Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 12/3000/40. The contractual start date was in October 2013. The final report began editorial review in September 2021 and was accepted for publication in May 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Goodwin et al. This work was produced by Goodwin et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Goodwin et al.
Chapter 1 Structure of the research and this report
The structure of the report is described below. Given the sheer number of data created from this research, a significant proportion is presented in the appendices for the main outcomes to be presented clearly and succinctly.
Chapter 2: dental caries and water fluoridation
Chapter 2 introduces the background evidence for how dental caries occurs and its impact. Chapter 2 describes water fluoridation (WF), how it works and why Cumbria was chosen for this type of study.
Chapter 3: history and implementation of water fluoridation as a public health intervention
Chapter 3 provides a thorough background of the history of WF, the early trials which resulted in the introduction and implementation of WF and, specifically, the history of WF in Cumbria where the current study was based.
Chapter 4: what we know now
Chapter 4 provides an overview of the epidemiology of dental caries and the current evidence about the clinical effectiveness and cost-effectiveness of WF.
Chapter 5: aims and objectives
Chapter 5 provides the specific aims and objectives of the study, which will be met within the report.
Chapter 6: methods
Chapter 6 presents the key elements of the study design, the setting, participants, variables and data sources, efforts to address potential bias and statistical/health economic methods.
Chapter 7: results – delivery of the intervention
Chapter 7 describes the delivery of the intervention over the study period.
Chapter 8: birth cohort clinical results
Chapter 8 provides the main results for the birth cohort and explores the full effects of WF on the presence/absence of decay in primary dentition, providing both the unadjusted and adjusted estimates and their precision. In addition, Chapter 8 provides secondary analysis on the count of decayed, missing or filled teeth (primary) (dmft) and dental general anaesthetics (DGAs), and explores the impact and interaction of WF and deprivation on decay.
Chapter 9: older school cohort clinical results
Chapter 9 provides the main results for the older school cohort and explores the topical effects on the presence/absence of decay in permanent dentition, providing both the unadjusted and adjusted estimates and their precision. In addition, Chapter 9 provides analysis on the count of DMFT and DGAs, and explores the impact and interaction of WF and deprivation on decay.
Chapter 10: health economic analysis
Chapter 10 provides the health economic analysis for both the birth cohort and the older school cohort. The measure for the economic analysis was summarised using incremental cost-effectiveness ratios (ICERs) [i.e. cost per quality-adjusted life-year (QALY) gained]. Estimates of net costs and outcomes were bootstrapped to generate cost-effectiveness acceptability curves that provided the probability of cost-effectiveness for a range of thresholds for willingness to pay for a QALY.
Chapter 11: discussion
Chapter 11 summarises the key results of the study, provides an interpretation, considering the study’s limitations, and links to the results of previous studies and reviews.
Chapter 2 Dental caries and water fluoridation
Introduction to dental caries
Dental caries remains a significant world-wide public health problem. Oral diseases are among the most prevalent globally, affecting more than 3.5 billion people around the world. Caries, in particular, can result in health and economic burdens, causing pain and sepsis and affecting quality of life. 1
In the UK, dental decay (Figure 1) is still one of the most common diseases affecting children, but there are recent indications that its prevalence is falling. The latest national survey2 reported obvious decay experience prevalence in 5-year-olds of 31% in England, 41% in Wales and 40% in Northern Ireland (data for Scotland were not reported); however, these figures had decreased from those reported in the 2003 survey3 (i.e. 41% in England, 52% in Wales and 61% in Northern Ireland). However, owing to changes in how parental consent is obtained, data from the last NHS surveys are not directly comparable and are difficult to interpret. 4
Tooth decay is strongly associated with poverty. Young children from poor families carry a disproportionate amount of the population disease burden. 5 The Child Dental Health Survey 2013, England, Wales and Northern Ireland reported that 18% of 5-year-olds in the highest deprivation quintile have severe or extensive decay compared with 4% of 5-year-olds in the lowest deprivation quintile. 2 More recent data from the 2019 NHS dental surveys reported a prevalence of dental decay of 23.4% in 5-year-old children in England,6 and a similar picture was reported when looking at inequalities, with a prevalence of dental decay of 13.7% in 5-year-olds living in the least deprived areas compared with a prevalence of dental decay of 34.3% in 5-year-olds living in the most deprived areas. A UK prospective cohort study of 3- to 6-year-olds7 showed that the disease, once developed, progresses rapidly. In addition, the disease can have a significant impact, as children with caries have each year a 25% risk of experiencing pain and an 11% risk of having an extraction. 7
FIGURE 1.
Extensive decay of the primary dentition in 7-year-olds.

If the disease is unchecked, multiple extractions under DGA are a common outcome. Dental extractions are the most common reason why young children have a DGA. Exact figures are difficult to quantify but Hospital Episode Statistics data, which capture only a proportion of extractions carried out in a hospital setting, show at least 60,000 hospital episodes associated with dental extraction each year in England alone. 8 We know that DGA extractions have a significant negative impact on young children and their families,9,10 and there is a strong association between dental extractions and dental anxiety, which can continue to affect individuals in later life. 11 The prevalence of disease in the permanent teeth has fallen rapidly over the last 30 years. The prevalence of obvious decay in 12-year-olds in England was 81% in 1983, 52% in 1993, 43% in 2003 and 34% in 2013. 3
The impact of caries and its treatment is cumulative, and their effects are felt as children mature into adulthood. The 2009 Adult Dental Health Survey12 reported that the prevalence of coronal caries in England fell from 46% to 28% between 1998 and 2009. There were reductions across all age groups, but the largest reduction (i.e. 21 percentage points) was seen in individuals aged 25–34 years. This picture of overall improvement in population prevalence masks significant inequalities in tooth decay experience within society. In addition, national surveys do not report disease statistics among vulnerable groups.
The costs to the NHS of treating tooth decay are very significant. In England alone, the NHS dental allocation in 2018–19 was approximately £3B. 13 Patient charges roughly make up one-quarter of the total primary care NHS budget and much of the NHS dental budget is consumed by the detection and treatment of dental caries.
There are significant inequalities in access and utilisation of dental services, with individuals with greatest need being least likely to access dental services. 14,15 This situation gives cause for concern, even more so when the main disease with which the service is concerned with (i.e. dental caries) is totally preventable.
Fluoride and water fluoridation
Dental caries should be totally preventable by limiting sugar intake and adopting a rigorous self-care regime, which includes regular plaque removal and optimal use of topical fluorides, most commonly in the form of fluoridated toothpaste. Indeed, the large decreases in caries seen within the UK over the last 40 years have been primarily attributable to the widespread uptake of fluoride toothpaste from the 1970s onwards. However, stringent self-care has not been adopted by significant numbers of the population, reflected in the high prevalence rates of dental caries, particularly in disadvantaged groups, leading to persistent inequalities in dental health.
Water fluoridation is widely advocated as the most cost-effective public health measure in addressing the caries challenge. The headline findings of the York systematic review16 of WF stated that the size of the benefit would be an approximate 15% increase (absolute difference) in the proportion of children with no experience of tooth decay, and a reduction in the mean number of teeth affected by decay of approximately 2.2 teeth. The review16 also concluded that the benefits of WF are in addition to the benefits derived from the use of fluoride toothpaste, a conclusion reiterated by a Cochrane systematic review of the effectiveness of fluoride toothpaste. 17
However, the York review16 also concluded that the evidence base for WF is limited, as most of the studies were conducted at a time before widespread use of fluoride toothpaste and before the significant fall we have seen in dental caries prevalence in the UK. The Medical Research Council (MRC) Working Group’s report18 recommended that:
Studies are needed to provide an estimate of the effects of water fluoridation on children aged 3–15 years against a background of widespread use of fluoride toothpaste, and to extend knowledge about the effect of water fluoridation by social class (or other relevant measures of socioeconomic status), considering potentially important effect modifiers such as sugar consumption and toothpaste usage.
Water fluoridation is believed to have a systemic effect. Constant exposure means that fluoride is incorporated into the mineral structure of the teeth as they develop in utero and in the first 5 years of life, and, subsequently, there is a topical effect once a tooth has erupted, creating an environment at the tooth surface that favours remineralisation. Research has suggested that it is the topical effect that is most important in reducing caries. 19 The MRC Working Group’s report18 also recommended that economic and quality-of-life outcomes need to be assessed in future studies. The case for fluoridation (a whole-population intervention) becomes more difficult to make, as dental disease levels in older children and adults continue to fall. A well-conducted study is required to assess the impact on health and the value for money of a WF scheme in the current context.
Cumbria and the York criteria
To satisfy the inclusion criteria set out in the York review16 for a high-quality study, a new scheme needed to be implemented and appraised or an operational scheme needed to be terminated. A unique set of circumstances in Cumbria provided an opportunity to conduct a high-quality evaluation of a reintroduced WF scheme. In addition, these circumstances satisfied the inclusion criteria stipulated by the York systematic review16 and could address the design issues identified in the MRC report. 18 There are two geographically contiguous WF schemes in West Cumbria (Figure 2), that is, Cornhow and Ennerdale (described in the remainder of this report as West Cumbria). The fluoride dosing plant at Cornhow (which serves zone 28) had been out of operation since April 2006 and the fluoride dosing plant at Ennerdale (which serves zone 31/32) had been out of operation since 2011 owing to failure of the plants; however, both plants were reinstated and began dosing again in 2013. There was a legal obligation for the responsible body, the North West Strategic Health Authority (SHA), to reinstate the scheme.
FIGURE 2.
Map detailing zones which are fluoridated in West Cumbria. Reproduced with permission from United Utilities. Maps were created using ArcGIS® version 10.4.1 (Esri, Redlands, CA, USA). ArcGIS® and ArcMap™ are the intellectual property of Esri and are used herein under license. Copyright © Esri. All rights reserved. For more information about Esri® software, please visit www.esri.com. Shape files provided by United Utilities.
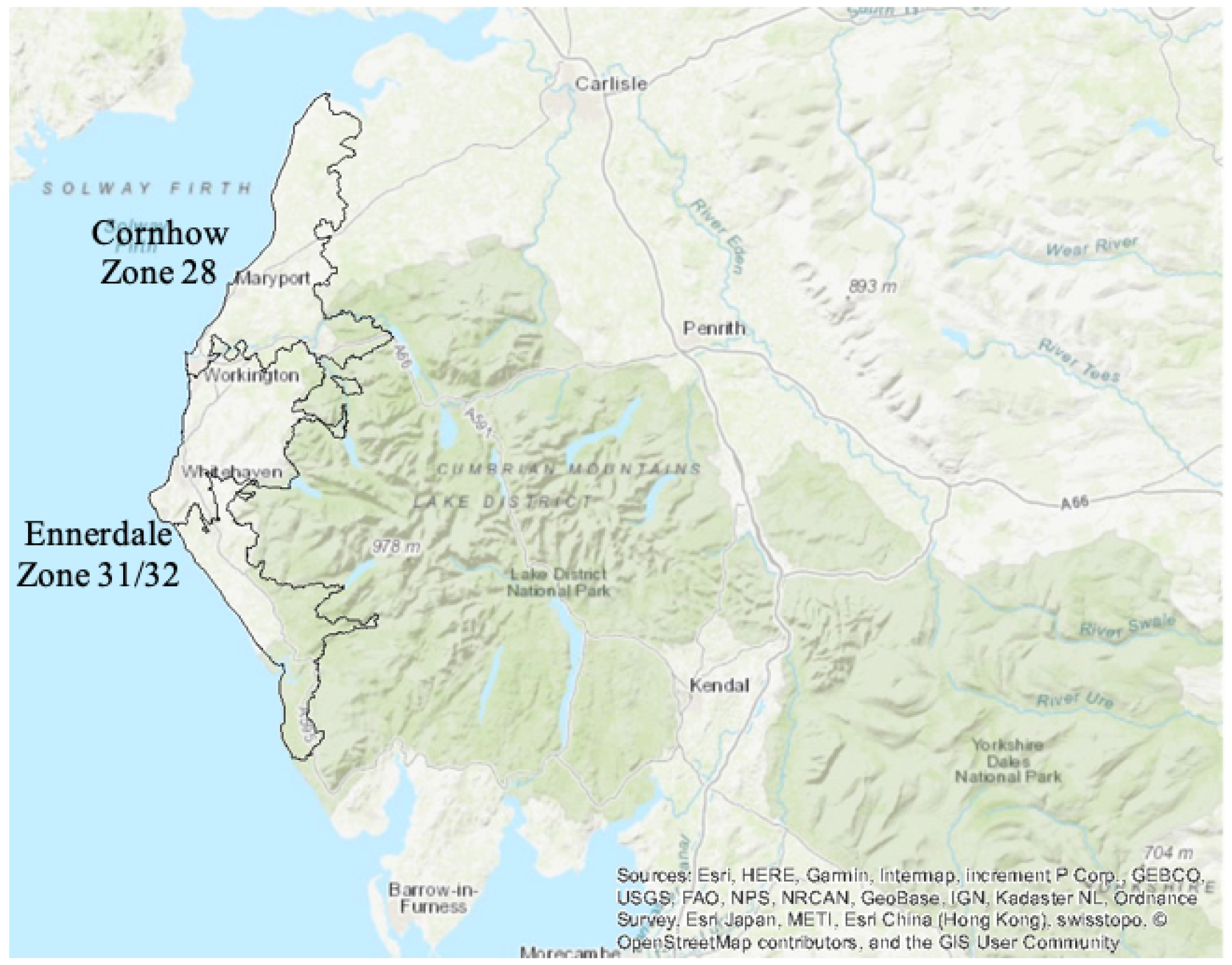
West Cumbria, therefore, presented an ideal study site, as there were no impediments to reinstating this paused scheme, there is low population mobility and there is a neighbouring, relatively homogeneous, sociodemographically similar control population (not receiving fluoridated water, referred to as North Cumbria in the remainder of the report). There were also no significant differences in oral health programmes taking place across the intervention and control groups that could have created additional confounding factors. Within Cumbria, the ‘Smile4Life’ programme is offered universally and focuses on facilitating healthier diets, regular and appropriate toothbrushing, adopting healthier lifestyles and regular access to dental services. In addition, all parents are provided a toothbrush pack when their child is approximately 6- to 9-months-old through the health visiting teams. The only targeted oral health programme is a supervised toothbrushing programme in early years settings; however, this began in 2020 and, therefore, would not have affected the children who took part in the CATFISH (Cumbrian Assessment of Teeth a Fluoride Intervention Study for Health) study.
One notable difference for the area receiving WF is that it occurs within a coastal community, whereas the control area (i.e. North Cumbria) is mainly inland. It has been noted that many coastal communities have some of the worst health outcomes in England, including low life expectancy and high rates of health issues such as diabetes, coronary heart disease and stroke. This was highlighted in the Chief Medical Officer’s Annual Report 2021: Health in Coastal Communities. 20 There are a variety of reasons for these health outcomes, including the economic, educational and connectivity disadvantages faced within seaside towns and villages. Deprivation levels [according to the Index of Multiple Deprivation (IMD)21] was taken into account within the analysis to account for some of these differences.
Within Cumbria, there was (1) a large salaried dental service already experienced in undertaking large dental epidemiological surveys in school and nursery settings, (2) strong support for the study from the public health community in Cumbria and the North of England and (3) a robust relationship with the water undertaker United Utilities (Warrington, UK).
Chapter 3 History and implementation of water fluoridation as a public health intervention
Dean and the 21 cities
The history of WF as a public health measure starts with reports from McKay, in Colorado, USA, in 1901, of widespread and unexplained staining of teeth. 22 McKay mapped the occurrence of the staining within Colorado and hypothesised that an element in the water supply might be responsible. Importantly, McKay also reported that dental caries prevalence and severity seemed to be much lower in communities exhibiting the dental staining than was the case in other communities with no staining. In 1925, in the UK, Ainsworth, in a MRC-sponsored descriptive epidemiological study of school children, found a statistical association between dental caries and the tooth staining, as described by McKay. Children living in areas with high prevalence of mottled teeth had lower levels of dental caries. 22
In the 1930s, chemical analysis of water supplies in Arkansas, USA, by Alcoa (the Aluminium Company of America) found that mottling and staining of teeth was associated with an elevated concentration of fluoride in the water supply. These findings were corroborated by Ainsworth in Essex, UK, who compared fluoride concentrations in the water supplies of the towns of Maldon, the population of which exhibited endemic staining, and Witham, the population of which did not show the staining. Fluoride concentrations in the Maldon water supply ranged from 4.5 to 5.5 parts per million (ppm), whereas the fluoride concentration in the Witham water supply was only 0.5 ppm. 22
Following these initial findings, the US Public Health Service appointed HT Dean to carry out his famous ‘21-city study’, which established a dose–response relationship between fluoride levels, the severity of mottling of the teeth and caries preventative effect. 23 The caries prevention was most evident at fluoride concentrations above 1 ppm. Following publication of Dean’s study, health authorities in the USA considered the possibility of artificially adding fluoride to the water supply at 1 ppm to reproduce the caries preventative effect found by Dean, but minimising the risk of development of mottling or fluorosis. Hence, the 1 ppm ‘optimal fluoride’ level was established.
Early studies of artificial water fluoridation
In 1945 and 1946, pilot WF schemes were introduced in the US towns of Grand Rapids, MI, Newburgh, NY, and Evanston, IL. 23 Dental caries rates of children living in these towns were monitored and compared with controls living in the non-fluoridated US towns of Muskegon, MI, Kingston, NY, and Oak Park, IL. In each of these pilot studies, significant reductions in dental caries rates were reported in children living in the fluoridated towns, with little or no change in children living in the control towns. Similarly designed studies in Canada (Brantford–Sarnia–Stratford, 1945–62), the Netherlands (Tiel–Culemborg, 1953–69) and New Zealand (Hastings, 1954–70) saw similar significant reductions in caries experience in artificially fluoridated areas to those found in the USA. 24,25
In 1952, on the recommendation of the MRC, the British government initiated a study into WF, with a view to advising on whether or not fluoride should be added to drinking water supplies in the UK. 26,27 As a result, in 1955, three sites were identified to pilot WF schemes, in Watford, Kilmarnock and part of Anglesey. Controls were selected in Sutton, Ayr and the remaining part of Anglesey. After 5 years of fluoridation, the prevalence of dental caries in 5-year-old children living in the fluoridated areas was approximately 50% lower than in children living in the control areas. Although clearly demonstrating a significant health improvement, the Kilmarnock scheme was discontinued in 1962 on the instruction of the local council. Likewise, Watford did not progress to a substantive scheme and, although the whole of Anglesey was fluoridated in 1964, in 1992 Welsh Water (Mid Glamorgan, UK) withdrew the scheme in Anglesey.
Implementation of water fluoridation programmes in the UK
Following the UK pilot schemes, the first substantive WF scheme in the UK commenced in Birmingham in 1964. The other large-scale fluoridated water scheme in England is in the north-east of England. Northumberland, Newcastle, Gateshead, North Tyneside and County Durham local authorities instituted schemes in the late 1960s and early 1970s. 28 In the north-west of England, three small scales schemes were introduced in the late 1960s and early 1970s (Table 1).
| Fluoridation schemes in the north-west of England | District council | Population covered (2020), n | Properties covered, n | Start date |
|---|---|---|---|---|
| Cornhow | Copeland and Allerdale | 62,798 | 30,421 | 1968 |
| Ennerdale | Copeland | 69,336 | 36,391 | 1971 |
| Hurleston | Crewe and Nantwich Borough Council | 148,552 | 71,110 | 1971 |
Following the implementation of schemes from the 1960s to the 1980s, further implementation stalled. This was, in part, due to the 1980 Strathclyde Court Case (the longest civil case in Scottish legal history). In 1978, the four health boards covering the Strathclyde Region of Scotland asked the regional council to fluoridate their water supplies, a request that was challenged by a local resident. At the end of the 2-year case, the presiding judge, Lord Jauncey, ruled that the process was ultra vires, that is, beyond the legal power of Strathclyde Regional Council.
Consequently, the Water (Fluoridation) Act29 was passed by Parliament in 1985 (later subsumed by the Water Industry Act30 in 1991). However, this new legislation put no obligation on water companies to fluoridate water supplies if requested to do so by health authorities. Over 60 health authorities went through the required consultation process, but none was successful in implementing a new WF scheme. The wording of the 1985 Water (Fluoridation) Act29 was revised in the Water Industry Act30 amendment in November 2003 from:31
. . . water authorities may add fluoride to the water supply following an application from the local health authority . . .
to
If requested to do so by a relevant authority, a water undertaker shall enter into arrangements with the relevant authority to increase the fluoride content of the water supplied by that undertaker to premises specified in the arrangements.
In England, this change in wording of the legislation obligated water companies to implement a new scheme if requested to do so by SHAs following completion of a consultation conducted in line with regulations set out in 2005. In November 2008, Southampton City Council voted to endorse the South-Central SHA’s proposed scheme to fluoridate the local water supply. In 2009, the Honourable Mr Justice Mitting gave limited permission for a judicial review of this decision. In February 2011, the judicial review upheld the decision made by NHS South-Central SHA, and the end of the legal process was confirmed in July 2011. However, both Hampshire County Council and Southampton City Council opposed the scheme and in 2014 Public Health England announced that it would not proceed with the proposed scheme without the backing of Southampton City Council, that is, the local authority where most of the population (approximately 160,000) affected by the proposed scheme lived. 32,33 Therefore, despite changes to relevant acts of parliament and lengthy and expensive legal proceedings, no new WF schemes have been implemented in the UK in the last 30 years.
History of implementation and provision of water fluoridation in Cumbria
Two schemes were implemented in Cumbria in the late 1960s and early 1970s, during the period in which WF schemes proliferated in England. The Cornhow scheme is referred to in an agreement (dated 1968) made between the West Cumberland Water Board and the Cumberland County Council. A consultation on the scheme was undertaken before the agreement was signed. The Cornhow water works is situated on the north-west margin of the Lake District, close to Loweswater and Crummock Water, and a few miles to the south-east of Cockermouth. The water works serves the north-west part of the Cumbria coastal plain, more specifically Workington, Seaton, High Harrington, Great Clifton, Silloth, down the coast to Maryport and Flimby, and inland to Cockermouth.
The Ennerdale scheme is referred to in an agreement (dated 1971) made between the South Cumberland Water Board and the Cumberland County Council. Again, a consultation on this scheme was made before the agreement was completed. The Ennerdale water works is situated on the shores of Ennerdale Water, serving residents living in Whitehaven, Arlecdon, St Bees, Frizington, Salterbeck, Egremont, Cleator Moor, Beckermet, Ravenglass and Bootle (see Figure 2 for map of areas covered). Although provided under different legal agreements, the supply of fluoridated water from these two plants is contiguous, and has been administered by the relevant water boards and, after privatisation, by the same water undertaker.
In England, each fluoridation scheme is the subject of a formal legal agreement between the parties to that scheme, that is, a public body acting as a ‘health body’ and the water utility company responsible for supplying water to the population served by the scheme. This is the case even for schemes such as Cornhow and Ennerdale, which predate specific legislation on fluoridation. The Water (Fluoridation) Act 198529 conferred ‘protected’ status on the agreements for both plants to enable fluoridation to continue at both sites. Since the schemes originated, there have been significant and multiple organisational changes to the bodies responsible for the public health of the localities in which schemes operate and to the organisations providing the fluoridated water supply. There have also been significant changes to the legislation governing fluoridation in that time.
Following privatisation of water utilities, North West Water (now United Utilities) emerged as the successor to the two water boards that were parties to the 1968 and 1971 agreements. The structure of the NHS and public sector bodies with responsibility for implementation, commissioning and oversight of WF has changed considerably and frequently since the agreements of 1968 and 1971. In Cumbria, North Cumbria Health Authority succeeded Cumberland County Council as the responsible body, with public health authority powers and duties. North Cumbria Health Authority affirmed its support for the two schemes when it came into existence in April 1996. In 2002, the NHS restructured and statutory responsibility for WF passed from North Cumbria Health Authority to the newly formed Cumbria and Lancashire SHA. From 1 July 2006, the number of SHAs reduced, and statutory responsibility passed from Cumbria and Lancashire SHA to the North West SHA.
Following the passage of the Health and Social Care Act34 in 2012, SHAs and primary care trusts were abolished on 31 March 2013. The Health and Social Care Act 201234 incorporates, in an amended form, the provisions of earlier acts of parliament, including the Water (Fluoridation) Act 198529 and the Water Industry Act 1991,30 as amended by the Water Act 2003. 31 Under the new arrangements, local authorities with public health responsibilities became the public bodies holding statutory responsibility for WF in their areas. Fluoridation proposals made by local authorities, such as whether or not to introduce new fluoridation schemes or terminate existing fluoridation schemes, are subject to public consultation. In 2013, a new body was established, Public Health England, an executive agency of the Department of Health and Social Care with operational autonomy. Public Health England has statutory duties regarding implementing the proposals made by local authorities that the Secretary of State for Health and Social Care has agreed are operable and efficient, entering into arrangements with water suppliers to give effect to those decisions and monitoring the health effects of WF schemes.
Disruption of supplies at Cornhow and Ennerdale in Cumbria that led to the CATFISH study
Following the dosing at the two treatment plants in Cornhow and Ennerdale, a unique set of circumstance led to the CATFISH study. There had been issues relating to the schemes at Cornhow and Ennerdale in terms of maintaining optimal and sustained dosing of fluoride to the water supply.
From October 1996 to June 2002, the Cornhow works had been non-operational after the installation of a treatment process to reduce the risk of cryptosporidium in the main water treatment process. The problem resulted in a requirement to cease fluoridation of the water supply at the plant. In 2002, when the fluoride dosing recommenced, leaks occurred, again, necessitating removal of the fluoride plant from service. In 2004, maintenance and refurbishments were undertaken and fluoride dosing recommenced. However, analysis of water samples showed that, almost from the outset of the commissioning, the plant struggled to achieve target dosing levels, although on no occasion did the concentration of fluoride exceed the statutory maximum. In April 2006, the dosing was suspended because of health and safety concerns relating to the fluoride stock tank and bund.
In 2004, Cumbria and Lancashire SHA commissioned the refurbishment of the equipment at Cornhow and commissioned a comprehensive refurbishment of the WF plant at Ennerdale. However, the dosing equipment performed suboptimally to specification from 2004 through to 2011, and from 2007 to 2010 the plant performed to specification for approximately only 20% of the time. As a result, the fluoridation plant at Ennerdale ceased dosing in November 2011.
NHS North West commissioned an independent review of the delivery of the scheme, recognising its legal obligations to reinstate the fluoride supply as quickly as possible. The SHA board agreed an action plan in July 2012, which included commissioning new equipment at both water treatment works and supporting a high-quality evaluation of the effects of reinstating the supply of fluoride.
Chapter 4 What we know now
This chapter provides an overview of the aetiology, pathogenesis and epidemiology of dental caries, the latter focused on children in the UK. The chapter then goes on to provide an overview of the current evidence of clinical effectiveness and cost-effectiveness of WF in the prevention of caries. The primary sources for this overview of the evidence for costs and effects of WF are seminal systematic reviews.
Aetiology and pathogenesis of dental caries
Dental caries is a chronic disease that affects the dental hard tissues, enamel, dentine and cementum. 35 The biochemical mechanisms of the aetiology and pathogenesis of caries are well understood. Dental caries is a localised phenomenon, with the disease process starting on the surface of susceptible dental hard tissues and, if untreated, leading to progressive destruction of the tooth. The initiation and progression of the disease requires the simultaneous presence of three elements:
-
a susceptible tooth surface
-
acidogenic bacteria in the biofilm (dental plaque) that covers the tooth surface
-
the presence of fermentable carbohydrates (sugars).
The acidogenic bacteria in dental plaque metabolise fermentable sugars ingested in the diet and produce acids, and this results in a lowering of pH in the plaque at the tooth surface, which promotes the loss of calcium and phosphate ions from the hard tissues of the tooth. This is a dynamic process: ions are exchanged between the tooth surface and the plaque biofilm, with an outflow of ions from the tooth surface at low pH and ingress of ions as pH rises. If there is frequent ingestion of fermentable sugars, then a low pH is maintained in the plaque for long periods, causing disruption in the balance of the ionic exchange and resulting in a net loss of mineral from the tooth.
At this early stage, when the process is confined to the enamel of the tooth, the loss of mineral is reversible. If demineralisation is not reversed and progresses, then the surface of the tooth becomes porous and is accompanied by loss of the organic material of the hard tooth tissues leading to cavitation, which is irreversible. If the disease is untreated, then the carious lesion advances through the dentine towards the pulp chamber of the tooth and the toxins released by the disease process promote an inflammatory reaction in the pulp. The release of inflammatory exudate within the confined rigid walls of the pulp chamber produces an increase in pressure, which results in pain (toothache) and eventually necrosis of the pulp. Subsequent infection of the pulp can spread through the foramina at the apex of the tooth root, resulting in a periapical or dental abscess.
Social, behavioural and environmental contributions to caries
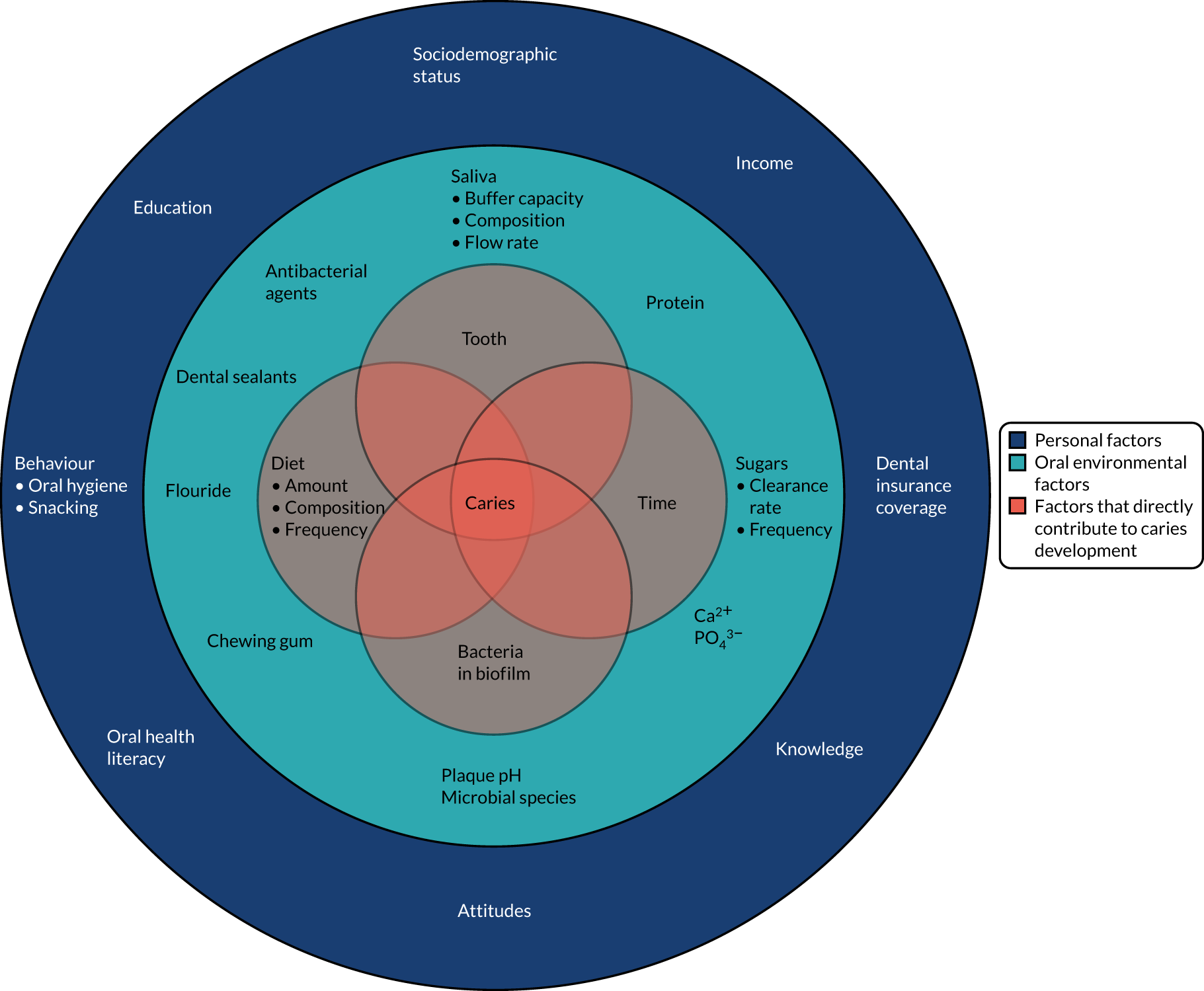
Dental caries is a complex disease and, although the three essential elements of tooth surface, cariogenic bacteria and fermentable carbohydrates must all be present for caries to develop, the initiation and progression of the disease is dependent on many factors that affect the influence of each of the three essential requirements and interact with one another. The so-called social determinants of dental caries, demonstrated in Figure 3,36 illustrate the interlinked biological, social and societal factors that contribute to the disease.
FIGURE 3.
Social determinants in caries. Reprinted from Lancet, 369, Selwitz RH, Ismail AI, Pitts NB, Dental caries, pp. 51–9, 2007, with permission from Elsevier.
Susceptibility of the tooth surface
The morphology of teeth influences susceptibility to caries, and the pits and fissures of teeth are more susceptible than the smooth surfaces of teeth. The presence of dental restorations or prostheses makes tooth surfaces more susceptible to caries, whereas professionally applied preventative sealants form a barrier between the tooth surface and the biofilm. Professionally prescribed preventative treatments using high concentration fluoride preparations, such as fluoride varnish, also provide a degree of protection.
Time
Caries is usually a slow-progressing disease that is characterised by periods of demineralisation and remineralisation of the tooth surface as intraoral conditions change in response to cariogenic challenges. Only when the balance of the demineralisation–remineralisation dynamic is weighted towards demineralisation does caries occur. If there is constant exposure to refined carbohydrates, then the disease can progress rapidly.
The structure and content of the dental biofilm
Disruption of the biofilm through oral hygiene-related behaviour (e.g. toothbrushing and professionally provided cleaning) reduces susceptibility. Likewise, an increase in the availability of fluoride within dental plaque helps prevent dental caries.
Diet and dietary behaviour
Reduction in the frequency of consumption of fermentable carbohydrates reduces caries risk.
Saliva
Saliva acts as a buffer and can limit falls in plaque pH. If saliva flow is impaired due to disease or, more commonly, as a side effect of medications, then the buffering action can be lost, favouring demineralisation and, therefore, increasing caries risk.
Epidemiology of dental caries internationally and in the UK
Dental caries is the most common disease experienced by mankind and affects more than one-third of the global population. 37 It is universal, affecting all populations, regardless of age, sex, ethnicity, occupation and geographical location. In the distant past, caries was a disease of the wealthy, as the wealthy were more able to afford and consume foods and drinks containing refined sugar. Post-World War II, refined sugar consumption expanded considerably and over the last 70 years caries has become a disease associated with social deprivation, especially in developed nations like the UK. 5,38
The international picture
Internationally, the prevalence of dental decay varies from country to country. Information from the World Health Organization Oral Health Data Bank shows that at a global level there has been a trend for an overall decrease in the prevalence of caries. 39 Numerous epidemiological studies3,40,41 have shown a reduction in caries in developed countries. In the immediate post-war period, the increased availability of sugar was associated with the increased levels of caries, but by the 1970s and 1980s a decrease in the prevalence of caries was being widely reported internationally in the literature. 3,41–43 Most commentators attribute this decrease to the rapid expansion in the availability and widespread use of fluoride toothpaste first introduced in the 1970s. 44 It is likely that other factors, such as government dental health polices, shifts in dietary patterns and improved oral hygiene, have also played a contributory role in this fall in disease. Primarily, the decrease in caries was reported in developed countries, with some studies41,45 conducted in a number of developing countries indicating a rise in the levels of caries. However, a recent systematic review46 looking at data from 1970 and 2004 concluded that the perception that dental caries rates are increasing in developing countries could not be supported.
UK trends in caries epidemiology
Caries in the primary dentition
Every 10 years, since the 1970s, a national child dental health survey has been conducted in the UK. The last national survey, conducted in 2013, reported that obvious caries prevalence in 5-year-olds varied between home countries, from 31% in England to 40% in Northern Ireland and 41% in Wales; however, these figures had decreased from those reported in the 2003 survey3 (i.e. 41% in England, 52% in Wales and 61% in Northern Ireland) (note that data for Scotland were not reported3). The 10-yearly national surveys are supplemented by more frequent local surveys in England, which have been recently coordinated by Public Health England. These surveys are reported on a lower-tier local authority level and are conducted by trained and calibrated examiners following a standardised national protocol similar to that used in the national surveys.
The latest survey recording caries in the primary dentition was conducted in 2019. 6 The survey reported dental caries prevalence in 5-year-old children in England of 23.4%. 6 However, prevalence varied significantly at a regional level, ranging from 17.6% in the south-east of England to 31.7% in the north-west of England. Dental health within Cumbria sits just above the national average, with a slightly higher proportion (24.2%) of children having had decay experience. When looking at severity of disease, data also demonstrate that children have a similar mean number of teeth with experience of decay in Cumbria [0.8 teeth, 95% confidence interval (CI) 0.68 to 0.86 teeth] compared with England (0.8 teeth, 95% CI 0.78 to 0.81 teeth). The same survey reported significant differences in caries prevalence in England according to ethnic grouping, with a prevalence of 36.9% in a group categorised as Asian/Asian British compared with a prevalence of 20.6% in a group categorised as white British. Socioeconomic status measured using the national IMD 201921 demonstrated significant health inequalities and a social gradient in caries prevalence, which was unsurprising given the well-documented association between deprivation and dental caries. The prevalence of dental caries was 13.7% in 5-year-olds living in the least-deprived quintile compared with a prevalence of dental caries of 34.3% in 5-year-olds living in the most-deprived quintile.
Caries in the permanent dentition
National child dental health surveys show that the prevalence of caries in permanent teeth has fallen rapidly over the last 40 years in the UK, with the prevalence of obvious decay experience in 12-year-olds in England falling from 81% in 1983 to 52% in 1993, and further to 43% in 2003 and 33% in 2013. 3 The last local survey of 12-year-olds was coordinated by the NHS Dental Epidemiology Programme for England and was conducted in 2008/9. The survey reported that 33.4% of pupils were found to have experience of caries in their permanent teeth at a national level. 47 Again, there was significant variation at a regional level, with the highest prevalence in Yorkshire and the Humber (44%) and the lowest on the south-east coast of England (25%). The report compared trends in disease over time using data on 12-year-olds from the 1973, 1983 and 1993 national child dental health surveys and the NHS Dental Epidemiology Programme surveys of 12-year-olds conducted in 1993, 1997, 2001 and 2009, and concluded that caries levels in 12-year-olds continued to decline between 1993 and 2009, but not as steeply as the fall documented between the national surveys of 1973 and 1993.
Health inequalities
In common with many other communicable and non-communicable diseases, dental caries is more common in deprived communities. From the 1990s, a new type of caries distribution among children in developed countries was being reported in the literature. 3 Most of the disease was being assigned to small, high-risk, socially and economically disadvantaged communities within the population, and this was initially described as the 80–20 phenomenon, that is 20% of the children harboured 80% of the caries in the population. 48 However, population segmentation analyses using area measures of socioeconomic status reported that about half of the population disease was confined to a minority of the population, but not to the extent of 80% of the disease in 20% of the population. These analyses also found that, although children with caries were more likely to be found in areas of social deprivation, caries was not confined exclusively to a small number of such areas, and caries prevalence exhibited a shallow gradient from poor to more affluent localities. This gradual fall in disease prevalence from most to least deprived illustrated the social gradient of disease model, as described in the Marmot review. 49
The national child dental heath surveys document the association between caries and deprivation in the UK. 50 The 2013 survey showed that children who were from lower-income families (defined as eligible for free school meals) were more likely to have caries than their more affluent peers. One-fifth (21%) of the 5-year-olds who were eligible for free school meals had severe or extensive tooth decay, compared with 11% of 5-year-olds who were not eligible for free school meals. Looking at the permanent dentition, one-quarter (26%) of 15-year-olds who were eligible for free school meals had severe or extensive tooth decay, compared with 12% of 15-year-olds who were not eligible for free school meals.
Significant inequalities are also evident in access to, and utilisation of, dental services. Individuals with the greatest need are least likely to access and utilise dental services. The 2013 child dental health survey reported that income deprivation is negatively associated with dental attendance. 50 Children aged 12–15 years from more deprived families were more likely to report their dental visiting to be triggered by symptoms, rather than attending asymptomatically for check-ups. Children from disadvantaged backgrounds were also more likely to report that they had never been to the dentist at the age of 12 years.
Caries risk
Caries risk or susceptibility to caries is largely dependent on how an individual’s lifestyle and behaviour (see Figure 3) influence the main aetiological determinants of caries (i.e. susceptible tooth surfaces, presence of cariogenic bacteria and consumption of refined sugars). Widespread availability of fluoride toothpaste and regular exposure to fluoride via toothbrushing as a social norm is believed to be behind the significant reductions in population caries prevalence described above. Toothbrushing with fluoride toothpaste both loads the biofilm with fluoride, protecting the tooth surface, and disturbs the biofilm, reducing its bacterial load. A recent behavioural study,51 conducted in parallel with the National Institute for Health and Care Research (NIHR)-funded Northern Ireland Caries Prevention In Practice (NIC-PIP) trial,52 on a population of 2- to 3-year-olds followed for 3 years provided useful insights as to why some children developed the disease and others remained caries free. The study51 reported that toothbrushing was widely adopted from a very young age and quickly became an ingrained or automatic behaviour for most families. However, frequent, between-meal sugar consumption was highly prevalent, and many parents struggled to control their child’s sugar consumption. The authors51 hypothesised that use of fluoride to prevent caries is limited in its effectiveness in the presence of unrestricted and frequent sugar consumption.
Over the last 10 years, a growing number of caries risk or caries prediction models and/or tools have appeared in the literature, such as the Caries Management By Risk Assessment (CAMBRA) and the Veterans Affairs Caries Risk Assessment (VA CRA) tool. 53,54 Despite these tools having numerous questions linked to ‘predictive factors’, the most accurate predictive risk factor for caries development at new sites is existing presence of the disease, typically over a 12-month period. A longitudinal observational study55 of caries development and progression in 7- to 16-year-olds in the north-west of England provides useful information for the disease trajectory of dentinal caries in permanent teeth as children move into adolescence (the study population55 is also geographically similar to the CATFISH study population). Clinical data were available from 6651 children over four time points. Caries prevalence was 16.7% at the first clinical examination (at ages 7–9 years), increasing to 31.0%, 42.2% and 45.7% at subsequent examinations. Children with caries in their primary dentition had a much steeper trajectory of disease in their permanent dentition than their caries-free contemporaries. The decayed, missing or filled teeth (permanent) (DMFT) count as pupils aged was significantly higher (4.49 times, 95% CI 3.90 to 5.16 times) in pupils with caries in their primary dentition than in pupils with a caries-free primary dentition. This study55 highlighted the importance of prevention in early years to reduce the risk of development of caries in late childhood into adolescence.
Caries in adults
In addition to the 10-yearly UK child dental surveys, the Department of Health and Social Care in England, the Welsh Assembly Health Department, and the Department of Health and Personal Services in Northern Ireland have commissioned 10-yearly national adult dental health surveys since 1968. Data from successive adult dental health surveys show a clear and rapid declining trend in the prevalence of edentulism (i.e. complete absence of teeth) in the population. 56 The first adult dental health survey in 1968 revealed a prevalence of edentulism of 37%, compared with just 6% in 2009, as many more people are retaining their teeth into old age. The UK has an ageing population, and over the past 40 years the proportion of population aged ≥ 65 years has increased from 13.8% to 17.7%. Furthermore, it is predicted that over the next two decades the proportion of people aged> 65 years will further increase to around 24% of the population. Retention of teeth into older age is associated with a need for more complex and, therefore, expensive dental care to maintain ageing, usually heavily filled, dentitions. This complex dental care, coupled with an expanding older population, suggests that there will be an increased burden on NHS dental services to meet the oral health needs of older people over the next 20 years. Observational studies and surveillance programmes56,57 show that, in the last four decades, dental caries in permanent teeth as a public health problem has evolved from a rapidly progressing disease of childhood, which results in early tooth loss, to a slowly progressing disease, where much of the burden is increasingly experienced by older adults.
Burden of disease
Tooth decay can cause pain, sleepless nights, sepsis, loss of function, social embarrassment, excessive use of antibiotics and the loss of productive workdays/school attendance. 9 As the disease claims progressively more tooth tissue throughout the life cycle, its effects are cumulative and can lead to complete tooth loss, and is one of the leading global causes of years lost to disability. 56 Dental treatment of caries is an uncomfortable experience and can provoke severe anxiety for some people. Thirty per cent of UK adults report that having a tooth drilled would make them very or extremely anxious. 12 Dental care is also very costly to society. Across the 28 European Union countries, treatment costs are higher than costs for Alzheimer’s disease, cancer and stroke, with only diabetes and cardiovascular disease costing more. 58
Looking at young children, a UK prospective cohort study of 3- to 6-year-olds showed that once a child develops caries it progresses rapidly and has a significant impact on their lives and their families. 7 Children with caries had a 25% risk of experiencing pain and an 11% risk of having an extraction each year. If the disease is unchecked in young children, multiple extractions under DGA are common. Dental extractions are the most common reason why young children in the UK have a DGA. Exact figures are difficult to quantify, but Hospital Episode Statistics data, which captures only a proportion of extractions carried out within a hospital setting, estimates at least 60,000 hospital episodes each year within England alone. 8 DGA extractions have a significant negative impact on young children and their families, and there is a strong association between dental extractions and dental anxiety, which can continue to affect individuals in later life. 59,60 Unsurprisingly, given the well-documented social gradient in dental caries, inequalities in extractions under DGA are also clear, with children from disadvantaged backgrounds more likely to have experience of DGA for exodontia than their more affluent peers. 61
Effects of fluoride
Since the mid-twentieth century, there has been an understanding that fluoride can prevent the development, and reduce the severity, of dental caries. 25 Over much of this time, there has been a debate about how fluoride exerts its preventative effects, particularly whether the mechanism bestowing protection is systemic in nature, arising from ingestion of the fluoride and incorporation into the developing tooth germ, or if fluoride’s mode of action is topical, influencing the ion exchange at the tooth surface–biofilm interface.
Systemic effects
Teeth develop in utero and during infancy. 62 Within tooth germs, specialised secretory cells called ameloblasts and odontoblasts lay down enamel and dentine, respectively. Development of enamel has a secretory stage when the organic and mineral content of the enamel matrix is produced and a maturation phase when the composition of the enamel is modulated. During this phase, matrix proteins are removed from extracellular space, and mineralisation increases to form a fully mineralised enamel matrix.
The maturation stage of enamel is considered the stage most susceptible to fluoride exposure. Under chronic fluoride exposure the outer surface of the enamel is thought to progressively hypermineralise during the maturation stage, providing greater protection to the tooth surface. However, studies have shown that the differences in fluoride concentration in surface enamel between permanent teeth from individuals from areas with no or low fluoride levels and fluoridated areas are minimal, and there is a contemporary consensus that the preventative effect of fluoride is almost exclusively post-eruptive and topical rather than systemic. 63 There does, however, remain uncertainty regarding the role of systemic fluoride in the delayed eruption of the permanent teeth. It is argued by anti-fluoridation groups that such a delay, possibly resulting in teeth erupting after peak risk periods, could explain the lower caries prevalence and severity of individuals exposed to WF. 64,65
Topical effects
The preventative effect of fluoride is thought to occur at the tooth–plaque interface by influencing the dynamics of ion exchange. Fluoride impedes demineralisation of the tooth surface in several ways, namely by reducing bacterial acid production, by reducing the solubility of apatite crystals that make up the inorganic content of the enamel matrix and by the fluoridation of apatite crystal surfaces, interacting with hydroxyapatite to form fluorapatite, which is less susceptible to erosion by acid-producing oral bacteria. 66
From available evidence,67 the latter process seems to have the most important effect. The effect depends on the presence of sufficiently high fluoride concentrations in the plaque to maintain tooth surface fluoridation. 67 Fluoride also promotes remineralisation of hard tissues (even at low concentrations) and, therefore, slows or prevents overall mineral loss. The formation of intraoral fluoride reservoirs capable of supplying ions for a prolonged period is crucial to the success of topical treatments. Such reservoirs include calcium fluoride, formed mainly at tooth surfaces, and fluoride associated with organic components of plaque and oral soft tissues. Fluoride is thought to delay the point of (irreversible) cavitation at a given sugar intake, thereby slowing the progression of the disease. However, when consumption of sugar is higher than 3% of total energy intake, caries will steadily accumulate throughout life, even in populations that are widely exposed to fluoride. 68
Risks of overexposure to fluoride
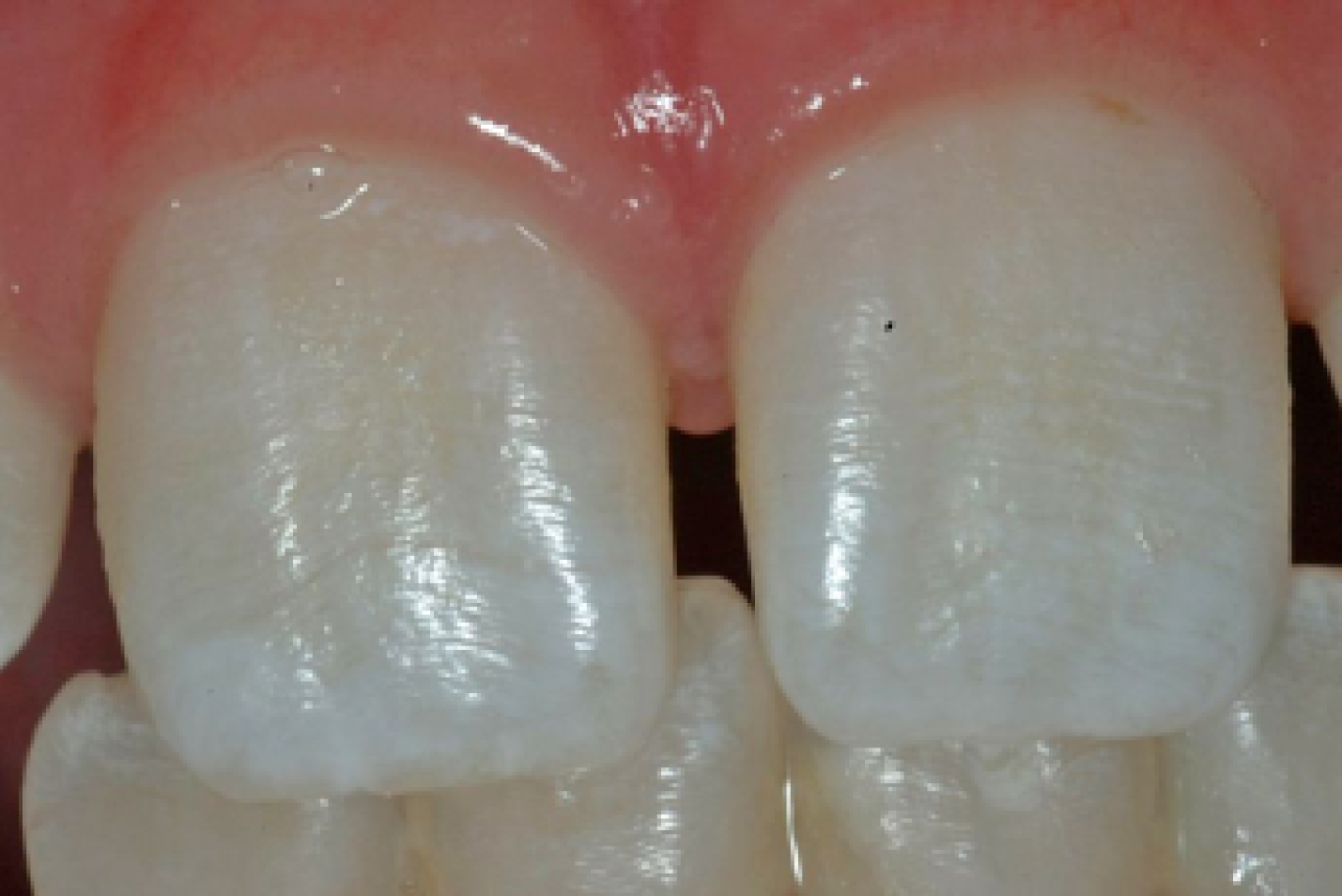
Fluoride is a single highly electronegative ion that interacts with the cells and developing enamel matrix within the tooth germ at the different stages of enamel formation. Both fluoride dose and duration of exposure have important influences on outcomes. 69,70 Intermittent exposure to high concentrations of fluoride over a prolonged period is believed to have the biggest impact on the developing tooth germs, leading to dental fluorosis (Figure 4), which is a condition that ranges from mild and barely discernible mottling of the tooth surfaces to pitting and softening of the enamel surface with hard tissue loss. 70 Dental fluorosis of aesthetic concern is an established risk related to WF, but it is dose dependent. For a fluoride level of 0.7 ppm, the chance of developing dental fluorosis is 12%, and the odds increase by 2.9% with each 1-ppm increase in fluoride level. 71 Supraoptimal levels of fluoride (e.g. high concentrations in water supplies occurring naturally) have been linked not only to severe dental fluorosis, but also to skeletal fluorosis. 72,73
FIGURE 4.
An example of mild fluorosis of the type seen in English studies.
Dental fluorosis is the only scientifically recognised impact of fluoride at the levels found in England’s WF schemes. However, it should be noted that fluorosis is seen in children who do not, or have ever, lived in a fluoridated area. 74,75 Public Health England has published the WF health monitoring report every 4 years (as a requirement of the Water Act 200331), which covers dental caries, fluorosis and other conditions that have been, arguably spuriously, linked to higher levels of fluoride, and the last publication of the report was in 2022. 76 Anti-fluoridation groups will point to studies showing the impact of fluoride on intelligence quotient (IQ) levels, thyroid toxicity and kidney damage; however, many of these studies are scientifically flawed, were conducted in areas where fluoride levels are extremely high (e.g. in some southern Indian states or in provinces in China) or fail to demonstrate any link between the fluoride levels observed and the supposed adverse health outcome. 77
More recently, however, a number of well-respected groups in the USA and Canada have reported ‘associations’ between artificially provided fluoride (in salt, as well as water) and adverse health outcomes, including IQ. 78 Although the authors78 correctly state that their work is preliminary findings and, therefore, not conclusive, it would be inappropriate to disregard such work as junk science, as has been done in the past.
Concerns are also raised by dental academics regarding the levels of fluoride that individuals will be exposed to, for example with the increased use of fluoride varnish for younger children and the availability of high-concentration fluoride toothpastes for individuals at risk of caries. Contested reports from the US National Health and Nutrition Examination Survey suggest not only that the prevalence of fluorosis is rising, but also that its severity is increasing. A near doubling of the most severe levels of fluorosis is reported in the work by Neurath et al. 79 and these risks need to be placed into two contexts:
-
In most cases, fluorosis is purely aesthetic and many adolescents report preferring the appearance of mildly fluorotic teeth as teeth often look ‘whiter’. In addition, evidence suggests that the appearance of milder forms of fluorosis reduces with age.
-
The burden of risk is carried by individuals with little risk of caries, and, in England, this represents the majority of children.
The current research does not seek to explore the risks of fluorosis or any other health-related condition because of the limited follow-up period of the birth cohort (fluorosis is usually measured after the permanent incisors erupt after 9 years of age) and is, instead, focused on the dental health benefits. However, in considering these benefits, should they be proven, the reader should be aware that they are not achieved without some risk (e.g. there is a low recognised risk of fluorosis) to the population.
Effectiveness of water fluoridation
We have known for over 90 years that fluoride can prevent tooth decay, and the improvement in oral health seen over the past 30 years is attributed mainly to the introduction of fluoride on a mass scale via fluoridated toothpaste. 44,80 The oldest method of administering fluoride is via the water supply, typically at a concentration of 1 ppm. Early studies of WF in the 1940s and 1950s showed very dramatic falls in caries; however, since the widespread introduction of fluoride toothpaste in the 1970s, it has been increasingly difficult to separate out the effect of WF programmes from that of exposure to fluoride from other sources, primarily toothpastes, but also mouthwashes and professionally applied gels and varnishes. 81
In the UK, over the last 20 years, three key documents have considered the effectiveness of WF. The first document is the so-called York review, published in 2000,16 which was commissioned by the chief medical officer and led by the NHS Centre for Reviews and Dissemination, University of York, and was a landmark systematic review of the effects of WF. The second document was the Medical Research Council Working Group’s report on Water Fluoridation and Health, which was published in 2002. 18 Most recent is the Cochrane systematic review Water Fluoridation for the Prevention of Dental Caries, which was published in 2015. 71
The York review
The aim of the York review16 was to assess the evidence on the positive and negative effects of population-wide drinking WF strategies to prevent caries. The report was critical of the then current research base for fluoridation, commenting that for such a high profile and contentious subject there was a surprising lack of high-quality research due to the high risk of bias in available studies. The York review16 had five distinct objectives, the first of which was to evaluate the effects of the fluoridation of drinking water supplies on the incidence of caries. The authors reported a median difference in the proportion of caries-free children of 14.6% (lower quartile 5.05%, upper quartile 22.1%) and a median difference in dmft/DMFT score of 2.25 teeth (lower quartile 1.28 teeth, upper quartile 3.63 teeth).
The second objective was to identify if WF has beneficial effects over and above alternative interventions. Nine studies, all completed after 1974, following the introduction of fluoride toothpaste, were included in this part of the review. 16 The quality of these studies was, again, assessed as suboptimal and limited the ability of the team to confidently answer the research question. However, the review team reported that a beneficial effect of WF was still evident, even in the presence of exposure to fluoride from other sources. The third objective was to examine the effect of WF on inequalities in caries experience. Again, the quality of studies was judged to be limited and analysis was hampered by heterogeneity in the measurement of socioeconomic status. Although the team reported that there appeared to be some evidence that WF reduces the inequalities in caries experience in 5- and 12-year-old children, using the dmft/DMFT index, this effect was not seen in the proportion of caries-free children among 5-year-olds, and the team urged caution in interpreting these results because of the small number and poor quality of available studies. 16
The fourth objective assessed negative effects of fluoridation and primarily dealt with risk of fluorosis. A significant dose–response relationship was identified: the prevalence of (any) fluorosis was estimated to be 33% (95% CI 26% to 41%) at a water fluoride concentration of 0.4 ppm, 48% (95% CI 40% to 57%) at a water fluoride concentration of 1.0 ppm and 72% (95% CI 62% to 80%) at a water fluoride concentration of 4 ppm. The review16 reported a lack of evidence for other postulated harms of WF, such as cancer and bone fractures. The final objective concerned potential differences in the effects of natural and artificial WF. This section of the review16 was substantially limited by the lack of studies comparing the effects or natural and artificially fluoridated water supplies. The team could find no major differences, but the evidence was not sufficient to make a conclusion regarding this objective.
Several areas of the evidence base were criticised by the report. 16 One major concern was the presence of observer bias in the measurement of caries and fluorosis within populations, especially as the examiners were aware of the fluoridation status of the participants. Weaknesses were also attributed to subjective instruments (visual indices) used to measure the presence or absence of caries and fluorosis. One of the most valuable outcomes of the review16 was a set of recommendations to improve the quality of future research projects. The following review study inclusion criteria have assumed a special importance as a marker of quality of research into WF (for the evaluation of impact on caries):
-
at least two populations compared
-
different fluoride levels in different populations
-
prospective study design, assessing two points in time
-
start of study < 1 year since change in fluoridation status
-
measurable outcomes reported (e.g. dmft/DMFT scores).
In addition, it was recognised that it is important to adequately adjust for effect modifiers, such as socioeconomic status, frequency of sugar consumption, total exposure from all sources of fluoride, the number of erupted teeth per child and the level of spending on dental health promotion and primary care services, and to report variance data. It was recommended that blinding of observers measuring outcomes should be attempted and that standardisation of the assessment methods is essential in future studies to reduce the risk of observer bias. The need for appropriate measures of socioeconomic status and and to consider longitudinal changes in socioeconomic status was also highlighted.
The Medical Research Council Working Group’s Water Fluoridation and Health
Following publication of the York review16 and the criticism of the quality of evidence, the Department of Health and Social Care approached the MRC to review the conclusions and recommendations of the York review16 and to consider what further research might be required to improve the evidence base in fluoride and health. A working party was established with the following terms of reference:
-
provide advice on current scientific evidence regarding the health effects of fluoride in the context of WF
-
consider what further research in this area might be required and what priorities should apply to usefully inform public health policy in this area.
