Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 10/90/04. The contractual start date was in April 2012. The final report began editorial review in February 2016 and was accepted for publication in August 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ian M Anderson reports personal fees from Alkermes, Janssen, Lundbeck-Otsuka and Takeda, outside the submitted work. Rebecca Elliot reports grant support from Genzyme Therapeutics, Pfizer and Johnson & Johnson and personal fees from P1vital, all outside the submitted work. Colleen Loo reports lecture fees from MECTA Corporation, outside the submitted work. Rajesh Nair reports personal fees from Lundbeck and Sunovion, outside the submitted work. R Hamish McAllister-Williams reports personal fees from Roche, Ferro Group, Sunovion, Pulse, Janssen, My Tomorrows, Lundbeck, AstraZeneca, Bristol-Myers Squibb, Cyberonics, Eli Lilly, Servier, Saudi Pharmaceutical Industries & Medical Appliances Corporation, Otsuka and Pfizer, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Anderson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and objectives
Scientific background
Between 2000 and 2012 unipolar depressive disorders ranked ninth in the world, and third in Europe, among causes of health-related disability, making up 2.8% and 3.8%, respectively, of the total disease burden. 1 Bipolar disorder, in which time spent in a depressed state is at least three times greater than for mania,2 accounted for a further 0.5% of total disability both worldwide and in Europe during this period. 1 In the UK around 3% of the population meet the criteria for major depression at any one time3 and this high prevalence of depression is combined with a significant proportion of patients failing to respond adequately to treatment. In the largest study to date examining patient outcomes in major depression, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, only one-quarter to one-third of patients remitted (i.e. had recovered or had minimal symptoms) after the first antidepressant and this fell to about one in seven in patients who had failed two or more treatments; overall, about one-third of patients failed to remit even after four sequential pharmacological treatments. 4
Clinical use of electroconvulsive therapy
The National Institute for Health and Care Excellence (NICE) recommends electroconvulsive therapy (ECT) as a treatment option for patients with severe depression that is life-threatening or for those with moderate or severe depression who have not responded to multiple drug treatments and psychological treatment. 5 ECT involves inducing a therapeutic generalised seizure by passing an electric current across the brain and predates the first antidepressant drugs by nearly two decades. 6 In modern practice ECT is given under a brief general anaesthetic with a muscle relaxant to minimise the physical seizure and potential harm from this. It is given two or three times a week for a typical course of about eight treatments over 3–4 weeks. ECT has been demonstrated to have greater acute treatment efficacy than pharmacotherapy [with a large effect size (ES) of –0.8]7 and it achieves acute remission rates of just under 50% in patients who have failed to respond to previous drug treatments. 8 The latest audit data from the Scottish ECT Accreditation Network (SEAN) reported that about 75% of depressed patients, most of whom have been resistant to previous treatment, had a good clinical response to ECT in 2014. 9 ECT has equal efficacy in both unipolar and bipolar depressed patients9,10 and in depression with and without psychosis. 9,11
Despite the robust evidence base demonstrating the efficacy of ECT, its use has fallen dramatically in recent decades. The number of patients receiving ECT in England and Wales was approximately 20,000 per year in the 1980s12 and this had fallen to < 5000 by 2006. 13 SEAN audit data show a small but continuing decrease in the number of people receiving ECT in Scotland between 2006 and 2014, from just over eight per 100,000 to just under seven per 100,000 of the population. 9 It is likely that there are a number of reasons for the reduction in ECT usage, including public and professional concerns about the nature of the treatment, negative perceptions of ECT, lack of consensus on its use and resource limitations. 14 A major contributing factor is concern, from both a professional15 and a patient16 perspective, about a poor risk–benefit balance because of adverse cognitive side effects following ECT.
Cognitive adverse effects of electroconvulsive therapy
Electroconvulsive therapy is incontrovertibly associated with significant acute objective impairments in cognitive function. A meta-analysis of 84 studies, involving 24 cognitive variables, found that ECT affected the majority of them when measured up to 3 days after the last treatment. 17 ESs ranged from –1.10 to –0.21; the largest effect was on verbal delayed recall, with significant impairment in all tests of anterograde memory, executive function and cognitive processing speed. However, as time passes after the last ECT treatment there is a rapid reversal of these deficits, and moderate to large improvements above baseline are seen in most measures after 1–2 weeks. 17 However, one study has shown deficits in spatial recognition memory enduring at 1 month after the end of treatment18 and retrograde amnesia or loss of autobiographical memories may also persist,19 although not all studies find this. 20,21 In addition, the methodology of the assessment of autobiographical memory impairment has been questioned, complicating interpretation of these data. 21,22 Nevertheless, a systematic review of seven studies assessing patient self-reports of memory effects following ECT found that 29–55% reported persistent, and often distressing, loss of important past memories,16 and an association has been reported between subjective memory impairment and objective autobiographical memory impairment both immediately after an ECT course and 6 months later. 23 Therefore, although the prevalence, severity and persistence of retrograde amnesia (including autobiographical memory loss) remains unclear, from a patient perspective it can be experienced as a distressing after-effect of ECT.
A survey across multiple hospitals suggested that the severity, incidence and persistence of memory and cognitive dysfunction after ECT are related to treatment approach and, in particular, to the use of a sine-wave stimulus to induce seizures. 24 Current practice is to use brief-pulse ECT (constant current delivery with square waves of 0.5- to 1.5-millisecond pulse width), which triggers seizures more efficiently than sine-wave stimulation. In addition, a number of studies have investigated whether or not differences in electrode placement, or ultra-brief pulses (< 0.5-millisecond pulse width), are associated with less cognitive impairment. There is consensus that both cognitive impairment and efficacy are related to stimulus dose, especially for right unilateral (RUL) ECT. 5,7,25 However, differences in cognitive impairment associated with different electrode placements for brief-pulse ECT when optimised for efficacy appear absent or modest. 26,27 There have been recent claims for marked cognitive benefit from RUL ultra-brief-pulse ECT compared with both bilateral ultra-brief-pulse ECT and all types of brief-pulse ECT without sacrificing efficacy;19,28 however, a recent meta-analysis of six studies, although confirming the cognitive advantage, found that it came at the price of lower efficacy than for bilateral brief-pulse ECT. 29
The role of glutamate in depression and the effects of electroconvulsive therapy
The precise mechanisms underlying the effects of ECT are not fully understood, but altered synaptic functioning in neural circuits involved in mood and cognition is thought to have a key role; these circuits include the hippocampus, amygdala, anterior cingulate cortex (ACC) and prefrontal cortex. 30,31 Neural circuits are dependent on the structure, function and connections of the neurones they contain. It is at this level that somatic treatments such as ECT are presumed to exert their effects, with evidence for alterations in neurotransmitter, neuromodulator and hypothalamic–pituitary–adrenal axis function and in gene expression, neurogenesis and synaptic plasticity. 30 Of particular interest is evidence that ECT affects glutamate function. Glutamate is an amino acid neurotransmitter with a role in both neuroplasticity and excitotoxicity, which acts on a variety of receptors in the brain, the most widespread being N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors. 32 Preclinical evidence supports a role for glutamate in mood regulation and in the action of antidepressant treatments;32,33 in particular, decreased NMDA-mediated and increased AMPA-mediated neurotransmission has been proposed. 33 Studies in depressed patients have found decreased frontal cortical measures of glutamate measured in vivo by magnetic resonance spectroscopy (MRS), which normalise with effective treatment. 32 In studies involving ECT, reduced pretreatment glutamate and glutamate-related concentrations have been found in the dorsolateral prefrontal cortex34 and ACC35,36 and these either normalised after ECT34,36 or predicted treatment response. 35 Glutamate also has a central role in cognition, especially learning and memory, through its role in synaptic plasticity and the signalling pathway involved in long-term potentiation in the hippocampus. 37 It has been proposed that the memory impairment caused by ECT is a consequence of indiscriminate activation, or saturation, of glutamate receptors at the time of the seizure, leading to a disruption of hippocampal plasticity involved in memory. 38 Autobiographical memory impairment may occur through disruption of the reconsolidation of ‘fragile’ reactivated (i.e. recalled) memories. 39
Ketamine is a dissociative anaesthetic, analgesic and psychotomimetic that inhibits NMDA receptors but also stimulates glutamate release and potentiates activity at non-NMDA receptors such as AMPA receptors. 40 Recent studies have shown a rapid antidepressant effect after a single dose of intravenous ketamine alone, or as an adjunct to antidepressants, in both unipolar and bipolar depression. 41 Repeated administration maintains improvement but relapse occurs rapidly on stopping treatment. 42
Given acutely, ketamine can cause cognitive impairment,43 particularly in manipulating information in working memory (WM) and in encoding into episodic memory. However, preliminary human data from retrospective and non-randomised studies have suggested that anaesthesia with ketamine, compared with other anaesthetic agents, improves reorientation44,45 and word recall46 after ECT. This apparent contradiction is likely to be attributable to whether or not ketamine is present in the brain. Acute antagonism of glutamate receptors by ketamine will alter synaptic function, resulting in cognitive effects that disappear after ketamine is eliminated from the body; however, the same antagonism during ECT may protect neurones from excessive stimulation. Ketamine anaesthesia may also result in a more rapid clinical response to ECT. 47 Subsequent small randomised controlled trials (RCTs) have produced mixed results, including impaired reorientation48,49 and improved50 or unchanged48 Mini Mental State Examination (MMSE) scores with ketamine. A further study found that ketamine augmentation produced no reduction in cognitive impairment after ultra-brief RUL stimulation on a range of tests;51 however, this technique is associated with less cognitive impairment than standard bilateral ECT (see Cognitive adverse effects of electroconvulsive therapy), which limits interpretation. Two recent meta-analyses looking at efficacy, and including the same four placebo-controlled RCTs in which ketamine has been combined with ECT, have reached different conclusions. When continuous measures were pooled a moderate to large advantage of ketamine was reported in one study,41 but no benefit was found for response, remission or continuous measures in another. 52 The latter meta-analysis also reported higher rates of confusion/disorientation/prolonged delirium with ketamine than with placebo. At present, the small heterogeneous studies of ketamine administered with ECT, either as the sole anaesthetic agent or alongside a conventional anaesthetic, emphasise the need for further, larger-scale trials.
Frontal cortical function in depression and after electroconvulsive therapy
Depressed patients have deficits in executive function, attention, psychomotor speed and memory,53 which may be associated with a poorer response to antidepressants. 54 Current neurobiological models of depression emphasise abnormalities in fronto-limbic and fronto-striatal neural circuits55,56 and it has been proposed that mood disorders involve altered reciprocal interactions between a ‘ventral circuit’, including ventral prefrontal and limbic areas (involved in affective cognition, mood and autonomic/neuroendocrine function), and a ‘dorsal circuit’, including dorsal prefrontal areas, dorsal ACC and parietal cortices (involved in executive, attentional and memory functions). 55,57 It has been suggested that unipolar depression is associated with underactivity of the dorsal circuit and overactivity of the ventral circuit, with recovery being dependent on ‘normalisation’ of these circuits through their modulation by the rostral ACC. 55 Broadly consistent with this, a meta-analysis of depression studies found significant decreases in resting cerebral blood flow/glucose metabolism in areas corresponding to the dorsal circuit and increases corresponding to the ventral circuit. 58 Effective treatment with antidepressants has been found to be associated with normalisation of pretreatment abnormalities. 58,59
Electroconvulsive therapy differs from antidepressants in that it improves mood but impairs cognition. Although limited by small sample sizes, imaging studies of the effects of ECT most consistently find a reduction in cerebral metabolism in the bilateral anterior and posterior frontal cortex. 60,61 Temporal lobe and dorsal frontal lobe decreases in cerebral blood flow have been associated with impairments in learning, attention, WM and autobiographical memory. 62 Although the picture is less clear for mood improvement, associations have been reported with a reduction in frontal blood flow,62,63 although also with increased subgenual ACC and hippocampal metabolism. 64 One hypothesis is that ECT, instead of reciprocally ‘rebalancing’ dorsal and ventral networks as is seen with antidepressants, produces a combined suppression of both networks, leading to the initial picture of mood improvement with impaired cognition. After the ECT course has ended the dorsal network recovers and cognition improves. If ketamine prevents cognitive deficits with ECT then a test of this hypothesis is whether or not ketamine prevents ECT-induced suppression of frontal cortical function.
Assessment of frontal cortex function: the use of near infrared spectroscopy
Verbal fluency (VF) and WM tasks consistently activate networks involving areas of the dorsal and lateral prefrontal cortex, but vary in the subcortical components involved. Using functional magnetic resonance imaging (fMRI), VF tasks activate predominantly left-sided areas including the inferior frontal gyrus (IFG) (Broca’s area), premotor and medial supplementary motor areas, dorsal ACC, anterior insula, temporal and parietal cortices, caudate nucleus and hippocampus, the last particularly in semantic tasks requiring associative memory. 65–67 In patients with depression, decreased responses have been found in the left lateral prefrontal cortex compared with control subjects in some studies using fMRI or single-photon emission tomography,68,69 with another study finding no difference70 and a further study reporting decreased responses only with recurrent, but not first-episode, depression. 71 Most studies also showed concurrent impaired performance. 68–70
Tests of WM, such as the n-back task, activate a network used in maintaining task-relevant information during a delay, involving rehearsal and goal-directed attention. This network includes the dorsolateral and ventrolateral prefrontal cortex, premotor area, dorsal ACC, parietal and temporal cortices and cerebellum. 72,73 Some imaging studies in depressed patients have found increased dorsolateral/ventrolateral prefrontal activation in the absence of impaired performance,74–76 but others have found no change77–79 or decreased activation. 77 One interpretation is that depressed patients recruit more brain regions in an attempt to maintain function. 74 A greater degree of improvement with antidepressant treatment has been associated with less dorsolateral prefrontal cortex hyperactivation before treatment,75 consistent with the dorsal–ventral network interaction theory of antidepressant response. 55
These two types of task, both activating the prefrontal cortex and dorsal ACC, offer the opportunity to investigate overlapping networks. The WM network is predominantly cortical, whereas the VF network includes the hippocampus and is involved in memory formation and recall. Encoding of memories activates the bilateral IFG and left hippocampus with the left IFG playing a pivotal co-ordinating part in associative encoding. 80 Autobiographical, episodic and semantic memory retrieval share a common functional network, which includes the left hippocampus, bilateral IFG, ACC and posterior cingulate cortex. 81 Therefore, although VF has traditionally been viewed as a ‘frontal’ function, it involves a memory network, and a recent fMRI study found hippocampal connectivity with a semantic network during semantic (category), but not phonetic (letter), fluency. 82 VF is strongly impaired by ECT, whereas WM is relatively unaffected by ECT. 17 Semantic VF is therefore a possible functional probe of the associative memory network, which includes the hippocampus, and is sensitive to the effects of ECT, whereas WM tasks appear more restricted to a cortical network that is less affected.
Functional near-infrared spectroscopy (fNIRS) is a portable brain imaging technique that can be used at the bedside or in a person’s home. It uses the differential light absorption properties of oxyhaemoglobin (HbO) and deoxyhaemoglobin (HbR) for near-infrared light when it passes through the scalp, skull and superficial layers of the cortex from a light source placed on the scalp. The change in the light absorption spectra, measured with detectors placed nearby on the scalp, can be converted into changes in concentrations of HbO and HbR. When these changes occur in response to a cognitive task, they can be used as a haemodynamic response to that task in the region being illuminated. 83 The neurovascular coupling principle links these haemodynamic changes with the underlying neural activity. 84 When a brain area is active, neuronal firing increases metabolic demand, which results in a localised increase in blood flow. The increase in blood flow is higher than the actual oxygen consumption by the firing neurones and, therefore, an increase in HbO and a decrease in HbR is expected in the active cortical areas. Hence, it is possible to use fNIRS as a measure of functional changes in cortical blood flow secondary to neuronal activation,85 analogous to fMRI using blood oxygen level-dependent (BOLD) changes. In addition, it is possible to use a spatially resolved spectroscopy technique, employing a modification of the diffusion equation of light transport, to provide an absolute measure of tissue oxygenation [the tissue oxygenation index (TOI)]. This index is the absolute percentage of total haemoglobin in the field of view that is oxygenated. 86 Its reliability and validity have yet to be established;87 however, it does provide a potential absolute measure with which to assess cerebral tissue metabolism across groups and within subjects. 88
Functional near-infrared spectroscopy has been extensively used to study frontal cortex activation using VF and WM tasks with a high degree of reliability with repeated administration. 83,89–93 Depressed subjects reliably demonstrate decreased lateral prefrontal cortex–anterior temporal cortex haemodynamic responses compared with control subjects during a VF task,94–106 including in bipolar depression. 107–109 There is some uncertainty over whether this is a state or trait abnormality, as some studies have found restored activation to a VF task following treatment110 and increased activation has been seen in hypomanic patients,108 whereas blunted responses have been found in remitted depressed patients111 and euthymic bipolar patients109 or failure to normalise after antidepressant treatment in depressed patients. 99 WM has been less investigated using fNIRS, but studies have also shown a decrease in lateral frontal haemodynamic responses in depression,112–114 which were associated with decreased performance in one study. 112 We hypothesised that severely ill depressed patients will have decreased lateral frontal activation to a VF task, which will correlate with clinical measures, and that ketamine will alleviate the further suppression of frontal activation by ECT, in line with its benefits in preserving cognitive function.
Objectives
The primary aim of the Ketamine-ECT study was to investigate, in a RCT, the effect of ketamine, given as an adjunct to conventional anaesthetics, on cognitive dysfunction caused by ECT in severely depressed patients who had consented to receive ECT as part of their usual care in NHS secondary care settings. The hypothesis was that adjunctive ketamine, compared with saline, would reduce ECT-induced cognitive impairment. Secondary aims were to (1) investigate the efficacy, tolerability and acceptability of adjunctive ketamine (we hypothesised that ketamine treatment would lead to a more rapid improvement in depressive symptoms with fewer ECT treatments needed to achieve remission) and (2) identify whether or not ketamine modified the effect of ECT on frontal cortical function using fNIRS during the performance of cognitive tasks (we hypothesised specifically that patients receiving ketamine, compared with saline, would show greater cognitive activation to a category VF task during an ECT treatment course).
Primary clinical objective
The primary objective was to determine whether or not intravenous ketamine (0.5 mg/kg), compared with placebo (saline), given immediately before the anaesthetic at each ECT treatment, would ameliorate impairment in anterograde amnesia caused by ECT. The primary outcome was delayed verbal recall in the Hopkins Verbal Learning Test – Revised (HVLT-R)115 at baseline and after four ECT treatments (mid-ECT), comparing patients randomised to ketamine with those randomised to saline who received at least one ECT treatment. This task was chosen given the evidence that delayed verbal memory is the most impaired cognitive function seen following ECT17 and because memory problems are a key complaint in patients receiving ECT.
Secondary objectives
Clinical objectives
-
To determine whether or not patients receiving ketamine, compared with saline, would show better/less impaired:
-
VF, autobiographical memory, visual memory and attention/WM at mid-ECT and at the end of treatment with ECT
-
anterograde memory at the end of treatment
-
autobiographical memory at 1 and 4 months after the last ECT.
-
-
To determine whether or not patients receiving ketamine, compared with saline, would show:
-
greater improvement in depression, anxiety and quality of life at mid-ECT
-
more rapid improvement in depression as shown by earlier time to remission and response and fewer ECT treatments needed to achieve remission.
-
-
To assess the safety and tolerability of ketamine augmentation of ECT as shown by:
-
adverse events (AEs) and adverse reactions (ARs)
-
reorientation after ECT treatments
-
psychotic and manic symptoms.
-
Mechanistic objectives
-
To determine whether or not patients receiving ketamine, compared with saline, would show, at mid-ECT and at the end of treatment, better/less impaired:
-
haemodynamic prefrontal cortex responses to a VF task using fNIRS, related to changes in VF performance
-
prefrontal cortex metabolism (TOI) using fNIRS, related to changes in VF performance.
-
-
In addition, although not in the original protocol, we wished to take the opportunity to compare patients and controls on:
-
measures of neuropsychological function
-
haemodynamic prefrontal cortex responses to VF and WM tasks using fNIRS
-
prefrontal cortex metabolism (TOI) using fNIRS.
-
(Note: in the original protocol mechanistic studies involving fMRI and MRS had been planned, but they were not able to be pursued because of insufficient recruitment.)
Chapter 2 Methods
Overview of the trial
The Ketamine-ECT study was a multicentre, two-arm (1 : 1 allocation), parallel-group, patient-randomised, placebo-controlled superiority trial of ketamine added to standard ECT during anaesthetic induction in severely ill depressed hospitalised patients or outpatients who have been referred for ECT by their psychiatrist. Assessment and ECT treatment was blind to treatment allocation but the anaesthetists administering the study medication were not blinded for reasons of safety. The primary outcome was neuropsychological function, with secondary efficacy outcomes and a mechanistic fNIRS substudy of prefrontal cortex function. ECT was administered twice a week until a patient’s depression had remitted, as determined by the patient’s treating clinical team, and patients were followed for 4 months after the end of ECT treatment.
All assessments were undertaken by trained research personnel under the supervision of the clinically trained principal investigators. There was no planned interim analysis and no ‘stopping rules’ for the study as a whole. Individual patients were withdrawn from the study medication if it appeared that to continue it would be deleterious to their mental health or safety. This was determined by the treating clinician and/or the research team in discussion with the patient. Withdrawal from study medication did not necessitate withdrawal from the study.
The study was registered on 27 December 2012 (ISRCTN14689382) under the public title ‘Ketamine-ECT study’. Clinical trial authorisation was given by the Medicines and Healthcare products Regulatory Agency (MHRA) (EudraCT 2011–005476–41) on 18 May 2012. Ethical approval was granted by the North West – Liverpool East Research Ethics Committee (reference number 12/NW/0021) on 25 January 2012. Recruitment commenced in December 2012 and an extension to the study and to recruitment was granted by the funder in 2014, with the study finishing in August 2015. The trial protocol is available at www.nets.nihr.ac.uk/projects/eme/109004 (accessed 25 August 2016).
Patient and public involvement
There was patient and public involvement at all stages of the study, with regular meetings with a service user group throughout the study. Input was especially valuable in the design and content of all information sheets, with regard to issues related to informed consent, safety and patient experience and in response to challenges in the trial (see Chapter 3, Challenges to recruitment). There was service user representation on the trial management group, which met monthly to oversee the trial, and on the trial steering committee (TSC). The service user group took a central role in developing and delivering the survey of patient experiences in the trial (reported in Chapter 5).
Participants
Patients
Inclusion criteria
-
Consent given for ECT as part of standard clinical care.
-
Male or female aged ≥ 18 years.
-
Current Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)116 diagnosis of a major depressive episode, moderate or severe as part of a unipolar or bipolar mood disorder, diagnosed by the Mini International Neuropsychiatric Interview (MINI). 117
-
American Society of Anesthesiologists score (excluding mental health considerations in the scoring) of 1, 2 or stable 3 and judged as suitable to receive ketamine by an anaesthetist.
-
Verbal intelligence quotient (IQ) equivalent to ≥ 85 and sufficiently fluent in English to validly complete neuropsychological testing.
-
Capacity to give informed consent.
-
Willingness to undertake neuropsychological testing as part of the study.
Exclusion criteria
-
DSM-IV diagnosis of a primary psychotic or schizoaffective disorder, current primary obsessive–compulsive disorder or anorexia nervosa.
-
History of drug or alcohol dependence (DSM-IV criteria116) within the past year.
-
Electroconvulsive therapy in the past 3 months (to avoid confounding the assessment of cognitive outcomes – this was 6 months in the initial protocol but was shortened to 3 months after the commencement of the trial; see Chapter 3, Challenges to recruitment) or previously received ECT in the current trial.
-
Known hypersensitivity or contraindication to ketamine or excipients in the injection, including significant cardiovascular disease, uncontrolled hypertension, glaucoma, cirrhosis or abnormal liver function or liver disease.
-
Known hypersensitivity or contraindication to concomitant medications used for ECT [propofol, thiopental (thiopental sodium) and suxamethonium (suxamethonium chloride) or excipients in the injections].
-
Evidence of organic brain disease including dementia, neurological illness or injury or medical illness that may significantly affect neuropsychological function.
-
Detained under the Mental Health Act (MHA) 1983 (as amended in 2007)118 or unable to give informed consent.
-
Pregnancy, or at risk of pregnancy and not taking adequate contraception, or breastfeeding.
-
Score of < 24 on the MMSE. 119
Healthy control subjects
Healthy control subjects (HCs) were prospectively sex- and age-group matched as far as possible with patients.
Inclusion criteria
-
Male or female aged ≥ 18 years.
-
Psychiatrically well, confirmed through the MINI,117 and on no current psychotropic medication.
-
Good physical health.
Exclusion criteria
-
Personal history of psychiatric disorder, as revealed by the MINI. 117
-
First-degree family history of major psychiatric illness requiring treatment.
-
Significant physical illness including organic brain disease, neurological illness or injury that could interfere with the interpretation of the results.
-
Psychotropic medication or other medication that could interfere with the interpretation of the results.
-
Score of < 24 on the MMSE. 119
Study withdrawal criteria
Patients withdrawing from study medication but continuing to receive ECT were encouraged to remain in the study and to complete assessments if possible, and consent remained valid. Reasons for study discontinuation were:
-
withdrawal of patient consent or decision to discontinue for any reason (e.g. AE) without having to give a reason
-
loss of capacity to provide continuing consent or detention under the MHA
-
local principal investigator or clinical team decision based on collaborative decision for safety reasons (e.g. serious AE or AR) or patient deterioration.
Recruitment
Recruitment was from secondary care settings, both inpatient and outpatient, in trusts in the north of England (see Table 2). Patients were identified through direct contact with inpatient wards and referrals to ECT suites. Potential eligibility was assessed through discussion with clinical teams and case note information obtained by authorised staff, and patients were invited to meet a member of the research team to have the study explained. HCs were recruited through advertisements in Manchester and the Volunteer Database in Newcastle, as well as by inviting relatives of participating patients.
Study design
Visits and assessment schedule
These are outlined in Table 1 and described further in the following sections.
| Assessments | Main study visits | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening and randomisation | Baseline | Week 0 | Visit 1, week 1 | Visit 2, mid-ECT week 2 | Visit 3a, 3b, etc. (weekly to end of treatment) | Visit 4, end of treatment | Visit 5, 4 weeks after end of treatment | Visit 6, 16 weeks after end of treatment | |||||
| Treatments | ECT 1 | ECT 2 | ECT 3 | ECT 4 | ECT 5 to final ECT | ||||||||
| Timing | 4 weeks–1 day pre ECT 1 | 2 weeks–1 day pre ECT 1 | 1–3 days after ECT 2 | 1–3 days after ECT 4 (± 1 ECT) | 1–3 days after even-numbered ECT | 1–5 days after final ECT | 3–5 weeks after final ECT | 12–20 weeks after final ECT | |||||
| Informed consent | ✓ | ||||||||||||
| Pretreatment assessmentsa | ✓ | ||||||||||||
| Safety measuresb | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Trial blinding assessment | ✓c | ✓c | |||||||||||
| Neuropsychological testsd | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Efficacy ratingse | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Near-infrared spectroscopyf,g | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
Screening visit
This occurred once a potentially eligible patient had consented to ECT and had agreed to see a member of the research team. Written informed consent was obtained after full explanation of the study. Eligibility was determined through a mixture of case note information and a semistructured interview to obtain demographic and background details and by using the MINI to determine diagnosis. Particular care was taken to determine potential physical reasons for exclusion from the study through the results of the standard clinical work-up for ECT including blood tests (full blood count, urea and electrolytes, liver function tests), an electrocardiogram (ECG) and physical examination. IQ and handedness were determined (see Assessments).
Baseline visit
At baseline the neuropsychological tests and efficacy assessments were carried out and, in those who consented to the mechanistic study, this was followed by near-infrared spectroscopy (NIRS) testing. This could be carried out over one or two visits according to patient wishes and practicality.
Randomisation
Randomisation occurred as close to the first ECT as possible to try to minimise dropout, as the modified intention-to-treat (mITT) population was based on patients starting ECT. In practice, this usually meant the day before ECT to allow for the study medication to be prepared and delivered to the ECT suite. Patients were randomised in a 1 : 1 ratio to ketamine or saline using permuted block randomisation (varying randomly from four to eight), stratified by NHS trust by the Christie Hospital Clinical Trials Unit (CTU). The relevant randomisation code was held by the CTU and also provided to the local site pharmacies. When a patient required randomisation the site research assistant (RA) contacted the CTU by telephone with the participant details; the CTU then contacted the local site pharmacy to prepare the drug in blinded, tamper-proof, secondary packaging according to the randomisation code.
Timing of neuropsychological and near-infrared spectroscopy assessments
Neuropsychological assessments were yoked to ECT sessions rather than to time from randomisation because of not-infrequent delays or missed ECT sessions as a result of patient (e.g. infections, failing to fast on the morning of ECT) or organisational (bank holidays, unavailability of staff) factors, as well as the need to carry out the tests within a specified time after the ECT session to capture cognitive impairment. Assessments were carried out at least 24 hours after an ECT session to avoid acute post-seizure and post-anaesthetic effects. The primary outcome assessment was carried out after four ECT sessions (usually just over 2 weeks after the first ECT session), when the effects of ECT should be apparent on cognition anterograde memory. 120 Given the individual variation seen in the number of ECT treatments administered, this time point was chosen to capture as many patients as possible before they stopped ECT, either because of dropout or improvement. Further assessments were carried out after the last ECT session (end of treatment) and at follow-up at 1 and 4 months after the last ECT session.
For those patients who had consented to the NIRS substudy, the imaging was carried out after the neuropsychological assessments.
Timing of efficacy assessments
Efficacy assessments were carried out weekly until the end of treatment, with the week-2 assessment mostly coinciding with the mid-ECT neuropsychological assessment. The primary efficacy measure was improvement in the Montgomery–Åsberg Depression Rating Scale (MADRS),121 an observer-rated measure of depression severity.
Timing of safety assessments
Safety assessments were carried out during and after each ECT session in accordance with standard good practice in the administration of ECT and included monitoring of vital signs and pulse oximetry. Degree of reorientation 30 minutes after ECT was used to assess recovery after ECT and staff were instructed to record any problem during the recovery period as an AE or possible AR. Weekly mania and psychosis ratings were carried out as part of the efficacy assessments.
Interventions
Electroconvulsive therapy
Standard ECT protocols based on the Royal College of Psychiatrists’ (RCPsych) ECT Handbook122 were agreed between centres, with ECT treatments scheduled twice weekly. For each ECT treatment, after pre-oxygenation with 100% oxygen, anaesthesia consisted of propofol (or rarely thiopental if clinically indicated) combined with the muscle relaxant suxamethonium. Etomidate was not permitted as an induction agent because of its action in elevating blood pressure. Anaesthetic drugs remained the same for all ECT treatments unless change was required for clinical reasons. ECT treatment was given after motor end-plate depolarisation was determined by cessation of muscle fasciculation in the feet. Electrode placement was standard bifrontotemporal (bilateral) or RUL (D’Elia placement) and the treatment consisted of constant-current brief-pulse (0.5-millisecond pulse width) stimuli to induce seizure. The pulse width could be increased to 1.0-millisecond if clinically indicated. Treatment dose was determined by rapid stimulus titration to determine the seizure threshold in the first session treatment, based on that given in the RCPsych ECT Handbook. 122 Target treatment doses were 1.5 times threshold for bilateral and 4–6 times threshold for RUL electrode placement and the stimulus parameters remained the same until after the fourth treatment unless change was required for clinical reasons. Standard operating procedures were agreed between treatment centres to determine criteria for an adequate seizure, and for re-stimulation and dosage adjustments later in the course, based on the RCPsych ECT Handbook. 122
Oral psychotropic medication was continued by the clinical team and remained unchanged for the first four ECT treatments and, if possible, until the end of ECT, unless changes were required for safety or clinical reasons.
The goal was to treat patients to remission (MADRS score of ≤ 10) in accordance with NICE guidance5 but the final decision to finish ECT treatment rested with the clinical team in consultation with the patient and the ECT team.
Study medication and blinding
The experimental intervention was 0.5 mg/kg of intravenous ketamine or an equal volume of normal saline (0.9% sodium chloride solution), given in accordance with the randomisation schedule by the anaesthetist as a slow bolus directly before the anaesthetic induction agent and before suxamethonium at each ECT treatment. Ketamine (at a concentration of 10 mg/ml) or saline was provided as a clear solution in ampoules retaining their original labelling, provided in secondary tamper-proof trial packaging by the local clinical trials pharmacy. The anaesthetic team broke the seal away from the psychiatric ECT team, drew up the trial medication into a syringe and disposed of the ampoule without revealing which drug was being administered. Members of the research team responsible for outcome assessment did not attend ECT sessions to minimise the risk of treatment allocation being inadvertently revealed.
Assessments
Screening and baseline tests
Mini International Neuropsychiatric Interview
The MINI is a brief structured interview for the major axis I psychiatric disorders in the DSM-IV,116 with good concordance with more comprehensive diagnostic interviews for mood disorders. It can be used with little training by clinicians but can be used by lay interviewers after training (provided in this study by IMA) and takes approximately 22 minutes to administer. 117
Mini Mental State Examination
The MMSE is a 30-item screening tool for dementia with scores of < 24 being considered abnormal. 119
Wechsler Test of Adult Reading
The Wechsler Test of Adult Reading123 is a language test to assess premorbid intellectual functioning and is thought to be relatively insensitive to cognitive deterioration. It uses vocabulary level as a correlate of IQ with the patient being presented with irregularly spelled words and prompted to pronounce each one. It consists of 50 irregularly spelled words of progressive difficulty and is discontinued after 12 consecutive incorrect pronunciations. Each correct pronunciation is given a score of 1 and the raw score is then standardised by age and compared with the scores predicted for the patient’s demographic classification to provide an estimate of premorbid IQ.
Modified Edinburgh Handedness Questionnaire
The modified Edinburgh Handedness Questionnaire124 asks participants to indicate which hand they use for eight common tasks, with responses ranging from ‘always left’ to ‘always right’ on a five-point scale. This is scored to determine right-, left- or mixed-handedness.
Massachusetts General Hospital Scale
The Massachusetts General Hospital Scale (MGHS)125 is a method for staging pharmacological treatment of depression that generates a continuous variable representing the degree of treatment resistance, taking into account the intensity and optimisation of each previous treatment. Non-response to an adequate trial of a marketed antidepressant (≥ 6 weeks at an adequate dose) scores 1 point per trial, with 0.5 points per trial added for each optimisation strategy. ECT increases the score by 3 points. In one study a score of 3 was found to confer a < 20% chance of remission with the next treatment, which diminished as the score increased further. 126
Other assessments
Other assessments consisted of those carried out by the clinical team as work-up for ECT and included a physical examination, including height and weight, cardiovascular system and blood pressure, an ECG and blood tests, including liver function tests.
Safety assessments
Monitoring before, during and after anaesthesia and electroconvulsive therapy treatment
Safety measures were those applied in standard practice, consisting of monitoring heart rate, blood pressure and peripheral oxygen saturation using pulse oximetry and, if indicated, capnography.
Reorientation
Reorientation was used as a measure of recovery from ECT and is a routine clinical assessment. To provide a standardised measure, reorientation 30 minutes and 60 minutes after eye opening was recorded, defined as being correct in four out of five orientation items (name, place, day of the week, age and birthday). 127 In addition, the number of correct orientation items at these time points was recorded. Reorientation usually occurs before 30 minutes49 and a delay beyond 60 minutes is extremely rare. The 30-minute time point was used to compare treatment arms.
Agitation and psychosis
This was not formally assessed during the recovery period but ECT staff were asked to record events that were unusual or more severe than expected as AEs, as well as any suspected ARs. The 18-item Brief Psychiatric Rating Scale (BPRS),128 with an additional 19th item for elevated mood, was used to assess symptoms of psychosis (sum of items 3, 4, 7, 8, 11, 12, 15 and 16) and mania (sum of items 8, 10, 17 and 19) outside the ECT sessions, at the same time as efficacy assessments.
Neuropsychological tests
The neuropsychological tests were chosen to be sensitive to the effects of ECT27,129,130 and to have a duration short enough to be acceptable to patients (total of 35–40 minutes).
Hopkins Verbal Learning Test – Revised
The HVLT-R115 is a word-list learning task in which the participant is asked to listen as a word list consisting of 12 words is read out to them and then to recall as many words as possible; this is carried out three times. Following an interval of 30 minutes the participant is given an unannounced recall task for the words previously learnt and a forced-choice recognition task in which the previously presented words are mixed with 12 words not heard before. Although repeated administration means that the delayed recall is no longer a surprise, this does not appear to affect performance. 131 The sum of the number of words recalled after the three presentations is used as a measure of learning and the number of words recalled after the delay is used as a measure of delayed recall (the primary outcome in this study). In addition, a retention score is calculated (the number of words on trial 4 divided by the highest score on trials 2 and 3 expressed as a percentage), as well as a recognition discrimination index (the number of true positives minus the number of false positives).
It is available in six alternative forms, which were used at the different time points in the study, with two different orders of presentation assigned according to even or odd participant numbers.
