Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5001/09. The contractual start date was in June 2013. The final report began editorial review in June 2015 and was accepted for publication in January 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Shaun Treweek reports a grant from the National Institute for Health Research during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Hubbard et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
In this chapter, the rationale for rehabilitation for people with colorectal cancer (CRC) is provided. In particular, evidence of the benefits of physical activity for people with CRC is presented. Arguments are also presented for the use of cardiac rehabilitation as a rehabilitation model to aid the recovery of people with CRC. Finally, the importance of conducting feasibility and pilot studies as preparation for large-scale effectiveness trials is discussed.
Rationale for cancer rehabilitation
Increasing 5- and 10-year survival rates mean that many cancers are now considered chronic diseases. There are approximately 28 million people living with and beyond cancer in the world1 and many of these cancer survivors face ongoing challenges from the post-treatment care standpoint. In this report, we use that term, cancer survivor, to refer to someone who is living with and beyond cancer; we recognise, however, that not all cancer survivors would identify themselves using the term ‘survivor’. 2 In the UK, there are over 2 million people living with and beyond cancer, a figure which is rising by 3% per annum. 3 As such, supporting cancer survivors represents one of the largest UK and global health challenges.
Colorectal cancer is also called bowel cancer and includes large bowel cancer (colon cancer) and cancer of the back passage (rectal cancer or cancer of the rectum). CRC is the second most frequently diagnosed cancer in women and the third most frequently diagnosed cancer in men, accounting for 1.23 million new CRC cases in 2008 worldwide. 4 CRC is the fourth most common cancer in the UK and there are approximately 244,000 people living with and beyond CRC. 5 In 2011 in the UK, 41,581 people were diagnosed with CRC (13% of all cancer cases); 57% of adults who were diagnosed with CRC (56% of men and 57% of women) in 2010–11 in England and Wales were predicted to survive ≥ 10 years. 6 Addressing the post-treatment needs of this group is, therefore, a UK public health priority.
Colorectal cancer survivors report ongoing and persistent physical and psychological impairments. 7–9 Physical symptoms include fatigue, physical discomfort and bowel function problems (e.g. diarrhoea, frequency of bowel movement and incontinence), and these may be present in up to 72% of survivors. 9 The clinical rationale for cancer rehabilitation is to support the management of late and long-term effects of cancer and its treatment, increase chances of survival and improve general health and quality of life.
The American Cancer Society and the World Cancer Research Fund recommend that cancer survivors would benefit from following lifestyle recommendations for secondary cancer risk reduction (e.g. taking a nutrient-dense diet, increasing levels of physical activity, ceasing smoking, reducing alcohol intake and avoiding excess body fat). 10,11 The adoption of lifestyle recommendations may also reduce CRC survivors’ risk of other diseases, such as cardiovascular disease, which is a common comorbidity in people diagnosed with CRC,12 the aetiology of which is also attributed to lifestyle factors. 13
However, most CRC survivors are not meeting these lifestyle recommendations. 14–18 A study conducted in Australia, for instance, shows that, at 12 months post diagnosis, approximately 8% of CRC survivors are smokers, 22% are high-risk drinkers, 62% are insufficiently physically active and 61% are overweight/obese. 14 CRC survivors have been found to have the lowest physical activity rates of any cancer group. 19 There is a strong case, therefore, for the provision of rehabilitation for cancer survivors, and, in particular, for people with CRC. However, at the time the protocol for this current study was written (2012), cancer rehabilitation was not usual care in the UK or, indeed, elsewhere. 20 A challenge facing the NHS, therefore, is integrating rehabilitation into standardised models of care for cancer survivors.
The National Institute for Health and Care Excellence guidance, Improving Supportive and Palliative Care for Adults with Cancer, states that:
Rehabilitation attempts to maximise patients’ ability to function, to promote their independence and to help them adapt to their condition. It offers a major route to improving their quality of life, no matter how long or short the timescale. It aims to maximise dignity and reduce the extent to which cancer interferes with an individual’s physical, psychosocial and economic functioning. 21
Cancer rehabilitation is a care specialty that comprises the full spectrum of rehabilitation fields, including the physical, psychosocial and socioeconomic, and can include physical activity, diet, nutrition and psychosocial components. 22 As we have already pointed out, cancer rehabilitation is an often-neglected aspect of cancer care in terms of health policy and infrastructure. 20 Reasons for this include clinicians remaining unconvinced or unaware of evidence of patient benefit from rehabilitation, and challenges around the implementation of rehabilitation in current cancer care pathways. The following section explains why rehabilitation for people with CRC should include physical activity as a core component.
Evidence for increasing physical activity
Physical activity is a key component of rehabilitation. In order for clinicians to prescribe physical activity, which is a non-pharmacological adjunctive therapy, for CRC survivors, there needs to be strong evidence of patient benefit. The clinical rationale for physical activity interventions for CRC survivors is derived from epidemiological observations of relationships between physical activity and survival, and evidence of cause and effect derived from randomised controlled trials (RCTs) about the benefits of physical activity on psychosocial domains, such as quality of life, fatigue, anxiety and depression. 23,24 We use the term ‘physical activity’ throughout this report, although we recognise that the term ‘exercise’ is often used interchangeably to refer to ‘A potential disruption to homeostasis by muscle activity that is either exclusively or in combination, concentric, eccentric or isometric’. 25
To assist the reader in interpreting the following evidence about associations between physical activity and clinical endpoints in people with CRC, and, perhaps more importantly, to recognise the level of physical activity required to achieve a health benefit, we describe current recommended UK guidance for physical activity for the general population and metabolic equivalents (METs) in Boxes 126 and 2, respectively. 27
At least 150 minutes (2 hours and 30 minutes) of moderate-intensity aerobic activity such as cycling or fast walking every week, and muscle-strengthening activities on 2 or more days a week that work all major muscle groups (legs, chest, shoulders, hips, back abdomen, and arms)
OR
75 minutes (1 hour and 15 minutes) of vigorous-intensity aerobic activity such as running or a game of singles tennis every week, and muscle-strengthening activities on 2 or more days a week that work all major muscle groups.
OR
An equivalent mix of moderate and vigorous-intensity aerobic activity every week (for example, two 30-minute runs plus 30 minutes of fast walking), and muscle-strengthening activities on 2 or more days a week that work all major muscle groups (legs, hips, back, abdomen, chest, shoulders and arms).
Also try to break up long periods of sitting with light activity as sedentary behaviour is now considered an independent risk factor for ill health, no matter how much exercise you do. 26
Reproduced from NHS Choices (www.nhs.uk). Contains public sector information licensed under the Open Government Licence v 3.0 (www.nationalarchives.gov.uk/doc/open-government-licence/version/3/).
A metabolic equivalent, or MET, is a unit useful for describing the energy expenditure of a specific activity. A MET is the ratio of the rate of energy expended during an activity to the rate of energy expended at rest. One MET is the rate of energy expenditure while at rest. A 4-MET activity expends four times the energy used by the body at rest.
The health benefits of physical activity demand a range of 500–1000 MET minutes per week.
Physical activity and survival
Three separate meta-analyses23,28,29 including the same six observational studies30–35 were published in 2013–14. Owing to different cut-off values for level of physical activity and different statistical analyses used, there is slight variation in the results; what is evident, however, is that all three studies show that a higher level of physical activity is associated with an increase in cancer-specific and overall survival.
A meta-analysis28 reported that three32,34,35 out of the six prospective cohorts assessing post-diagnosis physical activity found a statistically significant increase in cancer-specific survival among patients with a high level of physical activity, compared with patients with a low level. Overall, higher post-diagnosis physical activity was significantly associated with an improved cancer-specific survival [hazard ratio (HR) cancer-specific survival = 0.61, 95% confidence interval (CI) 0.44 to 0.86; random-effects model; p < 0.001]. The meta-analysis also found that higher post-diagnosis physical activity level was associated with a significantly increased overall survival (HR overall survival = 0.62, 95% CI 0.54 to 0.71; fixed-effects model; p < 0.001). Five31–35 out of the six individual studies assessing the relationship between physical activity level and overall survival found a statistically significant increase in overall survival among patients with higher post-diagnosis physical activity levels.
Pooled relative risks from another meta-analysis23 among CRC survivors showed inverse associations between post-diagnosis leisure-time physical activity and mortality based on six prospective cohorts. 30–35 The authors conducted a meta-analysis for exerciser versus not exerciser, moderate level of physical activity versus low physical activity, and high level of physical activity versus low physical activity. Low physical activity (reference group) was defined as 0, < 3 and < 3.5 MET hours per week or sedentary and the highest category was defined as ≥ 18 and ≥ 8.75 MET hours per week or sufficiently active. All categories above the reference group were pooled to represent ‘exercisers’. Exercisers had a risk ratio of 0.74 (95% CI 0.58 to 0.95; p = 0.02) for CRC-specific mortality, compared with non-exercisers. The risk ratios of CRC-specific mortality for moderate versus low physical activity and high versus low physical activity were 0.82 (95% CI 0.61 to 1.10; p = 0.19) and 0.65 (95% CI 0.47 to 0.92; p = 0.01), respectively. Similarly, exercisers had a risk ratio of 0.68 (95% CI 0.60 to 0.78; p < 0.001) for all-cause mortality, compared with non-exercisers, and the risk ratios of all-cause mortality for moderate versus low physical activity and high versus low physical activity were 0.76 (95% CI 0.64 to 0.90; p = 0.001) and 0.61 (95% CI 0.52 to 0.71; p < 0.001), respectively.
The meta-analysis indicated that there may be a threshold ‘dose’ of physical activity that is necessary to yield a health protective effect. 23 In this meta-analysis, those who participated in both moderate amounts of physical activity and high amounts of physical activity after diagnosis had a 24% and 39% risk reduction in all-cause mortality, respectively, compared with those who participated in low amounts of physical activity. Thus, the researchers highlight a clinically relevant finding from the meta-analysis, which is that a moderate amount of physical activity participation (defined by the researchers as physical activities between 3 and 18 MET hours per week or between 3 and 8.75 MET hours per week, or between 1 and 150 minutes of physical activity per week) was associated with a 24% risk reduction in all-cause mortality, whereas previously reported individual studies33,34 had suggested that higher levels of more than 18 or 27 MET hours per week of physical activity participation were associated with favourable survival outcomes in CRC survivors. 23
Another meta-analysis29 of the same studies30–35 also found a dose response. Each 5-, 10-, or 15-MET hours per week increase in post-diagnosis physical activity was associated with a 15% (95% CI 10% to 19%), 28% (95% CI 20% to 35%) and 38% (95% CI 28% to 47%) lower risk of total mortality, respectively. The reviewers also found that the apparent protection from total mortality afforded by physical activity was not modified by tumour stage, cancer treatment, smoking or adiposity.
A study published after the above meta-analysis36 found that spending ≥ 7 hours per week in leisure time physical activity after a diagnosis of CRC was associated with a 31% lower risk of death (from any cause) than doing no leisure time physical activity (HR = 0.69, 95% CI 0.49 to 0.98; p for trend = 0.01; adjusted for pre-diagnosis leisure-time physical activity). Looking at specific causes of death, spending ≥ 7 hours in leisure time physical activity after diagnosis, compared with none, probably reduces risk of death from CRC (HR 0.53, 95% CI 0.27 to 1.03; p for trend = 0.04) but not death from cardiovascular disease (HR 0.89, 95% CI 0.42 to 1.86; p for trend = 0.38).
Only one of the studies included in the above meta-analyses reported on sedentary time and mortality among people with CRC31 and found that sitting for ≥ 6 hours per day compared with sitting for < 3 hours per day after diagnosis was associated with a 27% increased mortality risk (95% CI 0.99 to 1.64). This specific study has added to the growing literature on the health risks of sedentary time, which is defined as waking activities performed in a seated or reclining posture that require very low energy expenditure (< 1.5 METs). 37–40 For this reason, our study is designed to assess the feasibility and acceptability of obtaining objective measures of both sedentary time and physical activity among people with CRC.
Another recent meta-analysis adopted a slightly different approach from the three described above. 41 Rather than focusing on assessing the amount or categories of physical activity at one point of time with cancer outcome, the focus was on the impact of actual changes of physical activity over time. Physical activity was required to be assessed at least twice, before and after diagnosis, or during two follow-up periods after diagnosis. Patients who increased their physical activity level or remained active throughout the diagnosis and treatment phases had significantly higher quality-of-life scores than patients with reduced physical activity levels after treatment. Pooled analysis from two studies30,35 found a significant association between increases in physical activity in the post-diagnosis period and reduced CRC death, but increases from pre diagnosis to 5 months post diagnosis were not significant. However, the overall pooled HR estimate of 0.70 (95% CI 0.55 to 0.85) indicated that the change was significant. A similar pattern was observed for overall mortality (pooled HR 0.75, 95% CI 0.62 to 0.87). Pooled analysis from three studies42–44 revealed that, compared with decreased physical activity post diagnosis and/or post treatment, increased physical activity was associated with significantly higher quality-of-life scores [standardised mean difference (SMD) 0.74, 95% CI 0.66 to 0.82]. The impact of physical activity on quality of life for people with CRC is discussed further in the following section.
Physical activity and psychosocial outcomes
Recent systematic reviews and meta-analyses of multiple cancer types provide evidence that physical activity interventions can help to address the psychosocial effects of cancer and associated treatments. 45–47 These reviews have been chosen for this report because they have been published recently (i.e. since 2010) and therefore include most recent evidence. Below we summarise the results of these reviews relating specifically to our outcomes of interest (i.e. level of physical activity, quality of life, fatigue, anxiety and depression). In addition, any results and conclusions about the moderating influence of intervention characteristics are described. Signs of effect sizes described below are set so that negative effect sizes for fatigue, anxiety and depression and positive effect sizes for quality of life and level of physical activity indicate improvements in favour of intervention participants.
A meta-analysis of 82 unique studies of interventions (not conducted in a physical therapy setting or delivered by a physical therapist) concluded that there is a small to moderate effect on physical activity level (weighted mean effect size 0.38, 95% CI 0.22 to 0.54; p = 0.0001), overall quality of life (weighted mean effect size 0.29, 95% CI 0.03 to 0.54; p = 0.03), and fatigue (weighted mean effect size –0.54, 95% CI –0.90 to 0.19; p = 0.003) in post-treatment interventions, and a small to moderate effect on functional quality of life (weighted mean effect size 0.28, 95% CI 0.02 to 0.54; p = 0.04) and anxiety (weighted mean effect size –0.21, 95% CI –0.39 to –0.03; p = 0.02) during treatment interventions. 45 No significant effect of physical activity interventions on depression was found (18 studies assessed depressive symptoms). Although intervention characteristics are described, no analyses of moderating effects are reported.
A meta-analysis of 15 RCTs of moderate-intensity physical activity programmes found a small but significant effect on depression under a random-effects model [effect size reported as mean change scores (Cohen’s d) –0.2, 95% CI –0.43 to –0.009; p = 0.04]. 46 Characteristics of physical activity interventions were found to be significant. Home-based exercise was associated with increased depressive symptoms [effect size reported as mean change scores (Cohen’s d) 0.16, 95% CI –0.15 to 0.47], compared with an improvement in depressive symptoms from interventions in other locations (e.g. community facilities, laboratories and gyms) [effect size reported as mean change scores (Cohen’s d) –0.45, 95% CI –0.77 to –0.14] and was significant (p = 0.04). Supervised and partially supervised exercise produced reductions in depressive symptoms, whereas non-supervised activity was associated with a small increase in depressive symptoms [supervised: effect size reported as mean change scores (Cohen’s d) –0.67, 95% CI –1.11 to –0.23; mixed supervision: effect size reported as mean change scores (Cohen’s d) –0.32, 95% CI –0.50 to –0.14; and unsupervised: effect size reported as mean change scores (Cohen’s d) 0.25, 95% CI –0.01 to 0.50] and was significant (p = 0.01). Exercise bout durations of > 30 minutes had larger effects on depression than exercise bouts of ≤ 30 minutes [> 30 minutes’ bout: effect size reported as mean change scores (Cohen’s d) –0.57, 95% CI –0.91 to –0.23; ≤ 30 minutes’ bout: effect size reported as mean change scores (Cohen’s d) 0.01, 95% CI –0.20 to 0.22] and this was significant (p = 0.02).
A meta-analysis of 34 studies of physical activity interventions for patients after they had completed their main treatment (it was possible that patients were still undergoing hormonal treatment) found effects on fatigue, depression and quality of life. 47 Measured by the revised Piper Fatigue Scale, physical activity was associated with slightly reduced fatigue (mean difference −1.0, 95% CI −1.8 to −0.1; p = 0.03) in three comparisons from two studies on breast cancer, compared with the control. In survivors of mixed types of cancer, physical activity was associated with reduced depression (mean difference −4.1, 95% CI −6.5 to −1.8; p < 0.01) as measured by the Beck Depression Inventory. In survivors of mixed types of cancer, physical activity improved the Short Form Health Survey 36 items (SF-36) physical function (mean difference 3.0, 95% CI 0.7 to 5.3; p = 0.01), social function (mean difference 3.4, 95% CI 0.4 to 6.4; p = 0.03) and mental health scores (mean difference 2.4, 95% CI 0.7 to 4.1; p = 0.01) compared with the control group. Although intervention characteristics were described, no analyses of moderating effects were reported.
These meta-analyses suggest that physical activity interventions have a small to moderate effect for level of physical activity, quality of life, fatigue, anxiety and depression. Although these reviews demonstrate patient benefit, a note of caution is required because most of the studies included in these reviews involve people diagnosed with breast cancer (e.g. 83% of all studies in the review by Speck et al. 45 involved people with breast cancer) and the results of these studies cannot be automatically generalised to people with CRC. This is because people with CRC, relative to people with a diagnosis of breast cancer (i.e. the patient group most represented in the controlled trials of physical activity), are likely to present with more advanced disease, have different treatments and side effects, tend to be older and include equal numbers of men and women.
However, a 2014 systematic review and meta-analysis of physical activity interventions for people with CRC suggests that, with appropriate support, people with CRC can increase physical activity levels, with subsequent health-related benefits. 24 Only three RCTs were included in the meta-analysis:44,48,49 a pilot RCT involving 18 participants who had recently undergone surgery and completed chemotherapy treatment for CRC, randomised to either a 12-week programme of twice-weekly supervised exercise sessions for 6 weeks followed by 6 weeks’ home-based exercise and dietary advice or standard treatment;48 a RCT involving 102 CRC survivors randomised to either an exercise group where they were specifically advised to perform moderate-intensity exercise 3–5 times per week or a control group who were requested not to exercise;44 and a RCT involving 46 people who had completed treatment for CRC and who were randomised to either 3 months of telephone counselling to support home-based physical activity or a contact telephone call. 49 The meta-analysis found no evidence for effects on quality of life (standardised mean difference 0.18, 95% CI –0.39 to 0.76; p = 0.53) or fatigue (standardised mean difference 0.18, 95% CI –0.22 to 0.59; p = 0.26). There was, however, evidence for improvements in physical fitness (standardised mean difference 0.59, 95% CI 0.25 to 0.93; p < 0.01). Analysis of moderating effects of intervention characteristics was not possible. The authors acknowledged that the review comprised only three studies with a total sample size of 166 participants and therefore concluded that further studies on physical activity for people with CRC are warranted.
At the time this current study was being developed (2012), two large-scale trials featuring people diagnosed with CRC were in progress. 50,51 The multinational, multicentre Colon Health and Life-Long Exercise Change trial (CHALLENGE) will determine the effects of a 3-year structured physical activity intervention on disease-free survival in 962 survivors of stage II or III colon cancer. 50 The 3-year intervention, modelled on the theory of planned behaviour, will consist of a behavioural support programme (n = 48 sessions in total), focusing on strategies to promote the adoption and long-term maintenance of physical activity and supervised physical activity sessions (n = 48 in total, one to one or in group format), designed to address physical activity techniques and intensity and safety, both delivered by a physical activity consultant (professional discipline not stated). The CanChange trial, drawing theoretically on cognitive–behavioural approaches for telephone-based health coaching sessions, recently reported significant intervention effects for moderate physical activity (28.5 minutes per week; p = 0.023). 51
What kind of cancer rehabilitation could be delivered in the NHS?
Given the increasing number of studies showing the safety and benefits of physical activity, it should be part of standard care for all cancer survivors. One barrier to physical activity becoming standard care is difficulties around implementation. Thus, when designing this study, we paid particular attention to this issue. We wanted a physical activity intervention that was effective, sustainable, cost-effective and capable of being integrated into the routine care of cancer survivors. As far as we know, this study is novel in that it aims to test an existing, evidence-based and theory-driven cardiac rehabilitation service for people with CRC. Should this model of rehabilitation prove to be clinically effective and cost-effective in a large-scale definitive trial, referral pathways could be adapted to ensure that the model is integrated into existing cancer service frameworks.
One of the reasons cardiac rehabilitation may be a suitable model for CRC survivors is that cardiac rehabilitation multiprofessional teams have the expertise required to provide relevant rehabilitation, including monitored physical activity, to a wide variety of patients, such as those with a CRC diagnosis. Second, a comparison of studies of coronary heart disease (CHD) and the post-treatment needs of people with CRC suggests that there is reasonable justification for referring CRC patients to cardiac rehabilitation and running mixed classes involving people with CHD and people with cancer. Four qualitative studies of patients’ experiences of needs after coronary artery bypass graft (CABG),52–55 one case note review of needs of 521 patients surgically treated for CRC cancer56 and one population-based cohort study including 522 people with CRC57 all indicate that people with CHD and people diagnosed with CRC experience similar problems, including pain, fatigue, anxiety and depression, worry, appetite loss, sexual problems, sleep disturbance, and work and financial-related difficulties, and express a need for information about medication and self-management. Thus, the rehabilitation needs of people with CHD and those of people with CRC are likely to be similar, suggesting that a common rehabilitation programme may be appropriate. Moreover, cardiac rehabilitation may be particularly relevant for people with CRC because the estimated prevalence of cardiovascular disease is 59% at 5 months post diagnosis, and, 16% develop de novo cardiovascular disease within 36 months of completion of treatment. 58 In addition, common comorbid conditions in CRC survivors include congestive heart failure, diabetes mellitus and chronic obstructive pulmonary disease,59 which, again, may be managed by rehabilitation.
Pointing out the similarities in post-treatment experiences is not to deny that there are, of course, disease-related differences among different patient groups. For example, people diagnosed with, and treated for, CRC can experience physical discomfort, bowel function problems and urinary tract infections and need advice about abdominal pain and stoma care,9 problems almost certainly not experienced by those with CHD unless they have comorbidities. There is a need, however, to meet the long-term rehabilitation needs of cancer survivors as well as a need to identify cost-effective and sustainable models of rehabilitation. This is why researching the effectiveness of rehabilitation to mixed classes of people with CHD and people with CRC is justifiable.
A further reason why it is worthwhile finding out if cardiac rehabilitation is a feasible, acceptable and effective model for CRC survivors is that cardiac rehabilitation is standard clinical care for patients after a cardiac event and is widely available throughout the UK. Comprehensive cardiac rehabilitation consists of supervised exercise, behavioural change, lifestyle risk factor management, education and psychological support. 60,61 In 2010–11 there were 276 cardiac rehabilitation centres in England, Northern Ireland and Wales62 and 37 in Scotland. 63
Cardiac rehabilitation is defined as:
The coordinated sum of activities required to influence favourably the underlying cause of cardiovascular disease, as well as to provide the best possible physical, mental and social conditions, so that the patients may, by their own efforts, preserve or resume optimal functioning in their community and through improved health behaviour, slow or reverse progression of disease. 61
Traditionally, the provision of cardiac rehabilitation has been described using phases 1–4, as mentioned in the National Service Framework for CHD. 64 Figure 1 summarises each phase.
FIGURE 1.
Four phases of cardiac rehabilitation.
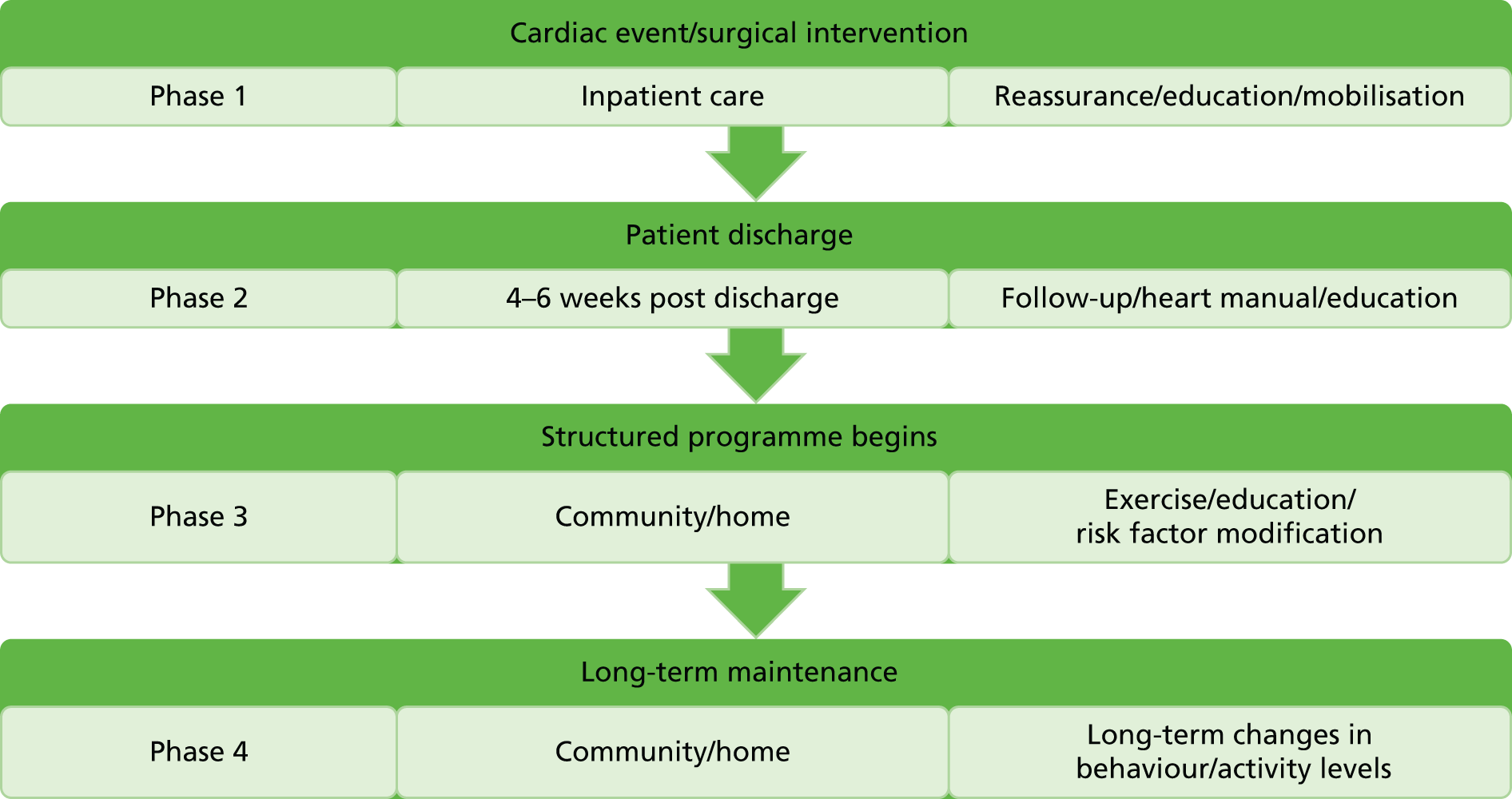
A more recent approach in the Department of Health’s commissioning pack on cardiac rehabilitation65 describes cardiac rehabilitation along a best practice care pathway, using stages 0–6 to reflect core stages in the cardiac rehabilitation pathway, as shown in Box 3.
Stage 0: identify and refer patient.
Stage 1: manage referral and recruit patient to cardiac rehabilitation programme.
Stage 2: assess patient for cardiac rehabilitation.
Stage 3: develop patient care plan.
Stage 4: deliver comprehensive cardiac rehabilitation programme.
Stage 5: conduct final assessment.
Stage 6: discharge and transition to long-term management.
Another reason why cardiac rehabilitation may be a good model for cancer rehabilitation is that supervised exercise is the cornerstone of cardiac rehabilitation. 60,61 Although phase 3 cardiac rehabilitation varies across the UK, exercise classes are usually offered to patients once-weekly for 6–10 weeks. There are 13 rehabilitation standards for cardiac rehabilitation physical activity and exercise. 66 These provide a benchmark for all cardiac rehabilitation programmes delivered throughout the UK and include standards about initial screening and assessment, goal planning, exercise programmes and health and safety. A recent audit found that at 12 months after participation in cardiac rehabilitation there was a 14 percentage point increase in the number of people exercising five or more times a week for 30 minutes and a 23 percentage point reduction in those who rarely/never took exercise. 62 These changes in levels of physical activity represent important milestones for achieving recommended physical activity levels associated with disease prevention. 26 There is no obvious reason why the observed increases in the amount of physical activity among people with CHD could not also be found among people with cancer attending cardiac rehabilitation.
Until the publication in 2012 of a study contesting the benefits of cardiac rehabilitation,67 the consensus had been that cardiac rehabilitation decreases mortality rates in people with CHD. After we had started our study, however, the Rehabilitation After Myocardial Infarction Trial67 involving 1813 patients was published, which reported that there were no significant differences between patients referred to rehabilitation and controls in mortality at 2 years (risk ratio 0.98, 95% CI 0.74 to 1.30) or after 7–9 years (0.99, 95% CI 0.85 to 1.15), cardiac events, quality of life or psychological general well-being. 67 Its publication has caused considerable debate in those providing cardiac rehabilitation services,61,68 not least as the results seem to contradict a 2011 Cochrane systematic review and meta-analysis, which included 47 studies with over 10,000 patients and showed that cardiac rehabilitation reduces death from any cause by 13% and cardiac deaths by 26%. 69 Our study, therefore, took place at a time when there was a degree of controversy about the health benefits of cardiac rehabilitation for people with CHD.
The importance of feasibility and pilot studies
Conducting a full-scale RCT and economic evaluation of cardiac rehabilitation versus usual care for CRC survivors requires the involvement of many sites and is likely to be resource intensive. As there are uncertainties regarding rates of eligibility, consent, recruitment, retention and participation in the intervention and uncertainties about the feasibility and acceptability of the intervention for patients and clinicians, it is important in the first instance to conduct feasibility and pilot work. Findings from feasibility and pilot work can then be used to optimise the design and conduct of any subsequent large-scale trial or, indeed, be used to judge whether or not it is even appropriate and ethical to proceed to such a trial.
Conducting pilot studies to iron out methodological bias in advance of a large-scale trial is critical if that larger trial is to become part of an evidence base that is then used for recommending policy and changing cancer care practice. The importance of addressing methodological bias was highlighted in a recent meta-analysis of 33 RCTs of physical activity interventions for people with breast cancer. 70 The meta-analysis found that RCTs rated at high risk of selection bias with the absence of random sequence generation, or at high risk of attrition bias with large attrition rate or the absence of intention-to-treat analysis, resulted in greater efficacy of physical activity on quality of life, anxiety or depression at the end of intervention in experimental group versus control. The reviewers call for exacting methodological standards in future trials to increase confidence in evidence about the benefits of physical activity for cancer survivors. A pilot trial in advance of a larger-scale trial may be one useful approach to improve methodological rigor and standards.
The Medical Research Council’s (MRC) recommended framework for the evaluation of complex interventions includes feasibility and piloting phases. 71 Currently, however, there are no internationally agreed definitions of feasibility and pilot work. 72 One definition of feasibility study and its differentiation from pilot study comes from the UK’s National Institute for Health Research (NIHR) Evaluation, Trials and Studies Coordination Centre:73
Feasibility Studies are pieces of research done before a main study in order to answer the question ‘Can this study be done?’ They are used to estimate important parameters that are needed to design the main study.
Pilot studies are a version of the main study that is run in miniature to test whether the components of the main study can all work together. It is focused on the processes of the main study, for example to ensure recruitment, randomisation, treatment, and follow-up assessments all run smoothly. It will therefore resemble the main study in many respects, including an assessment of the primary outcome.
Reproduced with permission from NIHR
Thus, whereas a feasibility study may examine a specific part of a trial, a pilot study is a dummy run, examining the trial as a whole in order to see if all of the parts work together as planned.
In a review of 54 pilot and feasibility studies,74 researchers found that pilot studies tend to have more rigorous methodological components, such as sample size estimation, randomisation and control group selection, and are more likely include a greater number of methodological components for testing than studies labelled as ‘feasibility’. Nevertheless, the reviewers drew the conclusion that the distinction between the two is not clear-cut. 74
Irrespective of what researchers actually call preliminary studies, it is important that they clarify why pilot and feasibility work is being carried out. Feasibility and pilot work can be conducted to evaluate the operational feasibility and acceptability of the intervention itself and the feasibility and acceptability of a trial’s protocol design. There seems little point in running large-scale (and therefore presumably expensive) trials of interventions – even those suggesting promise of effect – if these interventions are unlikely to ever see the light of day and be implemented in practice. Likewise, if a trial is unworkable, then results about effectiveness will not be forthcoming. Thus, for our study we explored the twin pillars of feasibility and pilot work by examining intervention implementation and trial methodology parameters.
Bowen et al. 75 recommend eight areas of focus to assess if a public health intervention is feasible (Table 1). Addressing each area can help in the assessment of the likelihood of an intervention being implemented as part of routine health care and as a future commissioned service. Their recommendations for areas of focus in feasibility studies share similarities with frameworks designed to identify public health impact of health promotion interventions and evaluate the extent to which it is implementable, such as RE-AIM. 76 Using this approach, feasibility and pilot work can be carried out to provide information that can be used to modify an intervention to enhance its future implementation, as well as inform decisions about whether or not it is sensible, from an implementation perspective, to progress to a large-scale trial.
| Area of focus | The feasibility study asks . . . |
|---|---|
| Acceptability | To what extent is a new idea, program, process or measure judged as suitable, satisfying, or attractive to program deliverers? To program recipients? |
| Demand | To what extent is a new idea, program, process, or measure likely to be used (i.e., how much demand is likely to exist)? |
| Implementation | To what extent can a new idea, program, process, or measure be successfully delivered to intended participants in some defined, but not fully controlled, context? |
| Practicality | To what extent can an idea, program, process, or measure be carried out with intended participants using existing means, resources, and circumstances and without outside intervention? |
| Adaptation | To what extent does an existing idea, program, process, or measure perform when changes are made for a new format or with a different population? |
| Integration | To what extent can a new idea, program, process, or measure be integrated within an existing system? |
| Expansion | To what extent can a previously tested program, process, approach, or system be expanded to provide a new program or service? |
| Limited efficacy | Does the new idea, program, process, or measure show promise of being successful with the intended population, even in a highly controlled setting? |
Another reason for conducting feasibility and pilot work is to evaluate trial methodology. Thabane et al. 77 propose four primary purposes for conducting pilot studies (Table 2). Although these key reasons were initially identified to guide the conduct of drug pilot trials, they have recently been adapted and used to guide the conduct of a rehabilitation intervention pilot trial. 78 Addressing these four areas will give some indication of the chances of a large-scale trial being successfully conducted.
| Process | This assesses the feasibility of the processes that are key to the success of the main study |
| Resources | This deals with assessing time and resource problems that can occur during the main study |
| Management | This covers potential human and data management problems |
| Scientific | This deals with the assessment of treatment safety, dose, response, effect and variance of the effect |
It is recommended that threshold criteria for claiming future success of a large-scale trial are established before feasibility and pilot work commences. 77,78 The criteria should be based on the primary feasibility objectives. 77 Examples include the acceptable proportion of participants being eligible, consenting and completing the intervention. Using these criteria, the outcome of a pilot study will be one of the following:
-
stop – main study not feasible
-
continue, but modify protocol – feasible with modifications
-
continue without modifications, but monitor closely – feasible with close monitoring
-
continue without modifications – feasible as is.
Using this approach, pilot and feasibility studies provide critical information for planning and designing large-scale trials and justification for whether or not to allocate large sums of money to such a trial.
It is generally recommended that feasibility and pilot studies descriptively evaluate a trial’s feasibility, acceptability and safety rather than test the effectiveness of the hypotheses of the planned main large-scale trial. 74,77,79,80 This is because the small number of effect data available in feasibility and pilot studies mean the degree of uncertainty is such that the chance of reaching inaccurate conclusions about intervention effect is high. Feasibility and acceptability assessments of trial components may also be misleading if only a limited number of highly motivated sites are included in a pilot study because these sites are unlikely to be representative of the multitude of sites involved in a large-scale trial. 80 Event rates such as recruitment and willingness to be randomised cannot be accurately estimated from small pilots and estimates of variance of the outcome variable to calculate sample size from small pilot studies are also likely to suffer from imprecision. 78–80 How many total participants are required to estimate a standard deviation (SD) for a sample size calculation is unclear, with suggestions ranging from 24,81 to 30,82 to 5083 and to 70. 84
Good trial design requires the magnitude of the clinically important effect size to be stated in advance, and at least some indication of the efficacy of the proposed intervention is often required to justify to funders that it is worth the effort and expense in conducting a large-scale trial. One strategy for reporting outcomes from pilot work is to declare ‘potential efficacy’ if the CI around the estimated effect of the intervention on a clinically important outcome includes a predefined minimal important difference and, conversely, to declare ‘potential harm’ if the harm effect lies outside the upper confidence limit for safety. 79 This approach acknowledges the limited power of pilot trials to confirm the benefits and/or harms of treatment, while at the same time minimises the likelihood of the abandonment of a large-scale trial on the basis of negative or positive results. 74 Nevertheless, conducting analyses to glean information about efficacy from pilot trials, although tempting, is misleading and unreliable. 76,78 As a consequence, any observed potential patient benefit ought to be reported extremely cautiously or not at all; robust and rigorous assessment of an intervention’s therapeutic implications must await adequately sized definitive pivotal trials. 80
Study aims
The CRIB (Cardiac Rehabilitation In Bowel cancer) study was funded by the NIHR Health Services and Delivery Research programme. The overall aims of the CRIB study were to assess whether or not using phase 3 cardiac rehabilitation is a feasible and acceptable model of rehabilitation to aid the recovery of CRC survivors (i.e. examine intervention implementation potential) and to test the feasibility and acceptability of the protocol design (i.e. examine methodological standard). Thus, the overall purpose of the study was to assess whether or not it is appropriate to progress to a larger-scale trial and, if so, to optimise the design and conduct of any such trial.
Chapter 2 Study design and governance
In this chapter, the study design, research questions and objectives are presented. The details about ethics committee and research management approvals are also provided. Finally, the trial registration information is reported.
Study design
The CRIB study was set up to evaluate the feasibility and acceptability of an innovative approach to aid the post-treatment recovery of people with CRC available in the NHS. Specifically, this involved an intervention study to test the feasibility and acceptability of the referral of people who had recently had surgery for CRC, who may or may not have been receiving adjuvant therapy, to cardiac rehabilitation. We undertook a phased programme of work comprising intervention testing and feasibility work (phase 1) and a pilot RCT (phase 2). The pilot trial was supplemented by a preliminary economic evaluation to consider the cost-effectiveness of providing the intervention compared with usual care. There was also a qualitative component to explore the views and experiences of patients and clinicians involved in the study. A description of the study protocol has already been published. 85
In phase 1, we sought to answer the following research questions:
-
What modifications, if any, are required to be made to existing cardiac rehabilitation (the intervention) to make it more relevant and acceptable to CRC patients and clinicians?
-
What modifications, if any, are required to be made to the training and support provided by the cancer-exercise specialist to make it more relevant and acceptable to cardiac physiotherapists running the cardiac rehabilitation exercise classes?
-
What modifications, if any, are required to be made to the proposed trial procedures to make the trial more feasible to conduct and to make the trial procedures more acceptable to CRC patients and clinicians?
In phase 2, we sought to answer the following research questions:
-
Are participating centres likely to recruit a sufficient number of patients to deliver a large-scale trial?
-
What are the likely eligibility, consent, recruitment, adherence and completion rates and speed of recruitment for a future large-scale trial and how can these be optimised?
-
Are patients allocated to the control group also increasing levels of physical activity (i.e. contamination)?
-
What are the likely completion rates at baseline and follow-up for the proposed outcome and process measures for a future large-scale trial and how can these be optimised?
-
What sample size is required to power a future large-scale trial?
-
Have practitioners delivering the intervention delivered it as intended and in accordance with the study protocol and how can intervention fidelity be optimised for a future large-scale trial?
-
What are the enablers and barriers that clinicians experience in delivering rehabilitation for patients and in conducting the trial?
-
What are the enablers and barriers that cancer patients experience in participating in the rehabilitation programme?
-
Can costs and health and outcomes be measured for this group using the patient-reported outcome tools for use in the economic evaluation?
Objectives
Phase 1: feasibility study objectives
-
To assess the feasibility of delivering rehabilitation to people with CRC within a cardiac rehabilitation setting.
-
To assess the acceptability of the intervention for patients and clinicians (cancer and cardiac).
-
To assess the acceptability and adequacy of the training and support provided by a cancer-exercise specialist for cardiac physiotherapists running the rehabilitation exercise classes.
-
To assess the feasibility and acceptability of the main trial components (e.g. recruitment procedures, rehabilitation referral procedures and proposed outcomes and process measurement tools) and proposed tools for measuring impacts on outcomes and costs.
Phase 2: pilot study objectives
-
To determine eligibility, consent, recruitment and retention rates and speed of recruitment.
-
To determine completion rates for proposed effect outcomes measurement tools at baseline and follow-up.
-
To determine likely contamination across trial arms (contamination occurs when controls reach exercise intervention goals. Contamination dilutes treatment effect and therefore increases the risk of false-negative conclusions).
-
To provide data for sample size calculations for a definitive RCT.
-
To assess intervention fidelity according to study protocol.
-
To assess the extent to which intervention and trial procedures can be integrated into routine clinical practice.
-
To conduct a preliminary economic evaluation of the cancer rehabilitation programme.
Ethics approval and research governance
An application for NHS ethics approval for the CRIB study was submitted using the electronic Integrated Research Application System. A submission was made on 20 January 2013, received by the NHS ethics committee on 25 January 2013 and reviewed by the committee at a meeting on 14 February 2013 [Research Ethics Committee reference 13/NS/0004; Integrated Research Application System project identification (ID) 121757]. The committee requested further information and submission of revised documentation. Hence, revised documentation was submitted to the chairperson of the ethics committee on 21 February 2013 and a favourable ethics opinion was given on 22 February 2013.
Applications for NHS Research Management approval, an additional approval required in the UK for research involving NHS patients, staff or premises, were made to the research and development office in each of the three health boards in which the study was conducted. Approval was given on the following dates:
-
site 1: 5 March 2013
-
site 2: 17 December 2013
-
site 3: 14 January 2014.
Trial registration
The trial was registered with the International Standard Randomised Controlled Trial Registry under the reference number ISRCTN63510637; and also with the UK Clinical Research Network Portal under the reference number 14092.
Chapter 3 Phase 1 methods
The purpose of phase 1 was to evaluate the feasibility and acceptability of the main trial procedures planned for phase 2 and also the feasibility and acceptability of delivering the intervention. The aim was to use the results of phase 1 to modify the methods and the intervention as appropriate for further testing in phase 2.
This chapter describes the phase 1 study design, participants, recruitment and consent procedures, intervention description, primary and secondary end points and methods for analysis.
Phase 1 design
Phase 1 was a before-and-after study; this is a rigorous design in which dependent variables are measured before and after an intervention has been delivered. 86 This design is suitable for assessing the feasibility of delivering an intervention and main trial procedures.
Participants
Colorectal cancer patients
The study sought to recruit people who had recently had surgery for CRC from an acute general hospital in Scotland.
Inclusion criteria
Patients were considered for inclusion if they:
-
were aged ≥ 18 years and had been diagnosed with primary CRC and were in the recovery period post surgery
-
were/were not receiving adjuvant chemotherapy/radiotherapy (note to reduce risk of infection, patients would have to wait 48 hours after each chemotherapy session before attending cardiac rehabilitation classes). 10
Exclusion criteria
The study excluded anyone:
-
with advanced disease
-
with failed clinical/risk assessment for rehabilitation and who were deemed unsafe to participate in exercise classes; for example, according to recent guidelines, those with severe anaemia should delay exercise and patients with compromised immune function should avoid public gyms and exercise classes10
-
with severe cognitive impairment and therefore are unable to give informed consent to participate in the study
-
unable to communicate in English, as this is the language used in the delivery of cardiac rehabilitation.
Clinicians
Cancer nurses involved in screening patients for eligibility and giving out study information or delivering the intervention (i.e. cardiac rehabilitation physiotherapists) were approached by an investigator and invited to attend a semistructured face-to-face interview about their experiences of the main trial procedures and the intervention.
Recruitment procedures
Recruitment took place over 5 months. The first participant was recruited on 12 August 2013 and the last participant was recruited on 26 November 2013.
The following recruitment procedures for phase 1 were employed.
A CRC clinical nurse specialist assessed all CRC patients admitted for surgery to determine their eligibility for the study. At a follow-up appointment, the nurse gave eligible patients an information sheet (see Appendix 1) about the study, talked them through it, and completed a screening and recruitment form (see Appendix 2) for all eligible patients. This form included, for instance, information about a patient’s demographic characteristics (e.g. age and gender), cancer diagnosis (e.g. rectal or colon), date and type of surgery (e.g. open surgery or laparoscopic), and neo-adjuvant and adjuvant therapies. The patient signed this form if they were willing to participate in the study and agreed to have their contact details forwarded to an investigator. If the patient agreed to participate, the nurse then referred the patient to cardiac rehabilitation by e-mail, fax or letter, and used a referral form (see Appendix 3) to advise cardiac rehabilitation services that the patient would be participating. Patients who, having read the study information, declined to participate were asked if they were willing to give their reasons for declining, which were recorded by the nurse on the ‘reasons for not participating’ form, and the patient was asked to sign a non-participation patient consent form (see Appendix 4) if they were willing to have information about them (e.g. age, gender, diagnosis, treatment) used by the investigators to assess if participants were representative of the study population.
An investigator contacted the patients who had signed the screening and recruitment form, agreeing to participate, and arranged a time for them to sign a consent form, which meant that they had formally consented, in writing, to participation in the study (see Appendix 5). Baseline assessments were conducted for consenting participants.
Once a CRC patient had consented to the study, a member of the cardiac multidisciplinary team (e.g. cardiac physiotherapist or nurse) contacted them and invited them to attend a cardiac rehabilitation clinical/risk stratification assessment to determine whether or not, from a cardiac clinical perspective, they were able to exercise safely; the team member also planned physical activity goals tailored to the individual patient’s needs. Patients deemed safe to exercise were invited to attend cardiac rehabilitation classes.
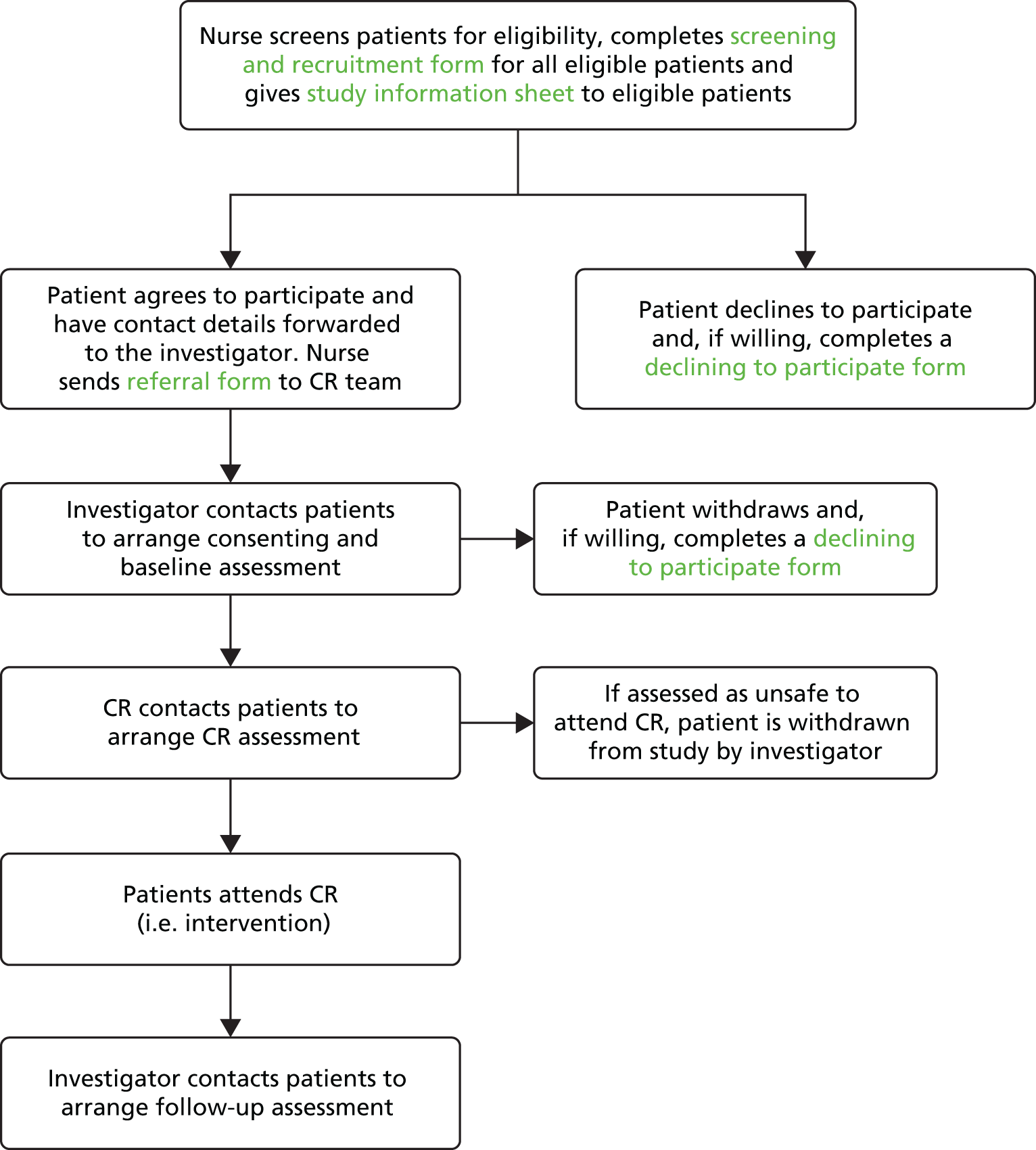
A flow chart outlining CRIB phase 1 recruitment and follow-up procedures is given in Figure 2.
