Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 15/70/26. The contractual start date was in September 2017. The final report began editorial review in December 2019 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 Lister et al. This work was produced by Lister et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Lister et al.
Chapter 1 Background
Severe mental illness
Definition of severe mental illness
Severe mental illness (SMI) describes a group of mental disorders that are persistent, cause serious functional impairment and substantially interfere with, or limit, major life activities. A key feature of SMI is the presence of psychosis, which is a loss of contact with external reality. Both the American Psychiatric Association1 and the World Health Organization2 (WHO) definitions of psychosis require the presence of hallucinations (i.e. perceptions occurring in the absence of corresponding external or somatic stimuli) and/or delusions (i.e. fixed false beliefs). These symptoms may be accompanied by cognitive impairment, disordered thinking and problems with motivation, energy and mood.
Although there is some debate about which conditions come under the SMI umbrella, there is consensus that the term includes schizophrenia, bipolar disorder and depression with psychotic symptoms. 3–5 Schizophrenia is characterised by diverse psychopathology including delusions, hallucinations and ‘negative symptoms’, such as impaired motivation, reduction in interest, limited spontaneous speech, social withdrawal and cognitive impairment. Psychotic symptoms tend to relapse and remit, although around 20% of people with schizophrenia may experience chronic unremitting residual symptoms. 6 Negative symptoms tend to be persistent and are associated with long-term effects on social functioning.
By contrast, bipolar disorder typically has an ‘episodic’ course, with recovery between relapses. It causes extreme mood swings ranging from mania or intense happiness, grandiosity, euphoria or irritability or decreased need for sleep, to low mood and depressive symptoms. Typically, a person with bipolar disorder cycles from one extreme to the other, while experiencing periods with few or no symptoms in between. Major depression is similarly a relapsing–remitting disorder, but it can have a chronic presentation. It is characterised by a persistent feeling of sadness or lack of interest in outside stimuli. When accompanied by psychotic symptoms, it is categorised as SMI.
Severe mental illness includes several other conditions, such as schizoaffective disorder and persistent delusional disorder, with symptoms that overlap with schizophrenia, bipolar disorder or severe depression. Apart from the presence of psychosis, these illnesses typically have a severe and enduring impact over the life course, affecting education, employment, relationships and wealth. 7–9
Global burden of disease due to severe mental illness
Although lifetime prevalence of SMI is < 5% (consistent across the world), these conditions contribute a large and increasing global burden of illness. The global age-standardised point prevalence of schizophrenia is estimated to be 0.28% [95% confidence interval (CI) 0.24% to 0.31%], but it contributes 13.4 (95% CI 9.9 to 16.7) million years lived with disability (YLD), equivalent to 1.7% of total YLD. 10 Similarly, the age-standardised prevalence rate of bipolar disorder is estimated to be 0.7% (95% CI 0.6% to 0.8%), contributing 1.3% of total YLD. 11
Treatment of severe mental illness
Treatment of SMI includes a combination of psychotropic medication, psychological therapies and psychosocial support.
In recognition of the high risk of comorbid physical conditions and the socioeconomic impacts of SMI, treatment guidelines also include provision of annual general practitioner (GP) health checks to monitor physical health; smoking cessation support; and supported employment, exercise and diet programmes. 12,13
The majority of people with SMI are likely to be prescribed antipsychotics, either typical (sometimes called ‘first generation’, developed in the 1950s) or atypical (sometimes called ‘second generation’, developed in the 1990s). 14 Although all antipsychotics have been associated with side effects such as drowsiness, tremors, muscle spasms and weight gain, atypical antipsychotics are thought to have fewer extrapyramidal (motor control) side effects, but some may be associated with greater metabolic side effects and weight gain. 14
Comorbid physical conditions and excess mortality and morbidity in severe mental illness
Burden-of-disease estimates do not account for the premature mortality and morbidity due to physical illnesses in SMI. People with SMI die, on average, 15–20 years earlier,15 with a death rate 3.7 times higher16 than the general population. Recent evidence indicates that this ‘mortality gap’ is widening for people with schizophrenia and bipolar disorder. 17,18 Suicide accounts for around 15% of the increased mortality, but the majority of premature deaths are due to physical disorders, including non-communicable diseases, such as diabetes. Rates of such disorders are increased in people with SMI, typically being two to three times higher. 4,19,20 It is estimated that as many as two out of three premature deaths among people with SMI are due to preventable physical illnesses. 15
Most of the risk factors for developing conditions such as diabetes are likely to be the same in people with SMI and the general population, but there are multiple additional reasons that could explain the high prevalence seen in SMI populations and the excess mortality from physical disorders. Mental and physical disorders have a complex bidirectional relationship, sharing individual and socioenvironmental risk factors (e.g. childhood adversity and social and economic disadvantage). 21,22 Medications for mental illness are often associated with adverse metabolic side effects, increasing the risk of cardiometabolic disorders. Sedentary behaviour is common because of the motivational deficits associated with these disorders, or the side effects of treatment such as tiredness and sedation. Additional health risk behaviours such as smoking, alcohol use and unhealthy diets are also elevated. Problems with cognition, energy and motivation also pose challenges for accessing and adhering to medical treatment for physical conditions. Moreover, people with SMI may be less likely to receive adequate treatment because of ‘diagnostic overshadowing’, whereby health-care services attribute reported physical symptoms to mental illness, and, consequently, fail to fully investigate and treat these symptoms. 23
Comorbid physical disorders such as diabetes can therefore both drive, and be a consequence of, the significant health and socioeconomic inequalities faced by people with SMI. A better understanding of the determinants, and improved prevention and treatment of physical illness, could help to tackle the inequalities for this group.
Diabetes mellitus
Diabetes mellitus is a complex metabolic disorder characterised by chronic hyperglycaemia as a result of relative insulin deficiency, resistance or both. In 2019, the International Diabetes Federation estimated that 463 million people (1 in 11 of the global population) had diabetes and estimated an increase to 700 million by 2045. Approximately 4 million people in the UK are currently living with diabetes. 24
Diabetes is associated with several short- and long-term complications that cause considerable morbidity, reduced quality of life and shortened life expectancy. These include acute metabolic perturbations (hypoglycaemia, diabetic ketoacidosis and hyperosmolar hyperglycaemic state), macrovascular disease (coronary artery disease, peripheral vascular disease and stroke) and microvascular disease (retinopathy, nephropathy and neuropathy). Diabetes was responsible for approximately 4.2 million deaths worldwide, or 11.3% of all deaths, in 2017, outnumbering the combined number of global deaths from human immunodeficiency virus/acquired immunodeficiency syndrome, tuberculosis and malaria. 24
Type 2 diabetes mellitus (T2DM), which results from a combination of insulin resistance and less severe insulin deficiency, is the commonest form of diabetes, accounting for 90–95% of all cases. The rapid rise in T2DM largely explains the global epidemic, which is driven by an ageing population, longer survival with T2DM, earlier age at onset and better diagnosis. The prevalence of T2DM has increased as rates of obesity have risen.
Several risk factors have been identified for T2DM. The major modifiable risk factors are poor-quality diet and reduced physical activity. Smoking, mental illness (and psychotropic medication) and decreased sleep have also been implicated. Environmental risk factors include urbanisation, poverty and toxins. Non-modifiable risks include family history, ethnicity, birthweight and fetal under- and over nutrition, and history of gestational diabetes. 25
It is estimated that, worldwide, approximately half of people with diabetes are unaware of their condition; however, this is lower in high-income countries, in part because of the introduction of screening programmes to systematically identify those with undiagnosed diabetes.
Once diabetes is considered, it is relatively easy to diagnose by the laboratory measurement of fasting plasma glucose (≥ 7.0 mmol/l), random glucose (> 11.0 mmol/l) or a 2-hour plasma glucose after a 75-g oral glucose tolerance test (> 11.0 mmol/l). The use of glycated haemoglobin (HbA1c) (≥ 48 mmol/mol) was introduced by the WHO as an alternative method in 2011.
Prevention of diabetes
Clinical trials have shown that it is possible to prevent or delay the development of T2DM through lifestyle and/or pharmacological interventions. 26–30 Lifestyle interventions aim to reduce body weight and dietary fat intake, in particular saturated fat, while increasing dietary fibre and moderate physical activity, to ≈ 30 minutes a day. The UK has recently launched a National Diabetes Prevention Programme to support at-risk individuals to implement these lifestyle changes through referral to a behaviour change programme. 31
Management of diabetes
For most people, diabetes is a lifelong condition. People will manage diabetes themselves (self-management) for most of the time, with only a few hours per year spent in contact with health-care professionals. Consequently, people must develop the skills to manage their condition effectively. Structured self-management education programmes have been developed and are recommended by the National Institute for Health and Care Excellence (NICE) as an integral component of diabetes management. 32,33
Diabetes is managed through a combination of lifestyle changes and, when needed, treatment with antidiabetes drugs or insulin. The latest guidance highlights the importance of certain foods and dietary patterns. 32 In common with the general population, people with diabetes should be encouraged to eat a healthy diet. For individuals who are overweight, losing weight is important; loss of 10–15 kg of body weight may trigger remission. 32,34 Increased physical activity has profound benefits, including improved fitness, reduced insulin requirement, better glycaemic control, lower cardiovascular risk and greater life expectancy.
Diabetes in people with severe mental illness
The prevalence of diabetes is two to three times higher in people with SMI, and around 10% of people taking antipsychotic medications live with diabetes. 35,36 A meta-analysis of 41 studies comprising 161,886 participants reported 9.0% (95% CI 7.3% to 11.1%) diabetes prevalence in SMI. 36 In people with multiple episodes of psychosis, the prevalence of diabetes was double that of the general population, with earlier age at onset. 37 The incidence rates of diabetes in SMI, however, are usually < 1%, meaning that the absolute risk for any one individual is small. 38,39 Although diabetes is rare in adolescents and young adults, the relative risk of diabetes in young people exposed to antipsychotic drugs appears to be increased, compared with healthy controls and people with psychiatric disorders but unexposed to antipsychotic drugs. 40 These increased risks are for T2DM; there is no evidence that the incidence of type 1 diabetes is increased in people with SMI. 41
There are multiple potential reasons for the higher incidence of T2DM in people with SMI, including living conditions, lifestyle and disease-specific factors, as well as the effect of antipsychotic medications on insulin secretion and action. 42–47 People with SMI tend to have a dietary pattern that includes increased intake of energy-dense foods that are rich in fat and refined sugars, with low levels of fruit and vegetable intake, and tend to have low levels of physical activity. 42,43 Smoking and social deprivation are also important risk factors for diabetes, and the risks of both are higher in people with SMI.
In the early 2000s, as the use of atypical antipsychotics rose, reports of substantial weight gain, diabetes and dyslipidaemia began to emerge. 48,49 It has now become apparent that rates of diabetes are higher among people taking typical or atypical antipsychotics than among the general population. Among people with SMI who have not yet started antipsychotic treatment, the prevalence of diabetes is low, but it rises rapidly after treatment initiation, suggesting that antipsychotics are involved in the aetiology of T2DM, with women and those with multiple episodes of psychosis being at higher risk. 44
Studies, including randomised controlled trials comparing different antipsychotics, indicate that the risk of developing diabetes differs between medications. 35,44,49–51 A consistently higher risk of diabetes is reported in people taking clozapine or olanzapine, with the lowest risks associated with aripiprazole, although all antipsychotics have been associated with increased risk of diabetes. 44 Clinical trial data are supported by real-world comparisons between atypical antipsychotics. 45,46 Diabetes risk also increases with the number and dose of antipsychotics prescribed. 47 Potential mechanisms mediating this increased risk include weight gain, which increases insulin resistance, and the direct effects of antipsychotics, which decrease insulin sensitivity of cells and also impair insulin secretory capacity. There is debate about the magnitude of increased diabetes risk, with estimates varying widely up to a 33-fold increase; typically, however, the relative risks are < 2. 52
Prevention of diabetes in people with severe mental illness
No diabetes prevention studies have been undertaken in people taking antipsychotics, but lifestyle interventions have been used to prevent weight gain or manage obesity. Short-term studies with follow-ups of < 6 months report weight loss of around 3.1 kg over a period of 8–24 weeks,53 but the results of longer-term studies are less consistent. 54 Similarly, there have been no studies of pharmacological interventions, but the use of metformin leads to a modest 3.3-kg reduction in body weight over 3–6 months, in association with improved insulin sensitivity. 53,55
Screening and diagnosis of diabetes in people with severe mental illness
Although regular screening for diabetes is recommended for people with SMI,56–58 many people taking antipsychotics are not screened regularly; further work is needed to understand why this simple measure has not been embedded into routine clinical practice. 59,60 In 2011, targets for monitoring blood glucose were included in the national Quality and Outcomes Framework (QOF) to incentivise general practices to screen patients with SMI for diabetes. However, these targets were removed in 2014. 61
Management of diabetes in people with severe mental illness
Diabetes appears to have a greater impact on people with SMI than on the general population through an increased incidence of acute metabolic emergencies and diabetes complications. 62,63 Current NICE guidance12 suggests that diabetes in people with SMI should be managed in a similar way to diabetes in the general population. 64 However, the guidelines do not take into account the implications of antipsychotic use or the unique challenges people with SMI may face in managing their diabetes.
Additional challenges faced by people with severe mental illness
Although the principles of diabetes management are the same as for the rest of the population, people with SMI face additional challenges in achieving optimal diabetes outcomes. These include, but are not limited to:
-
health-care systems that separate diabetes and mental health
-
overshadowing when physical health problems are considered to be caused by the mental illness
-
excessive weight gain caused by antipsychotics and effects on insulin secretion
-
psychotic symptoms
-
poor cognition that interferes with decisions about self-management
-
lack of social support.
Given the burden of illness, and health and health-care inequalities for this population, improving diabetes care for people with SMI is a high priority for the NHS. 65 Little is known, however, about how SMI and other risk factors and challenges combine to generate high diabetes prevalence and poor diabetes outcomes, and how the quality and quantity of health-care services and interventions can influence these risk factors and outcomes in people with SMI.
Understanding this is a first step in developing health-care interventions to improve outcomes for people with diabetes and SMI. Better prevention and management of diabetes have the potential to significantly reduce the risk of diabetes complications, deliver large cost savings to the NHS and help reduce health inequalities (including life expectancy and morbidity) experienced by people with SMI.
In this report, we have focused on T2DM, as it is the most common type of diabetes in people with SMI. When the term ‘diabetes’ is used without specifying type, this refers to T2DM.
Social inequalities
Socioeconomic factors are powerful determinants of health outcomes, and a theoretical framework based on socioeconomic conditions is, therefore, appropriate for this study, in which social inequalities are likely to play a key role. 66,67 Under this framework, health gaps in morbidity and mortality arise for people living in disadvantaged circumstances, from reduced access to the material, financial, social or structural resources that the advantaged population leverage to maintain or improve health. People with SMI are more likely to be socially disadvantaged than people without SMI. 20,68–73 For example, only 6% of people with SMI in England and Wales are employed, whereas 58% are on long-term sick leave or are disabled and receiving benefits. 73
There are three related explanations for this. First, there is evidence that social disparities, including racism and the multifactorial stresses of urban living, are among the causative factors for incident SMI. 74–77 Second, the presence of severe and enduring illness patterns, and the physical and social consequences of these, thwarts efforts to maximise and improve social conditions. For example, SMI is diagnosed most often in early adulthood, and prodromal symptoms that interfere with functioning can be present months or years prior to onset. 78 This may disrupt secondary education and limit educational progression, thus constraining the greater economic opportunities afforded by further and higher education. Third, if poor social conditions are experienced, these are likely to compound over time, for example due to individuals not having the resources to invest in social contact, high rates of housing precarity that places individuals into increasingly deprived neighbourhoods, and further restriction in income due to potential discrimination against mental illness in the benefits system. 79–81 Deprived neighbourhoods are more likely to have environments that are obesogenic, where access to safe physical activity is limited and accessibility of poor-quality, calorie-dense foods is greater, increasing the risk of poor health. 82,83 Stigma and discrimination, represented through labelling, stereotyping, separating, emotional reactions and status loss, and enacted through behaviours, in public attitudes towards people with SMI, internally held beliefs about how one is viewed by others, and structural barriers can thwart efforts to obtain health care and prevent illness, and can perpetuate social disadvantage. 84–87
Given these details, it is not surprising that there is evidence that social inequalities play a role in driving poor mental and physical health outcomes for people with SMI. 88–92 A complicating, and further disadvantaging, factor is an increased risk of developing diabetes, which is itself socially patterned. 93 These greater health-care needs, combined with socioeconomic disadvantage, create a vulnerability that demands increased levels of care while simultaneously decreasing the likelihood of successful navigation of the health-care system. 94 Social disadvantage also reduces one’s capacity to take advantage of health promotion opportunities, and creates more barriers to taking up interventions designed to prevent or treat illness. Further details about how we applied this framework are presented in Chapter 3, Theoretical framework.
Overview of routine health-care databases
This section describes the routine health-care databases used in this study; specific information about how they were used is provided in Chapter 5.
Clinical Practice Research Datalink
The Clinical Practice Research Datalink (CPRD) GOLD is the world’s largest computerised database of anonymised longitudinal medical records from primary care. The CPRD data set (originally the General Practice Research Database) was established in 1987. CPRD data include records of clinical events (medical diagnoses), referrals to specialists and secondary care settings, prescriptions issued in primary care, records of immunisations/vaccinations, diagnostic testing, lifestyle information (such as smoking and alcohol status), and all other types of care provided as part of routine general practice. Data have been collected on > 17 million patients from a network of 718 general practices throughout the UK, representing around 8% of the UK population registered with a GP. 95 A cross-sectional study of the regional distribution of clinical computer systems in primary care found that 9% (636/7526) of general practices in England used Vision® (In Practice Systems Ltd, London, UK) software, from which the CPRD primarily draws its data. 95
Clinical information is captured in the CPRD database using ‘Read codes’, which are recorded by primary care practice staff as part of routine data entry. Read codes96 are a comprehensive hierarchical coding system of clinical terms used in primary care to classify diseases, history and symptoms, patient characteristics, procedures and tests. 97 The purpose of this coding system is to provide a standard language to enable accurate recording of patient information and more efficient retrieval of information for clinical or research purposes. 97
The CPRD data set benefits from being a large, retrospective and prospective longitudinal primary care record. Patient characteristics in the database are broadly representative of the general UK population in terms of age, sex and ethnicity. 98 Although other clinical computer reporting systems are used more commonly across the UK than the Vision system, patterns of area deprivation (based on the locations of general practices) have not been found to differ between these different systems. 95 Practices using the Vision software system are, however, geographically concentrated in the south and north-west of England – particularly around London and Manchester – with relatively few practices based in Yorkshire and the Humber, and the north-east. 95,98
Individual patient data in the CPRD can be linked electronically to external data sources, including Hospital Episode Statistics (HES) data for hospital admissions, Office for National Statistics (ONS) data for dates and causes of death, and the Index of Multiple Deprivation (IMD) to assess area deprivation in the locality. The CPRD provides a single extract of linked HES/ONS/IMD anonymised data.
Hospital Episode Statistics
The HES database contains patient records for inpatient admissions, outpatient appointments and accident and emergency (A&E) attendances at NHS hospitals in England. 99 Admissions recorded in HES include those for physical and mental health problems where the patient was admitted to an acute hospital (but admissions recorded do not include specialist mental health facility admissions). HES Admitted Patient Care data contain information on patients’ demographics, admission sources and methods, discharge methods and destination, and primary and secondary diagnoses, as well as procedures conducted during the stay.
Diagnoses from hospital admissions are classified using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), codes. Procedures are classified using the Office of Population Censuses and Surveys Classification of Interventions and Procedures version 4 (OPCS-4) codes.
Office for National Statistics mortality data
The ONS mortality data include information from a person’s death certificate, such as cause of death, and date and place of death, for all registered deaths in England and Wales. 100 These data are the most complete source of information on deaths based on information from medical practitioners and/or coroners.
Index of Multiple Deprivation data
The English IMDs provide a relative measure of deprivation at the lower-layer super output area (LSOA) level. There are around 35,000 LSOAs in England, each containing an average of 672 households. 101 The IMD rank used for the linkage is from 2010, and is a combined index of seven domains: income, employment, health and disability, education, crime, barriers to housing and services, and living environment deprivation. 102
Chapter 2 Study aims and objectives
The overall aim of this study was to understand the determinants of diabetes and the variations in diabetes outcomes and care for people with SMI. We also aim to identify health-care interventions that are associated with better outcomes, which can be tested further. The study feeds into a wider research programme to improve diabetes outcomes for people with SMI.
Research questions
Our key research questions were as follows:
-
What are the sociodemographic and illness-related risk factors associated with –
-
diabetes developing in people with SMI?
-
variations in diabetes and mental health outcomes in people with SMI and diabetes?
-
-
How do physical and mental health outcomes differ between people with SMI and diabetes and people with –
-
SMI without diabetes?
-
diabetes but no SMI?
-
-
What factors are associated with access to and receipt of diabetes health care for people with SMI, and how are diabetes health-care interventions experienced by people with SMI?
-
How, and at what cost, is diabetes monitored and managed in people with SMI, compared with people without SMI?
-
What health-care interventions (e.g. medication, referrals and care pathways) are associated with better diabetes outcomes for people with SMI and diabetes?
These questions are addressed in two studies, with seven objectives (see Study objectives). The quantitative study (see Chapter 5) addresses objectives 1–4, 6 and 7; the qualitative study addresses objective 5 and also contributes to objectives 2 and 7 (see Chapter 6).
Study objectives
-
In people with SMI: to identify which sociodemographic, illness, family history and lifestyle factors are associated with the development of diabetes (see Chapter 5, Objective 1: factors associated with the development of diabetes in people with severe mental illness).
-
In people with SMI and diabetes: to identify which sociodemographic, illness, family history and lifestyle factors are associated with variations in diabetes and mental health outcomes (see Chapter 5, Objective 2: factors associated with variation in diabetes and mental health outcomes in people with severe mental illness and diabetes).
-
In people with SMI: to compare the health-care interventions and physical and mental health outcomes of people with diabetes with those of people without diabetes (see Chapter 5, Objective 3: comparing health-care interventions and health outcomes in people with severe mental illness with diabetes with those in people with severe mental illness without diabetes).
-
In people with diabetes: to compare the health-care interventions and physical and mental health outcomes of people with SMI with those of people without SMI (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness).
-
To understand the factors that are associated with access to, and receipt of, diabetes care for people with SMI, and to explore the experience of diabetes health care by people with SMI (see Chapter 6).
-
To compare diabetes care provision for people with and people without SMI, and to estimate costs for these (see Chapter 5, Objective 6: comparing diabetes care provision and estimating health-care costs for people with and people without severe mental illness).
-
To identify which health-care interventions (e.g. medication, referrals and care pathways) may be associated with better diabetes outcomes for people with SMI and diabetes (see Chapter 5, Objective 7: identifying health-care interventions associated with better outcomes for people with diabetes and severe mental illness).
Deviations from the study protocol
The protocols for the overall study and for the quantitative study (study reference: 17_161R) have been published and are publicly available. 103,104
There were some changes to the study protocol for the quantitative analyses.
First, we planned to stratify all analyses by ethnicity. However, the small numbers in minority ethnic categories precluded doing this for some analyses.
Second, for objective 2 (see Chapter 5, Objective 2: factors associated with variation in diabetes and mental health outcomes in people with severe mental illness and diabetes), the methods were changed from repeated measures to patient-level analysis, as the former did not add value.
Third, for objective 7 (see Chapter 5, Objective 7: identifying health-care interventions associated with better outcomes for people with diabetes and severe mental illness), following discussions with clinical experts at a research team workshop, we had planned to look at several physical health checks including blood pressure, cholesterol, HbA1c, body mass index (BMI), retinopathy screening, diabetes education and influenza vaccination. However, we were unable to explore in detail all health-care interventions that we originally planned as a result of inconsistencies in the recording of relevant activities in patient records. We therefore prioritised investigating the most common physical health checks, including blood pressure, cholesterol, HbA1c level and BMI, for which recording was more complete.
Fourth, we identified that a significant proportion of the study population had diabetes diagnosed before the onset of SMI. Therefore, we needed to explore any potential order of diagnosis effects; these investigations were added to the planned analyses.
Fifth, for the final integration of study findings, we did not develop a logic model as proposed in the protocol, because of the tentative findings about how diabetes care provision may be associated with outcomes in people with SMI and diabetes.
There were no deviations from the study protocol for the scoping of the literature or the qualitative study.
Chapter 3 Study framework
Theoretical framework
This study sought to address important gaps in evidence about which people with SMI experience poor diabetes outcomes and why, and how health-care services could be changed to improve physical and mental health outcomes.
For our theoretical framework, we conceptualised socioeconomic conditions as a fundamental contributor to health outcomes. 66 As described in Chapter 1, Social inequalities, people with SMI are more likely to be socially disadvantaged,68,69,74,79 leading to a reduced ability to take advantage of resources that improve health, or prevent or treat illness, which ultimately contributes to the significant inequalities in health outcomes seen in this population. We acknowledge that, as a ‘downstream’ determinant of health,67 the health-care system cannot completely remediate the social causes of poor health. However, using a social inequalities lens, we aimed to understand how health-care delivery could better respond to the physical health needs of people with SMI and diabetes, and identify areas for action in which health care and its organisation appear to generate further inequalities in this vulnerable population.
Study design
We used a concurrent triangulation mixed-methods design,105 comprising:
-
A quantitative longitudinal observational study.
The quantitative study interrogated CPRD-linked primary care and hospital records, along with linked HES, ONS and IMD data of a large sample of adults with SMI, adults with SMI and diabetes and a sample of matched controls with diabetes but no SMI.
-
A qualitative interview study.
The qualitative study sought to identify the health-care needs and health-care delivery concerns of this population through thematic analysis of semistructured interviews exploring how diabetes is managed alongside SMI, and how diabetes care is experienced by people with diabetes and SMI, their family members and informal supporters, and health-care professionals. The study drew on critical realism as a guiding methodological framework,106,107 which acknowledges that there is an external reality to be observed (e.g. through interrogation of data such as CPRD), but that there is also a lived reality that can be accessed only through the perspectives of individual actors who make sense of, and interact with, the world in which they live.
The mixed-methods design was underpinned by a pragmatic paradigm, which acknowledges that each data type provides a different, but equally important, worldview, and that, merged together, they will enable us to develop a more complete understanding of health inequalities in this population than would be possible from using either method alone. 105 In particular, this approach allowed for an exploration of how people with SMI and diabetes navigate the health-care system and the range of factors that might influence their health and access to health care.
The definitive design for the study was informed by the results of an initial expert consultation and scoping of the literature, which aimed to develop a better understanding of the potential factors that could influence diabetes outcomes for people with SMI (see Chapter 4). This was used to determine which variables were important to explore in both the quantitative and qualitative studies and consider how to include them.
In line with the concurrent triangulation model, the quantitative and qualitative studies were conducted in parallel, and findings were merged at the interpretation stages to develop a fuller understanding of the factors contributing to poor outcomes and the drivers of improved health outcomes in SMI and diabetes. As part of the integration process, we conducted two co-design workshops involving members of the patient and public involvement (PPI) panel and participants from the qualitative interview study, and a final research team workshop, to iteratively make sense of and interpret the study findings and translate findings into key messages and implications for research and practice (see Chapter 7). Following Medical Research Council guidance for the development of complex interventions,108 we also used the co-design workshops to assess the potential acceptability and feasibility of potential interventions and service improvements into routine health care.
Figure 1 illustrates the four distinct components of the study and how they inter-relate. Each component will be discussed in detail in the upcoming chapters of this report:
-
scoping of the literature and expert consultation (see Chapter 4)
-
CPRD patient record analysis (quantitative longitudinal observational study) (see Chapter 5)
-
qualitative interview study with people with diabetes and SMI, their family members and health-care staff (see Chapter 6)
-
study integration, including co-design workshops (see Chapter 7).
FIGURE 1.
The four components of this study and the relationships between them.

Study organisation and governance
The study research team, comprising all co-applicants, was responsible for day-to-day management of the project, and held bimonthly project management meetings. A Study Steering Committee (SSC) with an independent chairperson and service user, and clinical, academic and commissioning representation was established, meeting every 6 months. The study was sponsored by Bradford District Care NHS Foundation Trust, which was also represented on the SSC.
Patient and public involvement
Overview of patient and public involvement
This study has been supported by DIAbetes and Mental illness: improving Outcomes aND Services (DIAMONDS) VOICE, a PPI group that has provided ‘expert by experience’ input to the study, with the aim of ensuring that the research remains relevant to people with SMI and that the outcomes of the research have the potential to have a meaningful impact on care and services.
Established in 2015, DIAMONDS VOICE comprises service users living with SMI and diabetes, and family members who provide support. The group is facilitated by a PPI co-ordinator and has 12 members who have been involved throughout the study, and a further two who have joined more recently. Three of these are family members or carers of people with diabetes and SMI. The group has met quarterly to discuss study progress, to offer advice on challenges faced by the research team and to undertake activities to support and inform the study. In addition, individual members of the group have contributed beyond the regular meetings, attending the co-design workshops and helping to raise the profile of the study through their networks and at local and regional dissemination events.
John Radford is a member of DIAMONDS VOICE and also a co-investigator on this study. He has participated in study research team meetings and SSC meetings, as well as co-design workshops (see Chapter 7, Co-design workshops).
Patient and public involvement activities
DIAMONDS VOICE has been engaged in project-related activities, starting from the grant application stages and continuing throughout the study duration (described below). The group has led the write-up of this section of the report.
Pre project
During the planning stages, the group assisted with the prioritisation of research questions, expressing a need for a better understanding of how diabetes is prevented and managed in people with SMI. The group suggested that it was important to understand the lived experience of comorbid SMI and diabetes, which influenced the research team to integrate qualitative inquiry into the study.
DIAMONDS VOICE representatives were invited to join the study research team and the SSC (see Study organisation and governance for study governance details) to ensure that the service user perspective was incorporated into the management and governance of the study.
Year 1
Activities included reviewing patient- and public-facing documentation, providing advice on interview topic guides and publicity materials, and suggesting ways to ask sensitive questions relating to finances and health. One member acted as a practice interviewee, which enabled researchers to refine the topic guide and to assess participant burden. In addition, members publicised the project across their own networks and at NHS events, for example running a stall at the sponsoring trust’s research and development conference in May 2018. Membership of DIAMONDS VOICE also expanded as a result of these engagement activities.
Year 2
DIAMONDS VOICE was involved in advising on participant recruitment, interpreting findings and in engagement activities. The research team had experienced difficulties engaging family members in the study, and advice was sought from the group on how to overcome this challenge. Members increased promotion of the study and expanded the stakeholder network, using their personal networks to engage family members supporting people with SMI and diabetes. This resulted in the creation of an ongoing connection with organisations such as Roshni Ghar, a local charity group that was able to provide input into the research and raise the study’s profile in the Asian community.
DIAMONDS VOICE also participated in the study co-design workshops, which took place in May and July 2019 (see Chapter 7, Co-design workshops). In advance of the workshops, members were asked to review invitation documents sent to potential service user and family member participants to ensure that they were clear and engaging. Group members also offered to support service user attendees with no previous experience of contributing to research workshops. During the sessions, members offered valuable insights into the authenticity of the interpretation of the study findings.
Group members played an important role in dissemination and engagement activities. For example, John Radford was a co-author on the protocol paper published in the Journal of Medical Internet Research. 103 In addition, members continued to play an active role in engaging varied audiences, for example at a regional National Institute for Health Research (NIHR) event on multimorbidity research.
Ongoing patient and public involvement
DIAMONDS VOICE will continue to be involved after this study ends, for example through advising on key events for dissemination, giving feedback on materials used in dissemination of research to the public to ensure that they are effective and appropriate, and advising about future research priorities.
Impact of patient and public involvement on the research
Patient and public involvement has had a clear impact on the design, management and dissemination of this study. Importantly, the DIAMONDS VOICE group influenced the choice of mixed methods through their recommendation to explore the lived experience of SMI and diabetes in addition to conducting analyses of longitudinal patient records.
The study has benefited from PPI representation at study research team and SSC levels of governance, which has ensured that the research has continued to address questions that are meaningful to people living with SMI and diabetes. For example, although sleep difficulties were not identified as an important variable in the scoping of the literature, DIAMONDS VOICE representatives highlighted the significance of the relationship between sleep and the day-to-day management of SMI and diabetes. As a result, and in recognition that CPRD recording of sleep difficulties would be incomplete, a decision was taken to explore this issue through the qualitative interviews.
Input from DIAMONDS VOICE has also had an impact at the operational level of the study. Group members reviewed all patient- and public-facing project documentation, indicating ways to improve clarity and readability. They also advised on ways to make promotional materials engaging and appealing and have been instrumental in promoting the study across their mental health networks and expanding the stakeholder network. Topic guides and recruitment strategies were refined in accordance with advice from the group.
Reflections on the experience of participating in patient and public involvement
Researcher and member feedback on PPI involvement in this and other studies is collected annually by the PPI co-ordinator (see Appendix 1, Reflections on the experience of participating in patient and public involvement).
Chapter 4 Identifying potential variables: determinants, outcomes and health-care interventions for diabetes in severe mental illness
Introduction
In preparation for the quantitative and qualitative studies, we undertook an iterative expert consultation and rapid scoping of the literature to identify the following: factors that might be associated with the development of diabetes, diabetes outcomes and diabetes health-care interventions for people with SMI.
The aim was to create a ‘longlist’ of the range of factors potentially relevant to our study and map which explanatory factors had been theorised or empirically demonstrated to have a relationship with the outcomes. From this longlist, we considered which factors were feasible to explore, for example which variables were available in the CPRD or could be explored in an interview. We planned that this approach would assist in the construction of key explanatory, outcome and health-care intervention variables for the quantitative study, and shape the topic guides for qualitative interviews.
Methods
We iteratively searched and aggregated evidence from a range of sources between January and March 2018 using an inclusive approach to capture a broad range of potential factors. Systematic reviews and meta-analyses known to the research team provided a starting point. We also drew on primary quantitative studies, literature reviews, expert consultation and clinical guidelines, as well as results from a pilot interrogation of CPRD data of around 1000 people with SMI and diabetes. The list of sources was reviewed and added to by the study research team and the SSC. Additional sources were identified through iterative targeted searches of specific variables in key databases [e.g. MEDLINE, EMBASE™ (Elsevier, Amsterdam, the Netherlands), Cochrane Database of Systematic Reviews] for systematic reviews in the first instance, and, when these were not available, other study types. Only publications in the English language were considered.
Details of determinants (including social determinants), outcomes and interventions were extracted from each source to generate a longlist of potential variables. This was reviewed by the research team to ensure that relevant variables were included and appropriately labelled, and that duplicates removed or merged. Quality appraisal of sources was not undertaken. All evidence sources were imported into NVivo 12 (QSR International, Warrington, UK).
Tables containing each variable, type, titles and number of sources cited, and summary of justification (theory, research findings) were produced to inform plans for the quantitative and qualitative studies (see Appendix 2, Tables 28–31).
Under objective 7, we planned to explore which health-care interventions were associated with better diabetes outcomes for people with SMI and diabetes, subject to data availability in the CPRD and completeness of recording by practices. As these interventions needed to be from the UK, we identified candidate interventions from the following sources:
-
Diabetes and SMI QOF indicators that could be identified in CPRD data. The QOF is a national programme that offers financial incentives to general practices for meeting quality-of-care targets across a range of conditions, including SMI and diabetes. 109,110 These indicators include, for example, annual monitoring for key biological measurements such as blood glucose and blood pressure, dietary review, foot examination, retinal screening, and structured education and comprehensive care planning. 111
-
Diabetes interventions recommended by NICE. 32
-
Interventions identified from the systematic review and service user engagement completed in NIHR study 13/54/40,112,113 which identified a number of diabetes-related indicators of primary care quality (e.g. diabetes screening, monitoring concomitant antipsychotic medications, BMI and weight loss, retinal and foot examination, and education about nutrition and physical activity).
-
Interventions identified from the DIAMONDS systematic review114 and PPI consultation, and through ongoing consultation with the study research team, collaborators and steering committee, and the wider DIAMONDS research group, DIAMONDS VOICE panel, and virtual stakeholder network.
-
Results of our pilot interrogation of CPRD data carried out by the DIAMONDS research group to characterise the population and to develop and test clinical (Read code) lists in preparation for the study. 115
-
Interventions identified from systematic reviews as being potentially effective in the UK for reducing inequalities in diabetes or SMI care and outcomes. 116–121
Results
Fifty-six sources were used to identify variables, as shown in Table 1. The full list can be found in Appendix 2, Tables 28–31.
| Study type | Number |
|---|---|
| Systematic review and meta-analysis | 12 |
| Meta-analysis | 1 |
| Systematic review | 11 |
| Primary quantitative study | 30 |
| Literature review | 2 |
| Total | 56 |
The list of variables (Table 2) was reviewed by the study research team to check for clinical significance, duplication or missing variables, and to determine which variables could be investigated in CPRD data. Any that could not be identified or that would potentially be biased were considered for inclusion in the qualitative interview topic guides.
| Variable | Number of associated sources | Explored in quantitative study? |
|---|---|---|
| Factors associated with the development of diabetes in people with SMI | ||
| Sociodemographic variables | ||
| Age | ≥ 20 | Objectives 1–4, 6 and 7 |
| Ethnicity | 10–19 | Objectives 1–4, 6 and 7 |
| Sex | ≥ 20 | Objectives 1–3 |
| Poverty and disadvantage | 10–19 | Objectives 1–4, 6 and 7 |
| Regional variation/rurality and urbanicity | < 10 | Not explored (no linkage to these data) |
| Medication use | ||
| Antidepressant use | < 10 | Objectives 1–4, 6 and 7 |
| Antihypertensive use | < 10 | Objectives 1–4, 6 and 7 |
| Antipsychotic use | ≥ 20 | Objectives 1–4, 6 and 7 |
| Lipid-lowering medication | < 10 | Objectives 1–4, 6 and 7 |
| Illness features/physiological characteristics | ||
| Comorbidity or multimorbidity (e.g. comorbid depression, hypertension) | < 10 | Objectives 1–4, 6 and 7 |
| Cognitive functioning | < 10 | Objectives 1–4, 6 and 7 |
| First-episode vs. multi-episode psychosis | < 10 | Not explored |
| Genetic link/family history | 10–19 | Objectives 1–4, 6 and 7 |
| Gene–environment interaction | < 10 | Not explored |
| Hormonal imbalance, including HPA axis dysfunction and stress | 10–19 | Not explored |
| Immune dysfunction and chronic inflammatory state | < 10 | Not explored |
| Lipid dysregulation | < 10 | Objectives 1–4, 6 and 7 |
| Obesity | 10–19 | As above |
| Sleep | 10–19 | Not explored |
| Lifestyle factors | ||
| Lifestyle factors (general) | < 10 | Not explored |
| Alcohol use | < 10 | Objectives 1–4, 6 and 7 |
| Poor diet | < 10 | Not explored |
| Relational context | < 10 | Not explored |
| Sedentary lifestyle | < 10 | Not explored |
| Smoking | < 10 | Objectives 1–4, 6 and 7 |
| Substance use | < 10 | As above |
| Additional factors that may influence diabetes outcomes in people with SMI | ||
| Service providers’ adherence to care guidelines and quality of care | < 10 | Not explored |
| Care ambiguity | < 10 | Not explored |
| Non-adherence to diabetes medication | < 10 | Not explored |
| Non-treatment | < 10 | Not explored |
| Polypharmacy (antipsychotic or antidiabetes) | < 10 | Not explored |
| Underdiagnosis of metabolic dysfunction | < 10 | Not explored |
| Stigmatisation | < 10 | Not explored |
| Interventions that may influence diabetes outcomes in people with SMI | ||
| Medication | ||
| Antihypertensive medication | < 10 | Objectives 1–4, 6 and 7 |
| Antipsychotic switching | < 10 | Not explored |
| Antidiabetes medication | 10–19 | Objectives 1–4, 6 and 7 |
| Lipid-lowering treatment | < 10 | Objectives 1–4, 6 and 7 |
| Mood stabilisers | < 10 | Not explored |
| Monitoring and examinations | ||
| Monitoring: blood pressure, HbA1c, lipid profile, BMI | 10–19 | Objectives 1–4, 6 and 7 |
| Examinations: retinopathy screening, foot surveillance, nephropathy testing | 10–19 | Not explored |
| Self-management | ||
| Self-management and education (general) | 10–19 | Not explored |
| Outcome measures | < 10 | Not explored |
| Predictors of self-care behaviours | < 10 | Not explored |
| Social or family support | < 10 | Not explored |
| Theoretical frameworks of interventions | < 10 | Not explored |
We used the variables list to construct explanatory (e.g. age, obesity, type and severity of SMI) and outcome variables (e.g. complications, cardiovascular control, mental illness relapses) and to shape the topic guides for qualitative interviews (see Report Supplementary Material 1). Although it was important not to be constrained by preconceived notions about participants’ experiences, being aware of the potential influence of, for example, social determinants of diabetes in people with SMI steered researchers towards initiating discussion around topics such as financial constraints and barriers to service access.
Chapter 5 Interrogation of patient health records
Introduction
The interrogation of patient health records study addressed the following research questions:
-
What are the sociodemographic and illness-related risk factors associated with –
-
diabetes developing in people with SMI?
-
variation in diabetes and mental health outcomes in people with SMI and diabetes?
-
-
How do physical and mental health outcomes differ between people with SMI and diabetes and people with –
-
SMI without diabetes?
-
diabetes but no SMI?
-
-
What factors are associated with access to, and receipt of, diabetes care for people with SMI?
-
How and at what cost is diabetes monitored and managed in people with SMI, compared with those without SMI?
-
What health-care interventions (e.g. medication, referrals and care pathways) are associated with better diabetes outcomes for people with SMI and diabetes?
These correspond to study objectives 1–4, 6 and 7 (see Chapter 2, and the objective sections later in this chapter). For all objectives, we conducted analyses in line with the inequalities framework to quantify the relative effect of social inequalities on quality of care and outcomes. Specifically, we used deprivation and disadvantage markers, such as the IMD, as independent variables to estimate gap or gradient effects. We planned to stratify analyses by ethnicity, but were limited by the small numbers of people in minority ethnic categories for most analyses.
Data and methods
Ethics approval
A data-use agreement for CPRD records and linked HES and ONS mortality data was granted by the International Scientific Advisory Committee (reference: 17_161R).
Data sets
Three data sets were extracted from the CPRD for the quantitative analyses; the relationships between these data sets are shown in Figure 2. Data set A contains anonymised longitudinal health records of a cohort of 32,759 people with SMI. Records were extracted from the CPRD GOLD population cohort if they:
-
had at least one clinical or referral event with a SMI diagnostic code in their medical history (see Report Supplementary Material 2, Table S1, for the code list)
-
were actively registered with one of the contributing general practices in England in the study period from 1 April 2000 to 31 March 2016
-
were eligible for the linkages required (HES, IMD and ONS death)
-
were aged ≥ 18 years when SMI was diagnosed
-
had continuous health data up to research standard [up to standard (UTS)] in the study period. 98
FIGURE 2.
Venn diagram illustrating the relationship between CPRD data sets.
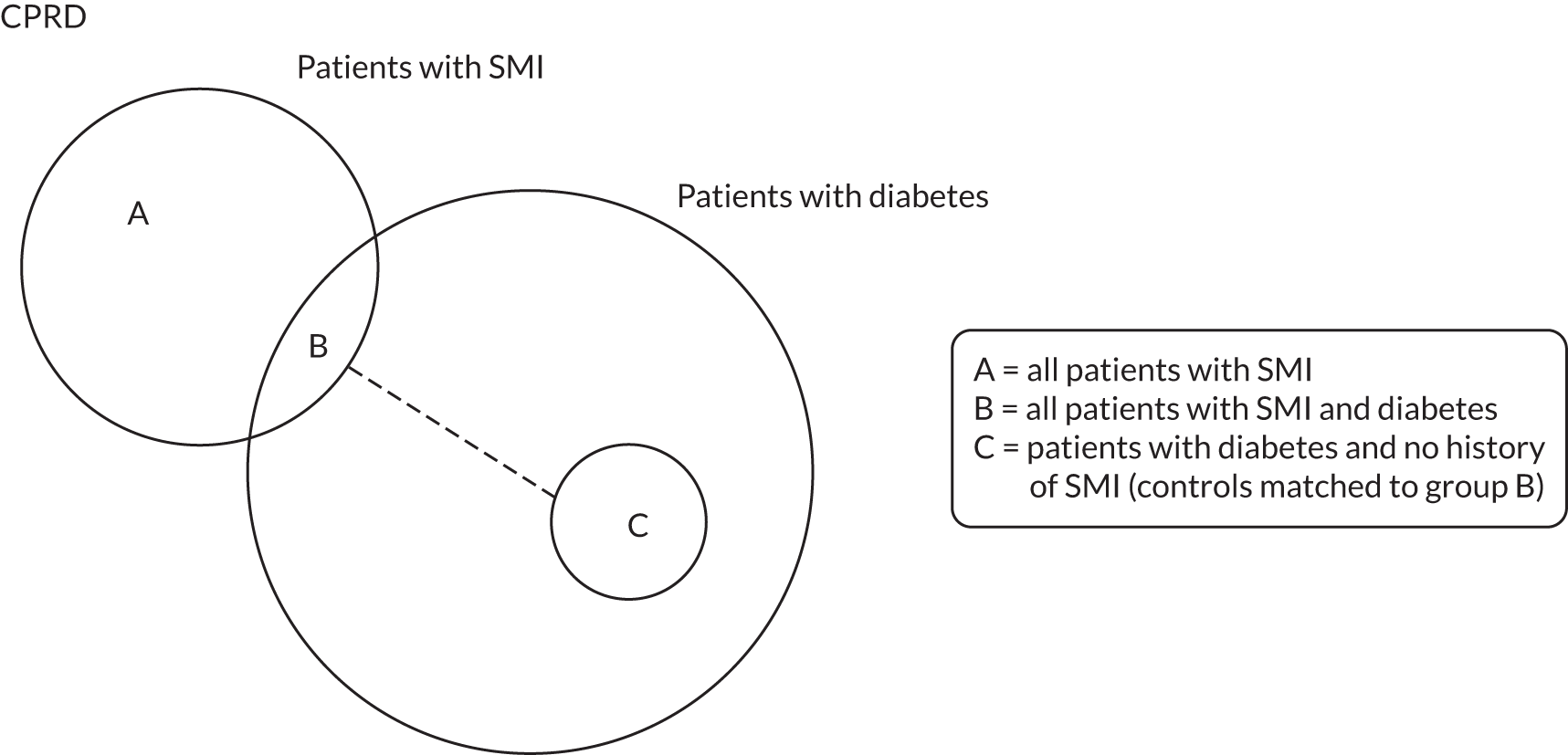
Data set B is a subset of data set A and contains records of 2761 people with SMI who also had T2DM. T2DM was defined as the presence of at least one clinical diagnostic code in a patient’s clinical or referral records (see Report Supplementary Material 2, Tables S2 and S3). We excluded people who had a record of type 1 diabetes mellitus after their diagnosis of T2DM.
Data set C comprises a cohort of 9573 control patients with a diagnosis of T2DM and no record of SMI who were matched to people in data set B (cases). These controls, with a diagnosis of T2DM and no record of SMI, were matched to cases based on age, sex and practice in a ratio of 4 : 1.
Variables
Using the longlist identified, as described in Chapter 4, Results, we constructed a range of candidate variables associated with the development of T2DM and with physical and mental health outcomes based on whether or not (1) the risk factor could be measured using the information in the CPRD and its linked data sets, (2) the data had been collected in the CPRD and its linked data sets and the quality of data recording was systematic enough for analysis and (3) it was likely to have an impact on the outcomes of interest.
Table 3 summarises the data sources and construction methods for the candidate risk factors that were used to measure patients’ baseline characteristics.
| Candidate explanatory variable | Type | Data sources and description |
|---|---|---|
| Demographic | ||
| Age | Continuous |
|
| Sex |
|
CPRD sex |
| Ethnicity |
|
CPRD and HES ethnicity |
| Socioeconomic status | ||
| Deprivation (patient level) | Categorical (by quintiles) | 2010 English IMDs102 at LSOA level, matched using patients’ residential postcodes |
| Status of SMI | ||
| Type of SMI |
|
|
| Duration of SMI | Continuous (years) |
|
| Status of diabetes | ||
| Duration of T2DM | Continuous (years) |
|
| Family history of diabetes | Binary |
|
| Comorbidities | ||
| Cardiovascular disease | Binary |
|
| Hypertension | Binary | As above |
| Dementia | Binary | As above |
| Learning disability | Binary | As above |
| Number of Charlson Index comorbidities | Count |
|
| Medication | ||
| Antidepressants | Binary |
|
| Antipsychotics | Binary (by typical and atypical) | As above |
| Antidiabetes | Binary | As above |
| Antihypertensive | Binary | As above |
| Lipid-lowering drugs | Binary | As above |
| Lifestyle factor | ||
| Smoking status |
|
|
| Alcohol intake status |
|
As above |
| Substance use | Binary |
|
| Biometric measure | ||
| BMI (kg/m2) |
|
|
| HbA1c (%) |
|
|
| Serum cholesterol (mmol/l) |
|
As above |
| Blood pressure (mmHg) |
|
As above |
It should be noted that patients’ follow-up periods varied for each objective, depending on the patient samples and research questions. We provide a detailed definition of follow-up period for each objective in the sections that follow.
Table 4 summarises the outcome variables under examination, along with their associated data sources, analysis methods and related objective(s).
| Outcome variables | Data sources and description | Type and analysis methods | Objectives |
|---|---|---|---|
| Physical health outcomes | |||
| Diabetes status |
|
Multilevel mixed-effects logistic regressions with practice-level random intercepts | 1 |
| Time to onset of diabetes |
|
Cox proportional hazards regressions | 1 |
| Glycaemic and cardiovascular control (HbA1c, serum cholesterol and blood pressure) |
|
Time trends plotted in graphs | 2–4 |
| Diabetes complications (hyperglycaemia and hypoglycaemia) |
|
Summarised using descriptive statistics | 2–4 |
| Microvascular complications (retinopathy, neuropathy and nephropathy) |
|
Cox proportional hazards regressions | 2 and 3 |
| Summarised using descriptive statistics | 4 | ||
| Macrovascular complications (myocardial infarction, peripheral vascular disease and stroke) |
|
Multilevel mixed-effects logistic regressions with practice-level random intercepts were applied on unmatched patient-level data | 2 and 3 |
| Conditional logistic regressions were applied on matched control data to generate ‘within’ estimators | 4 and 7 | ||
| Hospital admissions for macrovascular complications (ischaemic heart disease, peripheral vascular disease and cerebrovascular disease) |
|
Multilevel mixed-effects negative binomial regressions with practice-level random intercepts were applied on unmatched patient-level data | 2 and 3 |
| Negative binomial regressions with fixed effects by case–control clusters were applied on matched control data to generate ‘within’ estimators | 4 | ||
| All-cause mortality |
|
Cox proportional hazards regressions were applied on unmatched patient-level data | 2 and 3 |
| Cox proportional hazards regressions stratified by case–control clusters were applied on matched control data to generate ‘within’ estimators | 4 | ||
| Mental health outcome | |||
| SMI relapses |
|
Multilevel mixed-effects logistic regressions with practice-level random intercepts | 2 and 3 |
| Depression and anxiety |
|
Multilevel mixed-effects logistic regressions with practice-level random intercepts were applied on unmatched patient-level data | 2 |
| Conditional logistic regressions were applied on matched control data to generate ‘within’ estimators | 4 | ||
| Physical health checks | |||
| Glucose, HbA1c, serum cholesterol, blood pressure and BMI |
|
Multilevel mixed-effects negative binomial regressions with practice-level random intercepts were applied on unmatched patient-level data | 3 |
| Negative binomial regressions with fixed effects by case–control clusters were applied on matched control data to generate ‘within’ estimators | 4 and 7 | ||
| Resource use and costs | |||
| Resource use (consultations, drug prescriptions and diagnostic tests in primary care settings and inpatient stays at general hospitals) |
|
Generalised linear regressions with gamma distributions and log link functions | 6 |
| Medical costs (costs of consultations, drug prescriptions and diagnostic tests in primary care settings and inpatient stays at general hospitals) |
|
Generalised linear regressions with gamma distributions and log link functions | 6 |
Methods
To improve data quality, we developed and applied four common inclusion criteria to the extracted data set before carrying out analyses. These criteria were designed to account for the nature of SMI and diabetes, as well as for the features and limitations of this longitudinal data set:
-
First, we identified the diagnosis dates of SMI and diabetes. Diagnosis date is commonly identified as the date when the earliest diagnostic code was recorded in primary care. Because the first contact for (and diagnosis of) SMI can be in secondary care, we identified the diagnosis date for SMI as the earliest of the date of first GP diagnosis or the date of the first hospital admission for the condition. We also applied this method to the identification of diabetes diagnosis, although this condition is more commonly diagnosed in a primary care setting. This increased the number of people with SMI (data set A) to 32,781 and people with both SMI and T2DM (data set B) to 3448. Our matched controls (data set C), therefore, decreased in number to 9551 people.
-
Second, we excluded patients whose diagnosis of SMI and diabetes was recorded in the 90 days following registration with their current practice. As patients’ primary care records could be transferred between practices, we used this step to exclude those patients whose earliest primary care records of SMI and diabetes were likely to be updated from medical history because of changes of practices, rather than a new diagnosis.
-
Third, we excluded patients who were diagnosed with SMI and diabetes before the age of 18 years, using our modified diagnosis dates.
-
Fourth, we identified the patients with diagnosis codes of both type 1 diabetes and T2DM and removed people with potential type 1. For the patients with codes of both types, we followed an existing identification algorithm (from study NIHR 14_168R113), which categorised patients as potentially having type 1 diabetes if (1) their first diagnostic code of diabetes was recorded before 18 years of age or (2) there was a diagnostic code of type 1 recorded before 18 years of age or (3) they had been treated with insulin only.
Most analyses were conducted on patient-level data sets that typically had two levels of hierarchy: (1) patient-level observations nested within (2) practices. We accounted for the data structure by applying multilevel mixed-effects models with practice-level random intercepts.
Although a rich medical information source, these routine primary care data have been collected for administrative purposes, and the data quality depends on various factors such as the accurate and complete utilisation of the computerised recording system, standardised coding and the introduction of financial incentive schemes (such as the QOF) that reward general practices for performing incentivised Read-coded activities. We therefore identified an UTS follow-up period for each patient, which included comprehensive medical records for research. The start of the UTS period was defined as the later date between patient current registration and practice UTS dates; the end of this period was identified as the earliest among patient death, transfer-out and practice last collection dates.
We took a step-by-step forward approach to build the models from the most basic form (including only exposure variables) to expanded models with a wide range of adjustments. This approach was adopted to detect the potential confounding effect one variable might have on another, such as the association between SMI status and health outcomes attributed to older age. We adjusted for patient characteristics and time in all the final models as reported in this report (see Variables and statistical methods and Report Supplementary Material 3). Other candidate explanatory variables were included in the final models if they had significant association (p < 0.1) with the outcome of interest or their inclusion had improved the goodness of fit of models. We used c-statistics to compare logistic models and the Akaike information criterion and Bayesian information criterion (BIC) for negative binomial and Cox proportional hazards models. In the results tables (see Tables 6, 9, 13, 16, 22 and 23, and Report Supplementary Materials 4–12), we report a core model including only risk factors with good data quality and an extended model that also included covariates with less complete data, such as lifestyle and biometric measures.
Objective 1: factors associated with the development of diabetes in people with severe mental illness
Objective
The objective was to identify which sociodemographic, illness, family history and lifestyle factors are associated with the development of diabetes in people with SMI.
Study population
We used data set A (see Figure 2) containing records of 32,781 adults with SMI and applied the four common inclusion criteria, as outlined in Methods. From the remaining sample, people were included in the analysis if they (1) were diagnosed with SMI in their UTS continuous data period, (2) either had no record of T2DM in their medical history or had T2DM diagnosed only after SMI and (3) had a follow-up period of at least 1 day.
The follow-up period of eligible patients started from the later date between SMI diagnosis and UTS period start plus 15 months, to ensure that at least 15 months’ UTS continuous data were available for extracting baseline patient characteristics. The end of the follow-up period was identified as the end of the UTS continuous data period or the end of the study period, whichever was earlier. The final sample eligible for analysis included 14,838 people with SMI and without T2DM at baseline.
Variables and statistical methods
Descriptions of the outcome and candidate explanatory variables used in analyses for this objective, and their associated data sources and analysis methods, can be found in Tables 3 and 4. Further details on the statistical model specification have been provided in Report Supplementary Material 3.
For the status of diabetes, we adjusted for the length of follow-up (in years) and financial years to account for the effect of time in the multilevel logistic regressions. Robust standard errors for correlations by practices were specified in these regressions.
Results
Descriptive statistics
The distribution of patient characteristics at baseline was summarised according to diabetes diagnosis (Table 5).
| Patient characteristic | People with SMI (N = 14,838) | |
|---|---|---|
| Without T2DM | With T2DM | |
| Patients, n (%) | 14,131 (95.2) | 707 (4.8) |
| Diagnosis age (years), mean (SD) | ||
| SMI | 45.29 (19.38) | 50.70 (15.06) |
| T2DM | 55.56 (14.46) | |
| SMI type, n (%) | ||
| Schizophrenia | 7001 (49.5) | 340 (48.1) |
| Schizoaffective disorder | 429 (3.0) | 34 (4.8) |
| Bipolar disorder | 4893 (34.6) | 233 (33.0) |
| Depression and psychosis | 1433 (10.1) | 89 (12.6) |
| Other affective disorder | 337 (2.4) | 9 (1.3) |
| Mixed | 38 (0.3) | 2 (0.3) |
| Missing | 0 (0) | 0 (0) |
| Age at follow-up start (years), mean (SD) | 45.39 (19.36) | 50.82 (15.04) |
| Sex, n (%) | ||
| Male | 6794 (48.1) | 342 (48.4) |
| Female | 7337 (51.9) | 365 (51.6) |
| Ethnicity, n (%) | ||
| White | 11,907 (84.3) | 596 (84.3) |
| Asian | 469 (3.3) | 44 (6.2) |
| Black | 442 (3.1) | 30 (4.2) |
| Mixed | 168 (1.2) | 5 (0.7) |
| Other | 203 (1.4) | 11 (1.6) |
| Unknown | 942 (6.7) | 21 (3.0) |
| Deprivation (IMD), n (%) | ||
| 1st quintile (least deprived) | 2253 (15.9) | 79 (11.2) |
| 2nd quintile | 2595 (18.4) | 122 (17.3) |
| 3rd quintile | 2745 (19.4) | 124 (17.5) |
| 4th quintile | 3194 (22.6) | 176 (24.5) |
| 5th quintile (most deprived) | 3319 (23.5) | 206 (29.1) |
| Missing | 25 (0.2) | 0 (0) |
| Follow-up length (years) | ||
| Mean (SD) | 5.03 (4.57) | 9.17 (4.87) |
| Median (minimum–maximum) | 3.63 (0.003–25.46) | 8.85 (0.16–25.66) |
| Family history of diabetes, n (%) | 1105 (7.8) | 100 (14.1) |
| Comorbidities | ||
| Cardiovascular disease, n (%) | 823 (5.8) | 61 (8.6) |
| Hypertension, n (%) | 1617 (11.4) | 146 (20.7) |
| Learning disability, n (%) | 141 (1.0) | 3 (0.4) |
| Dementia, n (%) | 257 (1.8) | 6 (0.9) |
| Charlson Index score, mean (SD) | 0.36 (0.66) | 0.38 (0.65) |
| Medications, n (%) | ||
| Antidepressants | 7869 (55.7) | 419 (59.3) |
| Antipsychotics | ||
| Typical | 1530 (10.4) | 135 (19.1) |
| Atypical | 3976 (28.1) | 224 (31.7) |
| Antihypertensive | 2402 (17.0) | 212 (30.0) |
| Lipid-lowering drugs | 976 (6.9) | 86 (12.2) |
| Statins | 940 (6.7) | 84 (11.9) |
| Lifestyle factors, n (%) | ||
| Smoking | ||
| Non-smoker | 2355 (16.7) | 119 (16.8) |
| Ex-smoker | 1299 (9.2) | 63 (8.9) |
| Current smoker | 3423 (24.2) | 145 (20.5) |
| Missing | 7054 (49.9) | 380 (53.8) |
| Drinking | ||
| Non-drinker | 931 (6.6) | 47 (6.7) |
| Ex-drinker | 342 (2.4) | 19 (2.7) |
| Current drinker | 2717 (19.2) | 108 (15.3) |
| Missing | 10,141 (71.8) | 533 (75.4) |
| Substance use | 424 (3.0) | 13 (1.8) |
| Biometric measures | ||
| BMI (kg/m2), mean (SD) | 26.42 (6.24) | 31.57 (7.30) |
| < 20, n (%) | 594 (4.2) | 8 (1.1) |
| 20–24, n (%) | 1727 (12.2) | 42 (5.9) |
| 25–29, n (%) | 1426 (10.1) | 88 (12.5) |
| 30–40, n (%) | 989 (7.0) | 115 (16.3) |
| > 40, n (%) | 180 (1.3) | 39 (5.5) |
| Missing, n (%) | 9215 (65.2) | 415 (58.7) |
| HbA1c (%),a mean (SD) | 5.77 (1.19) | 7.18 (1.86) |
| ≤ 7.5, n (%) | 520 (3.7) | 41 (5.8) |
| > 7.5, n (%) | 19 (0.1) | 12 (1.7) |
| Missing | 13,592 (96.2) | 654 (92.5) |
| Cholesterol (mmol/l), mean (SD) | 5.19 (1.16) | 5.39 (1.36) |
| ≤ 5, n (%) | 1371 (9.7) | 84 (11.9) |
| > 5, n (%) | 1463 (10.4) | 115 (16.3) |
| Missing | 11,297 (79.9) | 508 (71.9) |
| Diastolic blood pressure (mmHg), mean (SD) | 77.39 (10.42) | 81.24 (10.56) |
| ≤ 80, n (%) | 5117 (36.2) | 239 (33.8) |
| > 80, n (%) | 2394 (16.9) | 188 (26.6) |
| Missing, n (%) | 6620 (46.9) | 280 (39.6) |
| Systolic blood pressure (mmHg), mean (SD) | 128.05 (18.50) | 134.59 (19.27) |
| ≤ 140, n (%) | 6054 (42.8) | 298 (42.2) |
| > 140, n (%) | 1457 (10.3) | 129 (18.3) |
| Missing, n (%) | 6620 (46.9) | 280 (39.6) |
Of the 14,838 people with SMI at baseline, 707 (4.8%) were diagnosed with T2DM in the follow-up period. This rate is lower than estimates from previous studies,44,122 and led us to further explore the sample characteristics.