Unsurprisingly, given the MRC’s interests, there was a significant biomedical flavour to the Working Party’s recommendations. In particular, the Working Party placed importance on a better understanding of the bioavailability and absorption of fluoride from naturally fluoridated and artificially fluoridated drinking water and the impact of water hardness on bioavailability. The Working Party recommended that WF should be a priority area for research funding in the future and also picked up many of the methodological difficulties identified by the York review. 16
The possible beneficial influence of WF on health inequalities was highlighted; however, it was recommended that further research should be conducted on this issue. 18 Both the York review16 and the MRC report18 stated that the link between caries and WF should be studied further and that contemporaneous data on possible effect modifiers, such as ‘discretionary’ fluoride (primarily toothpaste) use and dietary sugar consumption, should be collected in subsequent studies and included in analyses.
The Cochrane systematic review
Fifteen years after the York review,16 the Cochrane Oral Health Group published a systematic review, Water Fluoridation for the Prevention of Dental Caries. 71 The review71 was undertaken in response to the subject matter being identified as a priority topic in the Cochrane Oral Health Group’s international priority-setting exercise. The review team also acknowledged the passing of time since the York review,16 querying whether or not the conclusions were still relevant to contemporary society and noting that many of the caries studies presented in the York review16 were conducted prior to the widespread use of fluoride toothpastes, introduced in the 1970s, and exposure to other fluoride vehicles, such as fluoride varnish, which is now used extensively in primary care and community prevention strategies.
The Cochrane review is a key document for the CATFISH project, as it is the most recent comprehensive review of the effects of WF provided by a trusted source with a peerless track record of conducting high quality systematic reviews in the dental field. The Cochrane review updated the York review and aimed to contextualise the evidence to inform current national and international guidelines.
The Cochrane review71 was narrower in focus than the York review,16 concentrating solely on the effects of WF on preventing dental caries and increasing the risk of dental fluorosis. The Cochrane review71 made no distinction between artificial and natural WF and so did not investigate differences in the effects of natural and artificial WF. The authors71 confined their assessment of potential negative effects of WF to fluorosis and did not consider other possible adverse effects, such as bone fracture and cancer, which came under the York review’s16 remit. The inclusion criteria for caries and fluorosis in the Cochrane review71 followed those of the York review,16 illustrating the importance of the York inclusion criteria in guiding the design of evaluations of WF.
A total of 155 studies met the inclusion criteria of the review and 107 studies provided sufficient data to enable quantitative synthesis. 71 For caries severity, the review reported results in reductions in dmft because of WF [mean difference 1.81 teeth (95% CI 1.31 to 2.31)] and in DMFT [mean difference 1.16 teeth (95% CI 0.72 to 1.61)], translating to a 35% relative reduction in dmft and a 26% relative reduction in DMFT compared with control group mean values. The studies included in both analyses were assessed collectively as having a high risk of bias.
When assessing the impact on caries prevalence, increases in the percentage of caries-free children were identified in fluoridated compared with non-fluoridated populations. An increase of 15% (95% CI 11% to 19%) caries free in the primary dentition and 14% (95% CI 5% to 23%) in the permanent dentition were reported. The authors71 noted that most studies (71%) were conducted prior to the introduction and widespread use of fluoride toothpaste in 1975.
The Cochrane review,71 like the York review,16 found insufficient evidence to clearly determine whether or not WF affects inequalities in caries experience and/or prevalence. Like the York review,16 the Cochrane review71 noted the paucity of studies investigating the impact of WF on adult dental health and how no studies that aimed to evaluate the effectiveness of WF in adult populations met the review’s inclusion criteria. The review71 reported an estimated 40% (95% CI 35% to 44%) prevalence of fluorosis of any level and a prevalence of 12% (95% CI 8% to 17%) of fluorosis of aesthetic concern when WF occurred at a level of 0.7 ppm. The review71 noted that over 97% of the studies reporting fluorosis outcomes were at high risk of bias and there was substantial between-study variation in outcome measurement. The studies included in the review that examined dental fluorosis were generally more recent than those that evaluated caries and, consequently, the assessment of WF’s contribution to fluorosis risk could have been influenced by exposure to other sources of fluoride. 71
As WF is a contentious topic, it was unsurprising that the Cochrane review would be criticised. Rugg-Gunn et al.,82 writing in the British Dental Journal, from a pro-fluoridation stance, provided a critique of the review, which argued that the inclusion criteria were too narrow and restrictive for a public health intervention and that the risk-of-bias assessment was also limiting, preventing inclusion of studies that collectively would provide a fuller understanding of the effectiveness of WF. The critique argued that the findings and conclusions of the Cochrane review71 are at odds with the wider literature on WF, concerning its effectiveness in adults and its effectiveness in reducing inequalities.
The critique noted that assessment of WF’s impact on inequalities was not a stated objective of the Cochrane review71 and identified the narrow inclusion criteria as the reason why some relevant reports were excluded. The York review found that the evidence about reducing inequalities in dental health was of poor quality, contradictory and unreliable. 83 A secondary analysis of English national surveillance data suggested that the preventative effect of WF is greater in the most deprived communities. 77 Importantly, the critique pointed out that the absence of evidence, or the existence of poor-quality evidence, should not lead to the conclusion or implication of an absence of effect.
The Cochrane review71 authors noted, like the York review16 and the MRC report,18 the significant shortage of contemporary evidence meeting both the York and Cochrane reviews’ inclusion criteria,16,71 and that little had changed in the 15 years between the reports. In fact, the available data for both reports came predominantly from the same studies, which were conducted prior to fluoride toothpaste introduction in 1975, and so it is not surprising that the York review16 and the Cochrane review71 findings are similar. What is surprising, however, given that both the York and MRC reports called for WF to be a priority for research funding, is that very few studies meeting the ‘York criteria’ have been commissioned since the York review16 and MRC report. 71 In the UK, this is primarily due to the political difficulties experienced in implementing new schemes (i.e. one of the key inclusion criteria of the York review), the significant costs of longitudinal studies involving clinical examinations and the difficulties of identifying matched comparator populations, particularly in countries such as the USA, Ireland and Australia with widespread WF schemes in place.
The Cochrane review71 identified several research questions, which are still to be answered 20 years after being proposed within the York review. 16 We still do not have a contemporary understanding of the health risks and benefits of WF, nor of its impact on health inequalities. As caries is thought to be increasingly more of a public health problem in older, rather than younger, populations, it is worrying that there is still very little information of its effects on adult populations. Standardisation of diagnostic criteria and reporting, as well as adequate measurement and control of potential effect modifiers, were all (once again) highlighted by the Cochrane review,71 as the implications of the findings for further research were discussed. A single study cannot hope to provide definitive answers to all of these questions; however, a contemporary study conducted in the UK that meets the York inclusion criteria is probably more helpful to UK policy-makers than another review of less robust, mostly historical, studies, most of which have little relevance to the current epidemiological, social, financial and behavioural climate.
Health economics of water fluoridation
Neither the York review16 nor the Cochrane review71 assessed economic evaluations of WF; however, both reviews16,71 and the MRC report18 acknowledged the importance of assessing the costs, as well as the health effects, of WF schemes. The York review16 made recommendations for the approach to be taken in future health economic evaluations of WF scheme. A full accounting of costs of the intervention, both capital and revenue, and the costs of potential benefits, such as the number of dental visits, costs of dental procedures and impact on quality of life, should all be recorded. In the UK, over the last 20 years, the establishment of the National Institute for Health and Care Excellence (NICE) and its role of assessing the value of health technologies to the NHS, as well as the establishment of NIHR and its funding of applied research to support innovation in the NHS and public health, has increased the necessity and sophistication of economic evaluations, particularly for issues with a high policy profile.
Health economic evaluation of WF is important because of its high profile and because the cost to the NHS of treating tooth decay is very significant.
In England, the NHS spends around £3.4B per year on dental services and the value of the private market is estimated at approximately £3B per year. 13 Patient charges roughly make up one-quarter of the total primary care NHS budget. Much of the NHS dental budget is consumed by the detection and treatment of dental caries. With falling population levels of caries but increasing costs of NHS dental services, a health economic evaluation is now a requirement of any contemporary investigation of WF.
A key publication to assess the literature on economic evaluations of WF is the 2020 scoping review by Mariño and Zaror. 84 Mariño and Zaror84 identified 498 studies, of which 24 (in eight countries) met the inclusion criteria and formed the basis of a qualitative synthesis of the results. The studies included in the review were published between 1973 and 2017. A variety of health economic evaluation approaches were found in the studies that met the inclusion criteria, including 15 studies with a cost–benefit analysis, nine cost-effectiveness analyses and four cost–utility studies. The main outcome measure used in the included studies was caries averted and caries reduction, and effects of between 25% and 40% were reported. The cost savings of dental treatment was the next most common outcome reported.
The most used perspective was the payer’s perspective (n = 12), which includes only the costs that are directly related to the provision of the WF programme and the dental health service costs. Eleven studies used a societal perspective, attempting to include the payer’s costs and the costs incurred by patients and their families because of the intervention or due to the loss of the productivity. All studies incorporated the intervention costs, including one-off capital costs of the fluoride dosing plant, plus recurrent fixed costs (e.g. costs of maintenance, operation and monitoring) and variable recurrent costs (e.g. chemical and supplies costs). Social perspective studies also included costs for lost productivity due to the time off work spent attending dental services and attendant transportation costs.
The main methodological approach employed was building Markov models (n = 21) using data from observational studies retrieved from the literature and only one study analysed primary data from a cohort study. All studies concluded that WF was a cost-effective strategy when it was compared with non-fluoridated communities, independently of the perspective, time horizon or discount rate applied. The authors84 reached the following conclusion on WF:
. . . [WF] represents an appropriate use of communities’ resources, using a range of economic evaluation methods and in different locations.
The review84 provides a useful and timely overview of the literature on health economic evaluations of WF. It was a scoping review and had limitations, most notably that no risk-of-bias assessment was conducted, which was an important omission given the findings of the Cochrane review risk-of-bias assessment. Virtually all of the studies included in the review employed Markov models, using literature reviews of WF studies to determine the effect size of the intervention. Therefore, these studies are confined to using the same data from the same literature that was criticised by the York review16 and the Cochrane review71 as being of poor quality, with high risk of bias and out of date as far as contemporary context (widespread use of fluoride toothpaste and rapid falls in population disease) is concerned.
In some cases, differences between economic model predictions can arise simply because of calculation errors. In other cases, there may be more subtle reasons why the results of economic models vary in their findings, including differences in the complexity of the models, different underlying modelling assumptions and the use of different modelling techniques. The costs of the intervention and, particularly, the health-care and societal costs incurred because of the intervention will vary enormously depending on the context in which WF is delivered. Factors such as population disease prevalence and nature of the health-care system will have a significant influence on costs and effects. Therefore, applying findings from historical international studies to a contemporary UK context could result in seriously flawed policy decisions.
Conclusions and rationale for the CATFISH study
Public debate on WF tends to be highly polarised, with very strong and entrenched views held by both pro and anti lobbies. As described earlier, studies emerging from well-regarded groups have led to further questioning of the value of WF as a contemporary public health measure. There is, therefore, a growing group of scientists legitimately stating that there is equipoise surrounding WF and dental caries. Arguably, such equipoise has existed since the York report has been published. 16
The milestone documents relating to the effectiveness of WF in the UK have been the York and Cochrane systematic reviews16,71 and the MRC Working Group’s report on Water Fluoridation and Health. 18 These three documents16,18,71 reached similar conclusions, that is, although there is evidence to suggest that WF provides a benefit in caries reduction, the evidence is of poor quality and is limited in its relevance to contemporary UK context.
In the UK, there has been a legislative, political and, increasingly, financial impasse presenting formidable barriers to implementing new fluoridation schemes that would facilitate evaluation according to the inclusion criteria and recommendations made by the York review. 16 In West Cumbria, a WF scheme established in the 1960s had been offline for several years because of a need to refurbish the fluoride dosing plant. The refurbishment totally replaced the fluoride plant and equipment, and fluoridation resumed in West Cumbria in 2013, which provided a unique opportunity to study the contemporary impact of WF according to the recommendations made by the York review16 (i.e. for the start of the study to be < 1 year since a change in fluoridation status) and the Cochrane review. 71 This also allowed for the topical effect as well as the ‘full’ systemic and topical effect of WF to be studied in Cumbria, as children who were aged approximately 5 years when fluoridation resumed would receive a predominantly topical effect for their permanent teeth.
The CATFISH project aimed to provide robust evidence of the effects and costs of a ‘reintroduced’ WF scheme on the oral health of young children. A new study can contribute to our understating of the relationship between WF and socioeconomic status, as well as enable empirical evaluation of the cost-effectiveness of WF in a contemporary UK context.
Over the last 20 years, the epidemiology of caries has changed, with the disease experience and prevalence reducing significantly. With this fall in disease, the rationale for evaluating WF has subtly changed, that is, with falling prevalence fewer individuals in the population can potentially benefit from an intervention applied to the whole population and there is a larger number of individuals who will gain no benefit but will be at risk from harm. As an increasingly smaller population can benefit and an increasingly larger population is at risk for no benefit, the economics and return on investment, particularly as public finances are squeezed, have become much more important for policy-makers in a WF evaluation.
The authors of the Cochrane review71 also drew attention to the applicability of the evidence (much of it over 40 years old) to current lifestyles, particularly how water is consumed, the availability and consumption of sugar, toothbrushing behaviour and exposure to fluoride from multiple sources. Given these changes, the policy focus for providing a context-sensitive and contemporary evidence base for WF is perhaps shifting away from the question of ‘What improvement in dental health can we expect from a new fluoridation scheme?’ to a different question of ‘Do we still need to provide these services, and can the resources be better used elsewhere?’.
Chapter 5 Aims and objectives
Our aim was to assess the clinical effectiveness and overall cost impact of the introduction of a WF scheme on a contemporary population of children (with lower than historic disease levels), using a research design that meets the requirements of a new scheme evaluation described by both the York and MRC reviews. 16,18
Study objectives
Using a research design that meets the requirements of a new scheme evaluation described by both the York review16 and the MRC review,18 our objectives are as follows:
-
to assess the effects and costs of both systemic (i.e. exposure from in utero) and topical exposure to WF following the introduction of a WF scheme on a contemporary birth cohort of children, compared with a birth cohort of children not exposed to WF
-
to assess the effects and costs of topical exposure (i.e. exposure from approximately age 5 years onwards – those who are in their first year of school) to WF alone following the introduction of a WF scheme on a cohort of contemporary children (with falling disease levels), compared with a cohort of children not exposed to WF
-
to measure the impact of WF on social class inequalities in child dental health
-
to assess the cost-effectiveness of a WF scheme.
Chapter 6 Methods
Some text in this chapter has been reproduced from Goodwin et al. 85 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The tables and text produced include minor additions and formatting changes to the original text.
Study design
A pragmatic population-based cohort study was undertaken to examine the effects of WF on dental decay in children. Replacement of a WF plant in a paused scheme in Cumbria provided the opportunity to investigate the effects of reinstating WF to a defined population, which met the criteria for evaluation of WF set out by the York review. 16 Two cohorts were recruited and were followed for 5 years:
-
a birth cohort recruited at birth from September 2014 (i.e. after fluoride dosing in Cumbria had recommenced)
-
an older school cohort recruited in their first year of school (at age 5 years) in 2013 (i.e. immediately after fluoride dosing in Cumbria had recommenced).
In each cohort, outcomes in the exposed (to WF) population were compared with outcomes in a socially and geodemographically similar non-exposed control population.
The study design was peer reviewed and the study protocol was published in 2016. 85 The Liverpool Central NHS Ethics Committee (for the older school cohort) and the Cambridge South NHS Ethics Committee (for the birth cohort) provided a favourable ethics opinion (references 13/NW/0494 and 14/EE/0108, respectively).
Patient and public involvement (PPI) played an important role in shaping the design and management of the study, and the interpretation of our findings [note that the full involvement and impact of the PPI is discussed in Appendix 5 using the Guidance for Reporting Involvement of Patients and the Public 2 (GRIPP2) form]. Julie Fletcher at Barnardo’s (Ilford, UK) was the PPI lead and facilitated PPI feedback through groups of parents within Cumbria. In addition, several permanent PPI members made up of parents with young children in Cumbria met on a regular basis with the research team and provided advice at key stages of the research project. The research team also had representation from senior representatives of key stakeholders in Cumbria County Council and Public Health England. An independent Study Steering Group appointed by NIHR oversaw the conduct of the study.
Participants
Both age cohorts resided in Cumbria and were split into West Cumbria, where WF was provided to the community via two fluoridation plants, and the remaining non-fluoridated localities in Cumbria. Figure 2 presents a map of Cumbria with the WF areas defined. The fluoridated area in Cumbria covers a population of approximately 132,134 people in the areas of Allerdale and Copeland (but not all residents in Allerdale and Copeland receive WF). The other non-fluoridated districts in Cumbria are Carlisle, Barrow-in-Furness, Eden and South Lakeland. The populations of these districts are reported in Table 2, with data taken from the Cumbria Observatory. 86 The populated fluoridated and non-fluoridated areas are, for the most part, separated by sparsely populated upland areas.
| Population descriptive | District | |||||
|---|---|---|---|---|---|---|
| Allerdale | Copeland | Carlisle | Barrow-in-Furness | Eden | South Lakeland | |
| Population, n | 97,831 | 68,041 | 108,524 | 66,726 | 53,754 | 104,905 |
| Percentage of children living in low-income families | 13 | 14 | 13 | 17 | 8 | 7 |
| Percentage of children living in the most deprived decile | 6.7 | 6.1 | 7.4 | 22.4 | 0 | 0 |
Before the study began, for the older school cohort, it was estimated that there would be 1412 children available for recruitment in the fluoridated area of West Cumbria and 1816 children available in the non-fluoridated are of North Cumbria. 87 For the birth cohort, hospital statistics at the time indicated that 2931 children could be born in the hospitals managed by the North Cumbria University Hospital NHS Trust. 88 This trust runs Cumberland Infirmary (Carlisle, UK) (i.e. the non-fluoridated area) and West Cumberland Hospital (Whitehaven, UK) (i.e. the fluoridated area) where recruitment of the birth cohort took place. Although exact data were not given for births in each hospital, the split was expected to be similar to the older school cohort.
Birth cohort
A birth cohort was chosen because this cohort provided an opportunity to investigate the ‘full’ effect of WF, comprising systemic exposure where ingested fluoride is incorporated into the tooth germ during its development in utero, coupled with topical exposure of the tooth surface to fluoride after the teeth erupt into the mouth. Participants were recruited from maternity units within the two hospitals in Cumbria by the NIHR Clinical Research Network North East and North Cumbria. All new parents were approached during pregnancy and postnatally (at the 20-week scan, immediately after delivery and at health visits within the first 3 months of birth) (Figure 5).
FIGURE 5.
Recruitment outline for the birth cohort. Reproduced with permission from Goodwin et al. 85 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Trained recruiters, including clinical research assistants and research nurses, identified eligible women and began face-to-face recruitment during 20-week scans at hospital sites in May 2014. All eligible women were provided with the study information leaflet prior to their 20 weeks’ gestation scan or at the 20-week scan (see NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/phr/SHMX1584). Individuals could fully consent by providing written consent (see NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/phr/SHMX1584) at the time they were approached or leave their details to be contacted after the birth of their child. If any women were not contacted during this time, then they were approached face to face in the hospital/maternity ward after giving birth (starting from September 2014) or a consent pack was sent home. Recruitment ended in September 2015 and no children born after September 2015 were included (see Appendix 1, Figure 16).
Eligibility was assessed by the research nurses according to the following criteria.
Inclusion criteria
-
Owing to the population-based nature of the study, and for potential benefits to be accrued at a population level, the study had broad inclusion criteria, with only individuals with significant health issues at, or around, birth not eligible for inclusion in the study.
Exclusion criteria
-
Individuals who were planning to move from the area within the duration of the study.
-
Individuals who were unable or unwilling to provide consent.
-
Individuals with life-threatening conditions (maternal or foetal) identified at the time of recruitment.
Fluoride exposure status of recruited participants was determined after recruitment by reference to home postcode. Participants were not paid to consent to take part in the study. However, when participants were contacted about the 3-year-old clinical examination they were offered £15 compensation, as they had to travel to clinics to attend a dental examination. During the last round of questionnaires, participants were also provided with a £10 voucher along with their questionnaire.
Older school cohort
The older school cohort comprised 5-year-olds recruited from primary schools in West Cumbria (i.e. fluoridated) and a control group of 5-year-olds recruited in schools located in the non-fluoridated area of Cumbria. The older school cohort was chosen because this cohort provided an opportunity to investigate the topical effect of WF on caries to enable comparison of effect size with children who have systemic and topical exposure as the cohorts age.
We approached all primary schools in West Cumbria (i.e. the exposed population) and a comparable group across North Cumbria (i.e. the non-exposed population) and asked them to participate in the study. Parents of 5-year-old children attending participating schools were contacted over one academic year (from September 2013 to July 2014). A study information sheet was sent to each child’s home address through the school. Consent was obtained using parental written consent via a letter home through the school, with child assent gained at time of the clinical examination. For the information sheet and consent form see NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/phr/SHMX1584.
Eligibility criteria were assessed, and provision of consent was checked by the clinical examiners prior to clinical examination in school.
Inclusion criteria
-
All children attending state schools.
-
Children in their first year of school at the time of consent.
-
Children who live currently, and had been lifetime residents, in the predefined area of Cumbria.
Exclusion criteria
-
Children who were unable or unwilling to consent.
Consent was re-visited on each successive examination using an opt-out system after initial written consent was given.
Exposure status of recruited participants was determined by reference to their home postcode. Participants were not paid for their participation but in the final clinical examination were provided a £10 voucher (like the birth cohort) for the final questionnaire to be completed.
Table 3 summarises the recruitment process of the two cohorts.
| Group | Sample/design | Recruitment began | Data collection commenced | School year | Age of child at start of study |
|---|---|---|---|---|---|
| Systemic and topical (including control) | All children born across two hospitals in Cumbria invited to participate | May 2014 | September 2014 | First year of school in 2019 | From birth |
| Topical (including control) |
All schools in WF areas invited to participate Comparable schools in non-WF areas selected |
September 2013 | September 2013 | First year of school in 2013 | 4 or 5 years |
Study settings
Birth cohort
Recruitment took place in the maternity units of West Cumberland Hospital and Cumberland Infirmary. Examination of children at age 3 years took place at dental clinics across Cumbria. Participants could choose to attend clinics in Carlisle, Wigton, Workington, Cleator Moor, Penrith or Kendal. Participants could book in on certain days to have their dental examinations and were also provided with a questionnaire when they attended. Children were examined at age 5 years in their primary school. In total, 172 schools were approached and agreed to allow access for dental teams to carry out dental examinations for children to take part in the study. An additional eight schools with a total of 38 participants stated that they did not want the team to carry out the clinical examinations at the school. Children attending these schools were to be invited to clinics.
Older school cohort
Children were examined at 5, 7 and 11 years in their primary school. One hundred and thirty-five schools were included when the study started, and nine schools refused to participate at the time. Reasons for schools not wanting to participate in study included the school was small and had few children in each year (and it would have been disruptive for the rest of the class), and schools having building works and it not being appropriate, or there was limited space, to carry out dental examinations.
Intervention
United Utilities was, and remains, the water undertaker contracted by Cumbria County Council to supply fluoridated water to the defined population of the West Cumbria scheme. The fluoride dosing occurred in two water treatment works in West Cumbria (i.e. Cornhow and Ennerdale). The water supply zones served by the water treatment works are presented in Figure 2. United Utilities provided daily dosing figures to the team in monthly reports throughout the follow-up period. We conducted our own independent water supply fluoride concentration monitoring annually across five different addresses in the fluoridated water supply zones and our independent monitoring corroborated the data provided by United Utilities.
Following the completion of a new plant installation, fluoride dosing at Ennerdale recommenced on 8 October 2013 and at Cornhow on 4 November 2013. In December 2015, Cumbria experienced very severe and prolonged flooding, which resulted in a temporary cessation of fluoride dosing at the Ennerdale water treatment works from 8 December 2015 and dosing did not resume at optimum levels until 1 October 2016 (average dosing each month across both plants is presented in Chapter 7). This resulted in 391 (64% of intervention group) children in the birth cohort and 394 (69% of intervention group) children in the older school cohort having suboptimal fluoride exposure during the 5-year follow-up period. As the intervention was an operational programme and commencement or variation of fluoride dosing was not part of the consideration of the Ethics Committee, amendment to our ethics approval was not required. This was a pragmatic study, and instability of fluoride dosing is not uncommon89 and remains a feature of operational WF programmes. Our published protocol85 did not provide a priori plans to investigate the effects of suboptional dosing, and with a newly installed plant we did not foresee this eventuality. The study was also not powered to compare the effects of groups with optimal and suboptimal dosing with no exposure. However, because of this significant disruption to the intervention, we completed supplementary post hoc analyses comparing caries outcomes for subpopulations receiving optimal (1 ppm continuous) dosing and suboptimal (disrupted) dosing with the no-exposure controls in both age groups. The analytical approach for these supplementary analyses was the same as the approach used for the primary and secondary outcomes, described in Post hoc analysis following interruption to dosing at one plant (Ennerdale). Apart from this period when the fluoride dosing plant was switched off, fluoride dosing at Ennerdale and Cornhow WTW operated satisfactorily.
Control groups
The comparator groups were children in each cohort residing in Cumbria, born during the same period as children in the intervention group, who had not lived in a fluoridated area during the 5-year follow-up period.
Comparator groups and blinding
Choice of comparator group
The intervention was provided to a defined population based on the geography of the water supply and, therefore, randomisation of individuals to intervention and control groups was not possible. A whole population approach was taken to provide a comparator population, rather than matching individuals. It was important to identify a control population that was as socioeconomically and geodemographically similar to the intervention population (i.e. similar age, sex, deprivation and ethnic profile, similar access and utilisation of dental services). Therefore, we chose neighbouring non-fluoridated districts in Cumbria as the control population. For both cohorts, we compared the sociodemographic variables of intervention and control groups (i.e. age, sex, deprivation at baseline) to ensure that the groups were comparable, to ensure unbiased estimates and to minimise selection bias. If significant differences between the groups on key variables at baseline were found, then we planned to perform regression analysis to adjust for these variables.
Blinding
Owing to the geographical nature of the intervention, dental examiners who worked in the Cumbria community dental service were likely to be aware of the fluoride exposure status of participants from the school they attended. This potential source of bias, which was also recognised by the MRC report,18 is difficult to mitigate. There have been attempts in past studies to blind examiners to water fluoride exposure status by moving children to central examination centres or using examiners from out of area. 90,91 However, such approaches were not feasible for this study because of its size and because of the large size of Cumbria and its very rural nature. Therefore, we elected to accept that the clinical workforce would be aware of participants’ fluoride exposure and to assess the presence of any bias by taking high-resolution intraoral photographs [using the S950 Pal Sopro Care intraoral camera (Acteon Group, St Neots, UK)] and comparing examination of caries in photographs with traditional clinical caries examinations. Intraoral photos were taken following the clinical examination by the same dental examiner. For 5-year-olds, five photos were taken, namely of the two upper incisor teeth/area, upper-left molar teeth/area, upper-right molar teeth/area, lower-left molar teeth/area and lower-right molar teeth/area. For 7- and 11-year-olds, six photos were taken, namely of the upper-right incisor, upper-left incisor, upper-right molar, upper-left molar, lower-right molar and lower-left molar. Intraoral photos were examined remotely and blind to fluoride exposure by one expert examiner, who had previously scored caries from images for other large-scale caries studies. 92 Figure 6 provides an example of intraoral images taken during the study.
FIGURE 6.
An example of intraoral images from an 11-year-old participant captured during the CATFISH study. (a) Upper-right incisor; (b) upper-left incisor; (c) upper-right molar; (d) upper-left molar; (e) lower-right molar; and (f) lower-left molar.
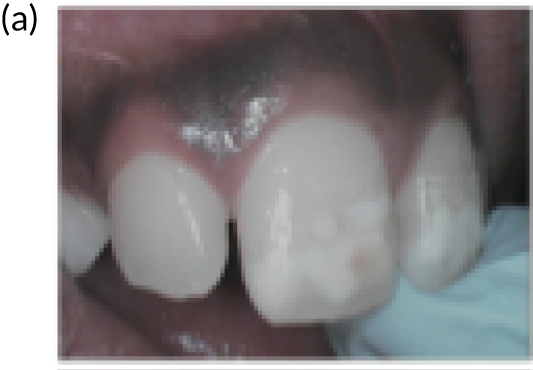
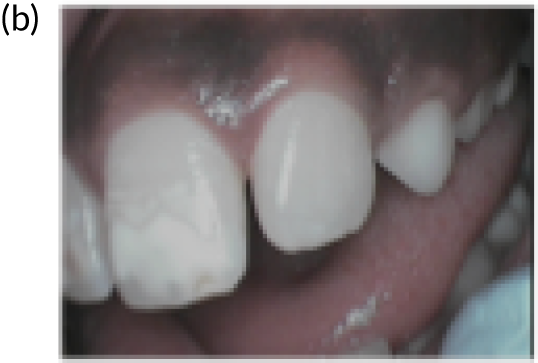
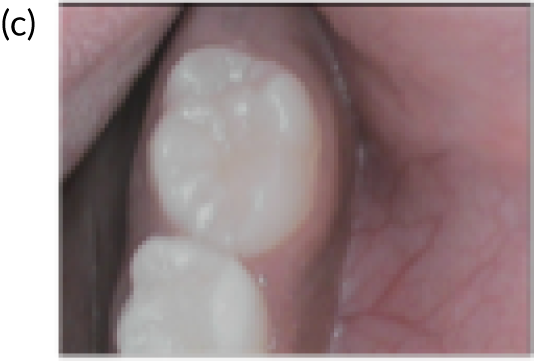
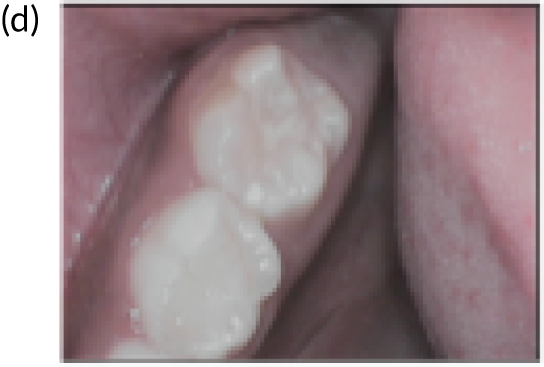
Images were stored on a secure database prior to being scored93,94 and then assessed in random order using the same caries classification scoring system used for the clinical examinations. 95 The difference in the proportion of participants with decay between test and control groups assessed using blinded photographs was compared with the difference in the proportion of participants with decay between test and control groups assessed using traditional unblinded clinical examinations to identify any systematic bias that may have been present due to examiner knowledge of exposure status.
Outcomes
Diagnostic protocol
The examinations were undertaken by trained and calibrated dentists working for the Cumbria community dental service. One of the key services provided by the community dental service is to undertake regular dental epidemiological surveys, which are conducted in schools and coordinated by Public Health England. We used the same diagnostic protocol and standard operating procedures as these surveys, that is, teeth were cleaned and dried using cotton wool rolls and a Daray lamp (Daray Ltd, Swadlincote, UK) was used to provide light. 95 Caries was recorded at the caries into dentine level for each tooth surface (final analysis was carried out at tooth level) and caries was considered present if there were one or more lesions into dentine present at the examination. Box 1 provides a list of the dental codes that were utilised (the full version of data collection forms can be found in NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/phr/SHMX1584).
Extracted caries: 6.
Unerupted or missing other: 8.
Surface codesSound: blank, ‘–’ or 0.
Hard, arrested caries: 1.
Decayed: 2.
Decay plus pulpal involvement: 3.
Roots only remaining: 3.
Filled and decayed: 4.
Filled: 5.
Filled, needs replacement: 11.
Obvious sealant restoration: 12.
Sealed surface: 13.
Crown: 10.
Trauma: 14.
Unrecordable: 9.
The same codes (see Box 1) were used for permanent teeth, but with an additional code of 7 for extracted due to ortho. Decay was recorded with any tooth, which had a code of 1, 2, 3, 4, 5, 6, 10, 11 or 12.
These codes enabled direct comparison with the Public Health England Child Dental Health Surveillance Programme outputs and ensured the schools and dentists used familiar consent, examination and data recording processes. Children’s teeth were cleaned and dried using cotton wool rolls prior to being scored clinically by trained and calibrated assessors.
Data were recorded on pseudoanonymised paper forms and were subsequently transferred to and processed within the Dental Survey Plus 2 software version 2.1 (University of Dundee, Dundee, UK; URL: www.nwph.net/dentalhealth), which is designed to record dmft. All data used unique identifiers to ensure anonymity when data were entered. These data were transferred and kept within locked cabinets at the main research centre (i.e. the University of Manchester).
Training and calibration
Calibration occurred every year there were clinical examinations, and this involved usual British Association for the Study of Community Dentistry (BASCD) practice, that is, a pre-training session was provided for any new examiners, children were screened the day before calibration to ensure sufficient numbers of children with decay experience and children who were caries free were included, and a training round occurred before calibration with at least two calibration rounds performed (for further information on calibration see the National Dental Epidemiology Programme: Training and Calibration Guide for Oral Health Surveys of Children96). Each dentist taking part and the reference examiner examined the same group of children for calibration (following the diagnostic protocol described above) and comparison of interexaminer differences were performed. Only dentists who achieved the minimum requirements for calibration set by BASCD took part in the CATFISH study.
Calibration results
To calibrate, dentists should have a sensitivity of 0.75 (minimum), a specificity of 0.90 (minimum) and a deviation from the mean of < 0.50. Tables 4–8 show the calibration results for each year of clinical examinations.
| Dentist | Sensitivity | Specificity | Deviation from benchmark |
|---|---|---|---|
| 1 | 0.91 | 0.98 | 0.14 |
| 2 | 0.91 | 0.98 | –0.23 |
| 3 | 0.89 | 0.99 | –0.09 |
| 4 | 0.83 | 1.00 | –0.15 |
| Dentist | Sensitivity | Specificity | Deviation from benchmark |
|---|---|---|---|
| 1 | 0.85 | 0.98 | 0.00 |
| 2 | 0.79 | 0.99 | –0.33 |
| 3 | 0.85 | 0.99 | –0.13 |
| 4 | 0.75 | 1.00 | –0.67 |
| 5 | 0.94 | 0.99 | 0.47 |
| Dentist | Sensitivity | Specificity | Deviation from benchmark |
|---|---|---|---|
| 1 | 0.78 | 0.99 | 0.10 |
| 2 | 0.87 | 0.99 | 0.40 |
| 3 | 0.86 | 0.99 | 0.00 |
| 4 | 1.00 | 0.99 | 0.00 |
| Dentist | Sensitivity | Specificity | Deviation from benchmark |
|---|---|---|---|
| 1 | 0.81 | 0.99 | –0.06 |
| 2 | 1.00 | 0.99 | 0.11 |
| 3 | 0.81 | 0.99 | –0.06 |
| Dentist | Sensitivity | Specificity | Deviation from benchmark |
|---|---|---|---|
| 1 | 0.80 | 0.98 | 0.11 |
| 2 | 0.80 | 1.00 | –0.11 |
| 3 | 0.90 | 1.00 | 0.00 |
Primary outcome
Birth cohort
The primary outcome for the birth cohort was the presence or absence of caries into dentine in the primary teeth of children aged approximately 5 years, at the end of the follow-up period. Clinical assessments were undertaken from September 2017 to August 2018, when children were 3 years old (referred to as wave 1), with the final examinations undertaken from September 2019 until March 2020, when children were approximately 5 years old (referred to as wave 2).
Older school cohort
The primary outcome for the older school cohort was the presence or absence of caries in permanent teeth (which had erupted after fluoridation began and were recorded at baseline, when children were approximately 5 years old) when children were approximately 11 years old, at the end of the follow-up period. Clinical assessments were undertaken from September 2019 until March 2020, when children were approximately 11 years old. The same diagnostic protocol and standard operating procedures used for the clinical examination of birth cohort were also used for children in the older school cohort. Children underwent a standardised clinical dental examination (referred to as waves within the results section) at ages 5 years (wave 1), 7 years (wave 2) and 11 years (wave 3), and which were conducted in their primary school by the same trained and calibrated assessors who undertook the birth cohort examinations.
Secondary outcomes
In the birth cohort, the clinical dental examinations described above were used to calculate the mean number of dmft in the primary dentition at 5 years. Likewise, in the older school cohort, the standardised clinical examinations at ages 7 and 11 years were used to calculate the mean number DMFT in the permanent dentition.
For the birth cohort, the number of erupted primary teeth could also be calculated at ages 3 and 5 years from the clinical examination.
Parents were asked at recruitment to provide consent for study researchers to collect data from relevant medical and NHS dental records. Therefore, in addition, we accessed activity data from the NHS Business Services Authority (BSA) and from the NHS Child DGA service operated by Cumbria Community Dental Service. The data from the former source were used primarily to estimate health services costs in the economic analyses (see Chapter 8), as well as to compare the numbers of dmft at 3 years old, given the low participant attendance for the 3-year-old wave of data collection within the CATFISH study.
Hospital data enabled us to identify children in both cohorts who had one or more teeth extracted under general anaesthesia during the follow-up period. Adverse reactions were measured by questionnaire in the birth cohort only. The questionnaire asked parents and guardians to detail any new chronic conditions.
Effect modifiers
Where randomisation of participants to intervention and control groups is not possible, the York review16 and the MRC report18 highlighted the importance of collecting data on potential effect modifiers to include as covariates in the analysis. These modifiers are largely behavioural factors associated with the aetiology of caries and its treatment, chiefly concerning sugar consumption, fluoride exposure from other sources and dental service use. This information was collected primarily by questionnaire.
Birth cohort
Parents were asked to complete questionnaires after the birth of their child and every 6 months until their child was 5 years old. Questionnaires were tailored to the age of the child over the 5-year follow-up period. A summary of the questionnaire administration schedule is provided (see Table 9). Parents had the options of completing questionnaires sent through the post, online or by telephone call. During the first year of the study, different methods of sending the questionnaire and different reminders (e.g. resending entire questionnaire, postcard reminder or telephone reminder) were trialled and the results of this nested study were subsequently published. 97
Details about the questionnaire and copies of the questionnaires are available via the NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/phr/SHMX1584. The content of the questionnaires can be categorised under the following headings.
Household data, demographics, water environment, attitudes and parental health status
Household data, demographics, water environment, attitudes and parental health status data were collected via a written parental questionnaire at recruitment (given after birth or at the first health-care visit after the birth of the child). The questionnaire included items to assess socioeconomic status and demographics, including:
-
number of household members
-
parental education
-
parental occupation
-
household income.
Equivalised household income was measured using the McClements’ equivalence scale,98 using data collected on gross household annual income and the number of adults and children resident at the child’s house. Equivalised household income was another measure collected that could be used to represent deprivation at the household, rather than area level.
The questionnaire also contained question items on water sources available to the household, including water consumed for drinking. Parents were also asked to self-report their use of oral health-care services, last dental treatment and general health and oral health status using the five-point Likert scale used within the UK adult dental health survey. 25
Health data
As well as reporting their own general and dental health, parents were also asked about their child’s health, and questions relating to hospital visits, pain experienced and trouble sleeping were asked every 6 months throughout the 5-year follow-up. A generic measure of the children’s health was obtained using the Child Health Utility 9-Dimensions (CHU9D) instrument, which was used primarily in the health economics analyses (see Chapter 8).
Anthropometry, diet and fluoride exposure
Parents were asked to provide their child’s weight and length/height recorded at each interval. In addition, a recent study99 examining WF in the north of England identified sugar consumption before bedtime as an important predictive risk factor for caries in children, and, therefore, this question formed the main dietary measure used in the study to avoid the need to complete lengthy diet diaries. Likewise, parents were asked about diet and weaning practices with milk formula, juice or other sugar-containing drinks consumed from either a bottle or cup, as well as frequency of consumption of sweets and chocolate each week.
Fluoride exposure from other sources
Fluoride exposure from other sources was captured in the following ways:
-
Information on fluoride exposure from dietary sources was collected via questionnaires (including water consumption).
-
Measures of non-dietary ingestion were estimated using pictorial information on how much toothpaste was used during toothbrushing, frequency of brushing, product name (which provided fluoride concentration level) and the age toothpaste use began. Although less common, we also collected information on exposure from professional prescription of fluoridated gels, tablets and varnishes.
Adverse events
Parents were asked about any new chronic conditions their child had developed. A chronic condition was described as one that is persistent or long-lasting.
Older school cohort
For the older school cohort, a shorter questionnaire (see the NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/phr/SHMX1584) on oral hygiene, fluoride exposure and dietary behaviours (including brushing, toothpaste use and what the child has eaten an hour before bed) was given during each year of data collection (completed in the first year by parents and subsequently completed by children during their dental examination).
Measures of deprivation and socioeconomic status
We used the IMD from 2010 to measure relative deprivation of participants. The IMD measures relative levels of deprivation in 32,844 small areas or neighbourhoods in England, called lower-layer super output areas. The IMD is used routinely in government reports, most notably the national 10-yearly UK children’s dental health surveys and was available for all participants via a look-up file, which matched their home postcode to the lower-layer super output area in which they lived. The IMD is converted into national quintiles (with 1 representing least deprived and 5 representing most deprived), which were the main measures used within this analysis. In addition to the small area measure of relative deprivation, we collected data on individual measures of socioeconomic status (e.g. parental income, occupation and education), as outlined in Household data, demographics, water environment, attitudes and parental health status.
Tables 9 and 10 provide summaries of the outcome and effect-modifier variables collected in the birth and older school cohorts, respectively, as well as the methods used in collecting the variables.
| Measure | Data collection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age in years | 0 | 1 | 2a | 3 | 5 | ||||
| Age in months | 0 | 6 | 12 | 18 | 30 | 36b | 48–60 | ||
| Child dental examination | ✓ | ✓ | |||||||
| Household environment and demographics | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Child-related questions | |||||||||
| Oral hygiene behaviours | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Child health | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| CHU9D | ✓ | ||||||||
| Diet: weaning practices | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Body mass index | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Access to fluoride | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Access to dental treatment/services | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Serious adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Occurrence of dental pain | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Hospital visits for dental treatment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| DGA extraction | ✓ | ||||||||
| Parent-related questions | |||||||||
| Oral hygiene behaviours | ✓ | ✓ | |||||||
| Self-reported oral health status | ✓ | ✓ | |||||||
| Fluoride levels (household water supply) | ✓ | ✓ | |||||||
| Attitudes and choice (water consumption) | ✓ | ✓ | |||||||
| Dental visits | ✓ | ✓ | |||||||
| Measure | Data collection | ||||||
|---|---|---|---|---|---|---|---|
| Age in years | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Child dental examination | ✓ | ✓ | ✓ | ||||
| CHU9D | ✓ | ✓ | |||||
| Oral hygiene behaviours | ✓ | ✓ | ✓ | ||||
| Parent-related questions | ✓ | ||||||
Changes to outcomes after trial commencement
No outcome measures were changed after the trial commenced; however, the frequency of questionnaire distribution was reduced from every 6 months to a maximum of once a year in response to PPI feedback regarding participant burden.
Sample size
The study design was a whole-population comparison between fluoridated and non-fluoridated birth and older school cohorts living in Cumbria. Consequently, one could argue that a sample size calculation is not required. However, to provide reassurance of adequate power to detect a difference if one truly existed, a sample size calculation was completed based on a more conservative effect size than that reported by the York and Cochrane reviews. 16,71
With a census approach, we planned to approach up to 3200 parents in each cohort and retain the parent–child dyads for the whole of the 5-year follow-up. With an anticipated consent rate of 84%, assuming that 7.5% of children refuse the dental examination (based on previous experience in this population from the Public Health England Dental Epidemiological Surveys100) and with a loss to follow-up of 12.5%, the number of participants available for the second clinical exam would be 1720. Based on 47% of ‘non-exposed’ children developing caries and 37% of ‘exposed’ children developing caries,44,47,71 to be adequately powered to detect a risk difference of 0.1 (risk ratio 0.8) at a significance level of 0.05 and with 90% power, a total sample size of 1044 children would be required, and this number was well within our anticipated recruitment and retention capabilities for both cohorts.
Statistical methods, including methods for additional analyses
The same analytical approach was taken for each cohort.
Recruitment and retention
Data were continuously collected on recruitment, ineligibility, examination refusals, withdrawals and loss to follow-up for intervention and control groups in both cohorts to enable compilation of STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) flow charts.
Baseline comparisons
For both cohorts, the distribution of baseline demographics was reported according to exposure group for age, sex and deprivation for the birth cohort and, additionally, dmft for the older school cohort, with 95% CIs for the between-group difference across the exposed and non-exposed groups.
Caries calibration analyses
Caries calibration was based on the BASCD guidance for caries calibration. 101 For each examiner, calibration utilised the dmft scores given to children. For examiners to successfully calibrate, the deviation from the benchmark should not be > 0.50, the sensitivity should be at least 0.75 when comparing dmft with the benchmark and specificity should be at least 0.90 when comparing dmft with the benchmark.
Evaluation of non-blinded examiners
The magnitude of difference between groups was assessed, comparing the difference between groups observed for the traditional clinical examinations (unblinded) and standardised photographs (blinded).
Caries analysis
In both cohorts, our primary outcome was presented as a cross-tabulation of presence or absence of caries with exposure or non-exposure to WF, with the risk ratio (with 95% CI), unadjusted odds ratio (OR) (95% CI) and adjusted OR (95% CI) obtained from a generalised linear model as the measure of effect. The ‘natural experiment’ in WF exposure implies an absence of confounders in this study (WF dosing in the exposed population is independent of social class, other fluoride sources, etc.). However, any significant differences between the groups on key variables identified as potential effect modifiers were accounted for by using appropriate regression models to adjust for these variables. Adjusted analysis of the primary outcome was undertaken using a generalised linear model with logit link function and adjusted for age, deprivation (quintiles) and sex as covariates. For the older school cohort, we were able to consider the baseline dmft caries levels between the two populations to identify any baseline imbalance in caries that required subsequent adjustment in the statistical analysis.
Secondary outcomes
Secondary outcome variables of dmft (for the birth cohort) and DMFT (for the older school cohort) were analysed using generalised linear models using the appropriate link function for the outcome, initially including only the exposure group and then additionally adjusting for baseline values of dmft, age, sex and deprivation (quintiles), and offset for number of erupted teeth.
Inequalities
In both cohorts, the primary outcome and secondary outcomes (dmft/DMFT) were compared across exposed and non-exposed groups by quintile of deprivation. Generalised linear models with the appropriate link function (negative binomial regression), and including an exposure by deprivation (quintiles) interaction term, were undertaken to determine the effects at different levels of deprivation. The regression examined the count of decay and the interaction between WF status and deprivation, and included the covariates age, sex and (for the older school cohort only) dmft at baseline, offset by the log of erupted teeth. This analysis was supplemented by the resulting marginal predicted probabilities for interpretability.
Changes to study design after trial commencement
After noticing a poor response rate at baseline, we took steps to address this problem by looking at ways to increase response rate. A nested trial within the birth cohort was undertaken with approximately one-third of participants, using their first questionnaires to determine the most effective way to remind participants to complete their questionnaire. 97 The nested trial resulted in only a marginal increase in return and so questionnaires for the birth cohort were reduced in frequency from every 6 months to annually after children reached 12 months of age, following feedback of responder fatigue. This was agreed within the team, given the need to capture the changes regarding weaning and diet in the first year of a child’s life. Following this, a yearly questionnaire provided sufficient information on the oral health behaviour and diet of children aged 1–5 years. Changes were implemented following approval from the Ethics Committee and the Oversight Committee.
Post hoc analysis following interruption to dosing at one plant (Ennerdale)
One water treatment plant (Ennerdale) was severely affected by the flooding in 2015/16, which resulted in an extended period when no, or suboptimal, fluoridation occurred (see Chapter 7 for average dosing across the two plants). Although the study was not powered to detect differences between treatment plants, it was agreed to provide additional separate results, showing the risk ratio/OR and 95% CIs for the presence/absence of caries, for individuals residing in the two treatment plants against the control, given this interruption.
Health economic evaluation methods
Approach
The framework for the economic evaluation was a within-study cost-effectiveness analysis to estimate the net cost per unit of health benefit gained by WF. A cost-effectiveness acceptability approach was used to estimate the likelihood that WF is cost-effective.
The population for the evaluation falls into two groups: (1) children from birth to age 5 years (i.e. the birth cohort) and (2) children aged 5–11 years (i.e. the older school cohort). The intervention is WF and was compared with no WF in the child’s area of residence (Cumbria).
The measure of health benefit was the QALY and the comparison of costs and benefits was generated using the ICER (i.e. cost per QALY gained). For the within-study analyses, the time horizon is 5 years for the birth cohort (i.e. ages 0–5 years) and 6 years for the older school cohort (i.e. ages 5–11 years).
The chosen perspective was that of both the health and care sector and the local authority, which incur the costs of dental treatment and of fluoridation, respectively.
Within-study analyses
Direct costs
The range of costs included the following:
-
The costs to the local authority of WF (both capital and running costs). These costs were obtained by requests to the water company and the local authority.
-
The costs of (both routine and emergency) dental treatment that fall on the NHS. These costs are captured by NHS BSA data on dental activity per child, and from hospital activity records obtained through North Cumbria Integrated Care Foundation Trust.
The direct costs were combined with the most recent national unit costs available at the time of data analysis. 102 Each item of service use was costed by multiplying the quantity of service used with the average unit cost for that item. Costs are presented in 2014 UK GBP (£) and costs beyond 2014 are discounted from year 2 at a discount rate of 3.5%, which is in line with NICE guidance for technology appraisal. 103
Water fluoridation costs
The cost of WF was allocated in two ways. First, the costs were distributed across the entire population residing in areas with fluoridation. Second, the costs of WF were distributed evenly across the population aged 0–12 years in areas with fluoridation. Capital costs were transformed into an equivalent annual cost with a discount factor of 3.5% and a time period of 6 years (a conservative approach that assumes the capital lasts for only 6 years).
Capital expenditure incurred by the local authority in the set-up of the fluoridation plant amounted to £1,643,889.60. This was the cost of a single capital works programme, covering both the Ennerdale and Cornhow schemes. Although operational beyond the study period, we took the conservative assumption that all capital expenditure covered 6 years (i.e. the longest period of follow-up over both cohorts).
Local authority running costs are provided in Table 11. In 2014 and 2016, the local authority paid a proportion of the final running cost, with the remainder covered by United Utilities. Table 11 gives the running costs and discounted running costs of WF over the study period.
| Year | Reported cost (£) | Discounted cost (£) |
|---|---|---|
| 2014 | 32,543.13 | 32,543.13 |
| 2015 | 104,119.50 | 100,598.55 |
| 2016 | 47,479.00 | 44,322.15 |
| 2017 | 84,272.94 | 76,009.36 |
| 2018 | 120,688.15 | 105,172.75 |
| 2019 | 104,000.00 | 87,565.21 |
| Total | 493,102.72 | 446,211.16 |
Water fluoridation costs per capita are provided in Table 12. The costs of WF were attributed to children in the sample in two ways: (1) on a per capita of the population and (2) on a per capita of the population aged < 12 years. 104 The latter approach is a stronger assumption that fluoridation is targeted at children only.
| Year | Cost (£) | Cost (£) per capita | Cost (£) per capita (0–12 years) |
|---|---|---|---|
| Capital cost | 1,643,889.60 | 9.88 | 73.78 |
| 2014 | 32,543.13 | 0.20 | 1.46 |
| 2015 | 100,598.55 | 0.60 | 4.52 |
| 2016 | 44,322.15 | 0.27 | 1.99 |
| 2017 | 76,009.36 | 0.46 | 3.41 |
| 2018 | 105,172.75 | 0.63 | 4.72 |
| 2019 | 87,565.21 | 0.53 | 3.93 |
| Total | 446,211.16 | 2.69 | 20.03 |
| EAC | 392,245.46 | 2.36 | 17.61 |
| Total: present value | 2,090,100.75 | 12.56 | 93.81 |
| Total: EAC | 2,353,472.78 | 14.14 | 105.63 |
The populations in Table 12 relate to fluoridated areas (i.e. Copeland and Allerdale), and no WF costs are attributed to non-fluoridated areas (i.e. Carlisle, Barrow-in-Furness, South Lakeland and Eden). We assume that children residing in specific postcode areas had exposure to fluoridated water.
Quality-adjusted life-years
Quality-adjusted life-years gained from baseline to end of follow-up were estimated as the number of days multiplied by the utility of observed survival. The utility values were estimated from the CHU9D questionnaire. The CHU9D is a generic health-related quality-of-life measure for children aged 7–17 years. 105–107 The CHU9D contains the following nine dimensions, with five levels:
-
worried
-
sad
-
pain
-
tired
-
annoyed
-
school work
-
sleep
-
daily routine
-
activities.
Children (or proxies for younger children aged 5–7 years) self-complete the questionnaire, with questions placed in the context of how the child is today/last night.
For the birth cohort, there is no baseline CHU9D utility measure and we assume that the utility value is equivalent across both fluoridated and non-fluoridated groups. A utility value of 1 (i.e. perfect health) is assumed in both groups. At follow-up, parents/carers completed the CHU9D for their child at age 5 years. Although the CHU9D was originally created and validated to be used for children aged 7–11 years, a proxy version for children aged 5–7 years has also been created and has been trialled. Early results indicate that a proxy- (parent-) reported CHU9D is appropriate. 108
For the older school cohort, parents/carers completed the questionnaire for their child at age 5 years and children self-completed the questionnaire at their final clinical assessment (at age 11 years).
The CHU9D responses are transformed to utility measures using preference weights obtained from a sample of 300 adults in the UK. 109 QALYs were then estimated as:
where U is utility value, i is individual, d is day of assessment and t is assessment point (1 = baseline, 2 = final).
Quality-adjusted life-years are discounted from year 2 at a discount rate of 3.5% in line with NICE guidance for technology appraisal. 103
Economic analyses
Descriptive analysis was used to summarise the CHU9D data, QALYs, service use and cost. Regression analysis was used to estimate incremental (net) costs and QALYs.
The following variables were selected as covariates for the analyses to estimate net costs and QALYs. The covariates used in the analysis of QALYs were:
-
sex
-
age
-
follow-up duration (years)
-
deprivation quintile of child’s residence
-
CHU9D utility measure at baseline.
Sex and age were included to reflect the fact that health and, therefore, utility typically vary across sex and decline with age. Deprivation quintiles were included because of the variation in deprivation observed between fluoridated and non-fluoridated areas in the study.
The covariates used in the analysis of costs were:
-
sex
-
age
-
follow-up duration (years)
-
deprivation quintile of child’s residence
-
CHU9D utility measure at baseline.
The CHU9D utility score at baseline was included as an overall measure of health and health need. For the birth cohort analyses of costs, there was no baseline CHU9D utility measure and so this is not adjusted for in the regressions for this cohort.
The primary measure for the economic analysis was the ICER. The ICER was estimated as the net cost divided by the net QALY estimates from the regression analyses.
To account for uncertainty in the parameter estimates, the estimates of net costs and outcomes from the regression were bootstrapped to simulate 10,000 pairs of net cost and net outcomes. These simulated pairs of net cost and net outcomes were used to generate cost-effectiveness acceptability curves, as recommended by NICE for health technology appraisals. 103 Based on the range of willingness-to-pay value thresholds (WTPTs) historically implied by NICE decisions,110 the simulated net QALY values from the bootstrap simulation were assigned a monetary value using a range of maximum willingness-to-pay values from £1 to £30,000 to gain 1 QALY.
A net benefit statistic for each pair of simulated net costs and net outcomes for each WTPT can then be calculated. For example, if the measure of health benefit is the QALY, then the net benefit of WF is estimated as:
This calculation was repeated for each WTPT. Cost-effectiveness acceptability curves plotted the proportion of bootstrapped simulations where the net benefit of WF is greater than zero for each WTPT111–114 and this provides a probability of cost-effectiveness that takes into account parameter uncertainty.
Missing data
Baseline data on the CHU9D were obtained via questionnaire [for the birth cohort, there is no baseline data and health-related quality of life utility is assumed to be identical in both groups, i.e. at 1 (perfect health)]. For the birth cohort, missing data can occur at follow-up via non-completion of the questionnaire or by incomplete CHU9D and/or resource (cost) data. For the older school cohort, missing data can occur via non-completion of the questionnaire at baseline or by incomplete CHU9D data at baseline and/or incomplete CHU9D data or resource (cost) data at follow-up.
For both cohorts, assessments were made of factors associated with questionnaire completion to inform whether or not there may be bias in the questionnaire data related to fluoridation group and/or IMD decile.
The approach taken to deal with missing data concerns missing data relating to incomplete CHU9D and/or resource (cost) data at baseline and/or follow-up.
Faria et al. 115 provide a guide to handling missing data. Faria et al. 115 propose identifying plausible assumptions for the missing data mechanism and applying methods that account for these reasons for missingness with sensitivity analyses that explores variations on the assumed mechanism. The suggested steps include (1) the identification of the missing data mechanism via descriptive analysis and logistic regression analyses on missing cost and QALY data against baseline measures, covariates and treatment, (2) the choice of methods to deal with the mechanism identified and (3) sensitivity analyses that relaxes/varies the assumed mechanism.
Identifying the missing data mechanism
The missing data mechanism is defined in line with Rubin’s classification. 116 Here, data are missing completely at random (MCAR) (i.e. where missing data are not associated with observed and unobserved values), covariate-dependent missing completely at random (CD-MCAR) (where missing data are not associated with observed outcomes but are associated with observed baseline covariates),117 missing at random (MAR) (where missing data are not associated with unobserved values but are associated with observed covariates and outcomes) and missing not at random (MNAR) (where missing data are associated with unobserved values).
Faria et al. 115 propose descriptive analysis of the missing data to infer the missing data mechanism and this includes:
-
an assessment of missing data by treatment group at each follow-up (as equivalent missing data rates provide some support for MCAR)
-
an assessment of missing data patterns (as patterns can help inform the methods to account for missing data)
-
assessments of associations between missingness and baseline variables (as any variable that predicts missingness suggests that the MCAR mechanism is not valid)
-
assessments of associations between missingness and observed outcomes (as any prior observed outcome variable that predicts missingness suggests that MAR is a more plausible mechanism).
Although these steps help inform the mechanism of missing data, the steps do not rule out MNAR.
Under MCAR, complete-case analysis (CCA) is valid. CCA is also valid when the analytical model includes baseline variables that predict outcome and missingness (i.e. CD-MCAR). However, to approach the analysis as intention to treat, individuals with missing baseline variables would need to be included.
The approach for identifying the missing data mechanism followed that suggested by Faria et al. 115 First, the proportions of missingness and of missing cost and QALY data are reported to observe whether or not there are differential rates of missing data across fluoridated and non-fluoridated groups. Second, logistic regressions of missing cost and QALY indicators are performed with a fluoridation group indicator, baseline covariates and baseline QALY as explanatory variables. If rates of missing data are similar and no baseline covariate or fluoridation indicator is a significant predictor of missing data, then the assumed missing data mechanism is MCAR and the CCA is the preferred model specification. If rates of missing data are similar and baseline covariates are significant predictors of missing data, then the assumed missing data mechanism is CD-MCAR and the CCA is the preferred model specification. If rates of missing data are similar and baseline QALYs are significant predictors of missing data, then the assumed missing data mechanism is MAR and imputation of missing data is the preferred methodological approach.
The study does not have multiple time points of follow-up, meaning that assessments of patterns of missingness and assessments of associations between missingness and observed outcomes are not applicable.
Missing data imputation
Missing at random was approached by multiple imputation, whereby missing data were replaced with predicted values. The chained equations approach was used to impute the data. Here, imputed values for variables were used to predict missing values in other variables. 118 Imputed values were obtained via predictive mean matching. The imputation was performed m times, where m was determined by the proportion of missing data (identified in the assessment of missing data above). The multiply imputed data sets account for uncertainty in the imputation. Each imputed data set was estimated and then combined to obtain a mean estimate and standard error (SE). 116
Imputation was performed by fluoridation group, with all baseline variables (i.e. age, sex and IMD quintile), costs (i.e. fluoridation costs, dental costs and DGA costs) and CHU9D domains included in the imputation model.
The multiply imputed costs and QALYs, domains of resource (costs) and CHU9D domains were compared with the CCA costs and QALYs to assess whether or not the imputation appeared to be valid. To obtain incremental cost and QALY estimates, seemingly unrelated regression models of the multiply imputed data sets were estimated.
Sensitivity analysis
Sensitivity analyses were used to explore the impact of changing the methods used on the estimates of whether or not WF was cost-effective, and this included re-running the analyses for the following:
-
analysis where multiple imputation of missing observations gives estimates for missing costs and outcomes
-
allocations of WF costs on a per capita of the population (all ages)
-
analysis of cost-effectiveness with –
-
the birth cohort:
-
the count of dmft is the outcome measure
-
presence of decayed teeth as an outcome measure.