FIGURE 2.
Flow chart outlining phase 1 recruitment and follow-up procedures. CR, cardiac rehabilitation.
Informed consent
Informed consent for patients with CRC was obtained at two stages of the recruitment process. First, nurses obtained written consent to forward a patient’s contact details to an investigator if trial eligibility was established. The patient signed a screening and recruitment form (see Appendix 2). Second, an investigator obtained written consent before undertaking the baseline assessment. The patient signed a consent form (see Appendix 5). The original signed and dated consent forms were held securely as part of the trial site file, with a copy in the clinical notes held securely at the hospital.
Thus, all participants gave written consent to participate in the study. Informed consent discussions for participants took place face to face with a nurse and an investigator, with the opportunity given for participants to ask questions. Patients were informed that they had no obligation to participate and their care would not be affected if they declined to participate. They were made aware that the results of the study would not directly give rise to changes in rehabilitation provision for CRC patients; rather, it would determine whether or not large-scale trials, which may give rise to change in rehabilitation, were feasible. If a patient’s consent to participate in the study was declined or terminated at any stage, that patient then entered usual follow-up care.
Participants had the right to withdraw from the study at any time for any reason, and without giving a reason. The investigator also had the right to withdraw patients from the study intervention if this was considered in the patient’s best interests. There were two withdrawal options:
-
complete withdrawal from both the study intervention (i.e. cardiac rehabilitation) and the provision of data
-
partial withdrawal, when the patient withdrew from participating in cardiac rehabilitation but continued to provide data.
Consent was sought from participants choosing option 1 to retain data collected up to the point of withdrawal. Participants were also asked if they would be willing to give their reasons for their decision to withdraw so that these could be recorded, as this would help to improve acceptability of the study in a large-scale trial. We also gathered data about patients with CRC who declined to participate in the study to explore their reasons for not giving consent, thereby helping us to make the study more acceptable to patients in a large-scale trial.
Intervention
Cardiac rehabilitation
The intervention was phase 3 cardiac rehabilitation (see Chapter 1 for a brief introduction to cardiac rehabilitation as practised in the UK). The cardiac physiotherapist contacted the patient and invited them to attend a cardiac rehabilitation clinical/risk stratification assessment, to determine whether or not, from a cardiac clinical perspective, the patient was able to exercise safely, and also planned physical activity goals tailored to individual patient needs. Patients deemed safe to exercise were given a date to attend cardiac rehabilitation sessions alongside cardiac patients. The participants were expected to attend once per week for 10 weeks. The weekly session consisted of approximately 60 minutes of aerobic and strength training delivered by a senior physiotherapist and physiotherapy assistant, followed by an educational session delivered by a range of clinicians for patients with CHD. Educational sessions included general risk factor advice for better health and some cardiac-specific sessions (e.g. medications and sessions with a cardiologist). Cardiac physiotherapists reinforced health behaviour theories by, for instance, discussing barriers to engaging in physical activity with patients and goal setting, in line with current behaviour change theory87–90 and cardiac rehabilitation guidance. 61 We have used the Template for Intervention Description and Replication (TIDieR)91 to describe, in more detail, cardiac rehabilitation in this site (Table 3). TIDieR is used to describe reasons for, and goals of, the intervention (why), materials and procedures used (what), personnel delivering the intervention (who), how the intervention is delivered (how), where the intervention is delivered (where), when the intervention is delivered and for how long (when and how much), if the intervention differs from one individual to the next (tailoring) and whether or not any changes were made to the original design (modifications).
| Item number | Item | |
|---|---|---|
| Brief name | ||
| 1 | Provide the name or a phrase that describes the intervention | Referral to a cardiac rehabilitation programme |
| Why | ||
| 2 | Describe any rationale, theory, or goal of the elements essential to the intervention | RATIONALE Physical activity in CRC patients has shown improvement in cancer-specific mortality and general mortality. Five recent systematic reviews of controlled trials indicate that physical activity interventions can help address the physiological and psychosocial effects of cancer and associated treatments in adult patients with cancer Cardiac rehabilitation may be an appropriate form of rehabilitation for patients with CRC because many of their needs post treatment are similar to those of individuals living with CHD. Studies on patients’ experiences of needs after CABG and patients with CRC indicate that patients with cardiac issues and cancer experience similar problems, including pain, fatigue, anxiety and depression, worry, appetite loss, sexual problems, sleep disturbance, and work and financial-related difficulties, and express a need for information about medication and self-management THEORY No one model best explains exercise behaviour, but the theory of planned behaviour is used most frequently in the research and literature. This proposes that patient behaviour is predicted by behavioural intent, and that their actions are based on that person’s intention to perform that behaviour: in this case, cardiac rehabilitation. Intentions are based on three factors: attitudes towards the behaviour; beliefs of friends/relatives about the behaviour; and level of control over their actions and behaviour. Health behaviour is, therefore, determined by a combination of these factors, and barriers to change |
| What | ||
| 3 | Materials: describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL) | Patients attending classes have access to an array of information booklets on behaviour change for smoking, diet, healthy living, physical activity and more. The majority of these can be accessed at www.bhf.org.uk/healthcare-professionals/resources-for-patients.aspx. There is also a folder with local information on exercise classes, walking groups, walking routes and other appropriate clubs and groups For intervention delivery, the cardiac rehabilitation team follows the BACPR core components and standards, available at www.bacpr.com/resources/46C_BACPR_Standards_and_Core_Components_2012.pdf A key component of cardiac rehabilitation is an exercise class (see section 4). Patients are talked through the circuit stations by staff, and each station has an illustration with varying degrees of difficulty to suit each participants needs during the class. Patients are also offered material to take home to continue their progress at home. These are individually tailored by the physiotherapist at the centre |
| 4 | Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities | A patient will attend for an initial consultation with the cardiac physiotherapist. This involves confirming details received on referral (past medical history, current medications), and includes an Incremental Shuttle Walk Test to establish fitness levels and suitability for patient to attend the exercise classes Once the patient is accepted into the intervention, they attend class once per week for 10 weeks, where they are put into a group of the most suitable level for their abilities; this includes a lower-level group who do activities at a lower intensity than the standard groups, to allow as many patients as possible to benefit from the intervention. Lower-level classes are run according to demand Standard cardiac rehabilitation sessions involve a 15-minute warm-up session involving range of motion exercises and pulse-raising exercises to gently prepare the body for the session. The main component involves a variety of exercise ‘stations’, which includes CV stations (e.g. shuttle walking, cycling) and strength stations, also known as ‘active recovery’ stations (e.g. exercise ball; dumbbell exercises; theraband movements). The main sessions lasts around 20 minutes, which consists of completing the circuit twice (2 × 10 minutes). Each station in the class has three varying levels of intensity, identified by an illustration at the station itself. This is followed by a 15-minute cool-down, in which feet are kept moving to maintain blood return to the heart, and to allow the heart rate to gradually reduce to resting, or near resting, values. A period of stretching exercises follows, and some sessions incorporate some relaxation techniques, if time allows Weekly information sessions are held for participants on a variety of behaviour change topics. Current classes are cardiac misconceptions, relaxation, consultant questions, healthy heart workshop, psychological health and a session on moving on |
| Who provided | ||
| 5 | For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, background and any specific training given | Cardiac physiotherapist is a band 7 NHS specialist physiotherapist with extensive experience working in cardiac rehabilitation Physiotherapy assistant is a band 3 NHS support member Cardiac rehabilitation co-ordinator is a band 6 NHS member with a background in cardiac nursing in a cardiac care unit |
| How | ||
| 6 | Describe the modes of delivery (e.g. face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group | Intervention is provided face to face by the cardiac rehabilitation team specified above. This is provided in a hospital gym in a group environment, with numbers at around 15–20 per class depending on demand. Initial consultations are given on a one-to-one basis with the specialist physiotherapist |
| Where | ||
| 7 | Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | Patients will attend their local hospital and perform their exercises in the Heartbeat centre, with its specialist cardiac rehabilitation gym with sprung flooring and temperature control. The hospital is the only district general hospital in the trust, providing care for 320,000 people over 32,500 km2 |
| When and how much | ||
| 8 | Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose | Patients will attend once per week for 10 weeks, plus an initial assessment appointment, for sessions that last between 60 and 75 minutes. Intensity of sessions is individualised to each patient by the physiotherapist. RPE scales are used to assess intensity, and heart rate monitors are used for checking pulses, not for intensity The class uses RPE Borg 6–20 scale, and asks participants to work at the range of 12–14 during classes. Patients are given information and explanations of the intensity required using the RPE scale |
| Tailoring | ||
| 9 | If the intervention was planned to be personalised, titrated or adapted, then describe what, why, when and how | Each patient is given targets that are agreed by the patient and the physiotherapist. These targets are individualised but will normally include attending as many sessions as possible, working at the agreed intensity, doing the exercises properly and achieving activity goals outside the classes. Goals are set with discussion with the patient and physiotherapist, but time allocation for this varies depending on other time pressures, so there is no standardised procedure. Ideally, patients see the physiotherapist after approximately 5 weeks to discuss how goals are going, but, again, this is time-dependent and not always achievable |
| Modifications | ||
| 10 | If the intervention was modified during the course of the study, describe the changes (what, why, when, and how) | The intervention is likely to remain constant throughout the study period. As the cardiac rehabilitation intervention is an existing service, there is no opportunity during this pilot study to make changes to the intervention, unless done so by the staff running the programme |
| How well (planned): If intervention adherence or fidelity was assessed, describe how and by whom, and if strategies were used to maintain or improve fidelity, describe them | Intervention adherence will be assessed using attendance at cardiac rehabilitation classes, giving a percentage attendance figure. This will be monitored by the cardiac rehabilitation team | |
| How well (actual): If intervention was assessed, describe the extent to which the intervention was delivered as planned | Average attendance over 10 sessions will be calculated. Of the patients allocated to the intervention group, we will calculate how many attended the programme, and the percentage of those who completed the programme | |
In addition, education sessions about cancer, delivered by a cancer nurse specialist (CNS), were planned to supplement the education sessions for cardiac patients. This was the only alteration to routine cardiac rehabilitation planned for the study. Education sessions were to be delivered either face to face to a group of CRC survivors or individually by telephone.
Cancer and exercise training
As described in Chapter 1, a multidisciplinary team, which includes qualified physiotherapists and nurses, delivers cardiac rehabilitation. Physiotherapists are registered with the Health and Care Professions Council (HCPC) and will have successfully completed a HCPC-approved programme in physiotherapy (offered as 3- or 4-year undergraduate degrees and 2-year postgraduate levels at various UK universities). The training involves both periods of theory and clinical experience gained by meeting and working with patients. The theory part of the course covers anatomy, physiology, physics and pathology. Cardiac rehabilitation physiotherapists are experienced in prescribing exercise for patients with a range of conditions. There are specialist physiotherapy areas, including cardiology, care of the elderly, rheumatology and women’s health. Given cardiac rehabilitation physiotherapists’ level of expertise, 1-day training in cancer and exercise was deemed to be sufficient to deliver the intervention for this study.
Cancer and exercise training sessions were delivered to clinicians in the phase 1 site and the two other sites that would be involved in phase 2. These sessions were delivered to clinicians (CRC nurses and cardiac rehabilitation team) by the director of CanRehab (www.canrehab.co.uk), a Skills Active-validated provider of Level 4 Cancer Exercise Rehabilitation training courses for fitness instructors and clinicians. The training session was delivered in 1 day, face to face in sites 1 and 2 and by video conferencing in site 3. The main aim was to provide cardiac rehabilitation clinicians with the appropriate evidence-based knowledge on current guidelines and contraindications in order to deliver safe effective and appropriate exercise classes to patients with cancer in a cardiac rehabilitation setting.
The content of the 1-day training included:
-
evidence of the benefits of exercise during and after CRC treatment
-
principles and guidelines of exercise prescription for cancer survivors
-
assessment tools for screening and monitoring CRC patients prior to and during exercise programme
-
contraindications, red flags and issues to monitor before and during exercise programme
-
examples of different types of exercise and FITT (frequency, intensity, time, type) principles
-
practical examples of circuit-based exercises, working at different levels of intensity
-
practical examples of seated exercise options
-
principles of exercise motivation and facilitating health behaviour change
-
methods of implementing the information in a cardiac rehabilitation circuit class.
Each attendee was given a report, produced by CanRehab, offering guidance about cancer and physical activity.
In phase 1, there was no control group.
Measures
The primary objective of a future large-scale RCT will be to test if cardiac rehabilitation is clinically beneficial for CRC survivors and cost-effective. As we have explained in Chapter 1, there is evidence that physical activity is associated with improved survival and quality of life, and with reduced anxiety, depression and fatigue. At the time of designing this study, the proposed primary outcome for a future large-scale trial would be the difference in measures of physical activity (e.g. minutes per week, MET hours per week, time spent sedentary and in moderate-intensity activity) between the intervention and usual care (control) groups, measured by accelerometer. The proposed secondary outcomes were self-reported measures of quality of life, anxiety, depression and fatigue. In phase 1, we assessed the feasibility and acceptability of data collection instruments for these proposed outcomes. The results of phase 1 informed decisions about which data collection instruments would be tested further in phase 2 (i.e. pilot RCT) or replaced. An economic evaluation was also planned for any future large-scale trial, and so questions for this economic evaluation were also assessed for feasibility and acceptability during phases 1 and 2 of this study.
The following accelerometer and patient-reported outcome measures were taken at baseline (T0) and approximately 2 weeks post intervention (T1). Information about the validity and scoring of each measure is provided below.
Proposed primary outcome
The proposed primary outcome is change in amount of weekly physical activity. This is based on the research described in Chapter 1, which shows health benefits (e.g. improved survival and quality of life) associated with increasing post-diagnosis physical activity.
Amount of physical activity
The amount of physical activity was assessed using the Actigraph GT3X+ triaxial accelerometer (Actigraph LLC, Pensacola, FL, USA). 92–94 It is designed to be worn around the waist and measures activity counts, steps, inclinometer, and light and moderate to very vigorous physical activity. Accelerometers record movement in such a way that it can be translated into a number of different outputs, for example total step count, bouts of physical activity at specified intensities or energy expenditure. Accelerometers were chosen because they are a robust method for identifying movement. A video-recorded study of 12 healthy adults wearing triaxial accelerometers found that the accelerometers demonstrated high validity, with sensitivity and positive predictive values of > 85% for sitting and lying and > 90% for walking and jogging. 95 Compared with self-report, accelerometers are also likely to provide a more accurate and objective assessment of physical activity in patients with cancer,96 including those diagnosed with CRC. 97 A recent study of 176 colon cancer survivors found that the total mean minutes per day spent in moderate to vigorous physical activity was 12 minutes based on accelerometer data and 26 minutes based on self-reported data (p < 0.01) and the proportion of participants meeting physical activity guidelines based on self-reported data and accelerometer data was 37.6 and 24.3%, respectively; agreement between the methods on this measure was poor (kappa = 0.32) with self-report overestimating level of physical activity. 97 Thus, participants in this study wore an accelerometer to provide an objective measure of the amount of physical activity undertaken. Every participant was offered an accelerometer to wear during waking hours for 7 consecutive days per week. At the end of the 7-day period, participants returned the monitors to the research team.
Initialisation of device
Accelerometer devices were initialised as follows:
-
Device recording of physical activity and sedentary behaviour was set for 10 days maximum, with the intention to gain at least 4 usable days of data for each participant (4 days is standard practice).
-
The date and time when the participant was scheduled to wear the device were set.
-
The sample rate was set to 30 Hz.
-
The unique participant ID was added to the specific device.
Device download
Once the device was returned by a participant, Actigraph software was used to download data, as follows:
-
The unit of measurement was set at 60-second epochs so that the data were automatically converted to minutes for analysis.
-
The ‘# of axis’ setting was set to 3, and ‘steps’, ‘lux’, ‘inclinometer’ and ‘low frequency extension’ were all selected.
-
The ‘limb’ setting was set to ‘waist’ and the ‘side’ setting was set to ‘right’.
Wear-time validation
The validation parameters and cut-off points described below have been used in cross-sectional40,97,98 and intervention studies49 that have measured physical activity and sedentary behaviour among people with CRC. Actigraph software wear-time validation was set to meet the following criteria:
-
Minimum number of valid days required = 4.
-
Non-wear-time was set at > 60 minutes of consecutive zeros.
-
Minimum number of wear hours per day required was set at > 10 hours (600 minutes).
In addition, each suggested non-wear-time was checked manually by an investigator to ensure that we minimised the chance of removing a sedentary period, which can be confused with a non-wear period, particularly among a population who have a high rate of sedentary behaviour, such as some clinical populations.
Cut-off points
Commonly accepted cut-off points for adults were used to differentiate activity intensity using Freedson et al. 92 adult cut-off criteria:
-
sedentary: < 100 counts per minute
-
light: 100–1951 counts per minute
-
moderate: 1952–5724 counts per minute
-
vigorous: > 5725 counts per minute.
In addition, a sedentary bout was set at 10 minutes.
Type of physical activity
Type of physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) long self-report version. This is a 27-item questionnaire used to establish levels of physical activity using 7-day recall. This was used in conjunction with the accelerometer to further explain the type of physical activity, which the accelerometer cannot pick up. 99 Activities are split into low, moderate and vigorous level of activity. The items in the IPAQ are structured to provide separate scores for walking, moderate in intensity and vigorous in intensity activity in each of the four domains (work-related, home and garden, recreation and transportation). The self-administered IPAQ (long version) has acceptable validity when establishing activity levels in healthy adults, and the scale ranks similarly to other self-report options, and produced repeatable data in adults in diverse settings. 100
Scoring
The ‘score’ can be calculated either in minutes per week in each intensity of activity or as a continuous variable in MET minutes per week. Total time spent in physical activity during the past 7 days can be obtained by multiplying the number of days of the activity by the amount of time spent in each activity and then summed according to the intensity of the physical activity.
Proposed secondary outcomes
Quality of life
The European Quality of Life-5 Dimensions (EQ-5D) instrument and SF-36 were chosen as measures of quality of life because they have strong evidence of reliability, validity, responsiveness and acceptability. 101 Both EQ-5D and SF-36 are used in health economics as a variable in the quality-adjusted life-year calculation to determine the cost-effectiveness of an intervention.
European Quality of Life-5 Dimensions
The EQ-5D is measure of health-related quality of life divided into two sections: the EQ-5D index and the EQ thermometer. 102 The EQ-5D index assesses health across five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The EQ thermometer is a single 20 cm vertical visual analogue scales with a range of 0 to 100, where 0 is the worst and 100 is the best imaginable health, and is completed by the user for their current health. A 2007 review shows a substantial and growing body of literature using the EQ-5D in cancer, and draws the conclusion that it is a valid and reliable instrument. 103 A 2010 review of patient-reported outcome measures for patients with CRC favourably summarises the EQ-5D and recommends its use for measurement of comprehensive general health status. 101
Scoring
Descriptive data from the five dimensions of the EQ-5D part 1 can be used to generate a health-related quality-of-life profile for the subject, created from the 1–5 scale for each question. This can be further divided into those reporting ‘problems’ or ‘no problems’, combining some of the subscales. Part 2 is scored from 0 (worst health state imaginable) to 100 (best health state imaginable). The score from part 2 can be used to track changes in health, on an individual or group level, over time. 104
Short Form Health Survey-36 items
The SF-36105 was also used to measure quality of life. The Medical Outcomes Study SF-36 is intended for application in a wide range of conditions and with the general population. The SF-36 is a validated health survey consisting of 36 questions that measure eight health concepts: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations secondary to emotional problems, and mental health. The content validity of the SF-36 has been compared favourably with that of other widely used generic health surveys. 106
Scoring
Two scores are available from the SF-36 results: a physical component and a mental component. The physical component score is derived from physical functioning, role limitations due to physical problems, bodily pain and general health. The mental component score is derived from the remaining four scales: vitality, social functioning, role limitations due to emotional problems, and mental health. For each participant, a number obtained from the responses indicates each health concept. A higher score indicates an improved level of function; for example, a high score in the pain category indicates low pain/improved pain.
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS), which consists of 14 questions, seven for anxiety and seven for depression, was used to measure anxiety and depression. 107 Test–retest scores for the anxiety subscale were reported as 0.84 at up to 2 weeks, 0.73 at 2–6 weeks and 0.70 at > 6 weeks, and for the depression subscale were reported as 0.85, 0.76 and 0.70 at the same intervals. 108 The scale has also demonstrated excellent internal consistency in both subscales using Cronbach’s alpha values (anxiety = 0.93; depression = 0.90) in a study of 568 cancer patients. 109 A meta-analysis suggests that HADS is sufficiently sensitive for identifying depression and anxiety in patients with cancer. 110
Scoring
Each variable is scored on individual subscales, with a maximum score of 21 on each scale. A higher score indicates higher levels of anxiety or depression. Six out of the 14 items on the scale are reverse scored, four of which are items related to the participant’s ratings of anxiety and the remaining two of which are measures of depression. Scores from 0 to 7 are considered normal, scores between 8 and 10 indicate borderline clinical disorder and scores of ≥ 11 represent possible clinical disorders.
Fatigue
Cancer-related fatigue was measured using the 13-item Fatigue Scale of the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system. 111 The FACIT measurement system is considered appropriate for use with patients with any form of cancer and, importantly, has been shown to be responsive to change in clinical and observational studies. 111 A 2008 systematic review of the scales used for the measurement of cancer-related fatigue shows that there is no accepted definition of cancer-related fatigue and no agreement on how it should be measured. 112 Nevertheless, the review recommends the use of FACIT questionnaires for measuring fatigue. The FACIT Fatigue Scale has been successfully used in studies investigating physical activity interventions for people with CRC. 38,44,49 In addition, FACIT questionnaires to measure fatigue have also been successfully used in a longitudinal study of 291 participants with early-stage CRC, 72 participants with metastatic disease and 72 healthy controls. 113 The study found that fatigue was self-reported by 52% of early-stage patients and 26% of healthy controls (p < 0.0001). 113
Scoring
Questionnaire responses are given on a Likert scale (0–4), with two items being reversed, giving a score range of 0–52. The higher the score, the better the quality of life; a score of < 30 indicates fatigue.
Proposed process variables
Self-efficacy and risk perception were measured to assess if they were predictive of cardiac rehabilitation attendance and of changes in health outcomes arising from the intervention.
Self-efficacy
Self-efficacy is predictive of both the adoption and the maintenance of physical activity. 114–116 Furthermore, there is experimental evidence that changes in self-efficacy can mediate the effects of behaviour change interventions on increases in objective measured physical activity behaviour. 117 General self-efficacy, which is the belief that one can perform difficult tasks or cope with adversity, was measured using a 10-item scale. 118 Physical exercise self-efficacy, which is the belief that one can engage in, and meet, physical activity goals, was also measured, using a 5-item scale. 119 High reliability, stability and construct validity of the general self-efficacy scale have been confirmed. 120,121
Scoring
Likert scoring (1–4 per questions) is used to give a score for self-efficacy (general self-efficacy range from 10 to 40; physical activity self-efficacy range from 5 to 20); the higher the score, the higher the self-efficacy.
Risk perception
According to the behaviour motivation hypothesis,122 perceived risk is positively and directly related to health behaviours. Risk perception of suffering from diseases has been found to play an important role in the development of intentions to perform physical activity among older adults123 and in explaining cancer-related behaviours. 124,125 Given lack of consensus about measuring risk perception, we measured cognitive (beliefs) and affective (feeling) risk perception and perceived severity. 126
Scoring
Risk perception was measured by six items and each item had a 5-point Likert scale. The two cognitive risk perception question scores were summed (‘If I don’t have a healthy lifestyle, my chances of getting colorectal cancer again at some point in my life are . . .’ and ‘If I have a healthy lifestyle my chances of getting colorectal cancer again at some point in my life are smaller . . .’). A higher score indicates that a respondent believed that a healthy lifestyle would have a protective health effect (range 0–10). The two affective risk perception question scores were summed (‘If I don’t have a healthy lifestyle, I feel . . .’ and ‘If I have a healthy lifestyle, I feel less vulnerable to getting cancer again at some point in my life . . .’). A higher score indicates that a respondent felt that a healthy lifestyle would have a protective health effect. The two perceived severity question scores were summed (‘Compared to other forms of disease, the consequences of bowel cancer are . . .’ and ‘Bowel cancer is more serious than other diseases I know . . .’). A higher score indicates that a respondent believed that bowel cancer was a more serious disease.
Proposed clinical variables
In addition, the following clinical confounding factors were reported on the screening and recruitment form (see Appendix 2):
-
colon or rectal surgery
-
surgical intervention (e.g. laparoscopic or open surgery)
-
temporary (a loop ileostomy) or permanent stoma or no stoma
-
chemotherapy or no chemotherapy.
Sample size justification
The sample size was based on two factors: (1) number of patients with CRC that was sufficient to address phase 1 objectives, that is, test the feasibility and acceptability of the intervention and main trial components before commencing phase 2 (i.e. a pilot RCT); and (2) estimated number of patients with CRC who could be recruited within a planned recruitment period of 2 months (the recruitment period was extended from 2 to 5 months).
As highlighted in Chapter 1, there is no clear guidance for how many participants are necessary for estimating event rates such as recruitment and willingness to be randomised in pilot RCTs. Similarly, there is no clear guidance for how many participants are required to assess the feasibility and acceptability of an intervention and study instruments. Thus, we aimed to recruit 12 patients with CRC, as this is the number of patients we thought that we could realistically recruit within the given time scale to meet phase 1 objectives.
Data collection and management
The original plan, as specified in the protocol, was to ask patients to complete the self-report questionnaires using pen and ink in the presence of an investigator who would guide them through it. However, all questionnaires were administered online (Bristol Online Survey) to save the time and expense of entering data.
Outcome measures
Baseline assessment
The investigator conducted baseline assessment at the cardiac rehabilitation facility. The investigator administered online questionnaires (IPAQ, EQ-5D, SF-36, HADS, FACIT Fatigue Scale, self-efficacy and risk perception questions) using Bristol Online Survey (http://survey.bris.ac.uk). The majority of questions were in a closed format, requiring participants to choose one option from a limited selection of discrete responses. Each question was read out by the investigator and answered by the participant. The investigator directly input the response to each question. The participant was also invited to wear the Actigraph GTX3+ accelerometer for 7 consecutive days (beginning the following day). All baseline assessments were conducted between 16 September 2013 and 26 November 2013.
Follow-up
Follow-up assessment coincided approximately with the end of the intervention delivery period (i.e. after the participant had attended the final cardiac rehabilitation class). The investigator conducted follow-up assessment at the cardiac rehabilitation facility. The investigator administered the online questionnaires that were completed at baseline using the same procedures. The participant was again requested to wear the Actigraph GTX3+ accelerometer for 7 consecutive days (beginning the following day). All follow-up data were collected between 27 April 2014 and 29 May 2014.
Process evaluation
Evaluation of cancer and exercise training
Clinicians attending the cancer and exercise training class completed a standard CanRehab evaluation form (see Appendix 6). The form included 18 questions covering pre-course information, course content, course venue and facilities. Questions were a combination of scaled questions (1–5: strongly agree 5, strongly disagree 1) and open-text questions.
Evaluation of measures and intervention
As described above, each instrument had already been independently tested for validity and reliability. However, we wanted to assess if completing the batch questionnaires was acceptable to participants. Immediately after the participant had completed the questionnaire, the investigator recorded any naturally occurring comments made by the participant as he or she answered questions. The investigator recorded comments about, for example, repetition (e.g. if the participant felt that questions were similar and he or she had already answered a similar question), relevance (e.g. if the participant felt that the questions applied to him or her) comprehension (e.g. if the participant asked the investigator to explain a question) and burden (e.g. if the participant commented on the length of the questionnaire). This approach is similar to the ‘think-aloud’ method, which involves the respondent completing the questionnaire and speaking aloud their thoughts as they reach each instruction and complete each item. 127 ‘Think aloud’ allows access to the participant’s genuine thoughts as they complete the instrument. 128
Face-to-face semistructured interviews were conducted with participants at the end of the intervention delivery period (i.e. after the patient had attended the final cardiac rehabilitation class) about the acceptability of main trial components and the intervention. Face-to-face semistructured interviews were also conducted with the three nurses involved in recruitment and the one cardiac physiotherapist delivering the intervention about the acceptability of main trial components and the intervention. Semistructured interviews were chosen because they allow flexibility in what sequence questions are asked, and in whether or not and how particular areas might be followed up and developed with different interviewees. 129 Interview schedules (see Appendix 7) were used to assist the investigator in gathering responses about the feasibility and acceptability of the intervention and trial procedures.
In addition, a focus group with 1 CRC nurse and 12 cardiac rehabilitation team members (eight physiotherapists/assistants and four nurses) in one of the other sites that would be involved in phase 2 was conducted to discuss the results of phase 1. An investigator recorded comments made by the clinicians at the focus group by making a set of notes. ‘Any group discussion may be called a “focus group” as long as the researcher is actively encouraging of, and attentive to, the group interaction.’130 A focus group was chosen because it was a practical method for involving cardiac rehabilitation clinicians in the process of developing the main trial components for testing in phase 2 and working collaboratively with the investigators.
Analysis
Descriptive statistics
To address phase 1 objectives (see Chapter 2), descriptive statistics were generated to summarise the main features of information from the screening and recruitment, declining to participate and evaluation forms to assess feasibility and acceptability of main trial components and the intervention. In addition, rates of missing data on the self-report questionnaires completed by patients and the accelerometers were analysed and reported.
Thematic analysis
Interviews and focus group data were also analysed to assess the feasibility and acceptability of the main trial components and the intervention. Audio-recorded interviews were transcribed verbatim and analysed thematically. The Framework approach, which is a rigorous method providing a structure within which qualitative data are organised and coded and themes are identified, was used to guide the analysis. 131 In brief, first the investigators became familiar with the interviews transcript data by reading and rereading transcripts and assigning interview and focus group data (sentences and paragraphs) to the two main themes, which were (1) the feasibility and acceptability of the main trial procedures, and (2) the feasibility and acceptability of the intervention. Second, subthemes were identified and a narrative summary of coded data was made under each subtheme. Third, the investigators referred to the original data to ensure that participant accounts were accurately presented to avoid misinterpretation.
Adverse events
Although this was not a clinical trial of an investigational medicinal product, the investigators adhered to Tayside Medical Science Centre standard operating procedure 11: ‘Identifying recording and reporting adverse events [AEs] for clinical trials of investigational medicinal products’. 132 The following serious adverse event (SAE) protocols were reported within 24 hours of the principal investigator or person delegated responsibility for recording SAEs becoming aware of them:
A SAE is any AE occurring that results in any of the following outcomes:
-
death
-
inpatient hospitalisation or prolongation of existing hospitalisation
-
persistent or significant disability/incapacity.
The following protocol exclusions applied:
-
hospitalisation for assault or accidental injury
-
hospitalisation for pre-planned surgery.
The above protocol exclusions were recorded in the AE log (see Appendix 8) for the study and line listings were reported annually to ethics and the sponsor.
Each hospital, and hence each cardiac rehabilitation programme, also had a reporting system for AEs, and cardiac rehabilitation operates a system of incident reporting. Thus, AE reporting of study participants by cardiac rehabilitation were also recorded by an investigator.
Chapter 4 Phase 1 results
The purpose of phase 1 was to evaluate the feasibility and acceptability of the main trial components planned for use in phase 2 and also the feasibility and acceptability of delivering the intervention in one site. In this chapter, phase 1 results are reported under two sections. The first section of this chapter reports the results that address the feasibility and acceptability of the main trial components. Descriptive statistics were generated to show the recruitment rate, participant and non-participant characteristics, completion rate and missing data. Findings from the interviews and focus group about the feasibility and acceptability of main trial components are reported thematically. Table 4 describes participants interviewed or involved in a focus group during phase 1. The second column of the table lists the key themes addressing the feasibility and acceptability of the main trial components that are reported in this chapter.
| Participants | Themes addressing feasibility and acceptability of the main trial components | Themes addressing feasibility and acceptability of the intervention |
|---|---|---|
Interviews (site 1)
|
|
Referral pathways to cardiac rehabilitation Importance of exercise for patients with CRC Cancer and cardiac patients exercising together Cardiac rehabilitation education sessions |
Focus group (site 2)
|
In this chapter, for quotations, ‘CNS’ refers to cancer nurse specialist; ‘CRP’ refers to cardiac rehabilitation professional; ‘P’ refers to participant, that is, a patient with CRC; and the number, for example, ‘001’, is given after the letters to uniquely identify the participant for the purposes of the study.
The second section of this chapter reports the results that address the feasibility and acceptability of delivering the intervention. Descriptive statistics were generated to show the number of clinicians who attended the cancer and exercise training and the results of the evaluation of this event. Descriptive statistics were also used to report cardiac rehabilitation attendance patterns of the four CRC patients who consented to the study. Findings from the interviews and focus group about the feasibility and acceptability of delivering the intervention are reported thematically. The third column of Table 4 lists the key themes addressing the feasibility and acceptability of the intervention that are reported in this chapter. The next chapter (see Chapter 5) presents the modifications made to trial procedures and the intervention based on phase 1 results for further testing on phase 2.
Feasibility and acceptability of main trial components
Colorectal cancer patient recruitment rate
Figure 3 presents patient flow through the study. In total, there were 34 new patient admissions. A nurse gave 24 (70% of all patient admissions) eligible patients a study information sheet. Nurses completed a screening and recruitment form for 17 (71% of those receiving a study information sheet) of these eligible patients. Nurses did not complete a screening and recruitment form for seven eligible patients who had been given study information, because the patient was too unwell, had been discharged or had moved wards. Ten (58% of completed screening and recruitment forms) patients signed a screening and recruitment form indicating their willingness to participate in the study and to have contact details forwarded to an investigator. Six patients who signed a screening and recruitment form indicating their willingness to participate withdrew from the study before signing a consent form and entering the intervention owing to ill health (n = 3) or travel problems (n = 2) or because they were subsequently unable to be contacted (n = 1). Four of these patients signed a consent form and started cardiac rehabilitation (i.e. the intervention), which is 17% of eligible patients. One of these patients withdrew owing to ill health.
FIGURE 3.
Participant flow.
Participant characteristics
Table 5 summarises the main characteristics of patients who signed a screening and recruitment form indicating their willingness to participate in the study and to have their contact details forwarded to an investigator (n = 10). The mean age was 71 years (range 50–89 years); 60% were men. Three patients were diagnosed with metastatic disease; eight patients had open surgery and two had laparoscopic surgery; five patients were receiving adjuvant therapy (one radiotherapy and four chemotherapy); and four patients had a stoma.
| ID | Age (years) | Gender | Diagnosis | Treatment | Reasons for withdrawal |
|---|---|---|---|---|---|
| 1a | 77 | F | Colon cancer | Open surgery, adjuvant chemotherapy | |
| 2 | 60 | M | Upper rectal cancer with lung and liver metastasis | Open surgery, permanent stoma | Travel distance |
| 3a | 84 | F | Caecal adenocarcinoma with liver metastasis | Laparoscopic surgery, adjuvant chemotherapy | |
| 4 | 75 | F | Lower rectal cancer | Open surgery, permanent stoma, adjuvant radiotherapy | Wound healing |
| 5a | 89 | F | Cancer of transverse colon | Open surgery | |
| 6a | 80 | M | Caecal cancer | Open surgery | |
| 7 | 61 | M | Rectal cancer | Open surgery, temporary stoma, adjuvant radiotherapy | Unable to be contacted |
| 8 | 69 | M | Rectal cancer | Open surgery, temporary stoma | Travel distance |
| 9 | 62 | M | Colon cancer, metastatic disease | Open surgery, adjuvant chemotherapy | Having chemotherapy |
| 10 | 50 | M | Rectal cancer | Laparoscopic surgery | More surgery scheduled |
Participant and non-participant characteristics
The characteristics of 10 eligible participants who were willing to participate and signed a screening and recruitment form and the seven eligible participants who declined to participate and signed a declining to participate form were compared. Differences by age, gender and type of surgery are shown in Table 6. The age range and the proportion of people diagnosed with colon and rectal cancer in each group were similar. There were proportionately more men and more people who had open surgery in the willingness to participate group than in the declining to participate group.
| Characteristic | Willing (n = 10) | Unwilling (n = 7) |
|---|---|---|
| Age (mean) | 71 years (range 50–89 years) | 69 years (range 51–90 years) |
| Gender | 60% male | 42% male |
| Diagnosis | 50% colon; 50% rectal | 57% colon; 43% rectal |
| Type of surgery | 80% open surgery; 20% laparoscopic | 57% open surgery; 43% laparoscopic |
Completion rates and missing data
As described in Chapter 3, outcomes and process variables were assessed by accelerometer and investigator-administered patient-reported outcomes questionnaires at two time points: pre intervention and post intervention.
Accelerometer
Participants were invited to wear an accelerometer for 7 consecutive days and for as long as possible on each day. Participants were asked to wear the device for an extra day or two if they felt that they had not worn it for long enough during the allotted time period, or if they had forgotten to wear the device on one of the days. Data were classed as valid if the device had been worn for at least 10 hours per day for 4 days (see Chapter 1). 133 Table 7 shows that participants wore the accelerometer as requested.
| Study ID | Baseline wear valid | Number of valid wear-days (out of 7) | Follow-up wear valid | Number of valid wear-days (out of 7) |
|---|---|---|---|---|
| 001 | Yes | 4 | Yes | 5 |
| 005 | Yes | 5 | Yes | 5 |
| 006 | Yes | 7 | Yes | 6 |
Participants did not report any difficulties wearing the accelerometer.
Right, and how did you find it wearing that [accelerometer]?
That was OK.
Any problems at all?
No.
P005
And how did you find it [wearing the device]?
Fine, no problem.
OK . . . em . . .
And I understood why I was wearing it, you know.
P001
Self-report questionnaires
As we pointed out in Chapter 3, the original plan was to ask patients to complete the self-report questionnaires using pen and ink in the presence of an investigator who would guide them through it. However, all questionnaires were administered online (Bristol Online Survey) with an investigador present, to save time and expense entering data. To administer online questionnaires, internet access was required, and hence permission was successfully obtained from the NHS to access the internet in the hospital where patients met the investigator to complete the questionnaire.
All items of the questionnaire were completed at baseline and at follow-up for the three patients completing the intervention. There were no missing data to report from the self-report questionnaires.
Participants reported that the questionnaire was repetitive. In particular, participants reported that there were similar questions being asked on a number of occasions about their quality of life (as described in Chapter 3, the EQ-5D and SF-36 were being used to measure quality of life). In addition, participants reported that they were not asked specifically about the impact of the cancer and cancer-related treatments on their quality of life, suggesting that general quality of life measures may not capture key domains relevant to patients with cancer. Participants reported that some questions about their physical activity were not relevant (as described in Chapter 3, the IPAQ was being used to measure types of physical activity). IPAQ included questions about work-related physical activity, and the majority of participants were not in paid employment. Self-efficacy and risk items were met generally with indifference and were completed with the only comment that questions were very similar. Participants did not report any difficulties answering questions about the costs associated with attending cardiac rehabilitation, which provided data for use in a health economic evaluation.
Clinicians’ experiences and perceptions of recruitment
The themes identified were interpretation of eligibility criteria, perceived barriers to participation, and time and place to recruit patients.
Interpretation of eligibility criteria
Nurses did not report difficulties applying exclusion and inclusion criteria. However, they used their personal judgement to exclude patients who they believed should not participate. For example, the criterion ‘advanced disease’ was not interpreted to refer to all patients with metastatic disease. In addition, one nurse reported that she did not approach a patient who met the inclusion criteria because she believed that the patient would be unable to participate.
We use our judgement all the time, like, I mean there is a lady in at the moment but she’s 88 and her husband’s got dementia, and she doesn’t drive and you think she’s, you know she’s not going to achieve anything.
CNS 002
Cardiac rehabilitation clinicians at the focus group discussed the challenges of including patients with metastatic disease in cardiac rehabilitation exercise classes and concluded that it was feasible to include these patients as long as the CRC clinical team confirmed that it was safe for these patients to exercise.
Perceived barriers to participation
Reasons given by eligible patients for non-participation (n = 7) or withdrawal (n = 6), and that were also perceived by clinicians as barriers to participation, were as follows:
-
travel distance from cardiac rehabilitation facility
-
returning to work and therefore unable to attend cardiac rehabilitation
-
feeling fit and well and therefore perceiving cardiac rehabilitation as unnecessary
-
having ongoing treatment and therefore not feeling well enough to attend cardiac rehabilitation
-
poor recovery from surgery (e.g. wound not healing).
Given how remote and rural some parts of the Scottish Highlands are, it was perhaps inevitable that some patients would decline to be involved in the study or withdraw from the study owing to the distance that they would have had to travel to attend cardiac rehabilitation.
The distance has been the big, the big stumbling block, but even some people, em, from a long way away have, have been very keen to take part.
CNS 001
The cardiac rehabilitation physiotherapist perceived that the main issue regarding the involvement of patients with CRC was their protracted recovery, which meant that they were not always able to attend cardiac rehabilitation immediately.
I think the biggest issue is time . . . eh, the effects on the cardiac service. The patients themselves weren’t a problem in any way. The only thing that we have established is that they don’t have a straightforward journey which has been the biggest problem . . . and which is probably going to have the biggest effect on how compliant they can be. But generally once we’ve, once we’ve sort of assessed the recruitment and screening and you’ve got appropriate patients, they’ve been quite keen.
CRP 001
A stoma was cited as a barrier to participation in cardiac rehabilitation; one nurse described why this might be the case:
It’s a big confidence issue; em, you know, living with a stoma, worried about whether the bag’s going to burst. And for them if they were in a class where they were doing a bit of exercise which they had been doing for a while and the bag was to fill up and you know maybe cause a problem that could be quite detrimental to their recovery. A disaster, you know, a leak or something, you know, they would maybe have to have spare clothes with them, em, you know, ensure that there’s toilet facilities so that they can empty their bag when and if necessary; there’s maybe changing facilities if they did have a problem, eh, you know, eh, with that. I suppose I mean you, you may get a cardiac patient that feels nauseous and is sick, so I mean there are different.
CNS 003
Four patients who signed a screening and recruitment form indicating their willingness to participate in the study had a stoma, suggesting that it was not a barrier to participation for all patients. However, none of the patients who had a stoma participated in the intervention (i.e. cardiac rehabilitation) because they decided to withdraw from the study. As Table 5 shows, their reasons for withdrawal were having travel difficulties (n = 2), being uncontactable (n = 1) and having wound problems (n = 1), suggesting that factors other than having a stoma were the barriers to participation.
Nurses cited age as a barrier to participation. One nurse, for instance, believed that older people were probably less likely or willing to change their lifestyle and, therefore, less likely to participate in the study.
And the older the people get they’re maybe not just quite so able or amenable to embracing drastic changes in their lifestyles.
CNS 002
Nevertheless, the average age of patients who signed a screening and recruitment form indicating their willingness to participate in the study was 71 years and the oldest patient was aged 89 years. The patient who was 89 years old participated in the intervention (i.e. cardiac rehabilitation), suggesting that age was not a barrier to study participation. Cardiac rehabilitation clinicians at the focus group did not perceive age as a barrier to attending cardiac rehabilitation and referred to the broad age range of people with CHD attending cardiac rehabilitation classes.
Time and place to recruit patients
Nurses did not believe it appropriate to raise the study with patients when they were being given their cancer diagnosis.
Not at diagnosis – you have to assess that patient at that time; are they able to take in, on board anything else that you’re going to say to them.
CNS 002
However, nurses believed that it was appropriate to give patients information about the study as early as possible so that they had enough time to think about participating.
I think it’s better to give it to them as early as possible in their diagnosis so that they can be thinking about it.
CNS 002
The original plan, as stated in the protocol and described in Chapter 3, was for nurses to approach CRC patients about the study after discharge from hospital, at the first follow-up appointment. This time and place was initially chosen because it avoided raising the issue of the study during diagnosis, but also was not too long after the diagnosis. In practice, however, nurses decided to raise the issue on patients’ admission to the surgical ward. Nurses found that this was appropriate because ways to support recovery from surgery were already being discussed with patients on the surgical ward, pre and post surgery. Thus, nurses gave patients a study information sheet and discussed participation in the study pre or post surgery on the ward.
As described in Chapter 3, nurses were expected to complete a screening and recruitment form for all eligible patients they approached about the study. Nurses said that screening and recruitment did not take up too much clinical time and the form was easy to complete.
No I think, certainly for the forms that we fill out, I mean it is very simple, it was just a tick box and eh, you know, it’s easy enough for us to do that.
CNS 003
It’s, it’s maybe 5 minutes per patient really. We’d introduce it and give them the leaflet to read and then, em, the forms themselves only take a few minutes to fill in, so it wasn’t any, it wasn’t any, eh, any hardship at all.
CNS 001
Feasibility and acceptability of the intervention
Cancer and exercise training
During phase 1, all clinicians across all three sites involved in phase 2 attended a 1-day cancer and exercise training course. All clinicians delivering the intervention (i.e. phase 3 cardiac rehabilitation) attended; that is, 10 cardiac physiotherapists/assistants and four cardiac nurses across all three sites were trained. In addition, all six CRC nurses involved in recruitment were trained. Fourteen (70%) evaluation forms from across all three sites were completed and returned; six (30%) forms were not returned.
The results of the evaluation are presented in Table 8. All scaled questions marked highly with a score of 4 or 5, with 5 being the maximum score. Additional free-text comments show that, overall, training was well received by all attendees who completed the evaluation forms (cancer and cardiac staff). Attendees reported that the training was excellent and enjoyable.
| Question | Average score (mean) (5: strongly agree; 1: strongly disagree) |
|---|---|
| Registration and pre-course information | |
| I received all the necessary information prior to starting the course | 4.3 |
| The directions to the venue were helpful and accurate | 4.5 |
| Additional comments for those trained via video conference | |
| Effective method of delivering courseOpportunity for asking questions over video link as we went alongNo practical session, but did not feel this was necessary | |
| Course content | |
| The content of the course was at the appropriate level | 4.3 |
| The course information was well presented | 4.6 |
| The content was well presented | 4.7 |
| The content of the practical session was well presented | 4.7 |
| Additional comments for course content | |
| Great booklet, presenter very knowledgeable and easy to listen toVery interesting and stimulatingNo practical givenInformation a little basicFelt session was more of a presentation than a training session | |
| Course venue and facilities | |
| Course teaching and rooms were of an adequate standard | 4.3 |
| The equipment used was of an adequate standard | 4.3 |
| The refreshments were of an adequate standard | 4.7 |
| The teaching room was set up and prepared | 4.6 |
| Each session started and finished on time | 4.7 |
| You were provided with sufficient breaks | 4.5 |
| Do you have any suggestion on how this course could be improved? | |
| Excellent introduction to exercise and cancerNeeds to be in person rather than teleconference | |
| Would you recommend this course to other colleagues? | |
| Yes – 65%; no – 0%; no response – 35% | |
| Please add any additional comments you wish to make | |
| Very enjoyable, great presenterNone, it was excellentI would like to learn moreSome examples given may have been patronising for staff doing classes for many yearsGood to learn that cancer patient risk factors are the same as cardiac so will benefit from most information sessionsEnjoyed the open discussion with colleagues | |
As described in Chapter 3, all sites received training separately. Two sites received cancer and exercise training face to face and the third site received training via group video conferencing (skypeTM, Microsoft Corporation, Redmond, WA, USA). One attendee in the site that received training via video conferencing wrote on the evaluation form that she would have preferred face-to-face training, whereas two other attendees reported that video conferencing was an effective method for delivering training.
Attendees reported that the course content was at the appropriate level (mean score of 4.3) and was well presented (mean score of 4.7). One attendee reported that the booklet about cancer and exercise that was handed out was ‘great’, and one attendee reported that the content was ‘interesting and stimulating’. However, one attendee reported that the information was a ‘little basic’ and another attendee reported that ‘some examples given may have been patronising for staff doing classes for many years’.
One attendee highlighted that she had learnt that many cancer and cardiac risk factors are similar and therefore patients with CRC would benefit from the education sessions organised for cardiac patients.
