Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0613-20001. The contractual start date was in October 2015. The final report began editorial review in May 2021 and was accepted for publication in June 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Gooberman-Hill et al. This work was produced by Gooberman-Hill et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Gooberman-Hill et al.
Results
Work package 1: systematic reviews
Our systematic review of post-operative risk factors for chronic pain after total knee replacement included 14 studies published up to October 2016, with data from 1168 people. Studies focused on acute pain, function and psychological factors. Risk factor measures and outcomes were heterogeneous. In a narrative synthesis we were unable to draw firm conclusions on potential interventions. The need for further prospective studies in representative populations was clear.
Research published up to December 2018 into pre-operative interventions mainly focused on exercise and education. In the eight trials, with a total of 960 people randomised, there was no association with these interventions and long-term pain outcomes. In the peri-operative setting, we identified 44 trials published up to February 2018, with a range of 10 to 280 people randomised. Unifactorial interventions including some forms of analgesia, early rehabilitation, electrical muscle stimulation and anabolic steroids were associated with improved long-term pain outcomes. However, studies were small and merit further evaluation. There was reassurance that some common peri-operative treatments are not associated with chronic pain. Post-operative interventions evaluated in 17 trials published up to November 2016, with a total of 2485 people randomised, mainly focused on physiotherapy. There was no strong evidence favouring one format of therapy over another.
There has been little research into treatments for chronic pain after total knee replacement. Considering interventions for general chronic post-surgical pain, we identified 66 randomised trials with a total of 3149 participants in our systematic review with searches up to March 2016. A more focused updated search including treatments for chronic pain after arthroplasty of the large joints was conducted in October 2020. Many unifactorial interventions have been evaluated, and specific nerve-focused treatments deserve further research.
Work packages 1 and 2: analysis of national databases
We undertook two analyses of linked databases to identify pre-, peri- and post-operative risk factors for chronic pain outcome. In the first analysis with NJR and HES data, the pre- and 6-month-post-operative Oxford Knee Scores (OKS) was available for 258,386 patients, 43,702 (16.9%) of whom were identified as having chronic pain at 6 months post surgery. Post-surgical predictors of chronic pain were mechanical complication of prosthesis, surgical site infection, readmission, reoperation, revision and an extended hospital stay. However, these post-surgical predictors explained only a limited amount of variability in chronic pain outcome.
In the second analysis, we analysed primary care data from CPRD and secondary care data from the HES–PROMs database and included 4570 patients. At 6 months after surgery, 10.4% of patients were classified as non-responders to surgery regarding their knee pain. Expressing the effects as absolute risk differences allowed us to quantify the relative importance of individual risk factors in terms of the absolute proportions of patients achieving poor pain outcomes. Pre-operative risk factors were having only mild knee pain symptoms, currently smoking, living in the most deprived areas, having a body mass index between 35 and 40 kg/m2 and having had previous knee arthroscopy surgery. Post-operative risk factors were revision surgery and manipulation under anaesthetic within 3 months after the operation, and use of opioids and antidepressants within 3 months after surgery.
Work package 2: long-term follow-up of COASt cohort and analysis of national databases
We characterised the long-term trajectory of chronic pain, including pain characteristics and resource use, through the 5-year follow-up of the COASt cohort of 1581 patients with total knee replacement, and analysis of the linked CPRD and HES databases.
We applied cluster analysis to data on 128,145 patients with primary total knee replacement included in the English PROMs programme to derive a cut-off point on the pain subscale of the OKS. A high-pain group was identified, defined as those with a score of ≤ 14 points on the OKS pain subscale 6 months after total knee replacement. About one in eight people experienced chronic pain 1 year after total knee replacement. Of these patients with chronic pain after surgery, after imputing significant missing data assumed to be missing at random, 65% experienced no-chronic-pain by year 5, 31% fluctuated and 4% remained in chronic pain. People with chronic pain in year 1 had worse quality of life to start with; this improved, but less rapidly than for those not in chronic pain. People with chronic pain reported slightly higher primary care consultation costs than those not in chronic pain but their prescriptions for analgesia were much more frequent, more costly to the health-care system and continued to grow even after surgery, especially prescriptions for opioids.
Work package 3: finalisation of an assessment protocol and care pathway
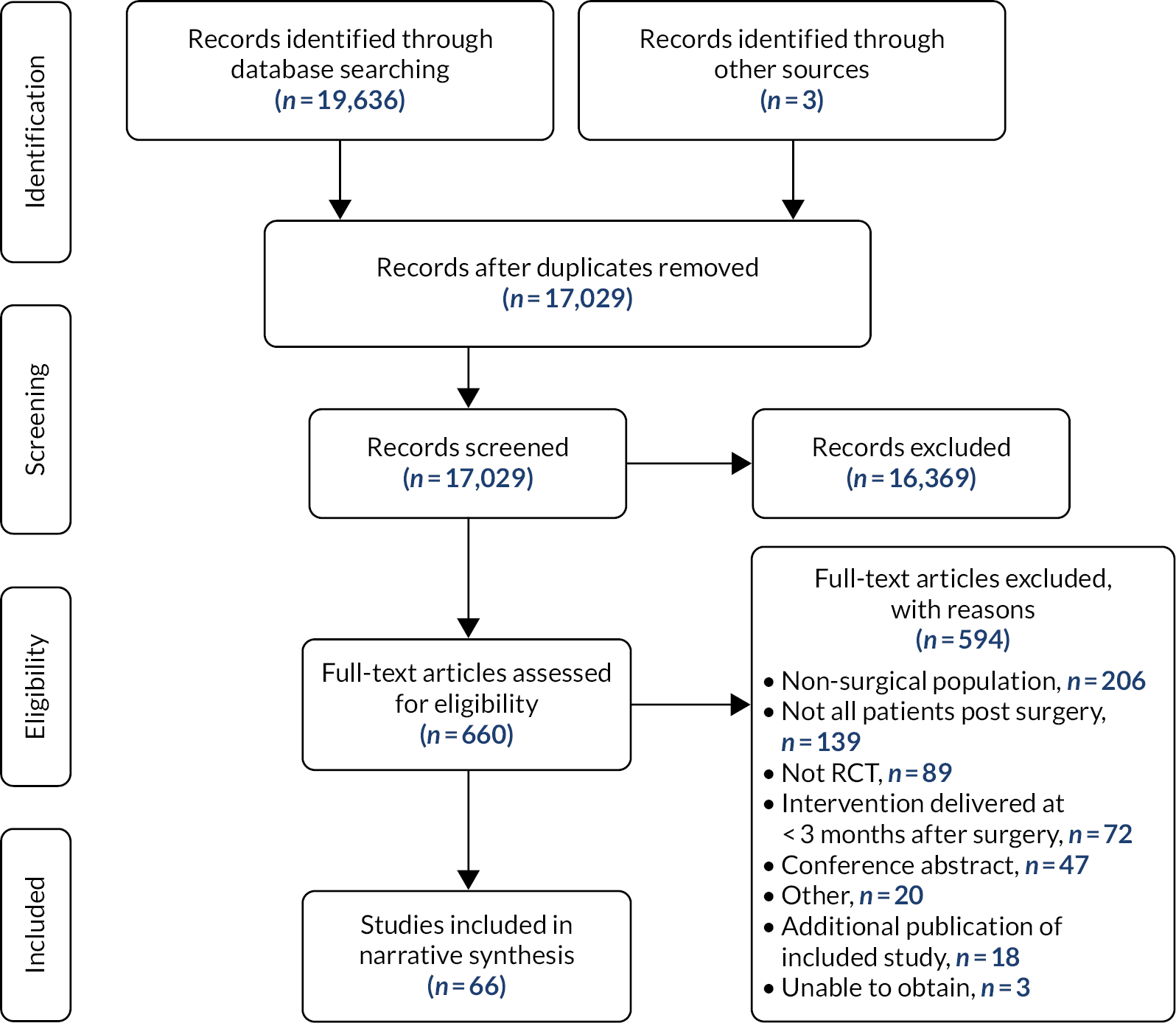
We refined and finalised the novel STAR care pathway and associated training materials. The STAR care pathway involves a clinic appointment for patients who have troublesome pain at 3 months after surgery. A specially trained extended scope practitioner (ESP) conducts a clinic assessment with the patient, comprising history, examination, radiography and questionnaire completion. Based on this assessment, which focuses on understanding the reasons for and impact of the pain, the patient is referred to the appropriate existing services for treatment, such as a surgeon, general practitioner (GP) or specialist, or receives ongoing monitoring. The ESP follows up with patients by telephone for up to 12 months.
Work package 4: randomised controlled trial
In a multicentre pragmatic, open randomised controlled trial, we evaluated the STAR care pathway. We screened 5036 people, randomised 363 patients with pain at 3 months after knee replacement from eight NHS Trusts in England and Wales and collected 12-month outcomes from 313 (85%) randomised participants. The sample had a mean age of 67 years, was 60% female and 94% white. Our analysis of clinical effectiveness indicated that at 12 months the intervention arm had lower mean pain severity and lower mean pain interference than the usual care arm. For pain interference at 12 months, the adjusted difference in means was –0.68 points on the Brief Pain Inventory pain interference scale [95% CI –1.29, –0.08; p = 0.026]. For pain severity at 12 months, the adjusted difference in means was –0.65 points on the Brief Pain Inventory pain severity scale [95% CI –1.17, –0.13; p = 0.014]. Our analysis of cost-effectiveness indicated that people receiving the STAR pathway from an NHS and personal social services perspective had lower costs (–£724, 95% CI -£1500 to £51) and more quality-adjusted life-years (QALYs) (0.03, 95% CI –0.008 to 0.06) than those receiving usual care. The STAR pathway was the cost-effective option: the incremental net monetary benefit at the £20,000-per-QALY threshold was £1256 (95% CI £164 to £2348). This was also the case from a patient perspective. Embedded qualitative research found that patients thought that the STAR pathway was acceptable, and patients described how it provided an opportunity for them to discuss their concerns and to receive more information about their condition while ensuring they received further treatment and ongoing support.
Work package 5: qualitative study
In semistructured interviews with 34 people, we found that people with chronic pain after total knee replacement who made little or no use of services did so because they became stuck in a cycle of appraisal of the validity of their need for help and concern that treatment may not be of benefit. Some were concerned that further treatment may even worsen their pain or cause further harm. When describing chronic post-surgical pain, some participants described sensations of discomfort including heaviness, numbness, pressure and tightness associated with the prosthesis, and some also reported a lack of felt connection with their knee as their movement was no longer natural and required deliberate attention, and that they had a lack of confidence in it.
Work package 6: implementation and dissemination
We found that health-care professionals involved in the delivery and implementation of the STAR care pathway valued its focus on the identification of neuropathic pain and psychosocial issues, enhanced patient care, formalisation and validation of referral practices and an increased knowledge of pain management. Stakeholders supported formal implementation of the STAR pathway. Whether or not this would be supported by hospital management was felt to be dependent on whether or not it was shown to be cost-effective.
Conclusions: implications for health care
After knee replacement, screening for pain with the OKS pain subscale beginning at 2 months after surgery can facilitate the delivery of targeted care from 3 months. Our findings indicate that the STAR care pathway can provide improved care and outcomes for people who have pain after knee replacement. To our knowledge, the STAR care pathway is the first multifactorial intervention for the treatment of post-surgical pain to have been evaluated in a randomised controlled trial. In database analyses and systematic reviews, we identified risk factors for and univariable interventions to prevent or treat chronic pain. After further research these may provide additional components to the care pathway.
Our work also indicates that people with pain could be empowered to seek health care and that health-care professionals can be encouraged provide support. This could include information for people living with chronic pain to inform them that health care may provide benefit and that seeking care is not futile. Informing patients of the likely outcomes after surgery may be a key part of pre- and post-surgical care.
Recommendations for research
We recommend that further research addresses the following points, numbered in descending order of priority:
-
How to implement the STAR care pathway into the NHS.
-
How to improve communication between patients and professionals before surgery.
-
Whether or not patient education and supportive care can enable earlier recognition of chronic pain.
-
The STAR care pathway showed benefit to patients for both pain and interference at 6 and 12 months. Further follow-up would describe the longer-term outcomes of this intervention and the health-care resources utilised by participants.
-
How to reshape the STAR pathway for other surgeries.
-
The STAR programme focused on care after surgery. Future research could make use of the recently developing evidence base about the time before surgery as an opportunity for intervention. Specifically, we now have a greater understanding of risk factors for poor outcome and using this understanding to design and evaluate pre-surgical intervention may prove of long-term benefit to patients and health-care systems.
-
How to better manage patient’s feelings of disconnectedness from the new knee and sensations of otherness to improve incorporation of the prosthesis.
-
Promising interventions, identified in systematic reviews and suggested by our risk factor studies, should be evaluated in appropriately powered high-quality randomised controlled trials.
-
New interventions with evidence of effectiveness in the treatment of chronic pain after knee replacement should be considered as new components of multifaceted personalised care as delivered in the STAR intervention.
Study registration
All systematic reviews were registered on PROSPERO (CRD42015015957, CRD42016041374 and CRD42017041382). The STAR randomised trial was registered as ISRCTN92545361.
Funding
This project was funded by the National Institute for Health and Care Research (NIHR) Programme Grants for Applied Research programme and will be published in full in Programme Grants for Applied Research; Vol. 11, No. 3. See the NIHR Journals Library website for further project information.
Background to the STAR programme
Total knee replacement
Osteoarthritis is the most common joint disease, affecting nearly 10% of adults in the UK1 and about 23% of adults in the USA. 2 The prevalence of knee osteoarthritis depends on its definition: international estimates vary from 8.2% (presence of symptoms) to 9.3% (self reported) to 31.7% (radiographic changes). 3
The primary reason that people choose to undergo total knee replacement is the expectancy of pain relief. 4 In 2019, over 100,000 primary total knee replacements were performed by the NHS,5,6 and it is estimated that about 11% of women and 8% of men will receive a knee replacement during their lifetime. 7
For many people with advanced osteoarthritis, total knee replacement is an effective treatment to relieve pain and improve function. However, some people experience continuing pain in the months and years following surgery.
Chronic post-surgical pain
Chronic post-surgical pain, defined as pain that occurs or increases in intensity at ≥ 3 months after surgery,8 is recognised after a range of surgeries. 9,10 After total knee replacement, average pain severity plateaus by 3 months,11 with overall clinical benefit achieved by 6 months. 12 People with bothersome pain at ≥ 3 months after surgery are often disappointed with their outcome. 4,13 We also know that people with chronic pain after total knee replacement may feel abandoned by health care,14 and struggle to make sense of ongoing pain. 15
In our systematic review bringing together longitudinal studies in representative populations, we found that 10–34% of patients reported unfavourable long-term pain outcomes after knee replacement. 16 The two UK studies included in the review showed that about 20% of patients with total knee replacement had persistent moderate-to-severe long-term pain in their operated knee. 17,18 More recent studies suggest that the prevalence of chronic pain has not changed, with estimates of 15–29%. 19–22 Furthermore, even among those eligible for fast-track total knee replacement, over one-third of patients may require analgesics 1 year after surgery with about half taking opioids. 23
Chronic pain has an impact on many areas of life and is associated with poor general health,24,25 interference with daily activities, disability24 and depression. 26 People with chronic musculoskeletal pain report lower satisfaction with life than the general population. 27–29 Older people with pain may become socially isolated, develop other health problems30 and have limited capacity to bring about change or seek help for their pain.
Economic impact
Chronic pain management has been estimated to account for 4.6 million general practitioner (GP) appointments per year in the UK, which is equivalent to the entire workload of 793 full-time GPs, at a cost of around £69 M. 31 In England in 2005, in addition to over-the-counter purchases, more than 66 million prescriptions were written for analgesic drugs, at a cost of about £510 M. 32 In Europe, the health-care and socioeconomic costs of chronic pain conditions represent 3–10% of gross domestic product, mainly owing to hospitalisations. 33 Although we have some understanding of the economic burden of chronic pain,34,35 up-to-date cost data on chronic pain after total knee replacement is needed.
Aetiology
Chronic pain after total knee replacement may be caused by biological and mechanical factors. Biological causes include the sensitising impact of long-term pain from osteoarthritis,36,37 the development of complex regional pain syndrome (CRPS),38–40 inflammation, infection and localised nerve injury. 41 Mechanical causes include altered gait, prosthesis loosening and ligament imbalance. 42,43 Psychological factors may also influence outcomes. 44–47
Risk factors
To prevent and manage chronic pain after knee replacement, patients, their treating surgeons and health-care professionals need to understand and target the risk factors for and causes of chronic pain after total knee replacement. 48
The potential value of pre-operatively identifying patients at risk of a poor outcome following total knee replacement and using targeted interventions is clear, and much research has focused on pre-operative predictors of outcomes. 45,46,49–51 Potentially modifiable risk factors include pain intensity,45,46,49,50 particularly on movement;52 presence of widespread pain;45,46 and anxiety, depression and pain catastrophising. 44–47,49,53 However, existing multivariable models have low predictive power for pain-related outcomes. 54,55
The operation itself is an important risk factor for chronic pain,56 and factors relating to the operation and recovery may be important. Early post-operative pain is associated with chronic pain,57 and new peri-operative analgesia regimens attempt to limit this. 58–60 In the context of major orthopaedic surgery, it is possible that other post-operative patient factors may be associated with the development of chronic pain.
Prevention
The targeted management of patients with pain after surgery may reduce the risk of longer-term pain and disability. 61 Interventions provided in the knee replacement pathway may have an impact on chronic pain through the modification of risk factors or provision of targeted care to specific patient groups.
Pre-surgical exercise and education interventions have focused on preparing patients for their knee replacement and hospital stay, reducing peri-operative pain, and facilitating early mobilisation and recovery. 49 However, randomised trials and meta-analyses published up to November 2015, when the Support and Treatment After joint Replacement (STAR) programme was developed, had not shown an impact on the key outcome of long-term pain. 49,62
Any treatment in the peri-operative period (including pain management, blood conservation, deep-vein thrombosis and infection prevention, and inpatient rehabilitation) could affect patient recovery and chronic pain. Direct mechanisms of treatments may be through prevention of nerve damage,40 post-thrombotic syndrome,63 reperfusion injury64 and articular bleeding. 65 For other treatments, the pathways leading to long-term pain may be indirect, possibly mediated through increased risks of adverse events. 66
Although early rehabilitation during the hospital stay focuses on regaining range of motion and improving mobility, after discharge treatment aims to enhance recovery through supporting a person to regain function and quality of life, optimising pain relief, and supporting reintegration into social and personal environments. 67 Although physiotherapy often focuses on functional health, another key outcome is the prevention of long-term pain. 68 A recent randomised trial found that a targeted outpatient rehabilitation programme after knee replacement does not improve outcomes in patients at risk of poor outcomes compared with a home-based exercise programme. 69 However, post-operative physiotherapy may be combined with other interventions to provide multidisciplinary comprehensive rehabilitation after knee replacement aimed at improving activity and participation, and reducing the severity of pain. 70
Management
Treatment is difficult once chronic pain is established, and the evaluation of treatments in combination or matched to patient characteristics is advocated. 71 Management of chronic post-surgical pain may focus on the underlying condition leading to surgery or the aetiology of the pain, or be multifactorial in recognition of the diverse causes of post-operative pain.
For patients with total knee replacement, surgical or prosthesis-related problems may require physiotherapy, bracing, arthroscopy or revision surgery. Nerve injury may respond to gabapentin or pregabalin,42 and nociceptive and regional pain may be treated with analgesic and opioid medication. Patients may also benefit from broader pain management approaches including psychological therapies, although high-quality evidence is lacking. 72
In our systematic review of randomised controlled trials published up to October 2014 evaluating interventions for the treatment or management of chronic pain after total knee replacement, we identified a single trial evaluating an intra-articular injection with antinociceptive and anticholinergic activity. 48 No trials of multidisciplinary interventions or individualised treatments were identified, and none was registered.
Health care
Management of chronic post-surgical pain is provided within primary and secondary care. However, not everyone will present at primary or secondary care for treatment of chronic pain. A European survey of almost 6000 adults with musculoskeletal pain suggested that over one-quarter had never sought medical help for their pain, despite many living with constant or daily pain. 73 Our research showed that 75% of adults aged > 35 years experiencing hip or knee pain had not sought help from a GP or allied health-care professional in the previous 12 months. 74 Half of adults with severely disabling knee pain may not consult a GP. 75 In this study, over 85% of participants had consulted about other illnesses and for each contact about knee pain there were 20 contacts relating to other conditions.
The provision of services for chronic pain may also be suboptimal. The 2012 National Pain Audit76 reported significant variation in access to specialist services for chronic pain and variation in levels of care. For example, 67% of services in England were below the minimum recommended levels of staffing, with a notable lack of provision for specialties including psychology and physiotherapy. Under-treatment is also apparent in the primary care setting: an interview study of over 500 GPs found that 81% believed patients received insufficient pain management. 77
Non-use of services is likely to be influenced by individual and social, structural and organisational factors. The average age of patients at knee replacement is 70 years,5 and older people may see pain as a normal part of ageing and may not present to health care. 78,79 Given the high prevalence of chronic post-surgical pain, there is potentially a large hidden population with an unexpressed need for care who are experiencing significant pain and disability.
The STAR programme
The overall aim of the STAR programme was to generate high-quality evidence about how to improve health care and outcomes for people with chronic pain after total knee replacement.
This programme provided the opportunity to conduct a major programme of work addressing multiple important themes. The programme comprised six interconnected work packages, which aimed to improve health care and outcomes for patients with chronic pain after total knee replacement. All work packages were underpinned by collaborative working with patients and full details of patient and public involvement (PPI) are reported in Patient and public involvement.
Specifically, the programme aims were as follows:
-
Synthesise evidence on the prevention of chronic pain after knee replacement and the treatment of chronic pain after diverse surgeries through systematic reviews of published studies. Identify post-surgical predictors of chronic pain after knee replacement through a systematic review and analysis of data from the National Joint Registry (NJR), linked to the Hospital Episode Statistics (HES) and Patient Reported Outcome Measures (PROMs) databases (work package 1).
-
Characterise the long-term trajectory of chronic pain, including an examination of pain characteristics and resource use, through extended follow-up of an existing cohort of patients with total knee replacement up to 5 years after surgery and analysis of the Clinical Practice Research Datalink (CPRD), linked to the HES database (work package 2).
-
Refine and finalise a novel care pathway for patients with chronic pain after total knee replacement through consensus work with health-care professionals. Test intervention delivery and acceptability to patients and evaluate views about implementation of the intervention at future trial centres using the Normalisation Measure Development (NoMAD) tool (work package 3).
-
Evaluate the clinical effectiveness and cost-effectiveness of the new care pathway for patients with chronic pain after total knee replacement in a pragmatic, open-label, parallel group, multicentre superiority randomised controlled trial with a 2: 1 allocation ratio and embedded economic evaluation and qualitative studies (work package 4).
-
Identify reasons for the non-use of services and how to improve access through a qualitative study with patients living with chronic pain after total knee replacement who make little or no use of formal health-care services (work package 5).
