Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as award number NIHR200625. The contractual start date was in June 2019. The draft manuscript began editorial review in January 2022 and was accepted for publication in August 2023. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Plappert et al. This work was produced by Plappert et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Plappert et al.
Synopsis
Background
Bipolar, schizophrenia and other psychoses are the single largest cause of disability in the UK,1 and yet probably one-quarter to half of people receive no specialist mental health care. 2 The prevalence of bipolar, schizophrenia and other psychoses in England, defined as the number of people on the general practice Quality and Outcomes Framework (QOF) registers for severe mental illness, was 0.8% for QOF year 2011–23 and has since risen to 1%. 4 Such numbers have a considerable impact on the economy, with total service costs for people with a diagnosis of schizophrenia and bipolar estimated as £3.8 billion in 2007 and likely to rise to £6.3 billion by 2026. 5
People with a diagnosis of schizophrenia, bipolar or psychosis have a significantly reduced life expectancy compared with the general population. 6 Two-thirds of the mortality gap can be explained by physical disorders. 7 This is primarily due to a combination of lifestyle factors and medication side effects contributing to cardiovascular and respiratory risk. Additionally, the diagnosis of other significant illnesses may be delayed because of diagnostic overshadowing.
The NHS England policy The Five Year Forward View for Mental Health highlights the low level of primary care engagement with this group: ‘We should have fewer cases where people are unable to get physical care due to mental health problems … we need provision of mental health support in physical health care settings – especially primary care’ (p. 11; Contains public sector information licensed under the Open Government Licence v3.0). 8
Poor continuity of care and lack of information exchange between primary and secondary care also create barriers to effective support. The PARTNERS1 review of primary care records found that approximately 31% of people with a diagnosis of schizophrenia, bipolar or other psychosis in the UK were seen only in the primary care setting and that those seen in secondary care received only minimal support. 2 Primary care practitioners find it difficult to effectively support patients with serious mental illness (SMI), often lacking the necessary time and training to address these patients’ mental health needs. 9–11 Furthermore, access to health prevention and promotion activities in primary care is reduced for people with SMI. 12,13
Recent UK policy has promoted joined-up care, including the integration of primary and secondary services to provide better care for harder-to-reach groups. 14 The recent NHS Community Mental Health Transformation policy15 also aims to address this problem by ensuring that all those with a diagnosis of schizophrenia, bipolar or other psychosis and requiring care are well supported, ideally by collaboration between primary care, secondary services and third-sector organisations. This is also consistent with person-centred care (e.g. the Comprehensive Model of Personalised Care),16 which offers an integrated approach to health care for people with complex needs and provides proactive support for physical health conditions.
A collaborative care model, whereby a specialist healthcare professional works in primary care forging collaboration between primary and secondary care, is a potential approach to achieving better integration between separate parts of the health and social care system. 1 Collaborative care has been shown to be effective in improving mental, physical and social functioning across a range of mental health conditions. 2,17,18
Most of the collaborative care evidence for SMI has been developed and evaluated in the USA, where the nature of service user populations and of service use differs from the way we fund, structure and use NHS England. 19 Although there is considerable evidence of effectiveness, this largely relates to depression. 18
While those with severe symptoms of psychosis generally receive significant attention from services, it is clear that for those at lower risk care is much more haphazard. This is aggravated further by the increases in discharges from specialist services over recent years. There have been sustainable approaches to providing specialist mental health input for those with psychosis in primary care in only a few settings, such as East London. 20
Studies over time report that primary care practitioners find it difficult to effectively support this group of patients, often lacking the necessary time and training. 9–11 There is often poor continuity within primary care teams and poor co-ordination with secondary care.
In light of the above, this research programme aims to further understanding of the nature of current care, decide which outcomes are important, and then develop and test a collaborative, person-centred intervention to address deficits in care. It focuses on the estimated 70% of adults with a diagnosis of schizophrenia or bipolar who are currently seen and treated in primary care alone, or those currently seen in secondary care with lower levels of risk (operationalised as diagnostic clusters 11 and 12). Risk in this context refers to self-harm, self-neglect, suicide or harm to others.
How to navigate the PARTNERS2 research programme report
The National Institute for Health and Care Research (NIHR)-funded PARTNERS2 programme ran between 2014 and 2021, across two phases consisting of a total of six workstreams (WSs) (Figure 1), all supported by three Lived Experience Advisory Panels (LEAPs).
FIGURE 1.
The PARTNERS2 programme.
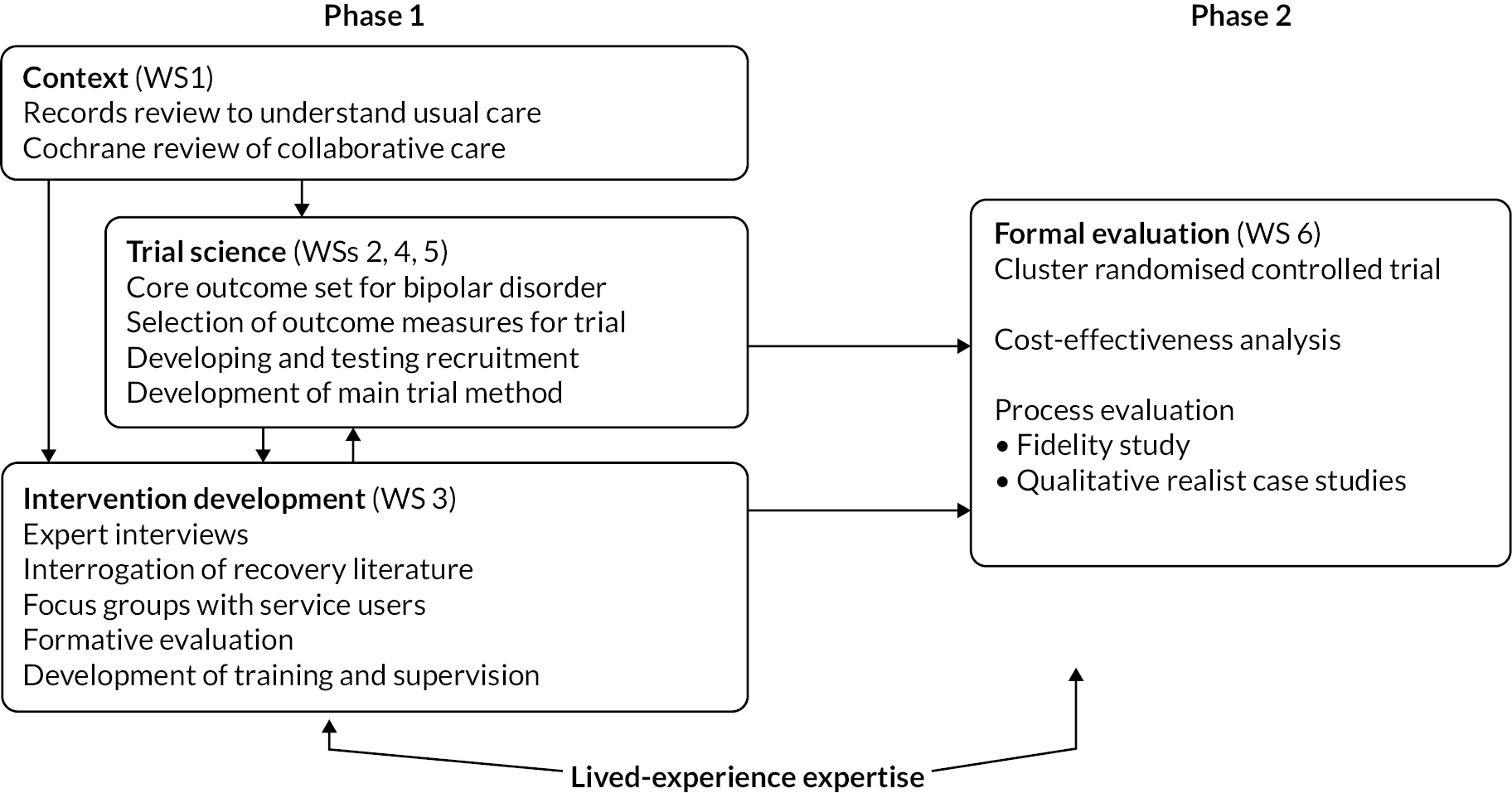
Phase 1: context of research (workstream 1)
-
an observational retrospective cohort study of primary and secondary care medical notes
-
an update of our original Cochrane review ‘Collaborative care approaches for people with severe mental illness’. 21
In parallel, we also developed the initial model of intervention (WS 3) through:
-
a systematic review of literature on collaborative care and personal recovery
-
interviews with key leaders in collaborative care and personal recovery
-
focus groups with service users
-
a formative evaluation of initial model of collaborative care to identify facilitators/barriers and refinement for implementation in main trial.
And trial methodology (WSs 2, 4 and 5):
-
development of a core outcome set (COS) for bipolar
-
development of recruitment methods.
Phase 2: randomised trial, cost-effectiveness study and integrated process evaluation (workstream 6)
In this stage we conducted:
-
a cluster randomised controlled trial (RCT)
-
a cost-effectiveness study
-
a mixed-methods process evaluation.
Public involvement underpinned all of the WS activity through the study LEAPs during the programme life cycle and the employment of service user researchers in the project team.
Alterations to the programme
The original programme proposed an external pilot trial of 300 participants from 60 general practitioner (GP) practices across three sites and did not include any effect sizes, power analysis or primary outcome. In 2016, a request was made to change the original design to carry out a definitive trial within the programme remit. The changes were agreed, converting the external pilot to an internal pilot trial with the following modifications:
-
agreement of a primary outcome and re-estimation of required sample size
-
reappraisal of sample size based io internal pilot
-
conversion to a definitive trial including stop–go rules set prior to trial onset [in conjunction with the Trial Steering Committee (TSC) and NIHR].
The trial arm of the programme [now split into two phases: development of feasibility stage (formative evaluation) and RCT (including with internal pilot)] is detailed in Table 1.
| Stage and key aims | Duration | Key dates |
|---|---|---|
| Develop feasibility stage: Aims: To develop and refine the intervention model To agree outcome measures To test recruitment processes |
24 months | 2014–6 |
| Convert internal pilot trial to full trial | VTC approved July 2016 | |
| RCT: internal pilot: Aims: To further test feasibility of both recruitment and the intervention delivery for patients and GP practices To assess recruitment against objectives: |
6 months | Trial started: 1 October 2017 Trial registered: 16 October 2017 First participant recruited: 8 June 2018 |
|
||
| To assess delivery and safety of intervention – e.g. intervention delivery (care partners in place) and adverse events such as crisis care (home treatment teams), admissions (psychiatric) To review initial sample size target of 336 participants |
||
| Change in CTU transition phase | 4 months | Decision November 2018. Handover period December 2018–February 2019 |
| Conversion to full trial | Stage 2 application submitted November 2018 Bridge funding period March 2019–May 2019 Funding award approved April 2019 Programme extension period June 2019–February 2021 |
|
| RCT (completion of RCT following internal pilot): Aim: To establish the clinical effectiveness and cost-effectiveness of primary-care-based collaborative care for people with a clinical diagnosis of schizophrenia, bipolar or other types of psychosis Note: revised recruitment target of 204 participants with a diagnosis of schizophrenia, bipolar or other types of psychosis from ≈34 clusters (GP practices), based on per-protocol sample size recalculation (December 2019) and funder requirements (January 2020) |
Completion of recruitment Follow-up: 10–12 months |
Recruitment end date: 28 February 2020 Follow-up end: 28 December 2020 Data collection end date: 31 March 2020 Study end date: 30 April 2021 |
Early in the programme, the COS work was expanded and streamlined. Instead of creating a COS for SMI, after consultation with service users, carers and practitioners a decision was made that both a COS for bipolar and a COS for schizophrenia were needed. This was because two outcome sets for bipolar and schizophrenia emerged during the preliminary qualitative work. The decision was made that the final COS would relate to bipolar only rather than to schizophrenia or to SMI generally (the PARTNERS2trial population). This was because of the larger number of available data on and greater research and advisory team expertise relating to bipolar. Within the resources of the PARTNERS2 programme, it was possible to create only one COS using robust methods and including user involvement. A COS for bipolar was selected and created.
During the formative evaluation phase, flexible approaches for recruitment were tested. However, Health Research Authority guidance changes meant that these processes were no longer permitted in the second phase due to confidentiality concerns. Additional changes in one of the host NHS trusts (Lancashire Care Foundation Trust) causing financial and time pressures meant that the site had to withdraw during the set-up phase. The programme was allowed to continue along with a change in the co-ordinating Clinical Trials Unit (CTU) (from Birmingham to Plymouth, in line with RB taking on a co-chief investigator role). At this stage it was also agreed that the health economics modelling and stated preference survey planned for phase 1 would not proceed, with resources reallocated to the trial. Alternative sites were recruited [Somerset Partnership Trust and Livewell Southwest Trust (Plymouth)].
Following slower-than-hoped recruitment, a re-estimation of sample size (as per protocol for internal pilot) and changes in power, requirements decided following input from the Programme Steering Committee and the funder meant that we were able to stop recruitment in January 2020, completing the trial within the funding envelope. Table 1 details the trial development, including changes.
After recruitment closed and during intervention delivery and follow-up, the COVID-19 pandemic and UK national lockdown necessitated significant programme changes. After consultation with the Programme Steering Committee and the funder, the research team adapted methods for intervention delivery, follow-up data collection and the ongoing process evaluation (Table 2). The changes were tested for an 8-week period. Once they were shown to be successful, and after risk assessments were conducted for both service users and practitioners, the decision was made to continue remote delivery, where possible, both of the intervention and of follow-up and process evaluation data collection, until the end of the trial.
| Challenges | Solutions |
|---|---|
| Adapting the intervention to remote delivery | Delivery via telephone or video conferencing software. Intervention practitioners received training on using video conferencing software and delivering interventions remotely. Coaching and goal setting were adjusted to be appropriate to a lockdown environment. The majority of participants found it acceptable to continue the intervention after adaptation to remote delivery. Practitioners delivering the intervention reported they were able to continue collaborating with primary care by remote means |
| Collecting data remotely | Follow-up data and process evaluation data collected via telephone, video conferencing or post. For the secondary outcome of time use, data collection was adjusted to include activities participants conducted remotely, for example attending church via video conferencing |
| Understanding the feasibility and acceptability of continuing the intervention, and collecting data, remotely. This included acceptability to participants and the feasibility of collaborating with primary care | 8-week trial phase, including rapid realist evaluation. This realist evaluation considered (1) the experiences of the intervention practitioners delivering the intervention during COVID-19 restrictions; and (2) during routine audio-assisted recall interviews with service users, exploring their experiences of engaging with the intervention by remote methods |
Phase 1: developmental and preparatory studies
Phase 1 included research to further understand the context (WS1), trial science work (WSs 2, 4 and 5) and intervention development (WS3).
Context-related research (workstream 1)
Assessment of local care pathways and current services
Background
Workstream 1 aimed to define the current status of integration and collaboration after the introduction of the QOF and identify where the strengths and weaknesses of integration lie. This was to inform better long-term solutions by describing the process of current care and help us target those who could benefit from collaborative care. It addressed a weakness in the PARTNERS1 study2 of primary care records that did not pick up all secondary care contacts. Three key questions addressed were:
-
What is the current level of primary care and secondary mental health care contact for those individuals with SMI who were taken on for specialist care?
-
What is the level of longitudinal continuity of care within primary and secondary care for those under secondary care?
-
What health risks were recorded and what physical healthcare monitoring was undertaken for this group?
This work has been published in Reilly et al. 22 (this is an open access article distributed under the terms of the Creative Commons CC BY licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited) and informed the development of the theoretical model for the PARTNERS2 intervention [see Development of theoretical model of the intervention (workstream 3)].
Methods
A multisite, epidemiological review of primary and secondary care contacts in participating mental health services was undertaken. Three host NHS trust sites were invited to participate (Birmingham, Lancashire and Devon). Five Community Mental Health Teams (CMHTs) and a subsequent 33 GP practices referring into these CMHTs were recruited. GP practices were stratified according to size. We aimed to identify 100 randomly selected eligible cases per secondary care site who met the inclusion criteria on 1 September 2014. This required individuals to be under secondary care and to have diagnoses of schizophrenia, bipolar or other psychoses.
Data were manually extracted from electronic secondary mental health care and primary care medical records between October 2014 and June 2016. The data extraction tools developed in the PARTNERS1 study were expanded for this purpose in consultation with our LEAP members and service user researchers.
Analyses were conducted using Stata version 13 (StataCorp LP, College Station, TX, USA). Descriptive statistics and measures of variance were derived relating to individual demographics, number and type of medications, number of comorbidities, direct service contacts and reasons for contacts. Continuity of care in primary care was measured using the Modified, Modified Continuity Index (MMCI), which measures the number of GPs seen; a higher continuity score occurs when there are larger numbers of visits with a smaller number of GPs. The type and frequency of contacts with primary and secondary care were also measured, as were the proportions of individuals who had no contact with primary care and the time between contacts in primary and secondary care.
Results
Among the 297 individuals included in the study, the average age was 47 years and 56% were male (see Table 1); 33% of individuals were from ethnic minority communities, and around half (53%) were smokers and 16% were ex-smokers; smoking cessation advice was reported to have been given to 66% of those who were smokers.
The majority of care in this group of individuals under specialist services at the eligibility point was provided by secondary care practitioners; of the 18,210 direct contacts recorded, 76% were from secondary care (median 36.5, interquartile range 14–68) and 24% were from primary care (median 10, interquartile range 5–20). There was evidence of poor longitudinal continuity; in primary care, 31% of people had poor longitudinal continuity (MMCI ≤ 0.5), and 43% had a single named care co-ordinator in secondary care services over the 2 years.
Thirty-seven (12%) individuals had been discharged to primary care within the 2-year period but were on the secondary care caseload when the sample was taken. Of these, 15 (41%) had been discharged more than once. A high proportion of individuals (44%) had seven or more GP contacts (25% had three to six and 17% had one or two contacts) and 7% of cases did not have any contact with a GP (6% were missing). The majority of primary care contacts were at the practice (72%) or by telephone (27%), of which 63% were with a GP, just over one-quarter (27%) were with a nurse and 10% were with another health professional.
The majority (88%) of individuals had had one or more contacts with a secondary mental health care professional, meaning that 12% had not. Over one-quarter of individuals had had a mental health admission over the 2 years and 16% had had a non-mental health admission.
In conclusion, three-quarters of all direct contacts recorded across primary and secondary care were from secondary care. For most individuals with SMI who are in contact with secondary mental health services, these services are central to their care. Individuals were seen on average every 2 weeks by specialist care practitioners, albeit with much variability. By contrast, these individuals were also seen on average every 6 weeks in primary care. Greater knowledge of how care is organised presents an opportunity to ensure some rebalancing of the care that all people with SMI receive when it is required.
Update of Cochrane review of collaborative care
Background
Collaborative care is a community-based intervention that promotes interdisciplinary working across primary and secondary care and typically consists of a number of components focused on improving the physical and/or mental health care of individuals, in this case those with SMI.
Since the publication of the original Cochrane review ‘Collaborative care approaches for people with severe mental illness’,21 there has been a substantial increase in the number of published and relevant RCTs, and a refinement in defining collaborative care and working models. The update to the original review has been conducted; it includes an additional seven studies and has been published in Reilly et al. 21
Objective
The objective was to assess the effectiveness of collaborative care approaches in comparison with standard care for people with a diagnosis of schizophrenia, bipolar or other psychosis who are living in the community. The primary outcomes of interest were quality of life (QoL), mental state, personal recovery and psychiatric admissions. These were selected by the review team and our LEAPs as the most suitable outcomes for all stakeholders.
Methods
Search methods
We searched the Cochrane Schizophrenia Group’s Trials Study-Based Register (18 April 2011; 20 February 2015; 12 August 2016; 28 January 2019; 28 January 2020; 10 February 2021) including clinical trial registries. We contacted 51 (in 2011) and 48 (in 2016) experts. We searched the Cochrane Common Mental Disorders Group (CCMD) controlled trials register (all available years to 6 June 2016).
Subsequent searches in Ovid MEDLINE, EMBASE and PsycInfo® together with the Cochrane Central Register of Controlled Trials (with an overlap) were run on 6 June 2020 and 17 December 2021.
We identified RCTs where interventions described as ‘collaborative care’ were compared with ‘standard care’ for adults (aged ≥18 years) living in the community with a diagnosis of SMI. SMI was defined as schizophrenia, other types of schizophrenia-like psychosis or bipolar affective disorder.
Data collection and analysis
Pairs of authors independently extracted and assessed the quality of data. The quality and certainty of the evidence was assessed using the Risk of Bias 2.0 (for the primary outcomes) and GRADE. Treatment effects were compared between collaborative care and standard care. We divided outcomes into short term (up to 6 months), medium term (7–12 months) and long term (over 12 months). For dichotomous data, we calculated the risk ratio (RR); for continuous data, we calculated standardised mean differences (SMD), presented alongside 95% confidence intervals (CIs). Random-effects meta-analyses were used because there were substantial levels of heterogeneity across trials. We created a ‘summary of findings’ table using GRADE. 23
Results
We included eight RCTs24–31 (1165 participants) in this review, two of which met a strict definition of collaborative care. The composition and purpose of the interventions varied across studies. Most outcomes provided low-quality or very-low-quality evidence.
We found three studies (28,29,31) assessing QoL of participants at 12 months. QoL was measured using the Short Form Questionnaire-12 items,28,29 and the World Health Organization Quality of Life Brief Version,31 and the mean end-point mental health component scores were reported for 12 months. Very-low-certainty evidence did not show a difference between collaborative care and standard care in medium-term QoL (mental health domain at 12 months: SMD 0.03, 95% CI −0.26 to 0.32; 3 RCTs, 227 participants) and nor did low-certainty evidence (physical health domain at 12 months between collaborative care and standard care: SMD 0.08, 95% CI −0.18 to 0.33; 3 RCTs, 237 participants).