The estimate above excludes those who developed diabetes before the onset of SMI. We found that, if we removed the criterion to have the recorded T2DM diagnosis after the diagnosis of SMI, in the 15,984 eligible patients whose SMI diagnosis was within the UTS data period, 1452 (9.1%) had a diagnosis of T2DM; of these, 782 (53.9%) had SMI diagnosed before T2DM and 670 (46.1%) had SMI diagnosed after or on the same day as T2DM. These figures are comparable to findings from the 2016–17 National Diabetes Audit (NDA),123 which reported that 34.9% of people with SMI and T2DM were diagnosed with SMI first, but are unexpected, as it is generally thought, from clinical experience, that most people with comorbid SMI and diabetes develop SMI first.
In the total sample of 29,281 people with SMI (not restricted to the UTS period), we found that 2984 (10.2%) patients had T2DM, and that 2209 (74.0%) of these had SMI before T2DM, which is more consistent with reported prevalence in the literature and with experience of diagnosis order from clinical practice. This demonstrated that, by restricting SMI diagnosis within the UTS data period in our initial analysis, we selectively excluded patients diagnosed with SMI at an earlier age; therefore, the patients remaining in the sample were more likely to have late-onset SMI.
Using the restricted UTS sample of 14,838, people who developed T2DM were diagnosed with SMI at an older age were more likely to be of Asian or black ethnicity and were more likely to live in the most deprived neighbourhoods than patients with no record of T2DM. Furthermore, people with T2DM had a higher baseline prevalence of cardiovascular disease and hypertension and were more likely to have been prescribed the medications under investigation.
Table 5 also shows that a large proportion of the sample had no baseline records for lifestyle factors and biometric measures. Based only on available data, the descriptive statistics showed that people with T2DM were more likely to be overweight; to have higher baseline levels of blood pressure, cholesterol and HbA1c; and to have a family history of diabetes.
Regression analyses results for the risk factors for development of type 2 diabetes
Regression results are summarised in Table 6, and Appendix 3, Figure 10, illustrates the non-linear impact of age on the risk of diabetes.
| Adjusted risk factor | Logistic regression | Cox proportional hazard regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core model | Extended model | Core model | Extended model | |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (years) (at follow-up start) | 1.171 | 1.135 to 1.207 | < 0.001 | 1.150 | 1.114 to 1.187 | < 0.001 | 1.159 | 1.125 to 1.194 | < 0.001 | 1.141 | 1.107 to 1.177 | < 0.001 |
| Age squared | 0.999 | 0.998 to 0.999 | < 0.001 | 0.999 | 0.999 to 0.999 | < 0.001 | 0.999 | 0.999 to 0.999 | < 0.001 | 0.999 | 0.999 to 0.999 | < 0.001 |
| Sex, reference = male | ||||||||||||
| Female | 0.879 | 0.751 to 1.028 | 0.106 | 0.791 | 0.673 to 0.930 | 0.004 | 1.109 | 0.880 to 1.397 | 0.381 | 0.986 | 0.783 to 1.243 | 0.907 |
| Ethnicity, reference = white | ||||||||||||
| Asian | 2.465 | 1.775 to 3.424 | < 0.001 | 2.431 | 1.751 to 3.377 | < 0.001 | 2.453 | 1.798 to 3.347 | < 0.001 | 2.294 | 1.681 to 3.131 | < 0.001 |
| Black | 1.853 | 1.247 to 2.753 | 0.002 | 1.850 | 1.203 to 2.844 | 0.005 | 1.828 | 1.282 to 2.605 | 0.001 | 1.735 | 1.189 to 2.531 | 0.004 |
| Mix, other and unknown | 0.767 | 0.553 to 1.065 | 0.113 | 0.781 | 0.563 to 1.084 | 0.140 | 0.778 | 0.569 to 1.063 | 0.115 | 0.770 | 0.565 to 1.050 | 0.099 |
| Patient IMD 2010, reference = 1st quintile (least deprived) | ||||||||||||
| 2nd quintile | 1.279 | 0.934 to 1.751 | 0.126 | 1.249 | 0.908 to 1.717 | 0.171 | 1.276 | 0.951 to 1.713 | 0.104 | 1.227 | 0.914 to 1.648 | 0.173 |
| 3rd quintile | 1.321 | 0.958 to 1.821 | 0.090 | 1.262 | 0.910 to 1.752 | 0.163 | 1.281 | 0.949 to 1.729 | 0.106 | 1.199 | 0.885 to 1.624 | 0.242 |
| 4th quintile | 1.602 | 1.182 to 2.173 | 0.002 | 1.507 | 1.105 to 2.054 | 0.010 | 1.550 | 1.167 to 2.058 | 0.002 | 1.441 | 1.084 to 1.915 | 0.012 |
| 5th quintile (most deprived) | 1.858 | 1.393 to 2.478 | < 0.001 | 1.677 | 1.247 to 2.254 | 0.001 | 1.782 | 1.364 to 2.329 | < 0.001 | 1.591 | 1.211 to 2.091 | 0.001 |
| Missing | 0.000 | 0.000 to 0.000 | < 0.001 | 0.000 | 0.000 to 0.000 | < 0.001 | ||||||
| SMI status | ||||||||||||
| Duration (years) | 1.212 | 0.911 to 1.612 | 0.187 | 1.126 | 0.828 to 1.533 | 0.450 | ||||||
| Type of SMI, reference = schizophrenia | ||||||||||||
| Schizoaffective disorder | 1.374 | 0.956 to 1.975 | 0.086 | 1.364 | 0.951 to 1.956 | 0.092 | 1.282 | 0.918 to 1.791 | 0.144 | 1.316 | 0.946 to 1.831 | 0.103 |
| Bipolar disorder | 0.917 | 0.764 to 1.101 | 0.355 | 0.869 | 0.722 to 1.046 | 0.137 | 0.904 | 0.759 to 1.076 | 0.256 | 0.866 | 0.729 to 1.029 | 0.103 |
| Depression and psychosis | 1.082 | 0.843 to 1.390 | 0.536 | 1.068 | 0.824 to 1.383 | 0.619 | 1.048 | 0.832 to 1.320 | 0.693 | 1.019 | 0.803 to 1.295 | 0.875 |
| Other affective disorder, mixed and missing | 0.515 | 0.300 to 0.886 | 0.017 | 0.437 | 0.240 to 0.795 | 0.007 | 0.532 | 0.315 to 0.899 | 0.018 | 0.429 | 0.243 to 0.759 | 0.004 |
| Comorbidities | ||||||||||||
| Cardiovascular disease | 1.410 | 1.028 to 1.934 | 0.033 | 1.360 | 0.985 to 1.877 | 0.062 | 1.376 | 1.024 to 1.848 | 0.034 | 1.250 | 0.927 to 1.684 | 0.143 |
| Hypertension | 1.800 | 1.422 to 2.278 | < 0.001 | 1.394 | 1.086 to 1.789 | 0.009 | 1.749 | 1.409 to 2.170 | < 0.001 | 1.415 | 1.133 to 1.767 | 0.002 |
| Medications | ||||||||||||
| Antipsychotics – typical | 1.215 | 0.984 to 1.499 | 0.070 | 1.209 | 0.973 to 1.503 | 0.087 | 1.055 | 0.831 to 1.339 | 0.662 | 1.030 | 0.814 to 1.304 | 0.804 |
| Antipsychotics – atypical | 1.391 | 1.162 to 1.666 | < 0.001 | 1.242 | 1.030 to 1.497 | 0.023 | 1.373 | 1.161 to 1.623 | < 0.001 | 1.238 | 1.043 to 1.471 | 0.015 |
| Family history of diabetes | 1.728 | 1.362 to 2.192 | < 0.001 | 1.708 | 1.378 to 2.118 | < 0.001 | ||||||
| Biometric measures | ||||||||||||
| BMI (kg/m2), reference = 20–25, normal | ||||||||||||
| < 20, underweight | 0.704 | 0.324 to 1.531 | 0.376 | 0.708 | 0.330 to 1.516 | 0.374 | ||||||
| 25–29, overweight | 2.268 | 1.526 to 3.372 | < 0.001 | 2.248 | 1.540 to 3.280 | < 0.001 | ||||||
| 30–40, obesity | 4.215 | 2.864 to 6.202 | < 0.001 | 3.781 | 2.628 to 5.440 | < 0.001 | ||||||
| > 40, severely obese | 10.936 | 6.608 to 18.098 | < 0.001 | 9.817 | 6.371 to 15.127 | < 0.001 | ||||||
| Missing | 1.725 | 1.217 to 2.446 | 0.002 | 1.632 | 1.184 to 2.250 | 0.003 | ||||||
| Blood pressure (diastolic) (mmHg), reference = ≤ 80 | ||||||||||||
| > 80 | 1.247 | 0.988 to 1.573 | 0.063 | |||||||||
| Missing | 0.988 | 0.804 to 1.214 | 0.905 | |||||||||
| HbA1c (%),a reference = ≤ 7.5 | ||||||||||||
| > 7.5 | 4.188 | 1.242 to 14.126 | 0.021 | 3.502 | 1.436 to 8.536 | 0.006 | ||||||
| Missing | 0.387 | 0.267 to 0.560 | < 0.001 | 0.363 | 0.255 to 0.518 | < 0.001 | ||||||
| Length of follow-up (years) | 1.221 | 1.190 to 1.254 | < 0.001 | 1.230 | 1.198 to 1.263 | < 0.001 | ||||||
| Financial year dummies | Yes | Yes | Yes | Yes | ||||||||
| Time-varying covariates | 0.020 | 0.034 | ||||||||||
| Sex – female | 0.956 | 0.921 to 0.993 | 0.051 | 0.960 | 0.925 to 0.997 | 0.058 | ||||||
| Medication – antipsychotics (typical) | 1.030 | 1.000 to 1.060 | 1.029 | 0.999 to 1.059 | ||||||||
| Patients | 14,806 | 14,806 | 14,829 | 14,829 | ||||||||
| Practices | 384 | 384 | ||||||||||
| c-statistic | 0.809 | 0.835 | ||||||||||
| Failures | 707 | 707 | ||||||||||
| Time at risk (years) | < 0.001 | < 0.001 | 74,328.397 | < 0.001 | 74,328.397 | < 0.001 | ||||||
The key risk factors for T2DM identified in the regression analyses were consistent across the four models, as were their estimated associations with the risk of T2DM. In the core model, results showed that older age appeared to be a risk factor for developing diabetes until around age 60 years [odds ratio (OR) 1.17, 95% CI 1.14 to 1.21], after which this impact would decrease, as suggested by the impact of quadratic age (see Appendix 3, Figure 10). For patients from an Asian minority background, the risk of T2DM increased by 147% (OR 2.47, 95% CI 1.78 to 3.42); for those from a black minority background, the risk of T2DM increased by 85% (OR 1.85, 95% CI 1.25 to 2.75). People who lived in the most deprived neighbourhoods were more likely, by around 86%, to develop diabetes (OR 1.86, 95% CI 1.39 to 2.48) than people from the most affluent areas.
After adjusting for patient sociodemographic characteristics and SMI status (e.g. type and duration), having comorbid hypertension and cardiovascular disease at baseline increased the risk of diabetes by 80% (OR 1.80, 95% CI 1.42 to 2.28) and 41% (OR 1.41, 95% CI 1.03 to 1.93), respectively. Patients who had been prescribed antipsychotics, particularly atypical antipsychotics, were more likely to have diabetes than people without these medications. All of these findings are consistent with expectations.
The extended model contained variables with higher proportions of missing values. The estimated results of this model, therefore, should be interpreted with caution. There seemed to be a strong association between BMI above the normal range and increased diabetes risk. This risk was doubled for patients who were overweight (OR 2.27, 95% CI 1.53 to 3.37), four times higher for those who were obese (OR 4.22, 95% CI 2.86 to 6.20) and 10 times higher for those who were severely obese (OR 10.94, 95% CI 6.61 to 18.10). Results from the Cox proportional hazards regressions generated a similar set of predictors for T2DM, the estimated impact of which was also comparable to that of the multilevel logistic regressions (see Table 6).
Summary of findings
Type 2 diabetes prevalence was 10.2% in the total sample of 29,281 people with SMI and 9.1% in the UTS-restricted sample (n = 15,984). The key predictors for developing T2DM in people with SMI included older age, ethnicity (Asian and black), socioeconomic deprivation, physical comorbidities (hypertension and cardiovascular disease) and antipsychotics. Potential risk factors may also include being overweight or obese, and glucose dysregulation.
Objective 2: factors associated with variation in diabetes and mental health outcomes in people with severe mental illness and diabetes
Objective
The objective was to identify which sociodemographic, illness, family history and lifestyle factors are associated with variations in diabetes and mental health outcomes among people with SMI and diabetes.
Study population
We used data set B containing records of 3448 patients with a clinical diagnosis of both SMI and T2DM, identified from either primary care records or hospital admissions (see Figure 2 for details of relationships between data sets). After applying the common eligibility criteria regarding registration, diagnosis age and diabetes type, there were 2984 patients in the sample.
The start of the follow-up period was identified as the latest date of the recorded SMI diagnosis, T2DM diagnosis or the start of UTS data period plus 15 months, so that patients were followed up after the second diagnosis and there was a 15-month window to obtain their baseline characteristics. The end of follow-up was identified as the end of the UTS data period or the end of the study period date, whichever was earlier. A total of 2754 patients with a follow-up length of at least 1 day were included for analysis.
Variables and statistical methods
Candidate risk factors are summarised in Table 3. The physical and mental health outcomes under examination included:
-
glycaemic and cardiovascular control (HbA1c, blood pressure and cholesterol levels)
-
diabetes complications (hyperglycaemia, hypoglycaemia, microvascular complications and macrovascular complications)
-
hospital admissions for macrovascular complications
-
mental health outcomes (SMI relapses and depression and anxiety)
-
mortality.
Details on the construction and statistical methods of these outcome variables are provided in Table 4, and further details on the methodology can be found in Report Supplementary Material 1.
Results
Descriptive statistics
Patient baseline characteristics are summarised in Table 7 and health outcomes are summarised in Figures 3 and 4, and Table 8. These descriptive statistics are reported for the whole eligible sample, as well as by diagnosis order.
| Characteristic | People with SMI and T2DM | ||
|---|---|---|---|
| Total | SMI before T2DM | SMI after T2DM (including same day) | |
| Patients, n (%) | 2754 (100) | 2019 (73.3) | 735 (26.7) |
| Diagnosis age (years), mean (SD) | |||
| SMI | 47.60 (17.76) | 41.88 (15.01) | 63.30 (15.05) |
| T2DM | 55.54 (13.75) | 54.98 (13.48) | 57.06 (14.37) |
| SMI type, n (%) | |||
| Schizophrenia | 1469 (53.3) | 1120 (55.5) | 349 (47.5) |
| Schizoaffective disorder | 150 (5.5) | 130 (6.4) | 20 (2.7) |
| Bipolar disorder | 862 (31.3) | 619 (30.7) | 243 (33.1) |
| Depression and psychosis | 228 (8.3) | 126 (6.2) | 102 (13.9) |
| Other affective disorder | 34 (1.2) | 18 (0.9) | 16 (2.2) |
| Mixed | 10 (0.4) | 5 (0.3) | 5 (0.7) |
| Missing | 1 (0.0) | 1 (0.1) | 0 (0) |
| Age at follow-up start (years), mean (SD) | 58.19 (14.26) | 56.08 (13.45) | 63.99 (14.81) |
| Duration (years), mean (SD) | |||
| SMI | 10.70 (12.05) | 14.33 (12.08) | 0.72 (2.63) |
| T2DM | 2.70 (5.05) | 1.12 (3.12) | 7.02 (6.60) |
| Sex, n (%) | |||
| Male | 1301 (47.2) | 993 (49.2) | 308 (41.9) |
| Female | 1453 (52.8) | 1026 (50.8) | 427 (58.1) |
| Ethnicity, n (%) | |||
| White | 2259 (82.0) | 1650 (81.7) | 609 (82.9) |
| Asian | 197 (7.2) | 140 (6.9) | 57 (7.8) |
| Black | 150 (5.5) | 116 (5.8) | 34 (4.6) |
| Mixed | 26 (0.9) | 18 (0.9) | 8 (1.1) |
| Other | 51 (1.9) | 42 (2.1) | 9 (1.2) |
| Unknown | 71 (2.6) | 53 (2.6) | 18 (2.5) |
| Deprivation (IMD 2010), n (%) | |||
| 1st quintile (least deprived) | 344 (12.5) | 235 (11.6) | 109 (14.8) |
| 2nd quintile | 438 (15.9) | 317 (15.7) | 121 (16.5) |
| 3rd quintile | 525 (19.1) | 382 (18.9) | 143 (19.5) |
| 4th quintile | 690 (25.1) | 514 (25.5) | 176 (24.0) |
| 5th quintile | 754 (27.4) | 570 (28.2) | 184 (25.0) |
| Missing | 3 (0.1) | 1 (0.1) | 2 (0.3) |
| Follow-up length (years) | |||
| Mean (SD) | 4.84 (4.09) | 5.16 (4.21) | 3.96 (3.62) |
| Median (minimum, maximum) | 3.78 (0.003, 23.80) | 4.14 (0.003, 23.80) | 2.84 (0.005, 18.78) |
| Comorbidities | |||
| Cardiovascular disease, n (%) | 396 (14.4) | 223 (11.1) | 173 (23.5) |
| Hypertension, n (%) | 978 (35.5) | 629 (31.2) | 349 (47.5) |
| Learning disability, n (%) | 33 (1.2) | 22 (1.1) | 11 (1.5) |
| Dementia, n (%) | 67 (2.4) | 33 (1.6) | 34 (4.6) |
| Charlson Index score, mean (SD) | 0.55 (0.80) | 0.48 (0.70) | 0.79 (1.00) |
| Medications, n (%) | |||
| Antidepressants | 1455 (52.8) | 992 (49.1) | 463 (63.0) |
| Antipsychotics | |||
| Typical | 553 (20.1) | 455 (22.5) | 98 (13.3) |
| Atypical | 1293 (47.0) | 1049 (52.0) | 244 (33.2) |
| Antihypertensive | 1347 (48.9) | 877 (43.4) | 470 (64.0) |
| Antidiabetics | 1098 (39.9) | 561 (27.8) | 537 (73.1) |
| Lipid-lowering drugs | 1114 (40.5) | 668 (33.1) | 446 (60.7) |
| Statins | 1083 (39.3) | 647 (32.1) | 436 (59.3) |
| Lifestyle factors, n (%) | |||
| Smoking | |||
| Non-smoker | 770 (28.0) | 512 (25.4) | 258 (35.1) |
| Ex-smoker | 508 (18.5) | 349 (17.3) | 159 (21.6) |
| Current smoker | 860 (31.2) | 699 (34.6) | 161 (21.9) |
| Missing | 616 (22.4) | 459 (22.7) | 157 (21.4) |
| Drinking | |||
| Non-drinker | 566 (20.6) | 420 (20.8) | 146 (19.9) |
| Ex-drinker | 187 (6.8) | 137 (6.8) | 50 (6.8) |
| Current drinker | 847 (30.8) | 648 (32.1) | 199 (27.1) |
| Missing | 1154 (41.9) | 814 (40.3) | 340 (46.3) |
| Substance use | 55 (2.0) | 41 (2.0) | 14 (1.9) |
| Biometric measures | |||
| BMI (kg/m2), mean (SD) | 32.40 (7.17) | 33.24 (7.31) | 30.41 (6.39) |
| < 20, n (%) | 29 (1.1) | 12 (0.6) | 17 (2.3) |
| 20–24, n (%) | 238 (8.6) | 138 (6.8) | 100 (13.6) |
| 25–29, n (%) | 574 (20.8) | 375 (18.6) | 199 (27.1) |
| 30–40, n (%) | 946 (34.4) | 707 (35.0) | 239 (32.5) |
| > 40, n (%) | 289 (10.5) | 233 (11.5) | 56 (7.6) |
| Missing, n (%) | 678 (24.6) | 554 (27.4) | 124 (16.9) |
| HbA1c (%),a mean (SD) | 7.67 (1.94) | 7.77 (2.00) | 7.49 (1.83) |
| ≤ 7.5, n (%) | 1094 (39.7) | 681 (33.7) | 413 (56.2) |
| > 7.5, n (%) | 678 (24.6) | 443 (21.9) | 235 (32.0) |
| Missing, n (%) | 982 (35.7) | 895 (44.3) | 87 (11.8) |
| Cholesterol (mmol/l), mean (SD) | 5.13 (1.41) | 5.32 (1.44) | 4.66 (1.19) |
| ≤ 5, n (%) | 1130 (41.0) | 725 (35.9) | 405 (55.1) |
| > 5, n (%) | 1044 (37.9) | 828 (41.0) | 216 (29.4) |
| Missing | 580 (21.1) | 466 (23.1) | 114 (15.5) |
| Diastolic blood pressure (mmHg), mean (SD) | 80.09 (10.61) | 81.37 (10.74) | 76.80 (9.54) |
| ≤ 80, n (%) | 1427 (51.8) | 929 (46.0) | 498 (67.8) |
| > 80, n (%) | 1013 (36.8) | 830 (41.1) | 183 (24.9) |
| Missing | 314 (11.4) | 260 (12.9) | 54 (7.4) |
| Systolic blood pressure (mmHg), mean (SD) | 134.44 (17.76) | 134.47 (17.72) | 134.36 (17.90) |
| ≤ 140, n (%) | 1748 (63.5) | 1262 (62.5) | 486 (66.1) |
| > 140, n (%) | 692 (25.1) | 497 (24.6) | 195 (26.5) |
| Missing, n (%) | 314 (11.4) | 260 (12.9) | 54 (7.4) |
| Family history of diabetes, n (%) | 430 (15.6) | 316 (15.7) | 114 (15.5) |
FIGURE 3.
Average cholesterol and HbA1c, 2000/1–2015/16. (a) Average cholesterol (mmol/l) and 95% CI; and (b) average HbA1c (%) and 95% CI.
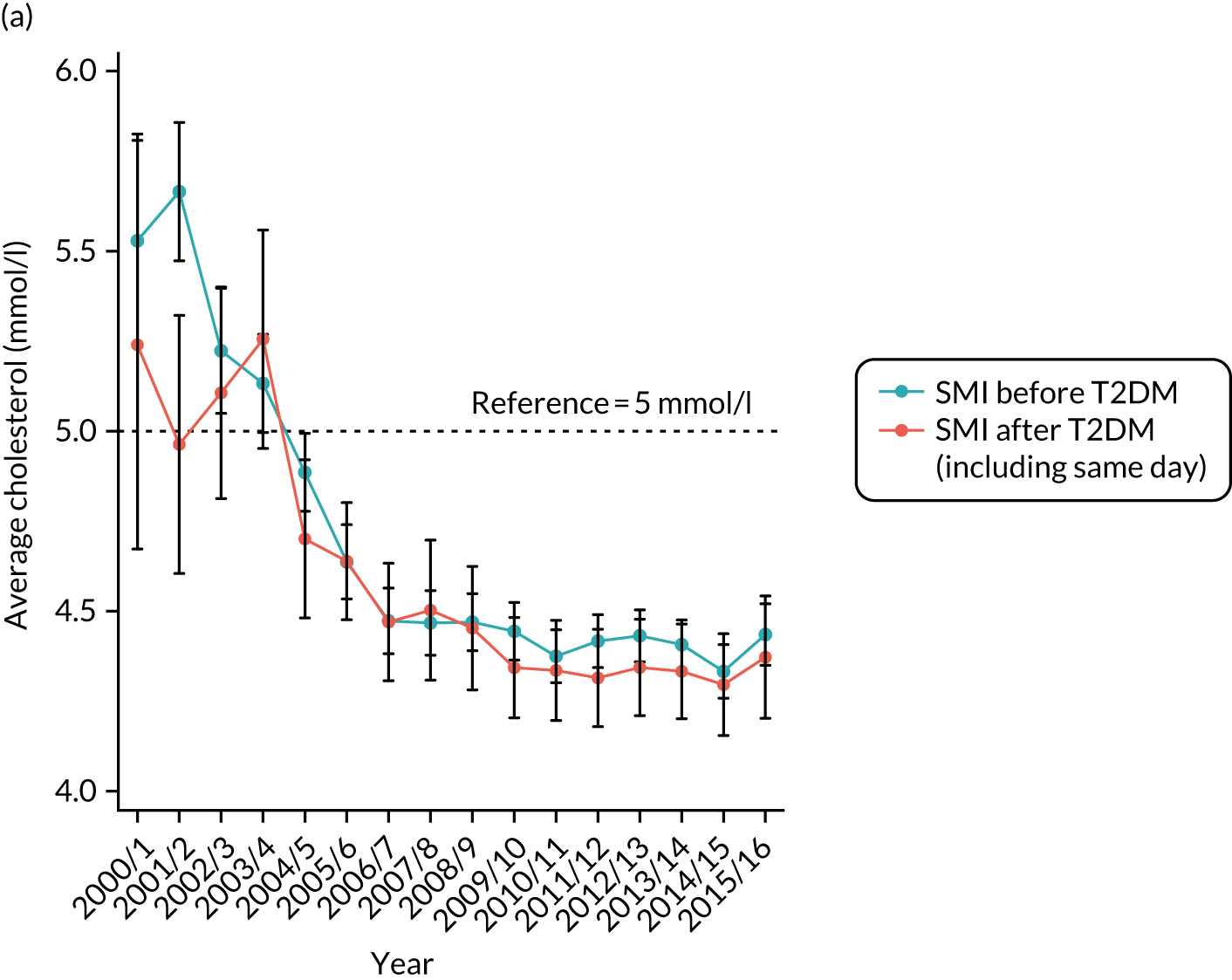
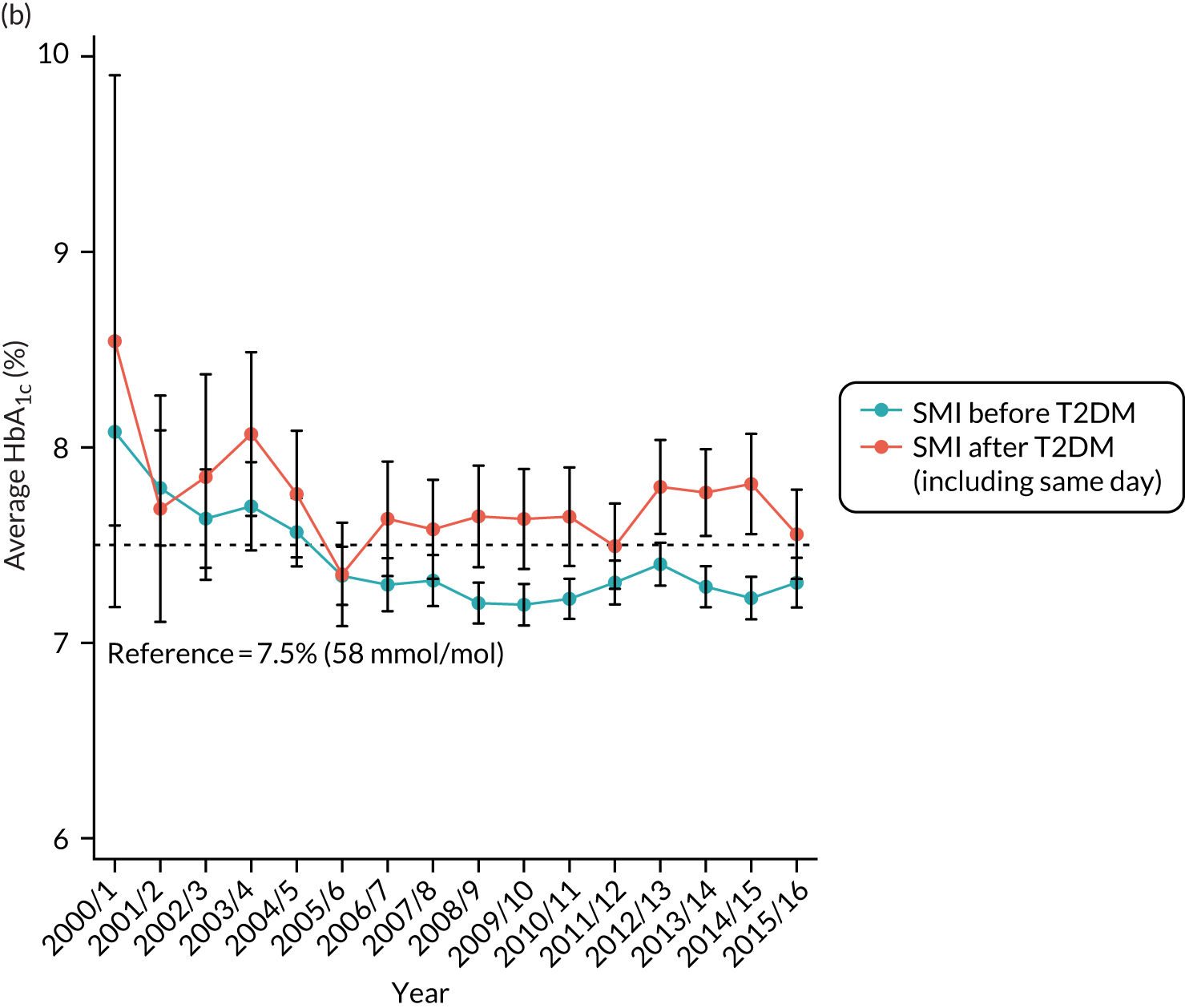
FIGURE 4.
Average blood pressure (mmHg), 2000/1–2015/16. (a) Systolic blood pressure and 95% CIs; and (b) diastolic blood pressure and 95% CIs.

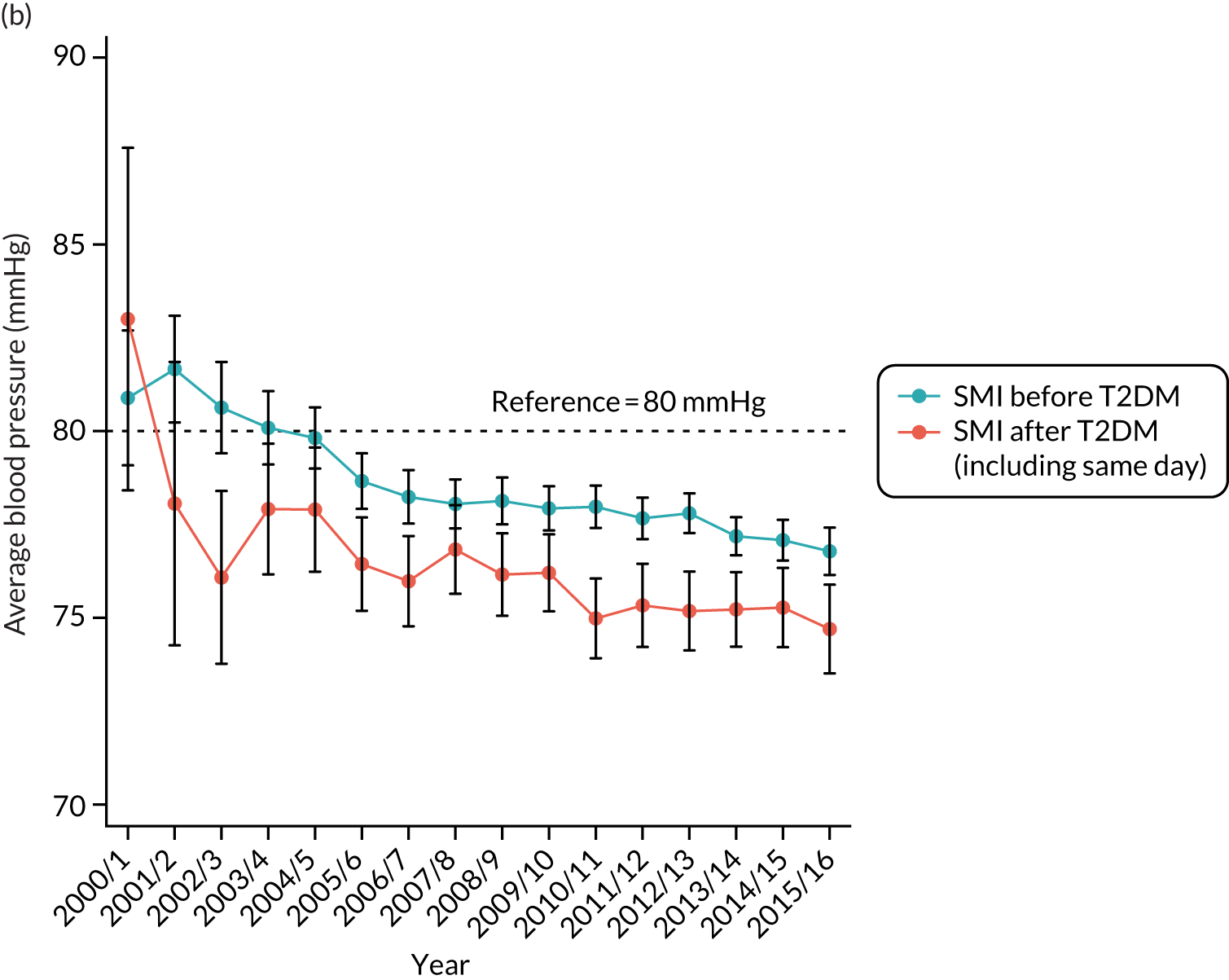
| Health outcome | People with SMI and T2DM | ||
|---|---|---|---|
| Total | SMI before T2DM | SMI after T2DM (including same day) | |
| Diabetes complications: primary care diagnosis, n (%) | |||
| Hyperglycaemia | 89 (3.2) | 67 (3.3) | 22 (3.0) |
| Hypoglycaemia | 115 (4.2) | 75 (3.7) | 40 (5.4) |
| Macrovascular complications (combined) | 187 (6.8) | 123 (6.1) | 64 (8.7) |
| Myocardial infarction | 74 (2.7) | 44 (2.2) | 30 (4.1) |
| Peripheral vascular disease | 55 (2.0) | 43 (2.1) | 12 (1.6) |
| Stroke | 73 (2.7) | 47 (2.3) | 26 (3.5) |
| Microvascular complications (combined) | |||
| Baseline | 229 (8.3) | 81 (4.0) | 148 (20.1) |
| New complications at follow-up | 461 (16.7) | 365 (18.1) | 96 (13.1) |
| Nephropathy | |||
| Baseline | 28 (1.0) | 12 (0.6) | 16 (2.2) |
| New nephropathy at follow-up | 62 (2.3) | 45 (2.2) | 17 (2.3) |
| Neuropathy | |||
| Baseline | 72 (2.6) | 26 (1.3) | 46 (6.3) |
| New neuropathy at follow-up | 82 (3.0) | 62 (3.1) | 20 (2.7) |
| Retinopathy | |||
| Baseline | 156 (5.7) | 48 (2.4) | 108 (14.7) |
| New retinopathy at follow-up | 385 (14.0) | 303 (15.0) | 82 (11.2) |
| Diabetes complications: admissions, mean (SD) | |||
| Macrovascular complications (combined) | 0.14 (0.58) | 0.13 (0.55) | 0.17 (0.67) |
| Ischaemic heart disease | 0.09 (0.50) | 0.09 (0.46) | 0.12 (0.58) |
| Peripheral vascular disease | 0.01 (0.17) | 0.01 (0.19) | 0.01 (0.10) |
| Cerebrovascular disease | 0.04 (0.22) | 0.03 (0.21) | 0.05 (0.26) |
| Mental health outcomes, n (%) | |||
| SMI relapses | 782 (28.4) | 559 (27.7) | 223 (30.3) |
| Depression and anxiety | 580 (21.1) | 413 (20.5) | 167 (22.7) |
| All-cause mortality, n (%) | 458 (16.6) | 296 (14.7) | 162 (2.0) |
Table 7 shows that 73.3% (2019/2754) of eligible patients had SMI diagnosed before T2DM, and 26.7% had T2DM diagnosed first (including same-day diagnosis). Patient characteristics differed between the two groups. Patients who had SMI before T2DM were diagnosed at an earlier age for both conditions: average diagnosis age was 41.9 years for SMI and 55.0 years for T2DM. In comparison, patients who had T2DM first were diagnosed with T2DM at around 57.1 years of age and with SMI at around 63.3 years of age. Patients who had SMI before T2DM were also more likely to be male and to live in the most deprived neighbourhoods.
Patients who had T2DM before SMI were more likely to have depression and psychosis as the type of SMI. The prevalence in this group was more than double the prevalence in people diagnosed with SMI first. These patients also had a higher prevalence of comorbidities and were more likely to be prescribed medications other than antipsychotics.
There were fewer missing data for baseline lifestyle factors and biometric measures in patients who had T2DM diagnosed first than in people diagnosed with SMI first. The latter were more likely to smoke cigarettes (although there were a large number of missing data in both groups, limiting definitive conclusions). People diagnosed with SMI first also had higher levels of HbA1c, cholesterol and blood pressure at baseline, and were more likely to be obese than people diagnosed with T2DM first.
For physical health outcomes, we examined the variations in glycaemic and cardiovascular control by plotting time trends against financial years (see Figures 3 and 4). There was a general trend for improved control among patients in both groups between 2000 and 2016. On average, people who had T2DM before SMI had better baseline control, and continued to have similar, or better, control for cholesterol and blood pressure in their follow-up periods. However, the average levels of HbA1c in this group were consistently above the NICE-recommended threshold of 58 mmol/mol (7.5%) in this period, whereas the average levels of HbA1c among people diagnosed with SMI first dropped (and remained) below this threshold from around 2004/5.
The apparent improvements in cholesterol, HbA1c and blood pressure might reflect improvements in care and control over time, but they might also reflect changes in the composition of the study population. Recorded prevalence of diabetes increased throughout the period and practices were financially incentivised for case-finding from 2004 onwards. As case-finding improved, less severe cases at earlier stages of the condition may have constituted an increasing proportion of the study population over time.
As summarised in Table 8, people who had SMI before T2DM had, in general, better physical and mental health outcomes. Patients in this group were less likely to develop macrovascular complications and had fewer hospital admissions for these complications in the follow-up period. These patients also had lower rates of SMI relapses and depression and anxiety, and had a lower mortality rate. The SMI before T2DM group had a lower baseline prevalence of microvascular complications, but were more likely to develop these conditions during the follow-up period than the other group. Those diagnosed with diabetes before SMI will have had diabetes for 5 or 6 years before entering the data set for this analysis; therefore, they would have had the ‘opportunity’ to develop complications in the pre-baseline period. As a result, they would be expected to have a higher baseline prevalence of microvascular complications (and fewer new microvascular complications during follow-up) than those diagnosed with SMI first.
Regression analyses results
The adjusted risk factors for physical health outcomes are reported in Table 9. Among the patient sociodemographic characteristics, older age was a significant predictor of macrovascular complications and more deprived socioeconomic status was also weakly associated with a higher risk of complications.
| Adjusted risk factor | Macrovascular complications | Microvascular complications | All-cause mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary care diagnosis | Hospital admissions | |||||||||||
| OR | 95% CI | p-value | IRR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at follow-up start (years) | 1.034 | 1.018 to 1.050 | < 0.001 | 1.211 | 1.108 to 1.324 | < 0.001 | 1.061 | 1.004 to 1.121 | 0.035 | 1.074 | 1.064 to 1.084 | < 0.001 |
| Age squared | 0.999 | 0.998 to 0.999 | < 0.001 | 0.999 | 0.999 to 1.000 | 0.041 | ||||||
| Sex, reference = male | ||||||||||||
| Female | 0.905 | 0.654 to 1.251 | 0.544 | 0.864 | 0.621 to 1.203 | 0.387 | 0.964 | 0.786 to 1.183 | 0.728 | 0.825 | 0.683 to 0.997 | 0.046 |
| Ethnicity, reference = white | ||||||||||||
| Asian | 0.703 | 0.365 to 1.354 | 0.292 | 1.168 | 0.645 to 2.114 | 0.608 | 1.075 | 0.769 to 1.503 | 0.673 | 0.477 | 0.299 to 0.762 | 0.002 |
| Black | 1.557 | 0.749 to 3.237 | 0.235 | 1.452 | 0.814 to 2.590 | 0.206 | 1.067 | 0.613 to 1.858 | 0.818 | 0.887 | 0.527 to 1.493 | 0.651 |
| Mix, other and unknown | 0.728 | 0.315 to 1.683 | 0.458 | 0.424 | 0.162 to 1.112 | 0.081 | 1.031 | 0.626 to 1.700 | 0.904 | 1.845 | 1.289 to 2.641 | 0.001 |
| Patient IMD 2010, reference = 1st quintile (least deprived) | ||||||||||||
| 2nd quintile | 1.094 | 0.566 to 2.116 | 0.790 | 1.122 | 0.624 to 2.017 | 0.700 | 1.376 | 0.967 to 1.959 | 0.076 | 1.184 | 0.827 to 1.695 | 0.357 |
| 3rd quintile | 1.594 | 0.920 to 2.761 | 0.097 | 1.587 | 0.909 to 2.771 | 0.104 | 1.153 | 0.815 to 1.632 | 0.422 | 0.959 | 0.663 to 1.387 | 0.824 |
| 4th quintile | 1.571 | 0.922 to 2.675 | 0.096 | 1.664 | 0.991 to 2.795 | 0.054 | 1.371 | 0.974 to 1.930 | 0.070 | 1.493 | 1.087 to 2.051 | 0.013 |
| 5th quintile (most deprived) | 1.346 | 0.761 to 2.379 | 0.307 | 1.589 | 0.913 to 2.764 | 0.101 | 1.154 | 0.787 to 1.694 | 0.463 | 1.377 | 0.992 to 1.911 | 0.056 |
| Missing | 0.000 | 0.000 to 0.000 | < 0.001 | 1.375 | 0.623 to 3.034 | 0.430 | 1.477 | 0.050 to 43.828 | 0.822 | |||
| SMI status | ||||||||||||
| Duration (years) | 0.989 | 0.977 to 1.002 | 0.087 | 1.008 | 0.999 to 1.016 | 0.068 | 0.990 | 0.983 to 0.998 | 0.017 | |||
| Type, reference = schizophrenia | ||||||||||||
| Schizoaffective disorder | 1.501 | 1.021 to 2.205 | 0.039 | |||||||||
| Bipolar disorder | 0.882 | 0.708 to 1.100 | 0.267 | |||||||||
| Depression and psychosis | 0.853 | 0.594 to 1.224 | 0.387 | |||||||||
| Other affective disorder, mixed and missing | 1.563 | 0.822 to 2.972 | 0.173 | |||||||||
| T2DM duration | 1.032 | 1.004 to 1.062 | 0.028 | 1.041 | 1.011 to 1.071 | 0.007 | ||||||
| Comorbidities | ||||||||||||
| Cardiovascular disease | 2.976 | 1.973 to 4.490 | < 0.001 | 2.706 | 1.868 to 3.920 | < 0.001 | 1.313 | 1.000 to 1.724 | 0.050 | |||
| Hypertension | 1.330 | 0.948 to 1.867 | 0.099 | |||||||||
| Dementia | ||||||||||||
| Learning disability | ||||||||||||
| Charlson Index comorbidities (count variable) | 1.241 | 1.056 to 1.458 | 0.009 | 1.155 | 1.041 to 1.283 | 0.007 | ||||||
| Medications | ||||||||||||
| Antidepressants | ||||||||||||
| Antipsychotics – typical | 1.272 | 1.018 to 1.590 | 0.034 | |||||||||
| Antipsychotics – atypical | 1.314 | 1.080 to 1.599 | 0.006 | |||||||||
| Antihypertensive | 1.529 | 1.103 to 2.119 | 0.011 | |||||||||
| Antidiabetics | 1.218 | 0.997 to 1.487 | 0.054 | 1.351 | 1.103 to 1.654 | 0.004 | ||||||
| Length of follow-up (years) | 1.158 | 1.102 to 1.217 | < 0.001 | 1.169 | 1.121 to 1.220 | < 0.001 | ||||||
| Constant | 0.004 | 0.001 to 0.016 | 0.000 | 0.000 to 0.001 | < 0.001 | |||||||
| Financial year dummies | Yes | Yes | Yes | Yes | ||||||||
| Patients (n) | 2628 | 2754 | 2524 | 2754 | ||||||||
| Practices (n) | 349 | 354 | ||||||||||
| c-statistic | 0.805 | |||||||||||
| Failures (n) | 460 | 458 | ||||||||||
| Time at risk (years) | 10,356.178 | 13,334.804 | ||||||||||
Diabetes duration of 1 additional year increased the odds for primary care diagnosis of macrovascular complications by around 3% (OR 1.03, 95% CI 1.00 to 1.06) and for hospital admissions by 4% [incidence rate ratio (IRR) 1.04, 95% CI 1.01 to 1.07]. The risk of these complications in patients with cardiovascular disease at baseline was more than double the risk in patients without this comorbidity; this increased by 198% (OR 2.98, 95% CI 1.97 to 4.49) for primary care diagnosis and by 171% (IRR 2.71, 95% CI 1.87 to 3.92) for hospital admissions. Risk predictors of macrovascular complications also include comorbid hypertension and the baseline prescription of antihypertensives. These two predictors are correlated because of their nature, and we included them separately in our regressions. We chose between alternative models using the c-statistic for the logistic regressions and BIC for negative binomial regressions. Furthermore, there was evidence suggesting that the presence of additional Charlson Index comorbidities was associated with increased hospital admissions.
The adjusted risk of microvascular complications was predicted by older age and baseline cardiovascular disease. There was also weak evidence that more deprived socioeconomic status, longer duration of SMI and baseline prescription of antidiabetes medications may also be associated with an increased risk of developing these complications. Ethnicity was not associated with risk of either macrovascular or microvascular complications.
All-cause mortality increased with demographic characteristics, including older age, male sex, white ethnicity and socioeconomic deprivation. Longer duration of SMI was associated with slightly reduced risk of mortality, and certain types of SMI, such as schizoaffective disorder, were associated with an increased risk of mortality, compared with having schizophrenia. One additional comorbidity of the Charlson Index increased the risk by 16% [hazard ratio (HR) 1.16, 95% CI 1.04 to 1.28]. Antipsychotics were associated with higher risk of mortality: those on typical antipsychotics had an increased risk of mortality of 27% (HR 1.27, 95% CI 1.02 to 1.59) and those on atypical antipsychotics had an increased risk of mortality of 31% (HR 1.31, 95% CI 1.08 to 1.60). The baseline prescription of antidiabetes medications also increased the risk by 35% (HR 1.35, 95% CI 1.10 to 1.65). We were not able to control for severity of mental illness or diabetes.
The regression results for mental health outcomes (SMI relapse and depression or anxiety) are summarised in Table 10. After adjustment, older age had a small but statistically significant impact on mental health outcomes; the risk reduced by ≈ 2% for each additional year of age [OR 0.98 (95% CI 0.98 to 0.99) for SMI relapses and OR 0.98 (95% CI 0.97 to 0.99) for depression and anxiety]. Female patients were more likely to have depression and anxiety (OR 1.27, 95% CI 1.03 to 1.58). Both SMI relapse and diagnosis of depression or anxiety were affected by SMI type. Patients with bipolar disorder were more likely to have a relapse of SMI in the follow-up period than patients with schizophrenia (OR 1.36, 95% CI 1.12 to 1.66) and had a similar increased risk for depression and anxiety (OR 1.37, 95% CI 1.07 to 1.77). Patients with a clinical diagnosis of depression and psychosis had an increased risk of depression and anxiety (OR 2.89, 95% CI 2.03 to 4.13) during follow-up. In terms of comorbidities, we found that the presence of dementia and learning disability was associated with reduced risk of SMI relapses. An additional Charlson Index comorbidity was associated with a lower risk of SMI relapses (OR 0.85, 95% CI 0.75 to 0.95) but a higher risk of clinical events of depression and anxiety (OR 1.20, 95% CI 1.05 to 1.37). Furthermore, baseline prescription of antidiabetes medications was a significant predictor for SMI relapses (OR 1.34, 95% CI 1.08 to 1.66).
| Adjusted risk factor | SMI relapses | Depression and anxiety | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age at follow-up start (years) | 0.984 | 0.977 to 0.992 | < 0.001 | 0.978 | 0.969 to 0.987 | < 0.001 |
| Sex, reference = male | ||||||
| Female | 1.125 | 0.943 to 1.343 | 0.192 | 1.272 | 1.025 to 1.578 | 0.029 |
| Ethnicity, reference = white | ||||||
| Asian | 1.073 | 0.731 to 1.575 | 0.718 | 1.421 | 0.960 to 2.104 | 0.079 |
| Black | 1.143 | 0.795 to 1.645 | 0.471 | 0.784 | 0.462 to 1.330 | 0.366 |
| Mix, other and unknown | 0.591 | 0.386 to 0.906 | 0.016 | 0.679 | 0.389 to 1.185 | 0.173 |
| Patient IMD 2010, reference = 1st quintile (least deprived) | ||||||
| 2nd quintile | 0.727 | 0.517 to 1.023 | 0.067 | 0.782 | 0.533 to 1.147 | 0.209 |
| 3rd quintile | 1.048 | 0.754 to 1.457 | 0.780 | 1.092 | 0.725 to 1.643 | 0.674 |
| 4th quintile | 0.863 | 0.617 to 1.208 | 0.390 | 0.732 | 0.497 to 1.079 | 0.115 |
| 5th quintile (most deprived) | 1.013 | 0.726 to 1.413 | 0.939 | 1.185 | 0.816 to 1.722 | 0.373 |
| Missing | 10.905 | 0.214 to 556.451 | 0.234 | |||
| SMI status | ||||||
| Type, reference = schizophrenia | ||||||
| Schizoaffective disorder | 1.017 | 0.681 to 1.519 | 0.934 | 1.110 | 0.697 to 1.767 | 0.660 |
| Bipolar disorder | 1.361 | 1.116 to 1.660 | 0.002 | 1.373 | 1.066 to 1.768 | 0.014 |
| Depression and psychosis | 1.019 | 0.710 to 1.461 | 0.920 | 2.893 | 2.029 to 4.125 | < 0.001 |
| Other affective disorder, mixed and missing | 0.502 | 0.201 to 1.249 | 0.138 | 2.165 | 1.157 to 4.051 | 0.016 |
| T2DM duration | 1.036 | 1.014 to 1.059 | 0.001 | |||
| Comorbidities | ||||||
| Dementia | 0.381 | 0.148 to 0.981 | 0.046 | |||
| Learning disability | 0.313 | 0.123 to 0.801 | 0.015 | |||
| Charlson Index comorbidities (count variable) | 0.845 | 0.749 to 0.953 | 0.006 | 1.197 | 1.048 to 1.366 | 0.008 |
| Medications | ||||||
| Antidepressants | 2.453 | 1.919 to 3.135 | < 0.001 | |||
| Antidiabetics | 1.336 | 1.076 to 1.658 | 0.009 | |||
| Length of follow-up (years) | 1.091 | 1.057 to 1.127 | < 0.001 | 1.158 | 1.114 to 1.203 | < 0.001 |
| Constant | 0.648 | 0.274 to 1.532 | 0.264 | 0.097 to 0.716 | ||
| Financial year dummies | Yes | Yes | ||||
| Patients (n) | 2751 | 2754 | ||||
| Practices (n) | 354 | 354 | ||||
| c-statistic | 0.738 | < 0.001 | 0.826 | < 0.001 | ||
In sensitivity analyses to investigate whether or not health outcomes and their association with risk factors differed by diagnosis order, we included diagnosis order in the regression models as an explanatory variable and tested its impact on outcomes as well as its interacting relationship with other risk factors. Adjusting for diagnosis order had a similar effect to adjustment for the duration of conditions. For instance, macrovascular complications were associated with longer duration and T2DM. When conducting regression analyses on a restricted sample that included only those patients who had SMI diagnosed first, the results were consistent with those obtained from the unrestricted models.
Results of the extended models can be found in Report Supplementary Material 4. In these models, we investigated the impact of potential risk factors, such as lifestyle, biometric measures and family history, on physical and mental health outcomes. The data on these variables were less complete and the patterns of missing data were unlikely to be random, meaning that these results should be interpreted with caution. The results showed that current smoker status increased the risk of macrovascular complications (primary care diagnosis), compared with non-smokers (OR 1.91, 95% CI 1.19 to 3.06); the risk of mortality was more than three times higher for patients who used substances at baseline (HR 3.30, 95% CI 1.58 to 6.90); and a baseline HbA1c level exceeding the recommended threshold was associated with a higher risk of developing microvascular complications (HR 1.66, 95% CI 1.33 to 2.08).
Summary of findings
The descriptive statistics showed that people diagnosed with SMI before T2DM differed from those diagnosed with T2DM first in characteristics including diagnosis age, types of SMI, socioeconomic status, comorbidities, medication and lifestyle, as well as baseline diabetes and cardiovascular controls.
After adjustment using regression analyses, we found that older age, socioeconomic deprivation and the presence of multimorbidity were the common risk predictors for physical health outcomes. The duration of T2DM and the use of medications were also associated with poorer status of some of these physical health outcomes. All-cause mortality risk increased with older age, male sex, white ethnicity and socioeconomic deprivation. The risk of poorer mental health outcomes, however, was primarily predicted by younger age, type of SMI and comorbidities.
Objective 3: comparing health-care interventions and health outcomes in people with severe mental illness and diabetes with those in people with severe mental illness without diabetes
Objective
The objective was to compare health-care interventions and physical and mental health outcomes in people with SMI and diabetes with those of people with SMI without diabetes.
Study population
We used data set A, containing records of 32,781 people with a clinical diagnosis of SMI (see Figure 2). After applying the common inclusion criteria regarding diagnosis date and age, practice registration, and type, 29,281 patients remained in the sample.
The start of follow-up was calculated as the later date of SMI diagnosis or the start of UTS data plus 15 months, so that each patient was followed up from (or after) the diagnosis of SMI, with a 15-month window for collecting baseline characteristics. The end of follow-up was identified as either the end of UTS data or the end of the study period, whichever occurred first. A total of 26,977 patients with a follow-up length of at least 1 day were included in the analyses.
Variables and statistical methods
The exposure variable for this objective was the status of T2DM. The other candidate explanatory variables are summarised in Table 3. The outcome variables under investigation were as follows (see Table 4 for further details):
-
glycaemic and cardiovascular control (HbA1c, blood pressure and cholesterol levels)
-
diabetes complications (hyperglycaemia, hypoglycaemia, microvascular complications and macrovascular complications)
-
hospital admissions for macrovascular complications
-
mental health outcomes (SMI relapses)
-
all-cause mortality
-
health checks (glucose, cholesterol, blood pressure and BMI).
For this objective, we used SMI relapse as the mental health outcome. Markers of depression and anxiety were not examined as the clinical Read codes for these overlapped with the diagnostic codes of depression and psychosis (a type of SMI).
Variation in receiving health-care interventions was explored using levels of health checks. The QOF61 rewards primary care providers for performing regular health checks and monitoring the physical health status for people with a wide range of long-term conditions, including SMI. We therefore selected four QOF-incentivised physical health checks (glucose, cholesterol, blood pressure and BMI) to examine their provision and association with T2DM status. For further details on the methodology, see Report Supplementary Material 3.
Results
Descriptive statistics
The baseline characteristics for eligible patients are summarised in Table 11. Of the 26,977 people with SMI, 2755 (10.2%) also had a diagnosis of T2DM. Patients with T2DM were diagnosed with SMI at an older age; the mean diagnosis age was 40.7 years for people without T2DM and 47.6 years for people with T2DM. People with T2DM were more likely to be female, to be of Asian or black ethnicity and to live in socioeconomically deprived neighbourhoods than people without T2DM. Furthermore, there was a higher probability of having a family history of diabetes in people with T2DM.
| Characteristic | People with SMI | ||
|---|---|---|---|
| Total | Without T2DM | With T2DM | |
| Patients, n (%) | 26,977 (100) | 24,222 (89.8) | 2755 (10.2) |
| Age at diagnosis (years), mean (SD) | |||
| SMI | 41.41 (18.06) | 40.71 (17.97) | 47.60 (17.75) |
| T2DM | 55.55 (13.77) | ||
| SMI type, n (%) | |||
| Schizophrenia | 14,166 (52.5) | 12,696 (52.4) | 1470 (53.4) |
| Schizoaffective disorder | 1213 (4.5) | 1063 (4.4) | 150 (5.4) |
| Bipolar disorder | 9264 (34.3) | 8402 (34.7) | 862 (31.3) |
| Depression and psychosis | 1837 (6.8) | 1609 (6.6) | 228 (8.3) |
| Other affective disorder | 424 (1.6) | 390 (1.6) | 34 (1.2) |
| Mixed | 72 (0.3) | 62 (0.3) | 10 (0.4) |
| Missing | 1 (0.0) | 0 (0) | 1 (0.0) |
| Age at follow-up start (years), mean (SD) | 46.71 (17.77) | 45.76 (17.79) | 55.02 (15.19) |
| Duration of SMI (years), mean (SD) | 5.38 (9.33) | 5.14 (9.08) | 7.54 (11.10) |
| Sex, n (%) | |||
| Male | 13,349 (49.5) | 12,048 (49.7) | 1301 (47.2) |
| Female | 13,628 (50.5) | 12,174 (50.3) | 1454 (52.8) |
| Ethnicity, n (%) | |||
| White | 22,790 (84.5) | 20,531 (84.8) | 2259 (82.0) |
| Asian | 982 (3.6) | 785 (3.2) | 197 (7.2) |
| Black | 1094 (4.1) | 943 (3.9) | 151 (5.5) |
| Mixed | 320 (1.2) | 294 (1.2) | 26 (0.9) |
| Other | 386 (1.4) | 335 (1.4) | 51 (1.9) |
| Unknown | 1405 (5.2) | 1334 (5.5) | 71 (2.6) |
| Deprivation (IMD 2010), n (%) | |||
| 1st quintile (least deprived) | 3912 (14.5) | 3568 (14.7) | 344 (12.5) |
| 2nd quintile | 4709 (17.5) | 4271 (17.6) | 438 (15.9) |
| 3rd quintile | 5249 (19.5) | 4724 (19.5) | 525 (19.1) |
| 4th quintile | 6333 (23.5) | 5643 (23.3) | 690 (25.1) |
| 5th quintile (most deprived) | 6733 (25.0) | 5978 (24.7) | 755 (27.4) |
| Missing | 41 (0.2) | 38 (0.2) | 3 (0.1) |
| Follow-up length (years) | |||
| Mean (SD) | 6.14 (5.35) | 5.93 (5.25) | 8.02 (5.83) |
| Median (minimum, maximum) | 4.58 (0.003, 26.21) | 4.33 (0.003, 26.21) | 7.10 (0.003, 25.91) |
| Family history of diabetes, n (%) | 2446 (9.1) | 2015 (8.3) | 431 (15.6) |
| Comorbidities | |||
| Cardiovascular disease, n (%) | 1550 (5.8) | 1212 (5.0) | 338 (12.3) |
| Hypertension, n (%) | 3199 (11.9) | 2423 (10.0) | 776 (28.2) |
| Dementia, n (%) | 424 (1.6) | 369 (1.5) | 55 (2.0) |
| Learning disability, n (%) | 252 (0.9) | 229 (1.0) | 23 (0.8) |
| Charlson Index score, mean (SD) | 0.33 (0.63) | 0.31 (0.61) | 0.45 (0.76) |
| Medications, n (%) | |||
| Antidepressants | 13,046 (48.4) | 11,603 (47.9) | 1443 (52.4) |
| Antipsychotics | |||
| Typical | 4637 (17.2) | 3970 (16.4) | 667 (24.2) |
| Atypical | 9088 (33.7) | 8143 (33.6) | 945 (34.3) |
| Antihypertensive | 4775 (17.7) | 3660 (15.1) | 1115 (40.5) |
| Antidiabetics | 1055 (3.9) | 86 (0.4) | 969 (35.2) |
| Lipid-lowering drugs | 2391 (8.9) | 1564 (6.5) | 827 (30.0) |
| Statins | 2304 (8.5) | 1502 (6.2) | 802 (29.1) |
| Lifestyle factors, n (%) | |||
| Smoking | |||
| Non-smoker | 4898 (18.2) | 4259 (17.6) | 639 (23.2) |
| Ex-smoker | 2555 (9.5) | 2211 (9.1) | 344 (12.5) |
| Current smoker | 7748 (28.7) | 7031 (29.0) | 717 (26.0) |
| Missing | 11,776 (43.7) | 10,721 (44.3) | 1055 (38.3) |
| Drinking alcohol | |||
| Non-drinker | 2733 (10.1) | 2321 (9.6) | 412 (15.0) |
| Ex-drinker | 938 (3.5) | 803 (3.3) | 135 (4.9) |
| Current drinker | 6862 (25.4) | 6182 (25.5) | 680 (24.7) |
| Missing | 16,444 (61.0) | 14,916 (61.6) | 1528 (55.5) |
| Substance use | 2551 (9.5) | 2354 (9.7) | 197 (7.2) |
| Biometric measures | |||
| BMI (kg/m2), mean (SD) | 27.59 (6.36) | 26.98 (6.05) | 31.36 (6.91) |
| < 20, n (%) | 969 (3.6) | 932 (3.9) | 37 (1.3) |
| 20–24, n (%) | 3662 (13.6) | 3434 (14.2) | 228 (8.3) |
| 25–29, n (%) | 3852 (14.3) | 3336 (13.8) | 516 (18.7) |
| 30–40, n (%) | 3098 (11.5) | 2372 (9.8) | 726 (26.4) |
| > 40, n (%) | 530 (2.0) | 351 (1.5) | 179 (6.5) |
| Missing, n (%) | 14,866 (55.1) | 13,797 (57.0) | 1069 (38.8) |
| HbA1c (%),a mean (SD) | 6.71 (1.85) | 5.78 (1.30) | 7.47 (1.88) |
| ≤ 7.5 | 1629 (6.0) | 894 (3.7) | 735 (26.7) |
| > 7.5 | 448 (1.7) | 35 (0.1) | 413 (15.0) |
| Missing | 24,900 (92.3) | 23,293 (96.2) | 1607 (58.3) |
| Cholesterol (mmol/l), mean (SD) | 5.16 (1.20) | 5.23 (1.15) | 4.92 (1.33) |
| ≤ 5, n (%) | 3160 (11.7) | 2372 (9.8) | 788 (28.6) |
| > 5, n (%) | 3283 (12.2) | 2681 (11.1) | 602 (21.9) |
| Missing, n (%) | 20,534 (76.1) | 19,169 (79.1) | 1365 (49.6) |
| Diastolic blood pressure (mmHg), mean (SD) | 77.68 (10.34) | 77.41 (10.30) | 79.50 (10.40) |
| ≤ 80, n (%) | 10,760 (39.9) | 9494 (39.2) | 1266 (46.0) |
| > 80, n (%) | 5216 (19.3) | 4458 (18.4) | 758 (27.5) |
| Missing, n (%) | 11,001 (40.8) | 10,270 (42.4) | 731 (26.5) |
| Systolic blood pressure (mmHg), mean (SD) | 127.82 (18.07) | 126.95 (17.88) | 133.79 (18.28) |
| ≤ 140, n (%) | 12,996 (48.2) | 11,521 (47.6) | 1475 (53.5) |
| > 140, n (%) | 2980 (11.1) | 2431 (10.0) | 549 (19.9) |
| Missing, n (%) | 11,001 (40.8) | 10,270 (42.4) | 731 (26.5) |
The baseline prevalence of cardiovascular disease and hypertension was substantially higher in people with both SMI and T2DM than in people without T2DM, consistent with the patterns of baseline prescriptions; the proportions of people receiving antihypertensive and lipid-lowering drugs were much higher in those with T2DM than in those without T2DM. People with both diagnoses were also more likely to receive other medications, including antidepressants, antipsychotics and antidiabetes medications.
