Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/1023/10. The contractual start date was in February 2013. The final report began editorial review in May 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Sahota et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background: transition care theories
The transition of care from hospital back into the community is a vulnerable stage in patient care and recovery. As acute hospital care ends and follow-up care in the community commences, the continuity of patient care can often become disrupted and ‘de-coupled’. This can lead to difficulties in sharing relevant knowledge or information about a patient at a given time, thereby leading to poorly integrated care planning and limited rehabilitation. This highlights the importance of timely, accurate and relevant communication or, more precisely, the ‘transition of care’ among different care providers. Transition of care involves more than the communication of information; rather, it is the exchange and use of meanings, assumptions, practices and know-how to engender shared understandings and collaborative practices. There is growing evidence that persistent occupational and organisational boundaries, especially between acute hospital care and the community, can hinder integrated care at the time of hospital discharge. For example, acute and community services are often characterised by different ways of working, team and resource configurations, care philosophies and service cultures, and ways of organising care. More significantly, it is often the case that those working in the community setting are not always involved in discharge planning, which can mean that early rehabilitation becomes delayed or community teams experience an initial ‘reactive’ phase in which they rapidly adjust or reformulate care plans.
A related area of theory elaborates how professional boundaries can influence the integration of different care sectors and organisations. The sociology of professions literature shows how expert occupations, such as medicine and nursing, are defined by well-established social, cultural and legal boundaries that determine their areas of expertise, responsibilities and remit of practice. These boundaries are historical in character and are linked to wider (societal) factors, including policy-making and professional associations, as well as more localised and everyday practices, including the customs of interaction and supervision between occupational groups. In many respects, this division appears settled with relatively permanent boundaries and hierarchies; for example, it is typically argued that medicine remains dominant in the clinical division of labour and there is a division of responsibility between acute and community care.
The boundaries and jurisdictions between health-care professions and organisations can also inhibit new or more integrated ways of working when the division of responsibilities between groups needs to be redrawn to better address the needs of patients. Closer examination of these boundaries reveals many points of contact and conflict, especially when new or extended roles are introduced and when the division of responsibilities is redrawn to better address the needs of patients. For example, many service innovations and new technologies extend the jurisdiction of one profession into the realm of another or bring into tension-established boundaries1,2 suggests that major workforce changes witnessed over the last 20 years have significantly transformed the boundaries between health-care professions, including new forms of specialisation, diversification, and forms delegation and substitution. For example, doctors readily delegate more routine work to other professions if it enables more specialisation within their given field. These debates are significant in the context of more integrated working between acute and community care, because they highlight the potential for established professional and sectoral boundaries to be redrawn and negotiated.
A ‘transitional care strategy’ is an intervention or a group of interventions initiated prior to hospital discharge with the aim of ensuring the safe and effective transition of patients from one setting to another. Hospital-based transitional care interventions aim to smooth the transition from secondary (hospital) to primary (community) care, avoid adverse events and prevent unnecessary readmissions back to hospital. In a Cochrane review by Shepperd et al. ,3 a structured discharge plan tailored to the individual patient was shown to bring about small reductions in hospital length of stay (LOS) and readmission rates for older people admitted to hospital with a medical condition. Twenty-one randomised controlled trials (RCTs) (7234 patients) were included. Fourteen trials recruited patients with a medical condition (4509 patients), four recruited patients with a mix of medical and surgical conditions (2225 patients), one recruited patients from a psychiatric hospital (343 patients), one recruited patients from both a psychiatric hospital and a general hospital (97 patients) and one recruited patients admitted to hospital following a fall (60 patients). Hospital LOS and readmissions to hospital were significantly reduced for patients allocated to discharge planning [mean difference in LOS –0.91 days, 95% confidence interval (CI) –1.55 to –0.27; readmission rates relative risk (RR) 0.85, 95% CI 0.74 to 0.97; 11 trials]. For elderly patients with a medical condition (usually heart failure) there was insufficient evidence of a difference in mortality (RR 1.04, 95% CI 0.74 to 1.46; four trials) or hospital LOS (RR 1.03, 95% CI 0.93 to 1.14; two trials). This was also the case for trials recruiting patients recovering from surgery and a mix of medical and surgical conditions. In three trials patients allocated to discharge planning reported increased satisfaction.
In another systematic review and meta-analysis of RCTs of inpatient rehabilitation specifically designed for geriatric patients,4 17 trials (4780 patients) comparing the effects of general or orthopaedic geriatric rehabilitation programmes with usual care were included. Compared with those in the control groups, the weighted mean length of hospital stay after randomisation was longer in patients allocated to general geriatric rehabilitation (24.5 vs. 15.1 days). Meta-analyses of effects indicated an overall beneficial effect on all outcomes at discharge [odds ratio 1.75 (95% CI 1.31 to 2.35) for function, RR 0.64 (95% CI 0.51 to 0.81) for nursing home admissions, RR 0.72 (95% CI 0.55 to 0.95) for mortality] and at the end of follow-up [odds ratio 1.36 (95% CI 1.07 to 1.71) for function, RR 0.84 (95% CI 0.72 to 0.99) for nursing home admissions, RR 0.87 (95% CI 0.77 to 0.97) for mortality]. Limited data were available on impact on health care or costs.
With respect to readmission, highly targeted disease-specific hospital-based transitional care strategies have shown modest success in reducing readmissions for chronic heart failure, chronic obstructive pulmonary disease and asthma, but little is known about effective transitional care strategies for frail older people, many of whom have multiple comorbidities. Four different types of transitional care strategies have been shown to be effective in reducing readmissions: Project Better Outcomes for Older adults through Safe Transitions (BOOST), Project Re-Engineered Discharge (RED), Care Transitions Intervention (CTI) and the Transitional Care Model (TCM). These programmes have several similarities and include bridging interventions with a dedicated transition provider, either a nurse or case manager, as the clinical leader. There is an over-riding emphasis on having an advocate to facilitate the co-ordination of care and outreach to patients following discharge from hospital.
Project Better Outcomes for Older adults through Safe Transitions
Project BOOST is a transitional care programme supported by the Society of Hospital Medicine. 5,6 This quality improvement collaborative has been implemented across the USA in different hospital settings, focusing on general medicine populations, both medical and surgical. Experts in the field of quality improvement and transitions in care facilitate the development and implementation of a BOOST site-specific programme that addresses the needs of each hospital.
The BOOST toolkit includes several interventions including risk assessment, a medication review, a discharge checklist and a multidisciplinary team-based approach to the discharge process. A recent study of 30 hospitals showed modest reductions (2–6%) in 30-day readmission rates. 5
Project Re-Engineered Discharge
Project RED is focused on a multidisciplinary approach to patient care co-ordinated by a nurse discharge advocate. 7 The discharge advocate engages patients during their admission to hospital and provides clinical information and an individualised and illustrated plan post discharge. Following discharge, a pharmacist performs the telephone follow-up including a medication review with direct communication to the primary outpatient provider. In a RCT there was a non-significant 6% reduction in the 30-day readmission rate and a significant 8% reduction in the 30-day visit to the accident and emergency (A&E) department post discharge. 7
Care Transitions Intervention
The CTI is a multicomponent programme designed to facilitate patient engagement and promote direct patient and caregiver involvement in self-management following hospitalisation, including providing the necessary skills to be able to navigate the health-care system. 8–11 There are four components of the CTI: (1) medication management, (2) development of a personal health record that is carried from site to site, (3) close follow-up with a primary care provider and (4) the identification of ‘red flags’ and indications that would prompt patients to contact providers. An advanced practice nurse ‘transition coach’ performs post-discharge home visits and makes telephone calls, emphasising patient engagement and self-management in the care of chronic diseases. The programme has been studied in several different acute care settings and has shown statistically significant reductions in 30-day readmission rates (4–6%)8–9,11 and 90-day readmission rates (6–22%). 9–10
The Transitional Care Model
The TCM is another nationally recognised transitional care programme and includes a strategy focusing on hospital-based discharge planning and home follow-up. 12,13 A transitional care nurse follows patients from hospital to home, facilitates communication with outpatient providers and performs a series of home visits and telephone follow-up calls in the post-discharge period. The TCM emphasises a multidisciplinary approach to patient care, led by the transitional care nurse who remains in contact with other providers including physicians, nurses, social workers, discharge planners and pharmacists. A reduction in the 90-day readmission rate of between 13% and 48% has been reported. 12,13
Report structure
Chapter 2 provides an introduction to the study and its relationship to current NHS policy. Chapter 3 describes the design and methods of the study and Chapter 4 describes the main RCT findings. Chapter 5 describes the qualitative study design and methods, followed by a summary of the main findings. Chapter 6 details the methods and results of the health economics analysis. Chapter 7 discusses the main findings and learning points from the study.
Chapter 2 Introduction
The number of people aged ≥ 75 years in the UK is expected to double by 2025, compared with a 12% growth in the overall population [see www.ons.gov.uk/ons/index.html (accessed 4 January 2016)]. The proportion of acute emergency medical admissions contributed to by this age group has seen a significant rise in the last 5 years from 9.5% to 14%14 and, with ageing trends, this is expected to increase significantly over the next 10 years. Compared with younger patients admitted to hospital, for older people the hospital LOS is much longer, the risk of hospital-acquired complications is much higher, discharge planning is more complex and 28-day readmission rates are much greater. 15
In some hospitals in the UK there have been significant reductions in hospital LOS but an increase in the 28-day readmission rate. Nationally, over the last 6 years the 28-day readmission rate has increased from 11% to 14%. 16 (DH, Emergency Admission Rates, 2008). More locally, in Nottingham the mean LOS across the medical elderly care wards over the period 2007–10 (five wards, 6924 patients) has decreased from 14 to 9 days through the use of ward discharge co-ordinators, but the 28-day readmission rate has increased from 14% to 19% (local audit 2011, unpublished data). The reasons for these readmissions are multifactorial, but an important component is the availability of appropriate resources in the community that are able to respond to the needs of these patients in a responsive manner. Patient safety is often compromised during this vulnerable period, with high rates of medication errors,17–20 incomplete or inaccurate information on transfer21 and lack of appropriate follow-up of care22 collectively leading to fragmented discharge planning and increased rates of recidivism to high-intensity care settings. 21
In England and Wales, to address the problem of rising readmission rates, the Department of Health has allocated £300M as part of the funding for reablement linked to hospital discharge funding stream [see www.gov.uk/government/uploads/system/uploads/attachment_data/file/215824/dh_123473.pdf (accessed 4 January 2016)]. This money was to be spent on developing local plans in conjunction with local authorities, foundation trusts/NHS trusts and community health services, to facilitate seamless care for patients on discharge from hospital and prevent avoidable hospital readmissions. Some Clinical Commissioning Groups (CCGs) have invested in early supported discharge at home schemes, some have invested in community-based rehabilitation schemes and some have invested very little at all. Reviews of the literature suggest that it is currently unclear which are the most effective and efficient structures and organisation of community/intermediate care services in relation to their purpose. 2,14 However, a recent retrospective review of discharges for stroke and other cerebrovascular diseases from a single academic medical centre found that 53% of the readmissions were potentially avoidable and reported that gaps in care co-ordination, lack of timely follow-up of care and inadequate instructions at discharge were the main problem areas. 23
To address this problem we undertook a structured literature review and identified systematic and Cochrane reviews of early supported discharge, discharge planning from hospital to home and care transition interventions. 3–4,9,18,23–27 The key components of successful service models included (1) more intensive rehabilitation; (2) working more closely with the patient and his or her relatives; and (3) bridging interventions with a dedicated transition provider, either a nurse or a case manager, as the clinical leader, with an over-riding emphasis on having an advocate to facilitate co-ordination of care and outreach to patients following discharge from hospital.
Our review was followed by a series of multiperspective focus group meetings with service users, experienced health-care professionals and service managers and led to the development of the Community In-reach Rehabilitation And Care Transition (CIRACT) service [consisting of a senior occupational therapist (transition coach), senior physiotherapist and assistant practitioner], linked directly to a social services practitioner and working more closely across multiple boundaries with patients and their carers. The CIRACT service was set apart from other models of community care because, although the CIRACT team was employed by a community NHS provider (NHS Nottingham CityCare Partnership), it was based on the hospital ward. By working across these boundaries, it was able to provide earlier contact with patients while they were still in hospital, assess care needs, work with hospital specialists in a more integrated way and, by staying with patients following discharge, follow them up in the community and facilitate community rehabilitation and personal care as needed.
Elaborating the theory of this model further, there is an underlying assumption that acute and community services are de-coupled or separated by occupational and organisational boundaries. By colocating community teams within the acute setting these boundaries can be mediated based on routine work interaction or functional proximity. This can in turn lead to enhanced knowledge sharing and mutual understanding of where community therapists bring, into the hospital setting, specialist information and understanding about community rehabilitation, the availability of community-based services and a profile of service demands and expectations within the community. At the same time, acute care teams are able to share knowledge about the organisation and configuration of hospital services, the profile of demands and expectations and the broader organisation of care. The two-way flow of knowledge is thus over time able to support mutual understanding of the respective work processes and contributions of each service to patient care, which in turn can foster enhanced integration or co-ordination of work. This has the potential to enable community therapists to work earlier with hospital-based care teams in discharge and care planning, especially through developing more holistic, patient-centred, ‘community-ready’ care plans. It also enables community therapists to participate earlier in direct patient care, including interaction with hospital-based specialists and patients, to determine the appropriate package of care, commence rehabilitation earlier, ensure that longer-term rehabilitation aligns seamlessly with acute care and provide earlier education and support to patients and families.
A pilot (before and after) 4-month study comparing the CIRACT service with the standard hospital rehabilitation service across two CCGs (Rushcliffe CCG and CityCare CCG) demonstrated a trend towards a reduction in LOS and reduced readmission rates. 28 The aims of this research were therefore to evaluate the clinical effectiveness, microcosts and cost-effectiveness of the CIRACT service compared with the traditional hospital-based rehabilitation (THB-Rehab) service through a high-quality RCT.
The primary objective of the CIRACT trial was to assess whether or not length of hospital stay among people aged ≥ 70 years admitted to hospital as an acute medical admission was different for the CIRACT service compared with the THB-Rehab service.
The secondary objectives were to assess the effects of the CIRACT service compared with the THB-Rehab service on:
-
the readmission rate within 28 and 91 days post discharge
-
super spell bed-days (total time in NHS care) at day 91
-
functional ability at day 91
-
comorbidity at day 91
-
health-related quality of life at day 91
-
microcosts and cost-effectiveness.
A parallel qualitative appraisal was undertaken to provide an explanatory understanding of the organisation, delivery and experience of the CIRACT service from the perspective of key stakeholders and patients.
Chapter 3 Study design and methods
Trial design
This trial was a single-centre pragmatic RCT (1 : 1 allocation ratio) with an integral health economic study and parallel qualitative appraisal.
Participants
The participants in the trail were older people admitted to the general medical wards as an acute medical emergency.
Eligibility criteria
Patients were eligible for the study if all of the inclusion and none of the exclusion criteria were met:
-
inclusion criteria:
-
age ≥ 70 years
-
general practitioner (GP) registered within the Nottingham City CCG catchment area only (catchment population 300,000)
-
-
exclusion criteria:
-
bed bound prior to admission or moribund on admission
-
receiving palliative care
-
previously included in the trial on an earlier admission
-
unable to be screened and recruited by the research team within 36 hours of admission to the study ward (a 36-hour deadline ensured that there was not a delay in the participant receiving therapy and enabled the recruitment of a large proportion of patients admitted over a weekend when the research team was not available)
-
nursing home residents.
-
Study setting
General medical elderly care wards at the Queen’s Medical Centre, Nottingham (1800-bed hospital, serving a population of 680,000), with community follow-up.
Study intervention
The trial had two arms: (1) the CIRACT service (intervention arm) and (2) THB-Rehab (standard care arm).
-
The CIRACT service provided a comprehensive assessment of each participant’s ability to perform certain tasks, which was completed within 24 hours of randomisation, enabling the formulation of a rehabilitation plan. While in hospital the participants were treated daily (7 days a week if appropriate). During the hospital stay, the team liaised with each participant and his or her carer(s) to enable a visit to the participant’s home to assess and provide recommendations for equipment and make adaptations and/or modifications as required. The CIRACT service utilised the team’s expertise in community working to form links with the appropriate services to ensure a smooth and effective discharge. In more complex cases the CIRACT team took the participant out of the hospital for a home visit prior to discharge. Following discharge, the CIRACT team visited the participant at home within 48 hours to assess the level of rehabilitation required and further follow-up visits were provided as deemed necessary.
-
The THB-Rehab service was provided by the ward therapy teams (usually a band 6 occupational therapist and a band 6 physiotherapist) on weekdays only. The team jointly conducted an assessment of each participant’s ability to perform certain tasks and provided recommendation for rehabilitation. The service referred the participants to the appropriate community-based services for provision of equipment at home, personal care and ongoing rehabilitation when appropriate at discharge. Once discharged from hospital, participants had no direct contact with the THB-Rehab service.
In either group, if a participant became medically unwell at any point to the extent that he or she was no longer able to undertake rehabilitation activities, the treating team withheld further rehabilitation until instructed by the ward doctor that it was safe to recommence rehabilitation activities. The nursing and medical care provided by the ward staff did not differ between the two groups.
Primary outcome measure
The primary outcome was hospital LOS from randomisation to discharge from the acute medical elderly care ward.
Secondary outcome measures
The secondary outcome measures were:
-
Unplanned readmission rates at day 28 and day 91.
-
Super spell bed-days (total time in NHS care including hospital care and intermediate care) from admission to 91 days’ follow-up.
-
Functional ability at 91 days as assessed by the Barthel Activities of Daily Living (ADL) index. 29 This 10-item index is scored out of 20, with a score of 20 indicating the ability to get up and down stairs unaided and in and out of the bath or shower independently.
-
Health-related quality of life as measured by the European Quality of Life-5 Dimensions three-level version (EQ-5D-3L)30 at 91 days post discharge. The EQ-5D-3L is a standardized measure of quality of life including five domains – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – each with three levels.
-
Comorbidity as measured by the Charlson index31 at 91 days post discharge. The Charlson index codes a total of 22 comorbid conditions into a single score.
-
Mean cost per patient of the CIRACT and THB-Rehab services estimated using microcosting methods and cost-effectiveness analysis from a NHS and Personal Social Services (PSS) perspective, using data collected from a modified Client Service Receipt Inventory (CSRI) questionnaire,32 with quality-adjusted life-years (QALYs) at 91 days post discharge.
Data collection
The research team collected demographic data [including age, sex, Mini Mental State Examination (MMSE) score33 and living circumstances] and outcome measures at baseline at face-to-face interviews and follow-up data through established hospital and community databases and participant telephone interviews [Nottingham Information System (NOTIS) hospital database: admission/discharge date data; Community System One: contacts with other services and equipment provision data; telephone interviews: outcome measure data].
Data were collected using trial data collection forms, which were monitored by the Nottingham Clinical Trials Unit (NCTU) for consistency, validity and quality. Missing data and data queries were referred promptly back to the recruiting site for clarification. For participants who withdrew from the study, data were collected up to that point (as specified in the consent form), with no further data collected. Participants were not replaced when they withdrew from the study. All reasonable attempts were made to contact any participants lost to follow-up during the course of the trial to complete the assessments.
Data management
All trial data were entered into a trial-specific database, with participants identified only by their unique trial number, date of birth and initials. The database was developed and maintained by the NCTU. Access to the database was restricted and secure. Data quality and compliance with the protocol were assessed throughout the trial by verification of trial data against clinical records and by data checking for accuracy and internal consistency.
Sample size calculation
The primary statistical analysis was to compare LOS for those allocated to receive the CIRACT service compared with LOS for those allocated to the THB-Rehab service (see Appendix 1 for the statistical analysis plan). Pilot data28 showed the log-transformed LOS to be normally distributed with a standard deviation (SD) of 0.9. Therefore, 111 patients per arm were required to detect a clinically important effect size of 3 days (equivalent to a geometric mean ratio of 0.7) with a 5% two-sided alpha and 80% power. Allowing for 5% non-collection of primary outcome data, 250 patients in total were recruited over a 13-month recruitment period.
Recruitment and consent
All eligible patients were made aware of the trial at the time of admission to hospital and invited to participate. Written informed consent was obtained by the research team in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice [see www.ich.org/products/guidelines/quality/article/quality-guidelines.html (accessed 4 January 2016)]. For patients who were confused (who had dementia/delirium such that they were unable to understand the nature of consenting to a research study and the study process), consent was obtained from a carer following an established framework34 used in previous ethically approved studies in older persons with dementia.