Furthermore, low-certainty evidence did not show a difference in medium-term mental state (binary) at 12 months between collaborative care and standard care (RR 0.99, 95% CI 0.77 to 1.28; 1 RCT, 253 participants); in medium-term mental state (depressive symptoms) at 12 months between collaborative care and standard care (SMD −0.17, 95% CI −0.53 to 0.18; 3 RCTs, 227 participants); in medium-term mental state (manic symptoms) at 12 months between collaborative care and standard care (SMD −0.08, 95% CI −0.38 to 0.22; 3 RCTs, 227 participants); or in the risk of being admitted to psychiatric hospital at 12 months in the collaborative care group compared with standard care (RR 5.15, 95% CI 0.67 to 39.57; 1 RCT, 253 participants). There was some low-certainty evidence of a reduction in the risk of psychiatric hospital admission at 2 years in the collaborative care arm compared with usual care (RR 0.75, 95% CI 0.57 to 0.99; 1 RCT, 306 participants), but no evidence of a difference in the risk of psychiatric hospital admission was observed at 3 years (RR 0.73, 95% CI 0.53 to 1.01; 1 RCT, 306 participants). One study indicated an improvement in disability (proxy for social functioning) at 12 months in the collaborative care arm compared with usual care (OR 1.69, 95% CI 0.98 to 2.91; 1 RCT, 253 participants); we deemed this low-certainty evidence.
Personal recovery and experience of care outcomes were not reported in any of the included studies. The data from one study indicated that the collaborative care treatment was more expensive than standard care [mean difference (MD) I$493.00, 95% CI I$345.41 to I$640.59] in the short term. Another study found the collaborative care intervention to be slightly less expensive at 3 years.
In conclusion, this review does not provide evidence that collaborative care is more effective than standard care in the medium term in relation to our primary outcomes. Dropout rates suggest no evidence of a difference between collaborative care and standard care in short-, medium- or longer-term treatment acceptability. However, our confidence in these findings is extremely limited due to the low certainty of evidence. Evidence would be improved by better reporting, higher-quality RCTs and an assessment of the underlying mechanisms of collaborative care. We advise caution when using the information in this review to assess the effectiveness of collaborative care.
Development of theoretical model of the intervention (workstream 3)
Initial programme theory development
The PARTNERS2 programme theory for the final intervention model was developed over three phases:32
-
drafting of an initial model and development into a prototype
-
refinement of the prototype model through formative evaluation into the trial intervention model
-
further refinement of the intervention model through the RCT with parallel process evaluation.
The process of developing the initial PARTNERS2 model has been published in Gwernan-Jones et al. (2019),32 which includes a supplementary file of the explanatory statements making up the intervention.
Stakeholder involvement events included regular consultation meetings with LEAP members over the full period of the research project (2014–21); two consultation meetings with LEAP members, researchers (including service user researchers), practitioners and policy-makers during the development of the initial model (November 2014 and January 2015); and a stakeholder meeting (October 2016) during the formative evaluation attended by researchers, practitioners delivering the partners service and LEAP members. Stakeholder input fed into model development by contextualising, shaping and providing feedback on the practicality and relevance of proposed aspects of the intervention.
An intervention was proposed in the original research programme funding application based on a recent systematic review of collaborative care for psychosis21 and the model of chronic care33 framed by concepts of personal recovery. 34 Using this proposal as a foundation, and drawing on a realist approach,35 a directory of 453 explanatory statements was iteratively created that articulated a rationale for why, how and for whom it was perceived the different aspects of the intervention would work. This directory of explanatory statements drew on a number of data sources:
-
literature on collaborative care for mental health, and personal recovery literature
-
11 telephone interviews with key leaders in collaborative care and personal recovery to explore their perceptions of best practice
-
six focus groups with 33 participants living with psychosis (13 women and 20 men) about their experiences of care.
Through cycles of discussion and consensus building, researchers and stakeholders debated the proposed approaches to delivering the intervention, and their decisions guided consolidation of 106 explanatory statements and graphic and written representation of the programme theory in the form of a prototype model. Finally, the prototype model was interrogated internally (by comparing the explanatory statements with the practitioner manual) and externally (by comparing the explanatory statements with National Institute for Health and Care Excellence (NICE) guidelines), and then through debate across the research team, refined to establish consistency and rigour within the model and across the supporting documents. A manual for practitioners (care partners and supervisors) and guides for service users and their friends and family were developed.
Formative evaluation of the PARTNERS service
Background
The prototype model was then tested during a pilot formative evaluation, conducted from November 2015 to April 2017, to:
-
assess the extent to which delivery of the intervention matched the model
-
identify issues that fostered or prevented delivery of the model as intended
-
identify any additional support for implementation required in the main trial
-
evaluate and refine the initial model by comparing the perceived effects of the intervention with the programme theory.
Methods
During the formative evaluation, the Partners Service (a name chosen by the LEAP) was delivered at three sites (Northern England, the Midlands and the South West; two urban sites and one urban/rural site) over a period of 8–10 months. Secondary care practitioners (‘care partners’) were trained to deliver a collaborative coaching model. We called these practitioners ‘care partners’ to emphasise the collaborative nature of the role. Thirty-seven semistructured interviews were conducted with care partners (n = 4), service users (n = 14), care partner supervisors (n = 4), friends and family of service users (n = 5), GPs (n = 4) and other primary or secondary care practitioners (n = 6). We also recorded eight care partner–service user sessions and followed these up by interviewing the care partner (n = 7) and service user (n = 7) individually to explore their experiences during the recorded session (interpersonal process recall). 37
Preliminary analysis of the data involved ongoing descriptive coding to identify issues that prevented delivery of the model as intended; these were fed back to the Partners Service delivery teams to improve the service during the pilot period. A secondary analysis, using the framework method,38 focused on the extent to which the actual provision aligned with the mechanisms and outcomes predicted by the PARTNERS prototype model. At the end, a stakeholder meeting in October 2016 informed the development of strategies to improve theory for both what should be delivered and the implementation strategies to be used for the main trial.
Results
Key components of the prototype model that were not delivered as intended by one or more of the care partners or in one or more of the sites included:
-
a lack of support from secondary care to care partners, including no protected time and irregular supervision
-
limited interaction and integration of care partners into primary care sites, including difficulties with access and poor evidence of record-keeping
-
a lack of evidence for some care partners of a collaborative approach to interacting with service users, consistent monitoring of mental health and/or follow-up of service users, and the use of intervention resources for coaching and motivational approaches.
Factors that prevented implementation according to the initial model included:
-
systemic problems with communication, for example within primary care
-
care partner understanding, capacity for and/or willingness to engage with a collaborative coaching and goal-setting approach
-
capacity for and/or understanding about how to provide PARTNERS2 supervision to care partners.
These findings prompted refinement for implementation, including:
-
support to increase the integration of care partners into primary care through researcher facilitation
-
improved care partner understanding and skills in relation to coaching, goal setting and working collaboratively, using increased levels of training and a revised, clearer practitioner manual. It was anticipated that, through better understanding of the Partners Service approach, care partners would be able to address barriers to service user motivation to work on goals.
Delivery of the prototype model was partial, and data availability limited the extent to which the operation of mechanisms and outcomes could be fully evaluated. Where there was evidence of intervention delivery as intended, mechanisms and outcomes seemed to operate as anticipated. Therefore, the programme theory was adapted not in relation to the way the intervention was understood to work, but only with regard to the way it was implemented. Further evaluation of the validity of the programme theory was conducted during the clinical trial and process evaluation.
Description of the PARTNERS2 intervention
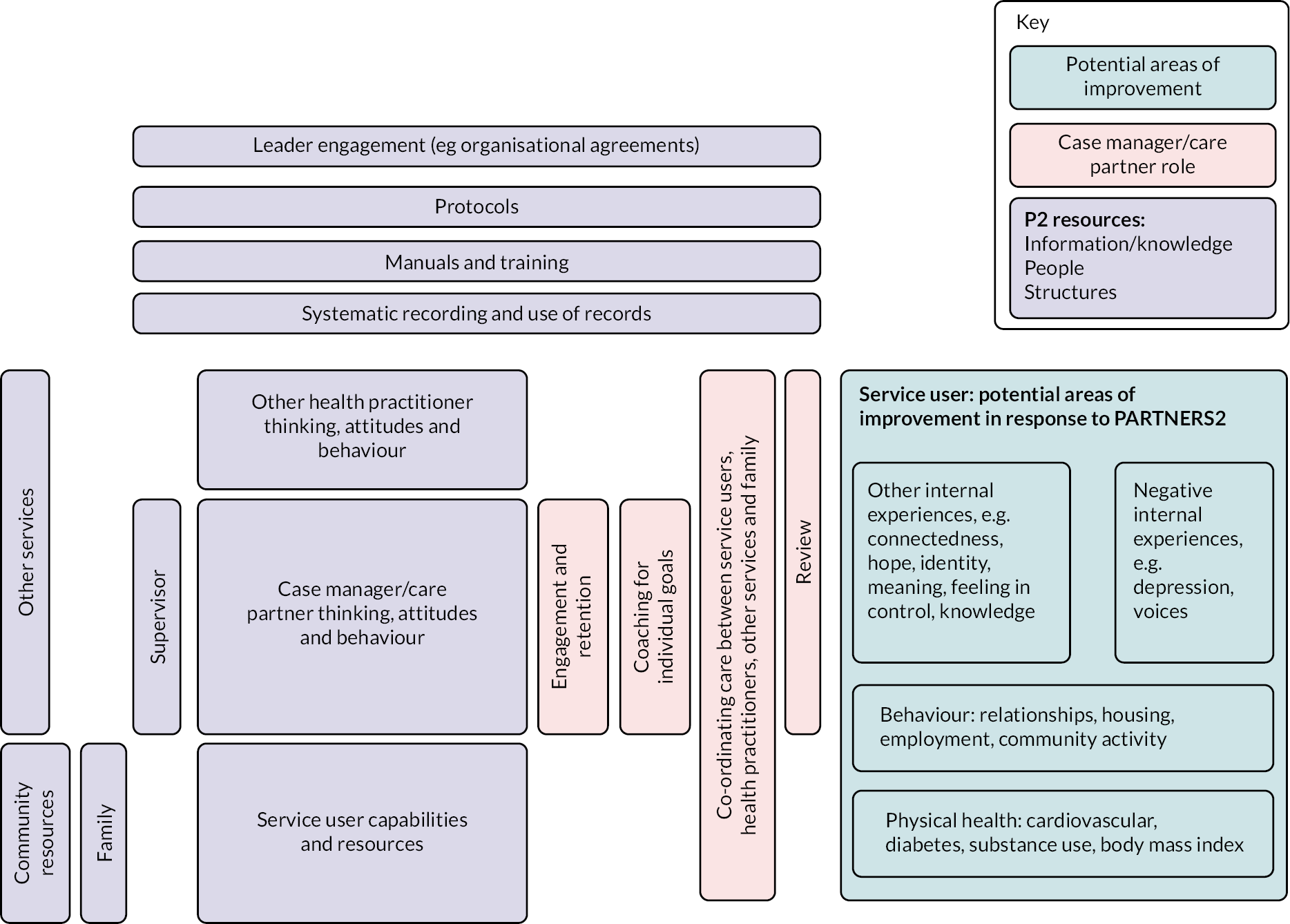
The intervention is complex, involving change at three levels, namely institutional level (secondary care trusts/CMHTs and primary care), practitioner level (care partners, supervisors, other primary and secondary care staff, third-sector and community organisational staff) and service user level (service users and friends and family, where there is consent). The 14 components are described in Table 3, and the relationships between contexts, resources, mechanisms (reasoning and reactions) and intermediate and intervention outcomes are shown in Figure 2. The manual is available as a supplementary file.
| PARTNERS2 intervention model component | Description |
|---|---|
| Underpinning conceptual models of collaboration | Wagner’s Chronic Care Model,33 the CHIME framework of personal recovery34 and coaching for mental health recovery39 |
| Identification of patients: method | Screening of patient records against inclusion criteria ensures a systematic approach to patient caseload |
| Identification of patients: setting | Primary and secondary care |
| Provider integration | Specialist mental health practitioner (a ‘care partner’) from a CMHT is sited in primary care practices |
| Multidisciplinary working | The care partner works alongside primary care practitioners under the supervision of a qualified mental health practitioner (from any mental health profession). The supervisor is based in a local secondary care CMHT. A linked psychiatrist is available if required |
| Systematic communication between providers | Care partners share patient records including progress notes and care plans; co-location supports face-to-face communication between care partners and primary care practitioners |
| Case management | Care partners co-ordinate care, liaising with other practitioners (primary and secondary care; third-sector and other community organisations) and friends and family to make sure an individual’s needs are met |
| Study protocols/treatment algorithms | Manuals (care partners, supervisors, GPs, service users, friends and family) describe the principles and approaches of the Partners Service, which includes flexible response to individual needs. A supervision protocol specifies clinical, caseload and pastoral guidance and support |
| Systematic monitoring and follow-up | Service users are reviewed regularly at negotiated intervals; session intensity and interval are varied according to an individual’s need. Minimal support is three telephone contacts per year; standard service involves more frequent face-to-face contact. Care partners routinely monitor mental health through standardised scales and/or patient notes |
| Pharmacological intervention | Pharmacological intervention is part of the PARTNERS model only when desired by the service user as a goal, and could involve an action plan or review by the linked psychiatrist or GP |
| Psychological intervention | Care partners follow principles of coaching to work with service users towards personally meaningful goals. These might necessitate more social or more medical care. Additionally, elements of motivational interviewing are included, for example to support individuals to consider changes in lifestyle. Individualised action plans identify relevant resources and agreed steps to support service users to take action to work towards goals |
| Education for mental health/primary care practitioners | Two-day training before start of practice for care partners and supervisors, based on the practitioner manual; regular follow-up training for care partners; training provided by research team including lived experience panel. Primary care induction for staff members to familiarise them with the PARTNERS model |
| Patient education/promoting self-management | Care partner provides information and draws on motivational interviewing approaches to increase knowledge of self-management strategies and motivation to improve physical and mental health |
| Collaborative relationship with patients | Care partners adopt a collaborative, egalitarian style of interaction with service users following coaching principles, to support the empowerment of service users in relation to CHIME (connectedness, hope, identity, meaning and empowerment) principles |
FIGURE 2.
Depiction of the PARTNERS2 initial model.
Development of trial methodology (phase 1: workstreams 2, 4 and 5)
Development of a set of outcomes for a randomised controlled trial to evaluate the effectiveness of the PARTNERS2 intervention (workstream 2)
Background
A COS was developed for use in community-based bipolar trials. The protocol and results papers have been published as Keeley et al. 40 and Retzer et al. 41 Our aim was to suggest a small number of agreed outcomes to be collected and reported in all trials within this research area.
Methods
The method was a three-stage process:
-
a long list of outcomes was derived from (1) focus groups with people with a bipolar diagnosis and friends/family, (2) interviews with healthcare professionals and (3) a rapid review of outcomes listed in bipolar trials in the Cochrane database
-
an expert panel of people with personal and/or professional experience of bipolar participated in a modified Delphi process; Reproduced with permission from Retzer et al. 41 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
-
a consensus meeting was held to finalise the COS.
Focus group and interview recordings were transcribed and analysed together as the purpose of analysis was to identify all possible outcomes. Transcripts were uploaded to Dedoose42 online qualitative data management software to manage and support data analysis. Dedoose was used to organise the qualitative data collected during the focus groups and one-to-one interviews to generate the outcome longlist, and descriptive accounts of the interviews and focus group discussions. Reproduced with permission from Retzer et al. 41 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text. The Cochrane database was accessed in March 2015, and two researchers independently performed a complete search of all pre-categorised titles listed under the bipolar reviews on the Cochrane database for systematic reviews. The outcome list was then reviewed during a multidisciplinary stakeholder meeting composed of academic researchers, LEAP members and a carer.
Participants from the UK were invited to complete a two-round Delphi survey co-developed with service user researchers. People with a bipolar diagnosis and carers were recruited nationally through local support groups, electronic advertisement via third-sector organisations and social media. Health and social care professionals and researchers were recruited through the professional networks of the PARTNERS2 research team. Purposive sampling was used to capture a range of professional roles and supplemented as required through snowball sampling. A screening tool was developed to monitor sample diversity and inform and direct recruitment. A paper-based version of the survey was available on request.
The Delphi survey was hosted by Delphi Manager software. 43 Participants were asked to rate each of the outcomes on a nine-point Likert scale and invited to suggest outcomes they considered were absent from the stage 1 and 2 longlist. Suggestions were automatically included for rating in round 2. Following the closure of round 1, the software internally calculated the stakeholder group's ratings of each outcome.
A consensus meeting44 was attended by the research team, LEAP members, participants of the Delphi survey and people who had been unable to participate in the Delphi but had expressed an interest in attending the meeting.
Results
Three focus group discussions were held, ranging in size from four to eight people between July 2014 and March 2015. Telephone interviews with healthcare professionals and researchers (n = 16) took place between July and November 2014. In total, 76 outcomes were identified (including 20 duplicates).
Data were extracted from 17 bipolar reviews in the Cochrane database, and a further 45 outcomes were identified. Following the multidisciplinary stakeholder meeting to review the 101 outcomes, 47 were merged and 12 more were added.
Fifty Delphi participants were recruited to participate in round 1 of the Delphi survey between September and December 2016, and round 2 was open from December 2016 to February 2017.
Sixty-six outcomes were included in the survey, and a further 13 were added by participants during round 1. Three of the suggested outcomes were rated as important by participants in round 2 and so were included in the consensus meeting discussion. The consensus meeting was attended by 14 people (six healthcare professionals, five people with a bipolar diagnosis, two carers and one researcher) and took place in September 2017.
The final COS comprised 11 outcome domains: personal recovery; connectedness; clinical recovery of bipolar symptoms; mental health; well-being; physical health; self-monitoring and management; medication effects; QoL; service outcomes; service user experience of care; and use of coercion.
Additional work to select primary outcome for PARTNERS2 trial
To account for the wider psychosis target population in PARTNERS2, and the nature of the intervention, we undertook an additional and separate more pragmatic stakeholder consultation to select outcomes and measures for use in the trial. This involved service users and carers, practitioners and researchers. A presentation of key decisions was followed by a discussion, ensuring that everyone’s views were registered. The aim was to select a set of outcomes, with associated validated measures, that reflected the needs of individuals and would also assess the effectiveness of the intervention model. We needed a primary outcome measure that was sensitive to change and had good psychometric properties and face validity for stakeholders; and also a set of measures that not only reflected outcomes desired by service users (as in COS work) but could be delivered by the intervention according to its internal logic.
Quality of life was selected. It is a common outcome in trials of SMI, although it was not prioritised in our COS work. Relevant measures of QoL were reviewed, and the Manchester Short Assessment of Quality of Life (MANSA) was selected because it was clinically relevant to the target population and potentially amenable to change by the intervention. The MANSA has good validity and reasonable internal consistency. 45 A range of other secondary measures were also agreed as described in the trial protocol paper,46 covering several of the COS domains identified, including QoL, recovery and experience of care.
Development of recruitment processes (workstream 4)
Background
Recruitment to trials is often slow, and feasibility needs to be tested. The PARTNERS collaborative care intervention necessitated the recruitment of secondary care providers, GP practice clusters and individual participants. In the feasibility phase, we tested the recruitment of practices and participants alongside the formative evaluation of the intervention (workstream 3).
Methods
Potential participants were identified by clinical research network (CRN) staff and clinicians screening patient lists in primary and secondary care. Those eligible from secondary care were approached by a clinician known to them. Those seen in primary care were sent an invitation letter with an expression-of-interest form by the GP practice. Those indicating interest were contacted by the research team. Recognising difficulties in recruiting the target population, we trialled two strategies to improve recruitment, both of which were acceptable to participants and improved response rates:
-
Those who did not respond to initial contacts received a telephone call from a clinician or the research team to discuss the study (Lancashire and Devon).
-
Those who did not respond to initial contact received a ‘rapid invite appointment letter’ inviting them to a short meeting with a member of the research team to discuss the study (Devon and Birmingham).
The research objective in this stage was to recruit participants to receive the intervention and to collect qualitative data to refine the intervention. Therefore, we did not test collection of quantitative outcome measures. Instead, trial outcome measures were piloted with LEAP members.
Results
Table 4 shows the feasibility stage recruitment numbers.
| Secondary care provider | GP practices | Individual participants | ||
|---|---|---|---|---|
| Number approached | Number recruited | Number eligible | Number recruited | |
| Birmingham and Solihull Mental Health Foundation Trust | 4 | 1 | Data missing | 6 |
| Lancashire Care Foundation Trust | 3 | 3 | 70 | 21 |
| Devon Partnership NHS Trust | 2 | 2 | 67 | 10 |
Pragmatic recruitment of secondary care providers utilised existing relationships, with the intention of covering a range of sociogeographic demographics. GP practice recruitment was also pragmatic, based on ease of location access and existing relationships. Where there was no existing relationship, practices were initially approached by letter and telephone call, with a follow-up face-to-face meeting with the practice team. Supporting this process was a study website built with our LEAP members, containing videos and information to describe the trial: www.partners2.net
Setting up the randomised controlled trial (workstream 5)
Transfer of both the intervention and the trial procedures to the RCT was not straightforward because of a combination of NHS pressures and the implementation of more stringent national research governance measures.
We had assumed that secondary care partners in the feasibility study would continue their participation into the RCT stage. However, changing NHS financial landscapes, staffing resource pressures, and GP (clusters) recruitment delays led to two trusts (Devon and then Lancashire) withdrawing. Consequently, we lost care partners who had been trained over a period of 2 years, including in the feasibility phase. Replacement sites to identify and train care partners were approached based on access considerations for the research team, and maintaining sociogeographic demographics (e.g. an urban/rural mix). We required substantially greater numbers of GP practices for the RCT. Although our approach mirrored the feasibility stage, it had not been possible to test the time and staff resource required or the local variation in response when recruiting a larger sample.