-
-
the older school cohort:
-
the volume of DMFT (including crowns) for teeth erupted post baseline
-
presence of decayed teeth for teeth erupted post baseline.
-
-
Chapter 7 Results: delivery of the intervention
Figure 7 shows the average fluoride dosing for the two treatment plants from when dosing resumed in 2013 until the study ended in 2020. It can be seen that Ennerdale experienced an extended period where no or suboptimal fluoridation occurred. A substantial amount of time where no or suboptimal fluoridation occurred was due to the severe flooding in Cumbria in 2015/16.
FIGURE 7.
Line graph of average fluoride dosing each month.

Chapter 8 Birth cohort clinical results
How the results are presented
The statistical analysis for this research is vast, given the length of the study and its various components. For simplicity, in the main body of the report, we are presenting only analyses relating to the main research objectives:
-
to assess the effects and costs of systemic and topical exposure to WF –
-
primary analysis on the proportion of children developing caries in each group (see Primary outcome)
-
secondary analysis on the count of dmft and experience of DGA (see Birth cohort: secondary data analysis)
-
-
to measure the impact of WF on social class inequalities in child dental health (see Birth cohort: inequalities data analysis).
Additional information on the analyses conducted and results and outcomes from the additional analyses, much of which are referenced in this section, are available in Appendix 2.
Subject recruitment and retention
Figure 8 provides details of the subject flow through the study, demonstrating the recruitment at the two principal hospital sites. Further information on recruitment is presented in Appendix 1, Figure 16.
FIGURE 8.
A STROBE flow diagram for the birth cohort. a, Invited to participate is based on area of recruitment; b, a child could be removed from the study if the study team was advised to remove them, the study team were informed they had moved out of the area or if they had died. Two children died who were part of the study, both were in the control area. This information was not treated as missing and not included within analysis as it was not connected to the study.

Figure 8 shows that the consent rate was higher in the non-fluoridated areas than in the fluoridated areas, despite an identical consent process, with most parents approached face to face when attending hospital for their scan or for the birth of their child. The numbers for ‘clinical examination 1’, which occurred when children were approximately 3 years old, were significantly smaller than expected. Examinations at age 3 years were particularly challenging, as examinations could occur within dental practices only, meaning that parents/guardians had to take their children to this appointment. Despite multiple sites and times being offered, with reminders over a month, as well as a being voucher offered as compensation, only 21% of children who had their final examination took part at ‘clinical examination 1’.
Examination status at the final clinical examination (from individuals who originally consented to take part) is presented in Table 13, according to fluoridated or non-fluoridated water status. There were fewer participants in the fluoridated area than in the non-fluoridated area at baseline (owing to the geographic restrictions of the fluoridated area). When comparing the percentage of participants who had a final examination, 67% of participants in the non-fluoridated group (of those originally recruited) completed their final examinations, compared with 78% of participants in the fluoridated group, with lack of access to schools in the non-fluoridated area (on account of COVID-19) being the principal source of the differential attrition. Further analysis on examination status is presented in Appendix 2, Table 61.
| Examination status | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| Examined (included in study) | 835 (66.9) | 609 (77.5) | 1444 (71.0) |
| Absent | 19 (1.5) | 25 (3.2) | 44 (2.2) |
| Child refused | 11 (0.9) | 17 (2.2) | 28 (1.4) |
| Child moved | 64 (5.1) | 23 (2.9) | 87 (4.3) |
| School not visited (COVID-19 closure) | 164 (13.1) | 8 (1.0) | 172 (8.5) |
| Withdrawn | 94 (7.5) | 51 (6.5) | 145 (7.1) |
| Removed for other reason | 30 (2.4) | 40 (5.1) | 70 (3.4) |
| Unable to trace | 32 (2.6) | 13 (1.7) | 45 (2.2) |
| Total | 1249 (100) | 786 (100) | 2035 (100) |
Demographics and behaviours were explored to assess if there were any differences between the fluoridated and non-fluoridated groups, and to look at differences between participants who had an examination and participants who did not (from participants who were consented). Table 14 shows that the relative proportions of males and females were similar in both groups. We observed that the proportion of people living in a most deprived area was higher in the WF group (30.2%) than in the no WF group (19.8%); however, the distributions across the quintiles of deprivation of people both examined and not examined were similar in the WF and no WF group. (See Appendix 2, Tables 62–111, for all other tables, descriptive statistics and analysis relating to potential confounders and bias.) When looking at potential confounders around behaviour that could affect dental health, there were only two variables that resulted in a statistically significant difference over time, and these were consumption of sweets/chocolate (i.e. sweets/chocolate consumed more than three times per week) and whether or not the child had ever visited the dentist. In the case of sweets consumption, the difference, of approximately a 10%, occurred after 12 months, with the prevalence of frequent sweets consumption (more than three times per week) being higher in the fluoridated area. These data were not included in the regression, as multiple (n = 11) behaviours were investigated, with the majority indicating no difference between groups over time. Therefore, we had to consider the risk of there being a type I error. In addition, the low response rate would have meant a reduced sample size and would have underpowered the final results.
| Characteristic | Exposure group, n (%) | |
|---|---|---|
| No WF | WF | |
| Age (years) | ||
| n | 773 | 562 |
| Mean (SD) | 4.88 (0.35) | 4.79 (0.29) |
| Sex, n (%) | ||
| Male | 448 (53.6) | 312 (51.2) |
| Female | 387 (46.4) | 297 (48.8) |
| Total, n | 835 | 609 |
| Chi-squared p-value | 0.363 | |
| Deprivation quintile, n (%) | ||
| 1 (least deprived) | 77 (9.2) | 41 (6.8) |
| 2 | 160 (19.1) | 79 (13.0) |
| 3 | 206 (24.7) | 124 (20.4) |
| 4 | 227 (27.2) | 179 (29.5) |
| 5 (most deprived) | 165 (19.8) | 184 (30.3) |
| Total, n | 835 | 607 |
| Chi square with trend | z = 5.37; p < 0.0001 | |
| Deprivation score | ||
| n | 835 | 607 |
| Mean (SD) | 23.4 (13.3) | 27.7 (15.5) |
| Median (LQ, UQ) | 20.2 (13.0, 31.4) | 23.3 (17.2, 36.5) |
| Minimum, maximum | 4.4, 58.5 | 2.9, 65.5 |
Primary outcome
The results are shown in a simple two-by-two format in Table 15. The proportion of children with decay was 17.4% in the intervention group and 21.4% in the control group (i.e. an absolute difference of 4%). The risk of developing caries for individuals living in an area served by WF was 81% of the risk of individuals living in non-fluoridated areas (risk ratio 0.81, 95% CI 0.65 to 1.01). From this unadjusted risk ratio, there is insufficient evidence of an effect on the presence or absence of decay from WF. An adjusted analysis using a logistic regression model was performed with covariates of deprivation (reference category lowest quintile, least deprived), age and sex (reference category male), all of which had a significant relationship with decay. This indicated that the odds of decay for children from a fluoridated area were 74% of the odds of decay for children from a non-fluoridated area, conditional on the values of the covariates (risk ratio 0.74, 95% CI 0.56 to 0.98; p = 0.038; n = 1333). Hence, when these variables are controlled for, there is evidence of an impact of WF on decay status (see Appendix 2, Tables 89 and 90, and Figure 17). Alternatively, it can be said that the odds of developing caries was 1.36 times (95% CI 1.02 to 1.81 times) higher for participants living in a non-fluoridated area than for participants living in a fluoridated area, conditional on the values of the covariates. The eruption of teeth was compared between groups, and no significant differences in the number of teeth present in each group were found (see Appendix 2, Table 88).
| Caries status | Exposure group | Total | |
|---|---|---|---|
| No WF | WF | ||
| Decay present, n (%) | 179 (21.4) | 106 (17.4) | 285 (19.7) |
| Decay absent, n (%) | 656 (78.6) | 503 (82.6) | 1159 (80.3) |
| N | 835 | 609 | 1444 |
| Risk ratio (95% CI) | 0.81 (0.65 to 1.01) | ||
| Unadjusted OR (95% CI) | 0.77 (0.59 to 1.01) | ||
| Adjusted OR (95% CI) | 0.74 (0.56 to 0.98) | ||
Birth cohort: secondary data analysis
Secondary outcome: decayed, missing or filled teeth (primary)
Table 16 details the count of dmft (generated from clinical scores, where the decayed component is into dentine) for each participant. The chi-squared with trend test demonstrated a statistically significant difference between the fluoridated and non-fluoridated areas when looking at count of dmft, with participants in a fluoridated area showing an, on average, a lower dmft score.
| dmft count (n) | Exposure group | Total (N = 1444), n (%) | |
|---|---|---|---|
| No WF (N = 835), n (%) | WF (N = 609), n (%) | ||
| 0 | 656 (78.6) | 503 (82.6) | 1159 (80.3) |
| 1 | 63 (7.5) | 36 (5.9) | 99 (6.9) |
| 2 | 27 (3.2) | 25 (4.1) | 52 (3.6) |
| 3 | 26 (3.1) | 16 (2.6) | 42 (2.9) |
| 4 + | 63 (7.5) | 29 (4.8) | 92 (6.4) |
| Chi-squared with trend | z = 1.98; p = 0.048 | ||
These data were further explored in a negative binomial regression with the count of dmft as the dependent variable, deprivation (quintile), age and sex entered as covariates and an offset for the number of erupted teeth. The regression shows that the incidence rate of dmft for children in a fluoridated region was 0.61 (95% CI 0.44 to 0.86) that of children living in a non-fluoridated region, holding other variables constant in the model. The rate of dmft was significantly lower for children in WF groups when controlling for these variables (see Appendix 2, Tables 94 and 95, and Figure 20).
Secondary outcome: dental general anaesthetic for extractions
There was no evidence of a difference in the DGA experience by exposure group, when controlling for deprivation (quintile) and sex as covariates (OR 0.759, 95% CI 0.397 to 1.468) (see Appendix 2, Tables 92 and 93 for further details).
Secondary outcome: questionnaire data – dentally related problem
Generalised estimating equations (GEEs) were used to determine if there was a difference between groups for a child experiencing a self-reported dentally related problem (e.g. pain, trouble eating, impact on sleep) over time (i.e. 5 years of questionnaire data). No significant difference was detected by groups over time (see Appendix 2, Tables 96–99, for further details).
Additional analysis from NHS Business Services Authority data
Analysis was performed on data collected by the NHS BSA. These data contain the charge to complete a course of treatment and the dmft/DMFT recorded following an examination at the dentist. Children were included if they were part of the CATFISH study and aged either 3 or 4 years at the time of dental examination. A dmft score was calculated from data collected by NHS BSA, which detailed the number of filled, missing or decayed teeth. The largest number of decayed, missing or filled teeth was taken from any examination from when a child was 3 years old. Data were available for 860 participants.
There was no evidence of an effect on presence/absence of dental decay (based on a dmft score of 0 or a dmft score of ≥ 1) from WF according to a logistic regression when adjusting for deprivation (quintile) and sex as covariates (OR 1.04, 95% CI 0.705 to 1.525) (see Appendix 2, Tables 101 and 102). The breakdown of deprivation for children included in these data is also presented in Appendix 2, Table 100, and is comparable to the breakdown for the overall CATFISH population.
Birth cohort: inequalities data analysis
Health inequalities: primary outcome
A logistic regression model, including an exposure by deprivation interaction term, was undertaken to determine the effects of WF on the presence or absence of decay at each level of the IMD. This analysis was supplemented by calculating the resulting marginal predicted probabilities for interpretability. A multidegree of freedom test of the interaction term following the logistic regression indicated that there was no evidence of an exposure by deprivation interaction (F = 4.78; p = 0.3109) (see Appendix 2, Tables 103–105 for further details). From our analysis, there is no evidence that deprivation influences the relationship between water exposure and the presence or absence of decay.
Figure 9 shows the resulting marginal predictive probabilities for decay (into dentine) or no decay in primary teeth based on a model that included age, sex and an interaction term for exposure by deprivation quintiles for the clinical assessment at the median age of 5 years. The predictive probabilities are displayed separately for males and females in Appendix 2, Figure 23.
FIGURE 9.
Adjusted predictions of decay or no decay including an interaction term for area across deprivation quintiles with 95% CIs at 5 years old. Decay is at tooth level, with the decay component being any decay into dentine.
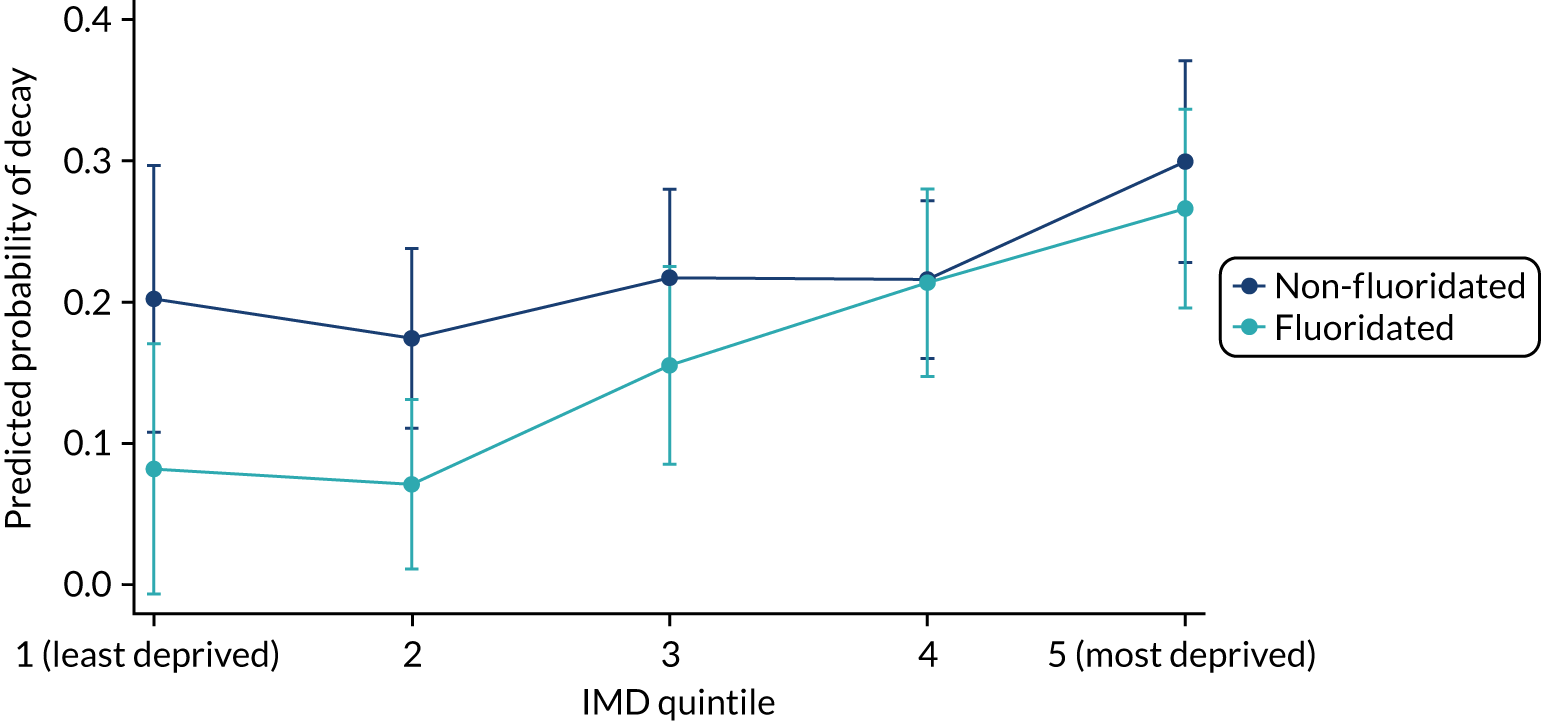
Health inequalities: secondary outcomes
A negative binomial regression, including an exposure by deprivation interaction term, was undertaken to determine the extent to which WF reduced health inequalities. This was achieved by examining the count of decay and the interaction between WF status and deprivation, and including the covariates age, sex and offset by the log of number of erupted teeth. This analysis was supplemented by calculating the resulting marginal predicted probabilities for interpretability. A multidegree of freedom test of the interaction term following the negative binomial regression indicated that there was insufficient evidence of an exposure by deprivation interaction (F = 1.29; p = 0.8639). From our analysis, there is no evidence that deprivation influences the relationship between water exposure and the severity of dental caries, as indicated by dmft. Full results from the model are reported in Appendix 2, Tables 106–108, and Figure 26.
Analysis for blinding: clinical versus photos
Examination of any potential bias in scoring was undertaken by comparing photo scores (where the examiner was blinded to the exposure status of the participant) and clinical scores (which were performed within a school setting and where an examiner may understand exposure status given the geographic location).
The photo scores (Table 17) show a very similar breakdown to the clinical scores [i.e. 21% vs. 17% decay for clinical scores and 22% vs. 18% for photo scores (across individuals receiving no WF vs. WF)].
| Caries status | Clinical scoresa | Photo scores | ||
|---|---|---|---|---|
| No WF | WF | No WF | WF | |
| Decay present, n (%) | 145 (21) | 85 (17) | 152 (22) | 92 (18) |
| Decay absent, n (%) | 552 (79) | 424 (83) | 545 (78) | 417 (82) |
| N | 697 | 509 | 697 | 509 |
| Difference in decay (%) | 4 | 4 | ||
The kappa agreement (Table 18) is substantial, with a kappa of 0.71 between scores achieved from photo examinations and clinical examinations.
| Photo | Clinical | |
|---|---|---|
| No decay | Decay | |
| No decay | 916 | 64 |
| Decay | 46 | 179 |
Post hoc analysis on separate water treatment plants
The post hoc analysis was carried out, splitting the participants by their water treatment supply, creating three principal groups [i.e. Ennerdale (interrupted dosing), Cornhow (more stable dosing) and non-fluoridated (control) supply]. Compared with the control area, there was insufficient evidence of a difference in dental caries experience in children who received their drinking water from Ennerdale, which had interrupted dosing (risk ratio 0.91, 95% CI 0.72 to 1.17), whereas a significant difference was observed in children served by Cornhow (risk ratio 0.62, 95% CI 0.43 to 0.89) (Table 19). These data should be treated with caution, given that the study was not powered to look at these differences.
| Caries status | Group | Total | ||
|---|---|---|---|---|
| Control | Cornhow (stable dosing) | Ennerdale (interrupted dosing) | ||
| Decay present, n (%) | 179 (21.4) | 29 (13.3) | 77 (19.7) | 285 (19.7) |
| Decay absent, n (%) | 656 (78.6) | 189 (86.7) | 314 (80.3) | 1159 (80.3) |
| N | 835 | 218 | 391 | 1444 |
| Risk ratio (95% CI), Cornhow (stable dosing) vs. control | 0.62 (0.43 to 0.89) | |||
| Risk ratio (95% CI), Ennerdale (interrupted dosing) vs. control | 0.91 (0.72 to 1.17) | |||
Chapter 9 Older school cohort results
How the results are presented
The statistical analysis for this research is vast, given the length of the study and its various components. For simplicity, in the main body of the report, we are presenting only analyses relating to the main research objectives:
-
to assess the effects and costs of systemic and topical exposure to WF –
-
primary analysis on the proportion of children developing caries in each group (see Primary outcome)
-
secondary analysis on the count of dmft and experience of DGA (see Older school cohort: secondary data analysis).
-
-
to measure the impact of WF on social class inequalities in child dental health (see Older school cohort: inequalities data analysis).
Additional information on the analyses conducted and results and outcomes from the additional analyses, much of which are referenced in this section, are available in Appendix 3.
Subject recruitment and retention
Figure 10 provides details of the subject flow through the study, demonstrating the recruitment in fluoridated and non-fluoridated areas.
FIGURE 10.
A STROBE flow diagram for the older school cohort. Potential participants included those attending reception in Cumbria schools in September 2013–August 2014. Invited to participate/identification was based on area of recruitment (school).
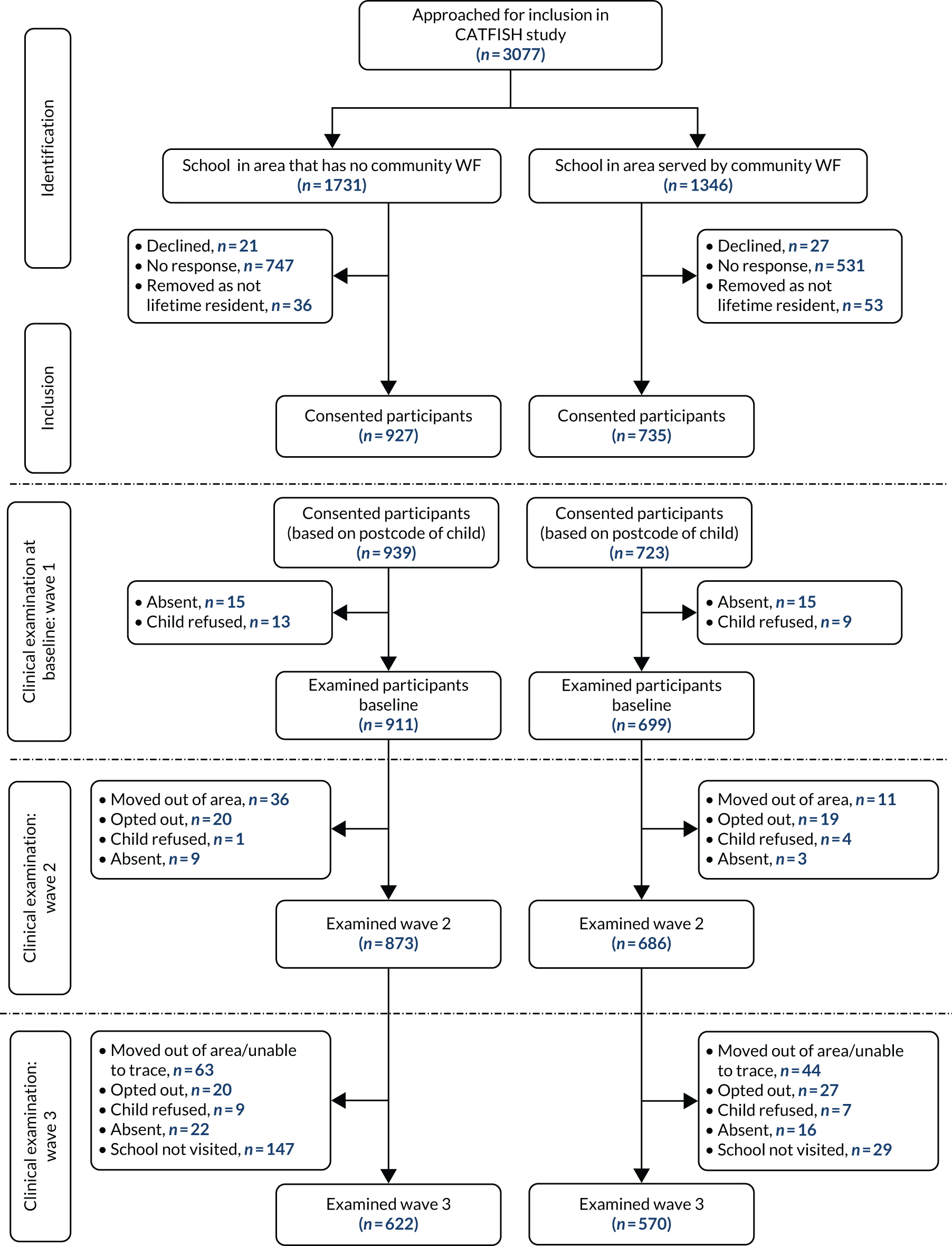
Examination status at the final clinical examination is presented in Table 20, according to fluoridated or non-fluoridated water status. There were fewer participants in the fluoridated area than in the non-fluoridated area at baseline (owing to the geographic restrictions of the fluoridated area). When comparing the percentage of participants who had a final examination, the non-fluoridated group had 66% of participants completing their final examinations (of those originally recruited) compared with 79% of participants in a fluoridated area, with lack of access to schools in the non-fluoridated area (on account of COVID-19) being the principal source of the differential attrition.
| Examination status | Exposure group, n (%) | Total | |
|---|---|---|---|
| No WF | WF | ||
| Examined | 622 (66.2) | 570 (78.8) | 1192 (71.7) |
| Absent | 22 (2.3) | 16 (2.2) | 38 (2.3) |
| Child refused | 9 (1.0) | 7 (1.0) | 16 (1.0) |
| Opted out | 40 (4.3) | 46 (6.4) | 86 (5.2) |
| Child moved | 99 (10.5) | 55 (7.6) | 154 (9.3) |
| School not visited (COVID-19 closure) | 147 (15.6) | 29 (4.0) | 176 (10.6) |
| Total | 939 (100) | 723 (100) | 1662 (100) |
Demographics and behaviours were explored to assess if there were any significant differences between the two groups (i.e. intervention and control). Table 21 details the demographics and deprivation for the fluoridated and non-fluoridated groups across each wave/clinical examination. Only children who had an examination at that time are included in Table 21. Ages were similar between groups at each wave. Deprivation followed the same pattern at each wave, with children in the fluoridated group having an, on average, higher (i.e. more deprived) deprivation score. In addition, we explored oral hygiene and related oral health behaviours periodically throughout the study (i.e. at baseline and then at each clinical examination). GEEs were carried out to determine if significant differences occurred between groups over this time period and whether or not they needed to be included in a regression analysis to adjust for these variables (see Appendix 3, Tables 112–149 for all tables, descriptive statistics and analysis relating to potential confounders and bias). Variables included within regression analysis were deprivation (quintiles), sex, dmft at baseline and age.
| Characteristic | Wave 1: baseline examination | Wave 2: second clinical examination | Wave 3: third clinical examination | |||
|---|---|---|---|---|---|---|
| No WF | WF | No WF | WF | No WF | WF | |
| Age | ||||||
| n | 896 | 693 | 858 | 680 | 611 | 562 |
| Mean (SD) | 5.06 (0.34) | 5.01 (0.34) | 7.00 (0.35) | 7.02 (0.35) | 10.80 (0.33) | 10.80 (0.32) |
| Median (LQ, UQ) | 5.05 (4.81, 5.33) | 5.01 (4.74, 5.28) | 7.01 (6.73, 7.28) | 7.02 (6.75, 7.30) | 10.80 (10.5, 11.10) | 10.80 (10.50, 11.00) |
| Minimum, maximum | 4.24, 5.81 | 4.27, 5.81 | 6.08, 7.79 | 6.13, 7.81 | 10.10, 11.80 | 9.90, 11.40 |
| Difference (95% CI) | 0.05 (0.02 to 0.08) | –0.18 (–0.05 to 0.02) | 0.06 (0.03 to 0.10) | |||
| Sex, n (%) | ||||||
| Male | 478 (54.0) | 390 (57.2) | 460 (54.2) | 382 (57.4) | 339 (54.9) | 320 (56.1) |
| Female | 407 (46.0) | 292 (42.8) | 389 (45.8) | 284 (42.6) | 278 (45.1) | 250 (43.9) |
| Total | 885 | 682 | 617 | 570 | ||
| Chi squared test | χ2 = 1.5699; p = 0.210 | χ2 = 1.5248; p = 0.217 | χ2 = 0.1719; p = 0.678 | |||
| IMD | ||||||
| n | 892 | 691 | 855 | 678 | 611 | 562 |
| Mean (SD) | 24.79 (15.32) | 27.75 (15.98) | 24.66 (15.34) | 27.69 (15.96) | 24.60 (15.50) | 28.20 (15.60) |
| Median (LQ, UQ) | 20.23 (12.81, 33.21) | 24.30 (16.39, 36.92) | 20.10 (12.6, 32.9) | 24.30 (16.4, 36.9) | 20.20 (12.60, 33.20) | 26.50 (16.50, 36.90) |
| Minimum, maximum | 4.37, 70.58 | 2.92, 65.45 | 5.06, 70.58 | 5.13, 65.45 | 4.40, 70.60 | 5.10, 65.50 |
| Difference (95% CI) | –2.96 (–4.52 to –1.41) | –3.03 (–4.60 to –1.45) | –3.50 (–5.30 to –1.80) | |||
| Deprivation quintile, n (%) | ||||||
| 1 (least deprived) | 87 (9.8) | 61 (8.8) | 88 (10) | 62 (9.1) | 74 (12.1) | 41 (7.3) |
| 2 | 180 (20.2) | 99 (14.3) | 175 (20.5) | 94 (13.9) | 119 (19.5) | 80 (14.2) |
| 3 | 199 (22.3) | 116 (16.8) | 189 (22.1) | 117 (17.3) | 127 (20.8) | 99 (17.6) |
| 4 | 227 (25.5) | 195 (28.2) | 213 (24.9) | 190 (28.0) | 153 (25.0) | 157 (27.9) |
| 5 (most deprived) | 199 (22.3) | 220 (31.8) | 190 (22.2) | 215 (31.7) | 138 (22.6) | 185 (32.9) |
| Total | 892 | 691 | 855 | 678 | 611 | 562 |
| Difference in deprivation quintile Chi square with trend | z = 4.77; p < 0.0001 | z = 4.86; p < 0.0001 | z = 5.04; p < 0.0001 | |||
| Decay dmft for wave 1 and DMFT (only teeth erupted after baseline included) for waves 2/3 | ||||||
| n | 911 | 699 | 873 | 686 | 622 | 570 |
| Mean (SD) | 1.18 (2.41) | 1.06 (2.16) | 0.04 (0.21) | 0.07 (0.34) | 0.40 (0.90) | 0.32 (0.77) |
| Median (LQ, UQ) | 0 (0, 1) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Minimum, maximum | 0, 14 | 0, 12 | 0, 2 | 0, 4 | 0, 5 | 0, 5 |
| Difference (95% CI) | 0.12 (–0.11 to 0.34) | –0.03 (–0.05 to 0.00) | 0.09 (–0.01 to 0.18) | |||
Primary outcome
The primary results are shown in a simple two-by-two format in Table 22. The risk of having dental caries for children living in an area served by WF was 87% of the risk of those living in non-fluoridated areas (risk ratio 0.87, 95% CI 0.698 to 1.095), with a 2.8% point difference. From this unadjusted risk ratio, there is insufficient evidence of a difference in the presence or absence of decay according to WF. A logistic regression with covariates of deprivation (reference IMD quintile 1), age, dmft at baseline and sex (reference male) was performed, and this indicated that the adjusted odds of decay for children from a fluoridated area was 80% of the odds of decay for children from a non-fluoridated area (risk ratio 0.8), conditional on the values of the covariates. The risk ratio’s 95% CI ranges from 0.58 to 1.09 and as this CI includes the null value of 1, we can conclude that there is insufficient evidence of an effect on the presence or absence of decay according to WF on permanent teeth conditional on the values of the covariates (see Appendix 3, Tables 134 and 135, and Figure 29). Eruption rates were not considered, as there was no systemic exposure to fluoride from water in this cohort.
| Caries status | Exposure group | Total | |
|---|---|---|---|
| No WF | WF | ||
| Decay present, n (%) | 136 (21.9) | 109 (19.1) | 245 (20.6) |
| Decay absent, n (%) | 486 (78.1) | 461 (80.9) | 947 (79.4) |
| N | 622 | 570 | 1192 |
| Risk ratio (95% CI) | 0.87 (0.698 to 1.095) | ||
| Unadjusted OR (95% CI) | 0.84 (0.64 to 1.12) | ||
| Adjusted OR (95% CI) | 0.80 (0.58 to 1.09) | ||
Older school cohort: secondary data analysis
Secondary outcome: dental general anaesthetic for extractions
In the intervention group, 2.1% of children experienced a DGA. In the control group, 2.8% of children experienced a DGA. There was no evidence of a difference in the DGA experience by exposure group when controlling for deprivation (quintile) and sex as covariates (OR 0.81, 95% CI 0.51 to 1.26) (see Appendix 3, Tables 136 and 137, for further details).
Secondary outcome: decayed, missing or filled teeth (permanent)
Table 23 details the count of DMFT (generated from clinical examination scores) for each participant.
| Caries into dentine (DMFT n) | Exposure group | Total (N = 1192), n (%) | |
|---|---|---|---|
| No WF (N = 662), n (%) | WF (N = 570), n (%) | ||
| 0 | 486 (78.1) | 461 (80.9) | 947 (79.5) |
| 1 | 67 (10.8) | 61 (10.7) | 128 (10.7) |
| 2 | 38 (6.1) | 32 (5.6) | 70 (5.9) |
| 3 | 17 (2.7) | 9 (1.6) | 26 (2.2) |
| 4 + | 14 (2.3) | 7 (1.2) | 21 (1.8) |
These data were further explored in a negative binomial regression in which deprivation quintile (reference category 1, least deprived quintile), age, dmft at baseline and sex (reference category male) were entered as covariates with an offset of number of erupted permanent teeth. The regression shows that the incidence rate of DMFT for children in a fluoridated region was 0.69 (95% CI 0.52 to 0.93) that of children living in a non-fluoridated region, when holding other variables constant in the model. The rate of DMFT is significantly lower for children in the WF group than for children in the no WF group when controlling for these variables (see Appendix 4, Tables 139 and 140 and Figure 32).
Older school cohort: inequalities data analysis
Health inequalities: primary outcome
A generalised linear model with logistic link function and including an exposure by deprivation interaction term was undertaken to determine the effects of WF on the presence or absence of permanent decay at different levels of the IMD. This analysis was supplemented by the resulting marginal predicted probabilities for interpretability. A multidegree of freedom test of the interaction term following the logistic regression indicated that there was insufficient evidence of an exposure by deprivation interaction (F = 2.68; p = 0.613). From our analysis, there is no evidence that deprivation influences the relationship between water exposure and the presence or absence of decay. Full results from the model are reported in Appendix 3, Tables 141–144.
Figure 11 shows the resulting predictive probabilities for decay or no decay in permanent teeth based on a model that included age, sex and an interaction term for exposure by deprivation quintiles for the median age of clinical assessment at 10 years.
FIGURE 11.
Adjusted predictions of decay or no decay including an interaction term for area across deprivation quintiles with 95% CI at 10 years old. Decay is at tooth level, with the decay component being any decay into dentine.
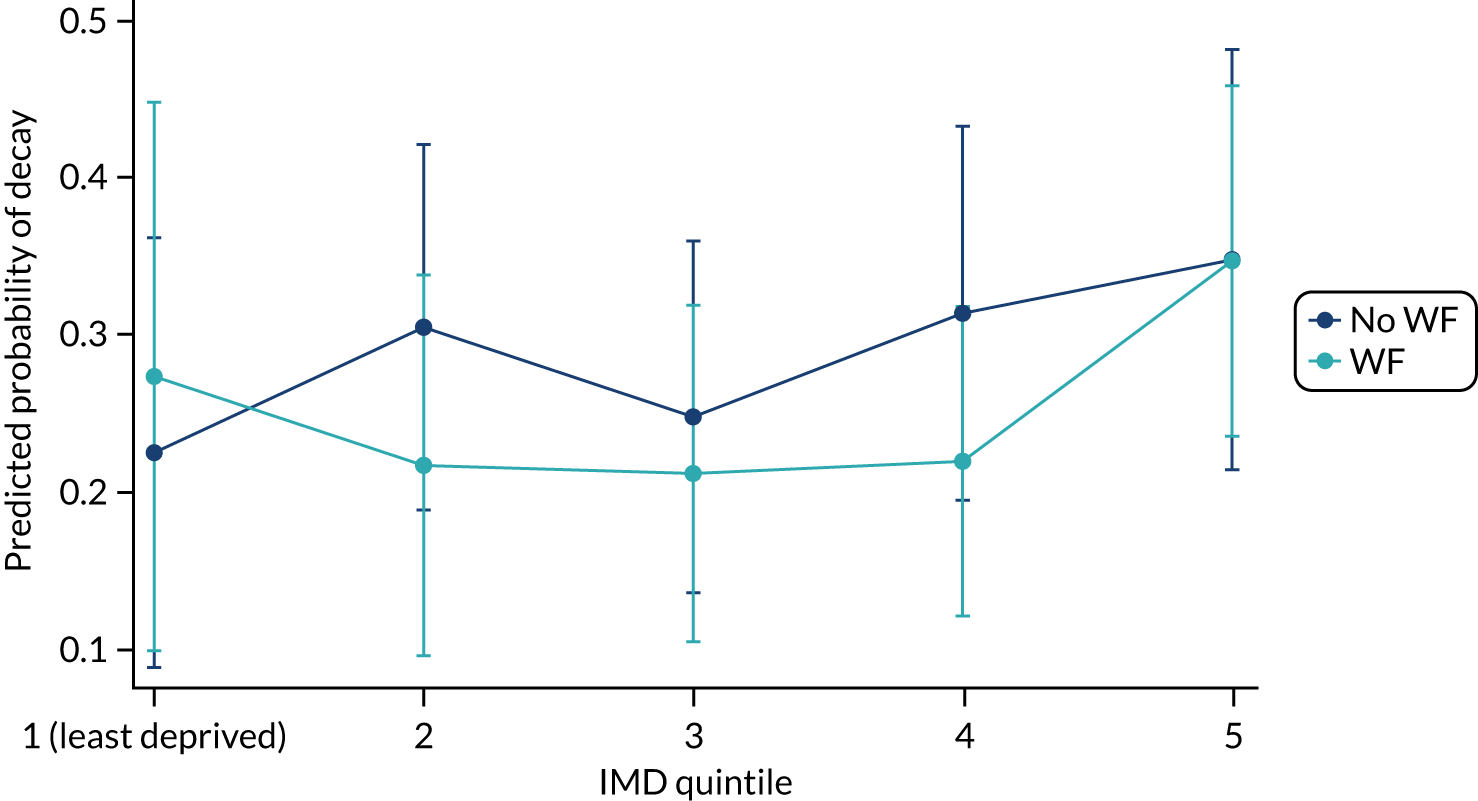
Health inequalities: secondary outcome
A negative binomial regression was undertaken to assess if WF contributes to reductions in health inequalities by examining the count of decay in permanent teeth and the interaction between WF status and deprivation, including age, dmft at baseline and sex as covariates, offset by erupted permanent teeth. A multidegree of freedom test of the interaction term following the negative binomial regression indicated that there was insufficient evidence of an exposure by deprivation interaction (F = 2.85; p = 0.5833). From our analysis, there is no evidence that deprivation influences the relationship between water exposure and the severity of dental caries as indicated by DMFT. Full results from the model are reported in Appendix 3, Tables 144–146 and Figure 35.
Analysis for blinding: clinical versus photos
Examination of any potential bias in scoring was undertaken by comparing photo scores (with the examiner blinded to the exposure status of the participant) and clinical scores (which were measured in a school setting, with the result that the examiner was likely to be aware of exposure status given the geographic location). The kappa value was highly significant when testing the hypothesis [H0, there is disagreement; H1, there is agreement (p < 0.00001)].
Table 24 illustrates the proportion of decay and no decay from both clinical and photographic scores. Both the clinical scores and photo scores showed a similar difference in decay between the fluoridated and non-fluoridated areas (3.5% for the clinical scores and 3.4% for the photo scores). Therefore, we can have confidence that the clinical scores can be utilised and were not unduly biased by the clinician in some cases being aware of the fluoridation status of a participant.
| Caries status | Clinical scoresa | Photo scores | ||
|---|---|---|---|---|
| No WF | WF | No WF | WF | |
| Decay present, n (%) | 122 (23.3) | 96 (19.8) | 175 (33.5) | 147 (30.1) |
| Decay absent, n (%) | 401 (76.7) | 390 (80.2) | 348 (66.5) | 339 (69.8) |
| N | 523 | 486 | 523 | 486 |
| Difference in decay (%) | 3.5 | 3.4 | ||
| Risk ratio (95% CI) | 0.85 (0.67 to 1.07) | 0.90 (0.75 to 1.08) | ||
Chapter 10 Health economic results
Birth cohort results
Sample
A total of 2035 children were consented into the study and 1444 children had an examination at school; however, three children had no identifier to link to the questionnaire data, giving a sample of 1441 children in the study. A total of 514 children completed the questionnaire at 5 years (927 children did not complete the questionnaire). Two children had no valid deprivation quintile score (one child with questionnaire data and one child with no questionnaire data), giving 513 children with and 926 children without questionnaire data on deprivation. Data were assessed to determine whether or not IMD and/or fluoridation status was associated with completing a questionnaire (see Appendix 4, Table 150). There was no evidence that questionnaire completion was associated with children residing in a fluoridated area (p = 0.291), but completing the questionnaire was associated with living in less deprived areas. Interacting fluoridation with deprivation quintile found that the relationship between deprivation and completion did not vary across fluoridation groups, implying that the potential bias caused by deprivation is a similar bias across fluoridation groups.
Of the 513 children with questionnaire data, eight had no follow-up dates (seven children because no birth date was provided and one child because no date of questionnaire completion was provided). In addition, one child had a follow-up date of zero, which was deemed a coding error. Subsequently, this gives a sample of 504 children in the birth cohort for the health economic analyses.
Missingness and complete-case sample
At least some CHU9D data were available for all of the 504 children in the final sample. Data across one or more domains were incomplete for 47 children, giving a final sample of 457 children with complete CHU9D data (i.e. 31.76% of 1439 children).
Of the 504 children in the final sample, all had data on DGA activity and costs; however, 165 children had no NHS BSA data activity and costs, resulting in a complete-case sample of 339 children (i.e. 67.26%). In some cases, no dental activity data at all were available; in others, the NHS BSA was unable to match respondents in the sample to its own data. In the 2013 Child Dental Health Survey,38 only 6% of children aged 5 years had never been to a dentist, which suggests that the vast majority of missing data are the result of an inability to match respondents, rather than actual zero activity, and, therefore, we assume that the data are missing rather than zero.
The rates of missing data in total and by fluoridation group are provided in Table 25. Complete data are similar across the groups, providing some support towards the missing data mechanism being MCAR.
| Data | Exposure group | All | |
|---|---|---|---|
| WF | No WF | ||
| Complete data | |||
| Baseline, n | 193 | 311 | 504 |
| Follow-up, n (%) | 117 (60.62) | 189 (60.77) | 306 (60.71) |
| Complete QALY data | |||
| Baseline, n | 193 | 311 | 504 |
| Follow-up, n (%) | 174 (90.16) | 283 (91.00) | 457 (90.67) |
| Complete cost data | |||
| Baseline, n | 193 | 311 | 504 |
| Follow-up, n (%) | 131 (67.88) | 208 (66.88) | 339 (67.26) |
Table 26 provides the estimates from logistic regressions of missing data against baseline covariates, outcomes and a fluoridation group indicator. No baseline variable was associated with missing data on either costs or QALYs, providing, again, some support towards the missing data mechanism being MCAR. Therefore, our preferred model specification for the birth cohort is that of CCA. MAR is explored as sensitivity analyses.
| Variable | Any missing data, OR (95% CI) | Missing cost, OR (95% CI) | Missing QALY, OR (95% CI) |
|---|---|---|---|
| Fluoridation group | 1.01 (0.69 to 1.46) | 0.95 (0.64 to 1.40) | 1.11 (0.59 to 2.06) |
| Age | 1.09 (0.68 to 1.74) | 1.14 (0.70 to 1.86) | 0.90 (0.41 to 2.00) |
| Female | 1.09 (0.76 to 1.56) | 1.13 (0.77 to 1.64) | 0.90 (0.49 to 1.65) |
| IMD (base = IMD quintile 5) | |||
| IMD quintile 1 | 0.93 (0.46 to 1.87) | 1.06 (0.52 to 2.17) | 1.14 (0.36 to 3.58) |
| IMD quintile 2 | 0.96 (0.52 to 1.77) | 0.95 (0.50 to 1.79) | 1.18 (0.43 to 3.22) |
| IMD quintile 3 | 1.09 (0.61 to 1.94) | 1.02 (0.56 to 1.86) | 0.97 (0.36 to 2.59) |
| IMD quintile 4 | 1.16 (0.64 to 2.08) | 1.16 (0.63 to 2.13) | 1.01 (0.59 to 2.06) |
| Baseline CHU9D | |||
| n | 504 | 504 | 504 |
Child Health Utility 9-Dimensions health status, utility and quality-adjusted life-years
The responses to the individual domains of the CHU9D are presented in Appendix 4, Tables 151 and 152, for children with complete CHU9D data and children with complete CHU9D data and cost data (i.e. the CCA sample). Table 27 includes the utility scores estimated from the CHU9D domains and published population weights. The utility scores are anchored at 0 (dead) and 1 (full health), with some states also valued as worse than death. There were no clear differences between the fluoridated and non-fluoridated groups at follow-up.
| Variable | CHU9D utility values, mean (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 189) | WF (n = 117) | ||
| Age 5 questionnaire | 0.9280 (0.0677) [0.9183 to 0.9377] | 0.9318 (0.0634) [0.9202 to 0.9434] | 0.004 (–0.0115 to 0.0191) |
Follow-up
Table 28 summarises the length of follow-up in years for children in the CCA.
| Variable | Follow-up duration (years), mean (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 189) | WF (n = 117) | ||
| Children with complete utility data | 4.87 (0.39) [4.82 to 4.92] | 4.81 (0.34) [4.75 to 4.87] | –0.06 (–0.14 to 0.03) |
Quality-adjusted life-years
Table 29 summarises the QALY measure and discounted QALY measure. There appears to be little evidence of differences in QALYs between the two groups.
| Analysis | Mean (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 189) | WF (n = 117) | ||
| QALY | 4.698 (0.411) [4.639 to 4.757] | 4.650 (0.377) [4.581 to 4.719] | –0.047 (–0.139 to 0.045) |
| Discounted QALY | 4.347 (0.351) [4.297 to 4.397] | 4.307 (0.322) [4.248 to 4.366] | –0.040 (–0.119 to 0.038) |
Service use and costs
NHS Business Services Authority and emergency hospital (dental) costs
Data from NHS BSA include data on units of dental activity (UDAs) attributed to a child for a course of treatment. Each child could have multiple courses of treatment over the 5-year period. The unit cost applied to a UDA varies depending on the dental practice providing the service. Dental practices are given a UDA target, which is the volume of UDAs the practice is commissioned to provide on behalf of the NHS. Each practice also has a UDA value, which is the actual amount the dental practice is paid. Dividing the value by the target provides a unit cost per UDA. Each course of treatment is allocated a UDA volume (band 1, 1 UDA; band 2, 3 UDAs; band 3, 12 UDAs; and urgent, 1.5 UDAs). The NHS commissioning and reimbursement of services is on the basis of UDAs and not per item (e.g. a band 1 covers 1 UDA but involves activity that includes examinations and scale and polish; however, the same UDAs are applied if a child received only an examination or an examination and scale and polish). Furthermore, there were no co-payments by the children’s parents or guardians.
General anaesthetic data included the number of DGAs provided. The unit cost applied here was £935 (sourced as the unit cost for multiple extraction of teeth as a day case within hospital, taken from NHS national cost collection data). 102
The average volume of treatments per band and DGAs per child are provided in Table 30. In addition, Appendix 4, Table 153, presents the data for children with complete cost data, irrespective of whether or not CHU9D data were complete. Most treatments were band 1, with low activity for other courses of treatment, urgent care and DGA over the period.
| Type of service | Exposure group, mean (SD) | |
|---|---|---|
| No WF (n = 189) | WF (n = 117) | |
| Dental activity: band 1 | 5.59 (2.34) | 4.38 (2.08) |
| Dental activity: band 2 | 0.19 (0.70) | 0.07 (0.29) |
| Dental activity: band 3 | 0.00 (0.00) | 0.00 (0.00) |
| Dental activity: urgent | 0.15 (0.48) | 0.04 (0.24) |
| Emergency hospital activity (dental) | 0.03 (0.18) | 0.03 (0.16) |
Table 31 summarises the cost of primary and community-based dental services (NHS BSA) and hospital-based dental services for those children with complete data. Overall, primary and community dental services were the highest cost component. Table 32 presents the total costs for children in the CCA.
| Type of service | Exposure group, mean cost (£) (SD) | |
|---|---|---|
| No WF (n = 189) | WF (n = 117) | |
| Dental activity | 136.94 (71.03) | 101.27 (54.64) |
| Emergency hospital activity (dental) | 24.88 (137.97) | 20.99 (130.64) |
| Total cost | Exposure group, mean cost (£) (SD) [95% CI] | Difference (95%CI) | |
|---|---|---|---|
| No WF (n = 189) | WF (n = 117) | ||
| Baseline to follow-up assessment | 161.82 (155.86) [139.45 to 184.18] | 136.41 (147.74) [109.35 to 163.46] | –25.41 (–30.78 to 9.96) |
Cost and quality-adjusted life-year differences and cost-effectiveness of water fluoridation
Table 33 reports the net costs and QALYs after adjusting for differences in covariates. The data suggest a net lower cost for WF and a small net gain in QALYs. It is important to note that both the net cost and QALY data are associated with wide CIs that include zero and, therefore, differences are not statistically significant.
| Analysis | Cost difference (£), mean (95% CI) | QALY difference, mean (95% CI) |
|---|---|---|
| Children with complete cost and health benefit data | –21.41 (–57.37 to 14.56) | 0.0110 (–0.0211 to 0.0431) |
Table 34 reports the bootstrap simulations of cost and QALY differences, as well as the results of the cost-effectiveness acceptability analyses. The cost-effectiveness plane (Figure 12) illustrates the scatter of the 10,000 simulated cost and QALY differences and Figure 13 shows the cost-effectiveness acceptability curve. Overall, there is no clear difference in costs or QALYs, with a mean net benefit of £246 (95% CI –£416 to £905). In this scenario, at a WTPT of £20,000, the probability that WF is cost-effective is 0.7698.
| Statistic | CCA |
|---|---|
| Cost (£) difference (95% CI) | –21.55 (–55.61 to 15.40) |
| QALY difference (95% CI) | 0.0112 (–0.0223 to 0.0445) |
| Incremental cost/QALY gained (£) | –1924.11 |
| Net benefit (95% CI): WTPT = £20,000 | 246 (–416 to 905) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.7698 |
FIGURE 12.
Cost-effectiveness plane of cost and QALY differences, primary analysis.

FIGURE 13.
Cost-effectiveness acceptability curve: probability that WF is cost-effective, primary analysis.
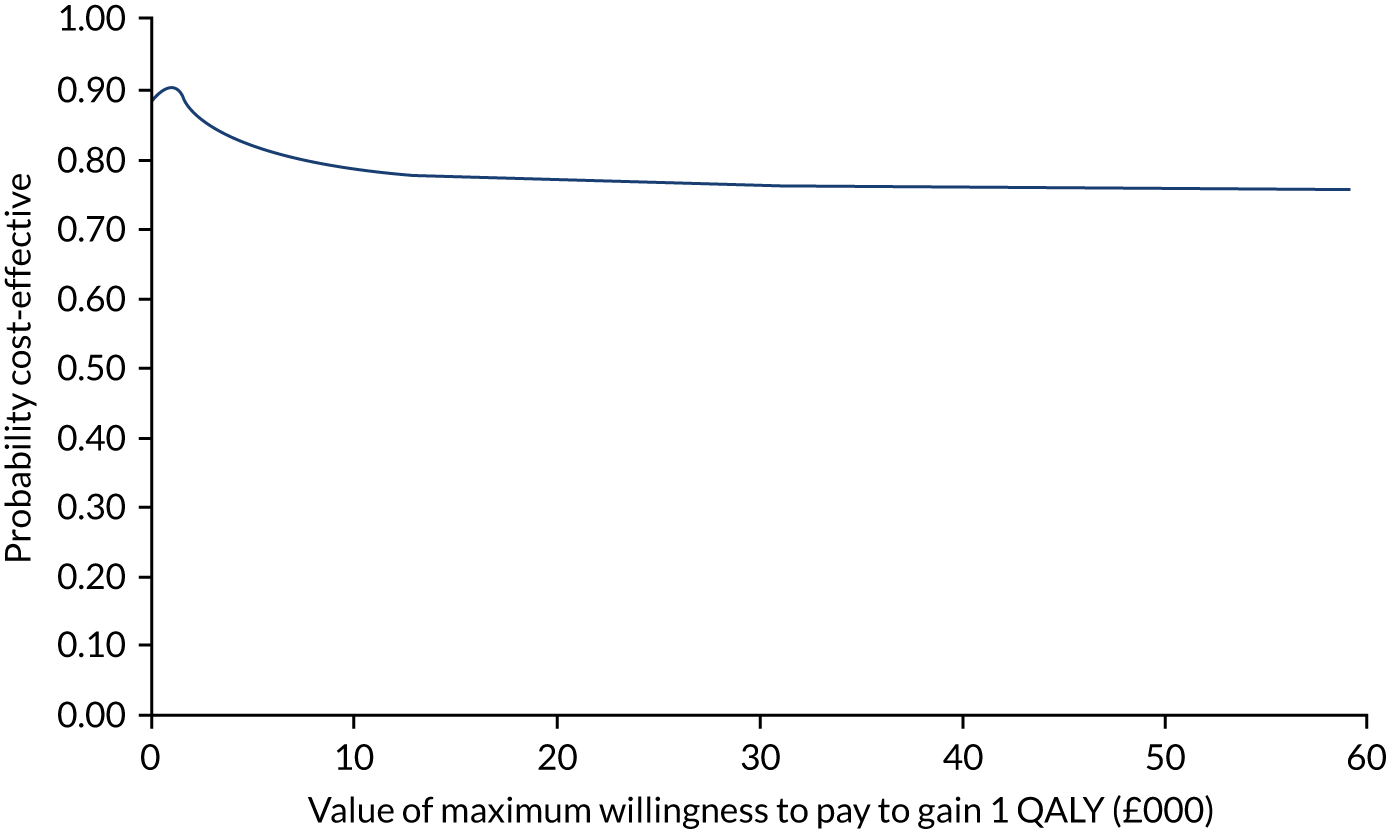
Sensitivity analyses
A range of sensitivity analyses were performed, including assuming that the missing data mechanism is MAR, attributing costs of fluoridation to the population aged 0–12 years, and an assessment with clinical measures of the volume of decayed, missing and filled teeth avoided and the presence of no decay.
Assuming a missing at random missing data mechanism
Multiple imputation using predictive mean matching via chained equations was performed to predict missing values at follow-up. The number of imputed data was set to 40 due to the percentage of missing data being 60.71% (see Table 25). Imputation was at the individual CHU9D domains and individual resource unit level (at which level missing data arose only in the NHS BSA dental cost data). The multiply imputed NHS BSA data are presented in Appendix 4, Table 154. Tables 35 and 36 present the discounted QALYs and total costs for the multiply imputed data, respectively. Table 37 presents the cost-effectiveness estimates from the imputed data. Multiple imputation had a minimal impact on costs but increased the net QALYs, and this increased the probability of fluoridation being cost-effective at a WTPT of £20,000 (from 0.7698 to 0.9649).
| Analysis | Exposure group, mean (SE) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 311) | WF (n = 193) | ||
| Discounted QALY | 4.343 (0.021) [4.303 to 4.382] | 4.322 (0.025) [4.273 to 4.371] | –0.021 (–0.085 to 0.043) |
| Total cost | Exposure group, mean (£) (SE) [95% CI] | Difference (95%CI) | |
|---|---|---|---|
| No WF (n = 311) | WF (n = 193) | ||
| Baseline to follow-up assessment | 165.32 (9.53) [146.67 to 183.98] | 140.68 (11.87) [117.37 to 164.00] | –24.64 (–54.71 to 5.43) |
| Statistic | Multiply imputed data analysis |
|---|---|
| Cost (£) difference (95% CI) | –20.70 (–48.80 to 9.71) |
| QALY difference (95% CI) | 0.021 (–0.0026 to 0.0441) |
| Incremental cost/QALY gained (£) | –986 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 433 (–40 to 921) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.9649 |
Assigning costs of fluoridation to the population aged 0–12 years
Table 38 presents the cost-effectiveness estimates when fluoridation costs are attributed to the population aged 0–12 years, rather than to all ages. Allocating fluoridation costs to a smaller population increases costs. These costs are sufficient to exceed the negative costs identified in the primary analysis. However, the probability that WF is cost-effective at a WTPT of £20,000 is reduced (from 0.7698 to 0.6789).
| Statistic | CCA |
|---|---|
| Cost (£) difference (95% CI) | 69.94 (35.88 to 106.89) |
| QALY difference (95% CI) | 0.0112 (–0.0223 to 0.0445) |
| Incremental cost/QALY gained (£) | 6245 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 154 (–507 to 813) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.6789 |
Volume of decayed, missing and filled teeth avoided and no decay
Sensitivity analyses were conducted with count of dmft avoided and the presence of no decay as alternative outcomes. There were 1422 children with valid age and deprivation data (all with complete dmft and any decay data). The rates of missing data in total and by fluoridation group are provided in Table 39. Complete data are similar across the groups, providing some support for the missing data mechanism being MCAR.
| Data | Exposure group | All | |
|---|---|---|---|
| WF | No WF | ||
| Complete data | |||
| Baseline, n | 816 | 606 | 1422 |
| Follow-up, n (%) | 481 (58.95) | 347 (57.26) | 828 (58.23) |
| Complete dmft data | |||
| Baseline, n | 816 | 606 | 1422 |
| Follow-up, n (%) | 816 (100.00) | 606 (100.00) | 1422 (100.00) |
| Complete decay data | |||
| Baseline, n | 816 | 606 | 1422 |
| Follow-up, n (%) | 816 (100.00) | 606 (100.00) | 1422 (100.00) |
| Complete cost data | |||
| Baseline, n | 816 | 606 | 1422 |
| Follow-up, n (%) | 481 (58.95) | 347 (57.26) | 828 (58.23) |
Table 40 provides the estimates from logistic regressions of missing data against baseline covariates, outcomes and a fluoridation group indicator. IMD quintile variables were associated with missing data on costs (with IMD quintile 3 having less odds of having missing data) and this supports the missing data mechanism of MCAR with IMD included as covariates in the analyses. Therefore, our preferred model specification for the clinical analyses in the birth cohort is CCA.
| Variable | Any missing cost data, OR (95% CI) |
|---|---|
| Fluoridation group | 1.02 (0.82 to 1.27) |
| Age | 1.09 (0.81 to 1.49) |
| Female | 1.05 (0.85 to 1.30) |
| IMD (base = IMD quintile 5) | |
| IMD quintile 1 | 0.71 (0.46 to 1.09) |
| IMD quintile 2 | 0.55 (0.39 to 0.78) |
| IMD quintile 3 | 0.75 (0.55 to 1.02) |
| IMD quintile 4 | 0.87 (0.65 to 1.16) |
| Baseline clinical measure | |
| n | 1422 |
Tables 41 and 42 present the cost-effectiveness estimates in which the clinical outcomes are the measures of effectiveness. Overall, the estimates show similar results to the primary (QALY) analysis, with either outcome having better oral health in the fluoridated group, as well as lower costs, with this, again, supporting a conclusion that WF is likely to be cost-effective.
| Statistic | CCA |
|---|---|
| Cost (£) difference (95% CI) | –19.73 (–41.20 to 2.36) |
| dmft avoided difference (95% CI) | 0.1262 (–0.0632 to 0.3206) |
| Incremental cost/dmft avoided (£) | –156.18 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 2544 (–1312 to 6539) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.9003a |
| Statistic | CCA |
|---|---|
| Cost (£) difference (95% CI) | –19.73 (–41.20 to 2.36) |
| No decay difference (95% CI) | 0.0204 (–0.0217 to 0.0631) |
| Incremental cost/no decay (£) | –967.15 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 428 (–427 to 1247) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.8398a |
Older school cohort results
Sample
There were 1662 children in the study, with 1192 children having an assessment at age 11 years. Nineteen children had missing data at follow-up, five children because follow-up was > 7 years (a clear coding issue) and 14 children because of incomplete follow-up or baseline assessment dates. Of the 1173 children with dates at follow-up, nine had no age recorded and a further 19 had no deprivation recorded and, therefore, this gave a sample of 1145 children. Of the 1145 children, 392 had CHU9D data at baseline (incomplete, n = 2) and 388 had CHU9D at follow-up (incomplete, n = 4). In total, 388 (33.89%) children had complete CHU9D data at both time points, and this gives a sample of 392 children in the older school cohort for the health economic analyses.
In the case of the 1145 children with complete dates, age and deprivation quintile score related to their postcode, we assessed whether or not IMD, age and/or fluoridation was associated with questionnaire completion (see Appendix 4, Table 155). There was some evidence that questionnaire completion was positively associated with children residing in a fluoridated area (p = 0.050) and/or in less deprived areas. Interacting fluoridation with deprivation quintile found that the relationship between deprivation and completion did not vary across fluoridation groups, implying that the potential bias caused by deprivation is an equivalent bias across fluoridation groups.
