Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 12/153/52. The contractual start date was in March 2014. The final report began editorial review in March 2015 and was accepted for publication in July 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Policy context
A number of agencies and countries, including the World Health Organization (WHO) and the Department of Health (DH) for England, have set a clear agenda for the future of public health. This agenda is focused on improving the healthy life expectancy of the population and, where possible, reducing or removing threats to this aim. 1,2 One strand within this agenda is to create accessible, multidisciplinary networks of public health professionals who work within communities and provide services to address key public health issues, health inequalities, and ultimately improve health and well-being. Worldwide, community pharmacies may be an important component of this agenda; WHO acknowledges that community pharmacies and their staff are easily accessible and, as such, could play a key role in public health initiatives. Interventions that aim to reduce obesity, smoking rates and alcohol misuse, led by community pharmacists and other service providers, have been identified by the DH as public health priorities. 3,4 Indeed, it is thought that the key characteristic through which community pharmacy-based public health interventions may have a positive impact on health equity relates to their access and acceptability.
Excessive alcohol intake, smoking and obesity are three of the most significant modifiable risk factors for morbidity and mortality in middle- and high-income countries. 5,6 Conditions that are caused or exacerbated by these risk factors include cardiovascular disease, type 2 diabetes mellitus, liver disease and lung cancer. Socioeconomic inequalities in the prevalence and treatment of these conditions are major contributors to overall inequalities in health and well-being.
The number of alcohol-related deaths in the UK is increasing and has almost doubled since 1991; higher rates of excessive alcohol intake and alcohol-related deaths are reported in those living in areas of social deprivation. 7 In addition, for men in unskilled low-paid occupations, the rate of alcohol-related mortality is around 3.5 times greater than in those in managerial and professional occupations. For women, this figure is even higher, with those in unskilled low-paid occupations at around 5.7 times greater risk of alcohol-related mortality than those in managerial and professional occupations. 8 In the UK, the highest number of preventable deaths are attributable to smoking,9 with approximately half of all life-long smokers dying prematurely, losing on average about 10 years of life. 10 It is estimated that up to 86,500 preventable deaths each year can be attributed to smoking in the UK. 11 In the UK, smoking rates declined to around 21% in 2007 and have since plateaued;12 smoking rates are greatest in low socioeconomic groups. 13 The prevalence of obesity in both children and adults remains relatively high in the UK compared with most other European countries,14,15 particularly in areas of social deprivation. The prevalence of obesity in women living in the UK is highest among those living in areas of social deprivation, but the association in men is less clear. 16
Existing relevant reviews were unable to assess the effectiveness of community pharmacy-delivered alcohol, smoking and weight management interventions because of a limited evidence base. 17–19 However, more interventions have been carried out since these reviews were conducted and the evidence base requires updating. In 2008, the DH2 stated it was crucial to develop ‘a sound evidence base that demonstrates how pharmacy delivers effective, high quality and value for money services’, and this systematic review aims to respond to this requirement.
Community pharmacies
Community pharmacies in the UK are often the most accessible and available health-care provider to the community, and higher numbers of community pharmacies are found in areas of high social deprivation. In England, there are over 10,500 community pharmacies, distributed across urban and rural areas,11 allowing the public to access health care without an appointment. These community pharmacies are open at convenient times, including evenings and weekends, allowing access for people who work a wide range of hours. This situation has consistently improved in recent years in England, with policy drives to improve access to medicines, including the promotion of ‘100-hour pharmacies’, which must open 100 hours per week for every week of the year. 2 Eighty-nine per cent of the population in England can access a pharmacy from home within a 20-minute walk. Importantly, in areas of highest deprivation, this value increases to almost 100%. 20 Estimates vary with regard to the reach of the community pharmacy network, but it is thought to be relatively high: a survey published in 2008 found that 95% of the population of Scotland make at least one visit during any 1 year. 21
Many community pharmacies now offer smoking cessation services and a few offer alcohol reduction and weight management services. 22 Currently, six local pharmaceutical committees (LPCs) have weight management services, 14 LPCs have alcohol services and there are 81 stop smoking services (some LPCs have more than one service covering different areas). These services are delivered by pharmacists, pharmacy technicians and counter assistants. The specific types of interventions are wide-ranging and include two main approaches: pharmaceutical related [e.g. supplying nicotine replacement therapy (NRT) for smoking cessation] and non-pharmaceutical related (e.g. providing advice on behaviour change strategies), or a combination of both approaches. At present, many of these services are commissioned by the local authority according to local need: all services are delivered to an agreed framework specification that allows for variations in the delivery of the service at a local level.
Summary
Excessive alcohol intake, smoking and obesity are three of the most significant modifiable risk factors for morbidity and mortality in the UK. The rates of excessive alcohol intake, smoking and obesity are all greater in lower socioeconomic groups, significantly contributing to overall inequalities in health. Within the UK, community pharmacies are potentially the ideal setting in which to deliver health-care interventions to reduce risk factors for disease. Community pharmacies are easily accessible; they are widely distributed, often in areas of highest deprivation, and many are open long hours. The unique access characteristics of community pharmacies may be more attractive to individuals who cannot, or choose not to, access conventional health-care providers. In addition to conventional health-care provision, community pharmacists can provide opportunistic health care. Community pharmacists can play a significant role in improving risk factors for disease through modifying health behaviours, such as through the management of alcohol consumption, smoking cessation and weight loss. All these factors taken together indicate that community pharmacists and the wider pharmacy team have the potential to deliver health-care interventions to those hardest to reach and arguably those most in need, and in so doing may reduce the socioeconomic inequalities in the prevalence and treatment of modifiable risk factors for disease.
The DH has identified interventions aimed at managing alcohol, smoking and weight, delivered by community pharmacists as public health priorities. We currently do not know how effective such community pharmacy-delivered interventions are. This systematic review assesses the effectiveness of such interventions and is of relevance to those responsible for policy and practice in many countries that are trying to tackle obesity, smoking and alcohol misuse, where one option is to deliver interventions through community pharmacies. Specifically, this review aims to help those commissioning public health services in the UK to determine which pharmacy-delivered interventions are effective, good quality and value for money.
Objectives
-
To assess the effects of community pharmacy interventions on health and health behaviours in relation to alcohol misuse, smoking cessation and weight management.
-
To explore if and how socioeconomic status (SES), sex, ethnicity and age moderate the effect of the interventions.
-
To describe how the interventions included in the review have been organised, implemented and delivered.
Chapter 2 Methods
The review was carried out using the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 23 The review protocol is published in BioMed Central’s Systematic Reviews24 and is registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD42013005943). A study advisory group that comprised patients, pharmacists and researchers with expertise in alcohol, smoking and obesity guided the research. The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)25,26 and Template for Intervention Description and Replication recommendations. 27
Interventions
The review included any type of intervention based in any country and in people of any age. The review included interventions that focused on alcohol misuse, smoking cessation and weight management. As there was no restriction on the type of intervention, interventions could include multiple lifestyle interventions that encompassed more than one component (e.g. smoking cessation and weight management). There was no restriction on the type of participant and so interventions could include participants with comorbidities such as cardiovascular disease or type 2 diabetes mellitus. There was no restriction on the type of comparator and could include a non-active control/usual care, or another type of active intervention set inside or outside the community pharmacy; the comparator could be an identical intervention carried out in a setting other than the community pharmacy. There was no restriction on study duration.
The setting of interest was the community pharmacy, which was defined as a pharmacy set in the community that is accessible to all and not based in a hospital, clinic or online. The participants could be recruited from outside the community pharmacy setting as long as the intervention was carried out from the community pharmacy. The intervention had to be led by the community pharmacist or the wider pharmacy team comprising the pharmacist, pharmacy assistant and/or pharmacy technician; however, the intervention could also include other deliverers as part of a multidisciplinary team. Interventions led by the pharmacist or the wider pharmacy team that took place outside the community pharmacy were excluded. Table 1 details the study eligibility criteria.
| Aspect of study design | Eligibility criteria | Examples and additional clarification |
|---|---|---|
| Population | People of all ages and in any country | Could include participants with comorbidities such as cardiovascular disease or type 2 diabetes mellitus |
| Intervention | Community pharmacy-delivered interventions for alcohol reduction, smoking cessation or weight loss | Could include multiple lifestyle interventions that encompassed more than one component |
| Comparator | Non-active control/usual care, or another type of active intervention | Could be carried out in a community pharmacy or in another setting |
| Outcome | Behavioural outcome (e.g. quit rates, change in alcohol intake). For weight loss interventions, studies had to report an anthropometric outcome (e.g. weight, BMI, waist-to-hip ratio) | No other limits (could be self-reported, observed, measured) |
| Setting | A community pharmacy was defined as a pharmacy set in the community which is accessible to all and not based in a hospital, clinic or online | Participants could be recruited from outside of the community pharmacy setting as long as the intervention was carried out from the community pharmacy |
| Provider | Had to be led by the community pharmacist or the wider pharmacy team comprising the pharmacist, pharmacy assistant and/or pharmacy technician | Could also include other deliverers as part of a multidisciplinary team |
| Study design | All studies with a control group (RCTs, nRCTs, CBAs, ITS and repeated measures studies) | No limit on study duration |
Study design
From the results of our initial scoping search it was anticipated that there would be insufficient evidence from randomised controlled trials (RCTs) alone and so all studies with a control group were included. Using the Cochrane Effective Practice and Organisation of Care study design criteria,28 the types of study design included in the review were as follows: RCTs, non-randomised controlled trials (nRCTs), controlled before-and-after studies (CBAs), interrupted time series and repeated measures studies. Before-and-after studies without a control group and all cross-sectional studies were excluded because it is impossible to attribute causation from such study designs.
Evidence from uncontrolled studies was excluded from this review, but has been identified for possible future research. Throughout the screening process, any reference that appeared to be an uncontrolled before-and-after study that otherwise seemed to fit the inclusion criteria were identified. It was considered important to identify these types of studies, which cannot inform issues of effectiveness but may inform future areas of research around issues such as the recruitment and retention of participants and the demographic and SES of participants accessing community pharmacy-based settings.
Search strategy
Ten electronic databases were searched (host sites given in parenthesis): MEDLINE (via Ovid), EMBASE (via Ovid), Cumulative Index to Nursing and Allied Health Literature (NHS Evidence Health Information Resources), PsycINFO (NHS Evidence Health Information Resources), Social Science Citation Index (Thomas Reuters’ Web of Science), Applied Social Sciences Index and Abstracts (via Cambridge Scientific Abstracts), International Bibliography of the Social Sciences (via EBSCOhost), Sociological Abstracts (via Cambridge Scientific Abstracts), Scopus (Elsevier) and the NHS Economic Evaluation Database (via the NHS Centre for Reviews and Dissemination).
Two reviewers developed the electronic searches (HJM and LS) using medical subject headings and text words using terms for pharmacy, alcohol, smoking cessation and weight. During development of the search we used the studies that were identified as relevant in our previous scoping search as a cross-check to see if the search strategy identified the same studies, this acted as a method of checking the sensitivity of the search strategy. All databases were searched from inception (e.g. MEDLINE starts in 1946) to May 2014. The MEDLINE search is detailed in Appendix 1. There was no restriction on publication date or language.
In order to capture all relevant evidence, various supplementary approaches were used to identify additional published, unpublished and ongoing studies. The electronic database searches were supplemented with website (Google) and grey literature searches (OpenGrey, Social Care Online, Prevention Information & Evidence eLibrary and Nexus UK). The International Standard Registered Clinical/Social Study Number registry and the National Research Register were also searched. The bibliographies of all included studies were hand searched; experts in the field were contacted as well as authors of ongoing studies.
Outcomes
The primary outcomes of this review were behavioural outcomes; a causal modelling framework29 was used to conceptualise behavioural outcomes. The framework contains four categories: (1) determinants of behaviour; (2) behavioural outcomes; (3) physiological and biochemical outcomes; and (4) health outcomes. Interventions for smoking cessation and alcohol consumption had to report a relevant behavioural outcome in order to be included (e.g. quit rates and change in alcohol intake, respectively). For weight loss interventions, studies had to report an anthropometric outcome (physiological) to be included [e.g. weight, body mass index (BMI), waist-to-hip ratio]. There were no other restrictions on study inclusion by type of outcome. Outcomes that were measured, observed or self-reported were included.
The secondary outcomes of this review were any differential effects of the interventions by sociodemographic status (age, ethnicity, sex) or SES (as measured by education, income, occupation, social class, deprivation or poverty), or interventions that were targeted at disadvantaged groups.
Contextual data on the organisation, implementation and delivery of interventions were extracted using the methodological tool for the assessment of the implementation of complex public health interventions in systematic reviews developed by Egan et al. 30 for the workplace and adapted by Bambra et al. 31 for obesity interventions. Examples of components of the organisation, implementation and delivery of interventions include theoretical underpinning and strategies used to change behaviour; implementation context; consultation and/or collaboration process; sustainability; stakeholder support; staff training and quality assurance; experience of the intervention team; and resources and other intervention-related costs. The Behaviour Change Wheel32 and the Nuffield Intervention Ladder33 were chosen to broadly describe the interventions by grouping and classifying the policy categories and intervention functions. We also provide a brief description of the theoretical models which underpinned the interventions and the behaviour change strategies used within each intervention, mostly paraphrasing the original papers.
Data extraction and quality appraisal
The initial screening of titles and abstracts was conducted by three reviewers (CM, HM and SS). The screening of full-text papers was conducted by two reviewers (CM and TB) with any disagreement or uncertainty about inclusion resolved through discussion with two other reviewers (AT and CS). Data extraction and quality assessment was conducted independently for each study by two reviewers (from AT, CM, CS, HM, LN, LS, SS and TB). The data extraction form is detailed in Appendix 2. Data were extracted on the study characteristics, service provider characteristics, outcomes, demographic and socioeconomic variables, and costs.
The quality of the included studies was appraised using the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies34 (see Appendix 3), which is recommended by the Cochrane Public Health Review Group. 35 Any discrepancies in the data extraction or quality assessment were either resolved through discussion or ultimately referred to a third reviewer for final assessment (TB). The quality assessment was used within the narrative synthesis to highlight variations between studies.
Analysis and synthesis
Owing to the heterogeneity of the studies, it was possible to conduct meta-analyses for the smoking cessation studies only. The analyses were performed using the R package meta (The R Foundation for Statistical Computing, Vienna, Austria). The smoking data were analysed using a binomial–normal random-effects model. In order to explain the observed heterogeneity between studies, four different meta-regression models were fitted, accounting for whether an active control group or usual care was used, duration of the intervention and the global quality assessment ratings. Q-statistics and the percentage of heterogeneity between studies were reported for each metaregression model. The optimum metaregression model was chosen using a minimum Akaike information criterion. Where meta-analysis could not be performed, as was the case with the weight data, the change data were described using a bar chart. Owing to the limited available data and lack of informative priors, the planned analysis as described in the protocol24 was not performed in R instead of Stata (StataCorp LP, College Station, TX, USA) or WinBUGS (MRC Biostatistics Unit, Cambridge, UK) for the same reason. A funnel plot for the smoking cessation RCTs was carried out to indicate (but not diagnose) the possible presence of publication bias: intervention effect estimates from individual studies were plotted on the horizontal axis and the standard error of the intervention effect estimate was plotted on the vertical axis. A triangular region was plotted, within which 95% of studies would be expected to lie in the absence of both biases and heterogeneity. 23 Narrative synthesis was conducted for all the included interventions.
Studies eligibility
The titles and abstracts of 14,011 records were screened for inclusion; 13,939 were excluded because inclusion criteria were not met. Inclusion criteria were all types of controlled trials set in a community pharmacy and delivered by the pharmacist or the wider pharmacy team with a focus on alcohol misuse, smoking cessation and weight management. Any type of intervention of any duration based in any country and in people of any age was included. Seventy-two records were obtained as full-text articles because on initial screening it appeared these records might fit the inclusion criteria. Twenty-four studies were finally included and full references are listed in Appendix 4. An additional three studies are ongoing and are listed in Appendix 5.
The process of inclusion and exclusion of studies is detailed in Figure 1. Of the 72 full-text articles that were screened, eligibility was unclear in 10 articles,36–45 and this was resolved through discussion among reviewers (AT, CO, CS, TB). Uncertainty mainly arose regarding whether the intervention was set in a community pharmacy or led by pharmacy staff, or the outcomes (e.g. where studies reported composite measures or where outcomes specific to the pharmacy-based element could not be picked out). The reasons for the exclusion of papers at the full-text stage (n = 40) are detailed in Appendix 6. Fifty-four studies that otherwise appear to fit the inclusion criteria were identified as uncontrolled before-and-after studies; these references are listed in Appendix 7.
FIGURE 1.
The PRISMA flow diagram for studies.
UK alcohol service evaluations
Our initial scoping search of the literature revealed a dearth of information from controlled studies of community pharmacy alcohol screening and brief intervention services. Therefore, a search was undertaken to identify any uncontrolled evaluations undertaken in the UK of community pharmacy alcohol screening and brief advice interventions.
Additional searches for these types of evaluations were carried out between March 2014 and July 2014 and included contacting (1) commissioners of such services; (2) providers of such services; and (3) experts in the academic community who have published in this field. Commissioners included all local authorities in England and all health boards in Scotland and Wales were contacted. The head of the Pharmaceutical Services Negotiating Committee (PSNC) was contacted. Key individuals responsible for commissioning alcohol services were contacted in NHS Scotland and NHS Wales. Providers including LPCs in England were contacted through the PSNC. An advert was taken out in the PSNC newsletter, which was then e-mailed to all LPCs. Similarly, Community Pharmacy Wales and Community Pharmacy Scotland were also contacted by e-mail. An advert was also taken out in The Pharmaceutical Journal asking for any evaluations in relation to community pharmacy and alcohol interventions to be sent to the research team. The Pharmaceutical Journal is sent to all pharmacists who are affiliated with the Royal Pharmaceutical Society (≈30,000 pharmacists) in the UK. Experts in the field including academics who have previously published in this area were contacted; information was also requested through Twitter (Twitter, Inc., San Francisco, CA, USA) and LinkedIn (LinkedIn, Mountain View, CA, USA). Authors were contacted of relevant conference abstracts from the Royal Pharmaceutical Society Conference (2010–14 inclusive), the Academic Health Services Research and Pharmacy Practice Conference (2010–14 inclusive) and the International Network on Brief Interventions for Alcohol and Other Drugs (2010–13 inclusive).
The results from these types of evaluations are reported separately and alongside the synthesis of effectiveness results from the included controlled interventions. Because these reports are not published in peer-reviewed journals and are uncontrolled service evaluations, they were not formally quality assessed (unlike the effectiveness interventions).
Chapter 3 Results
Study characteristics
Tables 2–5 provide study characteristics for each type of intervention focus (alcohol, smoking, weight and multicomponent, respectively). Twenty-four studies were included in this review (29 papers). There were two alcohol interventions,46,48 12 smoking cessation interventions,44,45,49–53,55–60,63 five weight-loss interventions39,64–67 and five multicomponent interventions that included pharmacotherapy and lifestyle changes in participants with comorbidities including dyslipidaemia (n = 1 study),71 hypertension72 and type 2 diabetes mellitus (n = 3 studies). 68–70 Four studies had multiple publications, one smoking cessation study had an English63 and a Danish73 publication; another smoking cessation study49 had two additional publications including a paper on cost-effectiveness51 and a paper on shorter-term follow-up. 50 Another smoking cessation study60 had an additional publication on cost-effectiveness. 61 One multicomponent intervention had an English72 and a Spanish publication. 74 We extracted data from all these additional publications with the exception of the Danish73 and the Spanish publication. 74 Three smoking cessation interventions52,55,60 appeared to be targeted at pharmacy staff as well as clients; the remaining 21 studies were targeted at pharmacy clients alone. There were 19 RCTs,44–46,48,52,53,55,57–60,63,64,66–68,70–72 three nRCTs39,56,69 and two CBAs. 49–51,65 There were 22 published journal articles39,44–46,49–53,55–60,63,64 and two reports. 48,65 Nine studies were conducted in the UK,46,48–51,56,65,66,68 four in the USA,39,44,52,64 two each in Australia,45,53 South America69,71 and Spain,69,70 and one each in Canada,55 Denmark,63 Japan,59 Thailand67 and the Netherlands. 57
| Study and funding source | Methods | Participants | Interventiona | Outcome measures |
|---|---|---|---|---|
| Dhital et al., 201546 Funding source: Hugh Linstead Fellowship Award; Royal Pharmaceutical Society of Great Britain and the Harold and Marjorie Moss Charitable Trust PhD award; Wellcome Trust Research Career Development fellowship (WT086516MA); Service Support Payment by North West London CLRN (UK Clinical Research Collaboration number 11920) |
Design: RCT Aim: to evaluate the effectiveness of a brief alcohol intervention by community pharmacists to reduce hazardous or harmful drinking Power: yes ITT: no |
Age (years), mean: I, 39.6; C, 40.5 Sex (percentage female): I, 47.8; C, 43.6 Ethnicity: 53.8% white British SES indicator: age, education, ethnicity, sex, age and sex of pharmacists Baseline AUDIT scores, mean (SD): I, 11.93 (3.24); C, 11.53 (3.19) Population: AUDIT score 8–19 Number: 407 Intervention setting: 16 community pharmacies in the London Borough of Hammersmith and Fulham Recruitment setting: community pharmacy Location: London, UK |
Who delivered: pharmacists (n = 17) Intervention: structured intervention aimed at promoting behaviour change. Ten-minute brief motivational discussion with pharmacist to encourage contemplation/change of drinking habits. Materials provided included Units & You booklet and ‘Unit/Calorie Calculator Wheel’ and an alcohol services leaflet Control/other: leaflet given, Alcohol: The Basics47 – not expected to change behaviour Duration: 3 months |
|
| Watson and Stewart, 201148 Funding source: Chief Scientist Office, Scotland |
Design: RCT (pilot cluster) Aim: to examine the provision of a brief alcohol intervention in community pharmacies, in terms of practical considerations, recruitment of pharmacists and client, uptake, potential effectiveness and acceptability to pharmacists and clients Power: no ITT: no |
Age: adult clients (≥ 18 years) Sex (percentage female): I, 48.1; C, 57.1 Ethnicity (percentage white): I, 96.6; C, 100 SES indicator: age, education, employment status, ethnicity, sex, IMD, marital status Baseline FAST score of ≥ 3 (%): I, 29.2; C, 24.6 Population: adult pharmacy clients with FAST scores of ≥ 3 Number: 69 Intervention setting: community pharmacies in Grampian (n = 20) Recruitment setting: community pharmacies Country: Scotland, UK |
Who delivered: pharmacists (n = 10) and pharmacy staff (n ≤ 10) Intervention: clients were provided with a brief alcohol intervention delivered by pharmacists specifically trained in brief alcohol intervention techniques Control/other: clients were provided with standard healthy living leaflets Duration: 6 months |
|
| Study and funding source | Methods | Participants | Interventiona | Outcome measures |
|---|---|---|---|---|
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 Funding sources: Glasgow Centre for Population Health, NHS Greater Glasgow and Clyde, and NHS Health Scotland |
Design: CBA Aim: to assess 1-year outcomes and a cost-effectiveness analysis of two NHS smoking cessation services: Smoking Concerns (SC) and Starting Fresh (SF) Power: unclear ITT: no |
Age (years), mean: SF, 44, SC, 49.8 Sex (percentage female): SF, 56.5; SC, 65.5 Ethnicity: NR SES indicator: age, sex, area, housing status, eligibility for free prescriptions, employment status, education, SES group score and marital status Baseline number of participants smoking 21+ cigarettes daily: SF, n = 396 (40.1%); SC, n = 169 (41.6%) Population: Smokers aged ≥ 16 years accessing stop smoking services Number: 1979 (SF, 1508; SC, 471) Intervention setting: over 200 community pharmacies (90% in Glasgow Health Board area) Recruitment setting: community pharmacies Country: Glasgow, UK |
Who delivered: pharmacists and pharmacist assistants (n = NR) Intervention: pharmacy-based smoking cessation intervention SF involving 12 weeks of one-to-one counselling with a pharmacist combined with the direct supply of NRT (in most cases the 16-hour Nicorette patch) Control/other: group-based support smoking cessation service, SC involved 7 weeks of group community-based behavioural counselling Duration: 12 months |
|
| Bock et al., 201052 Funding sources: Grants from the National Institutes of Health, National Cancer Institute (CA099881) and National Institute on Drug Abuse (DA022167) |
Design: RCT plus non-random control Aim: to test the effectiveness of a computer-tailored smoking cessation intervention Exper_Quit (EQ; version 1.0, BTTF Inc., AHleboro, MA, USA). EQ-assisted pharmacist counselling group EQ with or without nicotine transdermal patch Power: unclear ITT: no |
Age (years), mean: EQ group 1, 46.5; EQ+ group 2, 45.5; control group, 42.3 Sex (percentage female): 59 Ethnicity (percentage white): 91 SES indicator: age, education, ethnicity, sex and income Baseline number of cigarettes smoked/day: EQ, 17.9; EQ+, 18.2; control, 13.8 Population: pharmacy client over 18 years, current daily cigarette smoker (at least 5 cigarettes/day for ≥ 3 months), and no contraindications for nicotine patch use Number: 299 Intervention setting: pharmacies located within two large urban community health centres Recruitment setting: pharmacies Country: USA |
Who delivered: trained pharmacists (n = 6) Intervention: two intervention groups (1) EQ-assisted pharmacist counselling; and (2) EQ plus 8 weeks of nicotine transdermal patch. EQ is a computer-driven software system that provided individually tailored interventions to patients who smoke and matching tailored reports for pharmacists to help guide cessation counselling Control: observation only control Duration: 6 months |
|
| Burford et al., 201353 Funding sources: NR |
Design: RCT Aim: to test an intervention based on personalised vivid illustrations [using APRIL® Face Aging software (version 2.5; AprilAge Inc., Toronto, ON, Canada)] of smokers face among young smokers (aged 18–30 years) and to explore the value of an unfunded intervention within pharmacies Power: yes ITT: yes |
Age (years, mean): I, 24.2; C, 25.1 Sex (percentage female): I, 68.7; C, 56.2 Ethnicity: NR SES indicator: age, education and sex Baseline Fagerström score: I, 2.96; C, 2.87 Population: smokers aged 18–30 years accessing eight metropolitan community pharmacies Number: 160 participants Intervention setting: community pharmacies in Western Australia Recruitment setting: community pharmacies Country: Perth, Australia |
Who delivered: pharmacists (n = NR) Intervention: to assess whether or not the use of APRIL (face-ageing software) plus standard care (2-minute smoking cessation advice from pharmacist had an impact on self-reported quit rates of young smokers (aged 18–30 years) confirmed by CO testing Control: standard care (standardised 2-minute smoking cessation advice) Duration: 6 months |
|
| Costello et al., 201155 Funding source: Ontario Ministry of Health Promotion |
Design: RCT plus non-random control Aim: to evaluate the effectiveness of two models of pharmacist-led behavioural counselling for smoking cessation support provided by community pharmacists that included NRT Power: yes ITT: no |
Age (years): ≥ 18 years Sex (percentage female): group A, 54.4; group B, 54.9 Ethnicity: NR SES indicator: age, education, employment and sex Baseline Heaviness of Smoking Index, score of ≥ 3 (%): group A, 91.8; group B, 91.4 Population: Ontario residents, ≥ 18 years, self-report current daily smokers of 10+ cigarettes/day and willing to make a quit attempt within the next 30 days Number: 15,898 (6987 unique participants) Intervention setting: community pharmacies (n = 98) Recruitment setting: online Country: Ontario, Canada |
Who delivered: pharmacists (n = 113) Intervention: group A received three behavioural counselling sessions with a pharmacist and 5 weeks of free NRT. Group B received one individual counselling session plus 5 weeks of free NRT. Group B received all 5 weeks of NRT at their one session and group A received theirs over three sessions Duration: 5–12 weeks, mean 6.4 weeks |
|
| Crealey et al., 199856 Funding source: NR |
Design: CBA Aim: to determine the costs and effects associated with a community pharmacy-based smoking cessation programme in Northern Ireland, using the perspective of the payer in the main analysis Power: unclear ITT: unclear |
Age (years): NR Sex (percentage female): NR Ethnicity: NR SES indicator: NR Baseline measures: NR Population: 52 people entered the smoking-cessation programme (group 1), 48 bought nicotine gum and gave their address so that additional information could be sent and they could be followed up (group 2), and 60 people who expressed a wish to stop smoking were chosen on the basis that they matched, by age, sex, social status and disease status, those in group 1 Number: 160 Intervention setting: two community pharmacies Recruitment setting: NR Country: Belfast, Northern Ireland, UK |
Who delivered: pharmacists (n = NR) Intervention: smoking cessation advice from community pharmacist, the PAS model. Developed by the PAS group in association with the National Pharmaceutical Association in the UK, a written ‘contract’ between the patient and pharmacist (including a ‘stop date’), and a series of brief counselling meetings over approximately 6 months Control: participants received normal type of ad hoc, non-formalised advice that is currently given in community pharmacies Duration: 6 months |
|
| Hoving et al., 201057 Funding source: NR |
Design: RCT Aim: to test the effectiveness of a computer-tailored smoking cessation intervention distributed through GP surgeries and pharmacies Power: unclear ITT: yes |
Age (years), mean: I, 46; C, 47 Sex (percentage female): I, 53; C, 54 Ethnicity: NR SES indicator: age, education and sex Baseline number of cigarettes smoked per day (mean): I, 22; C, 21 Baseline stage of change (contemplator/preparer, %): I, 41/59; C, 41/50 Population: smokers accessing pharmacies and who had smoked within the last 7 days Number: 545 Intervention setting: Dutch community pharmacies Recruitment setting: Dutch pharmacies (n = 65) and GP surgeries (n = 75) Country: the Netherlands |
Who delivered: pharmacists (n = NR) Intervention: participants received computer-generated tailored advice/messages to aid smoking cessation Control: thank-you letter only Duration: 12 months |
|
| Howard-Pitney et al., 199944 Funding source: National Institutes of Health, Public Health Service, Grant from National Cancer Institute |
Design: RCT Aim: to examine the efficacy of a treatment programme combining nicotine patch with a minimal contact for chewing tobacco users. Behavioural intervention Power: unclear ITT: yes |
Age (years), mean: I, 36.3; C, 34.7 Sex (percentage female) I, 1; C, 1 Ethnicity (percentage white): I, 96; C, 93 SES indicator: age, education, ethnicity and sex Baseline number of cans chewed per week: I, 3.9; C, 4.1 Population: adult, non-smoking, chewing tobacco users of at least one can or pouch of chewing tobacco each week and scored 6 or higher on a 10-point scale rating their motivation to quit Number: 410 participants Intervention setting: community pharmacy (n = NR) Recruitment setting: telephone Country: USA |
Who delivered: pharmacists (n = NR) Intervention: 15-mg nicotine patch plus behavioural treatment including two pharmacy visits, two support calls, and self-help materials Control: placebo nicotine patch plus behavioural treatment plus behavioural treatment including two pharmacy visits, two support calls and self-help materials Duration: 6 months |
|
| Maguire et al., 200158 Funding sources: Medical Research Council and the Northern Ireland Department of Health and Social Services |
Design: RCT Aim: to evaluate whether or not a structured community pharmacy-based smoking cessation programme (the PAS model) would give rise to a higher smoking cessation rate compared with ad hoc advice from pharmacists Power: unclear ITT: yes |
Age (years), mean: I, 42; C, 38 Sex (percentage female): I, 40; C, 44 Ethnicity: NR SES indicator: age, sex Baseline number of participants smoking ≥ 20 cigarettes per day: I, 42; C, 53 Population: smokers attending community pharmacies Number: 484 Intervention setting: community pharmacies Recruitment setting: community pharmacies Country: Northern Ireland and London, UK |
Who delivered: pharmacists (n = 124) Intervention: the PAS intervention involved a structured counselling programme, an information leaflet and a follow-up, weekly for the first 4 weeks then monthly as needed. Smokers allocated to the PAS group received a leaflet and a one-to-one interview using the PAS flip chart. Follow-up advice given at weekly intervals for 4 weeks, then monthly for 3 months Control: participants accessed normal pharmaceutical service (including the provision of NRT) provided by the pharmacist. Smokers were not counselled using the PAS flip chart, not given a PAS leaflet and not asked to attend for follow-up interviews Duration: 12 months |
|
| Mochizuki et al., 200459 Note that the full paper is in Japanese with an English abstract: not all information available for extraction Funding source: unclear |
Design: RCT Aim: to evaluate whether or not pharmacists advice on smoking cessation would result in a higher smoking cessation rate using Nicorette Power: no ITT: no |
Age (years), mean: I, 44.1; C, 49.1 Sex (percentage female): I, 18.2; C, 18.8 Ethnicity: NR SES indicator: age and sex Baseline number of cigarettes smoked per day (mean): I, 23; C, 25.7 Population: smokers visiting pharmacies from 1 March 2002 through to 31 August 2002, aged ≥ 20 years, desire to quit smoking and smoke at least 11 cigarettes a day for last year Number: 28 participants Intervention setting: community pharmacies (n = 14) Recruitment setting: community pharmacies Country: Tokyo, Japan |
Who delivered: pharmacists (n = NR) Intervention: smokers received both regular instructions on Nicorette use and smoking cessation advice at first sale then follow-up advice prior to starting cessation and 1 week, 3 weeks, 8 weeks and 3 months thereafter Control: smokers received regular pharmacist instruction only Duration: 3 months |
|
| Sinclair et al., 199860 Sinclair et al., 199961 Funding: Department of Health Scottish Office |
Design: RCT Aim: to evaluate a training workshop for community pharmacy personnel to improve their counselling in smoking cessation based on stages-of-change model62 Power: no ITT: no |
Age (years), mean: I, 41.7; C, 41.5 Sex (percentage female): I, 61.2; C, 62.7 Ethnicity: NR SES indicator: age, sex and IMD Baseline nicotine dependence (mean Fagerström test score): I, 5.2; C, 5.2 Population: pharmacy customers who smoked Number: 492 participants Intervention setting: non-city community pharmacies (n = 62) Recruitment setting: community pharmacy Country: Aberdeen, Scotland, UK |
Who delivered: pharmacists (n = 40) and pharmacist assistants (n = 54) Intervention: customers were offered the pharmacist support model (to incorporate the stages-of-change model to improve counselling). As part of this programme customers were invited to register, were offered counselling and record keeping Control: control group customers were asked to register and continued to receive standard professional support Duration: 9 months |
|
| Sonderskov et al., 199763 Funding: partly funded by Ciba-Geigy (nicotine patch supplier) no further information given |
Design: RCT Aim: to estimate short-term smoking cessation rates among selected customers of nicotine patches at a number of pharmacies in Denmark and to evaluate smoking cessation on a long-term basis Power: unclear ITT: no |
Age (years), mean: I, 38.2/38.9; C, 39.1/39.9 Sex (percentage female): I, 51.7/48.3; C, 47.5/52.5 Ethnicity: NR SES indicator: age, education and sex Baseline Fagerström score: I, 6.1/6.1; C, 7.0/8.1 Population: pharmacy customers who smoked ≥ 20 cigarettes/day Number: 522 participants Intervention setting: community pharmacies (n = 42) Recruitment setting: community pharmacies in Aarhus and Copenhagen, Denmark Country: Denmark |
Who delivered: pharmacists (n = NR) and pharmacy staff (n = NR) Intervention (nicotine patches): customers who smoked ≥ 20 cigarettes per day were randomised to use one 21 mg/day patch per day during the first 4 weeks equivalent to one treatment period (active patches release 21 mg of nicotine in 24 hours), 14 mg/day patches (14 mg of nicotine/24 hours) during the second 4-week treatment period and 7 mg/day patches (7 mg of nicotine/24 hours) during the final 4 weeks. Smokers of < 20 cigarettes per day used 14 mg/day patches during the first two treatment periods (8 weeks), and 7 mg/day patches during the final treatment period Control: placebo patches Duration: 6 months |
|
| Vial et al., 200245 Funding source: Anti-Cancer Foundation of South Australia, The Queen Elizabeth Hospital Research Foundation and the University of South Australia |
