Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number NIHR127879. The contractual start date was in September 2019. The final report began editorial review in May 2021 and was accepted for publication in February 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Pritchard et al. This work was produced by Pritchard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Pritchard et al.
Chapter 1 Background
It is estimated that around 20 million adults in the UK are physically inactive (i.e. not active to the recommended levels). 1 Low levels of physical activity (PA) are associated with poorer physical and mental health. 2 Encouraging exercise through PA is thus a key part of the UK’s health promotion strategy,3 with levels of inactivity being an indicator within the current Public Health Outcomes Framework for England. 4 More than 4 million hospital admissions lead to surgery each year in England alone. 5 This presents an opportunity that has hitherto not been fully exploited. Aside from the benefit that judiciously applied and successfully delivered surgical interventions can bring in their own right, the perioperative health-care encounter offers the potential for substantial health gains in the wider sense and over the longer term. The perioperative period is typically defined as starting when a patient is first referred from primary care and ending at the point at which postoperative return to function is complete. The journey thus spans primary and secondary care (Figure 1). 6 However, we adopted a still wider view for the purposes of this evidence synthesis. The point at which surgery is first contemplated was a better place to start. A patient presenting to primary care with a potentially operable condition (e.g. a hernia or gallstone disease) may find that, if they are able to increase their PA levels, the problem improves such that surgery is no longer needed.
FIGURE 1.
The perioperative pathway.
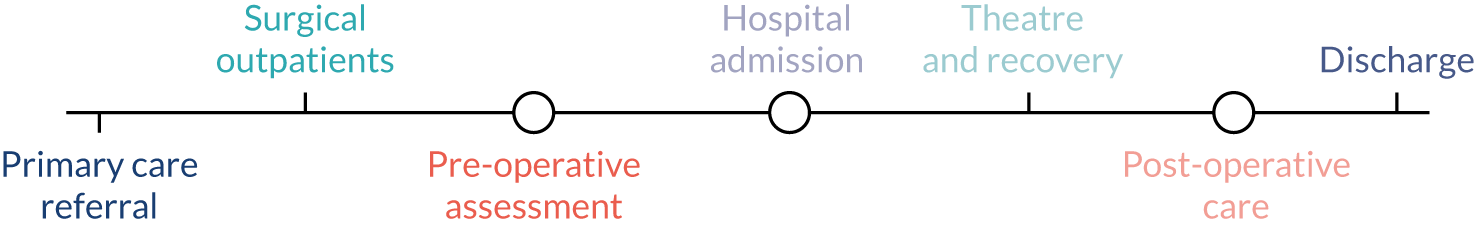
Likewise, a focus on discharge from hospital was insufficient to allow a proper appraisal of the benefits and drawbacks of PA in the medium to longer term (e.g. 12 months after surgery). This interpretation promoted an extended and more intensive involvement with primary health care than is currently considered within the perioperative pathway; this was a particular focus of our work.
This proposal aimed to explore the potential to use the perioperative encounter to promote PA and exercise in the medium to long term.
There has been some work on promoting exercise preoperatively, but this has focused on the use of physical exercise measurements to infer the risk of adverse outcomes after major surgery, and how this risk might be modulated by exercise training in the weeks before surgery. 7 Studies have focused on circumscribed groups of patients; for instance, the outcomes in the review by Loughney et al. 8 were physical fitness variables, but the participants were limited to people undergoing neoadjuvant cancer treatments. Loughney et al. 8 found that exercise interventions were safe and feasible in this patient group and that physical fitness was improved. To us, these findings demonstrate how the incentive of impending surgery, with an appropriate intervention, can provide people with motivation to adopt healthier lifestyle behaviours.
The concept of ‘perioperative medicine’ has gained ground in recent years. 9 This focuses on a wide package of measures to improve patients’ fitness before surgery; attend more closely to patients’ needs during procedures; and optimise recovery and rehabilitation to speed the return to work, home and family. This has been stimulated by two factors: first, an understanding that earlier involvement of a wider team of health-care professionals in the preoperative management of surgical patients brings substantial benefits. 10 For instance, ‘prehabilitation’ through smoking and alcohol cessation and optimisation of pre-existing diseases has improved surgical outcomes11,12 and may, in itself, reduce the need for surgical intervention. Second, the recognition that early postoperative problems can affect long-term outcomes has helped clinicians and researchers think further than 30 days postoperatively (the usual length of follow-up for many surgical outcome studies) to better promote longer-term health and well-being. 13
For any perioperative intervention to be effective, involvement of the necessary multidisciplinary team (MDT) as soon as surgery is contemplated is vital (rather than immediately before surgery). 14 In terms of health promotion (in this case, exercise promotion), a multidisciplinary approach allows for repetition and reinforcement of behaviour change messages and addressing barriers to change. General practitioners (GPs), specialist nurses, anaesthetists, psychologists, surgeons and physiotherapists15 would need to be included in a pathway that works between primary and secondary care, within an integrated care model. 16,17 Although there is some work on primary–secondary care co-ordination in general,18 and early interest in primary care intervention to improve postoperative outcomes,19 the potential for collaborative working to improve health in the longer term has not been studied in this context.
We urgently need to understand how to integrate models of care that optimise not only surgical outcomes, but also the longer-term health benefits of increased PA in a perioperative pathway, and understand why the successful models work. The current interest in a broader, cross-cutting approach to the medical needs of the surgical patient implied by the perioperative medicine ‘movement’ suggests that the time is right to explore this area more fully.
Our proposal explored the current evidence for using the perioperative encounter to promote exercise. However, it considered the context of this encounter and the model of care in which any interventions occurred. We focused on settings in which such interventions might be delivered, timings of interventions, incentives, staff20 and the types of interventions used (e.g. use of social media applications,21 web-based interventions22 and motivational interviewing23). Exercise promotion was the focus of this work, but it may be that something could also be learned from, for instance, interventions to promote alcohol and smoking cessation. 11,12 Williams and Glasby24 have argued that, in attempting to evaluate ‘what works’ in health and social care, too narrow a definition of valid ‘evidence’ has been used. They called for a notion of ‘knowledge-based practice’ that draws not only on research, but also on ‘the tacit knowledge of front-line practitioners and the lived experience of people using services’. 24 With this in mind, and drawing on our previous work on tacit knowledge in health-care practice,25,26 we proposed to expand our evidence synthesis by supplementing our appraisal of the peer-reviewed and ‘grey’ literature with a series of case examples.
Drawing on a previous mapping exercise we undertook,27 we had a working model of the ‘extended’ perioperative period (see Figure 1), and used this at the start of the project as a substrate for suggestion and development at the first advisory group meeting, and as the basis for a final overarching narrative synthesis of evidence against our contextual framework.
Rationale
Ageing populations and increased longevity, coupled with chronic health problems, have become global challenges, putting new demands on medical and social services. Delivering continuously improving care in the presence of increasing demand is perhaps the main challenge faced by health systems throughout the world. Reconciling the three aims of improving the patient experience, enhancing population health and reducing the per capita cost of care is a global problem, which the UK’s Sustainability and Transformation Partnerships broadly aim to embed into the NHS. 28
Enhancing public health through the promotion of physical exercise, whether at a community29 or, more commonly, an individual level,30 is a key public health priority. Current guidance is summarised by the National Institute for Health and Care Excellence (NICE)30 and centres around delivering brief interventions. Brief interventions can take many forms and, indeed, there remains some uncertainty about exactly what constitutes a ‘brief intervention’. There is evidence that they increase short-term self-reported PA, but there is still insufficient evidence about long-term impacts and factors influencing their effectiveness. 31 Longer interventions may include elements of motivational interviewing techniques that can be used by any health-care professional and for which there is strong evidence of increased PA. 23 There is also evidence that brief interventions to promote PA are likely to be cost-effective. 32
However, given that so many people are still not active to recommended levels, with the UK ranking poorly in a recent international study,33 new models of encouraging PA are needed. Consequently, it makes good sense to make the most of the public health potential of every health-care encounter. 34 The Chief Medical Officer’s Moving Medicine initiative (launched in October 2018)35 is part of this strategy, but is more targeted at specific physical conditions than at the perioperative period. 36
This proposal also addressed, and indeed expanded on, one of the key research questions within the NICE PA promotion guidelines,30 namely ‘What infrastructures and systems help increase the number of assessments of PA undertaken and the delivery of brief advice?’. In addition, it drew on the National Institute for Health and Care Research (NIHR)-supported James Lind Alliance Priority Setting Partnerships. 37 Cogent questions already prioritised through this engagement process with patients, carers and clinicians include ‘How can preoperative exercise of fitness training, including physiotherapy, improve outcomes after surgery?’.
Theoretical framework
This project involved a mixed-methods approach. We conducted a comprehensive systematic review38 to identify and appraise available peer-reviewed and ‘grey’ literature on exercise interventions that have been used during the perioperative encounter. We sought evidence on outcomes reflecting continued engagement in PA (e.g. at 12 months after the intervention) and also measures reflecting patients’ experiences of the interventions. Only when study designs and interventions were appropriately homogeneous was quantitative analysis conducted. 38
Taking a broad view of ‘evidence’ after Williams and Glasby,24 we also identified practical examples of relevant interventions to promote PA. For these practical examples we used case study methods to explore which aspects of the intervention, the individuals and teams, and the wider organisation (whether within primary or secondary care) influenced the adoption of the intervention.
By using multiple data collection sources in this phase of the project (document collection, interviews and brief observations), we intended to improve the accuracy and completeness of the current landscape in exercise innovations. 26 This also compensated for ‘lag time’ to publication, which is an inevitable consequence of a systematic review approach.
We used Bate et al. ’s39 ‘challenge’ framework to structure our examination of the contextual factors within the evaluation of exercise promotion interventions and models of care, both for the literature and practical case studies. A final integrative, interpretive synthesis explored relationships in the data between context, mechanisms and outcomes.
Research question/aim
What is the potential for promoting PA and exercise in the medium to long term among people undergoing elective surgery?
The aim was to examine a broad range of evidence and knowledge to identify, and place in context, interventions applied during the perioperative period to promote PA and exercise in the medium to longer term. We did this through comprehensive literature searching and synthesis (systematic review) supplemented by an analysis of relevant practical case studies.
Objectives
This research incorporated the following objectives:
-
a systematic search that included data extraction from, and quality appraisal of, published peer-reviewed and ‘grey’ literature
-
identification of, and the collection of data from, existing practical examples (i.e. case studies)
-
analysis of context, using the ‘challenge’ framework,39 and its role in the effectiveness of interventions
-
overarching narrative synthesis of existing and possible models of perioperative care that offer the greatest potential benefit for the promotion of PA.
Chapter 2 Systematic review
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi-randomised controlled trials (Q-RCTs); Q-RCTs use methods of allocation that are not random but are intended to produce similar groups when used to allocate participants, for example allocation according to date of birth or hospital record number.
We also included other study designs, or non-randomised studies (NRSs), in the review. We used study design features to categorise these studies according to Higgins et al. 38 (table 13.2.a), rather than study design labels used by study authors, and considered inclusion of non-randomised controlled trials, controlled before-and-after studies, prospective cohort studies, retrospective cohort studies, historically controlled trials, case–control studies, case reports and before-and-after comparison studies. These study designs represented a supplementary set in the review; we did not conduct risk-of-bias assessments of, or analyses on findings from, these studies.
We excluded small case studies in which there were fewer than 10 participants.
We also excluded crossover study designs in which participants in each group received both the intervention and the comparison at alternate times. This design requires a suitable washout period to avoid any carry-over effects of the initial intervention, and we did not expect a washout period to be achievable for studies meeting our review objectives.
Types of participants
We included adult participants who were at least 18 years of age.
To include a study population that most closely fitted the review objectives, we adopted population cut-off points when selecting studies; we acknowledge that these are arbitrary cut-off points. We included studies in which:
-
at least 60% of study participants were undergoing or had undergone a surgical procedure
-
participants were recruited within a mean time of 6 months after the completion of surgery.
We included oncological studies if they met the first two or the first and third of following criteria:
-
if the study authors specifically stated that participants had received surgery or adjuvant therapies (such as chemotherapy or radiotherapy)
-
if participants were recruited within a mean time of 6 months after surgery (or, if surgery was not specified, after completion of adjuvant therapies)
-
it could be inferred from the study report that surgery was likely to have been completed within 6 months (using information about adjuvant therapies and the time since diagnosis).
We excluded studies in which recruitment took place more than 6 months after completion of surgery/treatment. We also excluded studies that had an unclearly defined time since surgery/treatment, and that suggested a time of recruitment in which we expected that most participants (at least 60%) were likely to have been recruited after the aforementioned cut-off points.
Types of interventions
We included interventions that encouraged participants to engage in PA or exercise. We defined exercise and PA as a planned and structured activity that takes place regularly to improve physical fitness; some examples are walking, running, swimming, cycling, aerobics, Pilates and yoga.
We included interventions that took place as a group (such as a fitness class) or at a one-to-one level, or that were individualised to the participant or were for all participants. As well as interventions that required participants to engage in a specific activity (such as attendance at a clinic), we also included interventions that provided support or encouragement to engage in exercise or PA; these interventions could include counselling (face to face or remotely) or the provision of information or equipment to facilitate or motivate participants to engage in exercise or PA.
The interventions could be delivered on one or more occasions and could be delivered by one or more health-care professionals. We required the intervention to be initiated within the extended perioperative pathway up to the cut-off time points of participant recruitment described in Types of participants. However, the duration of the intervention could extend beyond this period.
We included studies in which interventions were given as part of a ‘package’ of measures aimed at promoting a healthier lifestyle; however, we required the promotion of engagement in exercise or PA to be a significant component of this package.
For studies in which a comparison group was included, we included comparisons that were ‘usual care’ or were another type of intervention. We accepted any type of usual care described by study authors, and acknowledge that this could vary considerably between countries and the time that the study was conducted. We included any other type of intervention as a comparison, which could be a less enhanced version of the main intervention, or could be the same intervention that was initiated at a later time point.
We excluded studies in which the intervention targeted a specific muscle group or followed surgery for a sporting injury.
Types of outcome measures
To ensure that studies addressed the research question, we included studies only if they measured and reported our primary outcomes (PA) at least 6 months after surgery (when the intervention was started post surgery), or 6 months after the beginning of the intervention (when the intervention was started pre surgery).
We accepted that measures of PA were not always the primary outcomes specified by study authors in their study objectives or in their sample size calculations. However, we excluded studies that did not measure and report our primary outcomes; we judged that these studies were not designed to evaluate our review objectives.
Primary outcomes
-
Physical activity: amount of PA/exercise conducted at the end of follow-up (such as mean number of steps measured using a step counter).
-
Physical activity: number of people who were engaging in PA at the end of follow-up (e.g. as measured in a self-reported questionnaire).
Secondary outcomes
-
Physical fitness: mean scores [and standard deviation (SD)] measured using standardised tools at the end of follow-up [cardiopulmonary exercise testing as first choice, but also included data for peak heart rate (beats/minute), peak oxygen consumption (ml/kg/minute), peak power output (W), 6-minute walk (m), bench press (kg), leg press (kg), handgrip (kg), sit and reach (cm), anaerobic or lactic threshold, peak oxygen uptake (VO2 peak) and minute ventilation to carbon dioxide output (VE/VCO2)]. 7
-
Quality of life at the end of follow-up: mean scores using a validated tool [e.g. Short Form questionnaire-36 items (SF-36), EuroQol-5 Dimensions (EQ-5D), the Centers for Disease Control and Prevention Health-related Quality of Life measure or condition-specific tools].
-
Cancellation of surgery because of improved health: number of participants who no longer needed surgery.
-
Participants’ experiences of participation: could include narrative summaries, measures on a continuous scale or number of people who were satisfied with the experience.
-
Adherence: may include attendance at PA classes or completion of motivational telephone calls.
-
Pain at the end of follow-up: mean scores using a validated tool [e.g. visual analogue scale (VAS)].
-
Adverse events: as described by study authors (both related and unrelated to the intervention).
Search methods for identification of studies
Electronic searches
We conducted electronic searches of a number of major databases, applying no restrictions on language or publication status (for search strategies, see Report Supplementary Material 1). We searched the following databases for relevant trials:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2020; Issue 10) via The Cochrane Library (searched on 22 October 2020)
-
MEDLINE (Ovid SP; 1946 to 22 October 2020)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost; 1981 to 22 October 2020)
-
EMBASE (Ovid SP; 1974 to 22 October 2020)
-
PsycINFO (EBSCOhost; inception to 22 October 2020)
-
SPORTDiscus (EBSCOhost; inception to 22 October 2020).
We searched the following clinical trial registers to identify ongoing studies and completed trials awaiting publication:
Searching other resources
We conducted forward citation searches of studies that met our inclusion criteria and backward citation searching of key articles and reviews using the Web of Science citation index. We examined grey literature, as defined by McGrath et al. 42 using ‘opengrey’43 for valuable contextual perspectives and up-to-date intelligence not yet available within peer-reviewed sources.
Data collection and analysis
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates. Using Covidence 2018 software (Melbourne, VIC, Australia), two of four review authors (SRL, SVG, MWP and AR) independently screened the results of the search of titles and abstracts to identify potentially relevant studies. Results were compared at regular intervals, and consensus was reached through discussion. During these discussions, we refined the inclusion and exclusion criteria to meet the specific objectives of the review. We sourced the full texts of all potentially relevant studies and two of four review authors (SRL, SVG, MWP and AR) considered whether or not they met the inclusion criteria; again, we reached consensus through discussion and refined the inclusion criteria as necessary. At this stage we reviewed abstracts, and included them in the review only if they provided adequate information and relevant results that contained denominator figures for the intervention and control groups. We recorded the number of papers obtained at each stage and include this information in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart (see Figure 2). In the review, we reported brief details of related but excluded papers.
Data extraction and management
We used Covidence 2018 software to collect and record details from individual studies to describe their context and the results of their outcome measures. We adapted a basic template from Covidence 2018 to collect the following information:
-
Methods – type of study design [for studies that were not RCTs, we collected study design features, as per Higgins et al. 38 (table 13.2.a), rather than using the study design labels used by the study authors], setting in which participant was initially recruited, dates of the study, funding sources and study author declarations of interest.
-
Participants – number of participants randomised to each group, type of surgery, reason for surgery and baseline characteristics for each group [to include age, gender, body mass index (BMI), weight, height, baseline level of fitness, current involvement in regular PA, relevant clinical variable and illness severity scores such as Acute Physiology and Chronic Health Evaluation II (APACHE II) or American Society of Anesthesiologists status].
-
Intervention – details of intervention and control (to include the type of PA, the location of the intervention, number of sessions, duration, person prescribing and/or providing the intervention, time point of initiation, group-based/individual, generic or individualised, resources used or special equipment provided, intensity of PA and provision of supplementary interventions or additional components).
-
Outcomes – all relevant review outcomes as measured and reported by study authors, including time points of measurement.
-
Outcome data – results of outcome data.
We considered the relevance of information from each study and the generalisability of data to the study population (i.e. the potential for indirectness in our work). In the event of finding associated publications from the same study, we created a composite data set based on all eligible publications.
Assessment of risk of bias in included studies
We conducted risk-of-bias assessments for RCTs and Q-RCTs using the Cochrane Risk of Bias tool. 44 Two review authors (AR, MWP or SRL) independently assessed study quality, study limitations and the extent of potential bias in each study. Consensus was reached through discussion. The following domains were assessed:
-
sequence generation (selection bias)
-
allocation concealment (selection bias)
-
blinding of participants, personnel and outcome assessors (performance and detection bias)
-
incomplete outcome data (attrition bias)
-
selective outcome reporting (reporting bias)
-
other potential risks of bias.
For each domain, we judged whether or not study authors had made sufficient attempts to minimise bias in their study design. We made judgements using three measures: high, low and unclear risk of bias. We recorded the judgements in risk-of-bias tables (see Report Supplementary Material 2) and present a summary risk-of-bias table in Appendix 8.
We did not conduct risk-of-bias assessments on the NRSs as these provided supplementary data, rather than primary data, for this review.
Measures of treatment effect
We collected both dichotomous and continuous data depending on the measurement methods, tools and scales used by the study authors. For example, for physical fitness, we collected data that reported the number of people engaged in PA at the end of follow-up, or the amount of PA using a tool such as a step counter. Where possible, we reported dichotomous data as risk ratios (RRs) to compare groups, and continuous data as mean differences (MDs) or standardised mean difference (SMDs); we reported 95% confidence intervals (CI). We also collected narrative data of individual participant experience.
Unit-of-analysis issues
We noted studies that had more than one intervention group. We did not combine data in these studies; instead, we reported data separately for each intervention or comparison group. We used sensitivity analysis to explore whether or not the choice of intervention group in the analysis influenced the effect estimate.
Dealing with missing data
We considered data to be complete if losses were reported and explained by study authors, and we combined no incomplete data in the meta-analysis. In the event that studies indicated that our primary outcomes were measured but not were not reported, we attempted contact with study authors via e-mail.
Assessment of heterogeneity
We assessed whether or not evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical and methodological heterogeneity by comparing similarities in our included studies between study designs, participants, interventions and outcomes, and used the data collected from the full-text reports. We assessed statistical heterogeneity by calculating the chi-squared test or I2 statistic and judged the level of heterogeneity according to the following I2 values:38
-
0–40% – might not be important
-
30–60% – may represent moderate heterogeneity
-
50–90% – may represent substantial heterogeneity
-
75–100% – considerable heterogeneity.
In addition to considering statistical results, we looked at point estimates and overlap of CIs. When CIs overlap, results are more consistent. However, although combined studies might display a large consistent effect, it might have significant heterogeneity. Thus we planned to interpret heterogeneity with caution. 45
Assessment of reporting biases
We sought published protocols for all included studies by utilising clinical trials registers. To assess the risk of selective reporting bias, we compared published protocols with published study results. We generated a funnel plot to assess the risk of publication bias if we identified sufficient studies reporting on an outcome (i.e. > 10 studies46). An asymmetrical funnel plot may suggest publication of only positive results. 47
Data synthesis
We completed meta-analyses of outcomes for which we had comparable effect measures from more than one study for each comparison group, and when measures of heterogeneity indicated that pooling of results was appropriate. We did not pool studies that had a high level of methodological or clinical heterogeneity. We used the statistical calculator in Review Manager (RevMan) 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) to perform meta-analysis.
We used the Mantel–Haenszel random-effects model to account for potential variability in participant conditions between studies.
We calculated CIs at 95% and used a p-value of ≤ 0.05 to decide if a result was statistically significant. We considered imprecision in the analyses’ results by assessing the effect measure’s CI; a wide CI would suggest a higher level of imprecision. Precision may also be reduced with a small number of studies. 48
For analyses where SMD indicated a statistically significant effect, we used Cohen’s d to judge the size of the effect. 49
We always used the latest time point reported by study authors in our analyses. Some studies reported data at more than one time point. In analysis, we used the latest time point reported by study authors because our intention was to establish the long-term effect of interventions. We reported the time points used in meta-analyses, alongside effect estimates, and whenever possible we used sensitivity analysis to reanalyse the data with time points that were more consistent.
If a study measured an outcome using different tools, we included data for only one of those tools. We clearly reported which tool we had used. Whenever possible, we aimed to select the tool that provided the most objective assessment, or which was the most commonly used tool in the analysis.
We included multiarm studies in the review. In analysis, we selected the intervention that we judged to be the most enhanced intervention; we selected the alternative intervention in sensitivity analysis.
Subgroup analysis and investigation of heterogeneity
We attempted to explore differences between the included studies using information collected during data extraction. To draw meaningful results from tests for subgroup interactions, we conducted subgroup analysis only when we had > 10 studies.
Our choice of subgroups was defined post hoc, after comparison of all the varying participant and intervention characteristics. We attempted subgroup analysis of the following characteristics:
-
duration of intervention – intervention undertaken for < 6 months or for at least 6 months
-
time of intervention commencement – intervention was given during the pre-surgery period or post surgery
-
type of surgery – participants underwent surgery for types of cancer or for other conditions
-
age – participants had a mean age of < 60 years or a mean age of at least 60 years
-
BMI – participants had a BMI of < 30 kg/m2 or of at least 30 kg/m2.
Sensitivity analyses
We used sensitivity analyses to explore the effect of decisions made during the review process. We compared the effect estimates from sensitivity analyses with those in the primary analyses; we used this information when assessing our confidence in the estimates. We conducted sensitivity analyses, if pooled analyses included at least two studies, as follows:
-
We excluded studies that measured the outcome immediately after the end of the intervention period.
-
Some studies used more than one measurement tool to report an outcome; we used the alternative measurement tools in sensitivity analysis.
-
Some studies were multiarm studies; we used data from the alternative intervention arm in sensitivity analysis.
-
We excluded studies that we judged to be at high risk of attrition bias (this was an outcome-specific judgement).
-
We excluded studies that we judged to be at high or unclear risk of selection bias (for sequence generation).
Grading of Recommendations Assessment, Development and Evaluation
Two review authors used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system to assess the certainty of the body of evidence associated with the following outcomes:50
-
amount of PA completed at the end of follow-up
-
number of people engaged in PA at the end of follow-up
-
level of physical fitness at the end of follow-up
-
health-related quality of life (HRQoL) at the end of follow-up
-
pain at the end of follow-up
-
adverse events
-
adherence to the intervention.
Based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed, the GRADE approach appraises the certainty of a body of evidence. Evaluation of certainty considers within-study risk of bias and risk of publication bias, heterogeneity of the data, directness of the evidence and precision of the effect estimates. We used the GRADE approach for both comparisons in the review: ‘intervention versus usual care’ and ‘intervention versus intervention’. We did not construct summary-of-findings tables, but instead presented these GRADE assessments narratively (see Effects of interventions).
Results
Results of the search
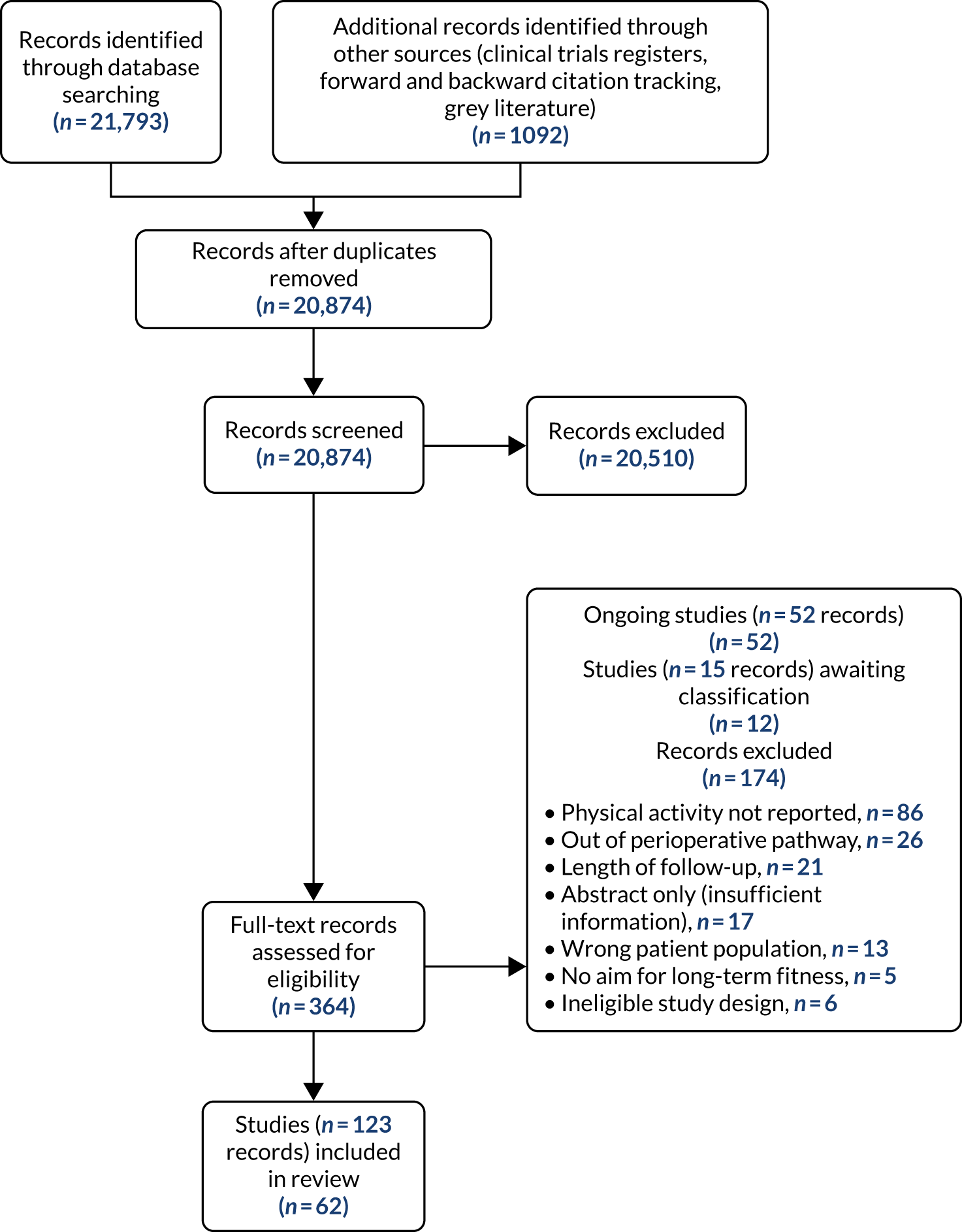
After the removal of duplicates from the search results, we screened 20,874 titles and abstracts, which included forward and backward citation searches and searches of clinical trials registers. We looked at the full text of 364 records and selected 62 studies for inclusion, based on review criteria (see Appendix 1, Table 7). We identified 52 ongoing studies (see Appendix 1, Table 9), found 12 studies for which we were not able to effectively assess eligibility (see Appendix 1, Table 8) and excluded 174 records (Figure 2).
FIGURE 2.
Study flow diagram for the review.
Included studies
We included 62 studies51–112 for which there are 124 references (see Appendix 1, Table 7). A total of 51 studies51–57,59–64,66,68–70,72,74–79,81–84,86–88,90,92–97,100–112 were RCTs, and two58,85 were Q-RCTs, all of which had a parallel design; 30 studies52–54,56,58,59,62,64,68,70,72,76–78,81,83,85,88,90,91,94–96,100,101,105–108,110 were based in a single centre, whereas 22 studies51,55,57,60,61,63,66,69,74,75,79,82,84,86,87,92,97,102–104,109,111 were multicentre. There is no information for one of the studies,93 as it exists as a conference abstract with only limited information.
Of the remaining nine studies, five were single-group, single-centre, before-and-after comparisons;65,67,71,89,98 two were single-group, multicentre, before-and-after comparisons;80,99 one was a parallel-design, multicentre before-and-after comparison;112 and one was a parallel-design, single-centre controlled before-and-after study. 73
In the RCTs and Q-RCTs, a total of 7939 participants were randomised. In the NRSs, 630 participants were included.
We collected and summarised the data on the population and intervention characteristics of each included study in characteristics tables (see Appendix 1).
Description of population characteristics, for randomised controlled trials
Surgical/non-surgical participants
Seven studies56,66,74,90,94,97,103 reported numbers of non-surgical participants; in all of these studies non-surgical participants accounted for no more than 40% of the total participants. The remaining 46 studies51–55,57–64,68–70,72,75–79,81–88,91–93,95,96,100–102,104–111 reported no non-surgical participants, or we judged them as being likely to include no, or an insignificant number of, non-surgical participants.
Age
All studies included only adult participants. One study93 did not report the average age of its participants, but did have an age requirement of between 55 and 75 years in its inclusion criteria. In the case of the study by Mundle et al. ,100 who neither reported the average age of participants nor specified an age requirement in their inclusion criteria, we assumed that only adult participants were included because all participants were undergoing a percutaneous coronary intervention or cardiac surgery. A number of studies included either older adults or elderly people only: Barnason et al. 55 included adults aged ≥ 65 years, Christiansen et al. 62 included adults aged > 45 years, Christiansen et al. 61 included adults aged between 50 and 85 years, Kummel et al. 91 included adults aged ≥ 65 years, Li et al. 93 included adults aged between 55 and 75 years, Losina et al. 96 included adults aged ≥ 40 years, Piva et al. 102 included adults aged ≥ 60 years, Santa Mina et al. 103 included adults aged between 40 and 80 years, Taraldsen et al. 107 included adults aged ≥ 70 years, Turunen et al. 108 included adults aged > 60 years and Turunen et al. 109 included adults aged ≥ 60 years.
Gender
Three studies76,88,103 included only male participants. Seven studies59,63,69,77,83,93,104 included only female participants. The remaining RCTs included a combination of male and female participants.
Type of condition
Eleven studies59,63,66,69,74–76,78,82,83,103 recruited participants undergoing treatment for types of cancer: breast cancer;59,63,69,83 prostate cancer;76,103 breast and prostate cancer;66 colorectal and prostate cancer;75 colorectal cancer;78,82 and prostate and breast cancer, as well as a number of unspecified tumours. 74
Nine studies55,56,70,88,90–92,105,111 recruited participants undergoing coronary artery bypass surgery. One study72 recruited participants undergoing myocardial revascularisation surgery. One study87 recruited a mix of participants, with some undergoing percutaneous transluminal coronary angioplasty and some undergoing coronary artery bypass surgery. One study100 did not specify the surgery that their participants underwent, describing it as cardiac surgery only.
Five studies53,57,64,85,94 recruited participants undergoing bariatric surgery. Three studies60,104,106 recruited participants undergoing Roux-en-Y gastric bypass surgery.
Six studies52,62,81,93,96,102 recruited participants undergoing total knee replacement (TKR). Four studies77,79,107,108 recruited participants undergoing total hip replacement. Two studies58,110 recruited a mix of participants, with some undergoing TKR, and some undergoing total hip replacement.
The treatments that the remaining 10 studies selected for were as follows: joint replacement and back surgery,109 lumbar spine fusion surgery,84 laminectomy,51 standard lumbar discectomy,86 surgery for degenerative lumbar spine disorder,95 lumbar fusion surgery,97 lumbar surgery,68 renal transplantation,101 major digestive surgery54 and dysvascular transtibial amputation. 61
Country
A total of 51% of the studies were conducted in Europe: four in the UK,52,76,82,87 five in Finland,70,84,91,108,109 two in Germany,58,77 four in the Netherlands,74,75,81,90 four in Norway,79,83,94,107 four in Sweden,86,95,97,104 two in Spain,54,85 one in Denmark106 and one in Switzerland. 56
A total of 42% of studies were conducted in North America: 15 in the USA;51,55,57,59–62,64,68,72,88,96,101,102,111 six in Canada;53,63,92,100,103,105 and one in both the USA and Canada. 66
Six per cent of studies were conducted in Australasia: all three in Australia. 69,78,110 One per cent of studies were conducted in Asia: in China. 93
Race/ethnicity
Fifteen studies51,57,59,60,64,66,76,83,85,87,96,101–103,111 reported baseline characteristics data on race and/or ethnicity. These studies reported a majority of participants of northern European and Iberian descent, with minority populations of African and Caribbean descent, and various Asian and autochthonous communities. We considered the ethnic make-up of these studies to be largely representative of the populations in these countries. We assumed that the study conducted in China93 included a majority of East Asian participants. The remaining studies did not report baseline characteristics data on race or ethnicity.
Body mass index
Seven studies53,57,60,64,85,94,104 reported a mean baseline BMI of ≥ 35 kg/m2 among participants. All these studies included participants undergoing surgery for obesity. The other study106 including participants undergoing surgery for obesity reported a mean baseline BMI of > 30 kg/m2.
In addition, four studies51,61,81,102 reported a mean baseline BMI of between 30 and 34.9 kg/m2. One study51 included participants undergoing surgery for spinal degenerative disorder, one study61 included participants undergoing dysvascular transtibial amputation and two studies81,102 included participants undergoing TKR.
Seventeen studies55,58,59,62,63,66,69,75,76,79,84,87,92,97,103,108,110 reported a mean baseline BMI of between 25 kg/m2 and 29.9 kg/m2.
Four studies54,77,78,101 reported a mean baseline BMI of between 20 kg/m2 and 24.9 kg/m2.
One study96 gave numbers of participants with a mean BMI of < 30.0 kg/m2, between 30.0 kg/m2 and 34.9 kg/m2 and ≥ 35.0 kg/m2.
The remaining 19 studies52,56,68,70,72,74,82,83,86,88,90,91,93,95,100,105,107,109,111 did not report baseline BMI data for their participants.
Education status
Twenty-six studies51,53,55,57,59,64,66,69,74–76,78,79,83–85,87,94,96,97,102–105,109,111 reported baseline characteristics data for education status. Because these studies were from a number of different countries, each having a different accreditation system, and they recorded these data in disparate ways, it was not viable to collate these data. However, when reported, we have included these data separately for each study in the population box of the characteristics of included studies tables (see Report Supplementary Material 2).
Economic status
Seven studies53,63,69,78,103,105,111 reported baseline characteristics data for economic status. Because these studies were from a number of different countries, each having a different currency, and they recorded these data in disparate ways, it was not viable to collate these data. However, when reported, we have included these data separately for each study in the population box of the respective characteristics of included studies tables (see Report Supplementary Material 2).
Other baseline characteristics
A number of studies reported baseline characteristics data for fitness, employment and amount of PA. Because these data were reported in disparate ways, and often sporadically, it was not viable to collate these data. However, when reported, we have included these data separately for each study in the population box of the characteristics of included studies tables (see Report Supplementary Material 2).
Description of intervention models, for randomised controlled trials
We describe the key intervention characteristics and approaches described by study authors. These are summarised for each individual study; see Report Supplementary Material 2.
We describe 67 interventions that were examined in 53 included studies. 51–64,66,68–70,72,74–79,81–88,90–97,100–111 Most studies examined one or more intervention in addition to a usual care comparator. However, this summary also includes 12 studies51,56,63,64,74,86–88,90,96,102,105 that compared two or more interventions, rather than an intervention and a control; in the cases of Boesch et al. 56 and Losina et al. 96 we describe three interventions.
Period of delivery
We refer to the interventions as falling into one of three clinical periods: preoperative (those that took place only in the period before surgery),54,57,95,103 postoperative (those that took place only in the period following surgery)51,52,55,56,58–63,66,68,69,72,75–79,81–88,90,92,93,96,100–102,104–109,111 or perioperative (those that include both preoperative and postoperative components). 53,64,70,74,91,94,97,110
Particularly for those interventions beginning post surgery, pinpointing exactly when interventions started was not always possible. Some studies simply noted that they began ‘after surgery’, whereas others indicated that the time of initiation was influenced by patients’ post-surgery recovery, or by the time taken to recruit participants to the study. In addition, some studies provided the mean time of the intervention, others a range. When a range was reported, we used the lower limit. Others provided time in months (we have equated 1 month to 4 weeks) or in days. We have rounded these figures to the nearest whole week. Therefore, these data should be interpreted as indicative. A summary chart is presented in Figure 3.
FIGURE 3.
Period of intervention delivery.
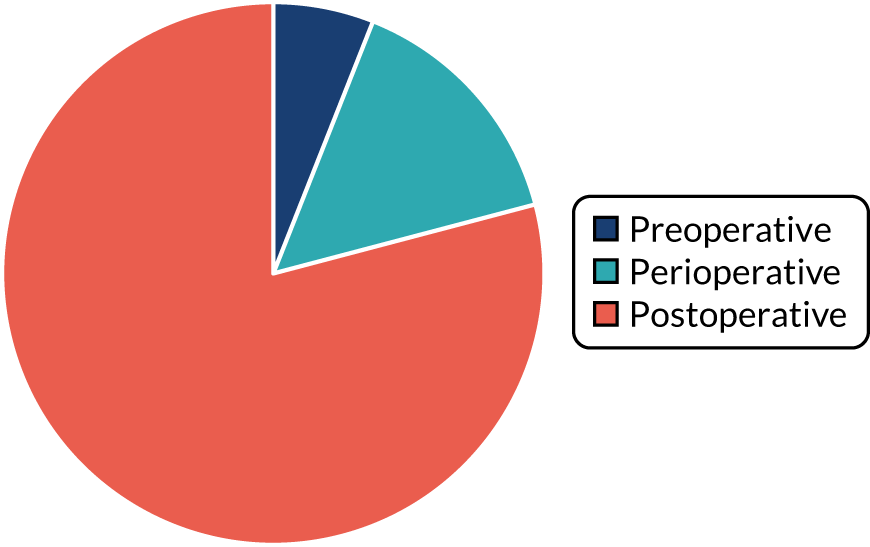
Post operative
Interventions overwhelmingly began post operatively (79.1%).
A number of studies55,59,62,63,66,78,83,90,100 were vague on start point, and, although we suspect that the majority began within a few weeks of surgery, as it was not clear, we have grouped them separately. Some studies indicated commencement a short time after surgery, for example ‘on entering’90 or ‘during’100 cardiac rehabilitation. A number of cancer studies described the intervention beginning before or during adjuvant therapies. 59,63,83 Two studies suggested that participants began interventions over a broader period of time (a mean of 6.3 months since colorectal cancer diagnosis78 and within 9 months of diagnosis66).
Of the other studies, roughly half of the interventions began in the immediate days or early weeks after surgery: 10 interventions began within a few days of, or during the first week after, surgery,72,77,81,82,111 including Kinsey et al. ,88 which involved two interventions, and Losina et al. ,96 which involved three interventions; and nine interventions began in the 2–4 weeks after surgery,58,85,93,109 including Boesch et al. ,56 which involved three interventions, and Johansson et al. ,86 which involved two interventions.
The remaining 23 interventions were delayed post surgery: in 17 studies, there was a delay of > 1 month to 3 months,52,60,68,69,75,76,79,84,101,104,108 including Archer et al. ,51 Jolly et al. 87 and Smith et al. ,105 which each involved two interventions; in six studies, the intervention began > 3 months after surgery,61,92,106,107 including Piva et al. ,102 which involved two interventions.
Perioperatively
Ten interventions (14.9%) were delivered perioperatively: five began between 2 and 4 weeks prior to surgery,70,91,110 including the two intervention arms of Creel et al. ;64 two began 6–8 weeks prior to surgery;94,97 one began 6 months prior to surgery;53 and the intervention arms of Goedendorp et al. ,74 which did not clearly state their initiation points (these were cancer interventions described as beginning at the time, or within days, of diagnosis).
Intervention approach
Interventions often involved multiple components or modes of delivery (55.2%). These components tended to fall into three categories (Figure 4): (1) education and advice (82.1%), including the provision of written or verbal information and advice, PA recommendations or a formal exercise prescription; (2) behavioural mechanisms (59.7%), which focused on behaviour change theories, usually through therapeutic approaches including counselling or motivational interviewing; or (3) direct PA instruction (44.8%) in the form of group classes or one-to-one sessions.
FIGURE 4.
Studies using each intervention approach.
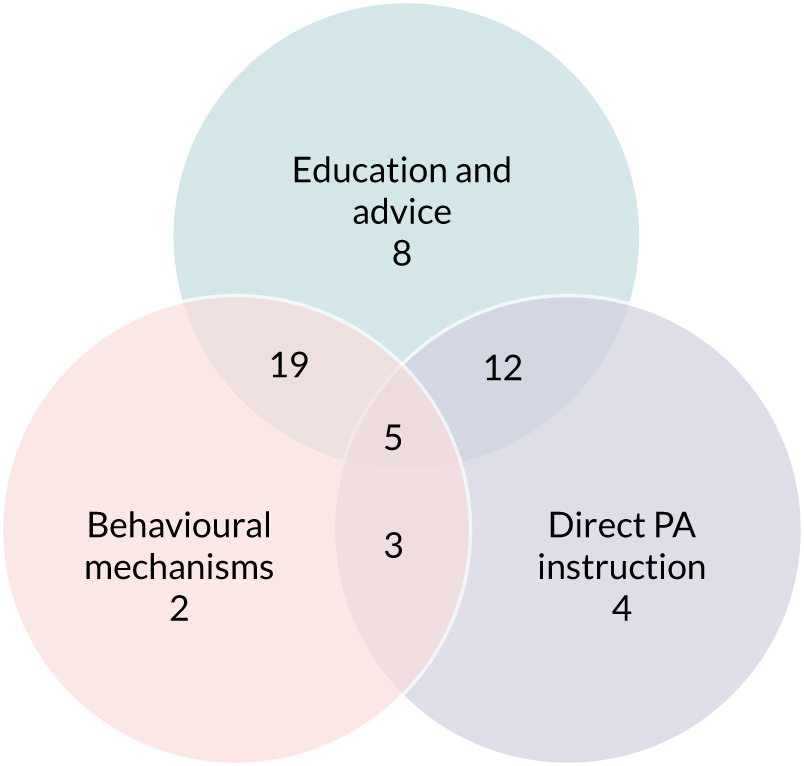
Education and advice
Fifty-five (82.1%) interventions described the encouragement of PA adoption through ‘education sessions’ – the provision of information, advice, recommendations, or a formal exercise prescription51–55,57–62,66,68–70,72,75,76,78,82–85,91–94,97,100–103,107–110 – including Boesch et al. ,56 which involved three interventions in this category, and Creel et al. ,64 Goedendorp et al. ,74 Johansson et al. ,86 Jolly et al. ,87 Kinsey et al. ,88 Kraal et al. ,90 Losina et al. 96 and Smith et al. ,105 which all involved two interventions in this category.
The content of these interactions tended to fall into one or more of the following groups:
-
Exercise recommendations, in which exercise prescriptions, advice for home exercise, recommendations or goals were described,52,54,55,57–59,62,66,68,69,72,75,78,83–85,87,92–94,97,100–102,108–110 including Creel et al. ,64 Goedendorp et al. ,74 Johansson et al. ,86 Kinsey et al. ,88 Kraal et al. ,90 Losina et al. 96 and Smith et al. ,105 which all involved two interventions in this group. These were sometimes provided in addition to, or following, a period of supervised PA. For example, in Artz et al. ,52 following 6 weeks of supervised classes, ‘participants were provided with a list of exercises, including their individual exercises, to continue with at home on a regular basis’. In others, recommendations might have been given at the start of an intervention: ‘the nurse explained how to break the negative spiral of low physical activity . . . consequently, patients were advised to increase their physical activity level stepwise’. 74 Alternatively, they might have been provided alongside sessions that supported the setting of PA goals. 57,62,69,75,96,109 Recommendations were, however, largely self-managed, although some interventions included a degree of practitioner contact, prompting adherence. For example, one intervention84 provided participants with booster sessions every second month, in which a physiotherapist would review the patient’s experience of the home-based exercise programme, provide instruction for new activities and define a new daily steps target; the option of telephone support from a physiotherapist was also on offer. The use of an exercise diary or activity log,54,59,64,92,94,96,108,109 or an activity device (such as a pedometer or wearable heart rate monitor)55,57,59,62,64,68,69,74,75,78,84,90,96 to monitor progress was also described. Barnason et al. 55 used a telehealth device that provided participants with daily strategies around rest, pain management and progressing PA. Recommendations were often personalised either in response to a baseline assessment (usually fitness) or would be reviewed and set again in response to progress over time. Those that were not personalised were sometimes noted as being based on national clinical guidelines.
-
Education or information materials, which comprised 20 interventions,52,59,61,66,68,69,75,76,82,84,91,103,108,109 including both the counselling and pedometer arms of Creel et al. ,64 the nurse and cognitive–behavioural therapy (CBT) arms of Goedendorp et al. ,74 the home-based intervention of Johansson et al. 86 and the home-based intervention of Jolly et al. 87 For these interventions, participants were provided with, for example, ‘a one-page information sheet . . . that also promoted the progressive attainment of 10,000 steps/day’,64 an exercise workbook,69 ‘pictorial and written instructions for [home] exercise’,84 or online role modelling or instruction videos for supporting home exercise adoption. 75,103 We also included here studies in which participants were provided with information about,108 or helped to identify,61 exercise opportunities in the local community.
-
Lifestyle education, which comprised 12 interventions,53,60,66,70,82,91 including all three intervention arms of Boesch et al. ;56 the home-based intervention of Jolly et al. ;87 and the financial incentive (FI) and financial incentive plus telephonic health coaching (FI + THC) intervention arms of Losina et al. ,96 in which the focus of contact with practitioners, or of resources, was placed on broader improvements to health, with PA just one strand of an emphasis on lifestyle factors that might also include diet, alcohol or smoking cessation. One study,53 for example, described a voluntary Motivator’s Club that explored PA, nutrition and psychological issues associated with weight management. Several interventions in cardiac and cancer studies framed sessions around risk factors for disease,70,82,87 whereas others touched on PA (goals, benefits, barriers, activity progression or diary-keeping) among a wider programme of sessions covering topics such as nutrition,53,60,70 use of medication,82,87 ‘pain management strategies’84,97,108 or mindfulness. 94 One study59 reported asking participants in both groups not to make significant changes in their dietary habits.
-
The value of PA, which comprised eight interventions,54,68 including the education arm of Archer et al. ,51 the nurse and CBT intervention arms of Goedendorp et al. ,74 the home-based arm of Johansson et al. ,86 and both the centre-based and home-based intervention arms of Jolly et al. 87 These studies described interventions highlighting the ‘importance’,53 the ‘benefits’68 or the ‘promotion’54 of exercise, or, as in the CBT intervention in Goedendorp et al. ,74 explained to participants the negative spiral of low PA and fatigue. This focus was often part of direct sessions in person, over the telephone, or, in one case, described as part of computer-tailored PA advice that patients could access from home. 75
Behavioural mechanisms
Therapeutic or behavioural approaches to supporting participants to engage in PA was adopted in over half (59.7%) of all interventions. The majority of these were delivered to the individual,54,55,57–59,61,62,68,69,75,76,78,81,83–85,91,92,95,97,108,109,111 including the CBT arm of Archer et al. ,51 the counselling arm of Creel et al. ,64 the CBT arm of Goedendorp et al. ,74 both the walking and cycling arms of Kinsey et al. ,88 the home-based arm of Kraal et al. ,90 and both the THC and the FI + THC arms of Losina et al. 96
A small number of these approaches was incorporated into group sessions of 6–10 people,94 and between 15 and 25 participants, depending on the site of delivery. 82 Baillot et al. 53 and Sellberg et al. 104 did not report group size. For Engblom et al. 70 and Boesch et al. ,56 which included three intervention arms (all residential models), and the centre-based arm of Jolly et al. ,87 it was not clear whether their residential models were delivered as groups or individually.
Fifteen of these approaches59,61,62,68,69,78,83,84,91,108,109 were delivered by telephone, including the CBT arm of Archer et al. ,51 the home-based arm of Kraal et al. ,90 and the THC and FI + THC arms of Losina et al. ;96 22 approaches53,54,57,58,70,81,82,85,92,94,95,97,104,111 (including all three intervention arms of Boesch et al. ,56 the counselling arm of Creel et al. ,64 the CBT arm of Goedendorp et al. ,74 the centre-based arm of Jolly et al. ,87 and both the walking and the cycling intervention arms of Kinsey et al. 88) were delivered face to face in a clinical, community or inpatient setting. Two interventions were delivered via smartphone55 or computer application,75 and another, which included motivational messaging, was delivered by participants’ method of choice (text message, telephone call, e-mail or post). 76 Therapeutic sessions were sometimes noted as being a ‘one-off’, but were usually reported as taking place over the course of several weeks or months.
Although the detail of sessions was generally limited, we identified several recurring topics or approaches in the content described:
-
Most53,54,58,61,62,68–70,75,76,78,81–85,91,92,94,95,97,104,108,109,111 (including the CBT arm of Archer et al. ,51 the counselling arm of Creel et al. ,64 the CBT arm of Goedendorp et al. ,74 the centre-based arm of Jolly et al. ,87 both the walking and the cycling arms of Kinsey et al. ,88 the home-based intervention arm of Kraal et al. ,90 and both the THC and FI + THC arms of Losina et al. 96) talked about behaviour change approaches, counselling or motivational interviewing, describing supporting participants, for example with the ‘restructuring of cognitions and beliefs’ (the CBT arm of Goedendorp et al. 74), or being ‘counselled to establish a home-based exercise programme’ (Lear et al. 92). Some of these interventions emphasised the use of goal-setting,57 or of focusing on participants’ experiences of their exercise programme to consider adaptions and new goals. 84 Details of the THC arm in Losina et al. 96 described coaches using ‘open-ended questions to elicit the participants’ own objectives’ and ‘while expressing empathy . . . helping to resolve any discrepancy between subjects’ PA goals and current behaviour’. Some studies described motivational interviewing techniques, sometimes adopted by PA coaches, and focused on barriers, the discussion of ‘obstacles, fears and solutions’ to PA (the CBT arm of Goendendorp et al. 74) or efforts to ‘allay harmful irrational beliefs and fears regarding activity’. 84
-
Improved self-efficacy and promoting self-management were also common foci in 12 interventions55,57,59,61,69,75,78,97 (including the CBT arm of Archer et al. ,51 the counselling arm of Creel et al. ,64 and both the THC and FI + THC arms of Losina et al. 96). This included self-management approaches ‘to reduce pain and disability, and improve PA’ (the CBT arm of Archer et al. 51) or to self-care and management of early recovery symptoms,55 but, more often, studies described a focus on encouraging participants to self-monitor PA,57,61,78 or on participants’ self-efficacy for exercise,59,69,75,108 which included both the THC and FI + THC arms of Losina et al. 96 Other studies used mechanisms such as exploring ‘self-rewards’. 62
-
Reference to the following was also common – lifestyle and health coaching68,81,92,94,104 (including the CBT intervention arm of Archer et al. ,51 all three intervention arms of Boesch et al. ,56 the CBT arm of Goedendorp et al. ,74 and both the walking and the cycling intervention arms of Kinsey et al. 88); active lifestyles, such as work-related, leisure-related and daily life activities;81 daily walking;56 or broader counselling approaches, which might include working on addressing fatigue, sleep or ‘risk factor counselling’. 92
Direct physical activity instruction
Thirty interventions (44.8%) provided face-to-face PA instruction,52–54,60,70,72,77,79,82,84,85,92,95,106,107,109,111 including all three intervention arms of Boesch et al. ,56 the aerobic and the resistance arms of Courneya et al. ,63 the centre-based arm of Jolly et al. ,87 the clinic-based arm of Johannson et al. ,86 both intervention arms of Kraal et al. ,90 both the clinic and the community arms of Piva et al. ,102 and the hospital and the home training arms of Smith et al. 105
These were more often one component of a broader approach52–54,60,70,72,82,84,85,92,95,107,109,111 (including all three intervention arms of Boesch et al. ,56 the clinic-based arm of Johansson et al. ,86 the centre-based arm of Jolly et al. ,87 both intervention arms of Kraal et al. ,90 the clinic-based arm of Piva et al. 102 and both arms of Smith et al. 105) that might also include education and advice52–54,60,70,72,82,84,85,92,107,109 (including all three intervention arms of Boesch et al. ,56 the clinic-based arm of Johansson et al. ,86 the centre-based arm of Jolly et al. ,87 both arms of Kraal et al. ,90 the clinic-based arm of Piva et al. 102 and both arms of Smith et al. 105) or therapeutic approaches53,54,70,82,84,85,92,95,109,111 (including all three intervention arms of Boesch et al. ,56 the centre-based arm of Jolly et al. 87 and the home-based arm of Kraal et al. 90). Several studies,52,53,60,77,79,106 (including the centre-based intervention arm of Kraal et al. ,90 the community arm of Piva et al. 102 and the hospital-based arm of Smith et al. 105) indicated that activity sessions took place in groups, although group size was infrequently described. When it was, group size ranged from two people79 to 25 people. 82
The majority of PA sessions52–54,60,72,77,79,82,85,95,106,107,111 (including all three intervention arms of Boesch et al. ,56 both arms of Courneya et al. ,63 the clinic-based arm of Johansson et al. ,86 the centre-based arm of Jolly et al. ,87 both intervention arms of Kraal et al. ,90 the community-based arm of Piva et al. 102 and the hospital training arm of Smith et al. 105) were provided at least weekly, often two or three times per week; all intervention arms of the Boesch et al. 56 study provided five sessions of cycling each week. Participants in this study56 also participated in walking twice daily; it was not clear if these sessions were supervised. In some interventions, the regularity of PA instruction varied over time, for example increasing progressively over 6 months;85 reducing in frequency, as in the clinic-based arm of Piva et al. ;102 or varying as in Baillot et al. ,53 in which participants received instruction (three sessions per week for almost 6 months) in the pre-surgery phase of the intervention only, with post-surgery intervention components focused on counselling only. In Lear et al. ,92 participants received up to eight cardiac rehabilitation exercise sessions over 12 months, and in the home training arm of Smith et al. ,105 participants were offered 2-hour exercise consultations with an exercise specialist at the beginning and after 3 months of home exercise.
Physical activity instruction usually took place in a clinical setting (including a hospital or physiotherapy clinic or gym)52–54,60,72,77,79,82,84,92,95,111 (including the clinic-based intervention arm of Johansson et al. ,86 the centre-based arm of Jolly et al. ,87 both the centre-based and the home-based arms of Kraal et al. ,90 the clinic-based arm of Piva et al. ,102 and both the hospital-based and the home-based arms of Smith et al. 105) but also in community settings (including a leisure centre, community centre or public gym)82,85,106 (including both the aerobic and resistance arms of Courneya et al. 63 and the community arm of Piva et al. 102), within a residential inpatient facility or during a hospital stay70,72 (including all three intervention arms of Boesch et al. 56) or as part of a home visit. 107,109
Six interventions77,79,106 (including both the aerobic and resistance arms of Courneya et al. 63 and the community-based arm of Piva et al. 102) described PA instruction only. In these studies, supervised training or classes took place two or three times per week, in a community or clinical setting, usually for ≥ 3 months.
Other components
Several studies noted additional features of intervention design. Artz et al. ,52 for example, reimbursed participants’ travel and parking costs, or supported access to transport. Losina et al. ,96 in two of their three intervention arms, used FIs, both in relation to the completion of PA logs and again if participants managed to increase their daily step count or minutes of moderate-to-vigorous physical activity (MVPA). Losina et al. ,96 for their THC arm, also reported providing coaching, always at a time most convenient to participants, and coaches were prepared to make several attempts at telephone contact; and Turunen et al. 109 described the use of volunteer students who would support frail participants (who could not manage this alone) to take walks outdoors to meet their activity goals. Additional design features in these studies could be seen as mechanisms to support engagement.
Nutritional counselling54 and a plant-based diet or lycopene supplements76 were noted in some studies; Boesch et al. 56 highlighted the provision of low-fat meals and the rural, high-altitude location of the inpatient facility in which participants spent 4 weeks, shortly after surgery.
Digital technologies
Digital and other technologies were integral components of protocols in just under one-third of interventions (29.9%). Most commonly this was through the use of a pedometer or other step activity monitor,57,62,68,69,75,78,84,90,100,110 including in Brandes et al. ,58 in which participants were blinded to the device, but the data were used by counsellors to encourage increases; in both the counselling and the pedometer arms of Creel et al. ;64 and in both the FI and THC arms of Losina et al. 96 However, also included were the use of heart rate monitors;59,60,90,110 an interactive online application for computer or smartphone (producing tailored advice);75 audio tapes (providing an abridged version of the intervention ‘Heart manual’, spoken in Punjabi for participants with a limited command of English) or online exercise videos, as featured in Santa Mina et al. 103 and the home-based arm of Jolly et al. ;87 and a telehealth device (an interactive device, requiring a telephone line but no internet access, providing individualised intervention sessions). 55
Pedometers in particular, and heart rate monitors, were often referred to as tools to support participants to engage in, or monitor, their activity. Although 22 studies51,53,55,60–62,64,74,75,81,82,85,90,93,96,97,102,104,106,107,109,111 also used movement accelerometers such as ActiGraph GT3X (ActiGraph, LLC, Pensacola, FL, USA) to measure study outcomes (e.g. bouts of MVPA or daily steps), these were generally not available for patients to use during the intervention period; instead, they were provided at specific time points, to be returned either side of measurement periods (usually 1 week). With the exception of five studies55,64,93,96,109 involving eight interventions, two64 of which had the screens on their devices obscured, all such measurement periods fell outside the intervention delivery period (usually prior to intervention commencement, at the immediate end of the intervention or at some point post intervention).
Other resources/equipment
In addition to digital technologies, such as pedometers, used as part of the intervention provision, 32 interventions provided other specific resources or equipment. This included printed materials, such as programme information, educational booklets, exercise workbooks (some of which were personalised) and motivational postcards, in 17 of the studies. 51,52,59,61,64,66,69,74,76,78,82,84,87,108,109
In 13 studies, interventions included the provision of, or the encouragement for participants to keep, exercise diaries, logs or similar; the purpose of these diaries/logs was sometimes described as a method to help participants keep track of progress and to provide additional motivation. 54,57,59–61,64,66,84,92,94,101,105
Participants in both the intervention and control groups in Mundle et al. 100 were asked to self-report daily estimates of their PA via a questionnaire.
In two studies, interventions included open access to fitness centres throughout the intervention period106 or, in both study arms of Courneya et al. ,63 for up to 1 month post intervention. Fitness equipment was provided to participants in five studies: resistance bands,83,84,108,109 and a stability ball and yoga mat, which participants in the study by Santa Mina et al. 103 were able to keep post intervention.
Figure 5 shows the number of interventions per resource used.
FIGURE 5.
Resources to support interventions.

Physical activity
Type of exercise/physical activity encouraged
Studies were frequently vague on the sorts of physical activities or exercises adopted or recommended. However, walking was the most frequently reported activity54,57,58,60–62,68,69,72,76,78,81,83,84,107,109–111 (including in the CBT intervention arm of Archer et al. ,51 all three arms of Boesch et al. ,56 the aerobic arm of Courneya et al. ,63 the counselling and the pedometer arms of Creel et al. ,64 both the nurse and the CBT arms of Goedendorp et al. ,74 the clinic-based and the home-based arms of Johansson et al. ,86 the centre-based and the home-based arms of Jolly et al. ,87 the walking and the cycling arms of Kinsey et al. ,88 the FI and the FI + THC arms of Losina et al. ,96 the clinic-based arm of Piva et al. ,102 and both the hospital-based and the home-based arms of Smith et al. 105), including outdoor walking or trekking; aerobic, brisk walking; running (one study81 only); short or functional walks; or the achievement of daily steps. Cycling, usually on a stationary bike, although also encouraged as a home-outdoor activity, was also described, and sometimes suggested as an alternative to walking54,60,70,81 (including in all three intervention arms of Boesch et al. ,56 the aerobic arm of Courneya et al. ,63 the nurse and the CBT arms of Goedendorp et al. ,74 the centre-based arm of Jolly et al. ,87 the cycling arm of Kinsey et al. 88 and the clinic-based arm of Piva et al. 102). For other interventions52–54,59,69,77,83,85,92,103 (including in the clinic-based arm of Piva et al. ,102 and both the hospital-based and the home-based arms of Smith et al. 105), authors simply reported aerobic training (including endurance activities, moderate-to-vigorous and high-intensity activities, and activities ‘designed to increase general fitness’52). Several studies described other activities such as swimming or pool activities,70,85,111 gymnastics,70 dance,85,111 rowing (in the centre-based intervention)87 and ‘traditional Spanish games’. 85
Resistance or strength training was often included52,61,69,77,83–85,103,106–109,111 (including in the resistance intervention arm of Courneya et al. ,63 the clinic-based and the home-based arms of Johansson et al. ,86 and both the clinic-based and the community-based arms of Piva et al. 102), which, with the exception of the resistance training arm of Courneya et al. ,63 was delivered or encouraged alongside one or more other activities. Ten interventions61,77,92,108,111 (including in both the clinic-based and home-based intervention arms of Johansson et al. ,86 the community-based arm of Piva et al. ,102 and both the hospital-based and the home-based arms of Smith et al. 105) were described as including stretch, flexibility and balance exercises.
Those studies that were less specific55,66,68,72,75,79,82,91,93–95,97,100,101,104,106 (including in both the CBT and the education intervention arms of Archer et al. ,51 the counselling arm of Creel et al. ,64 the home-based arm of Jolly et al. ,87 both the centre-based and the home-based arms of Kraal et al. ,90 and the THC arm of Losina et al. 96) tended to include reference to interventions supporting ‘activities of choice’, as in the home-based intervention for Jolly et al. ;87 ‘progressing physical activity’;55 participants being encouraged to engage in, for example, 30 minutes of daily exercise;94 or an exercise prescription (these, as in Painter et al. ,101 might include goal intensity markers, but there were no details on the activity or activities prescribed).
How much physical activity and at what sort of intensity?