Missingness and complete-case sample
Inference on the missing data mechanism was made on the sample of children with baseline data (n = 390; note that 2 of the 392 children in the sample had incomplete CHU9D data). Of the 390 children in the final sample with baseline data, four had incomplete data across one or more domains of the CHU9D at follow-up, giving a sample of 386 (98.97%) children with complete CHU9D data. All 390 children had data on DGA activity and costs; however, 116 children had no NHS BSA activity data and costs, resulting in a complete-case sample of 274 (70.26%) children. In some cases, no dental activity data at all were available; in others, the NHS BSA was unable to match respondents in the sample to its own data. In the 2013 Child Dental Health Survey,38 only 6% of children aged 5 years had never been to a dentist, which suggests that the vast majority of missing data are the result of an inability to match respondents, rather than actual zero activity, and, therefore, we assume that the data are missing rather than zero.
The rates of missing data in total and by fluoridation group are provided in Table 43. Complete data are similar across the groups, providing some support towards the missing data mechanism being MCAR.
| Data | Exposure group | All | |
|---|---|---|---|
| WF | No WF | ||
| Complete data | |||
| Baseline, n | 163 | 227 | 390 |
| Follow-up, n (%) | 112 (68.71) | 159 (70.04) | 271 (69.49) |
| Complete QALY data | |||
| Baseline, n | 163 | 227 | 390 |
| Follow-up, n (%) | 162 (99.39) | 224 (98.68) | 386 (98.97) |
| Complete cost data | |||
| Baseline, n | 163 | 227 | 390 |
| Follow-up, n (%) | 113 (69.33) | 161 (70.93) | 274 (70.26) |
Table 44 provides the estimates from logistic regressions of missing data against baseline covariates, outcomes and a fluoridation group indicator. No baseline variable was associated with missing data on either costs or QALYs (including baseline CHU9D) and this supports the missing data mechanism of MCAR. Therefore, our preferred model specification for the older school cohort is CCA. MAR is explored as sensitivity analyses.
| Variable | Any missing data, OR (95% CI) | Missing cost, OR (95% CI) | Missing QALY,a OR (95% CI) |
|---|---|---|---|
| Fluoridation group | 1.12 (0.71 to 1.78) | 1.12 (0.70 to 1.78) | 0.66 (0.06 to 6.78) |
| Age | 1.38 (0.71 to 2.69) | 1.23 (0.63 to 2.40) | 68.42 (1.01 to 4642.38) |
| Male | 0.75 (0.49 to 1.16) | 0.73 (0.47 to 1.14) | 2.80 (0.27 to 28.55) |
| IMD (base = IMD quintile 5) | |||
| IMD quintile 1 | 0.88 (0.42 to 1.87) | 0.95 (0.45 to 2.00) | |
| IMD quintile 2 | 0.82 (0.41 to 1.63) | 0.87 (0.44 to 1.75) | |
| IMD quintile 3 | 1.05 (0.53 to 2.07) | 0.99 (0.50 to 1.98) | |
| IMD quintile 4 | 0.94 (0.48 to 1.85) | 1.01 (0.51 to 1.99) | |
| Baseline CHU9D | 1.25 (0.04 to 38.56) | 0.96 (0.03 to 30.14) | 163.27 (–0.00 to 34,300,000,000) |
| n | 390 | 390 | 390 |
Child Health Utility 9-Dimensions health status, utility and quality-adjusted life-years
The responses to the individual domains of the CHU9D are presented in Appendix 4, Tables 156 and 157, for those with complete CHU9D data and Appendix 4, Tables 158 and 159, for those with complete CHU9D data and cost data (i.e. the CCA sample). Table 45 includes the utility scores estimated from the CHU9D domains and published population weights. The utility scores are anchored at 0 (dead) and 1 (full health), with some states also valued as worse than death. There were no clear differences between the fluoridated and non-fluoridated groups at baseline or follow-up.
| CHU9D utility value | Exposure group, mean (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 159) | WF (n = 112) | ||
| Age 5 years (baseline) | 0.9384 (0.0630) [0.9285 to 0.9483] | 0.9294 (0.0633) [0.9175 to 0.9412] | –0.0090 (–0.0244 to 0.0063) |
| Age 11 years (final clinical assessment) | 0.8918 (0.0806) [0.8791 to 0.9044] | 0.8964 (0.0853) [0.8804 to 0.9124] | 0.0046 (–0.0154 to 0.0247) |
Follow-up
Table 46 summarises the length of follow-up (years) for children with complete CHU9D data.
| Analysis | Follow-up duration (years), mean (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 159) | WF (n = 112) | ||
| Children with complete utility data | 5.83 (0.18) [5.80 to 5.85] | 5.93 (0.21) [5.89 to 5.97] | 0.1063 (0.0594 to 0.1532) |
Quality-adjusted life-years
Table 47 summarises the QALY measure for children with complete cost and CHU9D data at baseline and follow-up. There appears to be little evidence of differences in QALYs between the two groups.
| Analysis | Mean (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 159) | WF (n = 112) | ||
| QALY | 5.335 (0.371) [5.277 to 5.393] | 5.419 (0.377) [5.348 to 5.489] | 0.083 (–0.007 to 0.174) |
| Discounted QALY | 4.863 (0.325) [4.812 to 4.913] | 4.927 (0.329) [4.866 to 4.989] | 0.065 (–0.015 to 0.144) |
Service use and costs
NHS Business Services Authority and emergency hospital (dental) costs
The NHS BSA holds data on UDAs accredited to a child for a course of treatment. Each child could have multiple courses of treatment over the 6-year period. The unit cost applied to a UDA varies depending on the dental practice providing the service. Dental practices are given a UDA target, which is the volume of UDAs the practice is commissioned to provide on behalf of the NHS. Each practice also has a UDA value, which is the actual amount the dental practice is paid. Dividing the value by the target provides a unit cost per UDA. Each course of treatment is allocated a UDA volume (band 1, 1 UDA; band 2, 3 UDAs; band 3, 12 UDAs; and urgent, 1.5 UDAs). The NHS commissioning and reimbursement of services is on the basis of UDAs and not per item (e.g. band 1 covers 1 UDA but involves activity that includes examinations and scale and polish; however, the same UDAs are applied if a child received only an examination or an examination and scale and polish). Furthermore, there were no co-payments by the children’s parents or guardians.
General anaesthetic data included the number of DGAs provided. The unit cost applied here was £935 (sourced as the unit cost for multiple extraction of teeth as a day case within hospital from NHS national cost collection data). 102
The average numbers of treatments per band and DGAs per child are provided in Table 48. Appendix 4, Table 160, presents the data for children with complete cost data, irrespective of whether or not CHU9D data are complete. Most treatments were band 1, with low activity for other courses of treatment, urgent care and DGA over the period.
| Type of service | Exposure group, mean (SD) | |
|---|---|---|
| No WF (n = 159) | WF (n = 112) | |
| Dental activity: band 1 | 11.77 (4.44) | 8.90 (4.64) |
| Dental activity: band 2 | 2.11 (2.59) | 1.74 (2.25) |
| Dental activity: band 3 | 0.01 (0.08) | 0.02 (0.13) |
| Dental activity: urgent | 0.50 (0.96) | 0.39 (0.78) |
| Emergency hospital activity (dental) | 0.11 (0.32) | 0.10 (0.30) |
Table 49 summarises the cost of primary and community-based dental services (NHS BSA) and hospital-based dental services for children with complete data. Overall, primary and community dental services were the highest cost component. Table 50 presents the total costs for children in the CCA.
| Type of service | Exposure group, mean (£) (SD) | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 159) | WF (n = 112) | ||
| Dental activity | 432.73 (191.61) | 360.00 (200.20) | –72.73 (–120.14 to –25.32) |
| Emergency hospital activity (dental) | 102.20 (287.34) | 87.76 (267.55) | –14.45 (–82.29 to 53.40) |
| Total cost | Exposure group, mean (£) (SD) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 159) | WF (n = 112) | ||
| Baseline to follow-up assessment | 534.93 (342.79) [481.24 to 588.62] | 461.89 (340.05) [398.22 to 525.57] | –73.03 (–156.01 to 9.95) |
Cost and quality-adjusted life-year differences and cost-effectiveness of water fluoridation
Table 51 reports the net costs and QALYs after adjusting for differences in covariates. The data suggest a net lower cost for WF and a small net gain in QALYs.
| Analysis | Cost (£) difference, mean (95% CI) | QALY difference, mean (95% CI) |
|---|---|---|
| Children with complete cost and health benefit data | –63.03 (–152.66 to 26.59) | 0.0093 (–0.0421 to 0.0608) |
Table 52 reports the bootstrap simulations of cost and QALY differences, as well as the results of the cost-effectiveness acceptability analyses. The results show similar cost and QALY differences to the analysis adjusted for covariates. The cost-effectiveness plane (Figure 14) illustrates the scatter of the 10,000 simulated cost and QALY differences and Figure 15 shows the cost-effectiveness acceptability curve. Overall, there is no clear difference in costs or QALYs, with a mean net benefit of £255 (95% CI –£779 to £1250). In this simulation, with a WTPT of £20,000, the probability that WF is cost-effective is 0.6831.
| Statistic | Children with complete cost and health benefit data |
|---|---|
| Cost (£) difference (95% CI) | –64.01 (–150.11 to 20.13) |
| QALY difference (95% CI) | 0.0095 (–0.0412 to 0.0596) |
| Incremental cost/QALY gained (£) | –6737.89 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 255 (–779 to 1250) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.6831 |
FIGURE 14.
Cost-effectiveness plane of net costs and QALYs, primary analysis.
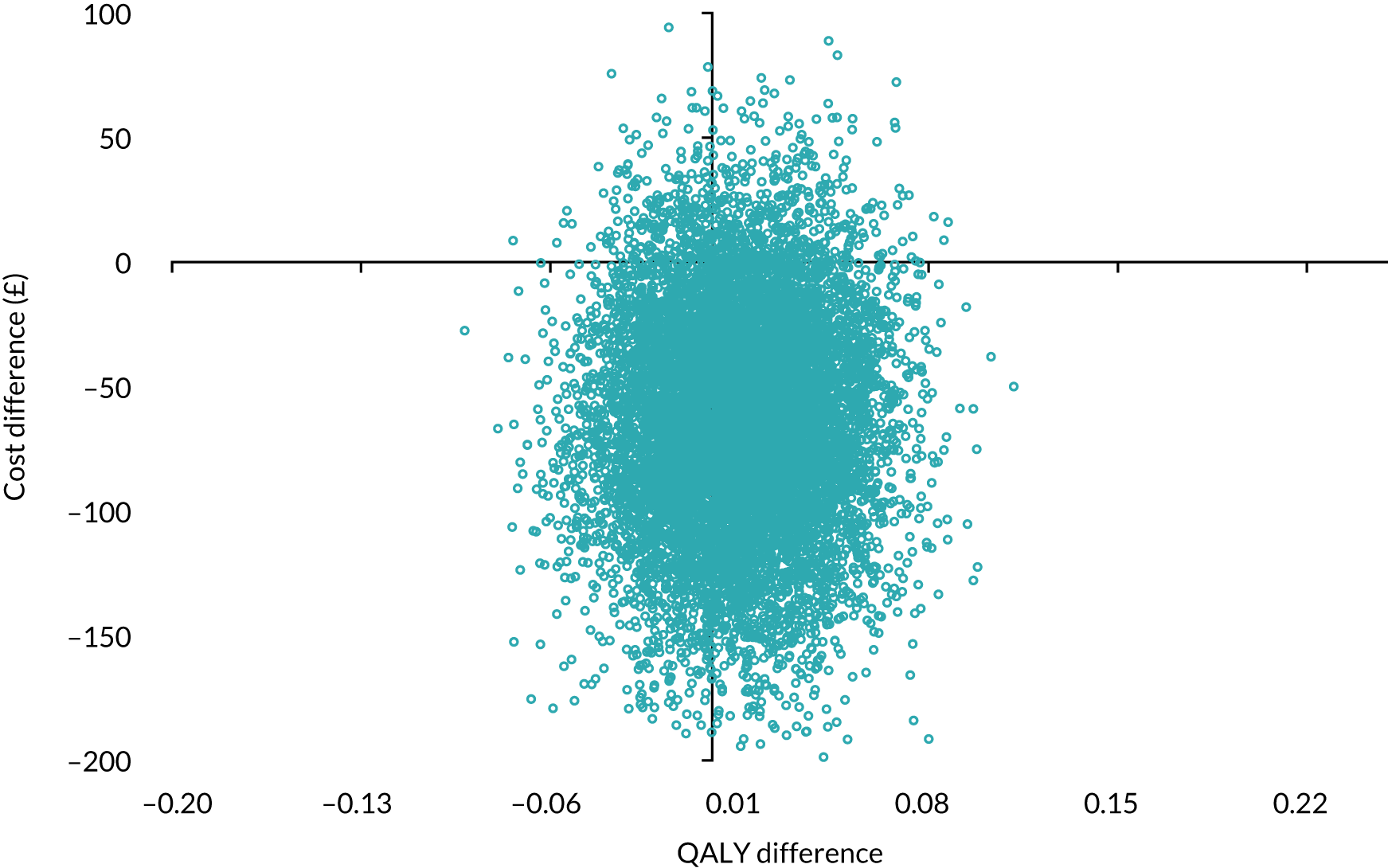
FIGURE 15.
Cost-effectiveness acceptability curve, probability that WF is cost-effective, primary analysis.

Sensitivity analyses
A range of sensitivity analyses were performed, including assuming that the missing data mechanism is MAR, attributing costs of fluoridation to the population aged 0–12 years, and an assessment with the clinical measures of the volume of decayed, missing and filled teeth avoided and the presence of no decay.
Assuming a missing at random missing data mechanism
Multiple imputation using predictive mean matching via chained equations was performed to predict missing values at follow-up and for baseline CHU9D (note that two children had incomplete CHU9D data at baseline). The number of imputed data was set to 31 because the percentage of missing data was 69.49% (see Table 43). Imputation was at the individual CHU9D domains level and individual resource unit level (where the only missing data were NHS BSA dental cost data). The multiply imputed data for the NHS BSA data are presented in Appendix 4, Table 161. Tables 53 and 54 present the discounted QALYs and total costs for the multiply imputed data, respectively. Table 55 presents the cost-effectiveness estimates from the imputed data. Multiple imputation had a minimal impact, increasing QALYs and cost differences slightly, and this increased the probability of fluoridation being cost-effective at a WTPT of £20,000 (from 0.6831 to 0.8089).
| Analysis | Exposure group, mean (SE) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 229) | WF (n = 163) | ||
| Discounted QALY | 4.871 (0.022) [4.829 to 4.914] | 4.924 (0.025) [4.876 to 4.972] | 0.053 (–0.012 to 0.118) |
| Total cost | Exposure group, mean (£) (SE) [95% CI] | Difference (95% CI) | |
|---|---|---|---|
| No WF (n = 229) | WF (n = 163) | ||
| Baseline to follow-up assessment | 532.02 (23.58) [485.61 to 578.43] | 461.37 (27.74) [406.81 to 515.93] | –70.65 (–142.20 to 0.91) |
| Statistic | Multiply imputed data analysis |
|---|---|
| Cost (£) difference (95% CI) | –66.91 (–148.90 to 15.36) |
| QALY difference (95% CI) | 0.0151 (0.0256 to 0.0551) |
| Incremental cost/QALY gained (£) | –4431.13 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 369 (–452 to 1132) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.8089 |
Assigning costs of fluoridation to the population aged 0–12 years
Table 56 presents the cost-effectiveness estimates where fluoridation costs are attributed to the population aged 0–12 years, rather than to all ages. Allocating fluoridation costs to a smaller population increases costs. These costs are sufficient to exceed the negative costs identified in the primary analysis. However, the probability that WF is cost-effective at a WTPT of £20,000 is only marginally impacted (reducing from 0.6831 to 0.6215).
| Statistic | CCA |
|---|---|
| Cost (£) difference (95% CI) | 27.95 (–57.90 to 114.40) |
| QALY difference (95% CI) | 0.0094 (0.0404 to 0.0597) |
| Incremental cost/QALY gained (£) | 2793.40 |
| Net benefit (£) (95% CI): WTPT = £20,000 | 160 (–878 to 1134) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.6215 |
Volume of decayed, missing and filled teeth avoided and no decay
Sensitivity analyses with the count of dmft avoided and the presence of no decay as alternative outcomes were conducted. There were 1145 children with valid age and deprivation data; however, 31 children had no baseline data on dmft, giving a baseline data set of 1114 children.
The rates of missing data in total and by fluoridation group are provided in Table 57. Complete data are similar across the groups, providing some support towards the missing data mechanism being MCAR.
| Data | Exposure group | All | |
|---|---|---|---|
| WF | No WF | ||
| Complete data | |||
| Baseline, n | 534 | 580 | 1114 |
| Follow-up, n (%) | 343 (64.23) | 387 (66.72) | 730 (65.53) |
| Complete dmft data | |||
| Baseline, n | 534 | 580 | 1114 |
| Follow-up, n (%) | 534 (100.00) | 580 (100.00) | 1114 (100.00) |
| Complete decay data | |||
| Baseline, n | 534 | 580 | 1114 |
| Follow-up, n (%) | 534 (100.00) | 580 (100.00) | 1114 (100.00) |
| Complete cost data | |||
| Baseline, n | 534 | 580 | 1114 |
| Follow-up, n (%) | 343 (64.23) | 387 (66.72) | 730 (65.53) |
Table 58 provides the estimates from logistic regressions of missing data against baseline covariates, outcomes and a fluoridation group indicator. Baseline dmft is associated with missing data and this supports the missing data mechanism of MAR. Therefore, our preferred model specification for the clinical analyses in the older school cohort is MAR.
| Variable | Any missing data, OR (95% CI) |
|---|---|
| Fluoridation group | 1.10 (0.85 to 1.42) |
| Age | 1.06 (0.73 to 1.54) |
| Male | 0.88 (0.68 to 1.12) |
| IMD (base = IMD quintile 5) | |
| IMD quintile 1 | 0.81 (0.50 to 1.30) |
| IMD quintile 2 | 0.70 (0.47 to 1.04) |
| IMD quintile 3 | 0.84 (0.58 to 1.23) |
| IMD quintile 4 | 1.01 (0.72 to 1.42) |
| Baseline clinical measure | 1.10 (1.04 to 1.17) |
| n | 1114 |
Tables 59 and 60 presents the cost-effectiveness estimates where the clinical outcomes are the measures of effectiveness. Overall, the estimates show similar results to the primary (QALY) analysis, with either outcome indicating better oral health in the fluoridated group and lower costs, again, supporting a conclusion that WF is likely to be cost-effective.
| Statistic | Multiply imputed data analysis |
|---|---|
| Cost (£) difference (95% CI) | –43.51 (–78.74 to –7.73) |
| dmft avoided difference (95% CI) | 0.1039 (0.0274 to 0.1810) |
| Incremental cost/dmft avoided (£) | –418.77 |
| Net benefit (95% CI): WTPT = £20,000 | 2122 (584 to 3652) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.996a |
| Statistic | Multiply imputed data analysis |
|---|---|
| Cost (£) difference (95% CI) | –47.03 (–82.73 to –11.61) |
| No decay difference (95% CI) | 0.0381 (0.0004 to 0.0751) |
| Incremental cost/no decay (£) | –1234.38 |
| Net benefit (95% CI): WTPT = £20,000 | 808 (24 to 1542) |
| Probability of WF being cost-effective if WTPT = £20,000 | 0.9803a |
Health economic modelling
An economic model is required to inform the longer-term impacts of WF. An economic model will only be as good as the data that populate it. A review of the existing evidence base was conducted to assess the feasibility of conducting an economic model.
A scoping review of economic evaluations of WF has recently been conducted. 84 The review comprised a systematic database search of MEDLINE, EMBASE, The Cochrane Library, LILACS (Latin American and Caribbean Health Sciences Literature), Paediatric Economic Database Evaluation and the NHS Economic Evaluation Database. The review identified 24 studies across eight countries.
The main factors relevant to determining the value of existing studies to inform an economic model of WF in Cumbria included the following:
-
timing of the study
-
setting of the study
-
outcome measure being investigated in the study
-
perspective of the study
-
time horizon of the study.
Timing and settings of the studies
Improvements in oral health over recent decades is a substantial confounder when assessing the appropriateness of existing models, and these improvements will vary over settings too. The setting of the studies is important, given that oral health and the provision of dental care will vary across settings. Two119,120 of the 24 studies were UK studies. The UK studies119,120 identified relate to data pre 1990 and, as such, are more than 30 years old.
Outcome measure being investigated in studies
Our prime focus for the study is in cost–utility analysis, using the QALYs outcome obtained via utility measures sourced from the CHU9D. Studies identified in the scoping review comprised 15 cost–benefit studies,121–135 nine cost-effectiveness studies120,132,133,136–141 and four cost–utility studies. 119,142–144 Of the four economic evaluations identified as cost–utility analyses, two142,143 measured disability-adjusted life-years, one119 measured quality-adjusted tooth-years and one144 measured QALYs. The two UK studies related to cost-effectiveness analysis (i.e. DMFT and DMFT averted)120 and cost–utility analysis (i.e. quality-adjusted tooth years). 119
Perspectives taken in studies
Two UK studies119,120 took the payer perspective. This aligns with the within-study evaluation in this evaluation, which took a payer perspective, assessing NHS and WF costs.
Time horizon in studies
The time horizon should be sufficient to capture all impacts of the intervention. Given that decay and DMFT are absorbing states (one cannot revert back to no decay of DMFT), a lifetime horizon is appropriate. The time horizon differed over the two UK studies,119,120 that is, one took a lifetime approach and the other adopted a time horizon of 14 years.
Conclusion
These two UK studies, given their setting, perspective and time horizon, appear most appropriate to inform an economic model of WF in Cumbria. However, when the UK studies were carried out, oral health was somewhat different from how it was during the time frame of this study, and beyond. Furthermore, the studies rely on the same literature as the York and Cochrane reviews,16,71 which were criticised for being of poor quality and at high risk of bias. The studies also have a different health outcome. The single study assessing QALYs was based in New Zealand144 and this suggests that there is insufficient data in existing studies to inform the long term-effects of WF.
Mariño and Zaror84 concur with this, noting limitations of the existing evidence base with regard to the current context of declining dental caries incidence, greater tooth retention and an ageing population. In addition, adverse effects, such as fluorosis, were considered to be negligible and the study did not include costs associated with this, prompting the authors to recommend future models to consider these effects.
The long-term impacts of WF need to be examined. Although the within-study evaluation finds evidence that WF is likely to be cost-effective, the study does not inform potential impacts on the adult population, nor does it incorporate potential adverse effects, such as fluorosis. Evaluations of the impacts of WF on adults in Cumbria are needed, as too are assessments of fluorosis both in adults and in the child cohorts of the within-study evaluation.
Chapter 11 Discussion
Introduction
Water fluoridation has been cited as one of 10 great public health interventions by the Centers for Disease Control and Prevention145 and, therefore, it is surprising that there are so few studies that have examined its utility in contemporary Western populations with low caries prevalence. The results of this study are timely, following, as they do, the publication of both the evidence evaluation report carried out by the National Health and Medical Research Council in Australia in 2016146 and the Cochrane systematic review of WF,71 which found that, although there was evidence to suggest effectiveness, the underlying research base was old, may not be not relevant to current population disease levels and had methodological weaknesses.
The current work sought to address these issues by examining the introduction of a ‘new scheme’ of WF in England, which was created by the failure, and subsequent re-commissioning, of two fluoride dosing plants in Cumbria. The study design utilised two cohorts of children: (1) a birth cohort, in which the effect of systemic and tropical fluoride was assessed and (2) a cohort of older children, in which topical effects alone were examined. In addition to addressing the issue of dental caries levels (a 20% prevalence of dental caries, rather than the 40–50% levels seen in older research), the study also provides a robust health economic evaluation and sought to address bias in clinical examinations (using remote photographic scoring) and to measure and control for the likely effect modifiers in both groups of children.
Principal findings
The birth cohort
As expected, the prevalence of caries in the primary dentition was lower than in previous studies (i.e. 21% compared with levels almost double this in studies included in the recent Cochrane review), demonstrating and emphasising the need for the study. The difference in the primary outcome measure (i.e. the proportion of children who developed dental caries) was statistically significant, with an adjusted OR of 0.74 (95% CI 0.56 to 0.98). Despite this, the difference in the proportion of children with dental caries was modest, with 21.4% of children developing dental caries in the non-WF group and 17.4% of children developing dental caries in the WF group and an absolute difference of 4% might be judged by some stakeholders to be not sufficiently large or of clinical or public health significance. Nevertheless, the absolute difference is in line with the latest results from Public Health England’s Water Fluoridation Health Monitoring Report for England 2022,76 which demonstrated an absolute reduction of 4.7% in the prevalence of dental caries experience of 5-year-olds between a WF area (above 0.7 ppm) and a non-WF area.
When levels of deprivation were considered, the analysis involving an interaction term within the regression model looked to see whether or not the interpretation of the effect of WF depended on the value of deprivation (and vice versa), with the null hypothesis that the intervention effect is equal across subgroups. The performance of WF on dental caries experience was seen to be similar across deprivation quintiles. It is important to note that the overall effect size for WF on dental caries experience was smaller than expected. Therefore, it is not surprising that differences were not observed across the deprivation quintiles. There was no clear pattern when looking at deprivation, WF and dental caries experience, with no evidence of a shift in benefit for either those most or least deprived.
Secondary outcomes in this cohort demonstrated a significant decrease in the count of dental caries according to dmft, but no significant difference in the number of children requiring extractions of teeth under DGA or reporting issues relating to poor oral health, which included pain, problems with talking, eating or sleeping and being upset.
The use of blinded remote scoring of dental caries by an independent examiner suggested no underlying systematic bias in the clinical scores used for the main analysis.
Questionnaires to capture modifying factors were poorly completed, thereby reducing their value. Eleven different factors were assessed, including, diet, weaning history, toothbrushing and toothpaste use. Of these, only two differed between the control and intervention groups [i.e. consuming sweets on ≥ 3 days in each week (which was higher in the WF group) and having ever attended a dentist (which was lower in the WF group)]. Given the direction of the statistical difference seen in the primary outcome and the possibility of type I error, and given that the low response rate would have resulted in a reduced sample size, these two factors were discounted in the analysis.
The older school cohort
The low caries levels seen in the primary dentition of the birth cohort at 5 years old were also seen in the permanent dentition of the older school cohort when children were approximately 11 years old, with caries in the permanent teeth of 21.9% prevalence in control group and 19.1% in the intervention group. For the primary outcome measure (i.e. the difference in the proportion of children who were caries free in each group), there was insufficient evidence of an effect on the presence or absence of decay in the permanent dentition according to WF, with an adjusted OR of 0.8 (95% CI 0.58 to 1.09). However, it should be noted that dental caries is a chronic, long-term condition and develops throughout life and, hence, it is plausible that, as the disease progresses, the small difference (of 3%) recorded between the two groups may widen. Again, there was no observed impact on health inequalities.
When the difference in DMFT between groups was assessed (which included only teeth that had erupted after the intervention), there was evidence to suggest that WF was likely to have a beneficial effect on DMFT count, but there was uncertainty surrounding the estimate, which ranged from a substantial benefit to a less clinically important benefit. No difference was seen in the numbers of children requiring extractions under DGA.
When considering the health questionnaire data, across all the modifying factors measured, the only significant difference was seen in post-brushing behaviour, with children in the intervention group more likely to rinse their mouth after brushing than to spit residual toothpaste out.
Stability of intervention
Community WF is defined as the artificial supplementation of fluoride to drinking water supplies at the required concentration in England of 1 ppm. This dosing concentration has usually been assumed to be consistent and optimal during studies assessing effectiveness of WF. However, we know from the work of Moore et al. 89 that this is not always the case, as supplies are often interrupted and dosing may not be provided consistently at the optimal level.
During the current study, there was substantial interruption to the dosing of water supplies caused, in part, by a series of major flooding events that hit Cumbria at the end of 2015 and start of 2016, as well as the innate fragility of the plants themselves.
Figure 7 (see Chapter 7) displays the dosing from each of the two treatment plants during the duration of the study and shows both the impact of the 2016 floods and the general picture of suboptimal dosing activity, even from these newly commissioned plants. The Cornhow supply was more stable and more resilient to the impact of the flooding event than Ennerdale. It is important to note the profile of dosing seen in these plants is by no means unusual,89 as most plants in England fail to dose consistently or optimally.
The analyses undertaken for both the primary and secondary outcomes followed an ‘intention-to-treat’ model in that the actual dosing was not considered within the models. This is a legitimate treatment of the study data, given the public health nature of the intervention and the wide variation in water treatment plant performance seen across fluoridation schemes in the UK.
However, for completeness, a further analysis was undertaken, splitting the participants by their water treatment supply and, therefore, creating the three principal groups of Ennerdale (interrupted dosing), Cornhow (more stable dosing) and a non-fluoridated (control) supply.
The results of this analysis are shown in Table 19 for the birth cohort and in Appendix 3, Tables 147–149, for the older school cohort. There was insufficient evidence of a difference in caries experience in those children who received their drinking water from Ennerdale (interrupted dosing), whereas a significant difference was seen in those served by Cornhow (more stable dosing) for the birth cohort. No significant difference was observed for the older school cohort between either of the water treatment plants and the control. For the birth cohort, this could indicate that, if dosing remains stable at the optimal level, then the results from the CATFISH study could slightly underestimate the effectiveness of a perfectly delivered dosing system. However, this is a post hoc ancillary analysis, comparing optimal and non-optimal intervention delivery with a control, and so the results should be treated with caution. From our previous work,89 we know that optimal performance in fluoride delivery is rare and unlikely to become commonplace.
Health economic findings
The economic evaluation was undertaken from an NHS and local authority perspective. Costs included the capital and running costs of WF, as well as NHS dental activity. The measure of health benefit was the QALY. The cost and QALYs were combined to estimate the ICER (i.e. cost per QALY gained). For the within-study analyses, the time horizon was 5 years for the birth cohort (i.e. ages 0–5 years) and 6 years for the older school cohort (i.e. ages 5–11 years). QALYs gained from baseline to end of follow-up were estimated as the number of days survived multiplied by utility values for health-related quality of life reported on the CHU9D questionnaire. There were a large number of missing data due to questionnaire non-response (31.76% in the birth cohort and 33.89% in the older school cohort). The estimates of net costs and outcomes were bootstrapped (10,000 bootstraps) to generate cost-effectiveness acceptability curves of the probability that fluoridation was cost-effective at a range of threshold values for the willingness to pay for a QALY.
Sensitivity analyses included imputation of missing data, where costs of WF are apportioned to only children aged 0–12 years, and the use of alternative clinical outcome measures, that is volume of decayed, missing and filled teeth avoided and presence of no decay. The sensitivity analyses provided a consistent picture of a high probability of WF being cost-effective. Our approach to apportion WF costs has been conservative, as we assume a capital life of 6 years and this would overstate the costs for the WF group. Additional sensitivity analyses could explore allocating a longer lifetime for capital, although this would increase the probability of cost-effectiveness only by decreasing costs for children in the WF group.
Water fluoridation represented a small proportion of costs, at £14.14 per capita (£105.63 when apportioned to each child aged 0–12 years), in comparison with NHS dental services, which were over 10 times this amount for the birth cohort and over three times this amount for the older school cohort. Changing the study perspective to one of the NHS only would only further the dominance found for WF.
For the birth cohort, there is a 77.0% probability of WF being cost-effective at a willingness to pay £20,000 per QALY. For the older cohort there is a 68.3% probability of WF being cost-effective. The figure of £20,000 was chosen because this is the standard threshold used to determine whether or not interventions constitute good value for money for the NHS. All of the economic analyses give a high probability (p > 0.62) that WF is cost-effective based on willingness-to-pay thresholds of £20,000 per QALY/DMFT avoided/decay avoided.
Principal contributions to the literature
The CATFISH study has, to the best of our knowledge, provided the first contemporary evaluation of WF in England since the publication of the York and MRC reports. 16,18 The CATFISH study has sought to directly address the weaknesses in the evidence base that were highlighted by these reviews,16,18 as well as those highlighted in the later Cochrane systematic review. 71
The caries levels seen in the examined populations reflect the widespread and significant reductions in disease levels seen in England since the late 1970s, resulting primarily from the almost universal availability and use of fluoridated toothpastes. The prevalence statistics we report are similar to statistics reported for Cumbria by Public Health England from dental epidemiological surveys100 and, therefore, we have more confidence that there is no significant bias in our sample according to dental caries levels. Therefore, the study provides new insights into the effectiveness of a universal public health measure that is delivered to all residents of a water supply zone, irrespective of their dental caries risk. Approximately 80% of all children in the study were caries free irrespective of their fluoridation status.
The intervention was shown to have a small, but statistically significant, effect on primary teeth when children had the full effect of WF through both systemic and topical exposure (i.e. the birth cohort). Further research is required to see if this benefit from systemic and topical exposure will also be seen in the permanent dentition. The effects of the intervention were not shown to be different across the five deprivation quintiles. When considering topical exposure-only effects in the permanent dentition of the older school cohort, no differences were detected between the control and intervention groups. This finding may challenge the current view that fluoridation works mainly by topical exposure, but may also reflect the differing anatomy of permanent and primary teeth and their relative caries risk. Caries progression is thought to be faster in primary teeth than in permanent dentition, and this could be because deciduous enamel is softer and more prone to fracture. 147,148 There may also be differences in the way in which permanent teeth are treated compared with primary teeth; for example, pit and fissure sealants have been used in the prevention and control of dental caries on permanent teeth for decades; however, the evidence for the use of sealants in primary teeth is less established. 149 WF is more effective in preventing smooth surface caries, which is more prevalent in the primary dentition, than pit and fissure caries, which are the most common sites for caries in the permanent dentition. 150 Without further research the question of WF’s main mode of action, that is systemic versus topical, remains unanswered.
Although the authors of the York and MRC reports16,18 highlighted the need to capture and consider a wide range of effect modifiers, there was little evidence in the current work to suggest that effect modifiers had a significant influence on the effectiveness of the intervention on the primary outcome, with deprivation being the main factor influencing caries levels within both cohorts, and within exposed and non-exposed groups.
The work presents a detailed and robust health economic evaluation, demonstrating the relatively low cost of the intervention when considered on a population basis, but the underlying assumption of the equal benefit across a population has not been established. However, when considering only children within the economic model, the intervention has a high probability of being cost-effective, which is in line with the current literature. 84 The model does not include all the costs that might be associated with the introduction of a new scheme, for example the significant costs of feasibility studies, public consultations and possible legal challenges, such as those seen in the failed Southampton scheme.
The potential cost savings of any new scheme must also be considered within the context of the payer/benefit model in England. Costs are borne by the local authority and yet savings would be potentially realised within NHS budgets. However, current NHS dental contracts in England are set at target levels that incentivise activity and are not sensitive to changes in population needs or disease levels.
Strengths
The study design, based on whole-population evaluation combined with the ‘new scheme’ introduction, a 5-year longitudinal design, examiner bias assessment, quantification of effect modifiers and the inclusion of an a priori health economic evaluation, was a major strength of the study. The sample size requirements for both cohorts were met and maintained throughout the study duration, with low levels of loss to follow-up. Control and intervention groups were well balanced, with comparable socioeconomic characteristics. The characteristics of the recruited population were consistent with those required by Public Health England when assessing if a population would be suitable for the introduction of a new WF scheme.
An assessment of consenting and non-consenting participants suggested no consent bias in relation to subject’s deprivation scores. The low levels of dental caries seen in the cohorts suggested that the study population was broadly representative of the current dental caries prevalence in England, and this was replicated with respect to other key metrics, such as ethnicity, health outcomes, dental attendance and employment status of parents.
The study protocol was peer reviewed and published at the start of the study,85 and there were no deviations from the protocol. In addition, the work was overseen by both an independent Study Advisory Group and a Data Monitoring Committee, and had input from PPI.
Weaknesses
As with all large-scale clinical studies, there were a number of challenges. The collection of questionnaire data completed by parents/guardians was problematic, with low response rates over the duration of the study. Low response rates are not unusual in oral health surveys/questionnaires. 151 Substantial efforts were made by the study team to increase and maintain response rates, but a combination of study fatigue and the participants not always understanding the rationale for data collection was difficult to resolve. The questionnaire data did reveal two areas of difference over time between the control and intervention group for the birth cohort: (1) dental attendance was lower in the WF group and (2) sweets consumption was higher in the WF group. These two areas of difference could have affected the prevalence of dental caries and the small difference seen between the fluoridated and non-fluoridated areas, as this behaviour in the fluoridated group could have led to increased caries experience. However the questionnaires were poorly completed and, hence, questionnaire data were not robust enough to be included within the final analysis, as this would have resulted in an underpowered study. The response rate for the clinical examination was, however, good and was above the required sample size, with a response rate of 71% of those originally consented for the birth cohort and 72% of those originally consented for the older school cohort.
During the study, there were several attempts by anti-fluoridation groups to disrupt the study. For example, letters were written to schools asking them to withdraw support for the clinical examinations. Despite this, as stated, the sample size requirements were met. However, although the sample size was met, the postulated prevalence of disease on which the sample size was based was not obtained, with a much lower prevalence of ≈ 20% within the CATFISH study. Although this figure is in line with other current dental surveys in England (a slight decreasing prevalence has been observed over previous dental health surveys2,6), the lower prevalence has resulted in reduced power. This low prevalence is an important feature of the CATFISH study, as the studies aim was to assess the effectiveness of WF in a contemporary population with lower caries levels. The fact these levels were even lower than expected, given the continued reduction of children experiencing caries, needs to be taken into account. When thinking about the future of this public health intervention, the prevalence levels of decay need to be considered against the modest benefit that was observed for the primary outcome.
The examinations of children aged 3 years was problematic, as nurseries were reluctant to engage with the study and parents reported that they did not want their children examined in these settings. Various attempts at examining children in dental clinics, including weekend appointments, failed to address this issue and, hence, these data are not reported. The lack of 3-year-old examination data in the study did not affect our consent to examine children in primary schools and, therefore, the main outcome examinations were conducted successfully, despite the advent of COVID-19 (when 172 children were not examined in the birth cohort and 176 children were not seen in the older school cohort because of school closures).
The primary analysis in the health economics analyses is the CCA; however, this is rarely sufficient in trials. 115 There are certain features of this study that help explain why MCAR is an appropriate missing data mechanism. First, there was only one follow-up time point in the data for the collection of both child health utility and cost data. Second, the absence of cost data is attributable to a third party (i.e. the NHS) and not to a lack of reporting by children or patients. However, the rates of missing NHS data were similar across fluoridation groups and did not appear to be associated with baseline covariates or outcomes. Third, missing child health utility in the birth cohort was mitigated because of the assumption of equal health at baseline. For the older school cohort, missing child health utility in the birth cohort was mitigated by providing the questionnaire at the time of the follow-up examinations. Sensitivity analyses where the missing data mechanism was assumed to be MAR helps support the MCAR approach, with minimal impact on the cost-effectiveness inference for both the birth cohort and the older school cohort.
The use of generic health-related quality-of-life measures for a dental intervention may not be the most appropriate outcome measure when considering cost-effectiveness because of the potential for these to be insensitive to changes in oral health. In the last two decades, the appropriateness of relating health-related quality of life to oral health has been largely endorsed, with the development and validation of site-specific oral health-related quality-of-life measures for adults and children, such as the Oral Health Impact Profile152 and the Child Perceptions Questionnaire153 for children. However, in children attending a dental examination in New Zealand, the CHU9D showed potential as an outcome measure when compared with the Child Perceptions Questionnaire. In addition, the chosen approach was taken to meet NICE conventions and to assist in comparisons with other public health interventions not limited to dental health. Further research could broaden the approaches taken here to include more relevant metrics, such as cost per tooth saved or cost of keeping a child caries free.
In the literature, there are number of limitations of the use of a generic measure of health-related quality of life for children. 154,155 First, it is argued that health state valuation in children is not ideal, as children are unlikely to be rational and informed and are not able to understand the tasks completely. Second, one might argue that society does not consider children as legal agents because they are not able to vote until they reach the age of 18 years and, therefore, they are not viewed as decision-makers in society. Furthermore, the study has a number of limitations with regard to the points at which health-related quality of life was measured, including (1) the necessity for parents or guardians to complete the CHU9D at follow-up for the birth cohort and at baseline for the older school cohort and (2) the subjective decision of assigning perfect health at baseline for the birth cohort.
The long-term effects of WF need to be examined. Although the within-study evaluation finds evidence that WF is likely to be cost-effective, the study does not inform potential effects on the adult population, nor does it incorporate potential adverse effects, such as fluorosis. Evaluations of the effects of WF on adults and the lifetime effect of WF is needed, as most people are at risk of developing dental decay during their life. WF has the potential to benefit adults, as they retain their teeth into older age, when the risk of dental disease increased.
The nature of WF provision in England is one of individual schemes commissioned by local authorities and results in geographically unconnected coverage. This means that the ‘full’ potential effect of WF, for example where fluoridated water is used in the commercial production of food and beverages, has not been assessed. Such an assessment would be possible only with national implementation of WF.
The current work considers only the benefits of WF and has not considered the broadly accepted risk of WF (e.g. fluorosis). This is because the birth cohort examinations concluded at 5 years of age, but fluorosis examinations are typically conduction around 12 years of age, when the anterior permanent teeth have erupted. Given the high levels of caries-free individuals in the population, it is essential that an assessment of fluorosis is undertaken to enable the very modest benefits seen in this study to be placed into context, again, providing policy-makers with all the information required to inform decision-making.
Conclusions
Considering both the strengths and weaknesses described above, the following conclusions can be drawn from the current work:
-
WF has a small beneficial impact on preventing caries in the primary dentition, but this is much smaller than the effect size reported by the York and Cochrane reviews. 16,71 This reduction in effectiveness is likely to be due to the low caries prevalence seen following the widespread use of fluoride toothpaste.
-
The benefit did not translate to a significant reduction in extractions undertaken in hospital or self-reported dental health outcomes for either cohort.
-
There was insufficient evidence of a benefit in the older school cohort for the permanent dentition when considering the proportion of children developing caries. Although there was a statistically significant difference in DMFT (secondary outcome), the magnitude of difference was small.
-
There was insufficient evidence to conclude the effects of the intervention across the deprivation quintile subgroups.
-
The intervention is likely to be cost-effective for both cohorts under the NICE willingness-to-pay threshold of £20,000 (with probabilities > 62% for each cohort).
Implications
For research
Research recommendation 1
The CATFISH study data should be included in any updating of the Cochrane systematic review71 to provide an evidence refresh for UK policy-makers. Given the requirements for a new scheme (or re-introduction of a halted scheme, such as in Cumbria) and the expense of long-term clinical studies, it is unlikely that further studies, such as the CATFISH study, will be possible soon and, hence, it will be important to ensure that these data are widely available and disseminated. Consideration should be given to future systematic reviews utilising either a temporal threshold related to the widespread use of fluoridated toothpaste or a caries prevalence level that more closely reflects the levels seen in contemporary populations.
Research recommendation 2
Follow-up of the birth cohort until at least 15 years old to determine the impacts on caries in the permanent dentition, combined with an assessment of fluorosis, will provide essential additional evidence on the effectiveness of the intervention. The work will need to continue to measure the impact of WF on socioeconomic inequalities on dental health. This information combined with further information on diet and other fluoride sources would be valuable.
In addition, and to support the health economic modelling (see Research recommendation 3), it will be important to understand the benefit, if any, that WF brings to adult populations, and to consider the complexities of lifetime residency in this assessment.
Research recommendation 3
Further research is required to complete health economic modelling beyond the ages observed in the cohorts. Future research should develop a decision-analytic model to determine the cost-effectiveness of fluoridation over a longer time horizon. This research should incorporate data from the current NIHR-funded programme, LOTUS (FLuOridaTion for AdUltS study),156 which is evaluating the impact of fluoridation in adults, as well as expert knowledge elicitation methods in areas where there is data paucity.
Research recommendation 4
Assessment of behaviours and parahealth-related outcomes is complex. In the current work, these elements were captured by questionnaires. Like many studies, response rates were low and, although a number of evidence-based interventions were deployed to increase returns, the rate remained lower than we would have hoped for. The evidence-based interventions included trialling different types of reminders (e.g. telephone calls, postcards or entire questionnaires being resent)97 and resending questionnaires was the most effective way to increase responses; however, calling individuals appeared to address the imbalance in deprivation in response rates. Following PPI feedback, questionnaires were reduced from every 6 months to annually, and a £10 voucher was sent with the final questionnaire as a thank you for completion. PPI was also useful in refining questions within questionnaires and maximising recruitment. The final questionnaire for the older school cohort was completed by children during the clinical examination and this resulted in a higher questionnaire response rate for this group, compared with previous years and the birth cohort (for whom questionnaires were sent home for parents to complete). Even with a high response rate, issues remain, such as recall bias and social desirability bias. Consideration of the use of proxy measures, routinely collected data or new technologies may help in future studies.
Research recommendation 5
Future research on WF should continue to reduce bias where possible, and this can be achieved by blinding clinical assessors with the use of remote scoring of images taken during clinical examinations and by recruiting the maximum number of participants to minimise selection bias and attrition.
For policy
Over the last 30 years, population caries levels have diminished very significantly so that even in this deprived population only 20% of children were at risk of developing caries into dentine. Although this figure is still high when we consider disease burden and demonstrates that further intervention is necessary to continue to reduce the number of children experiencing dental decay, it becomes much harder to make the case for a whole population intervention given these declining prevalence levels, especially when its benefits are small at best and could be outweighed by risks of fluorosis. The importance of continuing to follow up the birth cohort to determine the impact of both fluorosis and caries in the permanent teeth has been highlighted in this report. Prevalence levels are an important factor to take into account when considering new WF schemes and the applicability of the results of this study to areas with widely different caries levels. We suggest that, where prevalence is low, consideration should be given to targeted approaches that may be as or more effective, as this may attract less criticism from those who believe that WF is an inappropriate intervention. Such targeted approaches may include modification of known risk factors, such as sugar consumption, rather than relying on the biopharmaceutical effect of fluoride alone.
The ‘silver bullet’ approach that was effective in the 1950s is failing to address dental caries in the most deprived populations and, hence, a hybrid approach with behavioural elements should be considered, alongside considering the social determinants model described the Marmot review,49 to address what is a complex and multifactorial disease. Although the attractiveness of what appears to be a simple intervention to universally prevent disease is clear, it is also apparent that the changing epidemiological nature of dental decay requires further consideration in the future.
The inability of the public health authorities to introduce a new scheme in the UK in the last 30 years demonstrates the logistical and political problems of delivering this intervention. The history of WF over the last 30 years has resulted in resources being ploughed into trying to implement new WF schemes where resources may have produced greater benefit if they had been deployed in other areas. Into the future, assuming the continued use and availability of fluoride toothpaste, policy-makers will have to trade-off the costs of the introduction of new schemes against other public health interventions.
Acknowledgements
Oversight/Study Steering Committee members
-
Ivor Chestnutt (Cardiff University) (chairperson).
-
Deborah White (University of Birmingham).
-
Girvan Burnside (University of Liverpool).
-
Ji Hee Youn (University of Manchester).
Previous members
-
Paul Wilson (University of Manchester, formally University of York) left after moving to Manchester.
-
Brenda Gannon (University of Manchester) moved to Queensland, Australia, and could no longer be part of the committee.
Data Monitoring Committee
-
Gail Douglas (University of Leeds) (chairperson).
-
Rebecca Playle (Cardiff University).
-
Andrea Sherriff (University of Glasgow).
CATFISH research team and co-applicants
-
Iain A Pretty (University of Manchester) (principal investigator).
-
Tanya Walsh (University of Manchester).
-
Michaela Goodwin (University of Manchester).
-
Matt Sutton (University of Manchester).
-
William Whittaker (University of Manchester).
-
Mike Kelly (NICE, University of Cambridge).
-
Richard Emsley (King’s College London).
-
Martin Tickle (University of Manchester).
-
Colin Cox (Director Public Health – Cumbria).
-
Rebecca Wagstaff (Director Public Health – Cumbria).
-
Eric Rooney (Consultant in Dental Public Health – Cumbria).
Members of the dental team (remote photo scoring)
-
Steven Jones.
-
Uriana Boye.
-
Yvonne Halliwell.
-
Abigail Driver.
-
Kaveri Gupta.
-
Margaret Formby.
-
Ewa Stepnowska-Dabrowska.
-
Philippa Johnson.
-
Mike McGrady.
-
Cath Griffiths.
-
Hayley Crawford.
-
Kelly McMullen.
-
Amanda Newsome.
-
Laura Wilson.
-
Judith Rudd.
-
Diane Whitehead.
Further acknowledgements
The team would like to thank Gill Davies and Janet Neville (Public Health England) for their support with calibration and how to use the Dental Survey Plus 2 software.
The team would like to thank Sarah Procter, supported by Rose Carter, for her work as our reference standard during calibration.
The team would like to thank Dr Saima Bashir (University of Manchester).
The team would like to thank all at the North Cumbria University Hospitals NHS Trust’s research and development department who completed the birth cohort recruitment for the study.
Contributions of authors
Michaela Goodwin (https://orcid.org/0000-0002-0375-3118) (Lecturer in Dental Public Health) was involved in project management, conduct and analysis, and in the reporting phases.
Richard Emsley (https://orcid.org/0000-0002-1218-675X) (Professor of Medical Statistics and Trials Methodology) was involved in the design, conduct and analysis, and in the reporting phases.
Michael P Kelly (https://orcid.org/0000-0002-2029-5841) (Professor within Department of Public Health and Primary Care) was involved in the design, conduct and analysis, and in the reporting phases.
Matt Sutton (https://orcid.org/0000-0002-6635-2127) (Professor of Health Economics) was involved in the design, conduct and analysis, and in the reporting phases.
Martin Tickle (https://orcid.org/0000-0001-5348-5441) (Professor of Dental Public Health and Primary Care) was involved in the design, conduct and analysis, and in the reporting phases.
Tanya Walsh (Professor of Healthcare Evaluation) was involved in the design, conduct and analysis, and in the reporting phases.
William Whittaker (https://orcid.org/0000-0003-2530-0360) (Senior Lecturer in Health Economics) was involved in the design, conduct and analysis, and in the reporting phases.
Iain A Pretty (https://orcid.org/0000-0001-6298-4189) (Professor Public Health Dentistry) was the principal investigator and was involved in the design, conduct and analysis, and in the reporting phases.
Publications
Goodwin M, Emsley R, Kelly M, Rooney E, Sutton M, Tickle M, et al. The CATFISH study protocol: an evaluation of a water fluoridation scheme. BMC Oral Health 2016;16:8. https://doi.org/10.1186/s12903-016-0169-0
Goodwin M, Walsh T, Whittaker W, Emsley R, Sutton M, Tickle M, et al. Increasing questionnaire response: evidence from a nested RCT within a longitudinal birth cohort study. BMC Med Res Methodol 2020;20:163. https://doi.org/10.1186/s12874-020-01034-7
Goodwin M, Whittaker W, Walsh T, Emsley R, Sutton M, Tickle M, et al. Recruitment and consent in an observational study. Community Dent Health 2020;37:287–292. https://doi.org/10.1922/CDH_000682020Goodwin06
Data-sharing statement
The CATFISH study investigators are committed to furthering research by sharing, where possible, de-identified individual participant data. We are unable to share data collected by the NHS, as this was not within our data-sharing agreement. Participants were not expressly asked about sharing their data for further study and, therefore, this could only occur according to appropriate ethics approval, consent, previous obligations and publication timelines. For any data requests or queries please contact the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care.
References
- Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet 2019;394:249-60. https://doi.org/10.1016/S0140-6736(19)31146-8.
- Davies, Neville J, Rooney E. National Dental Epidemiology Programme for England: Oral Health Survey of Five-Year-Old Children – A Report on the Prevalence and Severity of Dental Decay 2013. http://qna.files.parliament.uk/qna-attachments/391268/original/HL%201655Oral%20Health%205yr%20old%20children%202012.pdf (accessed 29 August 2022).
- Murray JJ, Vernazza CR, Holmes RD. Forty years of national surveys: an overview of children’s dental health from 1973–2013. Br Dent J 2015;219:281-5. https://doi.org/10.1038/sj.bdj.2015.723.
- Davies GM, Jones CM, Monaghan N, Morgan MZ, Pine CM, Pitts NB, et al. The caries experience of 5 year-old children in Scotland, Wales and England in 2007–2008 and the impact of consent arrangements. Reports of co-ordinated surveys using BASCD criteria. Community Dent Health 2011;28:5-11.
- Harris R, Nicoll AD, Adair PM, Pine CM. Risk factors for dental caries in young children: a systematic review of the literature. Community Dent Health 2004;21:71-85.
- Public Health England . National Dental Epidemiology Programme for England: Oral Health Survey of 5-Year-Olds 2019 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/873492/NDEP_for_England_OH_Survey_5yr_2019_v1.0.pdf (accessed 20 July 2021).
- Tickle M, Blinkhorn AS, Milsom KM. The occurrence of dental pain and extractions over a 3-year period in a cohort of children aged 3–6 years. J Public Health Dent 2008;68:63-9. https://doi.org/10.1111/j.1752-7325.2007.00048.x.
- Public Health England . Hospital Tooth Extractions of 0 to 19 Year Olds 2020. www.gov.uk/government/publications/hospital-tooth-extractions-of-0-to-19-year-olds (accessed 10 August 2021).
- Goodwin M, Sanders C, Davies G, Walsh T, Pretty IA. Issues arising following a referral and subsequent wait for extraction under general anaesthetic: impact on children. BMC Oral Health 2015;15. https://doi.org/10.1186/1472-6831-15-3.
- Bridgman CM, Ashby D, Holloway PJ. An investigation of the effects on children of tooth extraction under general anaesthesia in general dental practice. Br Dent J 1999;186:245-7. https://doi.org/10.1038/sj.bdj.4800076.
- Tickle M, Jones C, Buchannan K, Milsom KM, Blinkhorn AS, Humphris GM. A prospective study of dental anxiety in a cohort of children followed from 5 to 9 years of age. Int J Paediatr Dent 2009;19:225-32. https://doi.org/10.1111/j.1365-263X.2009.00976.x.
- NHS . Adult Dental Health Survey, 2009 2009. http://discover.ukdataservice.ac.uk/Catalogue/?sn=6884=&typeData (accessed 20 November 2014).
- National Audit Office (NAO) . Dentistry in England 2020. www.nao.org.uk/wp-content/uploads/2020/03/Dentistry-in-England.pdf (accessed 24 August 2022).
- Milsom KM, Threlfall AG, Blinkhorn AS, Kearney-Mitchell PI, Buchanan KM, Tickle M. The effectiveness of school dental screening: dental attendance and treatment of those screened positive. Br Dent J 2006;200:687-90. https://doi.org/10.1038/sj.bdj.4813724.
- Steele J, White D, Rolland S, Fuller E. Children’s Dental Health Survey 2013. Report 4: The Burden of Dental Disease in Children 2015. https://files.digital.nhs.uk/publicationimport/pub17xxx/pub17137/cdhs2013-report4-burden-of-dental-disease.pdf (accessed 10 August 2012).
- McDonagh M, Whiting P, Sutton AJ, Wilson P, Chestnutt I. A Systematic Review of Public Water Fluoridation 2000. www.york.ac.uk/media/crd/crdreport18.pdf (accessed 10 August 2019).
- Marinho VC, Higgins JP, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;1. https://doi.org/10.1002/14651858.CD002278.
- Medical Research Council (MRC) Working Group . Water Fluoridation and Health 2002. www.mrc.ac.uk/pdf%20publications%20water_fluoridation_report.pdf (accessed 15 February 2015).
- Pizzo G, Piscopo MR, Pizzo I, Giuliana G. Community water fluoridation and caries prevention: a critical review. Clin Oral Investig 2007;11:189-93. https://doi.org/10.1007/s00784-007-0111-6.
- Whitty C. Chief Medical Officer’s Annual Report 2021: Health in Coastal Communities 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1005216/cmo-annual_report-2021-health-in-coastal-communities-accessible.pdf (accessed 27 February 2022).
- GOV.UK . English Indices of Deprivation 2019 2019. www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed 22 July 2022).
- Murray J, Rugg-Gunn AJ. Fluorides in Caries Prevention. London: Butterworth-Heinemann Ltd; 1982.
- Dean HT, Arnold FA, Jay P, Knutson JW. Studies on mass control of dental caries through fluoridation of the public water supply. Public Health Rep 1950;65:1403-8. https://doi.org/10.2307/4587515.
- Groeneveld A. Longitudinal study of prevalence of enamel lesions in a fluoridated and non-fluoridated area. Community Dent Oral Epidemiol 1985;13:159-63. https://doi.org/10.1111/j.1600-0528.1985.tb00434.x.
- Mullen J. European Association for Paediatric Dentistry. History of water fluoridation. Br Dent J 2005;199:1-4. https://doi.org/10.1038/sj.bdj.4812863.
- Department of Health and Social Security, Scottish Office, Welsh Office, Ministry of Housing and Local Government . The Conduct of the Fluoridation Studies and the Results Achieved After Five Years 1962.
- Department of Health and Social Security, Scottish Office, Welsh Office, Ministry of Housing and Local Government . The Fluoridation Studies in the United Kingdom and the Results Achieved After Eleven Years 1969.
- The British Fluoride Society . The Extent of Water Fluoridation 2012. https://7e13609e-2c80-44ea-a31e-5ae714793ae5.filesusr.com/ugd/014a47_0776b576cf1c49308666cef7caae934e.pdf (accessed 21 November 2019).
- Great Britain . Water (Fluoridation) Act 1985 1985.
- Great Britain . Water Industry Act 1991 1991.
- Great Britain . Water Act 2003 2003.
- Public Health England . Southampton Water Fluoridation Scheme 2014. www.gov.uk/government/news/southampton-water-fluoridation-scheme (accessed 10 August 2021).
- BBC News . Southampton Water Fluoridation Plans Scrapped 2014. www.bbc.co.uk/news/uk-england-hampshire-29803864 (accessed 7 March 2017).
- Great Britain . Health and Social Care Act 2012 2012.
- Fejerskov O, Nyvad B, Kidd E. Dental Caries: The Disease and its Clinical Management. Hoboken, NJ: Wiley-Blackwell; 2015.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369:51-9. https://doi.org/10.1016/S0140-6736(07)60031-2.
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. https://doi.org/10.1016/S0140-6736(18)32279-7.
- NHS Digital . Child Dental Health Survey 2013, England, Wales and Northern Ireland 2015. https://digital.nhs.uk/data-and-information/publications/statistical/children-s-dental-health-survey/child-dental-health-survey-2013-england-wales-and-northern-ireland (accessed 13 December 2020).
- Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol 2003;31:3-23. https://doi.org/10.1046/j.2003.com122.x.
- Jordan RA, Krois J, Schiffner U, Micheelis W, Schwendicke F. Trends in caries experience in the permanent dentition in Germany 1997–2014, and projection to 2030: morbidity shifts in an aging society. Sci Rep 2019;9.
- Lagerweij MD, van Loveren C. Declining caries trends: are we satisfied?. Curr Oral Health Rep 2015;2:212-17. https://doi.org/10.1007/s40496-015-0064-9.
- Paganelli APD, Constante HM, Sala FS, Bainha CC, Borges ÁLS, Bastos JL, et al. Trends in dental caries rates over 45 years (1971-2016) among schoolchildren in Florianópolis, southern Brazil. Int Dent J 2018;68:47-53. https://doi.org/10.1111/idj.12327.
- Sheiham A. Changing trends in dental caries. Int J Epidemiol 1984;13:142-7.
- Davies GM, Worthington HV, Ellwood RP, Bentley EM, Blinkhorn AS, Taylor GO, et al. A randomised controlled trial of the effectiveness of providing free fluoride toothpaste from the age of 12 months on reducing caries in 5–6 year old children. Community Dent Health 2002;19:131-6.
- Edelstein B. The dental caries pandemic and disparities problem. BMC Oral Health 2006;6.
- Cleaton-Jones P, Fatti P, Bönecker M. Dental caries trends in 5- to 6-year-old and 11- to 13-year-old children in three UNICEF designated regions – Sub Saharan Africa, Middle East and North Africa, Latin America and Caribbean: 1970–2004. Int Dent J 2006;56:294-300. https://doi.org/10.1111/j.1875-595x.2006.tb00104.x.
- NHS Dental Epidemiology Programme for England, North West Public Health Observatory . NHS Dental Epidemiology Programme for England Oral Health Survey of 12 Year Old Children 2007 2008 2010.
- Dugmore CR. The 80–20 phenomenon (80:20 distribution of caries) – myth or fact. Br Dent J 2006;201:197-8. https://doi.org/10.1038/sj.bdj.4813909.
- Marmot M. Fair Society, Healthy Lives: The Marmot Review 2010. www.instituteofhealthequity.org/resources-reports/fair-society-healthy-lives-the-marmot-review/fair-society-healthy-lives-full-report-pdf.pdf (accessed 4 July 2022).
- Health and Social Care Information Centre . Children’s Dental Health Survey 2013 2015.
- O’Malley L, Worthington HV, Donaldson M, O’Neil C, Birch S, Noble S, et al. Oral health behaviours of parents and young children in a practice-based caries prevention trial in Northern Ireland. Community Dent Oral Epidemiol 2018;46:251-7. https://doi.org/10.1111/cdoe.12357.