-
Conduct a process evaluation of the implementation of findings into clinical practice and distribute evidence-based information about the identification, assessment and management of chronic pain after total knee replacement (work package 6).
A research pathway diagram is provided in Figure 1.
FIGURE 1.
The STAR programme.
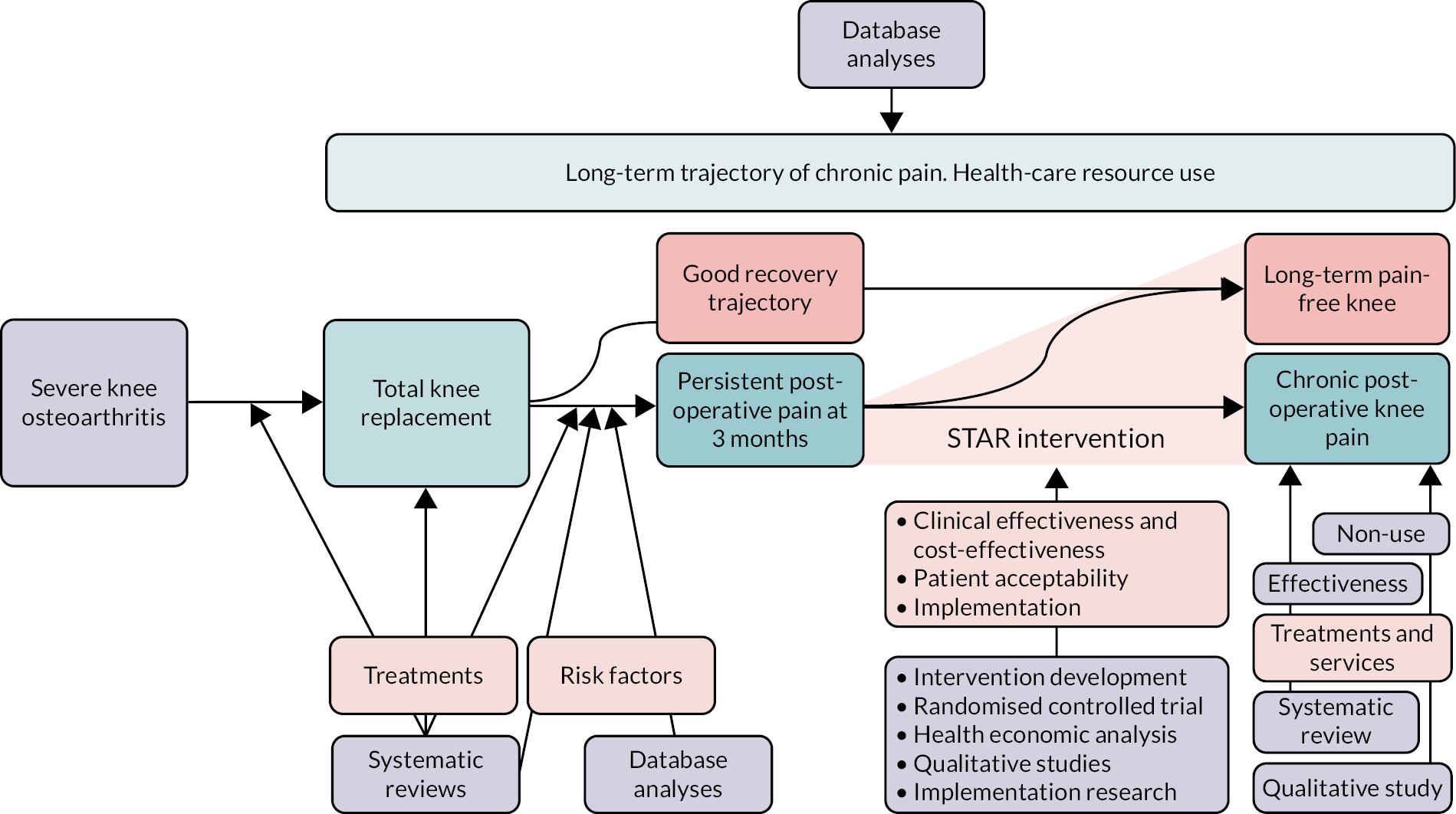
Changes to the programme and additional research
All planned research has been published or submitted to a journal, or is being written up. Some changes were made to the proposed research and additional investigations undertaken.
In work package 1, we originally planned an overview of systematic reviews looking at the effects of interventions in the knee replacement pathway. The reviews we identified did not focus on the outcome of long-term pain and so we undertook three new reviews of interventions in the pre-, peri- and post-operative setting.
In work package 2, we originally planned to conduct analyses of CPRD–HES data using an algorithm from our National Institute for Health and Care Research (NIHR) Programme Development Grant that indirectly identifies patients with chronic pain based on primary care resource use. Ultimately, we identified people with chronic pain directly from patient-reported pain outcomes. To do this, we requested two amendments to the CPRD protocol: (1) to use the CPRD to answer the programme’s specific research questions, and (2) to obtain linked HES–PROMs data that included the patient-completed Oxford Knee Score (OKS). 80 We conducted and published20 a study using publicly available HES–PROMs data between 2012 and 2015 and found that a cut-off of 14 points on the 28-point OKS pain subscale could be used to identify patients with chronic pain following knee replacement. We used this cut-off point in subsequent analyses of CPRD–HES linked patient-level data to evaluate the outcomes, resource use and quality of life of patients with and without chronic pain.
In work package 3 we used the NoMAD survey instrument81 rather than the Normalisation Process Theory (NPT) toolkit. The NoMAD tool had not been developed at the time of our original proposal and enabled us to collect more detailed information about implementation than the NPT toolkit. The questions in NoMAD are based around the four core constructs of NPT that represent different kinds of work that people undertake around implementing a new practice. The 23-item NoMAD survey instrument was developed by the same authors of the NPT toolkit and is a more flexible instrument for measuring implementation potential and implementation processes. To refine the intervention content, we conducted consensus questionnaires and facilitated meetings with health-care professionals. We proposed in the grant application to evaluate inter- and intra-observer reliability of intervention delivery using analysis of variance methods. Instead, we used a more narrative analysis of findings, which more closely aligned with our aims of ensuring the intervention was deliverable and allowed us to identify and address logistical issues with intervention delivery.
At the end of the STAR trial internal pilot phase described in work package 4, enrolment of participants was lower than anticipated. We developed a site feasibility assessment process and recruitment projection tool, increased the number of trial sites from four to eight, and extended the recruitment period for the trial by 3 months. This resulted in the achievement of the intended recruitment target. This change was approved by the Programme Steering Committee, NIHR and the NHS research ethics committee. We also made minor changes to the trial outcome measures at the trial design stage to minimise redundancy and participant burden, while ensuring that all items of the core outcome set for chronic pain after knee replacement were sufficiently represented. We did not include the WOMAC® (Western Ontario and McMaster Universities Osteoarthritis Index), as knee pain and function were assessed with the OKS. Temporal aspects of pain were measured with a single-item question rather than the Measure of Intermittent and Constant Pain, and we did not include single-item questions on pain duration, pain on kneeling or improvement in pain. Additional measures included were the Douleur Neuropathique-482 and a body pain map to assess widespread pain.
Results of a planned cohort-based Markov model to estimate 5-year costs and the (quality-adjusted) life expectancy of patients with chronic pain after total knee replacement under current practice in addition to the STAR intervention is not yet completed.
Impact of COVID-19
The COVID-19 pandemic impacted on the randomised trial and stakeholder engagement. Adaptations made to the research in relation to this are described in the relevant sections.
In work package 6, we planned to collaborate with local implementation teams in two participating centres to identify ways to deliver the programme findings in practice. Owing to COVID-19, this work was changed to an online meeting with key national stakeholders to communicate findings, including a short, animated video. Participants comprising NHS managers, heads of therapy, physiotherapists, surgeons, pain clinicians, representatives from relevant professional organisations, representatives from Versus Arthritis (Chesterfield, UK) and patients.
Patient and public involvement
Background patient and public involvement work leading to the programme
The STAR programme was developed in collaboration with the University of Bristol’s Musculoskeletal Research Unit patient involvement group, called the Patient Experience Partnership in Research (PEP-R). 49 PEP-R comprises people with experience of musculoskeletal conditions, including pain after surgery. STAR was also developed with input from a representative of Versus Arthritis, the UK’s largest charity dedicated to supporting people with arthritis. Ongoing collaboration with Versus Arthritis shaped the research and provided input into the design of the key implications for research and practice. The STAR programme was preceded by a 1-year NIHR Programme Development Grant that included PPI work. PPI informed the overall design of the programme, with specific input into the study of post-operative predictors and the trial design, including the acceptability of randomisation, timing of data collection, primary outcomes and questionnaire length. The PEP-R group also discussed and approved the PPI plans, specifically requesting a model of ‘taking the research to the patient’ rather than ‘taking the patient to the research’.
The approach to PPI was based on principles of coworking and partnership, in which we aimed to empower the individual patient partners by considering them part of the research team. We worked together to design PPI that resulted in patients having a considerable input into the relevance and quality of our research. 83,84 Our reporting of PPI is in keeping with the recommendations of the Guidance for Reporting Involvement of Patients and the Public 2 (GRIPP-2) short form. 85
Patient and public involvement during the programme
To complement PEP-R’s ongoing engagement, we established a dedicated forum for the programme, comprising four patients with experience of chronic pain after knee replacement. Forum members were offered support and training by an experienced PPI co-ordinator. They received training built into forum sessions, including overviews of qualitative research, systematic reviews, statistics and trial management. Patients were also given information about local PPI events.
During the programme, there were a total of 23 meetings of the STAR patient forum and two one-to-one discussions between the PPI co-ordinator and individual members. STAR was also discussed at six meetings of the PEP-R patient group. Patients were provided with reimbursement for their time in the form of shopping vouchers, as this was their preference. Travel was either reimbursed or arranged for patients to attend meetings. From April 2020 onwards, face-to-face STAR and PEP-R forum meetings were cancelled owing to COVID-19 and we continued to work with the PPI groups remotely. A member of PEP-R attended Programme Steering Committee meetings with the PPI coordinator. STAR PPI activity and its impact in each work package is summarised in Appendix 1 and Box 1.
Researchers worked with the programme’s PPI group to interpret the results of the systematic reviews and database analyses and contributed to summaries of the systematic reviews, which led to improvements in summary clarity and accessibility.
Work package 2The PPI group commented on the use and interpretation of routine health data to ensure that complex findings were accessible to patients. They also gave input into the plain English summary of the analysis of the OKS pain cut-off and its use to identify chronic pain.
Work package 3The PPI group developed and improved the study materials for the refinement of the care pathway, including patient information packs. They also discussed the feedback from 10 patients who attended the STAR clinic and improved the plain English summary of findings.
Work package 4In addition, the PPI group were involved in the development and refinement of trial recruitment and data collection materials. For the trial, this included the review and approval of the screening questions, questionnaire booklet, provision of contact details for support organisations and charities, development of the patient information booklet, trial questionnaire, recruitment and retention methods, and recommendations about the training day sessions for trial staff based at all trial sites. Recommendations included sending a postcard to participants to let them know that they would soon receive the questionnaire, telephoning patients who did not return the questionnaires rather than sending another questionnaire, and including a teabag with the questionnaire. For the economic evaluation work, this included input into revisions of the resource diary to ensure that it reflected the needs and experiences of patients. For the embedded qualitative research within the trial, this included input into the design of interview topic guides and the interpretation of findings, including discussions about the challenge of remembering one clinic among the many appointments a person might have had after a year had passed and how uncertainty about the reason for pain led to confusion and anxiety. PPI members also reviewed newsletters sent to participants.
Work package 5The PPI group gave input into the qualitative research study, including assisting in the design of patient information materials for recruitment such as plain English summaries to enhance clarity and work to interpret interim findings after nine qualitative interviews, in which group members helped to confirm key emerging themes alongside the qualitative researcher.
Work package 6The PPI group gave input into all dissemination through the review of all plain English summaries of findings sent to participants. For example, in work package 4, summaries of screening study findings sent to participants were designed in collaboration with the PPI group, who advised on the layout, wording and infographics. To communicate findings from work packages 3, 4 and 5, summaries for participants were developed in collaboration with PPI members. In addition, ongoing discussions with Versus Arthritis informed work on public dissemination. Finally, at group sessions towards the end of the programme in September and October 2020, patients and other stakeholders worked together to discuss the implementation of the STAR care pathway in practice and future priorities for research and care for chronic pain after knee replacement. One member was interviewed for the STAR film.
Summary of the value of patient and public involvement in the programme
Includes information published in Bertram et al. 86 and Bradshaw et al. 87
Patient and public involvement was an essential part of the programme. We involved patient partners at all stages and this had a significant impact on the study design, such as improvements to patient documents, recruitment and retention methods,86 the communication of results and the planning of next steps (Box 1). Their involvement ensured that patients’ voices were included in the design and delivery of this research and that the outputs were relevant and meaningful to them.
Our patient partners also had an impact on the design of the PPI, as we followed their advice to adopt an approach of ‘taking the research to the patient’, rather than having two PPI members simply attending less frequent and more formal management or advisory group meetings. This had a positive impact on PPI group members as they believed this resulted in improved equality of power and decision-making, an atmosphere of strong collaboration and respect, and the opportunity for them to have a real impact on research. In addition, the research benefited through allowing a greater number of patients with differing experiences to be involved, providing a wider insight into issues of greatest importance to people in chronic pain. The design of the PPI was an ongoing discussion between group members and the PPI coordinator. The STAR forum group members told us that they felt they were working in partnership with the research team and equality was achieved by having the researchers come to them. This approach also had an impact on the research team as they all had the opportunity to attend the regular PPI group meetings and work in collaboration with patients. For some researchers, this was their first opportunity to hear about the experiences of patients with long-term pain and explain their research in plain English face to face. We recommend this approach and continue to use it as our preferred approach to PPI within the Musculoskeletal Research Unit (Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK).
Chronic pain after total knee replacement: risk factors, prevention and management (work package 1)
Background
Although pre-operative risk factors for chronic pain after total knee replacement have been explored extensively, post-operative risk factors have not.
Treatments in the pathway through total knee replacement may potentially modify risk factors for poor patient outcomes and adverse events. These have been reviewed previously, but not with an emphasis on chronic pain.
Little research has focused on the treatment of chronic pain after knee replacement. 48 Interventions for the management of chronic pain after other surgeries may have value in the context of total knee replacement.
Risk factors for chronic pain after total knee replacement: systematic review
This section has been published as Wylde et al. 88
Aims
This systematic review aimed to identify early post-operative patient-related risk factors for chronic pain after total knee replacement.
Methods
A prospectively registered systematic review (PROSPERO CRD42016041374),89 was conducted and reported as recommended by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 90 We searched MEDLINE, EMBASE and PsycINFO from inception to October 2016 with no language restrictions (see Appendix 2, Systematic review search strategy as applied in MEDLINE on Ovid).
Eligible studies were longitudinal in design and met the following inclusion criteria:
-
patients observed within 3 months of knee replacement
-
intervention group – people with a risk factor
-
control group – non-exposed people
-
outcome – chronic pain at ≥ 6 months after knee replacement
Risk of bias was assessed with a non-summative checklist (Box 2),87 based on components of the methodological index for non-randomized studies (MINORS)91 and Newcastle-Ottawa Quality Assessment Scale. 92 Results were reported as a descriptive narrative analysis.
The following checklist components were rated as adequate, inadequate or not reported:
-
inclusion of consecutive patients (consecutive = adequate)
-
representativeness (multicentre = adequate)
-
per cent follow-up (> 80% = adequate)
-
minimisation of potential confounding (multivariate analysis = adequate).
Results
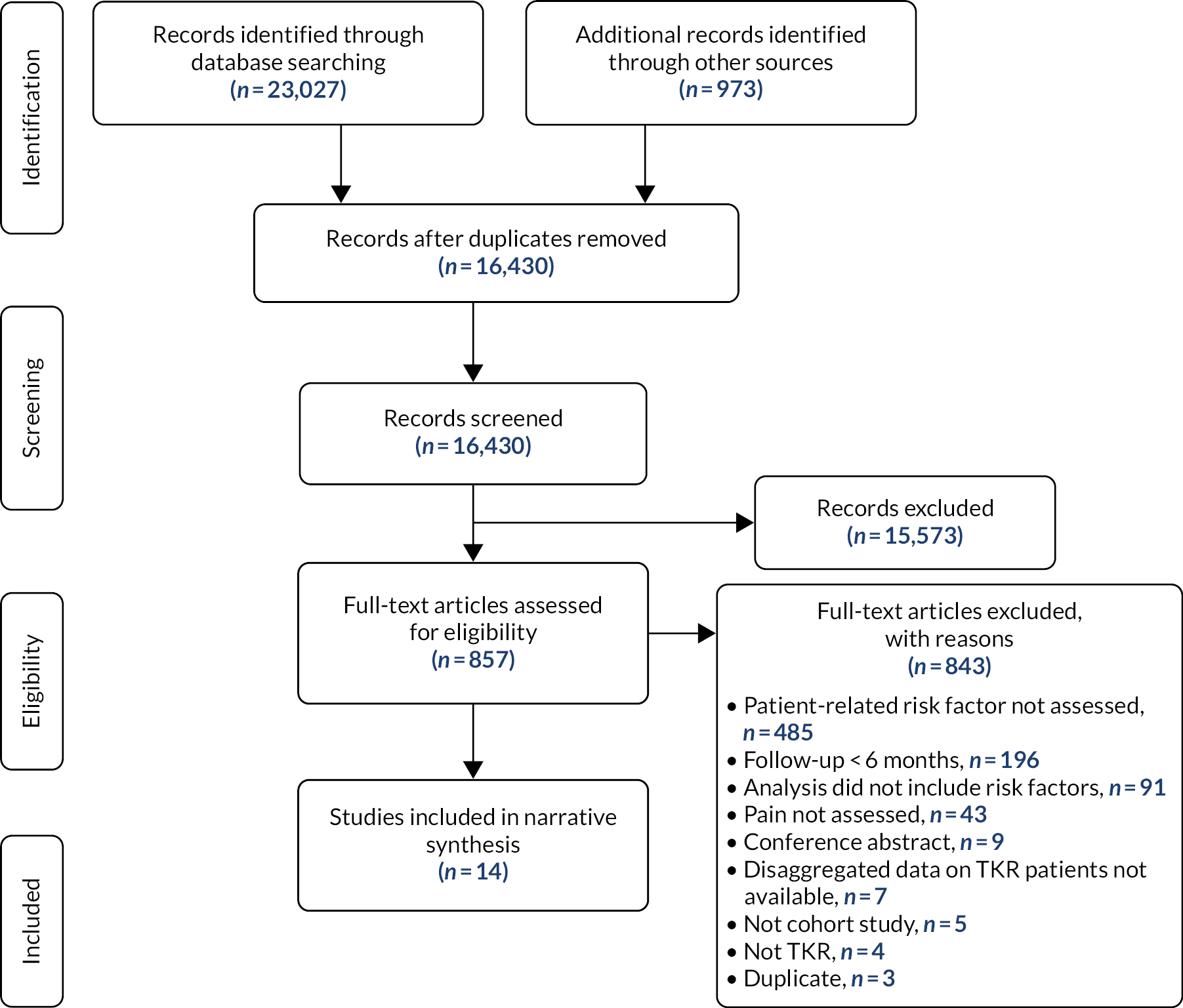
Searches identified 14 cohort studies (1168 patients) evaluating the association between patient-related factors in the first 3 months post operation and pain at ≥ 6 months after primary total knee replacement (see Appendix 2, Figure 2).
FIGURE 2.
Risk factors for chronic pain after total knee replacement: systematic review flow diagram. TKR, total knee replacement.
Post-operative patient-related factors evaluated included acute pain (eight studies), function (five studies) and psychosocial factors (four studies). In studies with no risk of bias other than patient selection, there was a suggestion that acute post-operative pain during the hospital stay was associated with chronic pain. 52,93 However, in one of these studies, the association was largely explained by pre-operative pain. 52 For all other post-operative patient factors, there was insufficient evidence to draw firm conclusions about an association with chronic pain after total knee replacement.
Risk factors for chronic pain after total knee replacement: database analyses
The NJR/HES analyses are published as Khalid et al. 94 and the HES/CPRD/PROMs analyses are published as Mohammad et al. 95
Aims
These analyses of national databases aimed to identify early risk factors for chronic pain after total knee replacement.
Methods: analysis 1
Primary knee replacements recorded in the NJR were linked with the HES and PROMs databases. We identified primary elective knee replacements performed between April 2008 and December 2016. Predictor variables within 3 months post surgery were surgical complications (i.e. fracture, patella tendon avulsion or ligament injury), medical complications (i.e. myocardial infarction, stroke, acute renal failure, deep-vein thrombosis or pulmonary embolism, surgical site infection, respiratory infection, urinary tract infection, wound disruption, mechanical complication of prosthesis, fracture, neurovascular injury, or blood transfusion), length of stay, readmission, reoperation or revision. The outcome was chronic pain measured using the OKS at 6 months after surgery. The associations of the predictors with the chronic pain outcome were explored using logistic regression modelling.
Methods: analysis 2
We conducted a retrospective observational study using anonymised data from the CPRD GOLD database linked to the HES and PROMs databases. Patients were identified using the CPRD GOLD database of individual patient data from electronic primary health-care records from practices across the UK. 96 The CPRD provides a detailed record of both primary and secondary care. 97 Primary care records from the CPRD were linked to secondary care admission records from HES Admitted Patient Care data and the Office for National Statistics mortality data. HES also provides PROMs data before and 6 months after knee replacements.
We included all patients receiving a primary knee replacement between 2009 and 2016. Inclusion in the analysis was limited to patients with HES-linked data (i.e. those in England only) who completed both the pre- and 6-month-post-operative OKS pain subscale (OKS-PS). 80
The treatment effect [(pre-treatment OKS-PS score – post treatment OKS-PS score)/pre-treatment OKS-PS score]98,99 was calculated for each patient. A treatment effect of ≤ 0.2 was used to classify patients as non-responders to surgery regarding their knee pain. Relative risk ratios were generated by fitting a generalised linear model with a binomial error structure and a log link function (log-logistic model) and adjusted risk differences (ARDs) estimated from marginal effects from the regression model.
Results: analysis 1
Pre- and 6-month-post-operative OKSs were available for 258,386 patients and 43,702 (16.9%) of these were identified as having chronic pain at 6 months post surgery. Within 3 months of surgery, complications were uncommon: there were surgical complications in 1224 (0.5%) patients, one or more medical complications in 6073 (2.4%) patients, readmissions to hospital in 32,930 (12.7%) patients, knee-related reoperation in 848 (1.5%) patients and revision knee replacement operation in 835 (0.3%) patients. Post-operative predictors of chronic pain were mechanical complication of prosthesis [odds ratio (OR) 1.56, 95% confidence interval (CI) 1.35 to 1.80], surgical site infection (OR 1.13, 95% CI 0.99 to 1.29), readmission (OR 1.47, 95% CI 1.42 to 1.52), reoperation (OR 1.39, 95% CI 1.27 to 1.51), revision (OR 1.9, 95% CI 1.64 to 2.25) and length of hospital stay ≥ 6 days (OR 1.48, 95% CI 1.35 to 1.63). Predictive ability of the model was fair, with an area under the receiver operating characteristic (ROC) curve of 0.71, indicating that in respect of discriminatory ability, post-surgical predictors explain a limited amount of variability in chronic pain outcome.
