Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 11/46/09. The contractual start date was in September 2013. The report detailing the set up phase and initial outcomes began editorial review in February 2015 and was accepted for publication in May 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report. Should the study progress further, the full report will be published in the PHR journal.
Declared competing interests of authors
Jennifer Mindell and Rachel Craig are funded by the Health and Social Care Information Centre to run the Health Survey for England.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Fragaszy et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Influenza is a major source of morbidity and mortality every year. Influenza A viruses are the most common type infecting humans and are characterised by the surface glycoproteins haemagglutinin (HA) and neuraminidase. Viral genetic reassortment can result in new influenza subtypes to which there is little or no population-based immunity. If these new strains are additionally transmissible among humans then the strain has pandemic potential.
Serum anti-HA antibodies remain the most consistent correlate of immunity to influenza virus infection. High antibody titres are protective against infection with homologous strains and drifted strains within the same subtype, but there is little evidence of heterologous protection across subtypes.
Mathematical models are used extensively to inform influenza control policies. A recent international consensus document highlighted the key importance of representative serological surveys as the only viable means to infer population-level susceptibility with any accuracy. 1 Measures of population-level susceptibility are central to mathematical models to predict future disease burden and guide response.
However, obtaining representative population samples including information on vaccination is problematic. Many serological studies rely on residual sera from samples taken for clinical purposes or from blood banks, as they can be obtained quickly and often do not require informed consent. 2 However, these samples often have limited clinical or epidemiological data, including vaccine history. 2,3 Surveys using residual clinical samples are biased towards people with chronic illness and therefore towards vaccinated individuals, whereas those using blood banks are biased towards healthy people who are less likely to be vaccinated. 2,3 Absence of information on vaccination is also an important challenge to validity, as serological tests cannot distinguish between naturally acquired infection and vaccination. Direct recruitment of representative population samples with information on vaccination exclusively for the purpose of sero-surveillance is expensive.
The 2009 H1N1 influenza pandemic highlighted limitations of conventional surveillance. Throughout the pandemic, particularly in the early stages, there was a need to assess the severity of disease to determine the proportionality of potentially highly expensive control measures. 4 Reporting of deaths was rapidly implemented but initial estimates of case fatality rates (CFRs) were inappropriately high owing to underestimation of the denominator (number of community cases). For example, the apparent CFR based on internationally reported deaths and laboratory-confirmed cases on the 16 May 2009 was 0.9%, with 95% confidence intervals (CIs) 0.7% to 1.0%. 5 Media reports of case numbers and deaths contributed to a distorted view of severity and political pressure for robust control measures. Attempts were made to identify community case numbers through prospective internet surveys (Europe)6,7 or retrospective telephone surveys (USA). 8,9
These helped to provide better denominator estimates for calculation of CFR. Resulting CFR estimates were considerably lower (0.005–0.04%), but showed approximately 10-fold variations with wide levels of uncertainty. 8–10 Later in the pandemic, a study of residual sera demonstrated that in some areas of the country a high proportion of younger age groups had become infected during the first wave of infection (period of widespread community infection and transmission), which occurred in the summer of 2009. Given the high infection rates, this indicated that the severity must be extremely low. 11 However, the results of this work were not available until around the time of the peak of the second wave (autumn/winter 2009), limiting the impact on policy.
In England, the Medical Research Council-funded Flu Watch study, which had followed community cohorts since 2006, gained additional funding to continue throughout the pandemic. The study was able to measure population rates of infection in vaccinated and unvaccinated individuals, and identified much higher clinical attack rates in the community than the rates observed through surveillance data. 12 These measures of population infection rates and clinical attack rates helped to establish that the true CFR for the pandemic strain was, in fact, very low. The work also demonstrated that most infections were asymptomatic. 12
Despite these successes, the experience of trying to rapidly expand the Flu Watch study in response to the pandemic highlighted the fact that recruitment via primary care was too expensive and too slow to enable rapid establishment of a large national cohort. This limited the study’s ability to provide timely information to inform decision-makers. After ethical permission was obtained, the process still involved recruitment of general practitioner (GP) practices, agreement of contracts with practices, training of practice staff, research and development approval for each practice from all primary care trusts, random selection of households from the practice register, preparation of individualised recruitment letters, sending out of recruitment letters by practices, and finding convenient appointment times for participants. In addition, conduct of the serological work in the National Reference Laboratory, which was already extremely busy providing an effective service response, led to further delays. This experience has prompted us to explore more efficient methods of measuring the community burden of infection and disease, which can be rolled out extremely rapidly.
Conservative estimates of the cost of efforts to control the 2009 swine flu pandemic in the UK are £1.24B. 4 Dame Deirdre Hine’s independent review of the response to the 2009 influenza pandemic highlights that plans ‘did not consider sufficiently the possibility that a pandemic might be far less severe than the one it envisioned’ (p. 47). 4 There was a high level of reliance on modelling but lack of data on the number of cases severely limited the reliability of these models. ‘The major difficulty with producing accurate models was the lack of a “denominator” – a relatively accurate idea of the total number of cases . . . This made calculating the clinical attack rates and the case fatality rates extremely difficult, which in turn made modelling more challenging . . .’ (p. 67). 4 The report states that ‘research into more effective early surveillance could pay considerable dividends through facilitating earlier decisions on scaling responses up or down, and thereby avoiding precautionary expenditure’ (p. 88). 4
Chapter 2 Aims and objectives
The study aims to establish an efficient end-to-end system allowing real-time assessment of population susceptibility, spread of infection and clinical attack rates.
Overall project objectives
-
Develop the Health Survey for England (HSE) as a tool for rapid population-based surveys of influenza infection and influenza-like illness rates.
-
Provide monthly measures of numbers of cases infected and weekly updates on numbers of influenza-like illnesses during the first two infection waves of a pandemic to act as denominators for national estimates of case fatality and hospitalisation rates.
-
Assess spread of the novel influenza strain geographically, by age and through time.
Chapter 3 Methods
Overall project methods
This research project has two components: a pre-pandemic component (currently running) designed to develop and assess a system to monitor population susceptibility, severity and spread of pandemic influenza; and a pandemic component designed to be triggered rapidly in the event of an influenza pandemic.