We intended to identify potential participants for the trial using the process trialled in the feasibility stage. However, local, changing, interpretations of research and information governance requirements meant that it was deemed inappropriate for the research team to view patient records prior to patient consent, even with permission from the practice and ensuring that no data left the practice. Therefore, primary/secondary care staff were required to identify potential participants. This is a time-consuming task, the reallocation of which led to a delay in participant recruitment. The onerous nature of this task also adversely affected practice recruitment. There were differences across regions in whether CRN-funded NHS research staff could and should support hard-pressed practice staff to carry out this screening of patient records.
We also sought ethics permission to send an invitation letter, followed by an accompanying telephone call, to potential participants who had not returned an expression of interest. The follow-up telephone call had to be undertaken by a clinician because of the governance changes noted above. Not all sites had the resource to provide this call; later in the programme, permission was obtained for NHS research staff to make these calls, which was associated with a boost in recruitment.
Phase 2
Phase 2 consisted of a RCT (initially an internal pilot trial), a health economics cost-effectiveness analysis and a mixed-methods parallel process evaluation.
Randomised controlled trial, cost-effectiveness study and process evaluation (workstream 6)
Cluster randomised controlled trial
Background
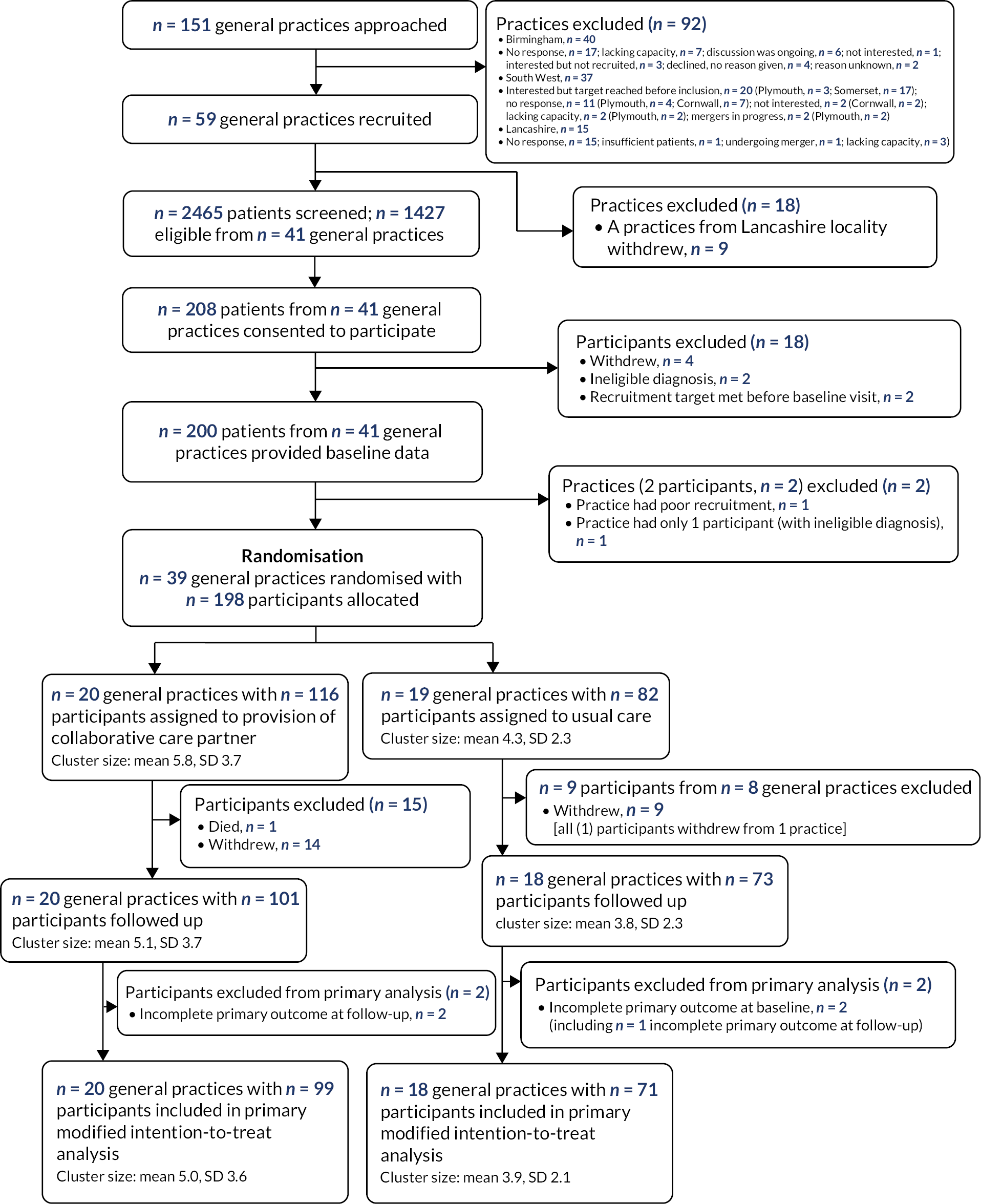
The aim of the definitive trial was to assess the effectiveness and cost-effectiveness of the developed primary-care-based collaborative model (PARTNERS) for people with a diagnosis of schizophrenia, bipolar or other psychoses on improving QoL. The protocol paper for the trial has been published as Plappert et al. 46 A paper detailing the trial results has been accepted for publication (Byng et al. ; Figure 3). 47
FIGURE 3.
CONSORT diagram. SD, standard deviation.
Method
The study used a cluster randomised controlled superiority trial design, the clusters being general practices in regions in England. Participants had to be consented and have their baseline measures collected before the practice was allocated (1 : 1) to either the PARTNERS (intervention) or the care as usual (control) group. Allocation was minimised on region and practice size.
The PARTNERS intervention (see Table 3) was compared with care as usual, which was the support being provided by primary and secondary care services at the time. Participants allocated to the PARTNERS2 intervention received up to 12 months of the intervention, including a 2-month transition back to care as usual. Care partners included nurses and support workers. Follow-up was planned at 10 months post unmasking but was brought forwards to 9 months for the final participants.
The primary outcome measure was the participant-reported overall MANSA score,45 measured at baseline and follow-up. Secondary outcome measures included Time Use Survey (TUS),50 Questionnaire about the Process of Recovery (QPR-15),49 the full and short version of the Warwick–Edinburgh Mental Wellbeing Scale [(S)WEMWBS],39 Brief-INSPIRE,51 ICEpop CAPability (ICECAP-A)52 and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L). 53 All participant-reported outcomes were collected at baseline and follow-up during an interview with a research assistant. Research assistants asked participants to also fill in self-complete measures; self-reported lifestyle outcomes included smoking, alcohol consumption, cannabis use and healthcare monitoring, as well as safety outcomes [number of psychiatric hospital admissions; number of days as an inpatient as a result of psychiatric admission; number of episodes under home treatment and total days under home treatment (crisis care); and serious adverse events (SAEs)].
Participants who experienced COVID-19-related restrictions during their study involvement were asked additional questions at follow-up to increase understanding of how lockdown and social distancing impacted on their mental health, access to physical health care and usual activities.
Primary analyses were on an intention-to-treat basis. The target between-group difference was 0.45 points in the overall MANSA score and assuming a standard deviation of 0.9. This is equivalent to a standardised effect size of 0.5. The original recruitment target was 336 participants from ≈ 56 clusters (each with a mean of six participants recruited) to achieve 90% power. This was revised to a target of 204 participants from ≈ 34 practices to achieve 80% power.
Analyses were prespecified in the statistical analysis plan,54 approved by the TSC prior to database lock. In summary, outcomes were analysed using a Gaussian random-effects regression models, including the cluster-level minimisation factors (region and practice size), individual-level baseline score as fixed-effects covariates, and GP practice as a random effect. Prespecified subgroup analyses of the primary outcome added the interaction effect of allocated group and the subgroup [(1) region, (2) practice size, (3) diagnostic group and (4) usual care provider at screening]. Four sets of sensitivity analyses of the primary outcome were originally planned; a further three were prespecified to explore the potential effects of the COVID-19 pandemic on the primary outcome and the secondary outcome of TUS.
Results
Thirty-nine general practices were recruited and randomised: 20 to the intervention group (116 individual participants recruited) and 19 to the care as usual group (82 individual participants). Around two-thirds of participants were under primary care for their mental health needs at the time of recruitment, just over one-fifth (22%) had a diagnosis of schizophrenia and 58% had a diagnosis of bipolar. Over 60% of participants were female and around 40% were single at recruitment. Only eight (4.1%) were black, five (2.5%) Asian, four (2.55%) mixed and one (0.5%) other.
The two groups were reasonably balanced in terms of individual-level baseline characteristics. Over 87% of recruited participants were followed up (101 in the intervention group and 73 in the care as usual group).
At baseline, the mean overall MANSA score in the intervention group was 4.29 (SD 0.88) points and 4.33 (SD 0.99) in the care as usual group. At follow-up, mean scores in both groups improved slightly, to 4.54 (SD 0.82) and 4.51 (SD 1.01) in the intervention and care as usual group, respectively. The change in overall MANSA (primary outcome) could be calculated for 99 (85%) participants in the PARTNERS2 intervention group [mean change 0.25 (SD 0.73)] and 71 (87%) participants in the care as usual group [mean change 0.21 (SD 0.86)]. The improvements in mean overall MANSA score did not differ significantly between the groups, with the fully adjusted mean between-group difference of 0.03 [95% CI (intervention minus care as usual) −0.25 to 0.33; p = 0.819]. All sensitivity analyses, including complier-average causal effect (CACE) analyses, were in agreement with the primary analysis. There was no evidence of a differential intervention effect in any of the four prespecified subgroup analyses.
There was no evidence of statistically significant differences between allocated groups in terms of the secondary outcomes. Numbers and patterns of missing data in the Brief-INSPIRE measure at both baseline and follow-up meant that these data were only summarised descriptively.
While there were some differences in the summary statistics of participants who completed the trial before COVID-19 and those who were followed up during the pandemic, there was no evidence of a statistically significant impact of COVID-19 from any of the associated, prespecified, sensitivity analyses for either MANSA or TUS. Due to COVID-19 restrictions, the planned review of primary care notes was possible for only 31 participants and so we were unable to obtain data on healthcare monitoring.
Seven participants had one recorded mental health episode each, of which three occurred after at least one recorded interaction with a care partner. Twenty-eight SAEs were reported in total, in 18 participants, with 11 categorised as mental health problems and none deemed to be related to PARTNERS2. Thirteen of the SAEs were reported in participants after they had had at least one recorded interaction with a care partner.
Health economics analysis
The health economics study comprised a set of cost-effectiveness analyses. This is described below and in Appendix 1.
Cost-effectiveness study
Background
The economic evaluation aimed to estimate the cost-effectiveness of PARTNERS2 compared with usual care, from the NHS and social care (costs) perspective, over the scheduled follow-up of 10 months.
Method
Quality-adjusted life-years (QALY) measured health benefit, as recommended by NICE. 55–57 Patient-level service use data were costed using national unit costs58,59 for 2019–20. The primary outcome was the incremental cost-effectiveness ratio (ICER), which combines service use costs and health benefit.
Participant-reported service use at follow-up was collected for a 3-month, rather than 10-month, recall period to reduce the burden to participants of recalling service use over a longer period and to balance complete service use data against incomplete recall, inconsistent or missing data, and limited resources for data collection. An audit of primary and secondary care notes was planned to collect (1) key high-cost psychiatric secondary and crisis care services that may not be used within the 3-month recall period at follow-up and (2) GP, practice nurse and other GP practice consultations. However, the latter was not feasible given the impact of COVID-19 on access to practices.
A generalised linear model (gamma, log) predicted a cost per day of participant-reported service use at follow-up for the pooled data, adjusting for baseline covariates. This was combined with the costs of mental health related admissions and crisis care from the secondary care audit to estimate the full cost from baseline to the end of follow-up. Missing baseline measures of cost, utility and clinical indicators were single imputed with indicators for missing demographic data60 costs and QALYs for the pooled data set were multiple imputed. 61
Results
Regression analysis estimated the net costs and QALYs of PARTNERS2, adjusting for key covariates. These estimated costs and outcomes were bootstrapped to estimate the probability that the PARTNERS2 intervention is cost-effective. Prespecified sensitivity analyses assessed whether alternative measures or analyses could change the conclusions of the economic analysis. Using the multiple imputed data, the average QALYs (usual care: mean 0.55, 95% CI 0.48 to 0.61; PARTNERS2: mean 0.51, 95% CI 0.45 to 0.57) and costs (usual care: mean £2689, 95% CI £999 to £4378; PARTNERS2: mean £1743, 95% CI £1149 to £2338) were similar for the two groups. Overall, the 95% CIs are wide and overlap, indicating a high level of variance and uncertainty. The net, bootstrapped QALYs (−0.007, 95% CI −0.086 to 0.071) and costs (−£213, 95% CI −£1030 to £603) were similarly inconclusive, with wide 95% CIs that overlapped zero. At the prespecified willingness-to-pay threshold (WTPT) of £15,000 to gain one additional QALY, the probability that the PARTNERS2 intervention is cost-effective is < 50% for the primary and all sensitivity analyses.
The major limitation to the economic evaluation is that the service use data available to generate cost estimates for the full follow-up period were restricted by the fact it was not possible to complete the service audit of primary care records. Consequently, the costs of the full follow-up period were predicted from the 3-month participant-reported costs combined with the secondary care audit. The data constraints and uncertainty in the data mean that there is insufficient evidence to draw conclusions about the overall cost-effectiveness of PARTNERS2.
Parallel process evaluation
Delivery and fidelity analysis
Background
The monitoring, measurement and assessment of intervention delivery and fidelity are important, as it has been demonstrated that fidelity can be a mediator of study outcomes. If interventions fail to produce a desired or expected outcome, this could be due to poor implementation rather than a lack of effectiveness of the intervention. In recent years, a science of intervention fidelity has grown, but a debate continues about the nature of the core elements to be measured. Behaviour change taxonomies have been developed62 in an attempt to standardise approaches when studying complex interventions in community settings.
Increasingly sophisticated work has identified five domains of fidelity: study design, training, intervention delivery, intervention receipt by participants and intervention enactment, defined as the extent to which participants apply the skills learnt. Receipt and enactment have been defined as ‘engagement’ by some authors. 63 Despite some real progress, no gold standard for engagement exists, and psychometric properties of fidelity scales are infrequently reported. 64
Method
Two methods were used. First, a set of research instruments was developed to capture the extent and reach of delivery by the care partners (see Appendix 2). These included details of when and how contacts were made, the key activities delivered in sessions and the extent of supervision. They were completed by care partners with support from researchers. We also documented periods of care partner absence.
Second, the PARTNERS2 Collaborative Care Fidelity instrument was designed to capture the intervention programme theory: what it was meant to do from the perspective of the individual receiving the intervention. The research team developed the instrument by following the steps outlined by Walton et al. 63 These steps include the following: (1) reviewing previous measures, (2) analysing intervention components and developing a framework outlining the content of the intervention, (3) developing fidelity checklists and coding guidelines, (4) obtaining feedback about the content and wording of checklists and guidelines and (5) piloting and refining checklists and coding guidelines to assess and improve reliability. Reproduced with permission from Walton et al. 63 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text. The instrument was piloted by LEAP members and contained 26 items relating to various aspects of intervention delivery and impact.
Results
A care partner was in place for at least 70% of the intervention period for 75% (15/20) of the intervention practices, and the majority of intervention group participants (91%) had at least one care partner interaction of any type. Care partners reported discussing goals with 101 participants (87%).
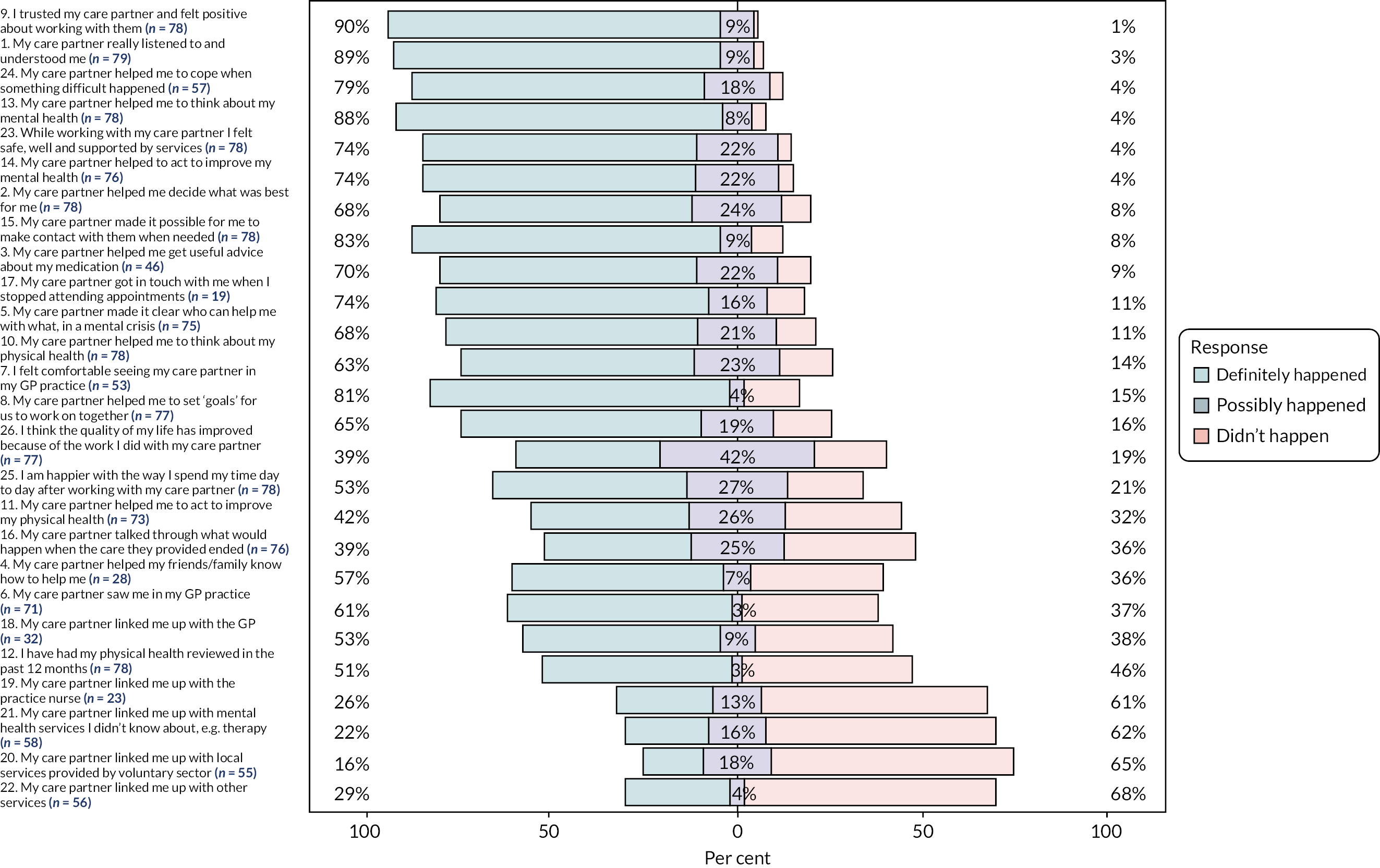
Of the 116 participants in the intervention group, fidelity questionnaire data were available for 81 (71%). The distribution of responses (frequencies and per cent) to individual items is reported in Table 11 (see Appendix 2). Figure 4, from which ‘not applicable’ responses were excluded, shows the proportion of those receiving the intervention (and who considered the question applicable to them) who recognised if they had received key components. Over half of the respondents to the fidelity instrument reported that 19 out of 26 items definitely happened. Overall, the data suggested that fidelity to the PARTNERS model was more likely to have occurred with interpersonal practices (e.g. listening and understanding, 89% of those who considered this question applicable to them, n = 70/79) than activities related to the co-ordination of care (e.g. linked to GP, 53% of those who considered this question applicable to them, n = 17/32).
FIGURE 4.
Participant responses to the fidelity questionnaire. Figure excludes ‘not applicable’ responses. Questions 1–14: 0 = didn’t happen, 1 = possibly happened, 2 = definitely happened; questions 15–26: 0 = no, 1 = to some extent, 2 = yes.
Future work will investigate the psychometric properties of the PARTNERS2 Collaborative Care Fidelity instrument, including divergent (or discriminant) validity, item homogeneity and data reduction analyses.
Realist process evaluation
Background
An integrated realist qualitative process evaluation was designed to assess delivery against programme theory, evaluate changes in care partner understanding and behaviour over time, and further develop the programme theory for implementation.
Methods
We purposively sampled care partners, supervisors, service users, friends and family members, and relevant health professionals from the four participating final trial sites. All care partners and supervisors were invited to take part. Service users were purposively sampled to capture geographical locations, demographics and interim data analysis.
Data collection comprised semistructured interviews; audio-recordings of intervention sessions between care partners and service users; tape-assisted recall interviews conducted separately with the participating care partners and service users; audio-recorded supervision meetings; reflective practice logs; and researchers’ observations and field notes, initial training and local top-up training sessions. COVID-19 pandemic restrictions led to changes in both remote data collection to incorporate more telephone interviews and recordings, and separate rapid realist evaluation of video-based interactions for remote delivery.
Data analysis involved the construction of case studies for care partners and service users, drawing on the above data sources. Evaluative coding and then within- and cross-case analyses enabled the exploration of delivery compared with the theory model, and the subsequent refinement of programme theory for implementation. LEAP members were involved in designing data collection tools, analysis and interpretation. A COVID-19 substudy examined delivery during the pandemic. Data collection and preliminary analysis were completed before the trial results were know in order to minimise interpretive bias.
Results
Semistructured interviews (n = 46) were conducted. Ten intervention sessions were recorded, followed by tape-assisted recall interviews with care partners and service users. Eight in-depth case studies for care partners and 15 in-depth case studies with service users were constructed.
We identified that practitioners need time to make changes to their practice in order to adopt more equitable relationships and shared understandings with service users. Having experience in reflective practice acted as a facilitator of making these changes. Previous experience working in mental health care may necessitate the unlearning of existing practice. Liaison with primary and secondary care was enabled by supplementary support, from existing relationships, strong introductions, or having a ‘PARTNERS champion’ within the primary or secondary care team. We have less information regarding supervision, because of inconsistent delivery, or the involvement of friends and family members, as most service users declined the involvement of these.