Results: analysis 2
Information was available for 4750 patients between 2009 and 2016. Patients had a mean age of 69 years (standard deviation 9 years) and 56.1% were female. At 6 months after surgery, 10.4% of patients were classified as non-responders. The strongest associations with a non-response to surgery were seen for pre-operative risk factors; these were having only mild knee pain symptoms at the time of surgery (ARD 18.2%, 95% CI 13.6% to 22.8%), smoking (ARD 12.0%, 95% CI 7.3% to 16.6%), living in the most deprived areas (ARD 5.6%, 95% CI 2.3% to 9.0%) and obesity class II (i.e. body mass index between 35 and 40 kg/m2; ARD 6.3%, 95% CI 3.0% to 9.7%). We also identified a range of other risk factors with more moderate effects, including a history of knee arthroscopy surgery (ARD 4.6%, 95% CI 2.5% to 6.6%) and the use of opioids within 3 months after surgery (ARD 3.4%, 95% CI 1.4% to 5.3%).
Effectiveness of interventions to prevent chronic pain after total knee replacement: systematic reviews
These reviews have been published as Wylde et al.,100 Beswick et al.,101 and Dennis et al. 102 A comprehensive overview has been published as Wylde et al. 103
Aim
These systematic reviews aimed to assess the effectiveness of pre-, peri- and post-operative interventions in preventing chronic pain in patients receiving total knee replacement.
Workshop
In March 2016, 57 invited experts and colleagues met with the STAR team at a workshop to discuss interventions for the prevention of chronic pain after knee replacement. Those attending included surgeons, anaesthetists, physiotherapists, nurses and former patients, as well as researchers with interests in randomised trials, health economics, qualitative studies, PPI, systematic reviews and cohort studies.
The systematic review work package lead explained that the aim of the systematic reviews was to identify interventions that may improve long-term pain outcomes after knee replacement. The following definition of an intervention was presented:
Any clinical treatment or public health measure designed to reduce the incidence or modify the effects of particular diseases.
Yarnell and O’Reilly 104
Workshop participants considered that aspects of pre-operative care were potential targets for an intervention to prevent long-term pain. Many patients receive pre-operative education at a single class and from information booklets. Changes to content and interventions to improve uptake of classes were seen as potentially valuable. Pre-operative interventions might include cognitive behavioural therapy, self-management and peer support, weight management, exercise, treatment of comorbidities, nutritional guidance, and intra-articular injections. A multimodal approach might include education, psychological support, management of comorbidities and other components.
In the peri-operative period, workshop participants considered effective pain management important, possibly using gabapentin, nerve blocks and multimodal approaches. Certain anticoagulants are associated with micro-bleeds, which may lead to long-term pain. The use of tourniquets may also be associated with long-term pain.
An enhanced recovery protocol was considered a possible intervention to limit long-term pain. After hospital discharge, workshop participants noted the potential value of mid-term rehabilitation, peer support groups, provision of contact points, an introduction to community services and online resources, and psychological support including cognitive behavioural and mindfulness therapies. Provision of physiotherapy in different formats was also recognised as worth evaluating. Other interventions suggested were intra-articular injections, weight management, podiatry and realignment.
Methods
The systematic reviews were registered prospectively as PROSPERO CRD42017041382. 105 We established a database of all randomised controlled trials and systematic reviews in total knee replacement. These were identified through searches of The Cochrane Library, MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature) and PsycINFO® (American Psychological Association, Washington, DC, USA) in November 2016 (updated February 2018 for peri-operative interventions and December 2018 for pre-operative interventions) (see Appendix 3, Systematic review search strategy as applied in MEDLINE on Ovid).
Eligible studies were randomised controlled trials that met the following inclusion criteria:
-
patients with osteoarthritis awaiting or who have received total knee replacement
-
intervention – treatment in the pre-, peri- or post-operative setting
-
control – usual care or alternative treatment
-
outcomes – chronic pain at ≥ 6 months after knee replacement (≥ 12 months for post-operative interventions), adverse events.
The database was screened, and interventions divided into pre-, peri- and post-operative contexts, and into intervention groups. Risk of bias was assessed using the Cochrane tool. 106
Results: pre-operative interventions
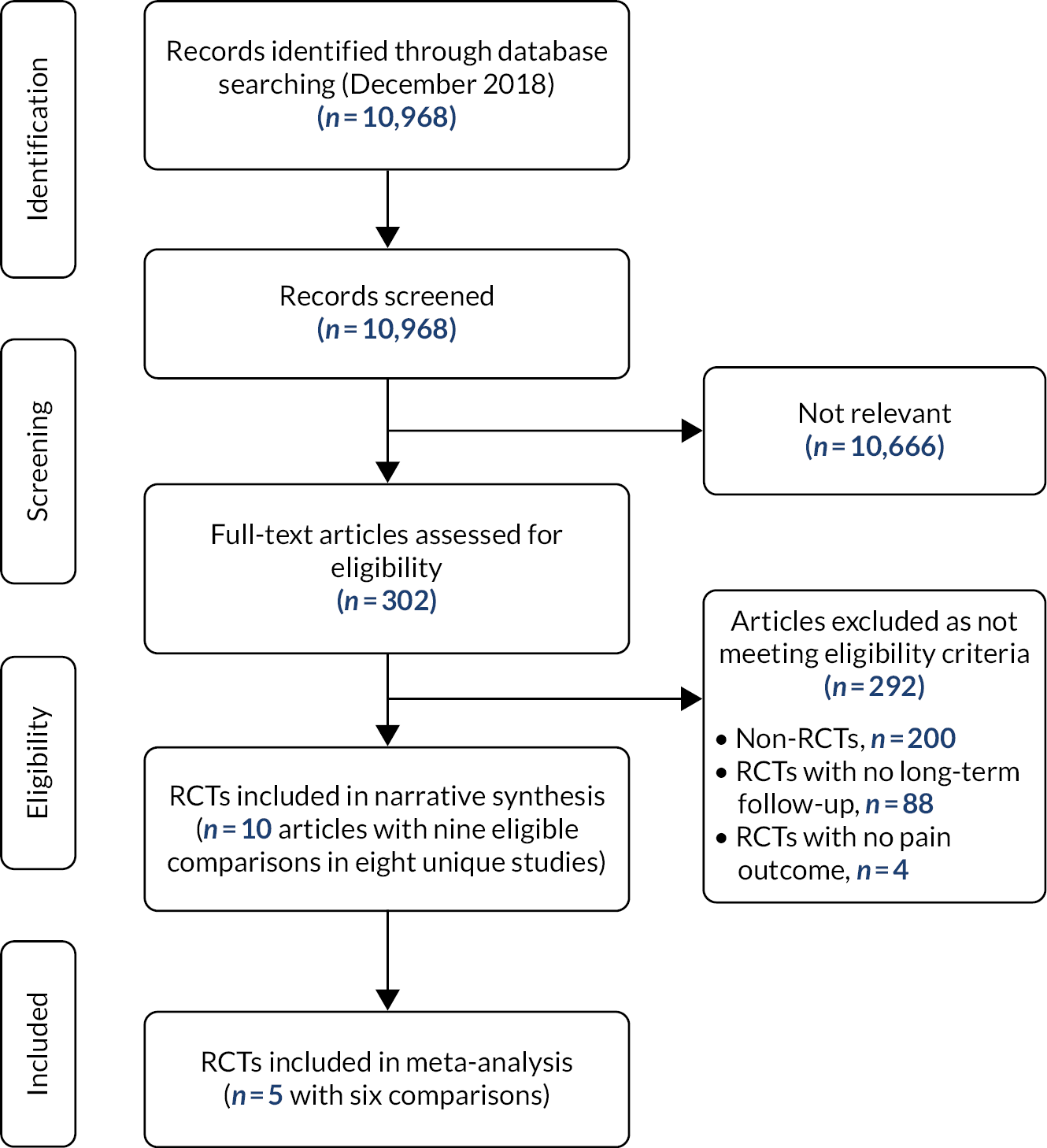
Eight randomised controlled trials with nine comparisons (960 patients) were eligible (see Appendix 3, Figure 3). There was moderate-quality evidence of no effect of exercise programmes on chronic pain after total knee replacement, based on a meta-analysis of six interventions with 229 participants (standardised mean difference 0.20, 95% CI –0.06 to 0.47; I2 = 0%). A sensitivity analysis restricted to studies at low risk of bias confirmed these findings. Studies evaluating a combined exercise and education intervention (one study) and education alone (one study) suggested similar findings.
FIGURE 3.
Effectiveness of pre-operative interventions: systematic review flow diagram. RCT, randomised controlled trial.
Results: peri-operative interventions
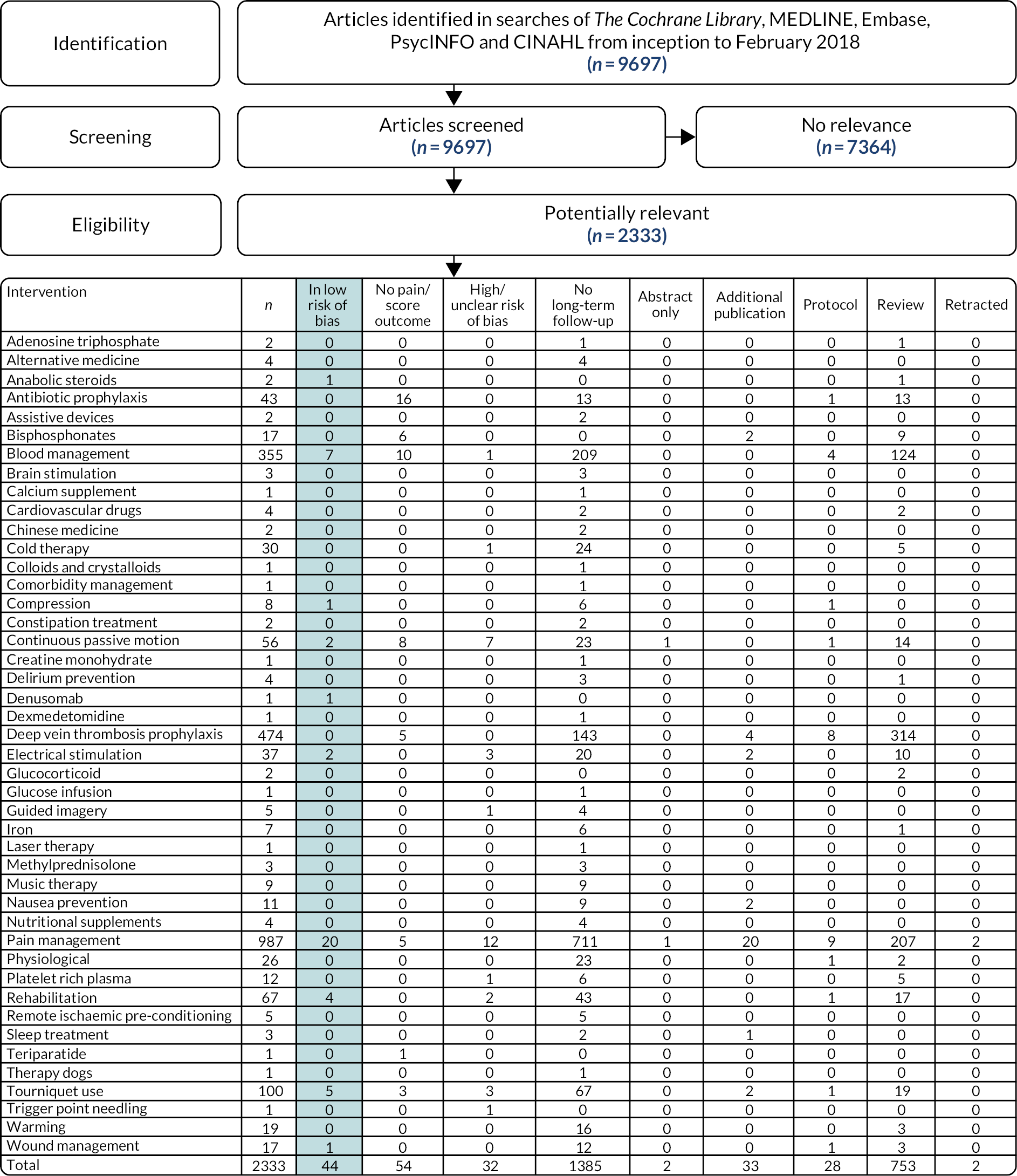
Forty-four randomised controlled trials at low risk of bias evaluated interventions in the peri-operative setting with a pain outcome or score with a pain component at ≥ 6 months’ follow-up (see Appendix 3, Figure 4). Intervention heterogeneity precluded meta-analysis. There was weak evidence for small reductions in chronic pain after total knee replacement in people who received peri-operative local infiltration analgesia (three studies), ketamine infusion (one study) or pregabalin (one study). Supported early discharge (one study) showed weak evidence of a small reduction in chronic pain. More clinically important benefits were seen for electric muscle stimulation (two studies), gait training (one study) and a course of anabolic steroids (one small pilot study).
FIGURE 4.
Effectiveness of peri-operative interventions: systematic review flow diagram.
For a range of peri-operative treatments there was no evidence linking them with unfavourable pain outcomes. For example, blood conservation with tranexamic acid during knee replacement was not associated with chronic pain. However, otherwise extensively researched interventions including venous thromboembolism prevention and tourniquet use have not been evaluated in relation to chronic pain.
Results: post-operative interventions
Randomised controlled trials of post-discharge interventions commencing in the first 3 months after total knee replacement were included (see Appendix 3, Figure 5). Seventeen trials with 2485 participants were included. All studies were at risk of bias because participants were not blinded to arm allocation, and five because of incomplete outcome data. Twelve trials evaluated physiotherapy interventions. Other interventions were nurse-led telephone follow-up, neuromuscular electrical stimulation and a multidisciplinary intervention. One study showed benefit for home-based exercises aimed at managing kinesophobia in reducing pain severity compared with no intervention. Otherwise, narrative synthesis found no evidence that one type of physiotherapy intervention is more effective than another. A 10-day multidisciplinary outpatient rehabilitation programme provided between 2 and 4 months after surgery showed no long-term benefit for pain or function. 107 For other interventions, there was insufficient evidence to draw conclusions about effectiveness.
FIGURE 5.
Effectiveness of post-operative interventions: systematic review flow diagram. RCT, randomised controlled trial; TKR, total knee replacement.

Effectiveness of interventions to manage chronic pain after surgery: systematic review
This review has been published as Wylde et al. 2017. 108
Aims
This systematic review aimed to assess the efficacy of interventions to treat chronic pain after non-cancer surgeries.
Methods
The systematic review was registered prospectively as PROSPERO CRD42015015957. 109 We searched MEDLINE, Embase, PsycINFO, CINAHL and The Cochrane Library from inception to March 2016 (see Appendix 4, Systematic review search strategy as applied in MEDLINE on Ovid). An update in October 2020 was timed to contextualise the STAR intervention.
Eligible studies were randomised controlled trials that met the following inclusion criteria:
-
patients with chronic pain after non-cancer surgery
-
an intervention for pain received by patients at a minimum of 3 months after surgery
-
a comparator of no treatment, placebo, usual care or alternative treatment
-
an outcome relating to pain.
Risk of bias was assessed using the Cochrane tool. 106
Results
As shown in Appendix 4, Figure 6, 66 trials with data from 3149 participants were included. Most trials included patients with chronic pain after spinal surgery (n = 25) or amputation (n = 21). Interventions were antiepileptics, capsaicin, epidural steroid injections, local anaesthetic, neurotoxins, opioids, acupuncture, exercise, spinal cord stimulation, further surgery, laser therapy, magnetic stimulation, mindfulness-based stress reduction, mirror therapy and sensory discrimination training. Opportunities for meta-analysis were limited by heterogeneity. For all interventions, there was insufficient evidence to draw conclusions on effectiveness but the review provided clear suggestions for future research.
FIGURE 6.
Effectiveness of interventions to manage chronic pain after surgery: systematic review flow diagram. RCT, randomised controlled trial.
Review update
To examine the STAR trial in a contemporary context, we updated searches with terms relating to arthroplasty of the large joints, post-surgical pain, and randomised controlled trials (see Appendix 5, Systematic review search strategy as applied in MEDLINE on Ovid). Systematic reviews and trial registries were checked for studies.
Eligible studies were randomised controlled trials that met the following inclusion criteria:
-
patients with chronic pain after arthroplasty of the large joints
-
intervention – treatment for chronic pain
-
control – no treatment, usual care or alternative treatment
-
outcome – pain.
Of 3901 articles identified and screened by one reviewer, 69 were potentially relevant (see Appendix 5, Figure 7). After detailed evaluation by two reviewers, four published randomised evaluations of treatments for chronic pain after knee replacement were identified in the original review and update (see Appendix 5, Table 1). No study was judged to be at high risk of bias. For people with general chronic pain, there were encouraging findings warranting further research into intra-articular botulinum toxin110 and denervation therapy. 111 Radiofrequency genicular nerve treatment showed similar outcomes to treatment with anaesthetic and corticosteroid. 112 No studies were found that evaluated a multifaceted intervention, but one study focusing specifically on treatment of neuropathic pain with topical lidocaine suggested that further research is merited for this personalised treatment. 113
| Study, country, date of recruitment | Inclusion criteria | Number randomised (intervention : control) | Intervention | Comparator | Risk of bias issues | Key results | |
|---|---|---|---|---|---|---|---|
| Published studies | |||||||
| Singh et al. 2010,110 USA, 2006–9 | Pain after total knee replacement for > 3 months, NRS pain intensity ≥ 6/10 | n = 54; 60 knees (30 : 30) | Single intra-articular botulinum toxin A injection | Single intra-articular injection of saline | Low risk of bias | Reduced pain intensity in botulinum A group after 3 months. Pain relief to around 40 days | |
| Ma et al. 2016,111 China, 2014–15 | Intractable pain of knee joint after total knee replacement | n = 100 (50 : 50) | Denervation therapy | Drug treatment | No losses to follow-up | Denervation therapy associated with improved symptoms | |
| Pickering et al. 2019,113 France, 2016 | Localised neuropathic pain after knee surgery | n = 36 (24 : 12) | 5% lidocaine-medicated plaster for 3 months | 5% plaster with no drug for 3 months | No losses to follow-up | Lidocaine plaster reduced localised neuropathic pain | |
| Qudsi-Sinclair et al. 2017,112 Spain, 2012–14 | Pain after total knee replacement | n = 33 (15 : 18; 14 : 14 received treatment) | Single radiofrequency genicular nerve block | Single analgesic block with corticosteroid | Some concerns: uneven follow-up in small study | Similar pain outcomes in both groups | |
| Wylde et al. 2022120 | Chronic pain 2 months after primary total knee replacement | n = 363 (242 : 121) | STAR intervention | Usual care | Low risk of bias | STAR was clinically effective and cost-effective in improving pain outcomes at 1 year | |
| From trial registries and published protocols | |||||||
| NCT02211534119 | Persistent post-operative pain following total knee replacement | NA | Pulsed electromagnetic energy field therapy | Sham pulsed electromagnetic field | NA | NA | |
| NCT02931435117 | Chronic knee pain despite total knee replacement at ≥ 6 months | NA | Nerve block with radiofrequency ablation | Sham radiofrequency ablation | NA | NA | |
| NCT03825965115 | Persistent post-surgical pain following total knee replacement | NA | Cannabinoids | Placebo | NA | NA | |
| NCT04100707118 | Knee pain 3 months after total knee replacement | NA | Genicular nerve blocks | Sham comparator | NA | NA | |
| NCT03973177116 Crossover |
Refractory chronic knee pain for more than 6 months after total knee replacement | NA | Phenol injection: neurolysis of genicular nerves | Methylprednisolone injection | NA | NA | |
| Larsen et al. 2020114 | Chronic pain after primary total knee replacement | NA | Neuromuscular exercise and pain neuroscience education | Pain neuroscience education | NA | NA | |
| NA, not applicable; NRS, numeric rating scale. | |||||||
FIGURE 7.
Interventions to manage chronic post-surgical pain update: systematic review flow diagram. RCT, randomised controlled trial.

In addition to the STAR trial, six studies were ongoing or not published (see Appendix 5, Table 1). These focus on exercise and education,114 cannabinoids,115 phenol neurolysis of genicular nerves,116 genicular nerve blocks117,118 and pulsed electromagnetic field therapy,119 all in people with general chronic pain after knee replacement.
Strengths and limitations
Our systematic reviews benefited from comprehensive literature searches and broad inclusion criteria to allow for evaluation of diverse interventions. Reviews were conducted according to appropriate guidelines and issues that may have introduced bias were considered. Meta-analysis was conducted, but only when appropriate. A narrative descriptive approach was used in circumstances of high heterogeneity of risk factors, interventions, outcomes and follow-up. Authors were contacted for clarification and missing information at all stages of the reviews. In the review of post-operative risk factors, a limited range of risk factors had been studied. In intervention reviews, our focus was on chronic pain as an outcome, which, although important, may not have been the specific outcome targeted by an intervention.
A strength of our database analyses was the large numbers of patients with linked data. Analyses included multiple centres and results should be generalisable throughout the NHS. Our analyses were limited by the content of data sets. Factors not recorded included implant positioning and surgical technique, pain management and medication use, and psychological, genetic, and environmental factors. Analyses were also limited by the single post-operative knee pain measure, which may not reflect established chronic pain.
Conclusions and inter-relationship with other parts of the programme
Before our comprehensive database analyses, knowledge of post-operative risk factors was limited to the observation that people with acute pain after knee replacement were more likely to report chronic pain. Risk factors identified in database analyses that may have potential for modification or use in the targeting of care included some patient and surgical factors, previous knee arthroscopy, use of opioids and surgical complications.
Randomised trials to assess long-term outcomes after pre-, peri- and post-operative interventions are feasible and necessary to ensure that patients receive care with reduced or no risk of chronic pain. Unifactorial interventions identified in systematic reviews and suggested by stakeholders merit further study.
Although some management strategies for chronic pain after diverse surgeries identified in our systematic review may have limited applicability outside the specific condition for which they were intended, others may be transferable regardless of the surgical procedure. Their value in the personalised prevention of chronic pain requires further research on their individual effectiveness and ultimately their potential as a component in a multifactorial care pathway.
Characterising chronic pain after total knee replacement (work package 2)
Background
Although chronic pain places considerable burden on health-care systems and individuals, little research has characterised chronic pain after total knee replacement and its impact on health-care resource use.
Aims
We aimed to characterise the natural history and course of chronic pain after total knee replacement, including health-care resource use, through additional follow-up of the Clinical Outcomes in Arthroplasty Study (COASt) cohort and analysis of the linked CPRD and HES databases.