The monitoring system will
-
collect representative population-level serological specimens with linked clinical and epidemiological data
-
conduct high-throughput serological assays
-
run automated data analysis and reporting programmes.
This will be achieved through a series of cross-sectional serological prevalence studies with retrospective ascertainment of vaccination and respiratory illness history in conjunction with the HSE.
Specimen and data collection
The HSE is series of annual surveys that have monitored the nation’s health since 1991. 13,14 All HSE surveys involve a stratified random probability sample of private residences and have covered the adult population aged ≥ 16 years, with children included every year since 1995. There are two parts to the HSE surveys: an interview visit, during which a trained interviewer collects information on health and health-related behaviours and measures height and weight, and, later, a nurse visit, during which additional measurements and biological samples (saliva, urine and blood for those aged ≥ 16 years) are collected. Each annual survey is planned well in advance with ethical permission obtained for each survey. Blood specimens and data are made available for analysis only after the end of each annual survey, meaning that, without the adaptations agreed for this project, the survey could not normally be used for real-time research.
Phase 1
In response to the pandemic, we included a special ‘swine flu’ section in the 2010 HSE survey, where we asked participants about influenza vaccination uptake and timing, and influenza-like illness, including timing, duration of sick leave and treatment.
Phase 2
For the purposes of this research project, the HSE research nurses collected an additional 5-ml blood sample (for serological analysis) from participants aged ≥ 16 years during the HSE home visits. The samples were transferred to the Newcastle General Hospital microbiology laboratory, which routinely receives and stores HSE samples. Another addition to the normal HSE process was the collection of additional data, recorded on separate forms that were transferred with the specimens. This included basic demographic data, as well as some additional questions on participants’ most recent influenza vaccination and whether or not they experienced a ‘flu-like’ illness in the previous month. These data were collected for all HSE participants (adults and children) regardless of whether or not they had an accompanying blood sample.
Phases 3 and 4
In order to ensure that blood specimens and data would be available immediately in the event of a pandemic, since 2013 this activity has been, and will continue to be, included in the annual HSE ethics application and planning procedures so that it can be rapidly triggered.
Serological analysis
Phase 2
The Newcastle General Hospital laboratory centrifuged specimens on arrival at the laboratory and prepared two aliquots of serum from each sample. Serum samples were frozen at –80 °C and transferred to the University College London Hospitals (UCLHs) microbiology laboratory.
Antibody concentrations in serum samples will be determined using the haemagglutination inhibition assay (HAI). HAI is a rapid, sensitive and inexpensive method for detecting subtype-specific antibody response to influenza A viruses in human sera. The test is based on the ability of the HA protein on the surface of influenza viruses to agglutinate red blood cells (RBCs). Specific attachment of antibody to the antigenic sites on the HA molecule interferes with the binding between the viral HA and sialic receptors on the RBCs. This effect inhibits haemagglutination and is the basis for the HAI. 15
Serial dilutions of sera are mixed with the virus of interest and RBCs. The antibody titres are defined as the reciprocal of the last dilution exhibiting inhibition and are expressed as haemagglutination units. 15
A working aliquot will be made and the original sample kept at –80 °C. Samples will be treated to remove non-specific inhibitors before being tested by HAI in batches of approximately 200 samples per week. Results will be verified by a second operator and logged on to the laboratory management software system.
Part of the pre-pandemic component of the research currently under way is the development of efficient protocols for high-throughput serological capacity to be tested on the 2012–13 sera. We will also develop protocols for rapid development and scale up of serological assays in the event of a novel influenza strain to allow roll-out of testing of HSE serological samples as early as possible.
Project analyses and outcomes by phase
Phase 1 (complete)
The availability of prospectively collected illness data from the Flu Watch study and retrospectively collected data from the same time periods for the 2010 HSE provided an opportunity to evaluate the HSE as a tool for monitoring levels of influenza-like illness. Monthly age-specific rates of illness and proportion vaccinated were calculated in both data sources and compared.
Phase 2 (in progress)
This phase has piloted the collection of specimens and data on recent illness and vaccination from the 2012 HSE and the transfer of these data and specimens to UCLH for further analysis. Serological protocols and assays are under development, as is the automated analysis and reporting of age-specific rates of influenza-like illness, monthly estimates of the age-specific proportion of the population with protective antibodies accounting for vaccination and the proportion vaccinated.
Phase 3 (ongoing)
In the 2013 HSE and onwards, this project was included in the annual HSE ethical approval and planning rounds. This enables the HSE to rapidly trigger the additional data and specimen collection required by the project in the event of a pandemic.
Phase 4 (ongoing)
In the event of a pandemic, the collection and transfer of specimens and data can be triggered within 1 working week. We will use the automated analytical routines developed in phase 2 to continually estimate pandemic severity, susceptibility and spread throughout the course of the pandemic. Reports will be sent fortnightly to Public Health England (PHE), and the estimates they contain can inform key parameters in the pandemic nowcasting and forecasting models. Additionally, these estimates can be used as denominators in measurements of pandemic severity including age-specific rates of influenza hospitalisations and the case fatality proportion. 16 Our findings will additionally be presented to key decision-makers through the researchers’ positions on government advisory committees and links with key policy-makers.
Phase 1: detailed methods
Data sources
Details of the 2010 HSE survey methodology have been described elsewhere. 13,14 The 2010 HSE included a child boost sample (an additional sample of children intended to increase the number in this age group) to increase accuracy of estimates in this age group. In the HSE interview visit, participants were asked if they had experienced a ‘flu-like illness, where you felt feverish and had a cough or sore throat?’ since May 2009. If they had, then the month and year of that illness was also recorded. They were also asked about influenza vaccinations. Since the interviews for the 2010 HSE took place over the 2010 calendar year, the participants would have been recalling illnesses and/or vaccinations anywhere from the past 9 months (those interviewed in January 2010) to the past 21 months (those interviewed in the December 2010). If the participant had more than one illness during the time period, they chose which one to report.