Service users sometimes framed their relationship with their care partner as that of a ‘professional friend’ or similar, and they valued the development of a collaborative relationship and shared understanding. These relational aspects of the PARTNERS service were important for fostering confidence, agency and identity, which were necessary conditions for thinking about and developing goals. Long-term poor agency and identity were barriers to working on goals.
The COVID-19 substudy identified that remote delivery was possible, although to be optimal it required a skilled care partner who had extensive experience of PARTNERS and was confident in using digital technologies, or who was comfortable modelling their vulnerability with technologies to service users. Some service users preferred telephone. Some individuals who lacked access to digital technologies appear to have been disenfranchised from the advantages of video.
Suggested theory refinement
Previous experience working in mental health care may act as a barrier to adopting care partner practice but as a facilitator of liaising. Care partner building of relationships with primary and secondary care can be facilitated by staff within these teams. Training and supervision should account for these factors. Core processes are the development of a collaborative relationship and shared understanding. Developing positive identity, improved agency and resilience are key interim outcomes for some service users. These are necessary for working towards goals and can be more important than goal attainment.
At an institutional level, supervisors need to be allocated sufficient time to understand the model and to support care partners. Care partners need not necessarily hold specific mental health experience; existing ways of working may act as a barrier to learning the model, whereas existing relationships with other practitioners may be a facilitator. This may be different if system-wide cultural changes in practice have taken place, as suggested by the new Community Mental Health Framework (CMHF) (NHS, 2019). 15 The difficulties in increasing identity, agency and resilience for long-term service users need to be acknowledged.
Patient and public involvement
Patient and public involvement (PPI) was embedded across the PARTNERS2 programme from the outset. Original co-applicants included both an applicant with bipolar and a McPin Foundation director. Patient involvement in PARTNERS2 has been an integrated element with clear decision-making responsibilities:65 LEAPs, service user research assistants (SURAs), TSC and Data Monitoring Committee (DMC) members. Across the research team, including at co-applicant level, others also drew on lived-experience expertise where appropriate. Reflections on this have been published by the PARTNERS 2 writing collective (2020). 66
To embed PPI, we co-produced a ‘ways of working’ document in collaboration with all members of the study team to frame and help develop working practices guided by the National Survivor User Network 4PIs. 67 PPI has been crucial in PARTNERS2. Some significant contributions are highlighted below.
Three LEAPs, consisting of service users and carers, were recruited in 2014, and included individuals with a range of diagnoses. Members were selected for their life skills as well as mental health experiences matched to a role description specification. Each study site – Lancashire, Birmingham and Devon – had its own LEAP, which met quarterly. An impact log was kept to track ideas and decisions. Members also attended full team sessions with the research and clinical team on an annual basis. Over time members took on more responsibilities for agendas and chairing meetings. LEAPs contributed to the development of the intervention and the trial design, as well as working across the different PARTNERS2 WSs. Meetings were held to problem-solve local issues, such as recruitment or engagement challenges, and to reflect on emerging findings. Membership remained stable throughout the study. Nineteen members worked collaboratively with the research team on a range of projects, developing recruitment materials, selecting the primary outcome and trial outcome measures, piloting outcome measures, reviewing data, and inputting to manuals for both service users and care partners. They also developed our study website design and content, contributing videos and scripts.
Three part-time SURAs, one per study site for the first 3 years of the study, worked alongside a PPI co-ordinator. Expertise from experience was used in various ways, including co-facilitating and co-chairing COS workshops and meetings; developing the intervention, including training care partners; writing recruitment materials, including a study leaflet containing SURA profiles; and recruiting trial participants. SURAs acted as a bridge between the LEAP members and the academic team, providing a dual perspective with their experiential expertise and research skills. We explored how SURAs, LEAP members and other members of the research team worked together in a peer-reviewed paper addressing PPI and co-production approaches. 67
In 2018, PARTNERS2 jointly won the NIHR Service User and Carer Involvement in Research Award in recognition of its PPI programme. We made some changes to the programme when we lost the Lancashire site in 2019, moving regional LEAP meetings to central sessions. During COVID-19 restrictions, the format of LEAP meetings was changed from face to face to online. Over time, research assistant staffing on the project changed as staff, including SURAs, left. We formally had only one SURA at the end of the study, but other team members drew on expertise from experience without carrying a specific peer or service user job title.
Discussion
Reflections on what was and was not successful in the programme
Key successes of the programme included iterative development of a theory-based intervention valued by individuals; an in-depth quantitative description of standard care; a COS for bipolar; an analysis of our partnership with service user researchers and the LEAPs; methodological innovations for complex intervention evaluation; and the delivery of a cluster RCT in adverse conditions. We report by WS and then more general reflections to provide a narrative over the life of the programme.
Workstream 1: understanding the context
We completed one of only a handful of quantitative descriptions of usual care for psychosis in the UK. However, hand-searching electronic health records from primary and secondary care to determine the nature of usual care was painstaking and time-consuming. The current lack of joined-up electronic health records both between secondary and primary care and within secondary care made this harder. We were unable to access social care and voluntary-sector records. We were able to provide new insights into the type of care received, showing that many people received high volumes of specialist mental health contacts and others much less, and also how the three locality health systems had distinct organisational patterns. The process of collecting the data, and delays in data transfer combined with the inconsistent nature of the data, meant that it was not possible to clearly identify pathways of care to develop the structure of the economic decision-analytic model as originally planned. Initial work indicated the need for a complex model that could account for the interactions between care providers in primary and secondary care as well as social care, and between participants and care providers. Limited data with which to populate such a model, combined with limited resources for the main trial, led to the decision to transfer the funding for this work to the trial.
The Cochrane review of collaborative care documented the range of interventions and outcomes measured and generated across diverse collaborative care type interventions for psychosis internationally. It is surprising how little knowledge there is about collaborative care – one of the only combined clinical and organisational models of care likely to support the integration for individuals with psychosis promoted by NHS policy. The review addresses this gap in knowledge, although the heterogeneity of studies limited clear conclusions about which components and underlying mechanisms may be responsible for benefit and which may be unhelpful or even counterproductive. The review also highlighted the low quality of existing evidence, underlining the importance of the PARTNERS2 trial.
Workstream 2: core outcome set work
We created a COS for individuals with a diagnosis of bipolar. This development took considerable effort to maintain the engagement of practitioners and individuals with lived experience to decide what outcomes matter most for individuals diagnosed with bipolar. Initially we had planned to also have a combined COS for those with schizophrenia. However, we decided that the groups needed to be separate and, therefore, to focus on bipolar.
Even though we were able to describe a set of outcome domains that were prioritised as most important for bipolar trials, this work was not directly linked to decisions about which outcome domains should be measured in our trial. This is because both the nature of the intervention (and what it was designed to achieve) and which outcomes are important to a wider group of people living with schizophrenia, bipolar and other psychoses need to be considered when selecting outcome domains to measure in a trial. A decision may also be influenced by the psychometric performance of measures available for the domain and the likelihood that they would detect a difference over time. Further problems include that standard measures with the most use can be old (e.g. use inappropriate language) and that measures may be composite (include overlapping domains). We therefore carried out a pragmatic process of reviewing measures related to the domains that emerged from our COS for bipolar, that were important to people in our LEAPs and that were part of the logic model of the intervention (i.e. could potentially be changed by the intervention). We then carried out a single consensus meeting, supporting individuals with lived experience to understand and fully engage in prioritising and making decisions for the trial. We believe this to have been a successful adaptation to the programme and a helpful method for selecting measures, but one that needs more formal development.
Workstream 3: development of the PARTNERS intervention
The development of the complex intervention, the PARTNERS intervention, was a significant enterprise; it brought together several data streams, including focus groups, expert interviews and focused literature reviews, and we tested out the prototype intervention in practice. Our expert interviews were a key and novel method of intervention development. They generated micro-theories that were not found in the literature, which we combined as realist ‘if–then’ statements and incorporated into the intervention programme theory. They guided our decisions about both what components of care should be included in the intervention and how we should bring about change in adverse real-world conditions, thus helping to address the implementation challenge. We needed to make judgements about which were relevant for the context of care partner delivery in the UK.
When selecting the psychological therapeutic model, we had little formal evidence to go on. None had been formally compared in either the original Cochrane review for psychosis21 or collaborative care reviews more generally. 68 We were influenced by two key interviews with practitioner intervention developers who provided a convincing rationale that a coaching approach with a motivational element would be best for individuals with psychosis, many of whom have low motivation, considerable anxiety and often low mood. 69
Bringing all the components together required a systematic approach with an overarching programme theory. This detailed the multistep causal chain from training and support of practitioners through to changes in their thinking and then their practices. These included working with individuals with psychosis, including thinking together about what personal goals and plans were most relevant for that individual and also undertaking liaison activities with other practitioners or teams. One of our key papers32 showed that while such a synthesis could have been carried out by one individual systematically, it actually involved a complex collaborative process of building relationships, engaging in disputation and understanding the limits of evidence.
The feasibility phase provided substantive evidence that both practitioners and individuals with lived experience found the model generally acceptable. Interpersonal process recall, also known as tape-assisted recall, was particularly useful in exploring more specifically the delivery of the PARTNERS2 service, and how practitioners and service users experienced it. 37 This demonstrated the difficulty that some practitioners have in shifting actual practice from a ‘fixing’, more medical, approach to one that involved deep listening to individuals’ real concerns and then supporting them to find their own solutions through coaching. We also identified a potential weakness in our intervention system: ensuring that supervision is carried out according to protocol despite competing pressures, and the need for top-up training and mentoring.
Workstreams 4 and 5: testing recruitment methods in preparation for the trial
The preparation for the trial included working out how best to recruit participants. During this period, we tested and adapted different methods of recruitment rather than formally piloting one method in an external pilot trial. The potential solutions were designed for recruiting both individuals who are seen mainly in secondary care and those in primary care only. The challenge is not insubstantial and contrasts with the recruitment of individuals with motivation and engagement in treatment regimes and investigation, such as cancer services. Interestingly, having practitioners who knew the patient make initial phone contact was found to be unreliable due to both biases (too much encouraging and discouraging were both informally reported) and workload pressures distracting them from the task. The techniques eventually used successfully included embedding NHS research staff within practices to identify individuals (rather than the usual method of paying GP staff to carry out complex searches and record reviews), GPs inviting individuals to appointments with set times in the practice, and having trained NHS researchers make telephone calls to those not responding.
Workstream 6: the cluster randomised controlled trial
Following the delays in starting the trial, and then problems with initial recruitment in two of the sites, the delivery of the trial in the COVID-19 context was possible only because of the goodwill, flexibility, hard work and sense of humour that the in-site researchers brought to the tasks of recruitment and follow-up. The qualitative team also worked through the pandemic collecting and analysing a range of data, including at interactional level, to complete what is one of a handful of in-depth process evaluations of complex interventions. The statistical team and workforce in the Peninsula CTU provided a very positive experience of controlled data input, cleaning and finally analysis, allowing us to report results on time.
The challenges we overcame fell into three broad areas: how to engage with individuals with lived experiences of schizophrenia, bipolar or other psychoses; how to work with NHS primary and secondary mental health care systems struggling with morale, workforce retention, a changing commissioning environment and the COVID-19 pandemic; and working through and bringing together the components of the NIHR and related research administration and governance. Motivation to continue the study came from hearing from practitioners putting the intervention into practice; our LEAP members’ commitment to changing services; an understanding of the relevance of further policies encouraging collaboration across the primary–secondary care interface for individuals with complex needs, such as those with psychosis;15 and the importance of person-centred approaches to care.
Our LEAP provided us with ideas about how best to work with individuals with varied experiences of psychosis and gave us inspiration to continue our quest to deliver the trial. They encouraged us to ensure that individuals with schizophrenia, bipolar and other psychoses had real opportunities to participate, and also that we should not take a lack of response to letters inviting involvement to mean that they would not wish to engage. As other studies working with vulnerable individuals have found, a balance needs to be struck between providing different opportunities for getting involved, such as letters, telephone calls or communication from trusted professionals, and the potential danger of vulnerable individuals feeling pressurised into joining research studies.
The research readiness of NHS services was constantly in our mind. In terms of primary care, initiating contact with GPs and practice managers was often hard. However, once their attention was gained, the opportunity to be involved in a trial supporting individuals with psychosis who had no mental health care was generally seen as positive and recruitment was often successful. Engaging and achieving this attention required tactics such as using senior clinicians to make contact as well as repeated assertive calls, and for researchers to be supported not to feel a failure or rejected. Working with practices to carry out recruitment procedures, such as doing searches, screening records and checking lists, was similarly problematic due to practice workload pressures.
We had planned to have NHS research staff embedded within general practices to carry out research procedures, but this was not initially allowed due to the prevailing governance issues around data protection. After the problems of asking practices to do all preconsent work were substantiated (causing delays), we requested an HRA amendment. Once this was in place, NHS research staff were able to carry out reliable screening of records and telephone approaches to individuals with psychosis from primary care, boosting recruitment.
Research readiness of secondary care was another significant issue in terms both of senior ‘sign-up’ and continuous delivery. While services could see that the intervention was in line with policy and the changes they wished to make, staff recruitment and retention problems meant that several services declined to participate, and those that did were unable to provide a supply of practitioners and supervisors able to engage fully in the intervention. The intervention delivery for the trial was often disrupted by changes in care partners and a lack of supervision. Problems included gaps in provision of care partners due to absence because of illness, and individuals moving on to other roles due to promotion or concerns about job insecurity. This meant that fidelity of the intervention was diminished. Despite this, practitioners who signed up to and were trained in the trial were highly motivated to deliver PARTNERS care and provided an inspiration through examples during their supervision and ongoing training of how they were putting PARTNERS into practice.
Although NIHR has developed a comprehensive range of systems to support different aspects of research, our experience was that it was not always easy to bring together the research team funded by one programme with personnel from various teams funded by the CRN in line with regulations governing research. We were particularly affected by (1) concerns about our multistep recruitment methods (which were deemed by some CRN and CTU managers as bordering on harassment but had support from the PPI LEAP members), and (2) our proposed practice of embedding CRN staff (or indeed university researchers with honorary contracts) in practices to relieve primary care of the burden of complex preconsent work. We were also affected by the assessments required by the CRN of ‘capability and capacity’ and training for each practice. Developing and implementing alternatives and then making changes (back towards our original proposals) led to many months of delays. In summary, there were delays and frustration as research systems designed mainly for preventing harm from medicinal products in motivated patients with no cognitive capacity problems collided with researchers (including LEAPs and equally frustrated CRN/CTU staff implementing the guidance) wanting more pragmatic approaches. We recommend clarity about further reduction in bureaucracy for low-risk studies and a different approach, with CRN-funded researchers alongside university researchers embedded into general practices carrying out the multiple tasks together as one team around the practice. This advice has been passed on to those developing the primary care strategy for the CRN and NIHR and has been approved as a strategy in a more recent study. 70 Additionally, as services are integrated and systems are subject to research, the old divisions of primary and secondary care become redundant for research governance.
Completion of the trial was possible only with compromise. We were able to demonstrate that recruitment had been possible and at a good rate in the Plymouth and Cornwall areas, and, following the appointment of a new lead, recruitment in West Midlands increased. The NIHR panel in January 2020 was convinced by the turnaround and a revised sample size calculation based on initial data showed that continuing to recruit for a couple more months would enable a reduced size trial to be carried out with a power of 80% to detect the prespecified between-group difference of 0.45 points in overall MANSA score. We were therefore able to continue recruiting rapidly and almost achieve the new target of 204.
The COVID-19 pandemic provided the final major challenge for the programme. Follow-up was affected as completion of recruitment to the study was subject to COVID-19 lockdown conditions. We quickly tested our ability to collect outcomes along with qualitative data by telephone and video. The care partners delivering the intervention were also able to ensure that individuals randomised to the intervention could continue to receive telephone calls and video contact to allow access to the PARTNERS person-centred coaching during COVID-19 conditions. Ongoing delivery of the trial was approved. The research team worked as an effective unit and, with telephone and video efficiencies, the trial was completed with an 85% follow-up rate for outcome collection and no decrease in contact rate during the pandemic.
Limitations
The limitations in phase 1 are covered above. Here we focus on the strengths and limitations of the trial, the process evaluation and the cost-effectiveness analysis.
The strengths of the trial included the real-life setting, with high proportions of recruited individuals from deprived rural, urban and coastal areas of England. We did not recruit as ethnically diverse a sample as we had hoped, with figures lower than those we found in WS1.
The cluster RCT design was considered necessary to avoid contamination within practices, but it resulted in an uneven randomisation of individuals because there was more variation in practice size (cluster size) than had been anticipated. The delivery of the intervention was suboptimal due to several care partner changes in employment and sickness affecting each site. We did not aim to quantify any differences in service user outcomes between care partners, but we did show clear differences in delivery between care partners in the case studies. Our process evaluation captured a wide range of data, and although we would have liked more data from interactions between the care partner and service user or the care partner and supervisor, and it is possible to get only a small window into intervention delivery over 2 years, we consider that the analysis revealed clear examples of potential benefit (and underlying mechanisms) in spite of the challenges of implementation, which were also revealed in detail.
The nature of psychosocial interventions and informed consent meant that blinding of participants was impossible. Although most of the primary trial outcome measures were carried out through self-report completion of questionnaires, some individuals required support in completing these, and, in a proportion of these for logistical reasons, the researchers were unblinded. There was also a problem in collecting two of the planned measures. First, the Brief-INSPIRE measure, which aimed to examine experiences of recovery-orientated care, was not analysed inferentially due to significant between-site differences in collection rates and both the numbers and patterns of missing data at both baseline and follow-up. Second, the physical healthcare process data were not completed due to reductions in permitted researcher presence in surgeries during COVID-19 pandemic conditions. This also limited the economic evaluation.
Despite outcome measure selection being based on an analysis of the intervention theory and predicted stages of change, it is recognised that health and QoL outcomes are often impacted downstream. It is notable that with person-centred interventions, changes may be highly idiosyncratic or individualised and these changes, however important to the individual, may be missed by standard outcome measures. Even though the delivery of PARTNERS was compromised, the process evaluation provided evidence that key elements were routinely delivered and that for some people meaningful benefits were evident. This adds weight to the possibility that, currently, trials of person-centred approaches are limited by our inability to measure initial unpredictable benefits that could lead to later changes in health status or QoL.
Conclusions for whole programme
The 7-year PARTNERS2 programme incorporated several substudies within two phases. Highlights of new knowledge gained are recorded here. During the first phase, a range of studies were carried out. The detailed analysis of care across three sites in England demonstrated significant variation in the proportion and extent of care. The Cochrane review of collaborative care provided limited evidence indicating that collaborative care may be more effective than standard care. However, our confidence in these findings is extremely limited because of the low certainty of evidence. Furthermore, the review pointed to a lack of detailed process evaluations of collaborative care.
The COS for bipolar was developed through engagement of stakeholders examining literature and a stepped Delphi approach. This provides a comprehensive set of 11 outcome domains, including symptom recovery, connectedness, patient experience, self-monitoring and the use of coercion.
The intervention development’s realist synthesis process led to a detailed programme theory for the PARTNERS2 intervention. This is laid out in an intervention manual (available via the website https://www.plymouth.ac.uk/research/primarycare/mental-health/partners2) and supported by the range of other documents co-produced with our LEAP. At the heart of the intervention is a coaching-based approach to working with individuals with SMI in a primary care setting. This approach helps individuals identify important personalised goals in the areas of physical health care, social outcomes or psychological problems, and supports achievement of these. This is in line with current policies but significantly different from the longstanding Care Programme Approach, which was used until 2022. A substantive implementation package involving training and top-up training, as well as supervision (in addition to the manual support), was developed to support delivery of the PARTNERS approach.
Phase 2 involved the main trial, including an analysis of quantitative outcomes, a realist process evaluation and a cost-effectiveness analysis. No significant differences were found using standard outcome measures for assessing QoL, mental health well-being, personal recovery or time use. The net costs and health benefits (QALY) were uncertain, with no indication of statistically significant differences between usual care and PARTNERS.
The delivery of the intervention was suboptimal, with significant absences of care partners for key periods, inconsistent supervision and a significant proportion of individuals choosing not to engage in the intervention; the last included when service users’ care passed to a new care partner, with engagement diminishing for some as a result.
The in-depth process evaluation also showed the extent to which several practitioners were able to practise the nuanced aspects of coaching and person-centred care. However, it also revealed the difficulty some individuals had responding to this and identifying and working on goals. Additionally, it showed, when care partners were available and individuals engaged, that for some individuals support led by goals generated short-term positive but not longer-term effects. We found a generalised mechanism, with some participants identifying how the care partner acted as a ‘professional friend’, offering support and encouragement through coaching. This aspect of the intervention effect was not covered in the measures used in the trial.
Recommendations for future research
PARTNERS2 implications for research build on the commentary above, outlining what went well and less well in this programme. In phase 1, the in-depth study quantifying care received by individuals with SMI demonstrated the lack of integration of electronic health records. It is inefficient to carry out case-by-case data extraction in the way we did to understand patterns of care; this points to the need for a new approach to routinely keeping track of care across and within systems. At a population level, we should be able to describe care received by individuals in different care clusters, including whether they are getting mental and/or physical health care input, and from which team. Support systems to organise the significant data already captured into a more usable form for such population-based monitoring would provide the basis for quantitatively monitoring care received in real time and context. This could supplement more qualitative assessment, either across large numbers of individuals using surveys or in-depth interviews for smaller numbers. We need research into how to reliably measure how much care groups of people sharing characteristics such as a diagnosis, poverty or ethnicity are receiving. Such a population-based approach is arguably more important than the more commonly used team- or service-based approaches, which miss out those not managed in secondary mental health services. This is in keeping with the move to population health management in Integrated Care Systems.