- Tickle M, O’Neill C, Donaldson M, Birch S, Noble S, Killough S, et al. A randomised controlled trial to measure the effects and costs of a dental caries prevention regime for young children attending primary care dental services: the Northern Ireland Caries Prevention In Practice (NIC-PIP) trial. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20710.
- Seelan Rg, Kumar A, Maheswari Su, Raja J. Caries management by risk assessment: a review on current strategies for caries prevention and management. J Pharm Bioallied Sci 2015;7. https://doi.org/10.4103/0975-7406.163436.
- Jurasic MM, Gibson G, Orner MB, Wehler CJ, Jones JA. Validation of a subjective caries risk assessment tool. J Dent 2021;113. https://doi.org/10.1016/j.jdent.2021.103748.
- Hall-Scullin E, Whitehead H, Milsom K, Tickle M, Su TL, Walsh T. Longitudinal study of caries development from childhood to adolescence. J Dent Res 2017;96:762-7. https://doi.org/10.1177/0022034517696457.
- Steele JG, Treasure ET, O’Sullivan I, Morris J, Murray JJ. Adult Dental Health Survey 2009: transformations in British oral health 1968–2009. Br Dent J 2012;213:523-7. https://doi.org/10.1038/sj.bdj.2012.1067.
- Moore D, Davies D. What is Known About the Oral Health of Older People in England and Wales: A Review of Oral Health Surveys of Older People. London: Public Health England; 2015.
- FDI World Dental Federation . The Challenge of Oral Disease – A Call for Global Action 2015.
- Milsom KM, Tickle M, Humphris GM, Blinkhorn AS. The relationship between anxiety and dental treatment experience in 5-year-old children. Br Dent J 2003;194:503-6. https://doi.org/10.1038/sj.bdj.4810070.
- Astramskaitė I, Poškevičius L, Juodžbalys G. Factors determining tooth extraction anxiety and fear in adult dental patients: a systematic review. Int J Oral Maxillofac Surg 2016;45:1630-43. https://doi.org/10.1016/j.ijom.2016.06.019.
- Moles DR, Ashley P. Hospital admissions for dental care in children: England 1997–2006. Br Dent J 2009;206. https://doi.org/10.1038/sj.bdj.2009.254.
- Ubelaker DH. Human Skeletal Remains: Excavation, Analysis, Interpretation. Chicago, IL: Aldine Publishing Co. Inc.; 1978.
- Hellwig E, Lennon AM. Systemic versus topical fluoride. Caries Res 2004;38:258-62. https://doi.org/10.1159/000077764.
- Künzel W. Influence of water fluoridation on the eruption of permanent teeth. Caries Res 1976;10:96-103. https://doi.org/10.1159/000260193.
- Jolaoso IA, Kumar J, Moss ME. Does fluoride in drinking water delay tooth eruption?. J Public Health Dent 2014;74:241-7. https://doi.org/10.1111/jphd.12053.
- Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc 2000;131:887-99. https://doi.org/10.14219/jada.archive.2000.0307.
- Ten Cate JM, Featherstone JD. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med 1991;2:283-96. https://doi.org/10.1177/10454411910020030101.
- Sheiham A, James WP. A reappraisal of the quantitative relationship between sugar intake and dental caries: the need for new criteria for developing goals for sugar intake. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-863.
- Fejerskov O, Manji F, Baelum V. The nature and mechanisms of dental fluorosis in man. J Dent Res 1990;69:692-700. https://doi.org/10.1177/00220345900690S135.
- Fejerskov O, Larsen MJ, Richards A, Baelum V. Dental tissue effects of fluoride. Adv Dent Res 1994;8:15-31. https://doi.org/10.1177/08959374940080010601.
- Iheozor-Ejiofor Z, Worthington HV, Walsh T, O’Malley L, Clarkson JE, Macey R, et al. Water fluoridation for the prevention of dental caries. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD010856.pub2.
- Everett ET. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 2011;90:552-60. https://doi.org/10.1177/0022034510384626.
- Mohammadi AA, Yousefi M, Yaseri M, Jalilzadeh M, Mahvi AH. Skeletal fluorosis in relation to drinking water in rural areas of West Azerbaijan, Iran. Sci Rep 2017;7. https://doi.org/10.1038/s41598-017-17328-8.
- Pereira AC, Da Cunha FL, Meneghim Mde C, Werner CW. Dental caries and fluorosis prevalence study in a nonfluoridated Brazilian community: trend analysis and toothpaste association. ASDC J Dent Child 2000;67.
- Mascarenhas AK, Burt BA. Fluorosis risk from early exposure to fluoride toothpaste. Community Dent Oral Epidemiol 1998;26:241-8. https://doi.org/10.1111/j.1600-0528.1998.tb01957.x.
- Public Health England . Water Fluoridation Health Monitoring Report for England 2022 2022.
- Public Health England . Water Fluoridation Health Monitoring Report for England 2018 2018.
- Green R, Lanphear B, Hornung R, Flora D, Martinez-Mier EA, Neufeld R, et al. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr 2019;173:940-8. https://doi.org/10.1001/jamapediatrics.2019.1729.
- Neurath C, Limeback H, Osmunson B, Connett M, Kanter V, Wells CR. Dental fluorosis trends in US oral health surveys: 1986 to 2012. JDR Clin Trans Res 2019;4:298-30. https://doi.org/10.1177/2380084419830957.
- McGrady MG, Ellwood RP, Pretty IA. Water fluoridation as a public health measure. Dent Update 2010;37. https://doi.org/10.12968/denu.2010.37.10.658.
- McGrady MG, Ellwood RP, Pretty IA. The water fluoridation debate. Dent Update 2011;38:12-2. https://doi.org/10.12968/denu.2011.38.1.12.
- Rugg-Gunn AJ, Spencer AJ, Whelton HP, Jones C, Beal JF, Castle P, et al. Critique of the review of ‘Water fluoridation for the prevention of dental caries’ published by the Cochrane Collaboration in 2015. Br Dent J 2016;220:335-40. https://doi.org/10.1038/sj.bdj.2016.257.
- McDonagh MS, Whiting PF, Wilson PM, Sutton AJ, Chestnutt I, Cooper J, et al. Systematic review of water fluoridation. BMJ 2000;321:855-9. https://doi.org/10.1136/bmj.321.7265.855.
- Mariño R, Zaror C. Economic evaluations in water-fluoridation: a scoping review. BMC Oral Health 2020;20. https://doi.org/10.1186/s12903-020-01100-y.
- Goodwin M, Emsley R, Kelly M, Rooney E, Sutton M, Tickle M, et al. The CATFISH study protocol: an evaluation of a water fluoridation scheme. BMC Oral Health 2016;16. https://doi.org/10.1186/s12903-016-0169-0.
- Cumbria Intelligence Observatory . Population 2020. www.cumbriaobservatory.org.uk/ (accessed 10 August 2021).
- Cumbria Children’s Services . A Summary List of Schools and Other Educational Establishments 2021.
- Hospital Episode Statistics Analysis, Health and Social Care Information Centre . NHS Maternity Statistics – England, 2013–14 2015.
- Moore D, Goodwin M, Pretty IA. Long-term variability in artificially and naturally fluoridated water supplies in England. Community Dent Oral Epidemiol 2020;48:49-55. https://doi.org/10.1111/cdoe.12502.
- Milsom K, Mitropoulos CM. Enamel defects in 8-year-old children in fluoridated and non-fluoridated parts of Cheshire. Caries Res 1990;24:286-9. https://doi.org/10.1159/000261284.
- Stephen KW, Macpherson LM, Gilmour WH, Stuart RA, Merrett MC. A blind caries and fluorosis prevalence study of school-children in naturally fluoridated and nonfluoridated townships of Morayshire, Scotland. Community Dent Oral Epidemiol 2002;30:70-9. https://doi.org/10.1034/j.1600-0528.2002.300110.x.
- McGrady MG, Ellwood RP, Maguire A, Goodwin M, Boothman N, Pretty IA. The association between social deprivation and the prevalence and severity of dental caries and fluorosis in populations with and without water fluoridation. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-1122.
- Boye U, Walsh T, Pretty IA, Tickle M. Comparison of photographic and visual assessment of occlusal caries with histology as the reference standard. BMC Oral Health 2012;12. https://doi.org/10.1186/1472-6831-12-10.
- McGrady MG, Ellwood RP, Srisilapanan P, Korwanich N, Taylor A, Goodwin M, et al. Dental fluorosis in populations from Chiang Mai, Thailand with different fluoride exposures – paper 2: the ability of fluorescence imaging to detect differences in fluorosis prevalence and severity for different fluoride intakes from water. BMC Oral Health 2012;12. https://doi.org/10.1186/1472-6831-12-33.
- Pitts NB, Evans DJ, Pine CM. British Association for the Study of Community Dentistry (BASCD) diagnostic criteria for caries prevalence surveys – 1996/97. Community Dent Health 1997;14:6-9.
- National Dental Public Health Team . National Dental Epidemiology Programme: Training and Calibration Guide for Oral Health Surveys of Children 2021.
- Goodwin M, Walsh T, Whittaker W, Emsley R, Sutton M, Tickle M, et al. Increasing questionnaire response: evidence from a nested RCT within a longitudinal birth cohort study. BMC Med Res Methodol 2020;20. https://doi.org/10.1186/s12874-020-01034-7.
- Anyaegbu G. Using the OECD equivalence scale in taxes and benefits analysis. Econ Labour Mark Rev 2010;4:49-54. https://doi.org/10.1057/elmr.2010.9.
- Goodwin M, Patel DK, Vyas A, Khan AJ, McGrady MG, Boothman N, et al. Sugar before bed: a simple dietary risk factor for caries experience. Community Dent Health 2017;34:8-13. https://doi.org/10.1922/CDH_3926Goodwin06.
- Dental Public Health Intelligence Programme . North West Dental Health Survey n.d. www.nwph.net/dentalhealth/ (accessed 21 May 2015).
- Pine CM, Pitts NB, Nugent ZJ. British Association for the Study of Community Dentistry (BASCD) guidance on the statistical aspects of training and calibration of examiners for surveys of child dental health. A BASCD coordinated dental epidemiology programme quality standard. Community Dent Health 1997;14:18-29.
- NHS England . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection (accessed 26 May 2022).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 10 August 2021).
- Office for National Statistics . Estimates of the Population for the UK, England and Wales, Scotland and Northern Ireland n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 26 May 2022).
- Stevens K. Developing a descriptive system for a new preference-based measure of health-related quality of life for children. Qual Life Res 2009;18:1105-13. https://doi.org/10.1007/s11136-009-9524-9.
- Stevens KJ. Working with children to develop dimensions for a preference-based, generic, pediatric, health-related quality-of-life measure. Qual Health Res 2010;20:340-51. https://doi.org/10.1177/1049732309358328.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Wolf RT, Ratcliffe J, Chen G, Jeppesen P. The longitudinal validity of proxy-reported CHU9D. Qual Life Res 2021;30:1747-56. https://doi.org/10.1007/s11136-021-02774-9.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. https://doi.org/10.1136/bmj.329.7459.224.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. https://doi.org/10.1002/hec.584.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- Sendi PP, Briggs AH. Affordability and cost-effectiveness: decision-making on the cost-effectiveness plane. Health Econ 2001;10:675-80. https://doi.org/10.1002/hec.639.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Little R, Rubin D. Statistical Analysis with Missing Data. New York, NY: Wiley; 1987.
- Roderick J, Little A. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc 1995;90:1112-21. https://doi.org/10.1080/01621459.1995.10476615.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Birch S. Measuring dental health: improvements on the DMF index. Community Dent Health 1986;3:303-11.
- Birch S. The relative cost effectiveness of water fluoridation across communities: analysis of variations according to underlying caries levels. Community Dent Health 1990;7:3-10.
- Davies GN. Fluoride in the prevention of dental caries. A tentative cost benefit analysis. Brit Dent J 1973;135:131-4. https://doi.org/10.1038/sj.bdj.4803044.
- Nelson W, Swint JM. Cost-benefit analysis of fluoridation in Houston, Texas. J Public Health Dent 1976;36:88-95. https://doi.org/10.1111/j.1752-7325.1976.tb02854.x.
- Carr S, Dooland M, Roder D. Fluoridation II: an interim economic analysis. Aust Dent J 1980;25:343-8. https://doi.org/10.1111/j.1834-7819.1980.tb03893.x.
- Doessel DP. Cost-benefit analysis of water fluoridation in Townsville, Australia. Community Dent Oral Epidemiol 1985;13:19-22. https://doi.org/10.1111/j.1600-0528.1985.tb00412.x.
- Millan MT, Galvez AJ, Gomez E, Garcia A, Fernandez-Crehuet J. Cost-benefit analysis of fluoridating the public water supply of the city of Malaga. Gac Sanit 1991;5:82-6. https://doi.org/10.1016/s0213-9111(91)71051-0.
- Murgueytio P, Estupinan-Day S. Evaluación De Costos Y Beneficios Anticipados Del Programa De Fluoruración Del Agua Potable Propuesto Para La VIII Region 1995. https://www1.paho.org/hq/dmdocuments/2009/OH_CHI_EvalCostBenProgFluorAgua1995.pdf (accessed 30 Aug 2019).
- Arjunan KC. Economic Evaluation of Fluoridation of Water Supply to Prevent Dental Caries in Remote Communities in Australia: A Public Health Investment Analysis 2000. https://ssrn.com/abstract=2975910 (accessed 30 Aug 2019).
- Griffin SO, Jones K, Tomar SL. An economic evaluation of community water fluoridation. J Public Health Dent 2001;61:78-86. https://doi.org/10.1111/j.1752-7325.2001.tb03370.x.
- Wright JC, Bates MN, Cutress T, Lee M. The cost-effectiveness of fluoridating water supplies in New Zealand. Aust N Z J Public Health 2001;25:170-8. https://doi.org/10.1111/j.1753-6405.2001.tb01841.x.
- O’Connell JM, Brunson D, Anselmo T, Sullivan PW. Costs and savings associated with community water fluoridation programs in Colorado. Prev Chronic Dis 2005;2.
- Campain AC, Mariño RJ, Wright FA, Harrison D, Bailey DL, Morgan MV. The impact of changing dental needs on cost savings from fluoridation. Aust Dent J 2010;55:37-44. https://doi.org/10.1111/j.1834-7819.2010.01173.x.
- Kroon J, van Wyk PJ. A model to determine the economic viability of water fluoridation. J Public Health Dent 2012;72:327-33. https://doi.org/10.1111/j.1752-7325.2012.00342.x.
- Kroon J, Van Wyk PJ. A retrospective view on the viability of water fluoridation in South Africa to prevent dental caries. Community Dent Oral Epidemiol 2012;40:441-50. https://doi.org/10.1111/j.1600-0528.2012.00681.x.
- Tchouaket E, Brousselle A, Fansi A, Dionne PA, Bertrand E, Fortin C. The economic value of Quebec’s water fluoridation program. Z Gesundh Wiss 2013;21:523-33. https://doi.org/10.1007/s10389-013-0578-3.
- O’Connell J, Rockell J, Ouellet J, Tomar SL, Maas W. Costs and savings associated with community water fluoridation in the United States. Health Aff (Millwood) 2016;35:2224-32. https://doi.org/10.1377/hlthaff.2016.0881.
- Manau C, Cuenca E, Martinez-Carretero J, Salleras L. Economic evaluation of community programs for the prevention of dental caries in Catalonia, Spain. Community Dent Oral Epidemiol 1987;15:297-300. https://doi.org/10.1111/j.1600-0528.1987.tb01738.x.
- Mariño R. Evaluación económica del programa de fluoración del agua de beber en Chile. Rev Chil Salud Pública 2013;17:126-33. https://doi.org/10.5354/0719-5281.2013.27092.
- Mariño R, Fajardo J, Morgan M. Cost-effectiveness models for dental caries prevention programmes among Chilean schoolchildren. Community Dent Health 2012;29:302-8. https://doi.org/10.1922/CDH_2893Marino07.
- Edelstein BL, Hirsch G, Frosh M, Kumar J. Reducing early childhood caries in a Medicaid population: a systems model analysis. J Am Dent Assoc 2015;146:224-32. https://doi.org/10.1016/j.adaj.2014.12.024.
- Fyfe C, Borman B, Scott G, Birks S. A cost effectiveness analysis of community water fluoridation in New Zealand. N Z Med J 2015;128:38-46.
- Atkins CY, Thomas TK, Lenaker D, Day GM, Hennessy TW, Meltzer MI. Cost-effectiveness of preventing dental caries and full mouth dental reconstructions among Alaska native children in the Yukon-Kuskokwim delta region of Alaska. J Public Health Dent 2016;76:228-40. https://doi.org/10.1111/jphd.12141.
- Ciketic S, Hayatbakhsh MR, Doran CM. Drinking water fluoridation in south East Queensland: a cost-effectiveness evaluation. Health Promot J Austr 2010;21:51-6. https://doi.org/10.1071/he10051.
- Cobiac LJ, Vos T. Cost-effectiveness of extending the coverage of water supply fluoridation for the prevention of dental caries in Australia. Community Dent Oral Epidemiol 2012;40:369-76. https://doi.org/10.1111/j.1600-0528.2012.00684.x.
- Moore D, Poynton M, Broadbent JM, Thomson WM. The costs and benefits of water fluoridation in NZ. BMC Oral Health 2017;17. https://doi.org/10.1186/s12903-017-0433-y.
- Centers for Disease Control and Prevention . Ten Great Public Health Achievements – United States, 1900–1999 1999. www.cdc.gov/mmwr/preview/mmwrhtml/mm4850bx.htm (accessed 2 September 2021).
- Jack B, Ayson M, Lewis S, Irving A, Agresta B, Ko H, et al. Health Effects of Water Fluoridation: Evidence Evaluation Report 2016.
- Low IM, Duraman N, Mahmood U. Mapping the structure, composition and mechanical properties of human teeth. Mater Sci Eng C 2008;28:243-7. https://doi.org/10.1016/j.msec.2006.12.013.
- De Menezes Oliveira MA, Torres CP, Gomes-Silva JM, Chinelatti MA, De Menezes FC, Palma-Dibb RG, et al. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc Res Tech 2010;73:572-7. https://doi.org/10.1002/jemt.20796.
- Ramamurthy P, Rath A, Sidhu P, Fernandes B, Nettem S, Fee PA, et al. Sealants for preventing dental caries in primary teeth. Cochrane Database Syst Rev 2022;2. https://doi.org/10.1002/14651858.CD012981.pub2.
- Singh KA, Spencer AJ. Relative effects of pre- and post-eruption water fluoride on caries experience by surface type of permanent first molars. Community Dent Oral Epidemiol 2004;32:435-46. https://doi.org/10.1111/j.1600-0528.2004.00182.x.
- Marshman Z, Dyer TA, Wyborn CG, Beal J, Godson JH. The oral health of adults in Yorkshire and Humber 2008. Br Dent J 2010;209. https://doi.org/10.1038/sj.bdj.2010.819.
- Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health 1994;11:3-11.
- Jokovic A, Locker D, Stephens M, Kenny D, Tompson B, Guyatt G. Validity and reliability of a questionnaire for measuring child oral-health-related quality of life. J Dent Res 2002;81:459-63. https://doi.org/10.1177/154405910208100705.
- Hill H, Rowen D, Pennington B, Wong R, Wailoo A. A review of the methods used to generate utility values in NICE technology assessments for children and adolescents. Value Health 2020;23:907-17. https://doi.org/10.1016/j.jval.2020.02.011.
- Rowen D, Rivero-Arias O, Devlin N, Ratcliffe J. Review of valuation methods of preference-based measures of health for economic evaluation in child and adolescent populations: where are we now and where are we going?. PharmacoEconomics 2020;38:325-40. https://doi.org/10.1007/s40273-019-00873-7.
- Moore D, Allen T, Birch S, . How effective and cost-effective is water fluoridation for adults? Protocol for a 10-year retrospective cohort study. BDJ Open 2021;7. https://doi.org/10.1038/s41405-021-00062-9.
- Nomis . Cumbria County Local Area Report 2011. www.nomisweb.co.uk/reports/localarea?compare=E10000006 (accessed 21 July 2021).
Appendix 1 Birth cohort recruitment
Figure 16 presents the recruitment graph for the birth cohort.
FIGURE 16.
Birth cohort recruitment graph. CIC, Cumberland Infirmary Carlisle (control); WCH, West Cumberland Hospital (intervention).
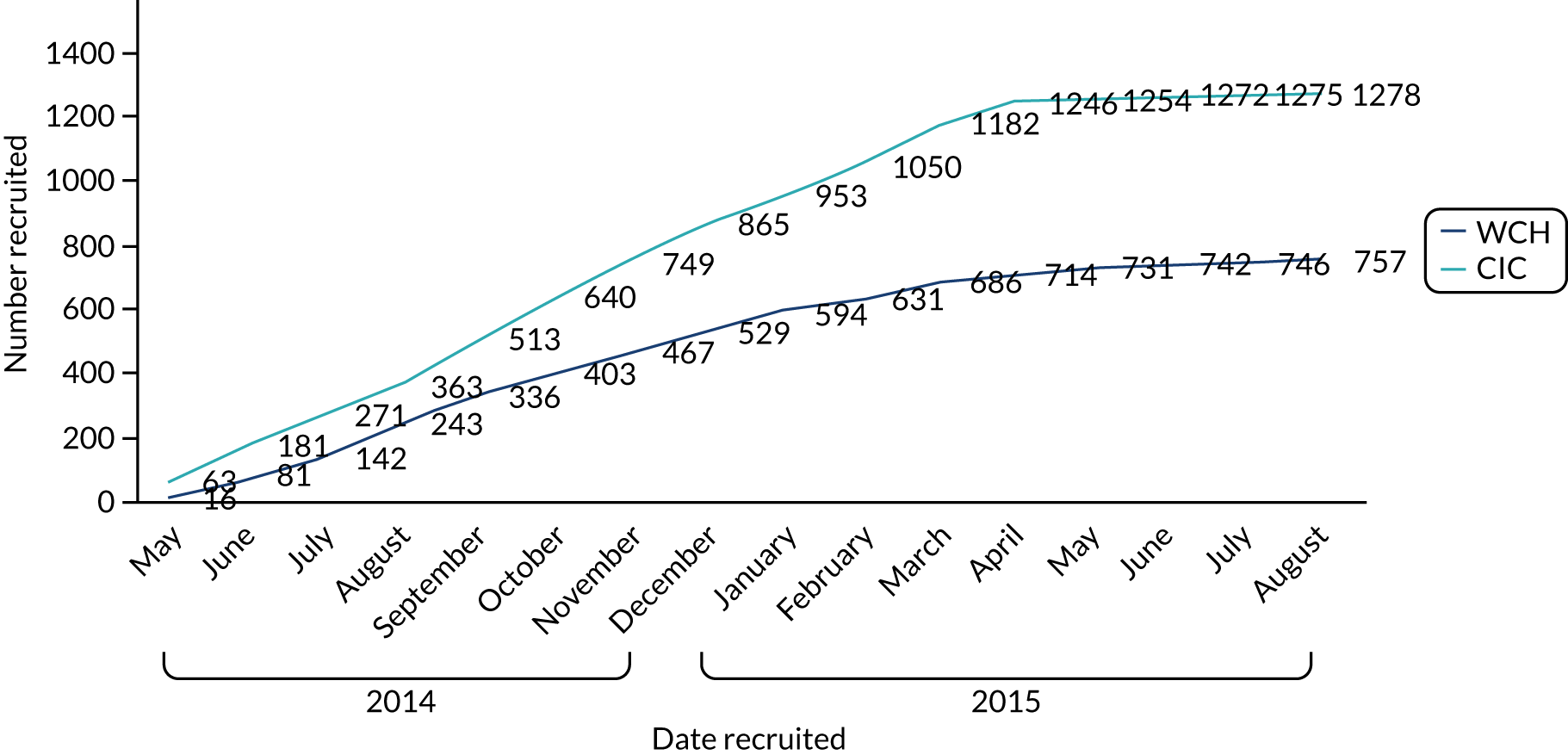
Appendix 2 Birth cohort additional analyses
This appendix presents the birth cohort additional analysis for the CATFISH study and is connected to Chapter 8 in the main body of the report. This appendix is divided into the following subsections:
-
-
This section provides additional analysis on examination status and any association with control/intervention group or deprivation.
-
-
Longitudinal data analysis of potential effect modifiers collected by questionnaires –
-
This section details the behavioural data collected through questionnaires during the course of the study. Data analysis consists of GEEs to determine if any behaviour differs between the groups (i.e. control and intervention groups) over time.
-
-
-
This section provides analysis on tooth eruption. If eruption rates differed across groups, then this would have been accounted for in further analysis.
-
-
-
Additional analysis linked to the primary outcome is presented here, including full logistic regression outputs.
-
-
-
Additional analysis linked to the secondary outcome is presented here, including full logistic and negative binomial regressions and accompanying predictive probabilities.
-
-
Health inequalities: interaction of deprivation and water fluoridation –
-
Additional analysis linked to the health inequalities data, which includes an interaction of deprivation quintiles and WF exposure within the regression, is presented here, including the accompanying predictive probabilities.
-
-
-
This section provides the analysis conducted outside the original protocol, following the unexpected interruption to dosing at one of the plants in Cumbria.
-
Examination status
A logistic regression on examination status between the two groups was performed. The logistic regression indicated that for children living in a fluoridated area the odds of being examined were 1.64 times as large as the odds for children living in a non-fluoridated area (Table 61).
| Variable | OR (95% CI) |
|---|---|
| Area no WF vs. WF (reference: no WF) | 1.64 (1.33 to 2.0) |
| Deprivation (reference: 1) | |
| 2 | 0.62 (0.39 to 0.96) |
| 3 | 0.82 (0.53 to 1.28) |
| 4 | 0.63 (0.41 to 0.97) |
| 5 | 0.71 (0.45 to 1.10) |
| Sex (reference: female) | 0.99 (0.81 to 1.20) |
Longitudinal data analysis of potential effect modifiers collected by questionnaires
This section details data collected from questionnaires that were completed by parents of children in the study. Seven questionnaires were provided during the 5-year study. The questionnaires were provided at the following approximate ages:
-
0 months (wave 1)
-
6 months (wave 2)
-
12 months (wave 3)
-
18 months (wave 4)
-
30 months (wave 5)
-
36 months (wave 6, provided when child attended for their dental examination)
-
48/54 months (wave 7, sent during the school year and so this could have been at any age between 4 and 5 years).
When participants were recruited, 24% of participants requested their questionnaire by post, 7% of participants requested their questionnaire by e-mail and 69% of participants stated either would be acceptable (or they did not provide a preference). Participants’ parents were contacted in accordance with their preferences. If no preference was stated, then the questionnaire was sent by post.
Table 62 provides descriptive data from the birth cohort baseline questionnaire, but for only participants who had a final clinical examination at 5 years old. Both groups provided a similar range of responses for most questions. One area where a slight difference was observed was breastfeeding. Although a slightly higher proportion of parents in the fluoridated group attempted to breastfeed (28% vs. 21%), a similar number were breastfeeding ‘now’ (when the child was approximately 1 month old), with 59% and 58% of parents breastfeeding at that time in the no WF and WF groups, respectively.
| Birth cohort questionnaire | Exposure group | |
|---|---|---|
| No WF | WF | |
| Age (years) of child when questionnaire completed | ||
| n | 413 | 273 |
| Mean (SD) | 0.13 (0.5) | 0.12 (0.6) |
| Did you ever try to breastfeed? | ||
| n | 419 | 280 |
| Yes/no (%) | 78/22 | 73/27 |
| Of those who tried was the child able to be breastfed? | ||
| n | 324 | 205 |
| Yes/no (%) | 94/6 | 97/3 |
| Breastfeed now? | ||
| n | 304 | 198 |
| Yes/no (%) | 59/41 | 58/42 |
| Child ever had formula? | ||
| n | 417 | 278 |
| Yes/no/do not know (%) | 75/24/1 | 75/25/0 |
| Child ever had sweetened drinks? | ||
| n | 412 | 277 |
| Yes/no (%) | 1/99 | 1/99 |
| Child ever had unsweetened drinks? | ||
| n | 410 | 277 |
| Yes/no/do not know (%) | 20/80/0 | 14/85/1 |
| Glasses of tap water consumed a day (250 ml): data for those who actually consumed water | ||
| n | 237 | 170 |
| Mean (SD) | 4.38 (2.06) | 4.50 (1.96) |
| Glasses of tap water consumed a day (250 ml) if water consumed or not (i.e. 0 included) | ||
| n | 416 | 278 |
| Mean (SD) | 2.46 (2.65) | 2.69 (2.66) |
| Pre/post birth history | ||
| n | 425 | 280 |
| Resuscitation/oxygen required (%) | 6 | 4 |
| Difficulty breastfeeding (%) | 10 | 7 |
| Antibiotic administered (%) | 6 | 12 |
| Premature (%) | 7 | 8 |
| Jaundice (%) | 23 | 28 |
| Colic (%) | 18 | 17 |
| Fluoride supplements (0) | 0 | 0 |
| Child attended hospital since birth? | ||
| n | 418 | 280 |
| Yes/no (%) | 24/76 | 26/74 |
| Child has any chronic conditions? | ||
| n | 415 | 276 |
| Yes/no (%) | 1/99 | 1/99 |
In the first questionnaire, the question on chronic illness (which was used to measure potential adverse events) showed the same proportion (1%) in both groups.
Table 63 provides descriptive data from questionnaires for the final questionnaire completed. Table 63 includes responses if a participant had a clinical examination. A similar distribution seems to be apparent between groups for consuming sweetened drinks in a bottle, sippy cup or cup (this is further explored in GEE analysis later in the appendix; see Tables 71 and 72). Regarding dental attendance, a higher proportion (14%) of children in the WF group’s last dental visit was for their first dental check-up compared with the no WF group (5%), with more children in the no WF group attending for a routine check-up.
| Birth cohort questionnaire | Exposure group | |
|---|---|---|
| No WF | WF | |
| Age (years) of child when questionnaire completed | ||
| n | 312 | 196 |
| Mean (SD) | 4.87 (0.38) | 4.83 (0.38) |
| Does child have sweetened drinks?a | ||
| n | 317 | 199 |
| Bottle (%) | 1 | 2 |
| Sippy cup (%) | 2 | 2 |
| Cup (%) | 64 | 65 |
| Does not have this drink (%) | 28 | 26 |
| Does child have unsweetened drinks?a | ||
| n | 317 | 199 |
| Bottle (%) | 2 | 2 |
| Sippy cup (%) | 3 | 3 |
| Cup (%) | 90 | 93 |
| Does not have this drink (%) | 6 | 4 |
| Does child have milk drinks?a | ||
| n | 317 | 199 |
| Bottle (%) | 4 | 4 |
| Sippy cup (%) | 4 | 5 |
| Cup (%) | 84 | 77 |
| Does not have this drink (%) | 7 | 11 |
| Glasses of water (tap) consumed a day (250 ml) if water actually consumed | ||
| n | 291 | 169 |
| Mean (SD) | 3.56 (1.55) | 3.66 (2.23) |
| Glasses of water (tap) consumed a day (250 ml) if water consumed or not | ||
| n | 316 | 199 |
| Mean (SD) | 3.28 (1.77) | 3.10 (2.43) |
| Child takes medications, supplements or vitamins? | ||
| n | 313 | 199 |
| Yes/no (%) | 35/65 | 37/63 |
| Child been to the dentist? | ||
| n | 316 | 199 |
| Yes (%) | 96 | 92 |
| If yes, what was their last visit for? | ||
| n | 299 | 184 |
| First check-up (%) | 5 | 14 |
| Routine check-up (%) | 93 | 85 |
| Emergency treatment (%) | 1 | 0 |
| Other (%) | 1 | 2 |
| If attend the dentist how long ago was the last visit (months) | ||
| n | 292 | 178 |
| Mean (SD) | 4.19 (2.93) | 5.37 (4.57) |
| Child ever had a dental-related visit to hospital? | ||
| n | 314 | 198 |
| Yes/no (%) | 3/93 | 2/98 |
| Of those who had a dental-related visit to hospital, were they in for a DGA? | ||
| n | 9 | 2 |
| Yes/no (%) | 44/56 | 0/100 |
| Had to attend hospital? | ||
| n | 315 | 198 |
| Yes/no (%) | 22/78 | 19/81 |
| Child has any new chronic conditions since last questionnaire? | ||
| n | 315 | 196 |
| Yes/no (%) | 12/88 | 12/88 |
| Has the child had a dental problem in the last 12 months? | ||
| n | 316 | 197 |
| Yes/no (%) | 7/93 | 4/96 |
| If yes, what was this?a | ||
| n | 23 | 7 |
| Pain (%) | 43 | 14 |
| Talking (%) | 4 | 0 |
| Eating (%) | 9 | 14 |
| Sleeping (%) | 9 | 14 |
| Being upset (%) | 21 | 14 |
| Other (%) | 35 | 71 |
| How often does child brush their teeth? | ||
| n | 319 | 198 |
| More than twice a day (%) | 3 | 4 |
| Twice a day (%) | 85 | 77 |
| Once a day (%) | 11 | 17 |
| Less than once a day (%) | 0.3 | 2 |
| Who brushes their teeth? | ||
| n | 314 | 198 |
| Child on own (%) | 9 | 10 |
| Child supervised (%) | 65 | 62 |
| Parent/carer (%) | 27 | 28 |
| After brushing does the child do the following? | ||
| n | 314 | 198 |
| Spit (%) | 53 | 52 |
| Rinse: wet brush (%) | 15 | 19 |
| Rinse: head under tap (%) | 3 | 2 |
| Rinse: using cup (%) | 4 | 3 |
| Rinse: using beaker (%) | 8 | 7 |
| Does not spit (%) | 15 | 16 |
| Other (%) | 1 | 1 |
| Toothpaste type: fluoridated/non-fluoridated | ||
| n | 234/2 | 143/1 |
| % | 99/1 | 99/1 |
The results in Table 64 are for the baseline questionnaire completed about the parents/guardians and their household. Demographics were similar between groups, with a high proportion (94–97%) being born in the UK and most participants selecting ‘white’ on the ethnicity question. One apparent difference between the groups was on dental attendance, with parents in the non-fluoridated area attending the dentist more regularly than those in the fluoridated area. In addition, the proportion of respondents selecting the highest household income band was higher in the fluoridated area (40%) than in the non-fluoridated area (17%), and this is reflected in the equivalised household income calculation (please note that the calculation is an approximation, as we could collect income only in bands and, hence, this was calculated from a median household income from each band following PPI feedback).
| Parent baseline questionnaire | Exposure group | |||
|---|---|---|---|---|
| No WF | WF | |||
| PC1 | PC2 | PC1 | PC2 | |
| Parent type: mother/father/other | ||||
| n | 304/4 | 4/269/1 | 182/3 | 3/173 |
| % | 99/1 | 2/98/1 | 99/1 | 2/98 |
| Born in the UK: no/yes? | ||||
| n | 19/289 | 15/259 | 7/178 | 5/170 |
| % | 6/94 | 5/95 | 4/96 | 3/97 |
| Ethnicity: white/other | ||||
| n | 302/3 | 272/3 | 184/1 | 172/4 |
| % | 98/2 | 99/1 | 99/1 | 98/2 |
| General health | ||||
| n | 308 | 274 | 185 | 175 |
| 1: very good (%) | 57 | 56 | 60 | 64 |
| 2: good (%) | 37 | 39 | 34 | 32 |
| 3: fair (%) | 5 | 4 | 6 | 3 |
| 4: bad (%) | 0.3 | 1 | 0 | 1 |
| 5: very bad (%) | 0 | 0 | 0 | 0 |
| Dental health | ||||
| n | 282 | 274 | 178 | 174 |
| 1: very good (%) | 37 | 37 | 35 | 32 |
| 2: good (%) | 41 | 41 | 45 | 44 |
| 3: fair (%) | 18 | 19 | 16 | 18 |
| 4: bad (%) | 3 | 3 | 3 | 5 |
| 5: very bad (%) | 0.4 | 1 | 0.5 | 1 |
| How often do you visit the dentist? | ||||
| n | 308 | 274 | 185 | 174 |
| 1: at least once every 6 months (%) | 57 | 50 | 49 | 33 |
| 2: at least once a year (%) | 33 | 30 | 36 | 41 |
| 3: at least once every 2 years (%) | 2 | 2 | 8 | 9 |
| 4: less frequently than every 2 years (%) | 3 | 5 | 1 | 2 |
| 5: only when I have trouble with my teeth (%) | 2 | 12 | 6 | 15 |
| 6: never been to the dentist (%) | 6 | 1 | 0 | 0 |
| Last visit to the dentist | ||||
| n | 308 | 274 | 185 | 174 |
| 1: a routine check-up (%) | 78 | 71 | 78 | 65 |
| 2: emergency or urgent treatment (%) | 8 | 8 | 5 | 10 |
| 3: other treatment (non-urgent) (%) | 11 | 15 | 15 | 21 |
| 4: not been to the dentist (%) | 1 | 3 | 1 | 2 |
| 5: other (please state) (%) | 2 | 2 | 2 | 2 |
| Employment status | ||||
| n | 308 | 274 | 184 | 173 |
| 1: full-time employee (> 30 hours) (%) | 30 | 88 | 28 | 87 |
| 2: full-time employee (< 30 hours) (%) | 50 | 3 | 54 | 4 |
| 3: school/full-time education (%) | 1 | 0 | 0 | 0 |
| 4: unemployed (%) | 1 | 1 | 1 | 2 |
| 5: retired from work (%) | 0 | 0 | 0 | 0 |
| 6: looking after the home (%) | 13 | 1 | 12 | 0 |
| 7: permanently sick or disabled (%) | 1 | 1 | 0 | 1 |
| 8: doing something else (%) | 5 | 7 | 3 | 6 |
| Job type | ||||
| n | 277 | 265 | 168 | 166 |
| 1: modern professionala (%) | 34 | 15 | 35 | 13 |
| 2: clerical and intermediate occupationsb (%) | 27 | 3 | 21 | 2 |
| 3: senior managers/administratorsc (%) | 8 | 13 | 7 | 15 |
| 4: technical and craft occupationsd (%) | 3 | 19 | 4 | 22 |
| 5: tool maker, electrician, gardener, train driver (%) | 8 | 14 | 5 | 6 |
| 6: semi-routine manual and service occupationse (%) | 8 | 15 | 3 | 12 |
| 7: routine manual and service occupationsf (%) | 3 | 9 | 9 | 5 |
| 8: middle or junior managersg (%) | 6 | 10 | 15 | 22 |
| 9: traditional professional occupationsh (%) | 2 | 1 | 1 | 1 |
| Household income (£) | ||||
| n | 288 | 176 | ||
| 1: up to 5199 (per annum)i (%) | 1 | 1 | ||
| 2: 5200–10,399 (per annum)j (%) | 1 | 4 | ||
| 3: 10,400–15,599 (per annum)k (%) | 6 | 5 | ||
| 4: £15,600–20,799(per annum)l (%) | 9 | 8 | ||
| 5: 20,800–25,999 (per annum)m (%) | 14 | 7 | ||
| 6: 26,000–31,199 (per annum)n (%) | 17 | 7 | ||
| 7: 32,200–36,399 (per annum)o (%) | 12 | 7 | ||
| 8: 36,400–51,999 (per annum)p (%) | 25 | 22 | ||
| 9: ≥ 52,000 (per annum)q (%) | 17 | 40 | ||
| Equivalised household income (£) [taking the median income from household income (above) and calculated using McClements equivalence scales] | ||||
| n | 240 | 153 | ||
| Mean (SD) | 552.55 (14.04) | 617.41 (238.07) | ||
| 95% CI | 524.89 to 580.20 | 579.39 to 655.43 | ||
| Number of children in household | ||||
| n | 274 | 167 | ||
| 1 (%) | 49 | 37 | ||
| 2 (%) | 38 | 46 | ||
| 3 (%) | 11 | 14 | ||
| 4 (%) | 1 | 2 | ||
| 5 (%) | 0.36 | 1 | ||
| 6 (%) | 0 | 0 | ||
| 7 (%) | 0.36 | 0 | ||
| Average number of cups of tap water consumed each day (including tea, squash) | ||||
| n | 303 | 265 | 177 | 168 |
| Mean (SD) | 6.71 (3.10) | 7.15 (3.25) | 7.34 (2.94) | 7.33 (3.29) |
The results presented in Table 65 from the final wave of data collection are comparable to the baseline results. The majority of participants filling out the questionnaire as primary carer 1 were white, mothers and born in the UK. More respondents in the non-fluoridated group than in the fluoridated group self-reported their dental and general health as ‘very good’; however, when the proportions responding ‘very good’ and ‘good’ are combined, the results are broadly similar in both groups. Parents in the non-fluoridated area attend the dentist more regularly. The proportion of participants who were employed full time was slightly higher in the fluoridated group. When looking at household income, the proportion of participants earning within the top band (i.e. ≥ £52,000 per annum) is higher in the fluoridated group (41%) than in the non-fluoridated group (26%). In addition, when primary carer 2 (usually the father) answered the questionnaire, there were more ‘middle managers’ in the fluoridated group.
| Parent final questionnaire | Exposure group | |||
|---|---|---|---|---|
| No WF | WF | |||
| PC1 | PC2 | PC1 | PC2 | |
| Parent type: mother/father/other | ||||
| n | 301/4/2 | 5/274/3 | 193/5 | 3/164/3 |
| % | 98/2/0.6 | 2/97/0.3 | 97/3 | 2/96/2 |
| Born in the UK: no/yes? | ||||
| n | 29/291 | 19/263 | 7/196 | 5/164 |
| % | 8/92 | 7/93 | 3/97 | 3/97 |
| Ethnicity: white/other | ||||
| n | 312/5 | 274/8 | 201/2 | 166/3 |
| % | 98/2 | 97/3 | 99/1 | 98/2 |
| General health | ||||
| n | 319 | 282 | 203 | 169 |
| 1: very good (%) | 61 | 60 | 54 | 52 |
| 2: good (%) | 32 | 35 | 41 | 43 |
| 3: fair (%) | 6 | 4 | 4 | 4 |
| 4: bad (%) | 1 | 1 | 1 | 1 |
| 5: very bad (%) | 0 | 0 | 0 | 0 |
| Dental health | ||||
| n | 319 | 282 | 203 | 170 |
| 1: very good (%) | 42 | 35 | 34 | 30 |
| 2: good (%) | 41 | 43 | 49 | 45 |
| 3: fair (%) | 16 | 17 | 16 | 19 |
| 4: bad (%) | 1 | 4 | 2 | 6 |
| 5: very bad (%) | 0 | 1 | 0 | 0 |
| How often do you visit the dentist? | ||||
| n | 318 | 282 | 202 | 168 |
| 1: at least once every 6 months (%) | 63 | 49 | 44 | 29 |
| 2: at least once a year (%) | 30 | 28 | 42 | 45 |
| 3: at least once every 2 years (%) | 2 | 5 | 7 | 11 |
| 4: less frequently than every 2 years (%) | 1 | 4 | 1 | 2 |
| 5: only when I have trouble with my teeth (%) | 4 | 13 | 6 | 12 |
| 6: never been to the dentist (%) | 0 | 1 | 0 | 0 |
| Last visit to the dentist | ||||
| n | 319 | 278 | 202 | 169 |
| 1: a routine check-up (%) | 80 | 78 | 83 | 70 |
| 2: emergency or urgent treatment (%) | 7 | 8 | 7 | 12 |
| 3: other treatment (non-urgent) (%) | 11 | 12 | 8 | 15 |
| 4: not been to the dentist (%) | 0.3 | 1 | 1 | 1 |
| 5: other (please state) (%) | 3 | 1 | 2 | 2 |
| Employment status | ||||
| n | 319 | 280 | 202 | 173 |
| 1: full-time employee (> 30 hours) (%) | 27 | 85 | 36 | 89 |
| 2: full-time employee (< 30 hours) (%) | 55 | 4 | 52 | 4 |
| 3: school/full-time education (%) | 1 | 0 | 1 | 0 |
| 4: unemployed (%) | 3 | 1 | 1 | 1 |
| 5: retired from work (%) | 0 | 0 | 0 | 0 |
| 6: looking after the home (%) | 9 | 1 | 9 | 2 |
| 7: permanently sick or disabled (%) | 1 | 1 | 1 | 0 |
| 8: doing something else (%) | 5 | 8 | 2 | 4 |
| Job type | ||||
| n | 293 | 271 | 186 | 162 |
| 1: modern professionala (%) | 39 | 19 | 40 | 13 |
| 2: clerical and intermediate occupationsb (%) | 24 | 4 | 19 | 3 |
| 3: senior managers/administratorsc (%) | 9 | 14 | 10 | 17 |
| 4: technical and craft occupationsd (%) | 1 | 21 | 2 | 27 |
| 5: tool maker, electrician, gardener, train driver (%) | 12 | 15 | 12 | 9 |
| 6: semi-routine manual and service occupationse (%) | 8 | 13 | 6 | 10 |
| 7: routine manual and service occupationsf (%) | 3 | 7 | 6 | 6 |
| 8: middle or junior managersg (%) | 5 | 7 | 5 | 15 |
| 9: traditional professional occupationsh (%) | 0 | 0 | 0 | 0 |
| Household income (£) | ||||
| n | 291 | 184 | ||
| 1: up to 5199 (per annum)i (%) | 1 | 2 | ||
| 2: 5200–10,399 (per annum)j (%) | 3 | 3 | ||
| 3: 10,400–15,599 (per annum)k (%) | 6 | 5 | ||
| 4: £15,600–20,799 (per annum)l (%) | 7 | 5 | ||
| 5: 20,800–25,999 (per annum)m (%) | 9 | 5 | ||
| 6: 26,000–31,199 (per annum)n (%) | 9 | 8 | ||
| 7: 32,200–36,399 (per annum)o (%) | 12 | 8 | ||
| 8: 36,400–51,999 (per annum)p (%) | 25 | 21 | ||
| 9: ≥ 52,000 (per annum)q (%) | 26 | 41 | ||
| Equivalised household income (£) [taking the median income from household income (above) and calculated using McClements equivalence scales] | ||||
| n | 289 | 179 | ||
| Mean (SD) | 589.81 (485.47) | 607.83 (419.43) | ||
| 95% CI | 533.60 to 646.02 | 545.96 to 669.69 | ||
We can compare data from these questionnaires overall with data collected on the population of Cumbria, including data from the 2011 Office for National Statistics census157 and data from the 2009 Adult Dental Health Survey12 on respondents in North West England, to determine if the population is representative of the wider area (Table 66).
| Demographic | Cumbria/North West England population | CATFISH study results: baseline | |
|---|---|---|---|
| PC1 (n = 615) | PC2 (n = 559) | ||
| Born in the UK:a no/yes? (%) | 3.7/96.3 | 5.9/94.1 | 5.0/95.0 |
| Ethnicity:a white/other (%) | 98.5/1.5 | 98.7/1.3 | 98.4/1.6 |
| General healtha (%) | |||
| 1: very good | 45 | 58.4 | 59.8 |
| 2: good | 34.6 | 35.6 | 35.7 |
| 3: fair | 14.4 | 5.9 | 4.0 |
| 4: bad | 4.7 | 0.2 | 0.5 |
| 5: very bad | 1.3 | 0 | 0 |
| How often do you visit the dentist?b (%) | |||
| 1: at least once every 6 months | 50 | 52 | 44 |
| 2: at least once a year | 20 | 35 | 32 |
| 3: at least once every 2 years | 4 | 4 | 5 |
| 4: less frequently than every 2 years | 10 | 2 | 4 |
| 5: only when I have trouble with my teeth | 15 | 6 | 13 |
| Employment statusa (%) | |||
| 1: employee full time | 43.0 | 51.5 | 87.0 |
| 2: employee part time | 18.7 | 29.1 | 3.8 |
| 3: full-time student | 2.9 | 0.3 | 0.0 |
| 4: unemployed | 3.9 | 1.5 | 1.3 |
| 5: retired from work | 21.0 | 0.2 | 0.0 |
| 6: looking after the home | 3.5 | 12.4 | 0.7 |
| 7: permanently sick or disabled | 5.2 | 0.3 | 0.7 |
| 8: doing something else | 1.8 | 4.7 | 6.5 |
Behaviour that could affect dental health was recorded through questionnaire response. To explore the impact of behaviours across each wave and to determine if there are differences between the WF and the no WF groups (taking into account multiple testing across waves), we used GEEs, using logit binomial with autoregressive correlation.
Generalised estimating equations were performed for the following behaviours:
-
drinks containing sugar consumed over time (5 years)
-
water consumed over time (5 years)
-
sweets/chocolate consumed more than three times per week over time (5 years)
-
fizzy drinks consumed over time (5 years)
-
cake, biscuits or pudding consumed over time (5 years)
-
drinks containing sugar consumed in the hour before bed over time (5 years)
-
snacks containing free sugars consumed in the hour before bed over time (5 years)
-
frequency of toothbrushing each day over time (5 years)
-
whether a child spits or rinses out their toothpaste over time (5 years)
-
if a child has ever attended a dentist over time (5 years)
-
if a child was breastfed over time (5 years).
Of these 11 behaviours, two produced a significant result: consuming sweets on three or more days each week and whether or not a child had ever attended the dentist. The two significant results are reported in Tables 67–70. All other non-significant results are provided in Tables 71–86.
Tables 67 and 68 show the GEEs and descriptive data for sweets/chocolate consumed three or more times per week over time (5 years – seven data points). The statistical test indicated a significant difference between the exposure groups (WF vs. no WF) over time in whether or not children consumed sweets/chocolate three or more times per week, with a risk ratio of 0.628 (95% CI 0.438 to 0.901). The proportion of children who consumed sweets/chocolate three or more times per week (based on those who answered the questionnaire) was higher in the fluoridated group than in the non-fluoridated group.
| Behaviour: ate sweets three or more times per week (yes/no) | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.628 | 0.116 | –2.530 | 0.011 | 0.438 to 0.901 |
| Deprivation quintile | 0.922 | 0.067 | –1.110 | 0.269 | 0.799 to 1.064 |
| Sex (male/female) | 1.014 | 0.183 | 0.080 | 0.940 | 0.712 to 1.444 |
| Age | 0.431 | 0.025 | –14.340 | 0.000 | 0.384 to 0.483 |
| Constant | 22.610 | 6.292 | 11.210 | 0.000 | 13.104 to 39.010 |
| Behaviour | Exposure group | Consumption | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Average sweet consumption each week | No WF | Three or more times per week | 0 | 2 (0.4) | 34 (8.5) | 94 (25.3) | 131 (46.4) | 122 (52.6) | 170 (57.4) |
| Less than three times per week | 568 (100) | 491 (99.6) | 364 (91.5) | 277 (74.7) | 151 (53.6) | 110 (47.4) | 126 (45.6) | ||
| WF | Three or more times per week | 0 | 7 (2.7) | 35 (16.4) | 61 (35.9) | 80 (55.4) | 87 (68.5) | 122 (63.9) | |
| Less than three times per week | 331 (100) | 249 (97.3) | 178 (83.6) | 109 (64.1) | 63 (44.1) | 40 (31.5) | 195 (36.1) | ||
Tables 69 and 70 show the GEEs and descriptive data for whether or not a child has ever attended the dentist over time (5 years – seven data points). There is a significant difference between the exposure groups (WF vs. no WF) over time in whether or not a child has ever attended the dentist, with a risk ratio of 0.411 (95% CI 0.267 to 0.634). The proportion of children who had ever attended the dentist was consistently lower in the fluoridated group than in the non-fluoridated group until wave 6, which would be approximately 3 years of age.
| Behaviour: ever been to the dentist | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.411 | 0.091 | –4.020 | 0.000 | 0.267 to 0.634 |
| Deprivation quintile | 0.817 | 0.069 | –2.400 | 0.017 | 0.692 to 0.964 |
| Sex (male/female) | 1.011 | 0.203 | 0.060 | 0.956 | 0.682 to 1.500 |
| Age | 5.295 | 0.599 | 14.740 | 0.000 | 4.242 to 6.609 |
| Constant | 0.135 | 0.038 | –7.180 | 0.000 | 0.078 to 0.233 |
| Behaviour | Exposure group | Ever been to the dentist? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Ever been to the dentist | No WF | No | – | 442 (89.8) | 275 (68.9) | 174 (46.5) | 53 (18.7) | 26 (11.1) | 13 (4.1) |
| Yes | – | 50 (10.2) | 126 (31.4) | 200 (53.5) | 231 (81.3) | 209 (88.9) | 306 (95.9) | ||
| WF | No | – | 245 (96.1) | 173 (81.6) | 111 (64.2) | 45 (31.3) | 23 (17.6) | 16 (7.8) | |
| Yes | – | 10 (3.9) | 39 (18.4) | 62 (35.8) | 99 (68.7) | 108 (82.4) | 188 (92.2) | ||
Tables 71 and 72 show the GEEs descriptive data for whether or not the participant had a drink that contained sugar in a bottle or cup (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time for this variable.
| Behaviour: sweet drink (yes/no) | OR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.056 | 0.173 | 0.330 | 0.739 | 0.766 to 1.456 |
| Deprivation quintile | 1.172 | 0.074 | 2.530 | 0.011 | 1.037 to 1.326 |
| Sex (male/female) | 0.735 | 0.113 | –2.010 | 0.045 | 0.545 to 0.993 |
| Age | 2.177 | 0.116 | 14.660 | 0.000 | 1.962 to 2.416 |
| Constant | 0.099 | 0.023 | –9.770 | 0.000 | 0.062 to 0.157 |
| Behaviour | Exposure group | Had a drink that contained sugar in a bottle or cup over the last 4 weeks? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Drank a sugary drink in last 4 weeks | No WF | No | 556 (98.4) | 420 (84.2) | 269 (66.6) | 221 (58.8) | 137 (47.4) | 81 (34.3) | 86 (28.8) |
| Yes | 9 (1.6) | 79 (15.8) | 135 (33.4) | 155 (41.2) | 152 (52.6) | 155 (65.7) | 213 (71.2) | ||
| WF | No | 325 (98.2) | 213 (82.2) | 118 (55.7) | 86 (49.7) | 50 (34.5) | 51 (38.9) | 52 (27.1) | |
| Yes | 6 (1.8) | 46 (17.8) | 94 (44.3) | 87 (50.3) | 95 (65.5) | 80 (61.1) | 140 (72.9) | ||
Table 73 shows the GEEs for water consumed (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time in the amount of water children consumed.
| Amount of water drank | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.944 | 0.067 | –0.820 | 0.414 | 0.821 to 1.085 |
| Deprivation quintile | 1.005 | 0.028 | 0.180 | 0.859 | 0.952 to 1.061 |
| Sex (male/female) | 0.985 | 0.066 | –0.220 | 0.825 | 0.863 to 1.124 |
| Age | 1.025 | 0.018 | 1.420 | 0.157 | 0.991 to 1.059 |
| Constant | 3.400 | 0.401 | 10.360 | 0.000 | 2.697 to 4.285 |
Tables 74 and 75 show the GEEs and descriptive data for fizzy drinks consumed (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time in the average number of fizzy drinks children consumed.
| Behaviour: fizzy drinks consumed three or more times per week (yes/no) | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.015 | 0.220 | 0.070 | 0.945 | 0.664 to 1.553 |
| Deprivation quintile | 0.853 | 0.065 | –2.080 | 0.038 | 0.734 to 0.991 |
| Sex (male/female) | 1.106 | 0.228 | 0.490 | 0.625 | 0.739 to 1.655 |
| Age | 0.663 | 0.034 | –8.030 | 0.000 | 0.599 to 0.733 |
| Constant | 15.882 | 4.816 | 9.120 | 0.000 | 8.766 to 28.774 |
| Exposure group | Fizzy drinks consumed | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| No WF | Three or more times per week | – | 22 (4.5) | 49 (12.4) | 72 (19.5) | 74 (26.3) | 72 (31.2) | 92 (31.3) |
| Less than three times per week | – | 469 (95.5) | 346 (87.6) | 297 (80.5) | 207 (73.7) | 159 (68.8) | 202 (68.7) | |
| WF | Three or more times per week | – | 15 (5.9) | 32 (15.1) | 35 (20.7) | 42 (29.4) | 39 (30.7) | 66 (34.6) |
| Less than three times per week | – | 241 (94.1) | 180 (84.9) | 134 (79.3) | 101 (70.6) | 88 (69.3) | 125 (63.5) | |
Tables 76 and 77 show the GEEs and descriptive data for consumption of cake, biscuits and pudding each week over time (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time in the consumption of cake, biscuits and pudding.
| Behaviour: ate cakes three or more times per week (yes/no) | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.849 | 0.146 | –0.950 | 0.341 | 0.606 to 1.190 |
| Deprivation quintile | 0.910 | 0.061 | –1.410 | 0.157 | 0.798 to 1.037 |
| Sex (male/female) | 1.221 | 0.202 | 1.210 | 0.228 | 0.883 to 1.690 |
| Age | 0.484 | 0.030 | –11.520 | 0.000 | 0.428 to 0.548 |
| Constant | 6.733 | 1.786 | 7.190 | 0.000 | 4.003 to 11.325 |
| Behaviour | Exposure group | Consumption of cake, biscuits and pudding each week | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Average cake, biscuit and pudding consumption each week | No WF | Three or more times per week | – | 46 (9.4) | 130 (32.8) | 191 (51.3) | 166 (59.1) | 132 (57.4) | 199 (67.7) |
| Less than three times per week | – | 445 (90.6) | 266 (67.2) | 181 (48.7) | 115 (40.9) | 98 (42.6) | 95 (32.3) | ||
| WF | Three or more times per week | – | 30 (11.7) | 71 (33.3) | 87 (51.2) | 87 (60.4) | 72 (56.3) | 124 (66.7) | |
| Less than three times per week | – | 227 (88.3) | 142 (66.7) | 83 (48.8) | 57 (39.6) | 56 (43.7) | 62 (33.3) | ||
Tables 78 and 79 show the GEEs and descriptive data for if a sugary drink is consumed in the hour before bed over time (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time for if a sugary drink is consumed in the hour before bed.
| Behaviour: sugary drink consumed in hour before bed | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.158 | 0.590 | 0.290 | 0.773 | 0.427 to 3.142 |
| Deprivation quintile | 2.060 | 0.472 | 3.160 | 0.002 | 1.315 to 3.227 |
| Sex (male/female) | 1.672 | 0.861 | 1.000 | 0.319 | 0.609 to 4.588 |
| Age | 1.826 | 0.206 | 5.330 | 0.000 | 1.463 to 2.278 |
| Constant | 0.000 | 0.000 | –7.740 | 0.000 | 0.000 to 0.003 |
| Behaviour | Exposure group | Sugary drink consumed in hour before bed? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sugary drinks consumed in hour before bed | No WF | No | – | 455 (99.8) | 371 (99.5) | 325 (98.8) | 182 (96.3) | 129 (92.8) | 194 (91.5) |
| Yes | – | 1 (0.2) | 2 (0.5) | 4 (1.2) | 7 (3.7) | 10 (7.2) | 18 (8.5) | ||
| WF | No | – | 220 (100) | 194 (98.0) | 142 (96.0) | 90 (97.8) | 70 (95.6) | 115 (83.9) | |
| Yes | – | 0 (0) | 4 (2.0) | 6 (4.0) | 2 (2.2) | 3 (4.1) | 22 (16.1) | ||
Tables 80 and 81 show the GEEs and descriptive data for if a snack containing free sugars is consumed in the hour before bed over time (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time for if a snack containing free sugars is consumed in the hour before bed.
| Behaviour: snack containing free sugars consumed in the hour before bed | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.584 | 0.487 | 1.500 | 0.135 | 0.867 to 2.894 |
| Deprivation quintile | 1.081 | 0.112 | 0.750 | 0.453 | 0.883 to 1.323 |
| Sex (male/female) | 0.791 | 0.243 | –0.760 | 0.444 | 0.433 to 1.444 |
| Age | 1.218 | 0.119 | 2.030 | 0.042 | 1.007 to 1.474 |
| Constant | 0.044 | 0.019 | –7.290 | 0.000 | 0.019 to 0.102 |
| Behaviour | Exposure group | Snack containing free sugar consumed in hour before bed? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Snack containing free sugar consumed in hour before bed | No WF | No | – | 439 (96.3) | 346 (92.8) | 301 (91.5) | 181 (95.8) | 133 (97.1) | 167 (79.2) |
| Yes | – | 17 (3.7) | 27 (7.2) | 28 (8.5) | 8 (4.2) | 4 (2.9) | 44 (21.9) | ||
| WF | No | – | 207 (94.1) | 173 (87.4) | 133 (89.9) | 75 (83.3) | 66 (90.4) | 97 (71.8) | |
| Yes | – | 13 (5.9) | 25 (12.6) | 15 (10.1) | 57 (16.7) | 7 (9.6) | 40 (29.2) | ||
Tables 82 and 83 show the GEE and descriptive data for how many times a participant brushes their teeth a day over time (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time in the number of times a participant brushes their teeth a day.
| Behaviour: brushing at least twice a day | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.856 | 0.171 | –0.780 | 0.436 | 0.579 to 1.266 |
| Deprivation quintile | 1.092 | 0.090 | 1.070 | 0.287 | 0.929 to 1.282 |
| Sex (male/female) | 1.056 | 0.201 | 0.290 | 0.774 | 0.728 to 1.532 |
| Age | 0.590 | 0.039 | –7.930 | 0.000 | 0.518 to 0.672 |
| Constant | 0.989 | 0.287 | –0.040 | 0.971 | 0.561 to 1.747 |
| Behaviour | Exposure group | Number of times a participant brushes their teeth a day | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Brushing habits (frequency) | No WF | Twice a day | – | 67 (45.3) | 197 (55.0) | 245 (65.5) | 202 (70.6) | 191 (80.9) | 262 (87.9) |
| Less than twice a day | – | 81 (54.7) | 161 (45.0) | 129 (34.5) | 84 (29.4) | 45 (19.1) | 36 (12.1) | ||
| WF | Twice a day | – | 35 (44.3) | 113 (58.6) | 112 (67.7) | 116 (80.1) | 111 (84.7) | 155 (80.7) | |
| Less than twice a day | – | 44 (55.7) | 80 (41.5) | 61 (35.3) | 28 (19.4) | 20 (15.3) | 37 (19.3) | ||
Tables 84 and 85 show the GEEs and descriptive data for whether a child spits or rinses out their toothpaste (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over whether a child spits or rinses out their toothpaste.
| Behaviour: spitting or rinsing after brushing | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.838 | 0.182 | –0.810 | 0.415 | 0.548 to 1.282 |
| Deprivation quintile | 1.224 | 0.106 | 2.330 | 0.020 | 1.033 to 1.451 |
| Sex (male/female) | 1.449 | 0.308 | 1.740 | 0.081 | 0.955 to 2.198 |
| Age | 1.073 | 0.064 | 1.180 | 0.240 | 0.954 to 1.207 |
| Constant | 0.131 | 0.047 | –5.690 | 0.000 | 0.065 to 0.264 |
| Behaviour | Exposure group | Brushing behaviour | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Brushing habits (frequency) | No WF | Spit/does not rinse out | – | – | – | 263 (74.5) | 199 (70.8) | 158 (67.5) | 215 (68.5) |
| Rinse | – | – | – | 90 (25.5) | 82 (29.2) | 76 (32.5) | 99 (31.5) | ||
| WF | Spit/does not rinse out | – | – | – | 127 (74.7) | 95 (67.9) | 97 (75.2) | 138 (68.3) | |
| Rinse | – | – | – | 43 (25.3) | 45 (32.1) | 32 (24.8) | 64 (31.7) | ||
Tables 86 and 87 show the GEEs and descriptive data for if a child was breastfed over time (5 years – seven data points). There is no difference between the exposure groups (WF vs. no WF) over time in whether or not a child was breastfed (at each time point).
| Behaviour: if a child is breastfed | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.005 | 0.206 | 0.020 | 0.982 | 0.673 to 1.500 |
| Deprivation quintile | 0.893 | 0.070 | –1.450 | 0.148 | 0.766 to 1.041 |
| Sex (male/female) | 0.973 | 0.187 | –0.140 | 0.886 | 0.668 to 1.418 |
| Age | 0.083 | 0.016 | –12.650 | 0.000 | 0.057 to 0.122 |
| Constant | 3.813 | 1.095 | 4.660 | 0.000 | 2.172 to 6.696 |
| Behaviour | Exposure group | Is the child breastfed? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Breastfed now (at time of questionnaire) | No WF | No | 174 (40.8) | 243 (67.3) | 233 (79.5) | 324 (93.6) | 264 (97.8) | 213 (97.7) | – |
| Yes | 253 (59.2) | 118 (32.7) | 60 (20.5) | 22 (6.4) | 6 (2.2) | 5 (2.3) | – | ||
| WF | No | 97 (42.5) | 100 (61.6) | 113 (76.4) | 150 (92.0) | 126 (94.0) | 113 (95.0) | – | |
| Yes | 131 (57.5) | 66 (38.4) | 35 (23.7) | 13 (8.0) | 8 (6.0) | 6 (5.0) | – | ||
Tooth eruption
The number of unerupted primary teeth was explored to determine if there was a difference in tooth eruption between the fluoridated and non-fluoridated groups. This was explored both in the 3-year-old data and in the 5-year-old data, using negative binomial regression to include both age and exposure status. There were no significant between-group differences in tooth eruption among either 3-year-olds [incidence rate ratio (IRR) 1.515, 95% CI 0.492 to 4.669] or 5-year-olds (IRR 1.001, 95% CI 0.977 to 1.025) (Table 88).
| Age (years) | Area: non WF vs. WF against count of unerupted primary teeth, IRR (95% CI) |
|---|---|
| 3 | 1.515 (0.492 to 4.669) |
| 5 | 1.001 (0.977 to 1.025) |
Primary outcome
Table 89 shows the results of the logistic regression for primary decay by exposure status (area), deprivation quintiles, age and sex.
| Regression analysis (n = 1333) | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.74 (0.55 to 0.98) |
| Deprivation quintile (reference 1) | |
| 2 | 0.84 (0.44 to 1.61) |
| 3 | 1.27 (0.70 to 2.30) |
| 4 | 1.47 (0.83 to 2.61) |
| 5 | 2.18 (1.23 to 3.90) |
| Age (centred) | 1.51 (0.99 to 2.29) |
| Sex (male/female) | 0.73 (0.55 to 0.96) |
The categorical variable ‘deprivation’ is statistically significant [χ2(4) = 19.92; p = 0.0005]. From the model, there is a negative association between fluoridation and decay. The odds of decay for children from a fluoridated area are 74% of the odds of decay for children from a non-fluoridated area. The 95% CI ranges from 0.56 to 0.98, with a p-value of 0.038.