Identification of patients with chronic pain after total knee replacement using the Oxford Knee Score
This work has been published as Pinedo-Villanueva et al. 20
We applied cluster analysis to data on 128,145 patients with primary total knee replacement included in the English PROMs programme to derive a cut-off point on the pain subscale of the OKS. A high-pain group was identified, defined by a score of ≤ 14 points on the OKS pain subscale 6 months after total knee replacement. This group comprised 15% of the patient sample and was characterised by severe and frequent problems in all pain dimensions.
Natural history of chronic pain after total knee replacement: extended cohort follow-up
This work has been published as Cole et al. 121
The COASt cohort (n = 1025) contains data from total knee replacement patients with comprehensive pre- and post-operative pain assessments. 122 Recruitment started in 2010 in Oxford and 2011 in Southampton. Patients were recruited pre-operatively and followed up post-operatively at 6 weeks and then annually. In the STAR programme, follow-up was extended to 5 years. This allowed the collection of detailed information on the course, qualities and variability of post-surgical pain. It also enabled the identification of pain patterns, such as late-onset or transient post-surgical pain.
Follow-up questionnaires were received from 580 patients 1 year following their primary total knee replacement and then from 500, 457, 390 and 336 patients for the 2- through 5-year questionnaires, respectively. The data were cleaned and analysed, and summary statistics generated for PROMs [EuroQol-5 Dimensions, three-level version (EQ-5D-3L) and OKS], chronic pain progression, resource use and (community and hospital) costs by chronic pain groups measured using the OKS-PS threshold applied to year 1 values. 20
There were 70 out of 580 participants with chronic pain at 1 year post surgery (12% of the full cohort). Their reported health utility index at 1 year post surgery was 0.39 compared with 0.79 for the non-chronic pain cohort, both reporting an important improvement over their baseline scores (0.27 and 0.48, respectively). Missing data (questionnaires not returned or questions not completed) increased with time to significant levels: 25% of the chronic pain cohort had missing OKS data at 5 years. A predicted means model approach was followed to impute answers to the pain subscale of the OKS and the EQ-5D-3L for all missing values to generate a complete picture of progression into and out of chronic pain over time and associated quality of life. Mean health utility was estimated to slowly improve for the chronic pain cohort, reaching 0.65 by year 5. The increase in the mean score was a reflection of many patients improving their OKS and EQ-5D-3L scores, although not all did. Although 65% of participants with chronic pain at 1 year were estimated to improve their OKS pain subscale beyond 14, never to drop below 14 again, 31% moved back and forth between chronic pain and non-chronic pain, and 4% remained in chronic pain over the 5 years.
In terms of health-care resources, having chronic pain was associated with greater use and costs than not having chronic pain. A larger proportion of participants in the chronic pain cohort than the non-chronic pain cohort reported seeing a GP (63 vs. 34%, respectively), physiotherapist, hospital doctor, nurse or alternative practitioner owing to their knee problem during the first year after surgery. This greater use of health-care services was maintained over the 5-year period. Costs reflected the difference between groups, with the chronic pain cohort reporting mean health-care costs of £1800 during the first year owing to their knee problem, compared with £500 for the non-chronic pain cohort; the gap was still present although significantly reduced at 5 years post surgery (£80 for the chronic pain cohort vs. £25 for the non-chronic pain cohort).
Health-care resource use: analysis of national data sets
This work has been published as Cole et al. 121
Analysis of the CPRD linked to the HES database was undertaken to characterise the natural history of chronic pain after total knee replacement, including resource use. Data from HES–CPRD data set were used to estimate the hospital and primary care costs associated with chronic pain after total knee replacement.
The analysis included patients in the CPRD–HES data set who reported post-operative (6-month) PROMs including the OKS so that they could be classified into chronic pain groups. The sample consisted of 5055 patients, 721 (14%) of whom reported OKS pain scores ≤ 14 and identified as in chronic pain. Hospital costs were estimated based on patient-level data about inpatient hospital stays for their total knee replacement as well as related complications, including revision surgery. Healthcare Resource Groups were generated based mainly on procedure and diagnostic codes reported for each spell. Primary care costs were estimated for reported consultations with GPs and other community health-care professionals as well as the prescription of analgesia.
Mean hospital costs for the primary total knee replacement were very similar for both groups (£6195 for the chronic pain group and £6055 for those not in chronic pain). Revision surgery also cost nearly the same to the NHS for both groups (£9188 for those in chronic pain and £9261 for those not in chronic pain), but its cumulative incidence at 7 years was significantly higher for those in chronic pain (nearly 10%) than the non-chronic pain group (just over 2%). Complications (i.e. myocardial infarction, venous thromboembolism and joint infection) were rare for both groups with less than 1.5% of patients reporting them, although venous thromboembolism and joint infection were more common in the chronic pain group than the non-chronic pain group (1.2 vs. 0.6% for venous thromboembolism, respectively, and 1.1 vs. 0.1% for joint infection, respectively).
In primary care, costs were consistently higher for those in chronic pain. Mean costs (adjusted for exposure) for all reported consultations with any health-care professional in the community and prescriptions of analgesia were estimated up to 10 years before primary total knee replacement and 8 years following surgery. Mean yearly consultation costs per patient for the chronic pain and non-chronic pain groups were, respectively, £244 and £182 at 10 years prior to surgery, £408 and £342 during the 12 months before surgery, £461 and £366 during the 12 months after surgery, and £426 and £325 8 years after primary total knee replacement. GP consultations accounted for 70–80% of the costs for those in chronic pain and generally slightly less for those not in chronic pain.
Prescriptions of antidepressants for pain management, paracetamol, non-steroidal anti-inflammatory drugs and opioids were more common (and hence leading to greater costs) for those in chronic pain throughout the 18-year period of analysis. Of particular interest is that those not in chronic pain reported only a small drop in the average yearly cost of analgesia per patient after surgery, only for it to slowly grow again afterwards, reaching levels similar to those immediately before the replacement by the eighth year. Remarkably, those in chronic pain reported a continuous increase of analgesia prescriptions costs from 10 years prior to 7 years after surgery, with an increasing cost of opioid prescriptions after surgery which accounted for 17% of all analgesia prescriptions costs 10 years before the replacement, 50% the year before, and 74% 7 years after.
Economic model
A cohort-based Markov model described in Appendix 6 was developed to estimate the 5-year costs and (quality-adjusted) life expectancy of patients with chronic pain after total knee replacement under current practice as well as the STAR intervention in order to assess the impact of the latter. The model was populated with evidence from the trial and the findings of analysis of both the COASt and CPRD cohorts described above, with scenarios including and excluding the impact on inpatient admissions identified during the trial.
Strengths and limitations
The cut-off point we identified on the OKS pain subscale was derived from patients who completed the post-operative PROMs questionnaires sent out by the NHS. Differences in rates of completion of PROMs questionnaires is known to lead to underrepresentation of some socioeconomic groups and potentially those who feel particularly unsatisfied about their surgery. Using data from over 120,000 patients reported over 4 years reduced this potential bias. Our findings, moreover, were consistent with those of other studies identifying patients with chronic pain.
Findings from the analysis of the COASt cohort were limited by missing data and the potential lack of representativeness from recruiting in just two centres. However, we applied an imputation method that clustered observations by patient and considered completed questionnaires by similar patients. The proportion of chronic pain patients in the cohort (12%) was similar to that found in the England-wide study used to derive the cut-off point (14%).
Although the CPRD population is known to be representative of that of the UK, it lacks long-term data on outcomes beyond the 6-month linked HES–PROMs. To better understand the long-term trajectories of patients in chronic pain following total knee replacement, we combined the representative picture of the CPRD–HES population with the long-term follow-up provided by the COASt cohort. 96
Conclusions and inter-relationship with other parts of the programme
We derived a cut-off on the OKS pain subscale that can be used for patient selection in research settings to design and assess interventions that support patients in their management of chronic post-surgical pain. Patients in chronic pain as identified by this method appear noticeably different from those who are not: their average quality of life (which also captures disability as it includes impact on mobility and the ability to self-care and carry out regular activities) is poorer prior to surgery and it improves after, but not as much as for those not in chronic pain. Most seem to leave the chronic pain category over the subsequent 5 years, but their average quality of life does not reach the level reported by those not in chronic pain just 12 months after their total knee replacement. Patients in chronic pain consume more health-care resources at hospital because they are much more likely to have revision surgery within 7 years, and the costs of their health care in the community are also greater than for those not in chronic pain. Although the average costs of GP consultations per patient plateau after surgery, the costs of opioid prescriptions continue to significantly grow for patients in chronic pain for up to 7 years after surgery. The analysis did not suggest any time points at which poor post-surgical outcome could predict long-term problems; there are, however, signs that long-term problems are associated with pre-operative pain and quality-of-life outcomes as well as health-care resource use, in particular prescriptions for strong analgesia.
Development of a complex intervention for patients with chronic pain after knee replacement: the STAR care pathway (work package 3)
This work has been published as Wylde et al. 123
Background
As part of our Programme Development Grant, we conducted preliminary studies to design an intervention to improve the management of chronic pain after total knee replacement. Studies included a systematic review, a survey of NHS practice, qualitative work with health-care professionals, an expert group meeting and PPI activities. 48,124–126 We found a lack of clear pathways and referral processes for patients with chronic pain after total knee replacement,124,125 hindering patients’ access to targeted and individualised care and highlighting the need for improved access to appropriate pain management interventions for this population.
Aim
The aim of this work package was to refine and finalise a new care pathway for patients with chronic pain after total knee replacement. Specific objectives comprised the following:
-
the refinement of intervention content
-
the testing of intervention delivery and acceptability
-
the evaluation of views about intervention implementation within a trial.
Methods
In line with Medical Research Council recommendations for the development of complex interventions,127 we undertook four phases of work:
-
a consensus questionnaire with 22 health-care professionals on the appropriateness of intervention components to refine content
-
four facilitated meetings with 18 health-care professionals to refine intervention content
-
delivery of the assessment clinic to 10 patients to evaluate intervention delivery and acceptability
-
a questionnaire based on the NoMAD tool with 10 health-care professionals at trial centres to assess views about intervention implementation.
Key findings
Consensus questionnaires and meetings with health-care professionals ensured that the intervention components were appropriate and informed substantive changes. Testing of intervention delivery identified that the intervention was acceptable to patients after small changes to assessment clinic processes. Engagement with stakeholders indicated that the intervention could be successfully implemented for evaluation within a trial setting. Based on this work, our novel intervention was refined and prepared for evaluation. Findings also informed the design and development of a comprehensive intervention training manual and training event.
An overview of the intervention is shown in Figure 8. The intervention involves patients reporting moderate-to-severe pain at 3 months after total knee replacement attending an assessment clinic with a trained Extended Scope Practitioner (ESP). The clinic uses a standardised procedure involving (1) clinical history; (2) a review of patient-reported outcome measures including measures of pain, depression and neuropathic pain; (3) a knee examination to evaluate knee tenderness, surgical wound healing, range of motion, alignment, stability, patellofemoral joint function, infection and signs of CRPS; (4) an evaluation of radiographs for evidence of fracture or concerns with alignment, fixation, sizing or implant position; and (5) a blood test for markers of infection. Based on the findings of the assessment clinic, patients are referred to the appropriate existing services for further treatment. This may include one or more of the following: an orthopaedic surgeon for surgical review, a physiotherapist for muscle strengthening and exercise, a GP for treatment of depression or anxiety, and pain specialists for treatment of neuropathic pain or CRPS (via GPs). The care pathway is individualised and flexible, and the number of referrals reflects the needs of the patient. Monitoring is also available if appropriate. As part of the intervention, patients receive telephone follow-up from the ESP up to six times over 12 months to ensure any referrals are being undertaken and discuss any further referrals based on clinical need. The intervention aims to enable appropriate onwards referral to existing services to ensure that underlying reasons for chronic pain are considered and that treatment is targeted at these to improve pain management and to reduce the impact of pain.
FIGURE 8.
The STAR care pathway. PFJ, patellofemoral joint. CRPS, chronic regional pain syndrome.
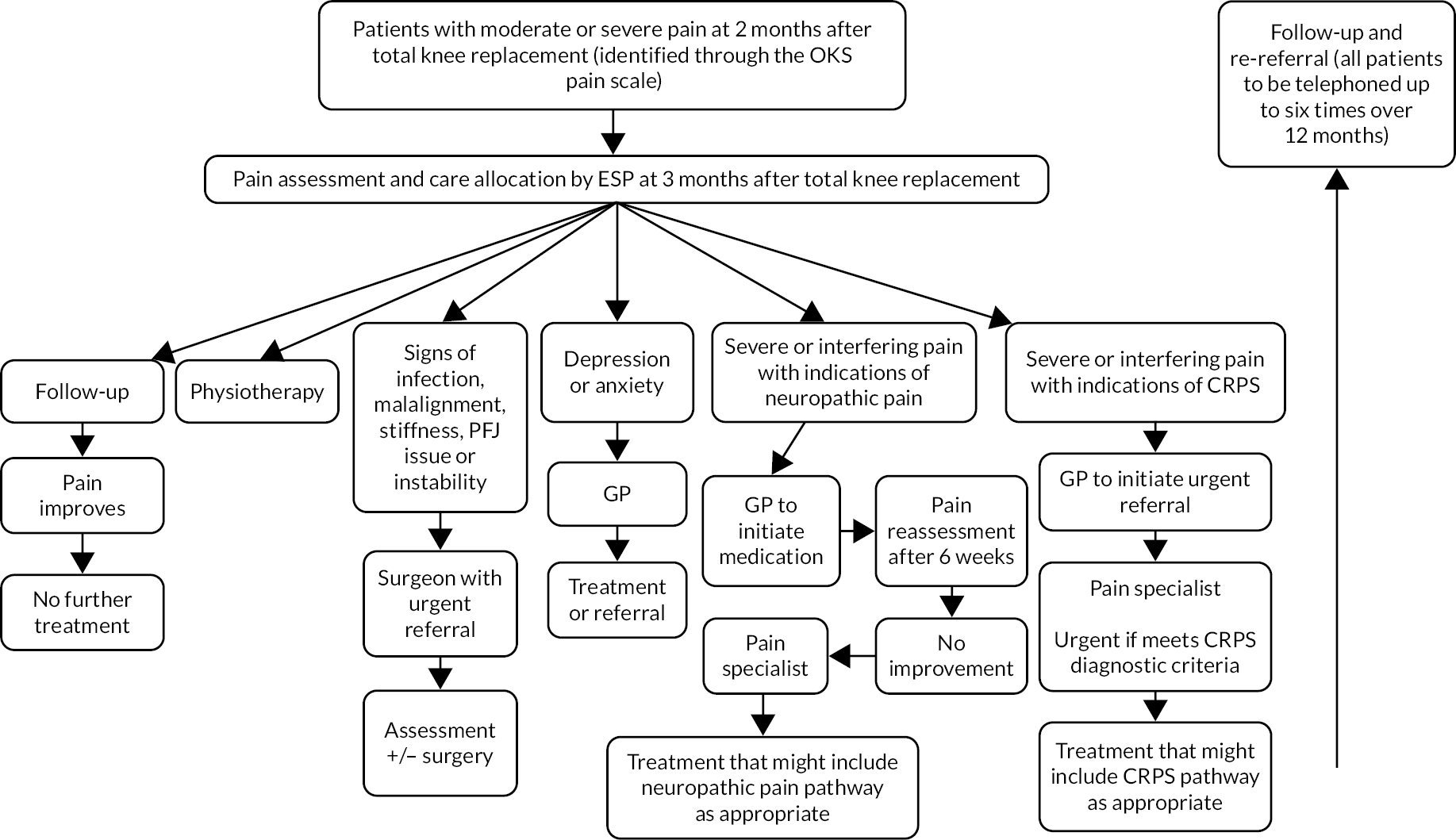
Strengths and limitations
We took a comprehensive approach to the development and refinement of a complex intervention, with a focus on ensuring that the intervention was deliverable, implementable and acceptable. Additional methods that could have been used in the development of this complex intervention include economic modelling, observation of intervention delivery and factorial screening experiments. Testing of intervention delivery was conducted in a single centre, thereby limiting generalisability.
Conclusion and inter-relationship with other parts of the programme
This work informed the development and refinement of a care pathway for people with chronic pain after total knee replacement, ready for robust evaluation in a multicentre randomised controlled trial.
Evaluation of the clinical effectiveness and cost-effectiveness of the STAR care pathway intervention (work package 4)
This work has been published as Wylde et al.,82,128–129 Bertram et al. 86 and Moore et al. 130
Background
After developing the STAR care pathway, evidence was required on its effectiveness, cost-effectiveness and acceptability.
Aim
This work package aimed to evaluate the clinical effectiveness and cost-effectiveness of the STAR care pathway compared with usual care for chronic pain after total knee replacement.
Methods
Study design
A pragmatic, open-label, parallel group, superiority, multicentre randomised controlled trial with a 2: 1 intervention-to-control allocation ratio was designed, with an economic evaluation and qualitative studies embedded. 128
Participants
Adults who received primary total knee replacement for osteoarthritis at a participating hospital were screened to identify patients who had pain in their operated knee at 2–3 months post surgery using the OKS pain component threshold score of ≤ 14, as validated in work package 2. Exclusion criteria comprised a lack of capacity to provide informed consent, previous participation in the STAR trial for the contralateral knee and participation in another study that would interfere with the STAR trial. Patients were recruited from eight NHS hospitals in Bristol, Cardiff, Exeter, Mansfield, Oswestry, Wrightington, Leicester and Birmingham.
Treatment allocation
After patients provided written informed consent and completed a baseline questionnaire, they were randomly allocated to the STAR pathway plus usual care or usual care alone. Randomisation, minimised by knee pain and stratified by hospital, was conducted remotely on a 2: 1 intervention-to-control basis by the Bristol Randomised Trials Collaboration. Masking of participants was not possible owing to the nature of the intervention. Trial personnel were not masked except for those collecting outcome measures over the telephone from participants who did not return a questionnaire.
Intervention: STAR care pathway
The STAR care pathway comprises assessment by a trained ESP, referral to appropriate existing services and telephone follow-up. Further details are provided in Development of a complex intervention for patients with chronic pain after knee replacement: the STAR care pathway (work package 3).
Control: usual care
Usual care at participating hospitals included a routine 6-week post-operative follow-up and some clinicians provided an additional 3-month appointment. All sites provided additional follow-up if requested but this did not include routine follow-up by pain specialists.
Outcome assessment
Assessments were conducted prior to randomisation (3 months post surgery) and then at 6 and 12 months after randomisation. The selection of outcomes was guided by our core outcome set for chronic pain after total knee replacement. 131 The coprimary outcomes were pain severity and pain interference, assessed using the Brief Pain Inventory (BPI) severity and interference scales,132 at 12 months after randomisation.
Secondary outcomes included the BPI at 6 months and the following measures at 12 months: the OKS,80 painDETECT,133 the Douleur Neuropathique 4,82 the Hospital Anxiety and Depression Scale,134 the Pain Catastrophizing Scale,135 the Possible Solutions to Pain Questionnaire,136 the Patient Satisfaction Scale,137 single-item questions on comparison of pain to pre-operative pain and pain frequency, ICEpop CAPability measure for Adults (ICECAP-A),138 the EQ-5D-5L,139 the Short Form-12,140 chronic widespread pain,141 serious adverse events related to the intervention and resource use. The 12-month questionnaire included free-text questions asking participants about what had and had not helped their knee pain.
Internal pilot phase
A 6-month internal pilot phase with embedded qualitative research was conducted at four hospitals to refine trial procedures. 86 On completion of the pilot, it was clear the planned sample size of 380 could not be reached with four recruiting sites. A projection equation was used to estimate potential recruitment for prospective sites, which included high volume centres from the NJR. A feasibility assessment was developed to ensure delivery was achievable by a site prior to addition. Nineteen sites were assessed for feasibility and five new sites ultimately joined the trial. Combined with refinements from the pilot qualitative work, this strategy successfully produced enough recruitment to provide a sufficiently powered sample.
Qualitative research in the pilot phase included audio-recording and thematic analysis of 31 recruitment consultations and 29 participant interviews. The analysis provided information that was used to refine trial recruitment processes (e.g. explanation of trial processes to patients), make improvements to patient information and make changes to standard operating procedures.
12-month qualitative study
After the 12-month follow-up, a purposive sample of 27 participants from the intervention group were interviewed about the STAR care pathway. The sample included 10 men and 17 women from six study sites, with sampling designed to include those with a range of outcomes based on BPI severity and interference. Interviews were audio-recorded and transcripts analysed thematically. 142
Statistical analysis
Full details of the sample size calculation and statistical analyses are provided in the published protocol128 and statistical analysis plan. 143 A sample size of 285 patients provided power of 80–90% to detect standardised differences of between 0.35 to 0.40 standard deviations (0.7–0.8 scale points) in the BPI between groups at 12 months after randomisation using a two-sided 5% significance level. Accounting for loss to follow-up of 25%, 381 participants were required.
Data analysis was conducted in accordance with CONSORT (Consolidated Standards of Reporting Trials) guidelines. The primary comparative analysis, performed on an ‘as randomised’ basis included all available primary outcome data at 12 months. Control and intervention groups were compared using linear regression models adjusted for the minimisation/stratification variables and the baseline values of the respective outcome, presented with both 95% CIs and p-values. Sensitivity analyses used multiple imputation with chained equation techniques to impute missing primary outcome data. The secondary outcomes were also analysed using regression. Subgroup analyses investigated variation in the treatment effect between orthopaedic centres and by pain severity, using interaction terms added to the relevant regression models. Per-protocol analysis was used to estimate the treatment effect in those patients who adhered to the protocol.
Cost-effectiveness analysis
Full details of the cost-effectiveness analyses are provided in the Health Economics Analysis Plan. 144 The primary cost-effectiveness analysis took an NHS and Personal Social Services (PSS) perspective. A secondary analysis took a broader perspective to include patients’ costs. Resources used in relation to the treatment of chronic pain were measured from randomisation to 12 months’ follow-up, the time horizon of the analysis. All resources were valued using routine data sources. All comparative analyses were conducted on an ‘as randomised’ basis and there was no discounting of costs or effects given the short duration of the study. The primary outcome for the economic evaluation was the quality-adjusted life-year (QALY), which was calculated from EQ-5D-5L responses and using the mapping function to the 3-level valuation set. 145 The difference in costs and QALYs between the arms was assessed on a multiply imputed data set using the net benefit framework, which uses seemingly unrelated regression models adjusted for the baseline values of the minimisation/stratification variables. Uncertainty was addressed using cost-effectiveness acceptability curves and sensitivity analyses.
Key findings
Between September 2016 and May 2019, 363 patients with pain 3 months after knee replacement were randomly assigned to receive either the intervention plus usual care (n = 242) or usual care alone (n = 121). Participants had a median age of 67 years (range 40–88 years) and 60% were female. Of those randomised to the STAR care pathway, 233 (96%) attended the clinic appointment and participants had a median of two (interquartile range 1–2) onward treatment referrals.
