekin P, White PM, James MA, Price CI, Flynn D, Ford GA. Incorporating evidence from recent trials in to estimates of stroke patients eligible for thrombectomy. Int J Stroke 2018;13:10–65.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke 2016;47:2331-8. https://doi.org/10.1161/STROKEAHA.116.013372.
- Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138-47. https://doi.org/10.1016/S1474-4422(16)30177-6.
- Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry 2017;88:38-44. https://doi.org/10.1136/jnnp-2016-314117.
- NHS England . Clinical Commissioning Policy: Mechanical Thrombectomy for Acute Ischaemic Stroke (All Ages) 2018.
- Stroke Unit Trialists Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;2013.
- King’s College London . Sentinel Stroke National Audit Programme 2016. www.strokeaudit.org (accessed 29 September 2021).
- Department of Health and Social Care . The National Stroke Strategy 2007.
- National Institute for Health and Care Excellence . Stroke: Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). Clinical Guideline 68 2008.
- Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014;7. https://doi.org/10.1002/14651858.CD000213.pub3.
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 5th Edition 2016.
- Morris S, Ramsay AIG, Boaden RJ, Hunter RM, McKevitt C, Paley L, et al. Impact and sustainability of centralising acute stroke services in English metropolitan areas: retrospective analysis of hospital episode statistics and stroke national audit data. BMJ 2019;364. https://doi.org/10.1136/bmj.l1.
- Elameer M, Price C, Flynn D, Rodgers H. The impact of acute stroke service centralisation: a time series evaluation. Future Healthc J 2018;5:181-7. https://doi.org/10.7861/futurehosp.5-3-181.
- Bray BD, Ayis S, Campbell J, Hoffman A, Roughton M, Tyrrell PJ, et al. Associations between the organisation of stroke services, process of care, and mortality in England: prospective cohort study. BMJ 2013;346. https://doi.org/10.1136/bmj.f2827.
- Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke 2003;34:71-6. https://doi.org/10.1161/01.STR.0000044170.46643.5E.
- Wojner-Alexandrov AW, Alexandrov AV, Rodriguez D, Persse D, Grotta JC. Houston paramedic and emergency stroke treatment and outcomes study (HoPSTO). Stroke 2005;36:1512-18. https://doi.org/10.1161/01.STR.0000170700.45340.39.
- Berglund A, Svensson L, Sjöstrand C, von Arbin M, von Euler M, Wahlgren N, et al. Higher prehospital priority level of stroke improves thrombolysis frequency and time to stroke unit: the Hyper Acute STroke Alarm (HASTA) study. Stroke 2012;43:2666-70. https://doi.org/10.1161/STROKEAHA.112.652644.
- Choi B, Tsai D, McGillivray CG, Amedee C, Sarafin JA, Silver B. Hospital-directed feedback to Emergency Medical Services improves prehospital performance. Stroke 2014;45:2137-40. https://doi.org/10.1161/STROKEAHA.114.005679.
- Wood K, Crouch R, Rowland E, Pope C. Clinical handovers between prehospital and hospital staff: literature review. Emerg Med J 2015;32:577-81. https://doi.org/10.1136/emermed-2013-203165.
- Iedema R, Ball C, Daly B, Young J, Green T, Middleton PM, et al. Design and trial of a new ambulance-to-emergency department handover protocol: ‘IMIST-AMBO’. BMJ Qual Saf 2012;21:627-33. https://doi.org/10.1136/bmjqs-2011-000766.
- Abbott TEF, Ahmad T, Phull MK, Fowler AJ, Hewson R, Biccard BM, et al. The surgical safety checklist and patient outcomes after surgery: a prospective observational cohort study, systematic review and meta-analysis. Br J Anaesth 2018;120:146-55. https://doi.org/10.1016/j.bja.2017.08.002.
- Djogovic D, Green R, Keyes R, Gray S, Stenstrom R, Sweet D, et al. Canadian Association of Emergency Physicians sepsis treatment checklist: optimizing sepsis care in Canadian emergency departments. CJEM 2012;14:36-9. https://doi.org/10.2310/8000.2011.110610.
- Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010;41:2254-8. https://doi.org/10.1161/STROKEAHA.110.592535.
- Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279-88. https://doi.org/10.1001/jama.2016.13647.
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-31. https://doi.org/10.1016/S0140-6736(16)00163-X.
- Ford G, James M, White P. Mechanical Thrombectomy for Acute Ischaemic Stroke: An Implementation Guide for the UK n.d. www.oxfordahsn.org/our-work/adopting-innovation/cardiovascular-disease/mt-guide/ (accessed 29 September 2021).
- Ismail M, Armoiry X, Tau N, Zhu F, Sadeh-Gonik U, Piotin M, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg 2019;11:14-9. https://doi.org/10.1136/neurintsurg-2018-014249.
- Turc G, Maier B, Naggara O, Seners P, Isabel C, Tisserand M, et al. Clinical scales do not reliably identify acute ischemic stroke patients with large-artery occlusion. Stroke 2016;47:1466-72. https://doi.org/10.1161/strokeaha.116.013144.
- Smith EE, Kent DM, Bulsara KR, Leung LY, Lichtman JH, Reeves MJ, et al. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 2018;49:e111-e122. https://doi.org/10.1161/STR.0000000000000160.
- Flynn D, Francis R, Robalino S, Lally J, Snooks H, Rodgers H, et al. A review of enhanced paramedic roles during and after hospital handover of stroke, myocardial infarction and trauma patients. BMC Emerg Med 2017;17. https://doi.org/10.1186/s12873-017-0118-5.
- Flynn D, Francis R, Lally J, Robalino S, Snooks H, Rodgers H, et al. A Systematic Review of Paramedics in Hospital for Acute Stroke, Acute Myocardial Infarction and Trauma Patients n.d. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42014010785 (accessed 30 September 2021).
- Lally J, McClelland G, Exley C, Ford G, Price C. Stakeholder engagement in the design of a novel pre-hospital acute stroke assessment. Emerg Med J 2016;33:e9-10. https://doi.org/10.1136/emermed-2016-206139.31.
- Glaser BG. The constant comparative method of qualitative analysis. Social Problems 1965;12:436-45. https://doi.org/10.2307/798843.
- Braun V, Clarke V, Hayfield N, Terry G, Liamputtong P. Handbook of Research Methods in Health Social Sciences. Singapore: Springer; 2019.
- McNarry AF, Goldhill DR. Simple bedside assessment of level of consciousness: comparison of two simple assessment scales with the Glasgow Coma scale. Anaesthesia 2004;59:34-7. https://doi.org/10.1111/j.1365-2044.2004.03526.x.
- Price CI, Shaw L, Dodd P, Exley C, Flynn D, Francis R, et al. Paramedic Acute Stroke Treatment Assessment (PASTA): study protocol for a randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-018-3144-z.
- Price CI, Shaw L, Islam S, Javanbakht M, Watkins A, McMeekin P, et al. Effect of an enhanced Paramedic Acute Stroke Treatment Assessment (PASTA) on thrombolysis delivery during emergency stroke care: a cluster randomised clinical trial. JAMA Neurol 2020;77:1-9. https://doi.org/10.1001/jamaneurol.2020.0611.
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. https://doi.org/10.1161/01.STR.20.7.864.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. https://doi.org/10.1161/01.str.19.5.604.
- Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified Rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011;42:2276-9. https://doi.org/10.1161/STROKEAHA.111.613273.
- Rao NM, Levine SR, Gornbein JA, Saver JL. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke 2014;45:2728-33. https://doi.org/10.1161/STROKEAHA.114.005135.
- Hsieh MJ, Tang SC, Chiang WC, Tsai LK, Jeng JS, Ma MH. Effect of prehospital notification on acute stroke care: a multicenter study. Scand J Trauma Resusc Emerg Med 2016;24. https://doi.org/10.1186/s13049-016-0251-2.
- Goldberg SA, Porat A, Strother CG, Lim NQ, Wijeratne HR, Sanchez G, et al. Quantitative analysis of the content of EMS handoff of critically ill and injured patients to the emergency department. Prehosp Emerg Care 2017;21:14-7. https://doi.org/10.1080/10903127.2016.1194930.
- Sujan M, Spurgeon P, Inada-Kim M, Rudd M, Fitton L, Horniblow S, et al. Clinical handover within the emergency care pathway and the potential risks of clinical handover failure (ECHO): primary research. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02050.
- McClelland G, Rodgers H, Flynn D, Price CI. The frequency, characteristics and aetiology of stroke mimic presentations: a narrative review. Eur J Emerg Med 2019;26:2-8. https://doi.org/10.1097/MEJ.0000000000000550.
- Bhattarai N, Price CI, McMeekin P, Javanbakht M, Vale L, Ford GA, et al. Cost-effectiveness of an enhanced Paramedic Acute Stroke Treatment Assessment (PASTA) during emergency stroke care: economic results from a pragmatic cluster randomized trial [published online ahead of print April 7 2021]. Int J Stroke 2021. https://doi.org/10.1177/17474930211006302.
- Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54. https://doi.org/10.1177/0272989X09349961.
- Curtis L, Burns B. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- National Institute for Care Excellence . Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management. NICE Guideline [NG128] 2019. www.nice.org.uk/guidance/ng128 (accessed 30 September 2021).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Guzauskas GF, Boudreau DM, Villa KF, Levine SR, Veenstra DL. The cost-effectiveness of primary stroke centers for acute stroke care. Stroke 2012;43:1617-23. https://doi.org/10.1161/STROKEAHA.111.648238.
- Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Clinical effectiveness, cost-effectiveness and acceptability of low-intensity interventions in the management of obsessive-compulsive disorder: the Obsessive-Compulsive Treatment Efficacy randomised controlled Trial (OCTET). Health Technol Assess 2017;21. https://doi.org/10.3310/hta21370.
- Lally J, Vaittinen A, McClelland G, Price CI, Shaw L, Ford GA, et al. Paramedic experiences of using an enhanced stroke assessment during a cluster randomised trial: a qualitative thematic analysis. Emerg Med J 2020;37:480-5. https://doi.org/10.1136/emermed-2019-209392.
- Munroe B, Curtis K, Considine J, Buckley T. The impact structured patient assessment frameworks have on patient care: an integrative review. J Clin Nurs 2013;22:2991-3005. https://doi.org/10.1111/jocn.12226.
- Evans SM, Murray A, Patrick I, Fitzgerald M, Smith S, Cameron P. Clinical handover in the trauma setting: a qualitative study of paramedics and trauma team members. Qual Saf Health Care 2010;19. https://doi.org/10.1136/qshc.2009.039073.
- Fitzpatrick D, McKenna M, Duncan EAS, Laird C, Lyon R, Corfield A. Critcomms: a national cross-sectional questionnaire based study to investigate prehospital handover practices between ambulance clinicians and specialist prehospital teams in Scotland. Scand J Trauma Resusc Emerg Med 2018;26. https://doi.org/10.1186/s13049-018-0512-3.
- Loseby J, Hudson A, Lyon R. Clinical handover of the trauma and medical patient: a structured approach. J Paramedic Practice 2013;5:563-7. https://doi.org/10.12968/jpar.2013.5.10.563.
- Morrison L, Cassidy L, Welsford M, Chan TM. Clinical performance feedback to paramedics: what they receive and what they need. AEM Educ Train 2017;1:87-9. https://doi.org/10.1002/aet2.10028.