Figure 17 demonstrates the difference in probabilities of margins data for those experiencing no decay or any decay, showing the difference across deprivation quintiles between participants living in a fluoridated area and a non-fluoridated area, while holding age at 5 years old. Figures 18 and 19 also show these data split by sex.
FIGURE 17.
Adjusted predictions of decay or no decay by area across deprivation quintiles with 95% CI, while holding age at 5 years old.
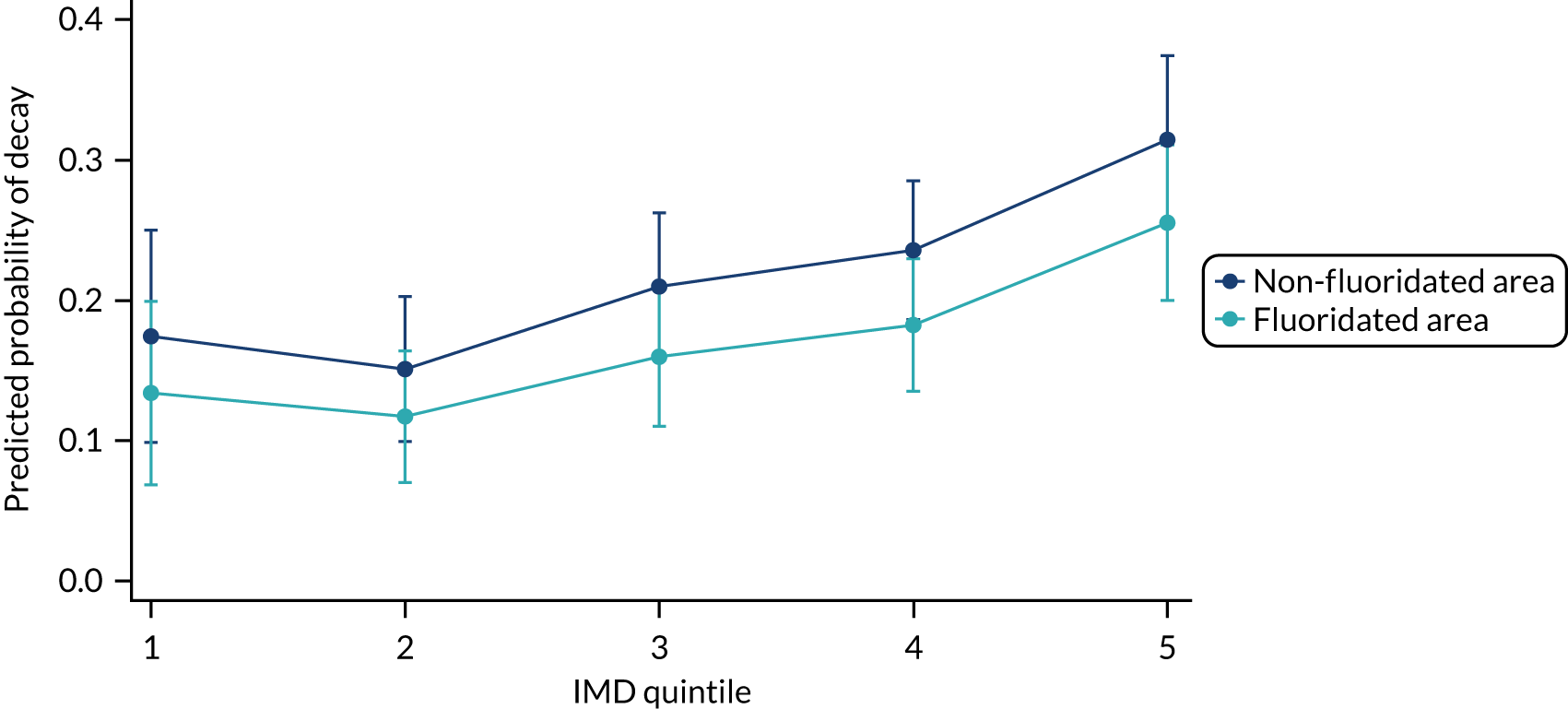
FIGURE 18.
Adjusted predictions of decay or no decay by area across deprivation quintiles with 95% CI, while holding age at 5 years old: males only.
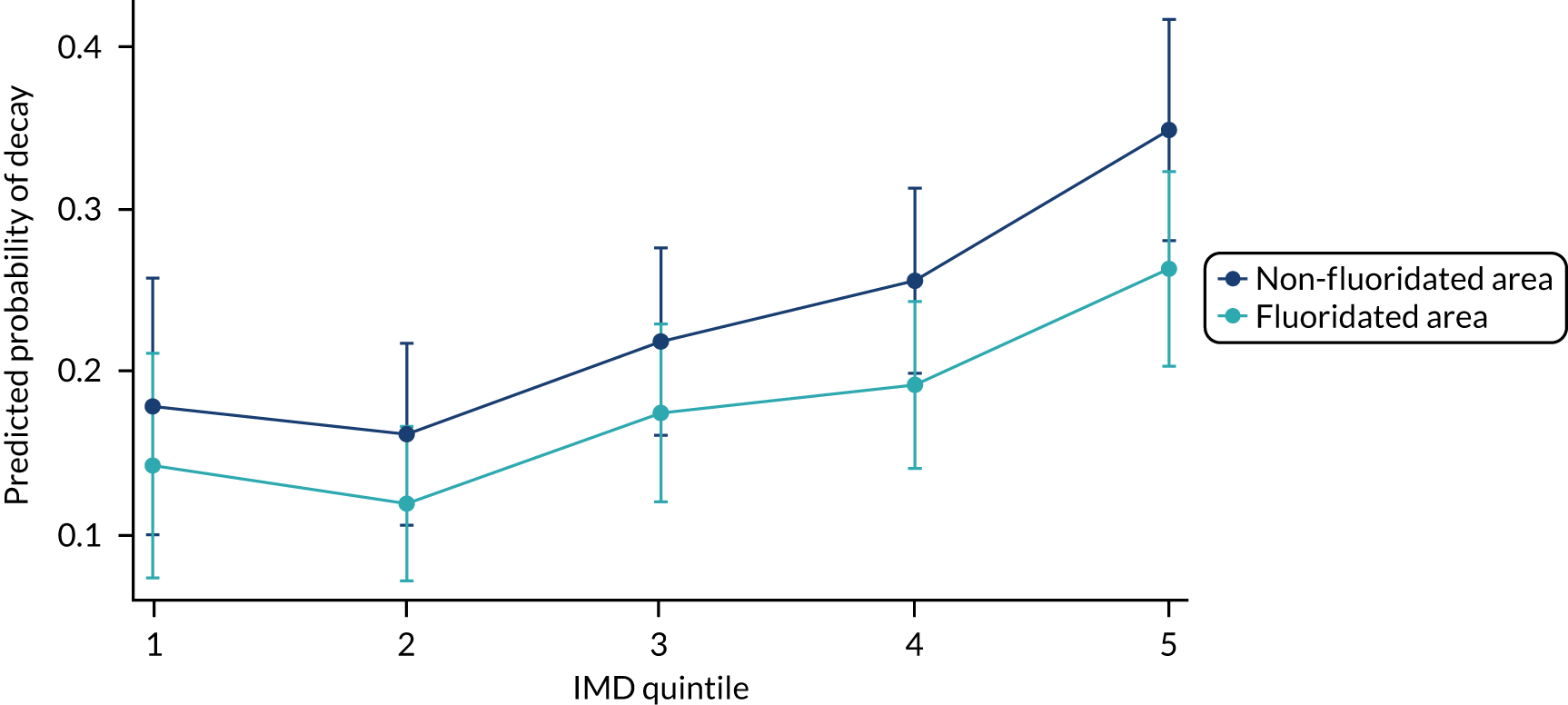
FIGURE 19.
Adjusted predictions of decay or no decay by area across deprivation quintiles with 95% CI, while holding age at 5 years old: females only.
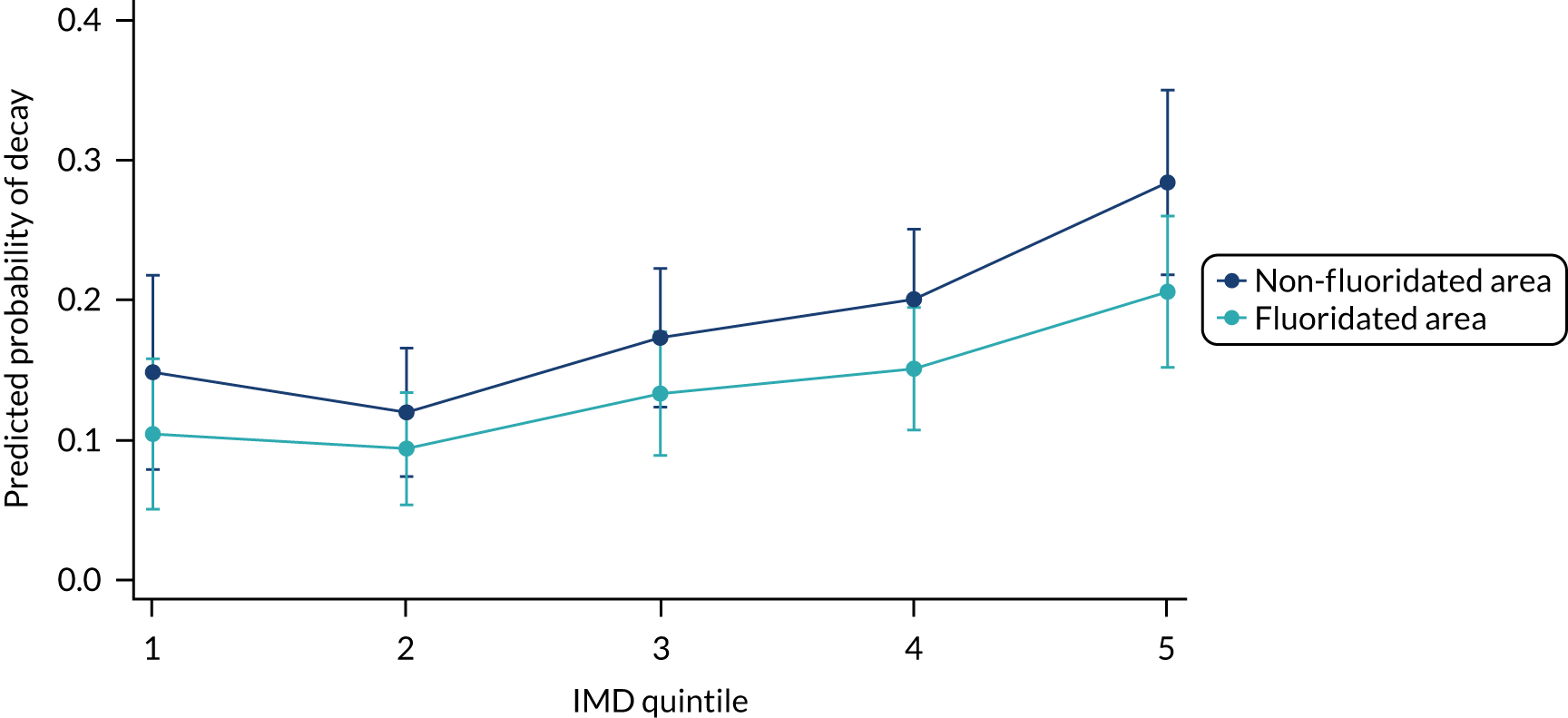
Table 90 shows the predictive probability along with the CIs, and displays the same data that are in Figure 17. These data show the predictive probability of decay at each level of deprivation, holding all other variables in the model at their means. The predicted probability of decay for deprivation quintile 1 in a fluoridated area is 0.134 (holding other variables at its mean). The predicted probability for deprivation quintile 2 in a fluoridated area is slightly lower at 0.117; however, after this it then increases for each deprivation quintile until deprivation quintile 5 (0.255) and so predicted probability at deprivation quintile 5 is 1.90 times the predicted probability for deprivation quintile 1 in the fluoridated area.
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Deprivation quintile 1 | ||
| Non-fluoridated area | 0.174 | 0.099 to 0.250 |
| Fluoridated area | 0.134 | 0.069 to 0.199 |
| Deprivation quintile 2 | ||
| Non-fluoridated area | 0.151 | 0.099 to 0.203 |
| Fluoridated area | 0.117 | 0.070 to 0.164 |
| Deprivation quintile 3 | ||
| Non-fluoridated area | 0.210 | 0.157 to 0.262 |
| Fluoridated area | 0.160 | 0.110 to 0.209 |
| Deprivation quintile 4 | ||
| Non-fluoridated area | 0.236 | 0.186 to 0.285 |
| Fluoridated area | 0.182 | 0.135 to 0.230 |
| Deprivation quintile 5 | ||
| Non-fluoridated area | 0.314 | 0.254 to 0.374 |
| Fluoridated area | 0.255 | 0.200 to 0.310 |
Table 91 shows the results of the logistic regression for where the exposure area is reversed to provide the adjusted OR when the intervention is the reference. The results of the logistic regression showed that individuals living in a non-fluoridated area were 1.36 times more likely to develop caries than individuals in the fluoridated area (conditional on the values of the covariates) (95% CI 1.02 to 1.81).
| Regression analysis (n = 1333) | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 1.36 (1.02 to 1.81) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 0.84 (0.44 to 1.61) |
| 3 | 1.27 (0.70 to 2.30) |
| 4 | 1.47 (0.83 to 2.61) |
| 5 | 2.18 (1.23 to 3.90) |
| Age (centred) | 1.51 (0.99 to 2.29) |
| Sex (male/female) | 0.73 (0.55 to 0.96) |
Secondary outcomes
Secondary outcome: dental general anaesthetic for extractions
A logistic regression was performed on DGA experience by exposure group, including deprivation and sex as covariates. No significant effect was found for exposure groups on experience of DGA when including deprivation and sex as covariates (OR 0.759, 95% CI 0.397 to 1.468) (Tables 92 and 93).
| Exposure status | DGA experience, n (%) | |
|---|---|---|
| No | Yes | |
| No WF | 1004 (97.2) | 29 (2.8) |
| WF | 648 (97.9) | 14 (2.1) |
| Logistic regression | OR | p-value | 95% CI |
|---|---|---|---|
| Fluoridation | 0.764 | 0.419 | 0.397 to 1.468 |
| Deprivation quintile (reference quintile 1) | |||
| 2 | 1.131 | 0.857 | 0.295 to 4.340 |
| 3 | 1.248 | 0.737 | 0.342 to 4.553 |
| 4 | 1.113 | 0.870 | 0.309 to 4.012 |
| 5 | 0.969 | 0.963 | 0.258 to 3.652 |
| Sex | 0.777 | 0.423 | 0.420 to 1.439 |
| Constant | 0.029 | 0.000 | 0.009 to 0.095 |
Secondary outcome: dmft
A negative binomial regression was performed on the count of decay (dmft) and included exposure status (area), deprivation quintiles, age and sex as covariates and offset for erupted teeth (Table 94).
| Regression analysis (n = 1333): dmft count | IRR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.61 (0.44 to 0.86) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.05 (0.50 to 2.19) |
| 3 | 1.70 (0.85 to 3.39) |
| 4 | 1.86 (0.95 to 3.66) |
| 5 | 2.76 (1.38 to 5.51) |
| Age (centred) | 1.84 (1.08 to 3.13) |
| Sex | 0.79 (0.57 to 1.10) |
| Log of erupted teeth (offset) | 1 (offset) |
The categorical variable ‘deprivation’ is statistically significant [χ2(4) = 16.31; p = 0.0026]. Individuals living in a fluoridated area, when compared with individuals in a non-fluoridated area and while holding the other variables constant in the model, are expected to have a rate 0.61 times less for the number of dmft.
Following on from Table 94, Table 95 details the predictive probabilities. We used the margins command to calculate the predicted counts at each level of deprivation, holding all other variables in the model at their means. The predicted number of events (dmft) for deprivation quintile 1 in a fluoridated area is 0.265 (holding other variables at its mean). The predicted number of events for deprivation quintile 2 in a fluoridated area is slightly higher, at 0.288, and this increases for each deprivation quintile until deprivation quintile 5 (0.771). Therefore, the predicted count at deprivation 5 quintile is 2.91 times the predicted count for deprivation quintile 1 in the fluoridated area.
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Deprivation quintile 1 | ||
| Non-fluoridated area | 0.439 | 0.166 to 0.712 |
| Fluoridated area | 0.265 | 0.089 to 0.441 |
| Deprivation quintile 2 | ||
| Non-fluoridated area | 0.478 | 0.272 to 0.684 |
| Fluoridated area | 0.288 | 0.144 to 0.431 |
| Deprivation quintile 3 | ||
| Non-fluoridated area | 0.744 | 0.466 to 0.102 |
| Fluoridated area | 0.449 | 0.256 to 0.641 |
| Deprivation quintile 4 | ||
| Non-fluoridated area | 0.836 | 0.553 to 1.118 |
| Fluoridated area | 0.507 | 0.316 to 0.698 |
| Deprivation quintile 5 | ||
| Non-fluoridated area | 1.268 | 0.801 to 1.735 |
| Fluoridated area | 0.771 | 0.494 to 1.047 |
Figure 20 is a visual representation of Table 95. Figures 21 and 22 also show these data split by sex. A similar pattern can be seen for both male and female participants across the deprivation quintiles.
FIGURE 20.
Predictive margins of decay by area across deprivation quintiles at 5 years old with 95% CI.
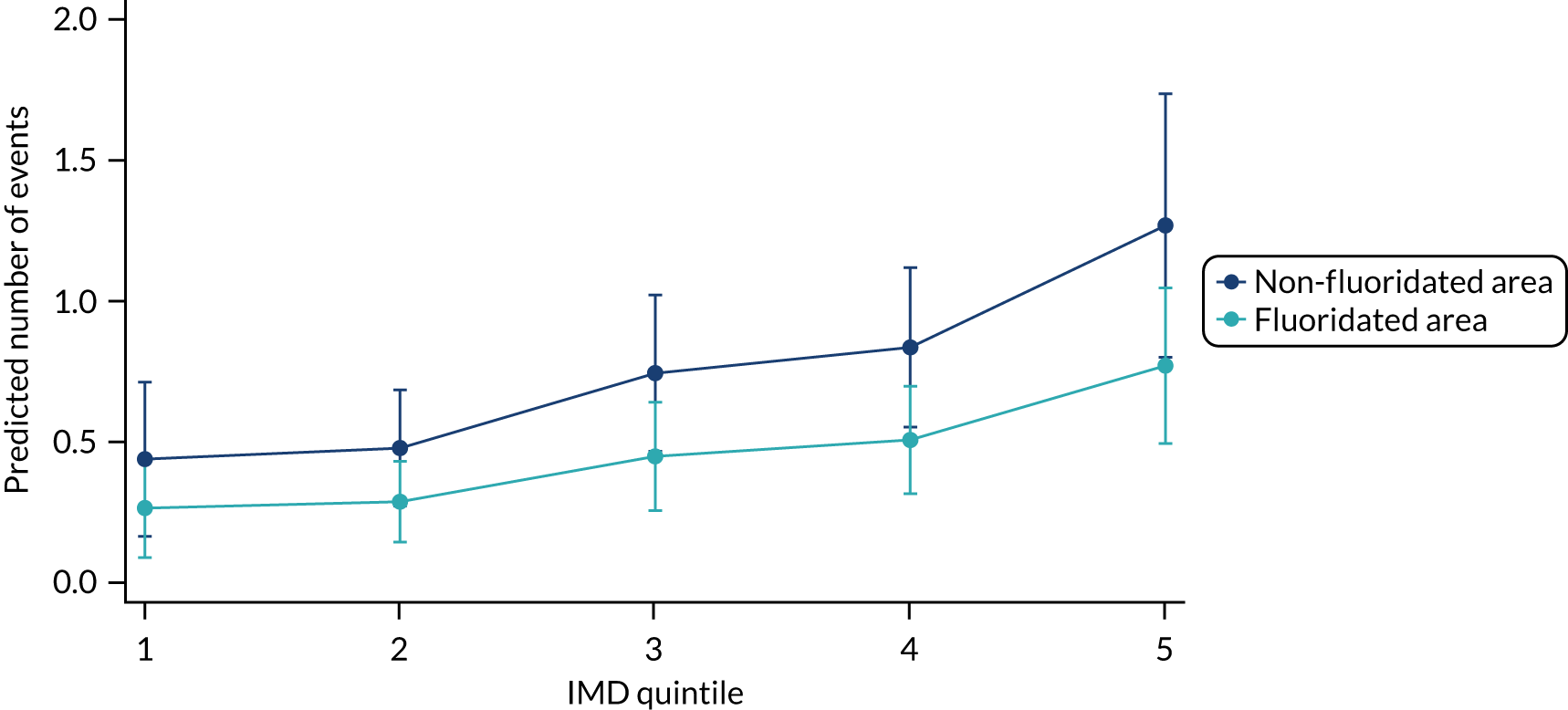
FIGURE 21.
Predictive margins of decay by area across deprivation quintiles at 5 years old with 95% CI: males only.
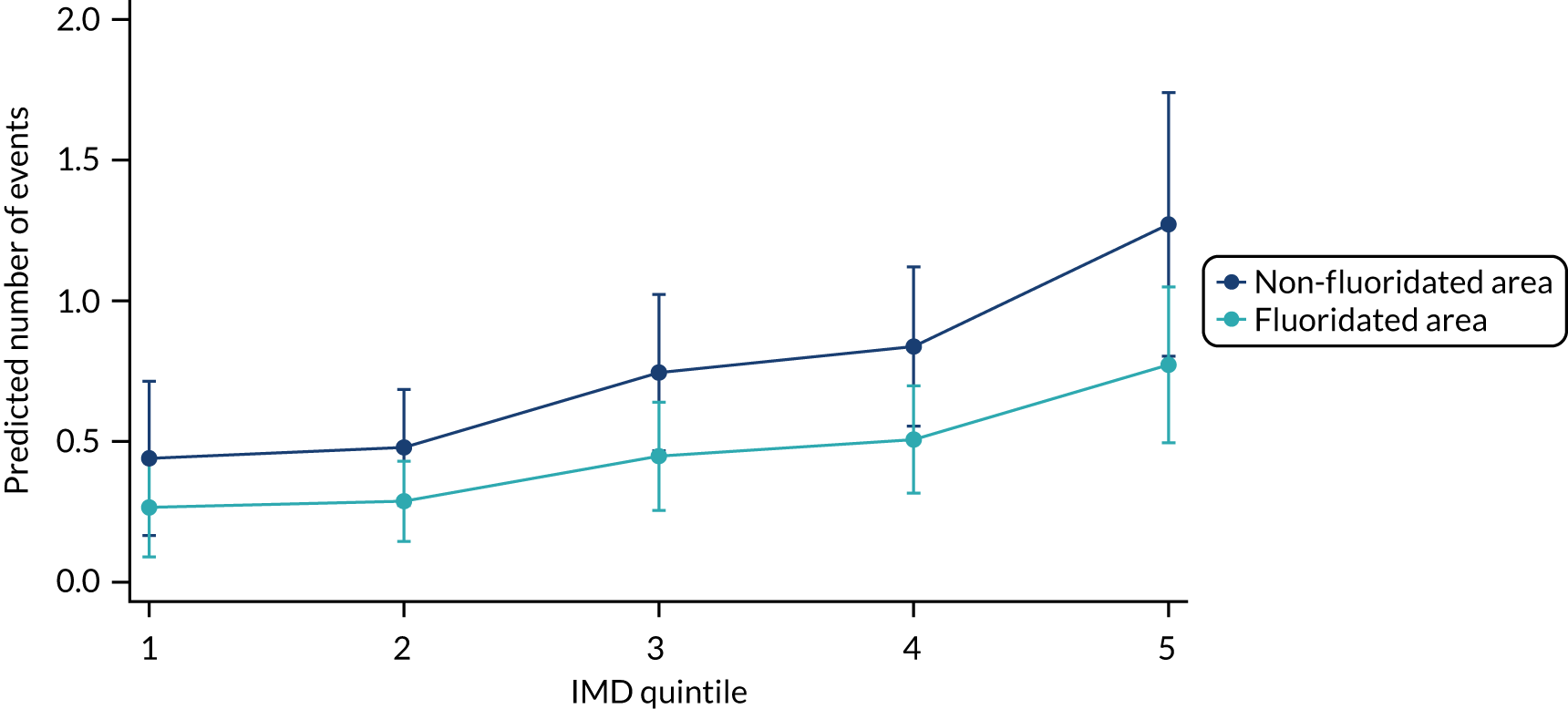
FIGURE 22.
Predictive margins of decay by area across deprivation quintiles at 5 years old with 95% CI: females only.
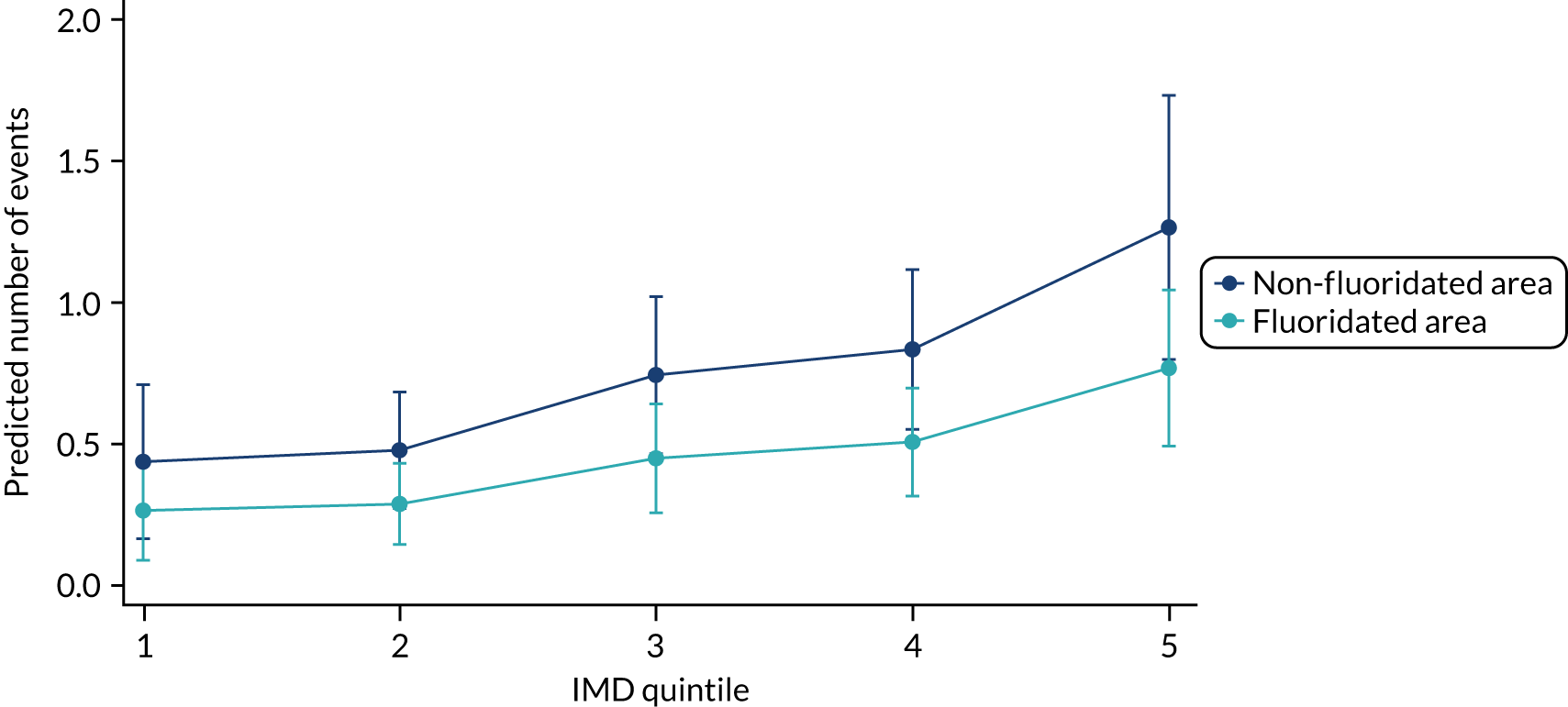
Secondary outcome: dental-related problem (questionnaire data)
Tables 96 and 97 detail the questionnaire responses to if a child had a dental problem, including problems with pain, talking, eating, sleeping or other, in the last 12 months (recorded by the parent).
| Outcome: dental problem in last 12 months | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.887 | 0.466 | –0.230 | 0.819 | 0.317 to 2.483 |
| Deprivation quintile | 1.352 | 0.270 | 1.510 | 0.131 | 0.914 to 2.001 |
| Sex (male/female) | 1.291 | 0.645 | 0.510 | 0.609 | 0.485 to 3.436 |
| Age | 1.783 | 0.224 | 4.610 | 0.000 | 1.394 to 2.280 |
| Constant | 0.001 | 0.001 | –8.420 | 0.000 | 0.000 to 0.006 |
| Outcome | Exposure group | Dental problem in last 12 months? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Dental problem in last 12 months (recorded on questionnaire) | No WF | No | – | 499 (99.2) | 402 (99.8) | 371 (99.2) | 281 (98.9) | 228 (97.0) | 296 (92.8) |
| Yes | – | 4 (0.8) | 1 (0.2) | 3 (0.8) | 3 (1.1) | 7 (3.0) | 23 (7.2) | ||
| WF | No | – | 251 (99.6) | 212 (99.5) | 173 (100) | 138 (96.5) | 128 (97.7) | 195 (96.5) | |
| Yes | – | 1 (0.4) | 1 (0.5) | 0 (0) | 5 (3.5) | 3 (2.3) | 7 (3.5) | ||
Secondary outcome: chronic illness – self-reported (questionnaire data)
Tables 98 and 99 detail questionnaire responses to if a child had a chronic illness, defined as a long-lasting illness (recorded by the parent). There was no difference between exposure groups (WF vs. no WF) over time for if a child had a chronic illness.
| Outcome: chronic illness recorded on questionnaire | IRR | Robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.843 | 0.277 | –0.52 | 0.602 | 0.443 to 1.604 |
| Deprivation quintile | 1.169 | 0.136 | 1.34 | 0.179 | 0.931 to 1.469 |
| Sex (male/female) | 0.999 | 0.315 | 0.00 | 0.997 | 0.538 to 1.86 |
| Age | 1.082 | 0.105 | 0.82 | 0.412 | 0.895 to 1.310 |
| Constant | 0.025 | 0.014 | –6.93 | 0.000 | 0.009 to 0.072 |
| Outcome | Exposure group | Chronic illness recorded? | Wave, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Chronic illness (recorded on questionnaire) | No WF | No | 546 (98.0) | 450 (94.9) | 355 (91.7) | 353 (97.3) | 267 (97.1) | 216 (96.9) | 274 (88.1) |
| Yes | 11 (2.0) | 24 (5.06) | 32 (8.3) | 10 (2.8) | 8 (2.9) | 7 (3.1) | 37 (11.9) | ||
| WF | No | 315 (97.8) | 234 (93.6) | 194 (93.3) | 168 (98.3) | 137 (97.9) | 131 (100) | 174 (87.9) | |
| Yes | 7 (2.2) | 16 (6.4) | 14 (6.7) | 3 (1.8) | 3 (2.1) | 0 (0) | 24 (12.1) | ||
Secondary outcome: additional analysis from NHS Business Services Authority data
Data from the NHS BSA were explored to determine if there were any differences in decay between the fluoridated and non-fluoridated groups at this age. To determine if the data available from NHS BSA were from a similar group as the full CATFISH data set, the distribution of deprivation was explored. For the NHS BSA data, the proportion of deprivation in each quintile at 3 years old followed a similar pattern to the deprivation quintiles for those involved in the final clinical examinations (Table 100).
| Deprivation quintile | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| 1 | 54 (9.9) | 20 (6.4) | 74 (8.6) |
| 2 | 123 (22.5) | 48 (15.4) | 171 (19.9) |
| 3 | 136 (24.8) | 66 (21.2) | 202 (23.5) |
| 4 | 141 (25.7) | 91 (29.3) | 232 (27.0) |
| 5 | 94 (17.2) | 86 (27.7) | 180 (20.9) |
| Total | 548 | 311 | 859 |
This follows a similar distribution to what we see in the final data for 5-year-olds (from the clinical data set), with slightly higher deprivation in the WF group (Table 101).
| Exposure status (n = 1421) | Exposure group, n (%) | |
|---|---|---|
| No WF | WF | |
| No decay (dmft) | 466 (84.88) | 259 (83.28) |
| Decay (dmft) | 83 (15.12) | 52 (16.72) |
There was no significant difference in decay experience between groups at 3 years old (see Table 101).
A logistic regression was performed with exposure, deprivation and sex. Note that age could not be entered, as data could have been taken across a number of appointments; however, all children would have been 3 years of age at the time (Table 102).
| Regression analysis (n = 859): dmft | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 1.04 (0.705 to 1.525) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.045 (0.473 to 2.313) |
| 3 | 0.780 (0,503, to 1.737) |
| 4 | 1.289 (0.608 to 2.733) |
| 5 | 1.818 (0.852 to 3.883) |
| Sex | 1.022 (0.705 to 1.482) |
Health inequalities: interaction of deprivation and water fluoridation
A logistic regression with an interaction (i.e. area by deprivation quintile) was carried out to explore the relationship between water exposure and the severity of caries, as indicated by decay/no decay (Table 103). Figures 23–25 and Tables 104 and 105 indicate that WF does not reduce inequalities. The interaction term is not significant, with no differential effect on decay according to deprivation.
| Regression analysis (n=1333) | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.350 (0.094 to 1.306) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 0.833 (0.401 to 1.729) |
| 3 | 1.095 (0.551 to 2.178) |
| 4 | 1.087 (0.555 to 2.129) |
| 5 | 1.690 (0.854 to 3.345) |
| Area by deprivation quintile interaction (reference WF by quintile 1) | |
| WF by quintile 2 | 1.029 (0.196 to 5.396) |
| WF by quintile 3 | 1.886 (0.436 to 8.155) |
| WF by quintile 4 | 2.815 (0.686 to 11.543) |
| WF by quintile 5 | 2.420 (0.593 to 9.879) |
| Age (centred) | 1.571 (1.027 to 2.404) |
| Sex | 0.726 (0.551 to 0.958) |
| Constant | 0.273 (0.150 to 0.494) |
FIGURE 23.
Adjusted predictions of decay or no decay including an interaction term for area across deprivation quintiles with 95% CI.
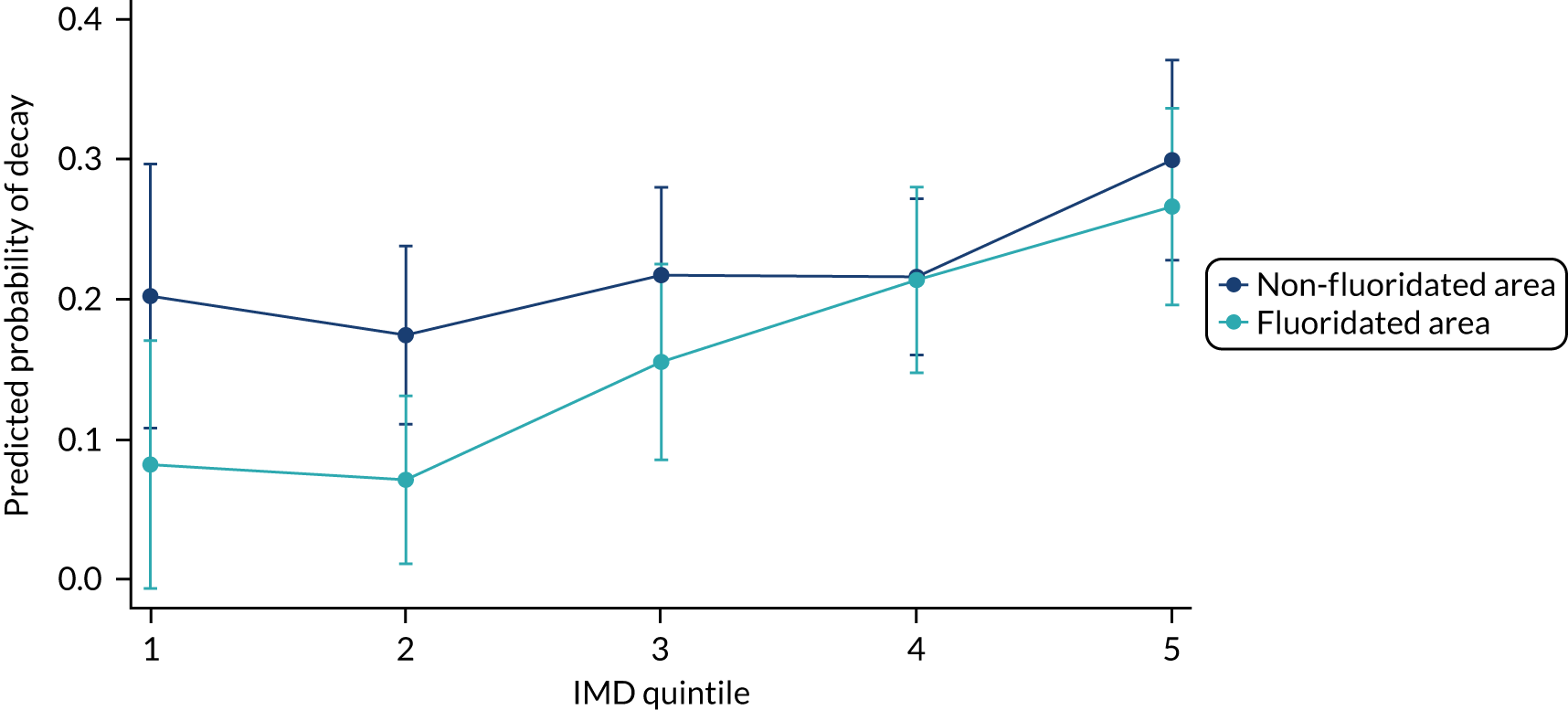
FIGURE 24.
Adjusted predictions of decay or no decay including an interaction term for area across deprivation quintiles with 95% CI: males only.
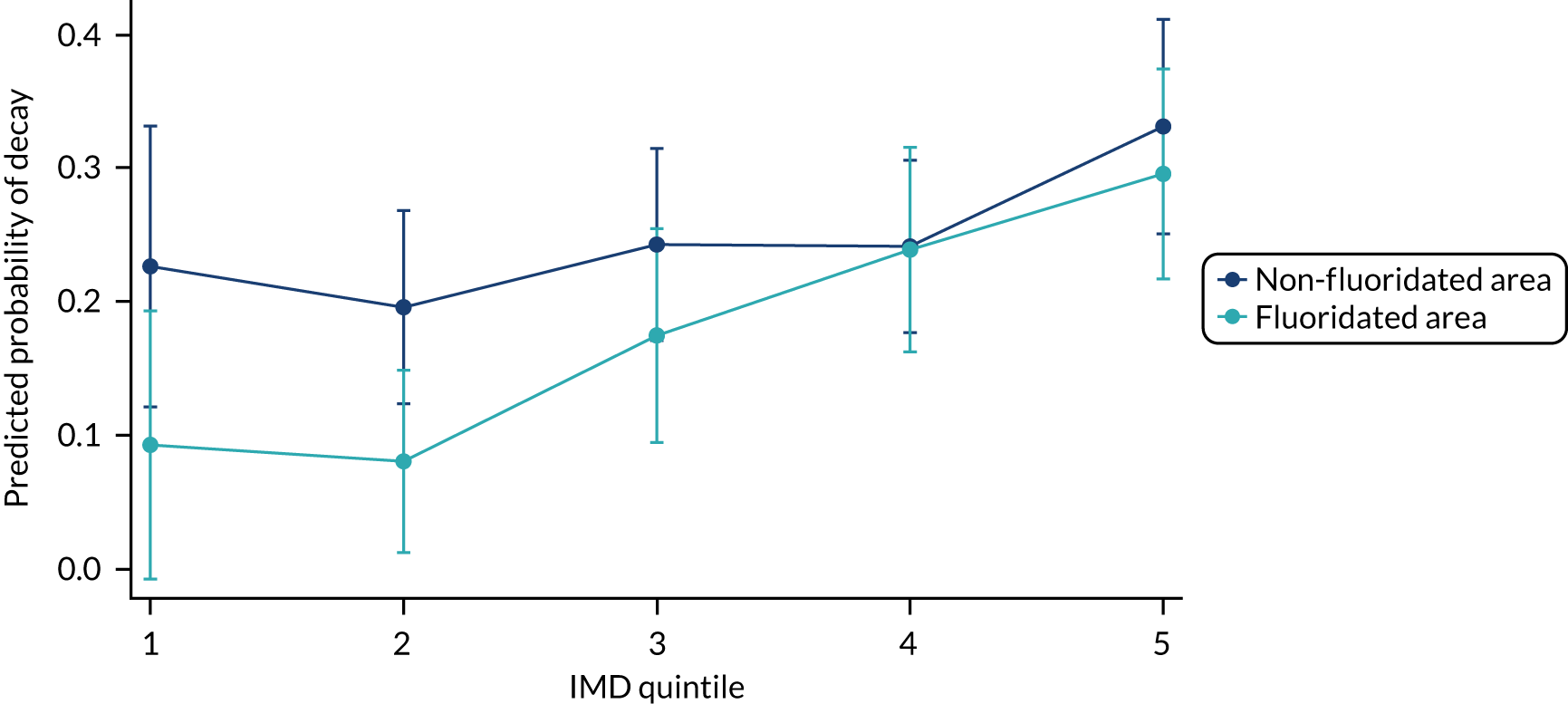
FIGURE 25.
Adjusted predictions of decay or no decay including an interaction term for area across deprivation quintiles with 95% CI: females only.
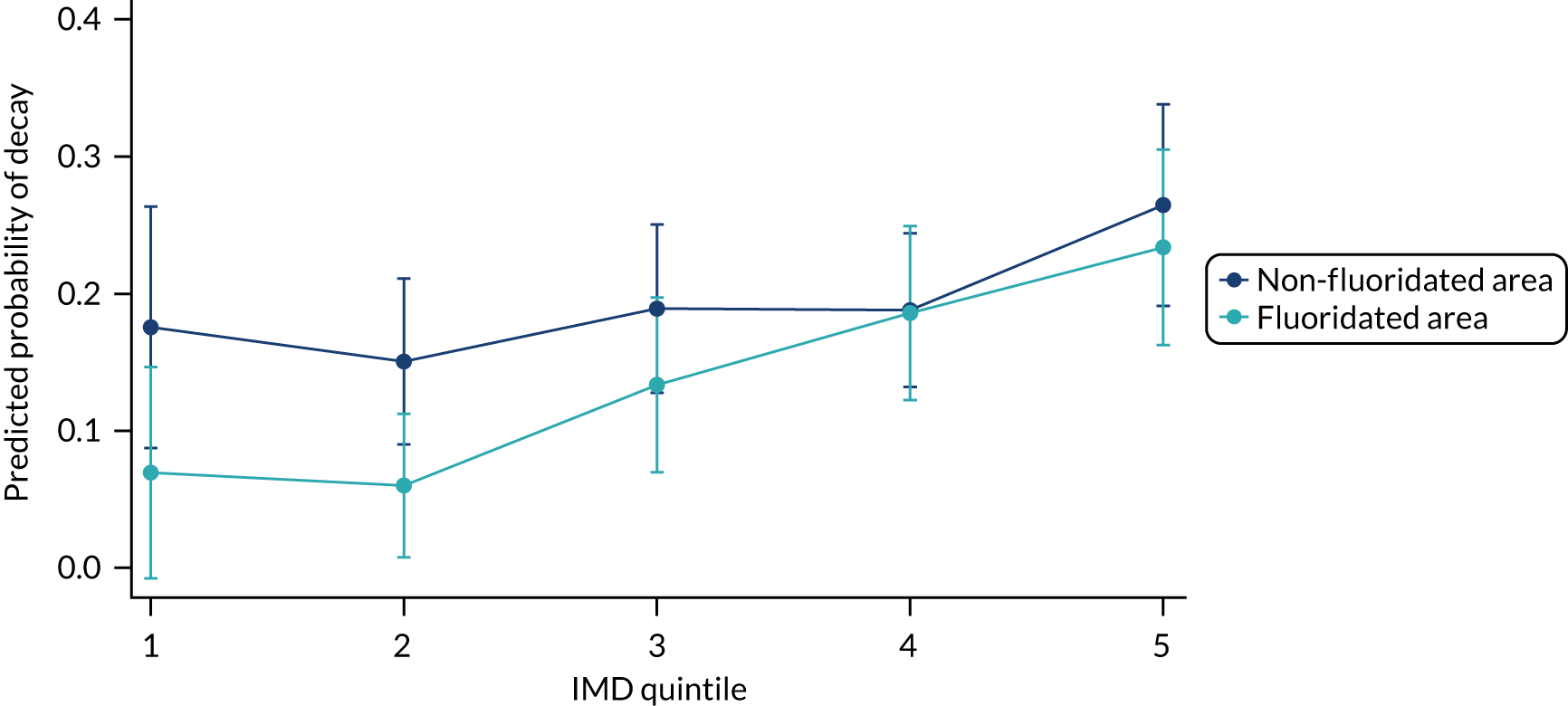
| Variable | df | F-value | p > F |
|---|---|---|---|
| Area (WF vs. no WF) | 1 | 7.36 | 0.0067 |
| Deprivation quintile | 4 | 21.83 | 0.0002 |
| Area by deprivation quintile | 4 | 4.78 | 0.3109 |
| Denominator | 1333 |
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Deprivation quintile 1 | ||
| Non-fluoridated area | 0.202 | 0.108 to 0.297 |
| Fluoridated area | 0.082 | –0.007 to 0.170 |
| Deprivation quintile 2 | ||
| Non-fluoridated area | 0.174 | 0.111 to 0.238 |
| Fluoridated area | 0.071 | 0.011 to 0.131 |
| Deprivation quintile 3 | ||
| Non-fluoridated area | 0.217 | 0.154 to 0.280 |
| Fluoridated area | 0.155 | 0.085 to 0.225 |
| Deprivation quintile 4 | ||
| Non-fluoridated area | 0.216 | 0.160 to 0.272 |
| Fluoridated area | 0.214 | 0.147 to 0.280 |
| Deprivation quintile 5 | ||
| Non-fluoridated area | 0.299 | 0.228 to 0.371 |
| Fluoridated area | 0.266 | 0.196 to 0.336 |
Table 104 demonstrates that there was no significant area by deprivation interaction overall.
A negative binomial regression was undertaken to assess if WF reduced health inequalities by examining the count of decay and the interaction between WF status and deprivation (Table 106). A table of contrasts (Table 107) for this negative binomial regression demonstrated a significant effect of both area and deprivation quintile, but no significant effect for area by deprivation quintile (interaction).
| Regression analysis (n = 1333) | IRR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.683 (0.187 to 2.494) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.199 (0.498 to 2.884) |
| 3 | 1.852 (0.799 to 4.294) |
| 4 | 1.87 (0.819 to 4.269) |
| 5 | 2.535 (1.074 to 5.985) |
| Area by deprivation quintile interaction (reference WF by quintile 1) | |
| WF by quintile 2 | 0.6134379 (0.124 to 3.027) |
| WF by quintile 3 | 0.7628808 (0.172 to 3.383) |
| WF by quintile 4 | 0.9520159 (0.227 to 3.985) |
| WF by quintile 5 | 1.10866 (0.264 to 4.664) |
| Age (centred) | 1.94 (1.122 to 3.355) |
| Sex (male/female) | 0.786 (0.562 to 1.1) |
| Log of erupted teeth (offset) | 1 |
| Variable | df | F-value | p > F |
|---|---|---|---|
| Area (WF vs. no WF) | 1 | 6.73 | 0.0095 |
| Deprivation quintile | 4 | 16.57 | 0.0023 |
| Area by deprivation quintile | 4 | 1.29 | 0.8639 |
| Denominator | 1333 |
Table 108 and Figure 26 show the margin probabilities for dmft, which includes an interaction term for exposure by deprivation quintiles. Table 108 and Figure 26 illustrate, again, that there is no significant interaction between deprivation and exposure when looking at decay, indicating that WF does not significantly reduce inequalities.
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Deprivation quintile 1 | ||
| Non-fluoridated area | 0.403 | 0.111 to 0.696 |
| Fluoridated area | 0.276 | –0.023 to 0.575 |
| Deprivation quintile 2 | ||
| Non-fluoridated area | 0.484 | 0.243 to 0.724 |
| Fluoridated area | 0.203 | 0.042 to 0.364 |
| Deprivation quintile 3 | ||
| Non-fluoridated area | 0.747 | 0.418 to 1.076 |
| Fluoridated area | 0.390 | 0.164 to 0.616 |
| Deprivation quintile 4 | ||
| Non-fluoridated area | 0.754 | 0.457 to 1.051 |
| Fluoridated area | 0.491 | 0.255 to 0.727 |
| Deprivation quintile 5 | ||
| Non-fluoridated area | 1.023 | 0.561 to 1.485 |
| Fluoridated area | 0.776 | 0.426 to 1.125 |
FIGURE 26.
Adjusted predictions of including an interaction term for area across deprivation quintiles with 95% CI.
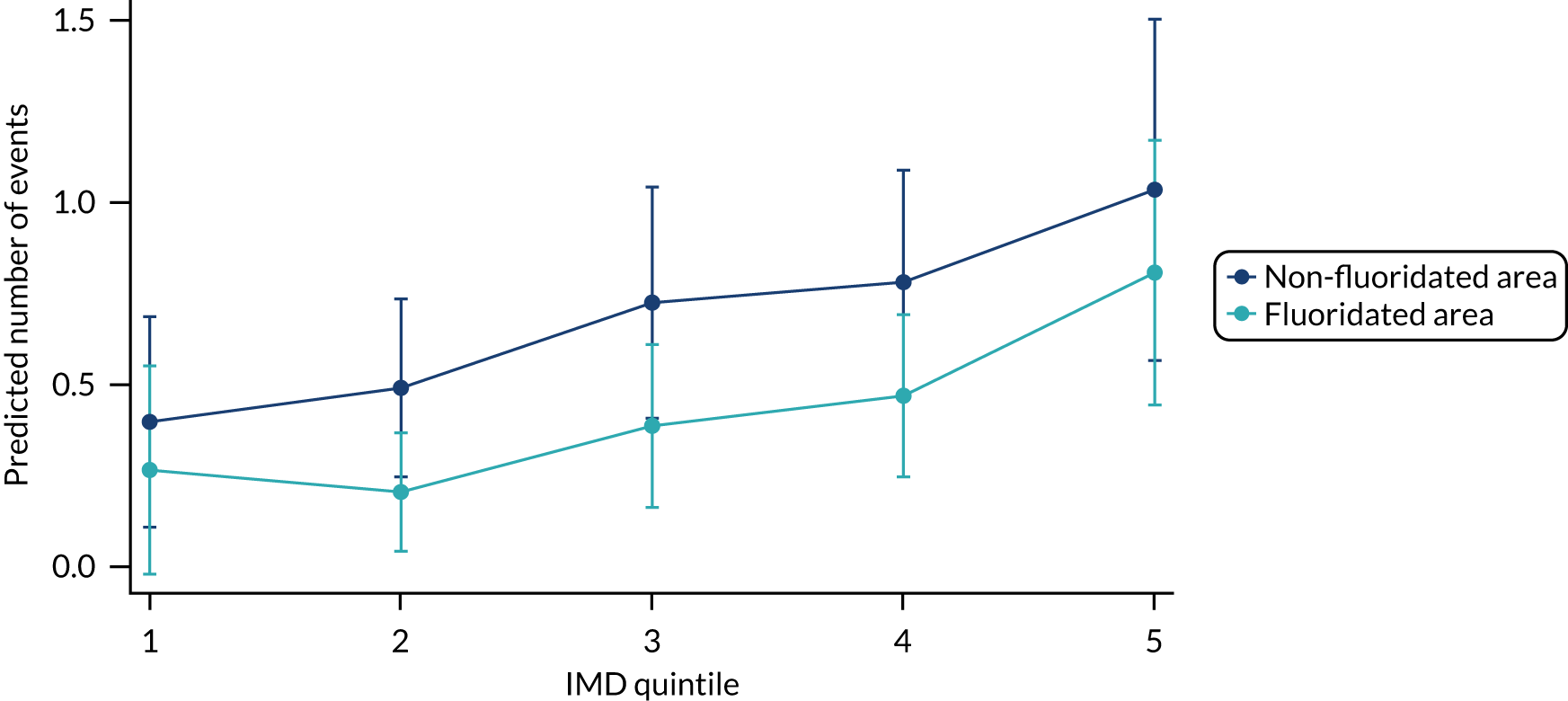
Figure 26 shows the resulting predictive probabilities for count of decay in primary teeth based on a model that included age, sex and an interaction term for exposure by deprivation quintiles for the median age at clinical assessment of 5 years and log of erupted teeth as an offset. The predictive probabilities are displayed separately for males and females in Figures 27 and 28.
FIGURE 27.
Adjusted predictions of including an interaction term for area across deprivation quintiles with 95% CI: males only.
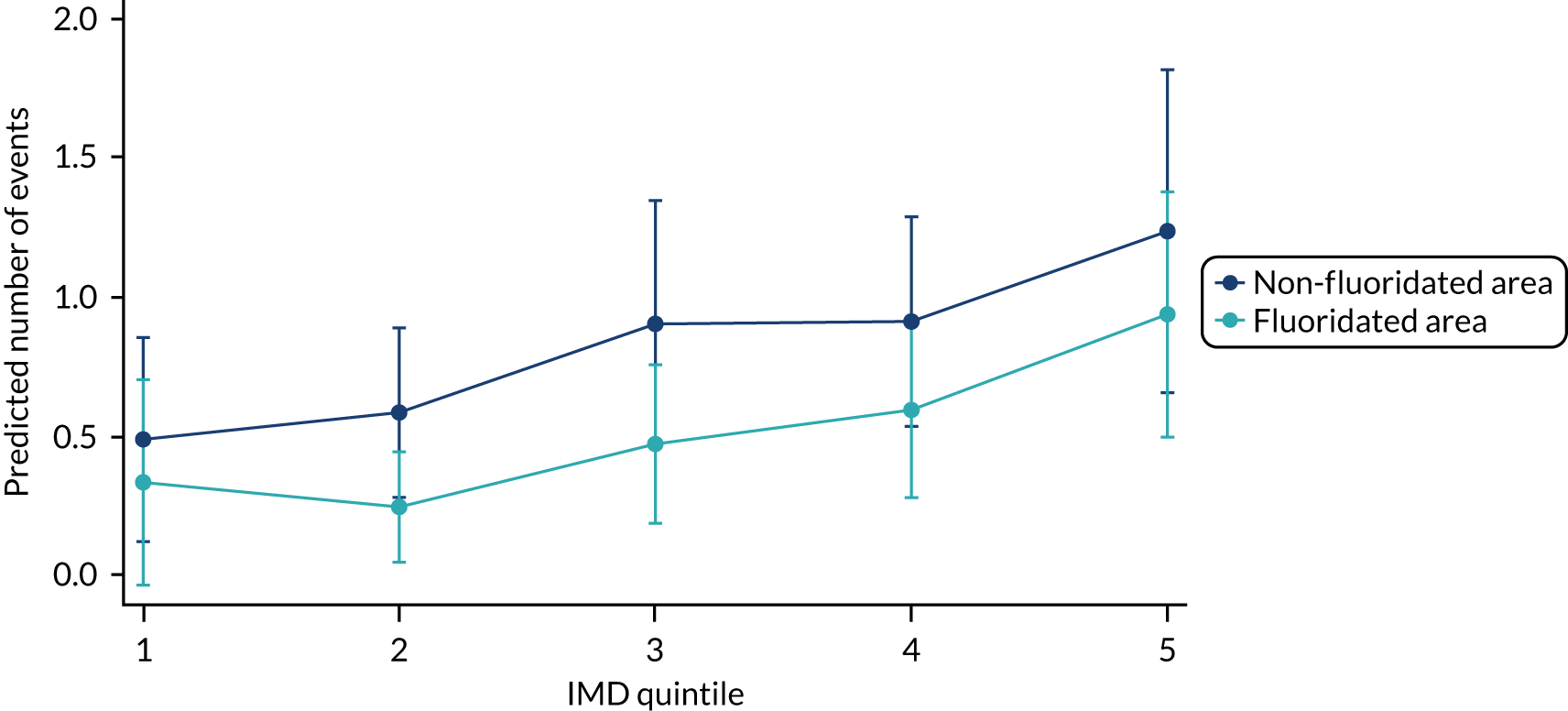
FIGURE 28.
