Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 12/3090/05. The contractual start date was in April 2014. The final report began editorial review in January 2017 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Kenning et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background and rationale
Background
Despite relatively high levels of employment among working-age adults in the UK, there is still a significant minority who are off work with ill health at any one time (so-called ‘sickness absence’). Figures for the UK show that 131 million days were lost as a result of sickness absences in 2013. 1 Although this is down from around 175 million days before the turn of the century,2 sickness absence still has huge economic implications.
More than 2.5 million people claim health-related benefits (Incapacity Benefit and Employment and Support Allowance – 2013/14 data), costing the government £12B a year. 3 Furthermore, employers pay around £9B per year in sick pay and associated costs. 3
Office for National Statistics figures show that, in 2013, minor illness (e.g. colds and coughs) accounted for around 27.4 million days lost, typically short-duration absences. The greatest numbers of days lost were attributable to musculoskeletal problems (30.6 million days of work lost) and mental health problems such as stress, depression and anxiety (15.2 million days of work lost). 1 Although most absences are of 4 weeks or less, many absences last longer than they need to, and every year over 300,000 people fall out of work and claim health-related state benefits. 3
People with long-term health conditions can and do work. Around one-quarter of the 28 million people in work in the UK have a long-term condition. 3 Employees who suffer significant periods of sickness absence are at increased risk of longer-term problems, with profound implications for their long-term health, wealth and social inclusion.
Policy and current initiatives
Dame Carol Black’s 2008 review of the health of Britain’s working-age population, Working for a Healthier Tomorrow,2 cast light on the scale and impact of sickness absence on the economy, as well as the personal impact on individuals. The report outlined the changes in attitudes to work and health that were required to manage the problem of sickness absence more effectively, and the organisational and service delivery challenges that such changes would be likely to introduce. 2
The report also listed a number of key priorities for the government. One of the recommendations from this review was the introduction of a new service to offer support for people in the early stages of sickness absence. Funded by the Department for Work and Pensions (DWP) and the Department of Health, a proof-of-concept pilot study was set up in 11 localities across the UK to test different locally determined models for delivering services to help employees to return to work. These were known as Fit for Work (FFW) services and the pilot ran from 2010 until 2013. The results of the pilot study were published by the DWP in June 2015. 4
In all pilots, the client journey included five separate stages, but practice at each stage varied from site to site. The stages were (1) referral, (2) screening, (3) assessment and case management, (4) support and (5) discharge.
Although not intended to be rolled out nationally, models of best practice from the pilot study were used to inform the implementation of the new national independent health and work advice and referral service (also named the FFW service) launched at the end of 2014.
Another recommendation from the Black review2 was the need to focus on the benefits of work for health and on getting away from the notion that a person needs to be 100% fit to work. Replacing the old ‘sick note’ system with a new ‘fit note’ system in 2010 was intended to encourage general practitioners (GPs) to include advice on how a person ‘may be fit’ to work with reasonable workplace adjustments.
Recommended by the Black–Frost review,3 the FFW scheme is a new independent assessment and advisory service aimed at getting people back to work and away from long-term sickness benefits. It is proposed that the scheme will save employers up to £160M a year in statutory sick pay and increase economic output by up to £900M a year. 5 Currently, only 10% of employees in small firms have access to an occupational health (OH) service, compared with more than half of staff in larger firms. The new service will enable employers of all sizes to access expert advice to help them manage sickness absence in the workplace.
Current evidence on return-to-work interventions
Research into the clinical effectiveness and cost-effectiveness of Employee Assistance Programmes (EAPs) commissioned by the British Occupational Health Research Foundation6 (BOHRF) concluded that there was a lack of evidence about the clinical effectiveness of EAPs. Despite the prevalence of EAPs, no studies were found that could empirically demonstrate that EAPs were more effective than no intervention on a range of outcomes, including sickness absence. However, EAPs have continued to be used, and a more recent review by Mellor-Clark et al. 7 provides some evidence towards the efficacy of these programmes. Looking at clinical improvement, the study included a data sample of 17,520 clients. For all clients with valid pre–post therapy Clinical Outcomes in Routine Evaluation Outcome Measure (CORE-OM) data, the mean pre-therapy clinical score was 17.40 [standard deviation (SD) 6.01] and the mean post-therapy clinical score was 8.80 (SD 6.09) (pre–post effect size 1.43). The results provide some evidence that EAP counselling provision may be an effective intervention for employees experiencing common mental health problems. However, this was a retrospective observational study with no comparator, so we cannot be sure how much of the observed effect was as a result of the intervention.
A review of long-term sickness absence interventions conducted for the National Institute for Health and Care Excellence (NICE)8 to support public health guidance in this area identified 45 evaluations of the effectiveness of interventions, targeting mainly musculoskeletal interventions. The evidence base was heterogeneous but identified three intervention strategies that merited further investigation: early intervention, multifaceted approaches and interventions with a workplace component. Economic modelling based on this review found that any intervention which returns at least an additional 3% of employees to work and costs less than an additional £3000 per employee is likely to be considered economically attractive compared with usual care, relative to other interventions routinely funded by the NHS. 9
A further review of the evidence for workplace involvement on return-to-work rates following long-term sickness absence10 found that only a particular type of workplace involvement intervention was consistent in achieving positive return-to-work results. The evidence was limited to employees with back pain and found that active, structured consultation among employee, employer and OH practitioners, and agreements regarding subsequent, appropriate work modifications, appear to be more effective at helping employees on long-term sickness absence to return to work than those interventions which lack such components. 11 This type of intervention was also more cost-effective than other workplace-linked interventions, including exercise. These findings are further confirmed in other reviews focusing on the characteristics of successful return-to-work interventions that highlight the importance of early intervention (i.e. in the first 6 weeks of absence) and the use of multifaceted interventions (particularly those including a workplace consultation component). 12,13
A report on vocational rehabilitation suggested that a variety of responses were required to better manage different patterns of workplace absence and the needs of different groups. Simple, low-cost workplace interventions might be sufficient for those with short-term absence, with effective vocational rehabilitation programmes combining health and occupational assistance for those with longer-term absences. 14 The delivery of a range of interventions of different intensity according to need echoes the adoption of ‘stepped-care’ services in the NHS to manage some long-term conditions, including depression. 15 The report also highlighted the need for systematic adoption of ‘basic principles’ related to the management of these problems, irrespective of whether they were work related or comparable health conditions. 14 However, the significant challenges associated with effective implementation of such principles in routine practice were also highlighted.
Since the current study began there have been a number of new reports published in this area. A review by Nieuwenhuijsen et al. 16 focused on return-to-work interventions for people with depression. The authors reported that adding a work-directed intervention to a clinical intervention reduced the number of days on sick leave compared with a clinical intervention alone [effect size –0.40, 95% confidence interval (CI) –0.66 to –0.14]. Another Cochrane review, by van Vilsteren et al. ,17 assessed the impact of workplace interventions compared with usual-care or clinical interventions. They reported that workplace interventions reduce time to first return to work (hazard ratio 1.55, 95% CI 1.20 to 2.01), and that workplace interventions reduce the cumulative duration of sickness absence (–33.33 days, 95% CI –49.54 to –17.12 days). However, the authors also reported a single study demonstrating that workplace interventions increased recurrences of sick leave (hazard ratio 0.42, 95% CI 0.21 to 0.82).
Current approaches to the management of people with long-term conditions
The call for adoption of core ‘basic principles’ is in line with current thinking in chronic disease (or ‘long-term condition’) management in health care. 18,19 There has been significant development in our understanding of the nature of long-term conditions. It is widely acknowledged that many long-term conditions raise common challenges for patients, and that the organisational and therapeutic interventions required involve the following common elements:
-
individualised assessment of behaviour
-
collaborative goal-setting
-
skills enhancement
-
proactive follow-up
-
self-management support for healthy behaviour change
-
access to resources. 6
As noted previously, the bulk of long-term sickness absence relates to musculoskeletal or mental health problems, and both of these areas have proven themselves amenable to adoption of these ‘basic principles’. 20,21 Depression and distress are common features of long-term sickness absence. The application of the principles of chronic disease management in depression has been demonstrated through the literature on so-called ‘collaborative care’ models. 22–24
Historically, conventional approaches to depression were oriented to the management of depression as an acute problem, where patients seek help when they deem it necessary, and professionals respond to those patients seeking help. 25,26 However, depression is a disorder that results in low motivation to seek, and adhere to, care, and services that respond only to patient presentations are unlikely to be optimal for managing depression in the community. 27 The full range of interventions employed in collaborative care models varies but generally includes education of primary care professionals (through short courses and provision of clinical guidelines), systematic screening to identify depression in the wider population, enhanced patient education and self-management support, and consultation between specialist and primary care provider to ensure that specialist and generalist approaches to management are aligned (a health-care analogue of the ‘workplace consultation’ identified in earlier reviews). However, a critical component is ‘case management’. Case management involves specific professionals taking responsibility for the assessment, support and follow-up of individual patients in an integrated and proactive fashion. 28
Given that the problems faced by employees on long-term sickness absence are likely to involve a complex mix of physical and psychological symptoms, this suggests that the broad ‘collaborative care’ model could be highly relevant to this population.
Collaborative care in occupational health
Although chronic disease management models and collaborative care for depression were developed in health settings, there is evidence for the relevance of these models in an OH context. Vlasveld et al. 29 developed a version of collaborative care including many of the conventional elements described above (6–12 sessions of problem-solving treatment, manual-guided self-help, and antidepressant management monitored by an occupational case manager and supported by a mental health specialist). The programme also included elements specific to the OH context, including workplace assessments and adjustments, with the case manager mediating between employee and employer. The study randomised 126 patients with depression to either collaborative care or usual care, and reported a significant difference between four groups in the proportion of clients achieving a 50% reduction in depression symptoms (50% in the collaborative care group and 28% in the usual-care group; odds ratio 2.50, 95% CI 1.04 to 6.10). However, there was less evidence of benefit in measures of return to work. 30
A second trial recruited 604 workers from diverse sectors of the US economy, and randomised them to a telephone-led case management programme or usual care (which included encouragement to enter existing treatment programmes). Case management included brief interventions direct from the case manager for patients who refused to seek help elsewhere, including eight sessions of cognitive–behavioural therapy (CBT) for those with persistent symptoms. 31 The results showed improvements in depression as a result of case management interventions similar in magnitude to existing evidence on collaborative care (approximately one-third of a SD), and better rates of recovery (31% vs. 21%) at 12 months. Patients in case management also reported two additional hours of work per week (approximately 2 weeks of additional work over a 12-month period). The potential of collaborative care models in OH has been demonstrated, but the case is far from proven. It is unclear whether or not these models will generalise to a UK OH context and whether or not the benefits found in patients with diagnosed depression will generalise to a broader mix of problems reported by employees currently on long-term sickness absence. A definitive trial of the potential of these models in the OH setting in the UK is thus indicated. The FFW pilot scheme also adopted a collaborative care model, the results of which were published during the course of this research. The findings are discussed later (see Chapter 5) in comparison with our own findings.
Summary
It is evident from the literature that employees who suffer significant periods of sickness absence are at increased risk of longer-term problems, with profound implications for their long-term health, wealth and social inclusion. Long-term absences also result in considerable financial implications for the government and for employers.
The body of evidence for intervention with people on or entering long-term sickness absence is growing, but results appear mixed. There is good evidence for collaborative care models in the care for long-term conditions and, as stated previously, around 25% of the working population currently have long-term conditions. Collaborative care in an OH setting has been trialled in the Netherlands and the USA, but a definitive trial has not taken place in the UK, which has a markedly different system.
Bringing this literature together, this study aims to adapt a collaborative care model for use in OH and conduct a pilot study to see how it might work in this setting. The pilot study will determine if it is feasible to recruit and deliver the new model to working adults on longer-term sickness absence and if it is acceptable to both employees and employers.
Research objectives
Phase 1: development
-
Adapt a collaborative case management intervention to the needs of UK employees, in a range of occupations and organisations, who are entering or experiencing long-term sickness absence.
Phase 2: internal pilot
-
Conduct a pilot study to test:
-
recruitment of employees on long-term sickness absence to a trial
-
delivery of the intervention in an OH setting
-
adherence and acceptability among employees on long-term sickness absence
-
appropriateness of inclusion criteria and outcome measures
-
evaluation of the rate of return to work in those receiving a collaborative case management intervention compared with those receiving care as usual.
-
Chapter 2 Phase 1
This chapter describes phase 1 (development), which was conducted to meet the objective of adapting a collaborative case management intervention to the needs of UK employees, in a range of occupations and organisations, who are entering or experiencing long-term sickness absence.
Scoping review
First, a scoping review was conducted to see what could be learned from previous trials. A database search was carried out using OVID and searching the following databases: CENTRAL (Cochrane Central Register of Controlled trials), MEDLINE, EMBASE and PsycINFO.
Search terms: the following broad search terms, key words and BOOLEAN operators were used in the searches: case management, collaborative care, co-ordinated care, collaboration, multidisciplinary care, employees, OH, workplace interventions, sickness absence, sick leave, return to work and absenteeism.
Suitable studies were selected and data extracted on methods, results and, in particular, on barriers to, and limitations of, the research.
The review looked at key existing research in three areas:
-
OH-based interventions that were not case management
-
case management interventions that were not based in OH
-
case management interventions that were based in OH.
Similarities and differences from the identified trials were considered. Key points were identified from the literature and discussed in relation to the content and delivery of an intervention.
Expert consultation
Second, a full trial meeting was held with all co-applicants and collaborators, with patient and public involvement and engagement (PPIE) input. The results of the review were presented to the group along with the existing intervention model that we proposed using. These were then discussed by the group to see how the model might need amending to better fit an occupational setting, and how to best set up and run the trial in light of the findings and expertise of those in the group.
Development of materials
Following the initial work, we developed materials for the intervention, including the intervention manual and case manager training.
Manual development
Following the meeting, the existing case management intervention was adapted to focus more on work issues and to include the option for workplace facilitation. Example case studies were written, in consultation with the FFW team, as real-life stories for the manual to help participants engage in the intervention. The manual was designed and sent to our PPIE representative for their feedback, and then amended where needed. A copy of the finalised manual can be found in Report Supplementary Material 1.
Case manager training
To support case managers, a 2-day training course was developed that introduced the principles of case management and provided training in the brief psychological interventions employed in the patient manual. The case managers took part in role play sessions to aid learning and were encouraged to ask questions. The course was based on similar courses run by applicant Karina Lovell for other trials,32 but was modified appropriately.
As well as training in the intervention, case managers also received training delivered by the Manchester Academic Health Science Centre Clinical Trials Units (MAHSC-CTU) (www.mahsc-ctu.co.uk/) in the trial methods and reporting procedures, and also completed Good Clinical Practice (GCP) training.
Summary of the results of the review
Occupational health-based interventions that were not case management
The studies carried out in OH settings33–40 were heterogeneous. They encompassed a wide range of interventions from psychological interventions, problem-solving and return on reduced hours, to interventions with occupational physicians and adherence to guidelines. The interventions were also aimed at a wide range of participants: some were sick-listed, some had recently returned from sickness absence, and other interventions were preventative and, therefore, not targeted to those on sickness absence. The index condition tended to be specific and aimed at common mental disorders or musculoskeletal disorders, with no studies targeting both, or other, conditions. Trial outcomes in those studies with sick-listed participants tended to focus on time to return to work, number of days’ absence and quality-of-life measures (Table 1).
| First author and date of publication | Title | Intervention | Sample | Outcome/results |
|---|---|---|---|---|
| Arends et al., 201433 | Prevention of recurrent sickness absence in workers with common mental disorders: results of a cluster-randomised controlled trial | Problem-solving intervention vs. usual care | 80 workers recently returned to work after sickness absence for CMDs | Incidence of recurrent sickness absence. Adjusted OR of 0.4 (95% CI 0.2 to 0.8) TG compared with control |
| Delivered by physicians | The Netherlands | Time to absence: adjusted hazard ratio of 0.53 (95% CI 0.33 to 0.86); TG compared with control | ||
| Aelfers et al., 2013;34 protocol only | Effectiveness of a minimal psychological intervention to reduce mild to moderate depression and chronic fatigue in a working population: the design of a randomized controlled trial | Over 4 months patients receive between 1 and 10 sessions | 124 workers with chronic mental fatigue or mild to moderate depression | Primary outcome: symptom measures; secondary outcomes: sickness absence, quality of life |
| Intervention: teaches workers to take responsibility for the day-to-day management of problems | The Netherlands | Protocol | ||
| Trained occupational nurse | ||||
| Feicht et al., 201335 | Evaluation of a 7-week web-based happiness training to improve psychological well-being, reduce stress, and enhance mindfulness and flourishing: a randomised controlled occupational health study | Web-based happiness training | 147 out of 1050 employees (15%) volunteered (not sick-listed) | Happiness (d = 0.93), satisfaction (d = 1.17) and quality of life (d = 1.06) improved; perceived stress was reduced (d = 0.64); mindfulness (d = 0.62), flourishing (d = 0.63) and recovery experience (d = 0.42) also increased significantly |
| Germany | ||||
| van Beurden et al., 2013;36 protocol only | Effectiveness of guideline-based care by occupational physicians on the return to work of workers with common mental disorders: design of a cluster-randomised controlled trial | Guideline-based training to improve occupational physicians’ understanding of and adherence to the national guidelines | 232 sick-listed workers with CMD | Protocol but primary outcome will be full RTW. Secondary: partial RTW, number of sick leave days, symptoms and work ability |
| The Netherlands | ||||
| Linden et al., 201437 | Reduction of sickness absence by an occupational health care management program focusing on self-efficacy and self-management | OHMP to improve the health status of employees, increase work ability and reduce absence time | Not clear | Rate of sickness absence in the intervention group decreased from 9.26% in the year before the OHMP to 7.93% in the year after the programme |
| Germany | ||||
| Rantonen et al., 201238 | The effectiveness of two active interventions compared to self-care advice in employees with non-acute low back symptoms: a randomised, controlled trial with a 4-year follow-up in the occupational health setting | Three groups: rehabilitation, exercise or self-care | 143 employees with LBP | Among employees with relatively mild LBP, both interventions reduced pain, but the effects on sickness absence and physical impairment were minor |
| Occupational physician | Finland | |||
| Lagerveld et al., 201239 | Work-focused treatment of common mental disorders and return to work: a comparative outcome study | Work-focused CBT vs. CBT | 208 workers on sick leave for CMD (168 included in analysis) | Duration to RTW. Full RTW occurred 65 days earlier for TG, partial RTW occurred 12 days earlier in TG |
| Delivered by psychotherapists | The Netherlands | |||
| Viikari-Juntura et al., 201240 | Return to work after early part-time sick leave due to musculoskeletal disorders: a randomized controlled trial | Randomised to part- or full-time sick leave (workload and work time reduced by about 50%) | 63 workers with MSDs and unable to perform regular work | Time to return to regular work > 4 weeks: shorter in part-time sick leave group (12 days vs. 20 days) |
Case care interventions that were not based in occupational health settings
The majority of collaborative care trials have been carried out in a health-care setting and they tend to be targeted at depression and anxiety disorders32,41–48 (Table 2). Accordingly, most outcomes were condition-specific measures. Although some looked at impact on disability and function, none reported on work-related outcomes.