Randomisation procedure
Once the research team had gained consent, patients were allocated to either the CIRACT service or the THB-Rehab service using the web-based randomisation service provided by the NCTU. Randomisation was determined by a computer-generated pseudorandom code using random permuted blocks of randomly varying size and held on a secure server. Participants were allocated with equal probability to either arm of the study. The randomisations were requested through a PC with Internet Explorer and internet access, located on a dedicated secure server within the University of Nottingham. All communications between the user’s PC and the server were fully encrypted (secure SSL 128 bit encrypted) and used a unique username and password.
Blinding
The research team collecting data and the research team analysing the data were blinded to treatment allocation. The participants and ward staff were not blinded to treatment allocation as the treating therapists liaised closely with ward staff to ensure optimal patient care. The 3-month follow-up data were collected by the research team blinded to the intervention.
Statistical analyses
Preliminary analyses
Preliminary analyses describing the proportions of participants who withdrew consent prior to discharge from hospital, died in hospital, were discharged from hospital and died post discharge from hospital were conducted.
Participants in the two trial arms were described separately with respect to age at inclusion, sex, Barthel ADL score, MMSE score, comorbidity scale and EQ-5D-3L health state score. Continuous data were summarised using mean, SD, median, lower and upper quartiles, minimum and maximum and number of observations. Categorical data were summarised using frequency counts and percentages.
Primary analysis
The primary analysis was hospital LOS for those who were discharged from hospital. Participants were analysed as randomised regardless of intervention received. The analysis was conducted using generalised linear regression modelling, with log-transformed LOS as a response. The primary effectiveness parameter was the LOS geometric mean ratio from admission to discharge between the two arms, along with the 95% CI and p-value.
Secondary analyses
We conducted the following additional analyses for the primary outcome:
-
including in the model participants in each arm who died prior to discharge, with a covariate specifying death or discharge
-
including in the model all randomised participants, with multiple imputation of missing LOS data
-
time to discharge analysed using Cox regression analysis including hospital death as a competing risk.
Secondary outcomes were analysed using appropriate generalised linear models, with choice of model and presentation of the estimated between-group effect dependent on outcome type (difference in means for normally distributed continuous outcomes, ratio of geometric means for log-normal continuous outcomes and risk differences for binary outcomes)
Qualitative appraisal
The parallel qualitative appraisal was concerned with understanding ‘how’ the CIRACT service:
-
was implemented, to develop evidence for future roll-out
-
was delivered and designed in terms of workforce configuration, to understand the barriers to and drivers of sustaining inter-occupational and inter-organisational working
-
interacted with other care processes and systems, to develop knowledge on their strategic alignment with existing care models
-
was experienced by clinicians, patients and families, to develop recommendations for improvement
-
impacted on established roles and relationships, to understand barriers to and drivers of change manifest in distinct professional knowledge, practice and cultural domains.
The appraisal was informed by a consolidated framework, drawing attention to a range of key factors that frame and are involved in the implementation of new practices. 35 The design of the qualitative appraisal involved a number of established methods of data collection and analysis, including non-participant observations, interviews and focus groups, which are further detailed in Chapter 5.
Health economic study
The integral health economic study was designed to evaluate the microcosts of service delivery and the cost-effectiveness of the CIRACT service compared with the THB-Rehab service. To determine the microcosts of service delivery, we proposed to use a time and motion study (TMS) microcosting methodology conducted through three phases (detailed in Chapter 6).
A TMS is used to understand existing work patterns with a view to informing a more efficient model and to aid microcost analysis. It directly observes and measures the time required to deliver a service. TMS methodology has its roots within business and was devised to improve work management. TMS was initially defined by Frederick Winslow Taylor36 in the 1900s and was designed to break down work into its component parts in order to streamline or redefine them to ensure maximum efficiency. TMS is increasingly being carried out within a health-care setting to ensure that systems are running as efficiently as possible, which in turn can lead to more cost-effective models for delivery. One part of this may be ascertaining resource use and valuing such resource use using microcosting, although other costing methods also exist. The microcosting method measures resource use and costs in detail at the individual patient level.
Differing methods of data collection for TMS have been used and include either self-report or observation. The choice of data collection method would depend on how intensive a job/role is and how many activities were being worked on over what period of time. Self-report TMS has been found to result in significantly less activities being described than with observer methods37 and therefore is regarded as being less accurate, although it is cheaper and more efficient as an observer is not required. For observation TMS there are two methods: work sampling or continuous observation. In work sampling the observer is external to the workforce and randomly records instantaneous observations. Because of this, observations can be made of multiple staff members, which may be required if the unit of work being observed is being carried out by a team rather than by an individual. Continuous observation is when either the person or the unit of work being observed is ‘shadowed’ by the observer and all predefined tasks of interest are noted using a data collection tool. Although the data collected with both observer methods are not significantly different,38 work sampling is a more economical and objective method (as the observer is more remote from the subject). However the choice of method used is dependent on the research question and resources available. To determine resource use in a TMS, a flow chart is usually drafted that includes all of the necessary steps to deliver the service. 39 This then allows the development of categories to be observed and systematic data collection. 40
Study harms/adverse events
Data were collected for each individual participant with regard to any falls that occurred while an inpatient on the ward until time of discharge. A fall was classed as an ‘adverse event’ and a fall resulting in a radiologically confirmed fracture was classed as a ‘serious adverse event’. The risks of taking part in the trial were regarded as minimal as the CIRACT service was not a different type of rehabilitation but a change in delivery of the THB-Rehab service that a participant would have received as part of his or her usual care.
Ethics
The study was approved by the National Research Ethics Service West Midlands – Staffordshire Research Ethics Committee (reference number 13/WM/0050) on 27 February 2013. The trial was conducted in accordance with ethical principles that have their origin in the principles of good clinical practice41 and the Department of Health Research Governance Framework for Health and Social Care. 42
Chapter 4 Main randomised controlled trial results
Flow of participants into the trial
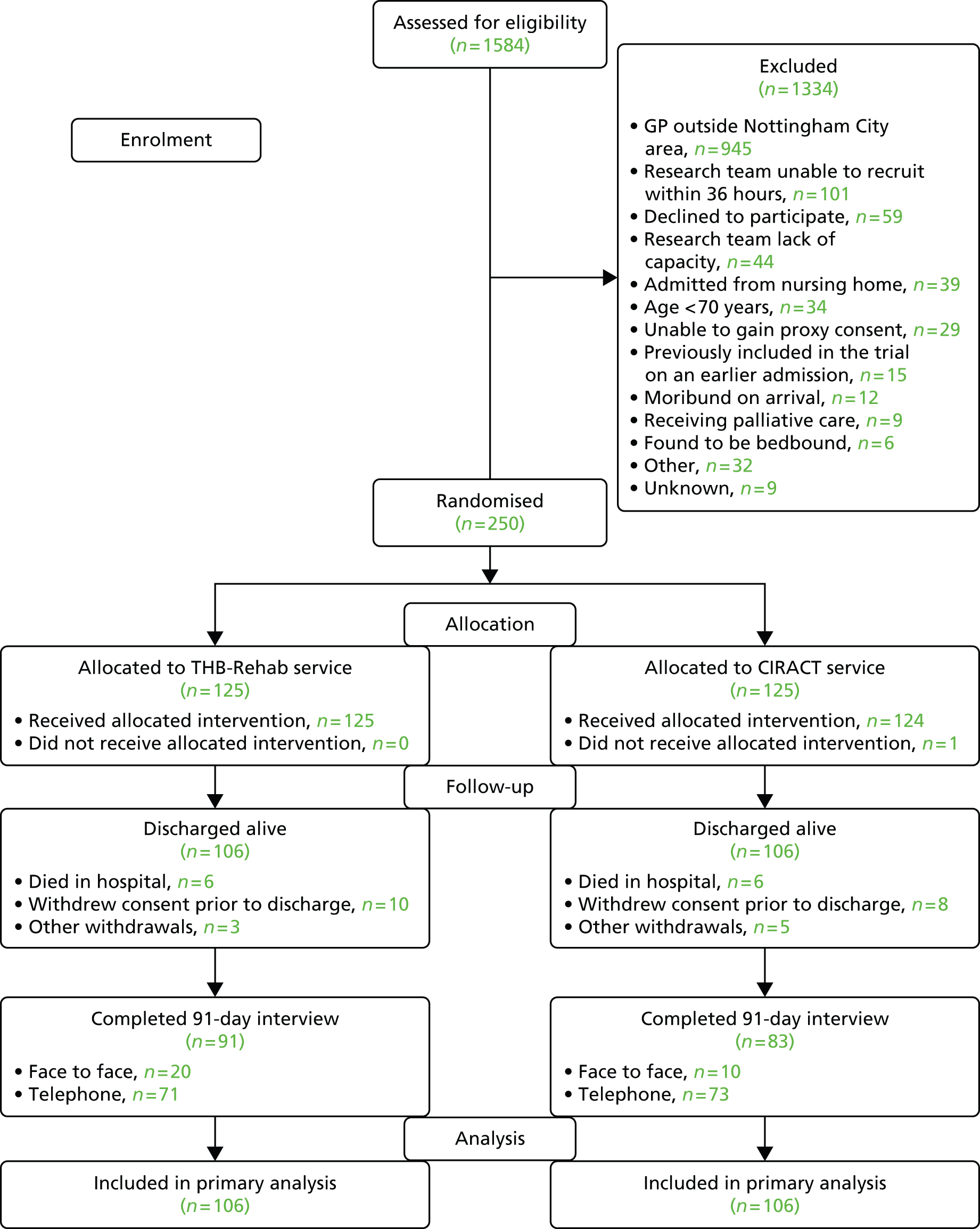
Study recruitment commenced on 23 June 2013 and ended on 31 July 2014, during which 1584 patients from three elderly care medical wards were screened for eligibility, of whom 250 were randomised into the trial. The dominant reasons for exclusion were GP registered outside the Nottingham City CCG catchment area, lack of research staff capacity and unable to gain consent from the participant (Figure 1). In total, 212 participants were followed up and included in the primary analysis.
FIGURE 1.
Participant flow.
Baseline characteristics of randomised participants
The baseline characteristics of the randomised participants are shown in Table 1. The mean age at randomisation was 84.1 years (range 67–99 years) and there was a slight predominance of women (64% of the total). The mean MMSE score was 21.7 out of 30 and the mean Barthel ADL score was 10.7 out of 20. There was a high prevalence of comorbidities among the participants, with a mean Charlson index score of 7.4. The groups appeared well balanced at baseline. This also held true between participants who had primary outcome data and those who did not within each arm, except for the Barthel ADL score, which was higher among those who had primary outcome data than among those who did not.
| Variable | Intervention arm | THB-Rehab | CIRACT | Total (n = 250) | |||
|---|---|---|---|---|---|---|---|
| Primary outcome collected | Primary outcome collected | ||||||
| THB-Rehab (n = 125) | CIRACT (n = 125) | Yes (n = 106) | No (n = 19) | Yes (n = 106) | No (n = 19) | ||
| Age at randomisation (years) | |||||||
| Mean (SD) | 84.5 (5.9) | 83.6 (6.6) | 84.3 (5.9) | 85.8 (5.7) | 83.8 (6.5) | 82.8 (7.4) | 84.1 (6.3) |
| Median (25th percentile, 75th percentile) | 85 (81, 89) | 84 (79, 89) | 84 (81, 88) | 86 (81, 90) | 84 (79, 89) | 85 (76, 88) | 84.5 (80, 89) |
| Min., max. | 70, 98 | 67, 99 | 70, 98 | 73, 94 | 70, 99 | 67, 93 | 67, 99 |
| n | 125 | 125 | 106 | 19 | 106 | 19 | 250 |
| Sex | |||||||
| Male | 46 (37) | 43 (34) | 38 (36) | 8 (42) | 33 (31) | 10 (53) | 89 (36) |
| Female | 79 (63) | 82 (66) | 68 (64) | 11 (58) | 73 (69) | 9 (47) | 161 (64) |
| Barthel ADL score | |||||||
| Mean (SD) | 10.5 (5.4) | 11.0 (6.1) | 11.4 (4.7) | 5.6 (6.5) | 12.1 (5.4) | 4.8 (6.1) | 10.7 (5.8) |
| Median (25th percentile, 75th percentile) | 10 (7, 15) | 12 (6, 16) | 11 (8, 15) | 4 (0, 13) | 13 (8, 16) | 1 (0, 12) | 11 (7, 16) |
| Min., max. | 0, 20 | 0, 20 | 1, 20 | 0, 17 | 0, 20 | 0, 16 | 0, 20 |
| n | 125 | 125 | 106 | 19 | 106 | 19 | 250 |
| Charlson comorbidity scale score | |||||||
| Mean (SD) | 7.3 (1.9) | 7.4 (2.2) | 7.2 (1.9) | 8.5 (1.8) | 7.4 (2.2) | 7.3 (2.1) | 7.4 (2.1) |
| Median (25th percentile, 75th percentile) | 7 (6, 9) | 7 (6, 9) | 7 (6, 9) | 8.5 (8, 9) | 7 (6, 9) | 7 (6, 9) | 7 (6, 9) |
| Min., max. | 4, 12 | 4, 13 | 4, 12 | 5, 12 | 4, 13 | 4, 11 | 4, 13 |
| n | 120 | 116 | 106 | 14 | 106 | 10 | 236 |
| MMSE score | |||||||
| Mean (SD) | 22.0 (6.2) | 21.4 (6.3) | 22.9 (5.3) | 16.2 (8.8) | 21.5 (6.4) | 20.2 (4.8) | 21.7 (6.2) |
| Median (25th percentile, 75th percentile) | 23 (19.5, 27) | 22 (19, 26) | 24 (20, 27) | 18 (8, 21) | 22.5 (19, 26) | 21 (17, 23) | 23 (19, 26) |
| Min., max. | 0, 30 | 1, 30 | 6, 30 | 0, 28 | 1, 30 | 14, 26 | 0, 30 |
| n | 80 | 87 | 70 | 10 | 82 | 5 | 167 |
| EQ-5D health state score | |||||||
| Mean (SD) | 54.5 (19.6) | 53.1 (22.7) | 54.7 (20.2) | 51.9 (6.5) | 52.9 (23.1) | 55.0 (15.5) | 53.8 (21.1) |
| Median (25th percentile, 75th percentile) | 50 (45, 0) | 50 (40, 0) | 50 (40, 0) | 50 (50, 7.5) | 50 (40, 0) | 50 (50, 5) | 50 (40, 0) |
| Min., max. | 10,00 | 0,00 | 10,00 | 40,0 | 0,00 | 30,0 | 0,00 |
| n | 114 | 111 | 106 | 8 | 104 | 7 | 225 |
Follow-up
In total, 212 participants were discharged from the hospital alive (106 in each arm), of whom 174 were followed up at 91 days post discharge (n = 91 from the THB-Rehab service and n = 83 from the CIRACT service) (Table 2). The main reason for not being followed up at 91 days post discharge was death post discharge.
| Outcome | Intervention arm | |
|---|---|---|
| THB Rehab (n = 125) | CIRACT (n = 125) | |
| Discharged alive | 106 (85) | 106 (85) |
| Followed up at 91 days | 91 (73) | 83 (66) |
| Not followed up at 91 days | ||
| Death post discharge | 11 (9) | 17 (14) |
| Withdrew consent post discharge | 1 (1) | 1 (1) |
| Loss to follow-up post discharge | 3 (2) | 1 (1) |
| Discontinued for other reasons post discharge | 0 | 4 (3) |
| Death in hospital | 6 (5) | 6 (5) |
| Withdrew consent prior to discharge | 10 (8) | 8 (6) |
| Discontinued for other reasons prior to discharge | ||
| Found to be bed bound | 1 (1) | 1 (1) |
| GP not Nottingham City CCG registered | 0 | 2 (2) |
| Age < 70 years | 0 | 1 (1) |
| Receiving palliative care | 0 | 1 (1) |
| Unable to gain consent | 1 (1) | 0 |
| Moved to end-of-life care | 1 (1) | 0 |
In total, 12 participants died in hospital prior to discharge and another 18 withdrew consent prior to discharge. Eight participants were discontinued from the study prior to discharge for various post-randomisation eligibility breaches (see Table 2). These are categorised as ‘other withdrawals’ (see Figure 1).
Primary outcome
The distribution of LOS for participants discharged is shown in Figure 2. This was skewed, with a peak proportion discharged at day 8 in the CIRACT group and at day 12 in the THB-Rehab group.
FIGURE 2.
Distribution of LOS for participants discharged alive. (a) THB-Rehab; and (b) CIRACT.
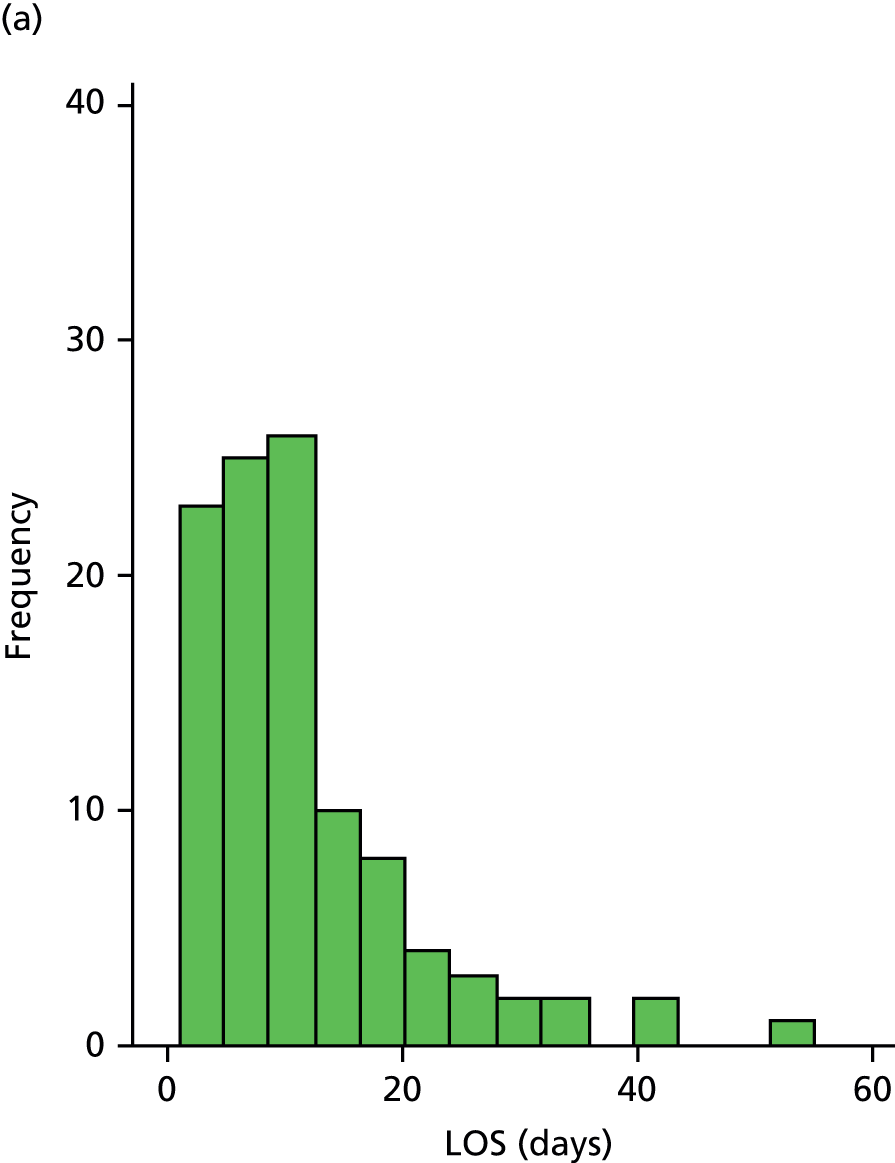
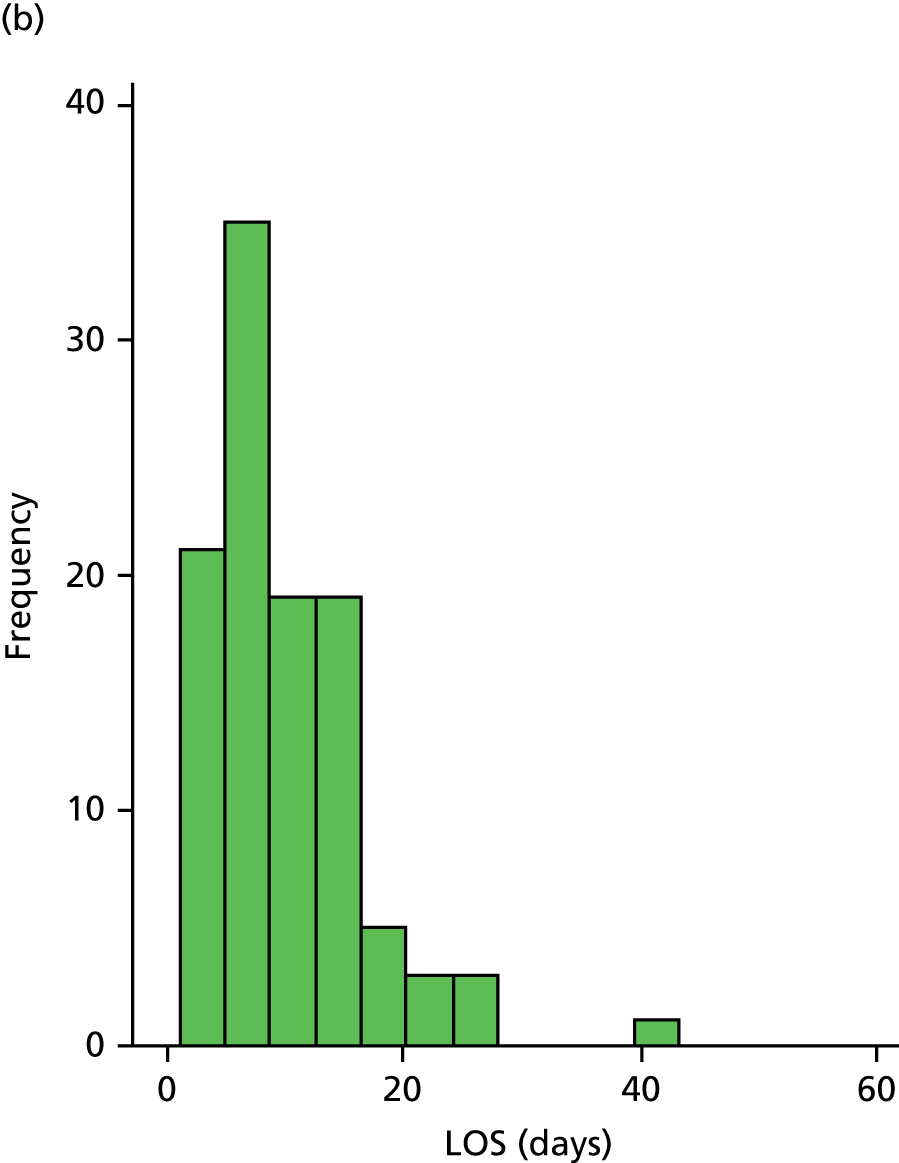
There was no significant difference in LOS between the CIRACT service and the THB-Rehab service (median 8 vs. 9 days; geometric mean 7.8 vs. 8.7 days, mean ratio 0.90, 95% CI 0.74 to 1.10), which was supported by the sensitivity analyses (Table 3).