It is interesting to note that the trial itself recruited twice as many people with bipolar as with schizophrenia. We might not have been able to clearly communicate the ideas behind PARTNERS to people with schizophrenia and other psychoses and sufficiently engage them so that they became interested enough in our proposition to meet us and consent to take part. While we involved service user researchers in developing protocols and personalising information booklets with messages from service users and carers, and went through several iterations of trying to make our approach more flexible, we think that the research community needs to rethink current ethics and governance standards/requirements, which may, through their concerns about preventing harm, inadvertently be contributing to the non-involvement of people with schizophrenia and other psychoses in research processes.
Realist methods for developing interventions are still relatively unusual, and our approach appeared to be capable of generating an intervention that was acceptable to practitioners and service users. Relatedly, the realist process evaluation alongside the trial showed the value of both depth and breadth of data from different sources, including interviews from a range of stakeholders as well as observation and video. We are aware, however, that trying to capture the process of engaging individuals in a complex intervention over a period of months across several regions is a challenge and, inevitably, biased in terms of both selection of participants and availability of data. The research process relies, from a capacity point of view, almost entirely on paid researchers who are distant from events, whereas it is the practitioners and service users who are present and potentially able to record what is happening in detail. New techniques are therefore required for process evaluation work to engage practitioners and patients in the research process, taking into account the former’s capacity and the latter’s busyness. There is potential for this to cross over with reflective practice and service user involvement and with co-production for services more generally. A potential hybrid between the formative and process evaluation techniques used to evaluate complex interventions and methods used in quality improvement could be developed for use, for example, in the implementation of the CMHF policy, where there are many uncertainties about how to proceed.
Lastly, given the weaknesses in intervention implementation, there is a question of whether a further RCT should be carried out to determine whether person-centred coaching approaches to collaborative care are helpful or not. We are cautious about suggesting further trials both because the current NHS context is not always supportive and because of doubts about whether a single primary outcome measure can capture the multiple possible outcomes of potential importance in person-centred care. An alternative is to shift the emphasis to a combination of learning from innovation and implementation, and the development of mixed-methods approaches where the quantitative measurement of ‘reach’ to poorly served populations, experience of care and cost of intervention delivery is combined with qualitative approaches. The latter could include both high-volume, low-intensity data and in-depth observational approaches. Randomisation could still be used but perhaps with ‘reach’ and equitable access or patient experience as the key outcomes rather than health or QoL. Indeed, our measure of intervention fidelity illustrated that the aspects of care more related to care partner action were more clearly recognised as having been delivered. Those aspects that were more related to liaison with local services and within practice were more likely not to have been delivered. Fidelity to the PARTNERS model was more likely to have occurred with interpersonal practices (e.g. listening and understanding, 89%, n = 70/79) than with liaison activities (e.g. linked to GP, 53%, n = 17/32).
Implications for practice and lessons learnt
The PARTNERS2 programme provides evidence in relation to four aspects of care for individuals with schizophrenia, bipolar and other psychoses: what kind of interactions are important to service users and what practitioners might undertake, how practitioners can be supported in this care, how care is organised, and how structures and processes might support such system-based changes.
The PARTNERS intervention is based on sets of practices, those involving direct contact with service users and those more related to liaison and co-ordination. It is possible that 16 components were too many. The coaching approach tested in PARTNERS2, while not shown to have a direct impact on composite and mental health outcomes, was often experienced as positive (by both care partners and service users) when engagement was achieved. While a minority of individuals did not wish to continue with the care that was offered, most of those responding to the fidelity questionnaire and process evaluation interviews suggested that PARTNERS care was valuable. The process evaluation suggested that rather than the active goal-oriented practice of coaching being key, some individuals most valued the presence and interactions of practitioners as ‘professional friends’: someone alongside them whom they could trust and who could really understand them. It is interesting to consider whether or not such relationships needed to be ongoing. The process evaluation also showed that a wide variety of practices and short-term outcomes resulted from the motivational coaching approach. Some individuals made significant gains towards social goals or goals more related to psychological well-being, and few engaged in more proactive care of physical conditions. There was evidence that for some people getting to know, trust and have ongoing contact with a practitioner was important, while for others doing some work and then coming back at a later point was also experienced as helpful. This is important for those developing services for people with SMI as part of the CMHF transformation programme, where systems may be incentivised to provide time-limited interventions. Perhaps most importantly, the qualitative results showed that having a practitioner really listening and providing support with what the individual cared about most, rather than focusing on a model of fixing deficits, was generally appreciated.
The other key component of clinical care is liaison, and while we were not able to directly observe liaison, we did learn how the key partnerships required with the GPs and nurses in primary care teams, and with a large variety of voluntary sector organisations working with both, was challenging for care partners. Support to do this through supervision, but also organisational relationship building with primary care and the voluntary sector, is vital to ensure a supportive context for collaborative work.
Supporting practitioners to shift from a focus on risk and/or fixing deficits is a challenge that needs to be addressed in many aspects of community-based care where individuals with complex needs such as frailty, dementia or homelessness are also being asked to take a different approach. The training we provided for PARTNERS2, in particular the ongoing top-up training and support provided by the research team, was generally valued as a way of helping individuals see what needed to change. Even this relatively basic combination of initial and top-up training is often absent in the NHS during system change. However, embedding supervision in the PARTNERS model was difficult, and more work is needed to address why supervision proved so hard to deliver.
The detailed tape-assisted recall work also showed that practitioners had a tendency to feel that they provided person-centred coaching approaches when they had not shifted from more paternalistic stances. The tape-assisted recall interviews can be seen as a way of supporting practitioners to understand what was happening in their interactions and has parallels with techniques used in some training programmes, such as observation of practice or the use of videos and subsequent reflective practice. While these were time-consuming to perform for the research team, and not all practitioners consented to participate, where achieved this is a thorough method of validating good practice and helping practitioners see where improvements can be made.
Supervision delivery was weak due to pressures on supervisors to manage teams or large caseloads, or because of illness, meaning the research team often had to step in. When supervision occurred, and particularly when cases were discussed in depth and supportive challenge was provided, supervision was perceived as helpful overall. We conclude, therefore, that PARTNERS2 probably demonstrates the need for a package of support for practitioners, which might include written manuals, training, top-up training, clinical discussions as a team, peer support and supervision. This challenge for shifting practice and developing a supportive culture focused on learning to change is particularly important during the implementation of the CMHF, which requires new roles and changes in thinking and practice.
The two key organisational shifts in PARTNERS care are a shift towards more proactive care for those receiving only general practice mental health care, and a shift from secondary care to primary-care-based care with a specialist input. While the trial did not show measured benefits, there was no evidence of deterioration in terms of the primary outcomes and the safety variables. In particular, there was no indication of an increase in crisis for those receiving the intervention. Therefore, although the trial results do not show that current policy would lead to benefits, they do provide evidence that it is unlikely to be unsafe to shift individuals with SMI who are progressing their recovery well to primary-care-based care with specialist support. The results from phase 1 demonstrated very great variation in the extent of current treatment as usual, with some people receiving large amounts of care and others very little secondary care. Similarly, in the trial, there were differences with engagement, pointing to a need for both flexibility and assertive engagement (rather than the current practice of discharge during disengagement). Such practices also potentially necessitate a shift in culture (shared thinking, language and behaviour) within services. Our study did not address this directly; it is likely that such changes can be bottom up through shifts in practitioner thinking but can also be supported by thoughtful leadership activities.
Our final reflection as a team is how important the way we have worked together has been for the study. We have people in varied roles as care partners, LEAP members, research assistants, co-applicants, CTU staff, administrative support; everyone has been vital. Learning together and delivering this complex intervention as a team has been crucial, and we remain committed to this work (with PARTNERS3 currently ongoing) so we can continue to test these ideas and achieve better outcomes for people with schizophrenia, bipolar and other psychoses across England.
Additional information
Acknowledgements
We would like to thank all service users and practitioners who participated in the PARTNERS2 process evaluation and randomised controlled trial. We had a large LEAP whose members contributed to this research project and are not authors of the report, but we would like to acknowledge the contribution of each member: Dawn Allen, Gail Benton, Lindsey Cree, Shaimaa El Naggar, Stewart Hendry, Beverly Jones, Hameed Khan, Dougie Mclellan, Mary Mancini, Manoj Mistry, Mary Nettle, Juliet Rawlins, Angela Sanders, Deb Smith and Diane Wright.
We acknowledge the work of the late Professor Helen Lester, who led the original application for the research, and whose ideas and ethos permeated the programme.
Contributions of authors
Humera Plappert (https://orcid.org/0000-0002-0959-2394): Department of Social Work and Social Care Programme Manager, University of Birmingham. Programme manager, programme design and management, intervention development, trial science development, randomised controlled trial and report writing.
Richard Byng (https://orcid.org/0000-0001-7411-9467): GP and Professor in Primary Care Research, University of Plymouth. Co-chief investigator, original conception, programme design and management, intervention development and formative evaluation, trial science development, randomised controlled trial, process evaluation and report writing.
Siobhan Theresa Reilly (https://orcid.org/0000-0003-4372-4415): Director – Applied Dementia Studies, University of Bradford. Site lead, original conception, programme design, Cochrane review, trial science development, randomised controlled trial, process evaluation and report writing.
Charley Hobson-Merrett (https://orcid.org/0000-0002-2990-2871): Research Fellow, University of Plymouth. Programme design, trial science development, randomised controlled trial, process evaluation and report writing.
Jon Allard (https://orcid.org/0000-0003-3778-0015): Honorary University Fellow, University of Plymouth. Intervention development, trial science development and report writing.
Elina Baker (https://orcid.org/0000-0001-9064-5897): Consultant Clinical Psychologist, Livewell Southwest. Intervention development and formative evaluation, and report writing.
Nicky Britten (https://orcid.org/0000-0002-7533-414X): Professor of Applied Health Care Research, University of Exeter. Original conception, lead qualitative analysis, programme design, intervention development and formative evaluation, process evaluation and report writing.
Melanie Calvert (https://orcid.org/0000-0002-1856-837X): Professor of Outcomes Methodology, University of Birmingham. Original conception, core outcome set lead, trial science development and report writing.
Michael Clark (https://orcid.org/0000-0003-4964-5005): Associate Professorial Research Fellow and Research Programme Manager, NIHR SSCR Care Policy and Evaluation Centre (CPEC), The London School of Economics and Political Science. Original conception, programme design, intervention development, randomised controlled trial, process evaluation and report writing.
Siobhan Creanor (https://orcid.org/0000-0002-7373-8263): Director of Exeter CTU; Professor of Medical Statistics & Clinical Trials, University of Exeter. Trial science development, randomised controlled trial, statistical analysis and report writing.
Linda Davies (https://orcid.org/0000-0001-8801-3559): Professor of Health Economics, University of Manchester. Original conception, trial science development, randomised controlled trial, health economic evaluation and report writing.
Rebecca Denyer (https://orcid.org/0000-0002-9984-2704): Research Assistant, University of Exeter. Data collection, randomised controlled trial data collection, process evaluation and report writing.
Julia Frost (https://orcid.org/0000-0002-3503-5911): Senior Lecturer Health Services Research, University of Exeter. Randomised controlled trial, lead qualitative analysis, process evaluation and report writing.
Linda Gask (https://orcid.org/0000-0002-7918-3967): Emerita Professor of Primary Care Psychiatry, University of Manchester. Original conception, programme design, intervention development and formative evaluation, intervention training lead, process evaluation and report writing.
Bliss Gibbons (https://orcid.org/0000-0002-1310-6838): Research Associate, University of Birmingham. Intervention development and formative evaluation, trial science development, randomised controlled trial and report writing.
John Gibson (https://orcid.org/0000-0003-1731-4869): Senior Service User Researcher, The McPin Foundation. PPI co-ordination, intervention development and formative evaluation, trial science development, randomised controlled trial data collection, process evaluation and report writing.
Laura Gill (https://orcid.org/0000-0001-6224-5693): Research Group Co-ordinator, University of Plymouth. Intervention development and formative evaluation, trial science development and report writing.
Ruth Gwernan-Jones (https://orcid.org/0000-0002-0223-6581): Complex Intervention Senior Research Fellow, University of Exeter. Programme design, intervention development and formative evaluation, process evaluation and report writing.
Joanne Hosking (https://orcid.org/0000-0001-7090-0205): Senior Research Fellow & BMBS Lead for Statistics & Numeracy, University of Plymouth. Trial science development, randomised controlled trial, statistical analysis and report writing.
Peter Huxley (https://orcid.org/0000-0001-5152-8030): Chair in Mental Health Research, Bangor University. Original conception, programme design, intervention development, trial science development, randomised controlled trial, process evaluation and report writing.
Alison Jeffery (https://orcid.org/0000-0001-7539-5957): Clinical Trials Manager, University of Plymouth. Trial management, randomised controlled trial, process evaluation and report writing.
Benjamin Jones (https://orcid.org/0000-0001-7374-1107): Postdoctoral Research Fellow in Medical Statistics, University of Exeter. Randomised controlled trial, statistical analysis, process evaluation and report writing.
Tom Keeley (https://orcid.org/0000-0003-1584-9093): Director, Patient Centred Outcomes (Value Evidence and Outcomes) at GSK. Core outcome set development, trial science development and report writing.
Richard Laugharne (https://orcid.org/0000-0001-9916-343X): Consultant Psychiatrist at Cornwall Partnership NHS Foundation Trust. Site lead, supervisor, randomised controlled trial and report writing.
Steven Marwaha (https://orcid.org/0000-0002-0303-9942): Clinical Professorial Fellow, Institute for Mental Health, University of Birmingham. Site lead, trial science development, randomised controlled trial and report writing.
Claire Planner (https://orcid.org/0000-0003-0938-0444): Research Associate, University of Manchester. Original conception, intervention development and formative evaluation, trial science development and report writing.
Tim Rawcliffe (https://orcid.org/0000-0001-7765-8052): Service User Researcher, Lancashire Care NHS Trust. Intervention development, trial science development and report writing.
Ameeta Retzer (https://orcid.org/0000-0002-4156-8386): Research Fellow, University of Birmingham. Intervention development and formative evaluation, trial science development and report writing.
Debra Richards (https://orcid.org/0000-0002-2368-6522): Research Assistant, University of Plymouth. Randomised controlled trial data collection, process evaluation and report writing.
Ruth Sayers: Senior Peer Researcher, The McPin Foundation. PPI lead, intervention development and formative evaluation, trial science development and report writing.
Lynsey Williams (https://orcid.org/0000-0002-2037-2093): Research Assistant, University of Plymouth. Trial science development, randomised controlled trial data collection, process evaluation and report writing.
Vanessa Pinfold (https://orcid.org/0000-0003-3007-8805): Research Director, The McPin Foundation. PPI lead, original conception, programme design and management, intervention development and formative evaluation, trial science development, randomised controlled trial, process evaluation and report writing.
Maximillian Birchwood (https://orcid.org/0000-0002-7476-0171): Professor of Youth Mental Health, University of Warwick. Co-chief investigator, original conception, programme design and management, intervention development and formative evaluation, trial science development, randomised controlled trial and report writing.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YAET7368.
Primary conflicts of interest: Richard Byng received additional support from the National Institute for Health and Care Research (NIHR) Applied Research Collaboration South West Peninsula. Maximillian Birchwood received additional support from the NIHR Applied Research Collaboration East Midlands and is partly supported by NIHR Applied Research Collaboration West Midlands. Richard Byng, Julia Frost, Vanessa Pinfold and Maximillian Birchwood received an NIHR Policy Research Programme grant to examine implementation of PARTNERS. Richard Byng is a member of the Health Technology Assessment (HTA) Prioritisation Committee A. Michael Clark was a member of HTA Funding Teleconference, NIHR CRRSU Funding Board, HTA Prioritisation Committee B Methods Group, HTA General Committee, and COVID-19 Reviewing. Siobhan Creanor was a member of Clinical Trials Units funded by NIHR.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the West Midlands-Edgbaston Research Ethics Committee (reference number 14/WM/0052). Local NHS approvals were obtained before the start of recruitment in each region from 27 February 2018. We had an independent Data Monitoring Committee and a Trial Steering Committee, as agreed with the funder. The trial is registered (ISRCTN95702682).
Information Governance Statement
University of Plymouth is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under Data Protection legislation (UoP) is the Data Processor; the many general practices and NHS Trusts involved in the study are the Data Controllers, and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights here: https://www.plymouth.ac.uk/students-and-family/governance/information-governance/information-security. The contact details for UoP’s Data Protection Officer is dpo@plymouth.ac.uk.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the PGfAR programme or the Department of Health and Social Care. This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publications
Keeley T, Khan H, Pinfold V, Williamson P, Mathers J, Davies L, et al. Core outcome sets for use in effectiveness trials involving people with bipolar and schizophrenia in a community-based setting (PARTNERS2): study protocol for the development of two core outcome sets. Trials 2015;16:47. http://doi.org/10.1186/s13063-015-0553-0
Baker E, Gwernan-Jones R, Britten N, Cox M, McCabe C, Retzer A, et al. Refining a model of collaborative care for people with a diagnosis of bipolar, schizophrenia or other psychoses in England: a qualitative formative evaluation. BMC Psychiatry 2019;19:7. https://doi.org/10.1186/s12888-018-1997-z
Baker E, Gwernan-Jones R, Britten N, McCabe C, Gill L, Byng R, Gask L. Using interpersonal process recall to understand empowerment processes in a collaborative care intervention for people with a diagnosis of psychosis. Psychosis 2019;11:350–61. http://doi.org/10.1080/17522439.2019.1640274
Gwernan-Jones R, Britten N, Allard J, Baker E, Gill L, Lloyd H, et al. A worked example of initial theory-building: PARTNERS2 collaborative care for people who have experienced psychosis in England. Evaluation 2020;26:6–26. https://doi.org/10.1177%2F1356389019850199
Retzer A, Sayers R, Pinfold V, Gibson J, Keeley T, Taylor G, et al. Development of a core outcome set for use in community-based bipolar trials – a qualitative study and modified Delphi. PLOS ONE 2020;15:e0240518. http://doi.org/10.1371/journal.pone.0240518
The PARTNERS2 writing collective. Exploring patient and public involvement (PPI) and co-production approaches in mental health research: learning from the PARTNERS2 research programme. Res Involv Engagem 2020;6:56. https://doi.org/10.1186/s40900-020-00224-3
Plappert H, Hobson-Merrett C, Gibbons B, Baker E, Bevan S, Clark M, et al. Evaluation of a primary care-based collaborative care model (PARTNERS2) for people with diagnoses of schizophrenia, bipolar, or other psychoses: study protocol for a cluster randomised controlled trial. BJGP Open 2021;5:BJGPO.2021.0033. https://doi.org/10.3399/BJGPO.2021.0033
Reilly S, McCabe C, Marchevsky N, Green M, Davies L, Ives N, Plappert H, et al. Status of primary and secondary mental healthcare of people with severe mental illness: an epidemiological study from the UK PARTNERS2 programme. Br J Psychiatry 2021;7:e53. https://doi.org/10.1192/bjo.2021.10
Byng R, Creanor S, Jones B, Hosking J, Plappert H, Bevan S, et al. The effectiveness of a primary care-based collaborative care model to improve quality of life in people with severe mental illness: PARTNERS2 cluster randomised controlled trial. Br J Psychiatry 2023;222:246–56. https://doi.org/10.1192/bjp.2023.28
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Fineberg NA, Haddad PM, Carpenter L, Gannon B, Sharpe R, Young AH, et al. The size, burden and cost of disorders of the brain in the UK. J Psychopharmacol 2013;27:761-70. http://doi.org/10.1177%2F0269881113495118.
- Reilly S, Planner C, Hann M, Reeves D, Nazareth I, Lester H. The role of primary care in service provision for people with severe mental illness in the United Kingdom. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0036468.
- NHS Digital . Quality and Outcomes Framework 2011–12 England Level n.d. www.hscic.gov.uk/catalogue/PUB08661 (accessed 16 November 2021).
- Chief Medical Officer’s Annual Report 2020 . Health Trends and Variation in England n.d. www.gov.uk/government/publications/chief-medical-officers-annual-report-2020-health-trends-and-variation-in-england (accessed 16 November 2021).
- McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price: The Cost of Mental Health Care in England to 2026. London: King’s Fund; 2008.
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52-77. https://doi.org/10.1002%2Fj.2051-5545.2011.tb00014.x.
- Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry 2017;211:175-81. https://doi.org/10.1192/bjp.bp.117.202606.
- Mental Health Taskforce . The Five Year Forward View for Mental Health 2016. www.england.nhs.uk/wp-content/uploads/2016/02/Mental-Health-Taskforce-FYFV-final.pdf (accessed 28 January 2024).
- Bindman J, Johnson S, Wright S, Szmukler G, Bebbington P, Kuipers E, et al. Integration between primary and secondary services in the care of the severely mentally ill: patients’ and general practitioners’ views. Br J Psychiatry 1997;171:169-74. http://doi.org/10.1192/bjp.171.2.169.
- Goodrich DE, Kilbourne AM, Nord KM, Bauer MS. Mental health collaborative care and its role in primary care settings. Curr Psychiatry Rep 2013;15. http://doi.org/10.1007/s11920-013-0383-2.
- Lester H, Tritter JQ, Sorohan H. Patients’ and health professionals’ views on primary care for people with serious mental illness: focus group study. BMJ 2005;330. http://doi.org/10.1136/bmj.38440.418426.8F.
- Daumit G, Pratt L, Crum R, Powe N, Ford D. Characteristics of primary care visits for individuals with severe mental illness in a national sample, 2002. Gen Hosp Psychiatry 2002;24:391-5. http://doi.org/10.1016/S0163-8343(02)00213-X.
- Osborn DPJ, Nazareth I, King MB. Risk for coronary heart disease in people with severe mental illness: cross sectional comparative study in primary care. Br J Psychiatry 2006;188:271-7. http://doi.org/10.1192/bjp.bp.104.008060.
- NHS . NHS Long Term Plan n.d. www.longtermplan.nhs.uk (accessed 16 November 2021).
- NHS England . The Community Mental Health Framework for Adults and Older Adults n.d. www.england.nhs.uk/publication/the-community-mental-health-framework-for-adults-and-older-adults/ (accessed 16 November 2021).