- Flynn D, Ford GA, McMeekin PJ, White P. Characteristics of intra-arterial thrombectomy service provision in England. Int J Stroke 2016;11:NP83-NP85. https://doi.org/10.1177/1747493016655363.
- National Institute for Health and Care Excellence . Mechanical Clot Retrieval for Treating Acute Ischaemic Stroke. Interventional Procedures Guidance (IPG548) No. IPG548 2016.
- Flynn D, Francis R, Halvorsrud K, Gonzalo-Almorox E, Craig D, Robalino S, et al. Intra-arterial mechanical thrombectomy stent retrievers and aspiration devices in the treatment of acute ischaemic stroke: a systematic review and meta-analysis with trial sequential analysis. Eur Stroke J 2017;2:308-18. https://doi.org/10.1177/2396987317719362.
- Flynn D, Francis R, McMeekin P, Price C, Ford G, Robalino S, et al. Intra-Arterial Mechanical Thrombectomy in the Treatment of Acute Ischaemic Stroke: A Systematic Review and Meta-Analysis n.d. www.crd.york.ac.uk/prospero/display_record.php?RecordID=16649 (accessed 30 September 2021).
- Balami JS, Sutherland BA, Edmunds LD, Grunwald IQ, Neuhaus AA, Hadley G, et al. A systematic review and meta-analysis of randomized controlled trials of endovascular thrombectomy compared with best medical treatment for acute ischemic stroke. Int J Stroke 2015;10:1168-78. https://doi.org/10.1111/ijs.12618.
- Badhiwala JH, Nassiri F, Alhazzani W, Selim MH, Farrokhyar F, Spears J, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015;314:1832-43. https://doi.org/10.1001/jama.2015.13767.
- Balami JS, White PM, McMeekin PJ, Ford GA, Buchan AM. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke 2018;13:348-61. https://doi.org/10.1177/1747493017743051.
- Goyal M, Demchuk AM, Menon BK. on behalf of the ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. https://doi.org/10.1056/NEJMoa1414905.
- Campbell BC, Mitchell PJ, Kleinig TJ. on behalf of the EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18. https://doi.org/10.1056/NEJMoa1414792.
- Berkhemer OA, Fransen PS, Beumer D. on behalf of the MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20. https://doi.org/10.1056/NEJMoa1411587.
- Jovin TG, Chamorro A, Cobo E. on behalf of the REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. https://doi.org/10.1056/NEJMoa1503780.
- Saver JL, Goyal M, Bonafe A. on behalf of the SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95. https://doi.org/10.1056/NEJMoa1415061.
- Halvorsrud K, Flynn D, Ford GA, McMeekin P, Bhalla A, Balami J, et al. A Delphi study and ranking exercise to support commissioning services: future delivery of thrombectomy services in England. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-2922-3.
- Hsu CC, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval 2007;12:1-7.
- Pearl G, Cruice M. Facilitating the Involvement of people with aphasia in stroke research by developing communicatively accessible research resources. Top Lang Disord 2017;37:67-84. https://doi.org/10.1097/TLD.0000000000000112.
- Stroke Association . Research Survey: Preferences for Delivery of Clot Retrieval Services for Stroke in the UK n.d. www.stroke.org.uk/news/research-survey-preferences-delivery-clot-retrieval-services-stroke-uk (accessed 27 September 2021).
- McMeekin P, White P, James MA, Price CI, Flynn D, Ford GA. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J 2017;2:319-26. https://doi.org/10.1177/2396987317733343.
- NHS National Services Scotland . Scottish Stroke Improvement Programme 2016.
- McMeekin P, White PM, James MA, Price CI, Flynn D, Ford GA. Incorporating evidence from recent trials in to estimates of stroke patients eligible for thrombectomy. Int J Stroke 2018;13.
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708-18. https://doi.org/10.1056/NEJMoa1713973.
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11-2. https://doi.org/10.1056/NEJMoa1706442.
- Hill MD, Demchuk AM, Goyal M. Alberta stroke program early computed tomography score to select patients for endovascular treatment: Interventional Management of Stroke (IMS)-III Trial. Stroke 2014;45:444-9. https://doi.org/10.1161/STROKEAHA.113.003580.
- Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 2018;17:895-904. https://doi.org/10.1016/S1474-4422(18)30242-4.
- NHS . Advisory Committee on Resource Allocation (ACRA) (2015) 36 – Costs of Unavoidable Smallness Due to Remoteness 2016. www.england.nhs.uk/publication/advisory-committee-on-resource-allocation-acra-2015-36-costs-of-unavoidable-smallness-due-to-remoteness/ (accessed 28 September 2021).
- Great Britain . The Air Navigation Order 2016 (SI 2016 765) 2016. www.legislation.gov.uk/uksi/2016/765 (accessed 17 November 2021).
- Balami JS, Coughlan D, White PM, McMeekin P, Flynn D, Roffe C, et al. The cost of providing mechanical thrombectomy in the UK NHS: a micro-costing study. Clin Med 2020;20:e40-5. https://doi.org/10.7861/clinmed.2019-0413.
- McMeekin P, Flynn D, Allen M, Coughlan D, Ford GA, Lumley H, et al. Estimating the effectiveness and cost-effectiveness of establishing additional endovascular thrombectomy stroke Centres in England: a discrete event simulation. BMC Health Serv Res 2019;19. https://doi.org/10.1186/s12913-019-4678-9.
- Coughlan D, McMeekin P, Flynn D, Ford GA, Lumley H, Burgess D, et al. Secondary transfer of emergency stroke patients eligible for mechanical thrombectomy by air in rural England: economic evaluation and considerations. Emerg Med J 2021;38:33-9. https://doi.org/10.1136/emermed-2019-209039.
- Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J, et al. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 4. Value Health 2012;15:821-7. https://doi.org/10.1016/j.jval.2012.04.013.
- McMeekin P, Gray J, Ford GA, Rodgers H, Price CI. Modelling the efficiency of local versus central provision of intravenous thrombolysis after acute ischemic stroke. Stroke 2013;44:3114-19. https://doi.org/10.1161/STROKEAHA.113.001240.
- Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P. Oxfordshire Community Stroke Project, the International Stroke Trial (UK) . Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ 2008;336:376-9. https://doi.org/10.1136/bmj.39456.688333.BE.
- Slot KB, Berge E, Sandercock P, Lewis SC, Dorman P, Dennis M. Oxfordshire Community Stroke Project . Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke 2009;40:1585-9. https://doi.org/10.1161/STROKEAHA.108.531533.
- Office for National Statistics . National Life Tables, UK: 2015 to 2017 2018.
- Knuth DE. The Art of Computer Programming, Volume 1: Fundamental Algorithms. Boston, MA: Addison-Wesley Publishing Company; 1973.
- Edwards JD, Kapral MK, Fang J, Swartz RH. Trends in long-term mortality and morbidity in patients with no early complications after stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2017;26:1641-5. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.09.038.
- Allen M, Pearn K, James M, Ford GA, White P, Rudd AG, et al. Maximising access to thrombectomy services for stroke in England: a modelling study. Eur Stroke J 2019;4:39-4. https://doi.org/10.1177/2396987318785421.
- Elwyn G, O’Connor AM, Bennett C, Newcombe RG, Politi M, Durand MA, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLOS ONE 2009;4. https://doi.org/10.1371/journal.pone.0004705.
- Balami JS, McMeekin P, White PM, Flynn D, Roffe C, Wiggam I, et al. Case costing of mechanical thrombectomy for acute ischaemic stroke in routine clinical setting: cost differences between mothership vs drip and ship. Int J Stroke 2018;13:10-65.
- NHS England . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk (accessed 29 September 2021).
- National Institute for Health and Care Excellence . Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management 2019.
- Helwig SA, Ragoschke-Schumm A, Schwindling L, Kettner M, Roumia S, Kulikovski J, et al. Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: a randomized clinical trial. JAMA Neurol 2019;76:1484-92. https://doi.org/10.1001/jamaneurol.2019.2829.
- ClinicalTrials.gov . Direct Transfer to an Endovascular Center Compared to Transfer to the Closest Stroke Center in Acute Stroke Patients With Suspected Large Vessel Occlusion (RACECAT) 2016. https://clinicaltrials.gov/ct2/show/NCT02795962 (accessed 30 September 2021).
- King’s College London . National Results – Clinical n.d. www.strokeaudit.org/results/Clinical-audit/National-Results.aspx (accessed August 2021).
- King’s College London . National Results – Organisational n.d. www.strokeaudit.org/results/Organisational/National-Organisational.aspx (accessed August 2021).
- Intercollegiate Stroke Working Party . National Clinical Guidelines for Stroke. n.d. www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-(1).aspx (accessed August 2021).
- NHS Digital . HRG4+ 2016 17 Reference Costs n.d. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper (accessed 30 September 2021).
- National Institute for Health and Care Excellence . Stroke (Update) Evidence Review D: Thrombectomy n.d. www.nice.org.uk/guidance/ng128/documents/evidence-review-4 (accessed August 2021).
- Dijkland SA, Voormolen DC, Venema E, Roozenbeek B, Polinder S, Haagsma JA, et al. Utility-Weighted modified Rankin scale as primary outcome in stroke trials: a simulation study. Stroke 2018;49:965-71. https://doi.org/10.1161/STROKEAHA.117.020194.
- Dewilde S, Annemans L, Peeters A, Hemelsoet D, Vandermeeren Y, Desfontaines P, et al. Modified Rankin scale as a determinant of direct medical costs after stroke. Int J Stroke 2017;12:392-400. https://doi.org/10.1177/1747493017691984.
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773-83. https://doi.org/10.1016/S0140-6736(05)67702-1.
Appendix 1 Additional results for Work package 1, Examination of the Paramedic Acute Stroke Treatment Assessment pathway intervention clinical effectiveness: a cluster randomised trial
This appendix includes additional information linked to the results of the PASTA clinical efficacy trial.