| First author and date of publication | Title | Intervention | Sample | Outcome/results |
|---|---|---|---|---|
| Coventry et al., 2015;32 | Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression or comorbid with diabetes or cardiovascular disease | Collaborative care that included patient preference for behavioural activation, cognitive restructuring, graded exposure, and/or lifestyle advice, medication management and relapse prevention | 387 primary care patients with diabetes and/or coronary heart disease and depressive symptoms | Mean depressive scores were 0.23 SCL-D13 points lower (95% CI –0.41 to –0.05 points) in the collaborative care arm, equal to an adjusted standardised effect size of 0.30 |
| Delivered by IAPT workers | UK | |||
| Stewart et al., 201441 | Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial | IMPACT: collaborative care programme involving antidepressants and psychotherapy | 235 primary care patients with depression or dysthymia with or without CVD (119 with) | Treatment × baseline CVD = significant interaction (p = 0.21). TG patients without CVD had a 48% lower risk of an event than UC |
| USA | ||||
| Von Korff et al., 2011 42 | Functional outcomes of multicondition collaborative care and successful ageing: results of randomised trial | TEAMcare: integrated treat to target programme | 214 patients with diabetes, CHD or both, and moderate depression (88% completed all six sessions) | Improvements from baseline on the Sheehan Disability Scale (−0.9, 95% CI −1.5 to −0.2; p = 0.006) and global quality-of-life rating (0.7, 95% CI 0.2 to 1.2; p = 0.005) were significantly greater at 6 and 12 months in patients in the intervention group |
| Intervention nurses and primary care physicians | USA | |||
| Richards et al., 201343 | Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial | Collaborative care including depression education, drug management, behavioural activation, relapse prevention and primary care liaison delivered by case managers | 581 primary care patients with depression | After adjustment for baseline depression, mean depression score was 1.33 PHQ-9 points lower (95% CI 0.35 to 2.31 PHQ-9 points; p = 0.009) in participants receiving collaborative care than in those receiving UC at 4 months, and 1.36 PHQ-9 points lower (95% CI 0.07 to 2.64 PHQ-9 points; p = 0.04) at 12 months |
| UK | ||||
| Fortney et al., 201344 | Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centres: a pragmatic randomized comparative effectiveness trial | Practice-based collaborative care delivered by on-site primary care provider and nurse care manager | 364 patients with depression | Significant group main effects were observed for both response (OR 7.74, 95% CI 3.94 to 15.20) and remission (OR 12.69, 95% CI 4.81 to 33.46) |
| USA | ||||
| Muntingh et al., 201445 | Effectiveness of collaborative stepped care for anxiety disorders in primary care: a pragmatic cluster randomised controlled trial | Collaborative stepped care (CSC) including guided self-help, CBT and antidepressants | 180 patients with panic disorder or generalised anxiety disorder | On the BAI, CSC was superior to CAU (difference in gain scores from baseline to 3 months: –5.11, 95% CI –8.28 to –1.94; 6 months: –4.65, 95% CI –7.93 to –1.38; 9 months: –5.67, 95% CI –8.97 to –2.36; 12 months: –6.84, 95% CI –10.13 to –3.55) |
| Care managers – 31 psychiatric nurses | The Netherlands | |||
| Oosterbaan et al., 201346 | Collaborative stepped care vs. care as usual for common mental disorders: 8-month, cluster randomised controlled trial | Collaborative stepped care | 163 patients with CMD | At 4-month mid-test CSC was superior to CAU: 74.7% (n = 68) vs. 50.8% (n = 31) responders (p = 0.003). At the 8-month post test and the 12-month follow-up no significant differences were found |
| GPs and psychiatric nurses | Holland | |||
| Morgan et al., 201347 | The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial | Nurse-led collaborative care model for depression in patients with diabetes or heart disease | 400 patients with depression, diabetes and CHD | Mean depression scores after 6 months of intervention for patients with moderate to severe depression decreased by 5.7 ± 1.3 points compared with 4.3 ± 1.2 points in control, a significant (p = 0.012) difference |
| Australia | ||||
| Huijbregts et al., 201348 | A target-driven collaborative care model for Major Depressive Disorder is effective in primary care in the Netherlands. A randomized clinical trial from the depression initiative | Web-based tracking and decision aid system that advised targeted treatment actions | 93 patients with major depression | CC more effective on achieving treatment response at 3 months (OR 5.20, 95% CI 1.41 to 16.09; NNT 2) and at 9 months (OR 5.60, 95% CI 1.40 to 22.58; NNT 3). Not statistically significant at 6 and 12 months |
| The Netherlands |
Case management interventions that were based in occupational health
There have been a of number collaborative care interventions carried out in OH settings, although none in the UK30,31,49–54 (Table 3). Interventions were generally targeted at specific conditions, including mental health problems such as depression30,31,49 or musculoskeletal disorders. 51–53 One study focused on women after gynaecological surgery50 and one study included people with a range of conditions. 54 Return to work was the primary outcome in most studies except for Vlasveld et al., which had clinical outcomes. 30 Content of the interventions varied but often incorporated a brief psychological intervention along with medical intervention and, in some cases, workplace intervention.
| First author and date of publication | Title | Intervention | Sample | Outcome/results |
|---|---|---|---|---|
| Vlasveld et al., 201330 | Collaborative care for sick-listed workers with major depressive disorder: a randomised controlled trial from the Netherlands depression initiative aimed at return to work and depressive symptoms | Collaborative care provided by OP. 6–12 sessions: problem-solving, self-help, workplace intervention, antidepressant medications (optional) | 126 sick-listed workers with MDD (4–12 weeks’ absence) | Outcome: depression response (reduction of symptoms by 50%) |
| The Netherlands | Shorter time to response by 2.8 months in TG. No difference in remission or RTW | |||
| Volker et al., 201349 | Blended E-health module on return to work embedded in collaborative OH care for common mental disorders: design of a cluster randomized controlled trial | E-health intervention (decision aid for OP and personalised modules for points) delivered as part of a collaborative care programme | 200 workers with common mental disorders (4–26 weeks’ absence) | Study protocol only |
| The Netherlands | Primary outcome = RTW | |||
| Vonk Noordegraaf et al., 201450 | A personalised eHealth programme reduces the duration until return to work after gynaecological surgery: results of a multicentre randomised trial | Described as multidisciplinary but not sure there is a case manager? | 215 women who had gynaecological surgery Secondary care |
Primary = RTW |
| The Netherlands | Mean 39 days TG, 48 days control | |||
| Jensen et al., 201151 | One-year follow-up in employees sick-listed because of low back pain: randomized clinical trial comparing multidisciplinary and brief intervention | Case management; one or more sessions depending on progress: tailored rehabilitation plan | 351 participants with LBP (3–16 weeks’ absence) | RTW achieved in 71% multidisciplinary intervention, 76% in brief intervention |
| Control = clinical examination, reassurance treatment and rehabilitation by GP | Denmark | |||
| Jensen et al., 201252 | Sustainability of return to work in sick-listed employees with low-back pain. Two-year follow-up in a randomised clinical trial comparing multidisciplinary and brief intervention | As above | As above: 2-year follow-up | 1 year RTW: multidisiplinary intervention, 61%; brief intervention, 66% |
| Denmark | 2 years RTW: multidisiplinary intervention, 58%; brief intervention, 61% | |||
| van Beurden et al., 201253 | A participatory return-to-work program for temporary agency workers and unemployed workers sick-listed due to musculoskeletal disorders: a process evaluation alongside a randomized controlled trial | Stepwise process guided by independent RTW co-ordinator | 79 sick listed as a result of musculoskeletal disorders | Satisfaction with RTW co-ordinator; barriers: administrative time investment, no clear information about programme |
| Feasibility study | The Netherlands | |||
| Martin et al., 201354 | Effectiveness of a coordinated and tailored return-to-work intervention for sickness absence beneficiaries with mental health problems | Co-ordinated and tailored RTW programme vs. conventional case management | 196 sick-listed workers (not employed – job centre intervention) | Time to RTW |
| Denmark | CRW: returned slower than CCM | |||
| Wang et al., 2007 31 | Telephone screening, outreach and care management for depressed workers and impact on clinical and work productivity outcomes | Telephone-delivered case management | 604 workers (not sick-listed) with evidence of depression and psychological distress | TG had significantly higher job retention, more hours worked and lower QIDS score (relative odds of recovery) |
| Master degree-level mental health clinicians | USA |
The majority of studies reported positive outcomes for the intervention group compared with control. However, two studies51,52,54 did not report improvement in primary outcomes for the intervention groups. Martin et al. 54 compared a co-ordinated and tailored return-to-work (CTRW) intervention to conventional case management, and reported that people in the conventional case management group returned to work more quickly than those in the treatment group. It may have been that, as the CTRW intervention was more in-depth, it took longer for employees to work through the different aspects of the intervention and return to work. It may have been useful if data had been collected on recurrent sickness absence in the groups to see if the CTRW intervention resulted in slower return to work but affected further sickness absence. The second study51,52 also showed slower return to work in the treatment group at both the 1- and 2-year follow-ups. The case management intervention in the Jensen et al. 52 trial did not include workplace intervention or any liaison between the employer and employees, which may have affected return-to-work rates.
These case management trials had many elements in common, such as consensus-based care plans and access to brief interventions such as problem-solving, self-help, pain management and brief psychotherapy. Not all interventions included a fully collaborative model including the employee, a general practitioner (GP)/occupational physician, employer and case manager. Another key point for many of the trials was the low adherence to the intervention by participants.
The key findings from the review are summarised in Box 1.
-
Participants: length of sickness absence varied but tended to be relatively short, with most between 2 and 12 weeks. Only one trial49 included employees absent for a longer period, and then only up to 26 weeks of absence.
-
Intervention: access to brief interventions such as problem-solving, self-help, pain management and psychotherapy was common but not all had a workplace component. Those that did include a workplace component identified it as a key aspect of the intervention.
-
Most studies agreed on the need for consensus-based action/care plans, making the intervention patient centred, and that the employee should be involved in all discussions about their health and capacity for work.
-
Many reported low adherence to intervention by participants.
-
Most trials focused on specific conditions, such as ‘common mental disorders’ or musculoskeletal disorders, but were not inclusive of both.
Developing the intervention
Phase 1 suggested several key aspects to the intervention. These constituted the need for:
-
an intervention that addresses multiple needs, supported by medical intervention where necessary
-
patient-centred assessments and care plans
-
a workplace component to the intervention
-
signposting to other services and support
-
interaction between employee, case manager, employer and GP/occupational physician.
The review and discussion from the meeting suggested the need for a three-component intervention that addresses multiple needs, supported by medical intervention where necessary. In usual care there may be little interaction between those involved in helping an employee return to work. The patient will have contact with their GP and/or an OH physician if their employer provides OH support. Other than producing a fit note, the GP will not have contact with the employer and, as a result of the format of the fit note, may not even know what their patient’s job role is. If the employer has OH support, then there may be interaction with the employer.
The usual sickness absence relationships are presented figuratively in Figure 1. A new model, based on that used in case management, was further adapted to include the workplace in the relationships (Figure 2). The model describes a cyclical route with the employee/patient at the centre, with co-ordinated contact and support provided by a case manager. As a cyclical model, other agencies or other support services could also be incorporated in it, for example if an occupational physician is employed by the employer.
FIGURE 1.
Usual sickness absence relationships.

FIGURE 2.
Collaborative care relationships.

As well as altering the usual sickness absence care relationships, the intervention aimed to improve employee feelings of well-being and to support return to work. A collaborative care approach was developed, comprising a client-centred approach including partnership working and proactive follow-up with integrated communication and care between the case manager, client, GP and employer.
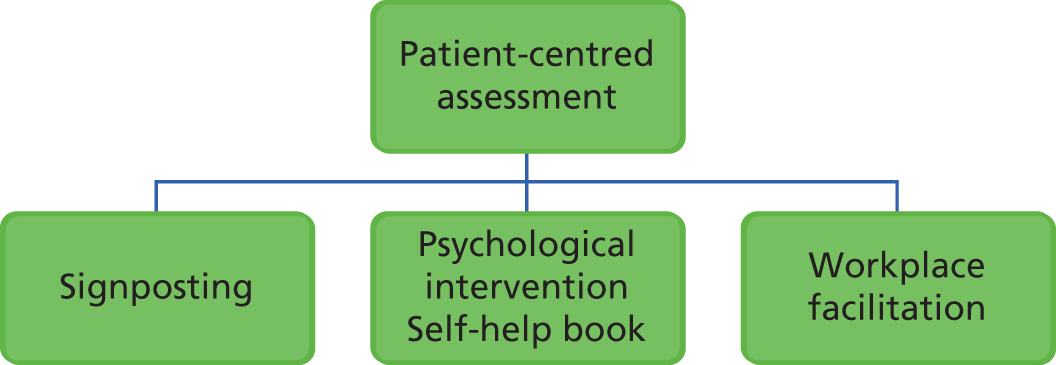
Adapted from an existing psychological intervention trialled previously in primary care, the intervention is client-defined and goal-orientated to improve mental and physical health outcomes. Within this framework, each employee is offered a client-centred assessment followed by a choice of intervention(s) including the psychological intervention, signposting and/or workplace facilitation (Figure 3).
-
Psychological interventions based on brief CBT interventions including behavioural activation and cognitive restructuring. The format is guided self-help, supported by a self-help client manual and telephone sessions with a case manager.
-
Workplace facilitation involving negotiation with the employer about workplace adjustments to assist the employee returning to work.
-
Signposting: providing information or encouraging employees to contact local/national agencies to help in other areas of their lives (e.g. debt advice, domestic violence services, self-help groups, health- or social-care services).
FIGURE 3.
Intervention model.
Intervention delivery
The intervention was to be delivered by a case manager specially trained for the trial and with specialist supervision. Case managers were provided with a therapist manual to support intervention delivery and adherence to the model (see Report Supplementary Material 2).
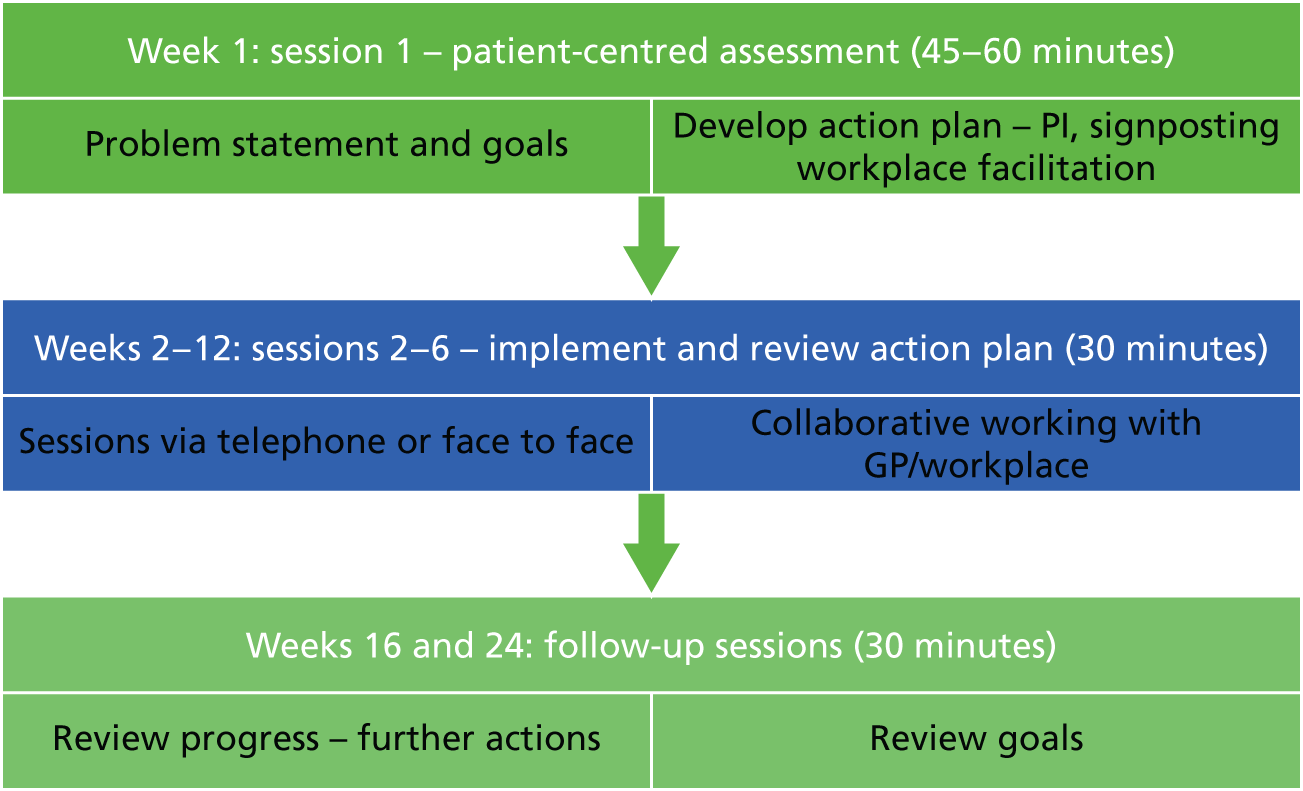
Participants would receive up to six telephone sessions with their assigned case manager, delivered over a 12-week period, with two follow-up sessions, at weeks 16 and 24, to check on progress (Figure 4).