- NHS England . Comprehensive Model of Personalised Care n.d. www.england.nhs.uk/personalisedcare/upc/comprehensive-model (accessed 16 November 2021).
- Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioural health care settings: systematic review and meta-analysis. Am J Psychiatry 2012;169:790-804. http://doi.org/10.1176/appi.ajp.2012.11111616.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002%2F14651858.CD006525.pub2.
- Bauer MS, Biswas K, Kilbourne A. Enhancing multiyear guideline concordance for bipolar disorder through collaborative care. Am J Psychiatry 2009;166:1244-50. https://doi.org/10.1176/appi.ajp.2009.09030342.
- Röhricht F, Waddon GK, Binfield P, England R, Fradgley R, Hertel L, et al. Implementation of a novel primary care pathway for patients with severe and enduring mental illness. BJPsych Bull 2017;41:314-9. http://doi.org/10.1192/pb.bp.116.055830.
- Reilly S, Hobson-Merrett C, Gibbons B, Jones B, Richards D, Plappert H, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev 2024;5. https://doi.org/10.1002/14651858.CD009531.pub3.
- Reilly S, McCabe C, Marchevsky N, Green M, Davies L, Ives N, et al. Status of primary and secondary mental healthcare of people with severe mental illness: an epidemiological study from the UK PARTNERS2 programme. Br J Psychiatry 2021;7. https://doi.org/10.1192/bjo.2021.10.
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias). J Clin Epidemiol 2011;64:407-15. https://doi.org/10.1016/j.jclinepi.2010.07.017.
- Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, et al. Cooperative Studies Program 430 Study Team. Collaborative care for bipolar disorder: Part II . Impact on clinical outcome, function, and costs. Psychiatr Serv 2006;57:937-45.
- Chatterjee S, Naik S, John S, Dabholkar H, Balaji M, Koschorke M, et al. Effectiveness of a community-based intervention for people with schizophrenia and their caregivers in India (COPSI): a randomised controlled trial. Lancet 2014;383:1385-94.
- Chwastiak LA, Luongo M, Russo J, Johnson L, Lowe JM, Hoffman G, et al. Use of a mental health center collaborative care team to improve diabetes care and outcomes for patients with psychosis. Psychiatr Serv 2018;69:349-52.
- Kilbourne AM, Goodrich DE, Lai Z, Clogston J, Waxmonsky J, Bauer MS. Randomized controlled pilot study of life goals collaborative care for patients with bipolar disorder and cardiovascular disease risk from community-based practices. Psychiatr Serv 2012;63:1234-8.
- Kilbourne AM, Goodrich DE, Lai Z, Post EP, Schumacher K, Nord KM, et al. Randomized controlled trial to assess reduction of cardiovascular disease risk in patients with bipolar disorder: The self-management addressing heart risk trial (SMAHRT). J Clin Psychiatry 2013;74:655-62.
- Mishra A, Krishna GS, Alla S, Kurian TD, Kurian J, Ramesh M, et al. Impact of pharmacist–psychiatrist collaborative patient education on medication adherence and quality of life (QOL) of bipolar affective disorder (BPAD) patients. Front Pharmacol 2017;8.
- Salman S, Idrees J, Anees M, Arifullah M, Al Waeel M, Ismail M, et al. Collaborative care for schizophrenic patients in primary care: a double blind, randomized clinical trial of efficacy and safety. El Mednifico J 2014;2:240-4.
- van der Voort TYG, Van Meijel B, Goossens PJJ, Hoogendoorn AW, Draisma S, Beekman A, et al. Collaborative care for patients with bipolar disorder: rRandomised controlled trial. Br J Psychiatry 2015;206:393-400.
- Gwernan-Jones R, Britten N, Allard J, Baker E, Gill L, Lloyd H, et al. A worked example of initial theory-building: PARTNERS2 collaborative care for people who have experienced psychosis in England. Evaluation 2020;26:6-26. https://doi.org/10.1177/1356389019850199.
- Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74:511-44.
- Leamy M, Bird V, Le Boutillier C, Williams J, Slade M. Conceptual framework for personal recovery in mental health: systematic review and narrative synthesis. Br J Psychiatry 2018;199:445-52. https://doi.org/10.1192/bjp.bp.110.083733.
- Pawson R. Evidence-based Policy: A Realist Perspective. London: SAGE Publications Ltd; 2006.
- Baker E, Gwernan-Jones R, Britten N, Cox M, McCabe C, Retzer A, et al. Refining a model of collaborative care for people with a diagnosis of bipolar, schizophrenia or other psychoses in England: a qualitative formative evaluation. BMC Psychiatry 2019;19. http://doi.org/10.1186/s12888-018-1997-z.
- Baker E, Gwernan-Jones R, Britten N, McCabe C, Gill L, Byng R, et al. Using interpersonal process recall to understand empowerment processes in a collaborative care intervention for people with a diagnosis of psychosis. Psychosis 2019;11:350-61. http://doi.org/10.1080/17522439.2019.1640274.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Bora R. Rethink Mental Illness. Empowering People. Coaching for Mental Health Recovery 2014. https://rethink.org/media/2423/empowering_people_-_coaching_for_mh_recovery.pdf (accessed 28 January 2024).
- Keeley T, Khan H, Pinfold V, Williamson P, Mathers J, Davies L, et al. Core outcome sets for use in effectiveness trials involving people with bipolar and schizophrenia in a community-based setting (PARTNERS2): study protocol for the development of two core outcome sets. Trials 2015;16. http://doi.org/10.1186/s13063-015-0553-0.
- Retzer A, Sayers R, Pinfold V, Gibson J, Keeley T, Taylor G, et al. Development of a core outcome set for use in community-based bipolar trials – a qualitative study and modified Delphi. PLOS ONE 2020;15. http://doi.org/10.1371/journal.pone.0240518.
- Dedoose: Great Research Made Easy n.d. www.dedoose.com/ (accessed 16 November 2021).
- COMET Initiative . Delphi Manager n.d. www.comet-initiative.org/delphimanager/ (accessed 16 November 2021).
- The McPin Foundation . Bipolar Research – Working Together to Decide Which Outcomes Matter n.d. https://mcpin.org/bipolar-research-working-together-to-decide-which-outcomes-matter/ (accessed 16 November 2021).
- Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry 1999;45:7-12. http://doi.org/10.1177%2F002076409904500102.
- Plappert H, Hobson-Merrett C, Gibbons B, Baker E, Bevan S, Clark M, et al. Evaluation of a primary care-based collaborative care model (PARTNERS2) for people with diagnoses of schizophrenia, bipolar, or other psychoses: study protocol for a cluster randomised controlled trial. BJGP Open 2021;5. https://doi.org/10.3399/BJGPO.2021.0033.
- Byng R, Creanor S, Jones B, Hosking J, Plappert H, Bevan S, et al. The effectiveness of a primary care-based collaborative care model to improve quality of life in people with severe mental illness: PARTNERS2 cluster randomised controlled trial. Br J Psychiatry 2023;222:246-56. https://doi.org/10.1192/bjp.2023.28.
- Gershuny J. Time Use Surveys and the Measurement of National Well-being. Centre for Time-Use Research. Oxford: University of Oxford; 2011.
- Neil ST, Kilbride M, Pitt L, Nothard S, Welford M, Sellwood W, et al. The questionnaire about the process of recovery (QPR): a measurement tool developed in collaboration with service users. Psychosis 2009;1:145-55. https://doi.org/10.1080/17522430902913450.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick–Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-63.
- Williams J, Leamy M, Bird V, Le Boutillier C, Norton S, Pesola F, et al. Development and evaluation of the INSPIRE measure of staff support for personal recovery. Soc Psychiatry Psychiatr Epidemiol 2015;50:777-86. https://doi.org/10.1007/s00127-014-0983-0.
- Flynn TN, Huynh E, Peters TJ, Al-Janabi H, Clemens S, Moody A, et al. Scoring the ICECAP-A capability instrument Estimation of a UK general population tariff. Health Econ 2015;24:258-69. https://doi.org/10.1002/hec.3014.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Jones B. The PARTNERS2 Study: Trial of Primary Care Based Collaborative Care for People With a Diagnosis of Schizophrenia, Bipolar or Other Types of Psychosis. Protocol Version 1.0 Dated 16 December 2020 n.d. http://hdl.handle.net/10026.1/16794 (accessed 28 January 2024).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 16 November 2021).
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 16 November 2021).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. http://doi.org/10.1016/j.jval.2012.02.008.
- NHS . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection/ (accessed 16 November 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. http://doi.org/10.1002/sim.1981.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. http://doi.org/10.1007/s40273-014-0193-3.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Walton H, Spector A, Williamson M, Tombor I, Michie S. Developing quality fidelity and engagement measures for complex health interventions. Br J Health Psychol 2020;25:39-60. https://doi.org/10.1111/bjhp.12394.
- Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor SJC. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003555.
- The McPin Foundation . Putting Lay Voices into Primary Care Research: PPI Strategies and Early Steps in the PARTNERS2 Study n.d. www.mcpin.org/wp-content/uploads/2021/04/Our20PPI20plan.pdf (accessed 16 November 2021).
- The PARTNERS2 writing collective . Exploring patient and public involvement (PPI) and coproduction approaches in mental health research: learning from the PARTNERS2 research programme. Res Involv Engagem 2020;6. https://doi.org/10.1186/s40900-020-00224-3.
- National Survivor User Network . Research and Resources n.d. https://nsun.org.uk/research-resources (accessed 16 November 2021).
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care: making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2018;189:484-93. https://doi.org/10.1192/bjp.bp.106.023655.
- Katon WJ, Lin EHB, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. New Eng J Med 2010;363:2611-20. https://doi.org/10.1056/NEJMoa1003955.
- University of Plymouth . D-PACT: Dementia-Person Aligned Care Team n.d. www.plymouth.ac.uk/research/primarycare/dementia/dementia-person-aligned-care-team (accessed 16 November 2021).
Appendix 1 PARTNERS2 trial: economic evaluation
Methods
The economic evaluation of the PARTNERS2 intervention was a trial-based analysis conducted as part of the RCT reported in Cluster randomised controlled trial in the main report.
Approach
The aim of the economic evaluation was to estimate the cost-effectiveness of the PARTNERS2 primary-care-based model of collaborative care (intervention) compared with usual practice (comparator) from the cost perspective of the NHS and social care (costs). The time horizon was the scheduled follow-up of 10 months (9 months for the final participants recruited) ± 1 month.
The measure of health benefit for the primary economic analysis was QALY estimated from the EQ-5D-5L and EQ-5D-5L Crosswalk Index Value Calculator as recommended by NICE. 44–46 QALYs were estimated as:
where U = utility and t = time at assessment. The time between assessments is the time from baseline to primary end-point follow-up (i = 1).
The economic analysis used patient-level service use data to estimate costs using published national unit costs47,48 for the year 2019–20. The primary outcome of the economic analysis was the ICER (which combines service use costs and health benefit):
The methods for cost and QALY estimation, handling of missing data and descriptive summaries, as well as the primary and sensitivity analyses, were defined and added to the statistical analysis plan for the trial prior to data transfer to the health economist.
Cost estimation
The PARTNERS2 intervention was developed to use existing resources by placing an experienced care partner from secondary care in the GP practice. Accordingly, it was assumed that the care partner represents a transfer of resources/costs from secondary to primary care rather than an additional cost of implementing the intervention. Additionally, it was expected that the level of contacts with the care partner would be captured in the primary care service use data collection and associated costs.
Participant-reported service use at follow-up was collected for a 3-month, rather than 10-month, recall period. This was because of concerns about the burden to participants of recalling service use over a longer period and the need to balance complete service use data with incomplete recall, potentially large numbers of inconsistent or missing data, and limited resources for data collection. Additional data collection from an audit of case notes in secondary mental health and GP practices was planned to cover the period from baseline to the end of scheduled follow-up.
The collection of service use data from case notes across the multiple care providers involved in providing services (primary, secondary and community health care and social care) is complex, highly resource intensive and costly in the absence of integrated record linkage for individual service users. Accordingly, the case note audit focused on (1) key high-cost psychiatric secondary and crisis care services that could be expected to differ if the intervention was effective but may not be used within the 3-month recall period at the end of scheduled follow-up and (2) GP, practice nurse and other GP practice consultations (mental and physical health). However, the latter was not feasible within the trial budget and the impact of COVID-19-related constraints on access and researcher time. Data were collected from a sample of 31 participants. A comparison of the cost per day for these participants showed no differences between the data collected from the primary care audit and those reported by participants.
For the primary analysis, regression was used to predict a cost per day for all service use for participants with complete 3-month service use data collected at the follow-up interview, for the pooled (masked to treatment allocation) data. A generalised linear model (gamma log) was used, adjusting for baseline covariates (categories of services used, mental health medications, MANSA score, locality, practice size and GP practice). The full cost from baseline to end of follow-up was passively estimated following multiple imputation of missing cost data (predicted participant-reported cost per day plus the cost per day of psychiatric secondary care, multiplied by the number of days’ follow-up from baseline).
Missing data
The cost and health benefit data were analysed by treatment allocated and include data for all participants whether or not they completed planned care. However, missing data are inevitable from loss to follow-up or missing observations. Single imputation was used for missing baseline measures of cost, utility and clinical indicators and indicators for missing demographic data. 49
Multiple imputation (predictive mean matching and sequential chained equations)50 from available data was used to generate 10 sets of estimates for costs and QALYs for the pooled data set. A literature review and a regression analysis of pooled baseline data (masked to treatment allocation) were used to identify key baseline and follow-up variables associated with costs and QALYs to include in the imputation models.
Primary analysis
The EQ-5D-5L and ICECAP-A data were summarised as proportions of participants reporting each level (n/N, %, 95% CI). Costs and summary health benefit measures were summarised descriptively (mean, standard deviation, 95% CI) for the intervention and comparator for the complete case and imputed data at baseline and the primary end point.
Regression analysis was used to estimate the net costs and QALYs of the intervention, adjusting for key covariates. These were identified from published literature and supplemented with an analysis of pooled (masked) baseline data. The trial cluster (GP practice) and stratification variables (locality and practice size) were included. The regression-based estimates of costs and outcomes were bootstrapped to replicate 10,000 pairs of net cost and QALY outcomes of the intervention. These were used to generate estimates of the net costs and QALYs for the PARTNERS2 intervention, the ICER, and the probability that the intervention is cost-effective for the primary and sensitivity analyses, as well as the cost-effectiveness plane and cost-effectiveness acceptability curve for the primary analysis. ICERs estimate the net cost per QALY gained by an intervention and raise the question of whether that cost is worth paying. To address this, the ICERs are compared with how much decision-makers may be willing to pay for an additional QALY. However, the UK has no universally agreed cost-effectiveness threshold. Reflecting this lack of consensus, the monetary value of simulated QALYs were estimated across the range of £0 to £30,000 WTPTs. To estimate the likelihood that the intervention is cost-effective for the primary analysis, a WTPT of £15,000 (the mid-point of the £0 to £30,000 range) was used.
Prespecified sensitivity analyses explored whether alternative measures or analyses could change the conclusions of the economic analysis. These included (1) using the ICECAP-A41 as an alternative measure to derive a QALY; (2) alternative methods of estimating 10-month costs from the 3-month data; and (3) complete-case analysis.
Additional exploratory analyses (not included in the statistical analysis plan) used the primary clinical outcome (change in MANSA score from baseline to follow-up) and hours of paid employment per week as alternative measures of health benefit with which to estimate an ICER. As these are not preference-based measures of health benefit, there is no defined range of willingness-to-pay values to gain an additional unit of outcome. Accordingly, the probability of cost-effectiveness was not estimated.
Stata SE version 16 (StataCorp LP, College Station, TX, USA) was used for data management, costing and all analyses.
Results
The demographic data are shown in the paper reporting the detailed clinical trial results. Tables 5–8 report unadjusted EQ-5D-5L, ICECAP-A, QALYs and costs as well as number of participants contributing to the estimates, using the available data. Tables 1 and 2 report the frequency of participants with no problems on the measures used to estimate QALYs for the primary (EQ-5D-5L) and sensitivity analysis (ICECAP-A). Tables 5 and 6 also report the average QALYs.
| Domain | Care as usual | PARTNERS2 intervention | ||
|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | |
| No problems with mobility | ||||
| Baseline | 42/82 | 51 (40 to 62) | 51/116 | 44 (35 to 53) |
| Follow-up | 35/72 | 49 (37 to 60) | 44/99 | 44 (34 to 54) |
| No problems with self-care | ||||
| Baseline | 51/82 | 62 (51 to 72) | 66/116 | 57 (48 to 66) |
| Follow-up | 39/72 | 54 (43 to 65) | 56/99 | 57 (47 to 67) |
| No problems with usual activity | ||||
| Baseline | 32/82 | 39 (29 to 50) | 42/116 | 36 (28 to 45) |
| Follow-up | 27/72 | 38 (27 to 49) | 32/99 | 32 (24 to 43) |
| No problems with pain or discomfort | ||||
| Baseline | 21/82 | 26 (17 to 36) | 36/116 | 31 (23 to 40) |
| Follow-up | 16/72 | 22 (14 to 43) | 30/99 | 30 (22 to 40) |
| No problem with anxiety or depression | ||||
| Baseline | 20/82 | 24 (16 to 35) | 23/116 | 20 (14 to 28) |
| Follow-up | 12/72 | 17 (10 to 27) | 27/99 | 28 (20 to 37) |
| QALY baseline to follow-up, mean, SE (95% CI) | 0.55, 0.03 (0.48 to 0.61); n = 67 | 0.52, 0.03 (0.45 to 0.59); n = 91 | ||
| Domain | Care as usual | PARTNERS2 intervention | ||
|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | |
| Feel settled and secure in all areas of life | ||||
| Baseline | 9/82 | 11 (6 to 20) | 9/116 | 8 (4 to 14) |
| Follow-up | 9/72 | 13 (7 to 23) | 4/98 | 4 (2 to 10) |
| A lot of love, friendship and support | ||||
| Baseline | 24/82 | 29 (20 to 40) | 30/116 | 26 (19 to 35) |
| Follow-up | 25/72 | 35 (25 to 47) | 30/98 | 31 (22 to 40) |
| Completely independent | ||||
| Baseline | 24/82 | 29 (20 to 40) | 24/116 | 21 (14 to 29) |
| Follow-up | 16/72 | 22 (14 to 34) | 18/98 | 18 (12 to 27) |
| Achieve and progress in all aspects of life | ||||
| Baseline | 6/82 | 7 (3 to 15) | 11/116 | 9 (5 to 16) |
| Follow-up | 5/72 | 7 (3 to 16) | 9/98 | 9 (5 to 17) |
| A lot of enjoyment and pleasure | ||||
| Baseline | 10/82 | 12 (7 to 21) | 14/116 | 12 (7 to 19) |
| Follow-up | 10/72 | 14 (8 to 24) | 18/98 | 18 (12 to 27) |
| QALY baseline to follow-up, mean, SE (95% CI) | 0.68, 0.04 (0.61 to 0.75); n = 66 | 0.62, 0.03 (0.57 to 0.68); n = 92 | ||
Tables 7 and 8 report the observed and predicted average participant-reported costs for the 3 months prior to the baseline and follow-up assessments that were used to estimate full costs for the primary and sensitivity analyses. The average mental health secondary care costs from the case note audit for the index mental health service are also included in Table 7. Table 9 reports the multiple imputed costs and QALYs for the full follow-up period. Overall, the 95% CIs for all the data are wide and overlap between the usual care and PARTNERS2 groups, indicating uncertainty in the estimates.