| Ambulance service | Hospital site | Average annual stroke admissions (April 2016–March 2018) | Average annual thrombolysis rate (%) (April 2016–March 2018) | Interventional neuroradiology on site | Telemedicine available for use in acute care | Number of consultants on thrombolysis rota | Percentage of stroke specialists on thrombolysis rota | National Clinical Guideline-compliant for specialist thrombolysis provision |
|---|---|---|---|---|---|---|---|---|
| 1 | A | 197 | 13 | No | No | 11 | 0 | No |
| 1 | B | 252 | 17 | No | Yes | 12 | 0 | No |
| 1 | C | 594 | 16 | Yes | Yes | 13 | 100 | Yes |
| 2 | D | 500 | 10 | No | Yes | 3 | 66 | No |
| 2 | E | 625 | 9 | Yes | Yes | 3 | 100 | No |
| 2 | F | 688 | 9 | No | Yes | 4 | 0 | No |
| 2 | G | 1081 | 10 | No | Yes | 7 | 100 | Yes |
| 2 | H | 1120 | 12 | No | Yes | 7 | 86 | No |
| 2 | I | 2073 | 9 | Yes | No | 13 | 100 | Yes |
| 3 | J | 608 | 13 | No | Yes | 6 | 66 | No |
| 3 | K | 656 | 11 | No | Yes | 6 | 100 | Yes |
| 3 | L | 749 | 12 | No | Yes | 7 | 100 | Yes |
| 3 | M | 817 | 14 | No | Yes | 5 | 80 | No |
| 3 | N | 911 | 14 | Yes | No | 6 | 100 | Yes |
| 3 | O | 1001 | 12 | No | Yes | 7 | 100 | Yes |
| Characteristic | Group | |
|---|---|---|
| PASTA intervention (N = 500) | Standard care (N = 714) | |
| Gender, n (%) | ||
| Male | 259 (51.8) | 365 (51.1) |
| Female | 41 (48.2) | 349 (48.9) |
| Age (years) | ||
| Median (IQR) | 76.5 (68.0–84.0) | 77.0 (67.8–84.0) |
| Pre-stroke mRS score, n (%) | N = 494 | N = 708 |
| 0 | 233 (47.2) | 341 (48.2) |
| 1 | 78 (15.8) | 126 (17.8) |
| 2 | 65 (13.2) | 79 (11.2) |
| 3 | 66 (13.4) | 97 (13.7) |
| 4 | 42 (8.5) | 47 (6.6) |
| 5 | 10 (2.0) | 18 (2.5) |
| 0–2 | 376 (76.1) | 546 (77.1) |
| 3–5 | 118 (23.9) | 162 (22.9) |
| Stroke severity at admission (NIHSS score) | N = 499 | N = 710 |
| Median (IQR) | 8 (4–17) | 9 (4–19) |
| Mean (SD) | 11.1 (8.7) | 11.5 (8.5) |
| Results of the first brain imaging, n (%) | N = 499 | N = 714 |
| Infarction | 409 (82.0) | 607 (85.0) |
| Primary intracerebral haemorrhage | 90 (18.0) | 106 (14.8) |
| Other | 0 | 1 (0.1) |
| Characteristic | Group | |||
|---|---|---|---|---|
| PASTA intervention | Standard care | |||
| Thrombolysed (N = 197) | Not thrombolysed (N = 303) | Thrombolysed (N = 319) | Not thrombolysed (N = 395) | |
| Gender, n (%) | ||||
| Male | 110 (55.8) | 149 (49.2) | 167 (52.4) | 198 (50.1) |
| Female | 87 (44.2) | 154 (50.8) | 152 (47.6) | 197 (49.9) |
| Age (years) | ||||
| Median (IQR) | 75 (63–82) | 78 (70–85) | 5 (64–82) | 79 (70–85) |
| Pre-stroke mRS score, n (%) | N = 195 | N = 299 | N = 318 | N = 390 |
| 0 | 117 (60.0) | 116 (38.8) | 184 (57.9) | 157 (40.3) |
| 1 | 26 (13.3) | 52 (17.4) | 55 (17.3) | 71 (18.2) |
| 2 | 24 (12.3) | 41 (13.7) | 35 (11.0) | 44 (11.3) |
| 3 | 18 (9.2) | 48 (16.1) | 31 (9.7) | 66 (16.9) |
| 4 | 10 (5.1) | 32 (10.7) | 12 (3.8) | 35 (9.0) |
| 5 | 0 | 10 (3.3) | 1 (0.3) | 17 (4.4) |
| 0–2 | 167 (85.6) | 209 (69.9) | 274 (86.2) | 272 (69.7) |
| 3–5 | 28 (14.4) | 90 (30.1) | 44 (13.8) | 118 (30.3) |
| Stroke severity at admission (NIHSS score) | N = 197 | N = 302 | N = 318 | N = 392 |
| Median (IQR) | 11 (6–19) | 7 (3–17) | 11 (6–18) | 7 (3–19) |
| Mean (SD) | 12.3 (7.5) | 10.4 (9.4) | 12.4 (7.4) | 10.8 (9.3) |
| Results of the first brain imaging, n (%) | N = 197 | N = 302 | N = 319 | N = 395 |
| Infarction | 196a (99.5) | 213 (70.5) | 319 (100) | 288 (72.9) |
| Primary intracerebral haemorrhage | 1 (0.5) | 89 (29.5) | 0 | 106 (26.8) |
| Other | 0 | 0 | 0 | 1 (0.3) |
| Blood pressure on admission | N = 196 | N = 301 | N = 317 | N = 395 |
| Systolic | ||||
| Median (IQR) | 152 (132–169) | 162 (140–185) | 151 (136–168) | 158 (138–182) |
| Mean (SD) | 151.0 (25.4) | 163 (33) | 152.7 (25.9) | 161.0 (32.0) |
| Diastolic | ||||
| Median (IQR) | 82 (70–94) | 86 (74–99) | 80 (71–89) | 85 (72–96) |
| Mean (SD) | 82.4 (17.4) | 86.9 (19.8) | 81.2 (16.6) | 85.7 (19.1) |
| Blood glucose on admission | N = 190 | N = 288 | N = 303 | N = 379 |
| Median (IQR) | 6.7 (5.7–7.8) | 6.7 (5.7–7.9) | 6.5 (5.7–7.8) | 6.7 (5.7–8.4) |
| Mean (SD) | 7.2 (2.6) | 7.5 (2.9) | 7.1 (2.3) | 7.7 (3.8) |
| Anticoagulation on admission, n (%) | N = 197 | N = 302 | N = 319 | N = 394 |
| Warfarin | 6 (3.0) | 36 (11.9) | 11 (3.4) | 40 (10.1) |
| Apixaban | 0 | 9 (3.0) | 0 | 24 (6.1) |
| Rivaroxaban | 1 (0.5) | 11 (3.6) | 3 (0.9) | 19 (4.8) |
| Dabigatran | 1 (0.5) | 2 (0.7) | 1 (0.3) | 1 (0.3) |
| Location of first hospital assessment, n (%) | N = 197 | N = 303 | N = 319 | N = 395 |
| Accident and emergency department | 135 (68.5) | 213 (70.3) | 213 (66.8) | 312 (79.0) |
| CT scan room | 9 (4.6) | 15 (5.0) | 16 (5.0) | 10 (2.5) |
| Acute stroke unit | 43 (21.8) | 53 (17.5) | 82 (25.7) | 55 (13.9) |
| Critical care (ICU, HDU, CCU) | 4 (2.0) | 0 | 0 | 1 (0.3) |
| Medical admissions unit | 6 (3.0) | 21 (6.9) | 8 (2.5) | 17 (4.3) |
| Unknown | 0 | 0 | 0 | 0 |
| Other | 0 | 1 (0.3) | 0 | 0 |
| Time interval | Group | Difference in mean (PASTA intervention minus standard care) (95% CI) | p-value | |
|---|---|---|---|---|
| PASTA intervention | Standard care | |||
| Stroke onset to 999 call (minutes) | n = 454 | n = 623 | –3.35 (–9.50 to 2.80) | 0.28 |
| Mean (SD) | 47.4 (51.7) | 50.8 (49.7) | ||
| Median (IQR) | 26 (9–67.0) | 32 (12–76.0) | ||
| 999 call to paramedic assessment (minutes) | n = 479 | n = 681 | 0.75 (–1.98 to 3.50) | 0.59 |
| Mean (SD) | 28.5 (23.3) | 27.7 (23.3) | ||
| Median (IQR) | 22 (14–36) | 20 (14–34) | ||
| Paramedic assessment to leave scene (minutes) | n = 451 | n = 615 | 1.61 (–0.20 to 3.42) | 0.08 |
| Mean (SD) | 26.0 (15.5) | 24.4 (14.4) | ||
| Median (IQR) | 24 (15–34) | 22 (14–31) | ||
| Leave scene to hospital admission (minutes) | n = 441 | n = 626 | 0.08 (–1.14 to 1.30) | 0.90 |
| Mean (SD) | 16.4 (9.2) | 16.3 (10.6) | ||
| Median (IQR) | 14 (10–20) | 14 (9–20) | ||
| Hospital admission to paramedic clear (minutes) | n = 445 | n = 616 | 8.80 (6.50 to 11.04) | < 0.001 |
| Mean (SD) | 39.9 (20.2) | 31.1 (15.6) | ||
| Median (IQR) | 36 (27–50) | 29 (20–38) | ||
| Total 999 call to paramedic clear (minutes) | n = 474 | n = 666 | 13.43 (9.42 to 17.44) | < 0.001 |
| Mean (SD) | 108.8 (36.3) | 95.3 (30.5) | ||
| Median (IQR) | 102 (85–123) | 90 (76–110) | ||
| Care component | Group | Comparison | |
|---|---|---|---|
| PASTA intervention | Standard care | ||
| Acute care, n/N (%) | OR (95% CI; p-value) | ||
| Referral for intra-arterial treatment | 13/499 (2.6) | 18/714 (2.5) | 1.03 (0.50 to 2.13; p = 0.93) |