Design: RCT Aim: to compare quit rates, initiated in hospital (as inpatient, on discharge) using nicotine patches and support, either in a hospital outpatients department, or a community pharmacy Power: no ITT: no |
Age (years), mean (range): 51 (23–81) Sex (percentage female): community pharmacy, 41; hospital, 54; and minimal intervention, 36 Ethnicity: NR SES indicator: income/education/occupation/area Baseline Fagerström score: pharmacy, 5.79; hospital, 5.94; minimal intervention, 6.33 Population: inpatients aged over 18 years who smoked 10 or more cigarettes per day Number: 102 (hospital, n = 35; community pharmacy, n = 34; and minimal intervention, n = 33) Intervention setting: community pharmacy Recruitment setting: Queen Elizabeth Hospital Country: Adelaide, Australia |
Who delivered: pharmacists Intervention: initial consultation with research pharmacist and begin nicotine patch treatment then followed with weekly counselling visits with either researcher in outpatient clinic or a community-based pharmacist Control: minimal intervention received written materials and advice on smoking cessation Duration: 12 months |
|
| Study and funding source | Methods | Participants | Interventiona | Outcome measures |
|---|---|---|---|---|
| Ahrens et al., 200364 Funding: grant from the Slim-Fast nutrition institute, West Palm Beach |
Design: RCT Aim: to compare a MR program with a conventional reduced-calorie diet for weight management using the pharmacy as the setting and the pharmacist as the point of contact for dietary advice Power: unclear ITT: no |
Age (years), mean: I, 47.8; C, 47.6 Sex (percentage female): 87 Ethnicity: NR SES indicator: age and sex Baseline BMI (kg/m2), mean: I, 29.5; C, 29.0 Population: patients with BMI between 25 kg/m2 and 32 kg/m2, aged 35–65 years Number: 95 participants Intervention setting: community pharmacies (n = 2) Recruitment setting: unclear Country: USA |
Who delivered: community pharmacists (n = 2) Intervention: phase 1, MR diet [SlimFast (SlimFast, Palm Beach Gardens, FL, USA)] (intervention group) patients drank one shake per day and ate two sensible meals of their choice; phase 2, patients told to self-regulate their caloric intake with the goal of maintaining their weight loss Control: phase 1, self-selected diet based on diabetic exchange; phase 2, conventional reduced-calorie diet whereby patients were instructed to return to a healthy diet of their choice and to control caloric intake as desired. Both programmes recommended increased physical activity Duration: 22 weeks |
|
| Bush et al., 201165 Funding: NHS Birmingham |
Design: CBA Aim: to reduce adult obesity levels; improve access to overweight and obesity management services in primary care; improve diet and nutrition; promote healthy weight and increased levels of physical activity in overweight or obese patients; and support patients to make lifestyle changes to enable them to lose weight through pharmacy or GP delivery Power: no ITT: no |
Age (years), mean: I, 32.9; C, 42.6 Sex (percentage female): I, 87; C, 85 Ethnicity: 80% black and minority ethnic groups SES indicator: age, sex, ethnicity, IMD and area (kg/m2) Baseline BMI (kg/m2), mean: I, 33.0; C, 35.6 Population: obese patients over 18 years old who have a BMI > 30 kg/m2 (> 25 kg/m2 in Asian patients) or > 28 kg/m2 (> 23.5 kg/m2 in Asian patients) in patients with comorbidities (diabetes mellitus, high blood pressure, cardiovascular disease) Number: 451 participants Intervention setting: community pharmacies within the Heart of Birmingham Teaching PCT (n = 12) Recruitment setting: I, community pharmacies; C, GP surgeries Country: Birmingham, UK |
Who delivered: pharmacists (n = NR) Intervention: pharmacy-delivered My Choice Weight management programme including: weekly weight and waist circumference measurements; lifestyle, behaviour, diet and activity assessment, completion of a food and exercise; diary; realistic weight loss targets, usually a maximum weekly weight loss of 0.5–1 kg, with the aim of a 5–10% reduction in initial weight; realistic targets for lifestyle, healthy eating and physical activity; and patients are empowered to develop skills for both losing weight and maintaining lost weight Control/other: GP-delivered My Choice Weight management programme (including same components as above) Duration: 9 months |
|
| Jolly et al., 201166 Funding: NHS South Birmingham |
Design: RCT (8 arm) Aim: to determine the effectiveness of a range of NHS and commercial weight loss programmes in an unselected primary care population Power: yes ITT: yes |
Age (years), mean: I, 48.94; C, 49.69 Sex (percentage female): I, 73; C, 75 Ethnicity (percentage white British/Irish): I, 87; C, 84 SES indicator: age, sex, ethnicity, IMD and area Baseline BMI (kg/m2), mean: I, 33.44; C, Population: obese or overweight men and women, over 18 years of age with a comorbid disorder identified from GP records Number: 740 participants Intervention setting: community pharmacies in Birmingham Recruitment setting: call centre (telephone)-based nurse-led recruitment Country: Birmingham, UK |
Who delivered: pharmacist (n = NR) and various others depending on assigned arm Intervention: pharmacy arm: 12 one-to-one weight management sessions in the pharmacy. Included key messages on diet and physical activity, doing a behavioural assessment, goal-setting, plans for change, dealing with resistance, enhancing motivation and weight maintenance. It included both practical tasks and informational components. Other arms: intervention varies depending on arm assigned to Weight Watchers™ (WeightWatchers.co.uk Ltd, Maidenhead, UK), Slimming World™ (Miles-Bramwell Executive Services Ltd, Alfreton, UK), Rosemary Conley™ (Rosemary Conley Online Ltd, Steyning, UK), group-based dietetics-led programme, general practice one-to-one counselling; all 12 weeks in duration Control: participants allocated to the comparator group were sent vouchers for 12 free sessions at a local authority-run leisure centre (a council-run facility open to all members of the public and usually consisting of a swimming pool, fitness suite, and other sports halls or courts). Participants were not given an appointment to attend and were given no individual advice or support on diet or physical activity Duration: 12 months |
|
| Malone and Alger-Mayer, 200339 Funding: NR |
Design: nRCT Aim: to evaluate the impact of pharmacist support + usual care for patients who were prescribed orlistat and attending an outpatient nutrition programme, compared with just usual-care (outpatient appointments every 4–6 weeks) terms of patient compliance with orlistat Power: no ITT: yes |
Age (years), mean: I, 44.9; C, 42.8 Sex (percentage female): I, 93; C, 80 Ethnicity: NR SES indicator: age and sex Baseline BMI (kg/m2), mean: I, 48.3; C, 42.8 Population: patients from a hospital outpatient clinic who were waiting to be initiated on to orlistat therapy Number: 30 participants Intervention setting: community pharmacies in patients area of residence (eight pre-selected pharmacists trained) Recruitment setting: university teaching hospital-based outpatient nutrition clinic Country: USA |
Who delivered: pharmacist (n = 8) Intervention: intervention group were familiarised with their local pharmacist to make contact when collecting their prescription for orlistat. At first visit, they had a consultation with the pharmacists; (involving support on weight loss/pharmacotherapy) they were encouraged by the pharmacist to sign up to the XeniCare support line (Roche, Nutley, NJ, USA). These patients returned to see the pharmacists after 2 weeks for a follow-up consultation. Unclear what the protocol was for number and frequency of consultations after this time. This intervention was in addition to usual-care 4- to 6-week appointments at outpatient clinic Control: usual care provided by the outpatient clinic Duration: 6 months |
|
| Phimarn et al., 201367 Funding: National Health Security Office Thailand Fund supported grant through Primary Care Practice Research Unit, Mahasarakham University |
Design: RCT Aim: to examine clinical outcomes, eating behaviours, and knowledge about being overweight and obesity; comparing the community pharmacy intervention with routine group weight management Power: yes ITT: no |
Age (years), mean: I, 59.12; C, 60.09 Sex (percentage female): I, 75.8; C, 84.8 Ethnicity: NR SES indicator: age, education, sex, income, marital status and occupation Baseline BMI (kg/m2), mean: I, 27.74; C, 27.49 Population: overweight and obese patients from one PCU diagnosed as overweight or obese by a doctor Number: 75 participants Intervention setting: single community pharmacy where there was an established network with one PCU in Mahasarakham province Recruitment setting: one selected PCU, Mahasarakham Country: Thailand |
Who delivered: pharmacists (n = NR) Intervention: 16-week intervention, sessions (lasting about 1 hour, one-to-one sessions provided by a pharmacist along with the weight-loss handbook for self-study. Sessions provided at 0 weeks, 4 weeks, 8 weeks and 16 weeks Control: group counselling with a focus on weight loss, which was routinely provided by the PCU staff. Typically, all overweight and obese patients. Group sessions lasted approximately 1 hour, covered information about healthy diet, principles of energy intake, food groups, portion size and exercise. The group counselling sessions were provided at weeks 0, 4, 8 and 16 Duration: 4 months |
|
| Study and funding source | Methods | Participants | Interventiona | Outcome measures |
|---|---|---|---|---|
| Diabetes mellitus (type 2) | ||||
| Ali et al., 201268 Funding: grants provided by the Department of Health, UK and Merck Sharp & Dohme Corp. |
Design: RCT Aim: to evaluate the impact of a pharmacist-led patient education and diabetes mellitus monitoring programme on HbA1c and other cardiovascular risk factors in the community Power: yes ITT: no |
Age (years), mean: I, 66.4; C, 66.8 Sex (percentage female): I, 56.5; C, 43.5 Ethnicity: 95.4% Caucasian SES indicator: age, ethnicity and sex Baseline BMI (kg/m2), mean: I, 30.84; C, 29.82 Population: patients with type 2 diabetes mellitus Number: 46 participants Intervention setting: community pharmacies in Hertfordshire (n = 2) Recruitment setting: pharmacies or by GP referral Country: Hertfordshire, UK |
Who delivered: pharmacist (n = NR) Intervention: patients in the intervention group received a pharmaceutical care package designed for patients with type 2 diabetes mellitus, with regular monitoring and consultations with the community pharmacist for 12 months. Patients were seen by the pharmacist every month for the first 2 months, and then every 3 months for the remainder of the 12 months, a total of six appointments Control: usual care as provided by the GP, practice nurse and community pharmacist Duration: 12 months |
|
| Correr et al., 201169 Funding source: NR |
Design: nRCT Aim: to evaluate the effects of PF on metabolic control and clinical outcomes in type 2 diabetic patients. The aim of identifying negative clinical outcomes and producing interventions to solve them Power: unclear ITT: no |
Age (years), mean: I, 58.1; C, 59.5 Sex (percentage female): I, 56; C, 50 Ethnicity: NR SES indicator: age, education and sex Baseline BMI (kg/m2), mean: I, 29.2; C, 27.6 Population: type 2 diabetic patients aged ≥ 30 years, using either oral hypoglyceamiants or insulin Number: 161 participants Intervention setting: community pharmacies in Curitiba, metropolitan region of Brazil (n = 4) Recruitment setting: community pharmacies Country: Brazil |
Who delivered: pharmacists (n = NR) Intervention: standard pharmacy care followed by PF (described as cognitive pharmacy service, where the pharmacist takes responsibility for a patient’s drug-related needs, and intervenes to improve quality of life) Control: standard pharmacy care with no PF Duration: 12 months |
|
| Fornos et al., 200670 Funding source: Bayer Spain, The Official College of Pharmacist of Pontevedra and the Pharmaceutical Northwest Wholesaler Cooperative (Cofano) |
Design: RCT Aim: to evaluate the improvement in metabolic control, the resolution of drug-related problems and the increase in patient awareness of diabetes mellitus as outcomes of a PF programme in type 2 diabetic patients Power: yes ITT: no |
Age (years), mean: I, 62.4; C, 64.9 Sex (percentage female): I, 57; C, 57 Ethnicity: NR SES indicator: age and sex Baseline BMI (kg/m2), mean: I, 31.0; C, 31.7 Population: type 2 diabetic pharmacy patients receiving treatment with oral antidiabetics for more than 2 months Number: 112 participants Intervention setting: community pharmacies in the province of Pontevedra (n = 14) Recruitment setting: community pharmacies Country: Spain |
Who delivered: pharmacists (n = NR) Intervention: patients receive pharmacotherapy follow-up in addition to usual community pharmacy care. It is a type of care that requires the involvement of the pharmacist in the outcomes of pharmacotherapy, in co-operation with the health-care team and the patient Control: usual community pharmacy care Duration: 13 months |
|
| Dyslipidaemia | ||||
| Paulos et al., 200571 Funding: NR |
Design: RCT Aim: to design a pharmaceutical care program for dyslipidaemia patients within a community pharmacy setting providing education in medication compliance and lifestyle modification while emphasising importance of achieving cholesterol goals to improve quality of life Power: unclear ITT: no |
Age (years), mean: I, 64; C, 66 Sex (percentage female): 81 Ethnicity: NR SES indicator: age, education and sex No baseline BMI results reported Population: aged ≥ 18 years with a clinical diagnosis of dyslipidaemia and currently being treated for the disorder Number: 42 participants Intervention setting: a community pharmacy, Santiago (with an established counselling area) Recruitment setting: community pharmacy Country: Chile |
Who delivered: pharmacists (n = 1) Intervention: the intervention comprised a comprehensive pharmaceutical plan and scheduled follow-up. Patients were surveyed for 16 weeks and interviewed on five occasions. Each interview lasted 20–25 minutes. The pharmacist intervention included obtaining total blood cholesterol and triglyceride levels as well as teaching patients about the role of cholesterol in illness and health, explaining risk factors associated with cardiovascular disease, and providing education/counselling regarding medication Control: patients were surveyed for 16 weeks and interviewed on two occasions only Duration: 4 months |
|
| Hypertension | ||||
| Zaragoza-Fernandez et al., 201272 Funding source: NR |
Design: RCT Aim: assess the impact of an intervention to modify patients’ diet lifestyle by pharmacists on blood pressure levels in hypertensive, treatment-compliant patients (not controlled with antihypertensive agents) Power: yes ITT: no |
Age (years), mean: I, 67.4; C, 69.3 Sex (percentage female): I, 57.9; C, 67.6 Ethnicity: NR SES indicator: age and sex Baseline BMI (kg/m2), mean: I, 30.8; C, 30.0 Population: patients collecting antihypertensive drugs from one of three pharmacies Number: 150 participants Intervention setting: community pharmacies (n = 3), Murcia, Spain Recruitment setting: community pharmacies Country: Spain |
Who delivered: pharmacists (n = NR) Intervention: participants had their blood pressure taken and patients were given a sheet with list of changes to be made in their diet and lifestyle in order to control their blood pressure. Participants were called on the same day for 3 consecutive weeks and on week 4 they were given an appointment for a personal interview. In the interview, participants were asked about the changes they had made and any problems they had encountered. Their blood pressure was taken and another form completed. Participants were telephoned for the next 3 weeks and had an interview and their blood pressure measured in week 8 Control: differences between control and intervention groups are not clearly described Duration: 2 months |
|
Seventeen studies recruited participants within the community pharmacy; other recruitment settings included hospital/primary care units, via telephone and a community health centre. The intervention setting was always a community pharmacy (for at least one intervention group); the types of pharmacies included single outlets, small chains and large chains. Pharmacies were set in rural, urban and a combination of both geographical settings. The number of pharmacies included within each study ranged from 1 to over 200. Participant sample size ranged from 28 to around 7000, resulting in approximately 14,000 service users in total.
All studies reported participant demographic characteristics and some reported socioeconomic details at baseline. Twenty-three studies reported age and sex, 13 studies reported education levels; seven studies reported ethnicity, two studies reported income, four studies reported employment/occupation and three studies reported marital status. Five studies (one alcohol management, two smoking cessation and two weight loss) reported the SES of participants using versions of deprivation scores. One smoking cessation study reported a ‘socioeconomic group score’ which was a composite measure of education levels, single parent status, housing status, employment status, sickness, free prescription eligibility and deprivation.
All studies were of adults and the mean age ranged from 24.2 to 67.4 years. Participants in a study of a photoageing intervention were much younger (mean age 24.2 years) than the majority of the participants because this intervention was specifically targeted at smokers aged between 18 and 30 years. In terms of sex, across all the studies there was a majority of female participants. Across all the studies the percentage of females ranged from 36% to 93%; however, when the weight loss trials were excluded, the percentage of females ranged from 36% to 69%. In the weight loss studies the majority of participants (> 70%) were female. There were two exceptions, both smoking cessation studies: one Japanese study59 reported 18.5% females (5/27) and in one US study44 99% were male; this reflected the target population, which comprised tobacco-chewing participants, who tend to be predominantly male.
The majority of participants in five44,48,52,66,68 out of the seven studies44,46,48,52,65–67 that reported ethnicity were white. In one weight loss intervention, four-fifths of participants were from black and ethnic minority groups. In one alcohol intervention46 based in the inner London Borough of Hammersmith and Fulham, 20% of participants were Asian, black, mixed, Chinese or other.
Three studies49–51,65,66 appeared to adopt a targeted approach to addressing inequality, recruiting a majority of participants from areas of high deprivation. All three studies compared a pharmacy-based setting with other settings. Bauld et al. 49–51 compared smoking cessation services that were group-based in the community with one-to-one pharmacy-based services; both services were attended by a larger proportion of women than men. The smaller number of clients who attended the group service were older, slightly more affluent (although still a relatively deprived group) and more likely to be women. Bush et al. 65 compared a weight management programme set in pharmacies versus the same programme set in general practitioner (GP) surgeries. GP surgery participants tended to be older than pharmacy participants and ethnic composition of the two groups differed significantly. The mean Index of Multiple Deprivation (IMD) score of participants at GP surgeries and pharmacies was 43.8 and 43.3, respectively. Jolly et al. 66 compared a range of NHS and commercial weight loss programmes including a pharmacy-based intervention; 73.4% of participants were from the two most socioeconomically disadvantaged quintiles of the population. The mean IMD score of participants in the GP surgery and pharmacy arms were 32.2 and 35.1, respectively; higher IMD scores indicate greater deprivation.
Eligibility criteria for participants in the two alcohol interventions46,48 included a minimum score that indicated possible harmful or hazardous alcohol consumption but not alcohol dependence. Eligibility criteria for participants in the smoking cessation interventions consisted of measures of nicotine dependence, which varied across the interventions. Four studies52,57–59 reported the baseline number of cigarettes smoked per day, and this ranged between 20 and 23 cigarettes per day for three studies;52,58,59 and one study reported a baseline of 42 cigarettes per day. 58 Another four studies reported only Fagerström scores (scored 0–10, with higher scores reflecting a greater dependence on nicotine), which ranged from 3 in a group of young adults with a mean age of 24 years53 to around 6 for three studies. 45,60,63
The degree of overweight or obesity varied across the five weight loss interventions; all interventions specified a minimum BMI at baseline and the mean BMI ranged from 27.7 kg/m2 to 44.9 kg/m2. 39,67
Intervention components varied considerably across all 24 studies and specific study intervention details are reported alongside the individual study results. Duration of follow-up (from baseline to end) ranged from 5 to 56 weeks. Where the duration of a study is referred to, this is defined as from baseline to final follow-up.
In terms of outcomes, both the alcohol interventions used self-reported questionnaires to evaluate change in alcohol behaviours. Half (6/12) of the smoking cessation interventions relied on self-reported change in smoking behaviours45,55,57,59,60,63 and half used biochemical measures44,49–53,56,58 [carbon monoxide (CO) or cotinine levels] to validate change in smoking behaviours. All five of the weight loss interventions39,64–67 and all five multicomponent interventions67–72 (pharmacotherapy plus lifestyle changes) measured weight. Five studies assessed health status or quality of life. 39,46,65,68,70 Eight studies reported cost outcomes. 48,49–51,56,60,65,66,69
Only four studies assessed whether or not certain demographic variables moderated the effect of interventions; all four studies48,60,65,66 assessed the differential effects of sex and one of these studies also assessed age. 65 No study assessed the differential effects of any measure of SES. Few studies used regression analysis to assess the influence of demographic or socioeconomic variables on change from baseline, in other words as potential predictors of outcomes within intervention groups or to explain retention.
Funding sources were stated in 16 studies;44–46,48–52,55,58,60,63–68,70 the types of funding sources included academic research bodies, health-related institutions, commercial organisations and pharmaceutical companies, with some studies receiving funding from a combination of different types of sources.
Study quality
Twenty-four studies39,44–46,48–53,55–60,63–72 were assessed for quality [three ongoing studies (see Appendix 5) were not quality assessed] using six criteria: (1) selection bias; (2) study design; (3) confounders; (4) blinding; (5) data collection methods; and (6) withdrawals/dropouts. Each study was given an overall (global) rating based on the ratings for the six criteria (Table 6).
| Study | Quality criteria | Global rating | |||||
|---|---|---|---|---|---|---|---|
| Selection bias | Study design | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | ||
| Alcohol | |||||||
| Dhital et al., 201546 | Moderate | Strong | Strong | Moderate | Moderate | Moderate | Strong |
| Watson and Stewart, 201148 | Weak | Strong | Weak | Moderate | Moderate | Weak | Weak |
| Smoking cessation | |||||||
| Bauld et al., 201149 Bauld et al., 200950 Bauld et al., 200951 | Weak | Moderate | Strong | Moderate | Strong | Weak | Weak |
| Bock et al., 201052 | Weak | Strong | Moderate | Moderate | Strong | Strong | Moderate |
| Burford et al., 201353 | Moderate | Strong | Strong | Weak | Strong | Moderate | Moderate |
| Costello et al., 201155 | Weak | Strong | Strong | Weak | Moderate | Weak | Weak |
| Crealey et al., 199856 | Weak | Strong | Weak | Moderate | Moderate | Weak | Weak |
| Hoving et al., 201057 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| Howard-Pitney et al., 199944 | Moderate | Strong | Weak | Strong | Strong | Strong | Moderate |
| Maguire et al., 200158 | Moderate | Strong | Weak | Weak | Strong | Strong | Weak |
| Mochizuki et al., 200459 | Moderate | Strong | Strong | Moderate | Moderate | Moderate | Strong |
| Sinclair et al., 199860 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| Sonderskov et al., 199763 | Moderate | Strong | Strong | Moderate | Moderate | Strong | Strong |
| Vial et al., 200245 | Weak | Strong | Strong | Weak | Moderate | Moderate | Weak |
| Weight loss | |||||||
| Ahrens et al., 200364 | Moderate | Strong | Strong | Weak | Strong | Weak | Weak |
| Bush et al., 201165 | Moderate | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Jolly et al., 201166 | Weak | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Malone and Alger-Mayer 200339 | Moderate | Strong | Weak | Moderate | Strong | Weak | Weak |
| Phimarn et al., 201367 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Multicomponent interventions (pharmacotherapy and lifestyle changes) | |||||||
| Diabetes mellitus – type 2 | |||||||
| Ali et al., 201268 | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Correr et al., 201169 | Moderate | Strong | Strong | Weak | Moderate | Weak | Weak |
| Fornos et al., 200670 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Dyslipidaemia | |||||||
| Paulos et al., 200571 | Moderate | Strong | Weak | Weak | Strong | Weak | Weak |
| Hypertension | |||||||
| Zaragoza-Fernandez et al., 201272 | Moderate | Strong | Weak | Moderate | Moderate | Strong | Moderate |
Selection bias
None of the studies was assessed as ‘very likely’ to have a representative study sample because participants were not obtained from randomly selected samples. Therefore, none of the studies was assessed as ‘strong’ for selection bias. In 13 studies39,52,56–59,64,65,67–69,71,72 the reviewers could not tell what percentage of selected individuals agreed to participate. Ten studies44–46,53,55,60,63,66,70 reported sufficient information, in two of which55,63 80–100% agreed to participate; in three studies44,53,60 60–79% agreed to participate and in five studies45–46,48,66,70 < 60% agreed to participate. In one study of the NHS Stop Smoking Service49–51 it was not applicable to assess the percentage of selected participants agreeing to participate, because smokers self-referred and did not require an invitation to access the services. Overall, in terms of selection bias, 16 studies39,44,46,53,57–60,63–65,67,69–72 were ‘somewhat likely’ (scoring moderate) and eight studies45,48–52,55,56,66,68 were ‘not likely’ (scoring weak) to have representative study samples.
Study design
Nineteen studies were RCTs,44–46,48,52,53,55,57–60,63,64,66–68,70–72 three studies39,56,69 were nRCTs and two studies49–51,65 were CBAs. All 21 RCT/nRCT designs were classed as ‘strong’ for quality and the two CBAs were classed as ‘moderate’. In two studies48,60 the pharmacies were ‘randomised’ rather than individual participants.
Confounders
Fifteen studies45,46,49–51,53,55,57,59,60,63,64,66–70 were classed as ‘strong’ for confounding, either because it was reported that there were no statistically significant baseline differences between the groups or because most differences were controlled for in the analyses. In some studies, even when groups were reported as comparable at baseline, potential confounders were adjusted for in subsequent analyses. One study52 scored ‘moderate’ for confounding, as it controlled for some baseline differences in the analyses. Eight studies39,44,48,56,58,65,71,72 were classed as ‘weak’ for confounding, either because it was not clear if there were baseline differences, or because there were baseline differences and it was not clear if confounders had been controlled for in the analyses, or < 60% of baseline differences were controlled for in subsequent analyses.
Blinding
One study44 reported that both the outcome assessors and the participants were blinded to the study intervention and was classed as ‘strong’ for blinding. Sixteen studies39,46,48–52,56,57,59,60,63,65–68,70,72 were classed as ‘moderate’ for blinding. In most of these cases, either the assessors were not aware of the intervention and it was not clear if the participant was aware or vice versa. One study46 clearly stated that outcome assessors were blinded and participants were not. Another study60 reported that pharmacists were aware of the intervention status but participants were not. Another study63 reported that blinding of participants had failed. Seven studies45,53,55,58,64,69,71 were classed as ‘weak’ for blinding because both the outcome assessor and the participants were aware of the intervention.
Data collection methods and tools
Fifteen studies39,44,49–53,57,58,60,64–68,70,71 report using valid and reliable data collection tools and were classed as ‘strong’ and nine studies45,46,48,55,56,59,63,69,72 reported using valid data collection tools; however, reliability was not explicitly reported and these studies were classed as ‘moderate’. It should be noted that as well as the robustness of the data collection tools, the outcome data varied in terms of the type of outcome (e.g. behavioural or clinical) and how it was measured (e.g. self-reported, observed or biochemically confirmed).
Withdrawals and dropouts
Eight studies44,52,58,63,67,68,70,72 reported a follow-up rate of 80% or more participants and were classed as ‘strong’, and seven studies45,46,53,57,59,60,66 reported a follow-up of 60–79% participants and were classed as ‘moderate’. Eight studies followed up < 60% of participants, and in one study71 dropouts were not reported; these nine studies were classed as ‘weak’.
Global rating
In terms of overall quality assessment, seven studies46,57,59,60,63,67,70 were rated ‘strong’, six studies44,52,53,66,68,72 as ‘moderate’ and 11 studies39,45,48–51,55,56,58,64,65,69,71 as ‘weak’. These quality ratings and the individual quality criteria of which they are composed should be borne in mind when evaluating the effectiveness data.
Intervention integrity
Thirteen studies44–46,52,55,57,58,60,63,65,66,69,72 reported measuring consistency of the intervention; however, sometimes this included only compliance rather than whether or not the intervention was carried out in a consistent manner (Table 7). One smoking cessation study60 used both quantitative and qualitative methods to evaluate the training of the pharmacists and the process of the intervention from the perspectives of both pharmacy personnel and participants; another smoking cessation study58 interviewed pharmacists to gain insight into the process of the intervention. An alcohol intervention study48 used follow-up focus groups to explore the actual experience of the service. Very few studies incorporated a process evaluation. In the majority of studies it was unclear whether or not the intervention was carried out as intended.
| Author, year | Study information | Intervention integrity | Appropriate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Design | Country | Number of pharmacies | Consistency | Contamination | ITT | Power | Impute/assume | Cluster | |
| Alcohol | |||||||||
| Dhital et al., 201546 | RCT | UK | 16 | Yes | No | No | Yes | Yes | Yes |
| Watson and Stewart, 201148 | RCT | UK | 20 | No | No | No | No | No | Yes |
| Smoking cessation | |||||||||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 | CBA | UK | > 200 | No | No | No | Unclear | Yes | No |
| aBock et al., 201052 | RCT | USA | 2 | Yes | No | No | Unclear | Yes | No |
| Burford et al., 201353 | RCT | Australia | 8 | No | Yes | Yes | Yes | Yes | No |
| aCostello et al., 201155 | RCT | Canada | 98 | Yes | No | No | Yes | Yes | Yes |
| Crealey et al., 199856 | nRCT | UK | 2 | No | No | Unclear | Unclear | Unclear | No |
| Hoving et al., 201057 | RCT | The Netherlands | 65 | Yes | Yes | Yes | Unclear | Yes | Yes |
| Howard-Pitney et al., 199944 | RCT | USA | 5 | Yes | No | Yes | Unclear | Yes | No |
| Maguire et al., 200158 | RCT | UK | 51 | Yes | No | Yes | Unclear | Yes | No |
| Mochizuki et al., 200459 | RCT | Japan | 14 | No | No | No | No | Yes | No |
| Sinclair et al., 199860 | RCT | UK | 62 | Yes | No | No | No | Yes | Yes |
| Sonderskov et al., 199763 | RCT | Denmark | 42 | Yes | No | No | Unclear | Yes | No |
| Vial et al., 200245 | RCT | Australia | 9 | Yes | No | No | No | Yes | No |
| Weight loss | |||||||||
| Ahrens et al., 200364 | RCT | USA | 1 | No | No | No | Unclear | Yes | No |
| Bush et al., 201165 | CBA | UK | 12 | Yes | No | No | No | No | No |
| Jolly et al., 201166 | RCT | UK | NR | Yes | No | Yes | Yes | Yes | Yes |
| Malone and Alger-Mayer, 200339 | nRCT | USA | NR | No | No | Yes | No | Yes | No |
| Phimarn et al., 201367 | RCT | Thailand | 1 | No | No | No | Yes | No | No |
| Multicomponent interventions (pharmacotherapy + lifestyle changes) | |||||||||
| Diabetes mellitus – type 2 | |||||||||
| Ali et al., 201268 | RCT | UK | 2 | No | No | No | Yes | No | No |
| Correr et al., 201169 | nRCT | Brazil | 6 | Yes | No | No | Unclear | No | No |
| Fornos et al., 200670 | RCT | Spain | 14 | No | Yes | No | Yes | No | No |
| Dyslipidaemia | |||||||||
| Paulos et al., 200571 | RCT | Chile | 1 | No | No | No | Unclear | No | No |
| Hypertension | |||||||||
| Zaragoza-Fernandez et al., 201272 | RCT | Spain | 3 | Yes | No | No | Yes | No | No |
Three studies53,57,70 reported on the issue of contamination; one study57 reported that contamination of the control group was possible (may have received a similar intervention from external sources). Another study70 reported the possibility of cross-contamination between study groups because participants were randomised within pharmacies. The final study53 reported on measures used to avoid contamination of groups: participants were randomised according to treatment being used that week at the pharmacy by week of attendance.
Appropriate analysis
Eight studies46,53,55,66–68,70,72 reported the power of the study samples, at either 80% or 90%, one68 of which was powered for the studies primary outcome of glycated haemoglobin (HbA1c) percentage rather than the outcomes of this review (alcohol behaviour, smoking cessation or weight reduction). It was unclear if 10 studies44,49–52,56–58,63,64,69,71 were sufficiently powered as power was not reported. Six studies39,45,48,59,60,65 were not sufficiently powered to detect significant differences in treatment effect between groups. One study55 comprised > 7000 participants and was assumed to be sufficiently powered.
Six studies39,44,53,57,58,66 conducted intention-to-treat (ITT) analyses (all randomised participants were assessed at final follow-up). Eleven smoking cessation studies44,45,49–53,55,57–60,63 carried out analyses with the assumption that those lost to follow-up had not stopped smoking. Another four studies39,46,64,66 imputed data for those lost to follow-up; Jolly et al. 66 used three types of analyses: (1) completers only; (2) ‘baseline observation carried forward’; and (3) ‘last observation carried forward’. One alcohol study46 performed sensitivity analysis, carrying baseline values forward for people with missing follow-up scores. Eight studies48,65,67–72 performed completer analyses only; however, the size of these studies varied, as did the percentage dropout. Three studies52,68,72 had a very low dropout rate, at less than 12%, and in two studies,65,69 using completer analysis only, dropout was very high (40% in one case69 and 93% in the other65), increasing the potential for attrition bias. In one study48 there was substantially higher attrition of intervention participants (78%) than of control subjects (67%).
Given that participants were nested within pharmacies, few studies used hierarchical modelling techniques to adequately adjust for potential pharmacy- or pharmacist-level effects on individual participant outcomes. Twenty studies39,44–46,52,53,55–59,63,64,66–72 randomised individual participants to groups; one study70 randomised participants within pharmacies. The two studies48,60 that randomised pharmacy settings (rather than individual participants) accounted for clustering within the analyses. Another two studies55,57 accounted for between-pharmacy variance using multilevel analyses in order to understand whether or not variability in participant outcomes existed between pharmacies. One alcohol study46 tested within-group changes in Alcohol Use Disorders Identification Test (AUDIT) scores from baseline to follow-up using a generalised linear mixed model with fixed effects for group and time (nested within group) and random effects for pharmacist and participant (nested within pharmacist). Another study66 performed secondary analyses of commercial weight loss programmes compared with primary care-based programmes (including pharmacy) and adjusted for clustering of participants within the intervention groups. One study52 adjusted for pharmacist and pharmacist sex in the analyses. The number of pharmacies within the studies ranged from 1 to over 200; seven studies52,56,64,67,68,71,72 used three or fewer pharmacies, which may limit the generalisability of the results.
Implementation of interventions
Implementation was evaluated using the tool30,31 for the assessment of the implementation of complex public health interventions in systematic reviews; this tool covered three key domains: organisation, implementation and delivery of interventions. In addition, the Behaviour Change Wheel32 and the Nuffield Intervention Ladder33 were used to broadly describe the interventions by grouping and classifying the policy categories and intervention functions.
Implementation
More details on implementation are reported in Appendix 9. Contextual subsections included political, economic, social and managerial factors. Interventions were categorised as ‘political’ if the primary purpose for developing and testing the intervention was the national political drive to extend the public health role of community pharmacies. Interventions were categorised as ‘economic’ if the primary purpose for developing and testing the intervention was to assess whether existing services could be delivered at a lower cost in pharmacies (and usually by pharmacists and pharmacy staff) than in other settings or by other service providers. Interventions were classed as ‘social’ if the primary purpose for developing and testing the intervention was to assess the reach of services to those most in need in pharmacies compared with similar services in other settings and offered by other service providers. Interventions were classed as ‘managerial’ if the primary purpose for developing and testing the intervention was to assess whether or not existing services set in pharmacies and delivered by pharmacists could be delivered equally effectively by pharmacy assistants.
The implementation context of the majority of interventions (n = 16) included in this systematic review was political. 39,45,46,48–52,55,58,60,63–67,69,70 For two of these 16 studies, the authors also performed a cost analysis. 49–51,61 The implementation context of three studies was economic. 53,56,69 For the remaining five included studies, the interventions had no specific implementation context.
Very few studies reported any degree of consultation or collaboration with stakeholders as part of the planning process or during delivery of the intervention. Watson and Stewart48 conducted focus groups with pharmacists and members of the public during the planning stages of their brief alcohol intervention, and their views and suggestions were incorporated into the intervention. Pharmacists were consulted in the planning of another brief alcohol intervention regarding an acceptable and feasible training period. 46 In two studies44,49–51 health authority staff provided assistance, but it is not clear whether or not this related to the implementation of the intervention. The study by Hoving et al. 57 collaborated with a national charity on smoking and health, and together they developed the intervention.
The study by Costello et al. 55 was nested within a ‘host’ study called Smoking Treatment for Ontario Patients (STOP), which collaborated with different community and regional partners in many different ways during the planning and delivery of the intervention, including tertiary care centres, public health units, mass distribution, community pharmacies, community health centres, STOP on-the-road workshops with primary health units, internet-based enrolment, family health teams and family physicians.
The issue of sustainability is particularly interesting for the interventions included in this review. In the majority of interventions, regardless of their target behavioural outcome, pharmacists received reimbursement for providing the intervention. The authors of a number of the studies highlighted that reimbursement to the pharmacist for providing the service is necessary in order for the intervention to be sustainable. 55,58
Organisation and delivery
More details on the organisation and delivery of interventions are reported in Appendix 10. All of the included studies were set in community pharmacies, although the nature of these varied in some countries. Twenty-one of the 24 interventions were delivered by the resident pharmacy staff; three smoking cessation interventions used other deliverers. One smoking cessation intervention53 was delivered by a research pharmacist employed by the local university, who delivered the intervention at all sites, and another smoking cessation intervention was delivered by a Master of Science student. 45 One smoking cessation intervention involved the postal delivery of a computer-generated letter. 57
Of the 21 interventions delivered by resident pharmacy staff, it was clear that most studies (n = 17) included standardised staff training, although this was usually brief (ranging from 2 hours to 2 days). Two of these studies, one of smoking cessation and one of quitting chewing tobacco, mentioned they also included role-play as part of the training44,52 and two weight loss studies reported that they also included ‘practical tasks’ as part of the training. 66,67
Of the four interventions delivered by resident pharmacy staff that did not include standardised staff training, we only had the abstract in English for one smoking cessation study59 at the time of writing this report. Another smoking cessation study63 included no mention of staff training but did state that the pharmacy staff were given instructions from the pharmaceutical company regarding procedures. The pharmacists responsible for delivering a weight management programme in another study64 did not receive any training, but were expected to carry out self-directed learning to prepare themselves to be able to counsel patents in dietary advice. Of note, in this study, a registered dietitian reviewed the dietary plan developed by the pharmacist before it was used with the patients, and was consulted as needed during the study. The weight management study by Zaragoza-Fernandez et al. 72 did not mention any staff training.