Studies were also not always clear on the frequency or intensity of PA that was being encouraged or delivered. Sometimes this was explicitly undefined: when participants were encouraged to focus on simply being more active95 or on the ‘quality of movement rather than quantity’. 52 Other studies focused on incremental progression, such as a 5% increase in daily steps58 or increasing ‘speed and duration . . . as tolerated up to a maximum 30 minutes’. 72 Some studies focused on perceived exertion, describing exercise classes ‘designed to be challenging for active older adults and safe for more frail individuals’, as in the community-based intervention for Piva et al. ;102 or the use of the Borg Scale to estimate participants’ training intensity, gradually increasing, for example from a score of 15 in the initial phase of the intervention to a Borg Scale score of 17. 106 Boesch et al. ,56 for both the self-regulation and the objective/subjective intervention arms, focused on a lower score of 12–14. A number of studies described activities of moderate to vigorous intensity, often also inclusive of bout-related goals, such as walking a ‘brisk pace for 30 minutes on at least five days a week’,76 or a goal of meeting a PA guideline of at least 150 minutes of MVPA each week, as in all three intervention arms in Losina et al. 96
A number of interventions described being guided by exercise testing, such as working (cycle ergometer, treadmill or elliptical trainer) at 60% of VO2 peak for 15 minutes and progressing to 80% of VO2 peak for 45 minutes, as found in the aerobic training arm of Courneya et al. 63 Similarly, the hospital training intervention arm in Smith et al. 105 used an exercise prescription based on a target intensity of 60% of VO2 peak for 3 months, revised, when appropriate, to 70% of VO2 peak for a further 3 months. Participants were advised to follow this regime using a cycle ergometer, arm cycle ergometer, treadmill and track-walking for 40 minutes, 5 days per week. Sessions would also include additional time doing warm-up and stretching exercises. Kinsey et al. ,88 in their walking programme, used metabolic equivalent of task (MET), encouraging short walks at a level of 3.3 METs three to five times weekly or, in their cycling programme, progressive cycling goals (also three to five times weekly) from 1.5 METs at the start of the intervention to 5.3 METs over 12 weeks. Yates et al. 111 encouraged a PA minimum goal of 150 minutes per week (but preferably activity all days of the week) at 3 METs, whereas Painter et al. 101 used an exercise prescription based on an intensity goal of 60–65% of maximal heart rate, increasing gradually to 75–80% of maximal heart rate, four times per week, over a 10-month period.
The total amount of PA either delivered or encouraged was sometimes hard to gauge from the limited details provided. However, we considered these as falling into one of three groups:
-
low – those encouraging PA once or twice per week (17.9%)52,61,62,79,82,106,107 (including in the centre-based intervention arm of Jolly et al. ,87 both the centre-based and the home-based arms of Kraal et al. ,90 and the clinic-based and the community-based arms of Piva et al. 102)
-
regular – those largely in line with typical PA recommendations of three (or four, as with Santa Mina et al. 103) times per week (11.9%)53,77,84,85,103,108 (including in the aerobic and the resistance intervention arms of Courneya et al. 63)
-
frequent – those encouraging PA on ≥ 5 days per week (46.3%)54,55,57,59,60,66,70,72,76,78,83,93,94,109–111 (including in all three intervention arms of Boesch et al. ,56 the pedometer arm of Creel et al. ,64 the CBT arm of Goedendorp et al. ,74 the clinic-based and the home-based arms of Johansson et al. ,86 the home-based arm of Jolly et al. ,87 both the walking and the cycling arms of Kinsey et al. ,88 all three intervention arms of Losina et al. ,96 and both the hospital-based and the home-based arms of Smith et al. 105).
Just under one-quarter of interventions (23.9%) did not provide this information58,68,69,75,81,91,92,95,97,100,101,104 (including in both the CBT and the education arms of Archer et al. ,51 the counselling arm of Creel et al. 64 and the nurse intervention arm of Goedendorp et al. 74).
Tailoring activity
Forty-one (61.2%) interventions took a personalised or tailored approach to PA. This was usually described in respect to participants’ capacities or fitness (such as tailored goals or activity intensity)54,57,58,60–62,66,68,69,72,75,77,79,81,82,84,92,95,101,103,107–109,111 (including in the CBT intervention arm of Archer et al. ,51 all three intervention arms of Boesch et al. ,56 the counselling arm of Creel et al. ,64 the CBT arm of Goedendorp et al. ,74 both the walking and the cycling arms of Kinsey et al. ,88 the centre-based and the home-based arms of Kraal et al. ,90 the clinic-based arm of Piva et al. ,102 and the hospital-based and the home-based arms of Smith et al. 105) and/or participant preferences (such as activity of choice)52,59,61,66,69,75,79,81,82,84,92,95,101,109,111 (including in the CBT intervention arm of Archer et al. ,51 the counselling arm of Creel et al. ,64 both the walking and the cycling arms of Kinsey et al. ,88 the centre-based and home-based arms of Kraal et al. ,90 all three intervention arms of Losina et al. ,96 the clinic-based arm of Piva et al. 102 and the home-based arm of Smith et al. 105). This included, for example, participants being asked to identify activities that they would like to return to following their surgery;52 activities that they wished to become better at;79 or PA plans developed around individuals’ interests, found in both intervention arms of Kinsey et al. 88 Other studies described interventions tailoring the number of sessions, intensity and progress of training (Barberan Garcia et al. 54); ‘the frequency and duration’ of rehabilitation components being ‘individualised to patient characteristics’;58 and ‘exercise parameters’ and ‘rate of progression’ that accounted for patients functional capacity, and treatment side effects (as well as their exercise preferences or previous exercise history). 69
Delivery
Home
Thirty-nine interventions took place in participants’ homes (or neighbourhood environment): 21 exclusively55,59,66,69,75,76,78,83,93,101,103,107–110 (including in both the CBT and the education arms of Archer et al. ,51 the home-based arm of Jolly et al. 87 and all three intervention arms of Losina et al. 96), whereas 19 included home-based components57,60–62,68,72,81,84,85,91,92,111 (including in both the clinic-based and the home-based intervention arms of Johansson et al. ,86 the walking and the cycling arms of Kinsey et al. ,88 the home-based arm of Kraal et al. ,90 the clinic-based arm of Piva et al. 102 and the home-based arm of Smith et al. 105). Of those taking place exclusively at home, the majority included some contact (usually weekly, but it was variable) with their clinical or intervention team via telephone, post or the participant’s method of choice, be that text message, telephone, post or e-mail, as in Hackshaw-McGeagh et al. 76 A small number had no ‘human’ contact, relying instead on support/guidance delivered through the use of a telehealth device,55 computer-generated messaging75 or a smartphone application. 110
Three of the exclusively home-based interventions included, in addition to telephone support, home visits from physiotherapists (delivering motivational interviewing with musculoskeletal patients108,109) or a nurse (supporting exercise goals among cardiac patients87). The addition of volunteer health sciences students to support frail participants, who were unable to go outdoors alone, to take outdoor walks was also offered to participants in one of these studies. 109
Clinical settings
The use of clinical settings, including hospitals and hospital gyms, outpatient and physiotherapy clinics, and health centres, was also common (49.3%), but was more usually accompanied by expectations for home exercise or further telephone support provided to participants at home52–54,57,60,62,68,72,77,79,81,82,84,91,92,95,97,104,111 (including in both the counselling and the pedometer arms of Creel et al. ,64 the nurse and the CBT arms of Goedendorp et al. ,74 the clinic-based and the home-based arms of Johansson et al. ,86 the centre-based arm of Jolly et al. ,87 the walking and the cycling arms of Kinsey et al. ,88 the centre-based and the home-based arms of Kraal et al. ,90 the clinic-based arm of Piva et al. ,102 and both the hospital-based and the home-based arms of Smith et al. 105).
Community
Community spaces (public or private leisure centres or gyms; local activity groups, such as walking groups; or community centres) were described less frequently (10.4%)61,82,85,106 (including in both the aerobic and the resistance intervention arms of Courneya et al. 63 and the community-based arm of Piva et al. 102). Some of those delivered in community settings were delivered as group activities, whereas some also provided access to leisure facilities outside (and, for a short period, beyond) the intervention delivery. One intervention61 that did not directly deliver PA described taking a therapeutic and goal-setting approach to behaviour change, and supported participants to identify walking opportunities within their local community.
Inpatient care
Six (9.0%) interventions had components set in inpatient care: one72 in a hospital setting and five in a residential rehabilitation centre,58,70 including in all three intervention arms of Boesch et al. 56 With the exception of Brandes et al. 58 (hip or knee replacement patients), these were all interventions aimed at cardiac patients. Stays lasted 2 weeks,72 3 weeks58 and 1 month,56 with one intervention involving three phases of admission that included a 2-day stay pre surgery, a 3-week stay 6–8 weeks post surgery and a further 2-day stay at 8 months post surgery. 70 The intervention in Foster et al. ,72 the only study to describe an intervention as part of their hospital stay, began within 48 hours of surgery, but continued in an outpatient clinic and as home exercise on discharge, after approximately 5 days.
Two further studies94,100 included no details on intervention setting. Figure 6 shows the number of interventions per setting.
FIGURE 6.
Settings used to deliver interventions. Two interventions are not included in this figure because the delivery setting is not described in the study reports. a, Participants also used other settings to complete PA.
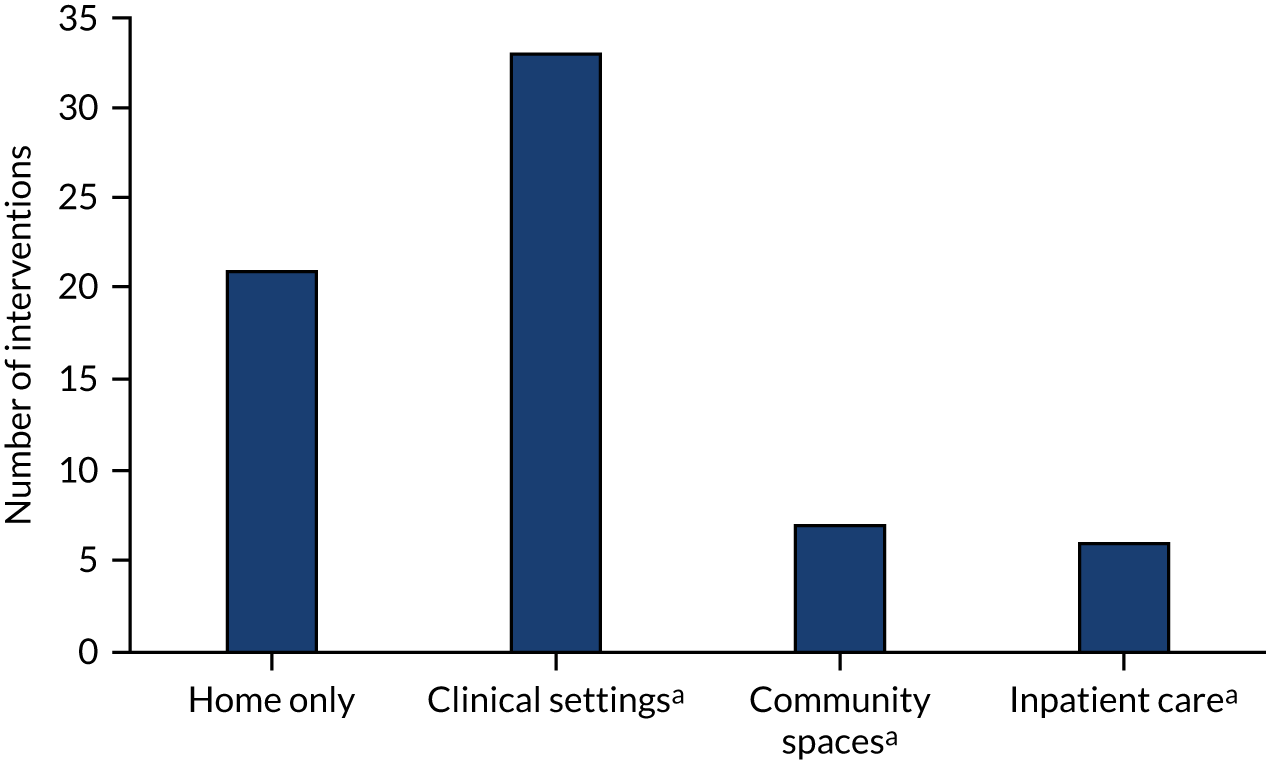
Remote delivery
Twenty-six interventions55,59,61,62,66,68,69,75,76,78,83,84,91,93,101,103,108–110 (including in the CBT and the education arms of Archer et al. ,51 the home-based arm of Jolly et al. ,87 the home-based arm of Kraal et al. 90 and all three intervention arms of Losina et al. 96) included some form of remote delivery. In 16 interventions55,59,66,69,75,76,83,93,101,103,110 (including in the CBT and education arms of Archer et al. 51 and all three intervention arms of Losina et al. 96) it was exclusively remote, reliant on contact with practitioners or study staff via telephone or post and/or supported by technology. Several studies,61,62,68,84,91 including in the home-based intervention arm of Kraal et al. ,90 took a blended approach, combining in-person/clinic-based support with remote (usually telephone) contact; three of these108,109 (including in the home-based arm of Jolly et al. 87) also included home visits in addition to telephone support.
Of those taking place exclusively at home, three interventions55,75,110 relied on no practitioner (or study) contact throughout the intervention period at all. Barnason et al. 55 was, for example, designed around a telehealth system that provided participants with daily strategies around rest, pain management and incremental progression of PA over the course of 6 weeks, but no direct contact with practitioners or the study team throughout that period. Similarly, Van der Walt et al. ,110 also a 6-week intervention, relied on predefined daily steps goals supported by the use of a Garmin Vívofit® (Olathe, KS, USA) activity tracker, but again included no interaction with practitioners throughout. Golsteijn et al. 75 was an application-driven intervention (although apparently a more enhanced package) providing computer-generated PA advice to participants at three time points (monthly intervals for 3 months) across the intervention period. The advice was generated automatically using a message library, questionnaire data and computer-based data-driven decision rules. The advice was also made available on paper and mailed to participants. This advice was provided alongside interactive content on the intervention website including role-modelling videos, home exercise instruction videos and a module for goal-setting using a pedometer. There was, however, an option to consult a physical therapist for additional information if needed (no details were provided as to whether or not participants selected this option).
Twenty-one59,61,62,68,69,78,83,84,91,93,101,103,108,109 (including in the CBT and the education arms of Archer et al. ,51 the home-based arm of Jolly et al. ,87 the home-based arm of Kraal et al. 90 and all three intervention arms of Losina et al. 96) of these remote delivery models predominantly relied on frequent (e.g. weekly) or irregular or less frequent (e.g. monthly, or reducing or variable over time) telephone support or coaching. Others were reliant on a smartphone, device or computer application guiding the intervention55,75,110 (including the home-based training intervention arm of Kraal et al. 90), or the exchange of workbooks and tailored newsletters via post (containing activity/lifestyle goals);66 just workbooks via post was a further mechanism for engaging with participants. 101 In Hackshaw-McGeagh et al. ,76 participants could choose the method of contact (text message, telephone, post or e-mail) through which they received motivational messages to encourage continuation of the intervention.
When remote and face to face was blended, some interventions appeared to begin with face-to-face contact and then move to a less frequent telephone model. For example, Christiansen et al. 62 provided weekly face-to-face therapeutic support (as an add-on to usual care rehabilitation) for the first 6–8 weeks, after which participants received monthly telephone contact with a physical therapist. In Ilves et al. ,84 participants received clinic booster sessions every second month, but otherwise exercised independently and, only if they needed it, had contact via telephone with a physiotherapist. However, Kraal et al. 90 was based on short but regular telephone-delivered motivational interviewing (of 10–20 minutes per session) each week in addition to in-person supervised PA training.
Practitioners and those involved in delivery
Interventions tended to be delivered by a single practitioner (62.7%): 12 by a physiotherapist52,54,60,69,79,84,95,106–108 (including in both the clinic-based and the home-based intervention arms of Johansson et al. 86), nine by study staff83,101,103,104,110 (including in both the aerobic and the resistance intervention arms of Courneya et al. ,63 the pedometer arm of Creel et al. 64 and the centre-based arm of Jolly et al. 87), nine by a physical therapist61,62,77,97 (including in the CBT and the education arms of Archer et al. ,51 the centre-based and the home-based arms of Kraal et al. ,90 and the clinic-based arm of Piva et al. 102), four by a health coach78 (including in all three intervention arms of Losina et al. 96), three by a nurse72,76 (including the nurse intervention arm of Goedendorp et al. 74), two by a PA specialist85 (including in the community-based arm of Piva et al. 102), one by a psychologist,74 one by a psychiatrist57 and one by a student. 58 Others (23.9%)53,70,81,82,91,92,94,109,111 (including in all three intervention arms of Boesch et al. ,56 the counselling arm of Creel et al. ,64 the home-based arm of Jolly et al. ,87 and both the hospital-based and the home-based arms of Smith et al. 105) were delivered by a MDT or provided no details55,59,66,68,75,93,100 (13.4%) (including in both the walking and the cycling intervention arms of Kinsey et al. 88) (Figure 7).
FIGURE 7.
Practitioners involved in the delivery of interventions.
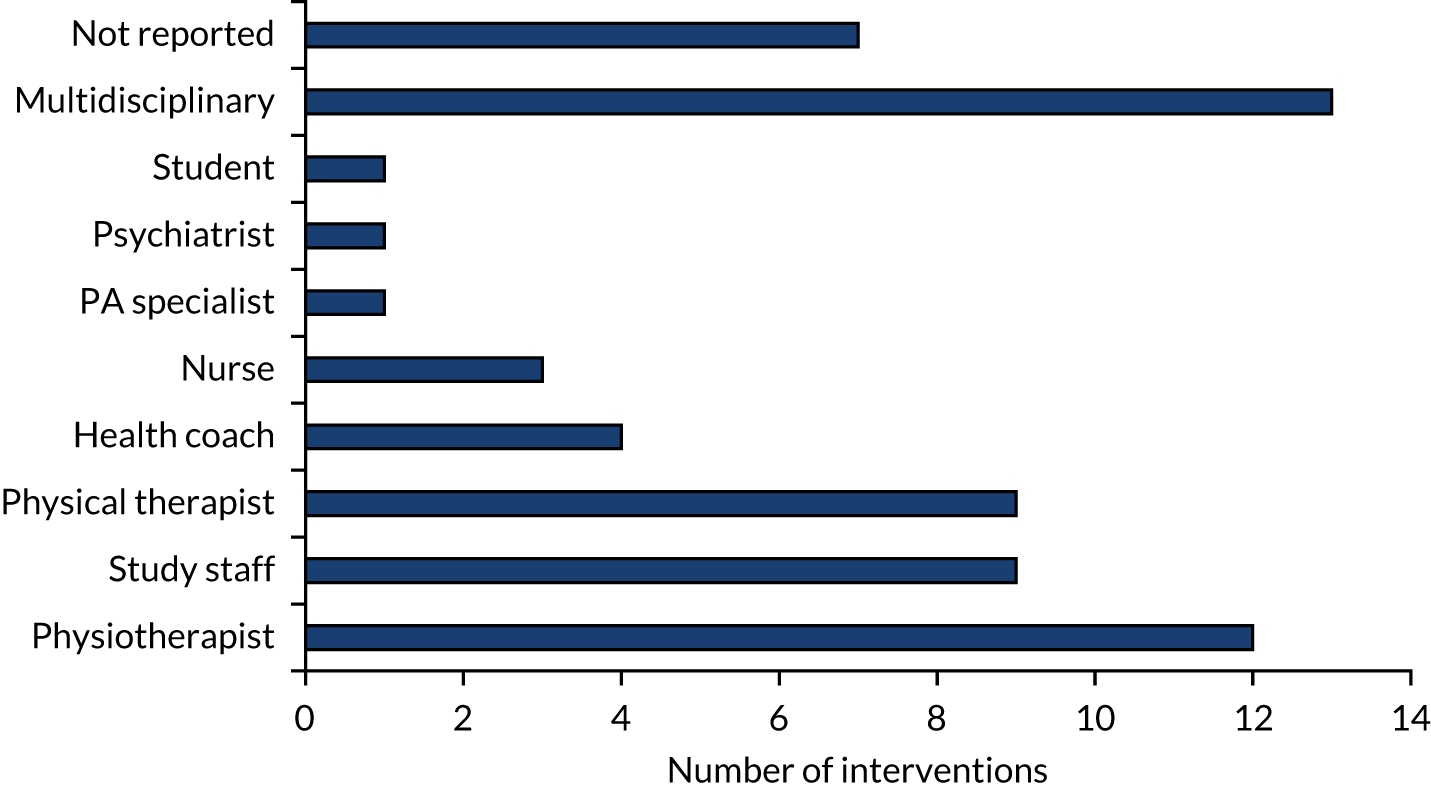
Multidisciplinary teams included staff from between two and six different specialties. Physiotherapists were involved with nine interventions81,82,94,109 (including in all three intervention arms of Boesch et al. ,56 and the home-based and the hospital-based arms of Smith et al. 105), PA specialists were involved with seven53,81,92,111 (including in the counselling arm of Creel et al. ,64 and both the home-based and the hospital-based arms of Smith et al. 105), physicians were involved with five70,111 (including all three intervention arms of Boesch et al. 56) and dietitians with four. 53,70,92,111 The following were also featured in interventions as part of these multidisciplinary approaches: psychologists in two interventions,70,94 physiotherapist assistants in one,82 students in one,109 a pharmacist in one111 and an occupational therapist in one. 81 The involvement of family or another key support person whom participants would invite to engage in activities111 or education sessions91 was mentioned in two studies.
Studies sometimes made reference to specific training or qualifications held by practitioners, including accredited qualifications, such as physical therapists with graduate diplomas in CBT,97 or exercise and sports science professionals with post-graduate qualifications in PA and health. 85 One study noted peer training, whereby physiotherapists and nurses had received training relating to cancer and exercise from a cancer exercise specialist,82 and several studies mentioned that practitioners had received some form of training from the study team69,78,85,87 or were following an accredited (intervention) course. 81 One study74 described regular supervision and therapists experienced in treating chronically fatigued cancer survivors, and two studies97,107 referenced staff with substantial clinical experience.
Interventions varied considerably in terms of the amount of practitioner contact or support they offered. This sometimes varied over time, or might be quite intense over a short period of time, for some interventions, or much more infrequent but over a much longer period of time in others. Therefore, when data were available, we considered the interventions as falling into one of three groups: 24 low contact (one to nine total contacts)52,54,57,58,66,68,76,81,83,84,91,93,94,97,103,104 (including in both the CBT and the education intervention arms of Archer et al. ,51 the counselling and the pedometer arms of Creel et al. ,64 the nurse arm of Goedendorp et al. ,74 both the home-based and the clinic-based arms of Johansson et al. ,86 and the home-based arm of Jolly et al. 87), 19 intermediate contact (10–20 total contacts)61,62,69,78,79,82,92,95,107–109 (including in the CBT arm of Goedendorp et al. ,74 the centre-based arm of Jolly et al. ,87 the home-based arm of Kraal et al. ,90 all three intervention arms of Losina et al. ,96 the clinic-based arm of Piva et al. 102 and the home-based arm of Smith et al. 105), and 17 high contact (≥ 21 total contacts)53,59,60,70,72,77,101,106,111 (including in all three intervention arms of Boesch et al. ,56 both the aerobic and the resistance arms of Courneya et al. ,63 the centre-based arm of Kraal et al. ,90 the community-based arm of Piva et al. 102 and the hospital-based arm of Smith et al. 105). We also looked at average weekly contacts. This ranged from 0.03 contacts (one contact across a 7-month pedometer-use intervention in the pedometer arm of Creel et al. 64) to 19 contacts (in which participants were residents at a residential facility providing several daily sessions, for 1 month, as in all arms of Boesch et al. 56). The mean number of contacts was 1.89 (median 0.97, mode 1.0). Excluding the 1-month residential intervention, which was an outlier, average weekly contacts ranged from 0.03 to 3.35 (mean 1.02, median 0.75).
Four studies were not included in the previous calculations; participants in Barnason et al. 55 received daily self-management messaging via the Health Buddy Telehealth device, but it was not clear whether this was entirely automated or whether physicians inputted messaging; Golsteijn et al. 75 provided automated computer-tailored advice at monthly intervals (3 months) across the intervention period (participants had the option to consult a physical therapist if they wished, although it was not clear whether or not participants took up this offer); and Van der Walt et al. ,110 an intervention based on daily steps goals, involved use of an activity tracker, but received no practitioner support. Mundle et al. ,100 an add-on to usual care cardiac rehabilitation, provided participants with accelerometers, but provided no details of the rehabilitation programme or any practitioner contact.
Duration of intervention
Interventions varied in duration from a mean of 19.4 (SD 1.4) days58 to 26 months. 94 Thirty-six interventions52,54,55,57,58,61,75,77,79,82,95,97,100,103,104,107,110,111 (including in both the CBT and the education intervention arms of Archer et al. ,51 all three arms of Boesch et al. ,56 the aerobic and the resistance arms of Courneya et al. ,63 the nurse arm of Goedendorp et al. ,74 the clinic-based and the home-based arms of Johansson et al. ,86 the centre-based and the home-based arms of Jolly et al. ,87 the walking and the cycling arms of Kinsey et al. ,88 the centre-based and the home-based arms of Kraal et al. ,90 and the clinic-based and the community-based arms of Piva et al. 102) lasted < 6 months, whereas 15 interventions72,76,78,85,93,106,109 (including in the CBT arm of Goedendorp et al. ,74 all three intervention arms of Losina et al. ,96 and the hospital-based and the home-based arms of Smith et al. 105) lasted 6 months. Seven studies108 took place over the course of 1 year or up to 2 years. One intervention83 was delivered throughout adjuvant therapy, and so varied in relation to patients’ treatment (Figure 8).
FIGURE 8.
Duration of delivery of interventions.
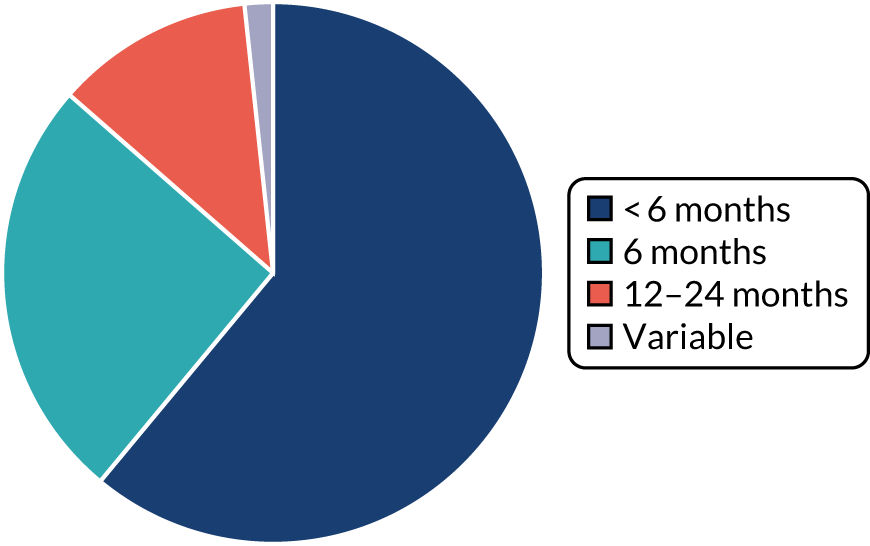
Usual care
Of the 45 studies involving usual care treatment as the study control, 21 studies54,58,62,68,70,77,79,81,85,91,95–97,100,102,104,107–111 provided details of what that provision involved. Although minimally described, this could be roughly grouped into the following categories:
-
Comprehensive. In two studies58,110 usual care could be described as comprehensive. One58 involved a period of inpatient rehabilitation including, for example, mobility and strength training, hydrotherapy and theoretical (usually group) classes. . In the other,110 following discharge from acute care (usually 5 days, including twice-daily physiotherapy sessions), the majority of patients received 7–10 days of inpatient rehabilitation, followed by 6 weeks of twice-weekly outpatient sessions.
-
Support. In 11 studies54,62,77,79,81,91,95,96,100,109,111 care was less intensive than ‘comprehensive’, but nevertheless included ongoing support, including PA recommendations, nutritional counselling and other lifestyle advice;54 outpatient physiotherapy79 or rehabilitation (e.g. Lindbäck et al. 95 and Mundle et al. 100, including a 6-week home exercise programme in Lindbäck et al. );95 or group education classes in nutrition, exercise, lifestyle modification, and disease and risk factors. 111
-
Advice. Three studies68,85,108 gave information about strategies such as ‘safe physical activity’68 or exercise recommendations focused on trying to maintain an active lifestyle (although without patient-specific information or exercise prescriptions). 85
-
Follow-up. Three studies70,97,104 provided follow-up such as ‘a single session with a physical therapist’;97 ‘consultancy with a dietician, nurse, or surgeon usually about medical complications, weight loss, and proper post-surgery diet’ at four points across a 2-year period;104 or clinic visits at 2, 6 and 12 months postoperatively, at which coronary heart disease risk factors, diet and medication were considered. 70
-
In addition, Piva et al. 102 indicated that their usual care (waiting list) control group received no rehabilitation support during the study period, explaining that, as participants were at least 2 months post TKR, there was an expectation that all usual care treatment had come to an end. However, any patients receiving ‘usual care’ were excluded from the study. Furthermore, Taraldsen et al. 107 reported that usual care varied between patients, but that no control group participants received extensive rehabilitation.
Description of population characteristics, for non-randomised studies
Surgical/non-surgical participants
The nine NRSs65,67,71,73,80,89,98,99,112 reported no non-surgical participants, or we judged them as being likely to include no, or an insignificant number of, non-surgical participants.
Age
The nine NRSs65,67,71,73,80,89,98,99,112 included only adult participants. However, Macchi et al. 98 included only adults aged ≥ 65 years.
Gender
Two studies71,112 included only male participants. The remaining NRSs included a combination of male and female participants.
Type of condition
Six studies67,73,80,89,99,112 recruited participants undergoing treatment for types of cancer: colorectal cancer,80,99 oesophageal cancer,67,89 prostate cancer112 and abdominopelvic cancer. 73
Two studies65,71 recruited participants undergoing coronary artery bypass surgery. One study98 did not specify the surgery the participants underwent, describing it only as cardiac surgery.
Country
Fifty-six per cent of studies were conducted in Europe: two in Germany,80,112 two in the UK67,99 and one in Italy. 98
Eleven per cent of studies were conducted in North America: the one71 in the USA.
Eleven per cent of studies were conducted in Australasia (i.e. the one73 in Australia), 11% were conducted in Asia (i.e. the one89 in Japan) and 11% were conducted in South America (i.e. the one65 in Brazil).
Race/ethnicity
These studies65,67,71,73,80,89,98,99,112 did not report baseline characteristics data on race or ethnicity.
Body mass index
Four studies73,98,99,112 reported a mean baseline BMI of between 25 kg/m2 and 29.9 kg/m2.
One study89 reported a mean baseline BMI of between 20 kg/m2 and 24.9 kg/m2.
The remaining four studies65,67,71,80 did not report baseline BMI data for their participants.
Education status
Three studies65,73,98 reported baseline characteristics data for education status. Because these studies were from a number of different countries, each having a different accreditation system, and they recorded these data in disparate ways, it was not viable to collate these data. However, when reported, we have included these data separately for each study in the population domain of the characteristics of included studies table (see Report Supplementary Material 2).
Economic status
One study99 reported baseline characteristics data for economic status. We have reported these data in the population domain of the characteristics of included studies table.
Other baseline characteristics
A number of studies reported baseline characteristics data for fitness, employment and amount of PA. Because these data were reported in disparate ways, and often sporadically, it was not viable to collate these data. However, when reported, we have included these data separately for each study in the population domain of the characteristics of included studies table.
Description of intervention models, for non-randomised studies
We describe below the key intervention characteristics and approaches described by authors. These are summarised for each individual study; see Report Supplementary Material 2.
Period of delivery
The nine NRSs fall into two clinical periods: postoperative65,71,73,80,98,112 (those that take place only in the period following surgery) and perioperative67,89,99 (those that include both preoperative and postoperative components). None of the NRSs was conducted in the preoperative period alone.
Similar to the RCTs, the majority (66.7%) of interventions began post surgery: within a few days or during the first week following surgery;65,98 ‘on-average’ 4 weeks following surgery;71 or, for two cancer studies, several months post surgery. 73,112 An additional cancer study80 simply described the intervention as beginning ‘after surgery’.
The three interventions67,89,99 delivered perioperatively were all cancer studies described as starting at the time, or within days, of diagnosis;99 on decision of surgery67 (but prior to commencement of adjuvant therapies when appropriate); or pre surgery. 89
Intervention approach
Similar to the RCTs, interventions often (77.8%) involved multiple components or modes of delivery65,67,73,89,98,99,112 and we summarised these designs using the same categories: (1) education and advice (77.8%) (including the provision of written or verbal information and advice, PA recommendations or a formal exercise prescription), (2) behavioural mechanisms (66.7%) (those that focused on behaviour change theories, usually through therapeutic approaches including counselling or motivational interviewing) and (3) direct PA instruction (55.6%) in the form of group classes or one-to-one sessions.
Education and advice
Seven interventions65,67,73,80,89,99,112 described the encouragement of PA adoption through ‘education sessions’: the provision of information, advice, recommendations or a formal exercise prescription.
The content of these interactions tended to fall into one or more of the following groups:
-
Exercise recommendations, including studies in which exercise prescriptions, advice for home exercise, recommendations or goals were described. 65,67,73,80,89,99,112 This sometimes involved the recommendation of, for example, 10,000 daily steps,73 the self-management of a personalised exercise programme67 or activity goals. 99 Sometimes advice was provided in addition to supervised PA; for example, in Zopf et al. ,112 participants were recommended to undertake an additional 60 minutes of weekly exercise on top of weekly supervised classes. Studies provided variable details on how PA recommendations were delivered or managed; in some cases, participants self-managed these expectations, sometimes through the use of a PA diary73,89 or an activity device that monitored or measured activity. 73,99 Doganay et al. 67 describes the weekly monitoring and modification of participants’ adherence to the exercise programme (recommendations) by the participants’ clinical team, and Macleod et al. 99 gave weekly feedback during the first weeks of the intervention from their lifestyle coach. Most recommendations were personalised in response to a baseline assessment (usually fitness) or to the participants’ personal goals, or would be reviewed and set again in response to progress over time.
-
Education or information materials, including studies in which participants were provided with exercise sheets;73 written resources with a focus on self-monitoring activity and goal-setting;99 or, as in Dantas et al. ,65 an ‘educational folder’ at hospital discharge, which detailed common physical and emotional experiences of patients following a coronary artery bypass graft (CABG), as well as information around the use of medication, activity progression and benefits, healthy diet, smoking cessation, sexual activity and managing weight.
-
The value of PA,65,67,73 including studies that reported participants being encouraged to recognise the importance of ‘exercise for well-being’;73 incorporating ‘routine exercise’ into daily life for ‘lasting benefit’67 was also described.
-
Lifestyle education – one study65 noted that the focus of education or advice was placed on broader improvements to health. This was delivered in the form of an ‘educational folder’, provided to patients on hospital discharge following CABG surgery (see previous list item ‘educational or information materials’).
Behavioural mechanisms
A therapeutic or behavioural approach to supporting participants to engage in PA was adopted in over half (66.7%) of interventions. 65,67,73,89,98,99 These sessions were delivered one to one, although in both Dantas et al. 65 and Macleod et al. 99 the participation of the patient’s family or a support person was encouraged.
Three interventions65,73,99 combined an initial period of face-to-face clinic contact with subsequent home telephone contact. For example, in Frawley et al. ,73 participants attended 8 weeks (two sessions per week) of in-person education sessions that used motivational interviewing and CBT techniques to discuss emotional management, diet and PA. These were followed by a series of six telephone motivational coaching sessions over the remaining intervention period (4 months). In Dantas et al. ,65 it appeared to be optional whether the latter counselling sessions, delivered at increasingly extended periods across the 6 months following hospital discharge, were provided at the clinic or on the telephone. Dantas et al. 65 also reported an invitation to patients or their families to call with any questions or problems, at any time.
The Komatsu et al. 89 intervention was delivered in a clinical setting; the Macchi et al. 98 intervention was delivered as part of a residential stay.
In Doganay et al. ,67 it was not clear from authors’ reporting where the intervention was delivered.
The finer detail of these sessions was limited. However, we identified some recurring topics or approaches in the content described. Five interventions67,73,89,98,99 talked about behaviour change approaches, counselling or motivational interviewing, such as coaching focused on removing bad habits or incorporating PA into participants’ daily routines,67 or motivational interviewing and CBT around emotional management, diet and PA. 73 As we saw in Frawley et al,73 these tended to overlap with references to lifestyle and health coaching,67,73,99 in which the focus might be on supporting participants to make broader behavioural changes, such as support from a lifestyle coach to reach ‘collaborative agreements’ towards achieving and maintaining, for example, smoking cessation, increased PA, caloric intake appropriate to weight status and a nutrient-dense diet. 99
Direct physical instruction
Five interventions71,73,80,98,112 (55.6%) provided face-to-face PA instruction. With the exception of Fontana et al. ,71 who described their intervention solely as medically supervised treadmill walking (three times per week), these sessions ran as one component of a broader approach that included the provision of education and advice73,80,112 and/or therapeutic behavioural mechanisms. 73,98 Activity sessions took place either individually71,98 or as group classes. 73,112 Among other activities (such as aerobic and resistance training), Zopf et al. 112 described ‘games and exercise’ ‘to promote interaction and communication’.
Physical activity sessions were provided at least weekly (although no details on the frequency of supervised training were provided in Heitkamp et al. 80). This ranged from once per week112 (over 15 months), to two or three sessions per week (for 8 weeks73 or 12 weeks71), to twice-daily sessions during a 3-week residential stay. 98
Physical activity instruction took place in a clinical setting71,73 or during a residential rehabilitation centre stay,98 or, as in Zopf et al. ,112 participants attended weekly ‘exercise classes’ in a local leisure centre. Again, no details were provided for Heitkamp et al. 80
Other components
Several studies65,67,99 reported additional features of intervention design. Dantas et al. ,65 for example, described efforts to improve communication among health teams and the patient and/or family and encouraged family participation in the patient’s health management. Macleod et al. ,99 too, noted the invitation for participants to engage a support person, such as a spouse, to assist in their adherence to the programme. Doganay et al. 67 described the reiteration of PA messaging by patients’ broader multidisciplinary clinical team at clinical follow-up appointments.
Digital technologies
There was limited utilisation of digital and other technologies to support delivery of interventions, or to provide objective outcome measures of participants’ PA. Two studies73,99 (22.2%) included the use of pedometers, provided to participants to support self-monitoring and goal-setting of PA99 and step count;73 this is a similar proportion of studies as in the RCTs.
Other resources/equipment
In addition to pedometers, the provision of specific resources or equipment was described in four studies. 65,73,99,112 This included the provision of an ‘educational folder’ containing information relating to the physical and emotional experiences often experienced by patients following CABG surgery, as well as guidance around the use of medication, activity progression and benefits, healthy diet, smoking cessation, sexual activity and body weight control;65 an exercise diary and home exercise sheets, provided to patients alongside a pedometer to support self-management of activity;73 and an ‘equipment toolkit’ for participants to use at home included a pedometer, resistance bands, digital versatile discs (DVDs) and booklets. 99 It is also worth noting that participants in Frawley et al. 73 had access to a well-equipped gym (aerobic and resistance training equipment including a treadmill, a stationary bike, a Pilates reformer, resistance bands and dumbbells).
Physical activity
Type of exercise/physical activity encouraged
Studies were often vague on the sorts of physical activities or exercises adopted by or recommended to participants. Some referred to aerobic training, including supervised aerobic training,73 aerobic exercise98,112 and counselling that aimed to facilitate at least 150 minutes of moderate-intensity activity per week. 99 Walking or the measurement of steps was described in three studies, including participants following a progressive programme of daily walking,65 treadmill walking71 and the encouragement of 10,000 daily steps. 73 Resistance or strength training,73,98,112 such as gentle calisthenic exercises to improve muscle strength or exercises for balance, was also reported,98 as were games and exercises focused on flexibility and relaxation,98,112 such as ‘gentle passive stretching’. 98 Both were in conjunction with other activities.
Doganay et al. 67 described a ‘personalised exercise programme’ with no details on the sorts of activities adopted by participants, except that the aim of the programme was to focus on PA that supported recovery and could be incorporated into daily routines. Similarly, Komatsu et al. ,89 noted counselling that encouraged PA, and that participants were to maintain a PA diary, but did not describe the sorts of activities participants undertook. Heitkamp et al. 80 reported supervised and home-based training, but provided no further details.
How much physical activity and at what sort of intensity
Studies reported varying approaches to the specification of the intensity at which participants should exercise or the frequency with which they should do it. In several studies65,67,99 this was largely undefined; rather, participants were encouraged to increase their activity such as by walking an additional 5 minutes each day up to a specified goal of 60 minutes,65 or to progress PA for lasting benefit. 67 Others used standard measures to focus intensity; Frawley et al. ,73 for example, stated participants were required to do at least 150 minutes of moderate-intensity activity at a Borg Scale score of ≥ 13. In Zopf et al. ,112 intensity was adjusted individually based on each patient’s condition and experience, but was aimed at moderate-intensity exercises of approximately 3.84 to 4.84 METs/hour/week. Similarly, for Fontana et al. ,71 target training intensity (for 35 minutes of treadmill walking each week) was tailored to each individual’s condition, to within 85% of maximal heart rate, determined by exercise testing at the start of, and 6 weeks into, the intervention period.
Although Macchi et al. 98 provided no details in relation to the aerobic activities, they referred to the adoption of ‘gentle passive’ stretching and ‘gentle’ strengthening exercises. Two studies80,89 provided no details.
The total amount of PA either delivered or encouraged tended to fall into one of three groups. The low-PA group comprised those studies encouraging PA once or twice per week, for example the recommendation of one supervised session and 60 minutes of home exercise each week. 112 The regular-PA group comprised studies largely in line with typical PA recommendations of three or four times per week. 71 Studies in the frequent group encouraged PA on at least five days per week. 65,67,73,99 Three studies80,89,98 did not provide this information.
Tailoring activity
The majority (88.9%) of interventions65,67,71,73,89,98,99,112 took some form of personalised or tailored approach to PA. This was usually65,71,73,98,112 described with respect to participants’ capacities or fitness, such as target heart rates determined by participants’ fitness,71 or the adjustment of intensity based on a patient’s condition and activity experience. 112 Two interventions, however, focused on participants’ preferences, such as determining specific action goals,99 or the collaborative development of an exercise programme between participants and their clinical team. 67
Delivery
Clinical settings
Over half of interventions65,71,73,89,99 took place in a clinical setting (outpatient clinic or rehabilitation centre), either exclusively71,89 or in conjunction with additional telephone contact with participants. Komatsu et al. 89 included expectations for self-directed home exercise.
Home
Four interventions65,73,80,99 took place in participants’ homes (or neighbourhood environment): coaching or counselling took place via telephone, in addition to some expectation of self-directed PA, and some face-to-face clinic contacts,73,99 including Dantas et al. 65 in which participants had the option to decide whether to meet in person or to receive telephone calls. Telephone contacts between participants and their clinical team (lifestyle coach, physiotherapists, research nurse) took place roughly every 3 or 4 weeks,73,99 or at months 1, 3 and 6 after hospital discharge. 65 Heitkamp et al. 80 noted home-based exercise training, but provided no further details.
Inpatient care
One intervention,98 for cardiac surgery patients, was delivered in a residential rehabilitation centre, across a 3-week stay.
Community
In Zopf et al. ,112 patients attended a weekly exercise class at a community sports centre. There was also an expectation for self-directed home exercise.
Although Heitkamp et al. 80 included home-based training, the authors provide no details as to where the supervised sessions took place. One further study67 provided no details on intervention setting.
Remote delivery
Three studies65,73,99 described some form of remote delivery. None was exclusively remote and instead combined either an initial or early face-to-face contact along with home resources, such as an education folder;65 exercise diaries, exercise sheets and pedometers;73 and subsequent telephone support (optional in-person in Dantas et al. 65). In Macleod et al. ,99 colorectal patients received a face-to-face session at the beginning of each phase of the three-phase, perioperative programme, followed by ongoing telephone contact to support participants’ progress. Each participant received an ‘equipment toolkit’, which included a pedometer, resistance bands, DVDs and booklets. Participants were also encouraged to engage a support person, such as a spouse, to assist in their adherence to the programme.
Practitioners and those involved in delivery
Interventions were often delivered by a MDT. 65,67,73,99 Specialisms within these teams varied between interventions; several included physiotherapists,65,73,99 with a lifestyle coach,99 dietitian,73 research nurse65 and psychologist73 also involved. In addition, Dantas et al. 65 and Macleod et al. 99 encouraged a family member or support person to actively support patients’ health management and adherence to programmes.
Doganay et al. 67 described a multidisciplinary clinical team, but provided no further details.
Two interventions were delivered by a single practitioner: one described as a nurse89 and one as a ‘qualified trainer’. 112 Fontana et al. 71 described the intervention being delivered under ‘medical supervision’. Heitkamp et al. 80 and Macchi et al. 98 provided no details.
Two studies mentioned specific training, qualifications or experience held by practitioners. Frawley et al. 73 described exercise physiologists who were experts in exercise prescription, with at least 2 years’ clinical experience, and Macleod et al. ,99 described lifestyle coaches with a ‘nursing background’; experience with cancer patient management; and having undertaken a 3-day bespoke training programme covering smoking cessation, increasing moderate PA, and brief interventions on alcohol and weight management.
Interventions varied considerably in terms of the amount of practitioner contact or support they offered. This sometimes varied over time, or might be quite intense over a short period of time for some interventions and much more infrequent over a much longer period of time in others. As a result, where data were available, we considered the interventions as falling into one of three groups:
-
low contact89 (25%) (one to nine total contacts), including Dantas et al. ,65 in which patients or family members were encouraged to call the MDT at any time if questions or problems arose
-
intermediate contact67 (25%) (10–20 contacts), including Macleod et al. ,99 in which a minimum of 12 contacts with the lifestyle coach was offered; they also reported that this might vary, depending on a patient’s individual treatment regime
As the duration of interventions varied considerably, we also looked at the average number of weekly contacts. This ranged from 0.15 contacts (one contact at hospital discharge with the provision of educational materials, followed by three contacts in person or on the telephone over a 6-month period, as in Dantas et al. 65) to 14 contacts (two sessions per day during a 3-week residential rehabilitation centre stay, as in Macchi et al. 98). The mean number of contacts was 2.98, the median was 0.92 and the mode was 1. Excluding the 3-week residential intervention, which was something of an outlier, average weekly contacts ranged from 0.15 to 3.0 (mean 0.95, median 0.85). This contact time was comparable to the interventions in the RCTs.
No details were provided in Heitkamp et al. 80
Duration of intervention
Interventions varied in duration from 3 weeks98 to 64 weeks. 112 With the exception of Macchi et al. 98 (3 weeks), all interventions lasted at least 3 months, although most lasted ≤ 6 months. 65,67,71,73,89,98 One intervention,67 delivered to oesophagogastric cancer patients, varied in duration, typically between 8 and 18 weeks, depending on a patient’s treatment plan; we have used a median point for the purpose of calculations.
Usual care
Neither of the two studies73,112 that included comparator groups provided details of the ‘usual care’ support that patients were offered. There was, however, some indication that, in Zopf et al. ,112 participants in the comparator group were able to access some form of rehabilitative sports groups, as these were described as accessible and open to all cancer patients in Germany.
Studies awaiting classification
During the search, we identified 12 studies that were completed without peer-reviewed published results. For completeness, we have summarised the characteristics of these studies (see Appendix 1, Table 8).
These include 12 RCTs and two NRSs. Half of the studies were conducted with cancer patients, and other studies included orthopaedic, bariatric, cardiac and renal transplantation surgical patients. Intervention approaches included education and advice, behavioural mechanisms and direct PA interventions.
Ongoing studies
We identified 52 ongoing studies that were potentially eligible for inclusion in our review (see Appendix 1, Table 9). Most of these are single-centre parallel-design RCTs with a hospital as the recruiting centre.
Most include cancer patients; others include bariatric, orthopaedic, transplantation, cardia, abdominal aortic aneurysm and major surgery patients. We noted that 30% of the interventions in these studies are being conducted during the preoperative period, 12% are perioperative and the rest are postoperative. The design of these studies largely reflects those of completed, included studies. Other design characteristics are also comparable with those included in this review (such as using education and advice, behavioural mechanisms and direct PA instruction).
Although all these studies aim to measure PA, only one-third of them have listed PA as the primary outcome. Most will gather PA data using digital technologies such as accelerometers and pedometers, and these data will be supplemented with responses from questionnaires completed by participants.
Risk of bias in included studies
See Appendix 8 for a summary of our risk-of-bias judgements. Blank spaces in the risk-of-bias table indicate that we did not conduct risk-of-bias assessments.
Allocation (selection bias)
We judged 33 studies51–54,57,59–61,63,66,69,74–76,78,82,84,86,87,90,92,94–97,102,104–107,109–111 as having a low risk of selection bias for sequence generation, because the study authors reported sufficient methods for randomisation. Two studies58,85 were Q-RCTs and performed quasi-randomisation by alternative allocation in blocks of 10. We judged these studies as having a high risk of bias for sequence generation. The remaining studies reported insufficient methods of sequence generation; therefore, we judged these to be at an unclear risk of bias.
We judged 24 studies51,54,59,61,63,64,66,74,78,81,82,84,86,87,90,94,95,97,102,103,105,107,109,110 as having a low risk of bias for allocation concealment, because the study authors reported sufficient methods for this judgement. We judged the two Q-RCTs58,85 as having a high risk of bias, as it was not feasible to conceal allocation because of the quasi-randomised methods to allocate groups. The remaining studies reported insufficient methods of allocation concealment; we judged these to be at an unclear risk of bias.
Blinding (performance bias and detection bias)
Because it was generally not feasible to blind participants and personnel to the intervention, we judged 52 studies51–64,66,68–70,72,74–76,78,79,81–88,90–97,100–111 as having a high risk of performance bias. One study77 was a placebo-controlled study, suggesting that participants were not aware of group allocation, but the study authors did not report details on personnel, although we assumed that they were aware of group allocation; we judged this study77 to be at an unclear risk of performance bias.
For detection bias for PA outcomes, we judged 26 studies52,54,56,59,66,69,70,72,76–79,83,84,86–88,91,92,94,95,101,103,105,108,113 that used subjective measurement tools as having a high risk of detection bias. One study53 reported data for two PA outcomes, one measured using subjective tools and one measured using objective tools. We judged this study53 to be at high risk of detection bias when outcomes were measured with subjective tools, and at low risk of detection bias when measured with objective tools. One study100 reported PA data using a combination of subjective and objective measurement tools; we judged this study to be at low risk of detection bias, but noted a high risk of bias for the self-reported data. The remaining 25 studies51,55,57,58,60–62,64,68,74,75,81,82,85,90,93,96,97,102,104,106,107,109–111 used only objective measurement tools for PA outcomes; we judged these to be at a low risk of detection bias.
Because all the studies53,60,61,72,77,79,83,87,90,97,101–103,105,107,109 that measured physical fitness used objective measurement tools, we judged these as having a low risk of detection bias for this outcome.
Conversely, all the studies51–54,58,59,63,66,69,75,76,78,79,82,84–87,90,95,97,101–107,110 that measured self-reported outcomes, including HRQoL, pain and participant experience, we judged as having a high risk of detection bias for these outcomes.
For detection bias for adverse events outcomes, we judged studies76,79 that used self-reported measurements to have a high risk of detection bias. Sixteen studies51,56,59,61,66,69,77,82,83,87,90,97,102–104,109 did not clearly specify their observation method for this outcome; we judged these studies to have an unclear risk of detection bias. In one study107 the outcome was reported by physiotherapists observing the intervention effects; we judged this study to have a low risk of attrition bias.
For detection bias for adherence outcomes, we judged studies59,83,84,103 that used self-reported measurements to have a high risk of detection bias. One study69 did not clearly report its measurement tool for this outcome; we judged this study to have an unclear risk of detection bias. The remaining 11 studies52,53,63,77,82,90,92,96,106,108,109 that reported data for this outcome used objective methods for its measurement; we judged these studies to have a low risk of attrition bias.
Incomplete outcome data (attrition bias)
We judged 21 studies51,53,54,56,59,61,66,69,70,76,77,79,84,86–88,92,93,97,102,111 as having a low risk of attrition bias because the study authors reported no participant losses or few losses (< 15%).
We judged 29 studies to have a high risk of attrition bias. One study72 did not report the number of participants randomised to each group, and losses were reported overall only; one study91 did not report the number of participants randomised to, or lost from, each group, and reported data for a subset of participants only; and the remaining studies52,55,57,58,60,63,64,74,75,78,81–83,85,90,94–96,101,103–110 reported a high number of participant losses (> 15%).
One study62 was an interim report and, although it reported a smaller number of participants at follow-up than at baseline, we were uncertain if this indicated participant loss or that data for the remaining participants were, as yet, unavailable. We could not ascertain losses in one study68 reported as an abstract because of insufficient information. In another study,100 authors did not report losses or numbers randomised to each group; thus, we were uncertain of attrition bias. Each of these studies was judged as having an unclear risk of attrition bias.
Selective reporting (reporting bias)
Eighteen studies prospectively registered with a clinical trials register and reported review outcomes consistent with the clinical trials register documents;51,61,69,75,78,79,82,84,85,90,95,96,102–104,107,109,111 we judged these studies to have a low risk of reporting bias. Two additional studies54,106 were also prospectively registered with a clinical trials register, but we noted discrepancies between the clinical trials register documents and the reported results; in these studies, we made risk-of-bias judgments at the outcome level. In Barberan-Garcia et al. ,54 we judged the risk of selective reporting bias to be high for PA because it was not listed in the clinical trials register, and to be low for HRQoL, which was listed in the clinical trials register. In Stolberg et al. ,106 PA (measured as number of steps) was judged to be at a low risk of reporting bias because it was listed in the clinical trials register; we judged both PA (measured as minutes per day) and HRQoL to be at a high risk of reporting bias because these outcomes were reported but not listed in the clinical trials register.
Seven studies52,57,58,64,74,97,108 retrospectively registered with a clinical trials register and we decided that it was not feasible to use these documents to effectively assess the risk of selective reporting bias. Twenty-one studies55,56,59,63,66,68,70,72,77,83,86–88,91–94,100,101,105,110 did not report clinical trial registration or study protocol publication; therefore, it was similarly not feasible to effectively assess the risk of reporting bias for these studies. One study54 prospectively registered with a clinical trials register, but we noted that the measurement of PA was not listed as a study outcome in the clinical trials report; we could not be certain of the risk of reporting bias for this outcome.
We judged five studies to have a high risk of reporting bias: Baillot et al. 53 did not include study dates in their study reports, which meant that it was unclear if registration was prospective, and not all reported outcomes were included in the clinical trials report; Carnero et al. 60 reported data from a secondary analysis and report outcomes that were not listed in the clinical trials document; Christiansen et al. 62 was an abstract that did not report all outcomes consistent with the clinical trials report; Hackshaw-McGeagh et al. 76 registered their study retrospectively and did not report PA data consistent with the clinical trials report; and, in Hoorntje et al. ,81 we noted that not all reported outcomes were included in the registration documents, which may indicate a high risk of reporting bias.
Other potential sources of bias
We could not be certain of other risks of bias in the four studies62,68,93,100 that were reported only as abstracts, but, because these studies had not been peer reviewed, we judged them to have a high risk of other biases.
Additional arms of one study76 explored nutritional interventions, for which participants were provided information, meaning that there may have been a crossover in behaviour, potentially diluting results; we judged this study to have a high risk of other biases. In Hubbard et al. ,82 the study authors reported a recruitment bias at baseline wherein most participants were already meeting the recommended level for MVPA; we judged this study to have a high risk of other biases.
We judged the remaining studies to have a low risk of other biases.
Effects of interventions
In this section, we report the analyses for combined data from RCTs and Q-RCTs, as well as effect estimates calculated from individual study data; we provide forest plots for these analyses (see Appendix 2). We also report data from studies for which we did not calculate an effect estimate. These data were reported without distribution values appropriate for our calculations; we provide data for these studies in tables (see Appendix 3, Tables 10–21).
We report the findings of subgroup analyses for outcomes that were included in at least 10 studies. We report the findings from sensitivity analyses only when these indicated a change in our interpretation of the effect; for the complete results from sensitivity analyses, see Appendix 4.
Intervention versus usual care
Amount of physical activity
Twenty-one studies57,59,60,62,64,66,74,75,78,81,82,85,94,96,97,100,104,106,107,109,111 used instruments to measure minutes of PA per day or per week. A combination of objective methods (such as using accelerometers) and subjective methods (such as using questionnaires) was used to record PA data in these studies.
Twelve studies59,66,74,75,78,82,85,94,96,100,104,109 reported PA as post-intervention values. Of these studies, Demark-Wahnefried et al. ,66 Hawkes et al. ,78 Lier et al. 94 and Sellberg et al. 104 measured the outcome at 12 months; Jiménez-Loaisa et al. 85 measured the outcome at 13 months; and the remaining studies measured the outcome at 6 months. When combining these studies, we found that people participated in more PA at the end of follow-up when they had received the intervention (SMD 0.15, 95% CI 0.04 to 0.27; participants, n = 1947; studies, n = 12; I2 = 24%; favours intervention) (Figure 9). This effect was likely to be small. 49
FIGURE 9.
Combined data: amount of PA at end of follow-up measured as minutes per day or week. a, Multiarm study: in this analysis, we combined CBT vs. usual care; b, multiarm study: in this analysis, we combined FI + THC vs. attention control. df, degrees of freedom; IV, inverse variance.
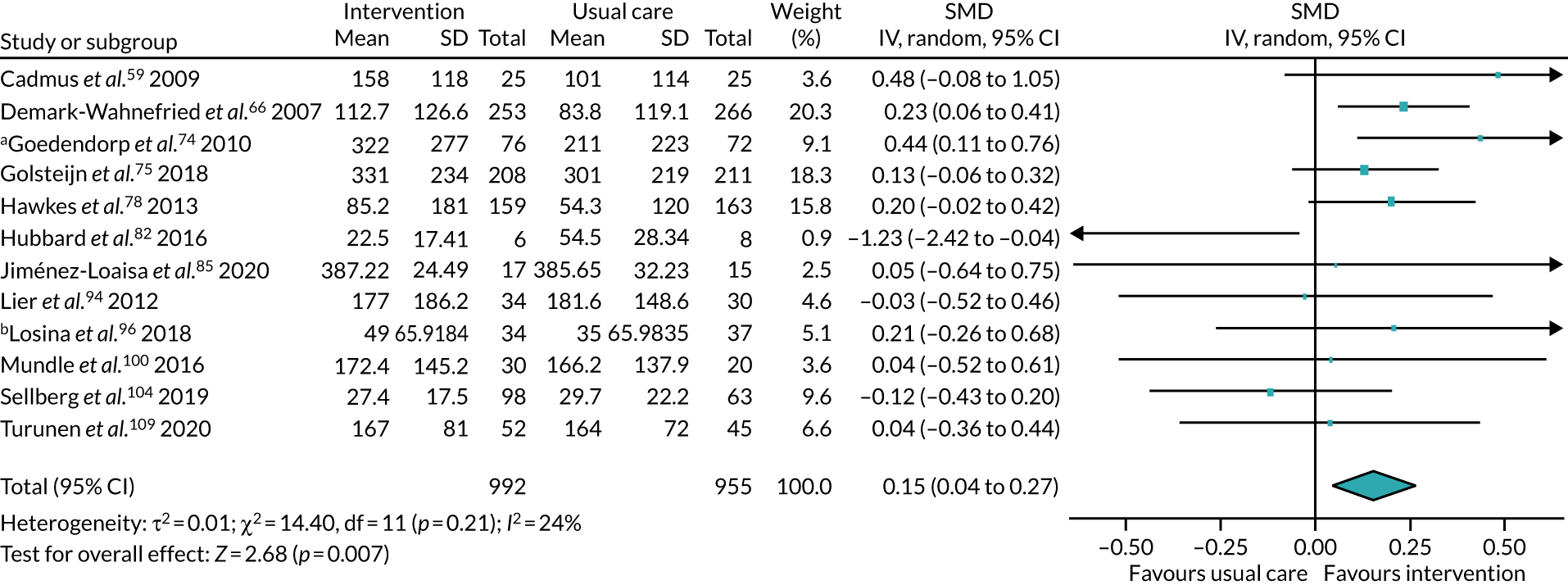
We conducted the following subgroup analyses on these data:
-
Duration of intervention. We subgrouped the studies according to whether the intervention was undertaken for < 6 months75,82,100,104 or for at least 6 months. 59,66,74,78,85,94,96,109 From our visual inspection of the data, we noted that, when the intervention was given for at least 6 months, people receiving the intervention appeared to participate in more PA at the end of follow-up (SMD 0.23, 95% CI 0.12 to 0.33; participants, n = 1303; studies, n = 8; I2 = 0%; favours intervention) (see Appendix 2, Figure 16). However, this difference was not supported by a formal test for subgroup interactions (p = 0.10).
-
Time of intervention commencement. We subgrouped the studies according to whether the intervention was given pre surgery74,94 or post surgery. 59,66,75,78,82,85,96,100,104,109 From our visual inspection of the data, we noted that, when the intervention was given post surgery, people receiving the intervention appeared to participate in more PA at the end of follow-up (SMD 0.14, 95% CI 0.02 to 0.25; participants, n = 1735; studies, n = 10; I2 = 18%; favours intervention (see Appendix 2, Figure 17). However, this difference was not supported by a formal test for subgroup interactions (p = 0.65).
-
Type of surgery. We subgrouped the studies according to whether the participants underwent surgery for types of cancer59,66,74,75,78,82 or for other conditions. 85,94,96,100,104,109 During our visual inspection of the data, we noted that, when the intervention was given to participants undergoing surgery for cancer, people receiving the intervention appeared to participate in more PA at the end of follow-up (SMD 0.22, 95% CI 0.07 to 0.38; participants, n = 1472; studies, n = 6; I2 = 45%; favours intervention) (see Appendix 2, Figure 18); we noted a moderate level of statistical heterogeneity in this effect. However, this difference was not supported by a formal test for subgroup interactions (p = 0.95).
-
Age. We subgrouped the studies according to whether the participants had a mean age of < 60 years59,66,74,85,94,104 or at least 60 years;75,78,82,96,109 one study100 did not report baseline characteristics for age. There was no statistically significant difference between the two subgroups (favours intervention; see Appendix 2, Figure 19).
-
BMI. We were unable to perform a subgroup analysis on the basis of BMI because of insufficient data.
We also conducted sensitivity analyses on this meta-analysis. When we excluded studies that measured this outcome immediately after the end of the intervention period, we found evidence of a possible increase, as well as a decrease, in amount of PA at the end of follow-up (SMD 0.11, 95% CI –0.03 to 0.26; participants, n = 1517; studies, n = 7; I2 = 35%); we noted a moderate level of statistical heterogeneity in this effect. For all sensitivity analyses for this outcome, see Appendix 4, Table 22.
The following additional data also provided evidence for this measure:
-
Two studies60,97 that used instruments to measure minutes of PA per day or per week reported their data based on changes from baseline; both measured the outcome at 6 months, and both used accelerometers to collect their data. We found little or no difference in the amount of PA according to whether an intervention or usual care was provided (MD –1.56, 95% CI –15.71 to 12.60; participants, n = 214; studies, n = 2; I2 = 76%; favours usual care) (see Appendix 2, Figure 20); we noted a substantial to considerable level of statistical heterogeneity in this effect.
-
Bond et al. 57 measured the amount of PA as mean bout-related MVPA minutes per day, but did not report a SD for these data, and did not clearly report the number of participants analysed at follow-up. The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.15) (see Appendix 3, Table 10).
-
Christiansen et al. 62 measured the amount of PA as total MVPA minutes per day, and reported their data as median [interquartile range (IQR)]. The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.55; participants, n = 22) (see Appendix 3, Table 10).
-
Creel et al. 64 reported their PA data as mean bout-related minutes per week, but did not include a SD with these data; the outcome was measured at 6.5 months. The study authors reported that people participated in more PA at the end of follow-up when they had received the intervention (p < 0.05; participants, n = 58) (see Appendix 3, Table 10).
-
Stolberg et al. 106 reported their data as the difference between groups at 24 months post surgery. The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p-value not stated; participants, n = 42) (see Appendix 3, Table 10).
-
Taraldsen et al. 107 reported their PA data as mean minutes per day of upright time, and based on between-group differences at 6 months post intervention. The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.346; participants, n = 113) (see Appendix 3, Table 10).
-
Yates et al. 111 reported their PA data based on changes from 3 to 6 months post surgery, and gave their data as a median (range). The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.79; participants, n = 34) (see Appendix 3, Table 10).
-
Hoorntje et al. 81 reported their data based on changes in the percentage of time spent active from baseline to 6 months post surgery. The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.59; participants, n = 97) (see Appendix 3, Table 10).
Thirteen studies53,57,58,60–62,64,93,96,97,104,106,110 used instruments to measure steps per day; all used some type of activity monitor to measure the number of steps taken.
Six studies53,58,61,93,96,104 reported PA data as a set of post-intervention value scores. Sellberg et al. 104 and Baillot et al. 53 measured the outcome at 12 months; the other four studies measured the outcome at 6 months. When analysing these studies, we found that people participated in more PA at the end of follow-up when they had received the intervention (MD 817.14 steps per day, 95% CI 82.95 to 1551.33 steps per day; participants, n = 378; studies, n = 6; I2 = 59%; favours intervention) (see Appendix 2, Figure 21); we noted a moderate to substantial level of statistical heterogeneity in this effect.
We had an insufficient number of studies to conduct a subgroup analysis. We conducted sensitivity analyses, and noted the following:
-
When we excluded studies deemed to be at high and unclear risks of selection bias (for random sequence generation), we noted that the effect indicated a possible increase, as well as a possible decrease, in the amount of PA at the end of follow-up (MD 642.76 steps per day, 95% CI –293.59 to 1579.12 steps per day; participants, n = 290; studies, n = 4; I2 = 58%) (see Appendix 4, Table 23); we noted a moderate to substantial level of statistical heterogeneity in this effect.