Adjusted predictions of including an interaction term for area across deprivation quintiles with 95% CI: females only.
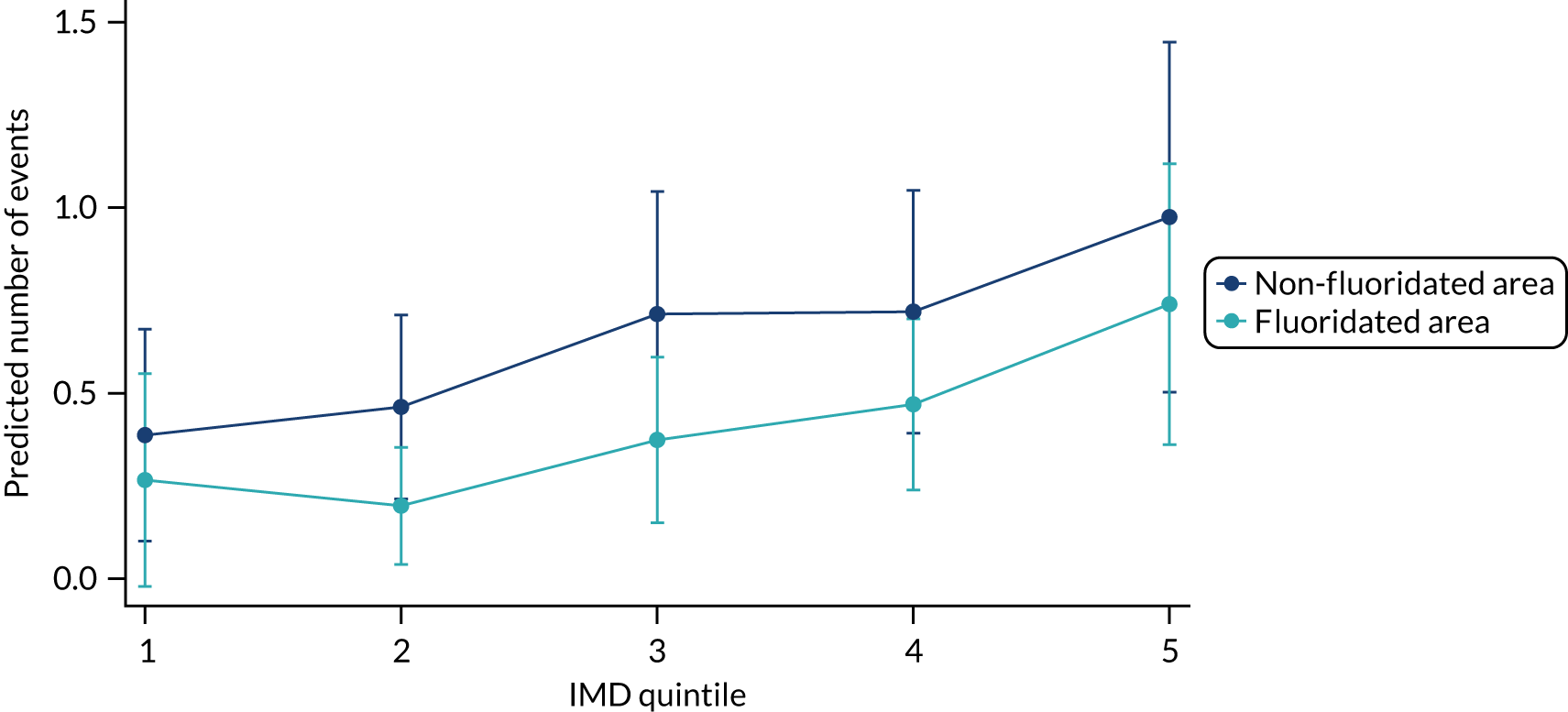
Post hoc analysis
There are three zones within Cumbria that are fluoridated. Zone 28 is fluoridated from Crummock (Cornhow), whereas zones 31 and 32 are fluoridated from Ennerdale. Post hoc analysis was undertaken because the fluoridation in Ennerdale experienced a significant interruption due to flooding in Cumbria, which resulted in fluoridation being suspended for a year.
Table 109 shows that similar numbers of individuals were examined across the three fluoridated zones in Cumbria in the final wave.
| Examination status | Total cohort (n = 2035) | Control (n = 1249) | Cornhow: zone 28 (n = 278) | Ennerdale: zones 31/32 (n = 505) |
|---|---|---|---|---|
| Examined | ||||
| n | 1444 | 835 | 218 | 391 |
| % | 71.0 | 66.7 | 78.4 | 77.4 |
| Absent | ||||
| n | 44 | 19 | 6 | 19 |
| % | 2 | 1.5 | 2.2 | 3.8 |
| Child refused | ||||
| n | 28 | 11 | 5 | 12 |
| % | 1.4 | 0.9 | 1.8 | 2.4 |
| Child moved | ||||
| n | 87 | 64 | 10 | 13 |
| % | 4.3 | 5.1 | 3.6 | 2.6 |
| Missed because of COVID-19 | ||||
| n | 172 | 164 | 4 | 4 |
| % | 8.5 | 13.1 | 1.4 | 0.8 |
| Withdrawn | ||||
| n | 144 | 94 | 19 | 31 |
| % | 7.1 | 7.5 | 6.8 | 6.1 |
| Removed for other reason | ||||
| n | 68 | 30 | 13 | 25 |
| % | 3.3 | 2.4 | 4.7 | 5.0 |
| Unable to trace | ||||
| n | 45 | 32 | 3 | 10 |
| % | 2.2 | 2.6 | 1.1 | 2.0 |
Table 110 indicates a distribution of demographics across the three fluoridated zones. For zone 28, a larger number of males, than females, were not examined (69% vs. 39%).
| Variable | Group A | Group B: zone 28 (N = 276) | Group B: zones 31/32 (N = 502) | |||
|---|---|---|---|---|---|---|
| Examined (n = 835) | Not examined (n = 388) | Examined (n = 218) | Not examined (n = 58) | Examined (n = 389) | Not examined (n = 113) | |
| Mean deprivation score (SD) | 23.33 (13.31) | 23.77 (13.00) | 26.51 (15.09) | 24.48 (11.94) | 28.38 (15.73) | 30.06 (16.33) |
| Variable | Group A | Group B: zone 28 (N = 277) | Group B: zones 31/32 (N = 504) | |||
| Examined (n = 835) | Not examined (n = 397) | Examined (n = 218) | Not examined (n = 59) | Examined (n = 391) | Not examined (n = 113) | |
| Sex | ||||||
| Male (%) | 448 (53.7) | 203 (51.1) | 106 (48.6) | 36 (61.0) | 206 (52.7) | 59 (52.2) |
| Female (%) | 387 (46.3) | 194 (48.9) | 112 (51.4) | 23 (39.0) | 185 (47.3) | 54 (47.8) |
Table 111 shows that only group B zone 28 showed a statistically significant effect, with the risk of having caries with optimal WF being 62% of the risk with no fluoridation (95% CI 0.43 to 0.89). The 95% CI does not include the null value and so there was a statistically significant effect.
| Caries status | Group A: control, n (%) | Group B: zone 28, n (%) | Group B: zones 31/32, n (%) | Total, n (%) |
|---|---|---|---|---|
| Decay present | 179 (21.4) | 29 (13.3) | 77 (19.7) | 285 (19.7) |
| Decay absent | 656 (78.6) | 189 (86.7) | 314 (80.3) | 1159 (80.3) |
| Total | 835 | 218 | 391 | 1444 |
Appendix 3 Older school cohort additional analysis
This appendix presents the older school cohort additional analysis for the CATFISH study. This appendix is divided into the following subsections:
-
-
This section provides additional analysis on examination status and any association with control/intervention groups or deprivation.
-
-
Longitudinal data analysis of potential effect modifiers collected by questionnaires –
-
This section details the behavioural data collected through questionnaires during the course of the study. Data analysis consists of GEEs to determine if any behaviours differ between the groups (i.e. control and intervention) over time.
-
-
-
Additional analysis linked to the primary outcome is presented here, including full logistic regression outputs.
-
-
-
Additional analysis linked to the secondary outcomes is presented here, including full logistic and negative binomial regressions and accompanying predictive probabilities.
-
-
Health inequalities: interaction of deprivation and waterfluoridation –
-
Additional analysis linked to the health inequalities data, which includes an interaction of deprivation quintiles and WF exposure within the regression, is presented here, including the accompanying predictive probabilities.
-
-
-
This section provides the analysis conducted outside the original protocol, following the unexpected interruption to dosing at one of the plants in Cumbria.
-
Examination status
Table 112 details the differences between individuals examined and not examined at the final examination (i.e. loss to follow-up) and this includes data collected at baseline for the whole sample and split by group. The data show that the proportions of males and females were similar in both groups. It can be observed that the proportion of people living in the most deprived area is higher in the WF group (31.9%) than in the no WF group (22.9%). Some differences were observed when looking at those examined and not examined across the quintiles of deprivation in both the WF and no WF groups, with a higher proportion of individuals in the least deprived quintile in the fluoridated group not having an examination. There was a similar level of dmft at baseline in the fluoridated and non-fluoridated areas. However, it was observed that, when looking at individuals who had an examination compared with individuals who did not have an examination, there was a higher level of dmft in both the fluoridated and non-fluoridated groups for those who did not have a final examination, indicating that participants lost to follow-up had, on average, more decay at baseline.
| Variable | Exposure group | |||||||
|---|---|---|---|---|---|---|---|---|
| No WF | WF | No WF and WF combined | ||||||
| Examined | Not examined | Total | Examined | Not examined | Total | Examined | Not examined | |
| Sex | ||||||||
| Male | ||||||||
| n | 339 | 153 | 492 | 250 | 51 | 301 | 659 | 236 |
| % | 54.9 | 51.7 | 53.9 | 56.4 | 61.9 | 57.2 | 55.5 | 54.9 |
| Female | ||||||||
| n | 278 | 143 | 421 | 320 | 83 | 403 | 528 | 194 |
| % | 45.1 | 48.3 | 46.1 | 43.9 | 38.1 | 42.8 | 44.5 | 45.1 |
| Total | ||||||||
| n | 617 | 296 | 913 | 570 | 134 | 704 | 1187 | 430 |
| % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Deprivation quintile | ||||||||
| Quintile 1 | ||||||||
| n | 74 | 16 | 90 | 41 | 23 | 64 | 115 | 39 |
| % | 12.1 | 5.2 | 9.8 | 7.3 | 15.0 | 8.9 | 9.8 | 8.5 |
| Quintile 2 | ||||||||
| n | 119 | 64 | 183 | 80 | 22 | 102 | 199 | 86 |
| % | 19.5 | 20.1 | 19.9 | 14.2 | 14.4 | 14.3 | 17.0 | 18.7 |
| Quintile 3 | ||||||||
| n | 127 | 79 | 206 | 99 | 21 | 120 | 226 | 100 |
| % | 20.8 | 25.7 | 22.4 | 17.6 | 13.7 | 16.8 | 19.3 | 21.7 |
| Quintile 4 | ||||||||
| n | 153 | 77 | 230 | 157 | 47 | 204 | 310 | 124 |
| % | 25.0 | 25.0 | 25.0 | 27.9 | 30.7 | 28.5 | 26.4 | 26.9 |
| Quintile 5 | ||||||||
| n | 138 | 72 | 210 | 185 | 40 | 225 | 323 | 112 |
| % | 22.6 | 23.4 | 22.9 | 32.9 | 26.1 | 31.5 | 27.5 | 24.3 |
| Total | ||||||||
| n | 611 | 308 | 919 | 562 | 153 | 715 | 1173 | 461 |
| % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Deprivation score | ||||||||
| n | 611 | 308 | 919 | 562 | 153 | 715 | 1173 | 461 |
| Mean (SD) | 24.6 (15.5) | 25.6 (15.3) | 24.9 (15.4) | 28.1 (15.6) | 26.0 (17.2) | 27.7 (16.0) | 26.3 (15.6) | 25.7 (15.9) |
| Median (LQ, UQ) | 20.2 (12.6, 33.2) | 20.2 (12.9, 33.2) | 20.2 (12.8, 33.2) | 26.5 (16.5, 36.9) | 22.9 (10.8, 34.5) | 24.3 (16.4, 39.9) | 22.9 (13.3, 36) | 21.5 (12.9, 33.7) |
| Minimum, maximum | 4.37, 70.58 | 4.37, 70.58 | 4.37, 70.58 | 5.13, 65.45 | 2.92, 65.45 | 2.92, 65.45 | 2.92, 70.58 | 2.92, 70.58 |
| % dmft baselinea | ||||||||
| dmft present | ||||||||
| n | 420 | 200 | 620 | 381 | 97 | 478 | 801 | 297 |
| % | 69.3 | 65.6 | 68.1 | 68.8 | 66.9 | 68.4 | 69.0 | 66.0 |
| No dmft recorded | ||||||||
| n | 186 | 105 | 291 | 173 | 48 | 221 | 289 | 153 |
| % | 30.7 | 34.4 | 31.9 | 31.2 | 33.1 | 31.6 | 31.0 | 34.0 |
| Total | ||||||||
| n | 606 | 305 | 911 | 554 | 145 | 699 | 1160 | 450 |
| dmft score at baseline | ||||||||
| n | 606 | 305 | 911 | 554 | 145 | 699 | 1160 | 450 |
| Mean (SD) | 1.1 (2.3) | 1.4 (2.6) | 1.2 (2.4) | 1.0 (2.1) | 1.2 (2.3) | 1.1 (2.2) | 1.0 (2.2) | 1.3 (2.5) |
| Median (LQ, UQ) | 0 (0, 1) | 0 (0, 2) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) |
| Minimum, maximum | 0, 14 | 0, 14 | 0, 14 | 0, 12 | 0, 12 | 0, 12 | 0, 14 | 0, 14 |
A logistic regression on examination status was performed, including exposure status, deprivation and sex (Table 113). The logistic regression indicated that for individuals living in a fluoridated area the odds of being examined were 1.86 (95% CI 1.45 to 2.39) times as large as the odds for individuals living in a non-fluoridated area (this was largely due to the COVID-19 pandemic, as data collection had almost finished in the fluoridated area, but there were almost 150 children to be seen in the non-fluoridated area when the COVID-19 pandemic stopped the study). However, given the geographic area, the no WF group always had a larger sample and, despite an increased likelihood of a final examination in the WF group, the number of participants remained larger in the non-fluoridated group.
| Logistic Regression analysis (n = 1994) | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 1.86 (1.45 to 2.39) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 0.92 (0.58 to 1.47) |
| 3 | 0.90 (0.57 to 1.42) |
| 4 | 0.91 (0.59 to 1.41) |
| 5 | 1.08 (0.69 to 1.69) |
| Sex (female) | 1.00 (0.79 to 1.27) |
| Constant | 2.49 (1.67 to 3.73) |
Table 114 illustrates the average DMFT scores for the fluoridated and non-fluoridated groups across each quintile for the final examination in wave 3. Mean DMFT scores between the groups appear to be similar at deprivation quintiles 1 and 5.
| Deprivation quintile | DMFT clinical score | Exposure group | Total | |
|---|---|---|---|---|
| No WF | WF | |||
| 1 | n | 74 | 41 | 115 |
| Mean (SD) | 0.27 (0.76) | 0.29 (0.78) | 0.28 (0.77) | |
| Median (LQ, UQ) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Minimum, maximum | 0, 4 | 0, 4 | 0, 4 | |
| 2 | n | 119 | 80 | 199 |
| Mean (SD) | 0.34 (0.76) | 0.2 (0.58) | 0.29 (0.70) | |
| Median (LQ, UQ) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Minimum, maximum | 0, 4 | 0, 3 | 0, 4 | |
| 3 | n | 127 | 99 | 226 |
| Mean (SD) | 0.28 (0.74) | 0.17 (0.45) | 0.23 (0.63) | |
| Median (LQ, UQ) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Minimum, maximum | 0, 4 | 0, 2 | 0, 4 | |
| 4 | n | 153 | 157 | 310 |
| Mean (SD) | 0.48 (1.05) | 0.21 (0.61) | 0.34 (0.87) | |
| Median (LQ, UQ) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) | |
| Minimum, maximum | 0, 5 | 0, 4 | 0, 5 | |
| 5 | n | 138 | 185 | 323 |
| Mean (SD) | 0.55 (1.01) | 0.54 (1.00) | 0.54 (1.01) | |
| Median (LQ, UQ) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | |
| Minimum, maximum | 0, 4 | 0, 5 | 0, 5 | |
Longitudinal data analysis of potential effect modifiers collected by questionnaires
During the 5-year study, there were three waves of questionnaires for the older school cohort that captured behaviour data that could affect dental health. The questionnaires were provided when the child was approximately:
-
5 years old (parent-completed questionnaire; wave 1)
-
7 years old (child-completed questionnaire; wave 2)
-
11 years old (child-completed questionnaire; wave 3).
Behaviours that could affect dental health were explored using binomial GEEs when there was a binary outcome across each wave to determine if there were differences in the fluoridated and non-fluoridated groups (taking into account multiple testing across waves).
The behaviours explored in the older school cohort were:
-
the toothpaste product used (fluoridated vs. non-fluoridated)
-
whether a child spit or rinses out their toothpaste
-
whether or not drinks containing free sugar are consumed in the hour before bed
-
whether or not snacks containing free sugars are consumed in the hour before bed
-
the toothpaste amount consumed (half of brush-large/large smear vs. small pea/smear).
The only significant result from GEE analysis over the three waves was the difference between groups for whether a child spits or rinses after brushing their teeth (Tables 115 and 116). The difference occurs in wave 2, with more children in the WF area (62%) than in the no WF area (45%) rinsing out the toothpaste.
| Behaviour: spitting vs. rinsing | IRR | Semi-robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.194 | 0.088 | 2.410 | 0.016 | 1.034 to 1.378 |
| Sex (male/female) | 1.184 | 0.084 | 2.370 | 0.018 | 1.030 to 1.362 |
| Age | 0.864 | 0.012 | –10.920 | 0.000 | 0.841 to 0.887 |
| Deprivation quintile | 1.145 | 0.031 | 4.990 | 0.000 | 1.086 to 1.207 |
| Constant | 1.929 | 0.268 | 4.730 | 0.000 | 1.470 to 2.533 |
| Behaviour | Exposure group | Behaviour after toothbrushing | Wave, n (%) | ||
|---|---|---|---|---|---|
| 1 (N = 1586) | 2 (N = 1538) | 3 (N = 1156) | |||
| Spitting vs. rinsing after brushing | No WF | Spit | 307 (34) | 475 (55) | 275 (69) |
| Rinse | 584 (66) | 390 (45) | 126 (31) | ||
| WF | Spit | 239 (34) | 258 (38) | 308 (56) | |
| Rinse | 456 (66) | 415 (62) | 246 (44) | ||
Tables 117 and 118 explores the difference in use of fluoridated and non-fluoridated toothpastes between individuals living in a fluoridated area and individuals living in a non-fluorinated area across time. There is no significant difference across the intervention and control groups over time in toothpaste product use (i.e. fluoridated vs. non-fluoridated toothpaste).
| Behaviour: fluoridated vs. non fluoridated toothpaste (yes/no) | IRR | Semi-robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 1.836 | 0.820 | 1.360 | 0.173 | 0.766 to 4.405 |
| Sex (male/female) | 0.733 | 0.280 | –0.810 | 0.415 | 0.346 to 1.549 |
| Age | 0.800 | 0.055 | –3.270 | 0.001 | 0.699 to 0.914 |
| Deprivation quintile | 0.960 | 0.132 | –0.290 | 0.769 | 0.734 to 1.257 |
| Constant | 735.704 | 648.620 | 7.490 | 0.000 | 130.694 to 4141.418 |
| Behaviour | Exposure group | Fluoridated toothpaste use | Wave | ||
|---|---|---|---|---|---|
| 1 (N = 1529) | 2 (N = 1459) | 3 (N = 1156) | |||
| Use of fluoridated vs. non-fluoridated toothpaste | No WF | Yes/no, % | 100/0 | 98/2 | 98/2 |
| Yes/no, n | 860/0 | 816/13 | 592/12 | ||
| WF | Yes/no, % | 99.5/0.5 | 99.7/0.3 | 99/1 | |
| Yes/no, n | 666/3 | 628/2 | 544/7 | ||
Tables 119 and 120 explore whether or not free sugars were recorded as consumed in a drink in the hour before bed (no free sugars could indicate that a drink was consumed without free sugars or no drink was recorded as being consumed). There were no significant differences over time between the groups. Over time, the majority of individuals in either group did not consume free sugars within a drink in the hour before bed.
| Behaviour: free sugars consumed within drink in the hour before bed | IRR | Semi-robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.813 | 0.233 | –0.720 | 0.469 | 0.464 to 1.424 |
| Sex (male/female) | 0.761 | 0.201 | –1.030 | 0.301 | 0.453 to 1.277 |
| Age | 0.974 | 0.052 | –0.490 | 0.621 | 0.878 to 1.081 |
| Deprivation quintile | 1.064 | 0.111 | 0.590 | 0.556 | 0.866 to 1.306 |
| Constant | 0.038 | 0.024 | –5.180 | 0.000 | 0.011 to 0.131 |
| Behaviour | Exposure group | No free sugars vs. free sugars in drinks | Wave | ||
|---|---|---|---|---|---|
| 1 (N = 1303) | 2 (N = 911) | 3 (N = 1174) | |||
| Free sugars in drinks consumed in the hour before bed | No WF | No free sugars/free sugars, % | 96/3 | 97/3 | 98/2 |
| No free sugars/free sugars, n | 601/27 | 272/9 | 598/12 | ||
| WF | No free sugars/free sugars, % | 98/2 | 97/3 | 97/3 | |
| No free sugars/free sugars, n | 659/16 | 610/20 | 547/17 | ||
Tables 121 and 122 explore whether or not free sugars from food as a snack were recorded as consumed in the hour before bed (no free sugars could indicate that food was consumed without free sugars, food was consumed but as part of a meal or no food was recorded as being consumed). There were no significant differences over time between the groups in whether or not free sugars from food as a snack were recorded as consumed in the hour before bed.
| Behaviour: free sugars in food consumed as a snack in the hour before bed | IRR | Semi-robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.951 | 0.118 | –0.410 | 0.684 | 0.746 to 1.212 |
| Sex (male/female) | 0.877 | 0.094 | –1.220 | 0.221 | 0.711 to 1.082 |
| Age | 0.980 | 0.018 | –1.110 | 0.266 | 0.946 to 1.015 |
| Deprivation quintile | 1.125 | 0.047 | 2.810 | 0.005 | 1.036 to 1.221 |
| Constant | 0.368 | 0.079 | –4.630 | 0.000 | 0.241 to 0.562 |
| Behaviour | Exposure group | No free sugars vs. free sugars in food | Wave | ||
|---|---|---|---|---|---|
| 1 (N = 1303) | 2 (N = 911) | 3 (N = 1174) | |||
| Free sugars in food consumed as a snack in the hour before bed | No WF | No free sugars/free sugars, % | 65/35 | 70/30 | 72/28 |
| No free sugars/free sugars, n | 406/222 | 196/85 | 439/171 | ||
| WF | No free sugars/free sugars, % | 69/31 | 67/33 | 71/29 | |
| No free sugars/free sugars, n | 470/205 | 425/205 | 402/162 | ||
Tables 123 and 124 explore whether or not toothpaste amount (half of brush-large/large smear vs. small pea/smear) differed between groups over time. There were no significant differences over time between the groups.
| Behaviour: amount of toothpaste used | IRR | Semi-robust SE | z-value | p > (z) | 95% CI |
|---|---|---|---|---|---|
| Exposure | 0.950 | 0.064 | –0.770 | 0.444 | 0.833 to 1.083 |
| Sex (male/female) | 0.984 | 0.065 | –0.240 | 0.812 | 0.865 to 1.121 |
| Age | 0.923 | 0.013 | –5.860 | 0.000 | 0.899 to 0.948 |
| Deprivation quintile | 0.960 | 0.025 | –1.570 | 0.116 | 0.913 to 1.010 |
| Constant | 2.937 | 0.411 | 7.690 | 0.000 | 2.232 to 3.865 |
| Behaviour | Exposure group | Small vs. large amount of toothpaste | Wave | ||
|---|---|---|---|---|---|
| 1 (N = 1556) | 2 (N = 1546) | 3 (N = 1179) | |||
| Amount of toothpaste used | No WF | Small amount/large amount, % | 35/65 | 44/56 | 48/52 |
| Small amount/large amount, n | 302/571 | 380/489 | 294/323 | ||
| WF | Small amount/large amount, % | 36/64 | 48/52 | 47/53 | |
| Small amount/large amount, n | 248/435 | 324/353 | 266/296 | ||
As only one behaviour showed a significant difference between the exposure groups for the older school cohort, which was spitting and rinsing out toothpaste after brushing, and that this happened during only one wave, when a higher proportion of the fluoridated group rinsed out their toothpaste (it is recommended to spit and not rinse to retain the effect of the fluoridated toothpaste), it was decided this one behaviour in one wave should not be included in the regression. However, given the known effects of deprivation, age and sex on the experience of dental decay, these effect modifiers have been included in logistic/negative binomial regression analyses when exploring the effect of WF on caries experience (see Appendix 3, Tables 134, 135 and 139–144).
Further data on behaviours collected during the final clinical examination in wave 3 can be found in Tables 125–132. Tables 125–132 provide greater detail of behaviours that were dichotomised for GEE analysis.
| Toothbrush head | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| Standard square | 52 (8.5) | 44 (7.9) | 96 (8.2) |
| Large triangle | 209 (34.0) | 192 (34.3) | 401 (34.1) |
| Small round brush | 291 (47.3) | 246 (43.9) | 537 (45.7) |
| Medium-angled brush | 63 (10.2) | 78 (13.9) | 141 (12.) |
| Total | 615 | 560 | 1175 |
| Toothpaste amount | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| Pea size | 9 (1.5) | 6 (1.1) | 15 (1.3) |
| Half of brush-large | 176 (28.6) | 170 (30.3) | 346 (29.4) |
| Thin smear | 285 (46.3) | 259 (46.2) | 544 (46.2) |
| Large smear | 146 (23.7) | 126 (22.5) | 272 (23.1) |
| Total | 616 | 561 | 1177 |
| Toothbrushing behaviour after brushing | Exposure group, n (%)a | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| Spit | 334 (55.6) | 307 (55.5) | 641 (55.6) |
| Rinse wet brush | 63 (10.5) | 77 (13.9) | 140 (12.1) |
| Rinse head under tap | 74 (12.3) | 75 (13.6) | 149 (12.9) |
| Rinse cup hand | 34 (5.7) | 22 (4.0) | 56 (4.9) |
| Rinse beaker | 89 (14.8) | 67 (12.1) | 156 (13.5) |
| Other | 7 (1.2) | 5 (0.9) | 12 (1.0) |
| Total | 601 | 553 | 1154 |
| Brushing frequency | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| More than twice a day | 37 (6.0) | 40 (7.1) | 77 (6.5) |
| Twice a day | 450 (72.7) | 387 (68.4) | 837 (70.6) |
| Once a day | 116 (18.7) | 125 (22.1) | 241 (20.3) |
| Less than once a day | 12 (1.9) | 10 (1.8) | 22 (1.9) |
| Never | 4 (0.7) | 4 (0.7) | 8 (0.7) |
| Total | 619 | 556 | 1185 |
| Fluoridated toothpaste | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| No fluoride | 12 (2.0) | 7 (1.3) | 19 (1.7) |
| Fluoride | 592 (98.0) | 543 (98.7) | 1135 (98.4) |
| Total | 604 | 550 | 1154 |
| Free sugars consumed before bed | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| Free sugar consumed before bed as a snack/drink | 179 (30.4) | 170 (30.4) | 349 (30.4) |
| No free sugar consumed before bed as a snack/drink | 410 (69.6) | 390 (69.6) | 800 (69.6) |
| Total | 589 | 560 | 1149 |
| Drink before bed | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| No drink before bed | 530 (89.8) | 476 (84.8) | 1006 (87.4) |
| Drink before bed n | 60 (10.2) | 85 (15.2) | 145 (12.6) |
| Total | 590 | 561 | 1151 |
| If a drink is consumed, does it contain free sugars? | |||
| No free sugars | 49 (81.7) | 67 (79.8) | 116 (80.6) |
| Free sugars | 11 (18.3) | 17 (20.2) | 28 (19.4) |
| Diet before bed | Exposure group, n (%) | Total, n (%) | |
|---|---|---|---|
| No WF | WF | ||
| No snack before bed | 317 (53.8) | 302 (53.9) | 619 (53.9) |
| Snack before beda | 272 (46.2) | 258 (46.1) | 530 (46.1) |
| Total | 589 | 560 | 1149 |
| If a snack is consumed, does it contain free sugars? | |||
| No free sugars | 102 (37.2) | 97 (37.5) | 199 (37.5) |
| Free sugars | 172 (62.8) | 162 (62.5) | 332 (62.5) |
Table 133 provides descriptive results from the parent questionnaire completed by parents during the last clinical examination.
| Parent questionnaire | Exposure group | |||
|---|---|---|---|---|
| No WF | WF | |||
| PC1 | PC2 | PC1 | PC2 | |
| Parent type: mother/father/other | ||||
| n | 179/7/1 | 4/147/10 | 151//15/2 | 14/115/4 |
| % | 97/2/0.05 | 3/91/6 | 89/9/2 | 11/86/3 |
| General health | ||||
| n | 197 | 161 | 170 | 132 |
| 1: very good (%) | 58 | 56 | 52 | 55 |
| 2: good (%) | 32 | 39 | 39 | 35 |
| 3: fair (%) | 8 | 5 | 6 | 9 |
| 4: bad (%) | 2 | 1 | 2 | 1 |
| 5: very bad (%) | 0 | 0 | 0 | 0 |
| Dental health | ||||
| n | 197 | 161 | 170 | 132 |
| 1: very good (%) | 39 | 32 | 32 | 29 |
| 2: good (%) | 48 | 48 | 50 | 42 |
| 3: fair (%) | 12 | 16 | 15 | 25 |
| 4: bad (%) | 2 | 4 | 2 | 3 |
| 5: very bad (%) | 0 | 1 | 1 | 1 |
| How often do you visit the dentist? | ||||
| n | 197 | 160 | 170 | 133 |
| 1: at least once every 6 months (%) | 71 | 58 | 44 | 40 |
| 2: at least once a year (%) | 21 | 27 | 44 | 42 |
| 3: at least once every 2 years (%) | 4 | 3 | 3 | 5 |
| 4: less frequently than every 2 years (%) | 2 | 2 | 1 | 3 |
| 5: only when I have trouble with my teeth (%) | 3 | 9 | 8 | 9 |
| 6: never been to the dentist (%) | 0 | 1 | 0 | 1 |
| Last visit to the dentist | ||||
| n | 197 | 160 | 170 | 131 |
| 1: a routine check-up (%) | 87 | 73 | 83 | 77 |
| 2: emergency or urgent treatment (%) | 4 | 13 | 6 | 10 |
| 3: other treatment (non-urgent) (%) | 8 | 12 | 8 | 12 |
| 4: not been to the dentist (%) | 0 | 3 | 2 | 1 |
| 5: other (please state) (%) | 1 | 0 | 1 | 0 |
| Employment status | ||||
| n | 186 | 146 | 159 | 126 |
| 1: full-time employee (> 30 hours) (%) | 31 | 94 | 36 | 89 |
| 2: full-time employee (< 30 hours) (%) | 56 | 3 | 50 | 4 |
| 3: school/full-time education (%) | 1 | 0 | 1 | 0 |
| 4: unemployed (%) | 1 | 1 | 2 | 2 |
| 5: retired from work (%) | 0 | 0 | 1 | 2 |
| 6: looking after the home (%) | 8 | 1 | 6 | 1 |
| 7: permanently sick or disabled (%) | 2 | 0 | 3 | 2 |
| 8: doing something else (%) | 2 | 2 | 1 | 1 |
| Job type | ||||
| 1: modern professionala (%) | 179 | 155 | 148 | 126 |
| 2: clerical and intermediate occupationsb (%) | 33 | 17 | 39 | 17 |
| 3: senior managers/administratorsc (%) | 29 | 3 | 20 | 4 |
| 4: technical and craft occupationsd (%) | 12 | 17 | 7 | 21 |
| 5: semi-routine manual and service occupationse (%) | 3 | 27 | 4 | 17 |
| 6: routine manual and service occupationsf (%) | 11 | 10 | 11 | 7 |
| 7: middle or junior managersg (%) | 5 | 7 | 7 | 13 |
| 8: traditional professional occupationsh (%) | 3 | 7 | 5 | 4 |
| 9: semi-routine manual and service occupationse (%) | 5 | 10 | 7 | 17 |
| Household income (£) | ||||
| n | 174 | 149 | ||
| 1: up to 5199 (per annum)i (%) | 1 | 1 | ||
| 2: 5200–10,399 (per annum)j (%) | 5 | 5 | ||
| 3: 10,400–15,599 (per annum)k (%) | 3 | 7 | ||
| 4: 15,600–20,799(per annum)l (%) | 6 | 5 | ||
| 5: 20,800–25,999 (per annum)m (%) | 7 | 7 | ||
| 6: 26,000–31,199 (per annum)n (%) | 10 | 7 | ||
| 7: 32,200–36,399 (per annum)o (%) | 13 | 3 | ||
| 8: 36,400–51,999 (per annum)p (%) | 26 | 26 | ||
| 9: ≥ 52,000 (per annum)q (%) | 29 | 38 | ||
| Equivalised household income (£) [taking the median income from household income (above) and calculating using McClements equivalence scales] | ||||
| n | 168 | 146 | ||
| Mean (SD) | 480.3.3 (175.5) | 487.2 (176.2) | ||
| 95% CI | 453.3 to 507.4 | 457.9 to 516.4 | ||
| Number of children in household | ||||
| n | 194 | 165 | ||
| 1 (%) | 16 | 16 | ||
| 2 (%) | 52 | 55 | ||
| 3 (%) | 23 | 24 | ||
| 4 (%) | 7 | 5 | ||
| 5 (%) | 1 | 1 | ||
| 6 (%) | 0 | 0 | ||
| 7 (%) | 0.5 | 0 | ||
| 8 (%) | 0.5 | 0 | ||
| Number of adults in household | ||||
| n | 186 | 166 | ||
| 1 (%) | 13 | 15 | ||
| 2 (%) | 82 | 80 | ||
| 3 (%) | 4 | 5 | ||
| 4 (%) | 1 | 1 | ||
| Average number of cups of tap water consumed each day (including tea, squash) | ||||
| N = 197 | N = 145 | N = 170 | N = 117 | |
| Mean (SD) | 5.32 (2.77) | 5.53 (3.94) | 5.38 (2.96) | 6.06 (3.06) |
Primary outcome
Table 134 shows the results of the logistic regression for permanent decay by exposure status (area), deprivation quintiles, age, dmft at baseline and sex.
| Logistic regression analysis (n = 1089) | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.80 (0.58 to 1.09) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.16 (0.61 to 2.23) |
| 3 | 0.95 (0.49 to 1.82) |
| 4 | 1.17 (0.63 to 2.17) |
| 5 | 1.78 (0.97 to 3.26) |
| Sex (male/female) | 1.01 (0.75 to 1.37) |
| Age (centred) | 0.55 (0.34 to 0.90) |
| dmft (baseline) | 1.21 (1.13 to 1.29) |
The categorical variable ‘deprivation’ is borderline statistically significant [χ2(4)=9.13; p = 0.0580]. From the model, there is a negative association between fluoridation and decay. The odds of decay for children from a fluoridated area are 80% of the odds of decay for children from a non-fluoridated area, but this is not statistically significant. The 95% CI ranges from 0.58 to 1.09, with a p-value of 0.155.
Figures 29–31 and Table 135 demonstrates the difference in probabilities of margins data for individuals experiencing no decay or any decay, showing the difference across deprivation quintiles between individuals living in a fluoridated and non-fluoridated area.
FIGURE 29.
Predictive margins of decay or no decay by area across deprivation quintiles, sex, age and dmft at baseline with 95% CI.
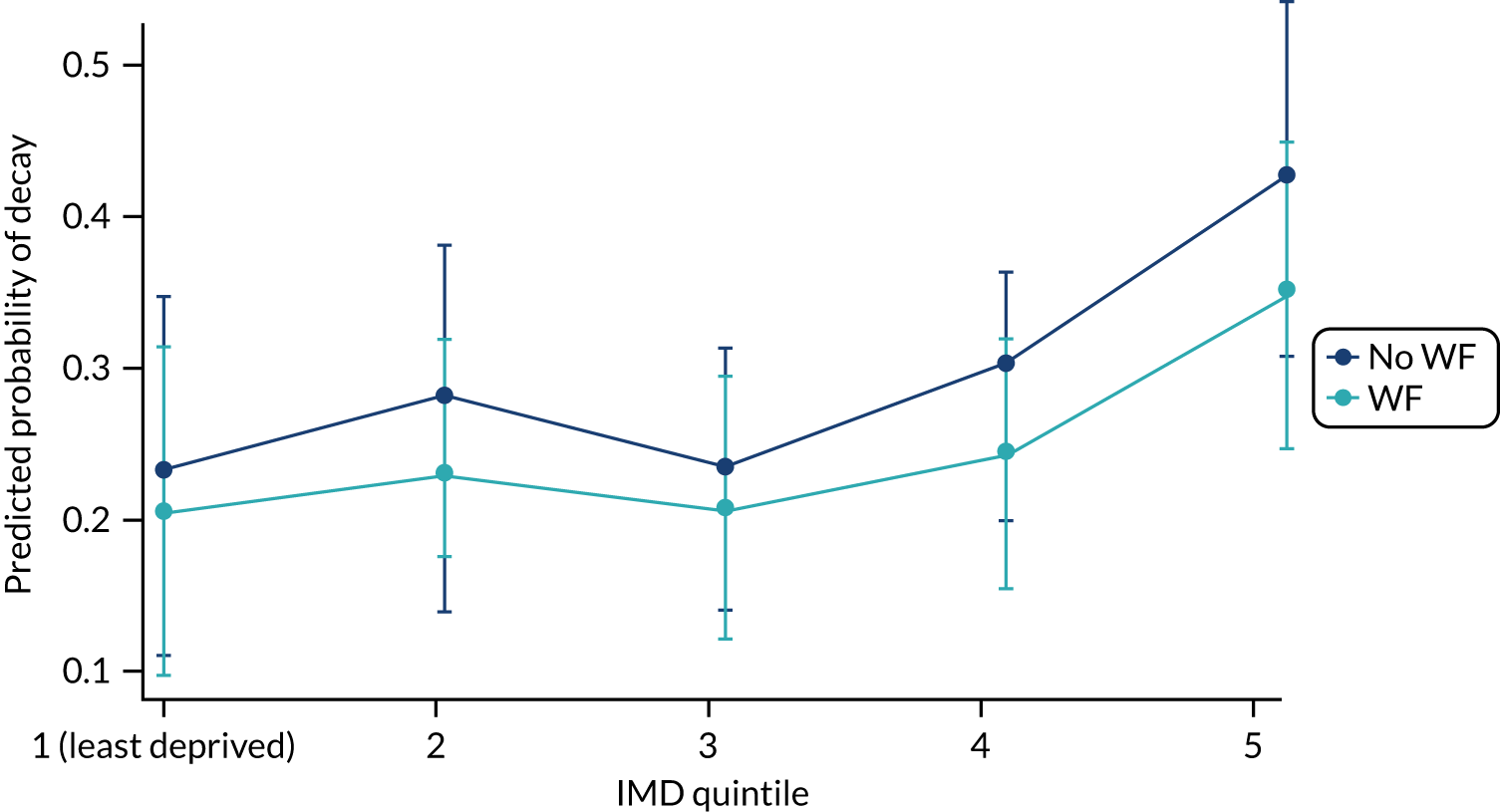
FIGURE 30.
Predictive margins of decay or no decay by area across deprivation quintiles, sex, age and dmft at baseline with 95% CI: males only.
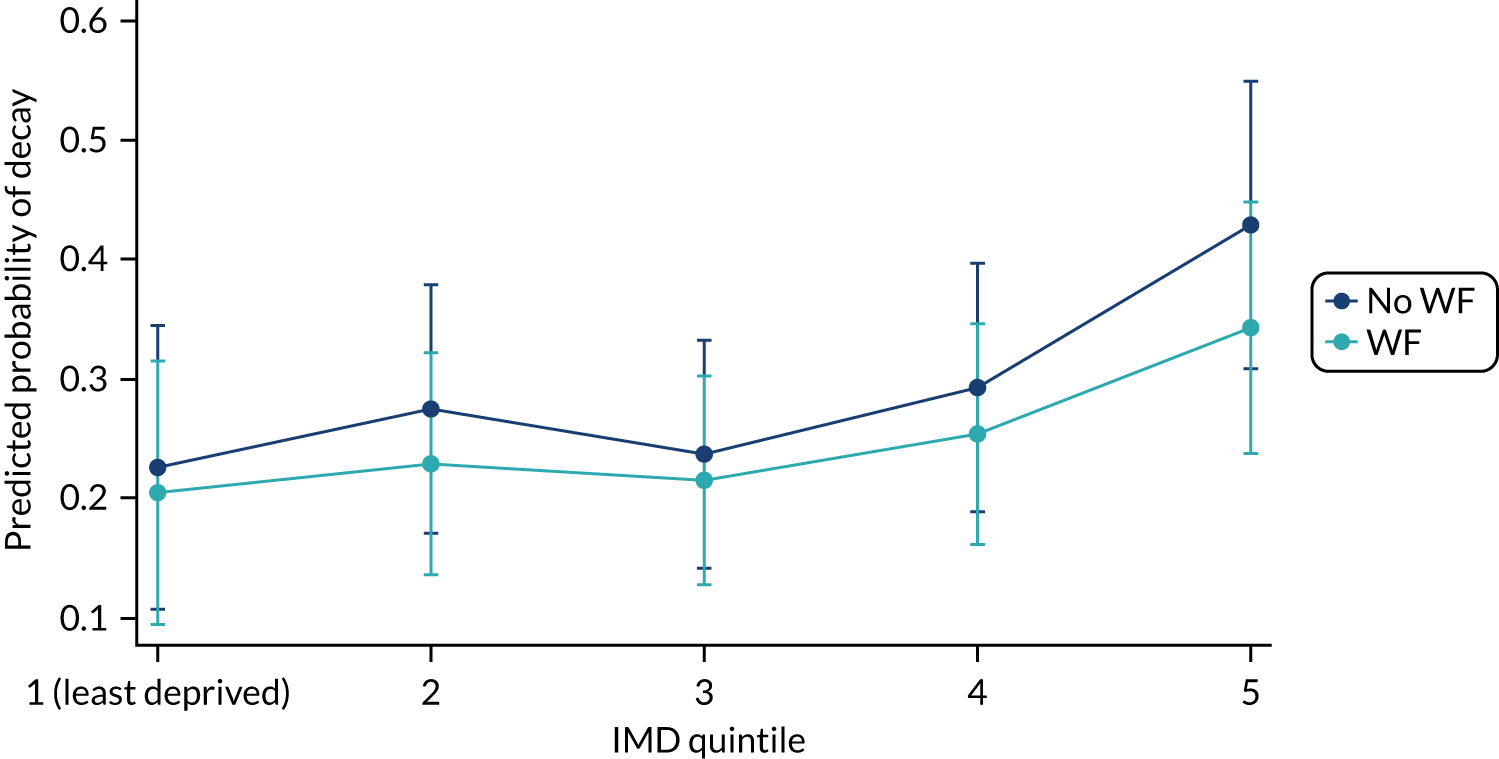
FIGURE 31.
Predictive margins of decay or no decay by area across deprivation quintiles, sex, age and dmft at baseline with 95% CI: females only.
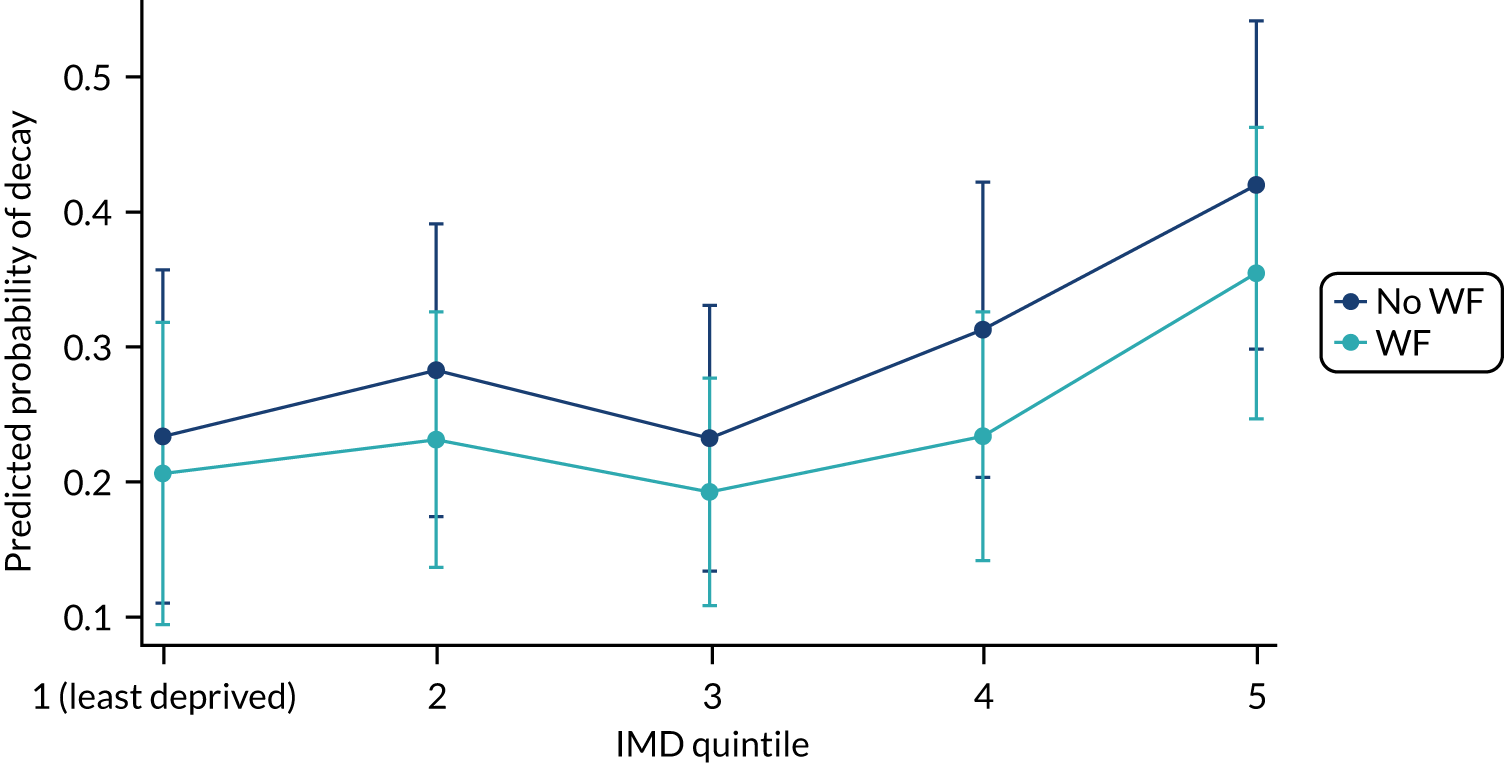
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Quintile 1 | ||
| Non-fluoridated area | 0.230 | 0.111 to 0.348 |
| Fluoridated area | 0.205 | 0.097 to 0.313 |
| Quintile 2 | ||
| Non-fluoridated area | 0.278 | 0.176 to 0.380 |
| Fluoridated area | 0.230 | 0.140 to 0.320 |
| Quintile 3 | ||
| Non-fluoridated area | 0.235 | 0.142 to 0.328 |
| Fluoridated area | 0.205 | 0.122 to 0.287 |
| Quintile 4 | ||
| Non-fluoridated area | 0.301 | 0.199 to 0.403 |
| Fluoridated area | 0.244 | 0.156 to 0.332 |
| Quintile 5 | ||
| Non-fluoridated area | 0.424 | 0.308 to 0.540 |
| Fluoridated area | 0.348 | 0.246 to 0.449 |
Secondary outcomes
A logistic regression was performed on DGA experience by exposure group, including deprivation, dmft at baseline and sex as covariates. No significant effect was found for exposure group on experience of DGA when including these covariates (OR 0.806, 95% CI 0.513 to 1.264) (Tables 136 and 137).
| Exposure status (n = 1421) | DGA experience, n (%) | |
|---|---|---|
| No | Yes | |
| No WF | 742 (92.9) | 57 (7.3) |
| WF | 585 (94.4) | 35 (5.7) |
| Logistic regression | OR | 95% CI |
|---|---|---|
| Fluoridation | 0.806 | 0.513 to 1.264 |
| Deprivation quintile (reference quintile 1) | ||
| 2 | 0.798 | 0.350 to 1.815 |
| 3 | 0.879 | 0.393 to 1.966 |
| 4 | 0.694 | 0.313 to 1.541 |
| 5 | 0.903 | 0.413 to 1.975 |
| Sex | 0.838 | 0.539 to 1.304 |
| dmft at baseline | 0.983 | 0.882 to 1.095 |
| Constant | 0.059 | 0.049 to 0.184 |
A negative binomial regression was performed on the count of decay (DMFT) and included exposure status (area), deprivation quintiles, age, sex, baseline dmft and offset for erupted teeth (Tables 138–140). Figure 32 is a visual representation of Table 140. Figures 33 and 34 show these data split by sex and similar patterns can be seen for both male and female participants across the deprivation quintiles.
| Negative binomial regression analysis (n = 1173): dmft count | IRR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.68 (0.52 to 0.90) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.02 (0.55 to 1.88) |
| 3 | 0.85 (0.46 to 1.57) |
| 4 | 1.25 (0.70 to 2.23) |
| 5 | 2.12 (1.21 to 3.68) |
| Regression analysis (n = 1127): DMFT count | IRR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.69 (0.52 to 0.93) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 0.87 (0.46 to 1.64) |
| 3 | 0.78 (0.39 to 1.46) |
| 4 | 1.09 (0.59 to 1.98) |
| 5 | 1.51 (0.83 to 2.78) |
| Age (centred) | 0.55 (0.36 to 0.87) |
| Baseline dmft | 1.18 (1.12 to 1.24) |
| Sex (male/female) | 0.96 (0.72 to 1.28) |
| Log of erupted teeth (offset) | 1 (offset) |
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Quintile 1 | ||
| Non-fluoridated area | 0.531 | 0.185 to 0.876 |
| Fluoridated area | 0.420 | 0.143 to 0.697 |
| Quintile 2 | ||
| Non-fluoridated area | 0.498 | 0.246 to 0.749 |
| Fluoridated area | 0.355 | 0.169 to 0.542 |
| Quintile 3 | ||
| Non-fluoridated area | 0.460 | 0.244 to 0.676 |
| Fluoridated area | 0.301 | 0.162 to 0.439 |
| Quintile 4 | ||
| Non-fluoridated area | 0.809 | 0.387 to 1.231 |
| Fluoridated area | 0.485 | 0.245 to 0.725 |
| Quintile 5 | ||
| Non-fluoridated area | 1.418 | 0.732 to 2.104 |
| Fluoridated area | 0.779 | 0.436 to 1.122 |
FIGURE 32.
Predictive margins for deprivation quintile with exposure area (negative binomial regression) at 10 years old.
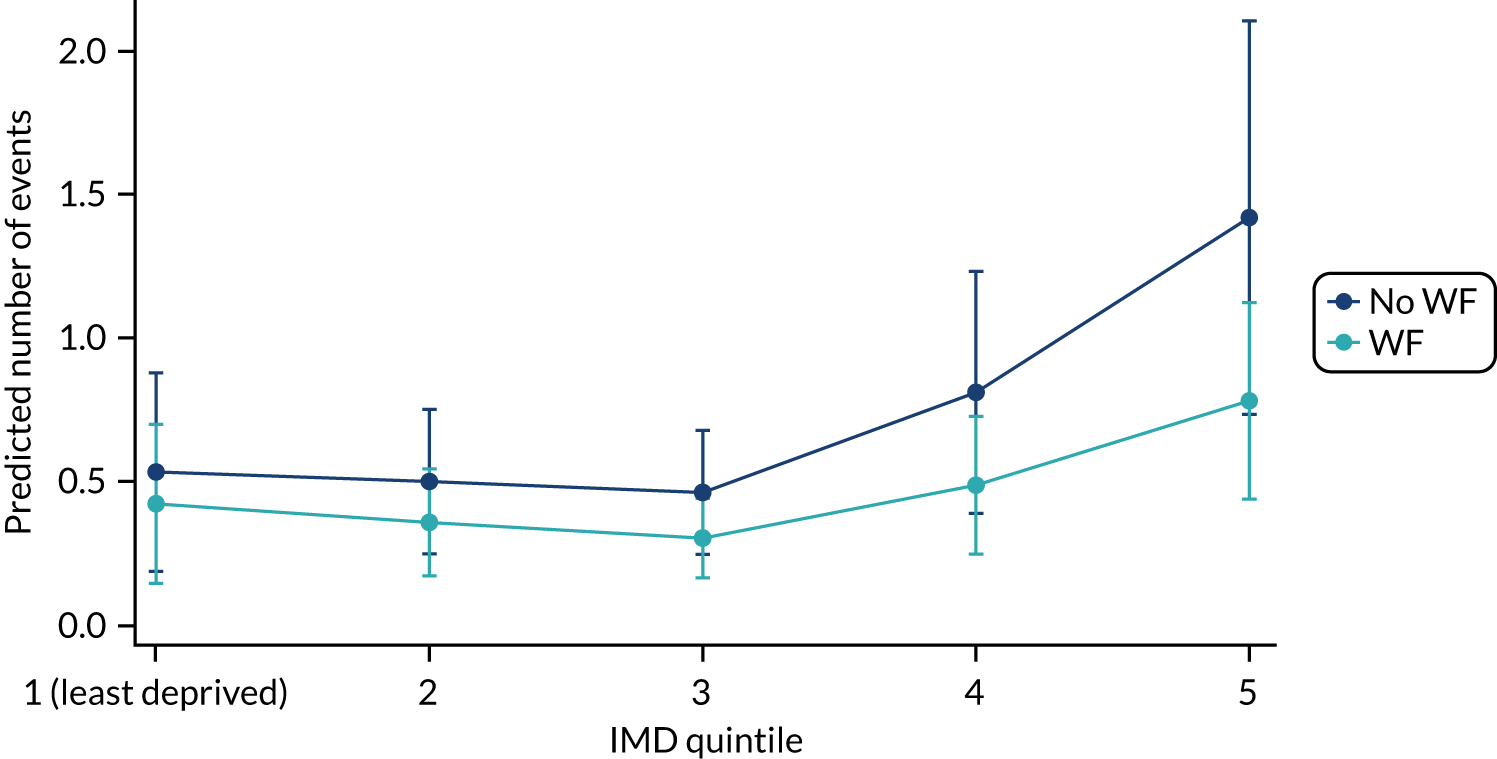
FIGURE 33.
Predictive margins for deprivation quintile with exposure area (negative binomial regression) at 10 years old: males only.
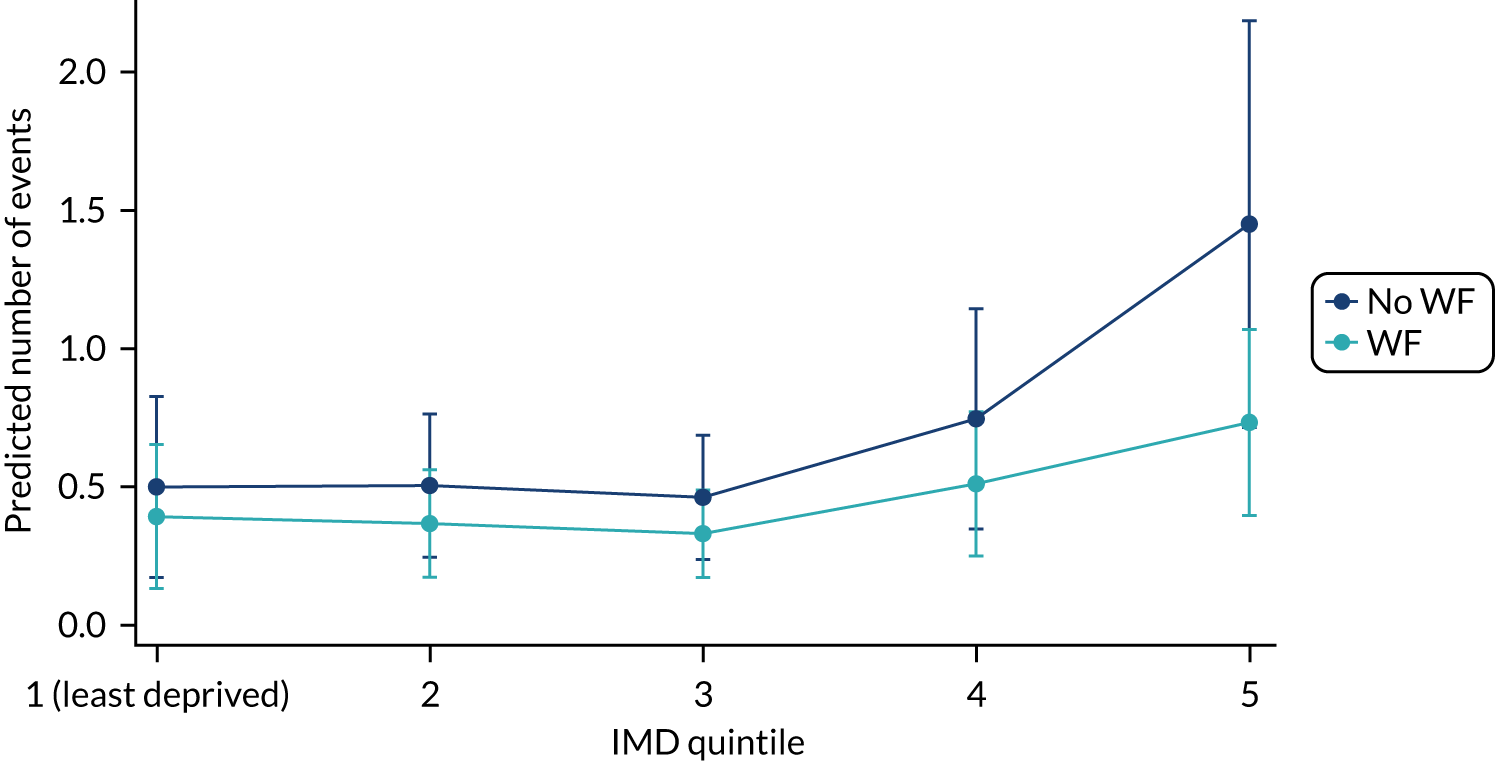
FIGURE 34.
Predictive margins for deprivation quintile with exposure area (negative binomial regression) at 10 years old: females only.