FIGURE 4.
Overview of the intervention sessions. PI, psychological intervention.
Case managers
The case managers were recruited from existing advisors at the two collaborating sites. Two were recruited from the OH provider site to deliver the intervention to trial participants for their customers, and one from FFW. We had intended to have at least two from each site to share the workload and to provide cover for annual leave or staff changes, but FFW was not able to facilitate this as it was a small team and they did not think it was necessary with the small number of cases (10) that they would be assigned.
All case managers attended an initial 2-day training course developed and delivered by applicant Karina Lovell. The training included an overview of collaborative care and specific sections on key psychological principles. The case managers took part in activities and role-playing sessions to facilitate their understanding. During these sessions, they were encouraged to ask questions and to reflect on aspects that were the same as, or different from, their current roles. Case managers were given copies of the therapist manual (see Report Supplementary Material 2) to support them, as well as the client manual (see Report Supplementary Material 1) so that they could support participants in using it.
Supervision
Case managers received supervision from applicant Karina Lovell. Sessions were delivered individually by telephone approximately every 2 weeks for around 15–30 minutes, dependent on the number of cases being managed by case managers. Supervision consisted of discussing problem summaries and goals, discussing selection and application of interventions, and problem-solving any difficulties or barriers that clients or case managers faced.
Chapter 3 Phase 2
In this chapter we present the methods for the second objective: to conduct a pilot study to assess feasibility for a full-scale trial and the acceptability of the newly developed intervention.
Internal pilot objectives
Conduct a pilot study to test:
-
recruitment of employees on long-term sickness absence to a trial
-
delivery of the intervention in an OH setting
-
adherence and acceptability among employees on long-term sickness absence
-
appropriateness of inclusion criteria and outcome measures
-
evaluation of the rate of return to work in those receiving the intervention.
Trial methods
The protocol for the pilot study was developed with input from the research team, the PPIE representative, MAHSC-CTU and the collaborating sites.
Design
The study was a two-arm randomised controlled trial evaluating a collaborative case management intervention for employees who have been on long-term sickness absence. The collaborative care intervention was delivered by existing OH staff with supervision from the research team.
Setting
The study was carried out with two partner organisations. One of our partners (OH provider) had links with several large commercial organisations with approximately 250,000 clients and up to 2000 new referrals per month. To access small and medium-sized enterprises (SMEs – with 250 or fewer employees), our other partner organisation was Leicester FFW.
Recruitment of sites: OH provider
Existing customers of the OH provider were approached and given information on the trial by the company, and were offered a consultation with the research team to explain the aims and methods of the trial in further detail. We aimed to recruit at least two large private or public sector companies.
Recruitment of sites: Fit for Work
As the FFW team currently utilises a similar case management approach for people in Leicestershire, but not in Leicester city (as a result of funding boundaries), it was decided that recruiting from Leicester city would provide a better context with less potential for contamination.
As FFW did not currently operate in the city, identification of people on long-term sickness absence was done through GPs in the area. Support costs were provided by East Midlands Clinical Research Network (CRN) to recruit up to 15 GP practices to conduct mail shots to eligible patients identified through fit notes.
Participants
Recruitment remains the major challenge to successful delivery of trials, especially in mental health,55–58 which highlights the need for the pilot.
Recruitment of patients with long-term conditions has been strongly supported by the method of screening routine records containing relevant information, followed by mass mailing of invitations to participants. 32,43,59 The proportion of patients entering the study can be low (10–30%), raising some concerns about external validity. However, this method is robust, facilitates the planning of larger trials and has been adopted by a number of National Institute for Health Research (NIHR)-funded trials in primary care. 32,43,59 The CAse Management to Enhance Occupational Support (CAMEOS) study was based on this model, and the pilot was adopted to test whether or not the model would translate to a new patient population (long-term sickness absence) and a new context (OH).
Eligibility
Employees experiencing or entering long-term sickness absence were identified using routine recording systems in their employing organisations, or through their GP. Long-term sickness absence was defined as having been off work for at least 4 weeks or being in receipt of a fit note from a GP for at least 4 weeks and up to 12 months.
Participants had to report a minimum level of baseline distress, defined as a score of 11 or more on the CORE-OM of general health and well-being. A minimum level of distress on the CORE-OM was required to ensure that there was significant room for improvement in outcomes associated with intervention.
Inclusion and exclusion criteria are shown in Box 2.
-
Adults aged 18–65 years.
-
Adults who have been off work for at least 4 weeks or who have been signed off for sickness absence for at least 4 weeks and for up to 12 months.
-
Minimum baseline distress level (CORE-OM score of ≥ 11).
-
Currently attending formal psychotherapy through NHS or private services.
-
Requires palliative care.
-
Absent because of bereavement.
-
Suffering from a severe and enduring mental disorder, or at risk of suicide, and requiring immediate care.
-
In advanced stage of pregnancy (defined as > 24 weeks’ gestation).
Identification: occupational health provider
Initial identification of participants was undertaken by human resources (HR) representatives of the employer. Each month their systems were updated to flag anyone who has been absent for 21 days or more. They were asked to check the system for employees who had been off for ≥ 4 weeks to match the inclusion criteria.
Once they had the list of names, HR staff screened the list to exclude those who they did not feel were eligible, those undergoing grievance proceedings or whose employment was to be terminated. The remaining employees were sent an information pack containing a participant information sheet (PIS), consent-to-contact form and participant consent form (Appendix 1).
Identification: Fit for Work service
Initial identification of participants was undertaken by GP Patient Identification Centre (PIC) sites facilitated by the Local Clinical Research Network (LCRN). General practices that agreed to take part were asked to identify patients requiring fit notes. A basic search strategy was developed by the LCRN to be run on practice databases (Box 3), to identify potentially eligible patients. The majority of fit notes are given for short periods of around 2 weeks, and they need to be reviewed and reissued after that time. This meant that identification of patients who had been absent from work for 4 weeks or more was problematic. Furthermore, GP records do not always record employment status of patients. Letters had to be sent to all patients with fit notes and then eligibility was assessed during screening. All patients with fit notes were sent an information pack containing a recruitment pack cover, PIS, consent-to-contact form and participant consent form (Appendix 1).
-
Age – > 18 years.
-
Event location – here (including branch site).
-
Select numerics – and numeric reading of duration of sickness certificate (days) (Read code: Y1712) > = 28days (**you can use the Read code search for MED3 – doctors statement, but that will include all MED3, i.e. less than 28 days as well.)
-
Run search – GP/staff to check MED3.
Participant consent
As information packs were mailed out, participants had as much time as needed to consider participation. The information pack asked them to either contact the research team directly by telephone or e-mail or to return the consent-to-contact form along with the consent form.
Once the employee details were received, a member of the research team contacted them by telephone. Participant consent to participate in the trial was taken after a full explanation had been given, with the opportunity to ask questions in accordance with the Research Governance Framework for Health and Social Care (RGFHS) guidelines. 60 The right of the participant to refuse to participate in the trial without giving reasons was emphasised and respected. If they had not done so already, they were asked to sign and return the participant consent form. If the participant consented, they were assigned a non-repeatable unique identifier (Screening Log ID).
Participants from both centres were also asked to indicate on the consent form if they would be willing to be considered to take part in an interview at the end of the study exploring their thoughts on, and experiences of, the intervention.
All participant materials including the PIS, response and consent forms were edited by the PPIE representative to ensure the language was easily understandable and clear.
Screening for eligibility
Once an employee contacted the research team, with the employee’s consent, they were screened for eligibility. This was done by telephone and took around 15 minutes. The researcher completed the screening case report form (CRF), which included key demographic eligibility criteria and also included the CORE-OM.
Employees who met all of the inclusion criteria were asked if they would like to take part in the trial, and were reminded that they may be randomly assigned to either care as usual or the trial intervention.
Outcome measures
Eligible consenting participants were sent a questionnaire containing the outcome measures (Box 4). Participants were also advised that they could complete these measures by telephone with the researcher if they preferred. Participants received a £20 gift voucher to thank them for completing the baseline questionnaire.
-
Clinical Outcomes in Routine Evaluation Outcome Measure. The CORE-OM is a 34-item measure of psychological distress and comprises four dimensions: subjective well-being, symptoms, functioning and risk. 61
-
The Short Form questionnaire-12 items (SF-12) (version 2) is a brief version of the well-known SF-36 (Short Form questionnaire-36 items). The scale uses 12 questions to measure functional health and well-being over the past 4 weeks. 62
-
Patient Health Questionnaire-9 (PHQ-9), which is a nine-item scale recording core symptoms of depression. 63
-
Work and Social Adjustment Scale (WSAS) is a short, five-item measure of impairment in functioning across five domains (work, home management, social leisure, private leisure and relationships). 64
-
Self-reported actual and effective working hours quantified by the absenteeism and presenteeism questions of the World Health Organization’s Health and Work Performance Questionnaire. 65
-
Client health- and social-care utilisation was included for cost-effectiveness calculations (adapted from the Client Service Receipt Inventory). 66
-
EQ-5D-5L (EuroQol-5 Dimensions, Five-Level version) measure of health-related quality of life. The five-item scale covers mobility; self-care; usual activities; pain; and anxiety and depression. Each has five levels of severity, and the scale provides a utility value based on a population tariff. 67
-
The Bayliss measure of multimorbidity was used to assess the impact of physical symptoms and associated long-term conditions. The measure assesses the presence and impact of 22 common problems (baseline only). 68
-
Client Satisfaction Questionnaire (CSQ-8): an eight-item assessment of client/patient satisfaction with mental health interventions (follow-up only). 69
The primary outcomes for the study were well-being and return to work. However, we collected data on a number of different measures, such as client health- and social-care utilisation (via the Client Service Receipt Inventory), although no economic analysis was planned during this pilot phase. The main function of economics in the pilot study was to explore our ability to collect relevant data.
Randomisation
Participants were randomised by the research team via a central telephone-based system provided by the MAHSC-CTU. The method of randomisation was permuted blocks within strata, with block sizes themselves varying randomly between prespecified limits. There were two stratification factors: partner organisation (OH provider, the FFW team) and baseline CORE-OM score (11–17.9, 18–23.9 and 24–40).
As a result of the nature of the intervention, it was not possible to blind participants to the arm of the trial they were in. After randomisation they were sent a letter informing them of the outcome: that they would either continue with their usual care or receive a copy of the patient manual along with an explanation that they would soon be receiving a telephone call from a case manager. As a small-scale pilot study, blinding of the single researcher involved in the study was not considered feasible.
Intervention: collaborative case management
The intervention was collaborative case management. The intervention involved core aspects of published ‘collaborative care’ models, including:
-
a 60-minute client-centred assessment
-
collaborative goal-setting (to agree on what support is needed)
-
evidence-based low-intensity interventions (such as behavioural activation, problem-solving and cognitive restructuring)
-
effective liaison and information sharing with key health-care personnel such as GPs and other primary care providers (where appropriate, and with patient consent).
These elements are central to all effective collaborative care interventions and the principles of effective chronic disease management. Following the assessment session, the intervention consisted of up to five 45-minute sessions to assess progress and solve problems that may arise in achieving their goals. To maximise the ‘reach’ of the intervention, the sessions were delivered by telephone. The intervention also involved the option for workplace facilitation, where the case manager (with client agreement) mediates between employer and employee to identify barriers to return to work. The case managers, in collaboration with the participants, were free to give the most suitable forms of interventions as described above.
Participants completing the 12-week intervention were monitored further and contacted by their case managers at 16 and 24 weeks after the start of the intervention. The case monitoring was considered part of the intervention and done through telephone calls to enquire about participant well-being and any progress made towards return to work, but no data were collected from these monitoring calls for this pilot trial.
Care as usual
The intervention was assessed against ‘care as usual’ in the organisations where we recruited. Variation in care as usual was expected, dependent on a number of factors such as reason for absence (predominantly physical, mental or work related), or whether they were receiving care mainly from primary care or through employer-provided OH packages. Although such variation in the trial reflects usual practice, data were collected about any care received during the 3-month intervention period on the follow-up questionnaire.
Follow-up measures
The outcome measures listed above were repeated, with the addition of the Client Satisfaction Questionnaire (CSQ-8), an eight-item self-administered questionnaire. Questionnaires were posted to participants at either 12 weeks (care as usual) or on completion of the intervention (treatment group). Participants were asked to complete and return the follow-up questionnaire in the pre-paid envelope provided to receive a £20 gift voucher as recompense for their time. A reminder was sent by post if the questionnaire was not returned within 2 weeks of posting.
Participants who dropped out of the intervention before 3 months were asked if they would still be willing to complete the measures and if they wanted to give a reason or take part in a short interview about why they had withdrawn from the study.
Nested qualitative study
The aim was to interview a subsample of around 20 participants who received the trial intervention to get feedback on their views and experiences of the intervention and trial participation. At the time of consent, participants were asked if they would be willing to be contacted for an interview. If they consented, a note was made and this was checked when selecting participants for interview. If they were selected, the research team then contacted them by telephone. The purpose of the interview was explained and they were asked for further verbal consent to confirm that they were happy to take part in the interview. With agreement, the interviews were recorded over the telephone and later transcribed or, if preferred, written notes were made by the researcher during the telephone interview.
Data management and analysis
Quantitative
Data were input into a database by MAHSC-CTU from the CRFs completed by a researcher and questionnaires completed and returned by participants.
Relevant data were recorded on the CAMEOS CRFs provided by the MAHSC-CTU. All entries on the CRF, including corrections, were made by an authorised member of trial staff. Screening and follow-up data collected by the research team were collected directly on to the CRF and, therefore, treated as source data. Participant-completed questionnaires were also treated as source data.
Data provided to the MAHSC-CTU were checked for errors, inconsistencies and omissions. If missing or questionable data were identified, the MAHSC-CTU requested that the data be clarified. On completion of relevant data management processes, the data were passed directly to the trial statistician for analysis (MH).
All data handling and analysis were conducted in line with RGFHS guidelines60 and the Data Protection Act 199870.
As a result of the limited data we were able to collect, analysis consisted of simple descriptive statistical analysis using Stata® [version 13 (StataCorp LP, College Station, TX, USA)].
Qualitative
All interviews were transcribed and analysed thematically.
This study was reviewed and approved by NHS Research Ethics Committee: North West – Greater Manchester Central on 25 July 2014 (reference number 14/NW/1008).
Chapter 4 Results
Phase 2 (internal pilot phase)
As well as feasibility and acceptability outcomes, baseline and follow-up data were collected through self-report questionnaires. The primary outcomes were well-being and return to work.
During this phase we tested the following:
-
recruitment of employees on long-term sickness absence to the trial
-
delivery of the intervention in an OH setting
-
adherence and acceptability among employees on long-term sickness absence
-
appropriateness of inclusion criteria and outcome measures
-
evaluation of the rate of return to work in those receiving the collaborative case management intervention compared with those receiving care as usual.
Recruitment to the trial
Evaluation of site recruitment: OH provider
The aim had been to recruit at least two large private or public sector companies that were current customers of the OH provider. Specifications for the companies were outlined and negotiations to begin recruitment were conducted with the OH provider at the first trial management group meeting in June 2014. Feedback from the OH provider was that they had difficulty recruiting suitable customers, and this was, at least in part, due to funding issues. Customers can purchase different levels of support for their employees but, in most cases, the delivery of the trial case management intervention resulted in increased time per employee, which, in turn, increased costs. In a NHS setting, this would be an ‘excess treatment cost’. These are often problematic for trusts and clinical commissioning groups (CCGs), but they are more used to them. Most companies would have had to invest financially if they agreed to take part in the trial. Five large organisations were approached and all felt unable to take on the extra costs associated with participating in the trial. This process took around 9 months and it failed to engage a company to take part.