| Outcome | Intervention arm | Analysis type | Ratio | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| THB-Rehab | CIRACT | |||||
| LOS | ||||||
| Geometric mean (95% CI) | 8.7 (7.5 to 10.1) | 7.8 (6.9 to 8.9) | (1) Primary analysis | 0.90 | 0.74 to 1.10 | 0.303 |
| Median (25th Q, 75th Q) | 9 (5, 15) | 8 (5, 13) | ||||
| Min., max. | 2, 55 | 1, 41 | ||||
| n | 106 | 106 | ||||
| LOS | ||||||
| Geometric mean (95% CI) | 8.9 (7.7 to 10.2) | 8.0 (7.0 to 9.2) | (2) As in (1) with deaths in hospital | 0.90 | 0.75 to 1.10 | 0.316 |
| Median (25th Q, 75th Q) | 9 (5, 15.5) | 8 (5, 14) | ||||
| Min., max. | 2, 55 | 1, 62 | ||||
| n | 112 | 112 | ||||
| LOS | ||||||
| Geometric mean (95% CI) | 9.1 (7.9 to 10.5) | 8.3 (7.2 to 9.5) | (3) As in (1) with missing data by imputation | 0.91 | 0.75 to 1.09 | 0.307 |
| Median (25th Q, 75th Q) | 9 (5, 16) | 8 (5, 14) | ||||
| n | 125 | 125 | ||||
The Kaplan–Meier estimates (Figure 3) similarly showed no significant difference in LOS between the groups and there was also no significant difference in LOS between the groups with in-hospital death as a competing risk (Table 4).
FIGURE 3.
Kaplan–Meier plot of time to discharge excluding in-hospital deaths.
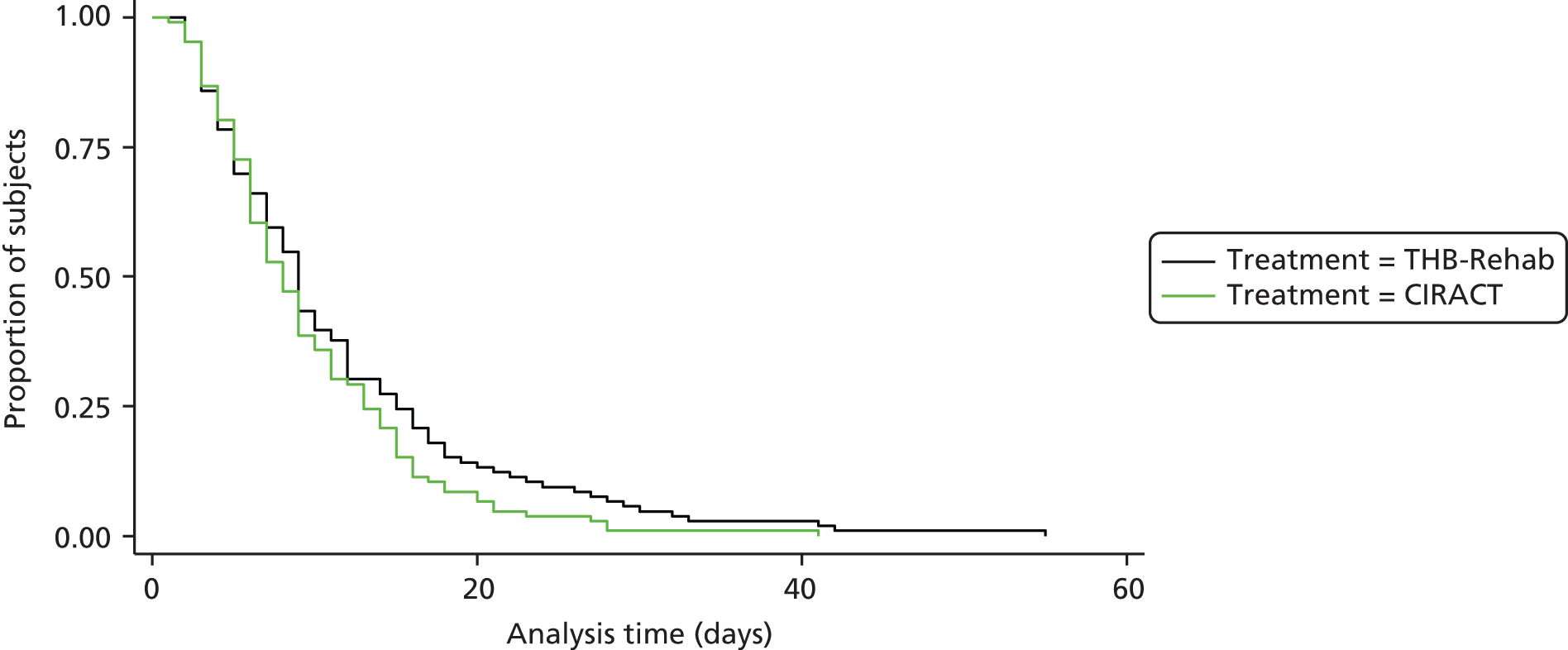
| Type of service | Sub-distribution hazard ratio | 95% CI | p-value |
|---|---|---|---|
| CIRACT vs. THB-Rehab | 1.14 | 0.88 to 1.48 | 0.327 |
Secondary outcomes
There were no significant differences in any of the secondary outcomes between the two arms (Table 5). There were a median of 15 and 17 super spell bed-days for the CIRACT and THB-Rehab groups respectively (geometric mean ratio 0.96, 95% CI 0.76 to 1.21). Of participants discharged from hospital, 17% and 13% were readmitted within 28 days post discharge from the CIRACT service and the THB-Rehab service respectively (risk difference 3.8%, 95% CI –5.8% to 13.4%) and 42% and 37%, respectively, were readmitted by 91 days post discharge (risk difference 5.7%, 95% CI –7.5% to 18.8%).
| Outcome | Intervention arm | Effectiveness parameter | 95% CI | p-value | |
|---|---|---|---|---|---|
| THB-Rehab | CIRACT | ||||
| Super spell bed-days | |||||
| Geometric mean (95% CI) | 15.6 (13.2 to 18.6) | 14.9 (12.4 to 17.9) | 0.961a | 0.76 to 1.21 | 0.713 |
| Median (25th Q, 75th Q) | 17 (9, 31) | 15 (7, 32) | |||
| Min., max. | 2, 112 | 2, 120 | |||
| n | 112 | 112 | |||
| Readmitted to hospital at 28 days post discharge | |||||
| n (%) | 14 (13) | 18 (17) | 3.8%b | –5.8% to 13.4% | 0.442 |
| N | 106 | 106 | |||
| Readmitted to hospital at 91 days post discharge | |||||
| n (%) | 39 (37) | 45 (42) | 5.7%b | –7.5% to 18.8% | 0.399 |
| N | 106 | 106 | |||
| Barthel ADL score | |||||
| Mean (SD) | 12.6 (5.7) | 14.3 (5.5) | 1.02c | –0.41 to 2.44 | 0.161 |
| Median (25th Q, 75th Q) | 14 (8, 17) | 16 (10, 18) | |||
| Min., max. | 0, 20 | 0, 20 | |||
| n | 90 | 83 | |||
| Charlson comorbidity score | |||||
| Mean (SD) | 7.5 (2.1) | 7.6 (2.1) | –0.06c | –0.31 to 0.20 | 0.663 |
| Median (25th Q, 75th Q) | 7 (6, 9) | 7 (6, 9) | |||
| Min., max. | 4, 13 | 4, 13 | |||
| n | 92 | 85 | |||
There were 15 protocol deviations in the CIRACT group and eight in the THB-Rehab group (Table 6). The deviations in the CIRACT group are shown in Appendix 2.
| Category | Intervention arm | |
|---|---|---|
| THB-Rehab (n = 125) | CIRACT (n = 125) | |
| Inclusion/exclusion criteria | 3 (2) | 5 (4) |
| Informed consent | 0 | 4 (3) |
| Other | 5 (4) | 6 (5) |
Adverse events
There were seven non-severe falls recorded from seven participants (n = 4 CIRACT service, n = 3 THB-Rehab service) (Table 7). No safety concerns were raised by the Trial Steering Committee (TSC).
| Patient number | Intervention arm | Adverse event | Severity | Relatedness to intervention | Outcome |
|---|---|---|---|---|---|
| 1096 | CIRACT | Fall | Mild | Not related | Recovered/resolved |
| 1097 | THB-Rehab | Fall | Mild | Not related | Recovered/resolved |
| 1132 | THB-Rehab | Fall | Mild | Not related | Recovered/resolved |
| 1138 | CIRACT | Fall | Mild | Not related | Recovered/resolved |
| 1164 | THB-Rehab | Fall | Mild | Not related | Recovered/resolved |
| 1190 | CIRACT | Fall | Mild | Not related | Recovered/resolved |
| 1193 | CIRACT | Fall | Mild | Not related | Recovered/resolved |
Chapter 5 Qualitative appraisal methods and results
In parallel with the main RCT we undertook a detailed qualitative appraisal, encompassing three main activities:
-
activity 1 – organisational profiling
-
activity 2 – study of the CIRACT and THB-Rehab services in action
-
activity 3 – patient tracking.
Activity 1: organisational profiling
Organisational profiling aims to understand the outer and inner context within which new interventions, such as CIRACT, are translated and implemented into practice. This can be seen as ‘setting the scene’ of enquiry or orientating the subsequent study of CIRACT ‘in action’. Accordingly, the objective of organisational profiling is to gather contextual data about local services in which new interventions or controls are in operation. This includes information on:
-
the spatial configuration of service areas
-
service goals, strategy and policy
-
staff and resource profiles
-
patient numbers, throughput and LOS
-
management and governance structures and processes
-
leadership roles and approaches
-
team structures and processes
-
financial and commissioning arrangements
-
performance management, including relevant data on performance levels.
Organisational profiling was carried out across two of the three wards during the initial months of the trial. In line with the consolidated framework and building on similar research carried out by the qualitative team,43 a profiling template was developed to guide these initial enquiries reflecting the themes listed above. This was completed through a series of linked research steps.
First, research gatekeepers (local sponsors) and key informants (service leaders) were engaged in ‘fact finding’ during which they were asked about the history of the service, the general configuration and current work pressures. Second, further semistructured qualitative interviews, lasting between 30 and 90 minutes, were carried out with six service leaders and key staff: service managers, clinical leaders, sisters and research co-ordinators. Third, service leaders provided ‘guided tours’ of each service area, including walking tours and introductory meetings with staff groups and attendance at scheduling staff meetings. Fourth, the qualitative study was introduced to clinical teams and groups working in the ward areas, usually during team briefings or rest breaks, at which more informal information and insight was provided about the service through a process of questions and answers. Finally, service leaders provided access to relevant documentary sources, including local policies and procedures, and performance-related data. Through these initial activities a broad contextual picture was developed of each service from which to direct subsequent and more fine-grained analysis.
Activity 2: study of the Community In-reach Rehabilitation And Care Transition and traditional hospital-based rehabilitation services in action
The second and largest period of data collection involved an in-depth ethnographic study of how the CIRACT and THB-Rehab services were organised, delivered and experienced as a situated, social process within a given context. This focused first on mapping and then understanding the organisation and delivery of each service in terms of (i) the structure and flow of the care pathway; (ii) the allocation of roles and responsibilities across the pathway; (iii) key decision-making and communication points across the pathway; (iv) variations in periods of care, support and education; and (v) handovers between care teams and interactions with external agencies. As such, data collection ‘zoomed in’ from the broader organisation of care and division of labour to a more focused analysis of clinical practices, interactions and care-giving processes.
In line with the ethnographic approach, this involved a combination of non-participant observations (122 hours) and semistructured and ethnographic (conversational) interviewing over a period of 6 months (see Appendices 3 and 4 for details of observations). Observations were made of different activities, building on the guided tours and on the rapport developed with service providers:
-
Work process observations. In-depth workplace observations were undertaken over a period of 2 months, which involved mapping the temporal and spatial organisation of daily work (schedule of ward rounds, meetings, handovers, discharge times), identifying key events and activities [multidisciplinary team (MDT) meetings, drug rounds], identifying key individuals or groups (discharge co-ordinators, clinical leads) and drawing together these data into a complex descriptive account of the social setting and care process. Observations were undertaken on a daily basis (3–4 days per week, including evenings and weekends) over 8 weeks, with additional observations undertaken in community settings with the CIRACT team.
-
In-depth observations of situational activities, tasks and settings. Prolonged follow-up observations were undertaken of key activities, tasks and settings to deepen knowledge of service delivery. Each setting or activity was observed at least three times and some, such as weekly MDT meetings, were observed up to 10 times. This included observations of:
-
morning handovers with the MDT on each ward
-
home visits prior to discharge
-
patient and family consultation meetings
-
social services assessments
-
use of information communication technology (ICT) and manual records about rehabilitation progress and discharge planning
-
referrals to multiple agencies by telephone and fax
-
ordering equipment and home adaptation, including telecare monitoring and adaptive devices
-
patient education and support
-
carer education and support
-
home visits after discharge
-
end-of-life care support across locations
-
referral to community agencies for longer-term support in person, by telephone or by fax.
-
-
Shadowing of individuals. To deepen the understanding of the roles and contributions of certain individuals or groups, shadowing observations were also undertaken with key individuals or representatives from professional groups. Observations ranged from several hours (ward clerks) to several days (discharge co-ordinators) as the individuals went about their day-to-day work. The individuals observed included:
-
CIRACT team leader (transition coach)
-
therapists
-
ward clerks and administrators
-
occupational therapists
-
physiotherapists.
-
All observations were recorded, first in hand-written field journals, including rich descriptions and separate interpretations, which were later typed up electronically as corresponding text and interpretations along with a summary overview of the key points. It is important to note that observations were not focused on intimate, personal or challenging patient care. All observations of patient–clinician interactions involved prior written and verbal consent and focused primarily on the activities and work of the clinical team member, not the patient.
As indicated earlier, alongside these observations members of staff were engaged in a large number of conversational-style ‘ethnographic interviews’. Ethnographic interviewing involved small conversations and interactions with clinicians and other study participants in the normal cause of their work or practice. They were usually short (i.e. 5–10 minutes) and were used to clarify observations, elaborate the reasons behind decisions or actions and gain reflective insight from participants about activities in ‘real time’. As these were informal, they were recorded only in field journals alongside observation records. It is estimated that 200 such short ethnographic interviews were carried out over the study.
More formal semistructured interviews were also carried out alongside observations. These aimed to develop more reflective accounts or narratives of the respective services (CIRACT and THB-Rehab) from the perspectives of different stakeholders. Interviews were carried out with 13 participants across the CIRACT and THB-Rehab services: two managers, six therapists, two discharge co-ordinators, one senior nurse, one social worker and one care home manager (Table 8). The interviews were arranged at the convenience of participants and, in most cases, were conducted in a private setting. They ranged from 40 to 90 minutes in length and most were digitally recorded. The interviews followed a broad guide that covered the following topics:
-
the implementation and development of the CIRACT service (or THB-Rehab service), including translation into practice, training and supporting and changes in design and delivery
-
the organisation and delivery of the CIRACT service (or THB-Rehab service), including service configuration, daily work planning, decision-making and communication, discharge planning and delivery of patient care
-
interprofessional and interorganisational working, including the barriers to and drivers of integrated working
-
the perceptions of therapy and the value of the CIRACT service from a professional viewpoint.
| Intervention arm | Interviewees |
|---|---|
| CIRACT | Service designer and academic adviser |
| Community care (NHS) manager | |
| Senior occupational therapist and team lead | |
| Senior physiotherapist (band 6) | |
| Social worker | |
| THB-Rehab | Senior occupational therapist |
| Senior physiotherapist | |
| Senior nurse (band 7) | |
| Physiotherapist | |
| Therapy assistant (band 3) | |
| Discharge co-ordinator ×2 | |
| Residential and nursing home manager |
Activity 3: patient tracking
Patient tracking aimed to develop a highly detailed and, importantly, patient-centred understanding of care processes and experiences. It focused on the interactions and care processes of a small sample of patients as their care progressed and as they moved between care teams and settings. As such, the method aimed to place the patient at the centre of analysis with the aim of understanding the web of interconnecting care processes that contribute to care planning, delivery and transition. This approach combined first-hand observations of care activities and processes, such as patient assessment, decision-making, communication and therapy, with a series of short structured interviews with patients as they moved along the care pathway and transitioned from hospital to a community setting. As such, it can lead to a highly developed, longitudinal (time–space) understanding of the patient experience.
In the first instance, the patient/family were approached in hospital, usually when the patient had been allocated to the CIRACT service or the THB-Rehab service. In collaboration with the patient’s designated care team, the research aims and methods and the patient tracking method were explained to the patient/family. Those who consented were then involved in a series of three to four observations of patient–clinician interactions on the ward and then at home, for example assessment, care planning, education and therapy. Each patient was also asked to participate in a series of short interviews to acquire further understanding of the patients’ experiences and views about their care. These started in hospital (usually two short conversations after observations of therapy on the ward) and then continued in the community at 1 week post discharge. Although every effort was made to schedule interviews at these times, in some instances they needed to be moved by ± 1 week because of other appointments. Furthermore, not all patients were able to participate in the full series of interviews because of withdrawal (their health had worsened), readmission to hospital, transfer to another care setting (i.e. left the CIRACT service) or death. The patient tracking data were initially recorded in reflective field journals and were then summarised within a common template to enable data management and comparison (see Appendix 5).
Data analysis
All data were managed in accordance with NHS and university research governance frameworks. All interview transcripts were anonymised with pseudonyms and all identifiable information, such as contact details, was securely filed. Hand-written ethnographic notes did not include identifiable names or locations and were archived within 48 hours into locked cabinets. Electronic data were stored within encrypted and secure external drives and back-up copies were kept within a locked location within the university.