| Cost category | Care as usual | PARTNERS2 | ||
|---|---|---|---|---|
| Used care, n/N (%) | Mean, SE (95% CI) | Used care, n/N (%) | Mean, SE (95% CI) | |
| Data collected from participant interview for 3 months preceding baseline/follow-up assessment | ||||
| GP practice | ||||
| Baseline | 64/82 (78) | £62, £8 (£47 to £78); n = 79 | 89/116 (77) | £58, £6 (£41 to £71); n = 115 |
| Follow-up | 52/70 (73) | £41, £5 (£30 to £51); n = 70 | 60/99 (61) | £41, £7 (£26 to £55); n = 99 |
| Other primary care services (physical health) | ||||
| Baseline | 28/82 (34) | £34, £8 (£17 to £51); n = 82 | 23/116 (20) | £21, £6 (£8 to £33); n = 114 |
| Follow-up | 22/72 (31) | £49, £16 (£16 to £82); n = 72 | 25/98 (26) | £30, £8 (£14 to £46); n = 97 |
| Community-based services | ||||
| Baseline | 36/82 (44) | £94, £21 (£52 to £136); n = 80 | 56/116 (48) | £128, £21 (£87 to £170); n = 114 |
| Follow-up | 25/71 (35) | £111, £29 (£54 to £168); n = 70 | 43/99 (43) | £100, £19 (£62 to £138); n = 98 |
| Social care services | ||||
| Baseline | 5/82 (6) | £12, £8 (< £1 to £28); n = 81 | 13/116 (11) | £38, £18 (£2 to £73); n = 115 |
| Follow-up | 6/71 (8) | £7, £3 (£1 to £14); n = 70 | 9/100 (6) | £24, £14 (< £1 to £51); n = 97 |
| Accident and emergency | ||||
| Baseline | 9/82 (11) | £26, £9 (£7 to £45); n = 82 | 11/116 (9) | £21, £6 (£8 to £33); n = 116 |
| Follow-up | 6/72 (8) | £18, £7 (£3 to £32); n = 72 | 6/99 (6) | £10, £4 (£2 to £19); n = 99 |
| Hospital outpatient services | ||||
| Baseline | 31/82 (38) | £83, £16 (£52 to £114); n = 81 | 32/116 (28) | £72, £19 (£35 to £109); n = 115 |
| Follow-up | 20/72 (28) | £60, £20 (£20 to £100); n = 72 | 26/100 (26) | £72, £16 (£40 to £104); n = 100 |
| Hospital day services | ||||
| Baseline | 2/82 (2) | £34, £31 (< £1 to £96); n = 82 | 6/116 (5) | £18, £9 (< £1 to £35); n = 116 |
| Follow-up | 3/71 (4) | £20, £12 (< £1 to £43); n = 71 | 5/99 (5) | £17, £8 (£1 to £32); n = 99 |
| Hospital inpatient stay (1 night or more) | ||||
| Baseline | 3/82 (4) | £69, £45 (< £1 to £158); n = 82 | 4/116 (3) | £44, £24 (< £1 to £91); n = 116 |
| Follow-upa | 3/72 (4) | £313, £296 (< £1 to £903); n = 72 | 4/100 (4) | £138, £98 (< £1 to £319); n = 100 |
| Total cost for 3 months preceding baseline/follow-up assessment | ||||
| Baseline | 77/82 (94) | £375, £54 (£268 to £482); n = 76 | 110/116 (95) | £402, £55 (£293 to £510); n = 112 |
| Follow-up | 58/68 (85) | £605, £327 (< £1 to £1259); n = 66 | 86/97 (89) | £405, £108 (£200 to £630); n = 93 |
| Secondary care case note audit, baseline to end of follow-up, index mental health service | ||||
| N = 77 | N = 109 | |||
| Inpatient careb | 1 (1) | £50, £50 (< £1 to £150) | 3 (3) | £617, £559 (< £1 to £1720) |
| Crisis carec | 2 (3) | £15, £11 (< £1 to £36) | 5 (5) | £106, £80 (< £1 to £263) |
| Other contacts | 37 (48) | £155, £18 (£118 to £191) | 8 (53) | £171, £15 (£141 to £202) |
| Total cost for secondary care case note audit | ||||
| Baseline-follow-up | 39 (51) | £220, £60 (£102 to £339) | 63 (58) | £894, £577 (< £1 to £2033) |
| Analysis | Usual care, mean, SE (95% CI) | PARTNERSb mean, SE (95% CI) | All participants,b mean, SE (95% CI) |
|---|---|---|---|
| Primary analysis | |||
| Predicted 3-month cost, baseline | £572, £149 (£269 to £481) n = 76 | £477, £75 (£328 to £625) n = 112 | £515, £75 (£367 to £663) n = 188 |
| Predicted 3-month cost, follow-up | £632, £216 (£204 to £1060) n = 62 | £424, £82 (£262 to £586) n = 92 | £508, £100 (£311 to £705) n = 154 |
| Multiple imputation 3-month cost, follow-up | £602, £176 (£255 to £949) n = 82 | £437, £77 (£284 to £590) n = 116 | £506, £86 (£335 to £676) n = 198 |
| Sensitivity analysis | |||
| Assumes costs constant, 3-month follow-up costs applied pro-rata to full follow-up period | |||
| Observed 3-month cost, follow-up | £605, £327 (<£1 to £1259) n = 66 | £405, £108 (£200 to £630) n = 93 | £494, £149 (£199 to £789) n = 159 |
| Multiple imputation 3-month cost, follow-up | £547, £264 (£26 to £1069) n = 82 | £436, £131 (£176 to £696) n = 116 | £482, £134 (£218 to £746) n = 198 |
| Assumes PARTNERS2 results in additional signposting and engagement with other services in first 3 months, not captured in final follow-up assessment | |||
| Additional cost, baseline to 3-month follow-up | – | £734, £341 (£52 to £1416) n = 93 | – |
| Multiple imputation, baseline to 3-month follow-up | £547, £264 (£26 to £1069) n = 82 | £1149, £108 (£935 to £1363) n = 116 | £923, £151 (£626 to £1221) n = 159 |
| Analysis | Usual care (n = 82) | PARTNERS2 (n = 116) |
|---|---|---|
| Mean, SE (95% CI) | Mean, SE (95% CI) | |
| Primary analysis | ||
| QALYs | 0.55, 0.03 (0.48 to 0.61) | 0.51, 0.03 (0.45 to 0.57) |
| Participant-reported costs | £2689, £856 (£999 to £4378) | £1743, £300 (£1149 to £2338) |
| Secondary care case note audit, index mental health service (single imputation) | £244, £57 (£131 to £357) | £877, £542 (< £1 to £1947) |
| Total cost | £2933, £855 (£1246 to £4620) | £2620, £647 (£1344 to £3897) |
| Sensitivity analyses | ||
| QALYs estimated from ICECAP-A | 0.66, 0.03 (0.60 to 0.72) | 0.62, 0.02 (0.60 to 0.72) |
| Total cost includes 3-month follow-up costs applied pro rata to full follow-up period | £2654, £1257 (£174 to £5133) | £2606, £743 (£1137 to £4075) |
| Total cost includes additional cost for baseline to 3-month follow-up | £2878, £814 (£1271 to £4484) | £3353, £646 (£2079 to £4626) |
Table 10 reports the bootstrapped net costs and QALYs and the probability that the PARTNERS2 intervention is cost-effective. Overall, the 95% CIs for the net costs and QALYs are wide and cross zero, indicating a high level of variance and uncertainty. Figure 5 illustrates the wide distribution of net cost/QALY pairs over the four quadrants for the primary analysis.
| Analysis | Net cost, SE (95% CI) | Net health benefit | ICER (£/QALY) | Probability PARTNERS2 is cost-effective if WTPT= | |||
|---|---|---|---|---|---|---|---|
| £0/QALY | £4000/QALYc | £15,000/QALYd | £30,000/QALY | ||||
| Primary analysis | −£213, £417 (−£1030 to £603) | −0.007, 0.040 (−0.086 to 0.071) | £29,495 saving per QALY lost | 0.62 | 0.51 | 0.30 | 0.24 |
| Sensitivity analyses | |||||||
| Complete-case analysis | −£100, £452 (−£986 to £785) | −0.004, 0.045 (−0.093 to 0.085) | £24, 947 saving per QALY lost | 0.52 | 0.44 | 0.34 | 0.32 |
| Assume 3-month follow-up costs constant over 10-month follow-up | £91, £508 (−£906 to £1087) | −0.007, 0.040 (−0.086 to 0.071) | Care as usual dominates | 0.29 | 0.21 | 0.16 | 0.17 |
| Assume additional costs for baseline to 3 months | £1402, £453 (£514 to £2290) | −0.007, 0.040 (−0.086 to 0.071) | Care as usual dominates | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| QALYs estimated from ICECAP-A data | −£213, £417 (−£1030 to £603) | −0.03, 0.04 (−0.11 to 0.05) | £7403 saving per QALY lost | 0.62 | 0.38 | 0.10 | 0.07 |
FIGURE 5.
Distribution of net cost–QALY pairs on a cost-effectiveness plane: primary analysis.
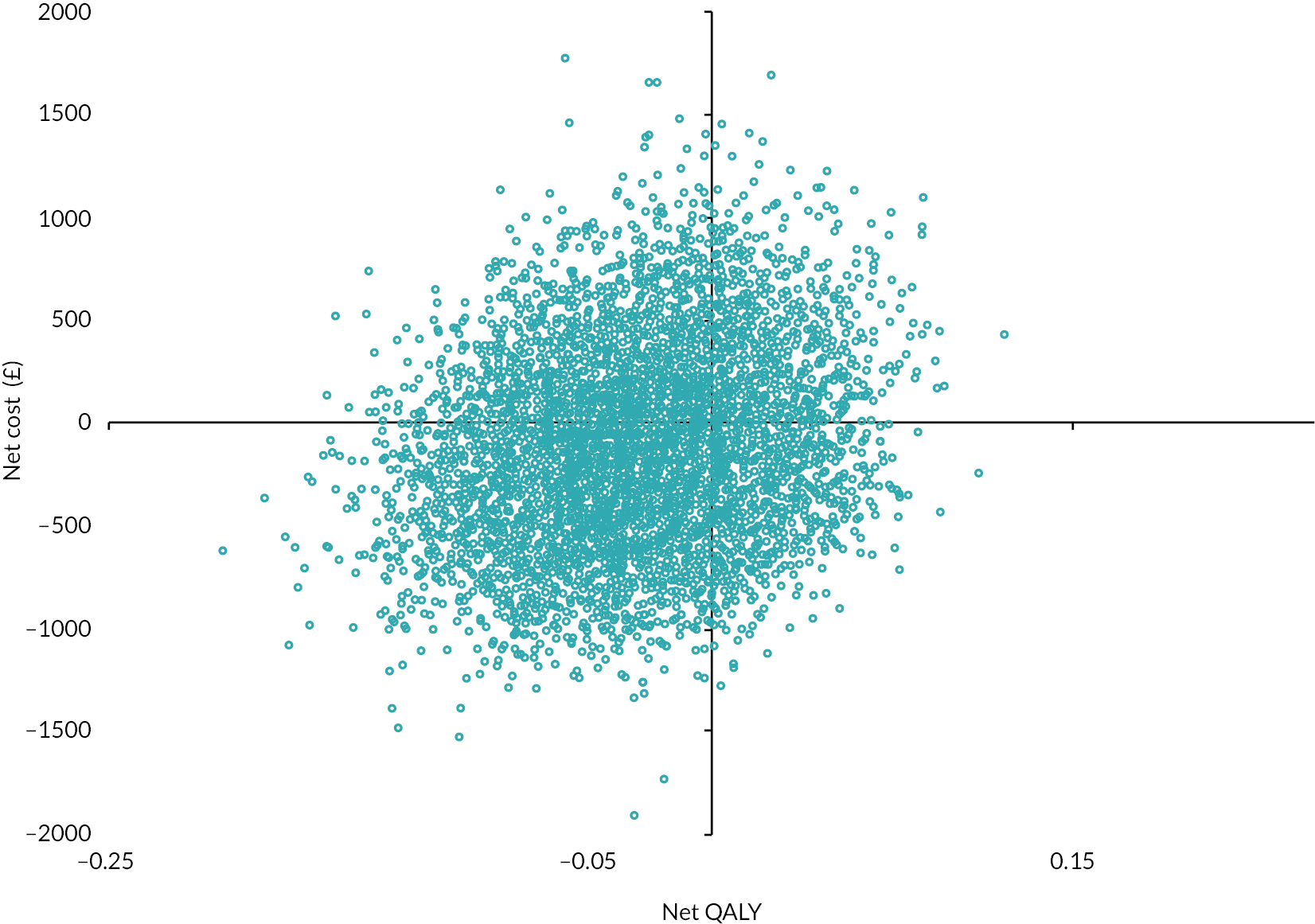
At the prespecified WTPT of £15,000 to gain one additional QALY, the probability that the PARTNERS2 intervention is cost-effective is < 50% for the primary and all sensitivity analyses. Figure 6 shows that the probability PARTNERS2 is cost-effective changes as the willingness to pay to gain one QALY increases. If decision-makers are prepared to pay £4000 to gain one additional QALY, then the probability that PARTNERS2 is cost-effective increases to just over 50% for the primary analysis, but not for the sensitivity analyses.
FIGURE 6.
Cost-effectiveness acceptability curve: primary analysis.
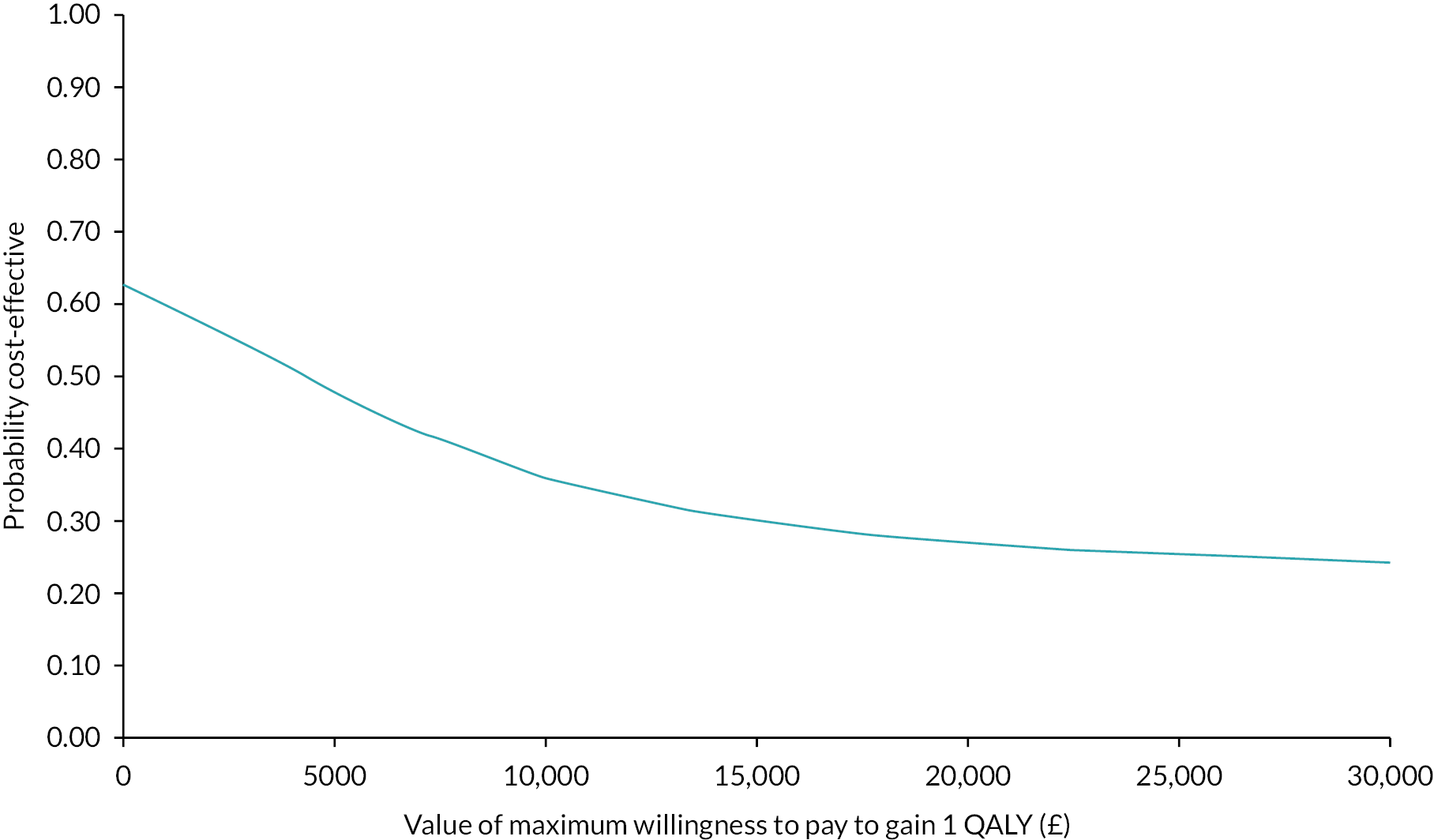
The bootstrapped net change in MANSA score (from baseline to follow-up) for participants in the intervention group was 0.01 (standard error 0.13, 95% CI −0.24 to 0.27). The bootstrapped net hours of paid work per week for participants in the intervention group showed a reduction of −1.52 hours per week (standard error 1.05, 95% CI −3.57 to 0.53).
Discussion
The economic evaluation shared the strengths and limitations of the trial. Although the trial achieved a high rate of follow-up, there were insufficient data to estimate QALYs and costs for 20% of participants. Overall, 77% of participants had complete QALY, participant-reported cost and secondary case note cost data at follow-up. Multiple imputation of missing data is important for intention-to-treat analysis, but larger numbers of missing data reduce the robustness of imputation.
The major limitation of the economic evaluation is that the service use data available to generate cost estimates for the full follow-up period were restricted. The collection of service use data from case notes across the multiple care providers involved in providing services is complex, highly resource intensive and costly in the absence of integrated record linkage for individual service users. As noted in the methods section of this appendix, participant-reported service use at follow-up was collected for a 3-month, rather than 10-month, period to minimise the burden to participants of recalling service use over a longer period. Additional data collection from an audit of case notes in secondary mental health and GP practices was planned to cover the period from baseline to the end of scheduled follow-up. It was assumed that the use of these services would be key cost drivers that the PARTNERS2 intervention could be expected to affect. However, the audit of GP practice case notes was not feasible within the trial budget and the impact of COVID-19-related constraints on access and researcher time. Additionally, a limited number of inpatient and crisis care episodes were reported in the secondary care audit. As a result, there was high variation in the costs estimated from the secondary care audit and no evidence of an association between these and the participant-reported costs. Consequently, the costs of the full follow-up period were predicted from the participant-reported costs for a 3-month period, baseline service use and the GP practice, locality and size. This increases uncertainty and reduces confidence in the robustness of the cost estimates and the overall cost-effectiveness analysis.
Conclusion
Overall, the results indicate that the cost and QALY estimates are uncertain, with insufficient evidence of differences between usual care and the PARTNERS2 collaborative care approach. Although the bootstrapped analysis suggests that PARTNERS2 is unlikely to be cost-effective if decision-makers are willing to pay £15,000 to gain one additional QALY (see Figure 6), limitations in the cost estimates mean that the robustness of these results is reduced.
Appendix 2 Fidelity measurement
This appendix includes the questionnaire used to capture the experiences of individuals receiving the intervention in terms of whether key components were perceived to have been delivered. It also includes the full table of results from those in the PARTNERS2 trial allocated to receive the intervention.
PARTNERS2 service user fidelity questionnaire
PARTNERS2 service user fidelity questionnaire version 1.0, 8 January 2020
Participant ID Date _______________
Your experiences working with your PARTNERS2 care partner
In this form we ask you some questions about what your care partner …… did when they were working with you.
Whatever you tell us is confidential; we will not pass this onto them. We know that not all of the things mentioned will have been done for everyone. We would like you to be honest about your PARTNERS2 experience so that we can improve the service for people in the future.
| Please tick | |||||||
|---|---|---|---|---|---|---|---|
| Definitely happened | Possibly happened | Didn’t happen | Not applicable | ||||
| Please tick | |||||||
| Yes | To some extent | No | Not applicable | ||||
| How they helped |
|
||||||
|
|||||||
|
|||||||
|
|||||||
|
|||||||
| Where you saw them |
|
||||||
|
|||||||
| Goals developed |
|
||||||
|
|||||||
| My health |
|
||||||
|
|||||||
|
|||||||
|
|||||||
|
|||||||
| Contact with my care partner |
|
||||||
|
|||||||
|
|||||||
| Linking with other services |
|
||||||
|
|||||||
|
|||||||
|
|||||||
|
|||||||
|
|||||||
| How I felt working with my care partner |
|
||||||
|
|||||||
|
|||||||
|
|||||||
In your own words please can you tell us about your experience of working with your care partner ……, what has been helpful or not? ………………………………
Results of responses for those receiving the intervention in the PARTNERS2 trial
| Item | Total number of responses | Not applicable | Definitely happened | Possibly happened | Didn’t happen | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % (of applicable) |
n | % (of applicable) |
n | % (of applicable) |
|||
| 1. My care partner really listened to and understood me | 81 | 2 | 2.5 | 70 | 88.6 | 7 | 8.9 | 2 | 2.5 | |
| 2. My care partner helped me decide what was best for me | 81 | 3 | 3.7 | 53 | 67.9 | 19 | 24.4 | 6 | 7.7 | |
| 3. My care partner helped me get useful advice about my medication | 81 | 35 | 43.2 | 32 | 69.6 | 10 | 21.7 | 4 | 8.7 | |
| 4. My care partner helped my friends/family know how to help me | 80 | 52 | 65.0 | 16 | 57.1 | 2 | 7.1 | 10 | 35.7 | |
| 5. My care partner made it clear who can help me with what, in a mental crisis | 81 | 6 | 7.4 | 51 | 68.0 | 16 | 21.3 | 8 | 10.7 | |
| 6. My care partner saw me in my GP practice | 81 | 10 | 12.3 | 43 | 60.6 | 2 | 2.8 | 26 | 36.6 | |
| 7. I felt comfortable seeing my care partner in my GP practice | 81 | 28 | 34.6 | 43 | 81.1 | 2 | 3.8 | 8 | 15.1 | |
| 8. My care partner helped me to set ‘goals’ for us to work on together | 81 | 4 | 4.9 | 50 | 64.9 | 15 | 19.5 | 12 | 15.6 | |
| 9. I trusted my care partner and felt positive about working with them | 81 | 3 | 3.7 | 70 | 89.7 | 7 | 9.0 | 1 | 1.3 | |
| 10. My care partner helped me to think about my physical health | 81 | 3 | 3.7 | 49 | 62.8 | 18 | 23.1 | 11 | 14.1 | |
| 11. My care partner helped me to act to improve my physical health | 80 | 7 | 8.8 | 31 | 42.5 | 19 | 26.0 | 23 | 31.5 | |
| 12. I have had my physical health reviewed in the past 12 months | 81 | 3 | 3.7 | 40 | 51.3 | 2 | 2.6 | 36 | 46.2 | |
| 13. My care partner helped me to think about my mental health | 81 | 3 | 3.7 | 69 | 88.5 | 6 | 7.7 | 3 | 3.8 | |
| 14. My care partner helped to act to improve my mental health | 80 | 4 | 5.0 | 56 | 73.7 | 17 | 22.4 | 3 | 3.9 | |
| 15. My care partner made it possible for me to make contact with them when needed | 81 | 3 | 3.7 | 65 | 83.3 | 7 | 9.0 | 6 | 7.7 | |
| 16. My care partner talked through what would happen when the care they provided ended | 79 | 3 | 3.8 | 30 | 39.5 | 19 | 25.0 | 27 | 35.5 | |
| 17. My care partner got in touch with me when I stopped attending appointments | 81 | 62 | 76.5 | 14 | 73.7 | 3 | 15.8 | 2 | 10.5 | |
| 18. My care partner linked me up with the GP | 80 | 48 | 60.0 | 17 | 53.1 | 3 | 9.4 | 12 | 37.5 | |
| 19. My care partner linked me up with the Practice Nurse | 81 | 58 | 71.6 | 6 | 26.1 | 3 | 13.0 | 14 | 60.9 | |
| 20. My care partner linked me up with local services provided by voluntary sector | 81 | 26 | 32.1 | 9 | 16.4 | 10 | 18.2 | 36 | 65.5 | |
| 21. My care partner linked me up with mental health services I didn’t know about, e.g. therapy | 80 | 22 | 27.5 | 13 | 22.4 | 9 | 15.5 | 36 | 62.1 | |
| 22. My care partner linked me up with other services | 79 | 23 | 29.1 | 16 | 28.6 | 2 | 3.6 | 38 | 67.9 | |
| 23. While working with my care partner I felt safe, well and supported by services | 81 | 3 | 3.7 | 58 | 74.4 | 17 | 21.8 | 3 | 3.8 | |
| 24. My care partner helped me to cope when something difficult happened | 80 | 23 | 28.8 | 45 | 78.9 | 10 | 17.5 | 2 | 3.5 | |
| 25. I am happier with the way I spend my time day to day after working with my care partner | 81 | 3 | 3.7 | 41 | 52.6 | 21 | 26.9 | 16 | 20.5 | |
| 26. I think the quality of my life has improved because of the work I did with my care partner | 81 | 4 | 4.9 | 30 | 39.0 | 32 | 41.6 | 15 | 19.5 | |
Contact sheets to document extent of delivery
Contact sheets to document extent of delivery of the PARTNERS intervention were developed during pilot work and adapted for use in the trial. The care partners were trained and supported to complete the contact sheets during the PARTNERS2 trial. These were also included in the PARTNERS manual version 1.8.