The comparison data came from the Flu Watch study, a household-based community cohort study of influenza in England. The study has been described in detail elsewhere. 12 In brief, individuals were randomly selected from participating GPs’ practice registers and their household was invited to participate in the Flu Watch study. The study took place every winter season and throughout the pandemic between 2006 and 2011, but for the purposes of this analysis we used data collected on illnesses occurring between May 2009 and February 2010, the same months for which the 2010 HSE collected illness data. All individuals in the participating households were contacted weekly to identify episodes of respiratory illness and influenza vaccination. Respiratory illnesses that included symptoms of cough and/or sore throat plus either a confirmed fever of ≥ 37.8 °C or a feeling of ‘feverishness’ were considered episodes of illness as that was the closest approximation to the HSE question about previous ‘flu-like’ illness.
The national influenza vaccination uptake estimates were taken from the Department of Health and the Health Protection Agency annual reports of influenza vaccine uptake. 17,18 In England, the 2009–10 seasonal influenza vaccine was offered to all of those aged ≥ 65 years and those patients aged 6 months to < 65 years in a clinical risk group. Carers who are in receipt of a carer’s allowance or are the main carer for an elderly or disabled person are also recommended to be offered seasonal influenza vaccine. 18
When the pandemic vaccine became available on 21 October 2009, it was initially offered to individuals aged 6 months to 65 years in the current seasonal influenza vaccine clinical risk group, all pregnant women, household contacts of immunocompromised individuals, and all of those aged ≥ 65 years who were also in the current seasonal vaccine clinical risk groups. In December 2009, the pandemic vaccination programme was expanded to healthy children aged from 6 months up to 5 years. 17
Statistical analysis
Statistical analyses were conducted in Stata statistical software, version 13 (StataCorp LP, College Station, TX, USA). Having an episode of illness during the month was the binary outcome variable used in the analysis. Episodes of illness were recorded by the month in the HSE and by the week in Flu Watch. For comparability, we collapsed the Flu Watch data by the month and created an analogous binary outcome variable for any illness that month.
We used Poisson regression to calculate monthly age-specific rates of illness separately for each data set in three broad age groups (0–15 years, 16–64 years and ≥ 65 years). Owing to the small number of events in some months, we were unable to stratify the monthly rates by more than three age categories. These regressions accounted for the studies’ sampling designs and survey response weights. To further investigate patterns of illness by age group, we calculated seasonal age-specific rates of illness in each data set during the first two waves of the pandemic for nine age groups (0–4 years, 5–15 years, 16–24 years, 25–34 years, 35–44 years, 45–54 years, 55–64 years, 65–74 years, ≥ 75 years) using the same regression techniques. We then compared these seasonal rates of illness between the two studies by calculating age-specific rate ratios in these nine age groups using Poisson regression, and graphed the results.
We hypothesised that the HSE seasonal illness rates during the first two waves of the pandemic might differ depending on the time of year the interview took place as a result of recall bias and/or the increasing possibility of multiple illnesses accruing as the follow-up time period increases. To investigate this we calculated age-specific rate ratios of illnesses occurring during the first two waves of the pandemic, comparing participants interviewed in the first quarter of 2010 with those interviewed in the second, third and fourth quarters of 2010, using Poisson regression and Wald tests.
We also calculated the month- and age-specific proportion vaccinated with any type of influenza vaccine (seasonal or pandemic) over the second wave of the pandemic (autumn–winter 2009–10) in the two data sets and compared these graphically to each other and to national estimates of seasonal and pandemic vaccine uptake.
In phases 2 and 4, the most important function of the vaccination history questions will be to inform the interpretation of individuals’ serology results; however, we will also take advantage of the available data and calculate the age-specific proportion vaccinated. We will not be able to use the HSE probability weights in these real-time analyses, as the weights will not be calculated until after the HSE data collection period has ended. In order to investigate whether or not our real-time estimate of vaccine uptake will be biased by the fact that we cannot account for the study design and response rates, we calculated the vaccine uptake proportions with and without these weights and compared graphically.
Chapter 4 Results
Phase 1: results
Table 1 describes the age breakdown of participants in the 2010 HSE and the 2009–10 Flu Watch study. The HSE interviewed a total of 14,112 individuals. There were 5692 children interviewed, of which 3618 came from the child boost sample. Flu Watch had 4452 participants, approximately 680 of which were followed up over the summer of 2009 (and the rest were recruited later in the autumn and winter).