-
When we excluded studies that measured the outcome immediately after the end of the intervention, we noted that the effect indicated a possible increase, as well as a possible decrease, in the amount of PA at the end of follow-up (MD 351.24 steps per day, 95% CI –287.96 to 990.44 steps per day; participants, n = 232; studies, n = 3; I2 = 15%) (see Appendix 4, Table 23).
-
Losina et al. 96 was a multiarm study. We replaced data from both alternative groups in a sensitivity analysis. We noted no evidence of a difference in the amount of PA according to whether an intervention or usual care was provided for either group (using data from the FI-only group: MD 658.57 steps per day, 95% CI –195.43 to 1512.56 steps per day; participants, n = 384; studies, n = 6; I2 = 70%; using data from the THC-only group: MD 572.99 steps per day, 95% CI –382.20 to 1528.17 steps per day; participants, n = 383; studies, n = 6; I2 = 76%) (see Appendix 4, Table 23); we noted a substantial to considerable level of statistical heterogeneity in these estimates.
The following additional data also provided evidence for this measure:
-
Two studies60,97 that used instruments to measure steps per day reported data based on change from baseline; both measured data at 6 months using accelerometers. When analysing these studies, we found little or no difference in the amount of PA according to whether an intervention or usual care was provided (MD 187.48 steps per day, 95% CI –410.09 to 785.06 steps per day; participants, n = 214; studies, n = 2; I2 = 0%; favours intervention) (see Appendix 2, Figure 22).
-
Bond et al. 57 did not report a SD for the mean value in their results section, and did not clearly report the number of participants analysed at follow-up. The study authors reported that people participated in more PA at the end of follow-up when they had received the intervention (p = 0.024) (see Appendix 3, Table 11).
-
Christiansen et al. 62 reported their data as median (IQR). The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.11; participants, n = 22) (see Appendix 3, Table 11).
-
Creel et al. 64 reported their PA data as mean steps per day, but did not include a SD with these data; the outcome was measured at 6.5 months. The study authors reported that people participated in more PA at the end of follow-up when they had received the intervention (p < 0.05) (see Appendix 3, Table 11).
-
Stolberg et al. 106 reported their PA data based on the difference between groups at 24 months post surgery. The study authors reported little or no difference in the amount of PA according to whether an intervention or usual care was provided (p-value not stated; participants, n = 42) (see Appendix 3, Table 11).
-
Van der Walt et al. 110 reported their PA data as the mean daily step count expressed as a percentage of the preoperative step count at 6 months. The study authors reported that people participated in more PA at the end of follow-up when they had received the intervention (p = 0.030; participants, n = 163) (see Appendix 3, Table 11).
Seven studies55,60,68,76,92,102,103 used instruments to measure energy expenditure.
Five of these studies55,76,92,102,103 reported PA data as sets of post-intervention value scores. Lear et al. 92 measured the outcome at 12 months; the remaining studies measured the outcome at 6 months. Two studies55,102 used an accelerometer to measure the outcome, one study76 used patient-reported outcome measures, one study92 used the Leisure Time Physical Activity questionnaire and one study103 used the Community Health Activities Model Program for Seniors (CHAMPS). When analysing these studies, we found little or no difference in the amount of PA using energy expenditure measures according to whether an intervention or usual care was provided (SMD 0.17, 95% CI –0.16 to 0.50; participants, n = 695; studies, n = 5; I2 = 76%; favours intervention) (see Appendix 2, Figure 23); we noted a substantial to considerable level of statistical heterogeneity in this effect.
We had an insufficient number of studies to conduct a subgroup analysis. Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 24).
The following additional data also provided evidence for this measure:
-
One study68 reported its data based on change from baseline at 12 months post intervention. It used the Paffenbarger Physical Activity and Exercise Index and reported data only for the intervention group. The study authors found that there was a significant increase in PA among the participants receiving the intervention (p = 0.03) (see Appendix 3, Table 12).
-
One study60 reported its data based on change from baseline at 6 months post intervention. Using these data, we found little or no difference in the amount of PA using energy expenditure measures according to whether an intervention or usual care was provided (MD –84.00, 95% CI –192.79 to 24.79; participants, n = 96; studies, n = 1; favours usual care) (see Appendix 2, Figure 24).
Five studies52,54,74,77,79 measured amount of PA using various questionnaires: two studies52,79 used the University of California, Los Angeles, activity scale (scale 1–10); one study54 used the Yale Physical Activity Survey (scale 0–13); one study74 used a Daily Observed Activity questionnaire (scale 0–16); and one study77 used a PA questionnaire for the elderly (total activity score). For each of these different scales, a higher score indicates more PA. Four studies52,54,74,77 measured the outcome at 6 months, and the remaining study79 measured the outcome at 5 years. When analysing these data, we found that people participated in more PA at the end of follow-up when they had received the intervention (SMD 0.47, 95% CI 0.24 to 0.70; participants, n = 304; studies, n = 5; I2 = 0%; favours intervention) (see Appendix 2, Figure 25). The effect size from the findings of these questionnaires is likely to be medium to large. 49
We had an insufficient number of studies to conduct a subgroup analysis. Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 25).
Three studies53,83,84 used the International Physical Activity Questionnaire-Short Form (IPAQ-SF) to measure amount of PA (in METs/minute/week).
Baillot et al. 53 and Husebø et al. 83 measured this outcome at 12 months and 6 months, respectively, and we found no evidence of a difference in the amount of PA according to whether an intervention or usual care was provided (MD 247.11 METs/minute/week, 95% CI –701.84 to 1196.05 METs/minute/week; participants, n = 78; studies, n = 2; I2 = 0%; favours intervention) (see Appendix 2, Figure 26). We had an insufficient number studies to conduct a subgroup analysis. Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 26).
In addition, Ilves et al. 84 measured this outcome at 12 months, and the study authors reported no evidence of a difference in the amount of PA according to whether an intervention or usual care was provided (p = 0.92; participants, n = 98) (see Appendix 3, Table 13).
Goedendorp et al. 74 used an actometer to measure mean daily activity counts at 6 months. We found little or no difference in the amount of daily activity counts according to whether an intervention or usual care was provided (MD 2.50, 95% CI –10.17 to 15.17; participants, n = 55; studies, n = 1; favours intervention) (see Appendix 2, Figure 27). Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 27).
Engagement in physical activity at the end of follow-up
Ten studies63,69,70,72,91,94,95,101,108,109 measured engagement in PA.
Seven of these studies69,70,72,91,94,101,109 measured the outcome at 12 months, one study63 measured the outcome at 6 months and one study108 measured the outcome at 24 months. When analysing these studies, we found little or no difference in the number of people engaged in PA at the end of follow-up when they had received the intervention (RR 1.19, 95% CI 0.96 to 1.47; participants, n = 882; studies, n = 9; I2 = 25%) (Figure 10). We had an insufficient number of studies to conduct a subgroup analysis. Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 28).
FIGURE 10.
Combined data: engagement in PA at the end of follow-up. a, Multiarm study: in this analysis, we compared aerobic exercise training group with usual care. df, degrees of freedom; M–H, Mantel–Haenszel.
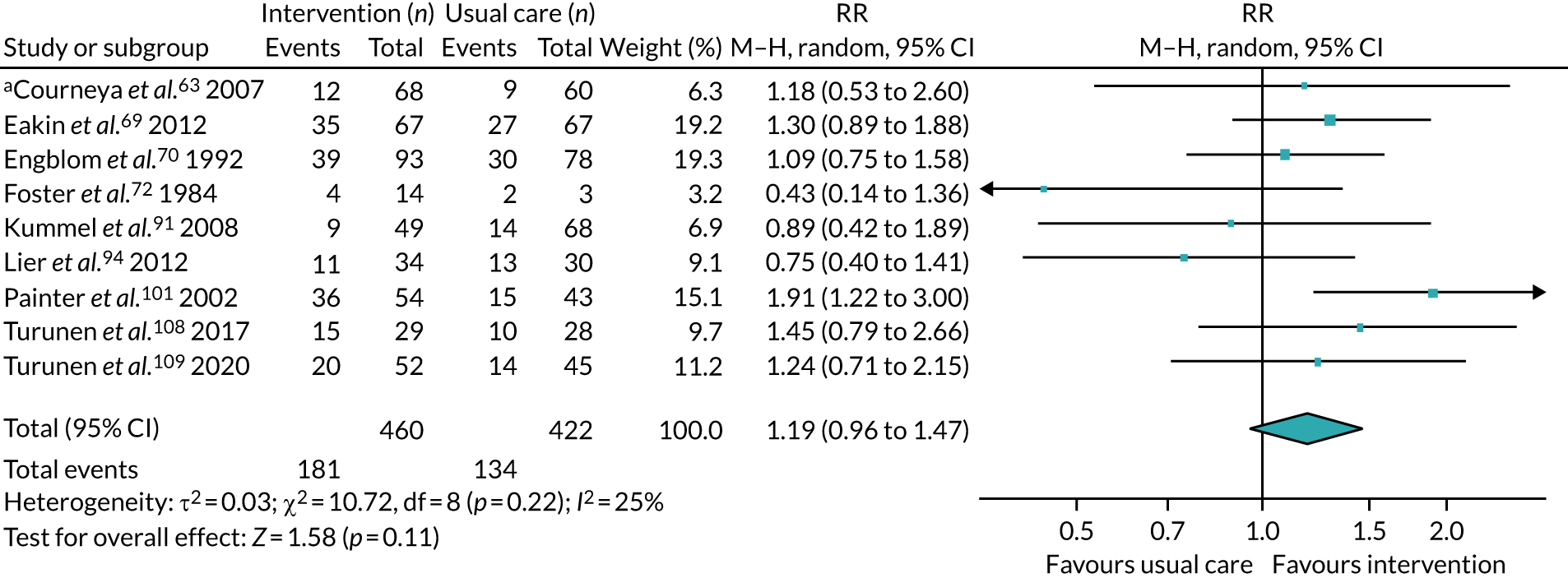
In addition, one study95 reported engagement in PA as analysed data at 12 months. The study authors reported that more people were engaged in PA when they had received the intervention (p = 0.020; participants, n = 197) (see Appendix 3, Table 14).
Physical fitness
Six studies53,61,79,83,97,103 measured physical fitness using walking tests.
Four studies61,79,83,103 reported physical fitness data as sets of post-intervention value scores. Of these, three studies61,83,103 measured the outcome at 6 months and one study79 measured the outcome at 5 years. Christiansen et al. 61 measured walking distance using the 2-minute walk test; the remaining studies79,83,103 used the 6-minute walk test (6MWT). We did not pool the studies for this measurement tool because we noted substantial statistical heterogeneity (86%); for data from individual studies, see Appendix 2, Figure 28.
We conducted sensitivity analyses on this effect (see Appendix 4, Table 29). We used data from a time point for Heiberg et al. 79 that was more consistent with other studies. We found evidence of improved physical fitness when participants received the intervention (SMD 0.99, 95% CI 0.47 to 1.51; participants, n = 215; studies, n = 4; I2 = 69%); we noted a moderate to substantial level of statistical heterogeneity in this effect. This effect was also consistent when we removed studies with unclear risks of selection bias (SMD 0.73, 95% CI 0.02 to 1.44; participants, n = 33, studies, n = 1); this estimate included only one small study.
Two studies53,97 that measured physical fitness using walking tests reported data based on changes from baseline. Baillot et al. 53 measured the outcome at 12 months and used the 6MWT. Lotzke et al. 97 measured the outcome at 6 months and used the 5-minute walk test. When analysing the Baillot et al. 53 study, we found that people achieved a greater increase in distance walked when they had received the intervention (MD 50.90, 95% CI 0.55 to 101.25; participants, n = 25; studies, n = 1; favours intervention) (see Appendix 2, Figure 29). When analysing the Lotzke et al. 97 study, we found no evidence of a difference between groups in this outcome according to whether an intervention or usual care was provided (MD 0.50, 95% CI –65.62 to 66.62; participants, n = 118; studies, n = 1; favours intervention) (see Appendix 2, Figure 30).
Three studies61,77,97 measured physical fitness using the timed up and go (TUG) test (measured in seconds).
Christiansen et al. 61 and Hauer et al. 77 reported their data as sets of post-intervention value scores, and measured the outcome at 6 months, whereas Lotzke et al. 97 reported these data based on change from baseline to 6 months. Because these studies used the same measurement tool for this outcome, we were able to combine their data using the unstandardised mean difference method. When analysing these data, we found little or no difference between groups in this outcome according to whether an intervention or usual care was provided (MD –0.09, 95% CI –0.98 to 0.80; participants, n = 175; studies, n = 3; I2 = 0%; favours usual care) (see Appendix 2, Figure 31). Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 30).
Two studies77,103 measured fitness using handgrip strength; both measured the outcome at 6 months. Hauer et al. 77 gave their data in kPa, whereas Santa Mina et al. 103 gave their data as kilogram-force. We did not pool the studies for this measurement tool because we noted substantial statistical heterogeneity (93%); for data from individual studies, see Appendix 2, Figure 32. Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 31).
Three studies53,72,111 measured cardiopulmonary fitness using an exercise tolerance test.
Baillot et al. 53 used a symptom-limited cardiac exercise; they reported these data as sets of post-intervention value scores, and measured the outcome at 12 months. Foster et al. 72 used a cardiopulmonary exercise capacity test, and measured the outcome at 6 months. We judged the measurement tools in these studies to be comparable and included the data in the same analysis. We found that people scored higher in this outcome at the end of follow-up when they had received the intervention (SMD 0.82, 95% CI 0.23 to 1.40; participants, n = 53; studies, n = 2; I2 = 0%; favours intervention) (see Appendix 2, Figure 33).
During a sensitivity analysis of this meta-analysis, we reanalysed the data using only Baillot et al. ,53 which was at low risks of selection and of attrition biases. With only this study, we found a change in the interpretation of the effect, finding little or no difference between groups in this outcome according to whether an intervention or usual care was provided (SMD 0.60, 95% CI –0.21 to 1.40; participants, n = 25; studies, n = 1) (see Appendix 4, Table 32).
In addition, Yates et al. 111 used a similar symptom-limited exercise test, but reported their data based on changes from 3 months to 6 months. For this study, we found no evidence of a difference between groups in this outcome at the end of follow-up according to whether an intervention or usual care was provided (p = 0.10; participants, n = 34) (see Appendix 3, Table 15).
Three studies measured physical fitness using performance-based tests: one study102 used a battery of six performance-based tests and reported their data as a composite z-score; the other two studies107,109 used the Short Physical Performance Battery and reported their data using a scale of 0–12.
Two studies102,109 measured their data at 6 months and reported their data as a set of post-intervention value scores. When analysing these studies, we found little or no difference between groups in this outcome according to whether an intervention or usual care was provided (SMD 0.19, 95% CI –0.08 to 0.45; participants, n = 240; studies, n = 2; I2 = 0%; favours intervention) (see Appendix 2, Figure 34). Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 33).
Taraldsen et al. 107 reported their physical fitness data based on between-group differences at 6 months post intervention. The study authors reported little or no difference between groups in this outcome according to whether an intervention or usual care was provided (p = 0.017; participants, n = 113) (see Appendix 3, Table 16).
One study53 measured fitness using a sit-to-stand test; it measured the outcome at 12 months. For this study we found little or no difference between groups according to whether an intervention or usual care was provided (MD 2.50, 95% CI –1.30 to 6.30; participants, n = 25; studies, n = 1; favours intervention) (see Appendix 2, Figure 35).
One study53 measured fitness using an arm-curl test; it measured the outcome at 12 months. For this study we found little or no difference between groups according to whether an intervention or usual care was provided (MD 0.50, 95% CI –3.86 to 4.86; participants, n = 25; studies, n = 1; favours intervention) (see Appendix 2, Figure 36).
One study77 measured physical fitness using a leg press, measuring the outcome at 6 months. For this study we found little or no difference between groups in this outcome at the end of follow-up according to whether an intervention or usual care was provided (MD 42.00, 95% CI –1.61 to 85.61; participants, n = 24; studies, n = 1; favours intervention) (see Appendix 2, Figure 37).
Two studies60,101 measured physical fitness using VO2 peak. Painter et al. 101 reported these data at 12 months, whereas Carnero et al. 60 reported these data based on change from baseline to 6 months. For Painter et al. ,101 we found little or no difference between groups in this outcome at the end of follow-up according to whether an intervention or usual care was provided (MD 3.60, 95% CI –0.22 to 7.42; participants, n = 95; studies, n = 1; favours intervention) (see Appendix 2, Figure 38). For Carnero et al. ,60 we found that people scored higher in this outcome at the end of follow-up when they had received the intervention (MD 188.00, 95% CI 55.57 to 320.43; participants, n = 96; studies, n = 1; favours intervention) (see Appendix 2, Figure 39).
Health-related quality of life
Twenty-two studies52–54,58,59,63,66,69,75,78,79,82,85,95,97,101–104,106,107,110 measured HRQoL.
Seventeen studies52–54,58,59,63,66,75,78,79,82,85,101–104,110 reported the outcome as a set of post-intervention values. Three of these studies measured the outcome using components of the Functional Assessment of Cancer Therapy (FACT): two studies66,103 used FACT-General and one study63 used FACT-Anaemia. Two of these studies63,103 measured the outcome at 6 months and one66 measured the outcome at 12 months. Eight studies54,58,59,78,85,101,102,104 measured the outcome using components of the SF-36; we extracted the data from the global score or general score for Brandes et al. ,58 Cadmus et al. ,59 Jiménez-Loaisa et al. 85 and Sellberg et al. ;104 for the remaining studies, we extracted the physical component summary (PCS). Of these studies, four54,58,59,102 measured the outcome at 6 months, three78,101,104 measured the outcome at 12 months and one85 measured the outcome at 13 months. One study52 measured the outcome, at 6 months, using the Knee injury and Osteoarthritis Outcome Score (KOOS). One study79 measured the outcome, at 5 years, using the Hip disability and Osteoarthritis Outcome Score (HOOS). One study53 measured the outcome using the Laval Questionnaire and reported their data as a weight-related quality-of-life score, measuring the outcome at 12 months. One study75 measured the outcome using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 Global (EORTC QLQ-C30 Global), and measured the outcome at 6 months. One study110 measured the outcome, at 6 months, using the EQ-5D. The remaining study82 measured the outcome using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), and measured the outcome at 6 months. When analysing these studies, we found a possible improvement in HRQoL at the end of follow-up with an intervention, as well as a possible reduction (SMD 0.12, 95% CI –0.03 to 0.26, favours intervention; participants, n = 2455; studies, n = 17; I2 = 60%; favours intervention) (Figure 11); we noted a moderate to substantial level of statistical heterogeneity in this effect. The effect size is likely to be small. 49
FIGURE 11.
Combined data: HRQoL at the end of follow-up. Measured using a range of measurement tools. a, Multiarm study: in this analysis, we compared aerobic exercise group with usual care; b, multiarm study: in this analysis, we compared clinic-based group with usual care. df, degrees of freedom; IV, inverse variance.
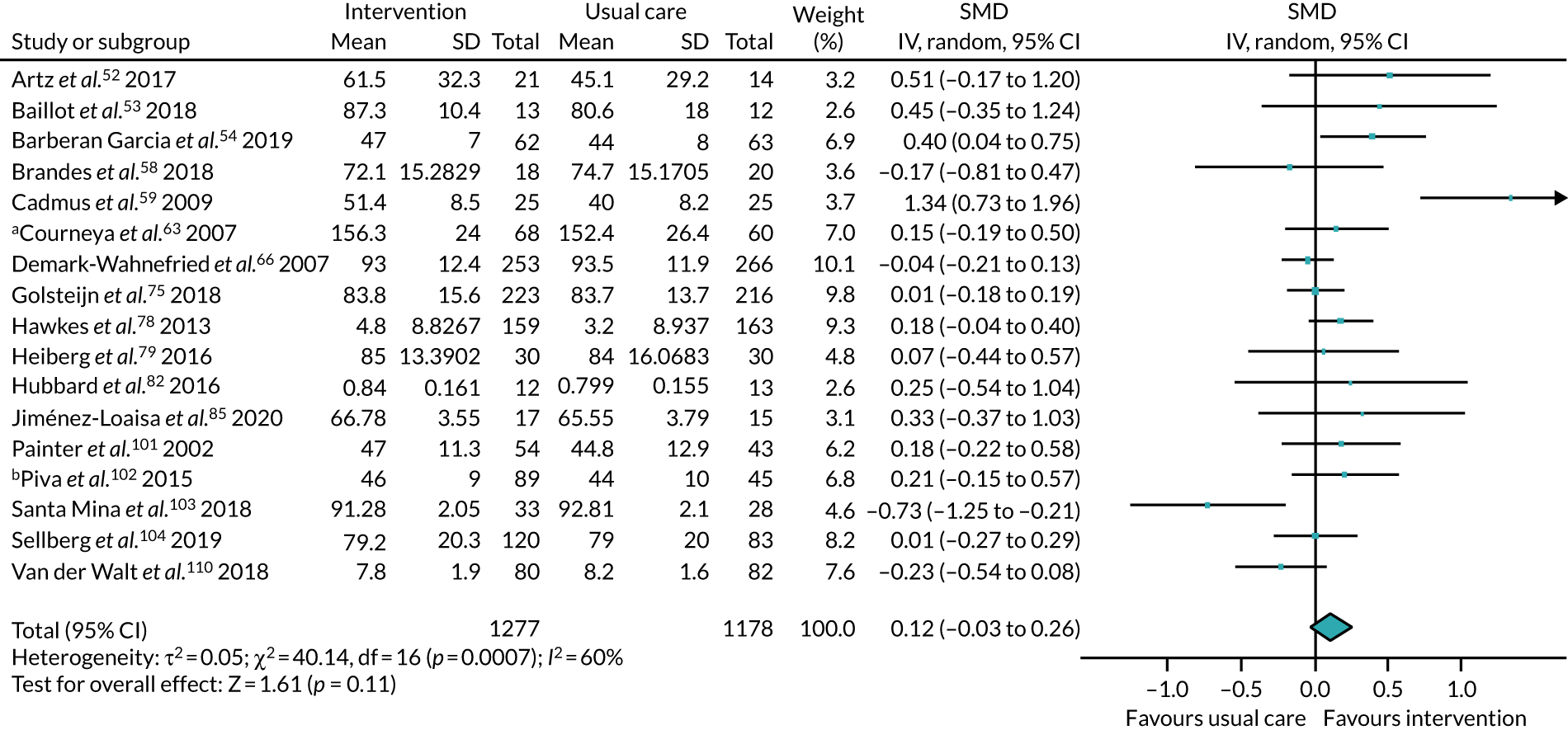
We conducted the following subgroup analyses on these data:
-
Duration of intervention. We subgrouped the studies according to whether the intervention was undertaken for < 6 months52,54,58,63,75,79,82,102–104,110 or for at least 6 months. 53,59,66,78,85,101 There was no statistically significant difference between the two subgroups. During our visual inspection of the forest plots, we noted that people receiving the intervention appeared to have improved HRQoL at the end of follow-up when the intervention was given for at least 6 months (SMD 0.32, 95% CI 0.01 to 0.62; participants, n = 1045; studies, n = 6; I2 = 75%; favours intervention); we noted a substantial to considerable level of statistical heterogeneity in this effect. However, this effect was not supported by a formal test for subgroup interactions (p = 0.11) (see Appendix 2, Figure 40).
-
Time of intervention commencement. We subgrouped the studies according to whether the intervention was given pre surgery53,54,103,110 or post surgery. 52,58,59,63,66,75,78,79,82,85,101,102,104 There was no statistically significant difference between the two subgroups (see Appendix 2, Figure 41).
-
Type of surgery. We subgrouped the studies according to whether the participants underwent surgery for types of cancer59,63,66,75,78,82,103 or for other conditions. 52–54,58,79,85,101,102,104,110 There was no statistically significant difference between the two subgroups (see Appendix 2, Figure 42).
-
Body mass index: we subgrouped the studies according to whether the participants had a mean BMI of < 30 kg/m2 (10 studies58,59,63,66,75,78,79,101,103,110) or of at least 30 kg/m2 (three studies85,102,104). Four studies52–54,82 did not report baseline characteristics for BMI. There was no statistically significant difference between the two subgroups (see Appendix 2, Figure 43).
-
Age. We subgrouped the studies according to whether the participants had a mean age of < 60 years53,59,63,66,85,101,104 or of at least 60 years. 52,54,58,75,78,79,82,102,103,110 There was no statistically significant difference between the two subgroups (see Appendix 2, Figure 44).
We also conducted sensitivity analyses on this meta-analysis (see Appendix 4, Table 34). We noted that the estimate clearly favoured the intervention group when we excluded studies that were deemed to be at a high or an unclear risk of selection bias for random sequence generation (SMD 0.17, 95% CI 0.01 to 0.33; participants, n = 2167; studies, n = 12; I2 = 63%), or we excluded studies at a high risk of attrition bias (SMD 0.34, 95% CI 0.00 to 0.68; participants, n = 913; studies, n = 6; I2 = 77%). We noted moderate to considerable levels of statistical heterogeneity in these estimates.
The following additional data also provided evidence for this measure:
-
Two studies95,97 measured the outcome using the EQ-5D and reported the outcome based on change from baseline. Lindbäck et al. 95 reported the outcome based on change from baseline to 12 months, whereas Lotzke et al. 97 reported the outcome based on change from baseline to 6 months. When analysing these studies, we found little or no difference between groups in this outcome according to whether an intervention or usual care was provided (MD –0.05, 95% CI –0.11 to 0.01; participants, n = 315; studies, n = 2; I2 = 0%; favours usual care) (see Appendix 2, Figure 45).
-
One study69 measured the outcome using the Functional Assessment of Cancer Therapy – Breast cancer plus arm subscale (FACT-B+4), and reported the outcome based on change from baseline to 12 months. For this study we found little or no difference between groups in this outcome according to whether an intervention or usual care was provided (MD 3.70, 95% CI –1.48 to 8.88; participants, n = 126; studies, n = 1; favours intervention) (see Appendix 2, Figure 46).
-
One study106 measured the outcome using the PCS of the SF-36; the outcome was reported as the difference between groups, and measured at 24 months post surgery. The study authors reported little or no difference in the outcome according to whether an intervention or usual care was provided (the study authors reported the p-value as not significant; participants, n = 42) (see Appendix 3, Table 17).
-
Taraldsen et al. 107 reported the outcome data based on between-group differences at 6 months post intervention. The study authors reported little or no difference in HRQoL according to whether an intervention or usual care was provided (p = 0.965; participants, n = 113) (see Appendix 3, Table 17).
Pain
Eleven studies52,58,59,79,84,95,97,103,104,106,110 measured pain using a range of measurement scales.
Seven studies52,58,59,79,103,104,110 reported pain as a set of post-intervention values. Three studies58,59,104 measured pain using the relevant subscore of the SF-36 HRQoL tool. Of these, Brandes et al. 58 and Cadmus et al. 59 measured the outcome at 6 months, whereas Sellberg et al. 104 measured the outcome at 1 year. Two studies52,110 measured pain using the relevant subscore of the KOOS HRQoL tool; both of these studies measured the outcome at 6 months. Heiberg et al. 79 measured pain using the relevant subscore of the HOOS HRQoL tool and measured the outcome at 5 years. Santa Mina et al. 103 measured pain using the Pain Disability Index, and measured the outcome at 6 months. Whereas all previously mentioned measurements report pain using a scale whereby a higher score indicates a better outcome, the Pain Disability Index reports pain using a scale whereby a higher score indicates a worse outcome. To avoid a unit-of-analysis error, we inverted the data for Santa Mina et al. 103 When analysing these studies, we found little or no difference between groups in pain according to whether an intervention or usual care was provided (SMD 0.19, 95% CI –0.14 to 0.53; participants, n = 602; studies, n = 7; I2 = 71%) (Figure 12); we noted a substantial level of statistical heterogeneity in this effect. Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 35).
FIGURE 12.
Combined data: pain measured at the end of follow-up using a range of pain scores. df, degrees of freedom; IV, inverse variance.
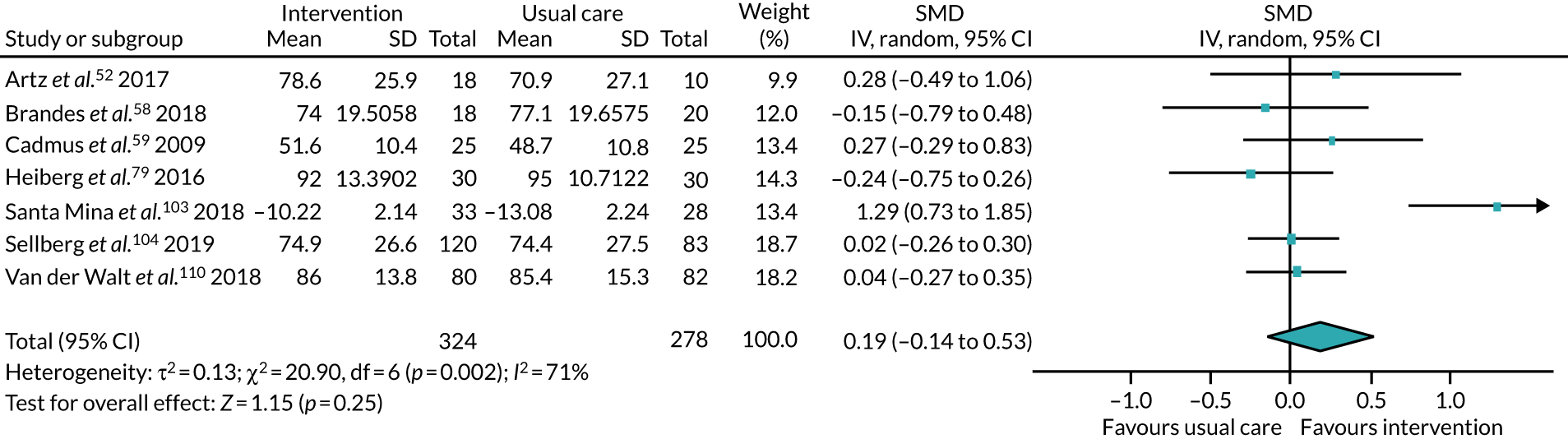
In addition, two studies95,97 measured back pain and leg pain using the region-specific VAS, and reported their data based on changes from baseline; in this scale, a lower score indicates less pain. Lindbäck et al. 95 measured the outcome at 12 months, whereas Lotzke et al. 97 measured the outcome at 6 months. When analysing the data for back pain, we found little or no difference between groups in this outcome according to whether an intervention or usual care was provided (MD 5.45, 95% CI –1.03 to 11.92; participants, n = 315; studies, n = 2; I2 = 0%; favours intervention). Similarly, when analysing the data for leg pain, we found little or no difference between groups in this outcome according to whether an intervention or usual care was provided (MD 2.00, 95% CI –6.19 to 10.18; participants, n = 315; studies, n = 2; I2 = 0%; favours intervention) (see Appendix 2, Figure 47). Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 35).
The following additional data also provided evidence for this measure:
-
Ilves et al. 84 measured back and leg pain using the region-specific VAS, and reported their data based on changes from baseline to 12 months post intervention; in this scale, a lower score indicates less pain. The study authors reported the data using median (IQR). They reported little or no difference in pain according to whether an intervention or usual care was provided (back pain, p = 0.76; leg pain, p = 0.40) (see Appendix 3, Table 18).
-
Stolberg et al. 106 measured bodily pain as a subscore of the SF-36 scale, and reported their data as a difference between groups 24 months post surgery. The study authors reported a non-significant difference (see Appendix 3, Table 18).
Adverse events
Seventeen studies51,56,59,61,66,69,76,77,79,82,83,97,102–104,107,109 reported information regarding adverse events. Of these, seven51,56,59,79,97,104,109 reported that no participants experienced adverse events that were related to the intervention.
Ten studies61,66,69,76,77,82,83,102,103,107 reported numbers of adverse events that were related or unrelated to the intervention, or were serious or not serious. Table 1 presents a summary of these events.
| Study | Data for adverse events | Related or unrelated to study |
|---|---|---|
| Christiansen et al.61 | Intervention group: 16 falls among 11 participants | No falls during study-related activity or exercise |
| Comparison group: 13 falls among 9 participants | ||
| Demark-Wahnefried et al.66 | Intervention group: participants reported 137 total events (35 serious and 102 non-serious) | Not reported |
| Comparison group: participants reported 142 total events (39 serious and 103 non-serious) | ||
| Eakin et al.69 | Intervention group: 3 (muscle soreness: 2; musculoskeletal injury: 1). None was serious and none required discontinuation of participation | Described as possibly related to the intervention |
| Hackshaw-McGeagh et al.76 |
|
Three events were ‘possibly related’ |
| Hauer et al.77 | No major health problems occurred during training or testing. Minor problems included aching muscles after initial training sessions, cramps, tenderness, knee pain and wound/scar aching. All these problems were resolved by adjustment of training and physiotherapy | Not reported |
| Hubbard et al.82 | One participant was unable to start cardiac rehabilitation owing to a torn knee ligament | Described as unrelated to the study |
| Husebø et al.83 | One participant in the intervention group reported knee discomfort and was referred to her primary physician for further evaluation. The participant stayed in the trial and completed the exercise prescription. Another participant in the intervention group experienced syncope during the walking exercise. This was related to a secondary chronic condition, and the patient was advised by her oncologist to withdraw from the trial | These events were considered as possibly related to the study |
| Piva et al.102 | Clinic based (n = 96 participants)
|
There were no serious adverse events related to study participationHowever, the events listed were described as being study related |
Community based (n = 96 participants)
|
||
Comparison group (n = 48 participants)
|
||
| Santa Mina et al.103 | Intraoperative adverse events were more common in the intervention group than in the control group. Most adverse events in the intervention group (3/^) were anastomotic leak | No serious events associated with the intervention. Any other adverse events lacked obvious plausible connection with the intervention |
| Taraldsen et al.107 | Adverse events were defined as any undesirable experience during the intervention and follow-up period reported to the monitoring committee by the physiotherapists responsible for the research intervention. A medical doctor determined the relatedness to the intervention of the events reportedSix adverse events were reported during the intervention period; two of these were described as serious | Described as unrelated to the intervention |
Adherence
Fifteen studies52,53,59,63,69,76,77,82–84,92,96,103,106,108,109 reported adherence data (Table 2).
| Study | Description of intervention group (n participants) | Adherence data (as reported by study authors) |
|---|---|---|
| Artz et al.52 | 6-week intervention (23) | 73% of participants attended classes; 57% of participants attended all six classes; 17% of participants attended five classes |
| Baillot et al.53 | Counselling every 6–8 weeks, exercise sessions three times per week; delivered by PA specialist (counselling and exercise sessions) and dietitian (counselling only) over 18 months (15) | Participants attended a median of 70% (45–90%) of the recommended sessions (three times per week); 47% of participants attended > 70% of sessions |
| Cadmus et al.59 | Telephone behavioural model for increasing or maintaining PA; self-selected MVPA (most selected walking) 5 days per week for 6 months (25) | 64% met the goal of 150 minutes of exercise per week. Participants returned a mean of 23.1 (SD ± 8.1) weekly logs; 72% of participants returned all 26 logs |
| Courneya et al.63 | Multiarm: AET group [use of cycle ergometer, treadmill or elliptical trainer, with goal of VO2 peak; three times per week throughout duration of chemotherapy (median 17 weeks)] and RET group [performing two sets of 8–12 repetitions of nine different exercises three times per week throughout duration of chemotherapy (median 17 weeks)], post surgery (AET: 68; RET: 73) |
|
| Eakin et al.69 | Sixteen calls of 15- to 30-minutes’ duration over 8 months (once per week for 2 months, once per fortnight for 2 months and once per month for 4 months) (73) | 93% completed a median of 14 out of 16 (range 5–16) calls with their exercise physiologist; 79% completed the majority (≥ 75%) of calls |
| Hackshaw-McGeagh et al.76 | Brisk walking at least 5 days per week in addition to usual PA, remote structured support via method of choice (text message, telephone, e-mail, post) from research nurse seven times over 6 months (42) | 53.8% (95% CI 38.6% to 68.4%; 21/39) had 90% adherence (defined as doing ≥ 30 minutes of walking on 18 of the 28 days) |
| Hauer et al.77 | 3 days per week, 36 sessions, approximately 1 hour and 45 minutes for 12 weeks (15) | 93.1% (SD ± 13.5%) adhered to the intervention [median 99% (range 55–100%)]; data available for those who started group sessions (13 participants) |
| Hubbard et al.82 | Exercise sessions once or twice per week over 6–12 weeks (21) | 62% completed the programme |
| Husebø et al.83 | Home-based exercise programme that combined strength and aerobic training performed throughout the time period of adjuvant chemotherapy (33) | 58% met the general recommendations of 150 minutes per week of MVPA; 17% adhered to the walking prescription of a minimum of 210 minutes per week of MVPA. Participants carried out approximately two sessions of resistance-band exercises per week, and 15% of the participants in the intervention group achieved the prescribed number of strength training sessions |
| Ilves et al.84 | Instructed to perform exercises at least two or three times per week for 12 months (52) | Frequency of back-specific exercises during first 2 months: median 2.5 (IQR 1.9–3.4). Frequency during last 2 months: median 1.4 (IQR 0.6–1.9) |
| Lear et al.92 | Cardiac rehabilitation exercise sessions with lifestyle and risk factor counselling and home exercise plan, delivered monthly with exercise specialist, dietitian and case manager over a 12-month period (151) | 83% attended exercise sessions |
| Santa Mina et al.103 | Aerobic and resistance training prescription (based on baseline assessment) and provision of exercise equipment (resistance band, stability balls, yoga mat), three or four times per week, pre surgery (typically 4–8 weeks) (44) | 68.4% met the minimum target exercise volume for the total exercise programme |
| Stolberg et al.106 | Twice-weekly physical training sessions, supervised by a physiotherapist, access to fitness centre, delivered over 26 weeks (32) | 59.4% had an acceptable compliance, defined as attending ≥ 50% of the planned training sessions |
| Turunen et al.108 | Combination of home visits, functional exercises and PA motivational counselling, delivered by a physiotherapist at approximately 10 time points across 12 months (40) | Compliance with the home-based physical exercises in the first 6 months was fair: strengthening, 61%; stretching, 53%; balance, 65%; and functional exercises, 69%. Thereafter, the values for the strengthening, stretching and balance exercises were 39%, 37% and 43%, respectively. Compliance with the face-to-face PA counselling session was 98% in the first 6 months and 88–90% thereafter |
| Turunen et al.109 | Seven physiotherapy home visits to supervise and adjust the exercise plan, with three telephone support calls, independent exercise and outdoor walking, delivered over 6 months (59) | 58% performed all the strengthening exercises; 53% performed balance exercises, 68% performed walking exercises, 15% visited a gym and 7% visited a swimming facility with the physiotherapist |
Participant experiences of the interventions
Four studies52,69,76,82 reported data on participants’ experiences of the interventions. Table 3 presents a summary of these data.
| Study | Description of intervention (n participants) | Experience data |
|---|---|---|
| Artz et al.52 | Postoperatively; a combination of education and advice, and direct physical instruction; generic aerobic and resistance; not frequently; to participants’ preferences; clinical setting with a physiotherapist; low contact for < 6 months (23) | A total of 17 participants provided feedback about the exercise class. All participants felt that the 1-hour duration of the session was the right amount of time to exercise, but three (18%) participants would have liked to receive more than six sessions. Overall, participants were satisfied with the range of exercises offered, with 15 (84%) participants reporting that they were ‘very satisfied. All patients felt that the exercise class met their individual functional needs and many provided positive feedback about the exercise classReproduced with permission from Artz et al.52 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 3.0 License (https://www.creativecommons.org/licenses/by-nc/3.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage)2016The Author(s)https://www.creativecommons.org/licenses/by-nc/3.0/ |
| Eakin et al.69 | Postoperatively; a combination of education and advice, and behavioural mechanisms; walking, and generic aerobic and resistance; to participant’s capacity and preferences; at home through remote delivery; with physiotherapist; intermediate contact (73) | In recovery for breast cancer the majority of participants in the telephone group provided positive feedback for each component of the intervention. For the telephone sessions in particular, > 90% of the participants rated them as helpful. In addition, most participants reported that had the EfH programme been delivered face to face or over the internet they would have been interested in taking part |
| Hackshaw-McGeagh et al.76 | Postoperatively; a combination of education and advice, and behavioural mechanisms; walking with frequent bout-related goals; at home through remote delivery; with nurse; low contact for 6 months (42) | Participants were predominantly positive with regards to the acceptability of the [. . .] physical activity interventions, with men indicating that they felt enabled to make the changes requested and to sustain them following completion of the trial. [. . .] Most felt that they were able to wear the physical activity monitors provided and record their daily steps. Two participants even stated that they found the monitors to be useful motivational tools and were purchasing their own at the end of the trial so that they could continue to monitor their daily steps. [. . .] Preferences made by some men to the type of physical activity they do, particularly to cycling, highlighted the importance of considering a choice of exercise regime or a more varied regime for future studies in this populationReproduced with permission from Shingler et al.114 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated2017The Author(s)https://creativecommons.org/licenses/by/4.0/ |
| Hubbard et al.82 | Postoperatively; a combination of education and advice, behavioural mechanisms, and direct physical instruction; non-specific, low frequency to participant’s capacity and preferences; clinical and community setting; MDT; intermediate contact for < 6 months (21) | Participants in the control and intervention group believed that rehabilitation was an important part of their recovery. [. . .] There were two main reasons why people with CRC agreed to participate in this study: they believed that it might help them and/or they believed that it might help others. In particular, some participants welcomed involvement in a study that was about physical activity because they believed physical activity was beneficial. [. . .] Given the favourable comments made about cardiac rehabilitation by those participants with CRC allocated to the intervention group, it is perhaps not surprising that the overall impression of cardiac rehabilitation was that it was very good, and that many participants would have liked to have remained on the programme for much longerReproduced with permission from Hubbard et al.82 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated2016The Author(s)https://creativecommons.org/licenses/by/4.0/ |
Grading of Recommendations Assessment, Development and Evaluation, and certainty of the evidence: intervention versus usual care
Amount of physical activity (37 studies, 4969 participants)
We found moderate-certainty evidence that PA interventions may increase the amount of PA in the ‘medium term’ after surgery. Most studies measured this outcome at either 6 or 12 months after surgery.
This outcome was evaluated using a range of measurement values that we could not combine in a single analysis: minutes of PA per day or week, energy expenditure measures such as kcal/kg/day, METs/minutes/week, steps/day, daily activity scores or results from activity-related questionnaires.
Physical activity was most commonly recorded as minutes per day or week (12 studies, 1947 participants), and analysis revealed a small increase in PA among participants who received the intervention. We similarly found a small increase in PA when reported as steps/day or from questionnaire data. Although energy expenditure measures, METs/minutes/week and the results of daily activity scores showed both a reduction and increase in PA when participants received the intervention, we noted that the effect size in all these analyses favoured the intervention. We believe that there was a consistent finding across all measures that the intervention may increase the likelihood that people would do more PA.
Engagement in physical activity (10 studies, 1097 participants)
We found moderate-certainty evidence that PA interventions probably slightly increase people’s engagement in PA, compared with usual care. For this outcome, we found that 60 more participants per 1000 would still be engaging in PA at the end of follow-up after receiving the intervention. However, we noted a wide CI for the data from this outcome, indicating that some people receiving the intervention will do less PA.
Physical fitness (15 studies, 1031 participants)
Although we noted that effects generally favoured the PA intervention, we found low-certainty evidence that PA interventions may improve physical fitness in the ‘medium term’ after surgery. Most studies measured this outcome at either 6 or 12 months.
This outcome was evaluated using various measures, and the differences in these measures of fitness may reflect the age of participants or the reason for surgery, or both. This outcome was measured using walking tests (such as the 6MWT), exercise tolerance tests (measured as METs maximum), sit-to-stand tests (such as the TUG test), handgrip strength tests, leg-press tests and VO2 peak tests. It was not feasible for us to combine the data when the measurement tools were so different, and most of our effects were derived from single studies or from two studies. In general, we noted a similar trend that suggested an improvement in fitness when people had received a PA intervention.
Health-related quality of life (22 studies, 3051 participants)
We found moderate-certainty evidence that PA interventions probably slightly increase HRQoL at the end of follow-up.
This outcome was evaluated using a range of measures that allowed for the combining of data using a SMD. The tools used were appropriate measures of quality of life, with some of these measures specific to the surgery within the study criteria (e.g. KOOS and FACT-Anaemia). Although the primary analysis showed a slight reduction in HRQoL as well as an increase, the findings more clearly favoured the PA interventions once we removed studies rated as having a high or an unclear risks of bias from the analysis.
Pain (11 studies, 1057 participants)
Again, the findings for pain tended to favour the intervention. However, the estimates were all imprecise and included possible benefits as well as harms; we judged the certainty of this evidence to be low.
Adherence (15 studies, 766 participants randomised to intervention groups)
Among studies clearly reporting this outcome, the level of adherence ranged from 47% to 93%. We noted that 93% of studies reported that adherence to the intervention was > 53% and that 20% reported that adherence was > 79%. However, the definitions of adherence varied between studies, and, because the designs of the interventions differed significantly, it was not reasonable to draw confident conclusions about adherence to all PA interventions; we judged the certainty of this evidence to be very low.
Adverse events (10 studies, 1410 participants)
We could not be certain of the risk of adverse events for interventions promoting PA because few studies reported adverse events data; we judged the certainty of this evidence to be very low. We noted that most events were described as not serious, and that most events were unrelated to the intervention. The few events described as possibly related to the intervention included muscle soreness, musculoskeletal injury, indigestion, abdominal bloating, knee pain/discomfort, syncope, arthralgia, back pain, myalgia, falls, musculoskeletal/connective tissue effects and skin/subcutaneous tissue effects; these events were reported for 30 participants.
Participant experience of intervention (four studies, 159 participants randomised to intervention groups)
Very few studies reported details of participants’ experiences. In the four studies that clearly reported this information, feedback was generally positive and participants were satisfied and/or felt that they had benefited from being able to engage with the intervention. We did not downgrade the certainty of this narrative evidence.
Reasons for downgrading the evidence
We used GRADE to assess the certainty of the evidence in this review.
Using our risk-of-bias assessments, we considered the impact of this on the data. Most outcomes included some studies that had an unclear risk of selection bias (often because of inadequate reporting in the studies of their methods for randomisation), and the Q-RCTs were at high risk of bias. In addition, we noted that some studies had high attrition rates for longer-term measures, which were not always adequately explained. We explored these risks of bias in sensitivity analyses. For most outcomes, we did not observe that these study limitations influenced the effect estimates of our primary analyses such that our interpretation of the findings was altered. For HRQoL, we found that removing studies in a sensitivity analysis made us more confident in the effectiveness of physical activities to slightly improve quality of life.
All the studies were inevitably at a high risk of performance bias and were also at a high risk of detection bias. We expected that being included in a trial that encourages a person to exercise could influence a participant’s reporting on the amount of PA or level of engagement with PA at the end of follow-up. We therefore downgraded all the evidence in this review by one level for serious risks of bias.
We considered consistency within an effect estimate (using evidence of statistical heterogeneity and observation of CIs in each study). We also considered whether or not effects were consistent when different measures were used for each outcome. We were not surprised by the high levels of statistical heterogeneity in some of our findings. We expected that some of this may have been caused by our decision to pool studies with different surgical population groups and a wide range of different types of interventions.
We noted some apparent inconsistencies in some of the analyses for ‘amount of PA’; for example, one analysis using energy expenditure measures produced potentially considerable statistical heterogeneity. We explored the possible causes of this heterogeneity, removing studies judged to have a high risk of both selection and attrition biases, and found that this reduced heterogeneity to an unimportant level; we are confident that our interpretation of the effect for this outcome was unaffected by inconsistency.
We downgraded for imprecision when we noted that the CI in most of the effect estimates for an outcome included harms as well as benefits. We therefore downgraded the evidence for measures of physical fitness and pain by one level for imprecision. Adverse events were reported by few of the included studies, and events were rare; we downgraded this outcome by two levels for imprecision. Similarly, the adherence was often not reported and the differences between studies and the descriptions for adherence meant that imprecision in the findings was inevitable; we also downgraded this outcome by two levels for imprecision.
We generated a funnel plot for outcomes with > 10 studies: only ‘amount of physical activity measured as minutes per day or per week’ and HRQoL included > 10 studies. When generating funnel plots, we noted very few outliers in terms of data distribution (Figures 13 and 14). However, we were unconcerned about publication bias as these were small studies with few participants, and the removal of their data reduced any statistical heterogeneity and increased the overall effect in favour of the intervention. Although we did not downgrade any of the evidence for publication bias in this review, we did not assess this in most of our outcome measures. Because these measures included several small studies, we could not confidently infer that the evidence did not include risk of publication bias or small-study effects.
FIGURE 13.
Amount of PA measured as minutes per day or per week. SE, standard error.
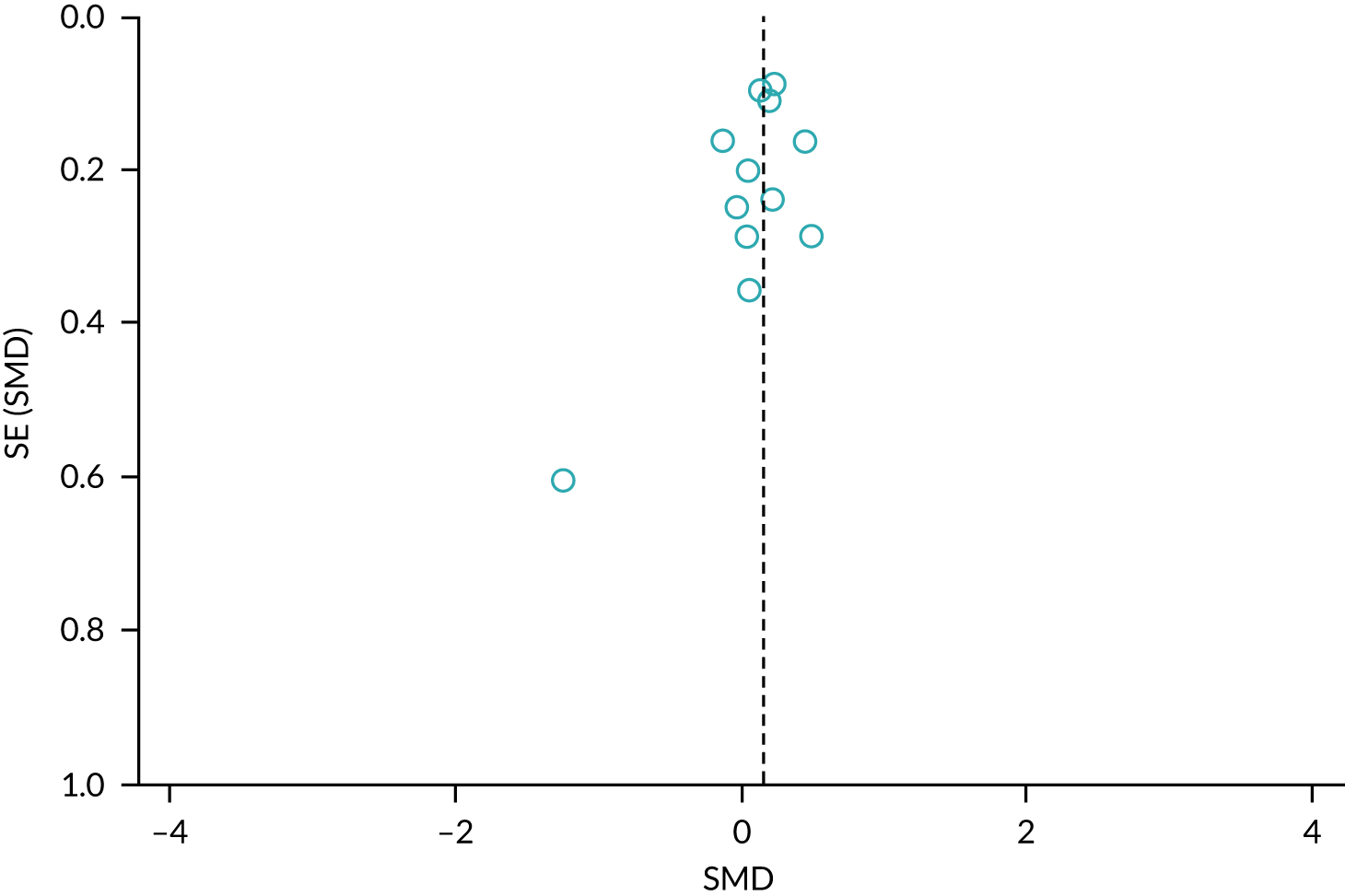
FIGURE 14.
Health-related quality of life measured using a range of measurement tools. SE, standard error.
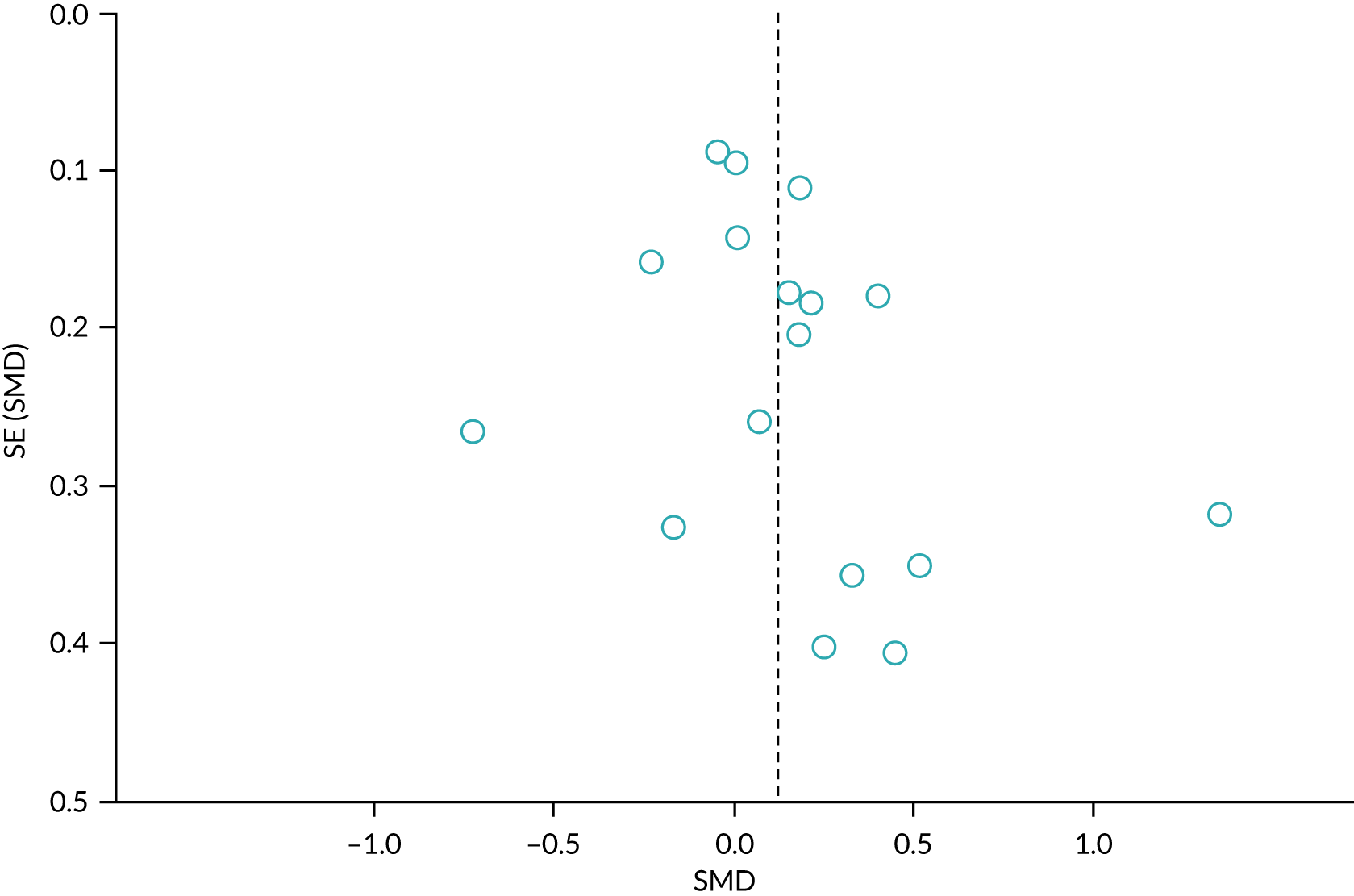
We included in the review only studies that recruited eligible participants and conducted relevant interventions. We therefore did not downgrade any of the evidence in the review for indirectness.
Intervention versus intervention
Amount of physical activity
In one three-arm study,56 participants exercised during indoor cycling sessions using a heart rate reserve method (with resistance controlled using a heart rate feedback mechanism), a self-regulation method (with participants using their own perception to determine intensity) or a combination of both. Study investigators measured the outcome at 2 years, and used a questionnaire modelled on the Harvard Alumni Study questionnaire to evaluate energy expenditure. In analysis, we compared the self-regulation method with the combined method and found little or no difference between these methods in the amount of PA at the end of follow-up (MD 723.00 kcal/week, 95% CI –409.33 to 1855.33 kcal/week; participants, n = 48; studies, n = 1; favours combined method) (see Appendix 2, Figure 48). Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 36).
One study51 measured PA in activity counts per minute. This study compared data from a group receiving a cognitive–behavioural physical therapy (CBPT) intervention with data from a group receiving an education intervention. Study investigators measured the outcome at 12 months. In this study, we found little or no difference in the number of activity counts per minute according to the type of intervention (MD –6.13, 95% CI –66.77 to 54.51; participants, n = 198; studies, n = 1; favours education intervention) (see Appendix 2, Figure 49).
One study87 measured amount of PA using the Godin–Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ). This study compared data from a group receiving a centre-based intervention with data from a group receiving a home-based intervention, and measured the outcome at 24 months. In this study, we found little or no difference in the amount of PA according to the type of intervention (MD –0.12, 95% CI –0.86 to 0.62; participants, n = 461; studies, n = 1; favours home-based intervention) (see Appendix 2, Figure 50).
One study90 measured the amount of PA using the Physical Activity Level (PAL) score; this measure combines data from an accelerometer with data from a heart monitor. The study compared data from a group receiving a home-based intervention with data from a group receiving a centre-based intervention, and measured the outcome at 12 months. In this study, we found little or no difference in the amount of PA according to which type of intervention was provided (MD 0.01, 95% CI –0.45 to 0.47; participants, n = 78; studies, n = 1; favours home-based intervention) (see Appendix 2, Figure 51).
Engagement in physical activity at the end of follow-up
This outcome was measured in three studies,86,88,105 which we did not pool because of differences in intervention designs.
Johansson et al. 86 compared a clinic-based intervention with a home-based intervention, and measured the outcome at 12 months. For this study, we found that more people were engaged in PA at the end of follow-up after receiving the clinic-based intervention (RR 1.25, 95% CI 1.03 to 1.52; participants, n = 57; studies, n = 1; favours clinic-based intervention) (see Appendix 2, Figure 52).
Smith et al. 105 compared a home-based intervention with a hospital-based intervention, and measured the outcome at 6 years. For this study, we found little or no difference in engagement in PA at the end of follow-up according to which type of intervention was provided (RR 1.30, 95% CI 0.90 to 1.87; participants, n = 108; studies, n = 1; favours home-based intervention) (see Appendix 2, Figure 53).
Kinsey et al. 88 compared a home walking intervention with a home cycling intervention, and measured the outcome at 4 years. The study authors did not report the data by group, and so we could not determine any differences between groups (see Appendix 3, Table 19).
Physical fitness
This measure was assessed in two studies. 90,105 Kraal et al. 90 compared a group receiving a home-based intervention with a group receiving a centre-based intervention, and measured the outcome at 12 months using a unit of measurement of ml O2/kg/minute. Smith et al. 105 compared a group receiving a home training intervention with a group receiving a hospital training intervention, and measured the outcome at 6 years using a unit of measurement of ml/minute. Because these studies both compared a home-based intervention with a centre-based intervention, we judged their designs to be similar, and pooled the data. We found little or no difference between home- and centre-based interventions with this measure of physical fitness (SMD 0.21, 95% CI –0.07 to 0.50; participants, n = 222; studies, n = 2; I2 = 11%; favours home-based intervention) (see Appendix 2, Figure 54). Exploration of the data in sensitivity analyses identified no differences in our interpretation of this effect (see Appendix 4, Table 37).
One study,87 which compared a centre-based intervention with a home-based intervention, used the Incremental Shuttle Walk Test to measure this outcome at 24 months. In this study, we found little or no difference between groups in distance walked according to which type of intervention was provided (MD –8.20 m, 95% CI –43.85 m to 27.45 m; participants, n = 342; studies, n = 1; favours home-based intervention) (see Appendix 2, Figure 55).
One study,105 which compared a home-based intervention with a hospital-based intervention, used a symptom-limited exercise test (METs maximum; a higher score indicates greater fitness) and measured the outcome at 6 years. In this study, we found that people scored higher in this outcome when they had received the home-based intervention (MD 0.50 METs maximum, 95% CI 0.09 to 0.91 METs maximum; participants, n = 144; studies, n = 1; favours home-based intervention) (see Appendix 2, Figure 56).
Health-related quality of life
Five studies51,86,87,90,105 measured HRQoL:
-
Smith et al. 105 measured the outcome using the PCS of the SF-36, and compared a group receiving a home training intervention with a group receiving a hospital training intervention; the study investigators measured the outcome at 6 years. In this study, we found little or no difference in HRQoL between the home and the hospital training groups at the end of follow-up (MD –2.30, 95% CI –5.70 to 1.10; participants, n = 144; studies, n = 1; favours centre-based intervention) (see Appendix 2, Figure 57).
-
Jolly et al. 87 compared a centre-based intervention with a home-based intervention and measured the outcome at 24 months using the EQ-5D. In this study, we found little or no difference in HRQoL between the centre- and the home-based intervention groups at the end of follow-up (MD 0.02, 95% CI –0.03 to 0.07; participants, n = 454; studies, n = 1; favours centre-based intervention) (see Appendix 2, Figure 58).
-
Kraal et al. 90 measured the outcome using the total score from the MacNew Heart Disease Health-Related Quality Of Life Questionnaire, which uses a scale from 1 to 7, whereby a higher score indicates a higher quality of life. The study investigators compared a group receiving a home-based intervention with a group receiving a centre-based intervention, and measured the outcome at 12 months. In this study, we found a small improvement in HRQoL at the end of follow-up when participants had received the home-based intervention (MD 0.32, 95% CI 0.27 to 0.37; participants, n = 78; studies, n = 1; favours home-based) (see Appendix 2, Figure 59).
-
Johansson et al. 86 measured the outcome using the EQ-5D and compared a clinic-based intervention with a home-based intervention and measured the outcome at 12 months. We did not calculate an effect estimate because the data were reported with median values. The study authors reported little or no difference between groups in HRQoL (p = 0.35; participants, n = 57) (see Appendix 3, Table 20).
-
Archer et al. 51 measured HRQoL using the PCS of the Short Form questionnaire-12 items compared a group receiving a CBPT intervention with a group receiving an education intervention and measured the outcome at 12 months. For this study we found little or no difference in the outcome according to whether a CBPT intervention or an education intervention was provided (MD 1.82, 95% CI –1.44 to 5.08; participants, n = 229; studies, n = 1; favours CBPT) (see Appendix 2, Figure 60).
Pain
Three studies51,86,87 measured pain using various components:
-
Archer et al. 51 measured back and leg pain using the region-specific Brief Pain Inventory, in which a higher score indicated a worse outcome. The study investigators compared a group receiving a CBPT intervention with a group receiving an education intervention, and reported the outcomes at 12 months. When analysing the data for back pain, we found little or no difference between CBPT and education intervention groups (MD 0.12, 95% CI –0.54 to 0.78; participants, n = 229; studies, n = 1; favours education). Similarly, when analysing the data for leg pain, we found little or no difference between the CBPT and the education intervention groups (MD –0.46, 95% CI –1.17 to 0.25; participants, n = 229; studies, n = 1; favours CBPT) (see Appendix 2, Figure 61).
-
Jolly et al. 87 measured pain using self-reported chest pain on movement; a higher score indicated a worse outcome. The study investigators reported the outcome at 24 months, and compared a group receiving a centre-based intervention with a group receiving a home-based intervention. When analysing these data, we found little or no difference between the centre- and the home-based intervention groups (MD 0.09, 95% CI –0.09 to 0.27; participants, n = 342; studies, n = 1; favours home-based intervention) (see Appendix 2, Figure 62).
-
Johansson et al. 86 measured the outcome using the region-specific VAS in which a lower score indicates a better outcome. The study investigators measured back pain and leg pain, and reported the outcomes at 12 months. They also compared a group receiving a clinic-based intervention with a group receiving a home-based intervention. We did not calculate an effect estimate for these data, which were reported as median values. The study authors reported a reduction in back pain for participants receiving the home-based intervention (p = 0.04; participants, n = 57) (see Appendix 3, Table 21).