Cross-sectional analyses of baseline data found that more severe pain at 3 months post surgery was associated with poorer general health, poorer physical health, more pain worry and lower satisfaction with surgery outcome. More severe functional limitation was associated with higher levels of depression, more pain worry, lower satisfaction with surgery outcome and higher pain acceptance. 129
Analysis of the primary outcome at 12 months after randomisation included 313 participants: 213/242 (88%) in the intervention group and 100/121 (83%) in the usual care group. The primary analysis yielded a difference in means in the BPI severity score between groups at 12 months after randomisation that favoured the STAR care pathway (–0.65 points, 95% CI –1.17 to –0.13; p = 0.014). The difference in means between groups from the primary analysis of the BPI interference score at 12 months also favoured the STAR care pathway over usual care (–0.68 points, –1.29to –0.08; p = 0.026).
At 6 months after randomisation, the mean BPI severity score also favoured the intervention group over the usual care group, with a difference in means of –0.55 points (95% CI –1.05 to –0.06; p = 0.028). There were similar results at 6 months for the BPI interference score, with a difference in means of –0.71 points (95% CI –1.28 to –0.15; p = 0.014). Repeated measures analysis showed no evidence of a difference in treatment effect at 6 months compared with 12 months, which was consistent with an effect at 6 months that was maintained at 12 months post randomisation for both the BPI pain and interference subscales. There was no evidence of a treatment effect on any of the secondary outcome measures at 12 months, with the exception of a better OKS in the intervention group, with a difference in means of 2.68 (95% CI 0.58 to 4.78; p = 0.013).
The analysis of costs from the NHS and PSS perspective showed that the intervention group dominated the usual care group, in that costs in the intervention group were £724 (95% CI –£1500 to £51) less and there were an additional 0.03 (95% CI –0.008 to 0.06) QALYs than the usual care group. This meant that the incremental net monetary benefit (iNMB) at a £20,000-per-QALY threshold was £1256 (95% CI £164 to £2348), indicating a 98.79% probability that the intervention is the cost-effective option. Similarly, from a patient perspective, and all perspectives combined, the intervention group remained dominant. The greater number of hospital admissions (for the NHS/PSS perspective), and the greater number of hours of unpaid leave (patient perspective) in the usual care group were the cost drivers of these results.
Free-text questions asked participants what they felt had and had not helped their knee pain during the 12 months of trial participation. Most responses for what had helped included physiotherapy and exercise activities such as walking, swimming and keeping moving (51%). Responses for what was not helpful included physical limitations such as not being able to bend the knee, stairs, stiffness and swelling (34%). Painkillers were included in both sections as helpful (16%) and unhelpful (14%).
Findings from the qualitative research in the pilot phase indicated that participants found the trial and randomisation acceptable. The pilot work also led to refinements in study materials, including improvements to the explanations of randomisation and usual care in the patient information leaflet, clarification that all participants would be able to access health care as usual regardless of group allocation, and clarification that patients would only attend one clinic appointment. We updated the standard operating procedures for recruiters to ensure that they made a clear distinction between completion of the questionnaire and the randomisation procedure, and modified the online version of the questionnaire to ensure that participants could select options more easily.
Findings from analysis of the qualitative interviews with participants who received the intervention indicated that they found it acceptable and that it provided patients with a sense of support and confidence in their ongoing recovery. Patients were reassured that referral was appropriate, and even when treatments did not fully resolve their pain, they valued the opportunity to discuss their knee problems with a health-care professional.
Strengths and limitations
Strengths of this trial included the pragmatic design, inclusion of multiple hospitals, use of a core outcome set, high adherence to the intervention, full cost-effectiveness analysis, and embedded qualitative work to optimise processes and explore patient experiences of the intervention. The demographics of the trial sample in terms of ethnicity (94% white), mean age (67 years) and gender (60% female) broadly reflects the population of individuals undergoing knee replacement in the UK at the time of the study (95% white, mean age 69 years and 57% female). 5,146 A limitation was that the postal screening process may have missed people with pain because nearly half of patients did not return the screening questionnaire. Another potential weakness was that it was not possible to mask participants to treatment allocation owing to the nature of the intervention, which could have contributed to an overestimation of the treatment effect. Missing questionnaire data were the main issue in the cost-effectiveness analysis, which meant it was not appropriate to conduct a complete-case analysis. However, the main cost driver related to hospital admissions, the data for which were obtained from the informatics departments of the treating hospitals. Other limitations include potential underreporting of participant-reported use of mental health services if participants had not considered these services as attributable to their pain, and not adjusting for patient-reported baseline costs, which potentially could have influenced the identified cost differences. However, given there were no substantial imbalances between trial arms, it is possible that the extra missing data that would have arisen from the inclusion of these baseline costs could have had more influence on the identified cost differences. Finally, although the difference in mean BPI scores between the groups was slightly lower than the published minimal clinically important difference of 1 point, the 95% CIs covered a clinically meaningful difference (95% CI –1.17 to –0.13 for BPI pain severity and –1.29 to –0.08 for BPI pain interference). It is widely acknowledged that chronic pain after knee replacement is a complex condition that is difficult to treat and in this context an improvement in both the BPI severity and interference scores of the observed magnitude, with the 95% CIs including the minimally clinically important difference, were interpreted as clinically important. The STAR intervention provides a model of care delivery that includes referrals to evidence-based treatments and we would anticipate that improvements in treatments and management strategies for chronic pain after knee replacement would lead to better outcomes for patients who receive the intervention.
Conclusion and inter-relationship with other parts of the programme
The trial was designed in collaboration with patients through PPI activities and was informed by our systematic reviews and intervention development work. The trial found that the STAR care pathway is a clinically effective and cost-effective intervention for reducing pain severity and interference for patients with troublesome pain at 3 months after knee replacement. Work to explore how to implement these findings is described in Implementation and dissemination of patient and health-care professional resources (work package 6).
Understanding non-use of services by people with chronic pain after total knee replacement (work package 5)
This work has been published as Moore et al. 147,148
Background
As with other chronic pain problems,75 people with chronic pain after total knee replacement may make limited or no use of formal services, and may believe that pain is inevitable and that little can be done. 78,149
Aim
We aimed to explore non-use of services by people with chronic pain after total knee replacement.
Methods
Participants were recruited from two high-volume NHS hospitals in central and south-west England. Individuals who had a total knee replacement ≥ 12 months ago were sent a screening questionnaire which included the OKS,80 elements of the Level of Expressed Need Scale150 and questions about health resource use. Potential participants were eligible for study inclusion if they answered ‘yes’ to the question ‘Are you currently troubled by pain in your replaced knee, either all the time or on and off, which has lasted for more than three months?’, scored ≤ 14 on the OKS pain component and described seeing GPs or other health-care professionals in relation to their pain as ‘rare’ or ‘never’ in the previous 12 months. A purposive sample of up to 40 people was planned as an approximation expected to achieve data saturation. 142 People who were eligible and interested in participating were contacted by a member of the research team to arrange a face-to-face interview.
All interviews took place in participants’ homes and were conducted by an experienced qualitative methodologist who was not known to any of the participants before the study. Participants provided written informed consent before the interview commenced. A semistructured topic guide was used to ensure that relevant issues were addressed, while also providing flexibility for the researcher and participant to explore and reflect on different areas. Topics included participants’ experiences of chronic pain after total knee replacement, characteristics of pain, comorbidities and their impact on chronic pain, participants’ management of their pain and the use of formal and informal health services. All interviews were audio-recorded with consent.
Interview recordings were transcribed, anonymised and uploaded to NVivo11 (QSR International, Warrington, UK) data management software. Inductive thematic analysis was undertaken. Line-by-line, focused, axial and theoretical coding took place, with independent double-coding to maximise interpretative depth. Analysis took place alongside data collection to inform subsequent interviews. After development of a descriptive account, a theoretical account and model was developed to highlight reasons for non-use of health care.
Key findings
Thirty-four interviews were completed, with 16 men and 18 women (mean age 74 years, range 55–93 years). Findings suggested a strong core theme that ‘nothing can be done’. Several subthemes are related to this core category.
The overarching theme that ‘nothing can be done’ reflected a common experience among participants. The subthemes explain why people came to this conclusion. Based on their initial experience of seeking support for chronic pain, many had stopped seeking help. This was due to a combination of inter-related factors, beginning with the response of health-care professionals, which was often discordant with their own experience. If no technical or mechanical reason for ongoing pain was evident, then surgeons’ assertions that the operation was a success left the patient uncertain about their pain and whether or not they could seek further care. Most patients seemed to want to avoid further medicine either because of unwanted side effects or a perception that medicines were ineffective. Patients expected to be told that nothing further could be done, having already had their knee joint replaced. Seeking further access to care was also thought to be risky: further surgery may worsen the pain or the likely outcome was not perceived to be worth the effort. For some people, other health conditions took priority. Added to this was a strong moral sense that seeking further care for chronic pain after total knee replacement could overburden an already overstretched health-care system or delay care for others in more need of care.
We applied Scott’s model of pathways to treatment151 and found it has limitations among individuals who have already sought treatment through knee replacement that left them with poor outcomes. People with pain are caught in a cycle of appraisal of symptoms which we have termed a ‘futility loop’, without moving on to health seeking.
Further analysis of the interviews found that some participants struggled with issues of embodiment, that is how one experiences and acts upon the world through the body. Some described feelings of discomfort rather than pain, and described their knee in terms of heaviness, numbness and tightness to the point of feeling uncomfortable and restricted. Pain and discomfort appear to be linked to a sense that the prosthesis was ‘alien’ or ‘foreign’, which is a sense of disembodiment. Participants described a disconnection from their knee that made it difficult to move, requiring deliberate conscious effort rather than natural subconscious flow. Some participants saw their replaced knee as unpredictable because of a lack of ‘conscious connection’ with it, which could sometimes lead to falling over without warning. Our findings suggest that rather than being a neurological issue, these separation experiences originate from a lost sense of ownership and agency over the limb. Thus, rehabilitation should focus not only on strengthening the joint and promoting full functional recovery, but also on modifying a person’s relationship with their new joint to achieve full embodiment or connectedness.
Strengths and limitations
The achievement of data saturation provides us with confidence that the findings are transferable to other people within the same population in the UK. Our study did not include the accounts of clinicians as we were interested in understanding the reasons why people with pain did not use health care. However, previous research by our team indicated that clinicians also feel that there is a lack of clarity about routes through care for people with chronic pain after knee replacement. 125
Conclusion and inter-relationship with other parts of the programme
Our findings explain why some people with chronic post-surgical pain after knee replacement do not seek health care. Our findings show that health-care professionals’ responses to reports of pain in initial follow-up appointments can powerfully affect patients’ beliefs about whether or not they have a legitimate reason to reconsult if that pain does not improve.
The findings from our study of embodiment suggest that post-operative rehabilitation may consider inclusion of rehabilitation strategies that focus on reincorporating the altered limb into the body.
Implementation and dissemination of patient and health-care professional resources (work package 6)
Background
Implementation science provides models to help ensure maximal uptake of new interventions and processes by health-care professionals and organisations. 152–154 NPT is an approach from implementation science that we used to assist optimal implementation of the care pathway in the trial and to understand how the pathway could be best put into practice if clinically effective and cost-effective. We also conducted dissemination activities, including conference presentations, journal articles, and communication for professionals, patients and the public.
Aims
This work package aimed to assess the implementation of the intervention into clinical practice in the trial and to distribute evidence-based information about identification, assessment and management of chronic pain after total knee replacement.
Evaluation of implementation
This work is described in detail in Appendix 7.
Qualitative study
Interviews were conducted with 14 participants involved in the implementation and delivery of the STAR care pathway. Participants comprised five consultant orthopaedic surgeons, one consultant rheumatologist and eight ESPs. Interviews were conducted after delivery of the intervention was complete. The interview topic guide used items from the NoMAD survey questionnaire81 to elicit stakeholders’ opinions about the implementation of the STAR care pathway delivered in the trial. The 23 items were designed to enable participants to provide information about their experiences of intervention delivery and their expectations of the implementation process. Items reflect the four core constructs of NPT (coherence, cognitive participation, collective action and reflexive monitoring), which represent the different kinds of work that people do when implementing a new practice. Data were audio-recorded, transcribed, anonymised and analysed using the NoMAD items as a coding frame for content analysis. 155
Participants quickly became familiar with the STAR care pathway. Many aspects of the pathway, including regular telephone follow-up calls and elements of the assessment, were additions to normal practice.
In terms of how participants made sense of the intervention (coherence), although the 60-minute clinic was longer than standard appointments (15–20 minutes), participants felt that patients benefited from the opportunity to talk about their surgery and recovery. Clinicians valued the protected time and training to address psychosocial factors and neuropathic pain. ESPs valued how the pathway had formalised a range of strategies for the management of ongoing pain, which enabled them to refer patients on for pain medicines, anxiety or depression, or to a GP, physiotherapist or surgeon. It was noted that life events impacted on some patients’ recovery and that this was not captured in the current protocol and may have been better dealt with by referral to a counsellor.
To build a community of practice around the STAR intervention (cognitive participation), key individuals were essential to enable intervention delivery. Research nurses and administrators were essential for the coordination and organisation of clinic appointments, senior managers and consultants for the motivation of multidisciplinary teams, and ESPs for clinic delivery. All participants supported the pathway and were open to working in new ways to implement the pathway should it be clinically effective and cost-effective.
Participants experienced some challenges in delivering the STAR clinics and follow-up calls (collective action). The intervention required more time and resources than normal assessment and follow-up practice. Participants felt that sensitivity was required when handling referrals back to the surgeons, so that the surgeons were fully aware that patients they had originally treated had been assessed in a STAR clinic and may be referred back to them. Participants also suggested that having information on patients’ pre-operative pain or depression scores may be useful in the future roll-out of the intervention as a way to provide a baseline reference by which to guide post-operative pain management.
Training and support for the delivery of the intervention was felt to be excellent, and ESPs reported feeling confident that they were supported. Future implementation of the STAR pathway into clinical practice and support by hospital management was felt to depend on whether the pathway was shown to be cost-effective.
Participants’ appraisal of how the intervention affected them (reflexive monitoring) suggested that they valued many elements of the STAR pathway. These included the focus on neuropathic pain and psychosocial issues, enhanced patient care, formalisation and validation of referral practices, and increased knowledge of pain management.
Stakeholder meeting
In September 2020, we conducted an online stakeholder meeting with NHS managers, heads of therapy, commissioners, surgeons, pain clinicians, representatives from relevant professional organisations, representatives from Versus Arthritis and patients. We shared programme findings to provide the basis for a discussion of the results, and shared specific findings from qualitative interviews to facilitate discussion about optimisation of implementation. For visual abstracts presented at the meeting, see Report Supplementary Material 1.
The event was attended by 55 stakeholders. Three breakout groups discussed the next steps for research, focusing on pain journeys and prediction, support for patients to seek care and for professionals to enable this, and how to move the care pathway from research into practice.
Based on this discussion, stakeholders identified two areas as future research priorities:
-
The pre-operative identification of patients who are most likely to have poor outcomes afterwards. This includes a tool to predict poor outcomes after surgery and pre-operative preventative interventions.
-
The development of evidence-based information for patients to inform, empower and manage expectations – including in the pre-operative phase of care – and to enable people with chronic pain after knee replacement to seek health care, provide information about forms of management that are available in the NHS, and encourage professionals to provide access and encouragement.
Dissemination
Journal publications and conference presentations
We disseminated the results of our research in journals and at national and international conferences, engaging audiences interested in pain and orthopaedics. We have published 14 articles from the programme and made 28 conference presentations.
Engagement with members of the public and patients
We worked in partnership with patients and Versus Arthritis to develop accessible, evidence-based information. These resources were disseminated through online information, web-based resources, written information and other appropriate outlets including an ‘NIHR Alert’. Engagement with members of the public and patients also took place through web material and Twitter (Twitter, Inc., San Francisco, CA, USA)) hosted at the University of Bristol. We have also made two short films about PPI and trial findings. All participants in work packages 2–6 received feedback on study findings, developed in partnership with patients.
Strengths and limitations
Using the NoMAD instrument to structure the topic guide allowed us to frame data collection and analysis around the four NPT constructs and to collect a rich data set that included multiple aspects of implementation. Fourteen out of a potential 20 ESPs and principal investigators (PIs) at trial sites took part and we achieved a good representation of stakeholders. Data collection and analysis only took place at centres involved in the trial because we wanted to collect data about the real experience of intervention delivery. Although this could be seen as limiting the transferability of these findings beyond the context of the trial, results suggest that the STAR pathway has good potential for implementation, and potential facilitators of and barriers to implementation have been identified. The stakeholder meeting also enabled us to address and discuss solutions to barriers, such as improved communication about the pathway between stakeholders and improving information for patients considering knee replacement.
Conclusion
Findings from the process evaluation showed that stakeholders supported delivery of the STAR care pathway and were willing to work in new ways to implement it if shown to be cost-effective. We recommend that sufficient time and resources are allocated to the implementation of the STAR care pathway and that information about the pathway and referral processes should be promoted widely and sensitively among those involved in its implementation. The stakeholder meeting identified priorities for future research.
Conclusions from the whole programme
The main reason that people undergo total knee replacement is to alleviate pain. However, a sizeable proportion of patients who have total knee replacement experience chronic pain after surgery. The programme addressed seven thematic areas.
Theme 1: how and when people with pain after total knee replacement should be identified
Our work recommends early post-operative screening, beginning at 2 months after surgery, to identify patients with troublesome pain after knee replacement. This can then facilitate targeted care delivery at 3–4 months post surgery when acute pain is transitioning to chronic pain, with the aim of preventing longer-term chronicity. To identify patients with troublesome pain after total knee replacement who would likely benefit from intervention, we have, to the best of our knowledge, developed and applied the first robust and standardised approach using a derived cut-off score on the OKS pain subscale.
Theme 2: predicting who will develop chronic pain after total knee replacement
Our database analyses confirmed the importance of pre-operative pain as a predictor of chronic pain after knee replacement. Those with the mildest pre-operative knee pain were more likely to move to a worse post-operative pain state. Patients taking opioids and antidepressant medications were more likely to have worse pain outcomes. Other risk factors, including smoking, obesity and comorbidities are potentially modifiable before surgery or may identify groups requiring additional care and monitoring.
Theme 3: prevention of chronic pain after total knee replacement
Randomised trials to assess long-term outcomes after pre-, peri- and post-operative interventions are feasible and necessary to ensure that patients receive care with reduced or no risk of chronic pain. Some unifactorial interventions merit further study and may have a role in the knee replacement pathway.
Theme 4: trajectory of chronic pain after total knee replacement
Although two-thirds of people with chronic pain 1 year after surgery appear to improve and no longer experience pain as the years pass, our estimates (after imputing significant missing data assumed to be missing at random) indicate that one in three still experience chronic pain 5 years after their operation or fluctuate in and out of pain. When describing chronic post-surgical pain, some people describe sensations of discomfort including heaviness, numbness, pressure and tightness associated with the prosthesis. Some people report a lack of felt connection with their knee and a lack of confidence in it.
Theme 5: how people manage chronic pain after total knee replacement
Chronic pain after total knee replacement is linked with slightly more visits to the GP, more physiotherapy and a greater consumption of stronger analgesia even years after the operation. People with chronic pain after knee replacement who make little or no use of services often feel nothing more can be done, or that further treatments may not be of benefit or cause further harm.
Theme 6: economic impact of chronic pain after total knee replacement
We found that chronic pain after total knee replacement is associated with an additional average expenditure of about £100 per patient in primary care consultations during the first year after surgery. This extra cost is sustained over time, for at least up to 8 years after the operation. Analgesia prescription is also more costly for patients with chronic pain and the associated extra cost increases after surgery primarily owing to the increased prescription of opioids. During the 8 years following knee replacement, pain analgesia prescription costs the NHS on average an additional £35 per patient per year for those in chronic pain. Additional costs associated with chronic pain, including primary and hospital care as well as private physiotherapy (but excluding prescription medication), can be as high as £1300 in the first year after surgery. This decreases over time, reaching £55 by the fourth year. Furthermore, chronic pain can be expected to be associated with increased formal and informal care as well as productivity losses, which would significantly increase its socioeconomic impact.
Theme 7: how to optimise the management of chronic pain after total knee replacement
Our trial showed that the STAR care pathway is a clinically effective and cost-effective intervention for reducing pain severity and pain interference over 1 year for patients with troublesome pain at 3 months after knee replacement. The novel STAR care pathway was designed to provide personalised care through a multifaceted treatment approach. The intervention addresses the need for clear pathways and referral processes to facilitate access to targeted care matched to individual patients’ pain characteristics.
The STAR care pathway provides patients with reassurance and confidence in their recovery and ensures that they receive the treatments they require. Patients valued the opportunities to discuss their concerns with a health-care professional and derived a sense of reassurance and encouragement from the STAR clinic and follow-up calls.
To the best of our knowledge, our trial is the first robust evaluation of a multifaceted and personalised intervention for patients with pain at three months after knee replacement.
For people with general chronic pain after knee replacement, there were encouraging findings from our systematic reviews that warrant further research into unifactorial interventions. However, there is currently insufficient evidence to support their use in clinical practice. This was also apparent in the more general literature reporting evaluations of interventions for chronic pain after diverse surgeries. The design of the STAR pathway will permit the incorporation of new evidence-based interventions into patient care.
Challenges in the programme as a whole
A strength of the STAR programme was the inclusion of work packages that delivered findings to build a broader picture of chronic pain after total knee replacement. The programme comprised inter-related studies and it was important that these were connected to one another.
The main challenges to the programme related to the implementation of strategies to improve trial recruitment and ensure the success of the trial. Differences between anticipated and actual recruitment related to the availability of patients at trial sites, including during periods when fewer patients had surgery such as elective surgery closures during winter bed pressures. Feasibility assessments and recruitment projections accounting for patient availability were developed to ensure realistic recruitment targets at each site. These methods of optimising recruitment were successful in producing a powered sample for analysis.
An opportunity presented by the programme was the multidisciplinary nature of the team and the research delivered. Over 5 years, the programme team worked together closely and shared progress, details of research methodologies and their clinical experience. As such, the programme served to develop UK health research capacity in knee replacement and pain research. The learning from one another’s approaches is intangible and difficult to define but is likely to deliver long-term benefit to team members’ individual capacity, future research leadership and potential to support high-impact research in the future.
Recommendations for future research
Based on the programme, we recommend the following areas for future research. These recommendations focus on moving from work on risk factors to future interventions, improvements to the STAR care pathway and enabling fuller engagement between people living with or at risk of pain after knee replacement and health care:
-
Research that identifies new interventions with evidence of effectiveness may need to be considered in future refinements of the STAR intervention to ensure that it remains current, evidence-based and able to deliver a personalised approach to the management of chronic pain after knee replacement.
-
Research to pre-operatively identify those patients who are most likely to have poor outcomes and to develop a tool for the prediction of poor outcomes after surgery.