People with T2DM, on average, had a higher baseline BMI and poorer glycaemic and blood pressure controls. The average cholesterol level, however, was lower in people with T2DM than in people without T2DM. In general, there were large numbers of missing data for lifestyle factors and biometric measures in people without T2DM, and the patterns of missing values for these variables differed between the two groups. Therefore, these comparisons should be interpreted with caution.
The patterns for the health outcomes of diabetes and cardiovascular control are shown in Figures 5 and 6. These figures show a common trend: that diabetes and cardiovascular control measures improved from 2000/1 through to 2015/16 for both people with and people without T2DM. The gap between these two groups, however, widened for average cholesterol and HbA1c levels.
FIGURE 5.
Objective 3: average cholesterol and HbA1c, 2000/1–2015/16 for people with SMI with T2DM and for people with SMI without T2DM. (a) Average cholesterol (mmol/l) and 95% CIs; and (b) average HbA1c (%) and 95% CIs.
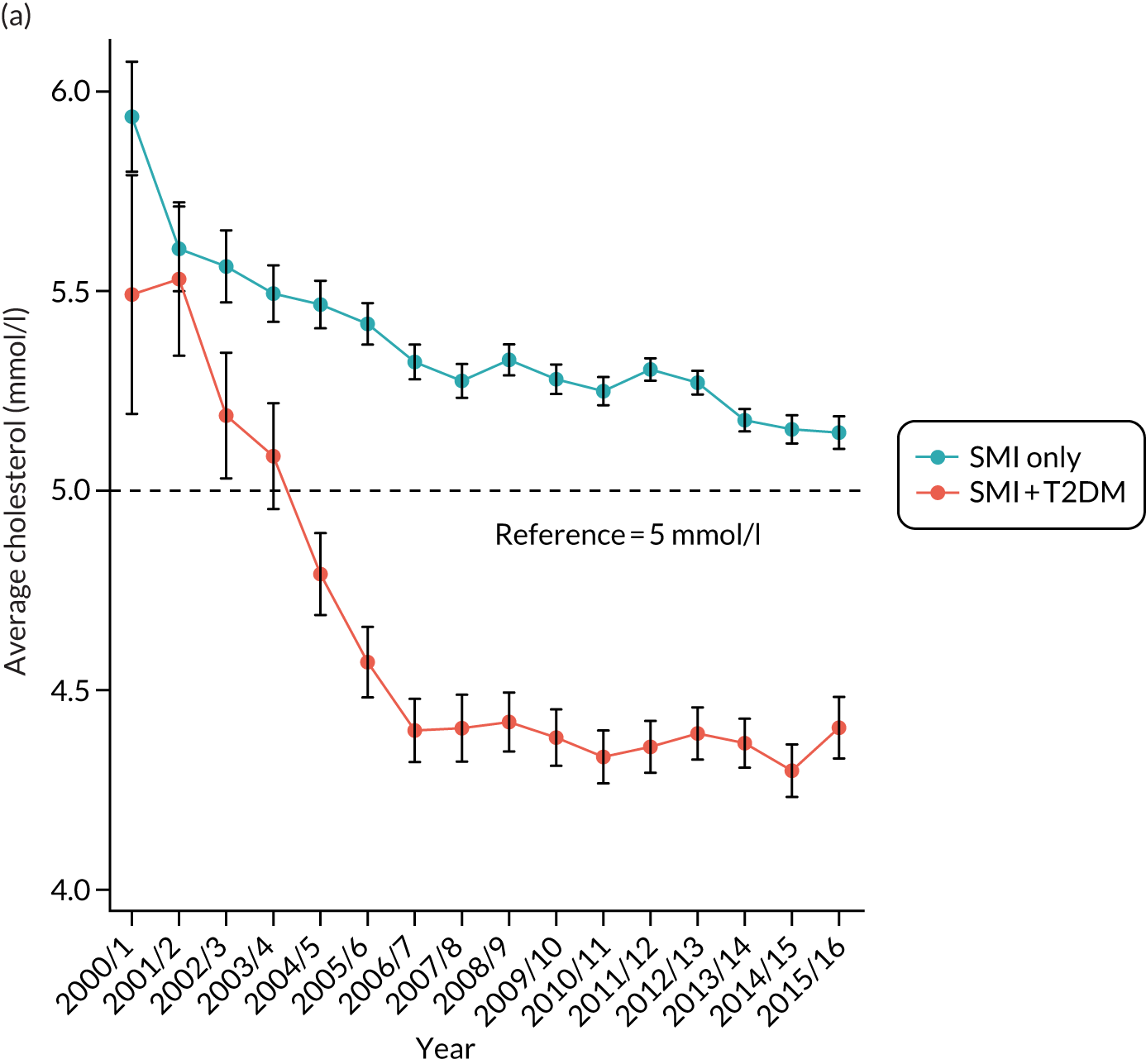
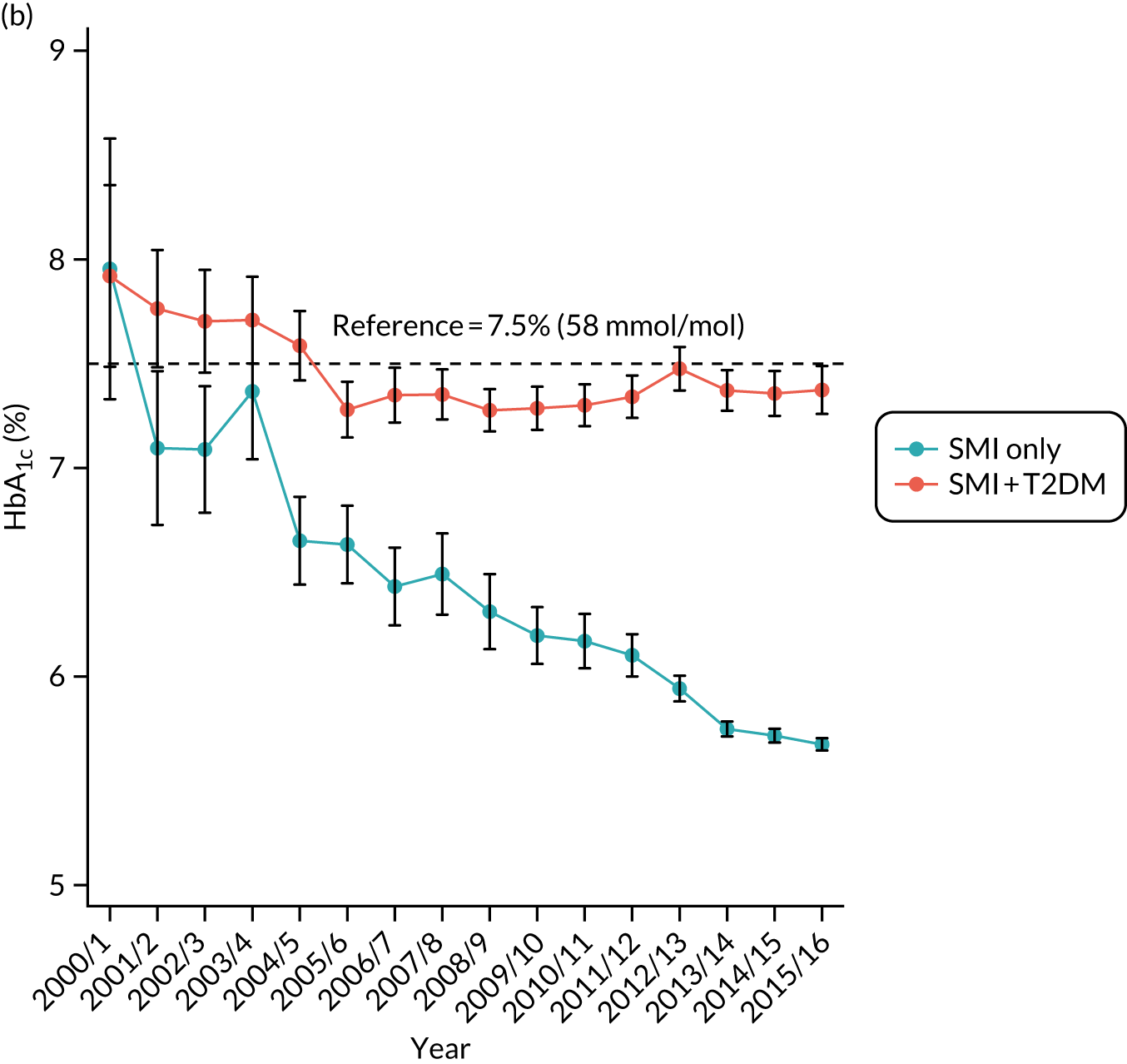
FIGURE 6.
Objective 3: average blood pressure (mmHg) and 95% CI, 2000/1–2015/16 for people with SMI with T2DM and for people with SMI without T2DM. (a) Systolic blood pressure and 95% CIs; and (b) diastolic blood pressure and 95% CIs.
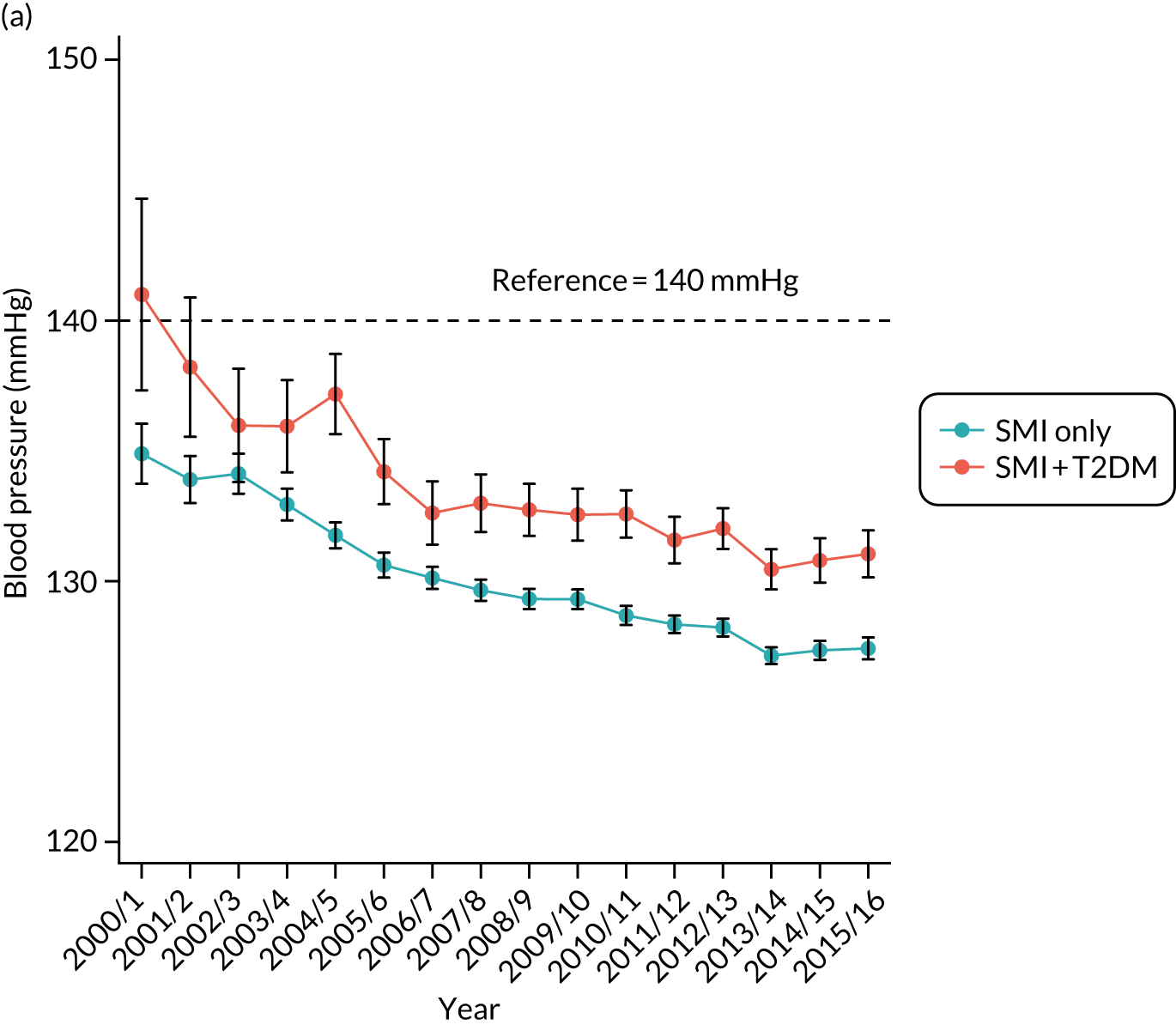
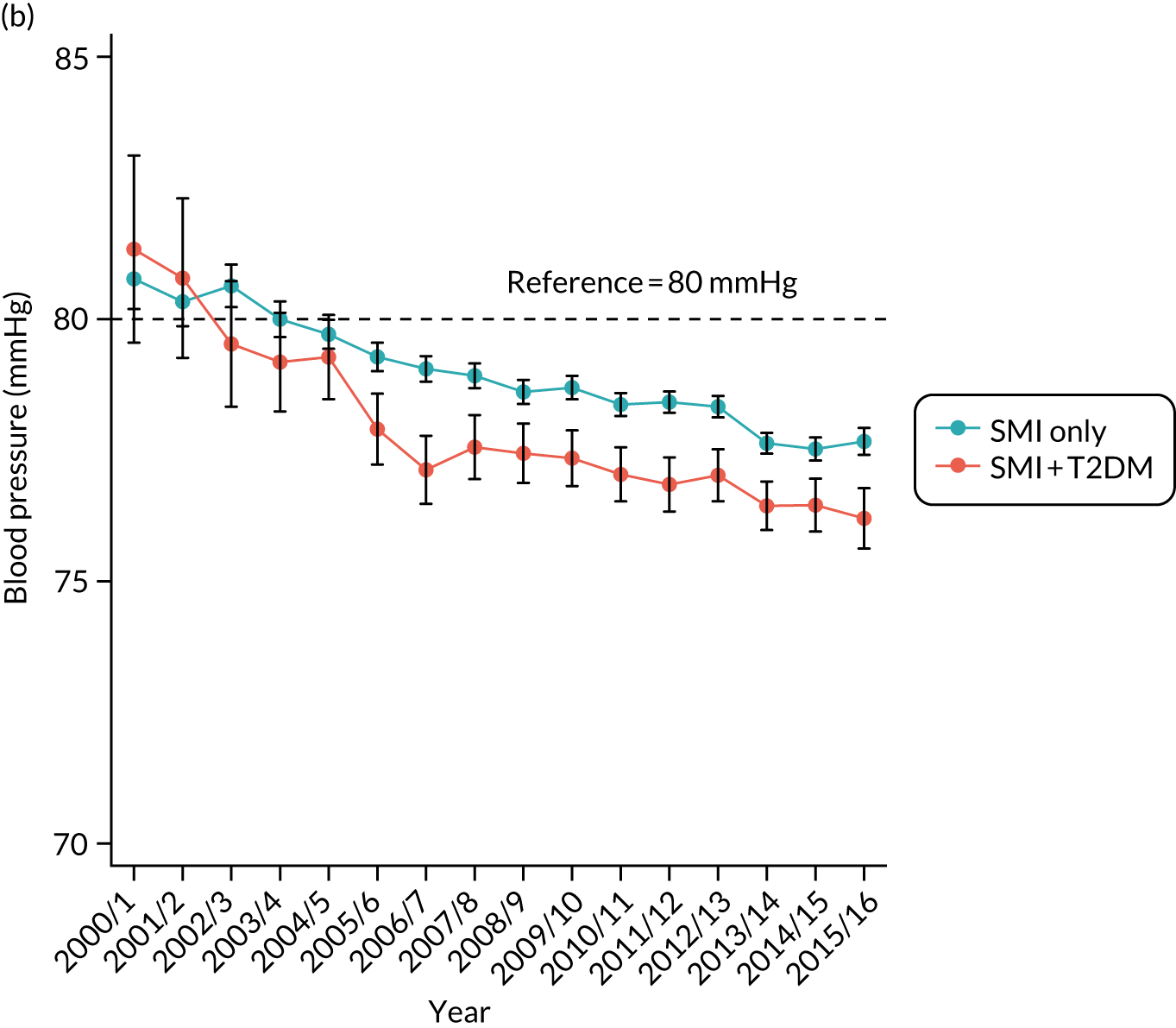
People with T2DM who had a lower cholesterol level at baseline continued to have better cholesterol control in the follow-up period than people without T2DM. The average level dropped below 5 mmol/l, the NICE-recommended threshold, after 2003/4 for people with T2DM, whereas the average level for people without T2DM remained above this threshold in this period.
The average level of HbA1c for people with T2DM was consistently higher than the average level for people without T2DM. There was a decreasing trend in people without T2DM, a pattern that might suggest that an increased number of T2DM patients had been identified among people with SMI through regular physical health checks, as incentivised under QOF.
For blood pressure, the gap between people with and people without T2DM remained constant over time. Systolic blood pressure was higher in people with T2DM throughout this period, a pattern consistent with the baseline measures of these two groups. Although diastolic blood pressure was higher in people with T2DM at baseline, results suggested better control for these patients during follow-up.
Descriptive statistics for the other health outcomes and physical health checks are summarised in Table 12. There was a general pattern that people with T2DM had higher crude risks for poorer physical and mental health outcomes, and received more frequent physical health checks, than people without T2DM.
| Descriptive statistic | People with SMI | ||
|---|---|---|---|
| Total | Without T2DM | With T2DM | |
| Diabetes complications: primary care diagnosis, n (%) | |||
| Hyperglycaemia | 147 (0.5) | 23 (0.1) | 124 (4.5) |
| Hypoglycaemia | 138 (0.5) | 18 (0.1) | 120 (4.4) |
| Macrovascular complications (combined) | 942 (3.5) | 717 (3.0) | 225 (8.2) |
| Myocardial infarction | 378 (1.4) | 292 (1.2) | 86 (3.1) |
| Peripheral vascular disease | 192 (0.7) | 123 (0.5) | 69 (2.5) |
| Stroke | 414 (1.5) | 323 (1.3) | 91 (3.3) |
| Microvascular complications (combined) | |||
| Baseline | 332 (1.2) | 122 (0.5) | 210 (7.6) |
| Follow-up | 690 (2.6) | 210 (0.9) | 480 (17.4) |
| Nephropathy | |||
| Baseline | 53 (0.2) | 31 (0.1) | 22 (0.8) |
| Follow-up | 171 (0.6) | 103 (0.4) | 68 (2.5) |
| Neuropathy | |||
| Baseline | 149 (0.6) | 82 (0.3) | 67 (2.4) |
| Follow-up | 192 (0.7) | 105 (0.4) | 87 (3.2) |
| Retinopathy | |||
| Baseline | 158 (0.6) | 11 (0.1) | 147 (5.3) |
| Follow-up | 398 (1.5) | 4 (0.0) | 394 (14.3) |
| Diabetes complications: admissions, mean (SD) | |||
| Macrovascular complications (combined) | 0.07 (0.40) | 0.05 (0.34) | 0.18 (0.76) |
| Ischaemic heart disease | 0.04 (0.35) | 0.03 (0.28) | 0.12 (0.68) |
| Peripheral vascular disease | 0.00 (0.05) | 0 (0) | 0.01 (0.17) |
| Cerebrovascular disease | 0.02 (0.18) | 0.02 (0.18) | 0.04 (0.24) |
| Mental health outcomes, n (%) | |||
| SMI relapses | 10,665 (39.5) | 9458 (39.1) | 1207 (43.8) |
| All-cause mortality, n (%) | 3038 (11.3) | 2580 (10.7) | 458 (16.6) |
| Number of health checks per year, mean (SD) | |||
| Glucose | 0.50 (0.81) | 0.44 (0.72) | 1.04 (1.27) |
| Cholesterol | 0.46 (0.65) | 0.38 (0.60) | 1.10 (0.74) |
| Blood pressure | 1.26 (2.85) | 1.14 (2.91) | 2.37 (2.02) |
| BMI | 0.77 (2.52) | 0.69 (2.60) | 1.53 (1.41) |
Regression analysis results for the impact of type 2 diabetes on health outcomes and health-care interventions
The impact of the exposure variable, the status of T2DM, on health outcomes and health-care interventions is summarised in Table 13 using results from the core models. The diagnosis of T2DM was associated with increased risk of poorer physical and mental health outcomes, as well as increased levels of physical health checks. After adjusting for patients’ sociodemographic characteristics, baseline comorbidities and medication use, people with T2DM had an increased risk of macrovascular complications compared with people without T2DM; clinical events recorded in primary care increased by 36% (OR 1.36, 95% CI 1.11 to 1.67) and hospital admissions increased by 62% (IRR 1.62, 95% CI 1.31 to 2.01). The risk of microvascular complications was more than 22 times higher (HR 22.23, 95% CI 17.79 to 27.77) in people with T2DM than in people without T2DM; the increased risk associated with T2DM was 24% for all-cause mortality (HR 1.24, 95% CI 1.12 to 1.38) and 10% for SMI relapses (OR 1.10, 95% CI 1.00 to 1.20).
| Health outcome | T2DM | ||
|---|---|---|---|
| Coefficient | 95% CI | p-value | |
| Physical health outcomes | |||
| Macrovascular complications: primary care diagnosis | OR 1.361 | 1.108 to 1.671 | 0.003 |
| Macrovascular complications: hospital admissions | IRR 1.624 | 1.314 to 2.007 | < 0.001 |
| Microvascular complications: primary care diagnosis | HR 22.229 | 17.792 to 27.772 | < 0.001 |
| All-cause mortality | HR 1.243 | 1.122 to 1.378 | < 0.001 |
| Mental health outcomes | |||
| SMI relapses | OR 1.096 | 1.002 to 1.199 | 0.045 |
| Health checks | |||
| Glucose | IRR 2.032 | 1.909 to 2.163 | < 0.001 |
| Cholesterol | IRR 2.284 | 2.195 to 2.376 | < 0.001 |
| Blood pressure | IRR 1.624 | 1.566 to 1.684 | < 0.001 |
| BMI | IRR 2.033 | 1.926 to 2.146 | < 0.001 |
The results also suggested that levels of physical health checks were associated with T2DM status. Compared with people without T2DM, people with T2DM received increased health checks by 103% for glucose (IRR 2.03, 95% CI 1.91 to 2.16), by 128% for cholesterol (IRR 2.28, 95% CI 2.20 to 2.38), by 62% for blood pressure (IRR 1.62%, 95% CI 1.57 to 1.68) and by 103% for BMI (IRR 2.03, 95% CI 1.93 to 2.15).
For full results of the core models, see Report Supplementary Material 5 and 6, and results of the extended models in Report Supplementary Material 7 and 8. Of the candidate explanatory variables, we found that older age was a significant predictor for poorer physical health outcomes and increased physical health checks, but was associated with a lower risk of SMI relapses. Female patients had reduced risk of poor physical health outcomes and increased risk of SMI relapse. They were also more likely to receive health checks (except for cholesterol tests) than males. People of Asian ethnicity had a reduced mortality rate and increased health checks. There was also a gradient in the impact of socioeconomic deprivation on health outcomes; people who lived in more deprived neighbourhoods had an elevated risk of macrovascular complications and mortality.
The core models also showed that physical comorbidities were generally associated with increased levels of physical health checks and poorer physical health outcomes. For instance, people with cardiovascular disease at baseline had an increased risk of both macro- and microvascular complications, and all-cause mortality. They also received more frequent health checks for diabetes and cardiovascular controls. Cardiovascular disease showed no impact on the risk of mental health outcomes. Dementia was associated with reduced risk of SMI relapse, as well as with receiving fewer physical health checks.
Of the medications we examined, antidepressants were associated with increased risk of macrovascular complications and a lower risk of SMI relapse. Typical antipsychotics were identified as a risk factor for SMI relapse and mortality. Furthermore, there was a general tendency towards physical health checks increasing with baseline medication uptake.
The results of the extended models were consistent with the results of the core models. Using additional information, we found that patients with a higher than normal BMI were more likely to receive physical health checks. Current smokers had a higher risk of macrovascular complications and SMI relapse. There was also evidence suggesting that poor diabetes and cardiovascular controls at baseline were not only risk factors of poorer health outcomes, but also predictors of more frequent physical health checks.
Summary of findings
A key finding is that, after adjustment, having diabetes increases the probability of receiving physical health checks and is a significant risk factor for both poorer physical and mental health outcomes.
The physical and mental health outcomes examined responded differently to variations in patient characteristics. For instance, advancing age was associated with increased risk of poorer physical health outcomes, but with reduced risk of mental health outcomes (SMI relapses); female sex was associated with a lower risk of diabetes complications and mortality, but a higher risk of SMI relapses. Type of SMI was a significant predictor for poorer mental health status, but had less influence on physical health outcomes, and antidepressant uptake reduced the risk of SMI relapses, but increased the risk of macrovascular complications.
The analyses also showed that receipt of the four physical health checks under investigation was affected by a wide range of factors, including older age, female sex, ethnicity, type of SMI, the presence of comorbidities and the use of various medications.
Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness
Objective
The objective was to compare health-care interventions and physical and mental health outcomes in people with diabetes and SMI with those of people with diabetes without SMI.
Study population
There was a cohort of 12,999 patients in the CPRD data who had a clinical diagnosis of diabetes. This cohort included 3448 patients in data set B who had been identified as ‘cases’ for having both SMI and T2DM. A total of 9551 patients in data set C (see Figure 2) had been identified as matched ‘controls’ based on age, sex and general practice. For this objective, we used data sets B and C so that patients with both SMI and T2DM (cases) could be compared with non-SMI patients with T2DM (controls) of the same age band, sex and general practice.
Patients with T2DM were included if (1) they satisfied the four common inclusion criteria regarding diagnosis date and age, practice registration and type of diabetes; (2) they had a follow-up length of at least 1 day, in which the start of follow-up was identified as the later date of T2DM diagnosis or the start of UTS data plus 15 months, and the end of follow-up was defined as the earlier of the end of UTS data or the end of study period date; and (3) they were nested within a matched case–control cluster. A total of 9965 patients remained in the final sample for analyses.
Variables and statistical methods
The exposure variable was SMI status. The candidate explanatory variables are listed in Table 3, with detailed descriptions. The outcome variables under examination were as described in Objective 3: comparing health-care interventions and health outcomes in people with severe mental illness and diabetes with those in people with severe mental illness without diabetes, Variables and statistical methods.
The description and analysis methods for physical and mental health outcomes are provided in Table 4. The outcome, mental health status, was identified by the markers of depression and anxiety only, because we were unable to include SMI relapse owing to its perfect correlation with SMI status. The four physical health checks were chosen using the QOF indicators for people with diabetes. We therefore examined the level of health checks for HbA1c, cholesterol, blood pressure and BMI.
The patient-level observations for this objective were nested within matched case–control clusters, an additional data hierarchy to the data used in previous objectives. We adopted statistical methods that examine variations within case–control clusters, ‘within’ estimators, to compare cases only with controls of the same age, sex and registered with the same practice. For further details on the methodology, see Report Supplementary Material 3.
Results
Descriptive statistics
The comparison of baseline characteristics between cases and controls, and by diagnosis order, are summarised in Table 14. In our sample of 9965 patients, 2192 (22%) cases were matched to 7773 (78%) controls. Just under 90% of cases had been matched to at least three controls. Cases had similar distribution in age and sex to controls. However, they differed for a range of other characteristics, and by diagnosis orders.
| Characteristic | SMI before T2DM | SMI after T2DM (including same day) | ||
|---|---|---|---|---|
| Cases (T2DM + SMI) | Controls (T2DM) | Cases (T2DM + SMI) | Controls (T2DM) | |
| Patients (n) | 1666 | 5883 | 526 | 1890 |
| Number of controls, n (%) | ||||
| 4 controls | 1200 (72.0) | 399 (75.9) | ||
| 3 controls | 255 (15.3) | 68 (12.9) | ||
| 2 controls | 107 (6.4) | 31 (5.9) | ||
| 1 control | 104 (6.2) | 28 (5.3) | ||
| Diagnosis age (years), mean (SD) | ||||
| SMI | 43.02 (15.09) | 63.70 (14.69) | ||
| T2DM | 56.36 (12.86) | 56.84 (12.47) | 58.22 (14.11) | 62.12 (13.36) |
| SMI type, n (%) | ||||
| Schizophrenia | 913 (54.8) | 248 (47.2) | ||
| Schizoaffective disorder | 96 (5.8) | 17 (3.2) | ||
| Bipolar disorder | 528 (31.7) | 173 (32.9) | ||
| Depression and psychosis | 110 (6.6) | 74 (14.1) | ||
| Other affective disorder | 15 (0.9) | 11 (2.1) | ||
| Mixed | 4 (0.2) | 3 (0.6) | ||
| Age at follow-up start (years), mean (SD) | 56.86 (12.81) | 57.55 (12.32) | 60.25 (13.73) | 63.08 (13.10) |
| Duration of T2DM (years), mean (SD) | 0.52 (2.13) | 0.74 (2.66) | 2.09 (4.65) | 0.99 (3.19) |
| Follow-up duration (years), mean (SD) | 5.39 (4.22) | 6.14 (4.39) | 8.02 (4.57) | 6.53 (4.52) |
| Family history of diabetes, n (%) | 245 (14.7) | 1123 (19.1) | 79 (15.0) | 319 (16.9) |
| Sex, n (%) | ||||
| Male | 822 (49.3) | 2861 (48.6) | 229 (43.5) | 846 (44.8) |
| Female | 844 (50.7) | 3022 (51.4) | 297 (56.5) | 1044 (55.2) |
| Ethnicity, n (%) | ||||
| White | 1376 (82.6) | 4657 (79.2) | 450 (85.6) | 1612 (85.3) |
| Asian | 108 (6.5) | 413 (7.0) | 31 (5.9) | 86 (4.6) |
| Black | 86 (5.2) | 213 (3.6) | 20 (3.8) | 44 (2.3) |
| Mixed | 16 (1.0) | 39 (0.7) | 5 (1.0) | 9 (0.5) |
| Other | 35 (2.1) | 118 (2.0) | 6 (1.1) | 29 (1.5) |
| Not stated/unknown | 45 (2.7) | 443 (7.5) | 14 (2.7) | 110 (5.8) |
| Deprivation (IMD 2010), n (%) | ||||
| 1st quintile (least deprived) | 195 (11.7) | 900 (15.3) | 84 (16.0) | 311 (16.5) |
| 2nd quintile | 271 (16.3) | 1101 (18.7) | 87 (16.5) | 401 (21.2) |
| 3rd quintile | 310 (18.6) | 1217 (20.7) | 105 (20.0) | 352 (18.6) |
| 4th quintile | 423 (25.4) | 1340 (22.8) | 119 (22.6) | 405 (21.4) |
| 5th quintile (most deprived) | 466 (28.0) | 1319 (22.4) | 129 (24.5) | 420 (22.2) |
| Missing | 1 (0.1) | 6 (0.1) | 2 (0.4) | 1 (0.1) |
| Comorbidities | ||||
| Cardiovascular disease, n (%) | 193 (11.6) | 922 (15.7) | 92 (17.5) | 384 (20.3) |
| Hypertension, n (%) | 521 (31.3) | 2655 (45.1) | 213 (40.5) | 929 (49.2) |
| Dementia, n (%) | 25 (1.5) | 19 (0.3) | 7 (1.3) | 13 (0.7) |
| Learning disability, n (%) | 18 (1.1) | 17 (0.3) | 1 (0.2) | 4 (0.2) |
| Charlson Index score, mean (SD) | 0.48 (0.71) | 0.51 (0.77) | 0.55 (0.78) | 0.62 (0.85) |
| Medications, n (%) | ||||
| Antidepressants | 817 (49.0) | 1156 (19.7) | 245 (46.6) | 367 (19.4) |
| Antipsychotics | ||||
| Typical | 361 (21.7) | 63 (1.1) | 73 (13.9) | 27 (1.4) |
| Atypical | 870 (52.2) | 41 (0.7) | 87 (16.5) | 11 (0.6) |
| Antidiabetics | 353 (21.2) | 1218 (20.7) | 168 (31.9) | 425 (22.5) |
| Antihypertensive | 723 (43.4) | 3200 (54.4) | 277 (52.7) | 1149 (60.8) |
| Lipid-lowering drugs | 517 (31.0) | 1981 (33.7) | 167 (31.8) | 696 (36.8) |
| Statins | 501 (30.1) | 1921 (32.7) | 164 (31.2) | 679 (35.9) |
| Lifestyle factors, n (%) | ||||
| Smoking | ||||
| Non-smoker | 410 (24.6) | 1691 (28.7) | 134 (25.5) | 540 (28.6) |
| Ex-smoker | 299 (18.0) | 1248 (21.2) | 91 (17.3) | 411 (21.8) |
| Current smoker | 550 (33.0) | 924 (15.7) | 100 (19.0) | 299 (15.8) |
| Missing | 407 (24.4) | 2020 (34.3) | 201 (38.2) | 640 (33.9) |
| Drinking | ||||
| Non-drinker | 325 (19.5) | 565 (9.6) | 64 (12.2) | 197 (10.4) |
| Ex-drinker | 112 (6.7) | 111 (1.9) | 16 (3.0) | 30 (1.6) |
| Current drinker | 519 (31.2) | 1549 (26.3) | 137 (26.1) | 511 (27.0) |
| Missing | 710 (42.6) | 3658 (62.2) | 309 (58.8) | 1152 (61.0) |
| Substance use | 31 (1.9) | 28 (0.5) | 8 (1.5) | 7 (0.4) |
| Biometric measures | ||||
| BMI (kg/m2), mean (SD) | 33.28 (7.08) | 32.77 (6.92) | 31.89 (6.57) | 31.91 (6.95) |
| < 20, n (%) | 8 (0.5) | 35 (0.6) | 4 (0.8) | 15 (0.8) |
| 20–24, n (%) | 102 (6.1) | 344 (5.9) | 43 (8.2) | 128 (6.8) |
| 25–29, n (%) | 303 (18.2) | 983 (16.7) | 98 (18.6) | 368 (19.5) |
| 30–40, n (%) | 582 (34.9) | 1770 (30.1) | 151 (28.7) | 501 (26.5) |
| > 40, n (%) | 181 (10.9) | 511 (8.7) | 40 (7.6) | 139 (7.4) |
| Missing, n (%) | 490 (29.4) | 2240 (38.1) | 190 (36.1) | 739 (39.1) |
| HbA1c (%),a mean (SD) | 7.85 (2.03) | 7.92 (1.96) | 7.71 (1.84) | 7.82 (1.89) |
| ≤ 7.5, n (%) | 506 (30.4) | 1758 (29.9) | 166 (31.6) | 568 (30.1) |
| > 7.5, n (%) | 350 (21.0) | 1335 (22.7) | 110 (20.9) | 393 (20.8) |
| Missing, n (%) | 810 (48.6) | 2790 (47.4) | 250 (47.5) | 929 (49.2) |
| Cholesterol (mmol/l), mean (SD) | 5.39 (1.42) | 5.30 (1.29) | 5.24 (1.42) | 5.22 (1.25) |
| ≤ 5, n (%) | 563 (33.8) | 2071 (35.2) | 159 (30.2) | 684 (36.2) |
| > 5, n (%) | 703 (42.2) | 2457 (41.8) | 189 (35.9) | 756 (40.0) |
| Missing, n (%) | 400 (24.0) | 1355 (23.0) | 178 (33.8) | 450 (23.8) |
| Diastolic blood pressure (mmHg), mean (SD) | 81.58 (10.59) | 82.20 (10.70) | 81.07 (11.16) | 81.36 (10.71) |
| ≤ 80, n (%) | 761 (45.7) | 2484 (42.2) | 250 (47.5) | 896 (47.4) |
| > 80, n (%) | 690 (41.4) | 2453 (41.7) | 184 (35.0) | 742 (39.3) |
| Missing, n (%) | 215 (12.9) | 946 (16.1) | 92 (17.5) | 252 (13.3) |
| Systolic blood pressure (mmHg), mean (SD) | 134.79 (17.48) | 139.59 (18.11) | 139.27 (19.91) | 141.75 (18.41) |
| ≤ 140, n (%) | 1026 (61.6) | 2973 (50.5) | 254 (48.3) | 901 (47.7) |
| > 140, n (%) | 425 (25.5) | 1964 (33.4) | 180 (34.2) | 737 (39.0) |
| Missing, n (%) | 215 (12.9) | 946 (16.1) | 92 (17.5) | 252 (13.3) |
In general, people with SMI were more likely to come from the most deprived neighbourhoods than people without SMI. This socioeconomic disadvantage was further increased if SMI was diagnosed before T2DM. We observed a similar pattern in the baseline prevalence of dementia and learning disability, which were more common in people with SMI than in people without SMI; people with SMI diagnosed before T2DM were most likely to be affected.
Recorded physical health status, including cardiovascular disease, hypertension and Charlson Index diseases, showed opposite patterns. People with SMI were less likely to have a recorded diagnosis of these physical comorbidities than people without SMI; prevalence of these diseases was lower in people with SMI diagnosed before T2DM than in people who had T2DM first. As expected, larger proportions of people with SMI had been prescribed antidepressants and antipsychotics than people without SMI. The prescription rates of antihypertensive and lipid-lowering drugs were lower in people with SMI than in controls, a pattern consistent with the lower prevalence of recorded hypertension and cardiovascular disease in people with SMI.
Factors including BMI, smoking status and alcohol use were better recorded for people who had SMI before T2DM than for the rest of the sample. Although results should be interpreted with caution, we found that people with SMI had a higher BMI and were more likely to have substance misuse at baseline. Furthermore, people with SMI tended to have better diabetes and blood pressure controls, but a higher cholesterol level, at baseline than people without SMI.
The health outcomes of diabetes and cardiovascular control were compared between people with and people without SMI (see Figures 7 and 8), and were analysed separately for people diagnosed with SMI first and diagnosed with T2DM first. These analyses showed a common decreasing trend in the average levels of cholesterol and blood pressure during the study period. The mean values of HbA1c remained stable and around the 7.5% (58 mmol/mol) threshold for both groups. People with SMI had lower HbA1c and blood pressure levels than people without SMI, a pattern consistent with the comparison of baseline levels. The cholesterol levels of people with SMI, particularly those with SMI diagnosed before T2DM, remained above the cholesterol levels of controls.
FIGURE 7.
Objective 4: average cholesterol and HbA1c, 2000/1–2015/16, for people with T2DM and SMI and people with T2DM without SMI. Average cholesterol (mmol/l) and 95% CIs for (a) SMI diagnosed before T2DM; (b) SMI diagnosed after T2DM (including same day); average HbA1c (%) and 95% CIs for (c) SMI diagnosed before T2DM; and (d) SMI diagnosed after T2DM (including same day).

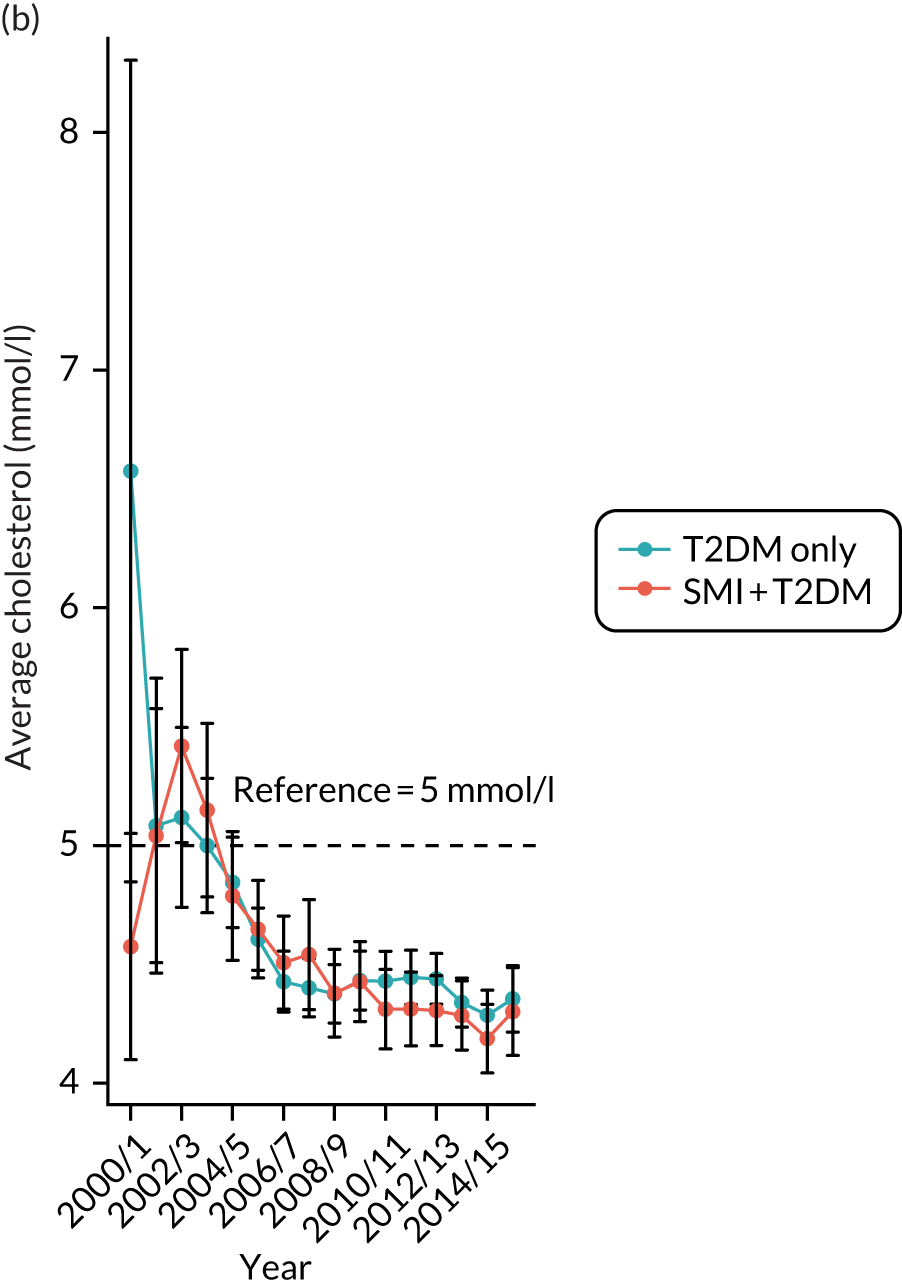
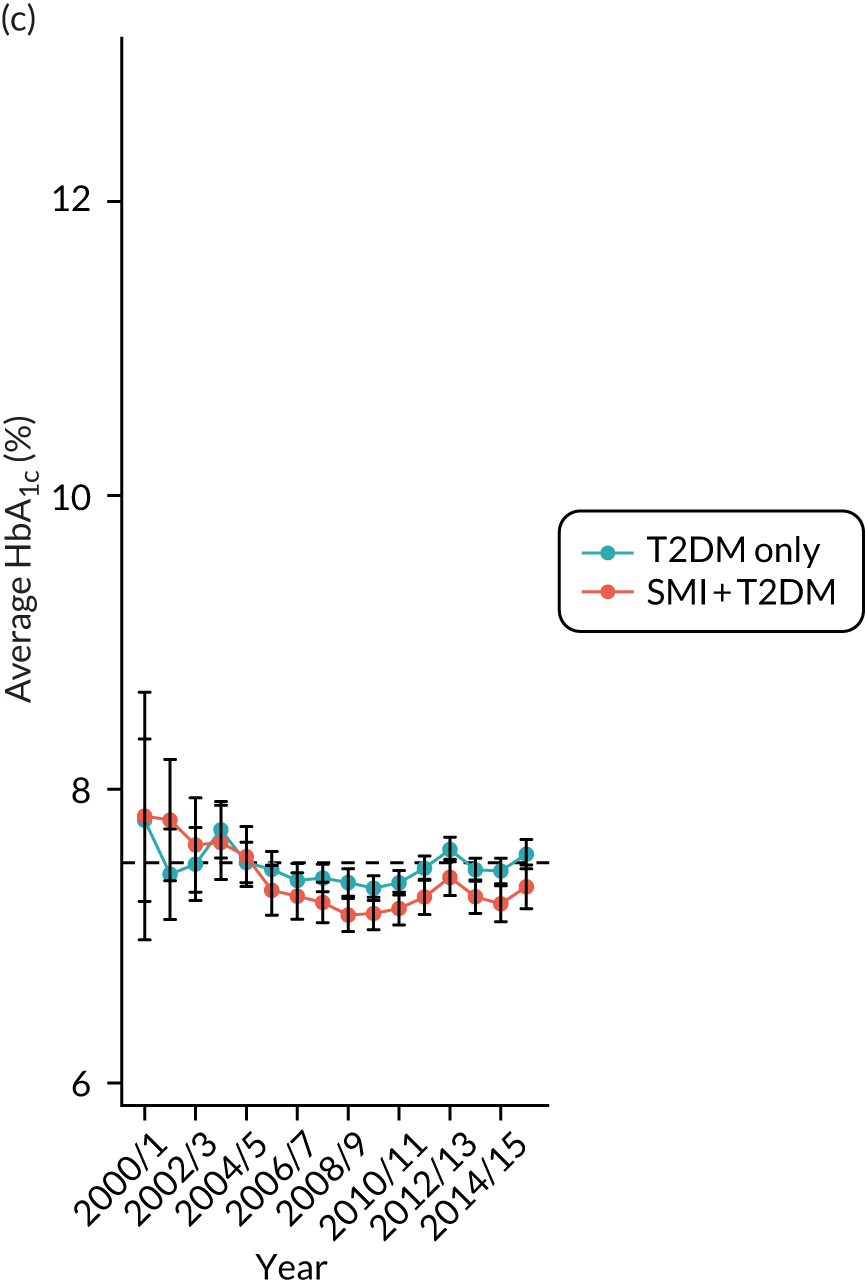
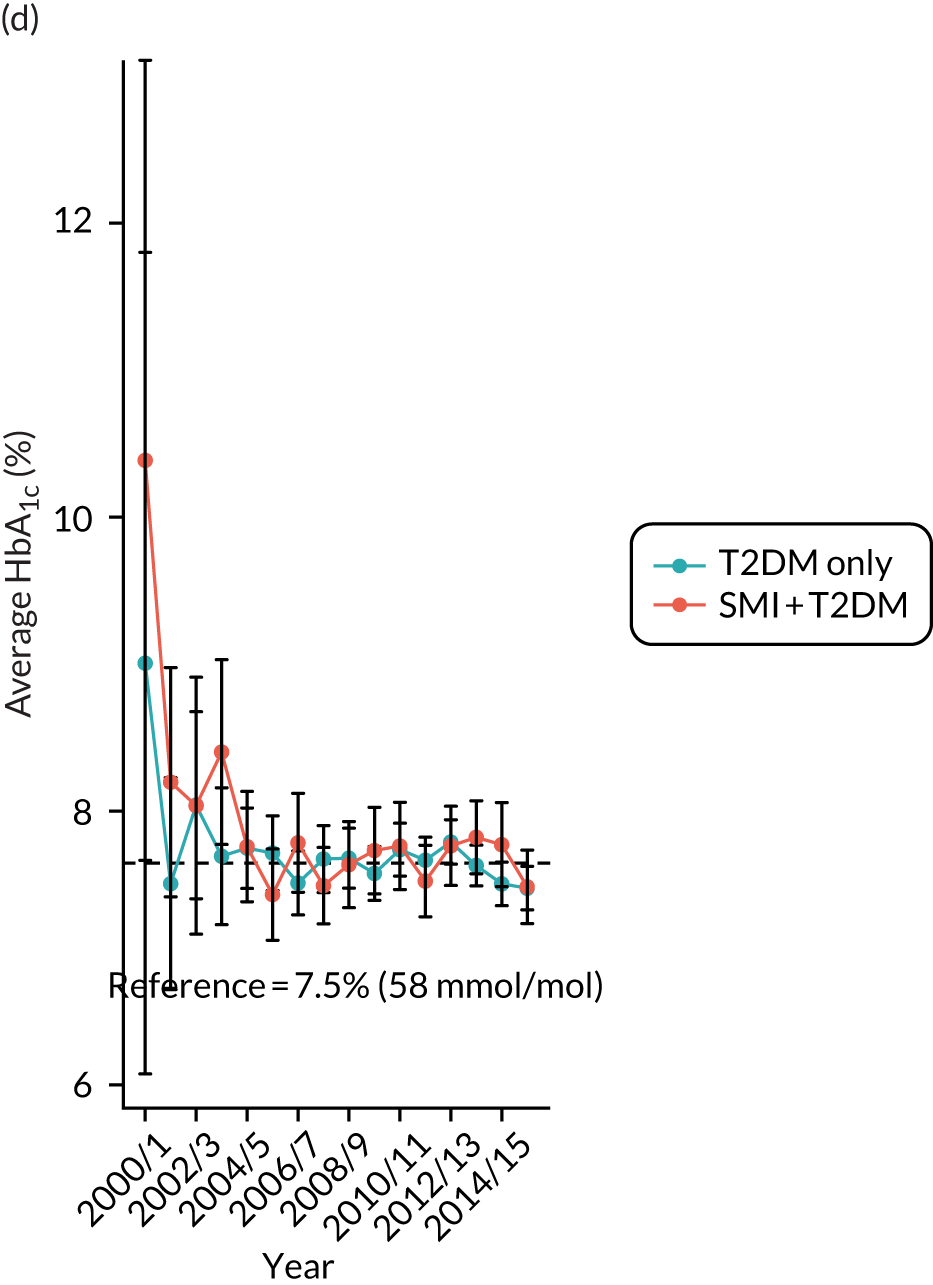
FIGURE 8.
Objective 4: average blood pressure, 2000/1–2015/16, for people with T2DM and SMI and people with T2DM without SMI. Systolic blood pressure (mmHg) and 95% CIs for (a) SMI diagnosed before T2DM; (b) SMI diagnosed after T2DM (including same day); diastolic blood pressure (mmHg) and 95% CIs for (c) SMI diagnosed before T2DM; and (d) SMI diagnosed after T2DM (including same day).
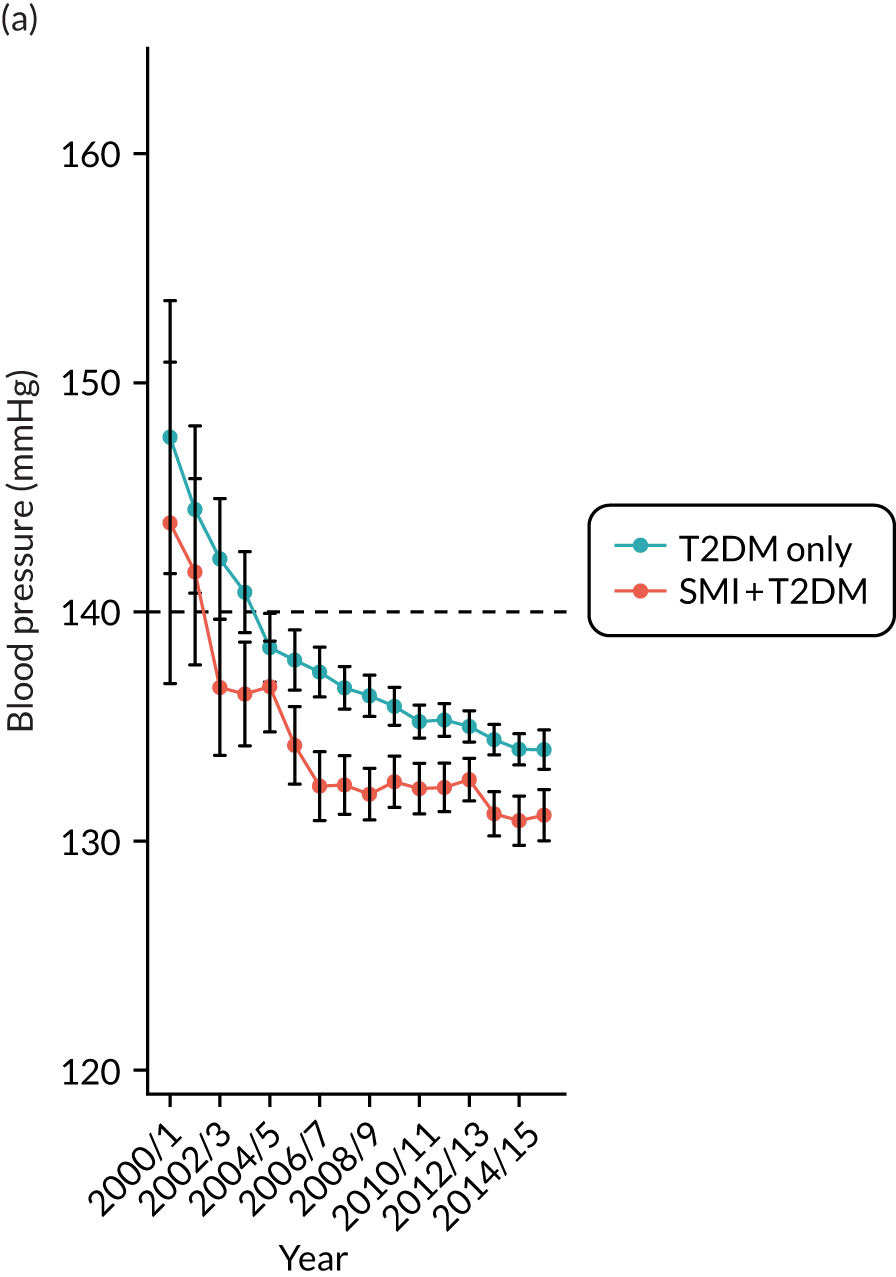
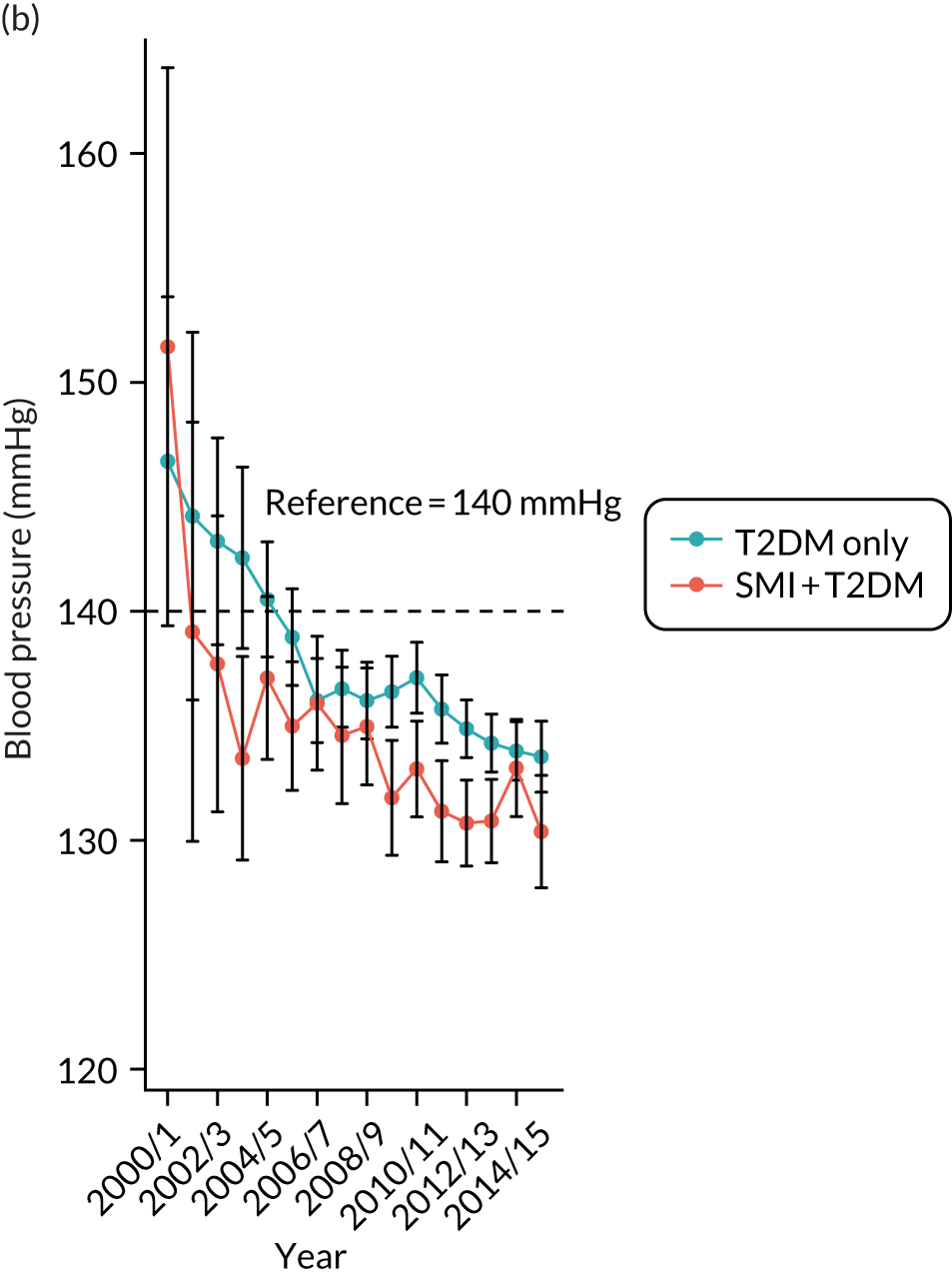
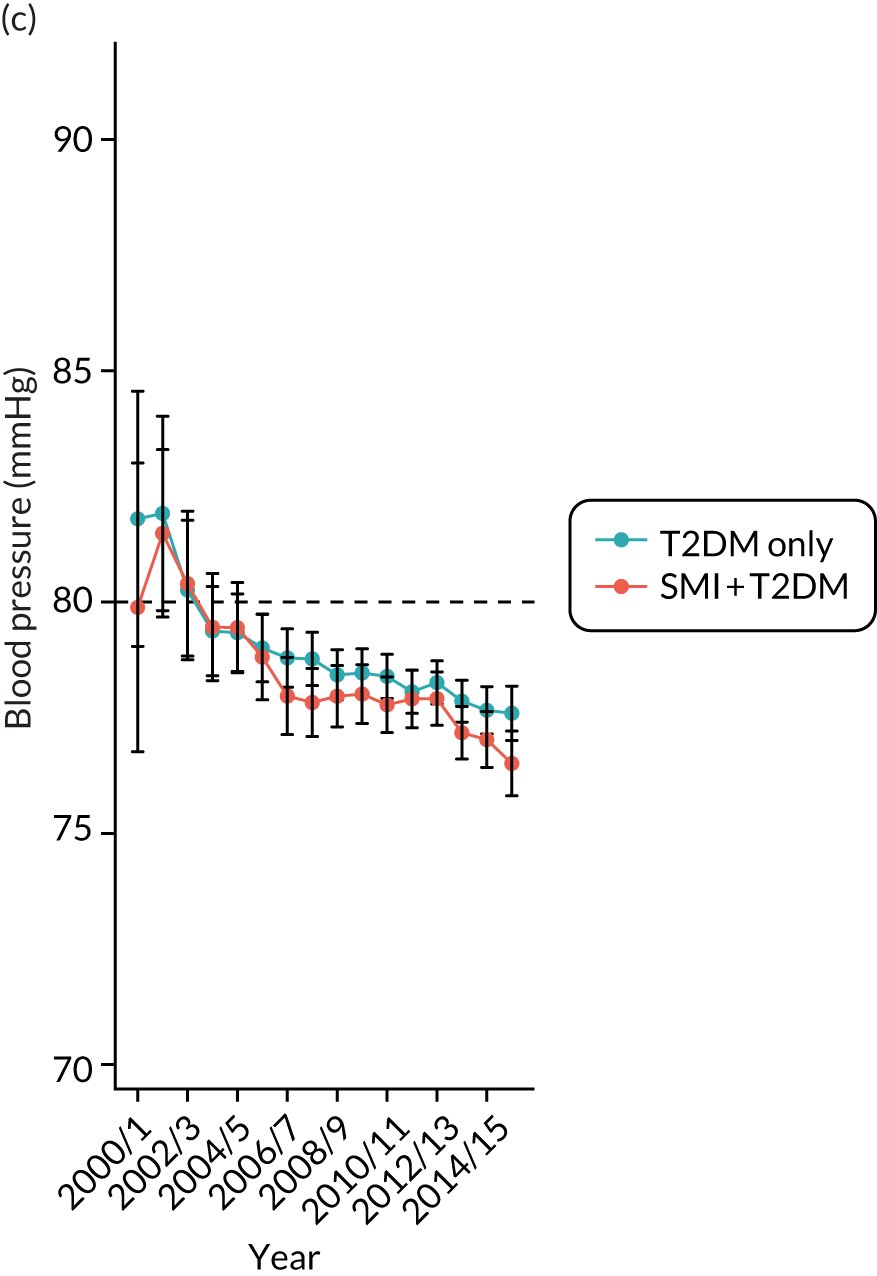

Descriptive statistics for the other outcome variables are provided in Table 15 and show that the pattern of diabetes complications differed between people with SMI and people without SMI, and that complications were also affected by diagnosis order. In general, the crude risks of both recorded macro- and microvascular complications were lower in people with SMI than in people without SMI, if SMI was diagnosed before T2DM. These crude risks were higher in people with SMI than in controls if T2DM was diagnosed first. Depression and anxiety were also more likely to be diagnosed in people with SMI than in people without SMI, and the crude mortality rate was higher in the SMI population. For the four physical health checks, we found that people with SMI tended to have more frequent checks than controls if SMI was diagnosed first, and less frequent checks if T2DM was diagnosed first.
| Descriptive statistic | SMI diagnosed before T2DM | SMI diagnosed after T2DM (including same day) | ||
|---|---|---|---|---|
| Cases (T2DM + SMI) | Controls (T2DM) | Cases (T2DM + SMI) | Controls (T2DM) | |
| Patients (n) | 1666 | 5883 | 526 | 1890 |
| Diabetes complications: primary care diagnosis, n (%) | ||||
| Hyperglycaemia | 52 (3.1) | 155 (2.6) | 31 (5.9) | 49 (2.6) |
| Hypoglycaemia | 60 (3.6) | 253 (4.3) | 41 (7.8) | 85 (4.5) |
| Macrovascular complications (combined) | 102 (6.1) | 481 (8.2) | 82 (15.6) | 203 (10.7) |
| Myocardial infarction | 37 (2.2) | 196 (3.3) | 33 (6.3) | 78 (4.1) |
| Peripheral vascular disease | 36 (2.2) | 183 (3.1) | 22 (4.2) | 64 (3.4) |
| Stroke | 38 (2.3) | 142 (2.4) | 34 (6.5) | 79 (4.2) |
| Microvascular complications (combined) | ||||
| Baseline | 45 (2.7) | 253 (4.3) | 39 (7.4) | 69 (3.7) |
| Follow-up | 310 (18.6) | 1416 (24.1) | 141 (26.8) | 514 (27.2) |
| Nephropathy | ||||
| Baseline | 6 (0.4) | 32 (0.5) | 4 (0.8) | 8 (0.4) |
| Follow-up | 35 (2.1) | 121 (2.1) | 24 (4.6) | 55 (2.9) |
| Neuropathy | ||||
| Baseline | 17 (1.0) | 88 (1.5) | 13 (2.5) | 24 (1.3) |
| Follow-up | 54 (3.2) | 251 (4.3) | 35 (6.7) | 103 (5.5) |
| Retinopathy | ||||
| Baseline | 25 (1.5) | 161 (2.7) | 28 (5.3) | 42 (2.2) |
| Follow-up | 257 (15.4) | 1227 (20.9) | 112 (21.3) | 433 (22.9) |
| Diabetes complications: admissions, mean (SD) | ||||
| Macrovascular complications (combined) | 0.13 (0.54) | 0.20 (1.12) | 0.32 (0.99) | 0.23 (0.74) |
| Ischaemic heart disease | 0.09 (0.45) | 0.15 (1.07) | 0.23 (0.90) | 0.17 (0.66) |
| Peripheral vascular disease | 0.01 (0.20) | 0.01 (0.16) | 0.02 (0.17) | 0.01 (0.11) |
| Cerebrovascular disease | 0.03 (0.19) | 0.03 (0.24) | 0.08 (0.33) | 0.05 (0.26) |
| Emergency | 0.09 (0.41) | 0.10 (0.45) | 0.21 (0.71) | 0.14 (0.52) |
| Elective | 0.03 (0.21) | 0.08 (0.93) | 0.07 (0.35) | 0.07 (0.37) |
| Mental health outcomes, n (%) | ||||
| Depression and anxiety | 343 (20.6) | 993 (16.9) | 232 (44.1) | 297 (15.7) |
| All-cause mortality, n (%) | 248 (14.9) | 672 (11.4) | 116 (22.1) | 348 (18.4) |
| Number of health checks per year, mean (SD) | ||||
| HbA1c | 1.94 (1.35) | 1.90 (1.07) | 1.73 (0.70) | 1.94 (1.08) |
| Cholesterol | 1.40 (1.07) | 1.34 (0.79) | 1.31 (0.58) | 1.38 (0.91) |
| Blood pressure | 2.94 (2.70) | 2.96 (2.21) | 2.92 (1.69) | 3.25 (8.60) |
| BMI | 2.16 (2.11) | 1.91 (1.74) | 1.80 (1.30) | 2.07 (8.57) |
Regression results for the impact of severe mental illness status on physical and mental health outcomes, mortality and health checks
The adjusted impact of SMI status on outcome variables based on our core models is summarised in Table 16. After adjustment, SMI status was not associated with macrovascular complications recorded in primary care data. However, we found a complex association with hospital admissions: having SMI was associated with an increased risk of emergency admissions for macrovascular complications (IRR 1.14, 95% CI 0.96 to 1.36) and with a reduced risk, by 36%, of elective admissions for macrovascular complications (IRR 0.64, 95% CI 0.47 to 0.88). Compared with controls, people with SMI were more likely to be diagnosed with depression and anxiety (OR 1.86%, 95% CI 1.63 to 2.12) and had an almost 90% increased risk of mortality (HR 1.89, 95% CI 1.59 to 2.26).
| Health outcome | SMI | ||
|---|---|---|---|
| Coefficient | 95% CI | p-value | |
| Physical health outcomes | |||
| Macrovascular complications: primary care diagnosis | OR 0.999 | 0.824 to 1.210 | 0.988 |
| Macrovascular complications: emergency admissions | IRR 1.141 | 0.956 to 1.362 | 0.143 |
| Macrovascular complications: elective admissions | IRR 0.644 | 0.470 to 0.881 | 0.006 |
| All-cause mortality | HR 1.893 | 1.589 to 2.256 | < 0.001 |
| Mental health outcomes | |||
| Depression and anxiety | OR 1.858 | 1.629 to 2.119 | < 0.001 |
| Health checks | |||
| HbA1c | IRR 0.997 | 0.978 to 1.016 | 0.762 |
| Cholesterol | IRR 1.054 | 1.033 to 1.075 | < 0.001 |
| Blood pressure | IRR 1.032 | 1.008 to 1.057 | 0.009 |
| BMI | IRR 1.085 | 1.058 to 1.113 | < 0.001 |
For physical health checks, people with SMI were more likely to receive tests for cholesterol (IRR 1.05, 95% CI 1.03 to 1.08), blood pressure (IRR 1.03, 95% CI 1.01 to 1.06) and BMI (IRR 1.09, 95% CI 1.06 to 1.11) than people without SMI. There was no difference between cases and controls in the chance of receiving a HbA1c test.