Controlled Oral Word Association Test
The Controlled Oral Word Association Test (COWAT)132 tests letter and category fluency, with participants asked to generate as many words as they can in 1 minute starting with the letters F, A or S and in a given category (animals or fruit and vegetables), with rules excluding proper names or variations of a word. The same letters were presented on subsequent tests and the two categories were alternated with a different order of presentation assigned according to even or odd participant numbers.
Autobiographical Memory Interview – Short Form
The Autobiographical Memory Interview – Short Form (AMI-SF)133 tests memory for personal events in the past with reference to an initial interview eliciting personal memories. This covers six areas with a score given to any events remembered with sufficient detail to be questioned about them again on subsequent occasions. On retesting, only items that had been mentioned the first time can be scored, with points deducted for missing items, so it is possible for scores to decrease even if a richer memory overall is recalled. The AMI-SF has been criticised as it does not allow for improvements to be detected, for its lack of normative data for the normal loss of consistency over time and for a lack of information about the independent effect of mood,22 although it has been defended against these criticisms. 19 An alternative extended scoring method has been recently proposed that allows more details to be scored at baseline, with points given for all items recalled. 134 A decrease in the consistency of memory of about 20% over 6 months in healthy volunteers has been found with the extended scoring method. 134 In this study we used both the standard and the extended scoring method to assess autobiographical memory. We did not administer the AMI-SF to HCs as we were not retesting them, and the baseline scores have little comparative value, although depressed patients do produce fewer episode-specific memories than healthy volunteers. 134
Medical College of Georgia Complex Figure Test
The Medical College of Georgia Complex Figure Test (MCGCFT)135 consists of first copying, and then reproducing from memory (immediately and after a delay), a complex line drawing with multiple elements, which tests not only visuospatial abilities but also memory, attention and planning. It is available in four alternative forms and different forms were used for the first four time points in the study, with two different orders of presentation assigned according to even or odd participant numbers.
Digit span
For the digit span task, strings of numbers of increasing length are read out slowly and in an even rhythm and participants are asked to repeat them back. 136 In the forward condition they are repeated in the same order as presented and in the backward condition they are repeated in reverse order. This task is a test of attention and being able to hold the numbers in WM to reproduce them, particularly for the backward condition. The number of digits remembered is reported (the ‘clinical’ digit span).
Self-reported Global Self-Evaluation of Memory
One of the problems in research into memory problems following ECT is the lack of agreement between the impact on subjective memory and objective memory measures. The self-rated Global Self-Evaluation of Memory (GSE-My)137 has been reported to correlate with AMI-SF scores after ECT. Patients are asked to rate their global memory and the effect of ECT on their memory on a 7-point scale.
Efficacy measures
Montgomery–Åsberg Depression Rating Scale
The MADRS is a 10-item observer-rated depression scale, which is sensitive to change and has internal consistency. 138 Each item is scored from 0 to 6 on the basis of defined anchor points; a score of ≤ 10 was taken as the cut-off for remission. 139 In addition to the standard MADRS, an alternative bespoke form was scored that included atypical depressive symptoms of increased appetite and sleep (MADRS atypical).
Clinical Anxiety Scale
The Clinical Anxiety Scale (CAS)140 is a 7-item observer-rated scale derived from the Hamilton Anxiety Scale for measuring the severity of anxiety. The seventh item is meant to be scored separately. Each item is scored from 0 to 4 on the basis of defined anchor points, and we calculated the total score and the total score of the first six items for this study.
Clinical Global Impression-Severity and Clinical Global Impression-Improvement
The Clinical Global Impression scale is a widely used 7-point observer-rated scale that is used to measure global severity [Clinical Global Impression-Severity (CGI-S)] judged in comparison to the overall range of depression seen in patients in clinical practice (1 = ‘normal, not at all ill’ to 7 = ‘among the most extremely ill patients’) and overall global improvement [Clinical Global Impression-Improvement (CGI-I)] compared with baseline (1 = ‘very much improved’ to 7 = ‘very much worse’). 141
Brief Psychiatric Rating Scale
The BPRS is an observer-rated instrument used to measure the overall range of psychiatric symptomatology. We used the 18-item version with an additional 19th item for elevated mood. Each item is scored from 1 to 7 on the basis of defined anchor points. Subscales were used to assess psychosis and mania as described under Safety assessments.
Quick Inventory of Depressive Symptomatology – Self Report
The Quick Inventory of Depressive Symptomatology – Self Report (QIDS-SR)142 is a 16-item self-rated depression scale that was used in the STAR*D study4 and rates the nine DSM-IV depression items, each on a scale of 0–3.
European Quality of Life-5 Dimensions three-level version
The European Quality of Life-5 Dimensions three-level version (EQ-5D-3L)143 is a self-rated quality-of-life instrument, with participants rating their degree of impairment or experience on five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) on a scale from 1 to 3. Based on standardised population studies, each item is weighted and an overall quality-of-life ‘index’ score is calculated to give a utility score ranging from 0 (death) to 1 (perfect quality of life); the lowest score is –0.59, that is, worse than death. In addition, a visual analogue scale (VAS) is scored from 0 (worst imaginable health state) to 100 (best imaginable health state).
Trial blinding assessment
A treatment allocation questionnaire was developed for the trial in which patients and research staff were asked to ‘guess’ their treatment allocation, indicate how certain they were on a 4-point Likert scale and identify the reason for their choice. The ratings were put in a sealed envelope and sent directly to the CTU for data entry to minimise the risk of those involved in study ratings being influenced by others’ beliefs.
Sample size
Original sample size and power calculations
Across the six NHS trusts originally involved in the study, 355 patients had received ECT over a 12-month period in 2009/10 (the most recent period available when planning the study). The most comparable recent study in the UK, using similar inclusion/exclusion criteria to ours,144 found that 41% of patients receiving ECT were eligible and 18% were randomised (to either ECT or transcranial magnetic stimulation). Given that the current study offered adjunctive rather than alternative treatment, a 22.5% recruitment rate was predicted. Based on these figures, recruiting 160 patients over a 24-month period was forecast as feasible, providing 152 patients for the primary end point assuming that 95% of patients could be assessed after four treatments.
The power calculation was based on a clinically useful benefit of a moderate standardised ES for the ketamine–saline difference (0.5–0.6). With 90% power, and a two-sided p = 0.05, based on independent t-tests, we would have been able to detect a standardised ES of 0.51 for the full intention-to-treat (ITT) analysis and 0.54 for a single primary cognitive outcome based on completer assessments. Using a Bonferroni correction for the originally proposed three interdependent measures for the primary cognitive outcome, HVLT-R delayed recall, COWAT category fluency and AMI-SF (i.e. assuming independence), gives 80% power to detect a standardised ES of 0.51 given a two-sided, corrected p = 0.05 for each measure.
The study aimed to recruit 100 patients (50 per treatment group) and 50 matched HCs to the mechanistic studies. This would have had 80% power to detect an ES of 0.5 with a two-sided alpha level of 0.05 between groups and an ES of 0.57 with a two-sided alpha level of 0.05 between groups. A meta-analysis of fNIRS studies in depressed patients145 found an ES difference compared with control subjects of 0.74 in frontal cortex HbO response to cognitive tasks activating the frontal cortex.
Revised power calculations
Following initial slow recruitment, the revised recruitment target for the clinical trial was adjusted to 100 patients, with 95 reaching the primary outcome time point, and the primary outcome was changed to the HVLT-R delayed recall alone. This sample size provided 80% power to detect an ES of 0.57 (ITT analysis) and 0.58 (completer assessments) with a two-sided p = 0.05.
In addition to the slow recruitment, there were practical difficulties with patients being offered fNIRS over a large geographical area, resulting in the target for recruitment to the mechanistic studies being decreased to a sample size of 20 patients (10 per treatment arm) and 50 control subjects. This has only a 21% power to detect an ES of 0.6 (with a two-sided p = 0.05) between the two treatment arms (i.e. 10 per arm) but a 70% power to detect the same size of effect of ECT itself (two-sided p = 0.05). Recruitment of 20 patients and 50 control subjects enables detection of an ES of 0.6 between groups with 60% power.
Analysis
Clinical trial
The full statistical analysis plan (SAP) (see Appendix 1) was agreed before the blind was broken by the data monitoring and ethics committee (DMEC) and signed off by the TSC. When there have been minor modifications to the SAP in the course of the analysis this is indicated with the reasons given.
Data analytical strategy
Statistical analysis of the randomised treatment groups was based on the mITT population subject to the availability of data; to be included a subject must have received electrical stimulus. Statistical analyses were carried out using Stata 13 (StataCorp LP, College Station, TX, USA) and IBM Statistical Product and Service Solutions (SPSS) Statistics version 22.0 (IBM Corporation, Armonk, NY, USA).
Pro-rating was used to calculate total scores when there were missing items for the efficacy scales. Values for the missing items were calculated by replacing the missing item with the mean of the other available items for that occasion provided at least 70% (75% for the BPRS mania subscale) of items had been completed. Pro-rating was not undertaken for the neuropsychological outcomes.
Analysis of the primary outcome measure and other neuropsychological secondary outcome measures
The SAP states that cross-sectional analysis of covariance (ANCOVA, i.e. linear regression) models would be fitted to the primary and secondary neuropsychological outcomes at the mid-ECT, end of treatment, follow-up 1 (1 month post ECT) and follow-up 2 (4 months post ECT) assessment times. However, with 15 outcomes this would mean fitting 60 separate models. Therefore, it was decided instead to fit a Gaussian repeated measures model to each of the 15 continuous neuropsychological outcomes using all of the available data and taking account of the correlation between measures on the same subject. These models were fitted using the ‘mixed’ procedure in which dropouts are assumed to be ‘missing at random’ (i.e. missing outcome data are conditional on observed data). This assumes that future behaviour, given the past, is the same for all, whether or not a subject drops out. This allows distributional information to be ‘borrowed’ from those who remain on the trial and applied to those who drop out, given that they have the same covariate set-up until the time of dropout. Therefore, the estimand of the treatment effect is what would be seen if all subjects had remained on the study until the end.
Before fitting the repeated measures models we examined the summary statistics for time from first ECT to each neuropsychological assessment (see Table 5). From this, the overall means of 2, 6, 10 and 22 weeks were used as the values for the discrete time variable in the repeated measures models. A time × treatment interaction was included to assess the treatment effect at each time point, adjusting for the covariates specified in the SAP, that is, age at randomisation, sex, baseline degree of treatment resistance, electrode placement (bilateral or unilateral) and baseline value of the outcome being evaluated.
The programmatic rules in the appendix to the SAP (see Appendix 1) were used to assign valid mid-ECT and end-of-treatment neuropsychological assessments. If the rules were not met then data for that assessment were set to missing, the only exception being that one patient who received six ECT treatments prior to the mid-ECT neuropsychology assessment was included, as one extra treatment would not be expected to have a major effect on the scores.
The use of the repeated measures analysis meant that no assessments could be carried forwards or backwards if there were missing data at critical time points, as stated in the appendix to the SAP. One exception was for a subject who responded to treatment after four ECTS [rule (e) of the appendix to the SAP], so that their end-of-treatment neuropsychology assessment was repeated for their mid-ECT value. All repeated measures model analyses involved the use of robust standard errors and associated confidence intervals (CIs) and p-values (allowing for non-normality and constraints in the ranges of some of the cognitive outcomes).
As this main outcome analysis did not assess whether or not there was a significant change in neuropsychological test scores during treatment (i.e. the presumed effect of ECT), post hoc exploratory univariate repeated measures analysis of variance (ANOVA) was carried out separately for the treatment-related assessments (baseline, mid-ECT and end of treatment) and during follow-up (end of treatment, follow-up 1 and follow-up 2) to describe the change over time.
Efficacy analyses
Continuous outcomes
The weekly efficacy data were analysed using a random-effects (random intercepts and slopes) ANCOVA model with time (in weeks) since the first ECT as a quantitative explanatory variable. To prevent bias from efficacy assessments being delayed, time from first ECT to assessment was included as a continuous variable in a longitudinal mixed-effect model rather than the notional assessment week recorded by the RA (RA week). Only data until the end-of-treatment assessment were included (i.e. excluding follow-up data). The fixed covariates included in each model were treatment, the time × treatment interaction and the same prespecified baseline participant characteristics as used for the neuropsychology repeated measures analyses. For each model, two correlated random-effects terms were included to capture between-subject variation in the intercept and slopes, assuming that people might have different rates of improvement with treatment. All models involved the use of robust standard errors and associated CIs and p-values.
A sensitivity analysis was performed on the primary efficacy outcome, the MADRS score, by fitting a longitudinal mixed-effect model using time since randomisation instead of time from first ECT.
Binary outcomes
To assess efficacy data up to the end of acute treatment for the remission and response binary variables, the frequency and cumulative frequency of first occurrence according to the week recorded by the RA (RA week) up to the end of treatment were tabulated. When the end of treatment occurred < 13 days after the previous week’s assessment it was designated as being 1 week after the last assessment; when it was ≥ 13 days then a gap of 2 weeks was assigned instead.
Kaplan–Meier plots and Cox analyses (adjusting for the same baseline variables as used for the repeated measures and longitudinal mixed-effect models, including baseline MADRS score) were undertaken. For these analyses the time to ‘event’ was defined as the difference between the first ECT date and the date of assessment. For censored subjects, their last known efficacy assessment date while on acute treatment relative to their first ECT date was used.
The proportion worsening at follow-up after acute treatment (MADRS increase of ≥ 4 points + CGI-S increase of ≥ 1 point to CGI-S ≥ 3 from the end-of-treatment assessment or, if not available, the last RA week assessment) was determined provided that there was a MADRS and CGI-S score at the end of treatment and at least one follow-up time point. The proportions worsening in each group were compared using Fisher’s exact test.
Near-infrared spectroscopy
Patients who consented to NIRS in addition to the main study neuropsychological tests were tested after they had undertaken the main tests. The intention had been to administer NIRS at the same time points as neuropsychological testing, but because of the low numbers recruited and dropouts, there were sufficient participants for analysis only at baseline and mid-ECT.
Data acquisition
Two purpose-built optical topography systems (NTS Optical Imaging System, Biomedical Optics Research Laboratory, Department of Medical Physics and Bioengineering, University College London, London, UK), each providing a 24-channel array for topographical coverage of the bilateral dorsolateral and ventrolateral prefrontal cortices, were deployed in Manchester and Newcastle.
The optode array utilised six light sources and two light detectors for each hemisphere, arranged in a star-like configuration to optimise the overlap between source–detector pairs (channels) and to provide adequate spatial coverage (Figure 1). Each light source emitted light at two wavelengths (780 and 850 nm) so that it is possible to recover, and differentiate between, HbO and HbR given their different absorption spectra at these wavelengths.
FIGURE 1.
The NIRS array layout used in the study. (a) Right hemisphere; and (b) left hemisphere. S, source; D, detector.
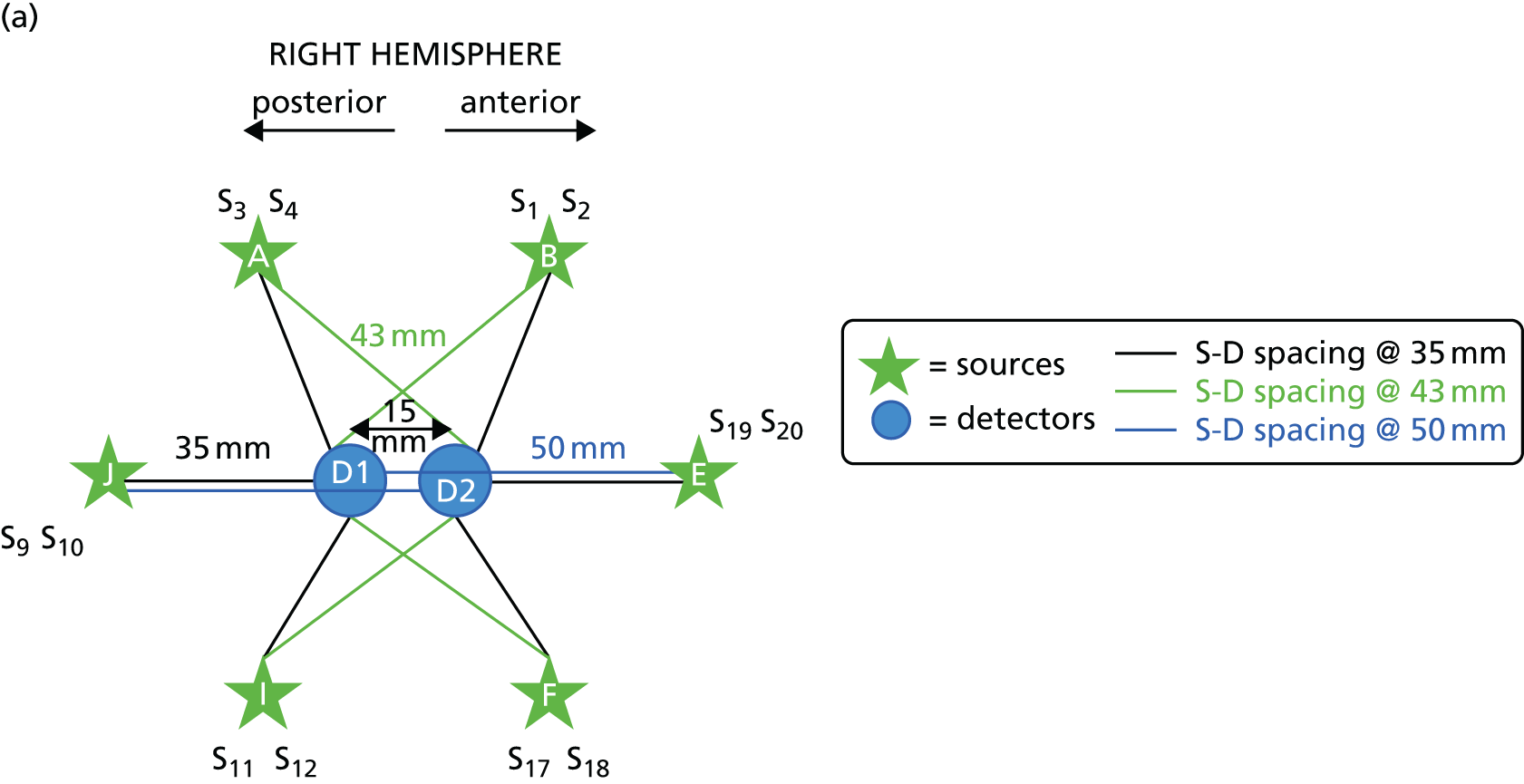

Near-infrared spectroscopy presents more challenges in adults than in its more widespread use in infants because of the light absorption properties of hair and the greater distance to the cerebral cortex from the skin surface. The distance between source and detector pairs can be varied to achieve an optimal signal for different depths below the skin. The shorter channel lengths offer the greatest signal-to-noise ratios but are less penetrating and, conversely, longer path lengths provide the greatest depth penetration but a poorer signal. In adults a channel distance of > 30 mm is typical. The array utilised in this study used a combination of 35-, 43- and 50-mm channels to try to optimise both penetration and signal quality (see Figure 1). The in-line arrangement of two sources and two detectors (including the two 50-mm channels) was designed to allow calculation of the TOI.
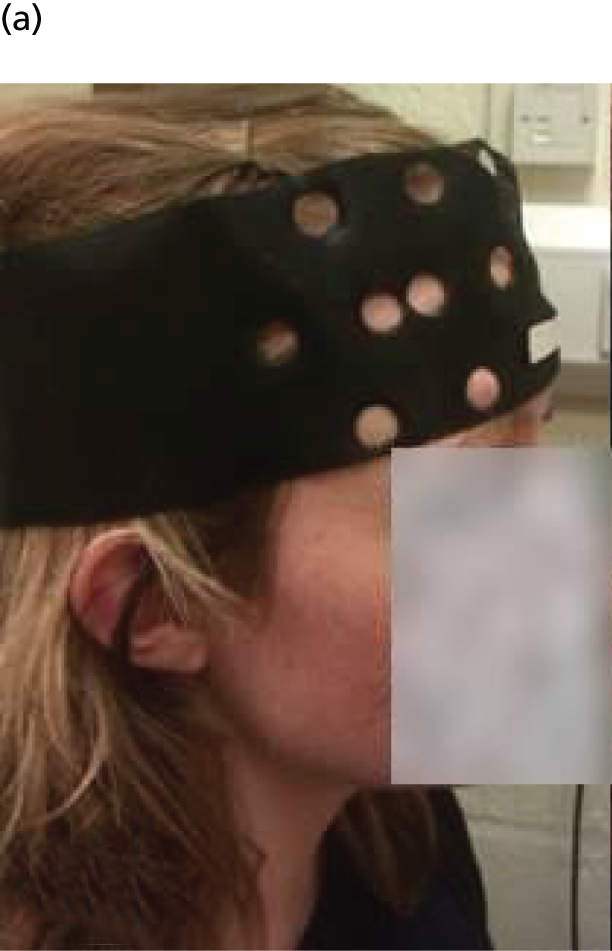
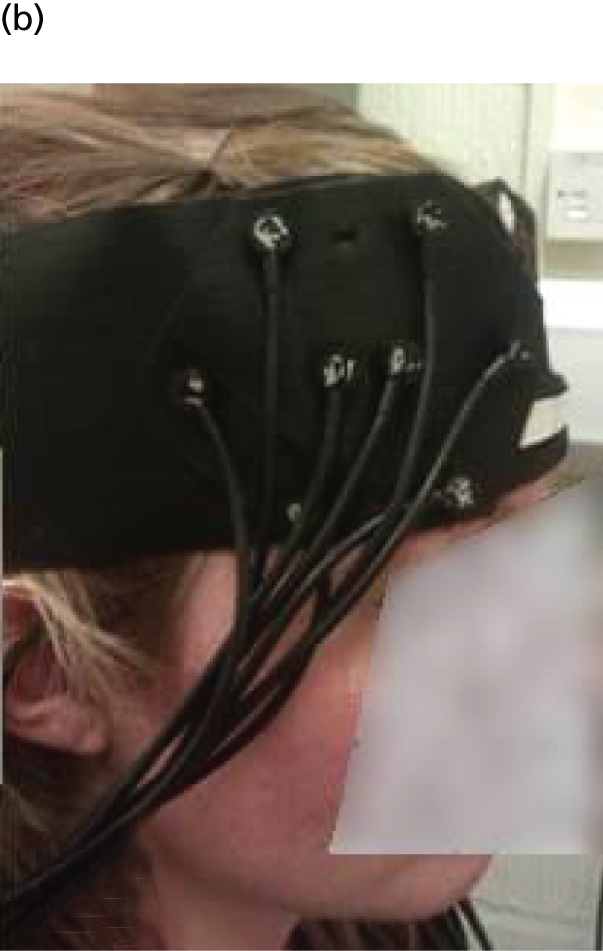
The bilateral arrays were built into left and right VELCRO® (Velcro Limited, Middlewich, UK) pads with fixed optode distances and configuration. The pads were attached to an optode placeholder (Figure 2a), which was positioned first on the head of participants in a consistent and reproducible way using reference points related to the 10/20 positioning system used in electroencephalogram (EEG) research. Participants were measured from each pre-auricular point, using the zygomatic notch as a reference, to ascertain the 10/20 position of Fpz above the nasion. The optode placeholder was adjusted according to the size of head to ensure that the detectors were in a standard position just below the F3/F4 EEG placement, with the lowest sources lying on the Fp1–T3 and Fp2–T4 lines. After moving hair obstructing the contact of the optodes with the skin, the array pads were attached (Figure 2b) and secured with further VELCRO straps to ensure contact with skin, prevent movement and exclude ambient light. To check the signal quality in each channel a read-out of each source–detector pair was checked using built-in software (mini NTS 24s 4d version 2.2, © UCL Medical Biophysics 2013, UCL, London, UK) and adjustments were made to the array if necessary to maximise the signal quality.
FIGURE 2.
Placement of NIRS array. Illustrated are (a) the array placeholder; (b) the array attached to the placeholder; and (c) fiducial markers used for magnetic resonance imaging co-registration. Photographs © Darragh Downey. Reproduced with permission.

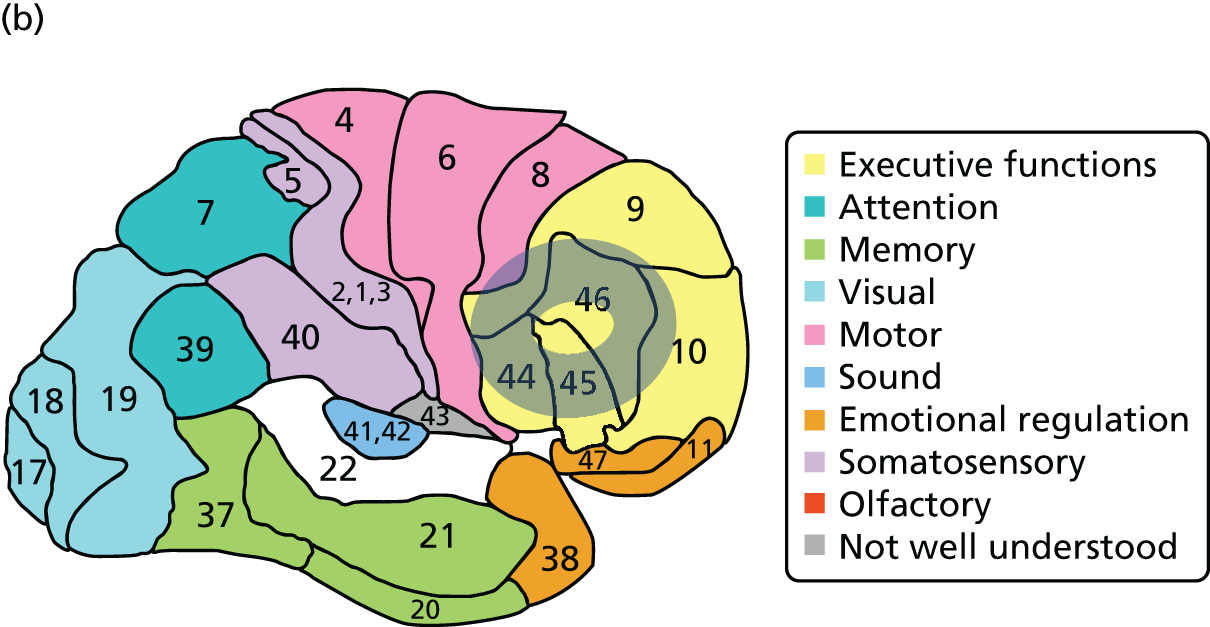
To confirm the location of the array with respect to the underlying cortex, a co-registered structural magnetic resonance imaging (MRI) scan was undertaken using cod liver oil capsules in the array pads as fiducial markers (Figure 2c) in a single participant on two occasions. Magnetic resonance T1-weighted structural images were acquired on a Philips 3T Achieva MRI scanner (Amsterdam, Netherlands) based at the National Institute for Health Research (NIHR)/Wellcome Trust Clinical Research Facility, Manchester [repetition time (TR) 9 milliseconds, echo time (TE) 4 milliseconds, voxel size 0.8 × 0.8 × 1.0 mm]. The fiducial markers were rendered using SPM12 [see www.fil.ion.ucl.ac.uk/spm/software/spm12/ (accessed 28 August 2016)] and MRIcroGL [see www.cabiatl.com/mricrogl/ (accessed 28 August 2016)]. The areas below the array correspond to Brodmann areas (BAs) 44, 45 and 46 and parts of BA 9 and BA 10 in the ventrolateral and dorsolateral prefrontal cortex (Figure 3). The within-subject reproducibility of the cortical area covered by the array was within a few millimetres. Data were acquired from all 24 channels at a rate of 10 Hz and manually synchronised with the onset of behavioural tasks using an event marker at the start of the task, triggered by the researcher.
FIGURE 3.
The areas of prefrontal cortex covered by the array. (a) A surface render of the fiducial markers on the right side with a shaded ‘doughnut ring’ indicating where the fNIRS signal was recorded; and (b) the shaded ring indicates the approximate BAs corresponding to the area of signal acquisition [BAs map reproduced with permission from BrainMaster Technologies, Inc. (see www.brainm.com/software/pubs/dg/BA_10-20_ROI_Talairach/functions.htm; accessed 28 August 2016), Brodmann Atlas].

Imaging tasks
The fNIRS tasks were administered after the main study neuropsychological tasks and, mindful of this, were designed to be as short and as straightforward as possible. The total time for testing was about 40–50 minutes (21 minutes performing the tasks).
Verbal fluency task
In the VF task participants were shown a category on a computer screen and asked to name out loud a word that matched that category (e.g. boys’ names, jobs, games and sports, vegetables). To minimise movement of the array caused by speaking, participants were instructed to verbalise through a fixed, or lightly locked, jaw, speak softly and remain as still as possible. The task was paced and consisted of prompts to produce the next word being given every 3 seconds in a block lasting 30 seconds (i.e. nine prompts per block). Pacing of the task was carried out to standardise, and to try and sustain, effort during the block, in an attempt to minimise expected performance differences between individuals, especially between patients and HCs. The category conditions were contrasted with rest blocks also lasting 30 seconds. A control naming task was not used so that the task was as short as possible while capturing the maximum signal–control difference in the haemodynamic response. The task consisted of an initial 30-second rest followed by 10 category naming blocks each separated by 10 rest periods. The total task duration was 10.5 minutes.
N-back task
The WM task was a shortened, adapted version of a standard blocked fMRI n-back task in which a series of letters were presented on a computer screen and participants were asked to respond to letters that matched ones that had appeared previously (in 1-back the letter matches the previous letter, in 2-back the letter matches the letter before last, etc.). This requires continuous updating of information held in WM. To maximise the signal, and to shorten the task, only the 2-back condition and rest were used. Participants were asked to attend to the letters and, if they saw a letter that was the same as one shown two letters previously, they were to press the keypad. They received a practice task to ensure that they understood the instructions. A total of 17 letters were displayed in a 30-second block, with four repeated (target) letters per block, followed by a 30-second rest block. Following an initial 30-second rest block, there were 10 2-back blocks each separated by 10 rest blocks, resulting in a task lasting 10.5 minutes.
Near-infrared spectroscopy analysis
Poor signal seen in the 50-mm channels meant that these data could not be used and the planned TOI analysis was not possible; the analysis method for this is therefore not described.
Functional near-infrared spectroscopy data were analysed with the Homer2 NIRS processing package146 based in MATLAB [see uk.mathworks.com (accessed 30 August 2016)]. For each participant, channels that measured a very low optical intensity (below the noise level of the device, e.g. because of poor optode–skin contact) were discarded from the analysis. Intensity data were then converted into optical attenuation data. For this preliminary analysis motion artefacts, that is, time points for which data exceeded selected changes in amplitude (AmpThresh) or standard deviation (SD) (StdThresh) over a period of 0.5 seconds, were identified in each channel. The choice of these parameters is data dependent, and a compromise between the number of motion artefacts identified in noisy channels and the number identified in less noisy channels is needed. In this study, AmpThresh = 10, StdThresh = 0.5 for all of the HC data and the patient mid-ECT data for the n-back task and AmpThresh = 8, StdThresh = 0.5 for all of the other patient data. The identified motion artefacts were corrected by applying a spline interpolation algorithm,147 which models the artefact with a cubic spline interpolation, subtracts it from the original signal and then corrects for the baseline shift caused by the subtraction. As some of the motion artefacts might still be present in the data even after this correction step, trials with remaining motion artefacts were rejected. A band-pass filter (third-order Butterworth filter) with cut-off frequencies of 0–0.5 Hz was applied to the data to reduce high-frequency noise. The filtered optical attenuation data were finally converted into concentration changes by applying the modified Beer–Lambert law,148 assuming a differential pathlength factor of 6.26. 149 Mean haemodynamic responses were calculated by block averaging all trials in an interval from 5 seconds before stimulus presentation to 60 seconds after stimulus presentation (hence containing both the 30-second task period and the 30-second rest period). Mean haemodynamic responses were baseline corrected by subtracting the mean value in the interval from –5 seconds to 0 seconds.
This produced one mean haemodynamic response for each channel in each participant, which was divided into three blocks of 20 seconds (0–20 seconds, 20–40 seconds and 40–60 seconds) (Figure 4). The integral of the mean haemodynamic response in each of these blocks was computed [area under the curve (AUC) for blocks 1, 2 and 3] for analysis.
FIGURE 4.
Example of mean haemodynamic response and block timing. The timings of the three 20-second blocks used in the analysis are shown (block 1: 0–20 seconds; block 2: 20–40 seconds; block 3: 40–60 seconds). The value of the integral in these blocks was used for fNIRS analysis. Hb, haemoglobin.
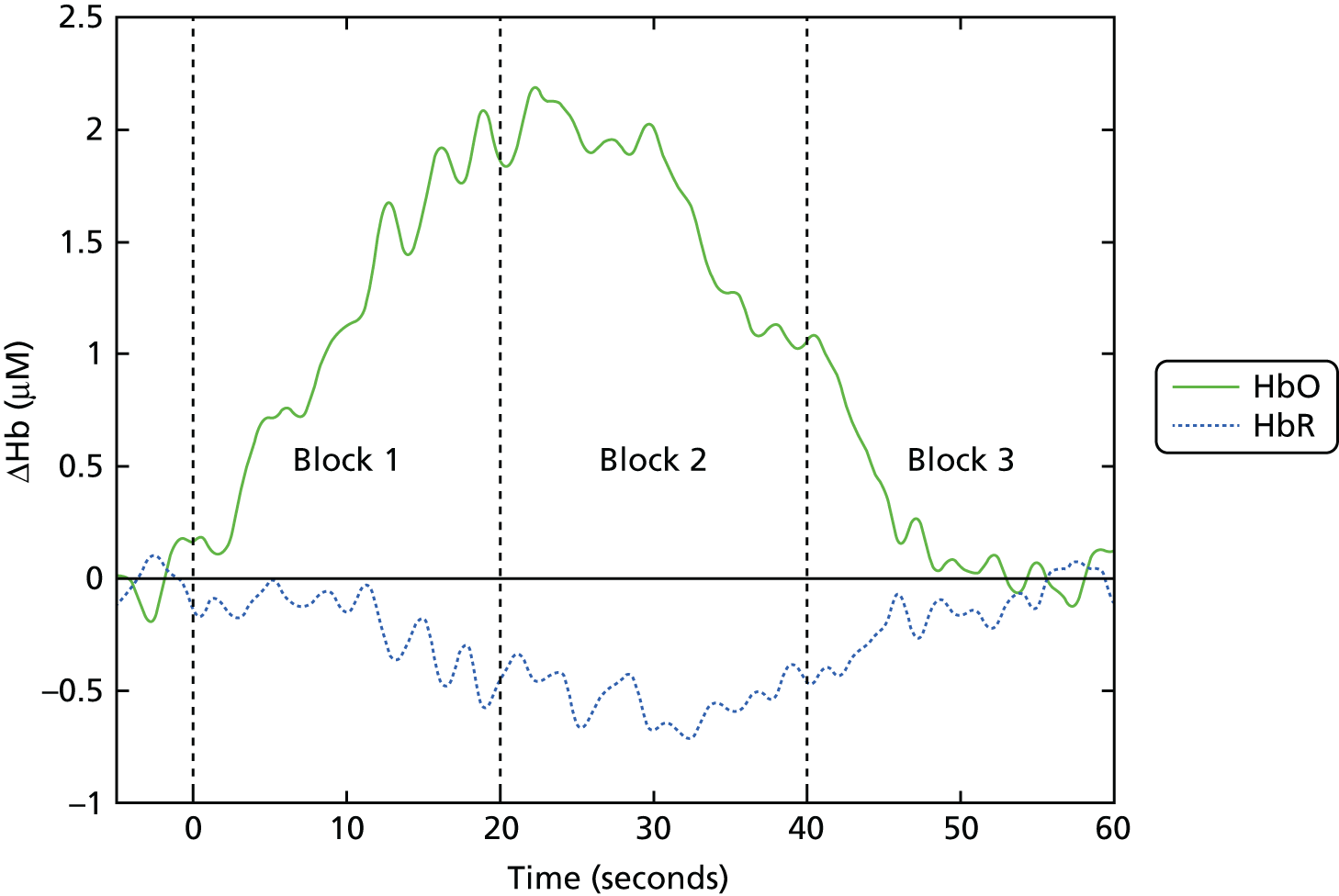
Given that the haemodynamic response typically lags behind neuronal activation by about 6 seconds, block 1 includes the onset of the haemodynamic response during the active task, block 2 includes the period of greatest sustained response during the task and block 3 includes the return of blood flow to rest level. As displayed in Figure 3, the areas of cortex in which HbO and HbR are measured by the array form a ring around the detectors. The data were collapsed to cover four regions, two on each side, an inferior and more posterior region consisting of BA 44, BA 45 and the inferior part of BA 9 [left ventrolateral prefrontal (LVF) and right ventrolateral prefrontal (RVF) regions] and a superior and more anterior region consisting of BA 46 and parts of BA 9 and BA 10 [left dorsolateral prefrontal (LDF) and right dorsolateral prefrontal (RDF) regions] (see Figure 3). This was carried out by calculating the mean of the channel values for that region [LVF: mean(D, C, L); LDF: mean(K, H, G); RVF: mean(F, I, J); RDF: mean(E, A, B); letters refer to channel sources shown in Figure 1] (see Figure 3). The long 50-mm channels had a poor signal and were excluded from the analysis, so that each region consisted of the mean of up to five channels (three 35-mm channels and two 43-mm channels).