| Transfer for intra-arterial treatment | 12/498 (2.4) | 15/713 (2.1) | 1.15 (0.53 to 2.5; p = 0.72) |
| Referral for neurosurgical assessment | 45/499 (9.0) | 46/714 (6.4) | 1.4 (0.94 to 2.21; p = 0.09) |
| Transfer for neurosurgical assessment | 4/499 (0.8) | 9/713 (1.3) | 0.63 (0.19 to 2.1; p = 0.44) |
| Intravenous blood pressure lowering pre thrombolysis | 27/197 (13.7) | 39/319 (12.2) | 1.14 (0.67 to 1.93; p = 0.63) |
| Intravenous blood pressure control in haemorrhagic stroke | 39/90 (43.3) | 47/106 (44.3) | 0.96 (0.55 to 1.79; p = 0.89) |
| Reversal of abnormal coagulation for haemorrhagic stroke | 13/90 (14.4) | 16/106 (15.1) | 0.95 (0.43 to 2.1; p = 0.90) |
| Received one or more of the above interventions | 133/500 (22.6) | 143/714 (20.0) | 1.16 (0.88 to 1.54; p = 0.28) |
| Time (minutes) | Difference in mean PASTA minus standard care (95% CI; p-value) | ||
| Paramedic assessment to brain imaging time | N = 490 | N = 700 | 3.60 (–9.5 to 16.71; p = 0.59) |
| Mean (SD) | 88.9 (127.7) | 85.4 (102.4) | |
| Median (IQR) | 65 (50–89) | 66 (50–90) | |
| Hospital arrival to brain imaging time | N = 450 | N = 648 | –0.61 (–14.05 to 12.84; p = 0.93) |
| Mean (SD) | 47.0 (123.8) | 47.6 (102.4) | |
| Median (IQR) | 23 (16–40) | 26 (15–45) | |
| Forms received | Participants (N = 227), n (%) |
|---|---|
| PASTA information 1: speech and vision | |
| How is speech? | |
| Normal | 31 (13.7) |
| Slurred | 79 (34.8) |
| With word finding difficulties | 41 (18.1) |
| Slurred with word finding difficulties | 63 (27.8) |
| Missing | 13 (5.7) |
| Is the speech issue new? | |
| Yes | 10 (4.4) |
| No | 171 (75.3) |
| Unknown | 8 (3.5) |
| Missing | 38 (16.7) |
| Known to have visual problems? | |
| Yes | 44 (19.4) |
| No | 133 (58.6) |
| Unknown | 36 (15.9) |
| Missing | 14 (6.2) |
| How is vision? | |
| Patient can see both left and right sides | 104 (45.8) |
| Patient can see left side only | 10 (4.4) |
| Patient can see right side only | 18 (7.9) |
| Patient unable to see both left and right sides | 4 (1.8) |
| Patient unable to understand instructions | 65 (28.6) |
| Missing | 26 (11.5) |
| PASTA information 2: anticoagulants | |
| Warfarin | |
| Yes | 23 (10.1) |
| No | 179 (78.9) |
| Unknown | 9 (4.0) |
| Missing | 16 (7.0) |
| Apixaban | |
| Yes | 4 (1.8) |
| No | 195 (85.9) |
| Unknown | 10 (4.4) |
| Missing | 18 (7.9) |
| Rivaroxaban | |
| Yes | 5 (2.2) |
| No | 192 (84.6) |
| Unknown | 11 (4.8) |
| Missing | 19 (8.4) |
| Dabigatran | |
| Yes | 2 (0.9) |
| No | 195 (85.9) |
| Unknown | 11 (4.8) |
| Missing | 19 (8.4) |
| Takes unknown anticoagulant | |
| Yes | 15 (6.6) |
| No | 117 (51.5) |
| Unknown | 0 |
| Missing | 95 (41.9) |
| PASTA information 3: surgery | |
| Had surgery or bleeding in last 3 months? | |
| Yes | 18 (7.9) |
| No | 186 (81.9) |
| Unknown | 8 (3.5) |
| Missing | 15 (6.6) |
| If yes, number of weeks ago | |
| Minimum | 0 |
| Maximum | 17 |
| Median | 4 |
| IQR (Q1–Q3) | 6 (2–8) |
| Mean | 5.1 |
| SD | 4.2 |
| Missing | 1 (5.6) |
| PASTA information 4: previous TIA/stroke | |
| Previous TIA | |
| Yes | 49 (21.6) |
| No | 152 (67.0) |
| Unknown | 13 (5.7) |
| Missing | 13 (5.7) |
| Previous stroke | |
| Yes | 37 (16.3) |
| No | 167 (73.6) |
| Unknown | 10 (4.4) |
| Missing | 13 (5.7) |
| Previous haemorrhage | |
| Yes | 6 (2.6) |
| No | 185 (81.5) |
| Unknown | 11 (4.8) |
| Missing | 25 (11.0) |
| PASTA information 5: assistance | |
| Walking | |
| Yes | 26 (11.5) |
| No | 196 (86.3) |
| Unknown | 2 (0.9) |
| Missing | 3 (1.3) |
| Eating | |
| Yes | 8 (3.5) |
| No | 211 (93.0) |
| Unknown | 5 (2.2) |
| Missing | 3 (1.3) |
| Pre-notification | |
| Accident and emergency | |
| Not attempted | 84 (37.0) |
| Unsuccessful | 0 |
| Successful | 81 (35.7) |
| Missing | 62 (27.3) |
| Stroke team | |
| Not attempted | 92 (40.5) |
| Unsuccessful | 4 (1.8) |
| Successful | 61 (26.9) |
| Missing | 70 (30.8) |
| Via dispatch/control | |
| Not attempted | 41 (18.1) |
| Unsuccessful | 0 |
| Successful | 129 (56.8) |
| Missing | 57 (25.1) |
| Total successful pre-notification recorded | 219 (96.5) |
| Pathway deviation | |
| Yes | 2 (0.9) |
| No | 214 (94.3) |
| Missing | 11 (4.8) |
| Handover | |
| Did the handover use FASTA PASTA CT format? | |
| Yes | 134 (59.0) |
| No | 8 (3.5) |
| Missing | 85 (37.4) |
| Paramedic actions | |
| Transfer directly to scan | |
| Yes | 43 (18.9) |
| No | 160 (70.5) |
| Missing | 24 (10.6) |
| Transfer to scan from accident and emergency/stroke unit | |
| Yes | 101 (44.5) |
| No | 73 (32.2) |
| Missing | 53 (23.3) |
| Intravenous cannula before admission | |
| Yes | 124 (54.6) |
| No | 97 (42.7) |
| Missing | 6 (2.6) |
| Intravenous cannula after admission | |
| Yes | 53 (23.3) |
| No | 114 (50.2) |
| Missing | 60 (26.4) |
| Weight measurement/estimation | |
| Yes | 69 (30.4) |
| No | 121 (53.3) |
| Missing | 37 (16.3) |
| Clarification/repetition of handover | |
| Yes | 190 (83.7) |
| No | 15 (6.6) |
| Missing | 22 (9.7) |
| Checklist | |
| Brain scan requested | |
| Yes | 184 (81.1) |
| No | 14 (6.2) |
| Missing | 29 (12.8) |
| Stroke team aware | |
| Yes | 192 (84.6) |
| No | 9 (4.0) |
| Missing | 26 (11.5) |
| Stroke team reviewed | |
| Yes | 167 (73.6) |
| No | 27 (11.9) |
| Missing | 33 (14.5) |
| Patient medical history known | |
| Yes | 201 (88.5) |
| No | 4 (1.8) |
| Missing | 22 (9.7) |
| Patient medication known | |
| Yes | 190 (83.7) |
| No | 14 (6.2) |
| Missing | 23 (10.1) |
| Blood clotting test requested | |
| Not relevant | 40 (17.6) |
| Yes | 73 (32.2) |
| No | 17 (7.5) |
| Missing | 97 (42.7) |
| Feedback | |
| Feedback received? | |
| Yes | 156 (68.7) |
| No | 44 (19.4) |
| Missing | 27 (11.9) |
| If yes | |
| Diagnosis feedback | |
| Yes | 138 (88.5) |
| No | 14 (9.0) |
| Missing | 4 (2.6) |
| Onset time feedback | |
| Yes | 113 (72.4) |
| No | 24 (15.4) |
| Missing | 19 (12.2) |
| Other feedback | |
| Yes | 43 (27.6) |
| No | 56 (35.9) |
| Missing | 57 (36.5) |
| Did the paramedic leave the patient early (i.e. < 15 minutes since handover) | |
| Yes: assistance no longer needed | 43 (18.9) |
| Yes: at control request | 4 (1.8) |
| No | 166 (73.1) |
| Missing | 14 (6.2) |
FIGURE 14.
Distribution of mRS scores at day 90 for patients who received intravenous thrombolysis.
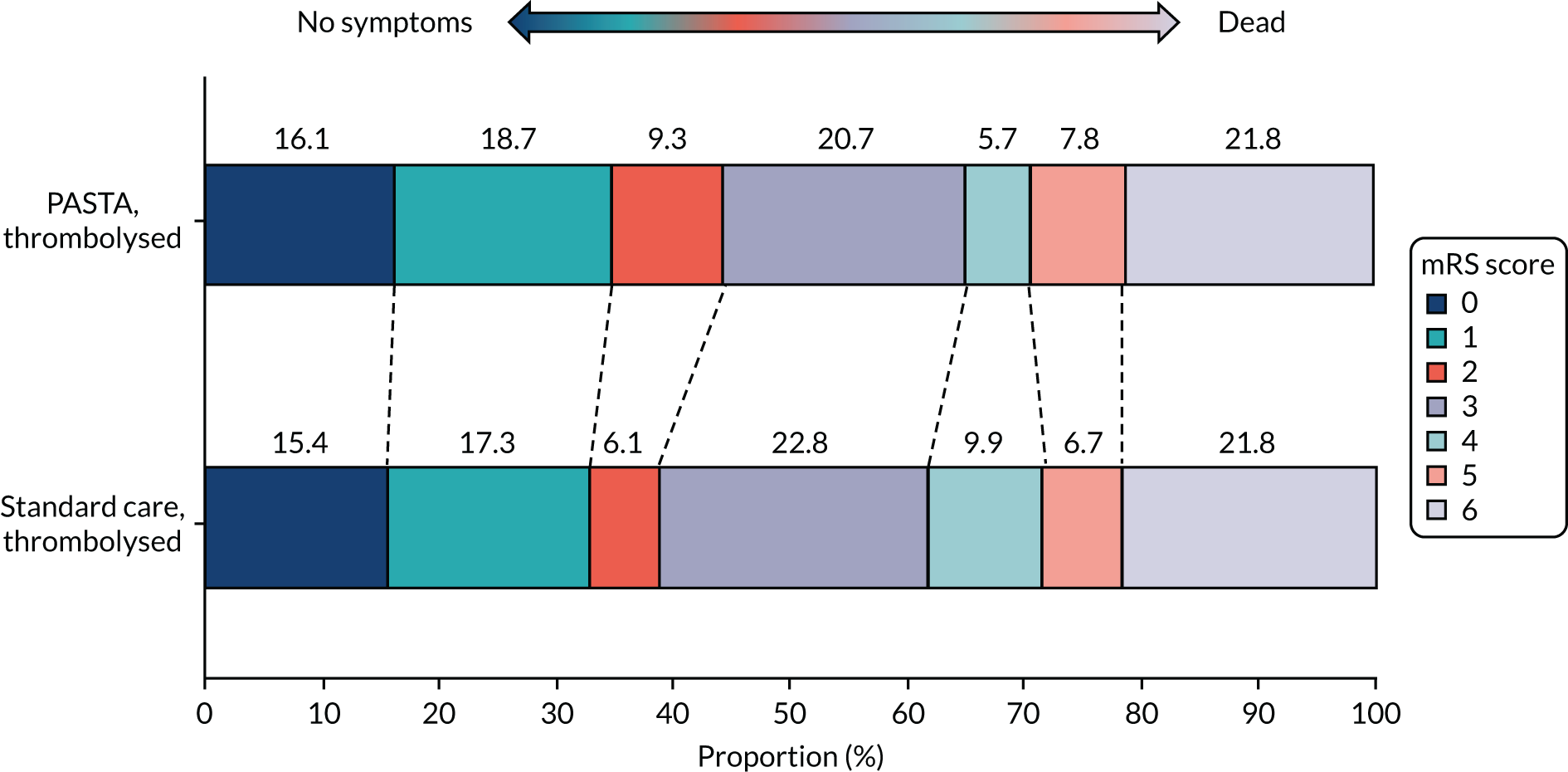
FIGURE 15.
Distribution of mRS scores at day 90 for patients who did not receive intravenous thrombolysis.