As suggested by the descriptive statistics, people who had SMI diagnosed before T2DM differed in many aspects from people with T2DM diagnosed first. We therefore included interactions in the regressions to investigate whether or not the impact of SMI could be modified by this diagnosis order. The impact of SMI was not significantly modified by diagnosis order for hospital admissions for macrovascular complications or physical health checks. For macrovascular complications recorded in primary care data, however, the risk of this outcome was reduced for people with SMI if SMI was diagnosed before T2DM, and increased with the presence of SMI if T2DM was diagnosed first. The risk of depression and anxiety was higher in people with SMI than in people without SMI; this risk increased by around 41% for people diagnosed with SMI first and by 245% for people diagnosed with T2DM first (results available on request).
For full results of the core models, see Report Supplementary Materials 9 and 10. These suggest that older age is associated with higher rates of emergency admissions and physical health checks. Socioeconomic disadvantage is associated with higher risk of depression and mortality and reduced chances of receiving regular health checks. The presence of physical comorbidities, particularly cardiovascular disease and high blood pressure, was found to be a risk factor for poorer physical health outcomes. The number of Charlson Index diseases was associated with increased risks of mortality and depression. People who had dementia were less likely to be identified for physical health checks, whereas people on lipid-lowering medications were more likely to receive checks for cholesterol, HbA1c and BMI. The results of the extended models confirmed these findings (see Report Supplementary Material 11 and 12). Furthermore, there is evidence suggesting that current smoker status and poor diabetes and blood pressure controls might be associated with poorer physical health outcomes. Obesity and abnormal diabetes and cardiovascular controls might increase levels of physical health checks.
Summary of findings
For this objective, we investigated the impact of SMI status on physical and mental health outcomes, as well as the utilisation of health-care interventions. We also explored whether or not health inequalities among people with SMI were associated with the order of diagnosis of SMI and diabetes.
People with SMI had tighter diabetes and blood pressure controls and better chances of receiving physical health checks than people without SMI. Despite this, for health outcomes, we found significant associations between SMI and increased risk for all-cause mortality and depression.
The results also suggested that elective admissions for macrovascular complications were significantly lower for people with SMI, whereas the rate of emergency admissions was elevated for this group (although the difference was not statistically significant). Further investigations indicated that most of these elective admissions had chronic ischaemic heart disease as the main diagnosis, and most emergency patients had been admitted for angina. These admission patterns might suggest a service gap; people with SMI are less likely to be referred by their GPs to specialist care for their cardiovascular disease and, when their symptoms deteriorate, they are more likely to be admitted as emergencies than people without SMI.
The analyses of the primary care data also supported this suggestion. Although the rate for recorded macrovascular complications was similar in people with and people without SMI, people with SMI were less likely to have a primary care record of chronic heart conditions, such as angina and chronic heart disease. This under-reporting/diagnosis pattern for people with SMI, which has been mentioned in the literature,124 could help explain the inequalities in specialist and hospital care experienced by people with SMI.
Objective 6: comparing diabetes care provision and estimating health-care costs for people with and people without severe mental illness
Objective
The objective was to provide a comparison of, and cost estimation for, diabetes health-care provision for people with and people without SMI.
Study population
We used the patient sample identified for objective 4 (see Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Study population) to compare health-care use and associated costs between people with SMI and T2DM (cases) and their matched controls (non-SMI, T2DM). These patients were included for the analyses of this objective if they (1) had a length of follow-up of at least 1 year and (2) belonged to a case–control cluster. The start and end of follow-up were identified using the same method as for objective 4.
Variables and statistical methods
The exposure variable in this objective was the status of SMI. The candidate explanatory variables are summarised in Table 3.
The outcome variables were health-care use and the associated costs, including:
-
primary care service use (consultations, prescriptions and diagnostic tests)
-
hospital admissions for physical conditions (number of admissions and length of stay)
-
hospital admissions for mental health problems
-
associated costs for this service use.
The data construction and analysis methods are provided in Table 4. Mental health-related hospital admissions were identified using Healthcare Resource Group (HRG) codes. 125 Primary care service use and hospital admissions were costed using a bottom-up approach. A&E attendance and the use of community mental health services were not included in the analyses, as linkages to these data sources were not available in the CPRD data set. For an overview of all sources of health-care utilisation data and unit costs (both primary and secondary care), see Report Supplementary Material 13. Health-care use and associated costs were aggregated on an annual basis.
For the consultation costs, we followed the approach adopted in Ride et al. 126 and multiplied the duration of consultations by the costs of 1 minute of staff time, which were extracted from Unit Costs of Health and Social Care 2018. 127 Multiple visits to the same staff on the same day were considered as duplicates, whereas visits to different staff in a day were counted as different visits. For details on the categorising of consultations, see Report Supplementary Material 14.
The costs of prescriptions were calculated as the number of prescriptions multiplied by unit costs in 2018, as extracted from the prescription cost analysis for 2018. 128 Prescription records were aggregated at British National Formulary subparagraph level; higher hierarchy levels (paragraph, section or chapter) levels were used if subparagraph codes were unavailable.
For diagnostic tests, we followed Ride et al. 126 and grouped the test records into the categories as set out in the NHS reference costs. Costs were then estimated using the levels of tests and the unit costs extracted from the NHS Reference Costs 2017/18. 129 For details of this approach, see Report Supplementary Material 15.
The use and cost of hospital care were calculated for admissions to general hospitals only (including non-specialist mental health providers), but not admission to specialist mental health facilities. Hospital activities, such as diagnoses and procedures, were firstly grouped into HRGs using the costing grouper that corresponds to the NHS Reference Costs 2017/18. The generated HRG codes were then used to link the inpatient episodes to the national average costs from NHS Reference Costs 2017/18. 129 The calculated costs were therefore expressed in 2018 prices. To compare people with SMI with people without SMI, we separated hospital admissions and associated costs by mental health- and physical health-related admissions using HRG codes.
We conducted generalised linear regression models to explore the impact of SMI status and other explanatory variables on health-care resource use and health-care costs, and to take into account the non-negative, highly skewed and leptokurtic characteristics of cost data. The choice of distributional family and link function were informed by the Park test130 and the Pregibon link test. 131
Results
Descriptive statistics
A total of 6383 patients were included in the analysis sample with 1,023,257 primary care and 22,253 hospital admission records. Table 17 shows the distribution of baseline characteristics in the sample. The mean age of the sample was 57.9 years, 48.3% were male, the majority (82.5%) were white, the most common physical comorbidity was hypertension (55%), 26.6% were on antidepressants and 39.8% were current or ex-smokers. Cases had distribution in baseline characteristics that was similar to that of controls, such as age, sex, ethnicity and deprivation. However, people with SMI were more likely to be prescribed medications, such as antidepressants, antipsychotics and antidiabetics. They were also more likely to have lifestyles including smoking and substance use, although this finding is limited by the quality of data recording and number of missing values.
| Characteristic | Total | Cases (T2DM with SMI) | Control (T2DM without SMI) |
|---|---|---|---|
| People, N (%) | 6383 (100) | 1620 (100) | 4763 (100) |
| Number of controls, n (%) | |||
| 1 | 158 (9.8) | ||
| 2 | 332 (20.5) | ||
| 3 | 579 (35.7) | ||
| 4 | 551 (34.0) | ||
| Age at T2DM diagnosis (years), mean (SD) | |||
| T2DM | 57.9 (12.6) | 57.4 (12.9) | 58.0 (12.5) |
| SMI | 47.8 (17.2) | ||
| Sex, n (%) | |||
| Male | 3080 (48.3) | 780 (48.1) | 2300 (48.3) |
| Female | 3303 (51.7) | 840 (51.9) | 2463 (51.7) |
| SMI diagnosis, n (%) | |||
| Schizophrenia | 850 (52.5) | ||
| Schizoaffective disorder | 83 (5.1) | ||
| Bipolar disorder | 524 (32.4) | ||
| Depression and psychosis | 140 (8.6) | ||
| Other affective disorder | 18 (1.1) | ||
| Mixed | 5 (0.3) | ||
| Ethnicity, n (%) | |||
| White | 5264 (82.5) | 1375 (84.9) | 3889 (81.6) |
| Non-white | 726 (11.4) | 203 (12.5) | 523 (11.0) |
| Unknown | 393 (6.2) | 42 (2.6) | 351 (7.4) |
| Deprivation (IMD 2010), n (%) | |||
| 1st quintile (least deprived) | 972 (15.2) | 217 (13.4) | 755 (15.8) |
| 2nd quintile | 1210 (19.0) | 275 (17.0) | 935 (19.6) |
| 3rd quintile | 1215 (19.0) | 281 (17.3) | 934 (19.6) |
| 4th quintile | 1475 (23.1) | 401 (24.7) | 1074 (22.6) |
| 5th quintile (most deprived) | 1505 (23.6) | 445 (27.5) | 1060 (22.3) |
| Missing | 6 (0.1) | 1 (0.1) | 5 (0.1) |
| Comorbidities | |||
| Cardiovascular disease,a n (%) | 2141 (33.5) | 510 (31.5) | 1631 (34.2) |
| Hypertension,a n (%) | 3513 (55.0) | 777 (48.0) | 2736 (57.4) |
| Dementia, n (%) | 27 (0.4) | 20 (1.2) | 7 (0.2) |
| Learning disability, n (%) | 29 (0.5) | 16 (1.0) | 13 (0.3) |
| Charlson Index diseases, mean (SD) | 0.51 (0.75) | 0.49 (0.73) | 0.51 (0.76) |
| Medications, n (%) | |||
| Antidepressants | 1696 (26.6) | 792 (48.9) | 904 (19.0) |
| Antipsychotics | |||
| Typical | 360 (5.6) | 307 (19.0) | 53 (1.1) |
| Atypical | 760 (11.9) | 733 (45.3) | 27 (0.6) |
| Antidiabetics | 893 (14.0) | 251 (15.5) | 642 (13.5) |
| Lifestyle, n (%) | |||
| Smoking | |||
| Non-smoker | 1764 (27.6) | 406 (25.0) | 1358 (28.5) |
| Ex-smoker | 1308 (20.5) | 285 (17.6) | 1023 (21.5) |
| Current smoker | 1234 (19.3) | 479 (29.6) | 755 (15.8) |
| Missing | 2077 (32.6) | 450 (27.8) | 1627 (34.2) |
| Drinking | |||
| Non-drinker | 673 (10.6) | 258 (15.9) | 415 (8.7) |
| Ex-drinker | 175 (2.7) | 95 (5.9) | 80 (1.7) |
| Current drinker | 1724 (27.0) | 484 (29.9) | 1240 (26.0) |
| Missing | 3811 (59.7) | 783 (48.3) | 3028 (63.6) |
| Substance use | 41 (0.6) | 24 (1.5) | 17 (0.4) |
| Biometric measures | |||
| BMI (kg/m2), mean (SD) | 33.0 (6.8) | 33.3 (7.1) | 32.9 (6.7) |
| < 20 (underweight), n (%) | 32 (0.5) | 7 (0.4) | 25 (0.5) |
| 20–24 (normal), n (%) | 328 (5.1) | 98 (6.0) | 230 (4.8) |
| 25–29 (overweight), n (%) | 1065 (16.7) | 286 (17.7) | 779 (16.4) |
| 30–39 (obese), n (%) | 1931 (30.3) | 532 (32.8) | 1399 (29.4) |
| ≥ 40 (severely obese) | 575 (9.0) | 171 (10.6) | 404 (8.5) |
| Missing, n (%) | 2452 (38.4) | 526 (32.5) | 1926 (40.4) |
| Family history of diabetes, n (%) | 1092 (17.1) | 222 (13.7) | 870 (18.3) |
| Average follow-up time (years)b | |||
| Mean (SD) | 7.0 (4.0) | 6.9 (4.0) | 7.1 (4.0) |
| Median (minimum, maximum) | 6.5 (1, 18) | 6.4 (1, 18) | 6.6 (1, 17.9) |
| Financial year at T2DM diagnosis, n (%) | |||
| 1998–9 | 51 (0.8) | 17 (1.1) | 34 (0.7) |
| 1999–2000 | 80 (1.3) | 19 (1.2) | 61 (1.3) |
| 2000–1 | 229 (3.6) | 55 (3.4) | 174 (3.7) |
| 2001–2 | 268 (4.2) | 70 (4.3) | 198 (4.2) |
| 2002–3 | 417 (6.5) | 97 (6.0) | 320 (6.7) |
| 2003–4 | 470 (7.4) | 118 (7.3) | 352 (7.4) |
| 2004–5 | 461 (7.2) | 119 (7.4) | 342 (7.2) |
| 2005–6 | 464 (7.3) | 127 (7.8) | 337 (7.1) |
| 2006–7 | 603 (9.4) | 149 (9.2) | 454 (9.5) |
| 2007–8 | 463 (7.3) | 139 (8.6) | 324 (6.8) |
| 2008–9 | 464 (7.3) | 115 (7.1) | 349 (7.3) |
| 2009–10 | 496 (7.8) | 106 (6.5) | 390 (8.2) |
| 2010–11 | 477 (7.5) | 119 (7.4) | 358 (7.5) |
| 2011–12 | 444 (7.0) | 117 (7.2) | 327 (6.9) |
| 2012–13 | 429 (6.7) | 113 (7.0) | 316 (6.6) |
| 2013–14 | 336 (5.3) | 84 (5.2) | 252 (5.3) |
| 2014–15 | 231 (3.6) | 56 (3.5) | 175 (3.7) |
Primary care costs results
Table 18 shows annual resource use and costs for people with diabetes and SMI (cases) and people with diabetes alone (controls). The crude results demonstrate that, on average, people with diabetes and SMI used significantly more primary care services every year than people with diabetes alone. The main driver of this difference was the number of consultations (12.140 and 8.657 contacts per year for cases and controls, respectively); drug prescription and test-related contacts were similar between the two groups.
| Resource use and cost | Crude | Adjusted | p-value | ||||
|---|---|---|---|---|---|---|---|
| Total | Cases (T2DM + SMI) | Control (T2DM only) | Total | Cases (T2DM + SMI) | Control (T2DM only) | ||
| N (people) | 6383 | 1620 | 4763 | 6383 | 1620 | 4763 | |
| Resource use, mean (SD) | |||||||
| Primary care contacts | 16.27 (10.64) | 20.07 (12.27) | 14.98 (9.69) | 16.75 (6.50) | 20.94 (7.76) | 15.33 (5.31) | < 0.0001 |
| Consultation only | 9.54 (7.12) | 12.14 (8.36) | 8.66 (6.41) | 9.81 (3.75) | 12.68 (4.39) | 8.84 (2.91) | < 0.0001 |
| Medicine/prescription related | 5.74 (4.63) | 6.88 (5.73) | 5.35 (4.12) | 5.92 (2.62) | 7.18 (3.20) | 5.49 (2.23) | 0.245 |
| Test related | 1.25 (1.48) | 1.31 (1.49) | 1.23 (1.48) | 1.29 (0.51) | 1.35 (0.52) | 1.27 (0.50) | 0.491 |
| Inpatient stays | |||||||
| Annual number of admissionsa | 0.61 (1.72) | 0.75 (2.00) | 0.56 (1.61) | 0.64 (0.58) | 0.87 (0.71) | 0.57 (0.51) | 0.001 |
| Mental health relatedb | 0.20 (0.14) | 0.07 (0.25) | 0.003 (0.05) | 0.04 (0.13) | 0.14 (0.22) | 0.0004 (0.01) | < 0.0001 |
| Non-mental health related | 0.59 (1.71) | 0.68 (1.96) | 0.56 (1.61) | 0.62 (0.40) | 0.74 (0.44) | 0.58 (0.38) | 0.018 |
| Annual number of inpatient days | 5.11 (24.63) | 11.60 (44.96) | 2.91 (10.33) | 6.46 (16.00) | 16.98 (28.01) | 2.89 (5.08) | < 0.0001 |
| Mental health related | 0.96 (9.50) | 3.36 (16.88) | 0.134 (4.63) | 2.34 (10.46) | 8.93 (19.30) | 0.10 (0.34) | < 0.0001 |
| Non-mental health related | 4.16 (22.41) | 8.24 (41.39) | 2.77 (9.10) | 4.72 (6.46) | 10.66 (9.60) | 2.70 (2.90) | < 0.0001 |
| Cost (£), mean (SD) | |||||||
| Total | 2618.7 (7214.8) | 4059.0 (12,230.8) | 2128.8 (4237.5) | 2708.6 (2705.2) | 4472.5 (3767.4) | 2108.6 (1887.8) | < 0.0001 |
| Primary care contacts | 618.0 (614.2) | 804.0 (785.5) | 554.8 (529.2) | 637.0 (331.1) | 849.2 (411.1) | 564.8 (263.1) | < 0.0001 |
| Inpatient stays | 2000.6 (7100.0) | 3255.0 (12,180.6) | 1574.0 (4049.7) | 2170.6 (3033.8) | 3883.3 (4543.6) | 1588.0 (1944.8) | < 0.0001 |
| Mental health related | 156.2 (1672.0) | 510.5 (2770.9) | 35.7 (1038.7) | 342.5 (1474.8) | 1271.0 (2719.9) | 26.7 (82.5) | < 0.0001 |
| Non-mental health related | 1844.4 (6834.3) | 2744.6 (11,770.5) | 1538.3 (3889.1) | 1982.4 (2561.6) | 3154.4 (3577.8) | 1583.8 (1953.5) | < 0.0001 |
Hospital costs results
A similar trend was found for hospitalisation. People with diabetes and SMI had more hospital admissions every year and stayed in hospital longer than those with diabetes only (controls). This was regardless of whether or not the admissions were mental health related. As expected, people with SMI had more mental health-related admissions and stayed in hospital longer following those admissions. However, we also found that, for admissions related to physical health conditions, people with SMI and diabetes spent significantly more days in hospital per year (p < 0.0001) than people with diabetes alone. The difference in the number of admissions every year was small (0.679 and 0.556 admissions per year for cases and controls, respectively), but statistically significant (p = 0.018).
We also found that people with SMI incurred more costs than those without SMI. This difference was observed across both primary and secondary care. The main cost driver was secondary care and hospitalisation, which accounted for 83.3% and 72.5% of overall health-care expenditure for people with diabetes and SMI and for people with diabetes alone, respectively.
Regression analysis results for adjusted health-care resource utilisation and costs based on baseline characteristics
To take sample heterogeneity into account, we conducted a series of generalised linear regressions to adjust resource use and cost results by people’s baseline characteristics. These results can be found in the ‘adjusted’ section in Table 18. Baseline characteristics that were controlled for included age at diabetes diagnosis, sex, ethnicity, deprivation, comorbidities, medication use, time since diabetes diagnosis and financial year of diabetes diagnosis. Generalised linear regression with gamma distribution and log-link function was chosen, based on the Park and Pregibon tests, because of the non-normality of the data. After adjustment, the differences in resource use and costs between people with and people without SMI remained significant, except for differences in numbers of prescription-related and test-related consultations.
Figure 9 demonstrates the 10-year trend of annual health-care cost per person for people with diabetes and SMI and people with diabetes but no SMI. As shown, people with SMI incurred significantly higher annual health-care costs across this 10-year horizon. Cases and controls followed a similar trend: higher costs were incurred in the first year after diagnosis, followed by a cost reduction in the second year; thereafter, the annual costs slowly increased year on year. This trend was more prominent in people with SMI and diabetes than in those with diabetes alone.
FIGURE 9.
Objective 6: trend for annual health-care cost per person with SMI and T2DM (cases) and matched person with T2DM but no SMI (controls).
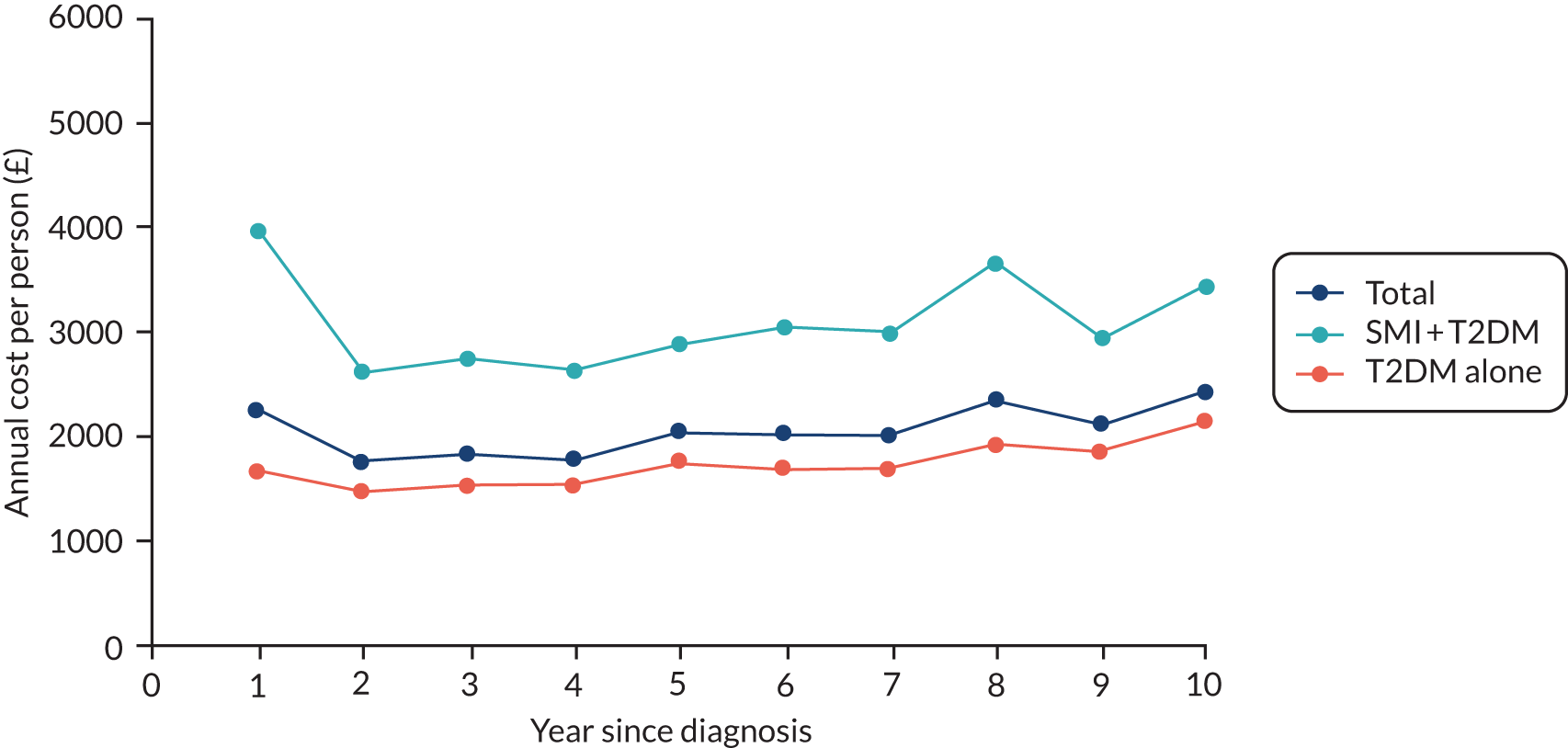
Table 19 shows the impact of SMI on total costs among people with diabetes. SMI increased the total costs by 82.6% (exp0.602 – 1), increased the primary care costs by 70.9% and increased secondary care costs by 110.9%.
| Adjusted risk factors | Total (n = 6383) | Primary care (n = 6383) | Secondary care (n = 6383) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | 95% CI | Coefficient | p-value | 95% CI | Coefficient | p-value | 95% CI | |
| Intercept | 7.848 | < 0.0001 | 7.450 to 8.247 | 6.281 | < 0.0001 | 6.090 to 6.474 | 7.619 | < 0.0001 | 7.004 to 8.234 |
| Age (years) at T2DM diagnosis | 0.013 | < 0.0001 | 0.010 to 0.017 | –0.001 | 0.357 | –0.003 to 0.001 | 0.020 | < 0.0001 | 0.015 to 0.026 |
| Sex | |||||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | 0.043 | 0.0325 | –0.042 to 0.127 | 0.178 | < 0.0001 | 0.137 to 0.219 | 0.027 | 0.678 | –0.102 to 0.156 |
| Case | |||||||||
| With SMI (case) | 0.602 | < 0.0001 | 0.502 to 0.702 | 0.253 | < 0.0001 | 0.204 to 0.301 | 0.746 | < 0.0001 | 0.594 to 0.899 |
| Without SMI (control) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Ethnicity | |||||||||
| White | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Non-white (Asian, black, other, mixed) | 0.151 | 0.031 | 0.014 to 0.289 | 0.062 | 0.064 | –0.004 to 0.128 | 0.180 | 0.094 | –0.031 to 0.390 |
| Unknown | < 0.0001 | –1.524 to –1.172 | –0.425 | < 0.0001 | –0.511 to –0.339 | –2.544 | < 0.0001 | –2.823 to –2.265 | |
| Deprivation (IMD 2010) | |||||||||
| 1st quintile (least deprived) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 2nd quintile | 0.175 | 0.017 | 0.032 to 0.319 | 0.107 | 0.003 | 0.037 to 0.177 | 0.135 | 0.228 | –0.084 to 0.354 |
| 3rd quintile | 0.143 | 0.050 | 0.000 to 0.286 | 0.164 | < 0.0001 | 0.095 to 0.234 | 0.105 | 0.344 | –0.113 to 0.324 |
| 4th quintile | 0.157 | 0.025 | 0.020 to 0.295 | 0.141 | < 0.0001 | 0.073 to 0.208 | 0.101 | 0.350 | –0.111 to 0.312 |
| 5th quintile (most deprived) | 0.136 | 0.054 | –0.002 to 0.275 | 0.155 | < 0.0001 | 0.088 to 0.223 | 0.059 | 0.589 | –0.155 to 0.273 |
| Missing | 0.233 | 0.736 | –1.121 to 1.588 | 0.462 | 0.170 | –0.199 to 1.123 | 0.009 | 0.993 | –2.052 to 2.070 |
| Comorbidities | |||||||||
| Hypertension | 0.126 | 0.007 | 0.034 to 0.218 | 0.126 | < 0.0001 | 0.082 to 0.170 | 0.132 | 0.066 | –0.008 to 0.272 |
| Charlson Index diseases, mean (SD) | 0.298 | < 0.0001 | 0.238 to 0.357 | 0.218 | < 0.0001 | 0.189 to 0.246 | 0.342 | < 0.0001 | 0.251 to 0.433 |
| Medications within 15-month window | |||||||||
| Antidepressants | 0.260 | < 0.0001 | 0.161 to 0.359 | 0.423 | < 0.0001 | 0.374 to 0.472 | 0.202 | 0.008 | 0.052 to 0.352 |
| Antidiabetes | 0.152 | 0.017 | 0.027 to 0.278 | 0.144 | < 0.0001 | 0.084 to 0.205 | 0.142 | 0.143 | –0.048 to 0.333 |
| T2DM duration | 0.062 | < 0.0001 | 0.045 to 0.079 | 0.158 | < 0.0001 | 0.149 to 0.166 | 0.024 | 0.074 | –0.002 to 0.050 |
| Financial year at T2DM diagnosis | |||||||||
| 1998–9 | 0.270 | 0.303 | –0.244 to 0.785 | –0.168 | 0.188 | –0.419 to 0.082 | 0.384 | 0.335 | –0.397 to 1.165 |
| 1999–2000 | 0.352 | 0.108 | –0.077 to 0.781 | 0.161 | 0.133 | –0.049 to 0.370 | 0.382 | 0.249 | –0.268 to 1.031 |
| 2000–1 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 2001–2 | 0.121 | 0.427 | –0.177 to 0.418 | 0.194 | 0.009 | 0.048 to 0.339 | 0.042 | 0.854 | –0.409 to 0.493 |
| 2002–3 | 0.094 | 0.499 | –0.179 to 0.368 | 0.124 | 0.067 | –0.009 to 0.257 | –0.004 | 0.984 | –0.421 to 0.412 |
| 2003–4 | 0.034 | 0.805 | –0.234 to 0.301 | 0.159 | 0.017 | 0.029 to 0.290 | –0.087 | 0.677 | –0.494 to 0.320 |
| 2004–5 | 0.019 | 0.891 | –0.252 to 0.289 | 0.215 | 0.001 | 0.084 to 0.347 | –0.071 | 0.736 | –0.483 to 0.341 |
| 2005–6 | –0.044 | 0.755 | –0.318 to 0.231 | 0.283 | < 0.0001 | 0.149 to 0.416 | –0.255 | 0.232 | –0.674 to 0.164 |
| 2006–7 | –0.150 | 0.262 | –0.413 to 0.112 | 0.300 | < 0.0001 | 0.172 to 0.427 | –0.401 | 0.051 | –0.804 to 0.002 |
| 2007–8 | –0.101 | 0.481 | –0.382 to 0.180 | 0.329 | < 0.0001 | 0.194 to 0.465 | –0.356 | 0.106 | –0.787 to 0.076 |
| 2008–9 | –0.349 | 0.015 | –0.631 to –0.068 | 0.316 | < 0.0001 | 0.179 to 0.453 | –0.646 | 0.003 | –1.075 to –0.217 |
| 2009–10 | –0.351 | 0.015 | –0.634 to –0.067 | 0.302 | < 0.0001 | 0.165 to 0.440 | –0.634 | 0.004 | –1.067 to –0.201 |
| 2010–11 | –0.442 | 0.003 | –0.734 to –0.150 | 0.250 | < 0.0001 | 0.110 to 0.391 | –0.807 | < 0.0001 | –1.257 to –0.357 |
| 2011–12 | –0.434 | 0.005 | –0.735 to –0.134 | 0.229 | 0.002 | 0.086 to 0.373 | –0.775 | 0.001 | –1.238 to –0.312 |
| 2012–13 | –0.928 | < 0.0001 | –1.231 to –0.625 | 0.163 | 0.029 | 0.016 to 0.310 | –1.460 | < 0.0001 | –1.927 to –0.993 |
| 2013–14 | –1.143 | < 0.0001 | –1.466 to –0.821 | –0.029 | 0.710 | –0.185 to 0.126 | –1.506 | < 0.0001 | –2.001 to –1.012 |
| 2014–15 | –1.503 | < 0.0001 | –1.854 to –1.151 | –0.180 | 0.037 | –0.350 to –0.011 | –2.152 | < 0.0001 | –2.693 to –1.610 |
Summary of findings
People with SMI and diabetes had higher costs than people with diabetes alone. We found that the main cost driver was secondary care, which accounted for 83.3% of overall health-care costs for those with diabetes and SMI and for 72.5% of overall health-care costs for people with diabetes alone. On average, people with diabetes and SMI use more primary care services every year than people with diabetes alone. The same trend applied to hospitalisation: people with diabetes and SMI tended to have more hospital admissions every year and to stay in hospital longer than those with diabetes only. This difference was regardless of whether or not the admissions were mental health related.
Objective 7: identifying health-care interventions associated with better outcomes for people with diabetes and severe mental illness
Objective
The objective was to identify which health-care interventions may be associated with better health outcomes for people with SMI and diabetes.
Study population
For objective 4, we combined data sets B and C (see Figure 2) and used comparisons between people with SMI (cases) and people without SMI (controls) to estimate the association between SMI status and the provision of physical health checks. For objective 7, we conducted further investigations to explore the association between SMI and health checks in the presence of macrovascular complications.
The development of macrovascular complications as a physical health outcome was defined as the presence of myocardial infarction, peripheral vascular disease or stroke during patients’ follow-up periods. Although under constant review, the QOF has been incentivising primary care providers to perform physical health checks as ongoing management for patients on the register for these conditions. For instance, regular checks of blood pressure and cholesterol levels were included as QOF indicators for patients with coronary heart disease (CHD) and stroke before 2011/12. These indicators were also incentivised by the QOF for patients with CHD, stroke and peripheral arterial disease in 2012/13 and 2013/14. Since 2014/15, however, only checks on blood pressure remain incentivised for patients with CHD, stroke or peripheral arterial disease, and regular checks for cholesterol levels are no longer required. 111 We hypothesised that physical health checks for blood pressure and cholesterol could influence people’s health outcomes, and would also be affected by the presence of macrovascular complications.
For this objective, we therefore applied restrictions on the patient sample used in objective 4 (see Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Study population) and compared only cases and controls with the same status of macrovascular complications (as a health outcome), so that the impact of these complications on health checks was equal within case–control clusters. A total of 8724 patients remained in this restricted sample after controls with a macrovascular complication status different from that of cases were excluded.
Variables and statistical methods
The exposure and explanatory variables and statistical methods for this objective were primarily based on the core and extended models, as reported in objective 4 (see Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness).
We explored the suitability of the CPRD data set for assessing individual interventions in terms of quality and consistency of recording by practices. Recording was not sufficiently comprehensive to allow detailed measurement of frequent and often complex interventions such as medication history and comprehensive care pathways. For referrals, we were not able to confirm attendance as we did not have linkage to outpatient data. However, we were able to ascertain whether or not lower frequency interventions such as health checks, diabetes education and influenza vaccination had been performed and we used these as the basis of our analysis.
For the impact of SMI on receipt of health checks, we re-estimated the core and extended models of blood pressure, cholesterol, HbA1c and BMI, as reported in objective 4. An interaction term between the status of SMI and macrovascular complications was added to these models to explore whether or not the impact of SMI could be modified by the status of complications.
For the impact of health checks on health outcomes, we separately estimated the association between the level of each health check, measured as the mean number per year, and markers of depression and anxiety using the core model reported in objective 4 (see Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness). We then added an interaction term between the level of health checks and SMI status to explore whether or not the association between health checks and depression could be modified by this.
We did not investigate the impact of health checks on physical health outcomes because of the confounding relationship between the two. We expected that the level of health checks would potentially be affected by the other physical health outcomes for reasons similar to why these health checks would be affected by macrovascular complications status. For instance, patients with poorer diabetes and cardiovascular control could potentially receive more frequent physical checks; death in the follow-up period would reduce health checks received, but deteriorated health status prior to death would increase physical health checks. Owing to this two-way causality, the estimated association between health checks and physical health outcomes would be biased using these patient-level data.
After initial investigation, we were unable to proceed with investigating the impact of retinopathy screening, diabetes education and influenza vaccination on health outcomes because of limitations in the overall data quality and the under-recording issue in primary care data for people with SMI. A descriptive summary of the provision of these health-care interventions can be found in Appendix 4, Table 32.
Results
Descriptive statistics
The baseline characteristics of the restricted sample are summarised in Table 20. Separating patients by macrovascular complication status, we found that patients without complications were diagnosed with SMI and T2DM at a younger age than patients with complications. The mean diagnosis age was 47.0 years for SMI and 56.2 years for T2DM for patients with SMI and without complications. Diagnosis ages were increased by > 10 years for patients with complications. In both groups, controls were closely matched to case patients in terms of age. There were more female patients in the group with complications. Socioeconomic deprivation was more common in people with SMI than in people without SMI.
| Characteristic | Without macrovascular complications | With macrovascular complications | ||
|---|---|---|---|---|
| Cases (T2DM + SMI) | Controls (T2DM) | Cases (T2DM + SMI) | Controls (T2DM) | |
| Patients (n) | 2000 | 6517 | 89 | 118 |
| Number of controls, n (%) | ||||
| 4 | 1031 (51.6) | 0 (0) | ||
| 3 | 595 (29.8) | 5 (5.6) | ||
| 2 | 234 (11.7) | 19 (21.4) | ||
| 1 | 140 (7.0) | 65 (73.0) | ||
| Diagnosis age (years), mean (SD) | ||||
| SMI | 46.99 (16.98) | 59.75 (19.94) | ||
| T2DM | 56.24 (13.12) | 56.90 (12.67) | 67.01 (11.28) | 68.72 (11.46) |
| SMI type, n (%) | ||||
| Schizophrenia | 1055 (52.8) | 50 (56.2) | ||
| Schizoaffective disorder | 105 (5.3) | 2 (2.3) | ||
| Bipolar disorder | 644 (32.2) | 29 (32.6) | ||
| Depression and psychosis | 168 (8.4) | 7 (7.9) | ||
| Other affective disorder | 21 (1.1) | 1 (1.1) | ||
| Mixed | 7 (0.4) | 0 (0) | ||
| Age at follow-up start (years), mean (SD) | 57.03 (13.05) | 57.63 (12.53) | 68.22 (10.57) | 69.98 (10.83) |
| Duration of T2DM (years), mean (SD) | 0.82 (2.91) | 0.74 (2.69) | 1.23 (2.96) | 1.33 (3.41) |
| Duration of follow-up (years) | ||||
| Mean (SD) | 5.76 (4.32) | 5.97 (4.33) | 8.91 (5.21) | 8.86 (5.06) |
| Median (minimum, maximum) | 4.88 (0.02, 24.25) | 5.25 (0.003, 24.81) | 8.92 (0.09, 25.84) | 8.54 (0.21, 21.67) |
| Family history of diabetes, n (%) | 297 (14.9) | 1253 (19.2) | 13 (14.6) | 19 (16.1) |
| Sex, n (%) | ||||
| Male | 963 (48.2) | 3114 (47.8) | 41 (46.1) | 51 (43.3) |
| Female | 1037 (51.9) | 3403 (52.2) | 48 (53.9) | 67 (56.9) |
| Ethnicity, n (%) | ||||
| White | 1657 (82.9) | 5164 (79.2) | 80 (89.9) | 103 (87.3) |
| Asian | 131 (6.6) | 443 (6.8) | 5 (5.6) | 8 (6.8) |
| Black | 98 (4.9) | 234 (3.6) | 3 (3.4) | 0 (0) |
| Mixed | 21 (1.1) | 43 (0.7) | 0 (0) | 0 (0) |
| Other | 38 (1.9) | 135 (2.1) | 0 (0) | 0 (0) |
| Not stated/unknown | 55 (2.8) | 498 (7.6) | 1 (1.1) | 7 (5.9) |
| Deprivation (IMD 2010), n (%) | ||||
| 1st quintile (least deprived) | 261 (13.1) | 1007 (15.5) | 7 (7.9) | 19 (16.1) |
| 2nd quintile | 330 (16.5) | 1278 (19.6) | 15 (16.9) | 18 (15.3) |
| 3rd quintile | 371 (18.6) | 1299 (19.9) | 20 (22.5) | 30 (25.4) |
| 4th quintile | 485 (24.3) | 1468 (22.5) | 25 (28.1) | 28 (23.7) |
| 5th quintile (most deprived) | 550 (27.5) | 1460 (22.4) | 22 (24.7) | 23 (19.5) |
| Missing | 3 (0.2) | 5 (0.1) | 0 (0) | 0 (0) |
| Comorbidities | ||||
| Cardiovascular disease, n (%) | 227 (11.4) | 895 (13.7) | 29 (32.6) | 47 (39.8) |
| Hypertension, n (%) | 645 (32.3) | 2890 (44.4) | 44 (49.4) | 64 (54.2) |
| Dementia, n (%) | 28 (1.4) | 24 (0.4) | 1 (1.1) | 0 (0) |
| Learning disability, n (%) | 19 (1.0) | 20 (0.3) | 0 (0) | 0 (0) |
| Charlson Index score, mean (SD) | 0.47 (0.69) | 0.50 (0.75) | 0.76 (1.09) | 0.86 (1.02) |
| Medications, n (%) | ||||
| Antidepressants | 982 (49.1) | 1256 (19.3) | 32 (36.0) | 21 (17.8) |
| Antipsychotics | ||||
| Typical | 393 (19.7) | 61 (0.9) | 15 (16.9) | 9 (7.6) |
| Atypical | 908 (45.4) | 43 (0.7) | 20 (22.5) | 0 (0) |
| Antidiabetics | 451 (22.6) | 1316 (20.2) | 34 (38.2) | 42 (35.6) |
| Antihypertensives | 887 (44.4) | 3505 (53.8) | 54 (60.7) | 86 (72.9) |
| Lipid-lowering drugs | 621 (31.1) | 2145 (32.9) | 32 (36.0) | 47 (39.8) |
| Statins | 604 (30.2) | 2089 (32.1) | 32 (36.0) | 44 (37.3) |
| Lifestyle factors, n (%) | ||||
| Smoking | ||||
| Non-smoker | 505 (25.3) | 1942 (29.8) | 17 (19.1) | 28 (23.7) |
| Ex-smoker | 361 (18.1) | 1342 (20.6) | 13 (14.6) | 34 (28.8) |
| Current smoker | 608 (30.4) | 1018 (15.6) | 13 (14.6) | 9 (7.6) |
| Missing | 526 (26.3) | 2215 (34.0) | 46 (51.7) | 47 (39.8) |
| Drinking | ||||
| Non-drinker | 365 (18.3) | 634 (9.7) | 7 (7.9) | 10 (8.5) |
| Ex-drinker | 118 (5.9) | 114 (1.8) | 3 (3.4) | 1 (0.9) |
| Current drinker | 610 (30.5) | 1736 (26.6) | 17 (19.1) | 32 (27.1) |
| Missing | 907 (45.4) | 4033 (61.9) | 62 (69.7) | 75 (63.6) |
| Substance use | 37 (1.9) | 31 (0.5) | 0 (0) | 1 (0.9) |
| Biometric measures | ||||
| BMI (kg/m2), mean (SD) | 33.15 (7.07) | 32.88 (6.98) | 29.94 (4.78) | 29.34 (4.61) |
| < 20, n (%) | 10 (0.5) | 42 (0.6) | 1 (1.1) | 1 (0.9) |
| 20–24, n (%) | 133 (6.7) | 359 (5.5) | 5 (5.6) | 14 (11.9) |
| 25–29, n (%) | 359 (18.0) | 1105 (17.0) | 22 (24.7) | 26 (22.0) |
| 30–39, n (%) | 688 (34.4) | 1947 (29.9) | 17 (19.1) | 34 (28.8) |
| > 40, n (%) | 215 (10.8) | 591 (9.1) | 2 (2.3) | 1 (0.9) |
| Missing, n (%) | 595 (29.8) | 2473 (38.0) | 42 (47.2) | 42 (35.6) |
| HbA1c (%),a mean (SD) | 7.84 (1.99) | 7.91 (1.96) | 7.38 (2.09) | 7.80 (1.90) |
| ≤ 7.5, n (%) | 617 (30.9) | 1991 (30.6) | 29 (32.6) | 26 (22.0) |
| > 7.5, n (%) | 427 (21.4) | 1468 (22.5) | 13 (14.6) | 23 (19.5) |
| Missing, n (%) | 956 (47.8) | 3058 (46.9) | 47 (52.8) | 69 (58.5) |
| Cholesterol (mmol/l), mean (SD) | 5.35 (1.39) | 5.28 (1.28) | 5.35 (1.35) | 5.11 (1.29) |
| ≤ 5, n (%) | 668 (33.4) | 2317 (35.6) | 25 (28.1) | 40 (33.9) |
| > 5, n (%) | 820 (41.0) | 2754 (42.3) | 30 (33.7) | 32 (27.1) |
| Missing, n (%) | 512 (25.6) | 1446 (22.2) | 34 (38.2) | 46 (39.0) |
| Diastolic blood pressure (mmHg), mean (SD) | 81.30 (10.47) | 82.23 (10.58) | 84.03 (14.76) | 79.75 (11.24) |
| ≤ 80, n (%) | 928 (46.4) | 2766 (42.4) | 38 (42.7) | 62 (52.5) |
| > 80, n (%) | 794 (39.7) | 2732 (41.9) | 38 (42.7) | 41 (34.8) |
| Missing, n (%) | 278 (13.9) | 1019 (15.6) | 13 (14.6) | 15 (12.7) |
| Systolic blood pressure (mmHg), mean (SD) | 135.07 (17.59) | 139.46 (17.81) | 145.71 (24.58) | 146.72 (22.71) |
| ≤ 140, n (%) | 1187 (59.4) | 3294 (50.5) | 41 (46.1) | 54 (45.8) |
| > 140, n (%) | 535 (26.8) | 2204 (33.8) | 35 (39.3) | 49 (41.5) |
| Missing, n (%) | 278 (13.9) | 1019 (15.6) | 13 (14.6) | 15 (12.7) |
Physical comorbidities, such as cardiovascular disease, hypertension and Charlson Index comorbidities, were more likely to be diagnosed in the group with macrovascular complications than in the group without complications, consistent with the pattern of prescription rates of antidiabetes, antihypertensive and lipid-lowering drugs. In these groups, patients with SMI had lower prevalence of physical comorbidities and lower prescription rates for antihypertensive and lipid-lowering drugs than people without SMI. This finding, however, may not suggest better baseline health status for people with SMI, but may reflect under-reporting or underdetection for this population (as previously discussed in Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness).
Lifestyle and BMI were better recorded in people with SMI and without macrovascular complications. Although there were a high number of missing values, limiting interpretation, results suggest that the group without macrovascular complications had greater BMI at baseline.
Table 21 shows descriptive statistics for the outcome variables. Without adjustment, the number of health checks for HbA1c and BMI were higher in the group without macrovascular complications and checks on blood pressure were higher in the group with complications. Within groups, people with SMI were more likely to receive checks for cholesterol level and BMI in the group without complications, and less likely to receive these checks in the group with complications, than people without SMI. For blood pressure and HbA1c, the crude rates of health checks were lower in people with SMI than in people without SMI in both groups. These statistics also showed that people with SMI were more likely to have depression and anxiety recorded in their primary care records.
| Outcome variable | Without macrovascular complications | With macrovascular complications | ||
|---|---|---|---|---|
| Cases (T2DM + SMI) | Controls (T2DM) | Cases (T2DM + SMI) | Controls (T2DM) | |
| Patients (n) | 2000 | 6517 | 89 | 118 |
| Number of health checks per year, mean (SD) | ||||
| HbA1c | 1.80 (1.30) | 1.81 (1.16) | 1.56 (0.66) | 1.76 (0.69) |
| Cholesterol | 1.39 (1.00) | 1.34 (0.84) | 1.21 (0.57) | 1.39 (0.61) |
| Blood pressure | 2.92 (2.56) | 2.97 (4.99) | 3.01 (1.79) | 3.70 (2.00) |
| BMI | 2.10 (1.99) | 1.98 (4.84) | 1.56 (1.24) | 1.68 (1.15) |
| Mental health outcome, n (%) | ||||
| Depression and anxiety | 506 (25.3) | 1032 (15.8) | 31 (34.8) | 30 (25.4) |
Regression analysis results for the adjusted impact of severe mental illness on levels of physical health checks
The adjusted impact of SMI on levels of physical health checks is reported in Table 22. These results suggest a significant association between SMI and health checks for blood pressure, cholesterol and BMI. These relationships differ by whether or not macrovascular complications have been diagnosed. Results from the core models show that people with SMI are more likely, by around 5%, to receive checks on blood pressure than people without SMI in the group without complications (IRR 1.05, 95% CI 1.03 to 1.08). This IRR is diminished by around 20% (IRR 0.80, 95% CI 0.70 to 0.91) in the group with complications; people with SMI are less likely, by around 16%, to receive this check [calculation of interaction between diagnosis of SMI and macrovascular complications (IRR 1.05 × 0.80)] than people without SMI in this group. A similar pattern was also found for the health checks for cholesterol and BMI, with HbA1c showing no significant association with the status of either SMI or macrovascular complications. Results from the extended models confirm these findings. The estimated impact of the other explanatory variables is consistent with the results for objective 4.
| Model | Health checks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood pressure | Cholesterol | HbA1c | BMI | |||||||||
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Core modelsa | ||||||||||||
| Diagnosis of SMI | 1.054 | 1.028 to 1.081 | < 0.001 | 1.069 | 1.047 to 1.092 | < 0.001 | 1.013 | 0.991 to 1.036 | 0.244 | 1.104 | 1.074 to 1.134 | < 0.001 |
| Macrovascular complications (outcome) | 0.969 | 0.703 to 1.334 | 0.847 | 0.523 | 0.245 to 1.118 | 0.094 | 1.055 | 0.574 to 1.937 | 0.864 | 0.804 | 0.547 to 1.182 | 0.267 |
| SMI*macrovascular complications | 0.796 | 0.700 to 0.905 | < 0.001 | 0.843 | 0.753 to 0.945 | 0.003 | 0.918 | 0.825 to 1.021 | 0.115 | 0.790 | 0.682 to 0.915 | 0.002 |
| Patients | 8697 | 8710 | 8706 | 8720 | ||||||||
| Case–control clusters | 2081 | 2082 | 2084 | 2087 | ||||||||
| Extended modelsb | ||||||||||||
| Diagnosis of SMI | 1.060 | 1.034 to 1.087 | < 0.001 | 1.070 | 1.048 to 1.093 | < 0.001 | 1.014 | 0.992 to 1.037 | 0.222 | 1.099 | 1.070 to 1.129 | < 0.001 |
| Macrovascular complications (outcome) | 0.983 | 0.710 to 1.362 | 0.918 | 0.579 | 0.254 to 1.318 | 0.193 | 1.047 | 0.568 to 1.930 | 0.883 | 0.765 | 0.521 to 1.122 | 0.170 |
| SMI*macrovascular complications | 0.802 | 0.706 to 0.910 | 0.001 | 0.840 | 0.752 to 0.940 | 0.002 | 0.921 | 0.828 to 1.024 | 0.129 | 0.811 | 0.700 to 0.939 | 0.005 |
| Patients | 8697 | 8710 | 8706 | 8720 | ||||||||
| Case–control clusters | 2081 | 2082 | 2084 | 2087 | ||||||||
The adjusted association between health checks and depression and anxiety is provided in Table 23. These results suggest that health checks for blood pressure and BMI are associated with increased recording of depression and anxiety after adjusting for SMI status and other risk factors. For instance, an additional check on blood pressure per year increases the probability of depression and anxiety being recorded by around 2% (OR 1.02, 95% CI 1.01 to 1.03); and one additional check on BMI increases this probability by around 1% (OR 1.01, 95% CI 1.00 to 1.02). The regressions with interaction terms show weak evidence that, in people with SMI, health checks for blood pressure have a bigger effect on the probability of depression and anxiety being classified than in people without SMI. An additional check per year increases this probability by around 1% for people without SMI (OR 1.01, 95% CI 1.01 to 1.02) and by around 6% [calculation of interaction effect (IRR 1.01 × 1.05)] for people with SMI. The impact of other health checks does not appear to be modified by the SMI status. The estimated impact of the other explanatory variables is consistent with the results for objective 4.
| Interaction | 1 | 2 | 3 | 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Without interactiona | ||||||||||||
| Diagnosis of SMI | 1.865 | 1.620 to 2.147 | < 0.001 | 1.858 | 1.612 to 2.141 | < 0.001 | 1.864 | 1.618 to 2.147 | < 0.001 | 1.860 | 1.616 to 2.142 | < 0.001 |
| Health checks (per year) | ||||||||||||
| Blood pressure | 1.016 | 1.005 to 1.026 | 0.004 | |||||||||
| Cholesterol | 1.031 | 0.908 to 1.170 | 0.637 | |||||||||
| HbA1c | 1.054 | 0.986 to 1.125 | 0.121 | |||||||||
| BMI | 1.010 | 1.002 to 1.018 | 0.014 | |||||||||
| Patients | 4912 | 4912 | 4912 | 4912 | ||||||||
| Case–control clusters | 1134 | 1134 | 1134 | 1134 | ||||||||
| c-statistic | 0.689 | 0.688 | 0.688 | 0.688 | ||||||||
| With interactiona | ||||||||||||
| Diagnosis of SMI | 1.598 | 1.254 to 2.037 | < 0.001 | 2.004 | 1.502 to 2.674 | < 0.001 | 1.939 | 1.373 to 2.737 | < 0.001 | 2.042 | 1.684 to 2.476 | < 0.001 |
| Health checks (per year) | ||||||||||||
| Blood pressure | 1.014 | 1.005 to 1.023 | 0.002 | |||||||||
| Blood pressure*SMI | 1.053 | 0.992 to 1.118 | 0.091 | |||||||||
| Cholesterol | 1.061 | 0.930 to 1.212 | 0.377 | |||||||||
| Cholesterol*SMI | 0.947 | 0.786 to 1.140 | 0.563 | |||||||||
| HbA1c | 1.059 | 0.999 to 1.123 | 0.054 | |||||||||
| HbA1c*SMI | 0.978 | 0.820 to 1.167 | 0.807 | |||||||||
| BMI | 1.012 | 1.004 to 1.020 | 0.002 | |||||||||
| BMI*SMI | 0.957 | 0.901 to 1.015 | 0.142 | |||||||||
| Patients | 4912 | 4912 | 4912 | 4912 | ||||||||
| Case–control clusters | 1134 | 1134 | 1134 | 1134 | ||||||||
| c-statistic | 0.689 | 0.688 | 0.688 | 0.688 | ||||||||
Summary of findings
We found that the association between SMI and physical health checks was influenced by the diagnosis of macrovascular complications. In the group without complications, people with SMI were more likely to receive health checks than people without SMI, whereas SMI was associated with reduced chances for receiving health checks in the group with complications.
The results also suggest that, in general, there is a positive association between the frequency of physical health checks and the likelihood that patients had depression and anxiety recorded. It is important to note that this finding is evidence of an association, rather than establishing causality; patients who saw their GPs more often would be more likely to have their depression and anxiety detected and recorded. This association, however, did not seem to differ between people with and without SMI.
Chapter 6 Qualitative interview study with people with diabetes and severe mental illness, family members and supporters, and health-care staff (objective 5)
Introduction
The qualitative study aimed to explore the experiences of people living with and managing diabetes alongside SMI. An overarching topic was the extent to which social circumstances (e.g. money for transportation, type of neighbourhood, carer role) enabled or hindered people’s ability to receive effective health care and to action preventative measures for diabetes and mental illness – eliciting both actual experiences of people with diabetes and SMI and the perceptions of the influence of these factors from health-care staff.
Objectives
This study focused on objective 5, to understand the factors that influence access to, and receipt of, diabetes care for people with SMI. We also aimed to explore factors perceived to be associated with variation in diabetes and mental health outcomes (objective 2) and perceived impacts of SMI on diabetes management, and vice versa.
As the quantitative analyses of patient records were limited to investigation of variables recorded in these data, we aimed to use the qualitative study to explore additional factors that may not be available from health-care records. We intended to develop a more in-depth understanding of the reasons for differences in diabetes care provision between people with and people without SMI observed in the observational study and to explore how health services may be supporting diabetes management. We also aimed to understand how people interacted with various health-care interventions for their diabetes and to explore perceived benefits associated with these interventions (objective 7). Insights were expected to also feed back into the quantitative study of health-care interventions (objective 7 analyses).
Methods
Ethics approval
Approval for this element of the study was given by the Greater Manchester West Research Ethics Committee (reference number 18/NW/0005).
Sample
We included people living with both SMI and diabetes; those providing informal care, such as family members and close friends; and health-care staff. Eligibility criteria and the sampling strategy for each of these participant groups were as follows.
People with diabetes and severe mental illness
-
Aged ≥ 18 years.
-
Recorded diagnosis of SMI (schizophrenia, schizoaffective disorder, bipolar disorder and manic episodes, or non-organic psychoses), excluding those experiencing an acute relapse.
-
Diagnosis of diabetes (excluding gestational diabetes).
-
Living in the community (including supported housing, but not admitted to acute hospital settings).
-
Had the capacity to provide informed consent to participate in the study.
We employed a maximum variation sampling strategy132 to include participants whose experiences were likely to differ, enabling us to explore the range of factors potentially affecting diabetes management, and to capture variations in diabetes care experiences.
From the expert consultation and scoping of the literature (see Chapter 4), we identified the following participant characteristics that were expected to affect diabetes experiences and management:
-
demographic (age, sex) and geographical (deprivation, region) characteristics
-
family composition and presence of carer/supporter
-
mental health and diabetes diagnoses
-
diabetes medication
-
diabetes control (determined by data obtained from health record)
-
presence of other comorbidities
-
provider of mental health care (e.g. primary or secondary mental health)
-
provider of diabetes health care (e.g. primary care, secondary care).
We initially adopted a convenience sampling strategy in recruitment sites because of recognised challenges to identifying and recruiting this patient population. 133 We continuously monitored variations in the sample, and worked with recruiting sites to increase diversity and include particular characteristics that were not represented. For example, we sought to target people with a history of diabetes complications to increase the sample variation. Our sampling strategy could not be informed by preliminary analysis of CPRD data, as originally planned, owing to delays in obtaining CPRD data.
To achieve diversity across the relevant characteristics, we aimed to recruit a minimum of 30 patients. In addition to adding participants who were under-represented in the study (e.g. those with poor diabetes control), we also monitored data saturation in the later stages of recruitment, and continued to recruit patients, with a stopping criteria of two, until we were satisfied that no new distinct ideas relevant to the study objectives were emerging. 134
Family members/supporters
We defined ‘family members/supporters’ as adults who were involved in the care of a person with diabetes and SMI taking part in the study. We expected that this would include a spouse/partner, parent or other family member, or close friend. They did not have to live with the person they supported, but they had to be identified by a participating person with diabetes and SMI as providing support for their health.
As people with SMI are more likely than the general diabetes population to have inadequate social support,135 we anticipated that the total sample would be 15–20. To reach these numbers, we aimed to monitor recruitment and adjust purposive sampling of people with diabetes and SMI accordingly (i.e. to include more people with diabetes and SMI living with other adults).
Health-care staff
We defined health-care staff as commissioners, clinicians, nurses and other staff who are involved in health-care services for SMI and diabetes. We used a purposive sampling strategy to ensure a mix of staff roles from differing organisations and services. We aimed to recruit a minimum of 15 staff to achieve this variation and continued to recruit participants, with a stopping criterion of one, until we achieved data saturation or a sufficient mix of staff roles (which we expected would affect perspectives and experiences of supporting this population).