Interpretative qualitative data analysis was undertaken to develop a descriptive and contextualised understanding of the respective care services, especially the implementation, organisation and delivery of the CIRACT service. This involved an iterative process of close reading of the data, coding, constant comparison, elaboration of emerging themes and re-engaging with the wider literature. In the first instance, one member of the research team independently reviewed a sample of transcripts and observation records to develop an initial case description and coding strategy. This was presented and discussed with the wider research team and qualitative methods advisors. Following feedback from these discussions, all data were systematically coded and categorised in line with the consolidated implementation framework and research questions. With regard to the reliability of the coding process, codes and categories were reviewed on a monthly basis by the wider team to ensure the accuracy of interpretation and the internal consistency of codes. Through this iterative process a number of common themes were developed in relation to four over-riding questions:
-
How was CIRACT designed, implemented and translated into practice?
-
How was CIRACT (and THB-Rehab) organised and delivered in context?
-
How was CIRACT (and THB-Rehab) experienced by participants and professionals?
-
How did CIRACT impact on clinical roles and how might services be developed?
Results
Service implementation
With regard to implementation, the study found that a combination of national priorities and pressures, together with innovations in local service delivery in the face of contextual pressures and drivers, contributed to development of the CIRACT service. National policies repeatedly call for more integrated, patient-centred and efficient care, especially when acute care is regarded as costly, and in recent months a number of initiatives were developed to better manage patient care in the community setting. Although many of these were targeted at reducing unplanned admissions, an important part of the problem remained delays in supporting discharge from hospital and, further still, inappropriate or poorly supported discharges that result in readmission. The CIRACT service directly addressed this challenge. Its design and development appeared to reflect the innovative practices of local service leaders, especially those in community health care, and also the willingness of both acute providers and care commissioners to support new ways of working:
So this service [CIRACT] I think is needed because there’s so many people that would fall through the gap otherwise. That don’t need four weeks of rehab, intense rehab, or they don’t need specific stroke goals, cognitive goals. They just need somebody to support them in that transfer home. Making sure the home is set up well, the hazards are removed. They’ve got everything they need.
CIRACT team lead and occupational therapist interview, 10/12/13 (lines 82–90)
When I was a hospital OT [occupational therapist] I was very frustrated a lot of the time that we’d try our best for a patient. We’d set them up as well as we thought, they could go home, and then you hand over maybe to the social worker in the hospital, you hand over to maybe a rehab team. That person is readmitted a few days later and you think, ‘We’ve done all these things. That was an unnecessary admission just because there isn’t that communication there.
CIRACT team lead and occupational therapist interview, 10/12/13 (lines 113–18)
Furthermore, the service was continually modified and adapted in the light of inner contextual factors, especially staffing and resource shortages and also ongoing feedback from service users. In addition, it is important to note that factors both in the acute hospital setting and in the community setting continue to exert an influence on the service, resulting in continuing elaborations and modifications, as seen during the winter pressures. Although it is useful to work towards a core service specification, it also remains useful to maintain a degree of flexibility in new services so that they can align where necessary with pre-existing ways of working.
The study found that there was a high degree of uncertainty about how the new service was communicated and introduced to pre-existing care providers. This resulted not only in role uncertainty and ambiguity but also in the potential for conflict over patients and tasks. As such, greater engagement and communication might be needed when implementing new services such as CIRACT. Additionally, local resource profiles, especially bed availability and staffing, influenced the implementation of the CIRACT service. Of note, increased winter bed capacity appeared to divert patients away from the CIRACT service, thereby rendering it marginal to service delivery, and, later, the lack of specialist staffing further reduced the capacity of the CIRACT service. Although the CIRACT service remained an important and innovative solution to the problems of hospital discharge, in this particular context it appeared to be an experimental solution to a specific set of service problems associated with undercapacity in both the acute and the rehabilitation sectors. Furthermore, it was not necessarily clear whether, as implemented, the CIRACT service was expressly concerned with fostering longer-term integration or closer working between acute and community settings.
Service organisation and delivery
In terms of how the CIRACT service was organised and delivered, the key differences between the CIRACT service and THB-Rehab are shown in Appendices 6 and 7. The study found that it represented a relatively discrete model and pathway of sustained and continuous patient care provided by a relatively small but specialist team, including skills in both acute rehabilitation and therapy but also service planning and co-ordination. The care pathway is distinctive because it demonstrates the spanning of the CIRACT service from the point of admission to as much as 12 weeks post discharge. Significantly, by working both within and across the hospital and community setting, the CIRACT team was better able to develop closer and more aligned working relationships with these distinct service providers, to close the gaps between these providers and to establish close working relations with the patient and family, leading to enhanced continuity of care.
Daily contact with patients supports progressive tailored planning for therapy and discharge; however, there can be a sense of isolation when the team ends its work with patients:
It’s somebody taking ownership of that patient and that patient’s journey through the acute service and once they’re at home, and I think that’s a really, really strong benefit of this service is that we get to know the patient really well. We get to draw them back home and support them there.
CIRACT occupational therapy lead interview, 10/12/13 (lines 103–6)
The integration of the CIRACT service within existing service configurations was found at times to be problematic, and competing demands and pressures within the wider health system could easily place unanticipated demands on the THB-Rehab service, which could impact on the CIRACT service. It might be important to establish clear lines of accountability for the CIRACT team, given that their work within and across the boundaries between acute and community care can make the service vulnerable to various local contextual pressures.
Furthermore, line management within the acute setting might be clearly demarcated from but, when relevant, integrated with other clinical accountability and reporting channels. For example, it often seemed unclear who the members of the CIRACT team were accountable to for their performance and they could easily be drawn into local ‘ward politics’. In addition, staff shortages and recruitment problems could easily disrupt the CIRACT service, especially as team members needed a particular set of competencies in different aspects of patient assessment, rehabilitation and care planning before, during and after discharge. In some respects, the unique skills and knowledge profile of the CIRACT team could make it vulnerable to future staffing problems and when reliance on locum staff was problematic.
Distinctions between the two service approaches are important because they offer different models of care and are characterised by different ways of organising work and care and subsequently meet patient needs in different ways. CIRACT can be interpreted as a boundary-spanning and responsive service. That is, it aimed to respond to longer-term patient needs beyond the single episode of acute hospital care and provide care across the boundaries between acute and community settings. For example, the service was premised on the assumption that the quality and effectiveness of post-hospital care could be increased if rehabilitation and support started earlier within the hospital, before discharge, and if the same care team provided ongoing support.
This resulted in two key service requirements that differentiated the CIRACT service from the THB-Rehab service. The first involved the provision of earlier intensive rehabilitation and therapy, with an explicit focus on the longer-term and holistic needs of the patient. The second was that this therapy should ideally be tailored to and, when possible, provided within the context of the patient’s home, where they could learn to manage their care in a relevant setting, rather than the more ‘artificial’ one of an acute hospital. To achieve these requirements the CIRACT service was designed to enable intensive therapy across and within acute hospital and community settings.
By contrast, the THB-Rehab service model was solely located in the acute hospital and demonstrated a model of care that sought to prepare the patient for the end point of discharge, rather than supporting longer-term care and therapy needs after discharge. The THB-Rehab service was predominantly task orientated, with an emphasis on getting jobs done, which limited the opportunities to deliver more holistic care. In particular, the THB-Rehab service gave limited scope for sustained knowledge sharing or a mutually beneficial learning relationship between the patient and therapists to develop, because of constraints on the frequency of contact alongside other ward-based duties. As THB-Rehab service therapy was confined to the acute hospital setting, home visits prior to discharge were exceptional (two a year per ward) compared with more frequent visits for the CIRACT patients. This meant that it was difficult to determine the extent of patient need following discharge, with decisions usually based on observed patient recovery and progress while on the ward:
Our stairs are completely different and just because they’re able to it here, doesn’t mean they’ll be able to do it at home, or if they can’t do it here, it doesn’t mean they won’t be able to do theirs at home. And we might change things or arrange things based on something that isn’t representative of what they’re able to do.
Senior physiotherapist interview, 31/01/14 (lines 51–5)
In terms of the organisation and planning of hospital care transition, it was also observed that hospital discharges in the THB-Rehab service were far from uniform across the week, with peaks of activity on Mondays and Fridays in response to the admission demands within the system. This uneven distribution of discharges had the knock-on effect of reducing standard service therapy sessions for inpatients and so many went without therapy from Thursday to Tuesday of each week, leading to a bottleneck of demand midweek. By contrast, the design of the CIRACT service across 7 days led to a more even distribution of therapy and discharge activities within the acute setting:
I think they [the trust] seem to forget the patients on the wards. It’s soon as they’re admitted, then they seem to be forgotten and it’s just all about the people who are coming in, not the people that are here already.
Discharge co-ordinator interview, 02/12/13 (lines 403–6)
Although the staffing capacity of the THB-Rehab service was greater than that of the relatively small CIRACT service, the THB-Rehab service teams of two therapists plus one assistant also managed the care needs of a greater number of patients (usually 28 per team). With this number of patients it was seen as being difficult to provide intensive therapy on a par with that provided by the CIRACT team. As such, it was acknowledged that the THB-Rehab service could not aspire to provide rehabilitative therapy beyond reaching the patient’s functional baseline. As such, the THB-Rehab service was predominantly resource led, with a focus on getting the older person ready to move on, whereas the CIRACT service was predominantly patient needs led, with a view to maximising resources for maximum patient outcomes.
Service experience
Patients
Patients’ early experiences of hospital admission, including the CIRACT service, were inevitably shaped by their expectations of care, including previous admissions, community care and being carers themselves. Patient expectations of hospital therapy and discharge planning were generally low, with patients expecting minimal contact with ward-based staff and little longer-term help or guidance for going home. Patients who had previously experienced a THB-Rehab service found it difficult to remember the standard service received, but social worker interventions, such as securing care packages, were more readily remembered. Carers recounted stories of minimal support or care packages that were so short that staff could not give the standard of expected care, such as bathing rather than strip washing.
Through observations of admission assessments, patients were receptive towards the CIRACT staff and most were able to express the reasons for their admission and their immediate and longer-term worries about the future. Some patients were very tired, and often in pain, and resented having to see another professional to explain their needs. Duplication of information giving was openly acknowledged by the team, who tended to focus on establishing core information related to abilities prior to admission rather than on a summary of medical information or home circumstances.
The continuous assessment element of the CIRACT service was also seen to be well received and experienced by patients as previous activities and plans were reviewed and progress towards recovery and discharged was monitored. Patients did not comment on this graduated information-seeking approach but did note that their interactions with the CIRACT team involved in-depth discussions about their goals and future plans, in addition to actual therapy. This was valued by the majority of patients, who felt able to disclose family issues that may not have been discussed during the initial assessment, such as ‘mentally ill carers and being left for days with no assistance’ (observation, 11/01/14). Patients also valued the continuous assessment approach to their care as it enabled them to express their anxieties and also raise issues as they happened. This was a benefit to carers who often felt able to ask if they were doing things right, including using equipment, meeting hygiene needs and maintaining safety. More broadly, the continuous assessment and interaction with the patient provided a more tangible sense of progress and recovery for the patient.
Patients were generally positive about the daily therapy approach operated by the CIRACT service and were happy to be regularly consulted about their therapy plans and progress. One patient suggested that confusing professional jargon was used by the therapists, such as ‘baseline’; this patient did not understand the term, thinking that it could relate to either anticipated deterioration or a return to minimum ability (observation, 02/03/14). Another patient could not fully hear the therapist when she had her head down, guiding her feet forward, and so was unsure of how to follow instructions. However, most patients liked the way in which the team found time to discuss progress and to decide on the next steps in therapy:
It’s nice to know there’s a service like that available. I didn’t know anything about that . . . it’s been more than adequate. They seemed to know what I needed more than I did myself. I’m a very independent sort of person and to be told that you needed this, that and the other. It was good advice.
Patient 6 interview, 02/03/2014 (lines 164–5)
Two male patients did not expect to receive daily therapy and thought this excessive as they regarded their prime task as a patient was to rest. One of the two did not want to leave hospital and thought the therapy to be an intrusion on his stay. In such cases the team balanced the intensity of therapy according to the ability of the patient on the day and were often seen to adapt and adjust to fluctuations in health, mood and motivation. Age was not considered a barrier to therapy by the team, who sought to maximise the identified potential of each patient. However, some family carers did consider that some of the therapy was too optimistic considering the extreme old age of their relative. Attitudinal barriers were usually addressed by banter and giving evidence of progress made. Fatigue was described as being an important factor in the daily therapy and could impact on information giving and the planning of care.
Patients tended to like the home visit because they identified it as a significant step towards hospital discharge. Patients were informed that the purpose of the visit was to identify any barriers to a successful discharge home, such as lack of equipment, safety measures, home carers and support in the community. Family carers, support staff and any other specific agency staff were invited to meet at the home during the home visit to enable collaborative planning within the context of the home. Patients were often more confident that they would manage their ongoing care after discussions in the home with the therapists. Therapists openly discussed how patients would be expected to continue to progress towards greater independence and that the intensity of agency support would be reduced as progress was made. For some patients the home visit also provided evidence of the need to change unrealistic perceptions of ability and accept the need for assistance with care in the home. Patients found the home visit very motivating; seeing pets often provided evidence of a commitment to return home.
Home visits helped the therapists and patients to address specific care issues. A common activity was to address the need for adaptations to the home, including a stairlift and wet room to enable a patient following stroke to maintain his or her independence and hygiene privacy. Similarly, intermediate equipment such as a commode and support with washing were planned as part of the discharge plan during the home visit, with assurances of referrals for the more extensive adaptations to be made. One home visit identified the need for collective planning with family carers within a large extended family and time was spent discussing the logistics around daily care and night sitting with regard to the other demands of each family member.
The majority of the observed CIRACT discharges progressed smoothly and as planned, with all medications, own clothes, notifications, transport and patient care in place. Although difficult for patients to compare discharge across different services, there were few instances when patients criticised or found problems with their discharge arrangements that could be directly linked to the CIRACT service. In comparison to standard care, few CIRACT patients were transferred to the discharge lounge as most were collected by the ambulance service on the ward and transferred directly to home. As noted above, a significant feature of the CIRACT service is the home visits pre discharge, which helped to identify potential risks or problems in the discharge processes to be addressed by the CIRACT service.
It was also usual for patients to have a post-discharge home visit, usually within 4 days of leaving hospital. Patients valued the continued therapy and the rapid supply of equipment and support as required. The process of obtaining new equipment was not always easy and often included lengthy discussions with family members to secure the best options:
The bed remains a problem and E [occupational therapist] discusses with the daughter use of another type of mattress but she is reluctant to do so. E agrees to go over to the Red Cross to see what they have there. She phones and arranges pick up. The reception and equipment staff obviously know E well and greet her with big smiles. She is informed about a new compressed style of mattress which can be easily carried into a home by the charity staff. They agree to let her have one immediately. E is pleased that she has secured this agreement and thanks the charity extensively.
Observation, home visit notes, 12/01/2014
These post-discharge home visits also gave an opportunity for family carers to demonstrate how they managed to give care to their relative. In the privacy of the home, many carers did agree to accepting more help in the form of practical equipment such as bath slides and perching stools, pendant alarms and social care services. Home visits also provided crucial information to the team about how home-alone patients had been managing and enabled the staff to discuss care with wardens and neighbours. Plans for increasing support were often instigated following the home visit.
The CIRACT service aimed to gradually withdraw therapy within a time frame of approximately 4 weeks post hospital discharge. This process involved a transition to the agencies already involved, including care agencies, and new referrals for local community services such as day centres or meal clubs. The process was graduated and the staff reminded patients of the plans for the withdrawal of their service. However, patients did find the cessation of the CIRACT service difficult and spoke of the subsequent isolation and loss of friends. It seemed that strong attachments were made by the patients to the therapy staff and that even when plans for social contact had been made they were acutely missed. This was felt even among patients who continued to receive daily support from social services.
Written plans to meet longer-term aims were discussed with patients on discharge from the service and tended to include personal goals. These were discussed along with any safety issues such as managing a frame through an external door:
I want to get out and do my pots in the garden. I’ve got a patio out here and my gardening and tidy things up and things like that. I miss doing that.
Patient 6 interview, 02/03/2014 (lines 385–6)
Long-term plans were regarded as valuable for the patient and especially carers, who felt that they were more supported. Carers spoke of the need for specific ‘benefits’ advice and felt that this was missing from the service. However, the majority of carers felt that the plans were comprehensive and thoughtfully devised. Carers were also pleased with the signposting information given to enable them to contact other services as required.
The majority of patients who returned home did so with above baseline abilities and as a consequence were motivated to continue their mobility exercises. One patient, aged 92 years, surmised that he was in the best shape in over 2 years and was very proud of his regained ability to put his slippers on:
I know I can’t go as far ever again but I would like to, you know, scramble around. They [CIRACT] were brilliant. They encouraged me. They tried so hard. I’m even better now. . . . They did what they say they was going to do and they delivered.
Patient 5 interview, 10/03/14 (lines 43, 49 and 100)
Another patient stated:
I felt more capable of doing things . . . I’ve slipped a bit now.
Patient 4 interview, 02/03/14 (lines 472 and 476)
Continuity in terms of the same team providing responsive plans of therapy and social care was greatly appreciated by patients and importantly by family carers. The team members were regarded as both therapists and conduits for other services and equipment. During the home observations of the intervention it was clear that a relationship-centred approach was taken, which included the central roles for spouses and other family carers in supporting the rehabilitative aims of the CIRACT patient. The approach taken by the individual team members was one of trying to sustain independence first and then offering support in terms of securing services or referrals if the patient or carer felt unable to do so themselves. Referrals made by the team were conducted with the patient present in the home to ensure accuracy of information and to keep the patient informed. This collaborative approach with the patient and carer helped maintain informed choices.
The CIRACT team were very sensitive towards those patients who were expected to deteriorate because of their prognosis. For example, equipment was gradually introduced at the point of need and not delivered on a pre-emptive basis, which the team considered to be negative for patient morale. This approach did seem to help maintain the often close professional relationships and enable even the most dependent patients to express their determination to continue to manage small but important tasks:
Coming up the stairs as well, you know, still getting out of breath and that, but he is managing the stairs equally if not – if anything he hasn’t got worse. They send the doctors out to see him now which is quite helpful.
Son and carer, patient 5 interview, 10/03/14 (lines 153–5 and 256)
Staff
In broad terms the CIRACT service was regarded as a positive way of providing care by the majority of professionals in both acute and community services. Within the acute hospital, most nurses and therapists providing standard care in the adult medical directorate were aware of the service, although comprehensive knowledge of the service was not uniform. Generally, when CIRACT staff were colocated with ward-based acute staff they were seen in more positive and supportive terms:
They’re a nice team and it’s quite interesting hearing what they do and getting advice occasionally.
Standard service occupational therapist interview, 03/12/13 (lines 516–17)
The whole idea about it is perfect because you see all these old people are so worried when they go home.
Senior ward nurse interview, 13/11/13 (lines 74–5)
They [patients] like independence but they are lonely and family live far. They value that and the faster we deal with them the better. There are some people who don’t want to go home because of their loneliness but then, you know, it [CIRACT] just encourages them to be at home with the extra help they get after the discharge.
Senior ward nurse interview, 13/11/13 (lines 294–2)
I think it works really well in being able to give the family a handover about this is what happened in hospital. This is what they’re going to do now when they’re at home. I think it alleviates a lot of their concerns. They don’t just feel that their relative is going home and may struggle with what do they do. They know they’ve got somebody to call on.
CIRACT occupational therapy lead interview, 10/12/13 (lines 90–6)
I was talking to the OT [occupational therapist] on the ward this morning and I said, ‘Oh our lady’s going home today. I’m going to follow her out and just check her mobility one last time because I just want to make sure the home can manage her’, and she went, ‘Oh that must be nice. I wish I could do that. That must really like put your mind at ease and things’. So I think when other people say to you, ‘Oh I wish I could do that’, you realise that that is a real good thing about the service that other people wish they were able to do.
CIRACT physiotherapist interview, 21/11/13 (lines 125–31)
At the same time, there was widespread ambiguity among ward staff about the types of tasks undertaken by the CIRACT team in the community, with very little concept of the continued intensive therapy. This suggests that organisational boundaries between acute and community care continue to influence the views of staff and that elements of the CIRACT team’s work were not always appreciated by those in the hospital setting, potentially leading to tension between groups in terms of workload:
I think probably every time it’s not the same person that goes back from the CIRACT team to home to see them. It could be different people. I think so. I’m not sure. I’m not sure how they follow it up when they get discharged.
Senior ward nurse interview, 13/11/13 (lines 84–8)
Health-care professionals openly voiced concerns about the erosion of professional roles and responsibilities as a result of how the CIRACT service reconfigured work within the hospital setting and allowed ‘outsiders’ to encroach onto ward work. Concerns were also raised about the dual role of therapists, that is, physiotherapists performing the work usually associated with occupational therapists. The CIRACT service was seen to challenge the established professional boundaries of practice among the hospital-based therapists:
My worry about the lower grades of staff doing that role is that you don’t expect a band 5 physio to be able to do the role of a full physio, never mind a physio and an OT [occupational therapist], and I know there’s a lot of generic band 4s and 3s, and you don’t expect someone qualified that hasn’t been at uni for three years or done placements and things, if you don’t expect them to be able to do both roles, how can we expect somebody less qualified with less experience? You do get the odd exception from that but we have had some band 4s that are generic and we’ve found it very tough to train them, and my worry is yes they may be able to do a very basic job, but things get missed because they haven’t got that depth of knowledge and experience. The elderly are very complex.