In terms of quality assurance, one alcohol reduction study provided a 2-hour evening follow-up training session during the intervention to address challenges and share learning across the pharmacists who were delivering the intervention. 46 In two smoking cessation studies,56,58 a researcher visited the pharmacists after the group training session to provide support and to address any queries they had in implementing the training. In one smoking cessation study that was organised by a pharmaceutical company63 the company contacted pharmacies at least once a week during the intervention. In a multicomponent study of people with type 2 diabetes mellitus,70 pharmacists had regular contact with the research team during the study and attended clinical sessions where results on drug-related problems were presented and discussed.
All studies reported information about the experience of the person/staff who delivered the intervention. In all cases this was the resident pharmacist, the research pharmacist or the pharmacy staff team (including the pharmacists and pharmacy technician/assistant). However, in most cases it was unclear who developed the intervention. Information on resources was documented for some of the studies, but the level of detail was variable. Seven studies (one alcohol reduction,48 four smoking cessation49–51,53,56,61 and two weight loss65,66) included a cost analysis (see Table 12).
Behaviour change
More details on the theoretical basis and behaviour change strategies of interventions are reported in Appendix 8. Fifteen studies44–46,48–52,55–58,60,65–67,70 reported the behaviour change strategy, model and/or theory of the intervention. A number of these studies reported using multiple behaviour strategies, models and/or theories. The most commonly reported was the transtheoretical (stages-of-change) model, which was reported by six studies,44,45,56,58,60,66 followed by motivational interviewing which was reported by five studies. 48,52,56,58,66,67 In addition, one intervention was informed by the I-change model,57 and another by the theory of planned behaviour. 67 Nine studies39,53,59,63,64,68,69,71,72 did not report using any type of behavioural counselling or support as part of the intervention.
The Behaviour Change Wheel32 and the Nuffield Intervention Ladder33 are both mainly descriptive models with broad aggregations and classifications on intervention strategies. These approaches were chosen as the descriptions available in included studies did not allow for coding specific aspects of theory and strategy used in the included interventions. Using the Behaviour Change Wheel32 the intervention functions of the majority of interventions identified in this review were ‘education’ and ‘enablement’. In addition, interventions that included the provision of NRT or commercial weight loss programmes or products, free of charge, were also deemed to include the intervention function ‘incentivisation’. One smoking cessation intervention, which included the use of face-ageing software,53 was deemed to include the intervention function ‘persuasion’. One weight loss intervention,64 which included the use of meal replacements, was deemed to include the intervention function ‘restriction’.
Using the policy category of the Behaviour Change Wheel32 all of the interventions were categorised as ‘service provision’. Six of these interventions also included ‘communication/ marketing’. 44,57,58,64,65,70 One intervention included computer-generated individually tailored advice in the form of a letter,57 and the other five interventions used various marketing recruitment strategies such as the involvement of the local press and supermarkets. No other policy categories were identified.
Using the Nuffield Intervention Ladder33 most interventions included in this review were coded as ‘enable choice’. 46,48–53,56–60,65–72 Those interventions which included the provision of NRT or commercial weight loss programmes or products, free of charge, were coded as ‘guide choice – incentives’. 44,45,52,55,63,66 Two interventions that lacked any detail about advice given, apart from the provision of educational information, were coded as ‘provide information’. 39,69 The meal replacement intervention64 for weight loss was coded as ‘restriction’.
Motivation/aim
All of the included studies clearly described the motivation (aim) behind the intervention investigated. Details can be found in Tables 2–5.
Follow-up and evaluation
All studies included information on follow-up response rate/retention (see Tables 2–5). However, in terms of implementation, there was very little useful information in the included studies. Six studies (four smoking cessarian49–51,53,56,60,61 and two weight management65,66) included some form of economic evaluation, but only one included a process evaluation. 63 One study included interviews and focus groups with the deliverers and participants which provided useful implementation information. 48
Intervention delivered as intended
None of the studies reported details about whether or not the intervention was delivered as intended, for example by observation of sessions, quality control audits, staff and researcher records. However, some studies included methods that were put in place to improve quality assurance (examples include standardised training and protocols/manuals, practice ‘role-play’ sessions with feedback, regular meetings with trainers/supervisors/more experienced members of the intervention team during the intervention). More information on methods to improve quality assurance is provided in Appendix 10.
Differential effects
The differential effects of the interventions, by age, sex, ethnicity or SES of the participants, are discussed within the main results section (see Differential effects by demographic or socioeconomic factors and see Table 13). In summary, very few of the studies included in this review reported the differential effects of an intervention by demographic variables and none evaluated any moderating effect of SES.
Effects of interventions
Study outcomes are summarised in Tables 8–11 for each type of intervention focus (alcohol, smoking, weight and multicomponent). Costs are summarised in Table 12. Demographic and socioeconomic variables are summarised in Table 13. See Appendix 11 for detailed outcomes for all studies.
| Study | Group | Duration (weeks) | AUDIT total scores | FAST total scores | Effective? I vs. C | Cost-effective? | Differential effects by demographic/SES? | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean change | 95% CI | n | Mean change | 95% CI | n | ||||||
| Dhital et al., 201546 | Pharmacy-based brief alcohol advice | 12 | 0.11 | –0.82 to 0.61 | 168 | ↔ | NR | NR | |||
| Pharmacy-based leaflet-only control | 12 | –0.74 | –1.47 to 0.00 | 158 | |||||||
| Watson and Stewart, 201148 | Pharmacy-based brief alcohol advice | 26 | 2.50 | 1.50 to 4.25 | 6 | ↔ | ? | NR | |||
| Pharmacy-based control | 26 | 3.50 | 2.00 to 7.50 | 14 | |||||||
| Study | Group | Duration (weeks) | Quits numerator | Quits denominator | Effective? I vs. C | Cost-effective? | Differential effects by demographic/SES? |
|---|---|---|---|---|---|---|---|
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 | Individual pharmacy-based NHS smoking cessation service + NRT | 52 | 38 | 1374 | ? | Yes both services | NR |
| Group-based NHS smoking cessation service + NRT | 52 | 26 | 471 | ||||
| Bock et al., 201052 | Smoking cessation training for pharmacists + tailored counselling using computer software + NRT | 26 | 28 | 100 | ↑ | NR | NR |
| Smoking cessation training for pharmacists + tailored counselling using computer software | 26 | 15 | 100 | ||||
| Observation only control (not randomised) | 26 | 8 | 99 | ||||
| Burford et al., 201353 | Smoking cessation advice + computer-generated photoageing | 26 | 11 | 80 | ↑ | Yes | NR |
| Smoking cessation advice | 26 | 1 | 80 | ||||
| Costello et al., 201155 | For 1 week then fortnightly pharmacy visits for NRT plus three sessions of brief behavioural counselling | 5 | 612 | 3503 | ↔ | NR | NR |
| 5 weeks of NRT at initial pharmacy visit plus one session of brief behavioural counselling at initial visit | 5 | 604 | 3350 | ||||
| Crealey et al., 199856 | Behavioural support (35/52 participants also had nicotine gum) | 26 | 24 | 52 | ↑ | Yes | NR |
| Nicotine gum only | 26 | 3 | 48 | ||||
| Control (expressed wish to stop smoking) | 26 | 0 | 60 | ||||
| Hoving et al. 201057 | Computer-generated tailored advice | 26 | 2 | 256 | ↔ | NR | NR |
| Thank-you letter control | 26 | 2 | 289 | ||||
| Howard-Pitney et al., 199944 | Advice and support + nicotine patch | 26 | 78 | 206 | ↔ | NR | NR |
| Advice and support + placebo patch | 26 | 69 | 204 | ||||
| Maguire et al., 200158 | Behavioural support, 87% (230/265) NRT | 52 | 38 | 265 | ↑ | NR | NR |
| Ad hoc advice on smoking cessation, 84% (183/219) NRT | 52 | 6 | 219 | ||||
| Mochizuki et al., 200459 | Nicotine gum plus advice on usage, initial and follow-up cessation advice | 12 | 5 | 11 | ↔ | NR | NR |
| Nicotine gum plus advise on usage | 12 | 5 | 16 | ||||
| Sinclair et al., 199860 Sinclair et al., 199961 | Training pharmacists/assistants in smoking cessation behaviour change + NRT | 36 | 26 | 217 | ↔ | Yes | NR |
| Standard professional pharmacy support + NRT | 36 | 19 | 257 | ||||
| Sonderskov et al., 199763 | 21-mg nicotine patches | 26 | 15 | 132 | ↑ | NR | No for sex |
| Placebo 21-mg patches | 26 | 6 | 142 | ||||
| 14-mg nicotine patches | 26 | 27 | 119 | ↔ | |||
| Placebo 14-mg patches | 26 | 23 | 125 | ||||
| Vial et al., 200245 | Pharmacy-based nicotine patches plus weekly counselling | 52 | 4 | 21 | ↔ | NR | NR |
| Hospital outpatient clinic nicotine patches plus weekly counselling | 52 | 5 | 21 | ||||
| Minimal intervention (written and verbal information at baseline) | 52 | 1 | 22 |
| Study | Group | n | Duration (weeks) | BMI (kg/m2) | WC (cm) | Weight (kg) | Effective? I vs. C | Cost-effective? | Differential effects by demographic/SES? | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD/(95% CI) | Mean | SD | Mean | SD/(95% CI) | |||||||
| Ahrens et al., 200364 | Pharmacy-based meal replacement diet | 45 | 22 | –8.08 | NR | –5.6 | NR | ↔ | NR | NR | ||
| Pharmacy-based low-calorie diet | 43 | 22 | –7.82 | NR | –5.2 | NR | ||||||
| Bush et al., 201165 | Pharmacy-based diet + PA | 60 | 15 | –1.3 | 0.4 | –6.5 | 1.6 | –3.4 | 1.1 | ? | Yes | Yes, but unclear because demographics of participants differed significantly between settings |
| GP diet + PA | 22 | 15 | –0.8 | 0.7 | –4.9 | 2.6 | –2.3 | 1.9 | ||||
| Jolly et al., 201166 | Pharmacy-based diet + PA | 70 | 52 | –0.31 | (–0.7 to 0.0) | –0.66 | (–1.7 to –0.4) | ↔ | No, commercial organisations more effective and lower cost than GP and pharmacy-based services | No for sex | ||
| Exercise only | 100 | 52 | –0.45 | (–0.8 to –0.1) | –1.08 | (–2.1 to –0.1) | ||||||
| Weight Watchers | 100 | 52 | –1.17 | (1.7 to –0.7) | –3.46 | (–4.8 to –2.1) | ||||||
| Slimming World | 100 | 52 | –0.71 | (–1.0 to –0.4) | –1.89 | (–2.9 to –0.9) | ||||||
| Rosemary Conley | 100 | 52 | –0.75 | (–1.1 to –0.3) | –2.12 | (–3.4 to –0.9) | ||||||
| NHS Size Down | 100 | 52 | –0.67 | (–1.0 to 0.0) | –2.45 | (–3.6 to –1.3) | ||||||
| GP | 70 | 52 | –1.32 | (–0.7 to 0.1) | –0.82 | (–2.0 to –0.4) | ||||||
| Own choice | 100 | 52 | –0.90 | (–1.3 to –0.5) | –2.15 | (–3.4 to –0.9) | ||||||
| Malone and Alger-Mayer, 200339 | Pharmacy support orlistat + usual outpatient care | 15 | 26 | –3.5 | 2.9 | ↔ | NR | NR | ||||
| Orlistat + usual outpatient care | 15 | 26 | –3.0 | 5.2 | ||||||||
| Phimarn et al., 201367 | Pharmacist individual support | 33 | 16 | –0.8 | 0.07 | 0.1 | 0.03 | –0.82 | 0.29 | ↔ | NR | NR |
| Primary care unit group support | 33 | 16 | 0.19 | 0.04 | –0.28 | 0.08 | 0.92 | 0.19 | ||||
| Study | Group | n | Weeks | Study primary outcome | BMI (kg/m2) | WC (cm) | Weight (kg) | Effective I vs. C (study primary outcome) | Effective I vs. C (BMI/WC/weight) | Cost-effective | Differential effects by demographic/SES | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD/(95% CI) | Mean | SD | Mean | SD/(95% CI) | Mean | SD | ||||||||
| Diabetes mellitus – type 2 | HbA1c (%) | ||||||||||||||
| Ali et al., 201268 | Diabetes mellitus monitoring and lifestyle modification counselling | 23 | 52 | –1.6 | NR | –3.86 | NR | ↑ | ↔ | NR | NR | ||||
| Usual care | 23 | 52 | –0.6 | NR | –1.09 | NR | |||||||||
| Correr et al., 201169 | Medicines management and patient education | 50 | 52 | –2.2 | (–2.8 to –1.6) | –0.2 | (–0.8 to 0.3) | 0.8 | (–0.7 to 2.4) | ↑ | ↔ | NR | NR | ||
| Usual care | 46 | 52 | –0.3 | (–0.8 to 0.2) | –0.1 | (–0.7 to 0.4) | 0.06 | (–2.0 to 2.1) | |||||||
| Fornos et al., 200670 | Medicines management and lifestyle advice | 56 | 56 | –0.5 | NR | –0.9 | NR | ↑ | ↔ | NR | NR | ||||
| Usual care | 56 | 56 | 0.7 | NR | –0.3 | NR | |||||||||
| Dyslipidaemia | TC (mg/dl) | ||||||||||||||
| Paulos et al., 200571 | Medication compliance plus lifestyle modification | 23 | 16 | –27.1 | 41.1 | 0.4 | NR | –1.0 | 1.3 | ? | ? | NR | NR | ||
| Usual care | 19 | 16 | –1.4 | 37.2 | NR | 1.1 | 2.6 | ||||||||
| Hypertension | SBP/DBP (mmHg) | ||||||||||||||
| Zaragoza-Fernandez et al., 201272 | Diet plus physical activity | 71 | 8 | –16.08/–9.95 | 9.46/7.46 | –0.4 | NR | –0.7 | NR | ↑ | ↔ | NR | NR | ||
| Usual care | 72 | 8 | 1.79/0.95 | 5.12/3.37 | –0.2 | NR | –0.6 | NR | |||||||
| Study | Intervention | Effectiveness | Cost data | Type of economic evaluation and perspective | Costs and consequences | Summary |
|---|---|---|---|---|---|---|
| Alcohol management | ||||||
| Watson and Stewart, 201148 | Brief alcohol intervention vs. usual care | No significant difference between FAST score for intervention vs. control at 3 or 6 months; recruitment and retention difficulties | Training costs annuitised over 3 years and staff costs based on fee payment to participating pharmacies | Costs only. Health service perspective | Cost per participant: £70.90 based on an average of 10 people screened for each brief alcohol intervention delivered | Not effective. Cost-effectiveness cannot be ascertained |
| Smoking cessation | ||||||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 | One-to-one pharmacist counselling or group community-based counselling vs. ‘self-quit’ scenario | Group-based service was significantly more effective in stopping smoking at 12 months compared with the pharmacy-based service (6.3% quits vs. 2.8% quits) using validated measures and adjusted for baseline differences In each service more smokers from deprived areas had lower cessation rates, although the trend relating socioeconomic score to cessation rate was significant only for the pharmacy service |
Direct cost to Glasgow NHS Health Board (NRT, professional time, materials and overheads) | Cost-effectiveness analysis, health service perspective | Incremental cost per QALY = £2600 for pharmacy one-to-one counselling; £4800 for group support Cost per pharmacy-based client was £79 based on 0.025 probability of 52-week quit |
Both services were considered to be highly cost-effective despite relatively low quit rates. Group service achieved a higher quit rate (6.3%) than the pharmacy service (2.8%), but was more intensive and required greater overhead costs. Pharmacy-based service attracted more clients Participants chose the service rather than being assigned by study investigators and, consequently, may have been a relatively more motivated sample |
| Burford et al., 201353 | Computer-generated photoageing service plus standard smoking cessation advice vs. standard smoking cessation advice only | Photoageing intervention was effective in stopping young people (mean age 24.2 years) smoking compared with control (13.8% vs. 1.3%) using validated measures at 6 months. Adjusted for baseline differences | Direct costs over and above providing standard cessation advice were calculated based on the time taken to provide the service and the cost to a pharmacy of purchasing tokens to use the online software to photoage participants. Potential cost offsets were based on the quit benefits model | Cost-effectiveness analysis, health service perspective | ICER = AU$46 per additional quitter or the equivalent of AU$74 per additional lifetime quitter Cost offsets of AU$2144 from a reduction in the health-care costs of quitters resulted in the intervention potentially generating net total cost savings of AU$1778. The mean cost of implementing the intervention was estimated at AU$5.79 per participant. The mean cost that participants indicated they were willing to pay for the digital ageing service was AU$20.25 (SD AU$5.32) |
Photoageing was effective and cost-effective in increasing quit rates |
| Crealey et al., 199856 | Pharmacist behavioural support based on the PAS model | At 6 months there was a statistically significant difference in cessation rates between intervention and control patients. Six-month CO-verified abstinence was 46% in the intervention group vs. 6% in the nicotine gum-only control and 0% in the control group that expressed a wish to stop smoking | Direct costs including fixed costs of PAS materials (manuals, flip charts, posters, etc.) and provision of training for all pharmacists involved in the programme and variable costs of time spent by pharmacists counselling patients on smoking cessation (1 hour over the 6-month period) at £30 per hour | Cost-effectiveness analysis, health service (payer) perspective | The cost-effectiveness of the PAS model was therefore measured in terms of cost per life-year gained for all patients who enter stage 3 of the PAS programme. The main cost-effectiveness analysis indicated that costs ranged from £196.76 to £351.45 per life-year saved for men, and from £181.35 to £772.12 per life-year saved for women, depending on the age at intervention Given the baseline assumptions (see table I) and on the basis of a 45-year-old smoker, the cost per successful intervention was £509.60 |
The PAS model appears effective and if the PAS smoking cessation programme were to be offered routinely by community pharmacists throughout Northern Ireland it would be cost-effective The cost of the programme was, however, sensitive to changes in the discount rate, variable costs and the success rate of the programme |
| Sinclair et al., 199860 Sinclair et al., 199961 | Training pharmacists and pharmacy assistants in the stages-of-change model of smoking cessation vs. standard professional pharmacist care | Intervention was associated with a favourable non-significant trend at 9 months based on self-reported continued abstinence (12% vs. 7.4%); outcome was not affected by sex, age or SES | Training costs included organising and operating costs of the training sessions and trainees’ out-of-pocket expenses, including staff costs and travel plus lost leisure time. Cost to the pharmacy was training time and counselling time. Cost to the NHS was organising and operating costs, pharmacy travel expenses and promotional materials and client documentation Participant costs included NRT and counselling time |
Cost-effectiveness analysis, societal perspective | The cost of producing one additional successful attempt to quit smoking by using intensive rather than standard pharmaceutical support was £300, or £83 per life-year | Intervention cost-effective despite non-significant trend for cessation |
| Weight loss | ||||||
| Bush et al., 201165 | Weight management programme (diet and PA) delivered through community pharmacies vs. GPs | Both groups appeared to reduce BMI, WC and weight at follow-up, statistical significance either from baseline to follow-up or between groups were not reported | Direct costs included training, initial test and appointments. Providers were reimbursed £300 for attending 2 days of training on recruitment of six participants; £30 for the initial assessment of each participant and £10 for each consultation after the initial assessment | Cost-effectiveness analysis, health service perspective | At session 12 each extra kilogram of weight loss per participant would cost £8.29 through pharmacy providers. Conversely, at session 15, each extra kilogram of weight loss per participant would cost £2.91 through GP providers. At the end of the intervention the ICER favoured the pharmacy Total costs were higher among GP providers (£26,970) than among pharmacy providers (£23,230). Costs per participant were higher through pharmacies (£126.90) than through GPs (£100.60). This was true throughout the course of the programme, but the gap in costs between pharmacy and GP providers narrowed as participants continued through the programme to the point where there was no statistically significant difference in costs between providers among participants attending session 15. Differences in costs was explained by number of participants The differences between providers were statistically significant; among participants attending session 12, the cost per kg of weight loss was £57.00 with costs being higher among pharmacy providers (£74.80) than among GP providers (£43.40). Among participants attending session 15, the opposite pattern was observed with costs being lower among pharmacy providers than GP providers for both measures (although these differences were not statistically significant) |
Unclear which provider type (pharmacy or GP) delivered the programme more cost-effectively because of different cost-effectiveness results at different time points. Pharmacies appear to provide more cost-effective delivery than GPs over entire intervention period. Participant demographics different between settings. Attendance rates on the programme were consistently better among pharmacy participants than among GP participants 93% attrition. Participants chose the service rather than being assigned by study investigators and consequently may have been a relatively more motivated sample |
| Jolly et al., 201166 | Pharmacy-based intervention vs. WW vs. SW vs. RC vs. Size Down vs. GP vs. participant own choice vs. exercise-only control | All except the GP and pharmacy groups resulted in significant weight loss at 1 year. At 1 year, only the WW group had significantly greater weight loss than did the control group (2.5 kg, 95% CI 0.8 kg to 4.2 kg). Mean weight loss at 1 year, with BOCF, was 0.8 kg (SD 4.7 kg) for primary care and 2.5 kg (6.2 kg) for commercial programmes BOCF 1-year weight change (kg): WW –3.46 (–4.8 to –2.1); SW –1.89 (–2.9 to –0.9); RC –2.12 (–3.4 to –0.9); Size Down –2.45 (–3.6 to –1.3); GP –0.82 (–2.0 to –0.4); pharmacy –0.66 (–1.7 to –0.4); choice –2.15 (–3.4 to –0.9); C –1.08 (–2.1 to –0.1) |
Direct costs to the primary care trust of each programme and of sending out invitation letters from practices. These included the costs of the provider’s service and the cost of the searches in general practice, invitation letters and provision of call centre support. The cost of the call centre that co-ordinated the service as an average per person, based on the cost of staff employed over a 12-month period and the number of clients who used the service over this time period. Costs to the participants were not included nor were any training costs for providers | Cost-effectiveness analysis, health service perspective | The primary care programmes were the most costly to provide In the most effective intervention (WW), participants lost 1.3 kg/m2, authors recalculated the life table on the basis of this reduced BMI. The difference in life expectancy was about 1 year. If we assumed that the people randomised to WW continued to weigh 1.3 kg/m2 less throughout life, then the cost per life-year saved was about £77 (not discounted) Cost per participant: WW, £76.87; SW, £71.37; RC, £76.87; Size Down, £91.87; GP, £112.73; pharmacy, £112.30 |
One-to-one GP and pharmacy-based were ineffective and most costly to provide. Commercial organisations more effective and lower cost than GP and pharmacy-based services |
| Study | Baseline | Within groups | Between groups | Retention |
|---|---|---|---|---|
| Alcohol management | ||||
| Dhital et al., 201546 |
|
NR | NR | Loss to follow-up was not related to whether a person was in the control or intervention group, their pharmacist, sex, ethnicity or education (all p > 0.05). Responders at follow-up were significantly more likely to be older than non-responders [42.1 years (SD 17.1 years) vs. 32.0 years (SD 12.2 years); p < 0.001] |
| Watson and Stewart, 201148 |
|
NR | FAST score by sex reported – not powered | NR |
| Smoking cessation | ||||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 |
Both services were attended by a larger proportion of women compared with men The smaller number of clients who attended the group service were older, slightly more affluent (although still a relatively deprived group) and more likely to be women |
Explored characteristics of quitters: at 52 weeks, older people were more likely to quit in either group, higher SES was associated with long-term abstinence for pharmacy clients. Sex did not predict quitting in either group. Group clients more likely to quit than pharmacy clients | NR | NR |
| Bock et al., 201052 |
|
NR | NR | NR |
| Burford et al., 201353 |
|
For the control group, there were no associations between change in score and age (p = 0.14), sex (p = 0.72). However, for the intervention group, age (p < 0.001) was significantly associated with change in score [Fagerström Smoking Dependence Scale (score from 0 to 10) www.quittersguide.com/fagerstrom-scale.shtml] whereas sex (p = 0.34) was not associated. Older participants were less likely to reduce their score than younger participants (p = 0.001), suggesting that the intervention may have a greater effect on the younger participants | NR | NR |
| Costello et al., 201155 |
|
Among 3-session completers, both younger and employed individuals were more likely to be abstinent than older and employed participants | NR | NR |
| Crealey et al., 199856 | NR | Cost-effectiveness analysis by age of onset of intervention | NR | NR |
| Hoving et al., 201057 |
|
NR | NR | NR |
| Howard-Pitney et al., 199944 |
|
Older chewers were less likely to relapse. Only examined active patch group | NR | NR |
| Maguire et al., 200158 |
|
Age and sex of pharmacist did not affect smoking cessation outcome | NR | NR |
| Mochizuki et al., 200459 |
|
NR | NR | NR |
| Sinclair et al., 199860 |
|
Trends in outcome were not affected by sex, age and SES | NR | NR |
| Sonderskov et al., 199763 |
|
NR | There were no differences in smoking cessation rates between men and women according to starting dose and treatment | NR |
| Vial et al., 200245 |
|
NR | NR | NR |
| Weight loss | ||||
| Ahrens et al., 201164 |
|
NR | NR | NR |
| Bush et al., 201165 |
GP participants tended to be older than pharmacy participants; ethnic composition of the two groups differed significantly |
There were no statistically significant relationships between sex, age, IMD quintile or ethnicity and percentage weight loss at session 12 within pharmacy or GP participants in the weight management programme | There were isolated statistically significant differences in weight loss between participants attending the programme at pharmacies and GPs. For example, female GP participants lost a larger proportion of their initial weight than female pharmacy participants. Similarly, participants aged 40–49 years lost a greater proportion of their initial weight at GP providers than at pharmacy providers | NR |
| Jolly et al., 201166 |
In one practice there was no difference in ethnicity, mean age or socioeconomic deprivation, but women were more likely to accept the invitation to take part |
In adjusted models, sex had no effect on weight loss at programme end or 1 year | No statistically significant interaction between sex and the weight loss programme | Participants who were lost to follow-up tended to be younger than those who were followed up, but they were similar in terms of BMI, sex, ethnicity and IMD score |
| Malone and Alger-Mayer, 200339 |
|
NR | NR | NR |
| Phimarn et al., 201367 |
|
NR | NR | NR |
| Multicomponent interventions (pharmacotherapy and lifestyle changes) | ||||
| Diabetes mellitus – type 2 | ||||
| Ali et al., 201268 |
|
NR | NR | NR |
| Correr et al., 201169 |
|
NR | NR | % women, greater among the dropouts (73.3% dropouts vs. 53.1% completers; p = 0.014) |
| Fornos et al., 200670 |
|
NR | NR | NR |
| Dyslipidaemia | ||||
| Paulos et al., 200571 |
|
NR | NR | NR |
| Hypertension | ||||
| Zaragoza-Fernandez et al., 201272 |
|
Only for blood pressure (age and sex) | NR | NR |
Alcohol interventions
There were two RCTs of brief alcohol interventions in adults compared with usual care or leaflet-only control; global ratings were strong for one study46 and weak for the other. 48 Table 8 summarises the results. Pharmacist training was provided in both studies. Both studies reported using behaviour change strategies and involved one-to-one contact with the pharmacist. Dhital et al. 46 encouraged self-directed behaviour change; the intervention included reflection and feedback of the AUDIT score.
Behavioural outcomes
Both studies had change in alcohol consumption as the primary outcome; however, different tools were used in each. Eligibility criteria for both studies were scores that indicated ‘possible harmful or hazardous alcohol consumption, but not alcohol dependence’ [indicated by an AUDIT score of 8–19 or a Fast Alcohol Screening Tool (FAST) score of 3–16]. One RCT46 used the AUDIT and reported a baseline AUDIT score of 11.93 and the other RCT48 used the FAST and reported 29.2% scoring ≥ 3 at baseline.
At 12 weeks there was no evidence in effectiveness of community pharmacist delivery of brief alcohol intervention. The AUDIT total change score did not differ significantly between the two groups and did not change significantly between baseline and follow-up in either the intervention or control group. The 12-week AUDIT between-group difference adjusted for pharmacist, sex, age, ethnicity and education was –0.57 [95% confidence interval (CI) –1.59 to 0.45].
There was no significant difference between FAST score for the intervention group compared with control at 3 or 6 months and adjusted for baseline FAST. The 6-month FAST between-group difference was –1.84 (95% CI –4.49 to 0.82). At 6 months there was substantially lower follow-up of intervention clients (22.2%) than of control clients (33.3%).
Meta-analysis
There were insufficient data to conduct meta-analyses.
Costs
One RCT48 reported on staff costs and training costs associated with the brief alcohol intervention. A baseline estimate used training costs annuitised (converted to a yearly rate) over 3 years and staff costs based on fee payment to participating pharmacies. Economic data were derived from financial records maintained by the research team and from pharmacy logs. The overall cost for delivering the brief alcohol intervention was £70.90, based on an average of 10 people screened for each brief alcohol intervention delivered. The costs were particularly sensitive to the staff time cost, the number of clients screened per pharmacy and the number of clients screened per brief intervention delivered. In addition, the time taken to deliver the brief intervention was very variable.
Differential effects by demographic or socioeconomic factors
Neither study evaluated differential effects by demographic or socioeconomic factors. One study48 reported change in FAST scores by sex; however, the numbers of participants were very small and the study was not powered to detect differences between the two groups.
UK service evaluations
Seventeen UK alcohol service evaluations were identified (see Appendix 12 for further details). All of the evaluations focused on alcohol screening and/or brief intervention. The number of pharmacies involved in the evaluations ranged from 4 to 240. The evaluation period (where reported) ranged from 8 weeks to 9 months. The service was delivered by pharmacists, technicians and support staff. The number of participants ranged from 30 to 2479. The majority of the services used either the AUDIT, AUDIT – Consumption or FAST. One evaluation used a ‘Drinkaware kit’78 that offered three resources [a plastic half-pint measuring glass, a cardboard wheel showing units and a booklet (which contained a drink diary)].
The length of the follow-up period was often not reported; where reported, it ranged from 2 weeks to 8 months. Some evaluations have followed up patients, but rather than ascertain alcohol consumption using the original screening tool, have explored patients’ alcohol intake from a qualitative perspective, which makes comparisons regarding change in alcohol consumption difficult. Other evaluations have followed up patients and have focused on satisfaction of the original intervention, rather than explore how alcohol consumption has changed. Of the evaluations found, only those done on a small scale have followed up patients using the original screening tool; the follow-up rates were also low with respect to the initial numbers accessing the services.
The evaluations do, however, show that community pharmacy is an appropriate place to screen patients and offer brief advice in relation to alcohol misuse. Indeed, of the evaluations obtained so far, over 50,000 patients have been screened in a community pharmacy setting – with over 20,000 reported as harmful or hazardous drinking. Significantly, a small proportion of these patients have been referred on to specialised alcohol services owing to intake that may suggest alcohol dependence. These results demonstrate the potential reach of the community pharmacy network.
Few evaluations reported the type of people accessing these services; one evaluation79 reported that service users who were female and aged over 60 years of age were more likely to access the service. Significantly, males had higher AUDIT scores than females and patients from more deprived areas had higher AUDIT scores. This evidence suggests that these types of services have the potential to reach those most in need.
It is not clear whether or not the screening and/or brief advice provided in the pharmacy setting reduces a patient’s consumption of alcohol over time. In addition, for the three main screening tools used in the community pharmacy, it was not clear which is the most appropriate to use in relation to patient outcome.
A summary of the evidence for alcohol interventions is provided in Box 1.
-
There was insufficient evidence for the effectiveness or cost-effectiveness of community pharmacy-based brief alcohol interventions.
-
It is not clear whether or not the UK alcohol screening services provided in the pharmacy setting reduce a patient’s consumption of alcohol over time.
Smoking cessation interventions
There were 10 RCTs,44,45,52,53,55,57–60,63 one nRCT56 and one CBA49–51 of smoking cessation interventions in adults. Table 9 summarises the results. Follow-up ranged from 5 weeks to 12 months. Quit rates within the pharmacies ranged from < 1% to 56%. Global ratings for studies varied: four studies57,59,60,63 were rated strong, three moderate44,52,53 and five weak. 45,49–51,56,58
Eleven smoking cessation studies44,45,49–53,55,57–60,63 carried out analyses with the assumption that those lost to follow-up had not stopped smoking. Details of the type of analysis used to measure effectiveness were not reported in one study. 56 Some studies also included completer analyses; however, we focus on results from the largest data set within each study and on the longest follow-up. Numbers of participants ranged from 2859 to approximately 7000,55 with the majority of studies including between 300 and 600 participants. Half (6/12) of the smoking cessation interventions relied on self-reported change in smoking behaviours45,55,57,59,60,63 and half used biochemical measures (CO or cotinine) to validate change in smoking behaviours. 44,49–53,56,58
Of the 12 smoking cessation studies, 10 included NRT (in either the intervention or control group or both). 44,45,49–51,55,56,58–60,63 Eleven of the studies44,45,49–53,55–60 included some form of behavioural support/advice/counselling. There was only one study63 which specifically reported that no psychological or behavioural support was added to the pharmacological treatment.
In terms of the component which is evaluated, seven studies52,55–60 evaluated some form of behavioural support, four of which compared behavioural support with a non-active control that received usual care. 52,57,58,60 In two studies,56,59 the behavioural support component was evaluated as an ‘additional’ element; the participants also received NRT in both the intervention and control arms. Another study55 compared 1 week of NRT then fortnightly pharmacy visits for NRT plus three sessions of brief behavioural counselling with 5 weeks NRT at the initial pharmacy visit plus one session of brief behavioural counselling at the initial visit.
Four studies44,52,56,63 evaluated a NRT component: one study compared NRT with placebo NRT,63 another study compared NRT with non-active control56 and two studies assessed NRT as an additional element (i.e. the participants also received behavioural support in both the intervention and control arms). 44,52 Three studies evaluated behavioural support plus NRT compared with non-active usual care. 45,52,56 Two studies evaluated the effect of the setting of the intervention; one study assessed behavioural support plus NRT provided in a hospital outpatient setting compared with pharmacy setting. 45 One study compared individual pharmacy-based behavioural support plus NRT with group support provided in a community setting. 49–51 One study53 evaluated the effect of a photoageing intervention in which both the intervention and control groups received pharmacist advice.
Behavioural outcomes
Eight studies45,52,53,56–58,60,63 evaluated an active pharmacy-based intervention in comparison with a non-active or usual-care control condition; five52,53,56,58,63 showed significant improvements compared with control. Five studies evaluated an additional element to an intervention:44,52,55,56,59 the additional elements included NRT in two studies44,52 and behavioural counselling/advice in another three studies. 55,56,59 One study52 showed significant improvements with the addition of NRT to tailored counselling and one study showed significant improvements with the addition of behavioural support to NRT. 56
Only two studies compared pharmacy-based setting with another setting. Bauld et al. 49–51 compared one-to-one pharmacist support with group-based smoking cessation clinics based in the community. All pharmacy clients had NRT, 84% of the group clients had NRT and the remaining 16% of the group clients received oral medication. The participants chose the service, rather than being assigned by study investigators. The group-based service attracted fewer clients but was significantly more effective than the pharmacy-based service in terms of the proportion of participants who were not smoking at 12 months (6.3% vs. 2.8%), determined using validated measures. However this study was observational only and the effectiveness results should not be directly compared in any formal manner. Vial et al. 45 compared pharmacy counselling with outpatient counselling and there was no significant difference in smoking cessation between the groups.
A RCT52 of training pharmacists to provide a tailored counselling service with and without NRT compared with a non-randomised control group that received observation only showed a significant increase in validated 7-day point prevalence at 6 months (28% for counselling and NRT, 15% for counselling only, 8% for control).
One RCT53 compared the addition of a computer-generated photoageing service (demonstrating the detrimental effects on facial physical appearance of smoking) with standard smoking cessation advice in a pharmacy. The photoageing intervention was more effective than the control based on the proportion of participants (young people with a mean age of 24 years) who were not smoking at 6 months [13.8% (n = 11/80) vs. 1.3% (n = 1/80)], determined using CO-validated measures. This difference between groups remained statistically significant after adjustment for small differences between groups in sex and nicotine dependence.
Another RCT55 evaluated three sessions of pharmacist counselling to one session based on the 5-A model for brief behavioural counselling in addition to both groups receiving 5 weeks of free NRT. There was no significant difference between intervention groups for self-reported 7-day point prevalence at 5 weeks, controlling for covariates [odds ratio (OR) 0.96, 95% CI 0.86 to 1.08]. At 5 weeks, the self-reported 7-day point prevalence was 17.5% for participants receiving three sessions of counselling and 18% for those receiving just one session. Approximately 50% of ‘three-session’ participants completed all three sessions, and among these participants quit rates were significantly higher than among the group of ‘one-session’ participants (27.7% vs. 18%).