| Age group (years) | HSE | Flu Watch | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 0–4 | 1611 | 11 | 235 | 5 | 1846 | 10 |
| 5–15 | 4081 | 29 | 638 | 14 | 4719 | 25 |
| 16–24 | 855 | 6 | 236 | 5 | 1091 | 6 |
| 25–34 | 1188 | 8 | 281 | 6 | 1469 | 8 |
| 35–44 | 1463 | 10 | 579 | 13 | 2042 | 11 |
| 45–54 | 1499 | 11 | 610 | 14 | 2109 | 11 |
| 55–64 | 1365 | 10 | 916 | 21 | 2281 | 12 |
| 65–74 | 1084 | 8 | 672 | 15 | 1756 | 10 |
| ≥ 75 | 966 | 7 | 284 | 6 | 1250 | 7 |
| Total | 14,112 | 100 | 4451 | 100 | 18,563 | 100 |
Survey response in the 2010 HSE is described elsewhere. 19 In brief, at the household level, 66% of eligible households from the general population sample and 70% of eligible households from the boost sample were interviewed. Interviews were obtained with 86% of adults and 93% of children in participating households. 19 In Flu Watch, roughly 10% of invited households participated in the cohort study. 12
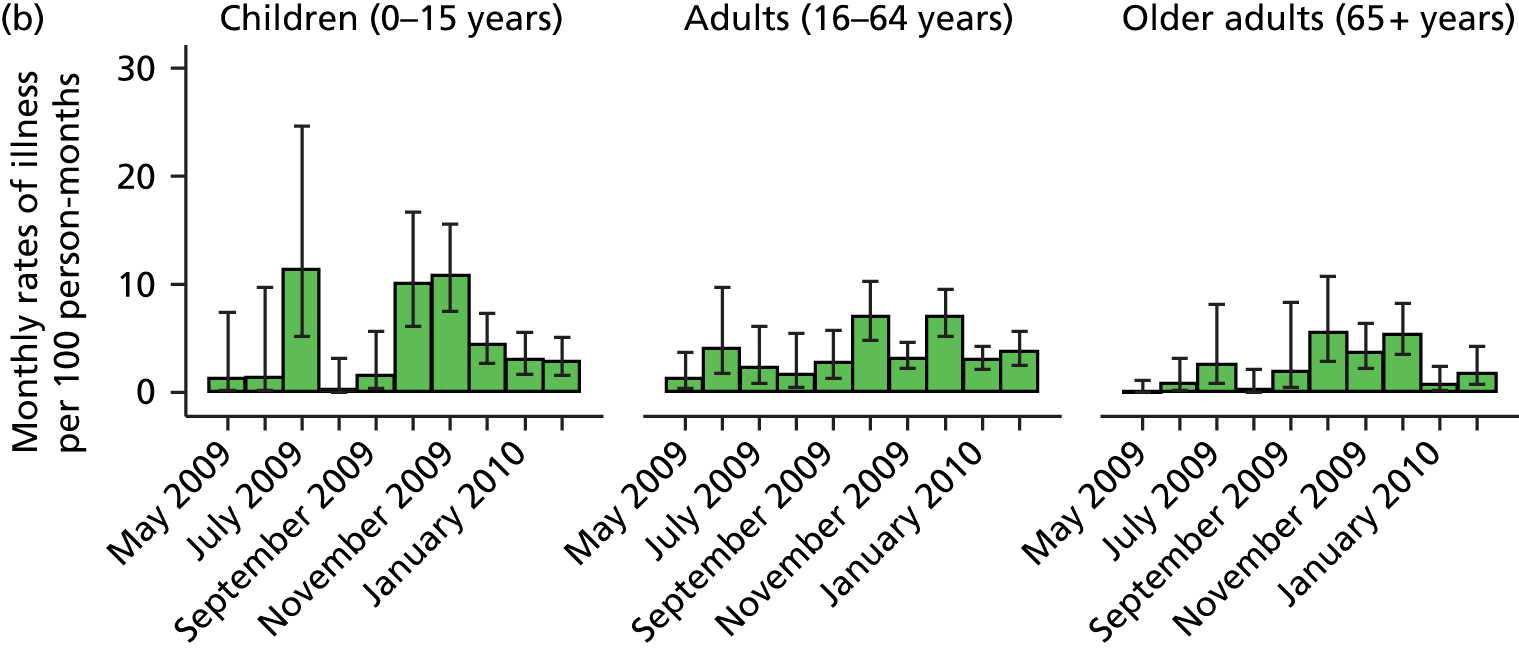
The illness rates seen in the Flu Watch study were generally much higher than those reported in the HSE (Figure 1 and Table 2). Illness rates were highest in children and young adults, and gradually decreased as age group increased. The general patterns of illness by age group were similar between the two studies in the older children and adult population, but less so in the youngest (0–4 years) and oldest (≥ 65 years) age groups where the Flu Watch study captured much higher rates than the HSE (see Table 2 and Figures 1 and 2). Both studies show the characteristic two-wave pattern of the UK pandemic experience, with rates peaking during the summer of 2009 (wave 1) and again in October and November 2009 (wave 2).
FIGURE 1.
Monthly rates of illness and 95% CIs by three age groups and study. Note differences in scales between the two studies. (a) HSE; and (b) Flu Watch.

| Age group (years) | Flu Watch | HSE | ||||
|---|---|---|---|---|---|---|
| Rate | LCI | UCI | Rate | LCI | UCI | |
| 0–4 | 8.28 | 5.79 | 11.85 | 0.70 | 0.57 | 0.86 |
| 5–15 | 4.38 | 3.33 | 5.78 | 0.80 | 0.70 | 0.92 |
| 16–24 | 6.36 | 4.61 | 8.76 | 0.97 | 0.76 | 1.25 |
| 25–34 | 4.39 | 3.10 | 6.23 | 0.80 | 0.64 | 0.99 |
| 35–44 | 5.77 | 4.00 | 8.33 | 0.64 | 0.52 | 0.79 |
| 45–54 | 3.99 | 2.86 | 5.56 | 0.53 | 0.42 | 0.66 |
| 55–64 | 3.48 | 2.80 | 4.32 | 0.40 | 0.31 | 0.53 |
| 65–74 | 3.00 | 2.05 | 4.39 | 0.17 | 0.11 | 0.27 |
| ≥ 75 | 1.99 | 1.13 | 3.47 | 0.20 | 0.12 | 0.32 |
FIGURE 2.
Age-specific rate ratios and associated CIs comparing Flu Watch with HSE seasonal rates of illness during the first two waves of the pandemic.

We found evidence that the rates of illnesses reported as occurring during the first two waves of the pandemic were associated with the timing of the participant interview. In general, the later in 2010 the interview took place, the lower the rates of illness reported during the first two waves of the pandemic (Table 3). There was strong evidence of the association between the rates of illness and interview quarter in children and adults (both p-values of < 0.001). The stratum-specific rate ratios in the elderly were in the right direction; however, there was no statistical evidence to support the association (p = 0.610).