Standard service senior physiotherapist interview, 31/01/14 (lines 436–45)
By contrast, ward nurses considered the service to be a positive in addressing the increasingly complex and growing needs of older people. Their concerns lay within the logistics and litigation prospects of working across the acute/community boundary. One senior nurse considered that the CIRACT team would benefit from the inclusion of a dedicated qualified nurse to provide specialist monitoring care such as interpreting blood pressures. This is an interesting finding as it highlighted the potential challenges of rolling out a service in which professional boundaries of practice could frequently overlap or seem ambiguous.
Changing cultures of care
The majority of health and social care professionals took a positive view towards the CIRACT service and considered it a positive step towards comprehensive care for older people with complex needs. There were divergent opinions about the impact that the service made to the entire NHS service locally but generally it was recognised as a small pilot service trying to make a difference. The majority of professionals who commented on the CIRACT service could only do so from within their own working boundary, which tended to be either acute hospital or community based. With the exception of the community managers and the academic lead, professionals could form opinions only of the parts of the CIRACT service drawing mostly on their own work with the team members and their previous experiences of community care.
There were concerns about the changing grades of staff and overlaps within physiotherapy and occupational therapy by the standard service teams:
I think in principle more senior staff doing a bit of OT [occupational therapy] and OT doing a bit of physio, I think it is helpful. You can do the basics . . . in concept taking on these roles, we’re half-way there anyway.
Standard service senior physiotherapist interview, 31/01/14 (lines 454–60)
Hospital-based staff perceptions of the types of service provided by CIRACT within the community locations were more constrained than community-based staff perceptions. Hospital-based therapists tended to view the CIRACT service as providing an emotionally supportive role towards patients on discharge rather than continuing intensive therapy and securing longer-term goals of care with other agencies. There was a sense of the CIRACT service undertaking some monitoring of well-being in the home although what this consisted of remained unclear:
I’m not able to follow them [patients] up and actually hold their hand when they get in [home]. I think that if they had that reassurance, just knowing that someone cared. And we do care. It’s just our actions suggests that we’re not there.
Standard service occupational therapist interview, 02/03/14 (lines 96–9)
There was mixed evidence of professional reflection about the CIRACT service in terms of changing the cultures of organisation. Nursing professionals considered the service as non-viable in terms of fitting in with the current financing and target-driven nature of the NHS as a whole. They did consider that the service design matched patient need and that the service acted as a more proactive rather than a reactive service.
There was no evidence of the emotional labour required to sustain such an intensive therapy service. The nature of the CIRACT service entails regular contact with patients and it was inevitable that close relationships were formed. Staff were observed to be genuinely upset when informed of a patient having died after the end of therapy and were often openly moved by the conversations of suffering and loss among families. This aspect is probably worthy of further work because the team did not seek extensive emotional support from each other but seemed to draw on their spiritual beliefs.
The THB-Rehab therapists tended to take a more sceptical view of the transferability of the service across the whole directorate and were concerned about their own terms of employment rather than it challenging the organisation as a whole. The service was not regarded as meeting patient need in any more ways than the THB-Rehab service except in relation to extending therapy beyond the inpatient stay. The community health-care professionals tended to regard the service as valuable but exclusive because of its limited capacity. There were some barriers to accessing GP services by the CIRACT team because of the lack of knowledge about the service among some practices.
Chapter 6 Health economic study methods and results
Cost analysis
A detailed microcost analysis of the two services was considered using a three-phase TMS. Phases 1 and 2 were used to determine the TMS methodology and phase 3 consisted of the TMS itself:
-
Phase 1 – Structured literature review (see Phase 1 systematic review).
-
Phase 2 – Observations and semistructured interviews. In-depth workplace observations and semistructured interviews were undertaken as part of the qualitative appraisal (see Chapter 5) to map out key events and activities in the provision of the CIRACT service and the THB-rehab service, with additional observations undertaken in community settings.
-
Phase 3 – Using the information from phases 1 and 2, the microcosting analysis (TMS) was defined and then conducted to identify, measure and value resource use and costing from a NHS perspective. Eleven patients in the CIRACT arm and 11 patients in the THB-rehab arm were recruited through purposive sampling over a 6-month period. Within each intervention arm, as far as possible, half of the participants had a baseline admission Barthel ADL score of < 14 and the other half had a Barthel ADL score of > 14 to ensure that a wide range of abilities were observed.
The staff costs (midpoint pay bands) for time spent on both services were recorded. Annual gross rates were converted into rates per minute assuming 42 weeks (37.5 hours per week) a year of work after annual leave and sick leave. All costs were calculated in UK pounds sterling (2014 prices) and all staff unit costs were inflated by 15% to take account of salary on-costs faced by employers.
Cost-effectiveness analysis
A cost (utility) analysis was undertaken in line with the National Institute for Health and Care Excellence (NICE) reference case44 to compare NHS and PSS costs with QALYs using established methods. 45 The economic evaluation was based on all costs and benefits incurred during the 12 months following randomisation. The EQ-5D-3L scores were converted into QALYs using linear interpolation and area under the curve methods for the trial period. 46 These were adjusted for baseline differences between the groups. 47 The results were presented using the incremental cost-effectiveness ratio (ICER). The costs and benefits were not discounted as the study was carried out over a period of 12 months.
Primary and community care costs included those for:
-
GP consultations (telephone and home visits)
-
nurse consultations (practice nurse and district nurse)
-
dietitians
-
community health workers
-
referrals to a complementary therapist
-
pharmacists
-
the rehabilitation team (physiotherapist/occupational therapist)
-
inpatient stays
-
outpatient visits (including A&E visits, NHS walk-in centre visits)
-
A&E visits
-
visits to a NHS walk-in centre.
Direct costs to PSS included costs for:
-
social workers
-
home help/community care assistants
-
admissions to a residential/nursing home
-
visits to a day centre
-
Meals on Wheels
-
community equipment and household adaptations paid by social services.
Measurement of resource use
A modified CSRI questionnaire32 was designed to obtain resource use data, with data collected through established hospital and community databases and participant telephone interviews.
Unit costs for PSS were obtained from the Unit Costs of Health and Social Care,48 NHS reference costs49 (see Appendix 8), salaries based on the NHS Agenda for Change scale50 and other published sources as appropriate. These were then combined with resource use data to produce a cost per patient. All costs were valued in UK pounds sterling at 2014 prices.
Results
Microcosts analysis
Phase 1: systematic review
The search was conducted using the following databases: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and The Cochrane Library including the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) database. No date limits were set and so searches were conducted from the date of database inception to the date that the searches occurred (22 July 2013). The search strategy included keywords to encompass:
-
TMS methodology: i. exp ‘time and motion studies’; ii. *’workflow’; i, or ii
-
setting: i. hospitals; ii. community; iii. general; iv. private; v. public; vi. rural; vii. satellite; viii. special; ix. teaching; x. urban; xi. tertiary care centers; i, or ii, or iii, or iv, or v, or vi, or vii, or viii, or ix, or x, or xi
-
therapy: i. home care services; ii. hemodialysis, home; iii. home infusion therapy; iv. home nursing; v. parenteral nutrition; vi. hospices.
A term from each section was included in all searches, that is, a + b + c.
Studies were limited to those in the English language, those in humans and those in which continuous observations were made, in either an inpatient or a community health-care rehabilitation setting. As the rationale for this review was to inform the methodological design of the TMS, papers were also excluded if they did not state the total observation time, the observation pattern, the length of time continuously observed or the type of observer recruited (n = 34 papers excluded on this basis). No restriction was placed on the type of study to be included as long as a TMS formed at least part of the study. Two members of the research team independently screened the titles and abstracts of all retrieved citations for potential inclusion based on the above criteria. For articles lacking an abstract but having a relevant title, the full text was obtained to determine eligibility. Full-text versions of the papers were then acquired and reviewed by at least two of the four reviewers from the research team to determine the final list of articles.
Data extraction was completed separately by two members of the research team and any disagreements were resolved by consensus; when needed, a third reviewer’s opinion was sought. Data extraction forms were standardised and based on the suggested TMS proposed by Zheng et al. 40 The data extraction forms included setting, methodology (e.g. pattern, total time), observer characteristics (e.g. training and number of observers), tasks observed and data recording (see Appendix 9). As this review focused on the TMS design only, no tools were used to assess for research bias. So as not to exclude potentially well-documented TMS designs, all studies that had incorporated a TMS into their methodology were reviewed.
After removing duplicates, 235 titles and abstracts of papers were screened. Of these, 142 papers were excluded based on the title and abstract, leaving 93 full-text papers to be screened for eligibility. Of these, 75 papers were excluded as they did not fit the inclusion criteria and two papers were unobtainable, even though interlibrary requests were submitted and the authors were contacted, leaving 16 papers51–66 included in the review (Table 9).
| Author (year) | Participants in the TMS (n) | TMS aims | Total duration (time observed) | Observation pattern | Continuous observation time (no. of observations) | Observers (n) | Task categories (n) | Collection tool |
|---|---|---|---|---|---|---|---|---|
| Douglas 201351 | RGNs: AICU (n = 78); CICU (n = 65); PICU (n = 26); NICU (n = 66) total (n = 235) | Observe activities performed by the RGNs and compare time spent on various tasks across four ICUs | 3 months out of 12 months (147 hours: AICU 37 hours; CICU 35 hours; PICU 37 hours; NICU 38 hours) | All shifts and days of the week | 1.5–3 hours (n = 58) | MSc RGN; human factors engineering PhD student (n = 2) | Direct patient care, care co-ordination, indirect patient care, non-patient care (n = 4; 17 subcategories) | Logger software on tablet |
| DeClifford 201264 | Clinical pharmacists assigned to general and surgical wards (n = 9) | To quantify the time that clinical pharmacists spend on various activities over their working day prior to the implementation of an EMMS | 1 month out of 12 months (265.3 hours) | Working day (0830–1700) | Average of 8 hours and 50 minutes (not stated) | Pharmacy students (n = 2) | Tasks separated into clinical and non-clinical activities (n = 7; 18 subcategories) | Laptops |
| Chisholm 201155 | Physicians (n = 85) | Evaluation of activities of physicians in academic and community EDs | 2 months out of 12 months (406 hours) | Non-randomised observation of all shifts, including weekends and nights | 2 hours (203 2-hr observations) | Trained observers (n = 2) | Direct/indirect patient care, personal activities (n = 3) | Standardised data collection form |
| Dasgupta 201154 | Nurses (n = 8) | Evaluation of workflow variables related to bedside barcode technology for medication administration | 60 days (517 hours) | Morning at 0700 | 4–5 hours (n = 106) | Data collector (n = 1) | Direct/indirect care, administration (bedside and non-bedside), miscellaneous, other activities (n = 4) | Paper data collection; two stopwatches |
| Hauschild 201153 | Anaesthetists (n = 20) | Evaluation of anaesthetists’ workload | 60 days (517 hours) | Three shifts on 3 different weekdays during the day | Average 8.37 hours (not stated) | RA (n = 1) | Ward round, history taking, communication in meetings or with papers, administration, intensive care, anaesthesia, either local or spinal or termination, induction, monitoring, research, education, emergency, problems, breaks, miscellaneous, transit, supervision (n = 20; 200 subcategories) | Mobile computer using specially designed software |
| Mache 201157 | Haematology and oncology doctors (n = 21) | To assess how much time oncologists and haematologists spend on their various work tasks | 6 months out of 12 months (626.2 hours) | Weekdays during the day | Average 9 hours, 56 minutes (n = 63 days) | RA (n = 2) | Internal/patient communication, indirect/direct patient care, ward rounds, walking time, admissions, breaks, supervision, teaching, miscellaneous activities, non-value-added work (n = 12; 70 subcategories) | PDA |
| Weigl 201156 | Doctors in internal medicine and surgery (n = 22) | Workflow interruptions, i.e. an intrusion of an unplanned/scheduled task causing a discontinuation of tasks | 32 shifts (277.4 hours) | Day shifts | Average 8 hours, 40 minutes (n = 32 shifts) | Trained observers (n = 2) | Patient/staff/others communication, diagnostic activities, therapy, documentation, other activities (n = 10 interruptions, seven activities) | Paper recording sheets |
| Westbrook 201152 | Nurses (n = 57) | Work patterns of RGNs on wards and how these changed over 2 years | 4 months out of 12 months (191.3 hours) | Weekdays (0700–1900) | 1 hour (not stated) | Experienced RGN and medical doctor (n = 2) | Direct/indirect care, medication tasks, documentation, professional communication, ward-related activities, in transit, supervision, social, other (n = 10) | PDA |
| Westbrook 201058 | Physicians in the ED (n = 44) | ED physicians’ rates of interruption and task completion times and rates | 6 months out of 12 months (210.45 hours) | Weekdays 0800–1800 | 131 sessions (9588 events) | Trained observer (n = 1) | Direct/indirect care, medication, documentation, communication, administrative, in transit, supervision, social, pager (n = 10) | PDA |
| Weigl 200959 | Physicians: internists and surgeons (n = 23) | Evaluation of activity patterns, time allocation and simultaneous activities of hospital physicians | 35 day shifts (303.1 hours) | Weekdays | Average 8 hours, 39 minutes (1757 surgeon activities, 2493 physician activities) | Psychologist and medical student (n = 2) | Direct/indirect patient contact, all patient-related activities, other professional/personal activities (n = 4) | Not stated |
| Yen 200960 | Physicians and nurses (n not stated) | Effect of computer physician order entry on ED staff | Not stated (252 hours before, 120 hours after) | Each participant two shifts evenly distributed (weekdays and weekends) | 21 observation periods both before and after | Trained observers (n not stated) | Direct/indirect patient care, other (n = 3 major) | Data sheet and timer |
| Keohane 200865 | Nurses (n = 108) | To evaluate nurses’ work patterns | 6 months out of 12 months (232 hours) | 2 hours per shift (0730, 0930, 1130, 1400, 1800) | 2 hours (n = 116) | RAs (n = 2) | 112 activities in 12 major categories, i.e. information retrieval, doctor’s requests, waiting, medication – obtaining and verifying, delivery, documentation; patient care, communication, computer, miscellaneous | Tablet |
| Westbrook 200861 | Doctors (n = 19) | Evaluation of time spent on work tasks and with health professionals and patients | 6 months out of 12 months (151 hours) | Weekdays 0830–1900 | 10.5 hours (n = 6243 tasks recorded) | Experienced RGNs (n = 2) | Direct/indirect care, medication, documentation, communication, administrative, in transit, supervision/education, social, pager (n = 10) | PDA |
| Ampt 200762 | Nurses (n = 44) | To ascertain the amount of time nurses spend on routine activities, with emphasis on medication tasks following EMMS | 8 months out of 12 months (15 hours) | Weekday shift, average 8.75 hours | Average 1 hour (not stated) | Experienced nurses (not stated) | Medication task, direct, indirect, documentation, professional communication, ward-related activities, in transit, supervision, social, other (n = 10; three medication subcategories) | PDA |
| Lo 200766 | Specialists (n = 17) | Time changes pre and post EHR implementation | 2 months out of 12 months (109 hours) | Outpatient urban speciality clinics within a hospital setting | 3.6 hours (30 observations) | RAs (n = 5) | Direct/indirect, other, administrative, miscellaneous (85 activities in five categories) | Touch screen computer running Access |
| Morton 200463 | Surgical residents (n = 12) | To describe what on-call surgical residents do | 7 months out of 12 months (144 hours) | 0700–1900 | 12-hour shift (n = 6) | MD/PhD students (not stated) | ADL, patient evaluation, communication, miscellaneous, pages, procedures (n = 6) | Stopwatch and PDA |
Data were extracted from the articles using the TMS design template (see Appendix 9).
These articles described the observation of several types of participants including nurses, physicians, pharmacists and anaesthetists. The majority of the papers used a TMS to describe work flow efficiency; others focused on interruptions or workflow changes because of a new computer system being implemented. The TMS methods varied across the studies with the time of continuous observation ranging from 1 to 12 hours, most often scheduled according to shift patterns. The number of participants being observed also varied between eight and 235 with a mean of 48.27 participants. The observations were conducted most often by researchers (n = 9); other observers were students (n = 4) or health professionals (n = 3). The number of observers collecting the data ranged from one to five with a mean of two. The most cited tool for inputting the data from the observations was a hand-held computer/tablet or laptop (n = 11). The task categories that were used in the studies were broadly categorised into direct and indirect patient care, with half of the studies breaking the general tasks down into further categories and subcategories (n = 8). The number of categories ranged from 3 to 20 (mean 8.19) and the number of subcategories ranged from 7 to 200 (mean 54.88).
In all of the studies, TMS data were collected within a hospital setting, with little mention of data collection within a community setting. This literature review offered insight into the various nuances of continuous observation TMSs that are currently being used within research, although because of the variability among the included literature firm conclusions were not possible, calling for a more standardised approach to conducting and reporting TMSs.
Phase 2: observation and semistructured interviews
The observations and semistructured interviews conducted are outlined in Chapter 5.
The main findings from the phase 1 systematic review and the phase 2 observations and semistructured interviews related to the health economics study were as follows:
-
The extreme variability in how TMSs were conducted, what data were collected and how they were analysed and reported made it difficult to pool the findings across studies.
-
90% of the included studies conducted their observations during normal daytime working hours.
-
Direct patient care was defined as time when the health-care professional was interacting with the patient and indirect patient care was defined as any non-face-to-face interactions that took place, that is, pre and post consultations, handover observations, ordering of equipment, liaisons with family/other care providers, travel and any other time spent working on behalf of the patient while not in face-to-face contact.
-
Although the participant was the focus of the observation, continuous 24-hour observations were not possible and observations at fixed intervals may not result in observation of tasks completed at other times and not in the participant’s presence.
-
Self-reporting through a research team liaising with therapists was quantifiably different from observational methods, with the reporting of fewer activities and longer mean activity times or logs not being completed.
-
Home data collection was very complex and difficult.
We proposed a work-sampling observational methodology TMS to record therapy interactions with the participants (both face-to-face and indirect) to enable microcosts to be calculated. This would be conducted by the research team contacting the THB-Rehab team or the CIRACT team (depending on allocation) on a twice-daily basis. The research team would ascertain how much time therapists spent in direct face-to-face contact with participants and how much time they spent indirectly, through non-face-to-face contact, that is, ward rounds, telephone calls, referrals, etc. To capture the contacts with therapists post discharge, the research team would ascertain from the therapists whether or not participants had been referred on for further therapy and, if so, would contact the treating therapists in the community to gather contact time [face to face and indirect (including travel time)] for up to 2 weeks following discharge from hospital (see TMS case record form in Appendix 10). However, this method meant that the wider NHS costs, that is, hospital care and primary care costs, were not captured, nor the social services detailed costs, and therefore it would not be possible to use the costs from the TMS to inform the economic evaluation as originally planned. The TMS was thus only able to inform the cost of the delivery of the two types of services.
Phase 3: time and motion study cost analysis
The mean time per face-to-face in-hospital participant contact was 4 hours 9 minutes for the THB-Rehab service (SD 2 hours 55 minutes, minimum 1 hour, maximum 8 hours 35 minutes) and 4 hours 48 minutes for the CIRACT service (SD 3 hours 4 minutes, minimum 1 hour 10 minutes, maximum 9 hours 10 minutes). The mean time per indirect in-hospital participant contact was 4 hours and 37 minutes for the THB-Rehab service (SD 2 hours 40 minutes, minimum 45 minutes, maximum 8 hours 45 minutes) and 4 hours for the CIRACT service (SD 3 hours 4 minutes, minimum 1 hour 5 minutes, maximum 11 hours 30 minutes).