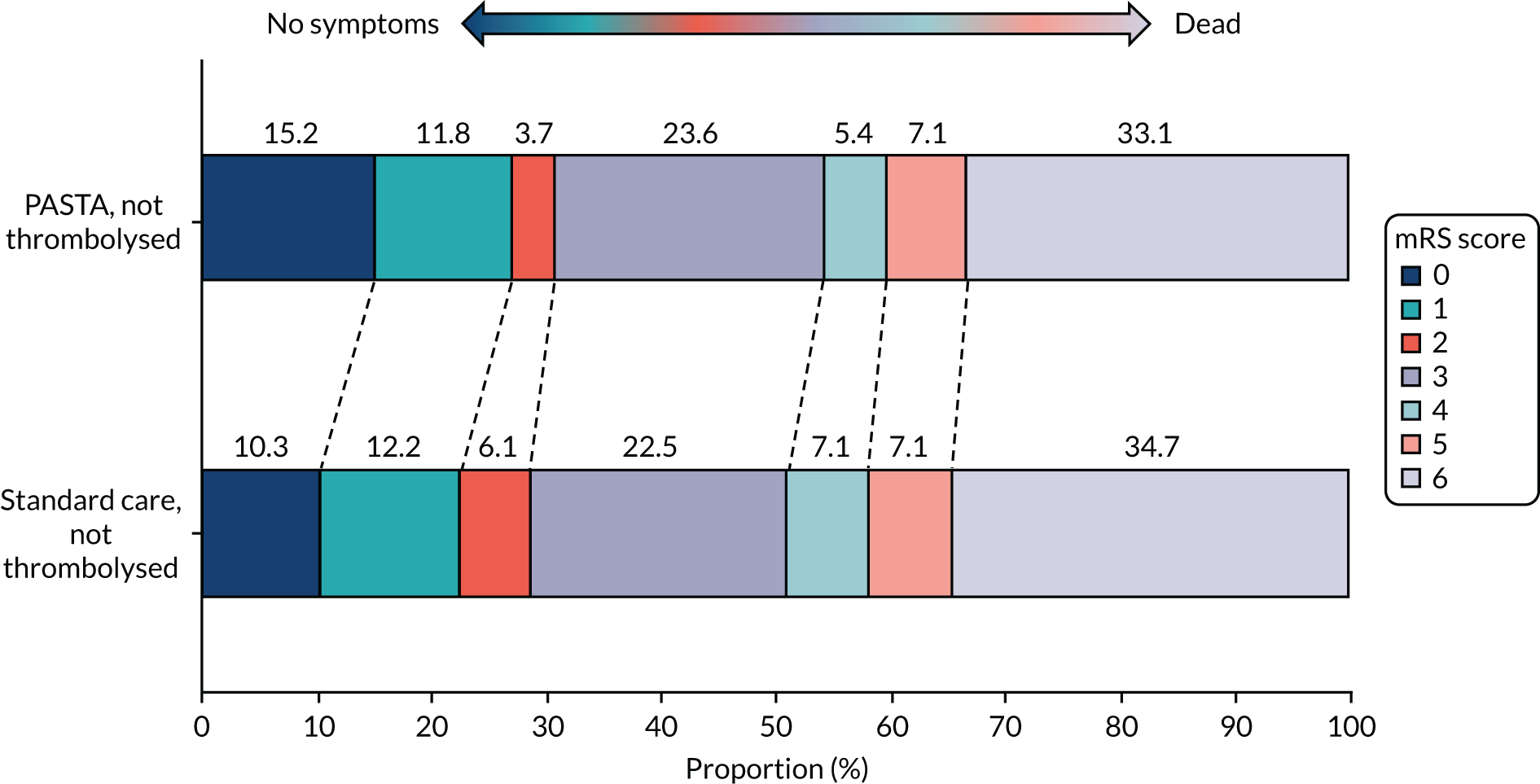
Appendix 2 Additional information and results for Work package 1, Examination of the Paramedic Acute Stroke Treatment Assessment pathway intervention cost-effectiveness
This appendix includes additional information linked to the results of the PASTA cost-effectiveness evaluation.
| Time point | Measure | Group | Difference | 95% CI | |
|---|---|---|---|---|---|
| PASTA intervention | Standard care | ||||
| Baseline | mRS score | 1.263 (n = 494) | 1.205 (n = 690) | 0.058 | –0.109 to 0.226 |
| Utility score | 0.760 (n = 494) | 0.768 (n = 708) | –0.008 | –0.036 to 0.02 | |
| 90 days | mRS score | 3.245 (n = 489) | 3.359 (n = 690) | –0.114 | –0.366 to 0.138 |
| Utility score | 0.438 (n = 489) | 0.421 (n = 690) | 0.017 | –0.0274 to 0.0615 | |
| QALYs | 0.109 (n = 489) | 0.104 (n = 690) | 0.005 | –0.006 to 0.0157 | |
| Total cost (£) | 11,809 (n = 398) | 13,217 (n = 562) | –1408 | –2695 to –121 | |
| Cost (£) | Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PASTA intervention | Standard care | |||||||||
| Obs. (n) | Cost (£) | Obs. (n) | Cost (£) | |||||||
| Mean | SD | Minimum | Maximum | Mean (£) | SD | Minimum | Maximum | |||
| Paramedic intervention training | 500 | 3.20 | 0.00 | 3.20 | 3.20 | 714 | 0.00 | 0.00 | 0.00 | 0.00 |
| Paramedic time per admission | 433 | 302.40 | 135.40 | 106.80 | 2160.90 | 612 | 255.20 | 87.80 | 8.20 | 649.10 |
| Hospital length of stay | 474 | 6685.40 | 8148.80 | 456.00 | 29,920.00 | 664 | 7122.20 | 8431.30 | 456.00 | 29,920.00 |
| Acute brain imaging | 499 | 88.40 | 3.20 | 88.20 | 138.00 | 714 | 88.30 | 2.60 | 88.20 | 138.00 |
| Intravenous thrombolysis | 500 | 2234.00 | 2773.30 | 0.00 | 5670.00 | 714 | 2533.20 | 2820.90 | 0.00 | 5670.00 |
| Intra-arterial treatments | 499 | 178.80 | 1192.10 | 0.00 | 8111.00 | 713 | 147.90 | 1086.00 | 0.00 | 8111.00 |
| Other acute stroke treatments | 500 | 53.50 | 188.90 | 0.00 | 1336.00 | 714 | 45.00 | 178.60 | 0.00 | 1336.00 |
| Early supported discharge care | 470 | 325.00 | 797.30 | 0.00 | 3288.00 | 662 | 541.40 | 993.20 | 0.00 | 3288.00 |
| Community rehabilitation care | 469 | 706.40 | 1247.80 | 0.00 | 3288.00 | 662 | 654.10 | 1214.60 | 0.00 | 2906.00 |
| Paid carer visits to private residence | 468 | 244.50 | 737.60 | 0.00 | 3444.50 | 657 | 211.30 | 682.10 | 0.00 | 3444.60 |
| Residence in a care home | 476 | 616.40 | 2487.20 | 0.00 | 14,817.60 | 664 | 882.00 | 2914.60 | 0.00 | 14,653.00 |
| Hospital re-admission | 468 | 570.50 | 2080.10 | 0.00 | 22,061.00 | 659 | 562.50 | 1956.82 | 0.00 | 16,456.00 |
| Total for complete-case data | 398 | 11,808.90 | 9603.08 | 654.20 | 41,476.70 | 562 | 13,217.00 | 10,290.96 | 692.10 | 47,627.20 |
| Difference in mean total costs (PASTA intervention minus standard care) | –1408.10 (95% CI –2695.30 to –120.90) | |||||||||
Unit costs were obtained from (1) NHS Reference Costs in the financial year 2016/17103 for ambulance provision (£247), paramedic training time (£26), brain imaging (CT costing £85.56 and magnetic resonance imaging costing £138), thrombolysis (£1341.59), acute stroke unit days (£758.24), other inpatient days (£213.44 to £446 according to setting) and early support discharge team visits (£53–96 according to a therapist); (2) NICE’s 2018 Stroke (Update) Evidence Review D: Thrombectomy104 for thrombectomy (£8111); (3) Curtis and Burns47 for community rehabilitation referrals (£2906.49), care home days (£158), salaried carer visits (£26–260 according to mRS score) and general practice visits (£38); and (4) production of intervention training materials (£6000).
FIGURE 16.
Cost-effectiveness acceptability curve for imputed data.
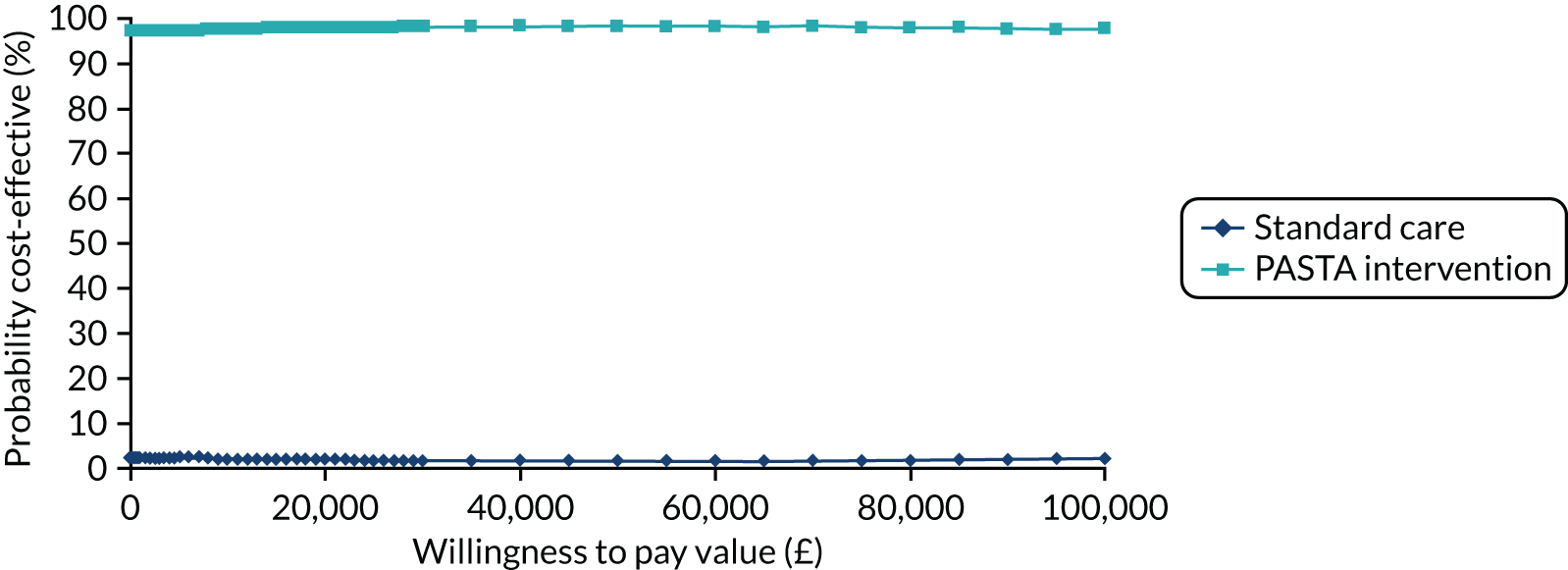
| Outcome | Hospitals compliant with thrombolysis rota guidelines, mean (95% CI) | Hospitals not compliant with thrombolysis rota guidelines, mean (95% CI) | ||
|---|---|---|---|---|
| PASTA intervention | Standard care | PASTA intervention | Standard care | |
| QALYs | 0.098 (0.089 to 0.107) | 0.103 (0.091 to 0.115) | 0.103 (0.090 to 0.115) | 0.112 (0.100 to 0.124) |
| ΔQALY | 0.005 (–0.008 to 0.018) | 0.009 (–0.008 to 0.025) | ||
| Total costs (£) | 12,542 (11,528 to 13,603) | 12,119 (10,629 to 13,512) | 14,213 (12,747 to 15,713) | 11,262 (10,053 to 12,579) |
| ΔTotal costs (£) | –423 (–2220 to 1362) | –2952 (–4988 to –917) | ||
FIGURE 17.
Cost-effectiveness plane for compliant hospitals (n = 7).

FIGURE 18.
Cost-effectiveness plane for non-compliant hospitals (n = 8).