Recruitment
People with diabetes and severe mental illness
We identified and recruited potential participants with diabetes and SMI through three routes:
-
Community mental health teams in NHS mental health trusts, which conducted electronic searches of registers or searched the case loads of psychiatrists and care co-ordinators. Learning from an earlier study,133 we prioritised the second strategy because of limited recording of diabetes diagnoses in patient records.
-
SMI and diabetes QOF registers61 in general practices. Practices identified patients who appeared on both the SMI and diabetes QOF registers using a query in the electronic patient record system.
-
Networks at the University of York, such as participants from another study who had consented to be contacted about taking part in future studies. 136
Mental health trusts (n = 7) in two regions of England (North West, and Yorkshire and the Humber) and general practices (n = 10) in Yorkshire and the Humber were chosen to include rural and urban patient populations, areas of wealth and deprivation and areas where minority ethnic groups are well represented. We expected that individual general practices would yield low participant numbers, compared with mental health trusts. However, as previous research indicates that around 20–30% of people with SMI are treated in primary care only and not in specialist mental health services,137 it was important to recruit from both settings as support for diabetes may differ. 138
Care co-ordinators and practice staff were asked to check that potential participants met the inclusion criteria for the study. Eligible participants were provided with an information pack about the study, containing an invitation letter from participating sites, a participant information sheet and response form (with prepaid envelope), and were asked to return a completed form to the research team if they were interested in taking part. A researcher telephoned those who were interested to introduce the study and answer any questions. Eligibility and capacity to consent were assessed during the telephone call, and a suitable date and time for an interview was arranged.
Family members/supporters
All participants with diabetes and SMI were asked to identify a family member/supporter during their interview. After the interview, the researcher explained that they would like to invite this person to take part in an interview as well and gave participants an invitation pack to provide to their named family member/supporter. Interested family member/supporter participants were asked to contact the research team or to return a completed response form. This approach was used successfully in the National Audit of Schizophrenia (involving the researcher DS) and avoids additional consent issues for service user participants. 139
We aimed to recruit 15 family members/supporters. However, many participants with diabetes and SMI reported not having a family member or friend who provided support for their health, or the family member was unwilling to participate in the study. Recruiting trusts were therefore encouraged to renew recruitment efforts and we promoted the study more widely (including through the McPin Foundation, an organisation dedicated to engaging people experiencing mental health difficulties to participate in research), DIAMONDS VOICE members leveraged their networks, and we contacted family member participants from another study who had consented to be contacted about taking part in future studies. 136
Health-care staff
Eligible health-care staff participants were identified through University of York networks, or by asking staff supporting the study in participating sites to identify people involved in diabetes care for people with SMI, and invite them to the study. Potential participants were invited by e-mail and provided with an information sheet about the study and asked to contact the study team if they wished to take part.
Written or audio-recorded verbal consent for all participants was taken at the time of interview. For telephone interviews and for participants with a limited understanding of written English, the researcher read out the statements contained in the study consent form to obtain verbal consent.
Data collection
We used in-depth semistructured interviews to explore individual experiences and perceptions of the management of diabetes in people with diabetes and SMI. Participants were offered the choice of a face-to-face or telephone interview; the former were conducted at home or another private venue, according to participant preference. For health-care staff, it was expected that interviews would be conducted at their workplace, although they were given the option of another suitable venue if preferred.
A standardised semistructured interview format was used for all participants, tailored in length to reflect the number of topics to be covered and to minimise participant burden. Key topics to explore aligned with study objectives and were guided by a social inequalities framework (see Report Supplementary Material 1). Separate interview guides for the three participant groups were developed in partnership with study co-investigators and DIAMONDS VOICE members (see Chapter 3, Patient and public involvement), following key principles outlined by Arthur and Nazroo. 140 Interview guides were also informed by the scoping of the literature and expert consultation process. This process led to the inclusion of new topics to explore (e.g. sleep difficulties), and to the rephrasing of questions to aid understanding and facilitate appropriate responses from participants. The patient topic guide was piloted with a DIAMONDS VOICE member and feedback was used to refine the guide before commencing data collection.
Topic guides were designed so that interviews with people with diabetes and SMI and with family members/supporters would last approximately 45–60 minutes to allow for sufficient exploration while minimising participant burden. This was particularly important for people with diabetes and SMI, some of whom were likely to have cognitive and attention difficulties. 2 To enable staff to take part during working hours, interviews were designed to last approximately 30 minutes (fewer topics were included in these). Interview guides were employed flexibly (e.g. ordering of topics and wording of specific questions were tailored to individual participants), but ensured that key topics were covered.
Data were collected by Sue Bellass, an experienced qualitative researcher with experience of working with people with cognitive and attentional difficulties and with people who may have fluctuating mental capacity. Sue Bellass was supported by Najma Siddiqi and Jo Taylor throughout the data collection period.
We planned to audio-record interviews, but allowed participants to consent separately to this. Two participants with diabetes and SMI and four staff members declined audio-recording. Recorded interviews were transcribed (intelligent verbatim) for analysis by a subcontractor with experience in transcribing audio for academic research. For interviews that were not recorded, the researcher made extensive field notes immediately after the interview. We sought to include non-English speakers (our primary research sites include significant South Asian populations, who are at greater risk of diabetes and SMI) and used a translator for one interview with a service user from the South Asian community.
Data analysis
The framework method was used to analyse interview data, to allow for inductive analysis and analysis of a priori themes and to enable us to move iteratively through the different stages in the analytical process to develop explanatory accounts and facilitate mixed-methods integration. 141,142
NVivo version 12 was used to manage and code anonymised interview transcripts, to test and apply the coding frameworks, to identify and describe themes, and to explore potential differences between participant groups.
Analysis involved two key stages (incorporating the five steps of framework analysis). 141
Stage 1: developing and applying the coding frameworks [framework step 1 (‘conceptual scaffolding’), step 2 (‘indexing’) and step 3 (‘coding’)]
This involved identifying and extracting recurring ideas and concepts from across the data and developing thematic coding frameworks (step 1), which incorporated a priori descriptive themes based on the study objectives (including diabetes care experiences, and diabetes knowledge and education), as well as those identified during analysis. To explore differences between participant groups, we analysed these data separately, starting with people with diabetes and SMI and then applying and refining the coding framework for the analysis of family members and health-care staff.
The coding frameworks were tested and modified as follows (step 2):
-
The coding framework for people with diabetes and SMI was tested with eight interview transcripts, chosen to represent a range of participants: two males and two females with bipolar disorder, and two males and two females with schizophrenia.
-
For family members, the diabetes and SMI participants’ framework was adapted and tested at the same time because of the small number of participants.
-
For staff, the diabetes and SMI participants’ framework was adapted and tested using four interviews, drawn from different staff groups to ensure that the diversity of experiences was accounted for.
Once the frameworks were finalised, all interviews were coded using NVivo (step 3). A record of how each framework was modified during development and testing was logged (manually for people with diabetes and SMI and in NVivo for family members and staff). The final frameworks can be found in Appendix 5, Boxes 1–3.
Stage 2: identifying cross-cutting themes and exploring relationships between themes [framework step 4 (‘descriptive analysis’) and step 5 (‘explanatory analysis’)]
We then explored the coded data (by theme and subtheme) to identify thematic patterns and classify findings into higher-order themes and to describe these with reference to relevant coded data (step 4). Again, we started with the data from participants with diabetes and SMI to develop themes and mapped the findings from family members and supporters onto these to explore similarities and differences and to refine the meaning and descriptions of themes. This was an iterative process involving regular discussion among the qualitative study team, using mind-mapping to explore links between themes (step 5) and continuous checking against the coded data to ensure that themes represented the accounts of all participants. Deviant cases were analysed to understand the variation in managing diabetes in the sample of people with diabetes and SMI, and coding matrix queries were used in NVivo to explore potential differences in diabetes experiences between people with schizophrenia and bipolar disorder.
In a final step, a table with the main themes and subthemes mapped to coded data (see Appendix 6, Tables 33–40) was shared with the wider research team for sense-checking and interpretation, and to explore further the relationships between themes and the meaning of findings in the context of the social inequalities framework that underpinned the study as a whole (step 5).
Ensuring quality of data analysis
To ensure consistency, the key steps of the data analysis were carried out by the same person for each group (participants with diabetes and SMI, and staff data: JL; family member data: Lyndsey Kramer, with guidance from JL). To ensure reliability, coding was regularly discussed with Jo Taylor and Sue Bellass, who also checked random samples of coded transcripts during the initial stages. To ensure credibility and authenticity of findings, preliminary findings were discussed with people with diabetes and SMI, family members and staff at co-design workshops (see Chapter 7, Co-design workshops) and at regular meetings involving John Radford (service user co-investigator).
Results
In total, we interviewed 39 people with diabetes and SMI, nine family members and 30 health-care staff.
Participant characteristics
Of the 39 people with diabetes and SMI, 22 (56%) were men and 17 (44%) women (Table 24). Schizophrenia was the most common SMI diagnosis (n = 22, 56%), followed by bipolar disorder (n = 13, 33%), schizoaffective disorder (n = 2, 5%) and depressive psychosis (n = 2, 55%). Most participants (n = 36, 92%) had a diagnosis of T2DM; three (8%) had type 1 diabetes. Twenty-three (59%) participants had received a diagnosis of SMI before their diabetes diagnosis, two (5%) had received the diabetes diagnosis first and two (5%) had received it at the same time; for the remainder, diagnosis order was unknown (n = 12, 31%). The mean age of participants was 53 years (range 28–71 years), with women being older than men (females, mean 55 years; males, mean 52 years). Most participants identified as white (n = 27, 69%), with others identifying as Asian/Asian British (n = 2, 5%), black/African/Caribbean/black British (n = 3, 8%) and mixed/multiple (n = 2, 5%) ethnic groups. Information on ethnicity was missing for five (13%) participants. The highest education level varied, with participants ranging from having no qualifications (n = 9, 23%) to 10 (26%) having qualifications beyond General Certificate of Secondary Education (GCSE) level. Data on education were missing for three (8%) participants. Only two (5%) participants were working, whereas 32 (82%) were unemployed and the remainder (n = 5, 13%) were retired.
| Participant ID | Primary diagnosis | Diagnosis order | Diabetes type | Age (years) | Sex | Ethnic group | Highest education | Employment status |
|---|---|---|---|---|---|---|---|---|
| ES-D1-05 | Depressive psychosis | SMI–DM | T2 | 69 | Male | White | No qualifications | Unemployed |
| ES-G2-01 | Bipolar disorder | SMI–DM | T2 | 45 | Male | White | Degree | Employed |
| ES-G3-01 | Schizophrenia | Not clear | T2 | 47 | Male | White | No qualifications | Unemployed |
| ES-G4-01 | Schizophrenia | SMI–DM | T2 | 55 | Male | White | GCSE/O levels | Unemployed |
| ES-G4-02 | Bipolar disorder | Same time | T2 | 67 | Female | White | NVQ/OND/other | Retired |
| ES-G7-01 | Bipolar disorder | DM–SMI | T1 | 61 | Female | White | No qualifications | Unemployed |
| ES-G8-01 | Bipolar disorder | SMI–DM | T1 | 63 | Male | White | Degree | Retired |
| ES-G9-01 | Schizophrenia | SMI–DM | T2 | 38 | Male | Asian/Asian British | GCSE/O levels | Unemployed |
| ES-SP-02 | Bipolar disorder | SMI–DM | T2 | 49 | Female | Asian/Asian British | No qualifications | Unemployed |
| ES-T2-02 | Bipolar disorder | SMI–DM | T2 | 63 | Female | White | No qualifications | Unemployed |
| ES-T2-03 | Bipolar disorder | Not clear | T2 | 51 | Male | White | Masters/PhD | Unemployed |
| ES-T2-04 | Schizophrenia | Not clear | T2 | 68 | Female | Not recorded | Not recorded | Unemployed |
| ES-T2-05 | Schizoaffective disorder | SMI–DM | T2 | 59 | Female | White | Masters/PhD | Retired |
| ES-T2-06 | Bipolar disorder | Not clear | T2 | 59 | Female | White | GCSE/O levels | Unemployed |
| ES-T2-07 | Schizophrenia | Not clear | T2 | 44 | Male | Black/African/Caribbean/black British | GCSE/O levels | Unemployed |
| ES-T2-09 | Schizophrenia | SMI–DM | T2 | 44 | Male | White | GCSE/O levels | Unemployed |
| ES-T2-16 | Schizophrenia | SMI–DM | T2 | 65 | Female | White | Degree | Unemployed |
| ES-T2-18 | Schizophrenia | SMI–DM | T2 | 48 | Male | White | GCSE/O levels | Unemployed |
| ES-T3-03 | Bipolar disorder | DM–SMI | T2 | 34 | Female | White | NVQ/OND/other | Unemployed |
| ES-T3-04 | Bipolar disorder | SMI–DM | T2 | 67 | Female | White | GCSE/O levels | Retired |
| ES-T3-07 | Bipolar disorder | Not clear | T2 | 71 | Female | White | GCSE/O levels | Unemployed |
| ES-T3-09 | Schizophrenia | Not clear | T2 | 37 | Female | White | GCSE/O levels | Unemployed |
| ES-T3-11 | Schizophrenia | SMI–DM | T2 | 60 | Male | White | GCSE/O levels | Unemployed |
| ES-T4-01 | Schizophrenia | Not clear | T2 | 39 | Female | Mixed/multiple | A levels | Unemployed |
| ES-T4-02 | Schizophrenia | SMI–DM | T2 | 53 | Female | White | BTEC | Unemployed |
| ES-T4-09 | Depressive psychosis | SMI–DM | T2 | 60 | Female | White | Degree | Unemployed |
| ES-T4-10 | Bipolar disorder | SMI–DM | T2 | 41 | Male | White | No qualifications | Unemployed |
| ES-T4-12 | Schizophrenia | SMI–DM | T2 | 41 | Female | White | GCSE/O levels | Employed: volunteer |
| ES-T4-13 | Schizophrenia | SMI–DM | T2 | 53 | Male | White | A levels | Unemployed |
| ES-T5-05 | Schizophrenia | SMI–DM | T2 | 35 | Male | Not recorded | GCSE/O levels | Unemployed |
| ES-T5-08 | Schizophrenia | SMI–DM | T2 | 51 | Male | White | No qualifications | Unemployed |
| ES-T5-09 | Schizophrenia | Not clear | T2 | 61 | Male | Not recorded | No qualifications | Unemployed |
| ES-T5-10 | Schizophrenia | SMI–DM | T2 | 64 | Male | White | GCSE/O levels | Unemployed |
| ES-T5-11 | Schizophrenia | SMI–DM | T2 | 59 | Male | Not recorded | Not recorded | Unemployed |
| ES-T6-05 | Schizoaffective disorder | Same time | T2 | 39 | Female | Not recorded | A levels | Unemployed |
| ES-T6-07 | Schizophrenia | SMI–DM | T2 | 60 | Male | White | No qualifications | Unemployed |
| ES-T7-02 | Schizophrenia | Not clear | T2 | 28 | Male | Mixed/multiple | Not recorded | Unemployed |
| ES-T7-03 | Schizophrenia | Not clear | T2 | 53 | Male | Black/African/Caribbean/black British | GCSE/O levels | Unemployed |
| ES-T7-04 | Bipolar disorder | Not clear | T1 | 65 | Male | Black/African/Caribbean/black British | A levels | Retired |
As shown in Table 25, among the nine family members in the final sample, six were female; ages ranged from 37 to 73 years (mean 59 years) and all participants who identified an ethnic group were white British (n = 8); one person’s ethnicity information was not recorded. All participants with recorded education data were educated to GCSE level or above, with six educated beyond degree level. Two participants had missing education data. Most family members were married to the person they supported (n = 6); of the remainder, two were parents and one was the adult child of the participant with diabetes and SMI.
| Participant ID | Sex | Age (years) | Ethnic group | Highest education | Relationship to person with diabetes and SMI |
|---|---|---|---|---|---|
| ES-D1-02 | Female | 71 | White British | College certificate | Parent |
| ES-D1-03 | Female | 73 | White British | GCSE/O levels | Spouse |
| ES-T2-08 | Female | Not recorded | Not recorded | Not recorded | Parent |
| ES-T2-17 | Male | 67 | White British | Not recorded | Spouse |
| ES-T2-20 | Female | 47 | White British | Masters/PhD | Spouse |
| ES-T2-21 | Male | 59 | White British | Masters/PhD | Spouse |
| ES-T3-08 | Female | 37 | White British | Postgraduate degree | Adult child |
| ES-T4-03 | Male | 61 | White British | Foundation degree | Spouse |
| ES-T5-12 | Female | 56 | White British | Batchelor’s degree | Spouse |
As shown in Table 26, the 30 staff members were from a variety of disciplines and had varied training and experience. Twelve (40%) participants in this group had their key training in mental health, 10 (33%) in physical health and five (17%) in a combination of the two. The remaining participants had training in management (n = 2, 7%) and social work (n = 1, 3%). Four (13%) participants were GPs, 11 (37%) were mental health nurses, three (10%) were physical health nurses, two (7%) were dietitians, two (7%) were pharmacists, four (13%) were psychiatrists and the remaining four staff members comprised an NHS commissioner, a diabetologist, a practice manager and a recovery support worker. Two (7%) of the nurse participants also worked as care co-ordinators.
| Participant ID | Role | Key training | Areas of expertise/experience/responsibility |
|---|---|---|---|
| ES-PC-01 | GP | Physical health | Some psychiatry experience |
| ES-PC-02 | GP | Physical health | Used to be diabetes lead |
| ES-PC-03 | Practice manager | Management | – |
| ES-PC-04 | Practice nurse | Physical health | – |
| ES-PC-05 | GP | Physical health | Diabetes lead |
| ES-PC-06 | GP | Physical health | – |
| ES-T1-01 | Psychiatrist | Mental health | Diagnosis and management of SMI |
| ES-T1-02 | Community mental health nurse | Mental health | – |
| ES-T1-03 | Community mental health nurse | Mental health | – |
| ES-T1-04 | Community mental health nurse | Mental health | – |
| ES-T1-05 | Nurse prescriber/care co-ordinator | Mental health | Some previous training in diabetes |
| ES-T2-01 | Mental health nurse | Mental health | Diabetes and physical health care |
| ES-T2-12 | Psychiatrist | Mental and physical health | – |
| ES-T2-13 | Pharmacist | Physical health | Training in psychiatric therapeutics |
| ES-T2-14 | Dietitian | Physical health | Working in mental health |
| ES-T2-15 | Psychiatrist | Mental and physical health | – |
| ES-T3-01 | Pharmacist | Physical health | Training in psychiatric therapeutics |
| ES-T3-02 | Community mental health nurse | Mental health | – |
| ES-T3-05 | Psychiatrist | Mental and physical health | – |
| ES-T4-06 | Recovery support worker | Social work | – |
| ES-T5-01 | Mental health nurse | Mental health | – |
| ES-T5-02 | Mental health nurse | Mental and physical health | Prescribing course |
| ES-T5-03 | Nurse | Mental and physical health | Nurse prescriber |
| ES-T5-04 | Mental health nurse – professional lead for nursing | Mental health | Previously ran team focused on physical health for SMI |
| ES-T6-01 | Mental health nurse | Mental health | Experience of work involving physical health for SMI |
| ES-T6-02 | Dietitian | Physical health | – |
| ES-T6-03 | Care co-ordinator/psychiatric nurse | Mental health | Training in physical health |
| ES-T6-04 | Commissioner | Management | – |
| ES-T7-01 | Mental health nurse | Mental health | Senior practitioner for physical health |
| ES-X1-01 | Diabetologist and endocrinologist | Physical health | – |
The analysis of interviews identified eight key themes, which are described in the following sections, accompanied by supporting quotations. A supplementary table containing further quotations for each theme and subtheme can be found in Appendix 6, Tables 33–40.
Theme 1: mental illness affects everything
Some of the participant quotations in this section have been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The pervasive effect of severe mental illness
Participants with diabetes and SMI described the pervasive effect of their mental health problems on their daily lives, for example on their ability to make or maintain friendships, retain a driving licence, engage in personal or household care, leave the house or keep appointments. SMI appeared to have a withering effect on social worlds, as one participant described:
I’m just getting worse actually, not going out, putting myself off, not going to the shops or anything like that. I make appointments and then I cancel them, I used to have friends, but I haven’t got any any more, they’ve all deserted me.
ES-G7-01, female, aged 61 years, bipolar disorder
In addition, their mental illness affected their ability to work and many participants relied on state benefits for financial support, a system that was perceived to disadvantage them through disregarding the disabling effect of their enduring mental illness. Several staff described time-consuming attempts to support service user applications for benefits, which were not always successful. When asked directly about the impact of their financial situation on their health, however, many participants with diabetes and SMI tended not to highlight their limited resources, perhaps normalising their constrained circumstances. By contrast, the impact of lack of employment and reduced economic resources on healthy dietary choices was discussed by several staff participants:
. . . patients with mental health . . . often are not in employment and are living on very very much reduced resources, and when you are discussing what foods to buy and prepare, often it’s very difficult for them to afford a lot of what you are asking them to [buy].
ES-PC-02, GP
Foregrounding mental illness
For all three participant groups, the effects of having SMI were perceived to overshadow the experience of diabetes and other physical health conditions. Participants spoke of diabetes self-management as being ‘governed’ by mental health (ES-T2-02, female, aged 63 years, bipolar disorder), or of mental health problems being ‘more all-consuming’ (ES-T5-03, nurse). Similarly, a family member noted that:
I know you’re looking at diabetes and mental health . . . but, from my point of view, the issues are, without a doubt, the mental health issues, not the diabetes.
ES-T2-17, male, aged 67 years, spouse
The foregrounding of SMI and associated overshadowing of physical health was particularly acute when experiencing a SMI relapse, as the following service user explained:
. . . the sort of GP service had tried to do a diagnosis, tried to tell me it [diabetes] was important, which was never going to have any impact, ‘cause when you’re mad as a hatter, you don’t take any notice. They’re just noise in a corner.
ES-T2-03, male, aged 51 years, bipolar disorder
Being unwell with mental illness was perceived to be a significant barrier to diabetes self-management, with some staff participants remarking that stable mental health was an important factor in physical health management. One participant suggested that, at times of poor mental health, successful self-management needed to be redefined:
If someone is unwell with their mental health they are just trying to survive . . . they don’t want to hear the advice on smoking and diet, they just need to get through the day, and if they are eating something, it’s a victory, not necessarily eating a healthy meal, it’s just eating a meal.
ES-PC-01, GP
Persistence and powerlessness
A dominant feature of their mental health problems for many participants was the persisting, unrelenting nature of their condition, which could leave them feeling powerless. One participant reported that their SMI is:
. . . something actually inside of your head all the time, and it’s like you’re carrying around mental illness . . . it’s just like having a cold, you want to get rid of it but you can’t. [. . .] it just keeps going round and re-playing and re-playing . . . and when am I going to get off the circle? And you can’t because it’s mental . . . I mean, it’s not like a diet . . . you can change your diet. You can’t change your mental illness.
ES-G3-01, male, aged 47 years, schizophrenia
Despite feelings of helplessness, participants described efforts to manage their mental illness, including engaging in physical exercise and practising religion. Most participants described taking at least one medication (often many more) for their SMI; for many, this was an unquestioned part of their lives. For some, however, this represented an unpleasant aspect of their daily reality, adding to their feelings of powerlessness as they disliked having to take medication but felt that they had no choice:
I mean, I take around 20 tablets a day . . . and I hate that, I hate that, I hate taking all of them, but it’s got to be done.
ES-T2-05, female, aged 59 years, schizoaffective disorder
. . . you wouldn’t take them if you had the choice. I mean, I am desperate, so I take my medication.
ES-T2-16, female, aged 65 years, schizophrenia
Theme 2: not just two illnesses – multimorbidity and diabetes management
Poor physical health and everyday life
Many participants with diabetes and SMI described living with multiple health conditions and problems, including cardiovascular and respiratory illnesses, such as angina, chronic obstructive pulmonary disease (COPD) and asthma; musculoskeletal problems, such as osteoarthritis or inflammatory arthritis; and other conditions, such as urinary incontinence, difficulties with eyesight, sleep problems or gastrointestinal conditions. Staff participants described the complexity of multimorbidity in terms of risk for the SMI and diabetes population:
I mean, definitely, the rates of the diabetic complications are higher in our group of SMI patients, as they will often have cardiac disease or cardiovascular disease at an earlier onset, I would say, than our non-SMI patients.
ES-PC-06, GP
This increased risk of multimorbidity could lead to polypharmacy, as one nurse noted:
. . . and normally if they are on diabetes tablets, they are on blood pressure tablets, and if they are on blood pressure tablets, they are on cholesterol tablets, if they are on cholesterol tablets, you know, they’ll be on something else . . .
ES-PC-04, practice nurse
Rather than describing their health conditions in terms of associated risk, participants with diabetes and SMI tended to illustrate how their health problems created limitations in their everyday lives. For one person, the combination of their conditions placed significant restrictions on their freedom:
My COPD only allows me to go to the bottom there, and I’m coughing and weeing myself and everything [. . .] I wear nappies now, I daren’t go out. I only go somewhere, where my scooter will take me.
ES-T2-02, female, aged 63 years, bipolar disorder
The constraints placed on people with diabetes and SMI by their poor physical health often affected their ability to exercise, and hence manage their diabetes, even when motivated to do so, as one person explained:
I’m waiting for a knee operation, I can’t get about as well as I used to [. . .] they won’t do the operation unless the leg muscle is strong. That is why I try and swim to keep the strength in the leg muscles. I walk as well. But unfortunately, I can’t do as much as I would like.
ES-D1-05, male, aged 69 years, psychosis
Precedence-taking in the context of multimorbidity
One of the key challenges for participants managing SMI and diabetes alongside other chronic conditions was deciding which to prioritise. Some staff described their patients as taking a ‘fire-fighting’ approach by attending to the problem that was causing the most difficulty at that moment:
The condition that’s causing the most immediate difficulty is going to be the one that gets the attention, so somebody has daily pain, that pain is going to take more attention than the diabetes, which isn’t causing any immediate pain but is a long-term complication and consequence. It’s a silent killer.
ES-T2-12, psychiatrist
Some participants described how they did not see immediate consequences when they did not manage their diabetes well. One participant, for example, did not feel the need to improve their diabetes management because they had ‘not had to hit that point yet’ (ES-G2-01, male, aged 45 years, bipolar disorder), and another emphasised the lack of feedback from the condition:
It’s a funny one, diabetes, I mean, you just don’t know until it’s too late how it’s affecting you.
ES-T2-16, F, 65, schizophrenia
This lack of visibility of diabetes may explain why physical and mental health conditions that are having a more salient effect on everyday life are prioritised.
Alternatively, having multiple health conditions may become so overwhelming that it feels easier not to manage any of them, as one family member observed:
I also think she gets quite confused, because she’s got things going on, not just the diabetes but she’s got other ailments, you know, [. . .] I think sometimes it will come as a bit overwhelming because she thinks, ‘well, there’s too many things that I’ve got to think about what I can and can’t eat. I’m just going to ignore it’, if you know what I mean.
ES-T2-08, female, aged 37 years, daughter
Theme 3: interacting conditions, overlapping symptoms
Some of the participant quotations in this section have been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Many participants had noticed an overlap in their conditions. People described struggling to identify whether their diabetes or their mental health was causing symptoms such as low mood, anxiety or fatigue, and some suggested that it could be a combination of the two:
. . . if I go on a high, sometimes, they’ve got to check my blood sugars, because they don’t know if it’s the blood sugars, that are causing me to go a bit loopy. Or it’s my mental illness.
ES-T4-10, male, aged 41 years, bipolar disorder
Many participants also stated that their conditions could affect each other, with one person describing a ‘direct correlation between the two’ (ES-G2-01 male, aged 45 years, bipolar disorder). This close link meant that poor diabetes control had a direct impact on participants’ mental health and vice versa:
Oh yeah, definitely, because you need to be careful what you eat, being overweight. I mean, that makes me anxious. That does upset me. And knowing that I’m diabetic, knowing I’m overweight, I am. That upsets me and that makes my anxiety worse and that makes the hallucinations worse. There’s definitely a connection.
ES-T3-09, female, aged 37 years, schizophrenia
One participant who had access to blood sugar monitoring equipment described seeing their blood sugars increase as a result of anxiety:
When I’m hyper, I’m, like, showing the meter to my partner, and stuff, and he’s like, ‘well why is that high?’ But it’s how my body reacts. It’s like as if the anxiety makes it go up as well.
ES-T3-03, female, aged 34 years, bipolar disorder
Not all participants with diabetes and SMI agreed that there was a link between their conditions; those who felt that there was no overlap or interaction mostly had a diagnosis of schizophrenia or psychosis. One participant described being uncertain if a link existed:
I don’t know. If my blood sugar’s dodgy, I do feel ill. But I’m not quite sure if there’s a link to my mental illness and diabetes really.
ES-T4-12, female, aged 41 years, schizophrenia
Staff participants had also noticed the interaction and overlap between diabetes and SMI, and suggested that symptoms such as irritability or manic episodes could also be linked to poor diabetes control, as one psychiatrist described:
And, on the other hand, if people are diabetic and they’re not maintaining their blood sugars right, it can give rise to symptoms which are synonymous with anxiety and low mood, so there is a big interface between diabetes and mental illness.
ES-T1-01, psychiatrist
A nurse also noted that behavioural symptoms could be interpreted by staff as a warning sign for poor diabetes control:
When it’s not managed well, when they’re not taking their insulin or taking their metformin when they should, when the blood sugars are high, they become more aggressive, argumentative, that’s often a sign that they’re not managing it properly and it might not just be a sign of their mental health deteriorating, because it could be a physical cause like diabetes.
ES-T1-02, community mental health nurse
This suggests that people with diabetes and SMI may be facing an additional barrier to effectively managing their diabetes, in that they need to understand the underlying cause of their symptoms before they are able to manage them, but this may be difficult or confusing. Most participants in our sample did not have access to blood sugar monitoring equipment, which could help to distinguish between symptoms of diabetes and SMI. If this group interpret symptoms as being linked to mental illness when they are actually physical, and vice versa, this could lead to conditions not being managed appropriately and exacerbating each other in a vicious cycle.
Mental health medication and diabetes management
As outlined in theme 1 (see Theme 1: mental illness affects everything), many participants in this study discussed the side effects of their antipsychotics and the impact that these had on their physical health and diabetes management. Participants, especially those with schizophrenia and psychosis, described extreme and rapid weight gain, as well as a constant hunger, as a consequence of their medication, which caused difficulties in diabetes management. Some participants felt that medication had caused their diabetes in the first place:
I got the schizophrenia and then I got the Clozaril [®; Mylan Products Ltd, Potters Bar, UK] and then I got the weight gain and then I got the diabetes.
ES-T3-09, female, aged 37 years, schizophrenia
Participants also emphasised the lethargy caused by SMI medication, which could affect people’s ability to manage diabetes:
It is and the medication that I’m taking. It makes me hungry and it makes me tired. Quite normal for me to have a couple hours’ sleep during the day, but if I didn’t take the tablets, I wouldn’t be snoozing like that.
ES-T2-18, male, aged 48 years, schizophrenia
Staff participants and family members also highlighted the issue of mental health medication and its effects on physical health and well-being. As one nurse explained:
. . . the medication is a big thing, they might feel slowed down, the less they do, so that adds to the weight gain.
ES-T1-02, community mental health nurse
A parent also described the extreme weight gain and ‘slowing down’ experienced by their son as a result of his medication:
. . . [son] weighed 79 kg; at his largest he went up to 180 kg from being on the mental health drugs, which is as a result of the type of medicine they give you. Because, you understand, the medicine slows down your mind. But it slows down all parts of your body.
ES-T2-08, female, parent
Theme 4: the effect of mood on diabetes management
Some of the participant quotations in this section have been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Fluctuating moods, fluctuating management
Participants described experiencing fluctuating moods that differed from SMI relapses, and were often part of their daily life. Low mood could lead to poor motivation, low self-esteem and pessimism, which, as participants explained, could derail their efforts to manage their diabetes and their health generally:
. . . it’s mostly the exercise, but it’s also diet as well. You just eat more poorly and put weight on, and it’s just all those things that you associate with depression, are actually also associated with poor physical health.
ES-G2-01, male, aged 45 years, bipolar disorder
Participants linked low mood and anxiety to lethargy, not wanting to exercise or engage in self-care, and a reluctance to leave the house, all of which affected diabetes management. Eating habits were prominent in participants’ accounts, with many people describing ‘comfort eating’ foods that they knew could negatively affect their diabetes when they felt anxious or had low mood:
I think definitely there is a link there, in if your blood sugar is low and you feel depressed or fat or you’re anxious or feeling paranoid, then you want comfort and I find that in naughty foods like crisps and chocolate.
ES-T2-05, female, aged 59 years, schizoaffective disorder
Family member participants had also noticed the effects of low mood on diabetes management, as the daughter of one participant with diabetes and SMI explained:
If she’s stressed or frustrated or angry or just not feeling 100%, she won’t be disciplined, especially with diet and what [she] should be doing to help, I suppose, control the diabetes.
ES-T3-08, female, daughter
Staff also acknowledged these links, and described low mood leading to diabetes taking a ‘back seat’, as one GP described:
I’ve seen patients who so, when their mental health deteriorates their eating deteriorates, so they may start to comfort eat, make the wrong food choices and so they lose their diabetic control. I’ve got a patient who, when her mother died, you know you can date things back, she said ‘I’ve got dreadful glycaemic control’ and this dates back to the death of her mother and she is still in a bereavement phase and the diabetes is just not important for her.
ES-PC-05, GP
More extreme drops in mood were also described, which could cause suicidal feelings and a sense of total hopelessness. At these times, participants did not look after their physical health at all:
. . . when I’m depressed, there’s nothing that you could say that would, ‘cause you stop caring, it’s like, if you stop caring about yourself, or what happens to you, it’s very difficult then, for someone to say, ‘well you need to stop eating these, these and these’. It’s very, very difficult.
ES-G2-01, male, aged 45 years, bipolar disorder
. . . so a lack of motivation, you know. ‘I don’t want to be here; therefore, why should I bother about this because I don’t care’. I forget the complications because I might not be here long enough to get them.
ES-PC-05, GP
A psychiatrist also outlined the ongoing difficulties that this link between low mood and poor diabetes management could cause further down the line:
They’re relapsing but they’re not risky. They’re like, well . . . just see your GP, would be the answer. In the meantime, they’re not eating or they’re eating chocolate for five meals a day, because it’s easy and they’re not sleeping any more. Their weight is ballooning or shrinking, they’re getting more physically unwell, their blood sugars are raging. That person is 6 months down the line and they’re acutely psychotic. At that point, they’ve got retinopathy, their blood sugars are all over the place, then you’ve given yourself two big problems to manage, what would have been one small problem.
ES-T2-12, psychiatrist
Worries about diabetes
Despite the foregrounding of SMI previously described, many participants with diabetes and SMI were aware of the severity of diabetes, and some reported that this caused them anxiety and stress, affecting their mental health more generally:
It does affect me badly because I worry. I worry about going to bed. I worry about sleeping because I think, ‘am I going to wake up or am I not going to wake up?’
ES-T3-03, female, aged 34 years, bipolar disorder
The accumulation of these worries could have a negative effect on participants’ mental state. A family member participant described the effect that the additional burden of diabetes had on the mental health of the person they supported:
. . . being diagnosed with diabetes and it’s another thing that she’s got to think about and, you know, sort of contend with. So, I do think it has affected her mental health in that it’s an additional thing for her to worry about.
ES-T3-08, female, aged 37 years, daughter
Theme 5: ‘the most critical person in my care’ – the role of informal support networks
The importance of family, friends and others
Many people with diabetes and SMI highlighted the role of family, friends and other informal supporters in helping them to manage their lives, emotionally and practically. Participants explained how these supporters provided essential emotional support as well as helping with everyday activities such as shopping, personal care and finances:
I go to church on a Sunday. My partner drives me to church and drops me and my daughter off while I’m at church and then picks me up. On a Monday, my mam and sister take me to Asda and then we go and have a coffee and then go and do our shopping. And then on a Tuesday and a Thursday, my mam’s now started taking me to the gym.
ES-T3-09, female, aged 37 years, schizophrenia
This type of support affected participants in a variety of ways. For example, one person described how their daughter could detect worsening mental health problems in their regular conversations and could ‘tell by my voice when I’m not right’ (ES-T3-04, female, aged 67 years, bipolar disorder), which would prompt her to arrange for professional support. Another participant described how sharing their difficulties helped them through a period of anxiety:
That’s what helped me through the anxiety a couple of years ago, was a girl at work who I was real close friends with, had said that she used to suffer from depression and anxiety [. . .] And she’d had the same thing, and talking to her, and I said, ‘how did you get through it?’
ES-G2-01, male, aged 45 years, bipolar disorder
Participants also described how their informal supporters were able to step in and support them with their self-management. One participant described how their parent was able to ensure that they had a ‘dosette box’ (a medication container with multiple compartments) to organise their medications:
I have a dosette box because, the second time being in hospital, they put me on all these tablets, I’m not on as many now, but I couldn’t cope with the . . . and my dad was really good, he sorted that out for me.
ES-T3-03, female, aged 34 years, bipolar disorder
The emotional support provided by family and friends was key for many people with diabetes and SMI. One person described how visiting the mosque with friends gave them ‘another motivation, [to] carry on’ (ES-G9-01, male, aged 38 years, schizophrenia). In extreme circumstances, this support could make the difference to participants deciding whether or not to continue living. One person summed up the huge impact and importance of this type of support:
Who helps me the most in continuing to be here at all? ‘Cause there’ve been occasions when that’s definitely been a possibility not to be true. My wife. So my unpaid carer, a 24-hour-a-day, unpaid carer, is the most critical person in my care.
ES-T2-03, male, aged 51 years, bipolar disorder
Family history of diabetes was also discussed by some participants, and family members with diabetes were sometimes able to provide additional support and knowledge based on their own experiences, as this participant described:
. . . my relatives are important as well because people with diabetes in my family, we discuss issues and medication and the best way forward to manage it, so they are important connections.
ES-T2-05, female, aged 59 years, schizoaffective disorder
Health-care staff too highlighted the ongoing and permanent nature of family support, compared with staff who ‘may think we’re very important but actually we may spend a couple of hours a week with the service user’ (ES-T6-01, mental health nurse). The different nature of peer relationships, compared with service user/staff relationships, was also acknowledged, with one staff member suggesting:
. . . they’re most probably more likely to listen to them [peers], than they would a professional.
ES-T1-02, community mental health nurse
Although many participants with diabetes and SMI highlighted the importance of informal support, some also discussed a lack or loss of support and the impact this could have. Several participants had relied on their parents for many years, for example, but, as their parents aged, they were now losing that support as a result of illness or death, leaving them isolated:
I’ve got family, although my mum’s passed away and my dad’s getting on a bit and he’s had heart surgery, so they can’t look after . . . they can’t help me any more, I’m on my own.
ES-T4-01, female, aged 29 years, schizophrenia
Participants also discussed losing support for other reasons, including the nature of their mental illness making social relationships difficult, poor physical health being a barrier to socialising outside the house and the loss of services they used to rely on such as day centres. One psychiatrist explained how a lack of support can make life more difficult for people with diabetes and SMI:
If they are lacking in the understanding, both from the physical perspective, but also from the emotional perspective, it makes it so much harder for the patient because the people immediately around them don’t get it.
ES-T2-12, psychiatrist
Although staff members acknowledged the vital role informal support plays in the lives of people with diabetes and SMI, they also discussed the potentially negative effects that family and friends can have on diabetes management. Staff participants highlighted examples of family members bringing unhealthy foods to hospital when visiting inpatients, or using food as a means of pacification when people became difficult. Staff and family member participants also identified the negative effect that a close supporter can have on motivation to manage diabetes:
If you’ve got somebody who is dismissive of the attempt or knocks it down constantly, then that can be very unhelpful. And if you’ve got somebody who’s encouraging them to get the takeaway or, ‘don’t bother with that, it’s raining outside’. If your own motivation is a bit shaky to begin with, that’s probably not going to help.
ES-T7-01, mental health nurse
. . . it’s easier having both of us having to watch our diet. If I munch away at huge bits of cake and he’d have to watch me – that would be awful.
ES-D1-02, female, aged 71 years, parent
The effects of being a supporter
Although the family members in our study made it clear that they loved the people they were supporting, and relationships were usually seen as reciprocal (with the label of ‘carer’ being forcibly rejected by two participants), interviews also demonstrated the toll that supporting someone with SMI and diabetes can take.
Family member participants explained how their supporter role had negatively affected their education, ability to work and their relationship with the person they supported. Some felt unable to have a break from their role, as they were reluctant to leave the person with diabetes and SMI for long periods. They also described times of crisis that had caused immense stress and upset:
I was waiting and waiting for a phone call back trying to calm him down, trying to stop him going out and, sort of, trying to meet his attackers or whatever, you know, with a knife in hand or whatever. You just don’t know what to do and you’re absolutely terrified for hours. And that shouldn’t happen, really.
ES-D1-02, female, aged 71 years, parent
Theme 6: diabetes health care
Some of the participant quotations in this section have been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Diabetes health care was an a priori theme identified as being of interest at the beginning of this study. Although nearly all participants reported receiving diabetes care, they tended to talk about it in simple and descriptive terms and were much less concerned with discussing their diabetes care than their mental health care. Most participants, when asked about diabetes care, talked about diabetes checks, which were most commonly carried out every 6–12 months, although some participants described having more regular appointments when necessary:
They’re 3 months at the moment, yeah. Three months at the moment, normally 6 months. [. . .] I do tend to go for the eye tests every year, and I do all the things that they ask me to do, and I do all the blood tests. And, if it’s more frequent, because they’re more worried, then I will go in.
ES-G2-01, male, aged 45 years, bipolar disorder
For participants in this study, diabetes care was predominantly carried out in primary care, with people reporting attending their diabetes checks with diabetes nurses or doctors at their GP’s surgery. These appointments were described as including a blood test, along with health checks such as weight and height measurements. Some participants reported being asked questions about their health more generally, and being given advice about diet, weight and exercise in these appointments, but not all received this. One participant described their experience of a typical appointment:
I have a blood test every 3 months, approximately, for the diabetes, specifically to look at my blood sugar levels, and I also have my feet checked and a chat with the nurse about the results, about a couple of weeks after the blood test, where we discuss the results and any changes that need doing vis-à-vis diet, medication or exercise.
ES-T2-05, female, aged 59 years, schizoaffective disorder
Despite the limited discussion around diabetes care, participants with diabetes and SMI did highlight a desire for more intensive diabetes management support, and explained how a lack of ongoing support and large gaps between diabetes checks could negatively affect their diabetes management:
. . . in diabetes, what you’re missing is the physiological feedback, and a consultation can, to a degree, give you some of the feedback that you lack on a minute-to-minute basis; even if it’s only three times a year. [. . .] Because you can lose the plot over the course of a year, whereas I think if you have an horizon of 4 months, that gives you an end point in sight.
ES-G8-01, male, aged 63 years, bipolar disorder
Another participant with diabetes and SMI described his desire for more support for people with diabetes for financial budgeting and dietary planning to help with self-management:
It should be a lot more help. Not just from GPs and nurses, but there should be teams going out into communities, and people what are really overweight and really obese, they should be sitting them down and going over a budget plan and a plan to lose weight.
ES-G3-01, male, aged 47 years, schizophrenia
One GP described how this type of more intensive, ongoing support could lead to great successes in diabetes management for people with SMI:
I think most of it, the credit will probably have to go to the health-care assistants that we’ve got who often see these patients almost week in, week out, monitoring weight and blood pressures and that type of thing, and just able to develop some really really nice relationships of, kind of, trust and really plugging away at the health understanding behind, kind of, some of the lifestyle management with diabetes, and we’ve had some really good successes in terms of really impressive weight loss.
ES-PC-06, GP
Theme 7: diabetes knowledge and education
Knowledge of diabetes
Knowledge of diabetes and diabetes management among participants with diabetes and SMI varied widely; some were very knowledgeable, whereas others gave incorrect information or simply said that they did not know about it:
I don’t know nowt about diabetes, and understand it.
ES-T2-02, female, aged 63 years, bipolar disorder
Staff participants also identified the variation in knowledge among the people with diabetes and SMI that they worked with:
I think there is a huge variation, some people are very engaged in their health and will often know more than you do about, you know, diet [. . .] but you always have this big group that know what they should be doing, but just don’t do it for whatever reason, and, some people, they have so many other problems that it is just a last thing on their minds.
ES-PC-01, GP
Staff members highlighted a lack of knowledge about healthy diets among people with diabetes and SMI whom they encountered, especially in relation to diabetes, for example those who ‘think that they can eat as much fruit as they want’ (ES-T5-02, mental health nurse). Staff participants’ expectations of knowledge among their patients also varied, with one nurse suggesting that:
. . . even with the people with mental health issues [. . .] People know what they should be eating and what they shouldn’t be eating these days.
ES-T5-03, nurse
Another, however, acknowledged that these kinds of expectations may be too high for people with SMI, and identified barriers to what may usually be considered general knowledge:
I thought [it] was general knowledge, but perhaps isn’t, perhaps, actually, it’s a lot to do with educational opportunities, having family that eat healthily, whatever.
ES-T2-15, psychiatrist
Sources of information
Participants with diabetes and SMI reported that the information they had received about diabetes came from various sources, both formal (e.g. from health-care professionals, NHS leaflets or education courses) and informal (e.g. family members):
I’ve done my own research on YouTube and asking people who’s had diabetes, or people who know what good food are [. . .].
ES-G3-01, male, aged 47 years, schizophrenia
[At diabetes appointments] They give me booklets about sugar diabetes and things like that. They tell me what to eat.
ES-T2-04, female, aged 68 years, schizophrenia
Family members also provided participants with diabetes and SMI with information. Some participants, for example, had family members with diabetes who shared their own tips on management, whereas other family member participants reported educating themselves on diabetes to provide better support.
Although many participants with diabetes and SMI reported receiving information about their diabetes, the varying quality of this information was identified as a concern by staff participants:
I mean one of my clients does use an online forum where she can kind of go for support, but the problem, as I say, in some ways with online is who is monitoring that? Who is providing the information, you know? How accurate is it?, and so forth.
ES-T1-05, nurse/care co-ordinator
Several participants reported receiving information about healthy diets and achieving weight loss from commercial programmes such as Slimming World (Alfreton, UK):
I’ve done all sorts of diets in the past and if you’re on your own, you don’t lose well. So yes, they took me [to Slimming World]. And I’ve lost nearly two stone.
ES-T3-07, female, aged 71 years, bipolar disorder
One nurse participant, however, highlighted how the dietary information given in these groups may be misleading for people with diabetes:
. . . even, like, being at Slimming World, you know, you’ll get people that come in and they’ll say, they have, like, a great big bowl of pasta with cheese on and it’s just like, ‘yeah you can have as much pasta as you like’, no you can’t. There’s only certain amounts of pasta you should actually be eating.
ES-T5-03, nurse
In contrast to many comments from staff participants about the types of information they provided about diabetes, and the varying ways in which they attempted to educate people on diabetes management, many participants with diabetes and SMI in our study reported receiving only perfunctory information such as leaflets or links to websites that they had to navigate themselves, rather than explanations that helped them understand their condition:
He [GP] never really sat me down and explained. I got all the leaflets and things, but I’m not one to read such things.
ES-T2-06, female, aged 59 years, bipolar disorder
This discrepancy between staff and service user reports on the provision and usefulness of different sources of information may indicate a need for a more considered approach to providing diabetes management information.
Diabetes education courses
Participants with diabetes and SMI described a range of experiences of formal diabetes education: some had attended formal courses; some had been offered a place on a course but were unable to attend; and some reported that this had not been discussed, or offered to them. Participants who had attended courses gave positive feedback, mentioning the valuable information they had gained about diabetes and its effects, as well as how to better manage the condition, for example through diet and exercise:
And that was by far and away the best thing and the most useful thing, in terms of management. [. . .] it was the intervention of the community trust, with their education, that was provided by a dietitian, that really, really, made a difference.
ES-T2-03, male, aged 51 years, bipolar disorder
[From interviewer notes] She reported having ‘zero knowledge’ before the course, it opened her eyes to diabetes, what it is, what it can do, and how it can be controlled. It was really interesting, and was successful because it was a friendly atmosphere where everyone wanted to join in.
ES-T6-05, female, aged 39 years, schizoaffective disorder
Despite this positive feedback, some specific barriers to attending education courses were identified by staff participants, although very few participants with diabetes and SMI discussed these. One GP suggested that the group-work element of diabetes education courses could cause difficulties for people with SMI, for example:
. . . often, I think, a barrier to the structured education is the group work, so I don’t know if it would help if the group was people with similar other comorbidity, so other mental health problems, or whether a bit more one to one.
ES-PC-05, GP
This was echoed by a participant with diabetes and SMI, who explained that ‘the actual getting out of the house and mixing, is a bit nerve-wracking’ and who had found they were ‘scared to death’ and ‘a shaking mess’ (ES-T3-09, female, aged 37 years, schizophrenia) when they did attend an education class. One participant with diabetes and SMI also discussed the fact that he had been offered an education course and was prevented from attending by his SMI symptoms:
Now, I was offered that by the GP service, and I went, ‘no thanks, I’m too busy being mad’.
ES-T2-03, male, aged 51 years, bipolar disorder
Staff participants suggested that the duration of sessions or complexity of content may be difficult for some people with SMI as a result of literacy issues or problems with concentration, and one GP highlighted a lack of tolerance a person with diabetes and SMI had experienced at an education class:
And our services users classically tell us that some of them have problems with literacy, so they go along, they can’t read the information. The information is information overload. If you think that a lot of our service users have problems with cognition, with attention, with cognitive deficit. They can’t take on board the information; they don’t understand the information.
ES-T6-01, mental health nurse
I sent a chap on a DESMOND [Diabetes Education and Self Management for Ongoing and Newly Diagnosed] course [T2DM education programmes provided by the NHS] recently, you know the diabetes education course, and he got kicked off because they didn’t like his behaviour, well you know he’s a chap with schizophrenia and actually even getting him there was massive.
ES-PC-06, GP
These findings suggest that there is a need for a more tailored approach to diabetes education for this group, both for the provision of information and in structured education classes.
Getting everyone on board
Staff participants discussed the importance of diabetes management education for supporters and family members of people with diabetes and SMI. Participants gave examples of family members who were not aware of aspects of diabetes management, such as blood glucose monitoring or dietary requirements, for example. One staff member highlighted a parent who said they ‘know nothing about diabetes really’ (ES-T3-02, community mental health nurse), despite having diabetes themselves.
A GP participant highlighted the important role that informal supporters play in helping people with diabetes and SMI to manage their diet, medication and lifestyle, and emphasised the need for this group to be educated on the topic:
I think that it is important that they have the awareness and understanding of diabetes as well, for the importance of it. The education as well, they might be the ones cooking, so just make the health choices, understand if they are blood glucose testing you know the values, when to be worried, when to react, sometimes teaching the family member to give insulin. They need as much education as the patient.
ES-PC-05, GP
Theme 8: person-centred care
Some of the participant quotations in this section have been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The value of person-centred care
Participants with diabetes and SMI identified many different health-care professionals whom they saw for their health conditions and expressed the most positivity when talking about professionals who focused on them as a whole person, understood their life circumstances as well as their multiple conditions and responded to emotional and social difficulties that fell outside their diagnosed condition management. This type of care is referred to as ‘person-centred care’ in the findings that follow, although it encompasses wider concepts such as continuity of care and relationships with staff who know people’s histories and the multiple challenges they face, and have the time and knowledge to help navigate complex systems.
To illustrate the person-centred care they valued, participants tended to focus on the informal conversations and relaxed interactions with these care providers, rather than the more practical or health-related aspects of their care. This emphasised the value they placed on having a relationship with their health-care provider(s):
My care co-ordinator’s brilliant, she comes every fortnight and we’ll just out for a cup of coffee and walk around the garden centre.
ES-T3-09, female, aged 37 years, schizophrenia
[About community psychiatric nurse (CPN)] We have a chat, see how I’m doing, she’s very supportive, sometimes she tells us about her family to give us a bit of amusing gossip.
ES-T4-02, F, 63, schizophrenia
Continuity of care was also described as important by participants, as it allowed them to build these relationships with health-care staff:
[About nurse] Yeah, she was great. [. . .] She was absolutely fantastic. She could tell . . . When we said hello to each other, she could tell my mood straight away. [. . .] She asked me in depth about the problems, the real problems and what was at the back of them. Sometimes she would recommend some medication to the psychiatrist. And that was a big help. I knew she cared. I knew she was listening, because there was a follow-up, so to speak.
ES-D1-05, male, aged 69 years, depressive psychosis
A family member participant described the difficulties they had experienced when there was a lack of continuity of care:
We’ve got a whole team of doctors down here – you never get to see the same one twice, but you used to just have one face, GP you knew really well [. . .] There is a lot of difference between somebody knowing you and just seeing different people each time.
ES-D1-02, female, aged 71 years, parent
Participants with diabetes and SMI described the powerful impact that person-centred care, focused around positive relationships, could have on their mental and physical health, as well as the support they received from valued care providers for wider issues such as navigating the financial benefits system:
Most people over the years have always been supportive [. . .] And all these services are very good because, without these people, we would be a lot worse than what I am now. [. . .] Without these people, people wouldn’t be sane.
ES-T5-11, male, aged 59 years, schizophrenia
When it first came out, when they were first looking at benefit, it was looking like I would have to go for interview. And whoever was my consultant psychiatrist at the time intervened, so I never went for an interview.
ES-T2-16, female, aged 65 years, schizophrenia
Although the importance of building relationships between health-care providers and people with diabetes and SMI was emphasised by participants, the lack of time available to build these relationships was also identified as a barrier to this type of person-centred care. Participants acknowledged the time pressures health-care staff face and the limitations this puts on care, while expressing their disappointment that this was the case:
Because when you go to your doctor you’ve got 10 minutes, and that’s what the problem is. Many of the guys [people with SMI], by the time they’re reaching the stage, like, you’re talking, guys with diabetes, they’ve got lots of things going on with them. And they’re trying to tell the doctor and it becomes like . . . So the doctor doesn’t give them . . . he just says ‘come for your annual diabetes check, come for your tablet, we have to check your liver and your kidneys. We’ll do that once a year’.
ES-T2-08, female, parent
I think GPs and practice nurse haven’t got the time to give that support, and it’s nothing against them, they just . . . their workload is so under pressure, do you know what I mean?
ES-T3-03, female, aged 34 years, bipolar disorder
These findings illustrated a difficult dichotomy in care for this group. People want and value person-centred care from the professionals who treat them and the trust that this brings, but this may not always be possible within the restrictions of the current health-care system.
Separation of mental and physical health care
Despite valuing person-centred health care, participants with diabetes and SMI often viewed health-care professionals as having defined roles based in either physical or mental health and described seeking help on this basis. Some participants chose to keep these areas separate, despite acknowledging the links between the conditions. One participant, for example, felt that she would be wasting her GP’s time discussing mental health:
I’m seeing my GP this Wednesday with physical things. And the two will interact, but I can’t go and sit in a GP’s surgery and go on about my paranoia because it would take up an hour of a GP’s time and that’s not fair, not fair on the GP, not fair on the other patients. So I don’t do that.
ES-T2-16, female, aged 65 years, schizophrenia
Other participants felt that mental health teams did not have the knowledge or understanding of their physical conditions and vice versa, and that health-care professionals focused on their own areas:
Their focus is on diabetes, yeah. Whenever I do anything that’s specifically for diabetes, obviously, on the blood tests through the GP, and the results go through the diabetic nurse at the GP’s surgery, but when I go for the eye test and things like that, they never ask me about mental health problems, it’s never considered.
ES-G2-01, male, aged 45 years, bipolar disorder
I don’t think the mental health side of things are necessarily that clued on diabetes.
ES-T3-09, female, aged 37 years, schizophrenia
Many staff member participants also recognised the separate nature of mental and physical health services and the lack of joined-up care that they saw in their practice:
I suppose it’s everybody is managing a certain part of that person’s either physical or mental, you know, needs but not really coming together to discuss it in a more holistic approach.
ES-PC-02, GP
I mean, we’re aware of the impacts of the comorbid conditions, but we don’t tend to actually deal with those, other than via the GP.
ES-T3-05, psychiatrist
Some participants, however, did feel that there was crossover in their care. One participant, for example, suggested that, although her doctor was just trying to find out the ‘one thing that’s wrong with you’, her mental health team understood that ‘it is a big picture, that it is the diabetes and everything’ (ES-T3-03, female, aged 34 years, bipolar disorder). Several people also suggested that their GP had knowledge and understanding of their mental health conditions.
It was clear from many comments from staff and participants with diabetes and SMI that physical and mental health-care systems were viewed and functioned, in the main, as separate entities.
Co-ordinating care
All participant groups talked about poor co-ordination of care and limited information-sharing between services as contributing to the separation of care for mental health and diabetes. Staff discussed experiencing time delays in getting health information about people with diabetes and SMI, and the difficulties of having to get access to information through GPs as they could not access it directly:
. . . if we are sending the bloods from our site, then they come to us, so can see blood sugars and we’ll have access to that. But the diabetic plan, as such, we don’t have access to it and it goes to the GP.
ES-T1-01, psychiatrist
One community mental health nurse also highlighted how people with diabetes and SMI were often the only source of information available on their own physical health treatments:
No, you just find out from the patient, you don’t get any information passed back to you. It’s when you say to them ‘how comes you’ve come on metformin? When did this happen?’ that you find things out.
ES-T1-02, community mental health nurse
The lack of co-ordination between mental and physical health care was particularly highlighted by staff participants, despite their awareness of the links between SMI and physical health. One member of staff described the risk of health-care professionals becoming ‘blinkered’ by remaining ‘within their own branch’ (ES-T1-05, nurse/care co-ordinator) and identified the importance of taking in all available information to provide the best possible care. Another staff member described health providers’ responsibility to understand the nature of difficulties experienced by people with SMI and diabetes:
Everyone who’s involved with that has responsibility to understand both sides. The psychiatrist needs to understand the physical impact of both the condition and the treatment. The GP needs to understand the interactions between the two. Any physician, medical professional, acute hospital needs to understand that having the two together is going to make things more complicated. There isn’t a uniform answer, that one-size-fits-all approach.
ES-T2-12, psychiatrist
Family member participants also described experiencing difficulties during their involvement in the care of people with diabetes and SMI and their frustrations at dealing with a system that they felt did not always understand the links between the conditions:
And I’m saying why if psychiatry caused the diabetes, why must be the GP be doing it? Why is mental health not interested enough to see what’s going on with the guys? Because very seldom does your GP talk to your psychiatrist. Maybe once in a while. They don’t even share the same computer system.
ES-T2-08, female, parent
Now I remember saying to the hospital, ‘look, my wife has mental health issues. Please make sure there is provision’. And you get the impression that . . . just in one ear and out of the other. No mental health provision. No-one came to see her with regards to that issue.
ES-T2-17, male, aged 67 years, spouse
These findings suggest that the lack of co-ordination in health care for people with diabetes and SMI may often occur at a systemic level, with many staff participants being aware of and frustrated by the inability to deliver the person-centred care that was identified as being the most valued by all parties.
Training needs
Many staff member participants identified their own lack of knowledge as a barrier to delivering person-centred care. Physical and mental health-care staff participants identified gaps in their training that would help them deliver care that accounted for the way conditions interacted and affected each other.
Several mental health support staff in our sample, for example, suggested that they would benefit from an improved understanding of diabetes care to better help people with diabetes and SMI to manage their conditions:
A better understanding of the types of diabetes, how to look for sign and symptoms of diabetes because it’s a bit ad hoc, what I remember and just generally managing it [. . .] So, it would be beneficial to all of us to have some education on it even if they’re not nurses, anybody.
ES-T1-02, community mental health nurse
It was also suggested by some staff participants that physical health staff could benefit from having a better understanding of mental health to improve care:
I think it’s very easy to focus on physical side of things, but the mental health is just a massive side of it and [. . .] I think if we were more trained and more aware of the mental health of a patient, I think it could probably alleviate a lot of the problems for both patients and the staff.
ES-PC-04, practice nurse
Examples of diagnostic overshadowing, whereby physical symptoms are attributed to a mental disorder rather than a somatic one, were given by participants with diabetes and SMI and staff member participants. These accounts highlight the consequences of diagnostic overshadowing and further illustrated the need for an understanding of both mental and physical health among health-care staff:
I’ve had patients on the wards who have complained endlessly about abdominal pain and it’s put down as delusional. When they actually collapse with a perforated bowel or ruptured appendix [. . .] or worse, then it’s like ‘oops, we didn’t look at that’.
ES-T5-03, nurse
I don’t know, he [GP], probably thinks, because I’ve got a mental illness, I’m just making everything up. Because I’ve faced that a lot over the years.
ES-T4-12, female, aged 41 years, schizophrenia
Finally, several staff participants noted the importance of any training that they did receive being mandatory and encouraged by management, or it would be unlikely to be attended because of time pressures.
Summary of findings
Themes from the qualitative enquiry, which were taken forward to the mixed synthesis (see Chapter 7) can be summarised as follows:
-
Mental illness affects everything.
-
Managing mental illness and diabetes is not just about coping with two illnesses – there are additional problems for diabetes management in multimorbidity.
-
The conditions interact and have overlapping symptoms.
-
Mood influences diabetes management.
-
Informal support networks are important, and often lacking.
-
There are gaps in diabetes health-care provision (a priori theme).
-
Diabetes knowledge and education vary (a priori theme).
-
Person-centred care and continuity of care are valued.
Chapter 7 Integration of quantitative and qualitative findings: understanding the relationship between severe mental illness and diabetes
Introduction
A key component of the mixed-methods concurrent triangulation study design involved the convergence of results from the quantitative and qualitative studies. In this chapter, we present the integrated themes that represent the study findings. First, the methods for integration are described, and the challenges to achieving this are discussed.
Methods for integration
The primary method for integration was ‘convergence’, which involved bringing together the results from each study to generate a more complete understanding of the relationship between SMI and diabetes. Co-design workshops formed part of this process to ensure that study findings, and the integration of these, were interpreted by service users and health-care providers, in addition to the research team. ‘Embedding’ techniques were also used to ensure that, when possible, each study informed the other throughout the research.
Embedding
Although we used a convergent design, in which the integration primarily occurs when the study findings are brought together, we also considered how the studies could be integrated at the data collection and analysis stages.
The following strategies were employed:
-
The scoping of the literature and expert consultation (see Chapter 4) informed the selection of a priori variables to explore quantitatively, qualitatively or in both studies when this was deemed important and was possible. For example, sleep was identified as an important determinant of diabetes self-management, but was not possible to explore reliably in CPRD data, and instead was added as a topic for the qualitative study.