Cardiac rehabilitation adherence
Table 9 shows that the average number of days between a patient signing a screening and recruitment form indicating agreement to participate in the study and starting cardiac rehabilitation was 70. Participants were expected to attend 10 consecutive cardiac rehabilitation exercise classes. Table 10 shows that one participant attended 10 cardiac rehabilitation classes over a period of 13 weeks, one participant attended 6 out of 10 classes over a 14-week period, one participant attended half of all classes over a 7-week period and another was unable to attend any classes owing to ill health.
| ID | Screening date | First rehabilitation class | Time (days) |
|---|---|---|---|
| 001 | 12 August 2013 | 5 November 2013 | 54 |
| 003 | 16 August 2013 | 28 November 2013 | 69 |
| 005 | 5 September 2013 | 5 September 2013 | 84 |
| 006 | 23 September 2013 | 23 September 2013 | 73 |
| Patient ID | First session | Last session | Sessions attended | Number of weeks | Completion (%) | Sessions per week equivalent |
|---|---|---|---|---|---|---|
| 001 | 7 November 2013 | 19 December 2013 | 5 | 7 | 50 | 0.7 |
| 003 | 28 November 2013 | Unable to attend owing to non-related medical reasons | ||||
| 005 | 28 November 2013 | 6 March 2014 | 6 | 14 | 60 | 0.4 |
| 006 | 5 December 2013 | 6 March 2014 | 10 | 13 | 100 | 0.8 |
Patients’ and clinicians’ experiences and perceptions of cardiac rehabilitation
The themes were referral pathways to cardiac rehabilitation, importance of exercise for patients with CRC, cancer and cardiac patients exercising together, and cardiac rehabilitation education sessions.
Referral to cardiac rehabilitation
The cardiac rehabilitation physiotherapist did not find the referral procedures of CRC patients into the service acceptable and so these were changed. The original plan, as stated in the protocol85 and described in Chapter 3, was that nurses would refer patients to cardiac rehabilitation. In the absence of a study, this is how patients would be referred if the intervention were implemented as part of routine care. In this study, however, the investigator referred patients to cardiac rehabilitation. The reason for this was to minimise the workload of the cardiac rehabilitation team. If we had kept to the original procedure (i.e. nurses directly referring patients to cardiac rehabilitation), the onus would have been on the cardiac rehabilitation team to continuously check with the patient when they felt ready to attend cardiac rehabilitation. The difficulty was that patients with CRC varied in terms of their recovery and readiness to start cardiac rehabilitation following surgery, which meant that the time to starting cardiac rehabilitation after surgery could not be strictly regimented. To save the team repeatedly contacting CRC patients to find out if they were ready to start the programme, telephone calls by an investigator were introduced instead. It was only once a CRC patient informed an investigator that he or she was ready to attend cardiac rehabilitation that an investigator informed the cardiac rehabilitation team about that patient. A member of the cardiac rehabilitation team could then contact the patient to invite him or her to attend the first appointment to conduct a risk assessment and discuss the programme. A physiotherapist explained why the original procedure was problematic.
It was slightly cumbersome, I think with the to-ing and fro-ing between when we wanted to fit them in, checking with you [the researcher] when you were available [to consent the patient and collect baseline measures before the patient started cardiac rehabilitation] and regrouping, em, and also time wasted initially, em, but once we got round to you phoning them in advance it was fine.
CRP 001
The new procedure to be implemented for the purposes of the study was also endorsed by the focus group. In particular, the cardiac rehabilitation team was concerned about the amount of time required of them to include CRC patients in their service and so welcomed any procedure that would reduce this.
Nevertheless, all of the clinicians involved in phase 1 reported that direct referrals from the cancer care team to cardiac rehabilitation would be possible in the future if this model of rehabilitation were to be implemented as part of routine care. Figure 4 illustrates key differences between referral and enrolment procedures in the study and the procedures if this model of cardiac rehabilitation were to be rolled out as part of routine aftercare.
FIGURE 4.
Research and practice cardiac rehabilitation referral pathways. CR, cardiac rehabilitation.
The referral form sent to the cardiac rehabilitation team about a new patient was modified in the light of interviews and focus group discussions with clinicians. The additional information requested is given in Table 11.
| Information | Reason for request |
|---|---|
| Names and address of GP | This was included (with participants’ prior consent) in case it was felt necessary to inform the GP that a patient on their register was participating in cardiac rehabilitation |
| Date of surgery | This information was included because CRC surgeons suggested that it was safe to exercise 4–6 weeks post laparoscopic surgery and 6–8 weeks post open surgery. Thus, cardiac rehabilitation practitioners wished to know the date of surgery |
| Current medication | This was included for safety reasons in case the patient became unwell during exercise classes |
| Relevant past medical history | This was included for safety reasons so that cardiac physiotherapist could assess whether or not the patient was safe to attend exercise classes |
Importance of exercise for patients with colorectal cancer
All three CRC nurses were supportive of cardiac rehabilitation for patients with CRC because it would help with patient recovery.
The more exercise you do post-op, you do, you have a much better recovery rate.
CNS 001
I like to try and encourage a healthy approach to living anyway so I think it [cardiac rehabilitation] formalises what I encourage.
CNS 002
Nurses mentioned the benefit of physical activity when recruiting patients to the study.
[The nurse said] it’s good for you, do it.
P005
Nevertheless, patients with CRC reported that they had not been informed about the role of physical activity to reduce the risk of cancer:
Yes. And are you aware of any things that you can do yourself to reduce the risk of the cancer coming back?
No, I haven’t been told about anything.
P005
And, how do you think you can reduce the risk of the cancer coming back, have you been given any steps that you can take?
No.
P006
Patients with CRC welcomed the opportunity to attend cardiac rehabilitation for three reasons: physical activity is generally beneficial, it is difficult to exercise independently, and rehabilitation provides an environment in which patients can learn how to exercise safely. These beliefs in the benefits of cardiac rehabilitation are likely to have influenced patients’ willingness to participate in the study.
Two patients with CRC said that being physically active would be good for them.
I’m sure that any exercise is good for us . . . em, sitting is maybe the worst thing you can be doing.
P006
You’ve got to be willing to try different things, it’s for your own good really, you want to try as much as you can.
P001
One patient with CRC said that she was not very good at exercising on her own at home.
. . . I mean, I do know it [exercise] does [help], but I’m not very good at doing it at home by myself [laughs].
Right, right, so you enjoy coming to a class to do it?
Eh, yes, it’s the getting here isn’t it but yes, it’s OK once I’m here it’s good for me.
P005
One patient with CRC welcomed the opportunity to attend cardiac rehabilitation because he learnt how to safely pace himself when being physically active.
It lets you know you can safely push yourself a bit.
P006
Cancer and cardiac patients exercising together
The intervention was referral of patients with CRC who had recovered from surgery to cardiac rehabilitation. Thus, as the physiotherapist pointed out, it is important to explain why this particular model for rehabilitation for patients with cancer was being researched.
Initially I just thought why involve them with cardiac rehab, why not set up a pilot geared towards cancer patients?
CRP 001
The physiotherapist found it straightforward to slot patients with CRC into existing classes.
They have come in as normal patients . . . they just slipped in and joined in.
CRP 001
Patients with CRC did not perceive mixing with cardiac patients during rehabilitation as problematic:
And how do you feel about it being a mixed group?
Oh, that didn’t bother me at all . . .
At all?
No, no.
No, OK.
I quite enjoy the various company of people, yes.
P005
And what about it being a group with cardiac patients as well?
You do talk to other people . . . most were heart, I think I was the only one with cancer . . . eh, maybe not.
Maybe, OK.
. . . Meeting other people and sitting having a cup of tea helps.
P001
Another patient summarised the mixed groups simply:
You just accept it, and they [cardiac patients] accept you.
P006
Cardiac rehabilitation clinicians attending the focus group also endorsed mixed patient exercise classes.
Cardiac rehabilitation education sessions
As described in Chapter 3, the intervention comprised a 60-minute exercise session followed by an education session. These education sessions were designed to provide information useful to cardiac patients. Nevertheless, according to the cardiac physiotherapist, patients with CRC attended not only the exercise sessions but also the education sessions.
And how do you feel that the patients that have come through have got on generally?
Well, all three of them have fitted in in fact very well, em, in fact, one of them, well they’ve just gone in to all the cardiac talks regardless.
One of the patients with CRC thought that the education sessions were the most useful part of the rehabilitation programme. This patient had recently had a stroke and so may have found the education sessions particularly relevant. However, given increasing comorbidity in older age, education sessions designed for cardiac patients may be relevant to patients with cancer.
. . . and the lecture is one of the best things about it.
Right, so what things, what particular lectures have you found, did you find most helpful?
Eh, drugs and eh, eh, resuscitation, although I knew something about resuscitation. But eh, eh, just em, the, letting you know about your condition and eh, eh, how to eh be a good boy and take care of it.
Not all education sessions, however, were regarded as useful. One patient with CRC said that the session on smoking cessation was not relevant because most patients, including cardiac patients, had given up smoking.
. . . I don’t know whether, it may have applied, most people had given up, there was only one person who was in the process of giving up and I really felt it wasn’t beneficial to anybody . . .
P005
As described in Chapter 3, it was planned that a CNS would deliver education sessions about cancer to patients with CRC. However, this did not happen. Thus, the cardiac rehabilitation programme was not altered in any way to accommodate CRC patients and patients with CRC attended classes alongside cardiac patients.
However, the cardiac physiotherapist believed that cancer education sessions should be provided:
I feel slightly for them that they’re getting all this cardiac stuff and not really any follow-up . . . there’s no support from the, from the cancer side as it was mooted about, eh, when we were talking about the project that, eh, if it was cancer-specific then they would maybe, eh, if it was cardiac-specific talks then they would maybe get some additional support and there’s just been absolutely nothing.
CRP 001
One patient also expressed a desire for cancer education:
. . . it’s more for heart than for cancer . . . I think that’s where it maybe falls down you know . . . but I don’t know how you will do that. When push comes to shove, they really didn’t know a lot about the cancer end of things, even [name of cardiac rehabilitation] said . . . eh . . . em, she didn’t know a lot about it [cancer].
P001
Chapter 5 Phase 1 discussion and recommendations for phase 2
In this chapter the key findings from phase 1 about the feasibility and acceptability of main trial components and intervention are presented. The chapter also describes the changes to improve trial procedures and the intervention for further testing in phase 2.
Summary of findings
The results for phase 1 are clear:
-
Nurses were willing to recruit patients with CRC to the study. The evidence is that nurses gave a study information sheet to 70% (n = 24) of all surgical CRC patients and completed a screening and recruitment form for 71% (n = 17) of these patients.
-
The majority of eligible patients were willing to participate in the study. The evidence is that 10 out of 17 eligible patients (58%) signed a screening and recruitment form indicating their willingness to participate in the study and have their contact details forwarded to an investigator.
-
The surgical ward, when patients with CRC were admitted or while they were waiting to be discharged from hospital, was an appropriate time to raise the study with patients with CRC and to give them a study information sheet. The evidence for this is that 10 out of 17 eligible patients (58%) signed a screening and recruitment form indicating willingness to participate in the study before being discharged from hospital.
-
There were barriers (e.g. travel and poor recovery from surgery) to patients participating in the intervention (i.e. cardiac rehabilitation), which had a detrimental impact on sample attrition. The evidence for this is that only 4 out of 10 (40%) patients who signed a screening and recruitment form then proceeded to start the intervention. These barriers and subsequent loss to the study of six participants are the main reasons why we did not meet our anticipated target of recruiting 12 patients.
-
A good cross-section of patients with CRC were interested in taking part. The evidence is that men and women from the ages of 50 to 89 years, with and without metastatic disease, having open surgery and laparoscopic surgery, with and without a stoma, and having and not having adjuvant therapy were willing to participate in the study (i.e. signed a screening and recruitment form).
-
An accelerometer was an acceptable objective method for assessing level of physical activity and sedentary behaviour. The evidence is that participants wore the accelerometer for a validated period of time.
-
Investigator administration of online patient-reported outcomes questionnaires was an acceptable method for collecting outcomes data and was not perceived as a major burden by participants. The evidence is that all questions were answered at baseline and follow-up and there were no missing data.
-
Some parts of the questionnaire were considered by participants as repetitive and irrelevant. Some participants found questions about quality of life very similar. Quality of life questions also failed to capture issues relating to the impact of CRC and treatments.
-
A cancer and exercise expert delivering 1-day cancer and exercise training face to face or by video conference to cancer nurses and cardiac rehabilitation practitioners was feasible and acceptable. The evidence is that the results of the evaluation of the training were excellent with all attendees, for instance, either agreeing or strongly agreeing that the information and course content was helpful and well presented.
-
Patient readiness to start cardiac rehabilitation varied owing to different rates of recovery and hence the length of time from date of surgery to the start of cardiac rehabilitation also varied (54–73 days).
-
Adherence was suboptimal. The evidence is that participants were expected to attend 10 exercise classes but the four participants attended 10, 6, 5 and 0 classes, respectively.
-
Cardiac rehabilitation for patients with CRC was feasible and acceptable. The evidence is that patients with CRC attended the exercise and cardiac-specific education sessions. However, it could be improved by introducing cancer-specific education sessions. In addition, cardiac and cancer clinicians were supportive of this model of rehabilitation and participated in the study.
Recommended changes to main trial components
Based on the results of phase 1, the following changes to the main trial components (Table 12) were recommended for further testing in phase 2.
| Procedure | Original protocol | Recommendation | Rationale |
|---|---|---|---|
| Recruitment procedures | |||
| Time and place when patient is first approached about the study | A nurse will give eligible patients at the first follow-up appointment post surgery an information sheet about the study and talk them through it | A nurse will give eligible patients when they are admitted on the surgical ward (pre or post surgery) an information sheet about the study and talk them through it | Nurses found that the surgical ward was an appropriate place to raise participation in a study about rehabilitation because ways to support recovery from surgery were being discussed with patients on the surgical ward pre and post surgery |
| Eligibility criteria | |||
| Clarification of eligibility criteria | The study will exclude anyone with advanced disease | Make it clear that advanced disease refers only to patients with metastatic and incurable disease | ‘Advanced disease’ is interpreted in different ways, with some definitions including all patients with metastatic disease and other definitions only including patients with incurable metastatic disease.134 The CRC multidisciplinary team decided that it was inappropriate to exclude patients who had metastatic disease but were expected to fully recover |
| Measures | |||
| Amount of physical activity | Accelerometer | Introduce guidance for patients on how to wear the accelerometer | Based on the investigator’s experience of giving verbal instructions to patients about wearing the accelerometer, we decided to also introduce written guidance for wearing the accelerometer. Guidance will include a photograph of how to wear the accelerometer and frequently asked questions, such as whether or not it is necessary to wear it at night in bed when asleep |
| Type of physical activity | IPAQ | SPAQ | IPAQ contains a large section on job-related activity, which is not relevant for the majority of study participants. In addition, SPAQ is shorter and therefore burden on patient significantly reduced but gathers relevant information135 |
| Quality of life | EQ-5D SF-36 |
EQ-5D FACT-C |
SF-36 repeats EQ-5D and, compared with EQ-5D, is relatively expensive to use (EQ-5D is freely available). Employing a CRC-specific measure, such as FACT-C, will mean that we will be able to assess general health-related quality of life and a cancer-specific quality of life |
| Fatigue | FACIT Fatigue Scale | No change | |
| Anxiety and Depression | HADS | No change | |
| Cancer risk perception | 6-item risk perception questionnaire | No change | |
| Self-efficacy | General self-efficacy (10 items) Physical activity self-efficacy (14 items) |
12 item-physical activity self-efficacy questionnaire | The general self-efficacy questions were too general and therefore participants did not see the relevance, especially when the focus is on physical activity. One of the coinvestigators is involved in the ActWell trial,136 which is a feasibility trial to reduce breast cancer risk factors by promoting lifestyle changes including physical activity. ACTWell includes a 12-item physical activity self-efficacy questionnaire. We decided to use this tool, as its questions were designed specifically to measure self-efficacy in the context of delivering a behaviour change intervention. The added advantage is that we can directly compare findings between two similar studies |
Recommended changes to the cardiac rehabilitation
Based on the findings of phase 1, the following changes (Table 13) to the intervention were recommended for further testing in phase 2.
| Procedure | Original protocol | Recommendation | Rationale |
|---|---|---|---|
| Cancer and exercise training | |||
| None | |||
| Cardiac rehabilitation | |||
| Referral procedures | The nurse will refer patients who have agreed to participate in the study to the cardiac rehabilitation team who will then contact the patient | After the patient is discharged from hospital an investigator will contact them by telephone to find out if they are ready to start cardiac rehabilitation. Only when a patient informs an investigator that they were willing and ready to start will the team be informed about the patient by the investigator | Were this model for rehabilitation to be implemented in routine aftercare, CRC nurses would refer patients to cardiac rehabilitation, hence our original plan However, the time to start cardiac rehabilitation after surgery cannot be strictly regimented for CRC patients. This is because patients vary in their recovery and readiness to start exercise classes following surgery. A key barrier to patients participating in cardiac rehabilitation is poor recovery and so to remove this barrier they need to be given time to recover To save the cardiac rehabilitation team repeatedly contacting CRC patients to find out if they are willing and ready to start, telephone calls by an investigator will be introduced. It is only once a CRC patient is willing and ready to attend that the cardiac rehabilitation team will be informed about the patient and subsequently invite the patient to attend the programme. Changing the time to refer to cardiac rehabilitation was also designed to address suboptimal intervention adherence |
| Referral form | Referral form | Include the following additional information: Name and address of GP |
GP information was included in case it was felt necessary to inform the GP that a patient on their register was participating in cardiac rehabilitation |
| Date of surgery | Date of surgery was included because CRC surgeons suggested that it was safe to exercise 4–6 weeks post laparoscopic surgery and 6–8 weeks post open surgery. Thus, cardiac rehabilitation practitioners wished to know date of surgery | ||
| Current medication | Current medication information was included for safety reasons in case the patient became unwell during exercise classes | ||
| Recent past medical history | Recent medical history was included for safety reasons so cardiac physiotherapist could assess whether or not the patient was safe to attend exercise classes | ||
| Exercise component | Patients with CRC attend exercise sessions | No change | |
| Education component | Patients with CRC attend cardiac-specific sessions delivered by cardiac clinicians and cancer-specific sessions delivered by cancer clinicians | No change but ensure that cancer-specific sessions are, in fact, delivered | |
Limitations
Phase 1 was a small study. The feasibility and acceptability of trial components and the intervention were tested on only one site and included fewer than 10 participants. The site was a relatively small hospital serving a rural and geographically dispersed population. It is not possible to determine if the study is feasible and acceptable in different contexts, such as large urban hospitals with a relatively large number of trials simultaneously taking place. It is important, therefore, to test feasibility and acceptability in more sites with more participants.
Conclusions
The feasibility and acceptability of trial components and the intervention were tested on only one site over a short period of 6 months, involving a very small number of patients and clinicians. Phase 1 results suggested that nurses were willing to recruit patients with CRC to the study, the majority of eligible patients indicated a willingness to participate in the study, the surgical ward was an appropriate place for nurses to give study information, a good cross-section of patients with CRC were interested in taking part, and no data were missing from accelerometers or questionnaires. Phase 1 results also highlighted barriers to CRC patient participation, a suboptimal consent rate (we did not meet our anticipated target of recruiting 12 patients), repetition and lack of relevance in the self-report questionnaires, a long period of time between CRC patients indicating a willingness to participate and actually starting cardiac rehabilitation classes, suboptimal intervention adherence and lack of cancer-specific education sessions and lifestyle advice.
A decision was reached among the research team, funder and advisory group to proceed to phase 2 with the following main modifications to trial procedures and the intervention.
Trial procedures
-
Nurses were requested to approach CRC patients about the study on the surgical ward.
-
A quality of life questionnaire was removed to minimise duplication, a physical activity questionnaire including questions about activity during work was removed and replaced with a more appropriate questionnaire for this age group, and general self-efficacy questions were removed and replaced with further physical activity self-efficacy questions.
Intervention
-
Referral of CRC patients to cardiac rehabilitation was changed so that a referral was made only when the patient had informed an investigator that he or she felt ready to begin exercise classes.
-
The referral form was changed to include further information about patients being referred, for example comorbidities, treatments, date of surgery and relevant previous medical history.
-
Colorectal cancer nurses were requested to provide cancer-specific education sessions and lifestyle advice to CRC patients to supplement the cardiac rehabilitation education sessions.
Chapter 6 Phase 2 methods
In this chapter, the design and methods used in phase 2 are described.
Phase 2 design
Phase 2 was a pilot RCT. As discussed in Chapter 1, and in accordance with the MRC’s framework for the evaluation of complex interventions,71 pilot work is valuable for helping to optimise study design and study procedures before proceeding to a large-scale trial. A RCT is also a robust method for reporting any preliminary effects of the intervention, with the proviso that these results are interpreted with great caution.
Participants
The study sought to recruit patients with CRC from two acute hospitals in Scotland and a large teaching hospital in Wales. Table 14 summarises characteristics for each hospital.
| Hospital | Classification | CRC CNSs | Surgeons | 2012 CRC surgical admissions |
|---|---|---|---|---|
| Site 1 | Urban and remote and rural | 3 | 4 | 127 |
| Site 2 | Urban and rural | 2 | 6 | 230 |
| Site 3 | Urban and rural | 2 | 7 | 215 |
Inclusion criteria
Patients were considered for inclusion if they:
-
were aged ≥ 18 years, had been diagnosed with primary CRC and were in the recovery period post surgery
-
were/were not receiving adjuvant chemotherapy/radiotherapy (patients would have to wait 48 hours after each chemotherapy session before attending cardiac rehabilitation classes). 10
Exclusion criteria
The study excluded anyone:
-
with advanced disease (includes patients with curable metastatic disease)
-
who failed clinical/risk assessment for rehabilitation and were deemed unsafe to participate in exercise classes; for example, according to recent guidelines, those with severe anaemia should delay exercise and patients with compromised immune function should avoid public gyms and exercise classes10
-
who had severe cognitive impairment and therefore was unable to give informed consent to participate in the study
-
unable to communicate in English, as this is the language used in the delivery of cardiac rehabilitation.
Recruitment procedures
Recruitment took place over 7 months. The first participant was recruited on 13 January 2014 (site 1) and the last participant was recruited on 29 July 2014 (site 2).
Based on phase 1 results (see Chapter 4), changes were made to the recruitment procedures and approved by the NHS Research and Ethics Committee (AM01 12/12/2013). The amended recruitment procedures in phase 2 were as follows.
A CRC clinical nurse specialist assessed patients admitted for surgery for CRC to determine their eligibility for the study. Eligible patients were given an information sheet (see Appendix 1) by the nurse (pre or post surgery) and the details of the study were discussed. If the patient agreed to participate in the study, the nurse asked them to sign a screening and recruitment form (see Appendix 2), before discharge from hospital, indicating their willingness and agreement to have their contact details given to an investigator. Patients who, having read the study information, declined to participate were asked if they would indicate their reasons for declining to participate on the screening and recruitment form; in addition, the patient was asked to sign a non-participation consent form (see Appendix 4) if they were willing to have information about them (e.g. age, gender, diagnosis or treatment) used by the investigators to assess if participants were representative of the study population.
After discharge from hospital, an investigator contacted patients who agreed to participate in the study, by telephone, to confirm the patient’s willingness to participate in the study and to establish if he or she was ready to attend cardiac rehabilitation. If the patient was willing and ready to attend cardiac rehabilitation, a mutually convenient time for the patient to meet with the investigator was arranged and formal written consent to participate in the study was sought. If the patient did not feel able to attend cardiac rehabilitation at this point in time (e.g. owing to poor recovery or transport difficulties) but was still willing to participate in the study and attend cardiac rehabilitation at some point in the future, then the investigator agreed to contact the patient again by telephone at a later date. Thus, at the first meeting with the investigator, eligibility was confirmed and written consent was obtained. If patients declined to give consent after hearing what the study involved, they were asked if they were willing to give their reason for no longer wishing to participate. Consented patients had baseline measures taken and were given an accelerometer to wear for a period of 7 days. They were given a FAQ (frequently asked questions) sheet, which listed answers to common queries (see Appendix 9). The patient was then randomised to the intervention or the control group (see Randomisation, concealment and blinding, which describes randomisation procedures).
Patients randomised to the control group were informed that they would not receive the intervention, but were given an information leaflet. They were also advised how and when to return their accelerometer to the investigator. Patients randomised to the intervention group were informed that they would be referred to cardiac rehabilitation.
The investigator completed a referral form and sent it on to the cardiac rehabilitation service. A member of the cardiac multidisciplinary team (e.g. a cardiac physiotherapist or nurse) then contacted the patient and invited them to attend a cardiac rehabilitation clinical/risk stratification assessment to determine whether or not the patient was able to safely exercise from a cardiac clinical perspective. Physical activity goals tailored to individual patient needs were also usually discussed at this time. Patients who were deemed safe to exercise were then given a date to start cardiac rehabilitation.
The recruitment process at site 3 was slightly different because a research nurse carried out all of the tasks conducted by the nurses and the investigator described above. Research nurses are nurses employed by hospitals in the UK to recruit to RCTs and can be working on a large number of trials simultaneously. Decisions about the use of research nurses for specific projects are decided by research and development managers in each NHS board. Site 3 had agreed that our study was able to make use of the research nurse, whereas the other two sites had not.
A flow chart outlining CRIB phase 2 recruitment and follow-up procedures is given in Figure 5.
FIGURE 5.
Flow chart outlining phase 2 recruitment and follow-up procedures. CR, cardiac rehabilitation.
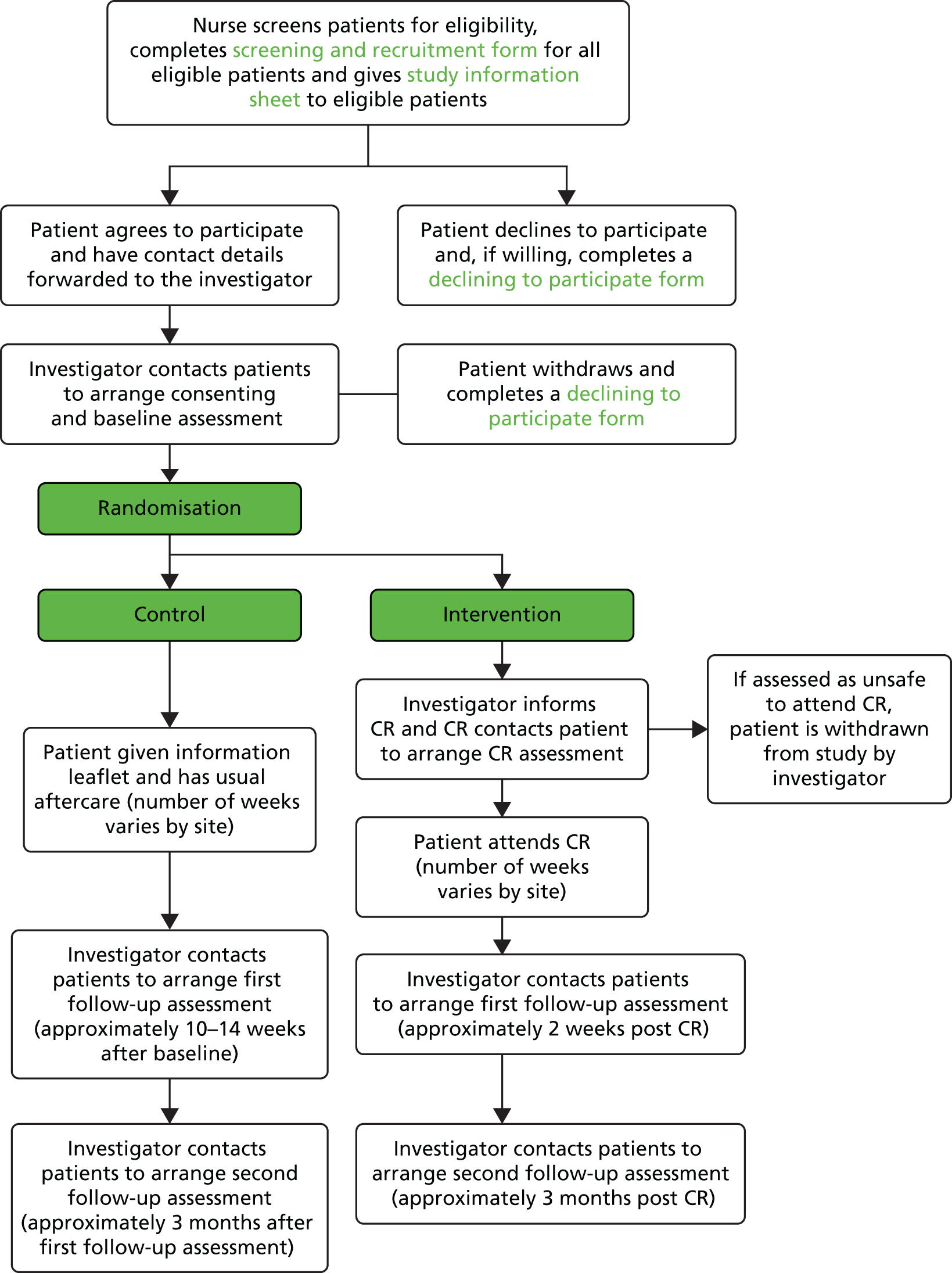
Informed consent
The phase 2 informed consent procedures were identical to those used in phase 1 and are described in Chapter 3.
Randomisation, concealment and blinding
Eligible and consenting patients were individually randomised at the end of their baseline assessment to one of two treatment groups: cardiac rehabilitation (i.e. the intervention) or usual care plus a booklet with information about lifestyle following CRC diagnosis and treatment (see Treatment group allocation).
To conceal the allocation of treatment from those conducting the research, the randomisation of individual participants to a particular treatment arm was undertaken using an automated online randomisation system, which was administered remotely and used a computer-generated code. The randomisation service was provided by Tayside Clinical Trials Unit, a UK Clinical Research Collaboration-registered trials unit.
Once the randomisation procedure had been completed, the outcome and further details about the allocated treatment were immediately communicated by the investigator to the participant. Because of the nature of the intervention, it was not possible to blind participants, investigators or the clinicians delivering the intervention (i.e. cardiac rehabilitation multidisciplinary team) to the treatment allocation.
Randomisation was stratified to take account of the three sites. This was to ensure equal distribution of intervention and control participants per site.
Treatment group allocation
Usual care
Individuals allocated to the usual care arm of the trial were advised to follow the current advice of their clinical team about physical activity if any advice was forthcoming. Individuals also received an information leaflet.
Intervention
The intervention was Phase III cardiac rehabilitation, which comprises exercise classes and cardiac-specific education sessions. Education sessions about cancer delivered by a CNS were planned to supplement the education sessions for cardiac patients. Education sessions were to be delivered either face to face to a group of patients with CRC or individually by telephone. This was the only alteration to routine cardiac rehabilitation planned for the study.
In each of the three sites, a cardiac physiotherapist contacted the patient and invited them to attend a cardiac rehabilitation clinical/risk stratification assessment to determine whether or not the patient was able to safely exercise from a cardiac clinical perspective, and also planned physical activity goals tailored to individual patient needs. Patients who were deemed safe to exercise were given a date to attend cardiac rehabilitation sessions alongside cardiac patients. We have used the TIDieR91 checklist to describe in more detail the components of the cardiac rehabilitation intervention in each site (see Table 3; Tables 15 and 16). Table 17 compares the three sites, highlighting key differences.
| Item number | Item | Description |
|---|---|---|
| Brief name | ||
| 1 | Provide the name or a phrase that describes the intervention | Referral to a cardiac rehabilitation programme |
| Why | ||
| 2 | Describe any rationale, theory, or goal of the elements essential to the intervention | Physical activity in CRC patients has shown improvement in cancer-specific mortality and general mortality. Eight recent systematic reviews23,24,28,29,41,45–47 of controlled trials indicate that physical activity interventions can help address the physiological and psychosocial effects of cancer and associated treatments in adult patients with cancer Cardiac rehabilitation may be an appropriate form of rehabilitation for patients with CRC because many of their needs post treatment are similar to those of individuals living with CHD. Studies on patients’ experiences of needs after CABG and patients with CRC indicate that patients with cardiac issues and cancer experience similar problems, including pain, fatigue, anxiety and depression, worry, appetite loss, sexual problems, sleep disturbance, and work and financial-related difficulties, and express a need for information about medication and self-management THEORY No one model best explains exercise behaviour, but the theory of planned behaviour is used most frequently in the research and literature. This proposes that patient behaviour is predicted by behavioural intent, and that their actions are based on that person’s intention to perform that behaviour, in this case cardiac rehabilitation. Intentions are based on three factors: attitudes towards the behaviour, beliefs of friends/relatives about the behaviour and level of control over their actions and behaviour. Health behaviour is, therefore, determined by a combination of these factors, and barriers to change |
| What | ||
| 3 | Materials: describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL) | Patients attending cardiac rehabilitation have access to an array of information on behaviour change for smoking, diet, healthy living, physical activity and more. The majority of these can be accessed at British Heart Foundation website (www.bhf.org.uk/healthcare-professionals/resources-for-patients.aspx) and from Chest, Heart and Stroke Scotland; however, some refer to very local events and groups and so will be specific to area (available on request). The patients are provided with a copy of the Scottish Borg Scale in a leaflet that also includes information about heart rate and how to check pulse rate. In addition, there are several wall charts and posters at each of the five different sections of the circuit with large photographs demonstrating each exercise. Patients are also given laminated cards depicting exercises that they can continue to complete at home between sessions For intervention delivery, the cardiac rehabilitation team follows local health board protocols, SIGN guidelines, ESC guidelines and ACPICR guidelines as well as the BACPR core components and standards, available at www.bacpr.com/resources/46C_BACPR_Standards_and_Core_Components_2012.pdf A key component of cardiac rehabilitation is an exercise class (see section 4). Patients are also invited to take part in information sessions on CHD, misconceptions of CHD, exercise, medications, diet, first aid, stress and relaxation, moving on and a general question-and-answer session that are provided once a week at the end of the exercise classes. The talks are done in both low impact and mainstream classes once a week for 30 minutes. Smoking cessation seminar is not provided, as patients who require this information are referred on to smoking cessation services |
| 4 | Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities | A patient will attend for an initial consultation prior to the exercise/education classes with the cardiac physiotherapist or cardiac specialist nurse. This involves confirming details received on referral (past medical history, current medications, any changes to symptoms, physical activity). Once the patient is assessed for the intervention, they attend class once or twice a week for 12 weeks, where they are put into a group of the most suitable level for their abilities; this includes a lower-level group who do activities at a lower intensity than the standard groups, to allow as many patients as possible to benefit from the intervention Standard cardiac rehabilitation sessions involve a 15-minute warm-up session involving range of motion exercises, and pulse-raising exercises to gently prepare the body for the session. The main component involves a variety of exercise ‘stations’ but includes CV stations (e.g. shuttle walking, cycling, step-ups) and strength stations, also known as ‘active recovery’ stations (e.g. sit–stand and dumbbell exercises, including bicep curls, shoulder press and upright row) and range of motion activities (such as toe backs, knee bends, shoulder lifts, half-jacks, side-to-side). The main sessions last approximately 30 minutes, depending on other time pressures. This is followed by a 10-minute cool-down, where feet are kept moving to maintain blood return to the heart, and to allow the heart rate to gradually reduce to resting, or near resting, values. A period of stretching exercises follows, along with some adapted tai chi Weekly information sessions are held for participants on a variety of behaviour change topics. These include CHD, misconceptions of CHD, exercise, medications, diet, first aid, stress and relaxation, moving on and a general question and answer session At the end of the intervention, patients are invited to continue to maintain their physical activity by moving on to different maintenance activities (e.g. Local Phase IV programme Healthy Hearts who have exercise classes, badminton, swimming, walking groups, aquacise, or Active Stirling, who provide a tailored exercise programme for the individual) |
| Who provided | ||
| 5 | For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, background and any specific training given | Cardiac physiotherapist is a band 7 NHS specialist physiotherapist with extensive experience working in cardiac rehabilitation Cardiac specialist nurse with extensive experience working in cardiac rehabilitation Band 6 physiotherapist and rotational physiotherapist are also part of the team One cardiac physiotherapist and one cardiac nurse always present at the class Numbers of staff vary depending on the number of participants. High-risk group staffing is 1 : 5. Lower-risk class 1 : 10 |
| How | ||
| 6 | Describe the modes of delivery (e.g. face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group | Patients are seen on the ward while an inpatient for initial cardiac event. Patients recovering from CABG or valve surgery are seen in a separate hospital, and are referred by tertiary centres rather than directly from ward staff Patients who are referred to cardiac rehabilitation are contacted by telephone to arrange for an initial consultation and the appointment is confirmed by letter. The initial consultation takes place just before the first exercise class on a one-to-one basis with the specialist physiotherapist or cardiac specialist nurse and this lasts approximately 30 minutes. The intervention is provided face to face by the cardiac rehabilitation team specified above. This is provided in either a hospital gym setting or a community sports centre (see section 7) in a group environment, with numbers around 15–25 per class depending on demand. Initial consultations are given on a one-to-one basis with the specialist physiotherapist or nurse |
| Where | ||
| 7 | Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | Patients attend either the main district hospital or the local community sports centre, depending on where they reside. The trust provides care for individuals across the Stirlingshire, Clackmannanshire, Falkirk and Kincardineshire regions |
| When and how much | ||
| 8 | Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose | Patients will attend either once or twice per week for 12 weeks, for sessions that last 60 minutes. Intensity of sessions is individualised to each patient by the physiotherapist or nurse. RPE scales are used to assess intensity Typical parameters for cardiac patients within a cardiac rehabilitation class are as follows RPE – using the 1–10 Borg CR10 scale to work at range 3–4. Staff use the ‘talk test’ to ensure the patient can speak in sentences; this is deemed an appropriate workload. Observational skills are also used by the experienced clinicians (e.g. facial colour; breathing rate; work intensity and speed) |
| Tailoring | ||
| 9 | If the intervention was planned to be personalised, titrated or adapted, then describe what, why, when, and how | Each patient is given a file and each week targets are set by the physiotherapist or nurse. These targets are individualised but will normally include attending as many sessions as possible, working at the agreed intensity, doing the exercises properly and achieving activity goals outside the classes. Goals set follow normal goal-setting procedure under the SMART principle |
| Modifications | ||
| 10 | If the intervention was modified during the course of the study, describe the changes (what, why, when, and how) | The intervention is likely to remain constant throughout the study period. As the cardiac rehabilitation intervention is an existing service, there is no opportunity during this pilot study to make changes to the intervention, unless done so by the staff running the programme |
| How well (planned): if intervention adherence or fidelity was assessed, describe how and by whom, and if strategies were used to maintain or improve fidelity, describe them | Intervention adherence will be assessed using attendance at cardiac rehabilitation classes, giving a percentage attendance figure. This will be monitored by the cardiac rehabilitation team | |
| How well (actual): if intervention was assessed, describe the extent to which the intervention was delivered as planned | Average attendance over 12 weeks (12 sessions for low impact or 24 sessions for the mainstream participants) will be calculated. Of the patients allocated to the intervention group, we will calculate how many attended the programme, and the percentage of those who completed the programme | |
| Item number | Item | |
|---|---|---|
| Brief name | ||
| 1 | Provide the name or a phrase that describes the intervention | Referral to a cardiac rehabilitation programme |
| Why | ||
| 2 | Describe any rationale, theory, or goal of the elements essential to the intervention | Physical activity in CRC patients has shown improvement in cancer-specific mortality and general mortality. Eight recent systematic reviews23,24,28,29,41,45–47 of controlled trials indicate that physical activity interventions can help to address the physiological and psychosocial effects of cancer and associated treatments in adult patients with cancer Cardiac rehabilitation may be an appropriate form of rehabilitation for patients with CRC because many of their needs post treatment are similar to those individuals living with CHD. Studies on patients’ experiences of needs after CABG and patients with CRC indicate that patients with cardiac issues and cancer experience similar problems, including pain, fatigue, anxiety and depression, worry, appetite loss, sexual problems, sleep disturbance, and work and financial-related difficulties, and express a need for information about medication and self-management THEORY No one model best explains exercise behaviour, but the theory of planned behaviour is used most frequently in the research and literature. This proposes that patient behaviour is predicted by behavioural intent, and that their actions are based on that person’s intention to perform that behaviour, in this case cardiac rehabilitation. Intentions are based on three factors: attitudes towards the behaviour, beliefs of friends/relatives about the behaviour and level of control over their actions and behaviour. Health behaviour is therefore determined by a combination of these factors, and barriers to change |
| What | ||
| 3 | Materials: describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL) | Patients attending cardiac rehabilitation have access to an array of information on behaviour change for smoking, diet, healthy living, physical activity and more. The majority of these can be accessed at www.bhf.org.uk/healthcare-professionals/resources-for-patients.aspx. A MI and surgery booklet is also given to patients with information on recovery Patients are given home exercise sheets, if appropriate A key component of cardiac rehabilitation is an exercise class (see section 4) |
| 4 | Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities | A patient will attend for an initial consultation with the cardiac physiotherapist and nurse. This involves confirming details received on referral (past medical history, current medications, any pending investigations, risk factor assessment), and includes a 6-minute walk test to establish fitness levels and suitability for patient to attend the exercise classes. The 6-minute walk test is repeated at the end of the programme. Patients are given a score of perceived fitness, and perceived confidence by the clinician completing the form. Occupational therapy assessments are also given, if appropriate. Referral to smoking cessation services is made if necessary Once the patient is accepted into the intervention, they attend class twice per week for 12 sessions over 6 weeks Standard cardiac rehabilitation sessions involve a 15-minute warm-up session involving range of motion exercises, and pulse-raising exercises to gently prepare the body for the session. This is followed by a 20- to 30-minute conditioning phase (10 CV and strengthening stations at 2 minutes each). Ten minutes are taken for the cool down. This is all in accordance with the ACPICR standards. A relaxation session is given once per week Weekly information sessions are provided with the following topics: physical activity, healthy eating, stress management and relaxation, CRC, cancer misconceptions and drug therapy |
| Who provided | ||
| 5 | For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, background and any specific training given | Two band 6 cardiac rehabilitation nurses for each class. Nurses check blood pressure and heart rate before and after each session. Medical condition is assessed to ensure that the patient is safe to exercise Band 7 physiotherapist to lead the exercise and to instruct patients to ensure that they are exercising safely |
| How | ||
| 6 | Describe the modes of delivery (e.g. face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group | Intervention is provided face to face by the cardiac rehabilitation team specified above. The maximum number of patients per class is 15. Pre-assessments are carried out one to one by a nurse and a physiotherapist |
| Where | ||
| 7 | Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | Patients attend the sessions at the local community leisure centre. The classes take place in the centre dance studio. The centre has lecture room facilities for the information sessions |
| When and how much | ||
| 8 | Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose | Patients will attend twice per week for 6 weeks, for sessions that last approximately 75 minutes. The physiotherapist individualises the intensity of sessions to each patient. Patients are taught the use of the Borg RPE scale and heart rate monitors are used to assess exercise intensity. Patients are given a heart rate target range to work within, and the nurse checks monitors after every exercise |
| Tailoring | ||
| 9 | If the intervention was planned to be personalised, titrated or adapted, then describe what, why, when and how | Each patient is given a pre-assessment to risk stratify and to establish fitness levels and suitability for classes. Patients are given an assessment of health behaviour and offered a treatment plan, including goal setting for health behaviour change. Regular reviews of progress are scheduled, and completed when time allows |
| Modifications | ||
| 10 | If the intervention was modified during the course of the study, describe the changes (what, why, when and how) | The intervention is likely to remain constant throughout the study period. As the cardiac rehabilitation intervention is an existing service, there is no opportunity during this pilot study to make changes to the intervention, unless done so by the staff running the programme. In this site, efforts have been made to provide cancer-specific sessions to intervention patients |
| How well (planned): if intervention adherence or fidelity was assessed, describe how and by whom, and if strategies were used to maintain or improve fidelity, describe them | Intervention adherence will be assessed using attendance at cardiac rehabilitation classes, giving a percentage attendance figure. This will be monitored by the cardiac rehabilitation team | |
| How well (actual): if intervention was assessed, describe the extent to which the intervention was delivered as planned | Attendance over 12 sessions will be calculated. Of the patients allocated to the intervention group we will calculate how many attended the programme, and the percentage attendance and programme completion | |
| Intervention component | Site 1 | Site 2 | Site 3 |
|---|---|---|---|
| What: materials | BHF booklets Local activities Home exercises |
BHF booklets Chest, Heart and Stroke Scotland resources Local activities Leaflet on exertion and pacing Home exercise cards |
BHF booklets A MI and surgery leaflet about recovery Home exercise sheets, if appropriate |
| What: procedures | 1 : 1 initial assessment Incremental Shuttle Walk Test Class: 15-minute warm-up; 20-minute stations (2 × 10 minutes); 15-minute cool-down Stretching and relaxation Weekly information seminars |
1 : 1 initial assessment Class: 15-minute warm-up; 30-minute stations; 10-minute cool-down Followed by stretching/tai chi Weekly information seminars |
2 : 1 initial assessment 6-minute walk test and given a score of perceived fitness and confidence by HP Class: 15-minute warm-up; 20-minute stations (2 × 10 minutes); 10-minute cool-down Relaxation session once per week Weekly information seminars |
| Who | Cardiac physiotherapist Physiotherapy assistant Cardiac rehabilitation co-ordinator |
Cardiac physiotherapist Cardiac specialist nurse Additional physiotherapist × 2 |
Specialist physiotherapist Two cardiac rehabilitation nurses |
| How | Group classes (15–20 patients per class) Hospital gym Low-level classes available |
Group classes (15–25 patients per class) Main district hospital and local community sports centre Low-level classes available |
Group classes (maximum of 15 patients) Leisure centre Dance studio facilities |
| When and how much (dose) | Frequency: once per week for 10 weeks (10 sessions) | Frequency: once or twice per week for 12 weeks | Frequency: twice per week for 6 weeks (12 sessions) |
| Intensity: 12–14 RPE (Borg 6–20 RPE scale) | Intensity: 3–4 RPE (Borg CR10 scale). ‘Talk test’ also used. Observation from health-care team | Intensity: RPE and heart rate monitor given, with patient-specific ranges to work within | |
| Time: 75-minute sessions (50-minute exercise component) | Time: 90 minutes (55-minute exercise component) | Time: 75 minutes (50-minute exercise component) | |
| Type: both CV and resistance/strength stations | Type: both CV and resistance/strength stations | Type: both CV and resistance/strength stations |
In addition, the investigators sought information about behaviour change techniques used by asking cardiac rehabilitation teams. The cardiac rehabilitation team was requested to complete a behaviour change technique taxonomy. 87 However, none of the sites achieved this because the task proved too difficult. Indeed, there is training on the use of the taxonomy (www.bct-taxonomy.com), so it was perhaps unreasonable to request that clinicians completed this task without additional training.
Measures
The primary objective of a future large-scale RCT will be to test if cardiac rehabilitation is clinically beneficial for CRC patients and also if it is cost-effective. As explained in Chapter 1, there is strong evidence that physical activity is associated with survival, improved quality of life and reduced levels of anxiety, depression and fatigue. At the time of designing this study, the proposed primary outcome for a future large-scale trial would be the difference in measures of physical activity (e.g. MET hours per week; sedentary and moderate activity) between the intervention and usual care (control) group measured by accelerometer. The proposed secondary outcomes in a future trial would be self-reported measures of quality of life, anxiety, depression and fatigue. An economic evaluation would also be conducted.
Based on phase 1 results, some data collection instruments were replaced for testing in phase 2 (see Chapter 4). All measures used in phase 2 are described below. A summary of outcomes and associated measures is presented in Table 18.
| Outcomes | Measures |
|---|---|
| Amount of physical activity Total mean minutes per day spent:
Number of participants meeting ≥ 150 minutes of physical activity per week |
Accelerometer |
| Type of physical activity Proportion of different types of physical activity (leisure time and at work) over 1 week |
SPAQ |
General quality of life
|
EQ-5D |
| Cancer-specific quality of life Subscale scores for the following subscales (maximum score of 28 for each subscale):
|
FACT-C |
Anxiety and depression
|
HADS |
| Fatigue Total score (maximum score of 52) |
FACIT Fatigue Scale |
| Physical activity self-efficacy Total score (maximum score of 120) |
Questionnaire developed by investigators for the ActWell trial136 |
Risk perception
|
Questionnaire designed by CRIB investigators |
Proposed primary outcome
Amount of physical activity
Amount of physical activity was assessed using the Actigraph GT3X+ accelerometer (Actigraph LLC, Pensacola, FL, USA). 92–94 Phase 1 (described in Chapter 3) procedures informed the introduction of one amendment, which was that written guidance for wearing the accelerometer was given to participants. Guidance included a photograph of how to wear the accelerometer and frequently asked questions, such as whether or not it is necessary to wear it in bed when asleep (see Appendix 9).