The optimal haemoglobin factor for detecting cortical activation remains unclear: HbO is regarded as the most sensitive and has been most commonly reported in tasks of cortical activation, HbR changes less and appears minimally influenced by motion-induced changes and the difference between HbO and HbR values (Hbdiff) may provide an overall ‘oxygenation index’. 150 Analysis was carried out using all three measures.
Analysis was carried out in IBM SPSS Statistics version 22.0. To identify whether or not a haemodynamic response occurred in each group, a repeated measures ANOVA was carried out in HCs and patients at baseline separately over the three time blocks for each task. The groups were compared (using patient baseline data) by analysing the individual mean AUC values over the three time blocks by a univariate repeated measures ANCOVA with one between-group factor (group) and two within-group factors (region and time). Age and sex were used as covariates given the group differences (see Table 16), which, although non-significant, may influence fNIRS signal. 151,152 The effect of ECT and drug were analysed in patients alone using a repeated measures ANOVA with one between-group factor [drug (saline vs. ketamine)] and three within-group factors [ECT (baseline vs. mid-ECT), region and time]. The Huynh–Feldt correction was applied to correct for sphericity violation, and post hoc exploration of individual regions was carried out by repeated measures ANOVA or ANCOVA by region. For the VF task, the LVF was identified a priori as a region of interest as fNIRS haemodynamic responses in this region have been shown to relate to fMRI BOLD signal change during a VF task. 153 For correlational analysis between regional haemodynamic responses and behavioural data, block 2 AUC values, the time of maximum task-related response, were used, employing Pearson’s product–moment correlation coefficient (r). Results are presented as mean (SD) or mean ± standard error of the mean (SEM) with unadjusted degrees of freedom for clarity and statistical significance at p < 0.05. Given the small patient numbers and exploratory nature of the analysis, trend significance (p = 0.05 to p < 0.1) is also reported and no correction was made for multiple comparisons.
Chapter 3 Results
Participant recruitment
Flow through the study
The flow of participants through the study is shown in Figure 5. The primary assessment took place after patients had received four ECT treatments (mid-ECT). A total of 628 patients, identified through ECT team referrals or records, received ECT in participating sites during the period of recruitment (from December 2012 to June 2015), with 31.2% (n = 196/628) deemed potentially eligible for the study based on the opinion of treating clinical staff, ECT team staff and case notes, although this could not be determined with certainty unless patients had a screening interview with a member of the research team. The main reason for patients being ineligible was detention under the MHA (n = 294/628, 46.8%). Impaired capacity in voluntary patients was the second most common reason (n = 44/628, 7%), followed by having received previous ECT within the exclusion period (n = 35/628, 5.6%). Other exclusion criteria (n = 33/628, 5.3%), including psychiatric or physical diagnoses or being unable to validly complete neuropsychological testing, were mostly identified in discussion with clinical teams and from case notes, with a small minority identified during the formal screening assessment (n = 6/33, 18.2%).
FIGURE 5.
Consolidated Standards of Reporting Trials flow diagram. Numbers are patients still in the study, not valid assessments at each time (given in results tables). a, Determined primarily from case records and by clinical teams – six patients had exclusion criteria identified at screening. b, Both patients received ECT before excluding diagnoses were identified (both leading to withdrawal of ECT) but are not included in the study patients starting ECT to avoid confusion. c, At mid-ECT one participant receiving saline completed all assessments except for the primary neuropsychology outcome (HVLT-R delayed recall) and one participant receiving ketamine had the neuropsychology assessment outside the specified time. Neither received a further assessment, which excluded them from analysis.

Of those thought to be potentially eligible, 78.1% (n = 153/196) were invited to see the research team, 53.6% (n = 105/196) agreed to do so and 40.3% (n = 79/196) were randomised. The final percentage of all patients receiving ECT who were randomised was 12.6% (n = 79/628), which was lower than the 22.5% predicted when planning the study. This low overall recruitment rate was due in part to the high proportion of patients who were ineligible. Of note, the majority (53.8%) of the total ECT population who were detained and/or who lacked capacity contrasts with the lower figure of 38% in Scotland in 2014. 9
The main reason for non-participation of those potentially eligible was patient decision (n = 69/196, 35.2%), with 70% declining the invitation to see the research team and 30% choosing not to participate after the study was explained. Insufficient time to seek consent and administer assessments before ECT commenced was an anticipated problem and, in addition, the research team was not notified of a few eligible patients, mostly early in the study; in total, 12.8% (n = 25/196) of potentially eligible patients could not be recruited because of lack of time or notification. Organisational reasons for non-recruitment were relatively rare and included lack of ketamine supply or research staff at crucial times and patients belonging to non-participating NHS trusts but being given ECT at a participating site.
The depressed patients who were potentially eligible for the study were significantly younger than ineligible patients by an average of 6.7 years [mean 57.9 years (SD 13.6 years) vs. 64.6 years (SD 15.3 years); p < 0.001], with more women than men in both groups (69.4% vs. 66.0%). Patients who were randomised were also significantly younger than potentially eligible patients who were not randomised [mean 55.2 years (SD 13.1 years) vs. 59.8 years (SD 13.7 years); p = 0.02], largely accounted for by the patients who declined participation compared with the rest of the potentially eligible patients [mean 61.0 years (SD 12.6 years) vs. 56.3 years (13.9 years); p = 0.024].
Table 2 shows the origin of patients in the study by site. The percentage of potentially eligible patients varied from 20% to 42% across the sites, reflecting differences in clinical practice, and the proportion of eligible patients randomised varied from 32% to 65%.
| Site | Patients receiving ECT (n) | Potentially eligible patients (% of all patients) | Randomised patients (% of potentially eligible patients) |
|---|---|---|---|
| Derbyshirea | 35 | 7 (20) | 4 (57) |
| Lancashireb | 98 | 41 (42) | 13 (32) |
| Leedsc | 159 | 43 (27) | 16 (37) |
| Manchesterd | 52 | 20 (38) | 13 (65) |
| Northumberlande | 183 | 51 (28) | 21 (41) |
| Stockportf | 62 | 25 (40) | 9 (36) |
| Darlington and Middlesbroughg | 39 | 9 (23) | 3 (33) |
| Total | 628 | 196 (31) | 79 (40) |
Given that the aim of the study was to determine whether or not ketamine given as an adjunct to ECT was beneficial, an mITT population was specified for efficacy assessments, consisting of randomised patients who received their first ECT (defined as receiving the electrical stimulus). The final mITT population was 88.6% (n = 70/79) of the randomised population (see Figure 5), with more patients in the ketamine arm than the saline arm not being included (7 vs. 2), a non-significant difference (Fisher’s exact test p = 0.15). The only reason patients were excluded from the mITT analysis was if they lacked valid post-baseline data; in one case (ketamine arm) the neuropsychology assessment was carried out outside the time specified in the SAP (6 days rather than the maximum of 5) and for the rest of the participants exclusion was because of missing data.
The dropout rate was comparable in the two arms, with 84.3% (n = 59/70) of participants retained at the end of ECT treatment, 67.1% (n = 47/70) retained at the 1-month follow-up and 52.9% (n = 37/70) retained at the 4-month follow-up. Nine patients (11.4%) dropped out because of loss of capacity, detention under the MHA 2007 or because they required further ECT, reflecting the severity of illness in the population studied.
Recruitment
The first patient was randomised in December 2012 and the last in June 2015. The last follow-up was at the end of August 2015.
Challenges to recruitment
Patient recruitment was slow, especially at first, and this was compounded by fewer sites than planned taking part initially and a relative low rate of ECT use within trusts; two of the six original trusts were not able to participate but this became apparent only after study initiation. Given that recruitment was from a fixed population of patients receiving ECT, the only way to increase numbers was by recruiting new sites. The initiation of new sites took considerable time because of uncertainties engendered by NHS reorganisations, the organisational complexity of ECT provision (varying at different ECT suites within each trust), practical difficulties and governance requirements in undertaking a clinical trial involving a controlled medicine and having to recruit sites with little research experience. By the end of the study seven mental health trusts had been recruited, involving 11 ECT suites; a further two ECT suites that had been initiated closed because of internal trust reorganisations during the trial (Figure 6). In spite of the predicted shortfall in recruitment it was not practically or financially feasible to recruit more sites; in discussion with the funder and the DMEC the primary outcome was revised to a single outcome (rather than three inter-related measures) to maintain as much statistical power as possible (see Sample size).
FIGURE 6.
Timeline of trusts and ECT suites starting in the trial.

At most trusts the referral process for ECT occurred with short notice, so that timely identification of patients to allow recruitment and assessment was challenging. Attempts to minimise this barrier involved working with the ECT suites so that they could accommodate the study within their individual referral pathways; however, organisational and time pressures, and clinical need, necessarily took precedence, given the severity of illness of the population. Particular emphasis was given to raising awareness of the study within participating trusts by the study research teams, supported by the Mental Health Research Network/Local Clinical Research Networks, which also provided cover for screening and assessments at a number of sites.
At an early stage it was recognised that a significant number of patients had received ECT in the last 6 months and were therefore being excluded. This criterion was reviewed in the light of the literature that cognitive function improved rapidly after the end of ECT and was altered to 3 months in a protocol amendment in May 2013.
A major hurdle to recruitment was the higher than predicted proportion of patients who were ineligible because of being detained under the MHA, even though a proportion might have had capacity to consent to ECT and therefore potentially to the study. This criterion was therefore reviewed carefully early in the study, in discussion with the service user group. The decision was made that it should remain in place given concerns that the principle of fully informed consent could be undermined if patients were detained in hospital against their will, that is, some patients might feel under an obligation to participate. In practice, this is unlikely to have greatly affected recruitment as most detained patients receiving ECT lacked capacity to consent to the study, either because of the severity of their depression or because of cognitive impairment. 9
In mid-2014 an open letter was published by a group of academics and clinicians requesting that the funder and ethics committee suspend the trial on the basis of risks to patients and the participant information being misleading (details can be found in the study team’s rebuttal on the Guardian newspaper’s headquarters site154). This request was deemed unfounded by the funder and the Health Research Authority and was therefore rejected; it did not appear to directly impact on recruitment.
Problems in ketamine supply were a major threat to the trial from early in the study when, in early 2014, Pfizer suspended the production of Ketalar, the only available ketamine preparation in the UK (supplies had not been resumed by the end of recruitment). Alternative sourcing was obtained to allow the trial to continue, first from Germany and then, when these supplies also dried up (because of clinical demand in the absence of Ketalar), by successfully importing a non-EU-licensed product from the USA. Re-sourcing ketamine was a time-consuming process, involving considerable uncertainty and substantial amendments to the MHRA Clinical Trial Authorisation. As a result, some sites temporarily ran out of ketamine (which could not be transferred between sites for governance reasons), but, fortunately, only two potentially eligible patients were missed for this reason.
End of study
The trial was stopped at the end of the study period, as agreed with the Efficacy and Mechanism Evaluation (EME) Board. Because of recruitment problems, this involved a 4-month extension and a revised recruitment target and curtailed follow-up of the last few patients to maximise the duration of recruitment for the primary outcome. Recruitment remained problematic to the end, largely because of the paucity of eligible patients; the final mITT sample of 70 gave 65% power to detect the effect (ES 0.57) hypothesised in the revised sample size determination.
Conduct of the trial
The trial assessments, including the fNIRS tasks, were generally well tolerated and most patients were able to complete them provided that there was sufficient time and flexibility in their scheduling; inevitably, some data, and occasionally assessments, were missed given the severity of illness and the priority of clinical requirements. The greatest challenge was clinical unpredictability in the conduct of ECT, which made carrying out the assessments within the specified time frames extremely challenging. It was not uncommon for individual ECT sessions to be missed or delayed because of either patient or organisational factors, and decisions about ending treatment were often made at short notice or sometimes only in retrospect. The most frequent protocol deviations that affected the study were, therefore, those related to missed assessments or the timing of assessments. This led to specifying broader assessment windows for analysis in the SAP (Appendix 1) than in the protocol, with a sensitivity analysis according to the original protocol timings.
The second notable protocol deviation was incorrect dosing of the trial drug (defined as outside a 10% margin of error based on the patient’s weight at the time of administration). Occasional miscalculations were identified at a few sites and remedial action was taken to ensure that checking procedures were followed. Unfortunately, at one site a systematic error occurred, with a fixed dose being administered over a period of months to six patients before it was identified. In total, 11 patients were affected by mis-dosing in the study, three of whom were in the ketamine arm (two for the whole ECT course and one for two doses). The three ketamine dosing errors consisted of under-dosing by 13–33%, with only one occurring at the site with the systematic error. Before the blind was broken this was referred to the sponsor and the DMEC for advice. After careful review the decision was that this error, although a major breach of good clinical practice, was not a serious breach as defined by the MHRA and had affected neither patient safety nor the scientific integrity of the study. Given that the majority of dosing errors were in saline-treated patients, it appears likely in retrospect that lack of blindness to treatment allocation by the anaesthetist may have led to less care being taken on occasions with regard to following trial procedures when they were administering saline (as the specific ‘dose’ was unimportant).
Clinical outcomes
Baseline data
Baseline demographic and clinical data for the mITT population are shown in Table 3. The majority of patients had recurrent, non-chronic, melancholic, unipolar major depression with a first-degree family history of mood disorder and had not responded to around four pharmacological treatments. About half had a comorbid anxiety disorder and a similar proportion had received ECT in the past. Most patients were on a combination of an antidepressant and an antipsychotic, with about one-quarter on combination antidepressants and a similar proportion on an antidepressant together with lithium or a mood stabiliser. The saline and ketamine groups were generally well balanced on key demographics. Compared with the saline group, however, those randomised to the ketamine group were a little younger on average and fewer were married or had a current partner. They were less likely to be an inpatient or to have psychosis, but had a longer duration of the current episode, which had been resistant to more pharmacological treatments (indicated by a higher MGHS score). Fewer recipients of ketamine had a family history of bipolar disorder but more had a family history of depression.
| Characteristic | Treatment arm | |||
|---|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | |||
| Mean (SD) or median (IQR) | n (%) | Mean (SD) or median (IQR) | n (%) | |
| Age (years) | 56.4 (12.4) | 52.5 (11.9) | ||
| Sex (female) | 22 (59) | 22 (67) | ||
| Years in full-time education | 13.5 (3.2) | 13.7 (4.0) | ||
| Ethnicity (white) | 35 (95) | 31 (94) | ||
| Married or current partner | 19 (51) | 13 (39) | ||
| Illness characteristics | ||||
| Inpatient | 21 (57) | 11 (33) | ||
| Bipolar disorder | 7 (19) | 4 (12) | ||
| Age at onset (years) | 32.2 (17.0) | 29.6 (14.0) | ||
| No. depressive episodes (lifetime) | 5.3 (5.2) | 4.9 (4.4) | ||
| No. of hypomanic/manic episodes | 0.5 (1.8) | 0.3 (1.0) | ||
| Previous ECT | 16 (43) | 18 (55) | ||
| Family history of depression | 18 (49) | 23 (70) | ||
| Family history of bipolar disorder | 10 (27) | 2 (6) | ||
| Current depressive episode | ||||
| Melancholia, no psychosis | 25 (68) | 26 (79) | ||
| Psychosis ± melancholia | 8 (22) | 3 (9) | ||
| Duration (months) median (IQR) | 8 (3.5–20.5) | 14 (7–38) | ||
| MGHS score | 4.0 (3.4) | 4.8 (2.6) | ||
| Comorbidity | ||||
| Anxiety disorder or secondary OCD | 18 (49) | 16 (48) | ||
| Other psychiatric disorder | 0 (0) | 1 (3) | ||
| Substance use | 3 (8) | 0 (0) | ||
| Medical illness | 9 (24) | 12 (36) | ||
| Physical signs | ||||
| Weight (kg) | 77.2 (17.7) | 82.2 (17.1) | ||
| BMI (kg/m2) | 28.8 (6.7) | 29.3 (5.8) | ||
| Systolic blood pressure (mmHg) | 126.1 (17.2) | 132.9 (18.0) | ||
| Psychotropic medication | ||||
| SSRI | 10 (27) | 10 (30) | ||
| SNRI | 16 (43) | 17 (52) | ||
| TCA | 7 (19) | 4 (12) | ||
| MAOI | 1 (3) | 0 (0) | ||
| Other antidepressant | 9 (24) | 13 (39) | ||
| Antipsychotic | 25 (68) | 21 (64) | ||
| Lithium | 5 (14) | 10 (30) | ||
| Antiepileptic mood stabiliser | 6 (16) | 5 (15) | ||
| Benzodiazepine | 24 (65) | 18 (55) | ||
The two groups were well matched on key neuropsychological test scores and efficacy ratings (Table 4). Just over half of patients believed that ECT would adversely affect their memory on the GSE-My, but about one-fifth believed that it would improve it, with a mean quantitative prediction that it would cause minor impairment (a score of 4 indicates no change). Overall, the patients rated themselves as having an extremely low quality of life.
| Measure | Treatment arm, mean (SD) | Possible range | |
|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | ||
| Observer rated | |||
| IQ | 109.9 (11.0) | 105.1 (11.3) | – |
| MMSE | 29.0 (1.2) | 28.8 (2.0) | 0–30 |
| HVLT-R | |||
| Total learning | 20.8 (6.7) | 20.0 (4.1) | 0–36 |
| Delayed recalla | 5.86 (3.63) | 6.12 (2.69) | 0–12 |
| Retention | 65.2 (32.9) | 73.3 (27.8) | – |
| Recognition | 8.16 (3.48) | 9.45 (2.05) | 0–12 |
| MADRSa | 35.2 (8.4) | 31.8 (7.4) | 0–60 |
| CGI-S | 5.30 (0.97) | 5.03 (0.85) | 1–7 |
| Self-rated | |||
| GSE-My | |||
| Present memory | 3.57 (1.32) | 3.73 (1.15) | 1–7b |
| Will ECT affect your memory?, n (%)c | |||
| Yes, better | 8 (22) | 7 (21) | |
| No | 9 (25) | 6 (18) | |
| Yes, worse | 19 (53) | 20 (61) | |
| Expectation of treatment effectc | 3.67 (1.29) | 3.64 (1.30) | 1–7b |
| EQ-5D-3L | |||
| Index | 0.351 (0.275) | 0.349 (0.268) | –0.59–1.00 |
| VAS | 24.2 (16.9) | 31.9 (14.6) | 0–100 |
Neuropsychological outcomes
Further details of the outcomes at each time point are provided in Appendix 2. The primary assessment point was mid-ECT after four ECT treatments. Table 5 shows the mean number of ECT sessions and the time between the last ECT session and the neuropsychological assessment for both mid-ECT and the end of treatment, as well as the time from the first ECT session and from randomisation for all four valid post-baseline assessments in the ITT population. The two arms were well matched on these variables.
| Assessment | Treatment arm, mean (SD) | |
|---|---|---|
| Saline | Ketamine | |
| Mid-ECT | ||
| Number of previous ECT treatments | 4.0 (0.5) | 4.2 (0.6) |
| Time between last ECT and NP (days) | 1.8 (1.0) | 2.2 (1.2) |
| Time from first ECT (weeks) | 1.9 (0.4) | 2.0 (0.3) |
| Time from randomisation (weeks) | 2.2 (0.5) | 2.6 (0.8) |
| End of treatment | ||
| Number of previous ECT treatments | 11.3 (4.5) | 11.4 (4.1) |
| Time between last ECT and NP (days) | 4.2 (2.4) | 4.0 (2.4) |
| Time from first ECT (weeks) | 6.2 (2.6) | 6.1 (2.2) |
| Time from randomisation (weeks) | 6.6 (2.6) | 6.7 (2.3) |
| Follow-up 1 | ||
| Time from first ECT (weeks) | 10.0 (2.7) | 10.3 (2.2) |
| Time from randomisation (weeks) | 10.4 (2.7) | 10.8 (2.4) |
| Follow-up 2 | ||
| Time from first ECT (weeks) | 21.8 (4.1) | 21.9 (3.4) |
| Time from randomisation (weeks) | 22.2 (4.2) | 22.5 (3.4) |
To qualify as a valid assessment for analysis, the mid-ECT assessment was required to take place after four ECT sessions (range 3–5) and between 1 and 5 days after an ECT treatment, with between 1 and 12 days allowed for the end-of-treatment assessment (see Appendix 1). One mid-ECT assessment in a patient receiving ketamine occurred after six ECT treatments but was included in the primary analysis (decided on the low likelihood of an important influence on the results). One patient in the ketamine arm was excluded from the analysis because of a missing mid-ECT assessment (the assessment occurred 6 days after the ECT session because of clinical uncertainty, with the patient then not continuing ECT). Six participants (two on saline, four on ketamine) did not have a mid-ECT assessment but were included in the primary analysis as they had subsequent assessments in the study. A sensitivity analysis on the primary outcome was undertaken, excluding assessments outside the stricter protocol specification.
Primary outcome
The primary outcome was HVLT-R delayed recall as a measure of anterograde verbal memory at mid-ECT. Figure 7 and Table 6 show the number of words recalled by visit over the course of the trial; the treatment effect at each assessment point is given in Table 6. No significant difference between treatment arms was found, with the estimate favouring saline. The sensitivity analysis excluded assessments carried out 4 and 5 days after the last ECT session at mid-ECT (n = 4) and assessments carried out 6–12 days after the last ECT session at the end of treatment (n = 11), with no substantial change in the results (mid-ECT –0.56, 95% CI –1.91 to 0.79; end of treatment –0.56, 95% CI –1.82 to 0.71; follow-up 1 –0.53, 95% CI –1.65 to 0.60; follow-up 2 –1.43, 95% CI –2.95 to 0.10).
FIGURE 7.
Hopkins Verbal Learning Test – Revised delayed recall scores by visit in patients receiving ECT randomised to saline or ketamine. Numbers refer to observed valid assessments at each time point. Values are means ± SEM.

| Measure | Treatment arm | Repeated measures analysisa | ||||
|---|---|---|---|---|---|---|
| Saline | Ketamine | |||||
| Mean (SD) | n | Mean (SD) | n | Treatment effect difference (95% CI) | p-valueb | |
| HVLT-R total learning | ||||||
| Baseline | 20.8 (6.7) | 37 | 20.0 (4.1) | 33 | ||
| Mid-ECT | 21.0 (5.4) | 36 | 20.2 (5.8) | 29 | –0.11 (–2.04 to 1.81) | 0.91 |
| End of treatment | 21.2 (5.6) | 32 | 19.8 (4.9) | 26 | –0.96 (–2.97 to 1.06) | 0.35 |
| Follow-up 1 | 23.5 (5.6) | 23 | 22.3 (4.7) | 23 | –0.50 (–2.54 to 1.54) | 0.63 |
| Follow-up 2 | 24.1 (6.1) | 18 | 21.6 (5.0) | 19 | –1.12 (–3.68 to 1.44) | 0.39 |
| HVLT-R delayed recallc | ||||||
| Baseline | 5.86 (3.63) | 37 | 6.12 (2.69) | 33 | ||
| Mid-ECT | 5.54 (3.42) | 35 | 5.17 (2.92) | 29 | –0.43 (–1.73 to 0.87) | 0.51 |
| End of treatment | 5.44 (3.18) | 32 | 5.69 (2.80) | 26 | –0.04 (–1.22 to 1.13) | 0.94 |
| Follow-up 1 | 7.26 (2.63) | 23 | 6.70 (2.67) | 23 | –0.53 (–1.66 to 0.60) | 0.36 |
| Follow-up 2 | 8.11 (2.83) | 18 | 6.63 (3.17) | 19 | –1.40 (–2.91 to 0.12) | 0.07 |
| HVLT-R retention | ||||||
| Baseline | 65.2 (32.9) | 37 | 73.3 (27.8) | 33 | ||
| Mid-ECT | 62.4 (31.8) | 35 | 58.6 (25.0) | 29 | –5.12 (–18.0 to 7.8) | 0.44 |
| End of treatment | 58.7 (26.4) | 32 | 69.4 (26.6) | 26 | 6.97 (–4.88 to 18.8) | 0.25 |
| Follow-up 1 | 77.1 (16.1) | 23 | 73.3 (24.0) | 23 | –3.22 (–13.3 to 6.9) | 0.53 |
| Follow-up 2 | 85.5 (18.7) | 18 | 74.2 (23.3) | 19 | –11.97 (–24.0 to 0.10) | 0.052 |
| HVLT-R recognition | ||||||
| Baseline | 8.16 (3.48) | 37 | 9.45 (2.05) | 33 | ||
| Mid-ECT | 8.54 (2.84) | 35 | 8.48 (2.81) | 29 | –0.64 (–1.72 to 0.44) | 0.25 |
| End of treatment | 9.66 (2.10) | 32 | 8.58 (2.90) | 26 | –1.59 (–2.76 to –0.42) | 0.008 |
| Follow-up 1 | 9.52 (2.33) | 23 | 9.52 (2.33) | 23 | –0.59 (–1.61 to 0.43) | 0.25 |
| Follow-up 2 | 9.56 (2.97) | 18 | 10.42 (1.43) | 19 | 0.51 (–0.78 to 1.80) | 0.44 |
| COWAT letter fluency | ||||||
| Baseline | 36.1 (14.3) | 37 | 33.8 (13.1) | 33 | ||
| Mid-ECT | 36.1 (13.2) | 36 | 31.5 (12.9) | 29 | –1.82 (–5.86 to 2.23) | 0.38 |
| End of treatment | 34.2 (13.5) | 32 | 33.0 (13.4) | 26 | –1.01 (–5.57 to 3.54) | 0.66 |
| Follow-up 1 | 39.5 (14.1) | 23 | 35.6 (13.4) | 23 | –1.52 (–5.74 to 2.70) | 0.48 |
| Follow-up 2 | 38.6 (10.7) | 18 | 37.6 (13.2) | 19 | –0.18 (–5.17 to 4.80) | 0.94 |
| COWAT category fluency | ||||||
| Baseline | 16.8 (5.3) | 37 | 15.8 (5.5) | 33 | ||
| Mid-ECT | 16.4 (4.2) | 36 | 15.9 (5.3) | 29 | 0.03 (–1.73 to 1.78) | 0.97 |
| End of treatment | 15.8 (4.2) | 32 | 14.3 (5.1) | 26 | –1.29 (–3.15 to 0.57) | 0.17 |
| Follow-up 1 | 17.1 (4.1) | 23 | 16.5 (5.6) | 23 | –0.23 (–2.43 to 1.96) | 0.84 |
| Follow-up 2 | 18.1 (5.3) | 18 | 17.8 (4.6) | 19 | –0.35 (–2.90 to 2.21) | 0.79 |
| AMI-SFd | ||||||
| Baseline | 44.2 (10.3) | 37 | 45.5 (9.2) | 33 | ||
| Mid-ECT | 38.0 (10.0) | 36 | 39.3 (9.0) | 29 | –0.67 (–3.16 to 1.81) | 0.60 |
| End of treatment | 34.8 (10.5) | 32 | 34.7 (9.8) | 25 | –0.11 (–3.63 to 3.41) | 0.95 |
| Follow-up 1 | 35.4 (10.4) | 23 | 35.1 (10.0) | 23 | –0.46 (–3.91 to 3.00) | 0.80 |
| Follow-up 2 | 38.9 (8.4) | 16 | 37.4 (10.1) | 19 | –0.70 (–3.93 to 2.54) | 0.67 |
Secondary neuropsychological outcomes
There was no benefit of ketamine for any secondary neuropsychological outcome (see Tables 6 and 7). Most effects were in the direction of favouring saline but were not significant, apart from two measures at different time points: end-of-treatment HVLT-R recognition and mid-ECT forward digit span. Self-evaluation of memory showed that the majority of patients reported a negative effect of ECT; the scores indicated that patients on average judged the effect of ECT to be mildly detrimental (Table 8).
| Measure | Treatment arm | Repeated measures analysisa | ||||
|---|---|---|---|---|---|---|
| Saline | Ketamine | |||||
| Mean (SD) | n | Mean (SD) | n | Treatment effect difference (95% CI) | p-valueb | |
| MCGCFT copy | ||||||
| Baseline | 33.4 (4.3) | 37 | 34.6 (2.4) | 33 | ||
| Mid-ECT | 34.1 (3.2) | 36 | 33.9 (4.0) | 29 | –0.66 (–2.27 to 0.95) | 0.42 |
| End of treatment | 34.4 (2.4) | 32 | 34.6 (2.0) | 26 | –0.54 (–1.66 to 0.57) | 0.34 |
| Follow-up 1 | 34.4 (3.0) | 23 | 35.3 (1.2) | 23 | –0.41 (–1.61 to 0.78) | 0.50 |
| Follow-up 2 | 35.0 (1.1) | 18 | 35.2 (1.1) | 19 | –0.49 (–1.32 to 0.34) | 0.25 |
| MCGCFT immediate recall | ||||||
| Baseline | 17.9 (7.6) | 37 | 19.0 (8.8) | 33 | ||
| Mid-ECT | 18.4 (7.2) | 36 | 18.6 (8.6) | 29 | –0.35 (–3.09 to 2.39) | 0.80 |
| End of treatment | 16.6 (6.2) | 32 | 19.0 (7.4) | 26 | 0.61 (–1.77 to 3.00) | 0.61 |
| Follow-up 1 | 19.7 (6.7) | 23 | 19.5 (6.0) | 23 | –1.96 (–4.43 to 0.50) | 0.12 |
| Follow-up 2 | 21.5 (6.4) | 18 | 23.8 (8.1) | 19 | –0.27 (–3.05 to 3.58) | 0.88 |
| MCGCFT delayed recall | ||||||
| Baseline | 17.6 (6.9) | 36 | 18.9 (7.7) | 33 | ||
| Mid-ECT | 17.7 (7.5) | 35 | 17.7 (8.2) | 29 | –0.77 (–3.64 to 2.10) | 0.60 |
| End of treatment | 15.3 (5.3) | 32 | 17.6 (5.9) | 25 | 0.21 (–1.94 to 2.36) | 0.85 |
| Follow-up 1 | 19.0 (6.5) | 23 | 18.9 (6.4) | 22 | –2.32 (–5.00 to 0.37) | 0.091 |
| Follow-up 2 | 20.8 (6.8) | 18 | 22.9 (9.7) | 19 | 0.29 (–3.79 to 4.36) | 0.89 |
| Digit span forward | ||||||
| Baseline | 5.84 (1.21) | 37 | 5.70 (1.07) | 33 | ||
| Mid-ECT | 6.00 (1.04) | 36 | 5.48 (0.95) | 29 | –0.46 (–0.81 to –0.11) | 0.009 |
| End of treatment | 5.84 (1.02) | 32 | 5.88 (1.34) | 26 | 0.003 (–0.49 to 0.50) | 0.99 |
| Follow-up 1 | 5.83 (1.11) | 23 | 5.91 (1.04) | 23 | 0.002 (–0.46 to 0.46) | 0.99 |
| Follow-up 2 | 5.72 (0.89) | 18 | 5.68 (1.20) | 19 | –0.18 (–0.64 to 0.27) | 0.43 |
| Digit span backwards | ||||||
| Baseline | 3.95 (1.10) | 37 | 3.64 (1.17) | 33 | ||
| Mid-ECT | 3.94 (1.01) | 36 | 3.86 (1.38) | 29 | 0.17 (–0.25 to 0.59) | 0.42 |
| End of treatment | 3.97 (0.97) | 32 | 3.88 (1.21) | 26 | –0.04 (–0.50 to 0.42) | 0.87 |
| Follow-up 1 | 4.13 (1.29) | 23 | 3.87 (1.25) | 23 | –0.14 (–0.70 to 0.43) | 0.63 |
| Follow-up 2 | 4.06 (1.06) | 18 | 3.89 (1.05) | 19 | –0.08 (–0.65 to 0.50) | 0.80 |
| Measure | Treatment arm | Repeated measures analysisa | ||||
|---|---|---|---|---|---|---|
| Saline | Ketamine | |||||
| Mean (SD) | n | Mean (SD) | n | Treatment effect difference (95% CI) | p-value | |
| Present memory | ||||||
| Baseline | 3.57 (1.32) | 37 | 3.73 (1.15) | 33 | ||
| Mid-ECT | 3.58 (1.13) | 36 | 3.61 (1.23) | 28 | 0.05 (–0.49 to 0.58) | 0.86 |
| End of treatment | 3.63 (1.13) | 32 | 3.27 (1.19) | 26 | –0.29 (–0.85 to 0.28) | 0.32 |
| Follow-up 1 | 4.13 (1.42) | 23 | 3.87 (1.22) | 23 | –0.28 (–0.99 to 0.44) | 0.45 |
| Follow-up 2 | 4.39 (1.50) | 18 | 3.53 (1.26) | 19 | –0.68 (–1.48 to 0.11) | 0.092 |
| Effect of ECTb | ||||||
| Baseline (expectation) | 3.61 (1.38) | 37 | 3.73 (1.15) | 33 | ||
| Mid-ECT | 3.17 (1.07) | 36 | 3.64 (1.29) | 29 | 0.13 (–0.35 to 0.61) | 0.60 |
| End of treatment | 2.94 (1.08) | 32 | 3.36 (0.87) | 26 | 0.27 (–0.35 to 0.89) | 0.40 |
| Follow-up 1 | 3.09 (1.11) | 23 | 3.12 (1.24) | 23 | –0.01 (–0.62 to 0.60) | 0.97 |
| Follow-up 2 | 3.53 (1.12) | 18 | 3.09 (1.04) | 19 | –0.53 (–1.33 to 0.27) | 0.20 |
| Has ECT affected your memory?, n (%) | ||||||
| Mid-ECT | ||||||
| Yes, better | 3 (8) | 36 | 1 (3) | 29 | ||
| No | 10 (28) | 13 (45) | ||||
| Yes, worse | 23 (64) | 15 (52) | ||||
| End of treatment | ||||||
| Yes, better | 2 (6) | 32 | 3 (12) | 26 | ||
| No | 7 (22) | 7 (27) | ||||
| Yes, worse | 23 (72) | 16 (62) | ||||
| Follow-up 1 | ||||||
| Yes, better | 2 (9) | 23 | 2 (9) | 23 | ||
| No | 5 (22) | 6 (25) | ||||
| Yes, worse | 16 (70) | 15 (65) | ||||
| Follow-up 2 | ||||||
| Yes, better | 2 (11) | 18 | 1 (5) | 19 | ||
| No | 9 (50) | 7 (37) | ||||
| Yes, worse | 7 (39) | 11 (58) | ||||
Change in neuropsychological function during the electroconvulsive therapy course
Inspection of test scores at mid-ECT and end of treatment showed only small changes, if any, from baseline in most scores. Repeated measures ANOVA showed that the only measures with a significant deterioration over the course of ECT were AMI-SF standard and extended scoring (both p < 0.001) and MCGCFT immediate recall (p = 0.026) and delayed recall (p = 0.001).
From the end of treatment to follow-up 2 (4 months after the end of ECT) repeated measures ANOVA showed significant improvements in most measures (HVLT-R delayed recall, p = 0.01; HVLT-R retention, p = 0.012; COWAT category fluency, p = 0.018; MCGCFT immediate recall, p < 0.001; MCGCFT delayed recall, p < 0.001; AMI-SF with standard scoring, p = 0.011), although a trend was seen only with the extended scoring method (p = 0.089). No change over time during the follow-up period was seen with digit span forwards or backwards or COWAT letter fluency. The pattern of improvement was for HVLT-R measures to improve between the end of treatment and follow-up 1, for COWAT category fluency and MCGCFT measures to improve progressively and for AMI-SR with standard scoring to improve only between follow-up 1 and follow-up 2. Multivariate repeated measures ANOVA for these six measures confirmed a significant measure × time interaction (Wilks’ lambda p = 0.012), supporting a different pattern of improvement over time between measures.
In the comparison with baseline, values were numerically better at follow-up 2 for all measures except for the AMI-SF. However, significant improvements (using paired t-tests) were seen only with MCGCFT immediate (p = 0.004) and delayed (p = 0.019) recall, digit span backwards (p = 0.046) and HVLT-R recognition (p = 0.016). A significant decrease was seen with AMI-SF standard and extended scoring (p < 0.001).
Efficacy outcomes
Symptom and quality of life ratings are shown in Figure 8 and Tables 9 and 10. Mean scores improved considerably with treatment, from severe illness at baseline to end-of-treatment scores in the mild illness range.
FIGURE 8.