-
Development of evidence-based information for patients to inform, empower and manage expectations – including in the pre-operative phase of care – and to enable people with chronic pain after knee replacement to seek health care, to provide information about forms of management that are available in the NHS and to encourage professionals to provide access and encouragement.
-
Research to explore and develop an intervention to identify how to support people to manage any feelings of disconnectedness from their replaced knee.
-
Longer-term follow-up of patients who received the STAR care pathway to describe longer-term outcomes.
-
Given the complexity of pain that extends or emerges after surgery, individualised multimodal interventions matched to pain characteristics after other surgeries – as evaluated in the STAR trial – merit development and evaluation.
-
The STAR programme focused on care after surgery. Future research could focus on the time before surgery as an opportunity for intervention. We have a greater understanding of risk factors for poor outcome and using this understanding to design and evaluate pre-surgical interventions may prove of long-term benefit to patients and health-care systems. For example, research could develop and evaluate an intervention to address opioid medication prescribing. There may be need for research into clearer expectation setting and communication between patients and professionals before surgery.
Reflections on work packages
Working with our PPI group has been valuable across all work packages. The group gave support to the STAR randomised controlled trial that helped with the inclusion, recruitment, retention and engagement of patients.
Trial recruitment originally aimed to recruit equal numbers of participants from four sites with a variety of patient characteristics. In an ‘internal pilot phase’, we increased the number of study sites from four to eight to ensure that we met the recruitment target.
Without a clear mechanism linking pre-operative and long-term post-operative pain, it proved hard to justify to peer reviewers that systematic reviews of trials of a broad range of pre-, peri- and post-operative interventions should include a focus on long-term pain as an outcome. However, pain should be included with other adverse events in long-term follow-up when changes are made to patient care in the knee replacement patient pathway. Direct effects may be clear through nerve damage and other biological mechanisms. However, associations with long-term pain may be indirect, mediated through other adverse events and, as we have shown, extended hospital stay and readmissions.
Challenges and successes
Successes for the programme included high-quality and robust results relating to patient care and outcomes after knee replacement. Although the research focused on the time after surgery, the programme provides findings are programmatic in nature and relate to the whole patient journey through knee replacement. These results are directly relevant to patients, health-care professionals and providers. We now have new important knowledge on impact, risk factors and management that will inform care.
Challenges included the need to improve recruitment and retention methods in the trial. Extending trial recruitment to additional sites proved a successful strategy. In addition, collaboration with the PPI group assisted in the improvement of recruitment and retention methods. This collaboration and the input provided by the PPI group was crucial to the success of the trial and the programme.
The COVID-19 pandemic fell during the follow-up phase of the main trial. Although this did not affect our follow-up response rate, concerns were raised over how responses may be affected by the national lockdown and limited availability of health-care services. Sensitivity analyses were implemented to assess this and did not find any significant effects.
Service developments
During the programme we developed, delivered and evaluated a new care pathway for people with chronic pain after knee replacement. This represents a service development that is acceptable, clinically effective and cost-effective and ready for implementation in health care.
Implications for practice and lessons learned
This programme was designed to inform and evaluate improvements to services and patient well-being. We discussed these with stakeholders towards the end of the programme, who expressed a desire to see the delivery of the STAR care pathway in the NHS.
The following key implications from the work relate to a pressing need to provide support and care for people experiencing chronic pain after knee replacement:
-
Access to an evidence-based care pathway, such as the STAR care pathway, can improve outcomes and is cost-effective. Delivery of the pathway should provide benefit.
-
People with chronic pain after knee replacement may benefit from clearer information about the likelihood that their pain may change over time, including that it might improve, and that engagement with health-care professionals provides an opportunity to address concerns about their replacement.
-
For people who do not make use of health care but who have chronic pain after knee replacement, providing them with information about the potential benefit of health care and encouraging professionals to enable them to seek care may have benefit by enabling these people to access evidence-based forms of management for their pain.
Taken together, the programme has provided evidence about what provides benefit and what is not known, and has identified directions of future research need. The programme has generated a large body of evidence about pain after total knee replacement and serves to raise awareness of the problem, pushing the issue higher up the agenda of research, practice and policy. The impact of the programme as a whole is beyond the sum of its parts: we have generated evidence and awareness of this important issue, and there is more work to be done.
Acknowledgements
We would like to thank all the patients, surgeons, health-care professionals, patient-partners from PEP-R and the STAR forum, and researchers who have contributed to the STAR programme. We acknowledge support from the NIHR Clinical Research Network. This study was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol.
Programme and Trial Steering Committees
We thank the members of the Programme and Trial Steering Committees for their support in the development, conduct and delivery of the programme as a whole:
-
Joy Adamson, chair, Programme Steering Committee; member, Trial Steering Committee
-
Paul Ewings, member, Programme Steering Committee; chair, Trial Steering Committee
-
Lizzy Betts, member, Programme Steering Committee; member, Trial Steering Committee
-
George Peat, member, Programme Steering Committee; member, Trial Steering Committee
-
Mark Rockett, member, Programme Steering Committee; member, Trial Steering Committee.
Researchers
Sophie Cole, Antonella Delmestri, Kirsty Garfield, Sara Khalid, Spyros Kolovos, Fiona MacKichan, Maria T Sanchez-Santos and Anushka Soni.
STAR site personnel
Shannon Biggs, Tim Board, Emma Bruce, Philip Buckley, Ben Burston, Kate Button, Joanna Clarke, Jean Denton, Vikram Desai, Andrew Emms, Colin Esler, Anne Evans, Jessica Falatoori, Jason Fletcher, Giles Head, Emma Hoskins, Natalie Jackson, Heather Jarvis, Phillips Jon, Debbie Jones, Dileep Kumar, Tracey Lee, Leigh Morrison, Gemma Munkenbeck, Adam Nelson, Claire Nicholas, Valerie Parkinson, Michael Parry, Charlotte Perkins, Kathryn Robinson, Paul Stanton, Rowenna Stroud, Joanne Taylor, John-Paul Whittaker, Matthew Williams, Deborah Wilson and Lucy Young.
Collaborators
Jo Adams, Aideen Ahern, Nicholas Ambler, Kate Button, Paul Dieppe, Peter Gladwell, Beverley Hopwood, Athene Lane, Helen Lewis-White, James Murray, Donna Noonan and Nick Smith.
Support
Elizabeth Bradshaw, David Carmichael, Beverley Evanson and Christine Hobson.
Contributions of authors
Rachael Gooberman-Hill (https://orcid.org/0000-0003-3353-2882) (Professor of Health and Anthropology) was chief investigator with responsibility for delivery of the overall programme and is the corresponding author.
Vikki Wylde (https://orcid.org/0000-0002-8460-1529) (Associate Professor in Musculoskeletal Health) was a co-applicant and led and conducted work in the systematic reviews, intervention design and randomised trial.
Wendy Bertram (https://orcid.org/0000-0001-8234-2052) (Senior Research Associate, Clinical Trials Manager) was involved in the intervention design, co-ordinated the pilot trial and was trial manager for the randomised trial.
Andrew J Moore (https://orcid.org/0000-0003-3185-1599) (Research Fellow in Qualitative Health Service Research) led the qualitative research throughout the programme and led the design and delivery of the qualitative implementation evaluation.
Rafael Pinedo-Villanueva (https://orcid.org/0000-0002-4723-5128) (Associate Professor in Health Economics) led the health economics analysis based on observational data.
Emily J Sanderson (https://orcid.org/0000-0003-2268-4194) (Research Associate in Medical Statistics) was the trial statistician and planned and conducted the statistical analysis in the trial.
Jane Dennis (https://orcid.org/0000-0001-9718-2653) (Senior Research Associate in Evidence Synthesis) undertook the systematic reviews and meta-analysis.
Shaun Harris (https://orcid.org/0000-0003-0698-1543) (Senior Research Associate in Health Economics) undertook the STAR randomised trial health economic analyses.
Andrew Judge (https://orcid.org/0000-0003-3015-0432) (Professor of Translational Statistics) was a co-applicant and led the analyses of routine data from the NJR and Clinical Practice Research databases, and from the COASt study.
Sian Noble (https://orcid.org/0000-0002-8011-0722) (Senior Lecturer in Health Economics) was a co-applicant and led the health economics analysis in the STAR randomised trial.
Andrew D Beswick (https://orcid.org/0000-0002-7032-7514) (Research Fellow, Systematic reviews) was a co-applicant, led the systematic reviews and co-ordinated the writing of the final report.
Amanda Burston (https://orcid.org/0000-0003-3480-4210) (Senior Research Associate, Public and Patient Involvement) was a co-applicant and led and coordinated PPI in the programme.
Tim J Peters (https://orcid.org/0000-0003-2881-4180) (Professor of Primary Care Health Services Research) was a co-applicant and led the statistical design and analysis of the STAR randomised trial.
Julie Bruce (https://orcid.org/0000-0002-8462-7999) (Professor of Clinical Trials) was a co-applicant and advised on the conduct and reporting of all aspects of the research.
Christopher Eccleston (https://orcid.org/0000-0003-0698-1543) (Professor of Pain Science) was a co-applicant and advised on the conduct and reporting of all aspects of the research.
Stewart Long (Versus Arthritis Director of Involvement, Influencing and Support) was a co-applicant and advised on the conduct and reporting of all aspects of the research.
David Walsh (https://orcid.org/0000-0002-6316-5692) (Professor of Rheumatology) advised on the conduct and reporting of all aspects of the research.
Nicholas Howells (https://orcid.org/0000-0002-8514-0322) (Consultant Orthopaedic Surgeon) was a named collaborator and principal investigator for the trial site at North Bristol NHS Trust.
Simon White (https://orcid.org/0000-0002-6264-2020) (Consultant Orthopaedic Surgeon) was a named collaborator and principal investigator for the trial site at Cardiff and Vale University Health Board.
Andrew Price (https://orcid.org/0000-0002-4258-5866) (Professor of Orthopaedic Surgery) was a co-applicant and provided specialist surgical input into the programme.
Nigel Arden (https://orcid.org/0000-0002-3452-3382) (Professor in Rheumatic Diseases) was a co-applicant and provided specialist clinical input into the programme.
Andrew Toms (https://orcid.org/0000-0002-8035-0494) (Professor of Orthopaedic Surgery) was a named collaborator and principal investigator for the trial site at Royal Devon and Exeter NHS Foundation Trust.
Candida McCabe (https://orcid.org/0000-0002-3593-0027) (Florence Nightingale Foundation Clinical Professor in Nursing) was a co-applicant and provided specialist clinical input into the programme.
Ashley W Blom (https://orcid.org/0000-0002-9940-1095) (Professor of Orthopaedic Surgery) was a co-applicant and provided specialist surgical expertise into the programme.
Publications
Articles
Pinedo-Villanueva R, Kolovos S, Maronga C, Delmestri A, Howells N, Judge A, et al. Primary care consultations and pain medicine prescriptions: a comparison between patients with and without chronic pain after total knee replacement. BMC Muskuloskelet Disord 2022;23:548. https://doi.org/10.1186/s12891-022-05492-6
Wylde V, Beswick AD, Dennis J, Gooberman-Hill R. Post-operative patient-related risk factors for chronic pain after total knee replacement: a systematic review. BMJ Open 2017;7:e018105. https://doi.org/10.1136/bmjopen-2017-018105
Wylde V, Dennis J, Beswick AD, Bruce J, Eccleston C, Howells N, et al. Systematic review of management of chronic pain after surgery. Br J Surg 2017;104:1293–306. https://doi.org/10.1002/bjs.10601
Pinedo-Villanueva R, Khalid S, Wylde V, Gooberman-Hill R, Soni A, Judge A. Identifying individuals with chronic pain after knee replacement: a population-cohort, cluster-analysis of Oxford knee scores in 128,145 patients from the English National Health Service. BMC Musculoskelet Disord 2018;19:354. https://doi.org/10.1186/s12891-018-2270-9
Wylde V, Bertram W, Beswick AD, Blom AW, Bruce J, Burston A, et al. Clinical- and cost-effectiveness of the STAR care pathway compared to usual care for patients with chronic pain after total knee replacement: study protocol for a UK randomised controlled trial. Trials 2018;19:132. https://doi.org/10.1186/s13063-018-2516-8
Wylde V, Beswick A, Bruce J, Blom A, Howells N, Gooberman-Hill R. Chronic pain after total knee arthroplasty. EFORT Open Rev 2018;3:461–70. https://doi.org/10.1302/2058-5241.3.180004
Wylde V, Dennis J, Gooberman-Hill R, Beswick AD. Effectiveness of postdischarge interventions for reducing the severity of chronic pain after total knee replacement: systematic review of randomised controlled trials. BMJ Open 2018;8:e020368. https://doi.org/10.1136/bmjopen-2017-020368
Wylde V, Howells N, Bertram W, Moore AJ, Bruce J, McCabe C, et al. Development of a complex intervention for people with chronic pain after knee replacement: the STAR care pathway. Trials 2018;19:61. https://doi.org/10.1186/s13063-017-2391-8
Bertram W, Moore A, Wylde V, Gooberman-Hill R. Optimising recruitment into trials using an internal pilot. Trials 2019;20:207. https://doi.org/10.1186/s13063-019-3296-5
Beswick A, Dennis J, Gooberman-Hill R, Blom AW, Wylde V. Are peri-operative interventions effective in preventing chronic pain after primary total knee replacement? A systematic review. BMJ Open 2019;9:e028093. https://doi.org/10.1136/bmjopen-2018-028093
Dennis J, Wylde V, Gooberman-Hill R, Blom AW, Beswick AD. Effects of presurgical interventions on chronic pain after total knee replacement: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2020;10:e033248. https://doi.org/10.1136/bmjopen-2019-033248
Moore AJ, Gooberman-Hill R. Why don’t patients seek help for chronic post-surgical pain after knee replacement? A qualitative investigation. Health Expect 2020;23:1202–12. https://doi.org/10.1111/hex.13098
Bradshaw E, Whale K, Burston A, Wylde V, Gooberman-Hill R. Value, transparency, and inclusion: a values-based study of patient involvement in musculoskeletal research. PLOS ONE 2021;16:e0260617. https://doi.org/10.1371/journal.pone.0260617.
Khalid S, Mohammad HR, Gooberman-Hill R, Garriga C, Pinedo-Villanueva R, Arden N, et al. Post-operative determinants of chronic pain after primary knee replacement surgery: analysis of data on 258,386 patients from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR). Osteoarthr Cartil Open 2021;3:100139. https://doi.org/10.1016/j.ocarto.2021.100139
Mohammad HR, Gooberman-Hill R, Delmestri A, Broomfield J, Patel R, Huber J, et al. Risk factors associated with poor pain outcomes following primary knee replacement surgery: analysis of data from the clinical practice research datalink, hospital episode statistics and patient reported outcomes as part of the STAR research programme. PLOS ONE 2021;16:e0261850. https://doi.org/10.1371/journal.pone.0261850
Moore A, Eccleston C, Gooberman-Hill R. ‘It’s not my knee’ – understanding ongoing pain and discomfort after total knee replacement through (re)embodiment. Arthritis Care Res 2021;74:975–81. https://doi.org/10.1002/acr.24534
Cole S, Kolovos S, Soni A, Delmestri A, Sanchez-Santos MT, Judge A, et al. Progression of chronic pain and associated quality of life and healthcare resource use over 5 years after total knee replacement: evidence from an observational cohort. BMJ Open 2022;12:e058044.
Moore A, Wylde V, Bruce J, Howells N, Bertram W, Eccleston C, Gooberman-Hill R. Experiences of recovery and a new care pathway for people with pain after total knee replacement: qualitative research embedded in the STAR trial. BMC Musculoskelet Disord 2022;23:451. https://doi.org/10.1186/s12891-022-05423-5
Wylde V, Bertram W, Sanderson E, Noble S, Howells N, Peters TJ, et al. The STAR care pathway for patients with pain at 3 months after total knee replacement: a multicentre, pragmatic, randomised, controlled trial. Lancet Rheumatol 2022;4:e188–e197. https://doi.org/10.1016/S2665-9913(21)00371-4
Wylde V, Sanderson E, Peters TJ, Bertram W, Howells N, Bruce J, et al. Screening to identify postoperative pain and cross-sectional associations between factors identified in this process with pain and function, three months after total knee replacement. Arthritis Care Res 2022;74:790–8. https://doi.org/10.1002/acr.24516
Moore AJ, Wylde V, Bertram W, Beswick AD, Howells N, Gooberman-Hill R. Healthcare professionals’ views on implementing the STAR care pathway for people with chronic pain after total knee replacement: A qualitative study. PLoS ONE 2023;18:e0284406. https://doi.org/10.1371/journal.pone.0284406
Other outputs
Noble SM, Harris SRS. Evaluation of a Care Pathway for Patients With Long-term Pain After Knee Replacement. Health Economic Analysis Plan. Version 1.0. Bristol: University of Bristol Publications Repository; 2020. URL: https://research-information.bris.ac.uk/files/281530345/STAR_HEAP_v1.0_June20_clean_pdf.pdf (accessed 22 December 2022).
Sanderson E, Peters T, Gooberman-Hill R. STAR: Support and Treatment After joint Replacement Statistical Analysis Plan. Bristol: University of Bristol Publications Repository; 2017. URL: https://research-information.bris.ac.uk/files/200553185/STAR_SAP_v.2.2.pdf (accessed 22 December 2022).
Conference posters and presentations
Work package 1
Beswick A, Wylde V, Dennis J, Howells N, Gooberman-Hill R. Interventions for the Management of Long-term Post-surgical Pain After Total Knee Replacement: A Systematic Review of Randomised Controlled Trials. Poster and presentation at the 13th Meeting of the Combined Orthopaedic Associations, Cape Town, South Africa, April 2016.
Beswick A, Wylde V, Dennis J, Howells N, Gooberman-Hill R. Interventions for the Management of Long-term Post-surgical Pain After Total Knee Replacement: A Systematic Review of Randomised Controlled Trials. Poster at the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Malaga, Spain, April 2016.
Dennis J, Wylde V, Beswick A, Bruce J, Eccleston C, Howells N, et al. Interventions for Chronic Pain After Surgery: A Systematic Review. Poster at the Population Health Early Career Researchers’ Event and Annual Symposium, Bristol, UK, December 2016.
Dennis J, Wylde V, Beswick A, Bruce J, Eccleston C, Howells N, et al. A Systematic Review to Inform a Trial of Comprehensive Pain Management for Patients With Chronic Pain After Total Knee Replacement: The STAR Experience. Poster at the International Clinical Trials Methodology Conference, Liverpool, UK, May 2017.
Wylde V, Dennis J, Beswick A, Bruce J, Eccleston C, Howells N, et al. Management of Chronic Pain After Surgery: A Systematic Review. Plenary podium presentation at the British Pain Society Conference, Birmingham, UK, May 2017.
Beswick AD, Dennis J, Wylde V, Bertram W, Blom AW, Gooberman-Hill R. The Effectiveness of Peri-operative Interventions in Preventing Chronic Pain in Patients Receiving Primary Total Knee Replacement: A Systematic Review. Poster at the European Orthopaedic Research Society Conference, Galway, Ireland, September 2018.
Dennis J, Wylde V, Murray J, Gooberman-Hill R, Beswick A. Use of Tourniquets in Knee Replacement Surgery and the Development of Chronic Pain: A Systematic Review of Randomised Controlled Trials. Poster at the European Orthopaedic Research Society Conference, Munich, Germany, September 2017 and North Bristol Trust Research Day, Bristol, UK, February 2018.
Khalid S, Gooberman-Hill R, Garriga C, Pinedo-Villanueva R, Arden N, Price A, et al. Post-surgical Predictors of Chronic Pain After Primary Knee Replacement. Poster at the Osteoarthritis Research Society International World Congress, Liverpool, UK, April 2018.
Work package 2
Khalid S, Wylde V, Gooberman-Hill R, Judge A, Pinedo-Villanueva R. An Oxford Knee Score Threshold to Identify Patients With Chronic Pain After Knee Replacement for a Complex Intervention Trial. Poster at the International Clinical Trials Methodology Conference, Liverpool, UK May 2017.
Pinedo-Villanueva R, Gooberman-Hill R, Judge A, Arden N. Healthcare Resource Use by Patients With and Without Persistent Pain After Total Knee Replacement: Findings From a Dual-centre English Cohort Study. Poster at the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Florence, Italy, March 2017.
Pinedo-Villanueva R, Gooberman-Hill R, Arden N, Judge A. Persistent Knee Pain After Total Knee Replacement: Patient-Reported Outcomes From a Dual-centre English Cohort Study. Poster at the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Florence, Italy, March 2017.
Pinedo-Villanueva R, Khalid S, Wylde V, Gooberman-Hill R, Judge A. A Cut-off Point in the Oxford Knee Score to Identify Patients With Chronic Pain After Knee Replacement. Poster presentation at the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Florence, Italy, March 2017.
Khalid S, Judge A, Villanueva R. An Unsupervised Learning Model for Pattern Recognition in Routinely Collected Healthcare Data. Paper at the 11th International Conference on Health Informatics, Funchal, Madeira, Portugal, January 2018.
Work package 3
Wylde V, Howells N, MacKichan F, Bruce J, McCabe C, Blom A, et al. Developing a Complex Intervention for Patients With Long-term Pain After Total Knee Replacement. Poster at the British Pain Society Annual Scientific Conference, Harrogate, UK, May 2016.
Wylde V, Moore A, Howells N, MacKichan F, Bruce J, McCabe C, et al. Developing a Complex Intervention for Patients With Long-term Pain After Total Knee Arthroplasty. Poster at the European Orthopaedic Research Society, Bologna, Italy, September 2016.
Wylde V, Howells N, MacKichan F, Bruce J, McCabe C, Blom A, et al. Developing a Complex Intervention For Patients With Long-term Pain After Total Knee Arthroplasty. Poster at the European Pain Federation Symposium, Dubrovnik, Croatia, September 2016.
Wylde V, Howells N, MacKichan F, Bruce J, McCabe C, Blom A, et al. Developing a Complex Intervention for Patients With Osteoarthritis Who Have Long-term Pain After Total Knee Replacement. Poster at the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Malaga, Spain, April 2016.
Wylde V, Howells N, Bertram W, Moore AJ, Bruce J, McCabe C, et al. Development of a Complex Intervention for Patients With Chronic Pain After Knee Replacement. Poster at the International Clinical Trials Methodology Conference, Liverpool, UK, May 2017.
Wylde V, Howells N, Bertram W, Moore AJ, Bruce J, McCabe C, et al. Refining a Complex Intervention for Patients With Chronic Pain After Total Knee Replacement. Poster at the British Pain Society Conference, Birmingham, UK, May 2017.
Work package 4
Bertram W, Moore AJ, Gooberman-Hill R. Optimising Informed Consent Within a Randomised Controlled Trial of a New Care Pathway for Chronic Post-Surgical Pain After Total Knee Replacement. Poster at the British Pain Society Conference, Birmingham, UK, May 2017.