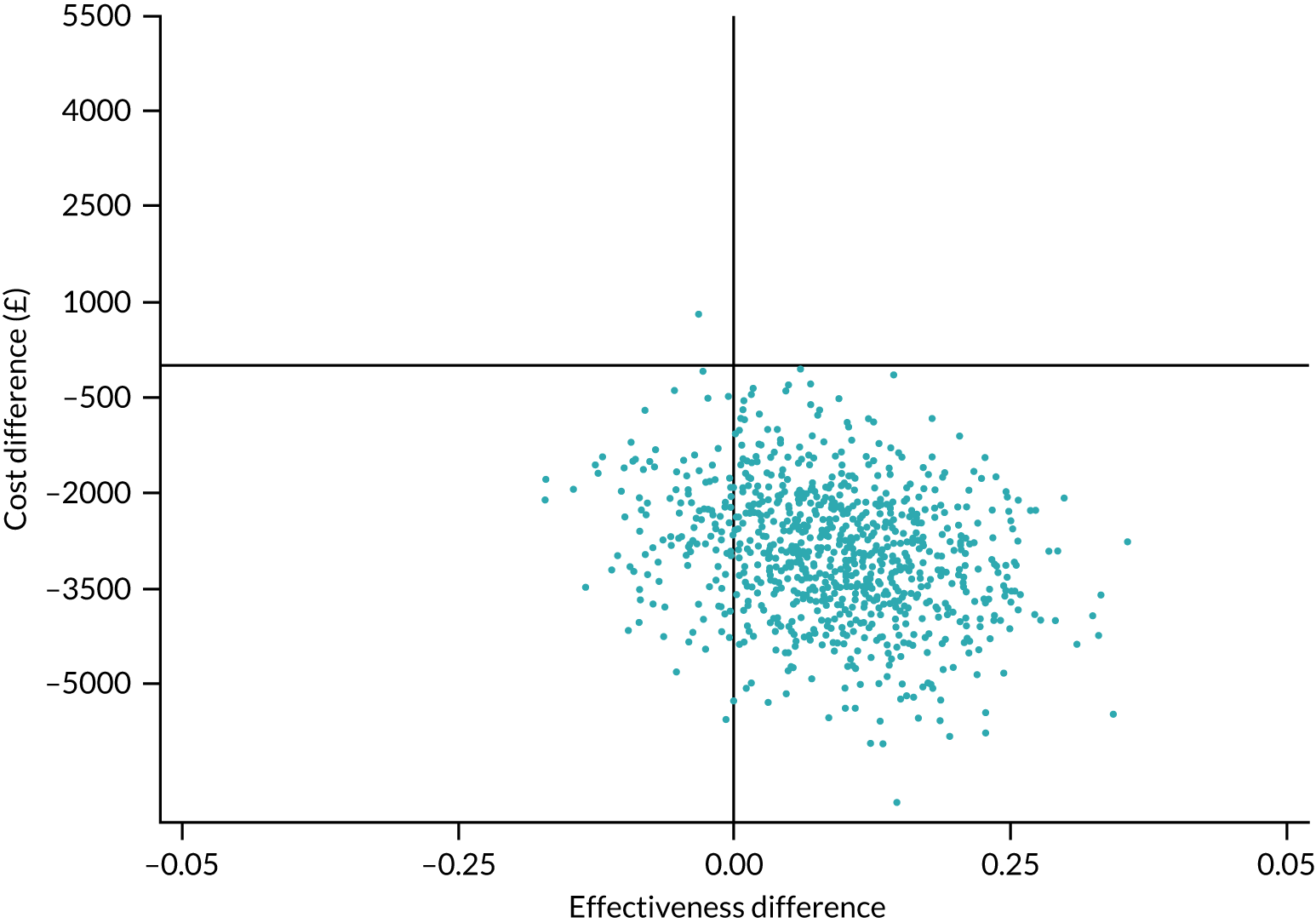
Appendix 3 Additional information and results for Work package 1, Impact of the Paramedic Acute Stroke Treatment Assessment intervention on ambulance response times
Tables 15 and 16 show the Red 1 and Red 2 ambulance response times for the PASTA and standard care groups.
| Call metric | Hour relative to the study ambulance call | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 hour before | 0–1 hours after | 1–2 hours after | 2–3 hours after | |||||
| PASTA intervention | Standard care | PASTA intervention | Standard care | PASTA intervention | Standard care | PASTA intervention | Standard care | |
| Number of Red 1 calls per study patient | ||||||||
| Mean | 1.60 | 1.85 | 1.67 | 1.77 | 1.94 | 1.81 | 1.67 | 1.70 |
| SD | 1.41 | 1.40 | 1.38 | 1.33 | 1.73 | 1.43 | 1.36 | 1.37 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 6 | 9 | 6 | 6 | 9 | 8 | 6 | 7 |
| Total | 275 | 569 | 287 | 544 | 333 | 558 | 288 | 523 |
| Compliant Red 1 responses | ||||||||
| Number | 195 | 384 | 198 | 378 | 228 | 377 | 196 | 365 |
| Percentage of total | 70.9 | 67.5 | 70.0 | 69.5 | 68.5 | 67.6 | 60.1 | 69.8 |
| Proportion of compliant Red 1 responses in cases with at least one Red 1 call | ||||||||
| Mean | 0.72 | 0.67 | 0.68 | 0.70 | 0.70 | 0.70 | 0.68 | 0.69 |
| SD | 0.35 | 0.38 | 0.36 | 0.37 | 0.34 | 0.35 | 0.39 | 0.36 |
| Call metric | Hour relative to the study ambulance call | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 hour before | 0–1 hours after | 1– 2 hours after | 2–3 hours after | |||||
| PASTA intervention | Standard care | PASTA intervention | Standard care | PASTA intervention | Standard care | PASTA intervention | Standard care | |
| Number of Red 2 calls per study patient | ||||||||
| Mean | 26.7 | 25.7 | 27.0 | 27.0 | 27.4 | 26.5 | 27.1 | 26.7 |
| SD | 8.5 | 7.4 | 12.1 | 7.5 | 21.7 | 13.9 | 17.9 | 16.5 |
| Minimum | 0 | 6 | 0 | 7 | 0 | 5 | 0 | 7 |
| Maximum | 51 | 41 | 147 | 88 | 291 | 241 | 237 | 285 |
| Total | 4241 | 7914 | 4651 | 8325 | 4707 | 8164 | 4653 | 8208 |
| Compliant Red 2 responses | ||||||||
| Number | 2592 | 4795 | 2837 | 5073 | 2847 | 5078 | 2824 | 5044 |
| Percentage of total | 61.1 | 60.6 | 61.0 | 60.9 | 60.5 | 62.2 | 60.7 | 61.5 |
| Proportion of compliant Red 2 responses in cases with at least one Red 2 call | ||||||||
| Mean | 0.61 | 0.61 | 0.61 | 0.61 | 0.60 | 0.62 | 0.59 | 0.61 |
| SD | 0.15 | 0.15 | 0.16 | 0.15 | 0.16 | 0.15 | 0.15 | 0.16 |
Appendix 4 Additional information and results for Work package 1, Process evaluation of the Paramedic Acute Stroke Treatment Assessment intervention
And to be honest, it’s [PASTA] probably stuff that we would have recorded, well I would have recorded anyway, but not necessarily in the right order. So I liked the structured approach, and I found it easy enough. It’s no different to what we would be doing with the patient. It’s just more structured and more organised, and more reportable.
P8
Quotation 2
There is very little difference from how I’ve always assessed a stroke. The visual things are different; getting them to follow your finger and things like that are a bit different. I wouldn’t usually be . . . I would be asking people their medical history and their medication, but I wouldn’t specifically be looking at whether they’re usually self-mobile and feed themselves. I wouldn’t be putting the surgical history and stuff in my handover and that.
P12
Quotation 3
We didn’t really do any visual disturbances or tests like cognition, and we didn’t differentiate between slurred speech and word finding difficulties. We knew it was important to figure out whether patients were on anti-coagulants or not.
P20
Quotation 4
I think it’s probably just helped to refine it and help it a little bit more, and give that focus to have a clear direction with your questioning. But I think having actual phrases that kind of nail it down quite succinctly . . . The checking of peripheral vision was not something that I’d ever really specifically checked for . . . But I think being aware, now, that it can specifically alter the field of view, and they may be having difficulty with vision at the peripheries was not only enlightening, but also a useful tool to bring into the history taking and observation.
P10
Quotation 5
I found that, as a sort of a memory aid, a tool, it helped to focus my history. I think it does really quite effectively help bringing a paramedic on-scene time down, which, at the end of the day, will then result in a faster onset to CT time then, won’t it, in itself.
P7
Normally the hospitals, when I ring them and say that I’m part of the PASTA trial, are really, really good. Yes, I’m more confident when I ring them as well because I know more stuff now so I can answer questions easier, if that makes any sense.
P10
Quotation 2
I have to say, they were, on the whole, very good. I think every time I’ve pre-alerted, there has either been somebody from the stroke unit in A&E [accident and emergency] waiting for me or they’ve arrived very quickly. So that’s been good.
P6
Quotation 3
But the trouble was that half the nurses you spoke to on the phone when you rang them to say, were just, basically they wouldn’t have a clue what you were talking about. It is becoming more apparent that there are some who do know about it. So you do get a better response from them. But some of them will try to cut you off in the middle of a sentence and say, ‘well I’m not interested in that, are they on anti-coagulants?’ Well if you let me work through it, you’ll know. That’s on the pre-alert.
P18
I found quite beneficial because it’s quite structured . . . Just having that in front of you, it’s much easier to fill that in on the way to hospital, and have that ready, then that gives you the structured handover and everything’s there.
P17
Quotation 2
When you do the handover as a PASTA handover it’s very . . . I don’t know the terms . . . I like doing it and you get all the information across, and I’ve had positive feedback every time I’ve asked about it. I feel like I give a lot of information very quickly, a lot more so than I used to when you’d pre-alert for a stroke. I think it’s a bit of both really. Because you are handing over in a specific way, like I say, in my handover I wouldn’t usually say about surgical history for a stroke, but I can see why that’s relevant. Whereas before it was just purely what the symptoms are and when it started, that was it.
P12
Quotation 3
To be honest, from my point of view, it made me feel more competent. I think it might be my imagination, but I think it makes us look a little bit more professional, if that makes sense.
P19
Quotation 4
I think the system does work well, and I think it could be applied to many things. I mean, the structured handover means that you don’t forget anything, you don’t miss anything out, and could be applied to every handover, really, in some respects. To have that structure would make it easier and actually, probably, would make it less daunting, when you’re coming to do a particular, like, the PASTA one. If you’re using that structure all the time, sort of thing, you just add the extra bits in that are relevant to stroke. So I did really like that. I think it helps.
P8
Quotation 5
Well as I said, the very first one they weren’t even aware of, as I say, the sister had heard of it but didn’t really know anything about it. So I spent most of my time there actually explaining what it was. She said ‘It’s good but then there’s no way they’ll ever meet the 15-minute CT time target’. Yes, so the difficulty is that you know all about the trial and you’re trying to hand it over to people that don’t necessarily know or understand the full picture.
P18
Quotation 6
Just having the knowledge, people not aware of it, just too busy to consider it – I think just because of the nature of things and how busy it is, there was often only a nurse to take handover, you know, not handing over to a doctor. I never handed over to anybody from the stroke team, even with the appropriate pre-alerts and everything else. That never happened. In fact, I never saw a stroke team coming down to a patient while I was there. I never actually handed over to a doctor or a nurse who knew about it. So that’s quite tricky.
P14
Quotation 7
But then the, sort of, having a bit of an extra hand in the ED, I didn’t really feel all that comfortable with that, really. I felt a bit like I was getting in the way, really, more than anything, with the ED nurse being there, and then the specialist nurse. I felt like I wasn’t really able to add very much, other than getting in the way.