After this delay we recruited a NHS trust that was an existing customer of the OH provider. We found that it was more willing to engage in research and had support costs available to it that facilitated participation. The process for getting the funding and contracts agreed for its participation took a total of 6 months. The trust was not as large as desired, with around 7500 full-time equivalent staff. It projected that around 4.4–6.0% of its workforce would meet our inclusion criteria, giving us a potential pool of 330–450 employees. Details of the study held on the CRN portfolio resulted in a number of expressions of interest from other NHS trusts, but unfortunately they were not existing customers of the OH provider and, therefore, could not take part in the pilot study. However, this showed that it is an area of research that interested employers, and that long-term sickness absence appears to be a significant concern in the NHS.
The difficulties associated with recruiting customers to the trial were what led to the 9-month delay in starting recruitment. There is a need to ensure that funding is available from the customer themselves or financial support is available from another source in order to get companies involved in this type of research.
Evaluation of site recruitment: Fit for Work
Funding was agreed with Public Health England to support the clinical activity involved in delivering this intervention through the FFW organisation, as it is a non-profit social enterprise. Therefore, there were no funding issues holding up recruitment. In order to identify potential participants for the FFW site, it was decided to use recruitment of patients through GPs in Leicester. This was how they often received referrals, and many of the GPs were familiar with this process. To support practices as PIC sites, we applied for support costs from East Midlands CRN. Using the network, they identified eight GP PIC sites. It was initially planned to recruit up to 15 sites in Leicester.
Although site set-up and recruitment through GPs was quickly arranged, we were unable to begin recruitment of participants as a result of the delays with the OH provider site. As we had designated a 6-month window for all recruitment, the clock would have started once we began with FFW, and as the OH provider site was not ready this would have resulted in the 6-month period expiring before recruitment could even begin with the OH provider. It was decided, therefore, to delay the start of recruitment until both sites were activated.
Recruitment of participants took place between October 2015 and September 2016. Follow-up took place 12 weeks after randomisation, ending in December 2016.
Evaluation of participant recruitment: OH provider
Delivery in an occupational health setting
Negotiations took place to discuss how best to set up the study within the participating organisation. Several points for intercepting employees on sickness absence were considered. However, it was decided that, rather than the OH provider recruiting participants directly, which would affect targets and agreed contractual obligations between the OH provider and customer, recruitment should instead take place at the host organisation. The HR department would identify employees who met the criteria of being absent for 4 weeks or more when they were updated on their monitoring systems, once a month.
As a result of the system update only occurring once a month, there were delays in sending out letters. In the first mailout there was a substantial delay of 5 weeks between the database being updated, the search being done and the letter being mailed out. The result of this delay was that, of the nine responses we received, all employees were ineligible (eight had returned to work and one had left employment). This resulted in us going back to the HR team to discuss how the process could be improved. It was agreed that searches of the database would happen as soon as possible after the database had been updated from the roster and that letters would be sent out immediately following the screening process.
Even with the implemented measures to try and speed up the process, it took 2–3 weeks before employees received their information packs.
Response rates
To assess likely response rates and assess the allocation of resources needed, an initial mailout restricted to 100 employees identified as meeting the study search criteria was conducted.
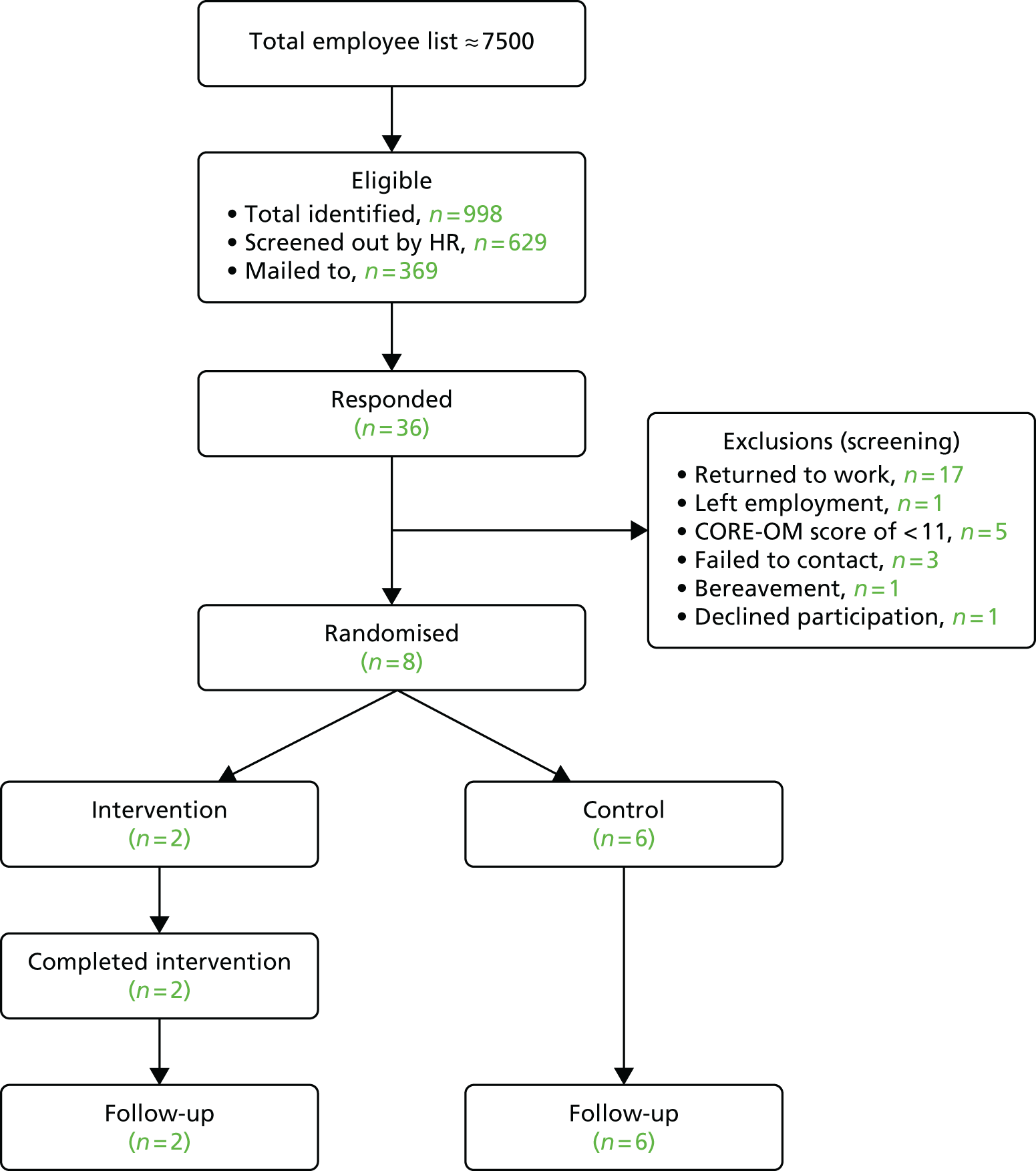
Screening identified 240 employees (3.2%, slightly below the projected 4.4–6.0%). Of the 100 letters sent out to employees, only nine responses were received, a rate of just 9% (in comparison to the 20% normally experienced with primary care studies). Testing the search and mailout process revealed that there were considerable delays that resulted in the information being outdated by the time responses were received from employees. As a result, none of the nine respondents was eligible to take part in the trial, mainly as a result of return to work. No participants were randomised from the initial mailout (see Table 4).
| Search number | Month/year | Identified, n | Screened out, n | Mailed to, n | Responses, n (%) | Randomised, n (%) |
|---|---|---|---|---|---|---|
| 1 | September 2015 | 240 | 140a | 100 | 9 (9) | 0 (0) |
| 2 | December 2015 | 166 | 65 | 101 | 9 (8) | 3 (3) |
| 3 | April 2016 | 132 | 56 | 76 | 8 (11) | 3 (4) |
| 4 | May 2016 | 101 | 67 | 34 | 6 (18) | 2 (6) |
| 5 | June 2016 | 96 | 74 | 22 | 0 (0) | 0 (0) |
| 6 | July 2016 | 111 | 96 | 15 | 1 (7) | 0 (0) |
| 7 | August 2016 | 152 | 131 | 21 | 3 (14) | 0 (0) |
| Total | 7 months | 998b | 629c | 369 | 36 (10) | 8 (2) |
A further mailout was conducted in December 2015, which identified an even smaller number of eligible employees (2.2% of staff). After excluding ineligible employees and those who had already been sent a letter in the first mailout, 101 letters were sent (see Table 4). Again, we received just nine responses, of which three were randomised.
At this point, we reviewed our methods and reported back to the Trial Steering Committee, which consisted of a triallist/methodologist, a statistician and a PPIE representative. At the meeting, we discussed the delays, likely causes of delays and possible courses of action to rectify the low identification and response rates. Following this meeting, a full trial management meeting was held with co-applicants, collaborators and a PPIE representative to feed back the steering committee’s comments and reflect on how the barriers could be addressed by incorporating the expertise of the wider team. An action plan was developed based on the outcome of these two meetings.
Key points from the action plan and how they were addressed
Improving the identification of potential participants:
-
We had hoped that it would be possible for weekly searches to be conducted by the HR team at the employer organisation but as a result of its internal procedures for updating its records, this was not possible. Negotiations with the employer organisation resulted in improving the system so that every month, as soon as its own systems were updated with absenteeism in the company, they would run the search and mail the invitations to eligible employees.
-
To widen the potential pool of participants, the search criteria were changed to identify employees who had been absent for 21 days and over (this is the internal marker for long-term sickness absence and thus integrates with their systems more effectively), instead of the 4 weeks or more previously agreed. The inclusion criteria for the trial remained the same.
-
The company would regularly send internal updates to managers and team leaders. Details about this research were to be included in those updates, raising awareness of the trial and asking senior staff to mention it to any staff who were currently on long-term sickness absence.
Increasing patient response rates and recruitment to the trial:
-
It was felt that some people may have been self-excluding from the study as they did not consider themselves ‘long-term’ after just 4 weeks’ absence. We amended the participant materials, looking at the language used, to make them more inclusive (see Appendix 2). The altered materials were again reviewed by a PPIE representative. These changes had to be approved by the NHS Research Ethics Committee.
-
Instead of sending a whole information pack to employees, a two-stage process was tested. Employees were initially contacted by the employer by letter with a brief flyer that also directed them to the intranet site. If they were interested or wanted more information, they could then contact the research team directly, and the PIS and consent forms were mailed out to them after a verbal explanation had been given and any queries were answered.
-
An internal website on the company’s intranet system was set up. The site was accessible to all employees and it provided them with information about the study. The site also encouraged employees to contact the research team, either through the site, by e-mail or by telephone, if they had any questions or wanted to know more.
-
The LCRN there also offered to provide an experienced band 5 research facilitator from the CRN team to make follow-up telephone calls to potential participants a week or so after they have been sent the recruitment materials. The aim of the call was to ensure that the employee had received the materials and to see if they would be willing to have a member of the research team contact them to discuss the study. As this was not part of our protocol, a further ethics amendment had to be submitted, which took some time to get approval.
The effects of the implemented changes
Following the changes to the methods as described above, we continued recruitment in April 2016 and five further mailouts were conducted (Table 4). Immediately following the changes, the response and randomisation rates both improved, peaking at an 18% response rate and a 6% randomisation rate. The improved response rates may have been a result of increased awareness of the trial following inclusion in briefings and the introduction of the website. However, this improvement appeared temporary and rates reduced again.
One of the main changes we made was the introduction of telephone calls from an independent party to aid the recruitment process. Unfortunately, because of a delay in getting ethics approval for this amendment, we were only able to implement it on the last mailout in August 2016. Telephone calls were made by a band 5 research facilitator from the CRN. Approximately 1 week after the letters had been sent to the 21 identified employees, the facilitator tried contacting them by telephone.
The information fed back from the research facilitator was that:
-
13 had answered neither their mobile nor their landline telephone
-
two had already returned to work – they were ineligible
-
two opted out – they did not want to take part in CAMEOS study
-
three stated they had not yet received the study pack – the information was sent again
-
one potential participant gave verbal agreement to forward personal details.
As Table 4 shows, a large proportion of potentially eligible employees were screened out by HR before mailout. The main reasons for this were that they were employees:
-
who had been sent an invitation in previous mailouts
-
who were involved in a grievance action with the employer
-
who were unlikely to remain with the employer, for example if their contract was ending and not likely to be renewed.
Recruitment had to close in September 2016 in order to meet the project end dates and allow delivery of the intervention. Any participants randomised to the intervention group required up to 12 weeks to complete participation. Therefore, the study closed with just eight participants randomised from OH Assist, reflecting just 10% of the total 80 we had intended to recruit (Figure 5).
FIGURE 5.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram 1: occupational recruitment.
Evaluation of participant recruitment: Fit for Work
Delivery by the Fit for Work team
As the FFW team was providing its own case management approach for clients, we had to recruit people from outside its coverage. To do this it was decided that recruitment through the GP, one of the ways they generally receive referrals, would be the most efficient method.
Recruitment of GP practices was slow, with East Midlands CRN able to identify only eight practices to take part. A further five practices were recruited from Nottingham. GPs seemed to show little interest in taking part in the research even though it was relatively light-touch with just a single screenshot and mailout. Some of the reasons for this may be the introduction of the new FFW service and the updated fit note system. It is possible that GPs felt that this increased the burden or they felt that working within these new systems provided their patients with enough support.
Response rates
During the first 4-month recruitment period we recruited seven GP PIC sites (1–7 in Table 5). As with the occupational recruitment, identification and response rates were lower than anticipated. From a patient population of just under 44,000, only 373 potentially eligible patients were identified and sent information about the study (0.85% of the population).
| Practice ID | List size (n) | Patients identified (n) | Screened out by GP (n) | Mailed to (n) | Responses (n) | Randomised (n) |
|---|---|---|---|---|---|---|
| 1 | 1344 | 3 | 0 | 3 | 0 | 0 |
| 2 | 1317 | 3 | 0 | 3 | 0 | 0 |
| 3 | 1867 | 5 | 0 | 5 | 0 | 0 |
| 4 | 3578 | 27 | 0 | 27 | 2 | 0 |
| 5 | 4263 | 18 | 5 | 13 | 2 | 0 |
| 6 | 5840 | 85 | 1 | 84 | 3 | 1 |
| 7 | 13,000 | 50 | 10 | 40 | 4 | 1 |
| 8 | 12,745 | 229 | 31 | 198 | 3 | 1 |
| 9 | 10,837 | 125 | 0 | 125 | 0 | 0 |
| 10 | 8764 | 277 | 192 | 85 | 4 | 1 |
| 11 | 8715 | 60 | 6 | 54 | 2 | 0 |
| 12 | 8999 | 87 | 0 | 87 | 4 | 3 |
| 13 | 6866 | 103 | 85 | 18 | 1 | 1 |
| Totals | 88,135 | 1072 | 330 | 742 | 25 | 8 |
Of the 373 sent an information pack, we received responses from only 14 potential participants (a rate of 3.8%), of whom only three were eligible and randomised to the trial.
Exploring possible reasons for the low response rate, we found that one reason may be the demographic of the population in Leicester. Leicester has one of the highest ethnic minority populations in the UK and the third highest number of non-English-language speakers. 71
Another possible, and more general, reason for the low response rates was the near-simultaneous announcement of government initiatives to provide what was perceived to be an analogous service to the target audience in the new FFW initiative.
As described above, we held a Trial Management meeting and a Trial Steering Committee meeting to try to identify and address the barriers to recruitment. An action plan was also created to address the issues of recruitment in primary care.
Key points from the action plan and how they were addressed
Improving recruitment of GP practices
-
To raise the profile of the research, contact was made with Leicestershire CCG through the lead of the FFW team, promoting links with the service.
-
The aim was to actively target the GPs who were consistent referrers to the FFW service. The FFW service has been operating in the Leicestershire area for several years and they have formed relationships with particular GPs.
-
The Leicester FFW service expanded to cover Nottingham. Permission was sought from the CCGs to extend to Nottingham City and Nottingham County. Recruitment was supported by Nottingham LCRN to identify suitable large GP practices. It was agreed that the case managers currently working in Leicester, and already trained for the trial, would also work with any people recruited from Nottingham.
Improving identification of potential participants
-
The search criteria being used by the practices were discussed with primary care colleagues, but no clear improvements were identifed. There were significant limitations in how patient information is recorded around length of fit notes, and the system is not currently set up for easily linking data on the patient records to fit our inclusion criteria. In order to improve identification of potentially eligible patients, hand-searching would need to be done, which is time intensive, and practices were not able to support this themselves.
-
We lost some patients from the trial because they had returned to work by the time the invitation reached them, as a result of the inevitable delays in running searches and sending letters. Furthermore, coding of fit note length was problematic using practice databases, and recent research has shown that GPs often underestimate the length of absence, giving patients multiple short fit notes. 72 The system was not set up to link up consecutive fit notes, so patients were being missed with the original search for notes for at least 4 weeks. To try to better suit the current recording system and avoid missing eligible patients, the search criteria were changed to identify any patients who had a current fit note for 2 weeks, rather than 4 weeks. Changing the search criteria allowed us to identify a larger pool of potential patients (fit notes of ≥ 2 weeks) and avoid potential losses as a result of delays in the mailing system. However, the inclusion criteria remained the same.
-
Quite a large number of patients were being screened out of the sample by the GPs, and one of the reasons for this was a diagnosis of cancer. However, the evidence does not support the need to exclude these patients; in fact, they are more likely to need support in getting back to work following successful treatment. 73 Practices were asked not to screen out cancer patients unless their treatment was unsuccessful and they were therefore unlikely to be returning to work, or if they required palliative care.