Computer-generated tailored advice, compared with a thank-you letter,57 did not increase self-reported 6-month abstinence in participants recruited from Dutch pharmacies (quit rates < 1% in either group). The pharmacists were involved only in making the questionnaires available to customers.
One study44 evaluated the addition of 6 weeks of free NRT (compared with placebo NRT) to pharmacist advice and support in participants who were tobacco chewers. Validated abstinence rates were relatively high at the 6-month follow-up but not significantly different between groups (38% for NRT group vs. 34% for placebo group).
A RCT58 evaluated the Pharmacist Action on Smoking (PAS) model compared with ad hoc smoking cessation advice in UK pharmacies; over 80% in each group also received NRT. The PAS intervention significantly increased validated smoking cessation compared with control at 12 months (14.3% vs. 2.7%).
A small Japanese study59 evaluated the additional effect of initial and follow-up cessation advice to nicotine gum (plus advice on usage). Both interventions appeared to increase cessation but it was not reported if there was a significant improvement from baseline to follow-up. There was no significant difference between groups in self-reported cessation at 3 months (45.5% vs. 31.2%).
A UK RCT60 compared training pharmacists and pharmacy assistants in the stages-of-change model of smoking cessation with standard professional pharmacist care. Intervention participants were significantly more likely than control participants to purchase an ‘anti-smoking’ product. The intervention was associated with a favourable non-significant trend at 9 months, but this was based on self-reported abstinence, and pharmacists were willing to participate before randomisation. Self-reported continued abstinence at 9 months was 12% in the intervention group versus 7.4% in the control group.
A Danish study63 evaluated the effect of two different strengths of over-the-counter nicotine patches compared with placebo. No psychological or behavioural support was added to the pharmacological treatment. Self-reported point prevalence included participants who had one episode of smoking (< 6 days). Those smoking ≥ 20 per day at baseline were randomised to 21-mg patches or placebo; those smoking < 20 per day at baseline were randomised to 14-mg patches or placebo. At 26 weeks the intervention was effective compared with placebo for those smoking ≥ 20 per day at baseline (11% vs. 4.2%) but not for lighter smokers (22.7% vs. 18.4%).
One RCT45 compared 16 weeks of nicotine patches plus weekly counselling delivered by pharmacies with the same treatment delivered in a hospital outpatient clinic; a minimal intervention control group received written and verbal information at baseline only. Participants were all former inpatients of a respiratory unit and the intervention commenced while participants were inpatients then continued after discharge (as either outpatient- or pharmacy-based treatment). Seven-day point prevalence, but not continuous abstinence, was significantly different in favour of the pharmacy- and outpatient-based intervention compared with control intervention at the 12-month follow-up. Self-reported continuous abstinence at 12 months was 19% in the pharmacy-based group versus 24% in the outpatient group and 4.6% in the control group.
A cost-effectiveness study56 carried out in two pharmacies in Northern Ireland compared a behavioural intervention based on the PAS model with a nicotine gum-only control. Another control group comprised participants who expressed a wish to stop smoking and who were chosen on the basis that they matched, in terms of age, sex, social status and disease status, those in the behavioural intervention group. The study was a cost-effectiveness analysis and did not report many participant details. At 6 months there was a statistically significant difference in cessation rates between intervention and control patients. The 6-month CO-verified abstinence was 46% in the intervention group versus 6% in the control group receiving only nicotine gum and 0% in the control group that expressed a wish to stop smoking.
Metaregression and meta-analysis
Table 14 shows the results of metaregression for the smoking interventions. In model 1, we fitted a random-effects model including all 10 RCTs. The pooled OR for the intervention effects was 1.85 (95% CI 1.25 to 2.75), an indication of positive effect of the interventions on participants smoking cessation. However, there was 72% unexplained differences between the studies. In model 2, we fitted a metaregression model accounting for whether a study had active control or usual care. The pooled ORs were 1.21 (95% CI 0.86 to 1.71) and 2.56 (95% CI 1.45 to 4.53) for the active control and usual care, respectively. As expected, there was a bigger effect for usual care than for the active control. The proportion of unexplained heterogeneity reduced to 52%. In model 3, we accounted for whether a study had active control or usual care and also the duration of the interventions; the unexplained heterogeneity reduced to 27.2% with a non-significant Q-statistic test (10.99; p < 0.2026). In model 4, we accounted for quality rating. The quality rating does not appear to contribute much to the model after accounting for intervention duration and whether a study had active control or care as usual.
| Model | Variables | AICa | Q-statisticb (quantile; p-value) | c I 2 |
|---|---|---|---|---|
| 1 | – | 27.63 | 35.78; p < 0.0001 | 72.0% |
| 2 | Active control (or usual care) | 26.21 | 18.73; p < 0.0276 | 52.0% |
| 3 | Active control + intervention duration | 23.69 | 10.99; p < 0.2026 | 27.2% |
| 4 | Active control + intervention duration + global rating | 26.07 | 8.14; p < 0.2277 | 26.3% |
The meta-analysis results by study groups (active control or usual care) and the overall pooled ORs, are presented in Figures 2 and 3, presents the same meta-analysis results by quality ratings. There is asymmetry in the funnel plot in Figure 4, which may be a reflection of publication bias. In the absence of bias, the plot should approximately resemble a symmetrical (inverted) funnel; however, Figure 4 shows a gap in one corner of the graph, which could indicate the presence of bias, including publication bias, with smaller studies without significant effects not being published. Such a pattern is compatible with publication bias, on the assumption that smaller studies with uninteresting effects are withheld from publication. However, the funnel plot must be interpreted with caution, taking into account that it contains only 11 studies which just exceeds the recommended study size threshold (n = 10) for creating such plots. 23
FIGURE 2.
Meta-analysis of smoking data accounting for whether active control or usual care. a, 21-mg nicotine patches/placebo; b, 14-mg nicotine patches/placebo.
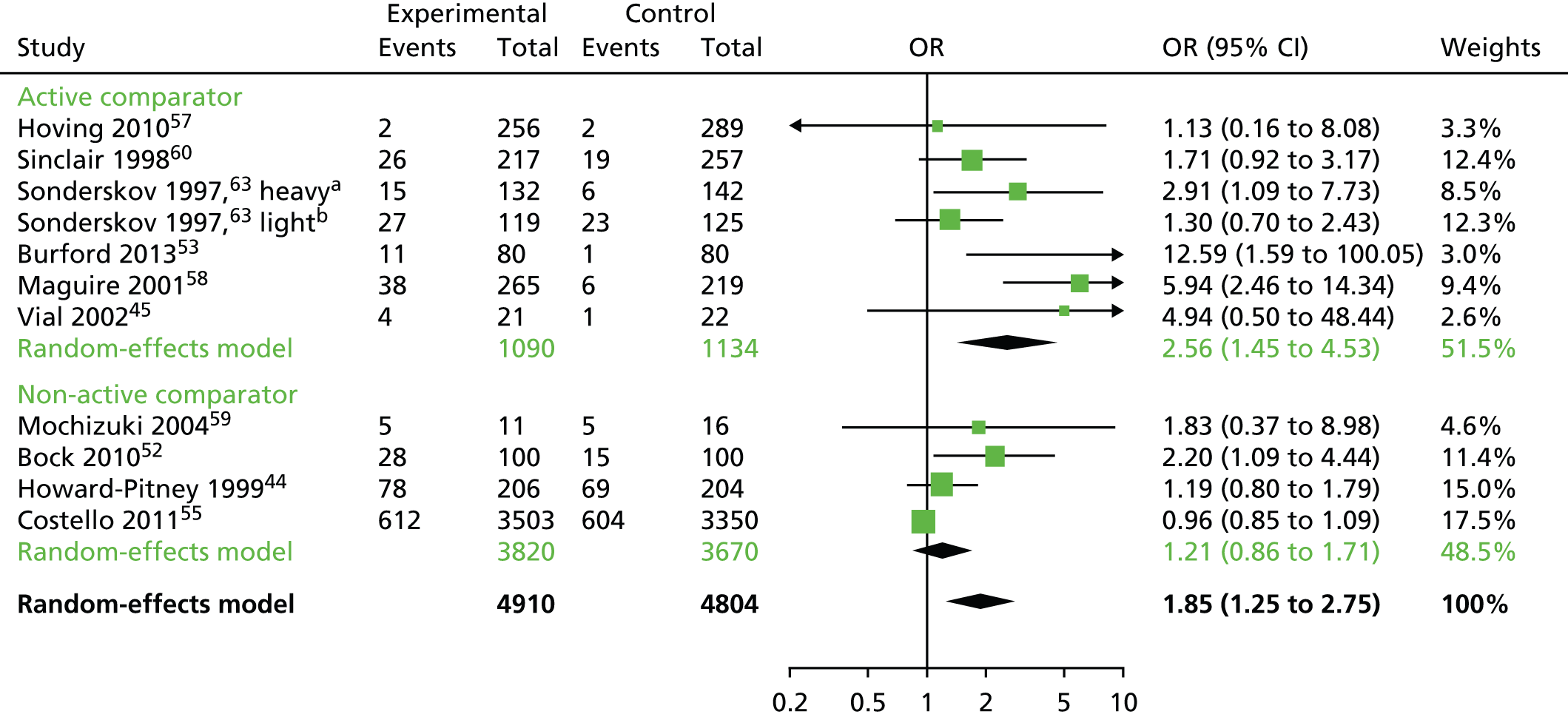
FIGURE 3.
Meta-analysis of smoking data accounting for global quality assessment ratings. a, 21-mg nicotine patches/placebo; b, 14-mg nicotine patches/placebo.
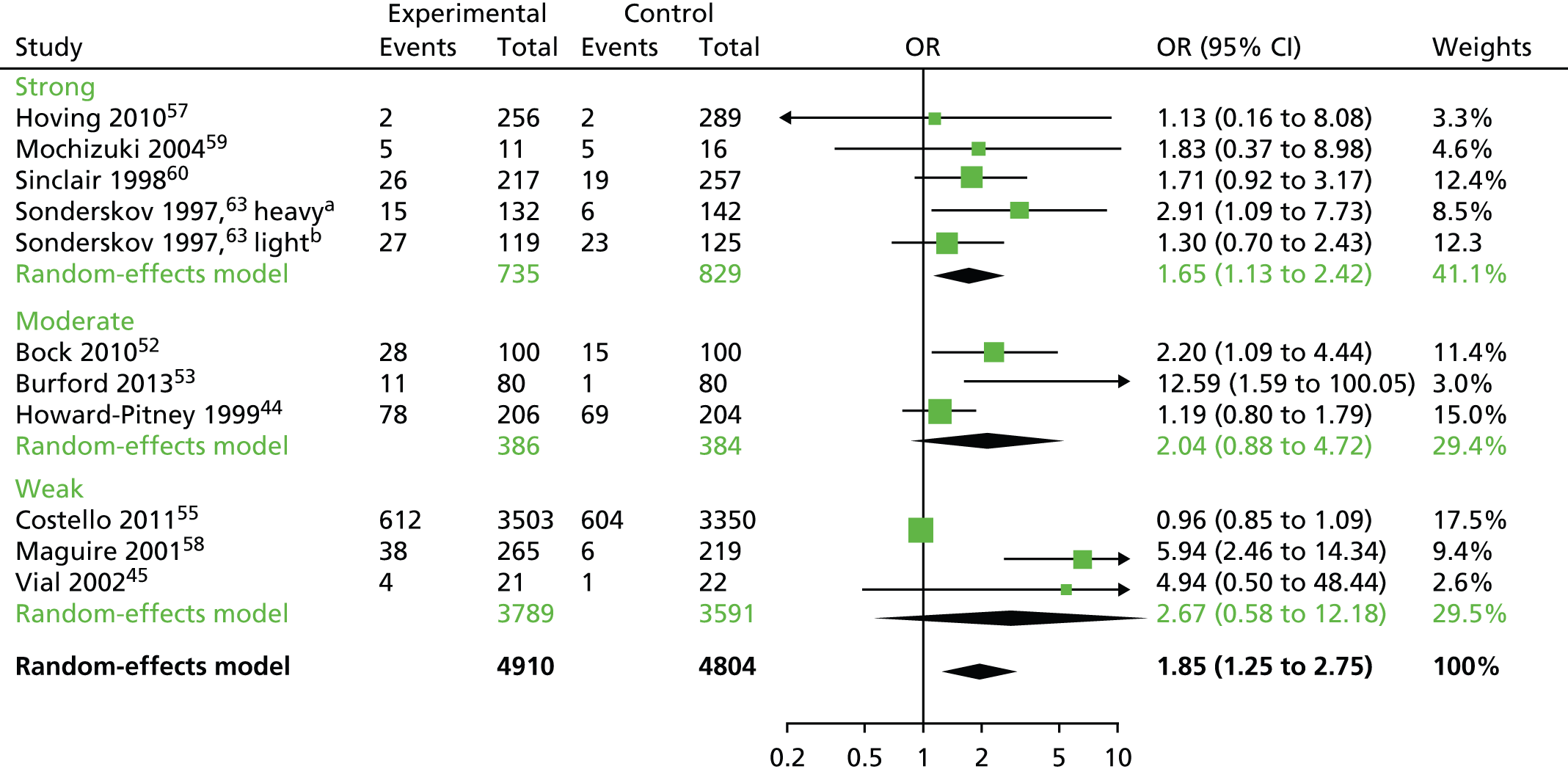
FIGURE 4.
Funnel plot.
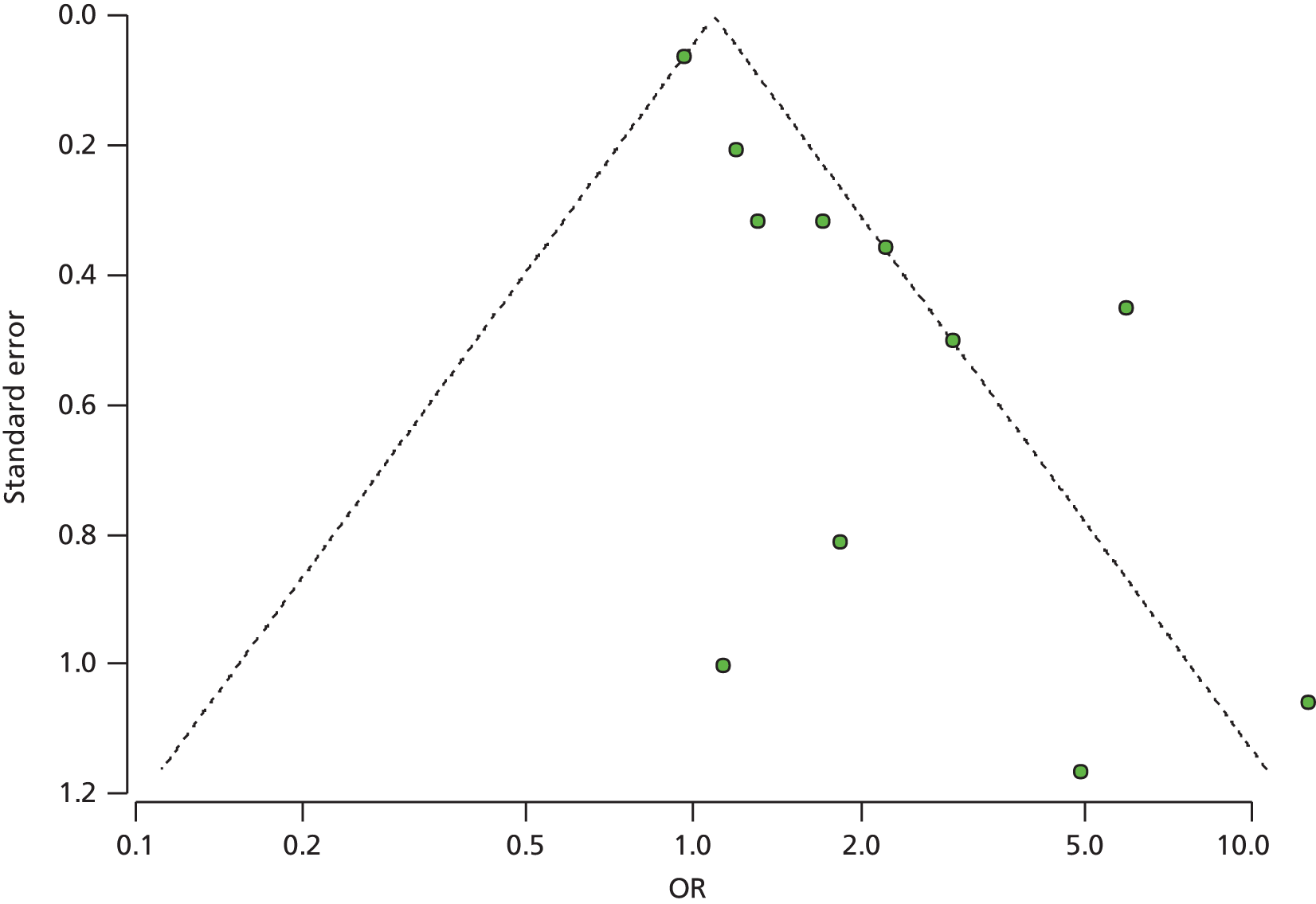
Costs
The CBA49–51 included a cost-effectiveness analysis, using validated quit rates for an economic evaluation of both the annual and the lifetime cost-effectiveness of the pharmacy- and group-based interventions in comparison with a baseline ‘self-quit’ scenario. At 52 weeks, the group service achieved a higher quit rate (6.3%) than the pharmacy service (2.8%) but was more intensive and required greater overhead costs. The Markov model estimated the potential lifetime outcomes in terms of cost per quality-adjusted life-year gained. The lifetime analysis resulted in an incremental cost per quality-adjusted life-year of £4800 for the group support and £2600 for pharmacy one-to-one counselling. Cost per pharmacy-based client was £79 based on a 0.025 probability of a 52-week quit. The paper reports that both services were considered to be highly cost-effective despite relatively low quit rates.
One RCT61 included a cost-effectiveness analysis using self-reported continued abstinence at 9 months (12% in the intervention group vs. 7.4% in the control group). Costs included those borne by the health service and pharmacies, but also by clients (societal perspective). Training costs included organising and operating costs of the training sessions and trainees’ out-of-pocket expenses, including staff costs and travel plus lost leisure time. Cost to the client included NRT and counselling time. Cost to the pharmacy covered training and counselling time. Any NRT purchased was a cost of the intervention to the client (total cost per user for NRT was £47.53). Cost to the NHS comprised organising and operating costs, pharmacy travel expenses and promotional materials and client documentation. The cost of producing one additional successful attempt to quit smoking by using intensive rather than standard pharmaceutical support was £300 or £83 per life-year.
A RCT53 of a photoageing intervention assessed the cost-effectiveness from a health sector perspective, in terms of the incremental cost per additional quitter and per additional lifetime quitter. Direct costs over and above providing standard cessation advice were calculated based on the time taken to provide the service and the cost to a pharmacy of purchasing tokens to use the online software to photoage participants. Potential cost offsets were based on the quit benefits model, which is a tool developed in Australia to predict the difference in health-care costs of smokers and non-smokers for males and females by age group after 10 years’ follow-up.
In the intervention group, 22 of 80 participants (27.5%) reported quitting, with 11 of 80 participants (13.8%) confirmed by CO testing. The difference between groups was significant even after adjustment for baseline differences. The incremental cost-effectiveness ratio (ICER) was AU$46 per additional quitter, or the equivalent of AU$74 per additional lifetime quitter. Cost offsets of AU$2144 from a reduction in the health-care costs of quitters resulted in the intervention potentially generating net total cost savings of AU$1778. The mean cost of implementing the intervention was estimated at AU$5.79 per participant. The mean cost that participants indicated they were willing to pay for the digital ageing service was AU$20.25 [standard deviation (SD) AU$15.32], which was more than the actual costs.
A cost-effectiveness study56 conducted in two pharmacies in Northern Ireland compared a behavioural intervention group based on the PAS model with a nicotine gum-only control group. The 46% quit rate of the intervention study was not used for cost-effectiveness analysis: a 10% quit rate was used to reflect the participants who entered stage 3 of the PAS programme (i.e. those who set a quit date) and who remained abstinent at 12 months. Various assumptions were also made, including uptake by pharmacies, recruitment of participants, natural cessation rate and relapse rates. The cost-effectiveness of the PAS model was therefore measured in terms of cost per life-year gained for all patients who entered stage 3 of the PAS programme. The main cost-effectiveness analysis indicated that costs ranged from £196.76 to £351.45 per life-year saved for men, and from £181.35 to £772.12 per life-year saved for women, depending on age at intervention. Given the baseline assumptions and on the basis of a 45-year-old smoker, the cost per successful intervention was £509.60. The PAS model appears effective and, if the PAS smoking cessation programme were to be offered routinely by community pharmacists throughout Northern Ireland, it would be cost-effective.
Differential effects by demographic or socioeconomic factors
Five smoking cessation studies44,49–51,53,55,60 reported examining demographic and/or socioeconomic characteristics as potential predictors of outcomes within intervention groups. One study was rated strong for global quality,60 one was rated moderate44,53 and three were rated weak. 49–51,55
Bauld et al. 49–51 compared smoking cessation services that were group based in the community with one-to-one pharmacy-based services; at 52 weeks, group-based clients were more likely to quit than pharmacy clients. Older people were more likely to quit in either the pharmacy-based or the group-based service, higher SES was associated with long-term abstinence for pharmacy clients. Sex did not predict quitting in either the pharmacy-based or the group-based service.
In another smoking cessation study of a photoageing intervention,53 there were no associations between change in Fagerström score (measures nicotine dependence) and age or sex in the control group. However, for the intervention group, age (but not sex) was significantly associated with a change in score. Older participants were significantly less likely to reduce their score than younger participants, suggesting that the intervention may have a greater effect on the younger participants. However, it should be noted that participants in this trial only included an age range of 18–30 years.
Another RCT55 evaluated three sessions of pharmacist counselling to one session based on the ‘5-A’ model for brief behavioural counselling in addition to both groups receiving 5 weeks of free NRT. Bivariate analyses showed that, among three-session completers, both younger and employed individuals were more likely to be abstinent than older and unemployed participants. A study that evaluated the addition of free NRT (vs. placebo NRT) to pharmacist support for tobacco chewers44 examined the active patch group for predictors of relapse: older chewers were less likely to relapse. A UK RCT,60 comparing trainee pharmacists and pharmacy assistants in the stages-of-change model of smoking cessation with standard professional pharmacist care, reported that trends in outcome (in favour of the intervention) were not affected by age, sex or IMD of the participants.
One smoking cessation study reported on demographic or socioeconomic characteristics as potential predictors of outcomes between intervention groups. A Danish study63 compared the effect of two different strengths of over-the-counter nicotine patches and placebo. There were no differences in smoking cessation rates between men and women according to starting dose and treatment.
A summary of the evidence for smoking cessation interventions is provided in Box 2.
-
Twelve studies of varied quality evaluated the effectiveness of community pharmacy-based smoking cessation interventions.
-
Pharmacy-based smoking cessation interventions including behavioural support and/or NRT are effective and cost-effective in helping adults to stop smoking, particularly when compared with usual care. A total of 10 RCTs were included in a meta-analysis; the pooled OR of the intervention effects for smoking cessation was 1.85 (95% CI 1.25 to 2.75). The pooled OR was 1.21 (95% CI 0.86 to 1.71) for intervention vs. active control and 2.56 (95% CI 1.45 to 4.53) for intervention vs. usual care.
-
Accounting for the type of comparator and the duration of the interventions reduced the unexplained heterogeneity to 27.2%, with a non-significant Q-statistic test (10.99; p < 0.2026).
-
Four smoking cessation studies included cost outcomes, but the methods of cost-effectiveness analyses differed, making comparisons difficult. However, three UK pharmacy-based interventions appeared cost-effective, despite relatively low quit rates in one case and a non-significant trend for cessation rates in another case. An Australian study appeared cost-effective (and effective) in increasing quit rates among young adults who were exposed to the detrimental effects on facial physical appearance of smoking using a computer-generated simulation.
-
The evidence was too heterogeneous to evaluate which specific types of smoking cessation interventions are the most effective or cost-effective.
Weight loss interventions
Five interventions were designed to evaluate weight loss (three RCTs,64,66,67 one nRCT39 and one CBA65) interventions in adults. Table 10 summarises the results. All but one study included advice regarding diet and physical activity; the other study39 included low-fat dietary advice within an intervention designed to improve adherence to orlistat therapy.
Three studies65–67 compared a pharmacy-based intervention with interventions in various other settings, including commercial weight loss programmes set in community venues, primary care settings such as GP practices, primary care units and outpatient clinics. One study64 compared a meal replacement diet with a conventional low-calorie diet (identical recommended total daily calorie intake); both interventions were set in a pharmacy. One small study39 assessed the added value of community pharmacy support for obesity management in addition to orlistat and an outpatient nutrition programme.
Meta-analysis
There were insufficient data to conduct meta-analyses and so the weight data are described in Figure 5.
FIGURE 5.
Bar chart of weight change from baseline to follow-up by treatment group. P, pharmacist; PA, physical activity.
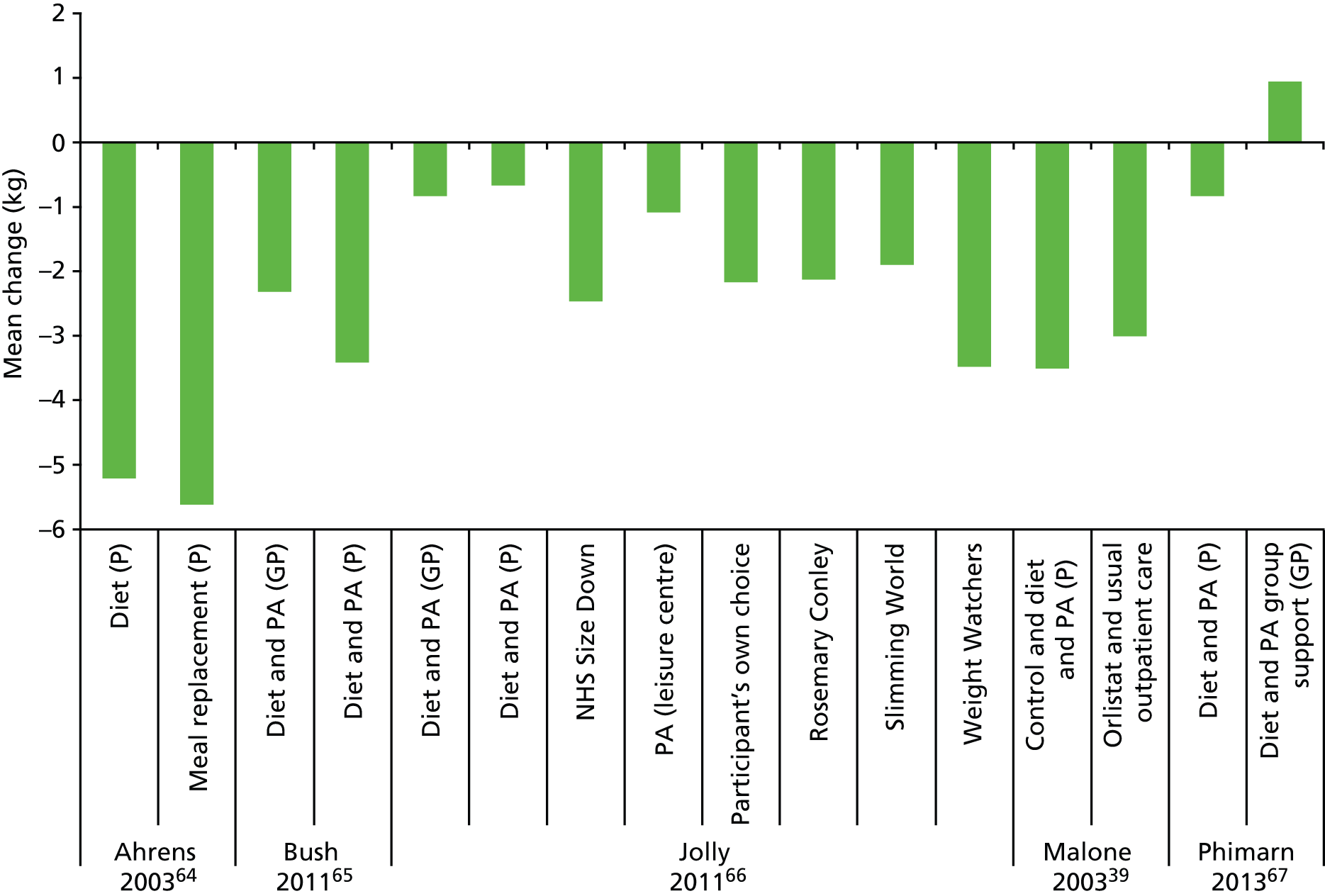
Behavioural outcomes
Three studies65–67 reported BMI, three studies64,65,67 reported waist circumference (WC) and all five studies39,64–67 reported weight. The largest improvement in BMI59 was –1.3 kg/m2; for WC it was –8.1 cm64 and for weight it was –5.6 kg. 64 None of the studies demonstrated a significant difference in favour of the pharmacy-based intervention compared with the control for any anthropometric outcome.
One UK RCT66 compared eight groups [Weight Watchers™ (WeightWatchers.co.uk Ltd, Maidenhead, UK), Slimming World™ (Miles-Bramwell Executive Services Ltd, Alfreton, UK), Rosemary Conley™ (Rosemary Conley Online Ltd, Steyning, UK), Size Down (a NHS community-based group), GP, pharmacy, participants’ own choice and an exercise-only control group]. All except the GP and pharmacy groups exhibited significant weight loss between baseline and 1-year follow-up. At 1 year, only the Weight Watchers group had significantly greater weight loss than the control group (mean 2.5 kg, 95% CI 0.8 kg to 4.2 kg). The commercial programmes (Weight Watchers, Slimming World and Rosemary Conley) achieved significantly greater weight loss than the primary care programmes (general practice and pharmacy-based interventions). At 1 year, the difference was 1.6 kg (0.3 kg to 2.9 kg; p = 0.06) in the adjusted model. Mean weight loss at 1 year, with baseline value used for imputation, was 0.8 kg (SD 4.7 kg) for primary care and 2.5 kg (SD 6.2 kg) for commercial programmes.
In one CBA study65 comparing diet and physical activity in a pharmacy with a GP-based intervention, in both groups BMI, WC and weight appeared to be reduced at follow-up. Statistical significance, either from baseline to follow-up or between groups, was not reported; there was very high attrition in this study (93%). In this CBA, the participants chose the service rather than being assigned by study investigators and, consequently, may have been a relatively more motivated sample.
In two studies,39,64 both the intervention and ‘control’ groups lost a significant but similar amount of weight between baseline and follow-up; participants in both the meal replacement and low-calorie diet groups64 lost a similar amount of weight (both based in the pharmacy) as those treated with orlistat on an outpatient basis,39 with or without additional pharmacy-based support. In one study,67 there was no significant loss of weight between baseline and follow-up in either the intervention or control group.
Costs
Two studies reported costs. 65,66 The CBA65 study reported that it was unclear which provider type (pharmacy or GP) delivered the programme more cost-effectively because of different cost-effectiveness results at different time points. Attendance rates on the programme were consistently better among pharmacy participants than among GP surgery participants. Direct costs included training, initial test and appointments. Providers were reimbursed £300 for undergoing 2 days of training once they had recruited six participants and then £30 for the initial assessment of each participant and £10 for each consultation after the initial assessment.
The total cost of delivering the My Choice Weight Management Programme was £50,200. Total costs were higher among GP providers (£26,970) than among pharmacy providers (£23,230). This difference can be explained by the remuneration structure for the intervention, as payments were based on the number of sessions hosted (number of sessions hosted by GPs = 1735; pharmacy = 1447).
Costs per participant were higher through pharmacies (£126.90) than through GPs (£100.60). This was true throughout the course of the intervention, but the gap in costs between pharmacy and GP providers narrowed and there was no statistically significant difference in costs between providers among participants attending the final session. Again, the difference in costs is a result of the larger number of participants recruited by GPs. It is important to note that the demographic and socioeconomic characteristics of the pharmacy and GP groups differed significantly: GP participants tended to be older than pharmacy participants and the ethnic composition of the two groups differed significantly.
The cost-effectiveness of the intervention is measured in terms of costs per kilogram weight loss and costs per 1% weight loss and ICER at session 12 and session 15. The differences between providers were statistically significant; among participants attending session 12, the cost per kilogram weight loss was £57.00, with costs being higher among pharmacy providers (£74.80) than among GP providers (£43.40). Among participants attending session 15 (final session), the opposite pattern was observed, with the costs of both measures being lower among pharmacy providers than among GP providers (although these differences were not statistically significant). At session 12 each extra kilogram weight loss per participant would cost £8.29 through pharmacy providers. Conversely, at session 15, each extra kilogram of weight loss per participant would cost £2.91 through GP providers. At the end of the intervention the ICER favoured the pharmacy.
Jolly et al. 66 evaluated the direct costs to the primary care trust of each programme and of sending out invitation letters from practices. These included the costs of the provider’s service and the cost of the searches in general practice, invitation letters and provision of call centre support. The cost of the call centre that co-ordinated the service as an average per person, based on the cost of staff employed over a 12-month period and the number of clients who used the service over this time period. Costs to the participants were not included, nor were any training costs for providers.
Assuming that participants randomised to the most successful intervention continued to have a BMI 1.3 kg/m2 less throughout life, then the cost per life-year saved was approximately £77. These benefits are not discounted and are based on many assumptions. The authors conclude that commercial organisations provide a more effective service at lower cost than primary care providers (GPs and pharmacists). One-to-one primary care-based programmes, including pharmacy-based programmes, were ineffective and most costly to provide (both £112.73 per participant based on a pool of 70 participants each).
Differential effects by demographic or socioeconomic factors
Two studies recruited participants from areas with high levels of socioeconomic deprivation. 65,66 The mean IMD score of participants in the GP surgery and pharmacy arms of the Lighten Up trial66 was 32.2 and 35.1, respectively. The study authors report that the characteristics of the participants reflected the population of the primary care trust well, with 23.5% of the participants being in the bottom 10% of socioeconomic deprivation, which is similar to that for the primary care trust, and 13% of participants being from a minority ethnic group, which is slightly lower than the local prevalence of 18%. The mean IMD score of participants attending the My Choice Weight Management Programme65 at GP surgeries and pharmacies was 43.8 and 43.3, respectively. Higher IMD scores indicate higher deprivation.
The Lighten Up trial66 evaluated a range of weight loss programmes in community and primary care settings; participants who were lost to follow-up tended to be younger than those who were followed up, but they were similar in terms of BMI, sex, ethnicity and IMD score.
The same two weight loss studies reported examining demographic and/or socioeconomic characteristics as potential predictors of outcomes within intervention groups. 65,66 Bush65 compared a weight management programme set in pharmacies versus the same programme set in GP surgeries and reported there were no statistically significant relationships between age, sex, IMD quintile or ethnicity and percentage weight loss at session 12 within pharmacy or GP surgery participants. In a study of weight loss programmes in various community and primary care settings, sex had no effect on weight loss. 66
These two weight loss studies reported examining demographic and/or socioeconomic characteristics as potential predictors of outcomes between intervention groups. 65,66 In a study of weight loss programmes in various community and primary care settings there was no statistically significant interaction between sex and the type of weight loss programme. 66
Bush et al. 65 compared a weight management programme set in pharmacies versus the same programme set in GP practices. Female participants who followed a programme based in a GP practice lost a significantly larger proportion of their initial weight than those following a pharmacy-based programme, and participants aged 40–49 years lost a greater proportion of their initial weight at GP providers than at pharmacy providers.
A summary of the evidence for weight loss interventions is provided in Box 3.
-
Five studies evaluated the effectiveness of community pharmacy-based weight loss interventions. The types of interventions were heterogeneous and the evidence limited; therefore, meta-analysis was not carried out.
-
None of the studies demonstrated a significant difference in favour of the pharmacy-based intervention compared with control for any anthropometric outcome.
-
Two studies reported cost-effectiveness; the costs associated with primary care interventions were broadly similar in the two studies. Cost-effectiveness varied at different time points and was influenced by the number of participants recruited (which differed by primary care setting). Commercial organisations provided a more effective service at lower cost than primary care providers.
-
Two studies recruited participants from areas with high levels of socioeconomic deprivation. In one study there was no difference in effectiveness by sex. In another study, where participants chose the intervention which varied by setting, there was variation in effectiveness by setting according to demographic characteristics (age and sex).