| Interview quarter | Rate ratio | 95% CI | p-value |
|---|---|---|---|
| Children (0–15 years) | |||
| Q1 | 1 | < 0.001 | |
| Q2 | 1.05 | 0.78 to 1.41 | |
| Q3 | 0.86 | 0.63 to 1.19 | |
| Q4 | 0.42 | 0.29 to 0.63 | |
| Adults (16–64 years) | |||
| Q1 | 1 | < 0.001 | |
| Q2 | 1.04 | 0.77 to 1.41 | |
| Q3 | 0.73 | 0.53 to 0.99 | |
| Q4 | 0.40 | 0.28 to 0.58 | |
| Older adults (≥ 65 years) | |||
| Q1 | 1 | 0.610 | |
| Q2 | 0.54 | 0.21 to 1.41 | |
| Q3 | 0.79 | 0.34 to 1.84 | |
| Q4 | 0.63 | 0.25 to 1.60 | |
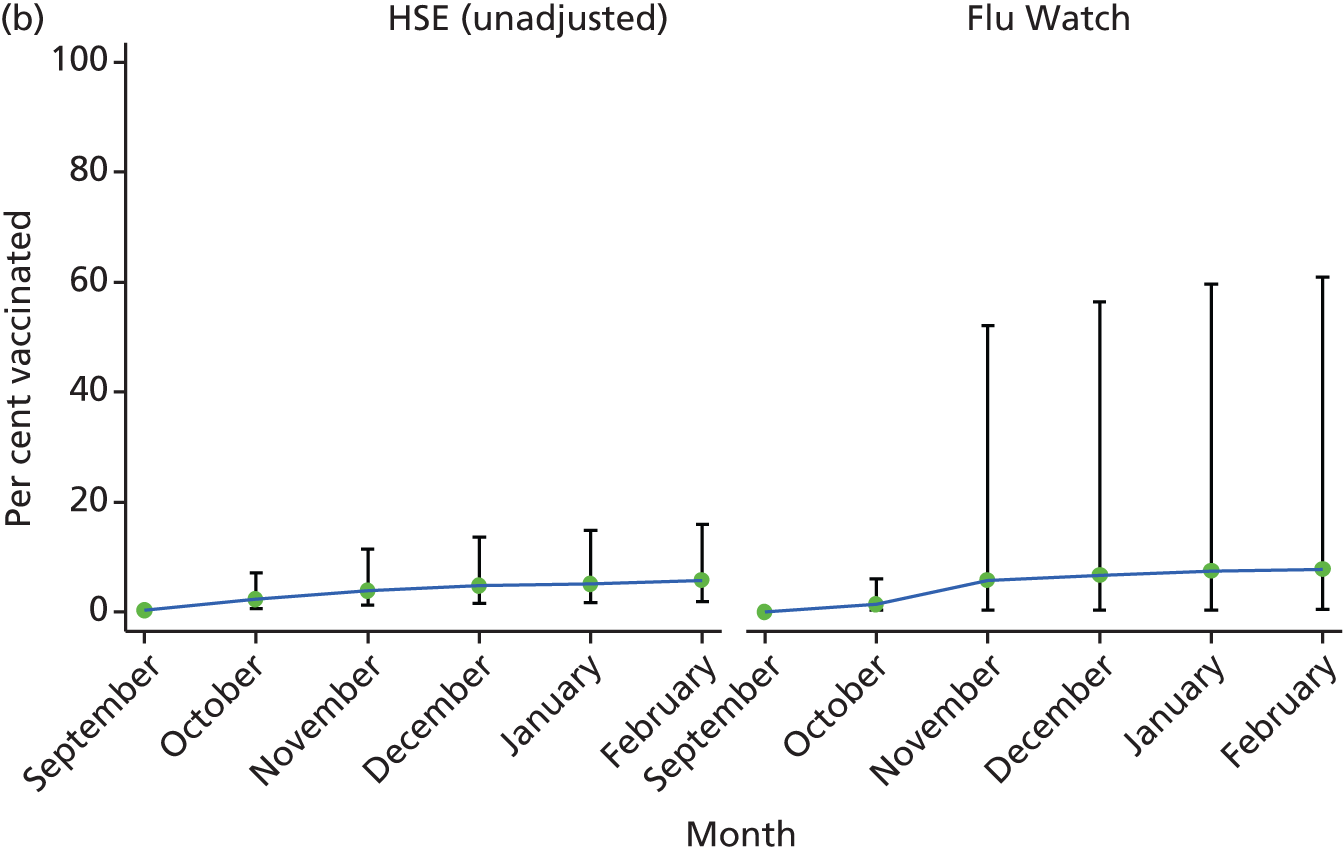
Uptake of any influenza vaccine by the end of February 2010 in the HSE was 14% (95% CI 2% to 55%) in children aged 0–4 years in the survey adjusted analysis and 17% (95% CI 3% to 59%) in the unadjusted analysis (Figure 3a). The CIs for these estimates were wide because of the small number of individuals in this age group and both estimates include the 24% national pandemic vaccine uptake estimate in those aged 6 months to < 5 years (measured at the end of March 2010)17 and the Flu Watch estimate of 40% (95% CI 2% to 96%). Both the HSE and Flu Watch data clearly show an increase in vaccination starting in December 2009, corresponding with the extension of the pandemic vaccination programme to include healthy children aged 6 months to < 5 years of age. 17
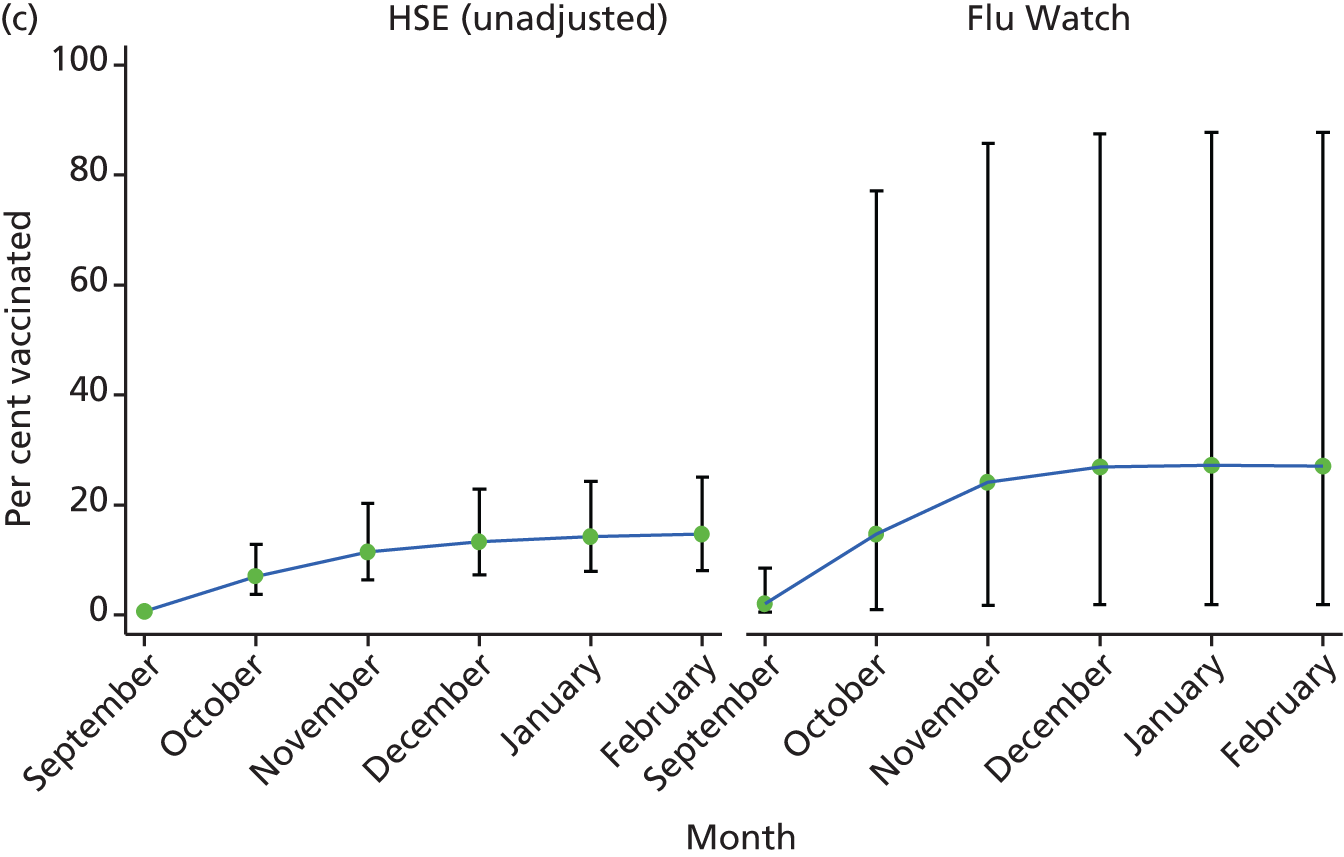
The HSE estimate in the older adults aged ≥ 65 years was 63% for both the adjusted and unadjusted analysis with 95% CIs of 44% to 78% and 48% to 76%, respectively (see Figure 3d). These CIs include the 72% national estimate for seasonal vaccine uptake in this age group. 18 The Flu Watch estimates for any influenza vaccination this age groups was 81% (95% CI 40% to 96%).
The age-specific time trends for vaccine uptake were similar in the HSE and Flu Watch data (see Figures 3a–d). The adjustment for study design and non-response weights in the HSE data set made a negligible difference to the point estimates and CIs regardless of age group or month. The Flu Watch vaccine uptake estimates had much wider CIs than the HSE estimates.
FIGURE 3.
Monthly proportion vaccinated and 95% CIs by age group and study. Vaccine uptake in (a) children aged 0–4 years; (b) children aged 5–15 years; (c) adults aged 16–64 years; and (d) adults aged ≥ 65 years.


Phases 2 and 3: initial results
Samples and data collected
Illness data and serological samples from 2018 participants have been collected as part of the 2012 and 2013 HSE and transferred to UCLH (Table 4).
| Month | Age group (years) | Total | |
|---|---|---|---|
| 16–64 | ≥ 65 | ||
| October 2012 | 270 | 90 | 360 |
| November 2012 | 275 | 116 | 391 |
| December 2012 | 126 | 63 | 189 |
| January 2013 | 307 | 87 | 394 |
| February 2013 | 246 | 81 | 327 |
| March 2013 | 246 | 111 | 357 |