Adverse events
Two studies87,90 reported information regarding adverse events. Both studies reported numbers of adverse events. These events are summarised in Table 4.
| Study | Data for adverse events | Related or unrelated to study |
|---|---|---|
| Jolly et al.87 | Centre based, n (%)
|
Not reported |
Home based, n (%)
|
||
| Kraal et al.90 | Centre based (n)
|
Not reported |
Home based (n)
|
Adherence
One study90 reported adherence data; Table 5 shows the data for this study.
| Study | Description of intervention groups (n participants) | Adherence data by group (as reported by study authors) | |
|---|---|---|---|
| 1 | 2 | ||
| Kraal et al.90 |
|
Exercise sessions completed, mean 22.0 (SD ± 6.8) | Exercise sessions completed, mean 20.6 (SD ± 4.3) |
Participant experience of intervention
One study87 reported data on participants’ experiences of the interventions; Table 6 presents this data.
| Study | Description of intervention (n participants) | Experience data |
|---|---|---|
| Jolly et al.87 |
|
Centre-based programme quotation:Patients enjoyed exercising in a group and mixing with other people was a positive experience for all the patients. They described this as like belonging to one community and being related to each other. Patients gained both motivation and support from other patients. [. . .] Patients described a sense of achievement and increasing confidence as they progressed through the exercise programme. [. . .] Views on the education sessions were more mixed and the quality of the talks was variable. The education sessions also appeared to be less well organised as the speakers did not always arrive as expected or talks were repeated and patients were given the same talk at more than one session. Comments included ‘some were boring, some were good’, ‘every week you learn something’, ‘a couple of times I was there and nobody came’, ‘too much talking’, ‘very informative’, ‘very helpful’ and ‘I enjoyed the talks’. However, in general patients thought the education programme helped them learn more about what had happened to them and how to improve their lifestyleJolly et al.87© Queen’s Printer and Controller of HMSO 2007. Contains public sector information licensed under the Open Government Licence v3.02007© Queen’s Printer and Controller of HMSOhttps://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/Home-based programme quotation:Patients thought the exercises were well planned and the gradual build-up helped to build confidence as initially patients found exercising difficult. [. . .] A few patients said they were worried about exercising on their own, especially early on in the programme, and were reluctant to push themselves. [. . .] Recording the exercises patients had completed could help with motivation as patients knew the nurse would be coming and would check up on them. Patients also found it encouraging to look back and see the progress they had made over the weeksJolly et al.87© Queen’s Printer and Controller of HMSO 2007. Contains public sector information licensed under the Open Government Licence v3.02007© Queen’s Printer and Controller of HMSOhttps://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/ |
Grading of Recommendations Assessment, Development and Evaluation, and certainty of the evidence: intervention versus intervention
Amount of physical activity (four studies, 785 participants)
We were uncertain whether or not there was any difference between the interventions in the amount of PA at the end of follow-up; we judged the certainty of the evidence to be very low.
In each study, the estimates indicated little or no difference between intervention groups when measured at 12 months or 24 months. The outcome was evaluated using a different measurement tool in each study: energy expenditure measures, activity counts per minute, the GSLTPAQ and the PAL score.
Engagement in physical activity at the end of follow-up (three studies, 204 participants)
We were uncertain whether or not there was any difference between the interventions in engagement in PA; we judged the certainty of the evidence to be very low.
In one study,86 the evidence indicated that more participants receiving a clinic-based intervention engaged in PA at 12 months than participants who had received a home-based intervention. However, this finding was not demonstrated in another study105 that compared a hospital-based intervention with a home-based intervention and found little or no difference between groups. Differences between the interventions in these studies meant that comparing the findings in these two studies was not appropriate.
Physical fitness (three studies, 564 participants)
We were uncertain whether or not there was any difference between the interventions in physical fitness; we judged the certainty of the evidence to be very low.
We pooled the data from two studies90,105 and found little or no difference between a home-based and a clinic-based intervention in VO2 peak. We similarly found little or no difference between interventions in the distance walked in a walking test. In one study,105 a home-based intervention showed improved results in exercise tolerance tests, compared with a clinic-based intervention.
Health-related quality of life (five studies, 962 participants)
We were uncertain whether or not there was any difference between the interventions in HRQoL; we judged the certainty of the evidence to be very low.
We did not pool the data from any of these studies because of the differences in the study designs. Only one small study90 (78 participants) noted an improvement in HRQoL when a home-based intervention was provided, rather than a centre-based intervention. Other studies, however, noted little or no difference in HRQoL between their intervention groups, and effects were often imprecise.
Pain (three studies, 628 participants)
We were uncertain whether or not there was any difference between the interventions in pain; we judged the certainty of the evidence to be very low.
Again, we did not pool the data from these studies. Only one small study86 (57 participants) noted an improvement in pain when a home-based intervention was provided, rather than a clinic-based intervention. However, this finding was not consistent with the other two studies, for which there appeared to be little or no difference between interventions in pain.
Adverse events (two studies, 615 participants)
We were uncertain whether or not any of interventions led to adverse events, and the certainty of this evidence was very low.
There were few adverse events reported in only two studies,87,90 and, because of limits in the reporting of these data, we could not ascertain whether these adverse events were related or unrelated to the interventions.
Adherence (one study, 90 participants)
It was not reasonable to draw confident conclusions about adherence to all PA interventions, as this was reported in only one study. 90 There appeared to be a comparable number of exercise sessions completed in both intervention groups in this study. We assessed the certainty of this evidence to be very low.
Participants’ experiences of the intervention (one study, 525 participants)
Only one study87 reported feedback on participants’ experiences. Participants in the centre-based intervention had enjoyed exercising in a group, and those who exercised at home were motivated by regular contact with a study nurse. We did not downgrade the certainty of this narrative evidence.
Reasons for downgrading the evidence
We judged the studies in this comparison to be at similar risks of bias to those in the intervention versus usual care comparison group, including some that had unclear risks of selection bias and high risks of attrition bias for some of the outcomes. All studies were also at a high risk of performance bias. We therefore downgraded all the evidence by one level for serious risks of bias.
Fewer studies contributed evidence in this comparison. Because of the differences in the types of interventions in both study arms, as well as the differences in measurement tools, it was often not feasible to pool the data. The effect estimates for most outcomes were often from single studies with a small number of participants. Effect estimates had wide CIs. We downgraded all the evidence by two levels for imprecision.
Consistency in the results was not of concern, largely owing to the absence of pooled data, and the limited number of studies meant that we did not formally assess risk of publication bias. The included studies in this comparison were all relevant to our review question. We therefore did not downgrade the certainty of the evidence for consistency, publication bias or indirectness.
Non-randomised studies
For a summary of the outcome data from the nine NRSs, see Appendix 5. We noted that the study authors reported statistically significant effects (p ≤ 0.05) in favour of the PA intervention for the following outcomes:
-
amount of PA at the end of follow-up (assessed using the IPAQ-SF) – Frawley et al. 73 (165 participants; at 6 months) and Heitkamp et al. 80 (50 participants; at 12 months)
-
amount of PA at the end of follow-up (assessed using GSLTPAQ) – Doganay et al. 67 (39 participants; at 13 months)
-
engagement in PA at the end of follow-up – Dantas et al. 65 (15 participants; at 6 months)
-
physical fitness at the end of follow-up [assessed using distance travelled (m) in walking tests] – Frawley et al. 73 (165 participants; at 6 months) and Macchi et al. 98 (131 participants; at 12 months)
-
physical fitness at the end of follow-up [assessed using work capacity (METs)] – Fontana et al. 71 (number of participants unknown; at 9 months)
-
physical fitness at the end of follow-up (assessed using handgrip strength) – Doganay et al. 67 (39 participants; at 13 months) and Frawley et al. 73 (165 participants; at 6 months)
-
physical fitness at the end of follow-up (assessed using VO2 peak) – Doganay et al. 67 (34 participants; at 13 months) and Heitkamp et al. 80 (50 participants; at 12 months)
-
HRQoL at the end of follow-up [assessed using the EORTC QLQ-C30 Global (0 to 100)] – Frawley et al. 73 (166 participants; at 6 months).
Because the NRSs provided a supplementary set of data to this review, we did not use GRADE to assess the certainty of this evidence.
Discussion
Brief summary of studies included in the review
We included 62 studies overall. Fifty-one were RCTs, and two were Q-RCTs; we included these within the description and data for RCTs. In addition, we found nine NRSs.
Of these studies, 46 RCTs compared one or more intervention with a usual care comparison, and seven RCTs compared an intervention with another intervention. We analysed these as two comparison groups, and we collected data for eight outcomes for these comparison groups.
We also reported the findings from the NRSs, but these data formed a supplementary set and we did not combine them with any data from the RCTs. We did not conduct risk-of-bias assessments on these studies or use GRADE to assess the certainty of the evidence for these study designs.
Overall completeness and applicability of evidence for the systematic review
The review included 8604 adult participants scheduled for, or who had recently had, elective surgery. In the RCTs, the types of surgery were cancer surgery (11 studies), cardiac surgery (12 studies), bariatric surgery (eight studies), and hip and knee surgery (12 studies), as well as individual studies for joint replacement and back surgery, lumbar fusion surgery, laminectomy, standard lumbar discectomy, surgery for degenerative lumbar spine disorder, lumbar surgery, renal transplantation, major digestive surgery and dysvascular transtibial amputation. Most studies included patients of a range of ages representative of the relevant surgical populations; seven studies recruited only participants who were at least 55 years of age.
Although the studies all described interventions that encouraged PA among people who were scheduled for, or had recently had, elective surgery, the design of interventions differed considerably. Among the RCTs, there were 67 distinct interventions overall, with some studies comparing two types of interventions, and two studies comparing three types of interventions. We explored the characteristics of the interventions and, although interventions typically involved multiple components or modes of delivery, we grouped the interventions into three broad categories: those that delivered education and advice, those that used behavioural mechanisms such as CBT, and those that involved direct PA instruction such as with group or one-to-one exercise classes. Differences in intervention strategies often related to a variety of contextual variables such as surgical procedure, illness type, population type and available resources.
Some interventions included additional resource components such as digital technologies, exercise diaries or other exercise equipment. Most interventions took place, in part, in participants’ homes, with others taking place in clinical settings, community settings (such as public or private gyms) or in inpatient care. One-third of interventions included some form of remote delivery. Most of the interventions were initiated after surgery, with only four taking place during the preoperative period. The duration of interventions ranged widely from 3 weeks to 26 months; half lasted < 6 months and only seven studies lasted > 1 year.
It is likely that services responsible for PA interventions had different motivators depending on the surgical indication. For example, after cardiopulmonary surgery, the aim may be to maintain and improve cardiopulmonary function, prevent cardiopulmonary events and improve function. In contrast, people managing their health following a cancer diagnosis may be engaged in PA to reduce symptoms of fatigue, improve quality of life and improve the effectiveness of their treatment. However, we inferred that the overarching purpose of PA in all studies was to improve overall general health, regardless of motivators in individual studies. We were unable to fully explore the impact of these potential different motivators on our findings because we had insufficient numbers of studies for all the clinical indications. We were similarly unable to explore the effect of the different intervention components in subgroup analyses, often because of a large overlap or because of a lack of information in published reports.
The types of measures for our outcomes (particularly for the amount of PA and physical fitness) were not consistent across studies, and we could not always combine data in analyses. We had only a few studies, with small numbers of participants, for many of these measures. We attempted to explore differences in our population (for oncological surgery vs. other types of surgery, for participants with a BMI of < 30 kg/m2 or > 30 kg/m2 and for participants who were aged < 60 years vs. those who were aged at least 60 years) or in the intervention characteristics (whether the intervention was initiated before or after surgery, or whether the intervention lasted < 6 months or at least 6 months). However, this was only possible in outcomes that had at least 10 studies (amount of PA measured as minutes per day or week, and HRQoL). In these subgroup analyses, we found no evidence of subgroup differences, but we could not be confident as to whether this reflected no real difference between subgroups or no evidence of a difference because of insufficient studies in each subgroup.
We did not limit our studies to those that specifically aimed to evaluate whether or not interventions enabled people to engage in PA in the long term. Limitations in reporting standards in many studies meant that the study objectives were not always clear (e.g. many did not include an ‘objectives’ statement or the outcome used in sample size calculation). Our evidence, therefore, included some studies that did not measure outcomes at a longer follow-up time point, and many did not include a delay between end of the intervention and measurement; this delay allowed us to establish if the intervention had been effective at self-regulated behaviour change. We expected that the lack of long-term follow-up in these studies could be explained by funding/resource limitations that mean that most studies have a short-term research period.
Relationship to existing literature: direction and magnitude of effect
The effect sizes of the interventions designed to promote PA were modest at best. However, the ‘headline’ MDs and RRs offer a deceptively simple account of a complex picture. This complexity stems from a number of factors:
-
The heterogeneity of care in the ‘usual care’ groups. Although the outcome measures express the difference between the result in one group (exercise intervention) with another group (usual care), the outcome measures are affected as much by the intervention in the ‘usual care’ groups as by the intervention in the activity promotion groups. As ‘usual care’ is not standardised between studies, this will affect the pooled differences between groups in a way that is unpredictable in both size and direction of effect.
-
The inclusion of more than one type of intervention component (education/advice, behavioural mechanisms and direct PA instruction) in many trials. As one component might be more or less effective than another (or, indeed, might worsen rather than improve intended outcomes), the net effect of this is also hard to predict.
-
The generally moderate recruitment rates in the studies. We do not know if there are random or systematic differences between the sorts of patients who are likely to participate in studies and those who do not. If there are differences, they are likely to have more influence the further that recruitment rates drop below 100%.
We are not aware of any other systematic reviews that have included such a broad surgical population or have considered such a range of interventions to promote PA. Similar to our findings for HRQoL, Coenen et al. 115 also found a slight improvement in quality life when ‘integrated programmes’ were used with orthopaedic surgical patients. Their systematic review included services that were additional to usual care provision, and also evaluated whether or not these programmes improved participation in PA; because of the smaller number of studies and the wider differences between interventions, data were not pooled for PA. The work by Steffens et al. ,116 for people undergoing cancer surgery, demonstrates an improvement in quality of life when there are higher levels of preoperative PA. Our own subgroup analysis, which included all indications for surgery, showed no evidence of overall improvement in HRQoL for preoperative interventions. A recent review conducted by van der Wardt et al. 117 found, in their meta-analysis of 24 studies of the effect of counselling about PA in primary care (not specific to surgical patients), that interventions showed a marginal effect on changing people’s PA behaviour. In a related area of practice, Mishra et al. 118 conducted a Cochrane systematic review of exercise interventions and quality of life among cancer survivors, published in 2012. Overall, the review included 3694 participants in 40 trials, and found beneficial effects of exercise on global HRQoL in a smaller number of participants at 12 weeks (SMD 0.48, 95% CI 0.16 to 0.81). The effect was still evident at 6 months, but data were available for only 115 participants; this is reflective of few clinical trials with long follow-up times.
Strengths and limitations
We used rigorous methods to search and independently assess study eligibility, extract data and assess risk of bias in the included RCTs. We reached consensus in decisions through discussion. We made changes to the methods section, which are described in Chapter 6. These changes were made once we had begun screening the results of our search. We identified a very large number of studies that met our broad criteria but did not fit with our review objectives. We therefore developed a more specific set of criteria that accounted for the time of initiation of the intervention and reported our primary outcomes (which we specified as ‘amount of PA’ and ‘engagement in PA’). Although this reduced the number of studies identified from our search, we believed that our criteria still allowed for the broad range of possible interventions initiated during the perioperative pathway. In addition, we reached the decision to present evidence from non-RCTs as a supplementary set of data; we were guided by Higgins et al. 38 We did not exclude the data from non-RCTs in our report and believe that we did not introduce bias from this change to protocol.
We expanded the methods for the review, as these were reported only briefly in the protocol; these were standard methodological considerations. Our choice of subgroup analyses was established post hoc. We had not anticipated such a broad range of interventions and participant conditions, and our selection of subgroups was based on those criteria that we believed may reveal differences in findings. It is possible that there are other relevant subgroups that we have not explored.
Chapter 3 Case studies
Objectives
We sought to identify existing services that were already promoting PA to people scheduled to undergo, or who had recently undergone, surgery. We aimed to identify promising and divergent intervention designs and use a series of online focus group discussions, supplemented by survey data, to explore how different interventions and services are embedded in practice, and into the ‘everyday lives’ of service users. We hoped to capture the practical experience of those who had introduced and developed the services and understand specific contextual features that appeared to make them successful.
Methods
Types of services
We aimed to identify services that promote PA and exercise to adults who were scheduled for, or had undergone, elective surgery. We used the following criteria to identify services of interest (although the primary focus was UK services, we did not at this stage exclude non-UK sites):
-
services that were overtly committed to longer-term behaviour change in PA
-
services that had a unique or novel design
-
services that provided a broad representation of different designs, delivery contexts and population/surgery groups.
We noted that services appeared to sit within at least one of six ‘models’ of care and we were keen to include representation of each of these:
-
those embedded across primary and secondary care as common/recommended practice
-
those embedded within specialist services
-
those that rely on partnerships between community non-health service providers and national health services
-
those that are community or patient led
-
those that are low-resource interventions yet appear to work
-
those that offer residential and/or extended (≥ 18 months) support.
Types of participants
We aimed to involve a broad range of perspectives in focus group discussions (including patients, different front-line practitioners, service managers and commissioners); we aimed to capture what goes on in each service, and understand the context of that service, rather than compare services with one another. We encouraged our service contacts to consider inviting colleagues in different roles within the service provision and across the perioperative pathway. We were also keen to include the perspective of service users, and we encouraged our service contacts to assist with the recruitment of service users from their own services.
Search methods to identify services
We used the following methods to select an initial list of possible services meeting our criteria:
-
We identified articles about possible services when screening the results of the database searches for the systematic review.
-
We sought knowledge and suggestions from the Advisory Group, the Study Steering Committee and its wider networks, and from clinicians in our local NHS trust.
-
We conducted web searches.
-
We used the study Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) account to ‘advertise’ what we were looking for.
Using our criteria (see Types of services), we selected a shortlist for our remote focus group discussions. We selected UK-based services only. We sought to include unique/novel services that concentrated on trying to bring about longer-term behaviour change, and which represented the various models mentioned previously with respect to design, delivery context and population/surgery groups.
Participant recruitment and ethics considerations
Health and Social Care Research Ethics Committee approval was granted.
Using e-mail and telephone, we invited contacts from our shortlist of services to take part in focus group discussions and/or individual online interviews. We encouraged the contacts to invite one or two of their patients to take part in the focus group discussions. We invited contacts to complete an online survey and to share an online patient survey with their patients. We also sought to recruit patients through our regional NIHR Clinical Research Network. Participant information sheets outlining the research process were provided to potential participants [see Report Supplementary Material 3 (staff) and Report Supplementary Material 4 (patients)], and informed consent [see Report Supplementary Material 5 (staff) and Report Supplementary Material 6 (patients)] was obtained prior to discussions/interviews.
Although we identify services involved in discussions, data collected from participants have been anonymised.
Focus groups
Group and individual discussions were conducted between 25 November 2020 and 19 January 2021. We conducted two online focus group discussions using Zoom (Zoom Video Communications, San Jose, CA, USA) (one with four participants and one with three participants) and two online individual discussions using Microsoft Teams (Microsoft Corporation, Redmond, WA, USA). Group discussions were co-facilitated by Amy Robinson and Antony Chuter [patient and public involvement (PPI) representative]. Individual discussions were conducted by Amy Robinson only. The focus groups lasted approximately 2 hours, and the interviews lasted 30 minutes. All discussions were recorded.
We developed a focus group topic guide (see Report Supplementary Material 7). This was a collaborative process with the research team and the PPI representative.
Patient and staff/service surveys
We sent, via e-mail, links to an online patient survey (see Report Supplementary Material 8) and an online staff/service survey to our service contacts (see Report Supplementary Material 9). Although service contacts initially thought it was feasible to complete these surveys, and to encourage their patients/clients to complete surveys, owing to COVID-19 constraints and service pressures (service contacts cited heavy patient engagement in relation to COVID-19 service adaptions, pressures on their time, and patients feeling overburdened and overwhelmed by online engagement), these were not completed.
Analysis of the results
One researcher (AR) transcribed the discussion recordings and produced a verbatim account of each event. Transcripts were read by Amy Robinson. Antony Chuter (the PPI representative) used an audio function to ‘listen’ to the transcripts. Following reading of/‘listening’ to each transcript, Amy Robinson and Antony Chuter engaged in four reflective discussions to consider, for example, common themes, divergent perspectives and comments of particular interest. Following these discussions, Amy Robinson produced an initial coding frame (see Appendix 6). Coding was undertaken by the copying of extracts of data from transcripts manually, without computer software, into separate computer files. Guided by our research aims and following principles of thematic analysis as set out by Braun and Clarke,119,120 these were developed iteratively into three overarching themes that we felt supported the utility of our research.
Results
During our initial search, we compiled a ‘directory’ of 61 services/interventions of interest. These programmes were designed for adults generally in a clinical pathway and included some form of PA promotion and behaviour change. Thirty-three were located in the UK. Although we aimed to engage services in the UK, we also found services of interest in other countries that we believed provided useful information to the global context (eight were in the USA and Canada, four in Denmark, two in Ireland, two in Australia, two in Norway and two in Germany). Each of the following locations contained one of these services: France, Italy, Sweden, Finland, Switzerland, Japan, Copenhagen, Gothenburg and Oslo, and one service is classed as being international. Twenty-six of the services or studies were for, or involved, cancer patients; five were for cardiac-related conditions; three were for either total knee arthroplasty or total hip arthroplasty; five were for weight-related health issues; and two were for people with diabetes. The remaining 20 services were for general patients, surgical and non-surgical, with various conditions, including social prescribing initiatives. The directory contains details about the intervention or service provided, as well as links to related websites and papers (see Report Supplementary Material 10).
Contacts from eight services (with nine participants) took part in online focus group discussions between November 2020 and January 2021. Services were chosen for their explicit commitment to long-term PA behaviour change, for their unique and creative methods of engaging with patients and for their different approaches to working within perioperative pathways and/or community health pathways. We have summarised these services in tables of service characteristics (see Report Supplementary Material 11).
Seven of these services were based in the north of England (Barrow, Leeds, Harrogate, Manchester and Greater Manchester, Middlesbrough and Stockport). One participant/service was based in Kilmarnock, Scotland, using, alongside other approaches, nationally available resources/provisions. Three services were unique to patients in a cancer pathway; two included people scheduled for, or who had undergone, any type of major surgery, one of which also included patients receiving cancer treatment. One service provided rehabilitation for surgical patients and supported people with long-term health conditions. Two services were general practice clinics: one with a focus on lifestyle medicine and the other an active practice.
Three services worked perioperatively and/or with patients during cancer treatment. Two services worked postoperatively (or post treatment) and one service delivered care preoperatively but could link to postoperative rehabilitation. The two general practices worked with patients at any point in their health-care journey.
Two services were co-ordinated and delivered by community-based organisations (a charity and a social enterprise). Other services were co-ordinated in primary and secondary care. Referrals to most services could be made via self-referral, but more generally were made from general practice, clinical teams or other cancer services. All but one service was located in areas of deprivation higher than the national average.
Each service promoted or enabled PA through information provision, scheduled PA classes or access to local gyms. Contact with patients was usually through group sessions but typically also included one-to-one contact, individualisation and support. Services all relied on in-person contact; however, many adapted their provision during COVID-19 lockdowns (see Digital delivery: the COVID-19 pandemic and beyond). Activities included regular high- and low-intensity exercise classes; taster sessions, including active core, Nordic walking, tai chi, ballroom dancing, strengthening and cardiovascular work; walking groups; and signposting to local leisure centres or other community provision.
Services tended to focus on physiological status as their primary concern, but this was consistently combined with an emphasis on people’s broader health and well-being, either through integrated provision or collaborative relationships with other care or programmes, such as nutritional, psychological and smoking cessation support.
We held two online focus group discussions, which each lasted approximately 2 hours: one discussion with four participants and one discussion with three participants. We also held two semistructured interviews lasting 30 minutes.
Focus group participants included two GP partners (one specialising in lifestyle medicine and the other in sports and exercise science). Five were managers or previous managers of the programmes, two were specialist PA trainers, one was a physiotherapist, two were health coaches and one was an anaesthetist and clinical director of a large hospitals trust. The majority of participants had been involved in the initial development and start-up of the services.
See Appendix 7 for an expanded thematic analysis of the qualitative contextual data from these events. Here, we present summary themes from the focus group discussions and individual interviews.
Narratives of physical activity promotion
Several narratives seemed to describe the impetus with which services and staff approached their work with patients. These centred largely around the idea that the patient was central, their needs were individual, and a broad wrap-around approach was necessary to both engage and support patients to make long-term changes to their health. This seemed to be grouped around three key principles: supporting a holistic, well-being approach; embedding PA in usual care; motivating and inspiring patients (around which PA was framed); the individual (as central to the sorts and ‘dose’ of activity promoted); and compassionate care (as a valued principle in services’ interactions with patients).
Framing of activity
Supporting a holistic, well-being approach
A ‘holistic’ approach, focused on a patient’s overall well-being, was frequently described, with many participants commenting on the link between PA and a patient’s psychological profile and ‘sense of self’. Several participants indicated that they frame the impact of their programmes to patients using the same narrative, encouraging patients to understand how activity might support them through their treatment, and help them manage stress and anxiety. This appeared to be seen as central to the efficacy of their approaches and an acceptable narrative with which patients could connect. This focus often included broader lifestyle aspects such as smoking and alcohol reduction; efforts to keep patients nutritionally ‘stable’; or working closely with dietitians, or with nurses around pain management, optimising medication and any ‘other elements of supporting patients’ that might become apparent. One participant described current efforts to find a way (perhaps using technology) to more systematically stratify the different aspects of patients’ physical, psychological and nutritional needs when they first meet a person, to ensure that every patient gets the right amount of support for each of their varying needs.
Well-being was often aligned with thinking about helping patients to feel supported, and this was often talked about in terms of making sure people ‘feel like they’re in a safe space’; one participant described feedback from patients that they had had ‘enough clinical appointments and don’t want this to be seen as clinical as well . . . so every effort is taken to create a more informal and relaxed service’. This was described as helping patients ‘open up’ much more than they might do with their clinical team.
Motivating and inspiring
Another common frame participants spoke about was driving ‘self-empowerment’ and motivating or inspiring people to make permanent changes; one participant explained that they focus on ‘self-efficacy for exercise because we know somebody with a higher self-efficacy for exercise are more likely to be independent once they’re discharged from our service’. Another talked about helping patients to understand ‘how to monitor their own body, [and] how to put together their own gym programme’. Attempts to motivate change sometimes included an element of education: group sessions, for example, focused around ‘physical activity and why it’s important and the kind of benefits of it’ or conversations, for example in which a patient’s anaesthetist might be talking to a patient about how their aerobic fitness is going to affect their care. This was seen as incentivising patients to concentrate on how they may be able to influence treatment outcomes and reduce side effects (e.g. of cytotoxic chemotherapy).
Several participants talked about providing information or guidance on how to find support and ‘the right exercise classes or activities’ available in patients’ local areas. This was described as ‘key for the long term’ and ‘massively important’ in making sure ‘they are able to motivate themselves, have the know-how and the confidence to keep active and address their activity needs’.
Several participants also commented on leading by example. One service, in working on becoming an active practice, described focusing first on getting their staff and GPs within the practice moving more. This included walking challenges and signing up to be a Park Run Practice (advertising local runs and a couple of staff going along). The participant felt that, on the whole, everyone now ‘feels more active’, more ‘willing’ and better placed to discuss PA more with their patients. Others too talked about role modelling, with one participant suggesting that it might be extremely helpful if activity was ‘something that is habitual’ and ‘health-care professionals themselves [are] physically active’.
Participants generally echoed a perception that their provision was ‘one area that [patients] do actually have control over . . . they can control what they come to and what they don’t come to . . . it’s a choice at the end of the day’. There was some understanding that ‘once you get into a hospital system, you don’t get a lot of control over many things . . .’ and that the programmes provided an opportunity to ‘actively participate in something’, regain an element of control over part of their lives among unfamiliar and unwelcome aspects of their illness, and that this feeling of choice can itself be enabling and ‘quite a kind of powerful thing for patients’.
Embedding physical activity in usual care
A further frame was that PA was effectively part of a patient’s treatment options, it was usual care or a prescription, and the ultimate aim of services was that their programmes were embedded in standard care. One participant explained that clinical teams at patients’ MDT appointments would ‘present a treatment plan’, and ‘this plan includes . . . “we’re going to send you to the exercise team” . . . it’s part of patients’ prescription . . . it’s just like them going to see the radiotherapist, they come and see us’. The message from participants was that PA must form an integral part of a patient’s treatment, and, whether implicit or overtly stated by those involved in patients’ care, having this reinforced was very helpful.
Activity: variety and dose
‘Physical activity; you’re not gonna do it, if you’re not gonna like it.’
Although the sorts of activities that services described sometimes differed, the need to be acutely in tune with patients’ individual contexts and needs was a recurrent message, and placing the individual at the centre by finding something that people enjoy and working on increasing the amount of PA people were doing, rather than driving particular activity guidelines, seemed to be the most important principles. This may be rekindling an old activity, starting a new one or finding something that means something to patients.
Participants talked about getting people ‘doing something that they love’ and finding that thing that ‘they enjoy in order to adopt a long and healthy lifestyle’ and make PA ‘a habit’. They also talked about testing possible activities with patients; one participant, emphasising that some people may not have been physically active before, suggested that this might require ‘building confidence to just try different things’ and ‘that what matters . . . is that people can just give stuff a go without feeling like they’re embarrassed or uncomfortable’. Finding what matters to the patient was often talked about as goal-setting. Examples described included patients wanting to get ‘back to kicking ball in the park with the grandkids’, ‘seeing their blood pressure decrease’ and ‘getting ready for their daughter’s wedding’.
There seemed to be a move away from relying on national guidelines recommending a particular amount and type of PA. As one participant explained, ‘guidelines don’t fit everybody. If we took everybody’s shoe size in this room and just gave you the average shoe size and said there you go, there’s your shoes for the next year, none of us would probably fit them’. In this respect, participants often talked about ‘every minute counts’ as the message they would convey to their patients and, from there, ‘building structure’.
One participant described, ‘intuitive exercise’, ‘it’s about . . . we listen to them, and their body and how their body responds . . . we learn how far we can push their exercise prescription before it starts to have a negative impact’, which might, they added, come afterwards rather than at the time of the activity. They described this as requiring collaboration between the exercise instructor and the patient, ‘listening to [the patient] and being flexible’ and there were frequent comments from participants that lesser ‘doses’ of activity, and expectations, can initially be tailored to what patients can realistically achieve.
The possibility of supporting patients in seeing the functional benefits of the programme was also mentioned, especially by relating it to everyday tasks, making the link between exercise and ‘real life’.
Timing was sometimes noted as important too; on the one hand, some services needed to be responsive enough to be able to perform useful work with patients within a few weeks (for cancer patients, this was typically the time between diagnosis and surgery or the beginning of treatment). As a result, in these services there was a heavy focus on the immediate benefits to patients’ physiological status, preparing them for treatment, the side effects of treatment and their initial recovery from surgery.
On the other hand, a number of participants commented on the need to recognise that some patients may simply not be ready to make changes in their lives relating to PA. They may, however, be ready to do so at some stage in the future, and participants felt that it was important that the initial contact with such patients remain positive, and that they ‘make sure that we’re doing all that we can’ and to have a ‘legacy with people’ should they wish to engage in the future. For those more reluctant patients, one participant mentioned their service’s ‘walk-and-talk’ sessions, volunteer-led events, with friends, family and dogs all invited, that provided an informal opportunity to talk about what the service could offer and see the facilities it provided. These were seen as useful in providing a taste of what was on offer; from here patients would usually go on to join the programme.
One respondent talked about cultural issues relating to PA and health perceptions that can be completely at odds with the approach they are taking. They suggested having cultural champions, giving the example of a young family from a particular ethnic background that seemed to reject all their suggestions. They suggested that one option here would be to work with local community leaders and in appropriately sited community centres to support particular patient groups.
The importance of the language used when talking to patients was raised by several participants. Although ‘exercise’ was a word readily used by participants when describing provision, there was some agreement that ‘physical activity’ was a better terminology to use with patients.
The clinical/non-clinical paradox
Participants were of the opinion that patients are most likely to engage in provision if the idea is presented to them as ‘part of their treatment’, endorsed by their clinical team. This could be anyone (e.g. physiotherapists, surgeons, anaesthetists, GPs, nurses), but ideally would be everyone, ‘singing from the same hymn sheet’. Several participants noted the benefit of this coming from a patient’s surgeon or consultant, suggesting that the person (e.g. a surgeon) who was going to ‘save someone’s life’ has ‘more of an influence than some random physio[therapist]’. Even if consultants/surgeons did not have a huge amount of time to explain to patients what prehabilitation was all about, if they ‘endorse the programme’ this was seen as making a significant difference; patients will have ‘almost bought into the idea’ and service staff felt that they could begin to build on the benefits from there.
However, despite this clinical endorsement, delivery appeared to work best in non-clinical settings delivered by people with very specific qualities.
There was strong belief that the acceptability of the setting and space in which services delivered sessions with patients is influenced both by the local context and by the patient group; ultimately it is important that the space is easily accessible and that it is not clinical (for instance, people do not want to go for exercise to the building where they were diagnosed with cancer). There was consensus that good-quality facilities support the success of programmes, but that the acceptability of a space is clearly subjective, and something to be considered by staff in relation to the patient group and the community in which their services are provided. One participant described their space (within a private sports and fitness centre, well equipped, with social spaces and cafes, and welcoming to families) as a ‘big draw’ in encouraging people to come, helping people to socialise and to relax. Others, however, talked about private gyms being rejected by patients, with local leisure centres, church halls or community hubs being preferred spaces.
Accessibility was also acknowledged by the majority of participants; venues needed to be ‘easy and local’ and accessible by public transport (‘if they’re on a bus then the bus needs to stop near the leisure centre . . . [not] down country lanes and stuff’) or by walking. One participant talked about the availability of parking: ‘if they can’t park their car . . . they’re not going to stop’.
Beyond the physical accessibility, the idea that ‘people like to go to their local centre’ seemed to also include an element of community affiliation, membership of a language: colloquial and centred around a narrative and life lived in a shared place and space. For example: ‘people like the fact that the receptionist has the same accent and uses the same terminology and the same dialect. They like the fact that my team are all from Greater Manchester and live in Greater Manchester and so we all have the lovely Greater Manchester twang . . . they like that sort of familiarity’. Another participant explained that a lot of the conversations patients and staff might have ‘aren’t necessarily about health and not about medical conditions [but] just about life’ and that ‘being able to talk about your local monuments . . . the local pub . . . or the local restaurant might seem quite small, but actually help, help a lot in terms of engaging people and keeping them wanting to stay within programmes or services’.
Several participants did also accept that, for some patients, familiarity is not always helpful; one service (in a small isolated town) trying to develop group lifestyle clinics found that ‘being in a small place, people didn’t want to engage a lot because they didn’t want their next-door neighbour or family to know what’s going on with them . . .’. Another participant described a small number of patients on their cancer programme ‘who don’t want to go to their local [sessions] because they don’t want to be seen as having cancer in their community. So, they prefer sometimes to travel’.
Family members too were seen as ‘strong influencing factor[s]’ in patients’ experiences. According to one participant, it is important that relatives, as well as providing practical help in getting to and from sessions, are enticed in: ‘patients have got all this stuff going on in their head, actually, if they bring husband, wife or whoever in with them, it’s another person to take information in, or ask a question that they’ve forgotten’. Several participants talked about inviting family members not just to appointments but also to activity or education sessions, with one describing ‘the big thing’ for them as ‘the opportunity to meet patients and the families’. Others talked about inviting family members into an exercise class: ‘they can actually learn the exercises and then they can actually do the exercises together at home. And that’s quite powerful’. Another participant said that there were often some great stories of families coaching patients: ‘you know, within an inch of their life basically but it was very good for the family, as well, because the family . . . it was often children coaching their relatives and they felt that they were really, really trying to give their relative the best chance’.
Plurality of benefit
Participants were clear that, although their services were nominally set up to promote PA, they in fact provided a wider range of support and benefits, both intended and unexpected. Focusing on PA in isolation was seen to miss the full potential of the perioperative encounter, and a ‘well-being focus to physical activity’ was seen as enabling conversations about nutrition; mental health; other lifestyle changes, such as smoking or alcohol reduction; and providing social support in the context of illness. One participant referred to their programme as a ‘safe space’ in which to broach topics patients might not have shared with anyone else. Several participants talked about efforts to forge greater links with psychology services and to build on colleagues’ existing ‘listening skills’, and some services provided counselling and psychological skills training to staff. In one discussion, a participant described a shared city-centre space with local public health teams as ‘a one-stop-shop’ enabling the joining up of support. Another participant emphasised the ‘luxurious position’ that their service was in, providing the ‘opportunity to help people, two or three times a week’, and viewed their frequent contact with patients as an opportunity to think much more broadly about patients’ health and well-being, and to utilise their colleagues or wider networks to access additional support or referrals depending on patient needs.
The significance of patients’ peers was commented on in a number of discussions, with the provision of time for patients to talk to other patients seen as supporting well-being. Several participants said that it was ‘the social side of things’ that patients ‘get a lot from’. In one (men’s-based) programme, there was a view that the programme had helped to create a ‘kind of lad-like feeling, that they find they can be a team together’. And in another service a participant described the benefit of people visiting the high-dependency unit (where a patient would wake up after surgery), and this creating an opportunity for patients to ‘see people that were like [them], who had cancer or were having major surgery’, to have a conversation and to see that ‘they’re all right’.
Setting up and running the service
Establishment and development
Most services were initiated by one person, or by a small number of enthusiasts. Individual characters seemed to have been the primary ‘drivers’ in many services, often then with the coming together of a small number of like-minded individuals. This might include someone with an academic background or interest in the science, including sports medicine, and/or strong experience in specialist PA training, cardiac rehabilitation or physiology, sometimes new to the NHS and not always with clinical experience.
Typically, services grew and evolved, with the majority of participants suggesting that soliciting and acting on the views of the people using the service was important in helping shape provision. One participant admitted, ‘We thought we knew the answers . . .. We thought that men would like to train with men and women would like to train with women. We were absolutely wrong about that. We were told absolutely not’. This influence from patients on how programmes evolved, from start-up and pilots to ongoing improvements, seemed to be rooted in ‘listening to them and, kind of, their concerns’. This was sometimes referred to as ‘a lot of co-designing’, with one participant suggesting that this meant that patients do ‘generally feel that [provision is] about them’. Another participant talked about patients ‘always at the table’, explaining that ‘at the end of the day they’re the ones we want to do it and we have to listen’, while another maintained that, ‘feedback makes anything possible and makes you kind of improve as you go along’. One participant suggested that acting on patient engagement was not always straightforward, describing it as being often ‘so personal’ and everyone having ‘extremely different’ opinions.
Framing and ‘marketing’
Bringing colleagues ‘on board’ was seen as essential. Having the understanding and support of management, whether at the level of a clinical lead within the trust, a senior GP or board-level leadership, was also seen as helpful in smoothing the path of service development. Relationships among broader colleagues, both within programmes but, critically, outside programmes and/or organisations, were frequently presented as important or as making things possible. Here, participants consistently presented a coherent message that ‘activity as treatment’ was a necessary message to engender that support, as well as being prepared to frame PA to the particular perspective of different health professionals. It was clearly a nuanced and gradual process, reliant on individuals, perseverance, good relationships, education and evidence.
Several participants echoed the benefits of having a proactive clinical lead, ‘who could get their colleagues on board’; another admitted that, for their service, it had probably helped that they had been in an increasingly managerially responsible role (now the clinical director for the intensive care unit and anaesthetics), that they already had personal relationship with hospitals across their trust and that they had a certain amount of ‘authority to say, well, we’re going to go in that direction . . .’. In another service, the participant explained that they had ‘hand-selected people’ who they knew ‘were already bought into the idea [of PA]’ and that they saw these ‘clinical champions’ (usually surgeons) as helping to ‘spread the word’. There was the impression too that patients also played a role in engaging their broader clinical team in the value of PA provision. One participant talked about this as having an almost cyclical effect in which patients were often reinforcing the positive effects of a programme, ‘how much they’ve enjoyed it’ and ‘how successful they’ve been’.
More generally, gaining clinical buy-in appeared to be a slow and ongoing process that took tenacity and perseverance, and that broader support from clinicians demanded ‘knock[ing] on the hospital doors a hell of a lot’ trying to ‘speak to a lot of nurses who were not too keen on sending their patients to anybody but a nurse’, and who ‘did not see the value in physical activity’. There was a clear message that ‘you’ve got to do the legwork . . . you’ve got to really promote . . . it’s like any sort of marketing: you get out what you put in . . .’.
One participant described as high on their agenda the need to ‘generate evidence or generate momentum that encouraging patients to be physically active should be viewed as a clinical intervention rather than something that’s kind of a well-being add-on to treatment’. Similarly, another recounted that the ‘big thing’ for them in developing their programme and gaining momentum had been being able to evidence impact (reductions in length of stay and in respiratory complications). For one service, a grassroots community programme having little direct connection with clinical teams, building trust with clinical colleagues and with patients appeared to have additional challenges. They talked about reputation (which they had ‘worked so hard to build’) and branding (their association with a rugby league club, with players acting as ambassadors for the programme) making ‘it easier’ sometimes ‘to get in the door somewhere’.
Resistance was often put down to a ‘lack of understanding’ of what services provide and practitioners are ‘qualified to do’ and that there was a need to ‘prove quite a lot’ to gain the trust and support of other clinical staff. There was broad acknowledgement that there is ‘a massive amount of work’ still to be done, as well as ‘a lot for primary and secondary care to learn about activity’. Encouraging dialogue about PA within health care in general was seen as important too; respondents noted that many health-care workers do not know how to talk about exercise, and need support with finding and using appropriate resources to integrate conversations about activity into routine clinical care.
Respondents recognised the benefits, but also the difficulties, of linking general practice, primary care and secondary care; one service had begun working with its local information technology (IT) team to try to develop a software-based solution for this.
Staff
Participants consistently portrayed staff as compassionate and prepared to do whatever it takes, praised them frequently, described a strong collegial environment, and acknowledged the responsibilities and challenges faced by their colleagues. A wide range of staff groups often took part in the provision of services. ‘Core teams’ tended to include physical trainers, physiotherapists (including oncology specialist physiotherapists), exercise scientists, nurses and administrative staff/receptionists. Several participants expressed that it was not so much the profession or experience that staff might have but the personal qualities that they bring. One participant suggested that these are ‘the keen individuals’ who bring a range of complementary skills. Several picked up on staff who are ‘very caring people’, ‘fantastic communicators’ and ‘good listeners’, and who ‘can pick up on’, ‘notice’ and ‘understand’ the needs of individuals.
Participants clearly had empathy for patients’ lived experiences: they often talked about pain, one suggesting that it is ‘probably one of the hardest things . . . for people to manage . . . living with pain, every single day . . . you know, the impact that has on mental health, as well as anxiety, depression, I’m not surprised people are not too enthusiastic about doing physical activity’. Participants also often acknowledged the ‘human’ aspects that their patients were likely to be experiencing, ‘the worry, the concerns, the anxieties related with the referral and “What’s my prognosis? Have I got weeks? Have I got months? Have I got years?”. . .’.
One participant explained that it is not just the attributes of staff directly delivering the programme, but everyone a patient may come into contact with that was important, a point that was echoed across other discussions, with one participant clarifying that ‘you need the receptionist at the facility to be kind to the person . . . see that they might be struggling a little bit and help them out’. Others who were key and, although perhaps not directly involved in a programme, were seen as clinical champions included nurses, cancer nurse specialists, care co-ordinators, anaesthetists, surgeons and oncologists. Broader collaborators, who took a greater or lesser part in providing the service depending on local needs and arrangements, included pharmacists, dietitians, clinical psychologists, GPs, staff at local gyms, volunteers and data analysts.
There was also recognition of the impact on staff of working with people in these contexts; one participant gave the example of colleagues working with newly diagnosed cancer patients, describing staff as ‘an outlet for a lot of stuff’, and conversations with patients that can be ‘emotionally draining’. Several services talked about making sure that there are systems in place to support staff, which in some cases included psychology provision, regular ‘debriefs’ and ‘continuously doing CPDs [continuing professional development courses]’.
Funding
Many services started with charitable or grant funding, or with one-off quality improvement money. The challenge then was to ‘mainstream’ the service within standard funding sources. Support from managers within NHS trusts, or commissioners outside, seems to help with this.
Data and outcomes
Some services started out as quality improvement projects, with data collection and monitoring an integral part of their work from the beginning. Others felt the need to gather data to evaluate their processes and outcomes, partly to help convince clinical and managerial colleagues of the utility of the service, but also to help secure further funding.
Data that appeared to be seen as important included objective physiological measures, such as the 6MWT, the sit-to-stand test, grip strength and more global measures of disability (e.g. WHO Disability Assessment Schedule), as well as measures related to efficiency of care; length of postoperative hospital stay was a primary outcome for one service, whereas for another fatigue was a primary outcome. Service-based measures such as recruitment and attendance at sessions were used and there were attempts/hopes to link this to patient records. Most services did not collect data on patients’ level of PA.
One service was described as being ‘heavily monitored’ on its recruitment of patients and getting clinical outcomes, ‘so our prehab[ilitation] is really heavily focused on getting people into the gyms, and really getting them exercising, to quite a high intensity’.
Participants also frequently mentioned patient feedback, often as something commissioners were particularly interested in seeing: ‘they want to know the patient impact, and the patients say that themselves, and they’re the best at saying that’. Video testimonials were also described as being useful for engaging both other patients and health professionals.
Several services were involved in formal service evaluations of their provision (see Report Supplementary Material 11).
Digital delivery: the COVID-19 pandemic and beyond
Although the COVID-19 pandemic presented substantial challenges for services, participants generally commented on the opportunities the period had presented to consider and explore new ways of working with patients. All services, to varying degrees, continued contact with patients during the period. Several services described providing information or linking patients to online and community-based activities, such as walking challenges. Participants also talked about a move towards the heavy use of social media, including service websites and YouTube channels (YouTube, LLC, San Bruno, CA, USA), to keep people moving. These tended to provide short active challenges or follow-along exercise videos, sometimes broken down into smaller parts than might be delivered in typical face-to-face sessions. Participants from one service also talked about the provision of an online exercise diary that people could complete, and that also linked with a PA trainer for feedback. Telephone and e-mail conversations and consultations were also described, as were some individual and group virtual interactions.
One service appeared to have been able to adapt particularly well to a digital and remote delivery model. Participants from this service talked about providing ‘the full spectrum’. People with ‘zero technical ability’ or those deemed ‘technically deprived’ (a high proportion of their patients) would receive home exercise packs, diagrams and descriptions, and resistance bands in the post, and then coaching sessions on a telephone. These were described as ‘a bit weird to get used to’ and ‘massively labour intensive’ but something they now ‘quite enjoy . . . it’s good fun, we’re enjoying it’. For others, they had a timetable of 15 online group classes each week, and, for some, the use of Myzone chest belts (digital heart rate monitors) (Myzone, Nottingham, UK), allowing trainers to track a patient’s heart rate during sessions. This enabled exercise trainers to visualise up to 10 people at one time doing an activity session, and provide encouragement, when appropriate, to ‘go a bit harder’, and also acted as a safety tool, whereby the trainer could ‘keep an eye’ on how people were doing. The approach also allowed the service to continue with much of its data collection.
Participants gave mixed views on the success of their service’s remote adaptions. Some felt that they had been ‘successful’, and, of these, some more so than others. One participant felt that, on the whole, remote delivery, ‘for the people we work with’ was not the ‘optimal method . . . to get the best outcomes from them’. There was agreement, however, that provision had supported well-being and prevented patients’ health deteriorating as much as it might have, although one described as ‘astonishing’ the sharp deterioration in fitness after the first lockdown, despite good adherence to the revised programme, and one service (the ‘full spectrum service’) reported gains.
Several participants suggested that virtual delivery had worked better for people who were already on a programme or known to the service, with one participant suspecting that had they ‘been delivering remote programmes from the off, it would have been very, very difficult to get the same level of engagement’ from patients whom they did not already know. One participant, however, described engagement as ‘really good’ from new and existing patients, the conjecture being that patients had responded well to the varying degrees of technicality/use of technology or resources that they offered: ‘nobody is excluded and I think that’s why the engagement rate is so high’.
Participants talked about remote sessions being particularly useful for particular patient groups: those with ‘caring responsibilities’ for example, or people who are on chemotherapy and radiotherapy, who ‘find it very hard to travel’. One participant described finding the way appointments were now being managed, many of them virtually, via e-mail or on the telephone, actually made it easier to bring in discussion of PA, and sometimes allowed for a bit more time to talk about broader issues with patients.
Although several services talked about efforts to ensure that there was an offer for everyone, regardless of the sorts of digital devices or internet access people might have, there was generally a heavy reliance on technology, mobile telephones, computers, devices for video contact and the internet, with services using various platforms for video sessions. Some participants emphasised how not having access to the technology ‘definitely hindered some people’; there was repeated discussion of the influence of deprivation and of technological literacy, with one participant describing their team as becoming ‘part-time IT consultants’. Several people commented, however, that many of the technical barriers had reduced as time had gone on; one felt that ‘older people and relatives have joined up and learnt technology’ in a way that, prior to COVID-19, ‘we could not have imagined’.
Most participants indicated a hope to move beyond COVID-19 with some form of a ‘hybrid model’ that might allow them to work more flexibly with different patients, or utilise remote delivery for certain aspects of their provision, such as an initial consultation, which ‘might actually engage somebody as a first step where before they wouldn’t have engaged at all. Because the thought of coming to a facility, to do their initial consultation, might have actually been a barrier’. These were seen as potentially reducing some barriers that some people experience in making a first step to coming along to a programme.
One participant suggested that the experience had shown that ‘remote rehab[ilitation] can be done’, although others suspected that people might ‘just crave that social interaction’, and one participant mentioned that they had ‘people begging us for turning things back to normal and back to face to face. They just cannot stand the virtual stuff’.
Several participants commented on how they felt that COVID-19 had ‘reinforced the importance of exercise’, ‘[be]cause we’ve been allowed out for an hour every day for 6 months, haven’t we, to exercise’ and that, now more than ever, it was such an ‘important time . . . to really push this forward’.
Health inequities and social determinants of health
Participants alluded to several conditions influencing both service capacity to reach patients and patients’ reception of, and capacity to engage in, provision. Primarily this related to the physical space/place in which services were delivered and access to digital technologies/the internet (during the pandemic period).
Accessibility was seen to be important, particularly for those in deprived areas, whom several participants suggested were much less likely to travel than patients in other areas. The quality of facilities in these areas was described as ‘probably the worst’, often with limited equipment, problems with heating and cold rooms, or ‘nicer rooms’ upstairs but there being no lift. This too was described as influencing engagement.
Most participants acknowledged that patients (sometimes a high proportion) might be deemed ‘technically deprived’ and attributed drops in attendance during the pandemic to people living in areas of high deprivation where people were often marginalised from access to certain technologies and/or access to the internet. Although some services had clearly attempted to adjust for these barriers, for example one service issued home exercise packs with no reliance on digital devices, such efforts were described as particularly labour intensive, and so raised questions about the long-term sustainability of those approaches.
One service had chosen to focus primarily on those they felt were most negatively affected by the pandemic, in terms of risk and isolation. Another service, during the second national lockdown, had, in fact (with the exception of those who were clinically extremely vulnerable), been able to reopen its in-person classes and face-to-face assessments, offering a full timetable that ran at > 70% capacity. This was unusual, but was made possible because, combined with the national drive for medical services to continue, it had been successful in ‘convincing’ its trust that its programme ‘is treatment’ and ‘part of normal cancer care’.
Finally, there was occasional reference to the use of public spaces: local green spaces, parks and outdoor park gym equipment, and cycle/walking routes in which some service contact took place or which patients might be encouraged to use. Although not explicitly discussed, this was clearly valued where it was available, which raises important questions around areas deprived of green space and inequality in access to good pedestrian/cycle ways, particularly given that people from low-income households and ethnic minority backgrounds are more likely to be affected by both these circumstances and inequalities in health. 121
Overall, participants appeared to be conscious of, and frustrated by, disparities in provision and the multiple disadvantages affecting many of their patients.
Discussion
Strengths and limitations of qualitative data from focus groups and interviews
We had initially planned a series of physical case study visits to the services of interest. However, the COVID-19 pandemic made this impossible, partly because of restrictions on travel and personal contact, but also because the way the services were delivered changed considerably. Further detail is given in Chapter 6. Nevertheless, we have been able to gather a great deal of contextual information to help others running, or considering setting up, a service to promote PA during the perioperative period. In addition, because many services have now incorporated elements of remote delivery into their work, we have been able to capture these experiences too, as health care moves into the post-pandemic period. As our project was already funded and running, we were able to respond promptly to the changes in service provision brought about by the pandemic. Unfortunately, the constraints posed by the pandemic meant that we were unable to recruit any patients to the online focus groups, and it must be noted that their perspective is not represented in our findings. We see this as a limitation of this study, and have recommended this as an area for further research.
We have compiled a compendium of 61 services/interventions of interest; 33 of these are based in the UK (see Report Supplementary Material 10). Choosing services from the list of candidate sites, we used a purposive sampling strategy, as outlined in Chapter 2, designed to identify sites that, collectively, could tell us as much as possible about different aspects of service provision. This shortlist was also shaped by the availability/capacity of services during the COVID-19 pandemic context. We had approached an additional four services, with initial agreement to take part from three of those services; however, participation was not feasible. We felt that the number of focus groups/interviews was relatively small compared with some qualitative studies;122 had we been able to include representation from all our intended services, we would have had a more complete sample that included broader service designs, surgical focus, profession positions and geographical location within the UK. We note that the work of Guest et al. 123 found that 80% of themes were discoverable during the first two or three focus groups, and also noted the problem of generating too many data if too many groups were run. Although time constraints prevented us from carrying out formal participant validation, we did benefit from the added analytical perspective of our principal PPI representative, and an informal ‘sense check’ from members of our Study Steering Committee.
We had planned to recruit additional PPI representatives, both at national and local levels. This was not progressed because of the COVID-19 pandemic, and we believe that this was a limitation of the project.
Chapter 4 Synthesising the two streams of the research
Our systematic literature review and the qualitative work with existing services offer two complementary perspectives on the same issue, namely the promotion of longer-term PA in the perioperative setting. Although their methodologies and intentions differ, they provide an opportunity to set the knowledge from the systematic review in context and highlight a number of aspects where their findings intersect and reinforce each other. These are now considered, under the following headings. It should be noted that neither data set took priority, and, although data did sometimes reinforce one another, comparable data were not always available.
Contextual features
Many features of study populations and care settings can influence process and outcomes in clinical trials, and in general service provision. These include the age and gender of patients, their diagnosis, the type of surgery and the health-care system. Ethnicity, education level and economic status of patients are also relevant, but are less obviously ‘clinical’ and thus may not be reported in clinical studies. For instance, only 15 of our included RCTs (28%) reported ethnicity, and only seven (13%) reported the economic status of participants. More reported educational level (26 studies, 49%), but this is still only roughly half of the trials in the data set, and the data are not comparable across studies because of global differences in education systems. We did not evaluate the effects of factors such as ethnicity, education level and economic status in the systematic review; differences between and within studies meant that this was not feasible.
Examination of the qualitative data, however, reveals that many other factors are at play in ensuring a successful outcome. These include staff enthusiasm, knowledge and personal qualities; the framing of PA; family and peer support; the broader ‘buy-in’ of colleagues; the physical state and location of facilities; and, for digitally delivered services, access to technology. These are seldom even mentioned in RCTs of even the highest methodological quality (e.g. Turunen et al. ,109 Piva et al. 102 and Lotzke et al. ;97 see Chapter 2), where the focus is on demographic and clinical characteristics. This makes it difficult to transfer the intervention from the study setting to another, as the ‘tacit’ elements making the intervention ‘work’24 are not described. Within our small-scale work with eight UK-based services, we identified and described a number of discrete ‘ways of understanding’ the promotion of PA.
Patient engagement and adherence
We did not capture data on recruitment rates within included studies in the review. Given that the complex information and consent process associated with research may discourage some patients who might otherwise have considered participating, we expect that the way the programmes were presented to potential participants was key to recruitment success. In our focus group discussions, ways of ‘framing’ activity were seen as important components of patient engagement. Participants wanted to motivate and inspire patients to engage in PA, with the intention of permanent behaviour change; this was often described as finding something people enjoy and building up levels of PA from there. This often included supporting broader lifestyle changes, helping patients to understand how they might benefit from being more active and helping patients to think about goals they might want to achieve. We found that more than half of the interventions in the included studies used behavioural mechanisms, and education and advice was a key component to most of the interventions; this included lifestyle education in 12 of these interventions.
Although data for adherence to interventions were reported less frequently than we had hoped in the included studies, it was apparent that some studies used interventions that enabled an adherence rate of > 90% (although, overall, this ranged from 47% to 93%). In the review, we did not explore further which intervention components had improved adherence levels; overlap of intervention components within and between studies meant that this exploration was not feasible. It is possible that the different health conditions, as well as the motivations of intervention delivery related to indication for surgery, may have affected people’s engagement and subsequent adherence in studies in the review. We noted in the focus group discussions that participants believed that an individualised model was essential; this included understanding an individual patient’s needs, listening to patients and noticing when they might need additional support, finding out what patients enjoy doing and supporting different options. This was also reflected in the included studies, with > 60% using a tailored approach.
Measures and metrics
The RCTs we included used a range of measures to present data for the outcomes. We selected only studies that measured the amount of PA or the number of people engaging in activity at the end of follow-up; thus, these studies were more clearly aligned with the aim of this research. We found that studies used a range of measures to present the amount of activity, and this was also the case for physical fitness, which used a number of different physiological and functional assessments. Less commonly reported were the effects of interventions on quality of life, adherence to the programme and participants’ experiences of the intervention. These last-named have been investigated in a number of studies and reviews, both within124–126 and outside127,128 the perioperative setting. Participants in the focus groups noted the importance of data collection, both to be able to demonstrate progress to programme participants, and also to be able to justify the programme to health-care funders, policy-makers and sceptical local clinicians. These different purposes require different metrics, making the business of data collection and analysis quite complex and time-consuming. It also needs skills in data management, and many services may therefore struggle to collect and process the necessary measures, especially when outside the framework of a formal funded quality improvement project. Some of the physiologically based measures used in the trials in the systematic review were used in practice. Service-based measures, such as recruitment and attendance at sessions, were also used in practice and there were attempts/hopes to link these to patient records. Notably, levels of patient PA were not collected in practice. In both the systematic review and services in practice, patients’ perspectives were sought formally or informally, or collected in the form of quotations and ‘testimonials’, or sometimes in discrete funded evaluations. In the systematic review, these data were not collected consistently, so it was not feasible to explore this further. We did not have access to these data from services in practice.
Inequity
Services in practice appeared conscious of, and frustrated by, disparities in provision and the multiple disadvantages affecting many of their patients, including access, for example to appropriate spaces and places for safe and comfortable activity (such as buildings and walking/cycle lanes), as well as to the internet/digital technologies and literacy. Although ethnicity is an essential consideration intersecting with these inequities, this was not explicitly discussed during our focus group discussions. However, digital exclusion, particularly in the context of evolving provision in response to COVID-19, was discussed, and some services went to sometimes substantial lengths to try to circumvent the impact of digital disadvantage. The studies included in the systematic review all predated the COVID-19 pandemic. However, we noted that more than one-third of the interventions incorporated remote delivery within their models, and that 16 delivered an exclusively remote service. These were mostly delivered by telephone or post, but some also used digital technologies and/or relied on access to the internet. Although we were unable to explore effects of remote delivery in our systematic review, we were able to capture some of the ways in which services had responded initially to having to deliver their programmes online/remotely in our qualitative focus group work. Although their understanding of this is still developing, the overarching message was that a blended approach that combines digital and remote contact with face-to-face provision, and that can be flexible to a patient and their changing needs, is a model of care many of them wished to continue exploring.
Organising for quality in health care: application of the ‘challenge framework’ to project findings
Bate et al. ’s39 ‘challenge framework’ provides a useful way of structuring our findings. Bate et al. 39 examined health-care organisations that had earned reputations for sustained achievement of quality improvement in an attempt to understand the process of improving quality. Although there are many different routes to sustained quality improvement, they found that the successful organisations they studied shared the ability to respond to multiple challenges. Therefore, we set out below, under Bate et al. ’s39 six domains, what we see as the main challenges and areas of focus that staff, services or commissioners might wish to consider within the area of perioperative PA; Figure 15 provides a summary.
FIGURE 15.
Challenges for perioperative PA promotion.
Structural
-
Good links primary and secondary care, local authorities, grassroots/community organisations and commercial exercise facilities. Co-working sites can be beneficial.
-
Starting early: GPs ‘sowing the seed’ as instigator, interested and involved, and connected with service (requires supported education and active collaboration).
-
Scoping new roles for the ‘perioperative practitioner’ focused on a core compassionate team with knowledge and capacity for empathy, kindness and excellent communication.
-
Intersecting inequalities and inequitable provision considered at all times (including, but not limited to, access to good and appropriate facilities, green space and good pedestrian/cycle ways; appropriate support and/or alternatives relating to digital exclusion; good public transport links; and consideration of cultural norms and practices).
Physical and technological
-
Places and spaces for PA:
-
accessibility (hyperlocal, identified green space, easy transport, parking)
-
warm, well-equipped, ‘nice’ spaces
-
non-clinical venues, part of patients’ community (consider local, sociocultural and socioeconomic contexts, and patient group).
-
-
Digital literacy and access:
-
people can learn (but only with support and patience and when motivated for change)
-
inequity in digital access (devices, internet/data)
-
alternative models with no compromise on quality
-
broader approaches to address physical, social and economic conditions driving marginality.
-
-
-
Collecting and sharing data (between services/departments, for evidence and service development).
-
Data-linking and codes between, for example primary and secondary care.
Political
-
Health-care policy:
-
health equity as central to policy interventions and their design, including consideration of the inter-related mechanisms and disadvantages influencing health and engagement in good health behaviours
-
new PA priorities that place individuals at the centre, take a broad wrap-around approach that aims to be embedded in usual care, and are motivating and inspiring to patients.
-
-
Every service relied on enthusiasts:
-
engendering the value of PA as treatment in health-care narratives (reducing reliance on enthusiasts to make services possible); it helps if this comes from the top, and it requires education and good relationships
-
opportunities for enthusiasts to pursue valuable ideas.
-
-
Encouraging dialogue about PA and supporting a transition to integrating conversations about PA into routine clinical care.
-
Acknowledging and supporting the shift from ‘usual practice’.
-
Supporting and valuing a learning and evolving culture: engaging health-care practitioners in evidence.
-
COVID-19 (evolving practice in response to new contexts, being open to change and adaption at pace and being better prepared for future pandemics).
-
Being prepared to work for and be open-minded about initial funding (seeking collaborations with charitable organisations and other sectors, quality improvement grants, a marketing approach to ‘selling’ ideas/service to commissioners).
Cultural
-
Shared understanding of service objectives, PA as integral ‘part of treatment’, endorsed by all, including patient-valued endorsement from senior clinical practitioners (e.g. patient’s surgeon). ‘Singing from the same hymn sheet’ with a consistent and considered approach from all clinical contacts.
-
Compassionate PA narratives that see patients as:
-
social rather than physiological beings
-
situated at the centre of their own care
-
with autonomy to choose what they enjoy (and could become habit)
-
understanding of what benefits they might experience
-
supported in the process.
-
-
Crossing boundaries and recognising collaborative benefits of the clinical versus non-clinical paradox (community health care at its best; professional medical objectives sharing space and priority with communities).
-
‘Patients at the table’ from start-up to developing and improving provision.
-
Structured supporting of valued staff; recognising the impact of working closely with patients, and sometimes with people with terminal illness, and seeking and acknowledging staff with exceptional compassion and communication skills who are good at noticing patient needs and concerns.
Educational
-
Importance of a learning culture and capacity to evolve.
-
Involvement of patients as co-developers.
-
Learning through measurement.
Emotional
-
Solidarity between patients and staff becoming active together.
-
Providing opportunities for beneficial peer support.
-
Involving families in patient care.
-
Addressing and supporting broader health and well-being through PA behaviour change and bringing back a sense of control in an otherwise difficult period (health agency).
-
Collegiate teams.
-
Compassionate staff who are empathetic, kind, caring and excellent communicators.
-
Glimmer of possibility: astute, patient and committed staff (from the receptionist, to the trainer, to management) able to notice and build on ‘a little glimmer’ of interest from patients.
-
‘Just not the moment’: accepting that patients may not be ready to change ‘just now’ but maintain a legacy for future engagement.
Chapter 5 Recommendations and conclusions
Recommendations for future research
The following recommendations are not presented in priority order; therefore, we give them equal merit.
Patient voice
To hear from patients (in different contexts, such as different communities and people with different surgical indications) about their experiences of PA promotion in the perioperative pathway, in particular patients who have engaged in specific perioperative PA programmes.
Practitioner and service manager voice
-
To further explore (using our expanded thematic analysis) identified tentative values and principles and present these. To study the utility and adoption of these further in the context of shaping new service development or quality improvement in relevant perioperative settings.
-
To further explore the role of the ‘prehabilitation or perioperative practitioner’ as part of a structured evaluation project.
Interventional studies
-
To develop a core outcome set for studies promoting PA (including in pre-surgical prehabilitation), to facilitate a structured approach to evaluation and to allow proper comparison and, if necessary, quantitative synthesis, of studies. We propose outcomes that reflect the wider benefits of PA programmes, for instance greater feelings of control and autonomy for patients, and reduction in symptoms (such as pain). We also recognise the need for adherence to be included.