P7
Quotation 8
Into the main stroke unit at night, and I handed over in the PASTA way. The whole team was stood there so I handed over to a nurse and the doctor at the same time. They just went, ‘That’s a fantastic handover. That’s everything we need to know’. But we have a lot of issues at our local stroke unit, as I say, it isn’t 24 hour, but, depending who’s on duty in there, they won’t see us until we’ve been triaged by the triage nurse, which, if we’re third ambulance in the queue, we’re triaged third in the queue and we could be there half an hour before we’re physically getting in and being able to do the PASTA handover to the team, at which point we’ve missed out on everything.
P2
No. That’s never happened [helped in hospital]. No, it hasn’t. I have never gone that far. I’ve never needed to – no. Yes. They have got health-care assistants in there. They have got a stroke nurse. They have got a stroke doctor. It is taken out of my hands pretty much straightaway, especially at this hospital.
P15
Quotation 2
They’re not going to be expecting us to then wait around, go to the scan with them and all that kind of thing. Whether it’s just that they don’t know about it as well and they aren’t expecting that to happen. I didn’t say it to them. I probably just didn’t feel it appropriate, to be honest and that probably says more about me in terms of not wanting to assume that that would be appropriate to do. It’s something worth thinking about though, the next time we go in.
P23
Quotation 3
Again, I’ve had no issues there. I would say, as a minimum, I’ve probably stayed 15 minutes with each patient I’ve taken in. Partly out of wanting to stay with your patient when you’ve built up that relationship. Partly because I’ve wanted to get a bit more experience, because obviously, when someone comes down from the stroke unit, they’re carrying out their assessment, which overlaps, slightly, with ours. But obviously, they have a wider range of assessment, and I’ve picked up certain little things.
P6
Quotation 4
So I wasn’t involved with anything; putting a cannula in, going round to CT, none of that happened whilst I was there. She did go to CT, but a porter came and took her – so I think it could have gone a lot better, but I think it was the stroke team not being aware of what was going on. So they weren’t too familiar with it.
P3
Quotation 5
I would be very happy to wait to know the outcome, but the pressure on ambulance staff is basically so hard that, honestly, they will ask me why I am waiting and I don’t do it, then I think that I try to wait sometimes, this is the reason why I’m trying to be quick in hospital. By waiting in the CT scan, of course, is satisfying my curiosity, and from the other half it’s not beneficial for the patients because I have provided all the information I know and it’s not beneficial, so at expense of ambulance service.
P16
Quotation 6
Yes. Our controller is aware of the PASTA trial and, obviously, we’re going to be delayed further in hospital because with the way the ambulance service go now, they want us to clear as quickly as possible so we’re available for the next job. So, we make our controller aware. We fill in the form and we get feedback, good feedback, from the doctors. We ask them, and we usually get the result of the scan and everything else, which is really good, because it’s always interesting to follow up a patient anyway.
P4
Quotation 7
I got feedback on the consultant’s initial feelings as to where it was going to go, but no feedback following on from the CT scan, because I was away by then. That everything that was done was right, and our diagnosis, they confirmed that they probably were having a stroke and they were rushing them off to CT, just confirming our diagnosis really, and that we had brought them into the department appropriately.
P17
I think they quite like that role. It gives them a little bit more credibility as well. Yes, and they’ve been with the patient for the last, sort of, however long, since they’ve picked the patient up, so they’ve built that rapport and they can take them over. But, we’ve got a good rapport with the crews, so when they are here on the unit we do have a good rapport here.
FG7: stroke nurse
Quotation 2
They seem very interested. They always like to know whether they are . . . You know, what the CT shows and whether we are going to treat it as a stroke or a mimic, or whatever.
FG7: stroke nurse
Quotation 3
Very informative, he had everything down. He could tell me exactly about onset time, past medical history, drugs, his written documentation, the examination, his FAST score, I thought was very good.
FG1: stroke nurse
Quotation 4
They were very comprehensive actually. It was an A-to-Z handover. They knew what they were talking about. They were highlighting specific parts at the handover like onset times and symptoms as well. You didn’t have to fish for the handover. It was, like, offered.
FG3: stroke nurse
Quotation 5
The time of transfer and the fact that the paramedics are escorting them down to CT as well, which means that one of our staff is not following them through, the medication has already been drawn up and they’re waiting for that result . . . so that time is saved as well.
FG7: AE nurse male
Quotation 6
It’s not a case of we’re not listening to them, but we do that for each other anyway, because conditions change.
FG3: stroke nurse
Quotation 7
But some of them do stay because they’re filling out their paperwork charting them and then at the end when they’re about to leave they usually say . . . ‘So can I just ask, was that right? Was the onset time right?’ As if they’re curious to know as well, because obviously then they can judge whether they’re doing it right or not.
FG1: stroke nurse
Appendix 5 Additional information for Work package 2, Updated estimates of certainty for intra-arterial thrombectomy effectiveness and safety
Below is the MEDLINE search strategy for the systematic review describing intra-arterial mechanical thrombectomy stent retrievers and aspiration devices in the treatment of acute ischaemic stroke. 60
Flynn D, Francis R, Halvorsrud K, Gonzalo-Almorox E, Craig D, Robalino S, et al. European Stroke Journal (volume 2, issue 4), pp. 308–18, copyright © 2017 by European Stroke Organization. Reprinted by permission of SAGE Publications.
-
1. exp brain ischemia/ or exp stroke/ or exp brain infarction/
-
2. exp “Intracranial Embolism and Thrombosis”/
-
3. (isch?emi$ adj6 (stroke$ or apoplexy$ or cerebral vasc$ or cerebrovasc$ or cva)).ti,ab.
-
4. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlu$ or hypoxi$ or accident?)).ti,ab.
-
5. stroke.ti,ab.
-
6. or/1-5
-
7. infusions, intra-arterial/ or injections, intra-arterial/
-
8. (Intra?arterial or intra arterial).tw.
-
9. (thrombol* or embolus or thrombus or endovascular device or thromboaspiration or embolectom* or thrombectom* or recanali?ation).ti,ab.
-
10. ((clot or thrombus or thrombi or embol$) adj5 (aspirat$ or remov$ or retriev$ or fragmentation or retract$ or extract$ or obliterat$ or dispers$)).ti,ab.
-
11. Thrombolytic therapy/ or exp plasminogen activators/ or “Intracranial Embolism and Thrombosis”/dt or thrombosis/dt
-
12. (tPA or t-PA or rtPA or rt-PA or plasminogen or alteplase or urokinase or reteplase or tenecteplase or streptokinase).ti,ab.
-
13. (“standard treatment?” or balloon*).ti,ab.
-
14. ((retrieval orextraction) adj5 device$).ti,ab.
-
15. endovascular procedures/ or radiography, interventional/ or radiology, interventional/ or stents/ or catheters, indwelling/ or thrombosis/su or “Intracranial Embolism and Thrombosis”/su
-
16. or/7-15
-
17. (mRS or rankin).tw.
-
18. (NIHSS or “National Institutes of Health Stroke Scale?” or “NIH Stroke Scale?” or “NIH Stroke Score?”).tw.
-
19. “Functional Independen* Measure*”.tw.
-
20. “Oxford Handicap Scale?”.tw.
-
21. (“Barthel Index” or “Barthel score?”).tw.
-
22. (EuroQoL* or EQ-5D or EQ5D).tw.
-
23. HRQoL.tw.
-
24. “quality of life”.tw.
-
25. ss-qol*.tw.
-
26. “stroke impact scale?”.tw.
-
27. “Stroke-specific Quality of Life”.tw.
-
28. “glasgow outcome scale?”.tw.
-
29. Treatment outcome/ or “quality of life”/
-
30. glasgow outcome scale/
-
31. (“clinical effectiveness” or safety).tw.
-
32. “Outcome Assessment (Health Care)”/
-
33. or/17-32
-
34. 6 and 16 and 33
-
35. (“clinical trial” or “clinical trial, phase i” or “clinical trial, phase ii” or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or “multicenter study” or “randomized controlled trial”).pt. or double-blind method/ or clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ or controlled clinical trials as topic/ or randomized controlled trials as topic/ or early termination of clinical trials as topic/ or multicenter studies as topic/ or ((randomi?ed adj7 trial*) or (controlled adj3 trial*) or (clinical adj2 trial*) or ((single or doubl* or tripl* or treb*) and (blind* or mask*))).ti,ab.
-
36. cohort studies/ or longitudinal studies/ or follow-up studies/ or prospective studies/ or retrospective studies/ or cohort.ti,ab. or longitudinal.ti,ab. or prospective.ti,ab. or retrospective.ti,ab.
-
37. or/35-36
-
38. 34 and 37
-
39. (cardiac or coronary or myocardi* or aorta or aortic).ti,ab.
-
40. 38 not 39
-
41. limit 40 to humans.
Appendix 6 Additional information for Work package 2, Expert consensus on preferred implementation option for intra-arterial thrombectomy services
-
Any local provider ‘ad hoc’
-
Any physician with some intra-arterial catheter skills delivers IAT as best they can when they can. There is no level 1 evidence (obtained from at least one properly designed and conducted RCT) for this option.
-
Any local provider delivers IAT on a formal rota
-
Interventional radiologists would likely be at the core of this option. There is no level 1 evidence for this option.
-
Transfer to nearest primary coronary percutaneous intervention unit and cardiology manage
-
There is no level 1 evidence for this option.
-
Transfer to nearest primary coronary percutaneous intervention unit and shared care with stroke physicians
-
Where a primary coronary percutaneous intervention unit and an acute stroke unit are geographically close enough to allow this to be feasible.
-
Ambulance bypass for all acute stroke patients of known time onset to comprehensive stroke unit where advanced imaging and ‘expert intra-arterial thrombectomy’ are available 24/7
-
According to data from SSNAP, 70% of acute stroke patients have known time onset and 60% of those reach hospital within 4 hours = 42%.
-
12% in SSNAP are haemorrhage not ischaemic strokes.
-
-
Local CT and transfer all patients with a NIHSS score ≥ 10 to the nearest neuroscience centre for interventional neuroradiologist delivered ‘expert thrombectomy’
-
This option is sometimes called a ‘drip and ship’ approach.
-
The neuroscience centre team might include interventional neuroradiology trained/mentored interventional radiologists or cardiologists to facilitate a 24/7 service.
-
Local CT/CTA then transfer all large artery occlusive stroke patients to nearest neuroscience centre for interventional neuroradiologist delivered ‘expert thrombectomy’
-
37% of all stroke patients arrive at hospital within 4 hours with ischaemic stroke of known onset time. ≈ 50% of patients have large artery occlusive strokes. So IAT currently potentially applies to almost 20% of acute disabling ischaemic strokes.
-
Adjunctive IAT approach is proven (level 1 evidence) to increase mRS 0–2 by 12% to 14% with benefit across the Rankin scale of shift to reduced disability.
-
Local advanced imaging then selective transfer to nearest neuroscience centre for ‘expert thrombectomy’
-
Selective brain tissue viability assessment approach to IAT is proven (level 1 evidence) to increase mRS 0–2 by 24% to 31% with benefit across the Rankin scale of shift to reduced disability.
-
All RCT results are based on expert interpretation of advanced imaging as triage for IAT
-
This option is a less time critical approach.