-
We had anticipated that the sampling criteria and interview topic guides for the qualitative study would be informed by the quantitative analysis for study objectives 1–3. However, this was not possible because of delays in obtaining CPRD data and operationalising key variables. Instead, we used preliminary analyses of a smaller CPRD data set carried out in preparation for the study to explore population characteristics to inform the sampling criteria. 115
-
Emerging results and observations from each study were discussed at each project meeting to identify opportunities for exploring these further; for quantitative results, using qualitative inquiry; and for qualitative findings, using quantitative interrogation of health records.
Co-design workshops
Two multistakeholder co-design workshops were used to make sense of emerging findings, to explore the potential for additional integration during analysis (e.g. re-analysing qualitative data to explain the finding from a quantitative model) and to situate the findings in the context of policy and practice from the perspective of both service users and providers. Owing to time delays in the quantitative study, the workshops primarily focused on emerging qualitative findings.
We invited study participants who had expressed an interest in the workshops and interested stakeholders who received newsletters about the study and wider research programme. To ensure service user engagement at the workshops, we also invited DIAMONDS VOICE members, who had an existing relationship with the study team and who helped to facilitate small group discussions and offered feedback during whole-group sessions.
Outputs from the workshops were used to help interpret the qualitative findings and were discussed with the study team to ascertain whether or not we could explore key points raised in the workshops in the quantitative study (e.g. the relationship between depression and diabetes outcomes). They also formed part of the final integration of study findings (see Convergence).
Workshop 1
Workshop 1 was attended by six service users and focused on learning more about two qualitative findings: (1) that mood affects diabetes management and (2) that it can be difficult to tell whether symptoms are due to SMI or diabetes. To explore the first finding, we presented two personas (David and Barbara; see Report Supplementary Material 16), based on findings from the qualitative study. We used an empathy map (see Report Supplementary Material 17 for an example) to discuss and record what the personas might think, do, say and feel when their mental illness affected them and how this affected their diabetes. For the second finding, we presented quotations from the study about the uncertainty regarding symptoms, and asked participants to write down feelings and symptoms on sticky notes and place them on a board to indicate whether they were associated with diabetes, mental illness or both.
Workshop 2
Workshop 2 was attended by nine service users, two informal supporters and seven staff (from services that support people with SMI and diabetes). We wanted to learn more about care provision for people with SMI and diabetes, in part to make sense of the passivity we had noted from participants about diabetes care, but also because of the limited data for exploring diabetes care in the CPRD (due to the unreliability of referrals data for diabetes education and specialist services).
We presented findings about care experiences and described the challenges to exploring the complexity of care provision in the quantitative study. Using a large board that represented 11 different types of health and social care professionals highlighted in the qualitative study, we asked participants to map their expectations for care against these in relation to SMI, diabetes, and health and well-being, and then to map their experiences (for an example, see Report Supplementary Material 18). In the final session, we asked participants to draw on their discussions to identify three changes that would improve diabetes care for people with SMI. In this workshop, service users and carers/informal supporters worked separately from health-care staff to explore any differences in expectations and experiences, and to enable service users to discuss their experiences together.
Convergence
To develop our understanding of the relationship between SMI and diabetes in this final phase of the study, we used principles of both convergence, which involved comparing and contrasting the study findings, and transformation, which involved translating the study findings into descriptive summaries and merging these when possible. 105 This final integration was underpinned by the study’s social inequalities theoretical framework, which considered how social and economic disadvantage can contribute to worse physical and mental health.
In practice, this process entailed a full-day workshop attended by the whole study team, and subsequent analysis of workshop outputs and discussions. Specifically, this involved (1) producing narrative and tabular summaries of findings from both studies, which were reviewed by the whole study team prior to the integration workshop; (2) mapping and synthesising study findings from the quantitative and qualitative studies to the study objectives in the integration workshop and subsequently into a table to allow for comparison (Table 27); (3) identifying key themes across the study findings and discussing these within a social inequalities framework in the workshop; and (4) developing the themes and writing integrated explanatory summaries for these to ensure that all findings, including any contradictory or unusual findings, fit within this framework. Although themes were derived through the integration process, they were not selected based on their potential for integration, but rather on their importance for understanding the relationship between SMI and diabetes, and the poor diabetes outcomes experienced in this population.
| Objective | Description | Quantitative data | Qualitative data | Conclusion |
|---|---|---|---|---|
| 1 | In people with SMI, to identify which sociodemographic, illness, family history and lifestyle factors are associated with the development of diabetes |
|
|
|
| 2 | In people with SMI and diabetes, to identify which sociodemographic, illness, family history and lifestyle factors are associated with variation in diabetes and mental health outcomes |
|
|
|
| 3 | In people with SMI, to compare health-care interventions and physical and mental health outcomes in those with diabetes with those of people without diabetes |
|
|
|
| 4 | In people with diabetes, to compare health-care interventions and physical and mental health outcomes in people with SMI with those of people without SMI |
|
|
|
| 5 | To understand the factors that are associated with access to, and receipt of, diabetes care for people with SMI, and to explore the experience of diabetes health care by people with SMI | This was a qualitative research question exploring personal experiences. The quantitative research was not able to address this objective | Key themes:
|
Experiences differed between participants; for some, having diabetes and a SMI is linked to numerous mental and physical barriers to self-care and diabetes self-management. The importance of personalised support from people who know you and understand all your conditions was emphasised |
| 6 | To compare diabetes care provision for people with and people without SMI, and to estimate costs for these |
|
|
|
| 7 | To identify which health-care interventions may be associated with better diabetes outcomes for people with SMI and diabetes |
|
|
Ongoing, regular support and continuity of care were discussed in the qualitative study as being key to diabetes and mental health management. The fluctuating nature of mood (not just SMI symptoms) and the close links between mental state and diabetes management suggest that closer monitoring of this group could help improve self-management and outcomes |
Results
Six themes were identified from the integration process. These are described in the following sections, with reference to individual study findings when relevant.
Diagnostic overshadowing
From the analysis of patient records, we found that, despite people with SMI and diabetes having more frequent contact with their GP and more diabetes checks (e.g. cholesterol, blood pressure, HbA1c, BMI) than people with diabetes alone, they still have a much higher risk of mortality (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results). We also found that, although the rates of macrovascular and microvascular complications were similar in both groups (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results), people with SMI are more likely to present to A&E for treatment (i.e. to have unplanned care) than people with diabetes alone, who have higher rates of elective care (see Chapter 5, Regression results for the impact of severe mental illness status on physical and mental health outcomes, mortality and health checks). Both of these findings suggest that, although there is a greater opportunity to detect and treat diabetes complications in people with SMI, this is not happening. Findings from the qualitative study suggest that diagnostic overshadowing, which occurs because of the foregrounding of SMI (see Chapter 6, Theme 1: mental illness affects everything) in a health-care system that treats mental and physical illnesses in silos (see Chapter 6, Separation of mental and physical health care), may help to explain this.
The qualitative study also found that diagnostic overshadowing is influencing patient, as well as staff, behaviours. So, although participants in all three groups advocated for care that was person-centred and co-ordinated (see Chapter 6, Theme 8: person-centred care), and described the separation of physical and mental health services as a barrier to this, they also expressed beliefs that primary care treats physical health and mental health services treat mental illness. Participants with SMI and diabetes also described encounters with staff during which these beliefs influenced what they spoke about as patients (see Chapter 6, Separation of mental and physical health care). Staff accounts reflected these beliefs too: although they agreed that SMI and diabetes interact, limited time and resources tended to restrict their focus according to these beliefs (see Chapter 6, Separation of mental and physical health care). Lack of training in both conditions reinforced the practice of focusing on a single condition (see Chapter 6, Training needs), and, in the co-design workshops, participants recommended that all health-care staff be trained in multiple conditions and multimorbidity.
The foregrounding of SMI affected patient and staff behaviours further, as they focused on addressing the greater impact of psychosis relative to diabetes (see Chapter 6, Foregrounding mental illness), which included addressing social issues, such as housing and benefits (see Chapter 6, The pervasive effect of severe mental illness). This, and the difficulties attributing symptoms to either illness (see Chapter 6, Theme 3: interacting conditions, overlapping symptoms), resulted in the symptoms of diabetes being hidden, and, for many participants, becoming a lesser priority. This may explain why we obtained such limited responses about diabetes care from service user participants (see Chapter 6, Theme 6: diabetes health care), which contrasted significantly with the detailed accounts of mental health care.
Depression and anxiety
In the context of SMI, this study suggests that, like diabetes, depression and anxiety may be overlooked because of the focus on managing and preventing psychotic symptoms, and living with the experience of these symptoms (see Chapter 6, Theme 1: mental illness affects everything). Depression and anxiety were highlighted by participants as being difficult to attribute to SMI or diabetes (see Chapter 6, Theme 3: interacting conditions, overlapping symptoms), and this was confirmed by co-design participants who consistently linked mood symptoms to both conditions. There was also a suggestion that these symptoms may not be prioritised for treatment (see Chapter 6, Fluctuating moods, fluctuating management); when we asked about this in the co-design workshop, service users reported generally not seeking help for low mood, and clinicians agreed that depression is not always considered as a potentially separate condition to manage.
This additional overshadowing is important, because many service user participants in the qualitative study described low mood, anxiety and stress as recurring features in their lives (see Chapter 6, Theme 4: the effect of mood on diabetes management), in addition to the symptoms of psychosis and mania that were associated with a relapse. In the quantitative study, we found that people with SMI who took antidepressant medications had an increased risk of macrovascular complications (see Chapter 5, Regression analysis results for the impact of type 2 diabetes on health outcomes and health-care interventions), even though having depression and anxiety was associated with better attendance for health checks that could help to prevent such complications (see Chapter 5, Regression analysis results for the adjusted impact of severe mental illness on levels of physical health checks). We also found that people with SMI and diabetes had an increased risk of depression and anxiety, compared with people with diabetes alone (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results). This risk was highest for those diagnosed with diabetes before SMI; for these patients, their SMI diagnosis was more likely to be depression and psychosis, rather than other types of SMI (see Chapter 5, Objective 2: factors associated with variation in diabetes and mental health outcomes in people with severe mental illness and diabetes, Results).
Service user participants seemed to relate their anxiety and low mood to worries about diabetes (see Chapter 6, Worries about diabetes) and the impact of poor physical health on their lives (see Chapter 6, Theme 3: interacting conditions, overlapping symptoms). At the same time, depression and low mood were perceived to contribute to worsening physical health, through lower motivation to manage diabetes and other health problems (see Chapter 6, Fluctuating moods, fluctuating management).
This complex relationship between depression and anxiety, SMI and diabetes suggests that low mood may result in poor diabetes outcomes in people with SMI and may help to explain why people with both conditions are more likely to experience a SMI relapse. Low mood is more likely to occur in people who are marginalised and less likely to be noticed in primary care, and people with SMI who develop T2DM are more likely to live in the most deprived neighbourhoods than those with SMI who do not develop T2DM (see Chapter 5, Objective 1: factors associated with the development of diabetes in people with severe mental illness, Results). This relationship may form part of the mechanism linking poor social conditions and worse physical and mental health.
Better diabetes control, or hidden fluctuation?
We found that, although people with SMI and diabetes have poorer outcomes, including increased risk of mortality, than people with SMI alone and people with diabetes alone, they consistently have lower HbA1c and blood pressure levels (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results), which are indicative of better diabetes management. Our integrated findings offer three potential explanations.
The first explanation relates to diagnostic overshadowing (see Diagnostic overshadowing); despite there being a greater opportunity to detect problems [through more testing and contact with GPs (see Chapter 5, Objective 6: comparing diabetes care provision and estimating health-care costs for people with and people without severe mental illness, Results)], there is disproportionately less action taken, with the consequence of underdiagnosis of complications and delayed referral to specialist services for people with SMI (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results). The second explanation is that diabetes control may fluctuate more widely in people with SMI because of the additional challenges to managing their diabetes. Such fluctuations would not be evident in primary care records without more regular monitoring of glucose. Co-design participants recommended glucose monitoring as a future intervention for people with SMI and diabetes to help increase awareness of whether a symptom was due to diabetes or SMI, and to improve diabetes management. The third explanation is that people with SMI who have the greatest risk of poor outcomes from diabetes may be the least likely to attend diabetes checks and receive specialist diabetes care. For example, we found that, among those with macrovascular complications, people with SMI were less likely to receive health checks than people with diabetes and no SMI (see Chapter 5, Objective 7: identifying health-care interventions associated with better outcomes for people with diabetes and severe mental illness, Results). Moreover, people with SMI and diabetes had more psychotic relapses than those without diabetes (see Chapter 5, Regression analysis results for the impact of type 2 diabetes on health outcomes and health-care interventions), and we know from the qualitative study that psychotic symptoms can lead to a foregrounding of mental illness and feelings of helplessness, and negatively affect self-care, engagement with services, social support and health management (see Chapter 6, Theme 1: mental illness affects everything, and Theme 5: ‘the most critical person in my care’ – the role of informal support networks).
Tied intimately to these potential explanations is that social and economic deprivation contribute to the health gap. People with SMI living in deprived areas or who are of black or Asian ethnicity were more likely to develop diabetes, and these factors were the strongest predictors of developing diabetes (see Chapter 5, Objective 1: factors associated with the development of diabetes in people with severe mental illness, Results). Compared with controls with diabetes only, people with SMI and diabetes lived in more deprived areas, which was a significant predictor of worse health outcomes (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results, Descriptive statistics). In addition, social deprivation was associated with fewer health checks (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results).
Multimorbidity
Both studies highlight the importance of situating comorbid SMI and diabetes within the framework of multimorbidity. People with SMI and diabetes had more comorbidities than people with SMI and no diabetes; this included depression and anxiety as well as physical illnesses such as cardiovascular disease and hypertension (see Chapter 5, Objective 3: comparing health-care interventions and health outcomes in people with severe mental illness and diabetes with those in people with severe mental illness without diabetes, Study population). When controlling for other key variables, comorbidities were also associated with both the onset of diabetes in people with SMI (see Chapter 5, Regression analyses results for the risk factors for development of type 2 diabetes) and poor outcomes for people with diabetes, whether or not they had SMI as well (see Chapter 5, Regression results for the impact of severe mental illness status on physical and mental health outcomes, mortality and health checks).
Surprisingly, we found that, when comparing outcomes between people with diabetes with SMI and people with diabetes without SMI, people with SMI had fewer physical comorbidities and similar rates of cardiovascular disease, even though they had a significantly increased mortality rate (see Chapter 5, Regression results for the impact of severe mental illness status on physical and mental health outcomes, mortality and health checks). Social deprivation may, in part, explain this, because people with SMI were more likely to live in a deprived area, and this was associated with a higher risk of mortality (see Chapter 5, Regression results for the impact of severe mental illness status on physical and mental health outcomes, mortality and health checks). Diagnostic overshadowing (see Diagnostic overshadowing) may also offer an explanation, with physical health problems being attributed to SMI; therefore, they are not diagnosed or treated. The qualitative study sheds light on other potential reasons too, as many service user participants talked about physical health problems such as poor sleep, musculoskeletal problems and poor mobility, which we could not reliably explore in the CPRD data, but which affected their ability and motivation to manage diabetes (see Chapter 6, Theme 2: not just two illnesses – multimorbidity and diabetes management). The sense of being overwhelmed by poor health from multiple conditions, each with their own treatment and management burdens (see Chapter 6, Theme 1: mental illness affects everything, and Theme 2: not just two illnesses – multimorbidity and diabetes management), led many participants to respond by prioritising SMI as their dominant concern, potentially to the detriment of optimal diabetes outcomes.
In the context of multimorbidity, this study additionally highlights the importance of ordering of diagnosis. Unexpectedly, we found some evidence that people who are diagnosed with diabetes first may have poorer health outcomes (see Chapter 5, Objective 2: factors associated with variation in diabetes and mental health outcomes in people with severe mental illness and diabetes, Results, and Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results). The qualitative study included very few participants who received their diagnoses in this order and offers little insights into how different their experiences are, but, considering the overwhelming nature of having SMI, it may be that the layering of psychosis onto poor physical health has a greater impact on an individual’s ability to manage their diabetes (see Chapter 6, Participant characteristics).
The role of antipsychotic medication
We found that, after controlling for other variables, treatment with antipsychotic medication increases the risk of developing diabetes in people with SMI (see Chapter 5, Objective 1: factors associated with the development of diabetes in people with severe mental illness, Results). For people with both conditions, use of antipsychotic medication increases the risk of mortality (see Chapter 5, Regression analyses results), and, for people with both conditions, atypical antipsychotics are associated with worse outcomes. The qualitative study also highlights concerns about the side effects of antipsychotic medication, including weight gain, increased appetite and lethargy (see Chapter 6, Mental health medication and diabetes management). Some participants associated these with both the onset and management of diabetes, and all three side effects were identified as barriers to eating healthily and being physically active (see Chapter 6, Mental health medication and diabetes management). Fewer people with diabetes and SMI than staff identified a link between antipsychotic medication and diabetes, and many expressed a resigned acceptance that living with the side effects that came with such medication was a necessity (see Chapter 6, Persistence and powerlessness). Very few participants talked about antipsychotic medications in relation to diabetes treatment; for example, no participant talked about addressing the side effects of antipsychotic medications to help their diabetes, or the possibility of switching medication as potential solutions (see Chapter 6, Theme 6: diabetes health care).
The value of relationship-based care that supports management of both conditions
The quality and quantity of relationships with others, both friends and family, and health-care providers, were identified as important resources for health management, and were also reported as being deficient by many participants (see Chapter 6, Theme 5: ‘the most critical person in my care’ – the role of informal support networks). When talking about person-centred care, the positive aspects that participants highlighted were often about the relationships they had established with individual mental health care co-ordinators, and the benefits of quite general support provided to help with their lives, such as getting out and about (see Chapter 6, The value of person-centred care). This mirrored the narrative about the benefits of interactions from informal social networks (see Chapter 6, Theme 5: ‘the most critical person in my care’ – the role of informal support networks), which included a focus on the whole person and help with navigating the care system.
In supporting people with SMI and diabetes, offering continuity and opportunities to build relationships (see Chapter 6, Theme 8: person-centred care), and compensating for the lack of informal support we observed among many participants, were highly valued by service users and staff in the qualitative study, and also by the co-design participants as they discussed recommendations for future care. Challenges to this were also identified, including lack of time and continuity to provide person-centred care, and limited knowledge among staff about either diabetes or SMI, which limited their ability to provide specialist advice or care for both conditions. Poor information-sharing between different services was identified as a further barrier to accessing specialist care and navigating the health-care system (see Chapter 6, Theme 8: person-centred care), and, although the unreliability of referral data limited our investigation of diabetes and mental health care provision, we did find that frequency of care alone (e.g. more GP contact, health checks, hospital stays) was not indicative of better care or health outcomes (see Chapter 5, Objective 4: comparing the health outcomes of people with diabetes and severe mental illness with the health outcomes of people with diabetes without severe mental illness, Results, Objective 6: comparing diabetes care provision and estimating health-care costs for people with and people without severe mental illness, Results, and Objective 7: identifying health-care interventions associated with better outcomes for people with diabetes and severe mental illness, Results).
In the final co-design workshop, service users and health-care staff took forward the study findings to make recommendations that they believed would improve outcomes for people with SMI and diabetes. Although they worked independently, all groups proposed similar changes and agreed on three key priorities: (1) relationship-based care that focuses on management of both conditions, (2) the sharing of patient records between primary care, mental health care and diabetes services and (3) access to specialist SMI and diabetes expertise for staff supporting patients, to increase knowledge of both conditions.
Chapter 8 Discussion
Summary of findings
We set out to identify the determinants of diabetes, to explore variation in health outcomes and mortality, and to investigate access to, and experiences of, diabetes health care for people with SMI and diabetes.
Underpinned by a social inequalities framework, we conducted a mixed-methods study, using a concurrent triangulation design, comprising:
-
a quantitative longitudinal observational study, using the health records of 32,781 people with SMI (2761 of whom had diabetes) and of 9573 ‘controls’ with diabetes and no mental illness, matched on age, sex and practice from the CPRD (GOLD) database, with individual linkages to HES, ONS and IMD data
-
a qualitative interview study with 39 adults with SMI and diabetes, nine family members and 30 health-care staff.
Emerging findings from each investigation iteratively informed the design and analysis of the other. Quantitative and qualitative results were integrated using charting and co-design methods to develop a more comprehensive and in-depth understanding of the relationship between SMI and diabetes.
Diabetes in people with severe mental illness
We found that the prevalence of T2DM was 10% in people with SMI. Sociodemographic factors associated with increased risk of diabetes in SMI included older age, older age at diagnosis of SMI, Asian or black ethnicity, living in areas of socioeconomic deprivation and family history of diabetes. Baseline cardiometabolic risks of overweight, cardiovascular disease, hypertension and higher baseline blood pressure, cholesterol and HbA1c levels were also associated with diabetes, as was being on antipsychotic medication. People with SMI who were prescribed atypical antipsychotics were 40% (OR 1.39) more likely to develop diabetes.
We observed a general trend of improved control of HbA1c, cholesterol and blood pressure from 2000 to 2016 in people with SMI and diabetes. These apparent improvements might reflect improvements in care, influencing better control over time, but may also be a function of changes in the composition of the study population. Recorded prevalence of diabetes increased throughout the period and practices were financially incentivised for case-finding from 2004 onwards. Less severe cases at earlier stages of disease may, therefore, have constituted an increasing proportion of the diabetes study population over time, as detection (and therefore correct categorisation) of diabetes ‘caseness’ improved.
Diabetes and mental health outcomes in people with severe mental illness and diabetes
Factors associated with poorer physical or mental health outcomes were complex and differed by outcome. For macrovascular complications, older age, longer diabetes duration, comorbidity, baseline cardiovascular disease, hypertension and prescription of antihypertensive agents were all risk predictors. A more deprived socioeconomic status was also weakly associated with a higher risk. Current smokers had almost double the risk compared with non-smokers, although missing data limit confidence in this finding.
Microvascular complications were predicted by older age, baseline cardiovascular disease and baseline HbA1c level exceeding the recommended threshold of 58 mmol/mol (7.5%). There was also weak evidence that more deprived socioeconomic status, longer duration of SMI and baseline prescription of antidiabetes medication increased the risk of developing these complications.
In contrast to the association with diabetes risk, ethnicity was not identified as a risk predictor for poor physical health outcomes in people with SMI and diabetes. This may be as a result of missing ethnicity data and small numbers recorded as being from ethnic minority populations in our data set.
For mental health outcomes, older age had a small but statistically significant protective effect. Women were more likely to have recorded depression and anxiety than men (which could indicate increased risk and/or increased recognition). Asian ethnicity increased the risk of depression, even after adjustment for baseline antidepressant prescriptions. People with bipolar disorder were more likely to have a relapse of SMI in the follow-up period than people with schizophrenia, and also had an increased risk of depression and anxiety. Clinical diagnosis of depression with psychosis, unsurprisingly, considerably increased the risk of depression and anxiety. An additional Charlson Index comorbidity was associated with reduced risk of SMI relapse but increased risk of depression and anxiety. Baseline prescription of antidiabetes medication was a significant predictor for SMI relapse, increasing risk by 34%. A possible explanation may be that being on such medication is associated with more severe/poorly controlled diabetes, which acts as a trigger for SMI relapse.
All-cause mortality in SMI and diabetes increased with older age, male sex, white ethnicity, Charlson Index comorbidity and socioeconomic deprivation; longer duration of SMI slightly reduced the effect on mortality. Schizoaffective disorder was associated with an increased risk of all-cause mortality, compared with schizophrenia. Baseline antidiabetes drug prescription increased all-cause mortality by 35%. Antipsychotics were also associated with higher mortality. Typical antipsychotics increased mortality risk by 27% and atypicals by 31%. These risks are likely to be confounded by higher severity of conditions (diabetes and SMI) requiring medication. Although missing data preclude definitive conclusions, people who used substances at baseline had a three times higher mortality risk than people with no substance use.
The impact of having severe mental illness for people with diabetes
We were interested in understanding the impact of having SMI for people with diabetes, and the impact of having diabetes for people with SMI.
We found evidence that people with SMI alongside diabetes had better control of diabetes and blood pressure and were more likely to receive physical health checks than people with diabetes alone. Despite this, we found that the risk of depression and all-cause mortality was increased, compared with people without SMI. We think this may be because of ‘service gaps’ in accessing elective or planned care that could improve health outcomes. This is supported by the findings on macrovascular complications, for which elective admissions were lower but emergency admissions higher, for people with SMI alongside diabetes than for people with diabetes alone. Similarly, as previously reported in the literature,124 there are likely to be gaps in recognition and reporting of health problems for this group. We found that the rate of macrovascular complications was similar in people with and people without SMI, but people with SMI were less likely to have a record of less acute conditions, such as chronic heart disease. Service gaps were also suggested by qualitative findings, which highlighted the additional barriers to receiving physical health interventions, structural inequalities and the sense of mental illness ‘overshadowing’ physical health needs from the perspective of both health-care providers and people with SMI. Challenges distinguishing between mental and physical illness symptoms, and the impact of low mood and anxiety on the importance given to, and the ability to manage, physical health may compound these difficulties.
The impact of having diabetes for people with severe mental illness
Diabetes alongside SMI increased the probability of receiving physical health checks, but was a significant risk factor for poorer physical and mental health outcomes than SMI alone. The risk of depression and anxiety was increased. From interviews, we found that worries about diabetes could make people feel depressed and anxious; this was also found to lower motivation and intentions to manage diabetes and physical health more generally in SMI. It could be challenging to distinguish between symptoms of mental illness relapse and symptoms of poor glycaemic control. Multimorbidity was common and posed a significant challenge; people with SMI frequently found it overwhelming to deal with several long-term conditions.
Ordering of severe mental illness and type 2 diabetes diagnosis
A surprising initial finding was the larger than expected proportion of people who had been diagnosed with T2DM before their SMI diagnosis. Although further investigation established that this was, in part, a function of using the necessary cut-off points to include only more reliable quality data (which had the unintended effect of selectively excluding people diagnosed with SMI at an earlier age), it was clear that the population diagnosed with T2DM before SMI constituted a significant proportion of those with comorbid diabetes and SMI. The NDA reported an even higher proportion of people with diabetes diagnosed before SMI (65%). 144
The approach to restrict analyses to UTS data is one that is widely used in studies interrogating large health-care data sets. The findings about how this affected the make-up of the study population, differentially excluding more people diagnosed with SMI at an earlier age, have wider applicability. Despite its potential to significantly affect study results, to our knowledge, this limitation of applying UTS criteria has not been reported elsewhere.
Although not part of the initial objectives, we conducted a limited exploration of how diagnosis order affected demographics, baseline characteristics and outcomes for the study population. People diagnosed with SMI before T2DM were more likely to be diagnosed at an earlier age for both conditions, be male, be overweight, live in the most deprived neighbourhoods and be current smokers. Baseline HbA1c, cholesterol and blood pressure levels were higher. They also had, in general, better physical and mental health outcomes, with lower risk of macrovascular complications and hospital admissions. Rates of SMI relapse, depression and anxiety, and mortality were all lower. The baseline prevalence of microvascular complications was lower, but they were more likely to develop new complications during the follow-up period than people diagnosed with T2DM before SMI.
People diagnosed with T2DM before SMI were more likely to have depression with psychosis, and a higher prevalence of comorbidities and prescribed medication other than antipsychotics. This group had had diabetes for, on average, 5 or 6 years; therefore, they had the ‘opportunity’ to develop complications in the pre-baseline period. A higher baseline prevalence of microvascular complications, and fewer new microvascular complications during follow-up, would, therefore, be expected (as found in the data), compared with those diagnosed with SMI first.
Health-care use and costs for people with severe mental illness and diabetes
To our knowledge, this is the first study to compare health-care use and costs across sectors for people with SMI and diabetes and people with diabetes alone using patient-level data. We found that use of primary care services, hospital admissions and duration of hospitalisation were all significantly higher for people with SMI alongside diabetes. Health-care costs were correspondingly higher (around double) than costs for diabetes alone. These costs are likely to be an underestimate as they exclude hospital admissions to specialist mental health facilities (which are likely to be higher for people with SMI).
Health-care interventions and outcomes for people with severe mental illness and diabetes
Our investigation of health-care interventions and how these influence health outcomes using routine health-care records was constrained by limited availability of information about these interventions in the data set, and by confounding between intervention and outcomes variables. However, interviews and co-design workshops provided important insights about the experience of negotiating health care for people with SMI and diabetes. These indicated that people accessed care separately for their conditions: diabetes care from their general practice and mental health care from specialist mental health services.
Formal and informal relationships with others, including social (family and friends) and health-care professionals, were highly valued as an important source of support for health management, although they were notably absent for many people. Such relationships and continuity of care were seen as essential to support health-care needs, particularly for dealing with the complex and overwhelming challenges of living with multimorbidity. These relationships helped with motivation, as well as providing more practical support to navigate health care. A case management approach to help people navigate across health service silos, sharing health records across different services and increasing staff knowledge about mental and physical conditions and management of multimorbidity, could be key to improving care and outcomes for people living with the double burden of SMI and diabetes.
Comparison with other research
These findings confirm results of previous research and also address some important evidence gaps.
The prevalence of diabetes in people with SMI has been consistently estimated to be two to three times higher than for the general population. Our estimate of 10.2% is similar to previously reported rates from meta-analyses (10.2%)44 and from interrogation of routine health-care data (8.0%). 145
A range of risk factors (which are similar to those in the general population) for developing diabetes in people with SMI have been previously described,25,146 but studies have been inconsistent. Stubbs et al. 122 reported only increasing age to be associated with risk of T2DM. A later meta-analysis implicated older age, longer illness duration and multiepisode (SMI) status, but not sex, ethnicity or smoking status;44 however, multivariable meta-regression analysis found that multiepisode (vs. first-episode) status was the only significant predictor for T2DM. These findings diverge from our results, which found a number of additional demographic and health variables, including Asian or black ethnicity, to be T2DM risk factors in SMI. Another health-care database study with a high minority ethnic population reported that increased risk of T2DM in SMI was exacerbated by Asian or Afro-Caribbean ethnicity;147 neighbourhood socioeconomic disadvantage has been reported as a risk factor in a previous study. 148 Antipsychotics, particularly atypical antipsychotics, are widely reported to be associated with increased risk of T2DM,64 which was confirmed by our results.
Most previous research has reported only on the population of people diagnosed with SMI before T2DM, has been cross-sectional or has not considered diagnosis order. We found that there were important differences between the two populations, people diagnosed with SMI before T2DM and people diagnosed with T2DM before SMI, and these differences warrant further exploration. Diabetes appears to increase the risk of poor mental illness outcomes. A cross-sectional study reported elevated risk for all mental illness in people with diabetes, with the greatest excess risk (around threefold) for schizophrenia. 149
Although relatively limited, there have been qualitative studies using focus groups, individual interviews and surveys to explore the experience of people living with comorbid SMI and diabetes. 150–153 A common theme reported in these studies is that mental illness care significantly overshadows diabetes care. Another strong theme across studies is that of feeling overwhelmed by multiple chronic comorbidities with differing self-care recommendations. Our study also found that mental illness symptoms and care needs dominated people’s day-to-day lives, casting a ‘shadow’ over everything else. Multimorbidity was also a consistent feature, making self-management and accessing health care extremely challenging. Furthermore, mood influenced the ability to manage, and importance given to managing, physical health. A further barrier to managing diabetes in SMI was that health care was experienced as being provided in condition-specific silos.
Previous studies on diabetes health-care interventions received by people with SMI (compared with people without SMI) have given conflicting results. The NDA144 found that, compared with the general diabetes population, people with SMI and T2DM were slightly less likely to receive NICE-recommended care checks, but there was no difference in treatment target achievement. Overall evidence suggests that levels of health checks and cardiovascular and metabolic control (e.g. cholesterol, blood pressure, HbA1c) are comparable (except for retinopathy checks). 154–156 However, concerningly, despite similar achievement of treatment targets as the general diabetes population, morbidity and mortality are increased for people with SMI. 154
Potential explanations for the NDA144 findings and the present study include limitations of data recording (see Limitations of this study). However, they can potentially also indicate problems with accessing health-care interventions for the same level of need. We found evidence suggesting service gaps, with similar rates of recorded macrovascular complications for people with SMI and those without SMI, but differing patterns of acute versus elective care between the two groups. A study investigating treatment of acute myocardial infarction in people with SMI157 found that they were less likely to receive revascularisation therapies than the general population, despite changes in care to improve this situation.
Strengths and limitations
Strengths of this study
We accessed a large, comprehensive longitudinal data set, CPRD (GOLD), with individual data linkages to HES, ONS and IMD data, allowing us to address important evidence gaps. Patient characteristics in the CPRD database have been shown to be broadly representative of the general UK population in terms of age, sex and ethnicity,98 meaning that our findings are likely to have wider generalisability.
There are a growing number of studies using routinely collected health-care data to explore mental and physical health, health care and outcomes for people with SMI. 145,147,155 The large numbers of records that are usually involved mean that such studies can address important applied health research questions. However, none, to date, has reported using longitudinal data combined with qualitative inquiry to develop a more in-depth understanding than can be achieved by cross-sectional designs or quantitative analyses alone.
Our mixed-methods design means we have been able to fill in ‘gaps’ from the quantitative study, which allows us to theorise further on the meaning of the findings. Moreover, an integrated approach in which emerging findings from both quantitative and qualitative inquiry informed further questions, allowed us to conduct a more detailed, in-depth exploration to address study objectives. The large qualitative sample size and inclusion of people with SMI and diabetes, their families and a variety of health-care staff ensured representation of a wide range of views in our data. Co-design workshops also ensured that, in addition to our multidisciplinary research team, people with SMI and diabetes, their families and health-care providers helped to interpret study findings. This strengthens the reliability and relevance of our findings, grounding them in the day-to-day experience of people living with and managing SMI and diabetes. Strong PPI and clinical input have also helped strengthen interpretation of findings and their relevance and application to practice.
Limitations of this study
As with all studies using routine health-care data, the quality of the data, in terms of accuracy and completeness, will influence the questions that can be addressed and the reliability of findings. As with all observational studies, it is not possible to control for unobserved confounders, and systematic biases in measurement errors might lead us to over- or underestimate associations of risk factors with outcomes for people with SMI. We also did not have access to contextual data on lifestyle, or to the environmental and social determinants of health that were not available in the health-care record or through data linkages.
Despite being representative of the UK population, the CPRD data set has some key limitations. Geographically, practices with large list sizes in urban areas in the south and north-west of England are over-represented, whereas practices in the north-east are under-represented. 98
Missing data in the CPRD limited some of the analyses. We found large numbers of missing data for lifestyle factors (e.g. smoking), which are coded less systematically than information on clinical diagnosis and prescriptions. It cannot be assumed that these data are missing at random, as missingness may be influenced by various factors, including the coding behaviour of primary care providers, a patient’s health status and the implementation of financial incentive schemes. Changes in QOF indicators, for example, are likely to change recording behaviour, not just practice. Therefore, it is difficult to adjust for missing data, as the potential impact of all of these factors is unknown.
Data for health-care interventions and pathways were limited in the CPRD (because of unreliability, e.g. of referral data for diabetes education and specialist services, and a lack of linked outpatient data in our data set). The ability to explore the influence of health-care interventions on health outcomes was, therefore, restricted. Related to this, when we found differences in provision of interventions for people with SMI, it was not possible to identify the level of the ‘service gap’ (referral or access) from the CPRD. We were also unable to measure severity of mental illness or diabetes, as this information is not routinely recorded, and cannot be reliably inferred from other parameters.
The study was also limited by the accuracy and completeness of the recording of diagnoses and activities by practices. The validity of the Read coding system used in practices’ electronic medical records varies over time and by diagnosis: in a 2010 review, estimates of the positive predictive value of recorded diagnostic codes in the General Practice Research Database system (precursor to the CPRD) were 98.6 (95% CI 92.2 to 100.0) for diabetes and 81.0 (95% CI 87.0 to 94.0) for schizophrenia. 158 However, completeness of recording in the CPRD has improved over time, particularly for conditions included in the QOF, for which standard reporting requirements (codes specified under QOF business rules) were introduced. This includes both diabetes and SMI. Although we based our diagnostic criteria on QOF business rules, we also extended our definitions to include patients for whom there was a reasonable presumption of having the condition (e.g. patients without a recorded diagnosis of diabetes receiving regular prescriptions of insulin or antidiabetic medication).
We estimated hospital use and costs based on the HES data set, which contains detailed information about admissions to general hospitals (including non-specialist mental health providers), but not admission to specialist mental health facilities. Use of community mental health care, outpatient attendances and A&E attendances was also missing from our data (because of limitations on how many data sets can be linked to avoid inadvertent identification of practices). A further limitation was that costs for diabetes care could not be separated from other costs.
The qualitative findings indicated fluctuating diabetes management because of changes in mental health or emotional states. However, this was not captured in the CPRD data because of large gaps between diabetes checks (often 6 or 12 months). Eligibility criteria for the interview study necessarily excluded people who were too unwell to participate and probably selected people with less severe SMI and better diabetes control. They were also less likely to be homeless or have unstable housing.
We were not able to achieve the target sample size for family members, as many participants with SMI and diabetes were not able to identify a family supporter. However, family members participated in the PPI group and co-design workshops, providing valuable insights.
Conclusions
We believe that this is the first study to combine interrogation of a large health-care data set with in-depth interviews to identify the factors associated with developing T2DM and those influencing mental and physical health outcomes for people with SMI and diabetes, and to estimate health-care costs and explore the experience of living with SMI and diabetes.
Implications for practice
The findings from this research indicate that the following five key messages are important for clinical practice.
Regular diabetes checks for people with severe mental illness
Check all people with SMI regularly to identify those with diabetes and those at high risk of developing diabetes and offer appropriate intervention.
Address the metabolic side effects of psychotropic medication
Although psychotropic medication forms a key part of the management of SMI, many of these medications are linked to weight gain and diabetes. Clinicians should therefore carefully consider the choice of psychotropics, discuss the side effects with patients, monitor for metabolic side effects and intervene when necessary.
Respond to the challenges of multimorbidity
Health-care professionals and services must take account of multimorbidity in the assessment and management of diabetes. 159 Possible solutions include a keyworker or case management approach to better integrate support for both mental illness and diabetes, and improved training for health-care staff on managing multimorbidity.
Provide tailored support for self-management
Provide a bespoke diabetes self-management education package for people with SMI that addresses the unique challenges to having these conditions together. These include managing side effects of antipsychotic medications, managing the impact of fluctuating mental health and mood on diabetes self-management, and distinguishing between mental and physical symptoms.
Address social concerns
Health-care services and professionals should identify difficulties related to diabetes management that stem from social issues, such as housing, discrimination, transport, poverty, access to benefits and social capital, given the complex interactions between socioeconomic factors and diabetes and SMI. When these are identified, patients should be signposted to appropriate services.
Implications for research
This study has implications for future research on SMI and diabetes, and also for research using similar methods.
Understand the relationship between health checks and outcomes
This study identified a paradoxical association between the number of health checks and poorer outcomes. There are a number of potential explanations for this finding that require further investigation, for instance reverse causality or lack of effective intervention. Exploration of this association using advanced statistical methods (such as instrumental variables regressions) and dynamic models is a research priority.
The relationship between severe mental illness and other long-term physical conditions
For this study, we constructed a large number of variables and will make the codes available for other researchers, reducing future resource needs for conducting research using these data sets. There is an opportunity, therefore, to replicate our methods to efficiently investigate other health conditions, such as cardiovascular disease, chronic lung disease and other chronic disorders.
Is diabetes a risk factor for older-age onset of psychosis?
An unanticipated finding of the study was that the diagnosis of diabetes often antedated the onset of SMI. We were able to conduct only limited analyses because of the nature of the data set. Further work is needed using a cohort of people with T2DM to fully understand this relationship.
Are tailored diabetes interventions clinically effective and cost-effective compared with generic interventions for people with severe mental illness?
Common across all of the recommendations for practice is the need for more intensive support, requiring additional resources for this high-risk and vulnerable group. This study design could not assess the effectiveness and cost-effectiveness of such approaches. Research is needed to develop and to determine the effectiveness and cost-effectiveness of such interventions (e.g. tailored diabetes self-management education, or changes to organisation of diabetes care for people with SMI) for improving diabetes and other outcomes.
Acknowledgements
The study team would like to thank all the participants with SMI and diabetes, their family members and clinicians who participated in this research.
We would also like to thank the following:
-
the members of our SSC for their invaluable support and advice
-
the PPI group, DIAMONDS VOICE, for their substantial input and guidance
-
Angela Ross, the DIAMONDS VOICE lead, for co-ordinating PPI meetings and supporting members
-
attendees at our co-design workshops
-
the administrator for the project, Nicky Traynor, for her invaluable assistance
-
Lyndsey Kramer for her assistance with coding the qualitative family member interview data.
Contributions of authors
Jennie Lister (https://orcid.org/0000-0002-2911-8331) (Research Fellow) attended study team meetings, completed the data analysis for the qualitative interview study (for staff members and people with SMI), supervised the analysis for family member qualitative interviews, drafted six of the eight themes in the qualitative interview study section, commented on draft versions of the report and led the compilation of the report.
Lu Han (https://orcid.org/0000-0001-7198-3380) (Research Fellow) attended study team meetings, contributed to the study design and protocol development, led the quantitative analysis of the CPRD data, wrote the draft of the quantitative interrogation of patient health records section and commented on draft versions of the report.
Sue Bellass (https://orcid.org/0000-0001-9383-4116) (Research Fellow) attended study team meetings; oversaw the protocol development; co-ordinated the PPI input throughout the study; conducted the scoping of the literature and expert consultation; collected all qualitative interview data; supervised the data analysis of the qualitative interviews with staff, family members and people with SMI; checked a random selection of qualitative interview coding during the initial stages of analysis to ensure reliability; drafted two of the eight themes in the qualitative interview study section; and commented on draft versions of the report.
Jo Taylor (https://orcid.org/0000-0001-5898-0900) (Lecturer in Applied Health Research) attended study team meetings; contributed to the study design and protocol development; assisted with PPI input; supervised the qualitative data collection and analysis of the qualitative interviews with staff, family members and people with SMI; checked a random selection of qualitative interview coding during the initial stages of analysis to ensure reliability; contributed to the draft of the qualitative interview study section; drafted the integration of quantitative and qualitative results section; and commented on draft versions of the report.
Sarah L Alderson (https://orcid.org/0000-0002-5418-0495) (Academic Clinical Lecturer in Primary Care) attended study team meetings, contributed to the study design and commented on draft versions of the report.
Tim Doran (https://orcid.org/0000-0001-7857-3704) (Professor of Health Policy) contributed to the study design, supervised data analysis for the quantitative components of the study and commented on draft versions of the report.
Simon Gilbody (https://orcid.org/0000-0002-8236-6983) (Director of the Mental Health and Addictions Research Group) attended study team meetings, contributed to the study design and commented on draft versions of the report.
Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Deputy Director, York Trials Unit) attended study team meetings, contributed to the study design, supervised data analysis for the quantitative components of the study and commented on draft versions of the report.
Richard IG Holt (https://orcid.org/0000-0001-8911-6744) (Professor in Diabetes and Endocrinology/Consultant Physician) attended study team meetings, contributed to the study design, drafted the diabetes section of the introduction section and commented on draft versions of the report.
Rowena Jacobs (https://orcid.org/0000-0001-5225-6321) (Professor of Health Economics) attended study team meetings, contributed to the study design, supervised data analysis for the quantitative components of the study and commented on draft versions of the report.
Charlotte EW Kitchen (https://orcid.org/0000-0002-9323-0061) (Research Fellow) attended study team meetings; drafted the abstract, plain English summary and scientific summary; and commented on draft versions of the report.
Stephanie L Prady (https://orcid.org/0000-0002-8933-8045) (Senior Research Fellow, Inequalities in Health) attended study team meetings, contributed to the study design, ensured that the social inequalities framework was applied throughout the research, drafted the social inequalities section of the introduction section, contributed to the draft of the integration of quantitative and qualitative results section and commented on draft versions of the report.
John Radford (Patient and Public Involvement Representative) attended study team meetings, contributed to the study design, attended co-design workshops and assisted in assuring the credibility of the qualitative interview findings.
Jemimah R Ride (https://orcid.org/0000-0002-1820-5499) (Research Fellow) provided code for the quantitative data analysis and commented on draft versions of the report.
David Shiers (https://orcid.org/0000-0003-2531-5837) (Honorary Reader in Early Psychosis/Honorary Research Consultant/Honorary Senior Research Fellow) attended study team meetings, contributed to the study design and commented on draft versions of the report.
Han-I Wang (https://orcid.org/0000-0002-3521-993X) (Research Fellow) attended study team meetings, led the quantitative section of comparing diabetes care provision and estimating health-care costs for people with and people without SMI analysis, drafted this section of the quantitative results and commented on draft versions of the report.
Najma Siddiqi (https://orcid.org/0000-0003-1794-2152) (Clinical Senior Lecturer in Psychiatry) attended study team meetings, led the project, conceived and designed the study, supervised the study (including the data collection in the qualitative interviews), drafted the SMI section of the introduction and discussion sections and editorially reviewed all sections of the report.
Publications
Bellass S, Lister JE, Kitchen CEW, Kramer L, Alderson SL, Doran T, et al. Living with diabetes alongside a severe mental illness: a qualitative exploration with people with severe mental illness, family members and healthcare staff [published online ahead of print May 4 2021]. Diabet Med 2021.
Han L, Doran T, Holt RIG, Hewitt C, Jacobs R, Prady SL, et al. The impact of severe mental illness on healthcare use and health outcomes for people with type 2 diabetes [published online ahead of print February 10 2021]. Br J Gen Pract 2021.
Bellass S, Taylor J, Han L, Prady SL, Shiers D, Jacobs R, et al. Exploring severe mental illness and diabetes: protocol for a longitudinal, observational, and qualitative mixed methods study. JMIR Res Protoc 2019;8:e13407.
Data-sharing statement
Owing to the sensitive and confidential nature of the qualitative and quantitative data used in this study, and the access permissions required, the data are not publicly available. Any queries should be addressed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) 2013. https://doi.org/10.1176/appi.books.9780890425596.
- World Health Organization (WHO) . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision 1992.
- The National Institute of Mental Health Information Resource Center . Mental Illness 2019. www.nimh.nih.gov/health/statistics/mental-illness.shtml#part_154784 (accessed November 2019).
- National Mental Health Intelligence Network . Severe Mental Illness (SMI) and Physical Health Inequalities: Briefing 2018.
- NHS England . 2019/20/General/Medical/Services/(GMS)/Contract/Quality/and/Outcomes/Framework/(QOF):/Guidance/for/GMS/Contract/201920/in/England 2019.
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet 2016;388:86-97. https://doi.org/10.1016/S0140-6736(15)01121-6.
- Thornicroft G, Tansella M, Becker T, Knapp M, Leese M, Schene A, et al. EPSILON Study Group . The personal impact of schizophrenia in Europe. Schizophr Res 2004;69:125-32. https://doi.org/10.1016/S0920-9964(03)00191-9.
- Breslau J, Lane M, Sampson N, Kessler RC. Mental disorders and subsequent educational attainment in a US national sample. J Psychiatr Res 2008;42:708-16. https://doi.org/10.1016/j.jpsychires.2008.01.016.
- Levinson D, Lakoma MD, Petukhova M, Schoenbaum M, Zaslavsky AM, Angermeyer M, et al. Associations of serious mental illness with earnings: results from the WHO World Mental Health surveys. Br J Psychiatry 2010;197:114-21. https://doi.org/10.1192/bjp.bp.109.073635.
- Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease study 2016. Schizophr Bull 2018;44:1195-203. https://doi.org/10.1093/schbul/sby058.
- Ferrari AJ, Stockings E, Khoo JP, Erskine HE, Degenhardt L, Vos T, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease study 2013. Bipolar Disord 2016;18:440-50. https://doi.org/10.1111/bdi.12423.
- National Institute for Health and Care Excellence (NICE) . Psychosis and Schizophrenia in Adults: Treatment and Management. NICE Clinical Guideline 178 2014. www.nice.org.uk/guidance/cg178/chapter/key-priorities-for-implementation (accessed November 2019).
- National Institute for Health and Care Excellence (NICE) . Bipolar Disorder: The Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care. NICE Clinical Guideline 185 2014. www.nice.org.uk/guidance/cg185 (accessed November 2019).
- Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull 2015;114:169-79. https://doi.org/10.1093/bmb/ldv017.
- Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 2014;13:153-60. https://doi.org/10.1002/wps.20128.
- NHS Digital . 1.5.I Excess Under 75 Mortality Rate in Adults With Serious Mental Illness (Formerly Indicator 1.5) 2016.
- Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry 2017;211:175-81. https://doi.org/10.1192/bjp.bp.117.202606.
- Siddiqi N, Doran T, Prady SL, Taylor J. Closing the mortality gap for severe mental illness: are we going in the right direction?. Br J Psychiatry 2017;211:130-1. https://doi.org/10.1192/bjp.bp.117.203026.
- Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6:675-712. https://doi.org/10.1016/S2215-0366(19)30132-4.
- Reilly S, Olier I, Planner C, Doran T, Reeves D, Ashcroft DM, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009010.
- Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav 1995:80-94. https://doi.org/10.2307/2626958.
- Coid J, Gonzalez Rodriguez R, Kallis C, Zhang Y, Bhui K, De Stavola B, et al. Ethnic disparities in psychotic experiences explained by area-level syndemic effects [published online ahead of print 30 October 2019]. Br J Psychiatry 2019. https://doi.org/10.1192/bjp.2019.203.
- Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017;16:30-4. https://doi.org/10.1002/wps.20384.
- International Diabetes Federation (IDF) . IDF Diabetes Atlas 2019.
- Holt RIG, Cockram C, Flyvbjerg A, Goldstein BJ. Textbook of Diabetes. Chichester: John Wiley & Sons Ltd; 2017.
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152-62. https://doi.org/10.1016/j.diabres.2004.06.010.
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783-9. https://doi.org/10.1016/S0140-6736(08)60766-7.
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention Programme (IDPP) . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289-97. https://doi.org/10.1007/s00125-005-0097-z.
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. https://doi.org/10.1056/NEJM200105033441801.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40. https://doi.org/10.1056/NEJMoa012512.
- NHS England . NHS Diabetes Prevention Programme (NHS DPP 2019. www.england.nhs.uk/diabetes/diabetes-prevention/ (accessed November 2019).
- National Institute for Health and Care Excellence (NICE) . Type 2 Diabetes in Adults: Management. NICE Guideline [NG28] 2019. www.nice.org.uk/guidance/ng28 (accessed April 2020).
- National Institute for Health and Care Excellence (NICE) . Type 1 Diabetes in Adults: Diagnosis and Management: NICE Clinical Guideline NG17 2016. www.nice.org.uk/guidance/ng17 (accessed April 2020).
- Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541-51. https://doi.org/10.1016/S0140-6736(17)33102-1.
- Holt RI, Mitchell AJ. Diabetes mellitus and severe mental illness: mechanisms and clinical implications. Nat Rev Endocrinol 2015;11:79-8. https://doi.org/10.1038/nrendo.2014.203.
- Vancampfort D, Wampers M, Mitchell AJ, Correll CU, De Herdt A, Probst M, et al. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry 2013;12:240-50. https://doi.org/10.1002/wps.20069.
- Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 2017;74:261-9. https://doi.org/10.1001/jamapsychiatry.2016.3803.
- Kessing LV, Thomsen AF, Mogensen UB, Andersen PK. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry 2010;197:266-71. https://doi.org/10.1192/bjp.bp.109.076935.
- Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology 2010;35:1997-2004. https://doi.org/10.1038/npp.2010.78.
- Galling B, Roldán A, Nielsen RE, Nielsen J, Gerhard T, Carbon M, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247-59. https://doi.org/10.1001/jamapsychiatry.2015.2923.
- Cohen D, Batstra MR, Gispen-de Wied CC. Immunological characteristics of diabetes in schizophrenia. Diabetologia 2005;48:1941-2. https://doi.org/10.1007/s00125-005-1879-z.
- McCreadie RG. Scottish Schizophrenia Lifestyle Group . Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry 2003;183:534-9. https://doi.org/10.1192/03-162.
- Holt RIG, Gossage-Worrall R, Hind D, Bradburn MJ, McCrone P, Morris T, et al. Structured lifestyle education for people with schizophrenia, schizoaffective disorder and first-episode psychosis (STEPWISE): randomised controlled trial. Br J Psychiatry 2019;214:63-7. https://doi.org/10.1192/bjp.2018.167.
- Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry 2016;15:166-74. https://doi.org/10.1002/wps.20309.
- Citrome L, Kalsekar I, Baker RA, Hebden T. A review of real-world data on the effects of aripiprazole on weight and metabolic outcomes in adults. Curr Med Res Opin 2014;30:1629-41. https://doi.org/10.1185/03007995.2014.908280.
- Yood MU, DeLorenze G, Quesenberry CP, Oliveria SA, Tsai AL, Willey VJ, et al. The incidence of diabetes in atypical antipsychotic users differs according to agent – results from a multisite epidemiologic study. Pharmacoepidemiol Drug Saf 2009;18:791-9. https://doi.org/10.1002/pds.1781.
- Yood MU, DeLorenze GN, Quesenberry CP, Oliveria SA, Tsai A-L, Kim E, et al. Association between second-generation antipsychotics and newly diagnosed treated diabetes mellitus: does the effect differ by dose?. BMC 2011;11. https://doi.org/10.1186/1471-244X-11-197.
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity . Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601. https://doi.org/10.2337/diacare.27.2.596.
- Holt RI, Peveler RC. Antipsychotic drugs and diabetes – an application of the Austin Bradford Hill criteria. Diabetologia 2006;49:1467-76. https://doi.org/10.1007/s00125-006-0279-3.
- Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382:951-62. https://doi.org/10.1016/S0140-6736(13)60733-3.
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010;123:225-33. https://doi.org/10.1016/j.schres.2010.07.012.
- Hirsch L, Yang J, Bresee L, Jette N, Patten S, Pringsheim T. Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Saf 2017;40:771-81. https://doi.org/10.1007/s40264-017-0543-0.
- Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res 2012;140:159-68. https://doi.org/10.1016/j.schres.2012.03.017.
- Naslund JA, Whiteman KL, McHugo GJ, Aschbrenner KA, Marsch LA, Bartels SJ. Lifestyle interventions for weight loss among overweight and obese adults with serious mental illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2017;47:83-102. https://doi.org/10.1016/j.genhosppsych.2017.04.003.
- de Silva VA, Suraweera C, Ratnatunga SS, Dayabandara M, Wanniarachchi N, Hanwella R. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-1049-5.
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 2009;24:412-24. https://doi.org/10.1016/j.eurpsy.2009.01.005.
- Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016;30:717-48. https://doi.org/10.1177/0269881116645254.
- Citrome L, Yeomans D. Do guidelines for severe mental illness promote physical health and well-being?. J Psychopharmacol 2005;19:102-9. https://doi.org/10.1177/0269881105059505.
- Morrato EH, Newcomer JW, Kamat S, Baser O, Harnett J, Cuffel B. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care 2009;32:1037-42. https://doi.org/10.2337/dc08-1720.
- Crawford MJ, Jayakumar S, Lemmey SJ, Zalewska K, Patel MX, Cooper SJ, et al. Assessment and treatment of physical health problems among people with schizophrenia: national cross-sectional study. Br J Psychiatry 2014;205:473-7. https://doi.org/10.1192/bjp.bp.113.142521.
- NHS Digital . Quality and Outcomes Framework (QOF) – 2014–15 2015. www.hscic.gov.uk/catalogue/PUB15751 (accessed May 2016).
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010;196:116-21. https://doi.org/10.1192/bjp.bp.109.067512.
- Jones LE, Clarke W, Carney CP. Receipt of diabetes services by insured adults with and without claims for mental disorders. Med Care 2004;42:1167-75. https://doi.org/10.1097/00005650-200412000-00003.
- Holt RIG. Association between antipsychotic medication use and diabetes. Curr Diab Rep 2019;19. https://doi.org/10.1007/s11892-019-1220-8.
- NHS England . Action for Diabetes 2014. www.england.nhs.uk/wp-content/uploads/2014/01/act-for-diabetes.pdf (accessed March 2017).
- Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav 2010;51:S28-40. https://doi.org/10.1177/0022146510383498.
- World Health Organization (WHO) . A Conceptual Framework for Action on the Social Determinants of Health 2010.
- Draine J, Salzer MS, Culhane DP, Hadley TR. Role of social disadvantage in crime, joblessness, and homelessness among persons with serious mental illness. Psychiatr Serv 2002;53:565-73. https://doi.org/10.1176/appi.ps.53.5.565.
- Cooper B. Schizophrenia, social class and immigrant status: the epidemiological evidence. Epidemiol Psichiatr Soc 2005;14:137-44. https://doi.org/10.1017/s1121189x00006382.
- Grigoroglou C, Munford L, Webb RT, Kapur N, Ashcroft DM, Kontopantelis E. Prevalence of mental illness in primary care and its association with deprivation and social fragmentation at the small-area level in England. Psychol 2020;50:293-302. https://doi.org/10.1017/S0033291719000023.
- Koenders JF, de Mooij LD, Dekker JM, Kikkert M. Social inclusion and relationship satisfaction of patients with a severe mental illness. Int J Soc Psychiatry 2017;63:773-81. https://doi.org/10.1177/0020764017737572.
- Wickham S, Taylor P, Shevlin M, Bentall RP. The impact of social deprivation on paranoia, hallucinations, mania and depression: the role of discrimination social support, stress and trust. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0105140.
- Royal College of Psychiatrists . National Clinical Audit of Psychosis – National Report for the Core Audit 2018 2018.
- Eaton WW, Mortensen PB, Frydenberg M. Obstetric factors, urbanization and psychosis. Schizophr Res 2000;43:117-23. https://doi.org/10.1016/S0920-9964(99)00152-8.
- Cooper B. Immigration and schizophrenia: the social causation hypothesis revisited. Br J Psychiatry 2005;186:361-3. https://doi.org/10.1192/bjp.186.5.361.
- Karlsen S, Nazroo JY, McKenzie K, Bhui K, Weich S. Racism, psychosis and common mental disorder among ethnic minority groups in England. Psychol Med 2005;35:1795-803. https://doi.org/10.1017/S0033291705005830.
- Harrison G, Gunnell D, Glazebrook C, Page K, Kwiecinski R. Association between schizophrenia and social inequality at birth: case–control study. Br J Psychiatry 2001;179:346-50. https://doi.org/10.1192/bjp.179.4.346.
- Goulding SM, Holtzman CW, Trotman HD, Ryan AT, MacDonald AN, Shapiro DI, et al. The prodrome and clinical risk for psychotic disorders. Child Adolesc Psychiatr Clin N Am 2013;22:557-67. https://doi.org/10.1016/j.chc.2013.04.002.
- Topor A, Andersson G, Denhov A, Holmqvist S, Mattsson M, Stefansson C-G, et al. Psychosis and poverty: coping with poverty and severe mental illness in everyday life. Psychosis 2014;6:117-27. https://doi.org/10.1080/17522439.2013.790070.
- DeVerteuil G, Hinds A, Lix L, Walker J, Robinson R, Roos LL. Mental health and the city: intra-urban mobility among individuals with schizophrenia. Health Place 2007;13:310-23. https://doi.org/10.1016/j.healthplace.2006.02.001.
- Pybus K, Pickett KE, Prady SL, Lloyd C, Wilkinson R. Discrediting experiences: outcomes of eligibility assessments for claimants with psychiatric compared with non-psychiatric conditions transferring to personal independence payments in England. BJPsych Open 2019;5. https://doi.org/10.1192/bjo.2019.3.
- Townshend T, Lake A. Obesogenic environments: current evidence of the built and food environments. Perspect Public Health 2017;137:38-44. https://doi.org/10.1177/1757913916679860.
- Jones A, Bentham G, Foster C, Hillsdon M, Panter J. Tackling Obesities: Future Choices – Obesogenic Environments – Evidence Review. London: Government Office for Science; 2007.
- Link BG, Phelan JC. Conceptualizing stigma. Annu Rev Sociol 2001;27:363-85. https://doi.org/10.1146/annurev.soc.27.1.363.
- O’Sullivan C, Thornicroft G, Layte R, Burfeind C, McDaid D, Salize H, et al. Background Document to the European Commission Thematic Conference. Lisbon: European Commission; 2010.
- Rüsch N, Thornicroft G. Does stigma impair prevention of mental disorders?. Br J Psychiatry 2014;204:249-51. https://doi.org/10.1192/bjp.bp.113.131961.
- Gronholm PC, Thornicroft G, Laurens KR, Evans-Lacko S. Mental health-related stigma and pathways to care for people at risk of psychotic disorders or experiencing first-episode psychosis: a systematic review. Psychol Med 2017;47:1867-79. https://doi.org/10.1017/S0033291717000344.
- Gallagher BJ, Jones BJ. Early-onset schizophrenia: symptoms and social class of origin. Int J Soc Psychiatry 2017;63:492-7. https://doi.org/10.1177/0020764017719302.
- Freeman D, Emsley R, Dunn G, Fowler D, Bebbington P, Kuipers E, et al. The stress of the street for patients with persecutory delusions: a test of the symptomatic and psychological effects of going outside into a busy urban area. Schizophr Bull 2015;41:971-9. https://doi.org/10.1093/schbul/sbu173.
- Glick ID, Stekoll AH, Hays S. The role of the family and improvement in treatment maintenance, adherence, and outcome for schizophrenia. J Clin Psychopharmacol 2011;31:82-5. https://doi.org/10.1097/JCP.0b013e31820597fa.
- Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, et al. Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 2009;70:1-46. https://doi.org/10.4088/JCP.7090su1cj.
- Preece J, Bimpson E. Housing Insecurity and Mental Health in Wales: An Evidence Review. Glasgow: UK Collaborative Centre for Housing Evidence; 2019.
- Moody A, Cowley G, Ng Fat L, Mindell JS. Social inequalities in prevalence of diagnosed and undiagnosed diabetes and impaired glucose regulation in participants in the Health Surveys for England series. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010155.
- Dixon-Woods M, Cavers D, Agarwal S, Annandale E, Arthur A, Harvey J, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-35.
- Kontopantelis E, Stevens RJ, Helms PJ, Edwards D, Doran T, Ashcroft DM. Spatial distribution of clinical computer systems in primary care in England in 2016 and implications for primary care electronic medical record databases: a cross-sectional population study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020738.
- NHS Digital . Read Codes 2018. https://digital.nhs.uk/services/terminology-and-classifications/read-codes (accessed November 2019).
- Chisholm J. The Read clinical classification. BMJ 1990;300. https://doi.org/10.1136/bmj.300.6732.1092.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- NHS Digital . Hospital Episode Statistics (HES) 2019. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed September 2019).
- NHS Digital . Linked HES–ONS Mortality Data 2018. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/linked-hes-ons-mortality-data (accessed November 2019).