-
To consider long-term follow-up of study participants beyond 12 months.
-
To provide more contextual information in study reports about how interventions were delivered, or at least make wider use of descriptive frameworks such as the Template for Intervention Description and Replication (TIDieR) checklist. Providing greater detail of the interventions proposed is likely to lead to making such features more explicit.
Inequities
-
To explore the interplay of ethnicity and PA in the perioperative setting, especially given that black and ethnic minority patients have been disproportionately represented in the numbers of COVID-19 deaths and hospital admissions. It will be important to explore cultural narratives, contextual factors and structural assumptions.
-
To map inequities in digital access, which appear to intersect with existing inequities including socioeconomic conditions, pre-existing illness and age. To document and explain corrective strategies from the research literature and exemplar services of relevance to perioperative service delivery. More broadly, to explore the physical, social and economic conditions driving health inequality and inequitable provision for the purpose of equitable ‘lifestyle’ health gains experienced across the population. This may include exploration of radical effective approaches.
Organisation
-
To consider, within service evaluations, the long-term benefits of provision, including social impact as an important measure to quantify the value of broader changes people (and communities) may experience. This could include measuring and acknowledging factors such as social relationships, self-confidence, the creation of ‘safe spaces’, accounting for people being more engaged in positive behaviours, raising awareness of green space depletion and equitable access to public space, and reduced demands on the public purse.
-
To develop and test a relevant service evaluation measures toolkit, to enable a standardised approach to data collection and service evaluation as programmes evolve.
-
To explore how best to flexibly support patients through the now evolving (post-COVID-19) blended (digital/home-based/face-to-face) approaches services are adopting. It would be helpful to understand the optimum blended approach and how patients can still receive the important peer support, compassionate relationships with practitioners, and individualised care and encouragement we identified as important.
-
To design and evaluate simple programmes for enhancing the educational skills of health professionals in the promotion of PA. Although clinical staff are knowledgeable about their own area of work, they are not always trained as ‘educators’, especially of patients and the public.
-
To explore broader implementation issues such as the challenging logistics of embedding such support in perioperative and cancer care pathways.
-
To explore the potential benefits of establishing a national PA ‘observatory’, extending beyond the perioperative setting to encompass PA more generally, initially by a scoping study examining similar initiatives either in other areas of health care in the UK, or relevant centres abroad.
Implications for decision-makers
We would encourage decision-makers to engage in our application of Bate et al. ’s39 challenge framework. However, in summary, the findings of this evidence synthesis support a focus by decision-makers on the following:
-
The repercussions of intersecting inequalities and inequitable access to both services and appropriate public spaces and resources appear to be key to effective engagement of patients/participants and the promising impact of interventions. This warrants, but is not limited to, a focus on good and appropriate facilities (non-clinical, hyperlocal, warm, well-equipped and easy to access), green space and good pedestrian/cycleways, appropriate support and/or alternatives relating to digital exclusion (technologies and access to data), good public transport links and close parking, and culturally accessible services (that consider the norms of all faiths, traditions and backgrounds).
-
Strong links, collaboration, co-working and multipurpose sites between primary and secondary care, local authority services and staff, grassroots/community organisations and commercial exercise facilities can be beneficial in terms of resources, patient care and patient engagement.
-
Seed funding is helpful for getting things going but, once established, a degree of funding security is necessary to ensure investment in staff (who are knowledgeable, excellent communicators and compassionate, and well-supported).
-
Design of services that takes seriously the inclusion of patients ‘at the table’ is essential because different patients, in different places, require different things.
Implications for policy and practice
We would encourage policy decision-makers and practitioners to engage in our application of Bate et al. ’s39 ‘challenge framework’. However, in summary, the findings of this evidence synthesis present the following implications for policy and practice:
-
Active collaboration with colleagues in primary and secondary care, local authorities, grassroots/community organisations and commercial exercise facilities makes programmes possible and supports participant engagement. This includes shared messaging that places conversations about PA into routine clinical care and engenders the value of PA as treatment in all perioperative narratives from receptionist to senior clinical practitioners (and particularly senior clinical practitioners). The sharing and linking of data, the acknowledging of and supporting of the shift from ‘usual practice’, and engaging health-care professionals in evidence may support adoption of this messaging.
-
A core compassionate team with knowledge and capacity for empathy, kindness and excellent communication supports the delivery of PA programmes. This, however, requires staff to be well supported and actively valued. Staff should be able to access training and have available specialist support that acknowledges the impact of often working closely with people with terminal illness. In addition, an active workforce can support solidarity with patients.
-
Policy and delivery of services must focus on reducing the inequalities in the determinants of health. These are interconnecting and far-reaching, but should specifically consider factors influencing engagement in, and maintenance of, good health behaviours, including digital literacy and access to appropriate spaces and facilities.
-
A holistic approach that sees patients as social rather than physiological beings, which places them at the centre of their own care, with autonomy to choose what they enjoy and are motivated to make a habit and with support to understand the benefits they might experience and changes they will need to make, appears to trump any focus on generalised PA goals of dose and intensity.
-
Services benefit from a learning culture that includes ongoing patient feedback and involvement and the capacity to evolve in changing contexts. Data and measurement appear to be key to a learning culture, as well as to buy-in from commissioners and senior management.
Conclusion
The research evidence base for interventions delivered in the perioperative setting, aimed at enhancing PA among patients in the longer term, suggests some overall benefit in terms of engagement, levels of activity, physical fitness and quality of life. Our detailed contextual enquiry complements the research literature by identifying many features that contribute to establishing and delivering successful programmes.
Chapter 6 Differences between protocol and completed project
The protocol for this project was published on PROSPERO. 129 We describe here the changes made during the project.
Systematic review
In the protocol, we described this part of the project as a comprehensive literature search. Given the rigorous methods used, we refer to this as a systematic review (or review) throughout this report.
Types of participants
We included people who had undergone surgery, as well as those who were scheduled to undergo surgery. The intention was always to capture people at any stage during the perioperative pathway. We found, however, that this criterion was too broad; therefore, we established a more specific criterion based on time since surgery, as well as a cut-off point to manage studies that had a mixed population of surgical and non-surgical participants. We adapted the criterion further for participants within an oncological treatment pathway, which was often a much longer pathway and did not always include surgery. We believed that it was not feasible to establish a specific time since surgery that captured the variable lengths of perioperative period for all potential studies; we acknowledge that cut-off points in the review were arbitrary. We reached decisions following discussion with clinical members of the Advisory Group.
Types of interventions
In the protocol, we stated that we would exclude interventions that were given as part of a ‘package’ of measures aimed at promoting a healthier lifestyle. However, once we began identifying potentially eligible studies, we judged this criterion to be too restrictive. We found that it was most likely that well-designed studies would combine educational provision or advice that included approaches to overall health management, as well as PA. In the review, we included these ‘packages’ of interventions, but required PA to be a dominant part of those packages; we used outcomes in the reported studies (see below) to assist the decision of whether or not PA was a dominant part of this package.
Types of outcomes
We found that many studies included PA interventions but did not have the intention to measure PA in the medium to long term. Whenever possible, we used the study objectives or a sample size calculation to determine the aim of the study. However, we found that these were often poorly reported. We adopted a criterion to include studies only if they measured and reported PA either as an amount of PA or as engagement in PA; the studies could describe these outcomes as primary or secondary outcomes. We also engaged with our PPI representative (AC) who asked that we also capture pain data and we added this to our list of outcomes; this outcome could indicate a benefit or harm of PA interventions. In the protocol, we did not present our outcomes in order of primary and secondary outcomes. When conducting the review, we provided a better structure for these outcomes and agreed that the primary outcomes were about how much PA people engaged in (either as an amount or as a number who were engaging in PA). The protocol included cancellation of surgery as an outcome. We identified no studies that measured or reported this outcome (most included studies initiated interventions after surgery), and so we removed this outcome.
Assessment of study quality and synthesis of findings
We did not conduct a quality assessment, such as the Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I), for non-RCT designs. When setting out the protocol, we did not expect to find a substantial body of evidence from RCTs. Given the more robust design of RCTs, we judged it more appropriate to consider the synthesis of findings from the RCTs as the main findings. However, we identified a number of non-RCTs during our search and, although we present a briefer overview of their findings in the report, we judged it relevant to still include these studies. The evidence from non-RCTs formed a supplementary set.
Data collection and analysis
We had presented only limited detail on our methods for managing data within the review. In this report, we present these methods in more detail. These methods are largely consistent with accepted systematic review methodology and allowed us to be transparent about all our approaches. We did not specify sensitivity analysis or exact subgroup analysis in the protocol. Our choice of subgroups was based on the broad range of interventions and participant conditions within our included studies and we were not able to anticipate this range prior to the search. Some sensitivity analyses were based on risk-of-bias decisions, and others on specific case-by-case decisions made during the review project (such as when more than one tool was used to measure an outcome).
Case studies
This project took place between 2019 and 2021. Plans for our case study investigation were halted in March 2020 because of the COVID-19 pandemic. At this stage, we had identified a broad list of possible services (these are presented in a directory of services; see Report Supplementary Material 10), we had approval from the Health Research Authority and we had engaged with a small number of services that were keen to work with us during site visits.
In the protocol, we stated an aim to collect qualitative data from services during site visits, which included interviews (most likely as focus group interviews with patients and one-to-one interviews with staff) and observations of practice. Our intention was to conduct these site visits in person.
As this was no longer viable, we worked with the funding body representatives, the Advisory Group and the Study Steering Committee, to create a new plan for this part of the project. We believed that there were still opportunities for us to collect useful information, and new approaches learnt as a consequence of the pandemic environment. We engaged with the identified services to see if the revised approach was acceptable and practical within the current context. We did not visit services, conduct face-to-face interviews or carry out any observations of practice.
Although we had sought preliminary agreement from services to engage their service users in our work, by the time that all approvals had been sought, and a survey agreed and produced, online services found that they were too overwhelmed to be able to support this element of the project. They cited efforts from their own teams to engage their patients in research around the COVID-19 response, pressures on their own time and patients citing that they were overburdened and overwhelmed by online engagement. As a result, this element of the project was not progressed.
Narrative synthesis
We stated within the original protocol that we would draw up a practical toolkit. During our research, it was apparent that we did not have the resources (in terms of time) to complete this part of the project within the time frame required by the funders; an edit was made to the protocol to reflect this, in consultation with the funders.
We did not present a graphical summary-of-findings statement. Combining the qualitative and quantitative results of this project within a template summary-of-findings table was not practical. Ideally, a summary of findings provides presentation summary statistics along with certainty of the evidence in a concise format. We found that our review outcomes were measured using many different approaches and these results could not easily be presented concisely. However, we believed that it was important to present certainty of the evidence; therefore, we used GRADE for the results of the systematic review and presented a narrative summary of these findings.
Patient and public involvement
We planned to work with two or three further PPI representatives. With the support of our PPI representative (AC), we identified the support of one national PPI representative. We were also preparing to recruit a local representative, from within our hospital trust, who was in the perioperative pathway. However, because of the impact of the COVID-19 pandemic, it was no longer feasible to draw on clinical colleagues to support local recruitment or to engage further with PPI representatives. We remained committed to working with Antony Chuter throughout the project.
Chapter 7 Stakeholder and patient and public involvement
We worked closely with Antony Chuter as our primary PPI representative. Antony Chuter lives with long-term conditions of pain, is chairperson of Pain UK and has extensive experience as a lay member on a variety of health-care research projects. Antony Chuter was a paid co-applicant on the study, and was involved in the project from bid-writing to completion.
We describe Antony Chuter’s involvement as it occurred throughout the report and we summarise Antony Chuter’s contributions below, including reflections from both Antony Chuter and the research team on the impact and efficacy of working with Antony Chuter on this project.
Primary PPI contributions included involvement in the following:
-
The early development of the project, including in the writing of the research funding application, developing the final research plan and recruitment of a researcher to the project (including sifting and scoring applications and conducting candidate interviews).
-
Encouraging the use of patient-focused language, for example advising against the original title of the project from ‘perioperative exercise project’ to ‘fit for surgery’. Antony Chuter continued to encourage patient-focused language throughout the duration of the project, both in written documentation (such as consent forms) and in conversational language among the research team.
-
Attending Advisory Group meetings, in person and via Microsoft Teams.
-
Selection of outcome measures within the systematic review. Antony Chuter influenced the decision to include pain measures as a secondary outcome of interest, emphasising the association between experiences of pain and an individual’s well-being and capacity/motivation to engage in PA.
-
Support with additional PPI recruitment (not progressed because of COVID-19).
-
Co-development/review of focus group topic guide, survey questions for patients and services, staff and patient consent forms, and participant information sheets.
-
Co-facilitation of two online focus group discussions.
-
Reflective discussion of quantitative data (interview/discussion group transcripts) to inform analytical framework.
-
Review of final report.
Towards the end of the project, Antony Chuter and one of the researchers engaged in a reflective meeting to consider the impact of PPI, the more nuanced contributions Antony Chuter provided and how the research team might seek to improve PPI in future research projects. A summary of the key points is included below:
-
Antony Chuter frequently emphasised to the research team how much language really matters. He described the reception to his encouragement in the early days of the project to consider more patient-focused language as ‘changing the atmosphere in the team’ and the ‘focus of the research’. Antony Chuter described this as accounting for a much broader perspective of the people who might benefit from activity and increased fitness. Antony Chuter also encouraged regular reflection on subjective perceptions of exercise for people with and people without a long-term health condition.
-
Antony Chuter would have benefited from being involved in a regular monthly meeting with the whole research team. This would have helped Antony Chuter to have ‘felt in touch’ and ‘more aware of the project as it evolved’.
-
Antony Chuter is an extensively experienced PPI representative and patient advocate with the associated confidence and understanding of ‘how health research works’. We discussed the different perspectives that Antony Chuter brought to the project, compared with someone who might be more of a ‘raw’ local surgery patient (representative). Although Antony Chuter felt able to be ‘a physical representation’ of patients, in part because of Antony Chuter’s experience of long-term conditions of pain and wide experience working with patient groups, there was some agreement that a combination of the two would have enhanced the project.
-
The research team felt that a central and constant commitment to PPI in our work and individual roles was needed to ensure that we were not using PPI as a ‘tokenistic’ gesture. During the project, we acknowledged that to do this effectively required allocation of our own time to account for effective and productive communication with the PPI representative in a way that ensured full involvement.
-
Antony Chuter commented that he felt ‘respected over a very short time’ and that there was agreement of good collegiate relationships with the research team, and that members, including Antony Chuter, brought ‘different skills to the table’. Antony Chuter described the principal investigator on the project as ‘a trailblazer for public involvement’, noting in particular the importance of being paid consistently throughout the project, from bid development to project completion (rather than at irregular times), and relationships in which members of the research team ‘gave time’ to Antony Chuter’s contributions.
-
One researcher felt particularly positive about Antony Chuter’s contribution to focus group discussions. As well as practical support, Antony Chuter was able to act as a pseudo patient, at times encouraging the discussion among focus group participants back to the patient and prompting interesting reflection among and between group participants. The researcher noted that there was a need for them (as co-facilitator) to be a bit more relaxed around lines of discussion that sometimes deviated from the discussion guide, but that, ultimately, they recognised the important conversations that Antony Chuter’s questions initiated.
Acknowledgements
We would like to thank the management and support team of the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) for their advice throughout this project, and for their flexibility in responding to the challenges we experienced conducting this project during the COVID-19 pandemic.
We are grateful for the valuable contributions from our Advisory Group (Phil Alderson, Gaynor Arnold, Kate Carey, Chris Coldwell, Wendy Craig, Margaret Cooper, Sir Muir Gray, Erica Janta and Nicola Swindlehurst) and our Study Steering Committee (Professor Alan Batterham, Mr Harry Gray, Professor Sandy Jack, Professor John Saxton and Professor Garry Tew).
We would like to thank all of the participants who attended focus group discussions and interviews, in particular for giving their time during what was a fraught and challenging period. Their energy and openness to engage in discussions was hugely valuable to this piece of work.
We would also like to thank the practitioners who kindly provided information about their services during our initial scoping and preparation for the case study observations.
Contributions of authors
Michael W Pritchard (https://orcid.org/0000-0002-6062-5016) (Research Associate) screened references, completed the full-text review, conducted data extraction and the synthesis of the quantitative data, and wrote the final report.
Amy Robinson (https://orcid.org/0000-0001-9850-4116) (Research Associate) screened references; completed the full-text review; conducted data extraction; completed scoping for the case studies; conducted focus group discussions and interviews; transcribed, and conducted the thematic analysis of, the qualitative data; and wrote the final report.
Sharon R Lewis (https://orcid.org/0000-0002-3408-3962) (Senior Research Associate) prepared search strategies, screened references, completed the full-text review, conducted data extraction, provided advice and guidance on systematic review preparation, and wrote the final report.
Suse V Gibson (https://orcid.org/0000-0002-1713-7180) (Research Co-ordinator) liaised with NIHR, prepared the protocol for PROSPERO, prepared search strategies and ran searches, screened references, completed the full-text review and wrote the final report.
Antony Chuter (https://orcid.org/0000-0002-0646-5939) (PPI representative, Trustee and Chairperson of Pain UK) provided guidance and advice throughout project, conducted focus group discussions, conducted the thematic analysis of the qualitative data and wrote the report.
Robert Copeland (https://orcid.org/0000-0002-4147-5876) (Professor of Physical Activity and Health) and Euan Lawson (https://orcid.org/0000-0002-4843-7733) (Director of Community Studies) provided guidance and advice throughout the project and advised on the final report.
Andrew F Smith (https://orcid.org/0000-0003-2650-9764) (Principal Investigator, Consultant Anaesthetist) conducted the analysis of the qualitative data, wrote the final report and was the guarantor.
Publication
Smith A, Lewis S, Gibson S, Robinson A, Lawson E. ‘Fit for Surgery’ or ‘Fit for Life’? Exploring the Potential of Using the Perioperative Encounter to Promote Regular Exercise and Physical Activity: An Expanded Evidence Synthesis. URL: www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=139008 (accessed 14 March 2022).
Data-sharing statement
Further data from either part of the project are available on request from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Moon YE, Lee SH, Lee J. The optimal dose of esmolol and nicardipine for maintaining cardiovascular stability during rapid-sequence induction. J Clin Anesth 2012;24:8-13. https://doi.org/10.1016/j.jclinane.2010.12.010.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Lancet Physical Activity Series Working Group . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219-29. https://doi.org/10.1016/S0140-6736(12)61031-9.
- Department of Health and Social Care . UK Physical Activity Guidelines 2019.
- Department of Health and Social Care . Public Health Outcomes Framework. Improving Outcomes and Supporting Transparency. Part 2: Summary Technical Specifications of Public Health Indicators 2016.
- Royal College of Surgeons of England . Surgery and the NHS in Numbers n.d. www.rcseng.ac.uk/news-and-events/media-centre/media-background-briefings-and-statistics/surgery-and-the-nhs-in-numbers/ (accessed 13 May 2021).
- Dhatariya K, Levy N, Flanagan D, Hilton L, Kilvert A, Rayman G, et al. Management of Adults with Diabetes Undergoing Surgery and Elective Procedures: Improving Standards. London: Joint British Diabetes Societies for Inpatient Care; 2016.
- Richardson K, Levett DZH, Jack S, Grocott MPW. Fit for surgery? Perspectives on preoperative exercise testing and training. Br J Anaesth 2017;119:i34-43. https://doi.org/10.1093/bja/aex393.
- Loughney L, West MA, Kemp GJ, Grocott MP, Jack S. Exercise intervention in people with cancer undergoing neoadjuvant cancer treatment and surgery: a systematic review. Eur J Surg Oncol 2016;42:28-3. https://doi.org/10.1016/j.ejso.2015.09.027.
- Royal College of Anaesthetists . Perioperative Medicine: The Pathway to Better Surgical Care 2015.
- Pucher PH, Aggarwal R, Singh P, Darzi A. Enhancing surgical performance outcomes through process-driven care: a systematic review. World J Surg 2014;38:1362-73. https://doi.org/10.1007/s00268-013-2424-8.
- Oppedal K, Møller AM, Pedersen B, Tønnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev 2012;7. https://doi.org/10.1002/14651858.CD008343.pub2.
- Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev 2014;3. https://doi.org/10.1002/14651858.CD002294.pub4.
- Moonesinghe SR, Harris S, Mythen MG, Rowan KM, Haddad FS, Emberton M, et al. Survival after postoperative morbidity: a longitudinal observational cohort study. Br J Anaesth 2014;113:977-84. https://doi.org/10.1093/bja/aeu224.
- Nicholson A, Coldwell CH, Lewis SR, Smith AF. Nurse-led versus doctor-led preoperative assessment for elective surgical patients requiring regional or general anaesthesia. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD010160.pub2.
- Lowe A, Gee M, McLean S, Littlewood C, Lindsay C, Everett S. Physical activity promotion in physiotherapy practice: a systematic scoping review of a decade of literature. Br J Sports Med 2018;52:122-7. https://doi.org/10.1136/bjsports-2016-096735.
- Department of Health and Social Care . Equity and Excellence: Liberating the NHS 2010.
- Goodwin N, Smith J, Davies A, Perry C, Rosen R, Dixon A, et al. Integrated Care for Patients and Populations: Improving Outcomes by Working Together. London: The King’s Fund; 2011.
- Powell Davies G, Williams AM, Larsen K, Perkins D, Roland M, Harris MF. Coordinating primary health care: an analysis of the outcomes of a systematic review. Med J Aust 2008;188:S65-8. https://doi.org/10.5694/j.1326-5377.2008.tb01748.x.
- Durrand J, McHardy F, Land E, Llewellyn Z, Norman C, Goh T, et al. PREP – The Preoperative Risk Education Package: an online educational resource for primary care clinicians to encourage community based prehabilitation prior to major surgery. Br J Gen Pract 2018;68. https://doi.org/10.3399/bjgp18X697277.
- Crisford P, Winzenberg T, Venn A, Schultz M, Aitken D, Cleland V. Factors associated with physical activity promotion by allied and other non-medical health professionals: a systematic review. Patient Educ Couns 2018;101:1775-85. https://doi.org/10.1016/j.pec.2018.05.011.
- Ferrer DA, Ellis R. A review of physical activity interventions delivered via Facebook. J Phys Act Health 2017;14:823-33. https://doi.org/10.1123/jpah.2016-0534.
- Jahangiry L, Farhangi MA, Shab-Bidar S, Rezaei F, Pashaei T. Web-based physical activity interventions: a systematic review and meta-analysis of randomized controlled trials. Public Health 2017;152:36-4. https://doi.org/10.1016/j.puhe.2017.06.005.
- O’Halloran PD, Blackstock F, Shields N, Holland A, Iles R, Kingsley M, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil 2014;28:1159-71. https://doi.org/10.1177/0269215514536210.
- Williams I, Glasby J. Making ‘what works’ work: the use of knowledge in UK health and social care decision-making. Policy Soc 2010;29:95-102. https://doi.org/10.1016/j.polsoc.2010.03.002.
- Pope C, Smith A, Goodwin D, Mort M. Passing on tacit knowledge in anaesthesia: a qualitative study. Med Educ 2003;37:650-5. https://doi.org/10.1046/j.1365-2923.2003.01581.x.
- Smith A, Goodwin D, Mort M, Pope C. Expertise in practice: an ethnographic study exploring acquisition and use of knowledge in anaesthesia. Br J Anaesth 2003;91:319-28. https://doi.org/10.1093/bja/aeg180.
- Smith A, Boult M, Woods I, Johnson S. Promoting patient safety through prospective risk identification: example from peri-operative care. Qual Saf Health Care 2010;19:69-73. https://doi.org/10.1136/qshc.2008.028050.
- NHS England, NHS Improvement . NHS Operational Planning and Contracting Guidance 2017–2019 n.d. www.england.nhs.uk/wp-content/uploads/2016/09/NHS-operational-planning-guidance-201617-201819.pdf (accessed 14 March 2022).
- Baker PR, Francis DP, Soares J, Weightman AL, Foster C. Community wide interventions for increasing physical activity. Cochrane Database Syst Rev 2015;1. https://doi.org/10.1002/14651858.CD008366.pub3.
- National Institute for Health and Care Excellence (NICE) . Physical Activity: Brief Advice for Adults in Primary Care 2013.
- Lamming L, Pears S, Mason D, Morton K, Bijker M, Sutton S, et al. VBI Programme Team . What do we know about brief interventions for physical activity that could be delivered in primary care consultations? A systematic review of reviews. Prev Med 2017;99:152-63. https://doi.org/10.1016/j.ypmed.2017.02.017.
- GC V, Wilson EC, Suhrcke M, Hardeman W, Sutton S. VBI Programme Team . Are brief interventions to increase physical activity cost-effective? A systematic review. Br J Sports Med 2016;50:408-17. https://doi.org/10.1136/bjsports-2015-094655.
- Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077-86. https://doi.org/10.1016/S2214-109X(18)30357-7.
- NHS Health Education England . Making Every Contact Count n.d. www.makingeverycontactcount.co.uk/ (accessed 13 May 2021).
- Faculty of Sport and Exercise Medicine UK . Moving Medicine n.d. https://movingmedicine.ac.uk/ (accessed 20 April 2022).
- Hellen N. Pick up thy bed and work out: GPs give rest cure its marching orders. The Sunday Times 2018.
- James Lind Alliance . Priority Setting Partnerships n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/ (accessed 13 May 2021).
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons Ltd; 2011.
- Bate SP, Mendel P, Robert G. Organising for Quality: The Improvement Journeys of Leading Hospitals in Europe and the United States. Oxford: Radcliffe; 2008.
- U.S. National Library of Medicine . ClinicalTrial.Gov n.d. https://clinicaltrials.gov/ (accessed 13 May 2021).
- World Health Organization . International Clinical Trials Registry Platform (ICTRP) n.d. www.who.int/ictrp/en/ (accessed 13 May 2021).
- McGrath Y, Sumnall H, Edmonds K, McVeigh J, Bellis M. Review of Grey Literature on Drug Prevention Among Young People. London: National Institute for Health and Care Excellence; 2006.
- OpenGrey . OPENGREY.EU – Grey Literature Database n.d. www.opengrey.eu/ (accessed 13 May 2021).
- Higgins JP, Churchill R, Chandler J, Cumpston MS. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017). London: The Cochrane Collaboration; 2017.
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence – inconsistency. J Clin Epidemiol 2011;64:1294-302. https://doi.org/10.1016/j.jclinepi.2011.03.017.
- Sterne JA, Egger M, Moher D, Boutron I, Higgins JP, Churchill R, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (Updated June 2017). London: The Cochrane Collaboration; 2017.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. J Clin Epidemiol 2011;64:1283-93. https://doi.org/10.1016/j.jclinepi.2011.01.012.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Archer KR, Coronado RA, Haug CM, Vanston SW, Devin CJ, Fonnesbeck CJ, et al. A comparative effectiveness trial of postoperative management for lumbar spine surgery: changing behavior through physical therapy (CBPT) study protocol. BMC Musculoskelet Disord 2014;15. https://doi.org/10.1186/1471-2474-15-325.
- Artz N, Dixon S, Wylde V, Marques E, Beswick AD, Lenguerrand E, et al. Comparison of group-based outpatient physiotherapy with usual care after total knee replacement: a feasibility study for a randomized controlled trial. Clin Rehabil 2017;31:487-99. https://doi.org/10.1177/0269215516642503.
- Baillot A, Vallée CA, Mampuya WM, Dionne IJ, Comeau E, Méziat-Burdin A, et al. Effects of a pre-surgery supervised exercise training 1 year after bariatric surgery: a randomized controlled study. Obes Surg 2018;28:955-62. https://doi.org/10.1007/s11695-017-2943-8.
- Barberan-Garcia A, Ubre M, Pascual-Argente N, Risco R, Faner J, Balust J, et al. Post-discharge impact and cost–consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: secondary results from a randomised controlled trial. Br J Anaesth 2019;123:450-6. https://doi.org/10.1016/j.bja.2019.05.032.
- Barnason S, Zimmerman L, Nieveen J, Schulz P, Miller C, Hertzog M, et al. Influence of a symptom management telehealth intervention on older adults’ early recovery outcomes after coronary artery bypass surgery. Heart Lung 2009;38:364-76. https://doi.org/10.1016/j.hrtlng.2009.01.005.
- Boesch C, Myers J, Habersaat A, Ilarraza H, Kottman W, Dubach P. Maintenance of exercise capacity and physical activity patterns 2 years after cardiac rehabilitation. J Cardiopulm Rehabil 2005;25:14-21. https://doi.org/10.1097/00008483-200501000-00004.
- Bond DS, Thomas JG, Vithiananthan S, Unick J, Webster J, Roye GD, et al. Intervention-related increases in preoperative physical activity are maintained 6-months after bariatric surgery: results from the bari-active trial. Int J Obes 2017;41:467-70. https://doi.org/10.1038/ijo.2016.237.
- Brandes M, Wirsik N, Niehoff H, Heimsoth J, Möhring B. Impact of a tailored activity counselling intervention during inpatient rehabilitation after knee and hip arthroplasty – an explorative RCT. BMC Musculoskelet Disord 2018;19. https://doi.org/10.1186/s12891-018-2130-7.
- Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psycho-Oncology 2009;18:343-52. https://doi.org/10.1002/pon.1525.
- Carnero EA, Dubis GS, Hames KC, Jakicic JM, Houmard JA, Coen PM, et al. Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity 2017;25:1206-16. https://doi.org/10.1002/oby.21864.
- Christiansen CL, Miller MJ, Murray AM, Stephenson RO, Stevens-Lapsley JE, Hiatt WR, et al. Behavior-change intervention targeting physical function, walking, and disability after dysvascular amputation: a randomized controlled pilot trial. Arch Phys Med Rehabil 2018;99:2160-7. https://doi.org/10.1016/j.apmr.2018.04.011.
- Christiansen MB, Thoma LM, Master H, Mathews D, Schmitt LA, White DK. Preliminary findings of a novel physical therapist administered physical activity intervention after total knee replacement. Osteoarthr Cartil 2018;26. https://doi.org/10.1016/j.joca.2018.02.663.
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 2007;25:4396-404. https://doi.org/10.1200/JCO.2006.08.2024.
- Creel DB, Schuh LM, Reed CA, Gomez AR, Hurst LA, Stote J, et al. A randomized trial comparing two interventions to increase physical activity among patients undergoing bariatric surgery. Obesity 2016;24:1660-8. https://doi.org/10.1002/oby.21548.
- Dantas RA, Aguillar OM, dos Santos Barbeira CB. Implementation of a nurse-monitored protocol in a Brazilian hospital: a pilot study with cardiac surgery patients. Patient Educ Couns 2002;46:261-6. https://doi.org/10.1016/S0738-3991(01)00162-8.
- Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol 2007;25:2709-18. https://doi.org/10.1200/JCO.2007.10.7094.
- Doganay E, Wynter-Blyth V, Halliday L, Mackinnon T, Osborn H, Moorthy K. Study of long-term follow-up of exercise levels following participation in a prehabilitation program in esophagogastric cancer. Rehabil Oncol 2020;38:110-15. https://doi.org/10.1097/01.REO.0000000000000205.
- Duculan R, Rigaud M, Cammisa FP, Sama AA, Hughes AP, Mancuso CA, et al. Fostering physical activity after complex lumbar spine surgery: long-term results of a randomized trial. Spine J 2020;20:S96-S7. https://doi.org/10.1016/j.spinee.2020.05.606.
- Eakin EG, Lawler SP, Winkler EA, Hayes SC. A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: exercise for health. Ann Behav Med 2012;43:229-38. https://doi.org/10.1007/s12160-011-9324-7.
- Engblom E, Rönnemaa T, Hämäläinen H, Kallio V, Vänttinen E, Knuts LR. Coronary heart disease risk factors before and after bypass surgery: results of a controlled trial on multifactorial rehabilitation. Eur Heart J 1992;13:232-7. https://doi.org/10.1093/oxfordjournals.eurheartj.a060152.
- Fontana AF, Kerns RD, Rosenberg RL. Exercise training for cardiac patients: adherence, fitness, and benefits. J Cardiopulm Rehabil 1986;6:4-15. https://doi.org/10.1097/00008483-198601000-00001.
- Foster C, Pollock ML, Anholm JD, Squires RW, Ward A, Dymond DS, et al. Work capacity and left ventricular function during rehabilitation after myocardial revascularization surgery. Circulation 1984;69:748-55. https://doi.org/10.1161/01.cir.69.4.748.
- Frawley HC, Lin KY, Granger CL, Higgins R, Butler M, Denehy L. An allied health rehabilitation program for patients following surgery for abdomino-pelvic cancer: a feasibility and pilot clinical study. Support Care Cancer 2020;28:1335-50. https://doi.org/10.1007/s00520-019-04931-w.
- Goedendorp MM, Peters MEWJ, Gielissen MFM, Witjes JA, Leer JW, . Verhagen CAHHVM . Is increasing physical activity necessary to diminish fatigue during cancer treatment? Comparing cognitive behaviour therapy and a brief nursing intervention with usual care in a multicenter randomised controlled trial. Oncologist 2010;15:1122-32. https://doi.org/10.1634/theoncologist.2010-0092.
- Golsteijn RHJ, Bolman C, Volders E, Peels DA, de Vries H, Lechner L. Short-term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: a randomized controlled trial. Int J Behav Nutr Phys Act 2018;15. https://doi.org/10.1186/s12966-018-0734-9.
- Hackshaw-McGeagh L, Lane JA, Persad R, Gillatt D, Holly JM, Koupparis A, et al. Prostate cancer – evidence of exercise and nutrition trial (PrEvENT): study protocol for a randomised controlled feasibility trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1248-x.
- Hauer K, Specht N, Schuler M, Bärtsch P, Oster P. Intensive physical training in geriatric patients after severe falls and hip surgery. Age Ageing 2002;31:49-57. https://doi.org/10.1093/ageing/31.1.49.
- Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol 2013;31:2313-21. https://doi.org/10.1200/JCO.2012.45.5873.
- Heiberg KE, Figved W. Physical functioning and prediction of physical activity after total hip arthroplasty: five-year followup of a randomized controlled trial. Arthritis Care Res 2016;68:454-62. https://doi.org/10.1002/acr.22679.
- Heitkamp M, Spanier B, Von Korn P, Halle M. Feasibility of a 12 months exercise intervention in colorectal cancer patients – the F-PROTECT study. Oncol Res Treat 2018;41.
- Hoorntje A, Witjes S, Kuijer PPFM, Bussmann JBJ, Horemans HLD, Kerkhoffs GMMJ, et al. Does activity-based rehabilitation with goal attainment scaling increase physical activity among younger knee arthroplasty patients? Results from the randomized controlled ACTION trial. J Arthroplasty 2020;35:706-11. https://doi.org/10.1016/j.arth.2019.10.028.
- Hubbard G, Munro J, O’Carroll R, Mutrie N, Kidd L, Haw S, et al. The use of cardiac rehabilitation services to aid the recovery of colorectal cancer patients: a pilot randomised controlled trial (RCT) with embedded feasibility study. Health Serv Deliv Res 2016;4. https://doi.org/10.3310/hsdr04240.
- Husebø AM, Dyrstad SM, Mjaaland I, Søreide JA, Bru E. Effects of scheduled exercise on cancer-related fatigue in women with early breast cancer. Scientific World Journal 2014;2014. https://doi.org/10.1155/2014/271828.
- Ilves O, Häkkinen A, Dekker J, Wahlman M, Tarnanen S, Pekkanen L, et al. Effectiveness of postoperative home-exercise compared with usual care on kinesiophobia and physical activity in spondylolisthesis: a randomized controlled trial. J Rehabil Med 2017;49:751-7. https://doi.org/10.2340/16501977-2268.
- Jiménez-Loaisa A, González-Cutre D, Beltrán-Carrillo VJ, Alcaraz-Ibáñez M. Changes in bariatric patients’ physical activity levels and health-related quality of life following a postoperative motivational physical activity intervention. Obes Surg 2020;30:2302-12. https://doi.org/10.1007/s11695-020-04489-1.
- Johansson AC, Linton SJ, Bergkvist L, Nilsson O, Cornefjord M. Clinic-based training in comparison to home-based training after first-time lumbar disc surgery: a randomised controlled trial. Eur Spine J 2009;18:398-409. https://doi.org/10.1007/s00586-008-0826-3.
- Jolly K, Taylor R, Lip GY, Greenfield S, Raftery J, Mant J, et al. The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Home-based compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11350.
- Kinsey MG, Fletcher BJ, Rice CR, Watson PH, Fletcher GF. Coronary risk factor modification followed by home-monitored exercise in coronary bypass surgery patients: a four-year follow-up study. J Cardiopulm Rehabil 1989;9:207-12. https://doi.org/10.1097/00008483-198905000-00005.
- Komatsu H, Watanuki S, Koyama Y, Iino K, Kurihara M, Uesugi H, et al. Nurse counseling for physical activity in patients undergoing esophagectomy. Gastroenterol Nurs 2018;41:233-9. https://doi.org/10.1097/SGA.0000000000000252.
- Kraal JJ, Peek N, van den Akker-Van Marle ME, Kemps HM. Effects and costs of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: the FIT@Home study. BMC Cardiovasc Disord 2013;13. https://doi.org/10.1186/1471-2261-13-82.
- Kummel M, Vahlberg T, Ojanlatva A, Kärki R, Mattila T, Kivelä SL. Effects of an intervention on health behaviors of older coronary artery bypass (CAB) patients. Arch Gerontol Geriatr 2008;46:227-44. https://doi.org/10.1016/j.archger.2007.04.003.
- Lear SA, Ignaszewski A, Linden W, Brozic A, Kiess M, Spinelli JJ, et al. The Extensive Lifestyle Management Intervention (ELMI) following cardiac rehabilitation trial. Eur Heart J 2003;24:1920-7. https://doi.org/10.1016/j.ehj.2003.08.015.
- Li Z, Jiang L, Lin J. The effect of education for daily physical activity level recovery of osteoarthritis patients after total knee arthroplasty. A prospective randomized controlled clinical trial using accelerometry. Osteoarthr Cartil 2015;23. https://doi.org/10.1016/j.joca.2015.02.686.
- Lier HØ, Biringer E, Stubhaug B, Tangen T. The impact of preoperative counseling on postoperative treatment adherence in bariatric surgery patients: a randomized controlled trial. Patient Educ Couns 2012;87:336-42. https://doi.org/10.1016/j.pec.2011.09.014.
- Lindbäck Y, Tropp H, Enthoven P, Abbott A, Öberg B. PREPARE: presurgery physiotherapy for patients with degenerative lumbar spine disorder: a randomized controlled trial. Spine J 2018;18:1347-55. https://doi.org/10.1016/j.spinee.2017.12.009.
- Losina E, Collins JE, Deshpande BR, Smith SR, Michl GL, Usiskin IM, et al. Financial incentives and health coaching to improve physical activity following total knee replacement: a randomized controlled trial. Arthritis Care Res 2018;70:732-40. https://doi.org/10.1002/acr.23324.
- Lotzke H, Brisby H, Gutke A, Hägg O, Jakobsson M, Smeets R, et al. A person-centered prehabilitation program based on cognitive–behavioral physical therapy for patients scheduled for lumbar fusion surgery: a randomized controlled trial. Phys Ther 2019;99:1069-88. https://doi.org/10.1093/ptj/pzz020.
- Macchi C, Polcaro P, Cecchi F, Zipoli R, Sofi F, Romanelli A, et al. One-year adherence to exercise in elderly patients receiving postacute inpatient rehabilitation after cardiac surgery. Am J Phys Med Rehabil 2009;88:727-34. https://doi.org/10.1097/PHM.0b013e3181b332a1.
- Macleod M, Steele RJC, O’Carroll RE, Wells M, Campbell A, Sugden JA, et al. Feasibility study to assess the delivery of a lifestyle intervention (TreatWELL) for patients with colorectal cancer undergoing potentially curative treatment. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-021117.
- Mundle JS, MacLeod JB, Hassan A, Lutchmedial S. Assessing the impact of accelerometry device use on exercise motivation and clinical outcomes in patients attending cardiac rehabilitation following percutaneous coronary intervention or cardiac surgery. J Cardiopulm Rehabil Prev 2016;36.
- Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM, et al. A randomized trial of exercise training after renal transplantation. Transplantation 2002;74:42-8. https://doi.org/10.1097/00007890-200207150-00008.
- Piva SR, Moore CG, Schneider MJ, Gil AB, Almeida GJ, Irrgang JJ. A randomized trial to compare exercise treatment methods for patients after total knee replacement: protocol paper. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0761-5.
- Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet-Dion G, Alibhai SMH, et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol 2018;27:289-98. https://doi.org/10.1016/j.suronc.2018.05.010.
- Sellberg F, Possmark S, Willmer M, Tynelius P, Berglind D. One-year follow-up of a dissonance-based intervention on quality of life, wellbeing, and physical activity after Roux-en-Y gastric bypass surgery: a randomized controlled trial. Surg Obes Relat Dis 2019;15:1731-7. https://doi.org/10.1016/j.soard.2019.07.001.
- Smith KM, Arthur HM, McKelvie RS, Kodis J. Differences in sustainability of exercise and health-related quality of life outcomes following home or hospital-based cardiac rehabilitation. Eur J Cardiovasc Prev Rehabil 2004;11:313-19. https://doi.org/10.1097/01.hjr.0000136414.40017.10.
- Stolberg CR, Mundbjerg LH, Bladbjerg EM, Funch-Jensen P, Gram B, Juhl CB. Physical training following gastric bypass: effects on physical activity and quality of life – a randomized controlled trial. Qual Life Res 2018;27:3113-22. https://doi.org/10.1007/s11136-018-1938-9.
- Taraldsen K, Thingstad P, Døhl Ø, Follestad T, Helbostad JL, Lamb SE, et al. Short and long-term clinical effectiveness and cost-effectiveness of a late-phase community-based balance and gait exercise program following hip fracture. The EVA-Hip randomised controlled trial. PLOS One 2019;14. https://doi.org/10.1371/journal.pone.0224971.
- Turunen K, Salpakoski A, Edgren J, Törmäkangas T, Arkela M, Kallinen M, et al. Physical activity after a hip fracture: effect of a multicomponent home-based rehabilitation program – a secondary analysis of a randomized controlled trial. Arch Phys Med Rehabil 2017;98:981-8. https://doi.org/10.1016/j.apmr.2017.01.004.
- Turunen KM, Aaltonen-Määttä L, Törmäkangas T, Rantalainen T, Portegijs E, Keikkala S, et al. Effects of an individually targeted multicomponent counseling and home-based rehabilitation program on physical activity and mobility in community-dwelling older people after discharge from hospital: a randomized controlled trial. Clin Rehabil 2020;34:491-503. https://doi.org/10.1177/0269215519901155.
- Van der Walt N, Salmon LJ, Gooden B, Lyons MC, O’Sullivan M, Martina K, et al. Feedback from activity trackers improves daily step count after knee and hip arthroplasty: a randomized controlled trial. J Arthroplasty 2018;33:3422-8. https://doi.org/10.1016/j.arth.2018.06.024.
- Yates BC, Norman J, Meza J, Krogstrand KS, Harrington S, Shurmur S, et al. Effects of partners together in health intervention on physical activity and healthy eating behaviors: a pilot study. J Cardiovasc Nurs 2015;30:109-20. https://doi.org/10.1097/JCN.0000000000000127.
- Zopf EM, Bloch W, Machtens S, Zumbé J, Rübben H, Marschner S, et al. Effects of a 15-month supervised exercise program on physical and psychological outcomes in prostate cancer patients following prostatectomy: the ProRehab study. Integr Cancer Ther 2015;14:409-18. https://doi.org/10.1177/1534735415583552.
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev 2007;16:2572-8. https://doi.org/10.1158/1055-9965.EPI-07-0413.
- Shingler E, Hackshaw-McGeagh L, Robles L, Persad R, Koupparis A, Rowe E, et al. The feasibility of the Prostate cancer: Evidence of Exercise and Nutrition Trial (PrEvENT) dietary and physical activity modifications: a qualitative study. Trials 2017;18. https://doi.org/10.1186/s13063-017-1828-4.
- Coenen P, Hulsegge G, Daams JG, van Geenen RC, Kerkhoffs GM, van Tulder MW, et al. Integrated care programmes for sport and work participation, performance of physical activities and quality of life among orthopaedic surgery patients: a systematic review with meta-analysis. BMJ Open Sport Exerc Med 2020;6. https://doi.org/10.1136/bmjsem-2019-000664.
- Steffens D, Beckenkamp PR, Young J, Solomon M, da Silva TM, Hancock MJ. Is preoperative physical activity level of patients undergoing cancer surgery associated with postoperative outcomes? A systematic review and meta-analysis. Eur J Surg Oncol 2019;45:510-18. https://doi.org/10.1016/j.ejso.2018.10.063.
- van der Wardt V, di Lorito C, Viniol A. Promoting physical activity in primary care: a systematic review and meta-analysis. Br J Gen Pract 2021;71:e399-405. https://doi.org/10.3399/BJGP.2020.0817.
- Mishra S, Scherer R, Geigle P, Berlanstein D, Topaloglu O, Gotay C. Exercise intervenions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD007566.pub2.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exerc Health 2019;11:589-97. https://doi.org/10.1080/2159676X.2019.1628806.
- Holland F. Out of Bounds: Equity in Access to Urban Nature. Birmingham: Groundwork UK; 2021.
- Carlsen B, Glenton C. What about N? A methodological study of sample-size reporting in focus group studies. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-26.
- Guest G, Namey E, McKenna K. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods 2017;29:3-22. https://doi.org/10.1177/1525822X16639015.
- Banerjee S, Semper K, Skarparis K, Naisby J, Lewis L, Cucato G, et al. Patient perspectives of vigorous intensity aerobic interval exercise prehabilitation prior to radical cystectomy: a qualitative focus group study. Disabil Rehabil 2021;43:1084-91. https://doi.org/10.1080/09638288.2019.1651907.
- Crandall K, Maguire R, Campbell A, Kearney N. A qualitative study exploring the views, attitudes and beliefs of patients and health professionals towards exercise intervention for people who are surgically treated for lung cancer. Eur J Cancer Care 2018;27. https://doi.org/10.1111/ecc.12828.
- Nadler M, Bainbridge D, Tomasone J, Cheifetz O, Juergens RA, Sussman J. Oncology care provider perspectives on exercise promotion in people with cancer: an examination of knowledge, practices, barriers, and facilitators. Support Care Cancer 2017;25:2297-304. https://doi.org/10.1007/s00520-017-3640-9.
- Ferreira V, Agnihotram R, Bergdahl A, Rooijen S, Awasthi R, Carli F, et al. Maximizing patient adherence to prehabilitation: what do the patients say?. Support Care Cancer 2018;26:1-7. https://doi.org/10.1007/s00520-018-4109-1.
- Midtgaard J, Hammer NM, Andersen C, Larsen A, Bruun DM, Jarden M. Cancer survivors’ experience of exercise-based cancer rehabilitation – a meta-synthesis of qualitative research. Acta Oncol 2015;54:609-17. https://doi.org/10.3109/0284186X.2014.995777.
- Smith A, Lewis S, Gibson S, Robinson A, Lawson E. ‘Fit for Surgery’ or ‘Fit for Life’? Exploring the Potential of Using the Perioperative Encounter to Promote Regular Exercise and Physical Activity: An Expanded Evidence Synthesis n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=139008 (accessed 14 March 2022).
- Augustin LSA, Libra M, Crispo A, Grimaldi M, De Laurentiis M, Rinaldo M, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer 2017;17. https://doi.org/10.1186/s12885-017-3064-4.
- Bruce J, Williamson E, Lait C, Richmond H, Betteley L, Lall R, et al. Randomised controlled trial of exercise to prevent shoulder problems in women undergoing breast cancer treatment: study protocol for the prevention of shoulder problems trial (UK PROSPER). BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019078.
- Gentry AL, Erickson KI, Sereika SM, Casillo FE, Crisafio ME, Donahue PT, et al. Protocol for Exercise Program in Cancer and Cognition (EPICC): a randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy. Contemp Clin Trials 2018;67:109-15. https://doi.org/10.1016/j.cct.2018.02.012.
- Kastelz A, Tzvetanov IG, Fernhall B, Shetty A, Gallon L, West-Thielke P, et al. Experimental protocol of a randomized controlled clinical trial investigating the effects of personalized exercise rehabilitation on kidney transplant recipients’ outcomes. Contemp Clin Trials 2015;45:170-6. https://doi.org/10.1016/j.cct.2015.10.002.
- McGregor G, Nichols S, Hamborg T, Bryning L, Tudor-Edwards R, Markland D, et al. High-intensity interval training versus moderate-intensity steady-state training in UK cardiac rehabilitation programmes (HIIT or MISS UK): study protocol for a multicentre randomised controlled trial and economic evaluation. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012843.
- O’Brien J, Hamilton K, Williams A, Fell J, Mulford J, Cheney M, et al. Improving physical activity, pain and function in patients waiting for hip and knee arthroplasty by combining targeted exercise training with behaviour change counselling: study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2808-z.
- van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-018-5232-6.
- Vasankari V, Halonen J, Husu P, Vähä-Ypyä H, Tokola K, Suni J, et al. Personalised eHealth intervention to increase physical activity and reduce sedentary behaviour in rehabilitation after cardiac operations: study protocol for the PACO randomised controlled trial (NCT03470246). BMJ Open Sport Exerc Med 2019;5. https://doi.org/10.1136/bmjsem-2019-000539.
Appendix 1 Characteristics of studies
| Study | Type of surgery (condition) | Total sample (n) | Follow-up | Intervention; duration | Comparator |
|---|---|---|---|---|---|
| Archer et al.51 | Laminectomy (spinal degenerative disorder) | 248 | 12 months post surgery | CBPT; 6 weeks | Education |
| Artz et al.52 | TKR (osteoarthritis) | 46 | 6 months post surgery | Physiotherapy exercise class; 6 weeks | Usual care |
| Baillot et al.53 | Bariatric surgery | 30 | 12 months post surgery | Counselling, exercise classes and education; 18 months | Usual care |
| Barberan-Garcia et al.54 | Major digestive surgery | 144 | 6 months post surgery | Motivational interviewing and exercise sessions; 4–6 weeks | Usual care |
| Barnason et al.55 | CABS | 280 | 6 months post surgery | Telehealth; 6 weeks | Usual care |
| Boesch et al.56 | CABS | 51 | 2 years post surgery | Objective/subjective regulation; 1 month | Heart-rate reserve |
| Self-regulated | |||||
| Bond et al.57 | Bariatric surgery | 80 | 6 months post surgery | Counselling sessions, monitored walking; 6 weeks | Usual care |
| Brandes et al.58 | Unilateral joint replacement | 65 | 6 months post intervention | PA counselling; mean 19.4 (SD ± 1.4) days | Usual care |
| Cadmus et al.59 | Surgery for breast cancer | 50 | 6 months post surgery | Telehealth with self-selected MVPA; 6 months | Usual care |
| Carnero et al.60 | RYGB | 128 | 6 months post intervention | PA and health education, and supervised exercise; 6 months | Lifestyle education sessions |
| Christiansen et al.62 | Unilateral TKR | 43 | 6 months post intervention | Fitbit (Fitbit LLC, San Francisco, USA) and telephone support; 7–8 months | Usual care |
| Christiansen et al.61 | Dysvascular TTA | 38 | 6 months post intervention | Behaviour change, walking and disease management; 12 weeks | Telephone session with physical therapist |
| Courneya et al.63 | Surgery for breast cancer | 242 | 6 months post intervention | Aerobic exercise training; 1 month | Usual care |
| Resistance training | |||||
| Creel et al.64 | Bariatric surgery | 150 | 6.5 months post surgery | Counselling; 26 weeks | Usual care |
| Pedometer | |||||
| Dantas et al.65 (NRS) | Cardiac surgery | 24 | 6 months post surgery | Lifestyle counselling with walking programme; 6 months | N/A |
| Demark-Wahnefried et al.66 | Surgery for breast and prostate cancer | 543 | 12 months post surgery | Personalised workbook and newsletters; 10 months | Non-tailored materials |
| Doganay et al.67 (NRS) | Oesophagogastric cancer surgery | 39 | 13 months post surgery | Personalised exercise programme; 6–8 weeks | N/A |
| Duculan et al.68 | Complex lumbar surgery | 230 | 12 months post intervention | Psychosocial intervention; education and support with goal-setting; 12 months | Information about safe PA |
| Eakin et al.69 | Lumpectomy and mastectomy | 143 | 12 months post surgery | Telephone-delivered aerobic and resistance training; 8 months | Usual care |
| Engblom et al.70 | CABS (severe coronary artery disease) | 201 | 12 months post surgery | Residential rehabilitation programme; 9 months | Usual care |
| Fontana et al.71 (NRS) | CABG | 50 | 9 months post intervention | Supervised exercise training programme; 12 weeks | N/A |
| Foster et al.72 | Myocardial revascularisation surgery | 40 | 6 and 12 months post surgery | Supervised exercise training; 6 months | Limited supervised exercise training |
| Frawley et al.73 (NRS) | Surgery for abdominopelvic cancer | 188 | 6 months post intervention | Exercise programme and education; 6 months | Usual care |
| Goedendorp et al.74 | Surgery for various cancers | 240 | 6 months post intervention | Brief nurse intervention; 3 months | Usual care |
| CBT; 6 months | |||||
| Golsteijn et al.75 | Colorectal and prostate cancer | 478 | 6 months post intervention | Personalised home-based lifestyle package; 3 months | Usual care |
| Hackshaw-McGeagh et al.76 | Radical prostatectomy | 81 | 6 months post surgery | Remote-supported walking (text message, telephone, e-mail, post); 6 months | Usual care |
| Hauer et al.77 | Hip surgery | 28 | 6 months post intervention | Group-based exercise training; 12 weeks | Placebo activities |
| Hawkes et al.78 | Surgery for colorectal cancer | 410 | 12 months post surgery | Telephone-delivered health coaching; 6 months | Usual care |
| Heiberg et al.79 | Total hip arthroplasty | 68 | 5 years post surgery | Group exercise classes; 6 weeks | Usual care |
| Heitkamp et al.80 (NRS) | Surgery for colorectal cancer | 50 | 12 months post surgery | Supervised and home-based exercise training; 12 months | N/A |
| Hoorntje et al.81 | Knee arthroplasty | 120 | 6 months post surgery | Goal-based physiotherapy and rehabilitation activities; 3–12 months | Usual care |
| Hubbard et al.82 | Surgery for colorectal cancer | 41 | 6 months post surgery | Cardiac rehabilitation including exercise sessions; 6–12 weeks | Usual care |
| Husebø et al.83 | Mastectomy or lumpectomy | 67 | 6 months post intervention | Exercise prescription with telephone support; mean 16.7 (SD ± 7.6) weeks | Usual care; mean 17.6 (SD ± 7.9) weeks |
| Ilves et al.84 | Lumbar spine fusion surgery | 104 | 12 months post intervention | Home exercise with clinic booster sessions and telephone support; 3 months | Usual care |
| Jiménez-Loaisa et al.85 | Bariatric surgery | 40 | 13 months post surgery | Personalised exercise sessions and plan; 6 months | Usual care |
| Johansson et al.86 | Standard lumbar discectomy | 59 | 12 months post surgery | Behavioural approach to physiotherapy (clinic based); 8 weeks | Behavioural approach to physiotherapy (home based) |
| Jolly et al.87 | Percutaneous transluminal coronary angioplasty/CABG | 525 | 24 months post surgery | Centre-based exercise and education programme; 8–12 weeks | Home-based lifestyle programme |
| Kinsey et al.88 | CABG | 48 | 4 years post surgery | Home walking programme; 12 weeks | Home cycling programme |
| Komatsu et al.89 (NRS) | Oesophagectomy (thoracic oesophageal cancer) | 29 | 6 months post surgery | One-to-one counselling; 3 months | N/A |
| Kraal et al.90 | CABG | 90 | 12 months post intervention | Centre-based group training; 12 weeks | Home-based training with telephone support |
| Kummel et al.91 | Acute CABG | 173 | 12 months post surgery | Group-based health counselling and education; 12 months | Usual care |
| Lear et al.92 | CABG | 302 | 12 months post intervention | Combined rehabilitation; exercise and lifestyle classes; 12 months | Usual care |
| Li et al.93 | Total knee arthroplasty | 50 | 6 months post surgery | Monthly telephone sessions; not stated | Usual care |
| Lier et al.94 | Bariatric surgery | 99 | 12 months post surgery | Semistructured group therapy; 6 weeks pre surgery then 24 months post surgery | Usual care |
| Lindbäck et al.95 | Surgery for degenerative lumbar spine disorder | 197 | 12 months post surgery | Physiotherapy and exercise sessions; 9 weeks | Usual care |
| Losina et al.96 | TKR | 202 | 6 months post surgery | THC; 6 months | FI + THC and attention control |
| FI | |||||
| Lotzke et al.97 | Lumbar fusion surgery | 118 | 6 months post surgery | Physiotherapy and counselling, and educational and therapeutic sessions; 14 weeks | Usual care |
| Macchi et al.98 (NRS) | Cardiac surgery | 143 | 1 year post intervention | Counselling and exercise sessions; 3 weeks, with a 1-year booster session | N/A |
| Macleod et al.99 (NRS) | Surgery for colorectal cancer | 22 | 31 weeks post surgery | Lifestyle intervention with PA focus; 31 weeks | N/A |
| Mundle et al.100 | Cardiac surgery | 50 | 6 months post surgery | Accelerometer wear; 30 days | Usual care |
| Painter et al.101 | Renal transplantation | 167 | 12 months post surgery | Independent home-based exercise prescription; 12 months | Usual care |
| Piva et al.102 | TKR | 240 | 6 months post surgery | Clinic-based physical therapy; 12 weeks | Usual care |
| Community-based exercise class | |||||
| Santa Mina et al.103 | Radical prostatectomy | 86 | 6 months post surgery | Home exercise programme; 4–8 weeks | Usual care |
| Sellberg et al.104 | RYGB | 259 | 1 year post surgery | Group-based well-being sessions; 4 weeks | Usual care |
| Smith et al.105 | CABG | 242 | 6 years post surgery | Centre-based supervised exercise sessions; 6 months | Home-based exercise consultation and training |
| Stolberg et al.106 | RYGB | 60 | 24 months post surgery | Supervised exercise classes; 26 weeks | Usual care |
| Taraldsen et al.107 | Hip fracture surgery | 143 | 6 months post intervention | Personalised exercise sessions; 10 weeks | Usual care |
| Turunen et al.108 | Surgery for hip fracture | 81 | 24 months post surgery | Home-based counselling and rehabilitation; 12 months | Usual care |
| Turunen et al.109 | Joint replacement and back surgery | 117 | 6 and 12 months post surgery | Physiotherapy home visits and telephone support | Usual care |
| Van der Walt et al.110 | Hip or knee arthroplasty | 202 | 6 months post surgery | Activity tracking; daily step goal and activity tracker; 6–8 weeks | Usual care |
| Yates et al.111 | CABG | 35 | 6 months post surgery | Individualised rehabilitation programme involving partner; between 3 days and 3 weeks | Usual care |
| Zopf et al.112 (NRS) | Radical prostatectomy or combination therapy (prostate cancer) | 85 | 15 months post intervention | Supervised group and home exercise; 15 months | Usual care |
| Study | Description |
|---|---|
| ACTRN12615000527561 | Pilot RCT investigating the effects of exercise on young cancer patients |
| Barker 2016 | RCT with a nested qualitative study and a health economic analysis |
| Klaassen 2017 | RCT with the aim of increasing PA, physical function and quality of life |
| NCT02083913 | Clinical trial, single group assignment investigating exercise training prior to bariatric surgery |
| NCT02381262 | RCT investigating how wearable technology can improve healthy lifestyle behaviour |
| NCT02650661 | RCT evaluating a programme designed to assist cancer patients cope with their illness proactively |
| NCT02720172 | RCT of a home exercise programme within first 6 weeks of surgery |
| NCT03498157 | RCT concerning a prehabilitation programme |
| NCT03902834 | Clinical trial, single group assignment looking at wearable technology |
| Short 2012 | RCT examining the effects of education, counselling and training to increase PA |
| Stammers 2015 | RCT of a cardiac prehabilitation programme |
| van Vulpen 2017 | Multicentre RCT investigating the effects of exercise on cancer patients |
| Study | Study name |
|---|---|
| ACTRN12617001267347 | Preoperative exercise medicine for prostate cancer (PEX) trial: efficacy of a pre- and postoperative exercise medicine intervention on sexual function and urinary incontinence in men with prostate cancer |
| ACTRN12617001283369 | Cancer And Physical ACtivITY (CAPACITY) trial: a RCT of exercise and self-management for people with lung cancer |
| ACTRN12618000930280 | Better knee, better me: effectiveness of two scalable health-care interventions supporting self-management for knee osteoarthritis – a RCT |
| ACTRN12618002020268 | A text message programme to support women’s physical and mental health after breast cancer treatments |
| Augustin 2017130 | Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial |
| Bruce 2018131 | Randomised controlled trial of exercise to prevent shoulder problems in women undergoing breast cancer treatment: study protocol for the prevention of shoulder problems trial (UK PROSPER) |
| CTRI/2017/10/009981 | Effect of yoga on recovery after heart surgery |
| DRKS00013972 | Process optimization by interdisciplinary and cross-sectoral care using the example of patients with hip and knee prostheses |
| Gentry 2018132 | Protocol for Exercise Program in Cancer and Cognition (EPICC): a randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy |
| Heiman Ullmark 2018 | PhysSurg-B: physical activity in relation to surgical operations – breast cancer |
| ISRCTN13543667 | Trial to test the feasibility of an exercise and metformin intervention for men with prostate cancer |
| ISRCTN16417174 | Can group therapy improve well-being and mental health of overweight women after gastric bypass surgery? |
| ISRCTN29770908 | PEP-TALK: a study investigating whether or not having group discussions in addition to physiotherapy improves the amount of PA following hip and knee replacement |
| ISRCTN82233115 | Supportive exercise programmes for accelerating recovery after major abdominal cancer surgery |
| ISRCTN96374224 | Cancer: Life Affirming Survivorship support in Primary care (CLASP): pilot and randomised controlled trial |
| Kastelz 2015133 | Experimental protocol of a randomized controlled clinical trial investigating the effects of personalized exercise rehabilitation on kidney transplant recipients’ outcomes |
| McGregor 2016134 | High-intensity interval training versus moderate-intensity steady-state training in UK cardiac rehabilitation programmes (HIIT or MISS UK): study protocol for a multicentre randomised controlled trial and economic evaluation |
| NCT02647021 | Therapeutic resistance group exercise training for head and neck cancer survivors (TARGET) |
| NCT02997618 | The AAA get fit trial: a pilot randomised controlled trial of community-based exercise in patients with abdominal aortic aneurysms |
| NCT03036007 | Physiotherapy after anterior cervical spine surgery |
| NCT03187028 | Diet and exercise after pancreatic cancer (PACE) |
| NCT03191630 | Increasing activity post kidney transplant with SystemCHANGE (CHANGE) |
| NCT03214471 | Evaluation of a lifestyle intervention after bariatric surgery |
| NCT03215537 | Development of patient-tailored guideline of physical activity for lung cancer |
| NCT03306992 | Precision-exercise prescription for lung cancer patients undergoing surgery: the PEP study |
| NCT03364673 | Stepping into survivorship: harnessing behavioral economics to improve quality of life in ovarian cancer |
| NCT03388983 | Effectiveness of prehabilitation for patients undergoing lumbar spinal stenosis surgery |
| NCT03452319 | Effects of increased PA before thoracoabdominal esophageal surgery |
| NCT03480464 | App technology to improve the level of PA after bariatric surgery |
| NCT03497546 | Exercise following bariatric surgery for severe/morbid obesity (EFIBAR) |
| NCT03502317 | Prehabilitation to improve cancer surgery outcomes (PICaSO) |
| NCT03509428 | The Wessex fit-4-cancer surgery trial (WesFit) |
| NCT03564171 | Prehabilitation for women undergoing preoperative chemotherapy for breast cancer |
| NCT03626610 | Prehabilitation of patients with oesophageal malignancy undergoing peri-operative treatment (Pre-EMPT) |
| NCT03641027 | Physical activity before obesity surgery (PABOS) |
| NCT03659123 | Prehabilitation and rehabilitation in oncogeriatrics: adaptation to deconditioning risk and accompaniment of patients with cancer (PROADAPT) |
| NCT03728257 | Lung transplant G0 (LTGO): improving self-management of exercise after lung transplantation (LTGO) |
| NCT03783481 | Distress reduction by activity tracking and activity enhancement by mobile support group in oncology (DRAAGON) |
| NCT03873597 | Physical activity telecoaching in lung transplant recipients |
| NCT03885817 | Physically active during cancer treatment (FAKT) |
| NCT03955627 | REJOIN Trial for Older Breast Cancer Survivors |
| NCT03963986 | Impacts of remote digital support on physical activity for patients in bariatric surgery (STIMUL) |
| NCT04044963 | The effect of a prehabilitation exercise program on physical functioning for patients undergoing kidney transplantation |
| NCT04046367 | Prehabilitation in bariatric surgery |
| NCT04054323 | The efficacy of physical activity on improving health outcomes for renal transplant patients and their caregivers |
| NCT04088968 | Against all odds: prehabilitation in urologic cancer surgery |
| NCT04103970 | The effect of Graded Activity and Pain Education (GAPE) for patients early after lumbar spinal fusion |
| NCT04190719 | Patient empowerment for major surgery preparation @ home (Paprika) |
| NCT04193397 | Effects of physical training on health markers of post-bariatric patients (obesity) |
| O’Brien 2018135 | Improving physical activity, pain and function in patients waiting for hip and knee arthroplasty by combining targeted exercise training with behaviour change counselling: study protocol for a randomised controlled trial |
| van Rooijen 2019136 | Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation |
| Vasankari 2019137 | Personalised eHealth intervention to increase physical activity and reduce sedentary behaviour in rehabilitation after cardiac operations: study protocol for the PACO randomised controlled trial |
Appendix 2 Data and analyses
Intervention versus usual care
FIGURE 16.