Multicomponent interventions (pharmacotherapy and lifestyle changes)
Five studies evaluated the effects of pharmacotherapy (medicines management) plus lifestyle advice in participants with comorbidities including type 2 diabetes mellitus,68–70 dyslipidaemia71 and hypertension. 72 Table 11 summarises the results. Global quality ratings varied among the studies; one was rated strong,70 two were rated moderate68,72 and two were rated weak. 69,71
Type 2 diabetes mellitus
There were three studies (two RCTs68,70 and one nRCT69) of interventions in adults with type 2 diabetes mellitus. In the nRCT,69 four pharmacies were assigned to intervention and two pharmacies were assigned to control; in addition, the pharmacists were willing to participate before randomisation. Two studies68,70 included lifestyle advice as well as pharmacotherapy, for example counselling in acute and chronic complications of diabetes mellitus, lifestyle (physical activity, healthy diet and smoking cessation), regular foot inspections, and correct use of drugs and self-monitoring of blood glucose. One study69 reported that the intervention included ‘patient education’, but no further details were reported. The studies were conducted in Brazil,69 Spain70 and the UK;68 the UK study was a small pilot study. All three studies had glycaemic control as the primary outcome and BMI was also a primary outcome in one study. 68
All three studies used completer analyses; dropout was minimal in two studies, but 60% in one study,69 which may have biased results. Follow-up duration was 12–13 months. Baseline BMI ranged from 28 kg/m2 to 32 kg/m2.
The small UK study68 demonstrated significant reductions in BMI in the intervention group as compared with no significant changes in the control group from baseline to follow-up, but no significant difference between groups at follow-up (–3.86 kg/m2 vs. –1.09 kg/m2, respectively). The intervention was also associated with significant improvement in HbA1c percentage, systolic blood pressure and blood glucose level as compared with the control group after the period of 12 months. Changes in lipids were mixed; triglycerides were non-significantly lower in the intervention group than in the control group. Low-density lipoprotein, high-density lipoprotein and total cholesterol levels were significantly higher in the intervention group than in the control group.
After 12 months of pharmacotherapy and patient education,69 BMI and WC remained similar in the intervention and control groups (–0.2 kg/m2 vs. 0.3 kg/m2, respectively for BMI). However, the intervention significantly improved glycaemic control, with participants in this group experiencing a greater reduction in HbA1c and fasting capillary glycaemia than those in the control group. The intervention was cost-effective in terms of costs per patient to reduce HbA1c values by 1%; however, there was 60% dropout.
After 12 months of pharmacotherapy and lifestyle advice,70 BMI was reduced in the intervention group, but not the control group (–0.9 kg/m2 vs. –0.3 kg/m2, respectively); however, BMI was not significantly different between groups. In the intervention group there were significant improvements in HbA1c levels, fasting blood glucose levels, total cholesterol levels and systolic blood pressure.
Dyslipidaemia
One small RCT71 set in one pharmacy in Chile evaluated pharmacotherapy plus lifestyle modification in adults with dyslipidaemia. Lifestyle modification included changes in eating habits, increase in physical activity and decrease in or cessation of other risk factors such as alcohol intake, smoking and excess weight.
At the end of the 16-week programme, weight within the intervention group decreased an average of 1.0 kg, while in the control group the average weight increased by 1.1 kg. There was a significant decrease in BMI of 0.4 kg/m2 in the intervention group from baseline to follow-up. It is assumed that BMI remained similar to baseline in the control group (data not reported). Baseline BMI and weight were not reported and the analysis is based on completers only; it is not clear how many participants entered the study. Cholesterol and triglycerides improved significantly from baseline to follow-up in intervention participants and there was no significant change in control participants.
Hypertension
One RCT72 compared a diet and physical activity intervention with usual care in hypertensive participants not controlled by antihypertensive medication despite compliance. There was no significant improvement in BMI or weight from baseline to follow-up in either the intervention or control groups. However, the main aim of this study was to improve control of hypertension rather than promote weight loss; the diet and physical activity intervention did significantly reduce blood pressure in intervention participants between baseline and the 8-week follow-up.
Differential effects by demographic or socioeconomic factors
None of the studies reported a differential effect by demographic or socioeconomic factors. One study of participants with type 2 diabetes mellitus69 reported that the percentage of female dropouts was significantly higher than the percentage of female completers (female/male dropouts were 73/27% and female/male completers were 53/47%).
A summary of the evidence for multicomponent interventions is provided in Box 4.
-
Five studies evaluated multicomponent interventions (pharmacotherapy and lifestyle changes) compared with usual care in participants with comorbidities (diabetes mellitus, dyslipidaemia and hypertension).
-
None of the studies demonstrated a significant improvement in anthropometric outcomes compared with control but they did show significant improvement in the relevant primary outcomes of blood pressure, glycaemic control and lipids.
Chapter 4 Discussion and conclusions
The objectives of the review were (1) to assess the effectiveness of community pharmacy interventions on health and health behaviours in relation to alcohol misuse, smoking cessation and weight management; (2) to explore if and how SES, sex, ethnicity and age moderate the effect of these interventions; and (3) to describe how the interventions included in this review have been organised, implemented and delivered. All three objectives have been met in terms of the extent to which the state of the evidence enabled us to do so. The extent to which objective 1 has been met is much stronger than the extent to which objectives 2 and 3 have been met. In order to satisfactorily meet objectives 2 and 3, more evidence is required from robust interventions that explore if and how SES, sex, ethnicity and age moderate intervention effects and report how such interventions are organised, implemented and delivered.
Objective 1: to assess the effectiveness of community pharmacy interventions on health and health behaviours in relation to alcohol misuse, smoking cessation and weight management
There was insufficient evidence for the effectiveness and cost-effectiveness of community pharmacy-based brief alcohol interventions. Evidence from two trials suggests lack of effectiveness. It is not clear whether or not the UK alcohol screening services or brief advice provided in the pharmacy setting reduces a patient’s consumption of alcohol over time. UK alcohol screening services demonstrate that the community pharmacy is an appropriate place to screen patients for alcohol misuse.
Twelve studies evaluated the effectiveness of community pharmacy-based smoking cessation interventions. Pharmacy-based smoking cessation interventions, including behavioural support and/or NRT, are effective and cost-effective in helping adults to stop smoking, particularly when compared with usual care; however, there was heterogeneity between the studies. Four smoking cessation studies reported cost-effectiveness analyses, but the methods differed and this made comparisons difficult. However, three UK pharmacy-based interventions appeared cost-effective, despite relatively low quit rates.
Five studies evaluated the effectiveness of community pharmacy-based weight-loss interventions and did not demonstrate significant between-group differences in weight. However, the majority of these studies were comparing a pharmacy-based intervention with another active intervention either within the pharmacy or in another setting. One UK RCT, Lighten Up, compared eight groups (Weight Watchers, Slimming World, Rosemary Conley, a NHS community-based group called Size Down, GP, pharmacy, participants’ own choice and an exercise-only control group). At 1 year, participants in only the Weight Watchers programme had significant weight loss compared with the control group and this intervention was associated with the highest attendance rate. Mean weight loss at 1 year was 0.8 kg (SD 4.7 kg) for primary care (GP and pharmacy) and 2.5 kg (SD 6.2 kg) for commercial programmes (Weight Watchers, Slimming World, Rosemary Conley). Two weight-loss trials reported on cost-effectiveness. The pharmacy-based arm of Lighten Up was not cost-effective compared with commercial programmes. Another study reported costs for two primary care-based weight-loss services (GP and pharmacy) and the costs were broadly similar to that of the pharmacy-based programme in the ‘Lighten Up’ trial.
Five studies evaluated multicomponent interventions (pharmacotherapy and lifestyle changes) compared with usual care in participants with comorbidities (diabetes mellitus, dyslipidaemia, hypertension). None of the studies demonstrated a significant improvement compared with control for anthropometric outcomes, but they did show significant improvement in the relevant primary outcomes of blood pressure, glycaemic control and lipids.
Objective 2: to explore if and how socioeconomic status, sex, ethnicity and age moderate the effect of these interventions
None of the studies examined the differential effects of any measure of SES. Three studies (one smoking cessation63 and two weight loss studies65,66) examined the differential effects of demographic variables. The smoking cessation study reported no differential effect by sex. One weight loss study reported no differential effect by sex. 66 The other weight loss study reported isolated statistically significant differences in weight loss between participants attending the intervention in pharmacies and GPs; for example, female participants attending a GP lost a larger proportion of their initial weight than females attending a pharmacy. 65 Similarly, participants aged 40–49 years lost a greater proportion of their initial weight at GP providers than at pharmacy providers.
The significance of these differences in terms of inequalities is unclear; participants chose the service they wanted to attend and the demographics of participants differed significantly between the two settings. Another smoking cessation study shows demographic and socioeconomic differences between participants who self-select treatment by setting. This evidence suggests that the people accessing pharmacies are different from those attending other settings for alcohol management, smoking cessation and weight loss.
Some studies examined demographic and/or socioeconomic factors at recruitment stage, as potential predictors of outcomes within group, and/or to explain differences in retention. Although these studies cannot inform if and how these interventions might impact on inequalities, they can help to inform how interventions can be targeted to improve access, success and retention. The UK alcohol service evaluations suggest that these types of services have the potential to reach those most in need.
Objective 3: to describe how the interventions included in this review have been organised, implemented and delivered
Few studies reported detailed information about the behaviour change strategies employed to deliver the interventions in order to enable more specific coding of the interventions. The most common behaviour change strategy used in the included interventions was the transtheoretical model (stages of change). The majority of included interventions were implemented within the political context of extending the pharmacists’ public health role. The overall poor descriptions of intervention content, mechanisms and procedures in most of the included papers limit the potential for knowledge implementation and replication of the interventions under review.
There was insufficient detailed information to examine any potential relationships between intervention effectiveness and behaviour change strategies, and whether or not any patterns existed between effective interventions and implementation components such as pharmacist training or resource intensity. The reporting of stakeholder involvement (consultation and collaboration) in the planning or during the delivery of the intervention was particularly poor (only reported in two studies). In terms of sustainability, a number of studies highlight that reimbursement is needed to the pharmacist for providing the intervention in order for it to be sustainable.
Strengths and limitations
In terms of the strengths and limitations of the included studies, a thorough and robust search of the literature was carried out to ensure that all types of community pharmacy-delivered alcohol, smoking and weight management interventions were captured. However, only 24 controlled studies were identified, of which 19 were RCTs. Most of the studies focused on smoking cessation interventions and there were only two interventions for alcohol misuse. No restriction was placed on the types of interventions included within the review; this meant that five included studies focused on disease states rather than health behaviours. These multicomponent interventions addressed a variety of lifestyle factors in adults receiving pharmacotherapy for comorbidities including type 2 diabetes mellitus, dyslipidaemia and hypertension. Evidence from these studies is specific to these subgroups of participants.
The purpose of this review was to evaluate the effectiveness of pharmacy-delivered interventions in three behaviour-related areas (alcohol, smoking and weight), which are each relevant for public health and have a clear evidence base for interventions outside pharmacy settings. Alcohol consumption and smoking cessation are both health behaviours (as well as outcomes), whereas obesity is an outcome, and intervention can target dietary or physical activity behaviours, or both. Therefore, the effectiveness of weight interventions cannot be directly compared with interventions to reduce alcohol consumption or for smoking cessation.
This review was primarily a review of effectiveness and not a review of economic evaluations. Any cost outcomes that were reported within the included studies were assessed; however, the methods of cost-effectiveness analyses differed between the included studies, making comparisons difficult. Some of the economic analyses modelled predicted costs of health care as well as observed costs. In addition, assumptions are made on modelling (such as weighing a certain amount less throughout life or how many people will relapse and start smoking again over the life course), which should be borne in mind when assessing the evidence.
Within the protocol it is reported that different types of interventions would not be combined within a meta-analysis. However, owing to the relatively small number of included RCTs and the mix of intervention types we felt that it was appropriate as a ‘first step’ to group together the smoking cessation studies that included behaviour support and/or NRT. The primary research objective was to assess the effectiveness of any type of intervention which is delivered and based within a community pharmacy setting. Therefore, by grouping all intervention types together in a meta-analysis, we can begin to assess the effectiveness of interventions based within the community pharmacy. There was, however, insufficient evidence to say which specific type of smoking cessation intervention is most effective.
In terms of the strengths and limitations of the included studies, two were pilot studies and, although they met the eligibility criteria, these types of studies are inherently different from full trials. Pilot studies do not aim to be sufficiently powered or to have the procedures fully developed, unlike full trials. Quality assessment might not reflect the inherent difference in aims between these types of study designs, and this should be borne in mind when comparing the evidence.
An area of ongoing debate among triallists is about the unit of randomisation in RCTs, that is whether or not it is more appropriate to randomise a cluster, in this case pharmacies, as opposed to randomising the individual pharmacy client. This is particularly relevant to behaviour change interventions within the pharmacy practice setting. Of the 19 included RCTs, 17 randomised individual pharmacy clients and only two randomised pharmacies. It could be argued that cluster RCT design more accurately reflects the real world and strengthens the external validity of a study, making the evidence more relevant.
In terms of intervention fidelity, we assessed whether or not the consistency of interventions was measured, whether or not the interventions were delivered as intended and if it was likely that contamination occurred. In the vast majority of included studies it was not possible to assess fidelity, as these measures were not reported. In studies in which participants using the same pharmacy were randomised to intervention or control groups, there is an increased risk of contamination. This was the case for many of the studies included in this review, yet, despite this, only two of the trials reported on the possibility of cross-contamination between intervention and control groups. Lack of reported information of intervention fidelity limited the review, in terms of assessing the strength of any causal relationships between intervention components and outcomes.
The review is strengthened by the attention paid to contextual factors including the organisation, implementation and delivery of interventions. Attention was paid to extracting information about pharmacist training, which will be particularly useful to policy-makers. Few studies reported detailed information about the behaviour change strategies employed to deliver the interventions, in order to enable more specific coding of the interventions; the Behaviour Change Wheel and intervention ladder approaches were therefore chosen to broadly describe the interventions. The overall poor descriptions in most of the included studies, of intervention content, mechanisms and procedures, limits the potential for knowledge implementation and replication of the interventions under review.
This review included only process evaluations that were included within the trial papers; we did not search for papers from the included studies that separately reported process evaluations. Many studies have also been done on processes outwith evaluations. A new search would be required to systematically capture all the evidence from trials that have published work on contextual findings around the organisation, implementation and delivery of pharmaceutical care service by community pharmacies. Therefore, we refer to process evaluations only to describe how the included interventions have been organised, implemented and delivered. Given the paucity of reported process data from the included interventions, it is important that future interventions clearly report contextual factors.
Conclusions
There is insufficient evidence to assess the effectiveness of pharmacy-based interventions for alcohol management; however, the evidence does show that community pharmacies can be appropriate places to screen patients for alcohol misuse. Pharmacy-based smoking cessation interventions, including behavioural support and/or NRT, are effective and cost-effective in stopping adults smoking, particularly when compared with usual care. Evidence suggests that pharmacy-based weight loss interventions are as effective as similar interventions in other primary care settings but not as effective or cost-effective as commercially provided weight management services in community settings. Very few studies explored if and how sociodemographic or socioeconomic variables moderated interventions effects. The information reported in the studies shed very little light on how best to organise, implement and deliver interventions in the pharmacy setting.
Implications for public health
Our review has found a relatively small international evidence base; more evidence is needed to assess the effectiveness of pharmacy-based interventions for alcohol and weight management. Nine studies were conducted in the UK,46,48–51,56,58,60,65,66,68 and so the study findings should be generalisable to the UK pharmacy context. Our review has demonstrated that pharmacy-based interventions are effective and cost-effective in helping adults to stop smoking. The review supports the commissioning of smoking cessation services in a community pharmacy setting.
The evidence shows a range of types of smoking cessation interventions that are feasible within community pharmacies, including behavioural support and/or NRT, but not which specific types of interventions and components are the most effective. A range of type of interventions in various different settings is required to suit different adults who want to manage their alcohol intake, stop smoking or lose weight. Evidence from this review suggests that pharmacy-based interventions for smoking cessation are suitable as part of a suite of interventions.
The review has shown that is feasible to recruit patients to an alcohol screening intervention within a community pharmacy setting, but there is insufficient evidence of the effectiveness of such screening and brief intervention and whether or not this reduces a patient’s alcohol consumption over time. What is not known, however, is the outcome for patients who are identified as hazardous/harmful drinkers within a community pharmacy and referred to other branches of health care (e.g. a GP or rehabilitation centre). Given the reach of the community pharmacy network, and that our review has shown it is feasible to recruit patients in this setting, it would be prudent to explore referral options for adults who screen positive for hazardous/harmful drinking.
There is a lack of evidence regarding the effect of pharmacy-based interventions on health inequalities. Very few studies targeted disadvantaged population groups; these types of studies can provide useful information about recruitment and retention of these high-risk groups. Given the potential reach of the community pharmacy network, more work is needed to ascertain how commissioning smoking cessation services may impact on inequalities in health. Inequalities in relation to interventions can result from both differential uptake of pharmaceutical services and differential effectiveness by demographic and SES.
This review concentrates on differential effectiveness by demographic and SES (and found little evidence reported) but acknowledges that inequalities in uptake are an equally important contribution to health inequalities. Pharmaceutical needs assessments80 include evaluation of the level of access to community pharmacies, the specific needs of individual localities and uptake of services compared with other regions, but not differential uptake. Evidence from weight management programmes based in community pharmacies shows that middle-aged women are more likely to join. A 2000 postal survey81 in a stratified random sample of 10,000 adults aged ≥ 35 years in North Staffordshire, UK, showed that female sex and older age were independently associated with collection of a prescription medicine. Female sex, younger age and higher social class were independently associated with over-the-counter purchase, while female sex and smoking were independently associated with seeking advice from the pharmacist.
Recruitment to pharmacy-based interventions may indicate possible differences in uptake of pharmaceutical services. There is some evidence of differences in recruitment to community pharmacy interventions according to sex. Across all the studies there was a majority of female participants recruited and this was the more pronounced within the weight loss studies. It is unclear how this might impact on the willingness of men to use weight loss services in pharmacies. More research is needed about whether or not access to community pharmacies, and uptake of their services, differs by sociodemographic and socioeconomic characteristics.
The authors are not aware of any other reviews which directly compare public health interventions across different primary or community health-care settings. This review attempts to do just that by including studies that compared a pharmacy-based intervention group with interventions based in other settings. However, only five of the included studies compared a pharmacy setting with another setting (two smoking cessation and three weight loss interventions). One smoking cessation study compared pharmacy with an outpatient setting and found no significant difference between groups for smoking cessation. Another smoking cessation study found that the NHS group-based service set in the community attracted fewer clients but was significantly more effective in stopping smoking at 12 months than the pharmacy-based service. Three weight loss studies compared different settings, GP, primary care units and pharmacy settings appeared to be of equal effectiveness, which was less than that of commercial services based in the community.
A direct comparison between public health interventions in different settings is difficult because of differences in the characteristics of the participants and the context in which the interventions are delivered. There are many other factors in addition to effectiveness that need to be considered, such as recruitment and attendance, which appear to be comparable between GP surgeries and pharmacies but better in community and commercial programmes. Current evidence shows that referral to commercial weight loss providers is more effective than GP surgery- and pharmacy-delivered interventions. In the choice arm of the Lighten Up trial,66 71 (71%) participants chose one of the commercial providers, 16 (16%) chose the Size Down programme, three (3%) chose general practice and 10 (10%) chose pharmacy provision. Women were more likely than men to choose one of the commercial providers [57 (81%) women, compared with 14 (47%) men]. Among those randomised, the rate of participants not taking up the invitation to attend was twice as high for pharmacy as for commercial providers and only the minimal contact control arm had lower uptake rate. In terms of programme attendance, pharmacy had the worst attendance records of all arms.
As well as possible differential uptake of pharmaceutical services by demographic and socioeconomic characteristics, there may be differential uptake relative to other community health-care settings such as primary care. If a distinct group of people is accessing pharmaceutical services who would not access services in other health-care settings even if effect sizes are smaller than in other settings, then public health interventions within community pharmacies need to be considered as an option within a suite of choices available for the general population that could positively impact on health inequalities at a population level. However, we found an absence of evidence in this regard.
There is insufficient evidence to examine the relationship between behaviour change strategies and effectiveness or evidence of consistent implementation factors or training components that underpin effective interventions. More information is needed about pharmacist training and the experience of those delivering the training, resources required and sustainability of pharmacy-based interventions.
Contextual factors are important when developing public health services in a community pharmacy environment. One such important consideration is the changing landscape of health-care services and the emphasis placed on expanding the role of community pharmacies. This has been acknowledged internationally: the WHO has described qualities of a future pharmacist82 – one of whom is a care-giver. Rather than using community pharmacies in their traditional role in dispensing and compounding medication, there are drivers in policy to extend the role of community pharmacists. This is also evident in the NHS, where the changing contract of community pharmacies is allowing pharmacists to become more involved in patient-focused health-care service delivery. Recently there have been campaigns to use community pharmacies to obtain certain health-care advice rather than other, perhaps more costly, primary care services. Clearly, how the contract for these services is structured is an important factor in how these services are implemented.
Another important factor in terms of service delivery is how patients perceive community pharmacies. Recent evidence from a qualitative study83 suggests that patients will not ‘trust’ pharmacies to deliver unfamiliar health-care services. Similarly, a systematic review undertaken by Eades et al. 84 showed that, despite the changing role of community pharmacies, most consumers did not expect a public health service by a pharmacist; they also had mixed views on a pharmacist’s ability to provide such services. The public’s perception of community pharmacies and the ability to provide public health services could be a significant barrier towards implementation unless strategies are put in place to promote this.
The role of community pharmacies differs across the world, with many pharmacies still solely used as a means of supplying medication. Although nine studies included in this review were UK based, there were studies set in a further 10 countries, inside and outside Europe. It is probable that the uptake of pharmacy services will be variable, based on consumers’ perceptions and experiences. The influences on people’s choice of community pharmacy versus GP surgery, self-management or commercial provider are likely to vary between countries where health care is delivered using different models. However, in view of the changing role of pharmacy, the concept of ‘pharmaceutical care’ has been introduced. Although this relates to the provision of drug therapy, it is associated with the outcomes of treating or preventing disease. This term is recognised internationally and used throughout the world by policy-makers relevant to health care.
Research recommendations
This review has prompted various suggestions for improvement, to contribute to a more useful and rigorous evidence base, which will enable the translation of research findings into effective public health approaches for managing alcohol, smoking and weight within the pharmacy setting.
-
Surprisingly, a relatively large proportion of the research so far has been carried out in the UK, which contributes to its generalisability to the UK pharmacy setting. However, the overall quality of the 24 included studies suggests that more research is required to improve recruitment and retention of participants to pharmacy-based interventions.
-
Only two studies evaluated the efficacy of pharmacy-based interventions in improving alcohol management. Evaluations of interventions are required in order to assess the effectiveness of pharmacy-based interventions for alcohol management.
-
More research is needed about how an intervention may impact on inequalities in alcohol misuse, smoking and obesity; and how this impact will be measured, in terms of socioeconomic variables and ethnicity. None of the studies examined the effect of interventions across the SES gradient. In a few cases where studies examined the differential effects of demographic variables, the significance of these differences on health inequalities is unclear. Future studies will need to be sufficiently powered to detect small changes and to measure equity effects of these small changes at a population level.
-
The implementation tool was useful in extracting descriptive data across a wide range of factors. However, it will need to be refined in the future, or a new tool developed, if it is to help gather more insight into why an intervention might or might not work. Other methods of review, such as a realist review, offer an alternative approach to synthesising information about implementation, and so any refinement or new tool might benefit from taking this approach into account. However, as one of the problems in our review, and in others that have assessed implementation, was the paucity of information in the primary studies about implementation factors. Therefore, we recommend that ‘implementation reporting’ guidelines be developed within public health so that this important information is included by researchers undertaking primary studies, in a more systematic way.
-
This review identified little evidence about the reach of pharmacy-based interventions. Targeted intervention studies provide some evidence that adults accessing pharmacies are a distinct subgroup that may not access other primary care or commercial services. This evidence is derived from participants who self-selected the intervention and setting. More research is required on the reach of the pharmacy setting.
Acknowledgements
We would like to thank the members of our review advisory group for their time and advice throughout the review: Claire Anderson (professor of social pharmacy, head of Division of Social Research in Medicines and Health, University of Nottingham), Christine Bond (professor, chairperson in general practice and primary care, University of Aberdeen), Mark Burdon (community pharmacist, member of PSNC), Carol Hall (retired, patient, member of the pharmacy-specific patient and public involvement group), Eileen Kaner (professor and director of the Institute of Health & Society, expertise in alcohol misuse, University of Newcastle), Elena Ratschen (lecturer in epidemiology/tobacco control, University of Nottingham), Pat Simpson (retired, patient, member of the pharmacy-specific patient and public involvement group) and Margaret Watson (senior research fellow, pharmacy, University of Aberdeen).
We would like to thank Julian Higgins (professor of evidence synthesis, University of Bristol) for advice on evidence synthesis. We would like to thank Nasima Akhter (post-doctorate, nutrition and epidemiology, Durham University) for statistical support.
We would also like to thank Sarah Smith (Doctor of Philosophy student, obesity, Durham University) for assistance with screening and data extraction, and Lucie Nield (lecturer in nutrition and dietitian, Sheffield Hallam University) for help with data extraction.
We would like to thank the following study authors for data clarification: Wiraphol Phimarn (professor of pharmacy, Mahasarakham University), John A Galdo (clinical assistant professor, University of Georgia College of Pharmacy) and James C McElnay (professor and pro-vice-chancellor, Queen’s University Belfast).
We would also like to thank Lisa Monkhouse (departmental secretary, pharmacy, Durham University) for administrative support.
Contributions of authors
Tamara J Brown (post-doctoral research associate, public health nutrition) provided methodological input and was responsible for management of the data collection, analysis and interpretation and, together with Carolyn D Summerbell, led the drafting of the report.
Adam Todd (programme director and senior lecturer in pharmacy practice) provided methodological and conceptual input, data collection, analysis and interpretation, and contributed to the drafting of the report.
Claire L O’Malley (research assistant, public health nutrition) contributed to data collection and the drafting of the report.
Helen J Moore (post-doctoral research associate, public health nutrition) designed and conducted the searches, contributed to the data collection and contributed to the drafting of the report.
Andrew K Husband (dean of pharmacy) provided methodological and conceptual input and contributed to the drafting of the report.
Clare Bambra (professor, public health geography) provided methodological input and contributed to analysis and interpretation in regards to health inequalities, and contributed to the drafting of the report.
Adetayo Kasim (statistician) conducted the meta-analysis and contributed to the drafting of the report.
Falko F Sniehotta (professor of behaviour medicine and health psychology) provided methodological input and contributed to the interpretation in regard to behaviour change interventions and to the drafting of the report.
Liz Steed (lecturer in health psychology) contributed to developing the search strategy, data collection and the drafting of the report.
Carolyn D Summerbell (professor, public health nutrition) was responsible for overall co-ordination and project management. She provided methodological and conceptual input, data collection, analysis and interpretation and, together with Tamara J Brown, led the drafting of the report.
Publications
Brown T, Todd A, O’Malley C, Moore H, Husband A, Bambra C, et al. Community pharmacy interventions for public health priorities: a systematic review of community pharmacy-delivered smoking, alcohol and weight management interventions. The Wolfson Research Institute for Health and Wellbeing 2015 Research Colloquium, Durham 15 April 2015.
Brown T, Todd A, O’Malley C, Moore H, Husband A, Bambra C, et al. Community pharmacy interventions for public health priorities: a systematic review of community pharmacy-delivered smoking, alcohol and weight management interventions. 22nd European Congress on Obesity 2015, Prague, 6–9 May 2015.
Brown T, Todd A, O’Malley C, Moore H, Husband A, Bambra C, et al. Community pharmacy interventions for public health priorities: a systematic review of community pharmacy-delivered smoking, alcohol and weight management interventions. International Society of Behavioral Nutrition and Physical Activity 2015 Annual Meeting, Edinburgh, 3–6 June 2015, poster number 1227.
Brown TJ, Todd A, O’Malley C, Moore HJ, Husband AK, Bambra C, et al. Community pharmacy-delivered interventions for public health priorities: a systematic review of interventions for alcohol reduction, smoking cessation and weight management, including meta-analysis for smoking cessation. BMJ Open 2016;6:e009828.
Data sharing statement
All data from this project can be acquired from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health.
References
- Global Strategy on Diet, Physical Activity and Health. Geneva: WHO; 2004.
- Pharmacy in England: Building on Strengths – Delivering the Future. London: DH; 2008.
- Choosing Health through Pharmacy. A Programme for Pharmaceutical Public Health 2005–2015. London: DH; 2005.
- Healthy Lives, Healthy People: Our Strategy for Public Health in England. London: DH; 2010.
- Bambra C, Joyce K, Maryon-Davies A. Strategic Review of Health Inequalities in England Post-2010 London; (Marmot Review) (Task Group 8: Priority Public Health Conditions: Final Report). London: University College London Institute of Health Equity; 2009.
- Marmot M. Fair Society, Healthy Lives (Marmot Review). London: University College London; 2010.
- Erskine S, Maheswaran R, Pearson T, Gleeson D. Socioeconomic deprivation, urban–rural location and alcohol-related mortality in England and Wales. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-99.
- Siegler V, Al-Hamad A, Johnson B, Wells C, Sheron N. Social inequalities in alcohol-related adult mortality by National Statistics Socio-economic Classification, England and Wales, 2001–03. Health Stat Q 2011;50:1-36. http://dx.doi.org/10.1057/hsq.2011.7.
- Action on Smoking and Health . Smoking Statistics: Illness and Death 2013. http://ash.org.uk/files/documents/ASH_107.pdf (accessed 30 June 2015).
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38142.554479.AE.
- Community Pharmacy: At the Heart of Public Health. London: PSNC; 2010.
- Action on Smoking and Health . Smoking Statistics: Who Smokes and How Much n.d. http://ash.org.uk/files/documents/ASH_106.pdf (accessed 30 June 2015).
- Smoking Cessation: Supporting People to Stop Smoking. London: NICE; 2013.
- National Obesity Observatory . International Comparisons of Adult Obesity Levels 2014. www.noo.org.uk/NOO_about_obesity/adult_obesity/international (accessed 30 June 2015).
- National Obesity Observatory . International Comparisons of Children’s Obesity Levels 2014. www.noo.org.uk/NOO_about_obesity/child_obesity/international (accessed 30 June 2015).
- Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Community Health 1998;52:399-405. http://dx.doi.org/10.1136/jech.52.6.399.
- Gordon J, Watson M, Avenell A. Lightening the load? A systematic review of community pharmacy-based weight management interventions. Obes Rev 2001;12:897-91. http://dx.doi.org/10.1111/j.1467-789X.2011.00913.x.
- Watson MC, Blenkinsopp A. The feasibility of providing community pharmacy based services for alcohol misuse: a literature review. Int J Pharm Pract 2009;17:199-205. http://dx.doi.org/10.1211/ijpp.17.04.0002.
- Sinclair HK, Bond CM, Stead LF. Community pharmacy personnel interventions for smoking cessation. Cochrane Database Syst Rev 2004;1. http://dx.doi.org/10.1002/14651858.cd003698.pub2.
- Todd A, Copeland A, Husband A, Kasim A, Bambra C. The positive pharmacy care law: an area-level analysis of the relationship between community pharmacy distribution, urbanity and social deprivation in England. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005764.
- NHS Scotland. Better Health, Better Care: Action Plan. Edinburgh: Scottish Government; 2008.
- Pharmaceutical Services Negotiating Committee . Service Specifications and Resources n.d. http://psnc.org.uk/service-search-results/?location-of-service=293&type-of-service=387&method-of-commissioning=&funding source=&wqsfsubmit=Filter (accessed 17 June 2015).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011.
- Todd A, Moore HJ, Husband AK, Bambra C, Kasim A, Sniehotta FF, et al. Community pharmacy interventions for public health priorities: protocol for a systematic review of community pharmacy delivered smoking, alcohol and weight management interventions. Syst Rev 2014;3. http://dx.doi.org/10.1186/2046-4053-3-93.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Welch V, Petticrew M, Tugwell P, Moher D, O’Neill J, Waters E, et al. PRISMA-Equity 2012 Extension: reporting guidelines for systematic reviews with a focus on health equity. PLOS Med 2012;9. http://dx.doi.org/10.1371/journal.pmed.1001333.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1687.
- What Study Designs Should be Included in an EPOC Review? EPOC Resources for Review Authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013.
- Hardeman W, Sutton S, Griffin S, Johnston M, White A, Wareham NJ, et al. A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res 2005;20:676-87. http://dx.doi.org/10.1093/her/cyh022.
- Egan M, Bambra C, Petticrew M, Whitehead M. Reviewing evidence on complex social interventions: appraising implementation in systematic reviews of the health effects of organisational-level workplace interventions. J Epidemiol Community Health 2009;31:4-11. http://dx.doi.org/10.1136/jech.2007.071233.
- Bambra CL, Hillier-Brown FC, Cairns JM, Kasim A, Moore HJ, Summerbell CD. How effective are interventions at reducing socioeconomic inequalities in obesity among children and adults? Two systematic reviews. Public Health Res 2015;3.
- Michie S, van Stralen M, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Public Health: Ethical Issues. London: Nuffield Council on Bioethics; 2007.
- Effective Public Health Practice Project . Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies n.d. www.ephpp.ca/tools.html (accessed 30 June 2015).
- Cochrane Public Health Group . Guidance for Authors n.d. http://ph.cochrane.org/review-authors (accessed 30 June 2015).
- Bond CM. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract 2007;24:189-200. http://dx.doi.org/10.1093/fampra/cml075.
- Botomino AR, Bruppacher S, Krahenbuhl S, Hersberger KE. Change of body weight and lifestyle of persons at risk for diabetes after screening and counselling in pharmacies. Pharm World Sci 2008;30:222-6. http://dx.doi.org/10.1007/s11096-007-9174-3.
- Castejon AM, Calderon JL, Perez A, Millar C, McLaughlin-Middlekauff J, Sangasubana N, et al. A community-based pilot study of a diabetes pharmacist intervention in latinos: impact on weight and hemoglobin A1c. J Health Care Poor Underserved 2013;24:48-60. http://dx.doi.org/10.1353/hpu.2014.0003.
- Malone M, Alger-Mayer SA. Pharmacist intervention enhances adherence to orlistat therapy. Ann Pharmacother 2003;37:1598-602. http://dx.doi.org/10.1345/aph.1D183.
- Moideen MM, Varghese R, Ramakrishnan P, Dhanapal CK. Patient education for overweight and obese patients on weight reduction in an urban community pharmacy and its outcome. Res J Pharm Biol Chem Sci 2011;2:392-405.
- Shakeshaft A, Doran C, Petrie D, Breen C, Havard A, Abudeen A, et al. The effectiveness of community action in reducing risky alcohol consumption and harm: a cluster randomised controlled trial. PLOS Med 2011;11. http://dx.doi.org/10.1371/journal.pmed.1001617.
- Zillich AJ, Ryan M, Adams A, Yeager B, Farris K. Effectiveness of a pharmacist-based smoking-cessation program and its impact on quality of life. Pharmacotherapy 2002;22:759-65. http://dx.doi.org/10.1592/phco.22.9.759.34073.
- Nieto L. A Comparison of Weight Loss Outcome Across Three Distinct Weight Loss Program Delivery Venues 2011.
- Howard-Pitney B, Killen JD, Fortmann SP. Quitting chew: results from a randomized trial using nicotine patches. Exp Clin Psychopharmacol 1999;7:362-71. http://dx.doi.org/10.1037/1064-1297.7.4.362.
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. J Pharm Pract Res 2002;32:57-62.
- Dhital R, Norman I, Whittlesea C, Murrells T, McCambridge J. The effectiveness of brief alcohol interventions delivered by community pharmacists: randomised controlled trial. Addiction 2015;110:1586-94. http://dx.doi.org/10.1111/add.12994.
- Kypri K, Mc Cambridge J, Wilson A, Attia J, Sheeran P, Bowe S, et al. Effects of Study Design and Allocation on participant behaviour-ESDA: study protocol for a randomized controlled trial. Trials 2011;12.
- Watson MC, Stewart D. Screening and Brief Interventions for Alcohol Misuse Delivered in the Community Pharmacy Setting: A Pilot Study 2011.
- Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, et al. One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services. Nicotine Tob Res 2011;13:135-45. http://dx.doi.org/10.1093/ntr/ntq222.
- Bauld L, Chesterman J, Ferguson J, Judge K. A comparison of the effectiveness of group-based and pharmacy-led smoking cessation treatment in Glasgow. Addiction 2009;104:308-16.
- Boyd KA, Briggs AH. Cost-effectiveness of pharmacy and group behavioural support smoking cessation services in Glasgow. Addiction 2009;104:317-25.
- Bock BC, Hudmon KS, Christian J, Graham AL, Bock FR. A tailored intervention to support pharmacy-based counseling for smoking cessation. Nic Tob Res 2010;12:217-25. http://dx.doi.org/10.1093/ntr/ntp197.
- Burford O, Jiwa M, Carter O, Parsons R, Hendrie D. Internet-based photoageing within Australian pharmacies to promote smoking cessation: randomized controlled trial. J Med Internet Res 2013;15.
- Droomers M, Schrijver CT, Mackenbach JP. Educational differences in the intention to stop smoking: explanations based on the Theory of Planned Behaviour. Eur J Public Health 2004;14:194-8.