Montgomery–Åsberg Depression Rating Scale score by visit in patients receiving ECT randomised to saline or ketamine. One patient randomised to saline who continued to have ECT beyond 10 weeks is excluded. Numbers refer to observed valid assessments at each time point. Values are means ± SEM. EoT, end of treatment; FU 1, follow-up 1 month after the end of treatment (follow-up 1); FU 2, follow-up 4 months after the end of treatment (follow-up 2).
| Measure | Treatment arm | Linear random-effects analysisa | ||||
|---|---|---|---|---|---|---|
| Saline | Ketamine | |||||
| Mean (SD) | n | Mean (SD) | n | Treatment effect difference (95% CI) | p-value | |
| MADRSb | ||||||
| Baseline | 35.2 (8.4) | 37 | 31.8 (7.4) | 33 | 0.44 (–1.03 to 1.91) 0.54 (–0.85 to 1.94) | 0.56 |
| Mid-ECT (2 weeks) | 25.9 (12.4) | 33 | 25.4 (9.8) | 31 | 0.45c | |
| End of treatment | 15.0 (10.4) | 32 | 17.2 (11.6) | 27 | ||
| Follow-up 1 | 14.8 (11.4) | 23 | 16.8 (13.6) | 24 | – | – |
| Follow-up 2 | 13.5 (13.9) | 18 | 18.0 (13.3) | 19 | – | – |
| CAS-6d | ||||||
| Baseline | 9.16 (5.23) | 37 | 7.79 (4.53) | 33 | 0.02 (–0.38 to 0.42) | 0.93 |
| Mid-ECT (2 weeks) | 7.48 (5.37) | 33 | 7.39 (5.29) | 31 | ||
| End of treatment | 5.16 (4.34) | 32 | 5.44 (4.04) | 27 | ||
| Follow-up 1 | 4.43 (3.89) | 23 | 4.96 (4.62) | 24 | – | – |
| Follow-up 2 | 3.67 (4.92) | 18 | 4.95 (4.73) | 19 | – | – |
| BPRSe | ||||||
| Baseline | 40.0 (8.6) | 37 | 37.3 (5.4) | 33 | 0.02 (–0.70 to 0.73) | 0.97 |
| Mid-ECT (2 weeks) | 35.4 (8.5) | 33 | 35.1 (6.9) | 31 | ||
| End of treatment | 28.3 (6.1) | 32 | 29.6 (6.9) | 26 | ||
| Follow-up 1 | 27.6 (6.4) | 23 | 28.8 (7.8) | 24 | – | – |
| Follow-up 2 | 27.1 (8.3) | 18 | 30.3 (8.3) | 19 | – | – |
| CGI-S | ||||||
| Baseline | 5.30 (0.97) | 37 | 5.03 (0.85) | 33 | 0.03 (–0.13 to 0.18) | 0.73 |
| Mid-ECT (2 weeks) | 4.24 (1.35) | 33 | 4.48 (1.12) | 31 | ||
| End of treatment | 2.88 (1.24) | 32 | 3.33 (1.33) | 27 | ||
| Follow-up 1 | 2.57 (1.27) | 23 | 2.79 (1.56) | 24 | – | – |
| Follow-up 2 | 2.17 (1.34) | 18 | 3.00 (1.60) | 19 | – | – |
| CGI-I | ||||||
| Baseline | 3.00 (1.09) | 33 | 3.03 (1.02) | 31 | –0.03 (–0.17 to 0.11) | 0.69 |
| Mid-ECT (2 weeks) | 2.25 (1.11) | 32 | 2.52 (1.31) | 27 | ||
| End of treatment | 2.00 (1.04) | 23 | 2.13 (1.26) | 24 | – | – |
| Follow-up 1 | 2.11 (1.41) | 18 | 2.42 (1.35) | 19 | – | – |
| Measure | Treatment arm | Linear random-effects analysisa | ||||
|---|---|---|---|---|---|---|
| Saline | Ketamine | |||||
| Mean (SD) | n | Mean (SD) | n | Treatment effect difference (95% CI) | p-value | |
| QIDS-SR | ||||||
| Baseline | 20.0 (3.9) | 37 | 17.9 (4.9) | 33 | 0.30 (–0.29 to 0.88) | 0.32 |
| Mid-ECT (2 weeks) | 16.0 (5.9) | 33 | 14.1 (5.6) | 31 | ||
| End of treatment | 11.0 (5.8) | 32 | 11.9 (6.2) | 27 | ||
| Follow-up 1 | 10.1 (6.2) | 23 | 12.0 (7.5) | 24 | – | – |
| Follow-up 2 | 9.4 (7.5) | 18 | 12.5 (7.7) | 19 | – | – |
| EQ-5D-3L index | ||||||
| Baseline | 0.35 (0.28) | 37 | 0.35 (0.27) | 33 | –0.01 (–0.03 to 0.01) | 0.43 |
| Mid-ECT (2 weeks) | 0.44 (0.38) | 33 | 0.55 (0.28) | 31 | ||
| End of treatment | 0.70 (0.27) | 32 | 0.66 (0.26) | 27 | ||
| Follow-up 1 | 0.71 (0.29) | 23 | 0.67 (0.33) | 24 | – | – |
| Follow-up 2 | 0.71 (0.34) | 18 | 0.60 (0.33) | 19 | – | – |
| EQ-5D-3L VAS | ||||||
| Baseline | 24.2 (16.9) | 37 | 31.9 (14.6) | 33 | –1.16 (–3.41 to 1.09) | 0.31 |
| Mid-ECT (2 weeks) | 33.6 (22.2) | 33 | 42.0 (18.6) | 31 | ||
| End of treatment | 52.2 (21.9) | 32 | 51.4 (22.3) | 26 | ||
| Follow-up 1 | 54.8 (30.2) | 23 | 53.0 (26.4) | 24 | – | – |
| Follow-up 2 | 62.4 (31.1) | 18 | 46.2 (24.6) | 19 | – | – |
The estimates of the time × treatment interaction (i.e. the difference in slope between the ketamine group and the reference saline group) from the linear random-effects model analyses are shown in Tables 9 and 10. For the primary efficacy outcome, the MADRS, there was a non-significant estimated difference in slopes of 0.44 in favour of the saline arm.
Extracting time point-specific treatment effects based on the MADRS from the time × treatment interaction, the marginal treatment effect in favour of the saline group over the ketamine group was 2.2 (95% CI –1.3 to 5.6) MADRS points at 2 weeks, 3.0 (95% CI –2.3 to 8.4) MADRS points at 4 weeks and 3.9 (95% CI –3.9 to 11.8) MADRS points at 6 weeks, none of which was statistically significant.
The same picture was seen with the secondary efficacy outcomes, with no significant differences between the two treatment arms but a slight numerical advantage to the saline arm. Quality of life improved considerably over the treatment course but this remained moderately impaired at the end of treatment and at follow-up.
In total, 13 out of 37 (35.1%) patients reached remission (MADRS score of ≤ 10) in the saline arm during the acute treatment phase compared with 13 out of 33 (39.4%) in the ketamine arm. A Kaplan–Meier plot of time to first remission by group is provided in Figure 9, which shows that, up to 5 weeks, the rate of remission was more pronounced in the saline arm. However, on fitting a Cox model, the estimated hazard ratio for ketamine compared with saline was 1.16 (95% CI 0.51 to 2.64; p = 0.73) in favour of remission in the ketamine arm, a non-significant difference.
FIGURE 9.
Kaplan–Meier plot of time from first ECT session to first remission by group. Remission was defined as a MADRS score of ≤ 10. The hazard ratio was 1.16 (95% CI 0.51 to 2.64; p = 0.73).
The mean number of previous ECT treatments to achieve remission was 7.0 (SD 3.6) and 10.0 (SD 4.7) in the saline and ketamine arms, respectively. Applying a linear regression model (adjusting for the prespecified baseline covariates and baseline MADRS score), the mean number of ECT treatments to achieve remission on ketamine was 0.83 (95% CI –3.2 to 4.9; p = 0.67) greater than on saline.
A non-significantly higher proportion of patients in the saline arm compared with the ketamine arm responded during treatment, as defined by a ≥ 50% decrease in MADRS score from baseline [22/37 (59.5%) vs. 16/33 (48.5%)]. A Kaplan–Meier plot of time to first response (Figure 10) shows an apparent faster rate of response in the saline arm; however, on controlling for baseline covariates in a Cox model the estimated hazard ratio was 0.99 (95% CI 0.49 to 1.99; p = 0.97).
FIGURE 10.
Kaplan–Meier plot of time from first ECT to first response by group. Response defined as a decrease in MADRS score of ≥ 50%. The hazard ratio was 0.99 (95% CI 0.49 to 1.99; p = 0.97).
In the saline group, eight out of 23 (35%) patients worsened during follow-up (MADRS score increase of ≥ 4 points + CGI-S score increase of ≥ 1 point to CGI-S ≥ 3 from the end-of-treatment assessment) compared with four out of 25 (16%) in the ketamine group; this was not significant (Fisher’s exact test p = 0.19). Over the follow-up period the pattern was for symptom ratings and quality of life to further improve in the saline arm, with a variable picture in the ketamine arm. A further five patients met remission criteria by the end of follow-up in the saline arm, and one more in the ketamine arm, bringing the numbers reaching remission by the end of the study to 48.6% and 42.4%, respectively.
Electroconvulsive therapy treatment variables
Variables related to ECT treatment are provided in Table 11. The large majority of patients received propofol as per protocol. In the ketamine arm two patients received thiopental from the start with a further two swapped to it from propofol during ECT; one patient in the saline arm was swapped to thiopental. A 16% lower dose of propofol was given in the ketamine arm than in the saline arm, as anaesthetists were not blind to randomisation arm and tended to reduce the propofol dose when ketamine was also given; this had been found in a previous study. 51 Otherwise, the two arms were comparable with regard to ECT stimulus dose, seizure duration and degree of reorientation. Nearly all patients were reoriented 30 minutes after ECT, with no difference between the treatment arms.
| Variable | Treatment arm | p-valuea | |||
|---|---|---|---|---|---|
| Saline | Ketamine | ||||
| Mean (SD) | n | Mean (SD) | n | ||
| Dose of drugsb | |||||
| Propofol (mg) | 151.4 (43.6) | 37 | 127.1 (33.9) | 31 | 0.01 |
| Thiopental (mg) | 150 | 1 | 334.9 (53.4) | 4 | – |
| Suxamethonium (mg) | 50.8 (14.3) | 37 | 49.8 (15.0) | 33 | 0.77 |
| Saline/ketamine (mg)c | 38.8 (8.1) | 37 | 40.8 (8.1) | 32 | 0.32 |
| ECT related | |||||
| Bilateral, n (%)d | 34 (92) | 37 | 28 (85) | 33 | 0.46 |
| Stimulus dose (mC)b | 276.5 (155.6) | 37 | 306.0 (213.6) | 33 | 0.51 |
| Motor seizure duration (seconds)b | 14.9 (5.2) | 37 | 15.2 (5.6) | 33 | 0.83 |
| EEG seizure duration (seconds)b | 26.2 (7.8) | 37 | 24.4 (10.4) | 33 | 0.42 |
| Reorientation after seizureb | |||||
| No. of items correct at 30 minutes | 4.69 (0.34) | 37 | 4.65 (0.50) | 33 | 0.69 |
| Proportion reoriented at 30 minutese | 0.94 (0.11) | 37 | 0.93 (0.14) | 33 | 0.75 |
Concealment of treatment allocation
Patients, and the researchers carrying out the assessments, completed questionnaires asking them to ‘guess’ their treatment allocation. At mid-ECT 28 out of 58 (48%) patient guesses and 35 out of 63 (56%) RA guesses were correct. At the end of treatment there were 30 out of 54 (56%) correct guesses for patients and 28 out of 55 (51%) correct guesses for researchers. These proportions did not differ significantly from chance.
Completion of the electroconvulsive therapy course
The reasons given by clinical teams for patients not completing their course of ECT are provided in Table 12. Nearly two-thirds of patients were deemed to have responded to treatment, with slightly more patients receiving ketamine than saline stopping because of a lack of efficacy, although this was not statistically significant.
| Main reason | Treatment arm | |||
|---|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | |||
| n | % | n | % | |
| Responded to treatment | 24 | 65 | 21 | 64 |
| Lack of efficacy | 4 | 11 | 7 | 21 |
| Patient deterioration | 0 | 0 | 1 | 3 |
| AE | 1 | 3 | 0 | 0 |
| Loss of capacity | 1 | 3 | 1 | 3 |
| Patient decision | 3 | 8 | 2 | 6 |
| Other | 4 | 11 | 1 | 3 |
Comparison of patients and healthy control subjects
Fifty-six HCs were recruited and their characteristics at baseline compared with those of the randomised patients are shown in Table 13. Patients rated their memory as being significantly worse than that of HCs and had a very low quality of life. Patients performed significantly less well on all neuropsychological tests (Table 14).
| Characteristic | Participants | p-valueb | |||
|---|---|---|---|---|---|
| HCs (n = 56) | Patients (n = 77)a | ||||
| Mean (SD) | n (%) | Mean (SD) | n (%) | ||
| Age (years) | 56.3 (12.0) | 54.4 (12.3) | 0.38 | ||
| Sex (female) | 34 (61) | 49 (64) | 0.86 | ||
| Years in full-time education | 14.6 (3.2) | 13.7 (3.6) | 0.16 | ||
| Ethnicity (white) | 55 (98) | 73 (95) | 0.40 | ||
| Handedness (left/mixed) | 2/4 (4/7) | 4/4 (5/5) | > 0.8 | ||
| IQ | 111.3 (10.2 | 107.7 (11.1) | 0.07 | ||
| MMSE | 29.8 (0.5) | 29.0 (1.6) | < 0.001 | ||
| GSE-My present memory | 4.5 (0.9) | 3.7 (1.3) | < 0.001 | ||
| MADRS | 0.9 (1.5) | 33.9 (7.9) | < 0.001 | ||
| EQ-5D-3L index | 0.96 (0.09) | 0.35 (0.27) | < 0.001 | ||
| EQ-5D-3L VAS | 82.2 (11.6) | 27.0 (16.2) | < 0.001 | ||
| Measure | Participants, mean (SD) | p-value | |
|---|---|---|---|
| HCs (n = 56) | Patients (n = 76) | ||
| HVLT-R | |||
| Total learning | 25.4 (4.6) | 20.4 (5.5) | < 0.001 |
| Delayed recall | 8.9 (2.5) | 6.05 (3.1) | < 0.001 |
| Retention | 87.0 (17.9) | 70.0 (30.0) | < 0.001 |
| Recognition | 10.3 (2.2) | 8.8 (2.9) | 0.002 |
| COWAT | |||
| Letter fluency | 45.5 (11.9) | 35.4 (13.6) | < 0.001 |
| Category fluency | 23.3 (4.5) | 16.5 (5.6) | < 0.001 |
| Digit span | |||
| Forwards | 6.7 (1.0) | 5.8 (1.2) | < 0.001 |
| Backwards | 4.9 (1.2) | 3.8 (1.1) | < 0.001 |
| MCGCFT | |||
| Copy | 35.6 (0.9) | 34.1 (3.4) | < 0.001 |
| Immediate recall | 25.9 (5.7) | 18.1 (8.3) | < 0.001 |
| Delayed recall | 25.2 (5.8) | 18.0 (7.6) | < 0.001 |
Table 15 shows results for patients assessed 4 months after the last ECT treatment according to remission status compared with results for HCs. The remitted patients (MADRS score of ≤ 10) had minimal symptoms on average, whereas non-remitted patients were still moderately depressed. The remitted patients had regained a very good quality of life, not significantly different from that of HCs, whereas for the non-remitted patients quality of life was still very poor. Remitted patients now rated their present memory no differently from HCs, with two-thirds saying that ECT either had not affected or had improved their memory. In contrast, non-remitted patients evaluated their memory as still poor, with the majority saying that ECT had made their memory worse. The neuropsychological test results did not show a uniform pattern. Verbal list learning and delayed recall (HVLT-R) returned to normal in remitted patients but remained impaired in non-remitted patients, whereas VF (COWAT) in remitted patients showed intermediate results between those of HCs and non-remitted patients. On the other tests, the remitted and non-remitted patients did not significantly differ; attention and WM as measured by digit span showed little improvement compared with baseline in either group of patients, but complex figure reproduction (MCGCFT) was better than at baseline in both groups.
| Measure | Participants, mean (SD) | One-way ANOVA p-valuea | ||
|---|---|---|---|---|
| HCs (n = 56) | Remitted patients (n = 18) | Non-remitted patients (n = 19) | ||
| MADRS | 0.9 (1.5) | 3.8 (3.2)* | 27.2 (8.8)**,††† | < 0.001 |
| EQ-5D-3L index | 0.96 (0.09) | 0.89 (0.17) | 0.42 (0.29)***,††† | < 0.001 |
| EQ-5D-3L VAS | 82.2 (11.6) | 78.0 (16.5) | 31.4 (16.8) ***,††† | < 0.001 |
| HVLT-R | ||||
| Total learning | 25.4 (4.6) | 23.4 (6.4) | 22.2 (4.8)* | 0.044 |
| Delayed recall | 8.9 (2.5) | 8.1 (3.4) | 6.7 (2.6)** | 0.009 |
| Retention | 87.0 (17.9) | 84.4 (21.8) | 75.3 (21.2) | 0.08 |
| Recognition | 10.3 (2.2) | 10.2 (2.1) | 9.8 (2.6) | 0.71 |
| COWAT | ||||
| Letter fluency | 45.5 (11.9) | 39.4 (10.4) | 36.8 (13.3)* | 0.013 |
| Category fluency | 23.3 (4.5) | 19.8 (4.4)* | 16.1 (4.7)***,† | < 0.001 |
| Digit span | ||||
| Forwards | 6.7 (1.0) | 5.6 (0.8)*** | 5.8 (1.3)** | < 0.001 |
| Backwards | 4.9 (1.2) | 3.8 (0.8)** | 4.1 (1.2)* | 0.001 |
| MCGCFT | ||||
| Copy | 35.6 (0.9) | 34.9 (1.2)* | 35.3 (1.0) | 0.038 |
| Immediate recall | 25.9 (5.7) | 21.5 (7.1)* | 23.8 (7.5) | 0.035 |
| Delayed recall | 25.2 (5.8) | 21.4 (7.8) | 22.3 (9.1) | 0.083 |
| AMI-SF | – | 37.1 (10.3) | 39.1 (8.3) | 0.53 |
| GSE-My | ||||
| Present memory | 4.5 (0.9) | 4.8 (1.4) | 3.2 (1.0)***,††† | < 0.001 |
| Has ECT affected your memory?, n (%) | ||||
| Yes, better | – | 3 (17) | 0 (0) | |
| No | – | 9 (50) | 7 (37) | |
| Yes, worse | – | 6 (33) | 12 (63) | |
| Effect of ECT | – | 3.7 (1.4) | 2.9 (1.0) | 0.071 |
Near-infrared spectroscopy study
The intention had been to administer NIRS at the same time points as for neuropsychological testing, but because of the low numbers recruited and dropouts there were sufficient participants for analysis only at baseline and mid-ECT (after four ECT sessions). As reported in Chapter 2, it was not possible to undertake the TOI analysis.
Population
The characteristics of patients and control subjects participating in the fNIRS study are shown in Table 16. Although patients were slightly younger and there was a lower proportion of female patients, these differences were not significant. There was no significant difference in IQ between the groups but patients had slightly but significantly lower MMSE scores and significantly higher MADRS scores than HCs.
| Characteristic | Participants | |||
|---|---|---|---|---|
| HCs (N = 51) | Patients (N = 18) | |||
| Mean (SD) | n (%) | Mean (SD) or median (IQR) | n (%) | |
| Age (years) | 56.2 (12.1) | 51.6 (15.6) | ||
| Sex (female) | 30 (59) | 8 (44) | ||
| Ethnicity (white) | 50 (98) | 17 (94) | ||
| Handedness (left/mixed) | 2/4 (4/8) | 1/0 (6/0) | ||
| Years in full-time education | 14.7 (3.3) | 13.5 (3.2) | ||
| Illness | ||||
| Bipolar disorder | – | 2 (11) | ||
| Episode duration (months) | – | 20.5 (7.75–46.5) | ||
| MGHS score | – | 4.4 (3.2) | ||
| IQ | 110.7 (10.2) | 108.6 (11.5) | ||
| MMSE | 29.8 (0.5) | 29.0 (1.2)* | ||
| MADRS | 0.9 (1.5) | 33.7 (7.3)*** | ||
| COWAT category fluency | 23.2 (4.7) | 15.7 (6.0)*** | ||
| Digit span backwards | 4.9 (1.2) | 3.8 (1.1)*** | ||
Verbal fluency task
Behavioural data
Although no behavioural data were collected during the fNIRS task, participants were tested on a similar category VF task using the COWAT and, as with the whole group, the fNIRS subgroup of patients performed significantly less well on this task than HCs (see Table 15). However, the COWAT task is not paced and therefore tests both the speed with which words are retrieved and the number of words that are able to be retrieved, unlike the fNIRS task; thus, the results may not reflect the patients’ performances while being scanned.
Haemodynamic response to the verbal fluency task in each group
Haemoglobin AUC data were available for 49–51 HCs and 16 or 17 patients depending on region (one patient did not complete the VF task).
The results for HbO values are shown in Table 17 and the mean effect of time averaged over all regions is illustrated in the group comparison in Figure 11. The repeated measures ANOVA in HCs showed a significant effect of time (F2,96 = 7.404, p = 0.002) and a trend to a region × time interaction (F6,288 = 2.408, p = 0.076), with changes over time in all regions. In patients there was only a trend to an effect of time (F2,30 = 2.868, p = 0.081) because of the values tending to rise over the blocks. ANOVAs of individual regions in patients showed a significant effect in the RVF only (see Table 17).
| Region | Block, mean (SD) (µM·second) | Post hoc ANOVA for individual regions (effect of time)a | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| HCs | ||||
| LVF | 1.7 (3.6) | 3.6 (5.2) | 0.6 (7.2) | F2,100 = 5.501, p = 0.014 |
| LDF | 2.1 (4.6) | 4.3 (7.3) | 2.8 (6.6) | F2,96 = 6.295, p = 0.003 |
| RVF | 2.5 (3.9) | 4.3 (6.1) | 2.1 (5.0) | F2,100 = 4.534, p = 0.018 |
| RDF | 1.9 (3.1) | 3.7 (5.2) | 2.8 (4.5) | F2,100 = 4.521, p = 0.016 |
| Patients | ||||
| LVF | –0.2 (2.8) | 1.3 (6.9) | 2.2 (3.8) | F2,30 = 1.829, p = 0.18 |
| LDF | 0.7 (4.1) | 1.0 (6.9) | 2.0 (4.7) | F2,30 = 0.765, p = 0.47 |
| RVF | –0.7 (3.5) | 2.4 (7.9) | 3.1 (5.2) | F2,32 = 3.685, p = 0.039 |
| RDF | 0.2 (3.1) | 2.6 (7.7) | 4.4 (8.2) | F2,32 = 3.304, p = 0.057 |
FIGURE 11.
Time course of HbO, HbR and Hbdiff AUCs in HCs and patients during the VF task. Responses are averaged over all regions. Values are adjusted means ± SEM (covaried for age and sex). (a) HbO all regions; (b) HbR all regions; and (c) Hbdiff all regions.
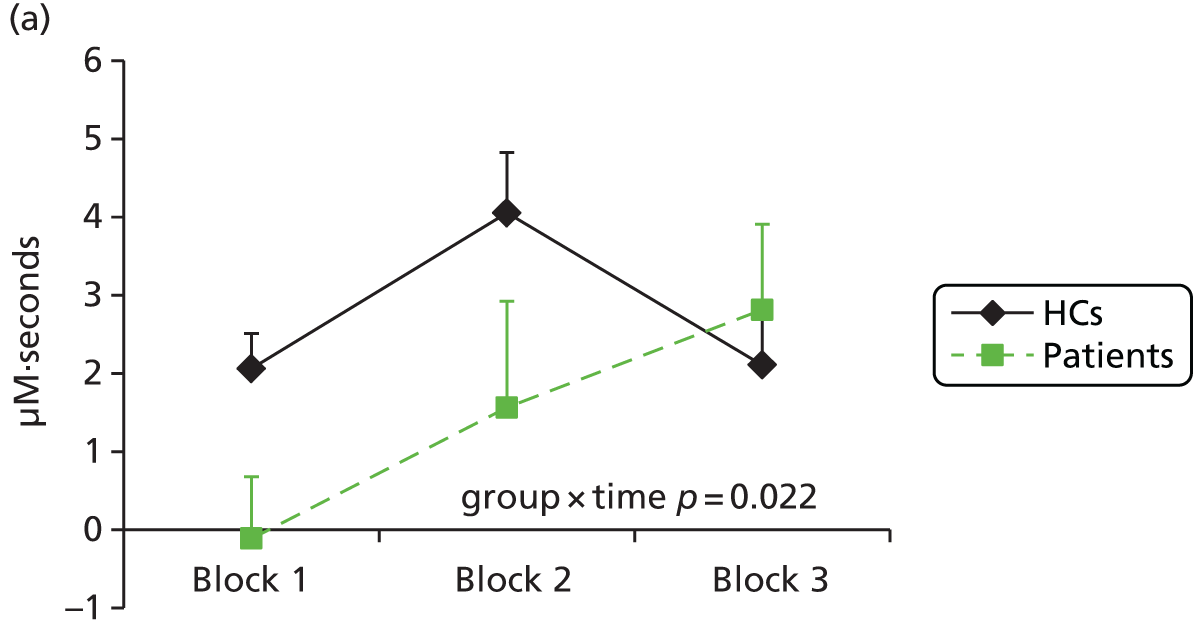
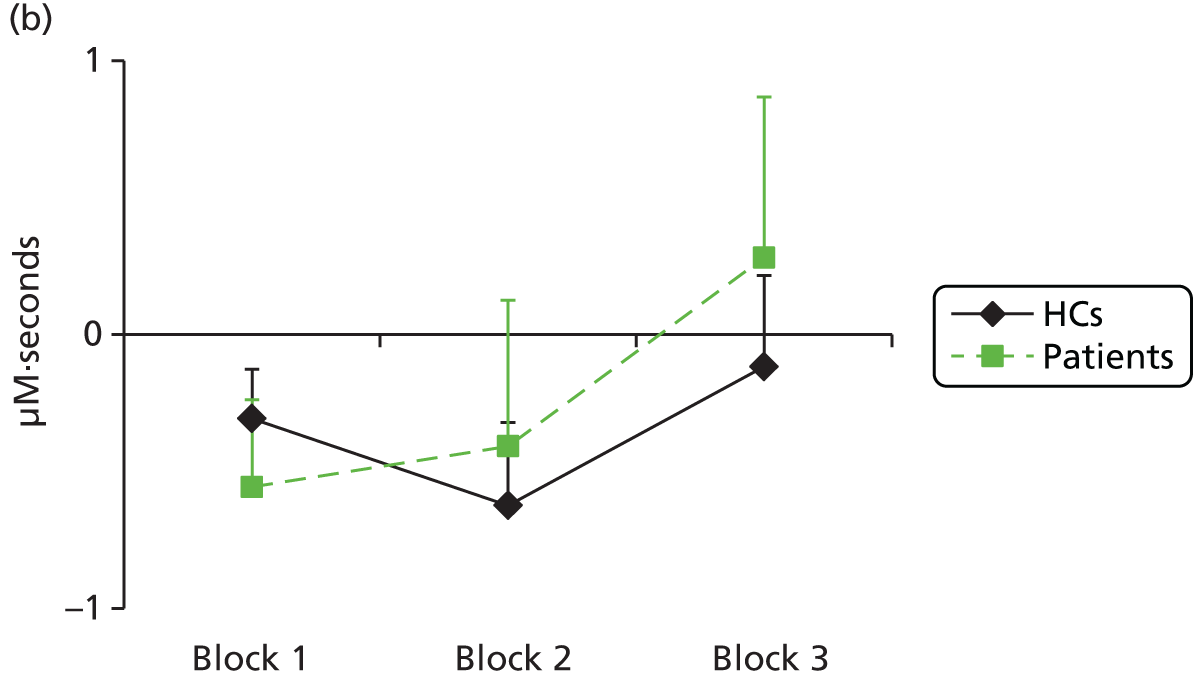

For HbR (Table 18 and see Figure 11), in HCs there was a trend to an effect of time (F2,96 = 3.116, p = 0.055) and a significant region × time interaction (F6,288 = 2.770, p = 0.037); a decrease in AUC was seen between block 1 and block 2 in all regions, with an increase in block 3 apart from in the LVF where it decreased further. All regions had significant (LDV, RVF) or trend significant (LVF, RDF) changes over time. In patients, although mean AUC values increased in all regions between blocks 2 and 3, no significant main effects or interactions were found.
| Region | Block, mean (SD) (µM·second) | Post hoc ANOVA for individual regions (effect of time)a | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| HCs | ||||
| LVF | –0.2 (2.0) | –0.7 (2.8) | –0.8 (1.8) | F2,100 = 2.821, p = 0.07 |
| LDF | –0.6 (2.5) | –0.8 (3.3) | –0.1 (3.7) | F2,96 = 3.492, p = 0.043 |
| RVF | –0.1 (1.8) | –0.6 (2.5) | 0 (2.8) | F2,100 = 3.401, p = 0.039 |
| RDF | –0.5 (1.5) | –0.5 (2.8 | 0.4 (4.2) | F2,100 = 2.818, p = 0.092 |
| Patients | ||||
| LVF | –0.3 (2.6) | –0.9 (3.4) | –0.5 (2.3) | F2,30 = 0.877, p = 0.43 |
| LDF | –0.5 (1.4) | –0.7 (2.6) | 0 (2.6) | F2,30 = 1.016, p = 0.37 |
| RVF | –1.1 (1.8) | –0.8 (2.8) | 0.1 (3.5) | F2,32 = 1.394, p = 0.26 |
| RDF | 0 (1.7) | 1.0 (4.9) | 1.8 (6.6) | F2,32 = 1.234, p = 0.29 |
For Hbdiff values (Table 19 and see Figure 11), in HCs there was a highly significant effect of time (F2,96 = 10.501, p < 0.001) with no significant effect of region or region × time interaction. The effect of time was due to a peak in block 2 seen in all four regions, confirmed by the post hoc regional analysis. In patients, although mean values increased over the blocks, no significant effect of time was seen in the ANOVA, although when regions were examined separately there was a trend in the RVF.
| Region | Block, mean (SD) (µM·second) | Post hoc ANOVA for individual regions (effect of time)a | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| HC | ||||
| LVF | 1.8 (4.3) | 4.2 (5.5) | 1.4 (7.4) | F2,100 = 7.297, p = 0.003 |
| LDF | 2.7 (5.1) | 5.1 (7.3) | 2.9 (5.2) | F2,96 = 8.638, p < 0.001 |
| RVF | 2.6 (3.8) | 4.9 (6.5) | 2.1 (5.8) | F2,100 = 6.071, p = 0.005 |
| RDF | 2.4 (3.5) | 4.2 (5.5) | 2.5 (3.8) | F2,100 = 6.785, p = 0.002 |
| Patients | ||||
| LVF | 0.1 (4.2) | 2.2 (8.7) | 2.7 (8.7) | F2,30 = 2.059, p = 0.15 |
| LDF | 1.2 (4.8) | 1.7 (8.4) | 2.0 (4.9) | F2,30 = 0.233, p = 0.78 |
| RVF | 0.4 (3.6) | 3.2 (8.9) | 3.0 (5.7) | F2,32 = 3.094, p = 0.075 |
| RDF | 0.2 (3.8) | 1.6 (8.6) | 2.5 (6.2) | F2,32 = 1.370, p = 0.27 |
Correlations between performance and mood and haemodynamic responses
No significant correlations were found in either all participants taken together or each group separately between COWAT category fluency and block 2 AUC values (chosen as the period encompassing the maximum response; see Figure 4). For MADRS scores a negative correlation was found in patients for the baseline block 2 Hbdiff AUC (r = –0.529, p = 0.035) in the LVF region (i.e. more severe depression was associated with lower responses). The corresponding correlation for the baseline block 2 HbO AUC in the LVF was in the same direction but not significant (r = –0.411, p = 0.11).
Group comparison of the time course of the haemodynamic response at baseline
Data were available in all regions for 49 HCs and 16 patients.
The ANCOVA of HbO AUC values, covaried for age and sex, showed a significant group × time interaction (F2,122 = 3.936, p = 0.022) with no other main effects or interactions. The time course for the mean AUC over all regions for the two groups is illustrated in Figure 11; patients had lower values in blocks 1 and 2 than HCs. Post hoc ANCOVA by region showed significant group × time differences in the LVF (F2,126 = 3.531, p = 0.041) and RVF (F2,128 = 3.575, p = 0.031).
For the HbR AUC ANCOVA no significant main effects or interactions were found (see Figure 11).
The Hbdiff AUC ANCOVA showed similar findings to the HbO analysis (see Figure 11) with a trend to a significant time × group interaction (F2,122 = 3.023, p = 0.053) and a significant effect of region (F3,183 = 3.295, p = 0.032) (because of higher overall mean AUC values in the LDF and RVF than in the LVF and RDF; see Table 19). Exploratory ANCOVA by region showed a significant group × time interaction in the LVF only (F2,126 = 3.191, p = 0.048).
The effect of electroconvulsive therapy and ketamine on the time course of the haemodynamic response in patients
Data were available for 11 patients at both baseline and mid-ECT (four of whom had received ketamine).
The ANOVA of HbO AUC values showed significant interactions for ECT × time (F2,18 = 8.240, p = 0.003) (Figure 12) and region × time (F6,54 = 3.111, p = 0.011), with a trend for an overall effect of time (F2,18 = 3.518, p = 0.051). There were no significant drug main effects or interactions. Post hoc ANOVA showed that all regions, apart from the RDF, had significant ECT × time interactions (i.e. the time course of the haemodynamic response was significantly different at mid-ECT compared with baseline). At mid-ECT the pattern was for lower AUC values in blocks 1 and 2 and higher values in block 3 than at baseline.
FIGURE 12.
Time course of (a) HbO, (b) HbR and (c) Hbdiff AUCs for the VF task in patients. Responses are averaged over all regions and are shown at baseline (pre ECT) and mid-ECT. Values are means ±SEM.