Increasing patient response rates and recruitment to the trial
-
The study PIS and recruitment flyer had been reviewed by our PPIE member and by the NHS Research Ethics Committee, but clearly they were not functioning well. As with the documents for the OH provider, we amended the participant materials, looking at the language used, to make them more inclusive (see Appendix 3). These were again reviewed by the PPIE representative. These changes had to be approved by the NHS Research Ethics Committee.
-
We are concerned that potential participants were being put off by the long PIS, so we revised the procedure to instead send just the flyer to patients (once it had been revised), and then follow it up with the PIS/consent form once they had responded. It was felt that, if an introductory telephone call could be made once the patient has expressed interest in the research, it may improve understanding of the study and make patients more willing to participate.
-
We also considered different methods of contacting patients, such as electronically in practices that use e-mail and SMS (short message service) reminders for patients. However, it was felt that this was not currently feasible for the practices.
The effects of the implemented changes
Following the changes to the methods as described above, we continued recruitment in July 2016. The delay was because of the time it took to get all the necessary permissions to conduct the research in Nottingham. A further five GP practices were identified in Nottingham (9–13 in Table 5). Following the changes, the identification rates remained the same at 0.84% (n = 369), and the response rate was a little lower with just 11 responses (a rate of 3%). The conversion rate improved to 45% (n = 5) of responses, perhaps showing better targeting of patients. However, these changes are highly sensitive to the small numbers and care should be taken with interpretation.
Recruitment had to close in September 2016 in order to meet the project end dates. Therefore, the study closed with just eight participants randomised from the FFW arm (five to intervention and three to control – see Figure 6). This reflected 40% of the total 20 we had intended to recruit.
FIGURE 6.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram 2: primary care recruitment.
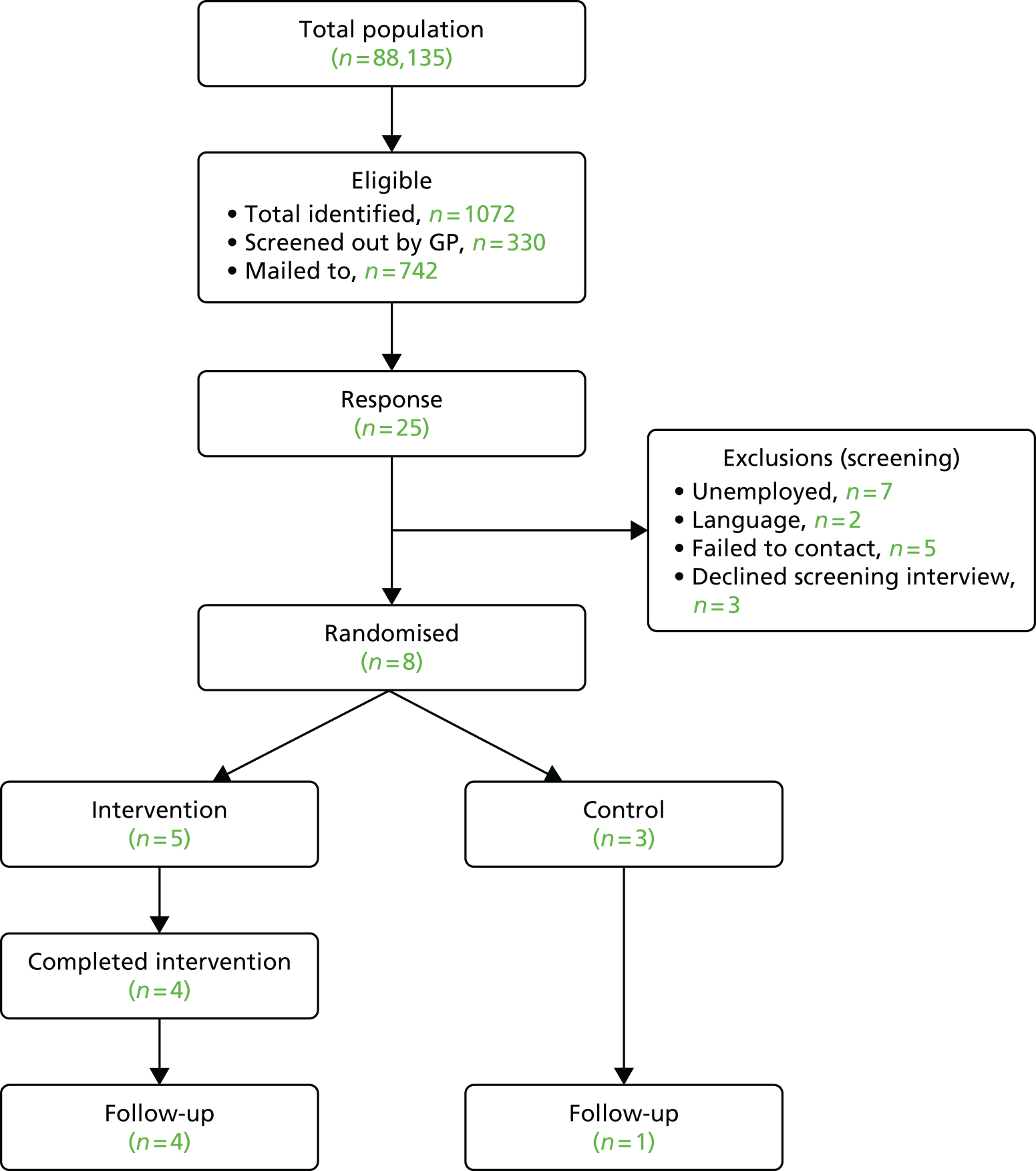
Delivery of the intervention in an occupational health setting
Evaluation of recruitment of case managers
The aim had been to have at least two trained case managers at each of the sites. This was possible for OH Assist as it is a large organisation. However, the FFW team could only release one member of staff for the training.
Both of the two case managers recruited from OH Assist received the full 2-day training along with a refresher training session. The refresher was thought necessary as a result of the long delay between the original training and the randomisation of the first cases. Although both were trained, only one of the case managers actually delivered sessions to participants as we had only two employees randomised to the treatment group in this organisation. This case manager remained in the study the whole time.
Only one case manager was recruited and trained from the second organisation, FFW. The case manager initially selected (case manager 1; CM1) attended the 2-day training session, but was not available for the refresher session 8 months later because of personal reasons. Another member of the FFW team (case manager 2; CM2) was selected and attended the refresher training, in addition to being sent all of the training materials from the first training sessions. This case manager worked on the study for 5 months but left on maternity leave. At this time, the first case manager (CM1) was back at work and took over the role. However, a few months later she left employment with FFW and a third case manager was selected (case manager 3; CS3). This case manager attended a full-day training session and remained on the study until its closure. The changes in staff meant that there were periods where there were no trained case managers at this site, which led to communication gaps and the 16- and 24-week follow-up telephone calls being missed.
Adherence and acceptability among employees on long-term sickness absence
Completion rates
All but one participant randomised to the intervention received sessions with a case manager.
Most (four) received the full six sessions, but one participant had five sessions, and one had four sessions, which they felt was enough.
One participant did not engage with the intervention at all and did not provide a reason for this.
Missed/rescheduled appointments
Few (three) appointments were missed or had to be rescheduled. Participants stated that receiving telephone calls at home was convenient for them and appointments were only missed if a medical appointment or holiday had been planned.
Only one appointment was rearranged as a result of the case manager cancelling the appointment for their own sickness absence.
Qualitative interviews
Brief telephone interviews were conducted with five participants who completed the case management intervention. One participant could not be contacted for interview and one participant did not complete any intervention sessions. This participant was asked if they would be willing to talk about why they had not engaged with the intervention but they did not respond.
Overall experience
All respondents were positive about the sessions they took part in:
I found it really helpful.
Well, I’m self-employed, can’t afford to be off – I have to work or I don’t get paid! Speaking to someone is good because I don’t work for someone so there is no one to go to, human resources or whatever.
016, 61 years old, male, CORE-OM score of 16.57
However, one participant felt that, because of the medical nature of their absence, they did not really need the psychological aspects of the intervention and that this was the main focus of the intervention:
The lady was nice and all that but I don’t think it would help a medical problem only the doctor can do that.
002, 44 years old, male, CORE-OM score of 18.8
Experience of the intervention components
Looking at what options participants engaged in, all participants developed a personal statement as well as setting goals and agreeing an action plan. All participants reported that this was a useful process to some degree, depending on the needs of the individual. For participant 001, who was managing a painful condition (lupus), it helped to focus on specific tasks:
It broke it all down and I could just work on certain things like doing a bit of housework or going to the shops, just the small things but that had become really difficult.
001, 60 years old, female, CORE-OM score of 21.4
The client manual described the use of specific psychological techniques such as goal-setting, behavioural activation and cognitive restructuring. All participants recalled using goal-setting as part of agreeing an action plan, but none of the participants talked about using behavioural activation or cognitive restructuring. Using these other models was elective based on whether or not the participant thought they might help them in their current situation. One factor in this may be that only one participant in the treatment group reported that they were absent from work because of mental health reasons. Although all participants had to register a certain level of distress on the CORE-OM, they may not have considered themselves as needing psychological interventions and instead focused on the physical aspects of their illness. Signposting to other services was also used in four cases, but there is no record of whether or not they made contact with those services.
Although workplace facilitation was seen as a key part of the intervention by the research team, only two participants opted for it. Both of these participants were from the site of the OH provider. It is likely that as the employer is a client of the OH provider, they were more used to input in workplace facilitation and had developed existing relationships and processes to facilitate this. However, there remained a barrier to facilitation where specific job roles were involved:
We talked about what help I needed to get back to work, which wasn’t much really. As I said I had tried to start back but my manager said I am either in work or off work I can’t just do some work. Anyway [the case manager] helped to arrange that. She spoke to my manager to see what could be done.
007, 56 years old, female, CORE-OM score of 11.18
Client Satisfaction Questionnaire (CSQ-8)
At follow-up, all participants were asked to complete the CSQ-8, which measures client satisfaction with services they have received (Table 6). Participants in the treatment group consistently scored higher on satisfaction with services than those in the usual-care group.
| Items on CSQ-8 | Trial group | |
|---|---|---|
| Collaborative care | Usual care | |
| Number randomised | 7 | 9 |
| Number of responses at follow-up | 6 | 7 |
| CSQ-8, mean score (SD); range | ||
| Quality of service you received | 2.8 (1.3); 1–4 | 1.7 (0.8); 1–3 |
| Kind of service you wanted | 3.0 (1.1); 2–4 | 1.9 (0.7); 1–3 |
| Programme met your needs | 2.3 (1.2); 1–4 | 1.6 (0.8); 1–3 |
| Recommend programme to a friend | 3.0 (1.3); 1–4 | 2.1 (0.7); 1–3 |
| Amount of help received | 2.8 (1.3); 1–4 | 1.7 (0.8); 1–3 |
| Deal more effectively with your problem | 2.7 (1.5); 1–4 | 1.9 (0.7); 1–3 |
| Overall, general satisfaction with service | 2.7 (1.5); 1–4 | 1.7 (0.8); 1–3 |
| Come back to programme if seek help again | 2.8 (1.2); 1–4 | 2.3 (0.8); 1–3 |
Acceptability
Although the intervention was generally considered acceptable to those who were randomised to receive it, the fact that we were unable to engage many employees to take part in the research suggests that the model may not have been highly valued. We do not have specific data to this effect and interpretation must be cautious as it is not clear whether the low participation related to low acceptability of the specific intervention under test, lack of interest in taking part in research or wider issues relating to the motivation of employees on sickness absence to engage in interventions which are targeted, at least partly, on returning them to work.
Appropriateness of inclusion criteria and outcome measures
Inclusion/exclusion criteria
The exclusion criteria are described in Box 1. The inclusion criteria were appropriate for the target population for this study but they also restricted the pool of eligible people who could be recruited to the trial.
Reviewing the reasons for exclusion in the OH provider recruitment, the majority were because the employee had already returned to work by the time they had received the information pack (n = 17). Even if employees returned for a few days before going back on sickness absence, they were ineligible to take part in the trial. Considering this, if an employee returns for less than a week before going back on sickness absence, then these people could potentially still be considered eligible. Although we could get no specific figures from the OH provider anecdotally, it said that short return to work followed by further sickness absence is not uncommon.
The main reason that potential participants were excluded from GP recruitment was that GP records did not record patients’ employment status. Seven patients who responded were currently unemployed and, therefore, ineligible for the trial.
Outcome measures
Return-to-work information was collected by the research team by telephone at follow-up. Therefore, this information was missing for the three participants whom we could not contact at follow-up.
Three participants did not complete the follow-up questionnaire: two from the control group and one from the treatment group. All were recruited through primary care rather than by their employer (Figure 6).
Generally, all outcome measures were well-completed at baseline except on the Work and Social Adjustment Scale (WSAS), in which one respondent (in the treatment group) did not complete all five items at baseline.
The primary outcome measure, the CORE-OM, was completed during the screening telephone call at baseline, so it was fully completed. The follow-up questionnaire was completed by mail and there were just three missing data points across this outcome.
Evaluation of the rate of return to work in those receiving collaborative case management intervention compared with those receiving care as usual
As a result of the low recruitment rate, it is not possible to conduct any meaningful statistical significance testing of the quantitative data. Therefore, we present descriptive statistics only, so that the data we have collected are at least available for later research or systematic review.
Participants
Seven participants were randomised to the treatment group and nine to the usual-care group (Table 7). The majority of respondents were female (63%, n = 10) and there were a higher proportion of females in the usual-care group. The participants were mainly of an older age group and of white British ethnicity.
| Participant demographics | Trial group | |
|---|---|---|
| Collaborative care | Usual care | |
| Number randomised | 7 | 9 |
| Demographics | ||
| Age at randomisation, mean (SD); range | 52 (8); 43–61 | 49 (9); 33–58 |
| Gender (female), n (%) | 3 (43) | 7 (78) |
| Marital status [married (vs. single/divorced/etc.)], n (%) | 4 (57) | 4 (44) |
| Ethnic group [white British (vs. other ethnic origins)], n (%) | 6 (86) | 8 (89) |
| Health related | ||
| Sickness duration (weeks), median (IQR); range | 15 (14, 29); 12–39 | 14 (12, 21); 9–52 |
| Reason for sickness, n (% of total) | ||
| Musculoskeletal | 1 (14%) | 3 (33%)a |
| Mental health | 1 (14%) | 6 (67%) |
| Recurrent condition | 1 (14%) | 0 |
| Acute condition | 1 (14%) | 2 (22%) |
| Other | 3 (44%) | 3 (33%) |
Sickness duration at the time of recruitment was similar for the two groups and ranged from 9 to 52 weeks. The main self-report reason for absence was mental health problems (44%; n = 7). However, only one participant randomised to the treatment group had reported that they were signed off primarily because of a mental health issue.
Although there are differences in the characteristics of the intervention and control participants, this is likely to be because of the small numbers randomised. If the full 100 participants had been randomised, these differences would be less evident.
Return to work
After 12 weeks or on completion of the intervention, participants were contacted to find out if they had returned to work. Of those contacted (three participants did not complete any follow-up), only one person in the treatment group reported having returned to work. Five people in the usual-care group reported having returned to work. However, looking at the data provided on the World Health Organization’s questionnaire (absenteeism and presenteeism) at follow-up, three people in the treatment group stated that they had worked some hours during the last 7 days. As mentioned in Appropriateness of inclusion criteria and outcome measures, people sometimes return to work briefly before going back on sickness absence. If this is the case, the answer at the time the follow-up was done would be that they had not returned. More detailed and sensitive data collection would be needed to see if people had returned to work briefly, how long they were back for and how many times they have been signed off. The fact that there was variation in responses between the two measures shows that consideration is needed when selecting the primary measure of return to work. Table 8 shows the rates of return to work as reported by the two different measures.
| Questions on return to work | Trial group | |
|---|---|---|
| Collaborative care | Usual care | |
| Number randomised | 7 | 9 |
| Number of responses at follow-up | 6 | 7 |
| Returned to work Y/N [Y], n (%) | 1 (14) | 5 (56) |
| Reported working some hours in last 28 days (WHO), n (%) | 3 (43) | 5 (56) |
Well-being
Well-being was measured using the CORE-OM. Descriptive data at both time points in the intervention and control groups are shown in Table 9.
| Sub-scales of the CORE-OM | Trial group | |||
|---|---|---|---|---|
| Collaborative care | Usual care | |||
| Baseline | Follow-up | Baseline | Follow-up | |
| Number randomised | 7 | 9 | ||
| Number of responses | 7 | 6 | 9 | 7 |
| CORE-OM, mean (SD); range | ||||
| W subscale | 1.71 (0.64); 1.00–2.75 | 1.33 (0.85); 0.00–2.25 | 2.94 (0.65); 2.00–4.00 | 2.46 (0.81); 1.25–3.25 |
| P subscale | 2.07 (0.48); 1.33–2.58 | 1.41 (0.95); 0.18–2.83 | 2.76 (0.43); 2.08–3.50 | 2.37 (1.03); 0.83–3.58 |
| F subscale | 1.67 (0.49); 1.08–2.33 | 1.01 (0.51); 0.42–1.83 | 2.00 (0.39); 1.50–2.67 | 1.93 (0.64); 1.17–2.92 |
| R subscale | 0.05 (0.08); 0.00–0.17 | 0.06 (0.14); 0.00–0.33 | 0.17 (0.30); 0.00–0.83 | 0.19 (0.28); 0.00–0.67 |
| All items | 1.53 (0.34); 1.12–1.88 | 1.02 (0.61); 0.21–1.97 | 2.05 (0.23); 1.68–2.32 | 1.84 (0.71); 0.85–2.62 |
| All items minus R subscale | 1.85 (0.41); 1.36–2.29 | 1.23 (0.72); 0.26–2.32 | 2.46 (0.25); 2.04–2.82 | 2.20 (0.81); 1.04–3.07 |
The ability to perform activities was measured using the WSAS. Descriptive data at both time points in intervention and control groups are shown in Table 10.
| Domains on the WSAS | Trial group | |||
|---|---|---|---|---|
| Collaborative care | Usual care | |||
| Baseline | Follow-up | Baseline | Follow-up | |
| Number randomised | 7 | 9 | ||
| Number of responses | 6 | 6 | 9 | 7 |
| WSAS, mean (SD); range | ||||
| Ability to work | 5.8 (1.5); 4–7 | 4.8 (2.8); 1–8 | 7.1 (0.9); 6–8 | 5.0 (2.6); 1–8 |
| Home management | 4.8 (2.2); 2–8 | 3.7 (1.8); 1–6 | 5.7 (1.8); 2–8 | 4.4 (2.6); 0–7 |
| Social leisure activities | 5.3 (2.4); 2–8 | 4.3 (2.5); 1–7 | 6.7 (1.2); 4–8 | 5.3 (2.4); 1–8 |
| Private leisure activities | 4.3 (2.6); 2–8 | 4.1 (2.5); 1–8 | 5.0 (2.1); 2–7 | 4.1 (2.6); 0–6 |
| Close relationship | 3.4 (2.6); 1–7 | 2.7 (1.0); 1–4 | 4.3 (2.4); 1–8 | 2.9 (2.6); 0–6 |
No harms or unintended effects were reported in either group.