- Costello MJ, Sproule B, Victor JC, Leatherdale ST, Zawertailo L, Selby P. Effectiveness of pharmacist counseling combined with nicotine replacement therapy: a pragmatic randomized trial with 6,987 smokers. Cancer Causes Control 2011;22:167-80. http://dx.doi.org/10.1007/s10552-010-9672-9.
- Crealey GE, McElnay JC, Maguire TA, O’Neill C. Costs and effects associated with a community pharmacy-based smoking-cessation programme. Pharmacoeconomics 1998;14:323-33. http://dx.doi.org/10.2165/00019053-199814030-00008.
- Hoving C, Mudde AN, Dijk F, de Vries H. Effectiveness of a smoking cessation intervention in Dutch pharmacies and general practices. Health Educ 2010;110:17-29. http://dx.doi.org/10.1108/09654281011008726.
- Maguire TA, McElnay JC, Drummond A. A randomized controlled trial of a smoking cessation intervention based in community pharmacies. Addiction 2001;96:325-31. http://dx.doi.org/10.1046/j.1360-0443.2001.96232516.x.
- Mochizuki M, Hatsugaya M, Rokujoh E, Arita E, Hashiguchi M, Shimizu N, et al. Randomized controlled study on the effectiveness of community pharmacists’ advice for smoking cessation by Nicorette – evaluation at 3 months after initiation. Yakugaku Zasshi 2004;124:989-95. http://dx.doi.org/10.1248/yakushi.124.989.
- Sinclair HK, Bond CM, Lennox AS, Silcock J, Winfield AJ, Donnan PT. Training pharmacists and pharmacy assistants in the stage-of-change model of smoking cessation: a randomised controlled trial in Scotland. Tob Control 1998;7:253-61. http://dx.doi.org/10.1136/tc.7.3.253.
- Sinclair HK, Silcock J, Bond CM, Lennox AS, Winfield AJ. The cost-effectiveness of intensive pharmaceutical intervention in assisting people to stop smoking. Int J Pharm Practice 1999;7:107-12. http://dx.doi.org/10.1111/j.2042-7174.1999.tb00957.x.
- Goldberg DN, Hoftman AM, Farinha MF, Marder DC, Tinson-Mitchem L, Burton D, et al. Physician delivery of smoking cessation advice based on the stages-of-change model. Am J Prev Med 1994;10:267-74.
- Sonderskov J, Olsen J, Sabroe S, Meillier L, Overvad OK. Nicotine patches in smoking cessation: a randomized trial among over-the-counter customers in Denmark. Am J Epidemiol 1997;145:309-18. http://dx.doi.org/10.1093/oxfordjournals.aje.a009107.
- Ahrens RA, Hower M, Best AM. Effects of weight reduction interventions by community pharmacists. J Am Pharm Assoc 2003;43:583-9. http://dx.doi.org/10.1331/154434503322452210.
- Bush J, Langley CA, Patel A, Harvey JE. Evaluation of the Heart of Birmingham Teaching Primary Care Trust (HoBtPCT) My Choice Weight Management Programme. Final Report 2011 2011.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d6500.
- Phimarn W, Pianchana P, Limpikanchakovit P, Suranart K, Supapanichsakul S, Narkgoen A, et al. Thai community pharmacist involvement in weight management in primary care to improve patient’s outcomes. Int J Clin Pharm 2013;35:1208-17. http://dx.doi.org/10.1007/s11096-013-9851-3.
- Ali M, Schifano F, Robinson P, Phillips G, Doherty L, Melnick P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomized controlled study. Diabet Med 2012;29:e326-33. http://dx.doi.org/10.1111/j.1464-5491.2012.03725.x.
- Correr CJ, Melchiors AC, Fernandez-Limos F, Pontarolo R. Effects of a pharmacotherapy follow-up in community pharmacies on type 2 diabetes patients in Brazil. Int J Clin Pharm 2011;33:273-80. http://dx.doi.org/10.1007/s11096-011-9493-2.
- Fornos JA, Andres NF, Andres JC, Guerra MM, Egea B. A pharmacotherapy follow-up program in patients with type-2 diabetes in community pharmacies in Spain. Pharm World Sci 2006;28:65-72. http://dx.doi.org/10.1007/s11096-006-9003-0.
- Paulos CP, Akesson Nygren CE, Celedon C, Carcamo CA. Impact of a pharmaceutical care program in a community pharmacy on patients with dyslipidemia. Ann Pharmacother 2005;39:939-43. http://dx.doi.org/10.1345/aph.1E347.
- Zaragoza-Fernandez MP, Gastelurrutia MA, Cardero M, Martinez-Martinez F. Intensive two-month intervention on diet and lifestyle in uncontrolled hypertensive patients in a community pharmacy. Lat Am J Pharm 2012;31:727-33.
- Sonderskov J, Olsen J, Meillier L, Overvad OK, Sabroe S. Nicotine patches in smoking cessation: a randomized trial among over-the-counter customers in Denmark. Ugeskr Laeger 1999;161:593-7.
- Fornos Perez JA, Guerra Garcia MM, Andres Rodriguez NF, Egea Ibernon B. Evaluacion de un programa de seguimiento farmacoterapeutico a diabeticos tipo 2. Aten Primaria 2004;34:48-54.
- Scottish Index of Multiple Deprivation. Edinburgh: Scottish Government; 2009.
- Carstairs V, Morris R. Deprevation and Health in Scotland. Aberdeen: Aberdeen University; 1991.
- The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- Evaluation of Drinkaware Resources. London: Shared Intelligence; 2014.
- Gray NJ, Wilson SE, Cook PA, Macridge AJ, Blenkinsopp A, Prescott L, et al. Understanding and Optimising an Identification/Brief Advice (IBA) Service about Alcohol in the Community Pharmacy Setting. Final Report 2012. Liverpool: Liverpool Primary Care Trust; 2012.
- Peterborough City Council . Peterborough Pharmaceutical Needs Assessment 2015. www.peterborough.gov.uk/upload/www.peterborough.gov.uk/healthcare/public-health/Healthcare-PublicHealth-PeterboroughPNA2015.pdf?inline=true (accessed 30 June 2015).
- Boardman H, Lewis M, Trinder P, Rajaratnam G, Croft P. Use of community pharmacies: a population-based survey. J Public Health 2005;27:254-62. http://dx.doi.org/10.1093/pubmed/fdi032.
- Wiedenmayer K, Summers RS, Mckie CA, Gous AGS, Everard M. Developing Pharmacy Practice. A Focus on Patient Care. Handbook – 2006 Edition. Geneva: WHO, Department of Medicines Policy and Standards, in collaboration with International Pharmaceutical Federation; 2006.
- Gidman W, Ward P, McGregor L. Understanding public trust in services provided by community pharmacists relative to those provided by general practitioners: a qualitative study. BMJ Open 2015;2. http://dx.doi.org/10.1136/bmjopen-2012-000939.
- Eades CE, Ferguson JS, O’Carroll RE. Public health in community pharmacy: a systematic review of pharmacist and consumer views. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-582.
- Corelli RL, Kroon LA, Chung EP, Sakamoto LM, Gunderesen B, Fenlon CM, et al. Staterwide evaluation of a tobacco cessation curriculum for pharmacy students. Prev Med 2005;40:888-95.
- Fiore C, Jaen CR, Baker TB. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008.
Appendix 1 Search strategy
MEDLINE (via Ovid)
Searched: May 2014.
Date of search: from inception to May 2014.
Search strategy
| # | Search term |
|---|---|
| 1 | exp Community Pharmacy Services/ |
| 2 | Pharmacies/ |
| 3 | exp Pharmacists/ |
| 4 | exp Pharmacists’ Aides/ |
| 5 | Pharmacy/ |
| 6 | chemist.tw. |
| 7 | (communit$ adj7 pharmac$).tw. |
| 8 | (office$ adj7 pharmacy$).tw. |
| 9 | ((pharmacy or pharmacist? or pharmacies) adj3 (community or counsel$ or advice or care)).tw. |
| 10 | (pharmacist? adj3 (front line or ’one to one’ or face to face)).tw. |
| 11 | (pharmacist? or pharmacy or pharmacies).tw. |
| 12 | ((pharmacist? or pharmacy) adj3 (aide or aides or assistant? or staff)).tw. |
| 13 | (Pharmacist? adj2 (care or delivered)).tw. |
| 14 | (pharmacist? adj3 (counsel$ or (patient? adj2 education$) or led or intervention? or public health or diagnos$)).tw. |
| 15 | or/1–14 |
| 16 | exp Obesity/ |
| 17 | exp Body Weight/ |
| 18 | exp Body Weight Changes/ |
| 19 | exp Weight Gain/ or exp Weight Loss/ |
| 20 | (obese or obesity).tw. |
| 21 | overweight.tw. |
| 22 | weight.tw. |
| 23 | diet$.tw. |
| 24 | nutrition$.tw. |
| 25 | (physical$ adj activ$).tw. |
| 26 | exercise$.tw. |
| 27 | lifestyle$.tw. |
| 28 | (bmi$ or (body adj mass ind$)).tw. |
| 29 | (waist adj6 circumference$).tw. |
| 30 | ((weight adj2 (control or reduction) adj2 (advice or counsel$ or program$ or intervention?)) or (weight adj manag$)).tw. |
| 31 | ((overweight or obese or obesity) adj4 (Advice or counsel$ or intervention? or program$)).tw. |
| 32 | or/16–31 |
| 33 | exp Smoking/ or exp Smoking Cessation/ |
| 34 | nicotine.tw. |
| 35 | cigarette$.tw. |
| 36 | (nicotine replacement therapy or NRT).tw. |
| 37 | smoking cessation.tw. |
| 38 | smok$.tw. |
| 39 | exp "Tobacco Use Cessation"/ |
| 40 | exp Smoking Cessation/ |
| 41 | (smoking cessation or (quit$ adj2 smok$)).tw. |
| 42 | ((reduce or reducing) adj3 (’tobacco use’ or cigarette? or smoking or addiction)).tw. |
| 43 | or/33–42 |
| 44 | alcohol.mp. |
| 45 | exp Alcohols/ |
| 46 | exp Alcohol Drinking/ |
| 47 | exp Alcoholism/ |
| 48 | exp Drinking Behavior/ |
| 49 | (drink$).tw. |
| 50 | beer.tw. |
| 51 | wine.tw. |
| 52 | ethanol.tw. |
| 53 | drunk.tw. |
| 54 | (addict$ or (alcohol adj2 (abus$ or misus$))).tw. |
| 55 | alcohol$.tw. |
| 56 | drunk$.tw. |
| 57 | intoxicat$.tw. |
| 58 | or/44–57 |
| 59 | 32 or 43 or 58 |
| 60 | (animals not humans).mp. |
| 61 | 59 not 60 |
| 62 | 15 and 60 |
| 63 | limit 62 to humans |
Appendix 2 Data extraction form
Project details
Author.
Year.
Project name.
Publication type.
Journal Volume (Issue) Pages.
Aims (rationale, theory or goal of the elements essential to the intervention).
Target population(s).
Country.
Intervention description (materials used and procedures).
Start date of project.
End date of project.
Date.
Reviewers initials of data extraction.
Cost to participant, pharmacy, local authority, or other organisation(s).
Throughput – number of participants per time period.
Resources (time, money, staff and equipment).
Theoretical basis/behaviour change techniques used.
Contact details.
Language.
Behaviour-change wheel.
Intervention function.
Behaviour-change wheel.
Policy category.
Nuffield Intervention Ladder code.
Staff training and quality assurance.
Was consistency of intervention measured?
Was the intervention delivered as intended?
Is it likely contamination occurred?
Delivery fidelity
Experience of intervention team.
Project details notes (if required).
Implementation context notes.
Study information
Study Focus.
Smoking/Alcohol Study: Measure of behaviour?
Weight Study: Measure of weight? If no, stop extraction. If yes, add details on measures taken and instruments used (including if validated). If no, stop extraction. If yes, add details on measures taken and instruments used (including if validated).
Setting (should be community pharmacy).
Study Design Type RCT: allocated to different groups using methods that are random.
nRCT: allocated to different groups using methods that are not random.
CBA: observations are made before and after an intervention, both in a group that receives the intervention and in a control group that does not.
Interrupted time series (ITS): observations are made at multiple time points before and after an intervention.
RMS: a ITS study where measurements are made in the same individuals at each time point.
Before–After: must have at least 1 measure before and after (stop extraction, and keep in a pile).
Level of intervention (individual, community, societal).
Approach to targeting inequality (targeted or universal?).
Unit of randomisation/allocation.
Unit of analysis.
Did the intervention deliverers receive any training related to the intervention, and if so, what?
Measure of inequality.
Sex, age, and individual or area-level measures of socioeconomic status (education, income, occupation, social class, deprivation, poverty).
Population details
Population targeted.
Method of sampling (volunteer, random, stratified, etc.).
Ethnicity.
Study design.
Total population (number who could take part/approached).
Time between baseline and follow-ups.
Confounding from attrition/non response explored?
Adjustments?
Intention-to-treat?
Imputation of missing data?
Population details notes (if required).
% female (baseline sample).
Mean age (years) SD Median age (years) range.
Baseline recruitment rate (%).
Baseline sample size.
Final sample size.
Follow-up response rate (%) Sample size of final analysis.
If data are reported in two groups:
If data are reported together:
% female (baseline sample).
Mean age (years) SD Median age (years) range.
Baseline recruitment rate (%).
Baseline sample size.
Final sample size.
Follow-up response rate (%) Sample size of final analysis.
Intervention/Group 1:
% female (baseline sample).
Mean age (years) SD Median age (years) range.
Baseline recruitment rate (%).
Baseline sample size.
Final sample size.
Follow-up response rate (%) Sample size of final analysis.
Control/Group 2:
Outcomes and results
Data collection methods.
Outcomes.
Outcome assessor(s) aware of intervention status?
Participants aware of research question?
Data collection tools valid?
Data collection tools reliable?
Results (evidence of effectiveness).
Acceptability to staff and customers.
Evidence of cost-effectiveness (where applicable).
Set up and running costs.
Funding source and length/security of funding.
Outcomes and results notes (if required).
Stakeholder support.
Sustainability.
Implementation
A) Motivation (Why was the intervention implemented?).
B) Theoretical basis/behaviour change techniques used and staff training and quality assurance.
C) Implementation context Notes.
D) Experience of intervention team (planners/implementers).
E) Consultation and/or collaboration processes.
F) Was the intervention delivered as intended?
G) Sustainability.
H) Stakeholder support.
I) Resources (time, money, staff and equipment).
J) Differential effects.
Implementation score.
Appendix 3 Quality assessment tool
Component ratings
(A) Selection bias
(Q1) Are the individuals selected to participate in the study likely to be representative of the target population?
Very likely
Somewhat likely
Not likely
Can’t tell
(Q2) What percentage of selected individuals agreed to participate?
80–100% agreement
60–79% agreement
< 60% agreement
Not applicable
Can’t tell
| RATE THIS SECTION | STRONG | MODERATE | WEAK |
|---|---|---|---|
| See dictionary | 1 | 2 | 3 |
(B) Study design
Indicate the study design
Randomized controlled trial
Controlled clinical trial
Cohort analytic (two group pre + post)
Case-control
Cohort (one group pre + post (before and after))
Interrupted time series
Other specify ____________________________
Can’t tell
Was the study described as randomized? If NO, go to Component C.
No Yes
If Yes, was the method of randomization described? (See dictionary)
No Yes
If Yes, was the method appropriate? (See dictionary)
No Yes
| RATE THIS SECTION | STRONG | MODERATE | WEAK |
|---|---|---|---|
| See dictionary | 1 | 2 | 3 |
(C) Confounders
(Q1) Were there important differences between groups prior to the intervention?
Yes
No
Can’t tell
The following are examples of confounders:
Race
Sex
Marital status/family
Age
SES (income or class)
Education
Health status
Pre-intervention score on outcome measure
(Q2) If yes, indicate the percentage of relevant confounders that were controlled (either in the design (e.g. stratification, matching) or analysis)?
80–100% (most)
60–79% (some)
< 60% (few or none)
Can’t tell
| RATE THIS SECTION | STRONG | MODERATE | WEAK |
|---|---|---|---|
| See dictionary | 1 | 2 | 3 |
(D) Blinding
(Q1) Was (were) the outcome assessor(s) aware of the intervention or exposure status of participants?
Yes
No
Can’t tell
(Q2) Were the study participants aware of the research question?
Yes
No
Can’t tell
| RATE THIS SECTION | STRONG | MODERATE | WEAK |
|---|---|---|---|
| See dictionary | 1 | 2 | 3 |
(E) Data collection methods
(Q1) Were data collection tools shown to be valid?
Yes
No
Can’t tell
(Q2) Were data collection tools shown to be reliable?
Yes
No
Can’t tell
| RATE THIS SECTION | STRONG | MODERATE | WEAK |
|---|---|---|---|
| See dictionary | 1 | 2 | 3 |
(F) Withdrawals and drop-outs
(Q1) Were withdrawals and drop-outs reported in terms of numbers and/or reasons per group?
Yes
No
Can’t tell
Not applicable (i.e. one time surveys or interviews)
(Q2) Indicate the percentage of participants completing the study. (If the percentage differs by groups, record the lowest.)
80–100%
60–79%
< 60%
Can’t tell
Not applicable (i.e. retrospective case–control)
| RATE THIS SECTION | STRONG | MODERATE | WEAK | ||||
|---|---|---|---|---|---|---|---|
| See dictionary | 1 | 2 | 3 | Not applicable | |||
(G) Intervention integrity
(Q1) What percentage of participants received the allocated intervention or exposure of interest?
80–100%
60–79%
< 60%
Can’t tell
(Q2) Was the consistency of the intervention measured?
Yes
No
Can’t tell
(Q3) Is it likely that subjects received an unintended intervention (contamination or cointervention) that may influence the results?
Yes
No
Can’t tell
(H) Analyses
(Q1) Indicate the unit of allocation (circle one).
Community organization/institution practice/office individual
(Q2) Indicate the unit of analysis (circle one).
Community organization/institution practice/office individual
(Q3) Are the statistical methods appropriate for the study design?
Yes
No
Can’t tell
(Q4) Is the analysis performed by intervention allocation status (i.e. intention to treat) rather than the actual intervention received?
Yes
No
Can’t tell
Global rating
Component ratings
| Please transcribe the information from the grey boxes on pages 1–4 onto this page. See dictionary on how to rate this section. A | SELECTION BIAS | STRONG | MODERATE | WEAK | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||
| B | STUDY DESIGN | STRONG | MODERATE | WEAK | |||||
| 1 | 2 | 3 | |||||||
| C | CONFOUNDERS | STRONG | MODERATE | WEAK | |||||
| 1 | 2 | 3 | |||||||
| D | BLINDING | STRONG | MODERATE | WEAK | |||||
| 1 | 2 | 3 | |||||||
| E | DATA COLLECTION METHOD | STRONG | MODERATE | WEAK | |||||
| 1 | 2 | 3 | |||||||
| F | WITHDRAWALS AND DROPOUTS | STRONG | MODERATE | WEAK | |||||
| 1 | 2 | 3 | Not applicable | ||||||
Appendix 4 List of included studies
Bold type indicates main study paper in cases of multiple study papers.
Ahrens RA, Hower M, Best AM. Effects of weight reduction interventions by community pharmacists. J Am Pharm Assoc 2003;43:583–9.
Ali M, Schifano F, Robinson P, Phillips G, Doherty L, Melnick P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomised controlled study. Diabet Med 2012;29:e326–33.
Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, et al. One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services. Nicotine Tob Res 2011;13:135–45.
Bauld L, Chesterman J, Ferguson J, Judge K. A comparison of the effectiveness of group-based and pharmacy-led smoking cessation treatment in Glasgow. Addiction 2009;104:308–16.
Boyd KA, Briggs AH. Cost-effectiveness of pharmacy and group behavioural support smoking cessation services in Glasgow. Addiction 2009;104:317–25.
Bock BC, Hudmon KS, Christian J, Graham AL, Bock FR. A tailored intervention to support pharmacy-based counseling for smoking cessation. Nicotine Tob Res 2010;12:217–25.
Burford O, Jiwa M, Carter O, Parsons R, Hendrie D. Internet-based photoageing within Australian pharmacies to promote smoking cessation: randomised controlled trial. J Med Internet Res 2013;15:e64.
Bush J, Langley CA, Patel A, Harvey JE. Evaluation of the Heart of Birmingham Teaching Primary Care Trust (HoBtPCT) My Choice Weight Management Programme. Final Report. Birmingham: Aston University; 2011.
Correr CJ, Melchiors AC, Fernandez-Limos F, Pontarolo R. Effects of a pharmacotherapy follow-up in community pharmacies on type 2 diabetes patients in Brazil. Int J Clin Pharm 2011;33:273–280.
Costello MJ, Sproule B, Victor JC, Leatherdale ST, Zawertailo L, Selby P. Effectiveness of pharmacist counseling combined with nicotine replacement therapy: a pragmatic randomised trial with 6,987 smokers. Cancer Causes Control 2011;22:167–80.
Crealey GE, McElnay JC, Maguire TA, O’Neill C. Costs and effects associated with a community pharmacy-based smoking-cessation programme. Pharmacoeconomics 1998;14:323–33.
Dhital R, Norman I, Whittlesea C, Murrells T, McCambridge J. The effectiveness of brief alcohol interventions delivered by community pharmacists: randomised controlled trial. Addiction 2015;110:1586–94.
Fornos JA, Andres NF, Andres JC, Guerra MM, Egea B. A pharmacotherapy follow-up program in patients with type-2 diabetes in community pharmacies in Spain. Pharm World Sci 2006;28:65–72.
Fornos Perez JA, Guerra Garcia MM, Andres Rodriguez NF, Egea Ibernon B. Evaluacion de un programa de seguimiento farmacoterapeutico a diabeticos tipo 2. Aten Primaria 2004;34:48–54.
Hoving C, Mudde AN, Dijk F, de Vries H. Effectiveness of a smoking cessation intervention in Dutch pharmacies and general practices. Health Educ 2010;110:17–29.
Howard-Pitney B, Killen JD, Fortmann SP. Quitting chew: results from a randomised trial using nicotine patches. Experi Clin Psychopharmacol 1999;7:362–71.
Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343:1035.
Maguire TA, McElnay JC, Drummond A. A randomised controlled trial of a smoking cessation intervention based in community pharmacies. Addiction 2001;96:325–31.
Malone M, Alger-Mayer SA. Pharmacist intervention enhances adherence to orlistat therapy. Ann Pharmacother 2003;37:1598–602.
Mochizuki M, Hatsugaya M, Rokujoh E, Arita E, Hashiguchi M, Shimizu N, et al. [Randomised controlled study on the effectiveness of community pharmacists’ advice for smoking cessation by Nicorette – evaluation at 3 months after initiation.] Yakugaku Zasshi 2004;124:989–95.
Paulos CP, Akesson Nygren CE, Celedon C, Carcamo CA. Impact of a pharmaceutical care program in a community pharmacy on patients with dyslipidemia. Ann Pharmacother 2005;39:939–43.
Phimarn W, Pianchana P, Limpikanchakovit P, Suranart K, Supapanichsakul S, Narkgoen A, et al. Thai community pharmacist involvement in weight management in primary care to improve patient’s outcomes. Int J Clin Pharm 2013;35:1208–17.
Sinclair HK, Bond CM, Lennox AS, Silcock J, Winfield AJ, Donnan PT. Training pharmacists and pharmacy assistants in the stage-of-change model of smoking cessation: a randomised controlled trial in Scotland. Tob Control 1998;7:253–61.
Sinclair HK, Silcock J, Bond CM, Lennox AS, Winfield AJ. The cost-effectiveness of intensive pharmaceutical intervention in assisting people to stop smoking. Int J Pharm Prac 1999;7:107–12.
Sonderskov J, Olsen J, Sabroe S, Meillier L, Overvad OK. Nicotine patches in smoking cessation: a randomised trial among over-the-counter customers in Denmark. Am J Epidemiol 1997;145:309–18.
Sonderskov J, Olsen J, Meillier L, Overvad OK, Sabroe S. [Nicotine patches in smoking cessation: a randomized trial among over-the-counter customers in Denmark.] Ugeskr Laeger 1999;161:593–7.
Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. J Pharm Prac Res 2002;32:57–62.
Watson MC, Stewart D. Screening and Brief Interventions for Alcohol Misuse Delivered in the Community Pharmacy Setting: A Pilot Study. Aberdeen: Chief Scientist Office; 2011.
Zaragoza-Fernandez MP, Gastelurrutia MA, Cardero M, Martinez-Martinez F. Intensive 2-month intervention on diet and lifestyle in uncontrolled hypertensive patients in a community pharmacy. Lat Am J Pharm 2012;31:727–33.
Appendix 5 List of ongoing studies
Zillich AJ, Corelli RL, Zbikowski SM, Magnusson LB, Fenlon CM, Prokhorov AV, et al. A randomized trial evaluating 2 approaches for promoting pharmacy-based referrals to the tobacco quit line: methods and baseline findings. Res Soc Admin Pharm 2013;9:27–36.
Taskila T, Macaskill S, Coleman T, Etter JF, Patel M, Clarke S, et al. A randomised trial of nicotine assisted reduction to stop in pharmacies – the RedPharm study. BMC Public Health 2012;12:182.
Petroni V, Serracino-Inglott A, Zarb-Adami M, Azzopardi LM. Pharmaceutical services in lifestyle modifications: overweight and obesity. Int J Clin Pharm 2013;2:897.
Appendix 6 List of excluded studies
| Reference | Reason for exclusion |
|---|---|
| Bond C. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Prac 2007;24:189–200 | O |
| Botomino A, Bruppacher R, Krahenbuhl S, Hersberger KE. Change of body weight and lifestyle of persons at risk for diabetes after screening and counselling in pharmacies. Pharm World Sci 2008;30:222–6 | D (x-sect) |
| Castejon AM, Calderon JL, Perez A, Millar C, McLaughlin-Middlekauff J, Sangasubana N, et al. A community-based pilot study of a diabetes pharmacist intervention in Latinos: impact on weight and hemoglobin A1c. J Health Care Poor Underserved 2013;24(Suppl. 4):48–60 | S |
| Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. Ann Pharmacother 2009;43:194–201 | S |
| Dent LA, Scott JG, Lewis E. Pharmacist-managed tobacco cessation program in Veterans Health Administration community-based outpatient clinic. J Am Pharm Assoc 2004;44:700–14 | S |
| Doescher MP, Whinston MA, Goo A, Cummings D, Huntington J, Saver BG. Pilot study of enhanced tobacco-cessation services coverage for low-income smokers. Nicotine Tob Res 2002;4(Suppl.1):S19–24 | D |
| Doucette WR, Witry MJ, Farris KB, McDonough RP. Community pharmacist-provided extended diabetes care. Ann Pharmacother 2009;435:882–9 | O |
| Fer T, Bluml BM, Ellis WM, Schaller CW, Garrett DG. The Diabetes Ten City Challenge: interim clinical and humanistic outcomes of a multisite community pharmacy diabetes care program. J Am Pharm Assoc 2008;48:181–90 | D |
| Fuller JM, Wong KK, Krass I, Grunstein R, Saini B. Sleep disorders screening, sleep health awareness, and patient follow-up by community pharmacists in Australia. Patient Educ Couns 2011;83:325–35 | D |
| Hammad EA, Yasein N, Tahaineh L, Albsoul-Younes AM. A randomized controlled trial to assess pharmacist-physician collaborative practice in the management of metabolic syndrome in a university medical clinic in Jordan. J Manag Care Pharm 2011;17:295–303 | S |
| Jacobs M, Sherry PS, Taylor LM, Amato M, Tataronis GR, Cushing G. Pharmacist assisted medication program enhancing the regulation of diabetes (PAMPERED) study. J Am Pharm Assoc 2012;52:613–21 | S |
| Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm 2012;18:516–26 | S |
| John EJ, Vavra T, Farris K, Currie J, Doucette W, Button-Neumann B, et al. Workplace-based cardiovascular risk management by community pharmacists: impact on blood pressure, lipid levels, and weight. Pharmacotherapy 2006;26:1511–17 | S |
| Kellow N. Evaluation of a rural community pharmacy-based Waist Management Project: bringing the program to the people. Aus J Prim Health Interchange 2011;17:16–22 | D |
| Kennedy DT, Giles JT, Chang ZG, Small RE, Edwards JH. Results of a smoking cessation clinic in community pharmacy practice. J Am Pharm Assoc 2002;42:51–6 | D |
| Khan N, Anderson JR, Du J, Tinker D, Bachyrycz AM, Namdar R. Smoking cessation and its predictors: results from a community-based pharmacy tobacco cessation program in New Mexico. Ann Pharmacother 2012;46:1198–204 | D |
| Lalonde L, O’Connor AM, Duguay P, Brassard J, Drake E, Grover SA. Evaluation of a decision aid and a personal risk profile in community pharmacy for patients considering options to improve cardiovascular health: the OPTIONS pilot study. Int J Pharm Prac 2006;14:51–62 | O |
| Lenz TL, Monaghan MS. Implementing lifestyle medicine with medication therapy management services to improve patient-centered health care. J Am Pharm Assoc 2011;51:84–188 | D |
| Lloyd KB, Thrower MR, Walters NB, Krueger KP, Stamm PL, Evans RL. Implementation of a weight management pharmaceutical care service. Ann Pharmacother 2007;41:185–92 | D, S |
| McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav 2006;3:1650–60 | I, S |
| McNamara KP, O’Reilly SL, Dunbar JA, Bailey MJ, George J, Peterson GM, et al. A pilot study evaluating multiple risk factor interventions by community pharmacists to prevent cardiovascular disease: the PAART CVD pilot project. Ann Pharmacother 2012;46:183–91 | D |
| Moideen MM, Varghese R, Ramakrishnan P, Dhanapal CK. Patient education for overweight and obese patients on weight reduction in an urban community pharmacy and its outcome. Res J Pharm Biol Chem Sci 2011;2:392–405 | D |
| Morello CM, Zadvorny EB, Cording MA, Suemoto RT, Skog J, Harari A. Development and clinical outcomes of pharmacist-managed diabetes care clinics. Am J Health Syst Pharm 2006;63:1325–31 | S |
| Nieto L. A Comparison of Weight Loss Outcome Across Three Distinct Weight Loss Program Delivery Venues. PhD Thesis. Ann Arbor, MI: TUI University 2011 | I |
| Nishita C, Cardazone G, Uehara DL, Tom T. Empowered diabetes management: life coaching and pharmacist counseling for employed adults with diabetes. Health Educ Behav 2013;40:581–91 | I |
| O’Neal KS, Crosby KM. Patients’ perceptions of a pharmacist-managed weight management clinic in a community setting. Res Soc Admin Pharm 2013;9:129–36 | D (x-sect) |
| Oyetayo OO, James C, Martinez A, Roberson K, Talbert RL. The Hispanic Diabetes Management Program: impact of community pharmacists on clinical outcomes. J Am Pharm Assoc 2011;51:623–6 | D |
| Paterson N, Wiest H, Fiscus L. Our success with a single-visit smoking cessation intervention. J Fam Prac 2013;62:334–6 | S |
| Patwardhan PD, Chewning BA. Effectiveness of intervention to implement tobacco cessation counseling in community chain pharmacies. J Am Pharm Assoc 2012;52:507–14 | O |
| Prokhorov AV, Hudmon KS, Marani S, Foxhall L, Ford KH, Luca NS, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Arch Int Med 2010;170:1640–6 | D (x-sect) |
| Roth MT, Westman EC. Use of bupropion SR in a pharmacist-managed outpatient smoking-cessation program. Pharmacotherapy 2001;21:636–41 | S |
| Scott A, Tinelli M, Bond C. Costs of a community pharmacist-led medicines management service for patients with coronary heart disease in England: healthcare system and patient perspectives. Pharmacoeconomics 2007;25:397–411 | O |
| Scott DM, Boyd ST, Stephan M, Augustine SC, Reardon TP. Outcomes of pharmacist-managed diabetes care services in a community health center. Am J Health Syst Pharm 2006;63:2116–22 | S |
| Shakeshaft A, Doran C, Petrie D, Breen C, Havard A, Abudeen A, et al. The effectiveness of community action in reducing risky alcohol consumption and harm: a cluster randomised controlled trial. PLOS Med 2014;11:e1001617 | O |
| Smith MD, McGhan WF, Lauger G. Pharmacist counseling and outcomes of smoking cessation. Am Pharm 1995;35:20–9 | D |
| Thavorn K, Chaiyakunapruk N. A cost-effectiveness analysis of a community pharmacist-based smoking cessation programme in Thailand. Tob Control 2008;17:177–82 | O |
| Tobari H, Arimoto A, Shimojo N, Yuhara K, Noda H, Yamagishi K, et al. Physician–pharmacist cooperation program for blood pressure control in patients with hypertension: a randomized-controlled trial. Am J Hypertens 2010;23:1144–52 | S |
| Tran MT, Holdford DA, Kennedy DT, Small RE. Modeling the cost-effectiveness of a smoking-cessation program in a community pharmacy practice. Pharmacotherapy 2012;22:1623–31 | D |
| Wollner S, Blackburn D, Spellman K, Khaodhiar L, Blackburn GL. Weight loss programs in convenient care clinics: a prospective cohort study. Am J Health Promot 2010;25:26–9 | D |
| Zillich AJ, Ryan M, Adams A, Yeager B, Farris K. Effectiveness of a pharmacist-based smoking-cessation program and its impact on quality of life. Pharmacotherapy 2002;22:759–65 | D |
Appendix 7 List of uncontrolled before-and-after studies
Anderson C, Mair A. Pro-change adult smokers program: Northumberland pilot. Int J Pharm Prac 2002;10:281–7.
Barbero Gonzalez JA, Quintas Rodríguez AM, Camacho JE. [Smoking cessation program from community pharmacy.] Aten Primaria 2000;26:693–6.
Boardman H, Avery A. Effectiveness of a community pharmacy weight management programme and its impact on blood pressure and waist circumference. Int J Pharm Prac 2012;20:56.
Ceric J. [Pharmacy project: education of overweight persons.] Farmaceutski Glasnik 2009;65:543–44.
Chang F, Gupta N, Smith L, Stringer D. Pilot community pharmacy-based diabetes program using health coaching principles. Can Pharm J 2012;145:S29.
Counterweight Project Team. The implementation of the Counterweight Programme in Scotland, UK. Fam Prac 2012;29(Suppl. 1):i139–44.
Didonato K, May J. The impact of wellness screening and monitoring services provided in a community pharmacy. J Am Pharm Assoc 2011;51:281.
Doescher MA, Whinston MA, Goo A, Cummings D, Huntington J, Saver BG. Pilot study of enhanced tobacco-cessation services coverage for low-income smokers. Nicotine Tob Res 2002;4(Suppl. 1):S19–24.
D’Silva J, Schillo BA, Sandman NR, Leonard TL, Boyle RG. Evaluation of a tailored approach for tobacco dependence treatment for American Indians. Am J Health Promot 2011;25(Suppl. 5):S66–9.
Fera T, Bluml BM, Ellis WM, Schaller CW, Garrett DG. The Diabetes Ten City Challenge: interim clinical and humanistic outcomes of a multisite community pharmacy diabetes care program. J Am Pharm Assoc 2008;48:181–90.
Fitzgerald N, McCaig DJ, Watson H, Thomson D, Stewart DC. Development, implementation and evaluation of a pilot project to deliver interventions on alcohol issues in community pharmacies. Int J Pharm Pract 2008;16:17–22.
Fuller JM, Wong KK, Krass I, Grunstein R, Saini B. Sleep disorders screening, sleep health awareness, and patient follow-up by community pharmacists in Australia. Patient Educ Couns 2011;83:325–35.
Gschwend P, Steffen T, Hersberger K, Ackermann-Liebrich U. [Smoking cessation in pharmacies – evaluation of the smoking cessation campaign ‘Tobacco Adieu!’ among pharmacists in Basle.] Sozial- und Praventivmedizin 1999;44:14–21.
Hertz S, Wichman K. Clinical tobacco intervention: a pilot project to assess the feasibility of pharmacists providing smoking cessation intervention. Can Pharm J 2002;135:24–9.
Hodges L, Gilbert H, Sutton S. Using computer-tailored smoking-cessation advice in community pharmacy: a feasibility study. Int J Pharm Prac 2009;17:365–8.
Hussein S, Mukherjee K, Bowden B. Evaluating the effectiveness of a pharmacist-provided smoking cessation program at a multisite, multispecialty medical group. J Am Pharm Assoc 2011;51:284.
Isacson D, Bingefors C, Ribohn M. Quit smoking at the pharmacy – an evaluation of a smoking cessation programme in Sweden. J Soc Admin Pharm 1998;15:164–73.
Jackson M, Gaspic-Piskoric M, Cimino S. Description of a Canadian employer-sponsored smoking cessation program utilizing community pharmacy-based cognitive services. Can Pharm J 2008;141:234–40.