Type of physical activity
Physical activity was assessed subjectively using the Scottish Physical Activity Questionnaire (SPAQ) to ascertain the types of activities participants engaged in. 135 SPAQ was used in phase 2 and replaced the IPAQ (see Chapter 5 for explanation). SPAQ measures 7-day recall of leisure and occupational physical activity. Respondents are asked to record the number of minutes for each day of the week spent undertaking each type of activity; leisure time physical activity, which includes minutes spent ‘walking outwith work’, ‘manual labour outwith work’, ‘active housework’, ‘dancing’, ‘participating in sport, leisure activity or training’ and ‘other physical activity’; and ‘physical activity at work’, which includes minutes spent ‘walking whilst at work’ and ‘manual labour whilst at work.’ The SPAQ was designed to include only activities classed as moderate to vigorous. Test–retest reliability is strong, with a correlation coefficient of 0.998, showing it to be significant (critical value 0.436, p < 0.01; 32 degrees of freedom). 135
Scoring
The sum of the total number of minutes for each type of activity is scored. The total sum of activity, for all types, is also calculated.
Proposed secondary outcomes
General quality of life
The EQ-5D was used to measure comprehensive general health status. This instrument was also used in phase 1 and is described in Chapter 3.
Cancer-specific quality of life
The Functional Assessment of Cancer Therapy – Colorectal (FACT-C) was used as a self-report measure of cancer-specific quality of life and replaced the SF-36 (see Chapter 5 for an explanation). A recent review of patient-reported outcome measures for patients with CRC summarises FACT-C’s reliability, validity, responsiveness and acceptability, and recommends its use for measurement of cancer-related health status. 101 FACT-C has been used to measure cancer-related quality of life in trials of physical activity interventions involving people with CRC. 44,48,49 FACT-C137 is a 37-item scale that supplements the general version (FACT-G). 138 Patients rate each item on the questionnaire from 0 to 4, where a higher score denotes a better quality of life. The instrument consists of five subscales: physical well-being, social and family well-being, emotional well-being, functional well-being and the Colorectal Cancer Subscale. However, following findings from phase 1 regarding respondent burden due to repetition of questions (see Chapter 4), we decided to remove the emotional well-being subscale, as this is covered by HADS and EQ-5D.
Scoring
Scores are produced for each of the subscales. A higher score indicates better quality of life (range 0–28).
Anxiety and depression
The HADS was used to measure anxiety and depression. This instrument was also used in phase 1 and is described in Chapter 3.
Fatigue
The FACIT Fatigue Scale was used to measure fatigue. This instrument was also used in phase 1 and is described in Chapter 3.
Proposed process variables
Physical activity self-efficacy
Physical activity self-efficacy was measured using a 12-item questionnaire developed by investigators of the ActWell trial. 136 We chose these questions as they were designed specifically to measure physical activity self-efficacy in the context of delivering a behaviour change intervention.
Scoring
Participants rate on a scale of 0 to 10 their confidence in being able to be physically active under the following conditions: when tired, in a bad mood, when they do not have time, during bad weather, during vacation; their confidence in following directions from an instructor, pacing, performing movements, checking how hard activity is making them work; their confidence in their ability to exercise regularly, overcome obstacles; and their confidence to make up times if they have missed regular exercise sessions. Scores are totalled. A higher score denotes higher physical activity self-efficacy (range 0–120).
Risk perception
Risk perception was measured using the same 6-item scale that was used in phase 1 and is described in Chapter 3.
Proposed clinical variables
In addition, the following clinical confounding factors were reported on the screening and recruitment form:
-
colon or rectal surgery
-
surgical intervention (e.g. laparoscopic or open surgery)
-
temporary (a loop ileostomy) or permanent stoma or no stoma
-
chemotherapy or no chemotherapy.
Sample size justification
The aim of the study was not to provide a definitive estimate of treatment effect, so we did not have a formal sample size calculation. Rather, the aim was to provide robust estimates of the likely rates of recruitment and retention, and to yield estimates of the variability of the primary and secondary outcomes to inform power calculations for a future large-scale effectiveness trial. As highlighted in Chapter 1, there is no clear guidance for how many participants are necessary for estimating event rates such as recruitment and willingness to be randomised in pilot RCTs. Our recruitment estimate for the pilot trial was based on three factors, which were the estimated:
-
number of patients admitted for surgery
-
number of patients likely to meet inclusion criteria
-
recruitment rates in previous similar studies (e.g. trials of physical activity interventions for people with cancer).
Based on information provided by the local NHS principal investigators of the number of patient admissions in the previous year (2012), we expected 250 patients, in total, to be admitted for surgery across the three sites over a 6-month period. Cancer clinicians involved in the study estimated that approximately one-third (n = 83) would be ineligible and, based on recruitment to a RCT about physical activity with patients with cancer in Scotland139 and a trial involving patients with CRC within 3 months of completing surgery conducted in Canada,44 we estimated that just over one-third of eligible patients would consent (n = 66) to take part. Thus, for the pilot RCT we estimated that we would recruit around 66 patients (40% of eligible patients). We estimated that sites 2 and 3 would each recruit 26 patients, and that site 1 would recruit 14 patients, as this site admitted fewer patients for surgery than the other two sites.
Data collection and management
Trial procedures
In phase 2, particular attention was paid to systematically collecting data about recruitment procedures, including reporting the number of patients screened and assessed for eligibility, eligibility rate, consent rate, retention rate, completion rate and intervention compliance. These are fundamental physical activity intervention metrics that are essential for understanding the effects of the intervention in any future large-scale trial. It was important, therefore, to evaluate in the pilot RCT if data on these key metrics could be systematically collected and if outcomes indicated that a large-scale trial was feasible. The following definitions, calculations and data collection methods were used:
-
‘Assessed for eligibility rate’ was defined as the number of people with CRC who were admitted for surgery and assessed for eligibility using inclusion/exclusion criteria. Information about all eligible patients with CRC considered for phase 2, and who were subsequently included or excluded, who withdrew or who were withdrawn, was recorded by site investigators on screening and recruitment forms and then entered into the OpenClinica (www.openclinica.com) data management system developed by Tayside Clinical Trials Unit. In addition, investigators maintained a local site record of all participants in the study, monitoring withdrawal.
-
‘Eligibility rate’ was calculated by dividing the number of people with CRC admitted for surgery by the number who met inclusion criteria using data entered into OpenClinica.
-
‘Consent rate’ was calculated by dividing the number of people with CRC who met inclusion criteria and were, therefore, eligible by the number who consented to participate in the study using data entered into OpenClinica.
-
‘Retention rate’ was defined as the number of participants who remained in the study, that is, the number of participants who did not formally drop out of the study, using data entered into OpenClinica.
-
‘Completion rate’ was defined as the number of participants who completed outcome measures: that is, the number of participants who completed the self-reported questionnaires and were given an accelerometer to wear at baseline and at first and second follow-up and who met the validation criteria for wearing an accelerometer. An investigator calculated accelerometer validity manually.
-
‘Missing data’ were defined as the number of participants not entered into analyses because of invalid accelerometer data.
-
‘Intervention adherence’ was measured by the total number of planned physical activity sessions/consultations attended by participants allocated to the intervention group. Adherence data were collected from the cardiac rehabilitation register of attendance. In addition, adherence data were collected by a weekly telephone call to participants allocated to the intervention group. The purpose of the call was to obtain information about attendance and also about the frequency, duration and type of exercise that the participant did at the cardiac rehabilitation class (see Appendix 10).
-
‘Intervention fidelity’ was also assessed during the weekly telephone call. In particular, participants were asked if they have received any lifestyle advice by a CRC nurse specialist as this was the main recommended change made to the cardiac rehabilitation intervention.
Adverse events
If a research participant experienced an AE or a SAE, the investigator submitted a report to the principal investigator and then the appropriate body was informed, depending on the nature of the event. Any event considered to be ‘related’ or ‘unexpected’ was reported to the REC and the study sponsor. These SAEs involved completion of the appropriate paperwork and submission within 15 days of the principal investigator becoming aware of the event, as per the National Research Ethics Service.
A SAE is any AE occurring that results in any of the following outcomes:
-
death
-
inpatient hospitalisation or the prolongation of existing hospitalisation
-
persistent or significant disability/incapacity.
The following protocol exclusions applied:
-
hospitalisation for assault or accidental injury
-
hospitalisation for pre-planned surgery.
The above protocol exclusions were recorded in the AE log (see Appendix 8) for the study and line listings were reported annually to the ethics committee and the sponsor. Each hospital, and hence each cardiac rehabilitation programme, also had a reporting system for AEs, and cardiac rehabilitation services operate incident reporting. Thus, the AE reporting of study participants by was also recorded by site investigators.
Outcome measures
All participants’ questionnaires were administered online (Bristol Online Survey). 140
Baseline assessment
The investigator conducted baseline assessment at the academic institution in site 1, at the academic institution or the participant’s own home depending on patient choice in site 2 and at the hospital in site 3. The investigator administered online questionnaires (SPAQ, EQ-5D, HADS, FACT-C, FACIT Fatigue Scale, self-efficacy and risk perception questions) using the Bristol Online Survey. 140 The majority of questions were of a closed format, requiring participants to choose one option from a limited selection of discrete responses. Each question was read out by the investigator and answered by the participant. The investigator directly inputted the response to each question. The participant was also invited to wear the Actigraph GT3x+ accelerometer for 7 consecutive days (beginning the next day). They were given an information sheet about wearing the accelerometer (see Appendix 9). All baseline assessments were conducted between 11 March 2014 and 29 September 2014.
Follow-up assessment
The first follow-up assessment coincided approximately with the end of the intervention delivery period, that is, after the participant had attended the final cardiac rehabilitation class. Follow-up measures were collected at the academic institution, hospital or participant’s own home. The investigator administered the online questionnaires that were completed at baseline using the same procedures. The participant was again requested to wear the Actigraph GT3x+ accelerometer for 7 consecutive days (beginning the next day). All first follow-up data were collected between 18 June 2014 and 20 February 2015.
The second follow-up assessment was approximately 3 months after the participant had finished cardiac rehabilitation. The same procedures as used for first follow-up assessment were adopted. All second follow-up data were collected between 30 October 2014 and 31 March 2015.
Analysis
Phase 2 had seven objectives. To meet objective 1 (see Chapter 2), descriptive statistics were used to summarise screening, eligibility, consent, adherence and retention rates. Descriptive data on sample demographic characteristics were reported as frequencies, means and SDs. Differences in categorical variables of sociodemographic and clinical characteristics were compared between those who signed a screening and recruitment form but withdrew from the study and those who signed a consent form and were randomised to the intervention or control group. The reasons why eligible people with CRC did not wish to participate or withdrew, for the purpose of analysis, were sorted into one of seven categories:
-
no longer eligible
-
distance/travel
-
stated that they are currently exercising and fit
-
clinical (e.g. poor recovery from surgery, receiving adjuvant therapy, comorbidity)
-
too much of a commitment
-
not/no longer interested
-
no reason given.
To meet objective 2, completion rates and rates of missing data were assessed by counting the number of missed responses on the self-report questionnaires completed by participants at baseline and at 2- and 12-week follow-ups. In addition, the amount of time that participants wore the accelerometer at baseline and at 2- and 12-week follow-ups was counted (number of days worn over maximum wear-time of 7 days and number of hours per day worn).
To meet objectives 3 and 4, inferential statistics were used to assess any differences in outcomes between baseline and 2- and 12-week follow-up. These analyses are described in full in the statistical analysis plan (see Appendix 11). If data were normally distributed, outcome measures were assessed by multiple linear regression, adjusted for baseline variables; when data are measured more than once during the study, repeated measures analysis was also used. Scoring for outcomes follows the scoring instructions given for each questionnaire. When no such instruction is present, the following approach was taken: if no more than 20% of questionnaire items are missing, the missing items will be replaced by the mean of the remaining items to build a sum score. When more than 20% of the items are missing, the sum score will be set to missing. Outcomes were analysed as baseline versus end of intervention and baseline versus 3 months after intervention. An intention-to-treat approach was used for these preliminary analyses. Differences were considered statistically significant at p < 0.05.
To meet objective 5, descriptive statistics were generated of the proportion of CRC participants allocated to the intervention group who received cancer-specific education sessions or lifestyle advice from the CRC nurse specialists.
Objective 6 is addressed in Chapter 9, which describes the embedded qualitative study, and objective 7 is addressed in Chapter 10, which describes the economic evaluation.
Chapter 7 Phase 2 trial results
This chapter presents the results of phase 2, the pilot RCT. Screening, eligibility and consent rates are reported alongside reasons for non-participation. We have already published our experiences of recruitment elsewhere. 141 Completion rates are also reported alongside results of analyses of the effect of the intervention on outcomes. These results are presented to inform decisions about conducting a larger multicentre trial to evaluate effectiveness.
Site recruitment
A total of six cardiac rehabilitation services and CRC clinicians located in the same hospital were initially approached to participate in the study. Two sites agreed to support the research. The reason why four sites did not wish to participate was due to cardiac rehabilitation concerns about capacity to include more patients in their classes. All CRC teams at the six sites were willing to collaborate in the study. Following contact with the NIHR Colorectal Cancer Clinical Studies Group, two further sites were identified and one of these sites was invited to participate in the study. Table 14 summarises the characteristic of the three sites involved in this study.
Flow of participants in the trial
In total, 41 individuals were recruited to the CRIB pilot trial, with 21 allocated to the intervention arm and 20 allocated to the usual care plus booklet group. Figure 6 presents the flow diagram for the trial and summarises patient throughput from referral to completion of the 1- and 3-month follow-ups. The diagram also reports the total numbers of patients who were given study information, did not meet inclusion criteria (sites 1 and 2 only), withdrew before randomisation, withdrew following randomisation or were lost to follow-up immediately following the intervention and at 3 months post intervention.
FIGURE 6.
Phase 2 total recruitment and sample attrition across all three sites.
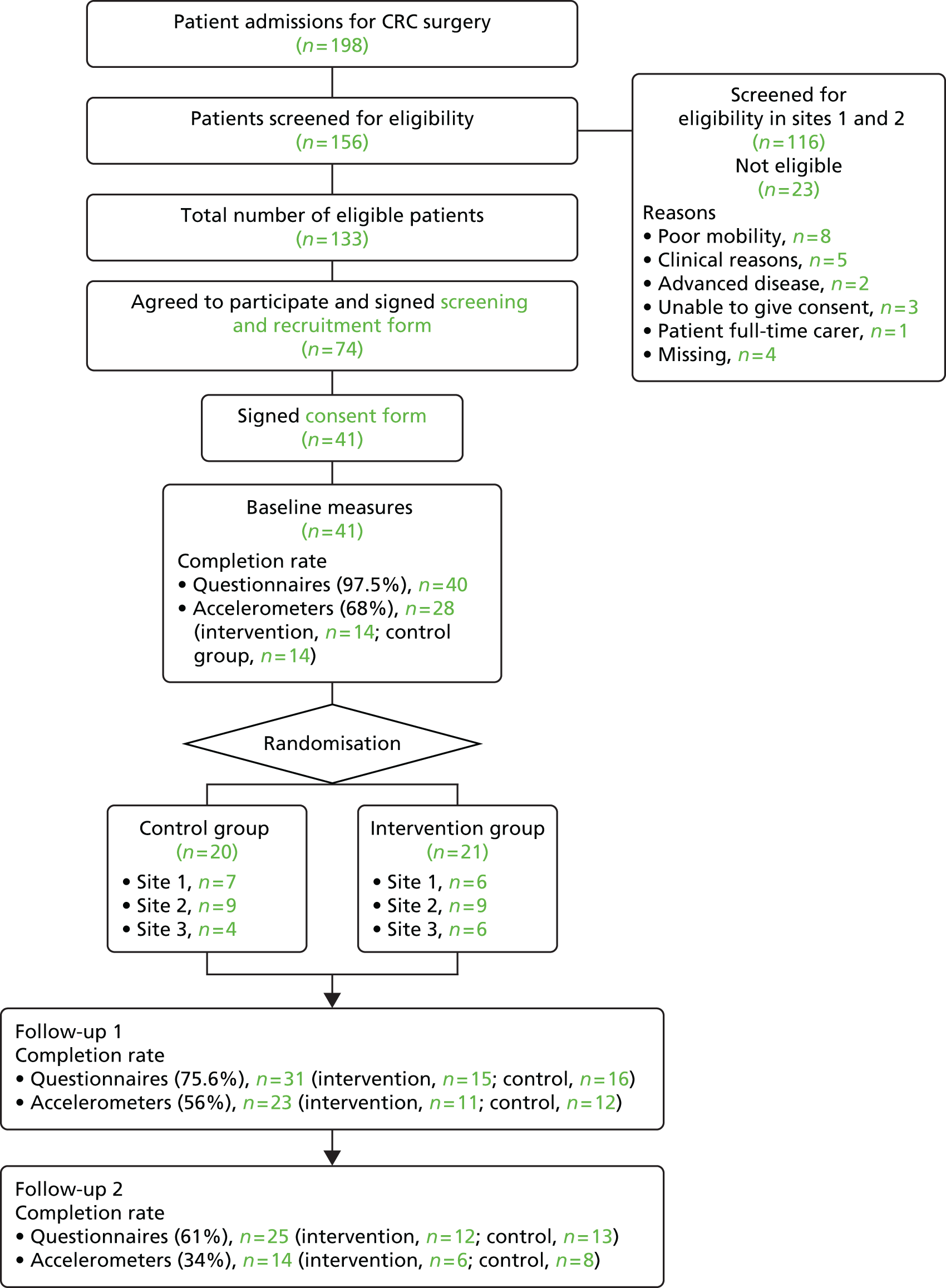
Screening rate
Screening rate was defined as the number of people with CRC who were admitted for surgery and assessed for eligibility. Across all three sites, there were 198 people admitted to hospital for CRC surgery and, of these, 156 were assessed for eligibility (79%). Table 19 shows screening rates by site and shows that the research nurse in site 3 assessed 65% of patients for eligibility, whereas the clinical nurse specialists at the other two sites reached more patients, assessing 86% and 91% of all patient admissions, respectively.
| Site | Actual admissions, N | Assessed for eligibility, n (%) |
|---|---|---|
| Site 1 | 58 | 50 (86) |
| Site 2 | 58 | 53 (91) |
| Site 3 | 82 | 53 (65) |
| Total | 198 | 156 (79) |
Eligibility rate
Eligibility rate was calculated by dividing the number of people with CRC admitted for surgery by the number who met the inclusion criteria. Out of 198 patient admissions for CRC surgery, 133 (67%) met the eligibility criteria. Table 20 shows that the proportion of patient admissions for surgery who were eligible for the study was almost identical across the three sites (67%, 67% and 65%, respectively).
| Site | Admissions, N | Eligible, n (%) |
|---|---|---|
| Site 1 | 58 | 40 (67) |
| Site 2 | 58 | 40 (67) |
| Site 3 | 82 | 53 (65) |
| Total | 198 | 133 (67) |
Reasons for ineligibility
Nurses at sites 1 and 2 recorded the reasons why patients were considered ineligible for the study. Reporting of reasons for ineligibility was not recorded at site 3; data were missing for 17% of cases in the other two sites. Table 21 summarises the main reasons why patients were considered to be ineligible. The table shows that over half (57%) of patients were excluded because of poor mobility or other health reasons. When these reasons are mapped to the exclusion criteria listed in Chapter 6, the main reason for ineligibility is shown to be criterion 2, that is, ‘Patients who fail clinical/risk assessment for rehabilitation and are deemed unsafe to participate in exercise classes’. Only 9% of patients were ineligible because they had ‘advanced disease’ (criterion 1) and only 13% of patients were ineligible because they were unable to provide informed consent (criterion 3). One patient was deemed ineligible because he or she was a full-time carer, although this was not listed as an exclusion criterion.
| Reason given by nurse | Number (%) of patientsa | Exclusion criterion (1 to 3) |
|---|---|---|
| Poor mobility | 8 (35) | 2 |
| Other health reason | 5 (22) | 2 |
| Advanced disease | 2 (9) | 1 |
| Unable to provide consent | 3 (13) | 3 |
| Patient is a full-time carer | 1 (4) | N/A |
| Unknown | 4 (17) | N/A |
Consent rate
In total, 74 out of 133 eligible patients signed a screening and recruitment form indicating that they were interested in participating in the study and willing to be contacted by an investigator (i.e. 56% of the number of people with CRC who met inclusion criteria).
The consent rate was calculated by dividing the number of people with CRC who met the inclusion criteria (n = 133) by the number who consented to participate in the study and were randomised. Forty-one people with CRC consented and were randomised to the intervention or usual care (control) group, which is a consent rate of 31%. Twenty-one patients were randomised to the intervention group and 20 were randomised to the control group.
Table 22 shows the difference between total estimated and actual patient admissions, eligibility and randomisation rates across all three sites.
| Variable | Site 1 | Site 2 | Site 3 | All sites | ||||
|---|---|---|---|---|---|---|---|---|
| Estimated | Actual | Estimated | Actual | Estimated | Actual | Original estimate | Actual | |
| Admissions | 74 | 58 | 134 | 58 | 125 | 82 | 250 | 198 |
| Eligible (% of admissions) | 49 (66) | 40 (69) | 88 (66) | 40 (69) | 82 (66) | 53 (65) | 165 (66) | 133 (67) |
| Randomised (% of eligible patients) | 20 (40) | 13 (32) | 35 (40) | 18 (45) | 33 (40) | 10 (19) | 66 (40) | 41 (31) |
Figure 7 shows graphically the difference between the total estimated and actual patient admissions at each stage of the recruitment process. ‘Admissions’ refers to the numbers of estimated and actual patients admitted to hospital for CRC surgery; ‘assessed for eligibility’ refers to the number of these patients who were assessed for eligibility for the study; ‘eligible’ refers to the number of patients who met eligibility criteria; ‘screened’ refers to the number of eligible patients who completed a screening and recruitment form; ‘consent to approach’ refers to the number of patients who indicated on this screening form that they were interested in participating in the study and willing to have an investigator contact them about the study; and ‘randomisation’ refers to the number of patients who signed a consent form and were randomised to the intervention group or the control group.
FIGURE 7.
Total differences between estimated and actual admission, eligibility and randomisation rates.
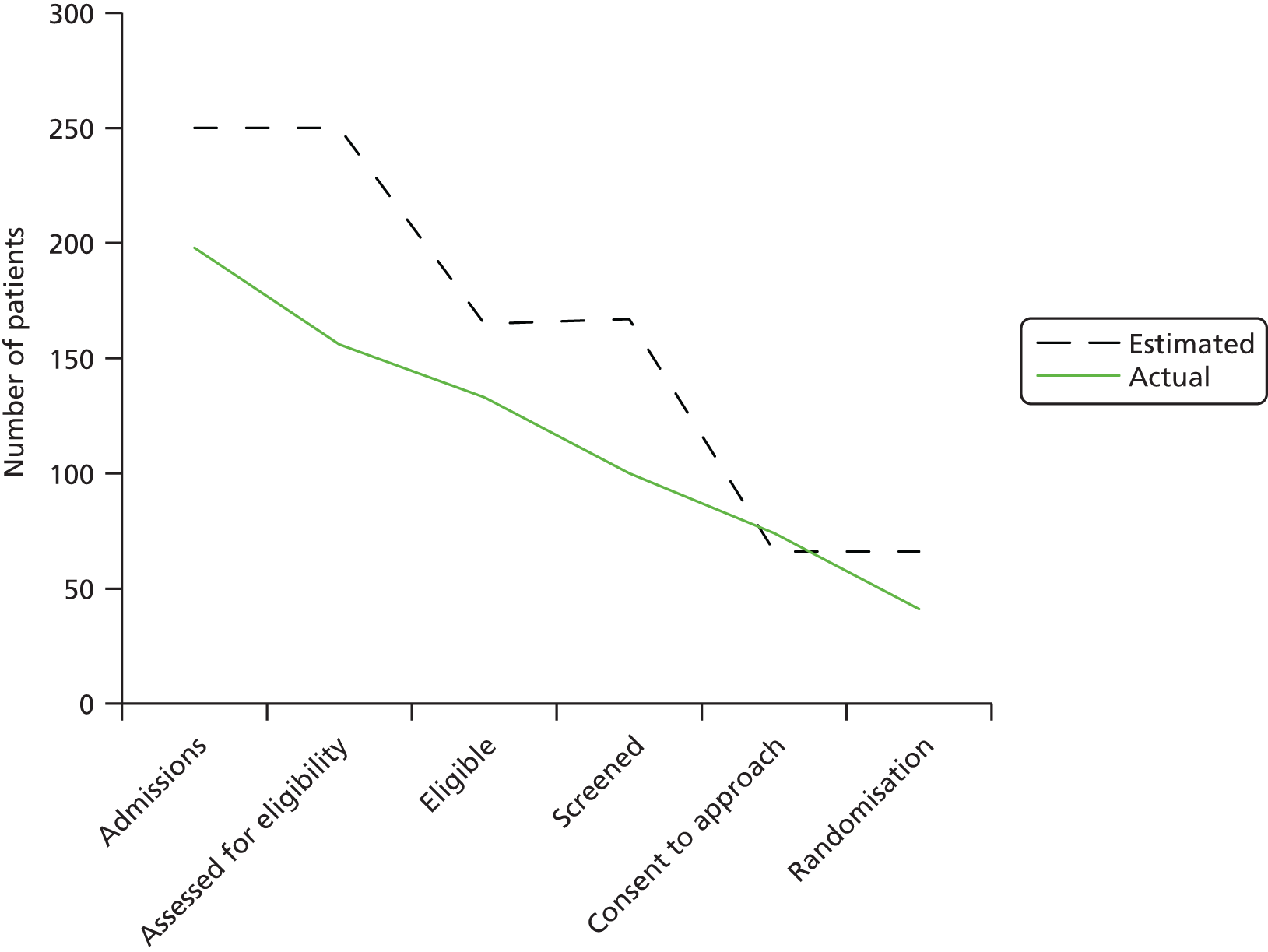
The number of actual surgical admissions was lower than the protocol estimate (198 vs. 250). The actual number of eligible patients was approximately two-thirds of surgical admissions, which was on a par with the protocol estimate. In total, 133 out of 198 (67%) actual patient admissions were judged as eligible for the study. Seventy-four eligible patients signed a screening and recruitment form indicating willingness to participate and 41 of these patients consented and were randomised to the intervention or control groups. Thus, 31%, as opposed to an estimated 40%, of eligible patients consented and were randomised.
Reasons for non-participation
Table 23 shows the reasons why eligible patients who signed a screening and recruitment form expressing willingness to participate in the study then withdrew before randomisation. Most commonly, reasons fell into the clinical category, which included poor recovery from surgery, comorbidity or receiving adjuvant therapy (15 out of 33; 46%).
| Reason | All sites, n (%) |
|---|---|
| Distance/travel barriers | 2 (6) |
| Return to normal activities | 3 (9) |
| Clinical (e.g. poor recovery from surgery, comorbidity) | 9 (28) |
| Other commitments/time | 2 (6) |
| Adjuvant therapy | 6 (18) |
| Study time limit | 3 (9) |
| Unable to contact | 1 (3) |
| Patient death | 1 (3) |
| Missing | 6 (18) |
Retention rate
The retention rate was defined as the number of participants who remained in the study, that is, the number of participants who did not formally drop out. As indicated above, 41 people with CRC consented and were therefore identified as study participants. Three (7%) of these participants formally left the study (two control and one intervention).
Completion rates and missing data
Completion rate was defined as the number of consenting and randomised participants (n = 41) who completed outcome measures, that is, the number of participants who completed the self-reported questionnaires and were given an accelerometer to wear. Missing data were defined as the number of participants who were given an accelerometer but did not provide validated accelerometer data (see Chapter 6 for definition of validity) at baseline and at first and second follow-up.
Number of participants completing the self-reported questionnaires
A total of 40 (97.5%) (20 intervention and 20 control) out of 41 participants completed the questionnaires (SPAQ, FACT-C, EQ-5D, FACIT Fatigue Scale, HADS) at baseline and 31 (75.6%) (15 intervention and 16 control) and 25 (61%) (12 intervention and 13 control) completed the questionnaires at the follow-up 1 and follow-up 2 time points, respectively.
Number of participants providing valid accelerometer data
Twenty-eight (68%) (14 intervention and 14 control) out of 41 participants provided validated accelerometer data to measure physical activity and sedentary behaviour at baseline and 23 (11 intervention and 12 control) (56%) and 14 (six intervention and eight control) (34%) participants provided validated accelerometer data at the follow-up 1 and follow-up 2 time points, respectively. These figures are broken down by site in Table 24.
| Site | Baseline, n (%) | Follow-up 1, n (%) | Follow-up 2, n (%) |
|---|---|---|---|
| Site 1 (n = 13) | 9 (69) | 9 (69) | 8 (62) |
| Site 2 (n = 18) | 11 (61) | 7 (39) | 2 (11) |
| Site 3 (n = 10) | 8 (80) | 7 (70) | 4 (40) |
| Total | 28 | 23 | 14 |
If all 41 participants had provided valid accelerometer data at each time point, there would have been a total of 123 accelerometer data sets. Sixty-five (53%) accelerometer data sets were provided and, of these, 20 (31%) were removed from analysis because data were invalid, which involved 14 different participants spread more or less evenly across the intervention and control groups. Table 25 shows reasons for invalid accelerometer data. The main reasons for invalidity were not wearing the device and not wearing it for a sufficient number of hours per day to meet the validation criteria.
| Reasons invalid | Intervention, n (n = 11) | Control, n (n = 9) | Total, n (%) |
|---|---|---|---|
| Days worn (< 4) | 0 | 3 | 3 (15) |
| Hours per day (< 10) | 2 | 3 | 5 (25) |
| Not worn at all | 5 | 2 | 7 (35) |
| Abnormal activity patterns | 4 | 1 | 5 (25) |
Figure 8 shows the number of completed questionnaires and valid accelerometer data at each time point.
FIGURE 8.
Number of participants completing self-reported questionnaires and valid accelerometer data.
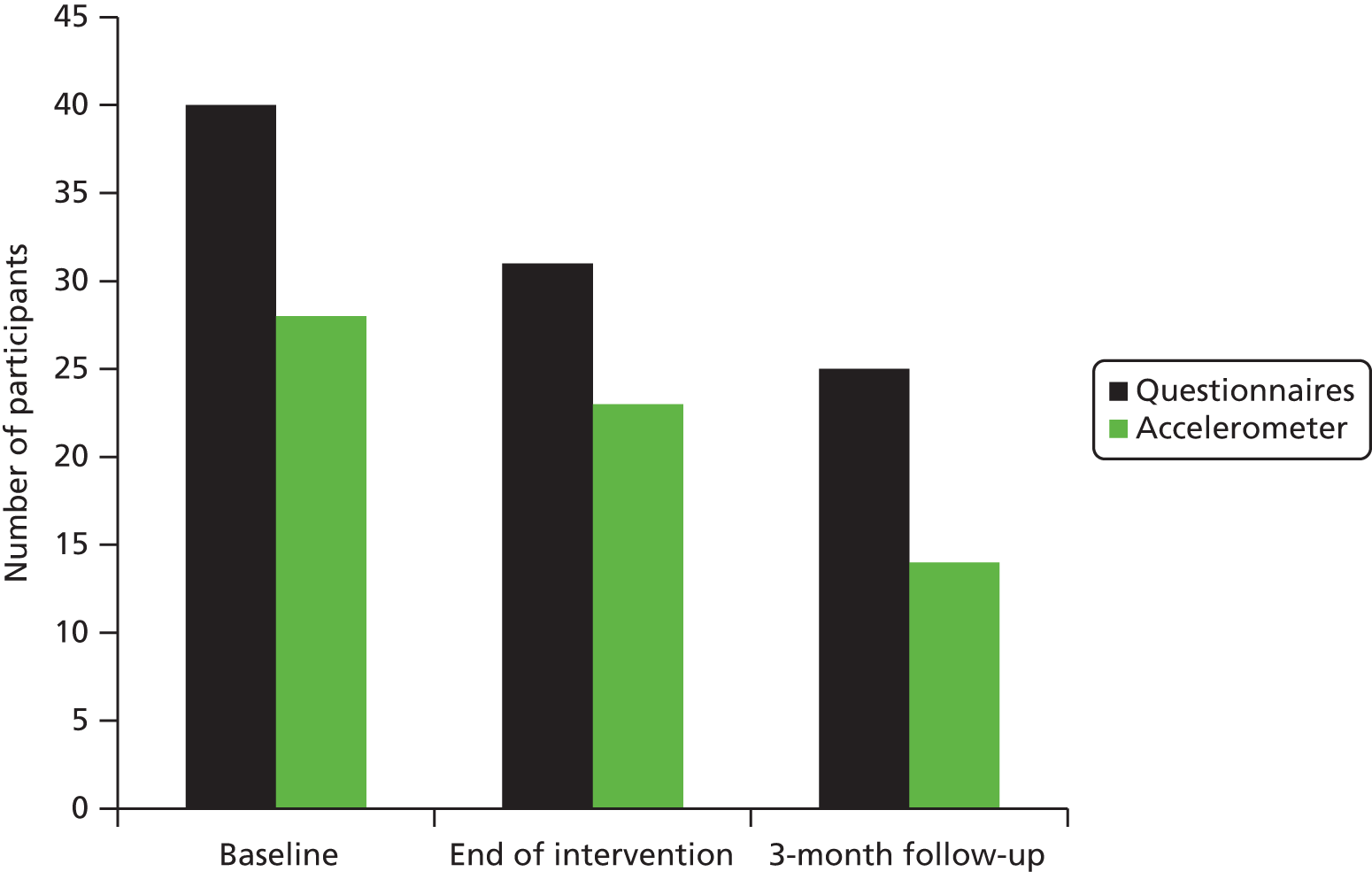
Adverse events
In phase 2, no AEs were reported. One participant was unable to start cardiac rehabilitation owing to a torn knee ligament that was unrelated to the study.
Intervention adherence
As described in Chapter 6, adherence data were collected from the cardiac rehabilitation register of attendance and by a weekly telephone call to the participant. Adherence rate was calculated in two ways:
-
attendance during the cardiac rehabilitation programme (i.e. site 1: once-weekly for 10 weeks = 10, site 2: twice-weekly for 12 weeks = 24, site 3: twice-weekly for 6 weeks = 12)
-
attendance from start and end date that participant attended cardiac rehabilitation, including breaks and absences owing to, for instance, treatment.
Thirteen out of 21 participants (62%) randomised to the intervention group completed the programme as per protocol. Three participants started but could not complete all classes and five did not begin (38%). Table 26 shows that the main barrier to starting or dropping out of cardiac rehabilitation was poor physical health. Table 27 shows that participants who were able to continue with the programme had high levels of attendance (range 75–142%).
| Study ID | Number of sessions | Reason for non-completion |
|---|---|---|
| Site 1 19 | 1 | Patient unable to complete owing to arthritis problems in his ankle. He was not suitable to attend the cardiac rehabilitation classes |
| Site 2 12 | 0 | Discharged by cardiac rehabilitation team owing to high blood pressure |
| Site 2 22 | 0 | Physically and mentally unfit at cardiac rehabilitation consultation |
| Site 2 26 | 2 | Further treatment – operation on liver |
| Site 2 14 | 6 | Found that the sessions were too easy for her and were not challenging enough |
| Site 3 01 | 0 | Neuropathic pain, other scans awaited. Wanted to follow own exercises at home |
| Site 3 18 | 0 | Torn knee ligaments |
| Site 3 20 | 0 | Unwell during follow-up therapies, and no longer wanted to participate in study |
| Study ID | Number (%) of sessions attended | First session attended | Last session attended | Number of weeks attended | Average number of sessions per week |
|---|---|---|---|---|---|
| Site 1 16 | 8 (80) | 1 May 2014 | 15 July 2014 | 12 | 0.7 |
| Site 1 11 | 12 (120) | 23 September 2014 | 9 December 2014 | 12 | 1.0 |
| Site 1 02 | 10 (80) | 15 May 2014 | 17 July 2014 | 10 | 1.0 |
| Site 1 13 | 11 (110) | 13 May 2014 | 26 August 2014 | 15 | 0.7 |
| Site 1 26 | 11 (110) | 6 November 2014 | 5 February 2015 | 13 | 0.8 |
| Site 2 19 | 22 (92) | 18 September 2014 | 16 December 2014 | 14 | 1.6 |
| Site 2 06 | 24 (100) | 26 May 2014 | 14 August 2014 | 11 | 2.2 |
| Site 2 15 | 24 (100) | 25 August 2014 | 8 January 2015 | 20 | 1.2 |
| Site 2 04 | 25 (104) | 22 April 2014 | 15 August 2014 | 16 | 1.6 |
| Site 2 04 | 34 (142) | 2 June 2014 | 4 September 2014 | 14 | 2.4 |
| Site 3 07 | 12 (100) | 17 June 2014 | 24 July 2014 | 6 | 2.0 |
| Site 3 30 | 12 (100) | 4 September 2014 | 23 October 2014 | 8 | 1.5 |
| Site 3 29 | 9 (75) | 4 September 2014 | 21 October 2014 | 8 | 1.1 |
Participant length of time in the study
Table 28 shows baseline, follow-up 1 and follow-up 2 dates for participants who completed all time points (intervention, n = 13; control, n = 12). The mean number of days between baseline and follow-up was 86 and 104 for control and intervention group, respectively; between follow-ups 1 and 2 it was 83 and 78 days, respectively. The total mean number of days involved in the study was 169 and 182 for control and intervention groups, respectively. Thus, there was minimal difference in the number of days between data collection points between the two groups.
| Subject ID | Date of surgery | Time (days) | Baseline | Time (days) | Follow-up 1 | Time (days) | Follow-up 2 |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| Site 1 15 | 23 January 2014 | 44 | 25 March 2014 | 62 | 18 June 2014 | 185 | 3 March 2015 |
| Site 1 23 | 17 March 2014 | 137 | 23 September 2014 | 56 | 9 December 2014 | 69 | 13 March 2015 |
| Site 1 32 | 7 July 2014 | 58 | 24 September 2014 | 85 | 20 January 2015 | 47 | 25 March 2015 |
| Site 2 05 | 24 January 2014 | 51 | 4 April 2014 | 71 | 11 July 2014 | 131 | 9 January 2015 |
| Site 2 07 | 18 February 2014 | 98 | 3 July 2014 | 101 | 20 November 2014 | 60 | 11 February 2015 |
| Site 2 08 | 4 March 2014 | 121 | 19 August 2014 | 134 | 20 February 2015 | 26 | 27 March 2015 |
| Site 2 09 | 4 March 2014 | 61 | 27 May 2014 | 97 | 8 October 2014 | 121 | 25 March 2015 |
| Site 2 10 | 4 March 2014 | 67 | 4 June 2014 | 74 | 15 September 2014 | 66 | 15 December 2014 |
| Site 2 16 | 29 April 2014 | 47 | 2 July 2014 | 105 | 25 November 2014 | 60 | 16 February 2015 |
| Site 2 17 | 5 May 2014 | 47 | 8 July 2014 | 134 | 9 January 2015 | 42 | 9 March 2015 |
| Site 3 02 | 11 February 2014 | 119 | 26 July 2014 | 13 | 13 August 2014 | 76 | 26 November 2014 |
| Site 3 09 | 19 March 2014 | 31 | 30 April 2014 | 99 | 15 September 2014 | 144 | 2 April 2015 |
| Site 3 32 | 4 August 2014 | 28 | 10 September 2014 | 86 | 7 January 2015 | 51 | 18 March 2015 |
| Intervention | |||||||
| Site 1 02 | 16 December 2013 | 66 | 17 March 2014 | 118 | 27 August 2014 | 114 | 2 February 2015 |
| Site 1 11 | 15 January 2014 | 145 | 5 August 2014 | 127 | 28 January 2015 | 42 | 26 March 2015 |
| Site 1 13 | 19 February 2014 | 48 | 25 April 2014 | 121 | 10 October 2014 | 90 | 12 February 2015 |
| Site 1 16 | 16 January 2014 | 51 | 27 March 2014 | 106 | 21 August 2014 | 139 | 3 March 2015 |
| Site 1 19 | 4 March 2014 | 143 | 18 September 2014 | 83 | 12 January 2015 | 57 | 31 March 2015 |
| Site 1 26 | 20 May 2014 | 86 | 16 September 2014 | 86 | 13 January 2015 | 53 | 26 March 2015 |
| Site 2 02 | 13 January 2014 | 88 | 14 May 2014 | 87 | 11 September 2014 | 84 | 6 January 2015 |
| Site 2 06 | 6 February 2014 | 49 | 15 April 2014 | 90 | 18 August 2014 | 143 | 4 March 2015 |
| Site 2 14 | 15 April 2014 | 45 | 20 June 2014 | 85 | 16 October 2014 | 87 | 13 February 2015 |
| Site 2 15 | 29 April 2014 | 53 | 10 July 2014 | 156 | 12 February 2015 | 32 | 27 March 2015 |
| Site 2 19 | 3 June 2014 | 57 | 20 August 2014 | 128 | 13 February 2015 | 30 | 26 March 2015 |
| Site 3 30 | 25 May 2014 | 63 | 20 August 2014 | 63 | 14 November 2014 | 70 | 19 February 2015 |
Comparability of characteristics of consenting and non-consenting eligible patients
Fifty-four out of 133 eligible patients who did not consent to participate in the study consented to have their demographic and clinical information used for the purposes of this study. Table 29 shows the characteristics of eligible consenting and not consenting patients. It shows no significant differences in age, gender and type of surgery (colon or rectal) between the two groups, but suggests that people with metastatic disease (T4 and N1 classification), people who have had open surgery and people with a stoma are more likely not to participate.
| Characteristic | Not consenting, n (%) (N = 54) | Randomised, n (%) (N = 41) |
|---|---|---|
| Age (years) | ||
| n | 54 | 41 |
| Missing | 0 | 0 |
| Mean | 65.6 | 66.0 |
| SD | 13.81 | 11.31 |
| Median | 65.5 | 67.0 |
| Sex | ||
| Male | 39 (72.2) | 27 (65.9) |
| Female | 15 (27.8) | 14 (34.1) |
| Primary tumoura | ||
| Missing | 13 (24.1) | 9 (22.0) |
| T0 | 2 (3.7) | 1 (2.4) |
| T1 | 1 (1.9) | 3 (7.3) |
| T2 | 8 (14.8) | 12 (29.3) |
| T3 | 20 (37.0) | 11 (26.8) |
| T4 | 10 (18.5) | 5 (12.2) |
| Regional lymph nodea | ||
| Missing | 19 (35.2%) | 13 (31.7) |
| Nx | 1 (1.9%) | 0 |
| N0 | 21 (38.9) | 22 (53.7) |
| N1 | 13 (24.1) | 6 (14.6) |
| Distant metastasisa | ||
| Missing | 48 (88.9) | 36 (87.8) |
| M0 | 5 (9.3) | 5 (12.2) |
| M1 | 1 (1.9) | 0 |
| Colon surgery | ||
| No | 21 (38.9) | 16 (39) |
| Yes | 33 (61.1) | 25 (61) |
| Rectal surgery | ||
| No | 35 (64.8) | 25 (61) |
| Yes | 19 (35.2) | 16 (39) |
| Laparoscopic surgery | ||
| Missing | 1 (1.9) | 0 (0) |
| No | 37 (68.5) | 32 (78) |
| Yes | 16 (29.6) | 9 (22) |
| Open surgery | ||
| Missing | 1 (1.9) | 0 |
| No | 21 (38.9) | 18 (43.9) |
| Yes | 32 (59.3) | 23 (56.1) |
| Temporary stoma | ||
| Missing | 3 (5.6) | 0 |
| No | 39 (72.2) | 35 (85.4) |
| Yes | 12 (22.2) | 6 (14.6) |
| Permanent stoma | ||
| Missing | 3 (5.6) | 0 (0) |
| No | 45 (79.6) | 34 (82.9) |
| Yes | 8 (14.8) | 7 (17.1) |
| Chemotherapy | ||
| Missing | 11 (20.4) | 7 (17.1) |
| No | 37 (68.5) | 27 (65.9) |
| Yes | 6 (11.1) | 7 (17.1) |
| Radiotherapy | ||
| Missing | 10 (18.5) | 7 (17.1) |
| No | 36 (66.7) | 29 (70.7) |
| Yes | 8 (14.8) | 5 (12.2) |
| Other treatment | ||
| Missing | 4 (7.4) | 3 (7.3) |
| No | 49 (90.7) | 35 (85.4) |
| Yes | 1 (1.9) | 3 (7.3) |
Baseline comparability of demographic and clinical characteristics of intervention and control group participants
Age and gender
No data about age and gender were missing. Twenty-seven men (65.9%) and 13 (34.1%) women were recruited to the study. The numbers of men allocated to the intervention and control groups were 13 (61.9%) and 14 (70%), respectively. The mean age of participants was 66 (SD 11.31) years; the youngest participant was aged 42 years and the oldest was aged 86 years. The mean ages of participants allocated to the intervention and control groups were 67.9 (SD 11.49) years and 64.2 (SD 11.10) years, respectively.
Colorectal cancer diagnosis
Table 30 shows participants’ CRC diagnosis using the tumour, node and metastases classification system. The table shows slight differences between participants in the intervention and control groups; for example, seven (33.3%) participants allocated to the intervention group and four (20%) allocated to the control group were classified as T3. However, it is difficult to make direct comparisons between the two groups because of missing data. The table shows that nine (22%) participants had missing information about tumour size, 13 (31.7%) had missing information about lymph nodes containing cancer cells and 36 (87.8%) had missing information about metastases. The most likely explanation for missing data is that the tumour, node and metastases classification was not known at the time when diagnosis was recorded on the screening and recruitment form completed by an investigator.
| Variable | Intervention, n (%) | Control, n (%) | Total, N (%) |
|---|---|---|---|
| Primary tumour | |||
| Missing | 3 (14.3) | 6 (30.0) | 9 (22.0) |
| T0 | 1 (4.8) | 0 (0.0) | 1 (2.4) |
| T1 | 1 (4.8) | 2 (10.0) | 3 (7.3) |
| T2 | 7 (33.3) | 5 (25.0) | 12 (29.3) |
| T3 | 7 (33.3) | 4 (20.0) | 11 (26.8) |
| T4 | 2 (9.5) | 3 (15.0) | 5 (12.2) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Regional lymph node | |||
| Missing | 5 (23.8) | 8 (40.0) | 13 (31.7) |
| N0 | 12 (57.1) | 10 (50.0) | 22 (53.7) |
| N1 | 4 (19.0) | 2 (10.0) | 6 (14.6) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Distant metastasis | |||
| Missing | 17 (81.0) | 19 (95.0) | 36 (87.8) |
| M0 | 4 (19.0) | 1 (5.0) | 5 (12.2) |
| M1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
Type of surgery and stoma
Table 31 shows no missing data about whether participants had colon or rectal surgery and that there was a near even split between participants allocated to the intervention and control groups who had colon surgery or rectal surgery.
| Variable | Intervention, n (%) | Control, n (%) | Total, N (%) |
|---|---|---|---|
| Colon surgery | |||
| No | 8 (38.1) | 8 (40.0) | 16 (39.0) |
| Yes | 13 (61.9) | 12 (60.0) | 25 (61.0) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Rectal surgery | |||
| No | 15 (71.4) | 10 (50.0) | 25 (61.0) |
| Yes | 6 (28.6) | 10 (50.0) | 16 (39.0) |
| Total | 21(100.0) | 20 (100.0) | 41 (100.0) |
| Laparoscopic surgery | |||
| No | 15 (71.4) | 17 (85.0) | 32 (78.0) |
| Yes | 6 (28.6) | 3 (15.0) | 9 (22.0) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Open surgery | |||
| No | 11 (52.4) | 7 (35.0) | 18 (43.9) |
| Yes | 10 (47.6) | 13 (65.0) | 23 (56.1) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Temporary stoma | |||
| No | 19 (90.5) | 16 (80.0) | 35 (85.4) |
| Yes | 2 (9.5) | 4 (20.0) | 6 (14.6) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Permanent stoma | |||
| No | 19 (90.5) | 15 (75.0) | 34 (82.9) |
| Yes | 2 (9.5) | 5 (25.0) | 7 (17.1) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
Table 31 shows that more participants allocated to the intervention group than to the control group had laparoscopic surgery [n = 6 (28.8%) vs. n = 3 (15%)], whereas more participants allocated to the control group than to the intervention group had open surgery [n = 13 (65%) vs. n = 10 (47.6%)]. Similarly, Table 31 shows differences between the two groups for stoma; four (19%) participants allocated to the intervention group had a temporary stoma or permanent stoma, whereas nine (45%) participants allocated to the control group had a temporary stoma or permanent stoma.