Changes to the protocol
Substantial amendment 1
Addition of safety reporting procedures
Substantial additions were made to the original research protocol (version 2 24 June 2014), which received approval as part of the Research Ethics Committee application, approved on 25 July 2014. These additions were made in consultation with the MAHSC-CTU.
The trial management and monitoring process required that the trial protocol provide clear instruction about safety monitoring for the trial. These additions did not change the original trial information, as previously reviewed by the REC committee, but resulted in additional measures to ensure that trial reporting was accurate and that there were procedures in place to monitor and report any harm to participants.
Amendment to the measured primary outcomes
At the request of one of our collaborators and with the agreement of the funders, the primary outcome measures for the pilot study were amended to specify return to work as well as the original well-being outcome. This change was seen as crucial to attain good levels of ‘buy-in’ by the collaborator’s customers who purchase those services specifically to support return to work for their employees. Wording on the participant and employer information sheets as well as the protocol were amended to reflect this change. The changes referred only to the stated primary outcomes and did not mean that there were any changes to the intervention delivery or the outcome measures that we were using.
Amendment to the recruitment protocol for the Fit for Work service
The FFW team provides a service for people who are either self-referred or GP-referred to their programme. It does not have contracts with employer companies as it is a not-for-profit social enterprise.
It was decided, in consultation with this service, that the study would be best served by identifying patients in areas that no longer received the FFW service (as a result of funding) but that have referred people to the service in the past. The aim was to provide a better comparator than those receiving the FFW service, which uses a similar biopsychosocial model.
The FFW service had close ties to GPs in the area involved with its original pilot scheme, which ran from 2010 to 2013. In the scheme, GPs were given referral cards. In consultations with patients requiring a sick note, GPs would complete the referral card, which contained contact information for the FFW service, and give it to the patient. The card could then be posted back to the team (and a member of the FFW team would then contact the patient to conduct an assessment), or the patient could contact the team directly.
Without having a direct contract with employers, this was thought to be the best way to recruit patients to the service and to allow access to employees of SMEs. Therefore, it was thought that this also offered the best way to recruit these employees to this trial.
Recruitment through GPs
Original wording
Initial identification of participants will be undertaken by occupational health and/or HR representatives through our host organisation partners.
New wording
Initial identification of participants will be undertaken by GPs in Leicester who have agreed to act as a recruitment site and who have worked with the FFW service in the past. The FFW service will contact GPs that they have worked with in the past, or are currently working with, to tell them about the pilot study. If they agree to take part in recruiting patients, they will be asked to identify employees requiring sick notes and ask if they would be interested in receiving information about the trial. If they assent, employees will be given an information pack containing a Recruitment Pack Cover, PIS, consent to contact Form and participant consent Form. The pack will also contain a cover sheet, which includes the FFW team contact details (as the local provider of the intervention and that with whom they may have had contact with in the past).
Oral consent for the FFW team to pass contact details to the research team
As mentioned in Amendment to the recruitment protocol for the Fit for Work service, it was decided to include contact details for the FFW team directly. If a suitable candidate enquired about the pilot study, we wanted to be able to get permission to pass on the potential participant’s contact details to the research team. It was hoped that this more direct contact (as opposed to relying on people to complete a form and post it back to the research team) might improve recruitment rates or at least speed up the process.
Original wording
Participants will be approached to seek consent for the trial by post or telephone by their OH provider, and will be given a PIS. If patients are interested they will be asked to return the consent to contact form.
New wording
If employees prefer to contact the FFW team directly they will be asked for verbal consent to pass their contact details on to The University of Manchester research team. Information packs will be posted to the participant by the FFW team for the patient to consider before making a decision. The University of Manchester research team would contact the potential participant and answer any queries the participant might have.
Amendment to the recruitment protocol for the OH provider company
The recruitment process for the OH provider remained the same, with the companies that receive their services being recruited directly and then employees being recruited on an individual basis.
However, as part of the pilot process, we were looking at the most efficient way to work with our partners to identify and recruit participants to the trial, while fulfilling their obligations to their customers (the companies receiving OH support). The OH provider was concerned that the use of mail in the initial stages of contact would severely affect their targets and their obligation to contact referrals to their services within a narrow timeframe. They required a process that ensured that the employee met the eligibility criteria, received the pack and gave consent for contact by the research team almost immediately so that they could be scheduled into the pilot trial or usual service provision straight away.
The OH provider suggested that after the initial screening to see if participants may be eligible (which would be conducted by telephone as part of their normal procedures), they post out the packs to those potential participants and obtain verbal consent to pass on their details to the research team, who would then telephone the potential participants to complete the screening and consent processes. Participants were not officially enrolled into the trial until their signed consent form was received by the research team.
Original wording
If patients are interested they will be asked to return the consent to contact form. They will then be contacted by a member of the research team and screened for suitability to take part in the intervention.
New wording
If patients are interested they will be asked for verbal consent for a member of the research team to contact them, with the view that this would be followed up by written consent if they wish to take part. OHA would then send out an information pack (PIS, consent to contact form, participant consent form) and pass on the employees’ details to the research team, who would answer any queries the person has and complete the screening and consent process.
Substantial amendment 2
The client handbook was submitted for approval. No changes were made to the protocol.
Substantial amendment 3
Following the evaluation of recruitment methods and subsequent creation of an action plan, we amended all of our patient recruitment materials and the study protocol.
Amendment to the information mailed out
As well as continuing with the original method of sending the flyer, PIS and consent form out together, we decided to try sending the information out in two stages. It was thought that, as a lengthy document, the full PIS might be off-putting or confusing because of all of the information we had to provide to potential participants. It was decided that sending advertising materials only (in the form of a flyer) might better engage employees and encourage them to contact us to find out more about the study.
Therefore, we decided to trial a two-stage process whereby a flyer and consent-to-contact form were sent to the participant. The flyer also provided e-mail and telephone contact details. If they then responded to the research team with a request for more information, the researcher would talk them through the information on the PIS. If the person was interested, they were then sent a copy of the PIS and consent form, and asked to return the signed consent form.
Amendment to the parameters for the GP database searches
The search criteria were changed to contact patients who had a current fit note for 2 weeks, rather than 4 weeks. This change was made as some patients were lost to the trial because they had returned to work by the time the study invitations reached them, as a result of the inevitable delays in running searches and sending letters. Furthermore, coding of fit note length appeared to be problematic in practice databases. The practice systems were not set up to link consecutive fit notes, so patients were being missed. The aim of changing the search criteria was to allow us to identify a larger pool of potential patients (fit notes of 2 weeks or more) and avoid losses as a result of administrative and postal delays. The inclusion criteria remained the same (people who were absent from work for 4 weeks or more), it was just the search criteria that changed.
Substantial amendment 4
Amendment to employee contact procedures
We had been working with a CRN to help facilitate recruitment at one of our sites. Its input had been limited to providing support costs for identification of potential participants. However, because of the poor response rates from the invitations we sent out to employees, the CRN offered to further support the study by making follow-up telephone calls to employees who had been identified and sent information packs, but who had not responded.
The calls were made by an experienced band 5 research facilitator from the CRN team who was independent from the employer and from the OH provider.
This amendment was originally rejected by the NHS Research Ethics Committee, as it felt that being contacted multiple times may be burdensome to employees. However, as we were not using any other active form of contact, this decision was reviewed and eventually approved, although after a considerable delay.
Original wording
Initial identification of participants will be undertaken by OH and/or HR representatives working for OH Assist. Participants will be informed about the trial by post or telephone by their OH provider. If they are interested, they will be asked for verbal consent for a member of the research team to contact them, with the view that this would be followed up by the written consent if they wish to take part.
New wording
Initial identification of participants will be undertaken by HR representatives of the employing organisation, who will mail out a flyer with a consent to contact form and return envelope. Approximately 1 week later they will receive a follow-up telephone call made by CRN. The employee will be asked if they received the information and if they may be interested in the trial. If they are interested, they will be asked for verbal consent for a member of the research team to contact them, with the view that this would be followed up by written consent if they wish to take part.
Chapter 5 Discussion
In this chapter we will review each of the study objectives and discuss the implications of the findings.
Phase 1: development of the intervention
Our first objective was to adapt a collaborative case management intervention to the needs of UK employees, in a range of occupations and organisations, who are entering or experiencing long-term sickness absence.
The intervention model was designed using evidence from the literature (see Chapter 2) and the expertise of the research team. The literature from the scoping review was insufficient for a formal analysis of ‘active components’ but was sufficient to identify some core principles, which were also supported by the expertise within the trial team. 74 The core principles of the intervention were to establish a collaborative care model based on case management for patients with long-term conditions, combined with factors specific to the occupational setting.
Key to the intervention were the initial patient-centred assessment and consensus-based action plan, agreeing the participants’ needs for support. As these took place in the initial sessions and provided the structure for the case management sessions, they were always completed, and participants reported liking the process and said that it did benefit them in some way.
The brief psychological interventions were featured in the participant handbook and were also supported by the case managers, but results show that no participants elected to use the psychological interventions during their case management sessions, although participants did report using the client manual. However, the majority of participants in the treatment group were not signed off for mental health reasons.
Workplace facilitation supports the fit note system introduced in 2010, which aims to focus on how a patient ‘may be fit’ if adjustments can be made to their working environment, hours or duties. Rather than the GP making recommendations and then leaving it to the patient to negotiate with their employer whether or not adjustments are possible, this model aimed to have the case manager act as an intermediary to facilitate the process. However, as not all patients ‘may be fit’ and need to be fully signed off from work, this process was not suitable for all participants. We found that only two participants elected to use this process and they were both employees of the OH provider’s clients. It is likely that, as a long-standing relationship exists between the client and provider, they are more readily able to facilitate this process. None of the participants recruited for the FFW team engaged in this process, and this may be because either the participants or the employer were not used to it. Grievance with the employer may also affect willingness to engage in workplace facilitation.
Signposting to external agencies to support other aspects of participants’ needs remained an important part of the intervention, utilised by all of the case managers for four of the participants. However, there was no clarification of whether or not participants then engaged with these services.
Overall, experience of intervention delivery suggests that it was broadly acceptable among the minority who opted to participate, and adherence was reasonably high. However, there were some barriers to the delivery of the psychological support and workplace facilitation that would need to be overcome if the model was to be maximally effective.
Phase 2: internal pilot
Recruitment
Although phase 2 had a number of objectives, recruitment remained key to assessing the viability of any definitive trial; (as with many randomised trials) this was by far the greatest challenge. 75 In the following section, we discuss the major obstacles to successful recruitment.
Working with partner organisations
We worked with two different partner organisations: a private company providing assessment and treatment for the employees of their customers and a non-profit social enterprise set up as one of the sites for the original FFW pilot.
Working with these different bodies allowed us to access employees of large companies, as well as employees of SMEs or self-employed people who rely on services provided by the NHS. Although recruitment at both of these sites was low, we did manage to reach participants from each of these groups.
Set-up of the study with the commercial partner faced challenges. Difficulties associated with recruiting customers to the trial (mainly because of problems associated with the financial burden of taking part in research) led to the 9-month delay in starting recruitment. The study depended on an agreement between the university and the OH provider and we were restricted to working with clients of that OH provider. When it proved difficult to recruit, we were unable to expand to other providers and their areas of coverage, which would be a common response in trials facing recruitment problems.
It is not unusual for companies to be reluctant to take on the burden of research. The BOHRF was a charity set up to support OH research and was dependent on financial contributions from sponsors with matched funding from organisations for particular research projects. The foundation folded in 2012 because it was unable to raise sufficient funding to continue. For the NHS sites, it is a requirement to be involved in research and the provision of support costs makes involvement in research much easier to organise, although excess treatment costs are still required. The incentives for commercial organisations to take part in research may be more limited. Although the case for many return-to-work schemes is economic in nature, companies were already paying for such services and needed stronger evidence that an enhanced scheme (at greater cost to them) would show a return on investment. Providing such evidence in the context of an early-stage feasibility trial is clearly difficult. There may be a need to identify innovative forms of support to help companies to get involved in this type of research.
The new FFW scheme includes an employer tax exemption for health-related interventions recommended by the service. The exemption is for £500 per employee per year. This exemption was introduced in 2013; however, knowledge of this remains low among employers. Looking at employer and GP knowledge of the FFW service and information such as the tax exemption, Paton76 reported that out of the 680 employers surveyed, only 14% had any knowledge of the exemption. Thus, where there may be some incentive for employers, awareness of these benefits remains low. We do not have information on whether or not this knowledge would have increased interest in this research from companies. However, as the main reason given by the OH provider as to why companies were not interested was the financial implication of taking part, raising awareness of this tax exemption may help future research.
Recruitment process
A common metaphor in the recruitment process concerns a ‘pipeline’ connecting a ‘pool’ of eligible participants with the study, with ‘leaks’ in the pipe representing losses at various stages of the recruitment process.
As noted earlier, difficulties in recruiting companies led to significant delays in beginning recruitment. Importantly, it also limited the available ‘pool’ of potentially eligible patients as a result of the restricted size of the NHS site we did recruit.
However, we eventually identified around 1000 potentially eligible participants through our recruitment schemes. The crucial parameter then becomes the ‘conversion rate’ from potentially eligible participants within that ‘pool’ to randomisation (via a formal assessment of eligibility and then patient-informed consent), which, in turn, depends on the losses at each stage of the process. Conversion rates vary by study, but a selection of collaborative care trials in primary care report rates from 0.9–11.5%. 30–32,43,49
We could have achieved close to our required sample size with a ‘conversion rate’ of 10%, which is lower than that of comparable trials, but not markedly so. However, the fundamental problem was that the conversion rate was very much lower than expected.
We also faced an additional limitation. Most studies using these methods can increase recruitment in the context of a low ‘conversion rate’ by extending the number of sites. However, the set-up of the study meant that we were restricted in this important regard in both the commercial and general practice settings, and could not easily seek additional ‘pools’ to overcome these problems. Our commercial provider provides services only for a limited number of companies; there were barriers to recruitment of some of these companies related to issues of cost and the focus of the intervention (on well-being as well as return to work). Our other provider was less restricted in some ways, but the study funding provided for only a limited number of cases through this route (20% of the target) and it was not possible to change that proportion.
We made various changes to study procedures to improve matters. However, the modest nature of these methods meant that such changes (for example, better wording of an information sheet) are unlikely to have a profound effect, given the very low rates of response to the initial invitations. 77 If our package of changes had led to an increase in recruitment of 25% (which would be a very large increase), the impact on recruitment targets would have been small.
One innovation that might be expected to have a large impact involved CRN staff calling patients,78 where there is a reasonable evidence base already, including a study testing this approach using employees on a sick list. 79 However, such methods are not available to university research staff because of governance issues. These methods are also resource intensive and were offered in only one setting (as that is where the study had CRN support) because of poor recruitment. This meant that by the time they were offered and implemented it was too late to have a large impact. It should be noted that, despite published evidence of effect, our experience of the impact of such telephone calls was not positive. This may, again, reflect the fact that, in the context of long-term sickness absence, the barriers to participation may be very different from those in other settings.
Understanding barriers to recruitment would have been enhanced by qualitative work. 80 However, the impact of such work may have been more limited than in other settings. Some of the most successful examples of qualitative research affecting recruitment have involved face-to-face recruitment methods (such as clinicians discussing trials with patients) where researchers have observed the process. However, the methods used in the current study provided no analogous discussions to observe, potentially limiting the utility of such methods. It is difficult to gain insights into barriers to participation from interviewing participants who have elected to enter the study, as it is likely that their experience will be very different. Although it is possible, there are barriers to accessing the views of non-participants, as they are, by definition, less likely to take part. 81 Ethics committees can also have concerns with accessing such patients if it is seen as requiring patients to justify non-involvement. Nevertheless, our understanding of the barriers to participation is limited, and it could be argued that an opportunity was missed to explore these barriers by not building in qualitative research of the process to the study from the outset.