The staff cost per minute was estimated and then multiplied by the length of time associated with face-to-face and indirect interactions on the ward and at home post discharge. The mean cost per participant for the CIRACT service was £302 (SD £196, 95% CI £185 to £418; n = 11) and the mean cost per participant for the THB-Rehab service was £303 (SD £129, 95% CI £226 to £379; n = 11). Table 10 shows the breakdown of mean cost per participant in the hospital and the community setting.
| Service | Mean (SD) face-to-face contact time; min., max. (hh:mm) | Mean (SD) indirect contact time; min., max. (hh:mm) | Mean (SD) cost face-to-face contact time (95% CI) (£) | Mean (SD) cost indirect contact time (95% CI) (£) |
|---|---|---|---|---|
| In-hospital CIRACT service | 4:48 (3:04); min. 1:10, max. 9:10 | 4:00 (3:04); min. 1:05, max. 11:30 | 104 (68) (61 to 146) | 86 (62) (47 to 124) |
| Community CIRACT service | 2:39 (2:02); min. 0:30, max. :00 | 3:14 (1:54); min. 0:45, max. 8:00 | 58 (49) (29 to 88) | 70 (47) (42 to 98) |
| Total cost | 302 (196) (185 to 418) | |||
| In-hospital THB-Rehab service | 4:09 (2:55); min. 1:00, max. 8:35 | 4:37(2:40); min. 00:45, max. 8:45 | 83 (58) (33 to 132) | 108 (72) (44 to 173) |
| Community rehabilitation (non-THB-Rehab service) | 5:53 (8:52); min. 0:00, max. 16:05 | 1:48 (2:33); min. 0:06, max. :24 | 92 (136) (7 to 177) | 29 (39) (5 to 54) |
| Total cost | 303 (129) (226 to 379) | |||
Cost-effectiveness analysis
The primary care, hospital and social care resource use is presented in Appendix 11. There was very little evidence of a difference in resource use between the CIRACT group and the THB-Rehab group.
Table 11 shows the mean costs and QALYs for the two groups. The mean cost (unadjusted for baseline differences) for the CIRACT intervention was £3683 and for the THB-Rehab service £3659, giving an incremental cost of £24 (95% CI –£1631 to £1583) for the CIRACT service. The estimated unadjusted mean QALYs for the CIRACT intervention were 0.417 and for the THB-Rehab service were 0.309 (mean QALY difference 0.108, 95% CI –0.0128 to 0.2281). The ICER was £222 per QALY (unadjusted)
| Analysis | Intervention arm | |
|---|---|---|
| CIRACT (n = 81) | THB-Rehab (n = 88) | |
| Total mean (SD) cost, unadjusted (£) | 3683 (5188) | 3658 (5396) |
| Total mean (SD) cost, adjusted for baseline difference (£) | 3744 (470) | 3603 (465) |
| Incremental cost (95% CI) (£) | 141 (–1645 to 1934) | |
| Mean (SD) QALYs, unadjusted | 0.417 (0.395) | 0.309 (0.397) |
| Mean (SD) QALYs, adjusted | 0.846 (0.109) | 0.806 (0.109) |
| Incremental benefit: QALY gain (95% CI) | 0.0404 (–0.0566 to 0.1375) | |
| ICER using bootstrap sample (95% CI) (£) | 2022 (–76,895 to 121,856) | |
When the baseline differences in the CIRACT and THB-Rehab services were adjusted, the difference in cost between the treatments was greater (£141, 95% CI –1645 to 1934) and the difference in QALYs was 0.0404.
Using a non-parametric bootstrap with replacement method and 1000 replications (see Appendix 12), the mean ICER for the CIRACT service compared with the THB-Rehab service was £2022 per QALY (£145/0.0404) (the discrepancy in the numbers is caused by a rounding effect). The net monetary benefit per patient per year (willingness to pay threshold of £30,000 per QALY) was £1932 (95% CI –£2134 to £5863) and the probability that the intervention is cost-effective was 0.909. This shows that the CIRACT service may be a cost-effective intervention for patients, although these results should be interpreted with caution given the small differences and wide CIs.
Table 12 shows the EQ-5D-3L scores at baseline and day 91. There were no significant differences between the two groups at either time point.
| Time point | Intervention arm | |
|---|---|---|
| CIRACT | THB-Rehab | |
| Baseline | 0.341 | 0.256 |
| Day 91 | 0.417 | 0.400 |
Chapter 7 Discussion and summary
The CIRACT service consisting of a senior occupational therapist ‘transition coach’, a senior physiotherapist and an assistant practitioner, linked directly to a social worker and working more closely across multiple boundaries with patients and their carers, did not shorten hospital LOS or reduce short-term readmission rates compared with THB-Rehab. Our findings are in contrast to those of the systematic review by Shepperd et al. ,3 which reported that a structured discharge plan tailored to the individual patient brought about small but significant reductions in hospital LOS and readmission rates for older people admitted to hospital with a medical condition (mean difference –0.91 days, 95% CI –1.55 to –0.27 days). However, our findings are similar to those in the review by Bachmann et al. ,4 which showed no significant difference in hospital LOS for inpatient rehabilitation specifically designed for geriatric patients.
Important factors in the interpretation of these reviews are the definition of the intervention, where it is delivered and the subsequent understanding of the relative contribution of each member within the team. It was acknowledged in the review by Shepperd et al. 3 that, although the authors of all of the trials provided some description of their interventions, it was not possible to assess how some components of the process or working make-up of the team members compared between trials. Nevertheless, a patient education component was included in the majority of these trials on discharge planning, which was not part of the CIRACT intervention. In the review by Bachmann et al. ,4 half of the studies included patients in community hospitals; this is in contrast to our study, which included patients undergoing acute hospital care.
The context in which an intervention such as discharge planning is delivered may play a role not only in the way that the intervention is delivered, but also in the way that services are configured, which may explain some of these differences. In the systematic review by Shepperd et al. ,3 10 of the trials included were based in the USA, five in the UK, three in Canada, one in Australia, one in Denmark and one in France. In the systematic review by Bachmann et al. ,4 of the eight general geriatric rehabilitation studies, only two were UK studies, both of which were undertaken in community hospitals. 67,68 In each country the orientation of primary care services differs, which may affect both the delivery of and the communication between services. Different perceptions of care by professionals in alternative care settings and country-specific funding arrangements may also influence timely discharge. Of the five UK studies in the review by Shepperd et al. ,3 three were studies of psychiatric inpatients, one was a study of stroke patients and only one was a study of general medical inpatients. The point in a patient’s hospital admission when discharge planning is implemented also varied across studies. 3,4 Two trials reported discharge planning commencing from the time that a patient was admitted to hospital69,70 and another study reported that discharge planning was implemented 3 days prior to discharge. 71 The timing of delivery of an intervention such as discharge planning, which depends on organising other services, will have some bearing on how quickly these services can begin providing care. Although the CIRACT service was different from the THB-Rehab service, both services fed into similar community-configured services and therefore bottlenecks in providing community services (e.g. community care support provided by social services) may have led to delays in both groups and potentially masked any significant differences in hospital LOS.
The readmission rate was a secondary outcome but was also recognised as an important outcome. Our findings are in contrast to those of recently published studies that have evaluated similar transition care models. The systematic review by Sheppard et al. 3 reported a 15% reduction in the 30-day readmission rate. The BOOST toolkit, which included a number of similar components to those in the CIRACT service (risk assessment, medication review, discharge checklist and a MDT-based approach to the discharge process), showed modest reductions (2–6%) in 30-day readmission rates. 5 The TCM, which incorporated a multidisciplinary approach to patient care, led by a transitional care nurse ‘transition coach’, who followed patients from the hospital to home, facilitated communication with outpatient providers and performed a series of home visits and telephone follow-up calls in the post-discharge period, showed a reduction in the 90-day readmission rate of between 13% and 48%. 12,13
Other models that have been successful, but whose key interventions were not included as part of our CIRACT service, included a more focused review of medicine management and the development of a portable personal health record. Project RED focused on a multidisciplinary approach to patient care co-ordinated by a nurse discharge advocate. 7 The discharge advocate engaged patients during their admission to hospital and provided clinical information and an individualised, illustrated plan post discharge. However, following discharge, a pharmacist performed a telephone follow-up including a medication review with direct communication to the primary outpatient provider. There was a non-significant 6% reduction in the 30-day readmission rate and a significant 8% reduction in 30-day visits to the A&E department post discharge. In the CTI, the four key components of CTI were defined as (1) medication management, (2) development of a personal health record that is carried from site to site, (3) close follow-up with a primary care provider and (4) the identification of ‘red flags’ and indications that would prompt patients to contact providers. An advanced practice nurse ‘transition coach’ performed the post-discharge home visits and telephone calls, emphasising patient engagement and self-management in the care of chronic diseases. This programme reduced 30-day readmissions by 4–6%9,10 and the 90-day readmission rate by 6–22%. 11,12
Another feature of many of the successful studies is the management of patients with specific chronic diseases, such as congestive cardiac failure, chronic obstructive airways disease and stroke disease,72–74 rather than patients who have a high prevalence of comorbidities, as in this study [mean age 84 years, mean MMSE score 22 out of 30 (significant cognitive impairment), mean Barthel ADL score 10.7 out of 20 (significant disability) and mean comorbidity score 7.4 (no comorbidities = 0)]. In disease-specific states, medication management may have a greater role in patient outcomes and interventions that address this may be more effective.
There are several possible reasons why the CIRACT service did not shorten hospital LOS or reduce short-term readmission rates compared with the THB-Rehab service, as explored in the qualitative phase of our study. The microcosting study showed that the time spent with participants, particularly during the inpatient stay, was not very different between the two services. Whether or not a more intensive intervention would have been of greater benefit is unclear. Interestingly, however, some of the CIRACT participants disliked the intensity of the CIRACT service provided. Many participants also found the period following cessation of the CIRACT service very difficult to manage, despite comprehensive attention being given to prepare for this transitional period. This may therefore have limited the benefits of the intervention. Additionally, it must be recognised that discharge from hospital is a complex process and, although the CIRACT service was able to provide better communication across agencies, local resource profiles, especially bed availability and staffing, influenced the implementation of the service. Local social services have seen a significant reduction in budgets over the last few years, which has resulted in a reduction in community care providers, a reduction in re-enablement services and a reduction in the availability of community residential rehabilitation. Therefore, although patients may have been ready for discharge at an earlier stage, or may possibly have required less support, the wait for community beds/social services may have led to unexpected delays that were outside the control of the CIRACT intervention. In addition, increased winter bed capacity appeared to divert patients away from the CIRACT service, thereby rendering it marginal to service delivery, and, later, the lack of specialist staffing further reduced the capacity of the CIRACT service.
Another potentially important reason why the CIRACT service did not reduce hospital LOS may be related to the difficulties at the interface between the CIRACT service and existing community services and therefore the introduction of an additional boundary. Rather than removing organisational boundaries, the CIRACT team may have introduced boundaries into the inpatient ward environment. A central tenet of multidisciplinary working is that the same team should make the assessment of need as the team that delivers the multidisciplinary intervention. Elements of patient care required but not available from the composition of the CIRACT team may have been more difficult to implement. In particular, nurses on the ward felt a little more excluded by the CIRACT team (as identified in the qualitative interviews) than when working with the THB-Rehab team.
With respect to readmissions, it is important to recognise that the reasons for readmissions in the frail elderly are multifactorial. A number of these have been previously discussed and include patient safety, with high rates of medication errors,17–20 incomplete or inaccurate information on transfer75 and lack of appropriate follow-up care. 22 The transition coach will have addressed many of these issues; however, the frailty of this group of patients cannot be underestimated. Poor compliance with medication and instability of chronic disease are common problems and may require ongoing medical input in the community, which was not part of the CIRACT service. Another factor may possibly be related to the ‘application of change model’. The CIRACT service does have the potential to mediate and transcend the usual boundaries between acute and community care sectors, based on closer and more continuous working with the patient, and to some extent enhanced communication with care providers in both acute and community settings. However, it was evident that this service did not lead to a more sustained integration between care settings and, without it bridging this gap, it remains possible that care services remained decoupled or poorly integrated. That being said, those patients enrolled into the CIRACT service did experience more integrated care based on earlier and more patient-centred care planning from a stable and consistent care team and, for these patients, rehabilitation and care appeared to be more seamless and enhanced patient recovery and experience.
A number of limitations are recognised in this study. First, there was no blinding between the groups. Both the CIRACT intervention and THB-Rehab services were conducted across the same wards, with participants individually randomised. Therefore, it is difficult to assess the extent to which contamination occurred between groups. However, bias is probably very limited in that early pilot work showed that both patients and staff seemed unaware which of the two services was responsible for patient rehabilitation, given the busy and fluid nature of the acute ward. In addition, patients readmitted back to the trial wards during the study had no recollection of the rehabilitation service that they had previously received. Second, patients were recruited from a single catchment area, with a high number of patients excluded who were not living in the study catchment area but who were admitted to the medical wards where participants were being actively treated. Thus, on some occasions the case workload of the THB-Rehab staff was almost twice that of the CIRACT team, although there were no significant differences in any of the outcomes. The third and perhaps the most important limitation was the power of the study. The study was powered to show a large difference in hospital LOS between the two groups of 3 days, based on our early pilot work. The lower CI would have been unable to exclude any significant differences of < 2.3 days, which clearly are important. Finally, the microcosting study excluded the estimation of the wider NHS and social care costs for the CIRACT and THB-Rehab services. It was not feasible to undertake a microcosting study to identify this resource use and cost over a wider NHS setting as the CIRACT service was a complex intervention that involved multifaceted processes involving a large number of different health-care professionals and services and differing times to deliver services. To capture such an intervention was a burdensome task to undertake. Therefore, we employed a combination of a gross costing method to estimate the cost to the NHS (primary, community and hospital care) and direct costs to PSS using standard cost questionnaires and the method of microcosting to cost the delivery of this intervention alone, as this is a new intervention and there was no evidence available to inform assumptions about expected resource use and costs to deliver the intervention.
Summary
In conclusion, the CIRACT service as a complex intervention did not reduce hospital LOS or short-term readmission rates, although it was highly regarded by those most involved with it compared with the THB-Rehab service. The estimated ICER appears to be cost-effective although it is subject to much uncertainty that spans all four quadrants of the cost-effectiveness plane such that caution should be used in interpreting this result. Microcosting (TMS) work-sampling observational methodology provides a useful means of estimating the cost of a service provision.
Chapter 8 Implications for practice
Organisational and contextual issues
-
Change in one service configuration impacts on the whole system, in this case both NHS and social care and across acute and community provision, which requires strong consistent leadership across the whole care pathway.
-
Readmission rates to hospital for frail older people remain high and should be targeted for further intervention.
Communications
-
It important to recognise the need for a lead to act as a key cross-team and cross-location influencer with localised knowledge of services and the capacity to establish and maintain networks as a core part of their everyday work.
-
Colocation of teams supports knowledge and information sharing in proactive ways among experts, although this may introduce barriers in other ways.
Costing methodology
-
The method of microcosting is the most precise method available to identify and measure resource use before quantifying this into monetary units. It is particularly useful in estimating the costs of a new service for which no, or little, previous cost estimates exist.
-
In addressing the research question, choice of costing method(s) is a balancing act between accuracy and feasibility. In the present study the most feasible costing approach was a mixed approach using the most precise method of microcosting for costing the services and a less accurate method to cost the wider resource implications.
Chapter 9 Future research agenda
-
The CIRACT service may work only for a particular group of patients; therefore, further studies are needed that redesign roles and team configurations to support broad and expert knowledge capacity among medical and allied health and social care professionals.
-
These studies need to be adequately powered to detect small changes in LOS and possibly use cluster randomisation to reduce contamination bias.
-
Further research is also needed to explore whether or not continued, longer-term individual case management with a dedicated care worker may have an effect on readmission rates.
-
It is important to explore both health and social care costs, which may require more complex methods of patient tracking.
Acknowledgements
The study team would like to acknowledge and express thanks for the support and participation of the Nottingham University Hospitals NHS Trust and the CityCare CCG, as well as all individuals and groups who agreed to take part. In addition, further acknowledgement is needed for the patients and families who shared with the study team their personal and often protracted experiences of hospital care and community follow-up. The study team would also like to express thanks for the support of the initial trial manager Lisa Chalesworth and the interim trial manager Margaret Childs and for the support and guidance of the TSC [Dr Anne-Louise Schokker (chair), Mr Edwin Sum, Ms Kate Robertson and Professor Lee Shepstone].
Data will be archived by the Nottingham University Hospitals NHS Trust.
Contributions of authors
Opinder Sahota (Professor of Orthogeriatrics and Consultant Physician and lead and corresponding author) designed and led the research project, managed the data analysis and wrote the final report.
Ruth Pulikottil-Jacob (Research Fellow in Health Economics) and Tracey Sach (Associate Professor in Health Economics) co-designed the health economics phase and contributed to data analysis and report writing.
Fiona Marshall (Research Fellow) conducted qualitative research, collected data from interviews, observations and patient tracking and contributed to data analysis and report writing.
Alan Montgomery (Professor of Medical Statistics) and Wei Tan (Research Fellow) designed and conducted the statistical analyses.
Pip Logan (Professor of Rehabilitation Research), Denise Kendrick (Professor of Primary Care Research) and Maria Walker (Occupational Therapist) provided valuable advice and guidance throughout the study.
Alison Watson (Research Fellow) conducted the quantitative research, collected data from interviews, observations and patient tracking and contributed to data analysis and report writing.
Justin Waring (Professor of Organisational Sociology and Improvement Science Fellow) co-designed the project, conducted qualitative research, collected data from interviews and contributed to data analysis and report writing.
Patient and public involvement
The study has benefited from strong patient and public involvement from Mrs Rosemary Clacy and Mr Peter Cass, who were involved from the inception of the trial and during conduct of the trial and data analysis. Both were able to attend regular steering group meetings and individual smaller groups meetings, which were critical at the inception stage of the trial.
Data sharing statement
Because of the nature of the study and condition of ethics approvals we are unable to share data related to this study. Please contact the lead author for further information.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Martin GP, Currie G, Finn R. Reconfiguring or reproducing intra-professional boundaries? Specialist expertise, generalist knowledge and the ‘modernization’ of the medical workforce. Soc Sci Med 2009;68:1191-8. http://dx.doi.org/10.1016/j.socscimed.2009.01.006.
- Nancarrow S, Moran A, Freeman J, Enderby P, Dixon S, Parker S, et al. Looking inside the black box of community rehabilitation and intermediate care teams in the United Kingdom: an audit of service and staffing. Qual Primary Care 2009;17:323-33.
- Shepperd S, Parkes J, McClaren J, Phillips C. Discharge planning from hospital to home. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd000313.pub3.
- Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 2010;340:1718-22. http://dx.doi.org/10.1136/bmj.c1718.
- Wilkinson ST, Pal A, Couldry RJ. Impacting readmission rates and patient satisfaction: results of a discharge pharmacist pilot program. Hosp Pharm 2011;46:876-83. http://dx.doi.org/10.1310/hpj4611-876.
- Hansen LO, Greenwald JL, Budnitz T, Howell E, Halasyamani L, Maynard G, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med 2013;8:421-7. http://dx.doi.org/10.1002/jhm.2054.
- Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178-87. http://dx.doi.org/10.7326/0003-4819-150-3-200902030-00007.
- Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Ger Soc 2004;52:1817-25. http://dx.doi.org/10.1111/j.1532-5415.2004.52504.x.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006;166:1822-8. http://dx.doi.org/10.1001/archinte.166.17.1822.
- Parry C, Min SJ, Chugh A, Chalmers S, Coleman EA. Further application of the Care Transitions Intervention: results of a randomized controlled trial conducted in a fee-for-service setting. Home Health Care Serv Q 2009;28:84-99. http://dx.doi.org/10.1080/01621420903155924.
- Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The Care Transitions Intervention: translating from efficacy to effectiveness. Arch Intern Med 2011;171:1232-7. http://dx.doi.org/10.1001/archinternmed.2011.278.
- Naylor MD, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial. Ann Intern Med 1994;120:999-1006. http://dx.doi.org/10.7326/0003-4819-120-12-199406150-00005.
- Naylor MD, Brooten D, Campbell R, Jacobsen, MS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999;281:613-20. http://dx.doi.org/10.1001/jama.281.7.613.
- Blunt IM, Bardsley M, Dixon J. Trends in Emergency Admissions in England 2004–2009: Is Greater Efficiency Breeding Inefficiency?. London: Nuffield Trust For Research and Policy Studies in Health Services; 2010.
- Sager MA, Franke T, Inouye SK, Landefeld CS, Morgan TM, Rudberg MA, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med 1996;156:645-52. http://dx.doi.org/10.1001/archinte.1996.00440060067008.
- Department of Health . Timeseries A&Amp;E Attendance Data 2009. www.hscic.gov.uk/catalogue/PUB02558/acci-emer-atte-eng-2008-2009-pla.xls.
- Beers MH, Sliwkowski J, Brooks J. Compliance with medication orders among the elderly after hospital discharge. Hosp Formul 1992;27:720-4.
- Moore CJ, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to outpatient setting. J Gen Intern Med 2003;18:646-51. http://dx.doi.org/10.1046/j.1525-1497.2003.20722.x.
- Coleman EAJ, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005;165:1842-7. http://dx.doi.org/10.1001/archinte.165.16.1842.
- Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz Z, Juurlink DN, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med 2005;165:424-9. http://dx.doi.org/10.1001/archinte.165.4.424.
- Van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med 2002;17:186-92. http://dx.doi.org/10.1046/j.1525-1497.2002.10741.x.
- Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hopsitalisation. Am J Med 2001;111:26-30S. http://dx.doi.org/10.1016/S0002-9343(01)00966-4.
- Grimmer KA, Moss JR, Gill TK. Discharge planning quality from the carer perspective. Qual Life Res 2000;9:1005-13. http://dx.doi.org/10.1023/A:1016693825758.
- Nahab F, Takesaka J, Mailyan E, Judd L, Culler S, Webb A, et al. Avoidable 30-day readmissions among patients with stroke and other cerebrovascular disease. Neurohospitalist 2012;2:7-11. http://dx.doi.org/10.1177/1941874411427733.
- Melin AL, Hakansson S, Bygren LO. The cost-effectiveness of rehabilitation in the home: a study of Swedish elderly. Am J Public Health 1993;83:356-62. http://dx.doi.org/10.2105/AJPH.83.3.356.
- Parry C, Coleman EA, Smith JD, Frank J, Kramer AM. The care transitions intervention: a patient-centred approach to ensuring effective transfers between sites of geriatric care. Home Health Care Serv Q 2003;22:1-17. http://dx.doi.org/10.1300/J027v22n03_01.
- Rennke S, Ranji S. Transitional care strategies from hospital to home: a review for the neurohospitalist. Neurohospitalist 2015;5:35-42. http://dx.doi.org/10.1177/1941874414540683.
- Walker M, Sahota O. An Evaluation of a Community In-Reach Rehabilitation and Care Transition Service n.d.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Med J 1965;14:61-5.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. http://dx.doi.org/10.1016/0021-9681(87)90171-8.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 1999.
- Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the Mini-Mental State Examination and the standardized Mini-Mental State Examination: implications for clinical practice. Appl Nurs Res 2000;13:209-13. http://dx.doi.org/10.1053/apnr.2000.9231.
- Berghmans RLP, Ter Meulen RHJ. Ethical issues in research with dementia patients. Int J Geriatr Psychiatry 1995;10. http://dx.doi.org/10.1002/gps.930100803.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50-5. http://dx.doi.org/10.1186/1748-5908-4-50.
- Taylor FW. The Principles of Scientific Management. New York: Harper & Row; 1911.
- Burke TA, McKee JR, Wilson HC, Donahue RM, Batenhorst AS, Pathak DS. A comparison of time-and-motion and self-reporting methods of work measurement. J Nurs Admin 2000;30:118-25. http://dx.doi.org/10.1097/00005110-200003000-00003.
- Wirth PL, Kahn H, Perkoff GT. Comparability of two methods of time-and-motion study used in a clinical setting: work sampling and continuous observation. Med Care 1977;15:953-60. http://dx.doi.org/10.1097/00005650-197711000-00009.
- Mogyorosy Z, Smith P. The Main Methodological Issues in Costing Health Care Services: A Literature Review. York: Centre for Health Economics, University of York; 2005.
- Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc n.d.;18:704-10. http://dx.doi.org/10.1136/amiajnl-2011-000083.
- Wikipedia . Declaration of Helsinki 1996 n.d. https://en.wikipedia.org/wiki/Declaration_of_Helsinki (accessed 4 December 2015).
- Department of Health . Research Governance Framework for Health and Social Care 2005. www.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf (accessed 4 January 2016).
- Waring J, Marshall F, Bishop S, Sahota O, Walker M, Currie G, et al. An ethnographic study of knowledge sharing across the boundaries between care processes, services and organisations: the contributions to safe hospital discharge. Health Serv Deliv Res 2014;2.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. http://publications.nice.org.uk/guide-to-the-methods-of-technologyappraisal-2013-pmg9 (accessed 4 January 2016).
- Drummond M, Schulpher M, Torrance GW, O’Brien BJ, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Department of Health . NHS Reference Costs 2013 to 2014 n.d. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 4 January 2016).
- NHS Employers . NHS Pay Scales for 2013: Pay Circular (AforC) 2 2013 2013. www.nhsemployers.org/∼/media/Employers/Publications/Pay%20circulars/Pay-Circular-AforC-2-2013.pdf (accessed 4 January 2016).
- Douglas S, Cartmill R, Brown R, Hoonakker P, Slagle J, Schultz Van Roy K, et al. The work of adult and pediatric intensive care unit nurses. Nurs Res 2013;62:50-8. http://dx.doi.org/10.1097/NNR.0b013e318270714b.
- Westbrook J, Duffield C, Li L, Creswick NJ. How much time do nurses have for patients? A longitudinal study quantifying hospital nurses’ patterns of task time distribution and interactions with health professionals. BMC Health Serv Res 2011;11:319-23. http://dx.doi.org/10.1186/1472-6963-11-319.
- Hauschild I, Vitzthum K, Klapp BF, Groneberg DA, Mache S. Time-and-motion study of anesthesiologists’ workflow in German hospitals. Wien Med Wochenschr 2011;161:433-40. http://dx.doi.org/10.1007/s10354-011-0028-1.
- Dasgupta, Sansgiry SS, Jacob SM, Frost CP, Dwibedi N, Tipton J. Descriptive analysis of workflow variables associated with barcode-based approach to medication administration. J Nurs Care Qual 2011;26:377-84. http://dx.doi.org/10.1097/ncq.0b013e318215b770.
- Chisholm CD, Weaver CS, Whenmouth L, Giles B. A task analysis of emergency physician activities in academic and community settings. Ann Emerg Med 2011;58:117-22. http://dx.doi.org/10.1016/j.annemergmed.2010.11.026.
- Weigl M, Müller A, Zupanc A, Glaser J, Angerer P. Hospital doctors’ workflow interruptions and activities: an observation study. BMJ Qual Saf 2011;20:491-7. http://dx.doi.org/10.1136/bmjqs.2010.043281.
- Mache S, Schöffel N, Kusma B, Vitzthum K, Klapp BF, Groneberg DA. Cancer care and residents’ working hours in oncology and hematology departments: an observational real-time study in German hospitals. Jpn J Clin Oncol 2011;41:81-6. http://dx.doi.org/10.1093/jjco/hyq152.
- Westbrook JI, Coiera E, Dunsmuir WTM, Brown BM, Kelk N, Paoloni R, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care 2010;14:12-6. http://dx.doi.org/10.1136/qshc.2009.039255.
- Weigl M, Müller A, Zupanc A, Angerer P. Participant observation of time allocation, direct patient contact and simultaneous activities in hospital physicians. BMC Health Serv Res 2009;9:110-14. http://dx.doi.org/10.1186/1472-6963-9-110.
- Yen K, Shane EL, Pawar SS, Schwendel ND, Zimmanck RJ, Gorelick MH. Time motion study in a paediatric emergency department before and after computer physician order entry. Ann Emerg Med 2009;53:462-8. http://dx.doi.org/10.1016/j.annemergmed.2008.09.018.
- Westbrook JI, Ampt A, Kearney L, Rob MI. All in a day’s work: an observational study to quantify how and with whom doctors on hospital wards spend their time. Med J Aust 2008;188:506-9.
- Ampt A, Westbrook JI. Measuring nurses’ time in medication related tasks prior to the implementation of an electronic medication management system. Stud Health Technol Inform 2007;130:157-67.
- Morton JM, Baker CC, Farrell TM, Yohe ME, Kimple RJ, Herman DC, et al. What do surgery residents do on their call nights?. Am J Surg 2004;188:225-9. http://dx.doi.org/10.1016/j.amjsurg.2004.06.011.
- DeClifford J-M, Blewitt P, Lam SS, Leung BK. How do clinical pharmacists spend their working day? A time-and-motion study. J Pharm Pract Res 2012;42:134-8. http://dx.doi.org/10.1002/j.2055-2335.2012.tb00151.x.
- Keohane CA, Bane AD, Featherstone E, Hayes J, Woolf S, Hurley A, et al. Quantifying nursing workflow in medication administration. J Nurs Adm 2008;38:19-26. http://dx.doi.org/10.1097/01.NNA.0000295628.87968.bc.
- Lo HG, Newmark LP, Yoon C, Volk LA, Carlson VL, Kittler AF, et al. Electronic health records in specialty care: a time–motion study. J Am Med Inform Assoc 2007;14:609-15. http://dx.doi.org/10.1197/jamia.M2318.
- Fleming SA, Blake H, Gladman JR, Hart E, Lymbery M, Dewey ME, et al. A randomised controlled trial of a care home rehabilitation service to reduce long-term institutionalisation for elderly people. Age Ageing 2004;33:384-90. http://dx.doi.org/10.1093/ageing/afh126.
- Young J, Green J, Forster A, Small N, Lowson K, Bogle S, et al. Postacute care for older people in community hospitals: a multicenter randomized, controlled trial. J Am Geriatr Soc 2007;55:1995-2002. http://dx.doi.org/10.1111/j.1532-5415.2007.01456.x.
- Parfrey PS, Gardner E, Vavasour H, Harnett JD, McManamon C, McDonald J, et al. The feasibility and efficacy of early discharge planning initiated by the admitting department in two acute care hospitals. Clin Invest Med 1994;17:88-96.
- Sulch D, Perez I, Melbourn A, Kalra L. Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke 2000;31:1929-34. http://dx.doi.org/10.1161/01.STR.31.8.1929.
- Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital admissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmissions. N Engl J Med 1996;334:1441-7. http://dx.doi.org/10.1056/NEJM199605303342206.
- Wakefield BJ, Ward MM, Holman JE, Ray A, Scherubel M, Burns TL, et al. Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E Health 2008;14:753-61. http://dx.doi.org/10.1089/tmj.2007.0131.
- Sorknaes AD, Madsen H, Hallas J, Jest P, Hansen-Nord M. Nurse teleconsultations with discharged COPD patients reduce early readmissions: an interventional study. Clin Respir J 2011;5:26-34. http://dx.doi.org/10.1111/j.1752-699X.2010.00187.x.
- Feltner C, Jones CD, Cené CW, Zheng Z-J, Sueta CA, Coker-Schwimmer EJL, et al. Transitional care interventions to prevent readmissions for persons with heart failure. A systematic review and meta-analysis. Ann Intern Med 2014;160:774-84. http://dx.doi.org/10.7326/M14-0083.
- Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med 2004;164:545-50. http://dx.doi.org/10.1001/archinte.164.5.545.
Appendix 1 Statistical analysis plan
The CIRACT (Community In-Reach And Care Transition) clinical and cost-effectiveness study Statistical Analysis Plan
Final Version 1.0 (20th Jan 2015).
Based on Protocol version 4.0 (dated 10 Mar 2014).
| The following people have reviewed the Statistical Analysis Plan and are in agreement with the contents | |||
| Name | Role | Signature | Date |
|---|---|---|---|
| Wei Tan | Author | ||
| Alan Montgomery | Statistical Reviewer | ||
| Opinder Sahota | Chief Investigator | ||
Abbreviations
| Abbreviation | Description |
|---|---|
| CIRACT | Community in-reach and care transition |
| NHS | National health service |
| RCT | Randomised controlled trials |
| THB-rehab | Traditional hospital based rehabilitation |
| TMG | Trial management group |
| TSC | Trial steering committee |
Introduction and purpose
This document details the rules proposed and the presentation that will be followed, as closely as possible, when analysing and reporting the main results from the NIHR-HSDRP funded randomised controlled trial of CIRACT service for older people.
The purpose of the plan is to:
-
Ensure that the analysis is appropriate for the aims of the trial, reflects good statistical practice in general, and minimises bias by preventing inappropriate post hoc analyses.
-
Explain in detail how the data will be handled and analysed to enable others to perform the actual analysis in the event of sickness or other absence.
-
Protect the project by helping it keep to timelines and within scope.
Additional exploratory or auxiliary analyses of data not specified in the protocol are permitted but fall outside the scope of this analysis plan (although such analyses would be expected to follow Good Statistical Practice).
The analysis strategy will be made available if required by journal editors or referees when the main papers are submitted for publication. Additional analyses suggested by reviewers or editors will, if considered appropriate, be performed in accordance with the Analysis Plan, but if reported the source of such a post-hoc analysis will be declared.
Amendments to the statistical analysis plan will be described and justified in the final report of the trial.
Synopsis of study design and procedures
Trial aims and objectives
The aim of the study is to compare the clinical effectiveness (hospital stay, readmission and health related quality of life), overall cost and cost-effectiveness of a community in-reach rehabilitation and care transition (CIRACT) service with usual hospital ward based rehabilitation service for unplanned hospital admission of older people (over 70 years).
Primary objective
To assess whether the CIRACT service reduces the length of hospital stay compared to the THB-rehab service for unplanned hospital admission of people of 70 years or older.
Secondary objectives
-
To assess whether CIRACT reduces the readmission rate within 28 days of discharge.
-
To assess whether CIRACT reduces super spell bed-days (total time in NHS: hospital care plus community care/intermediate care).
-
To assess whether CIRACT improves patient function (Barthel ADL index).
-
To assess whether CIRACT improves patient health related quality of life (EQ-5D-3L).
Trial design and configuration
A pragmatic, two-arm, parallel randomised controlled trial including an integral qualitative action and mechanism and health economic study.
Trial centres
Nottingham is the only site for this study.
Eligibility criteria
Inclusion criteria
-
Aged 70 years and over.
-
Admitted to hospital on the general medical elderly care ward as an unplanned medical admission.
-
Admitted to hospital from own home or residential care.
-
GP registered within Nottingham City PCT catchment area.
Exclusion criteria
-
Previously bed bound.
-
Receiving palliative care.
-
Moribund on admission.
-
Previously included in the trial on an earlier admission.
-
Admitted from a nursing home.
-
Not assessed by the study team within 36 hours of admission.
Description of interventions
CIRACT service
The CIRACT team will jointly conduct an assessment of the participant’s ability to perform certain tasks. Following the assessment a rehabilitation plan will be formulated which will be followed daily. The plan will focus on particular activities which are important to the participant. While in hospital the participants are treated every day (7 days a week) by the CIRACT team and the time of rehabilitation they receive will be dependent on their needs.
During the participant’s hospital stay the CIRACT team will liaise with the participant and their carer(s) to visit the participant’s home to carry out a home assessment in order to provide recommendations for equipment; make adaptations and/or modifications if required. In more complex cases the CIRACT team will take the participant out of the hospital for a home visit allowing assessment in the participants own home environment, if required.
Following hospital discharge, the CIRACT team will visit the participant at home within 48 hours of discharge. During this visit the level of rehabilitation required at home will be assessed and the CIRACT team will be able to undertake further follow-up visits as deemed necessary.
THB-rehab service
The THB-rehab service is provided by the hospital occupational therapy and physiotherapy services on weekdays only. Members of these teams jointly conduct an assessment of the participant’s ability to perform certain tasks. Following this assessment the team will provide recommendation for rehabilitation. Depending on this advice, the rehabilitation care starts in the hospital, for instance when physiotherapy exercises need to be learned and these are practices with the participant if time allows. Other rehabilitation care may only require some adaptation in the participant’s home and for these the ward team will be asked to refer the participant to the appropriate community-based services for provision of equipment at home, personal care and on-going rehabilitation where appropriate at the point of hospital discharge. Once discharged from hospital, the patient has no contact with the ward rehabilitation staff.
Randomisation procedures
Participants will be randomly allocated in a 1 : 1 ratio to the two intervention groups. Sequence generation will be using computer-generated random permutated balanced blocks of randomly varying size, created by the Nottingham Clinical Trials Unit in accordance with their standard operating procedure and held on a secure server. Randomisation will be done via a web-based system by a member of the research team.
Sample size and justification
The primary statistical analysis will compare duration of hospital stay between the trial arms. Pilot study data showed the log transformed length of stay to be normally distributed with standard deviation 0.9. Therefore, 111 participants per arm will be required to detect a clinically important effect size of 3 days (ratio of means 0.71) with alpha 0.05 and power 0.8. Allowing for 5% drop out rate at discharge 240 participants in total will be required. Further allowing for 25% exclusions and a 10% refusal rate, we will need to screen 350 participants over 12 months. There are five health care of older people’s general medical wards currently admitting 450 participants per ward each year and the current CIRACT service is able to manage 30 participants per month. We propose to conduct the trial at two wards which will give the required numbers within our proposed timetable for the study.
Blinding and breaking of blind
Allocation to trial arm will be concealed until after the participant baseline enrolment data set has been irreversibly entered into the trial randomisation system. This will minimise selection bias. The research assistant will not be aware of the rehabilitation allocation and the lead therapist will be informed by e-mail.
The participants and CIRACT service team will not be blind to the allocated rehabilitation arm. It will be ensured that the research assistant collecting the 28-day and 91-day data and performing the Activity of Daily Living (Barthel ADL index) remains blind to rehabilitation allocation. At follow-up at day 91 post discharge the research assistant may require face to face contact. To prevent unblinding, the research assistant will request the participants not to discuss any aspect of being involved with the trial.
Trial committees
Two committees will be assembled to ensure the proper management and conduct of the trial, and to uphold the safety and well-being of participants. The general purpose, responsibilities and structures of the committees are described in the protocol. However each committee will develop its own rules and procedures which may evolve with time.
-
Trial Management Group: The Trial Management Group (TMG) will oversee the operational aspects of the trial. The TMG will meet regularly to review the progress of the trial and address any issues arising.
-
Trial Steering Committee: The Trial Steering Committee will be set up with an independent Chairperson and will monitor, review and supervise the progress of the trial. The independent Trial Steering Committee will monitor blinded data to consider safety and efficacy indications. The TSC may recommend discontinuation of the study if significant ethical or safety concerns arise or if there is very clear evidence of benefit (clinical or statistical) prior to completion of the study. The TSC will meet independently prior to the start of the study and will agree terms of reference.
Outcome measures
Primary outcome
The primary outcome will be hospital LOS from admission to discharge from the general medical elderly care ward. This will be calculated from the date of admission to the date of discharge from the general medical elderly care ward.
Secondary outcomes
-
Super spell bed-days (total time in NHS: all hospital care + community care/intermediate care)
-
Unplanned re-admission rates at day 28 and day 91 post discharge from initial ward
-
Barthel ADL at 91 days post discharge from the acute hospital
-
EQ-5D-3L at 91 days post discharge from initial ward
Interim analysis
No formal interim analysis is planned for this study.
General analysis considerations
Analyses will be performed using Stata version 13 or above.
Analysis samples
Full Analysis set: all randomised participants for whom the primary end point is available.
Procedures for missing data
The primary analysis of length of stay will be conducted without imputation of missing data. Although we anticipate that primary outcome data will not be available for only a small proportion of participants, we will conduct a sensitivity analysis using multiple imputation of missing data.
Description of subject characteristics
Participant flow
Flow of participants through the trial will be summarised in a CONSORT diagram that will include the eligibility, reasons for exclusion, numbers randomised to the two treatment groups, number of participants with primary outcome, number of participants who withdrew and whose primary outcome was not available.
Baseline characteristics
Participants in the two treatments arms will be described separately with respect to age at inclusion, sex, Barthel ADL, MMSE, Co-Morbidity Scale and EQ-5D-3L health state score. Continuous data will be summarised in terms of the mean, standard deviation, median, lower and upper quartiles, minimum, maximum and number of observations. Categorical data will be summarised in terms of frequency counts and percentages.
Assessment of study quality
Eligibility checks
The numbers of participants falling into inclusion/exclusion criteria will be tabulated.
Withdrawals
The numbers (with percentages) of withdrawals (with reasons) will be summarised by treatment arm.
Protocol deviations
A listing of all participants with a protocol deviation will be presented, containing the deviation category and any additional information for the deviation.
Analysis of effectiveness
Primary analysis
Preliminary analyses describing the proportions of participants who withdrew consent prior to discharge from initial ward, died in initial ward, discharged from initial ward or died post discharged from initial ward will be conducted.
If the proportion of in-ward death is smaller than 5% and balanced between arms, the primary analysis will be length of hospital stay for those who were discharged from initial ward alive. The analysis will be conducted using generalised linear regression modelling, with log transformed length of stay as response. The primary effectiveness parameter will be the ratio of the geometric mean length of stay from admission to initial elderly care ward to discharge between the two arms, along with 95% confidence interval and p value.
Secondary analyses
Super spell days
Analysis for super spell days will follow the same approach described above for primary analysis.
Unplanned re-admission rate at 28 day and 91 day post discharge
Effectiveness will be assessed using logistic regression, with re-admission at 28 and 91 days as responses. The effectiveness parameter will be the odds ratio in unplanned re-admission at 28 and 91 day post discharge between the two treatment arms, along with 95% confidence interval and p value.