-
Local CT/CTA then transfer large artery occlusive stroke patients to nearest neuroscience centre for advanced imaging and ‘expert thrombectomy’
-
Advanced imaging performed locally but interpreted centrally by neuroradiology then selective transfer to nearest neuroscience centre for ‘expert thrombectomy’
-
Selective transfer to nearest on call neuroscience centre for ‘expert thrombectomy’
-
This entails networking of interventional neuroradiology units to deliver 24/7 cover sooner – with some longer transfer times, but does mean the efficacy data from RCTs can be applied (underpinned by data for UK centres from the PISTE trial).
-
Interventional neuroradiologist and necessary support team on standby in neuroscience centre – they transfer to patient’s hospital to deliver expert IAT when large arterial occlusion stroke is confirmed
-
This is provided by very few places worldwide.
-
This model of provision is clearly very expensive.
Reproduced with permission from Halvorsrud et al. 70 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Appendix 7 Additional results for Work package 2, Patient and public preferences on attributes of intra-arterial thrombectomy service organisation
| Characteristic | Overall | Stroke survivor | Relative/friend or carer of stroke survivor | Other member of the public |
|---|---|---|---|---|
| Age (years) | N = 146 | N = 27 | N = 51 | N = 67 |
| Median (IQR) | 49 (16) | 61 (14) | 47 (15) | 46 (16) |
| Range | 18–86 | 27–83 | 18–84 | 20–86 |
| Gender, n (%) | N = 147 | N = 27 | N = 50 | N = 69 |
| Male | 57 (39) | 19 (70) | 16 (32) | 22 (32) |
| Female | 90 (61) | 8 (30) | 34 (68) | 47 (68) |
| Region, n (%) | N = 147 | N = 27 | N = 51 | N = 68 |
| North-east England | 74 (50) | 10 (37) | 29 (57) | 34 (49) |
| North-west England | 6 (4) | 0 (0) | 2 (4) | 4 (6) |
| Yorkshire and the Humber | 1 (1) | 0 (0) | 1 (2) | 0 (0) |
| East Midlands | 6 (4) | 3 (11) | 2 (4) | 1 (2) |
| West Midlands | 22 (15) | 1 (4) | 4 (8) | 17 (25) |
| East of England | 7 (5) | 4 (15) | 2 (4) | 1 (2) |
| London | 9 (6) | 3 (11) | 4 (8) | 2 (3) |
| South East | 17 (12) | 5 (19) | 5 (10) | 7 (10) |
| South West | 5 (3) | 1 (4) | 2 (4) | 2 (3) |
Appendix 8 Additional results for Work package 2, Estimating the effectiveness and cost-effectiveness of establishing intra-arterial thrombectomy: a discrete-event simulation
| Parameter | Mean and uncertainty; distribution and parameters | Source |
|---|---|---|
| Cost of EVT (£) | 9116 (2519); gamma(554.86,16.42) | Balami et al.95 |
| Cost of category A ambulance per minute (£) | 6.86 | Curtis and Burns47 |
| Survival (years) following stroke at age 70 years, median (IQR) | ||
| mRS 0 | 8.4 (4.7–14.1) | Estimated from DESa |
| mRS 1 | 7.9 (4.3–13.2) | |
| mRS 2 | 7.2 (3.8–12.3) | |
| mRS 3 | 3.7 (1.4–7.0) | |
| mRS 4 | 2.7 (0.92–5.8) | |
| mRS 5 | 1.3 (0.42–3.6) | |
| mRS 6 | NA | |
| Utility parameters, interval; beta | ||
| mRS 0 | 0.95, 0.08; beta(48.4,2.55) | Dijkland et al.105 |
| mRS 1 | 0.93, 0.13; beta(128.04,9.64) | |
| mRS 2 | 0.83, 0.21; beta(222.24,45.52) | |
| mRS 3 | 0.62, 0.27; beta(173.70,106.46) | |
| mRS 4 | 0.42, 0.28; beta(173.15,239.11) | |
| mRS 5 | 0.11, 0.28; beta(6.07,49.12) | |
| mRS 6 | 0 | |
| Cost year 1 (£) | ||
| mRS 0 | 6620 | Dewilde et al.106 |
| mRS 1 | 11,196 | |
| mRS 2 | 18,929 | |
| mRS 3 | 35,771 | |
| mRS 4 | 60,118 | |
| mRS 5 | 60,458 | |
| mRS 6 | 0 | |
| Yearly cost thereafter (£) | ||
| mRS 0 | 2122 | Dewilde et al.106 |
| mRS 1 | 2836 | |
| mRS 2 | 4722 | |
| mRS 3 | 12,291 | |
| mRS 4 | 30,750 | |
| mRS 5 | 28,853 | |
| mRS 6 | 0 | |
| Proportion of all strokes presenting early with LAO and NIHSS score ≥ 6 (%) | 10.6 (SD 0.1) | McMeekin et al.74 |
| Monthly probability of deterioration before year 6b | Rothwell et al.107 | |
| 0 | 0.006 | |
| 1 | 0.004 | |
| 2 | 0.002 | |
| 3 | 0.001 | |
| 4, 5 | Not applicable – substitute with mortality | |
Appendix 9 Additional results for Work package 2, Patient, carer and public preferences
Service organisation
FIGURE 19.
Attributes and levels used in the BWS survey: service organisation.
Modelling outcomes
FIGURE 20.
Attributes and levels used in the BWS survey: modelling outcomes.
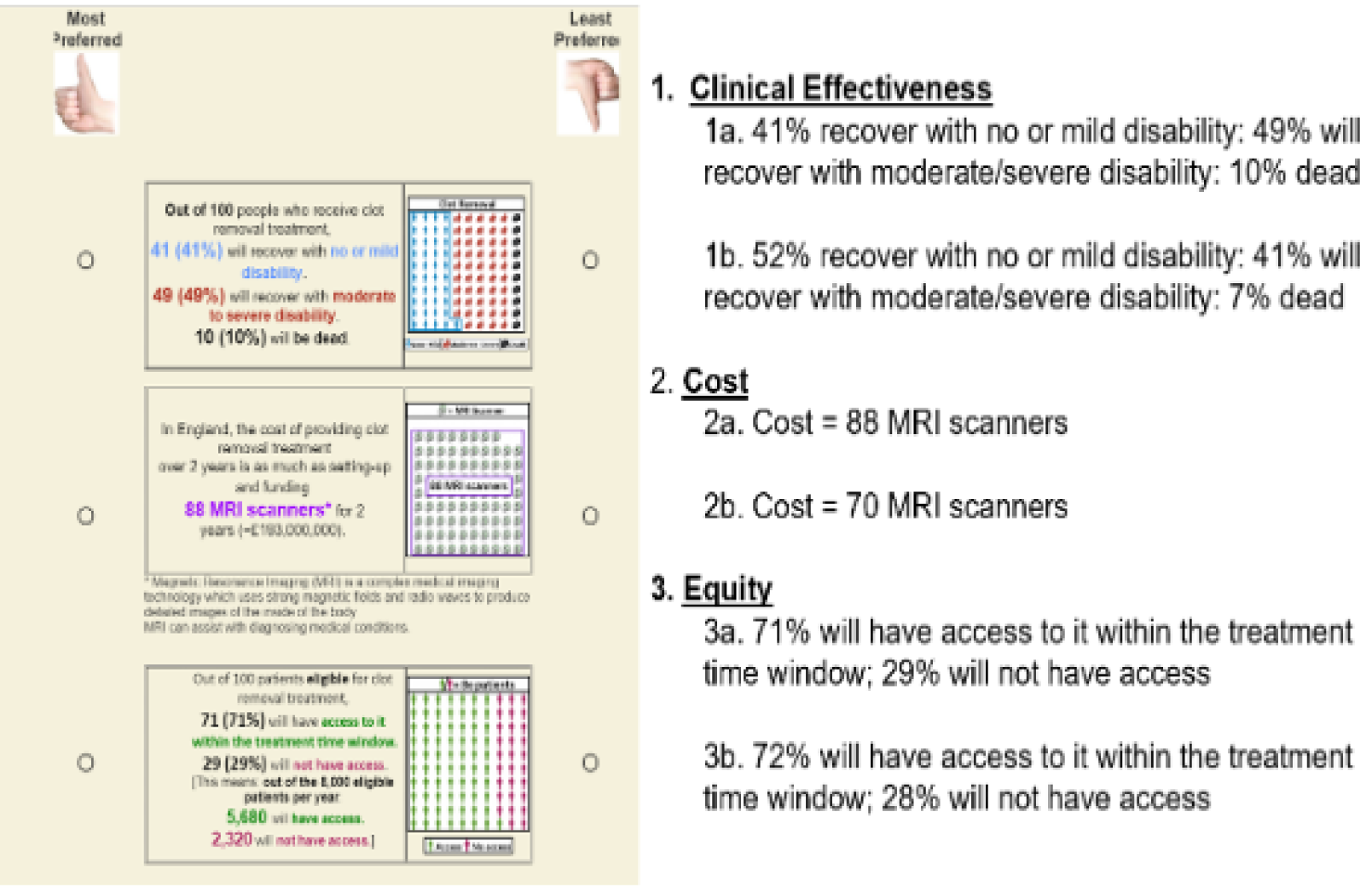
Appendix 10 Additional results for Work package 2, A commissioning decision support tool: the Interface for Thrombectomy Economic Modelling and outcomeS in stroke
To reduce the computational burden, ITEMS requires that patients similarly affected by reconfiguration are grouped into cohorts. It then compares outcomes in two alternative scenarios to estimate the marginal effects required by a health economic model.
FIGURE 21.
Conceptual model of ITEMS.
List of abbreviations
- 24/7
- 24 hours per day, 7 days per week
- aOR
- adjusted odds ratio
- AVPU
- Alert, Verbal, Pain, Unresponsive
- BASP
- British Association of Stroke Physicians
- BSNR
- British Society of Neuroradiologists
- BWS
- best–worst scaling
- CI
- confidence interval
- CLAHRC
- Collaboration for Leadership in Applied Health Research and Care
- CSC
- comprehensive stroke centre
- CT
- computed tomography
- CTA
- computed tomography angiography
- DES
- discrete-event simulation
- ED
- emergency department
- FAST
- Face, Arm, Speech Test
- HASU
- hyperacute stroke unit
- HDU
- high-dependency unit
- HEMS
- Helicopter Emergency Medical Services
- IAT
- intra-arterial thrombectomy
- ICU
- intensive care unit
- INR
- interventional neuroradiologist
- IQR
- interquartile range
- ITEMS
- Interface for Thrombectomy Economic Modelling and outcomeS in stroke
- LAO
- large artery occlusion
- mRS
- modified Rankin Scale
- NEAS
- North East Ambulance Service
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NIHSS
- National Institutes of Health Stroke Scale
- OR
- odds ratio
- OxVasc
- Oxford Vascular Study
- PASTA
- Paramedic Acute Stroke Treatment Assessment
- PEARS
- Promoting Effective and Rapid Stroke Care
- PGfAR
- Programme Grants for Applied Research
- PISTE
- Pragmatic Ischaemic Stroke Thrombectomy Evaluation
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SSNAP
- Sentinel Stroke National Audit Programme
- TIA
- transient ischaemic attack
- TSA
- trial sequential analysis
- TSC
- Trial Steering Committee
- WP
- work package
- WTP
- willingness to pay