In the 2013 HSE and onwards, this project has been included in the annual HSE ethics and planning rounds. A slight change to the illness data collection was included in the 2014 HSE and onwards. In the 2012 and 2013 HSE, the recent illness and vaccination questions asked were recorded on the blood sample dispatch notes that accompanied the blood samples to the Newcastle laboratories and then transferred to UCLH, allowing real-time access to both. Children are not asked to provide blood samples, although they are asked to provide other types of samples, such as saliva. In order to expand the age range of participants providing illness and vaccination data, these questions were added to all sample dispatch notes, not just the blood dispatch notes.
Chapter 5 Discussion
Phase 1
The HSE data showed the same broad patterns by age and over time as the Flu Watch data, but the overall magnitude of influenza-like illness was greatly underestimated. The large differences in illness rates between the HSE and the Flu Watch study highlight some of the challenges of retrospectively collecting population-level data on acute illnesses using a cross-sectional study design. The Flu Watch study was designed to accurately estimate the community burden of illness by prospectively collecting data through active weekly follow-up of a population-based cohort. For this reason, it is considered the ‘ground truth’ to compare against the HSE data. The ‘flu-like’ illness questions in the HSE underestimate the ground truth for a number of reasons. The questions asked about only one previous episode of illness, therefore missing any further respiratory illnesses that may have occurred in that same influenza season (allowing for multiple illnesses in Flu Watch data increases overall seasonal illness rates during the pandemic by about 2%). This may explain why in the youngest age group the HSE more severely underestimated the illness rates compared with the Flu Watch study, although it would not readily explain why the same thing happened in the oldest age groups. Recall bias was also likely to play a factor, as participants were asked to recall illnesses occurring many months previously, some of which may have been relatively mild. This may better explain the HSE’s more severe underestimation of rates in the oldest age groups, as their illnesses are, on average, milder and less likely to lead to a fever compared with younger adults, and, as a result, these illnesses may be less memorable many months later and subject to greater recall bias. Additionally, as the 2010 HSE interview schedule continued into the autumn and winter, a new influenza season had begun, thus some individuals began reporting the most recent illness instead of the illnesses occurring during the first two waves of the pandemic (our primary time period of interest).
To overcome the limitations of measuring acute respiratory disease incidence using a cross-sectional study design, we are taking the following two complementary approaches.