Barthel ADL score at 91 day post discharge
Effectiveness will be assessed using a generalised linear regression modelling, with Barthel ADL score at 91 days as response. The effectiveness parameter will be the difference in mean scores at 91 day post discharge between the two treatment arms, along with 95% confidence interval and p value.
EQ-5D-3L health status score at 91 day post discharge
Analysis for EQ-5D-3L health score will follow the same approach described above for Barthel ADL score.
Sensitivity analyses of primary outcome
Additional analyses for primary outcome will include:
-
Include all participants and add an additional covariate in the primary analysis model to specify whether a participant were discharged or died in the initial ward.
-
Repeat primary analysis model with imputation of missing length of stay data.
-
Repeat the primary analysis model adjusting for any baseline variables with marked imbalance between arms.
-
Time to discharge Cox regression analysis by including all in-ward deaths as competing risk.
Analysis of safety
Adverse events
Data shall be collected for each individual participant with regards to any fall that occurs whilst an in-patient on the ward until time of discharge. These data will be summarised using appropriate descriptive statistics.
Final report tables and figures
The following rules may be adopted when creating the summary tables:
-
Number of decimal places (DP):
-
For minimum and maximum the number of DPs will be the same as the raw data
-
For mean, median and SD the number of DPs will be one more than the raw data
-
Percentages – Round up to the nearest whole number
-
p-values – 3 decimal points will be presented
-
No more than 4 significant numbers will be used
-
-
Data Presentation:
-
Treatment group will be in columns with the visits in rows
-
Column headers in mixed case, with ‘(N = nn)’ below treatments to denote the denominator
-
Decimal places will be aligned
-
N(%) as a separate column rather than included in brackets for each element of the table
-
Categories (i.e. in column 1) in sentence case, in the order on the case report form
-
Ordering of statistics N, Missing, Mean, SD, coefficient of variation percentage, Minimum, Median and Maximum. Geometric Means inserted between Mean and SD for pharmacokinetcs.
-
Appendix 2 Protocol deviations in the Community In-reach Rehabilitation And Care Transition service group
Informed consent deviation details
| Patient number | Details of deviation |
|---|---|
| 1020 | Because of a miscommunication, the Personal Consultee Declaration Form was not given to the relative/carer for signing by the research nurse until 20 August 2013, which was outside the prescribed window. However, the Nominated Consultee Form was completed on 14 August 2013 as per protocol |
| 1028 | Because of a miscommunication, the Personal Consultee Declaration Form was not given to the relative/carer for signing by the research nurse until 20 August 2013, which was outside the prescribed window. However, the Nominated Consultee Form was completed on 14 August 2013 as per protocol |
| 1072 | Wife’s consent was not obtained until > 72 hours after nominated consent |
| 1086 | Wrong consent form used |
Details of other deviations
| Patient number | Details of deviation |
|---|---|
| 1002 | Participant’s health deteriorated rapidly after consent and she was too unwell to provide baseline data until 24 June 2013. PI said that this was acceptable in the circumstances |
| 1002 | Participant on holiday in Greece, husband answered on her behalf. Protocol deviation form completed |
| 1014 | 91-day follow-up completed outside the ± 3-day window |
| 1017 | 91-day interview conducted > 3 days post due date |
| 1017 | 91-day interview conducted over the telephone instead of face-to-face |
| 1023 | 91-day follow-up completed outside the ± 3-day window – had been booked at an earlier date with the care home but the care home cancelled at the last minute |
| 1024 | Patient refused visit so the 91-day interview was carried out by telephone |
| 1072 | Baseline data not collected from proxy until > 72 hours after nominated consent |
| 1118 | 91-day follow-up carried out at 82 days because of an administrative error (–9 days) when should be in a ± 3-day window |
| 1174 | 91-day follow-up completed 1 day early because of an administrative error |
| 1183 | MMSE data not collected – patient tired and then unavailable. RA intended to collect data on 14 April 2014 but the patient passed away the day before |
Appendix 3 Observation record (qualitative study)
| Date | Team | Time (minutes) | Running total (minutes) | Interviews |
|---|---|---|---|---|
| 16/09/13 | Ward visits | 180 | 180 | |
| 17/09/13 | Ward visits | 180 | 360 | |
| 01/10/13 | CIRACT | 200 | 560 | |
| 08/10/13 | Research team | 180 | 740 | |
| 14/10/13 | CIRACT | 120 | 860 | |
| 18/10/13 | THB-Rehab | 70 | 930 | |
| 23/10/13 | THB-Rehab | 120 | 1050 | ×1 |
| 30/10/13 | THB-Rehab | 120 | 1170 | ×1 |
| 05/11/13 | CIRACT | 200 | 1370 | ×1 |
| 06/11/13 | CIRACT | 240 | 1610 | ×3 |
| 13/11/13 | THB-Rehab | 120 | 1730 | ×1 |
| 19/11/13 | THB-Rehab/CIRACT | 120 | 1850 | |
| 27/11/13 | CIRACT | 70 | 1920 | |
| 02/12/13 | CIRACT | 120 | 2040 | |
| 05/12/13 | THB-Rehab/CIRACT | 200 | 2240 | |
| 10/12/13 | THB-Rehab/CIRACT | 240 | 2480 | |
| 06/01/14 | CIRACT | 180 | 180 | |
| 15/01/14 | CIRACT | 240 | 420 | |
| 16/01/14 | CIRACT | 120 | 540 | |
| 16/01/14 | THB-Rehab | 45 | 585 | |
| 20/01/14 | CIRACT | 60 | 645 | ×1 |
| 22/01/14 | CIRACT | 60 | 705 | ×1 |
| 27/01/14 | THB-Rehab | 180 | 885 | |
| 27/01/14 | CIRACT | 45 | 930 | |
| 28/01/14 | CIRACT | 80 | 1010 | |
| 29/01/14 | CIRACT | 60 | 1070 | |
| 29/01/14 | THB-Rehab | 120 | 1190 | |
| 31/01/14 | THB-Rehab | 120 | 1310 | ×1 |
| 04/02/14 | CIRACT | 45 | 1355 | |
| 06/02/14 | CIRACT | 300 | 1655 | ×1 |
| 11/02/14 | CIRACT | 40 | 1695 | |
| 13/02/14 | THB-Rehab | 70 | 1765 | |
| 20/02/14 | CIRACT | 60 | 1825 | ×1 |
| 20/02/14 | THB-Rehab | 180 | 2005 | ×1 |
| 21/02/14 | CIRACT | 300 | 2305 | |
| 24/02/14 | CIRACT | 240 | 2545 | |
| 25/02/14 | CIRACT | 180 | 2725 | |
| 27/02/14 | THB-Rehab | 65 | 2790 | |
| 03/03/14 | CIRACT | 180 | 2970 | ×1 |
| 06/03/14 | THB-Rehab | 60 | 3030 | ×1 |
| 10/03/14 | CIRACT | 60 | 3090 | ×1 |
| 11/03/14 | CIRACT | 120 | 3210 | |
| 13/03/14 | CIRACT | 65 | 3275 | ×1 |
| 25/03/14 | CIRACT | 60 | 3335 | ×2 |
| 08/04/14 | THB-Rehab | 120 | 3455 | |
| 09/04/14 | THB-Rehab | 240 | 3695 | |
| 29/04/14 | THB-Rehab | 60 | 3755 | ×2 |
| 29/04/14 | CIRACT | 120 | 3875 | ×2 |
| 30/04/14 | THB-Rehab | 70 | 3945 | ×1 |
| 02/05/14 | THB-Rehab | 120 | 4065 | |
| 07/05/14 | CIRACT/THB-Rehab | 240 | 4305 | |
| 13/05/14 | CIRACT/THB-Rehab | 180 | 4485 |
Appendix 4 Observational activities
| Type of activity | Examples | CIRACT service | THB-Rehab service |
|---|---|---|---|
| Direct patient care | Inpatient assessment | Yes | Yes |
| Ongoing care decisions | Yes | Yes | |
| Therapy | Yes | Yes | |
| Kitchen and stairs assessment | Yes | Yes | |
| Family meetings | Yes | Yes | |
| Discharge planning | Yes | Yes | |
| Home visits | Yes | No | |
| Indirect patient care | Multidisciplinary planning | Yes | Yes |
| Morning board round | Yes | Yes | |
| Discussion | Yes | Yes | |
| Record keeping | Yes | Yes | |
| Telephone contact | Yes | Yes | |
| ICT-based record keeping | Yes | No | |
| Equipment ordering | Yes | Yes | |
| Equipment collection | Yes | No | |
| Referrals | Yes | Yes | |
| Routine patient feedback | Yes | No | |
| Community networking | Social service providers | Yes | No |
| Community health | Yes | No | |
| Charities | Yes | No |
Appendix 5 Patient tracking template
| Pseudonym: | Data and source code | Reflective points |
|---|---|---|
| Service: | ||
| Consent checked in medical notes | ||
| Patient number for qualitative study | ||
| Age | ||
| Barthel ADL score on admission | ||
| Date of admission to study ward | ||
| Date of discharge from hospital | ||
| Discharge destination | ||
| Readmission within 48 hours of discharge | ||
| Summary admitting conditions | ||
| Communication difficulties | ||
| Summary biographical background | ||
| Previous community care provision | ||
| Therapist details | ||
| Physiotherapist | ||
| Occupational therapist | ||
| Therapy assistant | ||
| Social worker | ||
| Other | ||
| Key interactions | ||
| Assessment | ||
| Therapies | ||
| Equipment | ||
| Family meeting | ||
| Referral for funding care | ||
| Home visit | ||
| Discharge emphasis | ||
| Handover and communication | ||
| Day of discharge issues, e.g. medication, transport, timing | ||
| Discharge time and day of week | ||
| Continuity of care teams, e.g. same team | ||
| Therapist and patient interactions, e.g. levels of trust, sense of effective sharing of information, decision-making, kept informed | ||
| Therapist and hospital-based professional interactions, e.g. discharge co-ordinators, medics | ||
| Therapist and equipment-related interactions, e.g. Red Cross providers | ||
| Therapist and family interactions | ||
| Therapist and community professionals interactions | ||
| Therapist and other therapist professional interactions | ||
| Therapist and any other service provider interactions | ||
| Evidence sources and dates |
Appendix 6 Summary of the traditional hospital-based rehabilitation service
| Service details | Aspects of the service | Impacts of the service |
|---|---|---|
| Purpose of service | To provide therapy for older people during a hospital stay and discharge planning to enable early discharge and reduce placements in intermediate care or residential care; reduction in the readmission of patients within 30 days. This to be achieved by:
|
Impacts include adult directorate bed pressures, ward moves, managing risk and patient choice in the decisions made about care and discharge. Direct patient therapy not daily per patient and frequently every third day |
| Funding | Acute care NHS hospital trust | Continuous monitoring by separate therapy service management teams |
| Location | No distinction between city and county patients. Inner-city boundary with a population of those aged > 65 years of 38,800, of whom 19,013 live with a limiting long-term condition and 13,520 live alone at home [see www.poppi.org.uk/ (accessed 4 January 2016)]. County boundary with a population of those aged > 65 years of 158,000, of whom 78,010 live with a limiting long-term condition and 57,386 live at home (see www.poppi.org.uk/) | Wards included all patients regardless of funding eligibility/geographical boundary. In-reach reduces the number of city patients who receive standard care |
| Age of service | Since 2000 | Embedded |
| Term | Permanent contracts; agency staff for senior occupational therapy | Newly qualified staff tend to secure hospital-based work as first job. Difficulties with recruitment of occupational therapists |
| Capacity | Full capacity limited to a maximum of 12 therapy sessions per day per pair of therapists. Non-uniform distribution of demand with new patients on Mondays and discharges on Fridays | Small service compared with potential users of the service |
| Configuration | One senior occupational therapist lead (band 6), one senior physiotherapist (band 6), two physiotherapists (band 5), two occupational therapists (band 5), three assistant practitioners (band 3) | Senior staff provide expertise according to qualifications and experiences of working across acute hospital settings. Limited experience of community working or localised knowledge |
| Service availability | 5-day working from Monday to Friday. Emergency response across adult directorate during reduced hours Saturday and Sunday | Inpatients tend to not have any therapy at weekends and most have none until Tuesday |
| Patient eligibility criteria | Usual eligibility limited to those who will be going home or to an interim residential care home. Nursing home residents and those with mental health issues tend to have little if any direct therapy. End-of-life care supported as required to reduce pain and help with secretions | Limited patient inclusion to those most likely to benefit from therapy in preparation for discharge, leading to exclusion of some who may benefit |
| Decision-making | Larger team decision-making primarily by senior therapists and reassessed mid-day to support work delegation. Service decisions made by managers located with the NHS acute hospital trust as separate professional services | Delegation of work tended to be in the same small team on a specific ward with cross-directorate working as required |
| Bureaucratic structures | Embedded service with extensive bureaucratic frameworks but able to function with moderate levels of personal responsiveness, autonomy and professional accountability | Staffing levels hindered when recruitment in process as dependent on larger processes in the organisation. Hospital-wide structures impact on the implementation of any ward-based interventions beyond standard |
| Location | Three Health Care of Older People wards within the wider adult directorate within an acute NHS hospital and the community | Colocated with CIRACT therapy services across three wards |
Appendix 7 Summary of the Community In-reach Rehabilitation And Care Transition service
| Service details | Aspects of the service | Impacts of the service |
|---|---|---|
| Purpose of service | To provide intensive therapy for older people during a hospital stay and a seamless transition on discharge to enable early discharge and reduce placements in intermediate care or residential care; reduction in the readmission of patients within 30 days. This to be achieved by:
|
Wide inclusion criteria prevent patients who may be ineligible for re-ablement or intermediate care but who require support on discharge from returning home earlier and being supported by the in-reach team. In-reach designed to provide a ‘best fit’ service that optimises the chances of remaining at home and at least a return to baseline functionality for the patient. Impacts include managing risk and patient choice in the decisions made about care and discharge and disparate needs of carers and patients on discharge |
| Funding | Community NHS and social services collaborative funding with 12-month reviews of effectiveness. Fragile funding arrangement that is reliant on multiple local provider agreements | Continuous monitoring of service provides rationale for continuing funding. Arguably fragile funding arrangement linked to penalty system for readmissions by central NHS services |
| Location | Inner-city boundary with population of those aged > 65 years of 38,800, of whom 19,013 live with a limiting long-term condition and 13,520 live alone at home [see www.poppi.org.uk/ (accessed 4 January 2016)] | Wards included all patients regardless of funding eligibility/geographical boundary |
| Age of service | Since 2011 | Early service not fully embedded |
| Term | Fixed-term 12-month contracts. Perception that work is a means to secure early promotion. Staff headhunted by neighbouring services | Difficulties recruiting to fixed-term contracts. Embedding of service compromised by high staff mobility to other services |
| Capacity | Full capacity limited to 16 patients in total per day. Average during the trial of 10 patients, with six inpatients and four patients at home | Small service compared with potential users of the service |
| Configuration | One senior occupational therapist lead (band 7), one senior physiotherapist (band 6), one assistant practitioner (band 3), one assistant social worker practitioner part-time (Monday to Friday) (band 5) | Senior staff provide expertise according to qualifications and experiences of working across community and acute hospital settings. Dedicated social service input helps maintain responsive service |
| Service availability | 7-day working with reduced hours on Saturday and Sunday | 7-day working maintains momentum of intensive therapy and transitional planning. Weekend working and tendency to use single assistant practitioner |
| Patient eligibility criteria | Usual eligibility very broad and includes end-of-life care, confusion and mental health issues and social needs in addition to chronic physical needs. Carer needs also included in terms of emotional and health resilience to support patient on discharge | Trail study inclusion criteria excluded end-of-life care patients, which limited the range of service interventions for those patients not considered to be nearing the end of life |
| Decision-making | Core team decision-making primarily by senior therapists. Service decisions made by provider lead within the community NHS provider | High levels of autonomous working dependent on confidence and skills of the practitioners. Close working with lead manager supports implementation and changes in service |
| Bureaucratic structures | Small new service has minimal direct bureaucracy and so able to function with higher levels of personal responsiveness, autonomy and professional accountability | Implementation hindered when recruitment in process as dependent on larger processes in the organisation. This contrasts with usually working in more flexible and expansive ways because of limited bureaucracy |
| Location | Three Health Care of Older People wards within an acute NHS hospital and the community | Colocated with standard therapy services across three wards |
Appendix 8 Unit costs for primary, community health and social services
| Cost category | Unit cost (£) per visit | Source |
|---|---|---|
| GP (home) | 46 | Curtis48 |
| GP (surgery) | 46 | |
| Practice nurse (surgery) | 10.59 | |
| District nurse (community) | 12.91 | |
| Dietitian | 9.25 | |
| Social worker | 14.25 | |
| Rehabilitation team | 9 | |
| CIRACT team | 7.66 | |
| Pharmacist | 14.25 | |
| Paramedic | 11 | |
| Care home (per day) | 59.14 | |
| Day centre (per day) | 56 | |
| Home care (social care) (per minute) | 0.40 | |
| Meals on Wheels | 46 | |
| Outpatient appointment | 128 | Department of Health49 |
| NHS walk-in centre | 33 | |
| A&E | 69 |
Appendix 9 Time and motion study literature review data extraction form
Appendix 10 Time and motion study case record form
Appendix 11 NHS and Personal Social Services costs per patient by resource use and allocation group
| Cost category | Intervention arm, mean (SD) cost (£) | |
|---|---|---|
| CIRACT | THB-Rehab | |
| Baseline | ||
| GP (home) | 245 (180) | 223 (178) |
| GP (surgery) | 110 (79) | 121 (103) |
| Practice nurse (surgery) | 33 (39) | 23 (27) |
| District nurse (community) | 197 (354) | 299 (394) |
| Dietitian | 9 | 9 |
| Social worker | 24 (15) | 24 (12) |
| Rehabilitation team | 70 (97) | 170 (409) |
| Community In-Reach | 8 | 29 (14) |
| Pharmacy | – | – |
| Other (complementary therapist) | 116 | 103 |
| Hospital admission – elective | 3729 (3994) | 4749 (5201) |
| A&E visit | 91 (44) | 109 (109) |
| NHS walk-in centre | 33 (NA) | 41 (17) |
| Outpatient clinic | 300 (235) | 303 (180) |
| Social services | ||
| Admitted to residential home/care home | 580 (483) | 1070 (825) |
| Attended a day centre | 125 (76) | 117 (97) |
| Home help or community care assistant | 117 (86) | 25 (14) |
| Meals on Wheels | 20 (5) | 22 (10) |
| Help to buy equipment | 69 (128) | 85 (159) |
| Follow-up | ||
| GP (home) | 220 (155) | 215 (174) |
| GP (surgery) | 134 (125) | 85 (80) |
| Practice nurse (surgery) | 20 (25) | 28 (41) |
| District nurse (community) | 229 (445) | 288 (544) |
| Dietitian | 14 (7) | 33 (44) |
| Social worker | 24 (11) | 21 (10) |
| Rehabilitation team | 158 (195) | 134 (210) |
| Community In-Reach | 28 (19) | 27 (27) |
| Pharmacy | 62 (41) | 14 |
| Other (complementary therapist) | 116 | 171 |
| Hospital admission – elective | 5167 (5401) | 4661 (4839) |
| A&E visit | 101 (47) | 125 (90) |
| NHS walk-in centre | 231 (NA) | 33 (NA) |
| Outpatient clinic | 267 (162) | 285 (222) |
| Social services | ||
| Admitted to residential home/care home | 1097 (677) | 2359 (1628) |
| Attended a day centre | 96 (53) | 112 (80) |
| Home help or community care assistant | 186 (198) | 243 (236) |
| Meals on Wheels | 28 (NA) | 20 (3) |
| Help to buy equipment | 99 (186) | 148 (380) |
Appendix 12 Cost-effectiveness plot depicting a bootstrap sample (n = 1000)

List of abbreviations
- A&E
- accident and emergency
- ADL
- (Barthel) Activities of Daily Living
- BOOST
- (Project) Better Outcomes for Older adults through Safe Transitions
- CCG
- Clinical Commissioning Group
- CI
- confidence interval
- CIRACT
- Community In-reach Rehabilitation And Care Transition
- CSRI
- Client Service Receipt Inventory
- CTI
- Care Transitions Intervention
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- ICT
- information communication technology
- LOS
- length of stay
- MDT
- multidisciplinary team
- MMSE
- Mini Mental State Examination
- NCTU
- Nottingham Clinical Trials Unit
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RED
- (Project) Re-Engineered Discharge
- RR
- relative risk
- SD
- standard deviation
- TCM
- Transitional Care Model
- THB-Rehab
- traditional hospital-based rehabilitation
- TMS
- time and motion study
- TSC
- Trial Steering Committee