Please complete the table below for all contacts with the service user (please note: not all rows need to be completed at every contact).
| Contact | 1st contact | 2nd contact | 3rd contact | 4th contact | 5th contact | 6th contact | 7th contact |
|---|---|---|---|---|---|---|---|
| Date | |||||||
| Mode – phone (P), text exchange (T), face to face (F), letter (L), email exchange (E) | |||||||
| Please complete items below as necessary | |||||||
| SU attended (Y/N) | |||||||
| DNA/cancellation? (D/C) | |||||||
| Location [e.g. home (H), GP practice (GP), other (O)] | |||||||
| Are there any new conditions? (Y/N) | |||||||
| Healthy behaviours supported? (Y/N) | |||||||
| Physical health conditions discussed? (Y/N) | |||||||
| Social problems discussed? (Y/N) | |||||||
| Mental health issues discussed? (Y/N) | |||||||
| SU presentation (activity/engagement/behaviour) recorded? (Y/N) | |||||||
| Goals discussed? (Y/N) | |||||||
| CORE 10 score | |||||||
| Signposting to other services/professionals (Y/N) | |||||||
| Which service/professional signposted to? (See box 2 codes overleaf) | |||||||
| Meeting summarised with SU (Y/N) | |||||||
| Next meeting agreed? (Y/N) | |||||||
| Recorded on primary care notes (Y/N) | |||||||
| Recorded on secondary care notes (Y/N) | |||||||
| Session length (minutes) | |||||||
| Travel time (minutes) | |||||||
| Time taken taking and recording notes (minutes) | |||||||
Communication/liaison about this service user
Please complete after any other service user-related communication (except supervision – which will be recorded on a separate sheet)
| Box 1 codes – activity | Box 2 codes | ||||
|---|---|---|---|---|---|
| 1 = Liaison; 2 = Referral 3 = Other (please specify in table) |
Professional: | Service: | |||
| 1 = GP | 4 = Psychiatrist | 7 = Social Worker | 12 = Third sector (please specify) | ||
| 2 = Nurse | 5= Team manager | 8 = Occupational therapist | 10 = CAB | 13 = Social services (please specify) | |
| 3 = Practice manager | 6 = Support worker/STR worker/HCA | 9 = Other (specify in table) | 11 = Housing services | 14 = Other (please specify) | |
| Communication | 1st | 2nd | 3rd | 4th | 5th | 6th |
|---|---|---|---|---|---|---|
| Date | ||||||
| Type of activity (see box 1) | ||||||
| Service/professional (see box 2) | ||||||
| Mode of communication [phone (P), text (T), face to face (F), email (E), letter (L), if other please specify] | ||||||
| Reason (please add free text) |
Appendix 3 PARTNERS trial: realist process evaluation
The realist process evaluation used qualitative interviews from care partners, supervisors, service users, GPs, secondary care and researchers, observations of sessions, and records of care partner contacts to understand the extent to which each care partner delivered the model as expected. Delivery varied by care partner. Figures 7 and 8 demonstrate visually the extent to which delivery matched model for the two care partners in post the longest.
FIGURE 7.
The extent to which delivery matched model for care partner ‘Grace’. TAR, Tape | Assisted Recall.
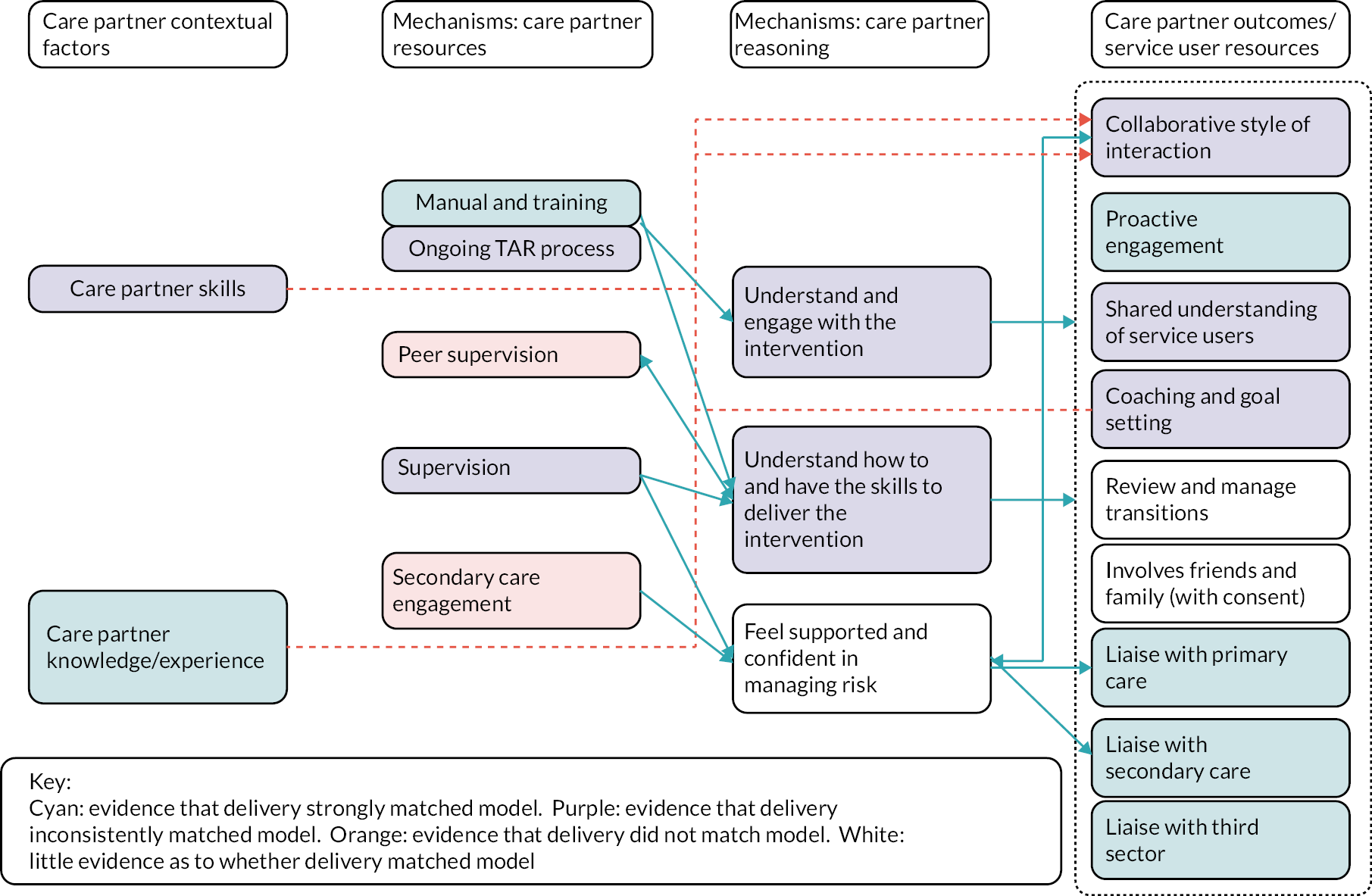
FIGURE 8.
The extent to which delivery matched model for care partner ‘Nora’.
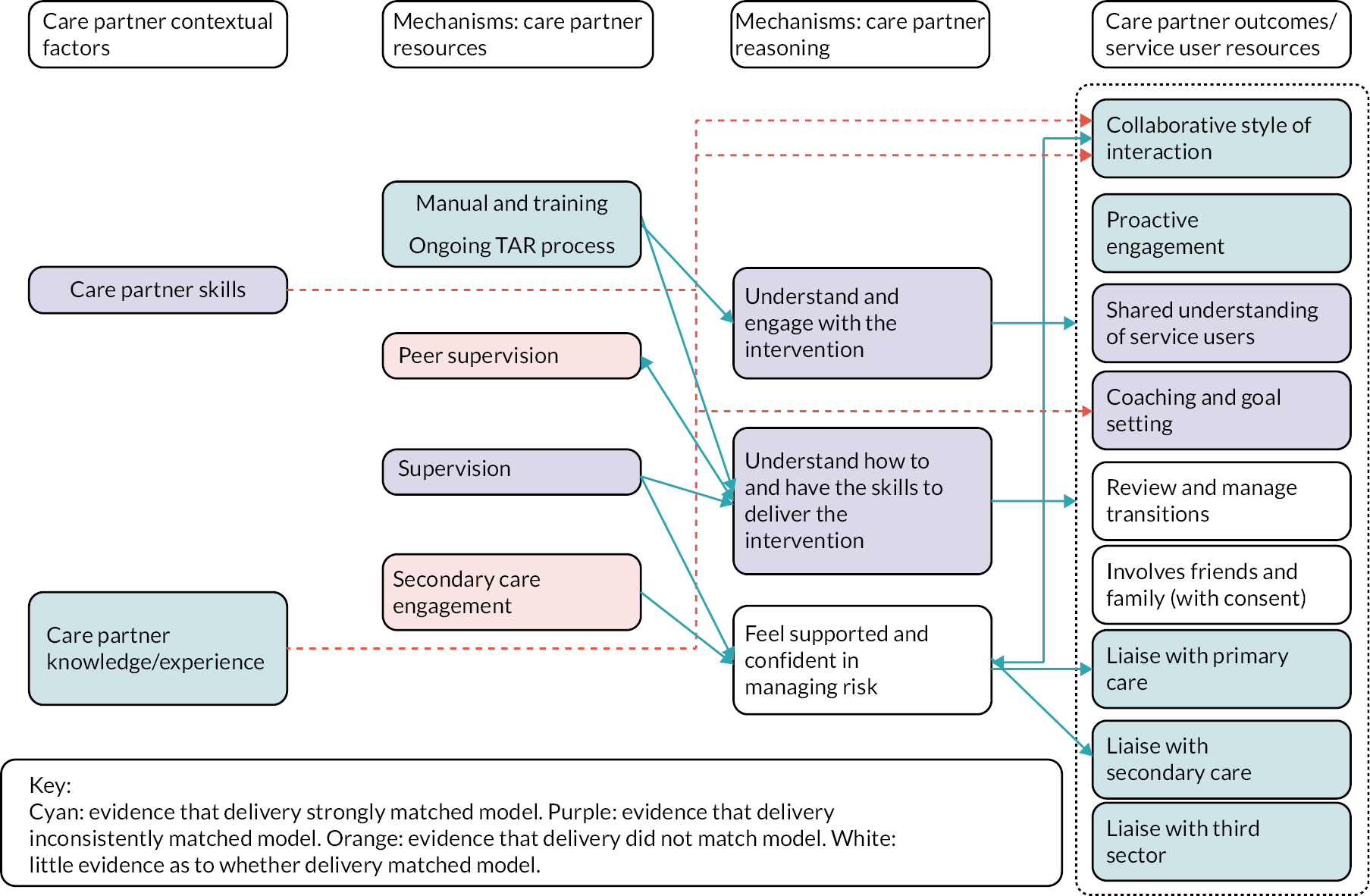
The realist process evaluation allowed us to explore why delivery varies between practitioners and to refine the programme theory regarding how, why and under what circumstances care partners are able to deliver the PARTNERS service. A refinement of this programme theory is represented visually in Figure 9. The main findings included that it takes time for care partners to deliver the model as intended, that care partners either needed existing experience and confidence liaising across hierarchies or support to achieve this, and previous experience working in mental health care could act as a barrier to understanding how and why to work collaboratively with service users.
FIGURE 9.
Refined PARTNERS programme theory: care partner level. CP, care partner; PCP, primary care practitioner; SU, service user.
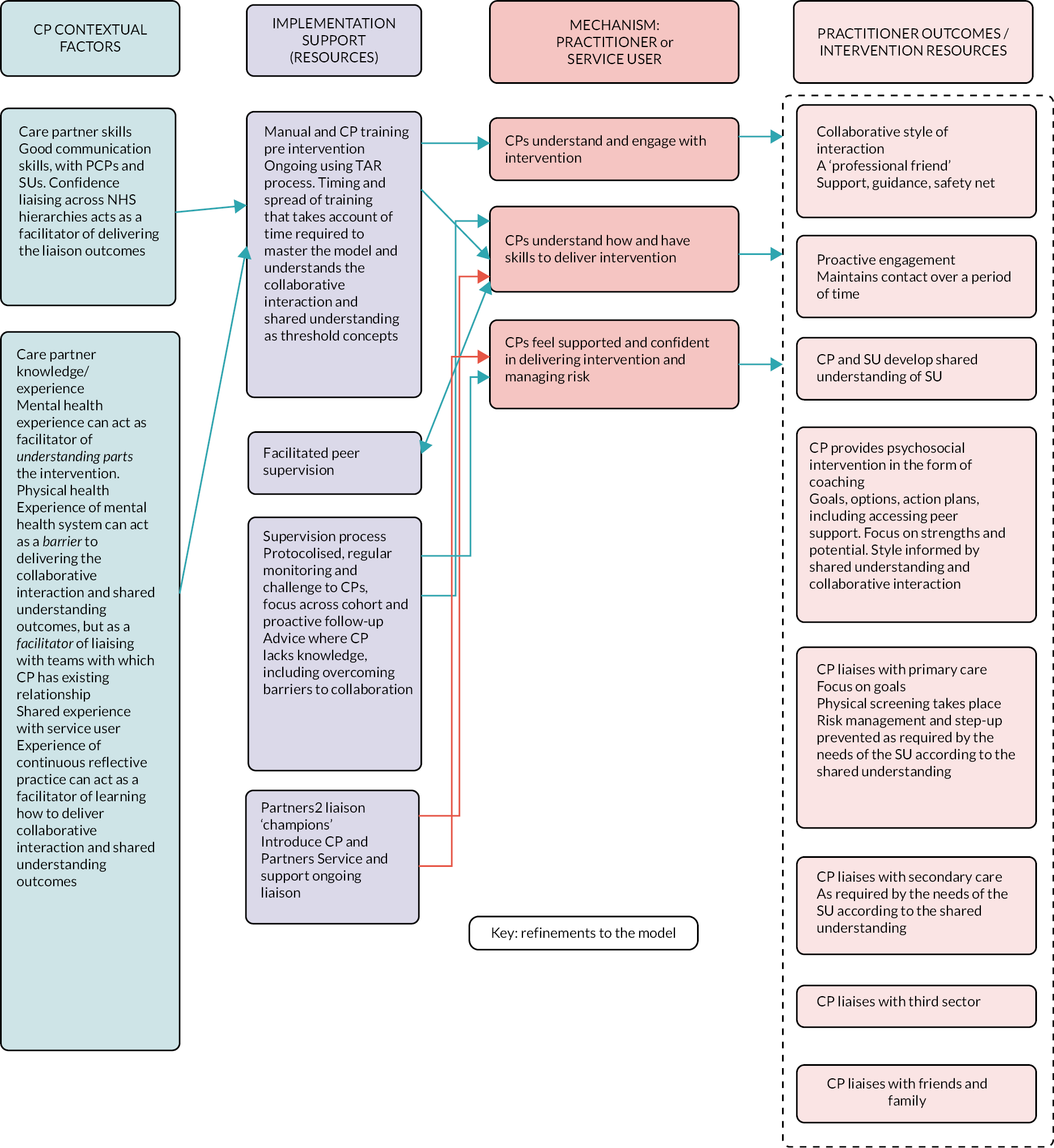
We also used the realist process evaluation to explore how, why and under what circumstances the provision of the PARTNERS service led to the expected changes in service user outcomes. A refinement of this programme theory is represented visually in Figure 10. The findings included that the length of delivery in the context of the trial was insufficient to lead to the expected outcomes; this supports the quantitative trial findings. This was because service users had long-term degraded senses of agency and identity that made it very difficult to complete goal-setting behaviours in the time frame of the trial. Many service users reported a sense of improved confidence and short-term increases in hope, positive identity and agency from working with their care partner. Some service users achieved ‘small’ goals that felt ‘big’ to them: walking to the shop and talking to the shop staff, taking their pet to their volunteering job, allowing themselves to sometimes prioritise their own needs. Service users valued the relationship with the care partner even more than anticipated by the model; seeing them as a ‘professional friend’ who had professional qualifications and with whom they could bounce ideas around, but who did not dictate what they should do.
FIGURE 10.
Refined PARTNERS programme theory: service user level. CP, care partner; SU, service user.
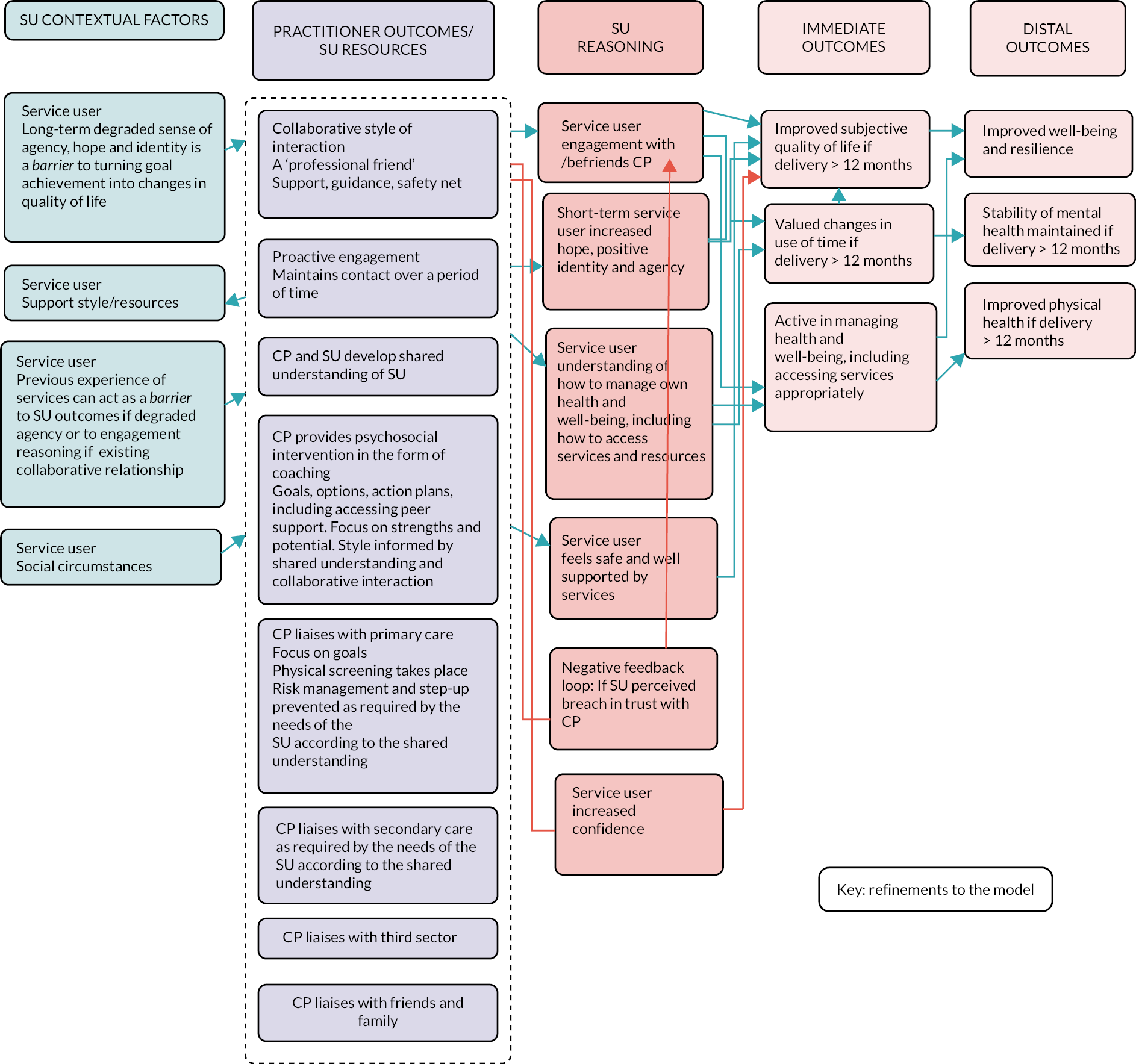
List of abbreviations
- CMHF
- Community Mental Health Framework
- CMHT
- Community Mental Health Team
- COS
- core outcome set
- CRN
- clinical research network
- CTU
- Clinical Trials Unit
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level
- GP
- general practitioner
- ICECAP-A
- ICEpop CAPability
- ICER
- incremental cost-effectiveness ratio
- LEAP
- Lived Experience Advisory Panel
- MANSA
- Manchester Short Assessment of Quality of Life
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SMD
- standardised mean difference
- SMI
- severe mental illness
- SURA
- service user research assistant
- TSC
- Trial Steering Committee
- TUS
- Time Use Survey
- WS
- workstream
- WTPT
- willingness-to-pay threshold