Amount of PA at the end of follow-up measured as minutes per day or week, subgrouped by duration of intervention. a, Multiarm study: in this analysis, we compared CBT with usual care; b, multiarm study: in this analysis, we compared FI + THC with attention control. df, degrees of freedom; IV, inverse variance.
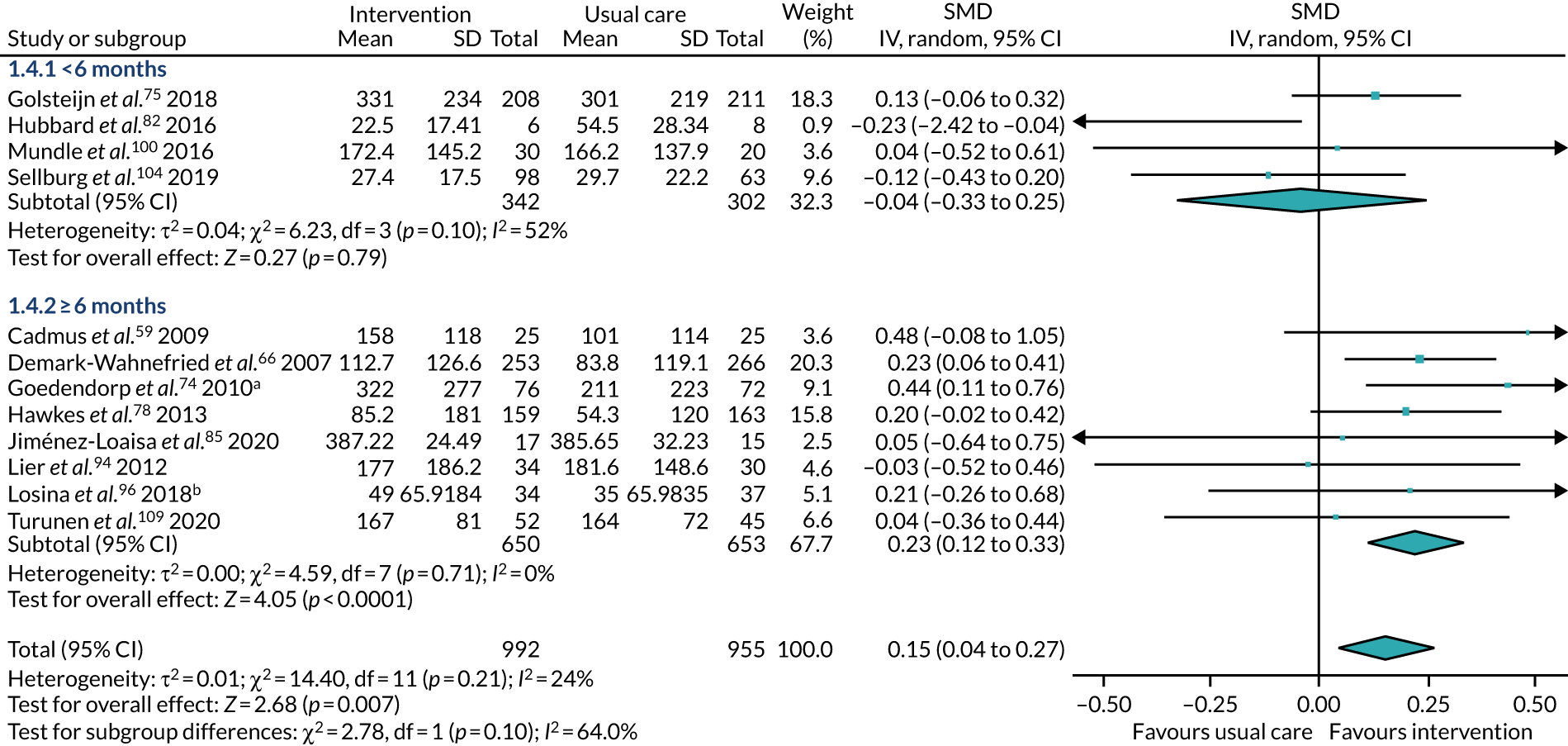
FIGURE 17.
Amount of PA at the end of follow-up measured as minutes per day or week, subgrouped by time of intervention commencement. a, Multiarm study: in this analysis, we compared CBT with usual care; b, multiarm study: in this analysis, we compared FI + THC with attention control. df, degrees of freedom; IV, inverse variance.
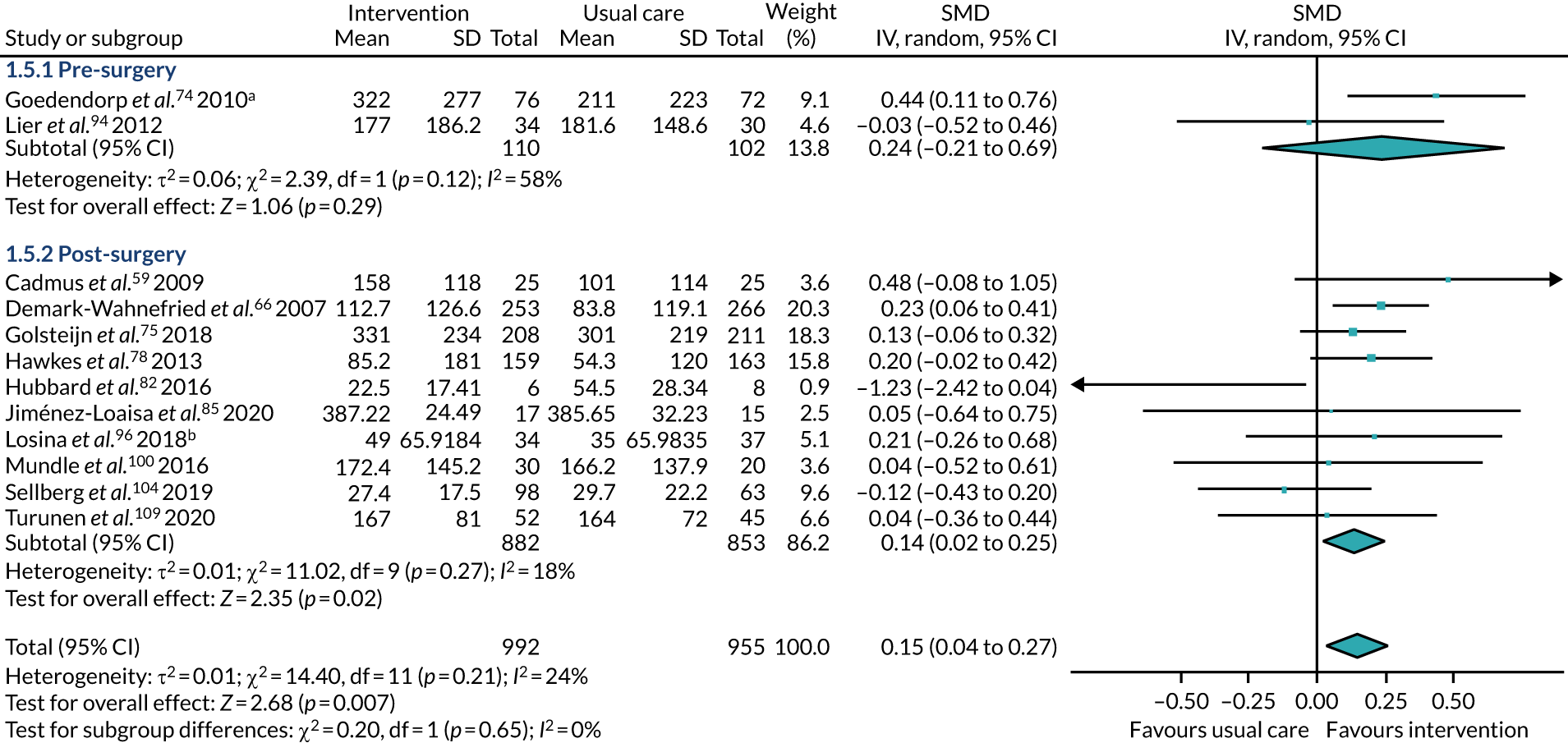
FIGURE 18.
Amount of PA at the end of follow-up measured as minutes per day or week, subgrouped by type of surgery. a, Multiarm study: in this analysis, we compared CBT with usual care; b, multiarm study: in this analysis, we compared FI + THC with attention control. df, degrees of freedom; IV, inverse variance.
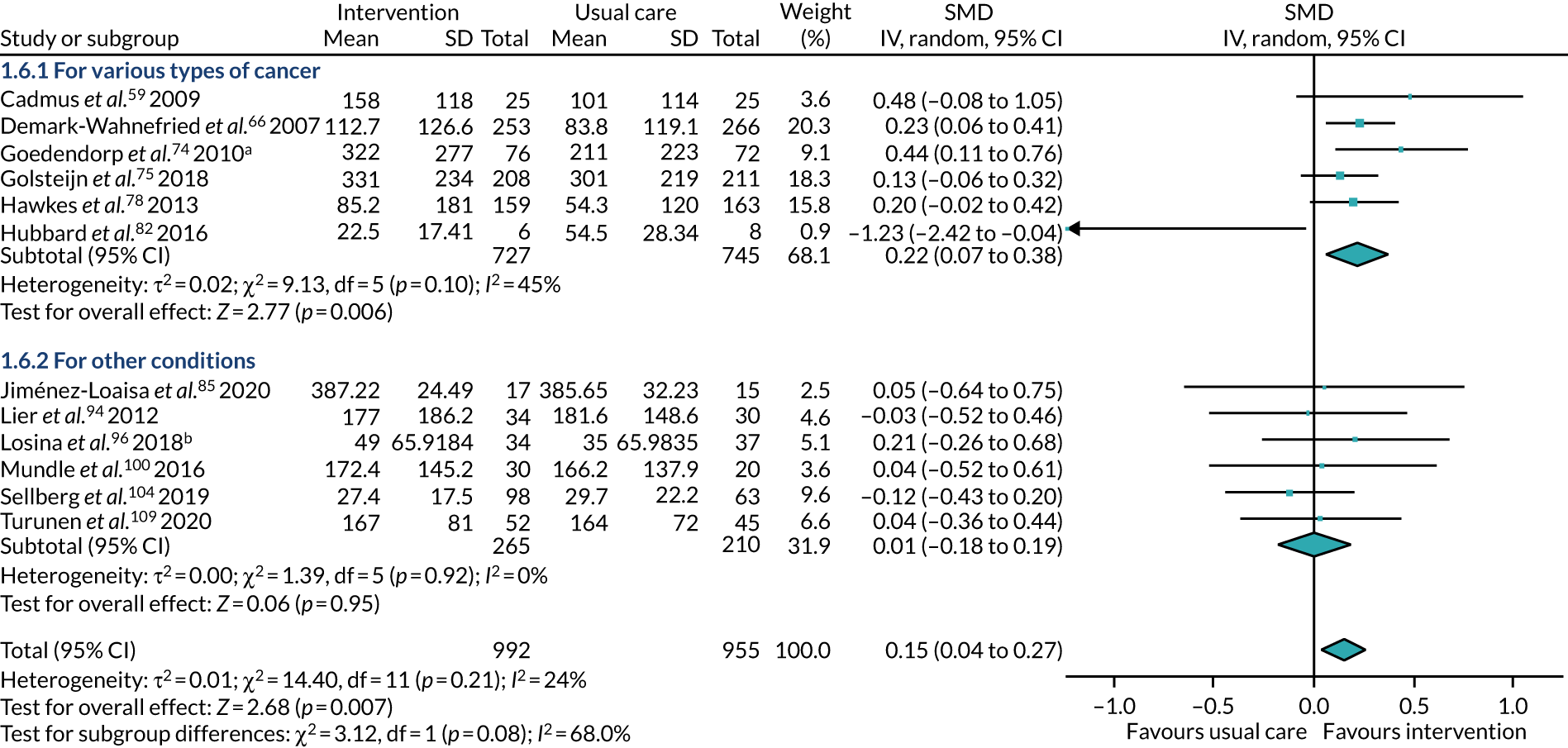
FIGURE 19.
Amount of PA at the end of follow-up measured as minutes per day or week, subgrouped by age. a, Multiarm study: in this analysis, we compared CBT with usual care; b, multiarm study: in this analysis, we compared FI + THC with attention control. df, degrees of freedom; IV, inverse variance.
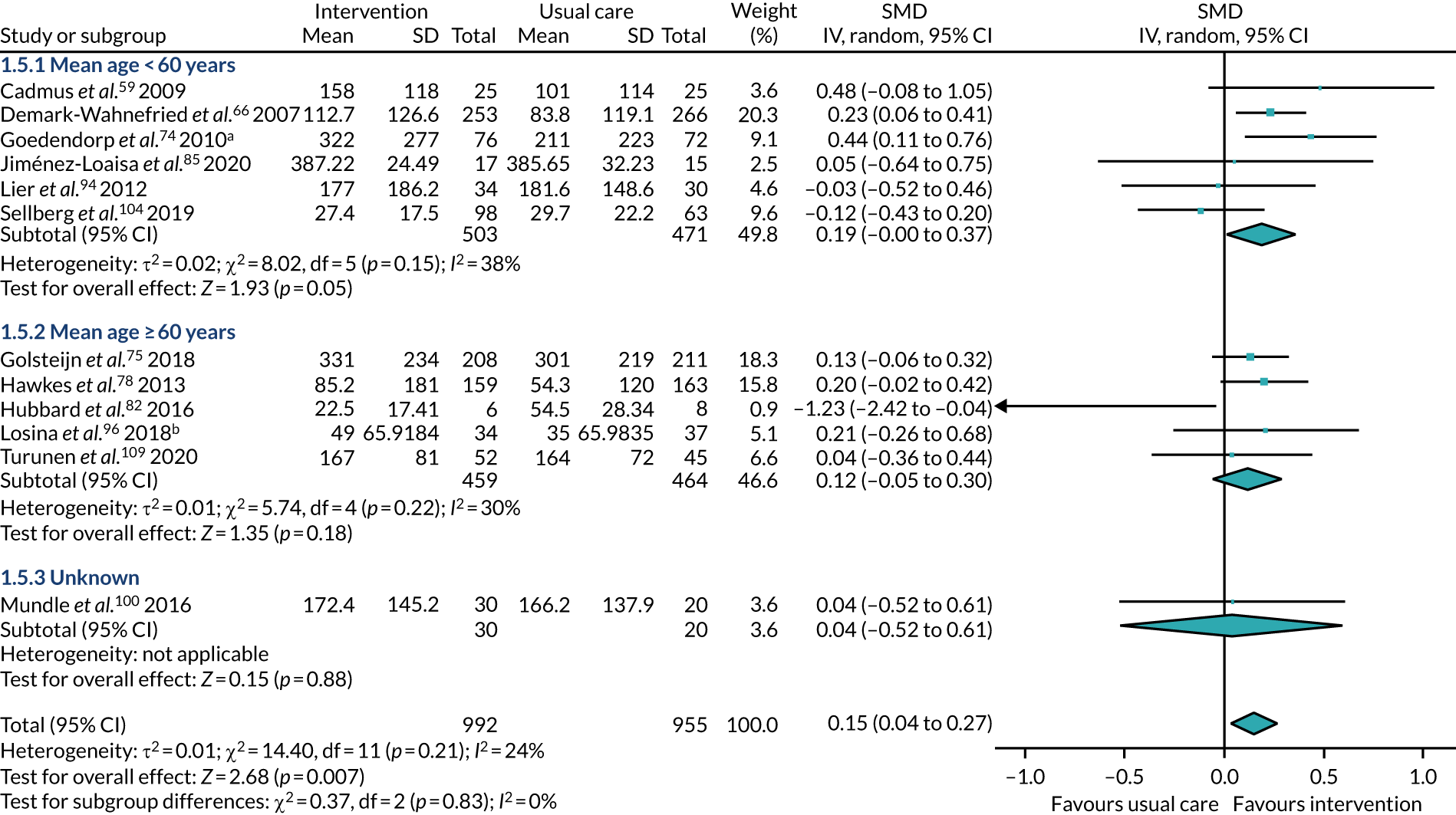
FIGURE 20.
Amount of PA at the end of follow-up measured as minutes per day or week: change from baseline. df, degrees of freedom; IV, inverse variance.
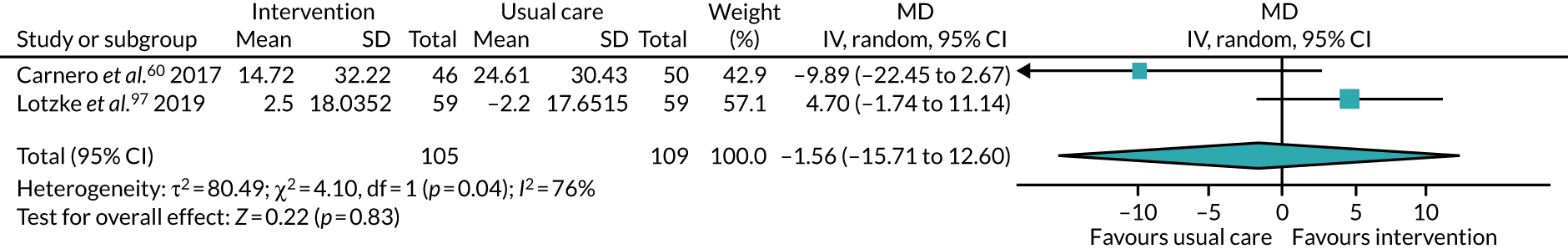
FIGURE 21.
Amount of PA at the end of follow-up measured as steps per day. a, Multiarm study: in this analysis, we compared FI + THC with attention control. df, degrees of freedom; IV, inverse variance.
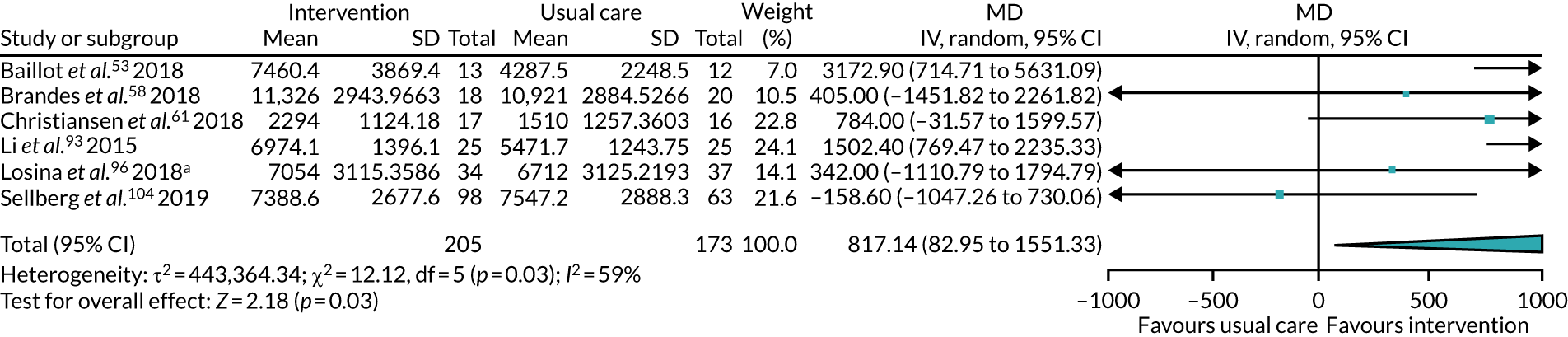
FIGURE 22.
Amount of PA at the end of follow-up measured as steps per day: change from baseline. df, degrees of freedom; IV, inverse variance.

FIGURE 23.
Amount of PA at the end of follow-up measured as energy expenditure values. a, Multiarm study: in this analysis, we compared a clinic-based group with usual care. df, degrees of freedom; IV, inverse variance.
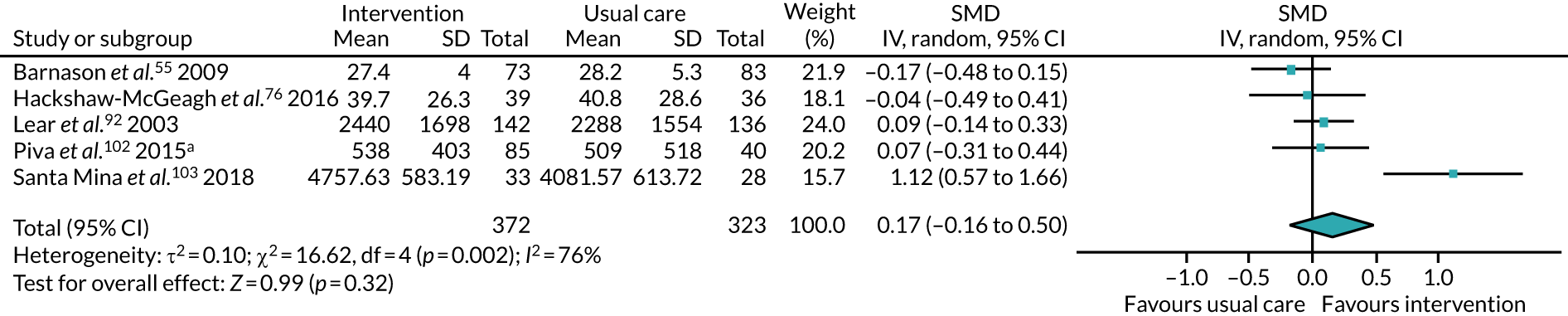
FIGURE 24.
Amount of PA at the end of follow-up measured as energy expenditure values: change from baseline. IV, inverse variance.
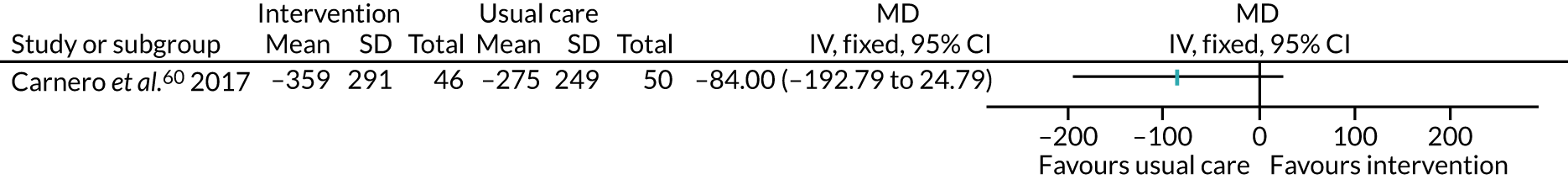
FIGURE 25.
Amount of PA at the end of follow-up measured using a range of questionnaires. a, Multiarm study: in this analysis, we compared CBT with usual care. df, degrees of freedom; IV, inverse variance.
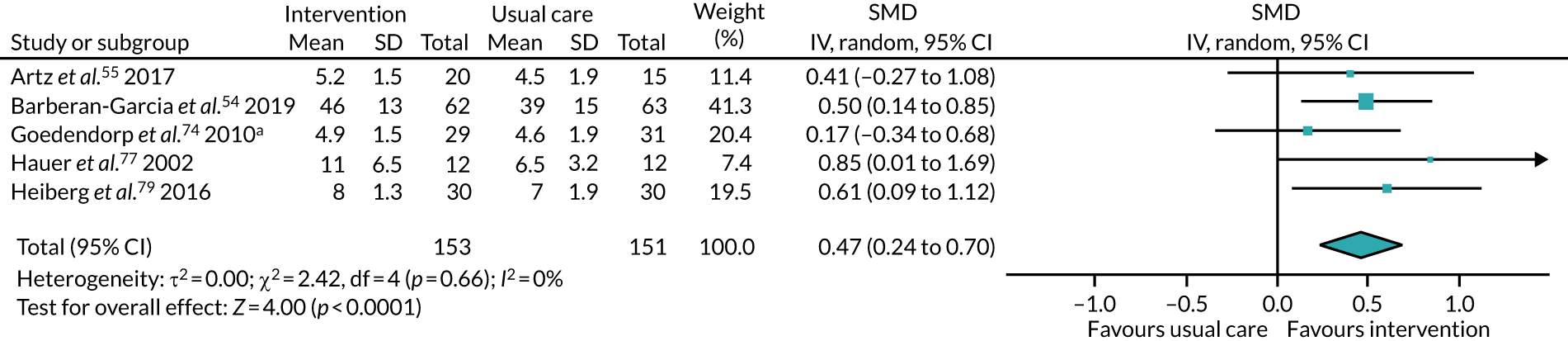
FIGURE 26.
Amount of PA at the end of follow-up using the IPAQ-SF (METs/minute/week). df, degrees of freedom; IV, inverse variance.
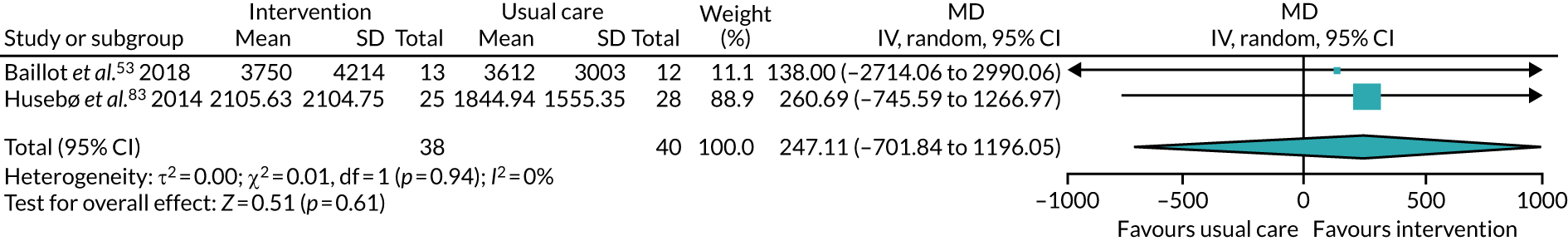
FIGURE 27.
Amount of PA at the end of follow-up measured using a daily activity score. a, Multiarm study: in this analysis, we compared CBT with usual care. IV, inverse variance.

FIGURE 28.
Physical fitness at the end of follow-up measured using walking tests (m). IV, inverse variance.
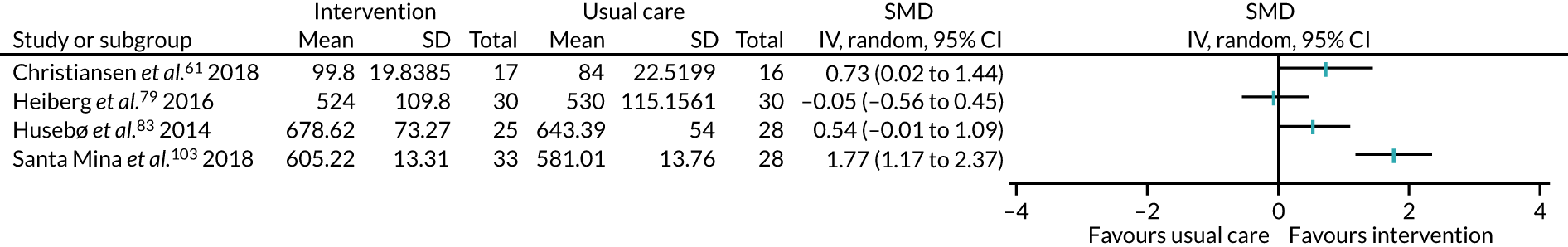
FIGURE 29.
Physical fitness at the end of follow-up measured using the 6MWT, based on change from baseline. IV, inverse variance.

FIGURE 30.
Physical fitness at the end of follow-up measured using the 5-minute walk test, based on change from baseline. IV, inverse variance.
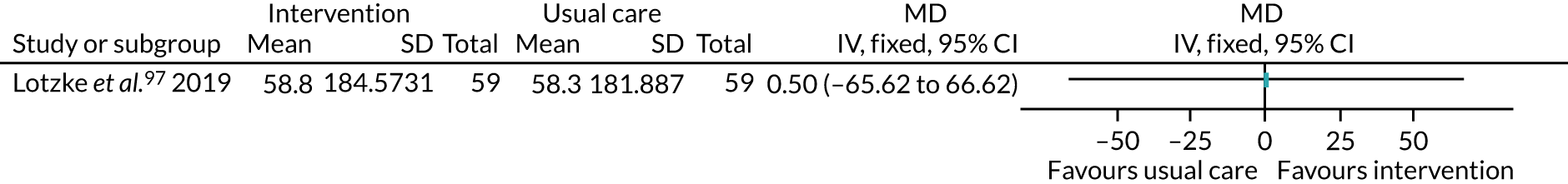
FIGURE 31.
Physical fitness at the end of follow-up measured using the TUG test. df, degrees of freedom; IV, inverse variance.
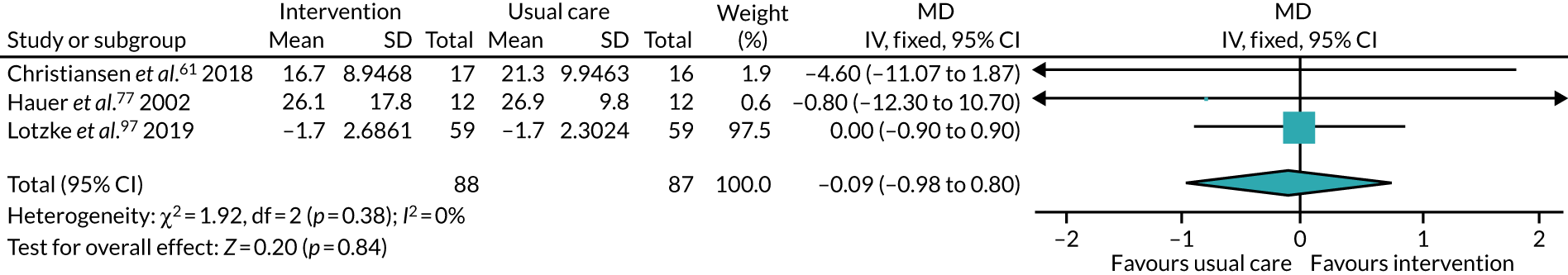
FIGURE 32.
Physical fitness at the end of follow-up measured using handgrip strength. IV, inverse variance.

FIGURE 33.
Physical fitness at the end of follow-up measured using an exercise tolerance test. df, degrees of freedom; IV, inverse variance.
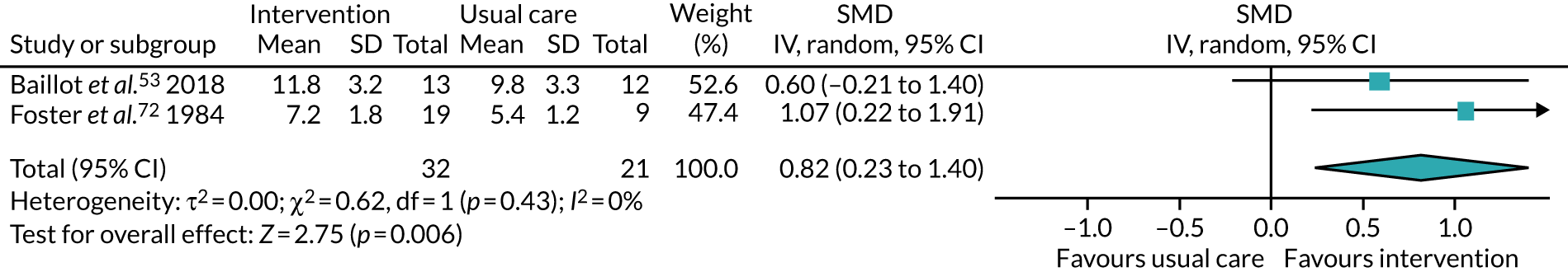
FIGURE 34.
Physical fitness at the end of follow-up measured using performance-based tests. a, Multiarm study: in this analysis, we compared clinic-based group with usual care. df, degrees of freedom; IV, inverse variance.
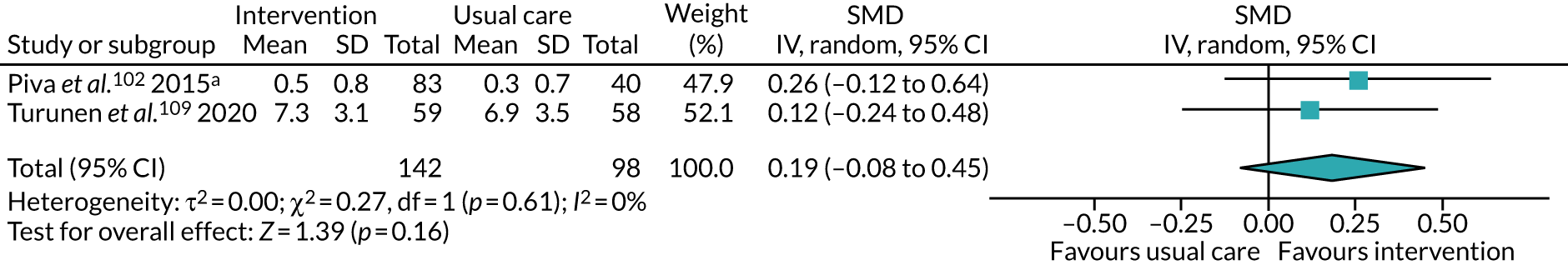
FIGURE 35.
Physical fitness at the end of follow-up measured using the sit-to-stand test. IV, inverse variance.
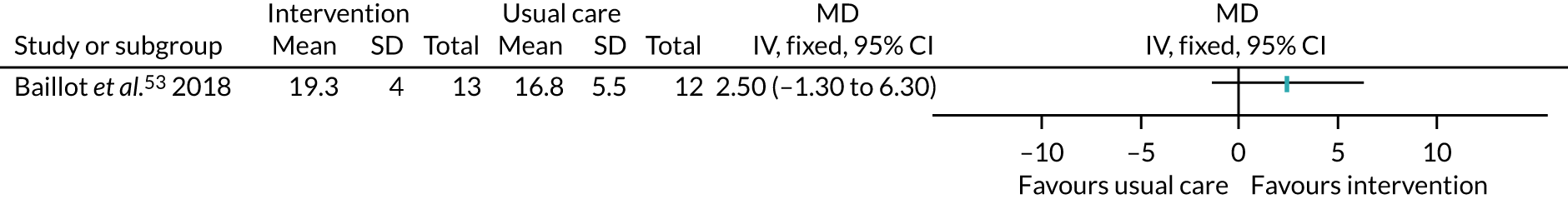
FIGURE 36.
Physical fitness at the end of follow-up measured using the arm-curl test. IV, inverse variance.
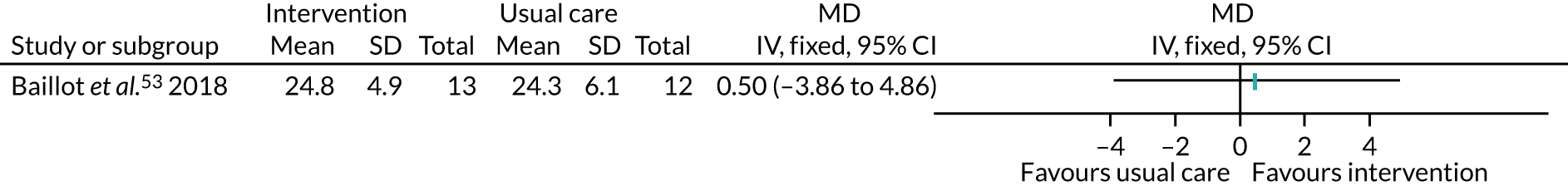
FIGURE 37.
Physical fitness at the end of follow-up measured using leg press, both legs, one repetition maximum (kg). IV, inverse variance.
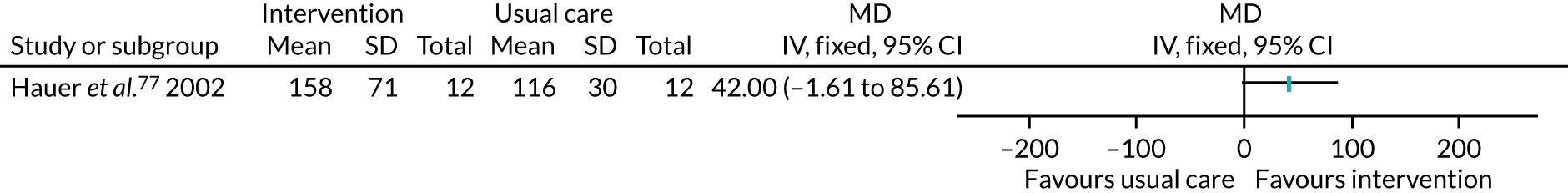
FIGURE 38.
Physical fitness at the end of follow-up measured using VO2 peak. IV, inverse variance.
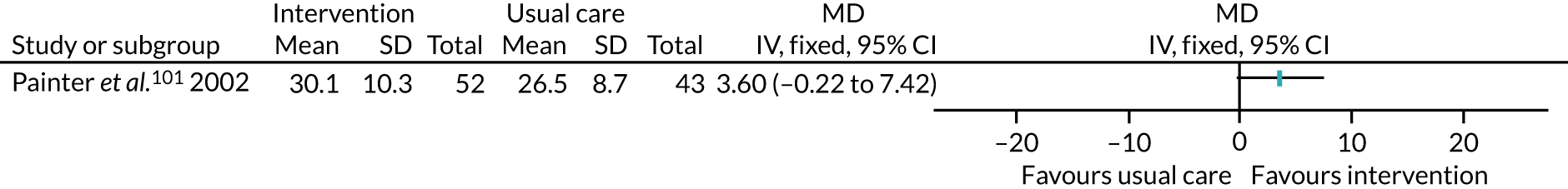
FIGURE 39.
Physical fitness at the end of follow-up measured using VO2 peak: change from baseline. IV, inverse variance.
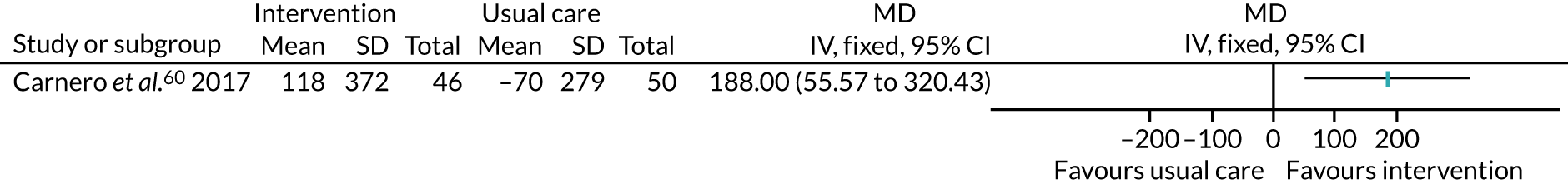
FIGURE 40.
Health-related quality of life using various components, subgrouped by duration of intervention. a, Multiarm study: in this analysis, we compared aerobic exercise training with usual care; b, multiarm study: in this analysis, we compared the clinic-based group with the usual care group. df, degrees of freedom; IV, inverse variance.
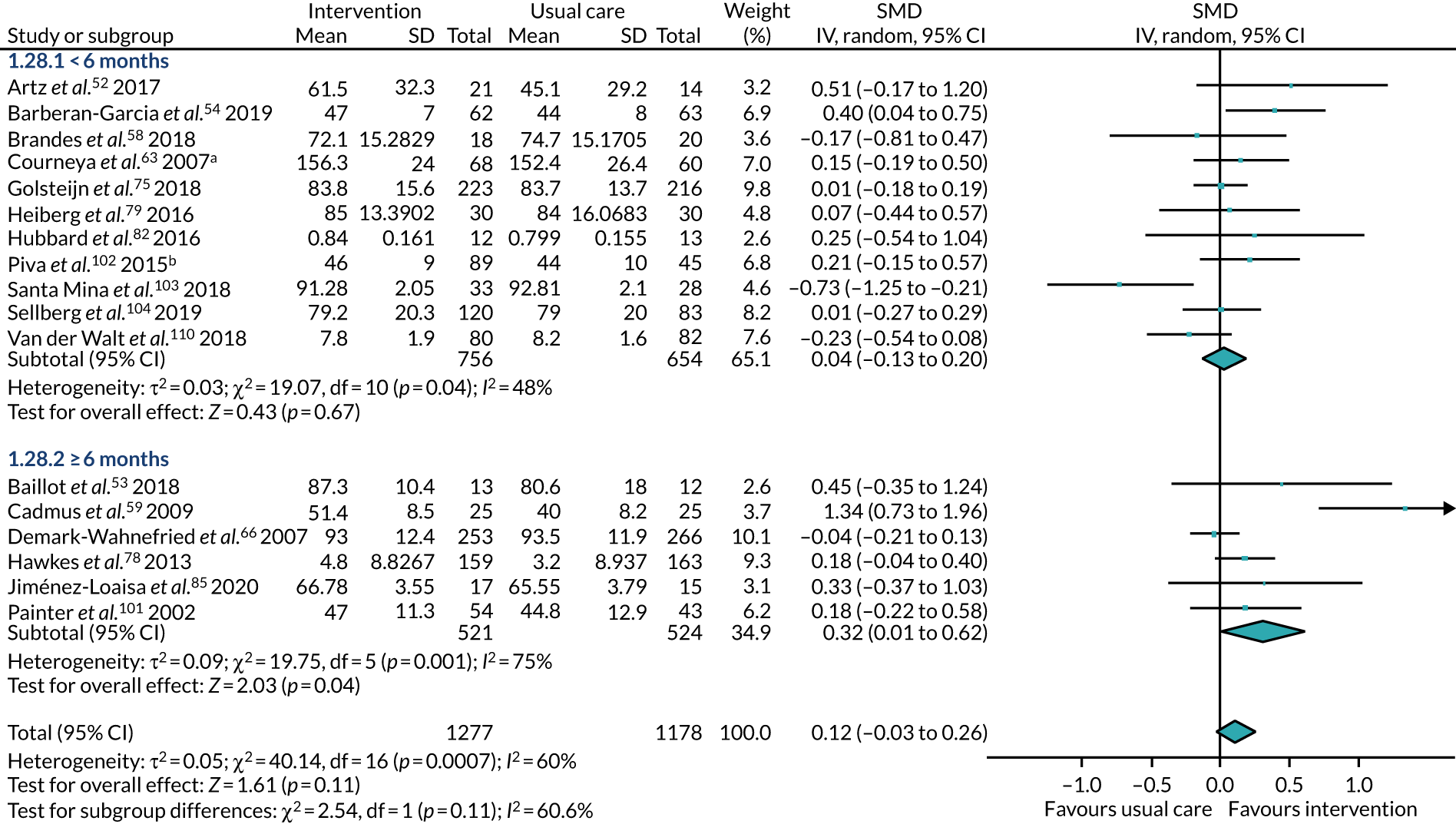
FIGURE 41.
Health-related quality of life using various components, subgrouped by time of intervention commencement. a, Multiarm study: in this analysis, we compared aerobic exercise training with usual care; b, multiarm study: in this analysis, we compared the clinic-based group with the usual care group. df, degrees of freedom; IV, inverse variance.
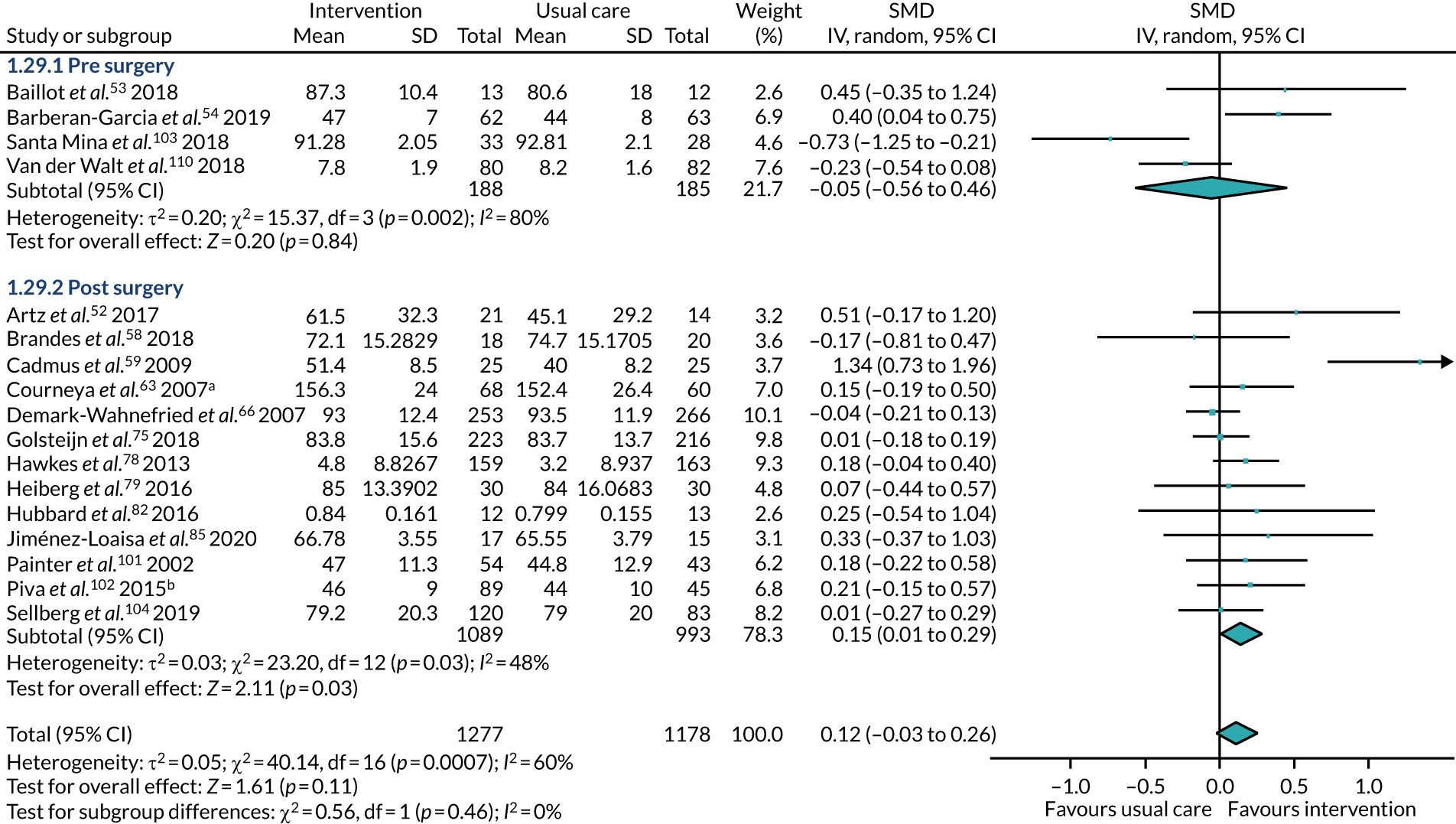
FIGURE 42.
Health-related quality of life using various components, subgrouped by type of surgery. a, Multiarm study: in this analysis, we compared aerobic exercise training with usual care; b, multiarm study: in this analysis, we compared the clinic-based group with the usual care group. df, degrees of freedom; IV, inverse variance.
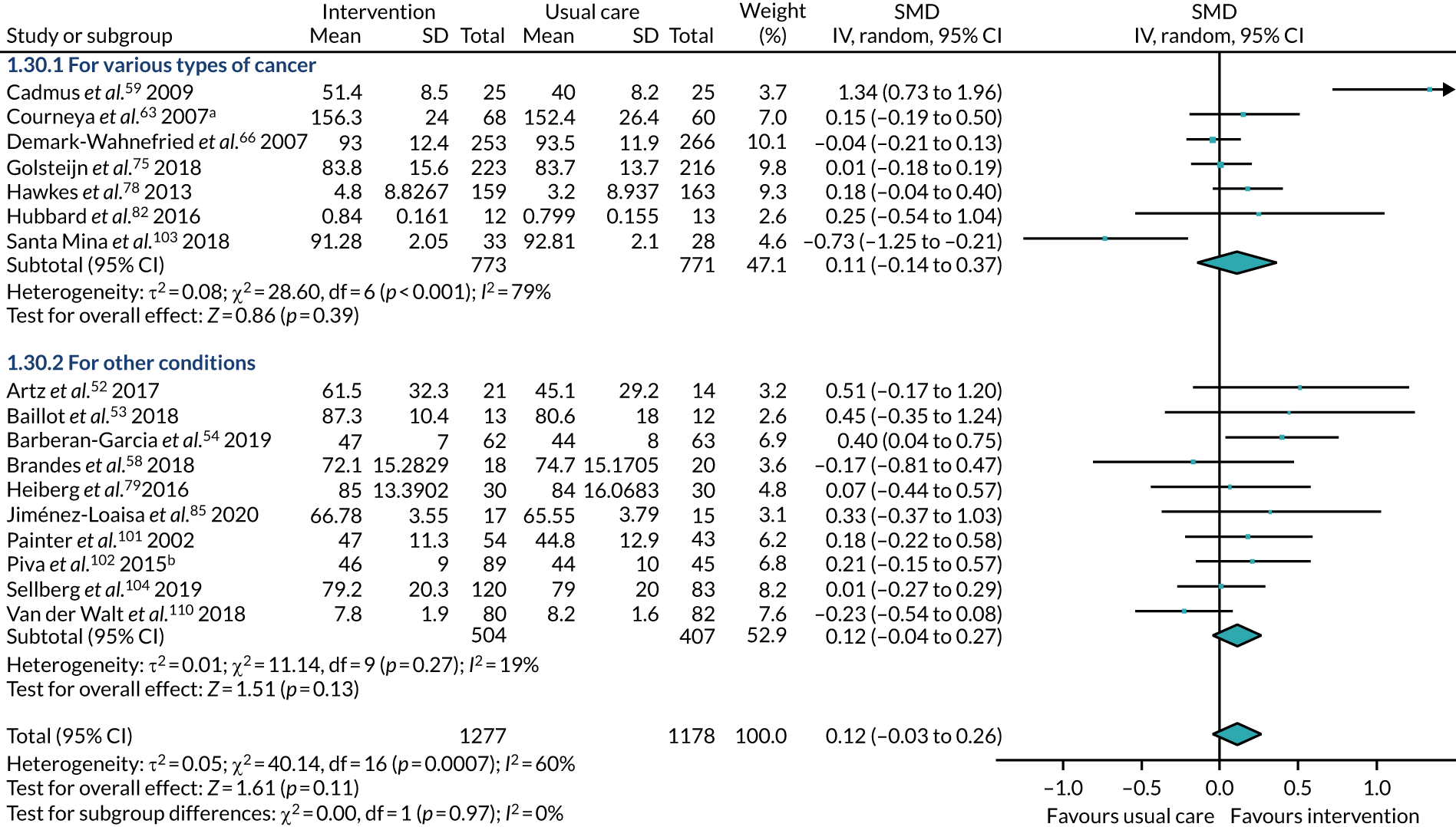
FIGURE 43.
Health-related quality of life using various components, subgrouped by BMI. a, Multiarm study: in this analysis, we compared aerobic exercise training with usual care; b, multiarm study: in this analysis, we compared the clinic-based group with the usual care group. df, degrees of freedom; IV, inverse variance.
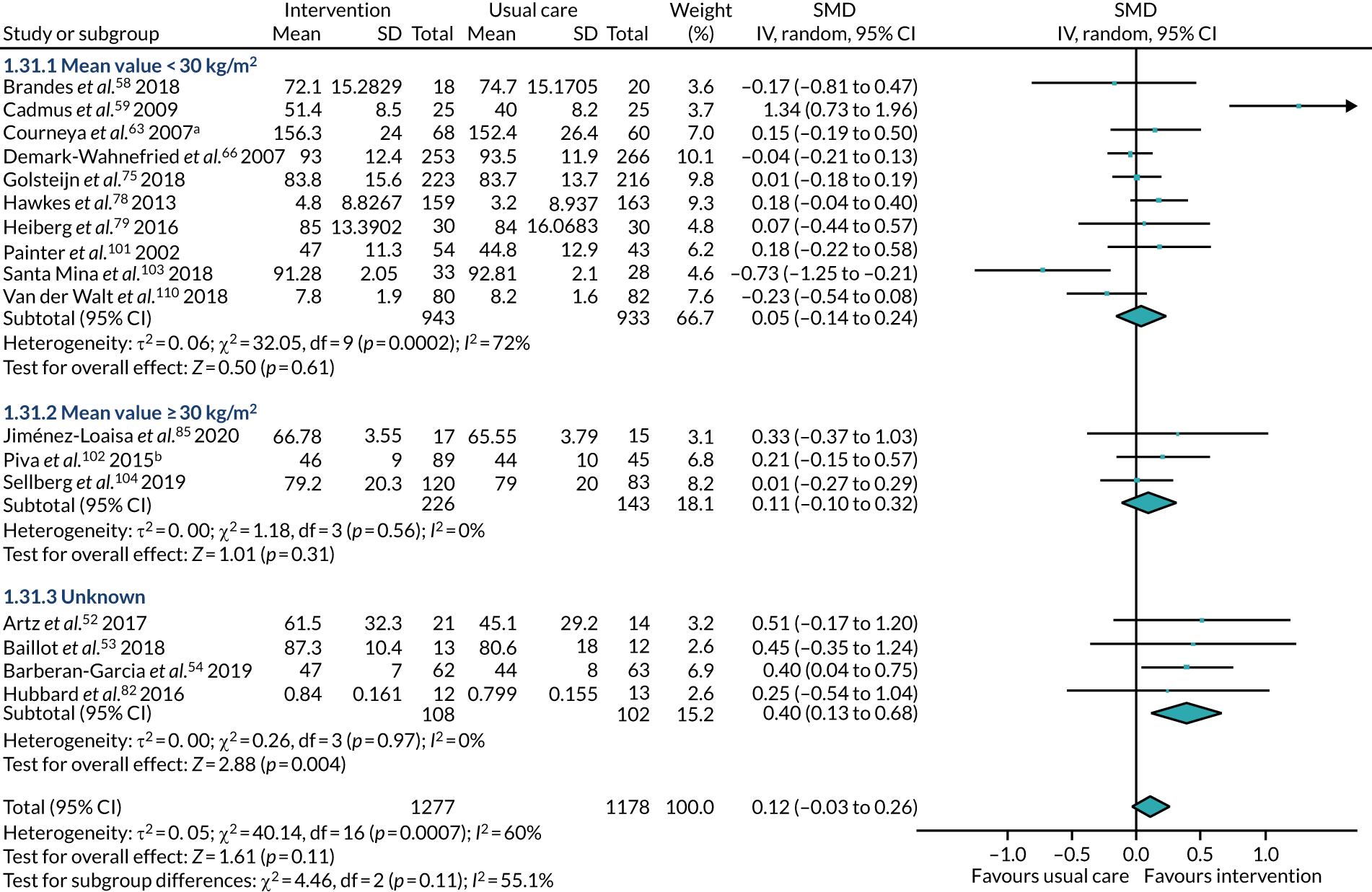
FIGURE 44.
Health-related quality of life using various components, subgrouped by age. a, Multiarm study: in this analysis, we compared aerobic exercise training with usual care; b, multiarm study: in this analysis, we compared the clinic-based group with the usual care group. df, degrees of freedom; IV, inverse variance.
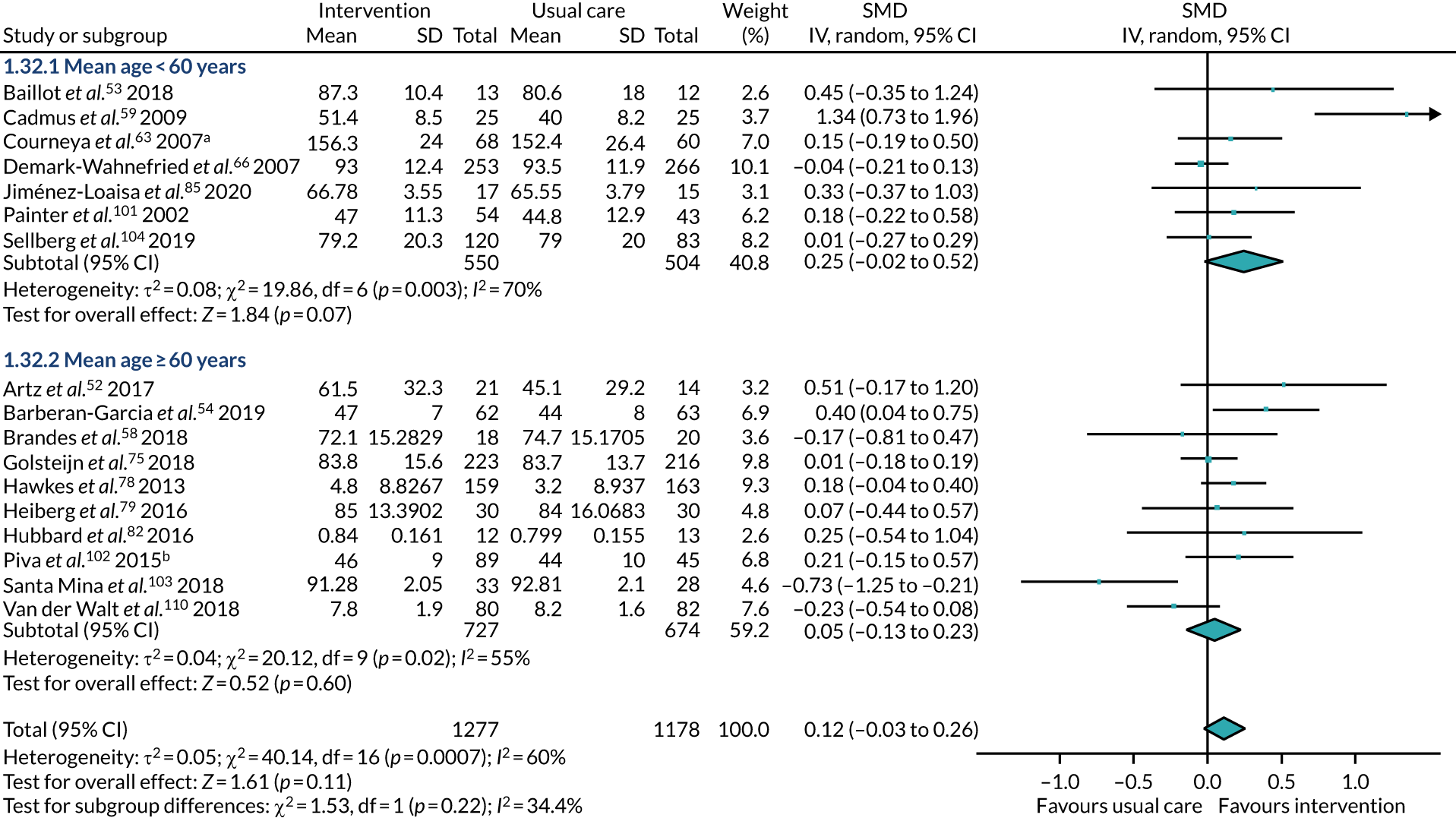
FIGURE 45.
Health-related quality of life at the end of follow-up measured using the EQ-5D: change from baseline scores. df, degrees of freedom; IV, inverse variance.

FIGURE 46.
Health-related quality of life at the end of follow-up measured using the FACT-B+4: change from baseline scores. FACT-B+4, Functional Assessment of Cancer Therapy – Breast cancer plus arm subscale; IV, inverse variance.
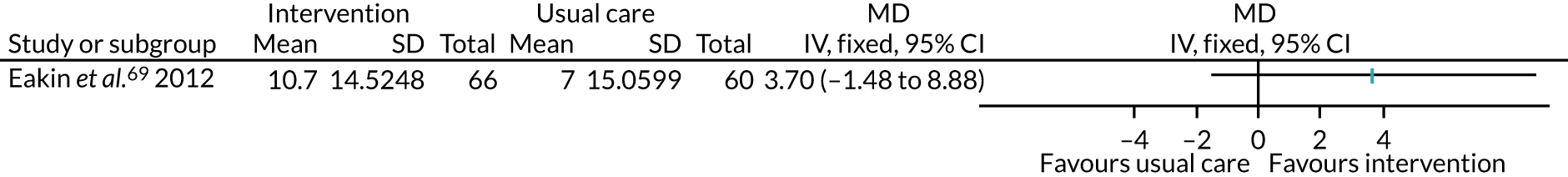
FIGURE 47.
Region-specific pain measured at the end of follow-up: change from baseline. df, degrees of freedom; IV, inverse variance.

Intervention versus intervention
FIGURE 48.
Physical activity at the end of follow-up using the Harvard Alumni Study questionnaire (kcal/week). a, Multiarm study: in this analysis, we compared self-regulation + heart rate reserve method used during exercise training with self-regulation alone. HR, heart rate; IV, inverse variance; self-reg, self-regulation.

FIGURE 49.
Amount of PA at the end of follow-up measured as activity counts per minute. IV, inverse variance.
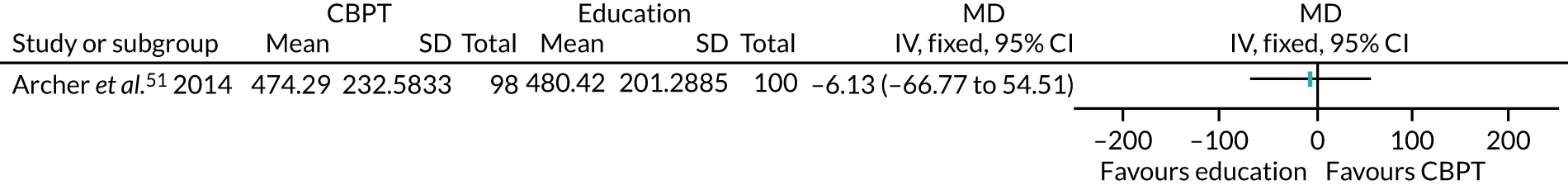
FIGURE 50.
Amount of PA at the end of follow-up using the GSLTPAQ. A higher score indicates more PA. IV, inverse variance.
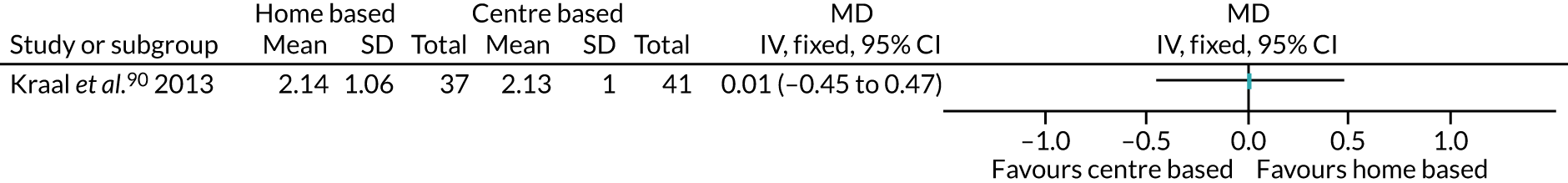
FIGURE 51.
Amount of PA at the end of follow-up using PAL score. IV, inverse variance.
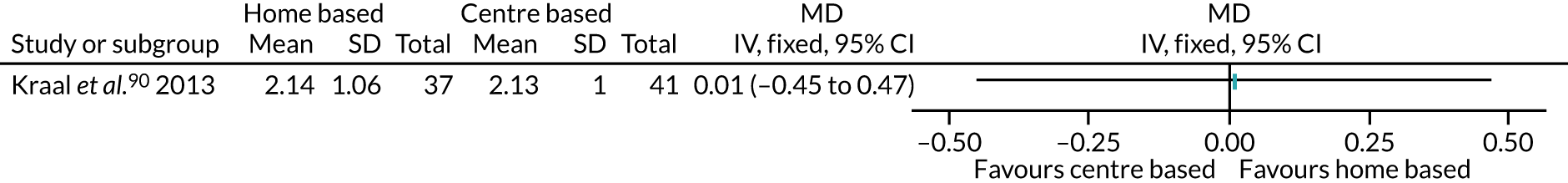
FIGURE 52.
Engagement in PA at the end of follow-up: centre based vs. home based. M–H, Mantel–Haenszel.
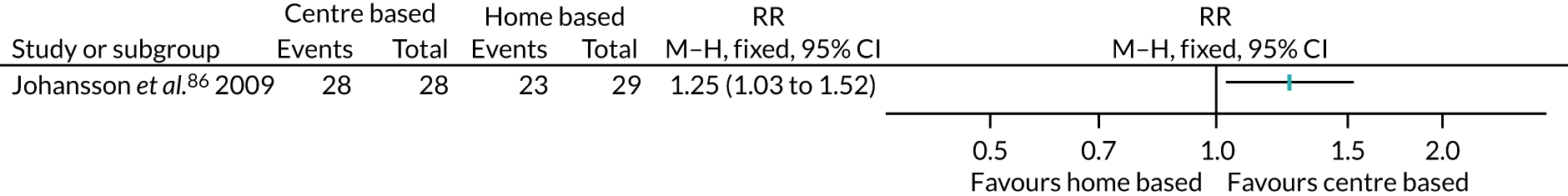
FIGURE 53.
Engagement in PA at the end of follow-up: home based vs. centre based. M–H, Mantel–Haenszel.

FIGURE 54.