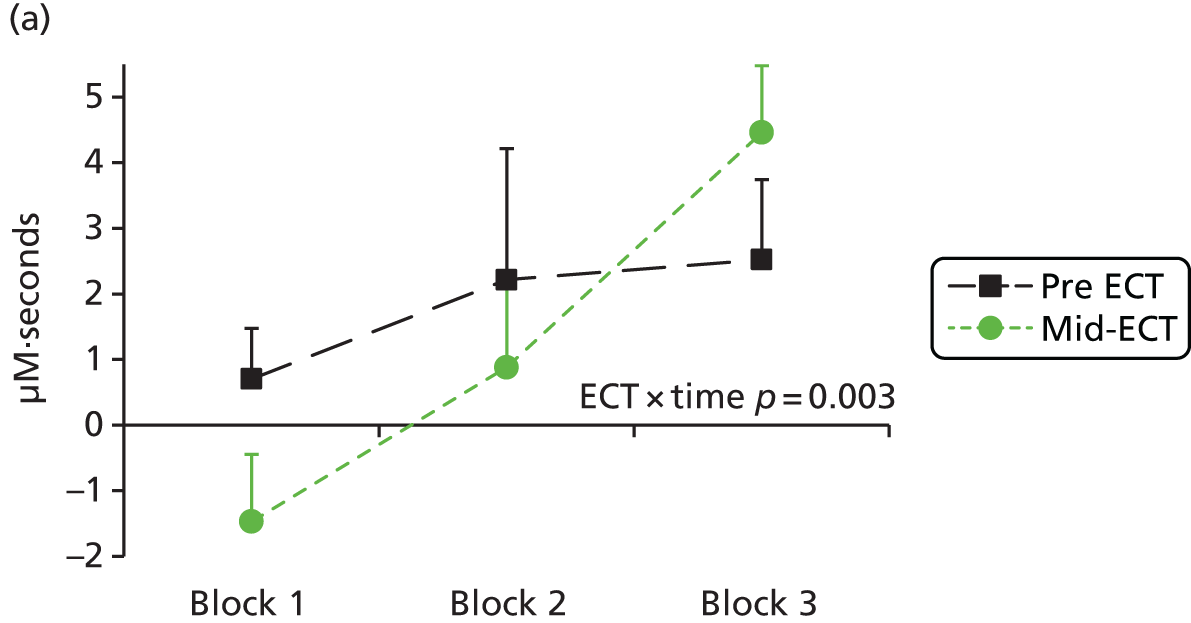
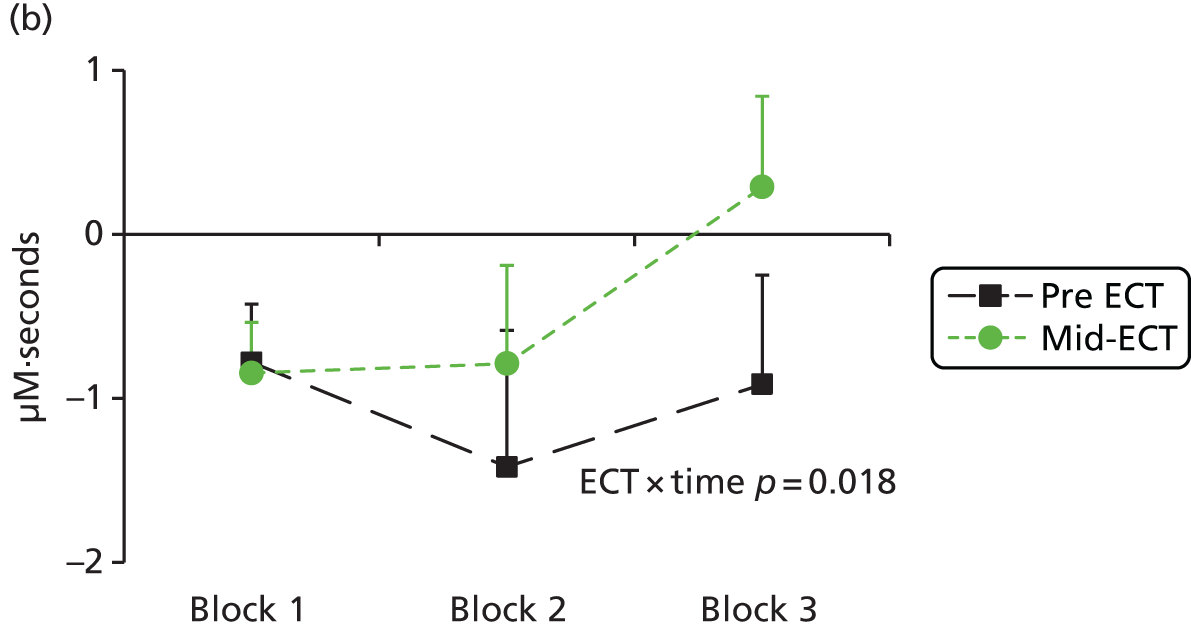
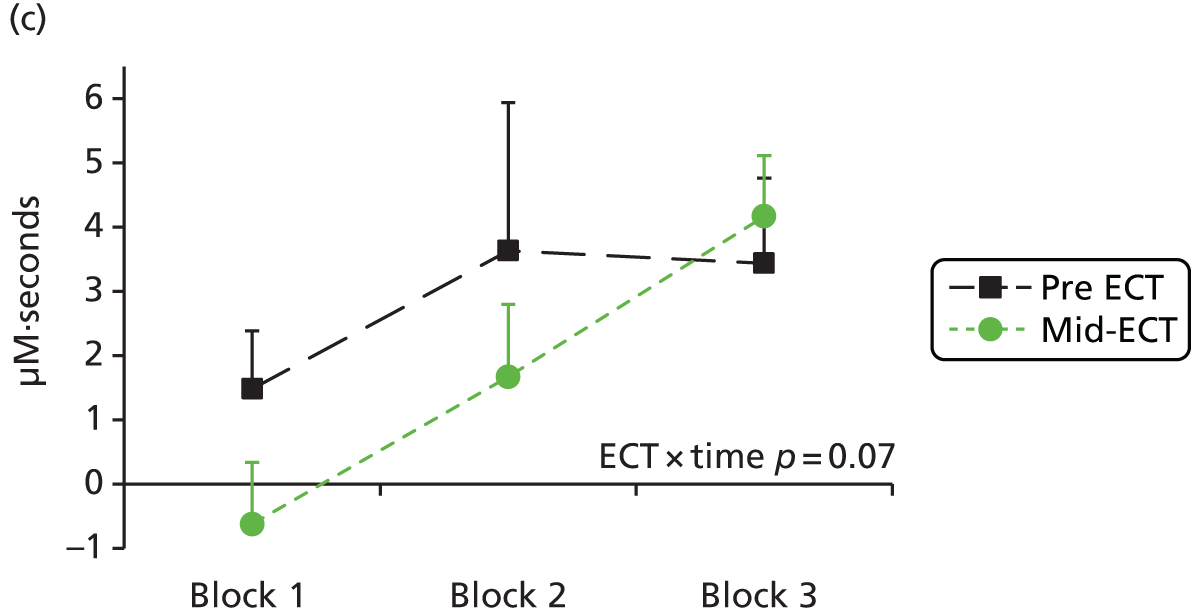
For HbR AUCs (see Figure 12) there was a significant interaction for ECT × time (F2,18 = 5.187, p = 0.018) and trend significance for ECT × drug (F1,9 = 3.612, p = 0.09) and ECT × drug × time (F2,18 = 3.069, p = 0.073). At mid-ECT there were smaller reductions in HbR than at baseline in blocks 2 and 3. Post hoc ANOVA showed a significant ECT × time interaction only in the LVF (F2,18 = 4.101, p = 0.036) and a significant ECT × drug × time effect in the RDF (F2,18 = 4.244, p = 0.031). The drug interactions were largely driven by baseline effects in patients receiving ketamine (Figure 13).
FIGURE 13.
Time course of HbR AUCs for the VF task in patients receiving (a) saline (n = 7) or (b) ketamine (n = 4). Responses are shown in the RDF cortex at baseline (pre ECT) and mid-ECT. ECT × drug × time interaction: F2,18 = 4.244, p = 0.031. Values are means ± SEM.

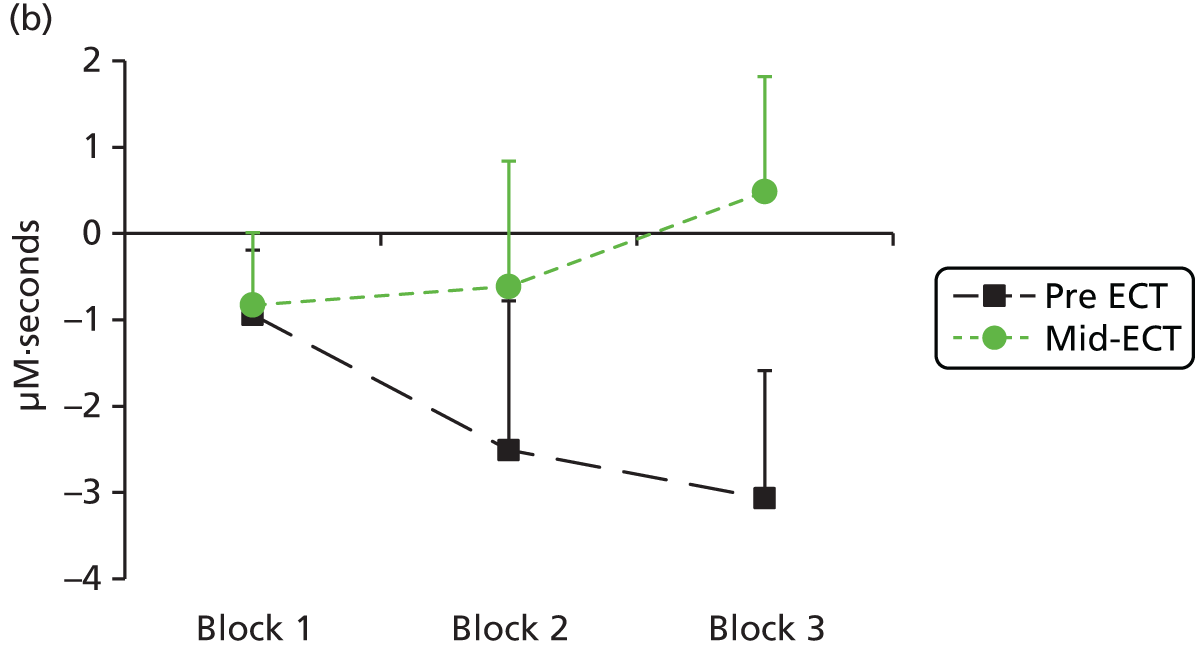
For Hbdiff AUCs there was a similar pattern to that seen with HbO (see Figure 12). The repeated measures ANOVA showed trends to effects of time (F2,18 = 3.287, p = 0.061), ECT × time interaction (F2,18 = 3.088, p = 0.07) and region × time interaction (F6,54 = 1.928, p = 0.096). There were no significant main or interaction effects of the drug. Exploratory ANOVA by region showed significant ECT × time interactions in the LDF (F2,20 = 3.854, p = 0.038) and RVF (F2,20 = 4.280, p = 0.034).
In summary, at mid-ECT, compared with baseline, there was an altered time course of the haemodynamic response, with blunting of the changes in HbO and HbR during the active task. No consistent effect of ketamine was found but the numbers in the two treatment arms were very small, meaning that no conclusions can be drawn.
N-back task
Behavioural data
Behavioural data from the n-back task were missing from one site for technical reasons and were available for only 20 HCs and 13 patients. Participants did not receive an n-back task as part of neuropsychological testing, but the digit span backwards provided an assessment of WM; its correlation with n-back accuracy was r = 0.39 (p = 0.025) in those receiving both. Excluding two outliers from the n-back data (one HC, one patient) increased the correlation to r = 0.57 (p = 0.001). As with the whole group, patients performed significantly less well on the tasks than HCs. The digit span backwards results are shown in Table 16; the fNIRS n-back accuracy was 87% (SD 19%) in HCs and 69% (SD 21%) in patients (p = 0.021).
Effect of the n-back task on the time course of the haemodynamic response in each group
Haemoglobin AUC data were available for 51 HCs and 18 patients.
In HCs some regions showed an increase in HbO AUC values over time, but the ANOVA was not significant (time: F2,100 = 1.199, p = 0.31) and no significant regional effects or interactions were seen (Table 20). The ANOVA in patients showed a trend towards an effect of time (F2,34 = 2.914, p = 0.068), reflecting decreases in mean AUC values in block 2 in all regions (Figure 14); no regions showed a significant effect of time on exploratory testing, although trends were seen in three regions (see Table 20).
| Region | Block, mean (SD) (µM·second) | Post hoc ANOVA for individual regions (effect of time) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| HCs | ||||
| LVF | 2.6 (3.3) | 0.5 (6.2) | 0.5 (4.4) | F2,100 = 0.163, p = 0.85 |
| LDF | 0.6 (3.1) | 0.8 (4.7) | 1.4 (4.2) | F2,100 = 1.403, p = 0.25 |
| RVF | 0.5 (3.1) | 1.0 (5.2) | 0.9 (3.8) | F2,100 = 0.660, p = 0.52 |
| RDF | 1.1 (3.4) | 1.4 (4.8) | 1.9 (4.4) | F2,100 = 1.342, p = 0.26 |
| Patients | ||||
| LVF | –1.4 (3.9) | –3.5 (8.4) | –0.4 (6.2) | F2,34 = 2.790, p = 0.077 |
| LDF | –1.0 (4.0) | –3.0 (6.8) | –0.6 (5.3) | F2,34 = 2.858, p = 0.071 |
| RVF | –0.7 (3.4) | –3.0 (8.3) | 0.4 (7.8) | F2,34 = 2.134, p = 0.13 |
| RDF | –0.2 (4.0) | –2.5 (6.9) | –0.3 (5.6) | F2,32 = 2.490, p = 0.098 |
FIGURE 14.
Time course of (a) HbO, (b) HbR and (c) Hbdiff AUCs for the n-back task in HCs and patients. Responses are averaged over all regions. Values are adjusted means ± SEM.



In HCs the ANOVA of HbR AUC values showed a highly significant effect of time (F2,100 = 10.151, p < 0.001) with no main effect of region or time × region interaction. Individual ANOVAs showed significant effects of time in all regions (Table 21) because of a dip in block 2 (see Figure 14). In patients there was also a significant effect of time (F2,34 = 5.946, p = 0.006) and significant effects of time in RVF and RDF considered individually (see Table 21).
| Region | Block, mean (SD) (µM·second) | Post hoc ANOVA for individual regions (effect of time)a | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| HCs | ||||
| LVF | –0.2 (1.4) | –1.5 (2.5) | –1.1 (2.3) | F2,100 = 10.093, p < 0.001 |
| LDF | –0.3 (1.2) | –0.9 (1.9) | –0.4 (2.3) | F2,100 = 3.521, p = 0.038 |
| RVF | –0.3 (2.2) | –1.6 (4.2) | –1.4 (6.2) | F2,100 = 4.153, p = 0.04 |
| RDF | –0.5 (3.0) | –1.7 (2.5) | –0.6 (3.0) | F2,100 = 7.159, p = 0.004 |
| Patients | ||||
| LVF | –0.4 (2.3) | –1.9 (4.2) | –1.4 (4.4) | F2,34 = 2.572, p = 0.105 |
| LDF | –0.4 (1.3) | –1.3 (2.5) | –0.4 (2.1) | F2,34 = 3.127, p = 0.057 |
| RVF | –0.6 (1.2) | –2.1 (2.5) | –0.9 (1.6) | F2,34 = 11.007, p < 0.001 |
| RDF | –0.7 (1.5) | –1.8 (3.0) | –0.7 (2.5) | F2,32 = 3.419, p = 0.044 |
For Hbdiff the repeated measures ANOVA in HCs showed a significant effect of time (F2,100 = 5.388, p = 0.006) because of a peak in block 2 seen in three of the four regions (Table 22), illustrated in Figure 14, with no significant time × region interaction. Exploratory ANOVAs by region found significant effects of time bilaterally in both ventrolateral prefrontal regions (see Table 22). In patients, although mean values dipped in most regions in block 2 and increased in block 3 (see Figure 14), the ANOVA showed no significant effects, although a trend to an effect of time was seen in the RDF in the ANOVA of individual regions (see Table 22).
| Region | Block, mean (SD) (µM·second) | Post hoc ANOVA for individual regions (effect of time)a | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| HCs | ||||
| LVF | 0.5 (3.5) | 1.9 (5.6) | 1.6 (3.5) | F2,100 = 3.918, p = 0.024 |
| LDF | 0.9 (3.2) | 1.7 (4.7) | 1.7 (2.9) | F2,100 = 1.482, p = 0.23 |
| RVF | 0.8 (3.9) | 2.6 (6.6) | 2.3 (6.4) | F2,100 = 4.529, p = 0.015 |
| RDF | 1.6 (3.6) | 3.1 (5.7) | 2.5 (4.9) | F2,100 = 2.877, p = 0.071 |
| Patients | ||||
| LVF | –1.0 (3.7) | –1.6 (8.9) | 1.0 (3.7) | F2,34 = 1.652, p = 0.21 |
| LDF | –0.7 (4.0) | –1.6 (6.6) | –0.2 (5.0) | F2,34 = 0.829, p = 0.45 |
| RVF | –0.1 (3.3) | –1.0 (7.9) | 1.3 (8.4) | F2,34 = 0.980, p = 0.38 |
| RDF | 0.5 (4.2) | –0.8 (6.4) | 0.5 (5.2) | F2,32 = 3.304, p = 0.057 |
Correlations between performance and mood and haemodynamic responses
No significant correlations were seen with the n-back behavioural data, but the number of participants with results was relatively low. For digit span backwards, in all participants taken together there were significant correlations with block 2 AUC haemodynamic responses in LDF (HbO r = 0.39, p = 0.001; Hbdiff r = 0.37, p = 0.002) and RDF (HbO r = 0.29, p = 0.018; Hbdiff r = 0.30, p = 0.011). In HCs the digit span backwards score correlated with block 2 haemodynamic responses in the LDF only (HbO r = 0.35, p = 0.012, Hbdiff r = 0.36, p = 0.011); there were no significant correlations in patients analysed alone. In HCs the MADRS score correlated negatively with the LVF block 2 HbO AUC (r = –0.29, p = 0.037) and the LDF block 2 HbO AUC (r = –0.31, p = 0.028); negative correlations of a similar magnitude were seen in patients but were not significant.
Group comparison of the time course of the haemodynamic response at baseline
Haemodynamic AUC data were available for 51 HCs and 18 patients.
The ANCOVA of HbO AUC data, covaried for age and sex, showed a significant effect of time (F2,130 = 6.213, p = 0.007), a trend to a significant effect of group (F1,65 = 3.304, p = 0.074) and a strong trend for a time × group interaction (F2,132 = 3.057, p = 0.050). In addition, there were significant effects of sex (F1,65 = 5.887, p = 0.018), time × age interaction (F2,130 = 3.270, p = 0.041) and time × sex interaction (F2,130 = 4.360, p = 0.015). The trend for a time × group interaction was due to a dip in block 2 in patients, whereas in HCs the values tended to rise over time (see Figure 14). Exploratory analysis by region showed a significant group × time effect in the RVF region (F2,130 = 3.959, p = 0.021) and a group difference in the RDF (F1,65 = 4.202, p = 0.044).
Deoxyhaemoglobin AUC values dipped in block 2 in both groups (see Figure 14) and there was a significant effect of time in the ANCOVA (F2,130 = 6.274, p = 0.004), with no other significant main effects or interactions, although trends were seen for both time × age (F2,130 = 3.226, p = 0.050) and time × sex (F2,130 = 2.513, p = 0.093) interactions. Exploratory analysis by region showed no group × time interactions.
For Hbdiff AUC values, although mean AUC values were lower in patients than in HCs, and increased in block 2 in HCs but decreased in patients, the repeated measures ANCOVA showed no significant effect of group (F1,65 = 2.714, p = 0.104) or group × time interaction (F2,130 = 2.034, p = 0.135) (see Figure 14). There were significant effects of time (F2,130 = 6.208, p = 0.003) and region (F3,195 = 3.272, p = 0.022) and also significant time × age (F2,130 = 5.789, p = 0.004) and region × age (F3,195 = 2.944, p = 0.034) interactions. Exploratory repeated measures ANOVA in individual regions showed a trend to a group effect in the LDF (F1,65 = 3.411, p = 0.069) and trends to both a group effect (F1,65 = 3.627, p = 0.061) and a group × time interaction (F2,130 = 2.436, p = 0.094) in the RDF.
The effect of electroconvulsive therapy and ketamine on the time course of the haemodynamic response in patients
Data were available from 12 patients at both baseline and mid-ECT (five of whom had received ketamine).
For HbO data the ANOVA showed a significant effect of time (F2,20 = 3.879, p = 0.039) and a drug × ECT × region interaction (F3,30 = 3.117, p = 0.041), with a trend to a drug × region effect (F3,30 = 2.485, p = 0.08). No other main effects or interactions were significant. The effect of time was due to a decrease in HbO in block 2 at both time points (Figure 15) and the drug × ECT × region interaction was driven by baseline differences in HbO between the two groups of patients, which were not present at mid-ECT (data not shown).
FIGURE 15.
Time course of (a) HbO, (b) HbR and (c) Hbdiff AUCs for the n-back task in patients. Responses are averaged over all regions and are shown at baseline (pre ECT) and mid-ECT. Values are means ± SEM.

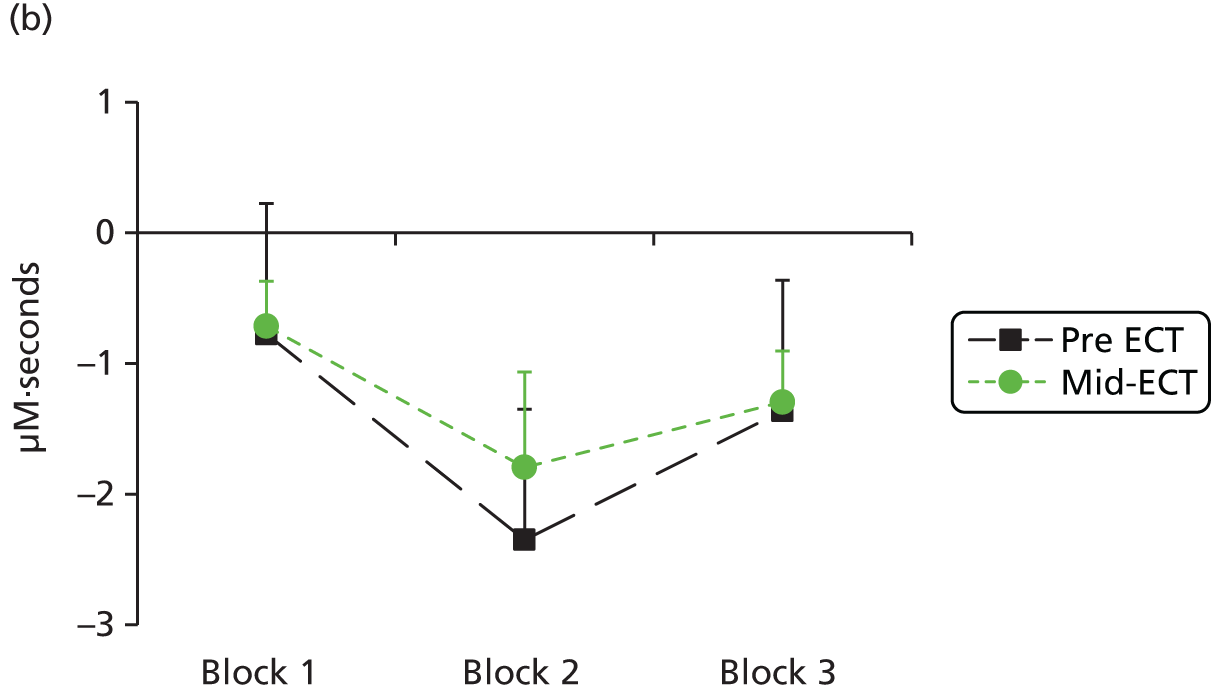

The analysis of HbR AUC values showed a significant effect of time (F2,20 = 4.719, p = 0.021) and a trend to a region × drug interaction (F3,30 = 4.607, p = 0.08) but no other significant main effects or interactions.
The ANOVA for Hbdiff AUC data showed a significant ECT × time interaction (F2,20 = 4.898, p = 0.019) with no other main effects or interactions. The time course for the mean AUC at baseline and at mid-ECT is illustrated in Figure 15. Post hoc analysis by region showed significant ECT × time interactions in the LDF (F2,20 = 3.703, p = 0.043) and RVF (F2,20 = 6.373, p = 0.007) with trends seen in the other two regions (p < 0.09).
In summary, at mid-ECT, compared with baseline, a significant altered time course was seen only for Hbdiff during the active task, which may be a measure of oxygenation; no consistent effect of ketamine was found but the numbers in the two treatment arms were very small, meaning that no conclusions can be drawn.
Haemodynamic responses and clinical improvement
Unfortunately, only 14 patients with fNIRS data (and only nine with baseline and mid-ECT data) had end-of-treatment clinical outcomes and so it was not possible to look at predictors of outcome with any confidence. When comparing patients who responded or remitted with those who did not, block 2 HbO and Hbdiff haemodynamic responses at baseline had a pattern of being higher, with greater decreases in haemodynamic responses at mid-ECT, but these were not significant. Defining responders as those rated as much or very much improved on the CGI-I, which provided the most even split in terms of patient numbers, an exploratory ANOVA of block 2 HbO in the VF task showed trends for significant ECT × responder status (F1,7 = 3.867, p = 0.09) and ECT × region × responder status (F3,21 = 2.897, p = 0.059) interactions. In the regions analysed separately there was a significant ECT × responder status interaction in the RDF (F1,7 = 9.366, p = 0.018) (Figure 16). Responders had higher HbO responses at baseline, which decreased at mid-ECT, whereas non-responders lacked HbO responses at baseline, which then tended to increase at mid-ECT.
FIGURE 16.
Haemodynamic response at baseline and mid-ECT during the VF task in responders and non-responders to ECT. Block 2 HbO AUC averaged over all regions (a) and in the RDF (b). Values are means ± SEM.
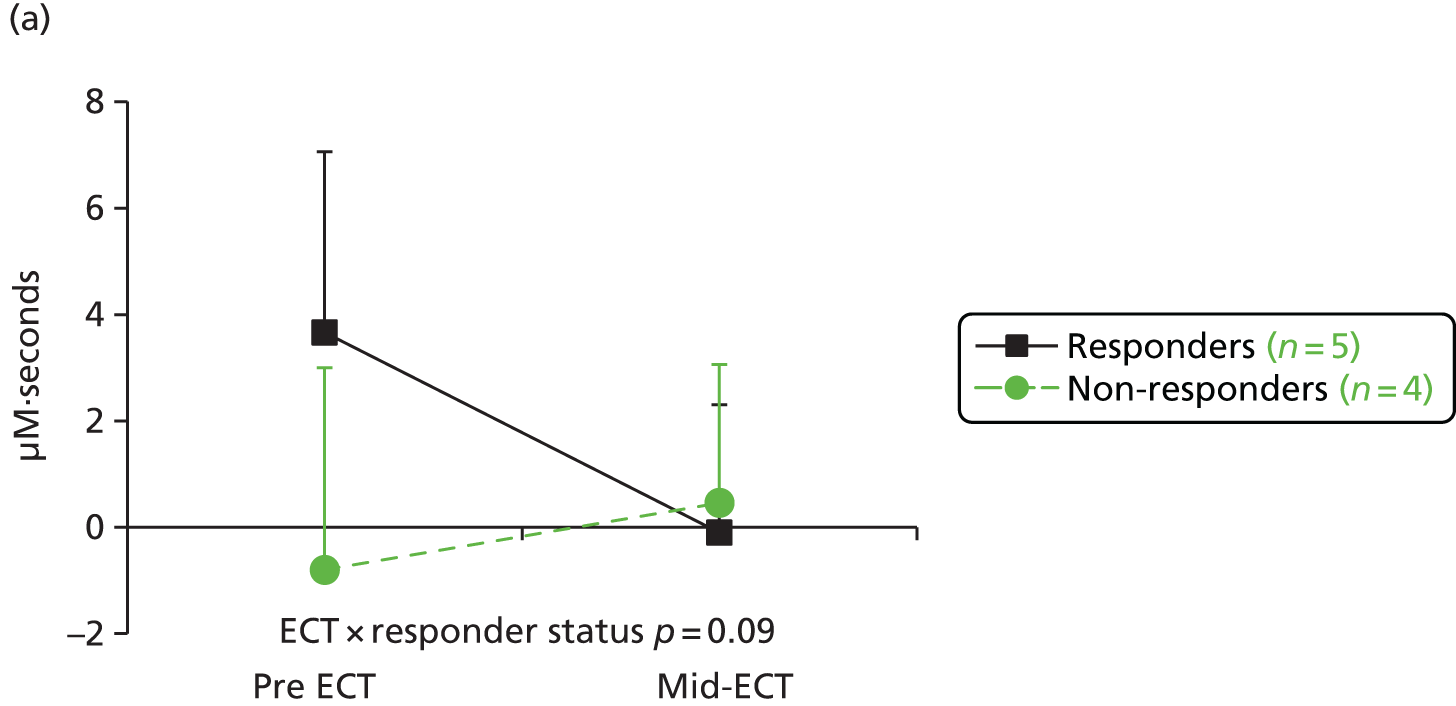
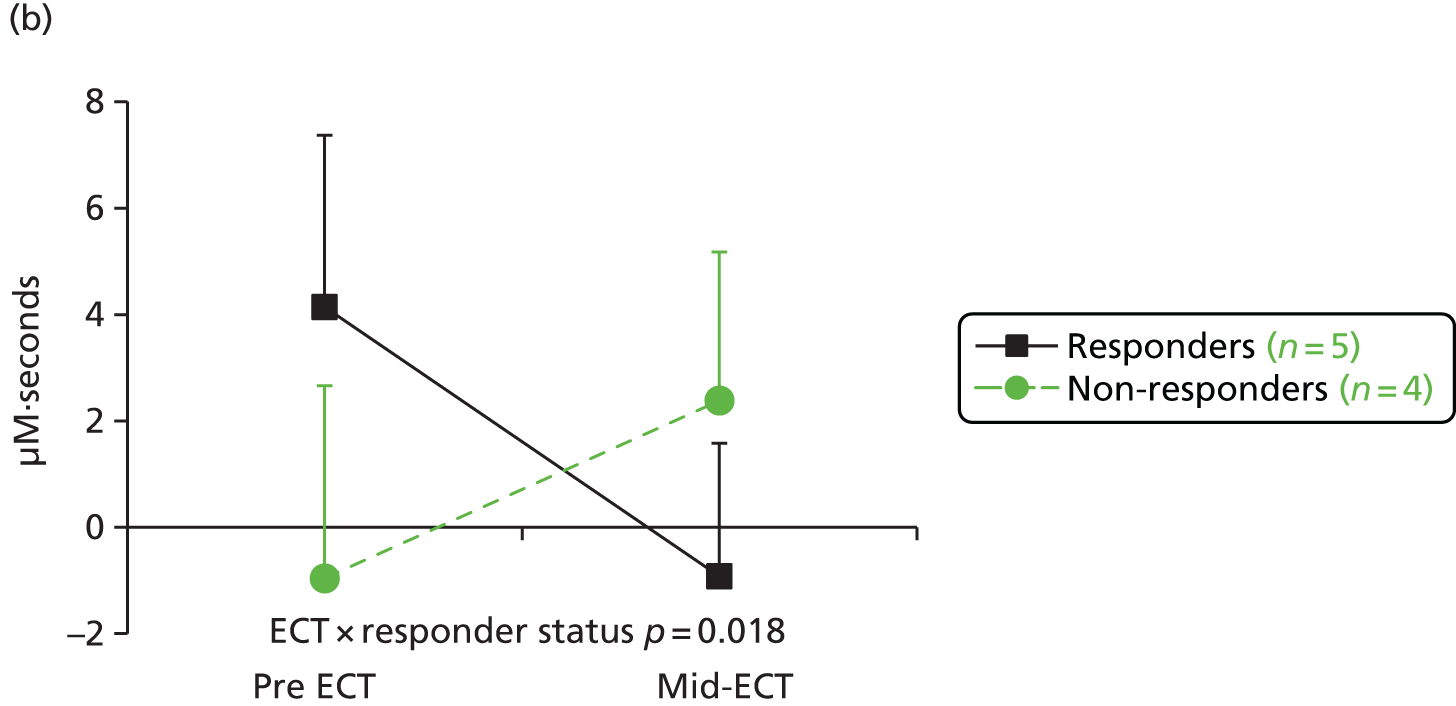
Safety
Two patients (both randomised to ketamine) were excluded from the study and further analysis after they were both found to have pre-existing excluding diagnoses after starting the study [both were originally recorded as severe adverse events (SAEs) in order to document and track the events]. One patient was discovered to have an advanced meningioma following headaches and vomiting (accounting for three additional AEs) and one patient, with a baseline MMSE score of 24, had persistent confusion and was re-diagnosed as suffering from dementia. These six AEs/SAEs, judged by the principal investigators as being related to the pre-existing conditions, are excluded from the following further description of AEs.
The independent members of the DMEC monitored the numbers of AEs, ARs and SAEs according to the two randomised arms (without the blind being broken); the research team managing the study were not aware of the breakdown by arm. The DMEC noted that there were imbalances in the events by arm but, given the small numbers and the nature of the events, did not consider that there was cause for concern and did not ask for the study blind to be broken. Two SAEs (persistent confusion in the re-diagnosed excluded patient and spontaneous seizures) were referred to the DMEC for discussion and advice and the decision that these were not related to study medication was supported.
Serious adverse events
Seven patients each experienced a single SAE. These are shown by randomisation arm in Table 23. No SAE was deemed to be related to the trial medication by the principal investigator in the treating centre and, although one patient sustained a fractured scapula, all resolved without permanent sequelae. No severe ARs or suspected unexpected serious ARs were reported.
| SAE | Allocation |
|---|---|
| Spontaneous seizure and status epilepticus between ECT treatments | Saline |
| Overdose resulting in hospital admission | Saline |
| Suicide attempt requiring general hospital admission | Saline |
| Suicide attempt on inpatient ward requiring intervention | Saline |
| Chest pain requiring admission to hospital overnight | Saline |
| Clinical deterioration with suicidal ideation requiring admission to hospital | Ketamine |
| Overdose requiring treatment in accident and emergency department | Ketamine |
Adverse events and adverse reactions
Including the SAEs, 35 events occurred in 25 of the 70 patients included in the study, all following the start of ECT treatment. Tables 24 and 25 show these events by treatment arm; there were more events in the ketamine arm than in the saline arm and more patients receiving ketamine than saline had at least one event, but this was not statistically significant (Fisher–Freeman–Halton exact test p = 0.33). Two patients who received ketamine experienced ARs after waking from ECT. One had a single occasion of altered sensory experiences of people’s faces that lasted until the next day, which did not recur with subsequent treatments. The other patient appeared distressed and inaccessible after the first ECT, but subsequently described it as a ‘pleasant alternative reality’, and then after the third ECT had a nightmare during recovery, with further nightmares on the two subsequent nights. The ECT course was continued without further incident.
| Type of event | Treatment arm, n | Total (n = 70) | |
|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | ||
| AE | 8 (4 mild, 4 moderate) | 16 (15 mild, 1 moderate) | 24 (19 mild, 5 moderate) |
| AR | 0 | 4 (3 mild, 1 moderate) | 4 (3 mild, 1 moderate) |
| SAE | 5 (3 moderate, 2 severe) | 2 (1 moderate, 1 severe) | 7 (4 moderate, 3 severe) |
| Total | 13 | 22 | 35 |
| Number of events | Treatment arm, n (%) | Total (n = 70) | |
|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | ||
| 1 | 7 (19)a | 9 (27)a,b | 15 (21) |
| 2 | 3 (8)a | 5 (15)b | 8 (11) |
| 3 | 0 (0) | 1 (3) | 1 (1) |
| Any number | 10 (27) | 15 (45) | 24 (34) |
The body system classification of the events [System Organ Class in the Medical Dictionary for Regulatory Activities (MedDRA); see www.meddra.org/ (accessed 29 August 2016)] is shown in Table 26. The most common AEs were psychiatric disorders followed by nervous system disorders. There were 10 psychiatric AEs/SAEs (i.e. not including the psychiatric ARs), of which seven (five on saline) involved self-harm or increased suicidal ideation. These were deemed likely to be related to the underlying clinical illness, although a triggering effect of ECT cannot be excluded. 155 Of interest, given that more of these events occurred on saline, there is preliminary evidence that ketamine may be an effective treatment in reducing suicidality,156 but analysis by treatment arm of the suicide items in the MADRS and QIDS-SR did not show any benefit of ketamine (data not shown). Of the nervous system disorder events, three were migraine-type headaches occurring in two patients on ketamine and one was a SAE involving spontaneous seizures; this may be a rare adverse effect of ECT,157 but concomitant treatment of the patient with quetiapine is also likely to be a cause. 158
| System Organ Class | Number of events | ||
|---|---|---|---|
| Treatment arm | Total (n = 70) | ||
| Saline (n = 37) | Ketamine (n = 33) | ||
| Blood and lymphatic system disorders | 1 | 1 | |
| Cardiac disorders | 1 | 1 | |
| Eye disorders | 1 | 1 | |
| Gastrointestinal disorders | 1 | 1 | |
| Hepatobiliary disorders | 1 | 1 | |
| Infections and infestations | 3 | 3 | |
| Musculoskeletal and connective tissue disorders | 3 | 3 | |
| Nervous system disorders | 1 | 4 | 5 |
| Psychiatric disorders | 7 | 6a | 13 |
| Renal and urinary disorders | 1 | 1 | |
| Respiratory thoracic and mediastinal disorders | 1 | 1 | 2 |
| Skin and subcutaneous tissue disorders | 2 | 2 | |
| Vascular disorders | 1 | 1 | |
| Total | 13 | 22 | 35 |
Other safety measures
There was no evidence of a difference between the ketamine arm and the saline arm with regard to confusion following ECT treatment or in the occurrence of manic or psychotic symptoms between treatments. As reported in Table 11, patients in the ketamine arm did not as a group have delayed reorientation assessed 30 minutes after ECT compared with saline-treated patients. BPRS mania and psychosis item scores did not differ between treatment arms (see Appendix 2). A potential concern raised following a change in the Ketalar summary of product characteristics was whether or not repeated administration of ketamine could lead to liver damage. 159 Liver disease was an exclusion criterion for recruitment to the study from the start, but this was widened to also specify elevated liver enzymes; the research team retrospectively collected the results of liver function tests carried out in trial patients by clinical teams. The data were reviewed by the independent members of the DMEC, who found no concerning pattern of increased liver enzymes in trial patients; one patient was noted to have a temporary increase in liver transaminases during ECT, which had resolved when tested 3 months later. After breaking the blind it was found that this patient had received ketamine, but it is not possible to draw conclusions from an isolated occurrence.
Chapter 4 Discussion
Summary of findings
The key outcome from this trial was that there was no benefit from the use of adjunctive ketamine at a dose of 0.5 mg/kg for the outcomes seen in patients being treated with ECT for their depression. The primary research question had been whether ketamine would improve cognitive function compared with saline and there was no indication that this was the case for the primary outcome or any of the secondary outcomes. In fact, there was a slight numerical advantage to the saline arm. A similar picture was seen with the efficacy outcomes, with no evidence for either better efficacy, or a more rapid onset of improvement, with ketamine.
In terms of potential harms of ketamine, two patients had transient psychological effects following isolated ECT treatments that were probably related to ketamine, but there was no evidence of serious tolerability or safety problems with ketamine given at this dose and in this way.
In comparison with HCs, the depressed patients were broadly, and highly significantly, impaired in neurocognitive function before receiving ECT, which is in agreement with other findings in the literature and consistent with their severity of depression. This emphasises the importance of any potential negative effect of ECT on already compromised cognitive function. Remitted patients, 4 months after their last ECT, had fully recovered in terms of verbal learning and recall, but remained impaired on other tests, although performance was better on most tests than at baseline.
The mechanistic fNIRS study, in spite of limitations because of low patient numbers, replicated the findings in the literature of impaired haemodynamic responses in the prefrontal cortex to a VF task in depressed patients; the data were less consistent on whether this also occurred with a WM task. There was provisional evidence for an inverse relationship between depression severity and the haemodynamic responses to the VF task in the LVF cortex in patients. ECT further reduced prefrontal cortex haemodynamic responses to the VF task in the absence of a behavioural effect on the corresponding neuropsychological task. Finally, very preliminary data suggest that prefrontal cortex haemodynamic responses to the VF task might be related to the outcome of treatment.