Treatments
Table 32 shows differences between participants allocated to the intervention and control groups who had chemotherapy or radiotherapy; for example, five (23.8%) and two (10%) participants allocated to the intervention and controls, respectively, had chemotherapy. However, it is difficult to make direct comparisons between the two groups because of missing data; for instance, there were missing data about chemotherapy for seven (17.1%) participants.
| Variable | Intervention, n (%) | Control, n (%) | Total, N (%) |
|---|---|---|---|
| Chemotherapy | |||
| Missing | 2 (9.5) | 5 (25.0) | 7 (17.1) |
| No | 14 (66.7) | 13 (65.0) | 27 (65.9) |
| Yes | 5 (23.8) | 2 (10.0) | 7 (17.1) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Radiotherapy | |||
| Missing | 3 (14.3) | 4 (20.0) | 7 (17.1) |
| No | 15 (71.4) | 14 (70.0) | 29 (70.7) |
| Yes | 3 (14.3) | 2 (10.0) | 5 (12.2) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
| Other treatment | |||
| Missing | 2 (9.5) | 1 (5.0) | 3 (7.3) |
| No | 17 (81.0) | 18 (90.0) | 35 (85.4) |
| Yes | 2 (9.5) | 1 (5.0) | 3 (7.3) |
| Total | 21 (100.0) | 20 (100.0) | 41 (100.0) |
Baseline physical activity self-efficacy and risk perception
A higher score indicates a higher level of physical activity self-efficacy (range 0–120). Table 33 shows that physical activity self-efficacy was high and skewed towards higher scores; at baseline the mean physical activity self-efficacy was 94.1 and 93.8 for intervention group and control group participants, respectively. A higher cognitive and affective risk perception score (range 0–10) indicates a greater perception that lifestyle behaviours have a protective health effect and a higher severity risk perception score (range 0–10) indicates a greater perception that CRC is a more serious disease. Risk perception scores were skewed towards higher scores. Table 33 shows little variation between the intervention and control groups; the table shows a mean cognitive risk perception score of 7.7 and 6.8, a mean affective risk perception score of 7.7 and 7.2 and a mean severity risk perception score of 6.1 and 5.6 at baseline for intervention and control group participants, respectively.
| Variable | Intervention | Control | Total |
|---|---|---|---|
| Physical activity self-efficacy total score | |||
| n | 19 | 18 | 37 |
| Missing | 1 | 2 | 3 |
| Mean | 94.1 | 93.8 | 93.9 |
| SD | 19.43 | 20.30 | 19.58 |
| 95% CI | 84.71 to 103.44 | 83.68 to 103.86 | 87.40 to 100.45 |
| Median | 93.8 | 99.5 | 95.0 |
| Cognitive risk perception | |||
| n | 20 | 20 | 40 |
| Missing | 0 | 0 | 0 |
| Mean | 7.7 | 6.8 | 7.2 |
| SD | 1.39 | 2.12 | 1.82 |
| 95% CI | 7.00 to 8.30 | 5.81 to 7.79 | 6.64 to 7.81 |
| Median | 8.0 | 7.0 | 8.0 |
| Affective risk perception | |||
| n | 20 | 20 | 40 |
| Missing | 0 | 0 | 0 |
| Mean | 7.7 | 7.2 | 7.4 |
| SD | 1.27 | 1.96 | 1.65 |
| 95% CI | 7.06 to 8.24 | 6.28 to 8.12 | 6.90 to 7.95 |
| Median | 8.0 | 8.0 | 8.0 |
| Perceived severity score | |||
| n | 20 | 20 | 40 |
| Missing | 0 | 0 | 0 |
| Mean | 6.1 | 5.6 | 5.9 |
| SD | 1.07 | 1.70 | 1.42 |
| 95% CI | 5.60 to 6.60 | 4.81 to 6.39 | 5.39 to 6.31 |
| Median | 6.0 | 6.0 | 6.0 |
Physical activity and sedentary behaviour
As discussed in Chapter 1, results about the effects of an intervention measured in a pilot study should not be reported at all or reported only with great caution. As highlighted above, the completion rates for accelerometer measurement were low. We therefore present descriptive data only for the primary outcome, physical activity, and for sedentary behaviour; descriptive data for quality of life are presented in Appendix 12. Inferential statistical analyses are not reported.
Moderate to vigorous physical activity
Per-day physical activity scores were calculated by dividing the total score by number of days worn. Table 34 shows that participants were meeting or close to meeting the recommended level for moderate to vigorous physical activity per day (i.e. 30 minutes of moderate physical activity per day). Mean moderate to vigorous physical activity per day scores were skewed towards lower scores at baseline but normally distributed at 3 months’ follow-up, indicating that those participants doing less physical activity at baseline increased their level of physical activity over time.
| Variable | Intervention | Control | Total |
|---|---|---|---|
| Baseline | |||
| n | 14 | 14 | 28 |
| Missing | 0 | 0 | 0 |
| Mean | 21.1 | 29.0 | 25.1 |
| SD | 11.68 | 35.90 | 26.50 |
| 95% CI | 14.40 to 27.89 | 8.27 to 49.72 | 14.80 to 35.34 |
| Median | 20.6 | 10.5 | 17.8 |
| End of intervention | |||
| n | 11 | 12 | 23 |
| Missing | 0 | 0 | 0 |
| Mean | 25.8 | 36.7 | 31.5 |
| SD | 17.57 | 39.00 | 30.52 |
| 95% CI | 14.00 to 37.61 | 11.90 to 61.46 | 18.28 to 44.68 |
| Median | 27.9 | 18.8 | 26.8 |
| End of intervention – baseline | |||
| n | 11 | 12 | 23 |
| Missing | 0 | 0 | 0 |
| Mean | 5.6 | 5.9 | 5.8 |
| SD | 12.35 | 17.21 | 14.75 |
| 95% CI | –2.69 to 13.91 | –5.03 to 16.84 | –0.62 to 12.14 |
| Median | 0.6 | 3.0 | 2.8 |
| 3 months’ follow-up | |||
| n | 6 | 8 | 14 |
| Missing | 0 | 0 | 0 |
| Mean | 22.5 | 54.5 | 40.8 |
| SD | 17.41 | 28.34 | 28.63 |
| 95% CI | 4.27 to 40.81 | 30.85 to 78.24 | 24.30 to 57.36 |
| Median | 23.9 | 56.3 | 35.6 |
| 3 months’ follow-up – baseline | |||
| n | 6 | 7 | 13 |
| Missing | 0 | 0 | 0 |
| Mean | 1.3 | 10.5 | 6.2 |
| SD | 15.04 | 28.37 | 22.79 |
| 95% CI | –14.51 to 17.06 | –15.74 to 36.73 | –7.53 to 20.01 |
| Median | 0.3 | 7.7 | 4.2 |
Sedentary behaviour
Per-day sedentary behaviour scores were calculated by dividing the total sedentary behaviour score by the number of days worn. Table 35 shows at baseline and follow-up that mean minutes of sedentary time per day for intervention and control groups was similar, normally distributed and decreased slightly over time. Change in total sedentary time per day between baseline and 3 months’ follow-up did not correlate with any demographic (e.g. age or gender), clinical (e.g. chemotherapy, type and location of surgery or stoma) or psychological (e.g. self-efficacy or risk perception) variables.
| Variable | Intervention | Control | Total |
|---|---|---|---|
| Baseline | |||
| n | 14 | 14 | 28 |
| Missing | 0 | 0 | 0 |
| Mean | 566.6 | 579.7 | 573.1 |
| SD | 96.83 | 80.12 | 87.46 |
| 95% CI | 510.68 to 622.50 | 533.42 to 625.94 | 539.22 to 607.05 |
| Median | 542.9 | 564.7 | 546.1 |
| End of intervention | |||
| n | 11 | 12 | 23 |
| Missing | 0 | 0 | 0 |
| Mean | 583.7 | 521.9 | 551.5 |
| SD | 127.75 | 99.58 | 115.63 |
| 95% CI | 497.86 to 669.51 | 458.65 to 585.19 | 501.46 to 601.46 |
| Median | 582.3 | 549.7 | 551.0 |
| End of intervention – baseline | |||
| n | 11 | 12 | 23 |
| Missing | 0 | 0 | 0 |
| Mean | 14.5 | –50.8 | –19.6 |
| SD | 119.50 | 81.85 | 104.66 |
| 95% CI | –65.78 to 94.78 | –102.80 to 1.21 | –64.82 to 25.69 |
| Median | 44.4 | –73.3 | –25.4 |
| 3 months’ follow-up | |||
| n | 6 | 8 | 14 |
| Missing | 0 | 0 | 0 |
| Mean | 513.4 | 479.0 | 493.7 |
| SD | 121.60 | 96.93 | 105.16 |
| 95% CI | 385.76 to 640.99 | 397.95 to 560.02 | 433.01 to 554.44 |
| Median | 513.5 | 520.6 | 520.6 |
| 3 months’ follow-up – baseline | |||
| n | 6 | 7 | 13 |
| Missing | 0 | 0 | 0 |
| Mean | –54.7 | –73.1 | –64.6 |
| SD | 184.66 | 86.49 | 134.31 |
| 95% CI | –248.49 to 139.08 | –153.10 to 6.88 | –145.78 to 16.55 |
| Median | –7.7 | –92.6 | –49.7 |
Type of physical activity
Table 36 shows that walking was the most common type of physical activity at baseline, followed by housework.
| Group | Walking | Manual labour | Active housework | Dancing | Sport | Other |
|---|---|---|---|---|---|---|
| Intervention | 357 | 142.5 | 244 | 0.25 | 23.7 | 79 |
| Control | 337 | 73 | 114 | 6 | 44 | 6 |
Intervention fidelity
All CRC participants allocated to the intervention group reported that they had not received any additional educational sessions about cancer or lifestyle advice from CRC nurse specialists during the intervention.
Chapter 8 Phase 2 discussion
In this chapter, a summary and a discussion of phase 2 results are presented.
Summary
Phase 2 shows that some of the key trial procedures were feasible and acceptable. The evidence for this is as follows:
-
Colorectal cancer nurses screened 79% (n = 156) of all surgical CRC patients for eligibility.
-
Sixty-seven per cent (133 out of 198) of all patient admissions for CRC surgery met eligibility criteria.
-
Fifty-six per cent (74 out of 133) of eligible patients signed a screening and recruitment form indicating their willingness to participate in the study and to have their contact details forwarded to an investigator; 31% (41) were consented and randomised to the intervention or control groups. This was short of our target of 66 patients.
-
A good cross-section of people with CRC were recruited. Men (n = 27; 65.9%) and women from the ages of 42 to 86 years (mean 66 years, SD 11.31 years), diagnosed with rectal (n = 16; 39%) or colon cancer (n = 25; 61%), having open surgery (n = 23, 56.1%) or laparoscopic surgery (n = 9; 22%), with (n = 13; 32%) and without a stoma, participated in the study.
-
Only 7% (2 out of 41) of participants formally dropped out of the study.
-
Forty (97.5%) out of 41 participants (20 intervention and 20 control) completed the questionnaires (SPAQ, FACT-C, EQ-5D, FACIT Fatigue Scale and HADS) at baseline, and 31 (75.6%) (15 intervention and 16 control) and 25 (61%) (12 intervention and 13 control) completed the questionnaires at follow-up 1 and follow-up 2 time points, respectively.
-
No SAEs were reported.
-
There was an insignificant difference in the number of days between data collection time points for participants in the intervention and control groups, and the mean numbers of days in the study were 182 and 169, respectively.
Phase 2 also shows potential threats to the internal and external validity of any future trial. In this chapter, we therefore make a number of recommendations to manage these threats. The evidence for potential risks to a future trial is as follows:
-
Thirty-three out of 74 (44.5%) of eligible participants who signed a screening and recruitment form indicating willingness to participate in the study withdrew before randomisation. The main barrier to going on to actually participate in the study was poor health (e.g. poor recovery from surgery).
-
Thirteen out of 21 participants (62%) who were randomised to the intervention group completed the programme. The main barrier to starting cardiac rehabilitation or stopping once starting to attend was poor physical health.
-
Completion rates for accelerometer devices decreased over time. Sixty-eight per cent (n = 28) of participants provided validated accelerometer data to measure physical activity and sedentary behaviour at baseline, and 56% (n = 23) and 34% (n = 14) provided validated accelerometer data at follow-up 1 and follow-up 2 time points, respectively. Across all time points, 31% (n = 20) of accelerometer data sets were assessed as invalid. The main reason for invalidity was that the device was not worn (35%).
-
Information about diagnosis (e.g. tumour size, lymph nodes and metastases) was not available for all participants, thereby making it difficult to compare diagnosis between intervention and control groups.
-
There was recruitment bias; at baseline, participants were meeting or nearly meeting recommended levels for physical activity (i.e. 30 minutes of moderate physical activity per day), had good self-reported quality of life and low levels of fatigue and low anxiety and depression, high physical activity self-efficacy and risk perception.
-
Analyses suggest no intervention effect on outcomes, but given that the study was not designed to evaluate health outcomes, had a small sample size and poor completion rate for the primary outcome (i.e. physical activity), this finding is not reliable.
-
Intervention fidelity was compromised because no additional cancer-related education sessions were provided for CRC participants attending cardiac rehabilitation.
Strengths and limitations of phase 2
People with CRC who agreed to participate in this study may be particularly keen to increase their level of physical activity, which means that the findings from CRIB may not be applicable to people with CRC who are likely to be less interested in being physically active to aid their recovery and reduce risk of recurrence. In addition, this pilot trial was small scale, recruiting patients with CRC from only three UK hospitals. Nonetheless, this is the first time that an already existing rehabilitation service (i.e. cardiac rehabilitation) has been pilot tested for people with cancer. In this respect, these findings are novel and can be used to inform future research directions. Importantly, phase 2 highlights ways in which trial procedures can be improved for a future large-scale trial to measure effectiveness.
Key trial parameters
In this chapter we discuss the CRIB study in relation to similar studies. Thus, Table 37 compares key trial parameters of physical activity interventions for people with CRC. Although we make comparisons between studies, CRIB was the only pragmatic trial. We tested an existing NHS service (i.e. cardiac rehabilitation) in real-world settings, whereas all of the other trials were experimental, with an intervention specifically designed and controlled by the research team. Studies use different definitions to report rates and so we applied the following definitions and calculations for the purposes of comparison:
-
Design of the study was defined as a RCT, a non-randomised trial or a before-and-after study of the intervention.
-
Mode of the intervention was defined as exercise classes, exercise counselling (telephone or face to face) or home-based exercise prescription (i.e. participants are given physical activity goals at the start of the intervention and expected to meet these goals without any or with minimal contact with an instructor).
-
Registered patients referred to the pool of people with CRC that potentially eligible participants were identified from through screening (e.g. in CRIB this was the number of people with CRC admitted to hospital for surgery).
-
Assessed for eligibility was defined as the number of people with CRC who were assessed for eligibility using study inclusion/exclusion criteria.
-
Eligibility rate was calculated by dividing the number of people with CRC who were assessed for eligibility by the number who met inclusion criteria (note this is different from how we defined eligibility rate in Chapter 7, which was calculated by dividing the number of people with CRC admitted for surgery by the number who met eligibility criteria). The calculation was changed for Table 37 because so few studies report the number of patients potentially eligible; instead, they report the number screened for eligibility, which is generally a considerably smaller number. Making the change allowed us to compare more studies.
-
Consent rate was calculated by dividing the number of people with CRC who met inclusion criteria (i.e. eligible) by the number who consented to participate in the study.
-
Completion rate was calculated by dividing the number of participants who had consented to participate in the study by the number of participants who completed outcome measures at different time-points (e.g. baseline and follow-ups).
-
Missing data were defined as the number of participants not entered into analyses because of invalid accelerometer data.
-
Intervention adherence was calculated by dividing the number of planned physical activity sessions/consultations by the number actually attended.
| Study and year | Design | Mode | Registered patients | Assessed for eligibility (% of registered patients) | Eligibility rate (% of those assessed for eligibility) | Consent rate (% n eligible) | Completion rate (baseline and first follow-up unless the study included a second or third follow-up) |
|---|---|---|---|---|---|---|---|
| CRIB | RCT | Exercise classes | 198 | 156 (79) | 133 (85) | 41 (31) Randomisation 21 intervention, 20 control |
Baseline 40 (97.5%) questionnaires (20 intervention, 20 control) 28 (68%) accelerometer (14 intervention, 14 control) Follow-up 1 31 (75.6%) questionnaires (15 intervention, 16 control) 23 (56%) accelerometer (11 intervention, 12 control) Follow-up 2 25 (61%) questionnaires (12 intervention, 13 control) 14 (34%) accelerometer (six intervention, eight control) |
| Allgayer et al. 2004142 | RCT | Exercise classes | – | – | – | 23 Randomisation 13 moderate intensity, 10 low intensity |
23 (100%) |
| Allgayer et al. 2008143 | RCT | Exercise classes | – | 90 | 49 (54) | 48 (98) Randomisation 29 intervention group and 19 in control group |
44 (92%) 27 high-intensity (note some switched groups) and 17 moderate-intensity group |
| Anderson et al. 2010144 | Single-arm trial | Counselling | – | 37 | 28 (76) | 20 (71) | 18 (90%) |
| Bourke et al. 201148 | RCT | Exercise classes | – | – | 180 | 18 (10) Randomisation Nine intervention, nine control group |
17 (94%) Eight intervention group (one loss), nine control group |
| Courneya et al. 200344 | RCT | Home-based exercise prescription | – | 366 | 295 (81) | 102 (35) Randomisation 69 intervention, 33 control group |
102 (91.2%) 62 intervention group (seven losses), 31 (two losses) control group |
| Grimmett et al. 2015145 | Single-arm trial | Counselling | – | 80 | – | 29 (36) | 23 (79%) |
| Hawkes et al. 2013146 | RCT | Telephone-delivered health coaching | – | 1141 | 523 (46) | 410 (78) Randomisation 205 intervention, 205 control |
Follow-up 1 347 (85%) (171 intervention, 176 control) Follow-up 2 322 (78.5%) (159 intervention, 163 control) |
| Lee et al. 2013147 | RCT | Home-based exercise prescription | 366 | 366 (100) | 186 (51) | 23 (12) Randomisation 12 intense, 11 less intense exercise group |
19 (82.6%) 10 intense exercise group, nine less intense exercise group (two losses from each group) |
| Lin et al. 2014148 | Two-arm trial | Exercise classes | – | 229 | 137 (60) | 45 (33) Randomisation 21 exercise, 24 control group |
39 (87%) One loss exercise group, five losses control group |
| Pinto et al. 201349 | RCT | Counselling | 315 | 315 (100) | 66 (18) | 46 (70) Randomisation 20 exercise group, 26 control group |
First follow-up Exercise group 19 (95%), control group 24 (92%) Second follow-up Exercise group 19 (95%), control group 23 (88%) Third follow-up Exercise group 19 (95%), control group 23 (88%) |
| Sellar et al. 2013149 | Single-arm trial | Exercise classes | – | 888 | 351 (39) | 29 (8) | 28 (93%) |
| Spence et al. 2011150 | Single-arm trial | Exercise classes | – | – | 10 | – | – |
Eligibility rate
The proportion of people with CRC who were assessed and found eligible in CRIB was 85% and the proportion of people with CRC who were found eligible from the total number of registered patients was 67%. As Table 37 shows, these figures compare favourably with other studies.
The studies reported in Table 37 have different inclusion and exclusion criteria, which will obviously influence the proportion of assessed participants who are judged as eligible to participate in a study. The ways in which these criteria are likely to impact eligibility rates and also likely to influence study bias and generalisability of results are discussed below.
Table 38 shows that 6 (including CRIB) out of 13 studies specified that only people aged ≥ 18 years were eligible;49,145,146,148 two studies excluded people who were aged > 75 years143,150 and another excluded people who were aged > 65 years. 142 Given that it is very rare for young people to have a CRC diagnosis, we can confidently assume that a lower age criterion does not have a decisive influence on eligibility rates. Nine studies (including CRIB) included language restriction as a criterion,44,49,142,143,145,146,149,150 suggesting a bias towards people who speak a country’s first language and thereby potentially limiting generalisability of the study findings to other groups of the population. In CRIB, no patients with CRC were excluded because they were unable to speak English, suggesting that this criterion did not have any bearing on the eligibility rate. However, language restriction may impact on eligibility rates in any future multicentred trials that are likely to include areas in which some people do not have English as their first language.
| Study and year | Age | CRC diagnosis | Time | Already physically active | Language | Contraindications for physical activity | Other |
|---|---|---|---|---|---|---|---|
| CRIB | ≥ 18 years | Primary CRC; excluded if advanced disease, included if metastasis curable | Not excluded if having AT | Excluded if unable to speak English | Excluded if failed clinical/risk assessment for rehabilitation and were deemed unsafe to participate in exercise classes according to recent guidelines | Excluded if cognitive impairment and unable to give informed consent | |
| Allgayer et al. 2004142 | Aged > 65 years excluded | Stage II or III | At least 4 weeks since surgery and radiation/chemotherapy completed | Excluded if a language barrier | Karnofsky index < 85.0%, serious comorbidity such as cardiovascular, lung, liver or metabolic diseases excluded and severe degenerative/inflammatory joint disease C-reactive protein (> 1 mg/ml) excluded | Smokers, and alcohol consumption (> 40.0 g per day) | |
| Allgayer et al. 2008143 | Aged > 75 years excluded | Excluded if metastasis | Patients with colorectal carcinoma after surgery and with completed radiation/chemotherapy (6–40 weeks) | Excluded if a language barrier | Karnofsky score < 80.0%, serious comorbidity such as heart, lung, liver disease, diabetes, acute infections, faecal incontinence, severe degenerative/inflammatory joint disease | Severe alcohol consumption | |
| Anderson et al. 2010144 | Up to 11 months post surgery and if completed curative treatment | Body mass index of > 25 kg/m2 | |||||
| Bourke et al. 201148 | Colon cancer (Dukes’ stages A–C) | Resected 6–24 months previously | Excluded if already physically active | Excluded if poor functional ability, unstable angina, uncontrolled hypertension, recent MI, or a pacemaker | |||
| Courneya et al. 200344 | Surgery within past 3 months | Only included if ready to engage in physical activity | Included if able to provide informed consent in English | No contraindications to exercise as determined by a submaximal cardiorespiratory fitness test | |||
| Grimmett et al. 2015145 | ≥ 18 years | Excluded if metastasis | Diagnosis within the last 6 months | Excluded if unable to speak English | Included if adequate mobility and no contraindications for unsupervised physical activity (e.g. without major health problems or subtotal or total colectomy or ileostomy) | ||
| Hawkes et al. 2013146 | ≥ 18 years | Primary diagnosis of CRC within previous 12 months; excluded if metastatic disease | Excluded if unable to speak and read English | Excluded if medical conditions limiting adherence to an unsupervised physical activity programme (as confirmed by their referring physician) | Included if access to a telephone | ||
| Lee et al. 2013147 | Stage II–III colon or rectal cancer | Excluded if having chemotherapy | Excluded if any condition unsuitable for participation in the study; ECOG performance status of 0 or 1; planned surgery anticipated during the 12-week intervention; pregnant or planned to be pregnant within 6 months | ||||
| Lin et al. 2014148 | ≥ 18 years | CRC (stages IIY– III), excluded if metastasis | Only included if having adjuvant chemotherapy | Excluded if physical/psychiatric impairments that would seriously impair physical mobility, known contraindications for exercise | |||
| Pinto et al. 201349 | ≥ 18 years | Colon or rectal cancer (stages I–III); excluded if prior history of cancer | ≤ 5 years since treatment completion | Excluded if already physically active | Excluded if unable to speak and read English | Excluded if unable to walk unassisted; medical or current psychiatric illness (e.g. orthopaedic problems) that could make compliance with the study protocol difficult or unsafe Patients with cardiovascular disease and/or diabetes were included if their treating physicians approved of their study participation |
Included if access to a telephone |
| Sellar et al. 2013149 | Stage II or III CRC (local disease only treated by surgery with or without adjuvant therapy) | ≥ 1 year since completion of AT | Exclude if unable to understand English | Any absolute contraindication to exercise testing or training; any other uncontrolled medical or psychiatric illness that would present completion of the exercise programme or interfere in study assessments; pass pre-screening assessments | |||
| Spence et al. 2011150 | 18–75 years | Stage I–III CRC treated with surgery followed by adjuvant chemotherapy. Excluded if metastatic or incurable cancer | Completed adjuvant chemotherapy within previous 4 weeks | Exclude if unable to read/write in English | Excluded if physical/psychiatric impairments that would seriously impair physical mobility; and known contraindications to exercise (assessed by pre-screening questionnaire) | Excluded if current smoker |
One inclusion criterion that is likely to influence generalisability of study results is level of physical activity. As Table 38 shows, two studies were specifically designed for people who were not currently physically active,48,49 whereas other studies did not apply physical activity behaviour as part of inclusion/exclusion criteria. Thus, most studies may have included people who were physically active, although as we highlighted in Chapter 1, evidence suggests that most people with CRC are unlikely to be meeting recommended levels for physical activity, which makes it probable that most participants in these studies were not physically active when they started the trial. Nonetheless, our baseline data show that most participants were already meeting or nearly meeting recommended levels for physical activity, suggesting that CRIB attracted people who are already physically active and therefore less in need of an intervention designed to increase people’s level of physical activity associated with health benefits (i.e. 30 minutes per day of moderate physical activity).
Another inclusion criterion that is likely to influence generalisability of study results is diagnostic inclusion criteria, which varied across studies. As Table 38 shows, CRIB excluded people with advanced disease but, as we discussed in Chapter 5, this is an ambiguous term. CRIB did not exclude people with CRC who had metastatic disease. Six studies specifically mentioned that people with metastatic disease would be excluded or only people with local disease would be included. 49,143,145,146,148–150 Thus, there appears to be a bias towards people with early-stage disease, thereby limiting generalisability of findings to people with metastatic disease.
Most studies included only people who had completed active treatment. Only one study was specifically designed for people who were on active treatment (i.e. receiving adjuvant chemotherapy);148 one study (CRIB) included people on or off active treatment, seven studies made it clear that people on active treatment would be excluded,49,142–144,147,149,150 and it was not clear in four studies if people on active treatment were included or not. 44,144–146 We are aware of only one current physical activity intervention trial for people with CRC on active treatment. 151 Thus, there appears to be a bias towards people who have completed active treatment. Our study suggests that there may well be good reason for only including people with CRC post treatment; one of the main reasons why participants who were interested in participating changed their minds was because they felt unable to partake in an exercise class while having chemotherapy. Thus, for practical reasons alone, a post-treatment trial may be more feasible.
Virtually all studies had exclusion criteria for contraindications for physical activity, which was the most common reason for exclusion. As shown in Chapter 7, most people with CRC who were excluded from CRIB fell into this category because they had a medical problem (46%). Moreover, as described in Chapters 4 and 9, CRC nurses were not approaching some people with CRC about the study because they believed that they were unable to attend, or would not be interested in attending, cardiac rehabilitation owing to poor health. The CHALLENGE trial has recently reported that staff did not approach people who ‘do not look like an exerciser’. 152 Similarly, most people who were excluded from a study conducted by Courneya et al. 44 fell into the clinical category (55% excluded owing to a medical condition). It is likely, therefore, that this particular exclusion criterion explains why most people are excluded from physical activity trials.
This criterion, therefore, ought to be clearly defined and explained to the clinicians and investigators involved in screening people with CRC for eligibility so that they know what the contraindications for physical activity are and can apply criteria competently. Training of recruiters is therefore required. Our study aimed to include people with a stoma, on active treatment and with a cardiac condition, even though these are known potential, albeit not automatic, contraindications for physical activity. Moreover, cardiac rehabilitation does accommodate people with poor mobility, wheelchair users and those who are very frail, and therefore we also did not set out to exclude people with these difficulties. If this exclusion criterion were to be applied too stringently, then people with CRC who can potentially benefit from the physical activity intervention would be excluded. As we showed in Chapter 7, our study suggests that cancer care nurses who screened patients were excluding people with poor mobility, suggesting that cancer care nurses may require additional information about the ways in which cardiac rehabilitation accommodates people who face mobility difficulties.
Excluding people from studies, however, is not inherently incorrect; what is important is that it is made clear which subgroups of the sample population are included and excluded and that reasons are given. To improve reporting of research and the ability to make reasonable judgements about the relevance of particular physical activity interventions for subgroups of the sample population, we recommend the development of standardised inclusion and exclusion criteria. Table 39 presents potential minimum inclusion and exclusion criteria for future trials of physical activity interventions for people diagnosed with CRC.
| Criterion | Description |
|---|---|
| Age | Indicate age range for inclusion |
| Time | Provide a time frame for inclusion. Consider the following times as reference points: date of diagnosis, date of surgery, date of active treatment completion |
| Diagnosis | Recommend use of AJCC system |
| Treatment | Make it explicit if people on active treatment (e.g. adjuvant chemotherapy) are included |
| Physical activity |
|
| Contraindications | Recommend use of American College of Sports Medicine exercise guidelines for CRC survivors.153 The following contraindications may be a reason for exclusion or may be used to guide exercise modifications (e.g. reduced impact, intensity, volume) and hence it should be made explicit if these subgroups of the sample population are included or excluded:
|
Consent rate
Table 37 shows that our study consented 31% of all eligible patients, which is slightly lower than an average of 40% (range 8–98%). It is also lower than our estimated target of 66 patients across the three sites. However, if 31% is a proxy for the number of people with CRC likely to take up the offer of cardiac rehabilitation, should this service be established as part of routine cancer care, then attendance of people with CRC would be 12% lower than the number of patients with CHD who attended phase 3 cardiac rehabilitation in 2011–12 (43%). 62 Given that cardiac rehabilitation for people with CHD is a well-established service that has been audited by the British Heart Foundation since 2004, a rate of 31% engenders optimism that uptake among people with CRC would eventually match attendance rates among people with CHD. Nevertheless, we acknowledge that consent rates for research and for an actual service are not directly comparable.
Table 37 suggests that consent rates are unrelated to research design or intervention mode. Not all studies included reasons why eligible participants declined to participate in a study, but, of those that did, ‘medical conditions’ and ‘not interested’ were the most common reasons given for eligible participants not consenting. Table 40 shows the reasons why eligible participants did not consent in those studies that provided these data.
| Reason | CRIB | Lin et al. 2014148 | Lee et al. 2013147 | Pinto et al. 201349 | Courneya et al. 200344 | Sellar et al. 2013149 | Hawkes et al. 2013146 |
|---|---|---|---|---|---|---|---|
| No response | 3 | 5 | 98 | 15 | 5 | 13 | |
| Medical condition | 28 | 26 | 25 | 28 | |||
| Adjuvant therapy | 18 | ||||||
| Travel | 6 | 9.5 | 15 | 7 | |||
| Not interested | 50 | 20 | 29.5 | 21 | 74 | ||
| Too busy | 6 | 9.5 | 1 | 25 | 17.5 | 16 | |
| Already active/returned to normal activities | 9 | 0.5 | 0.5 | 5 | 7 | ||
| No reason/missing | 18 | 0.5 | 0.5 | 5 | 3 | ||
| Other | 5 | 2 | 5 | 3 | |||
| Eligible but exceeded capacity/study time limit | 9 | 44 | |||||
| Deceased | 3 | 10 |
Based on our study and those of others, we anticipate that one of the main barriers to participation in physical activity interventions is a person’s health. In our study, for instance, some eligible participants were unwilling to participate because they were receiving adjuvant chemotherapy and did not perceive that they would feel well enough to attend an exercise class. Some of the health difficulties that people with CRC experience that may impede involvement in a physical activity intervention are discussed further in the next chapter, which reports and discusses the findings from our qualitative study.
Of note, many of the barriers that we found are not dissimilar to reasons why people with CHD do not attend cardiac rehabilitation. A recent audit of UK cardiac rehabilitation, for instance, found that 33% did not attend because they were not interested, 7% did not attend because of travel difficulties, 9% did not attend because of physical incapacity and 3% did not attend because they were too ill. 62 Addressing some of the barriers to people attending rehabilitation will, therefore, be relevant to people with CHD as well as people with CRC.
Retention, completion rates and missing data
Loss of consenting participants to a study may be due to a combination of factors, including participants formally dropping out of the study (retention rate), failing to complete outcome measures (completion rate) or failing to provide valid data (missing data). Loss of participants during trial follow-up can introduce bias and reduce power, affecting the generalisability, validity and reliability of results. 154 Thus, information about retention and completion rates and missing data is important for assessing bias. 155 It has been estimated that a 20% loss can threaten trial validity. 154 Some missing data can be dealt with statistically; nevertheless, the risk of bias due to missing data can remain156 and, therefore, should be reported alongside other rates.
Table 37 shows completion rates for CRIB and other studies. Our completion rate was slightly below average in comparison with other studies. The ongoing CHALLENGE trial has also experienced losses to the study;152 at first follow-up, 189 out of 250 (75%) randomised participants completed outcomes measures, and at second follow-up it was 141 out of 250 (56%) randomised participants. Our completion rate for patient self-reported questionnaires (i.e. most studies used self-report to measure outcomes) at first follow-up was 31 out of 41 randomised participants (75.6%), whereas other studies’ completion rates ranged between 79% and 95%. Our completion rate at second follow-up was 25 out of 41 (61%). This difference may be a reflection of differences between pragmatic and explanatory trials. CRIB was a pragmatic trial of an already existing service (i.e. cardiac rehabilitation), whereas all of the others were explanatory trials. In CRIB the intervention was independent of the actual study and it may be that participants were committed to the service (evidenced by high intervention adherence) but not to the actual study. Any future trial of CRIB should, therefore, introduce strategies to improve completion rates. A 2014 systematic review157 of 38 randomised retention trials evaluating six broad types of strategies to increase questionnaire response and retention in randomised trials concluded that no strategy had a clear impact on increasing the number of participants returning to sites for follow-up but found that the following strategies may improve questionnaire response: addition of monetary incentives for return of postal questionnaires, recorded delivery of questionnaires, and a ‘package’ of postal communication strategies with reminder letters. 157
Accelerometer validation
Our primary outcome was physical activity. Objective measures of physical activity and sedentary behaviour have been increasingly used to overcome limitations of self-report measures. Research conducted among the general population suggests that self-reported measures of physical activity and sedentary behaviour are inaccurate when compared with objective measurement from devices such as accelerometers. 158–160 A 2014 study comparing accelerometer-based and self-reported measures of recent moderate- to vigorous-intensity physical activity (MVPA) and sedentary time in colon cancer survivors found that total mean minutes per day spent in MVPA was 12 minutes based on accelerometer data and 26 minutes based on self-reported data (p < 0.01). 97 Correlation between the methods was fair (Spearman’s rank-order correlation = 0.51); however, agreement was poor [intraclass correlation coefficient (ICC) = 0.33]. Mean daily time spent sedentary was similar in both methods (≈ 8.5 hours); however, both correlation and agreement were poor (Spearman’s rank-order correlation = 0.19, ICC = 0.16). Pinto et al. 49 also found poor to fair agreement between self-reported and accelerometer-derived physical activity. Our research used objective (accelerometer) and self-reported measures of physical activity and also found poor agreement between the two measures.
Despite the advantages of obtaining an objective measure of physical activity and sedentary behaviour, there are few guidelines for using accelerometers in research161,162 and little guidance on improving participant compliance. 147 Our study shows that 31% of accelerometer data sets were invalid, mainly because participants did not wear the device. Some of the challenges of using accelerometers in research involving people with CRC are described in Chapter 9 and include difficulties wearing the device around the waist after abdominal surgery. Recommended approaches for improving compliance include a daily monitoring log filled out by participants, reminder telephone calls, adequate education about the monitor and its proper wear, and the identification of potential barriers to wearing with each participant. 163
Intervention adherence
Intervention adherence refers to the extent to which participants randomised to the intervention group follow specific treatment therapy instruction as per intervention protocol. Low adherence increases the risk of policy and service commissioners rejecting physical activity interventions that may actually be effective should compliance levels be high. Addressing the problem of adherence is, therefore, important. As reported in Chapter 7, our study shows that 62% of participants randomised to the intervention group completed cardiac rehabilitation and that the main reason for either not starting cardiac rehabilitation or stopping it was poor physical health. Table 41 shows adherence rates of physical activity interventions for people with CRC. It is difficult to draw direct comparisons because studies use different ways of measuring intervention adherence. Nonetheless, CRIB compares favourably with most studies that use exercise classes as the intervention mode.
| Study | Mode | Intervention adherence |
|---|---|---|
| CRIB | Exercise classes | 13 (62%) completed cardiac rehabilitation; attendance was 75–142% |
| Anderson et al. 2010144 | Counselling | 18 (90%) had three home visits |
| Bourke et al. 201148 | Exercise classes | 90% attendance of sessions |
| Courneya et al. 200344 | Home-based exercise prescription | 76% of exercise group met physical activity target, compared with 46% who met the intervention target in control group |
| Grimmett et al. 2015145 | Counselling | 18 (96%) completed all telephone consultations |
| Hawkes et al. 2013146 | Telephone-delivered health coaching | 81.4% of participants received at least 6 of 11 telephone sessions, whereas 77.3% received at least 8 sessions and 72.2% received all 11 telephone sessions. The median number of sessions was 10 (range 1–11), and median call length for all calls was 31.5 minutes (range 13.3–59.7 minutes) |
| Lee et al. 2013147 | Home-based exercise prescription | 19 (82.6%) completed intervention |
| Lin et al. 2014148 | Exercise classes | 73% of exercise sessions; 52.4% attending at least 75% attendance sessions |
| Pinto et al. 201349 | Counselling | A mean of 11.42 calls (SD = 1.39 calls) were delivered to the intervention group (maximum 12 calls) |
| Sellar et al. 2013149 | Exercise classes | Nine (31%) completed 100% of sessions; 20 (69%) completed ≥ 98% of sessions; 27 (93%) completed ≥ 80% of sessions |
Sample size calculation for a definitive randomised controlled trial
This study did not set out to measure the effectiveness of the intervention; rather, it was conducted to find out if cardiac rehabilitation is a feasible and acceptable rehabilitation service for people with CRC and to gather information to hone trial procedures for a future effectiveness trial. The feasibility work has provided process information that can inform a future trial but the effect data are insufficient to support a robust sample size calculation for a future definitive RCT. This is due to a small number of eligible participants being recruited (n = 41), decreasing completion rates at follow-up (61% at final follow-up), missing data (31%) and a recruitment bias of physically active and healthy participants.
Recommendations for improving trial procedures for a future effectiveness trial
Phase 2 suggests that it is feasible to conduct a definitive trial. Nonetheless, based on the results of phase 2, Table 42 presents proposed recommended changes to main trial components and their estimated impact.
| Parameter | Recommendations | Rationale | Impact |
|---|---|---|---|
| Screening | None | Screening rate compares favourably with those of other studies; CRC nurses screened 79% (n = 156) of all surgical CRC patients for eligibility | – |
| Eligibility | Remove language as exclusion criterion | A multicentred study is likely to include areas in which English is not the first language for some people | We do not envisage a significant impact on eligibility rate |
| Exclude people already meeting recommended levels of physical activity | People meeting recommended levels of physical activity will already be maximising their chances of obtaining the health benefits associated with post-diagnosis physical activity. It does not seem a good use of resource, therefore, to include these people | This change in criteria is likely to reduce the eligibility rate because studies suggest that approximately 40% of people with CRC are meeting recommended levels for physical activity In addition, our study shows that most participants were nearly meeting or were meeting recommended levels for physical activity and therefore greater efforts will have to be made to include those who are less active. To apply this eligibility criterion in any future study, patients could be screened using a self-report physical activity questionnaire to assess current physical activity |
|
| Include people with poor mobility | The study shows that the main reason nurses excluded people with CRC was poor mobility (35%). However, most cardiac rehabilitation services accommodate people with poor mobility, including people who use a wheelchair. Cardiac rehabilitation also accommodates people with poor physical health (e.g. a low-intensity class for people who are in poor physical health) | This change to criteria should impact on eligibility rate by approximately 35% | |
| Consent | Exclude people who are on active treatment, such as adjuvant chemotherapy | The study shows that one of the reasons why people with CRC who wanted to participate in the study changed their minds or why some participants who were randomised to the intervention group were unable to attend cardiac rehabilitation was because they did not feel able to exercise while on treatment. Changing the eligibility criteria should, therefore, remove this barrier to participation | This change to the criteria should not impact on eligibility rate but merely delay when people are invited to enter the study (i.e. post treatment). We estimate it will improve the consent rate by 20% (18% of those who were interested in participating changed their minds because they were having adjuvant therapy) and intervention adherence by 25% (25% of participants randomised to the intervention group did not complete cardiac rehabilitation because of ongoing treatment) |
| Completion | Introduce evidence-based strategies to improve completion rates at follow-up including: monetary incentive and a ‘package’ of postal communication strategies with reminder letters | The study shows that 61% of participants completed final follow-up measures | Evidence is lacking about the actual impact of these strategies on completion rates. Based on other studies’ completion rates (80–90%), we estimate an improvement of 20–30% |
| Missing accelerometer data | Introduce strategies to improve accelerometer wear-time, such as training investigators to explain the importance of these data for the study to participants and providing individual feedback on level of physical activity recorded by the device | The study shows that 31% of accelerometer data sets were assessed as invalid. The main reasons were not wearing the device or not wearing it for long enough | There was variation across sites and one site hardly had any invalid accelerometer data sets. One possible reason is that the investigator in site 1 had a sports science background and therefore was familiar with measurement of physical activity and could explain how to wear these devices properly. We estimate 10% invalid accelerometer data sets in any future trial |
| Missing diagnostic information | Ensure that investigators request this information once it becomes available | Diagnostic and treatment information was recorded at screening and therefore some of the diagnostic information was unavailable at this time | We estimate 100% of diagnostic information will be recorded in any future trial |
| Intervention adherence | None | There were genuine health-related reasons why people were unable to attend cardiac rehabilitation, and, of those who did attend, attendance rates were very good (range 75–142%) | – |
Chapter 9 Phase 2 qualitative study
This chapter presents the findings of the qualitative study that was nested within the pilot RCT (phase 2). Interviews and focus groups about the feasibility and acceptability of trial procedures and of the intervention (i.e. the feasibility and acceptability of using cardiac rehabilitation for people with CRC) are presented and discussed from the perspectives of people with CRC and people with CHD, and cancer and cardiology clinicians.
Introduction
Qualitative methods are an essential part of a trial’s evaluation and particularly apt for exploring the feasibility and acceptability of trial components and the intervention as opposed to measuring outcomes. Qualitative methods in RCTs can be used to understand and improve main trial components, such as recruitment. 164 Qualitative methods can also be used to explore processes, contextual factors or intervention characteristics and mechanisms that can aid the interpretation of trial outcomes. 165 Nevertheless, qualitative studies as an embedded component of RCTs remain uncommon,166 although there has been a recent growth in use of qualitative methods under the auspices of the MRC Collaboration and Innovation for Difficult or Complex Randomised Controlled Trials (ConDuCT) Hub. 167–170
The phase 2 qualitative study nested within the pilot RCT explored participants’ and clinicians’ views and experiences of the main components of the trial and the intervention (i.e. cardiac rehabilitation). The specific aims were to investigate:
-
the views and experiences of CRC participants of the main trial procedures (e.g. recruitment, randomisation)
-
the views and experiences of cardiac rehabilitation as a feasible and acceptable rehabilitation programme for CRC from the perspectives of CRC participants, people with CHD attending cardiac rehabilitation, and cardiac rehabilitation clinicians and CRC nurse specialists.
We have used the COnsolidated criteria for REporting Qualitative research, a 32-item checklist for interviews and focus groups,171 to guide the structure of this chapter.
Research team
Four investigators collected qualitative data by interview and focus group. All four investigators attended a 1-day training course in conducting qualitative interviews organised by the Social Research Association Scotland and one of the investigators involved in data analysis also attended 1-day training in qualitative data analysis. None of the investigators conducting interviews and focus groups was involved in providing patient care. Three were employed on the study as research assistants employed by the University of Stirling and one was a research nurse employed by the NHS trust.
Study design
Participant selection
Purposive sampling was used to select people for participation in the qualitative study to include people with CRC and people with CHD, and cancer and cardiac clinicians, across all three sites. In addition, if a participant with CRC nominated a family member, then that family member was also interviewed.
People with colorectal cancer
All trial participants (i.e. people with CRC) had provided informed consent at baseline to be approached by an investigator about being interviewed. All participants (randomised to intervention or control groups) were contacted by telephone and invited for interview. If a participant was willing to be interviewed, a mutually convenient time and place was arranged to conduct the interview. To minimise participant burden, this was often arranged at the same time as a follow-up measure was being assessed. Interviews were at either the first (i.e. within 2 weeks of the end of the intervention) or the second follow-up (i.e. approximately 3 months post intervention), depending on what was most convenient for participants.
Face-to-face interviews were conducted with patients with CRC at the end of the intervention delivery period for those allocated to the intervention group (i.e. after the patient had attended the final cardiac rehabilitation class) and for participants allocated to the control group at the first or second follow-up. Interviews were conducted in the cardiac rehabilitation facility in sites 1 and 3 and in the cardiac rehabilitation facility or the participant’s home in site 2, depending on participant preference. The mean duration of interviews can be seen in Table 43.
| Interview group | Mean duration in minutes (range) |
|---|---|
| Cardiac rehabilitation clinicians | 19 (13–23) |
| CRC clinicians | 24 (17–40) |
| Intervention patients | 41 (18–60) |
| Control patients | 17 (7–30) |
| Cardiac focus group | 14 (11–17) |
Patients with CRC participating in the trial were requested to nominate a family member to be interviewed by an investigator about the use of cardiac rehabilitation as a treatment for people with CRC. However, only one participant nominated a family member. Reasons given by some participants for not nominating a family member were as follows:
-
their partner was deceased
-
they had no partner or children
-
their partner was unwell
-
they did not feel comfortable nominating a family member.
Given that only one family member was nominated and interviewed, the views of family members are not included in this report.
People with coronary heart disease
Cardiac rehabilitation staff identified people with CHD and approached them about the study, inviting them to attend a focus group at a specific time and day. The investigator running the focus group consented those who attended the focus group before conducting the discussion. The focus group took place at the cardiac rehabilitation facility in each site. The mean duration of focus groups can be seen in Table 43.
Clinicians
An investigator approached CRC nurses involved in recruitment and cardiac rehabilitation physiotherapists and nurses delivering the intervention and invited them to attend a semistructured face-to-face interview. The CRC nurses at site 1 who were involved in identifying eligible patients with CRC for the study had already been interviewed in phase 1 and so were not interviewed a second time for phase 2; the perspectives of these clinicians about the trial are reported in Chapter 4. We did, however, interview the cardiac rehabilitation physiotherapist again at site 1. The main reason for conducting a further interview was because she was involved in actually delivering the intervention and so would have more experience of including people with CRC in her classes and might have changed her views as a consequence of further engagement with more people with CRC. No CRC nurses were interviewed at site 3 because none was involved in the trial; this is because a research nurse was employed instead to conduct recruitment and collect data at this site.
The face-to-face interviews were conducted with CRC nurses and cardiac rehabilitation clinicians delivering the intervention about the acceptability of main trial components and the intervention. The interviews were conducted at the end of the intervention delivery period either in the hospital or at the university. The mean duration of interviews can be seen in Table 43.
Data collection
All qualitative data were collected between 18 June 2014 and 9 April 2015. Semistructured interviews were chosen for collecting data from people with CRC and clinicians because they allow flexibility in terms of the sequence in which questions are asked, and whether or not and how particular areas might be followed up and developed with different interviewees. 129 Focus groups were chosen for collecting data from patients with CHD because they are a practical method for involving a group of patients.
The interview and focus group schedules were as open-ended as possible to enable participants to raise issues important to them. Interview schedules (see Appendices 8 and 9) were used to assist the investigator in gathering responses about the feasibility and acceptability of the intervention and trial procedures. However, the order of topics covered varied considerably between interviews to ensure that each interview and focus group was informal and open-ended to suit individual participants. Table 44 summarises the key topic areas explored with each group. With participants’ permission, interviews and focus groups were audio-recorded; no participants refused having the interview/focus group audio-recorded.
| Participant group | Topic area |
|---|---|
| Patients with CRC allocated to intervention group |
|
| Patients with CRC allocated to the control group |
|
| Patients with CHD |
|
| Cancer and cardiac rehabilitation clinicians |
|
Analysis
Thematic analysis – ‘a method for identifying, analysing and reporting patterns within data’172 – was used. Two investigators analysed qualitative data: one was involved in conducting the interviews and focus groups and the other was the principal investigator. Audio-recorded interviews were transcribed verbatim and analysed thematically. The Framework approach, which is a rigorous method providing a structure within which qualitative data are organised, coded and themes identified, was used to guide the analysis. 173 In brief, the investigators first became familiar with the interviews transcript data by reading and rereading transcripts and assigning data (sentences and paragraphs) to themes that related to the study objectives (e.g. barriers to participation in cardiac rehabilitation); second, a narrative summary of coded data was made under each theme and initial themes were refined; and third, the investigators referred to the original data to ensure that participant accounts were accurately presented to avoid misinterpretation. Using this method, participant quotations were summarised in tables. Tables were produced for each theme and contained summaries of each participant’s views and experiences. Finally, behaviour change theories and models were drawn on to facilitate interpretation of the data,174 and associations and patterns between themes were charted and, where relevant, mapped to theories to explain the findings.
Different investigators completed the qualitative analysis before analysis of the trial data so that the findings would not bias interpretation of the qualitative and quantitative material and vice versa.