The first approach is to change the way we ask about respiratory illnesses in order to limit recall bias and reduce the chance of multiple illnesses episodes over the reporting period. In phases 2–4 of this study, the ‘flu-like’ illness questions collect data on illnesses that occurred only in the past month, thus minimising recall bias and the likelihood of having multiple episodes of illnesses during that time period. Although shorter time periods could be used, this would reduce the number of events reported and therefore decrease the precision of the estimates.
Another approach we are investigating is to invite HSE participants into a follow-on Flu Watch-like cohort designed to prospectively collect illness data after the HSE interview. This could potentially provide an opportunity for participants to submit nasal swabs during future respiratory illnesses for virological confirmation of influenza and other respiratory viruses. This virological confirmation would greatly enhance the value of the influenza-like illnesses data by addressing one of its main limitations, the lack of specificity to influenza.
Many respiratory viruses can cause influenza-like illness, not just the influenza virus. The proportion of influenza-like illnesses that are due to influenza will vary over time, depending on how much influenza is circulating and the types and rates of other viruses circulating at the same time. The proportion may also vary by age and by influenza strain. For example, H3N2 strains are sometimes thought to be more severe than H1N1 strains. 12 As we are unable to predict these parameters in a future pandemic situation, we have not tried to account for them in this analysis nor will we account for them in our automated analyses in an attempt to estimate the number of influenza-like illnesses caused by influenza viruses. In the event of a pandemic, we will be sharing our results with PHE, who may include our findings in their weekly influenza surveillance reports. This would enable other researchers to use our data and adjust for these factors accordingly.
The retrospective reporting of influenza vaccination was more accurate than the retrospective reporting of illnesses. The 95% CIs for the HSE vaccine uptake estimates included the national point estimates in the age group at which vaccines were routinely offered and also often included the Flu Watch point estimates. This is probably owing to the fact that the HSE provides a ‘gold standard’ representative population sample. The vaccine uptake in those aged < 5 years was largely driven by pandemic vaccine uptake, as only at-risk children in this age group would have been offered seasonal vaccination. This is also evidenced by the boost in vaccine uptake occurring at the time of the extension of the pandemic vaccination programme to all healthy children in this age group. The high vaccine uptake seen in all age groups in the Flu Watch may be due to selection bias, for which those who agreed to participate in the time intensive Flu Watch study were more health conscious and thus more likely to get vaccinated than the general population, or a ‘priming’ effect of participating in Flu Watch increasing the parents’ awareness and/or views of salience of the influenza vaccination when offered this.
The differences in the HSE vaccine uptake estimates produced with and without adjustment for sampling design and non-response weights were almost identical, indicating that an unadjusted analysis of vaccine uptake during a pandemic would give fairly representative estimates.
Phases 2 and 3
The collection and transfer of samples and data from the 2012–13 HSE is complete. As the laboratory and statistical analysis of phase 2 continues, it is becoming clear that this pre-pandemic work will be crucial for a rapid, efficient and accurate research project should a pandemic arise. Having a test run for these systems allows the surfacing and resolution of any issues that inevitably arise when planning research on an unknown pandemic influenza virus arising at an unknown date. It also gives time to build links with the relevant researchers and stakeholders who would be involved in a future pandemic response. The preparatory work maximises the opportunity to provide timely results and inform the pandemic response.
The main limitation of the serological portion of this study is the lack of blood samples from children aged < 16 years. We could potentially overcome this limitation with the use of saliva samples (collected for this age group in the HSE) if methods to detect influenza antibodies in saliva become available. An additional limitation of serology data is that a small proportion of individuals do not elicit an antibody response when infected with influenza. In Flu Watch data, this proportion was relatively small [87.5% of individuals with laboratory-confirmed influenza A viral shedding (determined through polymerase chain reaction) had a fourfold rise in antibody titres, a further 1.4% had a twofold rise, and only 11.1% had no titre rise]. 12 However, there is evidence suggesting that the proportion of infections that lead to little or no antibody response may vary by virus subtype and season, therefore, a simple inflation based on previous estimates would be inappropriate. 20 We will acknowledge these limitations in our automated reports.
Chapter 6 Conclusions
We have demonstrated the feasibility of efficiently collecting large-scale representative population-level data and blood samples in real time during an influenza season, and we have established mechanisms to rapidly trigger this system in the event of a pandemic. Ongoing preparatory work includes protocols for development, and roll-out of serological assays for a novel influenza virus and automated processes for data analysis and reporting. Our phase 1 work has also demonstrated the importance of minimising recall bias in illness reporting by limiting recall periods. It has given us ideas of how we might improve measurement of respiratory disease incidence by building on the current study. It has also demonstrated the accuracy of retrospective reporting of vaccination and necessary unadjusted analyses of vaccine uptake.
Acknowledgements and disclaimer
Contribution of authors
Andrew C Hayward (Professor of Epidemiology) was principal investigator on the grant funding the work, and Ellen B Fragaszy (Research Associate, Epidemiology), Jennifer Mindell (Reader in Public Health, Epidemiology), Rachel Craig (Research Director of HSE), Judith Breuer (Professor of Virology) and Michael Kidd (Consultant Clinical Scientist/Honorary Senior Lecturer, Virology) were co-applicants.
Andrew C Hayward, Ellen B Fragaszy, Jennifer Mindell, Rachel Craig, Judith Breuer, Michael Kidd and Mark Quinlivan (Healthcare Scientist, Virology) contributed to the study design of the overall project.
Andrew C Hayward and Ellen B Fragaszy led the epidemiological and statistical side of the project.
Jennifer Mindell and Rachel Craig led the HSE side of the project and ensured the incorporation of the research project into the HSE ethics application and planning procedures.
Judith Breuer and Michael Kidd led the laboratory side of the project and the development of the serological methods and protocols with Mark Quinlivan and Stephanie Hutchings (Research Scientist, Virology).
Ellen B Fragaszy and Andrew C Hayward designed the phase 1 statistical analysis with input from Jennifer Mindell.
Ellen B Fragaszy conducted the statistical analyses.
Ellen B Fragaszy wrote the report with contributions from Andrew C Hayward and Mark Quinlivan.
All authors made contributions to manuscript review and approved the final version.
Disclaimer
No funder was involved in the design of the study, analysis of the data, the decision to publish it, or the wording of this report.