Clinical trial outcomes
The rationale for the clinical trial was to ameliorate the well-documented acute cognitive adverse effects of ECT. 17 In this study significant deterioration in cognitive function during the ECT course was seen only for the AMI-SR and the MCGCFT and not for the primary neuropsychological outcome. This contrasts with more striking verbal memory and fluency deficits seen in other similar studies using the same or equivalent measures;27,129 the lack of effect of ECT on digit span is, however, consistent with others’ findings. 129 This raises the question of whether or not a lack of effect of ECT on neuropsychological function could explain the lack of effect of ketamine.
The reason for the discrepancy with the literature with regard to the effect of ECT on verbal memory and fluency measures is not clear, but it should be noted that the standard pulse width used by the sites in our study was 0.5 milliseconds rather than 1–1.5 milliseconds, which is the ‘brief pulse’ width seen in many other studies. 28,129 We therefore gave ECT closer to the so-called ‘ultra-brief’ pulse width of 0.3 milliseconds, which, it has been claimed, has significantly fewer cognitive effects than 1- to 1.5-milliseconds stimuli. 28,129 However, a study using a 0.5-milliseconds pulse width has shown impairment in delayed verbal recall after three ECT treatments. 120 Memory deficits have been shown to improve rapidly following the immediate post-ECT period17 and, therefore, another potential reason for an apparent lack of effect on cognition might be delay between ECT and neuropsychological assessment. However, the mean gap for the mid-ECT assessment was only 2 days (see Table 5) and we did show significant impairment on the MCGCFT, which has been shown to rapidly recover in the 4–15 days after ECT. 17 It should also be noted that the patients receiving ECT started from a low baseline in their cognitive function compared with HCs (see Table 14) and their performance did not improve in line with clinical improvement during ECT, but did subsequently improve during follow-up on most tasks (without a major further change in depressive symptoms). It is arguable that ECT suppressed the expected improvement in verbal memory that one might expect with clinical improvement and this improvement was seen only after ECT was stopped. Although we cannot exclude that a lack of effect of ECT on verbal memory prevented the detection of any action of ketamine, it should be noted that no benefit from ketamine was found in a study in which HVLT-R delayed recall was impaired by ECT51 and that ketamine also showed no benefit in the current study for those neuropsychological measures that were impaired during ECT treatment.
There was little evidence that ECT, as practised by the NHS trusts in the study, caused permanent cognitive deficits compared with baseline on objective or subjective measures measured 4 months after ECT. A confounding issue in assessing cognitive function after ECT has been the contribution of persisting mood symptoms. Fully remitted patients, when asked 4 months after ECT, rated their present memory as comparable to that of HCs, with a majority saying that ECT either had not affected or had improved their memory, and the quantitative self-assessment of effect of ECT on memory was not significantly different from ‘no change’ [3.7 (SD 1.4) vs. 4 (the score for no change); p = 0.30] (see Table 15). On objective testing, comparing fully remitted and non-remitted patients 4 months after ECT, there was a mixed picture. Some deficits appeared to be clearly related to persisting depression, namely list learning and verbal memory and to some degree VF. In contrast, with tasks involving attention, WM and executive and visuospatial function, there was no difference between remitted and symptomatic patients, with both performing significantly less well than HCs. This is consistent with findings from other studies that have found impaired cognition in patients remitted from depression, particularly in executive function and attention (see the review by Woo et al. 160). Of interest, given the problematic nature of the assessment of autobiographical memory, the AMI-SF improved significantly following the end of ECT, suggesting that memories might return even if recall is impaired immediately after ECT, but again this did not depend on symptomatic state. As has been noted in Chapter 2, the AMI-SF has an inherent property, or some would say flaw, of decay over time, with healthy subjects showing a 20% drop in scores over 6 months. 134 At the end of treatment we found a little over a 20% decrease in AMI-SF score compared with baseline, which recovered to a 16% decrement at follow-up 2 (5–6 months after baseline). Therefore, we also have no objective evidence in this study of permanent autobiographical memory impairment after ECT. This does not, of course, exclude the loss of memories that were not evaluated in the objective tests, such as the memory ‘holes’ that have been described, or retrograde amnesia for impersonal memories, which may be more selectively impaired. 161 However, problems of this nature were not volunteered in our follow-up survey (see Chapter 5), although the sample was small.
Overall, the current study adds substantially to the current literature on the effect of adjunctive ketamine on cognitive outcomes with ECT. As discussed in Chapter 1 the results to date have been patchy and inconclusive (see also McGirr et al. 52). Pooling the results of this study together with those of Loo et al. ,51 which used ultra-brief RUL ECT and the same outcome measures, there is currently no evidence for a cognitive benefit of low-dose ketamine augmentation of ECT; the 95% CIs exclude more than a small-to-moderate advantage of ketamine for most measures (Table 27).
| Outcomes | Standardised ES for change scores (95% CI)a |
|---|---|
| Neuropsychological outcomes | |
| Mid-ECTb | |
| HVLT-R delayed recall | –0.17 (–0.54 to 0.21) |
| COWAT category fluency | 0.02 (–0.35 to 0.39) |
| AMI-SF | –0.04 (–0.41 to 0.34) |
| MCGCFT delayed recall | –0.0 (–0.38 to 0.37) |
| End of treatment | |
| HVLT-R delayed recall | –0.06 (–0.44 to 0.33) |
| COWAT category fluency | –0.08 (–0.46 to 0.31) |
| AMI-SF | –0.06 (–0.44 to 0.33) |
| MCGCFT delayed recall | 0.25 (–0.14 to 0.64) |
| Efficacy outcomes | |
| MADRS change at week 1 | –0.01 (–0.38 to 0.37) |
| MADRS change at end of treatment | 0.05 (–0.34 to 0.43) |
| Risk ratio (95% CI)a | |
| Response (≥ 50% decrease in MADRS) | 0.80 (0.58 to 1.11) |
| Remission (MADRS ≤ 10) | 1.15 (0.72 to 1.85) |
Patients improved substantially with ECT in the study, with 54% classified as responders and 37% classified as remitters at the end of treatment (a mean of 6 weeks after the first ECT session). This compares with a < 15% remission rate in the STAR*D study for subsequent antidepressant treatment in patients who had experienced two or more prospective treatment failures. 4 A recent UK RCT in patients with a similar degree of treatment resistance as in the patients in this study (mean MGHS score of 4.7), although with a lesser severity of depression (baseline mean MADRS score of 28), reported a 21% response rate and a 16% remission rate after 5 weeks in patients treated with placebo or metyrapone augmentation of ongoing antidepressant treatment. 162 This suggests that patients in this study received considerable clinical benefit attributable to ECT treatment. However, the degree of improvement was less than is often reported with ECT; for example, Kellner et al. 27 found a 65% remission rate with bilateral ECT, and a meta-analysis of seven cohort studies8 found a 65% remission rate for patients without previous pharmacotherapy failure and a 48% remission rate for those with treatment resistance. The mean MADRS score in this study at the end of treatment was 16, which is higher than the mean score of 13 after ECT found in patients with capacity in Scotland in 2014,9 who had a similar pretreatment MADRS score (35) to our patients. However, it is notable that the main reason (in 65% of cases) given by clinical teams for patients stopping ECT treatment was their having responded to treatment (see Table 12). The decision to end treatment was taken clinically and anecdotally it appeared that for many patients this was before full remission, in spite of NICE guidance to treat to full remission;5 we cannot therefore exclude the possibility that remission rates were not as high as potentially could have been achieved if patients had received further ECT treatments. This is consistent with the further improvement seen in some patients after ECT was stopped, so that by the end of follow-up 46% of patients had reached remission.
The lack of effect of adjunctive ketamine on efficacy is consistent with a meta-analysis of five small studies with differing methodology52 that showed no pooled benefit. Pooling this study with the largest other study, which also used a ketamine dose of 0.5 mg/kg and was the only one reporting a full course of ECT,51 there is no significant difference between ketamine and placebo in rates of response or remission or in continuous measures of depression either after 1 week or at the end of treatment (see Table 27).
The saline- and ketamine-treated groups differed slightly in baseline characteristics (see Tables 3 and 4), with the ketamine arm tending to have more features that might be associated with a poorer response to ECT, such as a lesser severity of depression, a lower rate of psychosis and a larger number of previously failed treatments. 8,9,11 However, most of these were adjusted for in the preplanned analysis and do not appear to account for the lack of benefit of ketamine.
In contrast to the meta-analysis by McGirr et al. 52 we did not find a significant increase in AEs or confusion, although 6% (n = 2/33) of ketamine patients experienced transient psychological reactions that were probably attributable to ketamine. Other studies found that ketamine alone, or at higher doses than used in this study, may be associated with slower reorientation and more AEs48,49,163 (although this appears to be attenuated when ketamine is used with propofol163,164). These effects have not been noted with the lower 0.5-mg/kg adjunctive dose51 as used in this study.
The dose of ketamine used in this study was derived from studies in which ketamine infusions have been shown to be effective in the rapid treatment of depression165 and, therefore, it is possible that a different dose of ketamine may have shown benefit in terms of cognitive, or possibly efficacy, outcomes. However, as discussed in the previous paragraph, it is likely that higher doses would be associated with a less favourable AE profile (see also McGirr et al. 52) and the data from studies using higher doses of ketamine with ECT,52 or as a pharmacological treatment,165 do not suggest an efficacy benefit.
Therefore, the conclusion from our study is that there appears to be no benefit from the adjunctive use of low-dose ketamine with ECT, although there is also no substantial harm.
Limitations
The limitations of our study need to be acknowledged. The most important one is the smaller than planned sample size. This means that we could detect an ES of only about 0.7 (with 80% power and p = 0.05) for the primary outcome. Our lack of power means that we cannot exclude either moderate benefit or moderate harm of treatment with ketamine. Nevertheless, the best estimate of effect did not numerically favour ketamine and this lack of effect is also consistent with other recent evidence. Although we did not see a differential dropout rate between treatment arms, 16% of patients had dropped out by the end of treatment, about one-third by the 1-month follow-up and almost half by 4 months’ follow-up. We therefore have limited ability to say what happened to patients after the end of treatment. The dropout rate was contributed to by the severity of illness in the population. The clinical realities of treatment also made strictly timed assessments difficult, but there were no systematic differences seen in assessment timing between the treatment arms. It is clear from examining the nature of the included patients that they are not fully representative of the population of patients receiving ECT as a whole; in particular, they were younger, less cognitively compromised and less severely ill. Although we have no reason to believe that the results would be different in the rest of the ECT population, the adverse effects of ECT are probably higher in patients with less cognitive reserve166,167 and we cannot assume that our results would generalise to them. Studies in such patients would, however, present considerable ethical difficulties.
Near-infrared spectroscopy study
Imaging severely ill psychiatric patients is difficult and the advantage of NIRS is that it is portable and can be taken to hospital wards or to patients’ homes. As the main study already included neuropsychological testing, a number of patients were reluctant to take part in this additional component. However, the experience of NIRS itself was generally positive and there were few problems encountered in patients who did consent, supporting its feasibility in this patient population.
As discussed in Chapter 2, NIRS is a more restricted imaging technology than MRI, in particular in the parts of the brain that are accessible for study, which are limited to superficial layers of the cortex in adults. In addition, there is still a lack of full understanding of exactly what the derived measures mean in terms of underlying brain function, the contribution of blood flow in larger veins and arteries and the exact localisation of the cortical areas being imaged (which is not possible to determine without a parallel MRI structural scan). There are technical difficulties in obtaining good signals from all channels in adults, especially when optodes are over hair, and therefore in ensuring good optode–skin contact. Further data cleaning and analysis methods are still relatively undeveloped compared with those in MRI and the preliminary analysis here is based on initial cleaning of the data. It is for these reasons that we decided to take a regional approach to analysis, collapsing the signal from a number of channels, rather than investigating individual channels. As shown in Figure 3 the array interrogated a relatively restricted area of the lateral prefrontal cortex, which on average is likely to be similar in different individuals, even if specific channels vary in position (as a result of anatomical, or array placement, variation). It also meant that we had results for nearly all regions, even though data from individual channels might be missing. An assumption in the analysis is that the missing data did not systematically vary between groups being examined and, indeed, we found no significant difference in the proportion of missing channels in the patient group compared with HCs (e.g. for the VF task it was 8.4% vs. 7.3%; p = 0.49).
The VF task resulted in haemodynamic responses in all four prefrontal regions in HCs and we did not find significant lateralisation as might initially be expected with a VF task activating Broca’s area (BA 44 and BA 45). However, other studies using fNIRS have also shown bilateral activation in VF tasks, with a variable degree of left-lateralisation. 89,91,93,153 We found similar, but weaker, bilateral effects with the n-back task in HCs, with the HbR and Hbdiff measures providing the most consistent changes. There is less extensive literature on using WM tasks with fNIRS, but, again, bilateral activation has been found. 92,113 Consistent with extensive literature,94–109,112–114 we found significantly decreased, and possibly delayed, prefrontal haemodynamic responses in depressed patients in the VF task and, less certainly, in the n-back task. For the VF task we found a significant negative correlation between MADRS score and the haemodynamic response in the LVF cortex in patients. The degree to which these reduced responses in patients are simply a reflection of impaired performance or effort remains unclear, but patients certainly performed significantly less well than HCs on the non-fNIRS VF and WM tasks (see Table 15).
The novel finding in this study is the further reduction in, and possible delaying of, prefrontal haemodynamic responses to the VF task after ECT treatment, in spite of unchanged behavioural performance (as measured by the COWAT). These results are purely observational but we believe that they are unlikely to be accounted for by order effects, as other studies have found reproducible haemodynamic responses with repeated administration of VF tasks. 89,93 In addition, they contrast with the lack of change that has been reported acutely with antidepressant treatment. 99 For the n-back task, a reduction in effect was seen only with the Hbdiff measure, a possible index of oxygenation. 150 The numbers of patients randomised to saline and ketamine were insufficient to statistically test whether or not ketamine modified this suppression by ECT; such interactions as were seen with drug condition were due to baseline differences.
These results, although provisional, are broadly consistent with the proposed hypothesis that ECT further suppresses a dorsal network involved in cognitive function that is already reduced in depressed patients compared with HCs. As we were unable to carry out MRI and MRS investigations as originally proposed, we could not further test hypotheses that this might be linked to altered glutamate function and reflect changes in connectivity between the dorsal and the ventral networks, especially between the hippocampus and the dorsolateral prefrontal cortex.
The haemodynamic responses seen in patients with the n-back task were unusual, with a pattern of apparent deactivation to the task seen. A similar pattern has been reported using a WM task in mild cognitive impairment, in which frontal and temporal, but not parietal, areas were deactivated in response to the task (in contrast to the activations seen with HCs) and were associated with poorer performance on the task than in HCs. 168 This suggests a possible parallel between mild cognitive impairment and the temporary cognitive impairment seen with ECT; the authors suggested that deactivation of frontotemporal areas of the network involved in WM may be associated with compensatory effects elsewhere.
Finally, we found that responders, compared with non-responders, showed greater suppression of the HbO response to the VF task and, moreover, tended to have numerically higher baseline haemodynamic responses than non-responders. Although these results are extremely preliminary, they are consistent with the finding that relatively maintained VF and response inhibition is associated with better clinical response to treatment,169 and with the findings of a recent fMRI study in which greater dorsolateral prefrontal cortex responses to a go/no go task predicted better response to antidepressants. 170 This suggests that it is relatively preserved prefrontal function, and its capacity for dynamic change, that might contribute to the ability to respond to treatment, including ECT treatment; however, this needs to be investigated prospectively in a suitably powered study using fNIRS.
Implications for clinical practice
The main clinical implication is a lack of evidence for the benefit of adjunctive ketamine at a dose of 0.5 mg/kg given with propofol for standard ECT as provided in the UK. We were not able to demonstrate any clinical benefit (or harm) from this regimen, although our study was underpowered to be able to conclude that ketamine has no effect or risk. However, when our results are taken in conjunction with those from the study by Loo et al. ,51 which used the same outcome measures, the 95% CIs of the pooled results suggest that if an effect exists it is likely to be in the small-to-moderate range.
Our study is helpful in quantifying the cognitive deficit pretreatment experienced by depressed patients undergoing ECT, although this is likely to be an underestimate as the most severe patients were excluded, and highlights that not only the effects of ECT but also the effects of depression itself need to be considered. Our results show some cognitive benefit for patients of achieving full remission compared with those not remitted, as 4 months after ECT remitted patients were performing close to the performance of HCs on verbal memory, although they still had deficits in VF and WM. This adds weight to the need to aim for symptomatic remission when using ECT to treat depression, something that did not occur in a substantial proportion of patients in our study; ECT was recorded as having been stopped because of the patient having responded to treatment in nearly two-thirds of cases, but < 40% had in fact achieved remission.
Although the effects of ECT itself were only observational in this study, we were not able to show any major lasting impact of ECT on cognition, but did demonstrate a large symptomatic benefit for a substantial proportion of patients. This provides some reassurance about the benefits compared with the risks of ECT for patients requiring this treatment. In carrying out this study we were struck by the considerable practical and organisational barriers faced by patients for whom ECT may be a treatment option following poor response to other therapies. An implication for mental health organisations is the need to assess whether or not their treatment pathways, particularly for outpatients, facilitate equitable access to ECT for those who might require it.
Implications for research
Although our study was underpowered and not able to give a definitive answer to the question of whether or not ketamine augmentation of ECT is of benefit, it adds to a literature in which there is little signal of substantial cognitive or symptomatic benefit from ketamine used in this way, suggesting that a further, adequately powered trial of similar design is not a priority for research. An updated systematic review of relevant clinical trials, including this study, would, however, appear warranted. Given the evidence that ketamine alone has a rapid antidepressant effect,41 it is notable in our study that a similar dose given with ECT did not appear to have a rapid antidepressant effect; in fact, no patients receiving ketamine remitted in the first week and only one out of 33 patients responded to treatment by this point (see Appendix 2, Tables 35 and 36). This raises an interesting question as to why ketamine given with ECT in this study appears to have different effects from when it is given alone. Further research is needed to address this; explanations could range from ECT blocking the effects of ketamine, suggesting involvement in a common pathway, to the antidepressant effect of ketamine requiring a slow infusion rather than a bolus or even requiring the subjective experience of ketamine’s dissociative effects (which did not occur in our patients). One retrospective study has found that intra-infusion dissociation predicts antidepressant response 4 hours and 7 days later. 171
The cognitive effects of ECT in our study were milder than might have been expected from the literature and it is possible that the now widespread use of a pulse width of 0.5 milliseconds for ECT in the UK results in less effect on cognition than 1-millisecond and 1.5-milliseconds pulse widths used historically (as has recently been proposed by others171). Although studies have been carried out comparing ultra-brief-pulse (0.25–0.3 milliseconds) and brief-pulse (typically ≥ 1 millisecond) ECT, we are not aware of a direct comparison between 0.5 milliseconds and longer-duration brief-pulse stimuli; this may be indicated to ascertain the optimal range of the pulse width used to minimise cognitive effects while maintaining efficacy.
The fNIRS substudy provided preliminary data suggesting that prefrontal cortex responses to a VF task may be sensitive to the effects of ECT and predict clinical response. There are important limitations to the results given the small numbers of patients, the lack of correction for multiple comparisons in the results presented and the need for further analysis using alternative methods for extracting the best signal from the data. Nevertheless, we have shown that applying NIRS at the ‘bedside’ is potentially feasible. Taking our results together with the increasing interest in the technology, which has a promising and expanding evidence base, further studies are warranted using NIRS not only as a research tool but also to explore its clinical potential in predicting and guiding treatment.
Conclusions
The main finding of the Ketamine-ECT study is that there was no evidence of benefit in terms of cognitive and efficacy outcomes from using low-dose ketamine as an adjunctive anaesthetic agent for ECT, as currently administered in the UK. Although no serious harms appear to be associated with its use at this dose, it may cause a transient psychological reaction in a small minority of patients.
Near-infrared spectroscopy is a promising portable brain imaging technique that appears potentially feasible in a seriously ill psychiatric population. Further research is warranted to determine how robust our findings are in clinical populations and validation is needed against more established imaging methods. The results from this study indicate that it may have potential as a clinical tool to guide treatment but this needs further investigation.
Chapter 5 Telephone survey of participants
Background
There is an increasing emphasis on patient and service user involvement in research but it still lacks an established evidence base. 172 In the UK, INVOLVE (funded by the NIHR) has published briefing notes for researchers emphasising the need for, and benefits of, service user involvement in all stages of the research cycle. 173
During the Ketamine-ECT study we became increasingly aware of the polarised views about ECT, which remains a controversial treatment that is declining in use in the UK12,13 (see discussion in Chapter 1). This was highlighted when a group of academics and clinicians raised a challenge to the study based on their concerns about the safety and efficacy of ECT and of its combination with ketamine and about the information provided to patients, which they believed was misleading. After correspondence, a freedom of information request and demands for the patient information sheet to be altered, the group published an open letter requesting that the funder and ethics committee suspend the trial (details of the open letter, the associated article in the Observer newspaper and the study team’s rebuttal are available on the Guardian newspaper’s headquarters website154).
Previous research has suggested that many patients feel that they do receive sufficient information about ECT, with some feeling coerced into having it. 174 A systematic review found that, by patient, groups report more harms from ECT than are reported in research carried out by professionals, and the authors suggested that patients may be reluctant to express their true views to clinicians on whom they depend for treatment. 174 However, research testing this hypothesis in patients detained under the MHA did not find a difference in information obtained by service user and non-service user interviewers. 175
Working through our service user group we used the opportunity provided by the Ketamine-ECT study to seek the views of participants, both about participation in the study and about their experience of ECT more generally. This was carried out using a telephone survey designed by the service user group to be delivered by interviewers with service user experience.
Methods
Participants
Forty-three of the 70 participants receiving ECT treatment in the study were invited to participate in the survey interview. The remaining patients either had been lost to follow-up in the main study or had withdrawn for other reasons (eight people who were lost to follow-up were, however, contacted on the advice of the RAs). Of the 43 participants invited, 18 returned consent forms, one of whom we were subsequently unable to contact, providing a final sample of 17 (24% of those treated).
Ethics approval for the survey was obtained on 12 February 2015 as a substantial amendment to the main study approval and informed consent was obtained.
Survey interview
The survey interview was developed by the Ketamine-ECT study service user group, chief investigator and project manager, with advice on content and format sought from experts in qualitative research and surveys (Professor Linda Claire, Bangor University, and Dr Ross Clark, a principal investigator on the study with experience in qualitative methods). A full qualitative study was not feasible within the resources available, but qualitative methods were employed as far as possible.
The interview consisted of 10 open questions and prompts relating to the Ketamine-ECT study and to ECT treatment. Each open question was followed by a quantitative question related to the open question topic, scored on a 6-point Likert scale ranging between the extremes of possible opinions or experience. A final opportunity was given for participants to add anything else that they had not had the chance to talk about. The reason for the combined methodology was to be able not only to identify themes related to the question topics but also to provide a description of the overall strength and distribution of opinions. For example, both positive and negative themes relating to participation in research may be identified, possibly within the same individual, and the quantitative measurement allows assessment of the degree to which, on balance, the experience of participation is negative or positive. The summary statistics provide a description of the majority views and the range of views within the group.
Given the uncertainty about whether or not the background of the person who collects data (e.g. professionals vs. users of a relevant service) impacts on the information provided (see Background), an item was added to the survey interview asking respondents their opinion about the involvement of service users in surveys such as this. Two members of the Ketamine-ECT service user group conducted 10 of the 17 surveys; the remaining surveys were conducted by the Ketamine-ECT study project manager, who had had no previous direct contact with the participants. The same survey interview was used for all participants and, with permission, interviews were recorded and transcribed to facilitate analysis and quality control. Interviews were recorded without identifying the participant in the recording and the transcriptions were anonymised.
Analysis
The interviews were transcribed and then coded by the project manager using NVivo 10 for Microsoft Windows (QSR International, Warrington, UK). Based on the codes identified, themes were identified and examined for further discussion as main themes based on their information content and coherence.
Quantitative data were analysed in IBM SPSS Statistics version 22.0 to provide a summary of the average (median) score, the score endorsed by most people (mode) and the extremes of the scores (range).
Results
In total, 19 codes were identified, some of which had subcodes. For example, ‘opinions of ECT’ was identified as a code, with four subcodes: family opinions, opinion based on previous ECT experience, fears and concerns and users’ opinions of ECT based on their own research. In total, 37 references were coded under ‘opinions of ECT’.
The codes were grouped into a total of seven themes as follows:
-
ECT experience, including memory problems and ECT staff
-
ECT information, including opinions of ECT, timing of ECT and decision to have ECT
-
study information, including the possibility of receiving ketamine, decision about the study and helping with future treatment
-
experience of the research study, including being approached about research and the follow-up survey
-
life since ECT, including use of other services
-
experience of depression, including medication for depression
-
opinions about the MHA and inclusion in research about ECT.
Balance of emerging themes
Of these identified themes, ‘opinions about the MHA’ was discounted as a main theme. The question of excluding patients being treated under a section of the MHA was discussed at various time points throughout the study because the inclusion of these patients might have provided more potentially eligible patients. However, our service user group was of the opinion that including patients detained under the MHA was potentially unethical on the basis that some might feel coerced to take part. The question had been included in the survey interview to seek the opinions of service users who themselves had received ECT. We found that the respondents on the whole did not have clear opinions either way about this issue and in many cases gave conflicting opinions that provided little rich data for discussion as a main theme. For example, although the response to the open question sometimes indicated that MHA patients should have been included, the answer to the quantitative item was that they definitely should not have been included. Several respondents said that they had never thought about the question and did not have an opinion at all.
The ‘experience of depression’ theme was also discounted as a main theme because of the relatively few references coded under this theme (n = 20), most of which were short and did not form a substantive narrative. The remaining five themes are discussed in the following section.
Main themes
The themes with the most coded references were ‘ECT experience’ (n = 93), ‘ECT information’ (n = 79), ‘study information’ (n = 77), and ‘experience of the research study’ (n = 74). Although these themes were those that were the most discussed, ‘life since ECT’ (n = 54) provided the broadest range of views and experiences.
Electroconvulsive therapy experience
Those surveyed reported a wide range of responses to ECT, with some people reporting that they felt it had changed, or saved, their life and others reporting that they did not feel any benefit; some respondents had felt initially better but had since required further ECT treatment. Only a few people reported that the experience of the ECT itself was unpleasant, with most people feeling that they would have preferred not to have had it but that they had not been particularly upset by it. Interestingly, almost all respondents focused on the practical arrangements of the treatment, with much discussion about travel arrangements, and, in particular, there was considerable strength of feeling expressed around not wanting to be in hospital. This was due to the disruption that it would cause to people, both emotionally and practically. It was also noted that support was still needed from a friend or a relative as an outpatient:
Because I didn’t want to go into hospital, we tried to do it as an outpatient and I just found . . . I found because I had to include people I didn’t want to . . . because I live on my own, I just found the whole thing too . . . I know it sounds silly, but too stressful and I just didn’t go again.
About half of the respondents reported memory problems after ECT treatment, with most saying that the problems were temporary and that their memory returned quite quickly after treatment. However, two people said that they felt that their memory was still poorer than before ECT treatment. Some people commented that their memory was poor anyway, which they attributed to their condition at the time, and so they struggled to assess whether or not their memory had been affected by ECT. One respondent felt frightened by this, saying:
Again, this is a little bit difficult, because my memory’s quite difficult from around that time anyway . . . so it’s very difficult thinking back to a lot of that time now, because there are so many blanks in it . . . I think a lot of what I was frightened of was the blanks in my memory, not being able to remember things. I think I found that quite difficult.
Electroconvulsive therapy information
There was a range of experiences and opinions when asking people to talk about the information they received about ECT, its timing and their decision to undergo the treatment. However, none of the respondents reported feeling rushed into having ECT or felt that they had not received sufficient information. Those that could remember the time before ECT generally recalled receiving a lot of information and a doctor being available to answer questions.
Opinions about ECT, before it had been suggested to them as a treatment, varied considerably and included the opinions of family and friends; some reported feeling that it was an old and outdated treatment, ‘from the Dark Ages’, whereas others knew about ECT either from their own profession or through the professions of people around them and felt it to be an effective treatment. Some people had received ECT previously and wanted the treatment again as they had found it useful in the past. However, the most striking responses came from people who had not previously had ECT, with the most common response when it had been suggested being fear:
. . . but it can impair permanently the function of the brain and it does harm the brain.
I think the fear about the treatment really was that it might affect my memory.
Fear, I think.
I think it’s always come across as being quite scary, sort of having an electric shock.
Well I was frightened.
I kind of felt it was a bit of a scary last resort type treatment.
So I was a bit scared of it, but when I thought it’s kind of reached the point where it’s the last resort almost.
As demonstrated by these last two quotations, several people felt that they were so ill that they had reached a point where they were willing to try anything that might help.
The following respondent felt that she wanted to try something because her illness was so severe:
Because I was so ill and no drugs seemed to be working, I was keen to try anything . . . but when you’re so ill and you think nothing is ever going to be right, and you want to die, and you think anything will help, so yes, I was quite happy about it [ECT].
Study information
Most of the respondents could remember being given information about the study but a small number reported not remembering because they were unwell at the time. One person said, ‘when I got this letter about . . . the survey, I thought I didn’t know I did a research programme, but I found my leaflets and realised, oh I did!’
All those who did remember the information provided recalled it as being set out well and feeling that they understood what the study was about. The most reported reaction to being asked to participate in the research was altruism; over half of respondents recognised the possible benefit for future patients undergoing ECT, with many feeling that participating in research is important for that reason.
None of the respondents felt concerned about the possibility of receiving ketamine, with the majority either having a knowledge of the clinical use of ketamine – ‘my son’s an anaesthetist, so I sort of valued his opinion on taking part’ – or feeling that they trusted the design of the study and the professionals involved – ‘well, I assumed it wouldn’t be harmful, so I was quite happy to go along with it, yeah’.
A number of respondents felt hopeful that they would receive the ketamine and that it might help their experience of ECT; the only people who reported any concern about not knowing which drug they would receive were those who wanted to be sure that they were getting the study drug, as exemplified by the statement: ‘I would have felt happier knowing that I was getting the ketamine, obviously, rather than a placebo’.
Experience of the research study
None of the respondents reported negative feelings about being approached about the research, with the majority having no particular feelings either way. In terms of taking part in the research, respondents reported feelings ranging from neutral (‘I think I was relatively OK with it’; ‘Neutral I guess. I wasn’t bothered’) to very positive (‘I was pleased to be able to help, so it was a positive and good feeling taking part’; ‘I felt good to think I was doing something useful’).
None of the respondents reported feeling concerned about the research activities and the most frequently reported feelings about the neuropsychological assessments were around wanting to know what the assessments showed; for example, this person looked to the assessments to monitor any improvement:
Fine really. I didn’t mind giving them. I think in a way it was good to like see whether you were improving or . . . .
Another respondent found them interesting in a different way:
They were fine. I found them interesting, because it was obvious from mine, that I had a mind that sort of had a number slant. I could remember numbers very easily, but not so much words . . . . The drawings from memory, I was absolutely atrocious on.
Nearly half of the respondents, without responding to a specific prompt, highlighted the research staff who carried out the assessments; all reported very positive experiences using terminology such as ‘brilliant’, ‘very nice and friendly’ and ‘very helpful’.
Life since electroconvulsive therapy
This theme included a broad range of experiences. Some respondents had had a further course of ECT since participating in the study and others felt that the treatment had not been successful at all, saying things like, ‘I’m still battling depression . . . I don’t think it’s been as effective as I thought it would have been’. Others felt that ECT had worked for them, with one person saying, ‘I’ve been very lucky’. Other people did not feel an immediate effect from the treatment; for example, one person stated that:
It was strange for me, because I had 13 of them and, at the end, I didn’t actually feel that [I] was much better. It was 2 days later; it was like a light switch went on . . . I thought nothing’s changed. But then, 2 days later, I remember waking up – it was 11 April.
The majority of respondents were still accessing mental health services following their ECT treatment, with most mentioning that they see their psychiatrist, some as often as once per month but others as little as twice a year. Most people felt that they had some support in the form of community teams or psychiatric nurses. A number of people reported accessing psychological therapy and feeling supported, with the most positive outcome being from a woman who was due to end therapy shortly: ‘I’m delighted. They’re thinking of discharging me in a couple of weeks’.
In contrast, a number of people also felt that they had only had medication offered to them and did not know about other services. One person said:
I’ve got a mental health worker who contacted me, but she didn’t offer me anything . . . I’d like to access any help that’s there, but I don’t know what’s there, because no one has ever told me.
Scored questions
The quantitative scores are presented in Table 28. Overall, patients’ views of taking part in the study were extremely positive, with no strongly negative scores. Most people reported that they were not concerned about not knowing whether or not they were to receive ketamine; only two people reported any concern. Similarly, the experience of ECT was scored as positive by the majority, who thought that they had been given enough information and time to decide about receiving it (however, scores were missing for four people). When asked to score their views on patients being detained under the MHA being offered the opportunity to take part in research if they were able to understand and consent, the majority view was positive (but, as noted earlier, this conflicted at times with their narrative response).
| Questions | Scoring | n | Median | Mode | Range |
|---|---|---|---|---|---|
| About taking part in the study | |||||
| How unhappy or happy did you feel at the beginning about being approached [for the study]? | 0 ‘very unhappy’ to 5 ‘very happy’ | 15 | 5 | 5 | 2–5 |
| Did you feel you knew fully about the study? | 0 ‘not understanding at all’ to 5 ‘fully informed’ | 15 | 5 | 5 | 3–5 |
| How concerned were you about not knowing whether or not you were receiving the study drug? | 0 ‘not at all concerned’ to 5 ‘very concerned’ | 15 | 0 | 0 | 0–5 |
| Was your experience of taking part in the research a negative or positive experience? | 0 ‘very negative’ to 5 ‘very positive’ | 17 | 5 | 5 | 2–5 |
| About ECT | |||||
| Did you think at the time that being offered ECT was the right thing? | 0 ‘strongly that it shouldn’t have been’ to 5 ‘strongly that it was the right thing’ | 16 | 4.5 | 5 | 0–5 |
| Did you feel that you were given enough information and time? | 0 ‘strongly that you were not provided with enough information/time’ to 5 ‘strongly that you were’ | 13 | 5 | 5 | 2.5–5 |
| How negative or positive was the overall experience of having ECT on this occasion? | 0 ‘very negative’ to 5 ‘very positive’ | 17 | 4 | 5 | 1–5 |
| Do you think you are receiving the right sort of help since having ECT? | 0 ‘not at all the right help’ to 5 ‘fully getting the right help’ | 16 | 4.75 | 5 | 0–5 |
| Other aspects | |||||
| Should we have offered patients on a section the opportunity to take part in the study, provided they were capable of understanding and consenting to it? | 0 ‘definitely no’ to 5 ‘definitely yes’ | 15 | 4 | 4 | 0–5 |
| Is talking to someone with personal experience of mental health services more negative or more positive than talking to someone who has not used services? | 0 ‘much more negative’ to 5 ‘much more positive’ | 14 | 4.5 | 5 | 3–5 |
Further discussion
Although necessarily somewhat constrained by the format of the survey interview, the responses to the open questions and the associated themes provided a richness to the responses that is not evident in the quantitative scores, or would not have been apparent in a standard questionnaire.
The fear expressed about having ECT from those who received it for the first time is notable and may be attributable to its controversial nature as well as misconceptions about its practice by the general public, even when it is felt to be necessary. This fear in anticipation contrasts with the mostly positive discussion about the experience in practice, including the information provided and the consent process (although there were some with a more negative experience). The high level of concern about lack of informed consent for ECT found in a previous report174 was not borne out in our sample. It is possible that clinical practice has improved and, in addition, the MHA was amended in 2007 making it unlawful for patients to receive ECT without their consent if they have capacity to make the decision. However, it is also likely to reflect our sample, given that study participants needed to have capacity to give informed consent for, and had chosen to take part in, a research project. An aspect of treatment that is often overlooked by clinicians and hospitals is how important the practical arrangements are for patients receiving ECT and the distress and disruption brought about by inpatient care when this is required. ECT was reported to have temporary effects on memory but our sample did not report the high prevalence of permanent memory effects reported by Rose et al. 16
The experience of taking part in the research study was positive in the sample, with most participants expressing an altruistic motivation. Researchers are often concerned about the burden of assessments in research such as this, but this did not emerge here; rather, patients valued them as an indicator of clinical progress.
In discussion about the benefits of having service users conducting the survey, there were mixed feelings. Some people felt that it ‘could’ be beneficial but was not necessary. Several people commented that the service user conducting the interview could have a very different condition or experience of services to that of the respondent and that any benefit would be dependent on the individual service user. A small number of people felt that the professionalism of the person conducting the survey was the most important characteristic and that the interview needed to be conducted dispassionately. Of those who felt that service users conducting the survey was a good thing, most felt that it was ‘nice to have’ but did not provide any specific benefits. One person, however, felt strongly that it would be easier to talk to a service user, stating that ‘people who haven’t suffered mental health issues don’t have a clue what it’s like’.