Bertram W, Wylde V, Beswick AD, Blom AW, Bruce J, Burston A, et al. Support and Treatment After Replacement: The STAR Trial. Poster at the North Bristol NHS Trust Research Day, Bristol, UK, February 2018.
Work package 5
Moore AJ, MacKichan F, Gooberman-Hill R. “I know they can’t get rid of my pain”: The Beliefs of Patients Who Do Not Seek Healthcare for Chronic Pain After Total Knee Replacement. Poster at the British Pain Society Conference, Birmingham, UK, May 2017.
Moore AJ, MacKichan F, Gooberman-Hill R. Chronic Post-Surgical Pain After Knee Replacement – Why Patients Do Not Engage With Health Services. Poster at the Health Services Research UK Conference, Nottingham, UK, July 2018.
Moore AJ, MacKichan F, Gooberman-Hill R. Why Patients Do Not Seek Healthcare for Chronic Post-surgical Pain After Knee Replacement. Poster at the North Bristol Trust NHS Research Day, Bristol, UK, February 2018.
Moore AJ, Gooberman-Hill R. Understanding Why People With Chronic Post-Surgical Pain Following Knee Replacement Do Not Consult Healthcare Professionals. Presentation at the 27th Annual Meeting of the European Orthopaedic Research Society, Maastricht, the Netherlands, October 2019.
Whole programme
Gooberman-Hill R, Wylde V, Bruce J, Beswick A, MacKichan F, Blom A, et al. Post-operative Prevention and Management of Persistent Pain After Knee Replacement: The STAR programme. Poster at the International Association for the Study of Pain World Congress, Yokohama, Japan, September 2016 and North Bristol Trust Research Day, Bristol, UK, February 2018.
Gooberman-Hill R, Bruce J, Wylde V, Judge A, Moore A, Beswick A, et al. Chronic Pain After Total Knee Replacement: The STAR Research Programme Investigating Management and Care for People with Chronic Post-Surgical Pain After Total Knee Replacement. International Association for the Study of Pain Conference, virtual presentation, August 2020.
Gooberman-Hill R, Bruce J, Wylde V, Judge A, Moore A, Beswick A, et al. Improving the Post-operative Prediction and Management of Chronic Pain After Total Knee Replacement: The UK STAR Programme. International Association for the Study of Pain conference, virtual presentation, June 2021.
Data-sharing statement
This project used data from numerous pre-existing databases such as the NJR, CPRD Gold, and HES and PROMS. For access to these data, data access applications will need to be made to the NJR Research Committee, Independent Scientific Advisory Committee for the Medicines and Healthcare products Regulatory Agency and NHS Digital, respectively. All anonymised data generated in work packages 3–6 is available from the University of Bristol Research Data Storage Facility upon completion of a data access agreement. For further information or any queries please contact the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
Appendix 1 Summary of STAR patient and public involvement activity during the programme
| Date of PPI activity | Meeting type | WP | PPI activity and actions |
|---|---|---|---|
| 10 February 2015 | PEP-R Forum | WP3 | Discussed whole programme. Refined the approach letter, patient information leaflet and screening questionnaire. Advised on logistics of the clinic. Discussed PPI plans |
| 15 September 2015 | PEP-R Forum | WP3 WP4 |
Discussed the trial and plans to interview patients who do not seek further treatment |
| 28 September 2015 | STAR Forum | WP4 WP5 |
Discussed whole programme. Approved screening questions, interview guides and plain English summary |
| 8 December 2015 | STAR Forum | WP4 | Reviewed questionnaire booklet and addressed how to provide contact details of organisations and charities |
| March/April 2016 | One-to-one | WP4 | Edited patient information booklet, resource diary and questionnaire |
| 22 March 2016 | PEP-R Forum | WP4 | Edited patient information booklet and interview topic guides. Discussed how to telephone patients |
| 28 June 2016 | STAR Forum | WP3 WP4 |
Discussed the feedback from the 10 patients who attended the STAR Planning clinics And CarE (PACE) clinic and improved plain English summary of findings. Made recommendations about trial data collection |
| 27 September 2016 | STAR Forum | WP4 | Reviewed patient information booklet and made recommendations about trial operating procedures |
| 5 December 2016 | STAR Forum | WP4 | Discussed sample size, randomisation, standard operating procedures and trial progress |
| 28 February 2017 | STAR Forum | WP1 WP4 |
Contributed to summaries of systematic reviews Refined and amended trial information and resource diaries |
| 6 June 2017 | STAR Forum | WP1 WP4 |
Reviewed summary of systematic review Made recommendations about patient involvement in the researcher training and recruitment standard operating procedures |
| 19 September 2017 | STAR Forum | WP4 WP6 |
Discussed trial recruitment, new sites and training day Discussed new grant application idea and STAR website Note: Stewart Long from Versus Arthritis attended |
| 28 November 2017 | STAR Forum | WP1 WP4 |
Reviewed systematic review plain English summary Reviewed the newsletter for STAR participants Made recommendations about STAR trial thank you letters |
| 27 March 2018 | STAR Forum | WP2 WP4 |
Discussed the plain English summary of the Oxford Knee Score pain cut off, and use and interpretation of routine health data Made recommendations for improving 12-month follow-up questionnaire |
| 8 May 2018 | STAR Forum | WP4 | Reviewed the feedback leaflet for screening project participants. Made recommendations about the newsletter for STAR participants and agreed to be featured in the next issue |
| 19 June 2018 | STAR Forum | WP4 WP6 |
Reviewed the newsletter for STAR participants Interviewed and photographed for ‘Take Part Be Involved in Research’ magazine. Discussed dissemination ideas |
| 23 October 2018 | STAR Forum | WP4 WP6 |
Discussed randomisation to usual care letter Approved the newsletter for STAR participants and the ‘Take Part Be Involved in Research’ article and leaflet. Discussed publicity of the article Discussed STAR website and films about PPI. Discussed James Lind Alliance’s (NIHR, School of Healthcare Enterprise and Innovation, University of Southampton, Southampton, UK) priority setting partnership on problematic knee replacements and made recommendations |
| 4 December 2018 | STAR Forum | WP4 WP5 |
Made recommendations for the letters to participants about their randomisation to usual care or STAR clinic. Discussed the results of the interviews carried out so far |
| 5 February 2019 | STAR Forum | WP4 | Welcomed new member to the group Discussed 3-month extension to trial and PPI plans |
| 9 April 2019 | STAR Forum | WP4 | Discussed how to measure mental health in pain trials Completed the James Lind Alliance Priority Setting Partnership on problematic knee replacement ranking survey |
| 16 June 2019 | STAR Forum | WP4 WP6 | Approved newsletter for participants Discussed public dissemination |
| 1 October 2019 | STAR Forum | PPI
WP4 WP6 |
Discussed PPI impact on STAR to date Reviewed the summary of findings for the screening project participants Discussed public dissemination and the use of infographics |
| 28 January 2020 | PEP-R Forum | WP4 WP6 |
Discussed the STAR trial Discussed the final report and infographics of STAR work packages |
| 8 February 2020 | STAR Forum | WP1 WP4 WP6 |
Discussed the systematic reviews Updated on the STAR trial update. Approved the summary of findings for the screening project participants Discussed the final report and infographics of STAR work packages |
| 1 July 2020 | STAR Forum | WP4 WP6 |
Discussed the STAR trial Discussed the final report |
| 2 October 2020 | PEP-R Forum | WP4 WP6 |
Discussed the results of the STAR trial Discussed the final report and infographics of STAR work packages/public dissemination |
| 6 October 2020 | STAR Forum | WP4 WP5 |
Discussed the results of the interviews with patients |
| 20 October 2020 | STAR Forum | WP4 WP5 WP6 |
Reviewed the summary of finding for trial participants Discussed the preliminary results of the costs to patients and community care Reviewed the summary of findings for interview participants Discussed implementation and public dissemination |
| 9 November 2020 | STAR Forum | WP6 | Interviewed for the film |
| 4 February 2021 | One to one | WP6 | Approved the film |
| 4 May 2021 | PEP-R Forum | WP4 | Discussed the final results of the costs to patients and community care |
Appendix 2 Post-operative patient-related risk factors for chronic pain after total knee replacement
Systematic review search strategy as applied in MEDLINE on Ovid
-
Epidemiologic Studies/
-
exp Case-Control Studies/
-
exp Cohort Studies/
-
Cross-Sectional Studies/
-
(epidemiologic adj (study or studies)).ab,ti.
-
case control.ab,ti.
-
(cohort adj (study or studies)).ab,ti.
-
cross sectional.ab,ti.
-
cohort analy$.ab,ti.
-
(follow up adj (study or studies)).ab,ti.
-
longitudinal.ab,ti.
-
retrospective$.ab,ti.
-
prospective$.ab,ti.
-
(observ$ adj3 (study or studies)).ab,ti.
-
exp clinical study/
-
randomized controlled trial/
-
15 not 16
-
adverse effect?.ab,ti.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 17 or 18
-
Arthroplasty, Replacement, Knee/
-
Knee Prosthesis/
-
(arthoplast$ adj3 knee$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
(knee$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
(knee adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
20 or 21 or 22 or 23 or 24
-
19 and 25
Appendix 3 The effectiveness of interventions applied in the pre-, peri- and post-operative setting in preventing chronic pain after total knee replacement
Systematic review search strategy as applied in MEDLINE on Ovid
-
randomized controlled trial/or randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
randomly.ab
-
trial.ab
-
randomised.tw
-
1 or 2 or 3 or 4 or 5 or 6 or 7
-
review/
-
‘systematic review$’.mp
-
9 or 10
-
8 or 11
-
Arthroplasty, Replacement, Knee/
-
Knee Prosthesis/
-
(arthoplast$ adj3 knee$).mp. [mp = title, abstract, original title, name of substance word,
-
subject heading word, keyword heading word, protocol supplementary concept word, rare disease
-
supplementary concept word, unique identifier]
-
(knee$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject
-
heading word, keyword heading word, protocol supplementary concept word, rare disease
-
supplementary concept word, unique identifier]
-
(knee adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject
-
heading word, keyword heading word, protocol supplementary concept word, rare disease
-
supplementary concept word, unique identifier]
-
13 or 14 or 15 or 16 or 17
-
12 and 18
Appendix 4 Interventions to manage chronic post-operative pain
Systematic review search strategy as applied in MEDLINE on Ovid
-
randomized controlled trial/or randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
randomly.ab.
-
trial.ab.
-
randomised.tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7
-
Pain, Postoperative/
-
((postoperative adj6 pain*) or (post-operative adj6 pain*) or postoperative- pain*).mp.
-
((post-operative adj6 analg*) or (postoperative adj6 analg*)).mp.
-
((post-surgical adj6 pain*) or (post surgical adj6 pain*) or (postsurgery adj6 pain*) or (post adj surg* adj pain*)).mp.
-
((post* adj pain*) or pain relief after or pain following surg*).mp.
-
((posttreatment adj6 pain*) or (pain control after adj6 surg*) or ((post-extraction or postextraction or post-surg*) and (pain* or discomfort))).mp.
-
((analg* adj6 postoperat*) or (analg* adj6 post-operat*) or (pain* adj6 after surg*) or (pain* adj6 after operat*) or (analgesi* adj6 after operat*)).mp.
-
((pain* or analg*) adj6 (“follow* operat*” or “follow* surg*”)).mp.
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
-
exp Neuralgia/
-
(neuralgia* or neurodynia).tw.
-
((neuropathic or nerve*) adj3 pain*).tw.
-
Amputation/
-
Phantom Limb/
-
Failed Back Surgery Syndrome/
-
18 or 19 or 20 or 21 or 22 or 23
-
(chronic* or constant* or continu* or persist* or longterm or long-term or longstanding or long-standing or long lasting or longlasting or phantom).mp.
-
exp Pain, Intractable/or exp Chronic Pain/
-
exp pain/and (chronic* adj5 pain*).mp.
-
25 or 26 or 27
-
17 or 24
-
24 or 28
-
8 and 29 and 30
Appendix 5 Interventions to manage chronic post-operative pain update
Systematic review search strategy as applied in MEDLINE on Ovid
-
controlled clinical trial.pt.
-
randomized controlled trial.pt.
-
clinical trials as topic/
-
(randomi#ed or randomi#ation or randomi#ing).ti,ab,kf.
-
(RCT or “at random” or (random* adj3 (administ* or allocat* or assign* or class* or cluster or crossover or cross-over or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kf.
-
placebo.ab,ti,kf.
-
trial.ti.
-
(control* adj3 group*).ab.
-
(control* and (trial or study or group*) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf.
-
((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf.
-
double-blind method/or random allocation/or single-blind method/
-
or/1-11
-
(systematic or structured or evidence or trials or studies).ti. and ((review or overview or look or examination or update* or summary).ti. or review.pt.)
-
(0266-4623 or 1469-493X or 1366-5278 or 1530-440X or 2046-4053).is.
-
meta-analysis.pt. or (meta-analys* or meta analys* or metaanalys* or meta synth* or meta-synth* or metasynth*).ti,ab,kf,hw.
-
((systematic or meta) adj2 (analys* or review)).ti,kf. or ((systematic* or quantitativ* or methodologic*) adj5 (review* or overview*)).ti,ab,kf,sh. or (quantitativ$ adj5 synthesis$).ti,ab,kf,hw.
-
(integrative research review* or research integration).tw. or scoping review?.ti,kf. or (review.ti,kf,pt. and (trials as topic or studies as topic).hw.) or (evidence adj3 review*).ti,ab,kf.
-
review.pt. and ((medline or medlars or embase or pubmed or scisearch or psychinfo or psycinfo or psychlit or psyclit or cinahl or electronic database* or bibliographic database* or computeri#ed database* or online database* or pooling or pooled or mantel haenszel or peto or dersimonian or der simonian or fixed effect or ((hand adj2 search*) or (manual* adj2 search*))).tw,hw. or (retraction of publication or retracted publication).pt.)
-
or/13-18
-
Arthroplasty, Replacement, Knee/
-
Knee Prosthesis/
-
(arthoplast$ adj3 knee$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(knee$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(knee$ adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(knee$ adj3 prosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(knee$ adj3 endoprosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
Arthroplasty, Replacement, Hip/
-
Hip Prosthesis/
-
(arthoplast$ adj3 hip$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(hip$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(hip$ adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(hip$ adj3 prosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(hip$ adj3 endoprosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
Arthroplasty, Replacement, Shoulder/
-
Shoulder Prosthesis/
-
(arthoplast$ adj3 shoulder$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(shoulder$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(shoulder$ adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(shoulder$ adj3 prosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(shoulder$ adj3 endoprosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
Arthroplasty, Replacement, elbow/
-
Elbow Prosthesis/
-
(arthoplast$ adj3 elbow$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(elbow$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(elbow$ adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(elbow$ adj3 prosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(elbow$ adj3 endoprosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
Arthroplasty, Replacement/
-
Wrist/or Wrist Joint/
-
48 and 49
-
(arthoplast$ adj3 wrist$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(wrist$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(wrist$ adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(wrist$ adj3 prosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(wrist$ adj3 endoprosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
Arthroplasty, Replacement, Ankle/
-
(arthoplast$ adj3 ankle$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(ankle$ adj3 replac$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(ankle$ adj3 implant$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(ankle$ adj3 prosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
(ankle$ adj3 endoprosthe$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
or/20-47
-
or/50-61
-
Pain, Postoperative/
-
chronic pain/
-
Pain, intractable/
-
(chronic* or constant* or continu* or persist* or longterm or long-term or longstanding or long-standing or long lasting or longlasting).mp. adj3 pain/[mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
((postoperative adj6 pain*) or (post-operative adj6 pain*) or postoperative- pain*).mp.
-
((post-operative adj6 analg*) or (postoperative adj6 analg*)).mp.
-
((post-surgical adj6 pain*) or (post surgical adj6 pain*) or (postsurgery adj6 pain*) or (post adj surg* adj pain*)).mp.
-
((post* adj pain*) or pain relief after or pain following surg*).mp.
-
((posttreatment adj6 pain*) or (pain control after adj6 surg*) or ((post-extraction or postextraction or post-surg*) and (pain* or discomfort))).mp.
-
((analg* adj6 postoperat*) or (analg* adj6 post-operat*) or (pain* adj6 after surg*) or (pain* adj6 after operat*) or (analgesi* adj6 after operat*)).mp.
-
((pain* or analg*) adj6 (“follow* operat*” or “follow* surg*”)).mp.
-
exp Neuralgia/
-
(neuralgia* or neurodynia).tw.
-
((neuropathic or nerve*) adj3 pain*).tw.
-
or/64-77
-
12 or 19
-
62 or 63
-
78 and 79 and 80
-
limit 81 to humans
-
limit 82 to yr = “2016 -Current”
-
This search strategy includes terms from Marques et al. 156 and Blom et al. 157
Appendix 6 Cost-effectiveness of the STAR intervention: an economic model
The aim of this analysis was to examine the expected cost-effectiveness of the STAR intervention compared with current practice over 5 years.
Model conceptualisation and structure
The target population was individuals classified as having chronic pain 3 months after a total knee replacement surgery. The model was designed as a cohort Markov model with time-dependent annual transition probabilities and a time horizon of 5 years. The cycle length was 1year. The setting of the economic evaluation was patients treated by NHS hospitals in England and the study perspective that of the NHS.
Simulated individuals in the model received either the pain management intervention (STAR) during the first year only or usual care. After the first year, simulated patients can either remain in chronic pain (CP) or move to a non-chronic pain (NCP) health state (Figure 9). Patients move between CP and NCP states at any point. Mortality was not included in the model given its short time horizon and because the intervention is assumed not to have any impact on the risk of death.
FIGURE 9.
Model structure. TKR, total knee replacement.
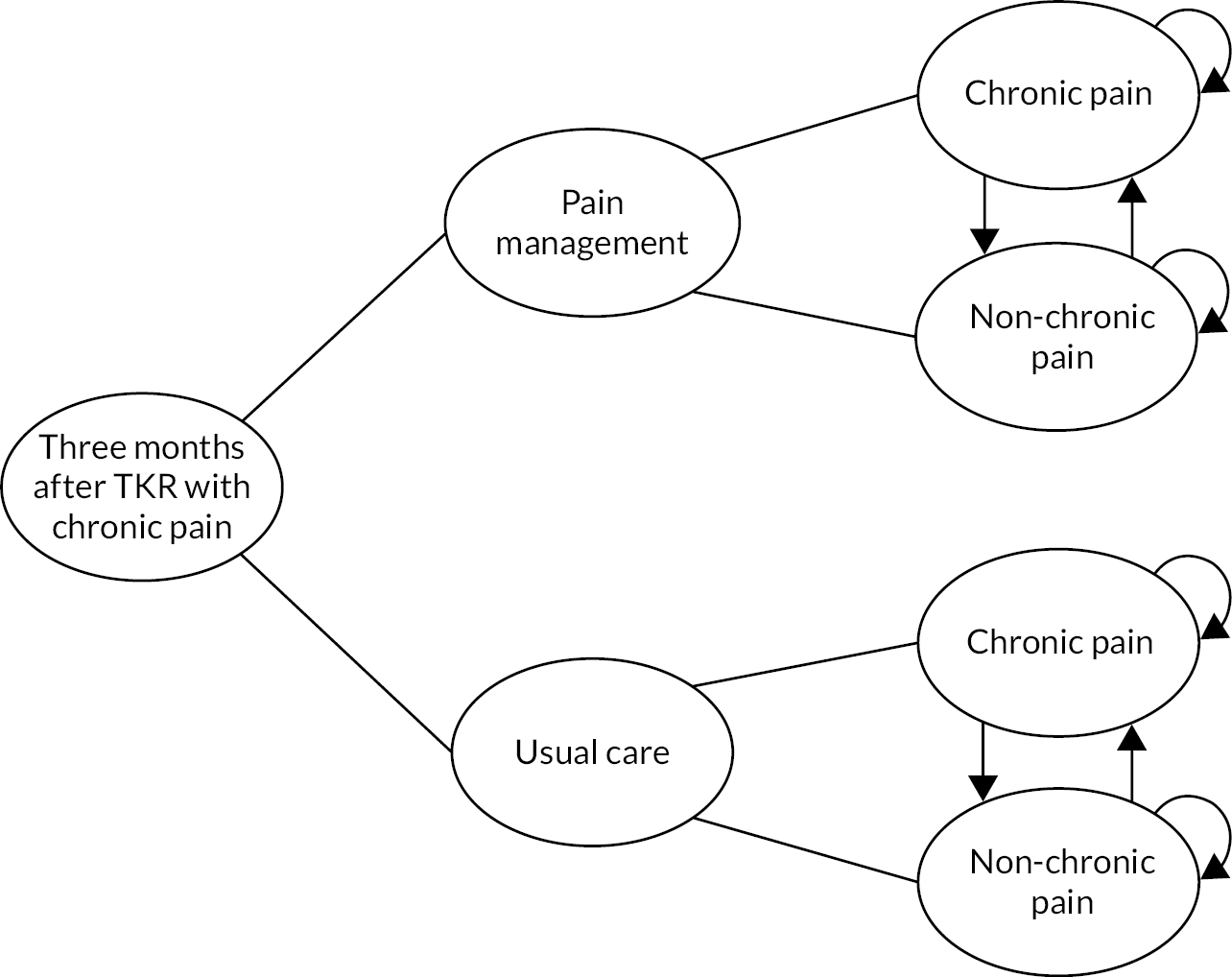
We discounted health utility and costs after the first year at 3.5% per year following National Institute for Health and Care Excellence guidelines. 158
Data sources
Model inputs were parameterised based on evidence from the STAR trial120 for the intervention comparator and real-world data from both a population-based cohort study (COASt)121 and the CPRD for the usual care comparator (Table 2).
Transition probabilities
Using evidence from the STAR trial, we estimated the probabilities of transitioning from baseline CP to CP or NCP in year 1 (the cycle during which the treatment, i.e. pain management, was given). Based upon the evidence from the STAR trial, these transition probabilities differed between the two arms, (i.e. usual care and pain management). Subsequent transition probability estimates (years 2–5) were calculated using data from COASt and were the same for both comparators.
Costs
The estimation of costs included medication prescriptions, primary care consultations, hospital admissions, and the cost of the STAR intervention. Costs were taken from the intervention and usual care trial arms for year 1. For years 2–5, we extracted the annual per cent changes observed in (1) CPRD for prescriptions and consultations, and (2) COASt for hospital admissions for both the CP and NCP groups. We then applied per cent changes to the respective year 1 parameter estimates for each of the pain groups. Costs from the trial and COASt cohort considered only those associated with the pain of the intervention and treatment of the knee, whereas CPRD costs considered all reported primary care consultations and the cost of prescriptions for pain. Medication prescriptions, consultations and hospital admission costs differed between arms (i.e. usual care and pain management) as identified in the trial; however, the difference in hospital costs identified in the trial was only applied to year 1 and assumed to be different for the CP and NCP health states only thereafter. The cost of pain management was applied during the first cycle only and to the pain management arm only. We estimated costs separately for each health state (i.e. CP and NCP).