Physical fitness at the end of follow-up measured as VO2 peak. df, degrees of freedom; IV, inverse variance.
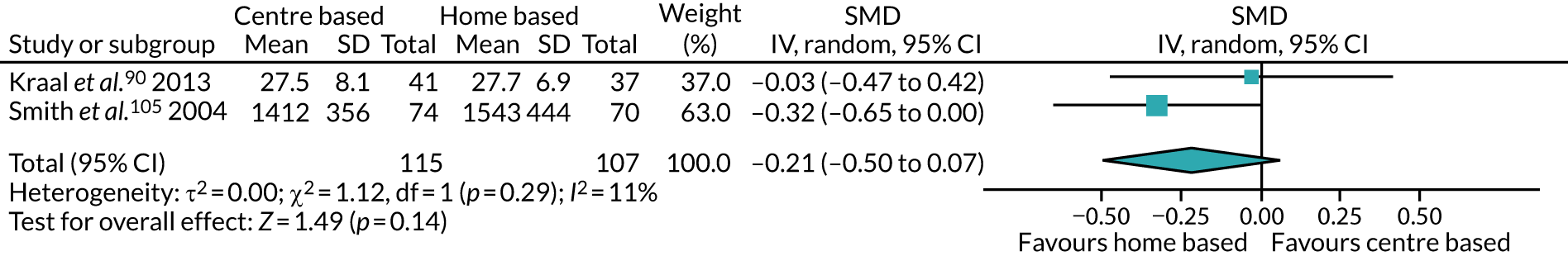
FIGURE 55.
Physical fitness at the end of follow-up measured using walking tests (m). IV, inverse variance.
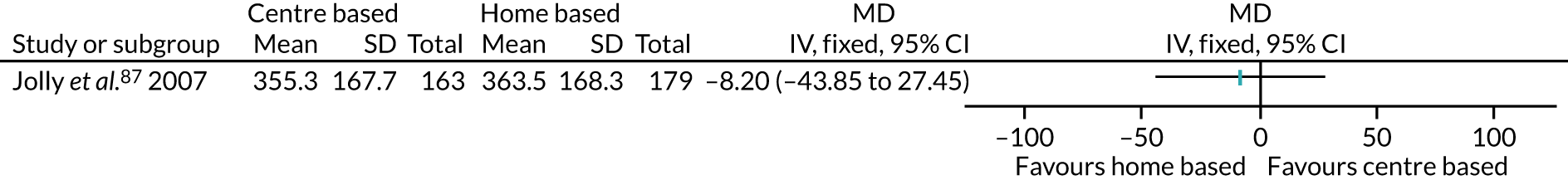
FIGURE 56.
Physical fitness at the end of follow-up measured using exercise tolerance tests (METs maximum). IV, inverse variance.
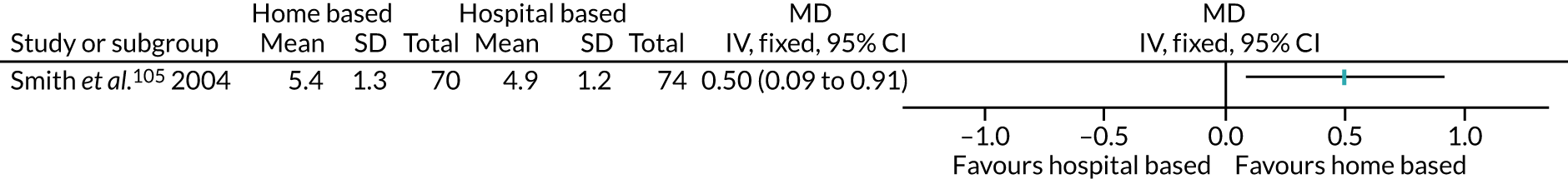
FIGURE 57.
Health-related quality of life at the end of follow-up using the PCS of the SF-36. IV, inverse variance.
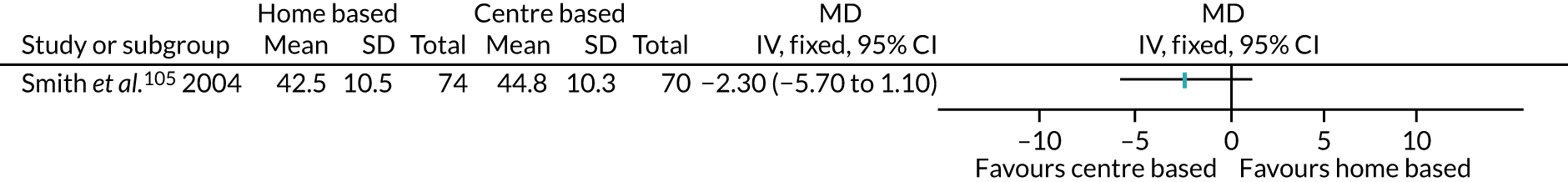
FIGURE 58.
Health-related quality of life at the end of follow-up using the EQ-5D. IV, inverse variance.
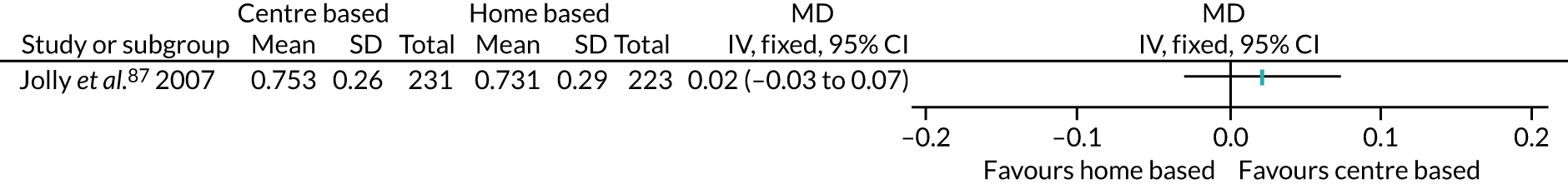
FIGURE 59.
Health-related quality of life at the end of follow-up using the MacNew Heart Disease Health-Related Quality Of Life Questionnaire (scale 1–7). IV, inverse variance.
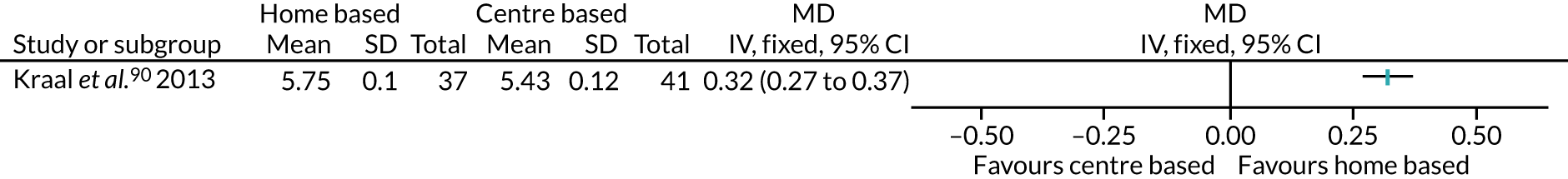
FIGURE 60.
Health-related quality of life at the end of follow-up using the PCS of the Short Form questionnaire-12 items. IV, inverse variance.
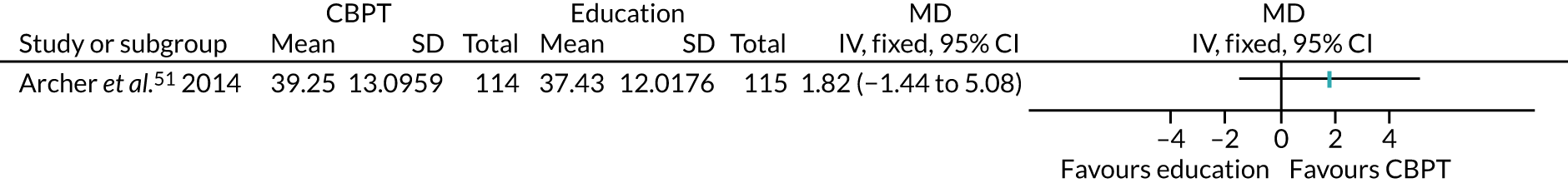
FIGURE 61.
Pain measured at the end of follow-up using the Brief Pain Inventory. IV, inverse variance.
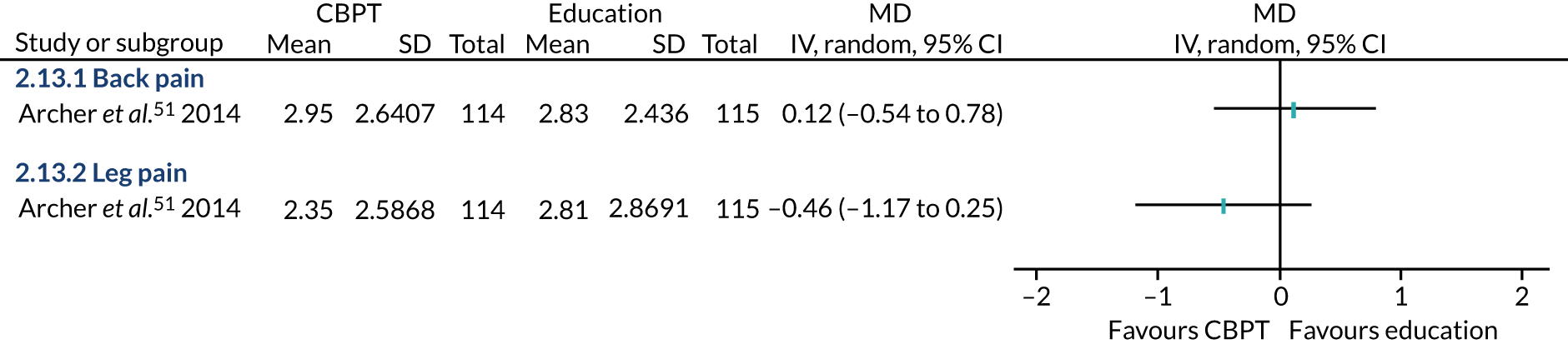
FIGURE 62.
Pain measured at the end of follow-up using self-reported chest pain on movement. IV, inverse variance.
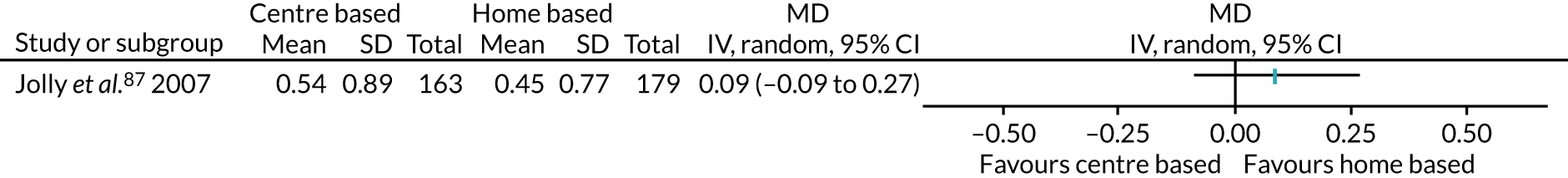
Appendix 3 Data not included in analysis
Intervention versus usual care
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Bond et al.57 | Pre surgery; a combination of education and advice, and behavioural mechanisms; frequent walking; to participant’s capacity; clinical with home-based component; with psychiatrist; low contact for < 6 months; measured 6 months post surgery; using a SenseWear® Armband monitor (BodyMedia, Pittsburgh, PA, USA) | Mean 28.7 bout-related MVPA minutes/day; number of available participants not clearly reported | Mean 18.5 bout-related MVPA minutes/day; number of available participants not clearly reported | p = 0.15 |
| Christiansen et al.62 | Postoperatively; a combination of education and advice, and behavioural mechanisms; low-frequency walking; to participant’s capacity; clinical with home-based component; with physical therapist with intermediate contact; measured 6 months post intervention; using an accelerometer | Median 15.9 (IQR 2.9–30.4) MVPA minutes/day; 11 | Median 3.4 (IQR 2.5–30.0) MVPA minutes/day; 11 | p = 0.55 |
| Creel et al.64 | Multiarm: counselling group vs. usual care group; perioperative; a combination of education and advice, and behavioural mechanisms; walking to participant’s capacity and preferences; clinical setting and low level of contact with MDT; measured 6.5 months post surgery (immediate); bout-related MVPA using an accelerometer | Mean 90 minutes/week (no SD given); 25 | Mean 40 minutes/week (no SD given); 33 | p < 0.05 |
| Hoorntje et al.81 | Postoperative, behavioural mechanisms only, running and cycling to participant’s capacity and preferences, clinical with home-based component, low level of contact MDT, based on changes in percentage of time spent active from baseline at 6 months post surgery, using an accelerometer | Mean 1.4% (SE ± 0.6%); 46 | Mean 0.6% (SE ± 0.6%); 51 | p = 0.59 |
| Stolberg et al.106 | Postoperative, direct physical instruction, generic resistance, community-based with physiotherapist, high level of contact for 6 months, difference between groups 24 months post surgery (delayed), MVPA using an accelerometer | Mean 2.4 (SEM ± 5.2) minutes/day; intervention: 22, control: 20 | Not significant | |
| Taraldsen et al.107 | Postoperative; a combination of education and advice, and direct physical instruction; low-frequency walking and generic resistance to participant’s capacity; home-based with physiotherapist; intermediate contact for < 6 months; based on between-group differences from baseline to 6 months post intervention; upright time using an accelerometer | Mean 12.07 (95% CI –12.67 to 37.54) minutes/day; intervention: 56, control: 57 | p = 0.346 | |
| Yates et al.111 | Postoperative; a combination of behavioural mechanisms and direct physical instruction; walking, swimming, dancing and other activities; frequent with measured intensity; to participant’s capacity and preferences; clinical with home-based component, with MDT; high level of contact for < 6 months; based on changes from 3 months to 6 months post surgery; total PA > 3 METs using an Actiheart monitor (CamNtech Ltd, Fenstanton, UK) | Median–24.5 (range –218.8 to 855.8) minutes/week; 17 | Median –11.4 (range –770.0 to 504.0) minutes/week; 17 | p = 0.79 |
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Bond et al.57 | Pre surgery; a combination of education and advice, and behavioural mechanisms; frequent walking; to participant’s capacity; clinical with home-based component; with psychiatrist; low contact for < 6 months; measured 6 months post surgery; using a SenseWear Armband monitor | Mean 7870 steps/day; number of available participants not clearly reported | Mean 5087 steps/day; number of available participants not clearly reported | p = 0.024 |
| Christiansen et al.62 | Postoperatively; a combination of education and advice, and behavioural mechanisms; low-frequency walking; to participant’s capacity; clinical with home-based component; with physical therapist with intermediate contact; 6 months post intervention (delayed); using an accelerometer | Median 5922 (IQR 3264–7415) steps/day; 11 | Median 3647 (IQR 2926–5419) steps/day; 11 | p = 0.11 |
| Creel et al.64 | Multiarm; counselling group vs. usual care; perioperative; a combination of education and advice, and behavioural mechanisms; walking to participant’s capacity and preferences; clinical setting and low level of contact with MDT; measured 6.5 months post surgery (immediate); using an accelerometer | Mean 6787 steps/day (no SD given); 25 | Mean 5253 steps/day (no SD given); 33 | p < 0.05 |
| Stolberg et al.106 | Postoperative, direct physical instruction, generic resistance, community based with physiotherapist, high level of contact for 6 months, difference between groups 24 months post surgery (delayed), using an accelerometer | Mean 291 (SEM ± 763) steps/day; intervention: 22, control: 20 | Not significant | |
| Van der Walt et al.110 | Perioperative, education and advice, frequent walking, home based with remote delivery from study staff, no support for < 6 months, mean daily step count expressed as percentage of preoperative step count at 6 months post surgery (delayed), using an accelerometer | 137% (amounts to 9526 steps/day); 81 | 117% (amounts to 8956 steps/day); 82 | p = 0.030 |
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Duculan et al.68 | Postoperative; a combination of education and advice, and behavioural mechanisms; walking to participant’s capacity; clinical with home-based component; low level of contact for up to 2 years; based on change from baseline to 12 months post intervention (delayed); using overall total kcal/week on the Paffenbarger Physical Activity and Exercise Index | Mean 642 (95% CI 75 to 1209) kcal/week; 110 | Data not reported for control group; 120 | p = 0.03 |
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Ilves et al.84 | Postoperative; a combination of education and advice, behavioural mechanisms and direct physical instruction; regular walking and generic resistance; to participant’s capacity and preferences; clinical with home-based component; low level of contact with physiotherapist for up to 2 years; measured 12 months post intervention (immediate); using the IPAQ-SF | Median 3190 (IQR 1150–6384) METs/minute/week; 48 | Median 3590 (IQR 1634–6485) METs/minute/week; 50 | p = 0.92 |
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Lindbäck et al.95 | Preoperative; a combination of behavioural mechanisms and direct physical instruction; to participant’s capacity and preferences; clinical with physiotherapist; intermediate contact for < 6 months; 12 months post surgery (delayed) | N/A; 99 | N/A; 98 | p = 0.020 |
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Yates et al.111 | Postoperative; a combination of behavioural mechanisms and direct physical instruction; walking, swimming, dancing, and other activities; frequent with measured intensity; to participant’s capacity and preferences; clinical with home-based component, with MDT; high level of contact for < 6 months; based on changes from 3 months to 6 months post surgery (delayed) | Median 0.45 (range –1.4 to 0.9) METs maximum; 17 | Median 0.5 (range –0.8 to 2.3) METs maximum; 17 | p = 0.10 |
| Study | Intervention details | Data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|
| Taraldsen et al.107 | Postoperative; a combination of education and advice and direct physical instruction; low-frequency walking and generic resistance to participant’s capacity; home-based with physiotherapist; intermediate contact for < 6 months; using SPPB (0–12); based on between-group differences from baseline to 6 months post intervention (delayed) | Mean 1.0 (95% CI 0.2 to 1.8); intervention: 56, control: 57 | p = 0.017 |
| Study | Intervention details | Data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|
| Stolberg et al.106 | Postoperative; direct physical instruction; generic resistance; community based with physiotherapist; high level of contact for 6 months; SF-36 (PCS); difference between groups 24 months post surgery (delayed) | Mean 1.8 (SEM ± 1.5); intervention: 22, control: 20 | Not significant |
| Taraldsen et al.107 | Postoperative; direct physical instruction; generic resistance; community based with physiotherapist; high level of contact for 6 months; EQ-5D-3L; based on between-group differences at 6 months post intervention (delayed) | Mean 0.0 (95% CI –0.1 to 0.11); intervention: 56, control: 57 | p = 0.965 |
| Study | Intervention details | PA intervention: data; no. of participants | Usual care: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Ilves et al.84 | Postoperative; a combination of education and advice, behavioural mechanisms and direct physical instruction; regular walking and generic resistance; to participant’s capacity and preferences; clinical with home-based component; low level of contact with physiotherapist for up to 2 years; using VAS back pain and VAS leg pain; based on change from baseline to 12 months post intervention (immediate) |
|
|
|
| Stolberg et al.106 | Postoperative; direct physical instruction; generic resistance; community based with physiotherapist; high level of contact for 6 months; using the SF-36 (bodily pain); difference between groups 24 months post surgery (delayed) | Mean 2.8 (SEM ± 2.7); intervention: 22, control: 20 | Not significant | |
Intervention versus intervention
| Study | Intervention details | Clinic-based intervention: data; no. of participants | Home-based intervention: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Kinsey et al.88 | Walking programme (postoperatively; a combination of education and advice, and behavioural mechanisms; walking; frequent, with measured intensity; to participant’s capacity and preferences; both clinic- and home-based components; for < 6 months) vs. cycling programme (postoperatively; a combination of education and advice, and behavioural mechanisms; walking and cycling; frequent; to participant’s capacity and preferences; both clinic- and home-based components; for < 6 months); measured 4 years post surgery (delayed) | Data given as overall number rather than by group; 16/39 participants regularly engaged in PA at the 4-year follow-up | Not reported | |
| Study | Intervention details | Clinic-based intervention: data; no. of participants | Home-based intervention: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Johansson et al.86 | Clinic based (postoperative; a combination of education and advice, and direct physical instruction; walking, generic resistance, stretch, flex and balance; frequent; clinic-based; with physiotherapist; low level of contact; for < 6 months) vs. home based (postoperative; education and advice; walking, generic resistance, stretch, flex and balance; frequent; home based; with physiotherapist; low level of contact; for < 6 months); 12 months post surgery (delayed) |
|
|
p = 0.35 |
| Study | Intervention details | Clinic-based intervention: data; no. of participants | Home-based intervention: data; no. of participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Johansson et al.86 | Clinic based (postoperative; a combination of education and advice, and direct physical instruction; walking, generic resistance, stretch, flex and balance; frequent; clinic based; with physiotherapist; low level of contact; for < 6 months) vs. home based (postoperative; education and advice; walking, generic resistance, stretch, flex and balance; frequent; home based; with physiotherapist; low contact; for < 6 months); 12 months post surgery (delayed) | Back pain
|
Back pain
|
Back pain: p = 0.04 |
Leg pain
|
Leg pain
|
Leg pain: p = 0.06 |
Appendix 4 Sensitivity analysis
Intervention versus usual care
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Cadmus et al.,59 Goedendorp et al.,74 Lier et al.,94 Losina et al.96 and Turunen et al.109 | Studies excluded for measuring the outcome immediately after the end of the intervention | SMD 0.11, 95% CI –0.03 to 0.26; participants, n = 1517; studies, n = 7; I2 = 35% | Using these data, we found a change in the interpretation of the effect, finding no difference between groups according to whether the intervention or usual care was provided |
| Creel et al.64 | This was a multiarm study. We prioritised the data from the group receiving a counselling intervention, compared with data from the group receiving usual care, as we judged the counselling intervention to be a more enhanced form of intervention. The other group received a pedometer intervention. The 6-month total for this group was 44 bout-related minutes per week; a SD for these data was not reported | p < 0.05; participants, n = 55 | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Goedendorp et al.74 | This was a multiarm study. We used the data from the CBT intervention, rather than the brief nurse intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the CBT intervention with those from the brief nurse intervention | SMD 0.15, 95% CI 0.05 to 0.24; participants, n = 1943; studies, n = 12; I2 = 6% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Goedendorp et al.,74 Golsteijn et al.,75 Hawkes et al.,78 Hubbard et al.,82 Jiménez-Loaisa et al.,85 Lier et al.,94 Losina et al.,96 Sellberg et al.104 and Turunen et al.109 | Studies excluded for high risk of attrition bias | SMD 0.24, 95% CI 0.08 to 0.40; participants, n = 619; studies, n = 3; I2 = 0% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Jiménez-Loaisa et al.85 and Mundle et al.100 | Studies excluded for high and unclear risk of selection bias (random sequence generation) | SMD 0.16, 95% CI 0.03 to 0.28; participants, n = 1865; studies, n = 10; I2 = 36% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Losina et al.96 | This was a multiarm study. We used the data from the FI + THC intervention, rather than the FI-only intervention or the THC-only intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the FI + THC intervention with those from the FI-only group. We also replaced the data from the FI + THC intervention with those from the THC-only group | Using data from the FI-only group: SMD 0.14, 95% CI 0.02 to 0.25; participants, n = 1953; studies, n = 12; I2 = 29% | No change for either analysis in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Using data from the THC-only group: SMD 0.13, 95% CI 0.00 to 0.25; participants, n = 1952; studies, n = 12; I2 = 33% | |||
| Based on change from baseline | |||
| Carnero et al.60 | This study measured the outcome in more than one way. We used the data for the MVPA measurement as this is the more commonly reported measurement for this outcome. However, this study also reported data for total daily PA. To explore this decision, we replaced the MVPA data with the total daily PA data for both the intervention group and the usual care group | SMD 0.05, 95% CI –0.38 to 0.48; participants, n = 214; studies, n = 2; I2 = 61% | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Carnero et al.60 | Study excluded for high risk of attrition bias | MD 4.70, 95% CI –1.74 to 11.14; participants, n = 118; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Carnero et al.60 | Study excluded for measuring the outcome immediately after the end of the intervention | MD 4.70, 95% CI –1.74 to 11.14; participants, n = 118; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Baillot et al.,53 Li et al.93 and Losina et al.96 | Studies excluded for measuring the outcome immediately after the end of the intervention | MD 351.24, 95% CI –287.96 to 990.44; participants, n = 232; studies, n = 3; I2 = 15% | Using these data, we found a change in the interpretation of the effect, finding no difference between groups according to whether the intervention or usual care was provided |
| Brandes et al.58 and Li et al.93 | Studies excluded for high and unclear risks of selection bias (random sequence generation) | MD 642.76, 95% CI –293.59 to 1579.12; participants, n = 290; studies, n = 4; I2 = 58% | Using these data, we found a change in the interpretation of the effect, finding no evidence of a difference in the amount of PA according to whether an intervention or usual care was provided |
| Brandes et al.,58 Losina et al.96 and Sellberg et al.104 | Studies excluded for high risk of attrition bias | MD 1369.36, 95% CI 502.95 to 2235.77; participants, n = 108; studies, n = 3; I2 = 51% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Creel et al.64 | This was a multiarm study. We prioritised the data from the group receiving a counselling intervention, compared with data from the group receiving usual care, as we judged the counselling intervention to be a more enhanced form of intervention. The other group received a pedometer intervention. The 6-month total for this group was 5325 steps per day; a SD for these data was not reported | p < 0.05 | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Losina et al.96 | This was a multiarm study. We used the data from the FI + THC intervention, rather than the FI-only intervention or the THC-only intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the FI + THC intervention with those from the FI-only group. We also replaced the data from the FI + THC intervention with those from the THC-only group | Using data from the FI-only group: MD 658.57, 95% CI –195.43 to 1512.56; participants, n = 384; studies, n = 6; I2 = 70% | Using these data, we found a change in the interpretation of the effect, finding no evidence of a difference in the amount of PA according to whether an intervention or usual care was provided |
| Using data from the THC-only group: MD 572.99, 95% CI –382.20 to 1528.17; participants, n = 383; studies, n = 6; I2 = 76% | |||
| Based on change from baseline | |||
| Carnero et al.60 | Study excluded for high risk of attrition bias | MD 364.30, 95% CI –465.50 to 1194.10; participants, n = 118; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Carnero et al.60 | Study excluded for measuring the outcome immediately after the end of the intervention | MD 364.30, 95% CI –465.50 to 1194.10; participants, n = 118; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Barnason et al.55 and Santa Mina et al.103 | Studies excluded for unclear risk of selection bias (random sequence generation) | SMD 0.06, 95% CI –0.12 to 0.25; participants, n = 478; studies, n = 3; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Barnason et al.55 and Santa Mina et al.103 | Studies excluded for high risk of attrition bias | SMD 0.06, 95% CI –0.12 to 0.25; participants, n = 478; studies, n = 3; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Hackshaw-McGeagh et al.76 and Lear et al.92 | Studies excluded for measuring the outcome immediately after the end of the intervention | SMD 0.30, 95% CI –0.36 to 0.96; participants, n = 342; studies, n = 3; I2 = 88% | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Piva et al.102 | This was a multiarm study. We used the data from the clinic-based intervention, rather than the community-based intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the clinic-based group with those from the community-based group | SMD 0.07, 95% CI –0.08 to 0.22; participants, n = 690; studies, n = 4; I2 = 77% | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Goedendorp et al.74 | This was a multiarm study. We used the data from the CBT intervention, rather than the brief nurse intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the CBT intervention with those from the brief nurse intervention | SMD 0.45, 95% CI 0.22 to 0.67; participants, n = 309; studies, n = 5; I2 = 0% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Goedendorp et al.74 | Study excluded for measuring the outcome immediately after the end of the intervention | SMD 0.54, 95% CI 0.29 to 0.80; participants, n = 244; studies, n = 4; I2 = 0% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Hauer et al.77 and Heiberg et al.79 | Studies excluded for unclear risk of selection bias (random sequence generation) | SMD 0.39, 95% CI 0.12 to 0.66; participants, n = 220; studies, n = 3; I2 = 0% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Artz et al.52 and Goedendorp et al.74 | Studies excluded for high risk of attrition bias | SMD 0.57, 95% CI 0.29 to 0.84; participants, n = 209; studies, n = 3; I2 = 0% | No change in the interpretation of the effect, which maintained that people participated in more PA when they had received the intervention |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Baillot et al.53 | Study excluded for measuring the outcome immediately after the end of the intervention | MD 260.69, 95% CI –745.59 to 1266.97; participants, n = 53; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Husebø et al.83 | Study excluded for unclear risk of selection bias (random sequence generation) | MD 138.00, 95% CI –2714.06 to 2990.06; participants, n = 25; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Husebø et al.83 | Study excluded for high risk of attrition bias | MD 138.00, 95% CI –2714.06 to 2990.06; participants, n = 25; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Goedendorp et al.74 | This was a multiarm study. We used the data from the CBT intervention, rather than the brief nurse intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the CBT intervention with those from the brief nurse intervention | MD –3.20, 95% CI –14.62 to 8.22; participants, n = 60; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups according to whether the intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Courneya et al.63 |
|
|
No change in the interpretation of either effect, which showed little or no difference in the number of people engaged in PA according to whether an intervention or usual care was provided |
| Foster et al.,72 Engblom et al.,70 Kummel et al.,91 Painter et al.101 and Turunen et al.108 | Studies excluded for unclear risk of selection bias (random sequence generation) | RR 1.16, 95% CI 0.89 to 1.50; participants, n = 423; studies, n = 4; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference in the number of people engaged in PA according to whether an intervention or usual care was provided |
| Courneya et al.,63 Foster et al.,72 Kummel et al.,91 Lier et al.,94 Painter et al.101 and Turunen et al.108,109 | Studies excluded for high risk of attrition bias | RR 1.19, 95% CI 0.91 to 1.54; participants, n = 305; studies, n = 2; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference in the number of people engaged in PA according to whether an intervention or usual care was provided |
| Kummel et al.,91 Lier et al.94 and Painter et al.101 | Studies excluded for measuring the outcome immediately after the end of the intervention | RR 1.18, 95% CI 0.96 to 1.46; participants, n = 604; studies, n = 6; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference in the number of people engaged in PA according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Heiberg et al.79 | This study measured the outcome at different time points. We used the data they measured at 5 years as this was the final time point at which they collected the data for this outcome. To explore this decision, we replaced the data measured at 5 years with the data measured at 12 months, as this was a time point more consistent with the other studies included in the analysis for this outcome | SMD 0.99, 95% CI 0.47 to 1.51; participants, n = 215; studies, n = 4; I2 = 69% | Using these data, we found a change in the interpretation of the effect, finding that people scored higher in this outcome at the end of follow-up when they had received the intervention |
| Heiberg et al.,79 Husebø et al.83 and Santa Mina et al.103 | Studies excluded for unclear risk of selection bias (random sequence generation) | SMD 0.73, 95% CI 0.02 to 1.44; participants, n = 33; studies, n = 1 | Using these data, we found a change in the interpretation of the effect, finding that people scored higher in this outcome at the end of follow-up when they had received the intervention |
| Husebø et al.83 and Santa Mina et al.103 | Studies excluded for high risk of attrition bias | SMD 0.30, 95% CI –0.46 to 1.06; participants, n = 93; studies, n = 2; I2 = 68% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Hauer et al.77 | Study excluded for unclear risk of selection bias (random sequence generation) | MD –0.09, 95% CI –0.98 to 0.81; participants, n = 151; studies, n = 2; I2 = 48% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Santa Mina et al.103 | Study excluded for high risk of attrition bias | SMD 0.44, 95% CI –0.37 to 1.25; participants, n = 24; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Baillot et al.53 | Study excluded for measuring the outcome immediately after the end of the intervention | SMD 1.07, 95% CI 0.22 to 1.91; participants, n = 28; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Foster et al.72 | Study excluded for unclear risk of selection bias (random sequence generation) | SMD 0.60, 95% CI –0.21 to 1.40; participants, n = 25; studies, n = 1 | Using these data, we found a change in the interpretation of the effect, finding little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Foster et al.72 | Study excluded for high risk of attrition bias | SMD 0.60, 95% CI –0.21 to 1.40; participants, n = 25; studies, n = 1 | Using these data, we found a change in the interpretation of the effect, finding little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Piva et al.102 | This was a multiarm study. We used the data from the clinic-based intervention, rather than the community-based intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the clinic-based group with those from the community-based group | SMD 0.12, 95% CI –0.14 to 0.38; participants, n = 233; studies, n = 2; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Turunen et al.109 | Study excluded for high risk of attrition bias | SMD 0.26, 95% CI –0.12 to 0.64; participants, n = 123; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Turunen et al.109 | Study excluded for measuring the outcome immediately after the end of the intervention | SMD 0.26, 95% CI –0.12 to 0.64; participants, n = 123; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Baillot et al.,53 Cadmus et al.59 and Painter et al.101 | Studies excluded for measuring the outcome immediately after the end of the intervention | SMD 0.05, 95% CI –0.07 to 0.17; participants, n = 2283; studies, n = 14; I2 = 41% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Courneya et al.63 | This was a multiarm study. We used the data from the aerobic exercise training intervention, rather than the resistance exercise training intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the aerobic exercise training intervention with those from the resistance exercise training intervention | SMD 0.11, 95% CI –0.03 to 0.25; participants, n = 2460; studies, n = 17; I2 = 60% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Heiberg et al.79 | This study measured the outcome at different time points. We used the data they measured at 5 years as this was the final time point at which they collected the data for this outcome. To explore this decision, we replaced the data measured at 5 years with the data measured at 12 months, as this was a time point more consistent with the other studies included in the analysis for this outcome | SMD 0.11, 95% CI –0.04 to 0.25; participants, n = 2463; studies, n = 17; I2 = 61% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Piva et al.102 | This was a multiarm study. We used the data from the clinic-based intervention, rather than the community-based intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the clinic-based group with those from the community-based group | SMD 0.11, 95% CI –0.03 to 0.25; participants, n = 2454; studies, n = 17; I2 = 60% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Brandes et al.,58 Heiberg et al.,79 Jiménez-Loaisa et al.,85 Painter et al.101 and Santa Mina et al.103 | Studies excluded for unclear and high risks of selection bias (random sequence generation) | SMD 0.17, 95% CI 0.01 to 0.33; participants, n = 2167; studies, n = 12; I2 = 63% | Using these data, we found a change in the interpretation of the effect, finding that people reported a greater quality of life when they had received the intervention |
| Artz et al.,52 Brandes et al.,58 Courneya et al.,63 Golsteijn et al.,75 Hawkes et al.,78 Hubbard et al.,82 Jiménez-Loaisa et al.,85 Painter et al.,101 Santa Mina et al.103 Sellberg et al.104 and Van der Walt et al.110 | Studies excluded for high risk of attrition bias | SMD 0.34, 95% CI 0.00 to 0.68; participants, n = 913; studies, n = 6; I2 = 77% | Using these data, we found a change in the interpretation of the effect, finding the people reported a greater quality of life when they had received the intervention |
| Based on change from baseline | |||
| Lindbäck et al.95 | Study excluded for high risk of attrition bias | MD –0.05, 95% CI –0.13 to 0.03; participants, n = 118; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Artz et al.,52 Brandes et al.,58 Santa Mina et al.,103 Sellberg et al.104 and Van der Walt et al.110 | Studies excluded for high risk of attrition bias | SMD –0.00, 95% CI –0.50 to 0.50; participants, n = 110; studies, n = 2; I2 = 44% | No change in the interpretation of the effect which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Brandes et al.,58 Heiberg et al.79 and Santa Mina et al.103 | Studies excluded for unclear and high risks of selection bias (random sequence generation) | SMD 0.07, 95% CI –0.12 to 0.26; participants, n = 443; studies, n = 4; I2 = 0% | No change in the interpretation of the effect which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Cadmus et al.59 | Study excluded for measuring the outcome immediately after the end of the intervention | SMD 0.19, 95% CI –0.19 to 0.57; participants, n = 552; studies, n = 6; I2 = 76% | No change in the interpretation of the effect which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Heiberg et al.79 | This study measured the outcome at different time points. We used the data they reported at 5 years as this was the final time point at which they collected the data for this outcome. To explore this decision, we replaced the data measured at 5 years with the data measured at 12 months, as this was a time point more consistent with the other studies included in the analysis for this outcome | SMD 0.23, 95% CI –0.09 to 0.54; participants, n = 610; studies, n = 7; I2 = 68% | No change in the interpretation of the effect which showed little or no difference between groups in this outcome according to whether an intervention or usual care was provided |
| Pain (region specific) using VAS (based on change from baseline) | |||
| Lindbäck et al.95 | Study excluded from both back pain and leg pain measurements for high risk of attrition bias | Back pain: MD 2.60, 95% CI –7.72 to 12.92; participants, n = 118; studies, n = 1; I2 = 0% | No change in the interpretation of either effect, which showed little or no difference between groups in these outcomes according to whether an intervention or usual care was provided |
| Leg pain: MD 4.00, 95% CI –14.35 to 22.35; participants, n = 118; studies, n = 1; I2 = 0% | |||
Intervention versus intervention
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Boesch et al.56 | This was a multiarm study. We compared the group receiving the combined intervention with the group receiving the self-regulation intervention, to avoid a unit-of-analysis error. To explore this decision, we replaced the data from the objective/subjective group with those of the heart-rate reserve group | MD 103.00, 95% CI –747.98 to 953.98; participants, n = 45; studies, n = 1 | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to which type of intervention was provided |
| Study | Reason for exchange or exclusion | New effect estimate | Change or no change in interpretation of effect |
|---|---|---|---|
| Smith et al.105 | This study measured the outcome at different time points. We used the data they measured at 6 years as this was the final time point at which they collected the data for this outcome. To explore this decision, we replaced the data measured at 6 years with the data measured at 12 months, as this was a time point more consistent with the other studies included in the analysis for this outcome | SMD 0.06, 95% CI –0.18 to 0.29; participants, n = 276; studies, n = 2; I2 = 0% | No change in the interpretation of the effect, which showed little or no difference between groups in this outcome according to which type of intervention was provided |
Appendix 5 Non-randomised study outcomes
| Study | Intervention details | PA intervention: data; n participants | Standard care: data; n participants | Effect estimate as reported by study authors |
|---|---|---|---|---|
| Amount of PA at the end of follow-up using the IPAQ-SF | ||||
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group-delivered, multicomponent, technology-assisted, remote capacity, post surgery; measured 6 months post intervention (delayed); total activity | Mean 3573.8 (SD ± 3145.5) MET-minutes/week; 73 | Mean 2887.2 (SD ± 3009.0) minutes/week; 92 | Between-group MD [rehabilitation minus comparator; T3 (6-month follow-up) minus T1 (baseline)]. Mean 1222.2 (95% CI 260.7 to 2183.6) minutes/week; p < 0.05 |
| Heitkamp et al.80 | In-person PA instruction, post surgery; measured 12 months post surgery (immediate) | Mean 36 (SD ± 31) METs/hour/week; 50 | N/A | p = 0.003 |
| Komatsu et al.89 | Therapeutic/counselling, advice/self-directed, advised only, multicomponent, pre and post surgery; measured 6 months post surgery (delayed); used the Japanese version of the International Physical Activity Questionnaire (IPAQ-SV) | Mean 1386.0 (95% CI 0 to 31,038); 22 | N/A | Not reported |
| Macleod et al.99 | Therapeutic/counselling, advice/self-directed, advised only, multicomponent, technology assisted, pre and post surgery; measured 31 weeks post surgery (immediate) | Median 840 (IQR 330–1260) minutes/week; 15 | N/A | Not reported |
| Amount of PA at the end of follow-up using the GSLTPAQ | ||||
| Doganay et al.67 | Advice/self-directed, advised only, delivered one to one, pre and post surgery; measured 13 months post surgery (delayed) | Median 15 (IQR 0–35); 39 | N/A | Significant change from baseline; p = 0.048 |
| Amount of PA at the end of follow-up using the Freiburger Questionnaire of Physical Activity (METs/hour/week) | ||||
| Zopf et al.112 | Advice/self-directed, in-person PA instruction, group-delivered, multicomponent, post surgery; 15 months post intervention (immediate) | Mean 55.86 (SD ± 47.44) MET-hours/week; 50 | Mean 63.69 (SD ± 47.90) MET-hours/week; 20 | Group difference in mean change from baseline to post test. Mean –0.93 (95% CI –43.58 to 41.72); p = 0.966 |
| Amount of PA at the end of follow-up measured as steps/day | ||||
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group-delivered, multicomponent, technology assisted, remote capacity, post surgery; measured 6 months post intervention (delayed); using a pedometer | Median 10,689.0 (IQR 8822.0–12,565.0); 31 | N/A | N/A |
| Engagement in PA at the end of follow-up | ||||
| Dantas et al.65 | Therapeutic/counselling, advice/self-directed, multicomponent, post surgery; measured 6 months post surgery (immediate) | Adherence to walking programme, n: 13; 15a | N/A | p = 0.031 |
| Doganay et al.67 | Advice/self-directed, advised only, delivered one to one, pre and post surgery; measured 13 months post surgery (delayed) | Classified as active or moderately active, n: 22; 39a | N/A | Not reported |
| Fontana et al.71 | In-person PA instruction, delivered one to one, post surgery; measured 9 months post intervention (delayed) | Undertaking regular exercise, n: 14; 38a | N/A | Not reported |
| Komatsu et al.89 | Therapeutic/counselling, advice/self-directed, advised only, multicomponent, pre and post surgery; measured 6 months post surgery (delayed) | Health-enhancing physically active, n: 4; 22a | N/A | Not reported |
| Macchi et al.98 | In-person PA instruction, delivered one to one, multicomponent, post surgery; 1 year post intervention (delayed) | Recreational PA, corresponding to at least 1 hour/day on 5 days, or 40–45 minutes/day on 7 days, each week, n: 85; 131a | N/A | Not reported |
| Physical fitness at the end of follow-up using walking tests (m) | ||||
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group delivered, multicomponent, technology assisted, remote capacity, post surgery; measured 6 months post intervention (delayed); using 6MWT | Mean 606.4 m (SD ± 87.4 m); 66 | N/A | MD within rehabilitation group [rehabilitation minus comparator; T3 (6-month follow-up) minus T1 (baseline)]. Mean 45.0 m (95% CI 31.0 m to 59.0 m); p < 0.05 |
| Macchi et al.98 | In-person PA instruction, delivered one-to-one, multicomponent, post surgery; 1 year post intervention (delayed); using 6MWT | Mean 473 m (SD ± 106 m); 131 | N/A | p < 0.001 |
| Physical fitness at the end of follow-up using work capacity (METs) | ||||
| Fontana et al.71 | In-person PA instruction, delivered one to one, post surgery; measured 9 months post intervention (delayed) | Mean 9.1 (SD 1.8) METs; number of participants unknown | N/A | Difference between baseline and 9 months; p < 0.0001 |
| Physical fitness at the end of follow-up using handgrip strength (kg) | ||||
| Doganay et al.67 | Advice/self-directed, advised only, delivered one to one, pre and post surgery; using a dynamometer; measured 13 months post surgery (delayed) | Median 31.3 kg (IQR 27–36.6 kg); 39 | N/A | Significant change from baseline to follow-up; p = 0.004 |
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group delivered, multicomponent, technology-assisted, remote capacity, post surgery; measured 6 months post intervention (delayed); dominant hand | Mean 38.4 kg (SD ± 10.9 kg); 70 | N/A | MD within rehabilitation group [rehabilitation minus comparator; T3 (6-month follow-up) minus T1 (baseline)]. Mean 2.1 (95% CI 0.2 to 3.9); p < 0.05 |
| Physical fitness at the end of follow-up using VO2 peak | ||||
| Doganay et al.67 | Advice/self-directed, advised only, delivered one to one, pre and post surgery; using the Chester Step Test; measured 13 months post surgery (delayed) | Median 21 (IQR 18.9–25.2) VO2 maximum, ml/kg/minute; 34 | N/A | Significant change from P4 to follow-up; p = 0.000 |
| Heitkamp et al.80 | In-person PA instruction, post surgery; measured 12 months post surgery (immediate) | Mean 24.5 (SD ± 6.7) ml/kg/minute; 50 | N/A | p = 0.002 |
| Zopf et al.112 | Advice/self-directed, in-person PA instruction, group delivered, multicomponent, post surgery; 15 months post intervention (immediate) | Mean 26.14 (SD ± 5.20) ml/kg/minute; 48 | Mean 24.91 (SD ± 6.47) ml/kg/minute; 16 | Group difference in mean change from baseline to post test. Mean 2.33 (95% CI –0.94 to 5.60); p = 0.160 |
| HRQoL at the end of follow-up using the EORTC QLQ-C30 Global (0–100) | ||||
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group delivered, multicomponent, technology assisted, remote capacity, post surgery; measured 6 months post intervention (delayed) | Mean 82.3 (SD ± 15.0); 73 | Mean 81.2 (SD ± 16.6); 93 | Between-group MD [rehabilitation minus comparator; T3 (6-month follow-up) minus T1 (baseline)]. Mean 6.1 (95% CI 1.2 to 11.1); p = < 0.05 |
| Komatsu et al.89 | Therapeutic/counselling, advice/self-directed, advised only, multicomponent, pre and post surgery; measured 6 months post surgery (delayed) | Mean 64.3 (95% CI 54.0 to 74.5); 22 | N/A | Not reported |
| Zopf et al.112 | Advice/self-directed, in-person PA instruction, group delivered, multicomponent, post surgery; 15 months post intervention (immediate) | Mean 74.83 (SD ± 16.37); 50 | Mean 67.92 (SD ± 25.26); 20 | Group difference in mean change from baseline to post test. Mean 5.08 (95% CI –5.25 to 15.41); p = 0.335 |
| Pain at the end of follow-up measured using various components | ||||
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group delivered, multicomponent, technology assisted, remote capacity, post surgery; using the EORTC QLQ-C30 pain (0–100; with higher scores indicating worse outcome); measured 6 months post intervention (delayed) | Mean 8.7 (SD ± 15.5); 73 | Mean 7.7 (SD ± 16.2); 93 | Between-group MD [rehabilitation minus comparator; T3 (6-month follow-up) minus T1 (baseline)]. Mean 3.9 (95% CI –3.0 to 10.8); p ≥ 0.05 |
| Komatsu et al.89 | Therapeutic/counselling, advice/self-directed, advised only, multicomponent, pre and post surgery; using the EORTC QLQ-OES18 (oesophageal pain); measured 6 months post surgery (delayed) | Mean 3.0 (95% CI 0.3 to 5.7); 22 | N/A | Not reported |
| Zopf et al.112 | Advice/self-directed, in-person PA instruction, group delivered, multicomponent, post surgery; using the EORTC QLQ-C30 pain (0–100; with higher scores indicating worse outcome); 15 months post intervention (immediate) | Mean 16.33 (SD ± 24.63); 50 | Mean 19.17 (SD ± 28.75); 20 | Group difference in mean change from baseline to post test. Mean –5.70 (95% CI –23.01 to 11.61); p = 0.515 |
| Study | Data | Related or unrelated to study |
|---|---|---|
| Adverse events | ||
| Frawley et al.73 | Seven serious adverse events occurred | These were considered by the local Human Research Ethics Committee not to be attributable to participation in the study |
| Macchi et al.98 | No clinically relevant adverse event occurred | N/A |
| Zopf et al.112 | No adverse events reported | Dropouts described as not being due to adverse effects of the training intervention |
| Adherence | ||
| Fontana et al.71 | Twenty-six (52%) of the 50 patients who lived within 40 miles of the medical centre and had qualified for the study attempted exercise training. Of these 26, 10 (39%) attended all 12 weeks and at least 75% of the sessions | – |
| Frawley et al.73 | 81% (n = 68/84) of the participants in the rehabilitation group attended 85 to 100% of 16 scheduled sessions. 56% (47/84) of the participants received telephone motivational coaching sessions between T2 and T3 and the mean number of phone calls received was 1.8. An average of 30% of the participants self-reported that they met the recommended PA guidelines of 150 min moderate-intensity PA plus two strengthening sessions per week at T2 and T3 | – |
| Participant experience | ||
| Study | Description of intervention; n | Experience data |
| Frawley et al.73 | Therapeutic/counselling, advice/self-directed, in-person PA instruction, group delivered, multicomponent, technology assisted, remote capacity, post surgery; 84 | Overall satisfaction with the program was 96% |
Appendix 6 Coding framework for the thematic analysis
1. Evolution/story of services (journey).
1.1. Patient participation.
1.2. Initial agenda including ‘buy–in’.
1.3. Funding.
1.4. Personal relationship.
1.5. Momentum/recognition/‘branding’/‘marketing’.
2. Key components of service.
2.1. Approach focus:
2.1.1. well-being/holistic/lifestyle approach
2.1.2. personalised care
2.1.3. outcome and/or commissioner driven
2.1.4. motivating/inspiring/supporting/change
2.1.5. other.
2.2. Staff: who is important (roles).
2.3. Staff: who is important (skills and attributes).
2.4. Family.
2.5. Resources (including funding and ‘luck’).
2.6. Systems: technology/communication (inter- and intraprofessional).
2.7. Organisational behaviours (reflective/supporting staff/interviewee’s manner).
2.8. Data and patient outcomes.
3. Framing and perceptions of service.
3.1. To patients/public.
3.2. To colleagues.
3.3. To broader organisation/funders.
4. Relationships (including challenges).
4.1. With patients.
4.2. Within service/broader organisation.
4.3. Outside service.
4.4. With commissioners.
5. The patient.
5.1. Voice, patient engagement, co-production (also check 2.1.2 and 2.1.4).
5.2. Meeting patients (between service/‘professionals’ and patient).
5.3. Relationships with each other (peer support, etc.).
5.4. Perceptions and observations of patient.
5.5. Perceived experiences.
5.6. Perceived benefits/language of impact/anecdotal.
5.7. Levels of physical activity.
6. Technology.
6.1. Technology use.
6.2. Remote delivery (general).
7. Context.
7.1. Type or surgery or condition.
7.2. Broader: local/culture/community (e.g. small or large)/deprivation/setting.
7.3. COVID-19.
8. Services (further details for summary).
8.1. Prehab4Cancer.
8.2. PARiS.
8.3. Active Beyond Cancer.
8.4. Moving Medicine.
8.5. Active Against Cancer.
8.6. The Family Practice.
8.7. ERAS+.
8.8. Prepwell.
Appendix 7 Expanded thematic analysis of qualitative contextual data from focus groups and interviews
Narratives of physical activity promotion
Attitudes of care
I think health care is much more about health care than it was 5 years ago, much more.
Although physiological status was often a primary focus, particularly during the prehabilitation phase of patients’ treatment, there were several narratives that seemed to be the impetus to how services and staff approached their work with patients. These largely centred around the idea that the patient was central, their needs were individual, and a broad wrap-around approach was necessary to both engage and support patients to make long-term changes to their health. These narratives were conveyed in relation to the general approach that services were taking, but were also talked about in the way they described the messaging to, and their interactions with, patients. This seemed to centre around four key considerations: supporting a holistic, well-being approach; the individual; motivating and inspiring; and compassionate care.
Supporting a holistic, well-being approach
Participants frequently referred to patients’ well-being, lifestyle medicine and holistic approaches, thinking about patients’ psychological profile and ‘how they feel about themselves’, often commenting too on the link between each of these and PA. Several participants indicated that they frame the impact of their programmes to patients around patients’ well-being, stress management and anxiety management, as well as how activity might support them through their treatment. This appeared to be seen as central to the efficacy of their approaches and an acceptable narrative with which patients could connect.
A ‘well-being focus to physical activity’ was often talked about as including broader lifestyle aspects, creating opportunities ‘for conversations around, smoking, [and] alcohol reduction’; efforts to keep patients nutritionally ‘stable’ or ‘sound’ or working closely with dietitians, or with nurses around pain management; and any ‘other elements of supporting patients’ that might become apparent. Several talked about support with ‘coping strategy stuff’ and there were clearly efforts in several services to forge greater links with psychology services and to build on colleagues’ existing ‘listening skills’, with some providing counselling and psychological skills training to staff. In one discussion, a participant described a shared city-centre space with local public health teams, ‘a one-stop shop’ enabling the joining up of well-being support alongside their own, such as counselling. Another participant described feeling ‘a little bit envious’ of this arrangement, recognising that their programme could better co-ordinate and utilise more local health and well-being provision. The same participant emphasised, however, the ‘luxurious position’ that they as a service were in, having the ‘opportunity to help people, two or three times a week’. They talked about it being highly unusual that a patient will, for example, ‘present with just cancer . . .. There are often a whole host of other things going on’, seeing their frequent contact with patients as an opportunity to think much more broadly about patients’ health and well-being, and to utilise their colleagues or wider networks to access additional support or referrals, depending on their needs. In a separate discussion, another participant said something similar, highlighting the ‘different aspects of [patients’] physical, psychological and nutritional needs’ that they consider when they first meet a person, and they talked about efforts they were taking to find a way to more systematically stratify their approach, perhaps using technology, to ensure that every patient gets the right amount of support for each of their varying needs.
Well-being was often aligned with thinking about helping patients to feel supported, and this was often talked about in terms of making sure people ‘feel like they’re in a safe space’; one participant described feedback from patients that they had had ‘enough clinical appointments and don’t want this to be seen as clinical as well . . . so every effort is taken to create a more informal and relaxed service’. This was described as helping patients open up to their colleagues much more than they might do with their clinical team. Similarly, other participants talked about delivery spaces and methods constructed ‘to support people’s well-being’ (e.g. sessions in local community gyms or in groups); although the exercise and activity might not be familiar, ‘they feel safe and secure because they’re in their surroundings’. One participant pointed out that ‘some people . . . have never done physical activity or structured exercise since school really and they just don’t know how to . . . they’re up for it . . . they just need us to support them’, and another commented that a supportive coaching approach ‘made sense . . . it actually works better rather than telling somebody to do something, doing it with them’.
In the same vein, the inclusion of family members and a consideration of the value of patients’ peers in supporting patients’ experiences and engagement was discussed by several participants. Family, particularly, was seen as a ‘strong influencing factor’ that was ‘overwhelmingly positive’. As well as being a practical help in getting to and from sessions, one participant explained that it was ‘important to entice them in:
. . . patients have got all this stuff going on in their head, actually, if they bring husband, wife or whoever in with them, it’s another person to take information in, or ask a question that they’ve forgotten. I think for some people they just want to get on and do it on their own and that’s fine, that’s their choice, but I think for a lot of people involving family in that activity is important.
Several participants talked about inviting family members not just to appointments but also to activity or education sessions; one described ‘the big thing’ for them as ‘the opportunity to meet patients and the families, we always invite the family member’. Others talked about inviting family members into an exercise class: ‘They can actually learn the exercises and then they can actually do the exercises together at home. And that’s quite powerful’. Group walk-and-talk sessions with families and friends were also described by one service as ‘really popular’. Another participant said that there were often some great stories of families coaching patients, ‘you know, within an inch of their life basically, but it was very good for the family as well, because the family . . . it was often children coaching their relatives and they felt that they were really, really trying to give their relative the best chance.’
The significance of patients’ peers was commented on in a number of interviews, with participants often framing group sessions as opportunities to ‘support people’s well-being’, for peer support and for providing time for patients to talk to other patients. One participant, referring to a focus group they had conducted with patients, explained that it is ‘the social side of things [that] they want’, and another that it is the ‘social aspect . . . for patients, which I think they get a lot from’. One participant explained that the familiar environment of a city rugby stadium (where patients may have been going to matches for years) helped create, for some of their men’s-based programmes, ‘that kind of lad-like feeling, that they find they can be a team together . . . they can almost say they’re going to train with the Rhinos . . .’.
Another participant described showing patients around the hospital high-dependency unit ‘so they would know where they would wake up . . . get looked after, after major surgery’. They explained that this also created an opportunity for patients to ‘see people that were like [them], who had cancer or were having major surgery’. They felt that ‘just seeing these people and they’re like, “I’m OK”, “they’re all right” ’, was an opportunity to have a conversation and relieve some anxieties. Similarly, other participants talked about that ‘patient-to-patient’ benefit: ‘You know me and my team are not the best people to necessarily encourage people to be more active . . . it is that peer-to-peer support that’s super important’. Group contexts were also talked about as lending themselves to ‘educational sessions’, and to a greater opportunity for conversations around broader lifestyle interventions, such as smoking, alcohol reduction and nutrition.
One participant explained that their service had attempted to trial video group clinics, but found that there was not much interest from patients, reflecting, ‘in a small place, people didn’t want to engage a lot because they didn’t want their next-door neighbour or family to know what’s going on with them’.
Despite this well-being narrative and language of support, one participant in particular made it clear that it is important that PA, including any broader approach that services were taking, be clearly seen as a ‘clinical intervention rather than something that’s a kind of a well-being add-on to treatment’ or ‘a nicety or a lifestyle thing’.
The individual
It’s that, kind of individual treatment of patients, isn’t it? Yes, they’re all there for a common reason, with us they’re all having surgery, so that’s the common factor, but other than that, they’re all individuals, so it’s kind of giving them what they need at whatever point in time.
A further frequent reference was the idea that to make this work – to get people moving – staff needed to be able to ‘understand’ the needs of the individual and pick up on what works for each person. They needed to ‘listen’: some people might be ‘absolutely I’ve kind of hit rock bottom so to speak and I know that I need to go up’; others might be ‘quite happy where they are’. One participant described this as ‘sometimes quite tough, tough to overcome’. A repeated idea was that staff needed to be ‘aware if someone’s struggling with something . . . offer them a different option’, recognise that individuals have their own way of looking at things and their own ‘individual motivations’. One participant explained that it was also important to encourage patients, ‘to listen to themselves . . . if their body’s saying no, then, then no, we don’t have to do it, sort of thing’.
Participants talked about finding the thing that people enjoyed, ‘what have they done before that I could possibly try and get them back into’, ‘Get[ting] somebody doing something that they love’ and then working on increasing that activity. ‘It’s finding what they want to do and what they enjoy in order to adopt a long and healthy lifestyle.’ Making the connection ‘between exercise and real life’, ‘making it a habit I suppose and making it something that you enjoy going to do’. There was some indication that, as a sector, PA specialists had moved away from narratives that ‘you have to do 30 minutes in one go of physical activity . . . you have to do it five times a week’ and that that this was viewed as really positive; 2 minutes for one person ‘might be absolutely enough and all you have to do. So, it’s about, I suppose, helping them pace their, their exercise. And just working with them to help them understand that . . .’. One participant explained that this might be slow, ‘kind of step-by-step’, ‘they might try something and not like it’ but that that is fine because there’s always something else that they can try.
Several participants talked about exploring what people might have done before: ‘and there’s always something like that to build on . . . and then we talk about adding structure into that activity that they do and things that are going to be beneficial for their type of surgery’. One participant talked about focusing on building ‘a bit of confidence [in patients] to just try different things’:
Some people haven’t even been physical[ly] active before they’ve come to us, so that’s kind of what matters to us is that people can just give stuff a go without feeling like they’re embarrassed or uncomfortable that they’re trying to do something that they don’t want to do.
The same service described different tasters of PA that are offered to individuals, each with a direct signposting route beyond into the local leisure centre, community provision or programmes within their service. This was seen as ‘really giv[ing] them a chance to find that thing that they enjoy’ and even when they do not find it during their time with the service, the participant felt that this gave people the confidence to go on to try something else by themselves.
Others talked about goal-setting (‘What matters to you [the patient]?’). ‘So rather than just being, “wouldn’t it be great if you got home on day 3?”, it’s more about “well, wouldn’t it be great if . . .?” ’, or as another participant put it, ‘what’s important for us, is trying to find what’s important for that person. You know . . . what is that, what is that thing that’s gonna help them, you know, carry on in the long term’. Participants relayed various examples that patients might describe, from ‘getting back to kicking ball in the park with the grandkids, brilliant’, ‘seeing their blood pressure decrease’, getting ready for their daughter’s wedding, ‘getting back to work’ or ‘back to 5k runs at Park Runs’. According to one participant, given that the patient has just been through a major trauma, ‘whatever is important to them is how we frame how the activity, nutrition and well-being will support them to achieve that and that’s what we do. We turn it to them and then we frame it to what their goals are.’
Motivating and inspiring
We can’t do that long term so educating them to do it themselves is a real light-bulb moment as well.
Another common narrative participants spoke about was driving ‘self-empowerment’ and motivating or inspiring people to make permanent changes, with one participant explaining that they focus on ‘self-efficacy for exercise because we know somebody with a higher self-efficacy for exercise are more likely to be independent once they’re discharged from our service’.
One participant felt that ‘somebody’s got to have something somewhere where they want to change’ but that all it needs is ‘a little glimmer [and] you can try and work with that and build on that’. They went on to explain that, for them, being able to ‘just give people the hope that things can improve, things can get better, their health can improve, their life doesn’t have to stay like it is at the moment if they don’t want it to be’ was really important. Several participants talked about their programmes as being ‘one area that [patients] do actually have control over. So they can control what they come to and what they don’t come to. And none of the cancer treatment is compulsory; it’s a choice at the end of the day. They get presented with the best possibilities for them and ours is a nice aspect of that treatment’. Others echoed this perception that their provision allowed for that ‘little bit of control . . .. You know, once you get into a hospital system, you don’t get a lot of control over many things . . . you know, they can actively participate in something, and choose to, or choose not to. Being in control of their destiny almost, so I think that’s quite a kind of powerful thing for patients’. Another participant added that it might be ‘fairly shortly after they’ve got some horrible news that they’ve got cancer and it does just tend to feel like life is falling apart . . . they’re just kind of lost, and bouncing around various different appointments’.
In several services, attempts to motivate change included an element of education; group sessions, for example, focused around ‘physical activity and why it’s important and the kind of benefits of it’ or conversations with, for example, a patient’s anaesthetist who might be talking to a patient about how their aerobic fitness is going to affect their care. One participant talked about placing ‘a real high value on educating the patient about exercise and why we’re asking them to do it’, as well as ‘how to monitor their own body, [and] how to put together their own gym programme’. Several talked about providing information or guidance on how to find support and ‘the right exercise classes or activities’ available in patients’ local areas. This was described as ‘key for the long term’ and ‘massively important’ to support patients to get to a point where, when they leave a service, they have ‘long-term strategies’ in place ‘to make sure that they continue to stay active, keep exercising and stay healthy’, and ‘that they are able to motivate themselves, have the know-how and the confidence to keep active and address their activity needs’.
A participant from one service in the early stages of developing provision described some concern from colleagues in framing too much around education, ‘concerned some patients wouldn’t respond to that and it should be more [about] inspiration’, recognising the challenges in getting the balance right. For another service, an hour’s session, known as ‘Surgery School’, preparing patients for major surgery (including the benefits of physical training, what they might expect and optimising medication) was also an opportunity for patients to meet members of their clinical team, including their anaesthetist; it provided, they said, ‘lots of opportunity for questions and answers.’ Another participant saw one of the issues being that ‘health-care professionals don’t even know the physical activity guidelines’, or that what they do know is out of date. A solution for this, they thought, was ‘some education . . . but I then don’t think we’re the right people to educate them. I think it has to be a role model within their setting that educates . . . so it’s a bit complicated really’.
Several participants commented on leading by example. One service ‘trying to become an active practice’, ‘so the idea was that we start with the staff and GP colleagues and everyone within the practice and then . . . if we’re all feeling it, we can then project that onto our patients’. There was a realisation that ‘none of us really move’ and they were ‘all sitting in front of the computer’, which led to practice walking challenges, ‘we all tried to walk as far as [we] could within a month . . . now currently we’re walking from Kilmarnock to Australia’. This also included signing up to be a Park Run Practice, by advertising local runs and a couple of staff going along. The participant felt that, on the whole, everyone now ‘feels more active’, more ‘willing’ and better placed to discuss PA more with their patients. Another GP talked about role-modelling being a promising way to frame the benefits of changing behaviours to their patients. Others also suggested that it might matter, or at least be extremely helpful, if activity was ‘something that is habitual to them’, the practitioner, if the ‘health-care professional themselves is physically active’. So as one participant explained, rather than it be a ‘secondary thought that they might see on a form and think, “Oh yeah like we can actually refer you to this physical activity programme” ’, they are personally invested in the idea that PA can provide all sorts of benefits, ‘they can answer questions’:
So I think almost a starting point would actually be offering a physical activity programme to the health-care professionals to then encourage them to realise that it’s a good thing, so then, for them to motivate their patients to get involved in it. ’Cause I definitely think those are more physically active I get referrals from. And I know that they do like the Park Runs and stuff like that so I think, that’s what we tend to see. I think if it’s something that you do, it’s something you more naturally talk about.
Another participant talked about their service’s walk-and-talk sessions; these were volunteer-led, with friends, family and dogs all invited, and were seen as:
[A] great session to get those people who were a little bit on the fence . . .. If we could persuade them to come to a walk and talk, and meet some of the staff, maybe have a look around the studio room that we use to deliver the exercise. It’s kind of like that first day of nursery school where you kind of go for a little bit of a visit first, get a little familiar with surroundings and generally those people who did come to a walk-and-talk session, we could then persuade them to come to one of the exercise classes as well.
Compassionate care
I think culturally. . . I think people that work in leisure facilities, I think, on the whole, they’re there because they want to help people and so I think there is a natural tendency that they are quite kind people, and people-orientated members of the organisations.
A fourth narrative clear from discussions was of staff who are compassionate and prepared to do whatever it takes and participants’ high opinion of their colleagues. Colleagues were frequently praised, with one describing their lead exercise trainer as ‘brilliant’, another attributed the perceived success of their programme to ‘having a good team to work with’ and a third talked about ‘a fantastic set of people, with a huge skill set’. They were empathetic to the responsibilities and challenges faced by staff, were respectful of the skills they had within their teams and portrayed a strong collegial environment.