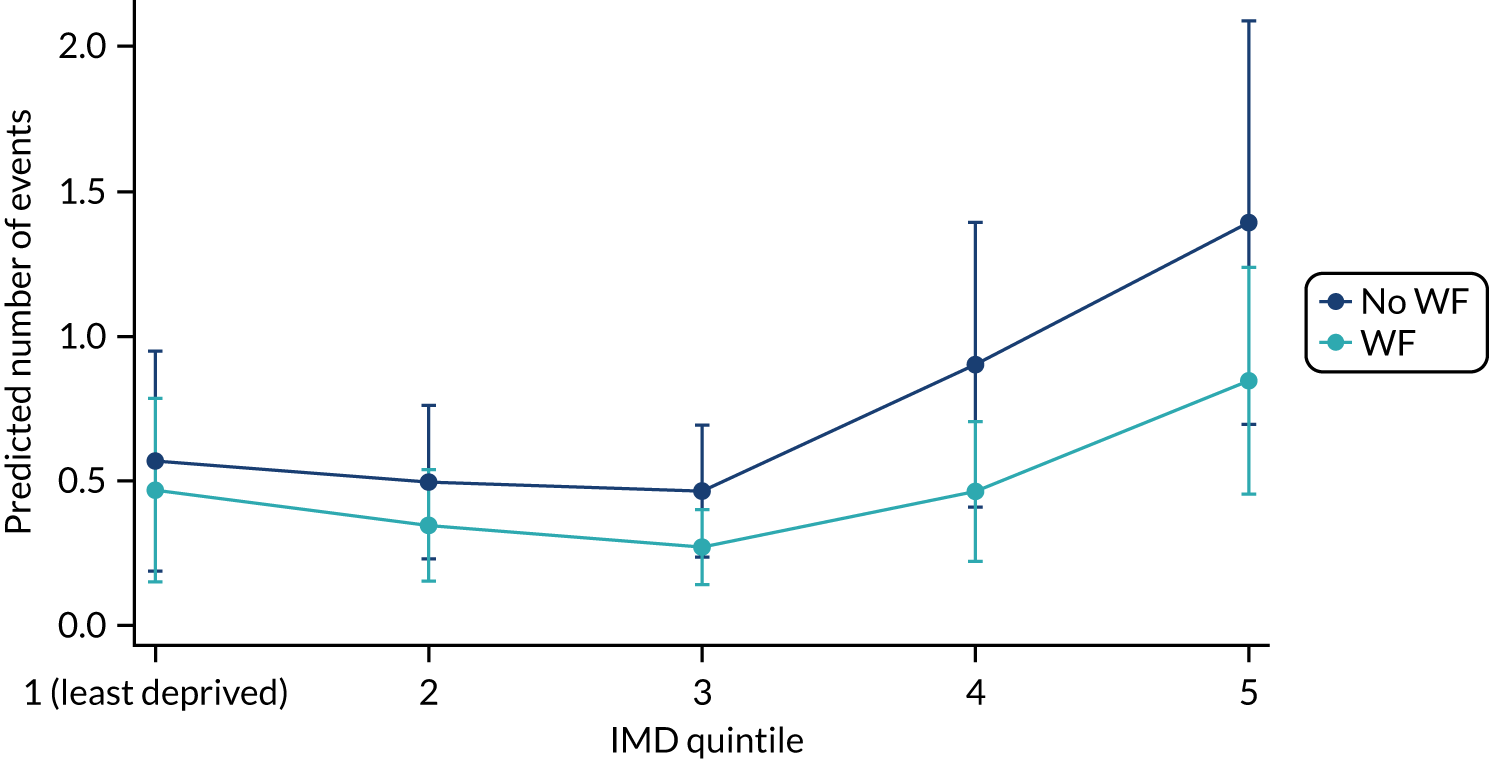
In Table 138, the categorical variable deprivation is not statistically significant [χ2(4) = 7.97; p = 0.092]. While holding the other variables constant in the model, those living in a fluoridated area had 0.68 times the rate of number of decayed, missing or filled teeth compared to those living in a non-fluoridated area. When looking at deprivation quintiles, no deprivation quintiles were significant when compared with the reference (i.e. deprivation quintile 1).
In Table 139, individuals living in a fluoridated area compared with individuals living in a non-fluoridated area, while holding the other variables constant in the model, had 0.69 times the rate of number of decayed, missing or filled teeth, with a p-value of 0.014. When looking at deprivation quintiles, no deprivation quintiles were significant when compared with the reference (i.e. deprivation quintile 1).
Health inequalities: interaction of deprivation and water fluoridation
A logistic regression with an interaction (area by deprivation) was carried out to explore the relationship between water exposure and the severity of caries, as indicated by decay/no decay (Table 141). Tables 142 and 143 indicate that WF does not reduce inequalities. The interaction term is not significant, with no differential effect on decay according to deprivation.
| Regression analysis (n = 1127) | OR (95% CI) |
|---|---|
| Area: no WF vs. WF | 1.312 (0.444 to 3.876) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.538 (0.665 to 3.557) |
| 3 | 1.141 (0.481 to 2.707) |
| 4 | 1.606 (0.713 to 3.615) |
| 5 | 1.886 (0.823 to 4.319) |
| Area by deprivation quintile interaction (reference WF by quintile 1) | |
| WF by quintile 2 | 0.472 (0.124 to 1.8) |
| WF by quintile 3 | 0.616 (0.164 to 2.319) |
| WF by quintile 4 | 0.459 (0.132 to 1.594) |
| WF by quintile 5 | 0.76 (0.226 to 2.556) |
| Age (centred) | 0.561 (0.345 to 0.911) |
| dmft at baseline | 1.212 (1.138 to 1.292) |
| Sex (male/female) | 1.02 (0.751 to 1.385) |
| Constant | 0.146 (0.071 to 0.299) |
| Variable | df | F-value | p > F |
|---|---|---|---|
| Area (WF vs. no WF) | 1 | 1.09 | 0.296 |
| Deprivation quintile | 4 | 8.34 | 0.0797 |
| Area by deprivation quintile | 4 | 2.68 | 0.6134 |
| Denominator | 1127 |
Table 142 also demonstrates no significant area by deprivation interaction overall.
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Quintile 1 | ||
| Non-fluoridated area | 0.225 | 0.088 to 0.362 |
| Fluoridated area | 0.273 | 0.099 to 0.448 |
| Quintile 2 | ||
| Non-fluoridated area | 0.305 | 0.188 to 0.421 |
| Fluoridated area | 0.217 | 0.096 to 0.338 |
| Quintile 3 | ||
| Non-fluoridated area | 0.248 | 0.136 to 0.360 |
| Fluoridated area | 0.212 | 0.105 to 0.319 |
| Quintile 4 | ||
| Non-fluoridated area | 0.314 | 0.195 to 0.433 |
| Fluoridated area | 0.219 | 0.121 to 0.318 |
| Quintile 5 | ||
| Non-fluoridated area | 0.348 | 0.214 to 0.481 |
| Fluoridated area | 0.347 | 0.235 to 0.459 |
A negative binomial regression was undertaken to assess if WF reduced health inequalities by examining the count of decay and the interaction between WF status and deprivation (Tables 144 and 145).
| Regression analysis (n = 1089) | IRR (95% CI) |
|---|---|
| Area: no WF vs. WF | 0.949 (0.314 to 2.869) |
| Deprivation quintile (reference quintile 1) | |
| 2 | 1.005 (0.424 to 2.384) |
| 3 | 0.925 (0.369 to 2.317) |
| 4 | 1.427 (0.617 to 3.303) |
| 5 | 1.449 (0.613 to 3.425) |
| Area by deprivation quintile interaction (reference WF by quintile 1) | |
| WF by quintile 2 | 0.701 (0.18 to 2.72) |
| WF by quintile 3 | 0.649 (0.171 to 2.462) |
| WF by quintile 4 | 0.516 (0.147 to 1.807) |
| WF by quintile 5 | 0.963 (0.286 to 3.241) |
| Age (centred) | 0.561 (0.356 to 0.882) |
| dmft at baseline | 1.179 (1.119 to 1.242) |
| Sex (male/female) | 0.968 (0.726 to 1.289) |
| Offset logs of teeth erupted | 1 |
| Variable | df | F-value | p > F |
|---|---|---|---|
| Area (WF vs. no WF) | 1 | 4.04 | 0.0455 |
| Deprivation quintile | 4 | 8.80 | 0.0664 |
| Area by deprivation quintile | 4 | 2.85 | 0.5833 |
| Denominator | 1089 |
Figure 35 and Table 146 show the predicted number of count of decay in permanent teeth including an interaction term for exposure by deprivation quintiles (and shown by males and females separately in Figures 36 and 37). Figure 35 and Table 146 illustrate that there is no significant interaction between deprivation and exposure when looking at decay, indicating that WF does not significantly reduce inequalities, as there are no overlapping CIs at each deprivation quintile.
FIGURE 35.
Adjusted predictions of number of counts of decay including an interaction term for area across deprivation quintiles with 95% CI.
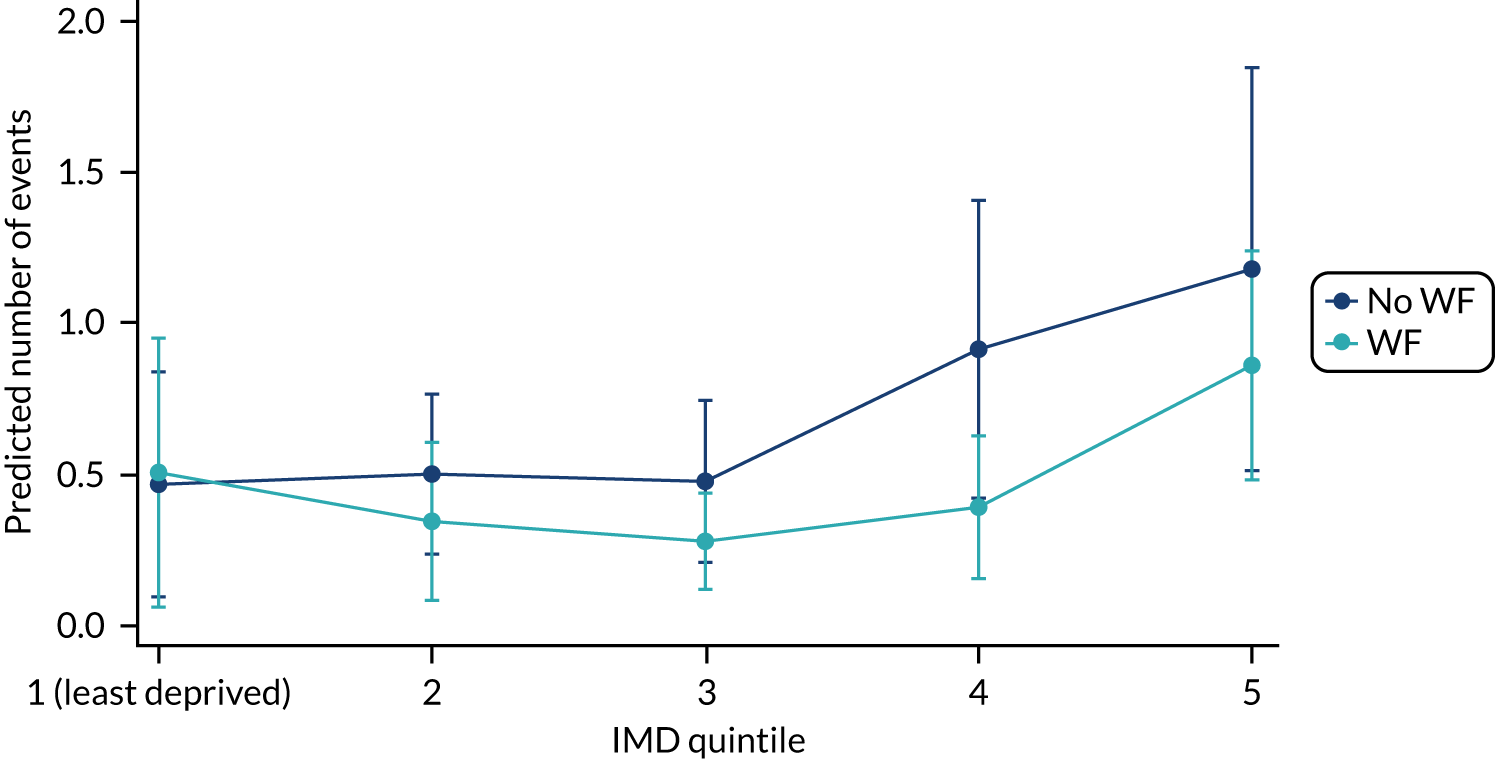
| Deprivation quintile by fluoridation status | Margin: probability | 95% CI |
|---|---|---|
| Quintile 1 | ||
| Non-fluoridated area | 0.465 | 0.093 to 0.838 |
| Fluoridated area | 0.504 | 0.059 to 0.950 |
| Quintile 2 | ||
| Non-fluoridated area | 0.499 | 0.234 to 0.764 |
| Fluoridated area | 0.343 | 0.082 to 0.604 |
| Quintile 3 | ||
| Non-fluoridated area | 0.475 | 0.207 to 0.743 |
| Fluoridated area | 0.276 | 0.117 to 0.436 |
| Quintile 4 | ||
| Non-fluoridated area | 0.912 | 0.419 to 1.406 |
| Fluoridated area | 0.390 | 0.153 to 0.626 |
| Quintile 5 | ||
| Non-fluoridated area | 1.178 | 0.511 to 1.845 |
| Fluoridated area | 0.859 | 0.480 to 1.238 |
FIGURE 36.
Adjusted predictions of number of counts of decay including an interaction term for area across deprivation quintiles, with 95% CI: males only.
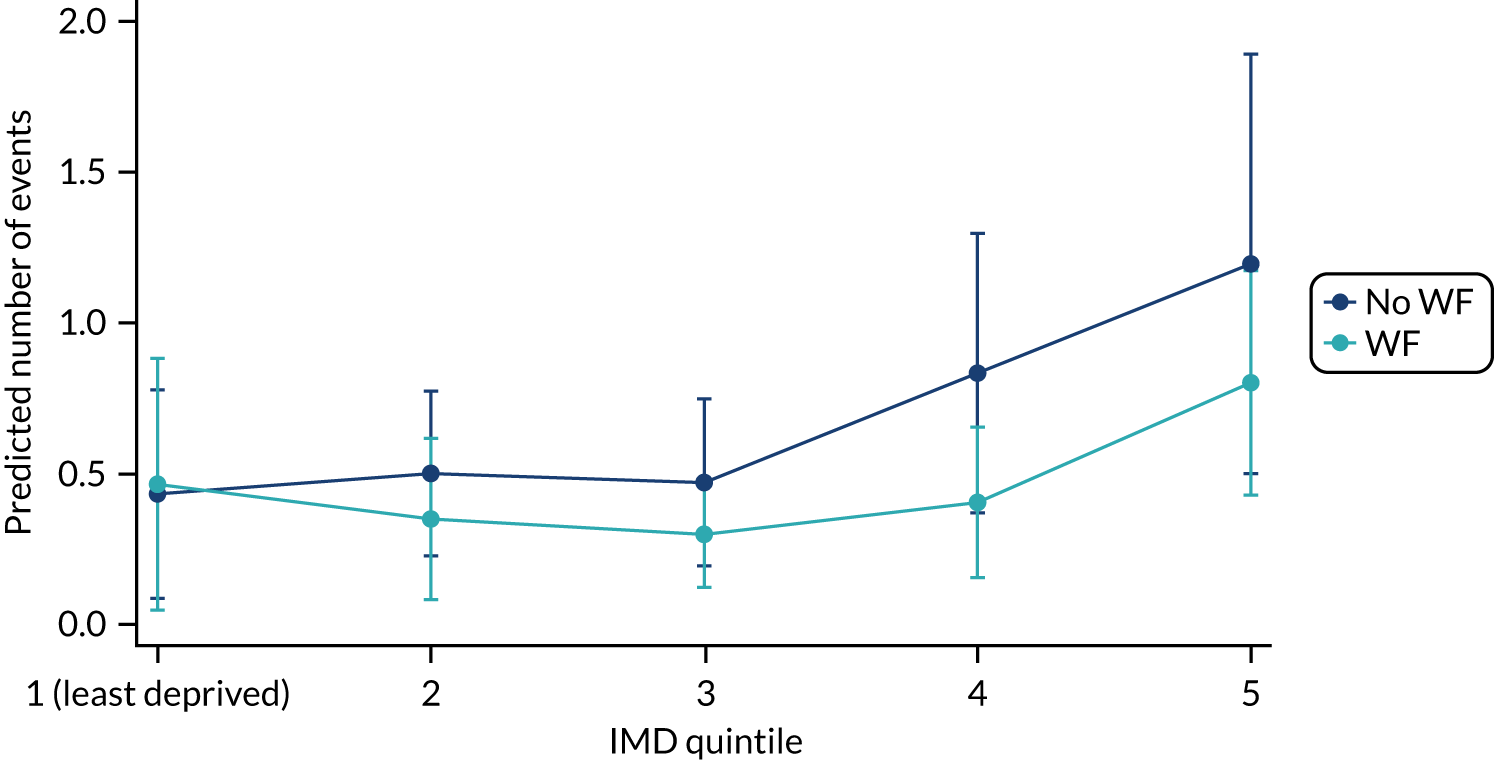
FIGURE 37.
Adjusted predictions of number of counts of decay including an interaction term for area across deprivation quintiles with 95% CI: females only.
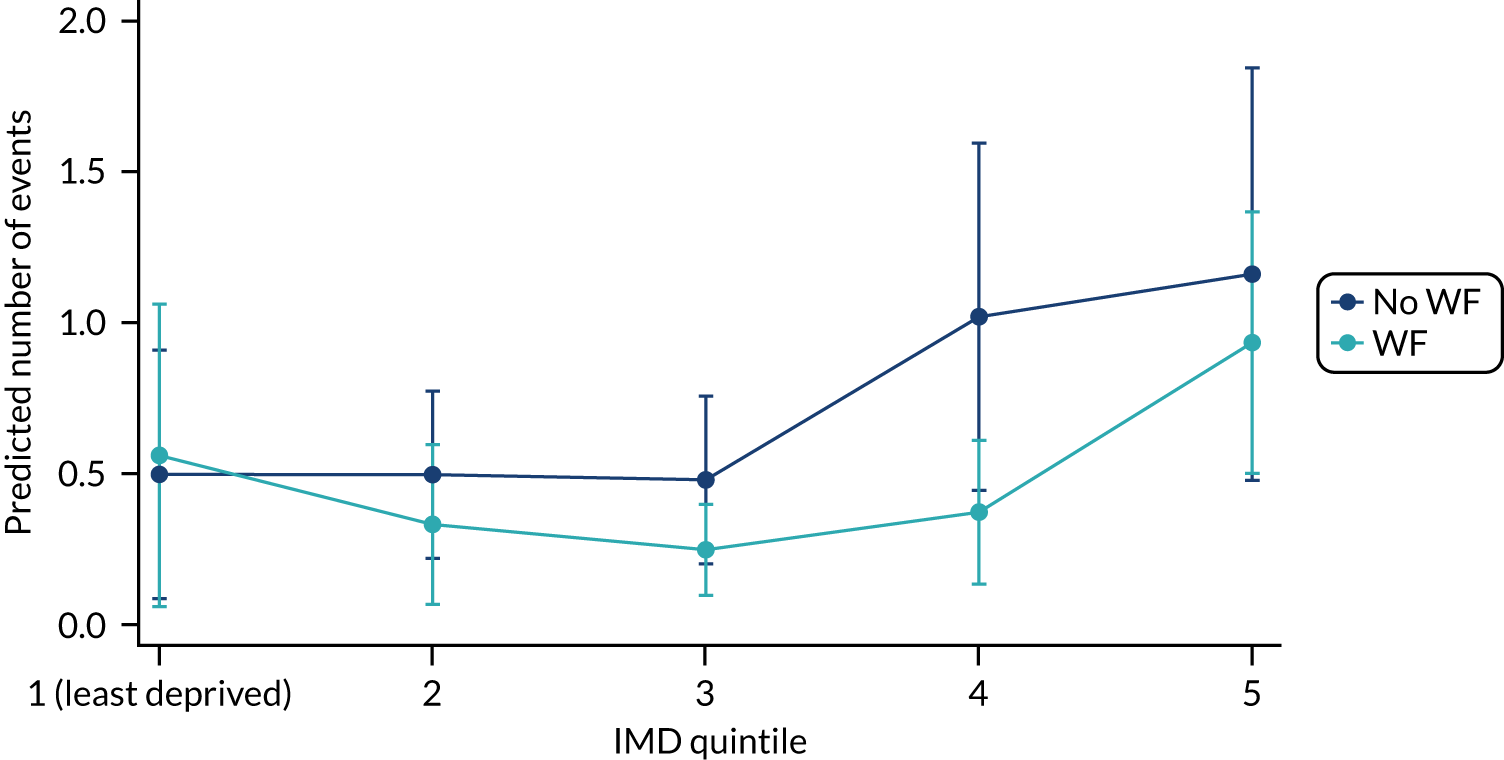
Table 145 demonstrates no significant area by deprivation interaction overall.
Post hoc analysis
Post hoc analyses were undertaken on account of fluoridation in Ennerdale experiencing a significant interruption due to flooding in Cumbria, which resulted in fluoridation being suspended for a year. Analysis was, therefore, split by fluoridation zones. There are three zones that are fluoridated within Cumbria. Zone 28 is fluoridated from Crummock (Cornhow), whereas zones 31 and 32 are fluoridated from Ennerdale (Table 147).
| Variable | Group A: control | Group B | ||||
|---|---|---|---|---|---|---|
| Cornhow: zone 28 (N = 244) | Ennerdale: zones 31/32 (N = 471) | |||||
| Examined (n = 835) | Not examined (n = 388) | Examined (n = 171) | Not examined (n = 73) | Examined (n = 391) | Not examined (n = 80) | |
| Mean deprivation score (SD) | 23.33 (13.31) | 23.77 (13.00) | 27.41 (14.97) | 22.41 (15.90) | 28.50 (15.89) | 29.21 (17.80) |
| Examined (n = 617) | Not examined (n = 296) | Examined (n = 171) | Not examined (n = 73) | Examined (n = 391) | Not examined (n = 80) | |
| Male | ||||||
| n | 339 | 153 | 102 | 36 | 211 | 39 |
| % | 54.9 | 51.7 | 58.6 | 60.0 | 54.5 | 61.90 |
| Female | ||||||
| n | 278 | 143 | 72 | 24 | 176 | 24 |
| % | 45.1 | 48.3 | 41.4 | 40.0 | 45.5 | 38.1 |
Table 148 shows that a larger number of individuals were examined in zones 31 and 32 than in zone 28 (despite zones 31 and 32 having a smaller number of participants overall).
| Examination status | Control | Cornhow: zone 28 | Ennerdale: zones 31/32 | Total cohort |
|---|---|---|---|---|
| Examined | ||||
| n | 622 | 176 | 394 | 1192 |
| % | 66.2 | 70.7 | 83.1 | 71.7 |
| Absent | ||||
| n | 22 | 4 | 12 | 38 |
| % | 2.3 | 1.6 | 2.5 | 2.3 |
| Child refused | ||||
| n | 9 | 2 | 5 | 16 |
| % | 1.0 | 0.8 | 1.1 | 1.0 |
| Opted out | ||||
| n | 40 | 18 | 28 | 86 |
| % | 4.3 | 7.2 | 5.9 | 5.2 |
| Child moved | ||||
| n | 99 | 22 | 33 | 154 |
| % | 10.5 | 8.8 | 7.0 | 9.3 |
| Missed because of COVID-19 | ||||
| n | 147 | 27 | 2 | 176 |
| % | 15.6 | 10.8 | 0.4 | 10.6 |
| Total | ||||
| n | 939 | 249 | 474 | 1662 |
Table 149 shows that none of the WF groups displayed a statistically significant effect in relation to the risk of having caries compared with no WF.
| Caries status | Group A: control, n (%) | Group B, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Zone 28 | Zones 31/32 | |||
| Decay present | 136 (21.9) | 32 (18.2) | 77 (20.5) | 245 (20.6) |
| Decay absent | 486 (78.1) | 144 (81.8) | 317 (79.5) | 947 (79.4) |
| Total | 622 | 176 | 394 | 1192 |
Appendix 4 Health economics
This appendix provides additional analysis completed for the health economic section of the report.
Birth cohort: completeness of data
Of the 1439 children with complete data on deprivation score (based on their postcode), we assessed if deprivation and/or fluoridation was associated with responding to the questionnaire. A linear regression was estimated against deprivation quintile dummies and a dummy for whether or not the child lived in a fluoridated area (Table 150). A logistic regression was also estimated; however, the results were qualitatively identical and, owing to the desire to test for differences across fluoridated areas, the linear regression approach was the preferred specification. There was no evidence that complete data were associated with children residing in a fluoridated area (p = 0.291), but completing the questionnaire was associated with living in a less deprived area. When interacting fluoridation with deprivation quintile, we found that the relationship between deprivation and completion did not vary across fluoridation groups, implying that the potential bias caused by deprivation was a similar bias across fluoridation groups.
| Covariate | Without WF and deprivation interaction (p-value) | With WF and deprivation interaction (p-value) |
|---|---|---|
| Deprivation quintile 1 (least deprived) | Reference | Reference |
| Deprivation quintile 2 | –0.0594 (0.261) | –0.0088 (0.892) |
| Deprivation quintile 3 | –0.0889 (0.078) | –0.0145 (0.817) |
| Deprivation quintile 4 | –0.2108 (< 0.001) | –0.1766 (0.004) |
| Deprivation quintile 5 (most deprived) | –0.2851 (< 0.001) | –0.2427 (< 0.001) |
| WF by deprivation quintile 1 | Reference | |
| WF by deprivation quintile 2 | –0.1462 (0.190) | |
| WF by deprivation quintile 3 | –0.2080 (0.048) | |
| WF by deprivation quintile 4 | –0.1054 (0.302) | |
| WF by deprivation quintile 5 | –0.1253 (0.228) | |
| Fluoridated area | –0.0268 (0.291) | 0.1048 (0.248) |
| Constant | 0.5262 (< 0.001) | 0.4805 (< 0.001) |
| n | 1439 | 1439 |
Table 151 summarises the proportion of children reporting responses to each of the CHU9D health domains. Cost data are presented in Tables 152–154.
| Domain responsea | Non-fluoridated area (N = 283), n (%) | Fluoridated area (N = 174), n (%) | Total (N = 457), n (%) |
|---|---|---|---|
| Worried | |||
| 1 | 253 (89.40) | 154 (88.51) | 407 (89.06) |
| 2 | 24 (8.48) | 13 (7.47) | 37 (8.10) |
| 3 | 6 (2.12) | 7 (4.02) | 13 (2.84) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Sad | |||
| 1 | 253 (89.40) | 155 (89.08) | 408 (89.28) |
| 2 | 27 (9.54) | 16 (9.20) | 43 (9.41) |
| 3 | 3 (1.06) | 3 (1.72) | 6 (1.31) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Pain | |||
| 1 | 256 (90.46) | 163 (93.68) | 419 (91.68) |
| 2 | 24 (8.48) | 8 (4.60) | 32 (7.00) |
| 3 | 1 (0.35) | 3 (1.72) | 4 (0.88) |
| 4 | 2 (0.71) | 0 (0.00) | 2 (0.44) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tired | |||
| 1 | 94 (33.22) | 62 (35.63) | 156 (34.14) |
| 2 | 129 (45.58) | 84 (48.28) | 213 (46.61) |
| 3 | 35 (12.37) | 19 (10.92) | 54 (11.82) |
| 4 | 21 (7.42) | 6 (3.45) | 27 (5.91) |
| 5 | 4 (1.41) | 3 (1.72) | 7 (1.53) |
| Annoyed | |||
| 1 | 217 (76.68) | 140 (80.46) | 357 (78.12) |
| 2 | 48 (16.69) | 27 (15.52) | 75 (16.41) |
| 3 | 14 (4.95) | 7 (4.02) | 21 (4.60) |
| 4 | 3 (1.06) | 0 (0.00) | 3 (0.66) |
| 5 | 1 (0.35) | 0 (0.00) | 1 (0.22) |
| School work | |||
| 1 | 252 (89.88) | 150 (86.21) | 402 (87.96) |
| 2 | 23 (8.13) | 18 (10.34) | 41 (8.97) |
| 3 | 6 (2.12) | 3 (1.72) | 9 (1.97) |
| 4 | 1 (0.35) | 1 (0.57) | 2 (0.44) |
| 5 | 1 (0.35) | 2 (1.15) | 3 (0.66) |
| Sleep | |||
| 1 | 225 (79.51) | 152 (87.36) | 377 (82.49) |
| 2 | 47 (16.61) | 16 (9.20) | 63 (13.79) |
| 3 | 7 (2.47) | 6 (3.45) | 13 (2.84) |
| 4 | 4 (1.41) | 0 (0.00) | 4 (0.88) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Daily routine | |||
| 1 | 242 (85.51) | 152 (87.36) | 394 (86.21) |
| 2 | 29 (10.25) | 16 (9.20) | 45 (9.85) |
| 3 | 9 (3.18) | 6 (3.45) | 15 (3.28) |
| 4 | 3 (1.06) | 0 (0.00) | 3 (0.66) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activities | |||
| 1 | 250 (88.34) | 161 (92.53) | 411 (89.93) |
| 2 | 25 (8.83) | 9 (5.17) | 34 (7.44) |
| 3 | 5 (1.77) | 2 (1.15) | 7 (1.53) |
| 4 | 2 (0.71) | 1 (0.57) | 3 (0.66) |
| 5 | 1 (0.35) | 1 (0.57) | 2 (0.44) |
| Domain responsea | Non-fluoridated area (N = 189), n (%) | Fluoridated area (N = 117), n (%) | Total (N = 306), n (%) |
|---|---|---|---|
| Worried | |||
| 1 | 171 (90.48) | 101 (88.32) | 272 (88.89) |
| 2 | 16 (8.47) | 11 (9.40) | 27 (8.82) |
| 3 | 2 (1.06) | 5 (4.27) | 7 (2.29) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Sad | |||
| 1 | 170 (89.95) | 105 (89.74) | 275 (89.87) |
| 2 | 17 (8.99) | 10 (8.55) | 27 (8.82) |
| 3 | 2 (1.06) | 2 (1.71) | 4 (1.31) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Pain | |||
| 1 | 170 (89.95) | 108 (92.31) | 278 (90.85) |
| 2 | 16 (8.47) | 7 (5.98) | 23 (7.52) |
| 3 | 1 (0.53) | 2 (1.71) | 3 (0.98) |
| 4 | 2 (1.06) | 0 (0.00) | 2 (0.65) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tired | |||
| 1 | 62 (32.80) | 40 (34.19) | 102 (33.33) |
| 2 | 86 (45.50) | 55 (47.01) | 141 (46.08) |
| 3 | 23 (12.17) | 16 (13.68) | 39 (12.75) |
| 4 | 15 (7.94) | 4 (3.42) | 19 (6.21) |
| 5 | 3 (1.59) | 2 (1.71) | 5 (1.63) |
| Annoyed | |||
| 1 | 147 (77.78) | 94 (80.34) | 241 (78.76) |
| 2 | 31 (16.40) | 18 (15.38) | 49 (16.01) |
| 3 | 9 (4.76) | 5 (4.27) | 14 (4.58) |
| 4 | 1 (0.53) | 0 (0.00) | 1 (0.33) |
| 5 | 1 (0.53) | 0 (0.00) | 1 (0.33) |
| School work | |||
| 1 | 172 (91.01) | 102 (87.18) | 274 (89.54) |
| 2 | 11 (5.82) | 12 (10.26) | 23 (7.52) |
| 3 | 5 (2.65) | 1 (0.85) | 6 (1.96) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 1 (0.53) | 2 (1.71) | 3 (0.98) |
| Sleep | |||
| 1 | 155 (82.01) | 103 (88.03) | 258 (84.31) |
| 2 | 25 (13.23) | 12 (10.26) | 37 (12.09) |
| 3 | 5 (2.65) | 2 (1.71) | 7 (2.29) |
| 4 | 4 (2.12) | 0 (0.00) | 4 (1.31) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Daily routine | |||
| 1 | 163 (86.24) | 99 (84.62) | 262 (85.62) |
| 2 | 16 (8.47) | 14 (11.97) | 30 (9.80) |
| 3 | 8 (4.23) | 4 (3.42) | 12 (3.92) |
| 4 | 2 (1.06) | 0 (0.00) | 2 (0.65) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activities | |||
| 1 | 167 (88.36) | 106 (90.60) | 273 (89.22) |
| 2 | 16 (8.47) | 7 (5.98) | 23 (7.52) |
| 3 | 4 (2.12) | 2 (1.71) | 6 (1.96) |
| 4 | 1 (0.53) | 1 (0.85) | 2 (0.65) |
| 5 | 1 (0.53) | 1 (0.85) | 2 (0.65) |
| Type of service | Non-fluoridated area (N = 208), mean (SD) | Fluoridated area (N = 131), mean (SD) |
|---|---|---|
| Dental activity band 1 | 5.60 (2.36) | 4.37 (2.10) |
| Dental activity band 2 | 0.18 (0.68) | 0.06 (0.27) |
| Dental activity band 3 | 0.00 (0.00) | 0.00 (0.00) |
| Dental activity urgent | 0.15 (0.47) | 0.05 (0.26) |
| Emergency hospital activity (dental) | 0.04 (0.19) | 0.03 (0.17) |
| Type of service | Non-fluoridated area (n = 311) | Fluoridated area (n = 193) |
|---|---|---|
| Dental activity, mean (£) (SE) | 137.24 (4.52) | 100.81 (4.54) |
Older school cohort: completeness of data
A linear regression was estimated against deprivation quintile dummies, age and a dummy for whether or not the child was in a fluoridated area (Tables 155–161). A logistic regression was also estimated; however, the results were qualitatively identical and, owing to the desire to test for differences across fluoridated areas, the linear regression approach was the preferred specification. There was some evidence that complete data were positively associated with children residing in a fluoridated area (p = 0.050) and with children in less deprived areas. When interacting fluoridation with deprivation quintile, we found that the relationship between deprivation and completion did not vary across fluoridation groups, implying that the potential bias caused by deprivation was a similar bias across fluoridation groups.
| Covariate | Without WF and deprivation interaction (p-value) | With WF and deprivation interaction (p-value) |
|---|---|---|
| Deprivation quintile 1 | Reference | Reference |
| Deprivation quintile 2 | 0.0814 (0.167) | 0.0505 (0.466) |
| Deprivation quintile 3 | 0.1594 (0.005) | 0.1678 (0.014) |
| Deprivation quintile 4 | 0.2512 (< 0.001) | 0.2458 (< 0.001) |
| Deprivation quintile 5 | 0.2838 (< 0.001) | 0.2990 (< 0.001) |
| WF by deprivation quintile 1 | Reference | |
| WF by deprivation quintile 2 | 0.0759 (0.504) | |
| WF by deprivation quintile 3 | –0.0186 (0.867) | |
| WF by deprivation quintile 4 | 0.0124 (0.907) | |
| WF by deprivation quintile 5 | –0.0242 (0.819) | |
| Age (years) | –0.0190 (0.637) | –0.0226 (0.579) |
| Fluoridated area | 0.0550 (0.050) | 0.0485 (0.597) |
| Constant | 0.5398 (0.009) | 0.5599 (0.009) |
| n | 1145 | 1145 |
| Domain responsea | Non-fluoridated area (N = 226), n (%) | Fluoridated area (N = 162), n (%) | Total (N = 388), n (%) |
|---|---|---|---|
| Worried | |||
| 1 | 204 (90.27) | 145 (89.51) | 349 (89.95) |
| 2 | 21 (9.29) | 15 (9.26) | 36 (9.28) |
| 3 | 1 (0.44) | 1 (0.62) | 2 (0.52) |
| 4 | 0 (0.00) | 1 (0.62) | 1 (0.26) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Sad | |||
| 1 | 211 (93.36) | 146 (90.12) | 357 (92.01) |
| 2 | 13 (5.75) | 16 (9.88) | 29 (7.47) |
| 3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 4 | 1 (0.44) | 0 (0.00) | 1 (0.26) |
| 5 | 1 (0.44) | 0 (0.00) | 1 (0.26) |
| Pain | |||
| 1 | 207 (91.59) | 143 (88.27) | 350 (90.21) |
| 2 | 18 (7.96) | 18 (11.11) | 36 (9.28) |
| 3 | 1 (0.44) | 1 (0.62) | 2 (0.52) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tired | |||
| 1 | 113 (50.00) | 64 (39.51) | 177 (45.62) |
| 2 | 88 (38.94) | 82 (50.62) | 170 (43.81) |
| 3 | 17 (7.52) | 12 (7.41) | 29 (7.47) |
| 4 | 4 (1.77) | 3 (1.85) | 7 (1.80) |
| 5 | 4 (1.77) | 1 (0.62) | 5 (1.29) |
| Annoyed | |||
| 1 | 192 (84.96) | 136 (83.95) | 328 (84.54) |
| 2 | 30 (13.27) | 23 (14.20) | 53 (13.66) |
| 3 | 3 (1.33) | 3 (1.85) | 6 (1.55) |
| 4 | 1 (0.44) | 0 (0.00) | 1 (0.26) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| School work | |||
| 1 | 188 (83.19) | 136 (83.95) | 324 (83.51) |
| 2 | 32 (14.16) | 17 (10.49) | 49 (12.63) |
| 3 | 2 (0.88) | 7 (4.32) | 9 (2.32) |
| 4 | 3 (1.33) | 2 (1.23) | 5 (1.29) |
| 5 | 1 (0.44) | 0 (0.00) | 1 (0.26) |
| Sleep | |||
| 1 | 186 (82.30) | 128 (79.01) | 314 (80.93) |
| 2 | 32 (14.16) | 26 (16.05) | 58 (14.95) |
| 3 | 7 (3.10) | 4 (2.47) | 11 (2.84) |
| 4 | 1 (0.44) | 4 (2.47) | 5 (1.29) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Daily routine | |||
| 1 | 192 (84.96) | 130 (80.25) | 322 (82.99) |
| 2 | 28 (12.39) | 26 (16.05) | 54 (13.92) |
| 3 | 5 (2.21) | 5 (3.09) | 10 (2.58) |
| 4 | 1 (0.44) | 1 (0.62) | 2 (0.52) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activities | |||
| 1 | 200 (88.50) | 140 (86.42) | 340 (87.63) |
| 2 | 21 (9.29) | 18 (11.11) | 39 (10.05) |
| 3 | 3 (1.33) | 3 (1.85) | 6 (1.55) |
| 4 | 2 (0.88) | 1 (0.62) | 3 (0.77) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Domain responsea | Non-fluoridated area (N = 226), n (%) | Fluoridated area (N = 162), n (%) | Total (N = 388), n (%) |
|---|---|---|---|
| Worried | |||
| 1 | 139 (61.50) | 117 (72.22) | 256 (65.98) |
| 2 | 65 (28.76) | 29 (17.90) | 94 (24.23) |
| 3 | 13 (5.75) | 7 (4.32) | 20 (5.15) |
| 4 | 7 (3.10) | 6 (3.70) | 13 (3.35) |
| 5 | 2 (0.88) | 3 (1.85) | 5 (1.29) |
| Sad | |||
| 1 | 185 (81.86) | 140 (86.42) | 325 (83.76) |
| 2 | 23 (10.18) | 18 (11.11) | 41 (10.57) |
| 3 | 17 (7.52) | 1 (0.62) | 18 (4.64) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 1 (0.44) | 3 (1.85) | 4 (1.03) |
| Pain | |||
| 1 | 154 (68.14) | 111 (68.52) | 265 (68.30) |
| 2 | 47 (20.80) | 38 (23.46) | 85 (21.91) |
| 3 | 17 (7.52) | 9 (5.56) | 26 (6.70) |
| 4 | 6 (2.65) | 3 (1.85) | 9 (2.32) |
| 5 | 2 (0.88) | 1 (0.62) | 3 (0.77) |
| Tired | |||
| 1 | 53 (23.45) | 46 (28.40) | 99 (25.52) |
| 2 | 90 (39.82) | 69 (42.59) | 159 (40.98) |
| 3 | 37 (16.37) | 20 (12.35) | 57 (14.69) |
| 4 | 24 (10.62) | 20 (12.35) | 44 (11.34) |
| 5 | 22 (9.73) | 7 (4.32) | 29 (7.47) |
| Annoyed | |||
| 1 | 180 (79.65) | 134 (82.72) | 314 (80.93) |
| 2 | 32 (14.16) | 21 (12.96) | 53 (13.66) |
| 3 | 10 (4.42) | 3 (1.85) | 13 (3.35) |
| 4 | 2 (0.88) | 2 (1.23) | 4 (1.03) |
| 5 | 2 (0.88) | 2 (1.23) | 4 (1.03) |
| School work | |||
| 1 | 174 (76.99) | 120 (74.07) | 294 (75.77) |
| 2 | 42 (18.58) | 30 (18.52) | 72 (18.56) |
| 3 | 7 (3.10) | 9 (5.56) | 16 (4.12) |
| 4 | 1 (0.44) | 1 (0.62) | 2 (0.52) |
| 5 | 2 (0.88) | 2 (1.23) | 4 (1.03) |
| Sleep | |||
| 1 | 143 (63.27) | 100 (61.73) | 243 (62.63) |
| 2 | 50 (22.12) | 41 (25.31) | 91 (23.45) |
| 3 | 20 (8.85) | 12 (7.41) | 32 (8.25) |
| 4 | 11 (4.87) | 6 (3.70) | 17 (4.38) |
| 5 | 2 (0.88) | 3 (1.85) | 5 (1.29) |
| Daily routine | |||
| 1 | 203 (89.82) | 142 (87.65) | 345 (88.92) |
| 2 | 22 (9.73) | 16 (9.88) | 38 (9.79) |
| 3 | 1 (0.44) | 4 (2.47) | 5 (1.29) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activities | |||
| 1 | 190 (84.07) | 133 (82.10) | 323 (83.25) |
| 2 | 24 (10.62) | 17 (10.49) | 41 (10.57) |
| 3 | 10 (4.42) | 3 (1.85) | 13 (3.35) |
| 4 | 0 (0.00) | 7 (4.32) | 7 (1.80) |
| 5 | 2 (0.88) | 2 (1.23) | 4 (1.03) |
| Domain responsea | Non-fluoridated area (N = 159), n (%) | Fluoridated area (N = 112), n (%) | Total (N = 271), n (%) |
|---|---|---|---|
| Worried | |||
| 1 | 143 (89.94) | 98 (87.50) | 241 (88.93) |
| 2 | 16 (10.06) | 12 (10.71) | 28 (10.33) |
| 3 | 0 (0.00) | 1 (0.89) | 1 (0.37) |
| 4 | 0 (0.00) | 1 (0.89) | 1 (0.37) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Sad | |||
| 1 | 146 (91.82) | 101 (90.18) | 247 (91.14) |
| 2 | 11 (6.92) | 11 (9.82) | 22 (8.12) |
| 3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 4 | 1 (0.63) | 0 (0.00) | 1 (0.37) |
| 5 | 1 (0.63) | 0 (0.00) | 1 (0.37) |
| Pain | |||
| 1 | 144 (90.57) | 100 (89.29) | 244 (90.04) |
| 2 | 15 (9.43) | 11 (9.82) | 26 (9.59) |
| 3 | 0 (0.00) | 1 (0.89) | 1 (0.37) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tired | |||
| 1 | 80 (50.31) | 45 (40.18) | 25 (46.13) |
| 2 | 60 (37.74) | 55 (49.11) | 115 (42.44) |
| 3 | 12 (7.55) | 8 (7.14) | 20 (7.38) |
| 4 | 4 (2.52) | 3 (2.68) | 7 (2.58) |
| 5 | 3 (1.89) | 1 (0.89) | 4 (1.48) |
| Annoyed | |||
| 1 | 135 (84.91) | 91 (81.25) | 226 (83.39) |
| 2 | 21 (13.21) | 19 (16.96) | 40 (14.76) |
| 3 | 2 (1.26) | 2 (1.79) | 4 (1.48) |
| 4 | 1 (0.63) | 0 (0.00) | 1 (0.37) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| School work | |||
| 1 | 135 (84.91) | 93 (83.04) | 228 (84.13) |
| 2 | 20 (12.58) | 14 (12.50) | 34 (12.55) |
| 3 | 0 (0.00) | 5 (4.46) | 5 (1.85) |
| 4 | 3 (1.89) | 0 (0.00) | 3 (1.11) |
| 5 | 1 (0.63) | 0 (0.00) | 1 (0.37) |
| Sleep | |||
| 1 | 130 (81.76) | 94 (83.93) | 224 (82.66) |
| 2 | 24 (15.09) | 14 (12.50) | 38 (14.02) |
| 3 | 4 (2.52) | 3 (2.68) | 7 (2.58) |
| 4 | 1 (0.63) | 1 (0.89) | 2 (0.74) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Daily routine | |||
| 1 | 134 (84.28) | 89 (79.46) | 223 (82.29) |
| 2 | 22 (13.84) | 19 (16.69) | 41 (15.13) |
| 3 | 3 (1.89) | 4 (3.57) | 7 (2.58) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activities | |||
| 1 | 139 (87.42) | 97 (86.61) | 236 (87.08) |
| 2 | 16 (10.06) | 13 (11.61) | 29 (10.70) |
| 3 | 2 (1.26) | 1 (0.89) | 3 (1.11) |
| 4 | 2 (1.26) | 1 (0.89) | 3 (1.11) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Domain responsea | Non-fluoridated area (N = 159), n (%) | Fluoridated area (N = 112), n (%) | Total (N = 271), n (%) |
|---|---|---|---|
| Worried | |||
| 1 | 97 (61.01) | 78 (69.64) | 175 (64.58) |
| 2 | 47 (29.56) | 20 (17.86) | 67 (24.72) |
| 3 | 9 (5.66) | 5 (4.46) | 14 (5.17) |
| 4 | 5 (3.14) | 6 (5.36) | 11 (4.06) |
| 5 | 1 (0.63) | 3 (2.68) | 4 (1.48) |
| Sad | |||
| 1 | 130 (81.76) | 95 (84.82) | 225 (83.03) |
| 2 | 16 (10.06) | 13 (11.61) | 29 (10.70) |
| 3 | 12 (7.55) | 1 (0.89) | 13 (4.80) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 1 (0.63) | 3 (2.68) | 4 (1.48) |
| Pain | |||
| 1 | 108 (67.92) | 81 (72.32) | 189 (69.74) |
| 2 | 31 (19.50) | 21 (18.75) | 52 (19.19) |
| 3 | 13 (8.18) | 6 (5.36) | 19 (7.01) |
| 4 | 5 (3.14) | 3 (2.68) | 8 (2.95) |
| 5 | 2 (1.26) | 1 (0.89) | 3 (1.11) |
| Tired | |||
| 1 | 34 (21.38) | 35 (31.25) | 69 (25.46) |
| 2 | 67 (42.14) | 45 (40.18) | 112 (41.33) |
| 3 | 23 (14.47) | 12 (10.71) | 35 (12.92) |
| 4 | 18 (11.32) | 14 (12.50) | 32 (11.81) |
| 5 | 17 (10.69) | 6 (5.36) | 23 (8.49) |
| Annoyed | |||
| 1 | 130 (81.76) | 90 (80.36) | 220 (81.18) |
| 2 | 18 (11.32) | 16 (14.29) | 34 (12.55) |
| 3 | 7 (4.40) | 3 (2.68) | 10 (3.69) |
| 4 | 2 (1.26) | 2 (1.79) | 4 (1.48) |
| 5 | 2 (1.26) | 1 (0.89) | 3 (1.11) |
| School work | |||
| 1 | 126 (79.25) | 86 (76.79) | 212 (78.23) |
| 2 | 25 (15.72) | 19 (16.96) | 44 (16.24) |
| 3 | 5 (3.14) | 5 (4.46) | 10 (3.69) |
| 4 | 1 (0.63) | 1 (0.89) | 2 (0.74) |
| 5 | 2 (1.26) | 1 (0.89) | 3 (1.11) |
| Sleep | |||
| 1 | 103 (64.78) | 67 (59.82) | 170 (62.73) |
| 2 | 30 (18.87) | 31 (27.68) | 61 (22.51) |
| 3 | 17 (10.69) | 8 (7.14) | 25 (9.23) |
| 4 | 7 (4.40) | 4 (3.57) | 11 (4.06) |
| 5 | 2 (1.26) | 2 (1.79) | 4 (1.48) |
| Daily routine | |||
| 1 | 140 (88.05) | 96 (85.71) | 236 (87.08) |
| 2 | 18 (11.32) | 12 (10.71) | 30 (11.07) |
| 3 | 1 (0.63) | 4 (3.57) | 5 (1.85) |
| 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 5 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activities | |||
| 1 | 133 (83.65) | 88 (78.57) | 221 (81.55) |
| 2 | 18 (11.32) | 14 (12.50) | 32 (11.81) |
| 3 | 7 (4.40) | 3 (2.68) | 10 (3.69) |
| 4 | 0 (0.00) | 5 (4.46) | 5 (1.85) |
| 5 | 1 (0.63) | 2 (1.79) | 3 (1.11) |
| Type of service | Non-fluoridated area (N = 163), mean (SD) | Fluoridated area (N = 113), mean (SD) |
|---|---|---|
| Dental activity band 1 | 11.74 (4.50) | 8.91 (4.62) |
| Dental activity band 2 | 2.18 (2.64) | 1.75 (2.24) |
| Dental activity band 3 | 0.01 (0.08) | 0.02 (0.13) |
| Dental activity urgent | 0.50 (0.95) | 0.39 (0.77) |
| Emergency hospital activity (dental) | 0.11 (0.31) | 0.10 (0.30) |
| Type of service | Non-fluoridated (n = 229) | Fluoridated (n = 163) |
|---|---|---|
| Dental activity, mean (£) (SE) | 437.63 (15.15) | 359.57 (17.39) |
Appendix 5 Patient and public involvement: Guidance for Reporting Involvement of Patients and the Public 2
Aim
The aim of PPI within the CATFISH study was to support the research to meet its full potential in understanding the effect of WF on children’s teeth. To do this, we needed to ensure maximum recruitment of and engagement with parents within Cumbria, as they would provide both their and their child’s permission to be involved in the study, as well as engagement in the study as it continued.
Methods
The CATFISH study utilised various networks to involve PPI within the development and running of the project. Several permanent PPI members (mothers of children mostly under the age of 11 years old) took part throughout the study; however, as the study lasted over 7 years, some members moved or lost contact during the project and, therefore, new members were recruited at various points during the study. PPI members fed back to the Oversight Committee through the project manager on all aspects of the study, including study design, how to increase engagement and dissemination to participants during the study.
The PPI lead Julie Fletcher (a senior member of Barnardo’s) facilitated further PPI and one-off large-scale PPI feedback sessions on aspects such as questionnaire development and participant information sheets. To explore the most appropriate way to recruit pregnant women onto the study and the information they would require, etc., PPI also took place at Cumberland Infirmary at Carlisle and at Whitehaven with individuals who were pregnant and attending their 20-week ultrasound.
Salford Citizen Scientist (Salford, UK) groups were also utilised to provide feedback on various parts of the project and to help pilot the questionnaire before it was finalised and distributed to participants.
Outcome of patient and public involvement/study results
The involvement of PPI throughout the study resulted in a data collection tool that was acceptable to parents. Certain questions were rephrased, updated or condensed based on PPI feedback. For example, during PPI, parents did not understand why there was a question on income and, therefore, this section was more fully explained within the questionnaire and how the response would be used within analysis. In addition, parents stated that they would not know their weekly income and so this was also illustrated as annual income. Parents were provided questionnaires as an online form or paper form, depending on preference. Further PPI feedback resulted in the questionnaire being provided annually rather than every 6 months.
Recruitment occurred at the 20-week scan or shortly after birth, as PPI feedback suggested that any recruitment at an earlier stage may result in a reluctance to sign up. Participants wanted a simple one-page piece of information about the project and an outline of what would be involved, as well as a more in-depth piece of information that they could take away with them, and so this was provided within the study.
Birthday cards sent each year and newsletters updating parents about the study were appreciated and facilitated engagement and retention. Following PPI feedback, the introduction of a voucher alongside the final questionnaire was implemented to increase response for this final data collection point.
The child questionnaire that was completed at the end of the study by 11-year-olds was tested among individuals of a similar age. No issues were identified and the questionnaire was successfully completed by most children who took part.
Discussion/conclusion
There was a concern that parents may be reluctant to answer certain questions posed within the data collection form. However, most questions were answered when a parent decided to complete a questionnaire. The only exception was with regard to the height and weight of a child; however, from feedback, this was purely as the parent did not have this information to hand.
Reflections and critical perspective
In the study, several parents did voice their concern or confusion about several of the questions asked (e.g. questions on income and employment status); however, these questions were thought to be beneficial to the study and so the reasons for inclusion were detailed rather than removing the questions. Further explanation needed to be balanced against having excessive text within the questionnaire, and too many questions. This is a particular issue with participants competing questionnaires on their own, rather than with a researcher who could explain or justify why questions are included. Questionnaires completed over the telephone were trialled, but the majority of participants did not answer their telephone. When participants did answer, the telephone call could act as a reminder, but few participants decided to complete the questionnaire at that time.
The project continuously attempted to recruit more PPI members; however, despite adverts online and utilising links through Barnardo’s/Sure Start and Cumbria County Council, this proved difficult and we had few individuals who wanted to engage to be permanent PPI members. What worked well was face-to-face large-scale feedback when attending Sure Start centres. Parents were very happy to contribute at that moment, but did not want to sign up to provide long term feedback on the project. Although this has implications for representativeness, the team believes that PPI made a significant contribution to the study and its outcome.
Glossary
- Caries
- Tooth decay, also known as dental caries or cavities, is the breakdown of dental hard tissues due to acids made by bacteria present in dental plaque as result of their metabolisation of dietary carbohydrates.
- Caries free
- In this report, ‘caries free’ refers to the absence of caries into dentine rather than the absence of any clinical caries, such as caries into enamel.
- Caries increment
- Count of new decayed, filled or extracted teeth on newly erupted surfaces (after baseline/recruitment). For the older school cohort, permanent teeth were not included in decayed, missing or filled teeth (permanent) if they had already erupted at baseline. For the birth cohort, as all children were recruited from birth, all teeth with decayed, missing or filled teeth (primary) resulted in a caries increment.
- Child Health Utility 9-Dimensions
- A generic health-related quality-of-life measure containing nine dimensions.
- Clinical Commissioning Group
- An NHS organisation set up by the Health and Social Care Act 2012 (Great Britain. Health and Social Care Act 2012. London: The Stationery Office; 2012) to organise the delivery of NHS services in England. Clinical Commissioning Groups replaced the primary care commissioning functions of primary care trusts.
- Commissioning
- According to the Department of Health and Social Care, the means to secure the best-value health care for the local population and taxpayers.
- Decayed, missing or filled surface (permanent)
- A measure of the condition of an individual’s or a population’s oral health in their permanent teeth. An individual tooth may have up to five surfaces that can be carious.
- Decayed, missing or filled surface (primary)
- A measure of the condition of an individual’s or a population’s oral health in their primary teeth. An individual tooth may have up to five surfaces that can be carious.
- Decayed, missing or filled teeth (permanent)
- A measure of the condition of an individual’s or a population’s oral health in their permanent teeth. Decayed teeth generally represent untreated disease.
- Decayed, missing or filled teeth (primary)
- A measure of the condition of an individual’s or a population’s oral health in their primary teeth. Decayed teeth generally represent untreated disease.
- Fluorosis
- A cosmetic condition that affects the teeth. Fluorosis is caused by overexposure to fluoride during the first 8 years of life, when most permanent teeth are being formed. After the teeth erupt, the teeth of individuals affected by fluorosis may appear mildly discoloured, with mottled areas or lines.
- FP17
- A form that needs to be submitted in order for dental care providers to claim payment for NHS activity under general dental service and personal dental service contracts. FP17 forms detail dental activity data. The data recorded on the FP17 show the patient charge collected, the number of units of activity performed and treatment banding information.
- General dental services
- The most widespread of the two main contract types for primary care dentistry. General dental service contracts are usually not time limited and contract holders are required to provide the full range of services described as ‘mandatory’. Patient charge revenue is collected and the units of dental activity are the currency of the contract.
- High-street dentists
- Also known as general dental practitioners, high-street dentists are the only clinicians who can contract directly with the NHS. In England, general dental practices are provided with a target for their clinical activity, known as the Annual Contract Value. NHS courses of dental treatment in England are categorised into three bands (i.e. band 1, band 2 and band 3) to reflect differing degrees of treatment complexity. Band 1 relates to examinations and preventative treatments, whereas bands 2 and 3 relate to invasive and irreversible dental procedures. Band 1 attracts 1 unit of dental activity, whereas bands 2 and 3 attract 3 and 12 units of dental activity, respectively. The Annual Contract Value is the annual target of units of dental activity that a dental team must provide. [Reproduced from Goldthorpe J, Walsh T, Tickle M, Birch S, Hill H, Sanders C, et al. An evaluation of a referral management and triage system for oral surgery referrals from primary care dentists: a mixed-methods study. Health Serv Deliv Res 2018;6(8). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.]
- Hospital Episode Statistics
- A database that contains details of all admissions to NHS hospitals and all NHS outpatient appointments in England.
- NHS Business Services Authority
- The authority that remunerates dentists based on FP17 form claims submitted, and provides dental statistics and key information to national, regional and local NHS organisations.
- Office of Population Censuses and Surveys codes
- A published procedural classification and coding of operations, procedures and interventions. This is a four-character code system. The first character is always a letter and the other three characters are numbers. All codes beginning with ‘F’ are related to the mouth.
- Patient charge revenue
- Revenue generated by the fees charged for dental treatment at bands 1, 2 and 3. The patient charge revenue is a co-payment scheme and certain individuals are exempted from paying based on their age or benefit status.
- Payment by Results
- The mechanism that NHS secondary care providers use to finance their service by reporting elements of care provided.
- Performer
- A qualified clinician who is contracted to perform the service and is registered on the national performer list.
- Primary care trust
- Part of the NHS in England that existed from 2001 to 2013. Primary care trusts were largely administrative bodies that were responsible for commissioning primary, community and secondary health services from providers. Until 31 May 2011, primary care trusts also provided community health services directly.
- Provider
- The contract holder to provide a service. In dentistry, this may be an individual, a legal partnership or, increasingly, a corporate body.
- Quality-adjusted life-year
- A measure of years in perfect health, comprising years of life multiplied by quality of life.
- Secondary Uses Service
- The service that holds patient-level information regarding service provision. This information can be used for health-care planning, commissioning services, Payment by Results and developing and enhancing national policy.
- Service-level Agreement Monitoring Data
- Sometimes called trading data, Service-level Agreement Monitoring Data are routinely sent from NHS hospitals to commissioning organisations in accordance with the provisions of the information schedule in the standard contract. Almost all acute trusts send trading data, but there is no standardised way of sharing the same information. Trading data are effectively a monthly invoice, aggregated and at patient level, sent per the national timetable of reconciliation and post reconciliation dates. [Reproduced from Goldthorpe J, Walsh T, Tickle M, Birch S, Hill H, Sanders C, et al. An evaluation of a referral management and triage system for oral surgery referrals from primary care dentists: a mixed-methods study. Health Serv Deliv Res 2018;6(8). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.]
- Strategic Health Authority
- Part of the structure of the NHS in England between 2002 and 2013. Each Strategic Health Authority was responsible for managing performance, enacting directives and implementing health policy, as required by the Department of Health and Social Care at a regional level.
- Unit of dental activity
- The contract currency for general dental service and personal dental service contracts in England. Each dental procedure has been classified into a banding structure, which determines what patients pay in NHS dental charges and the number of units of dental activity a dentist receives. Band 1 attracts 1 unit of dental activity, whereas bands 2 and 3 attract 3 and 12 units of dental activity, respectively. The Annual Contract Value is the annual target of units of dental activity that a dental contact must provide. The national average price for a unit of dental activity is approximately £25, but the unit of dental activity value is determined individually for each contract and, therefore, dental practices in the same locality, serving similar populations, are likely to have different unit of dental activity values.
List of abbreviations
- BASCD
- British Association for the Study of Community Dentistry
- BSA
- Business Services Authority
- CATFISH
- Cumbrian Assessment of Teeth a Fluoride Intervention Study for Health
- CCA
- complete-case analysis
- CD-MCAR
- covariate-dependent missing completely at random
- CHU9D
- Child Health Utility 9-Dimensions
- CI
- confidence interval
- DGA
- dental general anaesthetic
- DMFT
- decayed, missing or filled teeth (permanent)
- dmft
- decayed, missing or filled teeth (primary)
- GEE
- generalised estimating equation
- GRIPP2
- Guidance for Reporting Involvement of Patients and the Public 2
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IQ
- intelligence quotient
- IRR
- incidence rate ratio
- MAR
- missing at random
- MCAR
- missing completely at random
- MNAR
- missing not at random
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PPI
- patient and public involvement
- ppm
- part per million
- QALY
- quality-adjusted life-year
- SE
- standard error
- SHA
- Strategic Health Authority
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- UDA
- unit of dental activity
- WF
- water fluoridation
- WTPT
- willingness-to-pay value threshold