Findings
Number of participants
In total, 38 participants were involved in the qualitative study. Table 45 summarises the number of participants in each site.
| Site | Patients with CRC | Patients with CHD | CRC nurse | Cardiac rehabilitation clinician | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| 1 | 6 | 1 | 4 | 0 | 1 |
| 2 | 3 | 6 | 0 | 2 | 2 |
| 3 | 3 | 3 | 4 | 0 | 3 |
| Total | 12 | 10 | 8 | 2 | 6 |
Out of 41 consenting and randomised patients with CRC, 22 (54%) were interviewed, 3 (7%) formally withdrew from the study, and therefore were not invited for interview, and 16 (39%) declined an interview. Reasons for non-participation are presented in Table 46. Eight patients with CHD were involved in a focus group discussion. In total, two CRC nurses at site 2 and six cardiac clinicians across all three sites were invited for, and participated in, an interview.
| Reason | Number (n = 16) |
|---|---|
| Did not want to talk about cancer | 3 |
| Unable to be contacted | 3 |
| Not well enough to be interviewed | 2 |
| Did not attend | 1 |
| Too busy | 1 |
| Unable to enter study within study time period | 4 |
| Missing | 2 |
For all quotations, letters followed by a unique number are used as participant identifiers; letters indicate the following:
-
CR: cardiac rehabilitation clinician
-
CRCN: CRC nurse
-
CHDP: patient with CHD attending cardiac rehabilitation
-
control: participant allocated to control group
-
intervention: participant allocated to intervention group.
Key themes and subthemes
Unsurprisingly, key themes closely matched the interview and focus group topic guides shown in Table 44. There are five key themes: benefits for people with CRC attending cardiac rehabilitation, barriers for people with CRC attending cardiac rehabilitation, generic versus disease-specific rehabilitation, key concerns about including people with cancer in cardiac rehabilitation and barriers to involvement in a study about cardiac rehabilitation (CRC participants only). Themes and subthemes are presented in Table 47.
| Themes | Subthemes |
|---|---|
| Benefits for people with CRC attending cardiac rehabilitation |
|
| Barriers for people with CRC attending cardiac rehabilitation |
|
| Generic vs. disease-specific rehabilitation | |
| Key concerns about including people with cancer in cardiac rehabilitation |
|
| Barriers to involvement in a study about cardiac rehabilitation (CRC participants only) |
|
Clinician interviews
Benefits for people with colorectal cancer attending cardiac rehabilitation
Clinicians’ perceived main benefits of cardiac rehabilitation were that people with CRC would increase their level of physical activity, overcome fears about being physically active, gain in confidence and become more motivated to exercise, access peer support and forge new friendships and obtain psychosocial support from trained clinicians.
Cardiac rehabilitation clinicians perceived that people with CRC who attended their classes enjoyed them.
Certainly the patients that have attended the programme were very enthusiastic in fact as far as I know they enjoyed it.
CR 001
I think they’ve benefitted a lot from exercise. They all seemed keen.
CR 005
Delivered by health expert
Cardiac rehabilitation clinicians emphasised that a key advantage for people attending was the quality of support that they would receive from NHS clinicians.
I think it’s the same with any rehabilitation, it doesn’t matter what condition you have, it’s more that they’ve got support from NHS professionals. That they’ve got support from peers . . . em, the structure of the classes is that they had, they know that they’re coming to rehab[ilitation] you know, 2 days a week. It gives them confidence to go out and do the exercises themselves, and at what levels they can work at and how hard that they can push themselves. Em, so from that point of view, it’s similar to your cardiac patents and probably any patients that come for rehab. That early intervention is really, or short-term benefits are giving them the confidence in how much that they can do, giving them the advice, giving them, em, the structure about, em, advice about pacing their activities.
CR 003
They have the support obviously. It’s just having someone to ask isn’t it? It’s just having a link I think sometimes.
CR 005
Colorectal cancer nurses also perceived that a key benefit of this model of rehabilitation was that people with CRC were being guided and supported by trained clinicians. They were, therefore, completely reassured that people with CRC would be safely exercising under close supervision of and with support from qualified clinicians.
Well, it gives them a reason to get out of the house, erm, so it actually gave them a physical resource, it was good exercise, erm, because it was supervised by physiotherapists. You knew they wouldn’t be overdoing it, erm, which is quite important because people think they are actually better than they are, especially within the first few weeks after an operation . . . And there is a physiotherapist there and if there are any concerns they wouldn’t let them do it.
CRCN 006
Indeed, knowing that patients were being referred to a service delivered by clinicians was one of the reasons why this model of rehabilitation was attractive to CRC nurses as well as to patients with CRC. CRC nurses believed that some of the patients would not have attended a gym but were willing to attend cardiac rehabilitation because clinicians, who were able to offer them a greater degree of safety and understanding of their illness experiences, delivered it.
I think the thing that sold it was the fact that there was going to be physiotherapists and nursing staff there with the patients because they worry about hurting themselves and they were all quite happy to do whatever as long as they were under supervision and I, I got that from all the patients I spoke to. They would not have gone into a gym without something knowing what they had been through. And it gave them reassurance from them and that’s why some of them took it on when they were people who maybe did exercise anyway because they were worried about the wound and the work that had been done inside and so that, that was definitely a bonus.
CRCN 007
Benefits of physical activity
Many cardiac rehabilitation and CRC clinicians believed that any increase in physically activity, no matter how small, was of benefit to patients.
People think you need to be, you know, like be an athlete or really working full pelt to get benefit from exercise, but you know, absolutely not, wherever you start from and you’re increasing it gradually, it’s going to benefit.
CR 003
I mean some [patients] are very fit but others, erm, do need a wee bit of help so it’s good to be able to offer something.
CRCN 007
Additionally, it was pointed out by one CRC nurse that getting fitter was especially important for those patients who were likely to require further treatment such as further surgery or adjuvant therapy because their ability to cope and recover from treatment would be likely to be improved if their overall general health was better.
I suppose I think of the patients that I need to retreat or are involved with their retreatment. If we can get them their fitness back again it means that we have a healthier bunch of patients if ever in the future we need to give them chemotherapy or reoperate on people. You know there are people who are going to get recurrences and some people that won’t, but if they’re fitter it stands them in a better area to actually have treatment.
CRCN 007
Thus, cardiac rehabilitation was seen as helping patients to recover from past treatment but also preparing them for any future treatments. Indeed, cancer care clinicians were aware of the benefits of being fit, which was why they approved this study.
They [CRC surgeons] were all quite happy. We never had any of the surgeons saying, ‘No, don’t introduce that to my patients’. They were all quite happy that, I mean they wanted their patients fitter.
CRCN 007
Confidence
Cardiac rehabilitation gave people with CRC the confidence to start to become more physically active. Cardiac rehabilitation reassured them that they could be physically active following cancer diagnosis and treatment.
I think after any sort of event you’ve been through, it’s all, a lot about confidence isn’t it. And what our patients tell us they get from the programme the most is confidence.
CR 005
I think just enjoyment and inclusiveness, erm, they found quite helpful and you know, some of the supporting information, even just pacing and company, erm, was the most, yes, there were quite a few things that they seem to be, to enjoy about it . . . I think the reassurance, erm, it was actually the fear factor for exercising is one of the biggest things, erm, again company, hopefully motivating.
CR 001
Motivation
Cardiac rehabilitation clinicians also believed that their programme would motivate people to start becoming physically active. Cardiac rehabilitation provided a structure and regular opportunity to exercise, which was believed to motivate people to engage in physical activity. Moreover, cardiac rehabilitation motivated people to be physically active because by attending they experienced an improvement in their recovery.
I think when you’ve got that kind of slot where you must go, it helps to discipline people to do it. And then they begin to see the difference that it makes and that encourages them to, to do a bit more . . . when they actually, when they begin to see the improvement it does encourage them and it’s great, we see huge difference in people here, absolutely.
CR 002
Peer support
Cardiac rehabilitation and CRC clinicians believed that the intervention provided people with CRC with an opportunity for peer support. The interviews gave a sense that peer support arose from the shared experience of participating in group-based exercise. Thus, peer support did not emerge as a consequence of the shared experience of being diagnosed with, and treated for, the same condition (e.g. CHD or CRC), but was a consequence of the shared experiencing of recovering from illness and using the same rehabilitation service to aid recovery. Thus, in this sense, cardiac rehabilitation was a social opportunity for people to tap into support from their peers, as well as an exercise opportunity.
A lot of them get, erm, peer support from the folk round about them, ‘I’ve been through that. I was anxious like you, terrified to do anything’. And then they see people down the line, you know a lot better and it kind of gives them a wee bit of hope.
CR 002
I think once you exercise in a class situation you’re getting support inadvertently aren’t you? Even though they’re not talking about their cancer or their heart they’re working together in an exercise situation and chatting more generally I suppose rather than about their real condition.
CR 004
I think it’s a fantastic intervention. I think it will be really beneficial for people, not only from an exercise point of view but just even from a social aspect, getting out there and being social again. If their surgery, erm, meant that there was huge like body image changes to them that may, erm, it reinforces the fact that they can do the things that they might have thought that they were unable to do after their surgery, so I think it’s a very positive thing.
CR 008
I do think, it’s not just exercise that’s offered it’s the social side of it as well which I think is great . . . it’s certainly something that my own patients [people with CRC] have commented on.
CRCN 007
Furthermore, cardiac rehabilitation provided an arena in which friendships were forged based on mutual interest, as opposed to based on being diagnosed and treated for the same disease. A cardiac rehabilitation clinician described the friendship between two golfers attending the classes, one of whom had CHD and the other had CRC.
He’s going to meet up with one of the patients to play golf so he’s made a friend I suppose. They’re both keen golfers and they’re going to meet up now.
CR 005
Barriers for people with colorectal cancer attending cardiac rehabilitation
Colorectal cancer nurses, in particular, believed that cardiac rehabilitation should be available to as wide a group of patients as possible because of the benefits of physical activity. They perceived, however, that it might be less suitable for people who were very unfit or wheelchair-bound.
Everyone should have the opportunity to decide for themselves, em, no matter what their level of ability is, so you treat everyone sort of in the same respect when it comes to CRIB [name of this study] unless they were very much affected by their mobility, by that I mean wheelchair-bound or, you know, they’re just so unfit that even surgery was a struggle.
CR 008
There were, however, obstacles and concerns about including all patients, which are described below.
Travel and distance
Cardiac rehabilitation and CRC clinicians pointed out that travel distance acted as a barrier to attending, and perceived this as a general, as opposed to a disease-specific, barrier. In other words, people with CHD also encountered travel problems as a barrier to attending cardiac rehabilitation.
It can be difficult because this area covers, it’s wide you know it’s a huge distance for a lot of people to travel, so for some patients it is, it is a problem and we’ve had cardiac patients that won’t come because transport is a problem.
CR 002
The vast majority [of patients with CRC] were really interested [in participating in the study] but the distance was always an issue.
CRCN 006
Recovery from treatments
There were, however, some barriers and concerns that were seen to be unique or particularly prevalent among people with CRC. People with CRC were seen to experience protracted recoveries from treatment. As a consequence of protracted recoveries, there was a need for a flexible start date for being referred to, and attending, cardiac rehabilitation. Thus, the optimum time for people with CRC to start was difficult to determine. This is in contrast to people with CHD, for whom there is an expected time point following the cardiac event at which they start their rehabilitation (e.g. post myocardial infarction, 4 weeks; post angioplasty, 2 weeks; and post CABG surgery, 6 weeks). 175
They [people with CRC] were all very keen, very motivated . . . a couple of patients, I think, just with what they had gone through, were a wee bit hesitant about what they would be able to do. Certainly one lady that we’ve recently assessed, she’s still ongoing treatment and wound haven’t healed and things like that, so at her point in her treatment she’d not ready to start rehab but is very keen to obviously continue with you know the rehab programme when she’s, when she’s fit and able for it. But no, there was no sort of negative reaction, just, you know, em, initially it’s more, again it’s the advice that we give all our patients, it, take it at their own pace, don’t do anything that hurts them, em, and just to let us know if there’s any of the exercises that they wouldn’t be happy about.
CR 003
Colorectal cancer nurses recommended flexibility with regard to people with CRC starting rehabilitation because of protracted recoveries.
There were some patients who were fit and then something would happen to them and they basically crashed maybe a couple of, maybe a week or two after surgery. And you introduced them to the study but you know they were never going to go on it because they had kind of side effects and would infections and chest infection problems that, erm, it took maybe months, actually to get over.
CRCN 007
CRC clinicians also felt that a flexible start date was required because they believed that some people with CRC only realised that they might need help getting active again further on in the recovery process. It appears that some people need time to mentally adjust to the fact that they may require some help with their recovery.
I don’t think they realise how much stamina they lose postoperatively. They think they’re just going to go home and get right back out there and they cannot do it but it takes them a wee while to accept that.
CRCN 007
Chemotherapy was a concern for cardiac rehabilitation clinicians; they expressed uncertainty, for instance, about whether or not people receiving chemotherapy should be exercising at all.
What were your concerns about patients recovering from cancer surgery?
I think the biggest issue, I think, to being with, was the timing and chemo[therapy] and I suppose my main concern would still remain the chemo[therapy].
Side effects?
You know, the time of them and how it affected them coming or whether they should be here at all.
CR 001
And were you a little anxious about practical issue such as risk infection . . .?
The only thing about infection was, I was worried about if someone was maybe coming on chemotherapy or something like that and it was like the cancer patients that were going to pick up an infection. But that was all, there was nothing else, no, nothing else.
CR 002
Colorectal cancer nurses were aware that chemotherapy treatment could put people with CRC off being physically active. Nurses believed that the side effects of chemotherapy varied from one patient to the next, which was why they did not recommend excluding people undergoing adjuvant chemotherapy from the study. Indeed, nurses encouraged people on treatment to try to lead as normal a life as possible.
The chemo[therapy] is an issue because it affects people differently, so if you’re, erm, you are going to be missing with people who are because they are immune suppressed we would worry about that to some degree, but again, you know, they can’t, you would expect them to still be sort of living as normal a life as possible, but yeah, it is a concern.
CRCN 006
Despite voicing concerns about people with CRC exercising if they were also undergoing adjuvant chemotherapy, cardiac rehabilitation clinicians recognised the importance of early intervention if people with CRC were to gain the most benefit from the programme. This was because physical activity is a key component in the recovery and rehabilitation process.
I think the intervention for these patients [with CRC] should be sooner . . . should there be a timetable that, you know, try and get people in that wee bit sooner so that they’re going to get the maximum benefit from the rehab[ilitation]? So they know exactly you how to pace-up, what they’re doing, what exercise they can and cannot do . . . When is the best time for them to come to rehab? You know, obviously, with the cardiac it’s a sort of set times . . . should they [people with CRC] be starting 6 weeks post surgery or is it that it is 3 months down the line?
CR 003
The CRC nurses confirmed that referral of CRC patients to a cardiac rehabilitation programme should occur at least 6 weeks following surgery.
It would normally be about 2 months before they feel back to normal anyway or 6 weeks they should be starting to feel decent. They are not allowed to drive before 6 weeks anyway so that would stop some people coming, laparoscopic is about a fortnight.
CRCN 006
Stoma
A potential barrier to people with CRC participating in group-based rehabilitation is having a stoma. Two cardiac rehabilitation clinicians commented that one of the people with CRC in the group who had a stoma was very conscious about a possible odour, and this impacted on her interactions with others.
I’ve kind of noticed particularly that one of the ladies from the colorectal cancer group, she is just, she thinks that everybody can smell her . . . you know, she didn’t want to get too near people, erm, she would kind of change her position over, you know over the room in here because she thought there would be a smell and if she moved then people wouldn’t see it.
CR 002
A stoma was also a concern for cardiac rehabilitation clinicians because they were not sure what to do if there was a problem.
Did you have any fears looking after these patients?
My only fear was if we had one with a stoma and there was a problem with it.
Generic versus disease-specific rehabilitation
Cardiac rehabilitation clinicians recognised that there may be financial incentives in introducing a generic rehabilitation service for people with long-term conditions other than CHD, but they also perceived the need for specialist input. Indeed, their experience of including people with cancer led them to conclude that it was feasible to develop a much broader and more inclusive rehabilitation programme. Moreover, many programmes were already expanding their client group to include people with heart failure, and so the seed for including other patient groups had already been planted prior to this study.
This study suggests that all cardiac rehabilitation clinicians recognised that the programme’s exercise component was generic and applied to all participants regardless of their specific condition. Indeed, exercise was individually tailored by fitness level and not by the type of disease that a person was recovering from.
Did you tailor the classes for our patients?
No, not at all. Absolutely no difference whatsoever in the class. We tailor the exercises individually but not because they were cancer patients.
Similarly, CRC nurses recognised that offering healthy lifestyle advice was applicable to all patients, irrespective of the disease they were recovering from.
It doesn’t matter what, what the problem is you know a healthy diet is a healthy diet.
CRCN 007
There is, perhaps, an inevitable tension between the specialist and generic qualities of a rehabilitation service. On the one hand, cardiac rehabilitation clinicians recognised that they had the skills and knowledge that would be used to benefit other patient groups, and on the other hand, they recognised that specialist knowledge about a particular disease was essential for providing quality care.
I think, really, from a financial position, and I know that, erm, from a management point of view, I think we’re really being pushed toward generic classes.
And how do you feel about that?
And part of me doesn’t like the idea at all, but I would have to say, well, with this patient group, it’s not really been any different so it’s not caused problems, so I suppose I’m kind of, erm, [laughs] . . .
Has that reassured you slightly?
Yes, I mean, certainly from this group that’s been involved . . . it wasn’t a problem in itself, I suppose, erm, on your patient group, you know, if you are talking about having pulmonary in there as well, again, it just involves different expertise . . . which is always just a bit concerning, erm, and that’s why I’m slightly guarded with that because the you suddenly say, ‘Oh, we’ll just have all the diabetics and we’ll have this and we’ll have that’, erm, I mean, in fairness, we have a lot of diabetic patients and they have not generally been an issue either, but I’m not a specialist in diabetes.
Thus, when asked about developing a comprehensive rehabilitation programme for people with a range of long-term conditions, cardiac rehabilitation clinicians did not dismiss the idea, but they had reservations because of the need to retain a specialist disease component.
Moreover, alongside this recognition of the need for specialist support, there was reticence among cardiac rehabilitation clinicians about having to provide support to a group of patients other than people with CHD, because this was outside their specialist clinical domain. The interviews give the impression that some cardiac rehabilitation clinicians wished to maintain their ‘cardiology’ identity.
Would you be happy to have more patients?
Yeah definitely.
CR 004
And what are your opinions on mixing classes with different long-term conditions?
I really don’t know because my speciality is cardiac I don’t have the knowledge maybe that a lot maybe like psychological support for cancer patients . . . I’m not a counsellor by any manner of means but I can answer a lot of questions for folk with cardiac problems. Folk that came from the bowel cancer thing, I didn’t have that knowledge so I did feel a wee, I know that we can always phone and ask someone, but it’s quite good if there is somebody, you know, if you do know a wee bit . . . we do get quite specialists in out our own wee bit so I would be a bit apprehensive, I would need to look into it a wee bit more.
CR 002
My specialist area is obviously cardiac. Yes, we can give general advice about pacing and adapting exercises and things like that but . . . from a specialist support point of view I felt that the support was lacking from the bowel cancer side of things, you know, some of the issues that we’ve had to deal with . . . maybe we shouldn’t be having to deal with, you know, because we’re cardiac rather than bowel cancer . . . Yes, there’s obviously some certain similarities . . . when it comes to exercise and advice then, yes, you know, that’s something that is very general for everybody, but when it comes to tailored advice about medications or em, food and things like that, they, erm, then it really needs to be sort of tailored towards the specialist area.
CR 003
One cardiac rehabilitation clinician suggested that further information could be provided so that he or she could appropriately signpost people with CRC to specialist support. Thus, rather than providing psychological support or dietary advice for people outside their specialist area, their role would be to point the person with CRC in the right direction so that they could access specialist support. Signposting was something that the clinician already did for people with CHD, and so it was not unfamiliar.
What other resources are available to the bowel cancer patients? What else is available, what literature, what resources can they tap into as well would be useful for us to know about. You know, push them in the right direction of what information they needs. Obviously with the cardiac stuff you know we’ve got the British Heart Foundation, Chest, Heart and Stroke, you know, we know what websites are.
CR 003
Cardiac rehabilitation includes exercise and information sessions. Cardiac rehabilitation clinicians believed that some of the information sessions would be relevant to people with CRC as well as to people with CHD, including sessions about the benefits of exercise, stress management, relaxation and healthy lifestyle. Cardiac rehabilitation clinicians reported that people with CRC attended most of the information sessions.
They usually ended up coming to them all because they found them very interesting . . . Two of the cancer patients were on cardiac medications anyway so em, they’ve obviously found it really useful from that point of view.
CR 005
Nevertheless, the cardiac rehabilitation clinicians noted that they were unable to provide some specialist information for people with CRC because the information sessions were geared towards people with CHD.
The sessions that we have, em, some of them would be relevant to both. The benefits of exercise is relevant to both groups, stress relaxation would be relevant to both group. But we do medications, so we do all the cardiac drugs we do coronary heart disease and treatments, investigations, we do misconceptions, so there’s a whole chunk of our education that is very geared to folk, you know with cardiac problems. For folk coming from like a cancer point of view, healthy lifestyle, absolutely, you know it’s good for everybody, not just folk with health conditions, but there’s probably a lot of issues there that, you know, are just not relevant to, to folk in that group, and maybe they’ve go other things that would be more beneficial, you know, that could be covered for them that we’re not covering in our cardiac programme . . . the bowel cancer nurses maybe could have arranged a particular session for the with stuff that was relevant for them . . . if they had ileostomies, colostomies, certain foods that maybe upset them and different things.
CR 002
As the above quotation illustrates, dietary advice was a key omission in what cardiac rehabilitation was capable of providing for people with CRC. Another cardiac clinician also highlighted the lack of dietary advice available for those people with CRC attending the intervention.
We obviously offer dietetic input and a lot of the bowel cancer patients were interested in the dietetic side of things but they were having issues with the dietician because although it’s general healthy living, they feel that they need specific dietary advice . . . so that was, you know, a gap that you’re sort of noticing with the service. It’s maybe that you know they might need some sort of more dietary input as well to see what they can and cannot eat and what would be beneficial for them.
CR 003
Although CRC nursing teams were encouraged to provide information sessions as part of the cardiac rehabilitation programme, either on a one-to-one basis or for a group of patients, this did not happen in any of the sites. One of the reasons is a perception that people with CRC were already given plenty of information from nursing staff prior to taking part in the cardiac rehabilitation.
Nobody’s actually asked for anything, any more information. I mean, we do give out patients a lot anyway and it’s probably information overload.
CRCN 007
Key concerns about including people with cancer in cardiac rehabilitation
The key to this model of rehabilitation for people with cancer was the willingness and commitment of cardiac rehabilitation clinician to accept another group of patients into their care. Their concerns about this model of rehabilitation are now discussed in more detail.
Capability of clinicians
Cardiac rehabilitation clinicians were concerned that they would not have the relevant knowledge and skills to support people with cancer because their specialism was cardiology. Clearly, these clinicians wished to provide a high-quality service for any group of people using their service and, from their perspective, this meant possessing a level of knowledge and expertise about CRC.
My main concern was not being able to support them properly from a cancer point of view.
CR 001
I felt a wee bit out of my depth because not used to dealing with people, patients in that group [CRC patients], and I was just worried about are we saying the right things, doing the right things.
CR 002
Cardiac rehabilitation clinicians believed that it was important to obtain the medical history of anyone attending their service. This was because a good health service was one in which patients could safely assume that every clinician knew about their medical history, so that patients did not have to keep repeating their story. This applied equally to people with CHD and to people with CRC. Furthermore, knowing a person’s medical history equipped the clinician with the necessary information required to support that individual; one concern that arose, for instance, was being able to competently answer any questions. Cardiac rehabilitation clinicians articulated why knowing the medical history was important from their perspective as well as from the patient’s.
I don’t like dealing with patients when you know nothing about them . . . and you know, it’s important that if somebody is following a journey they don’t at every step of the journey get asked all the same questions over and over again.
CR 001
I know that we don’t necessarily need to know what type of tumour and things like that but from our point of view it’s quite interesting to know actually what these patients have gone through so that we can deal with any problems arising from that.
CR 003
Because you haven’t got any experience in that area. Just knowing what to expect isn’t it? What they’ve had done and any problems they might ask you about. That was all I was worried about really . . . Because otherwise you start off a pre-assessment and it’s a blind really, you don’t know anything about the patient.
CR 005
Anything we could have done differently to help you?
Slightly more information on the referral form. There was a couple of times we said, ‘Oh, what operation have you had?’.
However, cardiac rehabilitation clinicians had other concerns regarding cancer patients referrals. For instance, they wanted to know how they could safely support people to be physically active after abdominal or rectal surgery.
I was just a bit concerned, you know, will it affect them, are we going to give them something to do, they going to end up with hernias or something. The exercise is not that strenuous but sometimes, especially the guys, they come in and they really push themselves and they do more than we would like them to do.
CR 002
Similarly, they were uncertain about how they could support people with a stoma, or those having chemotherapy.
Our main concerns, really we thought they would be quite poorly, you know, with stoma, and because they might be having chemo[therapy] and we wouldn’t be able to answer any questions.
CR 005
The study highlights that cardiac rehabilitation clinicians did not feel that they necessarily had the right skills to provide psychological support for people with cancer. This suggests that, if cardiac rehabilitation services were to extend provision to other patient groups, training should not just cover issues relating to cancer, types of surgery and treatment, and exercise, but also equip cardiac rehabilitation clinicians with an awareness of the common psychosocial difficulties encountered by people with CRC.
One of the ladies who comes to the class, she was struggling so much with a whole lot of psychological issues so I did spend a lot of time with her. I don’t know whether it made any difference, but she was kind of needing, you know, a wee bit extra input, but I didn’t really have the expertise to give her . . . probably it would be good, you know, if we thought somebody needs a wee bit more kind of psychological help, if we knew if there was a way to help them.
CR 002
They’ve [people with CRC] obviously got different issues from our cardiac patients and what we’re finding is that they got a lot of psychological issues now that we’re having to deal with, whereas it probably would have been more relevant for, you know a specialist nurse in that area or possibly a physiotherapist in that area that probably could deal with their problems slightly better . . . we’ve got very minimum skills to do that.
CR 003
Capacity of cardiac rehabilitation
Alongside voicing concerns regarding their own capabilities to support people with CRC, cardiac rehabilitation clinicians were also concerned about the capacity to accommodate more patients. The inclusion of people with CRC inevitably added to the existing workload of the cardiac rehabilitation team.
At first I was a bit apprehensive because we’ve got quite a big workload, so it was the workload thing, erm, first of all that was the main concern.
CR 002
The main extra workload highlighted by cardiac rehabilitation clinicians was pre-assessments, which are routinely conducted prior to any patient attending.
Did it [including people with CRC] involve any extra time for you, personally?
It involved well, we always had to do the baseline assessments and that was the extra that was put on the service, erm, we didn’t have large numbers [of CRC referrals] so the perceived idea of having to run the extra classes never arose.
CR 001
We were having to go and do, you know, a full assessment on these patients ourselves which obviously takes up a lot of time as well, but obviously we want to make sure that everybody is safe to exercise.
CR 003
Overall, however, cardiac rehabilitation clinicians noted that the study and the inclusion of people with CRC did not create undue or impossible demands on their time or the service.
It’s not had a massive impact on our workload.
CR 003
In terms of workload was that a concern?
No not really because, erm, we were just going to do the same as we do really [with our CHD patients] . . . going to slot them [people with CRC] in.
Cardiac rehabilitation clinicians raised concerns about service capacity and whether or not including people with cancer would create a worse service for people with CHD.
Whether it would affect the numbers in the classes, whether we would have to run extra classes and whether my waiting lists would go up.
CR 001
There was acknowledgement that the referral of a different patient group to an already resource-stretched cardiac rehabilitation programme could lead to resentment and frustration among staff.
There is a certain group of folk, you know, with cardiac conditions that we don’t see because we don’t have the resources to see them so that was kind of, I was a wee bit probably protective of my own corner thinking, that’s not fair, you know, we don’t have resources to see our own folk, erm, however, that’s life [laughs].
CR 002
For the purposes of this study, two sites did not use ‘excess treatment costs’ to employ an assistant physiotherapist to accommodate the additional patients through the study, whereas one site did. A cardiac rehabilitation clinician from this last site explained how the extra funding made it feasible for them to be involved in the study.
My very first thought was they were sort of doing the study on the back of our service but then when we read the actual protocol it made sense then that then there was the funding for it, that’s what changed the picture because we thought we’d just have to absorb it and we were stretched enough as it was . . . we all thought it was a really good idea to be involved in something like this.
CR 004
Indeed, the appointed physiotherapist was a welcome addition to the team and meant that people with CRC could be invited to attend cardiac rehabilitation without it being to the detriment of people with CHD, which had been an initial concern.
It was really good having a colleague working with me. I enjoyed that very much. And that’s because you know we had our 12 cardiac patients and our powers that be said we couldn’t deny a cardiac patient a place.
CR 004
The interviews suggest that one site was not anticipating conducting the cardiac rehabilitation pre-assessments (e.g. fitness tests) themselves. Instead, they clearly thought that this would be done elsewhere. Nevertheless, these assessments did not appear to be too onerous a task.
At first we thought the patients would have had a kind of more thorough assessment . . . we really had to do our own assessment . . .
The assessment that you had to do, do you think that impacts on your daily workload?
We had to do like a half-hour assessment with each patient as though it was from scratch kind of thing so probably a bit, not a huge amount.
Colorectal cancer participant interviews
Benefits for people with colorectal cancer attending cardiac rehabilitation
Benefits of physical activity
Participants in the control and intervention group believed that rehabilitation was an important part of their recovery.
So if rehabilitation had been offered as part of normal NHS service would you have gone?
Absolutely, you’ve got to give yourself the best chance possible.
There were two main reasons why people with CRC agreed to participate in this study: they believed that it might help them and/or they believed that it might help others. In particular, some participants welcomed involvement in a study that was about physical activity because they believed physical activity was beneficial.
Well, I thought it would help other people and possibly help me too, because you do learn things as you go along, even about yourself . . . The one that attracted me, well the part that attracted me was the exercise. I thought that would be, that would have been beneficial. I’ve always been quite active you know.
Site 2 09 control
Well you don’t discover things unless you examine them and basically, if I can help by my experiences.
Site 2 17 control
If I can help somebody else erm, I’m happy to do that.
Site 1 23 control
Help others.
Site 1 19 control
If it helps somebody else I was quite willing to do it.
Site 2 07 control
Participants allocated to the intervention group described the exercises that they did during cardiac rehabilitation and the impact that these had on physical functioning, general health and well-being and daily living.
I was doing something before and I had to hold onto a chair and then suddenly I realised I didn’t have to, have to do it, so the exercises . . .
So they did strengthen then?
Yes, yes.
Site 3 30 intervention
When you are out and about you are more physically able, even for things like going and doing your weekly shop; doing bits and pieces about the house, things that needed done, a bit of decorating. I do think by doing the exercise programme that happens more quickly, because I reckon I would just have sort of sat at home and go for little walks round about where I live, but maybe not pushing myself so much, so getting back to your normal routine would probably have taken longer . . . And I also think when you come out of the class you’ve got a bit of a buzz . . . then also you see yourself sort of starting to tone and you think, ‘Oh, I could go out and buy myself something new to wear’, or go and get my hair done, or whatever, buy some new make-up – whatever it may be – and that again helps to your overall eh well-being.
Site 2 04 intervention
I mean, I enjoyed it like, but eh, it also helped me get a wee bit back tae [to] my, my fitness before the, well efter the operation, like ken [know], I sort o’ let things slide sort o’ thing ken, so it got me back tae being reason, reasonably fit . . . tae me it made me a wee bit mair [more] aware o’ what I can, what I can actually do like ken, as far as muscle-wise and, and, and eh being flexible.
Site 2 06 intervention
It is clear from some of these accounts that participants’ family members noticed the difference that cardiac rehabilitation made.
What were you expecting from cardiac rehabilitation and how were you hoping it would help?
Well that’s a laugh, ‘cause when I had the first just one-to-one with [name of physiotherapist] I went home and said, ‘Mmm, I think it’s going to be a bit easy, it sounds you know, little gentle exercises, we’re not to push ourselves’. And when I came back after the first class, my husband will never forget this, I was absolutely exhausted, so it was a, a real eye-opener to me, just how weak I was.
Site 1 13 intervention
Well, I was hoping that it would improve my activities, you know like – especially walking – and I was also hoping that eh, I would lose some weight over it, but I didn’t [laughs], but I certainly, after going to the, to the rehab[ilitation] my walking is greatly improved, and my wife tells me so. I tend, I tend to be a slow, slower and, you know I’m quite a brisk walker now . . . And there was a, I, I felt there was a purpose that I was doing it, you know there was . . . I felt, I felt that I needed to do, to do these exercises.
Site 1 02 intervention
Given the favourable comments made about cardiac rehabilitation by those participants with CRC allocated to the intervention group, it is perhaps not surprising that the overall impression of cardiac rehabilitation was that it was very good, and that many participants would have liked to have remained on the programme for much longer.
Was the length of the exercise programme the right time, so your 12 weeks, or could it have been longer?
I could have happily gone on.
Site 1 13 intervention
I would have been very happy if it was 20 weeks [laughs].
Site 1 02 intervention
Confidence
Participants believed that rehabilitation would instil confidence and remove some of the fears about becoming active again following major surgery for CRC.
I think it’ll stop them from being afraid to do things because it’s a controlled environment they’re encouraged to do as much as they can without overdoing it . . . so it gives you the confidence when you do go, you know, you think, ‘I can do this,’ and ‘I can do that’.
Site 2 17 control
So what did you get out of it the most do you think?
Confidence probably.
Confidence that you could exercise?
Yes, yes.
I think I was hoping it’d give me the confidence to go and do some physical activity, I think especially after you’ve had abdominal surgery and you’re not sure what you should and shouldn’t be doing, and I think they gave me that confidence and, they, they start you off doing things, you think, ‘Oh I wouldn’t, I wouldn’t have had the confidence to get on a, a spin bike for 5 minutes,’ so yeah I, from that point of view it’s been a good experience. You’re not really aware that the professionals are actually watching you, but they are, so you know that if anything goes wrong or you feel unwell that there’s somebody there on hand you know to help out, whereas I think if you went to a normal exercise class you wouldn’t feel that level of confidence.
Site 2 04 intervention
Motivation
Participants cited motivation to be physically active as one of the major benefits arising from attending a rehabilitation programme. In particular, they believed that a structured physical activity programme would motivate people to become physically active and help them to do so much quicker than would be the case if they had no assistance.
I would have enjoyed the exercise and it would have motivated me to do it . . . I mean obviously for the first couple of months I probably sat here most of the time; (a) I didn’t feel like doing anything and (b), well you just need that time to recover you know but after that you could, it would maybe help to get you back quicker.
Site 2 09 control
I’d be confident but just not motivated so I need somebody to give me a kick up the butt and say, ‘Come on, you’ve got to do this,’ and I will do it.
Site 3 02 control
My intentions are always good, and I think I’ve sort o’ mentioned to you before that I would need, I think I need to be in a, decide I’m going to a class, something that’s structured so that it makes me go.
Site 2 04 intervention
One participant allocated to the intervention group believed that cardiac rehabilitation had given her the motivation to continue being physically active after rehabilitation had ended.
Motivation, can I quote what I’ve written down, immediately, that I wrote immediately after the 12 sessions? It improved my motivation and discipline to do the exercises actually.
Site 1 13 intervention
Some participants allocated to the intervention group believed that one of the main benefits of cardiac rehabilitation was being shown exercises that they could do at home.
I, I found it helpful in so much that it, it gave me simple exercises that I, I continued to do at home. And, and, and there was a lot of em, information available eh, em, and some of it I read and I continue to, to read and also helpful in meeting the other members of the class and, and eh, how they were coping, who were all basically cardiac patients.
Site 1 11 intervention
Nevertheless, some of the participants allocated to the control group reported that they had started to become more active again, in absence of the intervention. The data suggest that many of these participants, however, were those who had been active before their surgery. This suggests that there may be a need for a tailored, individualised approach towards attendance at a rehabilitation programme to promote physical activity following surgery for CRC.
We’ve decided to go swimming . . . taking the [neighbour’s] wee dog out.
Site 2 17 control
My wife and I went on a 5-mile walk just a couple of days ago. I used to do as a regular thing before the operation . . .
We’re going to book a holiday and start going down the gym or going for long walks or a combination of the two, but yeah, I do want to get back to where I was.
Site 3 02 control
Similarly, those who had been typically more physically active before their surgery commented that the intervention had not made a difference to their level of physical activity post surgery.
In terms of the exercise, did you get anything out of it?
I don’t think so to be truthful with you. I was already into the golf before I went there.
Peer support
A key benefit of the rehabilitation programme was peer support. It was clear from interviews with participants in the intervention group that people attending cardiac rehabilitation provided companionship and that they encouraged each other to exercise.
And we all fell into the same trap: ‘Oh, did you do your exercises?’ [‘What do you mean, since last week?’] and, ‘Oh yes, last night’, you know [laughs], but, and then it got better, I got a bit more disciplined about it. But I’ve, an important point here, is the companionship during the sessions, but also before the sessions, ‘cause we were encouraged to meet sort of 10 minutes before the class so we were all there on time.
Site 1 16 intervention
So working in pairs it encouraged you to talk, so you chit-chatted away [laughs] and encouraged, not encouraged to exceed yourself, but it was an encouragement to say, I mean, I did, with one person say, ‘Hang on, don’t try and copy me, you know, remember what [name of physiotherapist] said and don’t push yourself’, and she didn’t.
Site 1 13 intervention
Social skills
Participants believed that cardiac rehabilitation provided an opportunity to meet other people and socialise, which they saw as important because they had lost confidence in their social skills. Cardiac rehabilitation provided an excuse to get out of the house, which was an important goal in their recovery.
To focus, get out of the house, see other people. And I mean, post cancer you can sit there and feel sorry for yourself as long as you like but the more chances you give yourself of opening your mind the better.
Site 1 19 control
It improves your social skills, because you can become quite, you know, isolated quite quickly, when you’re not out and about meeting people, so yeah, that was a good side of it as well.
Site 2 04 intervention
I think just by going along there and knowing you’re not the only one in that position and you met a good group of people and you had a laugh, and it was, you know, and you were getting better, but you didn’t realise you were getting better, do you know what I mean?
Site 3 07 intervention
So at least it’ll get me out the house twice a week; going somewhere, meeting other people.
Site 3 29 intervention
Generic versus disease-specific rehabilitation
Participants were asked if they could foresee any difficulties in having mixed patient groups for rehabilitation. None of the participants in the control group suggested that it would be a problem. Indeed, one participant did not even think it was worth even giving an opinion about.
I don’t see anything wrong in it.
Site 2 07 control
So it wouldn’t matter that they didn’t have stomas and they hadn’t had cancer?
Not in the slightest.
Site 3 02 control
I’ve got no opinion on that at all.
Site 1 19 control
Similarly, none of the participants in the intervention group believed that it was a problem either. Participants gave the impression that it was irrelevant which disease a person happened to have.
They said, ‘What are you?’ I said, ‘I had cancer’, and that was it.
Site 3 20 intervention
I think when you go to the class everybody is quite open, em, because I remember the first time somebody, I think it was the first or second class I’d been at, and a gentleman had said to me, ‘Was it a stent you had put in or . . .?’ and I went, ‘No, actually, no, I’m here because of bowel cancer’, and he just sort of looked at me, and, you know I, and then I explained to him about the programme as well, and I don’t know that I would have been quite, quite so open with somebody that I didn’t know before, but then I thought, well, everybody’s here for a reason, it’s not the same reason as me, but everybody’s here for a medical reason, so, yeah, probably yeah.
Site 2 04 intervention
Every, everybody mixed very well with me, you know, like when they knew what the, my problem was like you know, I mean they were surprised . . . like people saying, ‘What are you doing here?’ ‘Well, the reason I’m here is because I was invited to come here’, you know, and eh, em, that I would benefit from it, and I did benefit from me being there, yes . . . They asked the question . . . ‘What did you get? Did you get a stent?’ or whatever.
Site 1 02 intervention
Indeed, one of the advantages of having mixed patient groups was that it enabled one of the participants allocated to the intervention group to focus on their physical health without it necessarily being dominated by their cancer diagnosis.
Eh well, well they were’nae [weren’t] bothering aboot [about] me having the cancer, naebody [nobody] even spoke aboot that, because, as I say it was a heart thing that you were there for, it was a’ heart cases that were up there for their therapy, so it was right oot [out] ma’ [my] mind [slight laugh], the cancer.
Site 2 02 intervention
Key concerns about including people with cancer in cardiac rehabilitation
Nevertheless, participants did have some concerns about the use of cardiac rehabilitation for people with cancer.
One gap in support from cardiac rehabilitation, noted by a couple of participants, was cancer-specific advice about, for instance, bowel problems and stoma care.
They would ask how, how have you been, and I would say, ‘Well I’ve not been too great with my bowels.’ And he went, ‘We don’t have, that’s not our area of expertise’. You know. I didn’t need any follow-up treatment, but maybe for people who are having to have chemo[therapy] or whatever, maybe they would need to know a bit more about the medication and the effects of the medication. Well I don’t know what, what the situation will be going forward, whether they would be offered some kind of additional training on it or additional insight into em, cancer patients.
Site 2 04 intervention
Because [name of physiotherapist] was very clear with me in the introductory interview that you know she, she’s not a cancer nurse and she was worried about my stoma bag.
Site 1 13 intervention
Furthermore, participants suggested that they had not been given any advice about how to reduce the risk of recurrence and would have welcomed guidance on this.
And is there anything that you think that you can do to reduce your risk of recurrence of cancer?
Well it’s something I would like to know if I could [laughs]. No one has said you know, ‘Don’t do this’, or ‘Do this.’ It would certainly be helpful if there was a guideline, it might help.
Do you think there’s anything such as like food groups or anything like that?
Food group, exercise, em, again I think my age is against me because so many things come with age as opposed to just the cancer but yes I think if there were some guidelines it would be helpful.
Site 2 09 control
All of them, the consultant and the stoma nurse and my own doctor all said that I was to walk as much as possible but sensibly . . . but I walk the dog four times a day, every day, rain, hail or shine.
Site 1 23 control
However, other participants said that they had been given advice about what they could do to improve their health after surgery. Of particular relevance to this study was that some of them had been told by clinicians to keep physically active.
The consultants said [between diagnosis and surgery], ‘You want to get fit and do lots of walking and lose a bit of weight’. And so I went walking miles and miles and did lose a little bit of weight but not a lot and I did get a lot fitter.
Site 3 02 control
Barriers to involvement in a study about cardiac rehabilitation
Randomisation
For some participants, randomisation did not seem to be a major barrier to study participation because they did not mind which group they were allocated to.
Fine, I had no feelings one way or another. I was quite happy to participate one way or t’other [the other].
Site 1 23 control
Other participants, however, expressed disappointment being allocated to the control group.
I would have liked to have done it [physical activity] but I did it anyway but on my own back. It was my choice to do it, not, I didn’t do it because I was told to do it.
Site 2 17 control
In contrast, some other participants were pleased that they had been allocated to the control group.
I was quite happy in myself that I didn’t have to go through all that.
Site 2 16 control
Some participants were not clear about how randomisation worked and its implications. Furthermore, the impression given was that some participants allocated to the control group felt abandoned.
I didn’t get that, no . . . it would have seemed to have better to have gone right through with it rather than just cut me off like that . . . it was disappointing to be left for 12 weeks.
Site 2 07 control
I didn’t understand it to begin with. I thought the exercise was part of the whole thing.
. . . And I thought the age I am . . . I thought maybe it was, you know, an age thing and that my age they thought, ‘Well, she wouldn’t be interested in exercise anyway’.
Were you a little bit disappointed?
I was because I thought the exercise might help me.
Study information
When participants were asked about study information, most gave a perfunctory response. They either briefly replied that the information was clear or gave the impression that they could not remember what they had been given. Thus, although study information was not necessarily a barrier to participation, neither did it appear to promote participation.
Do you feel that all the information you were given was clear?
Yes I’m sure it was . . . I can’t remember reading the booklet. I think it was just verbal but maybe I should have read the booklet [laughs].
Can I ask you what verbal or written information were you given about the study?
I honestly can’t remember.
I got a big form with more information but I haven’t read it [laughs].
Site 2 17 control
I tell you, I would struggle to remember to be honest with you.
Site 1 23 control
Recovery from surgery and adjuvant therapy
None of the participants raised any concerns about being approached about the study on the surgical ward, either while waiting for surgery or while recovering from surgery and waiting to be discharged.
Was it an appropriate time to discuss the study?
Yeah.
There was nothing inappropriate about the timing or insensitive?
Not for me anyway.
Site 2 17 control
I didn’t feel it was an intrusion at any time.
Site 3 02 control
I didn’t see anything wrong in it.
Site 2 07 control
Participants allocated to the intervention group were expected to start cardiac rehabilitation about 6 weeks after the surgery. Those who were not receiving adjuvant chemotherapy felt that this was a good point at which to start rehabilitation.
OK, and do you feel that [6 weeks] was an appropriate time for somebody to bring it to your attention?
Yes, I think so, because beforehand you’ve got too many other things on your mind, and going to all these appointments and, whereas you’ve had the operation, you’re now looking ahead; you’re feeling extremely weak [laughs] so you want to get better as quickly as possible.
Oh yes, it came at the right time for me . . .
It wasn’t too early or too late?
No, no, far from it.
You felt it was quite . . .
Yes I, I was, I was ready for the classes.
Site 1 02 intervention
No, I think it came at quite a good time for me, because my wound had healed, I was back driving, so it probably did come at quite a good, quite a good point for me, because I needed to move onto the next stage of my recovery, which, as I said earlier, wouldn’t, I wouldn’t have had the confidence to do unless the programme had come along.
Site 2 04 intervention
Because I think after 6 weeks you, you’re fed up of staying in the house after 6 weeks, you’re looking for something to do and to be honest with you, something to get your out of the house for a change of scenery.
Site 3 07 intervention
Participants allocated to the intervention group who were receiving adjuvant therapy, however, believed that the appropriate time for them to start rehabilitation was at the end of adjuvant therapy.
Do you think that the class came at the right time for you, or is there a point that you think it might have fitted in better with your recovery?
No, no I think it, it basically em, had to come after the chemo[therapy]. I, I know I was still suffering some effects of the chemo/operation; I think if you put it further, any further back, em, there would be too long a period of time between the operation and, and starting on your rehab[ilitation]. Oh yeah, I wouldn’t have, no I wouldn’t have been, no, the chemotherapy makes you very tired.
One participant allocated to the control group described his post-surgical experience as a rollercoaster. His description of his experience highlights why attending rehabilitation can prove difficult, if not impossible, for some people, and especially for those receiving adjuvant therapy, which reinforces the need for a tailored, individualised approach.
It was very much like a rollercoaster from start to finish, it was big highs and big lows . . . I had the operation . . . then went home for about 3 days, then massive haemorrhage . . . and they found more cancer . . . it was another operation . . . when I came out the second operation I accepted the stoma a lot better than I thought . . . I’m finding everything’s affected by the chemotherapy . . . my whole plumbing system is a bit less predictable . . . I was totally knackered.
Any capacity for exercise?
None at all.
Being invited to attend cardiac rehabilitation once all primary treatment (surgery and adjuvant therapy) was over was seen as fortuitous by some participants because it came at a time when they felt abandoned by cancer services.
And I saw you before the operation . . . so was that about the right time, that would have been about 5 weeks after your discharge?
Yes, yes it was actually, ‘cause it, you felt, you hadn’t been abandoned.
And you felt ready to start doing something more . . .
Yes
Physical, then?
Yes, yes. Before that I had been very, very tired.
Participant burden: questionnaires
Some participants did not have any problems with the questionnaire, whereas others felt that it was perhaps too long and repetitive. Nevertheless, the overall impression given was that the questionnaire was not a major burden for participants to complete.
I’ve no great shakes about the questionnaires.
Site 3 02 control
Oh they’re fair, the questions are fair, yes, and eh, I mean, there’s nothing that I’m stumbling to answer, you know, it’s very simple and eh straightforward.
Site 1 02 intervention
Did you feel the questionnaire was possibly too long?
No.
Questions that you didn’t understand?
There were certain questions that I didn’t answer but I had no problems with that either but [name of investigator] said. ‘OK here’s the question, do you want to answer it?’ and I said ‘No’.
Site 2 10 control
Some participants commented on the length of the questionnaire.
Well, most of it was all right. It was long, I have to say. Em, some of the questions seemed, maybe it was just to me, em inapplicable. You know, it wasn’t, well, some of them were confusing as well to me, to be honest, but in general, I mean, they were OK.
Site 2 09 control
A few questions I wondered why they wanted . . . it was a bit lengthy [laughter].
Site 2 07 control
I think they’re quite long . . . Yeah, they were OK to understand.
Site 1 13 intervention
Other participants commented on question repetition.
Fine, no problems . . . Two or three had very similar answers . . . There would be no point having a very short 5-minute thing. If you want to gain something from it I think it’s got to be at least the length that you had.
Site 1 23 control
A wee bit long, a wee bit long, I’m saying eh, and, no, eh yae seemed tae be getting the same question, again and again.
Site 2 02 intervention
Participants were asked to recall how much physical activity they had done for each day of previous week, and one participant found this a difficult task.
Some of the questions that were asked, you know, how long every day did you do exercise, like cleaning and I don’t study my life, so I had to stop and think about just what I did.
Site 2 17 control
The interviews suggest that answering these types of questions (e.g. about quality of life) may provide therapeutic benefit for participants. One participant, for instance, felt that by answering the questions some of his or her worries had dissipated.
Do you know what. Questions that [name of spouse] and I never ever thought about and we have answered them and I think it takes it away, a lot of the worry from us as well.