In considering the results of this survey it is important to acknowledge that the participants cannot be taken as a representative sample of patients having ECT or even of those taking part in the study as a whole. From what was said in the interviews, about half were health-care or other professionals or were closely related to such a person. This may have influenced their decision to participate in the survey and could similarly have influenced their feelings towards the research and/or ECT. A possible further factor must also be considered: whether or not participation in the survey might have been based on a positive outcome from ECT treatment. However, the range of opinions provided about their experiences of ECT and its outcomes does not appear to support this.
Conclusions
Over the course of the Ketamine-ECT study the research team received input to and feedback on the trial from study investigators, the service user group, external professionals and committees overseeing its conduct. The survey was designed to gain an insight into the experiences and opinions of the participants themselves and it is interesting that some concerns raised from the research team’s side did not form an important part of the respondents’ narrative. For example, concern about the length of assessments was not raised by the patients who undertook them and there was a lack of strong opinions about whether or not patients being treated under a section of the MHA should be included.
Electroconvulsive therapy is a treatment that leads to extreme polarisation of opinions; this was not found in the views expressed by the participants interviewed, who provided thoughtful reflections, both positive and negative, about their experience. What the survey does highlight is the altruism that leads many people to take part in research, even when they are very ill. For most people ECT is a treatment that is not undertaken lightly, occurring as it does at a very difficult time, and is one that has a considerable emotional and practical impact on their lives.
Acknowledgements
The authors would like to thank the following for their invaluable help: Moruf Adelekan, Mark Appleton, Bev Austin, Victoria Baron, Hannah Bayes, Suzy Bourke, Vanda Brack, Jackie Brammer, Jonathan Burden, Karen Butler, Vinod Chaugule, Anthony Cleare, Richard Cree, Michael Dixon, Beatriz Duran, Ali Ford-Brown, John Green, Lloyd Gregory, Louise Golightly, Sharon Grieve, Matthew Haggarty, Lewis Halpin, Guy Harvey, Kathryn Hayes, Nicola Hermitage, Stephen Holgate, Keith Holt, Graham Hough, Jennie Hunter, Versha Jhinghan, Coral Jones, Farzana Kausir, Janet Kennedy, Chintaharan Kotur, Sean Lennon, Maureen Longstaff, Diane Lyons, Celia Marshall, Andy Mee, Kirsty Melia, Jane Newby, Apsara Panikkar, Susanna Piggott, David Ralph, Tim Ramsay, Sharron Robinson, Diane Ruddy, Darren Rusk, David Ryder, Elankathir Selvaraasan, Ram Singh, Amanda Spencer, Debbie Sutton, Albert Swana, Maxine Syme, Andrew Syndercombe, Emma Taylor, Alan Thomas, Aparna Trivedi, Seema Varshney, Rachel Ward, Stuart Watson, Rhiannon Whitaker, Chris White, Carolyn Whitley, Judith Wilkes, Kate Williams and Jan Wood. We would also like to express thanks to the Local Clinical Research Networks: East Midlands, Greater Manchester, North East and North Cumbria, Yorkshire and Humber. Essential support and guidance was provided by the Christie Hospital (now Manchester Academic Health Science Centre) CTU (Anne Bowers, Gemma Darby, Claire Goldrick and Simon Williams), the service user group (organiser Tim Rawcliffe), the TSC (chairperson David Baldwin) and the DMEC (chairperson Keith Matthews). Finally, and most importantly, we would like to thank all the patients who took part in the study and the follow-up survey.
Support
This project was funded by the Efficacy and Mechanism Evaluation (EME) programme, a Medical Research Council (MRC) and NIHR partnership (reference number 10/90/04). The study was sponsored by Manchester Mental Health and Social Care Trust and supported by the UK Clinical Research Network. The views expressed in this publication are those of the author(s) and not necessarily those of the MRC, NHS, NIHR or Department of Health.
Contributions of authors
All authors reviewed, revised and approved the final version of the manuscript.
Ian M Anderson* (Professor, Psychiatry) was the chief investigator.
Andrew Blamire (Professor, Magnetic Resonance Physicist) was a co-applicant and was involved in the design of the study.
Tim Branton (Consultant, Psychiatry) was a principal investigator for the Leeds site and was involved in the design of the study and recruitment.
Sabrina Brigadoi* (Postdoctoral Fellow, Bioengineering) was involved in the design and execution of the fNIRS analysis.
Ross Clark (Consultant, Anaesthetist) was a co-applicant and was involved in the design and supervision of the anaesthetic components of the study.
Darragh Downey* (Research Assistant, Neuroscience and Psychiatry) was involved in the design of the study, the fNIRS analysis and recruitment.
Graham Dunn (Professor, Biomedical Statistics) was a co-applicant, was involved in the design of the study and the trial statistical analysis plan and oversaw the statistical analysis for the clinical outcomes.
Andrew Easton (Consultant, Psychiatry) was a co-applicant and principal investigator for the Leeds site and was involved in the design of the study and recruitment.
Rebecca Elliott (Professor, Cognitive Neuropsychiatry) was a co-applicant and was involved in the design of the study.
Clare Elwell (Professor, Medical Physics) was a co-applicant and was involved in the design of the study.
Katherine Hayden (Consultant, Psychiatry) was a co-applicant and principal investigator for the Stockport site and was involved in the design of the study and recruitment.
Fiona Holland* (Statistician) was involved in the design of the trial statistical analysis plan and conducted the statistical analysis for the clinical outcomes for the study.
Salman Karim (Consultant, Psychiatry) was a principal investigator for the Lancashire sites and was involved in the design of the study and recruitment.
Jo Lowe* (Trial Manager) was involved in the design of the study and all aspects of the study’s management and monitoring.
Colleen Loo (Professor, Psychiatry) was involved in the design of the study.
Rajesh Nair (Consultant, Psychiatry) was a principal investigator for the Darlington and Middlesbrough sites and was involved in the design of the study and recruitment.
Timothy Oakley (Consultant, Psychiatry) was a co-applicant and principal investigator for the Newcastle site and was involved in the design of the study and recruitment.
Antony Prakash (Consultant, Psychiatry) was a principal investigator for the Derby site and was involved in the design of the study and recruitment.
Parveen K Sharma (Consultant, Psychiatry) was a co-applicant and principal investigator for the Manchester site and was involved in the design of the study and recruitment.
Stephen R Williams (Professor, Imaging Science) was a co-applicant and was involved in the design of the study.
R Hamish McAllister-Williams (Reader in Clinical Psychopharmacology, Psychiatrist) was a co-applicant and principal investigator for the Newcastle site and was involved in the design of the study and recruitment.
The Ketamine-ECT study team: Claire Blakeley, Katherine Crosby, Aisha Perkis, Graham Spencer, Liam Trevithick, Amanda Watson, Francesca Williams and Audrey Williamson.
(*Denotes members of the group who wrote the first draft.)
Publications
Anderson IM, Blamire A, Branton T, Clark R, Downey D, Dunn G, et al. Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine-ECT): a multicentre, double-blind, randomised, parallel-group, superiority trial [published online ahead of print 27 March 2017]. Lancet Psychiatry 2017. http://dx.doi.org/10.1016/S2215-0366(17)30077-9
Anderson I. ECT and Ketamine in the Treatment of Depression – a Response. 2014. URL: www.theguardian.com/science/head-quarters/2014/jun/30/electroconvulsive-therapy-ketamine-depression-treatment-ect (accessed 20 January 2016).
Trevithick L, McAllister-Williams RH, Blamire A, Branton T, Clark R, Downey D, et al. Study protocol for the randomised controlled trial: ketamine augmentation of ECT to improve outcomes in depression (Ketamine-ECT study). BMC Psychiatry 2015;15:257. http://dx.doi.org/10.1186/s12888-015-0641-4
Data sharing statement
Data are archived at the University of Manchester and can be obtained from the corresponding author, Professor Ian M Anderson (ian.anderson@manchester.ac.uk).
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health.
References
- World Health Organization . Health Statistics and Information Systems: Estimates for 2000–2012 – Disease Burden 2015. www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html (accessed 2 August 2016).
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002;59:530-7. http://dx.doi.org/10.1001/archpsyc.59.6.530.
- Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H. Psychiatric Morbidity among Adults Living in Private Households, 2000. London: The Stationery Office; 2001.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905-17. http://dx.doi.org/10.1176/ajp.2006.163.11.1905.
- National Institute for Health and Care Excellence . Depression in Adults (Update): Full Guideline 2009. www.nice.org.uk/guidance/cg90/evidence/full-guidance-243833293 (accessed 2 August 2016).
- Payne NA, Prudic J. Electroconvulsive therapy: part I. A perspective on the evolution and current practice of ECT. J Psychiatr Pract 2009;15:346-68. http://dx.doi.org/10.1097/01.pra.0000361277.65468.ef.
- UK ECT Review Group . Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003;361:799-808. http://dx.doi.org/10.1016/S0140-6736(03)12705-5.
- Heijnen WT, Birkenhäger TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol 2010;30:616-19. http://dx.doi.org/10.1097/JCP.0b013e3181ee0f5f.
- Scottish ECT Accreditation Network . Scottish ECT Accreditation Network Annual Report 2015: A Summary of ECT in Scotland for 2014 2015. www.sean.org.uk/docs/SEAN-Report-2015-web.pdf (accessed 2 August 2016).
- Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK. Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 2012;14:146-50. http://dx.doi.org/10.1111/j.1399-5618.2012.00997.x.
- Petrides G, Fink M, Husain MM, Knapp RG, Rush AJ, Mueller M, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001;17:244-53. http://dx.doi.org/10.1097/00124509-200112000-00003.
- Department of Health . Electro Convulsive Therapy: Survey Covering the Period from January 2002 to March 2002, England 2003. http://webarchive.nationalarchives.gov.uk/20130107105354/http:/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4023559.pdf (accessed 2 August 2016).
- Bickerton D, Worrall A, Chaplin R. Trends in the administration of electroconvulsive therapy in England. Psychiatr Bull 2009;33:61-3. http://dx.doi.org/10.1192/pb.bp.107.019273.
- Eranti SV, McLoughlin DM. Electroconvulsive therapy – state of the art. Br J Psychiatry 2003;182:8-9. http://dx.doi.org/10.1192/bjp.182.1.8.
- National Institute for Health and Care Excellence . Guidance on the Use of Electroconvulsive Therapy 2003. www.nice.org.uk/guidance/ta59/resources/guidance-on-the-use-of-electroconvulsive-therapy-2294645984197 (accessed 2 August 2016).
- Rose D, Fleischmann P, Wykes T, Leese M, Bindman J. Patients’ perspectives on electroconvulsive therapy: systematic review. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7403.1363.
- Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 2010;68:568-77. http://dx.doi.org/10.1016/j.biopsych.2010.06.009.
- Falconer DW, Cleland J, Fielding S, Reid IC. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB) to assess the cognitive impact of electroconvulsive therapy on visual and visuospatial memory. Psychol Med 2010;40:1017-25. http://dx.doi.org/10.1017/S0033291709991243.
- Sackeim HA. Autobiographical memory and electroconvulsive therapy: do not throw out the baby. J ECT 2014;30:177-86. http://dx.doi.org/10.1097/YCT.0000000000000117.
- Meeter M, Murre JM, Janssen SM, Birkenhager T, van den Broek WW. Retrograde amnesia after electroconvulsive therapy: a temporary effect?. J Affect Disord 2011;132:216-22. http://dx.doi.org/10.1016/j.jad.2011.02.026.
- Jelovac A, O’Connor S, McCarron S, McLoughlin DM. Autobiographical memory specificity in major depression treated with electroconvulsive therapy. J ECT 2016;32:38-43. http://dx.doi.org/10.1097/YCT.0000000000000267.
- Semkovska M, McLoughlin DM. Measuring retrograde autobiographical amnesia following electroconvulsive therapy: historical perspective and current issues. J ECT 2013;29:127-33. http://dx.doi.org/10.1097/YCT.0b013e318279c2c9.
- Brakemeier EL, Berman R, Prudic J, Zwillenberg K, Sackeim HA. Self-evaluation of the cognitive effects of electroconvulsive therapy. J ECT 2011;27:59-66. http://dx.doi.org/10.1097/YCT.0b013e3181d77656.
- Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology 2007;32:244-54. http://dx.doi.org/10.1038/sj.npp.1301180.
- Semkovska M, Keane D, Babalola O, McLoughlin DM. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res 2011;45:770-80. http://dx.doi.org/10.1016/j.jpsychires.2010.11.001.
- Kellner CH, Tobias KG, Wiegand J. Electrode placement in electroconvulsive therapy (ECT): a review of the literature. J ECT 2010;26:175-80. http://dx.doi.org/10.1097/YCT.0b013e3181e48154.
- Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry 2010;196:226-34. http://dx.doi.org/10.1192/bjp.bp.109.066183.
- Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 2008;1:71-83. http://dx.doi.org/10.1016/j.brs.2008.03.001.
- Tor PC, Bautovich A, Wang MJ, Martin D, Harvey SB, Loo C. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. J Clin Psychiatry 2015;76:e1092-8. http://dx.doi.org/10.4088/JCP.14r09145.
- Anderson IM, Fergusson GM, Waite J, Easton E. The ECT Handbook. London: RCPsych Publications; 2013.
- Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol 2009;219:20-6. http://dx.doi.org/10.1016/j.expneurol.2009.04.027.
- Mitchell ND, Baker GB. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr Scand 2010;122:192-210. http://dx.doi.org/10.1111/j.1600-0447.2009.01529.x.
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008;7:426-37. http://dx.doi.org/10.1038/nrd2462.
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med 2003;33:1277-84. http://dx.doi.org/10.1017/S0033291703007931.
- Merkl A, Schubert F, Quante A, Luborzewski A, Brakemeier EL, Grimm S, et al. Abnormal cingulate and prefrontal cortical neurochemistry in major depression after electroconvulsive therapy. Biol Psychiatry 2011;69:772-9. http://dx.doi.org/10.1016/j.biopsych.2010.08.009.
- Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res 2003;122:185-92. http://dx.doi.org/10.1016/S0925-4927(03)00003-9.
- Peng S, Zhang Y, Zhang J, Wang H, Ren B. Glutamate receptors and signal transduction in learning and memory. Mol Biol Rep 2011;38:453-60. http://dx.doi.org/10.1007/s11033-010-0128-9.
- Gregory-Roberts EM, Naismith SL, Cullen KM, Hickie IB. Electroconvulsive therapy-induced persistent retrograde amnesia: could it be minimised by ketamine or other pharmacological approaches?. J Affect Disord 2010;126:39-45. http://dx.doi.org/10.1016/j.jad.2009.11.018.
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci 2006;63:999-1008. http://dx.doi.org/10.1007/s00018-006-6025-7.
- Maeng S, Zarate CA. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep 2007;9:467-74. http://dx.doi.org/10.1007/s11920-007-0063-1.
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology 2014;231:3663-76. http://dx.doi.org/10.1007/s00213-014-3664-5.
- Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, et al. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol 2014;28:536-44. http://dx.doi.org/10.1177/0269881114527361.
- Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology 2006;188:408-24. http://dx.doi.org/10.1007/s00213-006-0572-3.
- Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci 2003;15:27-34. http://dx.doi.org/10.1176/jnp.15.1.27.
- Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci 2011;261:575-82. http://dx.doi.org/10.1007/s00406-011-0205-7.
- McDaniel WW, Sahota AK, Vyas BV, Laguerta N, Hategan L, Oswald J. Ketamine appears associated with better word recall than etomidate after a course of 6 electroconvulsive therapies. J ECT 2006;22:103-6. http://dx.doi.org/10.1097/00124509-200606000-00005.
- Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT 2010;26:223-7. http://dx.doi.org/10.1097/YCT.0b013e3181c3b0aa.
- Rasmussen KG, Kung S, Lapid MI, Oesterle TS, Geske JR, Nuttall GA, et al. A randomized comparison of ketamine versus methohexital anesthesia in electroconvulsive therapy. Psychiatry Res 2014;215:362-5. http://dx.doi.org/10.1016/j.psychres.2013.12.027.
- Yen T, Khafaja M, Lam N, Crumbacher J, Schrader R, Rask J, et al. Post-electroconvulsive therapy recovery and reorientation time with methohexital and ketamine: a randomized, longitudinal, crossover design trial. J ECT 2015;31:20-5. http://dx.doi.org/10.1097/YCT.0000000000000132.
- Yoosefi A, Sepehri AS, Kargar M, Akhondzadeh S, Sadeghi M, Rafei A, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder: a randomized, double-blind study. J ECT 2014;30:15-21. http://dx.doi.org/10.1097/YCT.0b013e3182a4b4c6.
- Loo CK, Katalinic N, Garfield JB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J Affect Disord 2012;142:233-40. http://dx.doi.org/10.1016/j.jad.2012.04.032.
- McGirr A, Berlim MT, Bond DJ, Neufeld NH, Chan PY, Yatham LN, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J Psychiatr Res 2015;62:23-30. http://dx.doi.org/10.1016/j.jpsychires.2015.01.003.
- Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol 2010;626:83-6. http://dx.doi.org/10.1016/j.ejphar.2009.08.046.
- McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry 2010;25:933-44. http://dx.doi.org/10.1002/gps.2431.
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003;65:193-207. http://dx.doi.org/10.1093/bmb/65.1.193.
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 2004;22:409-18. http://dx.doi.org/10.1016/j.neuroimage.2004.01.015.
- Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep 2007;9:512-20. http://dx.doi.org/10.1007/s11920-007-0070-2.
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 2008;29:683-95. http://dx.doi.org/10.1002/hbm.20426.
- Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res 2006;148:33-45. http://dx.doi.org/10.1016/j.pscychresns.2006.04.006.
- Nobler MS, Sackeim HA. Neurobiological correlates of the cognitive side effects of electroconvulsive therapy. J ECT 2008;24:40-5. http://dx.doi.org/10.1097/YCT.0b013e31815d6957.
- Schmidt EZ, Reininghaus B, Enzinger C, Ebner C, Hofmann P, Kapfhammer HP. Changes in brain metabolism after ECT-positron emission tomography in the assessment of changes in glucose metabolism subsequent to electroconvulsive therapy – lessons, limitations and future applications. J Affect Disord 2008;106:203-8. http://dx.doi.org/10.1016/j.jad.2007.06.009.
- Nobler MS, Sackeim HA, Prohovnik I, Moeller JR, Mukherjee S, Schnur DB, et al. Regional cerebral blood flow in mood disorders, III. Treatment and clinical response. Arch Gen Psychiatry 1994;51:884-97. http://dx.doi.org/10.1001/archpsyc.1994.03950110044007.
- Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, et al. Decreased regional brain metabolism after ECT. Am J Psychiatry 2001;158:305-8. http://dx.doi.org/10.1176/appi.ajp.158.2.305.
- McCormick LM, Boles Ponto LL, Pierson RK, Johnson HJ, Magnotta V, Brumm MC. Metabolic correlates of antidepressant and antipsychotic response in patients with psychotic depression undergoing electroconvulsive therapy. J ECT 2007;23:265-73. http://dx.doi.org/10.1097/yct.0b013e318150d56d.
- Allen MD, Fong AK. Clinical application of standardized cognitive assessment using fMRI. II. Verbal fluency. Behav Neurol 2008;20:141-52. http://dx.doi.org/10.3233/BEN-2008-0224.
- Whitney C, Weis S, Krings T, Huber W, Grossman M, Kircher T. Task-dependent modulations of prefrontal and hippocampal activity during intrinsic word production. J Cogn Neurosci 2009;21:697-712. http://dx.doi.org/10.1162/jocn.2009.21056.
- Pihlajamäki M, Tanila H, Hänninen T, Könönen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol 2000;47:470-6. http://dx.doi.org/10.1002/1531-8249(200004)47:4<470::AID-ANA10>3.0.CO;2-M.
- Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology 2003;47:21-6. http://dx.doi.org/68871.
- Audenaert K, Goethals I, Van Laere K, Lahorte P, Brans B, Versijpt J, et al. SPECT neuropsychological activation procedure with the Verbal Fluency Test in attempted suicide patients. Nucl Med Commun 2002;23:907-16. http://dx.doi.org/10.1097/00006231-200209000-00015.
- Videbech P, Ravnkilde B, Kristensen S, Egander A, Clemmensen K, Rasmussen NA, et al. The Danish PET/depression project: poor verbal fluency performance despite normal prefrontal activation in patients with major depression. Psychiatry Res 2003;123:49-63. http://dx.doi.org/10.1016/S0925-4927(03)00002-7.
- Takami H, Okamoto Y, Yamashita H, Okada G, Yamawaki S. Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am J Geriatr Psychiatry 2007;15:594-603. http://dx.doi.org/10.1097/01.JGP.0b013e31802ea919.
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46-59. http://dx.doi.org/10.1002/hbm.20131.
- D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond, B, Biol Sci 2007;362:761-72. http://dx.doi.org/10.1098/rstb.2007.2086.
- Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp 2008;29:490-501. http://dx.doi.org/10.1002/hbm.20414.
- Walsh ND, Williams SC, Brammer MJ, Bullmore ET, Kim J, Suckling J, et al. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 2007;62:1236-43. http://dx.doi.org/10.1016/j.biopsych.2006.12.022.
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord 2007;101:175-85. http://dx.doi.org/10.1016/j.jad.2006.11.017.
- Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry 2011;168:173-82. http://dx.doi.org/10.1176/appi.ajp.2010.09121718.
- Schöning S, Zwitserlood P, Engelien A, Behnken A, Kugel H, Schiffbauer H, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp 2009;30:2746-56. http://dx.doi.org/10.1002/hbm.20702.
- Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage 2006;29:203-15. http://dx.doi.org/10.1016/j.neuroimage.2005.07.002.
- Addis DR, McAndrews MP. Prefrontal and hippocampal contributions to the generation and binding of semantic associations during successful encoding. Neuroimage 2006;33:1194-206. http://dx.doi.org/10.1016/j.neuroimage.2006.07.039.
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 2010;49:865-74. http://dx.doi.org/10.1016/j.neuroimage.2009.08.066.
- Glikmann-Johnston Y, Oren N, Hendler T, Shapira-Lichter I. Distinct functional connectivity of the hippocampus during semantic and phonemic fluency. Neuropsychologia 2015;69:39-4. http://dx.doi.org/10.1016/j.neuropsychologia.2015.01.031.
- Irani F, Platek SM, Bunce S, Ruocco AC, Chute D. Functional near infrared spectroscopy (fNIRS): an emerging neuroimaging technology with important applications for the study of brain disorders. Clin Neuropsychol 2007;21:9-37. http://dx.doi.org/10.1080/13854040600910018.
- Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 2014;37:161-81. http://dx.doi.org/10.1146/annurev-neuro-071013-014111.
- Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009;103:i3-1. http://dx.doi.org/10.1093/bja/aep299.
- Wolf M, Ferrari M, Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt 2007;12. http://dx.doi.org/10.1117/1.2804899.
- Greisen G. Is near-infrared spectroscopy living up to its promises?. Semin Fetal Neonatal Med 2006;11:498-502. http://dx.doi.org/10.1016/j.siny.2006.07.010.
- Blasi A, Fox S, Everdell N, Volein A, Tucker L, Csibra G, et al. Investigation of depth dependent changes in cerebral haemodynamics during face perception in infants. Phys Med Biol 2007;52:6849-64. http://dx.doi.org/10.1088/0031-9155/52/23/005.
- Kono T, Matsuo K, Tsunashima K, Kasai K, Takizawa R, Rogers MA, et al. Multiple-time replicability of near-infrared spectroscopy recording during prefrontal activation task in healthy men. Neurosci Res 2007;57:504-12. http://dx.doi.org/10.1016/j.neures.2006.12.007.
- Kwee IL, Nakada T. Dorsolateral prefrontal lobe activation declines significantly with age – functional NIRS study. J Neurol 2003;250:525-9. http://dx.doi.org/10.1007/s00415-003-1028-x.
- Schecklmann M, Ehlis AC, Plichta MM, Fallgatter AJ. Functional near-infrared spectroscopy: a long-term reliable tool for measuring brain activity during verbal fluency. Neuroimage 2008;43:147-55. http://dx.doi.org/10.1016/j.neuroimage.2008.06.032.
- Molteni E, Butti M, Bianchi AM, Reni G. Activation of the prefrontal cortex during a visual n-back working memory task with varying memory load: a near infrared spectroscopy study. Conf Proc IEEE Eng Med Biol Soc 2008;2008:4024-7. http://dx.doi.org/10.1109/IEMBS.2008.4650092.
- Kakimoto Y, Nishimura Y, Hara N, Okada M, Tanii H, Okazaki Y. Intrasubject reproducibility of prefrontal cortex activities during a verbal fluency task over two repeated sessions using multi-channel near-infrared spectroscopy. Psychiatry Clin Neurosci 2009;63:491-9. http://dx.doi.org/10.1111/j.1440-1819.2009.01988.x.
- Matsuo K, Kato T, Fukuda M, Kato N. Alteration of hemoglobin oxygenation in the frontal region in elderly depressed patients as measured by near-infrared spectroscopy. J Neuropsychiatry Clin Neurosci 2000;12:465-71. http://dx.doi.org/10.1176/jnp.12.4.465.
- Matsuo K, Kato N, Kato T. Decreased cerebral haemodynamic response to cognitive and physiological tasks in mood disorders as shown by near-infrared spectroscopy. Psychol Med 2002;32:1029-37. http://dx.doi.org/10.1017/S0033291702005974.
- Pu S, Matsumura H, Yamada T, Ikezawa S, Mitani H, Adachi A, et al. Reduced frontopolar activation during verbal fluency task associated with poor social functioning in late-onset major depression: multi-channel near-infrared spectroscopy study. Psychiatry Clin Neurosci 2008;62:728-37. http://dx.doi.org/10.1111/j.1440-1819.2008.01882.x.
- Herrmann MJ, Ehlis AC, Fallgatter AJ. Bilaterally reduced frontal activation during a verbal fluency task in depressed patients as measured by near-infrared spectroscopy. J Neuropsychiatry Clin Neurosci 2004;16:170-5. http://dx.doi.org/10.1176/jnp.16.2.170.
- Ohta H, Yamagata B, Tomioka H, Takahashi T, Yano M, Nakagome K, et al. Hypofrontality in panic disorder and major depressive disorder assessed by multi-channel near-infrared spectroscopy. Depress Anxiety 2008;25:1053-9. http://dx.doi.org/10.1002/da.20463.
- Tomioka H, Yamagata B, Kawasaki S, Pu S, Iwanami A, Hirano J, et al. A longitudinal functional neuroimaging study in medication-naïve depression after antidepressant treatment. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0120828.
- Ohtani T, Nishimura Y, Takahashi K, Ikeda-Sugita R, Okada N, Okazaki Y. Association between longitudinal changes in prefrontal hemodynamic responses and social adaptation in patients with bipolar disorder and major depressive disorder. J Affect Disord 2015;176:78-86. http://dx.doi.org/10.1016/j.jad.2015.01.042.
- Akashi H, Tsujii N, Mikawa W, Adachi T, Kirime E, Shirakawa O. Prefrontal cortex activation is associated with a discrepancy between self- and observer-rated depression severities of major depressive disorder: a multichannel near-infrared spectroscopy study. J Affect Disord 2015;174:165-72. http://dx.doi.org/10.1016/j.jad.2014.11.020.
- Liu X, Sun G, Zhang X, Xu B, Shen C, Shi L, et al. Relationship between the prefrontal function and the severity of the emotional symptoms during a verbal fluency task in patients with major depressive disorder: a multi-channel NIRS study. Prog Neuropsychopharmacol Biol Psychiatry 2014;54:114-21. http://dx.doi.org/10.1016/j.pnpbp.2014.05.005.
- Tsujii N, Mikawa W, Akashi H, Tsujimoto E, Adachi T, Kirime E, et al. Right temporal activation differs between melancholia and nonmelancholic depression: a multichannel near-infrared spectroscopy study. J Psychiatr Res 2014;55:1-7. http://dx.doi.org/10.1016/j.jpsychires.2014.04.003.
- Kinou M, Takizawa R, Marumo K, Kawasaki S, Kawakubo Y, Fukuda M, et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res 2013;150:459-67. http://dx.doi.org/10.1016/j.schres.2013.08.026.
- Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Mitani H, et al. The relationship between the prefrontal activation during a verbal fluency task and stress-coping style in major depressive disorder: a near-infrared spectroscopy study. J Psychiatr Res 2012;46:1427-34. http://dx.doi.org/10.1016/j.jpsychires.2012.08.001.
- Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Yamada S, et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. J Affect Disord 2015;181:9-17. http://dx.doi.org/10.1016/j.jad.2015.04.010.
- Nishimura Y, Takahashi K, Ohtani T, Ikeda-Sugita R, Okada N, Kasai K, et al. Social function and frontopolar activation during a cognitive task in patients with bipolar disorder. Neuropsychobiology 2015;72:81-90. http://dx.doi.org/10.1159/000437431.
- Nishimura Y, Takahashi K, Ohtani T, Ikeda-Sugita R, Kasai K, Okazaki Y. Dorsolateral prefrontal hemodynamic responses during a verbal fluency task in hypomanic bipolar disorder. Bipolar Disord 2015;17:172-83. http://dx.doi.org/10.1111/bdi.12252.
- Mikawa W, Tsujii N, Akashi H, Adachi T, Kirime E, Shirakawa O. Left temporal activation associated with depression severity during a verbal fluency task in patients with bipolar disorder: a multichannel near-infrared spectroscopy study. J Affect Disord 2015;173:193-200. http://dx.doi.org/10.1016/j.jad.2014.10.051.
- Usami M, Iwadare Y, Kodaira M, Watanabe K, Saito K. Near infrared spectroscopy study of the frontopolar hemodynamic response and depressive mood in children with major depressive disorder: a pilot study. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0086290.
- Ikeda E, Shiozaki K, Ikeda H, Suzuki M, Hirayasu Y. Prefrontal dysfunction in remitted depression at work reinstatement using near-infrared spectroscopy. Psychiatry Res 2013;214:254-9. http://dx.doi.org/10.1016/j.pscychresns.2013.07.009.
- Pu S, Yamada T, Yokoyama K, Matsumura H, Kobayashi H, Sasaki N, et al. A multi-channel near-infrared spectroscopy study of prefrontal cortex activation during working memory task in major depressive disorder. Neurosci Res 2011;70:91-7. http://dx.doi.org/10.1016/j.neures.2011.01.001.
- Schecklmann M, Dresler T, Beck S, Jay JT, Febres R, Haeusler J, et al. Reduced prefrontal oxygenation during object and spatial visual working memory in unpolar and bipolar depression. Psychiatry Res 2011;194:378-84. http://dx.doi.org/10.1016/j.pscychresns.2011.01.016.
- Pu S, Yamada T, Yokoyama K, Matsumura H, Mitani H, Adachi A, et al. Reduced prefrontal cortex activation during the working memory task associated with poor social functioning in late-onset depression: multi-channel near-infrared spectroscopy study. Psychiatry Res 2012;203:222-8. http://dx.doi.org/10.1016/j.pscychresns.2012.01.007.
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: normative data and analysis of inter-form and test–retest reliability. Clin Neuropsychol 1998;12:43-55. http://dx.doi.org/10.1076/clin.12.1.43.1726.
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Arlington, VA: American Psychiatric Association; 2000.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Mental Health Act 2007. London: The Stationery Office; 2007.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Porter R, Heenan H, Reeves J. Early effects of electroconvulsive therapy on cognitive function. J ECT 2008;24:35-9. http://dx.doi.org/10.1097/YCT.0b013e31816207f0.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9. http://dx.doi.org/10.1192/bjp.134.4.382.
- The ECT Handbook Second Edition. The Third Report of the Royal College of Psychiatrists’ Special Committee on ECT. London: Royal College of Psychiatrists; 2005.
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001.
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97-113. http://dx.doi.org/10.1016/0028-3932(71)90067-4.
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003;53:649-59. http://dx.doi.org/10.1016/S0006-3223(03)00231-2.
- Petersen T, Papakostas GI, Posternak MA, Kant A, Guyker WM, Iosifescu DV, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol 2005;25:336-41. http://dx.doi.org/10.1097/01.jcp.0000169036.40755.16.
- Sobin C, Sackeim HA, Prudic J, Devanand DP, Moody BJ, McElhiney MC. Predictors of retrograde amnesia following ECT. Am J Psychiatry 1995;152:995-1001. http://dx.doi.org/10.1176/ajp.152.7.995.
- Overall JE, Gorman DR. The Brief Psychiatric Rating Scale. Psychol Rep 2011;10:799-812. http://dx.doi.org/10.2466/pr0.1962.10.3.799.
- Loo CK, Sainsbury K, Sheehan P, Lyndon B. A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. Int J Neuropsychopharmacol 2008;11:883-90. http://dx.doi.org/10.1017/S1461145708009292.
- Ingram A, Saling MM, Schweitzer I. Cognitive side effects of brief pulse electroconvulsive therapy: a review. J ECT 2008;24:3-9. http://dx.doi.org/10.1097/YCT.0b013e31815ef24a.
- Letz R, DiIorio CK, Shafer PO, Yeager KA, Schomer DL, Henry TR. Further standardization of some NES3 tests. Neurotoxicology 2003;24:491-50. http://dx.doi.org/10.1016/S0161-813X(03)00044-5.
- Benton LA, Hamsher K, Sivan AB. Controlled Oral Word Association Test. Multilingual Aphasia Examination. Iowa City, IA: AJA; 1994.
- McElhiney M, Moody B, Sackeim H. The Autobiographical Memory Interview – Short Form. New York, NY: New York State Psychiatric Institute; 1997.
- Semkovska M, Noone M, Carton M, McLoughlin DM. Measuring consistency of autobiographical memory recall in depression. Psychiatry Res 2012;197:41-8. http://dx.doi.org/10.1016/j.psychres.2011.12.010.
- Meador KJ, Moore EE, Nichols ME, Abney OL, Taylor HS, Zamrini EY, et al. The role of cholinergic systems in visuospatial processing and memory. J Clin Exp Neuropsychol 1993;15:832-42. http://dx.doi.org/10.1080/01688639308402599.
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corporation; 1981.
- Berman RM, Prudic J, Brakemeier EL, Olfson M, Sackeim HA. Subjective evaluation of the therapeutic and cognitive effects of electroconvulsive therapy. Brain Stimul 2008;1:16-2. http://dx.doi.org/10.1016/j.brs.2007.08.005.
- Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, et al. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med 2008;38:289-300. http://dx.doi.org/10.1017/S0033291707001730.
- Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery–Åsberg depression rating scale corresponding to the definition of remission on the Hamilton Rating Scale for Depression. J Psychiatr Res 2004;38:577-82. http://dx.doi.org/10.1016/j.jpsychires.2004.03.007.
- Snaith RP, Baugh SJ, Clayden AD, Husain A, Sipple MA. The Clinical Anxiety Scale: an instrument derived from the Hamilton Anxiety Scale. Br J Psychiatry 1982;141:518-23. http://dx.doi.org/10.1192/bjp.141.5.518.
- Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville, MD: National Institute of Mental Health; 1976.
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573-83. http://dx.doi.org/10.1016/S0006-3223(02)01866-8.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Eranti S, Mogg A, Pluck G, Landau S, Purvis R, Brown RG, et al. A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. Am J Psychiatry 2007;164:73-81. http://dx.doi.org/10.1176/ajp.2007.164.1.73.
- Zhang H, Dong W, Dang W, Quan W, Tian J, Chen R, et al. Near-infrared spectroscopy for examination of prefrontal activation during cognitive tasks in patients with major depressive disorder: a meta-analysis of observational studies. Psychiatry Clin Neurosci 2015;69:22-33. http://dx.doi.org/10.1111/pcn.12209.
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt 2009;48:D280-98. http://dx.doi.org/10.1364/AO.48.00D280.
- Scholkmann F, Spichtig S, Muehlemann T, Wolf M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol Meas 2010;31:649-62. http://dx.doi.org/10.1088/0967-3334/31/5/004.
- Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol 1988;33:1433-42. http://dx.doi.org/10.1088/0031-9155/33/12/008.
- Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, et al. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res 1996;39:889-94. http://dx.doi.org/10.1203/00006450-199605000-00025.
- Lu CF, Liu YC, Yang YR, Wu YT, Wang RY. Maintaining gait performance by cortical activation during dual-task interference: a functional near-infrared spectroscopy study. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0129390.
- Herrmann MJ, Walter A, Ehlis AC, Fallgatter AJ. Cerebral oxygenation changes in the prefrontal cortex: effects of age and gender. Neurobiol Aging 2006;27:888-94. http://dx.doi.org/10.1016/j.neurobiolaging.2005.04.013.
- Yang H, Wang Y, Zhou Z, Gong H, Luo Q, Wang Y, et al. Sex differences in prefrontal hemodynamic response to mental arithmetic as assessed by near-infrared spectroscopy. Gend Med 2009;6:565-74. http://dx.doi.org/10.1016/j.genm.2009.11.003.
- Murata Y, Sakatani K, Hoshino T, Fujiwara N, Katayama Y, Yamashita D, et al. Changes of evoked cerebral blood oxygenation and optical pathlength in the frontal lobe during language tasks: a study by multi-channel, time-resolved near-infrared spectroscopy and functional MRI. Adv Exp Med Biol 2010;662:213-18. http://dx.doi.org/10.1007/978-1-4419-1241-1_30.