- Office for National Statistics . 2011 Census: Population and Household Estimates for Small Areas in England and Wales, March 2011 2012.
- Lad M. The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- Bellass S, Taylor J, Han L, Prady SL, Shiers D, Jacobs R, et al. Exploring severe mental illness and diabetes: protocol for a longitudinal, observational, and qualitative mixed methods study. JMIR Res Protoc 2019;8. https://doi.org/10.2196/13407.
- National Institute for Health Research . Funding and Awards: Improving Diabetes Outcomes for People With Severe Mental Illness (SMI): A Longitudinal Observational and Qualitative Study of Patients in England n.d. www.fundingawards.nihr.ac.uk/award/15/70/26 (accessed December 2019).
- Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
- Blaikie N. Approaches to Social Enquiry: Advancing Knowledge. Cambridge: Polity; 2007.
- Fletcher AJ. Applying critical realism in qualitative research: methodology meets method. Int J Soc Res Methodol 2017;20:181-94. https://doi.org/10.1080/13645579.2016.1144401.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Gutacker N, Mason AR, Kendrick T, Goddard M, Gravelle H, Gilbody S, et al. Does the quality and outcomes framework reduce psychiatric admissions in people with serious mental illness? A regression analysis. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007342.
- Kontopantelis E, Reeves D, Valderas JM, Campbell S, Doran T. Recorded quality of primary care for patients with diabetes in England before and after the introduction of a financial incentive scheme: a longitudinal observational study. BMJ Qual Saf 2013;22:53-64. https://doi.org/10.1136/bmjqs-2012-001033.
- NHS Employers . 2016/17/General/Medical/Services/(GMS)/Contract/Quality/and/Outcomes/Framework/(QOF):/Guidance/for/GMS/Contract/2016/17 2016.
- Kronenberg C, Doran T, Goddard M, Kendrick T, Gilbody S, Dare CR, et al. Identifying primary care quality indicators for people with serious mental illness: a systematic review. Br J Gen Pract 2017;67:e519-e530. https://doi.org/10.3399/bjgp17X691721.
- Jacobs R, Aylott L, Dare C, Doran T, Gilbody S, Goddard M, et al. The association between primary care quality and health-care use, costs and outcomes for people with serious mental illness: a retrospective observational study. Health Serv Deliv Res 2020;8. https://doi.org/10.3310/hsdr08250.
- Siddiqi N, Lewis H, Taylor J, Mahmoodi N, Wright J, McDermid K, et al. A systematic review of pharmacological and non-pharmacological interventions for improving diabetes outcomes in people with serious mental illness. PROSPERO 2015 CRD42015015558 n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42015015558 (accessed August 2020).
- Smith R, Han L, Ali S, Prady SL, Taylor J, Hughes T, et al. Glucose, cholesterol and blood pressure in type II diabetes: a longitudinal observational study comparing patients with and without severe mental illness. J Psychiatr Ment Health Nurs 2019;26:347-57. https://doi.org/10.1111/jpm.12546.
- Peek ME, Ferguson M, Bergeron N, Maltby D, Chin MH. Integrated community-healthcare diabetes interventions to reduce disparities. Curr Diab Rep 2014;14. https://doi.org/10.1007/s11892-013-0467-8.
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007;64:101-56. https://doi.org/10.1177/1077558707305409.
- Ricci-Cabello I, Ruiz-Pérez I, Nevot-Cordero A, Rodríguez-Barranco M, Sordo L, Gonçalves DC. Health care interventions to improve the quality of diabetes care in African Americans: a systematic review and meta-analysis. Diabetes Care 2013;36:760-8. https://doi.org/10.2337/dc12-1057.
- Ricci-Cabello I, Ruiz-Perez I, Rojas-García A, Pastor G, Gonçalves DC. Improving diabetes care in rural areas: a systematic review and meta-analysis of quality improvement interventions in OECD countries. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0084464.
- Zeh P, Sandhu HK, Cannaby AM, Sturt JA. The impact of culturally competent diabetes care interventions for improving diabetes-related outcomes in ethnic minority groups: a systematic review. Diabet Med 2012;29:1237-52. https://doi.org/10.1111/j.1464-5491.2012.03701.x.
- Swinson Evans T, Berkman N, Brown C, Gaynes B, Palmieri Weber R. Disparities Within Serious Mental Illness. Technical Brief No. 25. Rockville, MD: Agency for Healthcare Research and Quality (US); 2016.
- Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr Scand 2015;132:144-57. https://doi.org/10.1111/acps.12439.
- NHS Digital . National Diabetes Audit. Report 1: Care Processes and Treatment Targets, 2016–17: Severe Mental Illness – Supplementary Information 2018.
- Smith DJ, Langan J, McLean G, Guthrie B, Mercer SW. Schizophrenia is associated with excess multiple physical-health comorbidities but low levels of recorded cardiovascular disease in primary care: cross-sectional study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002808.
- NHS Digital . ISB 0070: Healthcare Resource Groups (HRGs) 2018. https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb-0070-healthcare-resource-groups-hrgs (accessed December 2019).
- Ride J, Kasteridis P, Gutacker N, Aragon MJA, Jacobs R. Healthcare costs for people with serious mental illness in England: an analysis of costs across primary care, hospital care, and specialist mental healthcare. Appl Health Econ Health Policy 2020;18:177-88. https://doi.org/10.1007/s40258-019-00530-2.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- NHS Digital . Prescription Cost Analysis – England, 2018 [PAS] n.d. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed December 2019).
- NHS Digital . NHS Reference Costs 2017 18 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 18 November 2019).
- Manning WG, Mullahy J. Estimating log models: to transform or not to transform?. J Health Econ 2001;20:461-94. https://doi.org/10.1016/S0167-6296(01)00086-8.
- Pregibon D. Goodness of link tests for generalized linear models. Appl Stat 1980;29:15-24. https://doi.org/10.2307/2346405.
- Patton MQ. Qualitative Evaluation and Research Methods. Thousand Oaks, CA: SAGE Publications, Inc.; 1990.
- Taylor J, Lister J, Boehnke J, Holt R, Phillips A, Peyrot M, et al. The psychosocial impact of having diabetes alongside severe mental illness: comparing results from the Diabetes Attitudes, Wishes and Needs-Severe Mental Illness (DAWNSMI) and Diabetes Attitudes, Wishes and Needs Second (DAWN2) studies (conference abstract). Diabet Med 2019;36:9-11.
- Brookhart MA, Stürmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care 2010;48:114-20. https://doi.org/10.1097/MLR.0b013e3181dbebe3.
- Davis L, Brekke J. Social support and functional outcome in severe mental illness: the mediating role of proactive coping. Psychiatry Res 2014;215:39-45. https://doi.org/10.1016/j.psychres.2013.09.010.
- Holt RI, Nicolucci A, Kovacs Burns K, Escalante M, Forbes A, Hermanns N, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national comparisons on barriers and resources for optimal care – healthcare professional perspective. Diabet Med 2013;30:789-98. https://doi.org/10.1111/dme.12242.
- Reilly S, Planner C, Hann M, Reeves D, Nazareth I, Lester H. The role of primary care in service provision for people with severe mental illness in the United Kingdom. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0036468.
- Pendlebury J, Holt RI. Managing diabetes in people with severe mental illness. J Diabetes Nurs 2010;14:328-39.
- Royal College of Psychiatrists . Report of the Second Round of the National Audit of Schizophrenia (NAS) 2014 2014.
- Arthur S, Nazroo J, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Ritchie J, Spencer L, O’Connor W, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Bellass S, Lister JE, Kitchen CEW, Kramer L, Alderson SL, Doran T, et al. Living with diabetes alongside a severe mental illness: a qualitative exploration with people with severe mental illness, family members and healthcare staff [published online ahead of print May 4 2021]. Diabet Med 2021. https://doi.org/10.1111/dme.14562.
- NHS Digital . National Diabetes Audit, 2017–18. Report 1: Care Processes and Treatment Targets 2019. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-1-care-processes-and-treatment-targets-2017-18-full-report (accessed April 2020).
- Pearsall R, Shaw RJ, McLean G, Connolly M, Hughes KA, Boyle JG, et al. Health screening, cardiometabolic disease and adverse health outcomes in individuals with severe mental illness. BJPsych Open 2019;5. https://doi.org/10.1192/bjo.2019.76.
- NHS Digital . National Diabetes Audit. Report 2: Complications and Mortality, 2017–18 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-2--complications-and-mortality-2017-18 (accessed April 2020).
- Das-Munshi J, Ashworth M, Dewey ME, Gaughran F, Hull S, Morgan C, et al. Type 2 diabetes mellitus in people with severe mental illness: inequalities by ethnicity and age. Cross-sectional analysis of 588 408 records from the UK. Diabet Med 2017;34:916-24. https://doi.org/10.1111/dme.13298.
- Walsan R, Mayne DJ, Feng X, Pai N, Bonney A. Examining the association between neighbourhood socioeconomic disadvantage and type 2 diabetes comorbidity in serious mental illness. Int J Environ Res Public Health 2019;16.
- Wändell P, Ljunggren G, Wahlström L, Carlsson AC. Diabetes and psychiatric illness in the total population of Stockholm. J Psychosom Res 2014;77:169-73. https://doi.org/10.1016/j.jpsychores.2014.06.012.
- Cimo A, Dewa CS. Symptoms of mental illness and their impact on managing type 2 diabetes in adults. Can J Diabetes 2018;42:372-81. https://doi.org/10.1016/j.jcjd.2017.08.256.
- Stenov V, Willaing I, Knudsen L, Hansen DL, Joensen LE. Diabetes support needs in people with severe mental illness and diabetes. Diabetes 2019;68. https://doi.org/10.2337/db19-837-P.
- Mulligan K, McBain H, Lamontagne-Godwin F, Chapman J, Haddad M, Jones J, et al. Barriers and enablers of type 2 diabetes self-management in people with severe mental illness. Health 2017;20:1020-30. https://doi.org/10.1111/hex.12543.
- Mulligan K, McBain H, Lamontagne-Godwin F, Chapman J, Flood C, Haddad M, et al. Barriers to effective diabetes management – a survey of people with severe mental illness. BMC Psychiatry 2018;18. https://doi.org/10.1186/s12888-018-1744-5.
- Ashworth M, Schofield P, Das-Munshi J. Physical health in severe mental illness. Br J Gen Pract 2017;67:436-7. https://doi.org/10.3399/bjgp17X692621.
- Fleetwood K, Wild S, Smith D, Licence K, Mercer S, Sudlow C, et al. OP47 The impact of major mental illness on quality of care in people with type 2 diabetes in Scotland: an analysis of routinely collected health data. J Epidemiol Community Health 2019;A23. https://doi.org/10.1136/jech-2019-SSMabstracts.48.
- Brown K, Fortenberry K, Gren L, Gunning K, McAdam-Marx C. Achievement of adequate glycemic control in patients with type 2 diabetes and comorbid mental health conditions treated in a primary care setting. Diabetes Spectr 2017;30:277-87. https://doi.org/10.2337/ds16-0038.
- Schulman-Marcus J, Goyal P, Swaminathan RV, Feldman DN, Wong SC, Singh HS, et al. Comparison of trends in incidence, revascularization, and in-hospital mortality in ST-elevation myocardial infarction in patients with versus without severe mental illness. Am J Cardiol 2016;117:1405-10. https://doi.org/10.1016/j.amjcard.2016.02.006.
- Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010;60:e128-36. https://doi.org/10.3399/bjgp10X483562.
- National Institute for Health and Care Excellence (NICE) . Multimorbidity: Clinical Assessment and Management NICE Guideline (NG 56) 2016. www.nice.org.uk/guidance/ng56 (accessed April 2020).
- Taylor J, Stubbs B, Hewitt C, Ajjan RA, Alderson SL, Gilbody S, et al. The effectiveness of pharmacological and non-pharmacological interventions for improving glycaemic control in adults with severe mental illness: a systematic review and meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0168549.
- Gorczynski P, Firth J, Stubbs B, Rosenbaum S, Vancampfort D. Are people with schizophrenia adherent to diabetes medication? A comparative meta-analysis. Psych Res 2017;250:17-24. https://doi.org/10.1016/j.psychres.2017.01.049.
- Charles EF, Lambert CG, Kerner B. Bipolar disorder and diabetes mellitus: evidence for disease-modifying effects and treatment implications. Int J Bipolar Disord 2016;4. https://doi.org/10.1186/s40345-016-0054-4.
- McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: a comprehensive review. Schizophr Bull 2016;42:96-124. https://doi.org/10.1093/schbul/sbv101.
- McBain H, Mulligan K, Haddad M, Flood C, Jones J, Simpson A. Self management interventions for type 2 diabetes in adult people with severe mental illness. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD011361.pub2.
- McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Quality of medical care for persons with serious mental illness: a comprehensive review. Schizophr Res 2015;165:227-35. https://doi.org/10.1016/j.schres.2015.04.010.
- Ward M, Druss B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry 2015;2:431-51. https://doi.org/10.1016/S2215-0366(15)00007-3.
- Cimo A, Stergiopoulos E, Cheng C, Bonato S, Dewa CS. Effective lifestyle interventions to improve type II diabetes self-management for those with schizophrenia or schizoaffective disorder: a systematic review. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-24.
- Whitehead L. Self-management of type 2 diabetes and severe mental illness. Issues Ment Health Nurs 2017;38:606-7. https://doi.org/10.1080/01612840.2017.1328859.
- Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry 2004;47:S67-71. https://doi.org/10.1192/bjp.184.47.s67.
- Foley DL, Mackinnon A, Morgan VA, Watts GF, Castle DJ, Waterreus A, et al. Awareness of pre-diabetes or diabetes and associated factors in people with psychosis. Schizophr Bull 2016;42:1280-9. https://doi.org/10.1093/schbul/sbw027.
- Foley DL, Mackinnon A, Morgan VA, Watts GF, Castle DJ, Waterreus A, et al. Common familial risk factors for schizophrenia and diabetes mellitus. Aust N Z J Psychiatry 2016;50:488-94. https://doi.org/10.1177/0004867415595715.
- Rathmann W, Pscherer S, Konrad M, Kostev K. Diabetes treatment in people with type 2 diabetes and schizophrenia: retrospective primary care database analyses. Prim Care Diabetes 2016;10:36-40. https://doi.org/10.1016/j.pcd.2015.04.001.
- Wake DJ, Broughton P, Perera SM, MacIntyre DJ, Leese GP. Altered metabolic parameters in association with antipsychotic medication use in diabetes: a population based case-control study. Psychoneuroendocrinology 2016;66:214-20. https://doi.org/10.1016/j.psyneuen.2016.01.022.
- Wu CS, Gau SS. Association between antipsychotic treatment and advanced diabetes complications among schizophrenia patients with type 2 diabetes mellitus. Schizophr Bull 2016;42:703-11. https://doi.org/10.1093/schbul/sbv187.
- Wykes TL, Lee AA, McKibbin CL, Laurent SM. Self-efficacy and hemoglobin a1c among adults with serious mental illness and type 2 diabetes: the roles of cognitive functioning and psychiatric symptom severity. Psychosom Med 2016;78:263-70. https://doi.org/10.1097/PSY.0000000000000295.
- Foley DL, Mackinnon A, Morgan VA, Watts GF, Castle DJ, Waterreus A, et al. Effect of age, family history of diabetes, and antipsychotic drug treatment on risk of diabetes in people with psychosis: a population-based cross-sectional study. Lancet Psychiatry 2015;2:1092-8. https://doi.org/10.1016/S2215-0366(15)00276-X.
- Wu CS, Lai MS, Gau SS. Complications and mortality in patients with schizophrenia and diabetes: population-based cohort study. Br J Psychiatry 2015;207:450-7. https://doi.org/10.1192/bjp.bp.113.143925.
- Foley DL, Mackinnon A, Morgan VA, Watts GF, McGrath JJ, Castle DJ, et al. Predictors of type 2 diabetes in a nationally representative sample of adults with psychosis. World Psychiatry 2014;13:176-83. https://doi.org/10.1002/wps.20130.
- Chen SR, Chien YP, Kang CM, Jeng C, Chang WY. Comparing self-efficacy and self-care behaviours between outpatients with comorbid schizophrenia and type 2 diabetes and outpatients with only type 2 diabetes. J Psychiatr Ment Health Nurs 2014;21:414-22. https://doi.org/10.1111/jpm.12101.
- Mathur R, Hull SA, Boomla K, Robson J. Ethnic differences in primary care management of diabetes and cardiovascular disease in people with serious mental illness. Br J Gen Pract 2012;62:e582-8. https://doi.org/10.3399/bjgp12X653642.
- Schoepf D, Potluri R, Uppal H, Natalwala A, Narendran P, Heun R. Type-2 diabetes mellitus in schizophrenia: increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry 2012;27:33-42. https://doi.org/10.1016/j.eurpsy.2011.02.009.
- Becker T, Hux J. Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada. Diabetes Care 2011;34:398-402. https://doi.org/10.2337/dc10-1139.
- Vinogradova Y, Coupland C, Hippisley-Cox J, Whyte S, Penny C. Effects of severe mental illness on survival of people with diabetes. Br J Psychiatry 2010;197:272-7. https://doi.org/10.1192/bjp.bp.109.074674.
- Whyte S, Penny C, Phelan M, Hippisley-Cox J, Majeed A. Quality of diabetes care in patients with schizophrenia and bipolar disorder: cross-sectional study. Diabet Med 2007;24:1442-8. https://doi.org/10.1111/j.1464-5491.2007.02324.x.
- McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophr Res 2006;86:36-44. https://doi.org/10.1016/j.schres.2006.05.010.
- Dixon LB, Kreyenbuhl JA, Dickerson FB, Donner TW, Brown CH, Wohlheiter K, et al. A comparison of type 2 diabetes outcomes among persons with and without severe mental illnesses. Psychiatr Serv 2004;55:892-900. https://doi.org/10.1176/appi.ps.55.8.892.
- Koro CE, Fedder DO, L’Italien GJ, Weiss SS, Magder LS, Kreyenbuhl J, et al. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7358.243.
- Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163-80. https://doi.org/10.1002/wps.20420.
- Pillinger T, Beck K, Stubbs B, Howes OD. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br J Psychiatry 2017;211:339-49. https://doi.org/10.1192/bjp.bp.117.200907.
- Teasdale SB, Ward PB, Rosenbaum S, Samaras K, Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry 2017;210:110-18. https://doi.org/10.1192/bjp.bp.115.177139.
- Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 2017;16:308-15. https://doi.org/10.1002/wps.20458.
- Stubbs B, Firth J, Berry A, Schuch FB, Rosenbaum S, Gaughran F, et al. Schizophr Res 2016;176:431-40. https://doi.org/10.1016/j.schres.2016.05.017.
- Stubbs B, Williams J, Gaughran F, Craig T. How sedentary are people with psychosis? A systematic review and meta-analysis. Schizophr Res 2016;171:103-9. https://doi.org/10.1016/j.schres.2016.01.034.
- Hayes JF, Miles J, Walters K, King M, Osborn DP. A systematic review and meta-analysis of premature mortality in bipolar affective disorder. Acta Psychiatr Scand 2015;131:417-25. https://doi.org/10.1111/acps.12408.
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015;14:339-47. https://doi.org/10.1002/wps.20252.
- Rosenbaum S, Lederman O, Stubbs B, Vancampfort D, Stanton R, Ward PB. How can we increase physical activity and exercise among youth experiencing first-episode psychosis? A systematic review of intervention variables. Early Interv Psychiatry 2016;10:435-40. https://doi.org/10.1111/eip.12238.
- Happell B, Davies C, Scott D. Health behaviour interventions to improve physical health in individuals diagnosed with a mental illness: a systematic review. Int J Ment Health Nurs 2012;21:236-47. https://doi.org/10.1111/j.1447-0349.2012.00816.x.
- Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 2011;68:609-16. https://doi.org/10.1001/archgenpsychiatry.2011.2.
- Roberts SH, Bailey JE. Incentives and barriers to lifestyle interventions for people with severe mental illness: a narrative synthesis of quantitative, qualitative and mixed methods studies. J Adv Nurs 2011;67:690-708. https://doi.org/10.1111/j.1365-2648.2010.05546.x.
- Cabassa LJ, Ezell JM, Lewis-Fernández R. Lifestyle interventions for adults with serious mental illness: a systematic literature review. Psychiatr Serv 2010;61:774-82. https://doi.org/10.1176/ps.2010.61.8.774.
- Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009;194:491-9. https://doi.org/10.1192/bjp.bp.107.045732.
- Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry 2015;2:452-64. https://doi.org/10.1016/S2215-0366(15)00115-7.
- Nash M. Improving mental health service users’ physical health through medication monitoring: a literature review. J Nurs Manag 2011;19:360-5. https://doi.org/10.1111/j.1365-2834.2011.01244.x.
- Stubbs B, Chen LJ, Chung MS, Ku PW. Physical activity ameliorates the association between sedentary behavior and cardiometabolic risk among inpatients with schizophrenia: A comparison versus controls using accelerometry. Compr Psychiatry 2017;74:144-50. https://doi.org/10.1016/j.comppsych.2017.01.010.
- Das-Munshi J, Chang CK, Dutta R, Morgan C, Nazroo J, Stewart R, et al. Ethnicity and excess mortality in severe mental illness: a cohort study. Lancet Psychiatry 2017;4:389-99. https://doi.org/10.1016/S2215-0366(17)30097-4.
- Osborn D, Marston L, Nazareth I, King MB, Petersen I, Walters K. Relative risks of cardiovascular disease in people prescribed olanzapine, risperidone and quetiapine. Schizophr Res 2017;183:116-23. https://doi.org/10.1016/j.schres.2016.11.009.
- Stubbs B, Koyanagi A, Veronese N, Vancampfort D, Solmi M, Gaughran F, et al. Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low- and middle-income countries. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0734-z.
- Vancampfort D, Guelinckx H, Probst M, Stubbs B, Rosenbaum S, Ward PB, et al. Health-related quality of life and aerobic fitness in people with schizophrenia. Int J Ment Health Nurs 2015;24:394-402. https://doi.org/10.1111/inm.12145.
- Vancampfort D, De Hert M, Stubbs B, Ward PB, Rosenbaum S, Soundy A, et al. Negative symptoms are associated with lower autonomous motivation towards physical activity in people with schizophrenia. Compr Psychiatry 2015;56:128-32. https://doi.org/10.1016/j.comppsych.2014.10.007.
- Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013;368:1594-602. https://doi.org/10.1056/NEJMoa1214530.
- Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom's General Practice Research Database. Arch Gen Psychiatry 2007;64:242-9. https://doi.org/10.1001/archpsyc.64.2.242.
- Worswick J, Wayne SC, Bennett R, Fiander M, Mayhew A, Weir MC, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews – what does the evidence tell us?. Syst Rev 2013;2. https://doi.org/10.1186/2046-4053-2-26.
- Lind M, Odén A, Fahlén M, Eliasson B. A systematic review of HbA1c variables used in the study of diabetic complications. Diabetes Metab Synd 2008;2:282-93. https://doi.org/10.1016/j.dsx.2008.04.006.
- Steed L, Cooke D, Newman S. A systematic review of psychosocial outcomes following education, self-management and psychological interventions in diabetes mellitus. Patient Educ Couns 2003;51:5-15. https://doi.org/10.1016/s0738-3991(02)00213-6.
- Betteridge J. Benefits of lipid-lowering therapy in patients with type 2 diabetes mellitus. Am J Med 2005;118:10-5. https://doi.org/10.1016/j.amjmed.2005.09.013.
- Kontopantelis E, Springate DA, Reeves D, Ashcroft DM, Rutter MK, Buchan I, et al. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia 2015;58:505-18. https://doi.org/10.1007/s00125-014-3473-8.
- Nicolucci A, Kovacs Burns K, Holt RI, Comaschi M, Hermanns N, Ishii H, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 2013;30:767-77. https://doi.org/10.1111/dme.12245.
- Farmer A, Hardeman W, Hughes D, Prevost AT, Kim Y, Craven A, et al. An explanatory randomised controlled trial of a nurse-led, consultation-based intervention to support patients with adherence to taking glucose lowering medication for type 2 diabetes. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-30.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Diabetes Education and Self Management for Ongoing and Newly Diagnosed Collaborative. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89. https://doi.org/10.1056/NEJMoa0806470.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005;22:1379-85. https://doi.org/10.1111/j.1464-5491.2005.01644.x.
- Price HC, Ismail K. Royal College of Psychiatrists Liaison Faculty & Joint British Diabetes Societies (JBDS): guidelines for the management of diabetes in adults and children with psychiatric disorders in inpatient settings. Diabet Med 2018;35:997-1004. https://doi.org/10.1111/dme.13673.
- National Institute for Health and Care Excellence . Psychosis and Schizophrenia in Adults n.d. www.nice.org.uk/guidance/qs80 (accessed December 2019).
- National Institute for Health and Care Excellence . Type 2 Diabetes: Prevention in People at High Risk n.d. www.nice.org.uk/Guidance/PH38 (accessed December 2019).
- National Institute for Health and Care Excellence . Diabetes in Adults n.d. www.nice.org.uk/Guidance/QS6 (accessed December 2019).
Appendix 1 Reflections on the experience of participating in patient and public involvement
The DIAMONDS VOICE feedback on involvement in the study has been very positive, with members feeling that their contributions are both valuable and valued, and stating that they feel empowered by their involvement. Their commitment to the research is perhaps evidenced by the stability of the group membership, the core of which has endured for several years, despite the challenges created by comorbid SMI and long-term physical health problems.
Feedback from the DIAMONDS VOICE group indicated that members did not always understand how they could contribute at research meetings, so sometimes they felt that they were not needed. Some members of the research team used abbreviations and did not explain complex processes/methodology in lay language, which could make people feel overwhelmed and as if they had nothing to contribute. These issues could create barriers to discussion and engagement. Changes were made during the study because of this feedback. Group members are now briefed before and after meetings, and the PPI co-ordinator is always available to provide support if needed. Research team members chairing meetings are also asked to be aware of these issues and to ensure that the language and terminology are appropriate. Unfortunately, these efforts do not always eliminate these challenges. This has been an important learning point for the research team, which will continue to consider ways to optimise the contribution of PPI members to research in future studies.
Reflections on patient and public involvement from individual DIAMONDS VOICE members
Following each DIAMONDS VOICE meeting, comments were gathered by initial debrief and telephone calls. Two members of the panel agreed that their comments could be included in this report.
Member 1
This person took part in developing question prompts and tools.
-
Learning lots and big learning curve. Learning what research is and how it works.
-
Being and learning with others from a similar background. Like sharing with others, reaching out to other people.
-
Opens my eyes to different avenues and opportunities how [you] can care for yourself.
-
Explained well.
-
It’s getting me out.
Member 2
This person helped to identify research priorities, design question tools and prompts, and was involved in practice interviews. They also wrote notes, reviewed documents prior to submission for ethics approval and helped to prioritise questions, process some of the findings from the workshops and develop story boards. They are planning on being involved in study dissemination at research events and NHS trusts and user groups.
-
We feel valued as carers/patients and that our experiences count.
-
Been well informed and updated.
-
Feel empowered we have a voice.
-
Feel that people really care about how difficult it is to cope with SMI and diabetes.
-
It is interesting to see the processes in research and how it evolves.
-
Great to have peer support [and] share ideas. Made new friends who understand.
-
I have increased confidence [and a] sense of purpose.
-
Develop own knowledge, becoming expert by experience.
-
Getting out and about to new venues.
-
Always supported to attend meetings.
-
Being part of something exciting that gives others quality of life.
-
Being part of the team working with researchers, clinicians, professionals and stakeholders.
Appendix 2 Full list of sources included in the scoping of the literature
| Author(s) | Year of publication | Title | Journal |
|---|---|---|---|
| Systematic reviews and meta-analyses | |||
| Taylor et al.160 | 2017 | The effectiveness of pharmacological and non-pharmacological interventions for improving glycaemic control in adults with severe mental illness: a systematic review and meta-analysis | PLOS ONE |
| Pillinger et al.37 | 2017 | Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis | JAMA Psychiatry |
| Gorczynski et al.161 | 2017 | Are people with schizophrenia adherent to diabetes medication? A comparative meta-analysis | Psychiatry Research |
| Vancampfort et al.44 | 2016 | Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis | World Psychiatry |
| Galling et al.40 | 2016 | Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis | JAMA Psychiatry |
| Stubbs et al.122 | 2015 | The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis | Acta Psychiatrica Scandinavica |
| Systematic reviews | |||
| Charles et al.162 | 2016 | Bipolar disorder and diabetes mellitus: evidence for disease-modifying effects and treatment implications | International Journal of Bipolar Disorders |
| McGinty et al.163 | 2016 | Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: a comprehensive review | Schizophrenia Bulletin |
| McBain et al.164 | 2016 | Self-management interventions for type 2 diabetes in adult people with severe mental illness (review) | Cochrane Database of Systematic Reviews |
| McGinty et al.165 | 2015 | Quality of medical care for persons with serious mental illness: a comprehensive review | Schizophrenia Research |
| Ward and Druss166 | 2015 | The epidemiology of diabetes in psychiatric disorders | Lancet Psychiatry |
| Cimo et al.167 | 2012 | Effective lifestyle interventions to improve type II diabetes self-management for those with schizophrenia or schizoaffective disorder: a systematic review | BMC Psychiatry |
| Literature reviews | |||
| Whitehead168 | 2017 | Self-management of type 2 diabetes and severe mental illness | Issues in Mental Health Nursing |
| Bushe and Holt169 | 2004 | Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia | British Journal of Psychiatry |
| Primary quantitative studies | |||
| Brown et al.156 | 2017 | Achievement of adequate glycaemic control in patients with type 2 diabetes and comorbid mental health conditions treated in a primary care setting | Diabetes Spectrum |
| Das-Munshi et al.147 | 2017 | Type 2 diabetes mellitus in people with severe mental illness: inequalities by ethnicity and age. Cross-sectional analysis of 588 408 records from the UK | Diabetic Medicine |
| Smith et al.115 | 2019 | Glucose, cholesterol and blood pressure in type II diabetes: a longitudinal observational study comparing patients with and without severe mental illness | Journal of Psychiatric and Mental Health Nursing |
| Foley et al.170 | 2016 | Awareness of pre-diabetes or diabetes and associated factors in people with psychosis | Schizophrenia Bulletin |
| Foley et al.171 | 2016 | Common familial risk factors for schizophrenia and diabetes mellitus | Australian and New Zealand Journal of Psychiatry |
| Rathmann et al.172 | 2016 | Diabetes treatment in people with type 2 diabetes and schizophrenia: retrospective primary care database analyses | Primary Care Diabetes |
| Wake et al.173 | 2016 | Altered metabolic parameters in association with antipsychotic medication use in diabetes: a population based case–control study | Psychoneuroendocrinology |
| Wu and Gau174 | 2016 | Association between antipsychotic treatment and advanced diabetes complications among schizophrenia patients with type 2 diabetes mellitus | Schizophrenia Bulletin |
| Wykes et al.175 | 2016 | Self-efficacy and hemoglobin a1c among adults with serious mental illness and type 2 diabetes: the roles of cognitive functioning and psychiatric symptom severity | Psychosomatic Medicine |
| Foley et al.176 | 2015 | Effect of age, family history of diabetes, and antipsychotic drug treatment on risk of diabetes in people with psychosis: a population-based cross-sectional study | Lancet Psychiatry |
| Wu et al.177 | 2015 | Complications and mortality in patients with schizophrenia and diabetes: population-based cohort study | British Journal of Psychiatry |
| Foley et al.178 | 2014 | Predictors of type 2 diabetes in a nationally representative sample of adults with psychosis | World Psychiatry |
| Chen et al.179 | 2014 | Comparing self-efficacy and self-care behaviours between outpatients with comorbid schizophrenia and type 2 diabetes and outpatients with only type 2 diabetes | Journal of Psychiatric and Mental Health Nursing |
| Mathur et al.180 | 2012 | Ethnic differences in primary care management of diabetes and cardiovascular disease in people with serious mental illness | British Journal of General Practice |
| Schoepf et al.181 | 2012 | Type-2 diabetes mellitus in schizophrenia: increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up | European Psychiatry |
| Becker and Hux182 | 2011 | Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada | Diabetes Care |
| Nielsen et al.39 | 2010 | Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients | Neuropsychopharmacology |
| Vinogradova et al.183 | 2010 | Effects of severe mental illness on survival of people with diabetes | British Journal of Psychiatry |
| Whyte et al.184 | 2007 | Quality of diabetes care in patients with schizophrenia and bipolar disorder: cross-sectional study | Diabetic Medicine |
| McKibbin et al.185 | 2006 | A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial | Schizophrenia Research |
| Dixon et al.186 | 2004 | A comparison of type 2 diabetes outcomes among persons with and without severe mental illnesses | Psychiatric Services |
| Koro et al.187 | 2002 | Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case–control study | BMJ |
| Author(s) | Year of publication | Title | Journal |
|---|---|---|---|
| Systematic reviews and meta-analyses | |||
| Correll et al.188 | 2017 | Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls | World Psychiatry |
| Pillinger et al.189 | 2017 | Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis | British Journal of Psychiatry |
| Teasdale et al.190 | 2017 | Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness | British Journal of Psychiatry |
| Vancampfort et al.191 | 2017 | Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis | World Psychiatry |
| Stubbs et al.192 | 2016 | How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression | Schizophrenia Research |
| Stubbs et al.193 | 2016 | How sedentary are people with psychosis? A systematic review and meta-analysis | Schizophrenia Research |
| Hayes et al.194 | 2015 | A systematic review and meta-analysis of premature mortality in bipolar affective disorder | Acta Psychiatrica Scandinavica |
| Vancampfort et al.195 | 2015 | Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis | World Psychiatry |
| Rummel-Kluge et al.51 | 2010 | Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis | Schizophrenia Research |
| Systematic reviews | |||
| Rosenbaum et al.196 | 2016 | How can we increase physical activity and exercise among youth experiencing first-episode psychosis? A systematic review of intervention variables | Early Intervention in Psychiatry |
| Happell et al.197 | 2012 | Health behaviour interventions to improve physical health in individuals diagnosed with a mental illness: a systematic review | International Journal of Mental Health Nursing |
| Foley and Morley198 | 2011 | Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis | Archives of General Psychiatry |
| Roberts and Bailey199 | 2011 | Incentives and barriers to lifestyle interventions for people with severe mental illness: a narrative synthesis of quantitative, qualitative and mixed methods studies | Journal of Advanced Nursing |
| Cabassa et al.200 | 2010 | Lifestyle interventions for adults with serious mental illness: a systematic literature review | Psychiatric Services |
| Mitchell et al.201 | 2009 | Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies | British Journal of Psychiatry |
| Literature reviews | |||
| Henderson et al.202 | 2015 | Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses | Lancet Psychiatry |
| Nash203 | 2011 | Improving mental health service users physical health through medication monitoring: a literature review | Journal of Nursing Management |
| Primary quantitative studies | |||
| Hayes et al.17 | 2017 | Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014 | British Journal of Psychiatry |
| Stubbs et al.204 | 2017 | Physical activity ameliorates the association between sedentary behavior and cardiometabolic risk among inpatients with schizophrenia: a comparison versus controls using accelerometry | Comprehensive Psychiatry |
| Das-Munshi et al.205 | 2017 | Ethnicity and excess mortality in severe mental illness: a cohort study | Lancet Psychiatry |
| Osborn et al.206 | 2017 | Relative risks of cardiovascular disease in people prescribed olanzapine, risperidone and quetiapine | Schizophrenia Research |
| Stubbs et al.207 | 2016 | Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low and middle-income countries | BMC Medicine |
| Gutacker et al.109 | 2015 | Does the quality and outcomes framework reduce psychiatric admissions in people with serious mental illness? A regression analysis | BMJ Open |
| Reilly et al.20 | 2015 | Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK | BMJ Open |
| Vancampfort et al.208 | 2015 | Health-related quality of life and aerobic fitness in people with schizophrenia | International Journal of Mental Health Nursing |
| Vancampfort et al.209 | 2015 | Negative symptoms are associated with lower autonomous motivation towards physical activity in people with schizophrenia | Comprehensive Psychiatry |
| Daumit et al.210 | 2013 | A behavioral weight-loss intervention in persons with serious mental illness | New England Journal of Medicine |
| Reilly et al.137 | 2012 | The role of primary care in service provision for people with severe mental illness in the United Kingdom | PLOS ONE |
| Brown et al.62 | 2010 | Twenty-five year mortality of a community cohort with schizophrenia | British Journal of Psychiatry |
| Osborn et al.211 | 2007 | Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database | Archives of General Psychiatry |
| Author(s) | Year of publication | Title | Journal |
|---|---|---|---|
| Systematic reviews and meta-analyses | |||
| Ricci-Cabello et al.119 | 2013 | Improving diabetes care in rural areas: a systematic review and meta-analysis of quality improvement interventions in OECD countries | PLOS ONE |
| Ricci-Cabello et al.118 | 2013 | Health care interventions to improve the quality of diabetes care in African Americans | Diabetes Care |
| Systematic reviews | |||
| Worswick et al.212 | 2013 | Improving quality of care for persons with diabetes: an overview of systematic reviews – what does the evidence tell us? | Systematic Reviews |
| Zeh et al.120 | 2012 | The impact of culturally competent diabetes care interventions for improving diabetes-related outcomes in ethnic minority groups: a systematic review | Diabetic Medicine |
| Lind et al.213 | 2008 | A systematic review of HbA1c variables used in the study of diabetic complications | Diabetes and Metabolic Syndrome |
| Peek et al.117 | 2007 | Diabetes health disparities: a systematic review of health care interventions | Medical Care Research and Review |
| Steed et al.214 | 2003 | A systematic review of psychosocial outcomes following education, self-management and psychological interventions in diabetes mellitus | Patient Education and Counseling |
| Literature reviews | |||
| Peek et al.116 | 2014 | Integrated community-healthcare diabetes interventions to reduce disparities | Current Diabetes Reports |
| Betteridge215 | 2005 | Benefits of lipid-lowering therapy in patients with type 2 diabetes mellitus | American Journal of Medicine |
| Primary quantitative studies | |||
| Kontopantelis et al.216 | 2015 | Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study | Diabetologia |
| Nicolucci et al.217 | 2013 | Diabetes attitudes, wishes and needs second study (DAWN2TM): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes | Diabetic Medicine |
| Farmer et al.218 | 2012 | An explanatory randomised controlled trial of a nurse-led, consultation-based intervention to support patients with adherence to taking glucose lowering medication for type 2 diabetes | BMC Family Practice |
| Davies et al.219 | 2008 | Effectiveness of the diabetes education and self-management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial | BMJ |
| Holman et al.220 | 2008 | 10-Year follow-up of intensive glucose control in type 2 diabetes | New England Journal of Medicine |
| Peyrot et al.221 | 2005 | Psychosocial problems and barriers to improved diabetes management: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study | Diabetic Medicine |
| Author(s) | Year of publication | Title | Code |
|---|---|---|---|
| Price and Ismail222 | 2017 | The management of diabetes in adults and children with psychiatric disorders in inpatient settings | JBDS 13 |
| NICE223 | 2015 | Psychosis and schizophrenia in adults | QS80 |
| NICE32 | 2015 | Type 2 diabetes in adults: management | NG28 |
| NICE13 | 2014 | Bipolar disorder: assessment and management | CG185 |
| NICE12 | 2014 | Psychosis and schizophrenia in adults: prevention and management | CG178 |
| NICE224 | 2012 | Type 2 diabetes: prevention in people at high risk | PH38 |
| NICE225 | 2011 | Diabetes in adults | QS6 |
Appendix 3 Non-linear impact of age on the risk of diabetes
FIGURE 10.
Objective 1: age distribution of eligible patients (a) without diabetes; and (b) with diabetes.
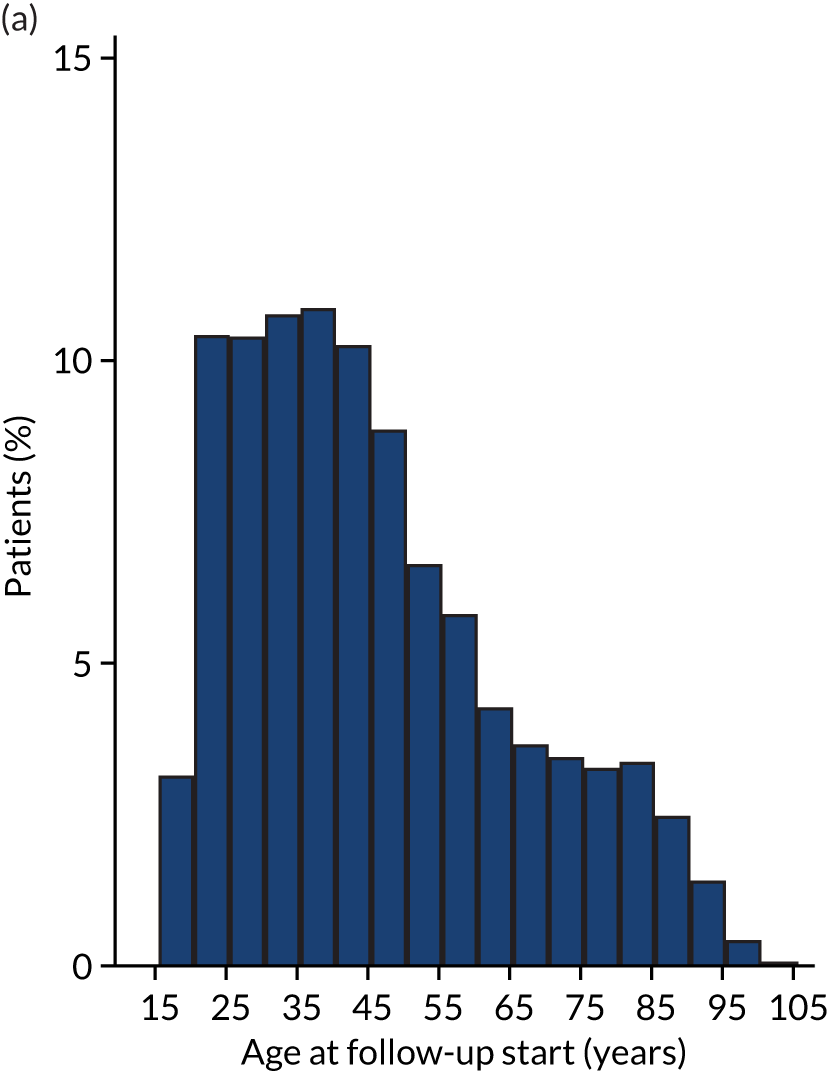
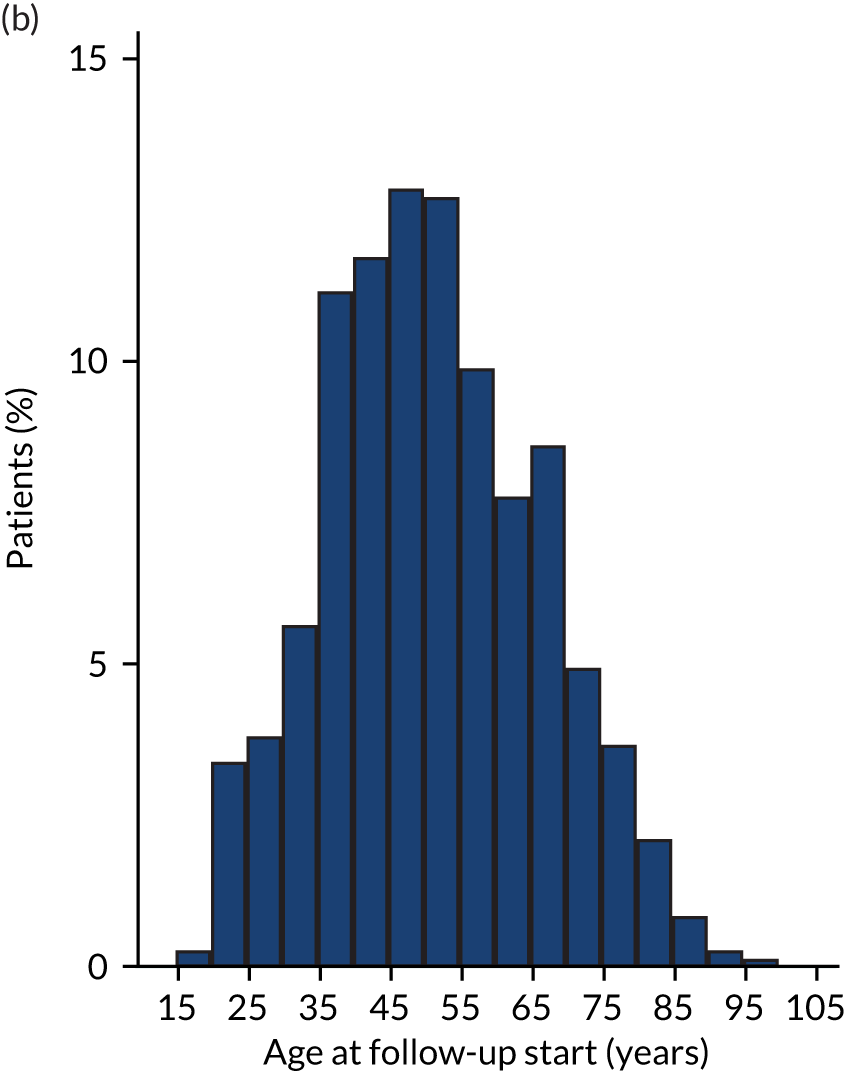
Appendix 4 Objective 7: a descriptive summary of provision of health-care interventions by diagnosis order for people with severe mental illness and diabetes
| SMI diagnosed before T2DM | SMI diagnosed after T2DM (including same day) | |||
|---|---|---|---|---|
| Cases (T2DM + SMI) | Controls (T2DM) | Cases (T2DM + SMI) | Controls (T2DM) | |
| Total, N = 9965 [cases, n = 2192 (22.0%); controls, n = 7773 (78.0%)] | ||||
| Patients (n) | 1666 | 5883 | 526 | 1890 |
| Number of additional health checks per year, mean (SD) | ||||
| Retinopathy screening | 0.76 (0.71) | 0.89 (0.85) | 0.73 (0.53) | 0.87 (0.87) |
| Diabetes education referral | 0.22 (1.12) | 0.25 (1.22) | 0.05 (0.16) | 0.34 (8.43) |
| Influenza vaccination | 0.32 (0.45) | 0.33 (0.51) | 0.20 (0.30) | 0.28 (0.39) |
| Total, N = 8905 [cases, n = 1968 (22.1%); controls, n = 6937 (77.9%)]a | ||||
| Patients (n) | 1456 | 5239 | 512 | 1698 |
| Number of additional health checks per year, mean (SD) | ||||
| Retinopathy screening | 0.79 (0.58) | 0.88 (0.57) | 0.73 (0.51) | 0.85 (0.56) |
| Diabetes education referral | 0.10 (0.26) | 0.10 (0.25) | 0.04 (0.12) | 0.07 (0.19) |
| Influenza vaccination | 0.30 (0.36) | 0.31 (0.35) | 0.19 (0.26) | 0.27 (0.32) |
Appendix 5 Coding frameworks for the qualitative data
This appendix has been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
There were 11 ‘parent’ codes in the framework (see Box 1), shown with their associated ‘child’ nodes. There were also two additional codes used to identify any other respondents speaking during the interview, and when a person exhibited some psychotic symptoms during interview.
-
Demographics and context:
-
age
-
caring responsibilities
-
current medication
-
diabetes type
-
diagnoses
-
ethnicity
-
family details
-
family health history
-
hobbies and interests
-
home and local environment
-
level of education
-
lifestyle
-
mobility
-
money and income
-
past trauma
-
personal history
-
relationships and social network
-
religious beliefs.
-
-
Diabetes education, knowledge and training:
-
access to education and knowledge
-
barriers to education and knowledge
-
being offered education
-
experience of education courses
-
impact of education and knowledge
-
knowledge of diabetes
-
knowledge of diabetes management
-
sources of information
-
specific education needs for this group.
-
-
Informal support and social contact:
-
activity groups
-
barriers to support
-
current support
-
experience of support groups
-
experiences of charities and organisations
-
impact of support
-
loss of support
-
rejecting support
-
types of support.
-
-
Having diabetes with SMI:
-
three-way interactions of diabetes, SMI and health behaviours
-
descriptions of interactions
-
diabetes takes priority
-
impact of diabetes on mental health
-
impact of mental health on diabetes
-
interactions between mental and physical care
-
mental health takes priority.
-
-
Experience of diabetes:
-
burden of diabetes
-
crisis points
-
diabetes and diet
-
diabetes control
-
duration or timings of diagnosis and treatment
-
experiences and perceptions of diabetes
-
family history
-
first port of call for concerns
-
good days and bad days
-
impact of diabetes
-
perceived causes
-
stigma and discrimination
-
symptoms and complications.
-
-
Experience of mental health care:
-
access to care
-
barriers to care
-
changes to care
-
current care
-
experience of medication
-
impact of care
-
involvement in care decisions
-
opinions on health care
-
personal experiences of mental health care
-
power dynamics
-
timing of care received
-
understanding of care received
-
wishes for and thoughts on improvements
-
worries about health care.
-
-
Other health problems:
-
effect on diabetes
-
effects on mental health
-
health worries
-
medication side effects
-
medications taken
-
types of health problem.
-
-
Employment:
-
barriers to working and employment
-
current working status
-
experience of working with SMI
-
impact of health on employment
-
past employment.
-
-
Experience of mental illness:
-
behaviours associated with mental illness
-
burden of mental illness
-
coping mechanisms
-
crisis points
-
current state of mental health
-
disclosing mental illness
-
duration or timings of illness and treatment
-
effect of outside influences
-
first port of call for concerns
-
good days and bad days
-
impact of mental illness
-
not feeling in control
-
others’ opinions and perceptions
-
perceived causes
-
perceptions of mental illness
-
personal experiences
-
stigma and discrimination
-
symptoms of mental illness
-
understanding and perceptions of own illness.
-
-
Experience of physical health care:
-
access to care
-
barriers to care
-
changes to care
-
current care
-
experience of medication
-
follow-up care
-
involvement in care decisions
-
opinions on care
-
personal experiences of physical health care
-
wishes and thoughts for improvement.
-
-
Self-management:
-
barriers to self-management
-
deciding to change
-
enablers of self-management
-
feeling in control
-
impact of self-management
-
poor self-management
-
self-management behaviours
-
self-management success
-
support for self-management
-
tools for self-management
-
worries about self-management.
-
-
Other respondents.
-
Psychosis during interview.
There were 13 ‘parent’ codes in this framework (see Box 2), each shown with their associated ‘child’ nodes. There was also an additional code used to identify when a respondent other than the participant was speaking (‘other respondents’).
-
Carer experiences:
-
carer dependence on main respondent
-
carer feelings of distress
-
carer opinion of medication
-
carer personal experience of medication
-
general carer experiences.
-
-
Demographics and context:
-
age
-
caring responsibilities
-
current medication
-
diabetes type
-
diagnoses
-
ethnicity
-
family details
-
family health history
-
hobbies and interests
-
home and local environment
-
level of education
-
lifestyle
-
mobility
-
money and income
-
past trauma
-
personal history
-
relationships and social network
-
religious beliefs.
-
-
Diabetes education, knowledge and training:
-
access to education and knowledge
-
barriers to education and knowledge
-
being offered education
-
experience of education courses
-
impact of education and knowledge
-
knowledge of diabetes
-
knowledge of diabetes management
-
sources of information
-
specific education needs for this group.
-
-
Employment:
-
barriers to working and employment
-
current working status
-
experience of working with SMI
-
impact of health on employment
-
past employment.
-
-
Experience of diabetes:
-
burden of diabetes
-
diabetes and diet
-
diabetes control
-
duration or timings of diagnosis and treatment
-
experiences and perceptions of diabetes
-
family history
-
first port of call for concerns
-
impact of diabetes
-
perceived causes
-
symptoms and complications.
-
-
Experience of mental health care:
-
access to care
-
barriers to care
-
changes to care
-
current care
-
experience of medication
-
impact of care
-
involvement in care decisions
-
opinions on health care
-
personal experiences of mental health care
-
power dynamics
-
timing of care received
-
understanding of care received
-
wishes for and thoughts on improvements
-
worries about health care.
-
-
Experience of physical health care:
-
access to care
-
barriers to care
-
changes to care
-
current care
-
experience of medication
-
follow-up care
-
involvement in care decisions
-
opinions on care
-
personal experiences of physical health care
-
wishes and thoughts for improvement.
-
-
Experience of mental illness:
-
behaviours associated with mental illness
-
burden of mental illness
-
coping mechanisms
-
crisis points
-
current state of mental health
-
disclosing mental illness
-
duration or timings of illness and treatment
-
effect of outside influences
-
first port of call for concerns
-
good days and bad days
-
impact of mental illness
-
not feeling in control
-
others’ opinions and perceptions
-
perceived causes
-
perceptions of mental illness
-
personal experiences
-
stigma and discrimination
-
symptoms of mental illness.
-
-
Having diabetes with SMI:
-
three-way interactions of diabetes, SMI and health behaviours
-
descriptions of interactions
-
diabetes takes priority
-
impact of diabetes on mental health
-
impact of mental health on diabetes
-
interactions between mental and physical care
-
mental health takes priority.
-
-
Informal support and social contact:
-
activity groups
-
barriers to support
-
current support
-
experience of support groups
-
experiences of charities and organisations
-
impact of support
-
loss of support
-
rejecting support
-
types of support.
-
-
Other health problems:
-
effect on diabetes
-
effects on mental health
-
health worries
-
medication side effects
-
medications taken
-
types of health problem.
-
-
Self-management:
-
barriers to self-management
-
deciding to change
-
enablers of self-management
-
feeling in control
-
impact of self-management
-
poor self-management
-
self-management behaviours
-
self-management success
-
support for self-management
-
tools for self-management
-
worries about self-management.
-
-
Other respondents.
There were 20 ‘parent’ codes in this framework (see Box 3), each shown with their associated ‘child’ nodes.
-
Barriers to delivering and receiving care.
-
Changes made to care.
-
Changes needed in, and recommendations for, care and support.
-
Demographics and context:
-
local area, ethnicity and socioeconomic status
-
personal and health issues
-
role and responsibilities
-
special interest or experience
-
training and career path.
-
-
Diabetes education, knowledge and training: patients –
-
access to education and knowledge
-
barriers to education and knowledge
-
education needs for this group
-
education offered
-
experience of education courses
-
impact of education and knowledge
-
knowledge of diabetes and management
-
sources of information.
-
-
Differences between disorders.
-
Employment – patients.
-
Enablers of delivering and receiving care.
-
Informal support: patients –
-
any other informal support
-
family support and interactions.
-
-
Interactions between disciplines and types of care.
-
Interactions of diabetes and SMI:
-
descriptions of interactions
-
diabetes takes priority
-
impact of diabetes on mental health
-
impact of mental health on diabetes
-
mental health takes priority.
-
-
Medication.
-
Opinions on care.
-
Other health problems: patients –
-
care for comorbidities
-
effect on diabetes
-
effects on mental health
-
types of health problem.
-
-
Other services and care providers.
-
Patient experience of diabetes.
-
Patient experience of mental illness.
-
Patient self-management:
-
barriers to self-management
-
enablers of self-management
-
impact of self-management
-
poor self-management
-
self-management behaviours
-
self-management success
-
support for self-management
-
tools for self-management.
-
-
Personal and general experiences in job.
-
Staff training and training needs:
-
types of care or service delivered and interactions with patients.