There was a suggestion that the occupational context is a slightly more contentious one for recruitment, as work-related issues (such as difficulties with work stress, or difficulties in work relationships with managers or colleagues) may be perceived to be a major contributor to sickness absence. An offer of assistance routed through the organisation may not be perceived as attractive, especially among employees where work-related issues are making a significant contribution to distress and for whom return to work is not an immediate priority. This relates to the wider issue of the incentives or disincentives for employees to return to work, and the potential impact on sick pay or incapacity benefits they receive. Although it is possible that such issues have an important effect on research in this setting, we do not have specific data from our study to support or refute this hypothesis.
Other trials in this field have experienced low recruitment rates, ranging from a 0.9% to 11.5%30–32,43,49. These problems are also mirrored outside the research context. One key finding from the evaluation of the FFW service report4 was that uptake was significantly lower than expected. In total, only 6726 people accessed the service offered, which was about 40% of the target 17,000. Also, rather than people being on sickness absence from work, two-thirds were people struggling at work with a health condition (presenteeism). Among the absentees, fewer than 30% had been off work for between 4 and 12 weeks, which was the target group for the FFW pilot. 4
Work around the poor recruitment rates for the FFW pilot showed that there were substantial difficulties in communicating with relevant agencies about the pilot service being offered. 76 A high percentage of employers, GPs and employees remained unaware of the service during the trial period. Once people were made aware of the service, there appeared to be some ambivalence about using it. Of note was the report that GPs who were aware of the service did not plan to use it; the main reason being given was that they believed it was the responsibility of the employer to address workplace absence, not theirs. 76
The report also discussed the difficulties experienced in trying to secure referrals from GPs. It was reported that it was difficult to access GPs and to gain and sustain their interest to ensure that the FFW service remained a prominent option. They also reported difficulties in engaging with employers, particularly at the outset. 4
Obviously, even the low level of recruitment demonstrated in this study could be scaled up with enough large employers and enough practices across England; a large trial could be conducted in principle, although this would be a major logistical undertaking, requiring a large number of organisations and general practices, and raising costs and risk. However, the rates found in this pilot also suggest significant concerns about the selection processes affecting recruitment. Although there are no ‘hard and fast’ rules on the level of recruitment required to provide reasonable levels of external validity, and many trials report rates that would raise major concerns about this bias, there would be significant concerns about generalising results from such a small proportion of eligible patients to the wider population struggling with long-term sickness absence.
Strong evidence to improve recruitment and retention is lacking. 78,82 In Alternative recruitment methods, we discuss possible solutions to the problems identified in the trial.
Process issues
As a result of contractual obligations between the client and the OH provider, we were unable to place recruitment within the normal referral processes. Sitting outside of usual processes made ‘interception’ of employees entering long-term sickness absence difficult.
We were working with the Leicester FFW service; thus recruitment was initially in this area. However, Leicester has a large ethnic minority population and high levels of non-English-language speakers. It was not possible to recruit non-English speakers as it was not possible to deliver the intervention in other languages. As a small team, they were able to release only one member of staff for training and to deliver the intervention and, therefore, there were periods when there were no trained case managers at that site.
Alternative recruitment methods
It is possible that face-to-face recruitment is a more effective method of engagement for some populations. However, we note that, in primary care, most researchers have moved away from face-to-face recruitment to postal recruitment. Although postal recruitment is less effective in terms of conversions (recruits compared with numbers invited), the ability to invite large numbers of patients more easily means that it is a reliable and consistent method of recruitment.
In contrast, although face-to-face recruitment can be effective in certain circumstances, it is very dependent on the skills and attitudes of individual GPs. The experience of many research teams is that this method can be highly unreliable. It was this previous experience that led the team to adopt the postal methods for this pilot.
Additionally, the use of face-to-face recruitment was not a practical option in this study. Face-to-face recruitment was not possible at the OH provider site as, by the time they were identified, those employees were not attending work at all. All OH support was provided by telephone from the company, meaning there was no opportunity at which we could see them face-to-face within the existing processes.
Likewise, with recruitment from GP PIC sites, the research was taking place in a different region from that of the researchers and it was not feasible to have the researcher repeatedly attend clinics in Leicester. Even if it had been feasible to attend GP surgeries, the number of patients attending surgery who met the inclusion criteria would have been very small, meaning this method was likely to have been inefficient. It is possible that we could have recruited GPs to raise the study during consultations with relevant patients, but, again, experience shows that this method is unreliable and does not scale well. 83,84
We had support costs for a single-shot mailout from each practice, so GPs were not actively recommending the trial to patients, and there was no follow-up to try and get patients engaged for the most part (although we did test telephone follow-up by a research nurse). Although more active follow-up may have improved issues,78 the effect would have to be quite profound in order to overcome the very low rates we identified.
Other options for research in this area would include cluster and stepped-wedge trials. 85 Randomising organisations (or departments within organisations) and then delivering new interventions within the organisations might overcome some of the problems identified. For example, once an organisation has been allocated to intervention, all employees could potentially be allocated to a new model of case management, while control clusters could continue with care as usual. Assuming appropriate ethics and governance agreements could be put in place, such a model could overcome some, but not all, of the recruitment issues, such as patient concerns about randomisation. Although employees would not face the decision about randomisation, they would still need to consent to data collection, so it cannot be assumed that all recruitment barriers would be overcome. Such a trial could forgo primary data collection and use routine data on return to work as the outcome, but that would severely restrict the scope of the analyses. A stepped-wedge design might have advantages in terms of convincing organisations to take part, as all would receive the new intervention.
However, moving away from an individually randomised trial does raise additional challenges, including ethics-related issues,86 potential for selection bias87 and the need for a large number of clusters. The effects of the last issue could be quite profound if the unit of allocation was the organisation, as recruitment of a large number of organisations would be a challenge. There might be options for smaller units of allocation within organisations (such as departments or teams), but that might be more difficult for organisations to justify in terms of equity of access among their staff. In addition, smaller units of allocation might struggle to recruit significant numbers of staff in the conventional recruitment window of a trial of this type.
Other options could include a shift in recruitment from the occupational setting to primary care. Although it was a challenge to identify long-term sickness absence by searching primary care records, focusing on health problems, such as depression and musculoskeletal problems, might provide an alternative to the recruitment of patients on long-term sickness absence. 88 However, that would be a significant change from the original research brief.
Eligibility criteria
One reason for the small number of participants identified as eligible could be that the original brief for the study excluded people who had returned to work at all within a 4-week period and anyone who was unemployed. Even with the much wider inclusion criteria in the FFW pilot, it was not possible to recruit to target.
It may be easier to recruit participants who have been off work for shorter periods, or those whose return to work is intermittent or who are suffering from degrees of presenteeism. However, this was not the original research brief and would have required a significant change in the aims of the research. Participants on long-term sickness absence are those who have the highest costs. Although it may be possible to recruit patients with shorter-term or more intermittent absence, showing that intervention would be cost-effective among such patients may be difficult given the fairly significant costs associated with the case management intervention.
Patient and public involvement and engagement
The study had PPIE involvement from the beginning and throughout. The study was presented to the PRIMER (Primary Care Research in Manchester Engagement Resource) group, before the funding application was made, for its feedback and input into the aims of the study and proposed methods. A PPIE representative became a dedicated member of the team, involved with all participant material development, including the language and images used; they also participated in the meetings to discuss ways to improve recruitment rates. However, the study continued to fail to engage employees.
There may have been benefits from holding a series of workshops with a large group of people with experience of long-term sickness absence, which could have fed directly into the design of the intervention at an early stage. Workshops could have been used to discuss how the study was marketed, to gauge if people would want this type of intervention (supported self-help model) and to discuss specific issues around gender, ethnicity or social class.
Limitations
The pilot showed that the chosen recruitment methods were not functional in the occupational context (which was one of the main aims of the study). One of the limitations of what we learnt from the pilot is that we were restricted to working with current clients of the single OH provider. Several additional NHS trusts contacted us with expressions of interest to host the research, but unfortunately we could only work with clients of the one OH provider. This made overcoming recruitment barriers difficult, despite interest from other sites, although it should be noted that those sites were likely to have faced the same problems with the recruitment process.
Responding to problems identified during the pilot faced the conventional delays in getting ethics and other agreements to change recruitment methods. Although some of these may have been managed with better planning, some could not be foreseen. For example, the offer to use CRN staff to call patients was made only in response to problems, when recruitment was still low after multiple mailouts. This meant that this ‘emergency’ procedure could only be used with a small number of participants.
Although the data we present suggest that the case management intervention was feasible and acceptable to respondents, the very low recruitment rates mean that the people in the study are unlikely to be representative of the target population. Therefore, no firm conclusions on feasibility and acceptability of the intervention can be made.
Consideration of the barriers
Engaging with more than one occupational health provider
Prior to funding, we had discussed with the provider the size of their client list and the numbers of employees referred to the service each month. At this point, the provider had not engaged with its customers, and thus its views on participation in this kind of research study had not been explored. With hindsight, earlier engagement with customers may have allowed us to identify some of the barriers earlier in the research process. It is worth noting that this was the first collaboration between the university and this commercial provider. The lack of previous collaborative research may have acted as a barrier to more active identification of issues.
Setting up the collaboration with this OH provider took many months. Although the collaboration had been agreed in principle before the funding had been agreed, it took significant time to get contracts completed. Therefore, at a later stage, when the recruitment methods were being reviewed and we explored the possibility of working with other OH providers, the earlier delays made this challenging.
Recruiting more customers of the occupational health provider
As noted above, the OH provider was unable to engage any commercial companies in the research. The NHS trust that we eventually recruited was eligible for research support funding, which enabled it to take part. A number of other NHS trusts were also interested but, as they were not customers of this OH provider, they were not eligible. To engage and agree participation with other OH providers would have taken many months and this could not be supported within our timelines, especially given existing delays with contracts.
Acceptability of the intervention
The poor response rate would suggest that people on long-term sickness absence either did not want to engage in the research or did not value the intervention being offered. More work could have been done to explore the needs of people on long-term sickness absence and their attitudes towards the intervention. It might be that aspects of the intervention, such as workplace facilitation, were off-putting to employees. Although workplace facilitation has been shown to be a key element for intervention in other studies, if the workplace is the reason for absence (e.g. work-related stress) then engagement with a line manager might be a reason not to take part in the study. We did explain in the literature and in screening interviews that this aspect of the intervention was optional, but it may still have acted as a barrier to participation. The current situation in a company, in relation to working conditions and job satisfaction, can have a significant effect on employees, and this may be particularly relevant in NHS trusts given current service pressures. There is evidence that links job satisfaction and working conditions to mental health issues such as depression, anxiety, burn-out and self-esteem, and also physical health. 89,90. Although these factors may affect research, they are difficult to foresee and mitigate, given the limited resources available to a research team.
Recruitment areas and populations
We have not explored issues around ethnicity in this pilot study, but it is possible that the large and diverse ethnic minority population in one of our recruitment locations presented additional challenges. First, language barriers may have affected some patients’ ability to respond to written invitations. Second, there is little research on attitudes of minority populations towards sickness absence, the type of intervention we were offering or participation in research in this area. All of these issues may have had an impact on response rates. Further research is needed into the attitudes and needs of minority populations in regard to sickness absence and intervention/support.
Research recommendations
Detailed qualitative research and more intensive patient and public involvement might have a role in better understanding the barriers to taking part in both interventions while on sickness absence and research. This may enable the development of more acceptable recruitment methods.
The feasibility of other trial methods, such as cluster and stepped-wedge trials, could be explored to see if they could overcome the barriers faced by this individually randomised trial, while remaining feasible in terms of ethics and cost.
Some of the barriers to recruitment in this study may reflect wider attitudes towards work, health and the role of research. 91 In the UK, clinical research of this type has achieved high levels of adoption in routine NHS services. Convincing employees and employers of the importance and contribution of high-quality research may be an important first step.
Equally, but probably more difficult to implement, is the need to convince employees of the importance of work to health and well-being2,3 and the possibilities for those who are not wholly fit to receive support to return to and maintain work. The role of health professionals in supporting patients on sickness absence to return to work requires further research. 92–95 There may be a need to examine incentives, and disincentives, to enter return-to-work schemes where there is evidence that interventions are effective in supporting return to work.
Conclusions
As a pilot study, this study’s main aim was to assess recruitment to a randomised trial for an intervention which may help to improve well-being and return-to-work rates in employees on long-term sickness absence.
This study shows that it was not possible to recruit enough patients to make a larger trial feasible with the conventional recruitment methods used.
The collaborative case management intervention appeared feasible and acceptable to employees, but given the problems with recruitment, the data need to be treated with appropriate caution.
Developing effective and acceptable ways of reducing sickness absence remains a high priority. We have discussed possible ways of overcoming these challenges in the future, including incentives for employers, alternative study designs and further modifications to recruitment methods.
Acknowledgements
We would like to acknowledge Public Health England for providing the excess treatment costs to allow us to work with the FFW service.
We would also like to thank:
Angela Ruddock, the patient representative from the PRIMER group, who worked with the team to help and advise on all aspects of the study, attended meetings and read participant materials to try and help improve our recruitment rates.
Professor Robbie Foy and Graeme MacLennan for their advice as part of the Trial Steering Group.
The case managers who agreed to take part in the study and deliver the intervention sessions to participants.
Andrew Kinder for his involvement, support and input during the study.
Simon Calvert (Leicester FFW team) for his involvement, support and input during the study.
East Midlands CRN for identifying and recruiting the GP PIC sites for the study.
Southern Health Research and Development for their input and support with participant recruitment.
The staff at the GP PIC sites for their work on identifying patients and sending out the information packs to patients.
The reviewers for their helpful comments on the draft report and their suggestions for additional research recommendations.
Contributions of authors
Cassandra Kenning (Research Associate) was responsible for the acquisition, analysis, and interpretation of data; drafting the work; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Karina Lovell (Professor of Mental Health) was responsible for substantial contributions to the conception and design of the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mark Hann (Research fellow, statistics) was responsible for the analysis and interpretation of data for the work; revising the work critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Raymond Agius (Professor of Occupational and Environmental Medicine) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Penny E Bee (Reader) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Carolyn Chew-Graham (Professor of General Practice Research) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Peter A Coventry (Senior Lecturer in Health Services Research) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Christina M van der Feltz-Cornelis (Professor of Social Psychiatry) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Simon Gilbody (Professor of Psychological Medicine and Health Services Research) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gillian Hardy (Professor of Clinical Psychology) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Stephen Kellett (Clinical Psychologist) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
David Kessler (Reader in Primary Care) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dean McMillan (Senior Lecturer, Health sciences) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
David Reeves (Reader, Research) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Joanne Rick (Honorary Fellow) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew Sutton (Professor of Health Economics) was responsible for substantial contributions to the conception and design of the work; revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Peter Bower (Professor of Health Sciences) was the principle investigator. Was responsible for substantial contributions to the conception and design of the work; supervising the running of the study; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreeing to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health.
References
- Sickness Absence in the Labour Market. London: Office for National Statistics; 2014.
- Black C. Working for a Healthier Tomorrow. London: The Stationery Office; 2008.
- Black C, Frost D. Health at Work – an Independent Review of Sickness Absence. London: TSO; 2011.
- Evaluation of the 2010–13 Fit for Work Service Pilots: Final Report. London: Department for Work and Pensions; 2015.
- Department for Work and Pensions and The Rt Hon Lord Freud . Press Release: New Support to Tackle Long-Term Sickness Absence 2013. www.gov.uk/government/news/new-support-to-tackle-long-term-sickness-absence (accessed 7 November 2016).
- Rick J, Carroll C, McGregor M, Bessey A, Squires H, Tighe C. Systematic Review of the Effectiveness and Cost Effectiveness of Employee Assistance Programmes. Sheffield: Institute of Work Psychology, University of Sheffield; 2012.
- Mellor-Clark J, Twigg E, Farrell E, Kinder A. Benchmarking key service quality indicators in UK Employee Assistance Programme Counselling: a CORE System data profile. Counsell Psychother Res J 2013;13:14-23. https://doi.org/10.1080/14733145.2012.728235.
- Hillage J, Rick J, Pilgrim H, Jagger N, Carroll C, Booth A. Review of the Effectiveness and Cost Effectiveness of Interventions, Strategies, Programmes and Policies to Reduce the Number of Employees who Move from Short-term to Long-term Sickness Absence and to Help Employees on Long-term Sickness Absence Return to Work. Brighton: Institute of Employment Studies, University of Sussex; 2008.
- Squires H, Rick J, Carroll C, Hillage J. Cost-effectiveness of interventions to return employees to work following long-term sickness absence due to musculoskeletal disorders. J Public Health 2012;34:115-24. https://doi.org/10.1093/pubmed/fdr057.
- Carroll C, Rick J, Pilgrim H, Cameron J, Hillage J. Workplace involvement improves return to work rates among employees with back pain on long-term sick leave: a systematic review of the effectiveness and cost-effectiveness of interventions. Disabil Rehabil 2010;23:607-21. https://doi.org/10.3109/09638280903186301.