Health utility estimates
For the first cycle, health utility was estimated using reported EQ-5D-5L (mapped to the three-level valuation set)145 from the STAR trial. We used the baseline, 6-month and 1-year health utility estimates from the trial to calculate the mean QALYs for the health states (i.e. CP and NCP) for each of the comparators using the area under the curve method. We then adjusted these QALY estimates by controlling for baseline health utility using multiple regression. 159 Estimates of QALYs for years 2–5 were generated by applying the per cent of potential change160 (PoPC) observed over time in those with CP/NCP in COASt (identified at the 12-month point) to the QALY estimates for year 1 from the trial (also identified at 12 months). Under the base-case analysis, QALYs differed between comparators (usual care and pain management) and between health states CP and NCP.
Scenario analysis
For the base-case analysis, patients were classified as being in CP at baseline (i.e. 3 months post surgery). QALY estimates for both health states (CP and NCP) are different between usual care and pain management; in both cases CP QALY estimates are lower than NCP. Some the assumptions made about findings in the trial and especially for the years beyond the trial were varied and their impact on results examined via scenario analysis.
Scenario 1
First, we removed the differences between the QALY estimates for CP (and NCP) across comparators so that CP would have a single QALY estimate for both usual care and pain management (and so would NCP). This was applied to the first cycle, and since parameters for the following years were based on PoPC, they were also the same across comparators for years 2–5. As transition probabilities still differed, each comparator would still accumulate different levels of QALYs based on the transition of simulated patients between CP and NCP. We examined how this change would impact results obtained in the base case.
Scenario 2
Secondly, we tested classifying the initial CP status of simulated patients at 10 weeks post surgery. This means that at baseline (12 weeks post surgery) some individuals would have left CP and begin the model in NCP. Results were then compared with the base case.
Scenario 3
Thirdly, considering that hospital inpatient admissions for those in the usual care arm of the trial were unexpectedly higher than for those in the STAR intervention arm, we recalculated the hospital admission costs for the CP and NCP groups in the trial without any differences based on trial arm and used those in the model for year 1. For the following years, we applied the same method used for the base case.
Probabilistic sensitivity analysis
To assess the impact of model parameter uncertainty on model results, we applied appropriate distributions to the transition probabilities, costs and health utilities. We ran 10,000 independent simulations of all input values. We used the results from the simulations to report the impact of parameter uncertainty on model results through a cost-effectiveness acceptability curve.
Results
Deterministic results indicate that the STAR pain management intervention would dominate usual care by leading to an expected 0.086 more QALYs over the 5 years (3.177 QALYs under the STAR pain management intervention compared with 3.091 for usual care) and £375 lower costs (£3189 and £3563, respectively). Assuming a cost-effectiveness threshold of £20,000 per QALY gained, this result would equate to an iNMB in favour of the STAR intervention of £2086 (95% confidence interval –£14,234 to £19,644]). The probabilistic sensitivity analysis suggests that, again at a cost-effectiveness threshold of £20,000 per QALY gained, the STAR intervention would have a 0.62 probability of being cost-effective (Figure 10).
FIGURE 10.
Base-case cost-effectiveness plane. PSA, probabilistic sensitivity analysis; WTP, willingness to pay.
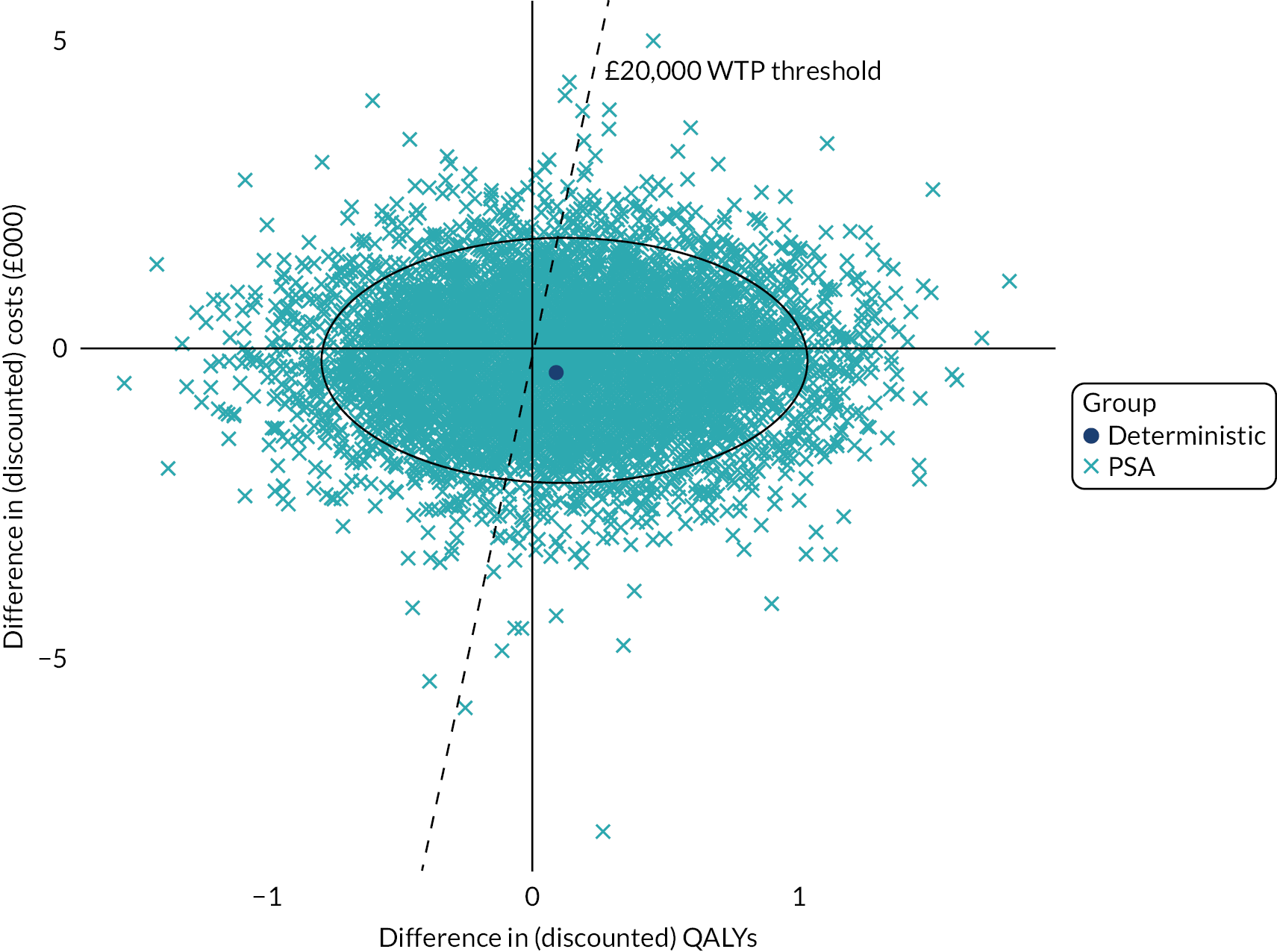
If QALY estimates for health states were the same regardless of comparator, the STAR pain management intervention would still be dominant under the deterministic analysis, with an iNMB of £782 and 0.64 probability of being cost-effective. If the CP status of modelled patients was classified earlier at 10 weeks (instead of 12) post surgery, the deterministic dominance of the STAR intervention would still prevail with an iNMB of £1,925 and a 0.61 probability of being cost-effective. Finally, if the STAR pain management intervention was assumed not to have any impact on hospital admissions, then it would still lead to higher QALY estimates (3.18 vs. 3.09 QALYs) but would cost £243 more than usual care over the 5 years, leading to a deterministic incremental cost-effectiveness ratio of £2839 per QALY gained, an iNMB of £1469 and a 0.59 probability of being cost-effective according to the probabilistic sensitivity analysis.
Discussion
The model results suggest that the STAR intervention is likely to be both cost-saving and more effective than usual care during the first 5 years of implementation. Expected gains in quality of life are small however (0.086 QALYs), well under the reported minimally clinically important difference for patients having a knee replacement (0.182, based on PROMs data from the English NHS PROMS). 161 The probabilistic analysis shows that it is most likely that the STAR intervention would be cost-effective, but also that there is an important level of uncertainty around these results. The 95% CI ellipse in the cost-effectiveness reaches all four quadrants, meaning that it is possible that both extra costs and QALY gains could favour either comparator.
The expected dominance of the STAR intervention appeared robust to different assumptions about quality of life and the starting point of the intervention. Identifying the CP status of individuals at 10 weeks furthermore suggests that the time of classification is an influencing factor in the cost-effectiveness of the STAR intervention. Though the difference was small, our scenario results suggest that it may be beneficial to wait until at least 3 months post surgery to classify the CP status of individuals to improve both the expected iNMB and the probability of the cost-effectiveness of the intervention. Expected dominance was however sensitive to hospital admissions being less costly under the STAR intervention than for usual care, as we found hospital costs to be a key driver of the STAR intervention being cost-saving. However, eliminating any effect on hospital admissions still showed the STAR intervention likely to be cost-effective.
Limitations of the economic model included sourcing the model input data from three different sources as well as differences in the time points at which chronic pain categorisation occurred in each. The trial showed that those who receive the STAR intervention move out of chronic pain at a faster rate and sooner than those in usual care; however, this was not incorporated into the model as it used a cycle length of 1 year and so changes in CP status could not be considered earlier. Model results are hence likely to underestimate the benefits of the STAR intervention.
In conclusion, modelling the 5-year costs and quality of life of the STAR pain management intervention compared with NHS usual care based on trial findings and long-term observational data suggests that the intervention is likely to be cost-effective and possibly dominant. Gains in both costs and quality of life are, however, both small and largely uncertain. Long-term follow-up of the participants involved in the trial would help refine assumptions and potentially reduce the uncertainty around these results.
| Input parameters | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Source |
|---|---|---|---|---|---|---|
| Base case | ||||||
| Transition probabilities | ||||||
| Usual care CP to CP | 0.494 | 0.368 | 0.388 | 0.400 | 0.406 | STAR trial (year 1), COASt (years 2–5) |
| Usual care CP to NCP | 0.506 | 0.632 | 0.612 | 0.600 | 0.594 | |
| Usual care NCP to CP | 0.000 | 0.050 | 0.042 | 0.031 | 0.037 | |
| Usual care NCP to NCP | 0.000 | 0.950 | 0.958 | 0.969 | 0.963 | |
| Pain management CP to CP | 0.355 | 0.368 | 0.388 | 0.400 | 0.406 | |
| Pain management CP to NCP | 0.645 | 0.632 | 0.612 | 0.600 | 0.594 | |
| Pain management NCP to CP | 0.000 | 0.050 | 0.042 | 0.031 | 0.037 | |
| Pain management NCP to NCP | 0.000 | 0.950 | 0.958 | 0.969 | 0.963 | |
| QALYs | ||||||
| Usual care CP | 0.465 | 0.556 | 0.607 | 0.642 | 0.698 | STAR trial (year 1), COASt (years 2-5) |
| Usual care NCP | 0.539 | 0.730 | 0.727 | 0.716 | 0.702 | |
| Pain management CP | 0.484 | 0.572 | 0.621 | 0.654 | 0.708 | |
| Pain management NCP | 0.560 | 0.742 | 0.739 | 0.728 | 0.714 | |
| Cost (£) | ||||||
| Pain management | 191 | 0 | 0 | 0 | 0 | STAR trial |
| Prescriptions CP | 100 | 88 | 91 | 91 | 91 | STAR trial (year 1), CPRD (years 2-5) |
| Prescriptions NCP | 22 | 18 | 17 | 17 | 18 | |
| Medical consultations CP | 166 | 143 | 137 | 137 | 125 | STAR trial (year 1), CPRD (years 2-5) |
| Medical consultations NCP | 62 | 51 | 48 | 46 | 48 | |
| Usual care hospital admissions CP | 2972 | 898 | 464 | 65 | 24 | STAR trial (year 1), COASt (years 2-5) |
| Usual care hospital admissions NCP | 1057 | 386 | 331 | 82 | 249 | |
| Pain management hospital admissions CP | 1265 | 898 | 464 | 65 | 24 | STAR trial (year 1), COASt (years 2-5) |
| Pain management hospital admissions NCP | 1302 | 386 | 331 | 82 | 249 | |
| Sensitivity analysis: scenario analysis | ||||||
| Transition probabilities | ||||||
| Usual care CP to CP | 0.500 | 0.368 | 0.388 | 0.400 | 0.406 | STAR trial (year 1), COASt (years 2–5) |
| Usual care CP to NCP | 0.500 | 0.632 | 0.612 | 0.600 | 0.594 | |
| Usual care NCP to CP | 0.000 | 0.050 | 0.042 | 0.031 | 0.037 | |
| Usual care NCP to NCP | 1.000 | 0.950 | 0.958 | 0.969 | 0.963 | |
| Pain management CP to CP | 0.357 | 0.368 | 0.388 | 0.400 | 0.406 | |
| Pain management CP to NCP | 0.643 | 0.632 | 0.612 | 0.600 | 0.594 | |
| Pain management NCP to CP | 0.222 | 0.050 | 0.042 | 0.031 | 0.037 | |
| Pain management NCP to NCP | 0.778 | 0.950 | 0.958 | 0.969 | 0.963 | |
| QALYs | ||||||
| CP | 0.477 | 0.566 | 0.616 | 0.650 | 0.704 | STAR trial (year 1), COASt (years 2-5) |
| NCP | 0.555 | 0.739 | 0.736 | 0.725 | 0.711 | |
| Cost (£) | ||||||
| Hospital admissions CP | 1935 | 898 | 464 | 65 | 24 | STAR trial (year 1), COASt (years 2-5) |
| Hospital admissions NCP | 1237 | 386 | 331 | 82 | 249 | |
Appendix 7 Assessing the implementation of the STAR care pathway for people with chronic pain after total knee replacement
Background
The STAR care pathway for people with pain at 3 months after total knee replacement comprised an assessment appointment with an ESP and up to six telephone follow-up calls over 12 months. 120 The pathway aims to identify the potential underlying causes for chronic pain and enable onward referral to appropriate treatment. The clinical effectiveness and cost-effectiveness of the STAR care pathway was evaluated in a multicentre randomised controlled trial. Here, we report on an evaluation of the experiences of the health-care professionals who mobilised the new pathway at different trial sites.
Normalisation Process Theory (NPT)162 can be used as a framework to explore the work involved in implementing the new pathway. Understanding implementation processes can provide an understanding of how and why some new interventions might become normalised and embedded within routine practice, while others do not. 162 NPT provides four key constructs to understand the different kinds of work that people do when implementing a new intervention:162,163
-
coherence – how people individually and collectively make sense of a new intervention
-
cognitive participation – how people build a community of practice around a new intervention
-
collective action – the operational work that people do to enact a new intervention
-
reflexive monitoring – the appraisal work that people do to understand how a new intervention affects them.
It is possible to assess the potential of a new intervention to become normalised as part of routine practice by identifying and assessing factors that are known to affect implementation processes. 164 These factors can be identified using the NoMAD instrument, which is a 23-item measure of normalisation based on the four core constructs of NPT. NoMAD can be used to identify and understand implementation processes from the viewpoints of those directly involved in implementing interventions in health care. 81 The aim of this study is to understand how the STAR care pathway was implemented within a multicentre randomised controlled trial.
Methods
Extended scope practitioners (ESPs) and PIs from each of the eight trial sites, who were involved in delivering the STAR care pathway during the trial, were invited to participate in a telephone interview. Before taking part, all participants provided consent to audio-recording and the publication of anonymous quotations.
We used the NoMAD questionnaire items as a framework to guide qualitative data collection and content analysis. 155 The interview topic guide used the NoMAD items to elicit stakeholders’ experiences of delivering the STAR care pathway. Interviews were conducted by the lead author (AJM; male, PhD) who has a background in health sociology and significant qualitative research experience.
Extracts from the interview transcripts were coded and assigned to themes according to the four core constructs of NPT and the linked NoMAD items. The content of these themes was explored with a focus on participants’ experiences of implementing the care pathway and any barriers to and facilitators of implementation.
We took a pragmatic approach to sample size and aimed to achieve representation across the eight trial sites. Twelve ESPs and eight PIs had experience of implementing the STAR care pathway. All were sent a study information pack.
Results
Eight ESPs and six PIs from seven trial sites participated (Table 3). We present the findings according to the three general items from the NoMAD questionnaire, which aim to elicit a general sense of familiarisation, and then the four core constructs from NPT.
| Participant study code | Occupation of participant and study site |
|---|---|
| EQ-5D-3L | EuroQol-3 Dimensions, three-level version 1 |
| COS1/S1 | Consultant orthopaedic surgeon, site 1 |
| ESP1/S1 | ESP/physiotherapist, site 1 |
| ESP2/S1 | ESP, site 1 |
| ESP1/S2 | ESP/physiotherapist, site 2 |
| COS1/S2 | Consultant orthopaedic surgeon, site 2 |
| ESP2/S2 | ESP/physiotherapist, site 2 |
| COS2/S2 | Consultant Orthopaedic surgeon, site 2 |
| ESP1/S3 | ESP/physiotherapist, site 3 |
| ESP1/S4 | ESP/physiotherapist, site 4 |
| ESP1/S5 | ESP/physiotherapist, site 5 |
| COS1/S5 | Consultant orthopaedic surgeon, site 5 |
| CR1/S6 | Consultant clinical rheumatologist and academic, site 6 |
| ESP1/S6 | ESP/physiotherapist, site 6 |
| COS1/S7 | Consultant orthopaedic surgeon, site 7 |
Familiarity with the intervention
Extended scope practitioners reported that they felt familiar with many aspects of the new care pathway in the trial, which had some aspects that were in addition to normal practice, including elements of the assessment process and follow-up telephone calls. ESPs felt the pathway could become part of normal practice if the trial found it to be clinically effective and cost-effective compared with usual care.
Coherence
Stakeholders identified some aspects of the new care pathway that were different from their usual practice. Regular telephone calls were felt to be a better use of time and resources for follow-up discussions than face-to-face clinics. The clinic was also much longer at 60 minutes than the standard 15–20-minute follow-up appointments and ESPs felt that patients benefited from having more time to talk about their surgery and recovery. Clinicians valued the protected time and training to address psychosocial factors and neuropathic pain. ESPs valued the way in which the pathway had formalised a range of strategies for the management of ongoing pain, which enabled them to refer patients on for pain medicines, anxiety or depression, or to a GP, physiotherapist or surgeon. It was noted that life events impacted on some patients’ recovery and that this was not captured in the current care pathway protocol. It was suggested that this may have been better dealt with by a referral to a counsellor.
Participants reported that the care pathway and training received as part of the trial changed some aspects of their work in ways that were of value to them. They noted the benefit of having protected time to address psychosocial factors and neuropathic pain, which could not be considered in the scope of usual care but that they thought played a part in post-operative knee pain. Participants reported feeling more confident and enabled to make decisions about referring patients to further treatment.
Cognitive participation
Participants noted the importance of key individuals to delivery of the intervention in the trial. These included staff to organise clinics, ESPs to deliver the intervention, consultants with overall responsibility and hospital managers with responsibility for funding.
Participants felt that the care pathway was a legitimate part of their role. They were open to working in new ways and suggested that they would continue to support it if it was shown to be clinically and cost effective. Some suggested that extra training may be needed to give ESPs the confidence to recommend prescribing for neuropathic pain, anxiety and depression.
Collective action
Participants experienced some challenges in delivering the STAR clinics and follow-up calls. The intervention required more time and resources than normal assessment and follow-up practice. Participants felt that sensitivity was required when handling referrals back to the surgeons so that the surgeons were fully aware that patients they had originally treated had been assessed in a STAR clinic and may be referred back to them. Participants also suggested that having information on patients’ pre-operative pain or depression scores may be useful in future roll-out of the intervention as a way to provide a baseline reference by which to guide post-operative pain management. In addition, one surgeon noted that involvement in the trial had contributed to the creation of a team of clinicians who were highly specialised in knee replacement and follow-up, and that this benefited patients. Training and support for the delivery of the STAR pathway was felt to be excellent.
Reflexive monitoring
Participants described how the intervention impacted on them. They valued many elements of the care pathway, including a focus on neuropathic pain, psychosocial issues, an increased knowledge of pain management, and the formalisation and validation of referral practices. Some ESPs noted that involvement in the trial and delivery of the care pathway had improved their own practice and management of patients and had increased their awareness of long-term pain.
Discussion
Our aim was to evaluate the implementation of the STAR care pathway, based on the views of professionals involved in its delivery within a randomised controlled trial. Allen et al. 165 have emphasised the importance of incorporating implementation into trials to enhance the translation of findings. We used the NoMAD instrument to frame our data collection and descriptive content analysis, with stakeholder responses mapped onto the four NPT core constructs.
Fourteen out of a potential 20 ESPs and PIs took part, and we were able to collect a rich data set including multiple aspects of implementation. We achieved good representation of stakeholders, including five consultant orthopaedic surgeons, one consultant rheumatologist, and eight ESPs.
Our findings indicate that participants quickly became familiar with the STAR care pathway, which differed from normal practice. Stakeholders valued the patient-centredness of the pathway and its ability to facilitate identification and management of neuropathic pain. The care pathway also formalised referral processes, enabling professionals to refer patients to appropriate services. Previous research has shown that clinicians often struggle to help patients with chronic post-operative pain because of a need for clear guidance and referral pathways. 125 Stakeholders were open to working in new ways and would continue to support the care pathway.
Potential challenges to implementation included the resources needed for intervention delivery. These were provided in the trial and would be needed to enable delivery in the NHS. A need for collective understanding and sensitivity in how referrals were handled was also identified, particularly when referring patients back to surgical teams. Support from hospital management in future implementation of the care pathway was felt to depend on whether or not the trial findings indicated that the STAR care pathway was clinically effective and cost-effective.
Conclusion
Our findings add to the wider evidence on identifying and managing chronic pain after knee replacement. We recommend that if the STAR care pathway is implemented, sufficient time and resources are allocated to enable delivery. Furthermore, understanding of the pathway and sensitivity in how referral processes are communicated should be promoted widely among colleagues involved in the pathway.
List of abbreviations
- ARD
- adjusted risk difference
- BPI
- Brief Pain Inventory
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COASt
- Clinical Outcomes in Arthroplasty Study
- CP
- chronic pain
- CPRD
- Clinical Practice Research Datalink
- CRPS
- complex regional pain syndrome
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESP
- extended scope practitioner
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- iNMB
- incremental net monetary benefit
- NCP
- non-chronic pain
- NIHR
- National Institute for Health and Care Research
- NJR
- National Joint Registry
- NoMAD
- Normalisation Measure Development
- NPT
- Normalisation Process Theory
- OKS
- Oxford Knee Score
- OKS-PS
- Oxford Knee Score pain subscale
- OR
- odds ratio
- PEP-R
- Patient Experience Partnership in Research
- PI
- principal investigator
- PoPC
- percentage of potential change
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- ROC
- receiver operating characteristic
- STAR
- Support and Treatment After joint Replacement
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/WATM4500).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