Jansen I, Keizers S. [Evaluation of a smoking cessation intervention in a community pharmacy.] Pharmaceut Week Wetenschappel Plat 2014;149:26–31.
Kellow N. Evaluation of a rural community pharmacy-based waist management project: bringing the program to the people. Aus J Prim Health Interchange 2011;17:16–22.
Kennedy DT, Giles JT, Chang ZG, Small RE, Edwards JH. Results of a smoking cessation clinic in community pharmacy practice. J Am Pharm Assoc 2002;42:51–6.
Kennedy DT, Small RE. Development and implementation of a smoking cessation clinic in community pharmacy practice. J Am Pharm Assoc 2002;42:83–92.
Khan N, Anderson JR, Du J, Tinker D, Bachyrycz AM, Namdar R. [Smoking cessation and its predictors: results from a community-based pharmacy tobacco cessation program in New Mexico.] Ann Pharmacother 2012;46:1198–204.
Khan NS, Norman IJ, Dhital R, McCrone P, Milligan P, Whittlesea CM. Alcohol brief intervention in community pharmacies: a feasibility study of outcomes and customer experiences. Int J Clin Pharm 2013;35:1178–87.
Kirby J, Frede S, Berry E, Heaton PC. The role of community pharmacy disease management programs in a value-based insurance design: results from Kroger pharmacy coaching programs. J Manag Care Pharm 2012;18:559.
Kjaer NT, Evald T, Rasmussen M, Juhl HH, Mosbech H, Olsen KR. The effectiveness of nationally implemented smoking interventions in Denmark. Prevent Med 2007;45:12–14.
Lenz TL, Monaghan MS. Implementing lifestyle medicine with medication therapy management services to improve patient-centered health care. J Am Pharm Assoc 2011;51:184–8.
Lloyd KB, Thrower MR, Walters NB, Krueger KP, Stamm PL, Evans RL. Implementation of a weight management pharmaceutical care service. Ann Pharmacother 2007;41:185–92.
Madison N, Ray L, Roberts D, Snyder M. The impact of a grocery store pharmacy-based fitness, nutrition, and weight loss program on patient-centred outcomes. J Am Pharm Assoc 2012;52:255.
McNamara KP, O’Reilly SL, Dunbar JA, Bailey MJ, George J, Peterson GM, et al. A pilot study evaluating multiple risk factor interventions by community pharmacists to prevent cardiovascular disease: the PAART CVD pilot project. Ann Pharmacother 2012;46:183–91.
Mistry AD, Lee KW, Machado MR. Developing a weight management program in a community setting. J Pharm Technol 2011;27:229–38.
Moideen MM, Varghese R, Ramakrishnan P, Dhanapal CK. Patient education for overweight and obese patients on weight reduction in an urban community pharmacy and its outcome. Res J Pharma Biol Chem Sci 2011;2:392–405.
Morrison D, McLoone P, Brosnahan N, McCombie L, Smith A, Gordon J. A community pharmacy weight management programme: an evaluation of effectiveness. BMC Public Health 2013;13:282.
Muller-Ehmsen J, Braun D, Schneider T, Pfister R, Worm N, Wielckens K, et al. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J 2008;29:1560–8.
Munarini E, Marabelli C, Marmotti A, Gardiner A, Invernizzi G, Mazza R, et al. Antismoking centers in Milan’s communal pharmacies: analysis of the 2010–2011 campaign. Tumori 2013;99:578–82.
Novak MC, Mrhar A. [Community pharmacists’ contribution to overweight management.] Farmacevtski Vestnik 2010;61:117–21.
Oyetayo OO, James C, Martinez A, Roberson K, Talbert RL. The Hispanic diabetes management program: impact of community pharmacists on clinical outcomes. J Am Pharm Assoc 2011;51:623–6.
Ozkan M, Ozcelikay G. [The role of community pharmacists on the lifestyle of diabetic patients.] Turk J Pharma Sci 2012;9:231–40.
Paluck EC, McCormack JP, Ensom MH, Levine M, Soon JA, Fielding DW. Outcomes of bupropion therapy for smoking cessation during routine clinical use. Ann Pharmacother 2006;40:185–90.
Poder N, Perusco A, Hua M. Subsidised nicotine replacement therapy in a community pharmacy setting. Health Promot J Aus 2005;16:151–4.
Sanchez-Benito JL, Pontes Torrado Y, Gonzales Rodriguez A. [Weight loss intervention has achieved a significant decrease of blood pressure and cholesterol.] Clin Invest Arterioscler 2012;24:241–9.
Sansgiry SS, Agrawal RN, Patel H, Mhatre SK, Hayes JD, Robertson K, et al. Community pharmacists’ impact on an employer-initiated diabetes disease management program. J Pharm Technol 2012;28:68–74.
Schwartz SM, Bansal VP, Hale C, Rossi M, Engle JP. Compliance, behavior change, and weight loss with orlistat in an over-the-counter setting. Obesity 2008;16:623–9.
Smith MD, McGhan WF, Lauger G. Pharmacist counseling and outcomes of smoking cessation. Am Pharm 1995;35:20–9.
Spittles J, Valdez G, Ashley J, Cervantes J, Montemayor D, Pope N. Effect of a community pharmacist-led walking club-based intervention on weight management, obesity, and various health conditions. J Am Pharm Assoc 2012;52:254.
Taylor J, Semchuk W, Neubauer S, Kuz G. Patient satisfaction with a smoking cessation program in community pharmacies. Can Pharm J 2003;136:30–4.
Thomas D, Leckbee G, Pope N, Montemayor D, Ashley J, Cervantes J. Assessment of community pharmacist intervention to improve outcomes in smoking cessation using a behavioral and pharmacotherapy approach. J Am Pharm Assoc 2012;52:247.
Tominz R, Vegliach A, Poropat C, Zamboni V, Bovenzi M. [Smoking cessation with the help of community pharmacists.] Epidemiol Prevent 2010;34:73–9.
Toubro S, Dahlager L, Hermansen L, Herborg H, Astrup AV. [Dietary guidelines on obesity at Danish pharmacies. Results of a 12-week course with a 1-year follow-up. Research Group on Human Nutrition, Frederiksberg.] Ugeskr Laeger 1999;161:5308–13.
Tran MT, Holdford DA, Kennedy DT, Small RE. Modeling the cost-effectiveness of a smoking-cessation program in a community pharmacy practice. Pharmacotherapy 2002;22:1623–31.
Villalon M, Cutillas L, Martinez-Martinez F, Lopez-Garcia De La Serrana H, Oliveras-Lopez MJ, Samaniego-Sanchez C. [Community pharmacy: a tool to determine the Mediterranean diet level of adherence of the population.] Ars Pharmaceutica 2012;53:19–25.
Wick M, Ackermann-Liebrich U, Bugnon O, Cerise C. [Evaluation of the Swiss Society of Pharmacists’ campaign ‘Future Non-Smoker’.] Sozial- und Praventivmedizin 2000;45:73–84.
Wollner S, Blackburn D, Spellman K, Khaodhiar L, Blackburn GL. Applied research brief: weight control: weight loss programs in convenient care clinics: a prospective cohort study. Am J Health Promot 2010;25:26–9.
Zillich AJ, Ryan M, Adams A, Yeager B, Farris K. Effectiveness of a pharmacist-based smoking-cessation program and its impact on quality of life. Pharmacotherapy 2002;22:759–65.
Appendix 8 Behaviour change
| Study | Behaviour change strategy used, and theoretical basis, of intervention delivered in a community pharmacy setting, where reported. Plus same information reported for the comparison group(s) (active and/or non-active control) | Behaviour Change Wheel | Nuffield Intervention Ladder code | |
|---|---|---|---|---|
| Intervention function | Policy category | |||
| Alcohol | ||||
| Dhital et al., 201546 | Brief alcohol intervention that was not motivational interviewing, but rather followed a structured protocol influenced by the motivational interviewing approach delivered in a 10-minute discussion; included reflection and encouraged self-directed behaviour change; feedback of the AUDIT score was also given Comparison group: a leaflet-only control |
Education, enablement | Service provision | Enable choice |
| Watson and Stewart, 201148 | Brief alcohol intervention based on motivational interviewing Comparison group: a general lifestyle leaflet control |
Enablement, education | Service provision | Enable choice |
| Smoking cessation | ||||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 | 12 weeks of medium-intensity behavioural counselling Comparison group: Maudsley hospital model of 7 weeks of intense group-based behavioural support (not delivered in a pharmacy setting, and delivered by an ‘advisor’) |
Education, enablement | Service provision | Enable choice |
| Bock et al., 201052 | All counselling approaches are aligned with the 5 A’s framework (ask, assess, advise, assist, arrange follow-up) which includes counselling on motivational issues. Pharmacists deliver counselling, supported by EQ. EQ is a software system that provides individually tailored feedback to patients who smoke cigarettes, and matches reports for the pharmacist to help guide cessation counselling. Contents of the tailored feedback address the domains of motivation, decisional making (pros and cons of quitting smoking) and perceived barriers to quitting, smoking triggers/cues, nicotine dependence and effective smoking cessation medications. The tailored feedback also addresses the relationship between quitting smoking and the experience of potential negative affect and/or depressive symptoms Comparison group: two intervention groups included the same counselling approaches (EQ); difference was ± free nicotine patches. The observation-only control group included no counselling |
For either intervention groups vs. control: education, enablement | Service provision | For EQ vs. control: enable choice For EQ + patches vs. EQ or control: guide choice – incentives (nicotine patches were provided free as part of the intervention) |
| Burford et al., 201353 | The intervention group participants were digitally photoaged by using the internet-based APRIL face ageing software so they could preview images of themselves as a lifelong smoker and as a non-smoker Comparison group: both the intervention and control groups received ‘standardised smoking cessation advice’ from the pharmacist |
Education, enablement, persuasion | Service provision | Enable choice |
| Costello et al., 201155 | Comparison of two interventions that used the same behavioural counselling strategy. The pharmacists used the ‘5-A’ model for brief behavioural counselling (see Bock et al. 201052) Comparison group: one intervention used one session and the other used three sessions of the behavioural counselling |
Both intervention groups: education, enablement, incentivisation | Service provision | Both intervention groups: guide choice – incentives (NRT was free as part of the intervention) |
| Crealey et al., 199856 | PAS is a structured intervention package based on the stages-of-change model and using motivational interviewing. It is designed to assist smokers to stop and to motivate and support them to stay stopped, delivered in a one-to-one counselling format with structured follow-up (a pilot study for the Maguire study listed below) Theoretical model: transtheoretical model (stages of change) Comparison group: matched controls who did not receive PAS |
Education, enablement | Service provision | Enable choice |
| Hoving et al., 201057 | Computer-generated advice in a 5- to 7-page coherent letter individually tailored, based on responses to a baseline questionnaire. Messages were selected through a theory-based algorithm to address aspects relevant to the individual participant (e.g. perceived advantages and disadvantages of smoking cessation and anticipated difficult situations to refrain from smoking) Theoretical model: I-change model incorporating several cognitive models such as the transtheoretical model and theory of reasoned action Comparison groups: a thank-you letter from a pharmacist; computer-generated advice in a letter from a GP that was individually tailored; a thank-you letter from a GP |
Education, enablement | Service provision, communication/marketing | Enable choice |
| Howard-Pitney et al., 199944 | Behavioural treatment comprising two visits to the pharmacy, support calls and self-help materials, including a 23-page, self-help quitting manual tailored for chewing tobacco users. The major sections in the manual took the chewer through typical stages in the quitting process: getting ready, quit date, dealing with urges, and recovery or staying off chew. [Note that it was unclear whether or not this intervention was based on the transtheoretical (stages-of-change) model] Comparison group: same behavioural treatment as intervention, but they received a placebo patch rather than a nicotine patch |
Both intervention groups: education, enablement, incentivisation | Service provision, communication/marketing | Both intervention groups (GP and pharmacy): guide choice – incentives (NRT was free as part of the intervention) |
| Maguire et al., 200158 | PAS is a structured intervention package based on the stages-of-change model and using motivational interviewing. It is designed to assist smokers to stop and to motivate and support them to stay stopped, delivered in a one-to-one counselling format with structured follow Theoretical model: transtheoretical (stages-of-change) model Comparison group: matched controls who did not receive PAS |
Education, enablement, | Communication/marketing, service provision | Enable choice |
| Mochizuki et al., 200459 | Structured support from the pharmacist (five times over 3 months) Comparison group: ad-hoc advice when asked for it by participant |
Education, enablement | Service provision | Enable choice |
| Sinclair et al., 199860 | Behavioural counselling in smoking cessation based on the stages-of-change model. The intervention group also received nicotine patches Theoretical model: transtheoretical (stages-of-change) model Comparison groups: same as intervention but, after an initial consultation with a community pharmacist they are followed up by a research pharmacist in a hospital outpatient clinic; advice from pharmacists who have not undergone training in behavioural counselling |
Education, enablement | Service provision | Enable choice |
| Sonderskov et al., 199763 | The intervention did not include any behavioural support. The intervention was nicotine patches Comparison group: same as intervention but placebo patches |
Enablement | Service provision | Guide choice – incentives (nicotine patches provided at half the retail cost) |
| Vial et al., 200245 | Behavioural counselling in smoking cessation based on the stages-of-change model Theoretical model: transtheoretical (stages-of-change) model Comparison groups: minimal intervention group who were provided with written material and advice only |
Education, enablement | Service provision | Guide choice – incentives (nicotine patches were provided at about half the retail cost) |
| Weight loss | ||||
| Ahrens et al., 200364 | Meal replacements Comparison group: normal low-calorie diet |
Education, enablement, restriction | Service provision, communication/marketing | Restrict choice |
| Bush et al., 201165 | My Choice Weight Management Programme delivered ‘through’ pharmacies. Based on the model used for the Counterweight Project, with weekly consultations. Compared with the Counterweight Project, there was more focus on goal-setting and the targets and less focus on portion control Comparison group: same as intervention but delivered ‘through’ GP surgeries |
Education, enablement | Service provision, communication/marketing | Enable choice |
| Jolly et al., 201166 | There were eight arms (six interventions) in this (Lighten Up) trial. Pharmacy (pharmacist led, one to one) was classed as the intervention group for this systematic review. The theoretical basis of the intervention was the stages-of-change model with use of motivational interviewing. Predominant behaviour change strategies included goal-setting, self-monitoring with food diaries, hunger scale, waist measurements and physical activity. Participants were encouraged to reward themselves for success Comparison groups
GP practice (nurse led, one to one): same as intervention but different setting Control (12 free vouchers for local leisure centre) Or a choice of one of the above |
Control: enablement Dietetic, GP and pharmacy: education, enablement WW, SW and RC: education, enablement, modelling, incentivisation |
Service provision | Dietetic, GP, pharmacy, control: enable choice WW, SW and RC: guide choice – incentives |
| Malone and Alger-Mayer, 200339 | Pharmacists delivering obesity management (following a training course) to patients prescribed orlistat Comparison group: orlistat plus usual care delivered by the pharmacist (who had not undertaken the training course) |
Education (but not enablement because no behavioural component to sessions) | Service provision | Provide information (rather than enable choice, because no behavioural component to sessions) |
| Phimarn et al., 201367 | Obesity counselling based on a obesity handbook comprising three parts: (1) an informational section which deals with healthy diet, principles of calorie intake, food groups, portion size and exercise; (2) a patient profile to record personal information and clinical outcomes; and (3) a daily food record for patients to record their daily meals Theoretical model: theory of planned behaviour Comparison group: a routine group-directed weight management service provided by staff in the GP practice |
Education, enablement | Service provision | Enable choice |
| Multicomponent interventions (pharmacotherapy and lifestyle changes) | ||||
| Diabetes mellitus – type 2 | ||||
| Ali et al., 201268 | Pharmacist-led patient education, lifestyle modification counselling and diabetes mellitus monitoring programme Comparison group: usual care |
Education, enablement | Service provision; communication/marketing | Enable choice |
| Correr et al., 201169 | ‘Pharmacotherapy follow-up’ in pharmacies. The key and differential component of pharmacotherapy follow-up compared with other cognitive services (e.g. medication review) is its focus on assessing the clinical outcomes resulting from the process of use of medicines, rather than evaluating this process of use, and thus ultimately identifying certain medication negative clinical outcomes Comparison group: usual care |
Education, enablement | Service provision | Enable choice |
| Fornos et al., 200670 | ‘Pharmacotherapy follow-up’ in pharmacies. This was a similar type of intervention as Correr et al.69 but also specifically included health education by the pharmacist with a view to achieving a healthier lifestyle Comparison group: usual care |
Education, enablement | Service provision | Enable choice |
| Dyslipidaemia | ||||
| Paulos et al., 200571 | A pharmaceutical care programme that provides education in the areas of medication compliance and lifestyle modifications Comparison group: usual care |
Education, enablement | Service provision | Enable choice |
| Hypertension | ||||
| Zaragoza-Fernandez et al., 201272 | ‘Individualised health education’ Comparison group: usual care |
Education (but not enablement as no behavioural component to sessions) | Service provision | Provide information (rather than enable choice, as no behavioural component to sessions) |
Appendix 9 Implementation
| Study | Implementation contexta | Consultation/collaboration processes during planningb | Consultation/collaboration processes during deliveryc | Sustainabilityd,e |
|---|---|---|---|---|
| Alcohol | ||||
| Dhital et al., 201546 | Political | Pharmacists were consulted in the planning of the trial regarding an acceptable and feasible training period | No information of relevance was reported | No information of relevance was reported |
| Watson and Stewart, 201148 | Political | Focus groups were convened before (and after) the study to (1) explore pharmacists’ perceptions of barriers and facilitators to delivering the intervention and (2) explore with members of the public their opinions/beliefs about the intervention in community pharmacy setting | No information of relevance was reported | No information of relevance was reported |
| Smoking cessation | ||||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 | Political, economic | The study authors acknowledge the assistance provided by NHS Greater Glasgow and Clyde staff and the study steering group, but it is not clear where (or how) they were involved during planning | The study authors acknowledge the assistance provided by NHS Greater Glasgow and Clyde staff and the study steering group, but it is not clear where (or how) they were involved during delivery | The authors do not discuss the sustainability of the pharmacy-led intervention, but they do conclude that it is appropriate that different cost-effective service configurations, such as pharmacy services, are available and can coexist to offer smokers choice and maximise accessibility |
| Bock et al., 201052 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Burford et al., 201353 | Economic | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Costello et al., 201155 | Political | This study was nested within a larger host study, that is the STOP study. During planning, the STOP programme collaborated with different community and regional partners in many different ways including:
|
During intervention delivery, the STOP programme collaborated with different community and regional partners in many different ways including:
|
Authors highlight that reimbursement is needed to the pharmacist for providing the service in order for it to be sustainable. (There was no financial reimbursement for the pharmacists’ professional services in this study) They also state that a secondary aim of the study was sustainability through training of pharmacists to provide counselling The authors state that they look forward to maintaining their existing partnerships as well as building new community connections into the future |
| Crealey et al., 199856 | Economic | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Hoving et al., 201057 | No specific implementation context (study focus was simply on testing the intervention) | The intervention was developed by the University of Maastricht in collaboration with the Dutch Foundation on Smoking and Health (STIVORO for a smoke-free future) | No information of relevance was reported | No information of relevance was reported |
| Howard-Pitney et al., 199944 | No specific implementation context (study focus was simply on testing the intervention) | The study authors acknowledge the assistance provided by the Shasta County Department of Public Health and Tehama County Health Agency, but it is not clear where (or how) they were involved during planning | The study authors acknowledge the assistance provided by the Shasta County Department of Public Health and Tehama County Health Agency, but it is not clear where (or how) they were involved during delivery | No information of relevance was reported |
| Maguire et al. 200158 | Political | No information of relevance was reported | No information of relevance was reported | Barriers emerging from the qualitative evaluation of this intervention included insufficient remuneration for pharmacists, which would impact on sustainability |
| Mochizuki et al., 200459 | No specific implementation context mentioned in the abstract (English abstract only) | No information of relevance was reported in the abstract | No information of relevance was reported in the abstract | No information of relevance was reported in the abstract |
| Sinclair et al. 199860 | Political, economic | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Sonderskov et al., 199763 | Political | The pharmaceutical company (Ciba-Geigy) provided instructions concerning trial procedure during planning | The pharmaceutical company (Ciba-Geigy) were in contact with pharmacies once a week during delivery | No information of relevance was reported |
| Vial et al., 200245 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Weight loss | ||||
| Ahrens et al., 200364 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Bush et al., 201165 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Jolly et al., 201166 | Political | No information of relevance for the pharmacy-led intervention was reported | No information of relevance for the pharmacy-led intervention was reported | No information of relevance for the pharmacy-led intervention was reported |
| Malone and Alger-Mayer, 200339 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Phimarn et al., 201367 | Political | The intervention (in Thailand) required a formal agreement between a pharmacy and a primary care unit | No information of relevance was reported | No information of relevance was reported |
| Multicomponent interventions (pharmacotherapy and lifestyle changes) | ||||
| Diabetes mellitus – type 2 | ||||
| Ali et al., 201268 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Correr et al., 201169 | Economic | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Fornos et al., 200670 | No specific implementation context | No information of relevance was reported | No information of relevance was reported | The authors suggested that a closer co-operation between GPs and pharmacists, than was in place for this study, for successful implementation of the intervention |
| Dyslipidaemia | ||||
| Paulos et al., 200571 | No specific implementation context | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
| Hypertension | ||||
| Zaragoza-Fernandez et al., 201272 | Political | No information of relevance was reported | No information of relevance was reported | No information of relevance was reported |
Appendix 10 Organisation and delivery of interventions
| Author | Type and location of pharmacy | Staff training and quality assurance | Experience of intervention team | Resources and other intervention-related costsa |
|---|---|---|---|---|
| Alcohol | ||||
| Dhital et al., 201546 | Community Pharmacies in the London Borough of Hammersmith and Fulham, London, UK | All trial pharmacists had been trained over 3.5 hours (by lead author) to deliver the intervention protocol, including flexible use of the discussion topics in ways influenced by the counselling approach of motivational interviewing. In such a brief training workshop it was not feasible to aim to train the pharmacists in motivational interviewing as this approach requires ongoing supervision of practice Quality assurance: a 2-hour evening follow-up training session was arranged 7 weeks after the start of the trial to address challenges and share learning across the group and was attended by 10 pharmacists |
Pharmacists and pharmacy support staff | Not reported |
| Watson and Stewart, 201148 | Community Pharmacies in Grampian, Scotland, UK | Two training sessions were delivered. One evening training session was delivered to describe the purpose of the study, the use of FAST and the study documentation. One pharmacist and up to one member of staff from each pharmacy were invited to attend. Pharmacies not represented at this event received a training visit from a research team member. A 1-day ABI training session was also delivered to pharmacists in the intervention group, attendance at which was compulsory for participation in the study. This training was provided by Create Consultancy and the research team Quality assurance: none reported |
Pharmacists and pharmacy support staff | Estimated cost for delivering one ABI was £70.90, based on an average of 10 people screened for each ABI delivered: £10.20 training costs, £50.00 staff time for screening, £10 staff time for delivering ABI, £0.70 for consumables |
| Smoking cessation | ||||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 | Community pharmacies (90% in Glasgow Health Board area), Glasgow, UK | Training of pharmacists varied from attending a Glasgow Health Board or online course, to observing sessions in the pharmacy Quality assurance: none reported |
Pharmacists and pharmacy support staff | Details are presented in the paper (in appendix). Cost-effectiveness (52-week quitter) of intervention per client: one-to-one pharmacy led intervention £79.00; group-based support (control) £368.00 |
| Bock et al., 201052 | Pharmacies located within large urban community health centres in the USA | A 3-hour training session for the pharmacists was conducted. Pharmacists were trained using the Rx for Change tobacco cessation programme (http://rxforchange.ucsf.edu (accessed 14 January 2015); Corelli et al.85) which focuses on fostering self-efficacy for counselling and includes role-playing and a hands-on workshop with the various US Food and Drug Administration-approved medications for smoking cessation. All counselling approaches were aligned with the 5 A’s framework (ask, assess, advise, assist, arrange follow-up) as described in the Clinical Practice Guideline (Fiore et al.86). The pharmacists were trained to assess readiness to quit, to focus their counselling on motivational issues for those not ready to quit, and, for those ready to quit, to offer practical advice regarding quitting, discuss the importance of obtaining social support, and evaluate the appropriateness of quit smoking medications and make recommendations (the primary difference between EQ and EQ+ conditions being the availability of free NRT). Additionally, the training addressed (1) study aims and the research protocol; (2) a demonstration of the EQ programme and examination of tailored intervention reports for the patient and pharmacist; and (3) role-playing with case scenarios that integrated output from the EQ system Quality assurance: none reported |
Pharmacists | No information provided except for incentivisation. All participants were compensated US$20 for their time and effort for completing the baseline survey and for returning the follow-up survey |
| Burford et al., 201353 | Community pharmacies located around Perth city centre in Western Australia | No training details provided Unclear whether it was the community pharmacists (who would have needed some training in the use of the face ageing software) or a single research pharmacist Quality assurance: none reported |
Unclear whether it was the community pharmacists or a single research pharmacist | The face ageing software (APRIL) was provided by the software company. Total costs of implementing the intervention from a health sector perspective were AU$463, or the equivalent of AU$5.79 per participant |
| Costello et al., 201155 | Pharmacies in Ontario, Canada | Pharmacists were trained in the intervention methodology during a 5-hour face-to-face session or a 3-hour pre-recorded online session plus 1-hour teleconference conducted by the STOP study staff. Training covered (1) the study protocol and documentation; (2) the ‘5-A’ model for brief behavioural counselling; and (3) an overview of NRT products and their use Quality assurance: none reported |
Community pharmacists | Resources listed (but not costed) included free NRT, training and pharmacists’ time |
| Crealey et al., 199856 | Pharmacies in Belfast, Northern Ireland, UK | Each study site pharmacist was sent a copy of the PAS model documentation, together with a written literature review on smoking cessation and asked to study the material. Between 2 and 3 weeks after receipt of the documentation, pharmacists attended a local workshop on smoking cessation (including detailed instruction on the study methodology). These workshops each lasted 3 hours and covered epidemiology, smoking statistics, the use of NRT, the cycle of change model and the PAS model Quality assurance: following the training, a researcher visited the pharmacists to provide support and to address any queries they had in implementing the model. This constituted the training for the intervention |
Community pharmacists | Fixed costs of the intervention are detailed in table II. Variable costs included pharmacist time – an average time of 1 hour (over the 6-month follow-up period) at £30 per hour The cost per life-year saved ranged from £196.76 to £351.45 for men and £181.35 to £772.12 for women (1997 values) |
| Hoving et al., 201057 | Pharmacies in the Netherlands | Note that training for pharmacy staff not relevant for this intervention. Computer-tailored letter (intervention) or a thank-you letter (control) Quality assurance: not relevant |
Not relevant | Not reported. One would assume the costs of setting up the system would be significant but, once set up, costs would be minimal (the cost of sending a letter) |
| Howard-Pitney et al., 199944 | Pharmacies in the USA | Pharmacists were trained initially during a 4-hour training session with investigators and field staff. Training included educating the pharmacists about chewing tobacco prevalence in their counties, study protocol, nicotine withdrawal symptom and role of nicotine patches in reducing physical withdrawal. In addition, field staff demonstrated the pharmacists’ role in the intervention protocol, and each pharmacist practised the intervention in a role-playing exercise Quality assurance: at the end of the training session, field staff, playing the role of a study participant, tested the pharmacists’ knowledge and ability to perform their intervention role. Pharmacists had to perform 80% of the steps adequately in each visit’s protocol before being certified to intervene with participants |
Pharmacists | Not reported, but NRT was offered free of charge |
| Maguire et al., 200158 | Pharmacies in London, England, Uk and Northern Ireland, UK | Each study site pharmacist was sent a copy of the PAS model documentation, together with a written literature review on smoking cessation and asked to study the material. Between 2 and 3 weeks after receipt of the documentation, pharmacists attended a local workshop on smoking cessation (including detailed instruction on the study methodology). These workshops each lasted 3 hours and covered epidemiology, smoking statistics, the use of NRT, the cycle of change model and the PAS model Quality assurance: following the training, a researcher visited the pharmacists to provide support and to address any queries they had in implementing the model. This constituted the training for the intervention |
Community pharmacists | Not reported but authors refer to their earlier paper (Crealey et al., 199852) which does report on costs and cost-effectiveness |
| Mochizuki et al., 200459 | Pharmacies in Japan | Note that this paper is written in Japanese and we only have the abstract in English. There is no mention in the abstract of pharmacists receiving training | Assume pharmacists | Not reported |
| Sinclair et al., 199860 | Non-city community pharmacies in Grampian, Scotland, UK | Delivery of a 2-hour training session to pharmacists and pharmacy assistants. The training did not include motivational interviewing techniques to encourage smokers to move from pre-contemplation to contemplation; however, it did include specific content and recommendations pertaining to preparation, action, maintenance and relapse. The training aimed to give participants an understanding of the stages in the stages-of-change model, and focused on brief questioning which could enable counsellors to assess the stage of individual customers and to subsequently increase the frequency and effectiveness of the counselling support by tailoring their advice to the current stage of the customer Quality assurance: not reported |
Pharmacists and pharmacy suport staff | The cost of producing one additional successful attempt to quit smoking by using intensive rather than standard pharmaceutical support was £300 (in 1995–7). Costs included training costs, NRT and counselling costs |
| Sonderskov et al., 199763 | Community pharmacies in the areas of Aarhus and Copenhagen, Denmark | No training of pharmacists as such, but they were given ‘instructions’ from the pharmaceutical company concerning trial procedures Quality assurance: pharmaceutical company contacted pharmacies at least once per week throughout the study period |
Pharmacists and pharmacy support staff | Not reported, but nicotine patches were provided free of charge |
| Vial et al., 200245 | Community pharmacies in Adelaide, Australia | Before the study commenced, participating community pharmacies were informed of all study-related procedures at a seminar. Brief information about stages of behaviour change and recommended interventions during smoking cessation were also included in the seminar Quality assurance: not reported |
MSc student in health science | Not reported, but nicotine patches were supplied at a reduced price |
| Weight loss | ||||
| Ahrens et al., 200364 | A community pharmacy: Travis Pharmacy in Shenandoah, Iowa, USA | The two pharmacists who participated in the study received no special training, although both used current literature and research to prepare themselves to be able to counsel patients in dietary advice. A registered dietitian reviewed the dietary plan developed by the pharmacist before it was used with the patients, and was consulted as needed during the study Quality assurance: not reported |
Community pharmacists | Not reported, but meal replacements were provided free of charge |
| Bush et al., 201165 | Community pharmacies in Birmingham, England, UK | Pharmacists did not received training, but intervention deliverers (in pharmacies and GP practices) were ‘trained health-care workers’, for example a pharmacy assistant working in a pharmacy, and they did receive training All intervention deliverers attended a 2-day training session organised by the PCT which provided deliverers with training material and resources . . .p. 67 The training included input from dietitians, GP and pharmacy staff All deliverers attended a 2-day training session, which was regarded as being useful and provided deliverers with training material and resources Quality assurance: not reported |
A trained health-care worker (for example, a health-care assistant, practice nurse or pharmacy assistant) | Costs per participant were higher in a pharmacy setting than in a GP surgery setting initially, but by the end of the programme (9 months) the costs were about the same because of the larger number of participants recruited by GPs (thus allowing for distribution of, for example, training costs across a larger pool of participants) |
| Jolly et al., 201166 | Community pharmacies, England, UK | There are eight arms (six interventions) in this (Lighten Up) Trial:
1–3: the group leaders were trained by the respective organisations 4–6: staff delivering these programmes had attended a 3-day training course on weight management in adults delivered by dietitians experienced in the management of obesity. This included key messages on diet and physical activity, doing a behavioural assessment, goal-setting, plans for change, dealing with resistance, enhancing motivation and weight maintenance. It included both practical tasks and informational components Quality assurance: not reported |
Variable depending on intervention For the pharmacy-led intervention, pharmacists delivered the intervention |
Resources and other intervention costs varied between the different weight loss interventions. Interventions 4–6 (primary care) were more costly than 1–3 (commercial) Provider costs:
Cost per participant (in addition to provider costs) = £10 for call centre, £3.54 for practices to search their lists and GPs to screen lists, £8.33 for invitation letters sent by practices (£1 per letter with 12% response rate) |
| Malone and Alger-Mayer, 200339 | Community pharmacies, USA | Pharmacists delivering the intervention were trained (a 1-day course) in ‘obesity management skills’. Training included various aspects of obesity but no mention of any behavioural support/skill training Quality assurance: not reported |
Community pharmacist | Not reported |
| Phimarn et al., 201367 | Community pharmacy, Thailand | The two community pharmacists who routinely provide weight loss advice received minimal training. The pharmacists developed the weight loss handbook. Information included was the same as the group advice provided by the primary care unit staff. The handbook is comprised of three parts: (1) an informational section, which deals with healthy diet, principles of calorie intake, food groups, portion size and exercise; (2) a patient profile to record personal information and clinical outcomes; and (3) a daily food record for patients to record their daily meals. Prior to the study, the handbook was provided to the two pharmacists as a standard guide for their use in counselling. Both community pharmacists practised giving advice with simulated patients Quality assurance: not reported |
Community pharmacists | Not reported |
| Multicomponent intervention (pharmacotherapy and lifestyle changes) | ||||
| Diabetes mellitus – type 2 | ||||
| Ali et al., 201268 | Community pharmacies, Hertfordshire, UK | The pharmacists who delivered the intervention undertook an 8-hour training programme provided by the School of Pharmacy at the University of Hertfordshire, involving workshop sessions with a consultant diabetologist and a diabetes mellitus specialist nurse, providing an update on diabetes mellitus management and referrals, an overview of the use of diagnostic equipment and the data collection formsQuality assurance: not reported | Community pharmacists | Not reported |
| Correr et al., 201169 | Community pharmacies, the Curitiba metropolitan region of Brazil | Pharmacists providing care for patients with type 2 diabetes mellitus in the PF group underwent a specific training provided by faculty staff of Federal University of Parana and University of Granada (Spain), including basic concepts and procedures of PF as well as diabetes mellitus care and glucose and blood pressure measurement Quality assurance: not reported |
Community pharmacists | An economic evaluation of the intervention estimated the annual cost of the reduction in 1% in HbA1c values in the PF group. However, no such analysis was conducted for a reduction in body weight or BMI |
| Fornos et al., 200670 | Community pharmacies, Province of Pontevedra in Spain | All the pharmacists involved in the study received 18 hours of training in the PF programme and in the proper use of the measuring tools, and followed a protocol which helped them to monitor patients Quality assurance: pharmacists had regular contact with the research team and attended clinical sessions where results on drug-related problems were presented and discussed |
Community pharmacists | Not reported |
| Dyslipidaemia | ||||
| Paulos et al., 200571 | Community pharmacy, Santiago, Chile | The study was conducted by a pharmacist who was dedicated solely to this study and who was trained specifically for the purposes of this study – no further details Quality assurance: not reported |
Community pharmacist | Not reported |
| Hypertension | ||||
| Zaragoza-Fernandez et al., 201272 | Community pharmacies, Murcia, Spain | No mention of any training for the pharmacists Quality assurance: not reported |
Community pharmacist | Not reported |
Appendix 11 Outcomes
| Study | Outcomes | Summary |
|---|---|---|
| Alcohol | ||
| Dhital et al., 201546 Brief alcohol intervention vs. leaflet-only control |
AUDIT baseline score, mean (SD): I (n = 205), 11.93 (3.24); C (n = 202), 11.53 (3.19) 12-week AUDIT score, mean (SD): I (n = 168), 11.80 (5.88); C (n = 158), 10.77 (5.54) 12-week AUDIT change in score: I (n = 168), 0.11 (95% CI –0.82 to 0.61); C (n = 158) –0.74 (95% CI –1.47 to 0.00) 12-week AUDIT between-group difference unadjusted: –0.63 (95% CI –1.69 to 0.43) 12-week AUDIT between-group difference adjusted for pharmacist, sex, age, ethnicity and education: –0.57 (95% CI –1.59 to 0.45) 12-week OR for AUDIT < 8 (control as reference): 0.87 (95% CI 0.50 to 1.51) Other outcomes:
|
There was no evidence of effectiveness of community pharmacist delivery of brief alcohol intervention. The AUDIT total change score did not differ significantly between the two groups and did not change significantly between baseline and follow-up in either the intervention or control group |
| Watson and Stewart, 201148 Brief alcohol intervention vs. control |
FAST total median score baseline: I (n = 27), 5.00 (IQR 3.00–6.00); C (n = 42), 5.00 (IQR 4.00–6.00) 3-month FAST median score: I (n = 10), 3.00 (IQR 1.00–4.25); C (n = 23) 4.00 (IQR 2.00–6.00) A reduction in FAST score of 0.93 (95% CI –2.84 to 0.97) was shown in the intervention group at 3 months (p = 0.32) 6-month FAST median score: I (n = 6) 2.50 (IQR 1.50–4.25); C (n = 14), 3.50 (IQR 2.00–7.50) 3-month FAST mean score change: Male: I (n = 4), 0.50 (SD 1.00); C (n = 9), –0.11 (SD 3.18) Female: I (n = 6), 1.67 (SD 2.73); C (n = 12), 1.17 (SD 1.90) 6-month FAST mean score change: Male: I (n = 4), 2.25 (SD 3.20); C (n = 4), –1.25 (SD 2.87) Female: I (n = 2), 0.50 (0.71); C (n = 8), 0.75 (SD 1.67) 6-month FAST between-group difference: –1.84 (95% CI –4.49 to 0.82) Other outcomes:
|
No significant difference was shown between FAST scores for the intervention group compared with control, at 3 or at 6 months At 6 months there was substantially lower follow-up of intervention clients (22.2%) compared with control clients (33.3%). Only adjusted for baseline FAST; not clear if there were baseline differences for other variables |
| Smoking cessation | ||
| Bauld et al., 201149 Bauld et al., 200950 Boyd et al., 200951 I: pharmacy-based NHS smoking cessation service (12-weeks one-to-one support) moderate intensity + NRT C: group-based NHS smoking cessation service (community-based 7 weeks behavioural support) high intensity + NRT/medication |
Baseline number of cigarettes/day > 21: I, 40.1% (396/987); C, 41.6% (169/406) 4-week CO-validated quitters: I, 255/1374; C, 146/411 of 1785 that set a quit date I, 255/1508; C, 146/471 of 1979 who accessed service and agreed to data usage but did not set quit date 52-week CO-validated quitters: I, 38/1374; C, 26/411 of 1785 that set a quit date I, 38/1508; C, 26/471 of 1979 who accessed service and agreed to data usage but did not set quit date Univariate analyses: in each service more deprived smokers (those in socioeconomic groups 5 and 6) had lower cessation rates, although the trend relating socioeconomic score to cessation rate was significant only for the pharmacy service In a multivariate model, restricted to participants (n = 1366) with data allowing adjustment for sociodemographic and behavioural characteristics and including interaction terms, users who accessed the group-based services (C) were almost twice as likely (OR 1.980, 95% CI 1.50 to 2.62) as those who used pharmacy-based support (I) to have quit smoking at 4-week follow-upOther outcomes:
|
Much larger sample size for pharmacy than group-based service (n = 1374 vs. n = 411). Clients could choose service. Group participants were older and of higher SES Pharmacy-based not as effective for smoking cessation but many more smokers access the pharmacy-based service All pharmacy clients had NRT, 84% group clients had NRT/16% medication This is secondary data analysis of an observational study so direct comparison between the pharmacy-based and group-based service is inappropriate |
| Bock et al., 201052 I1: smoking cessation training for pharmacists and use of a computer-driven software system, EQ, which provided individually tailored interventions and matching reports to the pharmacist to guide cessation counselling plus 8 weeks free NRT I2: same as above without NRT C: observation only |
Baseline number of cigarettes/day, mean (SD): I1, 18.2 (9.1); I2, 17.7 (8.3); C, 13.8 (8.6) 7-day point prevalence abstinence at 2 months [verified with CO (< 10 p.p.m.)]: I1, 39% (39/100); I2, 27% (27/100); C, 9% (9/99) 7-day point prevalence abstinence at 6 months (verified by saliva cotinine): I1, 28% (28/100); I2, 15% (15/100); C, 8% (8/99) 6-month quit between-group difference: I1 vs. C: OR 3.3 (95% CI 1.9 to 5.2) I2 vs. C: OR 1.49 (95% CI 1.2 to 3.6) I1 vs. I2: OR 2.3 (95% CI 1.5 to 3.9) Other outcomes:
N: EQ+, n = 100; EQ, 100; C, n = 99 |
Control group not randomised, but EQ and EQ+ groups were. There were significant baseline differences and it is not clear if these were controlled for in analyses of quit rates. Low attrition A tailored intervention combined with brief proactive counselling from pharmacist plus pharmacist training (EQ) was successful in increasing quit rates, with further increases among patients who also received free nicotine patches (EQ+) |
| Burford et al., 201353 I: standardised smoking cessation advice + computer-generated photoageing (demonstrating the detrimental effects on facial physical appearance of smoking) C: standardised smoking cessation advice only |
Baseline number of cigarettes/day > 21: I, 10% (8/80); C, 15% (12/80) 6-month CO-validated quitters (95% CI): I, 11/80 (13.8%, 7.8% to 22.9%); C, 1/80 (1.3%, 0% to 6.7%) This difference between groups remained statistically significant after adjustment small differences between groups in sex and nicotine dependence Other outcomes:
|
Photoageing intervention was effective in stopping young people smoking compared with control |
| Costello et al., 201155 I1: 1-week then fortnightly pharmacy visit for NRT plus three sessions of ‘5-A’ model for brief behavioural counselling I2: received 5-weeks NRT at initial pharmacy visit plus one session of ‘5-A’ model for brief behavioural counselling at initial visit C: 5-weeks NRT mailed directly to participants home |
Baseline Heaviness of Smoking Index score 5–6 (high): I1, 40.7% (1459/3588); I2, 40.1% (1364/3399); C, 39.4% (1823/4630) Self-reported 7-day point prevalence at 5 weeks: I1, 612/3503 (17.5%); I2, 604/3350 (18.0%) Self-reported 7-day point prevalence at 5 weeks (controlling for covariates): OR = 0.96 (95% CI 0.86 to 1.08) (n = 6809) Other analyses: Study models various confounders by abstinence and intervention group and also controls for covariates when modelling abstinence by intervention group Age and education were significant confounders: 25–39 years and 55+ years were more likely to be abstinent than 18–24 years, those completing some college/university were more likely to be abstinent than those who did not complete high school. Sex was not significant in ‘ITT’ analyses (n = 6809) Completer Multivariate analysis suggest when controlling for possible confounders and clustering across pharmacies group I1, three-session completers were more likely to quit compared with group I2 (OR 1.72, 95% CI 1.53 to 1.94) Other outcomes:
|
Control group not randomised, but intervention groups were. Control group only used in paper for baseline. Only completer analysis showed significant difference between groups. When participants assessed as assigned and with non-responders classed as still smoking there is no significant difference between intervention groups |
| Crealey et al., 199856 I: PAS model of behavioural support, 35/52 nicotine gum C1: Nicotine gum only C2: Control (expressed wish to stop smoking) |
Baseline cigarettes/day: NR 3-month CO-verified abstinence (and stopped using nicotine gum): I, 56%; C1, 16% 6-month CO-verified abstinence (and stopped using nicotine gum): I, 46%; C1, 6%; C2, 0% Other outcomes: NR N: I1, n = 52; I2, n = 48; C: n = 60 |
There was a statistically significant difference in cessation rates between intervention and control patients |
| Hoving et al., 201057 | Baseline cigarettes/day: I (n = 256), 22; C (n = 289), 21 3-month continued abstinence (having refrained from smoking between baseline and follow-up, yes/no): I, 37/256; C, 31/289 6-month continued abstinence (having refrained from smoking between baseline and follow-up, yes/no): I, 2/256; C, 2/289 Other outcomes:
|
At 3 and 12 months there was no significant difference between I vs. C in the pharmacy sample except for quit attempts at 12 months: responders in the experimental group were more likely to have had a quit attempt than the control group (OR 1.48, 95% CI 1.03 to 2.11; p < 0.05) controlled for number of previous quit attempts There was a pharmacy setting and a GP setting – treated as two separate trials. GP sample not extracted as follow-up is at different time periods than pharmacy sample – there is an intervention and control group for both pharmacy and GP settings (four groups) GP surgeries and 15 pharmacies used passive recruitment, 50 pharmacies used active recruitment |
| Howard-Pitney et al., 199944 I: pharmacist advice and support + nicotine patch (free 6-week 15-mg patches) C: pharmacist advice and support + placebo patch |
Baseline number of cans/week, mean (SD): I, 3.9 (2.4) n = 206; C, 4.1 (2.3) n = 204 7-day point prevalence at 6 months (verified by cotinine): I, 38% (78/206); C, 34% (69/204) In intervention group age was a significant predictor of relapse (older chewers less likely to relapse) Other outcomes:
|
Study of tobacco chewers. Abstinence rates relatively high at 6-month follow-up but not significantly different between groups |
| Maguire et al., 200158 I: PAS model, 86% NRT C: ad-hoc pharmacist advice on smoking cessation, 84% NRT |
Baseline number of cigarettes/day 1–10: I, 14/265; C, 26/219 10–20: I, 197/265; C, 121/219 20–30: I, 29/265; C, 33/219 > 30: I, 13/265; C, 20/219 12-month abstinence (self-reported abstinence since the intervention for 12 months supported by a negative urinary cotinine test at 12 months): I, 14.3% (38/265); C, 2.7% (6/219) Other outcomes:
|
The PAS intervention significantly increased smoking cessation compared with control. It is unclear how many of the participants actually reached 12 months of follow-up Pharmacists were willing to participate before randomisation |
| Mochizuki et al., 200459 I: nicotine gum plus advice on usage and initial and follow-up cessation advice C: nicotine gum plus advise on usage |
Baseline number of cigarettes/day: I (n = 11), 23.0 (6.75); C (n = 16), 25.7 (13.9) Self-reported complete cessation (no smoking and no use of nicotine gum) at 3 months: I, 45.5% (5/11); C, 31.2 (5/16); OR 1.83 (not statistically significant) Other outcomes:
|
Both interventions appear to increase cessation but not reported if significant improvement from baseline, no significant difference between groups at follow-up. Very small study |
| Sinclair et al., 199860 I: training pharmacists and pharmacy assistants in the stages-of-change model of smoking cessation C: standard professional support |
Baseline number of cigarettes/day: NR, Fagerström test for nicotine dependence: I (n = 224), 5.2; C (n = 263), 5.2 Self-reported continued abstinence at 9 months: I, 12% (26/217); C, 7.4% (19/257) Self-reported continued abstinence at 9 months between-group difference (95% CI): 4.6% (–0.8% to 10.0%) Outcome was not affected by sex, age and SES (Carstairs Morris deprivation score)69 Other outcomes:
|
The intervention was associated with a favourable non-significant trend at 9 months, but this was based on self-reported abstinence. Pharmacists were willing to participate before randomisation. The study failed to reach its recruitment target (about half); power was reduced to the 10% level |
| Sonderskov et al., 199763 I1: free 21-mg nicotine patches (12 weeks, dosage reduced) I2: free 14-mg nicotine patches (12 weeks, dosage reduced) C1: free placebo 21-mg patches (12 weeks, dosage reduced) C2: free placebo 14-mg patches (12 weeks, dosage reduced) No psychological or behavioural support was added to the pharmacological treatment |
Baseline number of cigarettes/day (n/N): 10–14: I1, 2/136; I2, 51/119; C1, 0/142; C2, 53/125 15–19: I1, 9/136; I2, 62/119; C1, 12/142; C2, 64/125 20–24: I1, 88/136; I2, 3/119; C1, 92/142; C2, 5/125 ≥ 25: I1, 37/136; I2, 0/119; C1, 38/142; C2, 0 Self-reported point prevalence at 26 weeks (no smoking during a 4-week treatment period or one episode of a slip defined as < 6 days of smoking within a 4-week period) (n/N): I1, 11% (15/132); C1, 4.2% (6/142); I2, 22.7% (27/119); C2, 18.4% (23/125) Prevalence proportion ratio (95% CI): I1; C1, 2.61 (1.04 to 6.53) Prevalence proportion ratio (95% CI): I2; C2, 1.23 (0.75 to 2.03) Other outcomes:
|
Self-reported point prevalence only which also includes participants who have one episode of smoking (< 6 days) Intervention effective for those smoking ≥ 20/day at baseline randomised to I1 (21-mg patches) vs. C1, but not effective for lighter smokers randomised to 12 (14-mg patches) vs. C2. However, it appears that both intervention and placebo 14-mg patch groups had more quitters than the 21-mg patch intervention and control groups (not statistically tested). Seems to be a placebo effect, especially in low-dose placebo group Non-compliance among successful quitters was low |
| Vial et al., 200245 I: community pharmacy-based nicotine patches plus weekly counselling (US$15.00 weekly patches × 16 weeks) I2: hospital outpatient clinic nicotine patches plus weekly counselling (US$15.00 weekly patches × 16 weeks) C: minimal intervention (written and verbal information at baseline) |
Baseline number of cigarettes/day: NR, Fagerström score mean (range): I1 (n = 34), 5.79 (3–9); I2 (n = 35), 5.94 (1–9); C (n = 33), 6.33 (1–9) Self-reported continued abstinence at 12 months (not smoked since discharge): I1, 19% (4/21); I2, 24% (5/21); C, 4.6% (1/22) Other outcomes:
|
Participants were all former inpatients of respiratory unit and intervention commenced for all participants while inpatients then continued after discharge (either as outpatient or pharmacy-based) Point prevalence, but not continuous abstinence, was significantly different in favour of either active intervention compared with control at 12-month follow-up |
| Weight loss | ||
| Ahrens et al., 200364 I1: meal replacement (free products) I2: conventional low-calorie diet both set in community pharmacy |
BMI baseline (kg/m2), mean (SD): I (n = 45), 29.5 (2.2); C (n = 43), 29.0 (2.6) BMI change: NR WC baseline (cm), mean (SD): I (n = 45), 89.1 (8.5); C (n = 43), 87.0 (8.2) 12-week WC change (cm): I (n = 45), –5.31; C (n = 43), –6.10 22-week WC change (cm): I (n = 45), –8.08 C (n = 43), –7.82 12-week WC between-group difference (95% CI): NR Weight baseline (kg), mean (SD): I (n = 45), 81.9 (11.1); C (n = 43), 78.3 (10.1) Weight 12 weeks (kg): I (n = 45), 77.0 (SE 1.6); C (n = 43), 74.0 (SE 1.6) 12-week weight change (kg): I (n = 45), −4.9 (SE 0.3); C (n = 43), –4.3 (SE 0.3) 12-week weight change (kg): I, −5.2 (SE 0.4); C, –4.3 (SE 0.4); n = 68 (I + C) 12-week weight between-group difference (kg) (95% CI): –0.9 (−2.0 to –0.1); n = 68 (I + C) 12–22-week weight change (kg): I, −0.7 (SE 0.4); C, –0.9 (SE 0.4); n = 68 (I + C) 12–22-week weight (kg) between-group difference (95% CI): 0.3 (−0.8 to –1.4); n = 68 (I + C) Other outcomes:
|
During the 12-week weight loss phase both groups lost a significant amount of WC and weight, the meal replacement group also lost a significant amount of weight between weeks 12–22, although no significant difference between the groups at 12 weeks or at 22 weeks High dropout especially during maintenance phase |
| Bush et al., 201165 Diet and exercise with a trained health-care worker (health-care assistant, practice nurse, pharmacy assistant): I: pharmacy-based C: GP-based |
BMI baseline (kg/m2): I (n = unclear), 33.0; C (n = unclear), 35.6 12-week BMI change (kg/m2): I (n = 91), –0.9 (95% CI ±0.2); C (n = 75), –1.4 (95% CI ±0.3) 15-week BMI change (kg/m2): I (n = 60), −1.3 (95% CI ±0.4); C (n = 22), –0.8 (95% CI ±0.7) BMI between-group difference (95% CI): NR WC baseline (cm): I, 105.1; C, 108.8 12-week WC change (cm): I (n = 91), –4.9 (95% CI ±0.9); C (n = 75), –6.0 (95% CI ±1.3) 15-week WC change (cm): I (n = 60), –6.5 (95% CI ±1.6); C (n = 22), –4.9 (95% CI ±2.6) WC between-group difference (95% CI): NR Weight baseline (kg): I (n = 186), 86.1; C (n = 268), 95.8 12-week weight change (kg): I (n = 91), −2.4 (95% CI ±0.6); C (n = 75), –3.8 (95% CI ±0.8) 15-week weight change (kg): I (n = 60), −3.4 (95% CI ±1.1); C (n = 22), –2.3 (95% CI ±1.9) Weight between-group difference (95% CI): NR Other outcomes:
|
Significant differences between groups at baseline. GP participants tending to be older than pharmacy participants. Large percentage of Asian participants recruited in pharmacies. Large dropout and small groups at last follow-up. Both groups appear to reduce BMI, WC and weight at follow-up, statistical significance either from baseline to follow-up or between groups is not reported. Pharmacy group appear to continue to improve between weeks 12–15 but the GP group outcomes do not There were no statistically significant relationships between sex, age, IMD quintile or ethnicity and per cent weight loss at session 12 among pharmacy or GP participants. Completer analysis only It is unclear which provider type delivered the programme more cost-effectively At session 12 each extra kg of weight loss per participant would cost £8.29 through pharmacy providers. Conversely, at session 15, each extra kg of weight loss per participant would cost £2.91 through GP providers Attendance rates on the programme were consistently better among pharmacy participants than among GP participants |
| Jolly et al., 201166 WW, SW, RC, Size Down (NHS community-based), GP, pharmacy, participants own choice vs. control (exercise) |
BMI baseline (kg/m2), mean (SD): WW, 33.96 (3.9); SW, 33.83 (3.8); RC, 33.38 (3.5); Size Down, 33.77 (3.9); GP, 33.06 (3.5); pharmacy, 33.44 (3.5); choice, 33.41 (3.4); C, 33.88 (4.4) BOCF 12-week BMI change: NR BOCF 1-year BMI change (kg/m2): WW, –1.17 (95% CI –1.7 to –0.7); SW, –0.71 (95% CI –1.0 to –0.4); RC, –0.75 (95% CI –1.1 to –0.3); Size Down, –0.67 (95% CI –1.0 to –0.3); GP, –1.32 (95% CI –0.7 to 0.1); pharmacy, –0.31 (95% CI –0.7 to 0.0); choice, –0.90 (95% CI –1.3 to –0.5; C, –0.45 (95% CI –0.8 to –0.1) BOCF BMI between-group difference adjusted for weight at baseline, physical activity at baseline, age, sex, and ethnic group (kg/m2): WW vs. C, −2.34 (95% CI −3.56 to –1.13); SW vs. C, −1.24 (95% CI −2.47 to –0.02); RC vs. C, −2.390 (95% CI −3.61 to –1.16); Size Down vs. C, −0.09 (95% CI −1.31 to 1.14 ); GP vs. C, 0.61 (95% CI −0.73 to 1.96); pharmacy vs. C, 0.12 (95% CI −1.51 to 1.27); choice vs. C, −1.33 (95% CI −2.55 to –0.11) WC baseline: NR BOCF 12-week weight change (kg): WW, –4.43 (95% CI –5.3 to –3.6); SW, –3.56 (95% CI –4.4 to –2.7); RC, –4.23 (95% CI –5.2 to –3.2); Size Down, –2.38 (95% CI –3.1 to –1.7); GP, –1.37 (95% CI –2.3 to –0.4); pharmacy, –2.11 (95% CI –3.2 to –1.0); choice, –3.32 (95% CI –4.1 to –2.5); C, –2.01 (95% CI –2.8 to –1.2) BOCF 1-year weight change (kg): WW, –3.46 (95% CI –4.8 to –2.1); SW, –1.89 (95% CI –2.9 to –0.9); RC, –2.12 (95% CI –3.4 to –0.9); Size Down, –2.45 (95% CI –3.6 to –1.3); GP, –0.82 (95% CI –2.0 to –0.4); pharmacy, –0.66 (95% CI –1.7 to –0.4); choice, –2.15 (95% CI –3.4 to –0.9); C, –1.08 (95% CI –2.1 to –0.1) Weight between-group difference adjusted for weight at baseline, physical activity at baseline, age, sex and ethnic group (kg): WW vs. C, −2.49 (95% CI −4.15 to –0.83); SW vs. C, −0.90 (95% CI −2.57 to 0.77); RC vs. C, −1.35 (95% CI −3.03 to 0.33); SD vs. C, −1.65 (95% CI −3.33 to 0.04); GP vs. C, 0.12 (95% CI −1.96 to 1.72); pharmacy vs. C, 0.06 (95% CI −1.84 to 1.96); choice vs. C, −1.47 (95% CI −3.13 to 0.20) Other analyses:
|
All programmes achieved significant weight loss ranging from –1.37 kg (GP) to –4.43 kg (WW) at 12 weeks All except GP and pharmacy groups resulted in significant weight loss at 1 year. At 1 year, only the WW group had significantly greater weight loss than did the control (exercise only) group (2.5 kg, 95% CI 0.8 kg to 4.2 kg) The commercial programmes (WW, SW and RC) achieved significantly greater weight loss than did the primary care programmes (general practice and pharmacy-based interventions) at 12 weeks and 1 year. At 1 year, the difference was 1.6 kg (95% CI 0.3 to 2.9 kg; p = 0.06) in the adjusted model. Mean weight loss at 1 year, with baseline value used for imputation was 0.8 kg (SD 4.7 kg) for primary care and 2.5 kg (SD 6.2 kg) for commercial programmes The primary care programmes were the most costly to provide. If assumed participants randomised to this intervention continued to weigh 1.3 kg/m2 less throughout life, then the cost per life-year saved was £77 (€88; US$122) |
| Malone and Alger-Mayer, 200339 I: orlistat + usual outpatient care + community pharmacy support C: orlistat + usual outpatient care |
BMI baseline (kg/m2), mean (SD): I, 48.3 (14.6); C, 42.8 (8.1) BMI change: NR WC baseline (cm), mean (SD): I, 128 (20); C, 127 (17) WC change: NR Weight baseline (kg), mean (SD): I, 130 (39); C, 124 (30) 26 weeks weight change (kg), mean (SD): I, −3.5 (2.9); C, –3.0 (5.2) Weight between-group difference: NR I, n = 15; C, n = 15 Other outcomes:
|
Very small study, high dropout and high baseline BMI. Both groups appeared to lose similar amount of weight at 26 weeks |
| Phimarn et al., 201367 I: community pharmacist individual support C: primary care unit group support |
BMI baseline (kg/m2), mean (SD), I, 27.48 × 3.14; C, 27.74 × 3.25 16 weeks BMI (kg/m2), mean (SD): I, 26.68 × 4.88; C, 27.93 × 3.30 BMI change (kg/m2), mean (SD): I, –0.80 × 0.07; C, 0.19 × 0.04 BMI between-group difference: NR WC baseline (inches), mean (SD): I, 36.26 × 3.50 ; C, 37.23 × 3.02 16 weeks WC (inches), mean (SD): I, 36.30 × 3.56 ; C, 37.12 × 3.01 WC (inches) change: I, 0.04 × 0.01; C, –0.11 × 0.03 WC change (cm), mean (SD): I, 0.1 × 0.03; C, –0.28 × 0.08 WC between-group difference: NR Weight baseline (kg), mean (SD): I, 66.80 × 7.44; C, 66.66 × 8.03 16 weeks weight (kg), mean (SD): I, 65.98 × 7.15; C, 67.58 kg × 7.98 Weight change (kg), mean (SD): I, –0.82 × 0.29; C, 0.92 × 0.19 Weight between-group difference (95% CI): NR I, n = 33; C, n = 33 Other outcomes:
|
Neither group showed significant improvement in clinical outcomes. Small study. Completer analysis only |
| Multicomponent interventions (pharmacotherapy and lifestyle changes) | ||
| Diabetes mellitus – type 2 | ||
| Ali et al., 201268 I: diabetes mellitus monitoring and lifestyle modification counselling C: usual care |
BMI baseline (kg/m2), mean (SD): I (n = 23), 30.84 (4.95); C (n = 23), 29.82 (5.46) 12 months BMI (kg/m2), mean (SD): I (n = 23), 26.98 (3.31); C (n = 23), 28.73 (4.06) BMI (kg/m2) change: NR BMI (kg/m2) between-group difference (95% CI): NR WC (cm) baseline: NR Weight (kg) baseline: NR Other outcomes:
|
Small study, low attrition. Significant reductions in BMI in the intervention group as compared with no significant changes in the control group from baseline to follow-up but no significant difference between groups at follow-up |
| Correr et al., 201169 I: pharmacotherapy including patient education C: usual care |
BMI baseline (kg/m2), mean (SD): I, 29.2 (4.9); C, 27.6 (4.4) 52-week BMI change (kg/m2), mean: I, –0.2 (95% CI –0.8 to 0.3); C, –0.1 (95% CI –0.7 to 0.4) BMI between-group difference (kg/m2): NR WC baseline (cm), mean (SD): I, 95.2 (11.4); C, 94.9 (10.2) 52-week WC change (cm), mean: I, 0.8 (95% CI –0.7 to 2.4); C, 0.06 (95% CI –2.0 to 2.1) WC between-group difference (cm) (95% CI): NR Weight baseline: NR Other outcomes:
|
BMI and WC remained similar between the groups at follow-up. Pharmacists were willing to participate before randomisation Analyses adjusted for baseline differences however the percentage of women, was greater among the drop-outs (73.3% vs. 53.1%; p = 0.014), and the initial SBP and DBP, were higher in the completing patients (p = 0.026 and p = 0.019, respectively) |
| Fornos et al., 200670 I: pharmacotherapy and lifestyle advice C: usual care |
BMI baseline (kg/m2), mean (SD): I, 31.0 (4.7); C, 31.7 (5.4) 13-month BMI (kg/m2), mean (SD): I, 30.1 (4.4); C, 31.4 (5.4) BMI change (kg/m2): NR BMI between-group difference (kg/m2): NR WC (cm) baseline: NR Weight (kg) baseline: NR Other outcomes:
|
Intervention group but not control reduced BMI from baseline to follow-up but BMI not significantly different between groups at follow-up |
| Dyslipidaemia | ||
| Paulos et al., 200571 I: medication compliance and lifestyle modifications C: usual care |
BMI baseline (kg/m2), mean (SD): NR 16-week BMI (kg/m2) change: I, –0.4 (0.5); C, NR BMI between-group difference (kg/m2): NR There was no significant difference in baseline BMI between the two groups, but there was a significant decrease of 0.4 kg/m2 within the intervention group WC baseline (kg), mean (SD): NR Weight baseline (kg), mean (SD): NR 16-week weight change (kg), mean (SD): I, −1.0 (1.3); C, +1.1 (2.6) Weight between-group difference (kg): NR Smoking status: One intervention participant smoked throughout the intervention (three Cigarettes per day); six control participants smoked at baseline, four of whom increased the number of cigarettes smoked per day by the end of the intervention Alcohol status: Fifty per cent of the intervention group and 72% of the control group did not drink alcoholic beverages. These numbers persisted until the end of the program, although 2 patients in the intervention group stopped drinking alcohol during the study period and 1 patient decreased his alcohol intake Other outcomes:
|
Appears to be significant difference from baseline to follow-up for weight in intervention group. Paper only reports change in BMI for intervention group (not control, assume it remains similar to baseline). Does not report baseline weight or BMI, only change in these variables. Completer analyses only |
| Hypertension | ||
| Zaragoza-Fernandez et al., 201272 I: diet and exercise C: usual care Hypertensive participants taking anti-hypertensive medication but not controlled |
BMI baseline (kg/m2), mean (SD): I (n = 76), 30.8 (3.9); C (n = 74), 30.0 (4.1) 8-week BMI (kg/m2), mean (SD): I (n = 71), 30.4 (4.0); C (n = 72), 29.8 (4.1) BMI change (kg/m2): NR BMI between-group difference (kg/m2): NR WC baseline: NR Weight baseline (kg), mean (SD): I (n = 76), 78.3 (14.4); C (n = 74), 74.9 (12.4) 8-week weight (kg), mean (SD): I (n = 71), 77.6 (14.8); C (n = 72), 74.3 (12.2) Weight change (kg): NR Weight between-group difference (95% CI): NR Other outcomes:
|
Short-term study but low attrition. Main focus is about controlling hypertension, BMI and weight appear fairly stable from baseline to follow-up and between groups (slight reductions) Reports alcohol reduction, but only for intervention group (as measure of adherence rather than effectiveness). Low attrition |
Appendix 12 UK alcohol service evaluations that were excluded from the systematic review
Reason for exclusion: these service evaluations did not meet the inclusion criteria for the systematic review.
| Reference | Location | Number of pharmacies | Design | Period | Duration of education | Delivery | Patients approached (A) and recruited (R) | Harmful/hazardous drinkers | Referrals | Follow-up | Method used |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Goodall T, Dawson P. A Feasibility Study: The role of Community Pharmacists in the Identification and Treatment of Hazardous Drinking. Leeds: Leeds PCT; 2006 | Leeds | 5 | ucB&A | 3 months | NR | Pharmacist | A: NR R: 352 |
105 | NR | 3 months | FAST |
| Anonymous. Lloyds Pharmacy Alcohol Identification and Brief Advice (IBA) Service for NHS Birmingham. Birmingham: Lloyds Pharmacy; 2010 | Birmingham | 40 | ucB&A | 3 years | NR | NR | A: NR R: ≈5000 |
≈2900 | Over 300 | 1 month | AUDIT |
| Blackwood L, Torrens J. NHS greater Glasgow and Clyde Alcohol Awareness Scratch Card Pilot. Glasgow: NHS greater Glasgow and Clyde; 2013 | Glasgow and Clyde | 30 | ucB&A | NR | 1 evening | NR | A: NR R: 1502 |
903 | 9 | NR | Scratch card, based on AUDIT |
| Parsons G. Evaluation of the Alcohol Identification and Brief Advice (IBA) Service in Plymouth Healthy Living Pharmacies (November to December 2011). Plymouth: Devon Local Pharmaceutical Committee; 2013 | Plymouth | 14 | ucB&A | 1 month | 1 evening plus e-learning | Pharmacy team | A: NR R: 515 |
191 | 8 | NR | AUDIT-C |
| Davies J, Gill J, Crisp M, Taylor D. Pan-London Pharmacy Alcohol Awareness Campaign. London: University College London School of Pharmacy; 2013 | London | 240 | ucB&A | 4 months | Online learning | Pharmacy team | A: NR R: 23,810 |
10,351 | NR | NR | AUDIT-C via scratch card |
| Fitzgerald N, Stewart D. Drinking Interventions in Pharmacies Study (DIPS). Development, Implementation And Evaluation of a Pilot Project to Deliver Interventions on Alcohol Issues in Community Pharmacies. Aberdeen: The School of Pharmacy, The Robert Gordon University; 2006 | Glasgow | 8 | ucB&A | 3 months | 2 days | Pharmacist | A: NR R: 70 |
37 | 1 | Various (4–8 months] | FAST |
| Bowhill J, Bowhill S, Evans D, Holden M, Nazar Z, Portlock J. An Interim Report on the Outcomes from the Portsmouth Health Living Pharmacy Initiative. Portsmouth: NHS Portsmouth; 2010 | Portsmouth | 32 (6-accredited HLPs) | ucB&A | 1 month | NR | Pharmacy team | A: NR R: 3649 |
More than 1450 | 29 | NR | AUDIT-C via scratch card |
| South East Alcohol Innovation Programme Evaluation Report. Lundbeck UK Limited & the Centre for Public Innovation | Windsor and Maidenhead | 19 | ucB&A | NR | NR | NR | A: NR R: 86 (62 MUR, 24 opportunistic) |
34 | NR | NR | AUDIT-C |
| Gray NJ, Wilson SE, Cook PA, Mackridge AJ, Blenkinsopp A, Prescott J, et al. Understanding and Optimising an Identification/Brief Advice (IBA) Service about Alcohol in the Community Pharmacy Setting. Final report. Liverpool: Liverpool PCT; 2012 | Wirral | 33 | ucB&A | 42 months | NR | Pharmacy team | A: NR R: 10,907 |
2461 | NR | NR | AUDIT |
| Gray NJ, Wilson SE, Cook PA, Mackridge AJ, Blenkinsopp A, Prescott J, et al. Understanding and Optimising an Identification/brief Advice (IBA) Service About Alcohol in the community Pharmacy Setting. Final report. Liverpool: Liverpool PCT; 2012 | Blackpool | 18 | ucB&A | NR | NR | Pharmacist and technicians | A: NR R: 522 |
142 | NR | 2 weeks and 3 months | AUDIT |
| Gray NJ, Wilson SE, Cook PA, Mackridge AJ, Blenkinsopp A, Prescott J, et al. Understanding and Optimising an Identification/Brief Advice (IBA) Service about Alcohol in the Community Pharmacy Setting. Final report. Liverpool PCT, 2012 | Bolton | 7 | ucB&A | NR | 1.5-hour evening session | Pharmacy team | A: NR R: 1035 |
159 | NR | NR | AUDIT |
| Gray NJ, Wilson SE, Cook PA, Mackridge AJ, Blenkinsopp A, Prescott J, et al. Understanding and Optimising an Identification/Brief Advice (IBA) Service about Alcohol in the Community Pharmacy Setting. Final Report. Liverpool PCT, 2012 | Knowsley | 17 | ucB&A | NR | E-learning | NR | A: NR R: 2479 |
819 | NR | 2 weeks and 3 months | AUDIT |
| Gray NJ, Wilson SE, Cook PA, Mackridge AJ, Blenkinsopp A, Prescott J, et al. Understanding and Optimising an Identification/Brief Advice (IBA) Service About Alcohol in the Community Pharmacy Setting. Final report. Liverpool PCT, 2012 | Sefton | 10 | ucB&A | NR | NR | NR | A: NR R: 398 |
101 | NR | NR | AUDIT |
| Dowds J. Community Pharmacy Alcohol Scheme Evaluation Report. Monmouthshire: Monmouthshire Local health board; 2008 | Monmouthshire | Not stated | ucB&A | 6 months | 2 days | Pharmacist | A: NR R: ≈30 |
NR | NR | NR | Unclear |
| Evaluation of Drinkaware Resources. Report by Shared Intelligence. 2014 | Berkshire | 151 | ucB&A | 8 weeks | NR | Pharmacy team | A: NR R: NR |
169 (from 300 who returned the questionnaire) | NR | Evaluation questionnaire | Drinkaware kit |
| Alcohol and EHC Brief Interventions & Alcohol Awareness with Brief Interventions. Hampshire and Isle of White Project LPC, 2009 | Hampshire | 50 | ucB&A | NR | Online training | NR | A: NR R: 214 (by EHC) |
102 | 7 | NR | Based on number of units |
| Alcohol and EHC Brief Interventions & Alcohol Awareness with Brief Interventions. Hampshire and Isle of White Project LPC, 2009 | Rushmoor and Hart | 10 | ucB&A | NR | Online training | NR | A: NR R: 794 |
420 | 41 | NR | Based on number of units |
| Hughes G. Alcohol Screening in Community Pharmacies Final Report 2011. Surrey: NHS Surrey; 2011 | Surrey | 6 | ucB&A | 6 months | 1 day | Pharmacist | A: NR R: 128 |
76 | 3 | 2 months | AUDIT-C and AUDIT |
| Dyoss M. HLP Report. Dudley: Dudley Public Health; 2013 | Dudley | 17 (part of HLP) | ucB&A | On-going (part of HLP) | Part of HLP training (Royal Society of Public Health Level 2) | Not stated | A: NR R: 280 |
82 | 2 | NR | AUDIT |
| Community Pharmacy Alcohol Identification and Brief Interventions Pilot Evaluation Report. London Borough of Richmond upon Thames, 2013 | Richmond Borough, London | 4 | ucB&A | 2 months | Not stated | Pharmacist | A: NR R: 119 |
39 | Not stated | NR | AUDIT-C and AUDIT |
| Templeton L. Windows of Opportunity: Reducing Alcohol-related Harm in Somerset. Evaluation of Alcohol Projects: Final Report. Somerset: Somerset Drug and Alcohol Partnership; 2013 | Somerset | 17 | ucB&A | 9 months | Not stated | Pharmacy staff | A: NR R: 574 |
234 interventions given to those ‘at high risk’ | 21 to Somerset single point of contact | NR | Reduced version of AUDIT |
| Khan NS, Norman IJ, Dhital R, McCrone P, Milligan P, Whittlesea CM. Alcohol brief intervention in community pharmacies: a feasibility study of outcomes and customer experiences. Int J Clin Pharm 2013;35:1178–87 | London | 26 | ucB&A | 5 months | Not stated | Pharmacist and support staff | A: 927 (663 were eligible) R: 141 |
91 identified as risky drinkers | Harmful drinkers (n = 20) were referred to GP/specialist alcohol services | 3 months | AUDIT–C |
List of abbreviations
- AUDIT
- Alcohol Use Disorders Identification Test
- BMI
- body mass index
- CBA
- controlled before-and-after study
- CI
- confidence interval
- CO
- carbon monoxide
- DH
- Department of Health
- FAST
- Fast Alcohol Screening Tool
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- ITT
- intention to treat
- LPC
- local pharmaceutical committee
- nRCT
- non-randomised controlled trial
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- PAS
- Pharmacist Action on Smoking
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSNC
- Pharmaceutical Services Negotiating Committee
- RCT
- randomised controlled trial
- SD
- standard deviation
- SES
- socioeconomic status
- WC
- waist circumference
- WHO
- World Health Organization