Site 2 16 control
Participant burden: accelerometers
Some participants reported no problems wearing the accelerometer.
Comfortable. I didn’t wear it in the shower, you say you can use it in the shower . . . but eh, that’s the only time I didn’t use it.
Site 1 02 intervention
I, I didn’t know I had it on half the, even when I was in work Monday, Tuesday, Wednesday, I didn’t realise I had it on, I sort of put it down by there.
Site 3 07 intervention
No problem at all.
Site 2 17 control
Easy to use. I think I wore it quite diligently.
Site 1 19 control
Oh no, it didn’t give me any bother.
Site 2 16 control
I knew what it was for and it was easy to put on and I had great fun with it telling everybody what it was or it wasn’t but that was just a bit of humour.
Site 1 23 control
Other participants reported problems wearing the accelerometer. The device proved particularly troublesome to wear for those who had a stoma or abdominal wound problems.
I couldn’t wear it because of the operation . . . it just wasn’t comfortable because of the hernia.
Site 2 07 control
The clip design was dreadful and eventually the clip came unglued from the actual accelerometer itself. The new one that I was given, I was given two, one with a strap round my tum, which I thought was not very good because I’ve got a bad down there and the other one was slightly better design clip but the actual accelerometer did fall off a couple of times.
Site 2 10 control
It was almost impossible to get on . . . trying to open it was just impossible. The belt one was uncomfortable. It would have been initially where my scar one, not my scar but the wound.
Site 2 09 control
Some participants appeared to be self-conscious when wearing the device.
Were you a little bit self-conscious of it possibly?
No, not really, eh, I mean, I’ve been wearing sort of loose tops, anyway.
I realised you had to do it [wear accelerometer] but I was putting up with so much with stitches round my rear end, stitches from here to there like top to bottom on my front, plus the [stoma] bag and all the rest.
Site 3 02 control
One participant forgot to wear the device.
Problem is that sometimes in the morning I would get up and I would go about my business and then I would go, ‘I forgot to put that on’.
Site 2 04 intervention
Focus groups with people with coronary heart disease
Generic versus disease-specific rehabilitation
None of the people with CHD said that they minded if people with cancer attended the cardiac rehabilitation classes.
I guess what I want to know is how you lot would feel about having those patients join you in a class?
Oh it’s fine.
Yeah.
Yes.
Site 3 CHDP3
So what are your initial thoughts when I say, ‘Putting cancer patients in your cardiac class’?
I don’t see why not, and if they’re just the same, why not?
Yeah.
The facilities can take it, I don’t see why not.
Site 1 CHDP3
What are your thoughts on having cancer patients in a class with you, as a cardiac patient?
I haven’t any problem with that, no.
It doesn’t bring anything up?
It wouldn’t make any difference.
Site 1 CHDP5
There were three main reasons why participants with CHD did not believe that mixed classes would be problematic. First, they believed that worries about becoming physically active were similar for both people with CRC and people with CHD who were recovering from treatment, and that both groups would, therefore, benefit from cardiac rehabilitation.
You have your own anxieties about starting to exercise, or starting to get back after major surgery, whatever that is, so you know.
I think the worries are the same for both.
Second, participants felt that people with CHD were not a homogenous group and that their different rehabilitation needs may not be too dissimilar to those of people with cancer.
Do you think the needs are different for yourselves, as cardiac patients, with people recovering from cancer surgery?
No. There are differences just within us anyway.
Third, cancer was not an unfamiliar disease for most people with CHD. They believed that many people attending cardiac rehabilitation would have family and friends who had been diagnosed with cancer, which meant that they had some understanding of the disease.
Do you think it would be difficult talking to these patients?
No.
Oh not at all.
Not at all.
No, fine.
Some people find cancer difficult to talk about . . .
They do, don’t they, yeah.
I think so, most people, well not most, but I think a lot of people these days have had experience of either friends or family.
Barriers for people with colorectal cancer attending cardiac rehabilitation
People with CHD were aware, from their own family and friendship networks, about some of the difficulties that people with CRC may face attending cardiac rehabilitation, such as having a stoma. Participants seemed to be particularly aware that a stoma could cause embarrassment.
I was only talking to a patient, well a pal of mine who was a patient some years ago, who again had eh colon cancer . . .
Oh really.
And eh, he came through it very well, he’s in great form. I have to say, he was very embarrassed when he had to have the bag put on.
Yes.
Yeah, and then he didn’t quite know what to do about it, especially when he was showering.
Yeah, I know several people who’ve got one, you don’t, you wouldn’t realise it.
No, not at all.
You don’t know.
Well, it depends on what they’ve had to eat.
[Laughter]
[laughs] The bag explodes!
My wife has had several bad experiences.
Really?
Has she, has she, oh yeah. But she still goes to the gym.
Key concerns about including people with cancer in cardiac rehabilitation
Participants with CHD believed that cardiac rehabilitation classes would probably need to expand should cardiac rehabilitation become routinely offered to people with cancer.
Do you think anything would need to be changed to incorporate my patients?
I think the thing you have to be aware of is how big the group gets.
Participants with CHD also believed that specialist input from cancer nurses may be required because people with CRC and people with CHD would be likely to ask different types of questions.
Sometimes when we have this talk it does overrun quite often and if there’s, if there’s more sets of questions to be asked relating to different issues [i.e. cancer], it may pull it back even further.
Yeah.
Yes, that’s true.
Will they have their own nurse then at all?
Discussion
Strengths and limitations
It is possible that some of the perceptions presented here represent those of a select cohort of people with CRC who were already motivated about, and interested in, being part of an intervention such as a cardiac rehabilitation programme. They may, for example, already have held positive views towards behaviour change, and, in particular, change in physical activity as a core component of their recovery. As demonstrated, however, we did uncover a broad spectrum of views, both positive and negative. In addition, the interviews were conducted by the same investigators involved in collecting baseline and follow-up measures from people with CRC, which might have influenced the extent to which participants were willing to criticise trial procedures. Nevertheless, these investigators were not involved in the direct care of participants, and, in particular, they were not involved in delivering the intervention (i.e. cardiac rehabilitation), and so participants might have been more candid about their views about the intervention itself. The generalisability of our findings, however, is limited, because the pilot was small scale, involving only 3 out of a possible 312 cardiac rehabilitation programmes throughout the UK62,63 and only small numbers of cardiac rehabilitation and CRC clinicians and people with CRC and CHD. The findings, nonetheless, provide valuable insights and a starting point for informing future research.
Feasibility and acceptability of cardiac rehabilitation for people with colorectal cancer
This qualitative study suggests that cardiac rehabilitation is an intervention that can motivate people with CRC to engage in physical activity. Clinicians perceived the main benefits of cardiac rehabilitation to be that people with CRC would increase their level of physical activity, overcome any fears about being physically active, gain in confidence and become more motivated to exercise. This was positive because they believed that physical activity was an important part of this patient group’s recovery. Similarly, participants with CRC believed that one of the main benefits of attending cardiac rehabilitation was that it would motivate people to get physically active following surgery. Motivation is a key construct in theories of behaviour change, including self-determination theory, and has been found to be associated with higher levels of physical activity among CRC survivors. 176 Motivation is, therefore, of particular interest to researchers and clinicians who are developing interventions to increase the level of physical activity among this group of the population.
This qualitative study indicates that cardiac rehabilitation gave participants the motivation to continue being physically active at home and once rehabilitation had ended. Whether motivation to be physically active is transitory or is maintained post rehabilitation requires further investigation. Being referred to rehabilitation may engender a feeling of ‘having to be’ rather than ‘wanting to be’ physically active. 177 There is a danger, therefore, that once rehabilitation stops, physical activity will also come to a halt. To sustain and maintain physical activity beyond rehabilitation, people would need to engage in physical activity because of its inherent satisfaction (e.g. because they enjoy it) and because they identify with the outcome (e.g. because it will improve personal health). 178 This suggests the need for a tailored and individualised approach to assessment of people’s physical activity needs and goals and their attendance at a rehabilitation programme.
According to self-determination theory, internalisation of the value (the benefits) of the outcomes of physical activity is likely to lead to greater persistence in being physically active. 179 This qualitative study, however, suggests that many participants were not informed of the benefits of being physically active. Indeed, recent studies indicate that provision of lifestyle advice from cancer care clinicians is low. 151,180–182 Yet clinicians are likely to play an important role in developing motivation because they can inform people with CRC about the associations between physical activity and health (see Chapter 1 for evidence about the benefits of physical activity for people with CRC). Developing this type of motivation may not prove to be too difficult because our qualitative study suggests that many participants with CRC were already aware of the benefits of adopting a healthy lifestyle. In addition, this study suggests that many cancer nurses were aware of the benefits of a healthy lifestyle for recovery. Diet appeared to be the main lifestyle behaviour that participants with CRC valued and associated with health benefit, suggesting that there is a need for greater promotion and awareness of the benefits of physical activity (see Chapter 1 for a summary of the benefits of physical activity for people with CRC).
None of the participants with CRC or participants with CHD had an issue with mixed CRC and CHD patient rehabilitation classes. Participants with CRC believed peer support to be a benefit of rehabilitation, a view that seemed to be expressed by people with CHD as well as people with CRC attending cardiac rehabilitation. The study shows that cardiac rehabilitation provided an arena for peer support from other people who had recently been diagnosed and treated with a life-threatening disease (CRC or CHD). Traditionally, peer support has been defined as support provided by people with the same disease. 183 Shared experience of the disease and experiential empathy is seen as crucial to the giving and receiving of support. 183,184 This study challenges the assumption that peer support for people with cancer can arise only from shared experience of the same disease. 185 Rather, our study suggests that people with CRC can obtain peer support from people with CHD in the context of rehabilitation. That peer support is not disease dependent opens up the possibilities of rehabilitation for mixed-disease patient groups. Moreover, our study raises the prospect of redefining peer support so that it is not confined exclusively to the shared experience of a specific disease.
A barrier to participation in cardiac rehabilitation was travel distance, which clinicians believed applied to people with CHD as well as to people with CRC. Clinicians believed that cardiac rehabilitation should be available to as many people with CRC as possible, but felt that those who had severe mobility difficulties may be unable to attend. Clinicians also recognised that recovery from CRC and its associated treatments was protracted and less straightforward than the recovery and rehabilitation pathway for people with CHD. Both clinicians and participants with CRC alike saw ongoing treatment, and, in particular, adjuvant chemotherapy, as a barrier to being physically active. A stoma was also felt to be a potential barrier, as well as a cause of embarrassment; steps would need to be taken to assure people with a stoma that cardiac rehabilitation was an environment in which they would be supported.
Clinicians understood that it was important for people with CRC to begin rehabilitation as early as possible if they were to reap and maximise the health benefits of cardiac rehabilitation. Early intervention fits with the enhanced recovery from surgery protocols developed by the Association of Surgeons of Great Britain and Ireland, which recommend ‘early and structured post-operative mobilisation’ for people undergoing surgery for CRC:186
A structured mobilisation plan should be in place. Patients should be helped to sit out in a chair on the evening of surgery and definitely by the first post-operative day. This should be followed by gentle assisted mobilisation either the same day or the next day.
p.14. 186 Reproduced with permission from the Association of Surgeons of Great Britain and Ireland
Early rehabilitation for patients with CRC also fits with the recommendations for patients with CHD. 187 This qualitative study suggests that cardiac rehabilitation provided a safe environment for people with CRC to increase their level of physical activity, and participants felt that they benefited from rehabilitation. On this basis, cardiac rehabilitation is an appropriate model of rehabilitation for people with CRC. The impression given was that clinicians delivering cardiac rehabilitation were able not only to support people to be physically active but, because of their professional training, also to provide a level of psychosocial support. This belief that clinicians should deliver rehabilitation aligns with patient views: people with cancer prefer to receive information about cancer from health-care providers. This model of rehabilitation to aid recovery may, therefore, be more acceptable to clinicians and people with CRC than, for instance, exercise referral to fitness centres.
Colorectal cancer nurses were enthusiastic about the prospect of integrating this model of rehabilitation within the cancer pathway. They believed that the surgical ward was a good place to talk to people with CRC about rehabilitation because at that point patients were already beginning to think about their recovery.
However, there was a degree of reticence about expanding cardiac rehabilitation to accept cancer patient referrals. Cardiac rehabilitation clinicians had two key concerns about including people with CRC in their service. They were concerned about their own capabilities for supporting people with CRC and also the capacity of cardiac rehabilitation to accommodate more patients. In particular, they were concerned that they would not have the relevant specialist knowledge and skills to support people with cancer because their specialism was cardiology. To improve their knowledge they wanted the full medical history of anyone using their service and wanted guidance and training on how people with CRC surgery who may be receiving adjuvant therapy, and, possibly, have a stoma, can safely exercise. In addition, cardiac rehabilitation clinicians wanted to know about the common psychosocial difficulties encountered by people with CRC because they viewed the provision of psychosocial support as part of their role. They also wanted a list of services for people with CRC so that they could of appropriately refer people with CRC to the relevant support. Finally, cardiac rehabilitation clinicians believed that a key gap in rehabilitation for people with CRC that they were unable to address was dietary advice. Thus, if cardiac rehabilitation was to be become the model of rehabilitation for people with CRC, additional input from CRC experts would need to be provided and fully integrated as part of the rehabilitation programme.
However, cardiac rehabilitation clinicians and people with CHD were concerned about the capacity of cardiac rehabilitation to accommodate more patients; their concerns regarded additional workload and whether or not the inclusion of people with cancer would create a worse service for people with CHD. Therefore, additional resources should be provided as an incentive for cardiac rehabilitation services to take cancer patient referrals. Indeed, if cardiac rehabilitation services perceive that the inclusion of other patient groups will be to the detriment of the service that they currently provide for people with CHD, then they are likely to withdraw their service for other patient groups, including people with cancer.
Feasibility and acceptability of trial procedures
Recruitment is critical to the success of a study. Finding out why people with CRC were likely to participate in this study was, therefore, an important study objective. Two of the main reasons people with CRC agreed to participate were altruism and personal benefit, echoing previous research findings about why people agree to participate in health research. 188,189 Any future trial should, therefore, consider advertising the benefits of rehabilitation and, in particular, physical activity for people with CRC in order to encourage participation and make people feel good about themselves for agreeing to participate because it may help others.
Randomisation was an issue for some, but not all, participants allocated to the control arm. A previous study found that about one-fifth of people with cancer who were surveyed about participation in clinical trials were worried about randomisation. 188 Thus, any future large-scale effectiveness trial would need to factor in the impact of randomisation on attrition rates when estimating the number of people who would need to be recruited for the study to be powered.
This qualitative study shows that participants either paid little heed to the participant information sheet (PIS) or could not recall its content. The UK Health Research Authority states that the guidance for producing PISs:
. . . should be considered as a framework, not a rigid template . . . One size does not fit all . . . The best way to make sure your consent documentation is fit for purpose is to test it with patient groups or other members of the public. 190
However, in our experience, PISs are often written to satisfy a NHS Research and Ethics Committee, whose members often request that the PIS includes, for instance, detailed procedures for making a complaint and a paragraph about how the research will be disseminated, and so on. The upshot of this is a very wordy and lengthy PIS that is not necessarily user-friendly or memorable. Any future study should, therefore, consider using different ways of presenting study information for participants so that it is easier to remember. More imaginative ways of presenting study information should be considered, including the use of technology (e.g. websites or mobile phone text messages). However, NHS Research and Ethics Committees would need to be convinced that less information and new approaches to giving information are ethical.
Our qualitative study suggests that the face-to-face investigator-administered self-report questionnaire using Bristol Online Survey (i.e. a non-paper-based questionnaire) was feasible to administer and generally acceptable to participants. Much has been written about the associations between length of questionnaire and response rates;191–193 some participants in our study found the questionnaire to be too long. Nevertheless, ‘how long is too long’ is likely to be subjective, and face-to-face administered surveys lasting over 1 hour have been found to be acceptable in some studies. 194 Moreover, a systematic review of strategies to improve retention rates in trials found that five trials (7277 participants) compared the effect of short and long questionnaires on postal questionnaire response rates and reported that there is only a suggestion that short questionnaires may be better (risk ratio 1.04, 95% CI 1.00 to 1.08; p = 0.07); based on one trial (of 900 participants), there is no clear evidence that long and clear questionnaires are more or less effective than shorter condensed questionnaires in terms of increasing questionnaire response rates (risk ratio 1.01, 95% CI 0.95 to 1.07; p = 0.86). 157
The repetition of questions, however, was an issue. There is a trade-off between minimising respondent burden, by the removal of questions, and making full use of a validated questionnaire. In our study, we did attempt to avoid repetition by removing some questions (see Chapter 5), but a future study should consider reducing this further if possible. Our qualitative study indicates that responding to questions yielded therapeutic benefit for some participants, which bears similarities to the findings of a survey about respondent satisfaction and burden. 195
Accelerometer use in physical activity and cancer research is becoming increasingly popular,96 but compliance with wearing the device is essential to avoid missing data. There is, however, only minimal guidance for the use of accelerometers among people diagnosed and living with cancer, and none specifically for people with CRC. 97 In this study, we suggested to participants that they wear the accelerometer around their waist rather than around their wrist, because wrist-worn accelerometers are not as accurate as hip-worn accelerometers at classifying activity and sedentary behaviour. 196 However, the interviews suggest that wearing the accelerometer in this way was a burden for some participants, especially for those with abdominal wound problems or a stoma. Thus, for the purposes of consistency in measuring the amount of physical activity, all people with CRC should be requested to wear the device on the wrist in future studies.
Chapter 10 Economic evaluation
Economic evaluation
The main aims of the economic evaluation component in the CRIB pilot study were to identify the main resource implications of delivering the rehabilitation programme and the subsequent impact on NHS care required by the participants; and devise and test approaches and tools to measure these.
To do this, the analysis looked at:
-
response rates to data collection tools
-
the cost of delivery for the rehabilitation groups
-
the health-care costs of participants in both trial arms
-
health-related quality of life through the calculation of utility values using the EQ-5D-5 Levels (EQ-5D-5L).
Feasibility
The main purpose of the CRIB pilot trial was to prepare the way for a full trial by testing methods of delivery and trial materials. In this pilot trial, response rates for the Health Resource Use questionnaire and EQ-5D-5L were high, and it appears that patients did not have problems filling in the questionnaires. A total of 40 (97.5%) (control, n = 20; intervention, n = 20) out of 41 participants, and 31 (75.6%) (control, n = 16; intervention, n = 15) and 25 (61%) (control, n = 13; intervention, n = 12) participants completed the questionnaires at the follow-up 1 and follow-up 2 time points, respectively. Overall, 60.9% of participants completed the trial.
Cost data
The overall cost of the programme is divided into two main components: the cost of delivery of the rehabilitation groups and the health-care resource use of all trial participants.
Rehabilitation groups
The content of the rehabilitation groups is described in Chapter 6. The key resources used for each group were the staff time, equipment and room hire. It was expected that the patients with CRC would attend existing classes being run for patients with CHD; therefore, the marginal cost per participant was expected to be negligible. However, each trial site was requested to provide an estimated cost per patient for running additional classes to accommodate the increased number of patients referred from cancer services. Table 48 shows the costs of providing cardiac rehabilitation for each site.
| Site 1 | Site 2 | Site 3 |
|---|---|---|
| £375.18 | £437.21 | £198.71 |
| Based on 180 patients | Based on 412 patients in 2013 | Based on 24 participants |
Sites 1 and 2 could provide only costs related to their usual cardiac rehabilitation team costs and not specific costs related to this trial. The above costs for these two centres would apply only if they had to set up a cardiac rehabilitation intervention from scratch. This means that NHS centres provided historical cost data that related to all costs with standard cardiac intervention. Box 4 presents the costs provided by sites 1 and 2.
Core staffing.
One administrative assistant: £17,803 pro rata 16 hours per week.
One physiotherapist: £34,876 pro rata 22 hours per week.
One rehabilitation nurse: £29,043 37.5 hours.
One physiotherapy assistant: £17,803 pro rata 22 hours per week.
Total costs: £67,533.
Annual patient numbers = 180.
Site 2 costs1.0 WTE senior physiotherapist (band 7).
3.6 WTE cardiology specialist nurses (band 6).
0.6 WTE physiotherapist (band 6).
0.3 WTE physiotherapist (band 5).
0.1 WTE support nurse (band 3).
Ad hoc (unquantified) clerical/medical records support.
Total cost per annum = £180,132.
Total number of patients assessed by the cardiac rehabilitation team in 2013 = 412.
Site 3’s cost data were the closest to marginal costs of adding patients with CRC to an existing class. Site 3 claimed £198 per trial participant to hire a physiotherapy assistant to accommodate additional CRC patients into the cardiac rehabilitation service. However, it is not clear if these would be recurring costs if this was an established NHS intervention. For a future larger-scale trial it would be necessary to establish if patients are being added to existing groups and the associated marginal costs of this or if new groups are required as this will probably impact on the overall cost-effectiveness of the service.
Health-care resource use
Resources used by the participants were recorded in the outcome questions. Participants were asked to record health-care resource use for the 3 months prior to the trial beginning to give us an indication of resource use prior to taking part in the trial (reported in Table 49). It would not generally be necessary to collect or report these data as we would assume that randomisation of participants would wash out any larger outliers, but given the small number of participants in the trial, it was important in this study to be aware of any large differences between the two groups prior to the start of the programmes.
| Service used | Intervention group | Control group |
|---|---|---|
| GP at surgery | 65 | 111 |
| Nurse at surgery | 58 | 65 |
| GP on the telephone | 10 | 17 |
| Nurse on the telephone | 20 | 29 |
| NHS Direct | 15 | 3 |
| GP at home | 7 | 15 |
| Nurse at home | 109 | 173 |
| Out-of-hours clinic | 7 | 1 |
| A&E visit | 2 | 6 |
| Outpatient department | 41 | 75 |
| Admitted to hospital | 11 | 47 |
| Emergency ambulance | 1 | 3 |
| Allied health professionals | 90 | 20 |
All contacts with NHS services and clinicians in the two follow-up periods, excluding the 3 months before baseline, are presented in Table 50.
| Service used | Rehabilitation group | Control group |
|---|---|---|
| GP at surgery | 51 | 69 |
| Nurse at surgery | 18 | 36 |
| GP on the telephone | 4 | 7 |
| Nurse on the telephone | 14 | 14 |
| NHS Direct | 7 | 2 |
| GP at home | 5 | 5 |
| Nurse at home | 19 | 47 |
| Out-of-hours clinic | 4 | 0 |
| A&E visit | 2 | 3 |
| Outpatient department | 25 | 35 |
| Admitted to hospital | 6 | 3 |
| Emergency ambulance | 0 | 0 |
| Allied health professionals | 76 | 13 |
Health-care costs
The information on resource use was combined with the unit cost of each resource to estimate the total cost of NHS resources used. Health service unit costs were valued using the most recent Department of Health resource cost data, at 2013–14 UK prices. 197 The NHS resources that were included and their unit costs are shown in Table 51 along with the source of cost information. The cost of drugs consumed by participants only includes drugs prescribed by participants’ general practitioner (this is not shown in the table). The British National Formulary200 was consulted for the unit cost of individual drugs prescribed to participants.
| Service | Unit cost (£) | Reference |
|---|---|---|
| GP at surgery | 45 | PSSRU198 |
| Nurse at surgery | 16 | PSSRU198 |
| GP on the telephone | 27 | PSSRU198 |
| Nurse on the telephone | 5 | PSSRU198 |
| NHS Direct | 8 | Richards et al. (2004)199 |
| GP at home | 114 | PSSRU198 |
| Nurse at home | 47 | PSSRU198 |
| Out-of-hours clinic | 66 | PSSRU198 |
| A&E visit | 124 | DH reference costs197 |
| Outpatient department | 111 | DH reference costs197 |
| Admitted to hospital | 1542 | DH reference costs197 |
| Emergency ambulance | 235 | DH reference costs197 |
| Allied health professionals | 22 | DH reference costs197 |
NHS resource use was combined with unit costs. The total mean costs for each trial arm are shown in Table 52.
| Rehabilitation group (£) | Control group (£) | |
|---|---|---|
| Mean cost at baseline | 1080 (1592) | 5206 (11,282) |
| Mean cost at end of intervention (follow-up 1) | 953 (1110) | 667 (1148) |
| Mean cost at follow-up 2 | 349 (603) | 399 (546) |
| Mean total cost | 826 (1217) | 2204 (7002) |
| Total cost excluding baseline | 685 (955) | 547 (924) |
NHS resource use and associated costs was similar in the two groups. There were some differences at baseline, but these were to be expected given the very serious nature of these patients’ condition. For example, two patients in the control group were admitted to hospital for a combined total of 41 times in the 3 months prior to baseline, inflating both resource use and mean costs in that group. At the first follow-up, resource use dropped in both groups, and it was much lower at follow-up 2, which was due to an improvement in participants’ health.
As the follow-up period of the study is < 1 year, the costs and outcomes are not discounted.
Patient costs
Information on patients’ out-of-pocket expenses was collected in the questionnaires. Patients were asked at baseline and follow-ups if they had bought medicines related to their condition. Most patients responded negatively to this question as they were likely to have received free NHS prescriptions and did not require extra medications. The patients who bought over-the-counter medicines spent an average of £5 each on items such as Corsodyl® mouthwash (GlaxoSmithKline, Brentford, UK), DioralyteTM sachets (Sanofi, Guildford, UK), Bio-Oil® (Pacific World Cosmetics, Aliso Viejo, CA, USA), heat gels, paracetamol, laxatives, Wind-Eze tablets (Forest Laboratories UK Ltd, Barnstaple, UK), vitamin D tablets, and herbal cleanse and other constipation-related treatments.
Patients were also asked if they had used any complementary therapies, such as reflexology, acupuncture or homeopathy. Again, most patients indicated that they had not used any such treatments. Three patients had acupuncture sessions, spending an average of £250 on these (this high figure was due to one patient in the control group spending £550 on acupuncture). Three patients had massage sessions, costing an average of £60. One patient reported having reflexology with no cost.
Questions 44–47 in the patient self-reported questionnaire asked patients if they had incurred costs specifically related to the cardiac rehabilitation of this trial. This means that these questions applied only to patients in the treatment group and should have been left blank by patients in the control group. Valid replies to these questions by patients in the rehabilitation group included the cost of having to purchase jogging pants, ankle splints and t-shirts, with less than £20 spent on each item. Six patients spent an average of £50 to travel to the cardiac rehabilitation classes. One patient required two extra gym sessions per week, costing an extra £4 per week. Finally, one patient had to take time off work to attend the cardiac rehabilitation classes, a total of 12 hours off.
Looking at the out-of-pocket expenses of patients in the rehabilitation group, it is clear these were not significant and should not affect costing considerations for this trial. If anything, it appears that patients in the control group spent more on alternative treatments than patients in the rehabilitation group.
European Quality of Life-5 Dimensions-5 Levels
Data for the EQ-5D-5L were collected at baseline and follow-up. Table 53 reports the mean utility score at baseline and follow-up by trial arm.
| Time point | Rehabilitation group | Control group |
|---|---|---|
| Mean EQ-5D baseline | 0.860 (0.150) | 0.799 (0.232) |
| Mean EQ-5D follow-up 1 | 0.855 (0.147) | 0.841 (0.254) |
| Mean EQ-5D follow-up 2 | 0.840 (0.161) | 0.799 (0.155) |
Discussion of economic evaluation findings
Economic evaluation can be defined as a comparison of alternative options in terms of costs and consequences. 201 As such, one can argue that we do not perform a full economic evaluation here as we mainly present costs and consequences rather than perform a formal comparison between interventions. Given the limited sample size and no difference in the primary clinical outcome measure and in measured health gain, as captured by the EQ-5D, between the two groups, we decided not to perform a full economic analysis in this pilot trial. In a future main trial, a cost-effectiveness analysis will be performed following methods as outlined by Drummond et al. 201 Costs and outcomes for each of the trial participants will be calculated and the mean incremental cost-effectiveness ratio (ICER) will be estimated as:
The primary economic outcome in this analysis will be the quality-adjusted life-year, using the EQ-5D-5L, measured at different time points during the trial, and the economic evaluation will estimate the incremental cost per quality-adjusted life-year associated with the treatment as stated in the protocol and shown above. The additional (incremental) costs associated with the treatment, when added to usual care, will be estimated using resource-use data collected within-trial, and unit costs for resource use from national published/NHS sources.
Hence, we are pleased to report that in this pilot all trial materials and questionnaires were tested successfully and proved suitable for economic evaluation purposes. There was a high response rate for Bristol Online Survey which included EQ-5D and other measures used in the trial. There were no missing data beyond people who did not participate in the follow-ups. We recognise that recall bias is a problem when using self-report to measure health service use. For the future we recommend that the larger full trial will need to clearly establish the ‘true’ costs of health service use using objective measurement and of the rehabilitation group and whether or not these can absorbed into current practice. It was initially planned that the CRC participants would enter existing classes, keeping costs at a minimum, so we need to establish if this is possible. A full economic evaluation during the main trial will require accurate cost data, so this issue must be addressed. Nevertheless, based on the data reported in this section and elsewhere in this report, we believe that a full trial is feasible and a full economic evaluation can be performed if similar materials and methods are employed in a future definite trial.
Chapter 11 Patient and public involvement
User involvement has been demonstrated to positively impact health research from the conceptualisation of research topic, through to data collection, interpretation and reporting. 202 The NIHR supports and encourages patient and public involvement in all the research that it funds, on the basis that this can lead to better research that is more focused on the needs of patients and can accelerate the transfer of research evidence into practice. A handbook for researchers about patient and public involvement (PPI) has been published by NIHR. 203 In addition, INVOLVE (www.invo.org.uk) is funded by the NIHR to support greater public involvement in NHS, public health and social care research.
Patient and public involvement can take different forms, including:
-
prioritisation of studies
-
design and management of studies
-
data collection and analysis
-
dissemination of findings.
In this chapter, we describe the PPI for this particular study.
Involvement of Bowel Cancer UK (Scotland)
The manager of Bowel Cancer UK (Scotland), Emma Anderson, was a member of our project advisory group. Two patient advisors who were recruited from Bowel Cancer UK (Scotland) also participated in the study. All three contributed towards the design of the study, advising on, for example, study information sheets and consent forms. One of the patient advisors moved abroad and so curtailed her involvement in the study. The other patient advisor, Gillian Sweetman, presented at the NIHR ‘welcome’ event, was a member of the project advisory group and conducted interviews with three people with CRC during phase 1. She participated in a 1-day training course so that she could conduct these interviews. Dr Gill Hubbard (the study’s principal investigator), who organised the event for members of the Service User Research Partnership of Breast Cancer Care, delivered the training. Gillian Sweetman was invited with agreement from Breast Cancer Care.
In March 2015, Gillian recounted her overall experience of being involved in the CRIB project (Box 5).
I was diagnosed with stage III bowel cancer in July 2008 with a solitary lung secondary. My immediate goal following bowel surgery was to be fit enough to have the lung surgery . . . and to then be fit enough for the chemotherapy. During chemotherapy I wanted to be able to have the next cycle on time.
I am a doctor, but it is so different helping patients to experiencing illness 24 hours a day. Getting out and walking helped me psychologically as well as physically. My background gave me the confidence to exercise.
Once treatment was complete, I looked around for voluntary work and found that Bowel Cancer UK needed volunteers to help with the Bowel Cancer awareness programme, and through that I became involved in the charity. If you are open about your own diagnosis you also start to meet people on a similar or harder journey with different types of cancer.
Sharing with others, there is a difficult stage when treatment finishes and the immediate link with hospital and more intensive treatment stops. There is little information given on self-help. I would have been very rich if I’d taken a pound for every time I was told to take it easy!
I was offered the opportunity to help with the CRIB project and from the proposal I could see immediately how this programme could fill this information gap and help people exercise with confidence.
I valued being able to help with writing the patient information sheets and with the initial presentation. I have participated as a member of the steering group. I have also helped with some feedback interviews from patients. The participants I met were very positive about the programme.
It has been a valuable experience being involved in this project. The intensive surgery and chemotherapy are very expensive. Rebuilding confidence that has been broken down by the diagnosis and treatment is important and it is this type of project that will show the best ways to do this. This will be both to the benefit of individuals and society.
Discussion and recommendations
In this study, we involved a leading UK CRC charity. We worked particularly closely with a full-time member of staff and a volunteer who had direct and personal experience of CRC as a patient. Their views about the study, in terms of its importance for people with CRC, were articulated at research group meetings, which instilled confidence that the study was worthwhile and would be supported by the charity at the national level. In addition, Gillian’s involvement demonstrated that, with training and support, volunteers can work closely with the researchers, helping, for instance, to collect data. This helped to situate Gillian as one of the team rather than as the patient representative on the team. The difference between the two approaches may be subtle, but by involving volunteers as co-researchers in this way, we developed a more inclusive team. Based on our experience, this pilot work can be used as a springboard for further PPI in any future trial.
Chapter 12 Discussion and conclusions
In this chapter we summarise key evidence and draw conclusions about the feasibility and acceptability of cardiac rehabilitation for people with CRC and the feasibility of running a future multicentre definitive trial.
Results
Is cardiac rehabilitation an acceptable and feasible rehabilitation service for people with colorectal cancer?
Bowen et al. 75 recommend eight areas of focus to assess if a public health intervention is feasible. Table 54 presents an assessment of the feasibility of cardiac rehabilitation as a rehabilitation model for people with CRC against these criteria. Overall, our feasibility work indicates that:
-
Cardiac rehabilitation is an acceptable rehabilitation service for people with CRC.
-
There is likely to be a demand for this service from people with CRC.
-
Cardiac rehabilitation physiotherapists and other members of the team can be trained in cancer and exercise and to support people with CRC to exercise safely.
-
Additional resources (e.g. the appointment of an assistant physiotherapist) are likely to be needed in order to expand cardiac rehabilitation so that the service can accommodate additional patients.
-
Cardiac rehabilitation exercise classes do not need to be adapted because physiotherapists are able to support people with CRC to exercise safely.
-
Cancer-specific educational sessions need to supplement the cardiac-specific educational sessions so that people with cancer attending cardiac rehabilitation have their psychosocial needs addressed.
-
Cardiac rehabilitation can be integrated into cancer pathways with minimal disruption.
| Area of focus | The feasibility study asks . . . | Evidence for CRIB |
|---|---|---|
| Acceptability | To what extent is a new idea, programme, process or measure judged as suitable, satisfying, or attractive to programme deliverers? To programme recipients? | The study shows that cardiac rehabilitation as a rehabilitation model for people with CRC is attractive and acceptable to the people with CRC. The study suggests that patients and clinicians believe that mixed patient rehabilitation classes are a good idea; nobody (people with CRC, people with CHD, or cancer and cardiac clinicians) had a problem with mixed rehabilitation classes for people with CRC and people with CHD. Physical activity was regarded as a key component in the recovery and rehabilitation process for people with CRC. The perceived main benefits of cardiac rehabilitation for people with CRC were that they would increase their level of physical activity, overcome fears about being physically active, gain confidence and become more motivated to exercise, access peer support and forge new friendships and obtain psychosocial support from trained clinicians |
| Demand | To what extent is a new idea, programme, process or measure likely to be used (i.e. how much demand is likely to exist?) | The study indicates that demand will be reasonably high because people with CRC believe there to be a range of benefits from attending cardiac rehabilitation; they believe that cardiac rehabilitation will help them to increase their level of physical activity, overcome fears about being physically active, gain confidence and become more motivated to exercise, access peer support and forge new friendships and obtain psychosocial support from trained clinicians We recommend, however, that barriers to cardiac rehabilitation attendance be addressed. In particular, people in poor physical health or with mobility difficulties should be encouraged to attend, and we recommend a flexible start date so that people with protracted recoveries following surgery can also attend. In addition, we recommend addressing travel difficulties by, for instance, offering outreach services or virtual classes |
| Implementation | To what extent can a new idea, programme, process or measure be successfully delivered to intended participants in some defined, but not fully controlled, context? | This study shows that it is possible to train cardiac physiotherapists and other members of the team in cancer and exercise so that they can support people with cancer to exercise safely. The evaluation of the cancer and exercise training was excellent, with all physiotherapists and nurses agreeing or strongly agreeing that the information and course content was helpful and well presented The study also shows that cardiac rehabilitation can be successfully delivered to intended participants (i.e. people with CRC); for instance, cardiac rehabilitation attendance was high, ranging from 75% to 142% (some people attended more sessions than planned). Moreover, cardiac rehabilitation is widely available throughout the UK and our study, conducted in three sites, suggests that cancer service pathways could be easily modified so that cancer nurses could refer patients to cardiac rehabilitation |
| Practicality | To what extent can an idea, programme, process or measure be carried out with intended participants using existing means, resources, and circumstances and without outside intervention? | The economic evaluation suggests that the costs of cardiac rehabilitation for people with cancer will depend on whether it involves adding people with CRC to an already existing service or setting up a completely new service. Costs would be marginal for the former The study suggests that there are concerns about capacity and it is likely, therefore, that, were this service to be offered to people with CRC, additional staff (e.g. a physiotherapy assistant) would need to be hired. Thus, we recommend the provision of additional resources so that cardiac rehabilitation staff can accommodate people with CRC without it having a detrimental impact on people with CHD |
| Adaptation | To what extent does an existing idea, programme, process or measure perform when changes are made for a new format or with a different population? | The study shows that existing cardiac rehabilitation can perform with a different population (i.e. people with CRC) However, the study suggests that cardiac rehabilitation clinicians do not feel competent providing specific CRC-related psychosocial advice and support. We believe that cancer services rather than the cardiac rehabilitation address gaps in rehabilitation support, such as stoma care and dietary advice, because they have the required expertise |
| Integration | To what extent can a new idea, programme, process or measure be integrated within an existing system? | This study suggests that referral pathways can be easily introduced so that CRC nurses can refer people with CRC to cardiac rehabilitation. In addition, CRC nurses can easily provide information (e.g. type of treatment, medication, comorbidities) about patients with CRC to the cardiac rehabilitation team so that they can support people with CRC to exercise safely |
| Expansion | To what extent can a previously tested programme, process, approach or system be expanded to provide a new programme or service? | The study shows that cardiac rehabilitation physiotherapists and other members of the cardiac rehabilitation team are able to support people with CRC to exercise safely. One AE was reported that was unrelated to the study, and attendance by people with CRC was high |
| Limited efficacy | Does the new idea, programme, process or measure show promise of being successful with the intended population, even in a highly controlled setting? | This pilot study did not set out to test if cardiac rehabilitation for CRC is effective |
Are trial procedures acceptable and feasible?
Thabane et al. 77 propose four primary purposes for conducting pilot studies. Table 55 presents an assessment of the feasibility of the main trial components against these set criteria. Our feasibility and pilot work indicates that some trial procedures worked particularly well, giving us good reasons and a solid foundation for moving forward. Nonetheless, our work also highlights a major challenge for a future trial; our recruitment strategy led to a sample of particularly healthy and active people with CRC. This, coupled with a small sample size, is the most likely reason why we did not find any differences in level of physical activity between the intervention and control groups at baseline or follow-up, which we could use to derive a sample size calculation for a future trial. Our recruitment strategy did not recruit people with CRC who needed it most, that is, those who were inactive and in poor health. We do not know if more targeted strategies to improve recruitment among people who are inactive or in poor health will work. Moreover, what this group can achieve may be different from that of an already active group of patients; it may not be feasible for the former group to reach a target of 150 minutes of moderate physical activity per week. Thus, perhaps a more achievable, but clinically relevant, end point for this group of patients would be a reduction in sedentary behaviour. Nevertheless, based on current evidence (presented in Chapter 1), which shows the health benefits (e.g. survival and quality of life) of increasing post-diagnosis physical activity, there is a strong argument for retaining change in amount of weekly physical activity as a primary outcome in future trials of structured physical activity interventions for CRC survivors. A key finding from phase 2 was the number of missing data from the accelerometers. However, given the limitations of using self-report to measure change in physical activity (discussed in Chapter 8), objective measurement is preferable. There are few studies that have used accelerometers with this clinical population and so it is premature to dismiss this method to measure physical activity. Instead, we believe that it is critical to develop and evaluate strategies to encourage participants to wear the device according to protocol.
| Area of focus | The feasibility study asks . . . | Evidence for CRIB |
|---|---|---|
| Process | This assesses the feasibility of the processes that are key to the success of the main study | This study suggests that a future trial is feasible for the following reasons:
Improving eligibility rate The main reason for nurses excluding people with CRC from the study was poor mobility (35%). However, most cardiac rehabilitation accommodates people with poor mobility, including people who use a wheelchair. Cardiac rehabilitation also accommodates people with poor physical health (e.g. there is a low-intensity class for people who are in poor physical health). We therefore recommend training clinicians involved in screening about the viability and importance of including people with poor mobility and health. We believe that the majority of patients excluded for poor mobility would, in fact, be candidates for the intervention, meaning that eligibility could potentially be increased by over 30% Addressing recruitment bias There was recruitment bias. At baseline, most participants were meeting or nearly meeting recommended levels of physical activity (i.e. 30 minutes of moderate physical activity per day). This is problematic for the NHS and for triallists. People meeting recommended levels of physical activity will already be maximising their chances of obtaining the health benefits associated with post-diagnosis physical activity. There seems little clinical value in providing a service to people who are already reaping health benefits arising from being physically active However, extra efforts will have to be made to recruit people who are inactive and in poor health because our study suggests that these people are less likely to wish to participate in a physical activity trial. Our study suggests that introducing motivational strategies, such as providing information about the benefits of being active, may help recruitment. Moreover, reducing the amount of sedentary behaviour may be a more acceptable and clinically meaningful end point for this group of patients. This could be achieved by exploring the use of alternative, emerging technology in the field of activity tracking devices Improving consent rate The study shows that 44.5% of eligible patients with CRC who wished to participate withdrew before randomisation. One of the reasons why people with CRC (who wanted to participate in the study) changed their minds was because they did not feel able to exercise while on treatment (e.g. adjuvant chemotherapy). We recommend, therefore, excluding people who are on active treatment. This change to the eligibility criteria should not impact on eligibility rate; it will merely delay when people are invited to enter the study (i.e. post treatment). Including only people with CRC post treatment should, however, increase the consent rate by approximately 20% (18% of those who were interested in participating changed their minds because they were having adjuvant therapy and did not wish to or did not feel able to exercise during this time) Improving completion rate
Across all time points, 31% (n = 20) of accelerometer data sets were assessed as invalid. The main reasons were participants not wearing the device or not wearing it for long enough. There was significant variation across sites and, importantly, one site had only one invalid accelerometer data set. The investigator in this site had a sports science background and was familiar with measuring level of physical activity and already well versed in the benefits of physical activity for people with CRC. We therefore recommend introducing strategies to improve accelerometer wear-time, such as additional training for investigators so that they can explain to participants the importance of these data for the study. We also recommend providing individual feedback on the level of physical activity recorded by the device to each participant so that they can actually see the data generated from these devices about their level of physical activity and sedentary behaviour. Evidence is lacking about the actual impact of these strategies on completion rates but we would expect positive reinforcement to increase compliance and data acquisition Improving intervention take-up Thirteen out of 21 participants (62%) randomised to the intervention group completed the cardiac rehabilitation programme as per protocol. Three participants started cardiac rehabilitation but could not complete all classes and five did not begin (38%). Two of these participants stopped attending because they did not feel well enough to exercise while having chemotherapy. As suggested, we therefore recommend excluding people on active treatment to address this barrier to attending cardiac rehabilitation (see above) |
| Resources | This deals with assessing time and resource problems that can occur during the main study | The study highlights the following potential resource problems:
|
| Management | This covers potential human and data management problems | The study involved three sites and a clinical trials unit. No data management problems were encountered that could not be easily addressed and the study was conducted according to protocol. Recruitment rates and the number of missing accelerometer data sets varied across the three sites and we therefore recommend regular monitoring of each site in parallel with data validation procedures in any future trial so that problems can be identified early in the study and addressed accordingly |
| Scientific | This deals with the assessment of treatment safety, dose, response, effect and variance of the effect | One AE was reported that was unrelated to the study, suggesting that the intervention and trial procedures are low risk We did not aim to measure effect on health outcomes because of our small sample size. That no differences were found between the intervention and control groups for physical activity and psychosocial outcomes is, therefore, neither surprising nor unusual for a feasibility study of this scale |
Limitations
This study was conducted to inform design and make decisions for a future full-scale trial of cardiac rehabilitation for cancer patients, including whether or not a full-scale trial should be done at all. This requires careful judgements about the limitations of this study and the impact those limitations will have on the applicability of what we have found to any future full-scale trial. These judgements are part of all feasibility and pilot studies. Broadly speaking, potential limitations fall into three categories:
-
Design and conduct challenges: challenges and problems encountered in the feasibility and pilot study that suggest changes are needed if a full-scale trial is to be feasible.
-
Unanticipated conduct failures: a planned part of the feasibility and pilot study was not possible because of some external event not linked to the intervention under evaluation (e.g. slow approvals, loss of trial staff).
-
Generalisability of the sites selected for the feasibility and pilot work: whether or not there is reason to suggest that the sites involved in the feasibility and pilot study are atypical of sites that would be part of a full-scale trial.
Item 1 is what feasibility and pilot studies are chiefly designed to identify: issues that make a full-scale trial either unfeasible or likely to be unfeasible without modification to what was planned and subsequently used in the feasibility study. In our study, there were many trial design issues falling into this category, including suboptimal eligibility, consent and completion rates and missing data. Issues relating to the intervention – cardiac rehabilitation – falling into this category include barriers to attending cardiac rehabilitation and the capability of existing cardiac rehabilitation clinicians to support people with cancer.
To address these challenges and study limitations we recommend induction training for staff involved in recruitment and data collection. The training, for instance, would address suboptimal recruitment by highlighting to staff the health benefits of cardiac rehabilitation for patients who have poor mobility with the aim of boosting the recruitment of those patients in relatively poorer health. The training would also aim to address missing data by increasing researchers’ competence to collect accelerometer data by for instance, emphasising the importance of these data for the study and showing staff how to explain to participants how and when to wear the accelerometer device. To improve the consent rate we propose to remove a major barrier to participation in the study, travel to and distance from cardiac rehabilitation, by including outreach services in any future trial. Offering cardiac rehabilitation outreach services would also address a limitation of the intervention, which was that cardiac rehabilitation was too far away for some people to travel. Another barrier to consenting to the study and also to attending cardiac rehabilitation highlighted by our feasibility and pilot work was poor recovery from surgery and ongoing treatments, especially adjuvant therapy. Indeed, there was a general reluctance by participants to start exercising during ongoing treatment. To improve the consent rate and address barriers to participating in the cardiac rehabilitation programme, we therefore suggest including only patients who are at the end of treatment. We also recommend adopting a strategy whereby patients are involved in the decision about their start date for attending cardiac rehabilitation. This is because a limitation of the study design, and also of the intervention, was that some patients needed more time post surgery to agree to participate than we provided. Additionally, questionnaire completion rates fell from baseline to first and then second follow up. To improve completion rates we recommend the adoption of evidence-based strategies such as monetary incentives to return questionnaires together with regular reminders. Finally, our study found that cardiac rehabilitation clinicians did not feel competent providing specific cancer-related psychosocial support to participants with CRC. Hence to address this limitation we propose that cancer clinicians should continue to provide cancer-specific psychosocial support while cardiac rehabilitation clinicians provide generic as opposed to disease-specific psychosocial support as well as supporting people to exercise.
Item 2 in the list of limitations differs from item 1, in that the limitation is not necessarily a result of the trial design, the intervention or its delivery, but of the inability of the study team to make something happen because of one or more unanticipated problems: generally an external event, such as the loss of a key member of staff. Whether or not these problems could have been anticipated is a moot point; the key issue is that they were not anticipated, the impact was substantial and, as a consequence, part of the study could not be carried out. In our pilot study, we were unable to meet one study objective, which was to provide data for sample size calculations for a definitive RCT. Our inability to provide data was a consequence of failing to meet our recruitment target of 66 (the recruitment challenges mentioned above meant that we recruited 41) and also of recruitment bias. Indeed, a key finding from the feasibility and pilot work, and also a good illustration of why feasibility and pilot work is important, was recruitment bias; most participants were already meeting or close to meeting the recommended level of physical activity for the adult population (i.e. 150 minutes per week of moderate physical activity), which is unusual for this patient group and, indeed, for the general population. 14–19 To address this study limitation we propose in a future trial to revise the eligibility criteria and exclude patients who are already physically active. This would also mean that the cardiac rehabilitation would be targeting those patients who need it most, that is, those who are currently not meeting the recommended level for physical activity associated with health benefits. This may, of course, extend the recruitment period of any full-scale trial, but this could be planned for from the start.