Data sharing statement
The 2010, 2012 and 2013 Health Survey For England data sets are available on the UK Data Archive at the University of Essex (http://discover.ukdataservice.ac.uk/series/?sn=2000021).
Data from the Flu Watch study are not publically available, but data sharing through strategic collaborations are welcome. Please contact the corresponding author for more information.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health.
References
- Van Kerkhove MD, Asikainen T, Becker NG, Bjorge S, Desenclos JC, dos Santos T, et al. Studies needed to address public health challenges of the 2009 H1N1 influenza pandemic: insights from modeling. PLOS Med 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000275.
- Broberg E, Nicoll A, Amato-Gauci A. Seroprevalence to influenza A(H1N1) 2009 virus – where are we?. Clin Vaccine Immunol 2011;18:1205-12. http://dx.doi.org/10.1128/CVI.05072-11.
- Hardelid P, Andrews NJ, Hoschler K, Stanford E, Baguelin M, Waight PA, et al. Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14550-03.
- Hine DD. The 2009 Influenza Pandemic. An Independent Review of the UK Response to the 2009 Influenza Pandemic. London: Cabinet Office; 2010.
- Global Alert and Response . Influenza A(H1N1) – Update 30 n.d. www.who.int/csr/don/2009_05_16/en/ (accessed 16 February 2015).
- Brooks-Pollock E, Tilston N, Edmunds WJ, Eames KT. Using an online survey of healthcare-seeking behaviour to estimate the magnitude and severity of the 2009 H1N1v influenza epidemic in England. BMC Infect Dis 2011;11. http://dx.doi.org/10.1186/1471-2334-11-68.
- Paolotti D, Carnahan A, Colizza V, Eames K, Edmunds J, Gomes G, et al. Web-based participatory surveillance of infectious diseases: the Influenzanet participatory surveillance experience. Clin Microbiol Infect 2014;20:17-21. http://dx.doi.org/10.1111/1469-0691.12477.
- Hadler JL, Konty K, McVeigh KH, Fine A, Eisenhower D, Kerker B, et al. Case fatality rates based on population estimates of influenza-like illness due to novel H1N1 influenza: New York City, May–June 2009. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0011677.
- Presanis AM, De Angelis D, Hagy A, Reed C, Riley S, . New York City Swine Flu Investigation T . The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000207.
- Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b5213.
- Baguelin M, Hoschler K, Stanford E, Waight P, Hardelid P, Andrews N, et al. Age-specific incidence of A/H1N1 2009 influenza infection in England from sequential antibody prevalence data using likelihood-based estimation. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0017074.
- Hayward AC, Fragaszy EB, Bermingham A, Wang L, Copas A, Edmunds WJ, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med 2014;2:445-54. http://dx.doi.org/10.1016/S2213-2600(14)70034-7.
- Mindell J, Biddulph JP, Hirani V, Stamatakis E, Craig R, Nunn S, et al. Cohort profile: the health survey for England. Int J Epidemiol 2012;41:1585-93. http://dx.doi.org/10.1093/ije/dyr199.
- Craig R, Mindell J. Health Survey for England 2010. Volume 2: Methods and Documentation. Leeds: The NHS Information Centre for Health and Social Care; 2011.
- Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol 2009;16:558-66. http://dx.doi.org/10.1128/CVI.00368-08.
- Garske T, Legrand J, Donnelly CA, Ward H, Cauchemez S, Fraser C, et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2840.
- Monica Sethi RP. Pandemic H1N1 (Swine) Influenza Vaccine Uptake Amongst Patient Groups in Primary Care in England 2009/10. London: Department of Health and the Health Protection Agency; 2010.
- Fateha Begum RP. Seasonal Influenza Vaccine Uptake Among the 65 years and Over and Under 65 Years at Risk in England. London: Department of Health and the Health Protection Agency; 2010.
- Craig R, Mindell J. Health Survey for England 2010. Volume 1: Respiratory Health. Leeds: The NHS Information Centre for Health and Social Care; 2011.
- Cauchemez S, Horby P, Fox A, Mai le Q, Thanh le T, Thai PQ, et al. Influenza infection rates, measurement errors and the interpretation of paired serology. PLOS Pathog 2012;8. http://dx.doi.org/10.1371/journal.ppat.1003061.
Appendix 1 Protocol
Project title
Pandemic Influenza: population susceptibility, severity and spread. Rapid research using the Health Survey for England (HSE)
Planned investigation
Study aim
The study aims to draw on our experience of large-scale community influenza studies, community surveys, surveillance and serological assays to establish an efficient system allowing real-time assessment of population susceptibility, spread of infection and clinical attack rates.
Research objectives
-
To develop the HSE as a tool for rapid population based surveys of influenza infection and influenza-like illness rates (Phases 1–3).
-
To provide monthly measures of numbers of cases infected and weekly updates on numbers of influenza-like illnesses during the first two waves of a pandemic to act as denominators for national estimates of case fatality and hospitalisation rates (Phase 4).
-
To assess spread of the novel influenza strain geographically, by age, and through time (Phase 4).
Design
Serial cross-sectional serological prevalence surveys with retrospective ascertainment of vaccination and respiratory illness history in conjunction with the National Health Survey for England (HSE).
Setting
Community household setting across England.
Target population
Households participating in the National Health Survey for England (HSE).
Inclusion criteria
All participants participating in the HSE who have a household interview date between 1 October 2012 and 31 March 2013.
Exclusion criteria
None.
Technology being assessed
Use of HSE as a rapid means of determining population-level immunity, severity and spread during the first two waves of a pandemic.
Proposed outcome measures
Antibody response to influenza strain of interest in vaccinated and unvaccinated individuals. Measurement of population rates of clinical flu-like illness. National hospitalisation and case fatality rates.
List of abbreviations
- CFR
- case fatality rate
- CI
- confidence interval
- GP
- general practitioner
- HA
- haemagglutinin
- HAI
- haemagglutination inhibition assay
- HSE
- Health Survey for England
- PHE
- Public Health England
- RBC
- red blood cell
- UCLH
- University College London Hospital