- Anderson IM. ECT and Ketamine in the Treatment of Depression - A Response 2014. www.theguardian.com/science/head-quarters/2014/jun/30/electroconvulsive-therapy-ketamine-depression-treatment-ect (accessed 2 August 2016).
- Munk-Olsen T, Laursen TM, Videbech P, Mortensen PB, Rosenberg R. All-cause mortality among recipients of electroconvulsive therapy: register-based cohort study. Br J Psychiatry 2007;190:435-9. http://dx.doi.org/10.1192/bjp.bp.106.026740.
- Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D 2015;15:37-43. http://dx.doi.org/10.1007/s40268-015-0081-0.
- Blackwood DH, Cull RE, Freeman CP, Evans JI, Mawdsley C. A study of the incidence of epilepsy following ECT. J Neurol Neurosurg Psychiatr 1980;43:1098-102. http://dx.doi.org/10.1136/jnnp.43.12.1098.
- Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry 2007;62:345-54. http://dx.doi.org/10.1016/j.biopsych.2006.09.023.
- Noppers IM, Niesters M, Aarts LP, Bauer MC, Drewes AM, Dahan A, et al. Drug-induced liver injury following a repeated course of ketamine treatment for chronic pain in CRPS type 1 patients: a report of 3 cases. Pain 2011;152:2173-8. http://dx.doi.org/10.1016/j.pain.2011.03.026.
- Woo YS, Rosenblat JD, Kakar R, Bahk WM, McIntyre RS. Cognitive deficits as a mediator of poor occupational function in remitted major depressive disorder patients. Clin Psychopharmacol Neurosci 2016;14:1-16. http://dx.doi.org/10.9758/cpn.2016.14.1.1.
- Fraser LM, O’Carroll RE, Ebmeier KP. The effect of electroconvulsive therapy on autobiographical memory: a systematic review. J ECT 2008;24:10-7. http://dx.doi.org/10.1097/YCT.0b013e3181616c26.
- McAllister-Williams RH, Anderson IM, Finkelmeyer A, Gallagher P, Grunze HC, Haddad PM, et al. Antidepressant augmentation with metyrapone for treatment-resistant depression (the ADD study): a double-blind, randomised, placebo-controlled trial. Lancet Psychiatry 2016;3:117-27. http://dx.doi.org/10.1016/S2215-0366(15)00436-8.
- Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT 2012;28:128-32. http://dx.doi.org/10.1097/YCT.0b013e31824d1d02.
- Yalcin S, Aydoğan H, Selek S, Kucuk A, Yuce HH, Karababa F, et al. Ketofol in electroconvulsive therapy anesthesia: two stones for one bird. J Anesth 2012;26:562-7. http://dx.doi.org/10.1007/s00540-012-1378-6.
- Xu Y, Hackett M, Carter G, Loo C, Galvez V, Glozier N, et al. Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2016;19:1-15. http://dx.doi.org/10.1093/ijnp/pyv124.
- Oudman E. Is electroconvulsive therapy (ECT) effective and safe for treatment of depression in dementia? A short review. J ECT 2012;28:34-8. http://dx.doi.org/10.1097/YCT.0b013e31823a0f5a.
- Hausner L, Damian M, Sartorius A, Frölich L. Efficacy and cognitive side effects of electroconvulsive therapy (ECT) in depressed elderly inpatients with coexisting mild cognitive impairment or dementia. J Clin Psychiatry 2011;72:91-7. http://dx.doi.org/10.4088/JCP.10m05973gry.
- Niu HJ, Li X, Chen YJ, Ma C, Zhang JY, Zhang ZJ. Reduced frontal activation during a working memory task in mild cognitive impairment: a non-invasive near-infrared spectroscopy study. CNS Neurosci Ther 2013;19:125-31. http://dx.doi.org/10.1111/cns.12046.
- Pimontel MA, Culang-Reinlieb ME, Morimoto SS, Sneed JR. Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry 2012;27:893-9. http://dx.doi.org/10.1002/gps.2808.
- Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A. Frontoparietal activation during response inhibition predicts remission to antidepressants in patients with major depression. Biol Psychiatry 2016;79:274-81. http://dx.doi.org/10.1016/j.biopsych.2015.02.037.
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects?. J Affect Disord 2014;159:56-61. http://dx.doi.org/10.1016/j.jad.2014.02.017.
- Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect 2015;18:1151-66. http://dx.doi.org/10.1111/hex.12090.
- Caress AL, Ford A, Roberts L, Turner K, Ward D, Williamson T. Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research 2012. www.invo.org.uk/wp-content/uploads/2014/11/9938_INVOLVE_Briefing_Notes_WEB.pdf (accessed 2 August 2016).
- Rose DS, Wykes TH, Bindman JP, Fleischmann PS. Information, consent and perceived coercion: patients’ perspectives on electroconvulsive therapy. Br J Psychiatry 2005;186:54-9. http://dx.doi.org/10.1192/bjp.186.1.54.
- Rose D, Leese M, Oliver D, Sidhu R, Bennewith O, Priebe S, et al. A comparison of participant information elicited by service user and non-service user researchers. Psychiatr Serv 2011;62:210-13. http://dx.doi.org/10.1176/appi.ps.62.2.210.
Appendix 1 Statistical analysis plan
Appendix 2 Numbers analysed and descriptive statistics
| Time point of assessment | Treatment arm, mean (SD) | |
|---|---|---|
| Saline | Ketamine | |
| Mid-ECT | 1.9 (0.4) | 2.0 (0.3) |
| End of treatment | 6.2 (2.6) | 6.1 (2.2) |
| Follow-up 1 | 10.0 (2.7) | 10.3 (2.2) |
| Follow-up 2 | 21.8 (4.1) | 21.9 (3.4) |
| Time point of assessment | Treatment arm, mean (SD) | |
|---|---|---|
| Saline | Ketamine | |
| Mid-ECT | 2.2 (0.5) | 2.5 (0.8) |
| End of treatment | 6.6 (2.6) | 6.7 (2.3) |
| Follow-up 1 | 10.4 (2.7) | 10.8 (2.4) |
| Follow-up 2 | 22.2 (4.2) | 22.5 (3.4) |
FIGURE 17.
Time from randomisation to first ECT session. (a) Saline; and (b) ketamine.
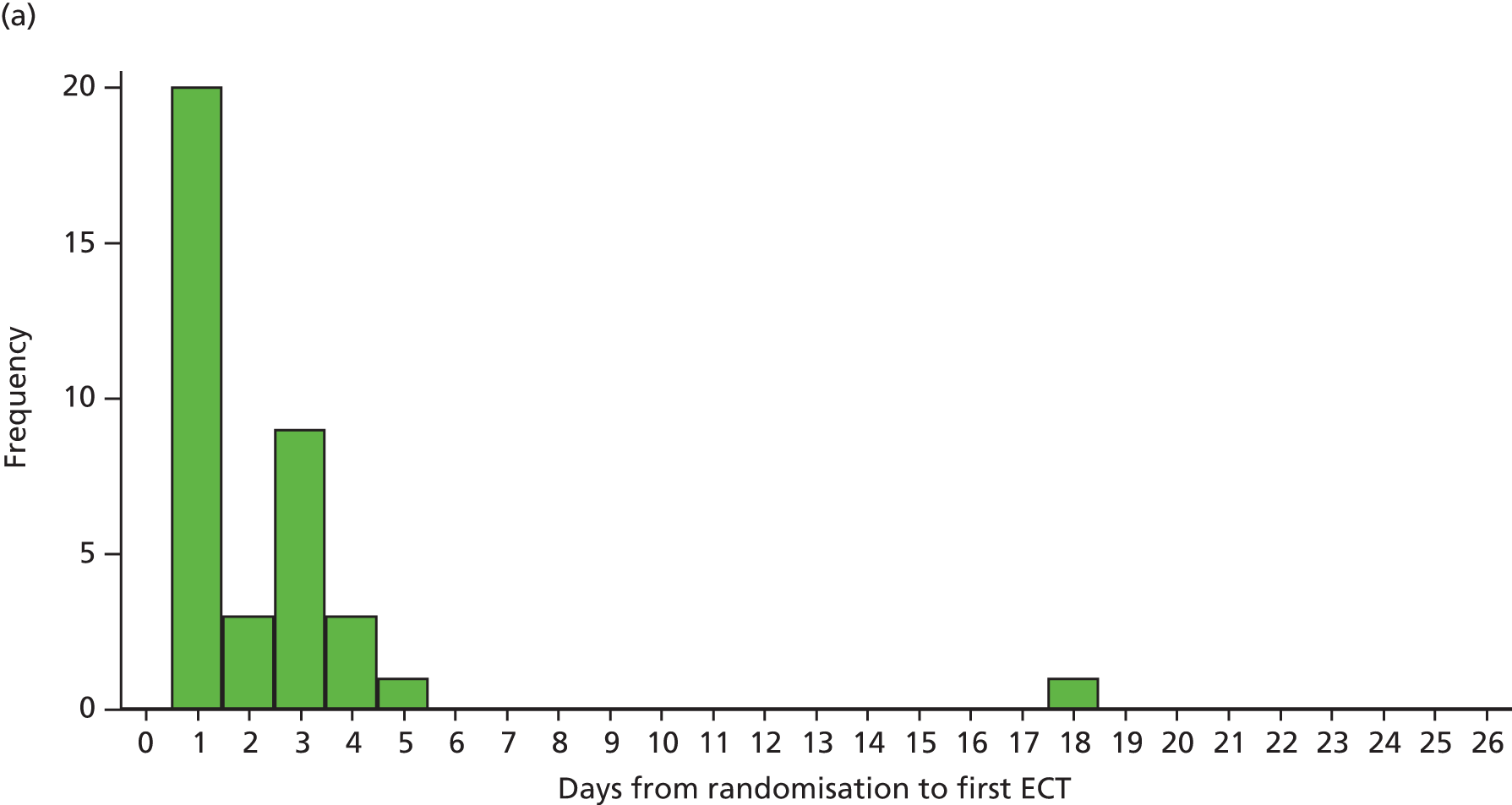

| Time point | Treatment arm | |||
|---|---|---|---|---|
| Saline | Ketamine | |||
| Mean (SD) | n | Mean (SD) | n | |
| Baseline | 45.5 (15.8) | 37 | 46.2 (16.1) | 33 |
| Mid-ECT | 39.1 (14.5) | 36 | 40.9 (16.0) | 29 |
| End of treatment | 36.9 (13.8) | 32 | 35.1 (15.8) | 25 |
| Follow-up 1 | 37.9 (14.8) | 23 | 36.5 (16.5) | 23 |
| Follow-up 2 | 39.4 (13.8) | 16 | 38.2 (16.9) | 19 |
| Time point | Treatment effecta | 95% CI | p-value |
|---|---|---|---|
| Mid-ECT | –0.41 | –2.89 to 2.06 | 0.744 |
| End of treatment | –0.30 | –3.42 to 2.81 | 0.849 |
| Follow-up 1 | –1.77 | –4.91 to 1.38 | 0.271 |
| Follow-up 2 | –0.90 | –4.16 to 2.37 | 0.590 |
| Outcome measure | Treatment arm | |||
|---|---|---|---|---|
| Saline | Ketamine | |||
| Mean (SD) | n | Mean (SD) | n | |
| MADRS | ||||
| Baseline | 35.2 (8.4) | 37 | 31.8 (7.4) | 33 |
| 1 | 30.1 (10.5) | 34 | 29.2 (7.7) | 33 |
| 2 | 25.9 (12.4) | 33 | 25.4 (9.8) | 31 |
| 3 | 22.9 (11.9) | 34 | 23.2 (10.2) | 29 |
| 4 | 21.8 (9.5) | 28 | 24.5 (10.5) | 28 |
| 5 | 23.7 (9.0) | 25 | 21.0 (11.8) | 24 |
| 6 | 19.7 (11.3) | 22 | 20.1 (11.7) | 19 |
| 7 | 23.5 (6.3) | 11 | 22.0 (9.2) | 11 |
| 8 | 21.6 (5.5) | 11 | 22.3 (9.3) | 6 |
| 9 | 20.8 (4.7) | 8 | 24.0 (4.5) | 4 |
| 10 | 20.3 (5.2) | 4 | 19.0 (10.4) | 3 |
| 11 | 22.0 | 1 | ||
| 12 | 14.0 | 1 | ||
| 13 | 16.0 | 1 | ||
| End of treatment | 15.0 (10.4) | 32 | 17.2 (11.6) | 27 |
| Follow-up 1 | 14.8 (11.4) | 23 | 16.8 (13.6) | 24 |
| Follow-up 2 | 13.5 (13.9) | 18 | 18.0 (13.3) | 19 |
| MADRS atypical | ||||
| Baseline | 36.5 (8.3) | 37 | 33.0 (6.8) | 33 |
| 1 | 31.0 (10.2) | 34 | 30.1 (7.8) | 33 |
| 2 | 27.1 (12.4) | 33 | 26.4 (9.9) | 31 |
| 3 | 24.1 (12.3) | 34 | 24.0 (10.2) | 29 |
| 4 | 22.9 (9.9) | 28 | 25.1 (10.5) | 28 |
| 5 | 24.6 (9.2) | 25 | 22.1 (12.1) | 24 |
| 6 | 20.6 (11.3) | 22 | 21.3 (11.7) | 19 |
| 7 | 24.1 (6.9) | 11 | 23.2 (8.9) | 11 |
| 8 | 22.2 (5.3) | 11 | 23.3 (9.2) | 6 |
| 9 | 22.1 (4.5) | 8 | 25.0 (5.2) | 4 |
| 10 | 20.8 (4.4) | 4 | 20.3 (11.9) | 3 |
| 11 | 22.0 | 1 | ||
| 12 | 14.0 | 1 | ||
| 13 | 16.0 | 1 | ||
| End of treatment | 16.0 (10.6) | 32 | 18.0 (11.7) | 27 |
| Follow-up 1 | 15.5 (11.7) | 23 | 17.6 (13.9) | 24 |
| Follow-up 2 | 14.2 (14.7) | 18 | 18.7 (13.1) | 19 |
| CAS-6 | ||||
| Baseline | 9.16 (5.23) | 37 | 7.79 (4.53) | 33 |
| 1 | 7.88 (5.78) | 34 | 8.03 (4.75) | 33 |
| 2 | 7.48 (5.37) | 33 | 7.39 (5.29) | 31 |
| 3 | 7.15 (5.40) | 34 | 6.59 (4.18) | 29 |
| 4 | 6.18 (3.76) | 28 | 7.00 (4.67) | 28 |
| 5 | 7.08 (3.90) | 25 | 5.83 (3.86) | 24 |
| 6 | 5.95 (3.98) | 22 | 5.53 (3.78) | 19 |
| 7 | 5.91 (3.53) | 11 | 6.18 (4.45) | 11 |
| 8 | 5.91 (3.96) | 11 | 6.67 (5.43) | 6 |
| 9 | 8.00 (4.72) | 8 | 6.25 (4.79) | 4 |
| 10 | 6.75 (6.40) | 4 | 5.67 (6.35) | 3 |
| 11 | 0.00 | 1 | ||
| 12 | 0.00 | 1 | ||
| 13 | 0.00 | 1 | ||
| End of treatment | 5.16 (4.34) | 32 | 5.44 (4.04) | 27 |
| Follow-up 1 | 4.43 (3.89) | 23 | 4.96 (4.62) | 24 |
| Follow-up 2 | 3.67 (4.92) | 18 | 4.95 (4.73) | 19 |
| CAS-7 | ||||
| Baseline | 10.27 (5.72) | 37 | 8.73 (5.09) | 33 |
| 1 | 8.94 (6.63) | 34 | 9.00 (5.52) | 33 |
| 2 | 8.24 (5.91) | 33 | 8.06 (5.95) | 31 |
| 3 | 8.03 (5.91) | 34 | 7.48 (4.77) | 29 |
| 4 | 6.61 (4.26) | 28 | 7.68 (5.25) | 28 |
| 5 | 7.84 (4.25) | 25 | 6.58 (4.48) | 24 |
| 6 | 6.45 (4.23) | 22 | 6.16 (4.48) | 19 |
| 7 | 6.45 (3.59) | 11 | 6.73 (5.04) | 11 |
| 8 | 6.91 (4.64) | 11 | 6.83 (5.71) | 6 |
| 9 | 9.50 (5.04) | 8 | 6.25 (4.79) | 4 |
| 10 | 7.25 (6.13) | 4 | 7.00 (7.81) | 3 |
| 11 | 2.00 | 1 | ||
| 12 | 2.00 | 1 | ||
| 13 | 2.00 | 1 | ||
| End of treatment | 5.84 (5.02) | 32 | 5.96 (4.43) | 27 |
| Follow-up 1 | 4.91 (4.18) | 23 | 5.67 (5.41) | 24 |
| Follow-up 2 | 4.17 (5.19) | 18 | 5.37 (5.27) | 19 |
| BPRS-19 | ||||
| Baseline | 40.0 (8.6) | 37 | 37.3 (5.4) | 33 |
| 1 | 36.9 (8.3) | 34 | 37.2 (6.1) | 33 |
| 2 | 35.4 (8.5) | 33 | 35.1 (6.9) | 31 |
| 3 | 33.5 (7.0) | 34 | 33.2 (6.3) | 29 |
| 4 | 32.2 (6.5) | 28 | 33.0 (6.9) | 28 |
| 5 | 32.8 (5.4) | 25 | 31.4 (7.5) | 24 |
| 6 | 30.4 (6.4) | 22 | 31.6 (7.8) | 19 |
| 7 | 31.5 (5.0) | 11 | 32.9 (7.0) | 11 |
| 8 | 32.0 (3.5) | 11 | 32.7 (3.6) | 6 |
| 9 | 33.3 (5.1) | 8 | 32.3 (4.0) | 4 |
| 10 | 31.8 (5.3) | 4 | 29.3 (5.5) | 3 |
| 11 | 33.0 | 1 | ||
| 12 | 31.0 | 1 | ||
| 13 | 32.0 | 1 | ||
| End of treatment | 28.3 (6.1) | 32 | 29.6 (6.9) | 27 |
| Follow-up 1 | 27.6 (6.4) | 23 | 28.8 (7.8) | 24 |
| Follow-up 2 | 27.1 (8.3) | 18 | 30.3 (8.3) | 19 |
| BPRS psychosis items | ||||
| Base | 14.4 (4.8) | 37 | 13.1 (3.7) | 33 |
| 1 | 13.0 (4.2) | 34 | 13.4 (3.9) | 33 |
| 2 | 12.7 (3.6) | 33 | 12.5 (4.1) | 31 |
| 3 | 11.9 (3.3) | 34 | 12.0 (3.2) | 29 |
| 4 | 10.9 (3.1) | 28 | 11.5 (3.5) | 28 |
| 5 | 11.1 (2.7) | 25 | 11.4 (4.7) | 24 |
| 6 | 10.5 (2.5) | 22 | 11.6 (3.6) | 19 |
| 7 | 10.7 (2.3) | 11 | 11.7 (2.6) | 11 |
| 8 | 11.5 (2.5) | 11 | 11.8 (1.0) | 6 |
| 9 | 11.5 (2.5) | 8 | 11.5 (1.3) | 4 |
| 10 | 11.8 (3.0) | 4 | 9.7 (1.5) | 3 |
| 11 | 14.0 | 1 | ||
| 12 | 14.0 | 1 | ||
| 13 | 14.0 | 1 | ||
| End of treatment | 10.1 (1.9) | 32 | 10.3 (2.7) | 27 |
| Follow-up 1 | 10.0 (2.6) | 23 | 10.7 (3.3) | 24 |
| Follow-up 2 | 9.8 (2.9) | 18 | 11.2 (3.8) | 19 |
| BPRS mania items | ||||
| Baseline | 4.22 (0.42) | 37 | 4.52 (1.00) | 33 |
| 1 | 4.26 (0.57) | 34 | 4.70 (1.13) | 33 |
| 2 | 4.42 (0.79) | 33 | 4.68 (0.91) | 31 |
| 3 | 4.21 (0.48) | 34 | 4.38 (0.68) | 29 |
| 4 | 4.36 (0.68) | 28 | 4.50 (0.75) | 28 |
| 5 | 4.32 (0.75) | 25 | 4.63 (1.88) | 24 |
| 6 | 4.18 (0.50) | 22 | 4.42 (0.84) | 19 |
| 7 | 4.18 (0.40) | 11 | 4.18 (0.40) | 11 |
| 8 | 4.45 (0.69) | 11 | 4.50 (0.84) | 6 |
| 9 | 4.13 (0.35) | 8 | 4.25 (0.50) | 4 |
| 10 | 4.00 (0.00) | 4 | 4.33 (0.58) | 3 |
| 11 | 4.00 | 1 | ||
| 12 | 4.00 | 1 | ||
| 13 | 4.00 | 1 | ||
| End of treatment | 4.25 (0.57) | 32 | 4.41 (0.64) | 27 |
| Follow-up 1 | 4.09 (0.29) | 23 | 4.58 (1.47) | 24 |
| Follow-up 2 | 4.61 (1.69) | 18 | 4.37 (0.68) | 19 |
| CGI-S | ||||
| Baseline | 5.30 (0.97) | 37 | 5.03 (0.85) | 33 |
| 1 | 4.76 (1.21) | 34 | 4.97 (0.92) | 33 |
| 2 | 4.24 (1.35) | 33 | 4.48 (1.12) | 31 |
| 3 | 4.24 (1.33) | 34 | 4.17 (1.28) | 29 |
| 4 | 4.03 (1.15) | 29 | 4.21 (1.10) | 28 |
| 5 | 4.00 (0.82) | 25 | 3.71 (1.52) | 24 |
| 6 | 3.45 (1.41) | 22 | 3.79 (1.40) | 19 |
| 7 | 4.18 (0.98) | 11 | 4.00 (1.00) | 11 |
| 8 | 4.18 (0.98) | 11 | 4.00 (0.89) | 6 |
| 9 | 3.88 (0.83) | 8 | 4.25 (0.50) | 4 |
| 10 | 4.00 (0.82) | 4 | 3.67 (1.53) | 3 |
| 11 | 4.00 | 1 | ||
| 12 | 4.00 | 1 | ||
| 13 | 4.00 | 1 | ||
| End of treatment | 2.88 (1.24) | 32 | 3.33 (1.33) | 27 |
| Follow-up 1 | 2.57 (1.27) | 23 | 2.79 (1.56) | 24 |
| Follow-up 2 | 2.17 (1.34) | 18 | 3.00 (1.60) | 19 |
| CGI-I | ||||
| 1 | 3.52 (0.87) | 33 | 3.71 (0.90) | 31 |
| 2 | 3.00 (1.09) | 33 | 3.03 (1.02) | 31 |
| 3 | 3.12 (1.07) | 34 | 2.83 (1.04) | 29 |
| 4 | 2.90 (0.98) | 29 | 2.93 (1.21) | 28 |
| 5 | 3.12 (1.13) | 25 | 2.78 (1.44) | 23 |
| 6 | 2.52 (1.17) | 21 | 2.74 (1.24) | 19 |
| 7 | 3.36 (0.92) | 11 | 3.00 (1.26) | 11 |
| 8 | 3.27 (1.42) | 11 | 3.33 (1.21) | 6 |
| 9 | 3.25 (1.16) | 8 | 3.33 (1.53) | 3 |
| 10 | 3.25 (1.26) | 4 | 3.33 (2.08) | 3 |
| 11 | 3.00 | 1 | ||
| 12 | 3.00 | 1 | ||
| 13 | 3.00 | 1 | ||
| End of treatment | 2.25 (1.11) | 32 | 2.52 (1.31) | 27 |
| Follow-up 1 | 2.00 (1.04) | 23 | 2.13 (1.26) | 24 |
| Follow-up 2 | 2.11 (1.41) | 18 | 2.42 (1.35) | 19 |
| QIDS-SR | ||||
| Baseline | 22.4 (2.6) | 37 | 21.0 (3.9) | 33 |
| 1 | 20.5 (4.0) | 33 | 19.4 (4.2) | 33 |
| 2 | 19.5 (4.7) | 33 | 18.0 (4.4) | 31 |
| 3 | 19.1 (4.3) | 34 | 17.3 (4.8) | 29 |
| 4 | 18.2 (4.6) | 27 | 17.6 (4.5) | 28 |
| 5 | 19.4 (4.0) | 25 | 17.7 (4.7) | 24 |
| 6 | 17.5 (4.6) | 22 | 16.3 (5.0) | 19 |
| 7 | 19.5 (2.8) | 10 | 16.5 (3.8) | 11 |
| 8 | 18.4 (4.4) | 11 | 18.2 (5.4) | 6 |
| 9 | 19.0 (3.2) | 8 | 18.8 (2.2) | 4 |
| 10 | 19.0 (2.9) | 4 | 19.0 (4.6) | 3 |
| 11 | 18 | 1 | ||
| 12 | 18 | 1 | ||
| 13 | 17 | 1 | ||
| End of treatment | 15.6 (4.8) | 32 | 16.3 (4.7) | 27 |
| Follow-up 1 | 15.4 (5.1) | 23 | 16.5 (5.9) | 24 |
| Follow-up 2 | 14.6 (6.0) | 18 | 16.9 (6.0) | 19 |
| Number of ECT sessions received at point of first remission | ||||
| By end of treatment | 7.0 (3.6) | 13 | 10.0 (4.7) | 13 |
| By end of follow-up | 8.3 (4.2) | 18 | 10.1 (4.5) | 14 |
| EQ-5D-3L index | ||||
| Baseline | 0.351 (0.275) | 36 | 0.349 (0.268) | 33 |
| 1 | 0.395 (0.346) | 33 | 0.475 (0.318) | 33 |
| 2 | 0.437 (0.383) | 33 | 0.553 (0.276) | 31 |
| 3 | 0.511 (0.389) | 34 | 0.624 (0.262) | 29 |
| 4 | 0.509 (0.361) | 27 | 0.573 (0.269) | 28 |
| 5 | 0.498 (0.329) | 25 | 0.634 (0.275) | 24 |
| 6 | 0.556 (0.361) | 22 | 0.653 (0.255) | 19 |
| 7 | 0.463 (0.335) | 10 | 0.667 (0.230) | 11 |
| 8 | 0.450 (0.379) | 11 | 0.580 (0.291) | 6 |
| 9 | 0.489 (0.360) | 8 | 0.691 (0.222) | 4 |
| 10 | 0.398 (0.490) | 4 | 0.494 (0.313) | 3 |
| 11 | 0.310 | 1 | ||
| 12 | 0.274 | 1 | ||
| 13 | 0.708 | 1 | ||
| End of treatment | 0.695 (0.272) | 32 | 0.656 (0.256) | 27 |
| Follow-up 1 | 0.711 (0.290) | 23 | 0.669 (0.330) | 24 |
| Follow-up 2 | 0.709 (0.340) | 18 | 0.598 (0.332) | 19 |
| EQ-5D-3L VAS | ||||
| Baseline | 24.2 (16.9) | 36 | 31.9 (14.6) | 32 |
| 1 | 29.1 (21.6) | 33 | 32.6 (14.6) | 33 |
| 2 | 33.6 (22.2) | 33 | 42.0 (18.6) | 31 |
| 3 | 37.9 (22.0) | 34 | 42.6 (17.4) | 29 |
| 4 | 38.9 (22.9) | 27 | 38.3 (20.2) | 28 |
| 5 | 33.6 (21.6) | 25 | 43.5 (20.3) | 24 |
| 6 | 45.3 (22.7) | 22 | 47.8 (21.5) | 19 |
| 7 | 40.1 (13.1) | 10 | 46.3 (21.2) | 11 |
| 8 | 36.6 (18.3) | 11 | 41.7 (30.8) | 6 |
| 9 | 45.0 (15.4) | 8 | 31.3 (19.3) | 4 |
| 10 | 41.0 (11.6) | 4 | 35.0 (26.5) | 3 |
| 11 | 40.0 | 1 | ||
| 12 | 80.0 | 1 | ||
| 13 | 60.0 | 1 | ||
| End of treatment | 52.2 (21.9) | 32 | 51.4 (22.3) | 27 |
| Follow-up 1 | 54.8 (30.2) | 23 | 53.0 (26.4) | 24 |
| Follow-up 2 | 62.4 (31.1) | 18 | 46.2 (24.6) | 19 |
| Outcome measure | Treatment effecta | 95% CI | p-value |
|---|---|---|---|
| MADRS atypical | 0.42 | –1.03 to 1.86 | 0.57 |
| CAS-7 | 0.0001 | –0.42 to 0.43 | 0.99 |
| BPRS psychosis items | –0.12 | –0.42 to 0.17 | 0.41 |
| BPRS mania items | –0.05 | –0.12 to 0.03 | 0.20 |
| Week | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | |||||
| n | Cumulative | n | Cumulative | |||
| n | % | n | % | |||
| 1 | 1 | 1 | 2.7 | 0 | 0 | 0.0 |
| 2 | 5 | 6 | 16.2 | 2 | 2 | 6.1 |
| 3 | 0 | 6 | 16.2 | 1 | 3 | 9.1 |
| 4 | 3 | 9 | 24.3 | 2 | 5 | 15.2 |
| 5 | 1 | 10 | 27.0 | 2 | 7 | 21.2 |
| 6 | 3 | 13 | 35.1 | 3 | 10 | 30.3 |
| 7 | 0 | 13 | 35.1 | 1 | 11 | 33.3 |
| 8 | 0 | 13 | 35.1 | 1 | 12 | 36.4 |
| 9 | 0 | 13 | 35.1 | 0 | 12 | 36.4 |
| 10 | 0 | 13 | 35.1 | 1 | 13 | 39.4 |
| Week | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Saline (n = 37) | Ketamine (n = 33) | |||||
| n | Cumulative | n | Cumulative | |||
| n | % | n | % | |||
| 1 | 2 | 2 | 5.4 | 1 | 1 | 3.0 |
| 2 | 5 | 7 | 18.9 | 2 | 3 | 9.1 |
| 3 | 6 | 13 | 35.1 | 5 | 8 | 24.2 |
| 4 | 2 | 15 | 40.5 | 2 | 10 | 30.3 |
| 5 | 4 | 19 | 51.4 | 3 | 13 | 39.4 |
| 6 | 3 | 22 | 59.5 | 0 | 13 | 39.4 |
| 7 | 0 | 22 | 59.5 | 2 | 15 | 45.5 |
| 8 | 0 | 22 | 59.5 | 0 | 15 | 45.5 |
| 9 | 0 | 22 | 59.5 | 1 | 16 | 48.5 |
| Assessment | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Ketamine | |||||||
| Guess saline | Guess ketamine | Guess saline | Guess ketamine | |||||
| n | % | n | % | n | % | n | % | |
| Accuracy | ||||||||
| RA completed | ||||||||
| Mid-ECT | 18 | 56.3 | 14 | 43.7 | 14 | 45.2 | 17 | 54.8 |
| End of treatment | 18 | 62.1 | 11 | 37.9 | 16 | 61.5 | 10 | 38.5 |
| Patient completed | ||||||||
| Mid-ECT | 14 | 43.8 | 18 | 56.2 | 12 | 46.2 | 14 | 53.8 |
| End of treatment | 14 | 46.7 | 16 | 53.3 | 8 | 33.3 | 16 | 66.7 |
| Mid-ECT | End of treatment | Mid-ECT | End of treatment | |||||
| n | % | n | % | n | % | n | % | |
| Certainty | ||||||||
| RA completed | ||||||||
| Pure guess | 24 | 75.0 | 18 | 62.1 | 19 | 61.3 | 19 | 73.1 |
| Slight suspicion | 7 | 21.9 | 10 | 34.5 | 8 | 25.8 | 4 | 15.4 |
| Moderately certain | 1 | 3.1 | 1 | 3.4 | 4 | 12.9 | 3 | 11.5 |
| Very certain | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 32 | 100.0 | 29 | 100.0 | 31 | 100.0 | 26 | 100.0 |
| Patient completed | ||||||||
| Pure guess | 16 | 50.0 | 16 | 53.3 | 15 | 57.7 | 14 | 58.3 |
| Slight suspicion | 10 | 31.3 | 11 | 36.7 | 7 | 26.9 | 8 | 33.3 |
| Moderately certain | 5 | 15.6 | 3 | 10.0 | 3 | 11.5 | 2 | 8.3 |
| Very certain | 1 | 3.1 | 0 | 0.0 | 1 | 3.8 | 0 | 0.0 |
| Total | 32 | 100.0 | 30 | 100.0 | 26 | 100.0 | 24 | 100.0 |
| Reason for choice | ||||||||
| RA completed | ||||||||
| No reason | 19 | 61.3 | 18 | 62.1 | 18 | 58.1 | 18 | 69.2 |
| Improvement | 7 | 22.6 | 3 | 10.3 | 2 | 6.5 | 4 | 15.4 |
| Cognitive impairment | 5 | 16.1 | 7 | 24.1 | 5 | 16.1 | 2 | 7.7 |
| Confusion | 0 | 0.0 | 0 | 0.0 | 4 | 12.9 | 0 | 0.0 |
| Revealed | 0 | 0.0 | 1 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| Other | 0 | 0.0 | 0 | 0.0 | 2 | 6.5 | 2 | 7.7 |
| Total | 31a | 100.0 | 29 | 100.0 | 31 | 100.0 | 26 | 100.0 |
| Patient completed | ||||||||
| No reason | 17 | 53.1 | 17 | 56.7 | 14 | 56.0 | 14 | 58.3 |
| Improvement | 2 | 6.3 | 4 | 13.3 | 0 | 0.0 | 2 | 8.3 |
| Cognitive impairment | 4 | 12.5 | 6 | 20.0 | 4 | 16.0 | 1 | 4.2 |
| Confusion | 5 | 15.6 | 2 | 6.7 | 5 | 20.0 | 4 | 16.7 |
| Revealed | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Other | 3 | 9.4 | 1 | 3.3 | 2 | 8.0 | 3 | 12.5 |
| Total | 32 | 100.0 | 30 | 100.0 | 25a | 100.0 | 24 | 100.0 |
Appendix 3 Telephone survey following participation
List of abbreviations
- ACC
- anterior cingulate cortex
- AE
- adverse event
- AMI-SF
- Autobiographical Memory Interview – Short Form
- AMPA
- α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- ANCOVA
- analysis of covariance
- ANOVA
- analysis of variance
- AR
- adverse reaction
- AUC
- area under the curve
- BA
- Brodmann area
- BOLD
- blood oxygen level dependent
- BPRS
- Brief Psychiatric Rating Scale
- CAS
- Clinical Anxiety Scale
- CGI-I
- Clinical Global Impression-Improvement
- CGI-S
- Clinical Global Impression-Severity
- CI
- confidence interval
- COWAT
- Controlled Oral Word Association Test
- CTU
- Clinical Trials Unit
- DMEC
- data monitoring and ethics committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- ECG
- electrocardiogram
- ECT
- electroconvulsive therapy
- EEG
- electroencephalogram
- EME
- Efficacy and Mechanism Evaluation
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- ES
- effect size
- fMRI
- functional magnetic resonance imaging
- fNIRS
- functional near-infrared spectroscopy
- GSE-My
- Global Self-Evaluation of Memory
- Hbdiff
- difference between oxyhaemoglobin and deoxyhaemoglobin values
- HbO
- oxyhaemoglobin
- HbR
- deoxyhaemoglobin
- HC
- healthy control subject
- HVLT-R
- Hopkins Verbal Learning Test – Revised
- IFG
- inferior frontal gyrus
- IQ
- intelligence quotient
- IQR
- interquartile range
- ITT
- intention to treat
- LDF
- left dorsolateral prefrontal
- LVF
- left ventrolateral prefrontal
- MADRS
- Montgomery–Åsberg Depression Rating Scale
- MCGCFT
- Medical College of Georgia Complex Figure Test
- MedDRA
- Medical Dictionary for Regulatory Activities
- MGHS
- Massachusetts General Hospital Scale
- MHA
- Mental Health Act
- MHRA
- Medicines and Healthcare products Regulatory Agency
- mid-ECT
- after four ECT treatments (primary assessment time point)
- MINI
- Mini International Neuropsychiatric Interview
- mITT
- modified intention to treat
- MMSE
- Mini Mental State Examination
- MRI
- magnetic resonance imaging
- MRS
- magnetic resonance spectroscopy
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIRS
- near-infrared spectroscopy
- NMDA
- N-methyl-D-aspartate
- QIDS-SR
- Quick Inventory of Depressive Symptomatology – Self-Report
- RA
- research assistant
- RCPsych
- Royal College of Psychiatrists
- RCT
- randomised controlled trial
- RDF
- right dorsolateral prefrontal
- RUL
- right unilateral
- RVF
- right ventrolateral prefrontal
- SAE
- severe adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SEAN
- Scottish ECT Accreditation Network
- SEM
- standard error of the mean
- SPSS
- Statistical Product and Service Solutions
- STAR*D
- Sequenced Treatment Alternatives to Relieve Depression
- TOI
- tissue oxygenation index
- TSC
- trial steering committee
- VAS
- visual analogue scale
- VF
- verbal fluency
- WM
- working memory