Item 3 is a challenge for all feasibility and pilot studies, and perhaps triallists have a tendency to overestimate the generalisability of findings from small-scale work carried out in a handful of sites to what might be many tens of sites in a future full-scale trial. There is, of course, a balance to be struck between the duration and cost of feasibility and pilot work and the duration and cost of a potential full-scale trial. For example, recruitment is still an important concern for all triallists; a recent study of NIHR- and MRC-funded trials found that about half of these trials met their targets despite some of the trial teams having done feasibility and pilot work. 204 An earlier study that looked explicitly at the impact of pilot work on recruitment did not find a clear link between doing pilot work and successful recruitment. 205 Our feasibility and pilot work suggests variation in sites regarding recruitment parameters (we have written about this in detail elsewhere141 as well as in this report) and we therefore anticipate that some sites will meet recruitment targets and others will not. Which sites are likely to perform well is difficult to judge in advance. It is for this reason, in particular, that we propose an internal pilot as part of any future multisite effectiveness trial. The internal pilot should have clearly defined stop–proceed rules based around recruitment targets. Another limitation of the study is that people with CRC who agreed to participate might have been particularly keen to increase their level of physical activity, which means that the findings may not be applicable to those people with CRC likely to be less interested in being physically active to aid their recovery and reduce risk of recurrence. As discussed above, we are proposing that any future trial should exclude those who are already physically active. Limitations on the ability to make generalisations about the intervention are that the costs associated with the referral of cancer patients to cardiac rehabilitation is likely to vary by site; the capacity of a cardiac rehabilitation service to take additional patients is also likely to vary from one site to the next. These variations are likely to be important information for those who are tasked with commissioning or managing services. Finally, we recommend embedding a process evaluation in any future trial so that contextual factors impacting the delivery of cardiac rehabilitation to patients with cancer (including the capacity to take more patient referrals) in each site are also highlighted. This is important given that there are over 300 cardiac rehabilitation services in the UK, and this and any future study is likely to include only a fraction of these sites.
In summary, this feasibility and pilot work highlights a range of trial design limitations, including suboptimal eligibility, consent and completion rates, missing data and recruitment bias. It also highlights the limitations of cardiac rehabilitation for patients with cancer, including capacity, costs and capability issues. To make a full trial feasible, we have made a series of recommendations to address the limitations we have identified, including an internal pilot with clear stop–proceed rules, induction training for staff and participant incentives. To clarify and aid interpretation of generalisation, we also recommend an embedded process evaluation so that each site’s contextual factors impacting cardiac rehabilitation for patients with cancer are illuminated.
Conclusions
Implications for health care
The main novel finding is that cardiac rehabilitation for cancer and cardiac patients together is feasible and acceptable, thereby challenging disease-specific rehabilitation models.
This feasibility and pilot study suggests that cardiac rehabilitation is, on the whole, an acceptable and feasible rehabilitation service for people with CRC and their clinical care teams, but there are key concerns regarding the capacity of cardiac rehabilitation services to accommodate additional patients with cancer and the capability of cardiac rehabilitation clinicians to provide cancer-specific psychosocial support. Before UK-wide implementation of the intervention, it is critical to address these concerns and then to find out if this model of rehabilitation has a health benefit. A major strength of this feasibility and pilot study, however, is that we evaluated an already widely available existing rehabilitation service, namely cardiac rehabilitation. The aim of this pragmatic trial was not to attempt to change and adapt cardiac rehabilitation, but to find out if it is feasible and acceptable to refer people with CRC to this service as currently configured. We were successful in achieving this aim.
Implications for future research
Research priorities and recommendations
To maximise the success of any future effectiveness trial, research priorities include addressing CRC patient barriers to attending cardiac rehabilitation and consenting to the study (e.g. travel, poor recovery), gaps in cardiac rehabilitation provision for cancer patients, such as cancer-specific psychosocial support, recruitment bias, missing accelerometer data and retention of control group participants, and marginal costs related to expanding cardiac rehabilitation provision to other patient groups.
To address concerns about capacity, we recommend that additional resources be given to cardiac rehabilitation services (if required) so that they can take more patients. To address concerns about the competence of cardiac rehabilitation clinicians to deal with cancer-specific issues, we recommend that the cancer team address cancer-specific needs and that cardiac rehabilitation attend to generic concerns of patients. To address travel barriers to attending cardiac rehabilitation, we recommend that outreach services should be offered. To address recruitment bias, we recommend that induction training should be provided to cancer clinicians about cardiac rehabilitation so that they refer and offer the service only to patients who need it most, that is, those who do not currently meet the recommended guidelines for physical activity. This training will also point out the ability of cardiac rehabilitation services to support people who, for instance, have a disability or are immobile, thereby encouraging referral of patients to the service who are frail, etc. To improve up-take of the service, we recommend that patients are part of the decision-making process about the start date for attending cardiac rehabilitation. This is so that those who wish to begin at the end of all active treatment, including adjuvant therapy, can still participate. If conducting a study, this strategy should also improve the consent rate. To improve completion rates, especially participants allocated to the control arm, we recommend that incentives are provided to remain in the study, such as monetary incentives and regular reminders. To reduce missing accelerometer data, we recommend that researchers are trained so that they communicate to participants the importance of these data and how to wear the device.
Next steps
A major strength and advantage of pragmatic trials is the testing of already existing services in real-world settings. It is very different, therefore, from an explanatory trial, in which the intervention is tightly controlled and managed by the investigating team. This is the first pragmatic pilot trial of a physical activity intervention for people with CRC. This study has highlighted threats to a future definitive trial and we have made a series of recommendations to manage these risks. Before proceeding to a full-scale multicentre trial to evaluate health benefits of cardiac rehabilitation for cancer patients, we recommend an internal pilot trial be conducted, incorporating the protocol modifications that we have recommended, and that screening, eligibility and consent rates, in particular, are closely monitored at each site during the pilot. This internal pilot should have clear ‘stop–proceed’ rules that are formally reviewed before proceeding to the full-scale trial.
Acknowledgements
The team would like to take this opportunity to thank everyone involved in making this project possible, and for all the help and advice along the way.
Thank you to our site research assistants, Aileen Ireland, Zoe Davies and Jillian Hart; to the CNSs who referred all our great participants over all three sites (thank you for your patience and input); to the cardiac rehabilitation teams who very kindly took our participants in, and who received nothing but excellent feedback from everyone who attended their classes; to the team at Tayside Clinical Trials Unit who guided us through our data management and provided us with our results; to Jon Godwin, Cam Donaldson, Sandra Campbell, Cara Taylor and Chrissie Lane for their input at stages throughout the research; to the Steering Group who assisted and guided us with the project – Dawn Storey, Margaret Johnstone, Morag Thaw, Debbie Provan, Emma Anderson, Mary Wells, Catherine Mondoa and Gillian Sweetman; and to Sue Pargeter, on behalf of our funder, NIHR, for dealing so well with us.
Finally, and most importantly, all of the lovely participants who gave up their time to be involved in this project, we thank you.
Contributions of authors
Dr Gill Hubbard is an experienced research project manager in cancer care and rehabilitation. Dr Hubbard was chief investigator and was involved in all aspects of the study, from project conception through to report completion and submission.
Ms Julie Munro is a researcher in exercise rehabilitation and was involved in trials management of the three sites throughout data collection. Ms Munro was also involved in drafting and editing all sections of the report.
Professor Ronan O’Carroll is a behavioural scientist who was involved in protocol development and contributed to edits of the final report.
Professor Nanette Mutrie (MBE) is a physical activity expert was involved in protocol development and contributed to edits of the final report.
Dr Lisa Kidd is a specialist in self-care in cancer and qualitative research, was involved in protocol development and contributed to edits of the final report.
Professor Sally Haw has expertise in public health, research methods and the evaluation of complex interventions, and was involved in protocol development and contributed to edits of the final report.
Dr Richard Adams is a senior lecturer and consultant in lower gastrointestinal oncology, with extensive experience in phase II and III colorectal clinical trials. Dr Adams was involved in protocol development, facilitating study set-up on one site with support and contributed to edits of the final report.
Professor Angus JM Watson is consultant in colorectal and general surgery with extensive research input and experience. Professor Watson commented on edits of the final report.
Professor Stephen J Leslie is consultant cardiologist with an extensive research portfolio. Professor Leslie was involved in protocol development and contributed to edits of the final reports.
Ms Petra Rauchhaus is an experienced clinical trials statistician who was involved in protocol development. Ms Rauchhaus produced the statistical analysis plan and completed data analysis for all report outcomes.
Dr Anna Campbell (MBE) is an expert in physical activity, with extensive experience in the field of cancer and exercise. Dr Campbell was involved in protocol development and contributed to edits of the final reports.
Dr Helen Mason is a senior lecturer in health economics. Dr Mason produced Chapter 10, the economic evaluation, for the report.
Dr Sarkis Manoukian is a researcher in health economics, involved in a number of clinical trials. Dr Manoukain produced Chapter 10, the economic evaluation, for the report.
Dr Gillian Sweetman was our patient advisor, who went on to contribute at steering group meetings. Dr Sweetman collected qualitative data and drafted and contributed to Chapter 11 of the report.
Professor Shaun Treweek is an experienced health services researcher with expertise in trial design, the development of complex interventions and trial recruitment. Professor Treweek was involved in protocol development and contributed to edits of the final report.
Publications
Munro J, Adams R, Campbell A, Campbell S, Donaldson C, Godwin J, et al. CRIB – the use of cardiac rehabilitation services to aid the recovery of patients with bowel cancer: a pilot randomised controlled trial (RCT) with embedded feasibility study. BMJ Open 2014;4:e004684.
Hubbard G, Campbell A, Davies Z, Munro J, Ireland AV, Leslie S, et al. Experiences of recruiting to a pilot trial of cardiac rehabilitation in patients with bowel cancer (CRIB) with an embedded process evaluation: lessons learned to improve recruitment. Pilot Feasibility Stud 2015;1:15.
Hubbard G, Adams R, Campbell A, Kidd L, Leslie S, Munro J. Is referral of postsurgical colorectal cancer survivors to cardiac rehabilitation feasible and acceptable? A pragmatic pilot randomised controlled trial with embedded qualitative study. BMJ Open 2016;6:e009284.
Hubbard G, O’Carroll R, Munro J, Mutrie N, Haw S, Mason H, Treweek S. The feasibility and acceptability of trial procedures for a pragmatic randomised controlled trial of a structured physical activity intervention for people diagnosed with colorectal cancer: findings from a pilot trial of cardiac rehabilitation vs. usual care (no rehabilitation) with an embedded qualitative study. BMC Pilot Feasibility Stud 2016; in press.
Data sharing statement
All available data can be obtained from the corresponding author: Gill Hubbard, Cancer Care Research Centre, School of Health Sciences, University of Stirling, Highland Campus, Centre for Health Science, Old Perth Road, Inverness, IV2 3JH. Telephone: + 44 (0) 1463 255649. E-mail: gill.hubbard@stir.ac.uk.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. http://dx.doi.org/10.1200/JCO.2005.05.2308.
- Hebdon M, Foli K, McComb S. Survivor in the cancer context: a concept analysis. J Adv Nurs 2015;71:1774-86. http://dx.doi.org/10.1111/jan.12646.
- Two Million Reasons – The Cancer Survivorship Agenda: Why We Need to Support People with or Beyond Cancer. London: Macmillan Cancer Support; 2008.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. http://dx.doi.org/10.3322/caac.20107.
- Macmillan Cancer Support . The Rich Picture of People Living With Colorectal Cancer n.d. www.macmillan.org.uk/Documents/AboutUs/Research/Richpictures/Richpicture-Colorectalcancer.pdf (accessed 21 July 2015).
- Cancer Research UK . Bowel Cancer Statistics n.d. www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/bowel-cancer/cancerstats-key-facts-on-bowel-cancer (accessed 19 December 2014).
- Foster C, Wright D, Hill H, Hopkinson J, Roffe L. Psychosocial implications of living 5 years or more following a cancer diagnosis: a systematic review of the research evidence. Eur J Cancer Care 2009;18:223-47. http://dx.doi.org/10.1111/j.1365-2354.2008.01001.x.
- Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psycho-Oncology 2001;10:19-28. http://dx.doi.org/10.1002/1099-1611(200101/02)10:1<19::AID-PON501>3.0.CO;2-6.
- Andreyev HJN, Davidson SE, Gillespie C, Allum WH, Swarbrick E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2011;61:179-92. http://dx.doi.org/10.1136/gutjnl-2011-300563.
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:242-74. http://dx.doi.org/10.3322/caac.21142.
- Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007.
- Van Leersum NJ, Janssen-Heijnen ML, Wouters MW, Rutten HJ, Coebergh JW, Tollenaar RA, et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995–2010. Int J Cancer 2013;132:2157-63. http://dx.doi.org/10.1002/ijc.27871.
- Diet, Physical Activity and Cardiovascular Disease Prevention in Europe. Brussels: European Heart Network; 2011.
- Hawkes A, Lynch BM, Owen N, Youlden DR, Aitken JF. Health behaviours of Australian colorectal cancer survivors compared to non-cancer population controls. Support Care Cancer 2008;16:1097-104. http://dx.doi.org/10.1007/s00520-008-0421-5.
- Grimmett C, Bridgewater J, Steptoe A, Wardle J. Lifestyle and quality of life in colorectal cancer survivors. Qual Life Res 2011;20:1237-45. http://dx.doi.org/10.1007/s11136-011-9855-1.
- Dennis DL, Waring JL, Payeur N, Cosby C, Daudt HML. Making lifestyle changes after colorectal cancer: insights for program development. Curr Oncol 2013;20:493-511. http://dx.doi.org/10.3747/co.20.1514.
- Chung JY, Lee DH, Park JH, Lee MK, Kang DW, Min J, et al. Patterns of physical activity participation across the cancer trajectory in colorectal cancer survivors. Support Care Cancer 2013;21:1605-12. http://dx.doi.org/10.1007/s00520-012-1703-5.
- Stevinson C, Lydon A, Amir Z. Adherence to physical activity guidelines among cancer support group participants. Eur J Cancer Care 2014;23:199-205. http://dx.doi.org/10.1111/ecc.12145.
- Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors. Cancer 2008;112:2475-82. http://dx.doi.org/10.1002/cncr.23455.
- Stubblefield M, Hubbard G, Cheville A, Koch U, Schmitz K, Dalton S. Current perspectives and emerging issues on cancer rehabilitation. Cancer 2013;119:2170-78. http://dx.doi.org/10.1002/cncr.28059.
- Gysels M, Higginson IJ, Rajasekaran M, Davies E, Harding R. Improving Supportive and Palliative Care for Adults with Cancer. Research Evidence Manual. London: National Institute for Health and Care Excellence; 2004.
- Scott DA, Mills M, Black A, Cantwell M, Campbell A, Cardwell CR, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev 2013;3. http://dx.doi.org/10.1002/14651858.cd007730.pub2.
- Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer 2013;133:1905-13. http://dx.doi.org/10.1002/ijc.28208.
- Cramer H, Lauche R, Klose P, Dobos G, Langhorst J. A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care 2014;23:3-14. http://dx.doi.org/10.1111/ecc.12093.
- Winter EM, Fowler N. Exercise defined and quantified according to the Systeme International d’Unites. J Sports Sci 2009;27:447-60. http://dx.doi.org/10.1080/02640410802658461.
- NHS Choices . NHS Physical Activity Guidelines for Adults n.d. www.nhs.uk/Livewell/fitness/Pages/physical-activity-guidelines-for-adults.aspx (accessed 19 December 2014).
- Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services; 2008.
- Des Guetz G, Uzzan B, Bouillet T, Nicolas P, Chouahnia K, Zelek L, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract 2013;2013. http://dx.doi.org/10.1155/2013/340851.
- Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 2014;25:1293-311. http://dx.doi.org/10.1093/annonc/mdu012.
- Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev 2011;20:1410-20. http://dx.doi.org/10.1158/1055-9965.EPI-11-0079.
- Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol 2013;31:876-85. http://dx.doi.org/10.1200/JCO.2012.45.9735.
- Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control 2012;23:1939-48. http://dx.doi.org/10.1007/s10552-012-0071-2.
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24:3535-41. http://dx.doi.org/10.1200/JCO.2006.06.0863.
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med 2009;169:2102-8. http://dx.doi.org/10.1001/archinternmed.2009.412.
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 2006;24:3527-34. http://dx.doi.org/10.1200/JCO.2006.06.0855.
- Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, Park Y, et al. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the National Institutes of Health – AARP diet and health study. J Clin Oncol 2014;58.
- Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123-32. http://dx.doi.org/10.7326/M14-1651.
- Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. Br J Cancer 2014;110:831-41. http://dx.doi.org/10.1038/bjc.2013.750.
- Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer 2013;119:1928-35. http://dx.doi.org/10.1002/cncr.28028.
- Vallance JK, Boyle T, Courneya KS, Lynch BM. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among colon cancer survivors. Cancer 2014;120:2919-26. http://dx.doi.org/10.1002/cncr.28779.
- Otto S, Korfage I, Polinder S, van der Heide A, de Vries E, Rietjens J, et al. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Support Care Cancer 2015;23:1237-50. http://dx.doi.org/10.1007/s00520-014-2480-0.
- Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Prospective relationships of physical activity with quality of life among colorectal cancer survivors. J Clin Oncol 2008;26:4480-87. http://dx.doi.org/10.1200/JCO.2007.15.7917.
- Courneya KS, Friedenreich CM. Determinants of exercise during colorectal cancer treatment: an application of the theory of planned behavior. Oncol Nurs Forum 1997;24:1715-23.
- Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care 2003;12:347-57. http://dx.doi.org/10.1046/j.1365-2354.2003.00437.x.
- Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2010;4:87-100. http://dx.doi.org/10.1007/s11764-009-0110-5.
- Craft L, Vanlterson E, Helenowski I, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;21:3-19. http://dx.doi.org/10.1158/1055-9965.EPI-11-0634.
- Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e70.
- Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, et al. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Arch Phys Med Rehabil 2011;92:749-55. http://dx.doi.org/10.1016/j.apmr.2010.12.020.
- Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psycho-Oncology 2013;22:54-6. http://dx.doi.org/10.1002/pon.2047.
- Kerry S, Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, et al. The colon health and life-long exercise change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol 2008;15:262-70.
- Hawkes AL, Pakenham KI, Courneya KS, Gollschewski S, Baade P, Gordon LG, et al. A randomised controlled trial of a tele-based lifestyle intervention for colorectal cancer survivors (‘CanChange’): study protocol. BMC Cancer 2009;9. http://dx.doi.org/10.1186/1471-2407-9-286.
- Lie I, Bunch EH, Smeby NA, Arnesen H, Hamilton G. Patients’ experiences with symptoms and needs in the early rehabilitation phase after coronary artery bypass grafting. Eur J Cardiovasc Nurs 2012;11:14-2.
- Leegaard M, Nåden D, Fagermoen MS. Postoperative pain and self-management: women’s experiences after cardiac surgery. J Adv Nurs 2008;63:476-85. http://dx.doi.org/10.1111/j.1365-2648.2008.04727.x.
- Theobald K, McMurray A. Coronary artery bypass graft surgery: discharge planning for successful recovery. J Adv Nurs 2004;47:483-91. http://dx.doi.org/10.1111/j.1365-2648.2004.03127.x.
- Goodman H. Patients’ perceptions of their education needs in the first six weeks following discharge after cardiac surgery. J Adv Nurs 1997;25:1241-51. http://dx.doi.org/10.1046/j.1365-2648.1997.19970251241.x.
- Harrison J, Young J, Auld S, Masya L, Solomon M, Butow P. Quantifying postdischarge unmet supportive care needs of people with colorectal cancer: a clinical audit. Colorect Dis 2011;13:1400-06. http://dx.doi.org/10.1111/j.1463-1318.2010.02478.x.
- Holm LV, Hansen DG, Johansen C, Vedsted P, Larsen PV, Kragstrup J, et al. Participation in cancer rehabilitation and unmet needs: a population-based cohort study. Support Care Cancer 2012;20:2913-24. http://dx.doi.org/10.1007/s00520-012-1420-0.
- Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population based cohort of colorectal cancer survivors. Eur J Cancer 2011;47:267-76. http://dx.doi.org/10.1016/j.ejca.2010.10.002.
- Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc 2006;54:1898-904. http://dx.doi.org/10.1111/j.1532-5415.2006.00973.x.
- Isles C. Cardiac Rehabilitation. Edinburgh: Scottish Intercollegiate Guidelines Network; 2002.
- The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2012. London: British Association for Cardiovascular Prevention and Rehabilitation; 2012.
- The National Audit of Cardiac Rehabilitation: Annual Statistical Report 2013. London: British Heart Foundation; 2013.
- Cardiac Rehabilitation Scotland . Cardiac Rehabilitation Finder 2014. www.cardiac-rehabilitation.net/Scotland.pdf (accessed 19 December 2014).
- National Service Framework for Coronary Heart Disease: Modern Standards and Service Models. London: Department of Health; 2000.
- Commissioning Pack for Cardiac Rehabilitation. London: Department of Health; 2010.
- Association of Chartered Physiotherapists in Cardiac Rehabilitation . ACPICR Standards for Physical Activity and Exercise in the Cardiac Population, 2009 2014. http://acpicr.com/sites/default/files/Acpicr%20standards_1.pdf (accessed 19 December 2014).
- West RR, Jones DA, Henderson AH. Rehabilitation After Myocardial Infarction Trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012;98:637-44. http://dx.doi.org/10.1136/heartjnl-2011-300302.
- Taylor RS. The RAMIT trial: its results in the context of 2012 Cochrane review. Heart 2012;98:63-3. http://dx.doi.org/10.1136/heartjnl-2012-301709.
- Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011;7. http://dx.doi.org/10.1002/14651858.cd001800.pub2.
- Carayol M, Delpierre C, Bernard P, Ninot G. Population-, intervention- and methodology-related characteristics of clinical trials impact exercise efficacy during adjuvant therapy for breast cancer: a meta-regression analysis. Psycho-Oncology 2015;24:737-47. http://dx.doi.org/10.1002/pon.3727.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Eldridge S, Bond C, Campbell M, Lancaster G, Thabane L, Hopwell S. Definition and reporting of pilot and feasibility studies. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-S1-O18.
- National Institute for Health Research . NIHR Evaluation, Trials and Studies Coordination Centre: Glossary 2012. www.netscc.ac.uk/glossary/#glos6/ (accessed 4 January 2015).
- Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-67.
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How we design feasibility studies. Am J Prev Med 2009;36:452-7. http://dx.doi.org/10.1016/j.amepre.2009.02.002.
- Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Pub Health 2013;103:38-46. http://dx.doi.org/10.2105/AJPH.2013.301299.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-1.
- Tickle-Degnen L. Nuts and bolts of conducting feasibility studies. Am J Occup Ther 2013;67:171-6. http://dx.doi.org/10.5014/ajot.2013.006270.
- Arnold DM, Burns KE, Adhikari NK, Kho ME, Meade MO, Cook DJ. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med 2009;37:69-74. http://dx.doi.org/10.1097/CCM.0b013e3181920e33.
- Loscalzo J. Pilot trials in clinical research: of what value are they?. Circulation 2009;119:1694-6. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.861625.
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287-91. http://dx.doi.org/10.1002/pst.185.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301-8. http://dx.doi.org/10.1016/j.jclinepi.2011.07.011.
- Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-264.
- Munro J, Adams R, Campbell A, Campbell S, Donaldson C, Godwin C, et al. CRIB – the use of cardiac rehabilitation services to aid the recovery of patients with bowel cancer: a pilot randomised controlled trial (RCT) with embedded feasibility study. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004684.
- Process and Methods Guides: Methods for the Development of NICE Public Health Guidance. London: National Institute for Health and Care Excellence; 2012.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986.
- Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice-Hall; 1977.
- Godin G, Kok G. The theory of planned behaviour: a review of its applications to health-related behaviours. Am J Health Promot 1996;11:87-98. http://dx.doi.org/10.4278/0890-1171-11.2.87.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1687.
- Freedson P, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777-81. http://dx.doi.org/10.1097/00005768-199805000-00021.
- Reilly JJ, Penpraze V, Hislop J, Davies G, Grant S, Paton JY. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch Dis Child 2008;93:614-19. http://dx.doi.org/10.1136/adc.2007.133272.
- Jovanovic JL, Hughes DC, Baum GP, Carmack C, Greisinger AJ, Basen-Engquist K. Accelerometry and self-report in sedentary populations. Am J Health Behav 2011;35. http://dx.doi.org/10.5993/AJHB.35.1.7.
- Lugade V, Fortune E, Morrow M, Kaufman K. Validity of using tri-axial accelerometers to measure human movement – part I: posture and movement detection. Med Eng Phys 2014;36:169-76. http://dx.doi.org/10.1016/j.medengphy.2013.06.005.
- Broderick JM, Ryan J, O’Donnell DM, Hussey J. A guide to assessing physical activity using accelerometry in cancer patients. Support Care Cancer 2014;22:1121-30. http://dx.doi.org/10.1007/s00520-013-2102-2.
- Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer 2014;23:1121-6. http://dx.doi.org/10.1007/s00520-014-2453-3.
- Vallance JK, Boyle T, Courneya KS, Lynch BM. Accelerometer-assessed physical activity and sedentary time among colon cancer survivors: associations with psychological health outcomes. J Cancer Surviv 2015:404-11. http://dx.doi.org/10.1007/s11764-014-0409-8.
- Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc 2014;46:99-106. http://dx.doi.org/10.1249/MSS.0b013e3182a0595f.
- Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 2006;9:755-62. http://dx.doi.org/10.1079/PHN2005898.
- Hadi M, Gibbons E, Fitzpatrick R. A Structured Review of Patient-Reported Outcome Measures (PROMS) for Colorectal Cancer, Report to the Department of Health. Oxford: Patient Reported Outcomes Measurement Group; 2010.
- EuroQol Research Foundation . EQ-5D Standardised Instrument for Use As a Measure of Health Outcome 2015. www.euroqol.org/eq-5d/what-is-eq-5d/eq-5d-nomenclature.html (accessed 16 May 2015).
- Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. PharmacoEconomics 2007;25:365-84. http://dx.doi.org/10.2165/00019053-200725050-00002.
- McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford: Oxford University Press; 1996.
- Ware JE. SF-36 Health Survey n.d. www.sf-36.org/tools/sf36.shtml (accessed 17 October 2012).
- Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey. Manual and Interpretation Guide. Lincoln, RI: Quality Metric Inc.; 1997.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Hermann C. International experiences with the Hospital Anxiety and Depression Scale: a review of validation data and clinical results. J Psychosom Res 1997;42:17-41. http://dx.doi.org/10.1016/S0022-3999(96)00216-4.
- Moorey S, Greer S, Watson M, Gorman C, Rowden L, Tunmore R, et al. The factor structure and factor stability of the Hospital Anxiety and Depression Scale in patients with cancer. Br J Psychiatry 1991;158:255-9. http://dx.doi.org/10.1192/bjp.158.2.255.
- Mitchell A, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. J Affect Disord 2010;126:335-48. http://dx.doi.org/10.1016/j.jad.2010.01.067.
- Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-79.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue. Ann Oncol 2008;20:17-25. http://dx.doi.org/10.1093/annonc/mdn537.
- Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol 2014;25:2404-12. http://dx.doi.org/10.1093/annonc/mdu448.
- Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-efficacy scales for healthrelated diet and exercise behaviors. Health Educ Res 1988;3:283-92. http://dx.doi.org/10.1093/her/3.3.283.
- Strachan SM, Woodgate J, Brawley LR, Tse A. The relationship of self-efficacy and self-identity to long-term maintenance of vigorous physical activity. J Appl Biobehav Res 2005;10:98-112. http://dx.doi.org/10.1111/j.1751-9861.2005.tb00006.x.
- Sallis JF, Hovell MF, Hofstetter CR. Predictors of adoption and maintenance of vigorous physical activity in men and women. Prev Med 1992;21:237-51. http://dx.doi.org/10.1016/0091-7435(92)90022-A.
- Darker C, French D, Eves F, Sniehotta F. An intervention to promote walking amongst the general population based on an ‘extended’ theory of planned behaviour: a waiting list randomised controlled trial. Psychol Health 2010;25:71-88. http://dx.doi.org/10.1080/08870440902893716.
- Schwarzer R, Jerusalem M. Generalized self-efficacy scale. Measures in health psychology: a user’s portfolio. Causal Control Beliefs 1995;1:35-7.
- Schwarzer R, Conner M, Norman P. Predicting Health Behavior: Research and Practice with Social Cognition Models. Buckingham: Open University Press; 1996.
- Leganger A, Kraft P, Røysamb E. Perceived self-efficacy in health behaviour research: conceptualisation, measurement and correlates. Psychol Health 2000;15:51-69. http://dx.doi.org/10.1080/08870440008400288.
- Schwarzer R, Mueller J, Greenglass E. Assessment of perceived general self-efficacy on the internet: data collection in cyberspace. Anxiety Stress Coping 1999;12:145-61. http://dx.doi.org/10.1080/10615809908248327.
- Rogers R. A protection motivation theory of fear appeals and attitude change. J Psychol 1975;91:93-114. http://dx.doi.org/10.1080/00223980.1975.9915803.
- Stephan Y, Boiche J, Trouilloud D, Deroche T, Sarrazin P. The relation between risk perceptions and physical activity among older adults: a prospective study. Psychol Health 2011;26:887-97. http://dx.doi.org/10.1080/08870446.2010.509798.
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med 2004;38:388-402. http://dx.doi.org/10.1016/j.ypmed.2003.11.012.
- De Vries H, Lezwijn J, Hol M, Honing C. Skin cancer prevention: behaviour and motives of Dutch adolescents. Eur J Cancer Prev 2005;14:39-50. http://dx.doi.org/10.1097/00008469-200502000-00006.
- Janssen E, Van Osch L, De Vries H, Lechner L. Measuring risk perceptions of skin cancer: reliability and validity of difference operationalizations. Br J Health Psychol 2011;16:92-112. http://dx.doi.org/10.1348/135910710X514120.
- Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage; 2005.
- Ericsson KA, Simon HA. Verbal reports as data. Psychol Rev 1980;87:215-51. http://dx.doi.org/10.1037/0033-295X.87.3.215.
- Mason J, Lewis-Beck M, Bryman AE, Liao TF. The Sage Encyclopedia of Social Science Research Methods. Thousand Oaks, CA: Sage; 2004.
- Kitzinger J, Barbour R. Developing Focus Group Research: Politics, Theory and Practice. Thousand Oaks, CA: Sage; 1999.
- Spencer L, Ritchie J, O’Connor W, Morrell G, Ormston R, Ritchie J, et al. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Warwick V, MacConnachie G, Martin D. Standard Operating Procedure for Identifying, Recording and Reporting Adverse Events for Clinical Trials of Investigational Medicinal Products. Dundee: NHS Tayside; 2013.
- Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the national health and nutrition examination survey, 2003–2006. Prev Chronic Dis 2012;9. http://dx.doi.org/10.5888/pcd9.110332.
- American Cancer Society . Topics: What Is Advanced Cancer? 2014. www.cancer.org/treatment/understandingyourdiagnosis/advancedcancer/advanced-cancer-what-is (accessed 5 January 2015).
- Lowther M, Mutrie N, Loughlan C, McFarlane C. Development of a Scottish physical activity questionnaire: a tool for use in physical activity interventions. Br J Sports Med 1999;33:244-9. http://dx.doi.org/10.1136/bjsm.33.4.244.
- Anderson AS, Macleod M, Mutrie N, Sugden J, Dobson H, Treweek S, et al. Breast cancer risk reduction – is it feasible to initiate a randomised controlled trial of a lifestyle intervention programme (ActWell) within a national breast screening programme?. Int J Behav Nutr Phys 2014;11. http://dx.doi.org/10.1186/s12966-014-0156-2.
- Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the functional assessment of cancer therapy-colorectal (FACT-C) quality of life instrument. Qual Life Res 1999;8:181-95. http://dx.doi.org/10.1023/A:1008821826499.
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9.
- Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39094.648553.AE.
- Bristol Online Surveys . Homepage n.d. www.onlinesurveys.ac.uk (accessed 2 February 2015).
- Hubbard G, Campbell A, Davies Z, Munro J, Ireland AV, Leslie S, et al. Experiences of recruiting to a pilot trial of cardiac rehabilitation in patients with bowel cancer (CRIB) with an embedded process evaluation: lessons learned to improve recruitment. Pilot Feasibility Studies 2015;1:1-12. http://dx.doi.org/10.1186/s40814-015-0009-z.
- Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detect Prev 2004;28:208-13. http://dx.doi.org/10.1016/j.cdp.2004.02.001.
- Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, et al. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol 2008;43:971-8. http://dx.doi.org/10.1080/00365520701766111.
- Anderson AS, Caswell S, Wells M, Steele RJ, MacAskill S. ‘It makes you feel so full of life’ LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Support Care Cancer 2010;18:409-15. http://dx.doi.org/10.1007/s00520-009-0677-4.
- Grimmett C, Simon A, Lawson V, Wardle J. Diet and physical activity intervention in colorectal cancer survivors: a feasibility study. Eur J Oncol Nurs 2015;19:1-6. http://dx.doi.org/10.1016/j.ejon.2014.08.006.
- Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol 2013;31:2313-21. http://dx.doi.org/10.1200/JCO.2012.45.5873.
- Lee DH, Kim JY, Lee MK, Lee C, Min JH, Jeong DH, et al. Effect of a 12-week home-based program on the level of physical activity, insulin, and cytokines in colorectal cancer: a pilot study. Support Care Cancer 2013;21:2537-45. http://dx.doi.org/10.1007/s00520-013-1822-7.
- Lin KY, Shun SC, Lai YH, Liang JT, Tsauo JY. Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nurs 2014;37:E21-9. http://dx.doi.org/10.1097/NCC.0b013e3182791097.
- Sellar CM, Bell GJ, Haennel RG, Au H, Chua N, Courneya KS. Feasibility and efficacy of a 12-week supervised exercise intervention for colorectal cancer survivors. Appl Physiol Nutr Metab 2013;39:715-23. http://dx.doi.org/10.1139/apnm-2013-0367.
- Spence RR, Heesch K, Brown WJ. Colorectal cancer survivors’ exercise experiences and preferences: qualitative findings from an exercise rehabilitation programme immediately after chemotherapy. Eur J Cancer Care 2011;20:257-66. http://dx.doi.org/10.1111/j.1365-2354.2010.01214.x.
- Williams GR, Alston SM. Physical Activity Intervention for Older Patients During Chemotherapy for Colorectal Cancer 2015. https://clinicaltrials.gov/ct2/show/NCT02191969 (accessed May 2015).
- Courneya KS, Vardy J, Gill S, Jonker D, O’Brien P, Friedenreich CM, et al. Update on the Colon Health and Life-Long Exercise Change trial: a phase III study of the impact of an exercise program on disease-free survival in colon cancer survivors. Curr Colorect Cancer Rep 2014;10:321-8. http://dx.doi.org/10.1007/s11888-014-0231-8.
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409-26. http://dx.doi.org/10.1249/MSS.0b013e3181e0c112.
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781-5. http://dx.doi.org/10.1016/S0140-6736(02)07882-0.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670-4. http://dx.doi.org/10.1136/bmj.319.7211.670.
- Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-003821.
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008;5. http://dx.doi.org/10.1186/1479-5868-5-56.
- Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults’ sedentary time in population-based studies. Am J Prev Med 2011;41:216-27. http://dx.doi.org/10.1016/j.amepre.2011.05.005.
- Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act 2012;9. http://dx.doi.org/10.1186/1479-5868-9-103.
- Murphy SL. Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Prev Med 2009;48:108-14. http://dx.doi.org/10.1016/j.ypmed.2008.12.001.
- Mâsse LC, Fuemmeler BF, Anderson CB, Matthews CE, Trost SG, Catellier DJ, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc 2005;37:S544-54. http://dx.doi.org/10.1249/01.mss.0000185674.09066.8a.
- Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:531-43. http://dx.doi.org/10.1249/01.mss.0000185657.86065.98.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7367.766.
- Glenton C, Lewin S, Scheel IB. Still too little qualitative research to shed light on results from reviews of effectiveness trials: a case study of a Cochrane review on the use of lay health workers. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-53.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3496.
- O’Cathain A, Thomas KJ, Drabble S, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002889.
- Drabble SJ, O’Cathain A, Thomas KJ, Rudolph A, Hewison J. Describing qualitative research undertaken with randomised controlled trials in grant proposals: a documentary analysis. BMC Med Res Methodol 2014;14. http://dx.doi.org/10.1186/1471-2288-14-24.
- O’Cathain A, Goode J, Drabble SJ, Thomas KJ, Rudolph A, Hewison J. Getting added value from using qualitative research with randomized controlled trials: a qualitative interview study. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-215.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomized controlled trials in health research: the QUAlitative Research in Trials (QUART) study–a mixed methods study. Health Technol Assess 2014;18.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. http://dx.doi.org/10.1093/intqhc/mzm042.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Norcross JC. Psychotherapy Relationships That Work: Therapist Contributions and Responsiveness to Patients. New York, NY: Oxford University Press; 2002.
- National Institute for Health and Care Excellence . NICE Commissioning Guides 2: An Integrated Approach to Commissioning High-Quality Integrated Cardiac Rehabilitation 2013. www.nice.org.uk/guidance/cmg40 (accessed 15 May 2015).
- Peddle CJ, Plotnikoff RC, Wild TC, Au HJ, Courneya KS. Medical, demographic, and psychosocial correlates of exercise in colorectal cancer survivors: an application of self-determination theory. Support Care Cancer 2008;16:9-17. http://dx.doi.org/10.1007/s00520-007-0272-5.
- Ryan RM, Williams GC, Patrick H, Deci EL. Self-determination theory and physical activity: the dynamics of motivation in development and wellness. Hell J Psychol 2009;6:107-24.
- Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act 2012;9. http://dx.doi.org/10.1186/1479-5868-9-78.
- Edmunds J, Ntoumanis N, Duda JL. A test of self-determination theory in the exercise domain. J Appl Soc Psychol 2006;36:2240-65. http://dx.doi.org/10.1111/j.0021-9029.2006.00102.x.
- Anderson AS, Caswell S, Wells M, Steele RJ. Obesity and lifestyle advice in colorectal cancer survivors – how well are clinicians prepared?. Colorect Dis 2013;15:949-57. http://dx.doi.org/10.1111/codi.12203.
- Miles A, Simon A, Wardle J. Answering patient questions about the role lifestyle factors play in cancer onset and recurrence: what do health care professionals say?. J Health Psychol 2010;15:291-8. http://dx.doi.org/10.1177/1359105309351245.
- James-Martin G, Koczwara B, Smith EL, Miller MD. Information needs of cancer patients and survivors regarding diet, exercise and weight management: a qualitative study. Eur J Cancer Care 2014;23:340-8. http://dx.doi.org/10.1111/ecc.12159.
- Hoey LM, Ieropoli SC, White VM, Jefford M. Systematic review of peer-support programs for people with cancer. Patient Educ Couns 2008;70:315-7. http://dx.doi.org/10.1016/j.pec.2007.11.016.
- Mastrovito R, Moynihan RT, Parsonnet L, Holland JC, Rowland JH. Handbook of Psychooncology: Psychological Care of the Patient with Cancer. New York, NY: Oxford University Press; n.d.
- Emslie C, Whyte F, Campbell A, Mutrie N, Lee L, Ritchie D, et al. ‘I wouldn’t have been interested in just sitting round a table talking about cancer’; exploring the experiences of women with breast cancer in a group exercise trial. Health Educ Res 2007;22:827-38. http://dx.doi.org/10.1093/her/cyl159.
- Khan S, Gatt M, Horgan A, Anderson I, MacFie J. Issues in Professional Practice. Guidelines for Implementation of Enhanced Recovery Protocols. London: Association of Surgeons of Great Britain and Ireland; 2009.
- National Institute for Health and Care Excellence . Myocardial Infarction: Cardiac Rehabilitation and Prevention of Further MI 2013. www.nice.org.uk/guidance/cg172/chapter/About-this-guideline (accessed April 2015).
- Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer 2000;82:1783-8. http://dx.doi.org/10.1054/bjoc.2000.1142.
- Townsend A, Cox SM. Accessing health services through the back door: a qualitative interview study investigating reasons why people participate in health research in Canada. BMC Med Ethics 2013;14. http://dx.doi.org/10.1186/1472-6939-14-40.
- UK Health Research Authority . Consent and Participant Information Sheet Preparation Guidance n.d. www.hra-decisiontools.org.uk/consent/ (accessed 15 May 2015).
- Burchell B, Marsh C. The effect of questionnaire length on survey response. Qual Quant 1992;26:233-44. http://dx.doi.org/10.1007/BF00172427.
- Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health 2011;14:1101-8. http://dx.doi.org/10.1016/j.jval.2011.06.003.
- Bradburn N. Respondent burden in Proceedings of the Survey Research Methods Section of the American Statistical Association. Health Survey Research Methods 1978;79:35-40.
- Sharp LM, Frankel J. Respondent burden: a test of some common assumptions. Public Opin Q 1983;47:36-53. http://dx.doi.org/10.1086/268765.
- Wenemark M, Frisman GH, Svensson T, Kristenson M. Respondent satisfaction and respondent burden among differently motivated participants in a health-related survey. Field Methods 2010;22:378-90. http://dx.doi.org/10.1177/1525822X10376704.
- Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating activity and sedentary behavior from an accelerometer on the hip or waist. Med Sci Sports Exerc 2013;45:964-75.
- Department of Health . Reference Costs 2013–14 2014. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 15 May 2015).
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Richards DA, Godfrey L, Tawfik J, Ryan M, Meakins J, Dutton E, et al. NHS direct versus general practice based triage for same day appointments in primary care: cluster randomised controlled trial. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38226.605995.55.
- British National Formulary. London: BMJ Group, Pharmaceutical Press and RCPCH Publications; 2014.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect 2014;17:637-50. http://dx.doi.org/10.1111/j.1369-7625.2012.00795.x.
- National Institute for Health Research . Patient and Public Involvement in Health and Social Care Research: A Handbook for Researchers n.d. www.nihr.ac.uk/funding/how-we-can-help-you/RDS-PPI-Handbook-2014-v8-FINAL.pdf (accessed 15 May 2015).
- Sully BGO, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-166.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. http://dx.doi.org/10.1186/1745-6215-7-9.
Appendix 1 Patient information sheet
Appendix 2 Screening form
Appendix 3 Referral form
Appendix 4 Non-participation consent form
Appendix 5 Consent form
Appendix 6 Training evaluation form
Appendix 7 Interview guides
Appendix 8 Adverse event log
Appendix 9 Accelerometer frequently asked questions sheet
Appendix 10 Weekly intervention log questions
Appendix 11 Statistical analysis plan
Appendix 12 Descriptive data for quality of life
| Variable | Rehabilitation | No rehabilitation | Total |
|---|---|---|---|
| Baseline | |||
| n | 20 | 20 | 40 |
| Missing | 0 | 0 | 0 |
| Mean | 58.8 | 53.3 | 56.1 |
| SD | 11.02 | 12.88 | 12.16 |
| 95% CI | 53.66 to 63.98 | 47.26 to 59.32 | 52.17 to 59.94 |
| Minimum | 38 | 23 | 23 |
| Q1 | 50.5 | 49.5 | 49.9 |
| Median | 60.0 | 57.0 | 57.5 |
| Q3 | 68.0 | 62.0 | 64.5 |
| Maximum | 80 | 70 | 80 |
| End of intervention | |||
| n | 15 | 16 | 31 |
| Missing | 0 | 0 | 0 |
| Mean | 63.2 | 58.2 | 60.6 |
| SD | 8.38 | 12.60 | 10.90 |
| 95% CI | 58.60 to 67.89 | 51.49 to 64.92 | 56.65 to 64.64 |
| Minimum | 48 | 26 | 26 |
| Q1 | 59.0 | 55.6 | 57.2 |
| Median | 67.7 | 61.3 | 64.0 |
| Q3 | 70.0 | 66.8 | 69.0 |
| Maximum | 71 | 71 | 71 |
| End of intervention – baseline | |||
| n | 15 | 16 | 31 |
| Missing | 5 | 4 | 9 |
| Mean | 3.6 | 2.6 | 3.1 |
| SD | 6.66 | 5.05 | 5.81 |
| 95% CI | –0.11 to 7.26 | –0.12 to 5.27 | 0.93 to 5.19 |
| Minimum | –9 | –4 | –9 |
| Q1 | –0.3 | –0.3 | –0.3 |
| Median | 3.8 | 0.3 | 2.0 |
| Q3 | 7.0 | 7.5 | 7.0 |
| Maximum | 18 | 12 | 18 |
| 3 months’ follow-up | |||
| n | 12 | 13 | 25 |
| Missing | 0 | 0 | 0 |
| Mean | 62.5 | 59.1 | 60.8 |
| SD | 9.20 | 12.98 | 11.23 |
| 95% CI | 56.69 to 68.39 | 51.30 to 66.98 | 56.14 to 65.41 |
| Minimum | 43 | 26 | 26 |
| Q1 | 57.5 | 57.5 | 57.5 |
| Median | 65.8 | 63.3 | 65.5 |
| Q3 | 68.0 | 68.0 | 68.0 |
| Maximum | 75 | 71 | 75 |
| 3 months’ follow-up – baseline | |||
| n | 12 | 13 | 25 |
| Missing | 8 | 7 | 15 |
| Mean | 1.2 | 5.0 | 3.2 |
| SD | 6.25 | 5.87 | 6.23 |
| 95% CI | –2.77 to 5.17 | 1.41 to 8.50 | 0.58 to 5.73 |
| Minimum | –9 | –4 | –9 |
| Q1 | –2.9 | 2.0 | –1.2 |
| Median | 2.0 | 3.4 | 3.0 |
| Q3 | 6.0 | 9.0 | 8.0 |
| Maximum | 11 | 14 | 14 |
| Variable | Rehabilitation | No rehabilitation | Total |
|---|---|---|---|
| Baseline | |||
| n | 20 | 20 | 40 |
| Missing | 0 | 0 | 0 |
| Mean | 39.2 | 36.9 | 38.0 |
| SD | 11.35 | 11.75 | 11.47 |
| 95% CI | 33.89 to 44.51 | 31.35 to 42.35 | 34.3 to 41.69 |
| Minimum | 16 | 14 | 14 |
| Q1 | 30.0 | 26.5 | 28.0 |
| Median | 40.5 | 39.5 | 40.5 |
| Q3 | 49.0 | 47.5 | 48.0 |
| Maximum | 52 | 51 | 52 |
| End of intervention | |||
| n | 15 | 16 | 31 |
| Missing | 0 | 0 | 0 |
| Mean | 44.1 | 41.5 | 42.7 |
| SD | 8.55 | 13.31 | 11.15 |
| 95% CI | 39.33 to 48.80 | 34.41 to 48.59 | 38.65 to 46.83 |
| Minimum | 23 | 9 | 9 |
| Q1 | 41.0 | 33.0 | 37.0 |
| Median | 46.0 | 47.5 | 47.0 |
| Q3 | 49.0 | 51.0 | 51.0 |
| Maximum | 52 | 52 | 52 |
| End of intervention – baseline | |||
| n | 15 | 16 | 31 |
| Missing | 5 | 4 | 9 |
| Mean | 4.7 | 2.1 | 3.4 |
| SD | 9.45 | 8.81 | 9.07 |
| 95% CI | –0.50 to 9.96 | –2.57 to 6.82 | 0.06 to 6.71 |
| Minimum | –10 | –23 | –23 |
| Q1 | –1.0 | 0.0 | 0.0 |
| Median | 2.0 | 2.5 | 2.0 |
| Q3 | 14.0 | 4.5 | 10.0 |
| Maximum | 20 | 20 | 20 |
| 3 months’ follow-up | |||
| n | 12 | 13 | 25 |
| Missing | 0 | 0 | 0 |
| Mean | 43.3 | 43.5 | 43.4 |
| SD | 10.56 | 11.18 | 10.66 |
| 95% CI | 36.54 to 49.96 | 36.78 to 50.30 | 39.00 to 47.80 |
| Minimum | 21 | 15 | 15 |
| Q1 | 41.0 | 41.0 | 41.0 |
| Median | 48.0 | 48.0 | 48.0 |
| Q3 | 50.0 | 51.0 | 50.0 |
| Maximum | 52 | 52 | 52 |
| 3 months’ follow-up – baseline | |||
| n | 12 | 13 | 25 |
| Missing | 8 | 7 | 15 |
| Mean | 0.7 | 4.7 | 2.8 |
| SD | 7.85 | 10.24 | 9.22 |
| 95% CI | –4.32 to 5.66 | –1.50 to 10.88 | –1.04 to 6.56 |
| Minimum | –16 | –17 | –17 |
| Q1 | –4.0 | 0.0 | –1.0 |
| Median | 0.5 | 3.0 | 3.0 |
| Q3 | 5.5 | 10.0 | 7.0 |
| Maximum | 13 | 24 | 24 |
List of abbreviations
- AE
- adverse event
- CABG
- coronary artery bypass graft
- CHD
- coronary heart disease
- CI
- confidence interval
- CNS
- cancer nurse specialist
- CRC
- colorectal cancer
- CRIB
- Cardiac Rehabilitation in Bowel cancer
- CRP
- cardiac rehabilitation professional
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-5L
- European Quality of Life-5 Dimensions-5 Levels
- FACIT
- Functional Assessment of Chronic Illness Therapy
- FACT-C
- Functional Assessment of Cancer Therapy – Colorectal
- HADS
- Hospital Anxiety and Depression Scale
- HCPC
- Health and Care Professions Council
- HR
- hazard ratio
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- IPAQ
- International Physical Activity Questionnaire
- MET
- metabolic equivalent
- MRC
- Medical Research Council
- MVPA
- moderate- to vigorous-intensity physical activity
- NIHR
- National Institute for Health Research
- PIS
- participant information sheet
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-36
- Short Form Health Survey-36 items
- SPAQ
- Scottish Physical Activity Questionnaire
- TIDieR
- Template for Intervention Description and Replication