- Brouwers E, Tiemens B, Terluin B, Verhaak P. Effectiveness of an intervention to reduce sickness absence in patients with emotional distress or minor mental disorders: a randomized controlled effectiveness trial. Gen Hosp Psychiat 2006;28:223-9. https://doi.org/10.1016/j.genhosppsych.2006.02.005.
- Hoefsmit N, Houkes I, Nijhuis F. Intervention characteristics that facilitate return to work after sickness absence: a systematic literature review. J Occup Rehabil 2012;22:462-77. https://doi.org/10.1007/s10926-012-9359-z.
- Seymour L, Grove B. Workplace Interventions for People with Common Mental Health Problems: Evidence Review and Recommendations. London: BOHRF; 2005.
- Waddell G, Burton A, Kendall N. Vocational Rehabilitation: What Works, for Whom, and When? 2008. www.dwp.gov.uk/docs/hwwb-vocational-rehabilitation.pdf (accessed November 2016).
- Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency: narrative literature review. Br J Psychiat 2005;186:11-7. https://doi.org/10.1192/bjp.186.1.11.
- Nieuwenhuijsen K, Faber B, Verbeek JH, Neumeyer-Gromen A, Hees HL, Verhoeven AC, et al. Interventions to improve return to work in depressed people. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD006237.pub3.
- van Vilsteren M, van Oostrom SH, de Vet HC, Franche RL, Boot CR, Anema JR. Workplace interventions to prevent work disability in workers on sick leave. Cochrane Database Syst Rev 2015;10. https://doi.org/10.1002/14651858.CD006955.pub3.
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness?. Eff Clin Pract 1998;1:2-4.
- Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff 2009;28:75-8. https://doi.org/10.1377/hlthaff.28.1.75.
- Epping-Jordan JE, Pruitt SD, Bengoa R, Wagner EH. Improving the quality of health care for chronic conditions. Qual Saf Health Care 2004;13:299-305. https://doi.org/10.1136/qhc.13.4.299.
- Wagner EH, Simon GE. Managing depression in primary care. BMJ 2001;322:746-7. https://doi.org/10.1136/bmj.322.7289.746.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. https://doi.org/10.1001/archinte.166.21.2314.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD006525.pub2.
- Williams JW, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry 2007;29:91-116. https://doi.org/10.1016/j.genhosppsych.2006.12.003.
- Katon W, Von Korff M, Lin E, Unützer J, Simon G, Walker E, et al. Population-based care of depression: effective disease management strategies to decrease prevalence. Gen Hosp Psychiat 1997;19:169-78. https://doi.org/10.1016/S0163-8343(97)00016-9.
- Gask L. Role of specialists in common chronic diseases. BMJ 2005;330:651-3. https://doi.org/10.1136/bmj.330.7492.651.
- Andrews G. Should depression be managed as a chronic disease?. BMJ 2001;322:419-21. https://doi.org/10.1136/bmj.322.7283.419.
- Von Korff M, Goldberg D. Improving outcomes in depression. BMJ 2001;323:948-9. https://doi.org/10.1136/bmj.323.7319.948.
- Vlasveld M, van der Feltz-Cornelis C, Adèr H, Anema J, Hoedeman R, van Mechelen W, et al. Collaborative care for major depressive disorder in an occupational healthcare setting. Br J Psychiat 2012;200:510-1. https://doi.org/10.1192/bjp.bp.111.095687.
- Vlasveld M, van der Feltz-Cornelis C, Adèr H, Anema J, Hoedeman R, Mechelen W, et al. Collaborative care for sick-listed workers with major depressive disorder: a randomised controlled trial from the Netherlands Depression Initiative aimed at return to work and depressive symptoms. Occup Environ Med 2013;70:223-30. https://doi.org/10.1136/oemed-2012-100793.
- Wang PS, Simon GE, Avorn J, Azocar F, Ludman EJ, McCulloch J, et al. Telephone screening, outreach, and care management for depressed workers and impact on clinical and work productivity outcomes: a randomized controlled trial. JAMA 2007;298:1401-11. https://doi.org/10.1001/jama.298.12.1401.
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350. https://doi.org/10.1136/bmj.h638.
- Arends I, van der Klink JJL, van Rhenen W, de Boer MR, Bultmann U. Prevention of recurrent sickness absence in workers with common mental disorders: results of a cluster-randomised controlled trial. Occup Environ Med 2014;71:21-9. https://doi.org/10.1136/oemed-2013-101412.
- Aelfers E, Bosma H, Houkes I, van Eijk J. Effectiveness of a minimal psychological intervention to reduce mild to moderate depression and chronic fatigue in a working population: the design of a randomized controlled trial. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-129.
- Feicht T, Wittmann M, Jose G, Mock A, von Hirschhausen E, Esch T. Evaluation of a seven-week web-based happiness training to improve psychological well-being, reduce stress, and enhance mindfulness and flourishing: a randomized controlled occupational health study. Evid Based Complement Alternat Med 2013;2013. https://doi.org/10.1155/2013/676953.
- van Beurden KM, Brouwers EPM, Joosen MCW, Terluin B, van der Klink JJL, van Weeghel J. Effectiveness of guideline-based care by occupational physicians on the return-to-work of workers with common mental disorders: design of a cluster-randomised controlled trial. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-193.
- Linden M, Muschalla B, Hansmeier T, Sandner G. Reduction of sickness absence by an occupational health care management program focusing on self-efficacy and self-management. Work 2014;47:485-9. https://doi.org/10.3233/WOR-131616.
- Rantonen J, Luoto S, Vehtari A, Hupli M, Karppinen J, Malmivaara A, et al. The effectiveness of two active interventions compared to self-care advice in employees with non-acute low back symptoms: a randomised, controlled trial with a 4-year follow-up in the occupational health setting. Occup Environ Med 2012;69:12-20. https://doi.org/10.1136/oem.2009.054312.
- Lagerveld SE, Blonk RW, Brenninkmeijer V, Wijngaards-de Meij L, Schaufeli WB. J Occup Health Psychol 2012;17:220-34. https://doi.org/10.1037/a0027049.
- Viikari-Juntura E, Kausto J, Shiri R, Kaila-Kangas L, Takala E-P, Karppinen J, et al. Return to work after early part-time sick leave due to musculoskeletal disorders: a randomized controlled trial. Scand J Work Environ Health 2012;38:134-43. https://doi.org/10.5271/sjweh.3258.
- Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med 2014;76:29-37. https://doi.org/10.1097/PSY.0000000000000022.
- Von Korff M, Katon WJ, Lin EH, Ciechanowski P, Peterson D, Ludman EJ, et al. Functional outcomes of multi-condition collaborative care and successful ageing: results of randomised trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d6612.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f4913.
- Fortney JC, Pyne JM, Mouden SB, Mittal D, Hudson TJ, Schroeder GW, et al. Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centers: a pragmatic randomized comparative effectiveness trial. Am J Psychiat 2013;170:414-25. https://doi.org/10.1176/appi.ajp.2012.12050696.
- Muntingh A, van der Feltz-Cornelis C, van Marwijk H, Spinhoven P, Assendelft W, de Waal M, et al. Effectiveness of collaborative stepped care for anxiety disorders in primary care: a pragmatic cluster randomised controlled trial. Psychother Psychosom 2014;83:37-44. https://doi.org/10.1159/000353682.
- Oosterbaan DB, Verbraak MJPM, Terluin B, Hoogendoorn AW, Peyrot WJ, Muntingh A, et al. Collaborative stepped care vs. care as usual for common mental disorders: 8-month, cluster randomised controlled trial. Br J Psych 2013;203:132-9. https://doi.org/10.1192/bjp.bp.112.125211.
- Morgan MAJ, Coates MJ, Dunbar JA, Reddy P, Schlicht K, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002171.
- Huijbregts KM, de Jong FJ, van Marwijk HW, Beekman AT, Adèr HJ, Hakkaart-van Roijen L, et al. A target-driven collaborative care model for Major Depressive Disorder is effective in primary care in the Netherlands. A randomized clinical trial from the depression initiative. J Affect Disord 2013;146:328-37. https://doi.org/10.1016/j.jad.2012.09.015.
- Volker D, Vlasveld MC, Anema JR, Beekman ATF, Hakkaart-van Roijen L, Brouwers EPM, et al. Blended E-health module on return to work embedded in collaborative occupational health care for common mental disorders: design of a cluster randomized controlled trial. Neuropsych Dis Treat 2013;9:529-37.
- Vonk Noordegraaf A, Anema JR, van Mechelen W, Knol DL, van Baal WM, van Kesteren PJ, et al. A personalised eHealth programme reduces the duration until return to work after gynaecological surgery: results of a multicentre randomised trial. BJOG 2014;121:1127-35. https://doi.org/10.1111/1471-0528.12661.
- Jensen C, Jensen OK, Christiansen DH, Nielsen CV. One-year follow-up in employees sick-listed because of low back pain: randomized clinical trial comparing multidisciplinary and brief intervention. Spine 2011;36:1180-9. https://doi.org/10.1097/BRS.0b013e3181eba711.
- Jensen C, Jensen OK, Nielsen CV. Sustainability of return to work in sick-listed employees with low-back pain. Two-year follow-up in a randomized clinical trial comparing multidisciplinary and brief intervention. BMC Musculoskel Dis 2012;13. https://doi.org/10.1186/1471-2474-13-156.
- van Beurden KM, Vermeulen SJ, Anema JR, van der Beek AJ. A participatory return-to-work program for temporary agency workers and unemployed workers sick-listed due to musculoskeletal disorders: a process evaluation alongside a randomized controlled trial. J Occup Rehabil 2012;22:127-40. https://doi.org/10.1007/s10926-011-9314-4.
- Martin MH, Nielsen MB, Madsen IE, Petersen SM, Lange T, Rugulies R. Effectiveness of a coordinated and tailored return-to-work intervention for sickness absence beneficiaries with mental health problems. J Occup Rehabil 2013;23:621-30. https://doi.org/10.1007/s10926–013–9421–5.
- Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-166.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-34.
- Rick J, Graffy J, Knapp P, Small N, Collier D, Eldridge S, et al. Systematic techniques for assisting recruitment to trials (START): study protocol for embedded, randomized controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-407.
- Adams CE. Many more reasons behind difficulties in recruiting patients to randomized controlled trials in psychiatry. Epidemiol Psychiatr Sci 2013;22:321-3. https://doi.org/10.1017/S2045796013000267.
- Gilbody S, Littlewood E, Hewitt C, Brierley G, Tharmanathan P, Araya R, et al. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ 2015;351. https://doi.org/10.1136/bmj.h5627.
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- Barkham M, Evans C, Margison F, Mcgrath G, Mellor-Clark J, Milne D. The rationale for developing and implementing core outcome batteries for routine use in service settings and psychotherapy outcome research. J Ment Health 1998;7:35-47. https://doi.org/10.1080/09638239818328.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. https://doi.org/10.1192/bjp.180.5.461.
- Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med 2003;45:156-74. https://doi.org/10.1097/01.jom.0000052967.43131.51.
- Beecham JK, Knapp MRJ, Thornicraft G. Measuring Mental Health Needs. London: Gaskell; 1992.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes 2005;3:51-8. https://doi.org/10.1186/1477-7525-3-51.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20. https://doi.org/10.1016/0149-7189(79)90094-6.
- Great Britain . Data Protection Act 1998 n.d. www.gov.uk/data-protection.
- Office for National Statistics . 2011 Census n.d. www.ons.gov.uk/census/2011census (accessed 22 August 2016).
- Hussey L, Money A, Gittins M, Agius R. Has the fit note reduced general practice sickness certification rates?. Occup Med 2015;65:182-9. https://doi.org/10.1093/occmed/kqu207.
- de Boer AG, Taskila T, Tamminga SJ, Frings-Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev 2011;16. https://doi.org/10.1002/14651858.CD007569.pub2.
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484-93. https://doi.org/10.1192/bjp.bp.106.023655.
- Smith CT, Hickey H, Clarke M, Blazeby J, Williamson P. The trials methodological research agenda: results from a priority setting exercise. Trials 2014;15:32-9. https://doi.org/10.1186/1745-6215-15-32.
- Paton N. Fit for Work service awareness low among employers and GPs. Occupational Health 2015;67.
- Man MS, Rick J, Bower P. Improving recruitment to a study of telehealth management for long-term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16. https://doi.org/10.1186/s13063-015-0820-0.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002360.
- Nystuen P, Hagen KBJ. Telephone reminders are effective in recruiting nonresponding patients to randomized controlled trials. Clin Epidemiol 2004;57:773-6. https://doi.org/10.1016/j.jclinepi.2003.12.015.
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan J. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12. https://doi.org/10.1186/1745-6215-12-78.
- Hughes-Morley A, Young B, Hempel RJ, Russell IT, Waheed W, Bower P. What can we learn from trial decliners about improving recruitment? Qualitative study. Trials 2016;17. https://doi.org/10.1186/s13063-016-1626-4.
- Brueton V, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.MR000032.pub2.
- Hetherton J, Matheson A, Robson M. Recruitment by GPs during consultations in a primary care randomized controlled trial comparing computerized psychological therapy with clinical psychology and routine GP care: problems and possible solutions. Prim Health Care Res 2004;5:5-10. https://doi.org/10.1191/1463423604pc168oa.
- Fairhurst K, Dowrick C. Problems with recruitment in a randomized controlled trial of counselling in general practice: causes and implications. J Health Serv Res Policy 1996;1:77-80.
- Raine R, Fitzpatrick R, Barratt H, Bevan G, Black N, Boaden R, et al. Challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Health Serv Deliv Res 2016;4.
- Taljaard M, Weijer C, Grimshaw JM, Eccles MP. The Ottawa Statement on the ethical design and conduct of cluster randomised trials: précis for researchers and research ethics committees. BMJ 2013;346. https://doi.org/10.1136/bmj.f2838.
- Eldridge S, Kerry S, Torgerson DJ. Bias in identifying and recruiting participants in cluster randomised trials: what can be done?. BMJ 2009;339. https://doi.org/10.1136/bmj.b4006.
- Bishop A, Wynne-Jones G, Lawton SA, van der Windt D, Main C, Sowden G, et al. Rationale, design and methods of the Study of Work and Pain (SWAP): a cluster randomised controlled trial testing the addition of a vocational advice service to best current primary care for patients with musculoskeletal pain (ISRCTN 52269669). BMC Musculoskel Dis 2014;15. https://doi.org/10.1186/1471-2474-15-232.
- Faragher EB, Cass M, Cooper CL. The relationship between job satisfaction and health: a meta-analysis. Occup Environ Med 2005;62:105-12. https://doi.org/10.1136/oem.2002.006734.
- Burgard SA, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci 2013;57:1105-27. https://doi.org/10.1177/0002764213487347.
- Wynne-Jones G, Webb K, Buck R, Cooper L, Button L, Main CJ, et al. What happens to work if you’re unwell? Beliefs and attitudes of managers and employees with musculoskeletal pain in public sector organisations. J Occup Rehabil 2011;21:31-42. https://doi.org/10.1007/s10926-010-9251-7.
- Welsh VK, Mallen CD, Wynne-Jones G, Jinks C. Exploration of GP’s views and use of the fit note: a qualitative study in primary care. Br J Gen Pract 2012;62:363-70. https://doi.org/10.3399/bjgp12X641483.
- Buck R, Porteous C, Wynne-Jones G, Marsh K, Phillips CJ, Main CJ. Challenges to remaining at work with common health problems: what helps and what influence do organisational policies have?. J Occup Rehabil 2011;21:501-12. https://doi.org/10.1007/s10926–011–9288–2.
- Wynne-Jones G, Mallen CD, Main CJ, Dunn KM. What do GPs feel about sickness certification? A systematic search and narrative review. Scand J Prim Health Care 2010;28:67-75. https://doi.org/10.3109/02813431003696189.
- Wynne-Jones G, Mallen CD, Main CJ, Dunn KM. Sickness certification and the GP: what really happens in practice?. Fam Pract 2010;27:344-50. https://doi.org/10.1093/fampra/cmp096.
Appendix 1 Original patient documents
Appendix 2 Modified patient documents (Occupational Health)
Appendix 3 Modified patient documents (Fit for Work)
List of supplementary material
- Report supplementary material 1
- Finalised Case Management to Enhance Occupational Support (CAMEOS) manual.
- Report supplementary material 2
- Therapist manual to support intervention delivery and adherence to the model
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/phr/12309005/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
List of abbreviations
- BOHRF
- British Occupational Health Research Foundation
- CAMEOS
- CAse Management to Enhance Occupational Support
- CBT
- cognitive–behavioural therapy
- CCG
- clinical commissioning group
- CI
- confidence interval
- CORE-OM
- Clinical Outcomes in Routine Evaluation Outcome Measure
- CRF
- case report form
- CRN
- Clinical Research Network
- CSQ-8
- Client Satisfaction Questionnaire
- CTRW
- co-ordinated and tailored return to work
- DWP
- Department for Work and Pensions
- EAP
- Employee Assistance Programme
- FFW
- Fit for Work
- GP
- general practitioner
- HR
- human resources
- LCRN
- Local Clinical Research Network
- MAHSC-CTU
- Manchester Academic Health Science Centre Clinical Trials Unit
- OH
- occupational health
- PIC
- Patient Identification Centre
- PIS
- participant information sheet
- PPIE
- patient and public involvement and engagement
- PRIMER
- Primary Care Research in Manchester Engagement Resource
- RGFHS
- Research Governance Framework for Health and Social Care
- SD
- standard deviation
- SME
- small and medium enterprise
- WSAS
- Work and Social Adjustment Scale