Several participants expressed that it was not so much the profession or experience that staff might have, but the personal qualities that they bring, ‘It’s kind of the keen individuals . . . that have got the right skill set . . . it’s a range of skills as well, that complement’. One participant suggested that, ‘as long as you’ve got frontline staff that are fantastic communicators, and can pick up on what works for that individual, then it seems to work well’. Another participant said that, ‘For me, the personal qualities of the person is much, much more important than, kind of, the background necessarily, where that person came from . . . it’s more, “are they good listeners? Can they communicate well?” ’. Several talked about staff ‘noticing’ and ‘understanding’ the needs of individuals, ‘are they aware if someone’s struggling with something they can offer them a different option . . . for me that’s much more important’. One participant described ‘learn[ing] very quickly who are the right people, and weren’t the right people, to deliver the exercise’. They described feeling they had ‘done a good job of that’ and that they feel they have ‘a really, really good team . . . they’re very caring people and that’s a real, a must, it’s an essential part of their qualities . . . and their knowledge is respected by their patients, and their colleagues’. One participant pointed out that ‘sometimes, it’s just that [patients] don’t want to change now’ that that ‘doesn’t necessarily always mean that they’re not going to want to change in the future . . . so I think we’re always keen as a service to make sure that we’re doing all that we can, even if they’re not going to be successful . . . in 2 years’ time they might’ so it was important, they felt, that staff ‘have that legacy with people’.
Participants clearly had empathy for patients’ lived experiences, noticing the particular difficulties they faced. They often talked about pain, with one participant suggesting that it is ‘probably one of the hardest things . . . for people to manage . . . people living with pain, every single day, you know, that’s just, you know, the impact that has on mental health, as well as anxiety, depression, I’m not surprised people are not particularly too enthusiastic about doing physical activity’. They described this as something they are still learning about, that they try to focus people on thinking about the after-effect of activity on their pain experience, and on ‘good pain and bad pain’. Another talked about pain as ‘one of the things that really, really can upset somebody’s adoption of physical activity, it’s a very subjective thing’. This tended to bring the discussion back to the importance of listening to somebody and of getting patients ‘to understand that it’s important to listen to themselves as well . . . like if their body’s saying no, then, then no, we don’t have to do it, sort of thing’.
Participants often acknowledged the ‘human’ aspects that their patients were likely to be experiencing, ‘the worry, the concerns, the anxieties related with the referral and “what’s my prognosis? Have I got weeks? Have I got months? Have I got years?”. . .’. There was also recognition of the impact on staff of working with people in these contexts; one participant gave the example of colleagues working with newly diagnosed cancer patients, describing staff as, ‘an outlet for a lot of stuff and, you know the guys have really, really deep conversations’. They talked about the importance of ‘looking after the staff in these services, and making sure that they are cared for’. They talked about the ‘stonking phone calls’ and even the ‘brilliant phone calls [being] just as emotionally draining’ and making sure there is a system in place for staff to talk to someone, ‘so yes, it’s looking after the staff’. Several services talked about psychology provision provided to staff, one talking about a ‘a monthly debrief with all the gym people because . . . they’re dealing with cancer all the time, people die and everything else like that’. They also talked about an effort more generally across their trust to support all health-care workers working with cancer patients, ‘to have better psychological skills’ through formal training.
One participant explained that it is not just the attributes of staff directly delivering the programme, but everyone a patient may come into contact with. A point echoed across other discussions was that you need ‘buy-in from your own organisation to sort of help . . . you can’t do it on your own’; ‘you need the receptionist at the facility to be kind to the person . . . see that they might be struggling a little bit and help them out’.
The patient
Patient involvement and the patient experience
A distinct learning culture, primarily in the context of seeking out, and acting on, patients’ views, was portrayed by a majority of participants. Often this was presented as explicit ‘exercises’ in which services sought the input of patients, described by one participant as ‘so important’ to be clear what patients want, and described by others as ‘a lot of co-designing’, and acting on patient feedback, with one participant suggesting that this meant that patients do ‘generally feel that it’s about them’.
One participant talked about several iterations in the early months of their programme, taking opportunities to meet patients and their families, ‘constantly asking patients, “What worked? What didn’t work? Was it too long? Was it too short? What do you want to hear about?” ’. Another participant admitted, ‘We thought we knew the answers . . . We thought that men would like to train with men and women would like to train with women. We were absolutely wrong about that. We were told absolutely not’. Others talked about patients being ‘key’ to the development of their services or discrete pieces of funding to work with different patient groups to explore, ‘what was important to them’, what their triggers were for accessing and prioritising a programme of support, the sorts of lifestyle intervention in which they would be prepared to engage. One participant talked about patient representatives on their programme board and steering group, explaining that patients help us shape our service to, to what they want. They signed off on everything we did to set up and they still sign off on everything that I change. I literally just presented to them this morning about a new bowel cancer pathway . . .’.
This influence from patients on how programmes evolve, from start-up and pilots to ongoing improvements, seemed to be rooted in ‘listening to them and, kind of, their concerns’, and as one participant maintained, ‘feedback makes anything possible and makes you kind of improve as you go along’. One participant commented on their service, moving activity sessions from a gym environment to a church hall, responding to feedback from patients who ‘felt quite uncomfortable being in a gym environment . . . people staring at them and maybe not knowing why they were coughing so much’. Another explained how their service had changed over the years, ‘I think the education sessions, they’ve changed massively based off patient feedback and what they found to be beneficial and what they’d rather not, kind of, discuss . . .’, and another talked about patients, ‘always at the table . . . we’re very lucky . . . we have a very active patient user involvement group . . . at the end of the day they’re the ones we want to do it and we have to listen’. Some participants talked about patient involvement in the recruitment of staff, ‘it is brilliant to actually go to the patient representative and just say, “How would you feel if this person was coaching you physical activity . . .” ’, adding that they felt that patients had added ‘massive value’ to the recruitment process.
One participant suggested that acting on patient engagement was not always straightforward, describing it often as ‘so personal’ and everyone having ‘extremely different’ opinions.
Perceived experiences: setting, accessibility and locale
There was a strong indication that the acceptability of the setting and space in which the services delivered sessions with patients was influenced both by the local context and by the patient group; ultimately, it was important that the space was easily accessible and that it was not clinical (people ‘did not want to revisit the building where they were diagnosed with cancer, repeatedly, for exercise’), and, in some cases, that the people involved were familiar and part of their community. Although there was consensus that good-quality facilities supported the success of programmes, the acceptability of a space was clearly subjective and something to be considered by staff in relation to the patient group and the community in which their services were working. One participant described a particular leisure centre as ‘a much more appropriate venue’ partly because of the available equipment, but also because it is ‘much cleaner, and nicer, and warmer’ and that was ‘massively important’. In contrast, other settings they said, could be problematic, because, in trying to be accessible and use spaces that are positioned across the city, in different communities, they had to rely on venues that were ‘colder’: ‘they don’t have heating that works as well’ and you ‘can’t use the nicer rooms because they’re up the stairs but there’s no lift’. These venues tended to be located in the more deprived areas, but people in those deprived areas were much less likely, one participant explained, to travel, than those in other areas, so ‘We need to be within those areas’ but ‘those kind of like [are] the things that do then impact how patients have experienced our programme . . . it would be nice if they got a refurb[ishment] it would do us a favour’. For another service this had been a similar stumbling block, overcome only recently because of local investment:
[T]he most deprived areas . . . had probably the worst facility. And then we wonder why people don’t access it; we wonder why the more deprived communities are the least active . . . there’s obviously loads of other things but you know, you can’t just, it’s not really fair that, is it. So we’re quite lucky that, that definitely helped in terms of engaging those, from those communities, into some of the programmes and physical activity in general.
One participant from another service, described their space (within a private sports and fitness centre, well equipped, with social spaces, cafes and welcoming to families) as a ‘big draw’ in encouraging people to come and in helping people to socialise and to relax: ‘You have to go in there with a flower in your hair because it’s so nice’. Others, however, talked about private gyms being rejected by patients, with local leisure centres, church halls or community hubs being preferred spaces.
Accessibility was a consideration acknowledged by the majority of participants. Venues were talked about as being ‘easy and local’ and accessible to patients, particularly by public transport, ‘if they’re on a bus then the bus needs to stop near the leisure centre . . . [not] down country lanes and stuff’, or by walking. One participant talked about the availability of parking: ‘if they can’t park their car there, they’re not going to stop. They have enough trouble trying to park the car at hospital car parks and we don’t need to compound that’. Another participant explained that their service was looking at outreach provision to overcome some of the challenges they recognised in reaching people across a wide geographical area.
Beyond the physical accessibility, the idea that ‘people like to go to their local centre’ seemed to also include an element of community affiliation, membership of a language, colloquial and centred around a narrative and life lived in a shared place and space:
People . . . like the fact that the receptionist has the same accent and uses the same terminology and the same dialect. They like the fact that my team are all from Greater Manchester and live in Greater Manchester and so we all have the lovely Greater Manchester twang to our accent as well . . . they like that sort of familiarity.
Another participant explained that a lot of the conversations patients and staff might have ‘aren’t necessarily about health and not about medical conditions [but] just about life’ and that ‘being able to talk about your local monuments . . . the local pub . . . or the local restaurant might seem quite small, but actually help, help a lot in terms of engaging people and keeping them wanting to stay within programmes or services’.
Several participants did also accept that, for some patients, familiarity was not always helpful; in one service (in a small isolated town), they found, in trying to develop group lifestyle clinics, that there was not ‘much interest from patients in our area unfortunately’, ‘with being in a small place, people didn’t want to engage a lot because they didn’t want their next-door neighbour or family to know what’s going on with them . . .’. Another described a small number of patients on their cancer programme ‘who don’t want to go to their local [sessions] because they don’t want to be seen as having cancer in their community. So they prefer sometimes to travel’.
The activity: relationships – a good interaction
Physical activity: you’re not gonna do it, if you’re not gonna like it.
The approach to getting people moving differed between services, but the finer detail of the PA narrative, the need to be acutely in tune with patients’ individual contexts and needs, was a recurrent message: ‘every patient is different, so it’s quite difficult to generalise . . . it’s trying to find, for each individual patient, what their priorities are and how we can use that as a persuasion, to persuade them to take up the offer of prehabilitation’.
You gonna what? You’re giving me exercise classes?
The majority of participants emphasised an approach, or moving towards an approach, that rooted their provision within patients’ treatment plans. What they wanted, and some had, was to not just be ‘encouraging the patient to engage in [PA], when they’re with you’, but for PA to be seen as usual care: ‘It’s about engaging those wider staff, so that they’re already having those conversations and what you’re telling them isn’t different or isn’t that disparate from what everyone else is telling them’. This was seen as crucial, that whole clinical teams ‘bought’ into the idea and therefore presented a unified message to patients who ‘see exercise as part of treatment just like anything else’, rather than ‘something you can go and do if you would like to, and if you kind of fancy it, it’s like no, this is part of your treatment and I think that’s where framing can be really important; ideally, we would have that from all health professionals’.
One participant explained that this might be an anaesthetist:
They’re already there talking to the patient about how their aerobic fitness is going to affect their care directly. So like, they’ve already, some of them, had that conversation before they come to us, so then we’re encouraging them to improve their physical activity, and improve their aerobic fitness, so it leads on really well from conversations they’ve already [had].
Another described clinical teams at MDT appointments presenting a treatment plan, and ‘this plan includes . . . “we’re going to send you to the exercise team” . . . it’s part of patients’ prescription . . . it’s just like them going to see the radiotherapist, they come and see us’. One participant talked about the surgeon ‘that’s going to save [a person’s] life’ having more of an influence than ‘some random physio[therapist] that’s ringing them up’, and another mentioned a particular surgeon who would often talk to patients about buying a bicycle . . . ‘he didn’t quite say . . . “I’m not going to operate [on] you” but he’s basically saying to them “this is so important for me, that you’re going to go and get a bicycle” ’. They described, anecdotally, that people would leave the clinic, they’d get a bicycle, and you’d see people coming back, ‘and their families had coached them, you know, within an inch of their life basically’. Even, it seemed, if consultants/surgeons did not have a huge amount of time to explain to patients what prehabilitation was all about, if they ‘endorse the programme’, this makes a significant difference, patients will have ‘almost bought into the idea’ and services can begin to build on the benefits from there.
In a service in which PA was being increasingly embedded in cancer treatment pathways, one participant talked about patients being presented with the best possibilities for them ‘and ours is a nice aspect of that treatment . . . we’re quite matter of fact about the fact that it is part of their treatment . . . it is good for them . . . [it] will help their recovery . . . and help to combat the effects of treatment’. Similarly, for the prehabilitation phase of another service, PA was described as being ‘very much framed around the physiological status of the patient . . . what the benefits of the programme would mean for them, in the immediacy . . . when they’re looking at the treatment and their initial recovery from surgery’.
On the whole, the indication from participants was that the messages around the value of PA are slowly getting through, both to patients and to clinical colleagues, ‘So we’re all kind of singing from the same hymn sheet now . . . and when you get that message, from lots of different places, people actually start to believe us’, ‘in their mindset it’s their treatment to come to us and that’s why I think we have such a high engagement rate’. There were clearly influences that supported that shift. A participant from one service emphasised the different experience for the patient and for their team when patients are referred from particular practitioners; they described the ‘valuable resource’ of a cancer care co-ordinator, who, in the early stages of a cancer referral, has an ‘opportunity to really sell [the] service, and sell the benefits’ so that when patients reach the service, they are ‘already primed, and it makes our job far easier to get them in and then to engage them. That initial small message really builds momentum . . . We know they’re going to engage, we know they’re going to come to classes’. Others talked about oncologists or surgeons ‘carry[ing] so much clout . . . You know they’re doing it; you know they’re bought in. You know they trust them and that’s really important’. For patients referred via other routes, participants gave examples of people arriving in front of them who might say, ‘Well I’ve no idea why I’m here’; in those contexts, in a finite time, it was much more difficult to engage patients in their programmes.
In other spaces, for example in a community-led service for which the participant described no ‘proper system that flows from, like, prehab[ilitation] to rehab[ilitation]’ and where their service is ‘not seen as part of treatment in the same way as it probably is [elsewhere]’, they see some people ‘a bit reluctant . . . kinda resistant to joining’ the programme. They acknowledged frustrations that cancer patients were not being directed to their service by clinical teams or ‘presented exercise as some sort of prescription’, and that, instead, it was then often about ‘timing’ and ‘quite personal’ to the patient whether or not they managed to engage individuals at all. One avenue to reach patients, they explained, was through a hospital Macmillan Centre, where they might ‘find a lot of people are just not ready for it . . . like at that moment in time . . . they’re just not ready because they might not have even told their own family, kind of, what they’re going through’. They described the need to get patients to the stage that they feel comfortable to then be able to join sessions, ‘and for some people, joining our sessions is getting them to the point where they feel comfortable to tell their family or something like that’. They talked about the capacity to reach patients being sometimes about ‘trust’; here it was less about placing trust in clinical leads endorsing PA, and more about practitioners in a service (the participant and their colleagues) being prepared and able to build trust with the patient over a period of time. They acknowledge too that their ‘brand’, associated with a professional rugby league club, probably also ‘helped engage people that wouldn’t normally access a service [such as this]’, particularly men, ‘because of the brand . . . because people see it and know it and trust it’.
One participant, a GP, voiced issues they’d experienced with patients’ perceptions, whereby they sometimes assumed ‘that we are asking them to do this to save money’, or even believed that a GP might ‘earn from it somehow’. Others mentioned concerns, particularly from the non-cancer patients, that their surgery was being put off, ‘your waiting lists are so long, are you just putting off my surgery’ and from orthopaedic patients, ‘worried, if they get too fit, if they’re able to walk further’ they would no longer be eligible for surgery. There was acknowledgement that services needed to balance these concerns, address the narrative ‘what is in it for me?’ and ensure that the framing from clinicians was clear that ‘nothing’s been put off’ and ‘this is going to actually help their treatment’.
Physical activity
As well as the generalised attitudes services appeared to take in their programmes (well-being, personalised, motivating and inspiring, and compassionate care), and although the sorts of activities differed slightly between some of the services, there was a common narrative that just ‘giving stuff a go’, finding something that people enjoy and working on increasing the amount of PA people were doing, rather than driving particular activity guidelines, was essential.
One participant described ‘intuitive exercise’ or ‘intuitive activity’, ‘it’s about . . . we listen to them, and their body and how their body responds . . . we learn how far we can push their exercise prescription before it starts to have a negative impact’, which might, they added, come afterwards rather than at the time of the activity. They described the need for collaboration between the exercise instructor and the patient, ‘listening to [the patient] and being flexible’.
There seemed to be a move away from generalised PA recommendations:
There are guidelines and guidelines don’t fit everybody. If we took everybody’s shoe size in this room and just gave you the average shoe size and said “there you go, there’s your shoes for the next year”, none of us would probably fit them.
One participant explained that they often say to patients, ‘You know if you do 2 minutes, that’s fine. ’Cause actually 2 minutes for you, might be absolutely enough’. Similarly, another participant explained that they had ‘[g]one very much with every minute counts. So you say to people, “It doesn’t have to be half an hour, it can be 5 minutes [at a time], as long as it adds up to 30 minutes”, encouraging patients to think about heart rate and not on running a 10k, half a marathon, that sort of malarkey’. Another participant described a focus on helping patients ‘pace’ their exercise, again to move away from any aim for a particular amount of MVPA per week and talk instead ‘about how, the actual movement, while there may be some pain, it’s the after-effect, do you feel better afterwards?’
One participant explained that, for some of their more reluctant patients, a meet-up known as a ‘walk and talk’, volunteer-led and less labour intensive from a staffing perspective, was a great introduction to movement and the service. Friends, family, dogs, anyone, are invited:
They were a great session to get those people who were a little bit on the fence. If we could persuade them to come to a walk and talk, and meet some of the staff, maybe have a look around the studio room that we use to deliver the exercise. It’s kind of like that first day of nursery school where you kind of go for a little bit of a visit first, get a little familiar with surroundings . . . generally those people . . . we could then persuade them to come to one of the exercise classes as well.
One participant noted that it was helpful to support patients to see the functional benefits of their programme or why ‘you’re telling them to do something in a certain way’, particularly when patients were experiencing pain. They used the example of a bicep curl, working on wrist positioning, getting it to that ‘perfect kind of point meant that that bicep curl was nowhere near as painful as it was previously’. They would relate this to everyday tasks, ‘when you’re lifting the kettle that’s exactly the same action as what we’ve just been doing . . . starting to just make that relation between exercise and real life and then listening to them and kind of their concerns, I’d say, is how I’ve kind of managed it’.
Several participants commented on working with individuals who had never been physically active before, with one participant explaining that they might ‘just need a bit of confidence to just try different things’ and another, that ‘they just need us to support them’. There was general agreement, however, that, although people might claim they do ‘absolutely nothing’, when services delve a little bit deeper, ‘people do actually more than what they think they do. And so that always gives us, there’s always something to build on. They always say, “Oh well I don’t do anything. Oh but I walk the dog three times a day”.
Although participants sometimes remarked on patients’ feelings of embarrassment or of being uncomfortable, or avoiding putting people in a position ‘to do something that they don’t want to do’, one participant acknowledged that ‘most health-care workers don’t know how to talk about exercise’ and described using Moving Medicine resources35 and consultancy to support education and training events and discussions with MDTs. The same resources were beginning to be adopted by a general practice, with staff being encouraged to look at and use the online resources geared at aiding the integration of conversations around PA into the routine clinical care provided by health-care professionals.
Language was something that came up during several discussions; although ‘exercise’ was a word readily used by participants when describing provision, there was some agreement that ‘activity’ was a better terminology to use with patients:
I think we’ve been doing it long enough to not go in, or not judge people and say, right, ‘How much exercise do you do?’ We talk about physical activity levels as part of the assessment and we say, I will say, ‘so what do you do to keep active now?’, ‘what things do you do?’ And the answer is sometimes, ‘absolutely nothing’, and then when we delve a little bit deeper, people do actually more than what they think they do. And so that always gives us, there’s always something to build on . . . and then we talk about adding structure into that activity that they do and things that are going to be beneficial for their type of surgery.
Activities included classes, taster sessions for various activities; strengthening and cardiovascular work; high- and low-intensity exercise classes; walking groups; in-person, one-to-one, group and online resources; and signposting to the local leisure centre, community provision or programmes within their service.
Perceived benefits
Participants frequently commented on what they believed mattered to patients, providing anecdotes of their interactions with patients or relating to patients’ experiences; they clearly enjoyed hearing these personal stories that patients provided, frequently referencing what they felt their services meant for people and indications of positive impact. One participant talked about the beneficial effect they felt that some patients experienced ‘just finally realising that they’ve done something positive for themselves even by coming for the first time’, and this was seen to motivate people to go on to try to make changes. Another talked about people who had come to their service who had been, ‘well, just completely destroyed individuals, to happy confident people who potentially have never exercised before, who are in their, let’s say latter years, and it’s completely transformed their lives’.
One participant felt that, as a service, they had been able to create ‘a safe space’, one in which people felt able to share things that they might not have shared with people outside of that environment before . . .. They speak to me about things that they might have never told anybody else . . .. That’s kind of what matters to us is that people can just give stuff a go without feeling like they’re embarrassed or uncomfortable that they’re trying to do something that they don’t want to do’. They went on to explain that sometimes, there’s a ‘kind of moment’ when people might realise that their lifestyle ‘isn’t something that’s good for me’ putting the shift down to people doing an activity that they realise ‘is actually something that I liked doing . . . it’s not because someone’s told me that I need to go for run, or a walk or whatever, it’s actually I want to do this because I like it, and it makes me feel good.’ Similarly, another participant described patients as very ‘accepting to other things that can help them’ once they are increasing their activity through their programme, ‘they often come to us and ask’ about other things, describing themselves as in a ‘great position’ to then refer or signpost to other support.
Several participants talked about the impression that their programme was able to bring patients an ‘element of control’ to what is otherwise a difficult time, and that ‘the time to talk to the other patients that are there . . . the time to talk to the trainer, it kind of, it grounds them a little bit . . . so that’s definitely a big positive that we’ve seen’. There was also an impression that, particularly for cancer patients, in prehabilitation programmes you might see them ‘responding really well . . . because they’re already in that fight or flight response and that’s what they want to do . . . they want to do it because they know that they want to improve their surgical outcomes so they’re very focused on doing the exercise for that gain’.
Several participants talked about clinical teams recognising the benefits in patients, including citing patients going back to clinicians telling them how a programme had made them feel, ‘how much they’ve enjoyed it’ and ‘how successful they’ve been’, and ‘what it had done for them both physically and psychologically’. This sort of feedback was seen as ‘massively important’, with one participant attributing it to a shift in how their service was perceived more broadly in their trust, from colleagues beginning to ‘see the actual value in physical exercise in terms of a treatment pathway’.
One participant talked about the importance of patients’ clinical teams noticing and taking an interest in how patients were getting on; it was ‘really important’ they said when, for example, GPs endorsed their programme, but what was ‘even more important, was that when patients went back to [the] GP for subsequent appointments, that the GP actually asked them how they were getting on’. They explained that often GPs did not do this; as a patient, they felt that staff might not follow-up on their progress and that was something that they felt that patients felt quite strongly, giving the example of a patient ‘dying to tell the GP that they’d lost weight and they’d done really well’ but never being asked or given the opportunity to tell.
There was an indication that, despite some people being reluctant or almost outraged when the programme was originally presented to them (‘You gonna what? You’re giving me exercise classes?’), one participant talked about some of those people still exercising with the programme 2 years on, ‘and they absolutely love it, and don’t ever try take it away from them’. Another mentioned patients, a number of years post surgery, continuing to use incentive barometers saying, ‘It’s helped me so much this thing’, suggesting that the spirometers had ‘become a little crutch for them, you know, and you think, that’s not harmful is it for them to do breathing exercises because that meant that they could do other exercise . . . it’s amazing stuff’. One service mentioned that people would often leave sessions ‘throwing tenners at us on the way out as if to say, why have I not paid for my appointment. They think it’s like private, which is brilliant, and it shouldn’t be private it should be NHS but I think it is a big draw for them to come there and it helps them socialise and relax when there’.
Evolving services
All participants talked about their provision as an evolutionary process, frequently portraying themselves as reflective organisations. They had largely begun small, ‘from a good idea’, focusing usually on one clinical group (typically cancer ‘because that was what everyone was doing’ or where the funding was), usually as a perioperative approach from the beginning, but moving through various iterations, learning from patients, learning from their own and colleagues’ practice and being prepared to learn more.
Individual characters seemed to have been the primary drivers, often then with the coming together of a small number of like-minded individuals. Often, but not exclusively, this would include someone with an academic background or interest in the sciences, often in sports medicine or something similar, and/or strong experience in specialist PA training, cardiac rehabilitation or physiology, sometimes new to the NHS and not always people with clinical experience.
Evolving
Bringing colleagues and other collaborators on board was seen as essential, ‘it can’t just be me just because I want to push something, you need other people’. This was always described as taking place over a period of time, sometimes 1 or 2 years, or more. One participant described almost a chance discussion, ‘and then you found that there were other people in the area starting to say the same things . . . And suddenly you’re kind of connected up with people . . . and that’s where it started’. There was frequent reference to patient involvement, with participants describing explicit efforts to hear from patients and a strong commitment to that feedback enabling continual improvement. One participant, claiming that they did not think they ‘were very good at it at the start’ but realising as their programmes evolved ‘the importance’ of continual engagement with patients as they went along, describing it as ‘really valuable’ and something that is now ‘there right from the get-go’ and ‘a voice in how the actual programme looks’ when they develop new provision.
In one service, the initial focus had been respiratory complications in surgery patients; they began to use incentive spirometers; provide patient education; and, over a period of several years, expand over several hospitals, initially under a quality improvement framework. Picking up small bits of funding along the way, some specifically related to collecting data, this enabled the service to evidence a reduction in length of stay for respiratory complications, giving them ‘a platform’ on which to then begin to build a broader focus on enhanced recovery, and then prehabilitation, exercise and nutrition, and now a comprehensive package with an interplay of specialists. The participant admitted that it probably helped that they had been in an increasingly managerially responsible role (now the clinical director for the intensive care unit and anaesthetics), that they already had personal relationships with hospitals across their trust, ‘through colleagues, and friends’, as well as a certain amount of ‘authority to say, well, we’re going to go in that direction . . . we can get things done’. There had also been an element of seeing a citywide, multihospital context as an opportunity, one that required acceptance that ‘they were all doing something slightly different’ but that working together, and trying to do things more collectively, was a great opportunity to improve on fairly poor health outcomes in the area. They talked about a bit of resistance to some of their ideas working, ‘surgeons and everybody else at the time poo-pooed it a little bit and said, “why would you do that?” We say, “well, why wouldn’t you?” ’.
Broader buy-in
I think it’s certainly, I’ve seen in 18 months in the trust there’s been a real shift in kind of ‘who are you, and what do you do?’ to seeing the actual value in physical exercise in terms of a treatment pathway. And I think that’s partly because of what we’ve done as physical trainers, with the patients but mainly because of what the patients are then going back to the clinicians and telling them.
Participants consistently presented a coherent message that activity as treatment is both a powerful message to patients and a necessary approach to engendering broader support from colleagues or organisations. The latter process, particularly, was clearly not straightforward and was highly nuanced; it was a gradual process, reliant on individuals, perseverance, good relationships, education and evidence.
One interviewee described feeling ‘very lucky’ that their trust had a ‘real proactive clinical lead’, something echoed by other participants, who described clinical leads ‘who were very supportive . . . who could get their colleagues on board’, or could make the transition sometimes ‘a little bit easier’ because often colleagues would ‘follow suit’.
In one trust, the example was given of a clinical lead who had ‘really promoted, and really pushed, the message . . . said “we’re great, we’re making a real difference, come and have look” ’. They talked about all sorts of ‘strange people doing classes’ turning ‘up in a suit when they should have turned up in trainers’, but that this was an excellent way to help commissioners and others nationally, to take an interest and come and see what they were doing. They also talked about ‘strong buy-in from senior management’, including their chief executive, ‘who values it, and that’s a really positive thing, that’s one of those, it’s a massive tick in the box because he likes what we do’.
One participant explained how, in their service, they had ‘hand-selected people’ who they knew ‘already bought into the idea [of PA]’ and that by focusing on only a small number of clinical pathways they hoped that ‘keen, engaged surgeons’ would help ‘spread the word’ and make the roll-out to usual care ‘a whole lot easier’. These people became known as ‘clinical champions’ and the participant recounted higher referral rates, and higher uptake, by trying ‘to create a little bit of competition’ between the clinicians: ‘My patients are getting this, why don’t you get your patients . . .’.
Commonly discussed were the roles patients played in terms of engaging health-care professionals in the value of PA provision: ‘I actually think patients, going back to their health-care professional, telling them how much they’ve enjoyed it and how successful they’ve been, is massively important’. One participant talked about this as having an almost cyclical effect whereby the messaging to staff (of the treatment benefits of PA) would be reinforced by patients ‘going back to the clinicians and saying “this is the effect I’m seeing”, and the clinicians are reinforcing what they’re saying to them, and not dismissing it out of hand. So that’s really positive’. Some participants suggested that they could capitalise on this more: ‘we very much learnt the importance of, like, patient feedback, and having those case studies and the quotes and things as we went along’. Similarly, another participant talked about ‘a big campaign around video testimonials, you know, the best people to talk about our service isn’t someone like me, it’s actually people that have been through it’. They had the support of a Public Health England PA champion, a nurse and a GP who had picked up the testimonials, using them when engaging clinical colleagues about PA; they described it also being used with patients, ‘You know me and my team are not the best people to necessarily encourage people to be more active, it is that peer-to-peer support that’s super important’. Another participant talked about interest from funders in service outcomes: ‘actually when you speak to them they want to know the patient impact . . . and the patients say that themselves . . . they’re the best at saying that’. They talked about presenting a recent commissioner report, and how ‘every single commissioner . . . they’ve ignored the first five pages, they’ve literally just gone straight to the back page because they know that’s where the patient quote or the patient case study [is]’.
More generally, gaining clinical buy-in appeared to be a slow and ongoing process that took tenacity and perseverance, and broader support from clinicians was, critically, not always a given: ‘knock[ing] on the hospital doors a hell of a lot’ trying to ‘speak to a lot of nurses who were not too keen on sending their patients to anybody but a nurse’, and who ‘did not see the value in physical activity’. There was a clear message:
you’ve got to do the legwork . . . you’ve got to really promote . . . you’ve got to get out there, and print the leaflets, and go and shout about it in different departments. And I guess it’s like anything. It’s like any sort of marketing; you get out what you put in . . .. It’s really tough and especially when you’ve got something that’s quite, well . . . that’s really groundbreaking and maybe a little bit witchcraft, like exercise.
For one service that had little direct connection with clinical teams, building trust with clinical colleagues and with patients appeared to be a continual process. Reputation (they described having ‘worked so hard to build ourselves a reputation of delivering health-care programmes’) seemed to help, and branding (their association with a rugby league club, the brand and the players as ambassadors for the programmes were seen as attracting some people), they felt, certainly made ‘it easier’:
to get in the door somewhere . . . somebody recognises the brand, so they’ll be more open to a conversation with you . . . I do find that just because I wear the kit and I’m around the hospital people actually approach me and speak to me just about rugby . . . so that’s quite nice in that sense, ’cause I think people almost see you as approachable just because you are wearing that brand and so that’s kind of definitely helped I think.
Resistance was often put down to a ‘lack of understanding’ of what services provide and practitioners are ‘qualified to do’, with broad acknowledgement that there is ‘a massive amount of work’ still to be done, as well as ‘a lot for primary and secondary care to learn about activity’.
A manager of one service described as high on their agenda the need to ‘generate evidence or generate momentum that encouraging patients to be physically active should be viewed as a clinical intervention rather than something that’s kind of a well-being add-on to treatment’. Another recounted how they were able to gradually move from a quality improvement project that began with post-surgery data and the use of incentive spirometers to introducing other elements, such as exercise and nutritional support. For them, the ‘big thing’ that had enabled this to gain momentum was showing an initial ‘length-of-stay reduction, a reduction in respiratory complications’.
One participant described, in working to engage GPs and practitioners more broadly, talking very little about the benefits of PA and instead ‘about reducing their patient visits, QOF [Quality and Outcomes Framework], things like that, that actually are not about the nuts and bolts of how physical activity actually benefits people but how it impacts their day-to-day life as a GP’. They acknowledged that it is worth thinking about framing PA to the particular perspective of different health professionals, because this will vary, giving a further example of physiotherapists who might use exercise referral programmes as an exit route from their own rehabilitation programmes.
Several participants spoke about having to ‘prove quite a lot’ to gain the trust and support of other clinical staff, with one explaining that, to get nurses to trust their message, ‘we had to prove that we were qualified to a certain level’. This seemed to be satisfied in part by qualifications, which included British Association for Cardiovascular Prevention and Rehabilitation qualifications; cancer and cancer rehabilitation qualifications; at least level 2 training for gym instructors, but usually level 3 or 4 training, which sometimes seemed to relate to whether they worked with less complex or more complex patients; and nutritional training that accounts for patients receiving chemotherapy.
A participant from one general practice talked about ‘teaching the local GP trainees about exercise prescription and physical activity in different conditions’ and this was seen as one way to begin those broader conversations, and engender them early on in clinical careers. Although still in its early days, this had moved on to talking more broadly with colleagues around becoming an ‘active practice’; with two existing colleagues ‘really interested in well-being and physical activity’; they had been able to push the movement agenda, engaging staff in ‘education’, ‘do we actually know how much physical activity we should be doing?’ ‘How we incorporate that into our practice when we’re talking to patients everyday?’. Realising that they themselves did not ‘really move’, they talked about needing to come up with ‘some inventive ideas’ to move more themselves. The thinking was that ‘the more we talk about it, the more it’s just second nature to us . . . And I think it does need a buy-in from everyone’. They felt that they had ‘done really well with all the staff’ and although there was a ‘wee cohort . . . “Oh, I’m not doing that” ’ there had been ‘a lot of engagement, and lots of support, and everyone feels more active . . . or most . . . people that have, like, joined in . . . and I think definitely would be willing now to discuss more with their patients’.
Participants frequently made reference to the language of activity being incorporated into routine clinical interactions, from the general practice to specialist care, ‘ “Do you smoke? How much alcohol do you drink?” I mean it should be, “how much do you move? Or how much physical activity do you do?” ’. There was also an impression that it might matter, or at least be extremely helpful, if activity was ‘something that is habitual to them’, the practitioner. So as one participant explained, rather than it be a ‘secondary thought that they might see on a form and think, “Oh yeah like we can actually refer you to this physical activity programme” ’, practitioners are personally invested in the idea that PA can provide all sorts of benefits, ‘they can answer questions’:
So I think that’s where the issue is . . . I’ve been in a lot of rooms where the health-care professionals don’t even know the physical activity guidelines. They know the old guidelines but they don’t know the new ones so it’s some education in that sense is probably needed. But I then don’t think we’re the right people to educate them. I think it has to be a role model within their setting that educates them so they can buy in to our programmes.
Relationships
They know our first names, we know their names so when the patient comes to see us the patients feels really safe; that we are part of their team . . . and I think that’s really important specially in that very scary moment before their treatment starts . . . they need to feel that everybody’s working together. They don’t need to be walking into an appointment with somebody who might not know who their clinical team is, or might not understand that they’ve been referred onto surgery. They don’t need that question. As soon as they have to explain it to that person, they’re going to lose confidence with that person, so I think collaboration in the whole system is needed.
The relationships between colleagues within programmes, but also, critically, outside programmes and/or organisations, were frequently presented as important or as making things possible. Services needed ‘other people’ to make their programmes a success, not just to engage patients, but also to ensure that their care was well supported by people working together.
One participant talked about their programme being ‘very much in collaboration with clinical teams’, suggesting that the key was that everyone involved saw patients’ interactions with the PA service as part of the ‘one team’ providing treatment and care. Good relationships, collaboration and trust enabled the PA to be rooted in that broader treatment; a patient’s clinical team knows the PA team and, importantly, the patient knows that they ‘communicate a lot’.
‘Obviously’ they explained, clinical teams are ‘really invested’ in ‘preparing the patients before surgery . . . really invested in what that patient is doing in their outcomes with us’. They described open communication, backward and forward, when any concerns or changes arise, and saw their responsibility to feed back to clinical teams as just as important as for clinical teams to ask how patients were getting on. This was seen to be enabled by open lines of communication; they talked about being ‘extremely lucky that we can just send an e-mail to a patient’s surgeon, and say “this patient turned up today and their heart rate didn’t respond as it was, we think that you should get them back in” ’. They talked about ‘surgeons really, really liking that . . . they know that in that 3 weeks leading up to surgery, we’re probably seeing their patients a couple times a week and they’re not . . . we are monitoring them very closely . . . they rely on us a lot.’
A participant from another prehabilitation service explained that, when they were setting up their provision, ‘we knew we wanted it to be kind of cross-sector’, they described already working ‘really closely alongside the public health team . . . so I kind of knew who they were, knew how they worked, so we’ve got that working relationship’. This, for them, had led to co-delivery of activity sessions as well as joined-up access to wider public health services. They also talked about working closely with the hospital dietitian and current efforts to work more closely with psychology services.
In the rehabilitation phase of one service, although they described much less contact with clinical teams than in prehabilitation (unless there were particular concerns with a particular patient), there was, nevertheless, an ongoing shared interest in how patients were getting on, with monthly meetings between the rehabilitation and the clinical teams to discuss patient outcomes as a cohort. At this stage they also had strong links with activity services in the community where they would typically discharge patients for ongoing support across each of their localities, ‘so we’re lucky enough to have that as well, as an exit strategy’. The surgery school described by another participant was presented as also fostering those relationships between colleagues; it was designed as an enhanced MDT preoperative event, ‘it does give you a chance to bring people together, so I think that’s important’, and it also provides an opportunity to meet patients, as some clinicians might not typically meet a patient until the morning of their surgery.
Some participants described more limited contact between their services and clinical teams. This was partly related to high numbers of self-referrals, but even for those referred by a clinician, unless a clinical need became apparent, or ‘something arises as part of their duty of care’, they described no natural lines of communication with clinical practitioners. One participant talked about this as an area in which they had ‘struggled’ and in which they ‘would like to see improved relationships . . . I suppose it works both ways . . . should health-care professionals be more interested in patient outcomes . . .’. Another described their relationship with clinical settings, as ‘quite poor’. They gave an example of the mixed messages they felt could result from this lack of collaboration, describing people who were receiving chemotherapy being encouraged to consider completely different diets depending on which service they were talking to.
On the occasion that a collaborative relationship had been there (usually when personal contacts were involved) ‘that feedback, backwards and forwards, it probably has led to some really good things happening’. The participant gave the example of someone really resistant to joining:
he had a slight little bit of interest in our programme, but still didn’t want to join, and it wasn’t until a year down the line, and it was only because of the backwards and forwards between myself and his nurse, that we managed to encourage him to come along. And then he came and he did the whole 12 weeks and he absolutely loved it and just carried on ever since, like joining in with things. So that, it clearly worked and it was because of the relationship that we had, but we don’t have enough of those relationships to mean that that unfortunately happens across the board.
They did, however, talk about beneficial relationships with ‘trusted partners’ across the city, including PA providers and local authority leisure centres, describing ‘better outcomes’ in the places where those relationships were good.
More broadly in conversations, there was a general recognition of the difficulties in linking general practice and primary and secondary care, of systems that ‘don’t talk to each other’, and being all ‘quite disjointed on the shop floor’. One service had been working with their local IT team to address some of the difficulties with traditional software in terms of monitoring patients and linking attendance at classes with their wider treatment record and primary care record. This was described as being ‘developed all the time’, ‘we’re still kind of in the process of figuring out the best way to do that’. In another service, software had managed to tie patients into their programme as part of a referral to MDT appointments. There was some suggestion that this was partly because the first step into the service was through surgery school, which had been framed to the trust as part of that MDT process. It was not clear if treatment and outcomes were also tied into this system (although a structured system of meetings seemed to be in place to also do this).
Referrals generally were talked about as coming from physiotherapists; consultants; cancer nurse specialists; GPs; and, for some programmes, from patients themselves. One participant talked about a steady increase in referrals from cancer nurse specialists, and felt that this demonstrated their success at ‘trying to embed the exercise part of their treatment into the cancer pathway’. There was some discussion among services as to the benefits of starting the discussion about raising activity levels in general practice. That time between a patient first presenting at a GP’s surgery and being referred to specialist care was seen as ‘quite a gap’, ultimately leaving sometimes only a short period of time to work with a patient prior to their surgery or beginning of treatment. One participant suggested that it ‘would be really, really positive’ if a ‘real point and effort is made to start to sow that seed’ with the GP. They saw this as an opportunity for people to begin to increase their PA levels, allowing for patients to make more headway before their surgery. Another participant talked about trying ‘all sorts of different ways when you go a bit earlier, to try and get some more time’, but explained that sometimes you would then have ‘people coming into the gyms who didn’t have a diagnosis. And they’re like, ”well why am I here’ sort of thing” ’; this seemed to be seen as a disruption to other patients. They also talked about spending quite a lot of time early in their programme’s development ‘talking about how we could communicate with GPs, getting them starting to manage things’; they explained that it was an area they planned to revisit and they maintained a GP as part of their broader team. There was some hesitation more generally from participants that, for cancer patients particularly, a referral direct from the GP would be more complicated: one participant described being ‘a bit worried’ that direct referrals at the point of a cancer query would be a significant workload for GPs, and another commented that ‘there are so few actual diagnoses of cancer in comparison to the amount of people presenting at a GP referral . . . from our service point of view, we would be absolutely over-run.’
However, there was agreement that, for all patients in surgery or potential cancer pathways, the period when ‘they are just waiting, they are just waiting for something to happen’ could be capitalised on and ‘certainly the message could definitely be sold by GPs then’.
Funding
Services generally began with some form of charitable or grant funding, including from cancer research charities, The Health Foundation, the Wellcome Trust and Sport England. Most services remained on short-term funding grants or contracts, with some describing the frustrations of funding that was often ‘piecemeal’ or ‘pots of money’ that would go ‘nowhere near actually delivering a service’. However, one service had been regularly funded for almost two decades. Participants acknowledged how ‘lucky’ they were, seeing this security as able to support longer-term planning, as well as being ‘massively important’ for the development of, and investment in, their staff, ‘so that you can lift them up, in terms of qualifications and experience’.
Despite frustrations, smaller pots of money were often seen as ‘a platform’, enabling services to get ‘off the ground’. One participant talked about ‘the reality’ that NHS trusts do not have money or the resource ‘to throw at things like this’, even if it is ‘the right thing to do for patients’. They described as ‘incredibly helpful’ an initial 2-year period of charitable funding that gave their service an opportunity to demonstrate what they could do, explaining that ‘it’s very hard to convince people in power, or people with the resource, that you can do this, and that it works’.
Although participants from one service, which was collaboratively funded with a local public health team, talked about flexibility in how its funding was spent, funding tended also, at least initially, to be fairly prescribed by funders: ‘money for data collectors’; or attached to objectives, such as ‘improving fatigue levels’; or service evaluation looking at specific patient outcomes (including physical and psychological markers); or the impact of the service across a trust. Some also talked about funding for a website, branding or pieces of public engagement work. Only one service described being explicitly funded to increase PA.
Services often talked about commissioners as ‘trusted partners’ or as ‘absolutely essential’. However, these relationships, or their ‘buy-in’ or interest had often taken considerable efforts to develop, requiring ‘a huge amount of work’ and a lot of perseverance, as well as a focus on trying to ‘prove’ what services were doing and find different ways to engage commissioners (as well as senior management) in their programmes.
Key staffing roles
Core team
-
Physical trainers (employed within and outside services).
-
Physiotherapists.
-
Oncology specialist physiotherapist (making assessments and supporting exercise).
-
Exercise scientists.
-
Nurses.
-
Allied health professionals.
-
Administrative staff/receptionists.
Clinical champions
-
Cancer nurse specialists.
-
Care co-ordinators.
-
Anaesthetists.
-
Surgeons.
-
Oncologists.
Broader collaborators
-
Pharmacists.
-
Dietitians.
-
Clinical psychologists.
-
Data teams.
-
Local gyms.
-
General practitioners (including trainees).
Data and outcomes
Some services started out as quality improvement projects, with data collection and monitoring an integral part of their work from the beginning. Others had the need to gather data to evaluate their processes and outcomes, partly to help convince clinical and managerial colleagues of the utility of the service, but also to help secure further funding.
One service was described as being ‘heavily monitored’ on its recruitment of patients and clinical outcomes prior to surgery. More generally, data that appeared to be seen as important included objective physiological measures, such as the 6MWT, the sit-to-stand test, the grip strength test; more global measures of disability (such as the WHO Disability Assessment Schedule); and measures related to efficiency of care; and length of postoperative hospital stay was a primary outcome for one service, whereas, for another, it was fatigue. Some services talked about well-being scales, such as the Warwick–Edinburgh Mental Wellbeing Scale, or other questionnaires that included dimensions of health, such as anxiety and depression (EQ-5D-5L and Hospital Anxiety and Depression Scale). Service-based measures including recruitment and attendance at sessions were also collected and some services talked about attempts or hopes to link this to patient records, although software challenges or systems not set up to recognise their service or its objectives or outcomes appeared to present barriers to this. Participants from several services talked about the difficulties for data systems to interact between primary and secondary care, with repeated comments around the benefits of being able to easily share patient engagement and improvements, as well as ‘monitoring patients’ conditioning’, with clinicians across clinical services. Participants acknowledged an increasing interest in measuring long-term benefits to patients, for example 2-year outcomes for cancer patients, as well as collecting quality-adjusted life-years (data that combine patient quality of life with health economics).
Although one participant talked about using the IPAQ self-reported PA measure, most services did not collect formal data on patients’ level of PA; however, participants from one service talked about self-efficacy for exercise as an important measure, explaining that ‘somebody with a higher self-efficacy for exercise’ tends to be more likely to continue exercising independently. Overall, data were usually collected at initial assessment and again when leaving provision; some were monitored throughout.
Participants frequently mentioned the importance of patient feedback, with one commenting that it was these ‘real-life stories that bring the data to life’. Often it was talked about as something commissioners were particularly interested in seeing: ‘they want to know the patient impact, and the patients say that themselves, and they’re the best at saying that’.
Video testimonials were described as being useful for engaging both other patients and health professionals: ‘you know, the best people to talk about our service isn’t someone like me, it’s actually people that have been through it’. Measurement of patient experience included small patient engagement projects, localised surveys (including a focus on venues and resources) and standardised patient-reported outcome data using questionnaires such as the Patient-Reported Outcome Measures.
Several services were involved in formal service evaluations of their provision, including some degree of health economic evaluation, with some services expecting these to include comparative elements reflecting the in-person versus remote adaption models.
Agendas
Several participants commented on local or regional initiatives that made possible, or enhanced, their approach. One participant talked about a regional agenda to ‘bring movement into people’s lives’, a collegiate partnership across Greater Manchester (GM Moving), with initiatives including active travel. They described this ‘whole-system thinking’ as helping to focus people’s mind on moving more, and how they felt that this helps; in the context of their service, patients’ could see more broadly that activity was being normalised and ‘reimagined’. ‘So then when you suggest more physical activity or exercise to them, “oh yeah I know that, I’ve got one of them live by walks near my house”. And so that’s helping and driving a lot in Greater Manchester’. The same regional approach was talked about as supporting the leisure industry to get more people having, and being ‘confident’ to have, conversations with people about their health diagnosis, to notice if ‘somebody’s struggling’, to signpost them to appropriate support. One participant framed this as ‘upskill[ing] the receptionist, the lifeguard, the level 2 gym instructor, the duty manager’ and talked about currently working with training organisations to deliver continuing professional development courses: ‘one is on cancer, one will be about musculoskeletal conditions so that focus, for example, of getting Stockport more active, more healthy, doesn’t just sit on a team of eight people’.
There was also a suggestion that broader agendas or collaboration can make more ambitious projects more feasible. One participant described as ‘fortunate’ that, at the same time as they were developing their provision, the local public health team was prioritising a wellness approach, setting up a city-centre space, which they described as ‘a beautiful facility’ with good gym facilities; an exercise studio; and access to smoking cessation teams, drug and alcohol teams and counselling services. This supported an approach that rooted PA within both a broader perspective on individuals’ health and a local priority; the challenge, they said, was replicating this in other areas.
Another participant talked about taking any opportunities they can to get involved in broader campaigns, research and just talking to people about their work. Several talked about ‘tying in’ with Public Health England initiatives, such as PA champions, with one describing a YouTube campaign, but there was also a view that services were ‘further ahead than what is being pushed by the national agenda’. Although participants acknowledged that people are now starting to see the value in their programmes, one participant described this as ‘a little bit frustrating’, particularly when it came to funding: pots of money to bid for that would go nowhere near actually delivering a service:
it might, you know, go somewhere to creating some leaflets to give out, it might go to, you know, an hour’s worth of staff training across the trust, but it feels like that’s a bit small-fry to actually putting something in place to treat patients in this way.
One participant talked about cultural aspects to exercise and PA, and health perceptions that can be completely at odds with the approach they are taking. They suggested having cultural champions as a way of bridging that gap:
there are areas where we might need that, I think that would be something to think about as well . . . There will be some people who would want to take up that kind of role . . . where they have like community centres and things if we have to make it popular, like X was saying. Just gently sow the seeds and I think those could be places we could start.
COVID-19
COVID-19 disrupted services across the board, including service evaluations, with services seeing staff directed elsewhere and participants seeing many of their colleagues furloughed, meaning that vital administrative resources were no longer ‘to hand’. However, often referenced was the learning that had resulted from the experience of adapting and providing care during this period: ‘we’ve done and learnt from it’, ‘it’s allowed us to take a step back from, look at our delivery models, and actually change them for the better’. It seemed to have presented an opportunity for services to consider and explore new ways of working with patients.
All services, to varying degrees, continued contact with patients during the period. Participants described a ‘great deal of consideration’ and ‘planning in adapting’ to the pandemic environment and ever-evolving provision to keep referrals open, keep supporting patients and transform provision to a remote delivery model. Several commented that, in shifting delivery, their focus remained on ensuring that they could produce a new model that was ‘acceptable’ to patients.
A participant from one service explained that it had quickly moved to an online timetable, but talked about focusing sessions primarily on those they felt were most negatively affected by the pandemic, in terms of risk and isolation, giving the example of ‘people that might be more afraid to go out because of their medical condition’. When lockdown restrictions had allowed, they provided one-to-one walk-and-talk sessions, using local green spaces, parks and outdoor park gym equipment; this was a new approach that they hoped would continue, although probably as group sessions, moving forward.
In one general practice, trainees had compiled a list of online and community-based activity resources, as well as books, for those with no access to the internet. They also described that, because of the way appointments were being managed, many of them virtually, via e-mail or on the telephone, it was actually easier to bring in discussion of PA: ‘it’s a bit more fluid in terms of time, so actually, I think if we could get organised, and remind people, then there’s no reason . . . Because before we were so quick, you just really didn’t have time’.
Another participant talked about initially focusing on WHO guidelines, ‘that every minute of exercise and activity counts, trying to give them small, fun things for them to do’. They talked about the ‘ad break’ and the ‘kettle boil’ challenges, breaking down full exercise sessions into smaller parts and posting activities on social media and on their website and YouTube channel, providing people access to an online exercise diary and some virtual group interaction. They described this as ‘somewhat a success . . . patients accepted that that’s what needed to happen’ and although it was not ‘something for everybody, I think a lot of people are accepting that, right now, it’s better than nothing’.
Despite maintaining this contact, the same participant talked about seeing a sharp deterioration in fitness after the first lockdown: ‘the regression of the patient’s fitness was astonishing’. They described, when starting to deliver classes again, having to ‘completely redo the timetable . . . and go right back to the beginning. Although they’d adhered to what we were asking them to do, it just wasn’t as good; it just wasn’t as effective.
During the second national lockdown, they described being ‘incredibly fortunate’ that, with the exception of those who were clinically extremely vulnerable, they had been able to keep their in-person classes running, and also deliver the majority of one-to-one assessments face to face, with a small number on the telephone, and have a full timetable of 26 sessions a week at > 70% capacity:
That’s because we’ve been quite successful in convincing our trust that this is part of normal cancer care and its treatment, not an add-on. So, kind of, with the national drive that all medical services should continue, we’ve been able to continue, which has been a huge, huge relief to lots of the patients who were using the service.
In another service, although having subsequently developed a virtual offer (beginning after the study period), they expressed disappointment that they had not been able to maintain full delivery of their service for their existing cohort (this under the direction of their commissioner and clinical colleagues): ‘It’s a shame because we’ve managed to do virtual sessions for other programmes that we offer’. For those already enrolled on their programme, they were able to continue to offer support through telephone conversations, e-mails and some virtual resources to support people to remain physically active, as well as a link to the broader provision, such as a citywide walking challenge, of the organisation.
Although several services talked about efforts to ensure that there was an offer for everyone, regardless of the sorts of devices or internet access people might have, there was generally a heavy reliance on technology, mobile telephones, computers, devices for video contact and the internet, with services using Microsoft Teams, Zoom and Attend Anywhere (Attend Anywhere, Melbourne, VIC, Australia) as the main platforms for video sessions. Some participants particularly emphasised how not having access to the technology ‘definitely hindered some people’; there were repeated discussions around the influence of deprivation and of technology literacy, with one participant describing their team as becoming ‘part-time IT consultants’. Several people commented, however, that many of the technical barriers had reduced as time had gone on, with one feeling that ‘older people [were] getting more and more used to using Teams, talking to their relatives and stuff’, and another that ‘older people and relatives have joined up and learnt technology’ in a way that, prior to COVID-19, ‘we could not have imagined’.
One service, talking about how they had tried to work around these barriers by providing ‘the full spectrum . . . zero technical ability . . . all the way up to using Microsoft Teams for online class.’ For those that had been deemed ‘technically deprived’ (a high proportion of their patients), people would receive home exercise packs, diagrams and descriptions, and resistance bands in the post, and then coaching sessions on a telephone. These were described as ‘a bit weird to get used to’ and ‘massively labour intensive’, but something they now ‘quite enjoy . . . it’s good fun, we’re enjoying it’. For others, they had a timetable of 15 online group classes each week, and, for some, they used Myzone chest belts (digital heart rate monitors), whereby trainers were able to live-track a patient’s heart rate during sessions. This enabled exercise trainers to visualise up to 10 people at one time doing an activity session, to see people ‘being active and how much’ and to say, ‘ “you can go a bit harder there”, “do a bit more”, that sort of thing’. This was described as a ‘really good’ approach for those people who ‘really like the digital home-based approach much more than the face to face’. It did rely on patients having a smartphone or computer. They also talked about a YouTube channel with follow-along exercise videos. The participant also described still being able to carry out much of their data collection, including sit-to-stand tests, remotely, ‘and it is showing that the improvements are still being made, the sit to stands are still being increased’.
Services generally felt that their adaptions had been ‘successful’, ‘the virtual challenges that we’ve done to engage people have been positive’, ‘we’ve helped mitigate their health . . . sort of well-being and their health actually deteriorating as much’; one participant suggested that this showed that ‘remote rehab[ilitation] can be done’. Several participants suggested that virtual delivery had worked better for people who were already on a programme or known to the service, with one participant describing referrals dropping ‘substantially’ and those that they did get, ‘people tended to want to wait until facilities had reopened’. In another service, a participant felt that:
from talking to people and patients . . . [that patients] were able to engage [with their service] better because they knew us and they had been there physically in person, first . . . I think had we just been delivering remote programmes from the off, it would have been very, very difficult to get the same level of engagement that we had with patients that we knew and we had regular contact with.
A drop-in attendance was also attributed to working with people in areas of high deprivation where people were marginalised with regard to access to the technology and/or the internet. One participant, however, described engagement as ‘really good’ from new and existing patients, the conjecture being that patients had responded well to the varying degrees of technicality/use of technology that they offered: ‘nobody is excluded and I think that’s why the engagement rate is so high’. Another participant talked also about remote sessions as being:
really useful for people that maybe had caring responsibilities, where in the past they would feel guilty about, or not able to leave someone to go and be active, they could actually do it, because they were at home, or the person they were caring for was able to, in some way, to join in. So it’s definitely been, definitely found . . . it’s been useful in some way to engage those that maybe previously would struggle to engage.
Despite mixed experiences in continuing provision, there was mutual agreement across discussions that services had been ‘almost a lifeline’ for people throughout the pandemic:
What it’s meant to people, particularly during COVID, that’s been massive, you know not having anybody, and in the gym people have filled this gap; their virtual system. They’ve had more than 600 people on their books during COVID just virtually looking after them, with weekly telephone calls and all that sort of stuff’.
Another participant talked about their provision as ‘positive’ because it is some of the only contact that ‘[people are] actually having with anybody throughout the week’. Another spoke of ‘the amount of testimonials’ they had received, from ‘people saying how much it was a lifeline for them, just to have that social interaction with people, never mind the exercise, over that COVID period, it was really, really beneficial’. In another service they talked about ‘one of the big things’ that they had found was ‘that we’re part-time exercise specialists, part-time IT consultant and part-time counsellors because there’s a lot of well-being that is needed at the moment’. They felt that this had been ‘compounded by the fact a lot of well-being services shut down . . . because they couldn’t offer face to face, and they were slow on the digital take-up, and the telephone take-up, and a lot of their staff was furloughed’. This was backed up by another participant, who talked about patients’ WHO Disability Assessment Schedule well-being scores being at ‘a lot higher baseline than they were before COVID . . . we’re still making the improvement, but what is very concerning is our baseline is so much worse than it was, pre COVID’. They attributed this to ‘peoples’ anxieties’ being ‘a lot higher . . . and people are deconditioning because they’re not just doing their daily active stuff. So when they’re asked if they are finding things difficult, they are’.
All participants acknowledged the opportunity the pandemic context had provided in allowing them to explore new ways of doing things. Although one participant said, ‘ideally, I think we are going to come out of this, we are going to get back to some sort of normality’, most participants indicated a hope to move forward with some form of a ‘hybrid model’. One participant talked about their virtual offer being ‘something we were wanting to try and do anyway, but with the businesses and everything else that was going on we never really got round to kind of offering it’. Another participant explained that they will ‘quite strongly argue that we will be a blended team, that we will do face to face and technology as well’, highlighting how a virtual offer would work particularly well for people who are on chemotherapy and radiotherapy, who ‘find it very hard to travel out into the day’ and would ‘really benefit’ from being able to log on at home. Another was interested to see how things would evolve, ‘whether the online stuff will stay as popular as it is, or whether people will just crave that social interaction and that face-to-face stuff . . . the remote aspect might not be . . . as big as it is now’.
In another service, they felt that they might continue a virtual offer, but only in certain areas:
with some services, we’ve basically people begging us for turning things back to normal and back to face to face. They just cannot stand the virtual stuff. But I think in other areas it’s made us take a step back from, looking at our delivery models, and actually change them for the better and add in that, we can do a consultation, and we don’t need to do it face to face with somebody, we can actually do it virtually if, if them accessing that consultation initially is the barrier to them then coming along to a programme, like. We can still do that using a virtual method. So I think there’s elements of it that we will definitely keep. I can’t say that [we] will probably keep a huge amount of it in terms of actual delivery because I just think for the people we work with, I don’t think it is the optimal method to, to get the best outcomes from them.
One participant suggested that initial telephone contact ‘might actually engage somebody as a first step where before they wouldn’t have engaged at all. Because the thought of coming to a facility, to do their initial consultation, might, might have actually been a barrier’, adding they ‘definitely hope to offer that’.
Several participants commented on how they felt that COVID had ‘reinforced the importance of exercise’, ‘ ’cause we’ve been allowed out for an hour every day for 6 months, haven’t we, to exercise’ and that, now more than ever, it was such an ‘important time now, you know, to really push this forward’.
Surgery
Participants suggested that patients’ priorities or motivations might vary, and, depending on their condition, they ‘might be slightly at odds with a clinical priority focused on physical activity’, as one participant explained, ‘it’s where that person is in terms of their mindset at that point in time’. That seemed to be particularly the case for cancer patients, and there was general concurrence across the discussions that there was perhaps a unique moment when patients might be in the early days of a diagnosis, facing particular challenges and perhaps holding particular motivations.
There was also awareness that, for cancer patients, at the point of diagnosis, it can be ‘information overload’, with patients overwhelmed by ‘stuff to read’; one participant described patients sat in front of them, sometimes not sure, or not sure in that moment, why they are there, and explained that they ‘try to be quite matter of fact . . . it is good for them . . . will help their recovery, it will help to combat the effects of treatment’.
Cancer patients often came with the recognised benefit of a care co-ordinator, highlighted by one service as a ‘highly valued’ bridge between patients, clinical teams and their programme. Although much of their role might be administrative, the care co-ordinator’s knowledge of a patient’s treatment plan, and their contact with the patient, was seen as a unique position from which they could begin to prepare patients for, and sometimes ‘sell the benefits’ of, the programme. The care co-ordinator’s wider contact with a patient’s cancer nurse specialist and clinical team helped hold together a perception of provision as one comprehensive pathway. In one discussion, participants suggested that the same relationship could be encouraged for other surgery pathways, by either utilising pre assessment or considering medical secretaries or staff in bookings or administrative team who could be trained to introduce ‘those ideas’.
Appendix 8 Summary of risk of bias assessments
| Study | Selection bias | Performance bias | Detection bias: blinding of outcome assessment | Attrition bias | Reporting bias | Other bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | PA outcomes | Fitness outcomes | Participant-reported outcomesa | Adverse events | Adherence | Incomplete outcome data | Selective reporting | ||
| Archer et al.51 | |||||||||||
| Artz et al.52 | |||||||||||
| Baillot et al.53 | |||||||||||
| Barberan-Garcia et al.54 | |||||||||||
| Barnason et al.55 | |||||||||||
| Boesch et al.56 | |||||||||||
| Bond et al.57 | |||||||||||
| Brandes et al.58 | |||||||||||
| Cadmus et al.59 | |||||||||||
| Carnero et al.60 | |||||||||||
| Christiansen et al.62 | |||||||||||
| Christiansen et al.61 | |||||||||||
| Courneya et al.63 | |||||||||||
| Creel et al.64 | |||||||||||
| Demark-Wahnefried et al.66 | |||||||||||
| Duculan et al.68 | |||||||||||
| Eakin et al.69 | |||||||||||
| Engblom et al.70 | |||||||||||
| Foster et al.72 | |||||||||||
| Goedendorp et al.74 | |||||||||||
| Golsteijn et al.75 | |||||||||||
| Hackshaw-McGeagh et al.76 | |||||||||||
| Hauer et al.77 | |||||||||||
| Hawkes et al.78 | |||||||||||
| Heiberg et al.79 | |||||||||||
| Hoorntje et al.81 | |||||||||||
| Hubbard et al.82 | |||||||||||
| Husebø et al.83 | |||||||||||
| Ilves et al.84 | |||||||||||
| Jiménez-Loaisa et al.85 | |||||||||||
| Johansson et al.86 | |||||||||||
| Jolly et al.87 | |||||||||||
| Kinsey et al.88 | |||||||||||
| Kraal et al.90 | |||||||||||
| Kummel et al.91 | |||||||||||
| Lear et al.92 | |||||||||||
| Li et al.93 | |||||||||||
| Lier et al.94 | |||||||||||
| Lindbäck et al.95 | |||||||||||
| Losina et al.96 | |||||||||||
| Lotzke et al.97 | |||||||||||
| Mundle et al.100 | |||||||||||
| Painter et al.101 | |||||||||||
| Piva et al.102 | |||||||||||
| Santa Mina et al.103 | |||||||||||
| Sellberg et al.104 | |||||||||||
| Smith et al.105 | |||||||||||
| Stolberg et al.106 | |||||||||||
| Taraldsen et al.107 | |||||||||||
| Turunen et al.108 | |||||||||||
| Turunen et al.109 | |||||||||||
| Van der Walt et al.110 | |||||||||||
| Yates et al.111 | |||||||||||
List of abbreviations
- 6MWT
- 6-minute walk test
- BMI
- body mass index
- CABG
- coronary artery bypass graft
- CBPT
- cognitive–behavioural physical therapy
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- DVD
- digital versatile disc
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FACT
- Functional Assessment of Cancer Therapy
- FI
- financial incentive
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- GSLTPAQ
- Godin–Shephard Leisure-Time Physical Activity Questionnaire
- HOOS
- Hip disability and Osteoarthritis Outcome Score
- HRQoL
- health-related quality of life
- IPAQ-SF
- International Physical Activity Questionnaire-Short Form
- IQR
- interquartile range
- IT
- information technology
- KOOS
- Knee injury and Osteoarthritis Outcome Score
- MD
- mean difference
- MDT
- multidisciplinary team
- MET
- metabolic equivalent of task
- MVPA
- moderate-to-vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NRS
- non-randomised study
- PA
- physical activity
- PAL
- Physical Activity Level
- PCS
- physical component summary
- PPI
- patient and public involvement
- Q-RCT
- quasi-randomised controlled trial
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SMD
- standardised mean difference
- THC
- telephonic health coaching
- TKR
- total knee replacement
- TUG
- timed up and go
- VAS
- visual analogue scale
- VO2 peak
- peak oxygen uptake
- WHO
- World Health Organization
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/NZPN0787).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