-
Appendix 6 Supplementary tables of quotations for the qualitative results
Some of the participant quotations in this section have been reproduced with permission from Bellass et al. 143 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
These supplementary tables give extra participant quotations for the eight key themes (with associated subthemes) from the qualitative study.
| Subtheme | Associated quotations |
|---|---|
| The pervasive effect of SMI, and persistence and powerlessness | It’s something actually inside your head all the time and it’s like you’re carrying around mental illness. I can’t describe it, but it’s like being, it’s like having a cold sometimes. Just for example, it’s just like having a cold. You want to get rid of it but you can’t and it’s in your head and it’s making you feel naff, but that’s how you feel like with mental illness. Not like a cold. I’m not saying like a cold, but like a feeling of being ill, you know, being constantly ill all the timeES-G3-01It just keeps going round and re-playing and re-playing and you’ve got to get off the boat sometimes. It keeps going round and round, this illness keeps going the same thing and when am I going to get off the circle? When am I going to get on? And you can’t because it’s mental, it’s a mental thing. It’s not, it’s not, it’s not life. . . . I mean it’s not like a diet. I mean, you can change your diet. You can’t change your mental illnessES-G3-01Well I don’t like going out on my own, I don’t like doing anything on my own, I wait for my husband, so I’m just getting worse actually, not going out, putting myself off, not going to the shops or anything like that. I make appointments and then I cancel them, I used to have friends, but I haven’t got any any more, they’ve all deserted meES-G7-01I’ve made a big effort to pull myself together and I’m trying to do things around my home. But for instance, I’ve been in my flat for about 2 and a half years and last week was the first time that I’ve cleaned the kitchen floor. [. . .] I’m delighted with the outcome but found it a tremendous barrier and effort to do itES-G8-1You’re not allowed to drive for 3 months after being manic, I think it is, and then you can make an application to carry on, and then they give you a 1-year temporary licence, and I’ve managed to get to a 3-year one nowES-T2-03Really lonely, really. I’ve been let down by so many people that I’m wary in making contactES-T2-06It could be something quite . . . something that triggers it off, like, if they got her going out and then she had a real bad experience one time when she was out, it was something totally unforeseen, something that most people would, sort of, treat as a bit annoying or a bit . . . not a good day, but you’d, sort of, take it in your stride, you’d be back again the next day. [. . .] Most days it doesn’t happen, that’s the sort of thing which when you . . . to someone with mental health issues, they become major incidents and set things going backwardsFamily member ES-T4-03If her attention starts wandering, she has, when we had the previous cooker with the rings for the hob, she put her hand on it and it made circular marks on her hand there, that was just through attention wanderingFamily member ES-T4-03[Talking about inpatient centre:] Guys are in there for months, spending months, eating the same stuff. You can be saying ‘listen, we hope we can make an impact here. We can be making an impact from the diabetic point of view, to show you there’s different things. Maybe a brown pasta, whatever it is, you can try . . . whatever. Just other things that you can eat and it’s really tasty and it looks good. But it’s not going to be as bad for you’Family member ES-T2-08So they say, ‘well, you’ve got diabetes so therefore you need to take something to lower your blood sugar levels because you’re more susceptible to stroke if you do. [sic]’. Where does that apply to mental health aspects, the other tablets that she takes? They will also be having an effect somewhere down the line. I don’t know. I’m not a chemist. I’m not a biologist even, so I don’t know, but there’s no doubt about it. The more you take, the more issues you’re going to haveFamily member ES-T2-17That’s more opportunistic, but, unfortunately, in [city] it’s again choose and book; so when you refer, they may be travelling 10/20 miles sometimes depending on where the dietitian is doing the session. [. . .] And imagine if they have got mental health, then it’s quite, in regards to the social deprivation, it is quite high here so they may not be having a car, they may not have the money to be travelling on bus and if they are quite disorientated and anxious they may not want to go to unfamiliar area but, unfortunately, for that, it’s already a non-starterStaff ES-PC-04I suppose, it’s not something I actively look out for, but you would, I suppose the effect of not having, you know, not being able to make healthy eating choices because you can’t afford itStaff ES-PC-05OK, I mean I think we’ve got the highest incidence of SMI in [city] in our population for a number of different reasons, but partly because of the area of the city that it is in, but also we’ve got quite a lot of hostels, homeless hostels and kind of supported accommodation, so we have a lot of people on our books with kind of very enduring mental health, and obviously along with that goes quite a lot of complex medical problems as well, so you know, there is certainly higher numbers for us. The difficulty we’ve got is in, kind of, accessing them and trying to get them to engage in follow-up and treatment, basicallyStaff ES-PC-06I think if they live in deprived areas and money is tight, I think it’s more difficult for them to get a healthy diet, they tend to grab what they can as opposed to being educated enough to manage living on healthier food reallyStaff ES-T1-02She needs the support but she can’t pay because, you know, she is on benefits, she is on PIP [Personal Independence Payment] but she has also got a car and she argues to say that she needs that car because if she doesn’t have the car it means that she can’t go out because of physical health problems, I think she has got problems with mobility . . . So she said ‘after paying about £100 for my car insurance every month then it means I am not left with enough money to even pay for my care’, so that means people like that will end up really struggling . . . because they need the care, but there is not enough funding in placeStaff ES-T1-03Well, they are, (a) they’re really time-consuming and (b) people are getting their benefits cut and you cannot believe that they’re getting them cut when we write really, kind of, explicit supporting letters as to what their complex needs are, but that’s another story I guess, we just have to then go through all the appeals, so it’s very time-consuming; however, we try and make sure that people get what they’re entitled toStaff ES-T1-04Oh, absolutely, oh completely, we’ve got another gentleman who’s not diabetic but I guess he’s a candidate, he’s on high-dose antipsychotics, his self-neglect is terrible, his diet is really poor, [. . .] and the PIP came along quite soon after I’d met him, he’d filled the form in himself, which I felt somebody should have read and thought, ‘this man is quite unwell, we’ll put an additional supporting letter in saying he’s got a consultant, he’s got Clozaril’, you know, all kind of nitty-gritty and they cut his benefit entirely, and he rang me out of the blue one day and said, ‘they’ve cancelled all my money so I’ll have to hang myself now’, and I was absolutely terrified thinking, ‘oh my word, what if he does?’Staff ES-T1-04Yeah most definitely and I suppose I’m coming from, I suppose my patients without mental health problems are coming from a reduced socioeconomic group here and the places that I work, so, even more so with patients with mental health, they often are not in employment and are living on very, very much reduced resources and when you are discussing what foods to buy and prepare, often it’s very difficult for them to afford a lot of what you are asking them to . . .Staff ES-PC-02And then again, there’s the financial implication of that for many people. It’s very difficult. And also many guys live on their own, they don’t care. They don’t know how to cook a healthy meal, put together something that’s . . . They’ve got £20 to buy food for the week and ‘how much can I get for this to survive?’Family member ES-T2-08 |
| Foregrounding mental illness | It’s mainly mental, it’s not really the diabetes, it isn’t too much of a problem at the moment, but it’s mainly the mental, I get a lot of anxiety sometimesES-T4-13I told [my CPN], I says, ‘I’ve nothing left, love’, I says, ‘I’ll have to go on my tablets, it’s the last resort, if I want to carry on. And I want to go on’, I says, ‘but I’ve got to do, ‘cause that’s the only option I’ve got’ES-T2-02Interviewer:What would you say has helped you the most with your mental health, would you say?ES-D1-05:[. . .] And then the medication without a shadow of a doubt. Without a shadow of a doubt the medicationI mean I take around 20 tablets a day, 14 in the morning, but for my diabetes, my mental health and my other conditions, and then I take about three or four in the evening and I hate that, I hate that, I hate taking all of them but it’s got to be doneES-T2-05I don’t think you quite understand what this is like. The side effects of the drugs are not good. You cannot be not genuine and take these things because you wouldn’t take them if you had the choice. I mean, I am desperate, so I take my medication, I never . . . well, as long as I remember to take them [laugh]. Well, I do usually remember to take them, but I would never skip on the medicationES-T2-16I got the schizophrenia and then I got the Clozaril and then I got the weight gain and then I got the diabetesES-T3-09. . . because the thing is, to them diabetes might not be that problem that they need to look at at the moment, because obviously their mental health may be overtaking that, so they may not see it as a factor or they may not be putting it into, they may not see it as a significant issue there and then, and, unfortunately for mental health, if the diabetes is quite bad, the mental health will be going bad as well and they won’t be focusing on that because they will be having acute episodes from the mental health so they will just think that’s just something that is going wrong at the momentStaff ES-PC-04They neglect themselves. They don’t take their medication as they should be. They don’t eat the right things and both ways, it’s there . . . it’s, sort of, like the mental illness, I think, becomes first, prior to the diabetes. They just don’t take any notice of it reallyStaff ES-T4-06I think it’s more all-consuming, the mental health problems. And actually, breaking through that, but then some people will take more care of the physical health and ignore the mental health issues. But then you’ve got the spiralling issues with that and I think, unless your mental health is very well controlled and very stable, then you’re not going to do very well with your physical health eitherStaff ES-T5-03[. . .] she got me to send a booklet, it didn’t really help me. You see, it’s governed by mental health, love. It’s governed by your mental health. You’ve been poorly. They all lean on each other, the conditions and illnesses, they all lean against itES-T2-02Whereas, I think for somebody who’s diabetic, it would be, your main focus would be on the diabetes, and you’d be trying to work out what you’re going to eat, and make sure, whereas for me, I’m always worried about the mental health side, and if I am in a really bad place, I don’t care anywayES-G2-01The sort of GP service had tried to do a diagnosis, tried to tell me it was important, which was never going to have any impact, ‘cause when you’re mad as a hatter, you don’t take any notice. They’re just noise in the cornerES-T2-03Like, I can eat healthy, I can do the exercise, I can stop the alcohol, I can take my medication, I can find ways to distract myself, I can sleep, I can do all the things, but then my diabetes and my mental health are always right there at the topES-T3-03If someone is unwell with their mental health, they are just trying to survive, aren’t they, day to day, and they don’t want to hear the advice on smoking and diet, they just need to get through the day, and if they are eating something, it is sometimes a victory, rather than, you know, not necessarily about eating a healthy meal, it’s just eating a mealStaff ES-PC-01Now I know you’re looking at diabetes and mental health, and . . . but, from my point of view, the issues are, without a doubt, the mental health issues, not the diabetes. A lot of people suffer from diabetes and you just have to resolve it in one way or anotherFamily member ES-T2-17 |
| Subtheme | Associated quotations |
|---|---|
| Multimorbidity | And my heart condition, I’ve got a leaking valve. [. . .] And then I’ve got chronic bronchitis, COPD, and asthmaES-T2-02Yeah, so you often get patients who just have several different things, where your diabetic might have ischemic heart disease or COPD or something like that, often with mental health and mental illness there is often smoking, which would list on to risk of COPD and other respiratory problems, so yeah, if they’ve got one thing, they are going to have anotherStaff ES-PC-01. . . and normally if they are diabetes tablets, they are on blood pressure tablets, and if they are on blood pressure tablets, they are on cholesterol tablets, if they are on cholesterol tablets, you know they’ll be on something else, so that’s five tabletsStaff ES-PC-04Yeah, I mean, definitely the rates of the diabetic complications are higher in our group of SMI patients, so they will often have cardiac disease or pseudovascular disease at an earlier onset, I would say, than our non-SMI patientsStaff ES-PC-06 |
| Impact of physical conditions on diabetes management | My COPD only allows me to go to the bottom there, and I’m coughing and weeing myself and everything, and I’ve got to be open and truthful with him, saying that I wear nappies now, I daren’t go out. I only go somewhere, where my scooter will take meES-T2-02ES-T2-04:Well I’ve got my knee, that’s difficult for a start-off. I’ve had [inaudible] for walking and that with it. Its cartilage trouble and osteoarthritisInterviewer:Oh right, OK. So what does that stop you doing?ES-T2-04:It stops me from doing a lot of walking what I used to like doingShe diagnosed me and I think my eyesight’s getting poor. You know, my eyesight seems to be getting poor. I’m seeming to look at my phone a lot harder with texting. I’m finding reading and writing, I like to read it, like put big writing on my text now, you know. So my eyesight is really taking a hit, you knowES-G3-01Well I’m waiting for a knee operation, I can’t get about as well as I used to. [. . .] They won’t do the operation unless the leg muscle is strong. That is why I try and swim to keep the strength in the leg muscles. I walk as well. But unfortunately, I can’t do as much as I would likeES-D1-05I’ve also been quite recently diagnosed with, with an inflammatory arthritis, which has made life quite difficult, but that’s coming into playES-G8-01. . . for people who have had it [diabetes] quite a long term and a number of years, unfortunately for them, it may mean that they may then be susceptible for things like having strokes, cardiovascular diseases, which then limit the validity then for what they were doing a couple of years ago if they are quite elderly, which means, if they have had a stroke then they may not have the quality of life that they did have before because, unfortunately, they have had series of events, such as strokes or heart attacks, and then that just further deteriorates, then, to access how, then, because they may be at home or they may not be able to get outStaff ES-PC-04I think their illnesses would take more precedence, it depends how extreme the other illness is and how much that impacts on their day-to-day life. I guess if you’re living with constant pain, your mobility is poor, you would be focused on that more than you would, than your diabetes.Staff ES-T1-02Yes, so the condition that’s causing the most immediate difficulty is going to be the one that gets the attention, so somebody has daily pain, that pain is going to take more attention than the diabetes which isn’t causing any immediate pain but is a long-term complication and consequence. It’s a silent killerStaff ES-T2-12So there are studies that have showed that other comorbidities, such as heart condition, etc., can also have a detrimental effect on SMI and, in particular, the control of or the lack of control of diabetes or poor control, inadequate control, if you like. So all of the conditions, we believe, sort of, work hand in hand . . .Staff ES-T3-01I also think she gets quite confused, because she’s got things going on, not just the diabetes but she’s got other ailments, you know, so she’s got irritable bowel and she’s got a fatty liver, there’s other sort of ailments that she suffers from. And obviously, there’s different diets attached to those and I think sometimes that gets a little bit overwhelming for her as well, in that she knows that there’s certain foods she should avoid and certain things she should and shouldn’t do in respect of her diabetes. But then also, the same could be said for the other ailments that she’s got, and I think sometimes it will come as a little bit overwhelming because she thinks, ‘well, there’s too many things that I’ve got to think about what I can and can’t eat. I’m just going to ignore it’, if you know what I meanFamily member ES-T3-08 |
| Subtheme | Associated quotations |
|---|---|
| Symptoms of diabetes and SMI can overlap and interact | And something I’ve noticed is there’s quite an overlap between feeling mentally low and feeling unwell because your blood sugar is upES-G8-01When I was poorly [. . .] it was trying to work out whether it was my diabetes or mental health. It was a mixture of bothES-T3-03If I go on a high, sometimes, they’ve got to check my blood sugars, because they don’t know if it’s the blood sugars, that are causing me to go a bit loopy. Or it’s my mental illnessES-T4-10And on the other hand, if people are diabetic and they’re not maintaining their blood sugars right, it can give rise to symptoms which are synonymous with anxiety and low mood, so there is a big interface between diabetes and mental illnessStaff ES-T1-01It’s difficult to separate out the effects of the SMI and the diabetesStaff ES-T2-13She were doing really well, but, for whatever reason, got it into her head that she was going to wake up having a hypo [an episode where the level of blood glucose drops too low], and didn’t want that, so stopped using this insulin on a night. And just went up and up and up, to what looked like quite a manic episode, but it’s not, it’s when the diabetes gets really unstableStaff ES-T3-02And you know, sometimes, the irritability that comes with a low blood sugar could be interpreted as part of somebody’s mental illness. And it’s diagnosed and mistreatedStaff ES-T5-03‘Cause they’re so closely linked for me, I can’t speak for other people, but for me it is, when one gets worse, the other one does tooES-G2-01Yes, I think if I’ve, sort of, got a low mood and I don’t want to eat, then I could get a low blood sugar, or if I was to eat too much sugar, I could get angry and uptight and that could affect my mood as wellES-T2-05When I’m not taking medication and I’m not taking that, then I’m obviously hyper and that affects my mental health, and they were linked together somehow, it’s the both set each other offES-T3-03Because you can’t think clearly, that’s . . . not being able to think clearly is hard, because then you’re not applying the right tools to address your diabetic situationES-T7-04If you were doing a link between my blood sugar and my depression, my mental health goes down as my blood sugar goes up. There’s a direct correlation between the twoES-G2-01I guess if their blood sugar is all over the place, that can potentially impact on their mental health as wellStaff ES-PC-01Well, just being ill with your diabetes I don’t think will add to stabilising your mental health, and having very, very high sugars, I think, will increase, will have a poor effect on your mental healthStaff ES-PC-02I mean, certainly, when they are acutely unwell, their mental health will really slide and often that can be either that their diabetes is not well controlled or, and then that leads on to picking up more infections and being susceptible to things. Quite often we will see them acutely because of a behavioural change that has happened as a result of an acute infection that has usually happened as a result of their diabetes not being brilliantly controlledStaff ES-PC-06When it’s not managed well, when they’re not taking their insulin or taking their metformin when they should, when the blood sugars are high, they become more aggressive, argumentative, that’s often a sign that they’re not managing it properly and it might not just be a sign of their mental health deteriorating, because it could be a physical cause like diabetes and it’s a good thing that we have these clinics where this can be monitored a bit, you know, blood glucoseStaff ES-T1-02Well, the diabetes, obviously the diabetic control has quite an impact on people’s overall well-being and feelings of energy, or lethargy can lead to depression and anxiety. People can put weight on, which leads to other problems and low self-esteemStaff ES-T3-05 |
| SMI medication is linked to poor physical health and difficulty with diabetes management | I’ve got lots of medication going on, so it makes me feel hungry. It makes me feel lazy. So some days I can sit here all day long with not radio or TV on and be lost in my own thoughts, and my thoughts can just be going round and round in my head and it does me no good whatsoever. It makes me worseES-G3-01Yeah. The one I’m on at the moment, the aripiprazole, doesn’t put . . . well, it does put a bit of weight on me, but the metformin takes it off again. So I’m fairly OK with my weight. But Dr [GP] has said anything else will put weight on me. Because it gives you an appetite, it just suppresses your feeling of being full. They all do, all the antipsychotic drugs do. And that’s a problem, that is a big problem. Because I’m not really a greedy person but when you get the hunger from the tablets, it is . . . you can’t ignore itES-T2-16It is and the medication that I’m taking. It makes me hungry and it makes me tired. Quite normal for me to have a couple hours’ sleep during the day, but if I didn’t take the tablets, I wouldn’t be snoozing like that. I’m feeling a bit drowsy and a bit tired. It’s the medication, if I’m going to carry on taking it, I’ve just got to put up with it I supposeES-T2-18. . . when I put all this weight on when I first started on my lithium and the Epilim, they put you a lot of weight onES-T3-04When I went on psychotic medication, I put 4 stone on in about 6 months. I were only about 9 stone and I went up to, at one stage, [to] 14ES-T3-04I went on the medication for 3 weeks and I put on at least two sizes of clothing in that time. It happened very quicklyES-T3-09I’ve gone to the psychiatrist, it’s pretty normal with the medication, and she asked how long it goes on for and I said maybe about 2 hours. Some people, they have it all night. And it stops them sleeping, which obviously makes your mental illness a lot worseES-T3-09I just slipped and fell on the desk like that and my head hit the desk, so I thought, ‘I can’t do this, I can’t do cooking, I can’t do anything active, or with machinery, because of the medication I’m on’.ES-T4-01On the flip side, the patient can begin as not diabetic, but is known to the services for a long time with severe and enduring mental illness and is on a range of antipsychotic medication, which then gives rise to . . . not, as such, direct weight gain, but an increase in diet, an increase in weight. Then, at some point, they can develop diabetes or poor blood sugar control, and that happens quite commonly. We call it metabolic syndromeStaff ES-T1-01I think it’s a lot to do with that and plus with the medication as well, the medication is a big thing, they might feel slowed down, the less they do, so that adds to the weight gain. Also, antipsychotics like olanzapine are renowned for people gaining immense weight, stones, rather than a few pounds and I’ve got a patient at the moment who is from an ethnic minority background, who has other health problems besides diabetes, but since they came to us, and went on olanzapine, he’s developed diabetesStaff ES-T1-02I think the difficulties that have been experienced often is that because of the mental health medication they are on, they have got a very big appetite so they end up eating the wrong foodsStaff ES-T1-03You know, that’s keeping him reasonably fit, except that the diabetes and the drugs – all the drugs say that they’re likely to put the blood sugar up and, as a result of taking the antipsychotics and the antidepressants or whatever the other one is – he takes metformin to keep the sugar downFamily member ES-D1-02 |
| Not everyone agrees that conditions are linked | I don’t know. If my blood sugar’s dodgy, I do feel ill. But I’m not quite sure if there’s a link to my mental illness and diabetes reallyES-T4-12. . . educating patients, as well, about how mental illness and physical illness are inter-related, because oftentimes it’s fixed in the patient’s mind, ‘oh, this is my diabetes, separate, and this is mental illness, and they don’t have anything to do’, but there is a bearing there, a strong bearing, in helping people to understand. And once they understand that, that helps us in our management plan as wellStaff ES-T1-01No, I wouldn’t say that there’s any connection with them at all. I think they’re separate matters. I don’t think her mental health problems makes her diabetes worse, but I don’t think her diabetes influences her mental health problems either. If there is any connection at all, basically, it’s lost in the mix of what creates our personalities and what creates our physiological conditions, but I can’t say there’s any difference, any connection, ratherFamily member ES-T2-01 |
| Subtheme | Associated quotations |
|---|---|
| Fluctuating moods affect diabetes management | I think definitely there is a link there, in if your blood sugar is low and you feel depressed or fat or you’re anxious or feeling paranoid, then you want comfort, and I find that in naughty foods like crisps and chocolate that are very bad because crisps are carbohydrates and they turn to sugar overnight and that’s not good and then the chocolate is full of sugar.ES-T2-05Depression. Call it comfort food, comfort drinking.ES-D1-05It’s mostly the exercise, but it’s also diet as well. You just eat more poorly and put weight on, and it’s just all those things that you associate with depression are actually also associated with poor physical health, if you want to put it that way.ES-G2-01But when you’re having a real bad episode all that goes out the window. And you’re not looking after yourself. You’re not looking after your diabetes either. And unless somebody is really on top of it with you, you can get into a mess.ES-T3-07When you’re having a bad day, you’re not so bothered about having salad for tea, you might be naughty.ES-T3-09When I’m not well, I’m just not capable of managing my own medication.ES-T4-09A packet of shortbreads, five Mars bars, that’s my diet, that’s because of this anxiety that takes me through sweetness, and all sorts of things.ES-T7-04I’ve seen patients who, so when their mental health deteriorates, their eating deteriorates, so they may start to comfort eat, make the wrong food choices, and so they lose their diabetic control. I’ve got a patient who, when her mother died, you know, you can date things back, she said ‘I’ve got dreadful glycaemic control’ and this dates back to the death of her mother and she is still in a bereavement phase and the diabetes is just not important for her.Staff ES-PC-05Obviously when a person is unwell, whether it is schizophrenia or bipolar or depression, everything will fall out of the window.Staff ES-T1-01They’re relapsing but they’re not risky. They’re like, ‘well . . . just see your GP’, would be the answer. In the meantime, they’re not eating or they’re eating chocolate for five meals a day, because it’s easy, and they’re not sleeping any more. Their weight is ballooning or shrinking, they’re getting more physically unwell, their blood sugars are raging. That person is 6 months down the line and they’re acutely psychotic. At that point they’ve got retinopathy, their blood sugars are all over the place, then you’ve given yourself two big problems to manage, what would have been one small problem.Staff ES-T2-12I suppose it depends how the impact that’s having on that person. So, if someone’s having quite a lot of negative symptoms, that might be affecting their motivation, but just purely their condition, I don’t think, would impact their diabetes management, not that I’ve seen.Staff ES-T2-14There are lots of reasons why people don’t or can’t buy the right food options, and then, usually, because of lack of motivation, either because of negative symptoms and/or because of low mood, there’s a lot of patients who, even if they’re . . . you know, I’ve got a patient who is trained as a chef, but from a motivational point of view, they can’t, actually, push themselves to go to the effort of preparing something.Staff ES-T2-15And especially if you’re depressed, you either don’t want to eat anything, or you want to eat a load of chocolate and chips and things like that, which is partly the fault of the medications that we use.Staff ES-T3-05And if you are depressed, if you are anxious, if you are experiencing hallucinations, you’re maybe not going to want to go to the gym, to the supermarket to look for healthy food. You’re maybe not going to have the motivation to cook for yourself, cook healthy meals. You’re maybe just going to be putting stuff into a microwave.Staff ES-T6-01If she’s stressed or frustrated or angry or just not feeling 100%, she won’t be disciplined, especially with diet and what should be doing to help, I suppose, control the diabetes, because it’s almost like a link with food. I mean, she does try but I do notice that when she’s particularly stressed, it might be, well she goes one or two ways, it’s either not eating very much at all, you know, so she’s not getting the correct nutrients and all the rest of it. Or, she eats things that she shouldn’t be eating, then it’s almost like . . . not that there’s an excuse, but she’s stressed and that what she fancies at that time, so, you know, she would probably go off the sort of diet that she should be on. Or care a little bit less I suppose.Family member ES-T3-08 |
| Extreme low mood and depression affect management further | I really don’t know, ‘cause when I’m depressed there’s nothing that you could say that would, ‘cause you stop caring, it’s like if you stop caring about yourself, or what happens to you, it’s very difficult then, for someone to say, ‘well you need to stop eating these, these and these, and you need to eat a portion size that looks like this, with brown rice on it’, and you get home and you just think ‘fish and chips’. It’s very, very difficult.ES-G2-01You’re mixed up because part of you wants to live but you want to . . . I mean, I said when I went to Slimming World I want to lose weight, because I want to live longer. You know, with diabetes, you’re liable for a stroke, a heart attack, **** knows what else. So if I do something right . . . on that, maybe . . . But other times, no, you don’t want to live. And it’s that, because you don’t want to live in this hell you haven’t created, but your mind is doing.ES-T3-07I’ve got another patient, when her mental health deteriorates, she often goes into crisis and it’s often related actually to benefits and things like that, so her self-harm increases, her diet increases, but she does just stop taking her tablets as well, just because she can’t be bothered with it really, so a lack of motivation, you know, ‘I don’t want to be here; therefore, why should I bother about this because I don’t care, I forget the complications because I might not be here long enough to get them.’.Staff ES-PC-05I don’t think if differentiates and I think the worst of all is depression. Because somebody’s proper clinically depressed and flat, they don’t even eat let alone go and see the GP or even get out of bed . . .Staff ES-T5-03 |
| Worries about diabetes can contribute to low mood | It frightens you, doesn’t it, how you feel, dizzy and . . . sick.ES-T2-02I get very worried, because of the eyesight and they took X-rays of my eyes, because you know what it is, you know another thing, you know when I’m looking at my phone, this is the main damage it’s done to me, looking at my phone from this distance, now when I want to send a text or something . . . so I am worried about my eyesight. I need to sort something out, but it does get me worried, yes.ES-G9-01I suppose it’s stress involved, knowing that I had a friend who were diabetic who died when I was 30, so I’m quite aware that it’s not something to ignore.ES-T2-06It does affect me badly because I worry. I worry about going to bed. I worry about sleeping because I think, ‘am I going to wake up or am I not going to wake up?’.ES-T3-03I’ve constantly got to think, ‘oh, I hope this next reading’s going to be OK’. Or I’m always like that, if you know what I mean, and it . . . that drives my anxiety and that drives, like, the bipolar type of thing in me.ES-T3-03But I just think generally my health has gone down since . . . my mental health has gone down since I’ve had diabetes. Because it’s another illness, you know, you get fed up having yet something else added.ES-T3-04Oh yeah, definitely, because you need to be careful what you eat, being overweight. I mean, that makes me anxious. That does upset me. And knowing that I’m diabetic, knowing I’m overweight, I am. That upsets me and that makes my anxiety worse and that makes the hallucinations worse. There’s definitely a connection.ES-T3-09I think sometimes, patients I suppose without mental illness as well, if they are injecting so they are on insulin, it’s just a constant reminder that they have diabetes and so that can have a negative impact on their mental health.Staff ES-PC-05Any physical illness, any long-term chronic physical illness can affect somebody’s mental health. If you are living a life where you have to constantly worry about an aspect of your physical health, whether it’s pain, whether it’s fatigue or whether it’s diabetes, and the difficulty with diabetes sometimes is that it isn’t tangible in the same way, so you either are worrying about it because you’re not sure what’s going on, you know that your sugars aren’t quite right but you haven’t got skills or the support to manage it, or you don’t know it’s a problem until it becomes such a big problem that you have the long-term consequences, you know? [. . .] You know, in the context of being diagnosed with the consequence of diabetes is incredibly overwhelming and then to then think about medications and then eventually insulin, you know, it’s devastating and incredibly stressful, and one stress begets another stress. So yes, those definitely are related both ways.Staff ES-T2-12. . . being diagnosed with diabetes and it’s another thing that she’s got to think about and you know, sort of contend with. So, I do think it has affected her mental health, in that it’s an additional thing for her to worry about.Family member ES-T3-08 |
| Subtheme | Associated quotations |
|---|---|
|
I talk to people in here and they help me [. . .] I’ve got some good friends.ES-T2-04I’ve got one really close friend who I talk to about it, and others that I don’t really mention it at all to. And that’s because, well sorry, two people I talk to about it.ES-G2-01I get pretty good support, me family supports me alright, they’re always concerned if I’m alright, and what have you.ES-G4-01Well he’s my carer, is my husband, he looks after me, he looks after everything, he does all the financial bills and everything to pay, he sorts holidays out, he pays all the bills.ES-G7-01Yes, it means a lot to me, like I said, I’ve been to the mosque about 2 days ago, and I feel really good with my friends, that’s giving me another motivation, carry on.ES-G9-01Who helps me the most in continuing to be here at all? ‘Cause there’ve been occasions when that’s definitely been a possibility not to be true. My wife. So my unpaid carer, a 24 hour a day, unpaid carer, is the most critical person in my care.ES-T2-03. . . my relatives are important as well because people with diabetes in my family, we discuss issues and medication and the best way forward to manage it, so they are important connections.ES-T2-05. . . because I am more open with [husband] my husband now [. . .] because I’ve got to be because he is my main support person and I haven’t got the support in CPN any more so in order to manage my mental health situation, I do need to be honest and open with him 100%.ES-T2-05Well, [partner]. Without her, I wouldn’t be around. I would have taken an overdose by now. She keeps me grounded.ES-T2-06And it’s like my family were trying to help me then and they couldn’t because I wouldn’t let them, but it’s like now, because I’m feeling better, I’m letting them in, I’m letting them help me.ES-T3-03So both my son, even though he’s got his own issues, but my daughter is the one, she can tell by my voice when I’m not right. So she rings me first thing in the morning and she’ll ring me at night, and I go over, as I say, every alternate weekend. [. . .] So she’s the one who would then ring [son] and say, ‘my mum’s not right’, and then they would get in touch with the CPN, or say to me, ‘mum, what’s on your mind, you know, what are you worrying about?’.ES-T3-04My mum and my sister take me to Asda on a Monday and I go to church on a Sunday. My partner drives me to church and drops me and my daughter off while I’m at church and then picks me up. On a Monday, my mam and sister take me to Asda and then we go and have a coffee and then go and do our shopping. And then on a Tuesday and a Thursday, my mam’s now started taking me to the gym.ES-T3-09So only the person I’ve got now is the advocate or friends, you know, Christian friends, you know because I don’t have a family. All my family passed away when I was in prison. My mum died, you know.ES-G3-01I don’t really have a lot of friends. [. . .] I have one friend who goes to MIND and I see him sort of once a month, once every couple of months, something like that. And, really, apart from that, I don’t really see people from one week to the next.ES-T2-06I’m surrounded by lots of people who I get along with, I’ve got friends, I’ve got family, although my mum’s passed away and my dad’s getting on a bit and he’s had heart surgery, so they can’t look after . . . they can’t help me any more, I’m on my own.ES-T4-01I still used to like to go with my friend and we’d go and have lunch. And now that’s gone out of my life because I can’t physically do it, and that’s because of my diabetes.ES-T3-04But yeah, I did really miss my dad and I found it hard, you know, to cope without him.ES-T4-09Yeah. I went to a place called the [day centre] and I was there for about 10 or 15 years and that was a godsend. And I loved going there. But, since it closed down, I’ve missed it.ES-T5-11I think the thing that helps them the most is if they’ve got a support worker whose working with them, who goes in regularly and prompts them or if they’ve got a relative who helps them because they’re at home.Staff ES-T1-02I think peer support is really important because there might be somebody else sat there with diabetes who can talk to them about it and they’re most probably more likely to listen to them, than they would a professional.Staff ES-T1-02Now, her aunt wasn’t the best in terms of discouraging the alcohol use because they both drank together, but her aunt was quite vigilant in making her bathe and look after herself and do her washing and they [inaudible] better between them when she was there and her aunt stayed with her a few weeks when her circumstances were difficult.Staff ES-T1-04No man is an island, as the saying goes, and family is so important in helping to prop somebody up when they’re struggling and if they are lacking in the understanding, both from the physical perspective, but also from the emotional perspective, it makes it so much harder for the patient because the people who are immediately around them don’t get it.Staff ES-T2-12Really important because we may think we’re very important, but actually we may spend a couple of hours a week with the service user and actually it’s the carers that are going to spend that long term and that the carers may be very different from a health point of view and may be able to influence things from a role model point of view. Or the carers and relatives may have the same kind of health needs, as we often find. So, again, you’re helping them as well as helping them to help the service users, so that you’re having almost a double impact. So I think it’s very important to work with carers and relatives as well as, because if you’re going to get that sustained behavioural change in people.Staff ES-T6-01 |
| Not all support is positive | In this case, classically, the culture aspect was that family of the patient was bringing huge amounts of food, raw cheese and chapattis and all those things, and obviously rice, so helping the family to understand as well. And why I am mentioning this case is that it is a classical case, because there was a lot of learned behaviour the patient had developed from the family. So since an early age, the family had developed this behaviour, to pacify this person, give food, so that the person doesn’t get [inaudible] this was a tall, huge lady, so she can be aggressive as well, so if they give food, she will be quiet. So that kind of coping mechanism, the family developed and obviously that impacted on her physical health as well.Staff ES-T1-01Sometimes you’ve got family who would support the patient, [. . .] whereas other families, they just leave everything to the patient, which is difficult if the patient becomes a bit unwell.Staff ES-T1-03Some people, some family members, are not particularly helpful and they don’t understand the illness. They often think that the person involved is being lazy or difficult and they should do this, that and the other, and they won’t do this, that and the other, and they believe that is just because they’re being awkward. So if they come to the clinic, then you have to sort of gently try and explain that this could be part of the actual illness, not a choice to be awkward.Staff ES-T3-05Obviously liaising with their family as well, because lots of the family bring in chocolate and full-sugar coke and loads of biscuits and so on. [. . .] Or bring tobacco, and they just bring all sorts of things that aren’t going to help anyone with diabetes.Staff ES-T5-01I think creating a culture within the home is helpful. So, if you’ve got somebody who is dismissive of the attempt of, knocks it down constantly, then that can be very unhelpful. And if you’ve got somebody who’s encouraging them to get the takeaway or, don’t bother with that, it’s raining outside. If your own motivation is a bit shaky to begin with, that’s probably not going to help.Staff ES-T7-01It’s easier having both of us having to watch our diet. If I munch away at huge bits of cake and he’d have to watch me – that would be awful.Family member ES-D1-02 |
| The effects of being a support person | Like I say, it does take over a bit, but, like I say, it’s good. It’s the best part of my life because, like I say, it wasn’t that happy before and I’m happy with [husband]. I just hope nothing else is thrown at us.Family member ES-T5-12Also when he was sick he believed he had acid on his body, and I was showering him actually 13 times a day. And then every time you shower him you’ve got to wash the washing. So it does, it puts a lot of strain on everybody in the house.Family member ES-T2-08I think she runs the risk and probably does to an extent become a recluse. It’s very easy for her to be reclusive. That will have a knock-on effect on me because if my wife’s reclusive then . . . I don’t really like to leave her for great long periods, although I have done.Family member ES-T2-17. . . in the past, it did impact on things like my education. Because things happened where she’s ended up in hospital with it before my finals. When I was doing my degree, things like that, where it’s had an impact in the past. And so, after I graduated, and I got a job, there was a few times where I had a call and it was like, ‘She’s been taken into hospital again, can you get down here?’. Just coming in the car and going straight down. So, in the past, it’s had a massive impact, I think. But not so much these days.Family member ES-T3-08It was very, very hard. There were times, even though you know someone’s ill, whether they threaten to kill you, which she did, is a very challenge to your relationship, isn’t it?Family member ES-T2-21But, you know, when you’ve got somebody who’s swearing blind that there are attackers around the corner and they’re coming. You know, to wait another hour for somebody to come back to you on the phone, is a nightmare. An absolute nightmare. I was waiting and waiting for a phone call back trying to calm him down, trying to stop him going out and sort of trying to meet his attackers or whatever, you know, with a knife in hand or whatever. You just don’t know what to do and you’re absolutely terrified for hours. And that shouldn’t happen, really.Family member ES-D1-02 |
| Getting everyone on board (supporters need diabetes education too) | I think that it is important that they have the awareness and understanding of diabetes as well for the importance of it. The education as well, they might be the ones cooking so just make the health choices, understand, if they are blood glucose testing, you know, the values, when to be worried, when to react, sometimes teaching the family member to give insulin. They need as much education as the patient.Staff ES-PC-05So if families are caring, explaining to families, because here, in [city], there’s a cultural variation, so people want to eat, so that also has a bearing. So, in that way, the whole family needs educating about what you’re getting and cooking in the household. So, yeah, so those are the bits, as I mentioned, so patient, carers and families, all these need to be educated alongside.Staff ES-PC-05But, in the course of that, she was staying with her mum, just to give her partner a break, and her mum said, ‘well it’s been interesting all this, ‘cause I know nothing about diabetes really’. And this is a woman who’s been taking metformin for years.Staff ES-PC-05So yeah, kind of, educating the family, as well, and around what foods to bring in on visits; and when patients go home on visits, not to make too many sugary and fatty meals for them. Just getting everyone on board to, kind of, manage the diabetes.Staff ES-T5-01You could have a very switched-on family member who wants to encourage their loved one to eat healthily, but I think it is more likely that that family member will just buy the person what they want to eat, if they’re the one buying the food or they eat the same food and if you’ve got somebody who hasn’t got mental health problems and isn’t diabetic, who are going to eat certain foods and if that person eats pie and chips and a packet of biscuits for their dinner at night, then that’s what their person with mental health issues and diabetes is also going to eat.Staff ES-T5-03We have had information and I actually had to go to a meeting – not a meeting but, I had somebody at the hospital who was, I think, a nutritionist or something and she had to give me a chat about being diabetic and what I should and shouldn’t do, etc. So, I did get a session and that was a couple of years ago, I think. Maybe more. We get a certain amount of information and, of course, if you want to know more you can ask. But, generally, just plodding along on routine check-ups and diabetic clinics.Family member ES-D1-02No, the first one-off was a group meeting at the hospital. At the diabetes unit but, there is one at our own practice quite frequently. Where a group of people with diabetes get together and exchange views and so on.Family member ES-D1-03 |
| Subtheme | Associated quotations |
|---|---|
| Diabetes health care | Interviewer:And who is it that you see for diabetes? [. . .]ES-T5-11:GPs, [nurse], you go to the nurse. [. . .] you’ve to go over a year, or every 6 months, I don’t know. [. . .] She does my finger, asks me what I’m eating, weighs me, my height.They give me booklets about sugar, diabetes and things like that. They tell me what to eat. [. . .] They told me to do my bloods regular.ES-T2-04Well yes, every 12 months. [. . .] They give me an eye . . . They did this thing, they examined the back of my eyes for blood clots or something like that. [. . .] I get the podiatrist every 3 months, bending my feet to keep a check on those.ES-D1-05They’re 3 months at the moment, yeah. Three months at the moment, normally 6 months. [. . .] I do tend to go for the eye tests every year, and I do all the things that they ask me to do, and I do all the blood tests. And, if it’s more frequent, because they’re more worried, then I will go in.ES-G2-01She [diabetic nurse] weighs me, which I hate. [. . .] She tests my feet, asks me questions, does my blood pressure, that’s it, I think.ES-G4-02I go and have my diabetes reviewed, each year, at the GP’s surgery.ES-T2-03I have a blood test every 3 months, approximately, for the diabetes, specifically to look at my blood sugar levels, and I also have my feet checked and a chat with the nurse about the results, about a couple of weeks after the blood test where we discuss the results and any changes that need doing vis a vis diet, medication or exercise.ES-T2-05But they keep monitoring it through blood tests and things. [Pause] I’m seeing lots of different specialists, hepatologists and dietitian, various others. So, I mean, every month I’m in hospital for something.ES-T2-06Interviewer:Thinking about the help that you’ve had regarding your diabetes [. . .] in terms of health-care staff, who would you say has helped you the most?ES-T2-06:No one. Well, the GP for pointing it out in the first place. Apart from that it’s just been getting weighed and blood tests, so I see the nurse.I get six-monthly checks and I go to the local health centre, which is in the village. [. . .] Well HbA1c, blood pressure, my kidney functions, thyroid function, vitamins sometimes, I think they do that once a year, because I’ve also got a high prolactin level, so I get my calcium and my vitamin D measured, I’m quite well looked after.ES-T4-02 |
| Subtheme | Associated quotations |
|---|---|
| Knowledge of diabetes | Not really, no. No one’s said anything why I’ve caught it. I mean, I don’t know how I’ve caught it. I just think it’s the sugar. I just think it’s the sugar so . . .ES-G4-01No, no. I think I’ve basically just been winging it. I know, like, if I eat a big carby dinner, I get very tired afterwards.ES-G3-01I don’t know nowt about diabetes, and understand it. I know you shouldn’t have ‘owt sweet, when they checked me down at the doctors, she said, [interviewee], ‘fruit, what is it?’. So I go, ‘sweet’. ‘Well, you shouldn’t have it.’ I said, ‘oh well I might as well not exist, [nurse]’.ES-T2-02. . . if I wasn’t exercising, and you start to poor eat, that’s when my diabetes get worse and starts to rise, the sugar levels and the fats as well, the lipids in my bloodstream as well, go up.ES-G2-01Diabetes is all about not being able to handle glucose properly, and one of the signs that you get is that once you get too much, your kidneys can’t handle it, and you end up peeing it out in the urine.ES-T2-03I’m aware of the complications like amputations and heart attacks and stroke and dementia.ES-T2-05There’s all these frightening things to do with circulation and glaucoma, and all the rest of it. People sort of think, ‘oh, diabetes, yeah, you’ve got to avoid sugar’, but actually it’s quite a bad one if you don’t take note of it.ES-T2-16I think there is a huge variation, some people are very engaged in their health and will often know more than you do about, you know, diet or they will have been on a diabetes education programme and the prevention programme as well [. . .], but you always have this big group that know what they should be doing but just don’t do it for whatever reason, and some people they have so many other problems that it is just a last thing on their minds.Staff ES-PC-01I think usually they don’t have that knowledge. They don’t have, I suppose, the area here that I am working don’t have the right skills, they are not cooking, they don’t cook at home, they don’t know what foods to buy, they don’t know what labels to look at when they are looking at foods, they don’t have, as I was saying, the cooking skills to be able to cook fresh foods, so there is a lot of processed foods, and also they don’t exercise.Staff ES-PC-02[About patient knowledge] It’s probably more frequent that it is inaccurate than it’s accurate. It’s usually things like ‘eating carbohydrates is bad, I can’t eat fruit if I’ve got diabetes.’.Staff ES-T2-14I think, from a food point of view, a dietary point of view, and this is based on my experience of lots of patients, while it still surprises me sometimes the lack of basic knowledge some patients have about the healthy food plate, or which things are high in fat, which things are high in calories. You know, basic things, which I suppose, wrongly, I thought was general knowledge, but perhaps isn’t, perhaps, actually, it’s a lot to do with educational opportunities, having family that eat healthily, whatever.Staff ES-T2-15Sometimes they have the wrong information – they think that they can eat as much fruit as they want, for instance, their level of knowledge may not be good.Staff ES-T5-02I mean, if you don’t know that you shouldn’t eat McDonald’s three times a day, then you’ve got your head buried in the sand, even with the people with mental health issues. People know what they should be eating and what they shouldn’t be eating these days, I’d say.Staff ES-T5-03 |
| Sources of information on diabetes and management | I’ve done my own research on YouTube and asking people who’s had diabetes, or people who know what good food are [. . .].ES-G3-01I did know quite a bit about diabetes, with my husband being a diabetic.ES-T4-09We are more involved in sort of promoting their general well-being, so we talk about alcohol and smoking and general health advice, and part of that is to do with weight management and if there HBA1C is within, then talking about a diet and that kind of thing.Staff ES-PC-01We’ve built up sort of a pack of leaflets to give to people to sort of explain what it is, complications, so I couldn’t tell you the source of those leaflets, and I use Diabetes UK, some of their patient information leaflets as well.Staff ES-PC-05We rely really heavily on good relationships that we’ve got with some of the carers and the managers of these places who, you know, they are absolutely fantastic in terms of trying to do the health promotion side of it and get them to their appointments.Staff ES-PC-06In the psychological education, there will be the consultations, and that’s very important, when you start prescribing, having a discussion with the patient, what are the side effects of the medication. And one of the clear discussions I have is that medication, as such, will not give rise to weight gain, but it can increase your diet. So watching what you are eating and exercise is a really important part in this.Staff ES-T1-01Yes, the diabetic nurse or the GP practice. That’s the, sort of, practical support that they get there, low-cost monitoring and the patient information and patient education from the diabetic nurse.Staff ES-T1-02I will routinely give people self-help workbooks, or YouTube I will quite routinely use because YouTube has some fabulous resources to simply educate people.Staff ES-T1-05I mean one of my clients does use an online forum where she can kind of go for support, but the problem, as I say, in some ways with online is who is monitoring that, who is providing the information, you know, how accurate is it and so forth, so yeah I always say to her, I’m glad you are looking for this information and you’re getting support, but, you know, just be mindful of where it is coming from’.Staff ES-T1-05And I’ve tried to, I mean, I’ve had to educate myself really. Because, before mum got diagnosed, it wasn’t something, I knew what it was, but I didn’t really know very much about it. So, you know, there’s been a process I suppose for myself and my mum where I’ve had to do some research into diabetes and the type of diet and what she can do to help herself with that condition. So, I’ve bought various books and you know, I’ve read through them, I’ve bought books for mum, diet books, sort of things that she can follow.Family member ES-T3-08Interviewer:So when you go and see the nurse every 6 or 7 weeks, or when you go to the hospital every 6 months, do they advise you on what kind of things to eat or what to do?ES-G3-01:Not really, no, I wish they did.It was the GP really pointed it out in the first place that it showed up in my regular blood test. He never really sat me down and explained. I got all the leaflets and things, but I’m not one to read such things.ES-T2-06The information I was given was no fat, no sugar, and that was it. [. . .] And you had to work out what has actually got fat in and what has actually got sugar in.ES-T3-07No, so she gave me this website and when I went on it, I was just struggling to find what I was trying to find.ES-T3-09 |
| Diabetes education courses | Now, I was offered that by the GP service, and I went, ‘no thanks, I’m too busy being mad’. So, 3 years later, I still haven’t done it. [. . .] So, I requested it. And there is a note in my notes saying that I requested it, and there’s a note in my notes, a month later, saying I requested it again. And there’s a note in my notes, saying, refused.ES-T2-03Interviewer:Have you ever been on an education course or been offered an education course about diabetes?ES-T3-11:No. But there’s lots of forms in the doctors that you can read.I’ve been given information, but I’ve not actually been on any courses.ES-T5-10So, in terms of the diabetes, when they are actually diabetes, the education course, they would be offered it when they come for their annual review, so the nurses would offer them it and refer them on it and we are encouraged to refer as many people and try and get everyone through it, so that’s done.Staff ES-PC-01So once you get put on the diabetic register because your blood indicates that you are diabetic, automatically you should be referred for dietary diabetic appointment with the doctor or the nurse and then an expert, which basically is a programme I think developed in the NHS to say ‘right, you’ve got diabetes that’s do an intense dietary programme and educational to try and reverse the effects’, so we are not then just leaving you to your own devices, and I think the uptake is very poor for that and I think that is just across the board with dietitian appointments.Staff ES-PC-04In [city] that is a current quality improvement programme to offer all type 2 diabetics, however long they’ve had it or even if they’ve been on a structured education programme again if that makes sense, there is some funding to promote that and we’ve now got a bigger choice of programmes so referring people. There is an online one that has been commissioned, a one-off 4-hour session and then the more traditional 6-hour structured education.Staff ES-PC-05We asked them on both sides, what support or what training or what information had they had about diabetes and I think they’d been offered the DESMOND but that was it. [. . .] Some of them haven’t been offered that. So I mean, the DESMOND is not really . . . Our patients aren’t going to sit through the DESMOND.Staff ES-T5-04I mean, I knew anyway but there was certain, I can’t remember now, but certain things that they said ‘oh, you know, you shouldn’t eat that’, I can’t remember what it was, but I was thinking, ‘oh, I didn’t realise that’. [. . .] So, it was interesting, it did help.ES-G4-02And that was by far and away the best thing and the most useful thing, in terms of management. So the GP has been completely useless in the management of my diabetes. They might do a bit of monitoring, badly, but actually it was the intervention of the community trust, with their education, that was provided by a dietitian, that really, really, made a difference.ES-T2-03Like, I’ve only ever been on the DAFNE [Dose Adjustment for Normal Eating] one, that’s the NHS one, and I found it was good. It taught me about food, and stuff, and it taught me about what it does to the body, it’s, like, I didn’t find that’s what I needed, to go on course, I found, like, I needed the support. [. . .] Like, going on the course and sitting there and listening, I could do that, but it was, like, putting it into action that I found hard.ES-T2-03Yeah, the DESMOND, it’s either a full day or 2 half days, I think. I did that years ago but that’s the only thing that I’ve ever done related to diabetes.ES-T3-09So doing the course did help, and it helped me with my husband as well, because, you know, it was people who knew what they were talking about, you know, giving advice.ES-T4-09I went on a Living with Diabetes course. So, that taught me quite a few things.ES-T4-12She reported having ‘zero knowledge’ before the course, it opened her eyes to diabetes, what it is, what it can do, and how it can be controlled. It was really interesting, and was successful because it was a friendly atmosphere where everyone wanted to join in.ES-T6-05. . . it’s not tailored to people with SMI so, you know, the courses aren’t necessarily run in places that are local to them, they are not run in a way that’s, kind of, made in any way enticing to them. I mean, for example, I sent a chap on a DESMOND course recently, you know, the diabetes education course and he got kicked off because they didn’t like his behaviour. Well, you know, he’s a chap with schizophrenia and actually even getting him there was massive, and, unfortunately, you know, he was never going to sit there and behave like everybody else, so yeah, kind of, getting them to access stuff is very multilayered I suppose, it’s not just getting them in the door, there are lots of other bits that need to happen to really make that work.Staff ES-PC-06. . . if we can get someone to commit to a DESMOND course, we’ll go with them, ‘cause that’s quite a long morning, and again, sometimes they’re quite big groups, and lots of people that are really interested, are asking loads of questions. So, that’s quite intimidating sometimes, isn’t it, so occasionally we’ve gone along to DESMOND courses with people . . .Staff ES-T3-02And our services users classically tell us that some of them have problems with literacy, so they go along, they can’t read the information. The information is information overload. If you think that a lot of our service users have problems with cognition, with attention, with cognitive deficit. They can’t take on board the information; they don’t understand the information. And I think some of them feel, in a big group, very inhibited. When some of them find out that it’s a big group, they don’t even go. It’s run in our local library, which is a lovely venue, it’s an old Victorian library, but a lot of them don’t even access the training because they just, when they know what they’re going to, they don’t even access it.Staff ES-T6-01. . . often, I think, a barrier to the structured education is the group work, so I don’t know if it would help if the group was people with similar other comorbidity, so other mental health problems, or whether a bit more one to one.Staff ES-PC-05Obviously, there are lots of patients who, for motivational reasons, paranoia reasons, difficulties using public transport reasons, lots of reasons, can’t contemplate the thought of going to [city] Market and doing the course.Staff ES-T2-15. . . we had the healthy-eating lady coming – a dietitian. But, saying, as well, that you can change things. I don’t think I realised how much the carbohydrate part was part of it. And I think that was the worst bit, you know, the rice and things. And me, bread. Chocolate’s bad and the biscuits I have with it, but I hadn’t realised that part. And like I said, it was very, very good, weren’t it? It included everything. Feet and showing what can happen to your feet and some of it were a bit horrific, but it was good. Like people who had been on holiday and maybe someone who was careful of their feet. But, someone just went like that on a cruise to come and have his massage. So, he walked across the deck and when he got back he blistered. He didn’t realise from walking across that he’s blistered his feet badly because he couldn’t feel with the diabetes. [. . .], but, now I do check my shoes and check my heels aren’t rubbing and I try and have the lace-up shoes, in general, when I’m out.Family member who also has diabetes ES-T5-12 |
| Subtheme | Associated quotations |
|---|---|
| The value of care that takes the whole person into account | Interviewer:I think you mentioned that you previously had a nurse as support, didn’t you?ES-D1-05:Yeah, she was great. She was an ex-policewoman. She was absolutely fantastic. She could tell . . . When we said hello to each other, she could tell my mood straight away. And she would speak to the psychiatrist before I got to the room, and probably brief him. [. . .] She asked me in depth about the problems, the real problems and what was at the back of them. Sometimes she would recommend some medication to the psychiatrist. And that was a big help. I knew she cared. I knew she was listening, because there was a follow-up, so to speak.[About psychiatric nurse] Well, she says things like ‘what do I want to do?, what would I like to do?’, make sure my tablets are the right ones that I’m taking and then I get some vitamins, I don’t know what she is, she’s not a social worker, she’s a nurse, a hell of a nurse, but she comes to the house about once a fortnight and she’ll take me out for a coffee. So, we go out in her car and we’ll go for a coffee, which isn’t far.ES-G7-01My care co-ordinator’s brilliant, she comes every fortnight and we’ll just out for a cup of coffee and walk around the garden centre.ES-T3-09Interviewer:How does he [care co-ordinator] help you?ES-T3-11:He’s an old punk. [. . .] I’m an old hippy. In the seventies, I was a hippy. So, we’re a bit . . . I like some punk, you know. I mean I like some of it. It were good fun. Yeah, we get on great. And we talk a lot about music. [. . .] So, we have a common bond.[About CPN] We have a chat, see how I’m doing, she’s very supportive, sometimes she tells us about her family to give us a bit of amusing gossip.ES-T4-02. . . if they work better in 10-minute bursts, I can see them like that, if they work better as, yeah, doing something a bit more involved and if they’re going to engage bet . . . I had a service user before that he wouldn’t really sit in a room and have a chat with me, but if we cooked a meal together, he’d talk about everything. So, our sessions would be less often, so I’d see him like once a month, but we’d make a meal and talk while we were cooking together, sit down, eat the meal together, and all that time we’re having conversations.Staff ES-T2-14We’ve got a whole team of doctors down here – you never get to see the same one twice, but you used to just have one face, GP you knew really well [. . .] There is a lot of difference between somebody knowing you and just seeing different people each time. If you want to speak to a particular person, you have to make an appointment. It can take a week or fortnight, you know, it depends on their schedule.Family member ES-D1-02She felt that doctors and nurses don’t seem to have as much time for you as her [care co-ordinator] does, but diabetes is a serious condition. They don’t give you the information that you need, that you were looking for. They don’t seem to be as friendly and have as much time, they seem to be rushed.ES-T6-05I get, with a GP, they haven’t got that time to understand mental health . . .ES-T3-03I think GPs and practice nurse haven’t got the time to give that support, and it’s nothing against them, they just . . . their workload is so under pressure, do you know what I mean?ES-T3-03Because when you go to your doctor, you’ve got 10 minutes, and that’s what the problem is. Many of the guys, by the time they’re reaching the stage, like, you’re talking, guys with diabetes, they’ve got lots of things going on with them. And they’re trying to tell the doctor and it becomes like . . . So the doctor doesn’t give them . . . he just says ‘come for your annual diabetes check, come for your tablet, we have to check your liver and your kidneys. We’ll do that once a year’.Family member ES-T2-08I didn’t always want to, in the first 2 or 3 or 4 years, discuss all my feelings with my husband, and if I was suicidal I wouldn’t want to necessarily tell him that because I wouldn’t want to frighten or worry him, but I could share that and I would share that with my CPNs.ES-T2-05I’ve had the CPN nurse involved it has helped me to understand that mental health, it’s not this horrible thing that people try and put it down that it is and it can be sorted, it can. You’ve just not got to think negatively about it.ES-T3-03I’m a lot more confident now, but that’s taken a lot of hard work by [. . .] occupational therapist . . .ES-T4-02Most people over the years have always been supportive – the wife and social workers and the psychiatrists and yourself, as well. And all these services are very good, because without these people, we would be a lot worse than what I am now.ES-T5-11Nowadays [inaudible] the things that gets me is the social workers will be cut down or yourselves being cut down. Without these people, people wouldn’t be sane.ES-T5-11So they all said, the psychiatrist to get my PIP highered, which I were there last year, February, in person. And I got it [. . .] I can show you [psychiatrist] letters, that she sent to the PIP.ES-T2-02. . . when it first came out, when they were first looking at benefit, it was looking like I would have to go for interview. And whoever was my consultant psychiatrist at the time intervened, so I never went for an interview.ES-T2-16The GP’s been very supportive, I think that’s been just amazing, from first getting diagnosed with diabetes, and first getting proper support for depression, they’ve been outstanding. And I think probably are the reason why I’m alive, to be honest, because I think, one or the other would probably have killed me, if I hadn’t have changed, to some extent.ES-G2-01[Discussing issues with the PIP:] Almost all of my patients are going through that, and even if they’ve got a lot of physical health problems as well, and lots of specialists involved, the GPs charge for doing those reports. Which, I’m sure they have to, but that’s the thing. So, they can’t get reports from GPs, we, obviously, do them for free, and I will do my utmost to try and . . . You know, almost all of my patients, I know without hesitation they should be getting these benefits, and I don’t have any qualms in writing increasingly angry letters explaining that, talking about the injustices of the system. And, I do that, not because I want to be difficult, but because I’m trying to advocate for my patients.Staff ES-T2-15I also go to the benefits assessments with them because very often I find – if we send a letter then they don’t go through for the assessment. But, if we send a letter and they still go to the assessment, I will go with them, so they’ve got that level of support.Staff ES-T6-03 |
| The separation of mental and physical health care | Their focus is on diabetes, yeah. Whenever I do anything that’s specifically for diabetes, obviously, on the blood tests through the GP, and the results go through the diabetic nurse at the GP’s surgery, but when I go for the eye test and things like that, they never ask me about mental health problems, it’s never considered.ES-G2-01Interviewer:Does the psychiatrist know about your diabetic care and vice versa?ES-T2-03:They don’t know doodly squat.But, if I’ve broken a leg, and I was in recovery, and I had angina, and I was being looked after, and had diabetes and being looked after, then all of that would be under one, under the GP. But psychiatry, it isn’t, and it’s not joined up.ES-T2-03Interivewer:And does he [psychiatrist] show any sort of interest in your diabetes or does he purely talk to you about your mental health?ES-T2-05:No, just about my mental health.The GPs I tend not to go with anything to do with up here, I treat the GPs with anything physical. But sometimes it does overlap. Sometimes I go to the GP and I say, ‘oh, I’m not particularly well’, and they refer me back to the health professionals, mental health professionals. So it does work, but I tend to keep one from the other.ES-T2-16I’m seeing my GP this Wednesday with physical [laugh] things. And the two will interact, but I can’t go and sit in a GP’s surgery and go on about my paranoia because it would take up an hour of a GP’s time and that’s not fair, not fair on the GP, not fair on the other patients. So I don’t do that.ES-T2-16Interviewer:Would you ever see your GP about your schizophrenia?ES-T2-18:No, not really. It’s not really their thing is it? [. . .] What could they do? There’s nothing they can do is there.But the mental health team seem to understand it all round, that it is a big picture, that it is the diabetes and everything, it is the anxiety, not just one thing that the doctor’s trying to get out of you, they just wanted to know what one thing what’s wrong with you.ES-T3-03I don’t think the mental health side of things are necessarily that clued on diabetes.ES-T3-09Interviewer:Right, OK. And do they [GP/nurse] ever help you with your mental health as well or . . .?ES-T7-03:No, I got a psychiatrist, haven’t I?So, yes, my experience on the mental health side, experiences on the diabetic side, because . . . and that is long term and now I’ve been referred to the diabetic centre, the specialist centre, I would suggest those are borne out of lack of understanding. They haven’t got anybody who mediates that’s in a mental health capacity there, I find that bizarre.ES-T7-04. . . so the majority of our annual review it isn’t really to do with the mental illness because often they are under secondary services for that.Staff ES-PC-01What is our role in supporting them . . . I think at the moment it appears that we are the only ones that are actually supporting them in managing their diabetes, because I think there is very little support from secondary care in the management of type 2 diabetes.Staff ES-PC-02They don’t tend to ask about it [diabetes] because they don’t see it as part of a mental health nurse’s role, I don’t think. It’s still very much about a physical illness, so it’s a GP and the diabetic nurse.Staff ES-T1-02I mean, we’re aware of the impacts of the comorbid conditions, but we don’t tend to actually deal with those, other than via the GP.Staff ES-T3-05 |
| Co-ordination of mental and physical health care | We would have to liaise, which can be really frustrating because it’s not a quick answer that you get, you’ve got the single point-of-access entry into mental health, and so if somebody isn’t under services at the moment and you are wanting to get some information on medication review or whatever, you have to go through a single point of access and they get assessed by the well-being team and then they get passed on to whoever they feel is most appropriate person to see them or the team, and it’s very long-winded. It can take up to 4/6 weeks to get a simple medication review or even to get some advice sometimes, which is not brilliant really. It makes it quite frustrating for the patient and for us.Staff ES-PC-01I don’t think there is much linking between the mental health services and the diabetic services as such, but would be referring to what we have available for any patient with diabetes and I suppose most people, I suppose, with mental health problems are already under the secondary care for their health problems. We do have a mental health nurse here in the practice that we have on a once a week basis, so she would be able to give me help specifically with someone with mental health problems, but she is not, I suppose we’ve got nothing that links both, if that’s what you are meaning.Staff ES-PC-02Information doesn’t necessarily come to us directly, it goes to the GP, and so we have to access the information from the GP. Blood results, if we are sending the bloods from our site, then they come to us, so can see blood sugars and we’ll have access to that. But the diabetic plan, as such, we don’t have access to it and it goes to the GP.Staff ES-T1-01Interviewer:. . . are you provided with information that someone would have been referred on for education?Staff ES-T1-02:No, you just find out from the patient, you don’t get any information passed back to you. It’s when you say to them, how comes you’ve come on Metformin, when did this happen that you find things out.’. . . just trying to encourage them to think about it and to give them suggestions, really, about this is what you could eat instead and maybe even a referral to the dietitian and getting them to go back to the GP, because we can’t refer to dietitians, and get the GP to refer to the dietitian to give them more patient advice about their diet as well.Staff ES-T1-02. . . difficult especially with GPs, it is a very difficult to get hold of GPs, it’s very hard to get hold of GPs, but other experience has been really positive, with other professionals it has been really positive.Staff ES-T1-03We need to have a really good communication between diabetic pain and the mental health pain because I think that is what is lacking, we don’t have that communication, we don’t liaise with each other.Staff ES-T1-03Nothing for GPs and pharmacists, I think they are OK, I don’t think they need any training, I think what they need to liaise with mental health services or liaise more with mental health services.Staff ES-T1-03I think that is a bit more disjointed, I think there is still very much a divide between mental and physical in particular [. . .] people tend to very much still sit within their own branch and it’s a shame because I think you become blinkered, you know, and you can kind of, if you tap into what is available around you, I think you can certainly deliver a far more holistic service.Staff ES-T1-05Everyone who’s involved with that has responsibility to understand both sides. The psychiatrist needs to understand the physical impact of both the condition and the treatment. The GP needs to understand the interactions between the two. Any physician, medical professional, acute hospital needs to understand that having the two together is going to make things more complicated. There isn’t a uniform answer, that one-size-fits-all approach.Staff ES-T2-12It sounds like, ‘oh, that’s a lot of work’, but actually, if you know your patient and you understand the patient enough that you’re treating them with medication, with psychotherapy, with OT [occupational therapy], whatever, then you should be able to put together a five-point, six-point, three-point plan that just sets it out for an overworked GP, and equally for a GP, every physician has patients with varying levels of complexity, and for a GP to spend 5 minutes understanding his or her most complex patients will pay dividends later on.Staff ES-T2-12Like, in 3 minutes he’ll drink three milkshakes, and then he’ll vomit. And then he can’t understand where it came from. And then they make us appointments with the stomach doctor. And I said the head doctor should be working on this, it’s got nothing to do with the stomach doctor. It’s because the psychiatrist does nothing. We’re ending up at the stomach doctor, who thinks I’m stupid because do you know, with that kind of intake you should be vomiting. I do know that. I know that, but what do you want me to do about it?Family member ES-T2-08But now since it’s out of hospital we have to get one dosette box from the GP and a separate dosette box from the psychiatry, with different medicine in it. And I’m saying why, if psychiatry caused the diabetes, why must be the GP be doing it? Why is mental health not interested enough to see what’s going on with the guys? Because very seldom does your GP talk to your psychiatrist. Maybe once in a while. They don’t even share the same computer system.Family member ES-T2-08I do find that information – even though technology is a wonderful thing and should make everything easier, sometimes things don’t get passed on that should get passed on. Certainly, it takes ages for any decisions about medication from the psychiatrist doctor at [mental health centre] – he changes his medication. It seems to take ages until they readjust.Family member ES-D1-02 |
| Training needs for staff | A better understanding of the types of diabetes, how to look for sign and symptoms of diabetes because it’s a bit ad hoc, what I remember and just generally managing it [. . .] So, it would be beneficial to all of us to have some education on it even if they’re not nurses, anybody.Staff ES-T1-02I think if everyone was given the [diabetes] training, then I think it would be really good for the benefit of our patients.Staff ES-T1-03I think there are massive training needs and gaps and I think there’s a lack of understanding, you know, within mental health nursing, in particular, and having worked very closely with the nursing staff in mental health, there’s a real lack of understanding of how diabetes should be managed.Staff ES-T3-01I remember sitting with one practice nurse, and advising someone to just eat a normal diet, and me thinking, ‘****, do you know what this person’s normal diet is like? Don’t say that’, you know. We’ve spent ages trying to get through to them about just basic things like sugary drinks, and not drinking tons and tons of orange juice, they think it’s got vitamin C in it, and it’s like, no. That sort of lack of awareness of our client group.Staff ES-T3-02I would say I think I wasn’t quite as aware as perhaps I should have been about the impact that medications do have on the patient’s weight, especially weight management aims, because the weight gain is so significant and I think if you were seeing somebody for weight management advice, [. . .] if you weren’t aware of the significance of that medication, you probably would just assume that they perhaps were not entirely being . . . they were withholding things from you.Staff ES-T6-02I think it’s very easy to focus on physical side of things, but the mental health is just a massive side of it and I think if a patient has, I think if we were more trained and more aware of the mental health of a patient, I think it could probably alleviate a lot of the problems for both patients and the staff.Staff ES-PC-04 |
Glossary
- DIAbetes and Mental illness: improving Outcomes aND Services VOICE
- The patient and public involvement group associated with the DIAbetes and Mental illness: improving Outcomes aND Services (DIAMONDS) research programme.
- Diabetes Education and Self Management for Ongoing and Newly Diagnosed
- A family of type 2 diabetes education programmes provided by the NHS.
- HbA1c
- Glycated haemoglobin; an integrated measure of average blood glucose concentration over the preceding 2–3 months.
List of abbreviations
- A&E
- accident and emergency
- BIC
- Bayesian information criterion
- BMI
- body mass index
- CHD
- coronary heart disease
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CPN
- community psychiatric nurse
- CPRD
- Clinical Practice Research Datalink
- DESMOND
- Diabetes Education and Self Management for Ongoing and Newly Diagnosed
- DIAMONDS
- DIAbetes and Mental illness: improving Outcomes aND Services
- GCSE
- General Certificate of Secondary Education
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IMD
- Index of Multiple Deprivation
- IRR
- incidence rate ratio
- LSOA
- lower-layer super output area
- NDA
- National Diabetes Audit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OR
- odds ratio
- PIP
- Personal Independence Payment
- PPI
- patient and public involvement
- QOF
- Quality and Outcomes Framework
- SMI
- severe mental illness
- SSC
- Study Steering Committee
- T2DM
- type 2 diabetes mellitus
- UTS
- up to standard
- WHO
- World Health Organization
- YLD
- years lived with disability
Notes
-
Objective 2: extra models investigating the impact of additional risk factors on physical and mental health outcomes
-
Objective 3: adjusted impact of type 2 diabetes on physical and mental health outcomes for people with severe mental illness (core models)
-
Objective 3: adjusted impact of type 2 diabetes on physical health checks for people with severe mental illness (core models)
-
Objective 3: adjusted impact of type 2 diabetes on physical and mental health outcomes for people with severe mental illness (extended models)
-
Objective 3: adjusted impact of type 2 diabetes on physical health checks for people with severe mental illness (extended models)
-
Objective 4: adjusted impact of severe mental illness on physical and mental health outcomes for people with type 2 diabetes (core models)
-
Objective 4: adjusted impact of severe mental illness on physical health checks for people with type 2 diabetes (core models)
-
Objective 4: adjusted impact of severe mental illness on physical and mental health outcomes for people with type 2 diabetes (extended models)
-
Objective 4: adjusted impact of severe mental illness on physical health checks for people with type 2 diabetes (extended models)
-
Objective 6: overview of measures of unitisation and unit costs
-
Objective 6: types, Healthcare Resource Group codes and unit costs of diagnostic tests
-
The two personas used in co-design workshop 1 to generate discussion
-
Example of the board representing care professionals used as a discussion point in co-design workshop 2
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr09100).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
